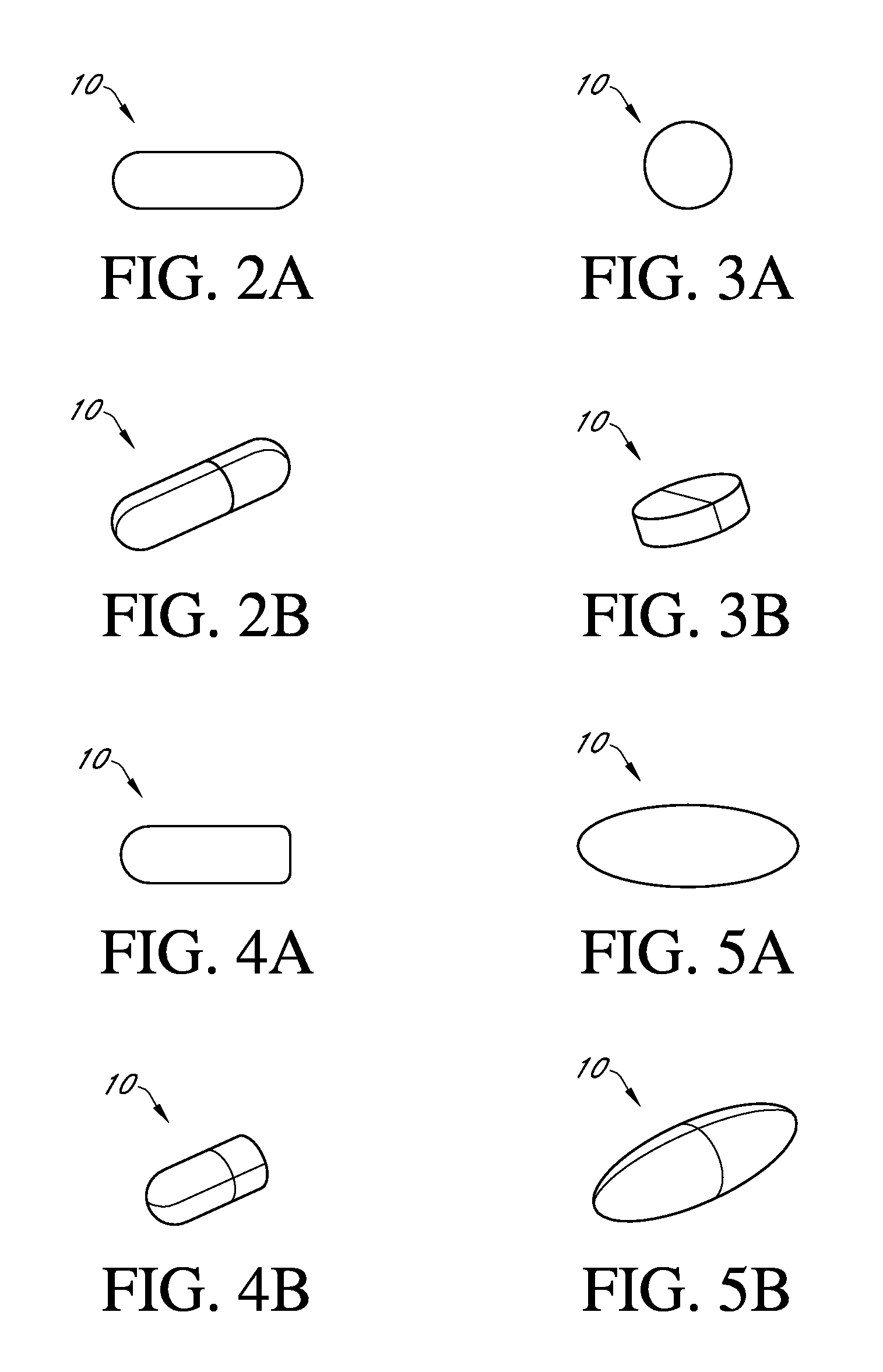
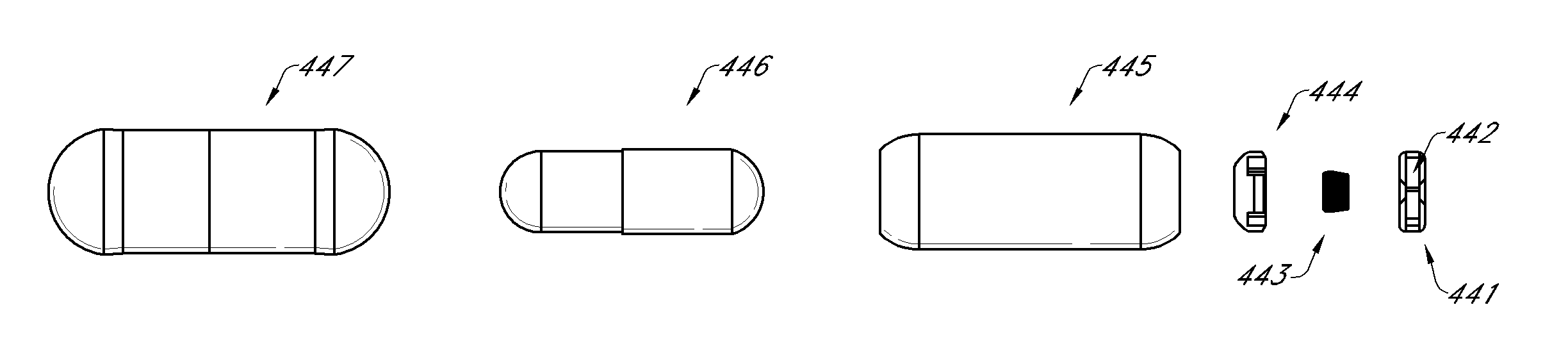
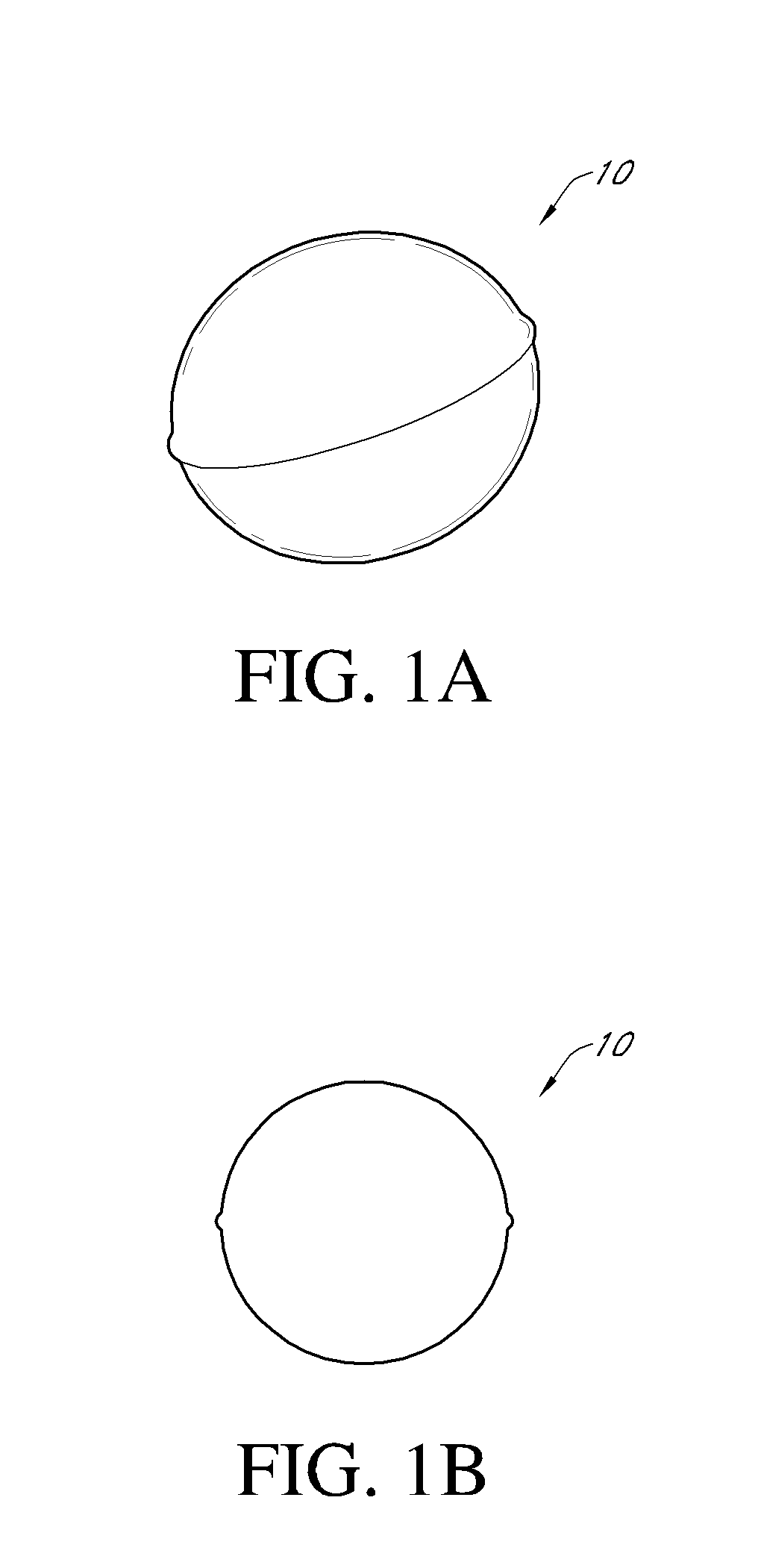
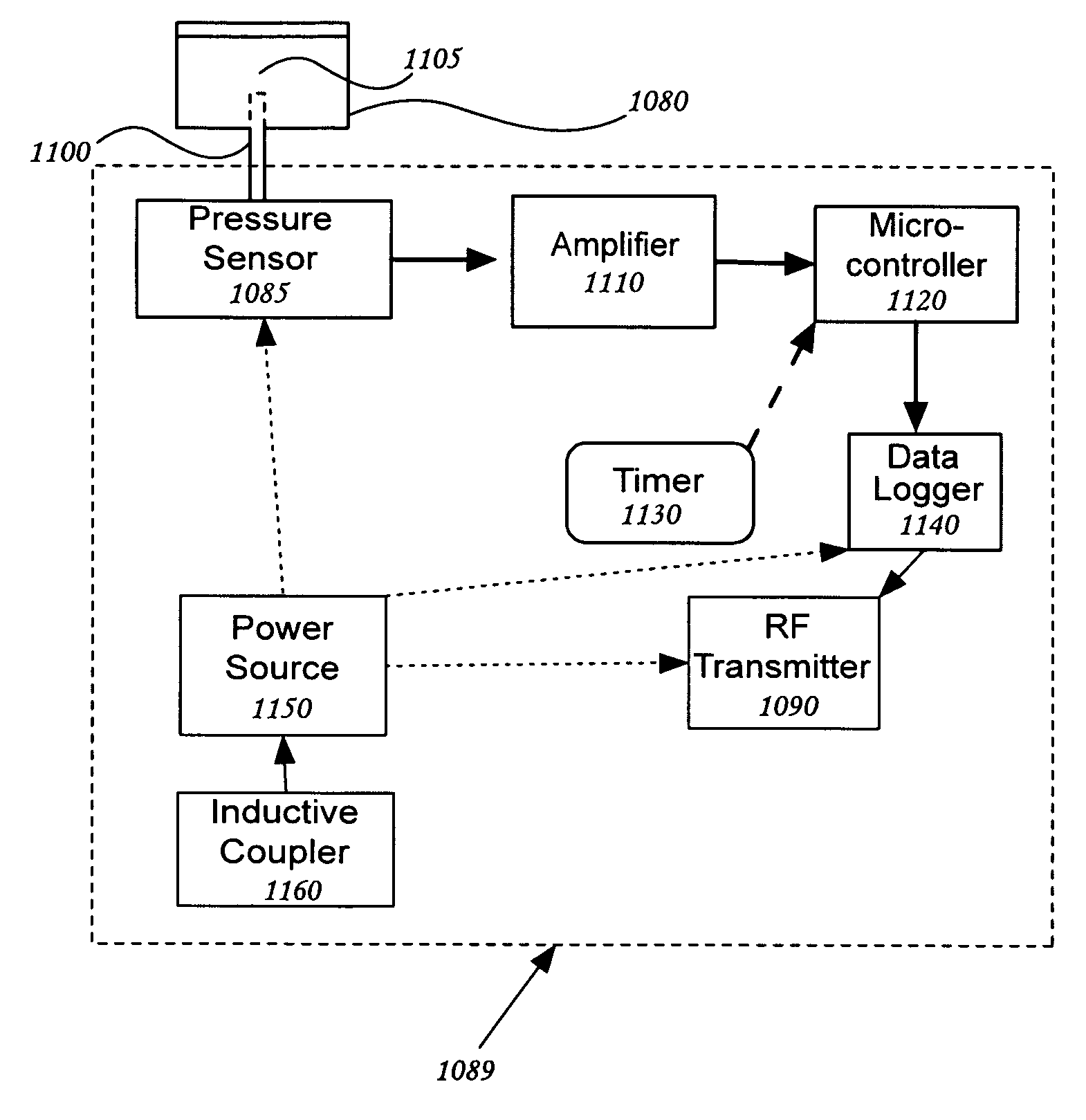
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1617 results about "Ingestion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ingestion is the consumption of a substance by an organism. In animals, it normally accomplished by taking in the substance through the mouth into the gastrointestinal tract, such as through eating or drinking. In single-celled organisms, ingestion can take place through taking the substance through the cell membrane.
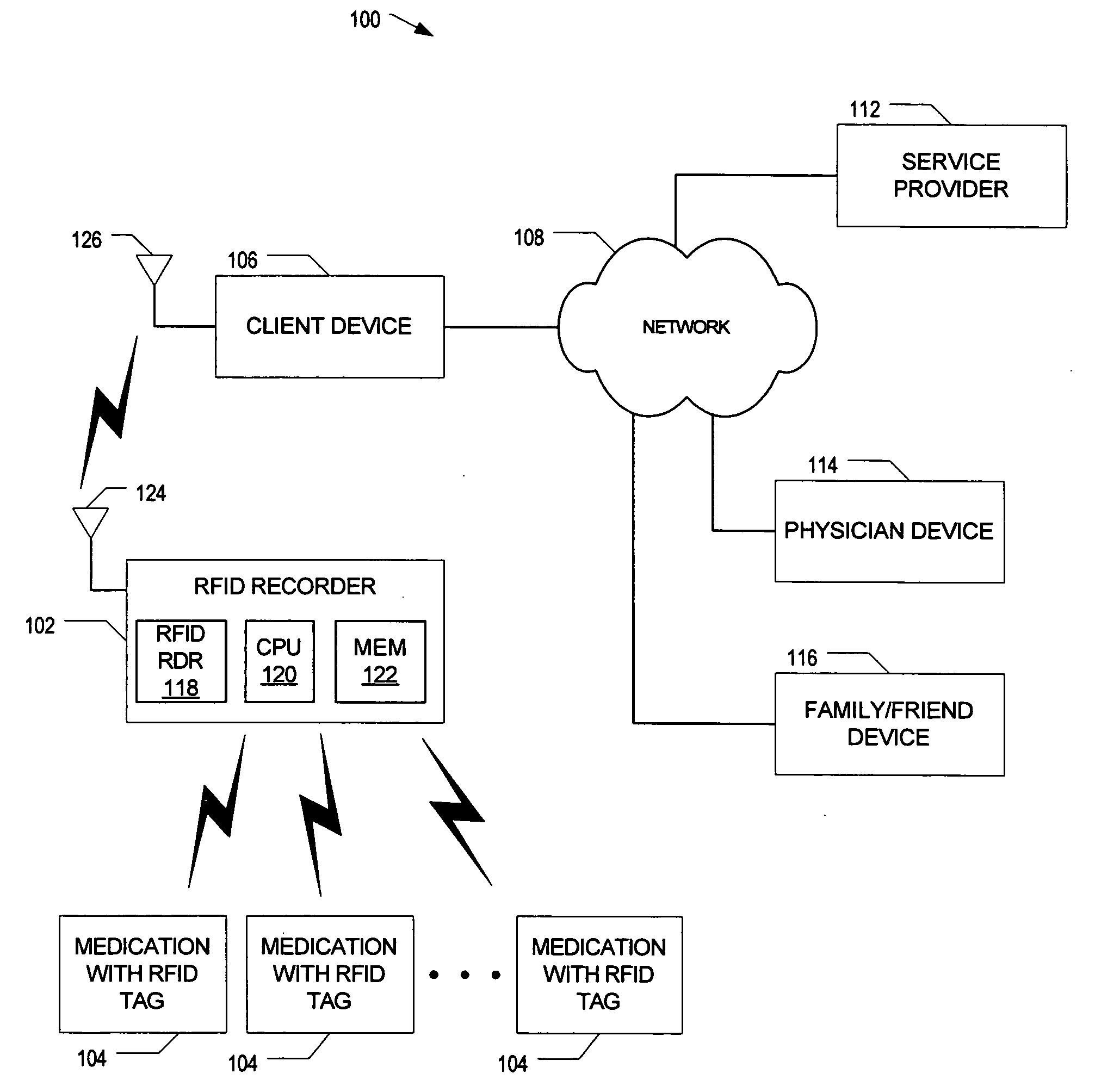
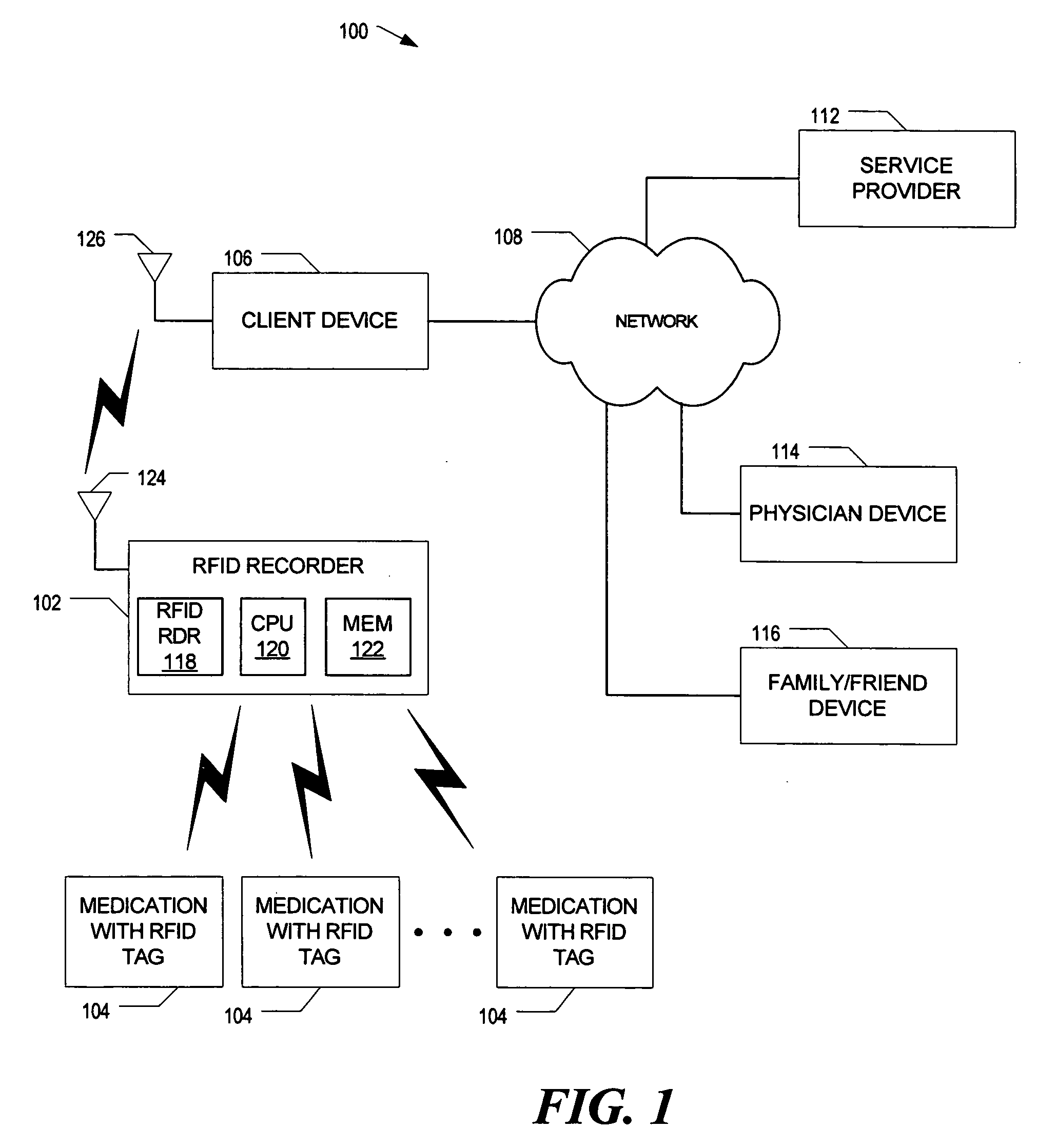
System to monitor the ingestion of medicines
InactiveUS20070008113A1Easy to manageEasy to testDigital data processing detailsEndoradiosondesComputer hardwareRadio-frequency identification
Owner:CARESTREAM HEALTH INC
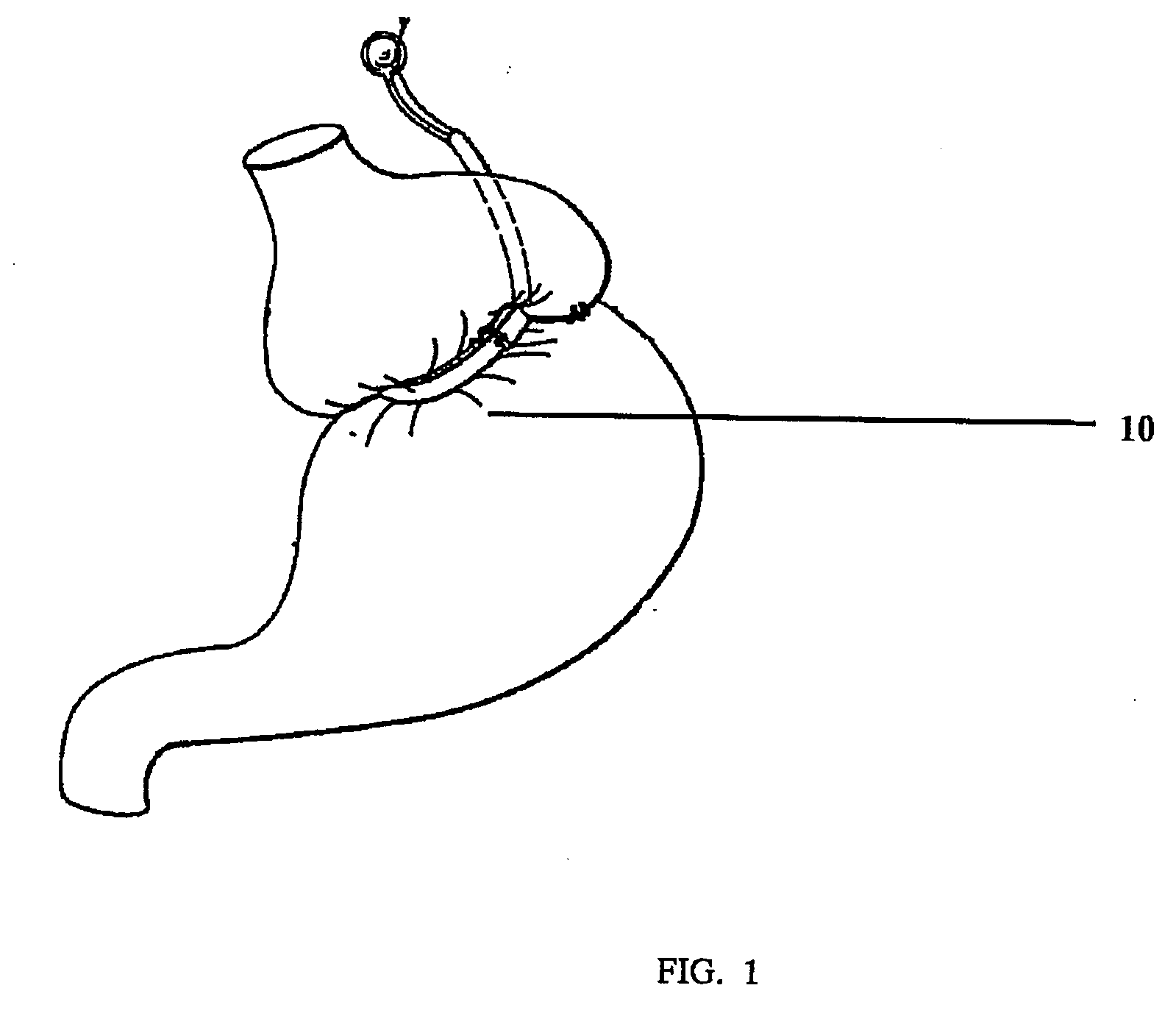
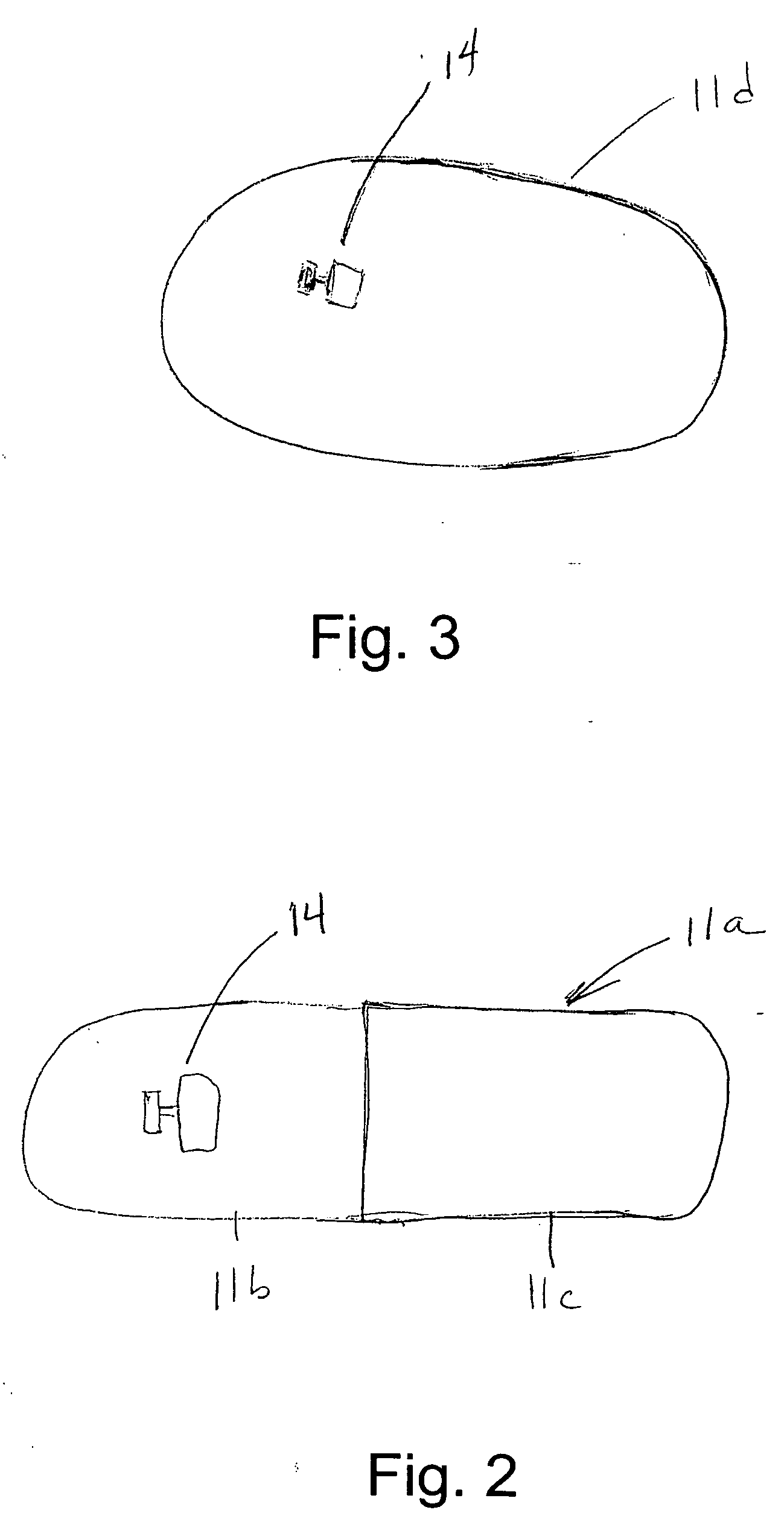
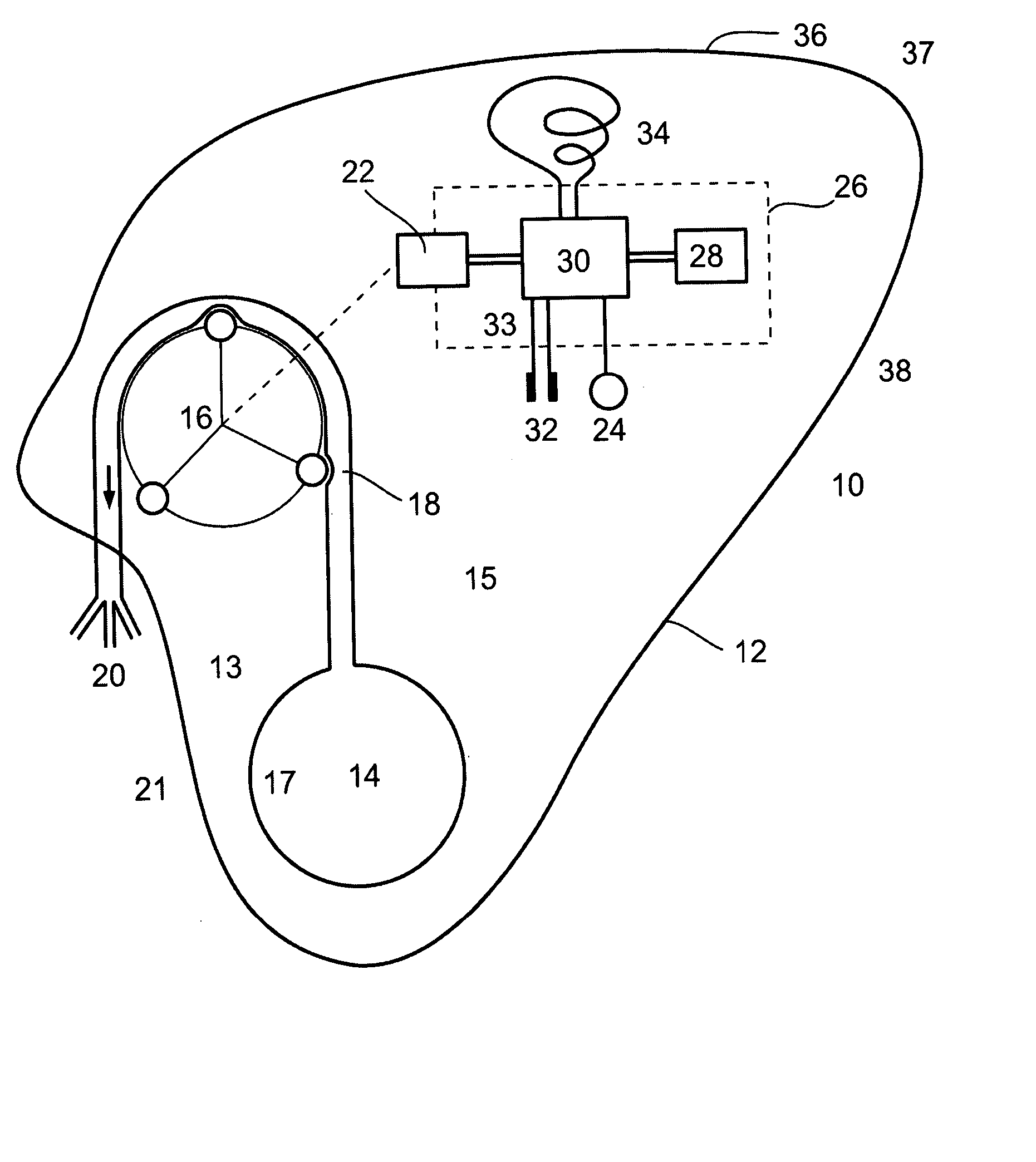
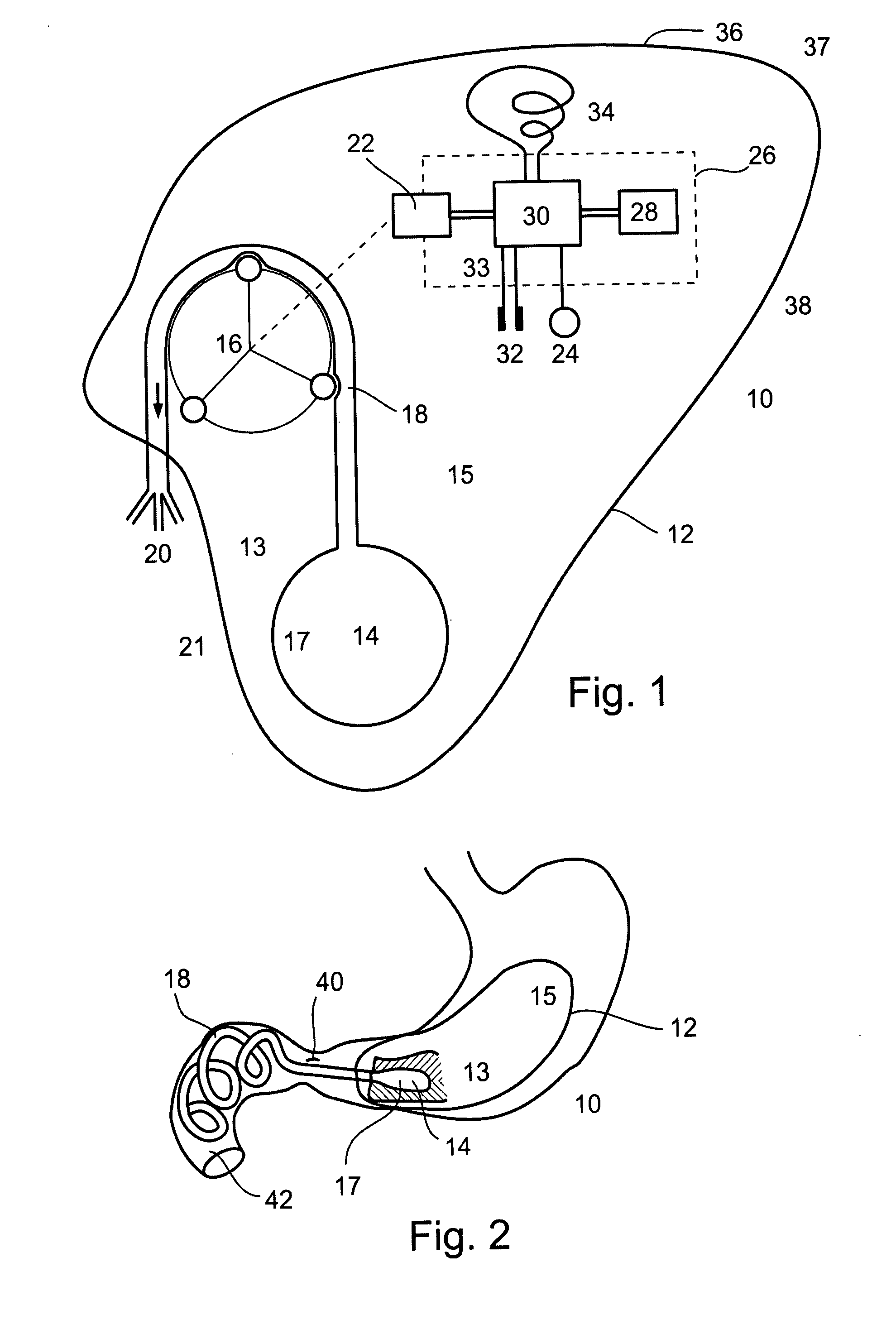
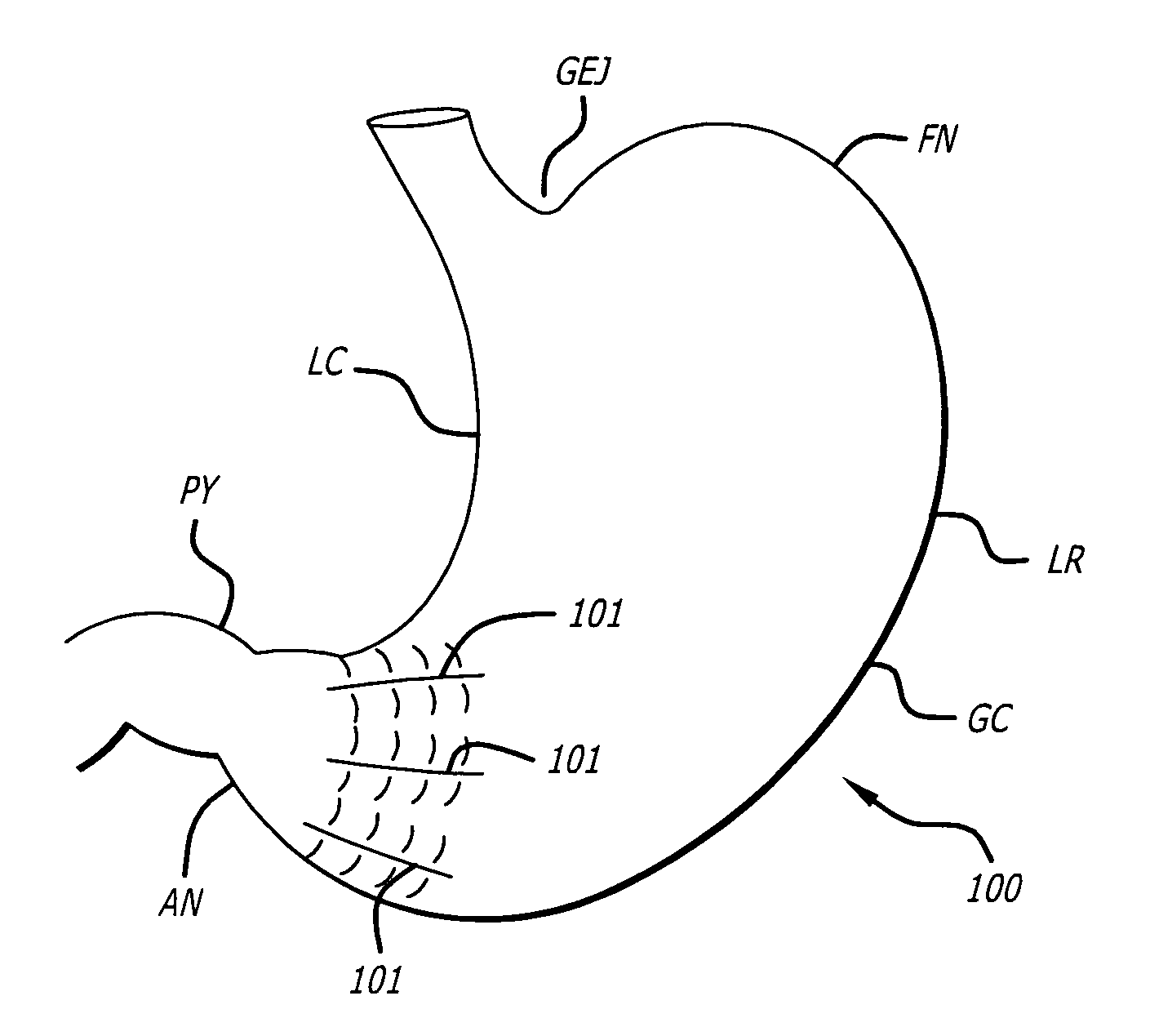
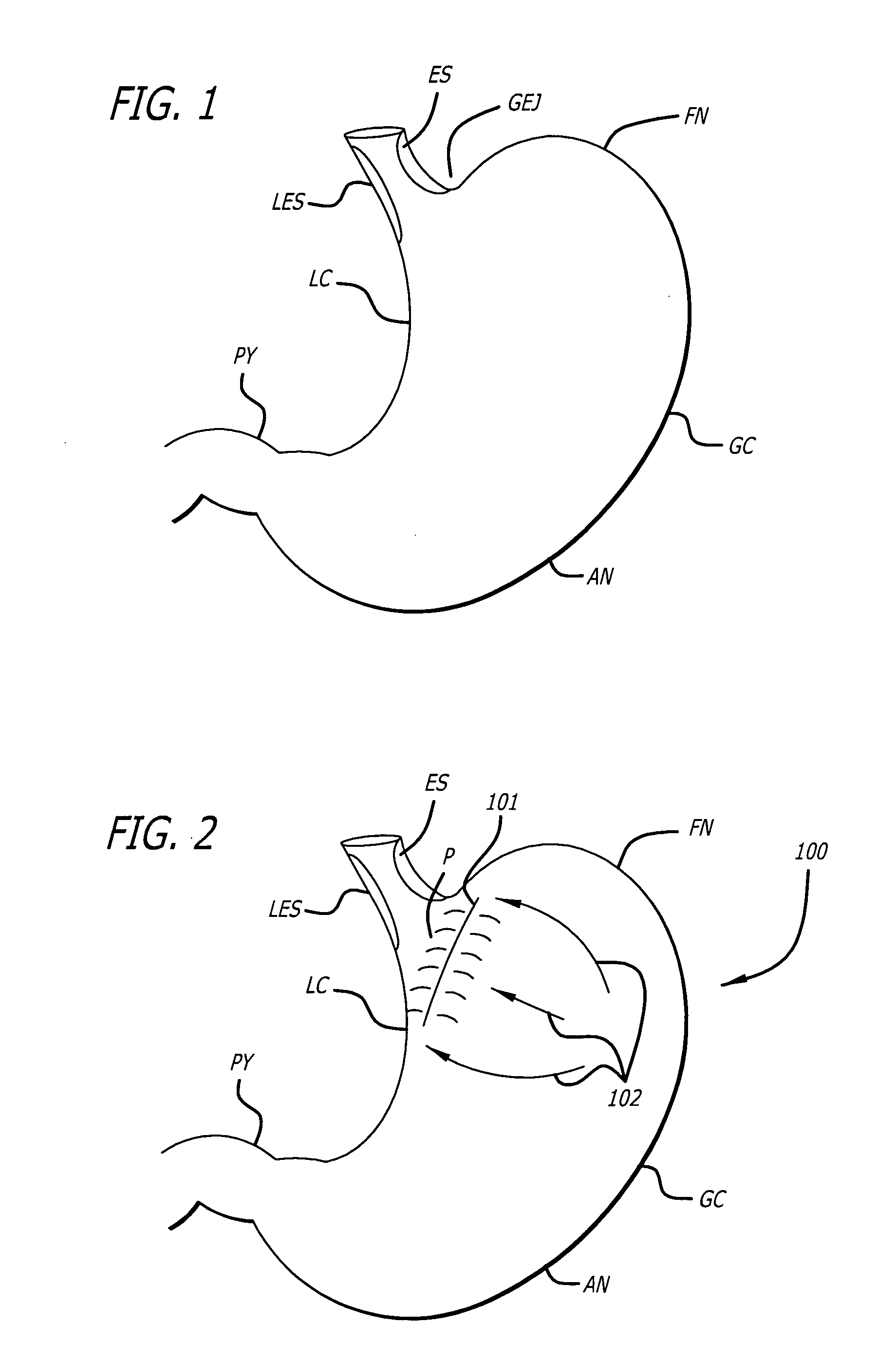
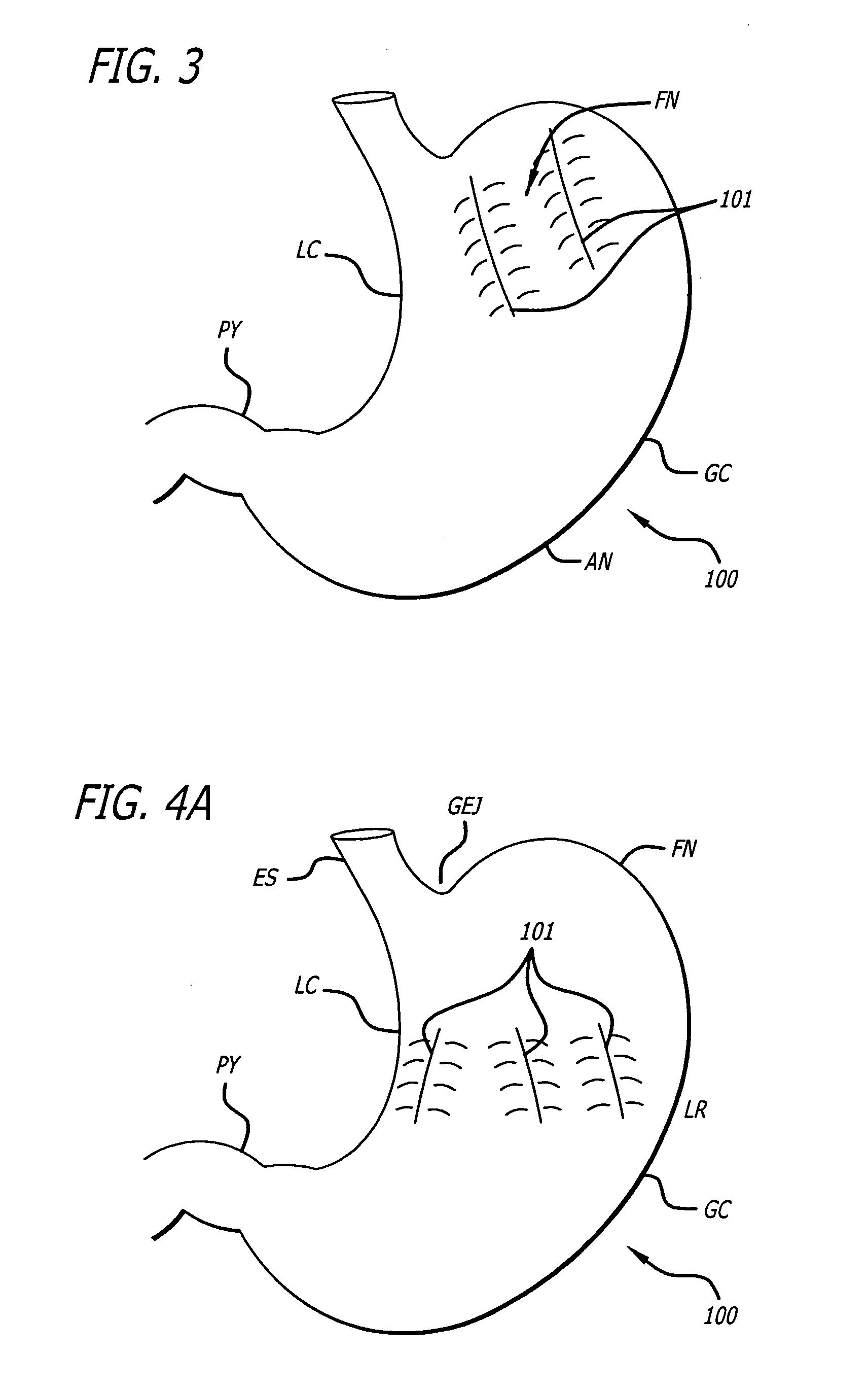
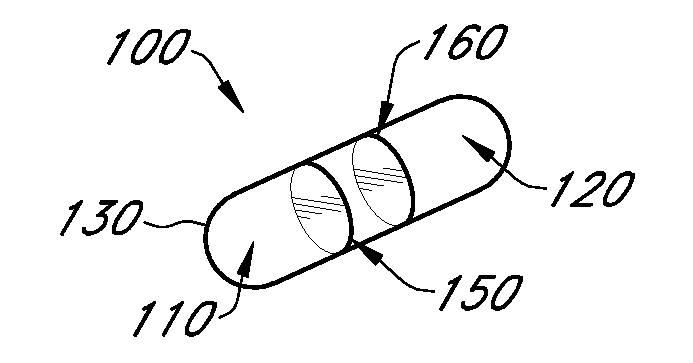
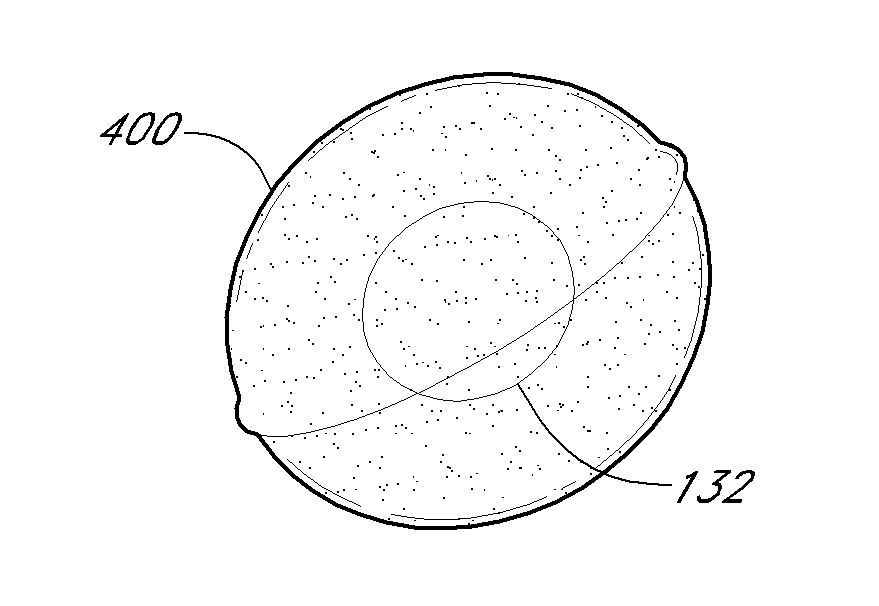
Gastric constriction device
ActiveUS20050119674A1Reduce volume potentialPrevent slippingNon-surgical orthopedic devicesObesity treatmentStomach wallsGeneral surgery
A gastric constriction device (160) comprises a sheet (131) extending over part of the wall of the stomach (24). Five bands (151) extend around the stomach (24) to fix the sheet (131) in position relative to the stomach (24). The lower two bands (151) extend from the first side (152) of the sheet (131) around the stomach (24) only partially towards the second side (153). These lower two bands (151) are not fixed to the second side (153). This arrangement results in an unconstricted portion of the stomach (161). In this manner, the device (160) restricts expansion of the majority of the stomach wall while facilitating expansion of this unconstricted portion (161). The unconstricted portion (161) is therefore free to expand or bulge outwardly upon ingestion. This expansion may trigger the feeling of satiation due to the presence of the vagal nerves in this portion (161) of the stomach (24).
Owner:PROXY BIOMEDICAL
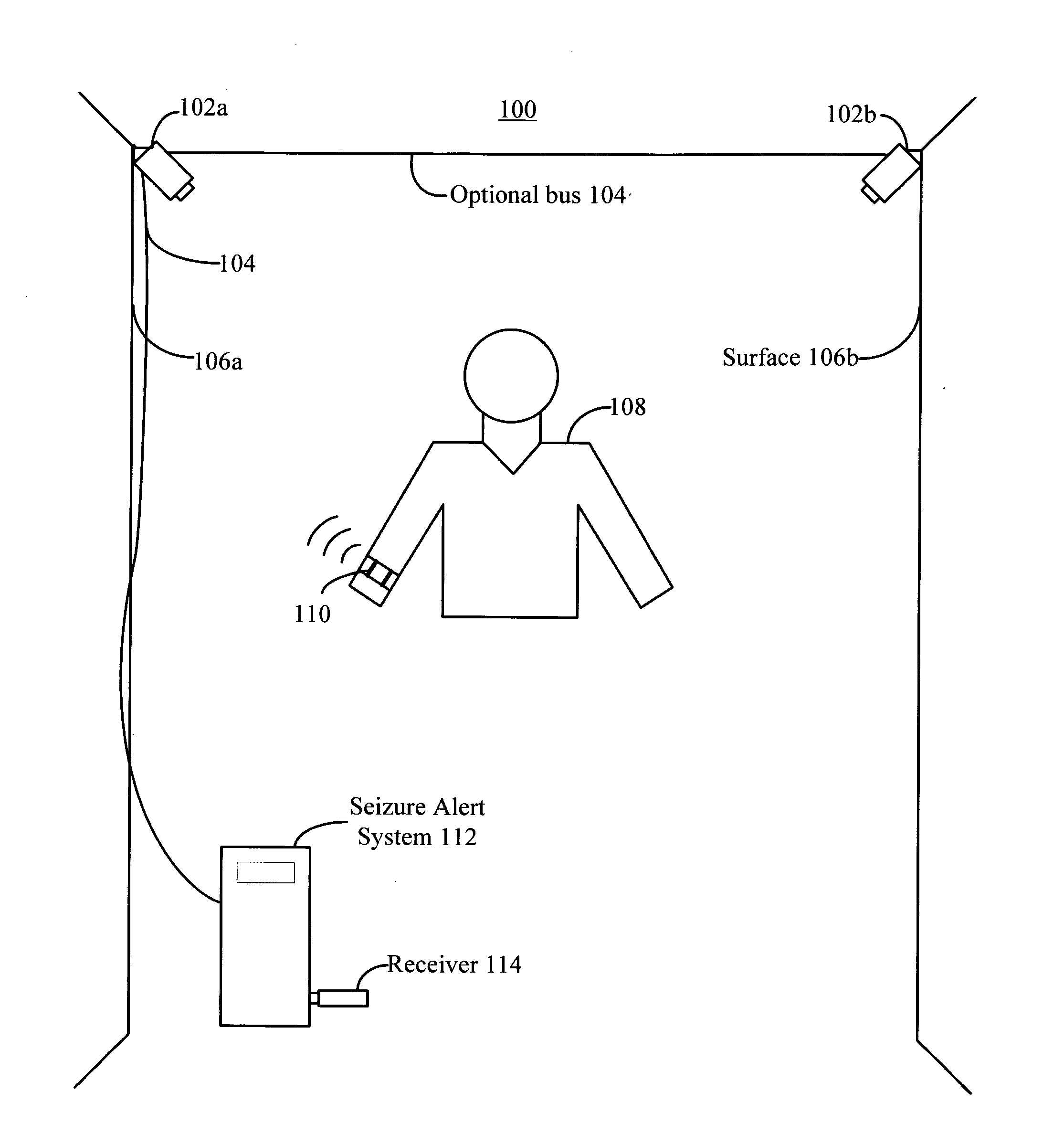
Abnormal motion detector and monitor
In an embodiment, a seizure monitor provides intelligent epilepsy seizure detection, monitoring, and alerting for epilepsy patients or people with seizures. In an embodiment, the seizure monitor may be a wearable, non-intrusive, passive monitoring device that does not require any insertion or ingestion into the human body. In an embodiment, the seizure monitor may include several output options for outputting the accelerometer / gyro or other motion sensor data and video data, so that the data may be immediately validated and / or remotely viewed. The device alerts and communicated to the outside care givers via wirelessly or wired medium. The device may also support recording of accelerometer or other motion sensor data and video data, which can be reviewed later for further analysis and / or diagnosis. The device and invention is also used and applicable for other body motion disorders or detection and diagnostics.
Owner:SMART MONITOR
Methods and compositions for the treatment and management of hemoglobinopathy and anemia
InactiveUS20050143420A1Good effectRelieve symptomsBiocidePeptide/protein ingredientsRed blood cellThalassemia
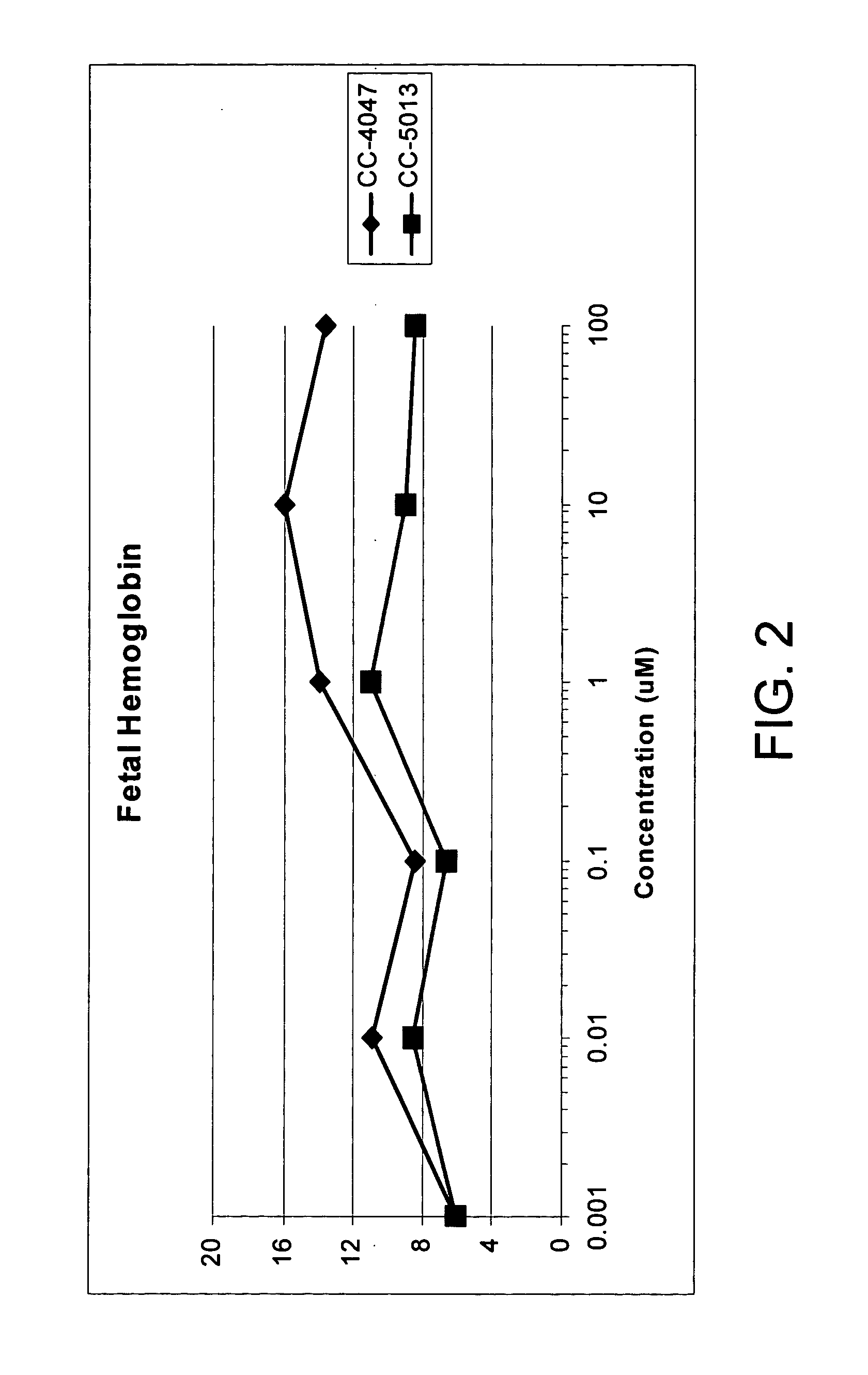
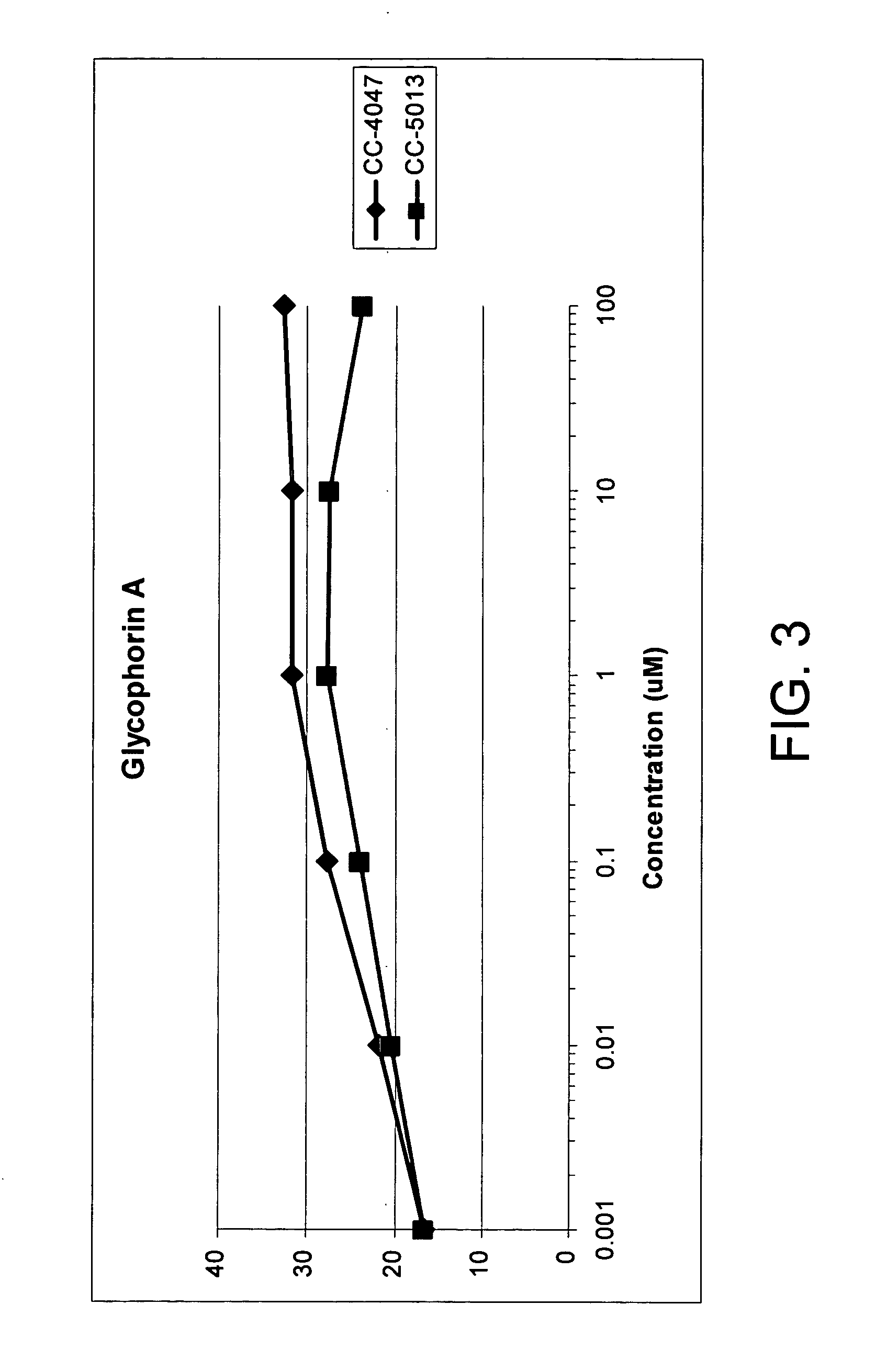
The present invention is directed to the use of immunomodulatory compounds, particularly members of the class of compounds known as IMiDs™, and more specifically the compounds 4-(Amino)-2-(2,6-dioxo(3-piperidyl))-isoindoline-1,3-dione and 3-(4-amino-1-oxo-1,3-dihydroisoindol-2-yl)-piperidine-2,6-dione, to induce the expression of fetal hemoglobin genes, genes essential for erythropoiesis, and genes encoding alpha hemoglobin stabilizing protein, within a population of CD34+ cells. These compounds are used to treat hemoglobinopathies such as sickle cell anemia or β-thalassemia, or anemias caused by disease, surgery, accident, or the introduction or ingestion of toxins, poisons or drugs.
Owner:SIGNAL PHARMA LLC
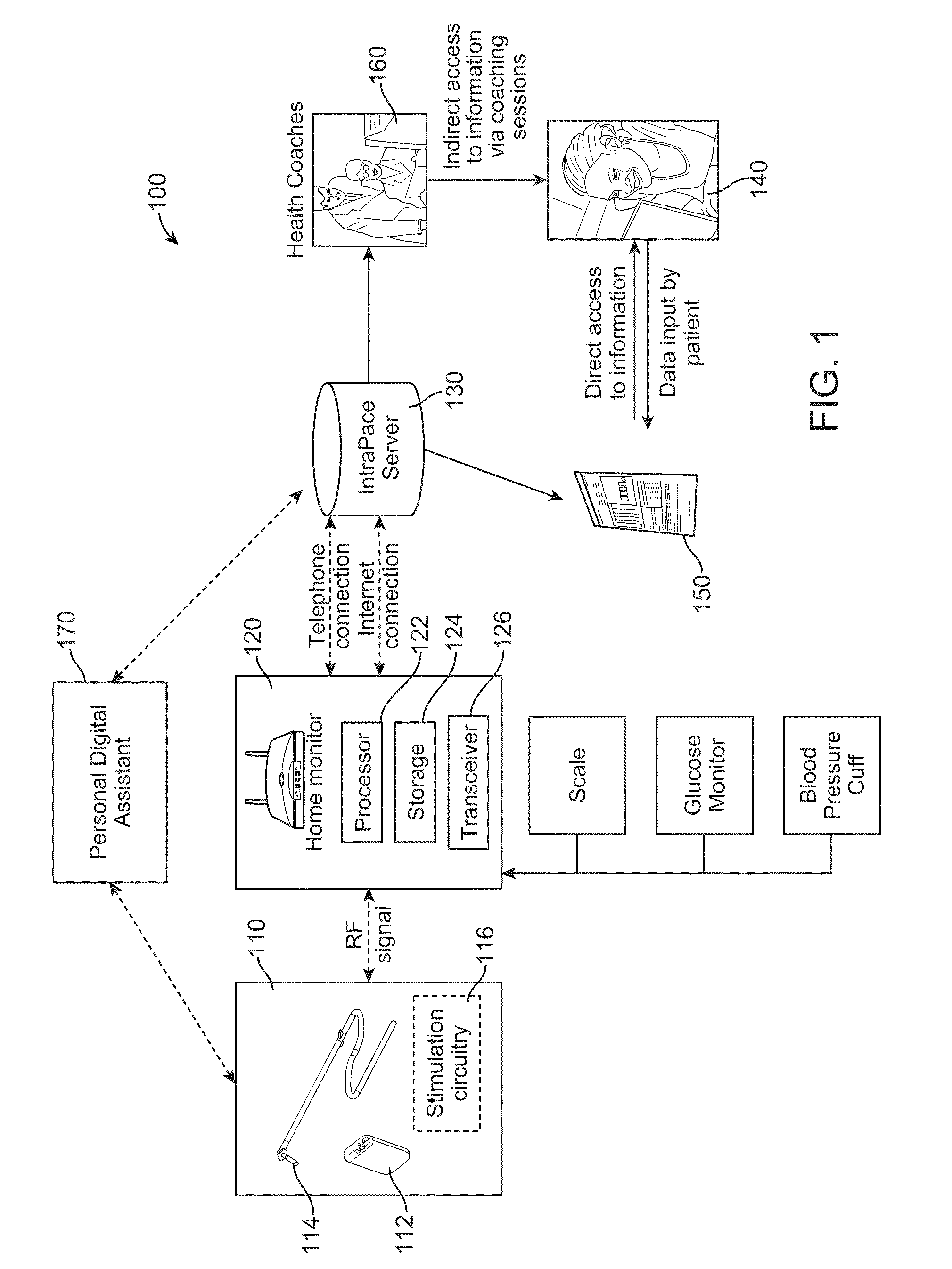
Method and apparatus for monitoring ingestion of medications using an implantable medical device
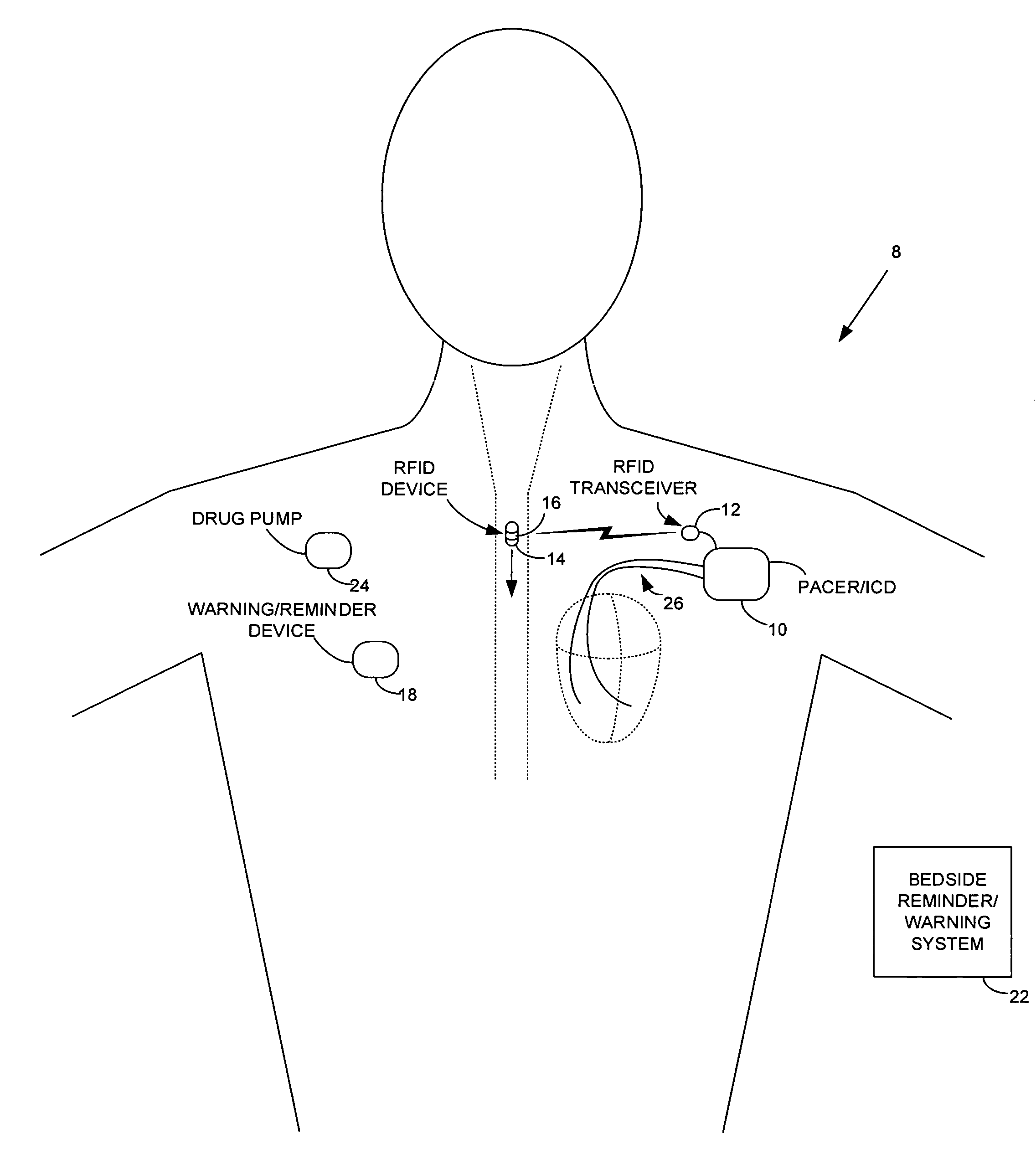
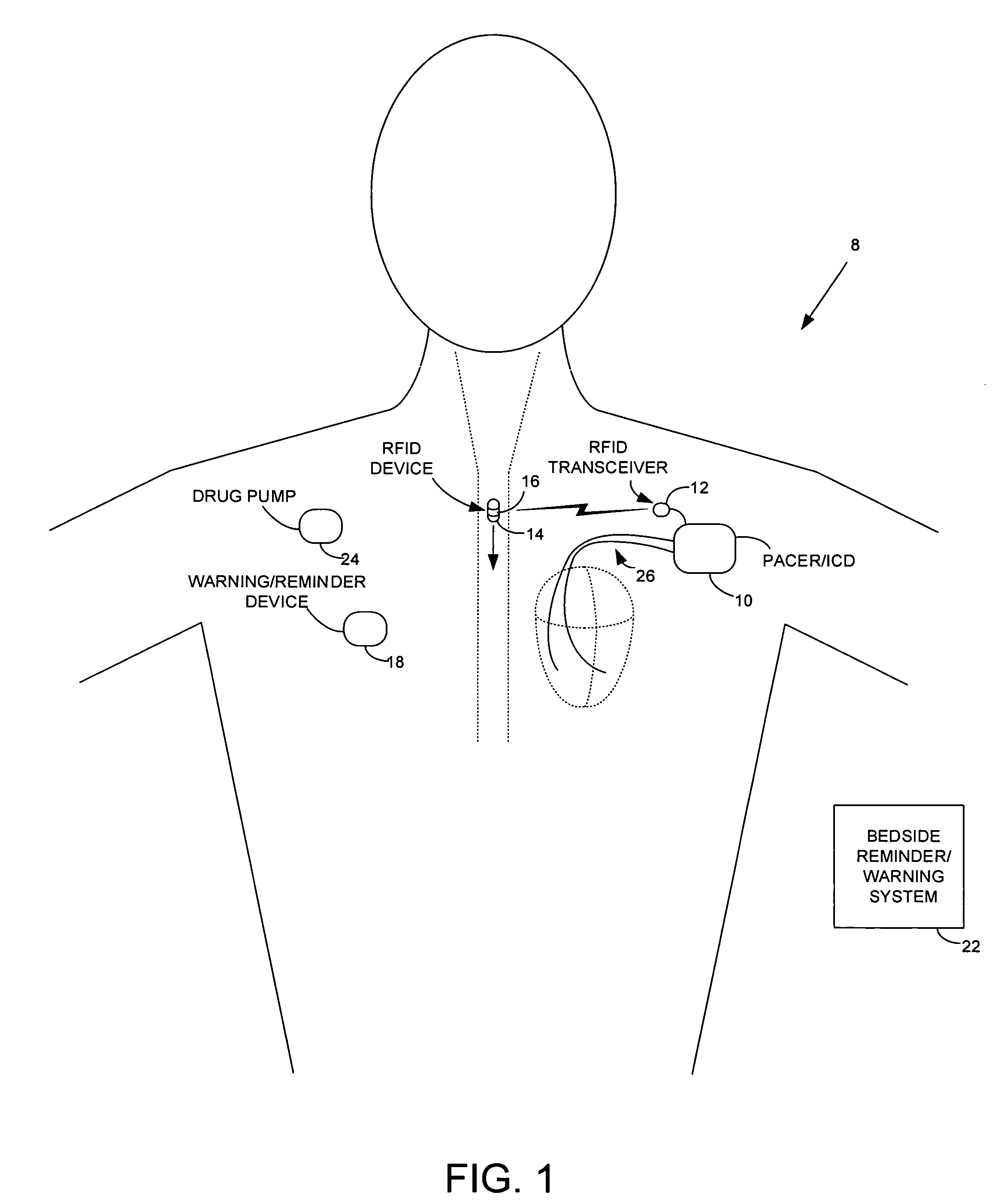
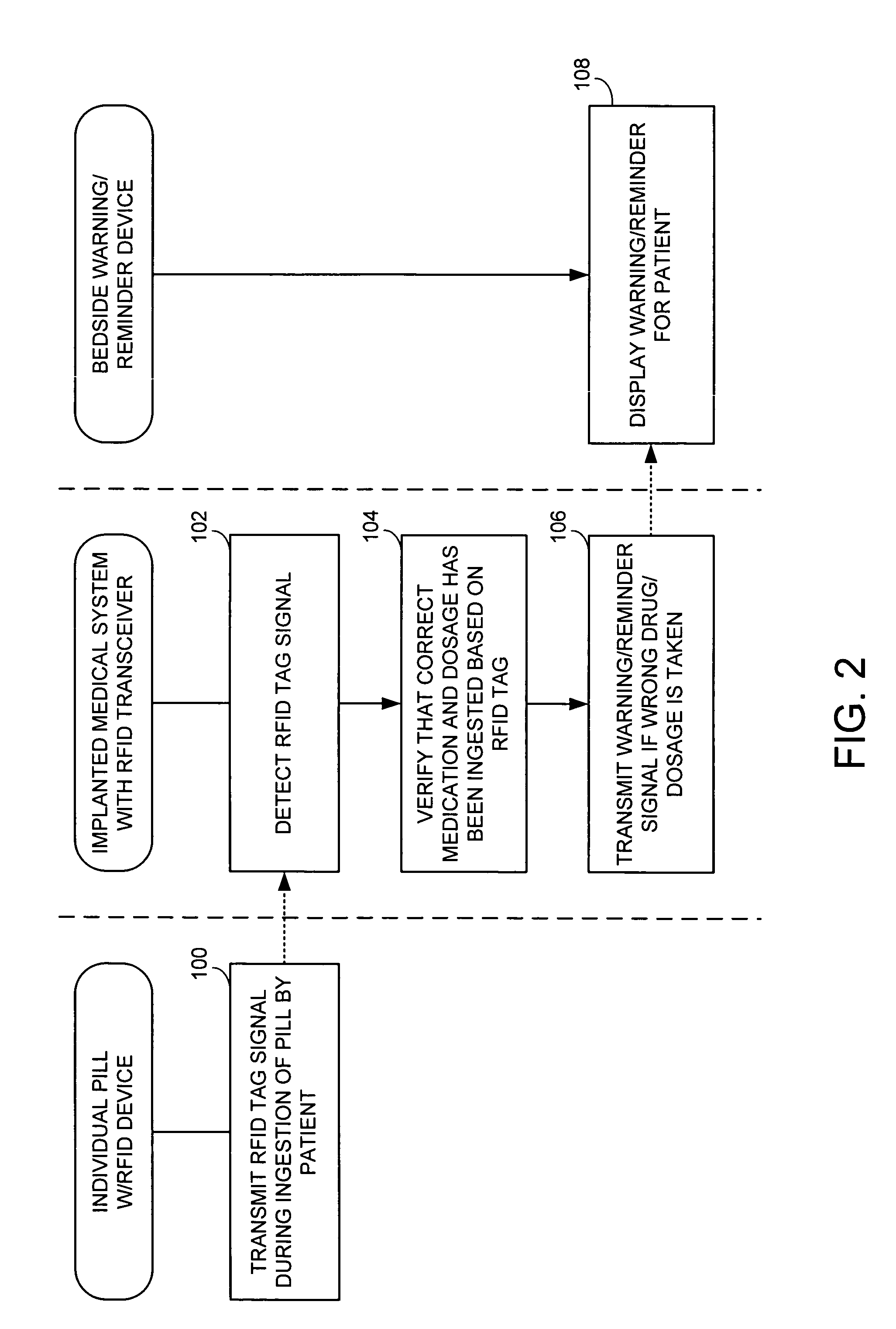
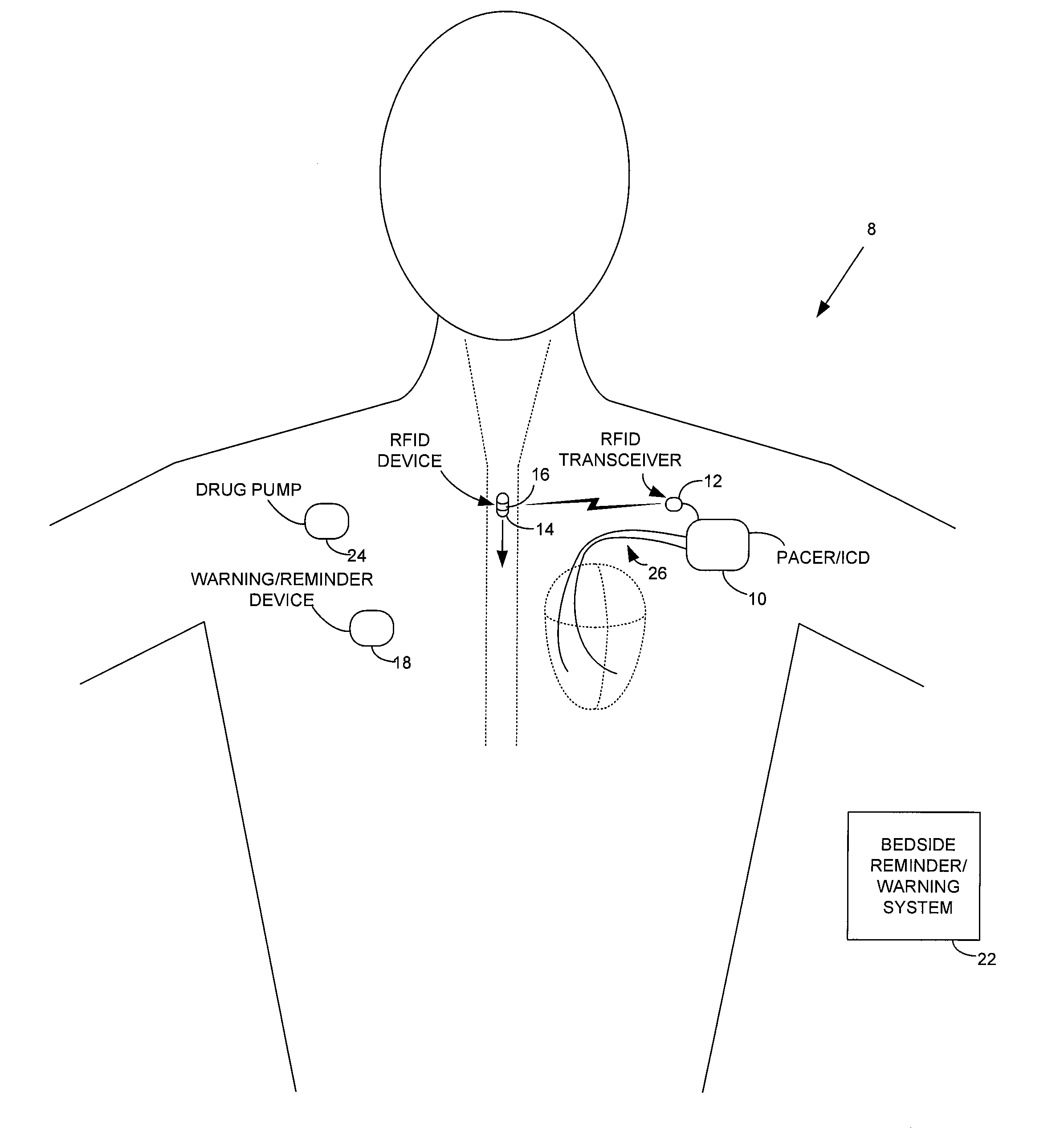
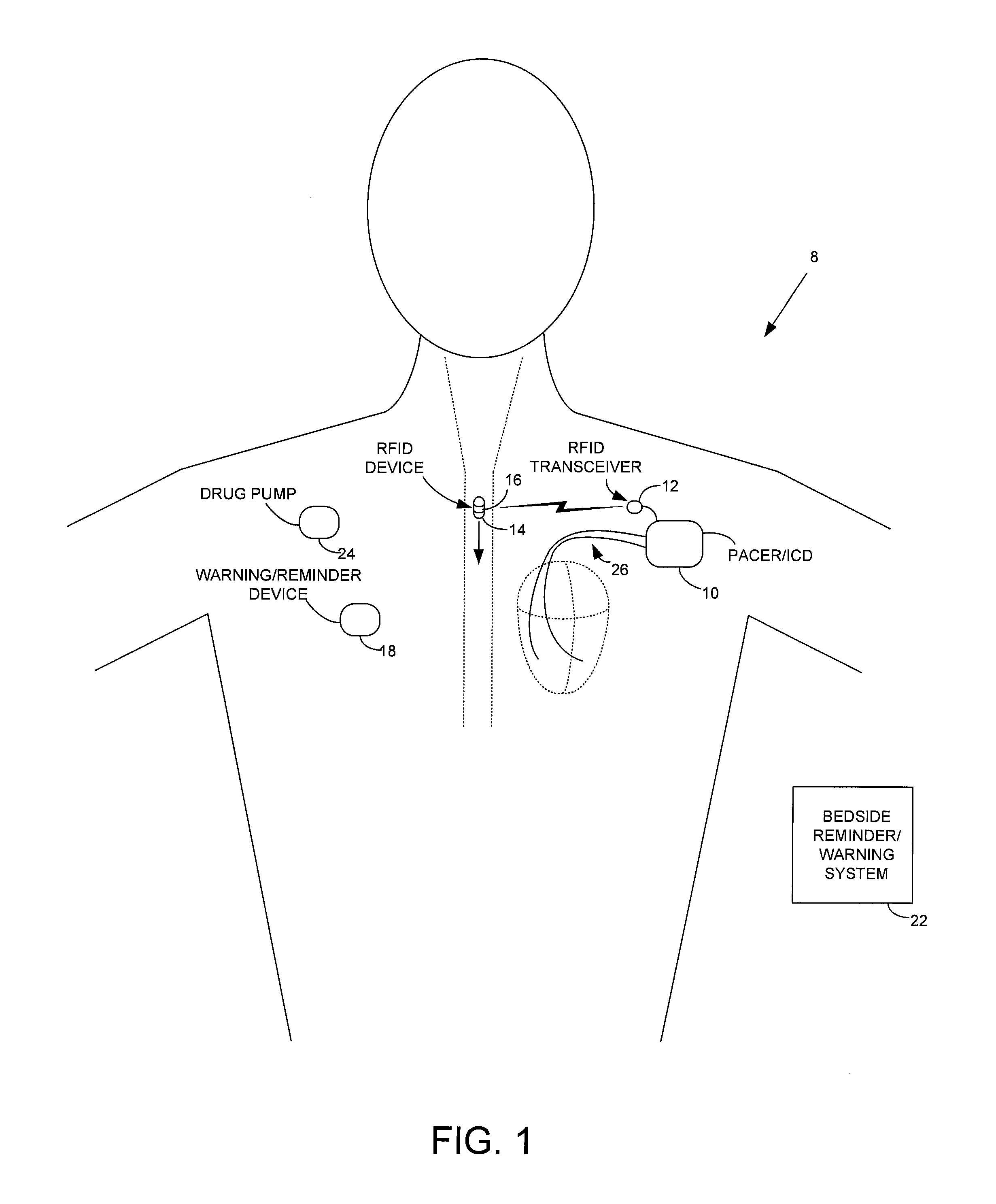
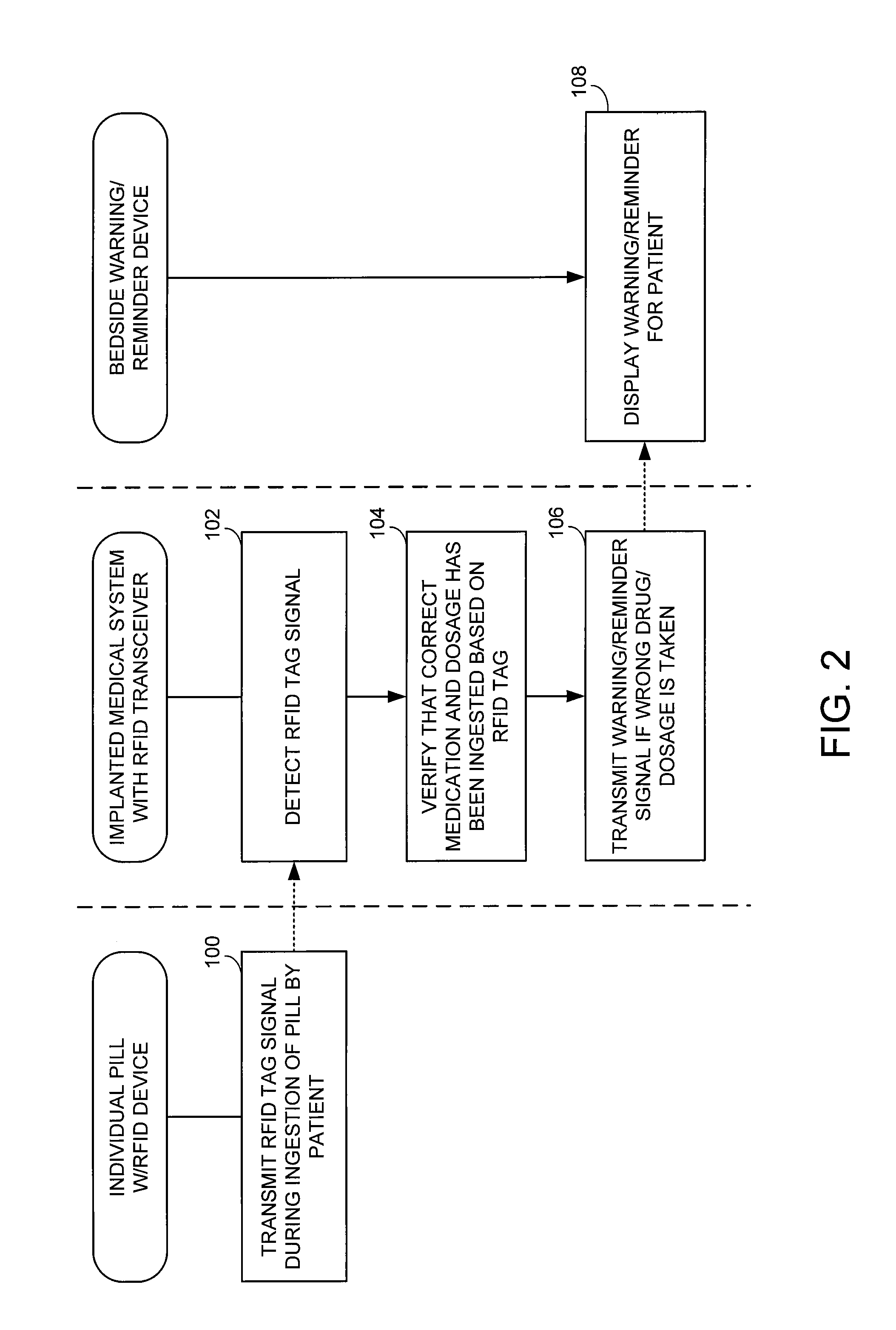
ActiveUS7414534B1Reduce the possibilityIncrease chances of survivalDrug and medicationsSurgeryTransceiverCardiac pacemaker electrode
An implantable medical device, such as a pacemaker or implantable cardioverter defibrillator (ICD), is configured to automatically detect ingestion of medications to verify that prescribed medications are taken in a timely manner and at the correct dosage. Briefly, individual pills are provided with miniature radio frequency identification (RFID) devices capable of transmitting RFID tag signals, which identify the medication contained within the pill and its dosage. The implanted device is equipped with an RFID transceiver for receiving tag signals from a pill as it is being ingested. The implanted system decodes the tag to identify the medication and its dosage, then accesses an onboard database to verify that the medication being ingested was in fact prescribed to the patient and to verify that the correct dosage was taken. Warning signals are generated if the wrong medication or the wrong dosage was taken. Therapy may also be automatically adjusted. Non-RF-based ID devices are also described, which instead transmit ID data via biphasic current pulses.
Owner:PACESETTER INC
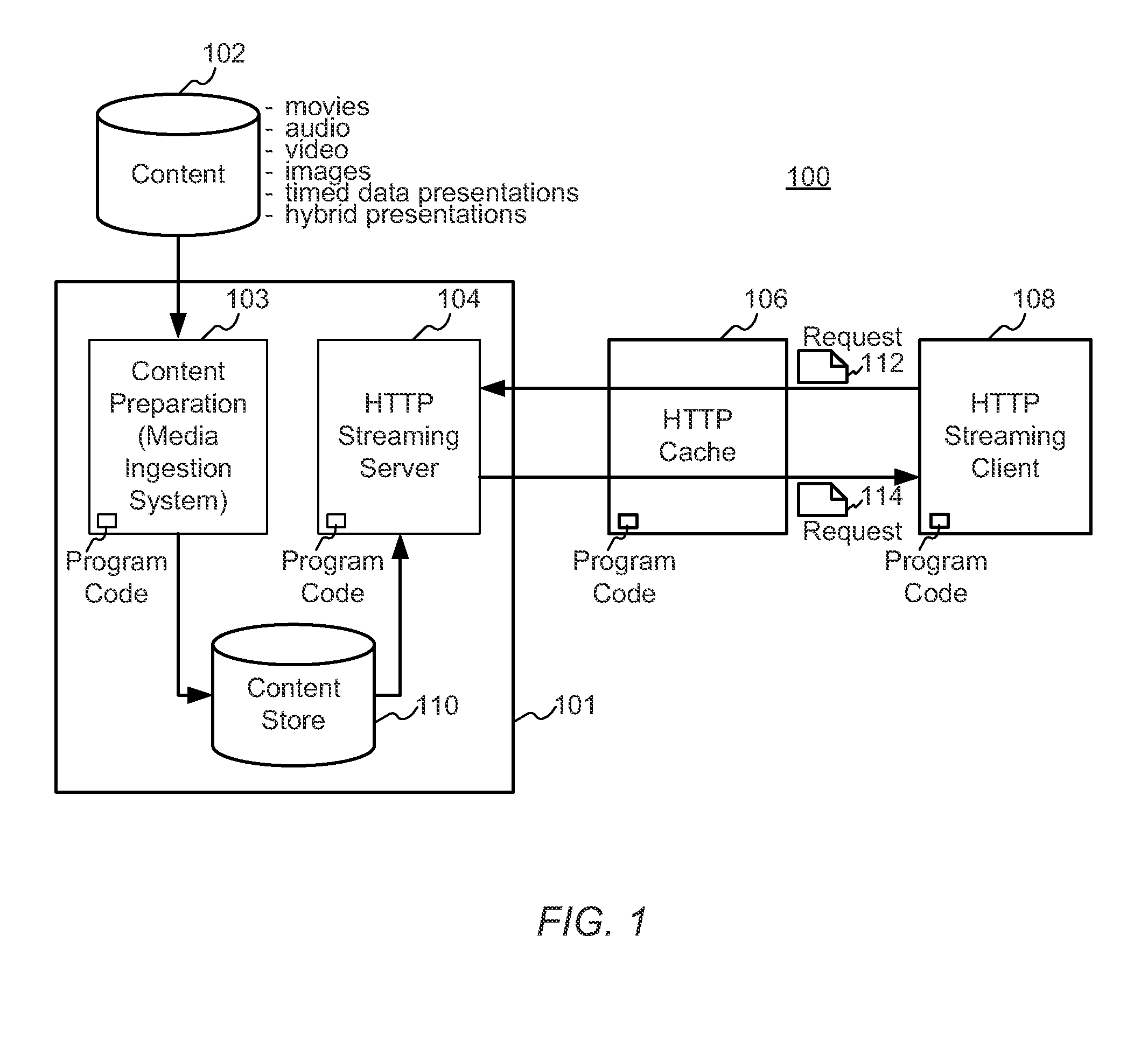
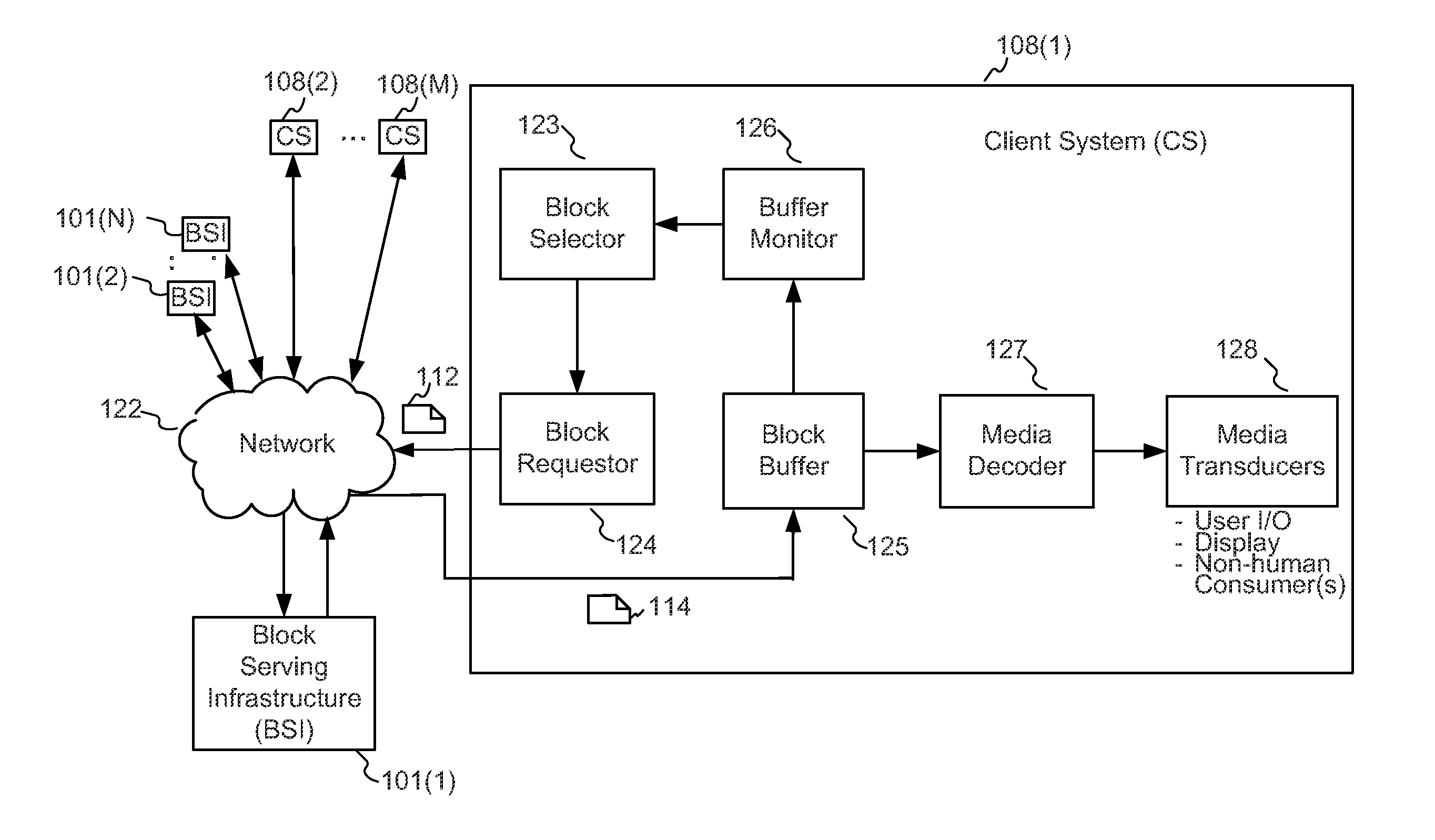
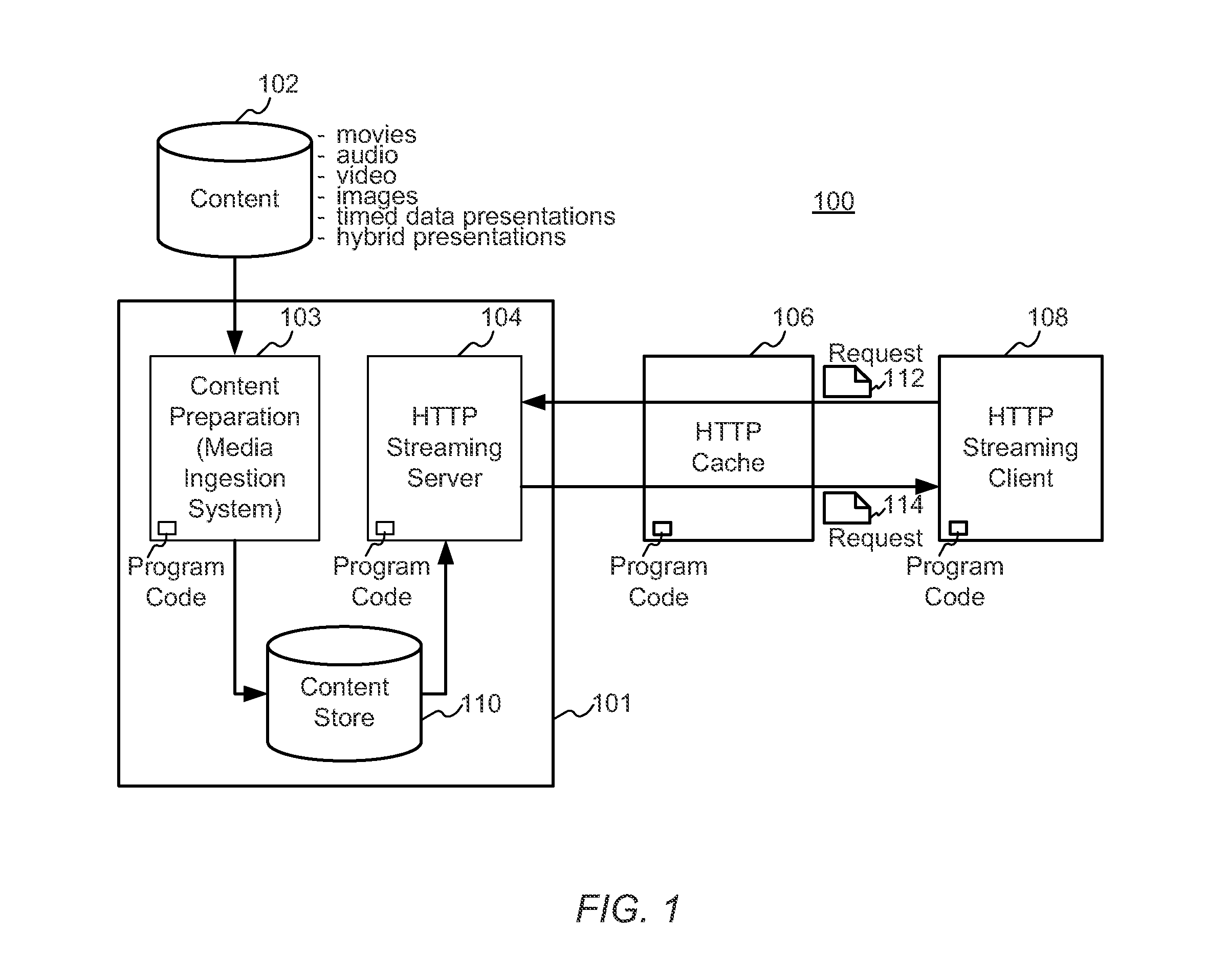
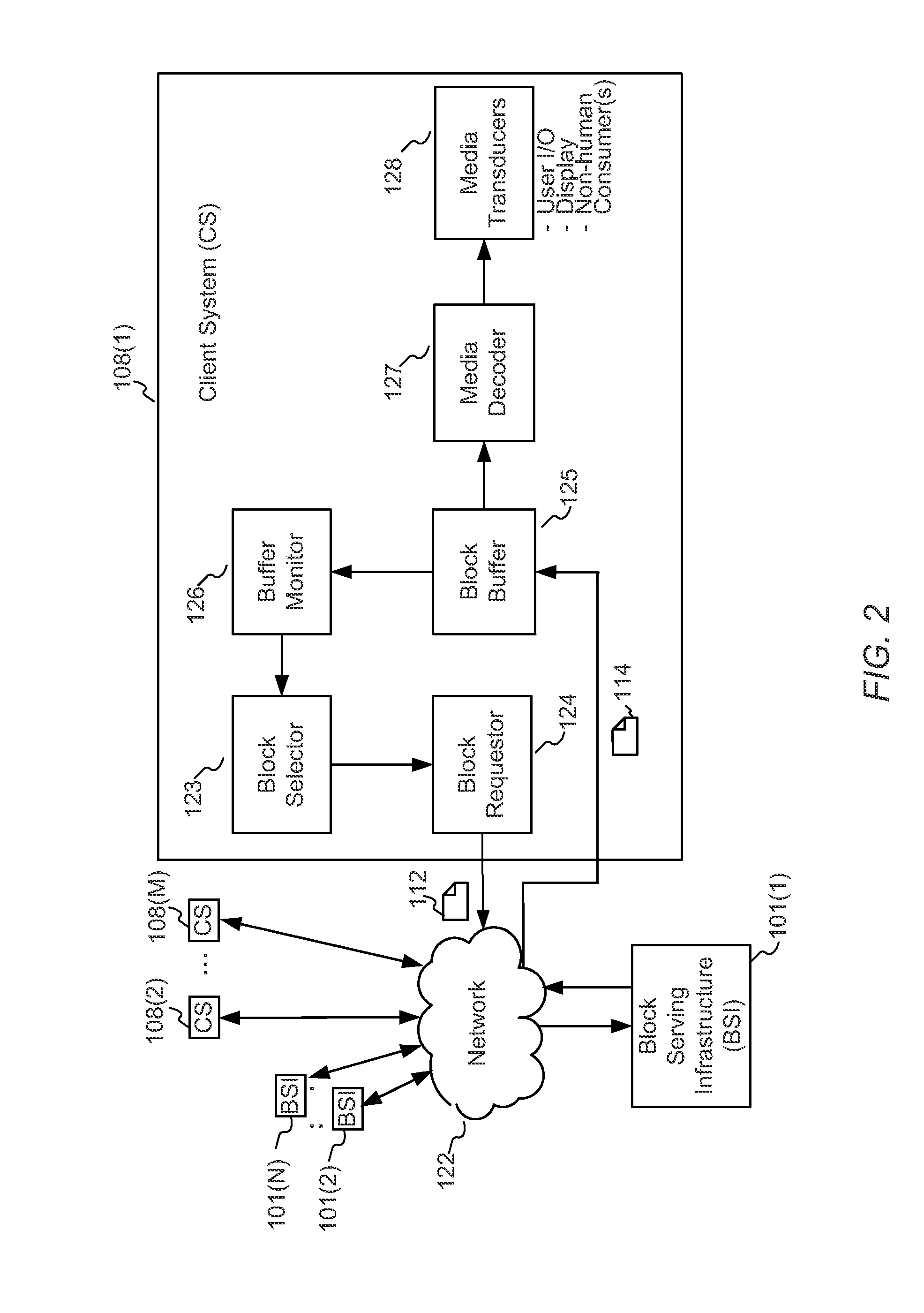
Enhanced block-request streaming system using signaling or block creation
ActiveUS20110238789A1Improve bandwidth efficiencyImprove user experiencePulse modulation television signal transmissionMultiple digital computer combinationsTransport systemPoint placement
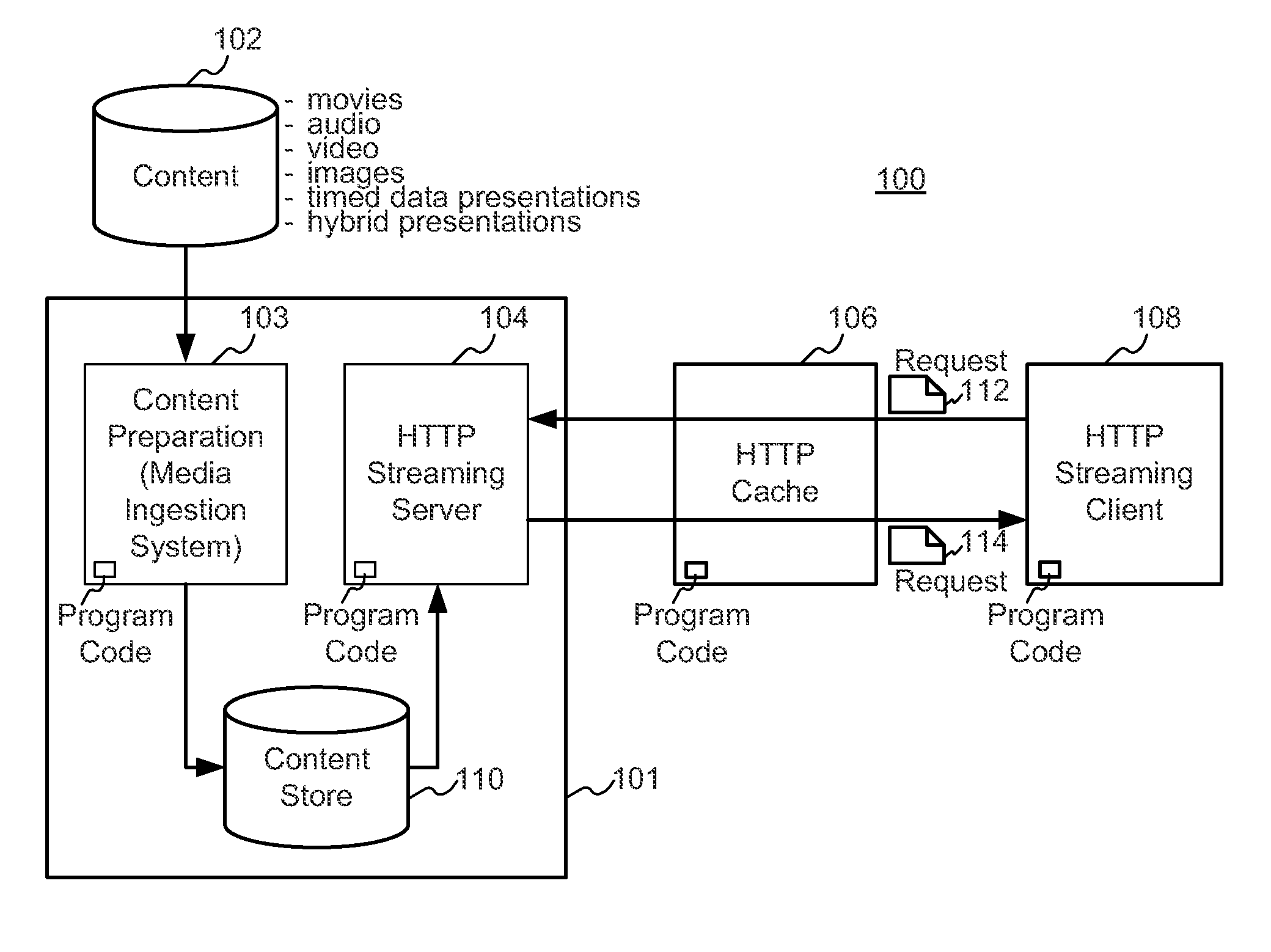
A block-request streaming system provides for improvements in the user experience and bandwidth efficiency of such systems, typically using an ingestion system that generates data in a form to be served by a conventional file server (HTTP, FTP, or the like), wherein the ingestion system intakes content and prepares it as files or data elements to be served by the file server. The system might include controlling the sequence, timing and construction of block requests, time based indexing, variable block sizing, optimal block partitioning, control of random access point placement, including across multiple presentation versions, dynamically updating presentation data, and / or efficiently presenting live content and time shifting.
Owner:QUALCOMM INC
Parenteral delivery systems
Hypertonic sugar compositions administered by other than ingestion and swallowing or intravascular injection, such as by intranasal spray or drops, intraocular drops or ointment, oral spray, intraotic spray or drops, lozenges, chewable tablet, chewing gum, or gargle, pulmonary inhalation, vaginal or rectal suppositories, or transdermal creams, ointments, lotions, or patches, are effective to open the blood-brain barrier to permit entry into the central nervous system of a co-administered chemical compound, such as a nutrient or a therapeutic or diagnostic agent. In this way, the compositions and methods of the invention increase the therapeutic or diagnostic efficacy of such chemical compounds.
Owner:NAITO ALBERT T
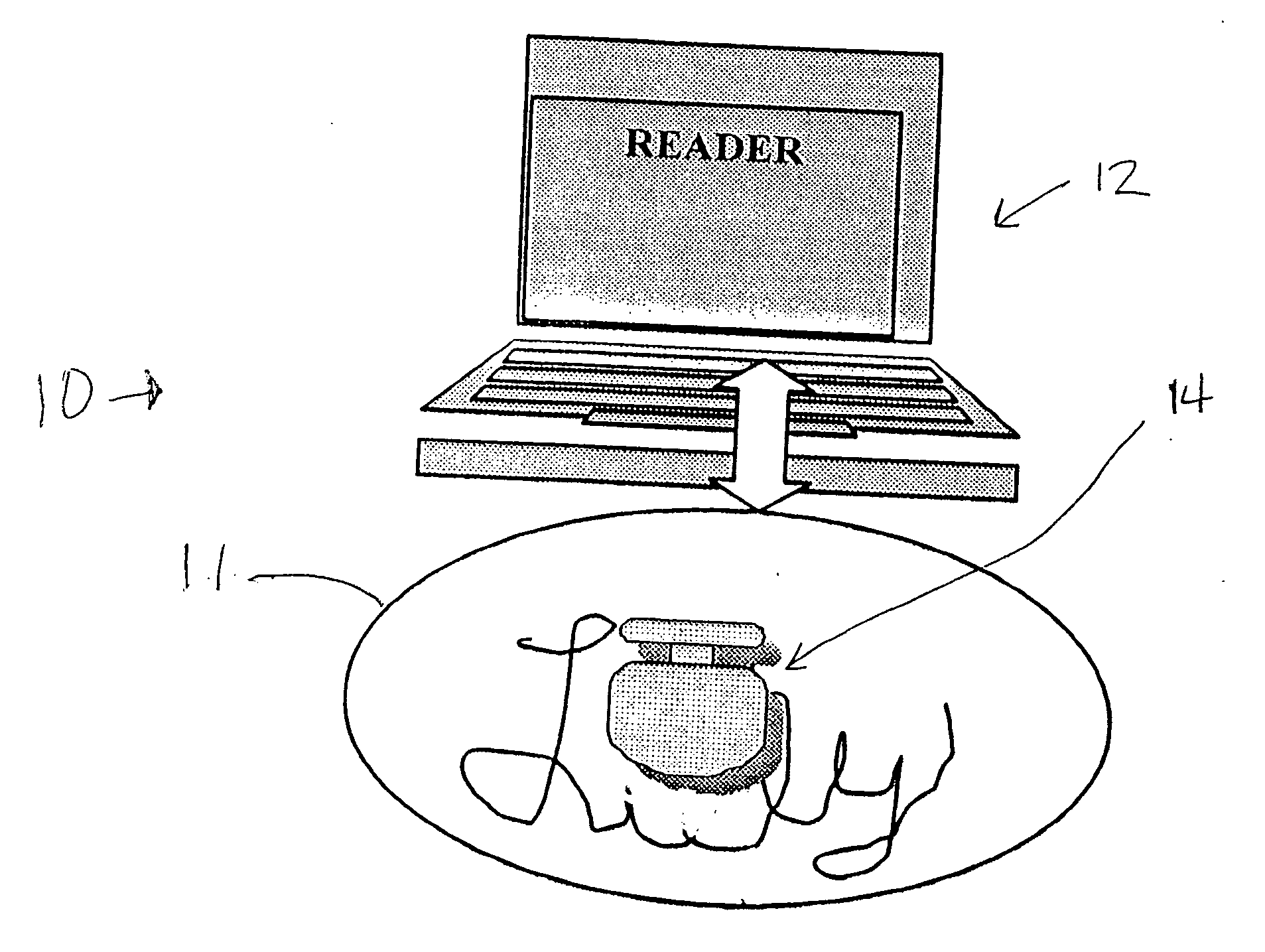
Trackable pills with electronic ID tags
InactiveUS20060061472A1Prevent the tablet from dissolvingIncrease in sizeMemory record carrier reading problemsPharmaceutical product form changeConstant powerConductive materials
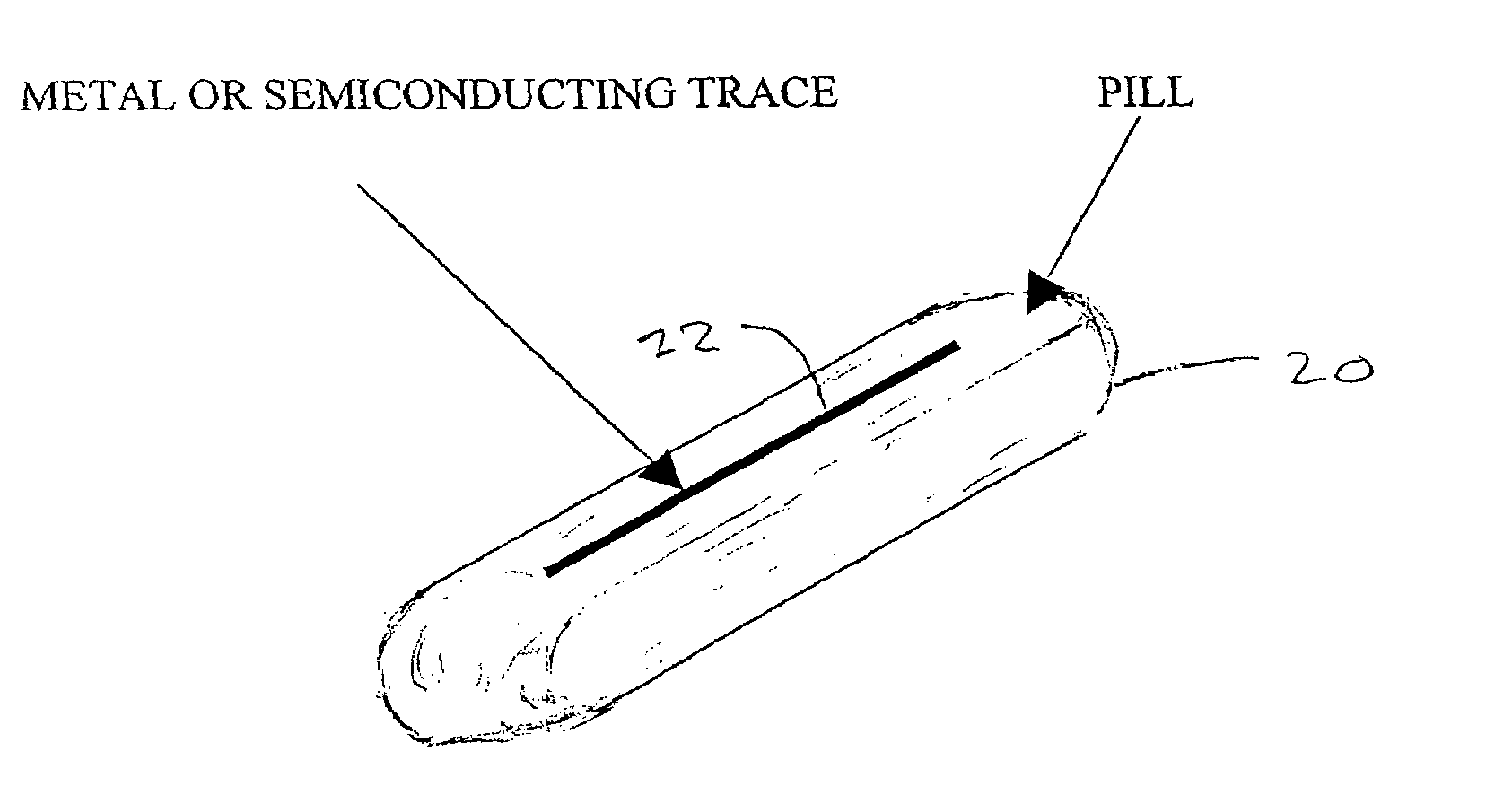
A medical pill intended for human or animal consumption includes an RF ID tag in or on the pill. The tag will respond to a nearby reader, the tag itself being without a battery or other constant power supply, capturing power from the reader's transmitted signal and storing a portion of that power in a power supply. An antenna for the RF ID tag may be integral with the tag or it may be transferred to the pill using conductive materials in the pill's coating, filler or binding agents, embedded within the pill, or printed onto the pill. If separate from the tag the antenna is electromagnetically coupled to the tag which has a small onboard antenna. The RF ID tag of each pill has data that are transmitted when the tag is interrogated by a signal from a reader. Incorporation of an ingestable ID tag is possible because of the tag's very small size compatible with ingestion and because the tag can contain an antenna within the pill that allows the tag to be read at a substantial distance. Several different methods for deactivating the RF ID tag after ingestion or use of the pill are disclosed. Medicaments other than oral pills can also have the ID tags.
Owner:TAGENT
Oral drug compliance monitoring using radio frequency identification tags
A device useful for oral drug delivery device consisting of: (a) a capsule, tablet or pill designed to disperse in the gastrointestinal system; (b) an RFID tag positioned in the capsule, tablet or pill, the RFID tag comprising an antenna; (c) an object selected from the group consisting of a magnet, a ferromagnetic object, a ferrite object and an electromagnetic shielding object positioned within, over or adjacent the antenna of the RFID tag to alter the antenna characteristics of the RFID tag so that if the RFID tag is interrogated before the capsule, tablet or pill disperses in the gastrointestinal system, the response of the RFID tag is sufficiently altered or attenuated to determine that the capsule, tablet or pill has not dispersed in the gastrointestinal system and so that if the RFID tag is interrogated after the capsule, tablet or pill has dispersed in the gastrointestinal system, the object separates from the RFID tag so that the response of the RFID tag is sufficiently detectable to determine that the capsule, tablet or pill has dispersed in the gastrointestinal system. Alternatively, a switch can be used to signal ingestion of the device, and change the response of the device. In another embodiment, the instant invention is a device useful for oral drug delivery, consisting of: (a) a capsule, tablet or pill designed to disperse in the gastrointestinal system; (b) a first non-anti-collision RFID tag positioned in the capsule; (c) a second non-anti-collision RFID tag positioned in the capsule, so that if the RFID tags are interrogated by an RFID reader before the capsule, tablet or pill disperses in the gastrointestinal system, the response of the RFID tags collide and so that after the dispersible material of the capsule has dispersed in the gastrointestinal system thereby allowing the first and second non-anti-collision tags to separate from each other, then the response of the RFID tags is sufficiently different from each other to determine that the capsule has dispersed in the gastrointestinal system
Owner:DOW GLOBAL TECH LLC
Device and Method for Treating Weight Disorders
An apparatus and a method for treating a weight disorder in a subject are provided. The apparatus comprising an implantable device such as an inflatable balloon and electrodes capable of sensing a physiological change associated with food ingestion or hunger and a mechanism adapted for directly stimulating a region such as the duodenum which is responsive to a gastrointestinal satiety agent, such a mechanism can be a drug reservoir containing a drug such as CCK or analogs thereof which is contained within an inflatable balloon being implantable in a stomach of the subject. The apparatus and method provided here combine synergistic approaches to limiting meal size, i.e., chemo and mechano receptor activation of vagal satiety stimuli, electric stimulation of specific vagal pathways and limitations of gastric space.
Owner:DUOCURE
Oral drug compliance monitoring using radio frequency identification tags
A device useful for oral drug delivery device consisting of: (a) a capsule, tablet or pill designed to disperse in the gastrointestinal system; (b) an RFID tag positioned in the capsule, tablet or pill, the RFID tag comprising an antenna; (c) an object selected from the group consisting of a magnet, a ferromagnetic object, a ferrite object and an electromagnetic shielding object positioned within, over or adjacent the antenna of the RFID tag to alter the antenna characteristics of the RFID tag so that if the RFID tag is interrogated before the capsule, tablet or pill disperses in the gastrointestinal system, the response of the RFID tag is sufficiently altered or attenuated to determine that the capsule, tablet or pill has not dispersed in the gastrointestinal system and so that if the RFID tag is interrogated after the capsule, tablet or pill has dispersed in the gastrointestinal system, the object separates from the RFID tag so that the response of the RFID tag is sufficiently detectable to determine that the capsule, tablet or pill has dispersed in the gastrointestinal system. Alternatively, a switch can be used to signal ingestion of the device, and change the response of the device.
Owner:DOW GLOBAL TECH LLC
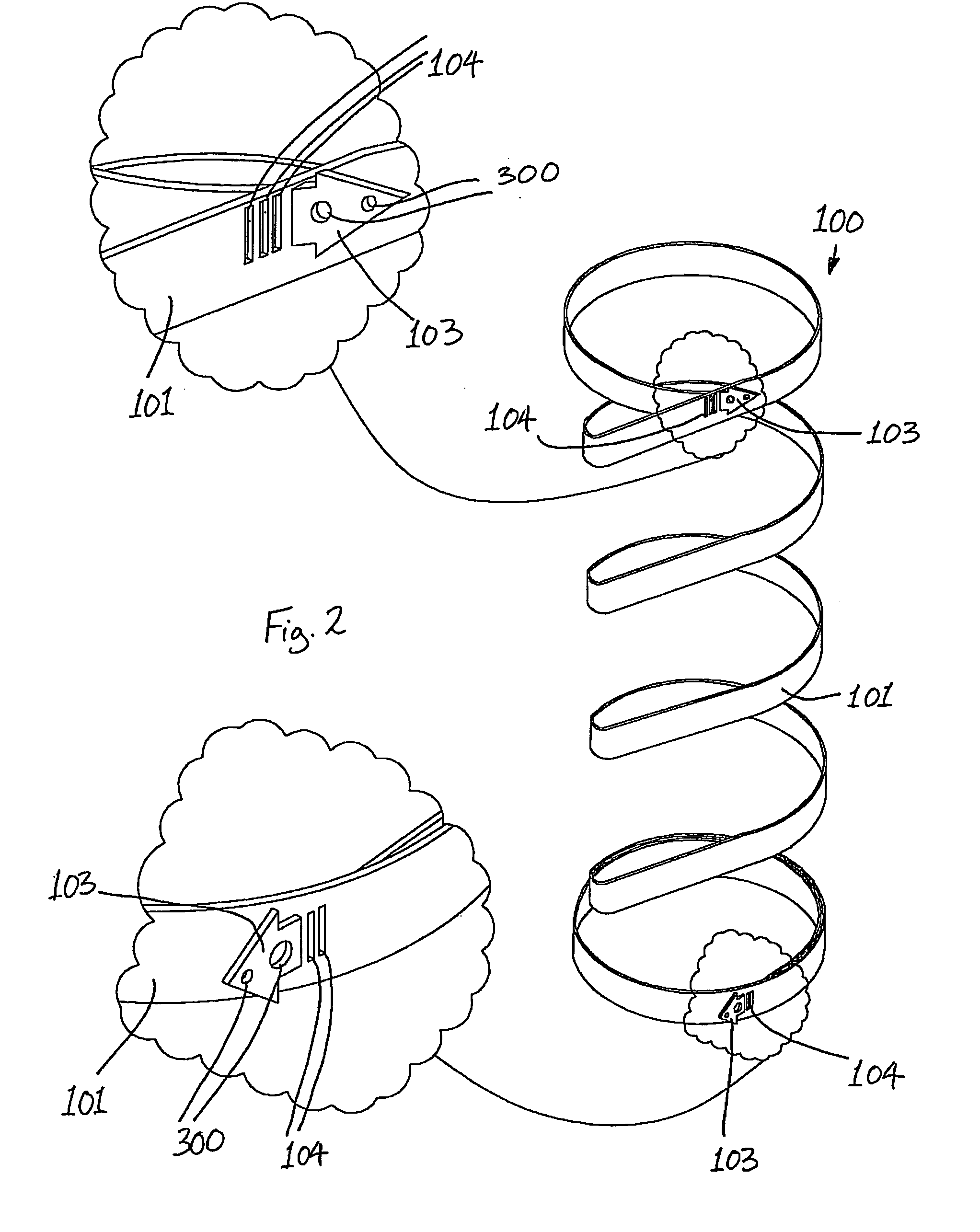
Method and devices for modifying the function of a body organ
InactiveUS7175638B2Prevent shortening and unrollingMinimize distensionSuture equipmentsNon-surgical orthopedic devicesBody organsIntestinal structure
Methods and devices for partitioning or plicating a region of a hollow body organ are described herein. These methods and devices relate generally to medical apparatus and methods and more particularly to devices and methods for affecting a change in the function of a hollow body organ, particularly a stomach, intestine or gastrointestinal tract. These changes can include reducing the volume capacity of the hollow body organ, disrupting or altering the normal function of the organ, functionally excluding certain sections of the organ either by affixing adjacent tissue or excising certain regions, or affecting or correcting the response of the organ to naturally occurring stimuli, such as ingestion.
Owner:ETHICON ENDO SURGERY INC
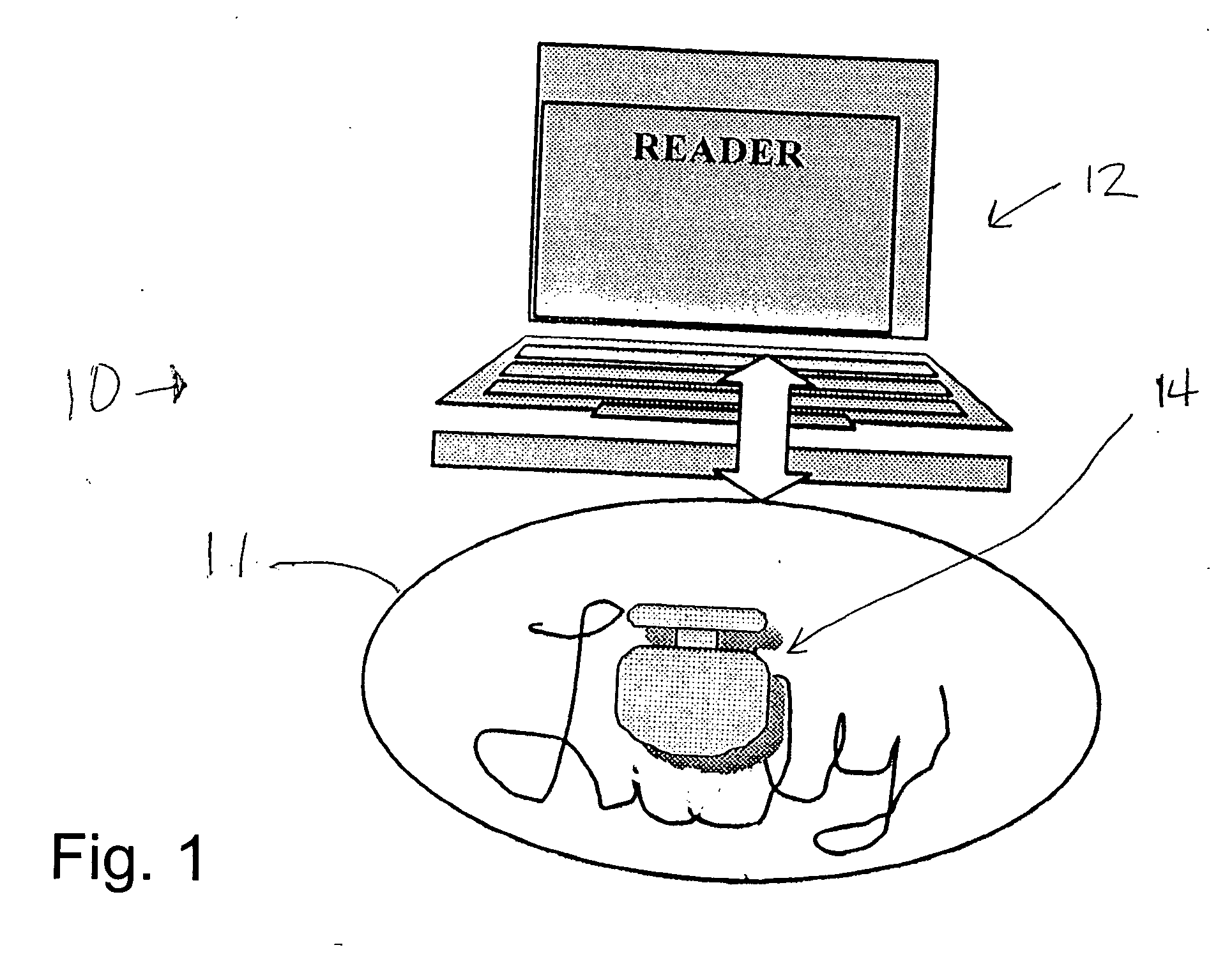
Trackable pills with electronic ID tags
InactiveUS7253716B2Prevent counterfeitingAccurate trackingMemory record carrier reading problemsPharmaceutical product form changeConstant powerEngineering
A medical pill intended for human or animal consumption includes an RF ID tag in or on the pill. The tag will respond to a nearby reader, the tag itself being without a battery or other constant power supply, capturing power from the reader's transmitted signal and storing a portion of that power in a power supply. An antenna for the RF ID tag may be integral with the tag or it may be transferred to the pill using conductive materials in the pill's coating, filler or binding agents, embedded within the pill, or printed onto the pill. If separate from the tag the antenna is electromagnetically coupled to the tag which has a small onboard antenna. The RF ID tag of each pill has data that are transmitted when the tag is interrogated by a signal from a reader. Incorporation of an ingestable ID tag is possible because of the tag's very small size compatible with ingestion and because the tag can contain an antenna within the pill that allows the tag to be read at a substantial distance. Several different methods for deactivating the RF ID tag after ingestion or use of the pill are disclosed. Medicaments other than oral pills can also have the ID tags.
Owner:TAGENT
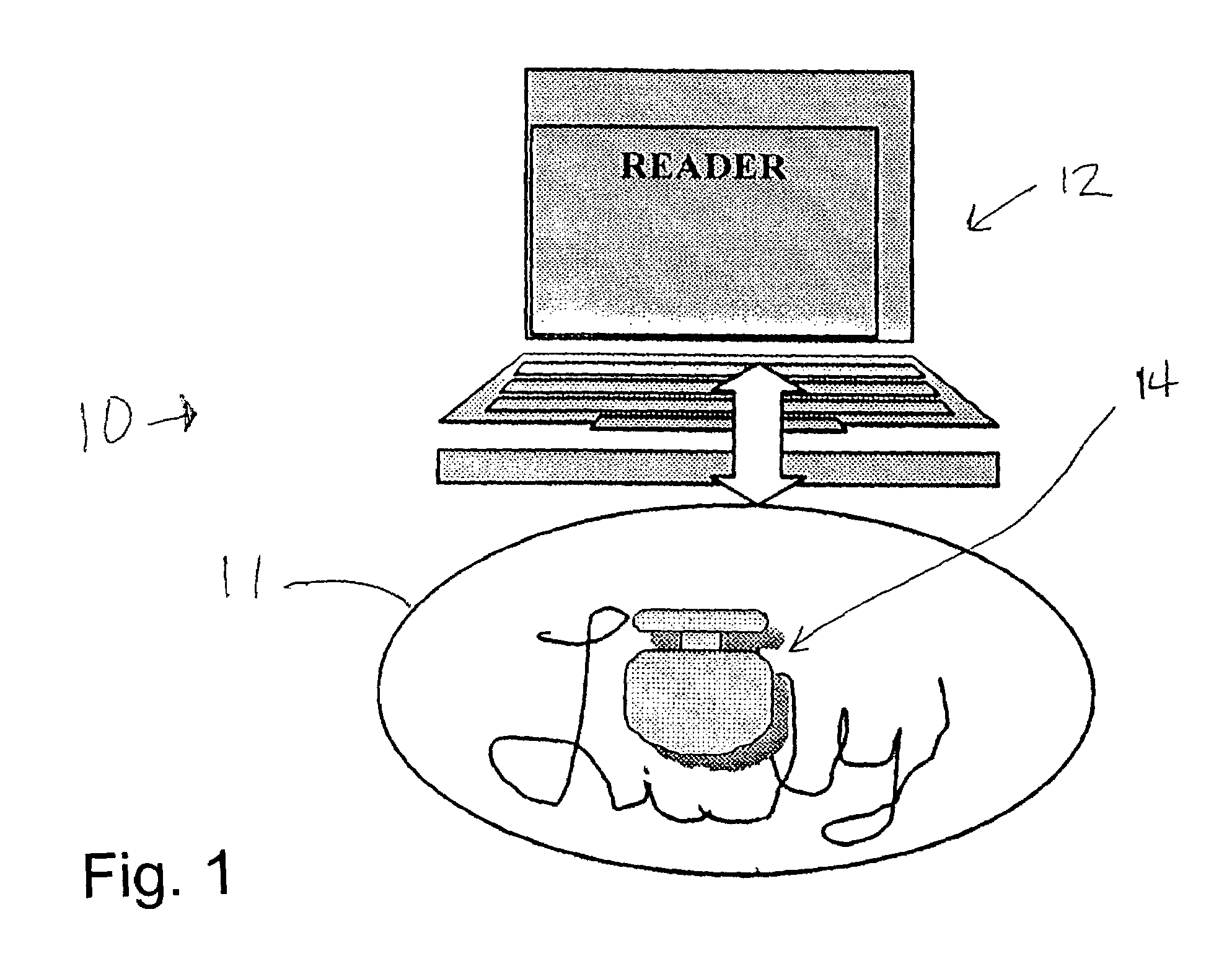
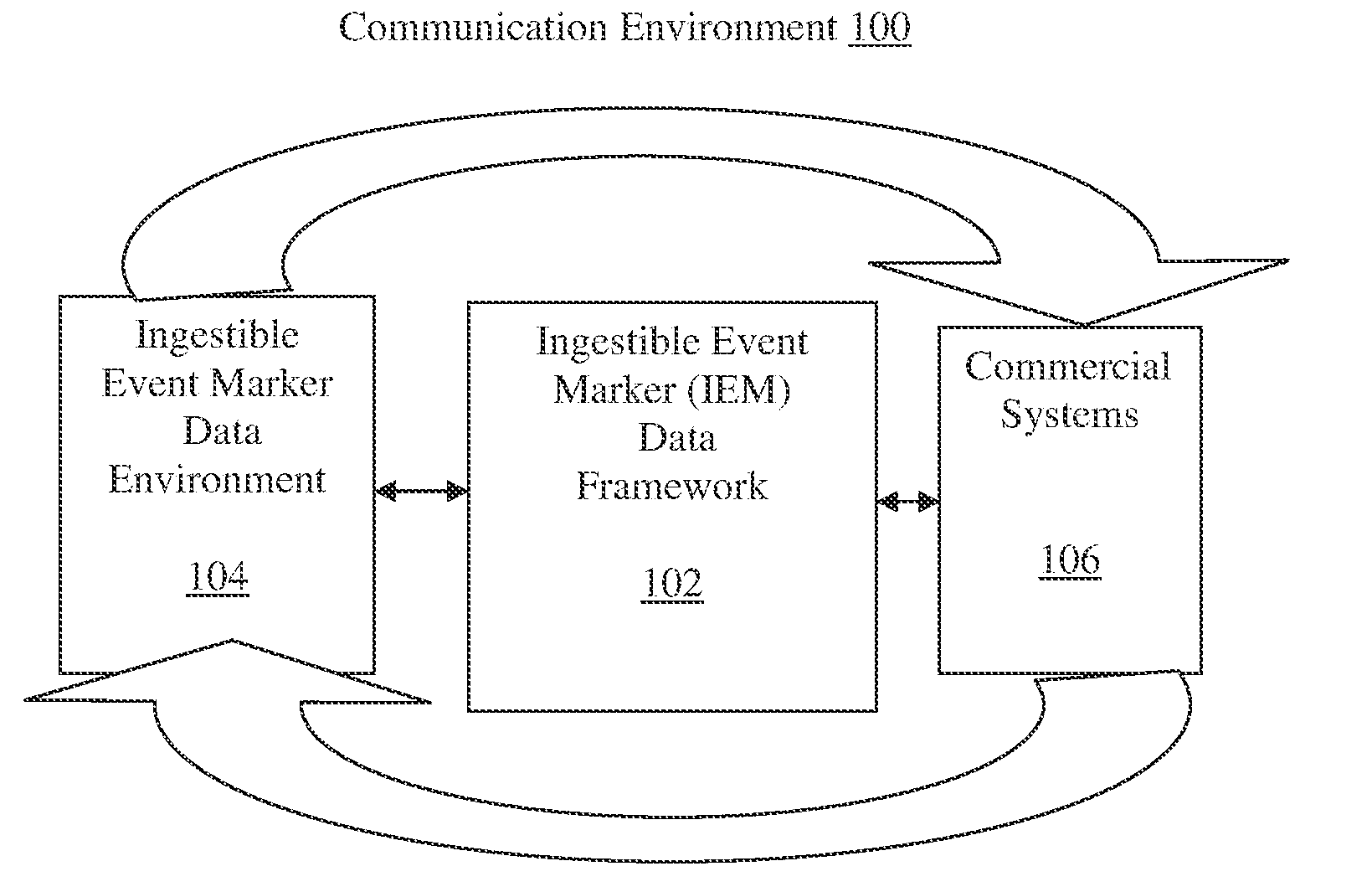
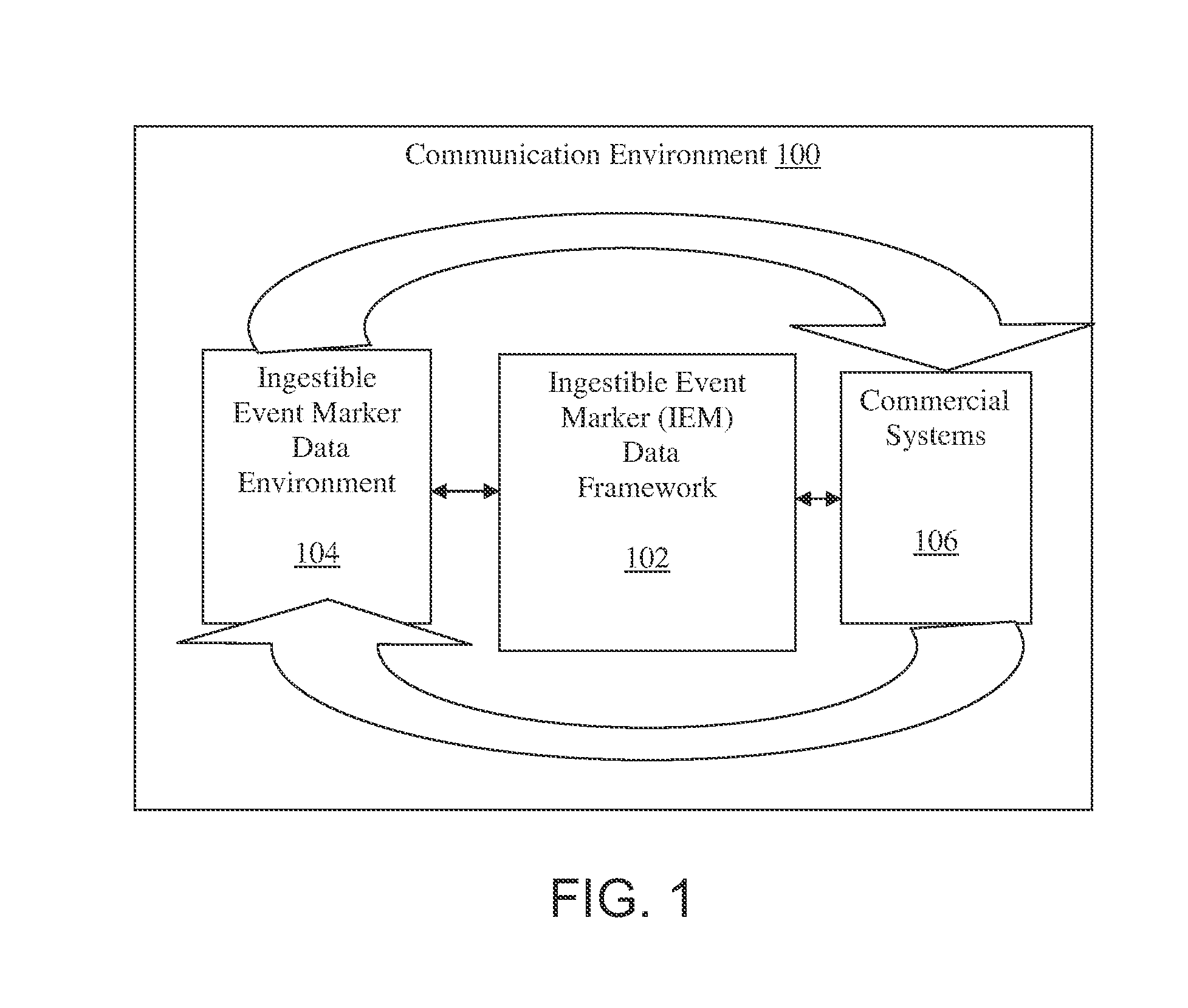
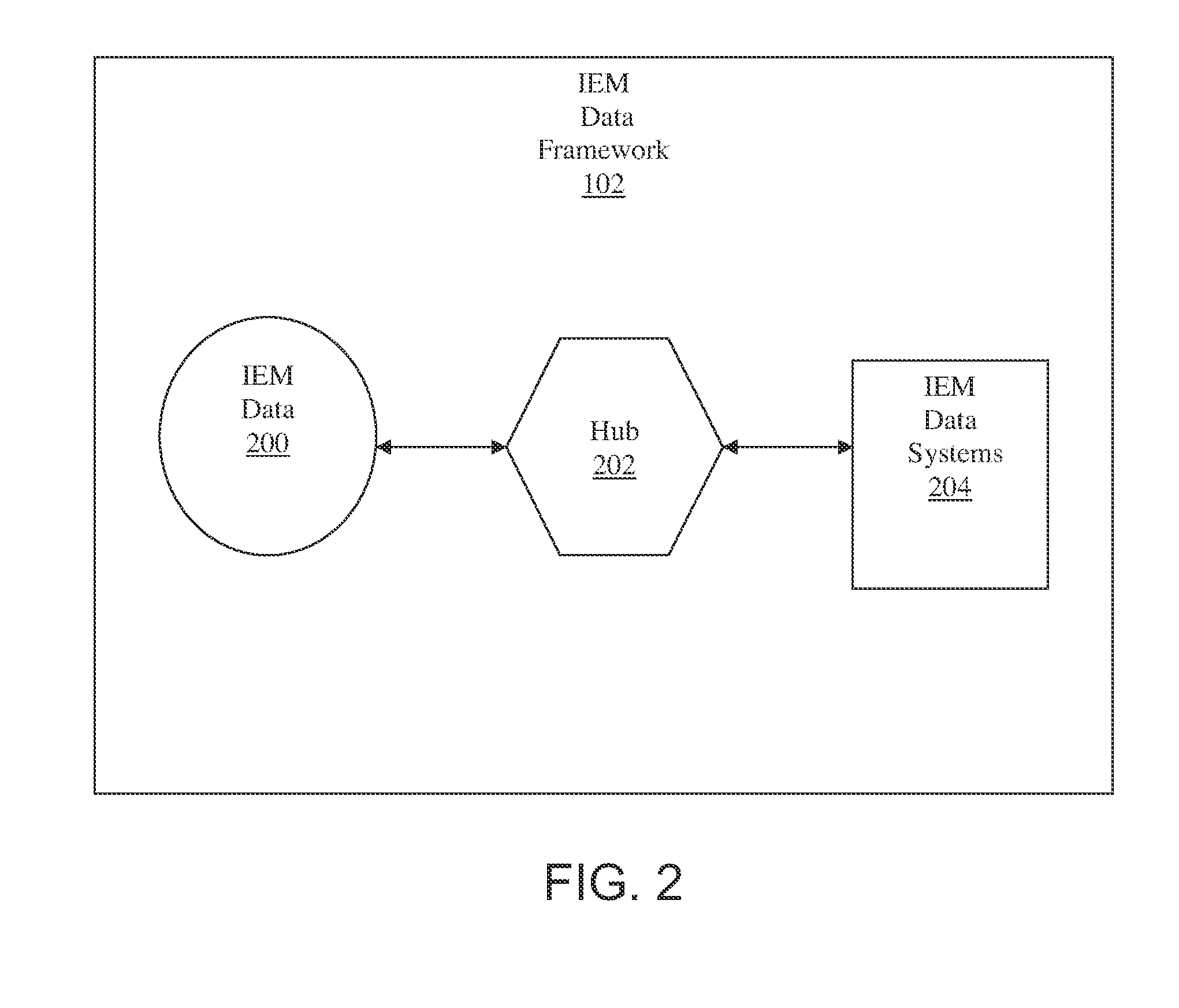
Ingestible event marker data framework
InactiveUS20110009715A1Physical therapies and activitiesDrug and medicationsLabeled dataData science
The ingestible event marker data framework provides a uniform, comprehensive framework to enable various functions and utilities related to ingestible event marker data (IEM data). The functions and utilities include data and / or information having an aspect of data derived from, collected by, aggregated by, or otherwise associated with, an ingestion event.
Owner:PROTEUS DIGITAL HEALTH INC
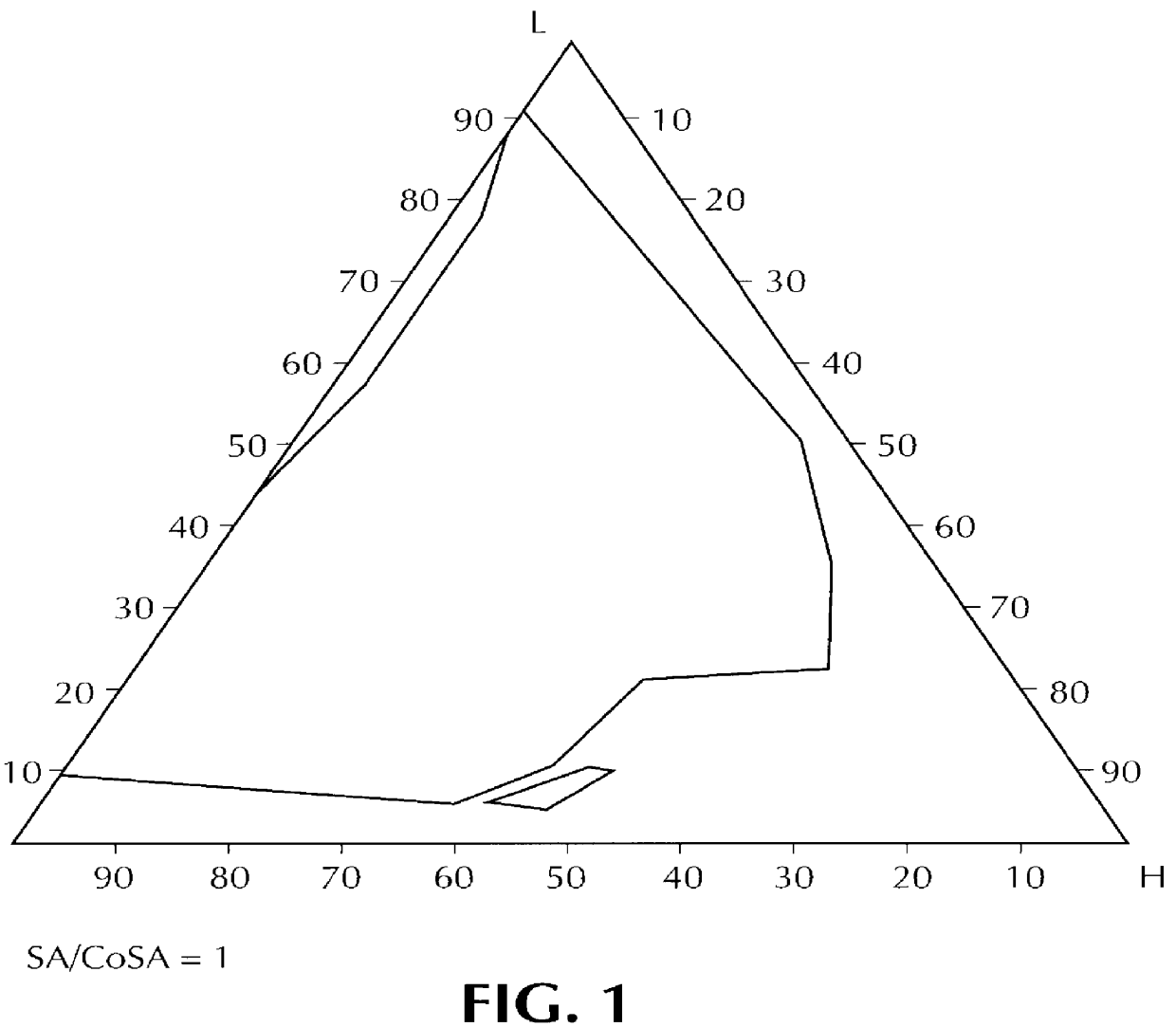
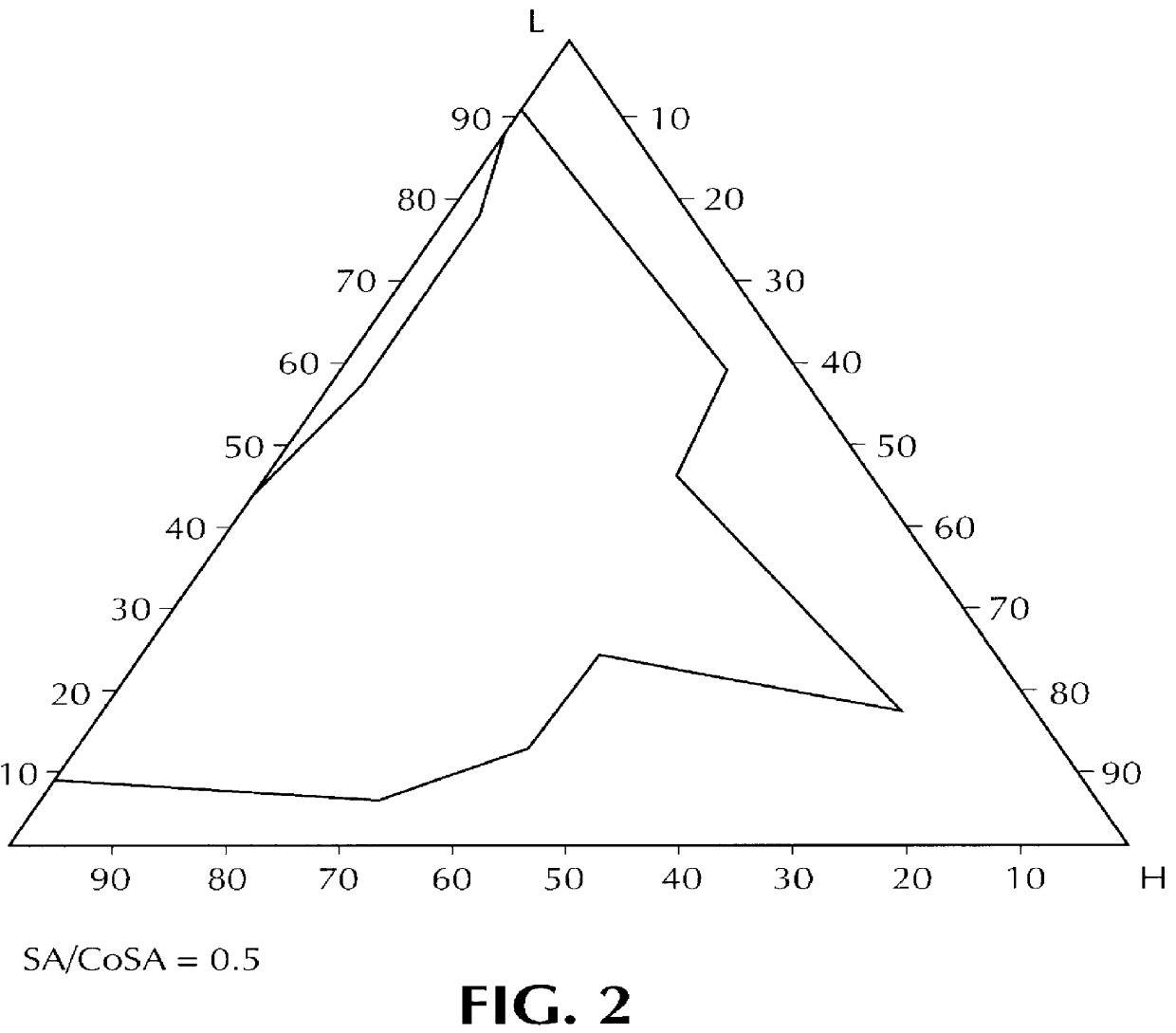
Orally administrable composition capable of providing enhanced bioavailability when ingested
InactiveUS6054136AImprove solubilityImprove bioavailabilityCosmetic preparationsToilet preparationsFatty acid esterIngestion
Composition for pharmaceutical or cosmetic use, capable of forming a microemulsion, comprising at least: an active principle, a lipophilic phase consisting of a mixture of fatty acid esters and glycerides, a surfactant (SA), a cosurfactant (CoSA), a hydrophilic phase, characterized: in that the lipophilic phase consists of a mixture of C8 to C18 polyglycolized glycerides having a hydrophilic-lipophilic balance (HLB) of less than 16, this lipophilic phase representing from 30 to 75% of the total weight of the composition; in that the surfactant (SA) is chosen from the group comprising saturated C8-C10 olyglycolized glycerides and oleic esters of polyglycerol, this surfactant having an HLB of less than 16; in that the cosurfactant (CoSA) is chosen from the group comprising lauric esters of propylene glycol, oleic esters of polyglycerol and ethyl diglycol; in that the SA / CoSA ratio is between 0.5 and 6; and in that the hydrophilic phase of the final microemulsion is supplied after ingestion by the physiological fluid of the digestive milieu.
Owner:GATTEFOSSE HLDG
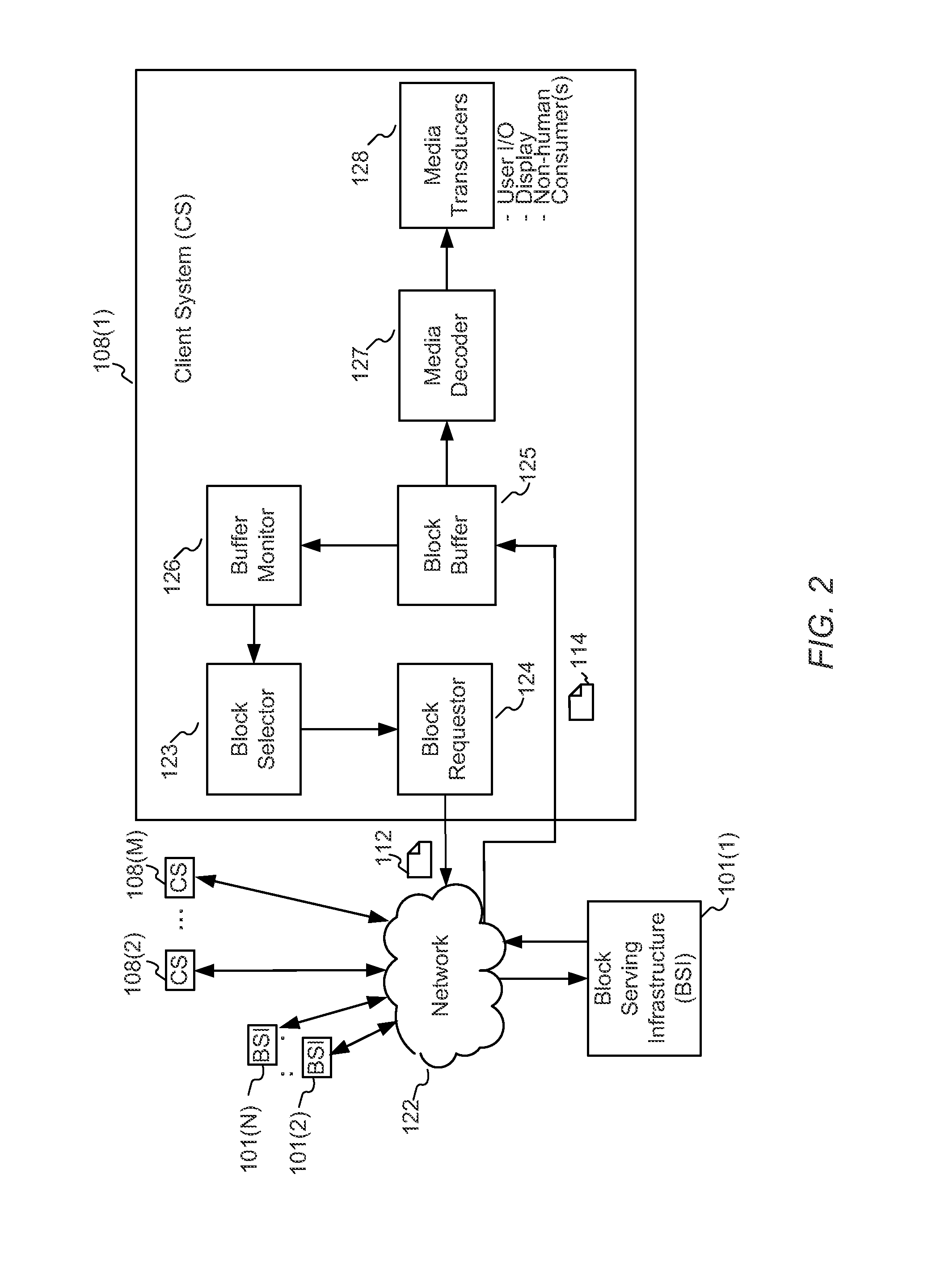
Enhanced block-request streaming using block partitioning or request controls for improved client-side handling
ActiveUS20110231569A1Improve bandwidth efficiencyImprove user experienceMultiple digital computer combinationsSelective content distributionTransfer systemClient-side
A block-request streaming system provides for improvements in the user experience and bandwidth efficiency of such systems, typically using an ingestion system that generates data in a form to be served by a conventional file server (HTTP, FTP, or the like), wherein the ingestion system intakes content and prepares it as files or data elements to be served by the file server. A client device can be adapted to take advantage of the ingestion process. The client device might be configured to optimize use of resources, given the information available to it from the ingestion system. This may include configurations to determine the sequence, timing and construction of block requests based on monitoring buffer size and rate of change of buffer size, use of variable sized requests, mapping of block requests to underlying transport connections, flexible pipelining of requests, and / or use of whole file requests based on statistical considerations.
Owner:QUALCOMM INC
Radio frequency identification based system to track consumption of medication
InactiveUS20050285746A1Drug and medicationsCo-operative working arrangementsDevice MonitorRadio frequency
A radio frequency identification (RFID) based system to track consumption of medicine is disclosed. An RFID device monitors for a signal pattern indicative of an RFID tag contained within medication and determines a status based on a presence or absence of the signal pattern. The signal pattern includes a medication identifier and a signal level over time. A detected signal pattern is compared to stored ingestion profiles to distinguish between consumed and non-consumed medication.
Owner:INTEL CORP
Optical sensor containing particles for in situ measurement of analytes
ActiveUS7228159B2Material analysis by observing effect on chemical indicatorMicrobiological testing/measurementAnalyteBiological organism
The invention relates to a sensor for the in vivo measurement of an analyte, comprising a plurality of particles of suitable size such that when implanted in the body of a mammal the particles can be ingested by macrophages and transported away from the site of implantation, each particle containing the components of an assay having a readout which is an optical signal detectable transdermally by external optical means, and either each particles being contained within a biodegradable material preventing ingestion by the macrophages, or each particle being non-biodegradable. The invention relates to a process for the detection of an analyte using such a sensor, comprising implantation of the sensor into the skin of a mammal, transdermal detection of analyte using external optical means, degradation of the biodegradable material, ingestion of the particles by macrophages, and removal of the particles from the site of implantation by macrophages.
Owner:MEDTRONIC MIMIMED INC
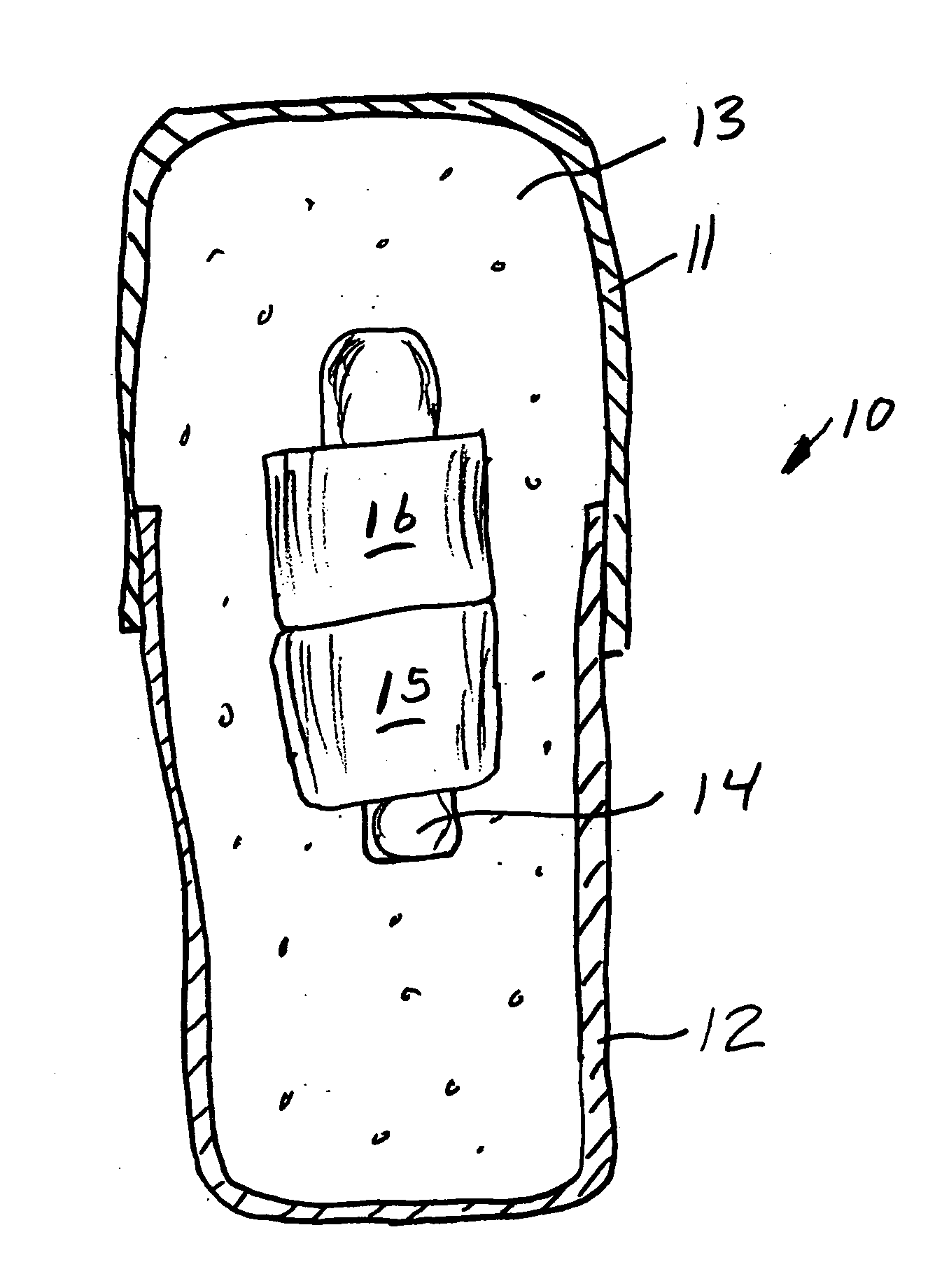
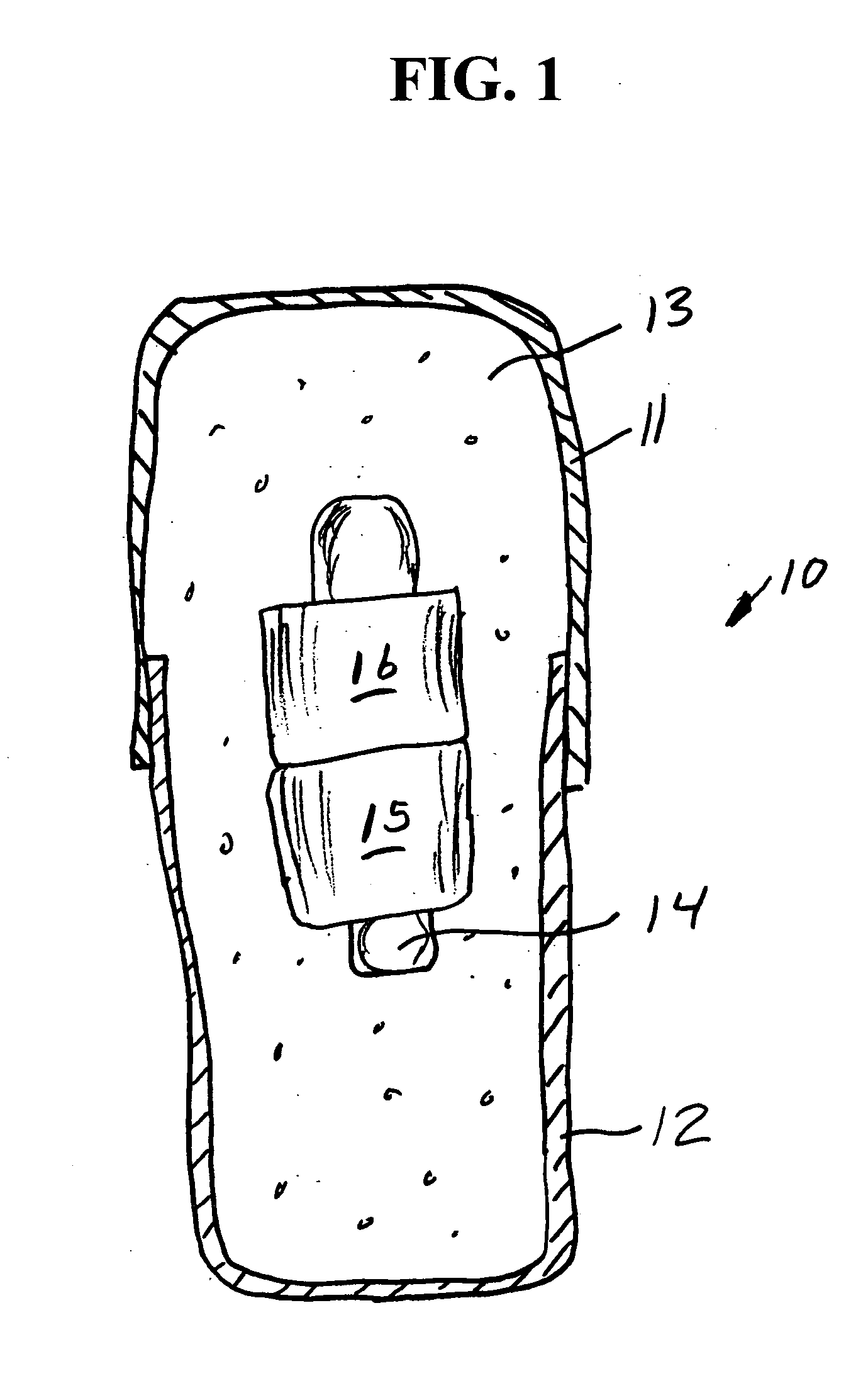
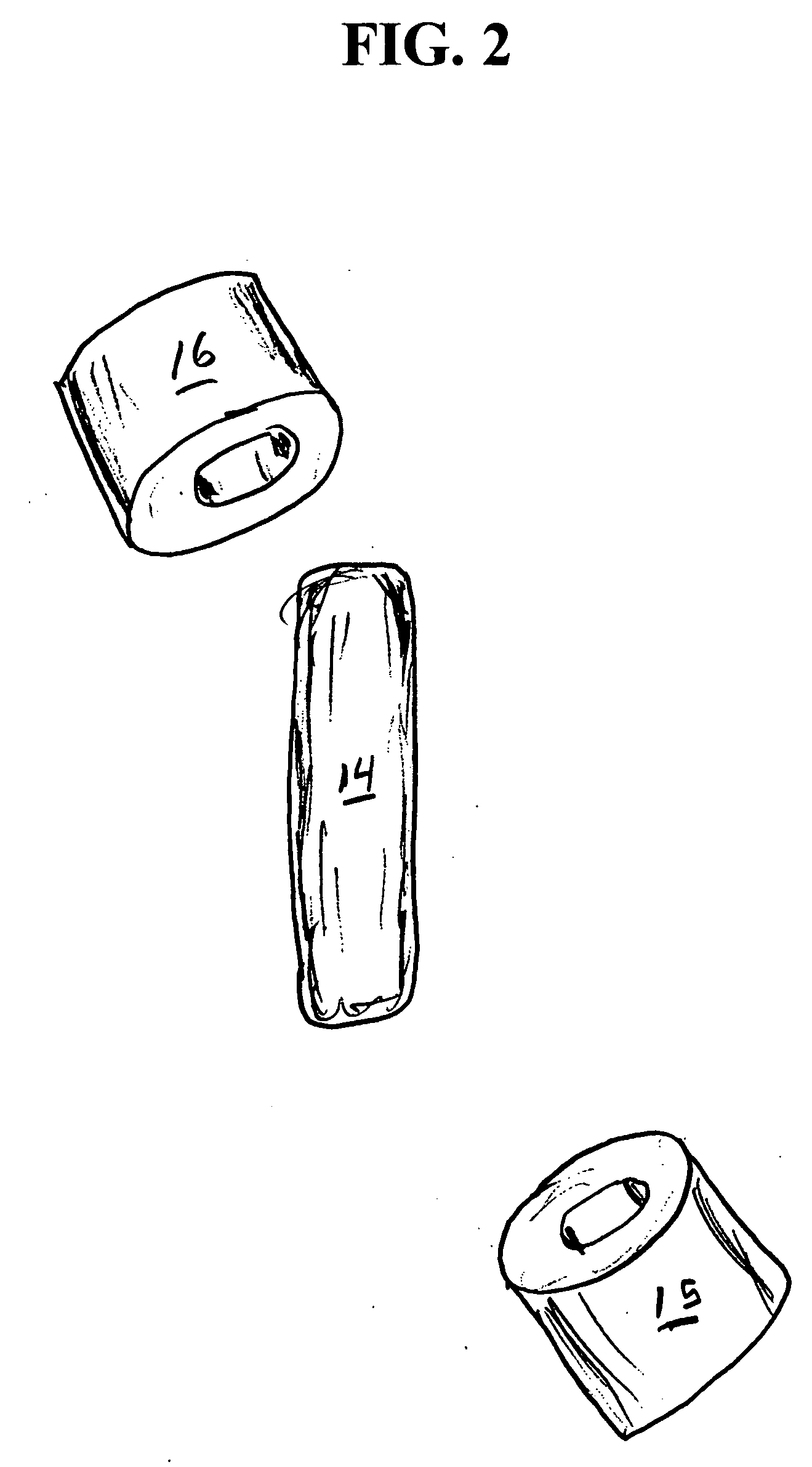
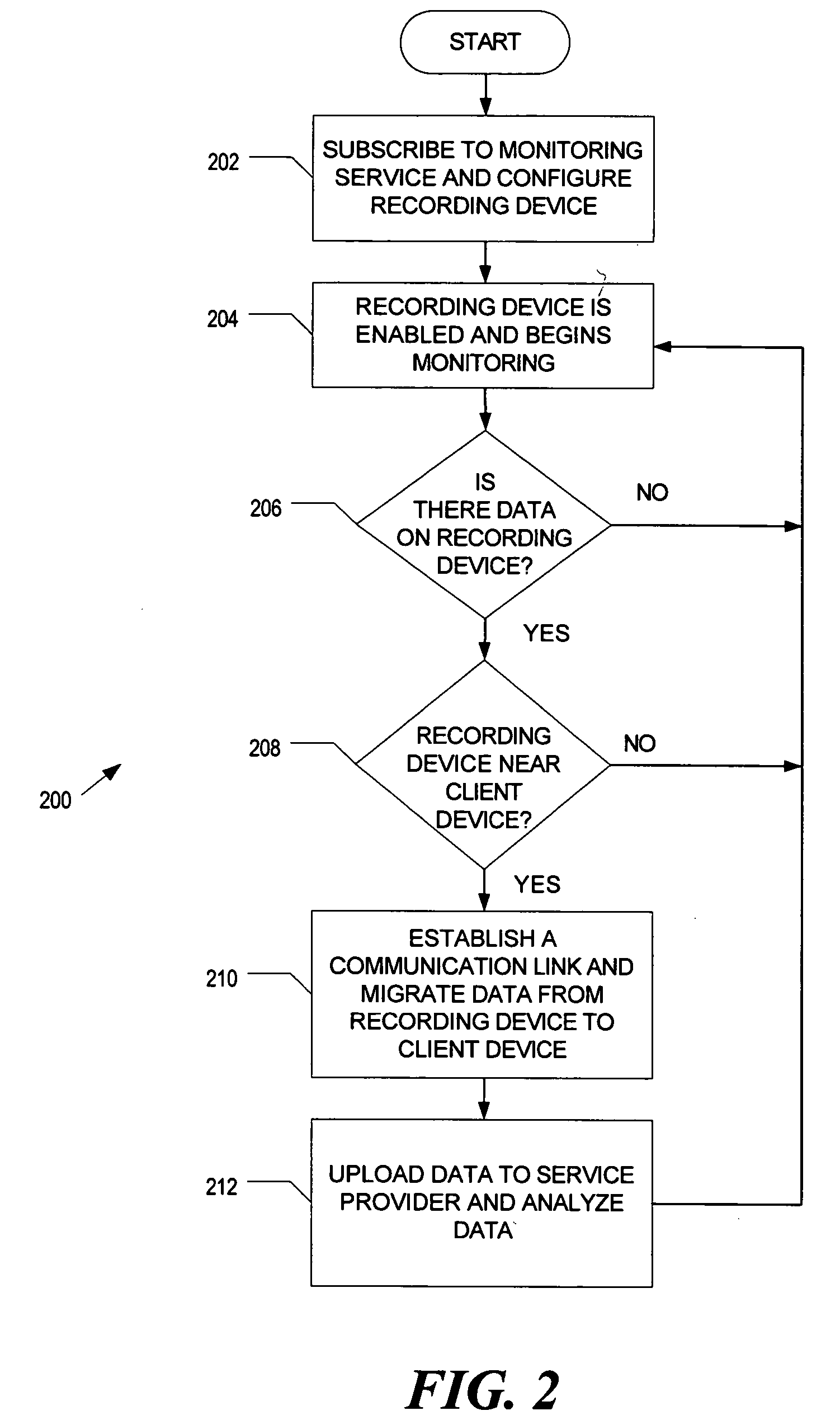
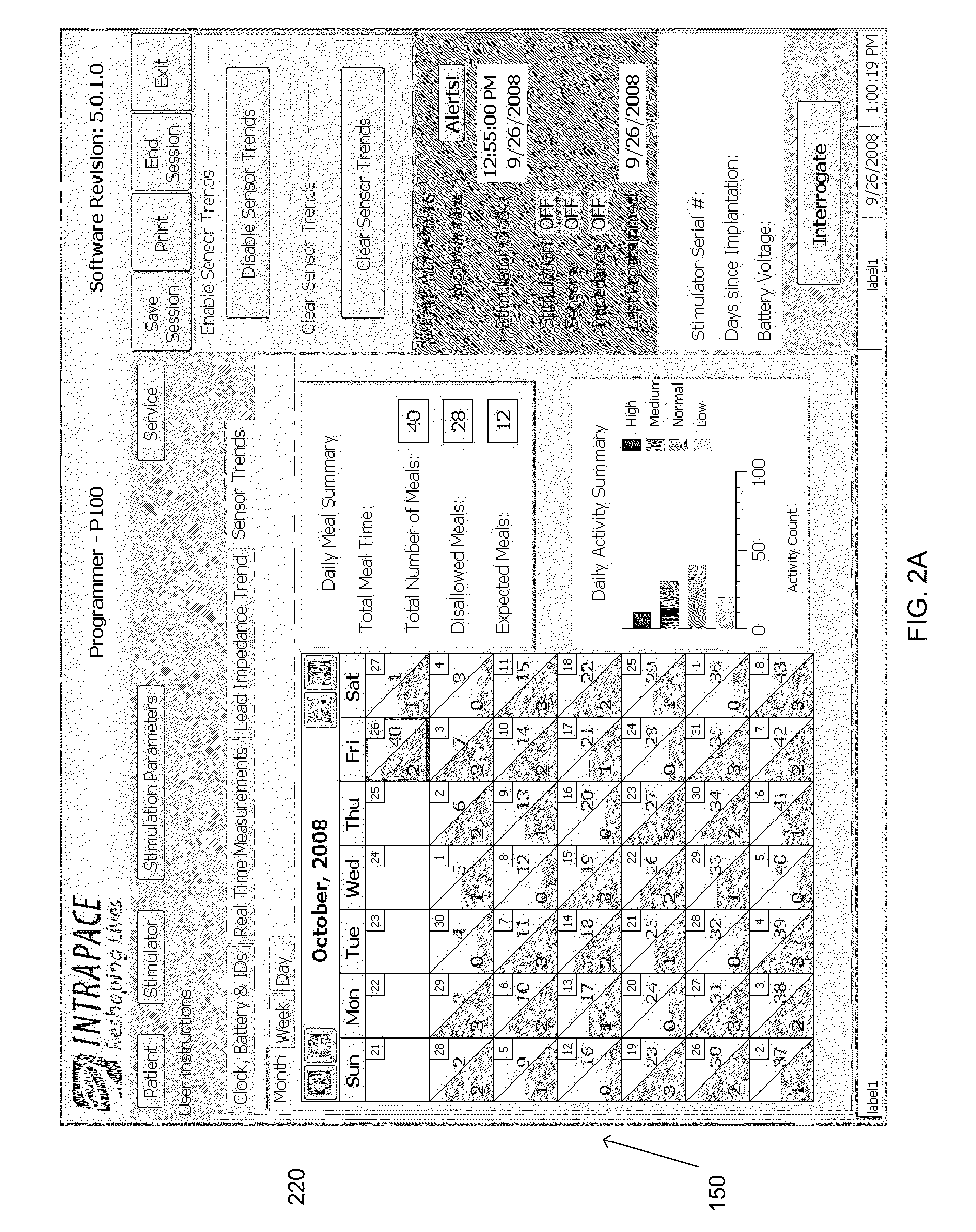
Medicine ingestion state management method, medicine and medicine ingestion state management device
ActiveUS20060145876A1Avoid overdosePrevent an excessive dosage of the medicine to the patientDrug and medicationsSurgeryMedication informationState management
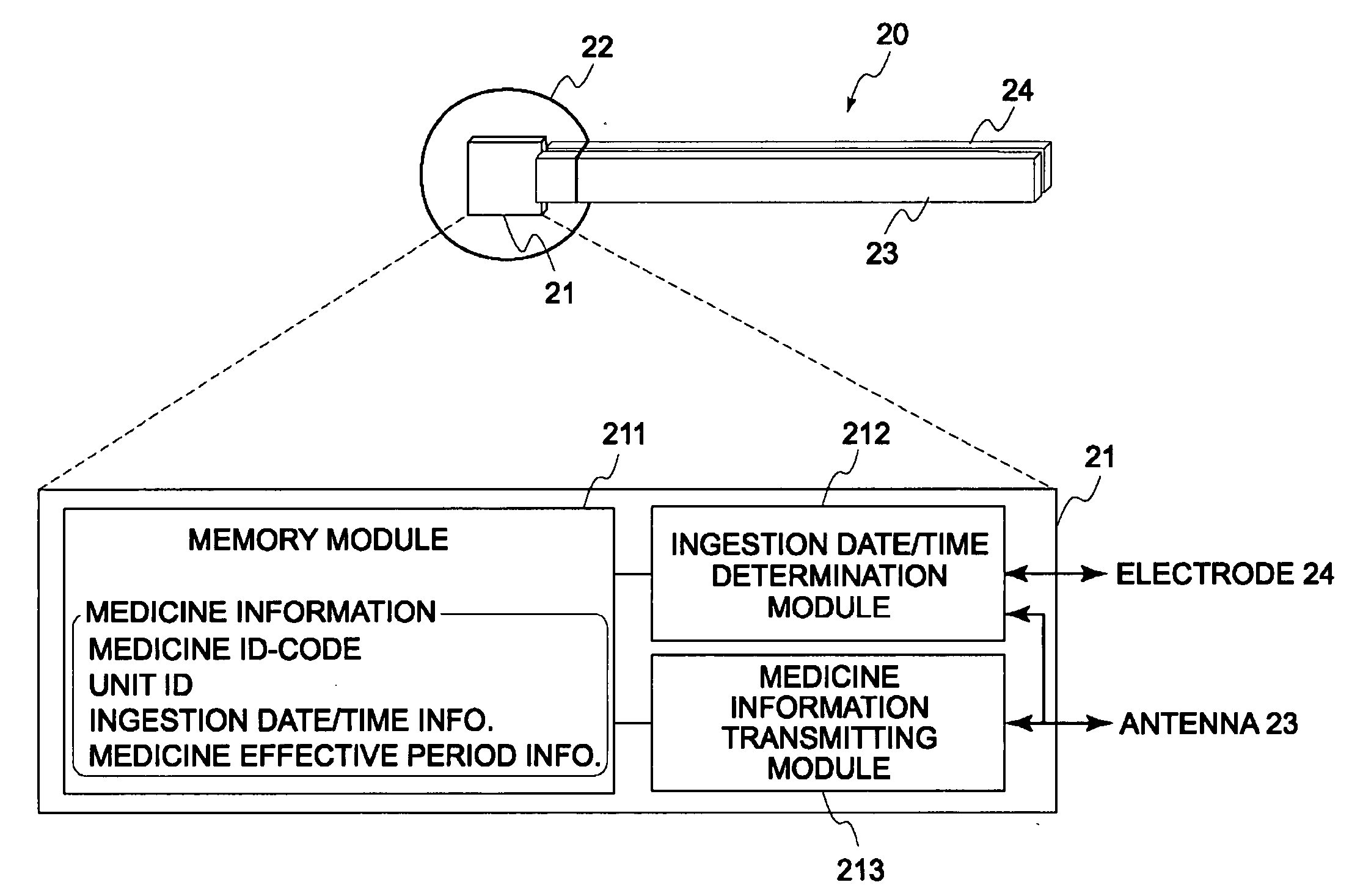
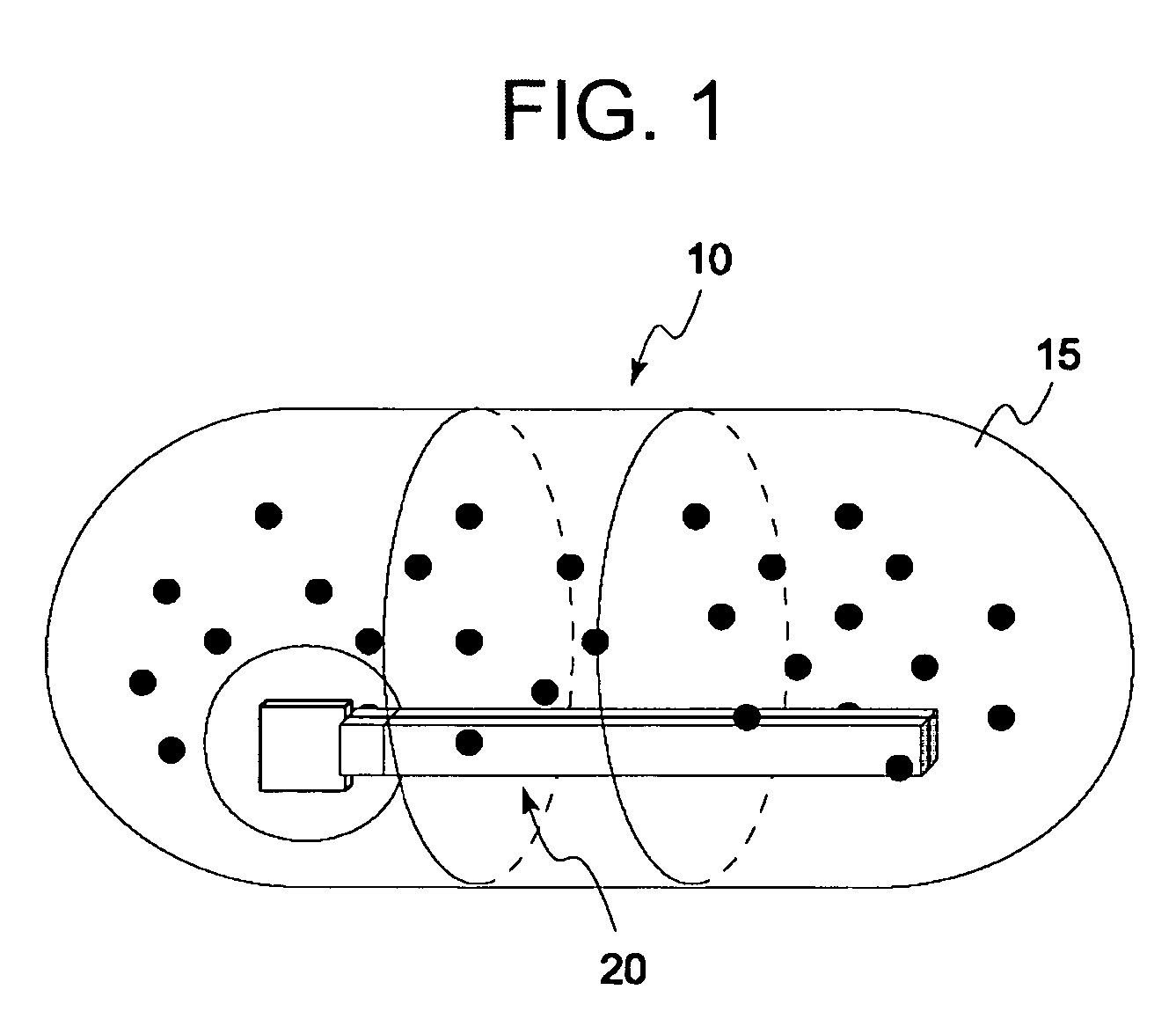
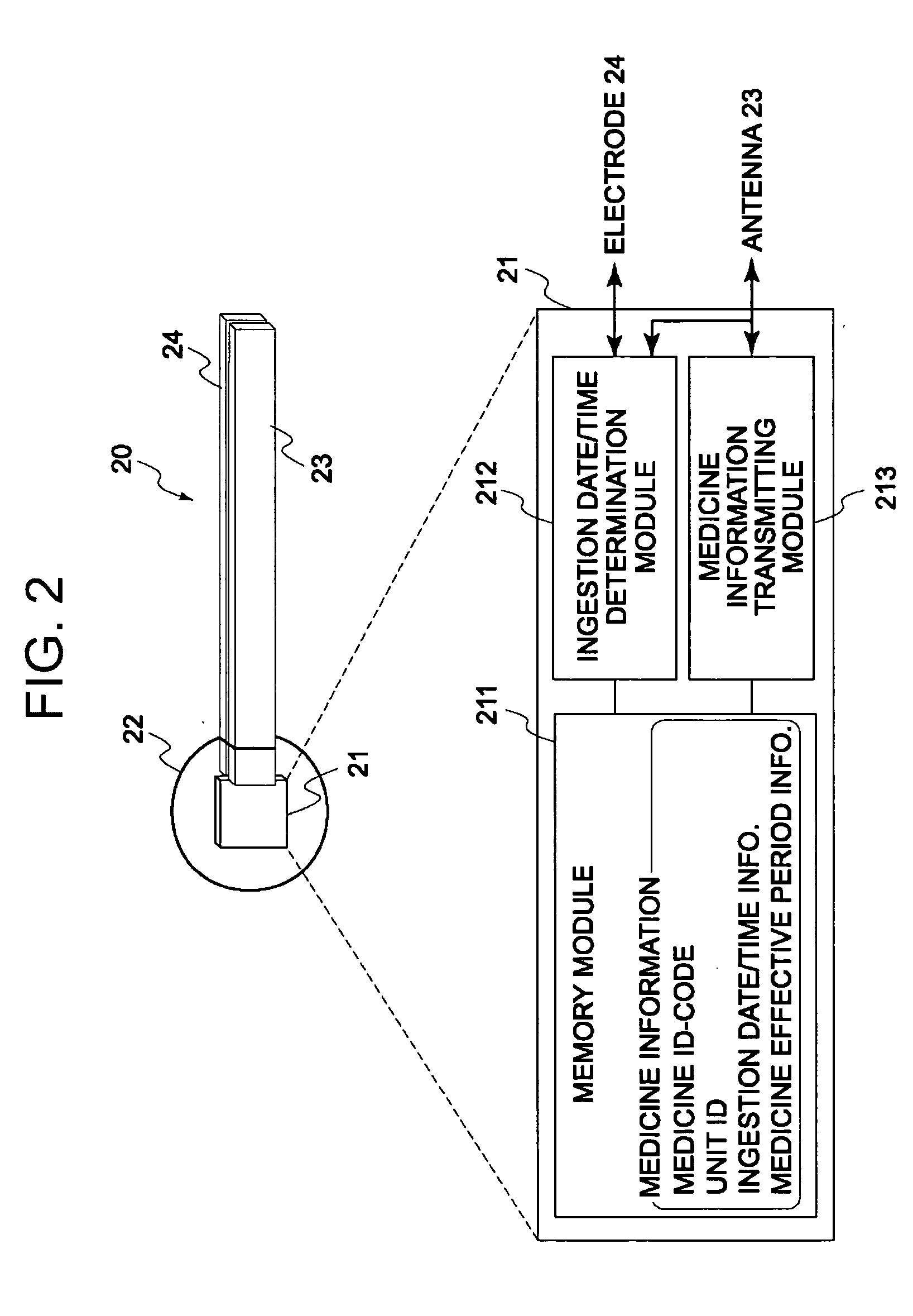
Disclosed is a medicine ingestion state management method capable of objectively managing medicine ingestion states of patients. The medicine ingestion state management method involves an operation of prescribing medicine (10) encapsulating, together with a medicament, a medicine information transmitting unit (20) having a function of transmitting medicine information capable of specifying a type and a quantity of the medicament to each individual patient, and an operation of grasping the medicine ingestion state of each patient by collecting the medicine information from each medicine information transmitting unit (20) in each patient.
Owner:FUJITSU LTD
Displaying Estimated Social Interest in Time-based Media
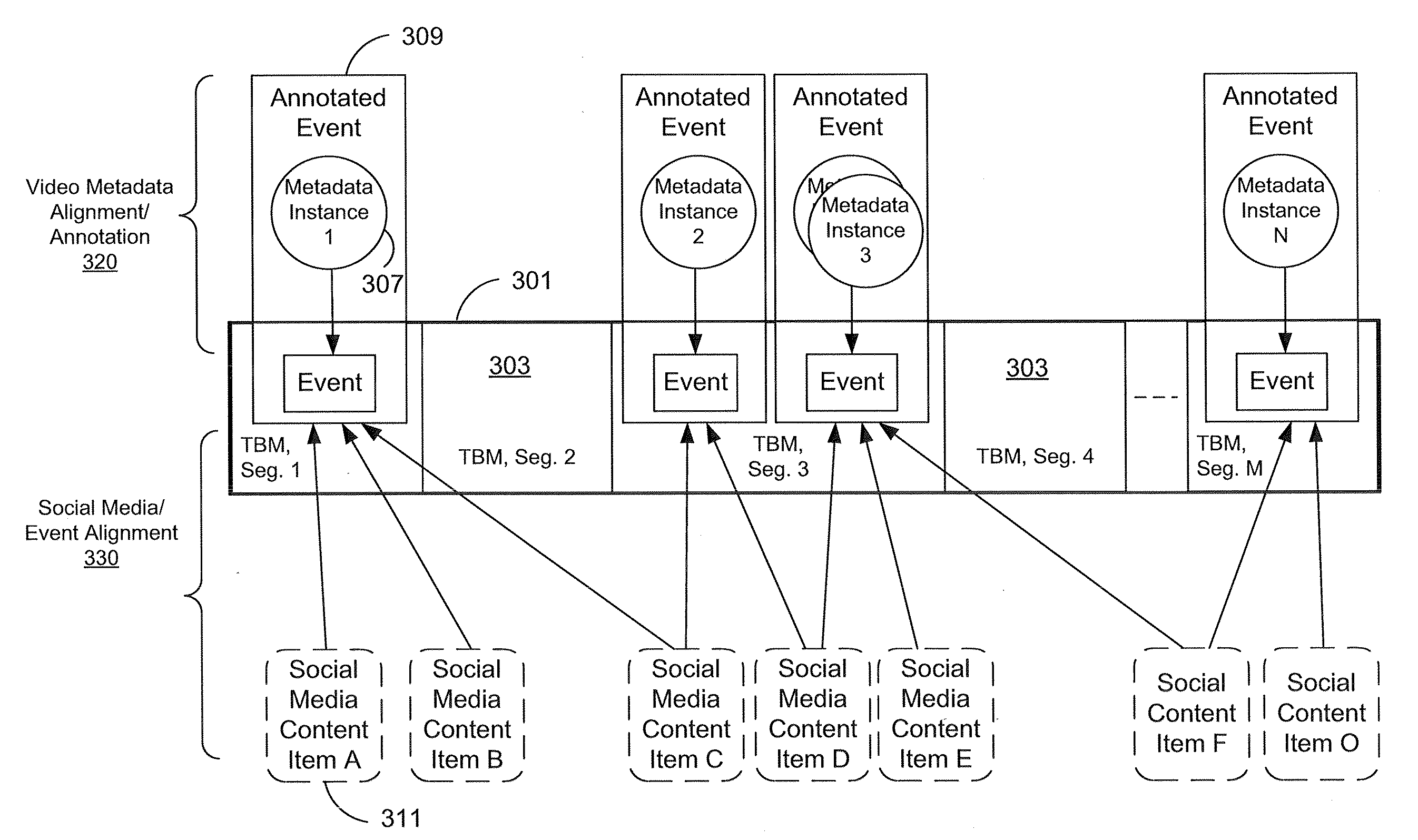
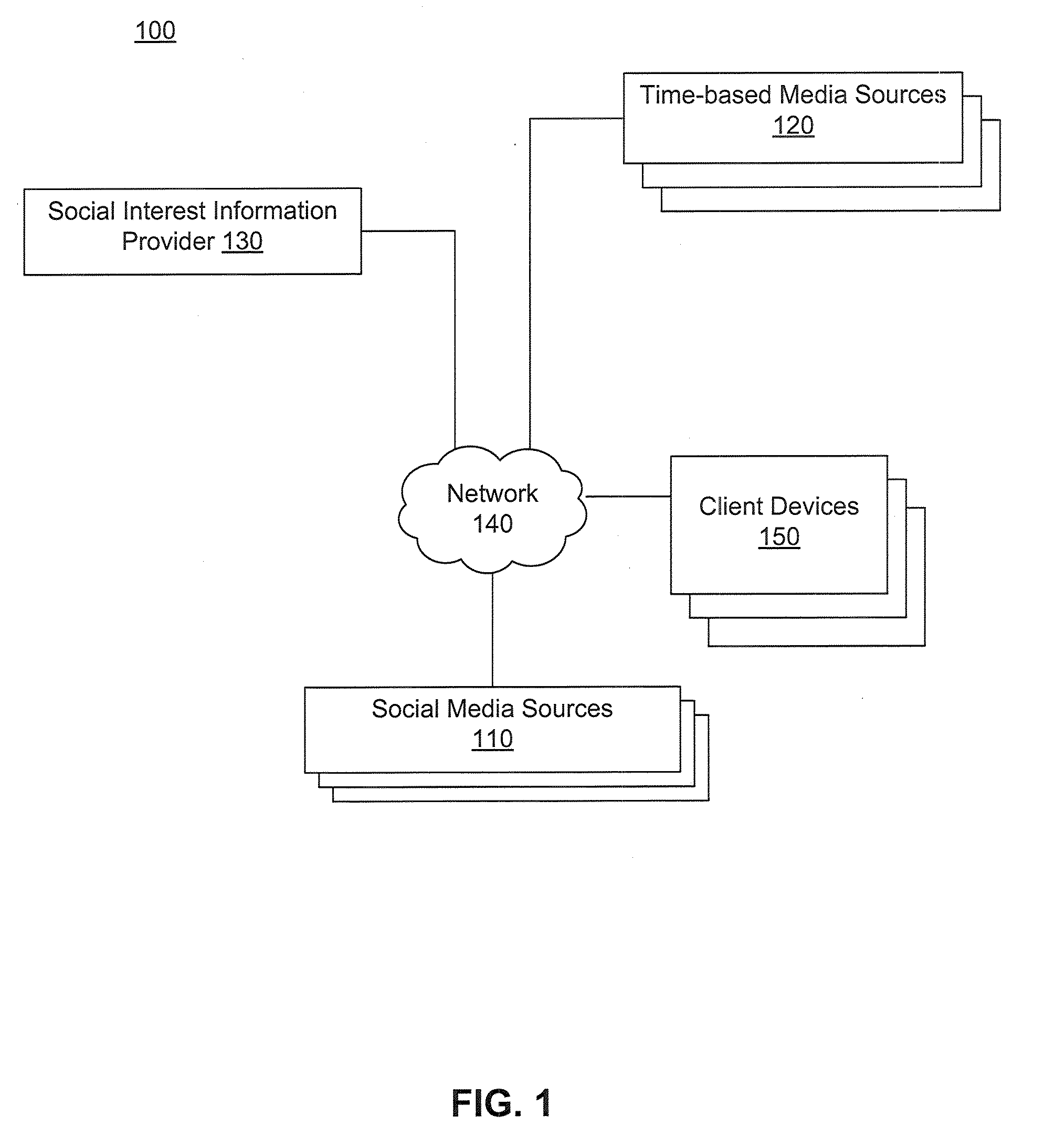
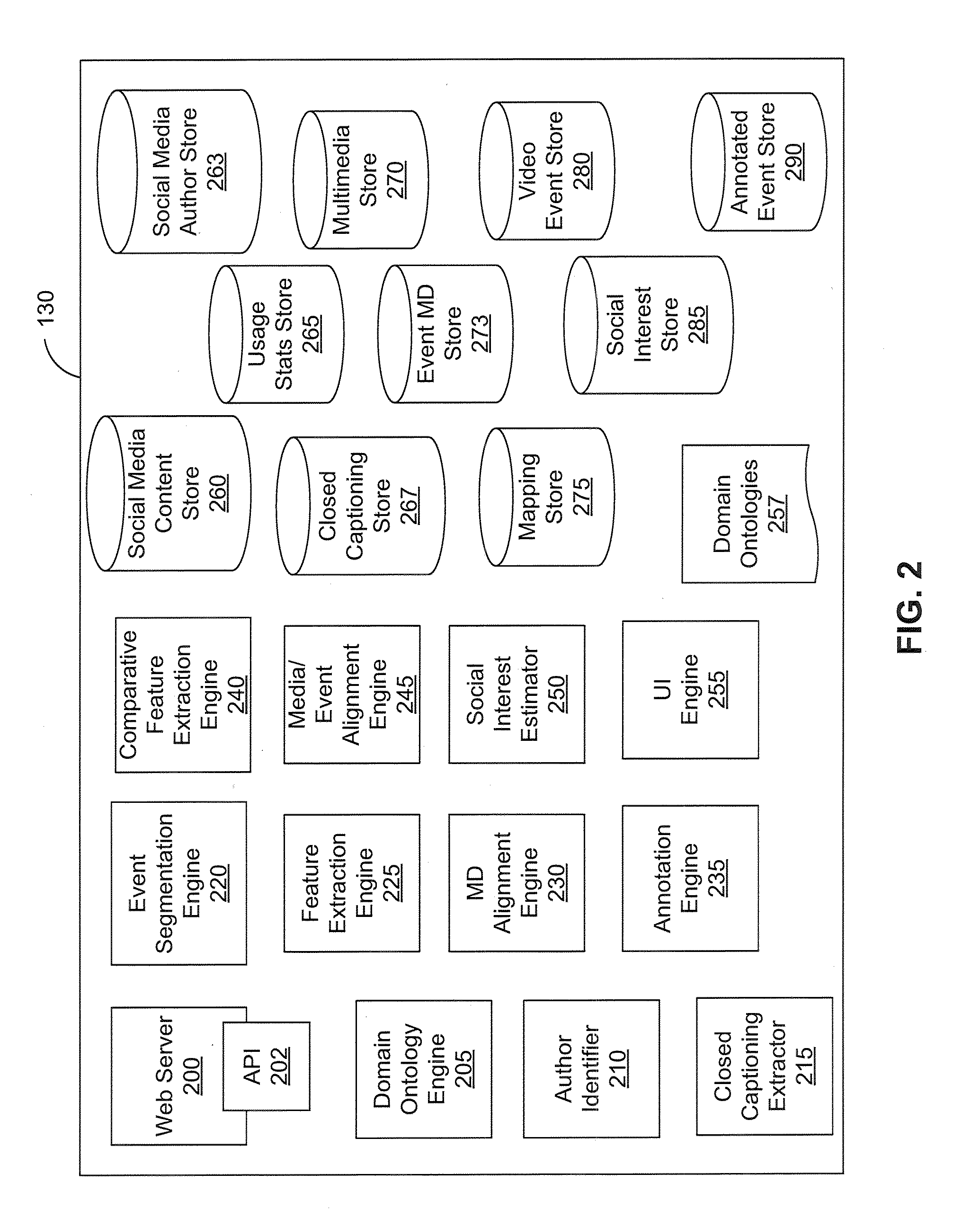
ActiveUS20110041080A1Input/output for user-computer interactionVisual data miningGraphicsData ingestion
Social media content items are mapped to relevant time-based media events. These mappings may be used as the basis for multiple applications, such as ranking of search results for time-based media, automatic recommendations for time-based media, prediction of audience interest for media purchasing / planning, and estimating social interest in the time-based media. Social interest in time-based media (e.g., video and audio streams and recordings) segments is estimated through a process of data ingestion and integration. The estimation process determines social interest in specific events represented as segments in time-based media, such as particular plays in a sporting event, scenes in a television show, or advertisements in an advertising block. The resulting estimates of social interest also can be graphically displayed.
Owner:BLUEFIN LABS
Intragastric device
An implant configured for ingestion by a patient. After the implant has been swallowed by the patient and is disposed within the target location, e.g. the patient's stomach, an inflation subcomponent causes the implant to expand from a compact delivery state to an expanded, volume-occupying, deployed state. In the deployed state the implant creates a sensation of satiety in the patient stomach and thereby aids in limiting food intake and obesity. After a predetermined time a deflation subcomponent is actuated and the implant reduces in size so as to allow it to pass through the remainder of the patient's digestive track. The device may further incorporate tracking and visualization subcomponents, as well as pharmaceutical delivery subcomponents.
Owner:RESHAPE LIFESCIENCES INC
Intragastric device
An implant configured for ingestion by a patient. After the implant has been swallowed by the patient and is disposed within the target location, e.g. the patient's stomach, an inflation subcomponent causes the implant to expand from a compact delivery state to an expanded, volume-occupying, deployed state. In the deployed state the implant creates a sensation of satiety in the patient stomach and thereby aids in limiting food intake and obesity. After a predetermined time a deflation subcomponent is actuated and the implant reduces in size so as to allow it to pass through the remainder of the patient's digestive track. The device may further incorporate tracking and visualization subcomponents, as well as pharmaceutical delivery subcomponents.
Owner:RESHAPE LIFESCIENCES INC
Obesity treatment systems
In some embodiments, an extragastric balloon is described in which the balloon is contoured to fit a portion of the stomach but not circumscribe the stomach. In some embodiments, the extragastric balloon comprises multiple material components. In some embodiments, the extragastric balloon comprises multiple inflateable compartments. In some embodiments, the extragastric balloon is filled with one or more of a gas, liquid, solid, or foams to create portions of the balloon with differing densities or buoyancies. In some embodiments, multiple anchors contact the stomach, each of which are inflateable with a fluid such as a liquid, gas, or gel. In some embodiments, a pressure sensor is described which is programmable from a place external to the patient and can be programmed to increase its sampling frequency when an ingestion event is detected.
Owner:GERTNER MICHAEL
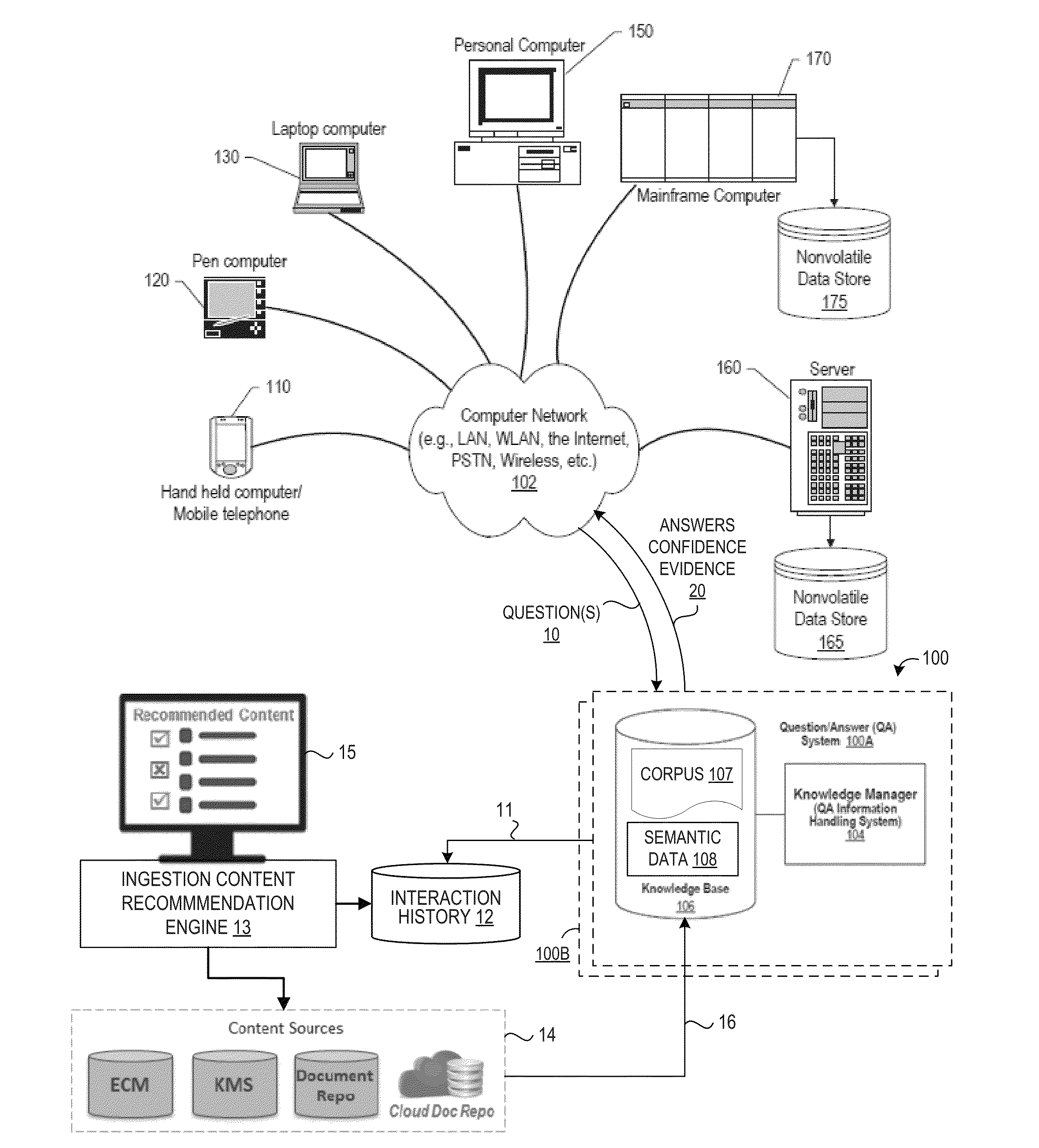
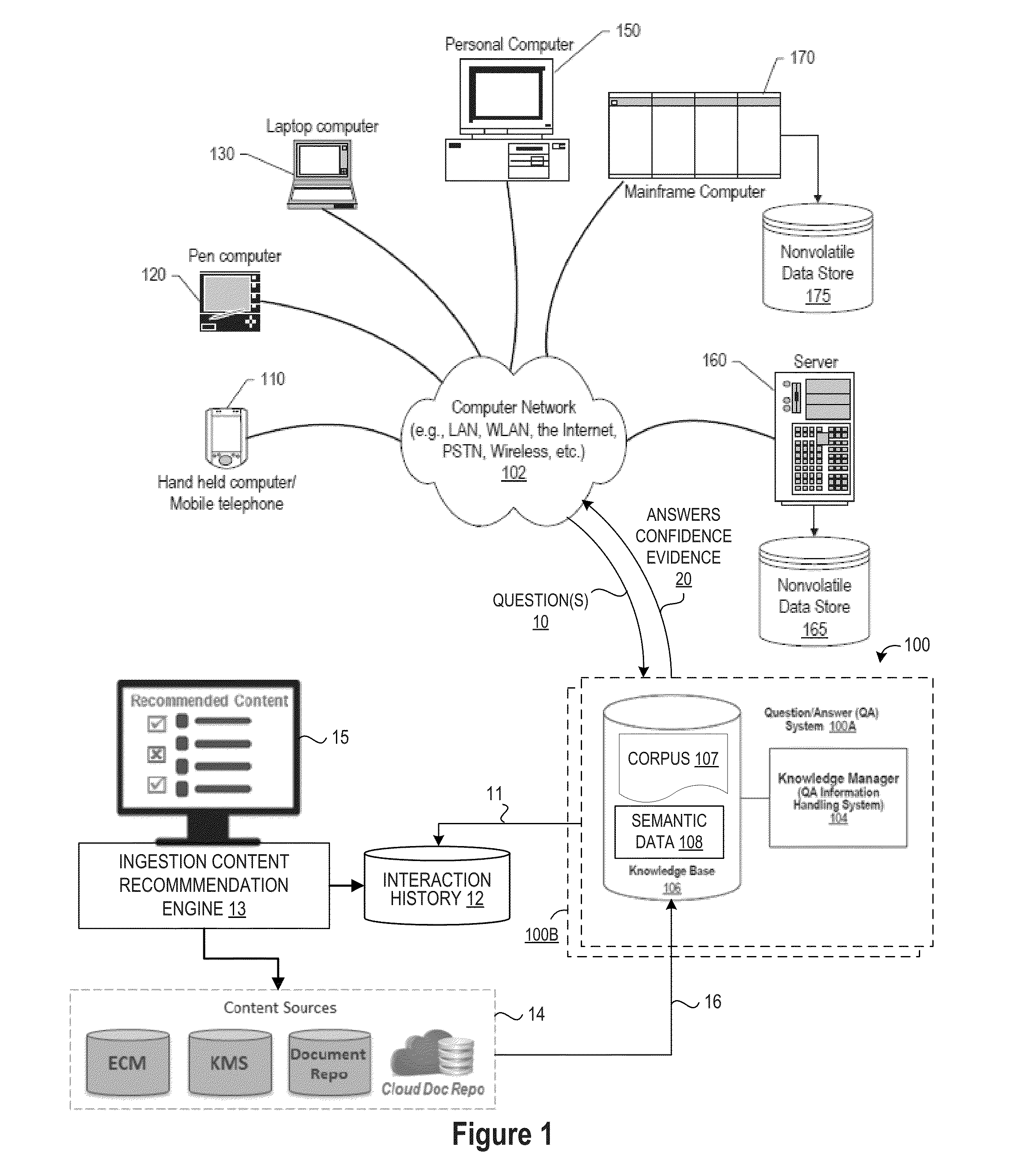
Method For Recommending Content To Ingest As Corpora Based On Interaction History In Natural Language Question And Answering Systems
InactiveUS20160196491A1Quality improvementNatural language translationSemantic analysisQuestions and answersA domain
An approach is provided for generating actionable content ingestion recommendations based on an interaction history that is mined to extract interaction context parameters from questions and answer results that meet specified answer deficiency criteria by searching one or more content sources using the extracted interaction context parameters to identify new content that is relevant to improving the first answer, and then presenting the new content in an actionable content ingestion recommendation list for display and review by a domain expert, where the actionable content ingestion recommendation list recommends the new content for ingestion in a knowledge base corpus.
Owner:IBM CORP
Method and apparatus for monitoring ingestion of medications using an implantable medical device
InactiveUS20080288027A1Reduce the possibilityIncrease chances of survivalDrug and medicationsSurgeryTransceiverCardiac pacemaker electrode
An implantable medical device, such as a pacemaker or implantable cardioverter defibrillator (ICD), is configured to automatically detect ingestion of medications to verify that prescribed medications are taken in a timely manner and at the correct dosage. Briefly, individual pills are provided with miniature radio frequency identification (RFID) devices capable of transmitting RFID tag signals, which identify the medication contained within the pill and its dosage. The implanted device is equipped with an RFID transceiver for receiving tag signals from a pill as it is being ingested. The implanted system decodes the tag to identify the medication and its dosage, then accesses an onboard database to verify that the medication being ingested was in fact prescribed to the patient and to verify that the correct dosage was taken. Warning signals are generated if the wrong medication or the wrong dosage was taken. Therapy may also be automatically adjusted. Non-RF-based ID devices are also described, which instead transmit ID data via biphasic current pulses.
Owner:PACESETTER INC
Intragastric device
Owner:RESHAPE LIFESCIENCES INC
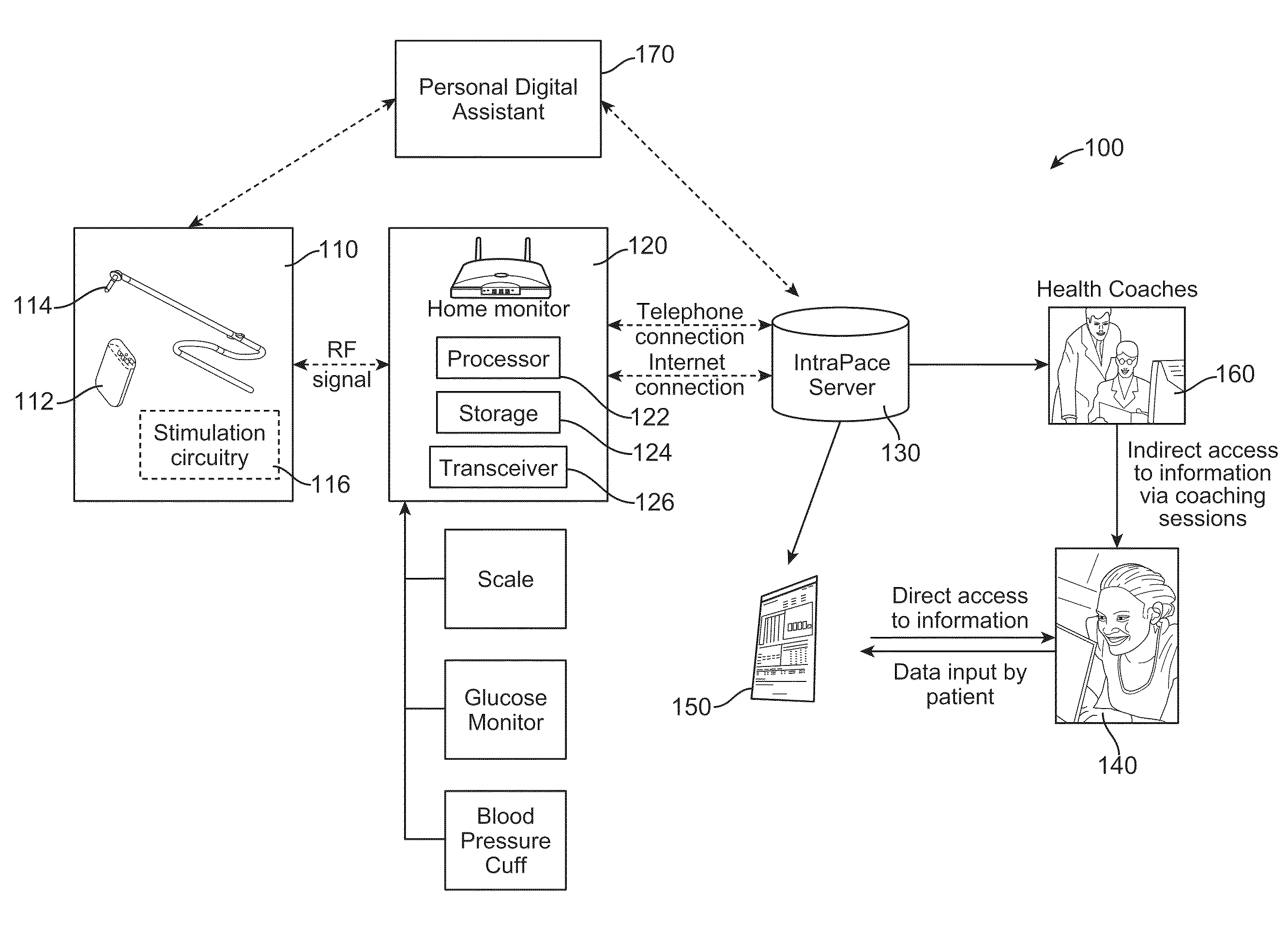
Feedback systems and methods for communicating diagnostic and/or treatment signals to enhance obesity treatments
InactiveUS20110087076A1Accurate and reliable behavior-modification feedbackGood curative effectPhysical therapies and activitiesElectrotherapyFeeding disabilityFeeding disorder
Owner:INTRAPACE
Methods of treating cutaneous flushing using selective alpha-2-adrenergic receptor agonists
InactiveUS20050020600A1Effective treatmentTreatment safetyBiocidePharmaceutical delivery mechanismQuinoxalineVasomotor instability
The present invention relates to a method of treating, reducing, inhibiting, preventing and / or reversing cutaneous facial flushing caused by abnormal, endogenously-induced vasomotor instability associated with, but not limited to acne rosacea, menopause-associated hot flashes, hot flashes resulting from orchiectomy or ingestion of substances capable of inducing a cutaneous facial flushing reaction (e.g.: alcohol, chocolate, spices) by topical dermatological application of an effective dose of a composition comprising at least one α2 adrenergic receptor agonist (such as a (2-imidazolin-2-ylamino) quinoxaline derivative such as brimonidine tartrate)and a suitable carrier.
Owner:GALDERMA LAB LP
System to monitor the ingestion of medicines
InactiveUS7616111B2Easy to manageEliminate needPharmaceutical product form changeMedical devicesComputer hardwareRadio frequency
Owner:CARESTREAM HEALTH INC
Alteration of microbial populations in the gastrointestinal tract
InactiveUS6348452B1Increase the number ofHigh activityBiocideBacteria material medical ingredientsMicroorganismResistant starch
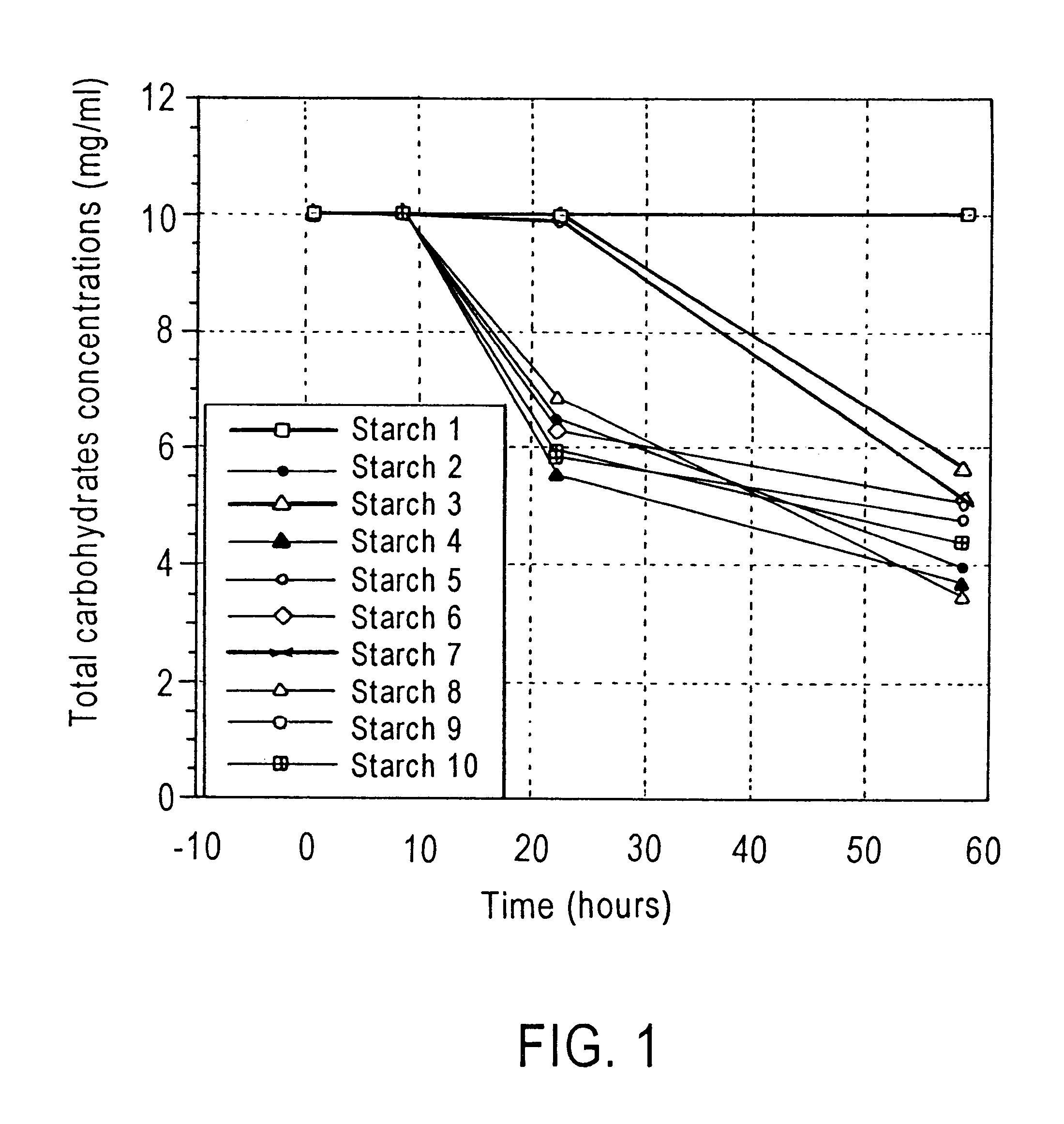
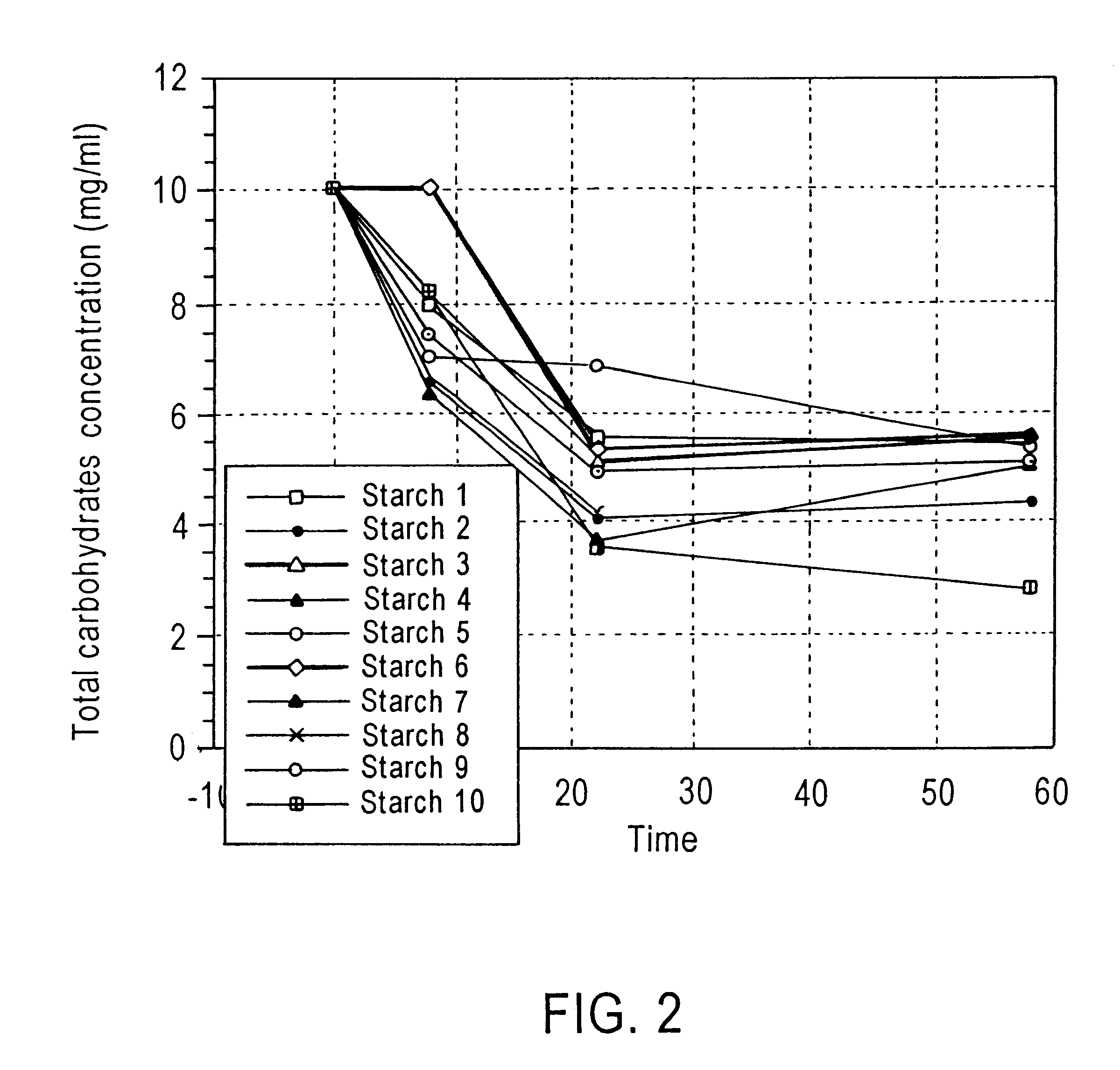
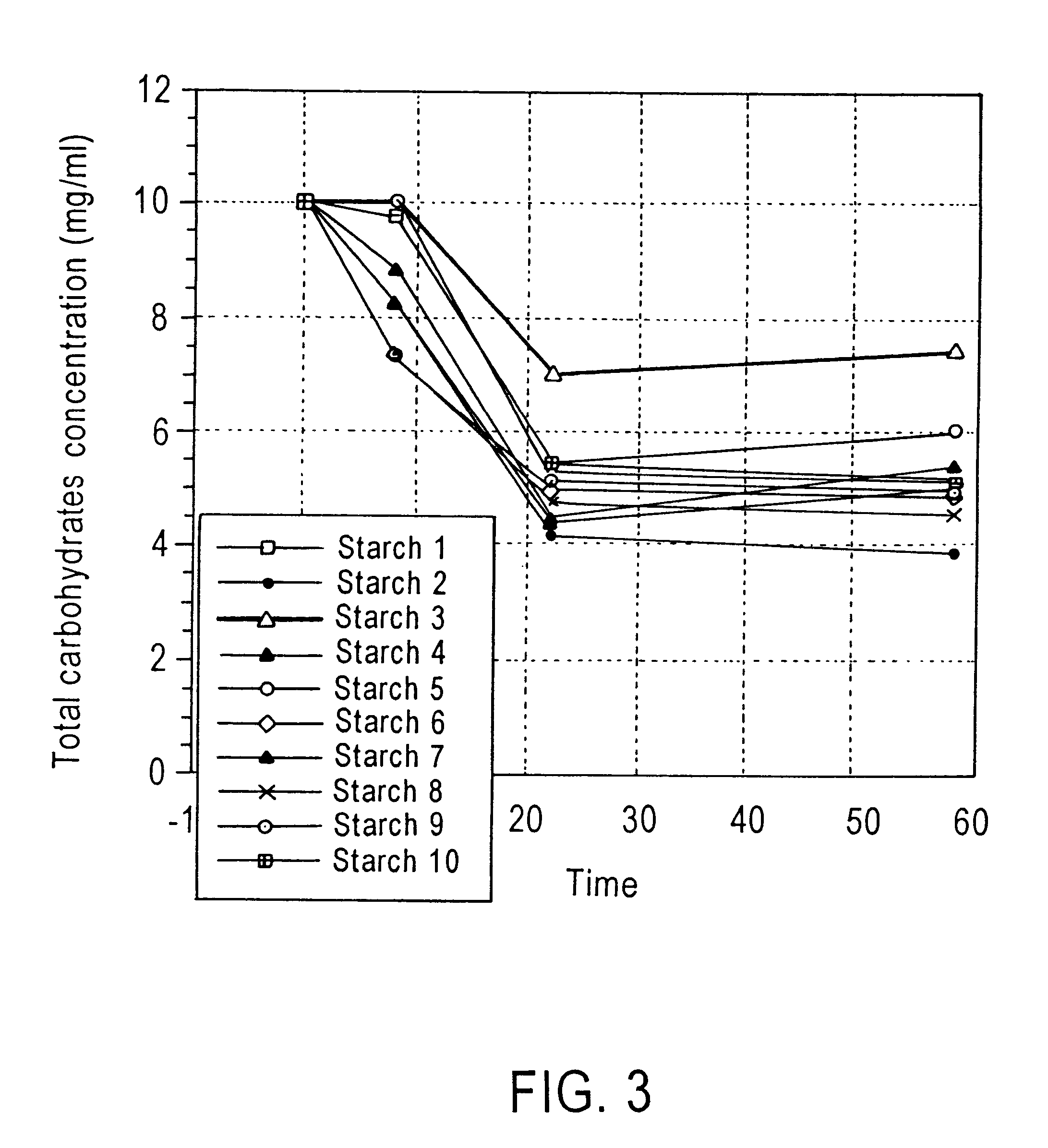
Method of enhancing a resident population of microorganism in a selected site of the gastrointestinal tract of an animal, the method comprising providing to the animal a selected modified or unmodified resistant starch or mixtures thereof in combination with one or more probiotic microorganisms such that upon ingestion the starch passes through the gastrointestinal tract substantially unutilized until it reaches the selected site where it is utilized by the resident and / or the probiotic microorganisms thereof causing an increase in number and / or activity of the microorganisms.
Owner:CORN PROD DEV INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com