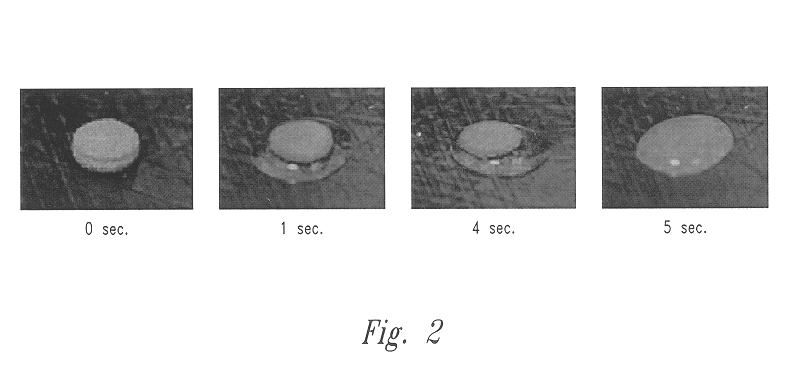
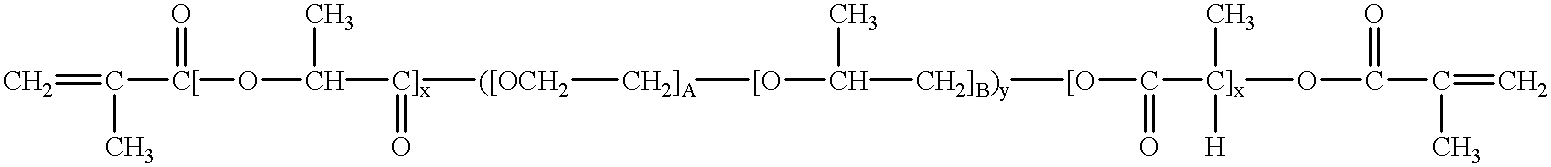
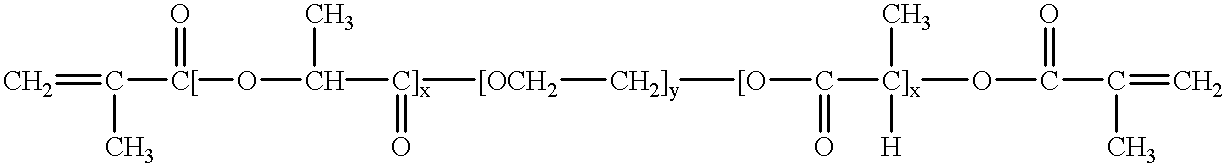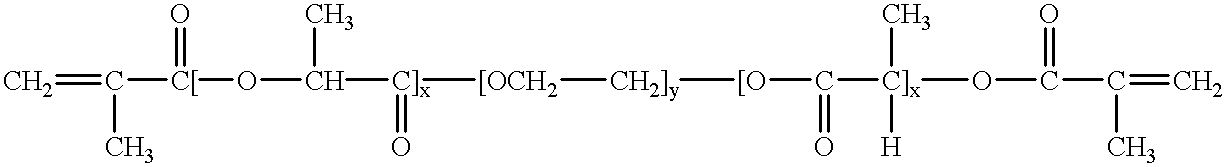Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35610results about "Pill delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
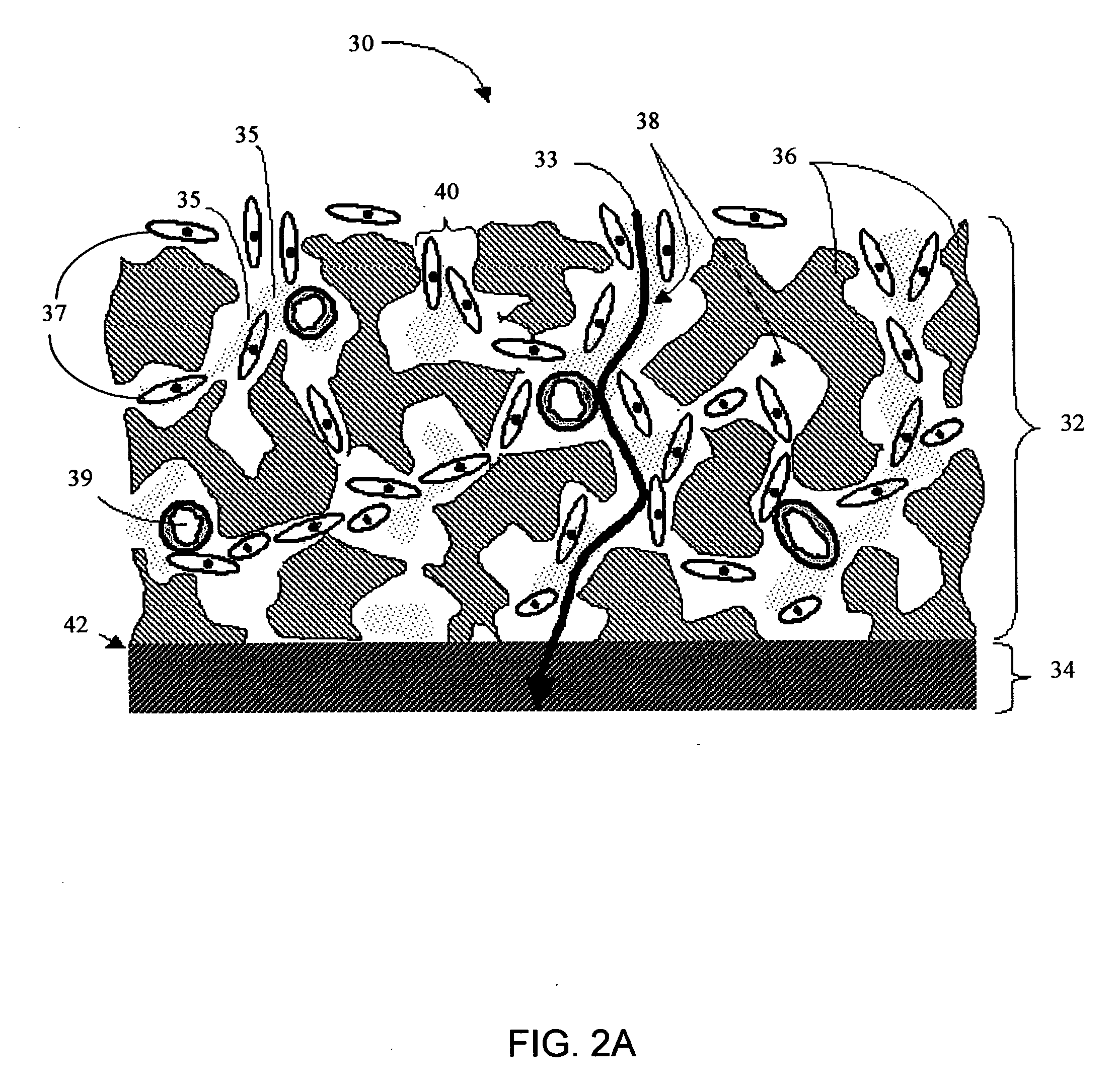
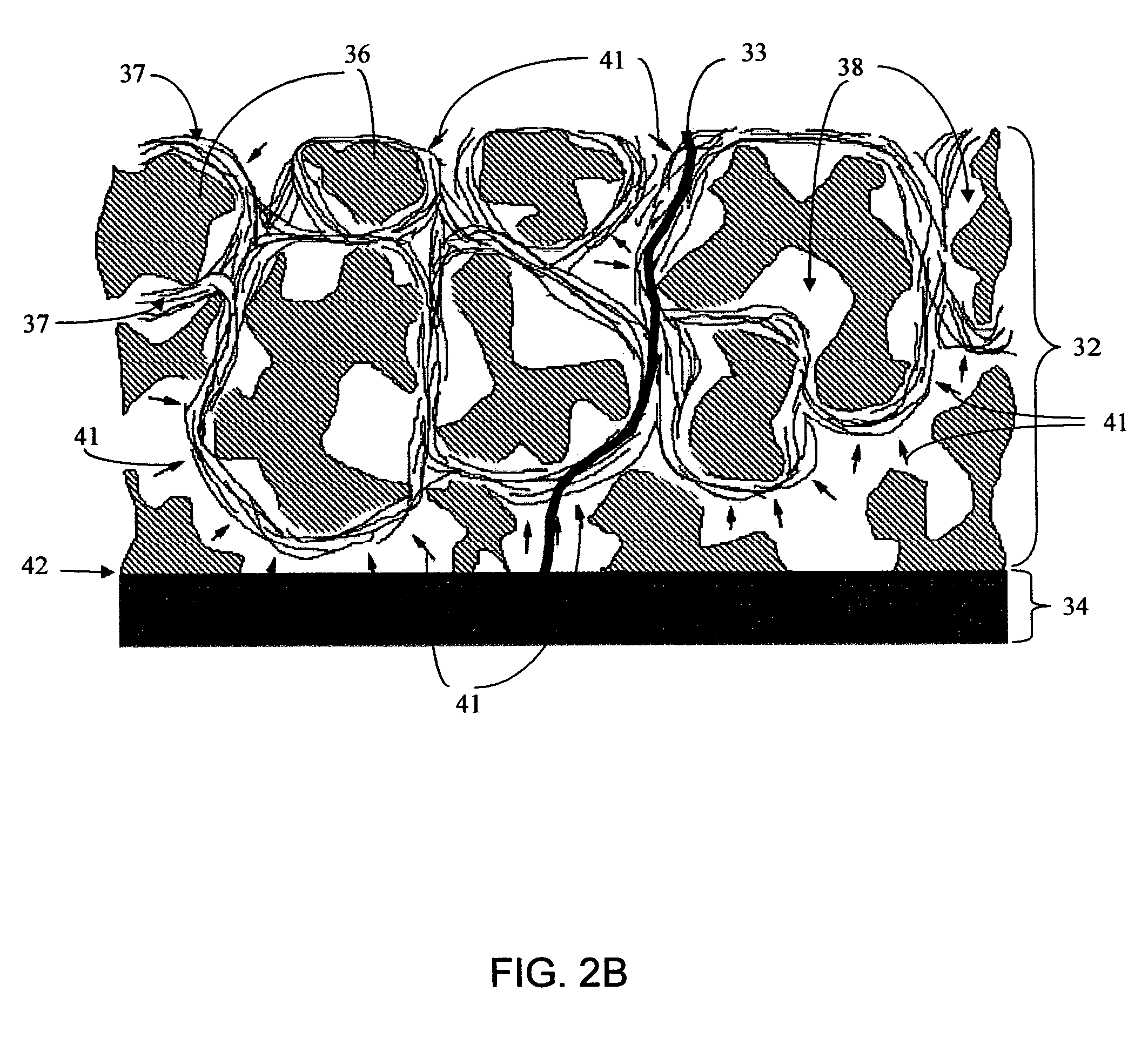
Biointerface membranes incorporating bioactive agents
InactiveUS20050031689A1Improve performancePowder deliveryAdditive manufacturing apparatusBiointerfaceActive agent
A biointerface membrane for an implantable device including a nonresorbable solid portion with a plurality of interconnected cavities therein adapted to support tissue ingrowth in vivo, and a bioactive agent incorporated into the biointerface membrane and adapted to modify the tissue response is provided. The bioactive agents can be chosen to induce vascularization and / or prevent barrier cell layer formation in vivo, and are advantageous when used with implantable devices wherein solutes are transported across the device-tissue interface.
Owner:DEXCOM
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions
InactiveUS6923988B2Rapid dissolvableMore solubilizedAntibacterial agentsOrganic active ingredientsDiagnostic agentTG - Triglyceride
The present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered. In one embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate. The encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides. In another embodiment, the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactants, lipophilic surfactants and triglycerides. The compositions of the present invention can be used for improved delivery of hydrophilic or hydrophobic pharmaceutical active ingredients, such as drugs, nutritional agents, cosmeceuticals and diagnostic agents.
Owner:LIPOCINE
Method of treating androgen independent prostate cancer
The present invention is directed to a method treating prostate cancer. The method comprises administering to a patient in need thereof at least one compound selected from N-methyl-Δ3,3′-dihydroindole-2,2′ diketone; N-1-(β-D-O-triacetyl-xylopranosyl)-Δ3,3′-dihydroindole-2,2′ diketone; and N-1-(β-D-O-triacetyl-xylopranosyl)-N′-methyl-Δ3,3′-dihydroindole-2,2′ diketone. Preferably the compound is in an amount sufficient to inhibit growth, invasion, and / or metastasis of prostate cancer cells.
Owner:NATROGEN THERAPEUTICS INT
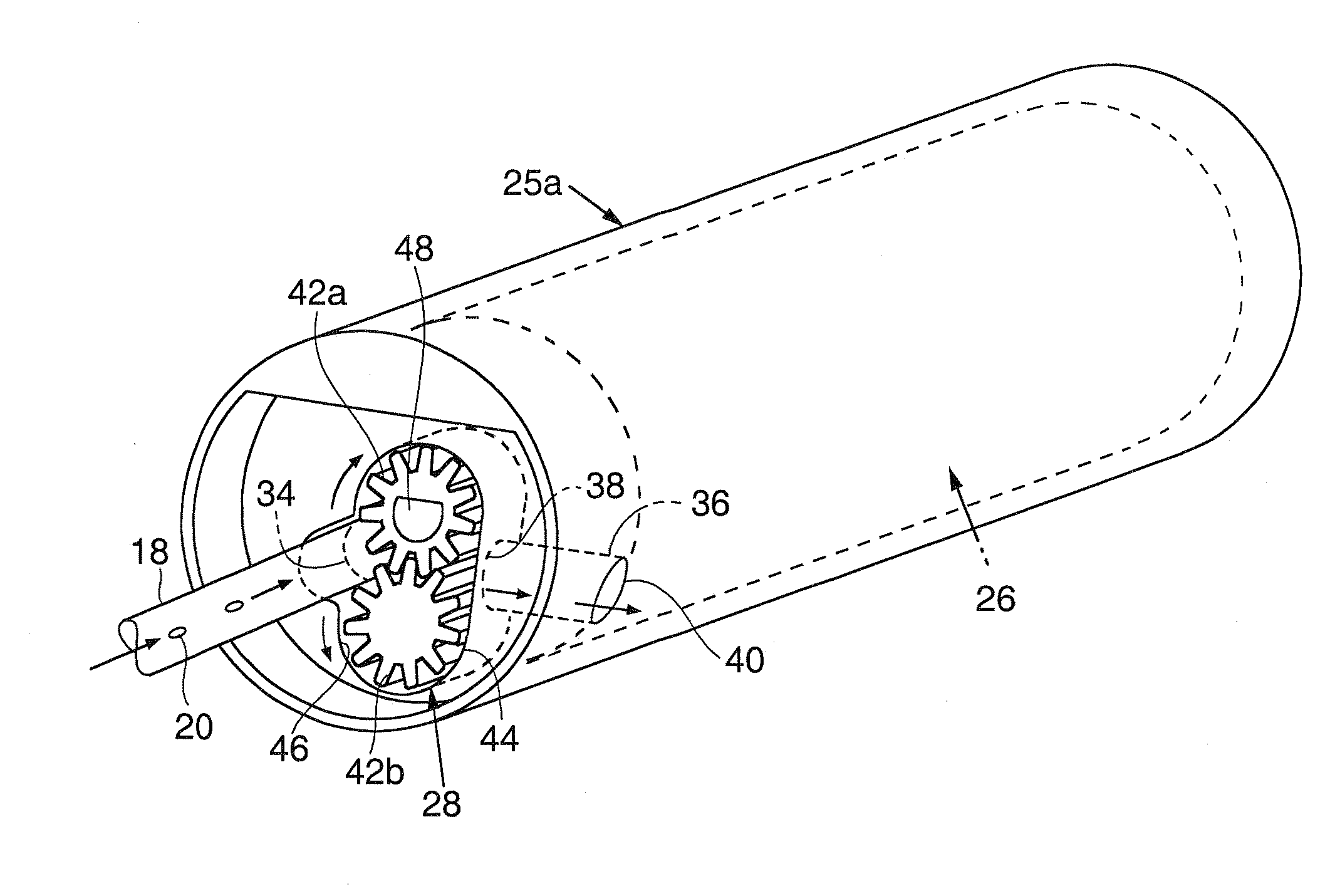
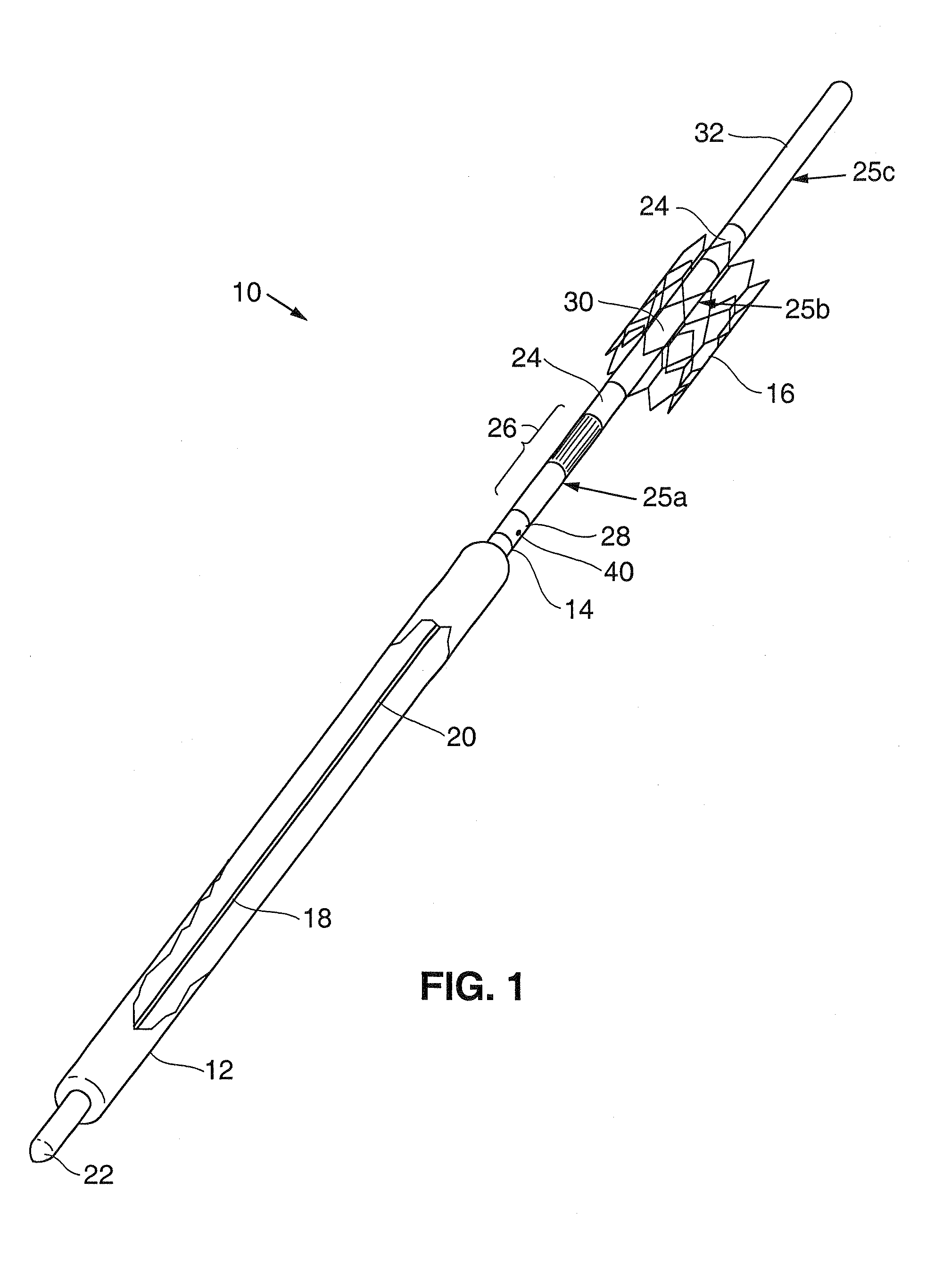
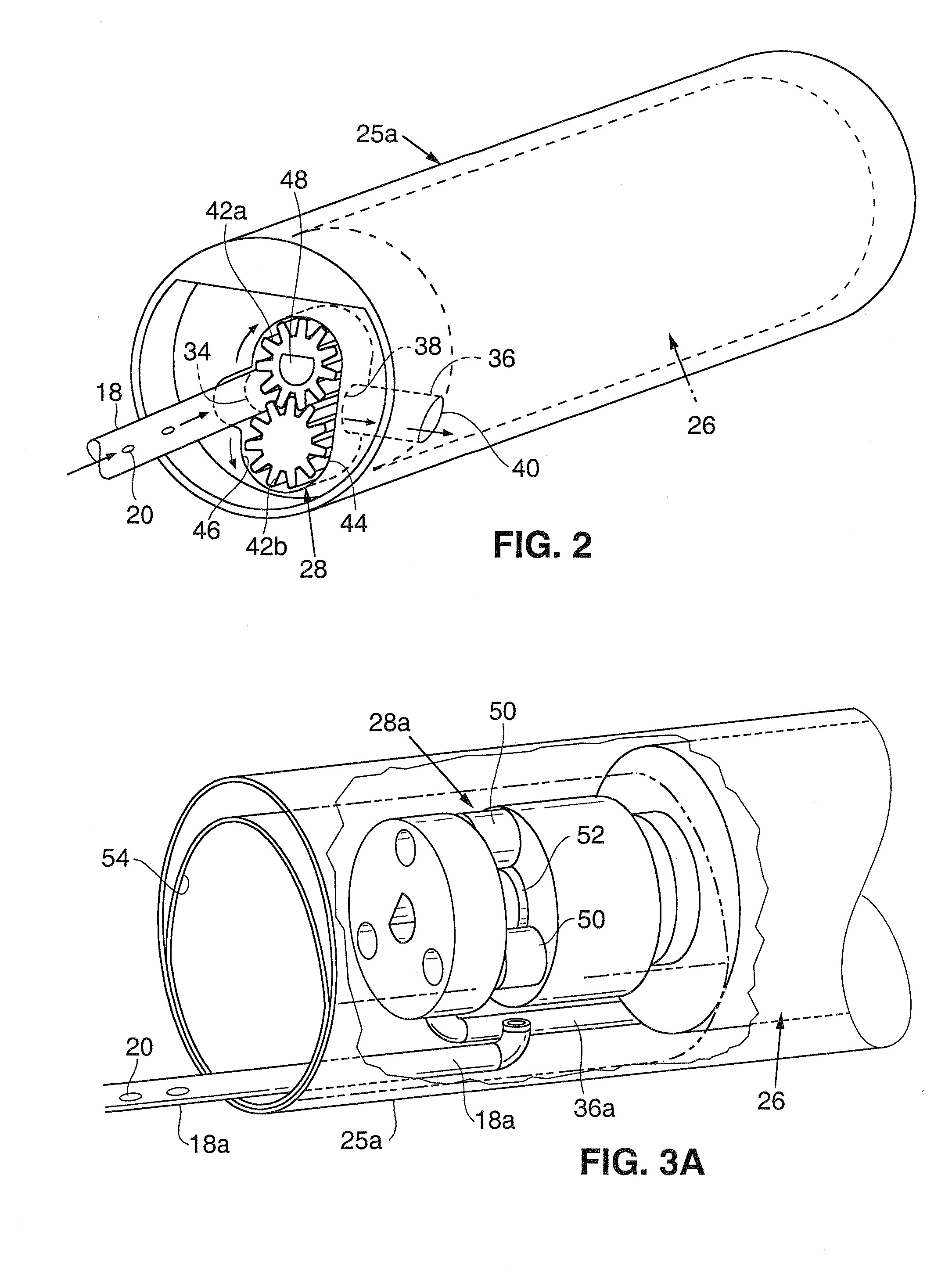
Intravascular delivery system for therapeutic agents
Described herein is a system for intravascular drug delivery system, which includes a reservoir implantable a blood vessel, an intravascular pump fluidly coupled to the reservoir and an anchor expandable into contact with a wall of the blood vessel to retain the system within the vasculature. Delivery conduits may be extend from the reservoir and are positionable at select locations within the vasculature for target drug delivery to select organs or tissues.
Owner:WILLIAMS MICHAEL S +2
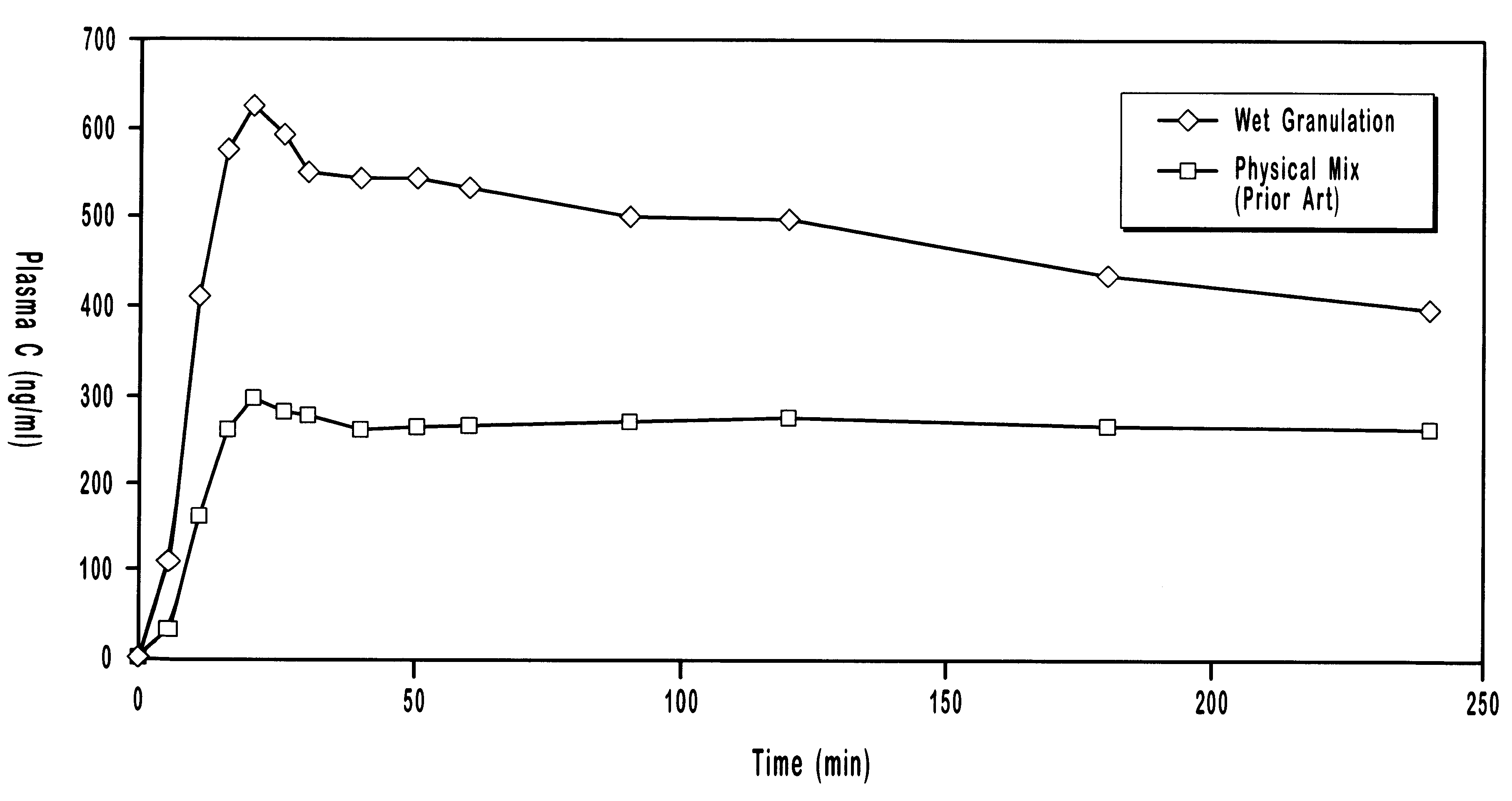
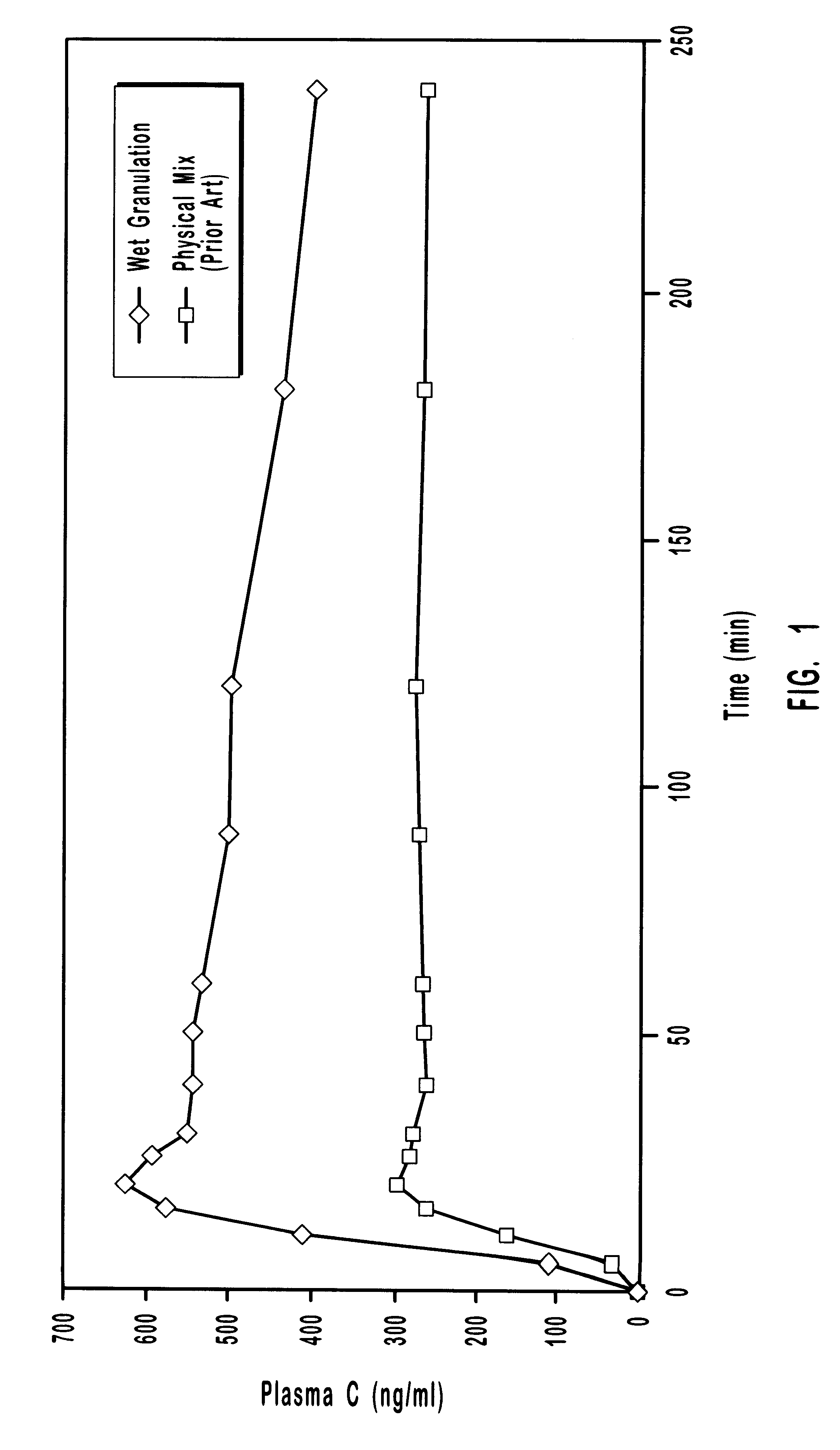
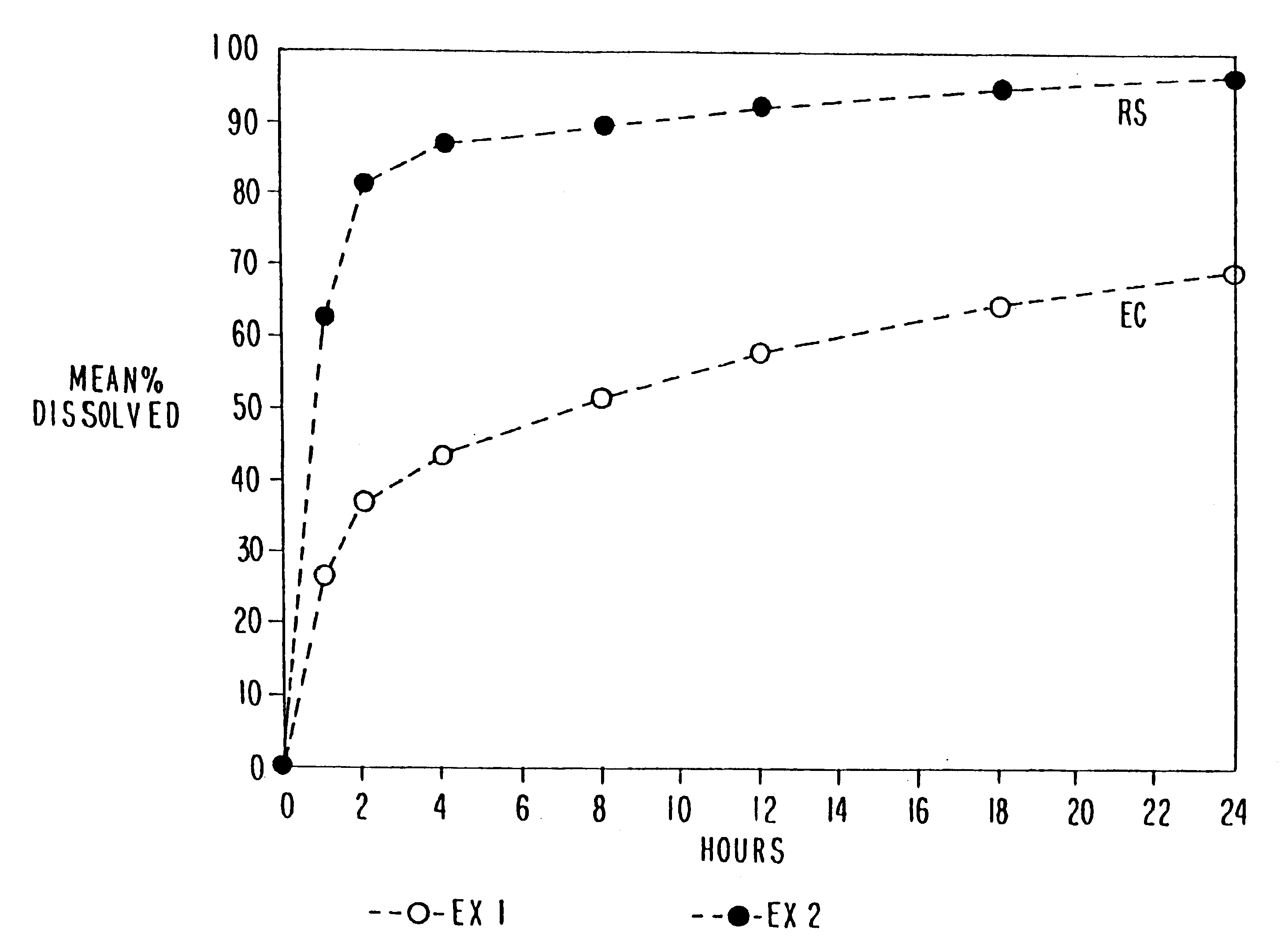
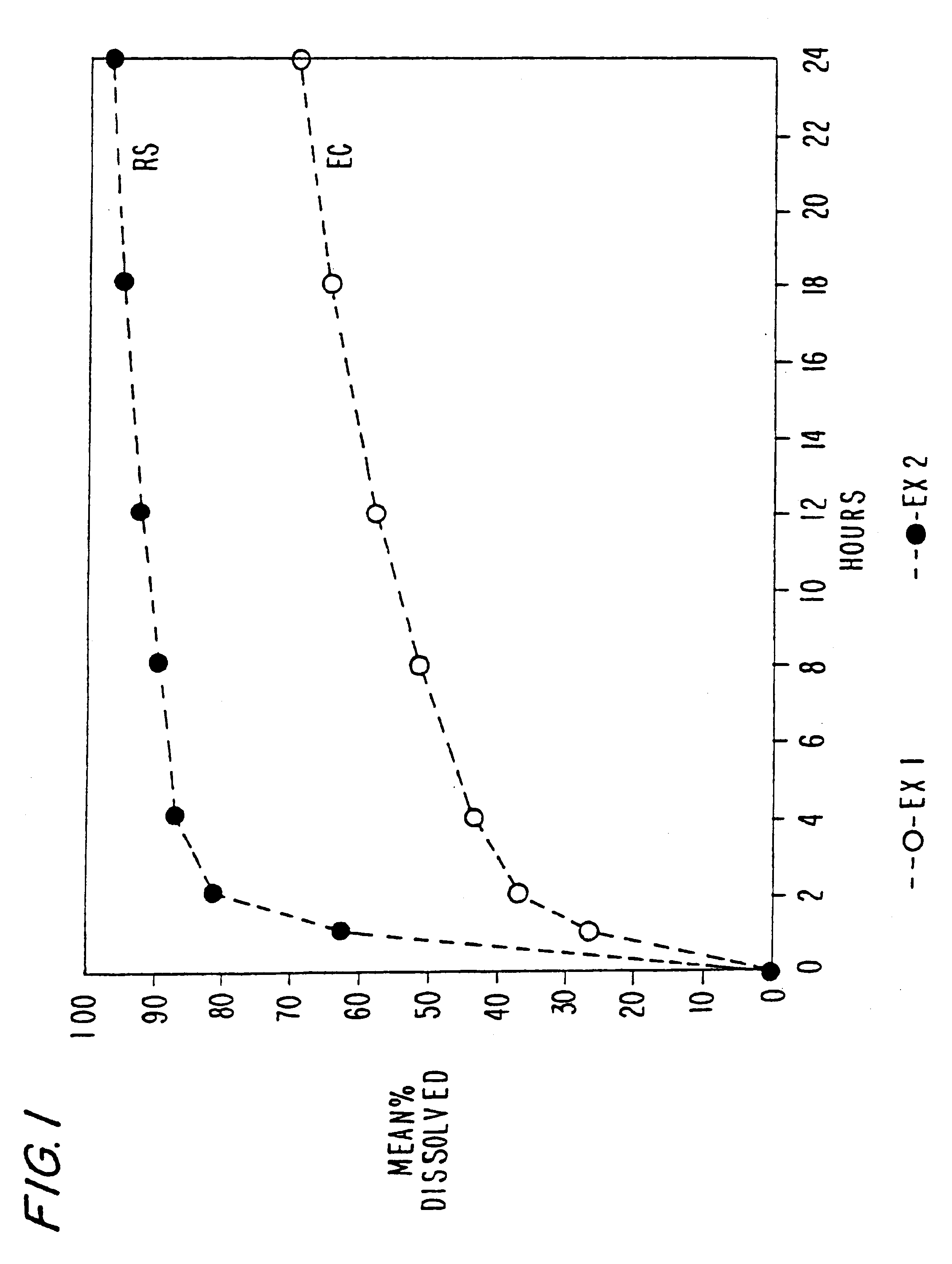
Multiparticulate modified release composition
The invention relates to a multiparticulate modified release composition that in operation delivers an active ingredient in a pulsed or bimodal manner. The multiparticulate modified release composition comprises an immediate release component and a modified release component; the immediate release component comprising a first population of active ingredient containing particles and the modified release component compnsimg a second population of active ingredient containing particles coated with a controlled release coating; wherein the combination of the immediate release and modified release components in operation deliver the active ingredient in a pulsed or a bimodal manner. The invention also relates to a solid oral dosage form containing such a multiparticulate modified release composition. The plasma profile achieved by the multiparticulate modified release composition is advantageous in reducing patient tolerance to the active ingredient and in increasing patient compliance by reducing dosage frequency.
Owner:ALKERMES PHARMA IRELAND LTD +1
Sustained release drug delivery devices, methods of use, and methods of manufacturing thereof
InactiveUS6375972B1Without of effectWithout riskOrganic active ingredientsSenses disorderBiological bodySustained release drug
A method and device for treating a mammalian organism to obtain a desired local or systemic physiological or pharmacological effect is provided. The method includes administering a sustained release drug delivery system to a mammalian organism in need of such treatment at an area wherein release of an effective agent is desired and allowing the effective agent to pass through the device in a controlled manner. The device includes an inner core or reservoir including the effective agent, an impermeable tube which encloses portions of the reservoir, and a permeable member at an end of the tube.
Owner:EYEPOINT PHARMA INC
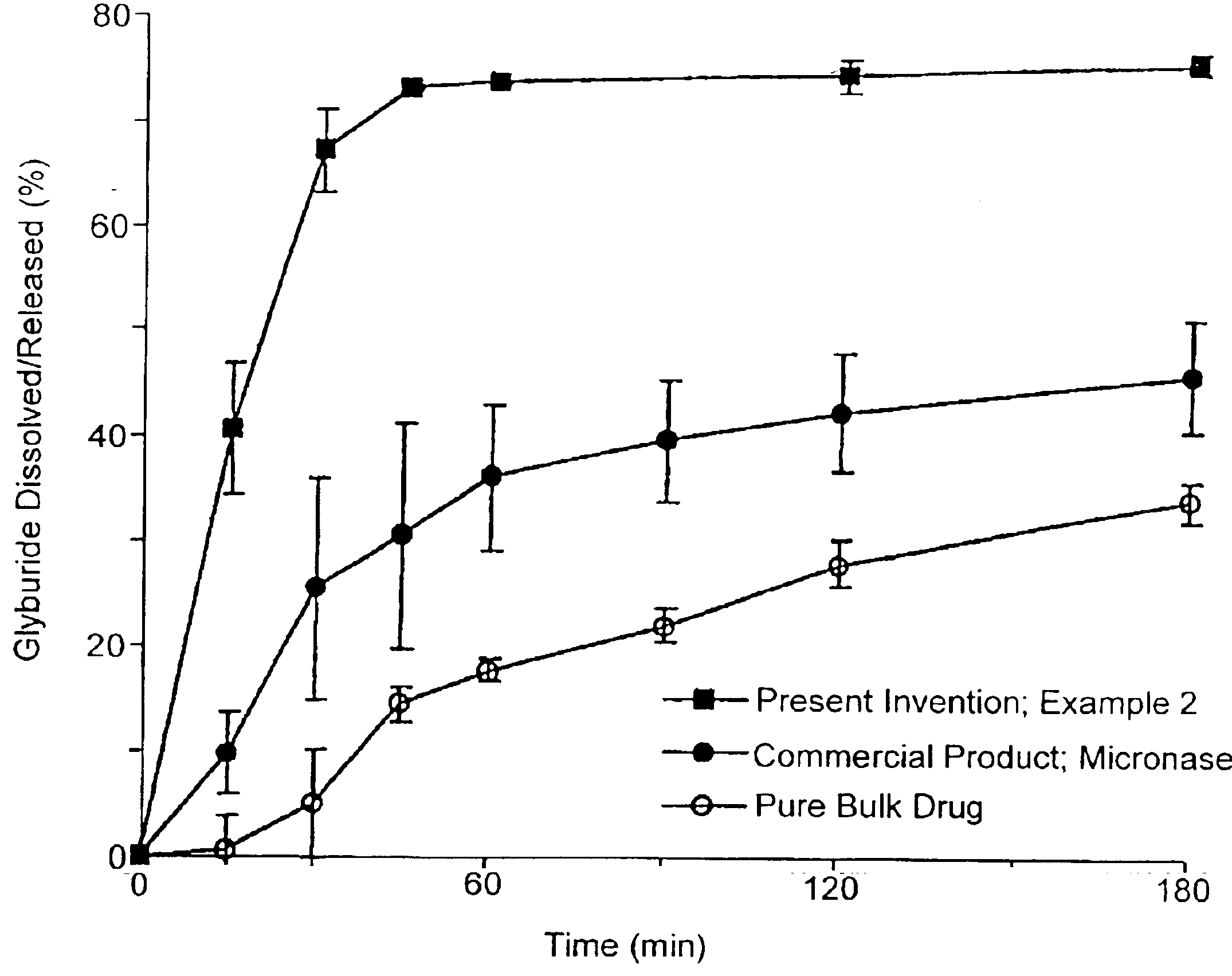
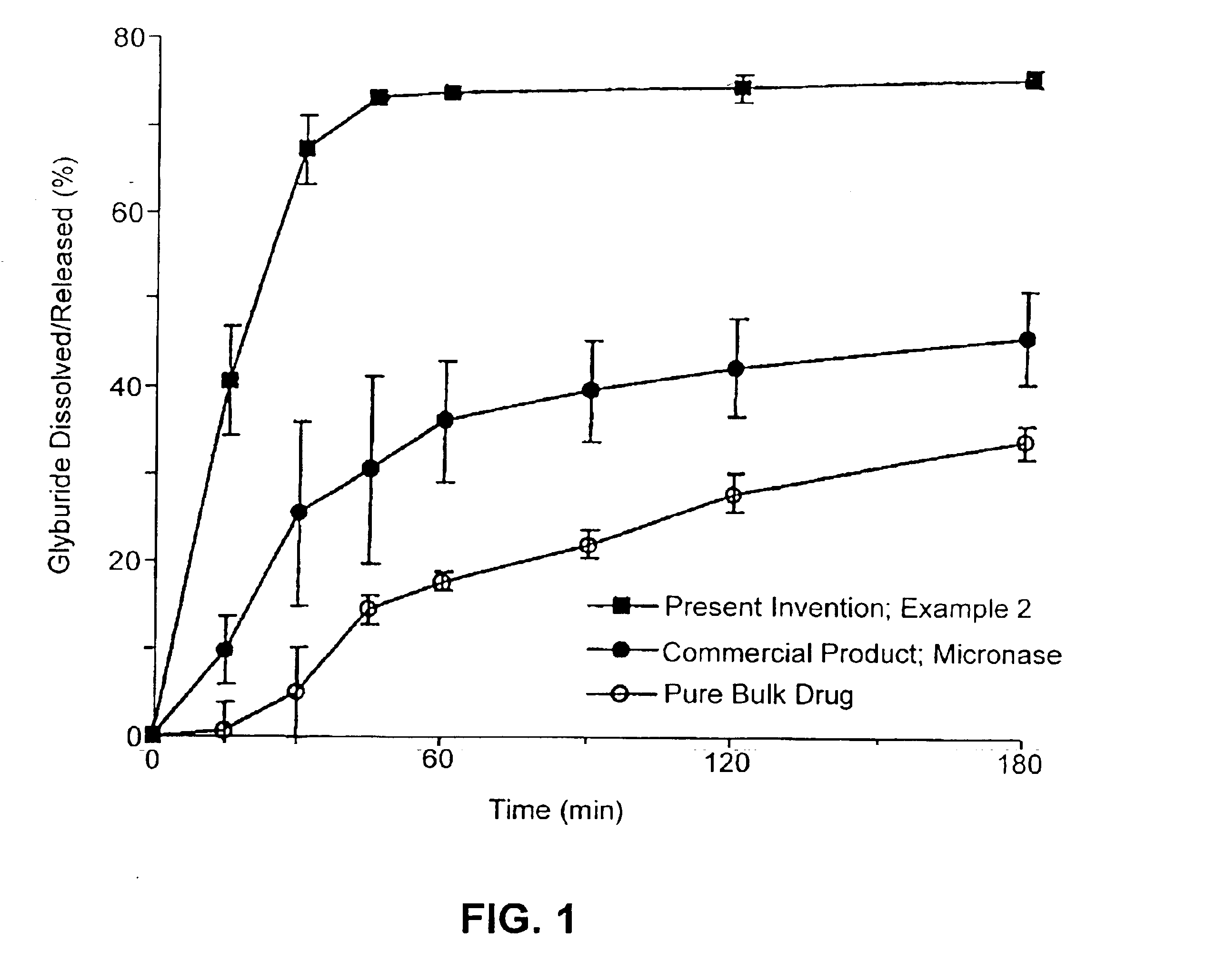
Oral transmucosal drug dosage using solid solution
InactiveUS6264981B1High dissolution rateEasy to usePharmaceutical non-active ingredientsPill deliverySolid solutionPharmaceutical formulation
The present invention is directed toward formulation and method for oral transmucosal delivery of a pharmaceutical. The invention provides a drug formulation comprising a solid pharmaceutical agent in solid solution with a dissolution agent. The formulation is administered into a patient's oral cavity, delivering the pharmaceutical agent by absorption through a patient's oral mucosal tissue. The formulation and method provide for improved oral mucosal delivery of the pharmaceutical agent.
Owner:CEPHALON INC
Methods of making conditioned cell culture medium compositions
InactiveUS6372494B1Eliminate wrinklesEliminate frown lineCosmetic preparationsPeptide/protein ingredientsReserve CellCell culture media
Novel products comprising conditioned cell culture medium compositions and methods of use are described. The conditioned cell medium compositions of the invention may be comprised of any known defined or undefined medium and may be conditioned using any eukaryotic cell type. The medium may be conditioned by stromal cells, parenchymal cells, mesenchymal stem cells, liver reserve cells, neural stem cells, pancreatic stem cells and / or embryonic stem cells. Additionally, the cells may be genetically modified. A three-dimensional tissue construct is preferred. Once the cell medium of the invention is conditioned, it may be used in any state. Physical embodiments of the conditioned medium include, but are not limited to, liquid or solid, frozen, lyophilized or dried into a powder. Additionally, the medium is formulated with a pharmaceutically acceptable carrier as a vehicle for internal administration, applied directly to a food item or product, formulated with a salve or ointment for topical applications, or, for example, made into or added to surgical glue to accelerate healing of sutures following invasive procedures. Also, the medium may be further processed to concentrate or reduce one or more factors or components contained within the medium.
Owner:ALLERGAN INC
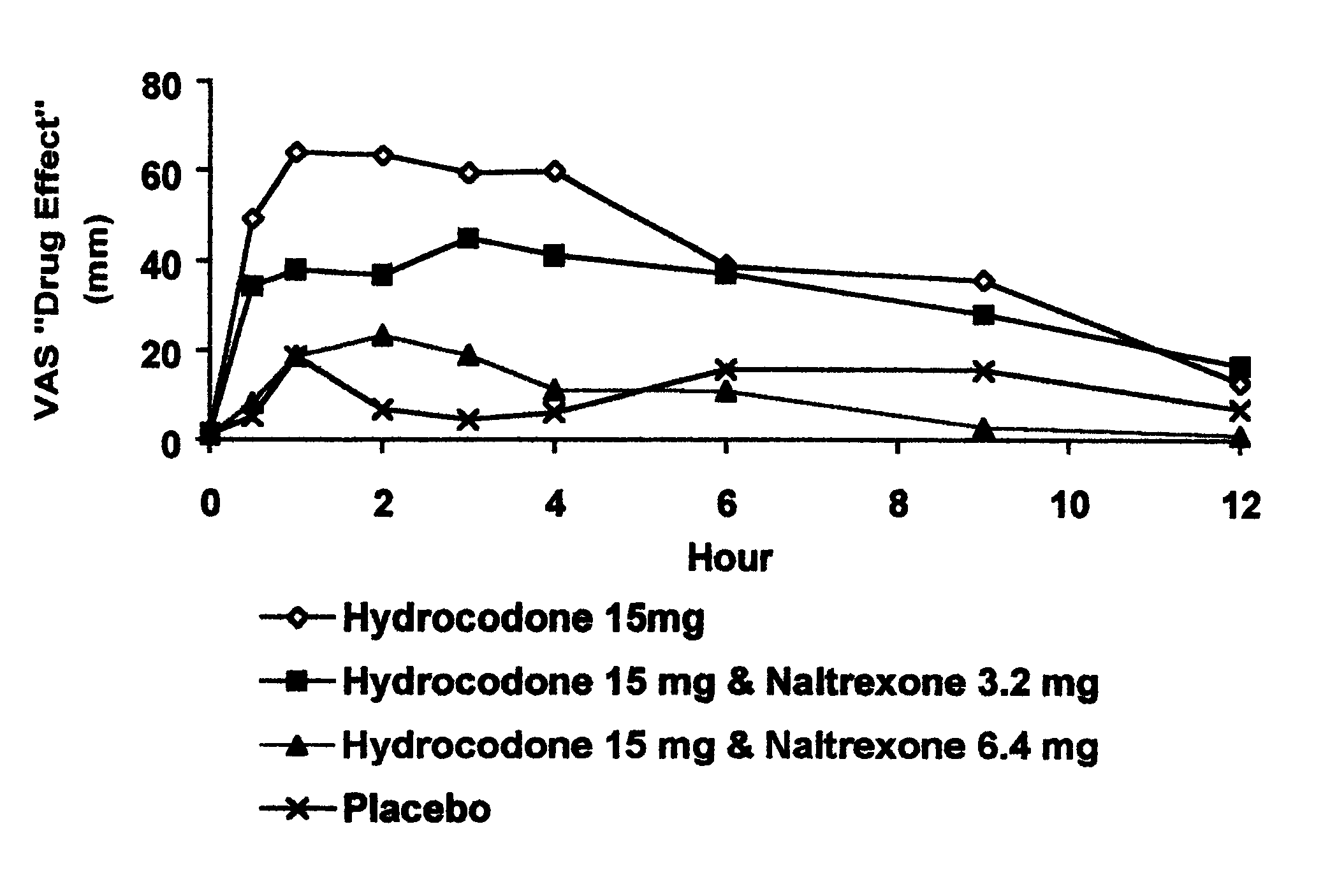
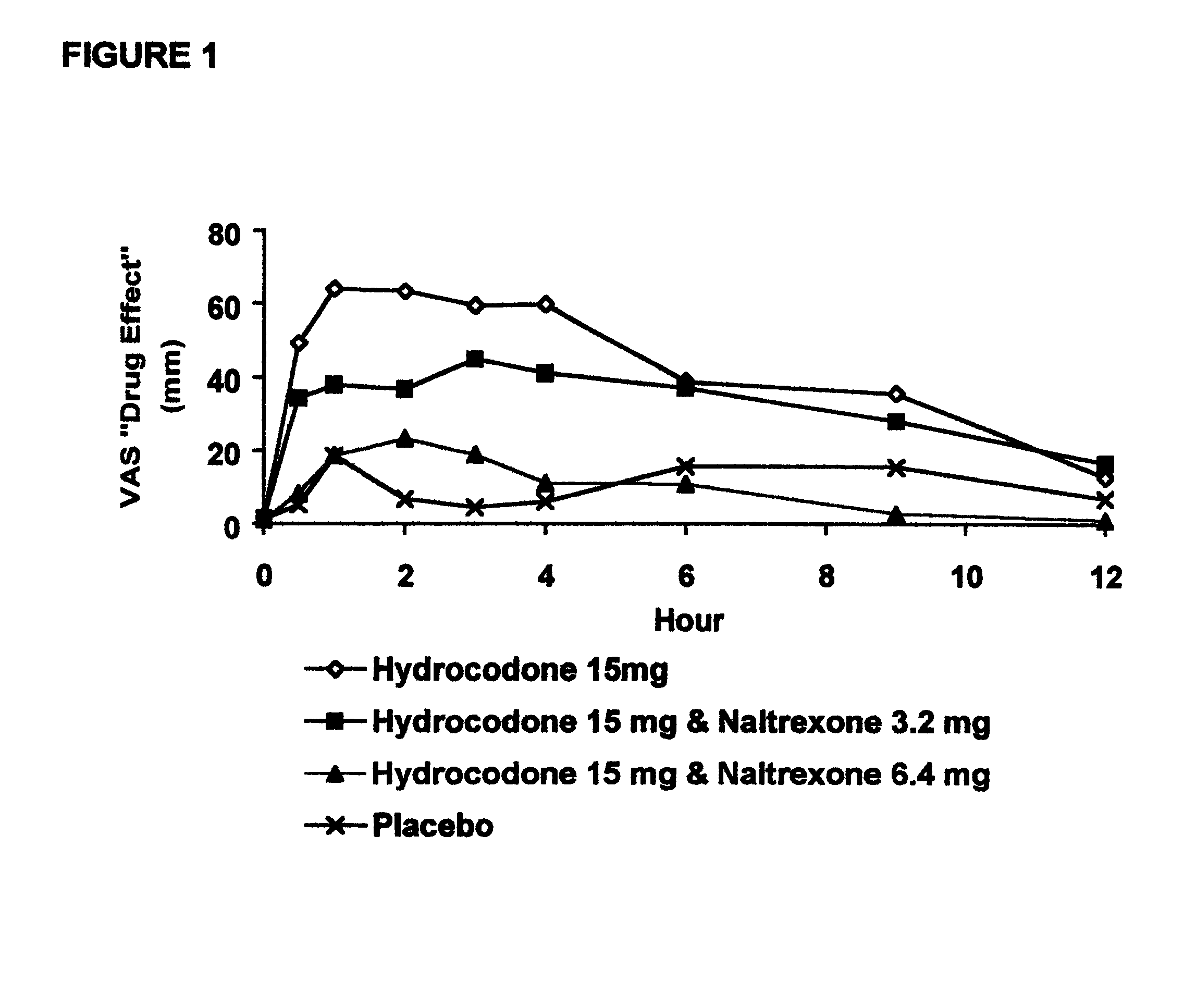
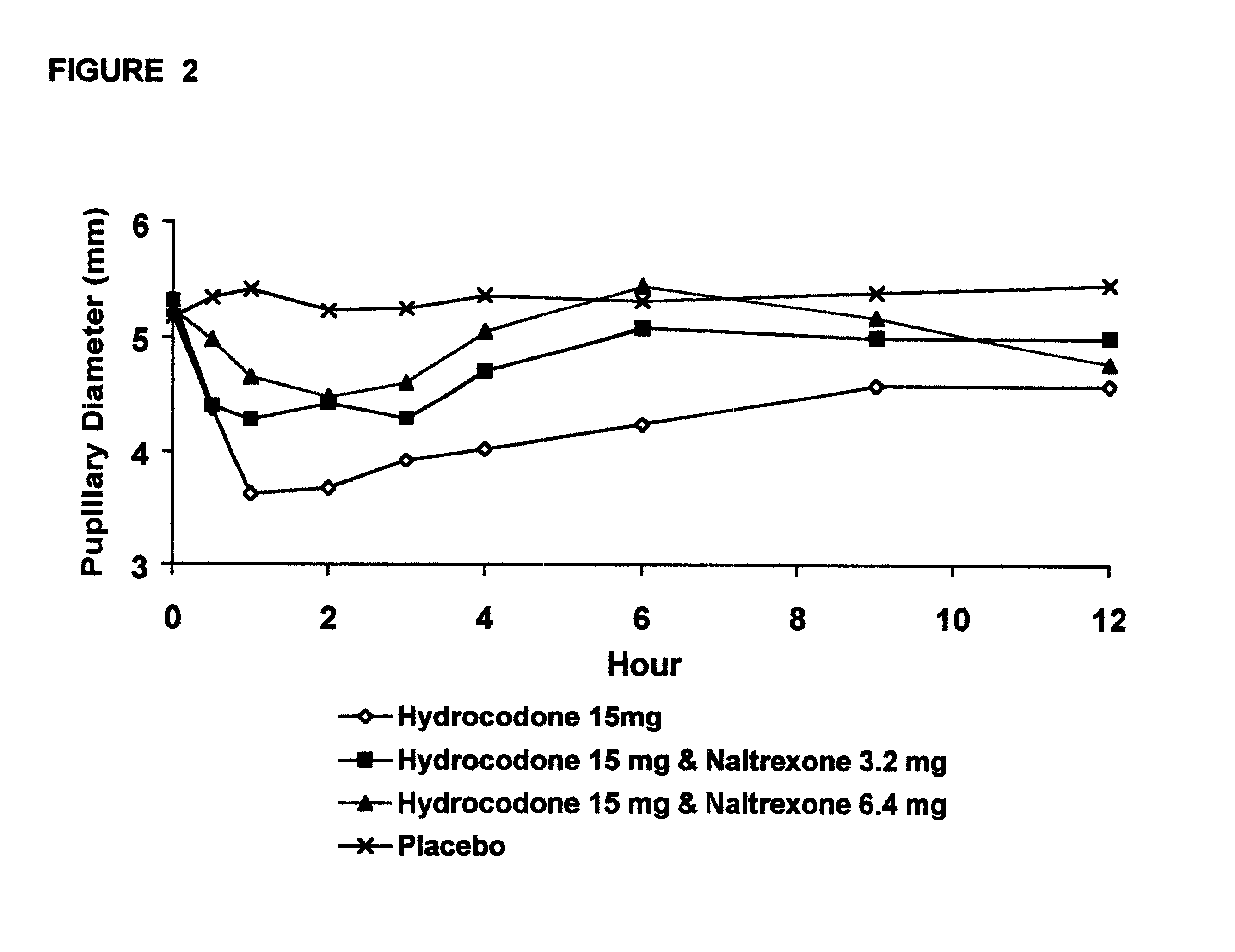
Opioid agonist/antagonist combinations
InactiveUS6277384B1Increase elasticityTrend downBiocideNervous disorderOpioid AgonistOpioid antagonist
The invention is directed in part to oral dosage forms comprising a combination of an orally analgesically effective amount of an opioid agonist and an orally active opioid antagonist, the opioid antagonist being included in a ratio to the opioid agonist to provide a combination product which is analgesically effective when the combination is administered orally, but which is aversive in a physically dependent subject. Preferably, the amount of opioid antagonist included in the combination product provides at least a mildly negative, "aversive" experience in physically dependent addicts (e.g., precipitated abstinence syndrome).
Owner:PURDUE PHARMA LP
Melt-extruded orally administrable opioid formulations
InactiveUS6261599B1Sustained effectSlow and control releaseBiocideOrganic active ingredientsMelt extrusionDosage form
Bioavailable sustained release oral opioid analgesic dosage forms, comprising a plurality of multiparticulates produced via melt extrusion techniques disclosed.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant
ActiveUS20030068392A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid AgonistOpioid antagonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
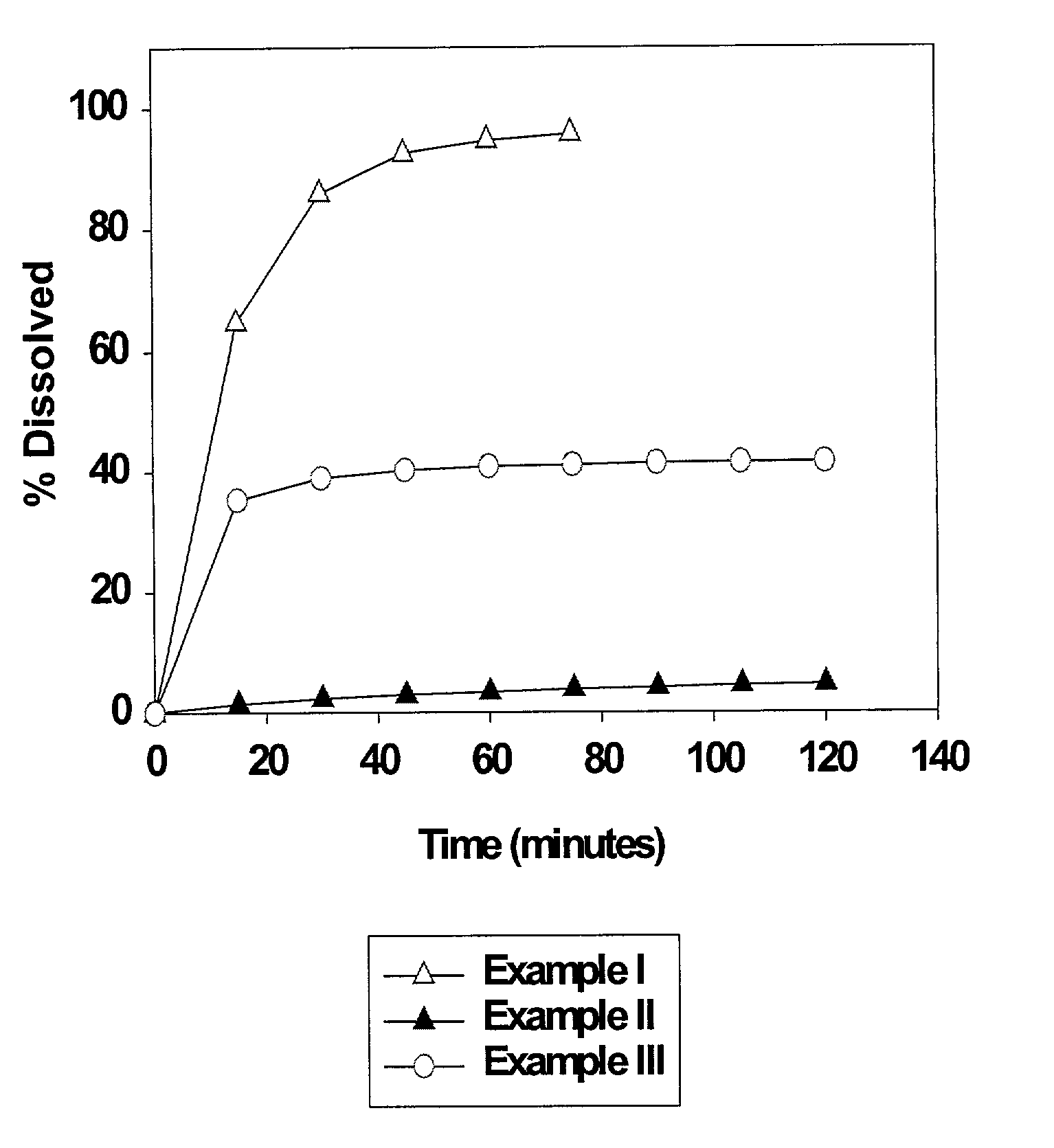
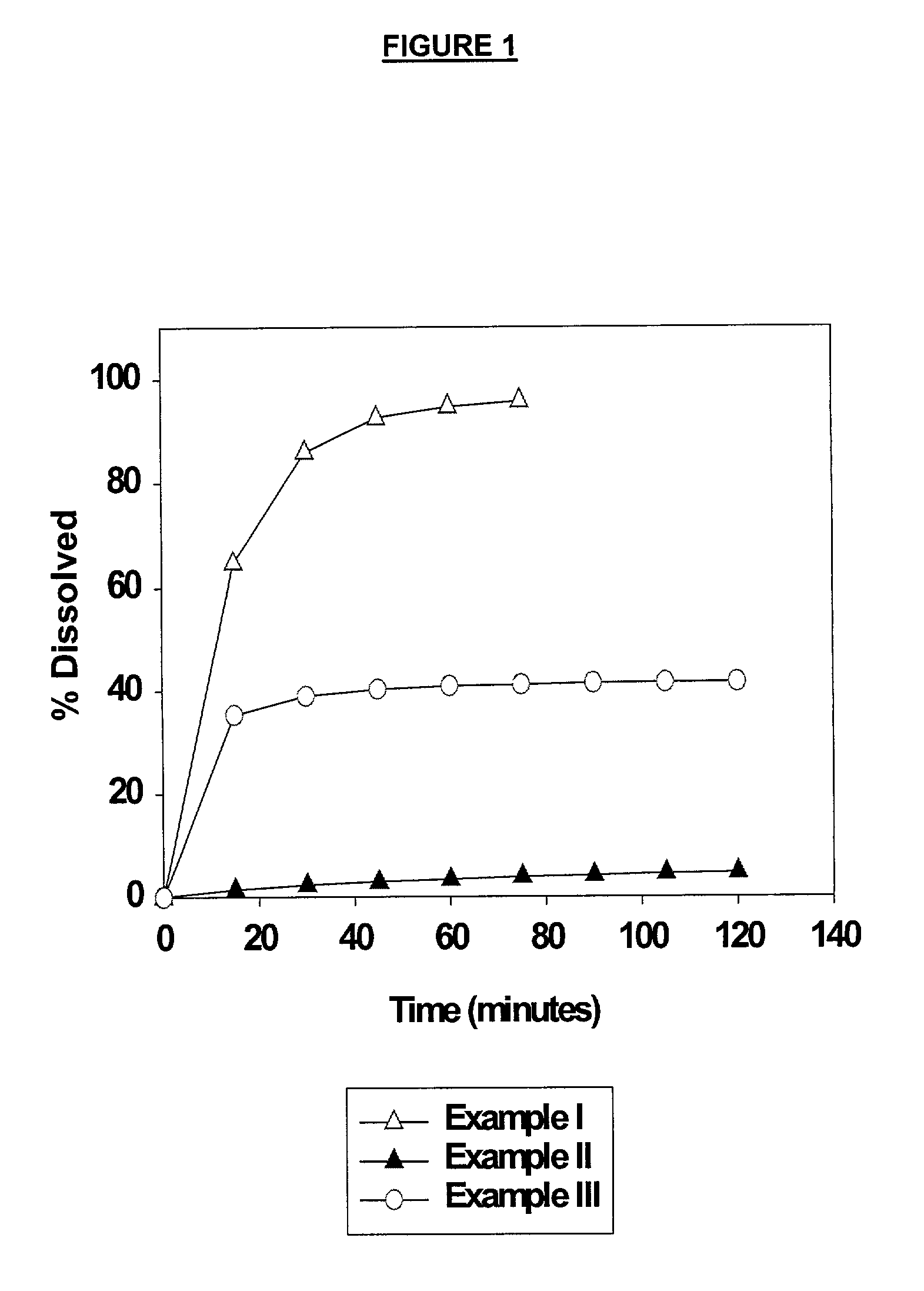
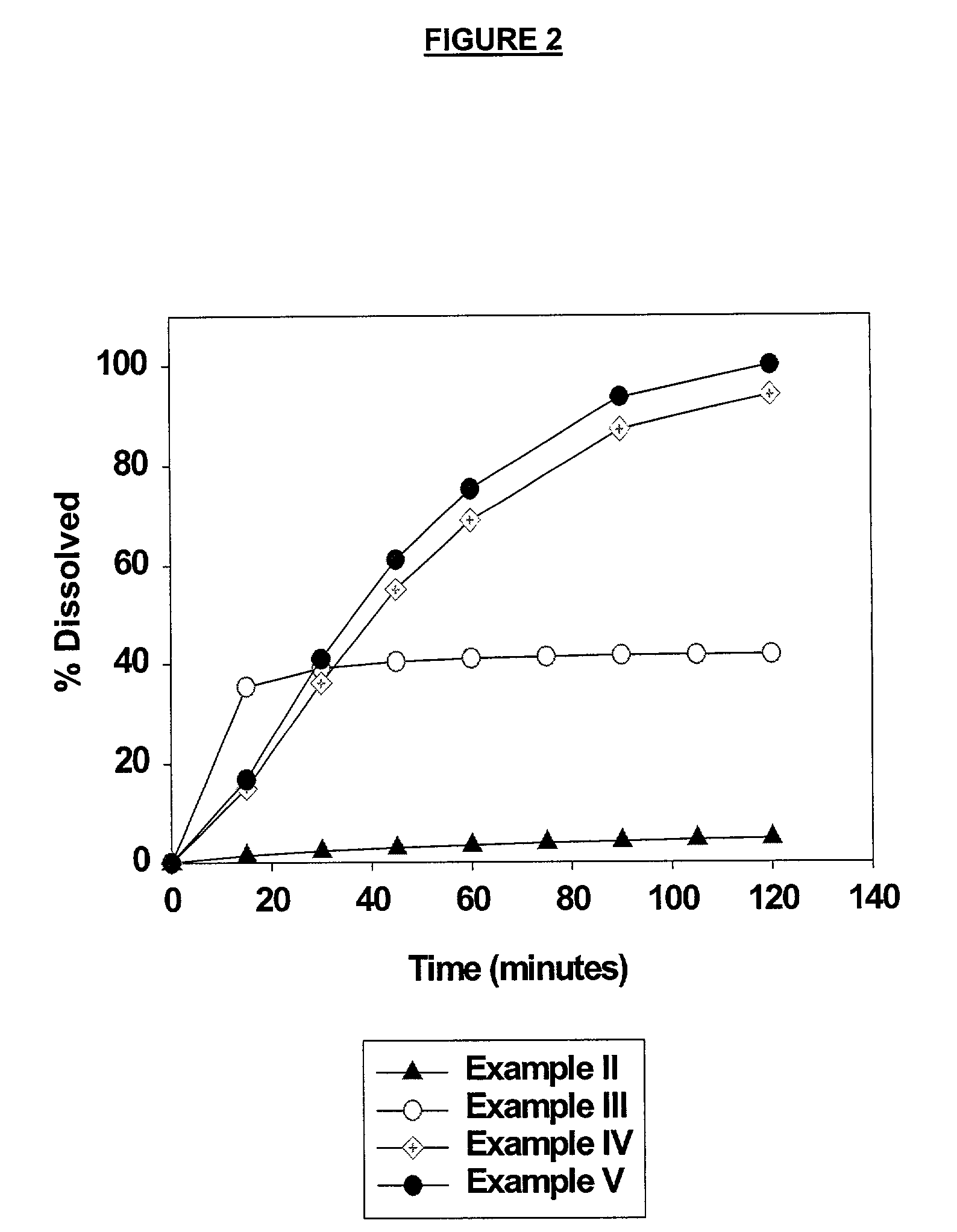
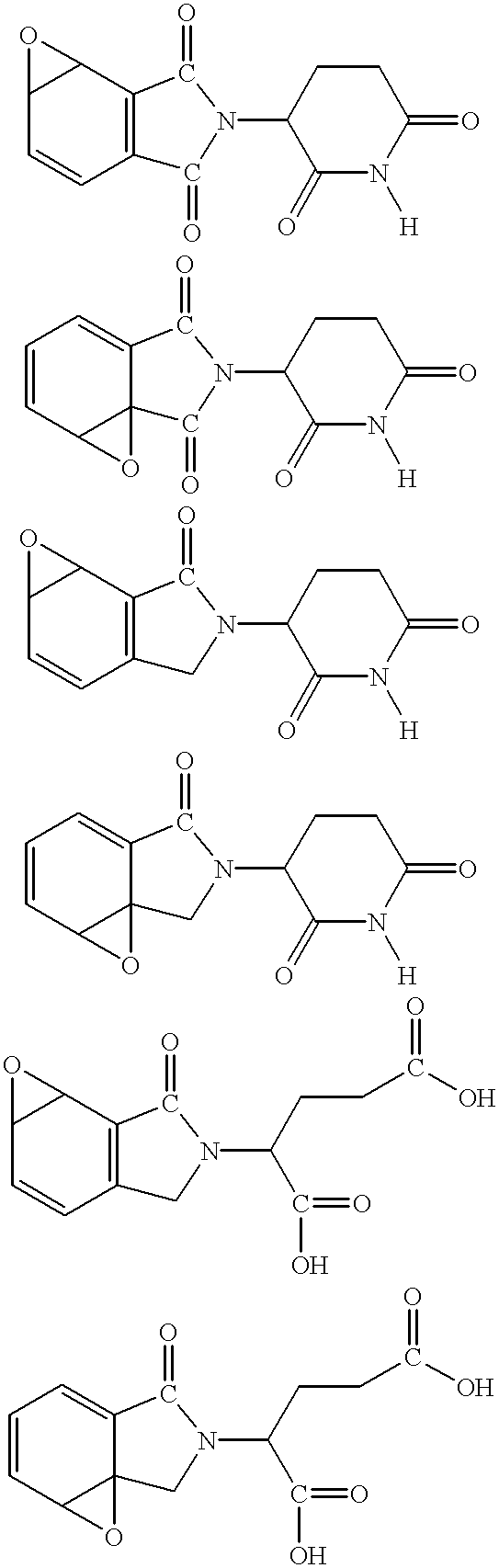
Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors
The present invention provides heteroaryl substituted pyrrolo[2,3-b]pyridines and heteroaryl substituted pyrrolo[2,3-b]pyrimidines that modulate the activity of Janus kinases and are useful in the treatment of diseases related to activity of Janus kinases including, for example, immune-related diseases, skin disorders, myeloid proliferative disorders, cancer, and other diseases.
Owner:INCYTE HLDG & INCYTE
Pharmaceutical formulation containing opioid agonist,opioid antagonist and gelling agent
InactiveUS20030068371A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Combination sustained release-immediate release oral dosage forms with an opioid analgesic and a non-opioid analgesic
InactiveUS20030092724A1Long durationConstant plasma levels of opioid and non-opioid analgesicsBiocidePill deliveryImmediate releaseTherapeutic effect
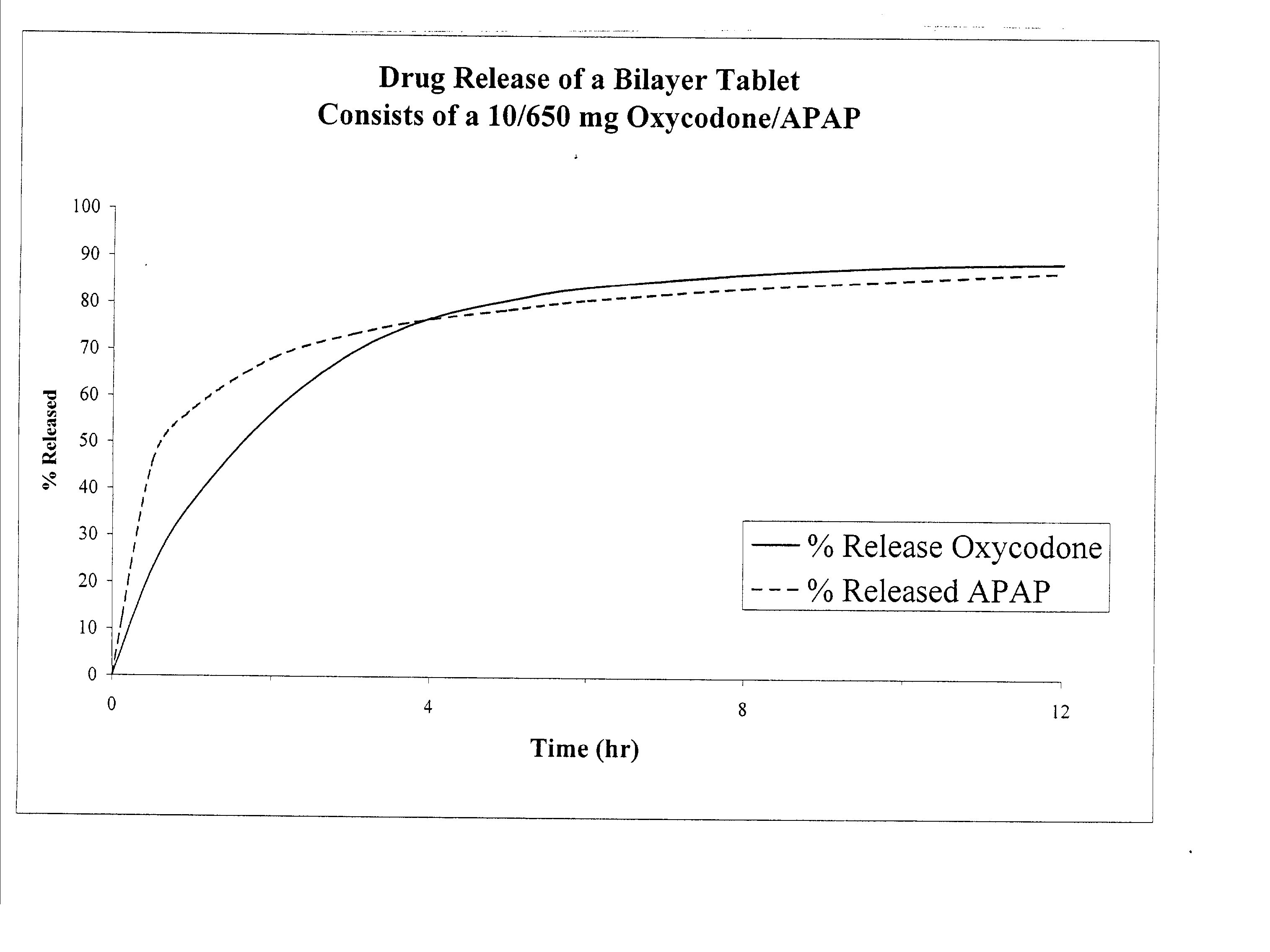
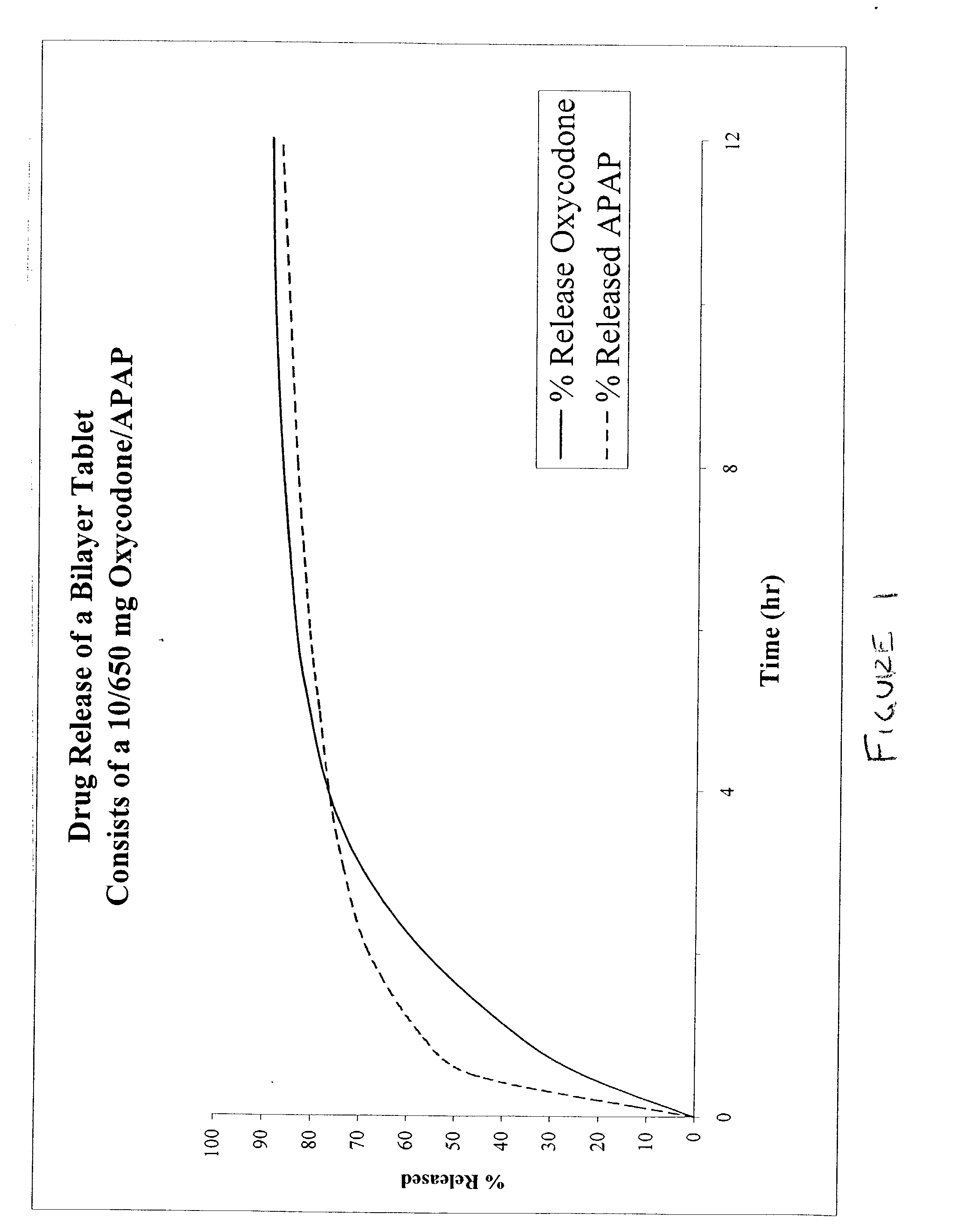
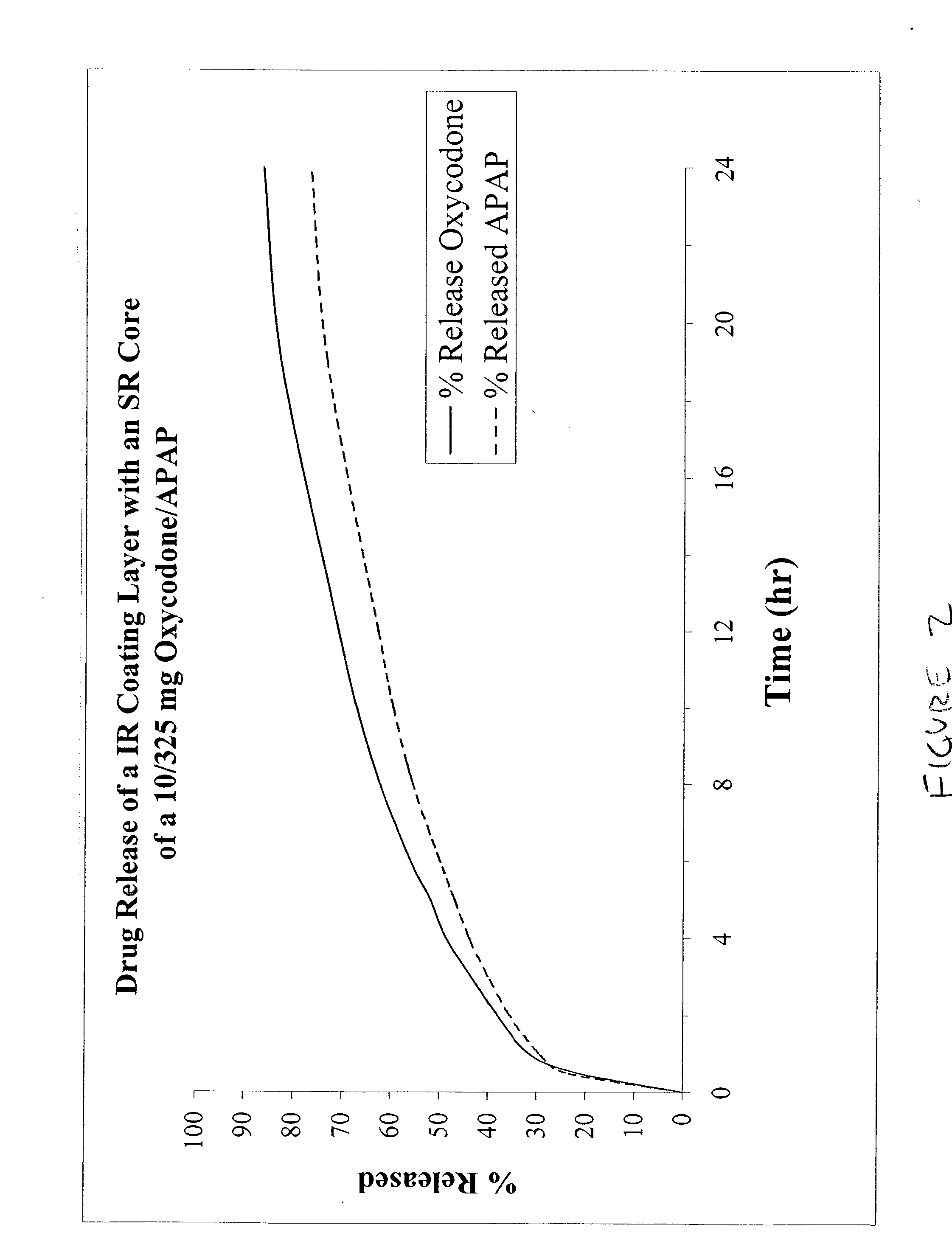
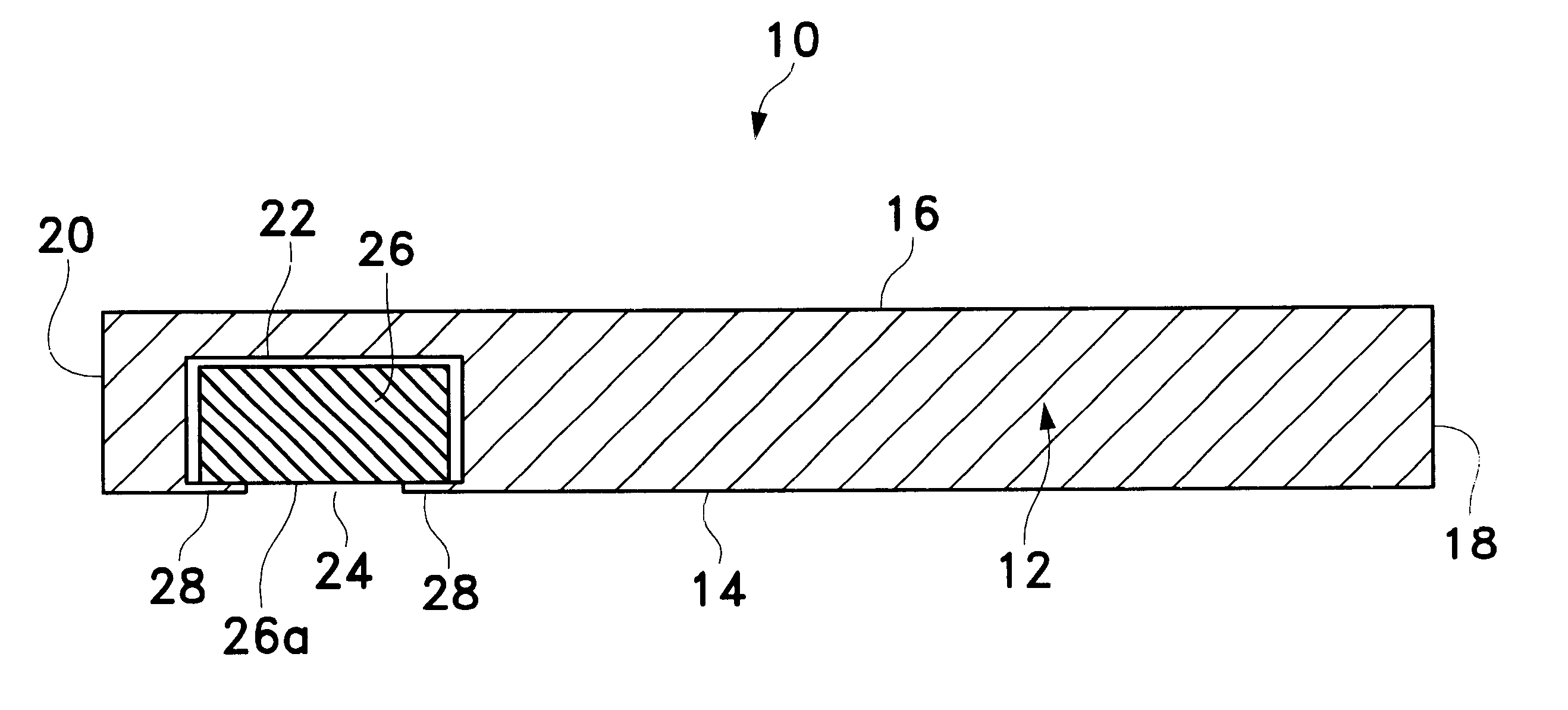
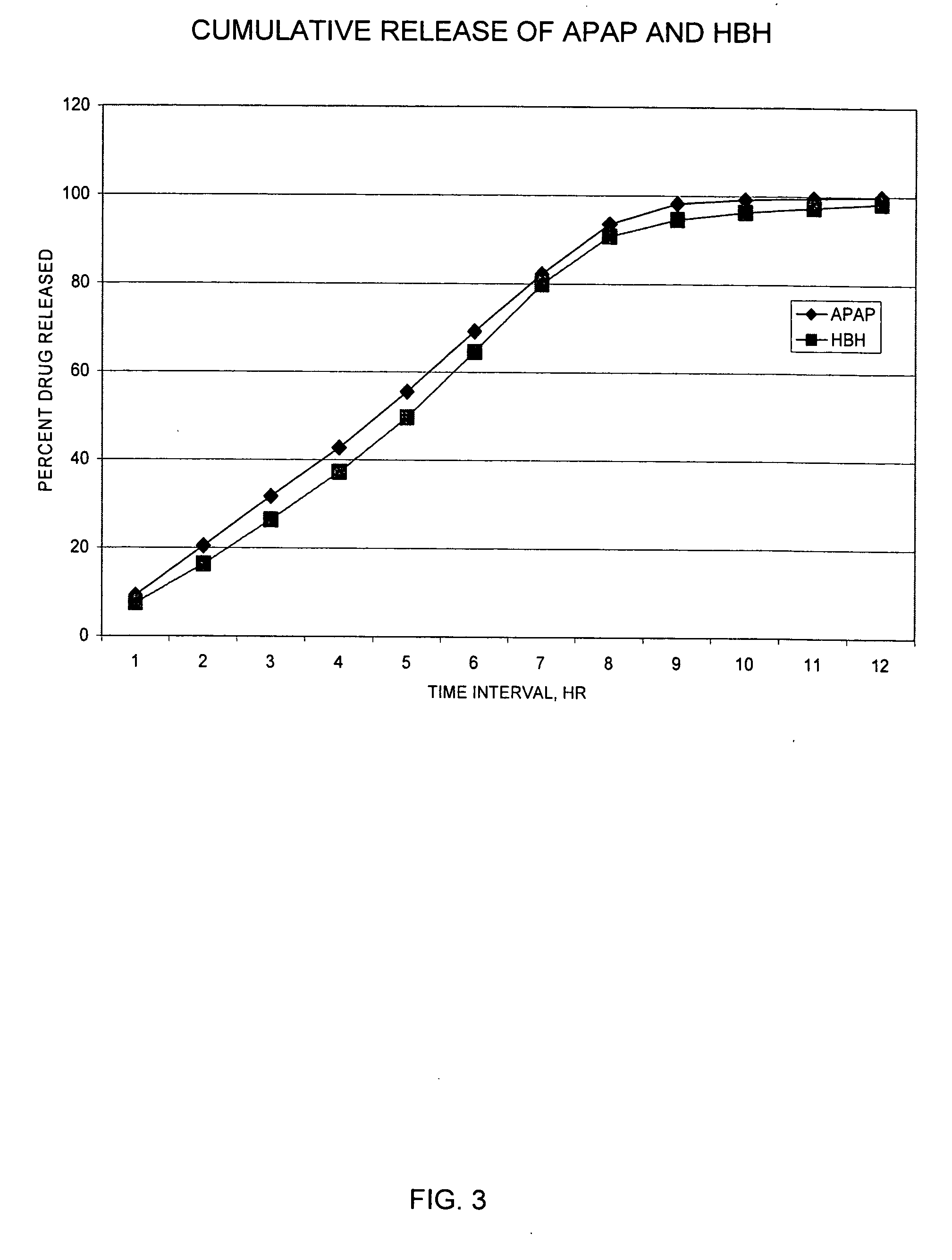
The present invention relates to new and useful oral tablet compositions which include an immediate release portion having an opioid analgesic and a non-opioid analgesic, providing for a rapid onset of therapeutic effect, and a sustained release portion of an opioid analgesic and a non-opioid analgesic, providing for a relatively longer duration of therapeutic effect. A multilayer oral dosage form containing a sustained release layer, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release layer containing the same active ingredients as the sustained release layer, is also disclosed. Also disclosed are oral tablet compositions, containing a sustained release core, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release coating containing the same active ingredients as the sustained release core, are also disclosed. In addition, methods of making and using such oral tablet compositions are disclosed.
Owner:ENDO PHARMA INC
Drug delivery device
Drug delivery devices, and methods of delivering pharmaceutically active agents to a target tissue within a body using such devices, are disclosed. One drug delivery device includes a body having an internal surface for placement proximate a target tissue and a well having an opening to the internal surface. An inner core comprising a pharmaceutically active agent is disposed in the well.
Owner:NOVARTIS AG
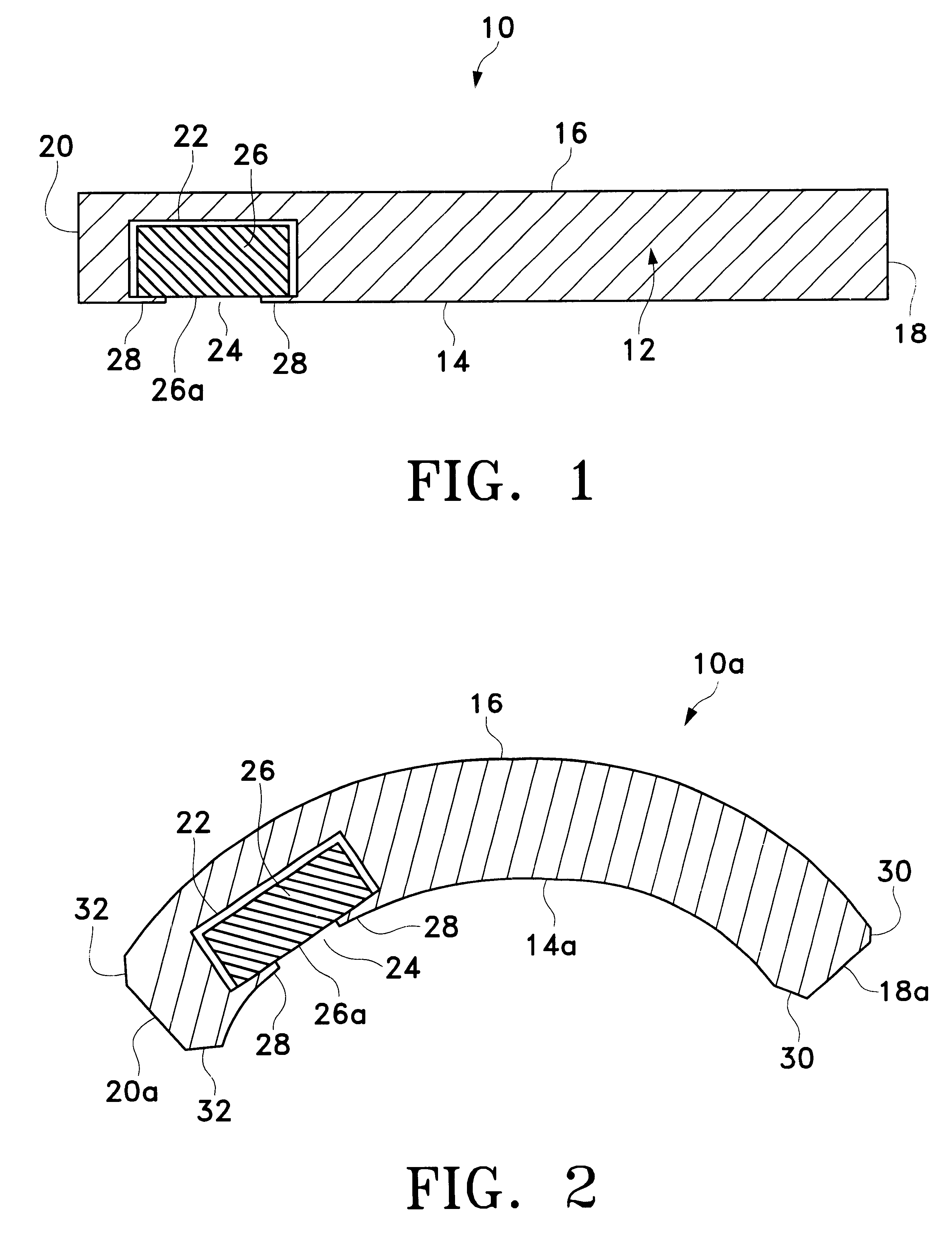
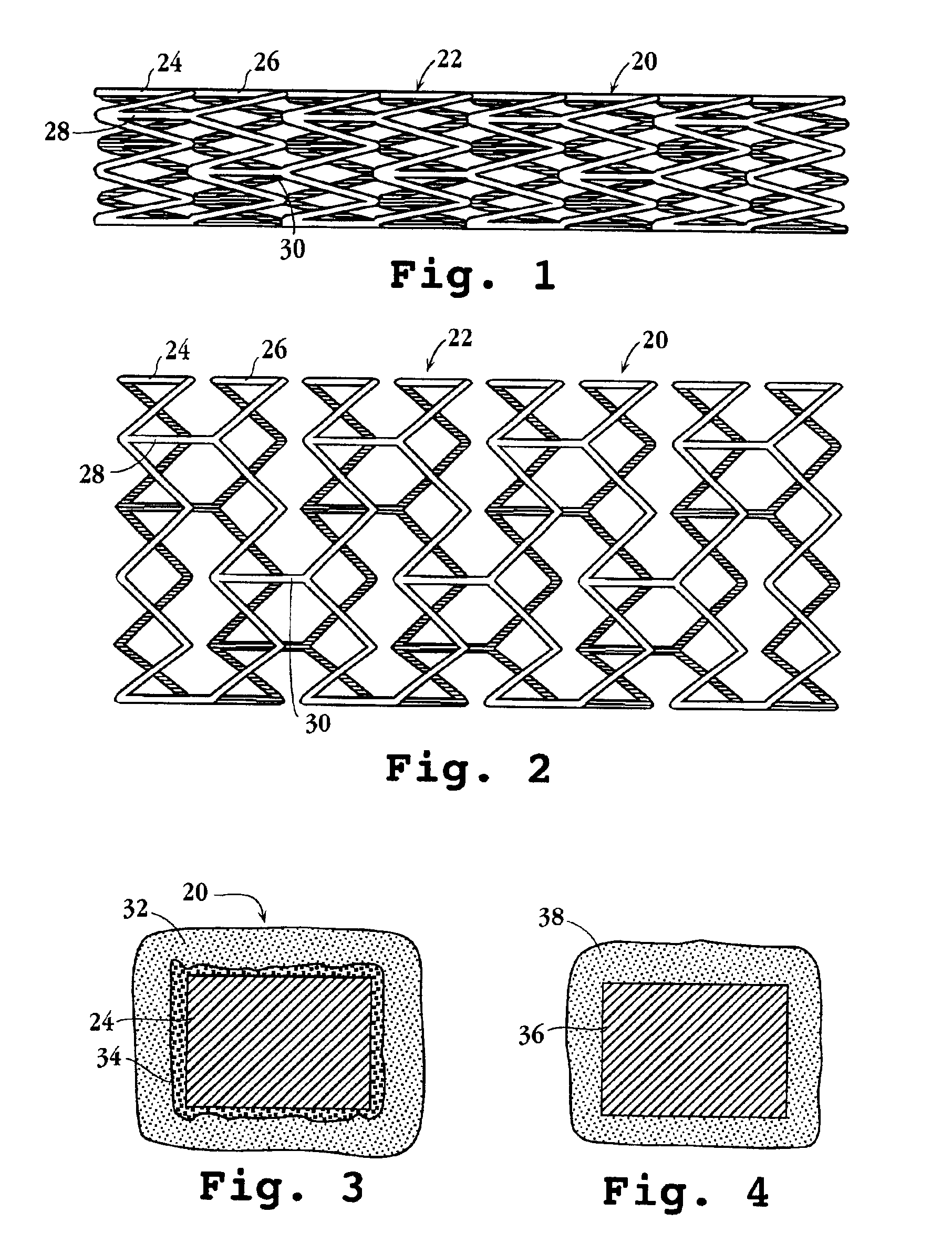
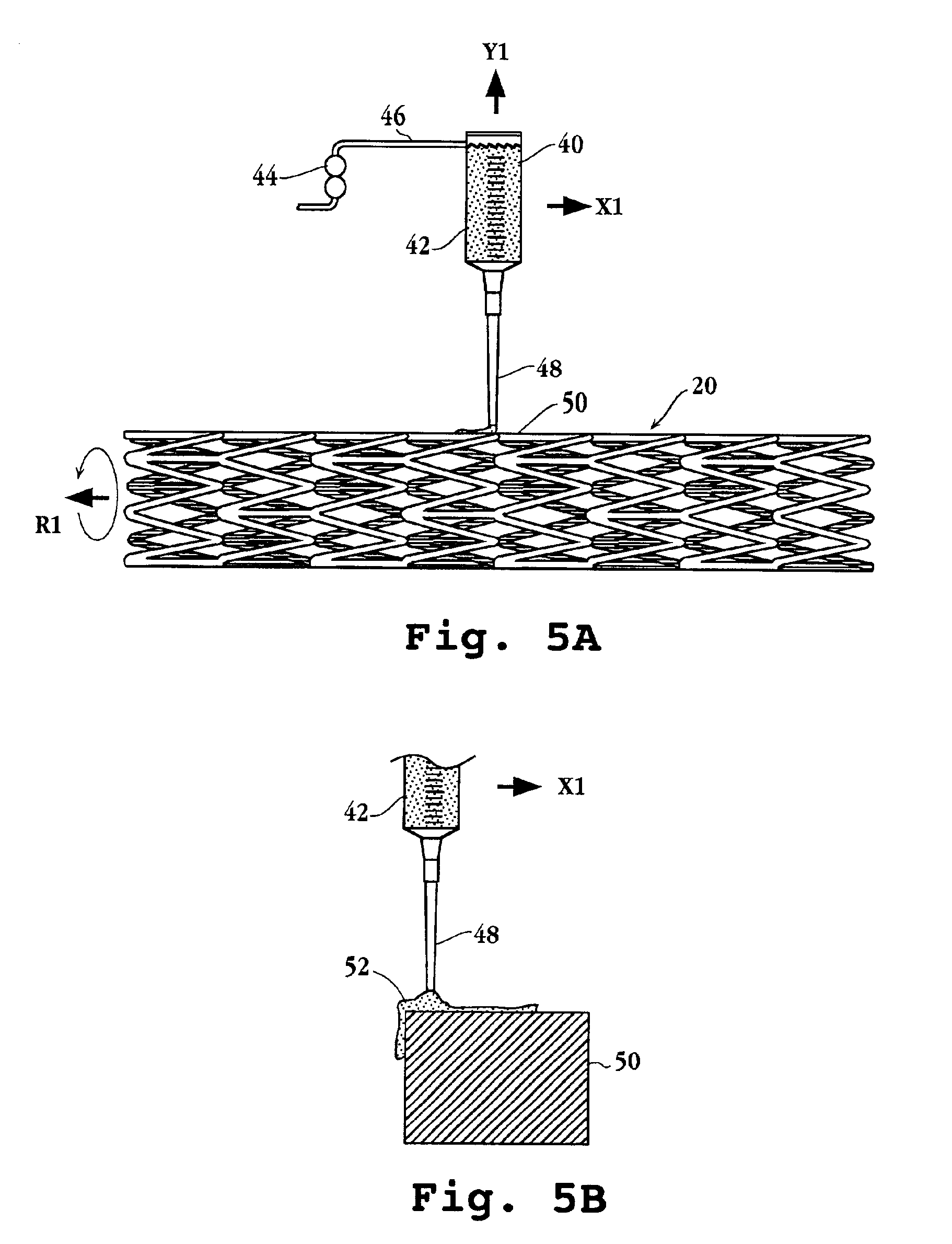
Drug-delivery endovascular stent and method for treating restenosis
InactiveUS6939376B2Efficient releaseOrganic active ingredientsOrganic chemistryRestenosisPoly dl lactide
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-dl-lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
Sublingual buccal effervescent
A pharmaceutical dosage form adapted to supply a medicament to the oral cavity for buccal, sublingual or gingival absorption of the medicament which contains an orally administerable medicament in combination with an effervescent for use in promoting absorption of the medicament in the oral cavity. The use of an additional pH adjusting substance in combination with the effervescent for promoting the absorption drugs is also disclosed.
Owner:CEPHALON INC
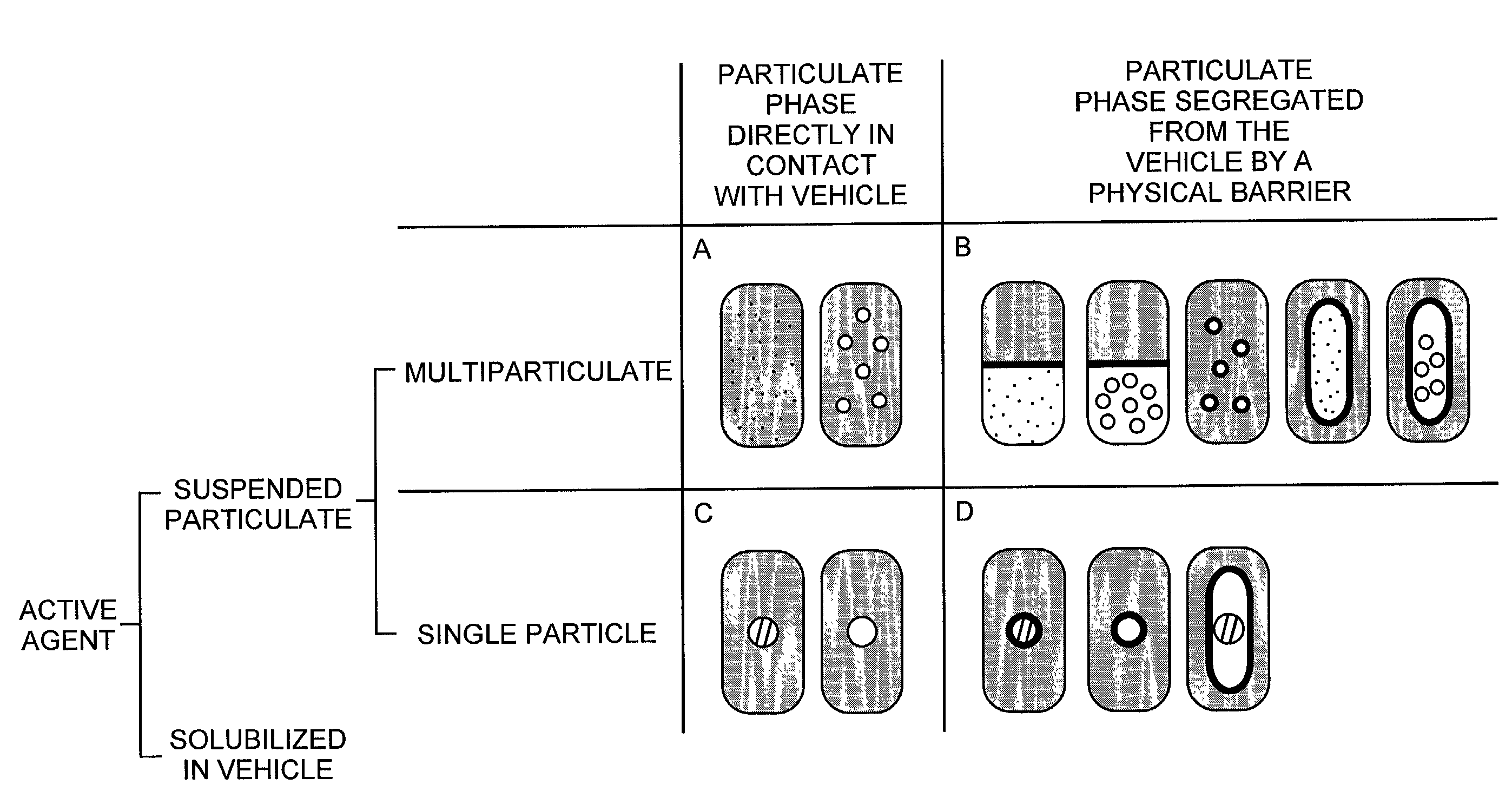
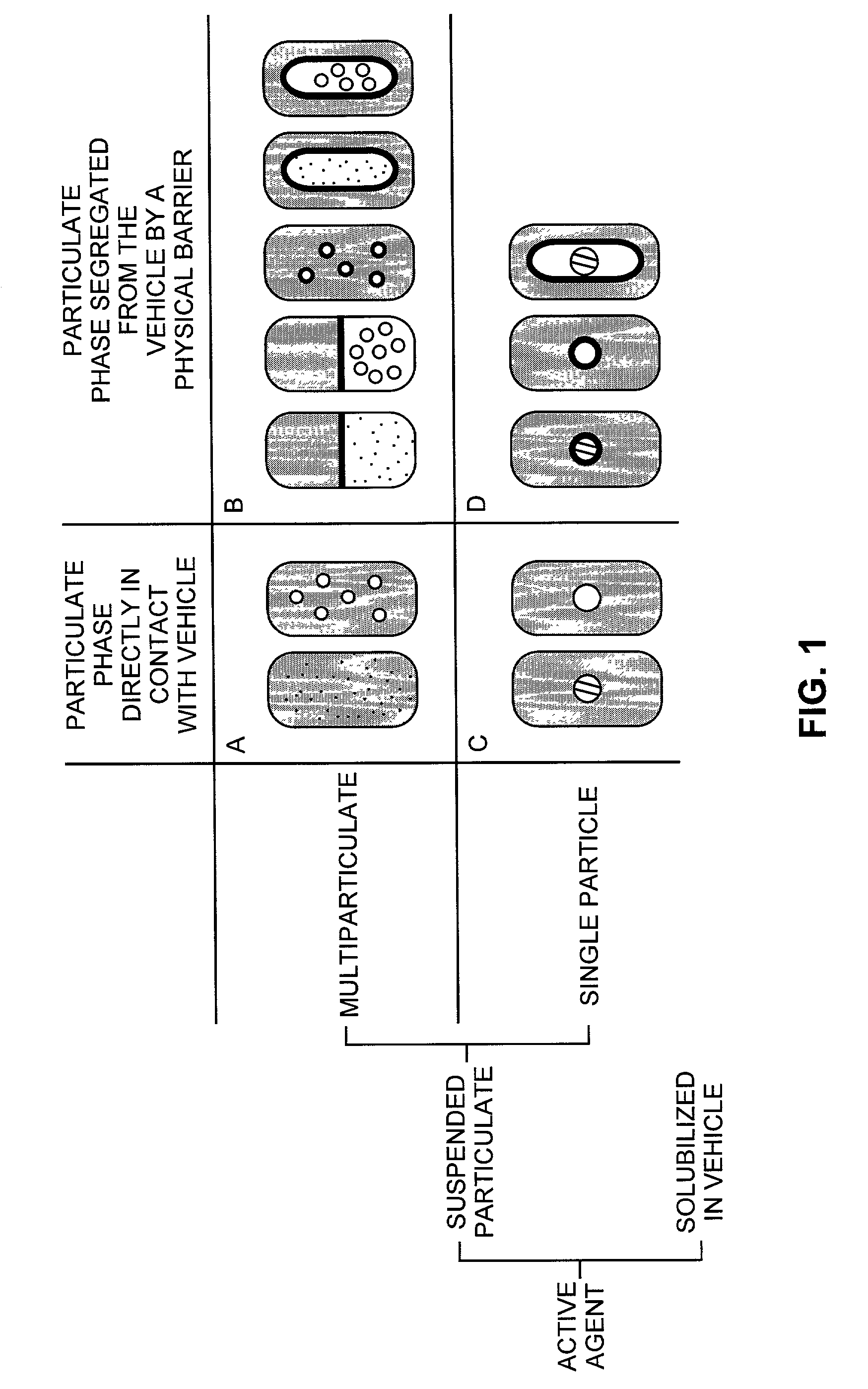
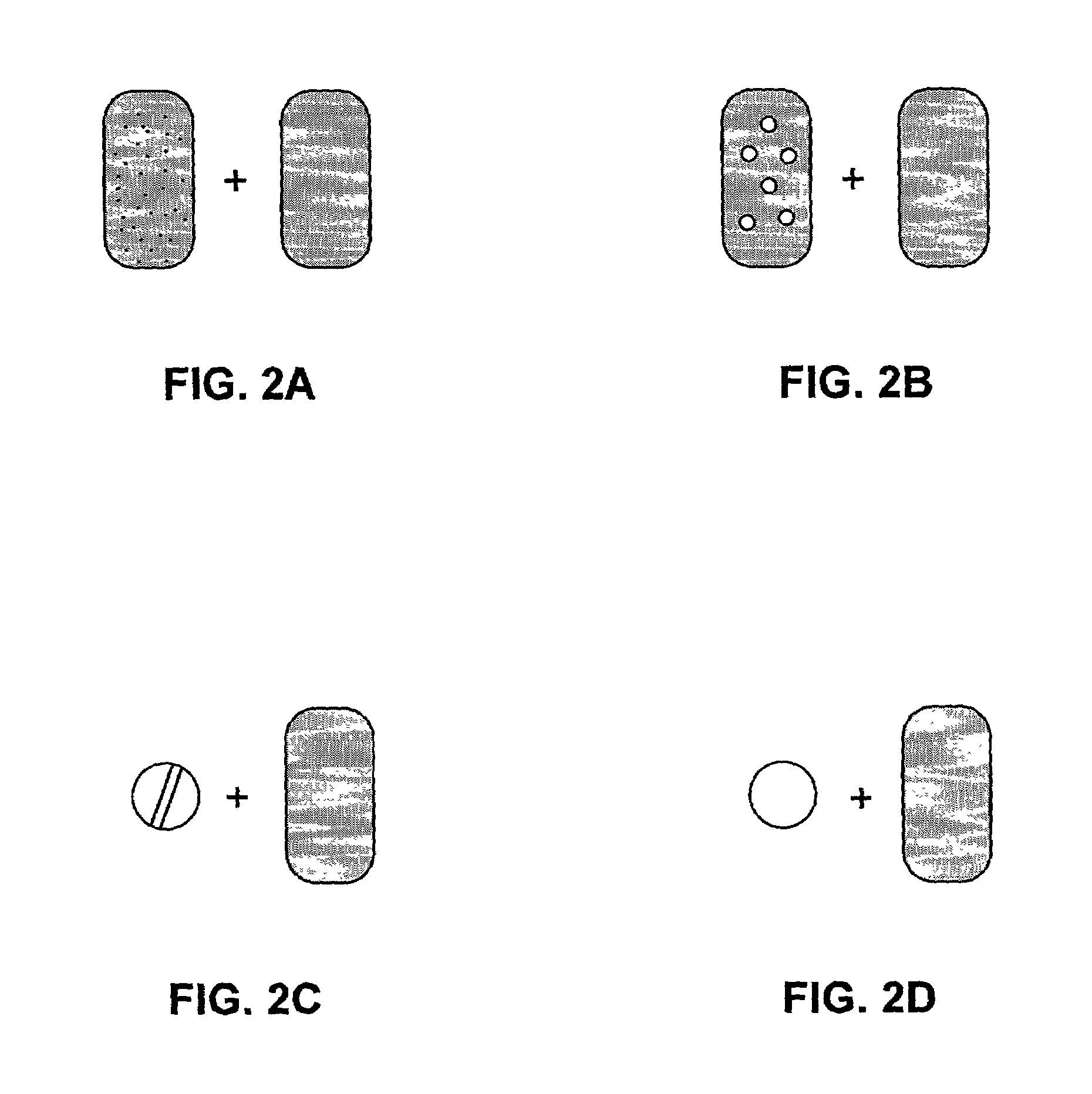
Pharmaceutical formulations and systems for improved absorption and multistage release of active agents
InactiveUS7374779B2Improve bioavailabilityLow variabilityPowder deliveryOrganic active ingredientsActive agentFast release
The present invention pertains to pharmaceutical formulations and systems for delivery of active agents, wherein a first fraction of an active agent is suspended in a vehicle and a second fraction of active agent is solubilized in the vehicle, with the suspended fraction representing about 5 wt. % to about 80 wt. % of the active agent and the second fraction representing about 20 wt. % to about 95 wt. % of the active agent. One or more additional active agents, which may be fully solubilized, partially solubilized, or suspended, may also be present. The first and second fractions of the active agent may or may not have different release profiles. Generally, a significant fraction of the solubilized drug will release rapidly, providing for rapid onset, while the suspended drug may be formulated for delayed and / or sustained release.
Owner:LIPOCINE
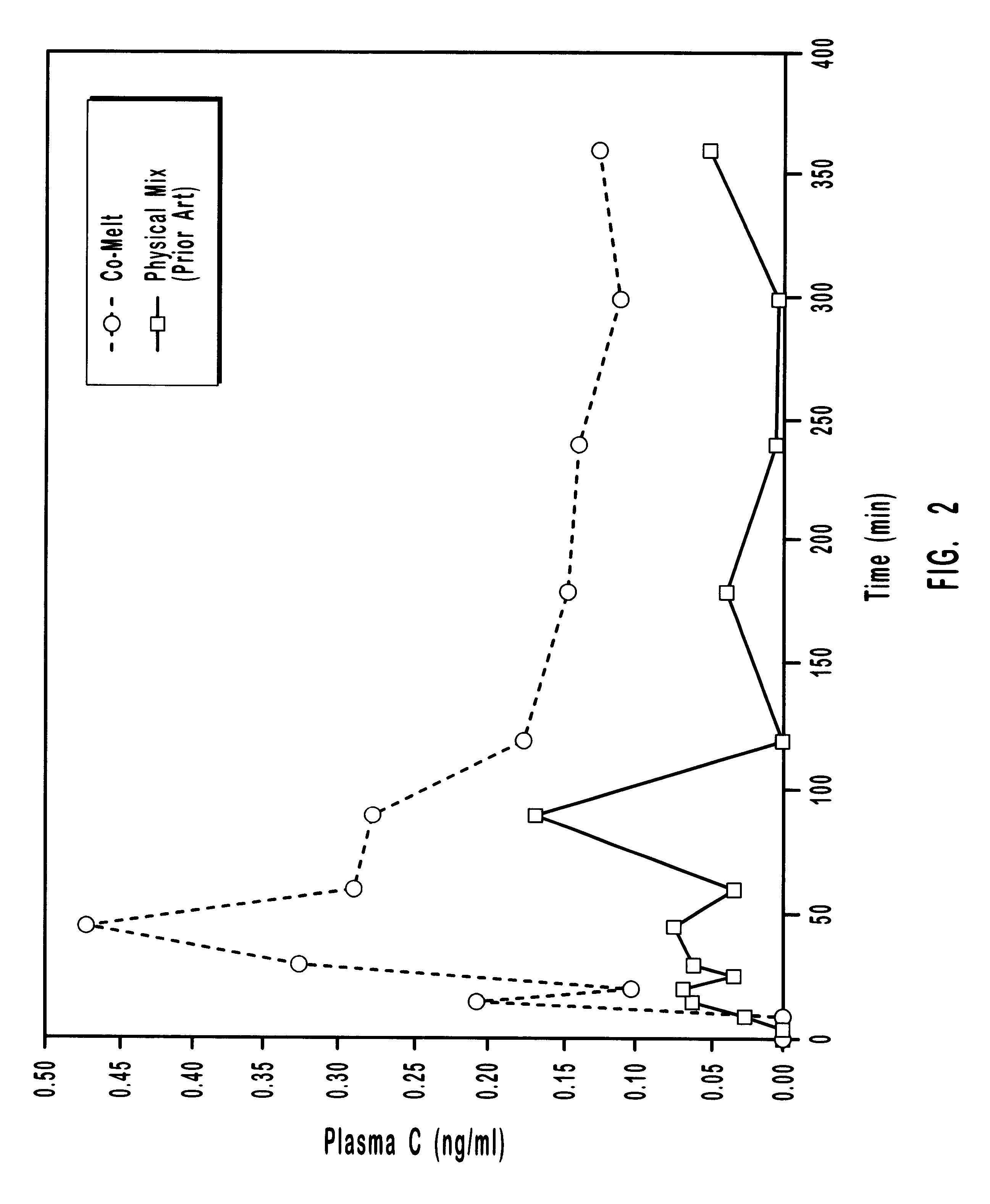
Abuse-proofed dosage form
ActiveUS8114383B2Pulverisation of the dosage form is considerably more difficultComplicating or preventing the subsequent abuseOrganic active ingredientsPowder deliveryBreaking strengthPhysiology
The present invention relates to an abuse-proofed, thermoformed dosage form containing, in addition to one or more active ingredients with abuse potential optionally together with physiologically acceptable auxiliary substances, at least one synthetic or natural polymer with a breaking strength of at least 500 N and to a process for the production thereof.
Owner:GRUNENTHAL GMBH
Controlled-release compositions containing opioid agonist and antagonist
InactiveUS6716449B2Good curative effectPatient compliance is goodBiocideNervous disorderOpioid antagonistOpioid Agonist
Controlled-release dosage forms containing an opioid agonist; an opioid antagonist; and a controlled release material release during a dosing interval an analgesic or sub-analgesic amount of the opioid agonist along with an amount of the opioid antagonist effective to attenuate a side effect of the opioid agonist. The dosage form provides analgesia for at least about 8 hours when administered to human patients. In other embodiments, the dose of antagonist released during the dosing interval enhances the analgesic potency of the opioid agonist.
Owner:PURDUE PHARMA LP
High dose solid unit oral pharmaceutical dosage form of amorphous nelfinavir mesylate and process for making same
InactiveUS7014866B2Satisfactory bioavailabilitySatisfactory dissolutionPowder deliveryBiocideHigh dosesNelfinavir mesylate
A solid unit oral pharmaceutical dosage form of amorphous nelfinavir mesylate is provided comprising amorphous nelfinavir mesylate in an amount of from about 400 mg to about 700 mg calculated as nelfinavir base, and a pharmaceutically acceptable water soluble, non-ionic synthetic block copolymer of ethylene oxide and propylene oxide, the copolymer having a melting point of at least about 45° C. and an HLB value at 25° C. of from about 18 to about 29, wherein the copolymer is present from about 40% to about 65% by weight of the nelfinavir mesylate. A hot melt granulation process for making the dosage form is provided.
Owner:F HOFFMANN LA ROCHE & CO AG
Methods and compositions for the prevention and treatment of atherosclerosis, restenosis and related disorders
Methods and compositions for the prevention and treatment of all forms of atherosclerosis are described. Administration of compounds such as thalidomide, its analogs, hydrolysis products, metabolites, derivatives and precursors as well as additional compounds capable of inhibiting tumor necrosis factor alpha (TNF-alpha) are used in the invention. Also disclosed is the coating of prosthetic devices, such as stents, with the compounds of the invention for the prevention and / or treatment of restenosis.
Owner:CELGENE CORP
Controlled release formulations of opioid and nonopioid analgesics
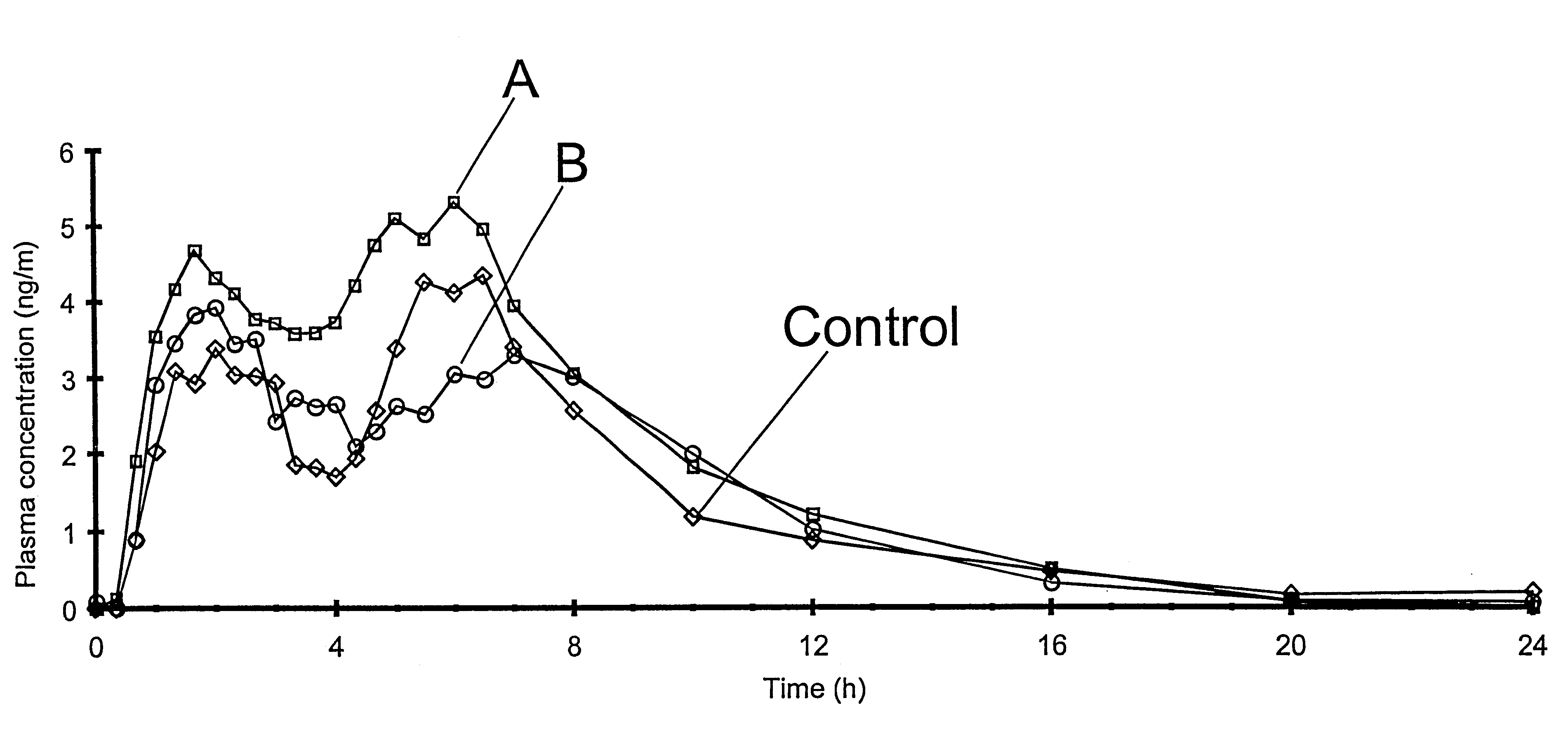
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
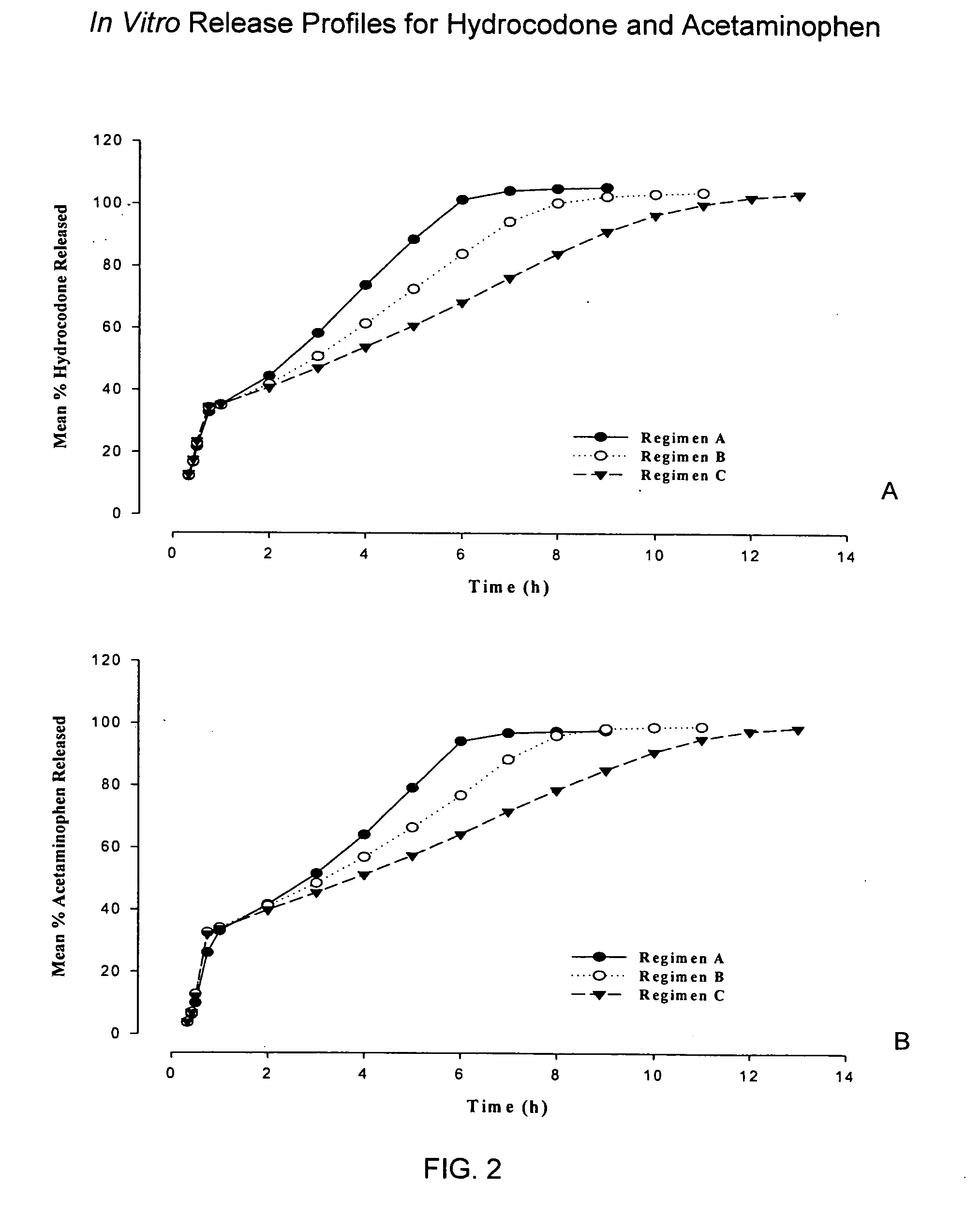
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Effervescent granules and methods for their preparation
InactiveUS6071539AMinimize degradationMelt and soften binderPowder deliveryPill deliveryPlasticizerHot melt
According to the present invent, effervescent granules having a controllable rate of effervescence are provided. Such granules comprise an acidic agent, an alkaline agent, a hot-melt extrudable binder capable of forming a eutectic mixture with the acidic agent and, optionally, a plasticizer. The effervescent granules are made by a hot-melt extrusion process.
Owner:ETHYPHARM SA
Compositions used in human treatment
A composition for treatment of a human body comprises a combination of at least one hormone, at least one amino acid, at least one enzyme and / or vitamin, and least one mineral. The relative proportions of the hormone, amino acid, enzyme and mineral in the combination are balanced with respect to each other so as to be present in effective amounts to substantially restore to optimal levels in the body the hormone, amino acid, enzyme and mineral The hormone, amino acid, enzyme and mineral in the combination further operate synergistically to provide both nutrients and command components to enable the body to effectively utilize the nutrients. The invention is also a method of forming a composition for the treatment of a human body.
Owner:COCHRAN TIMOTHY M
Pharmaceutical formulation containing opioid agonist, opioid antagonist and gelling agent
InactiveUS7842307B2Reducing abuse potential of dosage formLower potentialBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
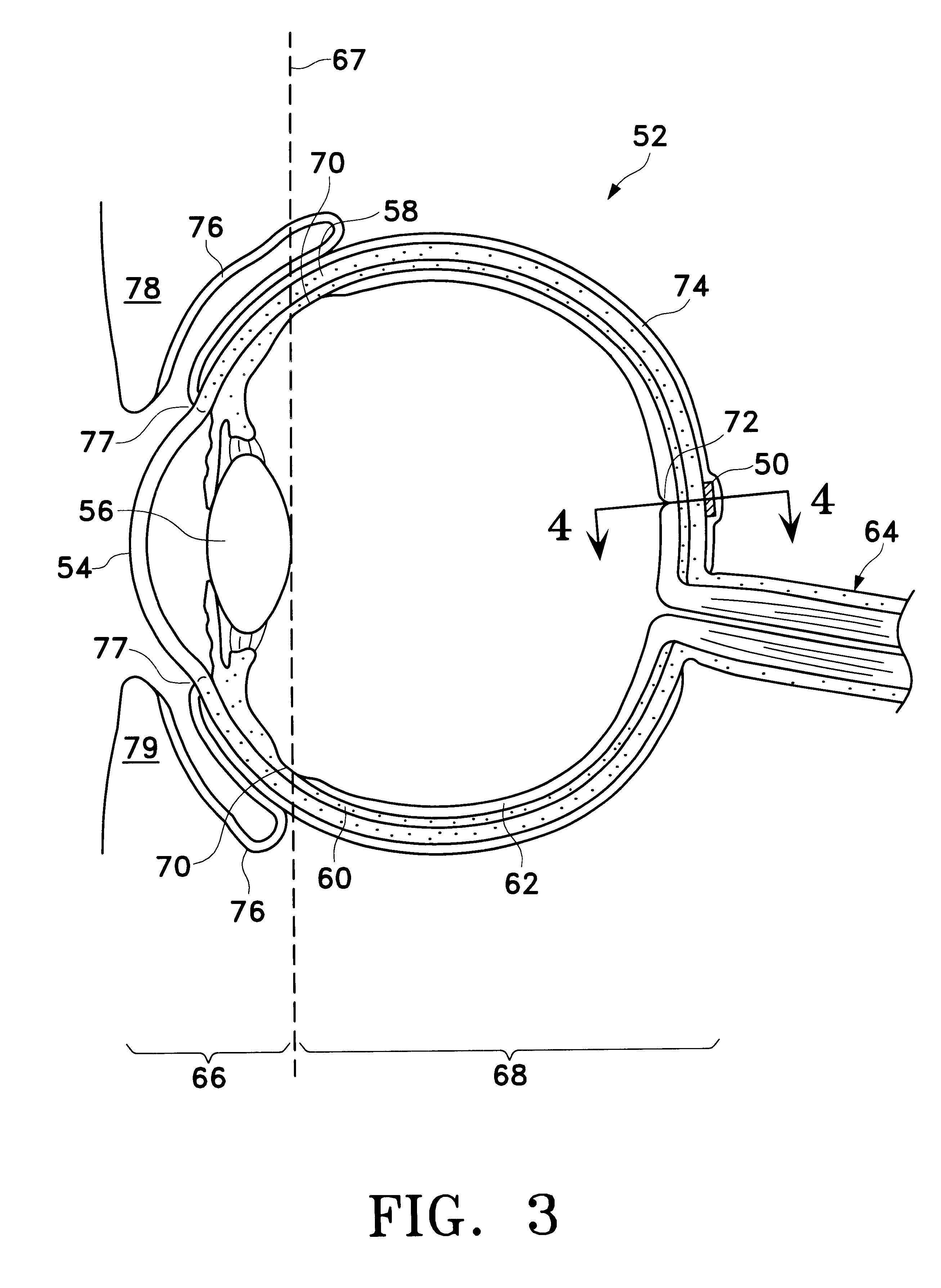
Extended release biodegradable ocular implants
Biodegradable implants sized and suitable for implantation in an ocular region or site and methods for treating ocular conditions. The implants provide an extended release of an active agent at a therapeutically effective amount for a period of time between 50 days and one year, or longer.
Owner:ALLERGAN INC
Dosage form exhibiting rapid disperse properties, methods of use and process for the manufacture of same
InactiveUS6471992B1Disperse fastHigh hardnessPowder deliveryAdditive manufacturing apparatusThree dimensional shapeBULK ACTIVE INGREDIENT
A rapidly dispersing dosage form is described, which releases its active ingredients within a period of less than about ninety seconds. These dosage forms exhibit a three-dimensional shape that is retained for adequate storage but is readily dispersed in the presence of excess moisture. Also disclosed are methods of administration of a medicament and a process for the preparation of rapidly dispersing dosage forms.
Owner:MASSACHUSETTS INST OF TECH +2
Bioresorbable hydrogel compositions for implantable prostheses
Crosslinked compositions formed from water-insoluble copolymers are disclosed. These compositions are copolymers having a bioresorbable region, a hydrophilic region and at least two cross-linkable functional groups per polymer chain. Crosslinking of these polymers can be effected in solution in organic solvents or in solvent-free systems. If crosslinking occurs in a humid environment, a hydrogel will form. If crosslinking occurs in a non-humid environment, a xerogel will form which will form a hydrogel when exposed to a humid environment and the resulting crosslinked materials form hydrogels when exposed to humid environments. These hydrogels are useful as components in medical devices such as implantable prostheses. In addition, such hydrogels are useful as delivery vehicles for therapeutic agents and as scaffolding for tissue engineering applications.
Owner:LIFESHIELD SCI
Compositions and methods relating to reduced mucoadhesion
InactiveUS20120121718A1Reduced mucoadhesionDiffusion fastOrganic active ingredientsPowder deliveryMedicineParticle composition
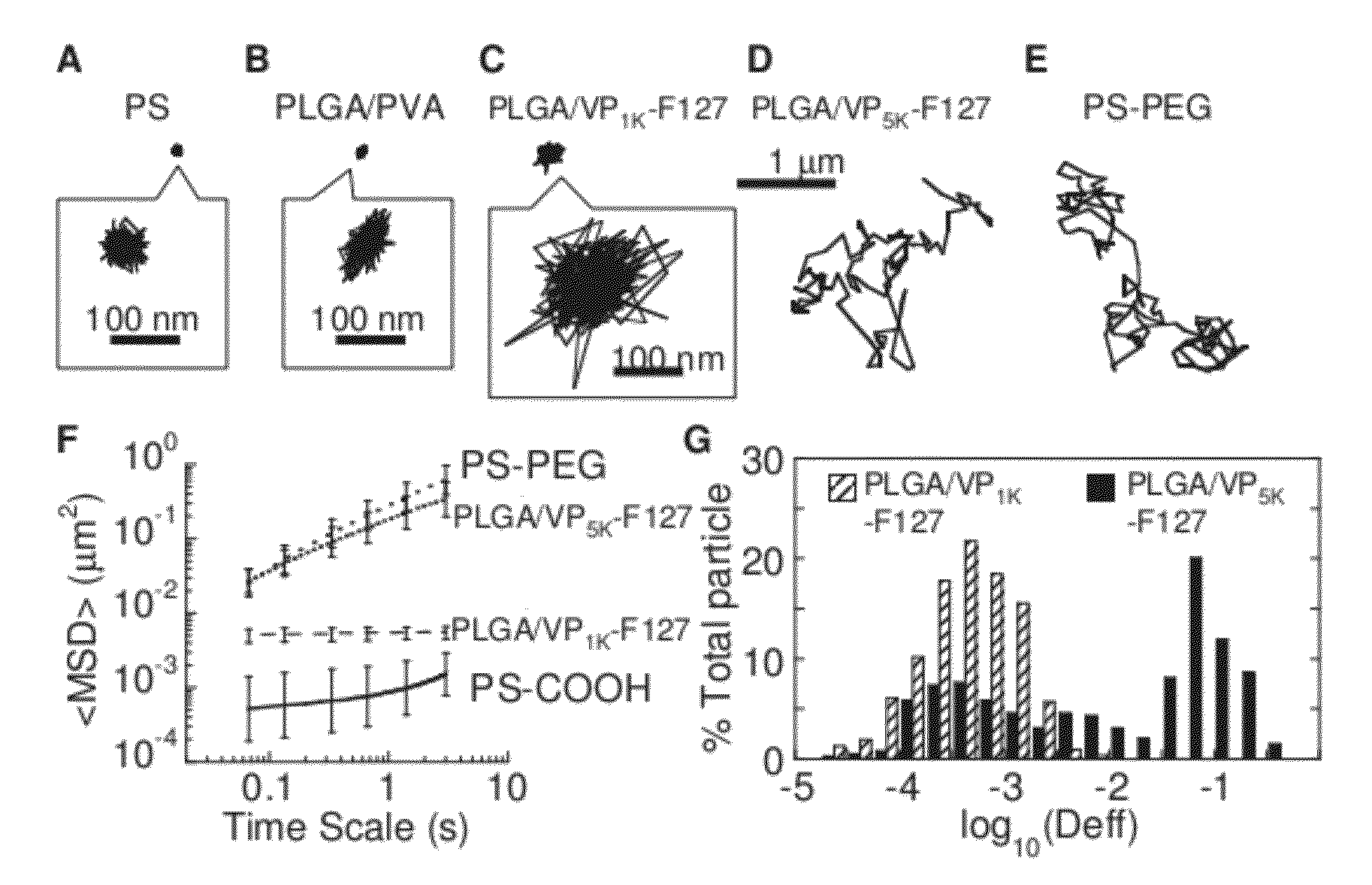
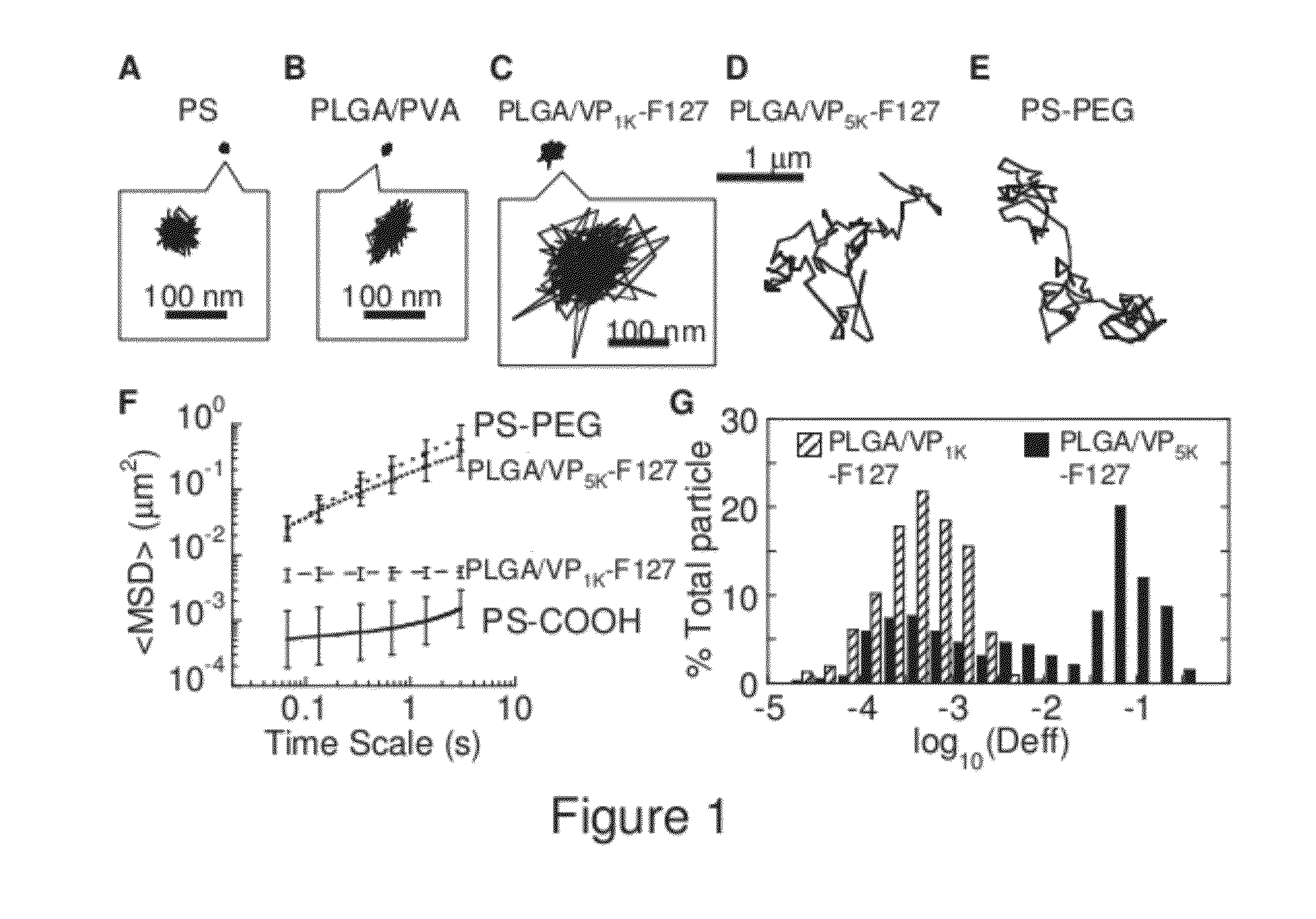
The present invention generally relates to reducing the mucoadhesive properties of a particle. In some embodiments, the particle is coated with and / or associated with a (poly(ethylene glycol))-(poly(propylene oxide))-(poly(ethylene glycol)) triblock copolymer. Methods for preparing inventive particles using a poly(ethylene glycol)-vitamin E conjugate as a surfactant are also provided. In some embodiments, methods are provided comprising administering to a subject a composition of particles of the present invention. Such particles with reduced mucoadhesive properties are useful in delivering agents to mucosal tissues such as oral, ophthalmic, gastrointestinal, nasal, respiratory, and genital mucosal tissues.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/78e0b760-3b4b-4848-b642-2e00beb09f2a/US20070135461A1-20070614-C00001.png)
![Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/78e0b760-3b4b-4848-b642-2e00beb09f2a/US20070135461A1-20070614-C00002.png)
![Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors Heteroaryl substituted pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidines as janus kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/78e0b760-3b4b-4848-b642-2e00beb09f2a/US20070135461A1-20070614-C00003.png)