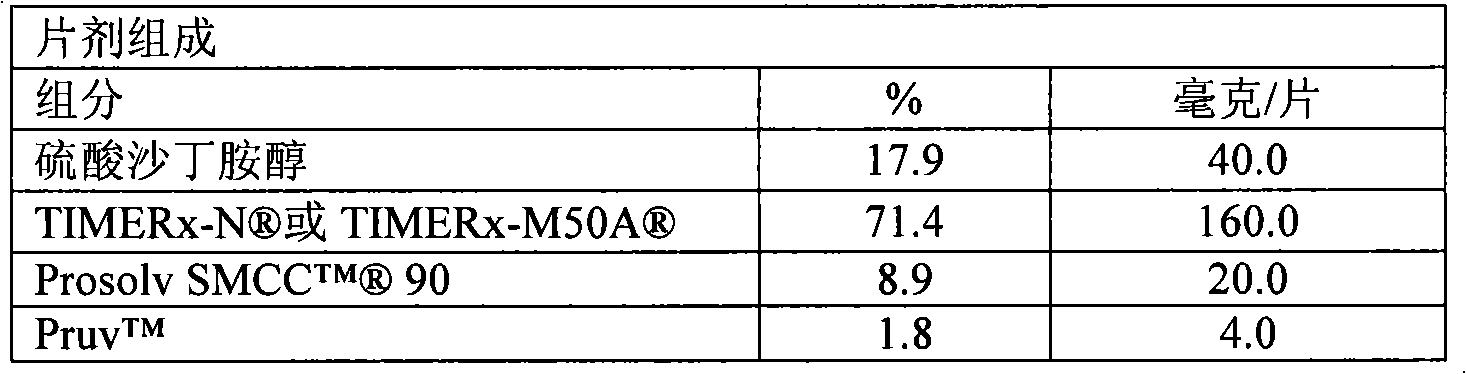
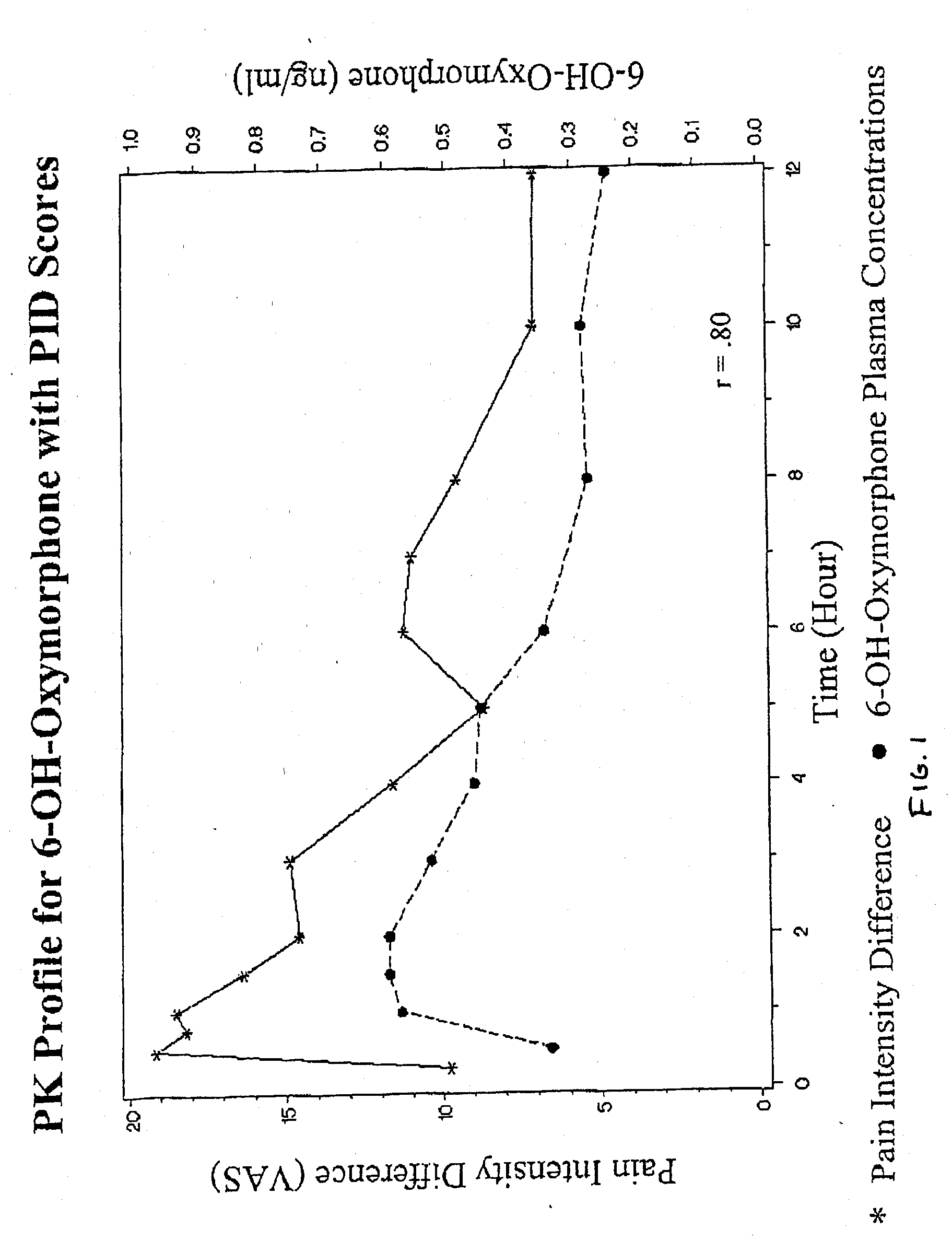
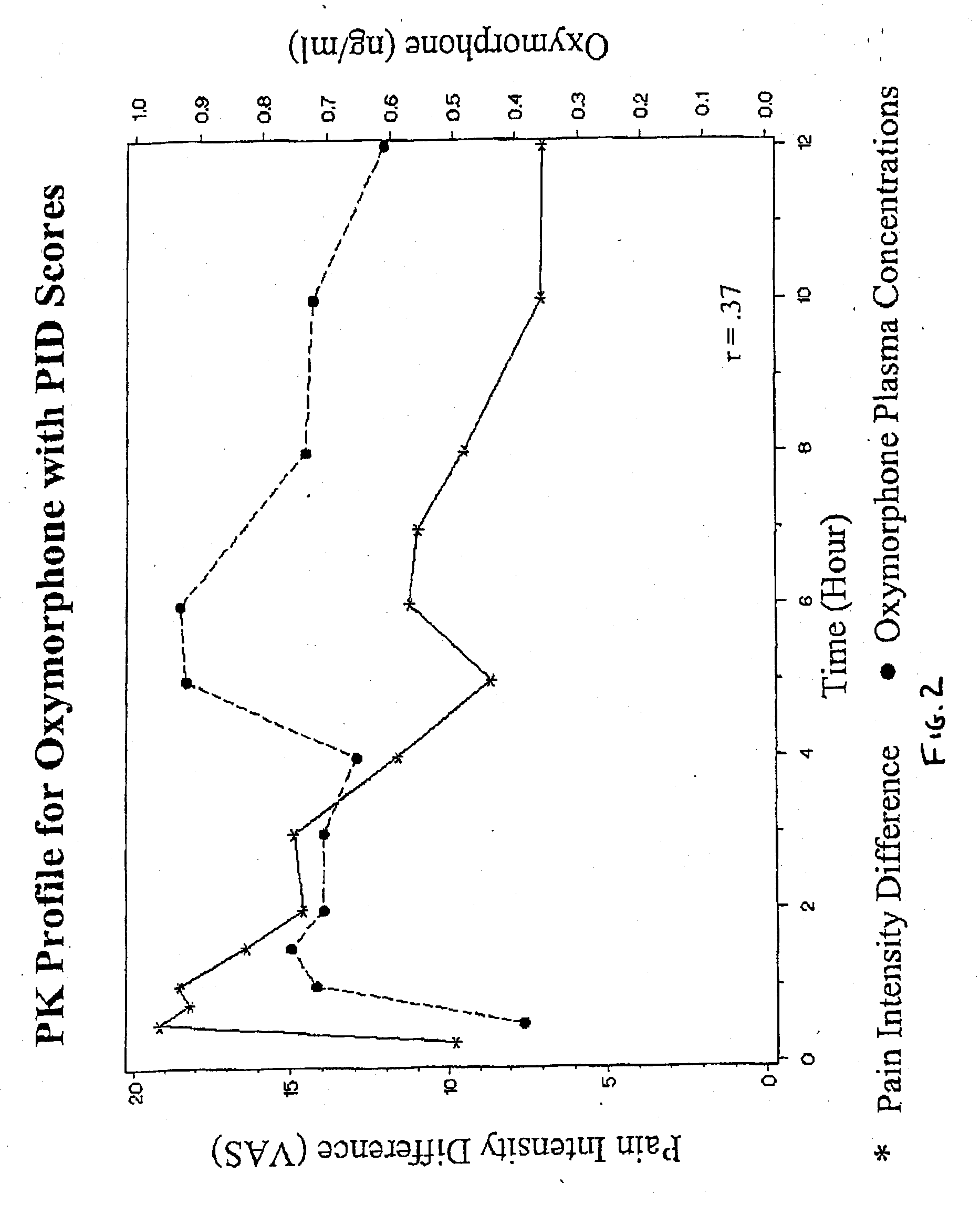
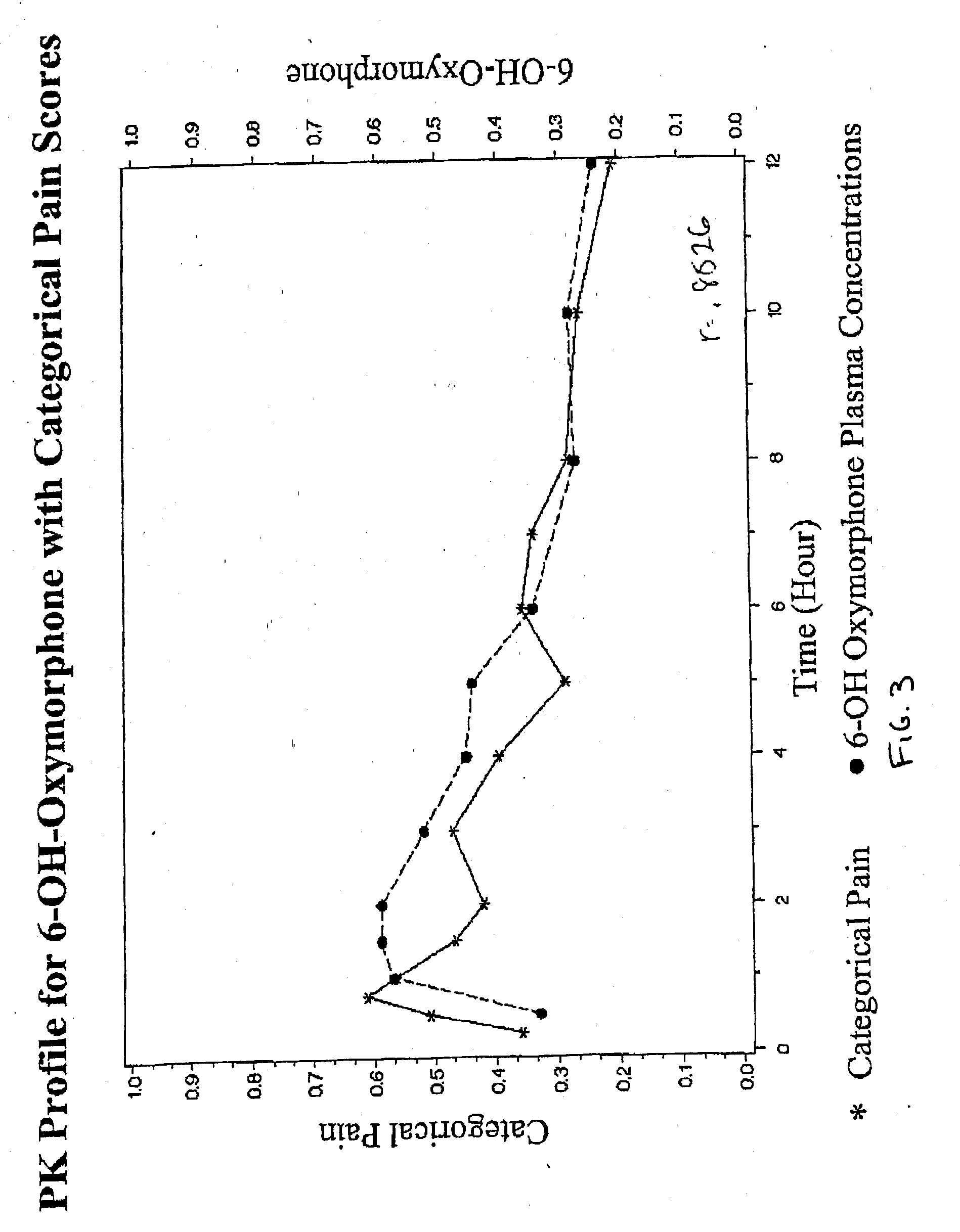
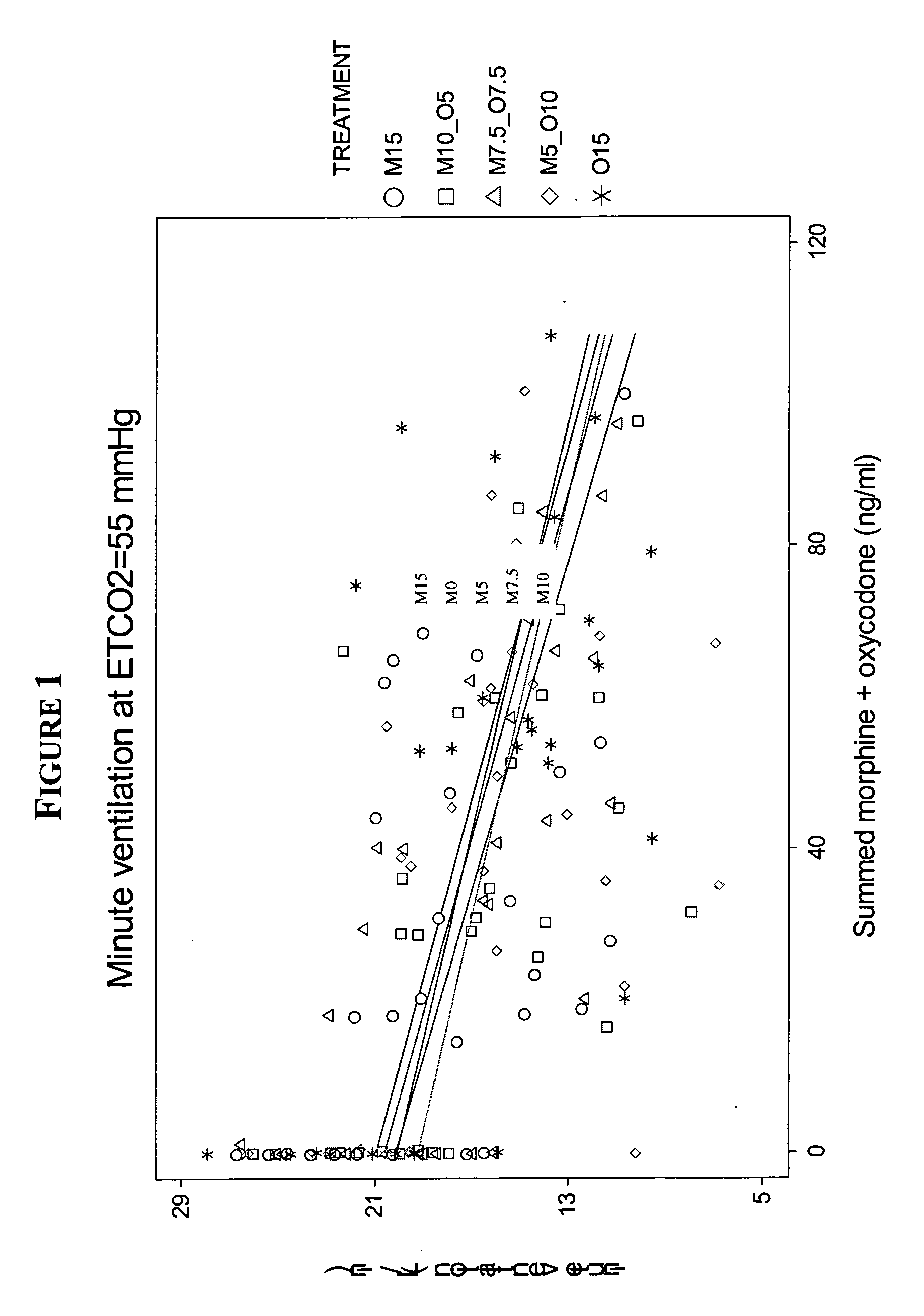
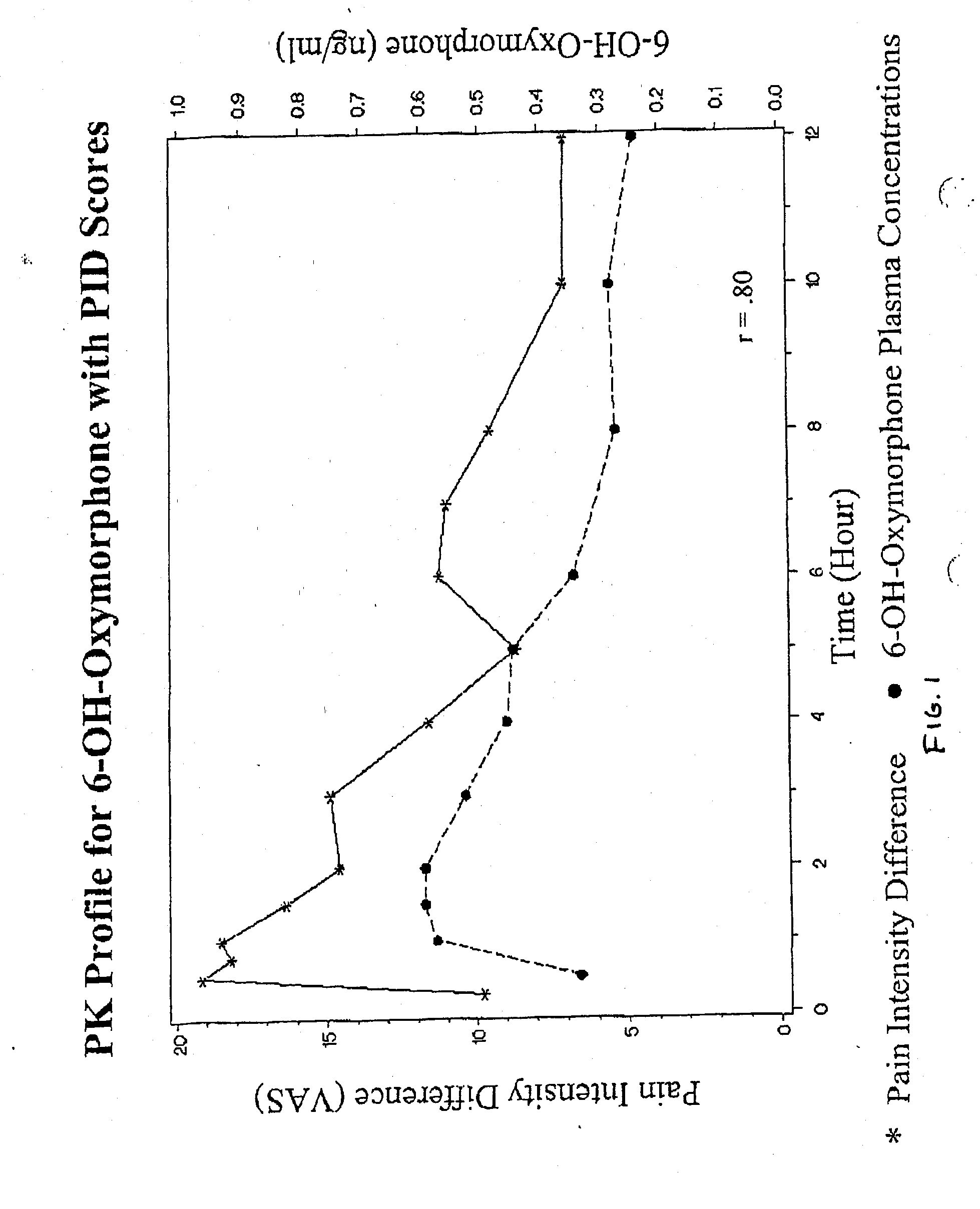
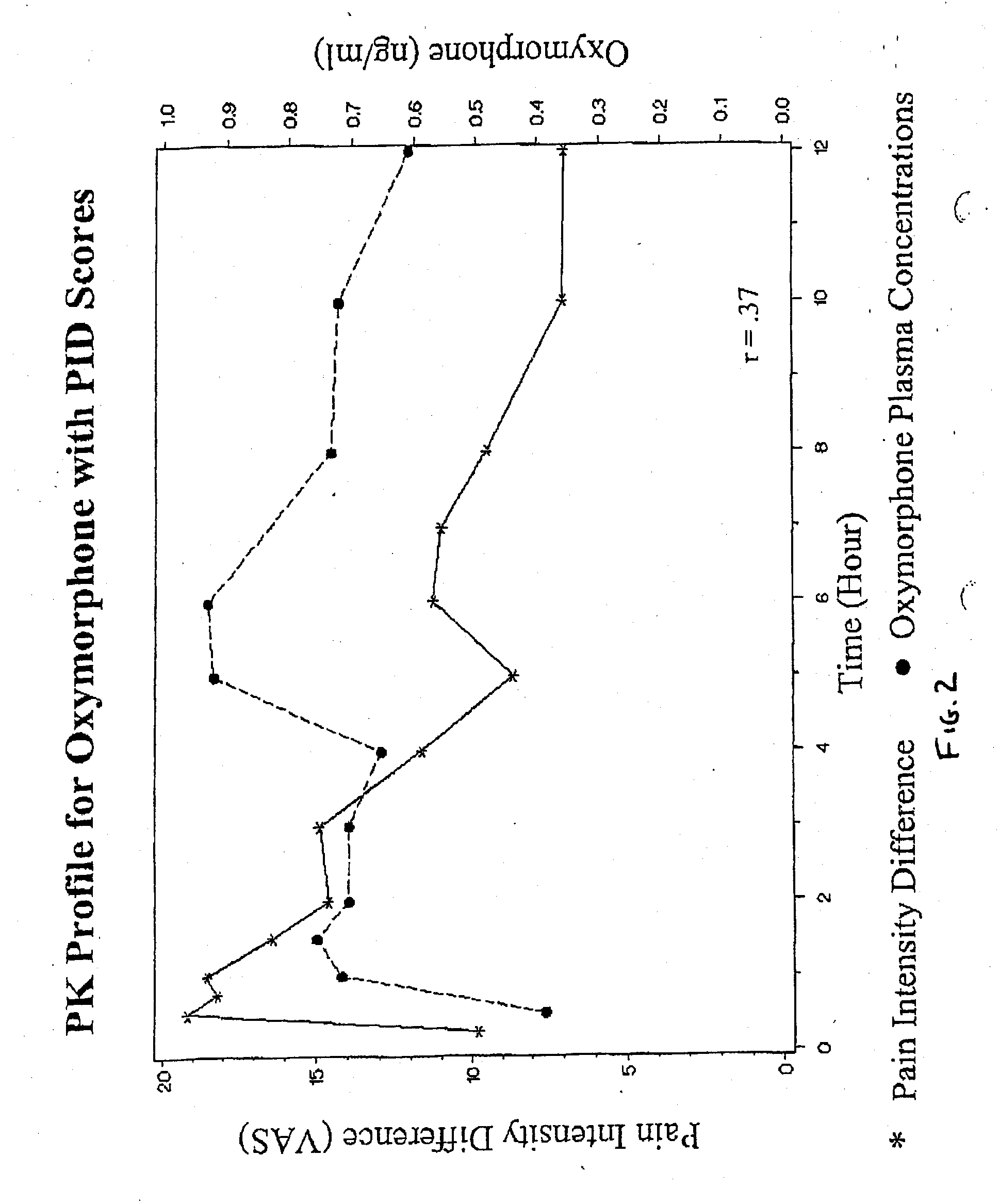
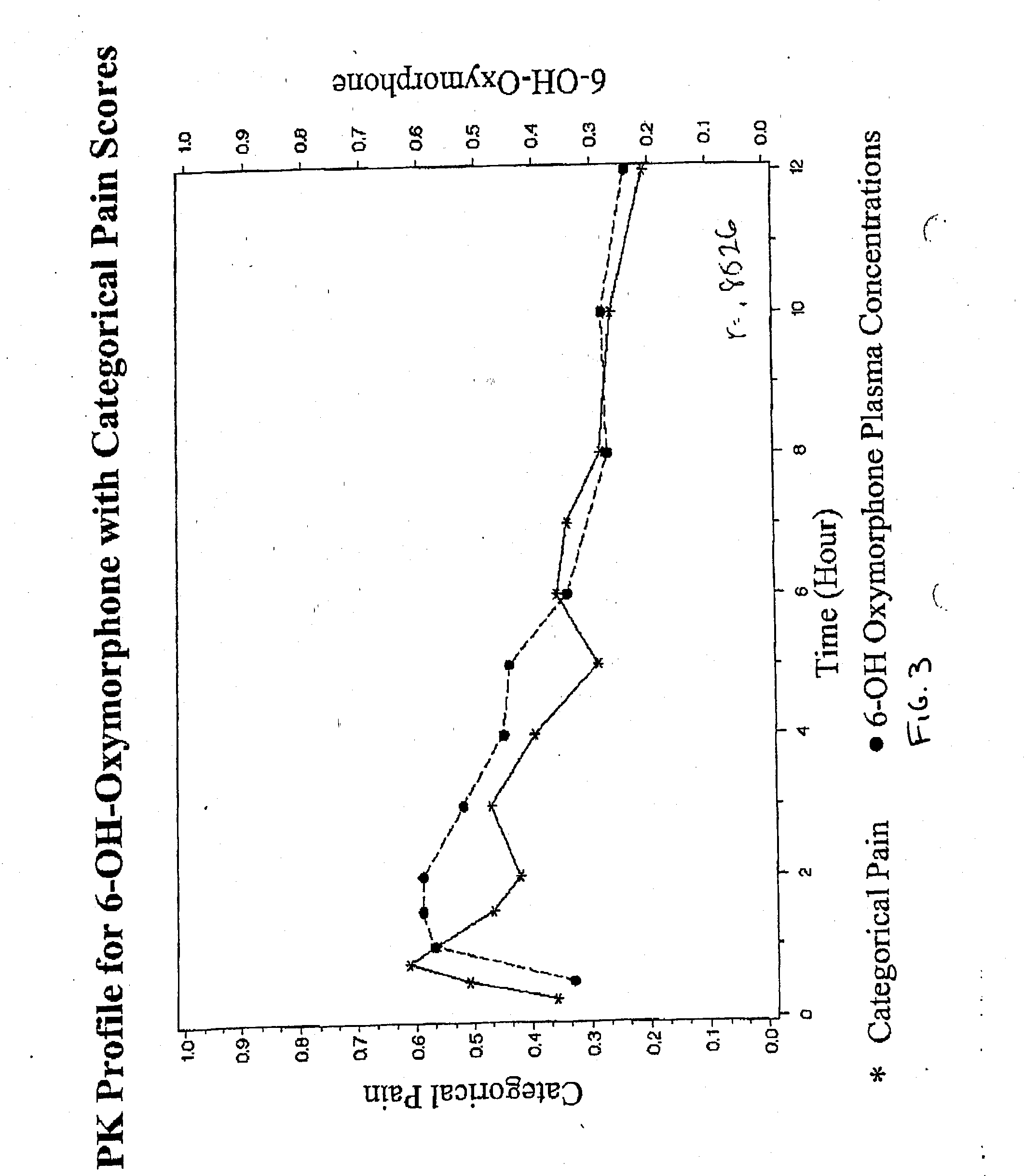
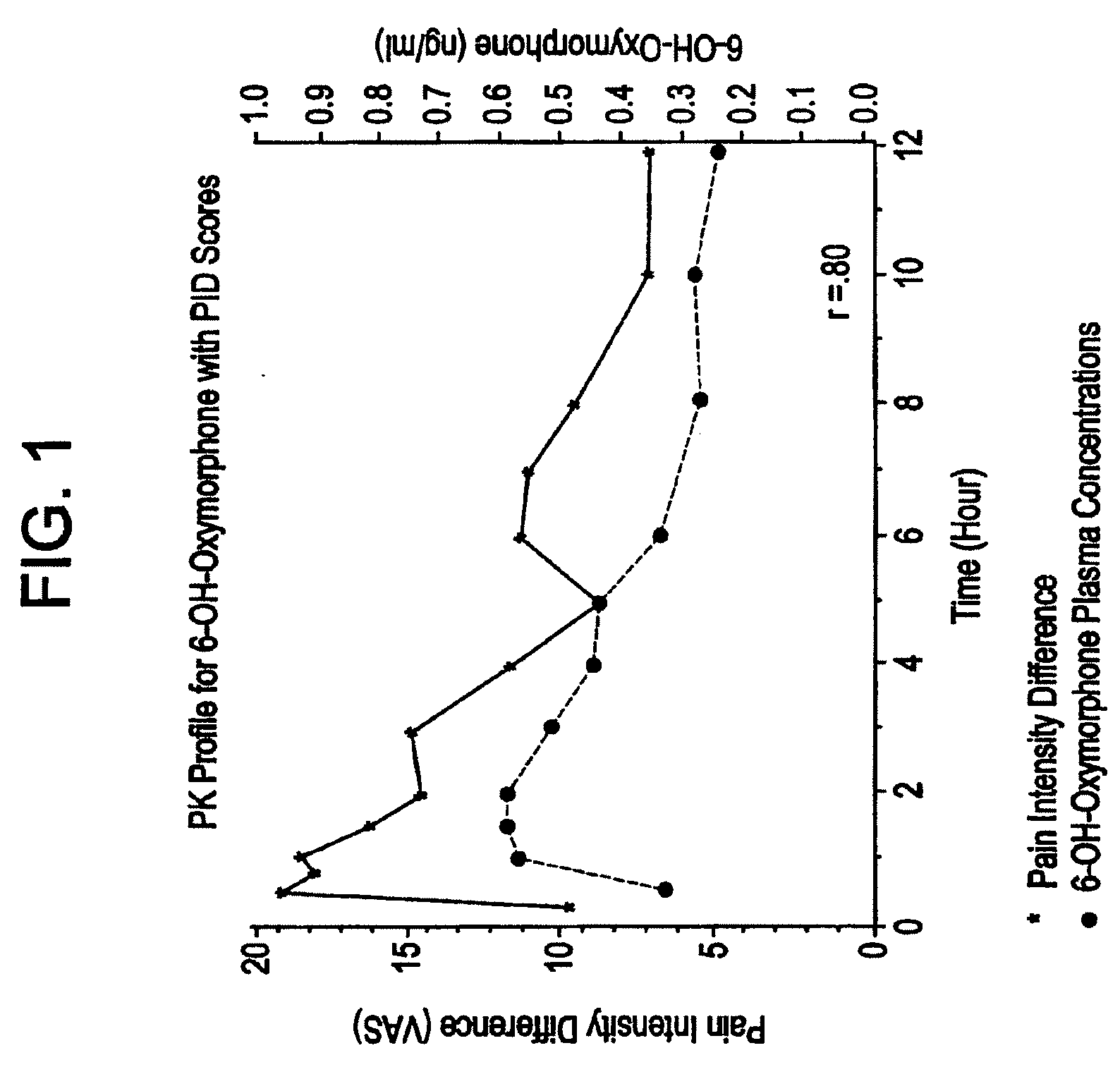
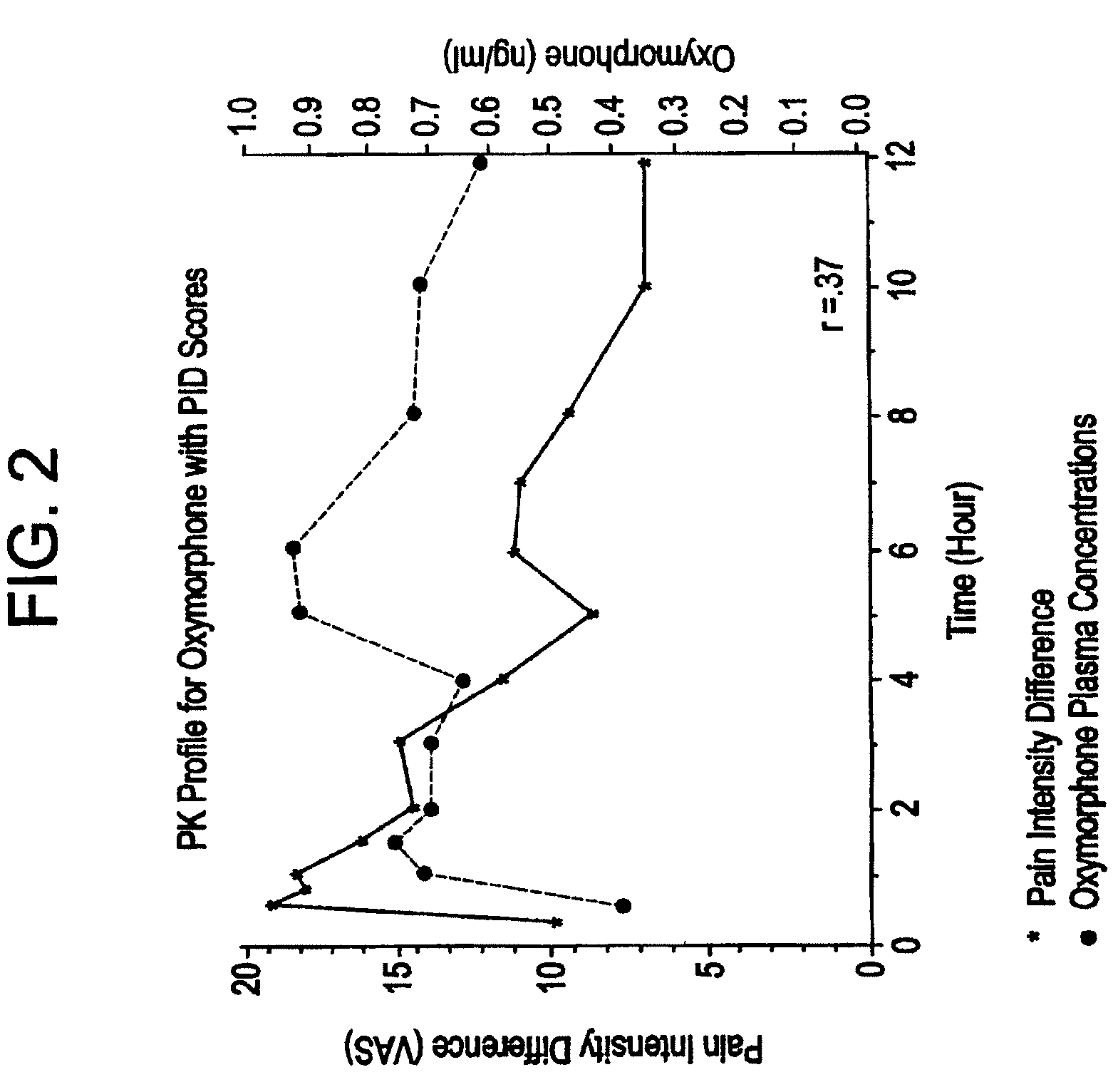
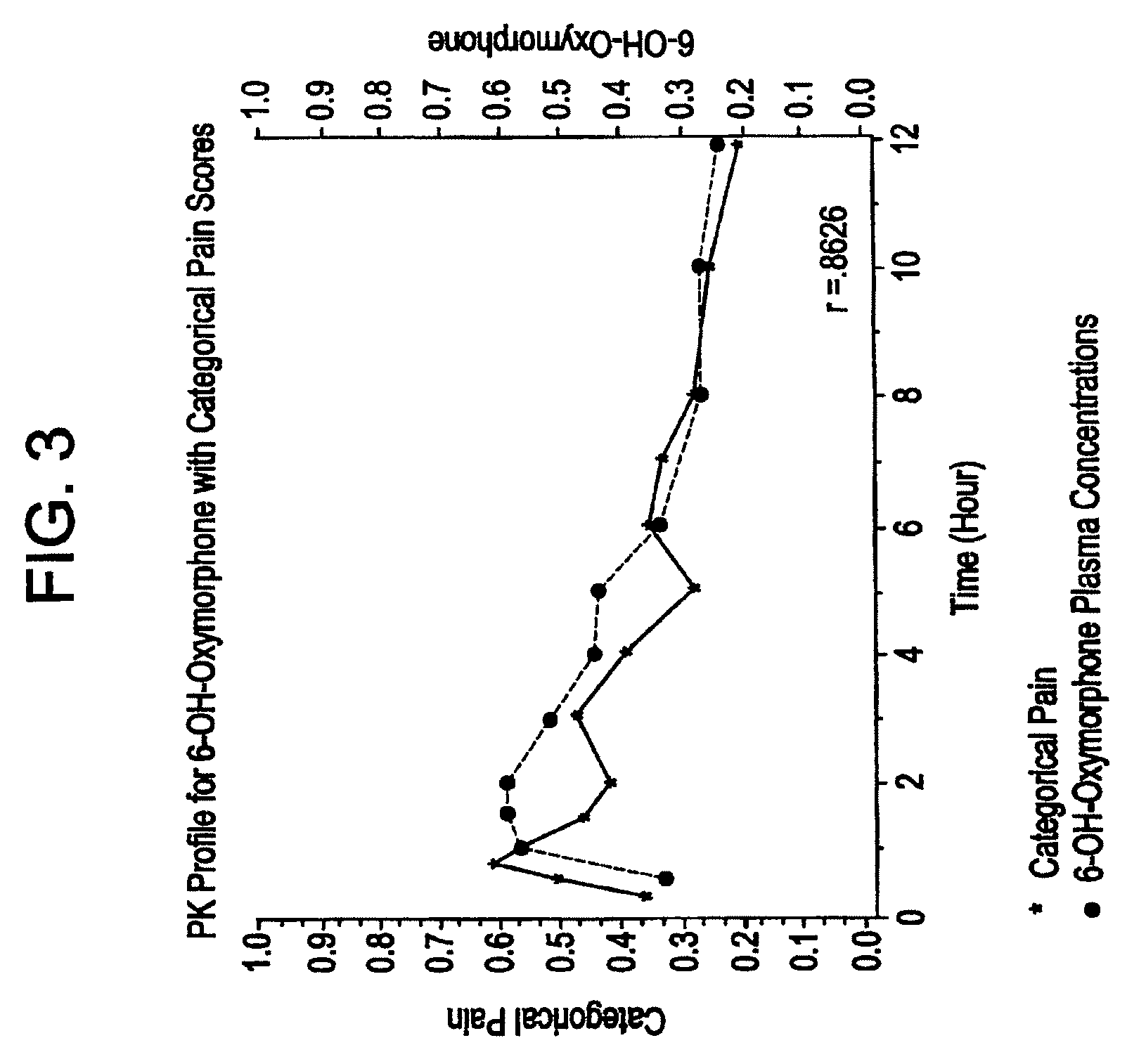
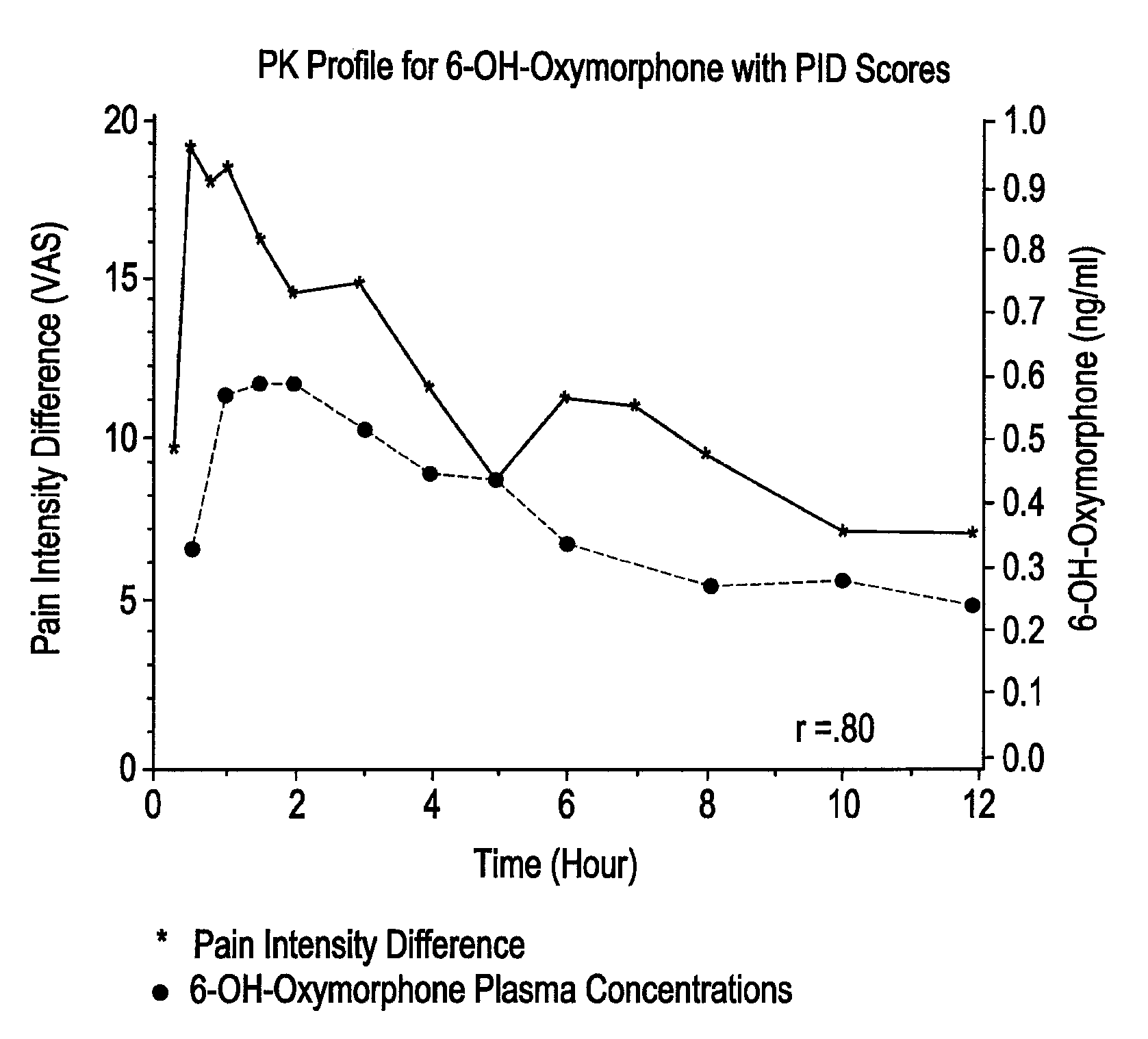
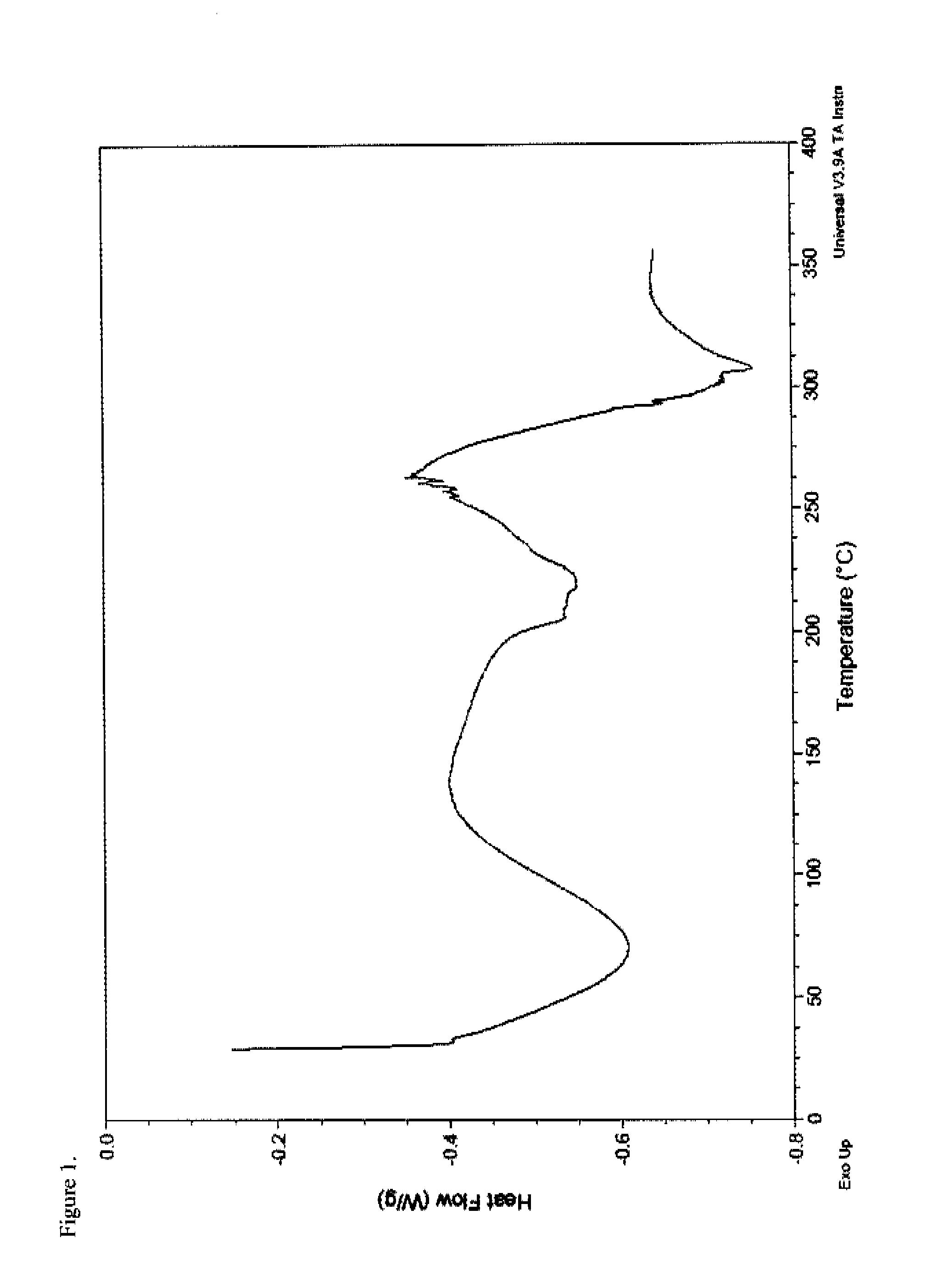
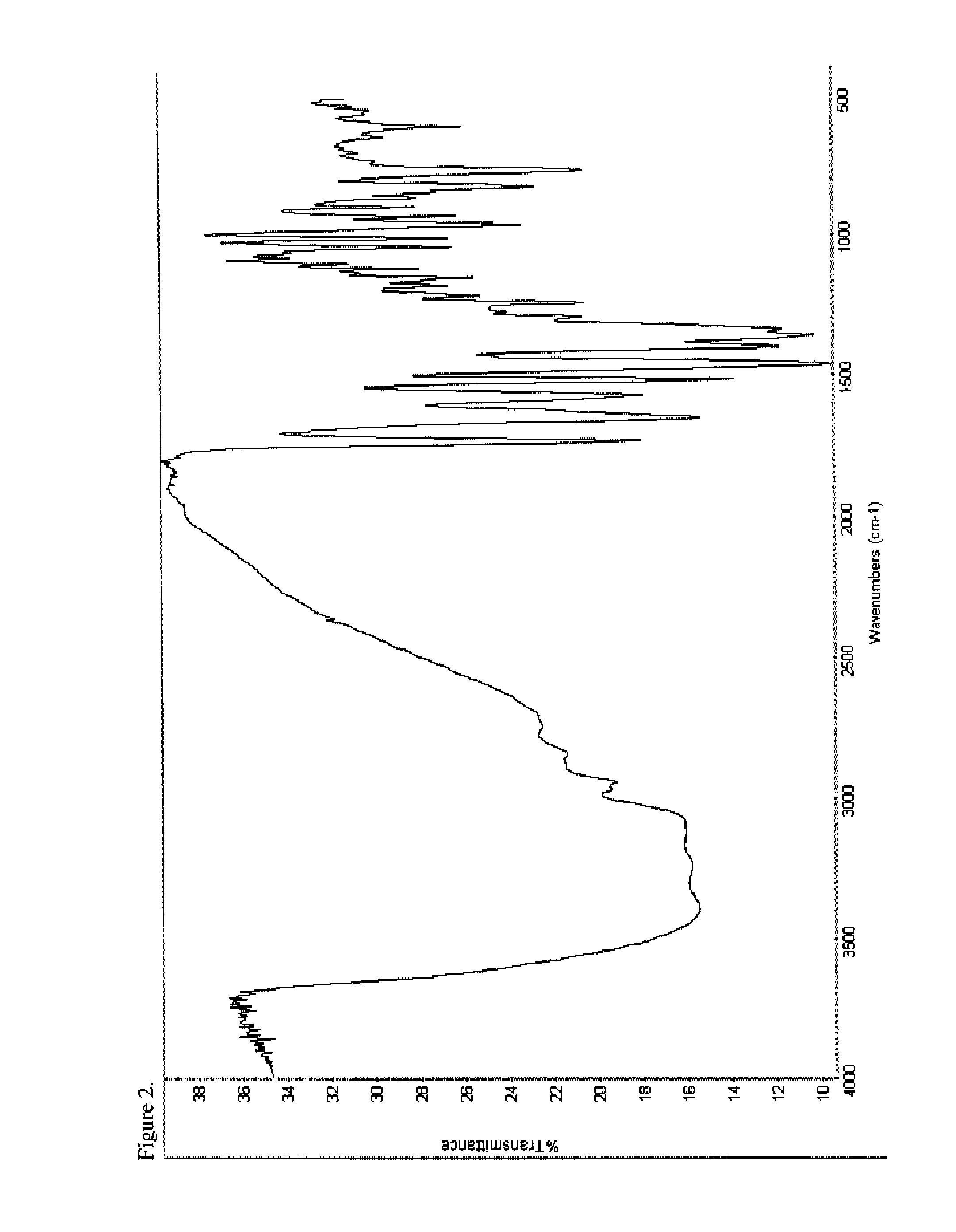
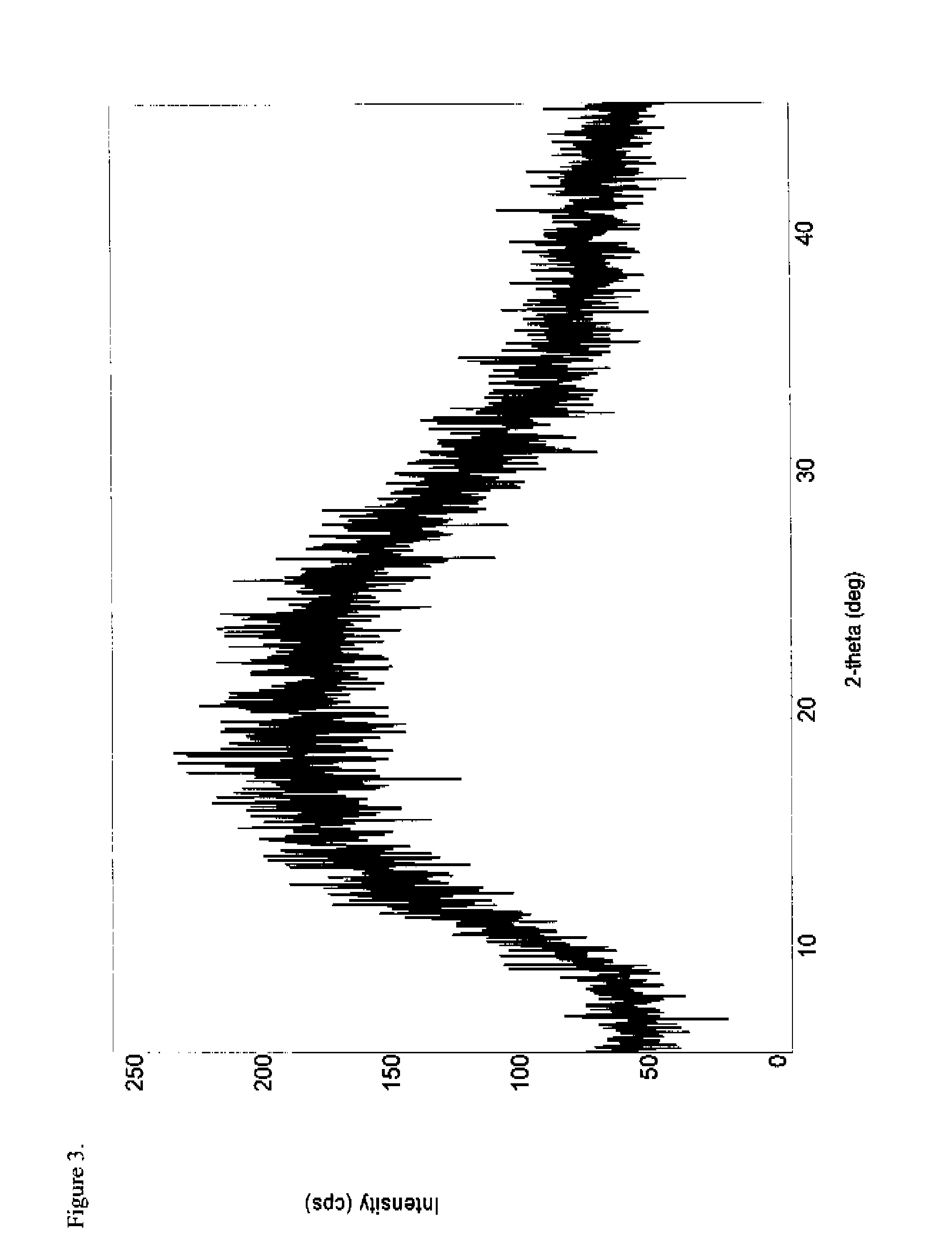
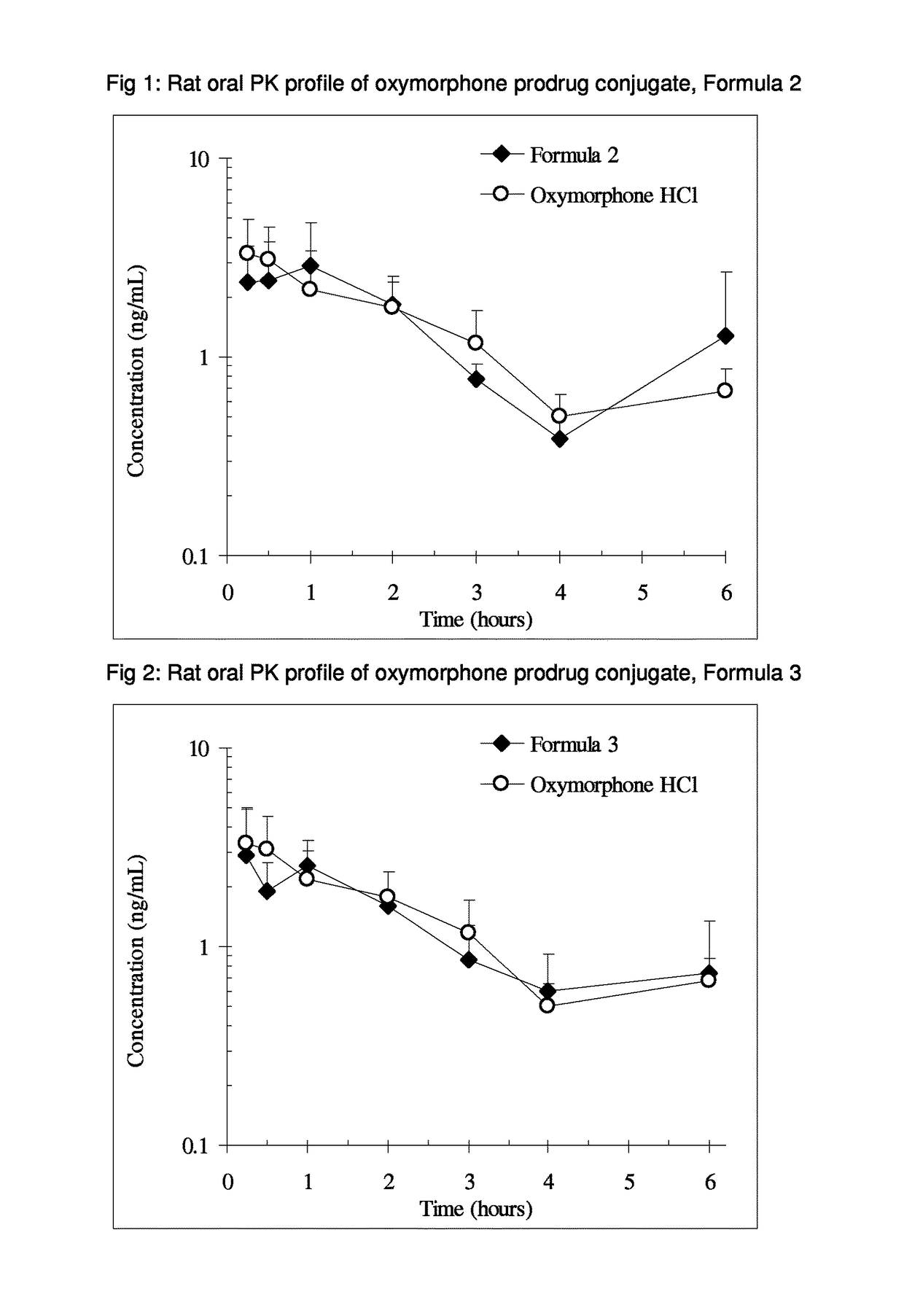
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "Oxymorphone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oxymorphone extended-release is used to help relieve severe ongoing pain.
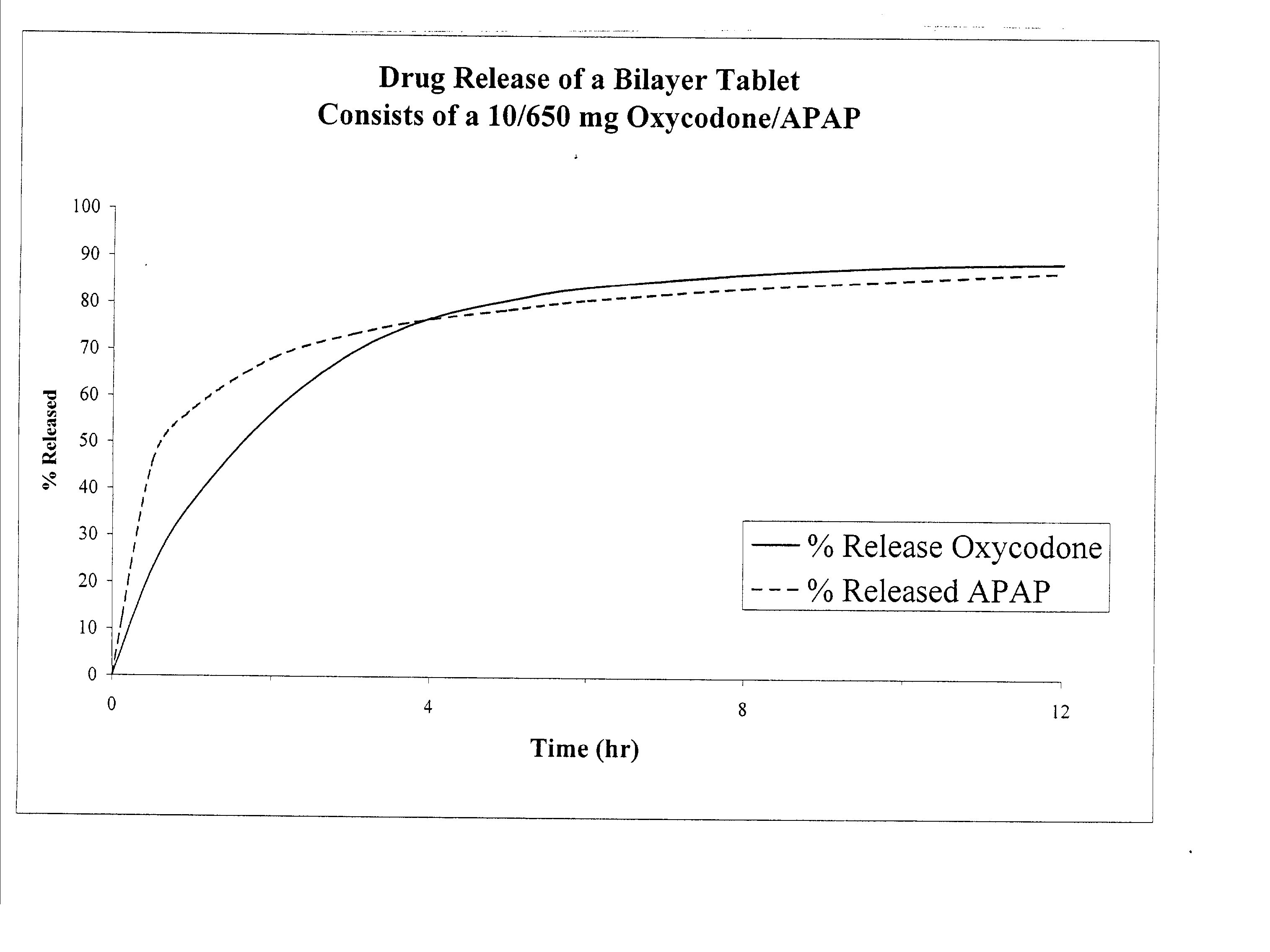
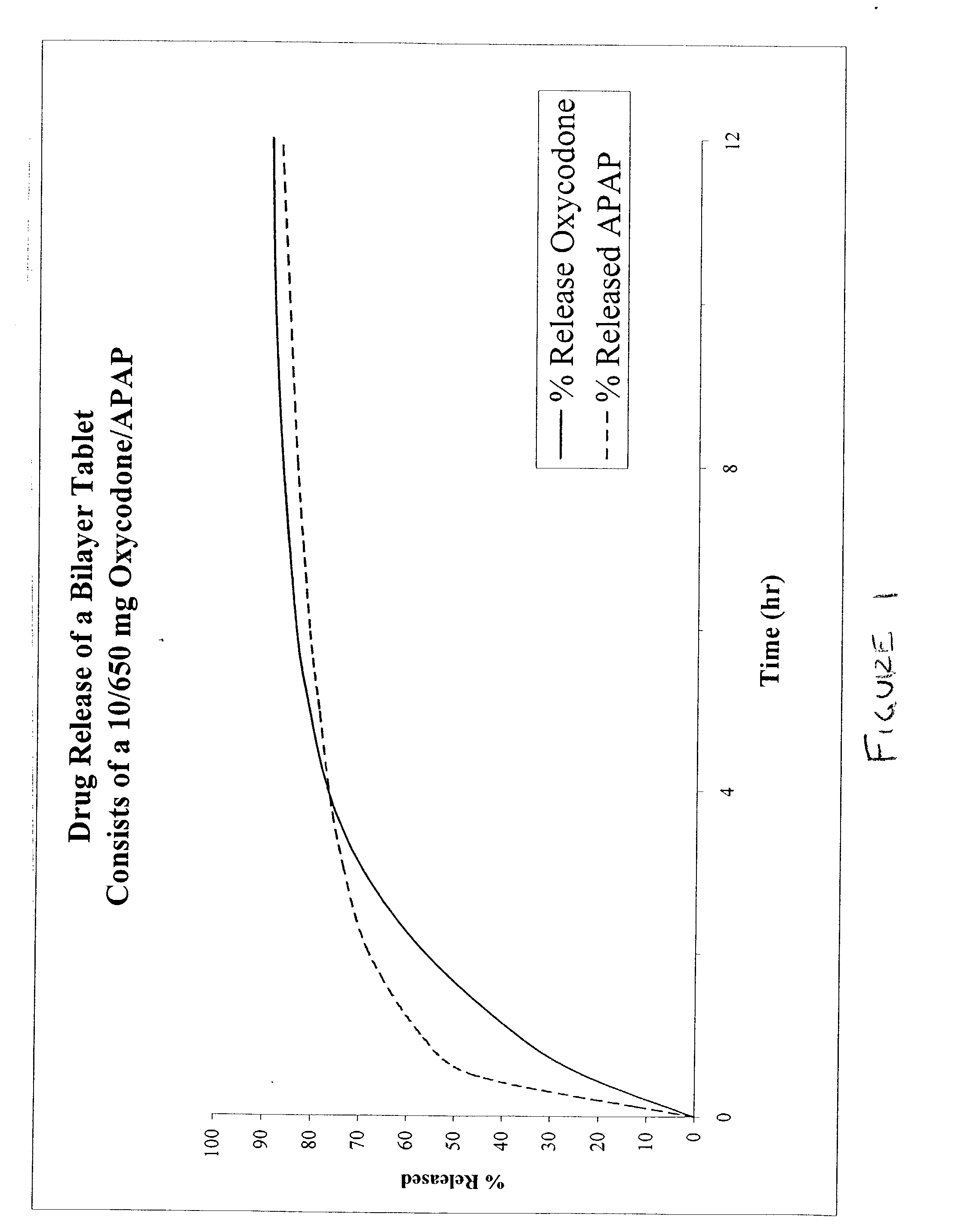
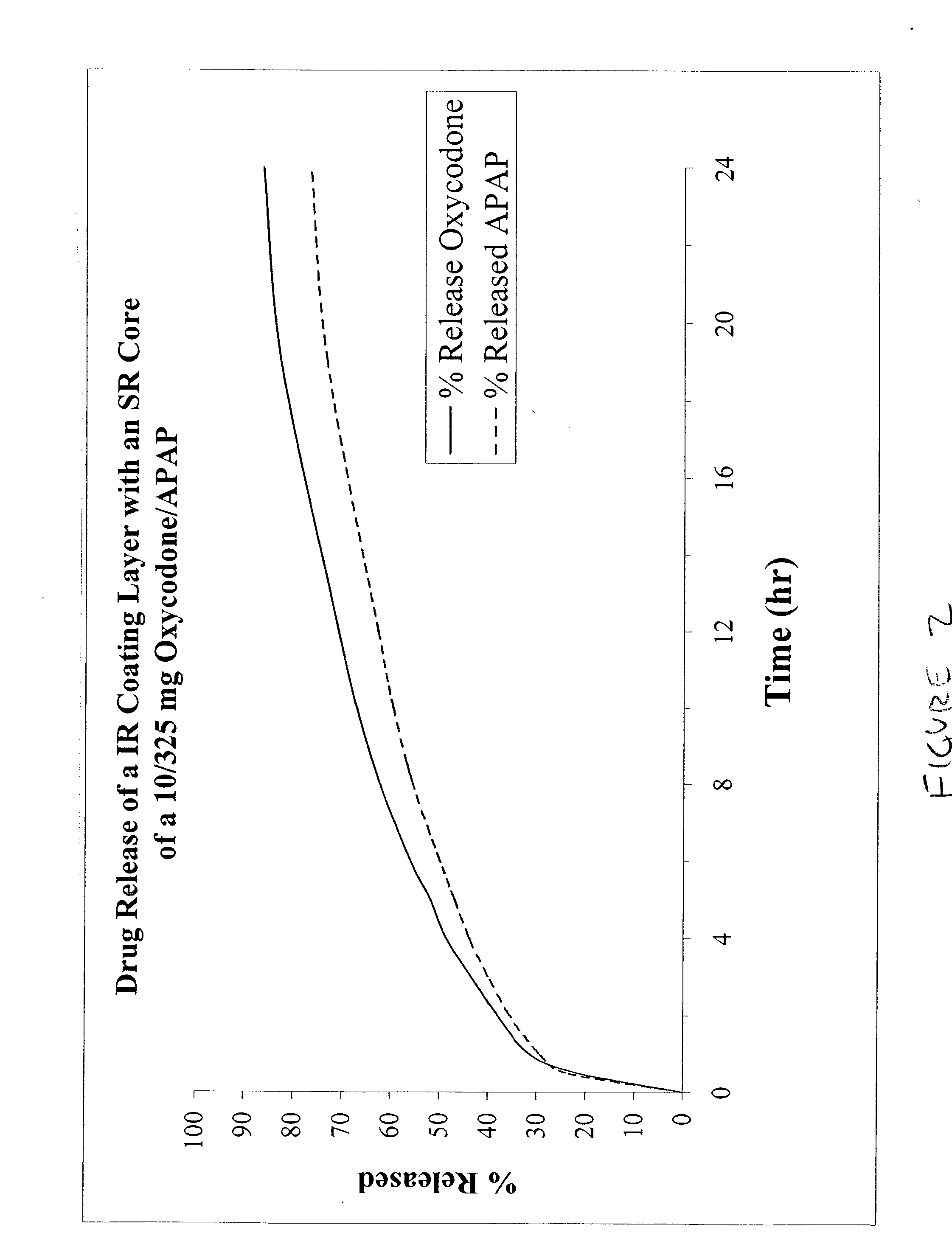
Combination sustained release-immediate release oral dosage forms with an opioid analgesic and a non-opioid analgesic
InactiveUS20030092724A1Long durationConstant plasma levels of opioid and non-opioid analgesicsBiocidePill deliveryImmediate releaseTherapeutic effect
The present invention relates to new and useful oral tablet compositions which include an immediate release portion having an opioid analgesic and a non-opioid analgesic, providing for a rapid onset of therapeutic effect, and a sustained release portion of an opioid analgesic and a non-opioid analgesic, providing for a relatively longer duration of therapeutic effect. A multilayer oral dosage form containing a sustained release layer, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release layer containing the same active ingredients as the sustained release layer, is also disclosed. Also disclosed are oral tablet compositions, containing a sustained release core, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release coating containing the same active ingredients as the sustained release core, are also disclosed. In addition, methods of making and using such oral tablet compositions are disclosed.
Owner:ENDO PHARMA INC
Robust sustained release formulations
InactiveUS20080085304A1Avoid dose dumpingHigh drug safetyPowder deliveryPill deliverySolid Dose FormOxymorphone
Robust sustained release formulations, solid dosage forms comprising robust sustained release formulations, and methods for making and using these formulations and solid dosage forms are provided. Robustness of the sustained release formulation is related to the particle size of the hydrophilic gum. Sustained release formulations resist dose-dumping when ingested with alcohol. The formulations are useful for treating a patient suffering from a condition, e.g., pain. The formulations comprise at least one drug. In one embodiment, the drug is an opioid, e.g., oxymorphone.
Owner:ENDO PHARMA INC
Sustained release formulations of oxymorphone
Sustained release formulations of oxymorphone or pharmaceutically acceptable salts thereof; methods for making the sustained release formulations of oxymorphone or pharmaceutically acceptable salts thereof; and methods for using the sustained release formulations of oxymorphone or pharmaceutically acceptable salts thereof to treat patients suffering from pain are provided.
Owner:ENDO PHARMA INC
Opiod tannate compositions
A composition comprising the tannate of an opioid. Suitable opioids include alfentanil, buprenorphine, butorphanol, carfentanil, cocaine, codeine, dezocine, diacetylmorphine, dihydrocodeine, dihydromorphine, diphenoxylate, diprenorphine, etorphine, fentanyl, heroin, hydrocodone, hydromorphone, beta-hydroxy-3-methylfentanyl, levo-alpha-acetylmethadol, levorphanol, lofentanil, meperidine, methadone, morphine, nalbuphine, nalmefene, o-methylnaltrexone, naloxone, naltrexone, oxycodone, oxymorphone, pentazocine, pethidine, propoxyphene, remifentanil, sufentanil, tilidine and tramadol. The opioid tannate may be readily prepared by reacting an opioid free base with tannic acid, either neat or in the presence of up to about 30 wt. % water, at a temperature of about 60 to about 150° C. and thereafter recovering the resultant opioid tannate. The opioid tannate may also be prepared by an alternative process that involves reacting the opioid free base with water at a temperature such that not more than about 10 wt. % of the opioid tannate will be decomposed and thereafter removing the water by freeze-drying.
Owner:JAME FINE CHEM
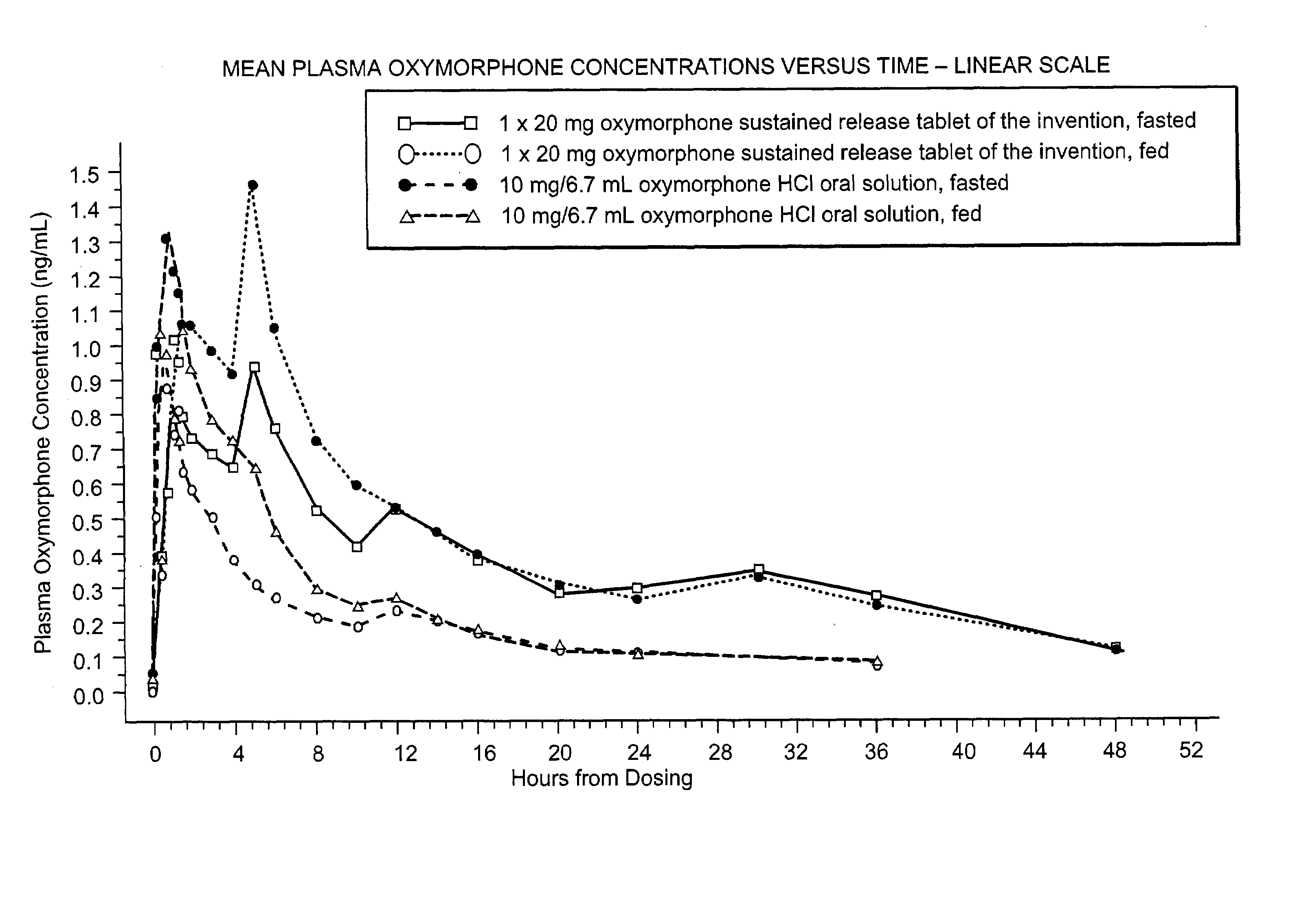
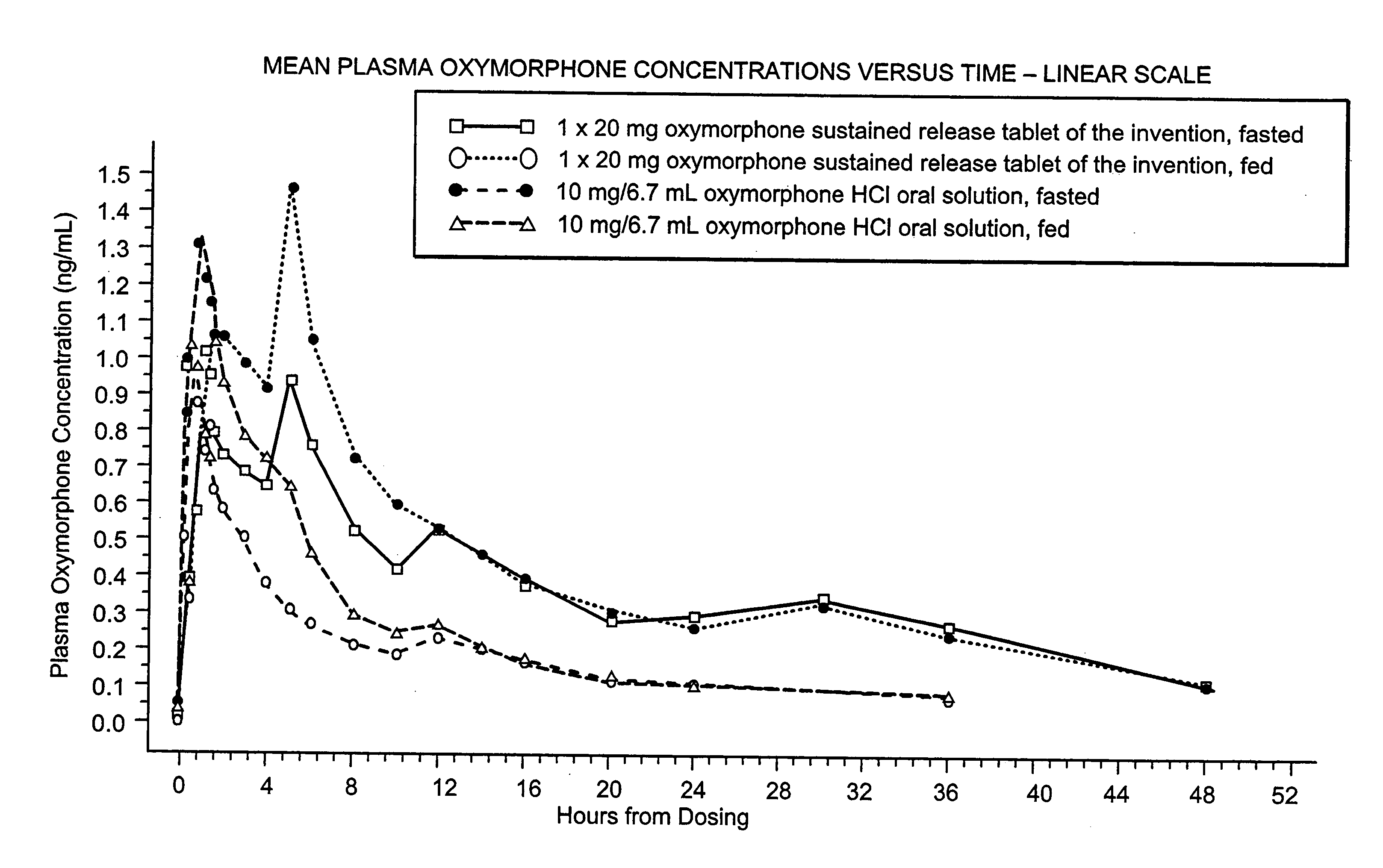
Oxymorphone controlled release formulations
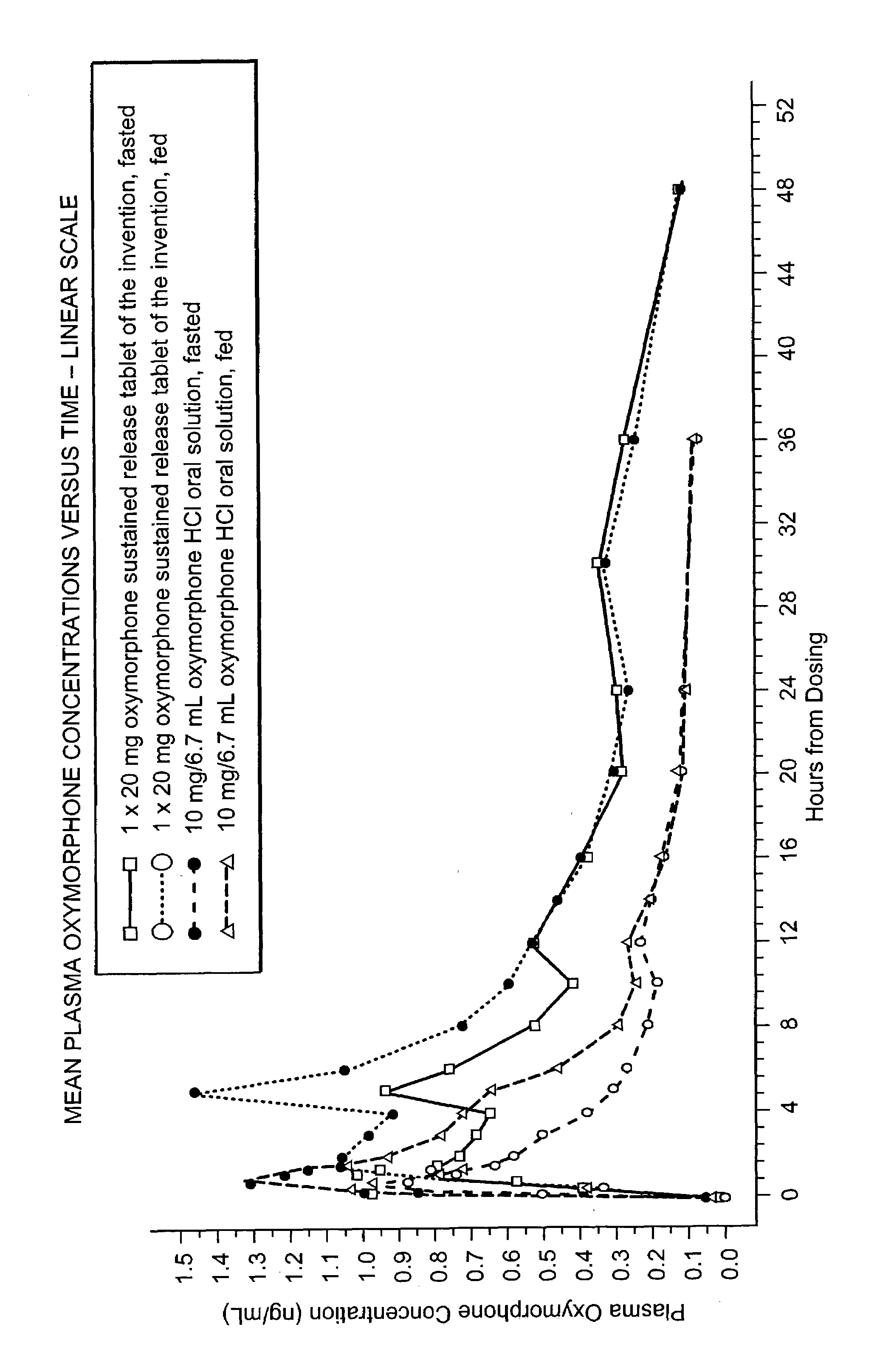
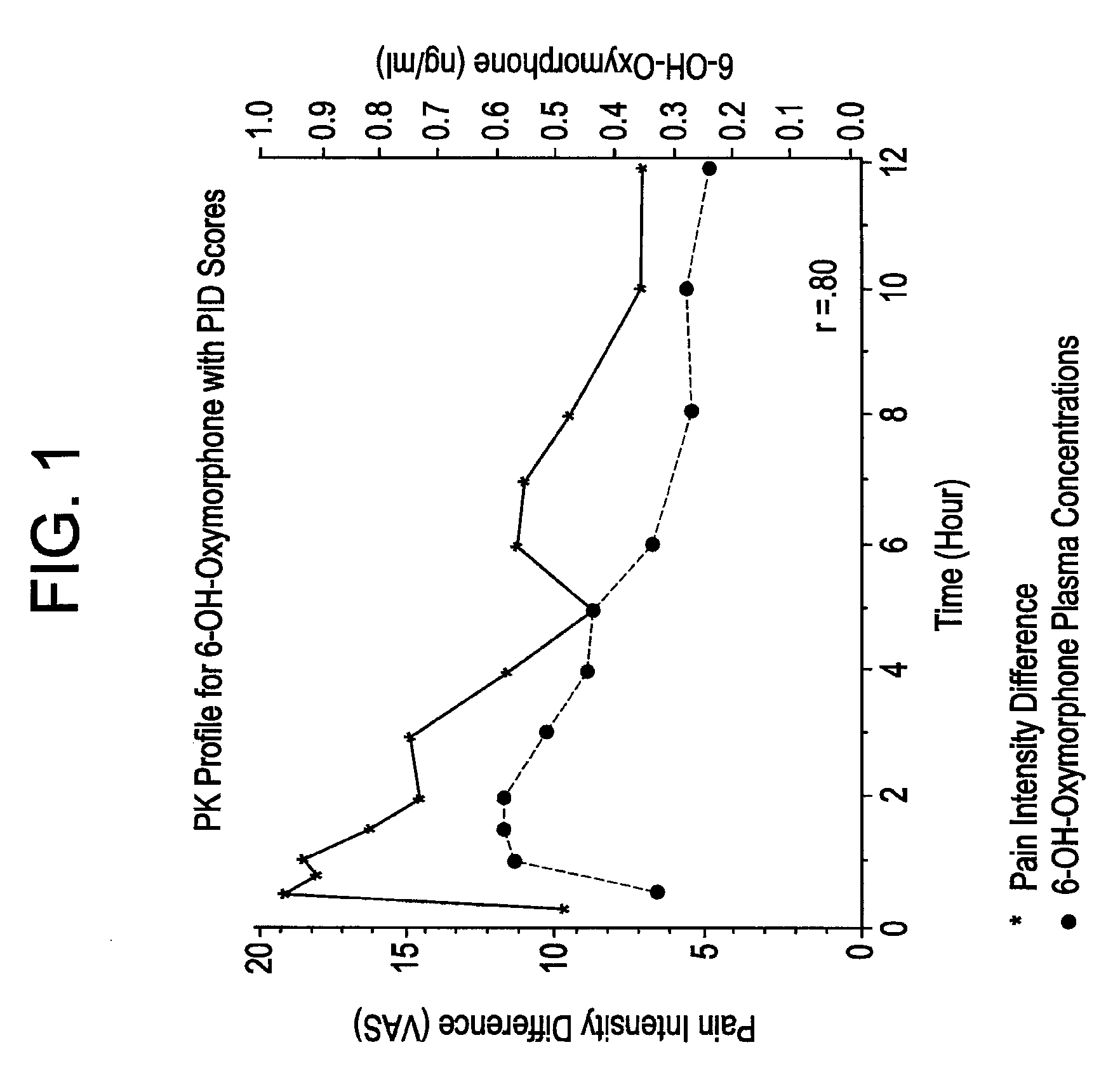
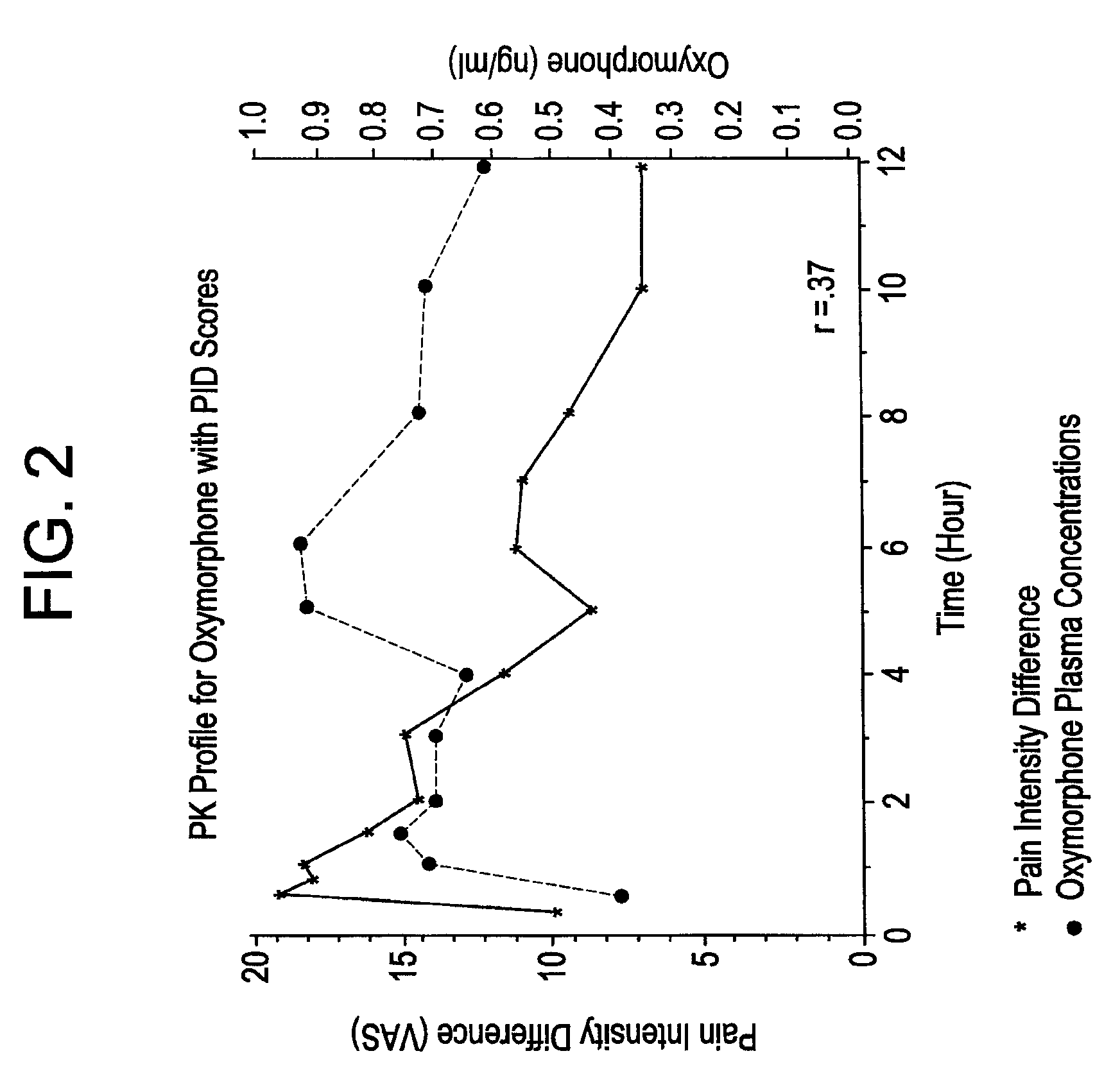
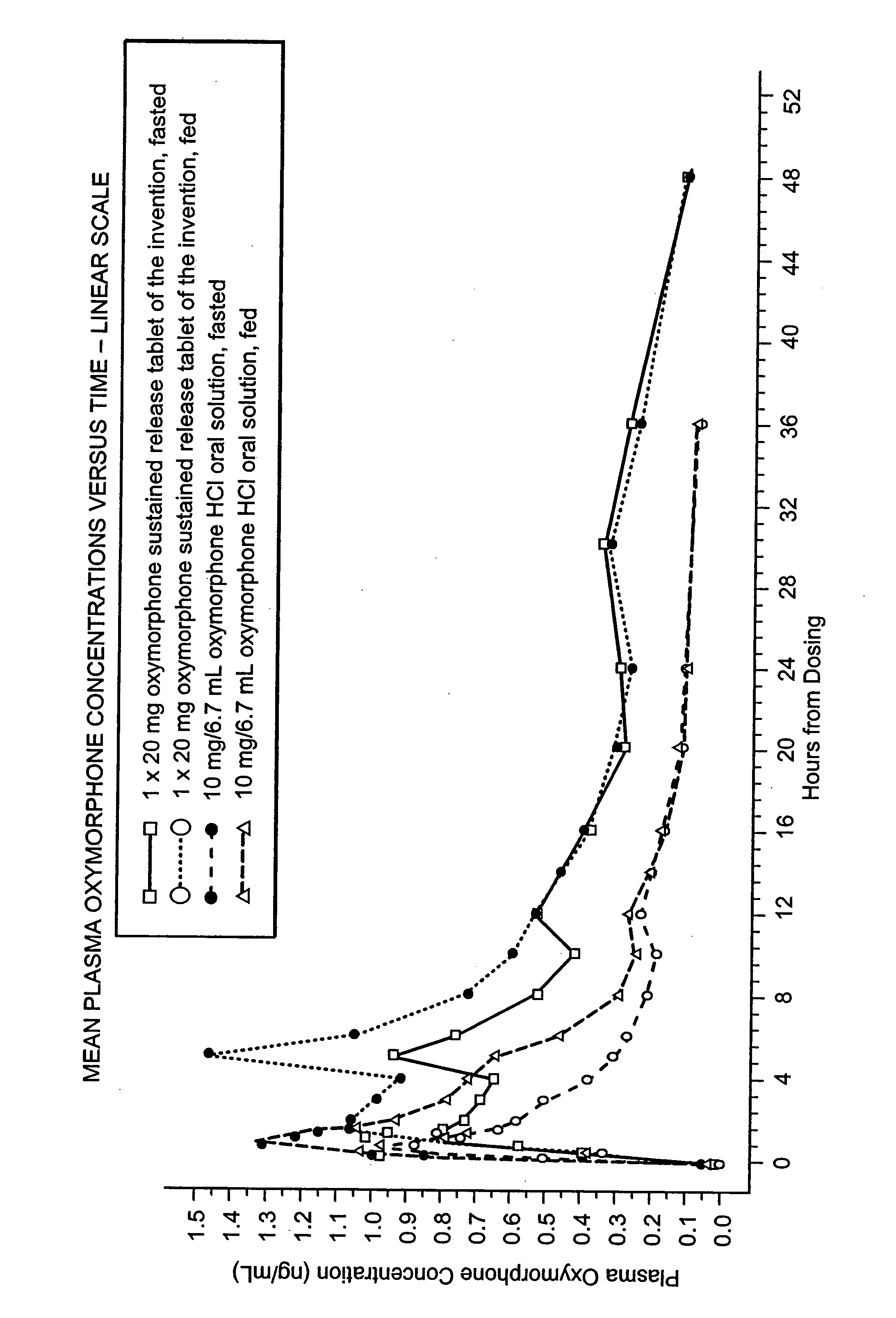
The invention pertains to a method of relieving pain by administering a controlled release pharmaceutical tablet containing oxymorphone which produces a mean minimum blood plasma level 12 to 24 hours after dosing, as well as the tablet producing the sustained pain relief.
Owner:ENDO PHARMA INC
Robust sustained release formulations of oxymorphone
InactiveUS20080085305A1Avoid dose dumpingHigh drug safetyPowder deliveryOrganic chemistryOxymorphoneSustained Release Formulations
Robust sustained release formulations, solid dosage forms comprising robust sustained release formulations, and methods for making and using these formulations and solid dosage forms are provided. Robustness of the sustained release formulation is related to the particle size of the hydrophilic gum. Sustained release formulations resist dose-dumping when ingested with alcohol. The formulations are useful for treating a patient suffering from a condition, e.g., pain. The formulations comprise at least one drug. In one embodiment, the drug is an opioid, e.g., oxymorphone.
Owner:ENDO PHARMA INC
Pharmaceutical composition
A pharmaceutical composition comprising an analgesic or analgesic combination and a stool softener is disclosed. The analgesic is selected from morphine, meperidine, fentanyl, hydromorphone, oxymorphone, oxycodone, hydrocodone, methadone, propoxyphene, pentazocine, levorphanol, codeine, acetaminophen and combinations of these analgesics. The composition is formulated for oral administration as a liquid or solid dosage form for immediate, slow, delayed or sustained-release characteristics.
Owner:BRANDED PRODS FOR THE FUTURE
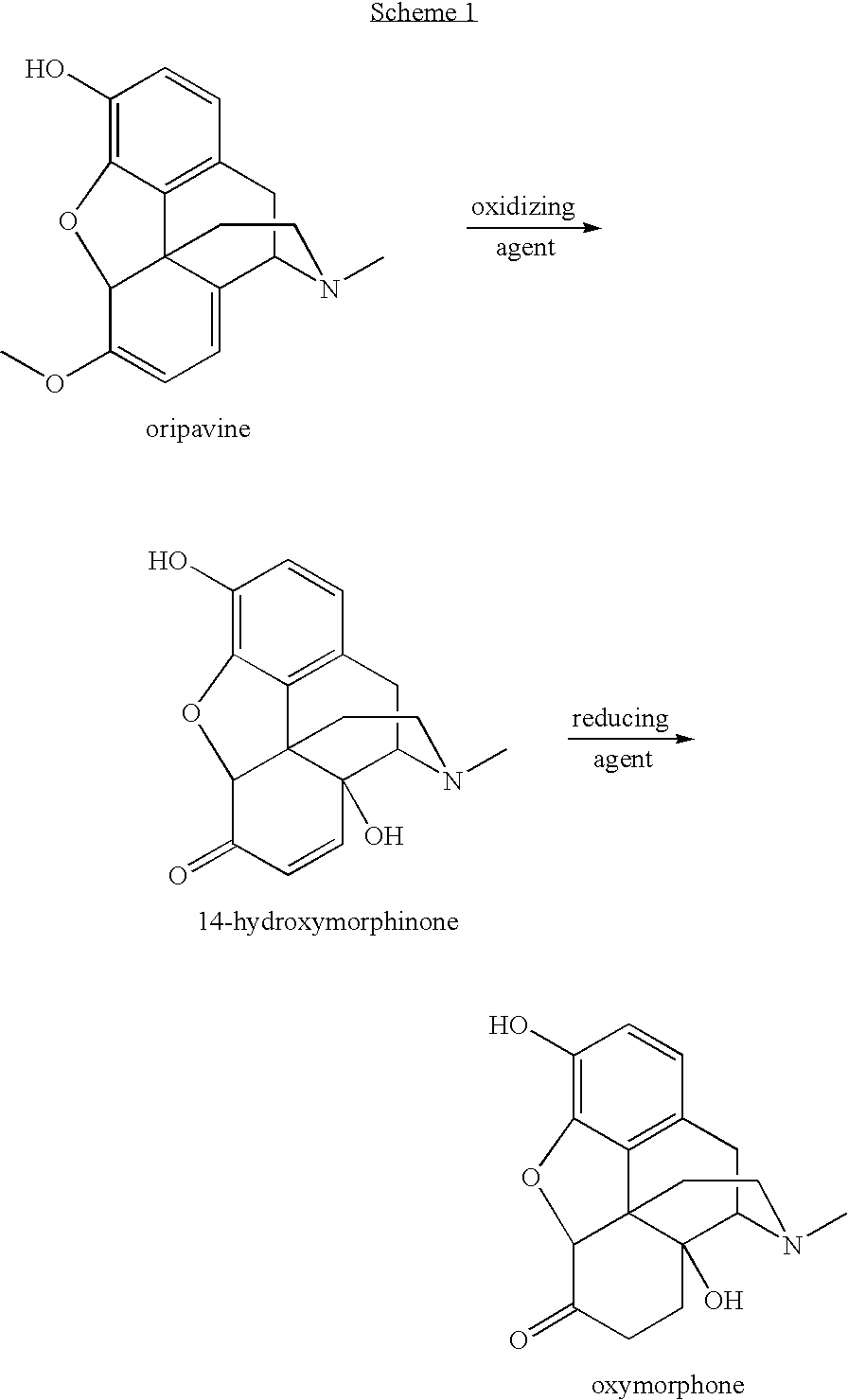
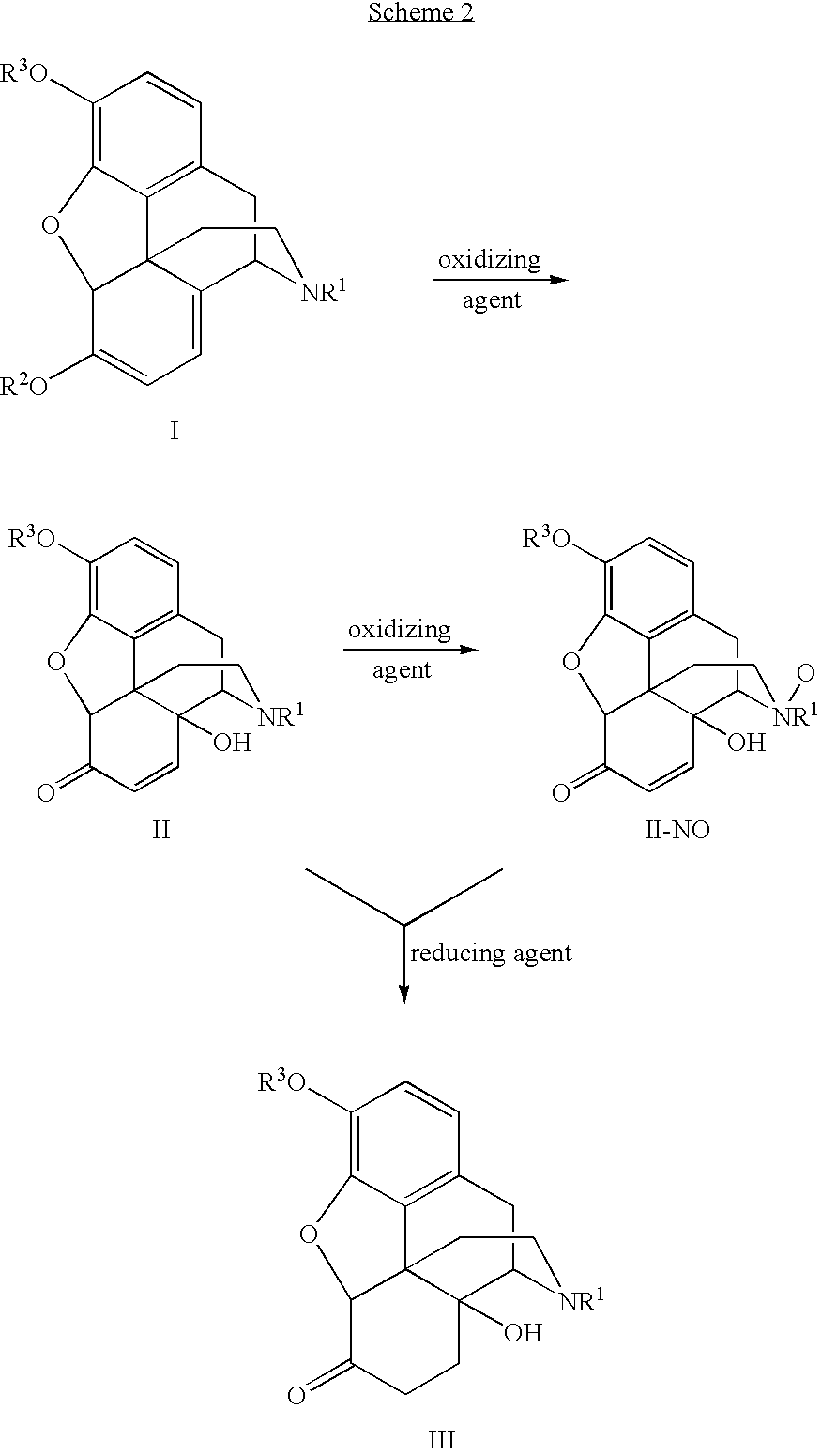
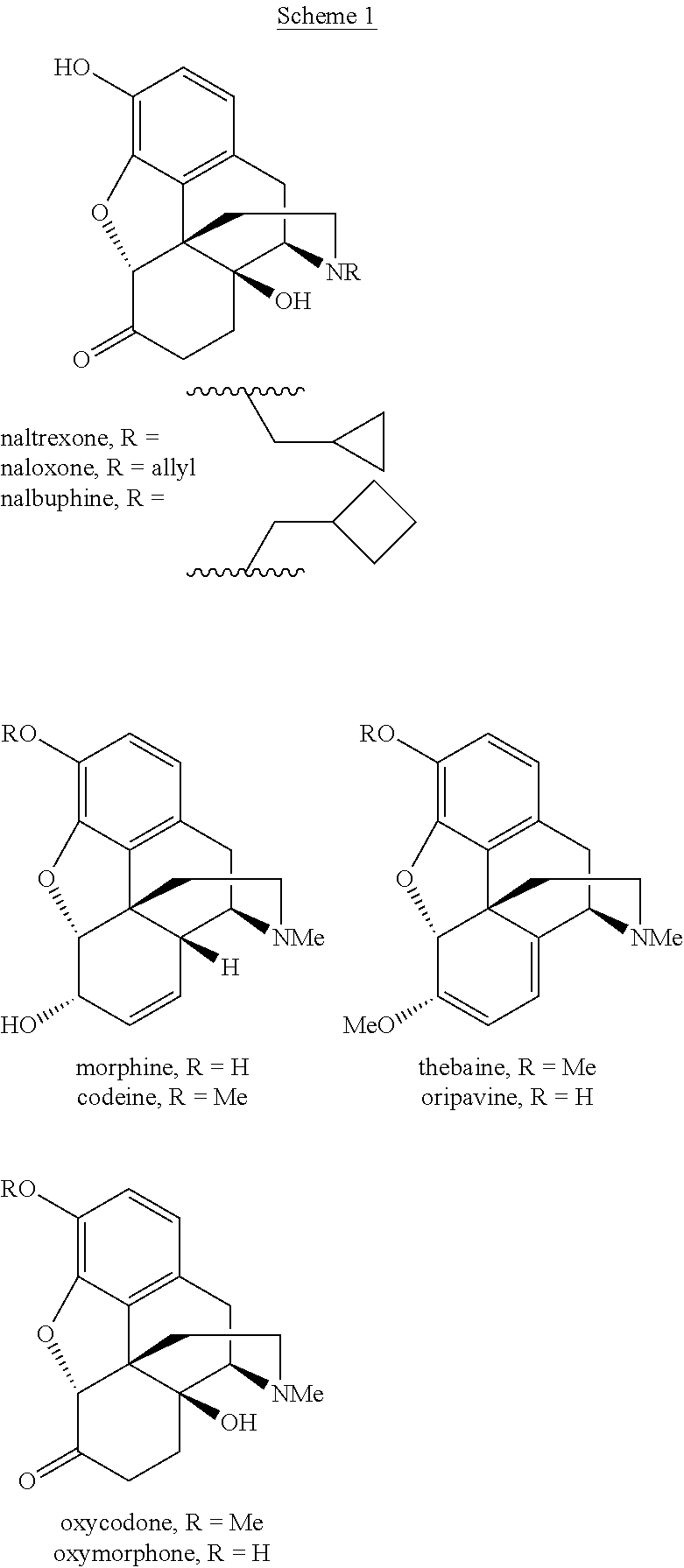
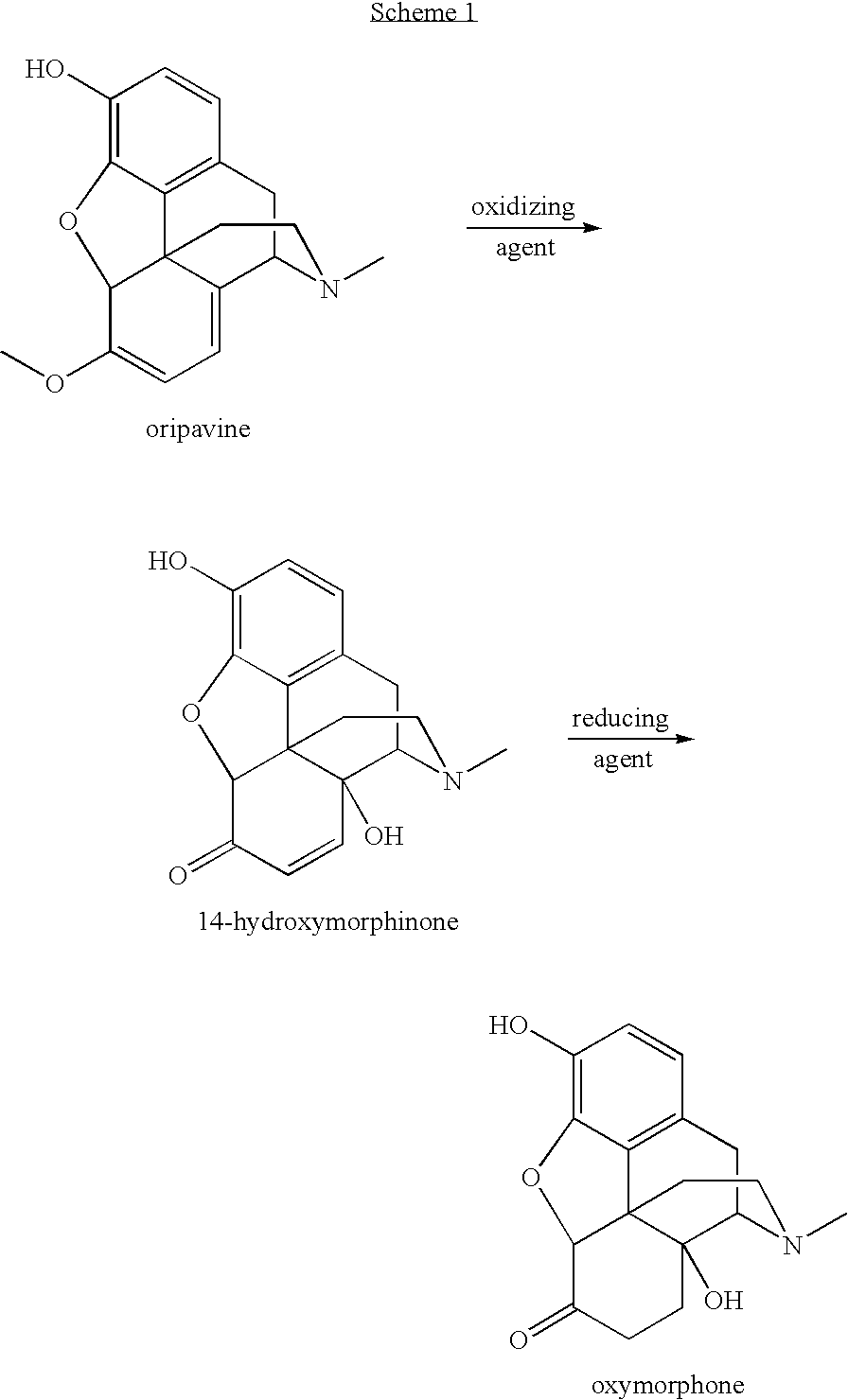
Preparation of oxymorphone from oripavine
An improved method for the preparation of oxymorphone from oripavine is provided. Oripavine is oxidized to form 14-hydroxymorphinone after which the oxidation reaction is quenched to prevent the formation of 1-1′-dimer side products. The 14-hydroxymorphinone is then reduced, typically by catalytic hydrogenation to form oxymorphone. The inventive method disclosed is further applicable to the production of morphinan derivatives.
Owner:SPECGX LLC
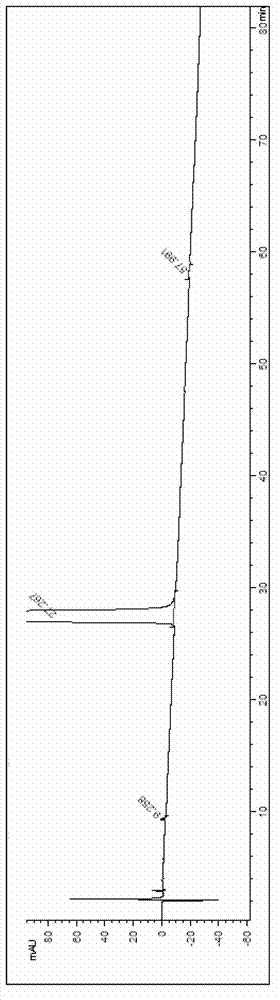
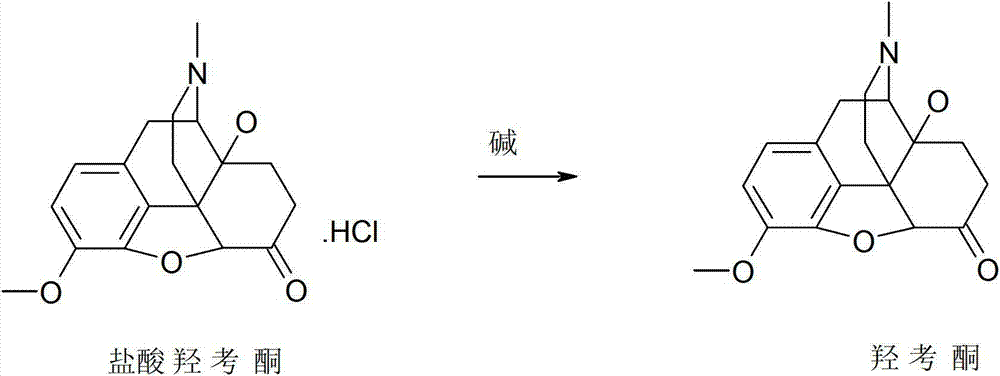
Synthesis method of oxymorphone hydrochloride
ActiveCN103113378AAvoid it happening againRaw materials are easy to getOrganic chemistryOxymorphone HydrochlorideHydrogenation reaction
The invention discloses a synthesis method of oxymorphone hydrochloride. The synthesis method mainly comprises the following steps of: 1) alkalifying to obtain free alkali by taking oxycodone hydrochloride as an initial a raw material; 2) carrying out a demethylation reaction to obtain oxymorphone; 3) carrying out a hydrogenation reaction on the oxymorphone under acidic condition; and 4) salifying the hydrogenated product with hydrochloric acid to obtain the target product oxymorphone hydrochloride. The synthesis method disclosed by the invention has the advantages of available raw materials, high product purity and is easy to operate and is applicable to industrial production.
Owner:北京华素制药股份有限公司
Robust sustained release formulations
Robust sustained release formulations, solid dosage forms comprising robust sustained release formulations, and methods for making and using these fopnulations and solid dosage forms are provided. Robustness of the sustained release formulation is related to the particle size of the hydrophilic gum. Sustained release formulations resist dose-dumping when ingested with alcohol. The formulations are useful for treating a patient suffering from a condition, e.g., pain. The formulations comprise at least one drug. In one embodiment, the drug is an opioid, e.g., oxymorphone.
Owner:PENWEST PHARMA CO
Oxymorphone controlled release formulations
The invention pertains to a method of relieving pain by administering a controlled release pharmaceutical tablet containing oxymorphone which produces a mean minimum blood plasma level 12 to 24 hours after dosing, as well as the tablet producing the sustained pain relief.
Owner:KAO HAUI HUNG +3
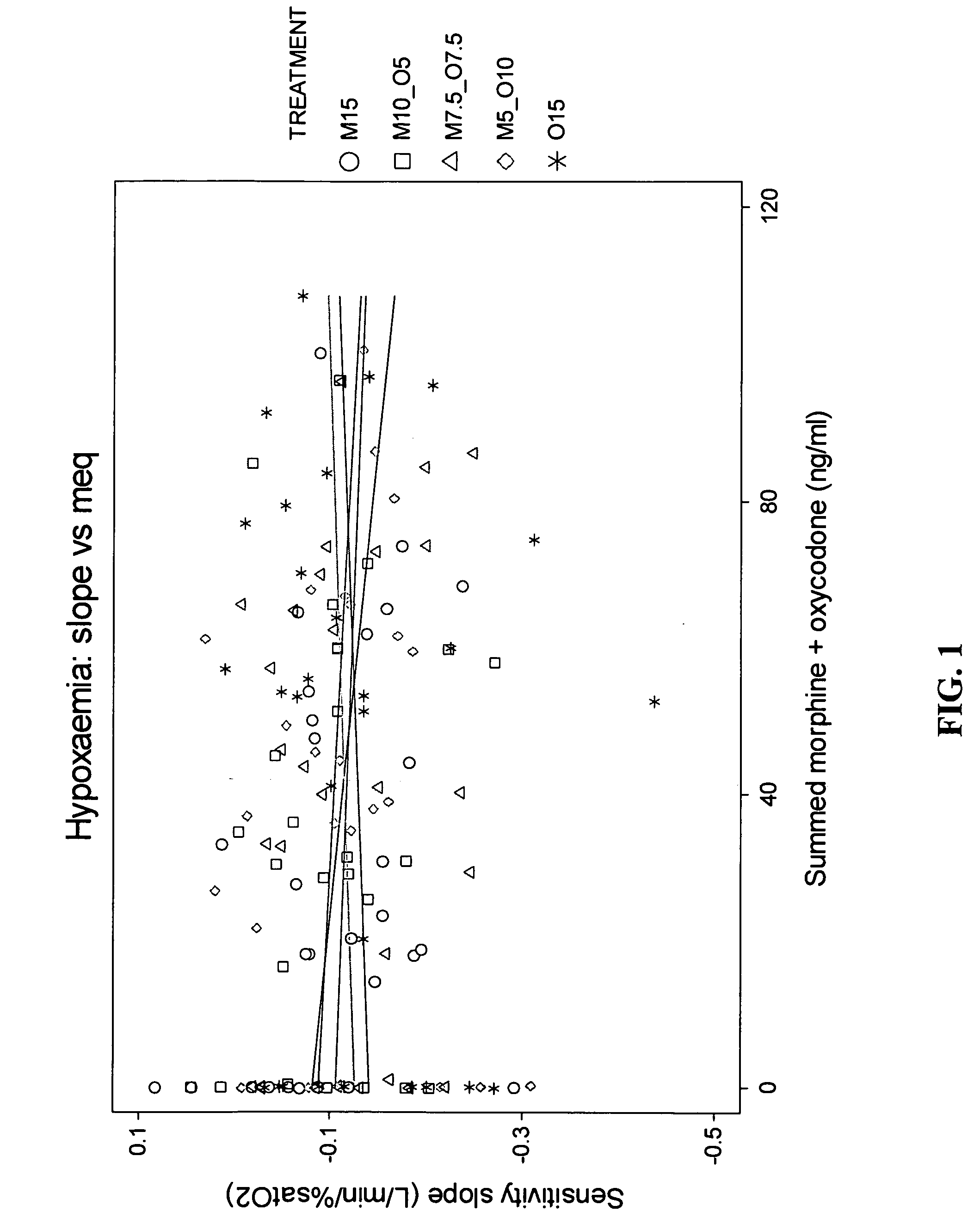
Methods and compositions for reducing the risk associated with the administration of opioid analgesics in patients with diagnosed or undiagnosed respiratory illness
The present invention relates to methods for reducing the risk associated with the administration of opioid analgesics in patients diagnosed or undiagnosed with respiratory illness by administering an analgesic composition comprising a sub-analgesic dosage of a μ-opioid agonist selected from the group consisting of morphine, fentanyl, sufentanil, alfentanil, oxymorphone and hydromorphone, or a pharmaceutically acceptable salt thereof, and a sub-analgesic dosage of oxycodone which is a κ2-opioid agonist or a pharmaceutically acceptable salt thereof.
Owner:QRXPHARMA
Oxymorphone controlled release formulations
The invention pertains to a method of relieving pain by administering a controlled release pharmaceutical tablet containing oxymorphone which produces a mean minimum blood plasma level 12 to 24 hours after dosing, as well as the tablet producing the sustained pain relief.
Owner:KAO HAUI HUNG +3
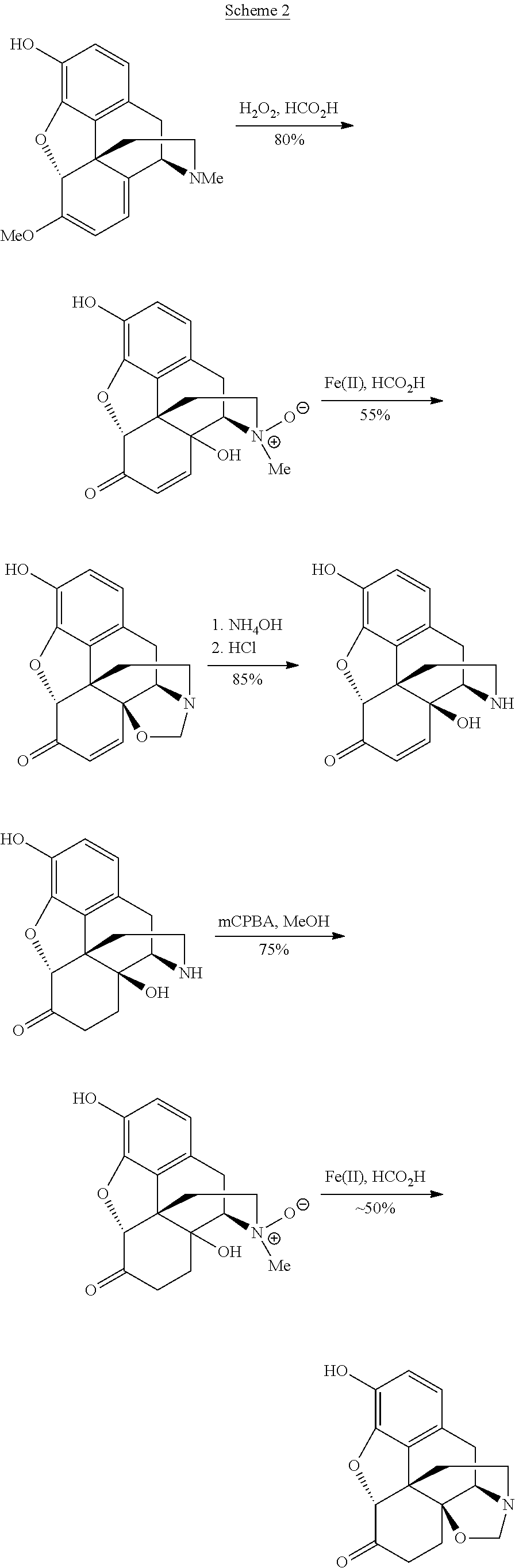
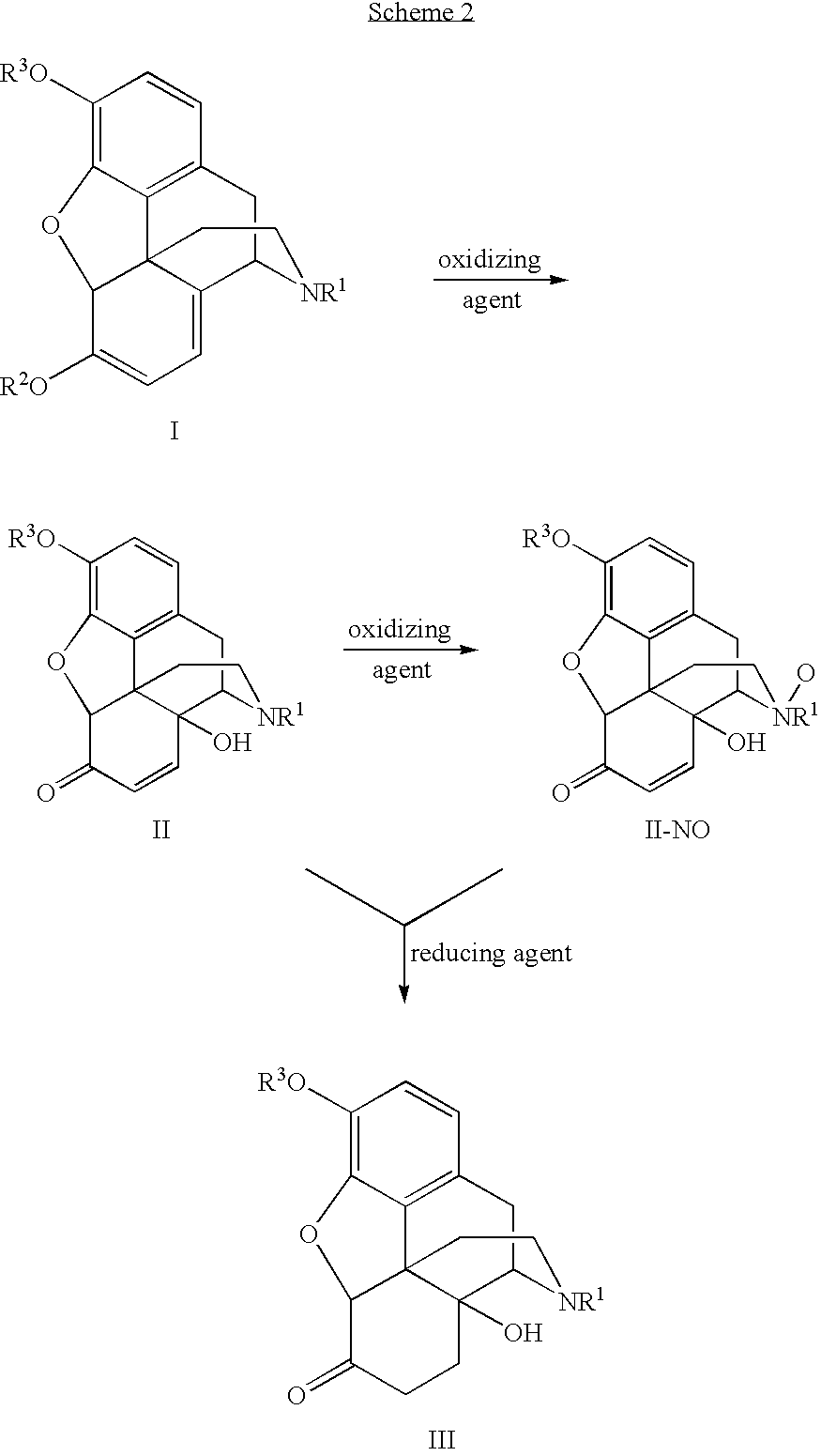
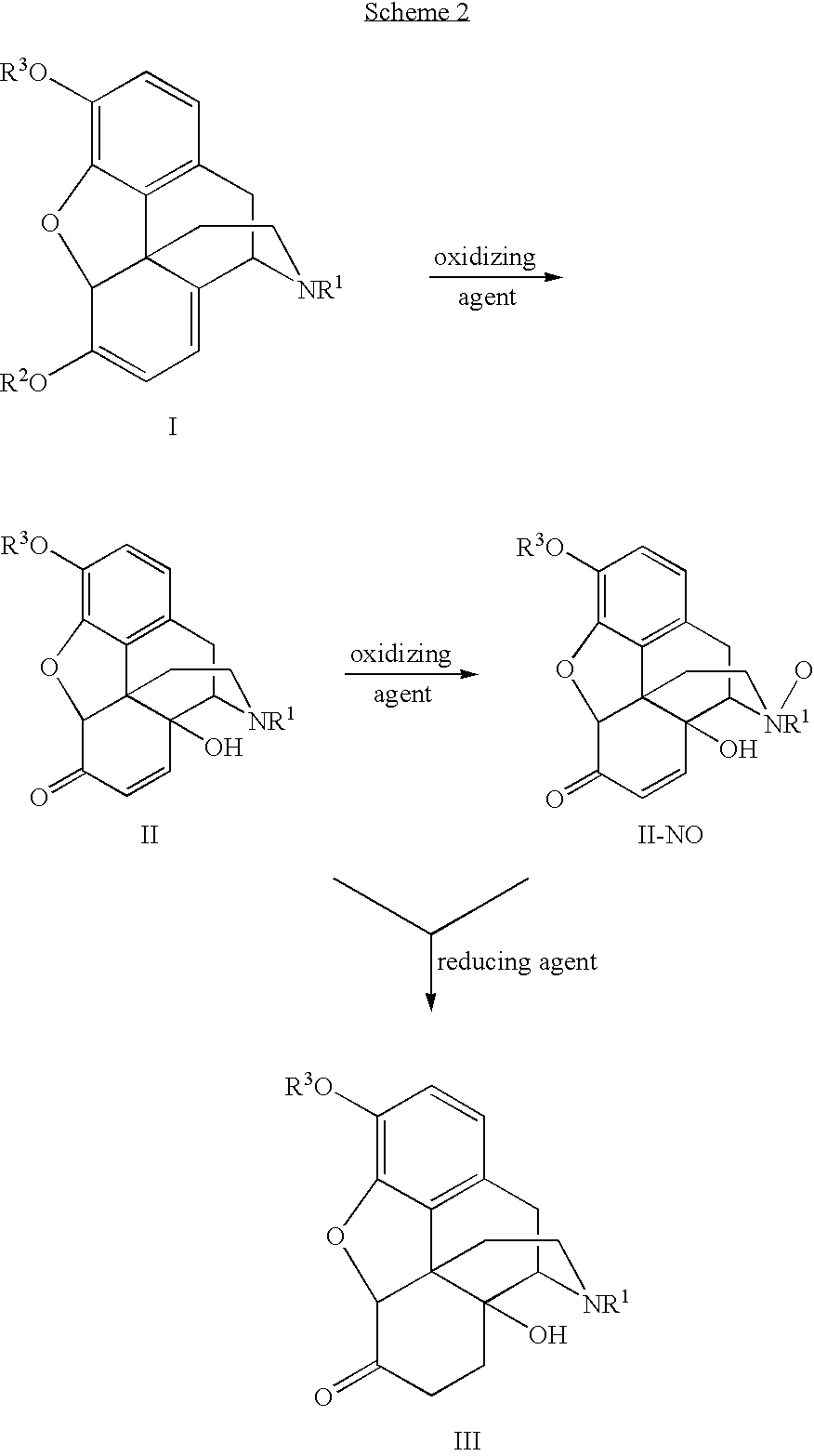
Processes and Intermediates in the Preparation of Morphine Analogs via N-Demethylation of N-Oxides Using Cyclodehydration Reagents
InactiveUS20120283443A1Efficient conversionOrganic chemistryBulk chemical productionMorphinansOxymorphone
A high-yielding method for the N-demethylation of oxycodone- and oxymorphone-N-oxides by the reaction of these compounds with cyclodehydration reagents has been performed. This method has been utilized to improve the synthesis of various morphine analogs, such as naltrexone, nalbuphone and naloxone.
Owner:BROCK UNIVERSITY
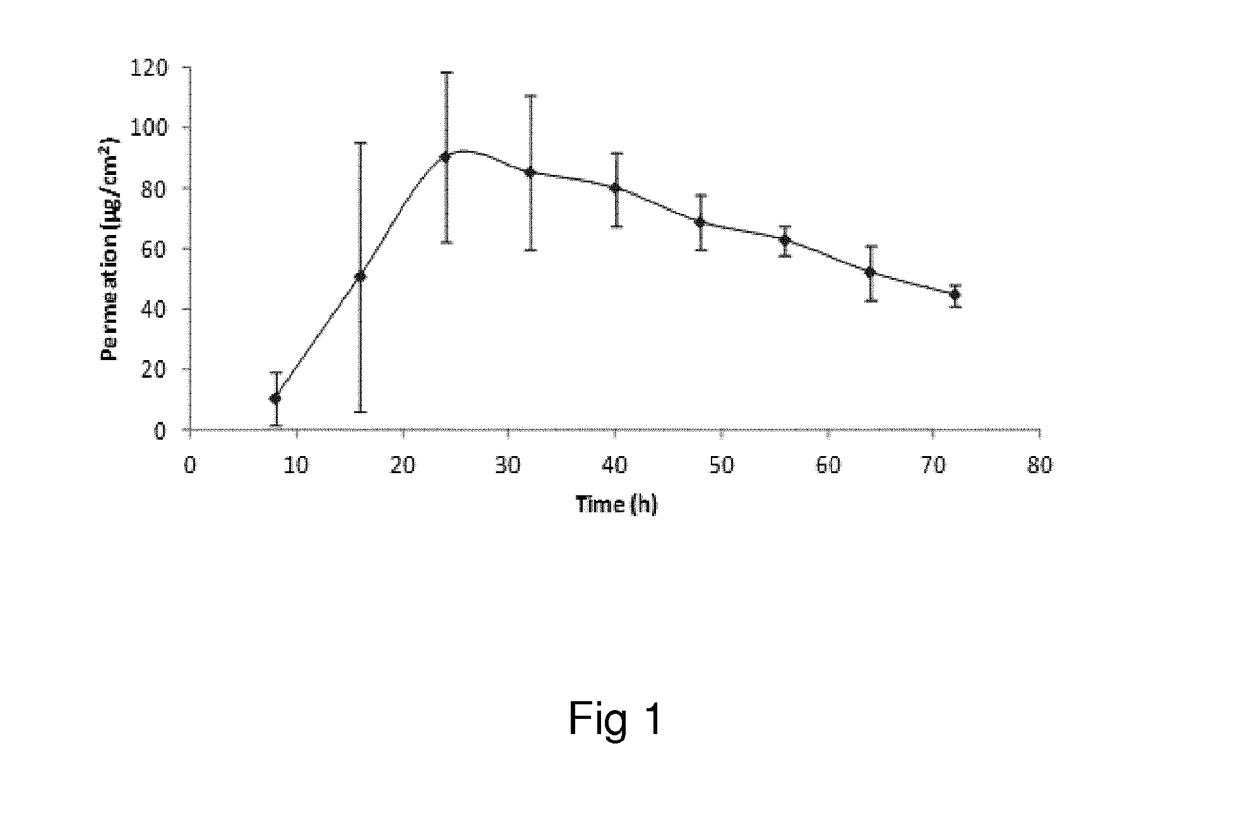
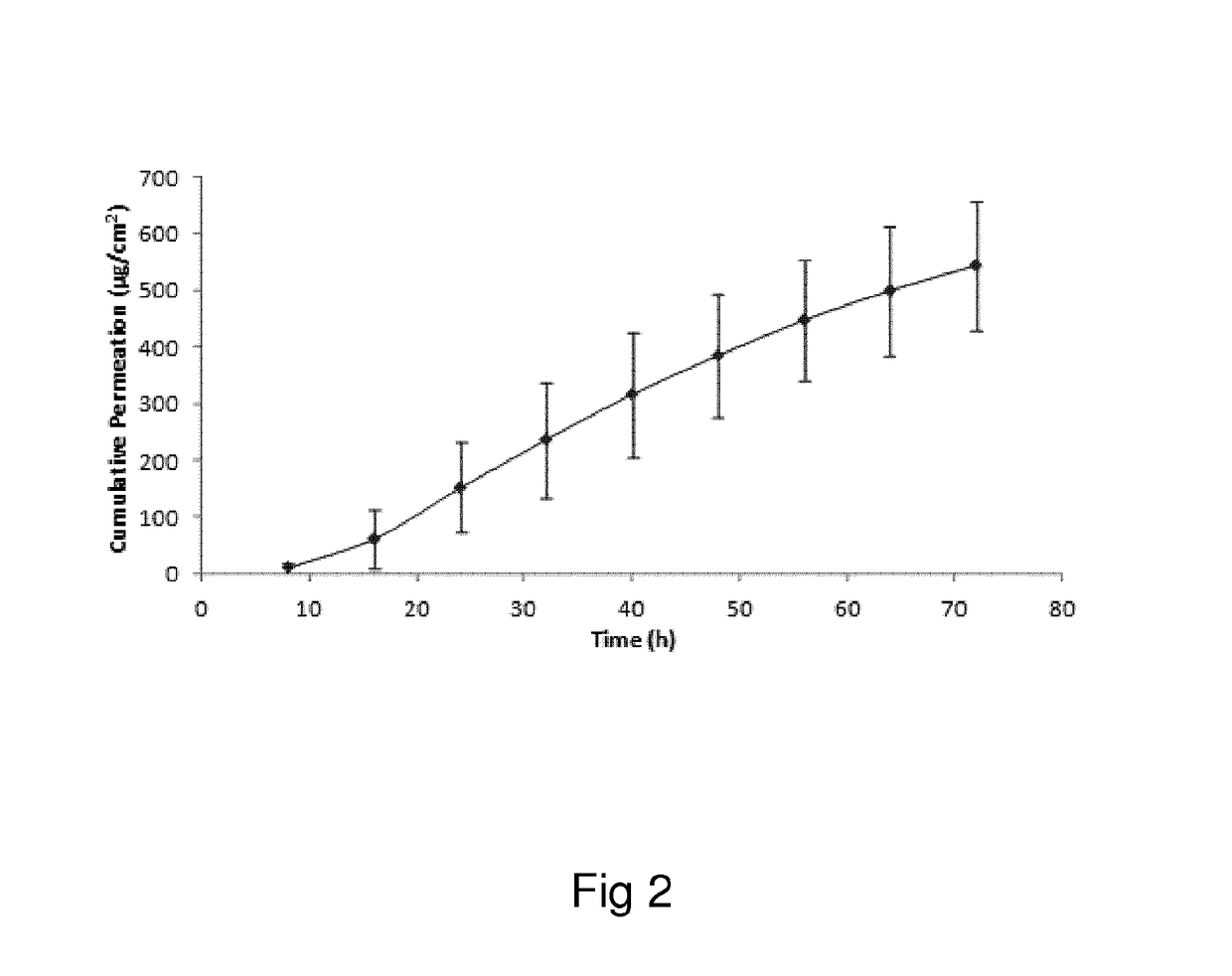
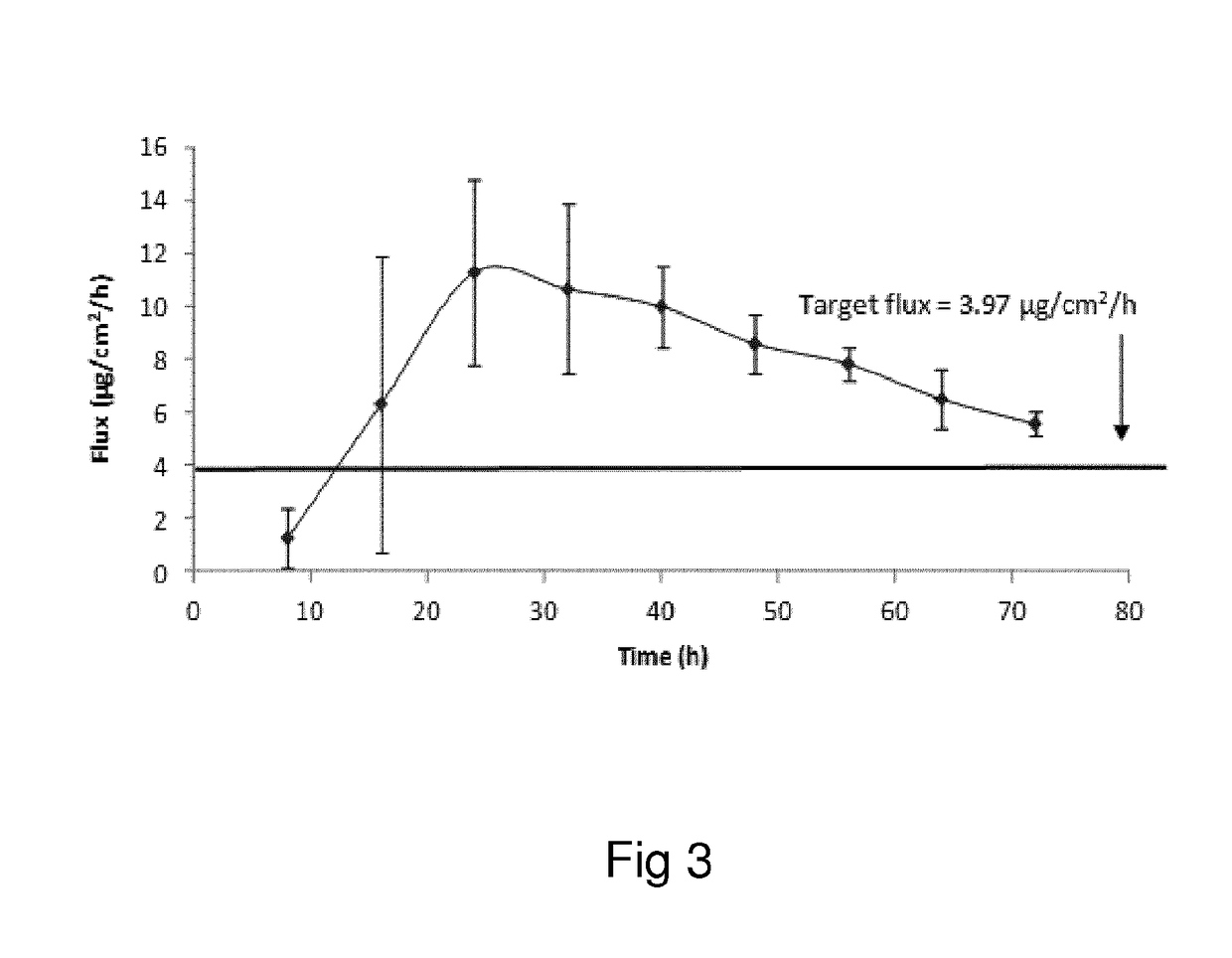
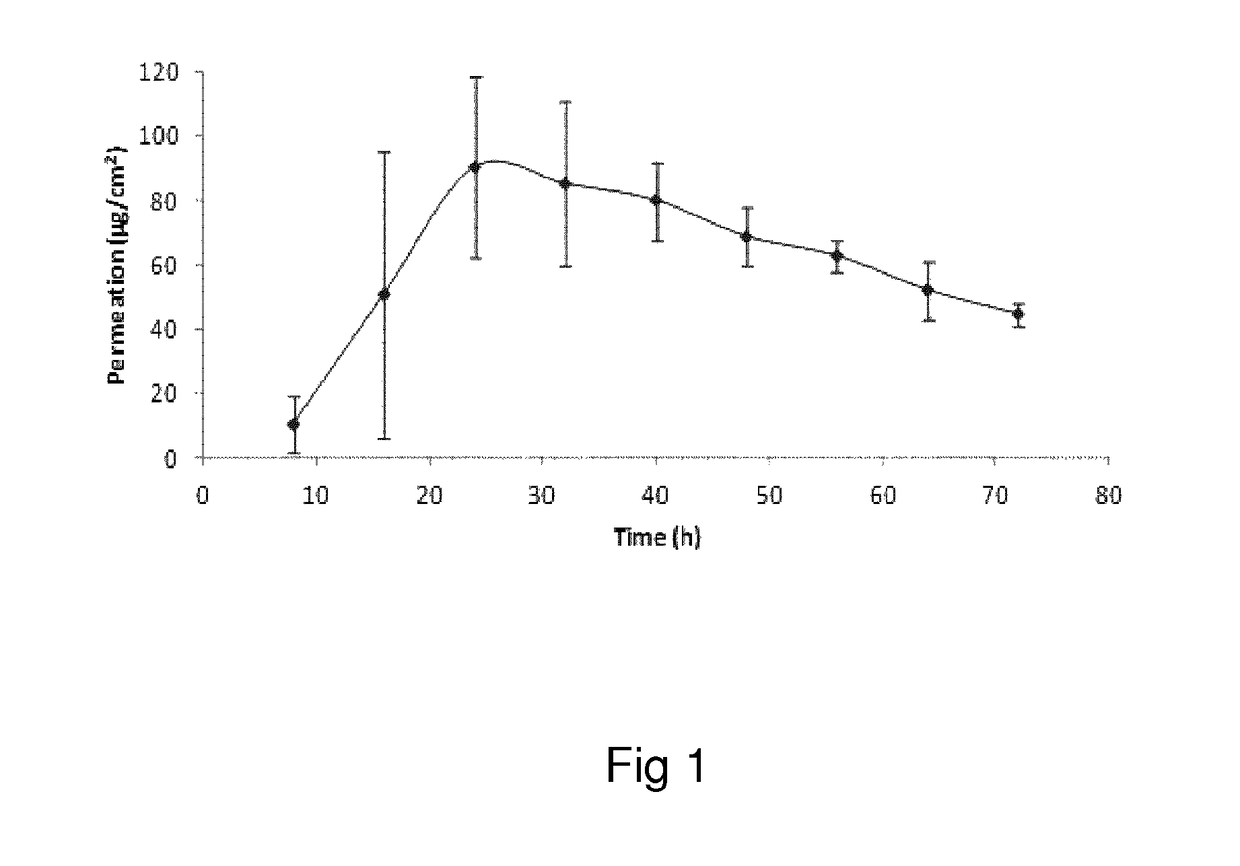
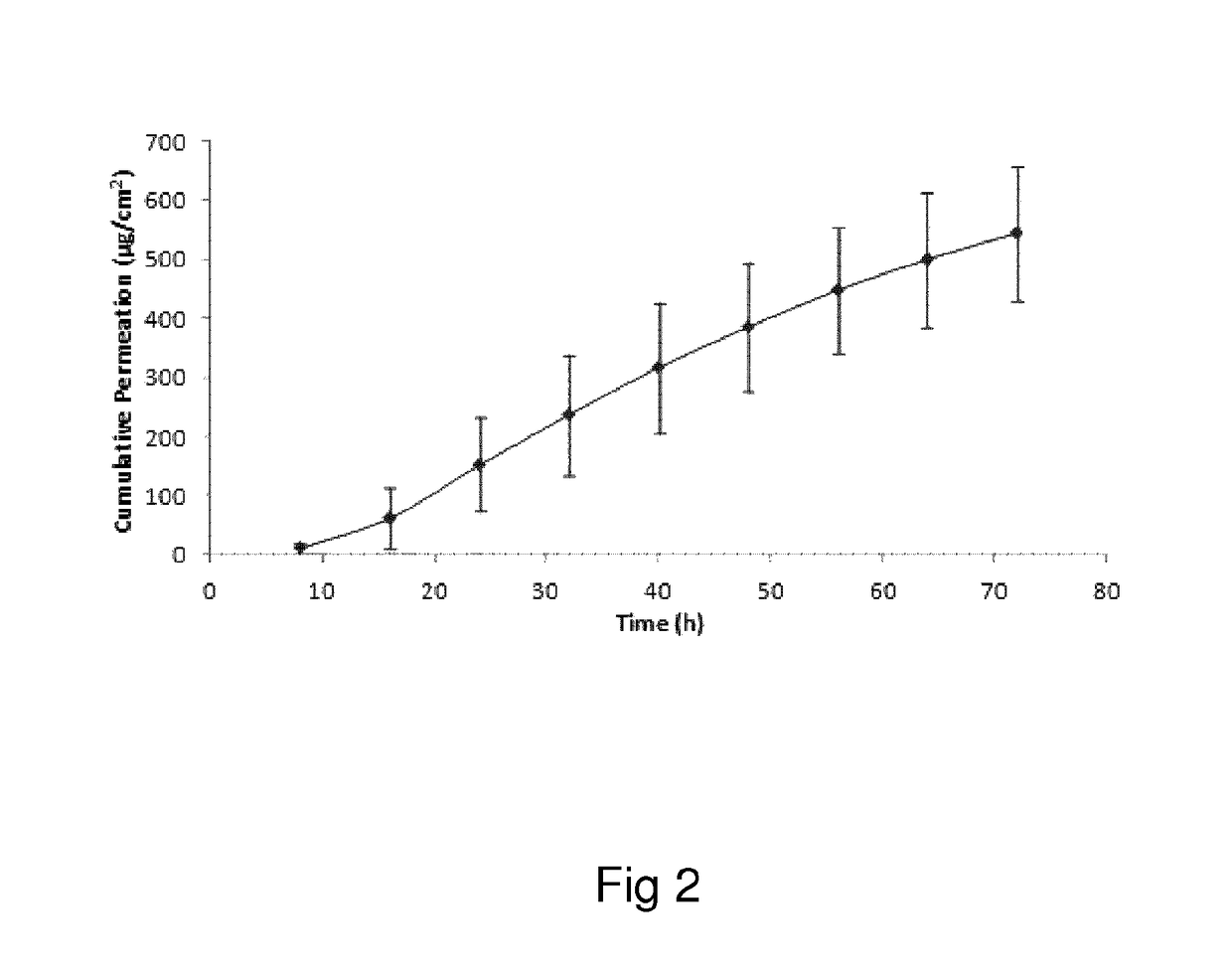
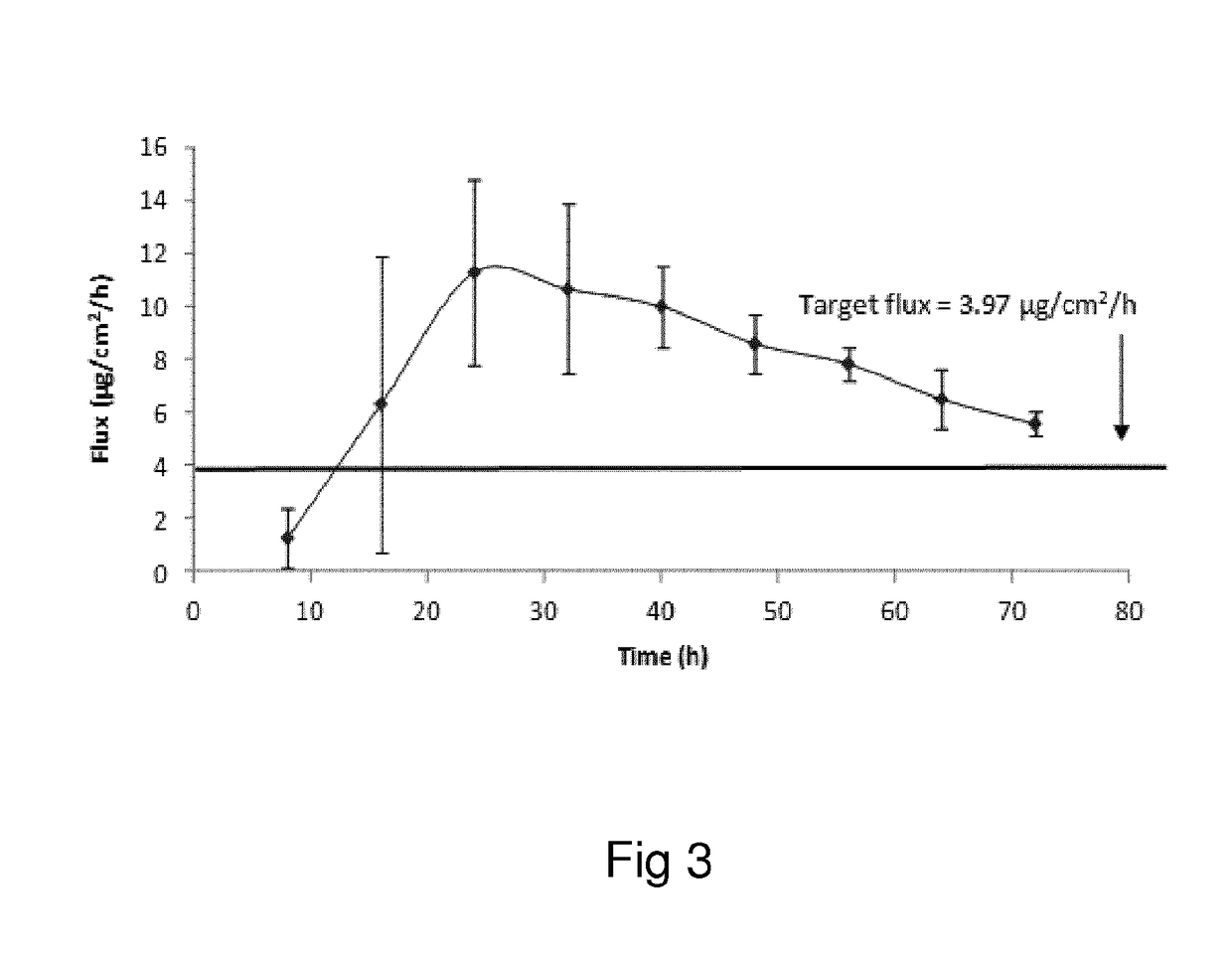
Oxymorphone transdermal patch
The present invention relates to a transdermal patch comprising oxymorphone. The present invention also relates to processes for the preparation of the transdermal patches defined herein, as well as to the use of these patches for the treatment of pain.
Owner:BUZZZ PHARMA
Oxymorphone controlled release formulations
Owner:ENDO PHARMA INC
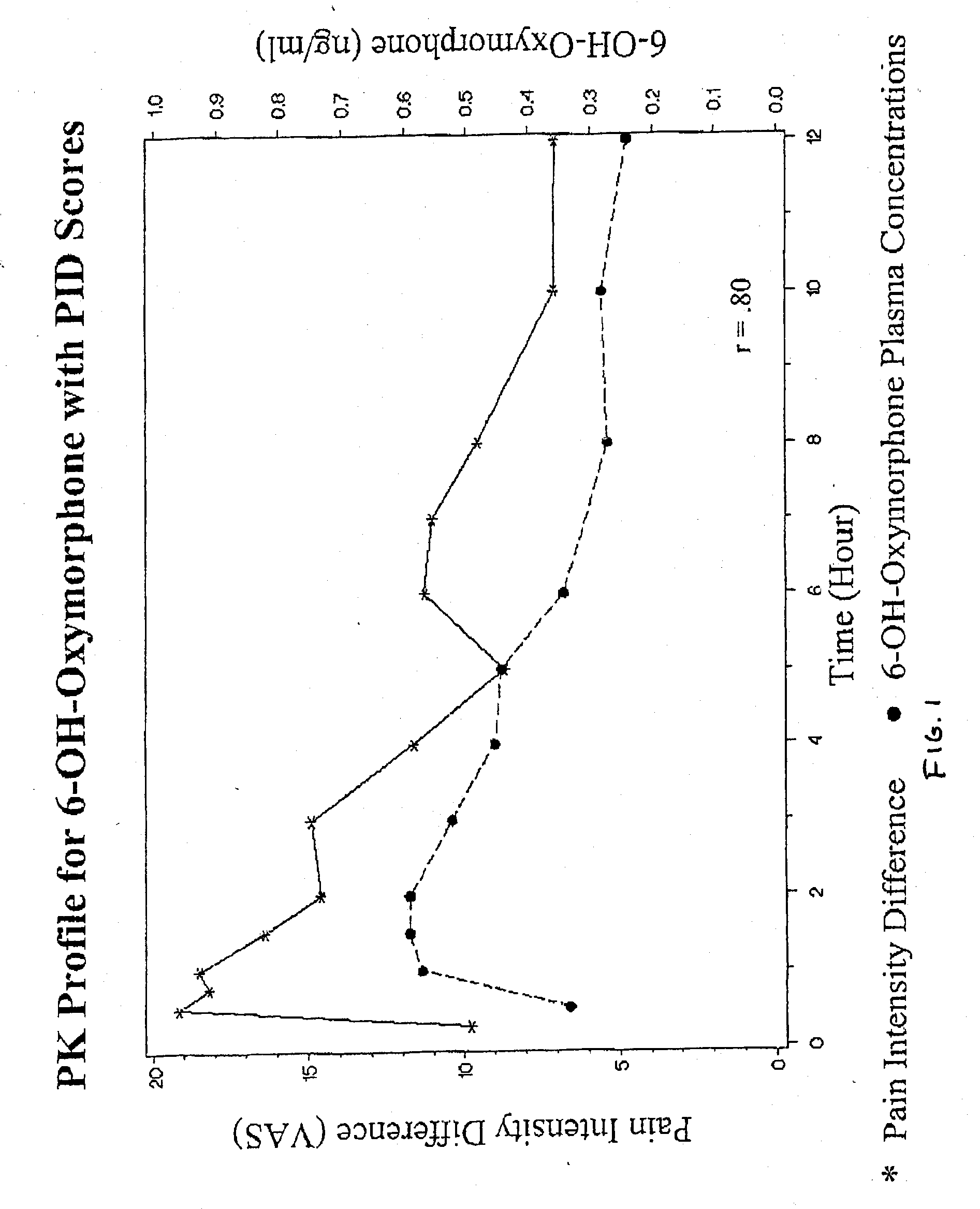
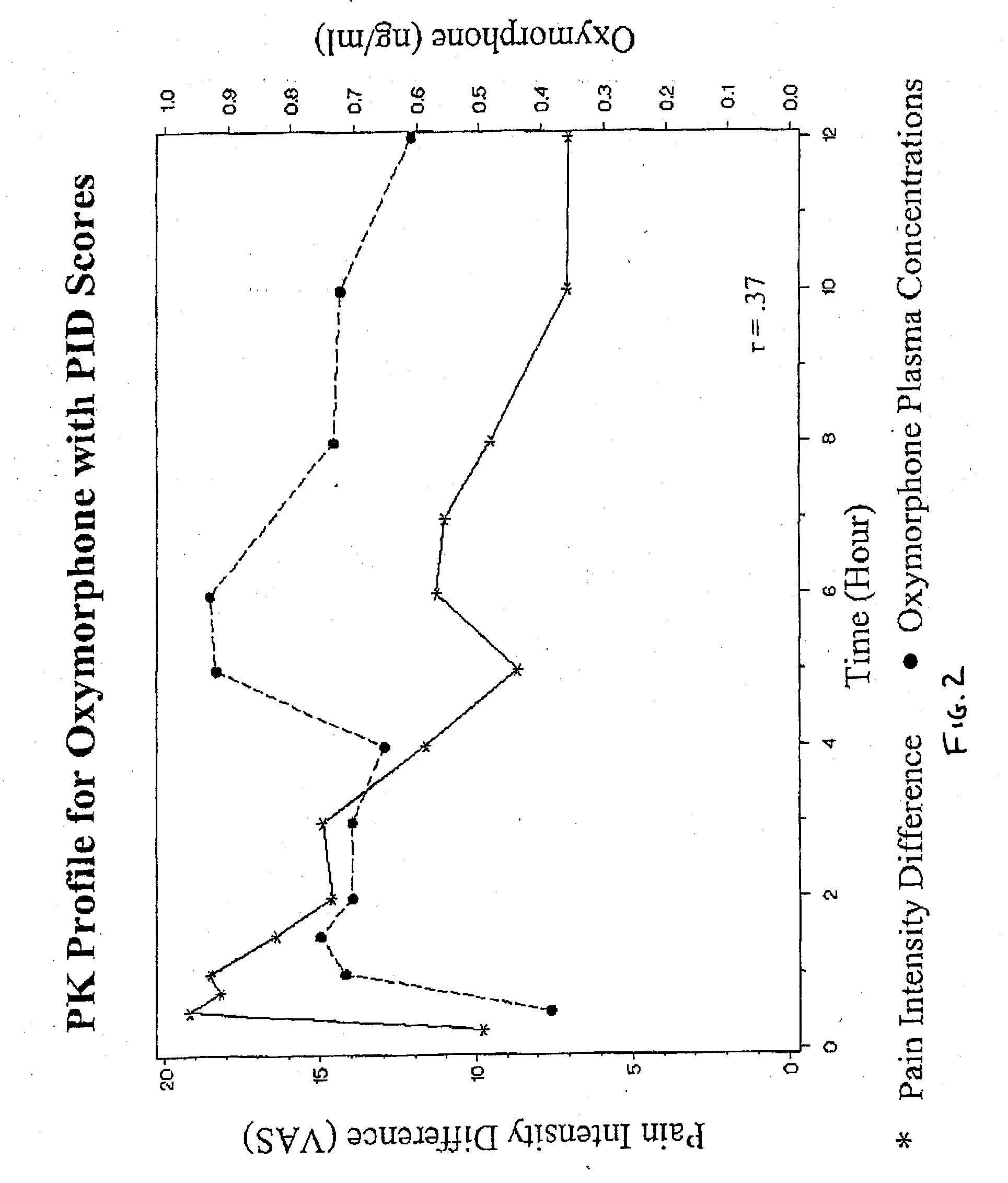
Method of Treating Pain Utilizing Controlled Release Oxymorphone Pharmaceutical Compositions and Instruction on Dosing for Hepatic Impairment
InactiveUS20080318993A1Improve bioavailabilityBiocideData processing applicationsControlled releaseHepatic impairment
The invention pertains to a method of using oxymorphone in the treatment of pain by providing a patient with an oxymorphone dosage form and informing the patient or prescribing physician that the bioavailability of oxymorphone may be increased in patients with hepatic impairment.
Owner:ENDO PHARMA INC
Method of Treating Pain Utilizing Controlled Release Oxymorphone Pharmaceutical Compositions and Instructions on Effects of Alcohol
The invention pertains to a method of using oxymorphone in the treatment of pain by providing a patient with an oxymorphone dosage form and informing the patient or prescribing physician of the effect of alcohol on the maximum concentration of oxymorphone.
Owner:ENDO PHARMA INC
Method of Treating Pain Utilizing Controlled Release Oxymorphone Pharmaceutical Compositions and Instruction on Dosing for Renal Impairment
InactiveUS20080318994A1Improve bioavailabilityBiocideNervous disorderControlled releaseRenal function
The invention pertains to a method of using oxymorphone in the treatment of pain by providing a patient with an oxymorphone dosage form and informing the patient or prescribing physician that the bioavailability of oxymorphone is increased in patients with renal impairment.
Owner:ENDO PHARMA INC
Methods and compositions for reducing the risk associated with the administration of opioid analgesics in patients with diagnosed or undiagnosed respiratory illness
The present invention relates to methods for reducing the risk associated with the administration of opioid analgesics in patients diagnosed or undiagnosed with respiratory illness by administering an analgesic composition comprising a sub-analgesic dosage of a p-opioid agonist selected from the group consisting of morphine, fentanyl, sufentanil, alfentanil, oxymorphone and hydromorphone, or a pharmaceutically acceptable salt thereof, and a sub-analgesic dosage of oxycodone which is a κ2-opioid agonist or a pharmaceutically acceptable salt thereof.
Owner:QRXPHARMA
Sustained release formulations of oxymorphone
Sustained release formulations of oxymorphone or pharmaceutically acceptable salts thereof; methods for making the sustained release formulations of oxymorphone or pharmaceutically acceptable salts thereof; and methods for using the sustained release formulations of oxymorphone or pharmaceutically acceptable salts thereof to treat patients suffering from pain are provided.
Owner:ENDO PHARMA INC
Robust sustained release formulations of oxymorphone and methods of use thereof
InactiveUS20080085303A1Avoid dose dumpingHigh drug safetyBiocidePowder deliveryOxymorphoneSustained Release Formulations
Robust sustained release formulations, solid dosage forms comprising robust sustained release formulations, and methods for making and using these formulations and solid dosage forms are provided. Robustness of the sustained release formulation is related to the particle size of the hydrophilic gum. Sustained release formulations resist dose-dumping when ingested with alcohol. The formulations are useful for treating a patient suffering from a condition, e.g., pain. The formulations comprise at least one drug. In one embodiment, the drug is an opioid, e.g., oxymorphone.
Owner:ENDO PHARMA INC
Process for improved oxymorphone synthesis
ActiveUS20170022209A1Reduce the amount requiredReduce amountOrganic active ingredientsNervous disorderTrifluoroacetic acidOxymorphone
Processes for preparing oxymorphone are provided. Said processes encompass a step which is a hydrogenation of an 14-hydroxymorphinone salt in the presence of trifluoroacetic acid and / or a glycol.
Owner:NORDBOTICS INC
Opioid Salts with Release Properties and Characteristics Useful for Abuse Deterrent Drug Product Formulations
InactiveUS20150164835A1Robust and stablePotential for abuse of eitherBiocideOrganic chemistryLevorphanolDrug product
A drug substance, and drug products comprising the drug substance, wherein the drug substance is selected from the group consisting of amorphous oxymorphone pamoate; polymorphic oxymorphone pamoate; oxymorphone xinafoate; amorphous codeine pamoate; codeine xinafoate; amorphous levorphanol pamoate; polymorphic levorphanol pamoate; levorphanol xinafoate; amorphous naltrexone pamoate; polymorphic naltrexone pamoate and naltrexone xinafoate.
Owner:PISGAH LAB
Pharmaceutical Composition
A pharmaceutical composition comprising an analgesic or analgesic combination and a stool softener is disclosed. The analgesic is selected from morphine, meperidine, fentanyl, hydromorphone, oxymorphone, oxycodone, hydrocodone, methadone, propoxyphene, pentazocine, levorphanol, codeine, acetaminophen and combinations of these analgesics. The composition is formulated for oral administration as a liquid or solid dosage form for immediate, slow, delayed or sustained-release characteristics.
Owner:BRANDED PRODS FOR THE FUTURE
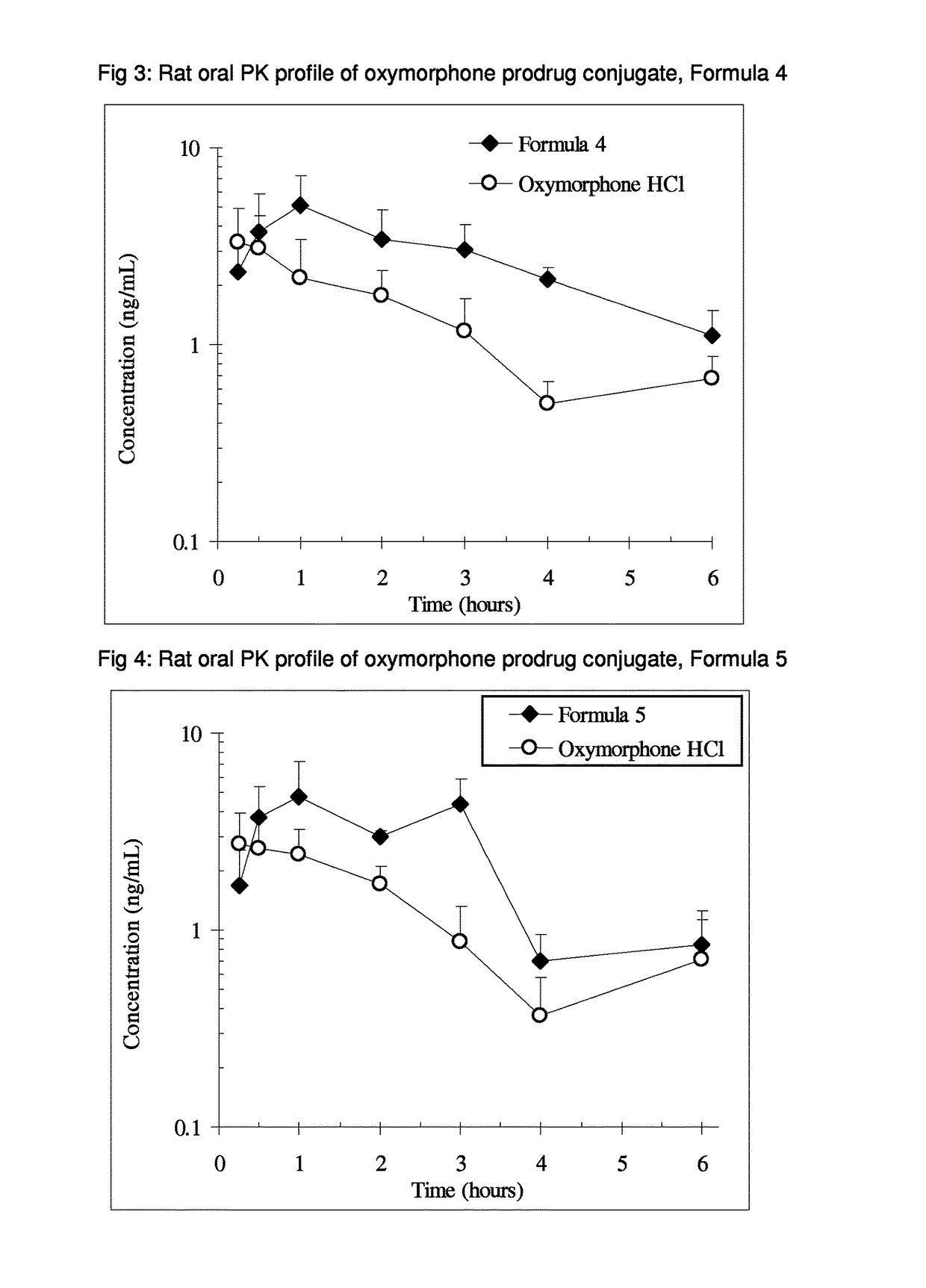
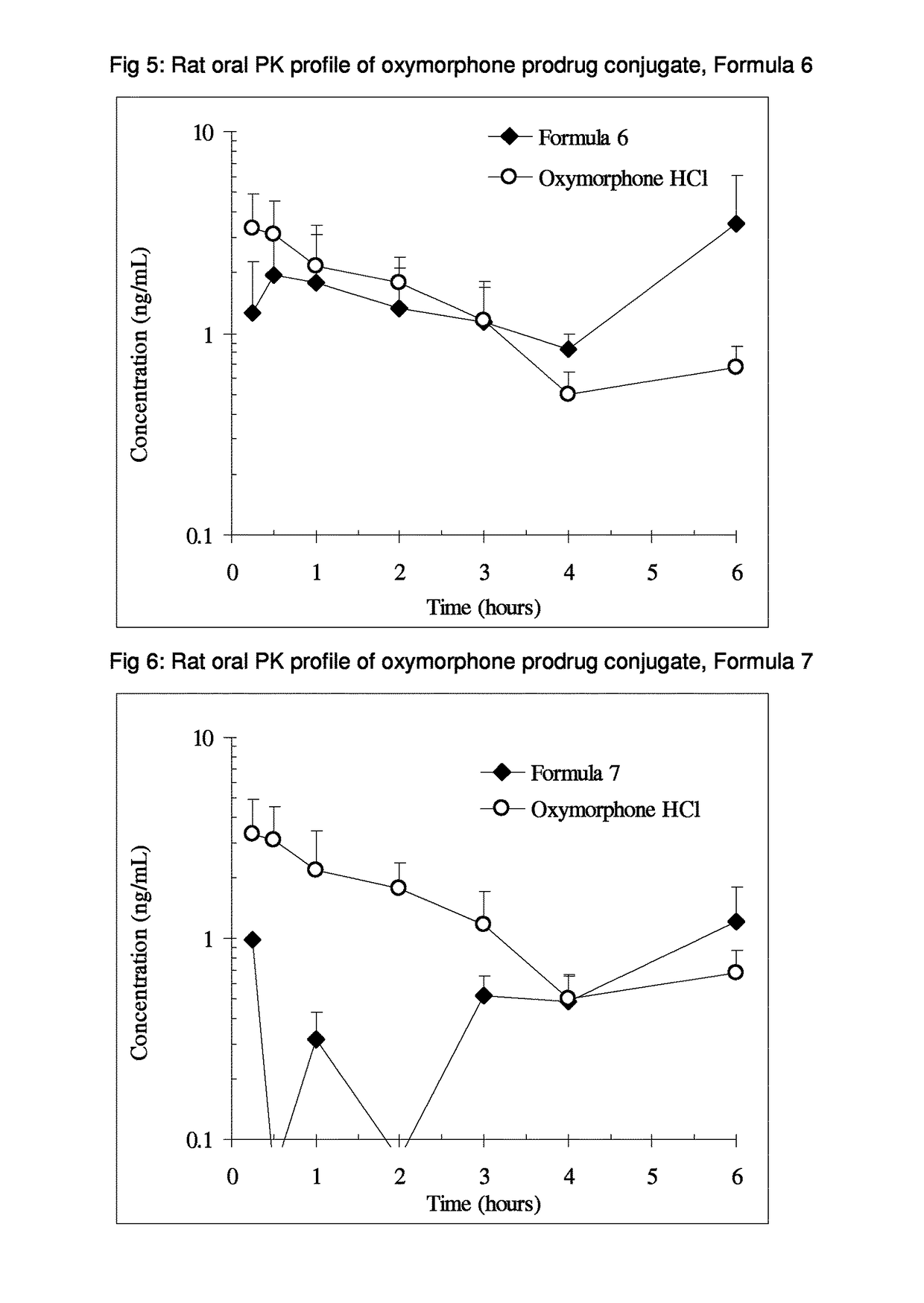
Alpha-hydroxy carboxylic acid and derivatives and other GRAS-based prodrugs of oxymorphone and uses thereof
The invention describes pharmaceutical compounds and compositions comprised of a ligand attached to the opioid oxymorphone, in a manner that substantially decreases or deters the potential for opioid abuse, addiction, illicit and illegal use, and overdose. When delivered at the proper dosage, the pharmaceutical composition provides therapeutic activity similar to that of the parent active agent.
Owner:3ST RES LLC
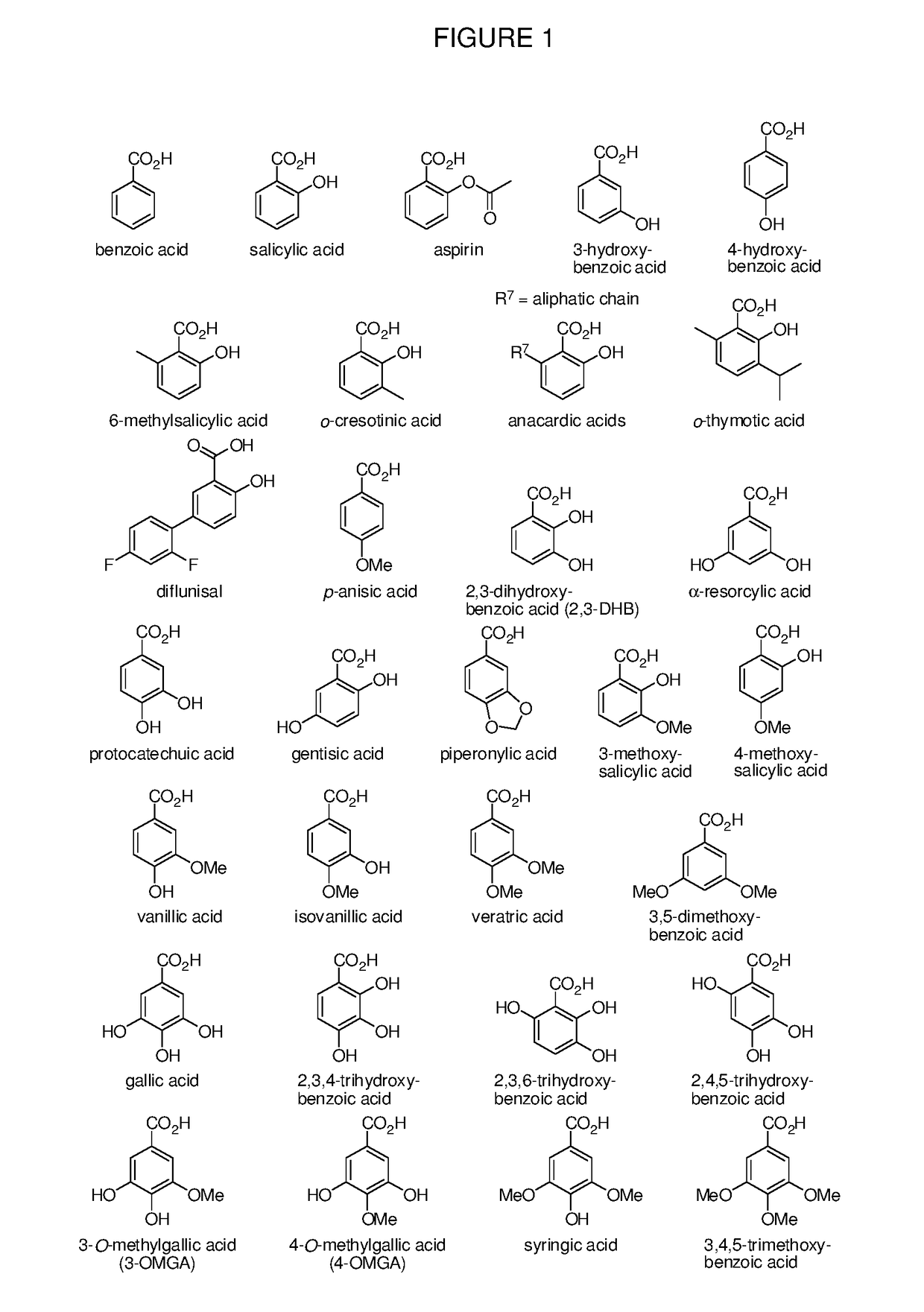
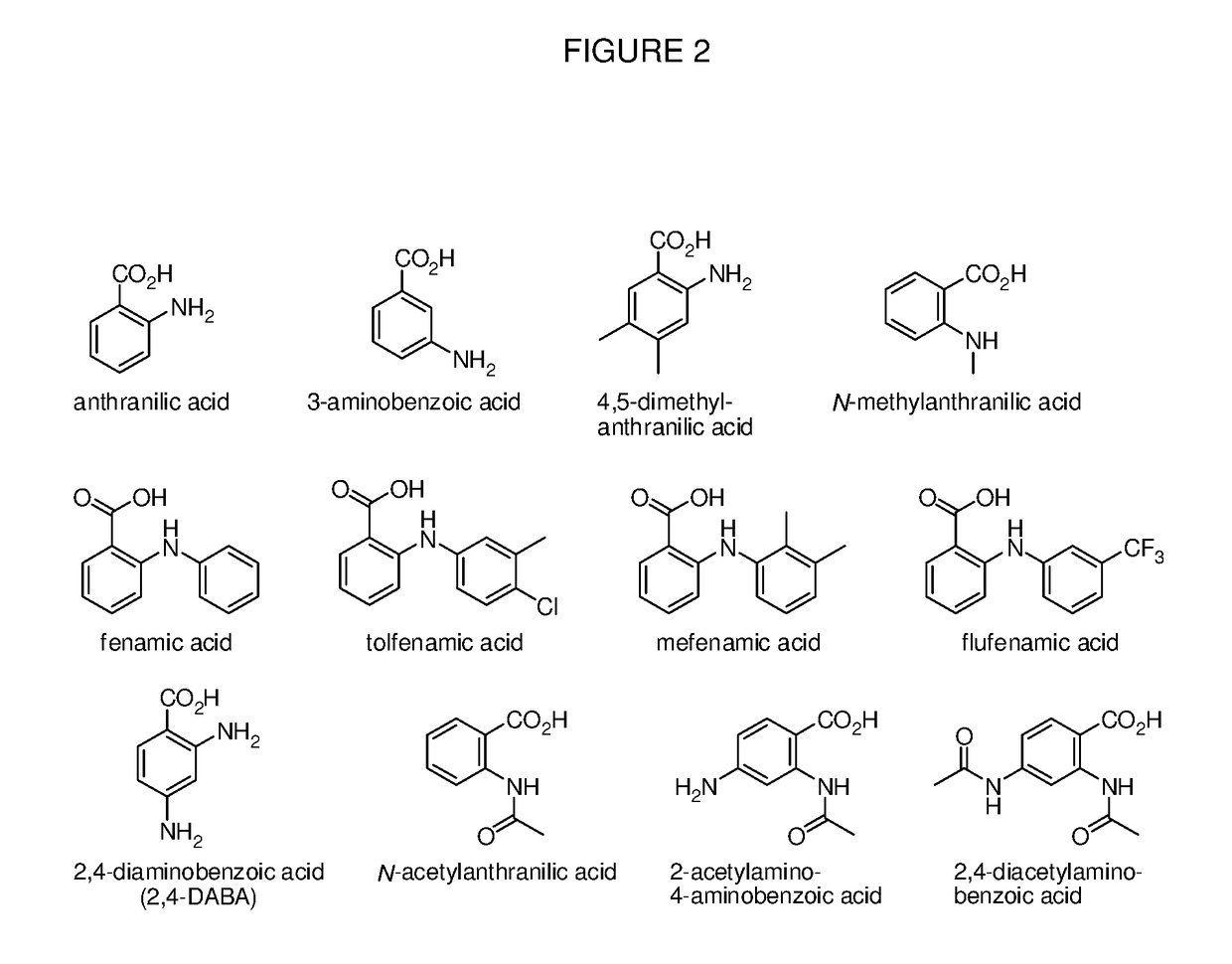
Benzoic acid, benzoic acid derivatives and heteroaryl carboxylic acid conjugates of oxymorphone, prodrugs, methods of making and use thereof
ActiveUS9682076B2Eliminate the effects ofReduce or prevent oral, intranasal or intravenous drug abuseNervous disorderOrganic chemistryBenzoic acidPropionate
The presently described technology provides compositions comprising aryl carboxylic acids and, for example NSAIDs, chemically conjugated to oxymorphone (4,5-α-epoxy-3,14-dihydroxy-17-methylmorphinan-6-one) to form novel prodrugs / compositions of oxymorphone, including benzoates, salicylates, propionates, fenamates, and acetates, which have a decreased potential for abuse of oxymorphone. The present technology also provides methods of treating patients, pharmaceutical kits and methods of synthesizing conjugates of the present technology.
Owner:KEMPHARM INC
Pharmaceutical composition
A pharmaceutical composition comprising an analgesic or analgesic combination and a stool softener is disclosed. The analgesic is selected from morphine, meperidine, fentanyl, hydromorphone, oxymorphone, oxycodone, hydrocodone, methadone, propoxyphene, pentazocine, levorphanol, codeine, acetaminophen and combinations of these analgesics. The composition is formulated for oral administration as a liquid or solid dosage form for immediate, slow, delayed or sustained-release characteristics.
Owner:BRANDED PRODS FOR THE FUTURE
Preparation of Oxymorphone from Oripavine
An improved method for the preparation of oxymorphone from oripavine is provided. Oripavine is oxidized to form 14-hydroxymorphinone after which the oxidation reaction is quenched to prevent the formation of 1-1′-dimer side products. The 14-hydroxymorphinone is then reduced, typically by catalytic hydrogenation to form oxymorphone. The inventive method disclosed is further applicable to the production of morphinan derivatives.
Owner:SPECGX LLC
Oxymorphone transdermal patch
The present invention relates to a transdermal patch comprising oxymorphone. The present invention also relates to processes for the preparation of the transdermal patches defined herein, as well as to the use of these patches for the treatment of pain.
Owner:BUZZZ PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com