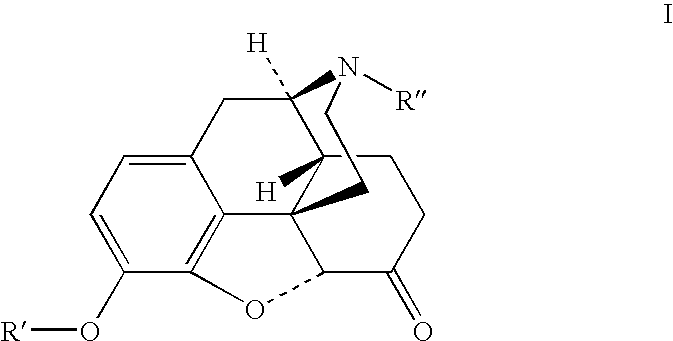
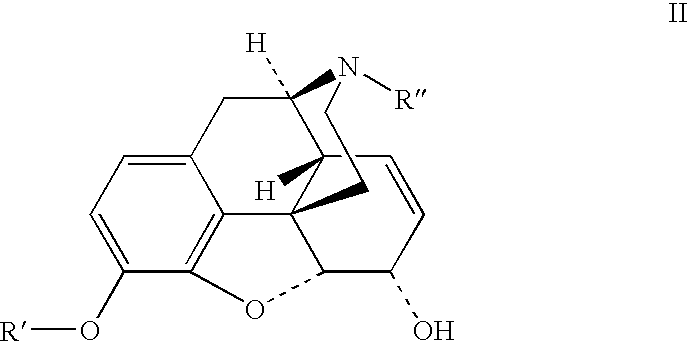
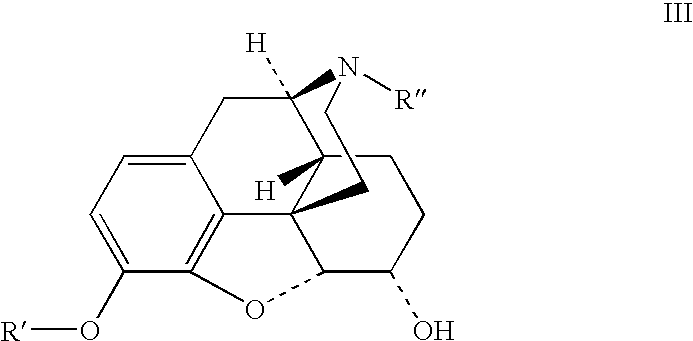
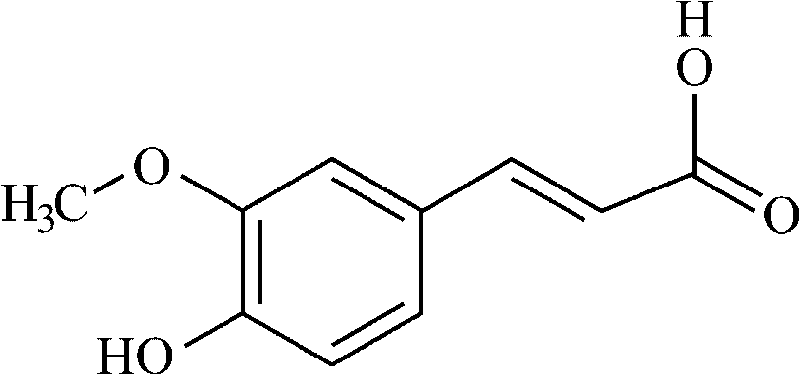
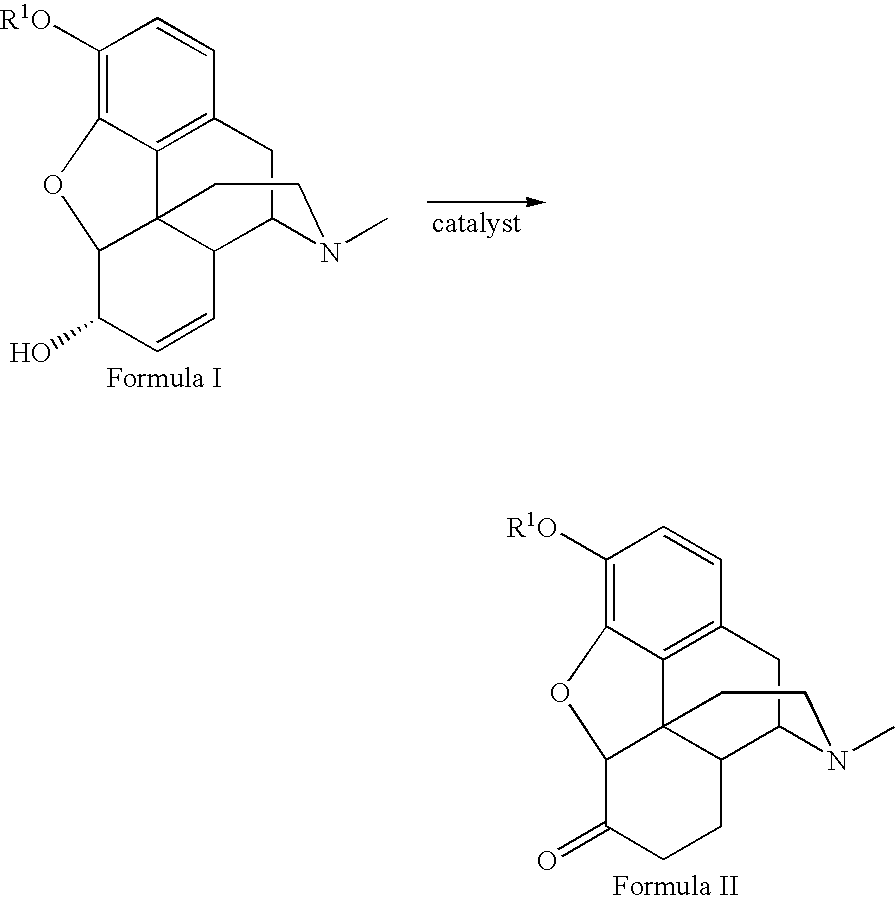
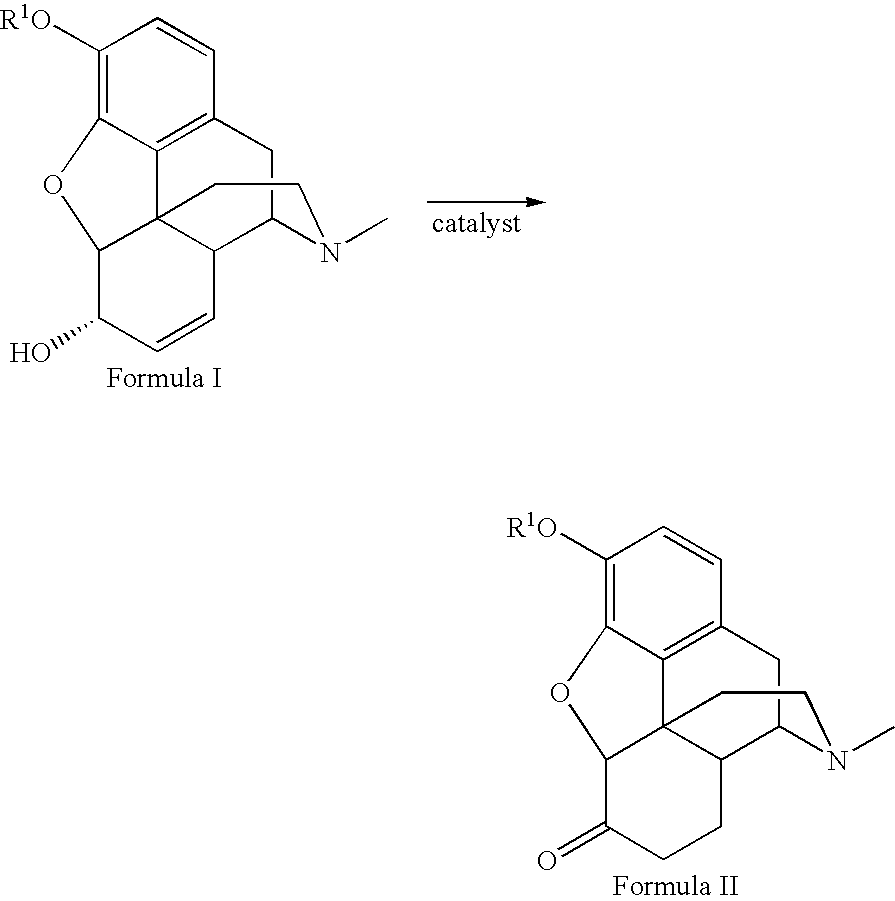
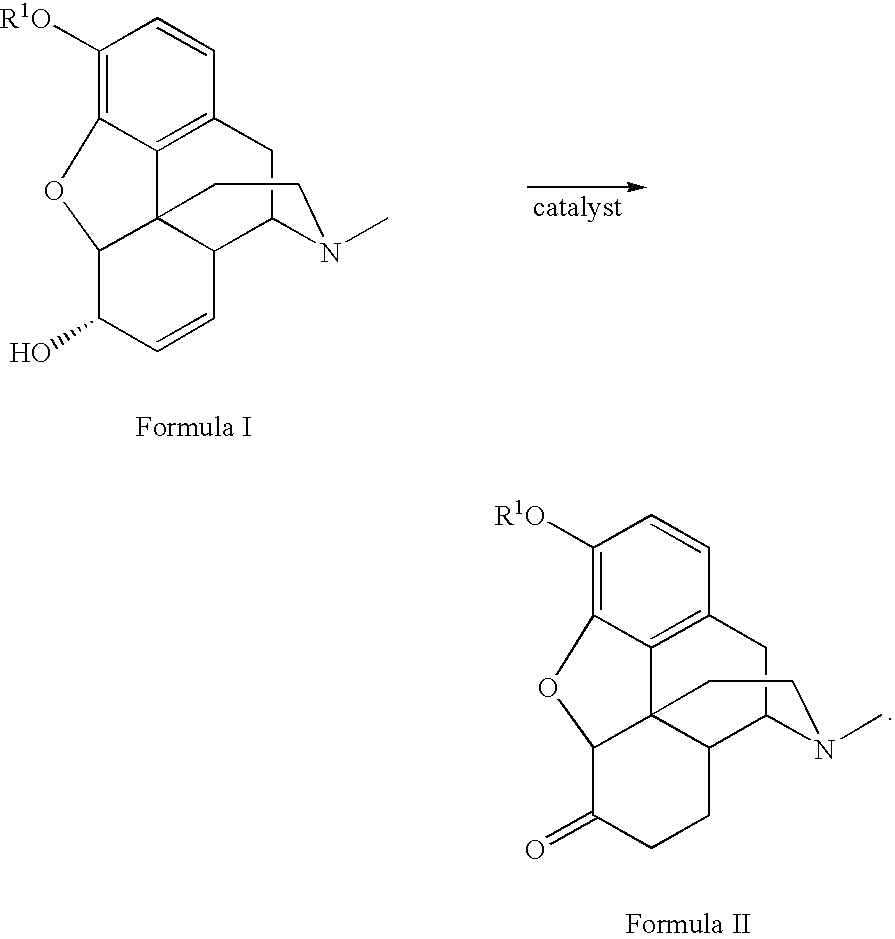
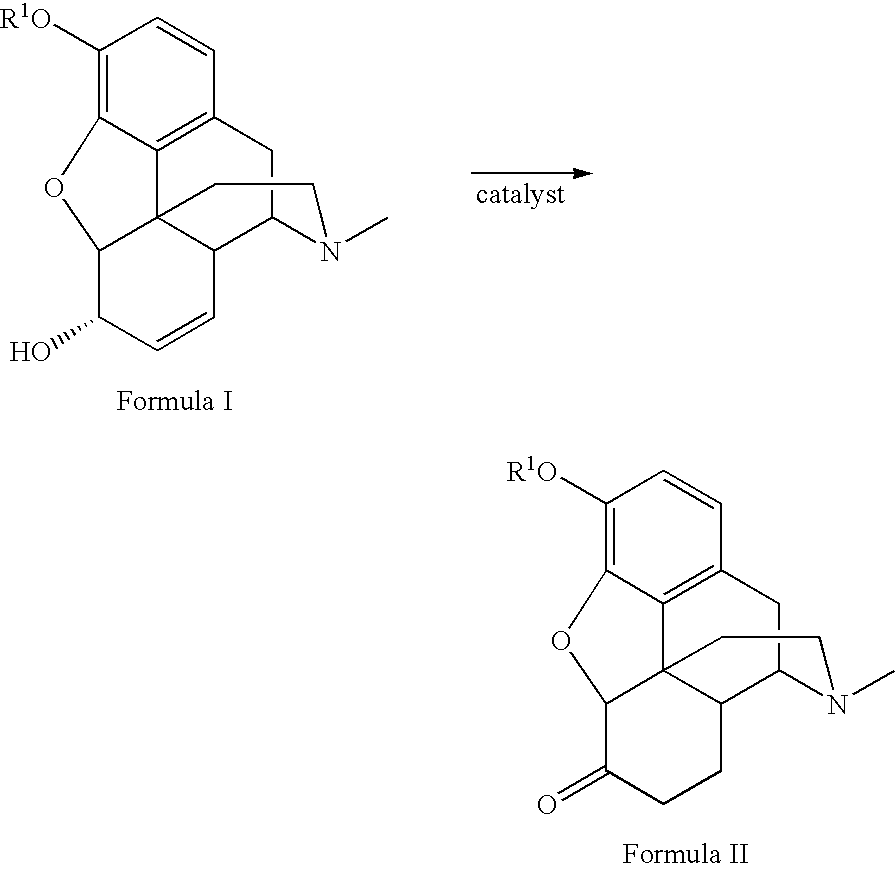
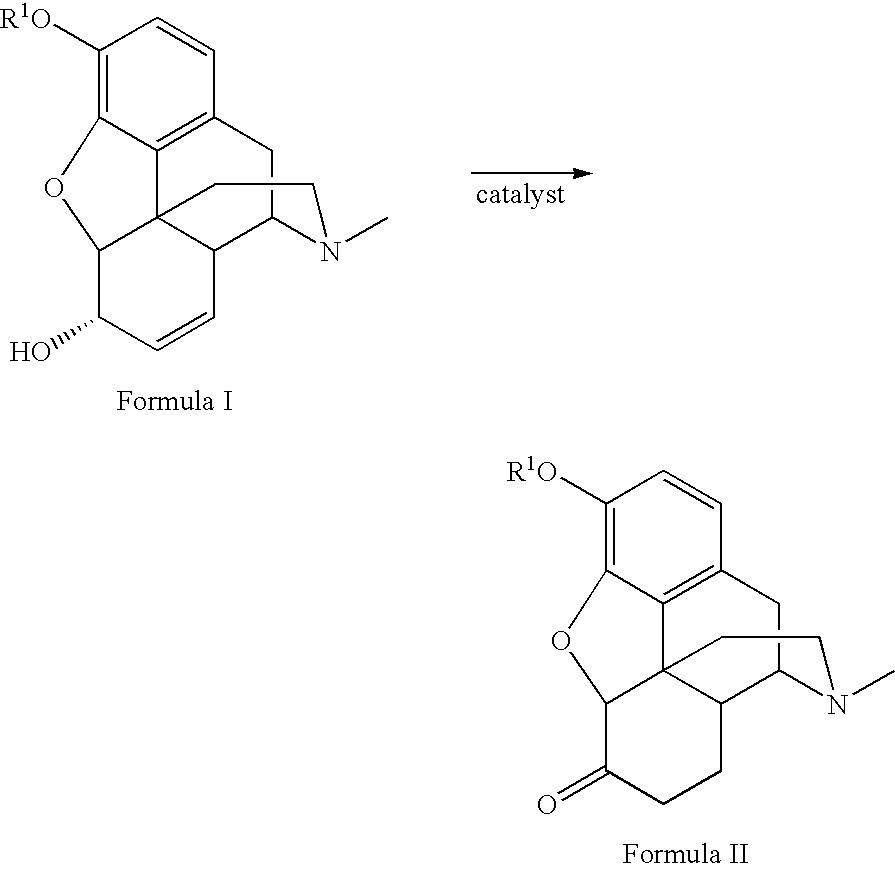
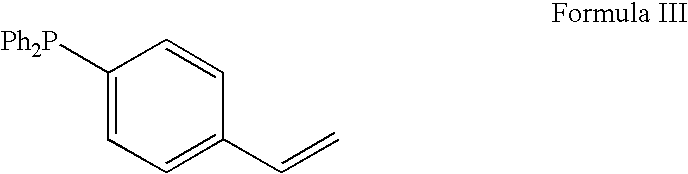
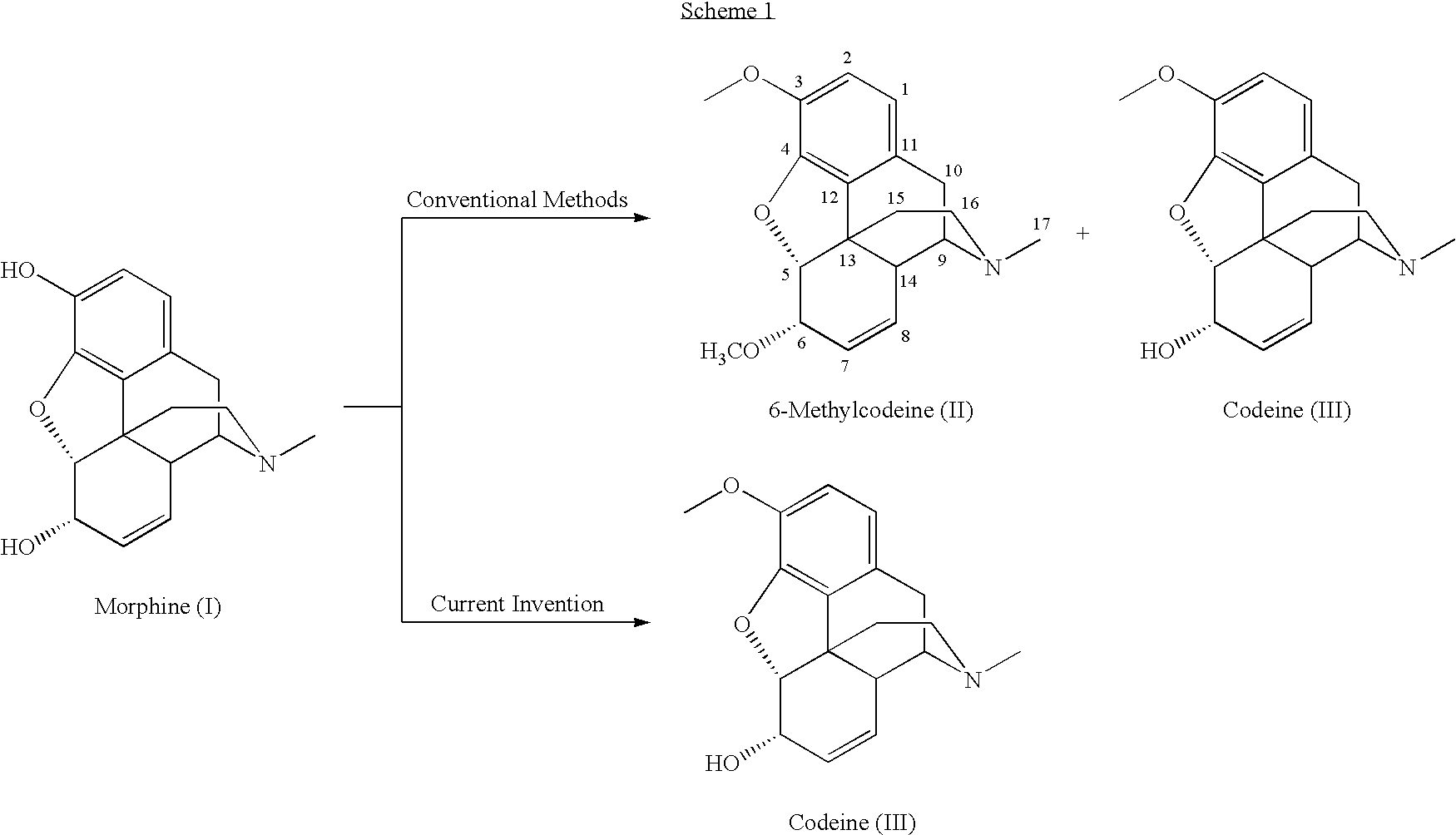
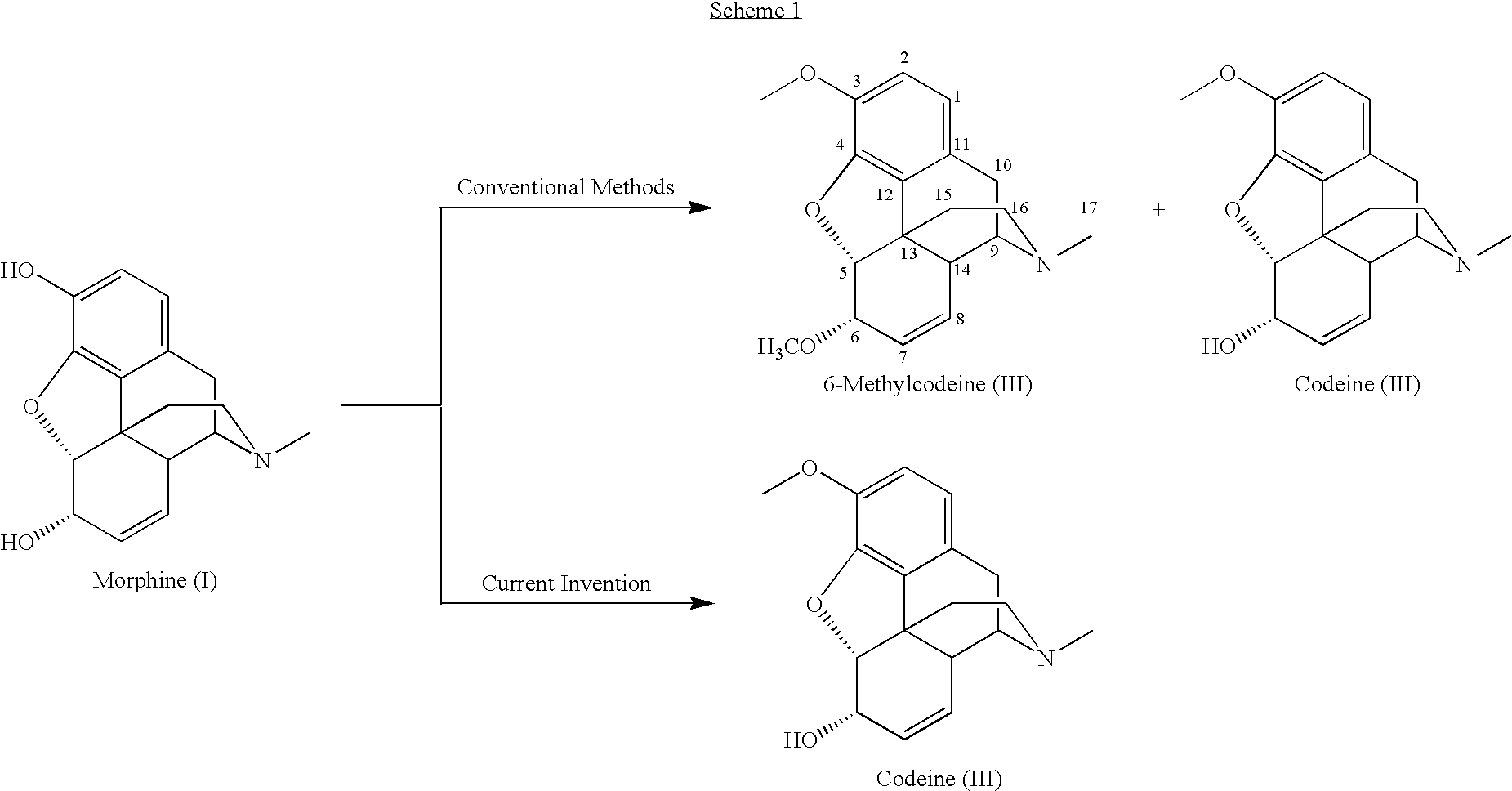
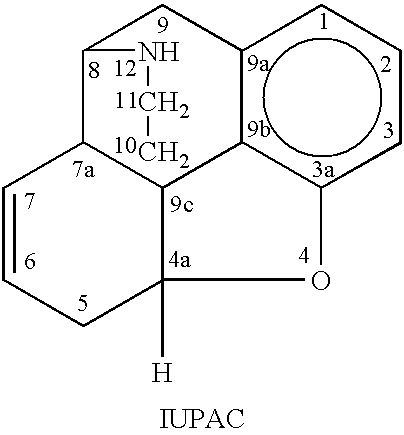
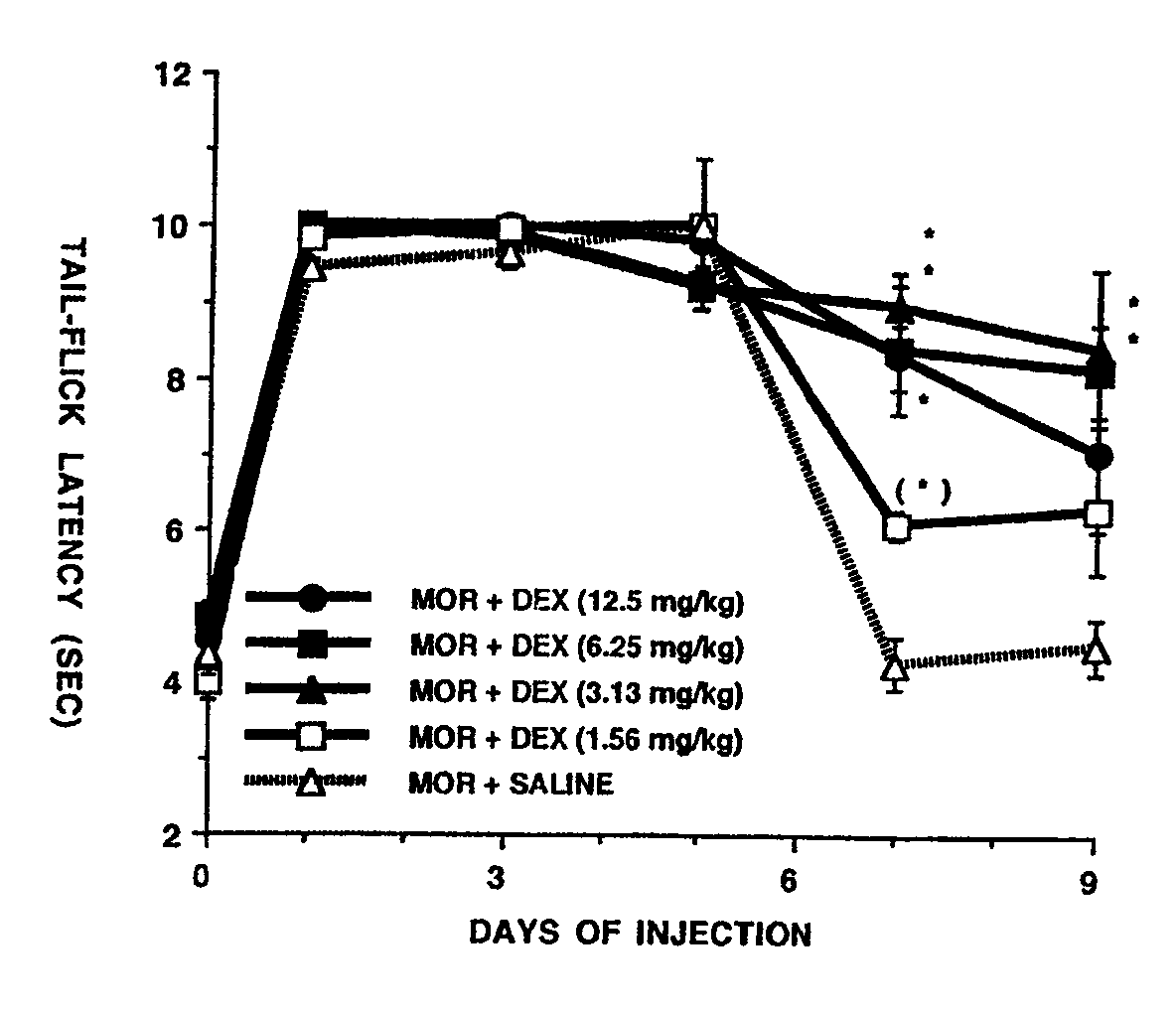
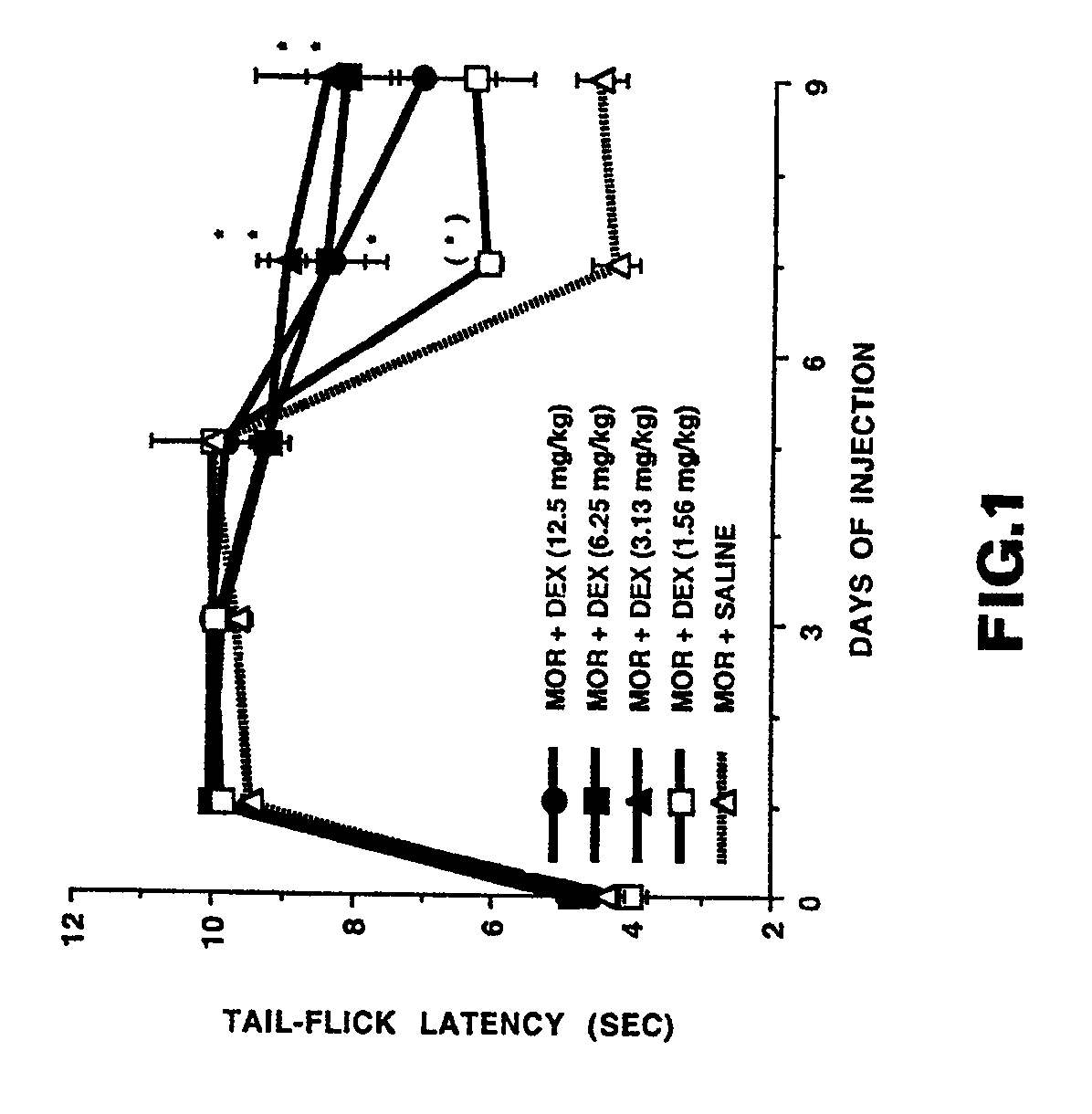
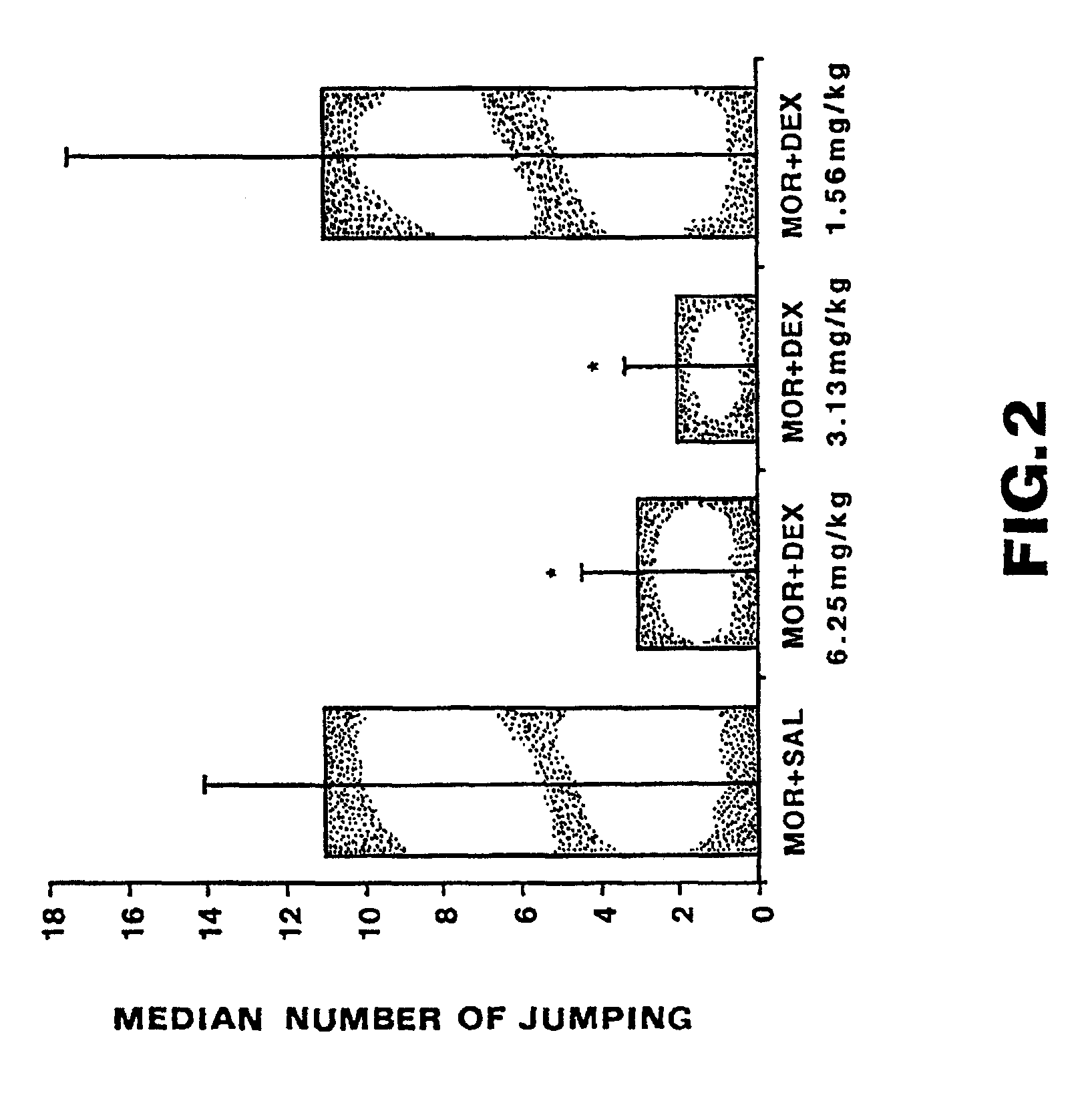
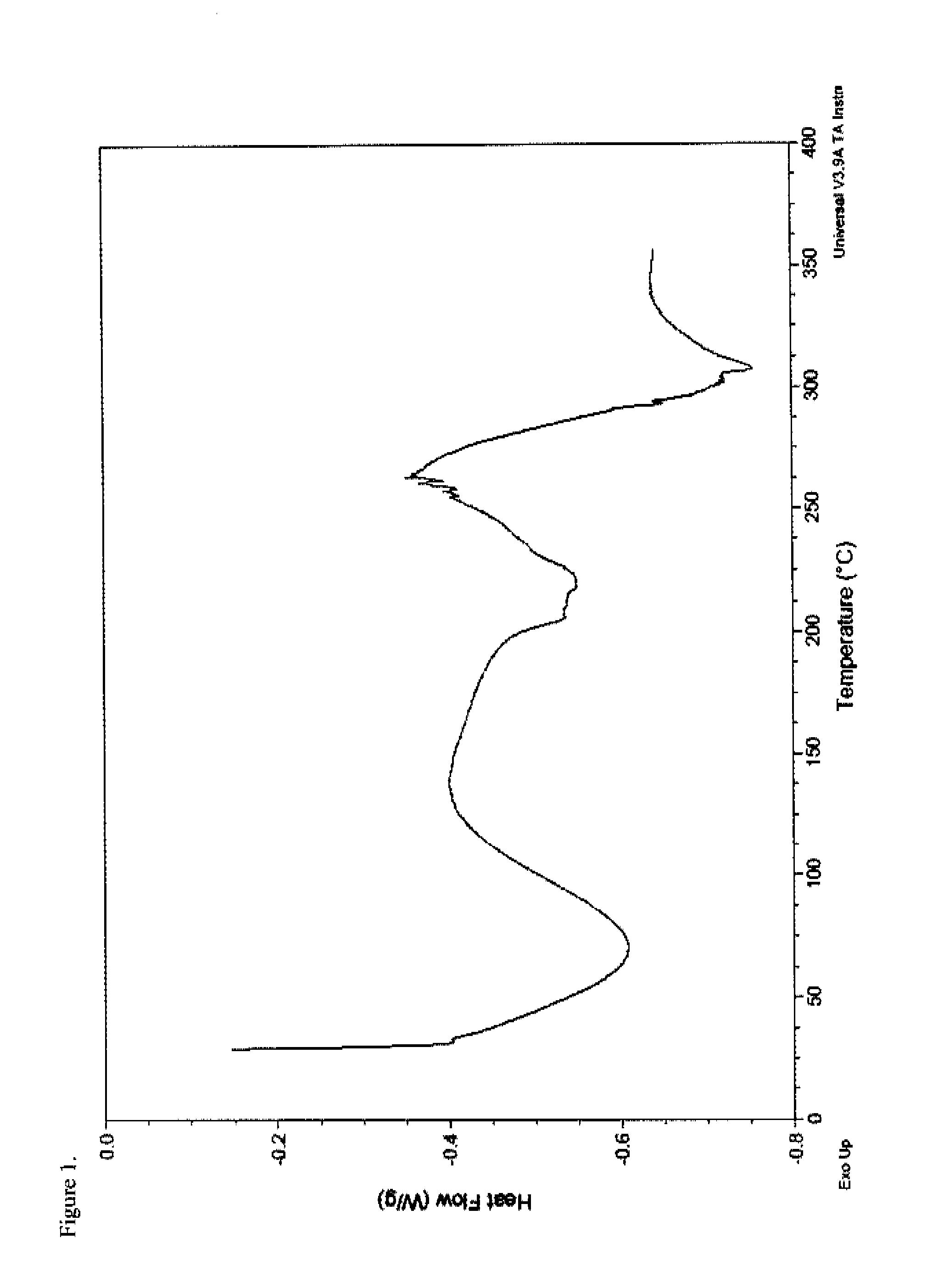
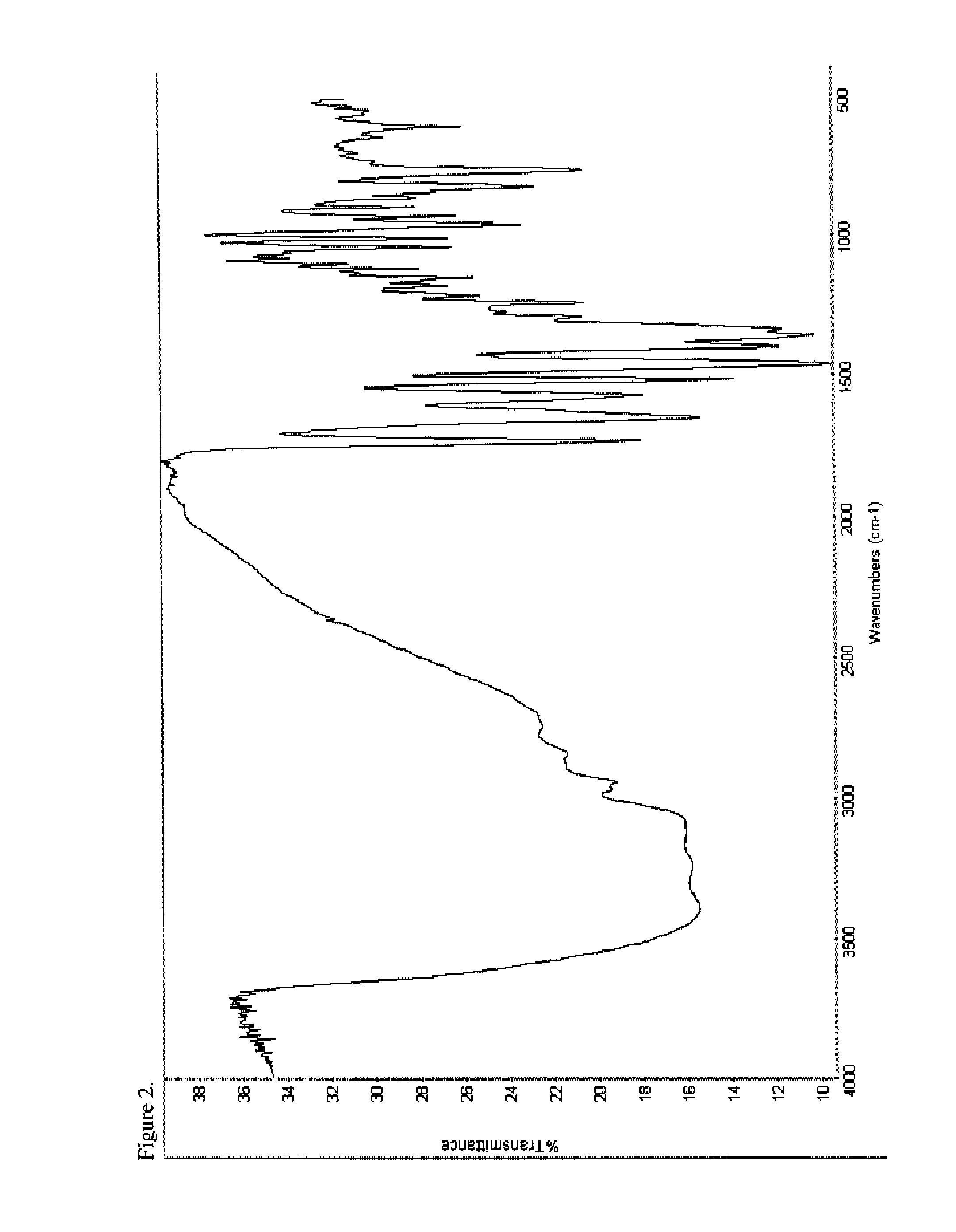
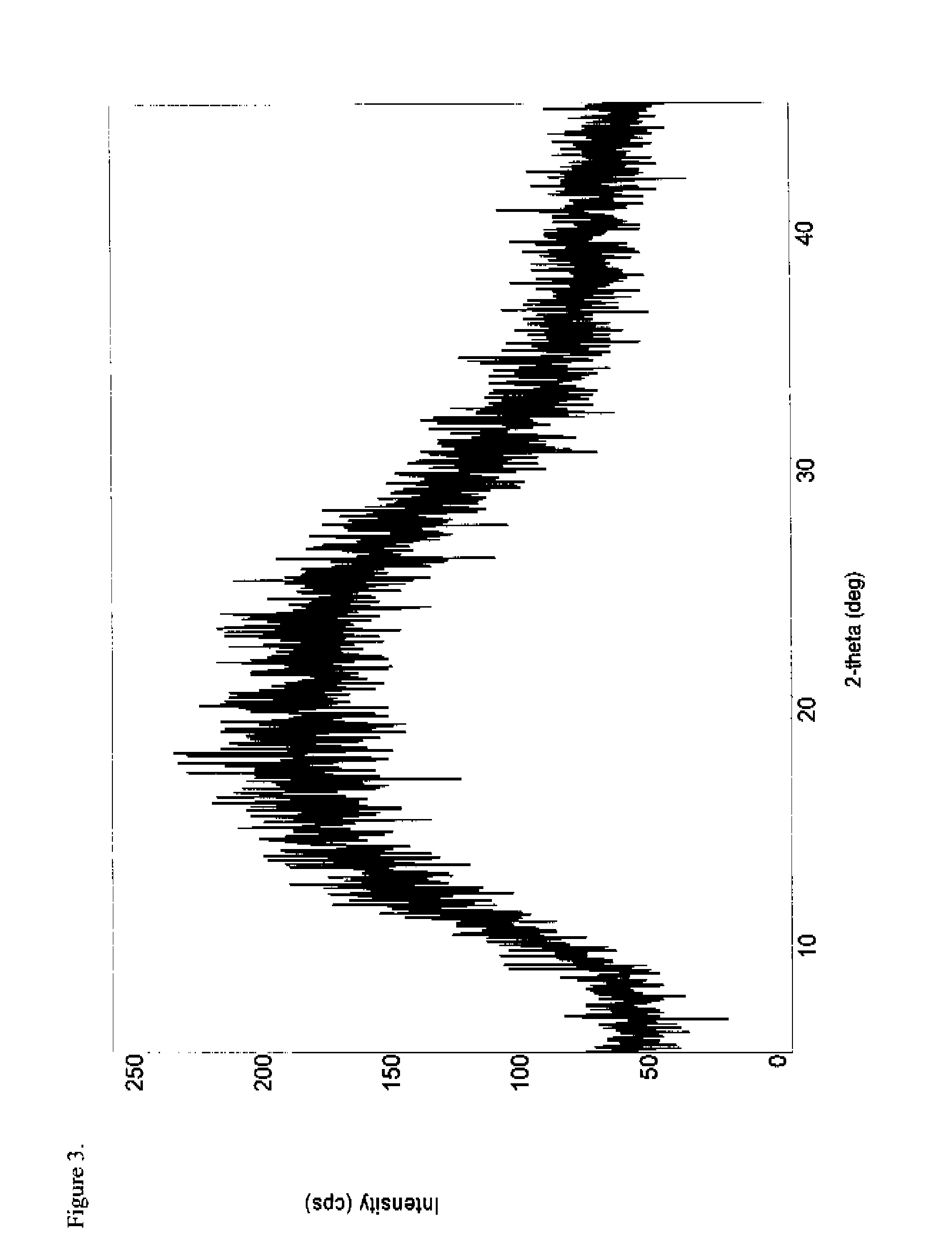
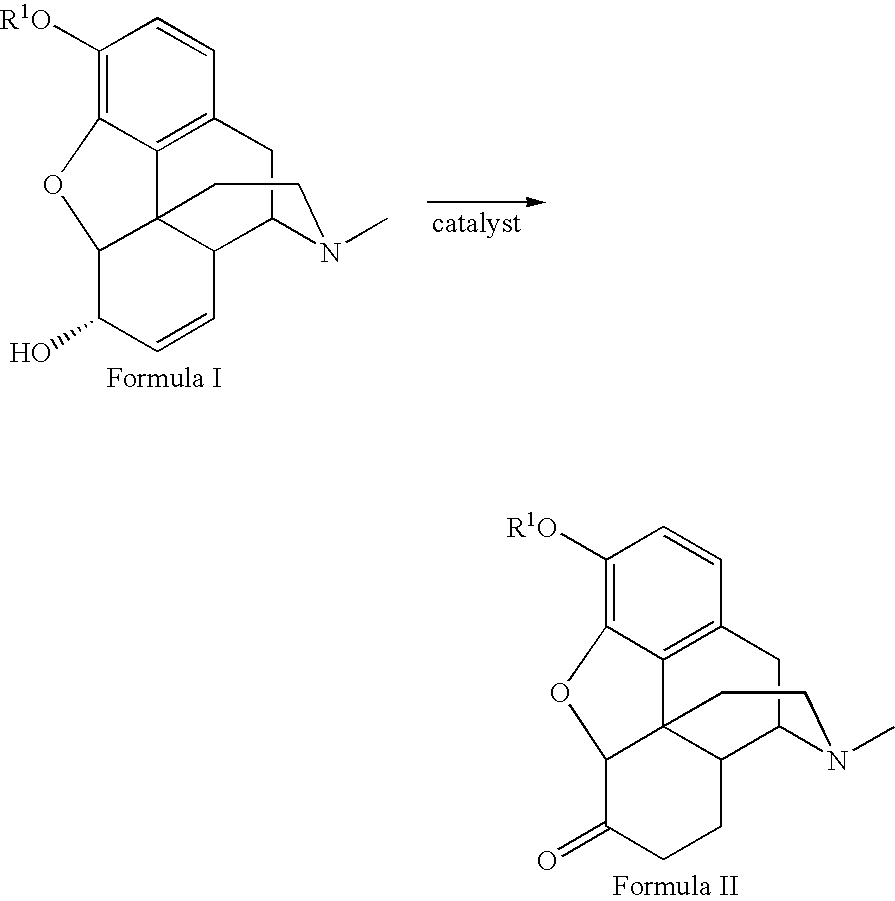
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
79 results about "Codeine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to help relieve mild to moderate pain. Codeine belongs to a class of drugs known as opioid (narcotic) analgesics. It works in the brain to change how your body feels and responds to pain..
Pharmaceutical formulations comprising ibuprofen, oxycodone, and 14-hydroxycodeinone
The present invention relates to pharmaceutical formulations of ibuprofen, oxycodone and 14-hydroxycodeinone and their use for the treatment of acute, moderate to severe pain.
Owner:FOREST LABORATORIES
Preparation of opioid analgesics by a one-pot process
A one-pot process for preparing opioid analgesics such as hydrocodone, hydromorphone, and analogues thereof by reacting codeine, morphine, and analogues thereof with hydrogen in a solvent system of benzophenone and neutral solvent in the presence of a metal catalyst followed by oxidation in the presence of potassium tert-alkylate.
Owner:ACURA PHARMA
Pharmaceutical composition
A pharmaceutical composition comprising an analgesic or analgesic combination and a stool softener is disclosed. The analgesic is selected from morphine, meperidine, fentanyl, hydromorphone, oxymorphone, oxycodone, hydrocodone, methadone, propoxyphene, pentazocine, levorphanol, codeine, acetaminophen and combinations of these analgesics. The composition is formulated for oral administration as a liquid or solid dosage form for immediate, slow, delayed or sustained-release characteristics.
Owner:BRANDED PRODS FOR THE FUTURE
Action of ferulic acid on enhancing drug effect of some medicaments and purpose thereof
InactiveCN101721400AOrganic active ingredientsPharmaceutical non-active ingredientsCatharanthineChemical reaction
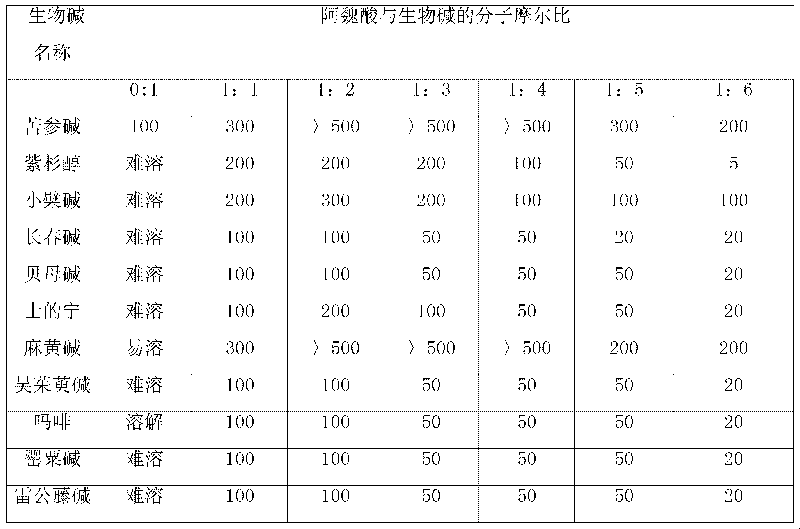
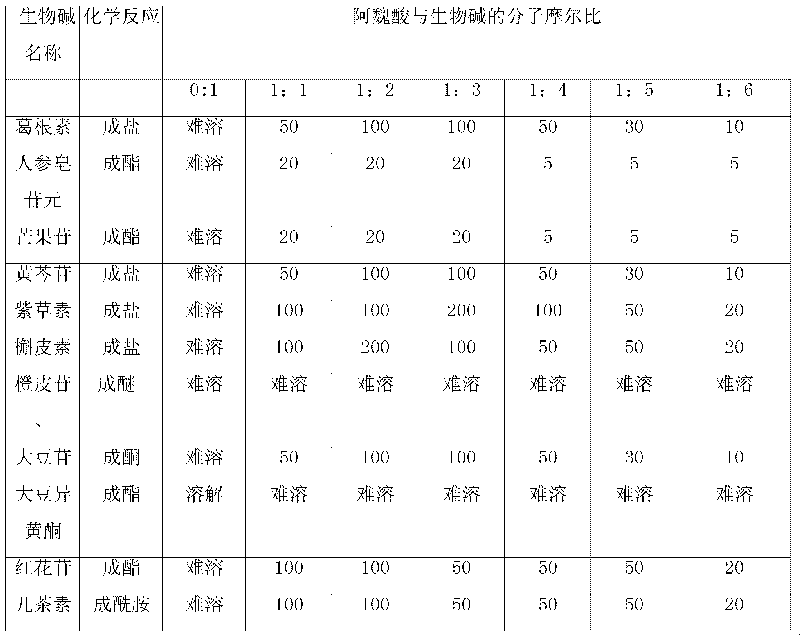
The invention relates to an action of ferulic acid on enhancing the drug effect of some medicaments and a purpose thereof, wherein the medicaments mainly comprise alkaloid medicaments including matrine, kushenin, sophocarpine, hyoscyamine, narceine, hanfangchin A, jamaicin, morphine, codeine, evodiamine, strychnine, catharanthine, vincristine, taxol, verticine, peimine, peiminine, ephedrine, pseudoephedrine, wilfordine, triptolide, tripdiolide and the like, and flavonoid medicaments including puerarin, ginsenoside, ginsengenin, mangiferin, scutelloside, alkannin, meletin, rutin, hesperidin, daidzin, soybean isoflavone, daidzein, carthamin, catechin and the like. The ferulic acid and the medicaments can form a compound or a medicament compound, or the ferulic acid and the medicaments can generate a chemical reaction (including salification, esterification, amidation, ketonization, etherification and the like), and / or the ferulic acid and the medicaments can generate a synergistic effect and an additive effect.
Owner:QINGDAO QIYUAN BIO TECH CO LTD
Pharmaceutical combinations of cox-2 inhibitors and opiates
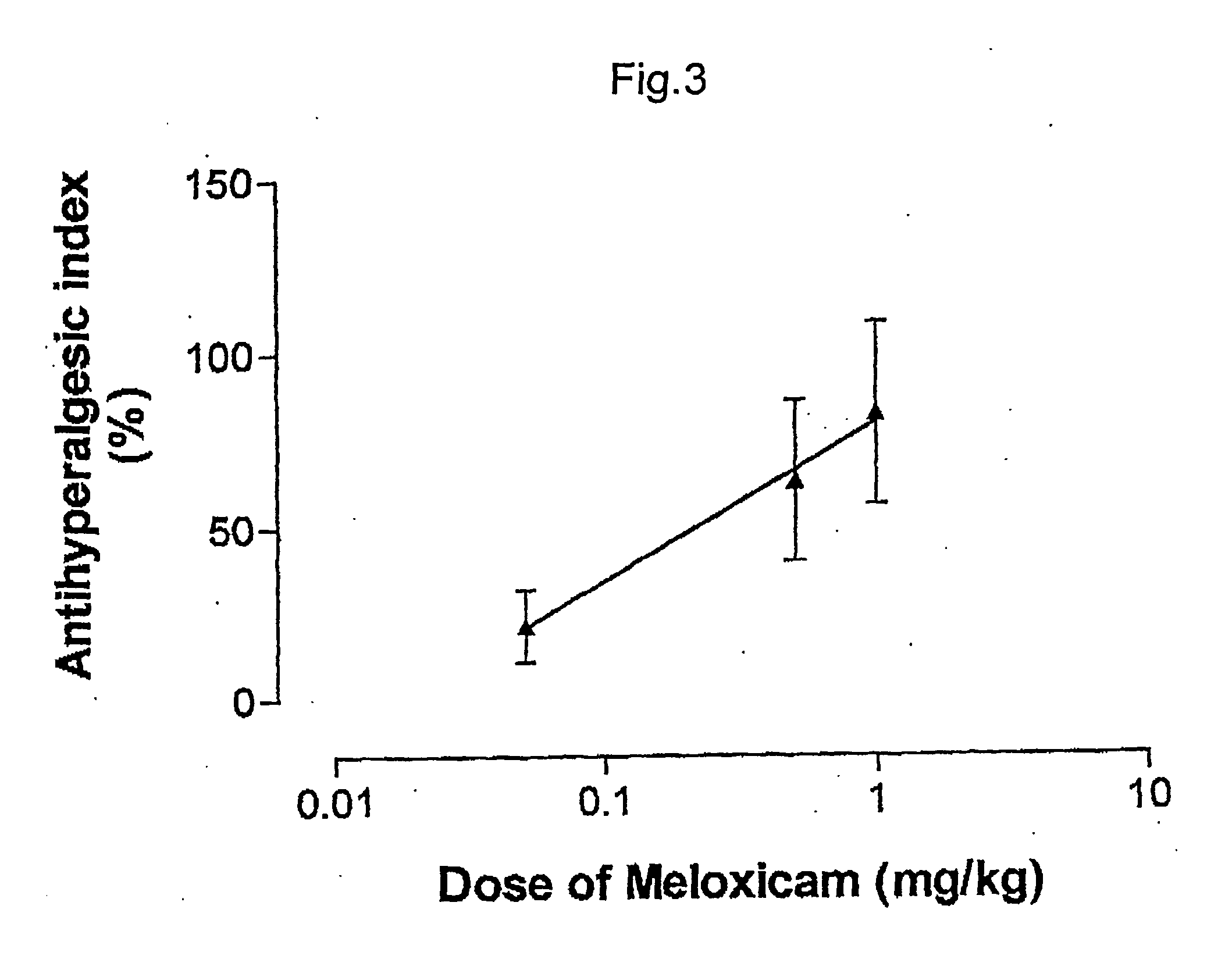
ABSTRACTA pharmaceutical composition comprises a combination of a selective or specific COX 2 inhibitor or a pharmaceutically acceptable salt or derivative thereof and an opiate or a pharmaceutically acceptable salt or derivative thereof, for example a combination of meloxicam and codeine, as active ingredients, and a pharmaceutically acceptable carrier. It may include a centrally-acting cyclo-oxygenase inhibitor such as paracetamol or its pharmaceutically acceptable salts or derivatives. The pharmaceutical compositions are used in methods of providing symptomatic relief or treatment of pain, in an algesic and / or hyperalgesic state, with or without fever, in particular that associated with inflammation such as that associated with trauma, osteoarthritis, rheumatoid arthritis, non-inflammatory myalgia or dysmenorrhoea
Owner:ADCOCK INGRAM LTD
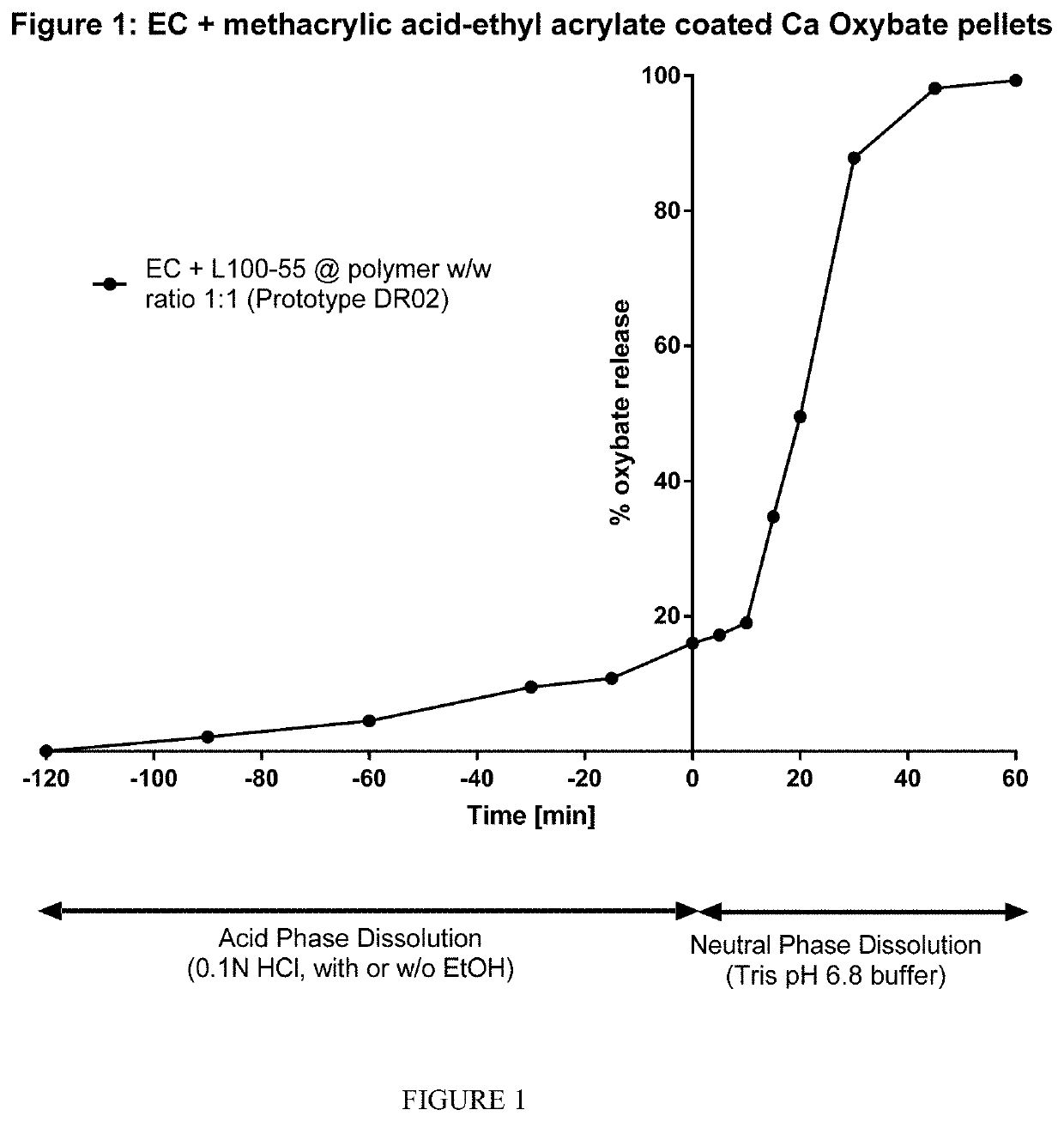
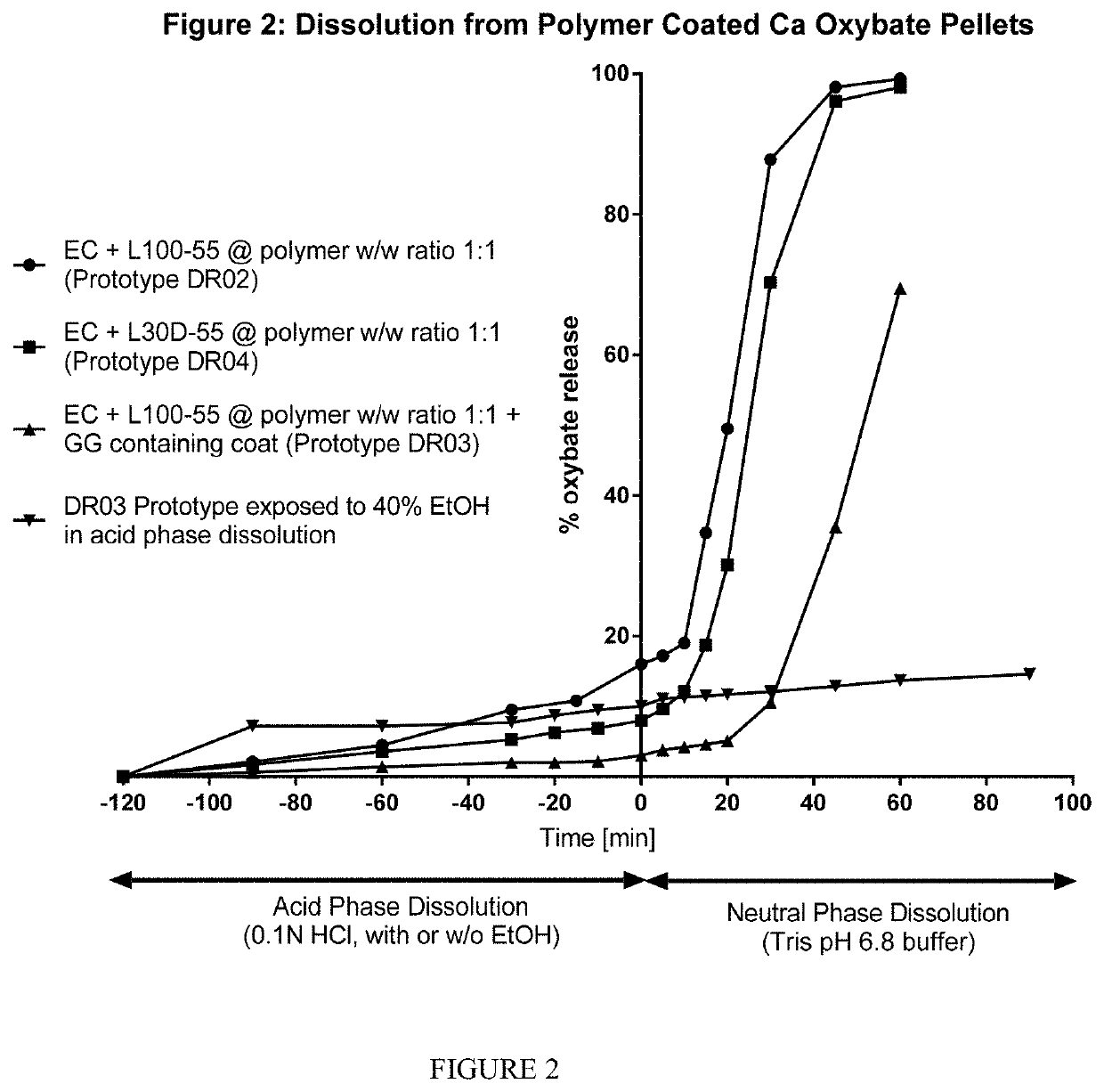
Alcohol-resistant drug formulations
ActiveUS20200330393A1Eliminate side effectsNervous disorderGranular deliverySleep arousalParacetamolum
The invention relates to modified release oral formulations of therapeutic agents, including gamma hydroxybutyrate (GHB), paracetamol, codeine or oxycodone, which are resistant to alcohol induced dose dumping. Provided are formulations that have improved resistance to rapid release of the active ingredient in the presence of increasing amounts of alcohol. Also provided are formulations that can reduce or prevent the release of the active ingredient following exposure to alcohol-containing media. The invention also relates to methods of making the formulations, and methods of their use for the treatment of sleep disorders such as apnea, sleep time disturbances, narcolepsy, cataplexy, sleep paralysis, hypnagogic hallucination, sleep arousal, insomnia, and nocturnal myoclonus.
Owner:JAZZ PHARMA IRELAND LTD
Method for the catalytic production of hydrocodone and hydromorphone
A method for the catalytic conversion of codeine, morphine or analogs thereof into hydrocodone, hydromorphone or analogs thereof utilizing a transition metal complex of a tertiary phosphine halide as catalyst.
Owner:MALLINCKRODT INC
Method for the catalytic production of hydrocodone and hydromorphone
InactiveUS7321038B2Organic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsArylHydrocodone
A method for the catalytic conversion of codeine, morphine or analogs thereof into hydrocodone, hydromorphone or analogs thereof utilizing a transition metal catalyst of the formula [M(PR3R4R5)nXm]p; wherein R1 is H, alkyl, aryl or acyl; M is a Group VIII transition metal; R3, R4 and R5 are selected from the group consisting of alkyl, aryl, alkoxyl, phenoxyl and combinations thereof; X is a halide or an anion; n is 1, 2, 3 or 4; m is 1 or 2; and p is at least 1.
Owner:MALLINCKRODT INC
Pharmaceutical formulation containing ibuprofen and codeine
The invention consists of a new formulation of ibuprofen and codeine in the form of a tablet, which comprises L-leucine in a concentration ranging between 4%-15% as a lubricant, in order to prevent the formulation mixture from adhering to the punches and to other elements of the compression machine during the compression process. The new formulation additionally comprises talc (0.5%-5.0%) and silicified microcrystalline cellulose (30%-80%). The formulation is preferably arranged in the form of a core that comprises the active principles and, amongst others, the L-leucine, part of the talc and the silicified microcrystalline cellulose; this core is coated with a composition that contains a copolymer of methacrylic acid and ethyl acrylate. The tablets of the invention do not exhibit flaking problems, have an adequate hardness with a convenient attrition to allow for subsequent coating, offer disintegration values of less than 5 minutes, with dissolution values for both active principles in accordance with those specified for rapid-release tablets.
Owner:FARMASIERRA MFG
Method for the catalytic production of hydrocodone and hydromorphone
InactiveUS20050124811A1Improve scalabilityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsArylAcyl group
A method for the catalytic conversion of codeine, morphine or analogs thereof into hydrocodone, hydromorphone or analogs thereof utilizing a transition metal catalyst of the formula [M(PR3R4R5)nXm]p; wherein R1 is H, alkyl, aryl or acyl; M is a Group VIII transition metal; R3, R4 and R5 are selected from the group consisting of alkyl, aryl, alkoxyl, phenoxyl and combinations thereof; X is a halide or an anion; n is 1, 2, 3 or 4; m is 1 or 2; and p is at least 1.
Owner:MALLINCKRODT INC
Process for the production of opiates
A morphine component, e.g., a concentrate of poppy straw, is converted into codeine in high yield and high purity and in a highly controlled manner. The conversion process involves the following steps: (a) providing a solution or suspension of a morphine component in an inert solvent or a mixture of solvents; (b) methylating the resultant solution or suspension with a methylating agent in the presence of an alkaline ingredient; and (c) recovering the resultant codeine as the free base or as a salt.
Owner:ACURA PHARMA
Non-Codeine Opioid Analgesic Process And Formulations
InactiveUS20090306119A1Prevent and minimise isolationAvoid isolationBiocideNervous disorderMedicineNon prescription
The invention is directed to the relief of pain using opioid analgesics other than codeine, preferably in combination with a non-steroidal anti-inflammatory agent. Treatment is by self-administration. Compositions for the treatments are provided. Compositions of opioid analgesics other than codeine adapted to avoid the problems of potential abuse are also provided. The compositions have potential use as both prescription and non-prescription medications.
Owner:KEANE MICHAEL
Oral liquor slow releasing preparation containing codeine and chlorophenamine and its preparing method
ActiveCN1820752AGood sustained release effectReduce the number of dosesOrganic active ingredientsAntipyreticSide effectOlder people
The present invention discloses oral slow released liquid preparation containing codeine and chlorophenamine and its preparation process. The preparation has improved effectiveness, safety and compatibility, and suitable for children and old people to take. The preparation includes codeine, chlorophenamine, ion exchange resin, hydrophilic or water soluble material, coating material, plasticizer, suspension assistant and pure water. The preparation process includes the following steps: preparation of resin containing medicine, coating of resin containing medicine, and preparing suspension of resin containing medicine. Compared with available technology, the present invention has obvious slow release effect, reduced medicine taking times, less side effect, convenient taking and good taste, and is used in treating dry cough, and acute and frequent cough.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Oral liquid sustained-release preparation containing codeine and chlorphenamine and preparation method thereof
ActiveCN101474148AControl release behaviorNo grittinessOrganic active ingredientsSolution deliveryAdditive ingredientIon exchange
The invention discloses an oral liquid sustained-release preparation containing codein and chlorphenamine and a preparation method thereof, aiming at solving the technical problem of realizing sustained-release micro-capsules coating of subparticle with the particle size being less than 100 microns. The oral liquid sustained-release preparation comprises the ingredients based on the following proportion by weight: 0.1-1.0% of codein, 0.01-1.0% of chlorphenamine, 0.1-10.0% of ion exchange resin, 0.5-30.0% of suspending aid, 0.001-5.0% of sustained-release material, 0.1-5% of acidity regulators, 0.01-5% of heavy metal ion complexing agent and the rest of water. The preparation method comprises: compound codein, chlorphenamine resin particles and medium are stirred to be prepared. Compared with the prior art, the invention adopts a surface-modified method to combine the sustained-release material with ion exchange property radical and empty radical which is obtained after the medicine is absorbed by the ion exchange resin, a layer of compact sustained-release film is formed at the outer layer of medicine resin to control the releasing action of the medicine, thus realizing the sustained-release micro-capsule coating of subparticle with the particle size being less than 100 microns.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Loxoprofen-containing pharmaceutical composition
InactiveCN102740854AGood storage stabilityReduce or inhibit damageOrganic active ingredientsPowder deliveryBromineLoxoprofen
Disclosed is a pharmaceutical composition containing loxoprofen or a salt thereof and codeine or the like, which has excellent storage stability. Specifically disclosed is a pharmaceutical composition which contains at least one component selected from the group consisting of codeine, carbinoxamine or a salt thereof, clemastine or a salt thereof, chlorpheniramine or a salt thereof, diphenylpyrraline or a salt thereof, bromhexine or a salt thereof, ambroxol or a salt thereof, lysozyme or a salt thereof and dextromethorphan or a salt thereof and loxoprofen or a salt thereof in such a manner that the at least one component and loxoprofen or a salt thereof are substantially not in contact with each other.
Owner:KOWA CO LTD
Process for the production of opiates
A morphine component, e.g., a concentrate of poppy straw, is converted into codeine in high yield and high purity and in a highly controlled manner. The conversion process involves the following steps: (a) providing a solution or suspension of a morphine component in an inert solvent or a mixture of solvents; (b) methylating the resultant solution or suspension with a methylating agent in the presence of an alkaline ingredient; and (c) recovering the resultant codeine as the free base or as a salt.
Owner:ACURA PHARMA
Lozenge for delivery of dextromethorphan
InactiveUS20050238695A1Powder deliveryOrganic active ingredientsDextromethorphan+diphenhydramineEthylmorphine
The present invention provides an organoleptically pleasing lozenge containing an antitussive selected from the group consisting of dextromethorphan, diphenhydramine, caramiphen, carbapentane, ethylmorphine, noscapine, codeine, and mixtures thereof, complexed with an ion exchange resin wherein the particle size of the resin is 38 μm or less in diameter. Also provided is a process for producing the lozenge and methods of administering the lozenge.
Owner:MCNEIL PPC INC +1
Novel method for detection of codeine based on ionic iridium complex electrochemiluminescence and molecular imprinting recognition
The invention discloses a method for rapid detection of codeine based on combination of ionic iridium complex electrochemiluminescence and molecular imprinting recognition technologies. The method comprises the steps: firstly, preparing a composite solution from a cation exchange agent Nafion, multi-walled carbon nanotubes and an ionic iridium complex, taking a proper amount of the composite solution, and dropwise coating the surface of an electrode with the composite solution, to obtain a [(bpq-OCH3)2Ir(dcbpy)]<+>PF6<-> / MWCNT / Nafion electrochemiluminescence modified electrode; and then preparing a molecularly imprinted layer on the modified electrode by using an electro polymerization method. Codeine is detected based on the ionic iridium complex electrochemiluminescence and electro polymerization molecular imprinting recognition for the first time, the sensitivity is relatively high, the selectivity is good, good linear property exists at 1.0*10<-11>-5.0*10<-9> mol*L<-1>, the linear equation is I[ECL]=11.21logC+127.98 (r=0.9962), and the detection limit reaches 9.23*10<-12> mol*L<-1> (S / N=3).
Owner:JIANGNAN UNIV
Asarum total lignan extract and extraction method thereof, and application of asarum total lignan extract in preparation of drugs used for mitigating or inhibiting cough
ActiveCN104027374AWiden the field of choiceSignificant antitussive effectRespiratory disorderPlant ingredientsDiseaseCough reflex
The invention discloses an asarum total lignan extract and a preparation method and application thereof, belonging to the technical field of medicines. The preparation method comprises the following steps: extracting the root and / or tuber of asarum with a polar solvent and then carrying out concentration and drying so as to obtain an asarum total extract; and dissolving the asarum total extract with water, injecting the dissolved asarum total extract into a macroporous adsorption resin, carrying out gradient elution by using an aqueous ethanol solution, collecting eluate, recovering the solvent and carrying out drying so as to obtain the asarum total lignan extract with a content of 60 to 80 wt%, wherein yield of the asarum total lignan extract is 8.4 wt%. Through a great number of pharmacodynamic tests, it is proved for the first time that the asarum total lignan extract has substantial cough-inhibiting and cough reflex sensitivity-lowering effects which are close to the effects of codeine, and it is found out that the asarum total lignan extract has effects on inhibiting airway inflammations, reducing coughing frequencies and prolonging the incubation period of cough, exerts good prevention and treatment effects on a variety of coughs and can be used for preparation of drugs used for treating diseases related to cough. The asarum total lignan extract has the advantages of definite activity, usage of original plants as experimental materials and low cost.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV (GUANGZHOU RESPIRATORY CENT) +1
Method for preparing oxycodone
InactiveUS6469170B1Easy to synthesizeHigh degreeNervous disorderOrganic chemistry methodsAcetic acidEther
A method for the preparation of oxycodone, and salts thereof, from codeine comprising oxidation of codeine to codeinone, formation of an dienolsilyl ether congener of codeinone in strong amine base, oxidation of the dienolsilyl ether congener using peracetic acid, and hydrogenation of the resulting 14-hydroxycodeinone product.
Owner:BOEHRINGER INGELHEIM ROXANE
Inhibiting the development of tolerance to and/or dependence on an addictive substance
InactiveUSRE39300E1Alleviate withdrawal symptomsRelieve symptomsBiocideCarbohydrate active ingredientsNR1 NMDA receptorTolerability
Nontoxic substances that block the N-methyl-D-aspartate (NMDA) receptor, e.g., a morphinan such as dextromethorphan or dextrorphan, or that block a major intracellular consequence of NMDA-receptor activation, e.g., a ganglioside such as GM1 or GT1b, a phenothiazine such as trifluoperazine or a naphthalenesulfonamide such as N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide, inhibit the development of tolerance to and / or dependence on addictive drugs, e.g., narcotic analgesics such as morphine, codeine, etc.
Owner:VIRGINIA COMMONWEALTH UNIV MEDICAL COLLEGE
Opioid Salts with Release Properties and Characteristics Useful for Abuse Deterrent Drug Product Formulations
InactiveUS20150164835A1Robust and stablePotential for abuse of eitherBiocideOrganic chemistryLevorphanolDrug product
A drug substance, and drug products comprising the drug substance, wherein the drug substance is selected from the group consisting of amorphous oxymorphone pamoate; polymorphic oxymorphone pamoate; oxymorphone xinafoate; amorphous codeine pamoate; codeine xinafoate; amorphous levorphanol pamoate; polymorphic levorphanol pamoate; levorphanol xinafoate; amorphous naltrexone pamoate; polymorphic naltrexone pamoate and naltrexone xinafoate.
Owner:PISGAH LAB
Method for the catalytic production of hydrocodone and hydromorphone
A method for the catalytic conversion of codeine, morphine or analogs thereof into hydrocodone, hydromorphone or analogs thereof utilizing a transition metal complex of a tertiary phosphine halide as catalyst.
Owner:MALLINCKRODT INC
Pharmaceutical formulation containing ibuprofen and codeine
The invention consists of a new formulation of ibuprofen and codeine in the form of a tablet, which comprises L-leucine in a concentration ranging between 4%-15% as a lubricant, in order to prevent the formulation mixture from adhering to the punches and to other elements of the compression machine during the compression process. The new formulation additionally comprises talc (0.5%-5.0%) and silicified microcrystalline cellulose (30%-80%). The formulation is preferably arranged in the form of a core that comprises the active principles and, amongst others, the L-leucine, part of the talc and the silicified microcrystalline cellulose; this core is coated with a composition that contains a copolymer of methacrylic acid and ethyl acrylate. The tablets of the invention do not exhibit flaking problems, have an adequate hardness with a convenient attrition to allow for subsequent coating, offer disintegration values of less than 5 minutes, with dissolution values for both active principles in accordance with those specified for rapid-release tablets.
Owner:FARMASIERRA MFG
Column switching LC-MS/MS (Liquid Chromatography-Mass Spectrometry/Mass Spectrometry) analytical method for detecting morphine alkaloid in urine
ActiveCN101923081AEasy to handleHigh analytical sensitivityComponent separationHigh pressureLinearity
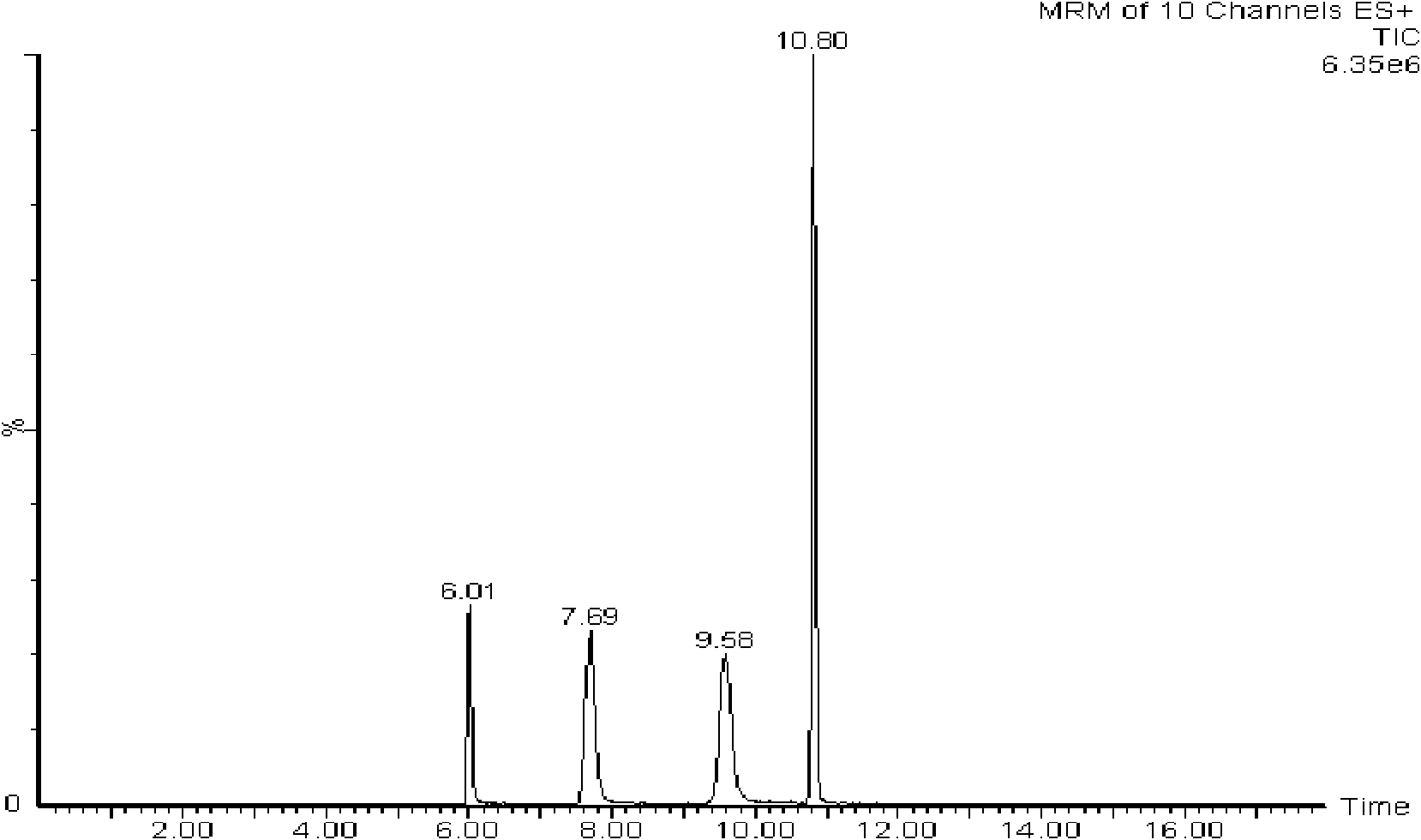
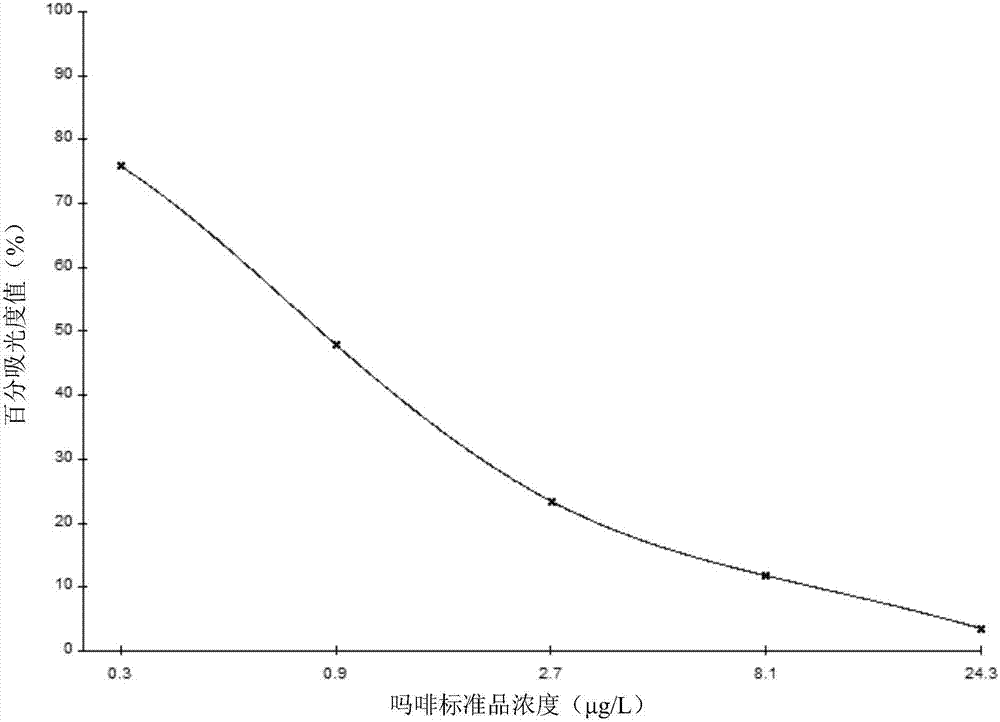
The invention discloses a column switching LC-MS / MS (Liquid Chromatography-Mass Spectrometry / Mass Spectrometry) analytical method for detecting morphine alkaloid in urine, comprising the following steps of: (1) configuring a double-pump transfusion system and a high-pressure flow path selecting valve system; (2) analyzing a sample according to the set LC-MS / MS condition; and (3) analyzing a result. The limit of detection of the method for morphine, O6-monoacetylmorphine, codeine and acetylcodeine in the urine sample is 0.3-1ng.mL-1, the related coefficient gamma is above 0.9991, and the linear relation is favorable. Meanwhile, the urine sample can be directly injected and analyzed without pretreatment so that the experimental step is simplified, and the cost is saved. The method has the advantages of rapidity, sensitivity, simplicity and reliability and can be widely applied to actual case analysis.
Owner:上海市公安局刑事侦查总队
Medicinal composition for diminishing inflammation and ease pain and preparation and use thereof
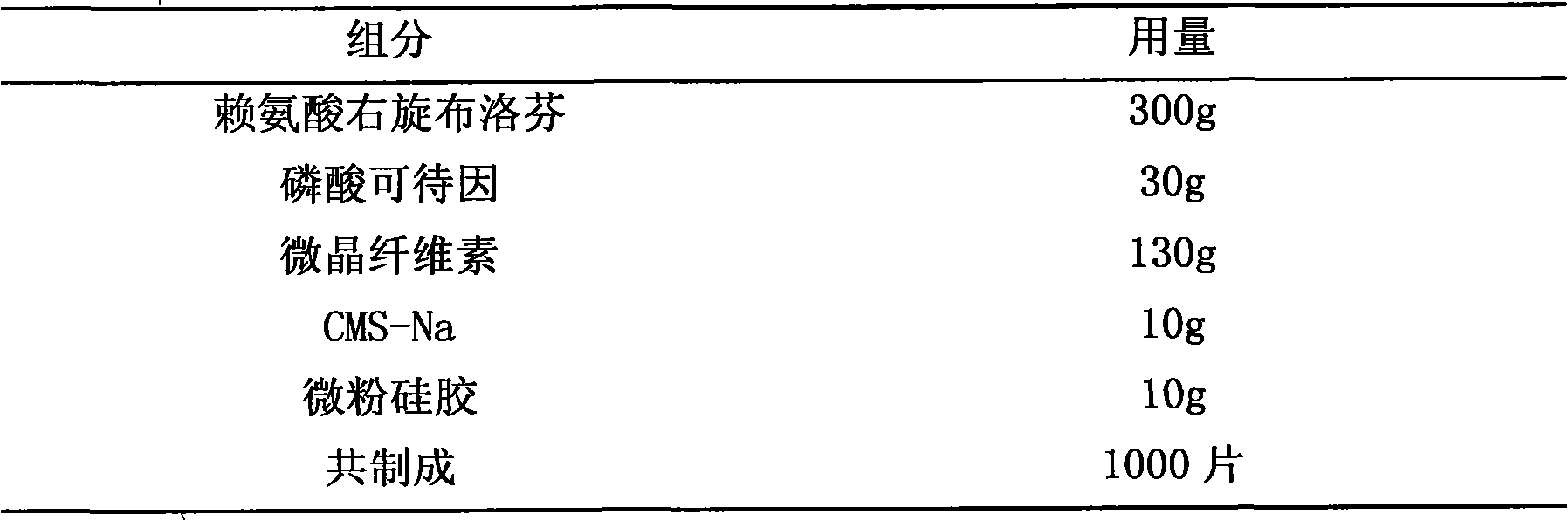
The invention provides a medicine composition for inflamination diminishing and pain easing, which is a composition formed by an active component and a medicinal carrier, and the active component is formed by one of right hand ibuprofen, ibuprofen or amino acid salt of right hand ibuprofen, right hand ketoprofen, ketoprofen or amino acid salt of right hand ketoprofen and another backbone pain easing medicine or quill. The preferred composition way: lysine right hand ibuprofen and phosphoric acid codeine, arginine right hand ibuprofen and caffeine, right hand ibuprofen and oxycodone, lysine right hand ketoprofen and hydrocodone, arginine right hand ketoprofen and diphenhydramine. The invention is prepared to various oral preparations to acquire better coordination effect.
Owner:FUKANGREN BIO PHARMA
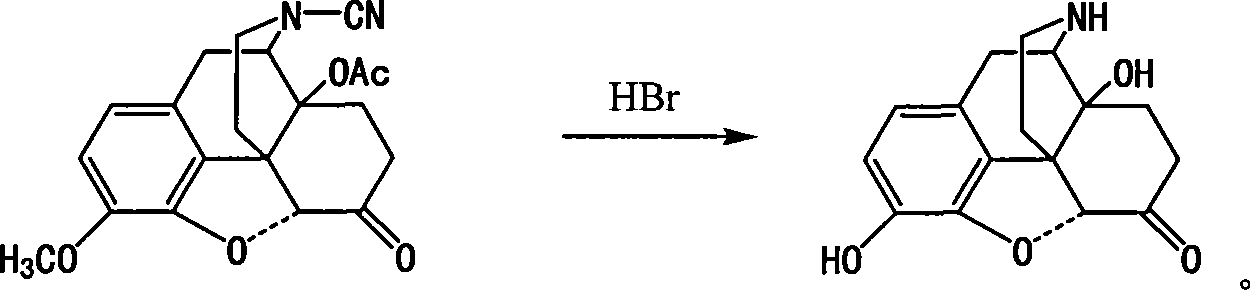
Method of preparing 14-hydroxy-7,8-dihydromorphone
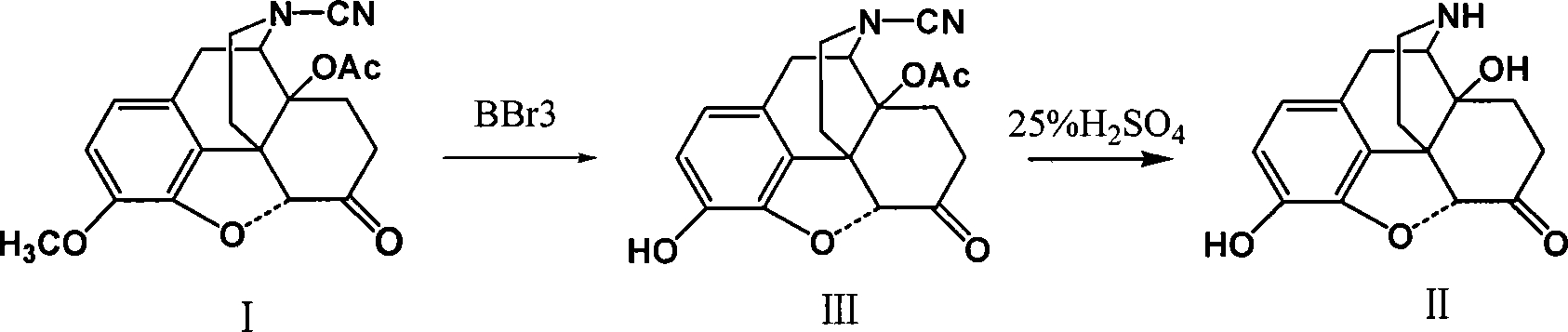
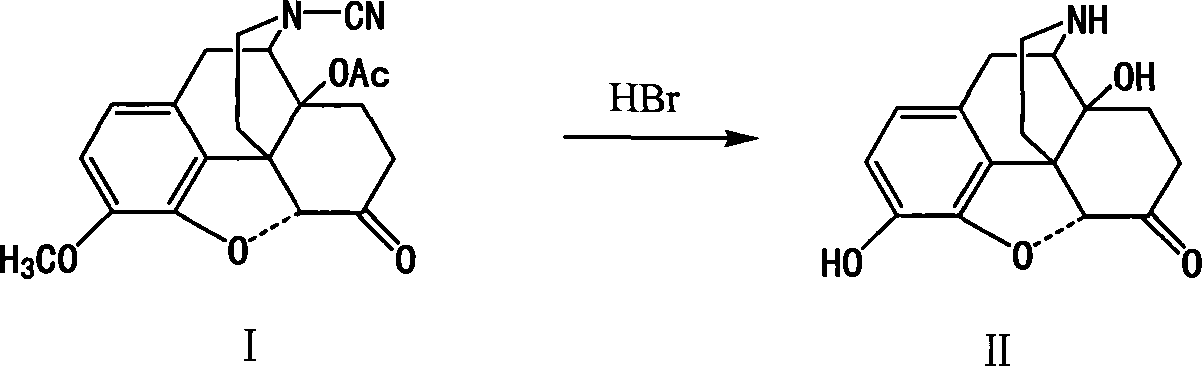
This invention discloses a method for preparing 14-hydroxide-7, 8-One drop of morphine dihydro, which takes 14-acyloxy-17- Cyano-7, 8-dihydro - codeine drop one as the raw material, hydro-bromic acid as the deoxidation methyl and hydrolyzation reaction reagent to process14-acyloxy-7, 8-dihydro-morphia ketone.
Owner:FUDAN UNIV
Pharmaceutical composition
A pharmaceutical composition comprising an analgesic or analgesic combination and a stool softener is disclosed. The analgesic is selected from morphine, meperidine, fentanyl, hydromorphone, oxymorphone, oxycodone, hydrocodone, methadone, propoxyphene, pentazocine, levorphanol, codeine, acetaminophen and combinations of these analgesics. The composition is formulated for oral administration as a liquid or solid dosage form for immediate, slow, delayed or sustained-release characteristics.
Owner:BRANDED PRODS FOR THE FUTURE
Enzyme-linked immunosorbent assay kit for detecting morphine and codeine, and applications thereof
ActiveCN107228939AQualitatively suitable forImprove accuracyMaterial analysisSite monitoringElisa kit
The present invention provides an enzyme-linked immunosorbent assay kit for detecting morphine and codeine. The enzyme-linked immunosorbent assay kit comprises an enzyme label plate coated with a morphine-conjugated antigen, a morphine monoclonal antibody, an enzyme-labeled anti-antibody, a morphine standard substance solution, a substrate coloring solution, a stopping solution, a washing liquid and a reconstituted solution. The invention further discloses a method for using the enzyme-linked immunosorbent assay kit to detect morphine and codeine, wherein the method comprises: pre-treating a sample, detecting with the kit, and analyzing the detection result. According to the present invention, the enzyme-linked immunosorbent assay kit can be used for detecting the residual morphine and the residual codeine in the hot pot bottom material and the chilli sauce, has characteristics of simple operation, low cost, high sensitivity and on-site monitoring, and is suitable for screening of a large number of samples.
Owner:BEIJING KWINBON BIOTECH +1
Combinations and methods
The present disclosure relates to novel methods of treating pain comprising administering to a human in need thereof an effective amount of (1R,5S)-(+)-1-(3,4-dichlorophenyl)-3-azabicyclo[3.1.0]hexane, in free and / or pharmaceutically acceptable salt form, substantially free of the corresponding (−) enantiomer, wherein the human is a heavy drinker and / or binge drinker and / or wherein the human has compromised liver function and / or wherein the human is a codeine non-responder. The present disclosure also relates to a method of treating pain comprising concurrently or sequentially administering to a patient in need thereof an effective amount of (a) (1R,5S)-(+)-1-(3,4-dichlorophenyl)-3-azabicyclo[3.1.0]hexane, in free and / or pharmaceutically acceptable salt form, and (b) acetaminophen. The present disclosure also relates to a method of treating pain comprising simultaneously or sequentially administering to a patient in need thereof an effective amount of (a) (1R,5S)-(+)-1-(3,4-dichlorophenyl)-3-azabicyclo[3.1.0]hexane, in free and / or pharmaceutically acceptable salt form, and (b) a non-steroidal anti-inflammatory drug.
Owner:ETHISMOS RES INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com