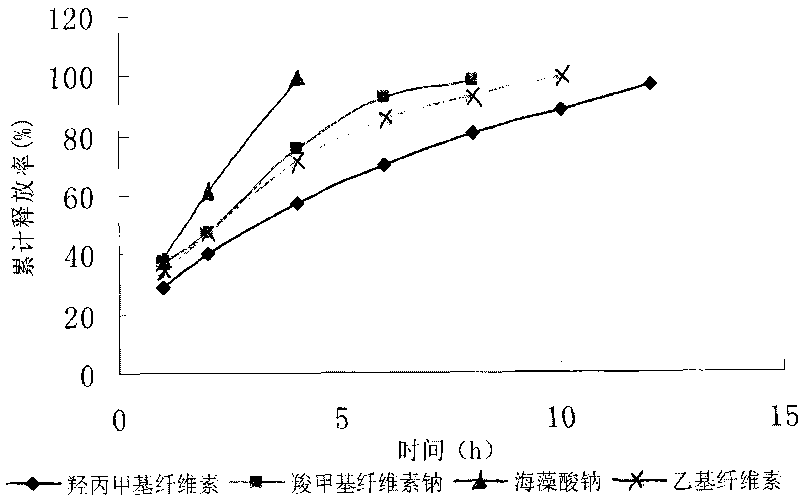
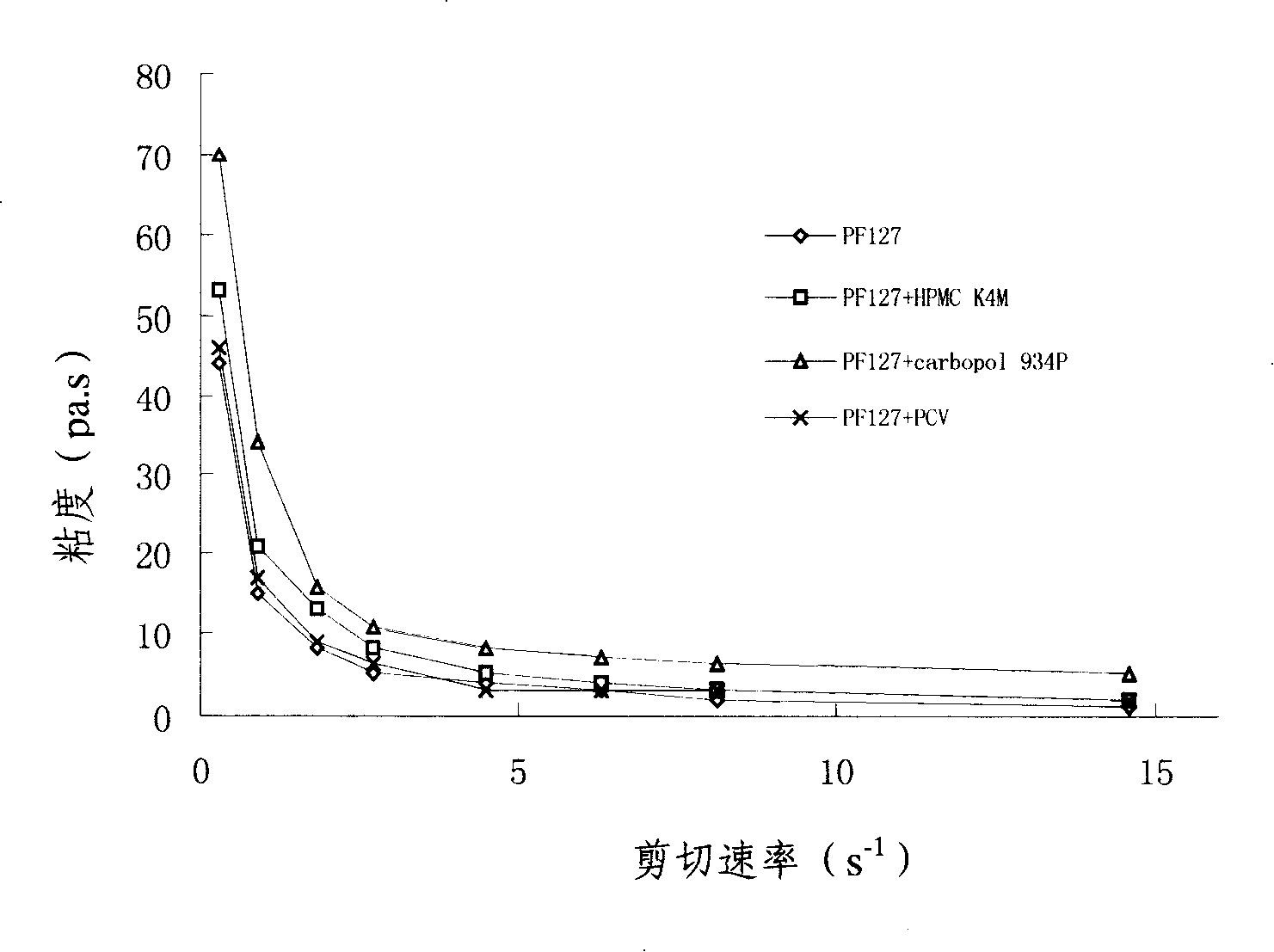
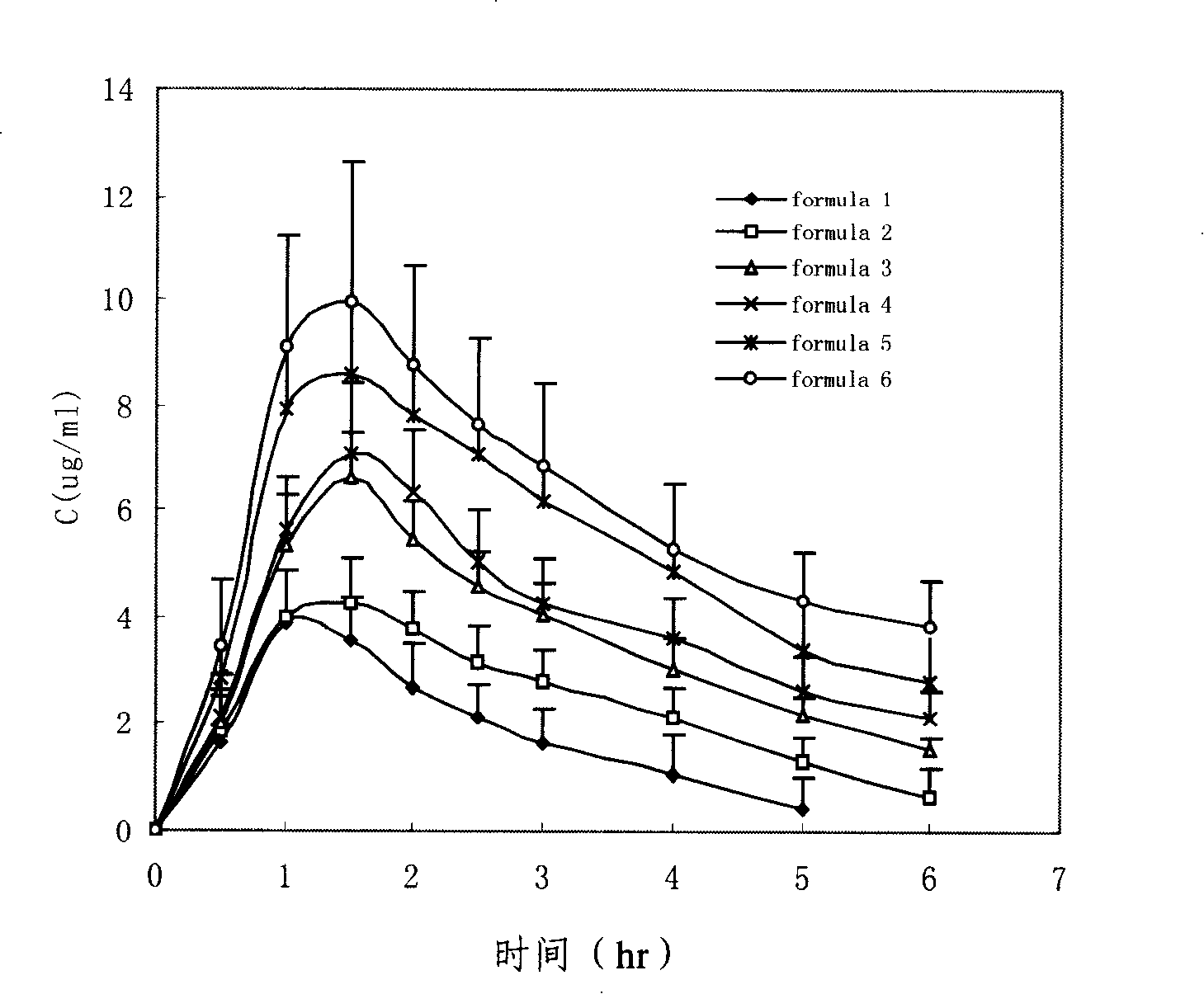
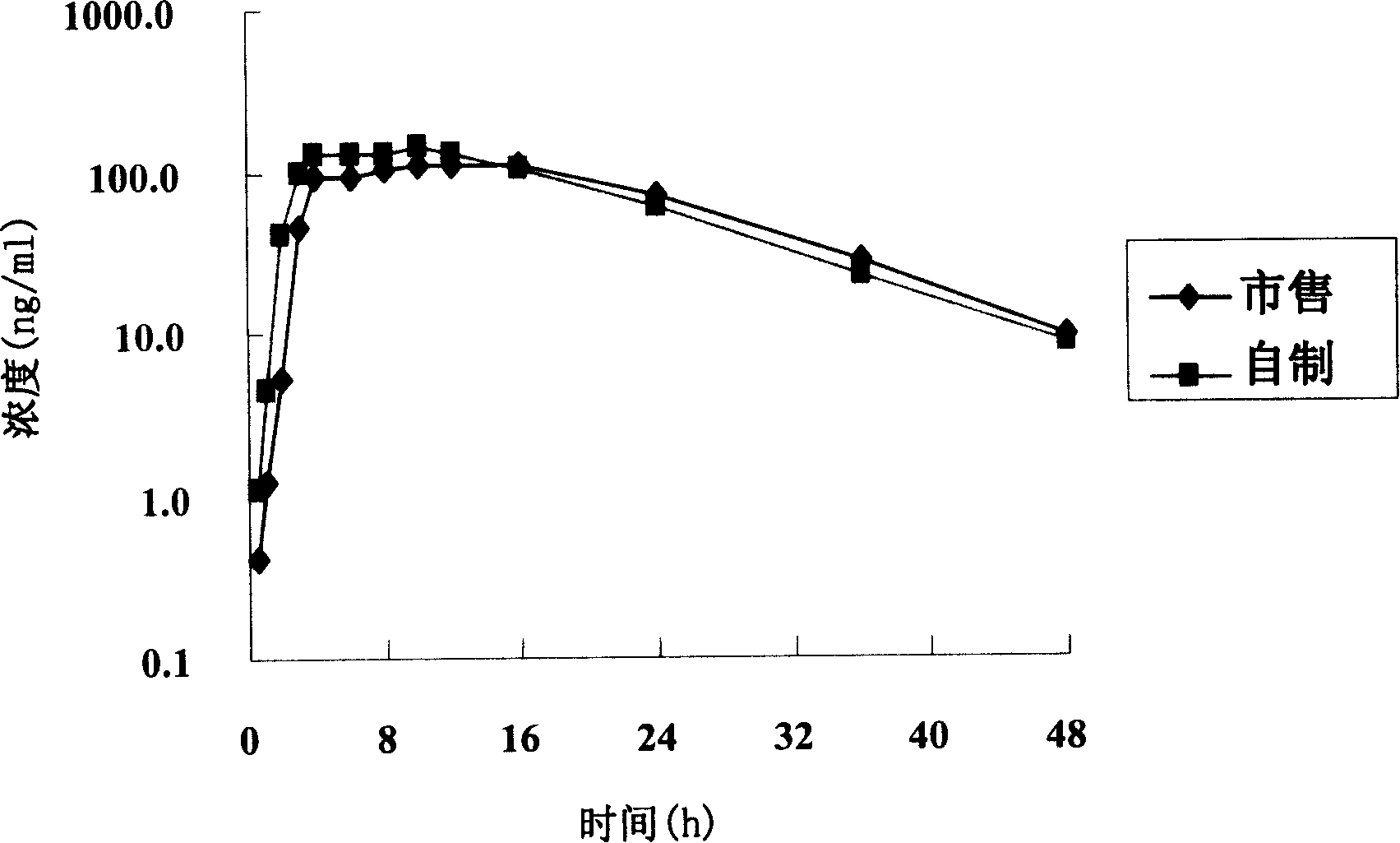
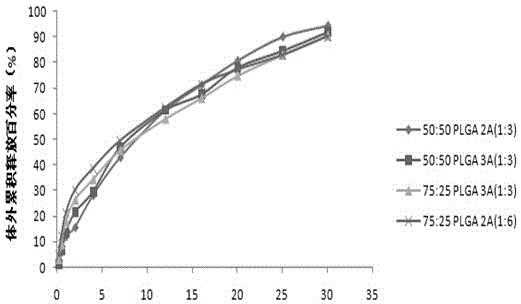
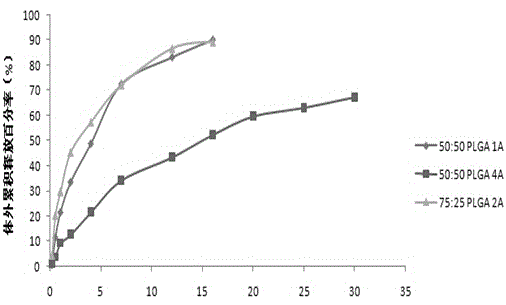
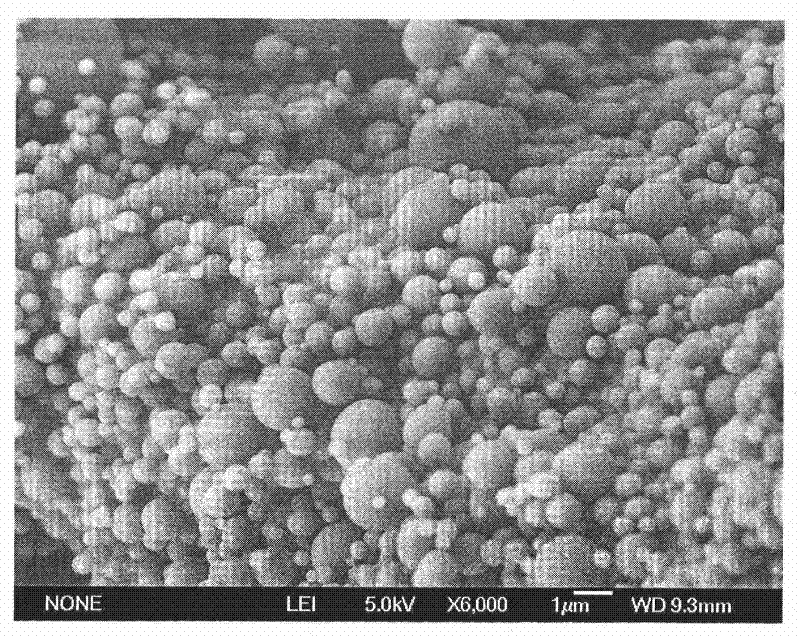
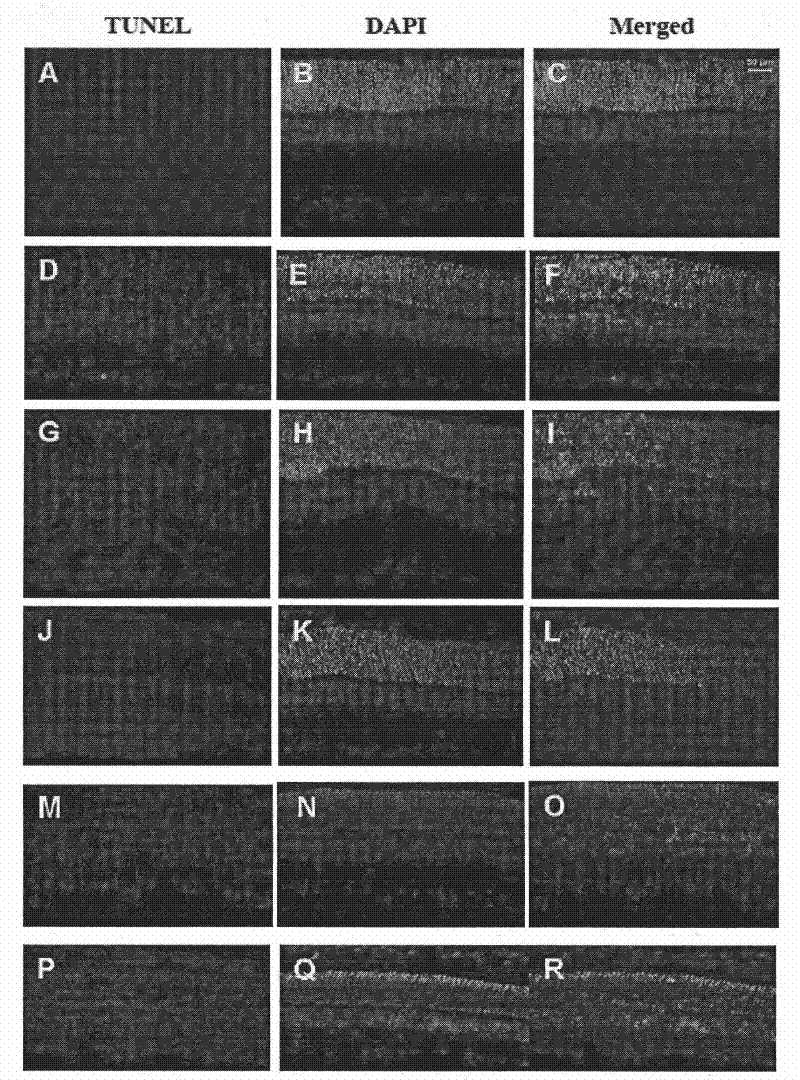
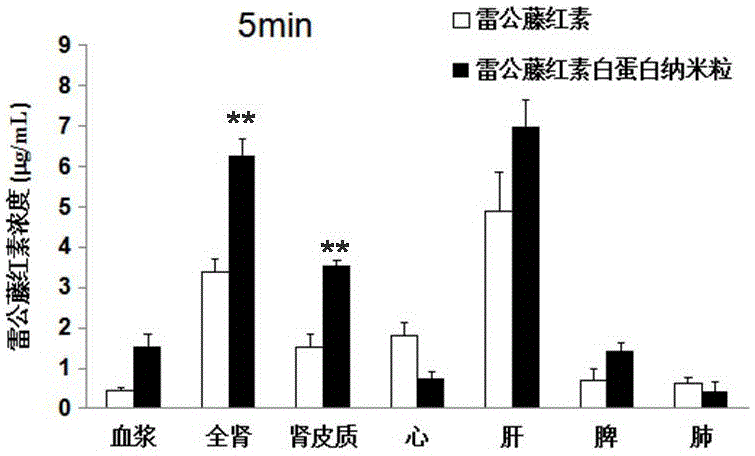
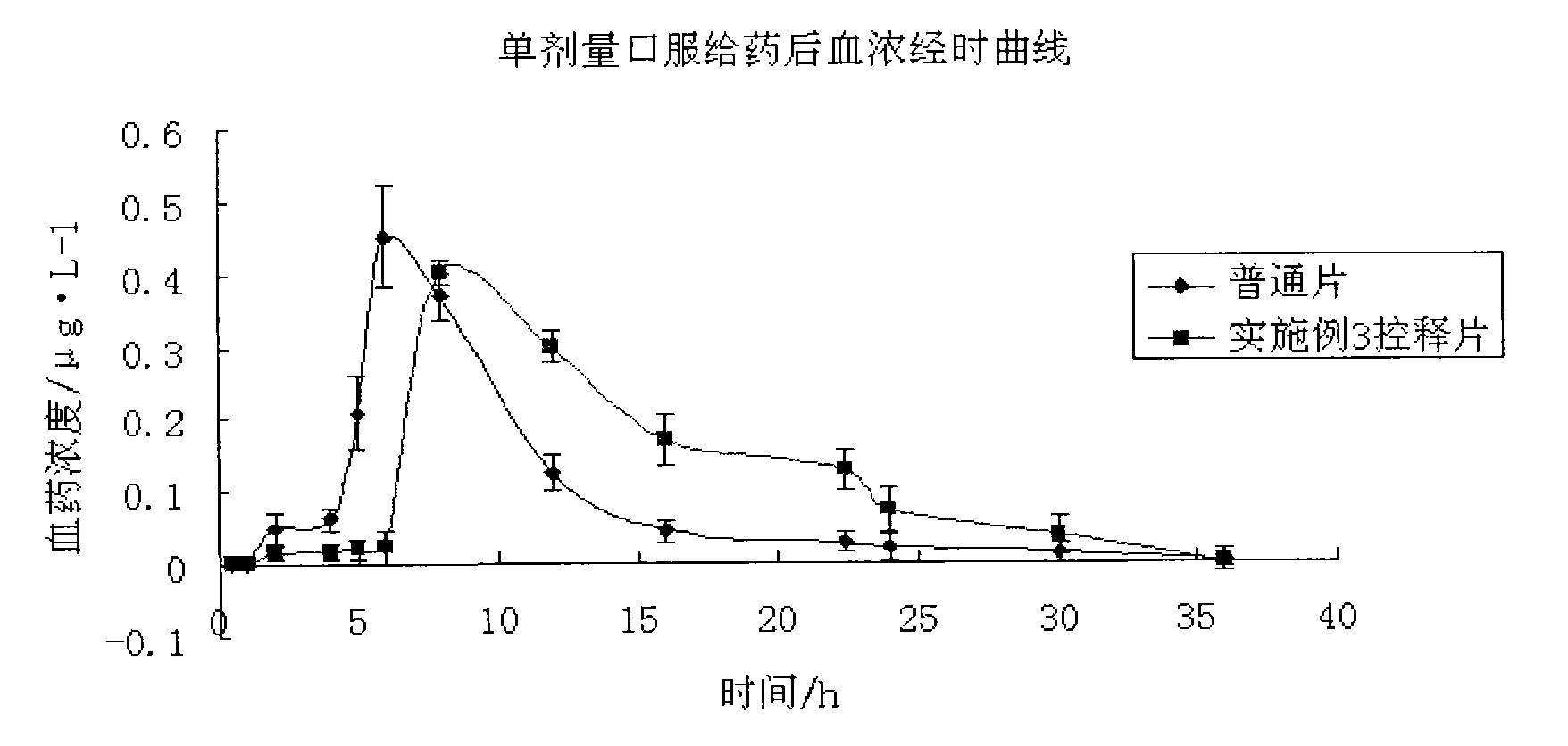
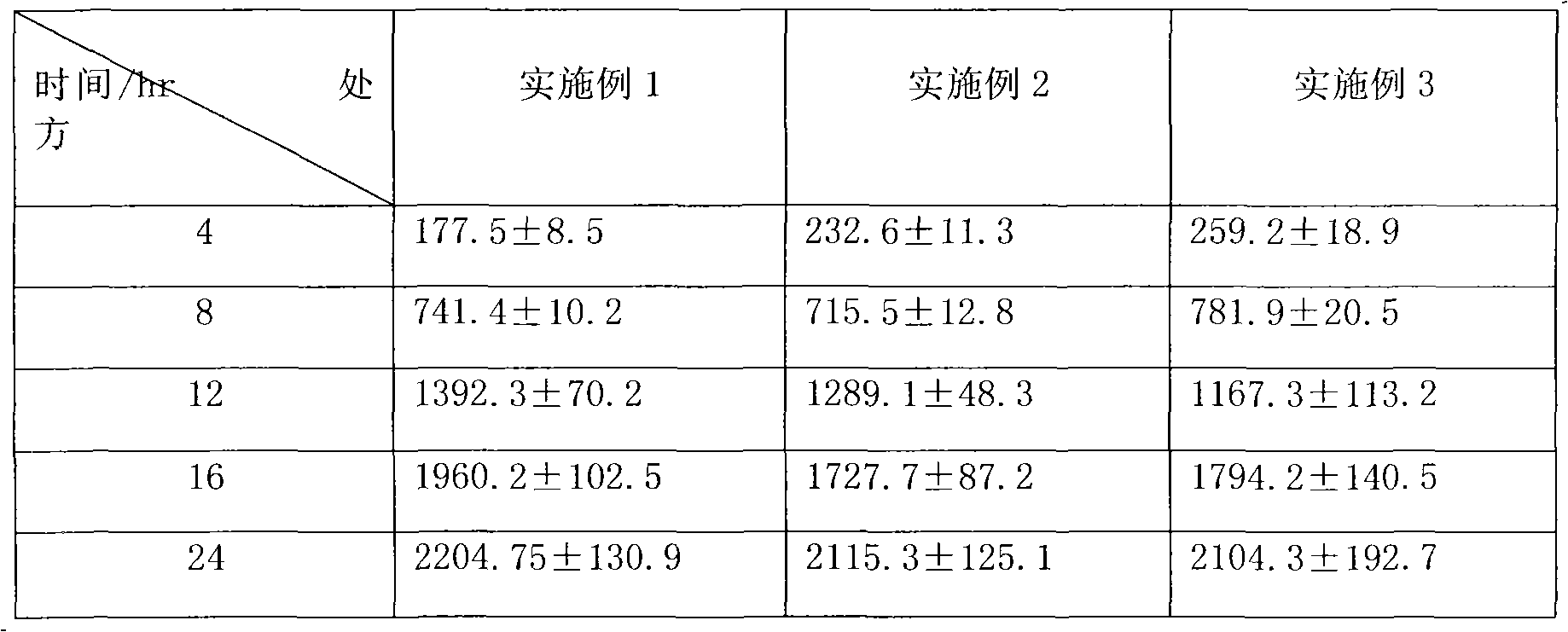
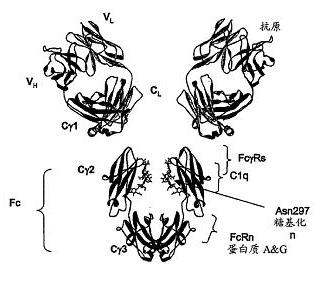
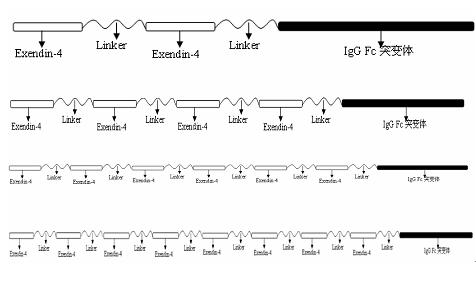
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1037results about How to "Reduce the number of doses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Components and preparation method of beta-lactam injection
ActiveCN101721366AReduce the number of dosesReduce stressAntibacterial agentsSolution deliverySterile environmentVegetable oil
The invention discloses the components and preparation method of beta-lactam injection. The beta-lactam injection comprises 5 to 20 percent of beta-lactam antibiotics, 0.05 to 5 percent of suspending agent, 0.005 to 0.3 percent of antioxygen, 0.1 to 0.2 percent of nonionic surfactant and the balance of vegetable oil or grease for injection. The injection can be used for preventing and curing animal bacterial infectious diseases and can be injected hypodermically or in muscle and be applied through breast for a few times. The preparation method provided by the invention comprises: firstly, making the antibiotics and the antioxygen into micro powder and making the suspending agent into fine powder; secondly, adding the vegetable oil or grease for injection, which is sterilized at high temperature, into the fine powder of the suspending agent, heating the mixture, uniformly mixing the mixture and keeping the mixture in a sterile environment to cool the mixture to room temperature for later use; and finally, transferring the prepared oil or grease added with the suspending agent to a colloid mill, adding medicament micro powder, the antioxygen and the non-ionic surfactant with stirring, and performing uniform mixing and sterilization to obtain the beta-lactam injection.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Bilayered osmotic pump type preparation of controlled release tablet of Breviseapini
InactiveCN1480147AGood drug release correlationReduce the number of dosesOrganic active ingredientsPharmaceutical delivery mechanismControlled Release TabletHydroxypropylmethyl cellulose
A dual-layer release-controllable osmotic breviscapine tablet for treating cerebral thrombosis, coronary heart disease and cardiovascular disease is prepared from breviscapine, release-controlling additive for medicine layer, and release-controlling additive for auxiliary layer. Its advantages are high and durable curative effect and low toxic by-effect.
Owner:SHENYANG PHARMA UNIVERSITY
Gingko leaf slow-releasing table and preparation process thereof
InactiveCN1371744AReduce the number of dosesStable blood concentrationUnknown materialsPill deliveryDiseaseAnhydrous ethanol
The preparation method of ginkgo leaf slow-released tablet mainly for curing cerebrovascular disease includes the following steps: 1. preparing gingko leaf extract; and 2. preparing slow-released tablet: using gingko leaf extract 120 portions, hydroxypropyl methyl cellulose 80-100 portions, lactose 50-80 portions and starch 30-80 portions, mixing them uniformly, adding anhydrous ethanol 0.8-1.2 portions to make granulation, then adding magnesium stearate 2-5 portions, uniformly mixing them and tabletting to obtain said invented product.
Owner:YABAO PHARMA GRP CO LTD
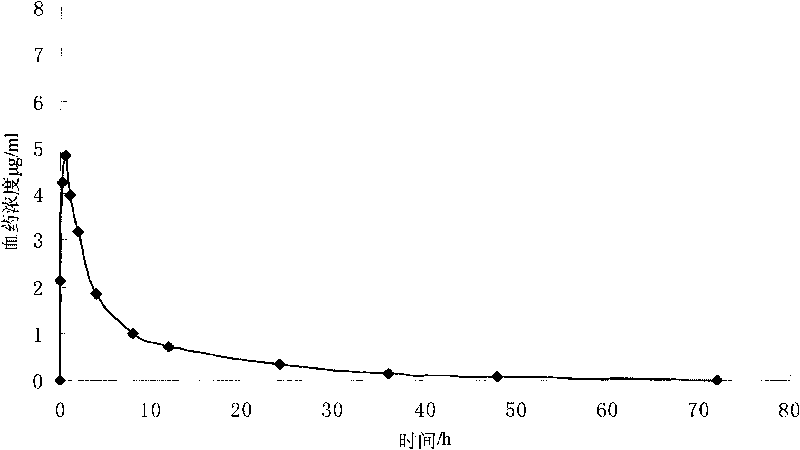
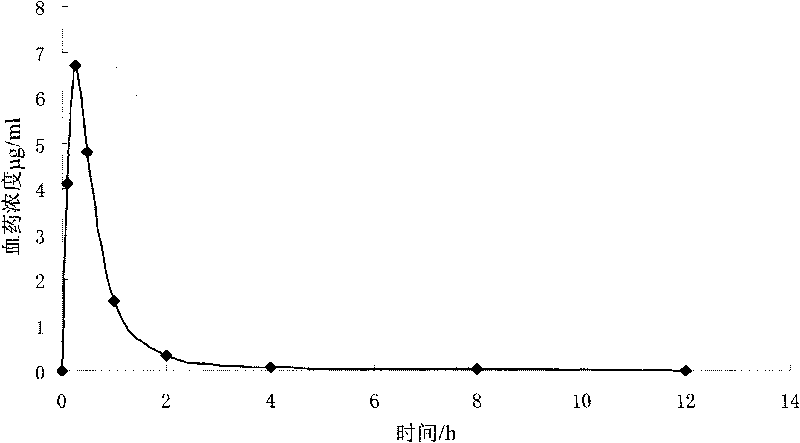
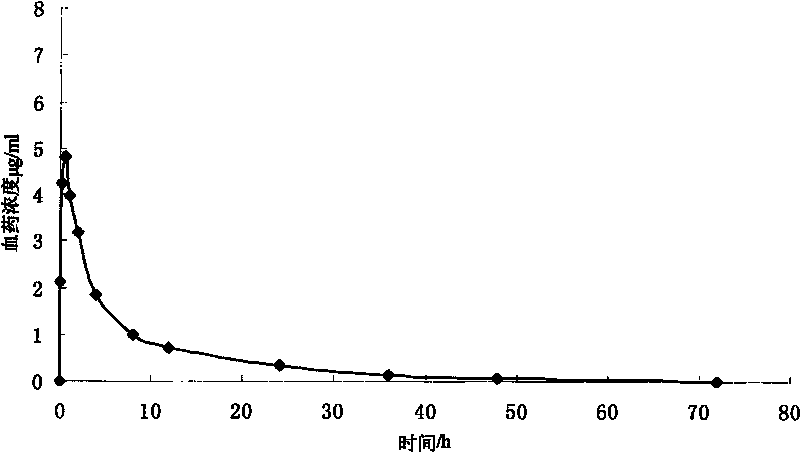
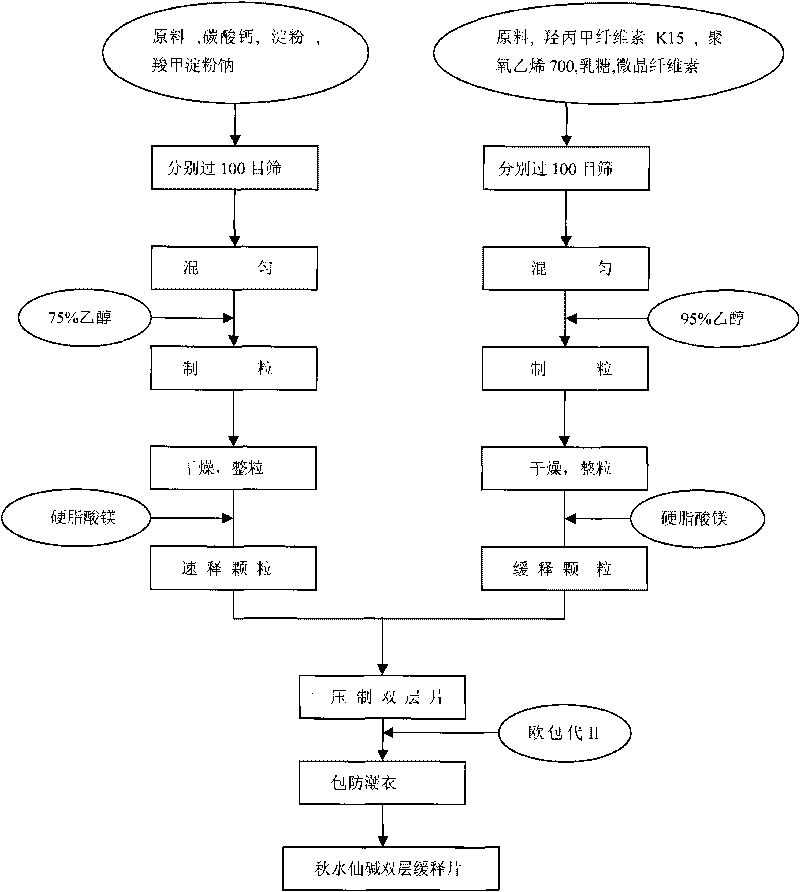
Colchicine bilayer sustained-release tablet and preparing method thereof
InactiveCN101732274AEffective plasma concentration stableStable blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletMedicine
The invention discloses a colchicine bilayer sustained-release tablet which comprises a quick release layer and a sustained-release layer, wherein the quick release layer mainly consists of colchicine, a disintegrant, a filler, a lubricant and an adhesive; the sustained-release tablet mainly consists of colchicine, a sustained-release matrix, a retarding agent, a filler and an adhesive; the weight ratio of the colchicine in the quick release layer to the colchicine in the sustained-release layer is (0.25-0.42) : 1; and the weight ratio of the quick release layer to the sustained-release layer is 1: 2 to 1: 4. The invention also discloses a method for preparing the colchicine bilayer sustained-release tablet. The colchicine bilayer sustained-release tablet releases the drugs through the quick release layer to quickly achieve the effective blood drug concentration, slowly releases the drugs through the sustained-release layer to exert and sustain the stable and uniform effective blood drug concentration, reduces the drug taking times and lightens the toxic or side function.
Owner:普尔药物科技开发(深圳)有限公司
Penciclovir ophthalmic temperature sensitivity in situ gel preparation and preparation method thereof
InactiveCN101185650AGood biocompatibilityMedication convenienceSenses disorderPharmaceutical delivery mechanismRetention timeBiocompatibility Testing
The invention provides an in situ eye gel preparation of penciclovir. The compositions and weight percentages thereof are 0.01-2.0wt% of penciclovir, 5-40wt% of poloxamer, 0.1-5wt% of tackifier high polymer base, 0.8-10wt% of osmotic regulation agent, 0.001-0.5wt% of antiseptics and the rest is distilled water. Meanwhile, the preparation method is also provided. The in situ gel preparation has good biocompatibility and glutinousness; compared with a liquid preparation, the invention can increase retention time of drug in the eyes and delay elimination, thus improving the bioavailability of the eyes and reducing the times of administration; meanwhile, the invention avoids weaknesses that operation is inconvenient during gel administration, the preparation is not easy to spread in eyes, the dosage can not be controlled accurately, etc.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Donepezils compound long-acting slow-releasing and controlled-releasing composition and preparation method thereof
InactiveCN101167697AReduce releaseImprove compliancePowder deliveryNervous disorderDonepezilProcess equipment
The invention discloses a long-acting sustained controlled release formulation of donepezil-like composition and a process for preparation. The composition is in the shape of micro-granular. According to the measurement by weight, the invention contains donepezil-like compounds 0.5-5 parts, carrier material of the sustained controlled release formulation 1-500 parts, and further contains other common excipient in pharmacy. The composition is used for anti senile dementia. The frequency of donepezil-like compounds is reduced, and the periodic time of administering medicament is prolonged, the compliance and conformability of the patient are improved, and the bioavailability and the therapeutic index of the donepezil are promoted. Meanwhile the invention discloses a plurality of processes for the preparation of long-acting sustained controlled release formulation of donepezil-like compounds. The process for preparation is flexible. Common processing equipment and commercial scale can be adopted. The production efficiency is high and the quality keeps steady. The invention can be directly or secondly used for making injection or medicament for oral administration.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Sodium alginate liver-targeted nanometer drug delivery system and preparing method thereof
InactiveCN101549158AHighly liver-targetedReduce dosagePowder deliveryOrganic active ingredientsSide effectLiver targeting
A sodium alginate liver-targeted nanometer drug delivery system uses sodium alginate as carrier material and uses glycyrrhetic acid as liver-targeted compound. The single-end-modified glycyrrhetic acid-ethylene diamine is prepared through the reacting between the carboxy group of glycyrrhetic acid and the ethylene diamine. The glycyrrhetic acid-sodium alginate is prepared through the reacting between the carboxy group of sodium alginate and the amino group of glycyrrhetic acid-ethylene diamine. Finally the sodium alginate liver-targeted drug delivery nanometer particles are prepared through crosslinking the glycyrrhetic acid-sodium alginate through calcium ions. The invention has a beneficial effect that the liver-targeted nanometer drug delivery system prepared by the invention has high liver targeting property. The hydrophilic anticancer drug and hydrophobic anticancer drug can be packaged simultaneously or a single anticancer drug is packaged. The sodium alginate liver-targeted nanometer drug delivery system of the invention has the advantages of sustained-releasing function of drug, reduced drug dosage, reduced drug taking times, reduced toxic-side effect of drug, increased drug effect, simple and practical preparing method, and excellent application prospect.
Owner:NANKAI UNIV
A explosive core composition of controlled release administer drug and controlled release preparation as well as its preparing method
ActiveCN101161242AImprove securityImprove effectivenessInorganic non-active ingredientsOsmotic deliverySolubilityMedicine
A medicine core composition used for low solubility active medicine controlling release administration is provided in the present invention, wherein, at least comprising medicine-containing layer and boosting layer, the medicine-containing layer comprises low solubility active medicine and hydrophilic polymer carrier, the boosting layer at least comprises penetration enhancing polymer, insoluble polymer and osmotic pressure promoter; a osmotic pump containing the medicine core composition is also provided in the present invention, wherein also comprising semi-transparent material film coating outer of the medicine core; the medicine core composition of the present invention can controlled speed release medicine, leading the preparation containing the medicine core to achieve aim of administration one time every day, that is about in 24 hours to release active medicine.
Owner:OCEAN STAR INT
Aripiprazole sustained-release microspheres and preparation method thereof
ActiveCN105310997AImprove complianceGood treatment effectOrganic active ingredientsNervous disorderAcetic acidMicrosphere
The invention relates to aripiprazole sustained-release microspheres and a preparation method thereof. The sustained-release microspheres include aripiprazole and a bio-degradable pharmaceutical high-molecular material PLGA, wherein the ratio of lactic acid to hydroxyacetic acid in the PLGA is 75:50-25:50. The PLGA is 25000-35000 Dolton in molecular weight. The addition weight ratio of the aripiprazole to the PLGA is 1:1-20. The aripiprazole accounts for 3.01-21.09% of total weight of the microsphere. The aripiprazole sustained-release microspheres have high drug embedding rate, is high in drug loading capacity, is smooth and round in surface and can release more than 90% of the drug in 30 days.
Owner:CHONGQING PHARMA RES INST
Loxoprofen sodium sustained release preparation
InactiveCN1628648AImprove securityImprove effectivenessPowder deliveryOrganic active ingredientsEmulsionSustained-Release Preparations
The invention provides a Loxoprofen sodium sustained release preparation, wherein the preparation comprises Loxiprofen sodium as active component and pharmaceutically acceptable carrier, the components include (1) skeleton type, membrane control type, or penetration pump type tablet or capsule, (2) microcapsule, small pill, microballoon, emulsion, and slow release preparation, (3) space or time delay type slow release preparation.
Owner:FUDAN UNIV
Preparation and applications of mesoporous silica/insulin nanoparticles modified by phenylboronic acid
InactiveCN106236734AStabilize blood sugar levelsAutomatic sensing of glucose concentration changesPeptide/protein ingredientsMetabolism disorderPhenylboronic acidNanoparticle
The invention relates to preparation and applications of mesoporous silica / insulin nanoparticles modified by phenylboronic acid, for effectively solving the problem that the traditional drug carriers release medicines unidirectionally. The technical scheme is as follows: the preparation comprises the following steps: firstly, synthesizing mesoporous silica nanoparticles, modifying amino groups on the surfaces of the mesoporous silica nanoparticles, loading medicine insulin in the mesoporous structure through physical absorption, and further modifying with phenylboronic acid and polysaccharide, thus obtaining the mesoporous silica / insulin nanoparticles modified by phenylboronic acid. The preparation and applications have the advantages that the synthesis process is simple, the prepared nanoparticles have the good biocompatibility, and the releasing valve of medicines can be repeatedly opened and closed, so that the sustained-release effect is achieved on the release of medicines; the mesoporous silica / insulin nanoparticles have a long-time circulation in the body, so that the administration times can be reduced, and thus the mesoporous silica / insulin nanoparticles belong to an innovation in diabetes treatment medicines.
Owner:ZHENGZHOU UNIV
Chitosan-based hepatic-targeted nano-particle drug delivery system and preparation method thereof
InactiveCN101642573AGood biocompatibilityFunctionalOrganic active ingredientsDigestive systemDiseaseSide effect
The invention relates to a chitosan-based hepatic-targeted nano-particle drug delivery system which takes a derivative of glycyrrhetinic acid-modified chitosan as a carrier material and is prepared byembedding an anti-cancer drug, the particle size of nano-particles is 50nm-300nm, and the drug-loading rate is 5-40%; and the carrier material is glycyrrhetinic acid-sulfate chitosan or glycyrrhetinic acid-carboxymethyl chitosan, and the embedded anti-cancer drug is doxorubicin, paclitaxel or hydroxycamptothecin. The chitosan-based hepatic-targeted nano-particle drug delivery system has the beneficial effects that the chitosan and the derivative thereof are non-toxic and have good biocompatibility and anti-tumor effect, the hepatic targeting tendency of glycyrrhetinic acid and excellent biological performance of the chitosan derivative are combined and a novel hepatic-targeted drug delivery system,is developed and prepared; and the hepatic-targeted nano-particle drug delivery system has drug sustained-release function and hepatic targeting property and can reduce the using amount of the drug and the administration times, reduce the toxicity or the side effects of the drug and improvethe efficacy by being used in the treatment of liver diseases, thereby having good application prospects.
Owner:NANKAI UNIV
Long-acting sustained release preparation for preventing or treating retinal damage, and preparation method thereof
ActiveCN102233129ARelieve painSolve the problem of long-term frequent injectionsSenses disorderPeptide/protein ingredientsMicrosphereRetinal ganglion
The invention belongs to the field of pharmaceutical preparations, and relates to a long-acting sustained release preparation for preventing or treating retinal damage, and a preparation method thereof. The long-acting sustained release preparation takes erythropoietin (EPO) as an active component, takes dextran as a protective agent for the active component, and takes poly(lactic-co-glycolic acid), polylactic acid or polycaprolactone as a coating component to prepare sustained release microspheres, wherein the erythropoietin is coated by the poly(lactic-co-glycolic acid), polylactic acid or polycaprolactone. Proven by animal experiments, the sustained release microspheres of the long-acting sustained release preparation provided by the invention have the same protective actions on the ganglionic cells of damaged retinas by single vitreous chamber injection and repeated EPO protein vitreous chamber injection, and the sustained release microspheres are capable of avoiding a series of complications caused by many times of injection and overcoming the defects of repeated intraocular injection administration and gene therapy. The long-acting sustained release preparation provided by the invention adopts intraocular local administration, can reduce the dosage and treatment cost, and can not generate adverse effects on other organs or tissues in vivo.
Owner:SHANGHAI JIAO TONG UNIV +1
Glomerulus-targeted protein nanoparticle pharmaceutical composition and application thereof
ActiveCN105944109ASmall toxicityIncrease concentrationPowder deliveryOrganic active ingredientsProtein targetSide effect
The invention provides a glomerulus-targeted protein nanoparticle pharmaceutical composition and an application thereof. The protein nanoparticle pharmaceutical composition is mainly prepared from a protein ingredient, a pharmacological active substance and a stabilizer, and the grain size of the protein nanoparticle pharmaceutical composition ranges from 10nm to 170nm. With the application of the pharmaceutical composition, passive targeted drug delivery of glomerular mesangial cells is achieved, and the aggregation concentration of a drug in a glomerulus is obviously improved or the aggregation duration of the drug in the glomerulus is prolonged, so that the curative effect of the drug is remarkably improved, and meanwhile, the toxic and side effects of the drug on a non-targeted part are greatly reduced.
Owner:SICHUAN UNIV
Transdermal plaster of rivastigmine and preparation process thereof
InactiveCN1994290AEasy to useClear curative effectOrganic non-active ingredientsEster active ingredientsTransdermal patchRivastigmine
The invention relates to a method for preparing kabalatin adhere agent for treating senile dementia, wherein said adhere agent can stably hold blood drug density and reduce feeding time, with high safety.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Trimetazidine dihydrochloride sustained-release tablet and preparation method thereof
InactiveCN102885795AThe solution is not easy to cleanSimple preparation stepsOrganic active ingredientsPharmaceutical non-active ingredientsTrimetazidine DihydrochlorideSustained Release Tablet
The invention discloses a trimetazidine dihydrochloride sustained-release tablet and a preparation method thereof. The trimetazidine dihydrochloride sustained-release tablet comprises the following constituents in percentage by mass: 5-60% of trimetazidine dihydrochloride, 10-25% of sustained-release framework material, 1-8% of adhesive, 20-80% of filler, 0.1-5% of glidant and 0.2-3% of lubricant. According to the trimetazidine dihydrochloride sustained-release tablet, medicine can be slowly and uniformly released by adding the sustained-release framework material, so as to achieve regulation and control for a release speed, reduce the peak-valley ratio of the medicine, improve the efficacy, reduce the toxic and side effects of the medicine, reduce daily medicine-taking times and enhance the compliance of the patient on the medicine. The preparation method of the trimetazidine dihydrochloride sustained-release tablet disclosed by the invention is simple in process, does not need specially process production equipment, and is low in cost and good for batch amplification and industrialized production for products.
Owner:AC PHARMA CO LTD
Nifedipine osmotic pump controlled release tablet and preparation method thereof
InactiveCN102138912AMaintain blood levelsGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismNifedipineControlled Release Tablet
The invention provides a nifedipine osmotic pump controlled release tablet comprising a drug-containing layer tablet core, a booster layer tablet core, a coating membrane and a single drug-release pore on the surface of the controlled release tablet at one side of the drug-containing layer tablet core. The nifedipine osmotic pump controlled release tablet provided by the invention has stable medicament release velocity, basically realizes zero drug release within 0-20h and basically fully releases drug; therefore, the dosing number of a patient is reduced, and more stable blood drug concentration can be realized after a patient takes the drug. The nifedipine osmotic pump controlled release tablet provided by the invention is a safe, effective, stable, controllable and conveniently-applied medicament new preparation for clinically treating hypertension.
Owner:CHINA PHARM UNIV
Fusion protein for treating diabetes and preparation method for fusion protein
InactiveCN102558362ACan stimulate secretionReduce physical burdenPeptide/protein ingredientsMetabolism disorderPancreatic hormoneChinese hamster
The invention relates to the technical field of medicines for treating diabetes, in particular to a fusion protein for treating diabetes and a preparation method for the fusion protein. The fusion protein is recombined by 2 to 8 Exendin-4-Linkers and a human IgGFc mutant; Linker is a flexible peptide fragment; and the human IgGFc mutant is an IgG1Fc, IgG2Fc or IgG4Fc mutant. The invention also discloses the preparation method for the fusion protein. The recombined fusion protein obtained by the steps of performing high-level expression in Chinese hamster ovary (CHO) cells, affiliating, and performing ion exchange and molecular sieve chromatography has the bioactivities of stimulating the secretion of insulin and inhibiting glucagon generated after dinner from being released which are possessed by Exendin-4, also has the characteristics of prolonging the half-life period of the Exendin-4 in serum, is used for treating type I and type II diabetes, can effectively reduce the psychological and physiological burden of patients, is safe in use and high in practicability, and can be massively produced and sold.
Owner:DONGGUAN JINLANG BIOTECH
Emulsifiable grains of emamectin benzoate and preparation method thereof
ActiveCN101731207APromote absorptionImprove quicknessBiocideAnimal repellantsEmamectin benzoateChemistry
The invention provides emulsifiable grains of emamectin benzoate and a preparation method thereof. Based on the total weight of the emulsifiable grains, the emulsifiable grains comprise 0.1 to 60 weight percent of emamectin benzoate, 0.1 to 20 weight percent of dispersant, 0.1 to 10 weight percent of wetting agent, 0.1 to 20 weight percent of emulsifying agent, 0.1 to 40 weight percent of adsorbent and 5 to 95 weight percent of filler. In the invention, the emamectin benzoate are made into emulsifiable grains, so the drawbacks of large dosage, low quick-action performance, short medicament effect duration and environmental pollution of the prior preparations of the emamectin benzoate are overcome, the dosage of the emamectin benzoate is reduced obviously, the quick-action performance and the medicament effect duration of the emamectin benzoate are improved, and environmental pollution is avoided.
Owner:北京富力特农业科技有限责任公司
Preparation method of ceftiofur long-acting injector
InactiveCN101416968AReduce stress responseGood curative effectAntibacterial agentsOrganic active ingredientsHalf-lifeTherapeutic effect
A ceftiofur long acting injection consists of the following substances: 2.5g to 5.0g of ceftiofur crude drugs, 0.5g to 3.0g of span 80, 0.1g to 0.3g of Vitamin E, 0.2g to 0.5g of aluminium stearate, 0.25g to 0.5g of chlorobutanol and soybean oil. The preparation method of the ceftiofur long acting injection comprises the following steps: (1) smashing the ceftiofur crude drugs till the particle size of the ceftiofur crude drugs is 5 Mum; (2) adding the span 80 into a mortar for grinding till the span 80 becomes even; (3) then, adding the aluminium stearate, the Vitamin E and the chlorobutanol into the mortar together; and (4) moving the mixture into a measuring vessel and adding the soybean oil into the measuring vessel till the total volume of the substances in the measuring vessel reaches 100ml. The ceftiofur long acting injection has the advantages of obvious therapeutic effect, long half-life period, less adverse reaction, reducing medication times, saving manual labor and time and the like.
Owner:PU LIKE BIO ENG
Tripterine nano structure lipid carrier and preparation method and application thereof
InactiveCN102225205AReduce systemic side effectsImprove bioavailabilityOrganic active ingredientsAntipyreticNano structuringTreatment effect
The invention relates to the field of Chinese medicine preparation, in particular to a method for preparing tripterine nano structure lipid carrier containing traditional Chinese medicine monomer and application of the tripterine nano structure lipid carrier in preparation of transdermal drugs used for treating psoriasis, rheumatoid arthritis, skin cancer and breast cancer. The tripterine nano structure lipid carrier is characterized by comprising the following components in parts by weight: 1 part of tripterine, 5-100 parts of mixed lipid, 0.5-10 parts of phospholipid, 0.1-15 parts of poloxamer-188 and 0.5-10 parts of vitamin E and tocopherol polyethylene glycol succinate, wherein the mixed lipid is composed of solid lipid monoglycerine and liquid lipid octylic acid / caprin according to the weight ratio of 1: 0.1-10: 1. Tripterine is prepared into the nano structure lipid carrier, the tripterine nano structure lipid carrier in a semi-solid or liquid preparation form is applied in a transdermal way, bioavailability of the tripterine can be improved, toxic response of tripterine can be reduced, and the nano structure lipid carrier provided by the invention has great clinical application value in the improvement of the treatment effect of tripterine.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Levetiracetam slow release pellet capsule preparation and preparation method thereof
InactiveCN101647789AImprove liquidityReduce or eliminate irritationNervous disorderPharmaceutical delivery mechanismMass compositionSide effect
The invention relates to a levetiracetam slow release pellet capsule preparation and a preparation method thereof. The levetiracetam slow release pellet capsule preparation comprises the following components in percentage by mass: 50-70 percent of levetiracetam, 15-30 percent of blank pellet, 5-10 percent of hydroxypropylmethyl cellulose or polyvidone, 7-15 percent of ethylcellulose or acrylic resin, 1-8 percent of talc powder and 0.5-2 percent of cataloid. The preparation method comprises the following steps: coating levetiracetam fine powder on the blank pellet by a binding agent to be madeinto a medicine-contained pellet; coating an isolating layer on the medicine-contained pellet; coating a slow release coating film on the medicine-contained pellet coated with the isolating layer to prepare a slow release pellet; and mixing the slow release pellet, the talc powder and the cataloid and filling the mixture into a capsule. The levetiracetam is made into the slow release capsule preparation by a pellet and slow release technology, and the slow release preparation can stabilize blood medicine concentration, reduce the generating frequency and degree of the side effect and fundamentally solve the problems of great influence on a tablet by gastric pyloric sphincter and big differences of gastric emptying individuals.
Owner:天津药物研究院药业有限责任公司
Dexibuprofen slow release pellet and preparation method thereof
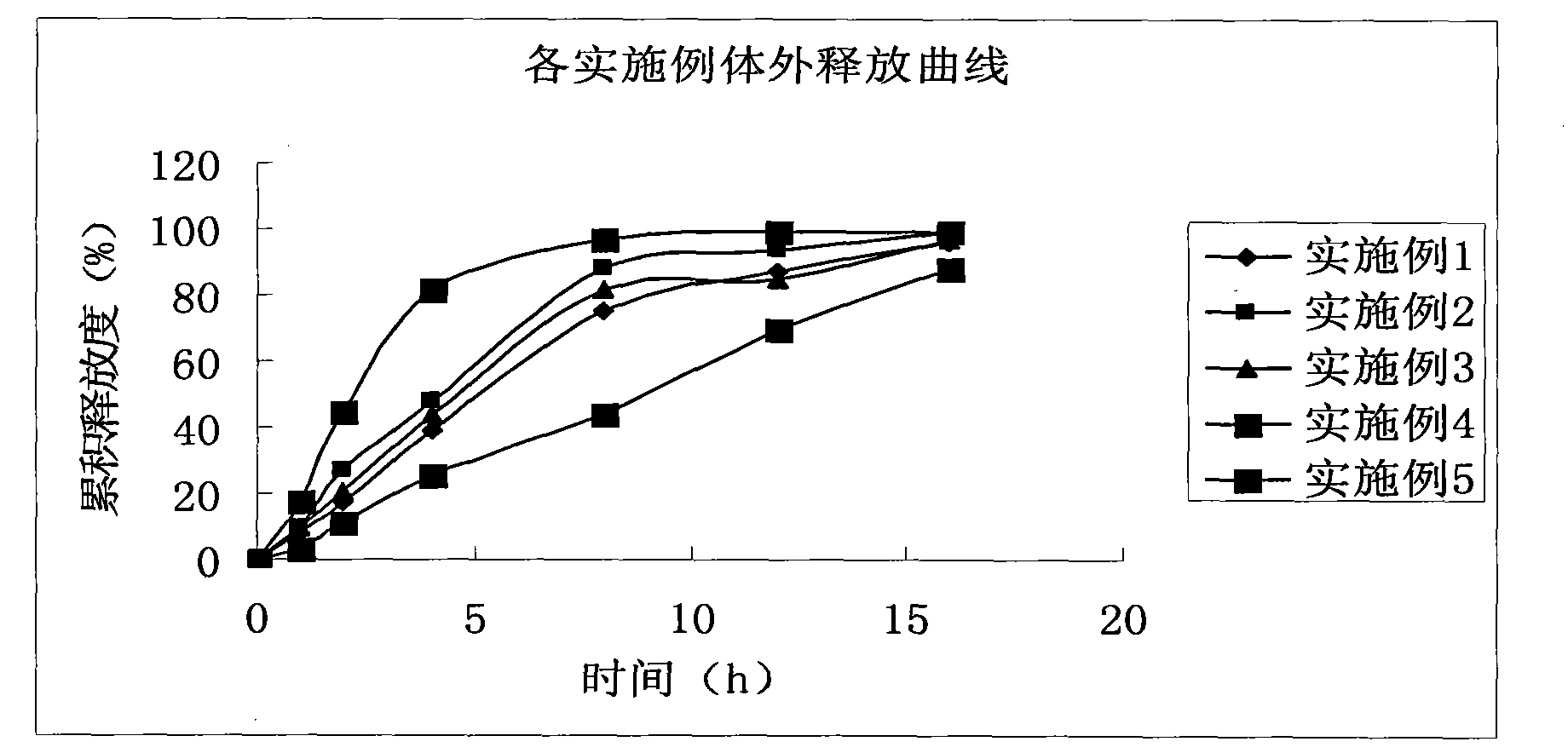
ActiveCN104739773AReduce the number of dosesImprove complianceOrganic active ingredientsAntipyreticAdhesiveIn vitro dissolution
The invention discloses a dexibuprofen slow release pellet and a preparation method thereof, and belongs to the field of medicinal preparations. The dexibuprofen slow release pellet comprises 50-80% of dexibuprofen, 0-5% of a slow release material, 0-2.5% of an adhesive, 8.75-47.5% of a filler, and 1.25-6.25% of a disintegrating agent, and all above percentages are based on the total weight of the pellet. The dexibuprofen slow release pellet has the advantages of good stability, stable quality, simple device and process, strong operationality, and suitableness for industrial large scale production. In vitro dissolution test shows that the accumulative release rate within 1h is 10-35%, the accumulative release rate within 2h is 25-55%, the accumulative release rate within 4h is 50-80%, and the accumulative release rate within 7h is 75% or above, so the dexibuprofen slow release pellet has a good release curve.
Owner:LUNAN BETTER PHARMA
Injection-use recombinant human Endostatin porous sustained-release microsphere and preparation method thereof
ActiveCN101536984AReduce the number of dosesImprove compliancePeptide/protein ingredientsPharmaceutical non-active ingredientsChemistryMicrosphere
The invention relates to a recombinant human Endostatin porous sustained-release microsphere for injection, and a preparation method thereof, in particular to an injection-use porous sustained-release microsphere which takes a biodegradable polymer of lactic acid and hydroxyacetic acid as a matrix and comprises recombinant human Endostatin and a pore-forming agent, and a preparation method thereof.
Owner:JIANGSU SIMCERE PHARMA
Degradable esophagus tubular intervention support and preparation method thereof
InactiveCN101513367AReduce first pass effectPromote absorptionStentsProsthesisX-rayBiomedical engineering
The invention relates to a degradable esophagus tubular intervention support which is characterized in that the degradable esophagus tubular intervention support is a tubular intervention support with various patterns obtained by hollowing out outer wall of a hollow tube made of mixture of degradable macromolecular material and medicament or X-ray developer, external diameter of the hollow tube is 1-100 millimeters, thickness of the tube wall is 0.05-10 millimeters, and medicament film or X-ray developer is coated on the tube wall of the degradable tubular intervention support.
Owner:中国人民解放军总医院第二附属医院
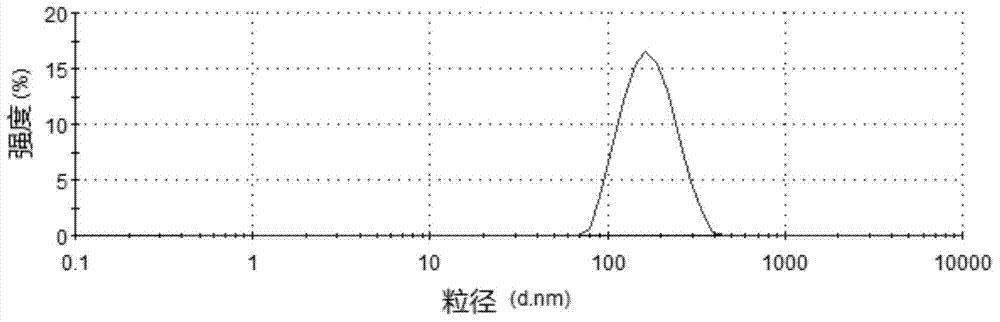
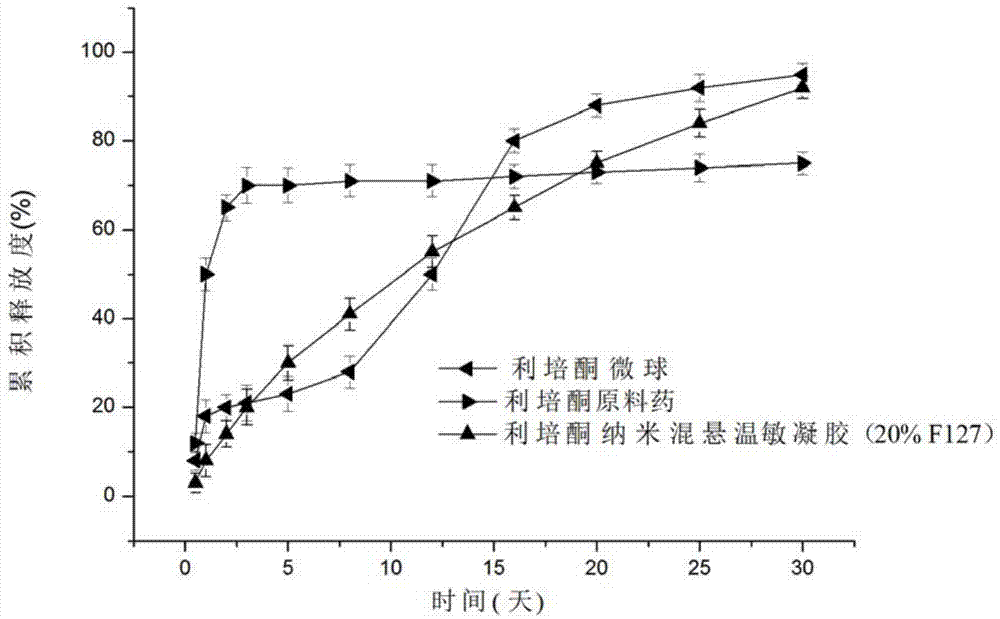
Risperidone nano-suspension temperature sensitive gel and its preparation method
InactiveCN104288091AImprove complianceIncrease dissolution rateOrganic active ingredientsNervous disorderWater basedPatient compliance
The invention discloses a risperidone nano-suspension temperature sensitive gel and its preparation method. Each 100ml of the nanosuspension temperature sensitive gel contains 0.1-10g of risperidone, 0.1-5g of a stabilizer, 1-50g of a temperature sensitive gel material, 0-5g of an additive, and the balance of a water-based solvent. The risperidone nano-suspension temperature sensitive gel is a temperature sensitive controlled-release in situ gel. The above dosage form makes risperidone highly dispersed, so the gel has the advantages of good drug load and good stability; after the gel is administrated in a liquid form, phase transition occurs in applied sites, and the liquid becomes non-chemically-crosslinked semisolid gel, so the gel has good histocompatibility and improves the drug dissolution rate; and the administrated gel has a long retention time in the applied sites as a drug reservoir, so the gel has a slow release effect, prolongs the drug release time, improves the drug bioavailability, reduces the administration frequency and improves the drug effect and the patient compliance.
Owner:HENAN UNIV OF SCI & TECH
LID-PEG-PLGA controlled-release nano microsphere and preparation method thereof
InactiveCN101961316AImprove hydrophobicityHigh encapsulation efficiencyPowder deliveryOrganic active ingredientsWater bathsMatrix solution
The invention discloses an LID-PEG-PLGA controlled-release nano microsphere and a preparation method thereof. The microsphere is the controlled-release microsphere which contains medicinal lidocaine and a degradable carrier. The degradable carrier contains polylactic-glycolic acid and PEG-2000. The mass percentage of the lidocaine in the controlled-release microsphere is 30 to 35 percent. The preparation method comprises the following steps of: preparing the carrier into matrix solution; dispersing the lidocaine into the matrix solution and preparing the lidocaine into an oil phase; mixing the oil phase and the aqueous solution of polyvinyl alcohol, and performing ultrasonic emulsification on the mixture under a water bath condition to obtain W / O-type protogala; mixing the W / O-type protogala and the aqueous solution of polyvinyl alcohol again, and further emulsifying the mixture into W / O / W-type complex emulsion; volatilizing the emulsion by reducing pressure at the normal temperature to obtain cured lidocaine-carried nano microsphere; and scattering, blending, packaging, freezing, sterilizing and the like. The medicament loading rate of the controlled-release nano microsphere can be up to 15 to 22 percent; the entrapment rate can be up to 68 to 78 percent; and the half-life period can be prolonged to 3 to 4 days. Therefore, the microsphere has relatively good effect of burst in the first day after the microsphere is taken and good effect of slow release in later days.
Owner:ARMY MEDICAL UNIV
Liposome nanometer carrier situ gel preparation used for eye epidermal growth factor
InactiveCN101057966AGood physical and chemical stabilityReduce exposureSenses disorderPeptide/protein ingredientsLipid formationGel preparation
The invention relates to a liposome nano carrier in-situ gelling preparation for eyes containing epidermal growth factor, which is prepared from epidermal growth factor, lipid material, emulsifying agent, thickening agent, isotonic conditioning agent, bacteria inhibitor, pH regulator and purified water, wherein non-irritating phosphatide is employed as the emulsifying agent, macromolecular material having ion and / or pH responsive characteristics is used as the thickening agent.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
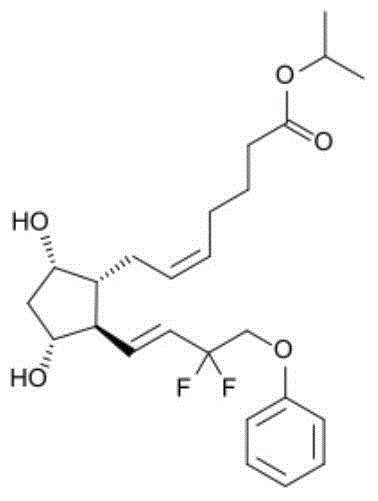
Eye drops containing tafluprost and preparation method thereof
ActiveCN104622798AGood bioadhesionImprove complianceOrganic active ingredientsSenses disorderTafluprostEnhanced bioavailability
The invention provides eye drops containing tafluprost. The eye drops comprise tafluprost and a hydrophilic polymer, wherein the hydrophilic polymer is selected from polycarbophil or carbomer and the content of the hydrophilic polymer is 0.4-1.0%. Medicament storage formed by the eye drops plays a role of slowly releasing medicaments, prevents medicaments from being rapidly eliminated, and improves the bioavailability. The preparation method of the eye drops is simple and feasible and suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Preparation method of test sample of musk Baoxinwan slow release gel for pharmacological research
ActiveCN105726464AReduce the number of dosesIncrease drug concentrationAmphibian material medical ingredientsHydroxy compound active ingredientsSolventDrug
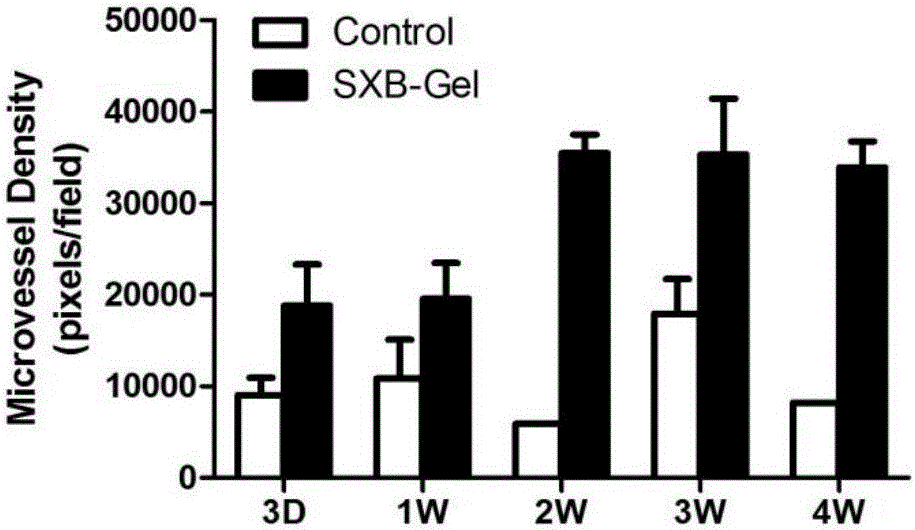
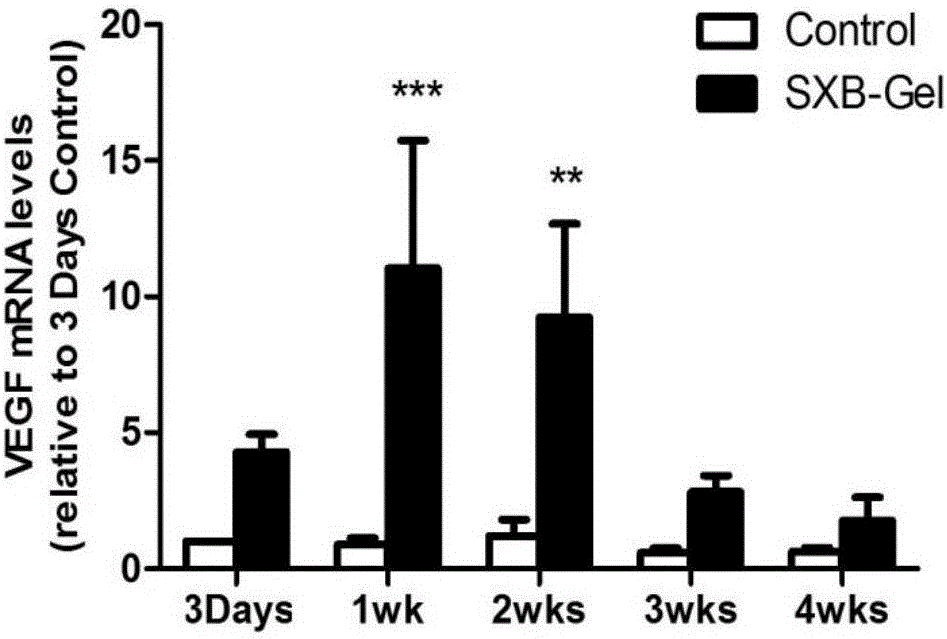
The invention belongs to the technical field of medicine and relates to a preparation method of a test sample of a musk Baoxinwan slow release gel for pharmacological research. The invention also provides a musk Baoxinwan slow release gel, prepared from the following components in percentage by weight: 0.1-30% of musk Baoxinwan powder, 0.9-30% of gel matrix agent and 40-99% of solvent. The invention further provides a preparation method of the musk Baoxinwan slow release gel and an application thereof in preparing a medicine for promoting angiogenesis. The invention also further provides an application of the musk Baoxinwan slow release gel in preparing a medicine for carrying out research on the mechanism of musk Baoxinwan in treating angiogenesis on in vitro animal models. The preparation method of the test sample of the musk Baoxinwan slow release gel for pharmacological research provided by the invention can ensure stable and lasting release of medicine, enables the medicine to play an effect on promoting therapeutic angiogenesis to the utmost extent, and provides a new dosage form for pharmacological research on the musk Baoxinwan.
Owner:HEHUANG PHARMA SHANGHAI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com