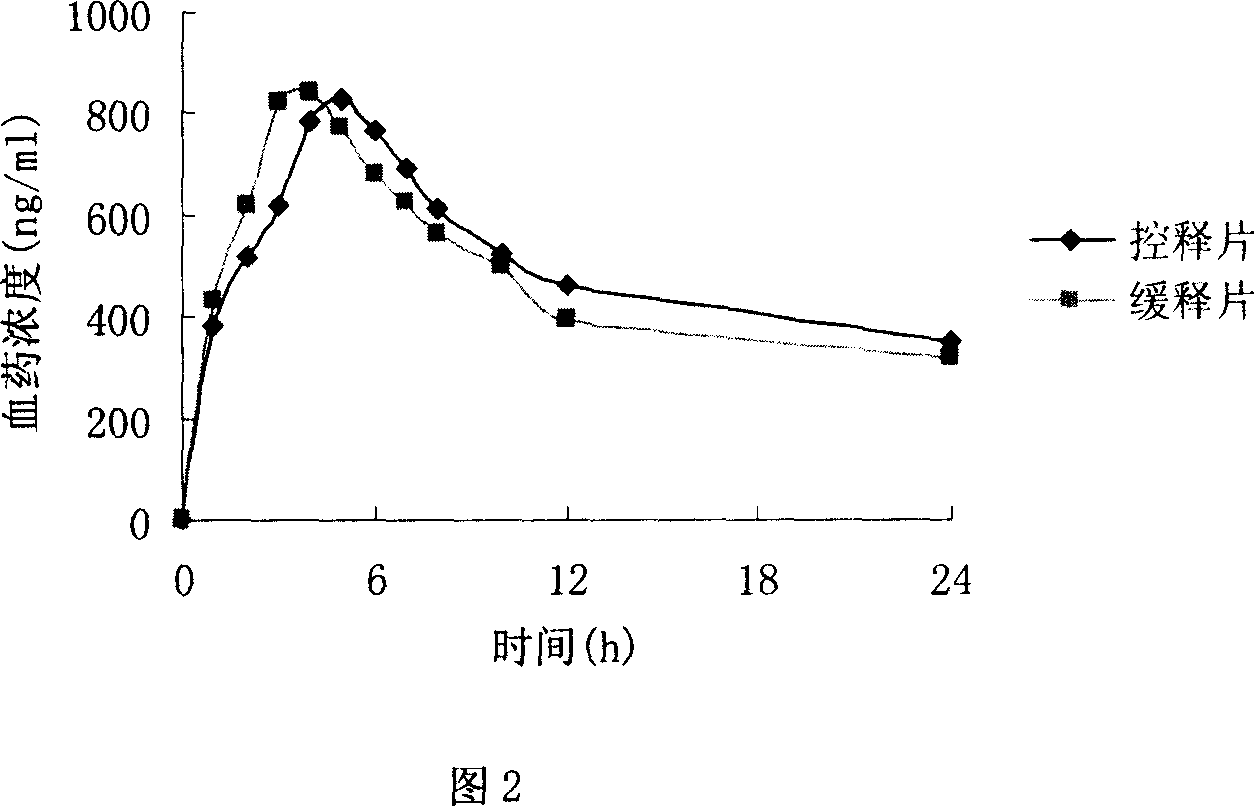
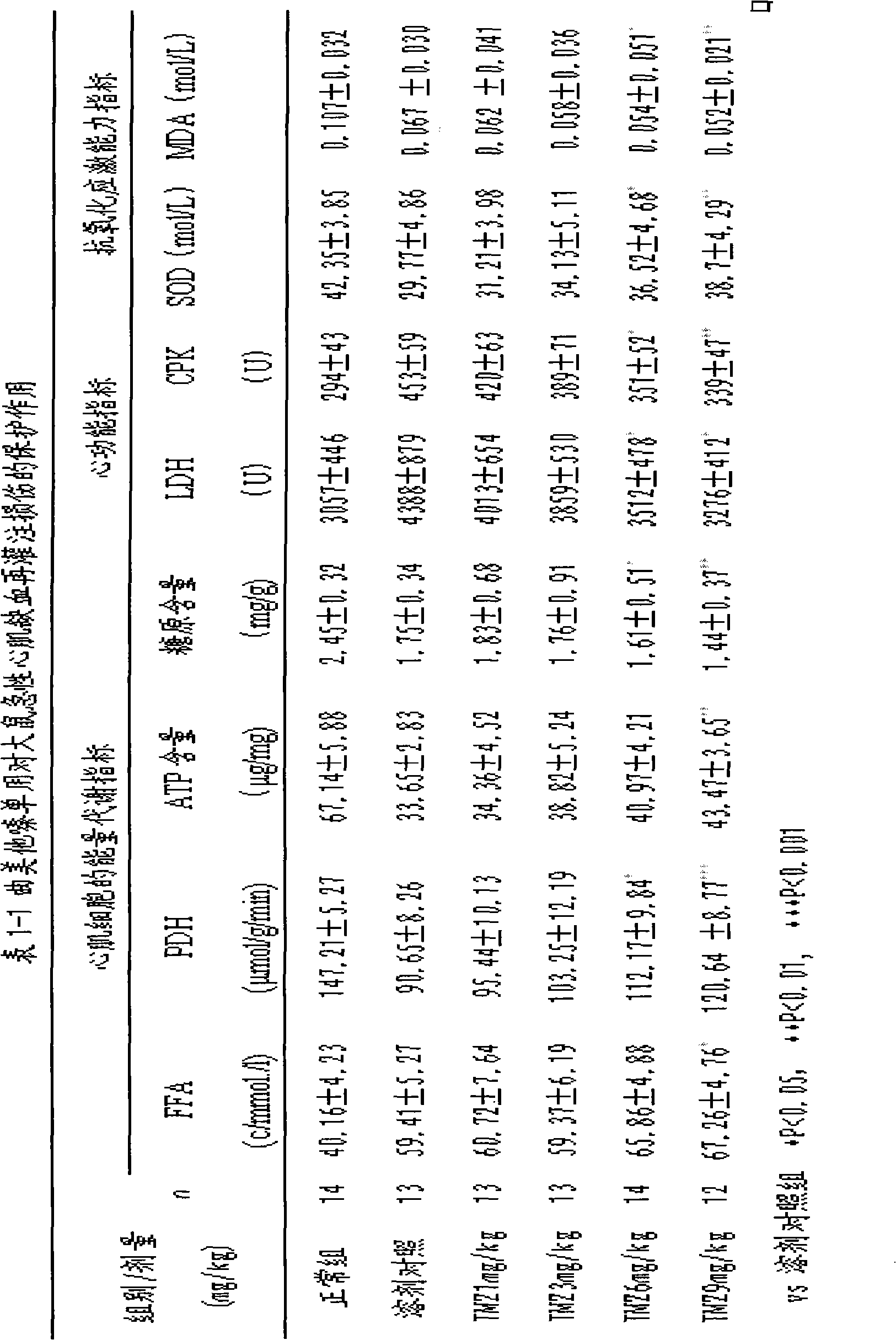
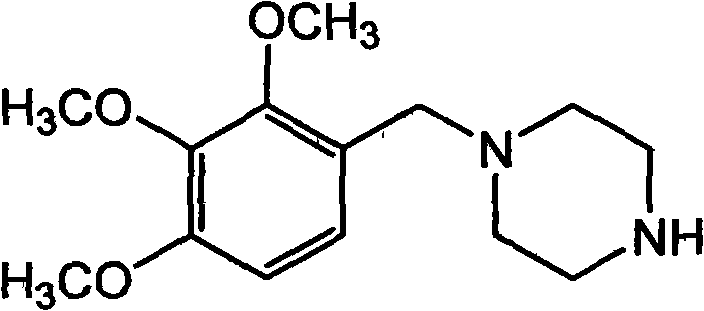
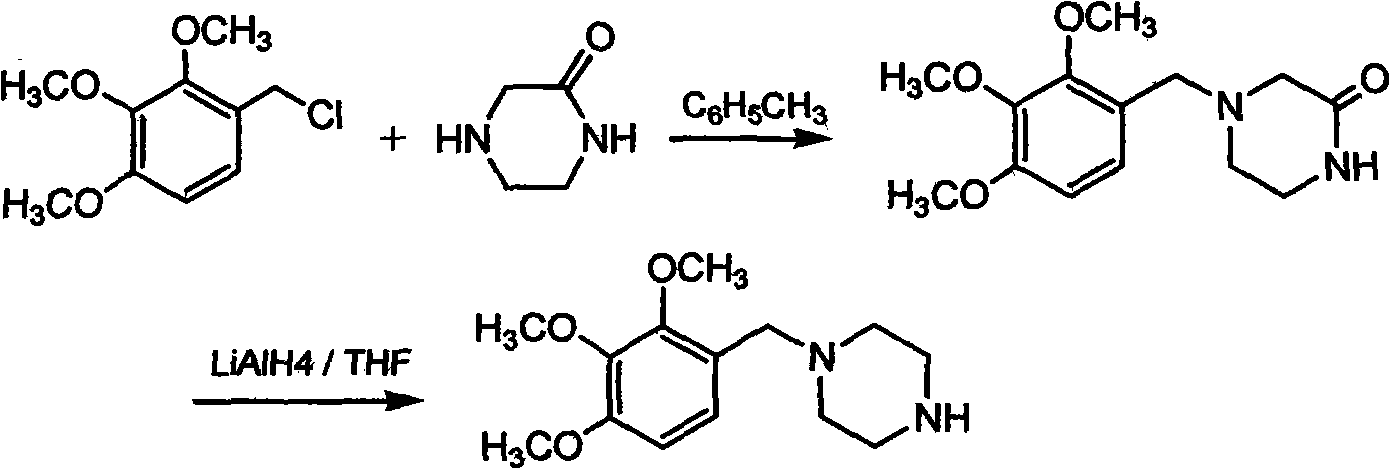
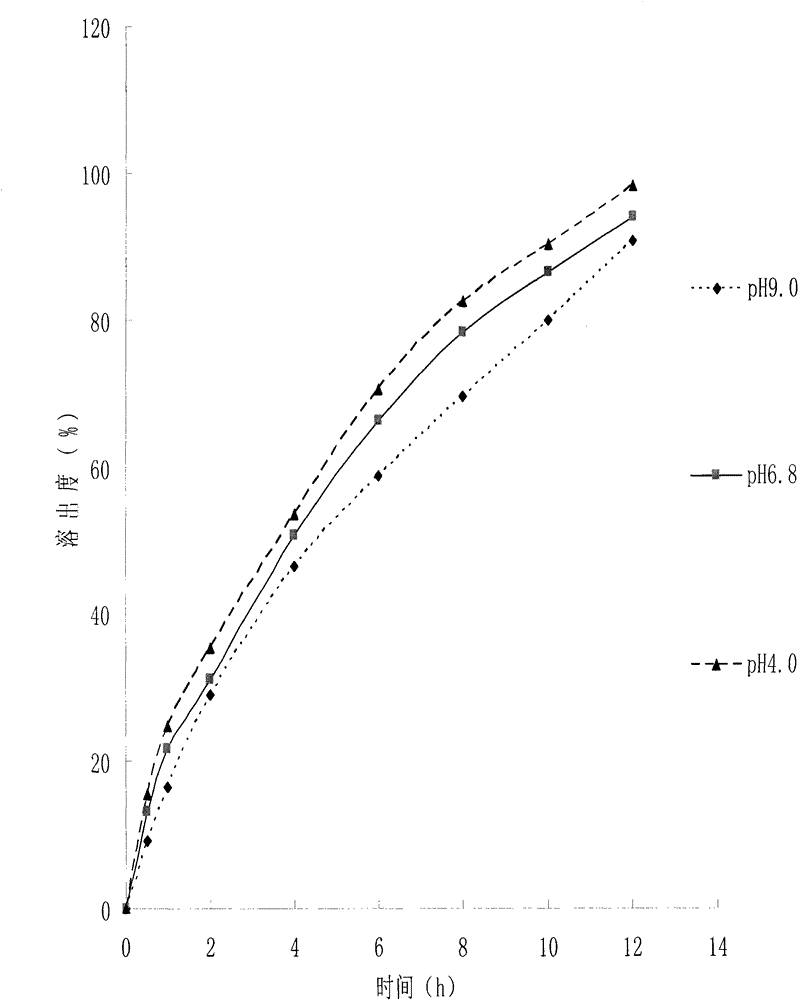
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "Trimetazidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
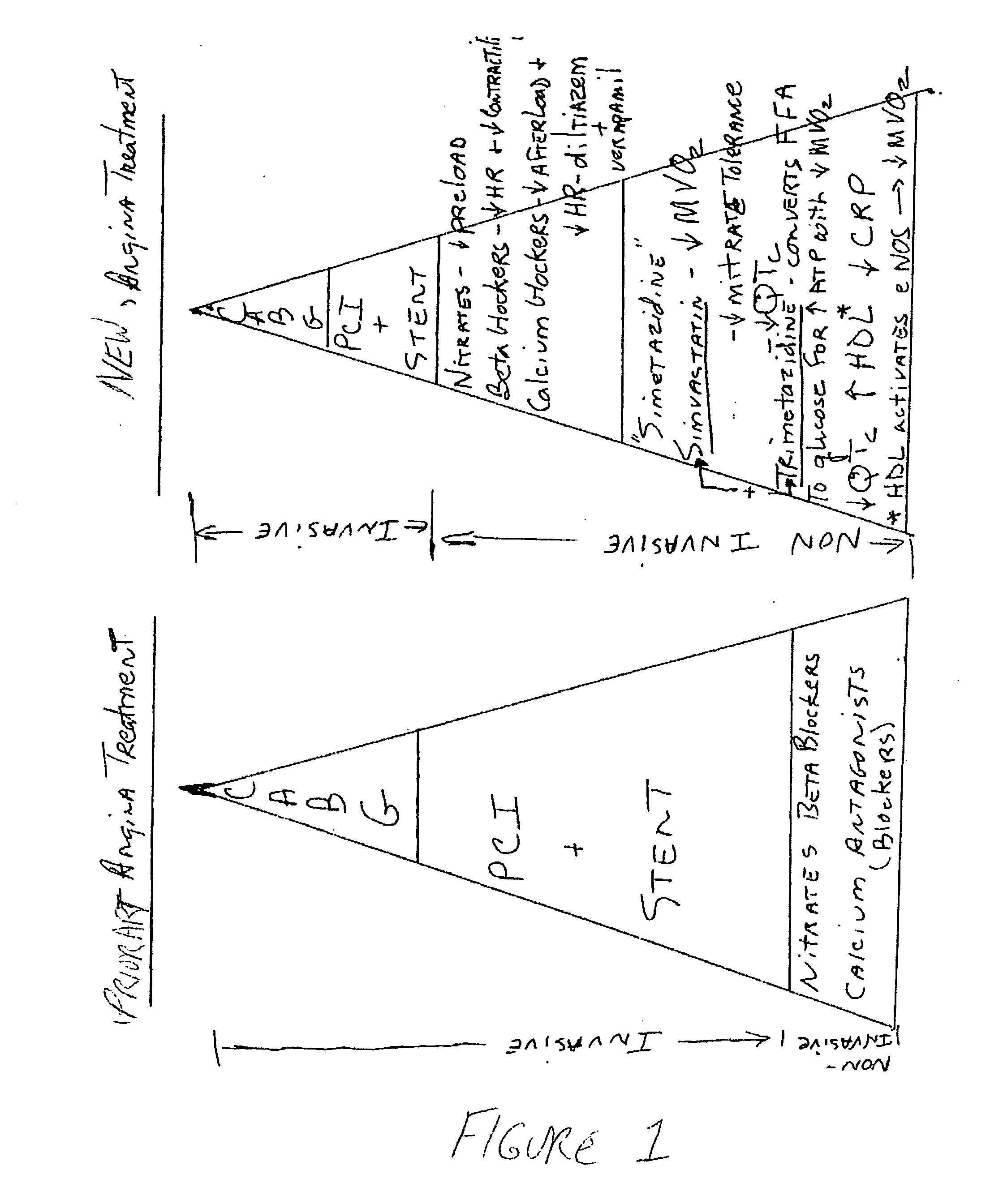
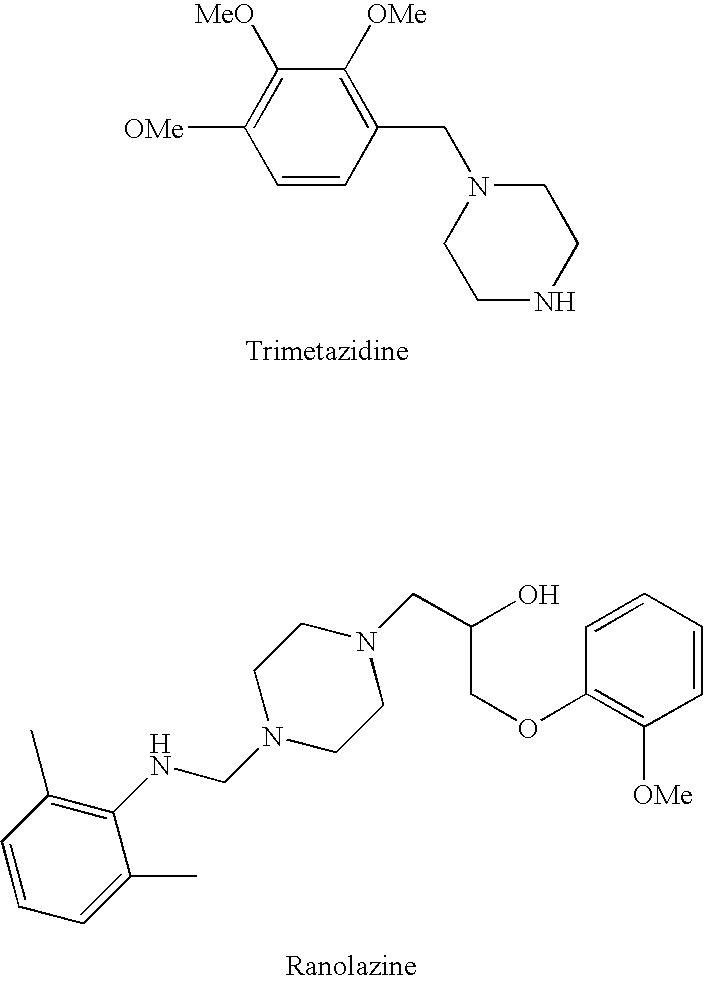
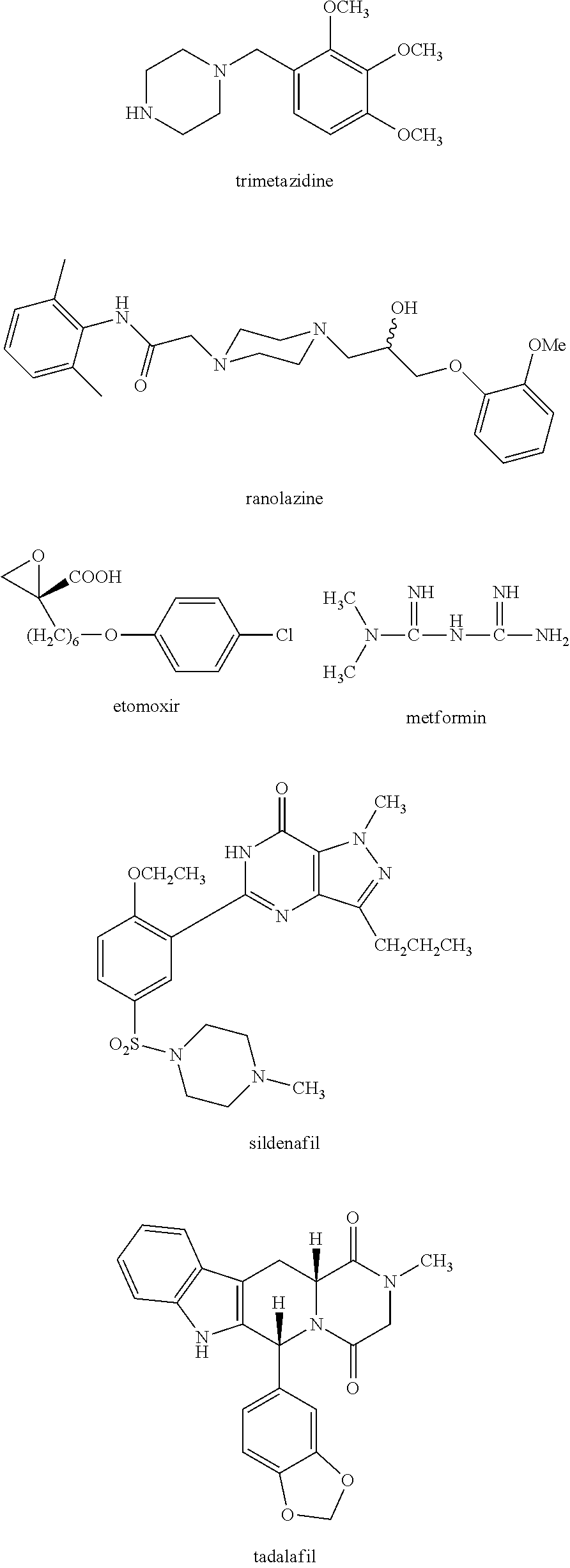
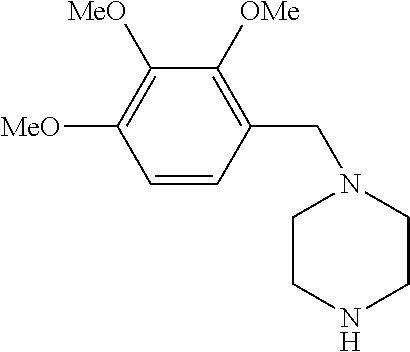
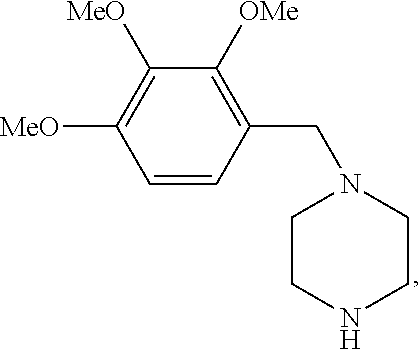
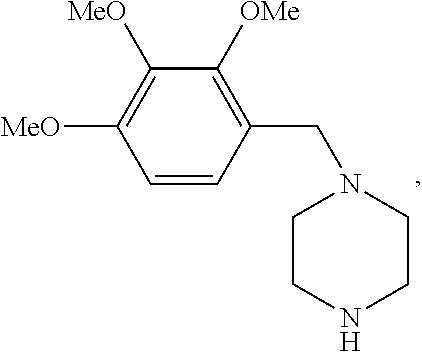
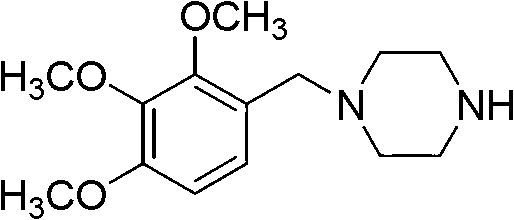
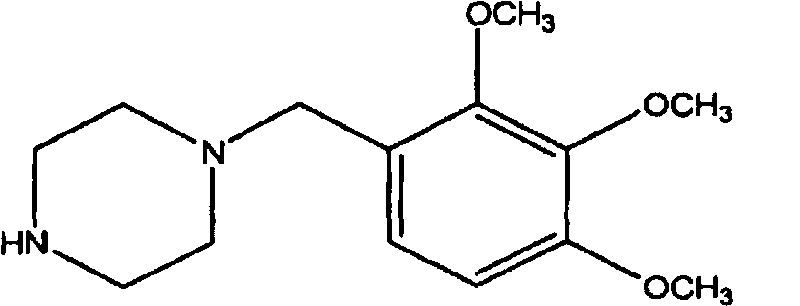
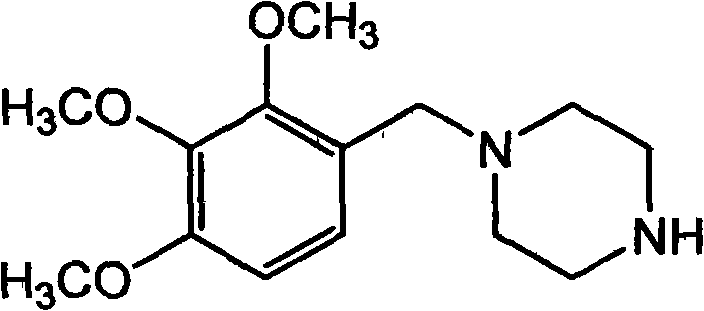
Trimetazidine is a drug for angina pectoris sold under many brand names. Trimetazidine is described as the first cytoprotective anti-ischemic agent developed and marketed by Laboratoires Servier (France). Trimetazidine is an anti-ischemic (anti-anginal) metabolic agent, which improves myocardial glucose utilization through inhibition of fatty acid metabolism, also known as fatty acid oxidation inhibitor.
Combination therapy for endothelial dysfunction, angina and diabetes
InactiveUS20060205727A1Control blood sugar levelsIncrease productionBiocideMetabolism disorderHMG-CoA reductaseTrimetazidine
The combination of a HMG CoA reductase inhibitor like a statin, such as simvastatin, with a pFox inhibitor such as trimetazidine (“Simetazidine”) is particularly advantageous for treatment of end-stage complications, such as acute coronary syndrome (ACS) and chronic angina, especially in type II diabetics. The combination therapy is also useful in the treatment and / or prevention of chronic heart failure (CHF) and peripheral arterial disease (PAD). The combination of a nitric oxide (NO) mechanism with increased NO production with pFox inhibition simultaneously treats both the effect and the cause of angina. One or more oral hypoglycemic compounds (biguanides, insulin sensitizers, such as thiazolidinediones, α-glucosidase inhibitors, insulin secretagogues, and dipeptidyl peptidase IV inhibitors), protein kinase C (PKC) inhibitors, and acetyl-CoA carboxylase inhibitors can also be used in combination with the HMG CoA reductase inhibitors and / or pFox inhibitors, especially in type II diabetics, to control glucose levels and treat endothelial dysfunction. The drugs can be given in combination (e.g. a single tablet) or in separate dosage forms, administered simultaneously or sequentially. In the preferred form the statin is given in a dose of between 5 and 80 mg / day in two separate doses, and the pFox inhibitor is administered in a sustained or extended dosage formulation at a dose of 20 mg three times a day or 35 mg two times a day. The dose of the oral hypoglycemic, PKC inhibitor, or acetyl-CoA carboxylase inhibitor varies with the type of drug used.
Owner:HONG KONG NITRIC OXIDE
Orally taken control released trimetazidine medicine composition
InactiveCN1931143AImprove effectivenessImprove securityOrganic active ingredientsPharmaceutical non-active ingredientsTrimetazidinePlasticizer
The present invention relates to one kind of orally taken control released trimetazidine medicine composition and its preparation process. The trimetazidine medicine composition consists of trimetazidine or its physiologically acceptable salt in effective dosage and pharmaceutically acceptable medicine excipient capable of reaching release controlling osmotic pump effect. The medicine excipient is one or several selected from osmotic pressure active matter, permeation assistant, diluent, colorant, lubricant, moistener or adhesive, filming material, pore creating agent, plasticizer and solvent.
Owner:GUANGZHOU PUIS PHARMA FACTORY
Pharmaceutical composition for treatment of abnormal energy metabolism and application thereof
ActiveCN102058888ATo promote metabolismPromote recoveryOrganic active ingredientsNervous disorderDiseaseDiabetes mellitus
The invention relates to a pharmaceutical composition for the treatment of abnormal energy metabolism, which is the pharmaceutical composition containing a) a medicine for promoting fatty acid oxidation or derivatives thereof and b) a medicine for promoting glucose oxidation or pharmaceutical salts thereof. The pharmaceutical composition can comprehensively balance the metabolisms of fatty acid and glucose, optimize the productivity of fatty acid and glucose, reduce the jeopardy of abnormal aggregation of acidic metabolites, including free fatty acid or lactic acid and the like on organism, treat mitochondrial dysfunction, improve the defect that trimetazidine and the like are likely to create the accumulation of free fatty acid owing to the suppression on the fatty acid oxidation, and can be used for the auxiliary treatment for a plurality of diseases featured by imbalanced energy metabolism, such as acute and chronic ischemia, traumas, serious infection, diabetes, tumors, etc.
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
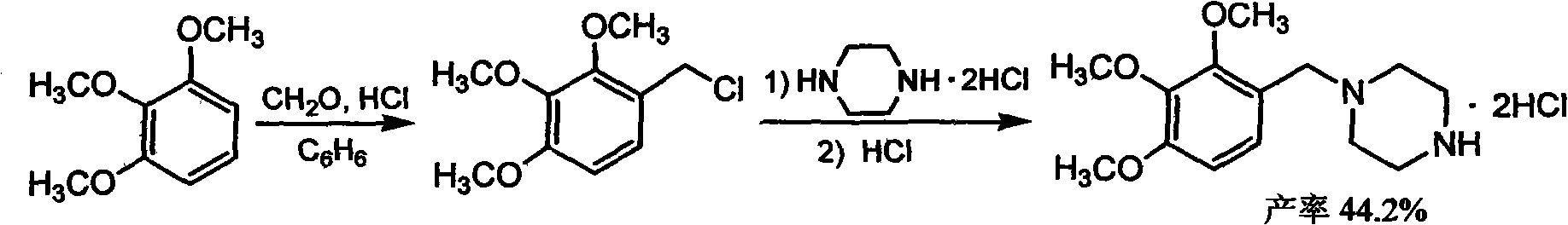
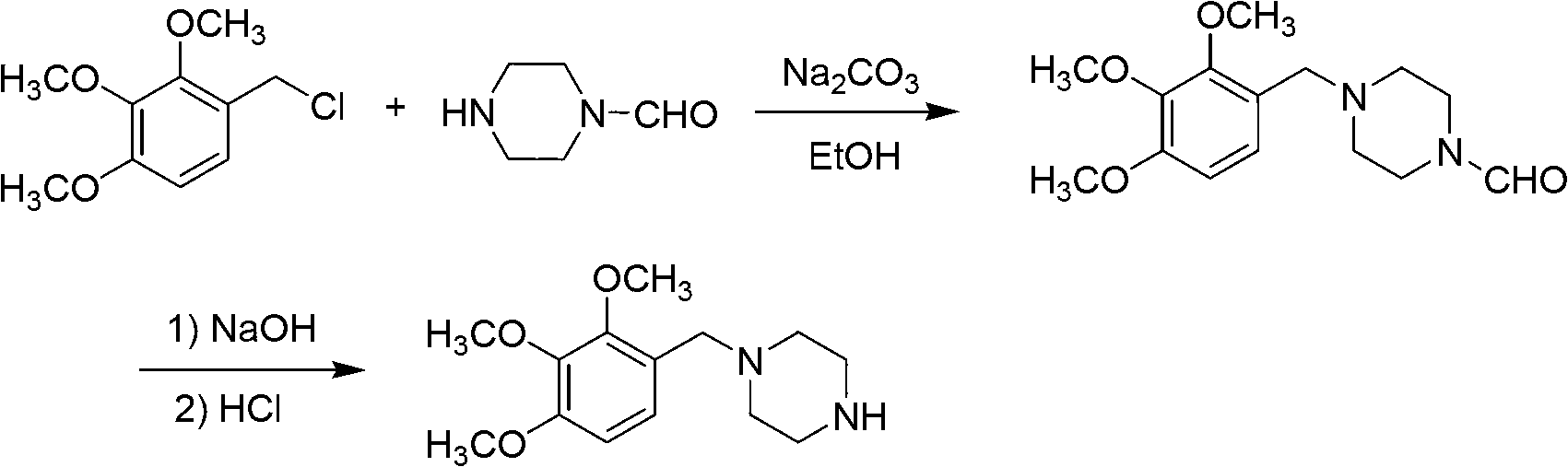
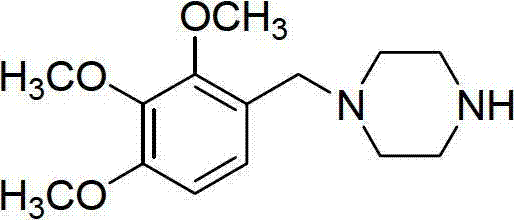
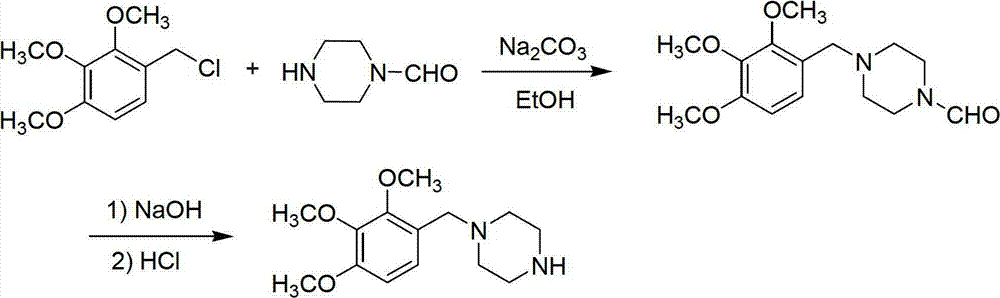
Trimetazidine and production method of hydrochloride thereof
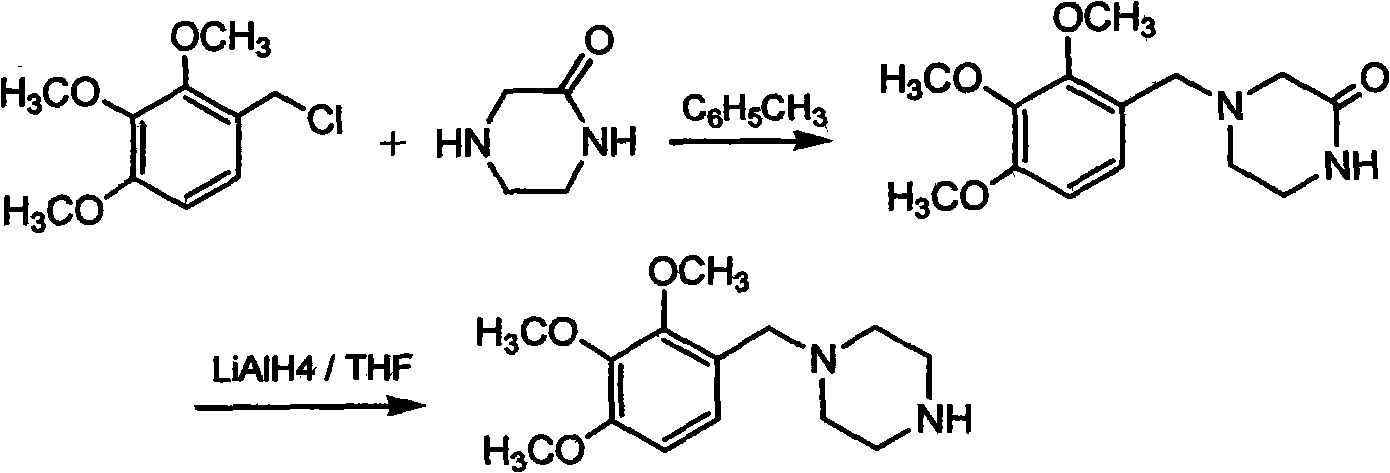
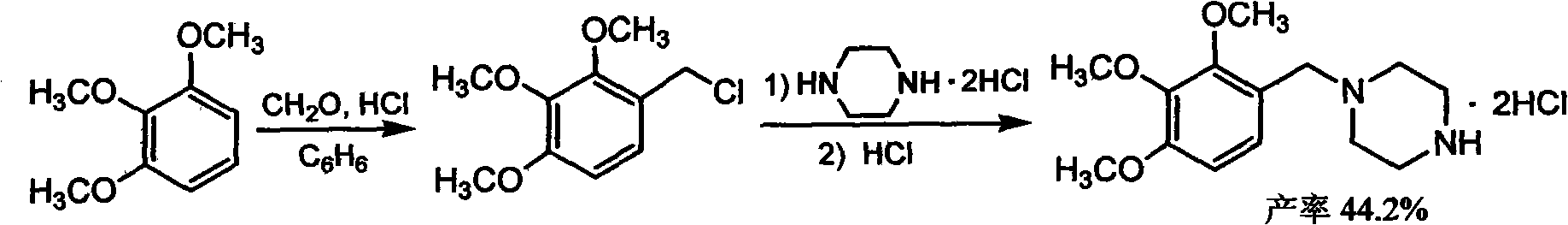
The invention discloses trimetazidine and a production method of hydrochloride thereof, which belongs to the technical field of chemical synthesis and is characterized by adopting 2,3,4-3-methoxybenzaldehyde and piperazine as raw materials. The method comprises the following steps of: adding a solvent, 2,3,4-3-methoxybenzaldehyde and anhydrous piperazine with the molar ratio of 1:1 to 1:3, and a nickel-based catalyst accounting for 3 to 10% of the mass percentage of the 2,3,4-3-methoxybenzaldehyde and the anhydrous piperazine to a pressure kettle; using nitrogen for purging before hydrogen is led in, with hydrogen pressure maintained within the range of 0.7MPa to 2.0MPa, reaction temperature within the range of 50 DEG C to 95 DEG C, reaction time within the range of 4 hours to 10 hours, and pH value regulated to 3 to 4; separating out organic phase; and recovering the solvent. The water phase is washed with chlorinated hydrocarbon extraction, and the pH value is regulated to 12, then the water phase is extracted with aromatic hydrocarbon, and the aromatic hydrocarbon in the aromatic hydrocarbon extraction liquid is steamed out, thus obtaining the trimetazidine. The invention has the advantages of low production cost, high yield, and good environmental protection.
Owner:BEIJING JIALIN PHARM INC
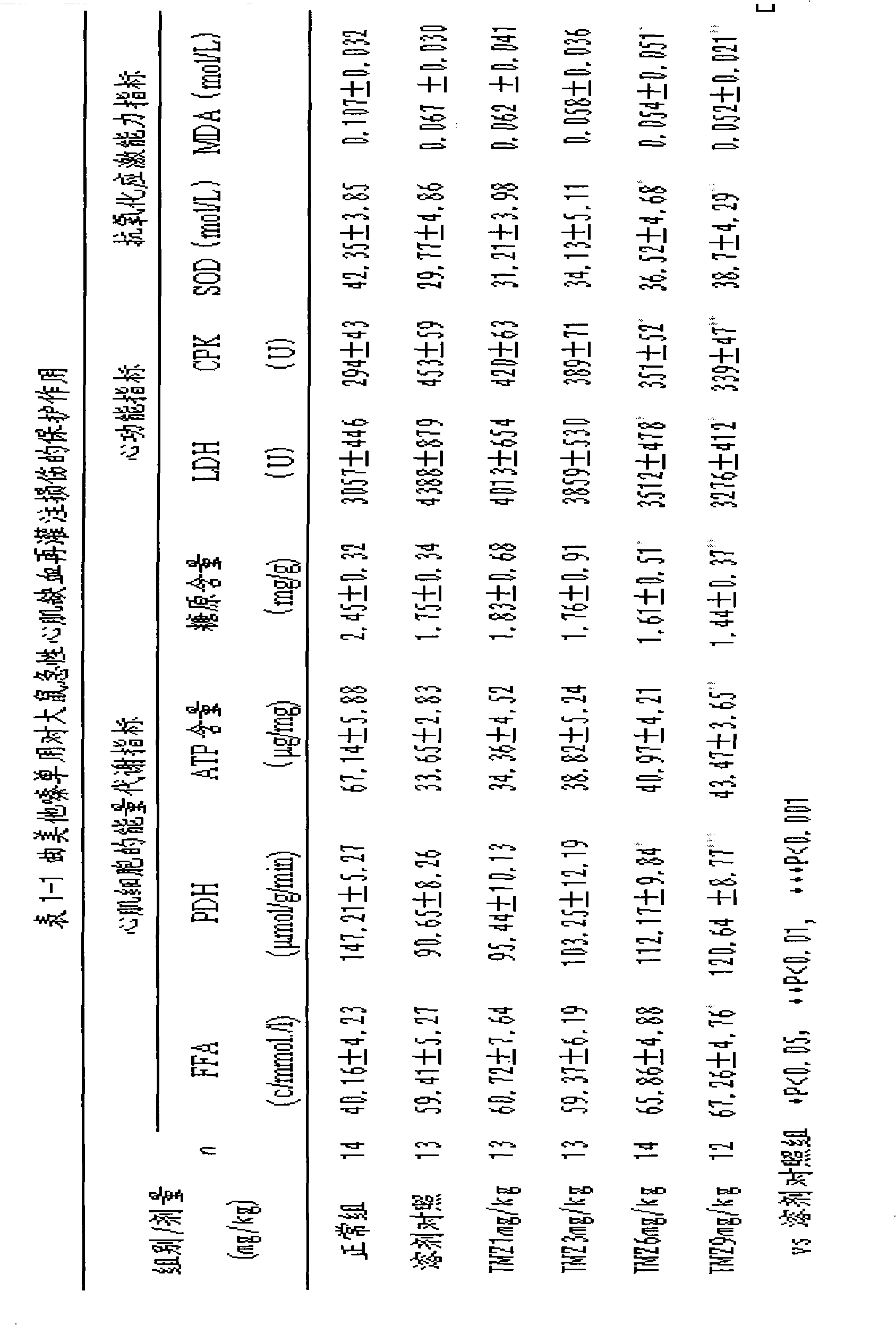
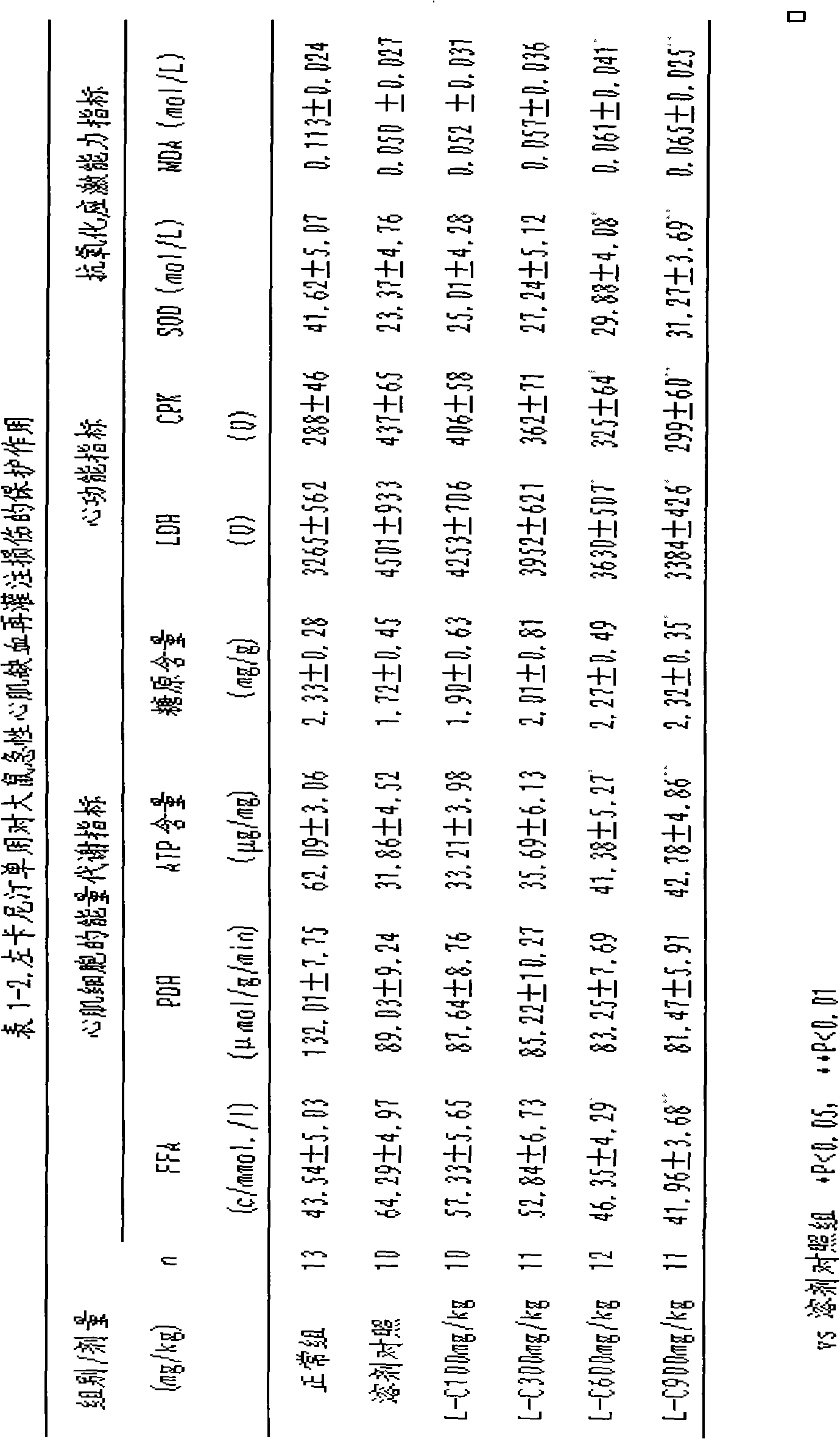
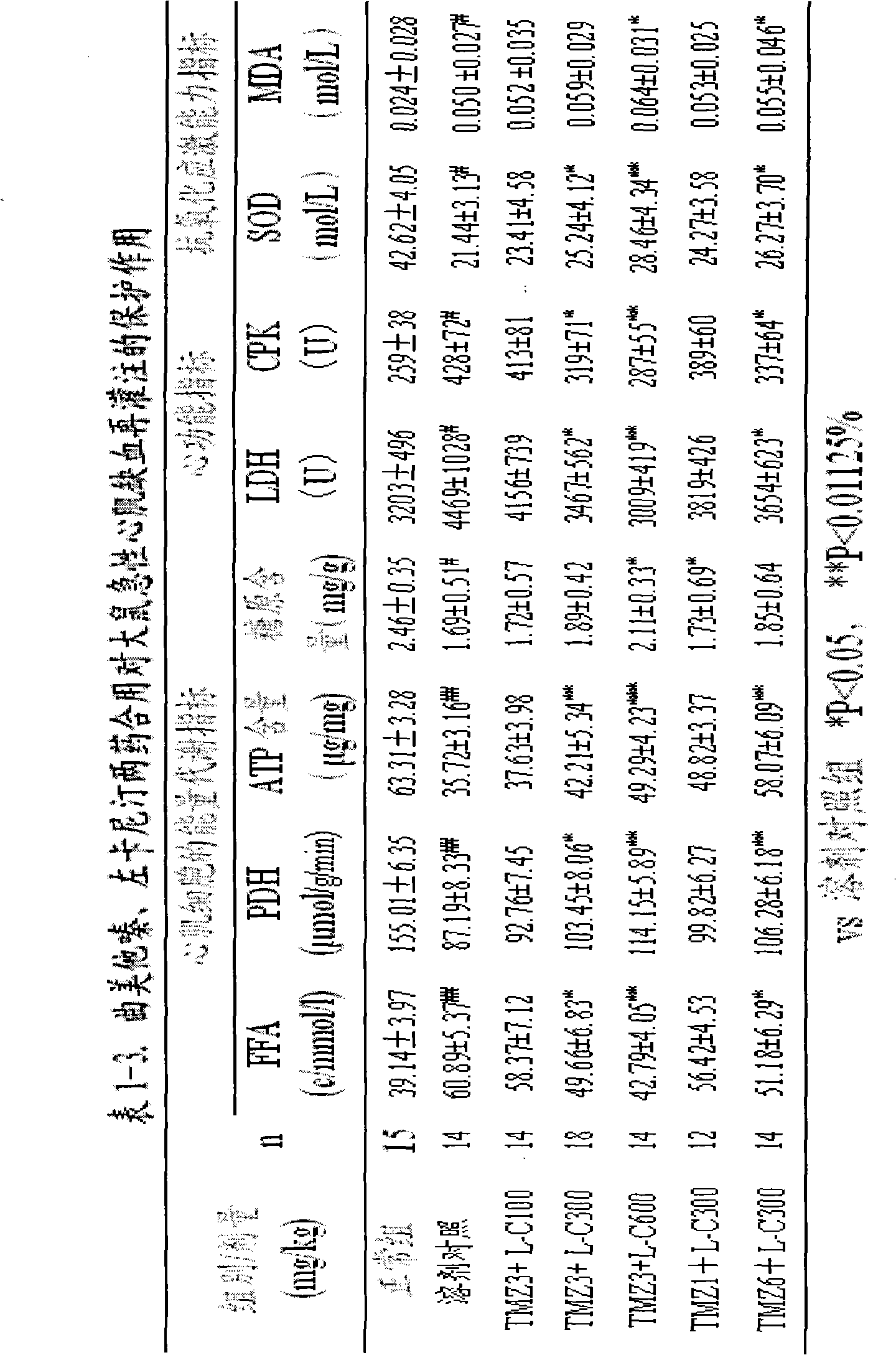
Pharmaceutical composition for reducing the area of myocardial infarction and its use
This invention relates to a pharmaceutical composition for preventing and curing myocardium ischemia and reducing area of myocardial infarction, its pharmaceutical preparation and applications. The composition includes (a) levocarnitine or its derivatives, and (b) trimetazidine or its medicative salts. The quantity of levocarnitine or its derivatives, and trimetazidine or its medicative salts in the composition is effective amount for treating myocardial ischemia and reducing the area of myocardial infarction.
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
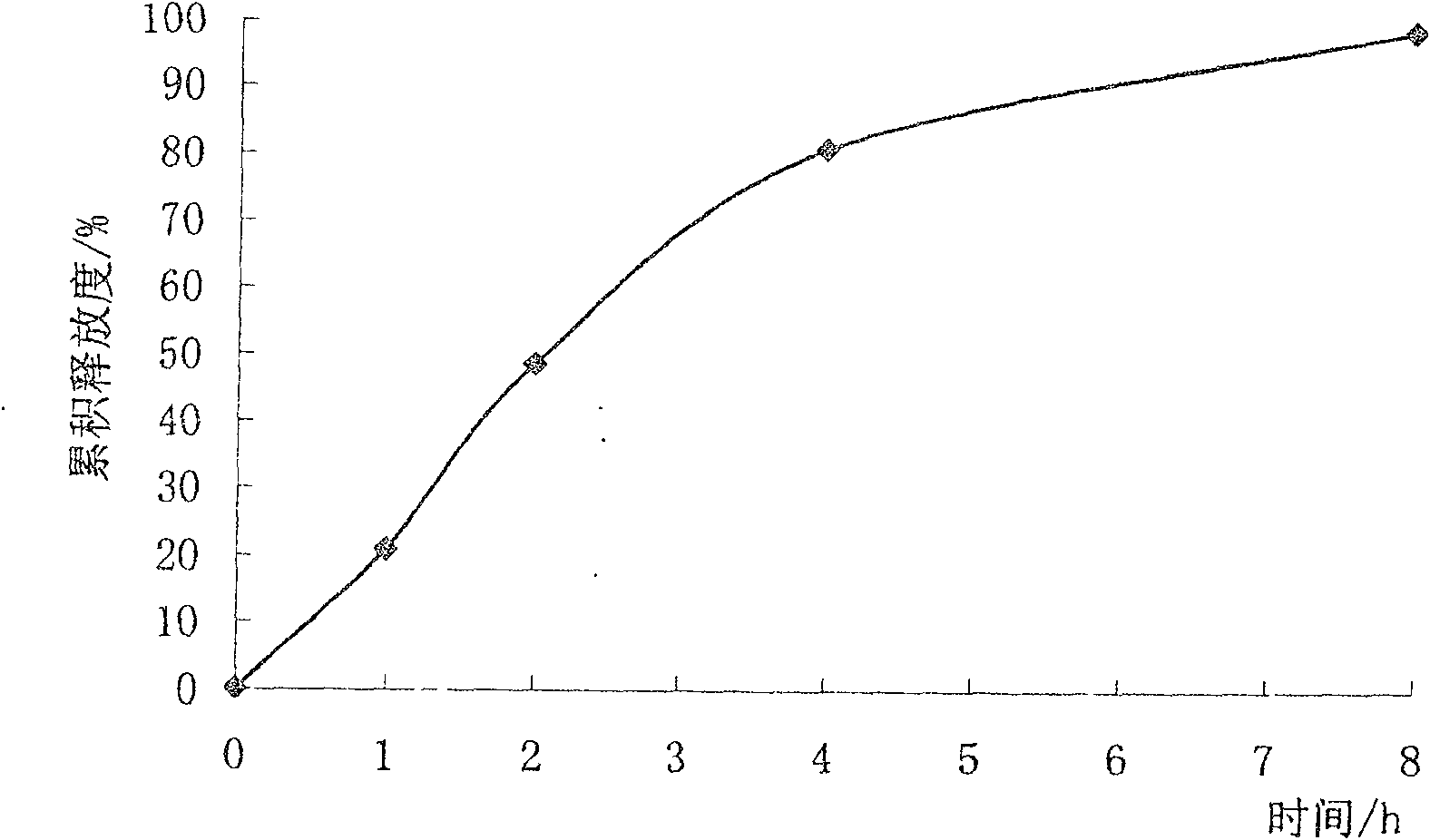
Trimetazidine sustained-release mini-pill composition and method for preparing same
ActiveCN105616358AImprove solubilityPoor sustained releaseOrganic active ingredientsSenses disorderMedicineTrimetazidine
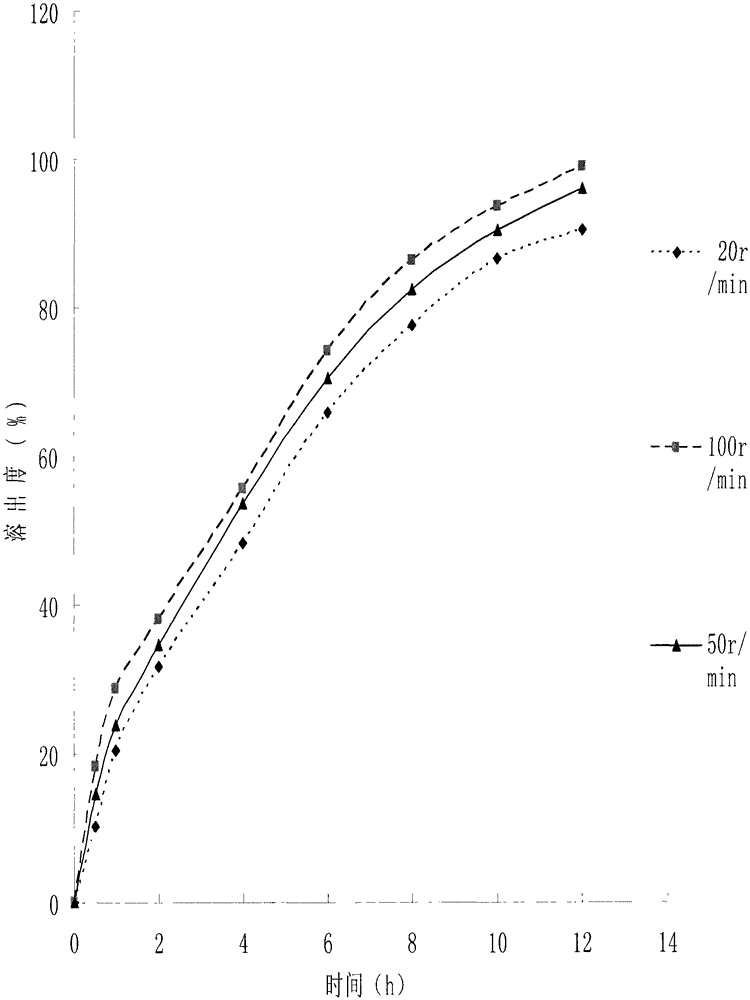
The invention discloses a trimetazidine sustained-release mini-pill composition. The trimetazidine sustained-release mini-pill composition comprises sustained-release pills A and C or sustained-release pills A, B and C. The sustained-release pills A, B and C have different increased weights. The trimetazidine sustained-release mini-pill composition has the advantages that two or three types of film-controlled sustained-release mini-pills with different increased weights are proportionally mixed with one another to obtain the trimetazidine sustained-release mini-pill composition, accordingly, zero-order release effects can be realized by the trimetazidine sustained-release mini-pill composition, and the trimetazidine sustained-release mini-pill composition can be released in a long-acting and sustained manner.
Owner:南京卓康医药科技有限公司
Fatty Acid Oxidation Inhibitors Treating Hyperglycemia and Related Disorders
The invention relates to a methods, compositions and kits for treating hyperglycemia and related disorders, such as type 2 diabetes mellitus, impaired glucose tolerance, diabetic retinopathy, diabetic nephropathy and diabetic neuropathy, and to methods, compositions and kits for treating erectile dysfunction. The methods comprise administering an inhibitor of fatty acid oxidation to a subject in need thereof. In some embodiments, trimetazidine and metformin or a phosphodiesterase 5 inhibitor are administered.
Owner:SYMCOPEIA
A pharmaceutical composition treating severe high-altitude diseases
InactiveCN104138377AEffective treatmentOrganic active ingredientsAntinoxious agentsDiseaseTrimetazidine
The present invention provides a pharmaceutical composition used for treating severe altitude sickness, characterized by a composition consisting of trimetazidine or pharmaceutically acceptable salt thereof and L-carnitine or derivative thereof or pharmaceutically acceptable salt thereof, and the weight ratio of the above being 1:200.
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
A sustained release agent improving anoxia endurance
InactiveCN104138376AIncrease blood oxygen saturationOrganic active ingredientsAntinoxious agentsTrimetazidinePerylene derivatives
The present invention discloses a slow-release agent for increasing hypoxia tolerance; the slow-release agent is a composition formed by combining trimetazidine or pharmaceutically acceptable salt thereof and L-carnitine or derivative thereof or pharmaceutically acceptable salt thereof, according to the ratio 1:1.6-6400.
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
Trimetazidine for use in the treatment of fibromyalgia syndrome and related conditions
The present invention provides a method for treating certain rheumatic conditions such as fibromyalgia syndrome, chronic fatigue syndrome, myofascial pain syndrome, and Gulf War syndrome, among others, by administration of trimetazidine, along with related compositions and kits.
Owner:MINTAILS
Pharmaceutical composition for reducing the area of myocardial infarction and its use
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
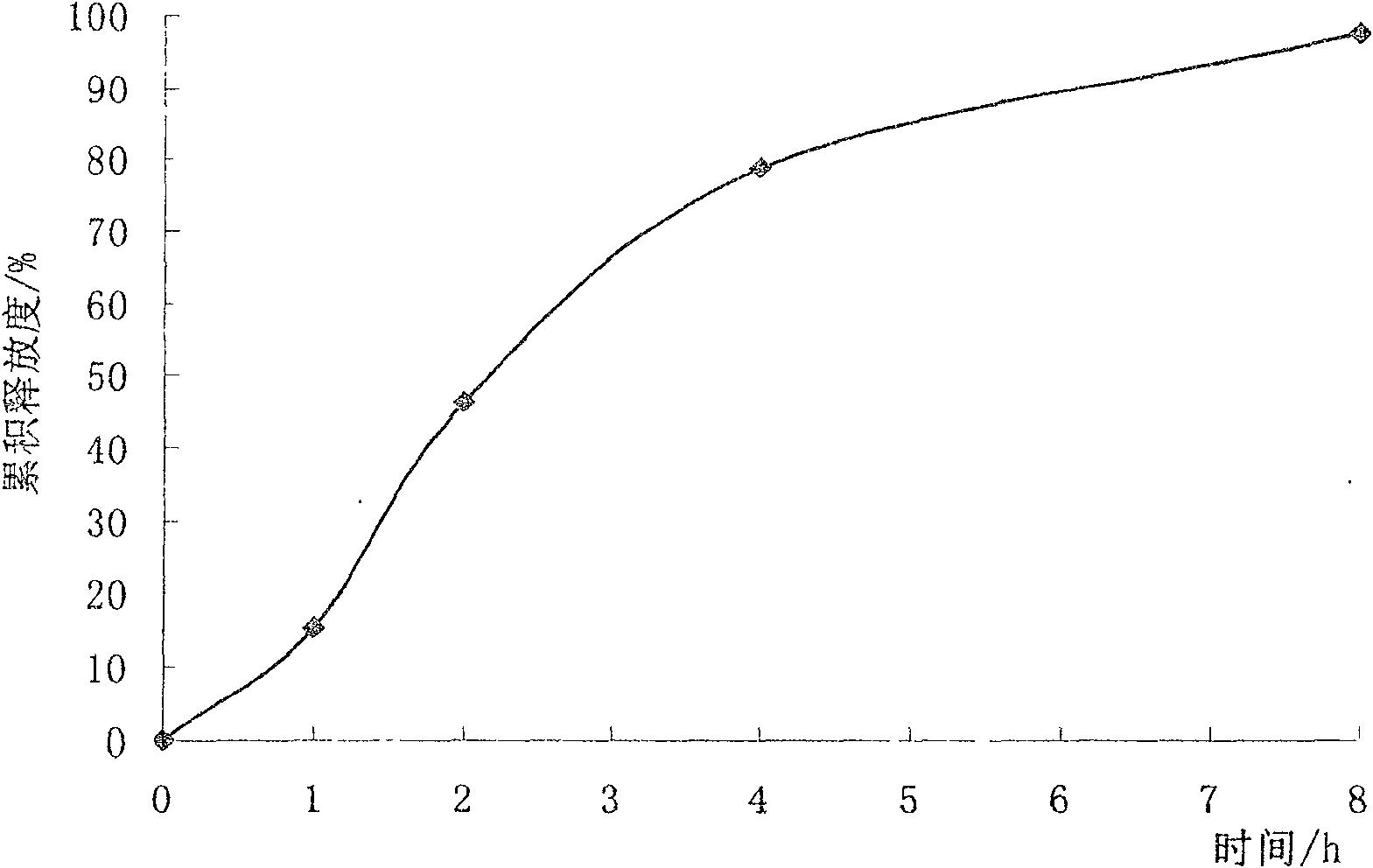
Trimetazidine sustained release capsule and preparation method thereof
InactiveCN102552217APromote absorptionImprove complianceOrganic active ingredientsSenses disorderControl releaseSustained Release Capsule
The invention relates to a trimetazidine or salt sustained release capsule and a preparation method thereof. The trimetazidine sustained release capsule comprises the following components in percentage by weight: 5-50 percent of trimetazidine, 5-30 percent of auxiliary materials playing a role in sustained release and 45-85 percent if other auxiliary materials. The trimetazidine sustained release capsule and the preparation method thereof are characterized in that (1), a capsule content is a pellet; (2), the medicated pellet is firstly prepared, and a sustained release preparation is then prepared; and (3), the release of a drug is controlled through a controlled release membrane enwrapped by the pellet.
Owner:TIANJIN KAIWEN BIO TECH
Antihypoxic pharmaceutical composition and application thereof
ActiveUS20160081966A1Obvious adverse effectQuality improvementBiocideNervous disorderVinpocetineTrimetazidine
The present invention discloses an antihypoxic pharmaceutical composition and application thereof. The pharmaceutical composition contains vinpocetine and L-carnitine or a derivative thereof and a pharmaceutically acceptable salt thereof, and also can contain trimetazidine or a pharmaceutically acceptable salt thereof.
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
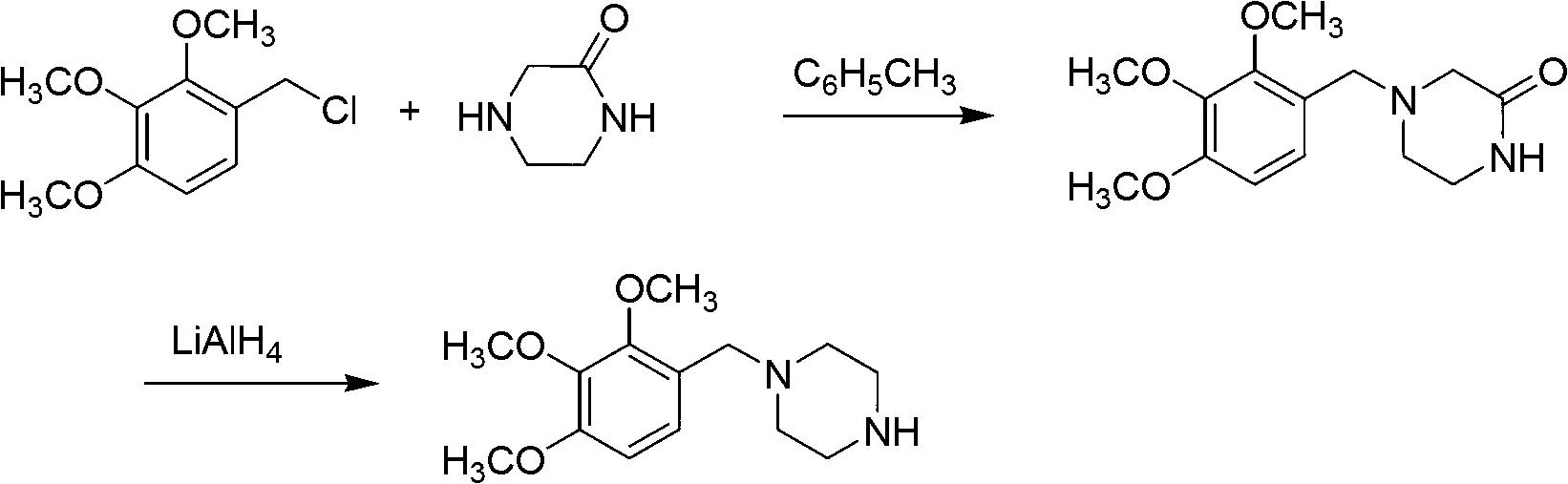
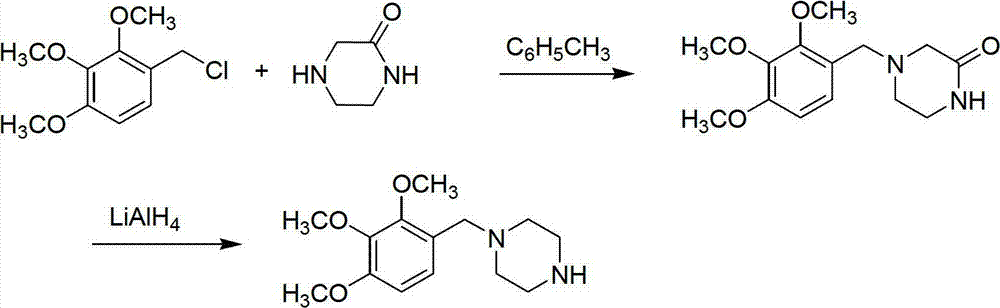
Preparation method of trimetazidine
The invention relates to a preparation method of trimetazidine, belonging to the preparation field of medicine compound. The method comprises the steps of: by taking 2, 3, 4-trimethoxy benzaldehyde and piperazine as raw materials and formic acid as a catalyst; adding a solvent and formic acid in the 2, 3, 4-trimethoxy benzaldehyde and the piperazine for reaction; removing the solvent by steaming, and adjusting the pH value of reaction liquid to be 11-13; and then, carrying out reflux, acidification and rotary steaming to obtain the trimetazidine. In the method, the reductive amination reaction is adopted and the trimetazidine is synthesized in one step under the condition that the formic acid is taken as the catalyst, and the yield reaches up to 83-92%; meanwhile, precious metal palladium catalyst is not used, so that the synthesis cost is reduced. The LiAlH4 and NaBH4 which are high in danger are not used, so that the experimental safety is improved; and the method is beneficial to the large-scale production of the trimetazidine.
Owner:REYOUNG PHARMA
Therapy for hyperglycemia, related disorders and erectile dysfunction
InactiveCN101702884AOrganic active ingredientsMetabolism disorderAcute hyperglycaemiaDiabetic retinopathy
The invention relates to a methods, compositions and kits for treating hyperglycemia and related disorders, such as type 2 diabetes mellitus, impaired glucose tolerance, diabetic retinopathy, diabetic nephropathy and diabetic neuropathy, and to methods, compositions and kits for treating erectile dysfunction. The methods comprise administering an inhibitor of fatty acid oxidation to a subject in need thereof. In some embodiments, trimetazidine and metformin or a phosphodiesterase 5 inhibitor are administered.
Owner:SYMCOPEIA
Modified release solid pharmaceutical compositions of trimetazidine and process thereof
There is provided a modified release solid pharmaceutical composition comprising Trimetazidine and polyethylene oxide, wherein the composition does not include any lubricant.
Owner:MICRO LABS
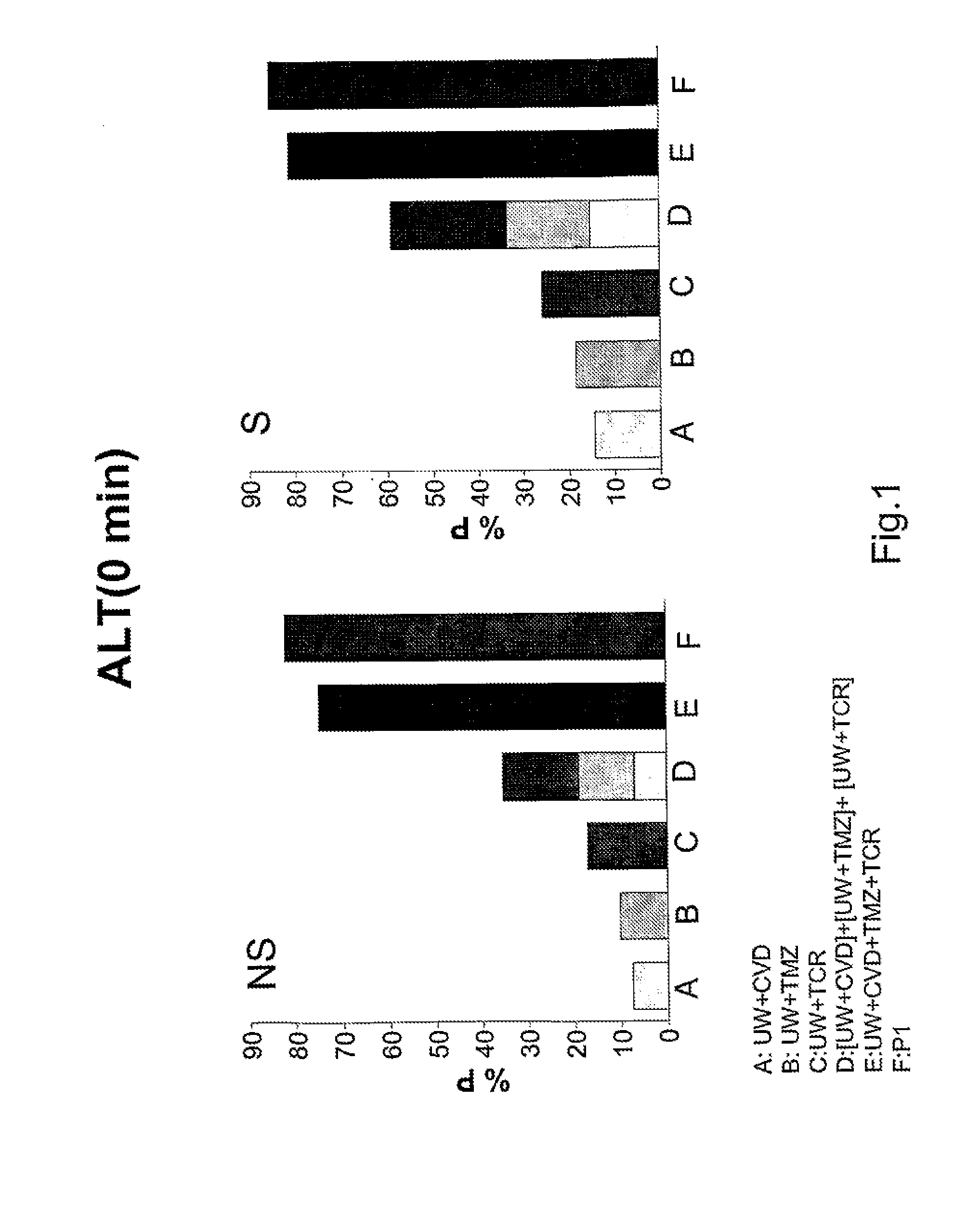
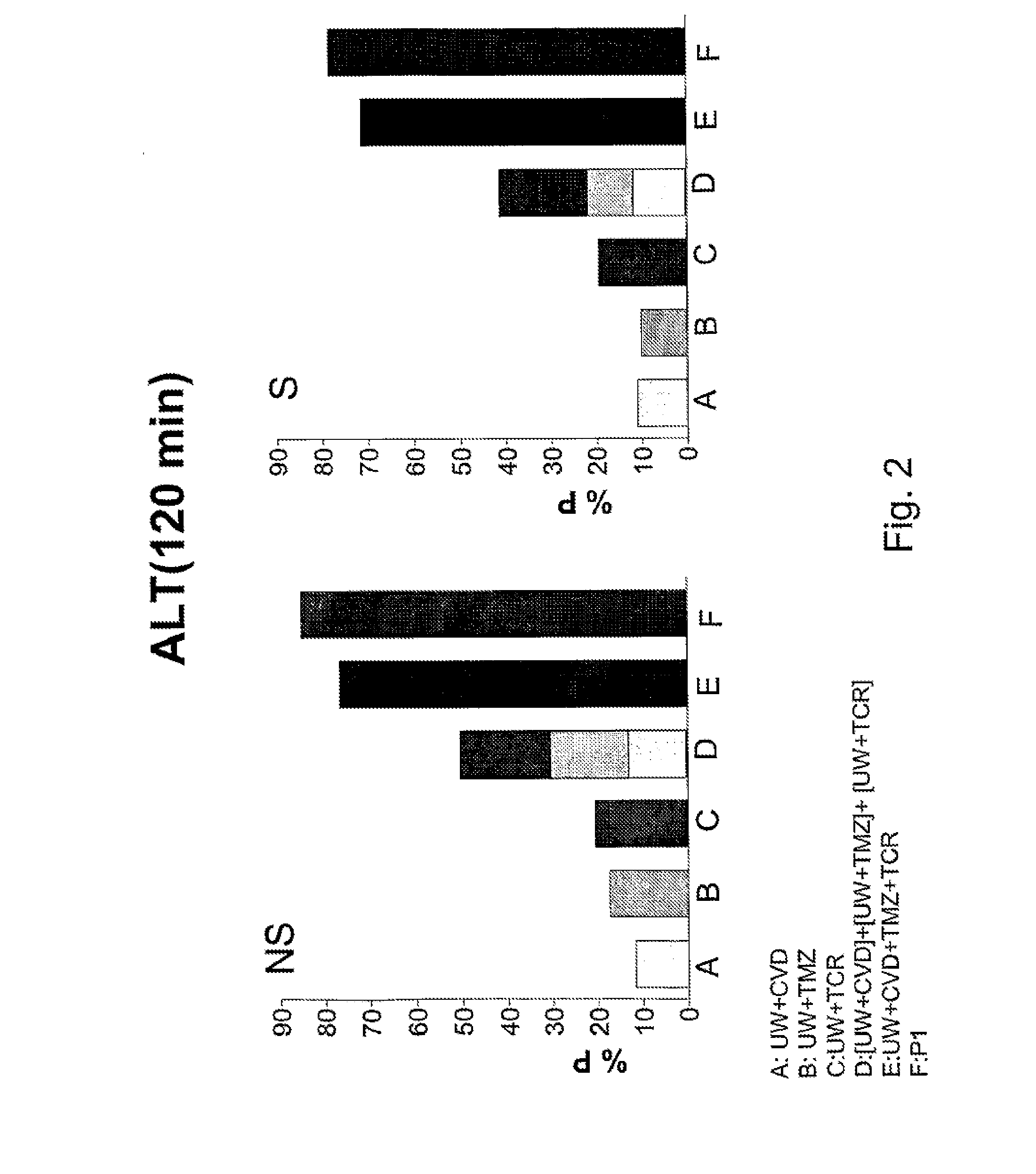
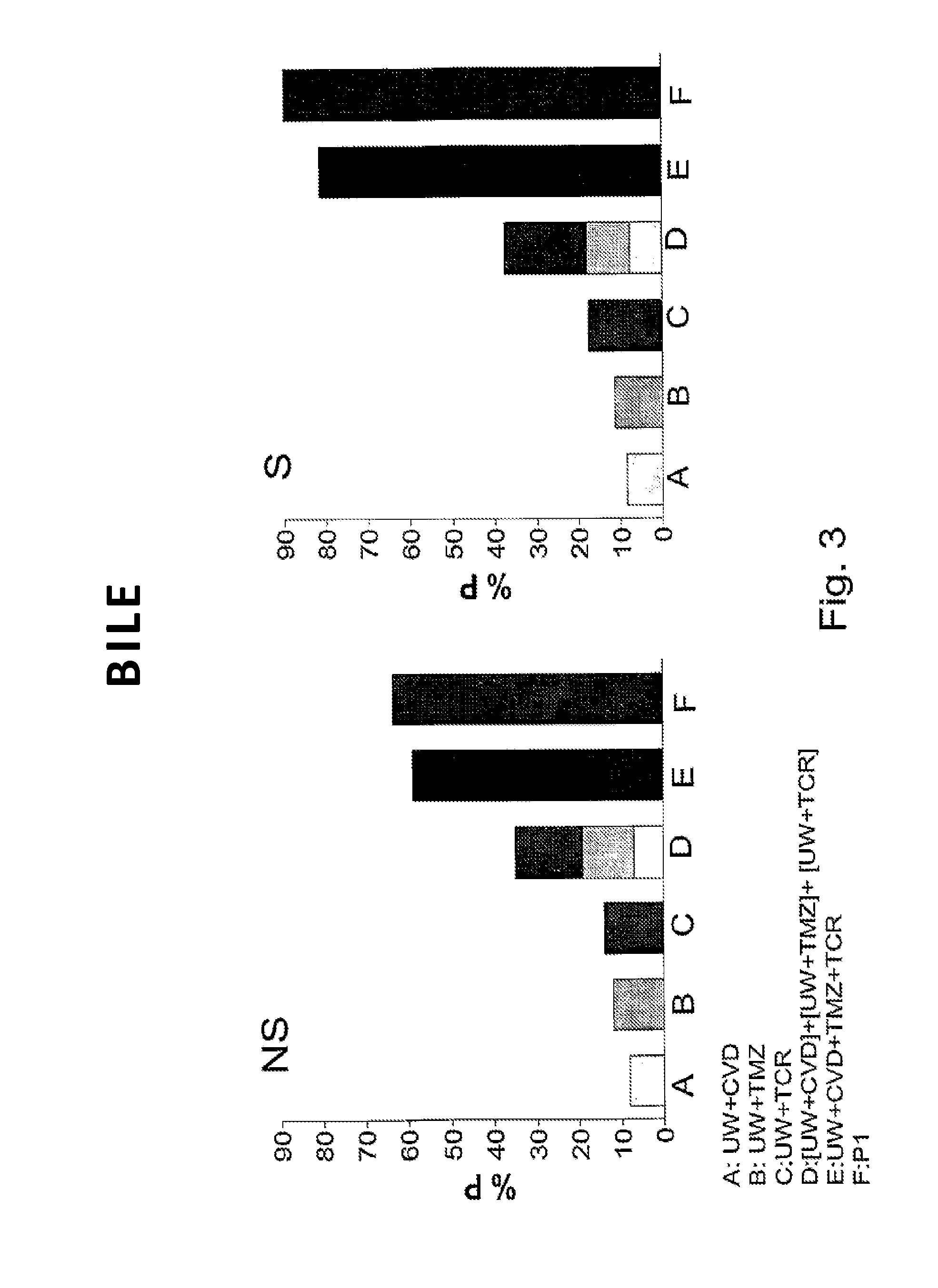
Aqueous solution for the preservation of tissues and organs
ActiveUS20130059285A1Improve initial conditionsIncrease capacityArtificial cell constructsDead animal preservationFatty liverTrimetazidine
It is provided an improved aqueous solution for the preservation of tissues and organs comprising carvedilol, tacrolimus, and trimetazidine. A synergistic effect is observed for this preservation solution which is particularly effective in marginal organs, such as steatotic livers.
Owner:UNIV DE BARCELONA +2
Pharmaceutical composition for treatment of abnormal energy metabolism and application thereof
ActiveCN102058888BTo promote metabolismPromote recoveryOrganic active ingredientsNervous disorderDiseaseDiabetes mellitus
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
Method convenient for myocardial metabolism development
PendingCN111904381AImprove accuracyImprove image qualityMedical imagingDiagnostic signal processingOral glucoseTrimetazidine
The invention discloses a method convenient for myocardial metabolism development. The method comprises the following steps of S1, determining a research population; S2, regulating blood sugar and performing developing; S3, evaluating image quality; S4, performing visual analysis; S5, performing quantitative analysis; S6, performing quantitative analysis on heart function and myocardial viability;and S7, performing counting. According to the method, trimetazidine can increase the glucose metabolism level of normal or ischemic myocardium of a diabetic patient to different extents, remarkably improve the image quality of 18F-FDG myocardial metabolism imaging (MMI), potentially improve the accuracy of gated PET quantitative parameter measurement, is safe and effective, and is combined with an oral glucose load / intravenous injection insulin method for use. Compared with a normal blood sugar hyperinsulinism holder technology, the method disclosed by the invention is more convenient to operate, and clinical application and popularization are facilitated.
Owner:THE FIRST PEOPLES HOSPITAL OF CHANGZHOU
Preparation method of trimetazidine
Owner:REYOUNG PHARMA
Antihypoxic pharmaceutical composition and application thereof
ActiveUS9421181B2Prevention and treatmentPromote oxidationOrganic active ingredientsNervous disorderMedicineTrimetazidine
Owner:CHANGZHOU HI TECH DISTRICT MULTIPLE DIMENSION IND TECH INST
A kind of medicinal gelatin microsphere and preparation method thereof
ActiveCN102475684BMeet the needs of delayed release and prolonged efficacyImprove efficacyOrganic active ingredientsPharmaceutical product form changeCross-linkMicrosphere
Disclosed are a gelatin microsphere for pharmaceutical use and a preparation method thereof, with the active ingredient being trimetazidine or pharmaceutically acceptable salts thereof. The microsphere is prepared by a chemical cross-linking method comprising the steps of emulsifying, removing oil and water, chemical cross-linking, and washing and drying. Using particle size and appearance, encapsulation rate and drug loading rate of the microsphere as indexes, reagents such as carrier, oil phase, water removing and oil removing agent and cross-linking curing agent suitable for the microsphere of the present invention are optimally selected. The microsphere prepared according to the present invention has a particle size in the range of 50 to 200 µm and a drug loading rate of 30 to 35%.
Owner:CHANGZHOU SIMM DRUG RES & DEV CENT
Prevention and/or treatment of contrast-induced acute kidney injury
PendingUS20210401832A1Preventing contrast-induced acute kidney injuryReduce and preventOrganic active ingredientsUrinary disorderOXFENICINETrimetazidine
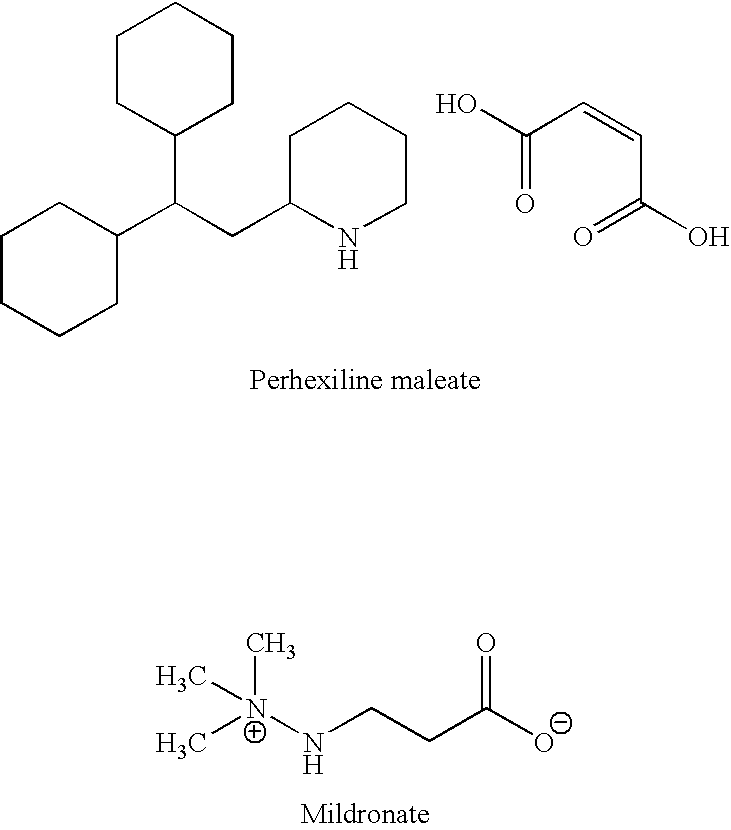
Methods are provided for preventing, reducing, and / or treating contrast-induced acute kidney injury which include administering an inhibitor of fatty acid oxidation to a patient in need thereof. Also provided are methods involving use of trimetazidine or pharmaceutically acceptable salts thereof for the prevention and / or treatment of contrast-induced acute kidney injury. Methods are also provided for preventing and / or treating contrast-induced acute kidney injury which include administration of one or more of trimetazidine, etomoxir, oxfenicine, perhexiline, mildronate, or ranolazine, or pharmaceutically acceptable salts of any of the preceding.
Owner:SAGHMOS THERAPEUTICS INC
A kind of solid pharmaceutical composition containing trimetazidine or its salt and its preparation method
ActiveCN108721235BSimple preparation processControllable parametersOrganic active ingredientsPharmaceutical non-active ingredientsMedicineTrimetazidine
The invention relates to a solid pharmaceutical composition containing trimetazidine or a salt thereof and a preparation method thereof. Specifically, the solid pharmaceutical composition of the present invention contains trimetazidine or a pharmaceutically acceptable salt or a solvate thereof as an active ingredient, and two or more sustained-release matrix materials. The solid pharmaceutical composition of the present invention has the characteristics of gentle release over a long period of time.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Production method of trimetazidine and its hydrochloride
The invention discloses trimetazidine and a production method of hydrochloride thereof, which belongs to the technical field of chemical synthesis and is characterized by adopting 2,3,4-3-methoxybenzaldehyde and piperazine as raw materials. The method comprises the following steps of: adding a solvent, 2,3,4-3-methoxybenzaldehyde and anhydrous piperazine with the molar ratio of 1:1 to 1:3, and a nickel-based catalyst accounting for 3 to 10% of the mass percentage of the 2,3,4-3-methoxybenzaldehyde and the anhydrous piperazine to a pressure kettle; using nitrogen for purging before hydrogen isled in, with hydrogen pressure maintained within the range of 0.7MPa to 2.0MPa, reaction temperature within the range of 50 DEG C to 95 DEG C, reaction time within the range of 4 hours to 10 hours, and pH value regulated to 3 to 4; separating out organic phase; and recovering the solvent. The water phase is washed with chlorinated hydrocarbon extraction, and the pH value is regulated to 12, then the water phase is extracted with aromatic hydrocarbon, and the aromatic hydrocarbon in the aromatic hydrocarbon extraction liquid is steamed out, thus obtaining the trimetazidine. The invention has the advantages of low production cost, high yield, and good environmental protection.
Owner:BEIJING JIALIN PHARM INC
Medicine composition for treating heart failure and application thereof
InactiveCN104434920AReduce adverse reactionsProtect mitochondriaOrganic active ingredientsCardiovascular disorderTrimetazidineCardiac muscle
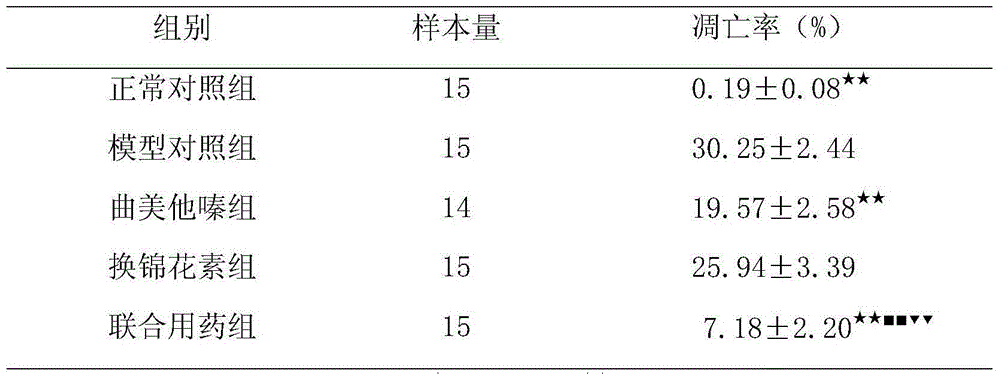
The invention discloses a medicine composition for treating heart failure and application thereof. The medicine composition is prepared from an active ingredient and medicinal auxiliaries, wherein the active ingredient comprises (1) tortuosine and (2) trimetazidine or medicinal salt thereof. The medicine composition can be used for synergistically resisting myocardial apoptosis and protecting cardiac muscle mitochondria so as to achieve the aim of preventing and treating heart failure.
Owner:QINGDAO MUNICIPAL HOSPITAL
Pet health food formula for enhancing heart vitality and preparation method thereof
InactiveCN112273536AEnhance myocardial contractilityReduce myocardial oxygen consumptionAnimal feeding stuffAccessory food factorsFormularyDisease
The invention discloses a pet health food formula for enhancing heart vitality and a preparation method thereof. The formula consists of the following components: astragalus membranaceus, ginseng, lucid ganoderma, honey-fried licorice roots, salviae miltiorrhizae, metoprolol and trimetazidine. The preparation method comprises the following steps: 1) cleaning; 2) crushing; 3) decocting; and 4) mixing. Compared with the prior art, the formula has the advantages that: the formula is simple, convenient to prepare and capable of increasing myocardial contractility of pets, reducing myocardial oxygen consumption of the pets and solving the problems of weakness and shortness of breath of the pets, so that exercise tolerance is improved, and disease resistance of the pets is enhanced. The formulahas good market popularization values.
Owner:王志全
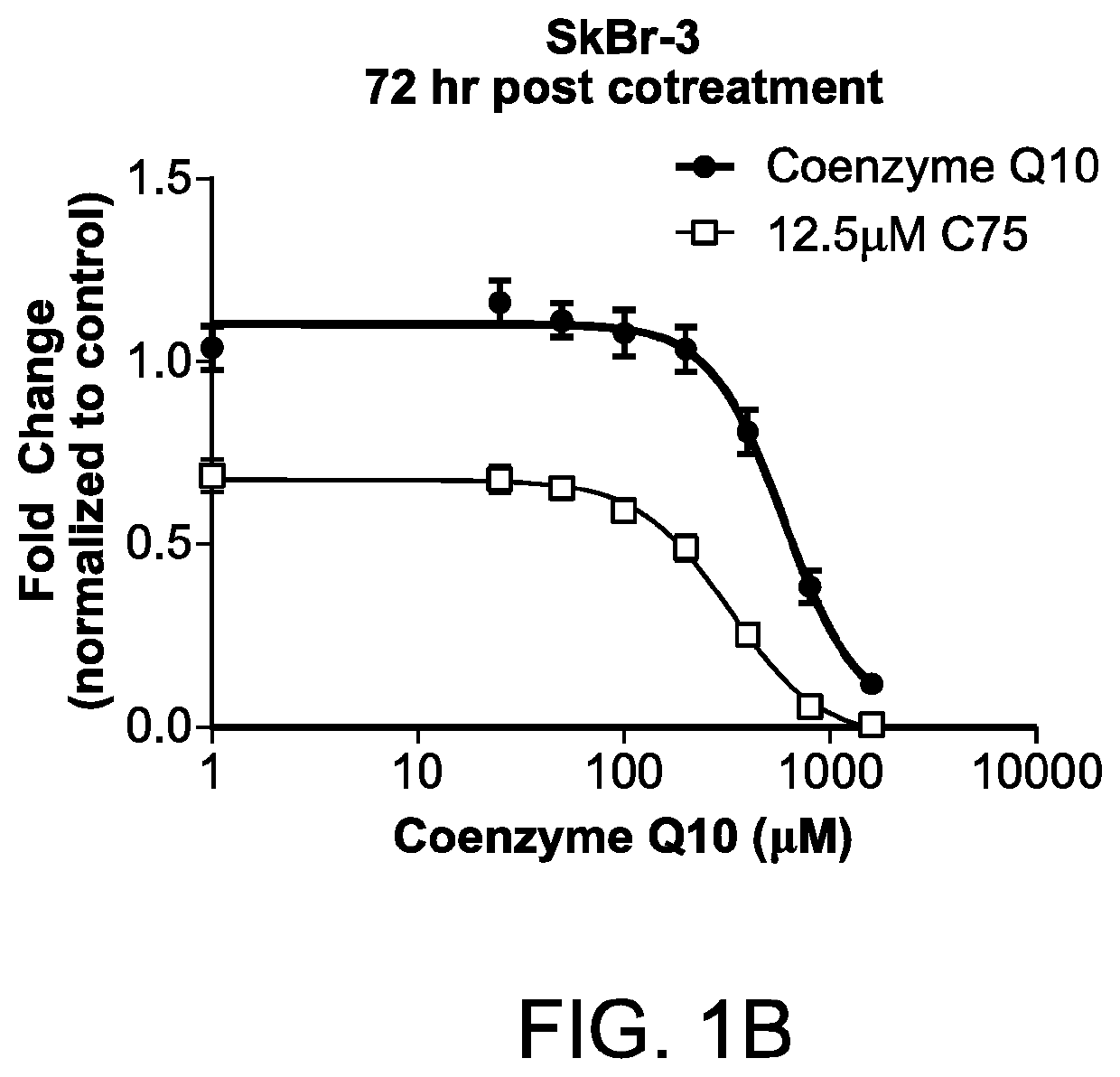
Methods for the treatment of cancer using coenzyme q10 and fatty acid metabolism inhibitors
InactiveUS20200330400A1Promote degradationHigh activityPharmaceutical delivery mechanismPharmaceutical non-active ingredientsLipidomeTrimetazidine
Presented herein are methods for the treatment of oncological disorders by the co-administration of CoQ10 compositions and at least one fatty acid metabolism inhibitor. In one embodiment, the CoQ10 compositions are lipid-containing compositions. The fatty acid metabolism inhibitor may be an inhibitor of fatty acid synthesis, storage, transport or degradation. The fatty acid metabolism inhibitor may also be a modulator of fatty acid structure, for example a fatty acid desaturase or elongase. The fatty acid inhibitor may inhibit any molecule involved in fatty acid metabolism, such as fatty acid synthase (FASN), carnitine palmitoyltransferase 1 (CPT-1), long-chain 3-ketoacyl-CoA thiolase, or stearoyl-CoA desaturase-1 (SCD-1). In embodiments, the fatty acid metabolism inhibitor may be C75, Etomoxir, trimetazidine or A939572.
Owner:BERG
Sustained-release micro-pellet of trimetazidine and preparation process thereof
InactiveCN100571703CImprove liquidityUniform absorption rate in the bodyOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsMedicine
The invention relates to a slow-release micro drop whose active component is Humeitashen or other salt, wherein it is formed by element and film layer that controlling the drug release, whose weight ratio is 20:1-5:1; the Humeitashen content of element is 10-60%. The invention mainly uses protrusion method to prepare drop, and uses fluidize bed to pack.
Owner:SHANDONG INST OF PHARMA IND
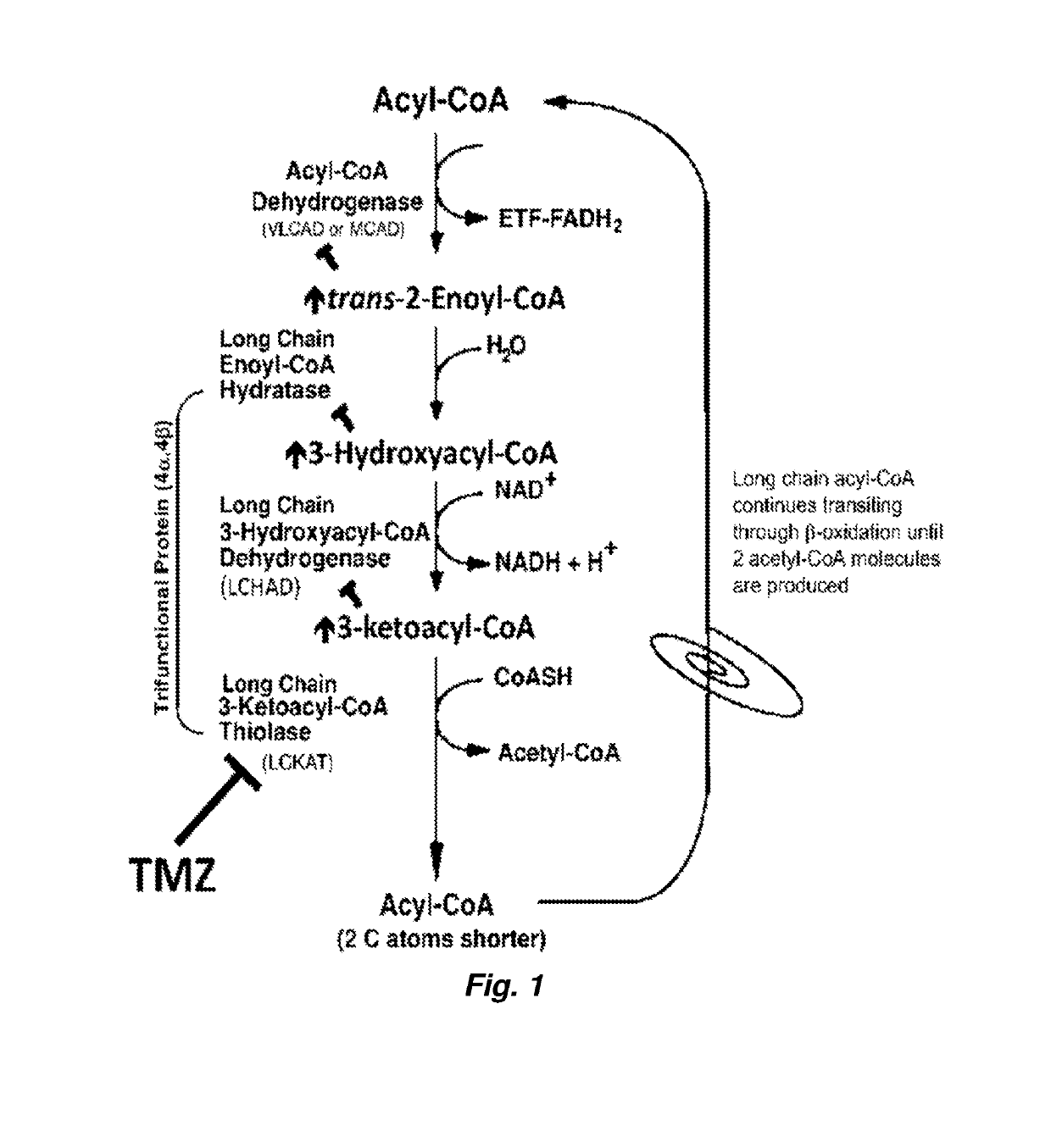
Therapy for Mitochondrial Fatty Acid Beta-Oxidation and Transport Disorders
ActiveUS20190290642A1High activityLoss of activityOrganic active ingredientsPeptide/protein ingredientsMutated proteinTrimetazidine
Methods of treating mitochondrial fatty acid b-oxidation and / or transport disorders arising from mutant proteins in the mitochondrial fatty acid β-oxidation and transport metabolic pathways in patients are provided. The methods modulate the mitochondrial fatty acid β-oxidation pathway at the last step so that the product of the mutant protein accumulates and stabilizes the mutant protein and / or the substrate(s) / product(s) of the down stream reactions accumulate and possibly bind to allosteric sites on the mutant protein to stabilize it. Trimetazidine pharmacodynamics function as such in the β-oxidation pathway. Further, a synergistic effect is observed where trimetazidine and PPARδ agonist combination enhanced enzyme activity and presence significantly more than either alone.
Owner:UNIVERSITY OF PITTSBURGH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com