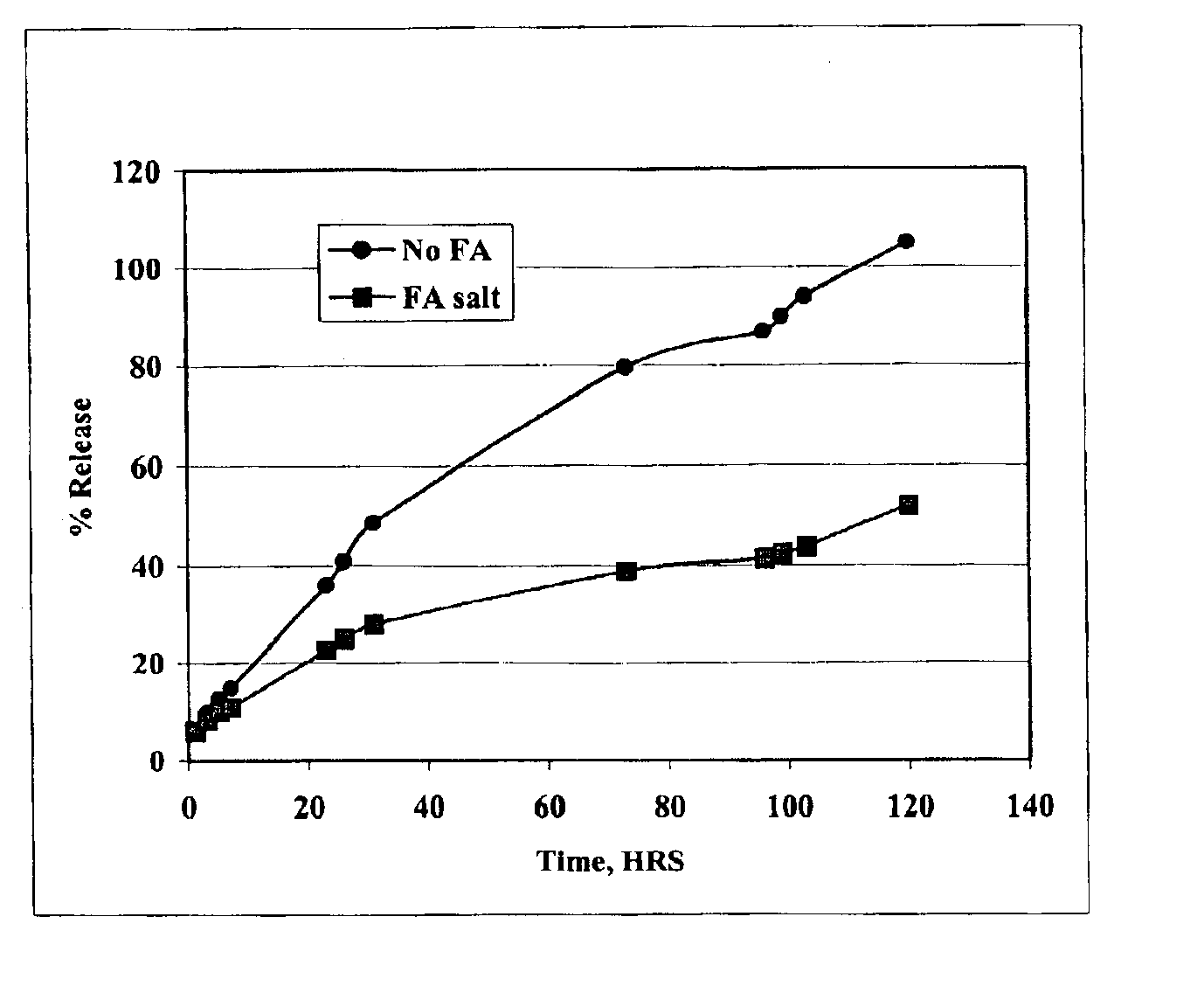
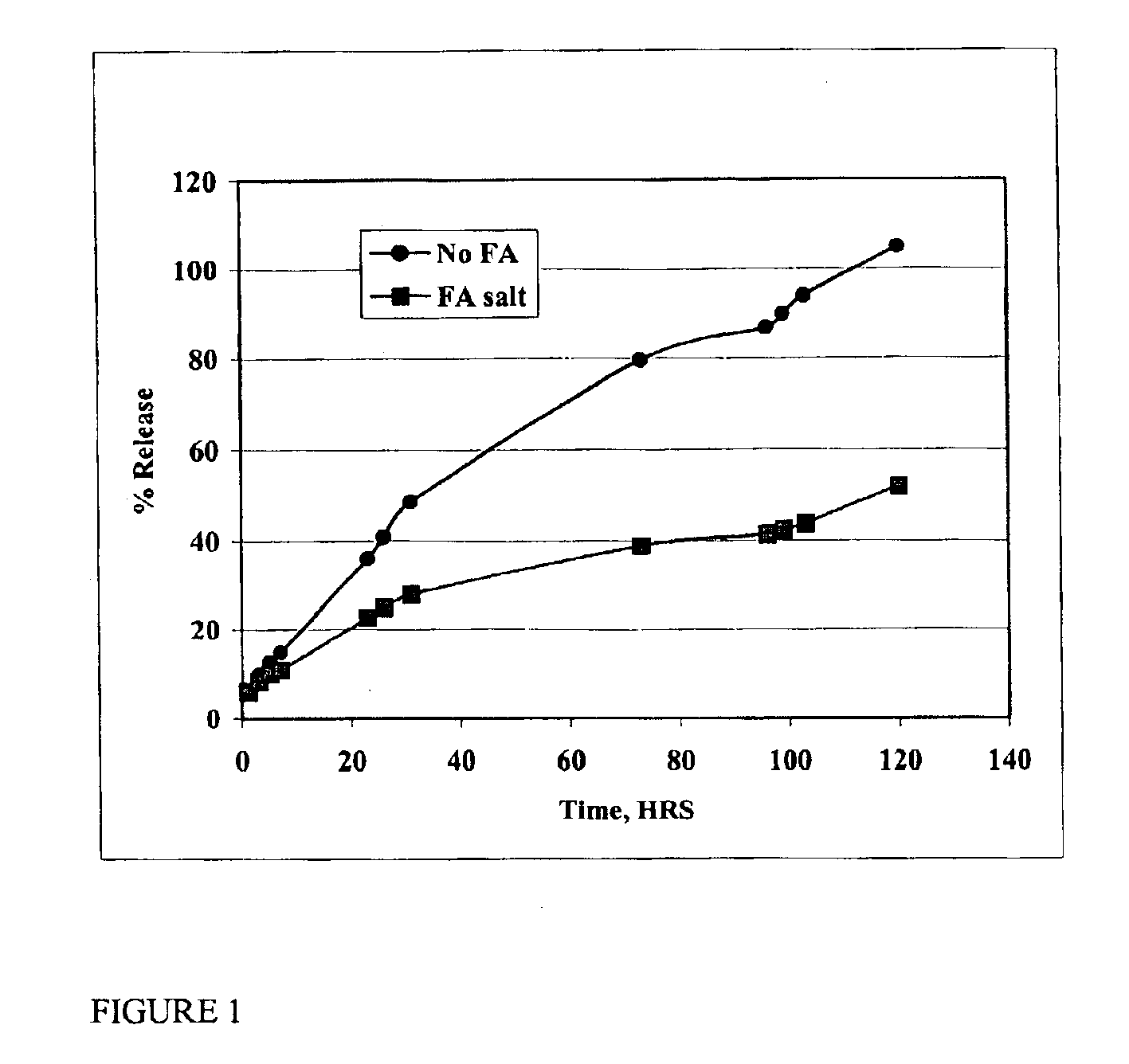
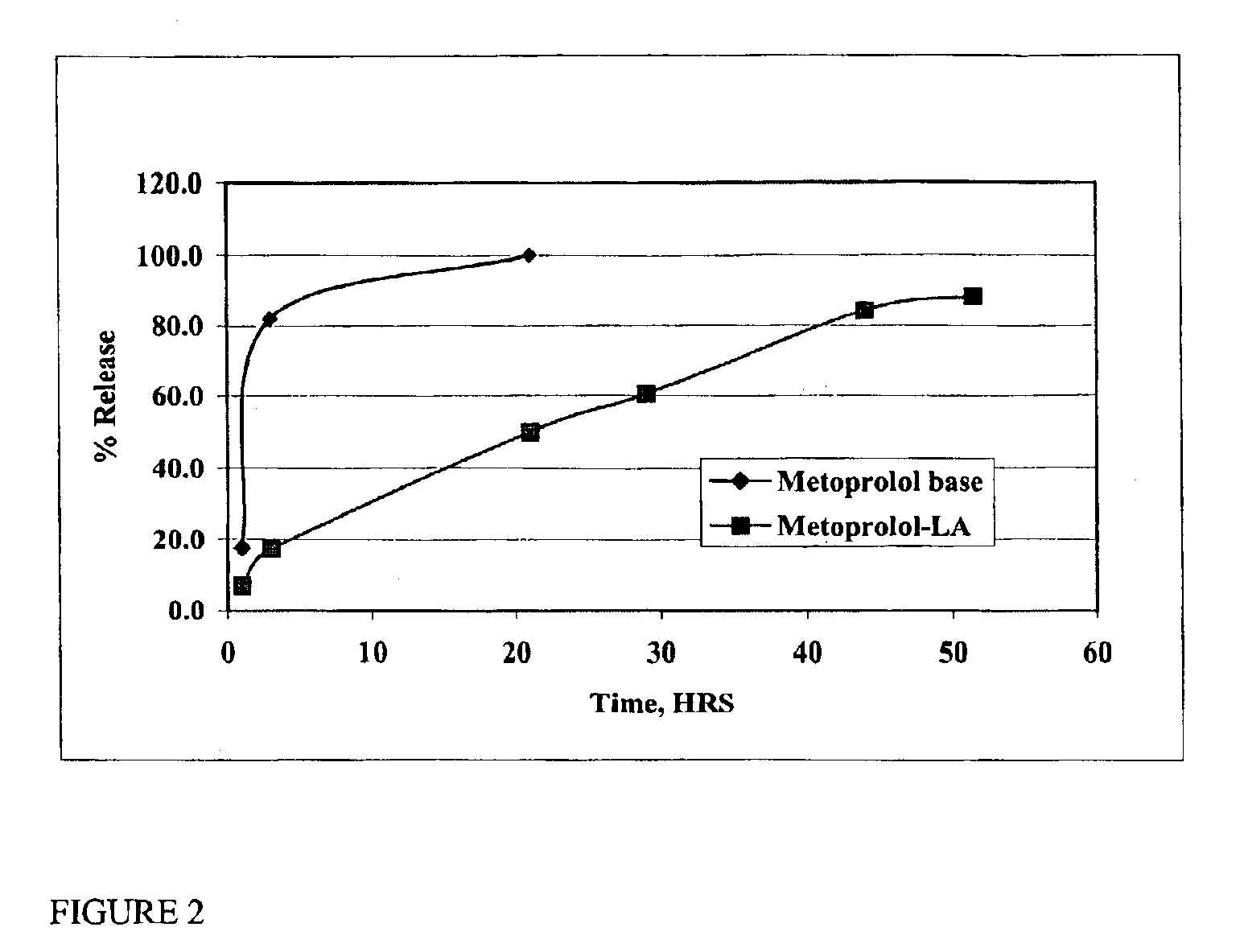
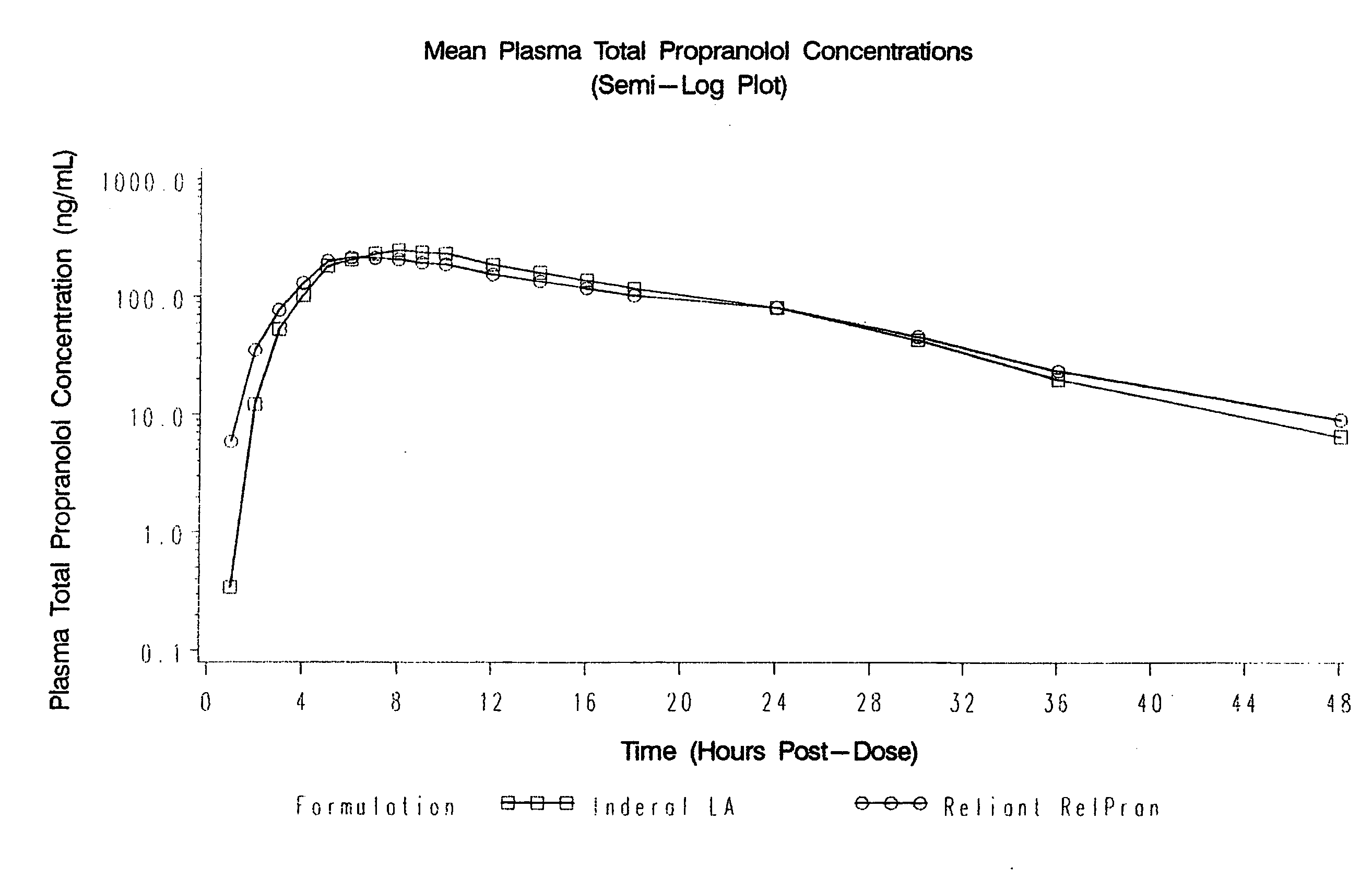
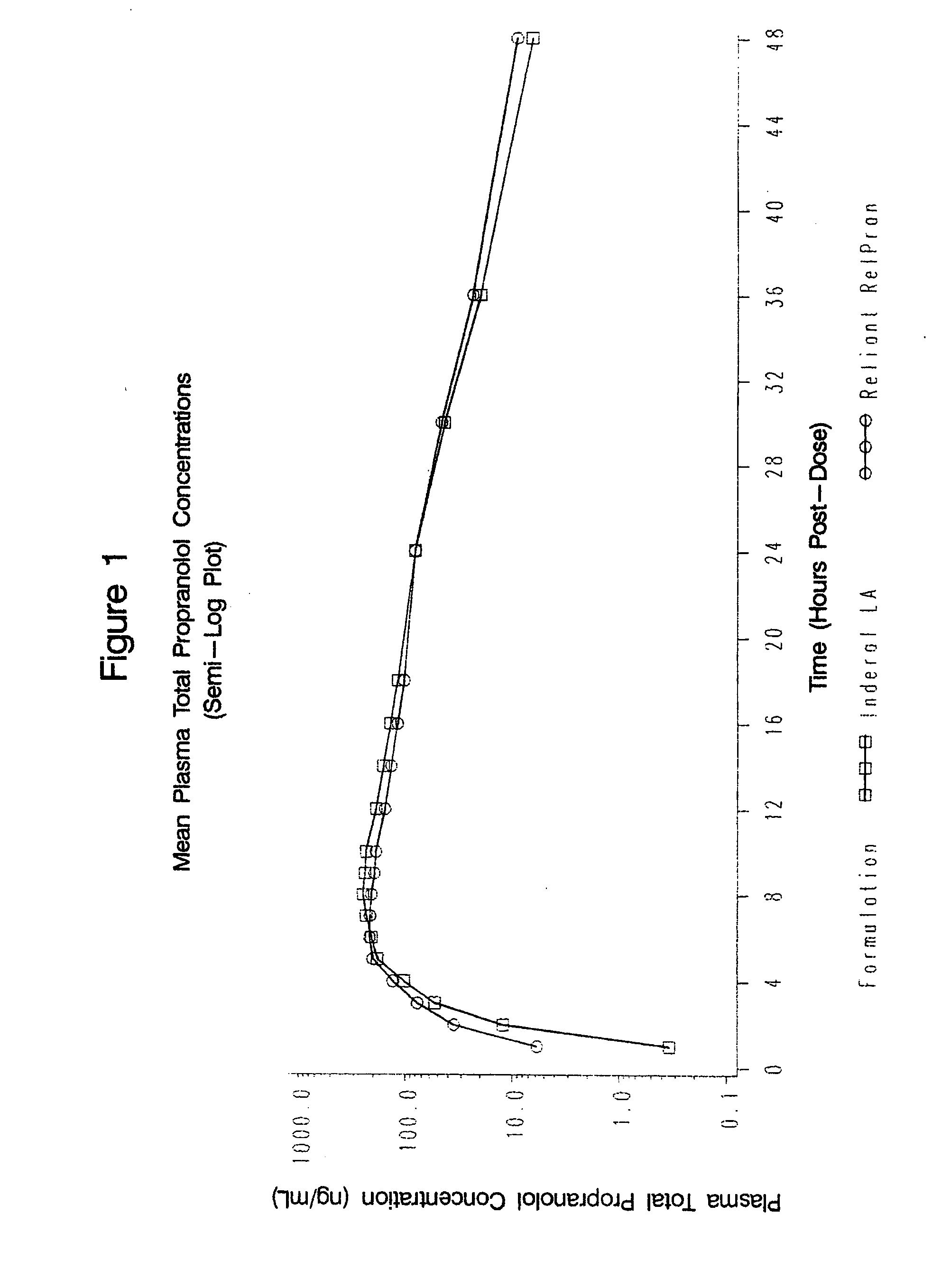
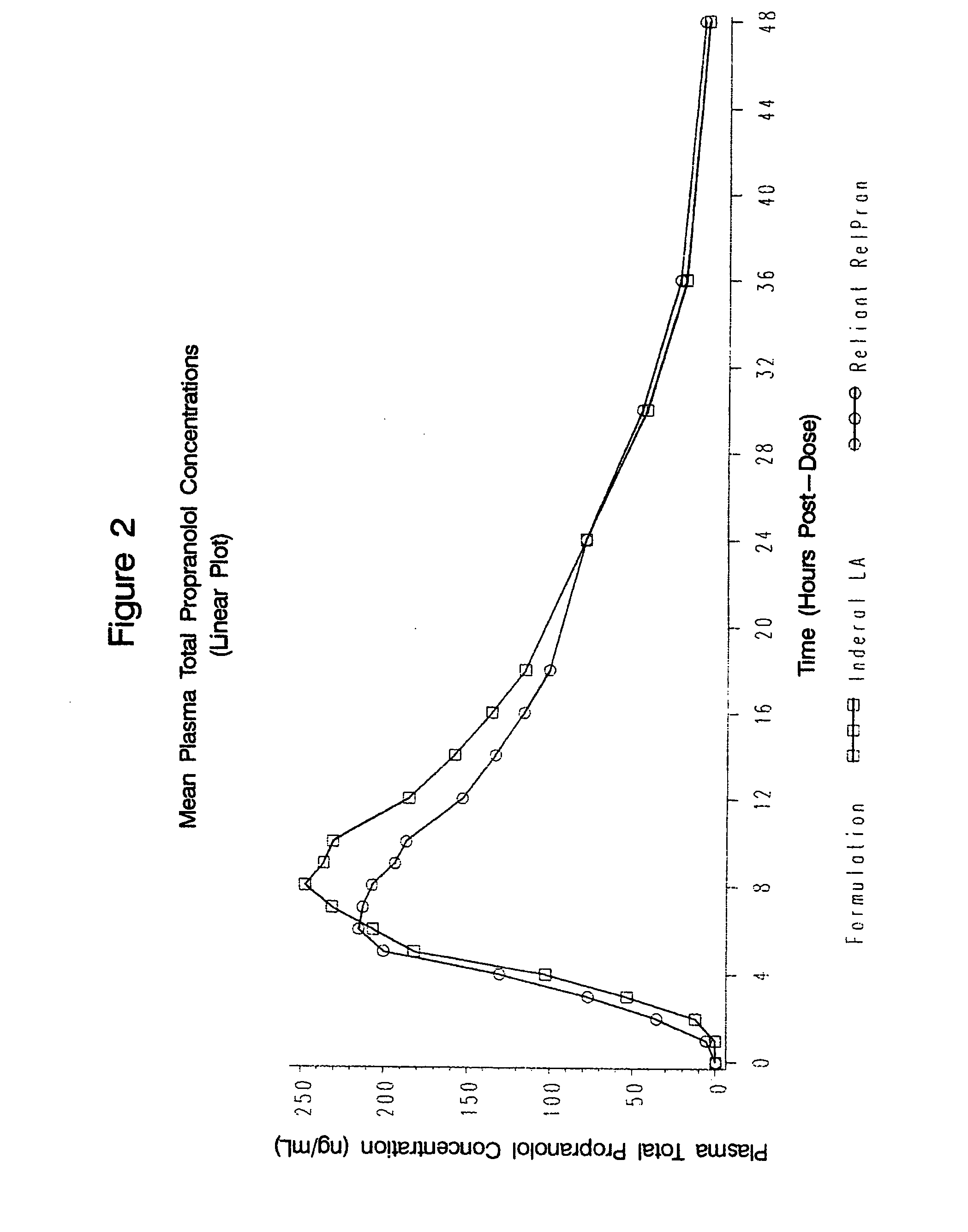
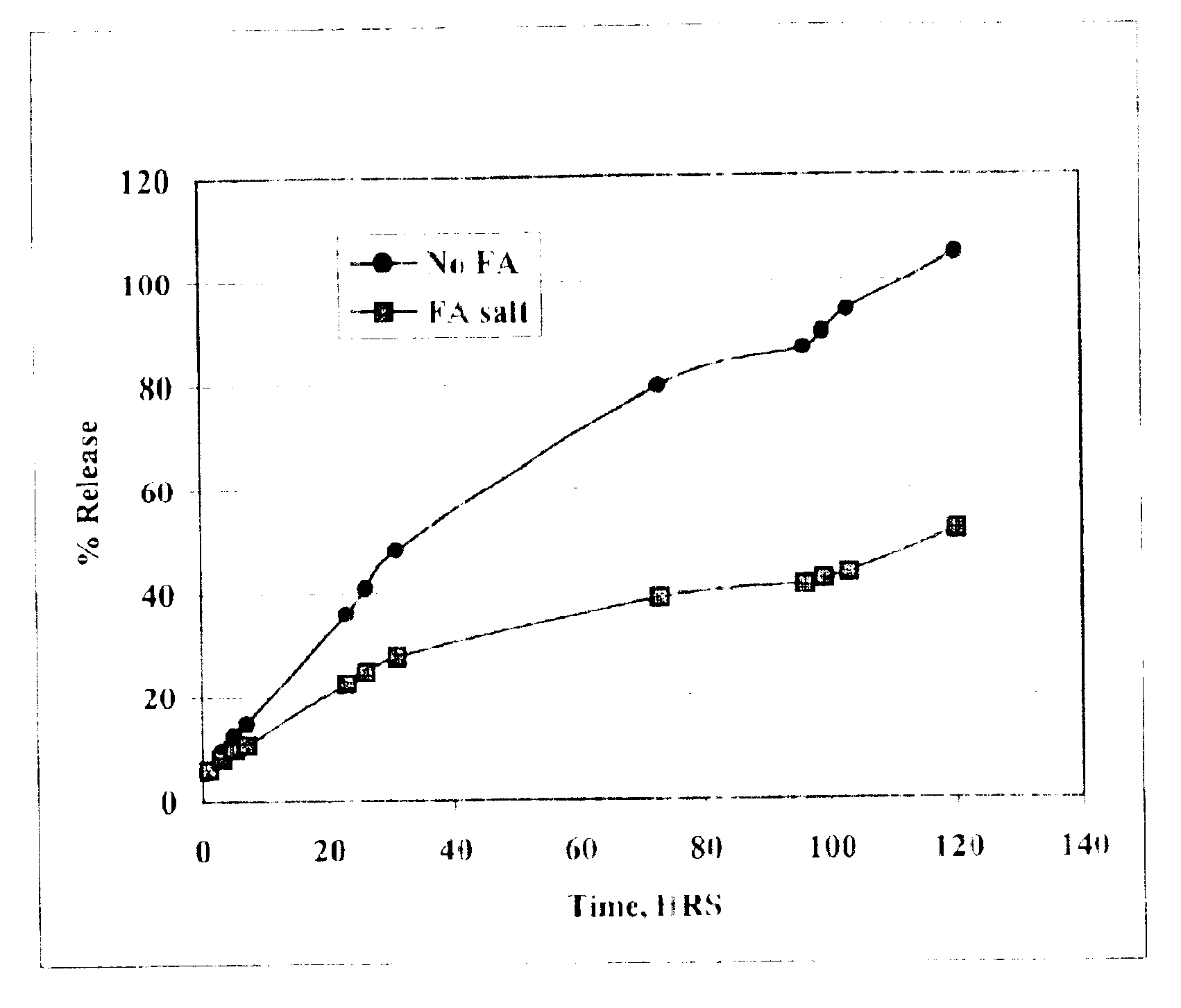
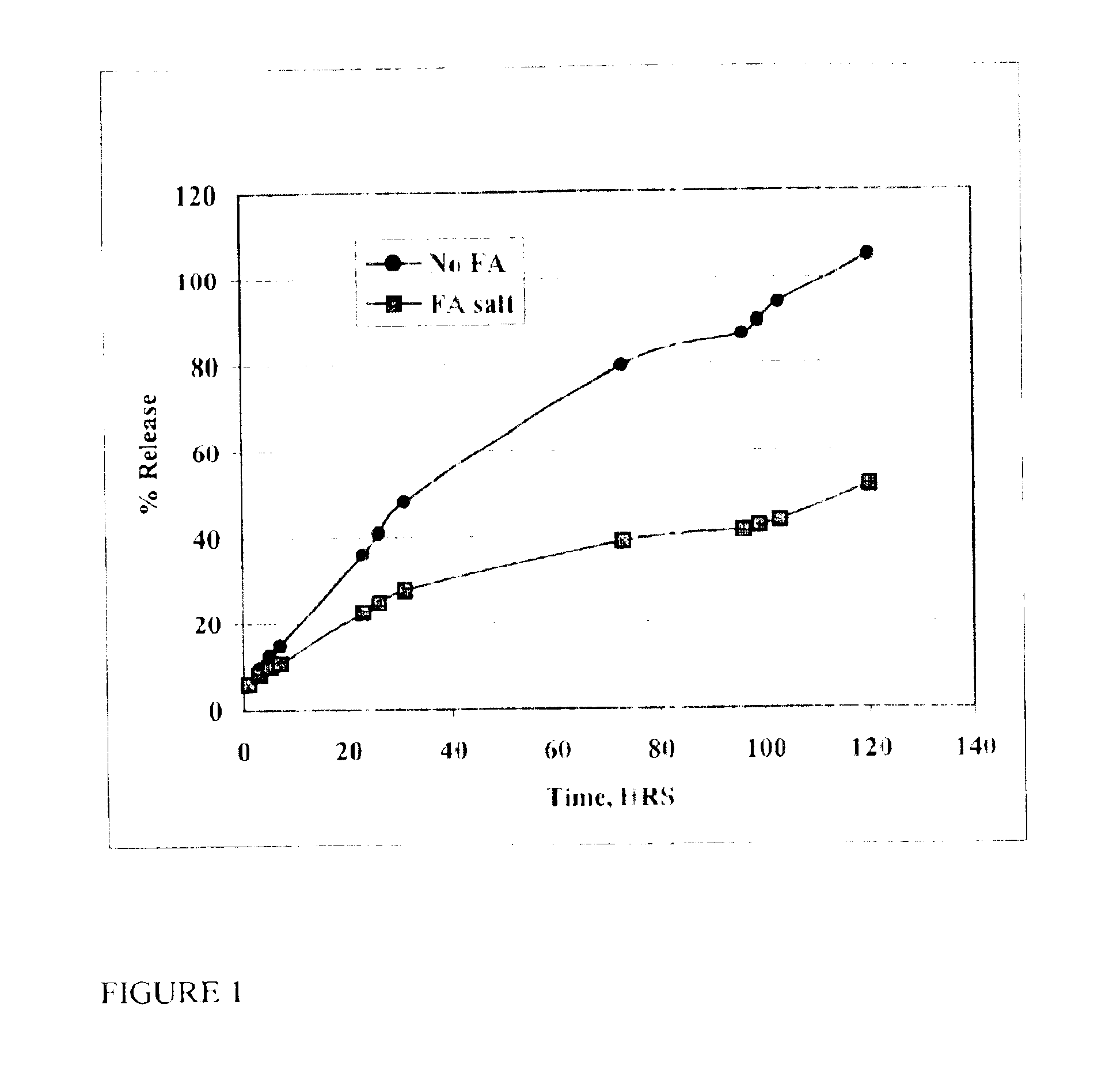
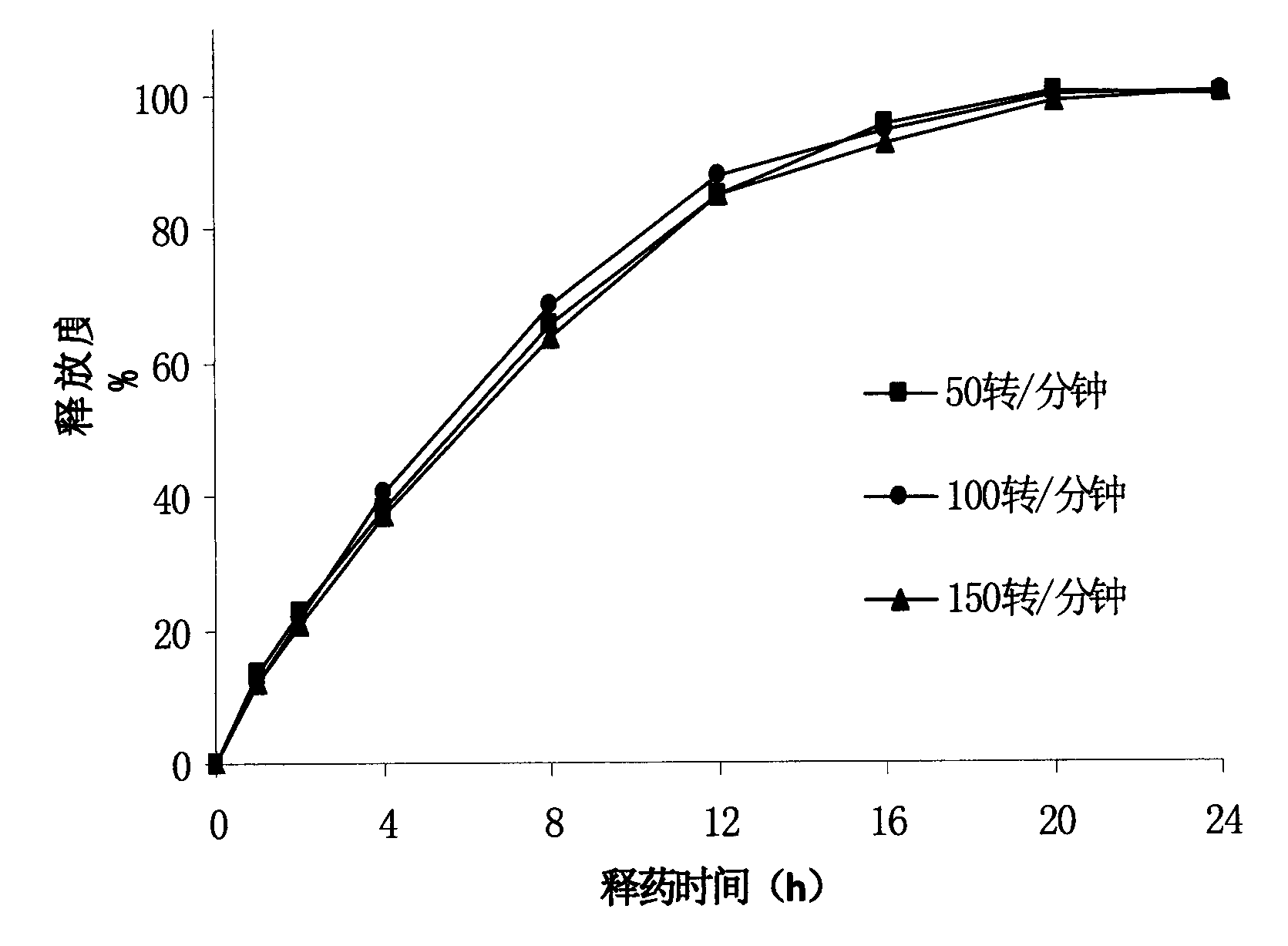
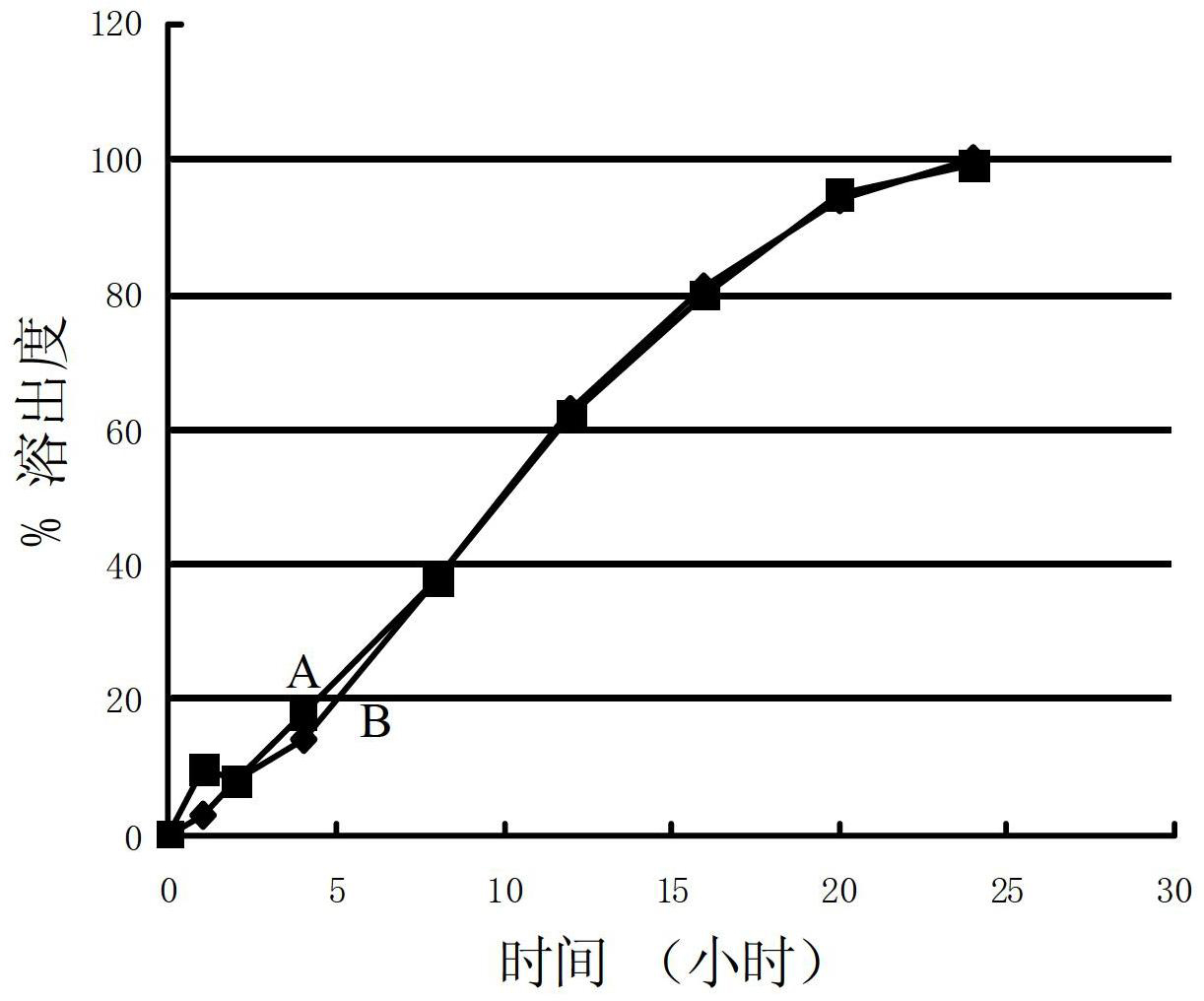
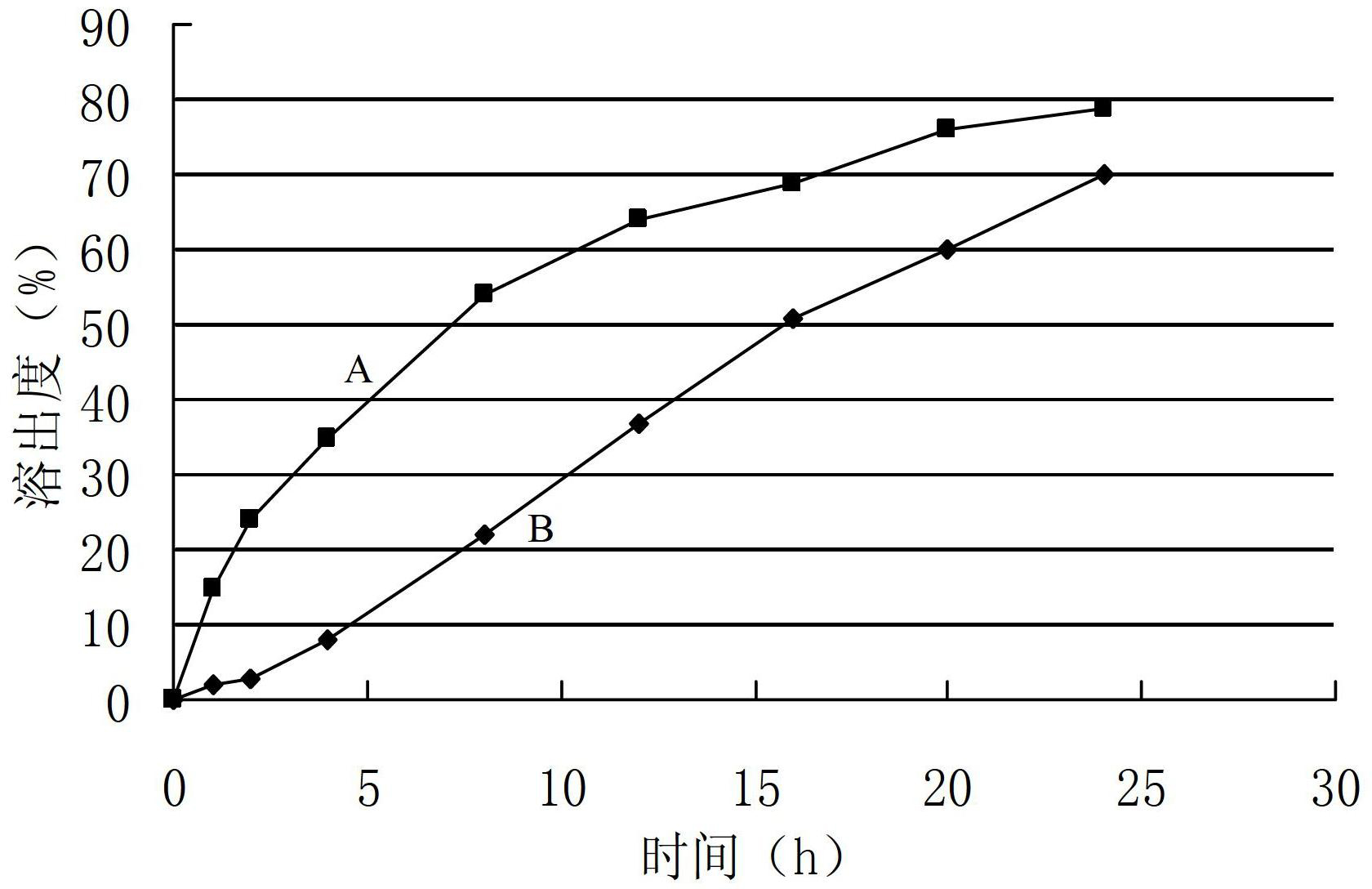
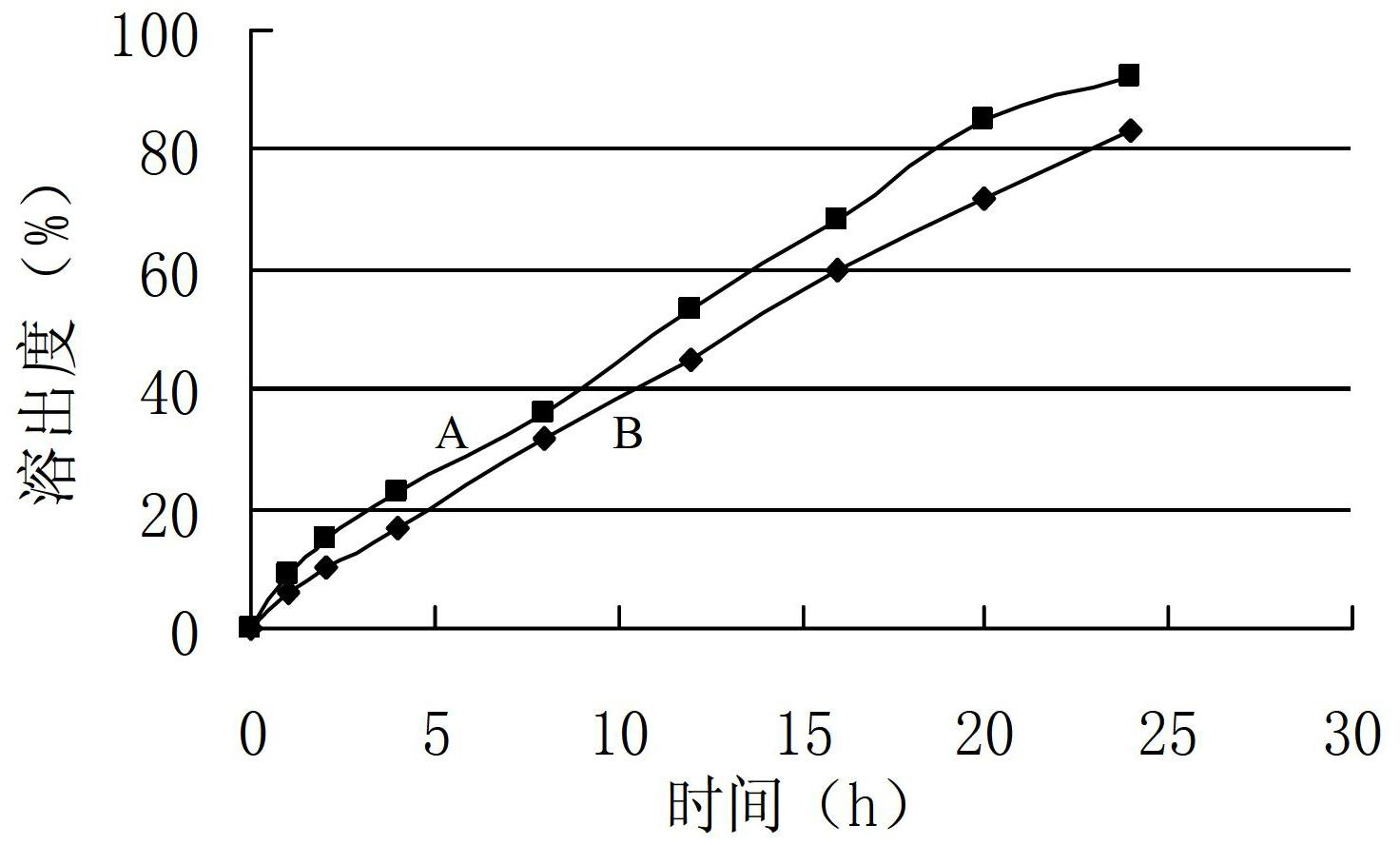
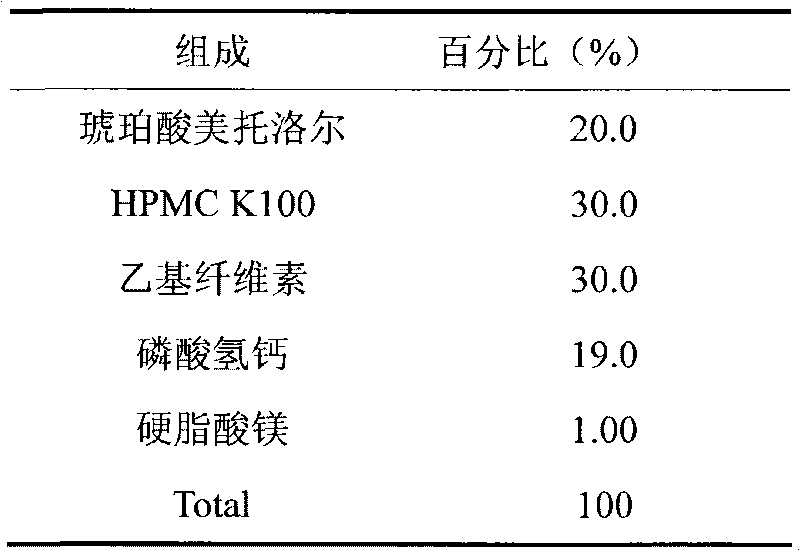
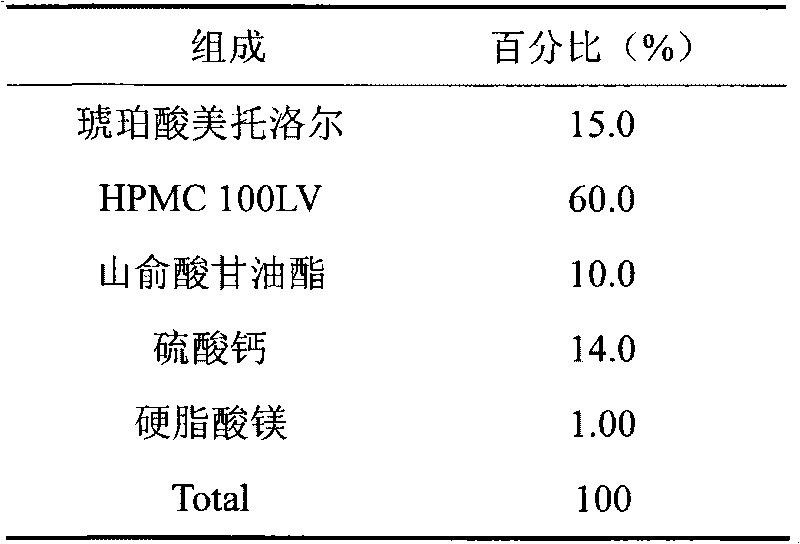
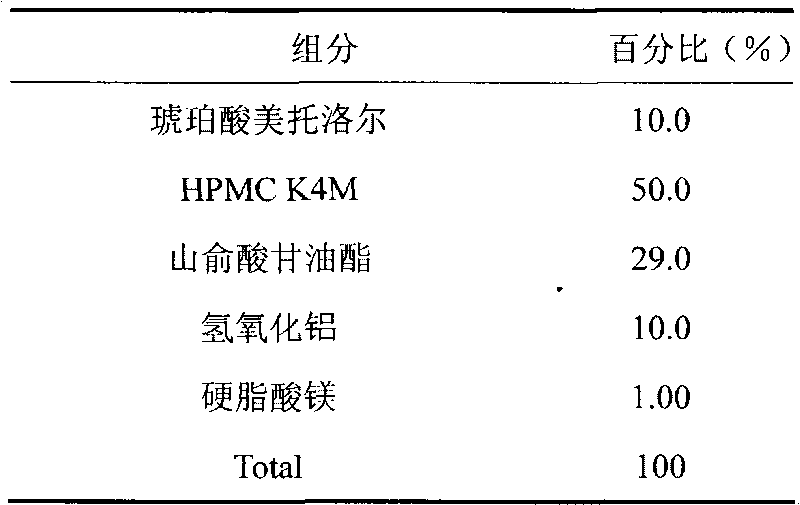
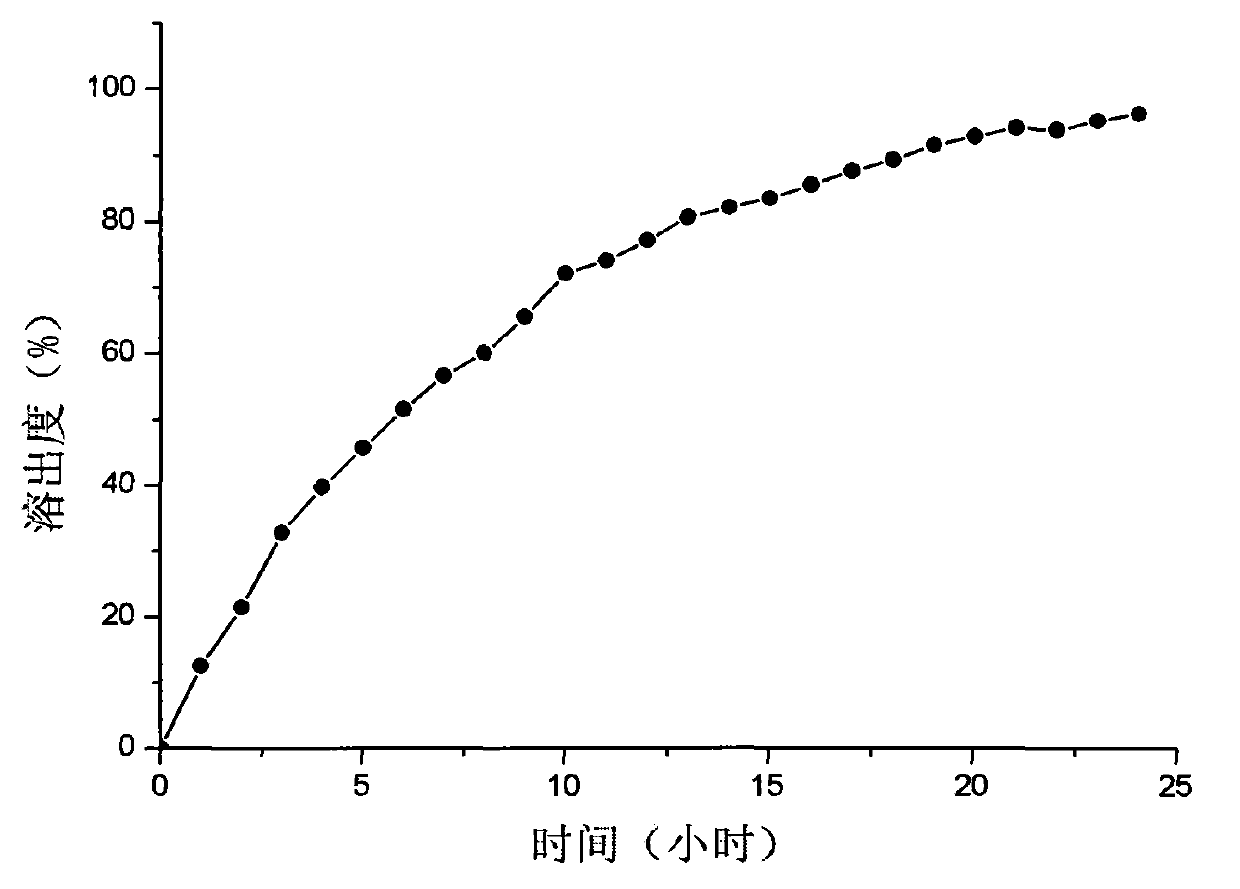
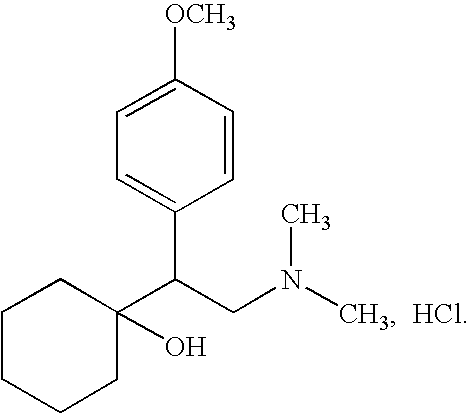
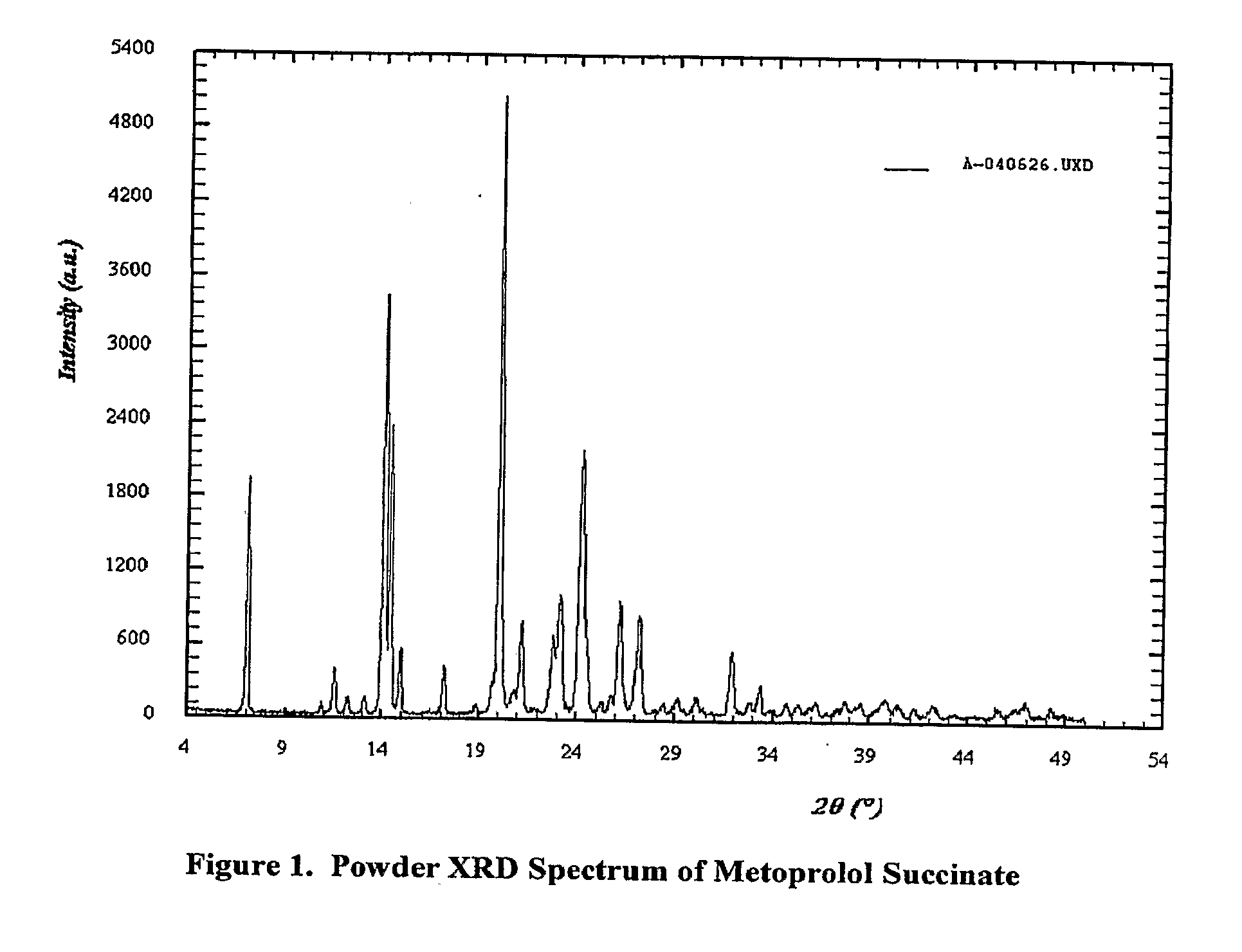
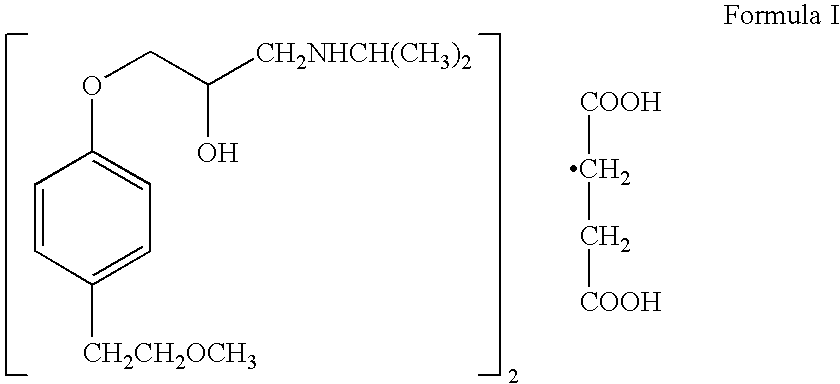
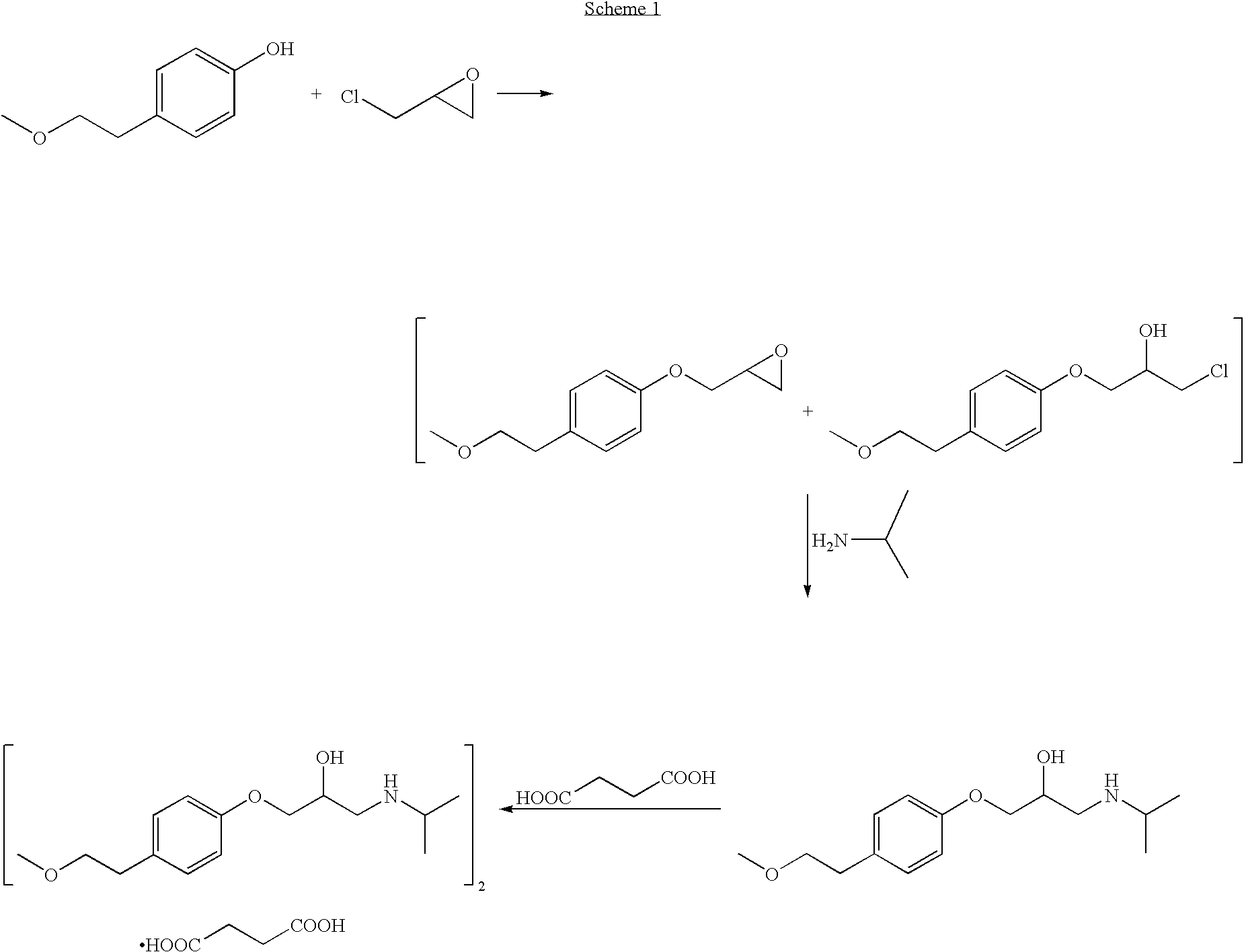
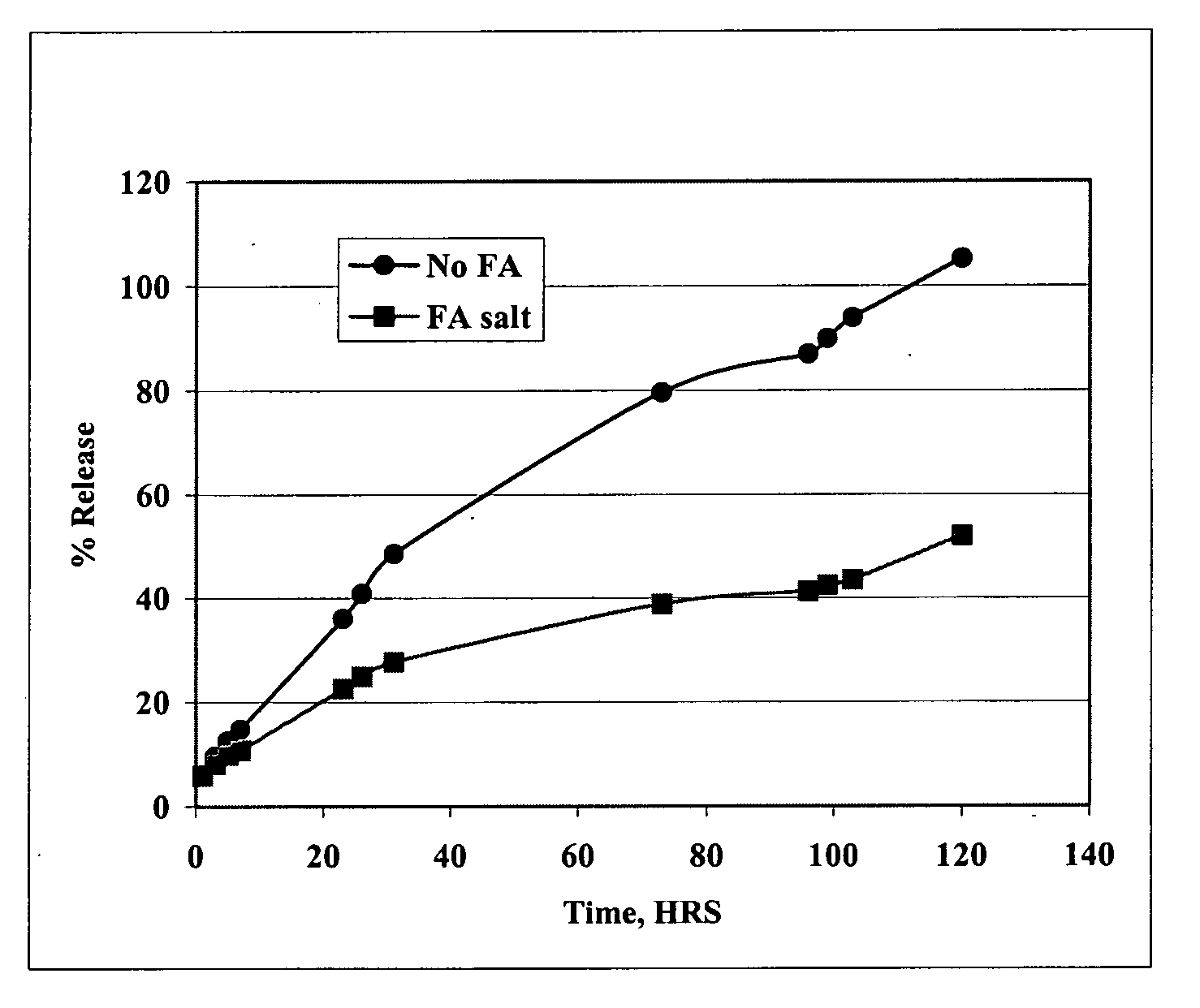
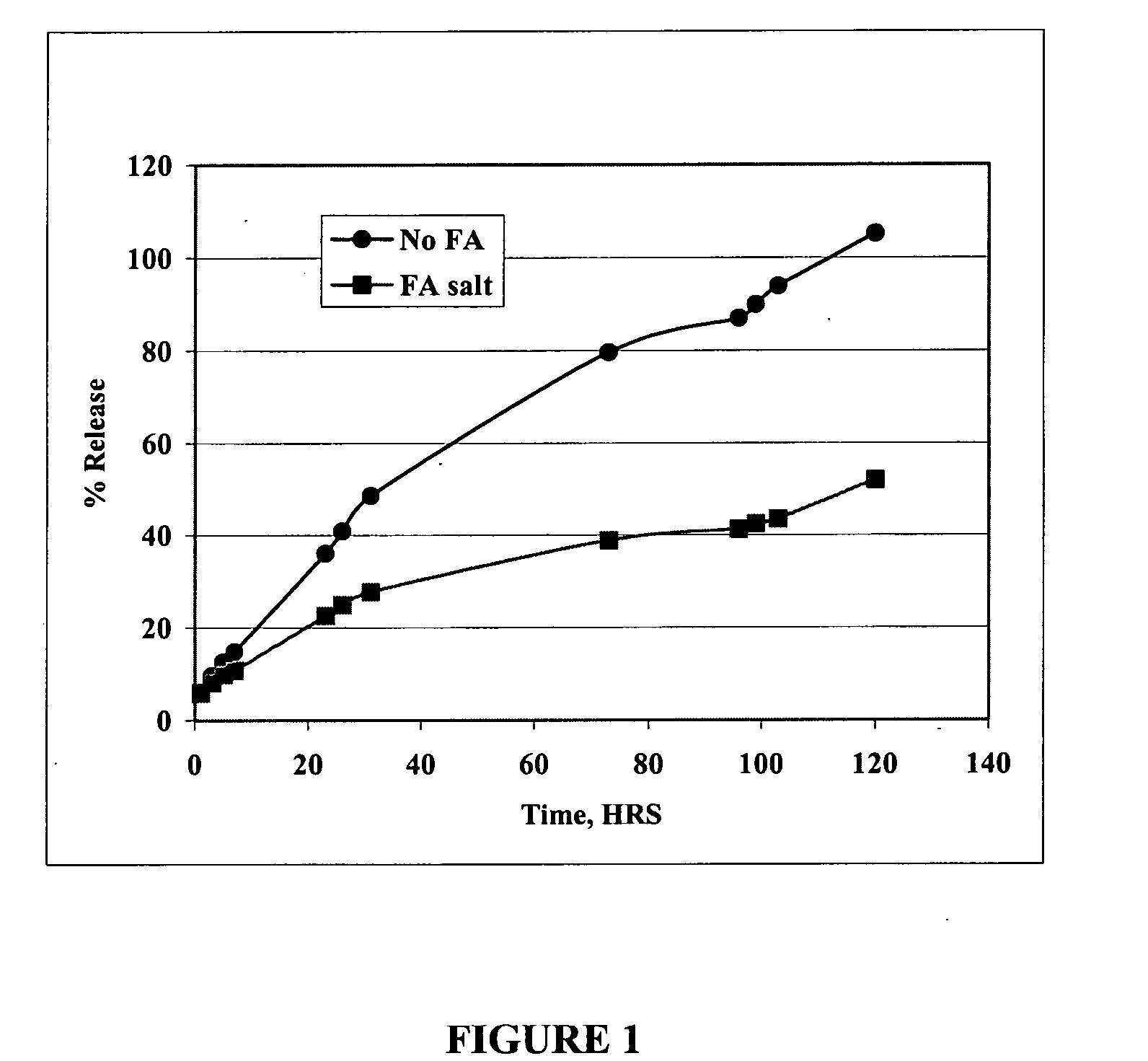
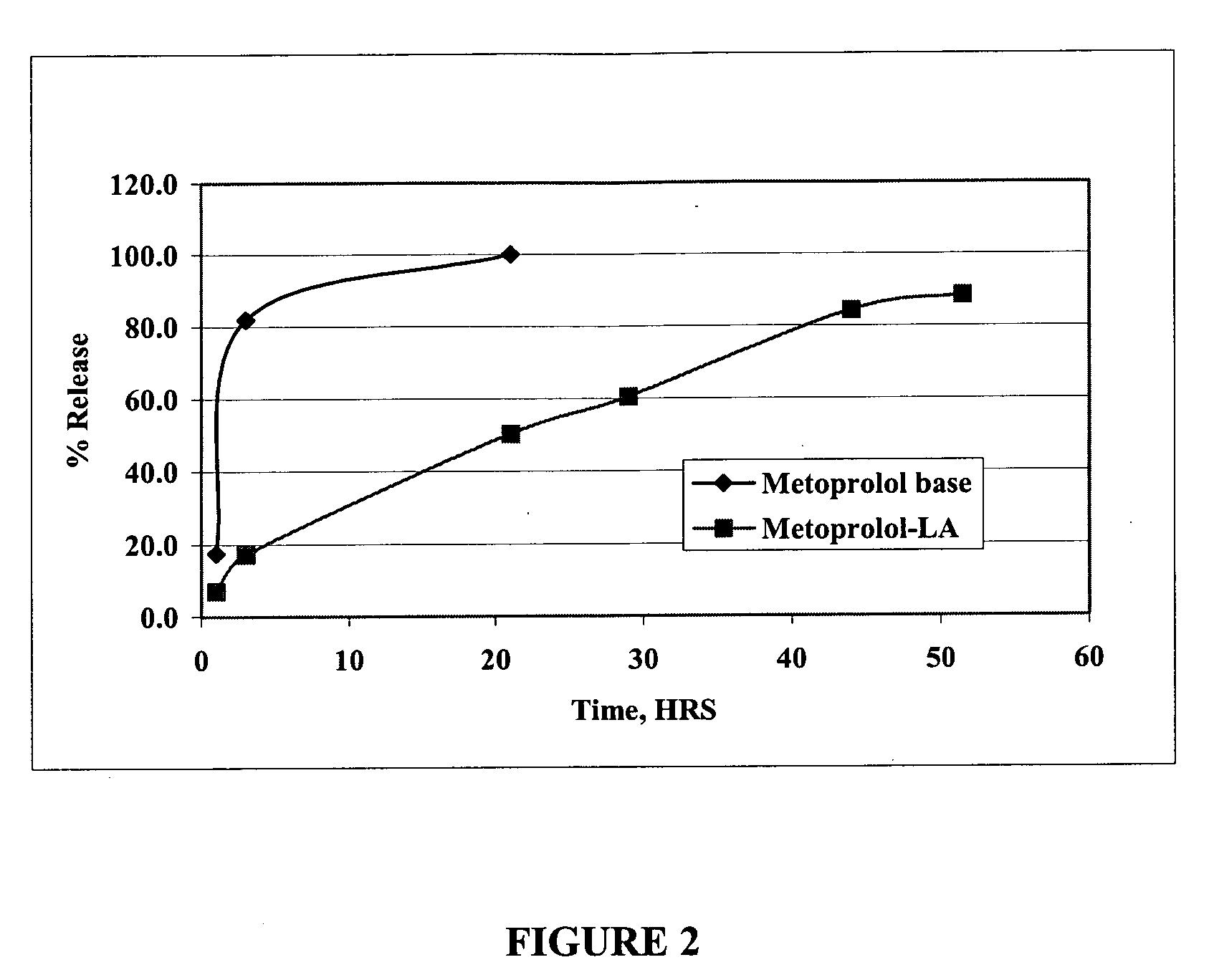
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
127 results about "Metoprolol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
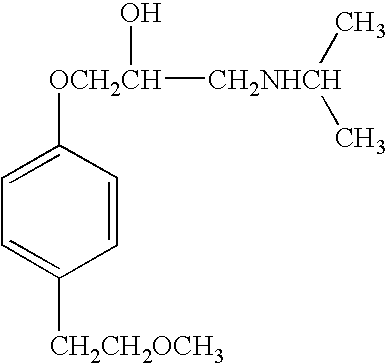
Metoprolol, marketed under the tradename Lopressor among others, is a medication of the selective β₁ receptor blocker type. It is used to treat high blood pressure, chest pain due to poor blood flow to the heart, and a number of conditions involving an abnormally fast heart rate. It is also used to prevent further heart problems after myocardial infarction and to prevent headaches in those with migraines.
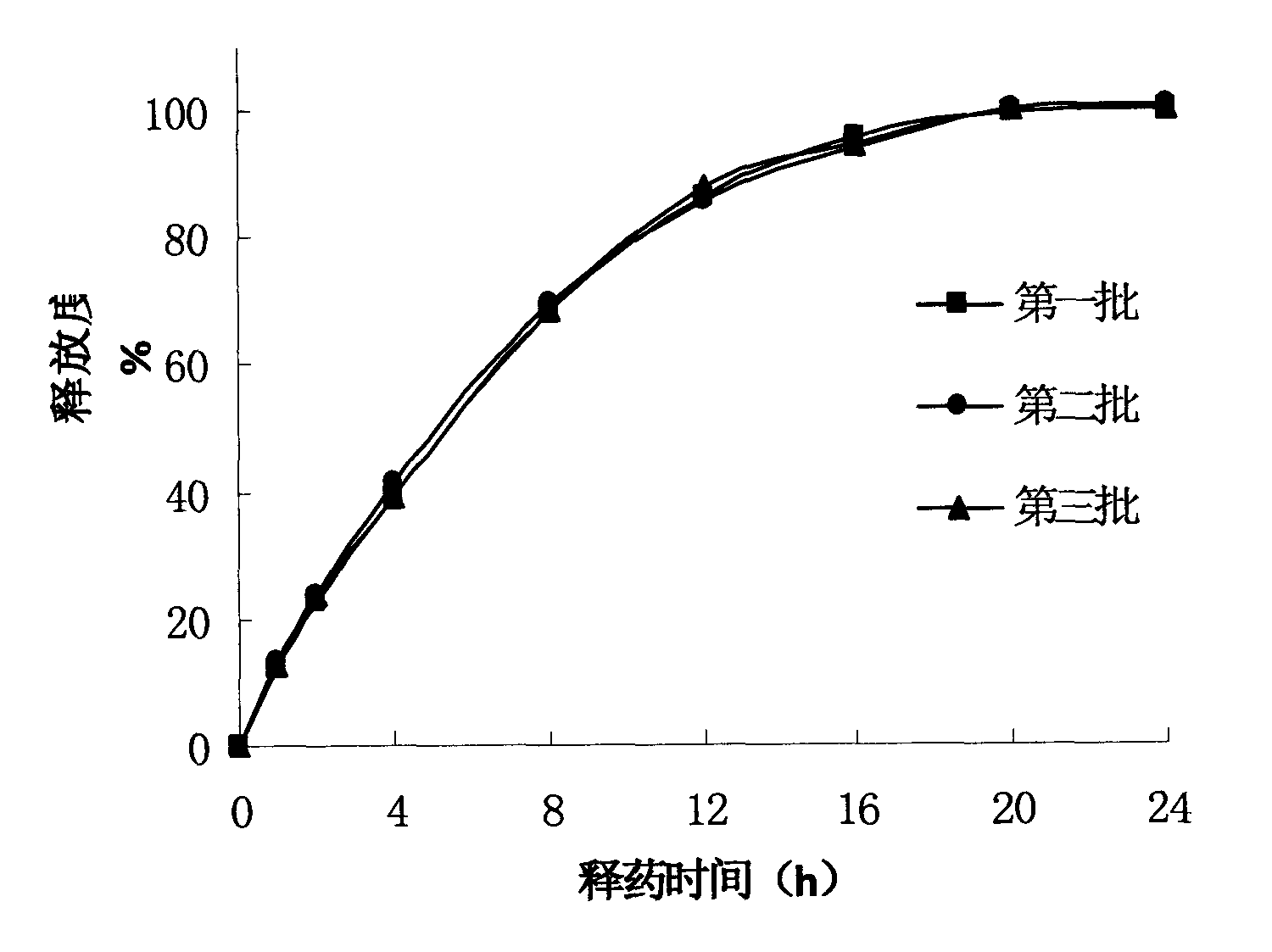
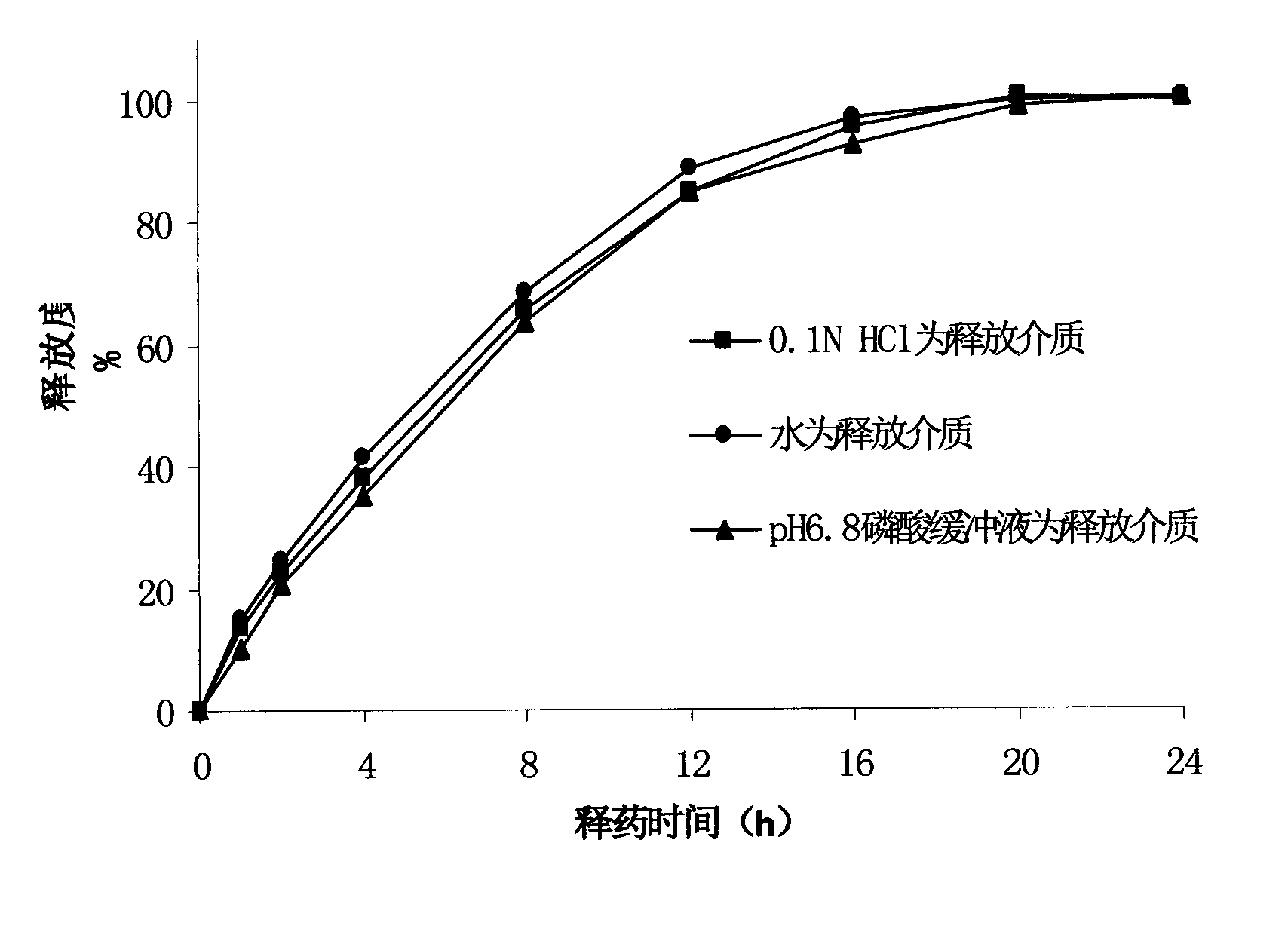
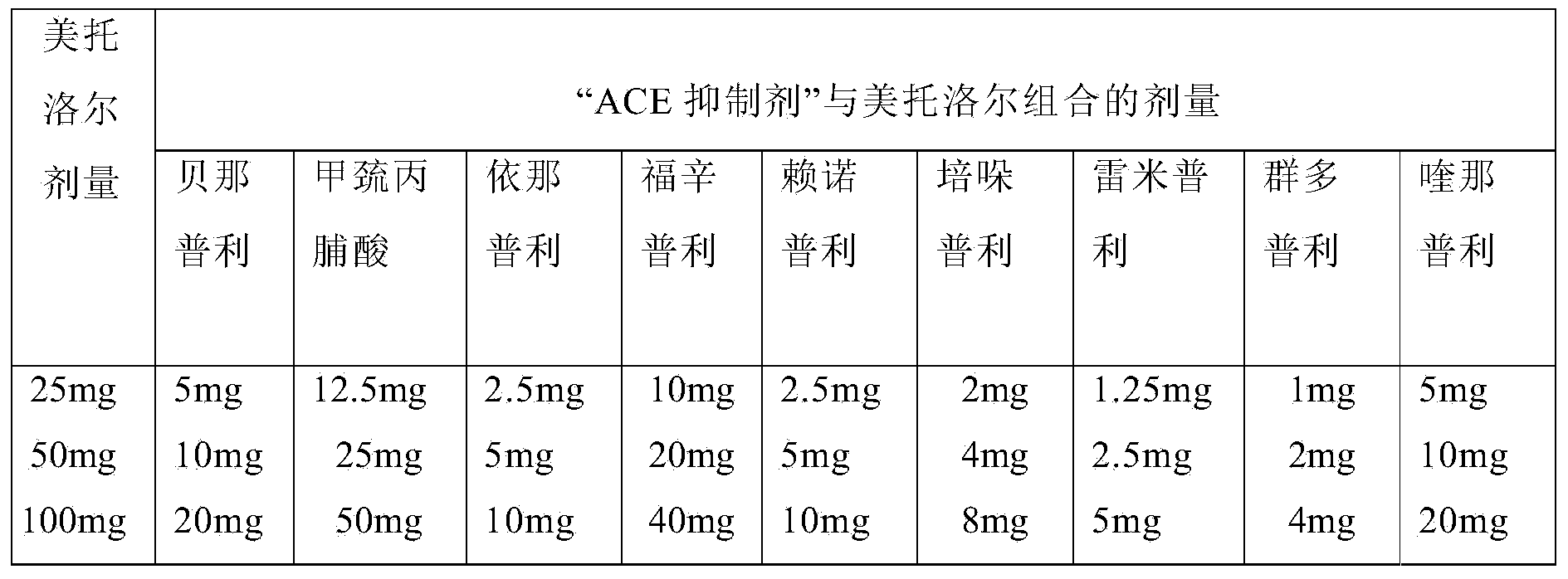
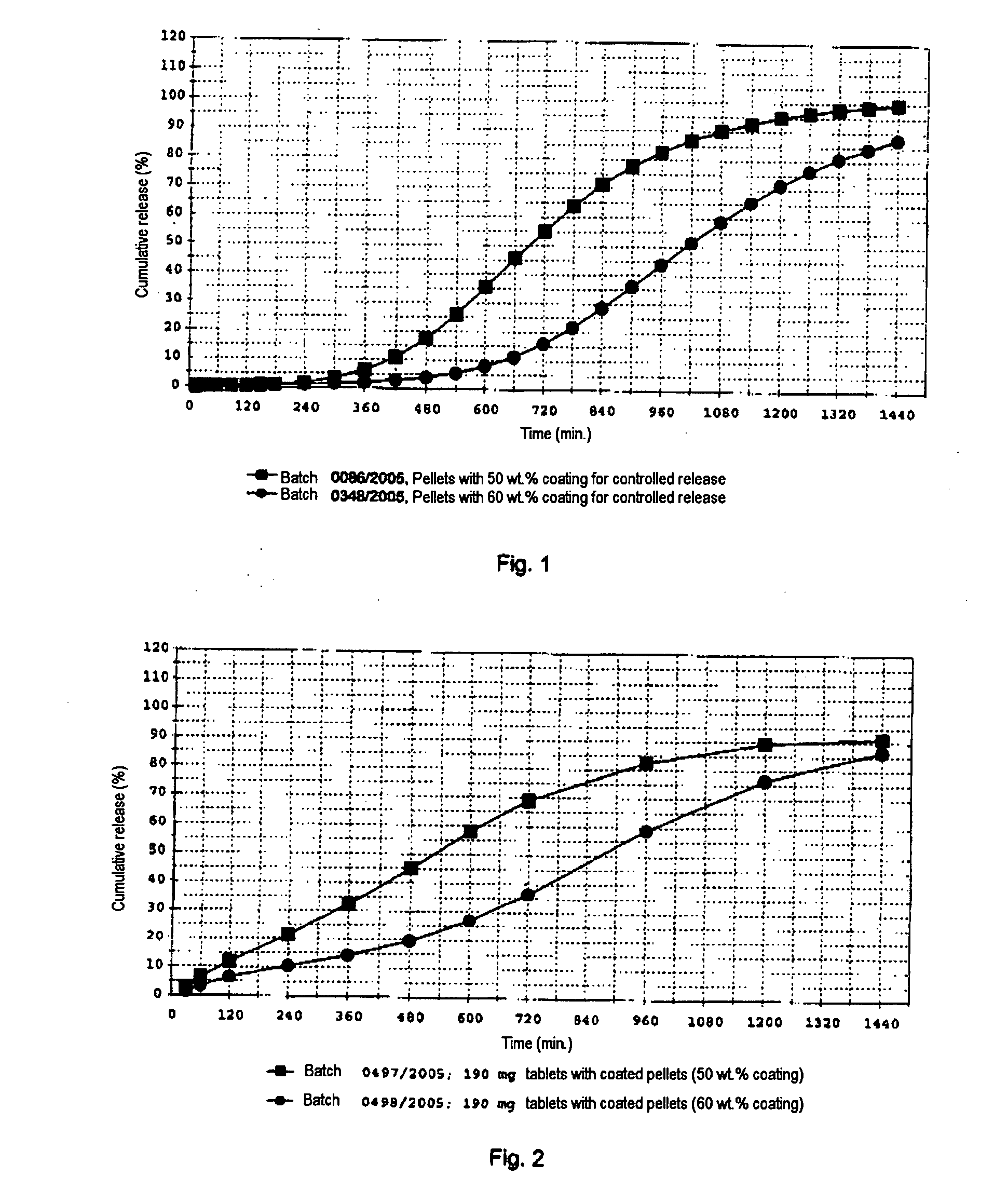
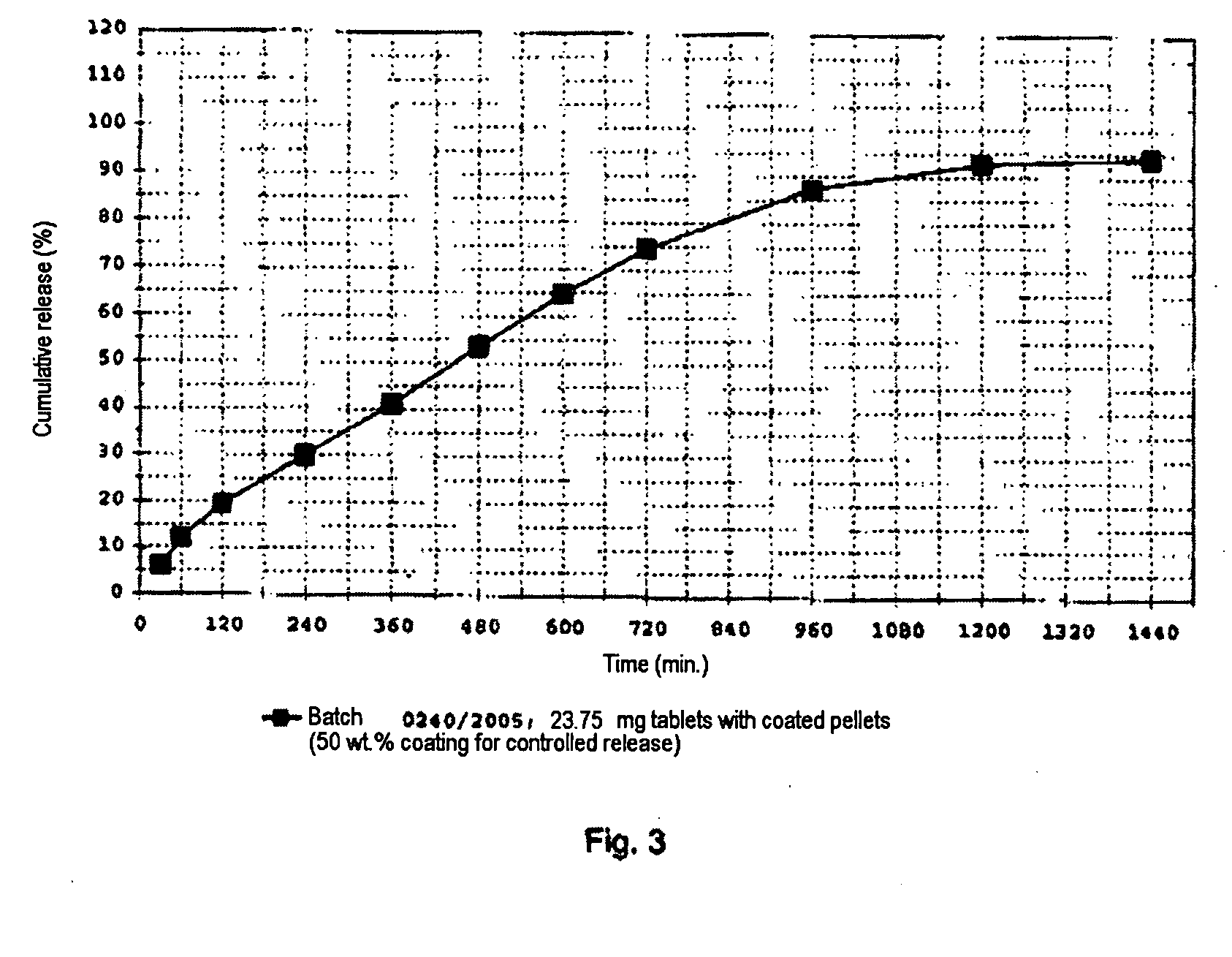
Formulation and process for drug loaded cores
ActiveUS20050008701A1Process environmental protectionBiocideOrganic active ingredientsControlled releaseMedicine
The present invention relates to a controlled release pellet of metoprolol and its pharmaceutically acceptable salts that uses a water soluble or a water swellable inert starting seed or core.
Owner:ANDRX PHARMA INC
Injectable compositions for the controlled delivery of pharmacologically active compound
InactiveUS6887487B2Extend posting timeControl doseAntibacterial agentsBiocideRoxithromycinRelease time
The present invention provides compositions and methods for extending the release times and lowering the toxicity of pharmacologically active compounds. The compounds comprise a salt of the pharmacologically active compound with a lipophilic counterion and a pharmaceutically acceptable water soluble solvent combined together to form an injectable composition. The lipophilic counterion may be a saturated or unsaturated C8-C22 fatty acid, and preferably may be a saturated or unsaturated C10-C18 fatty acid. When injected into a mammal, at least a portion of the composition precipitates and releases the active compound over time. Thus, the composition forms a slowly releasing drug depot of the active compound in the mammal. Therefore, the present invention enables one to provide a controlled dose administration of the active compound for a periods of up to 15 days or even longer. Many compounds can be administered according to the present invention including, but not limited to, tilmicosin, oxytetracycline, metoprolol, fluoxetine, roxithromycin, and turbinafine.
Owner:IDEXX LABORATORIES
Time-sustained-release formulations comprising a beta-blocker
InactiveUS20080131517A1Providing therapyAvoid problemsPowder deliveryOrganic active ingredientsCarteololBeta blocker
The present invention relates to compositions and methods of treating human subjects with a beta-adrenergic receptor blocking agent (“beta-blocker”) provided in a time-sustained-release delivery system. The time-sustained-release drug delivery systems includes at least three populations of beads, where each population of beads includes a beta-blocker. The beads may be selected from immediate-release beads, enteric-release beads, sustained-release beads, and time-sustained-release beads. The beta-blocker may be selected from acebutolol, atenolol, betaxolol, bisoprolol, esmolol, metoprolol, nebivolol, butoxamine, carteolol, carvedilol, labetalol, nadolol, oxprenolol, penbutolol, propranolol, pindolol, sotalol, and timolol. According to presently preferred embodiments, the beta-blocker is propranolol. The dosage forms of the present invention are useful for treating conditions including hypertension, angina pectoris due to coronary atherosclerosis, hypertrophic subaortic stenosis, congestive heart failure, arrhythmias, angina, anxiety, glaucoma, migraines, esophageal varices, alcohol withdrawal syndrome, irregular heartbeat, tachycardia, tremor, and neuroleptic-induced akathisia. They are also useful in the prophylaxis of migraine headaches.
Owner:RELIANT PHARMACEUTICALS INC
Formulation and process for drug loaded cores
The present invention relates to a controlled release pellet of metoprolol and its pharmaceutically acceptable salts that uses a water soluble or a water swellable inert starting seed or core.
Owner:ANDRX PHARMA INC
Methods for the controlled delivery of pharmacologically active compounds
InactiveUS6946137B2Low toxicitySmall investmentBiocideTetracycline active ingredientsRoxithromycinRelease time
The present invention provides compositions and methods for extending the release times and lowering the toxicity of pharmacologically active compounds. The compounds comprise a salt of the pharmacologically active compound with a lipophilic counterion and a pharmaceutically acceptable water soluble solvent combined together to form an injectable composition. The lipophilic counterion may be a saturated or unsaturated C8-C22 fatty acid, and preferably may be a saturated or unsaturated C10-C18 fatty acid. The compounds precipitate in aqueous environments. When injected into a mammal, at least a portion of the composition precipitates and releases the active compound over time. Thus, the composition forms a slowly releasing drug depot of the active compound in the mammal. Therefore, the present invention enables one to provide a controlled dose administration of the active compound for a period of up to 15 days or even longer. Many compounds can be administered according to the present invention including, but not limited to, tilmicosin, oxytetracycline, metoprolol, fluoxetine, roxithromycin, and turbinafine.
Owner:IDEXX LABORATORIES
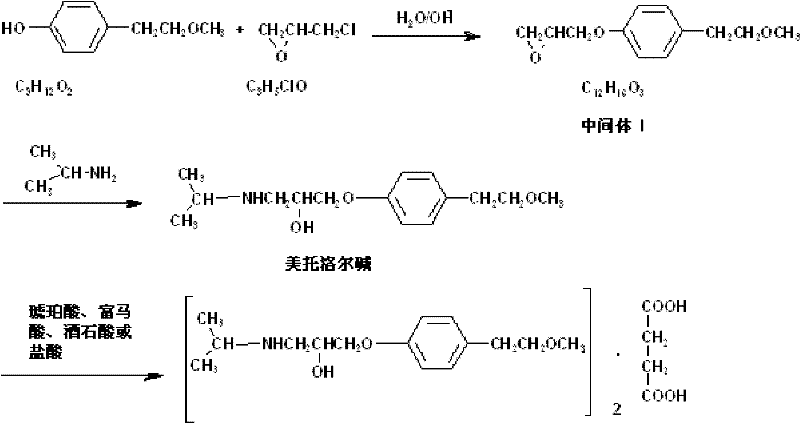
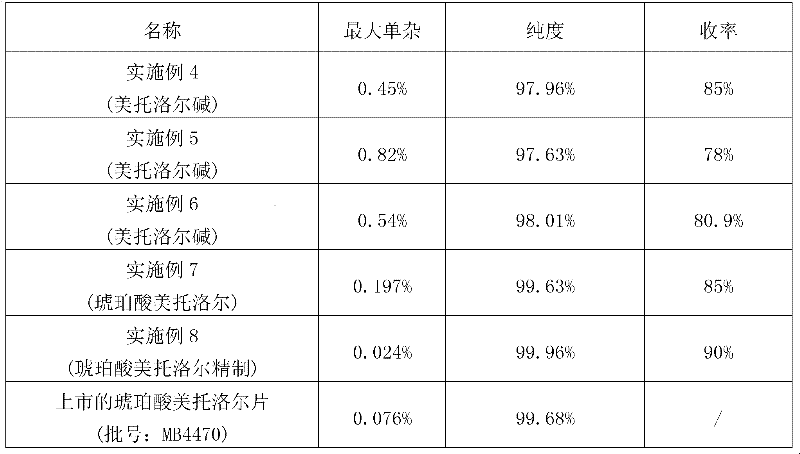
Preparation method for metoprolol salt
ActiveCN102503843AReduce pollutionReduce processing costsOrganic compound preparationCarboxylic acid salt preparationEpoxyCoronary heart disease
The invention discloses a preparation method for metoprolol salt, which includes the steps of utilizing p-(2-methoxyethyl) phenol and epoxy chloropropane as raw materials and water or ethanol as dissolvent, and obtaining 3-[4-(2-methoxyethyl phenoxy)]-1,2-epoxypropane under the action of strong alkali; and utilizing water as dissolvent, carrying reaction between the 3-[4-(2-methoxyethyl phenoxy)]-1,2-epoxypropane and isopropylamine so that metoprolol alkali is obtained, and then carrying out reaction between the metoprolol alkali and saturated monohydric alcohol liquor of organic acid or saturated monohydric alcohol liquor of hydrochloric acid, so that the metoprolol salt is obtained. Clinically, hydrochloride, succinate, fumarate or tartrate of the compound is utilized as common medicinefor treating hypertensive disease, coronary disease, congestive heart failure and arrhythmia. The raw materials of the preparation method are cheap and easy to obtain, the water and the ethanol are utilized as the dissolvent so as to hardly cause pollution, the method is convenient in operation, high in purity of the prepared metoprolol salt, high in yield, lower in cost and extremely suitable for mass production.
Owner:SHANDONG JINHE DRUG RES DEV
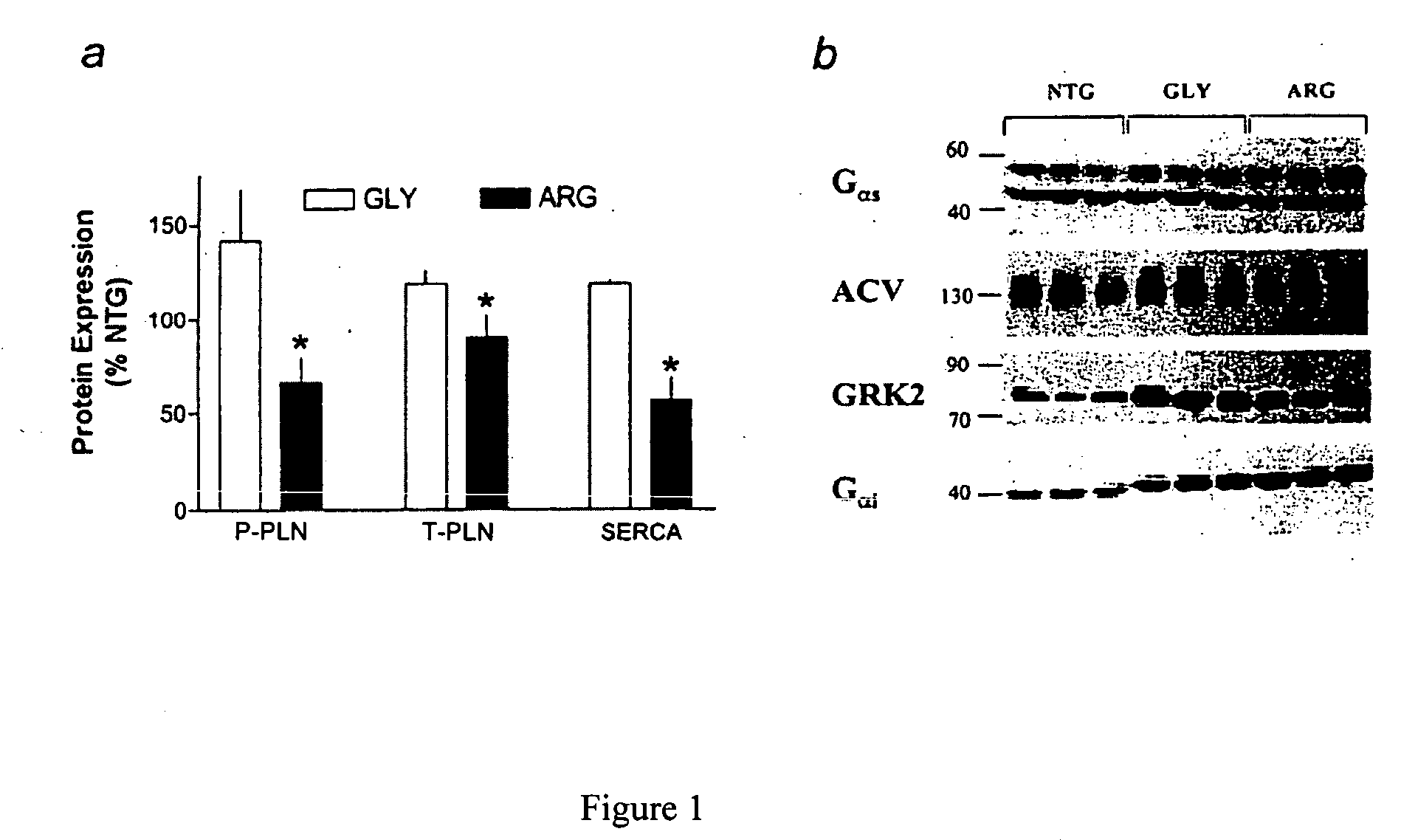
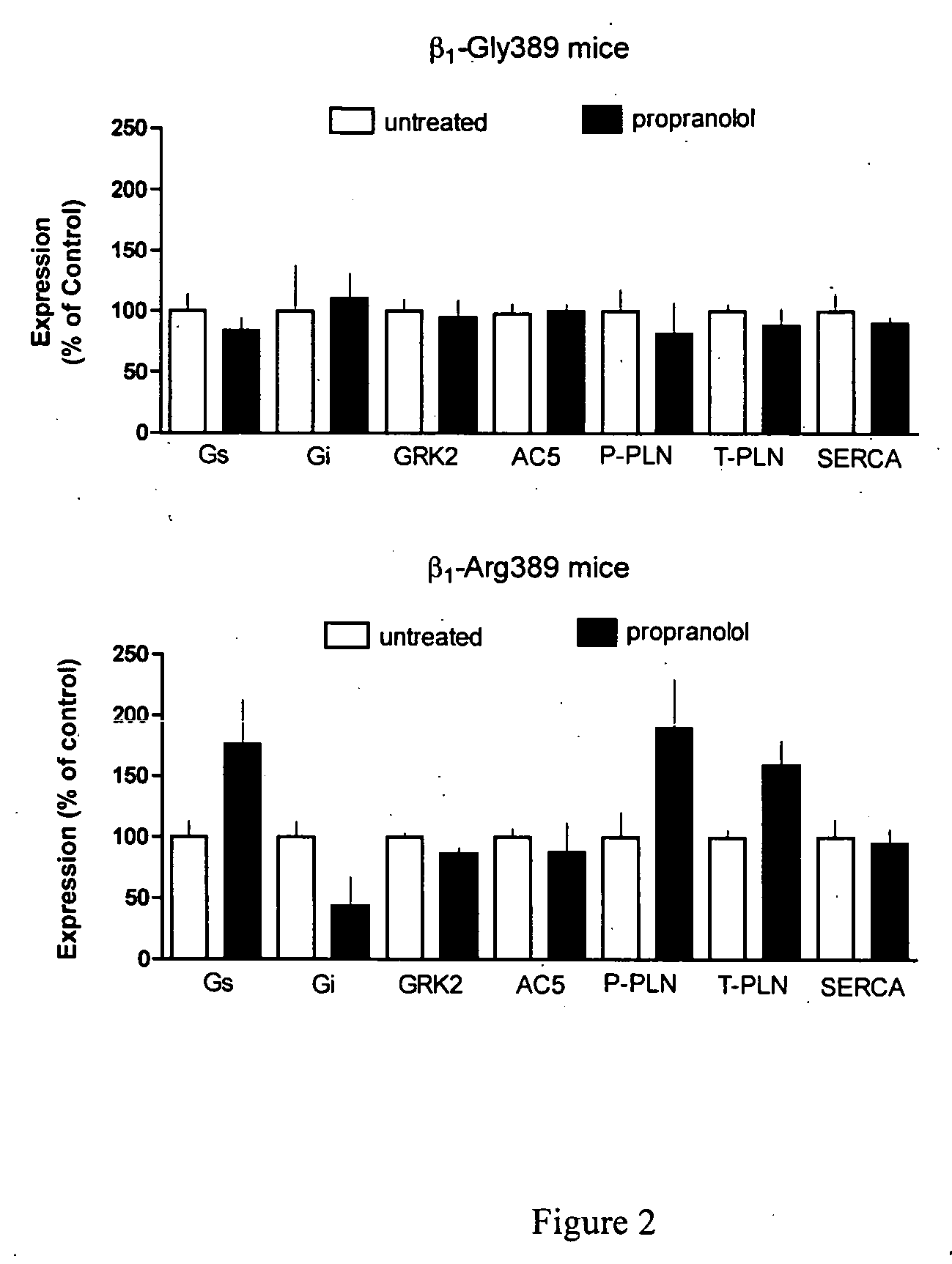
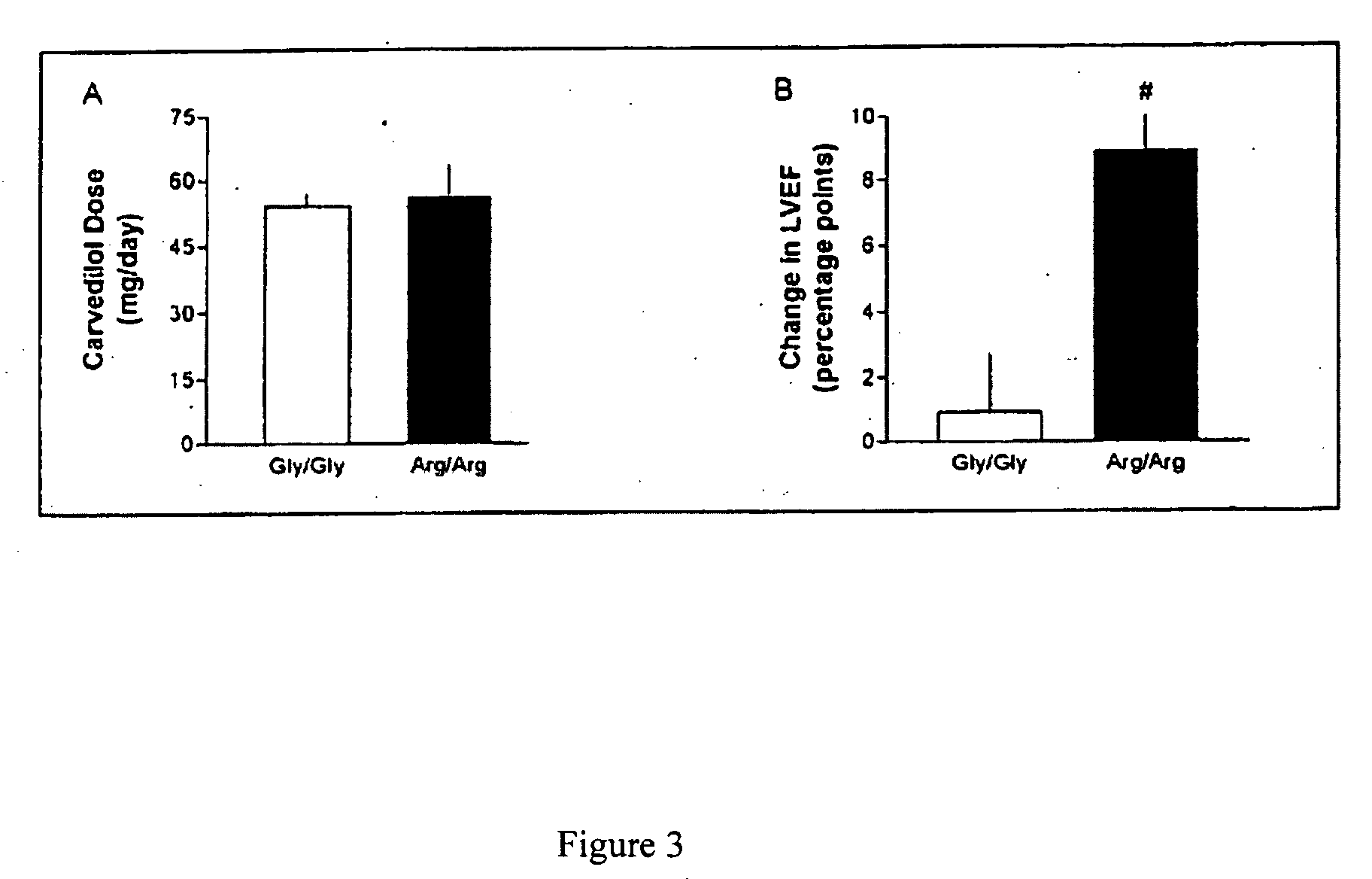
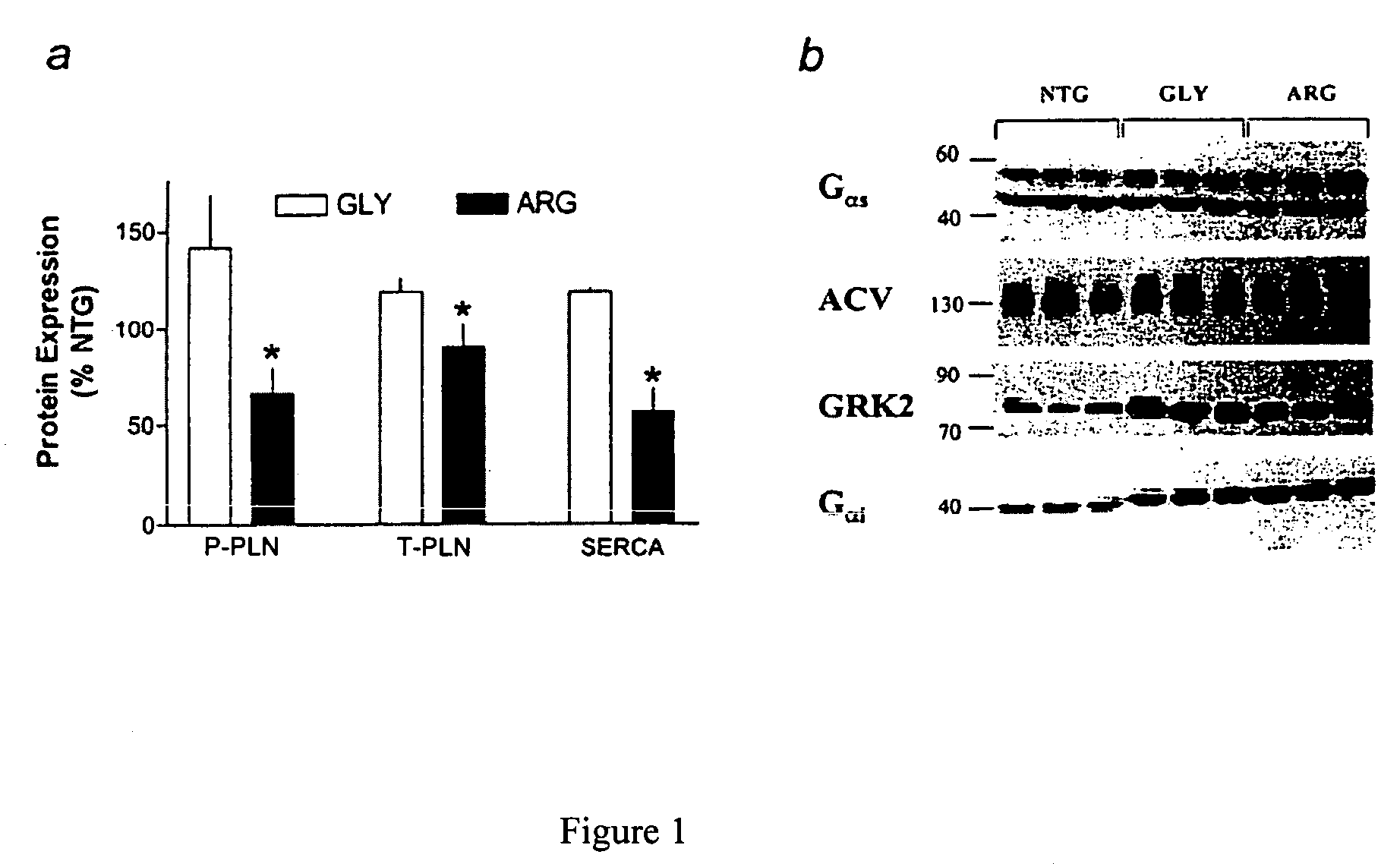
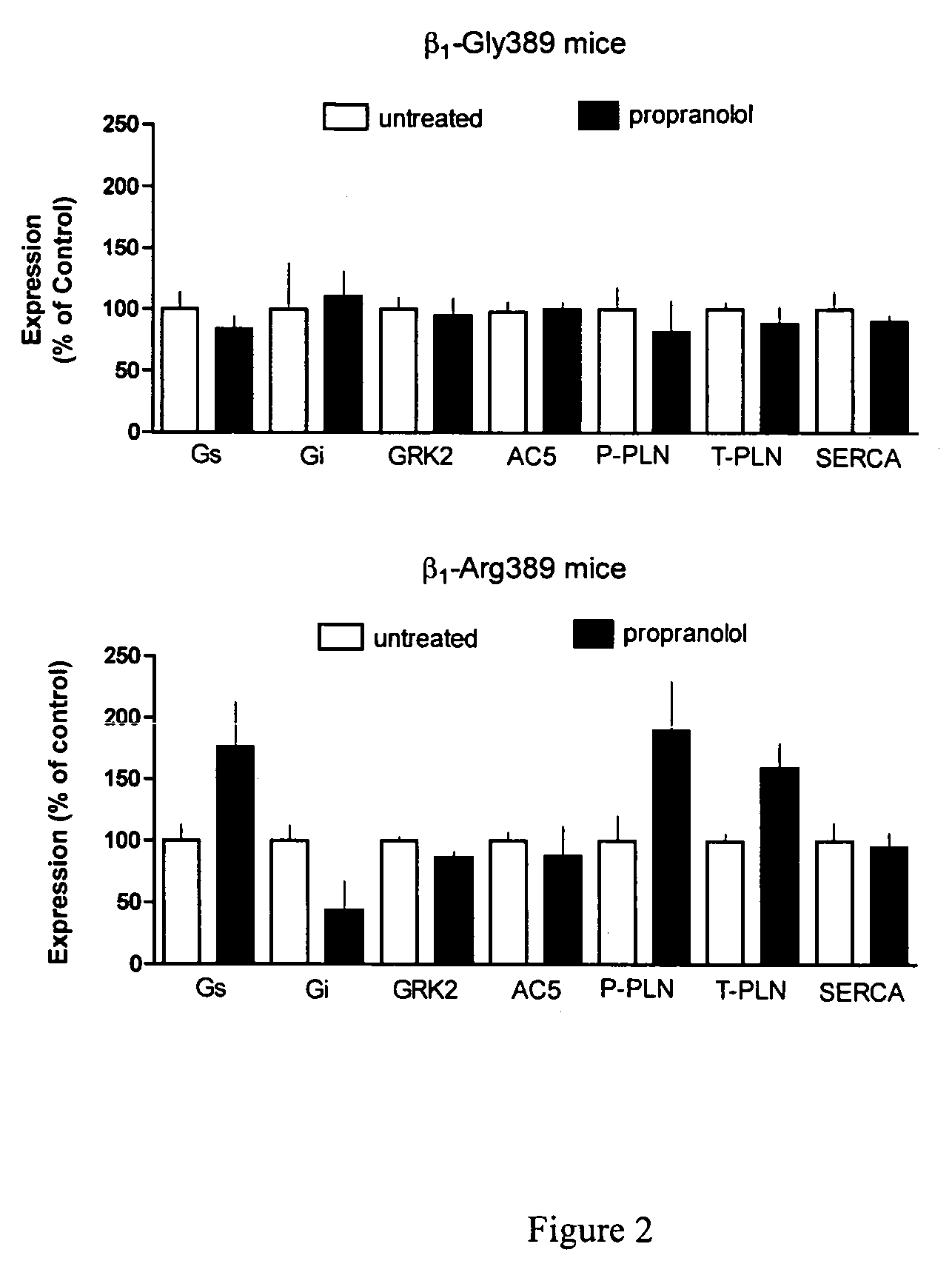
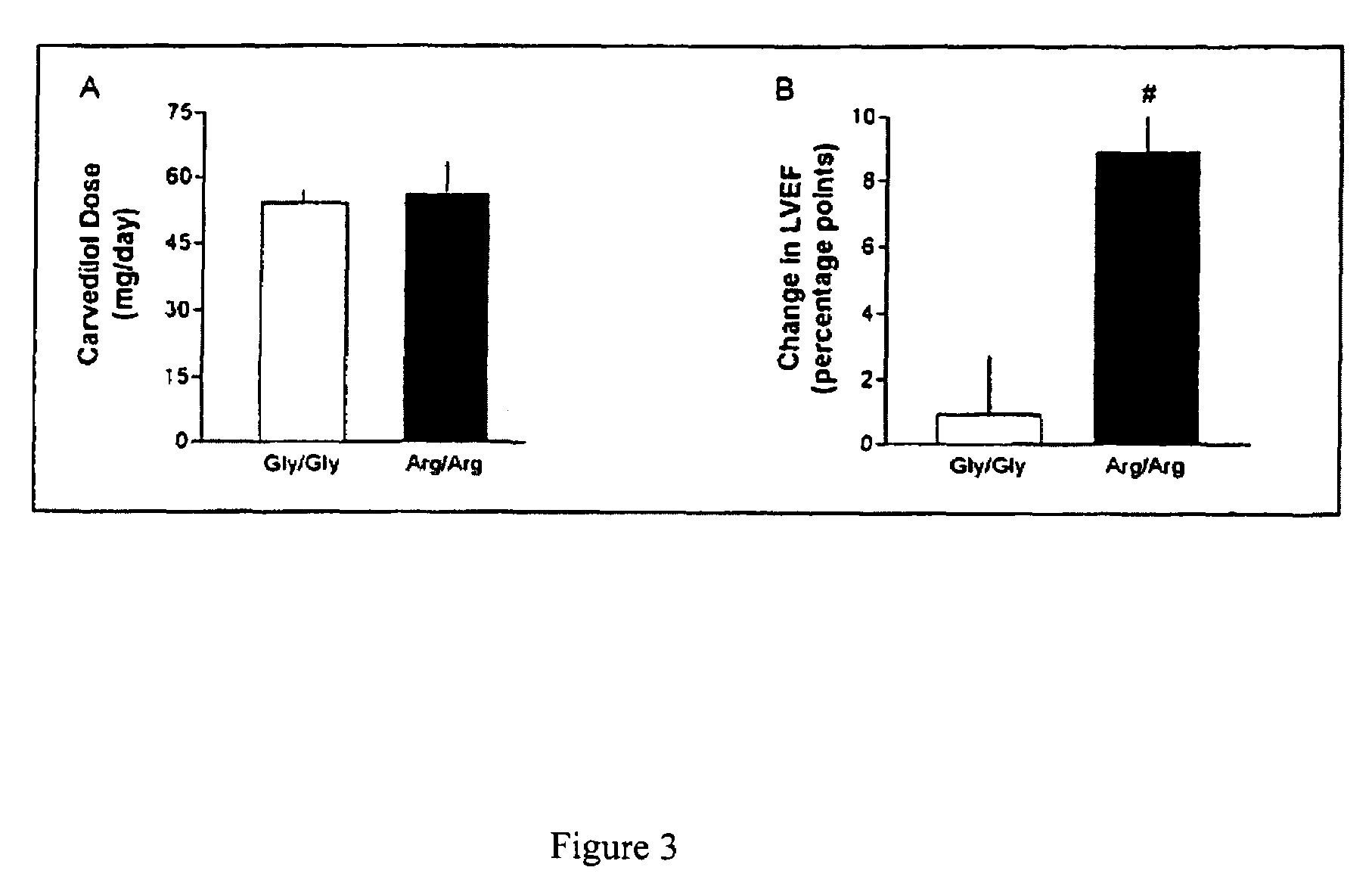
Methods for diagnosis, prediction and treatment of heart failure and other cardiac conditions based on beta1-adrenergic receptor polymorphism
Methods and compositions for the detection, diagnosis, and prevention of cardiac conditions are provided. Polymorphisms of β1-adrenergic receptor are provided. The Gly389 β1-adrenergic receptor variants are not as responsive to treatment β blockers such as carvedilol, metoprolol or bisoprol. Thus, genotyping β1-adrenergic receptor polymorphisms is useful for predicting relative responsiveness to treatment with beta blockers. The Gly389 polymorphism also may be used, alone or in conjunction with other adrenergic receptor polymorphisms, to predict relative risk of developing cardiovascular diseases such as heart failure or to predict relative survival rate in patients with heart failure or other cardiovascular diseases. Also provided are transgenic mice and transgenic cells expressing the β1-adrenergic receptor polymorphisms, and their use in identifying therapeutic agents.
Owner:UNIVERSITY OF CINCINNATI
Metoprolol salt oral administration impulse pellet preparation
InactiveCN101269056AAchieve therapeutic effectSimple preparation processOrganic active ingredientsInorganic non-active ingredientsDiseaseTime lag
The invention discloses the ingredients and the preparation method of an oral pulse pellet pharmaceutical preparation of metoprolol salts. The pharmaceutical preparation is a multi-layered coated pellet which has a basic structure of an immediate-release pellet core, an alkaline layer and a retardation layer containing remedium cardinale of 20 to 100 mg from inside to outside, wherein, the retardation layer contains polyacrylic resin 3, the weight increment of which is 100 to 240 percent of the immediate-release pellet core; the alkaline layer is a medicinal inorganic alkaline adjuvant, the weight of which is 10 to 30 percent of the immediate-release pellet core. After being taken, the pulse pellet begins to release immediately till a complete release is obtained after a time lag of 3 to 4 hours and achieves the effect of one pulse release. The oral pulse pellet pharmaceutical preparation can effectively prevent angina pectoris, hyperpiesis and other diseases from being triggered due to the rise of blood pressure and heart rate within a few hours after a patient awakens and wakes up in the early morning.
Owner:北京华禧联合科技发展有限公司
Methods for predicting relative efficacy of a beta blocker therapy based on a B1-adrenergic receptor polymorphism
InactiveUS7449292B2Sugar derivativesMicrobiological testing/measurementBeta blockerRelative efficacy
Methods and compositions for the detection, diagnosis, and prevention of cardiac conditions are provided. Polymorphisms of β1-adrenergic receptor are provided. The Gly389 β1-adrenergic receptor variants are not as responsive to treatment β blockers such as carvedilol, metoprolol or bisoprol. Thus, genotyping β1-adrenergic receptor polymorphisms is useful for predicting relative responsiveness to treatment with beta blockers. The Gly389 polymorphism also may be used, alone or in conjunction with other adrenergic receptor polymorphisms, to predict relative risk of developing cardiovascular diseases such as heart failure or to predict relative survival rate in patients with heart failure or other cardiovascular diseases. Also provided are transgenic mice and transgenic cells expressing the β1-adrenergic receptor polymorphisms, and their use in identifying therapeutic agents.
Owner:UNIVERSITY OF CINCINNATI
Slow-release pharmaceutical composition of metoprolol and preparation method of pharmaceutical composition
The invention discloses a metoprolol slow-release composition. The composition comprises: a, a blank sucrose pill core with the particle size of 200-350mu m; b, an active medicine layer containing metoprolol, wherein the active medicine layer is positioned on the surface of the blank pill core; and c, a slow-release coating layer containing ethyecellulose and hydroxypropylcellulose, wherein the slow-release coating layer is positioned outside the active medicine layer. The invention also discloses a method for preparing the pharmaceutical composition.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A +1
Metoprolol slow-release tablet and preparation method thereof
ActiveCN102626396ARelease stabilityAvoid burst phenomenonOrganic active ingredientsNervous disorderPlastic materialsPlastic property
The invention relates to the technical field of pharmaceutics and discloses a metoprolol slow-release tablet and a preparation method thereof. The preparation method provided by the invention comprises the following steps: preparing a metoprolol active drug and a first plastic material into a pastille pill; obtaining slow-release pastille pill by coating a slow-release layer; and tabletting together with a tablet agent containing at least 90wt% of second plastic material. During the process of preparing the metoprolol slow-release tablet according to the invention, the specific material is added to the active drug and the tablet accessory, so that the crushing degree of the slow-release pastille pill is relieved, the stable releasing of the active drug of the slow-release tablet is ensured and the sudden releasing phenomenon of the drug is avoided.
Owner:华益泰康药业股份有限公司
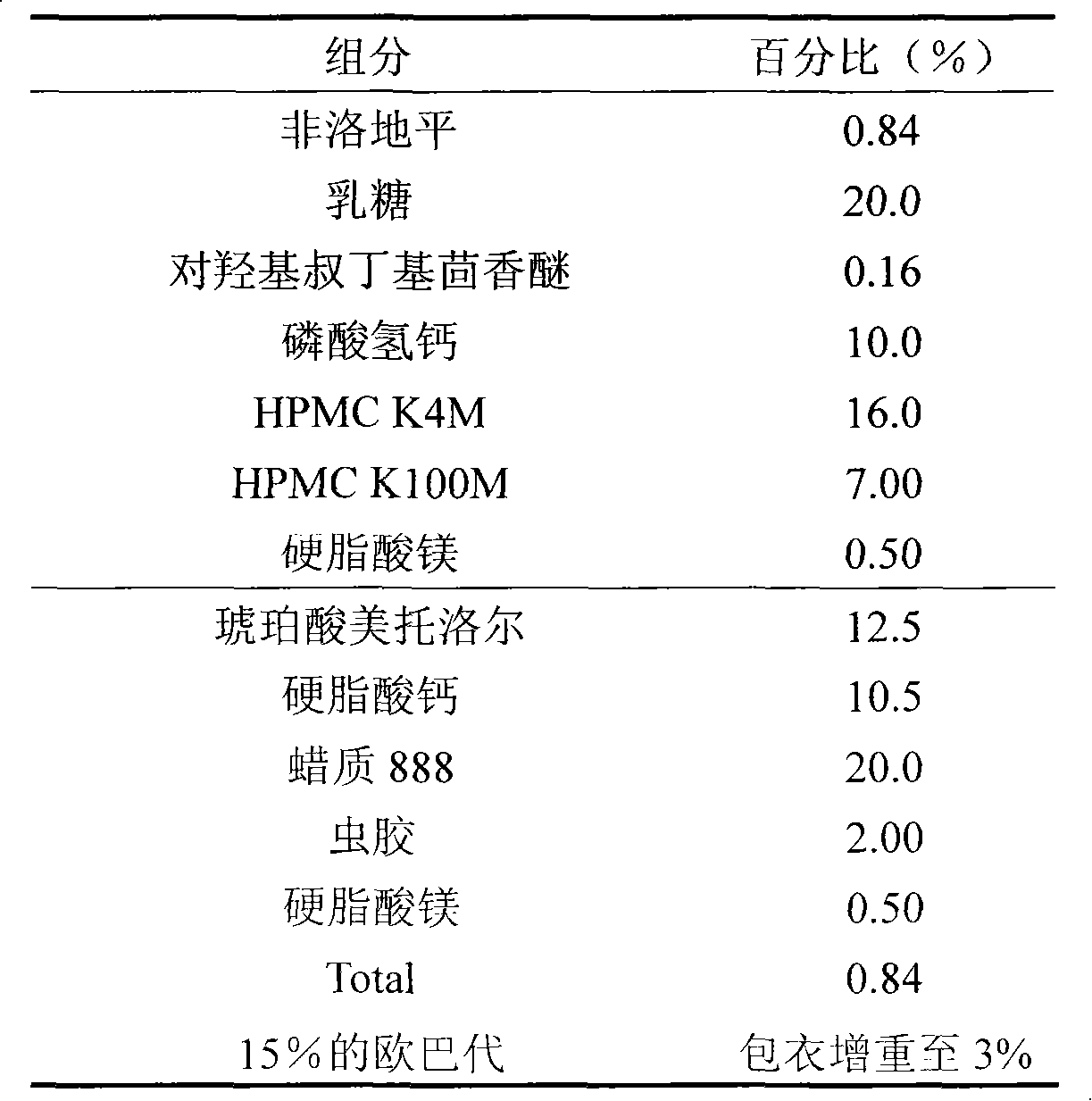
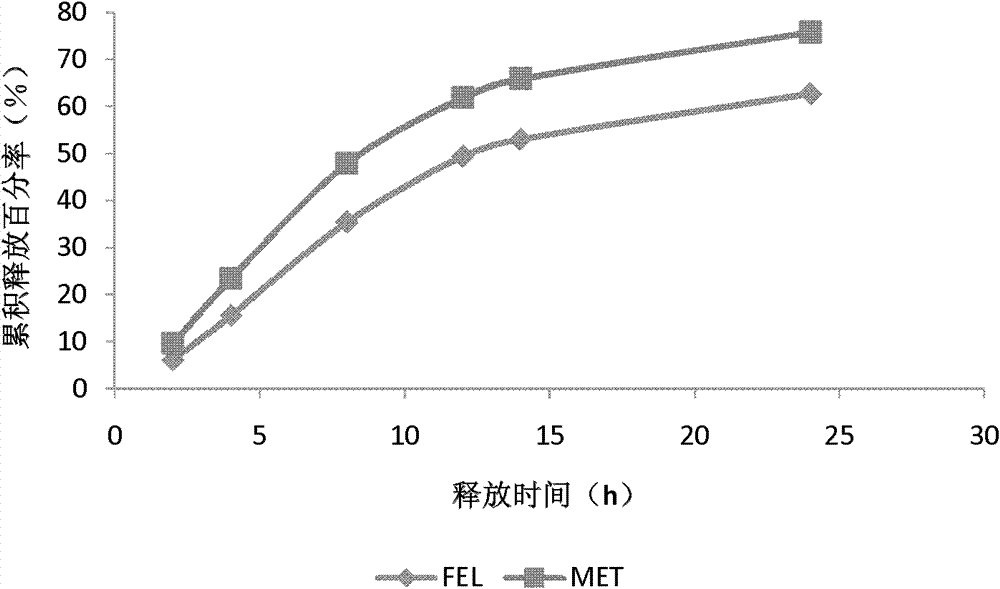
Medicinal composition containing felodipine and metoprolol
The invention discloses a medicinal composition containing felodipine and metoprolol, which contains the felodipine and the metoprolol with a grain diameter D90 of between 10 and 200mu m, wherein the felodipine and the metoprolol are double-layer tablets prepared by dispersing in respective pharmaceutically acceptable carriers respectively in the medicinal composition. The composition can be steadily released, has good stability at the same time, and is used for the hypertension which has poor treatment effect by other medicaments.
Owner:BEIJING D VENTUREPHARM TECH DEV
Method to prepare microparticles metoprolol that contain
InactiveUS20040116392A1Reduce brittlenessHigh mechanical strengthBiocidePhosphorous compound active ingredientsFluidized bedMicroparticle
A method for the preparation of homogenous microparticles containing metoprolol by a fluidized bed process. The microparticles have a size distribution of less than 250 mum and contain at least 80% by weight of metoprolol.
Owner:ASTRAZENECA AB
Methods for treating cardiovascular disorders
There is provided a once-a-day therapeutically synergistic pharmaceutical dosage form for treatment of cardiovascular disorders, wherein the dosage form comprises a fixed dose combination of metoprolol in extended release form and one or more calcium channel blocker, angiotensin II receptor blocker or angiotensin converting enzyme inhibitor along with one or more rate controlling excipient.
Owner:WOCKHARDT LTD
Slow-release tablets containing felodipine and metoprolo salt, and preparation method thereof
ActiveCN102727460ASimple processOrganic active ingredientsPharmaceutical delivery mechanismFluidized bedMedicine
The present invention provides slow-release tablets containing felodipine and a metoprolo salt, and a preparation method thereof. The slow-release tablets comprise a tablet core, a drug-containing layer, a slow-release coating layer and a film coating layer from the inside to the outside, wherein the tablet core is prepared by tableting a metoprolol salt, an insoluble slow-release framework material, and other pharmaceutically-acceptable excipients, and the drug-containing layer comprises micronized felodipine, a solubilizing agent and a binder. According to the present invention, the process is simple and feasible, the characteristic of 24-hour continuous release is provided, the direct tablet core coating manner is adopted to prepare the slow-release tablet, the production process can be completed in the traditional production workshop, and disadvantage of requirement of the fluidized bed and other equipment in the original process is overcome.
Owner:SHANDONG UNIV +1
Metoprolol oral drug composite and preparation method thereof
InactiveCN101716157AOrganic active ingredientsPharmaceutical delivery mechanismTreatment hypertensionMetoprolol
The invention discloses an extended action tablet containing metoprolol and a preparation method thereof. The drug mainly contains metoprolol, a hydrophobic retarding agent and a gel-type retarding agent. The invention enables the drug to achieve 24-hour slow release effect by mainly adopting a mode of taking the hydrophobic retarding agent and metoprolol as a binding agent after being dissolved or melted together; then mixing the binding agent and the gel-type retarding agent, pelletizing and tabletting. The extended action tablet has simple preparation process and high stability and is mainly used for treating high blood pressure.
Owner:BEIJING D VENTUREPHARM TECH DEV
Novel composing prescription sustained-release preparation for treating high blood pressure and preparation method thereof
InactiveCN101185624AQuick-acting and long-actingReduce the frequency of takingPharmaceutical delivery mechanismHeterocyclic compound active ingredientsDissolutionDrug release
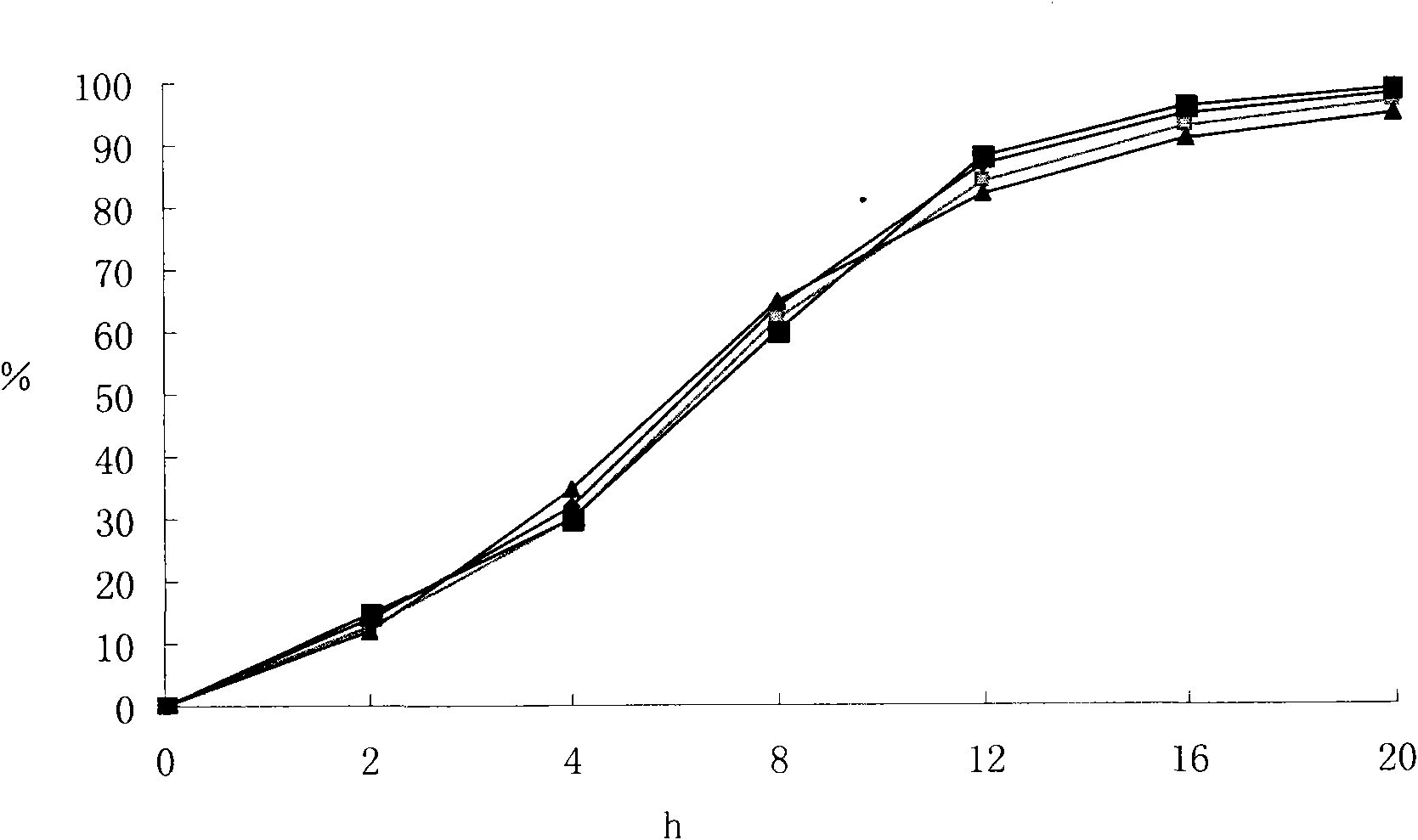
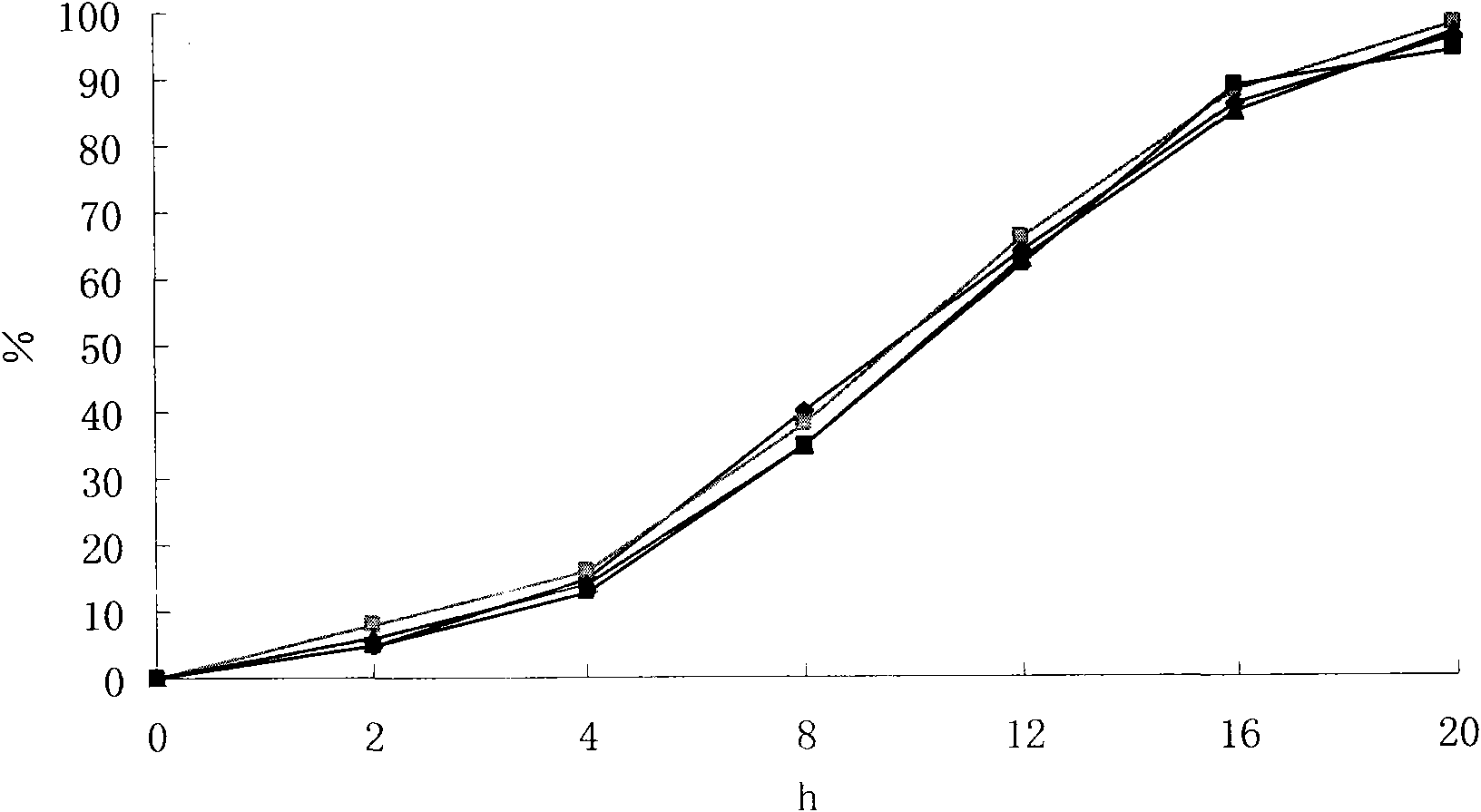
The invention relates to a sustained release preparation of a novel prescription for treating hypertension and the preparing method thereof. The novel prescription comprises a heart selective Beta1 receptor blocker metoprolol and an angiotensin II receptor antagonist (sartan drugs). The sustained release preparation consists of delayed release part and rapid release part, wherein the heart selective Beta1 receptor blocker metoprolol is the delayed release part, with first hour releasing 25-45%, fourth hour releasing 40-75% and eighth hour releasing over 75%; the angiotensin II receptor antagonist (sartan drugs) is the rapid release part, with 45 minutes dissolution over 75%. The composition has both rapid and prolonged action. The invention discloses in vitro drug release characteristics and preparation method thereof.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Metoprolol controlled release mixed matrix tablet and preparation method thereof
InactiveCN101904828AControl release speedGuaranteed uniformityOrganic active ingredientsPharmaceutical delivery mechanismControlled releaseBlood concentration
The invention discloses a metoprolol controlled release mixed matrix tablet, belonging to the pharmaceutical field, comprising the following components: 4-60% of metoprolol or metoprolol salt, 0.05-10% of lauryl sodium sulphate, 15-60% of high viscosity hydrophilic polymer and 2-60% of non-disintegration matrix material; the non-disintegration matrix material forms a matrix system with equal gaps; the mixture of the metoprolol or metoprolol salt and the high viscosity hydrophilic polymer is filled in the matrix system. The metoprolol matrix tablet can slowly release for 24 hours and can be taken one time per day; the blood concentration is in the range of the therapeutic window of the metoprolol, which is suitable for the request of the treatment. Simultaneously, the invention provides a method for preparing the metoprolol controlled release mixed matrix tablet; the other auxiliary materials and pharmaceutical preparations are mixed with the insoluble non-disintegration matrix material; the insoluble non-disintegration matrix material can form rigid matrix and effectively control the release speed of the drugs.
Owner:GUANGZHOU HANFANG PHARMA
Resolution method for preparing optically pure metoprolol
ActiveCN102070469AReduce lossesHigh split efficiencyOrganic compound preparationAmino-hyroxy compound preparationSolubilityEnantiomer
The invention discloses a resolution method for preparing optically pure metoprolol, which belongs to the field of racemic compound resolution. Chiral cyclic phosphoric acid is taken as a resolving agent for preparing the optically pure metoprolol, and the chiral cyclic phosphoric acid resolving agent can be used independently or in a mixed mode. The method comprises the following steps that: racemic metoprolol and the chiral cyclic phosphoric acid resolving agent form diastereoisomer salt in a solvent, the diastereoisomer salt with low solubility is filtered, and a filter cake is dissociated to obtain the optically pure metoprolol; and filtrate is dissociated to obtain another diastereoisomer of the metoprolol. The method is characterized in that: the optically active metoprolol has high optical purity and yield, the resolving agent can be recycled, and the method is easy to operate and suitable for industrialized amplification.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
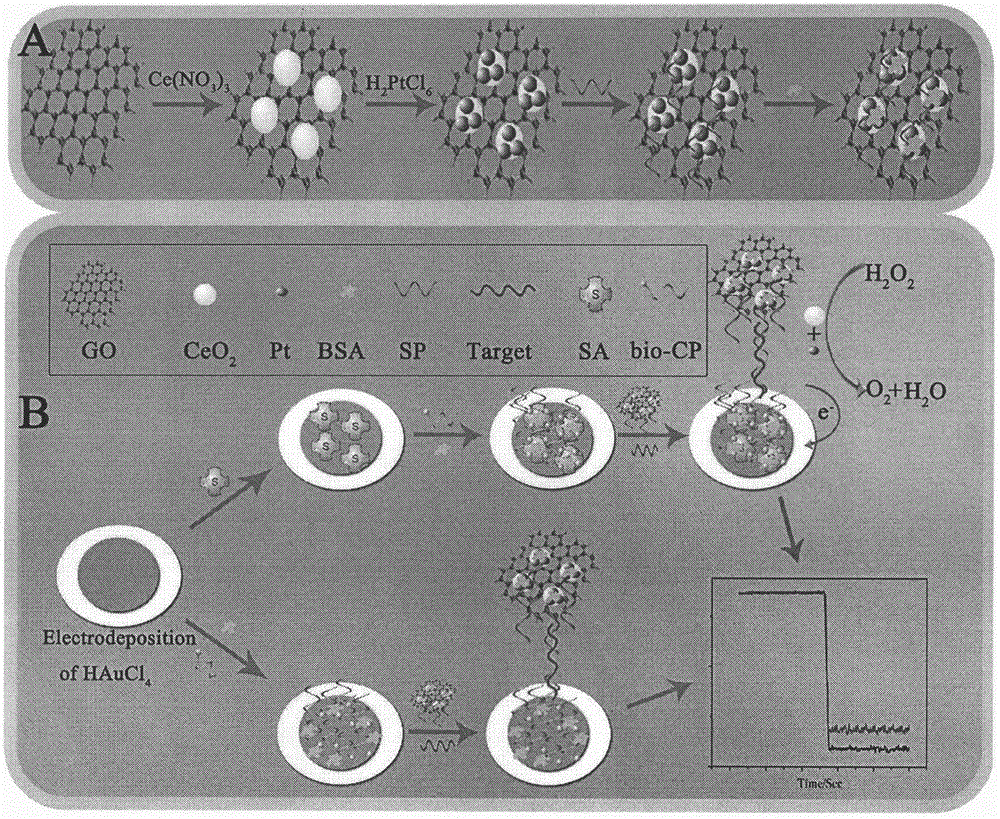
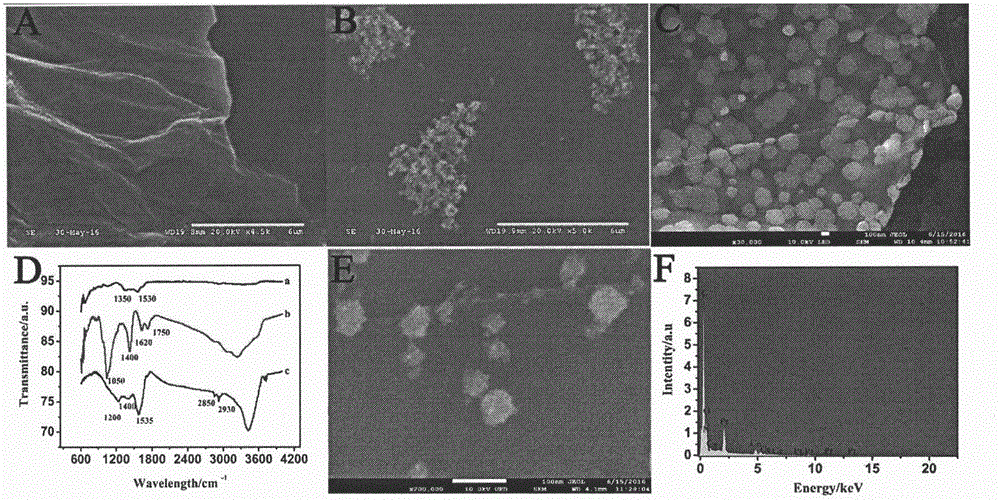
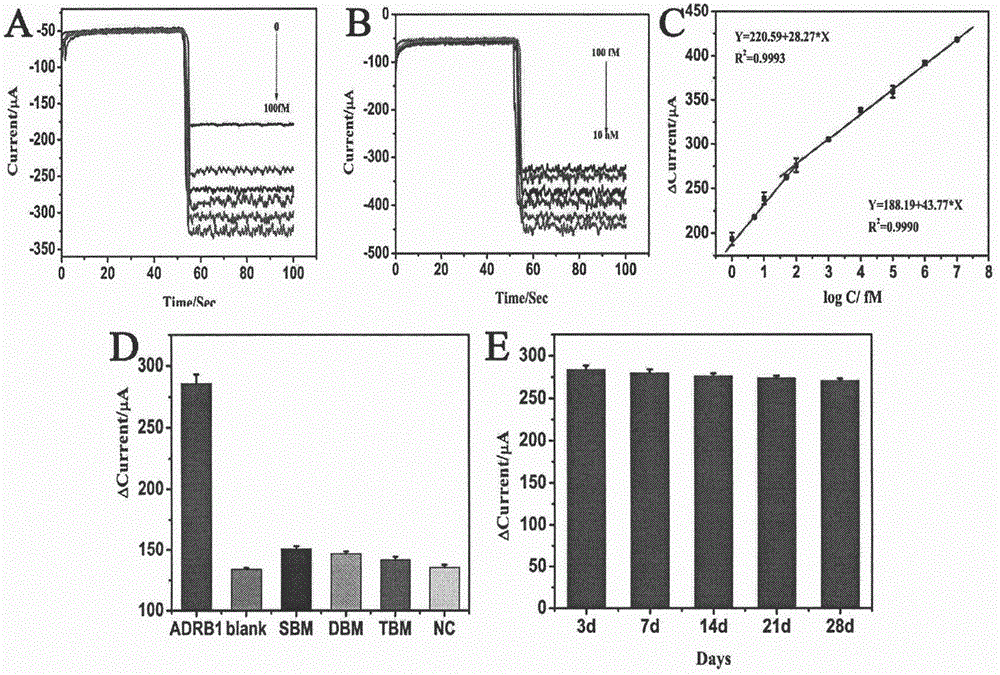
Preparation method of electrochemical sensor for detecting polymorphism of ADRB1-115G>C gene
ActiveCN106290521AHigh catalytic activityHigh sensitivityMaterial analysis by electric/magnetic meansCeriumSingle strand dna
The invention relates to a preparation method and an application of an electrochemical sensor for detecting the polymorphism of metoprolol personalized medicine gene-beta1 adrenergic receptor (ADRB1), and belongs to the technical field of electrochemical detection. The preparation method is characterized by comprising the following steps: firstly reducing cerium dioxide (CeO2) onto graphene oxide (GO) to obtain a GO-CeO2 composite material, reducing platinum nano particles on the surface of the composite material, and mixing a single-chain DNA probe with the composite material to obtain a detection probe; self-assembling nano gold and avidin to fix the biotinylation single-chain DNA capturing probe, thereby preparing the electrochemical sensor for detecting the polymorphism of the ADRB1-1165G>C gene. The sensor is successfully used for detecting the single base mutation of the ADRB1 gene. The electrochemical sensor for detecting the polymorphism of the ADRB1-1165G>C gene has the advantages of high sensitivity, high specificity, and rapidness and convenience in detection. A novel detection method is provided for the metoprolol personalized medicine.
Owner:CHONGQING MEDICAL UNIVERSITY
Methods for treating cardiovascular disorders
There is provided a once-a-day therapeutically synergistic pharmaceutical dosage form for treatment of cardiovascular disorders, wherein the dosage form comprises a fixed dose combination of metoprolol in extended release form and one or more calcium channel blocker, angiotensin II receptor blocker or angiotensin converting enzyme inhibitor along with one or more rate controlling excipient.
Owner:WOCKHARDT LTD
Single layer osmotic pump controlled release preparation containing metoprolol and felodipine
ActiveCN102784143ASimple manufacturing processOrganic active ingredientsPharmaceutical delivery mechanismPharmaceutical formulationFilm coating
The present invention belongs to the field of pharmaceutical preparations, and relates to a single layer osmotic pump controlled release preparation containing metoprolol and felodipine. Specifically the controlled release preparation comprises a tablet core and a semipermeable film coating, wherein the tablet core comprises the following components, by weight, 50-100 parts of metoprolol or a pharmaceutically acceptable salt thereof, 5-10 parts of felodipine, 3-10 parts of hydroxy propyl methyl cellulose, 5-25 parts of povidone, and 5-25 parts of sodium carboxymethyl cellulose. The present invention further relates to a preparation method and a use of the preparation. With the controlled release preparation of the present invention, synchronous and constant speed release of metoprolol and felodipine can be achieved. Compared with preparation of double layer osmotic pump tablets, the preparation method of the present invention has a characteristic of substantial simplification of the preparation process, wherein the method comprises that metoprolol is encapsulated in a pill core, and then conjointly preparing into tablets with felodipine.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Felodipine metoprolol succinate sustained-release tablet and preparation method thereof
InactiveCN104434844AEasy to acceptSimple preparation processOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsMetoprolol
The invention relates to the field of pharmaceutical preparations and particularly relates to a felodipine metoprolol succinate sustained-release tablet and a preparation method thereof. The felodipine metoprolol succinate sustained-release tablet is prepared by the following steps: preparing metroprolol succinate into sustained-release pellets; mixing with felodipine sustained-release granules; and adding other auxiliary materials and tabletting. The sustained-release tablet is characterized in that the preparation comprises metoprolol pellets, felodipine sustained-release granules and other auxiliary materials. By adjusting the components and proportion of the felodipine sustained-release granules, not only can the release of felodipine be controlled, but also the release of metroprolol succinate can be adjusted. By adopting a special preparation process of pellet tablets, as the components in the formula are properly selected and proportioned, the felodipine metoprolol succinate sustained-release tablet is simple in preparation process, good in repeatability and easy for industrial production.
Owner:NANJING KEKANG BIOTECH
Metoprolol sustained release capsule and preparation method thereof
ActiveCN106727435AEasy to swallowAvoid influenceOrganic active ingredientsPharmaceutical non-active ingredientsHard CapsuleSustained Release Capsule
The invention provides a metoprolol sustained release capsule and a preparation method thereof. The sustained release capsule is composed of drug-containing filler, a gelatin capsule shell and sustained release coating. The preparation method of the sustained release capsule comprises the following steps: uniformly mixing metoprolol, microcrystalline cellulose and lactose, placing the mixture in a hollow gelatin capsule shell to prepare a drug-containing capsule inner core; and carrying out dry powder coating on the drug-containing capsule inner core by using an inner adding method or an outer adding method of a plasticizer, and carrying out aging at 60-70 DEG C for 2-12h to obtain the metoprolol sustained release capsule. The metoprolol sustained release capsule provided by the invention represents first-order drug release behavior in 24h drug release in vitro, and provides a product convenient to swallow for treating cardiovascular diseases. The metoprolol sustained release capsule is prepared by dry powder coating, no aqueous or organic solvent is used in the preparation process, so the operation is convenient, and the problems of instability, energy consumption, time consumption, environmental pollution and the like of hard capsules can be solved.
Owner:ZHEJIANG UNIV OF TECH
Controlled porous osmotic pump tablets of high permeable drugs and the preparation process thereof
ActiveUS20100291208A1Improve securityEasy to makeBiocideOrganic active ingredientsPorosityVenlafaxine
The present invention relates to controlled porous osmotic pump tablets of high permeable drugs and the preparation process thereof. The controlled porosity osmotic pump tablets do not need to be drilled by laser, but provides controlled porosity for drug release by adding a suitable quantity of pore-forming agents into the semipermeable membrane. In specific embodiments, the present invention relates to controlled porous osmotic pump tablets comprising venlafaxine or metoprolol or pharmaceutically acceptable salts thereof.
Owner:COSCI MED TECH CO LTD
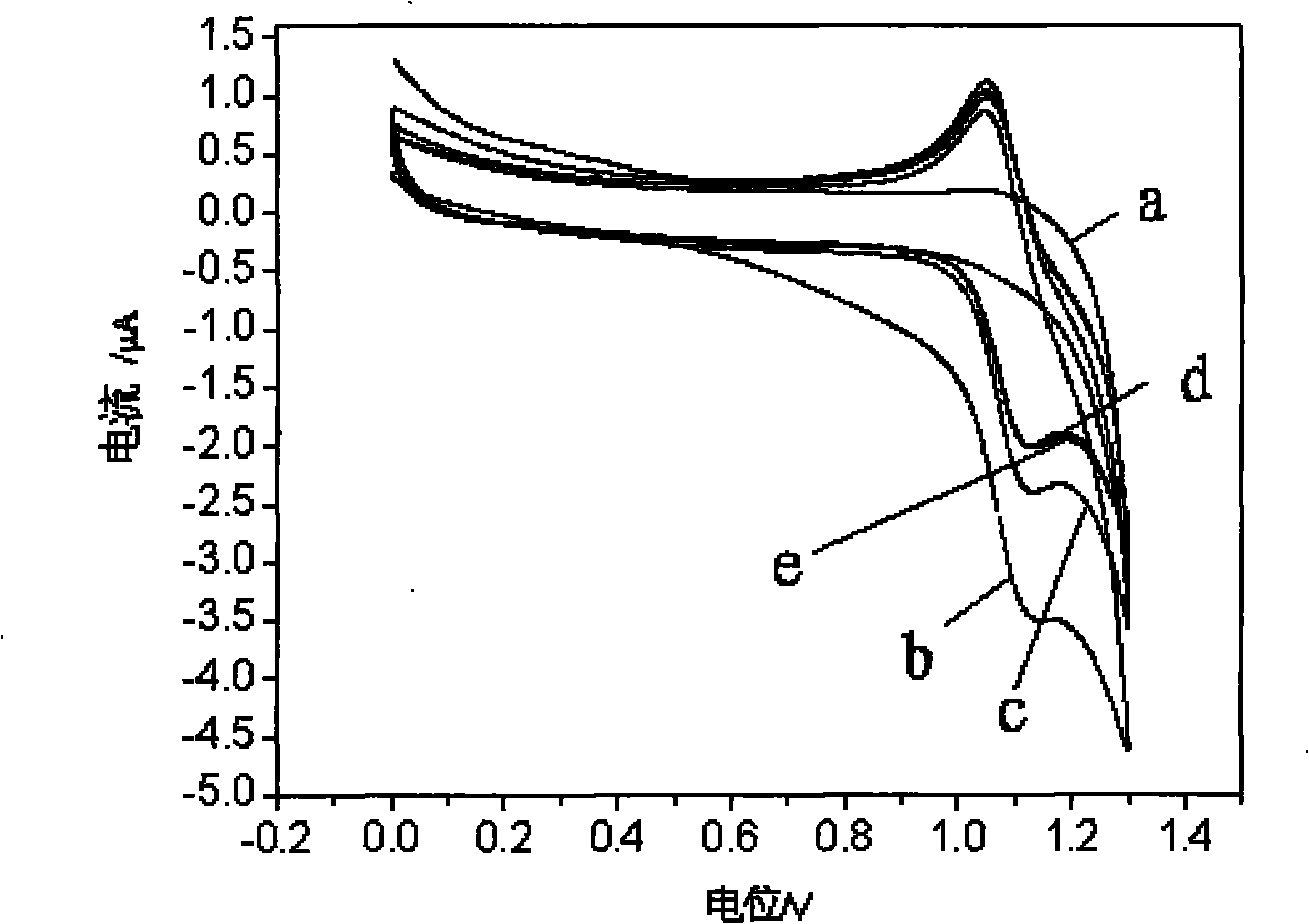
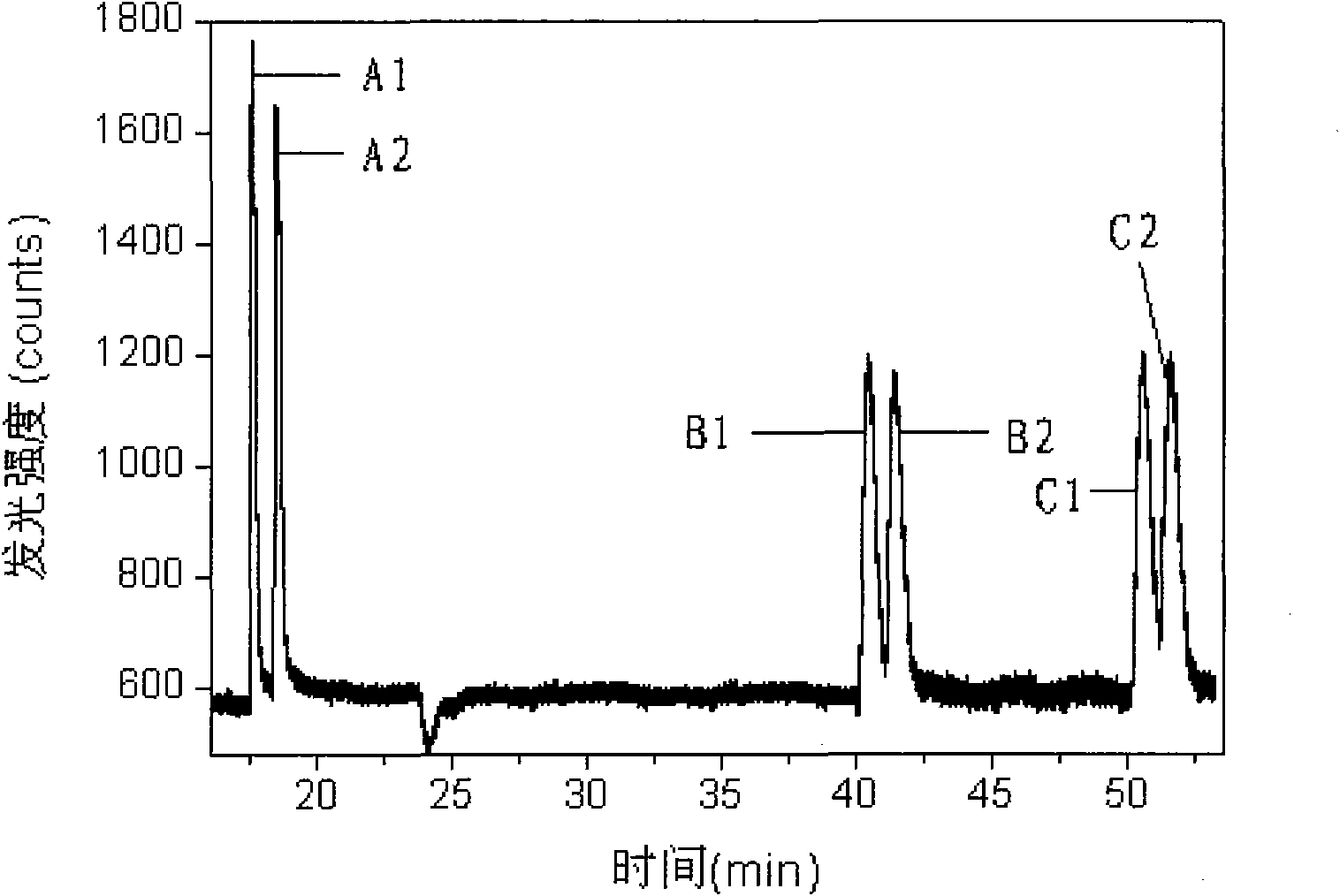
Method for simultaneously carrying out chiral separation analysis on anisodamine, atenolol and metoprolol
ActiveCN101788490ARealize simultaneous separation detectionReduce usageChemiluminescene/bioluminescenceMaterial analysis by electric/magnetic meansElectrochemistryLuminescence
The invention provides a method for simultaneously carrying out chiral separation analysis on anisodamine, atenolol and metoprolol, which comprises the following steps: putting an anisodamine sample, an atenolol sample and a metoprolol sample in a sample cell of a capillary electrophoresis electrochemistry luminescence detection hyphenated instrument and applying an injection voltage to make the samples enter a capillary of the instrument; applying a separation voltage to make the samples carry out electrophoretic separation in the capillary, wherein the capillary is filled with separation solution comprising carboxymethyl-beta-cyclodextrin and the pH value of the separation solution is between 3.0 and 6.0; and making the separated samples enter a detection cell of the instrument and applying a detection voltage to carry out detection, wherein the detection cell is filled with detection solution comprising tris (bipyridine) ruthenium and the pH value of the detection solution is between 6.0 and 9.0. Compared with the prior art, the method for carrying out chiral separation analysis, which is provided by the invention, has the advantages of less reagent use, lower detection cost, higher separation efficiency and higher detection sensitivity.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Oral Preparation With Controlled Release
ActiveUS20090311319A1Control releaseOrganic active ingredientsBiocideControlled releasePh independent
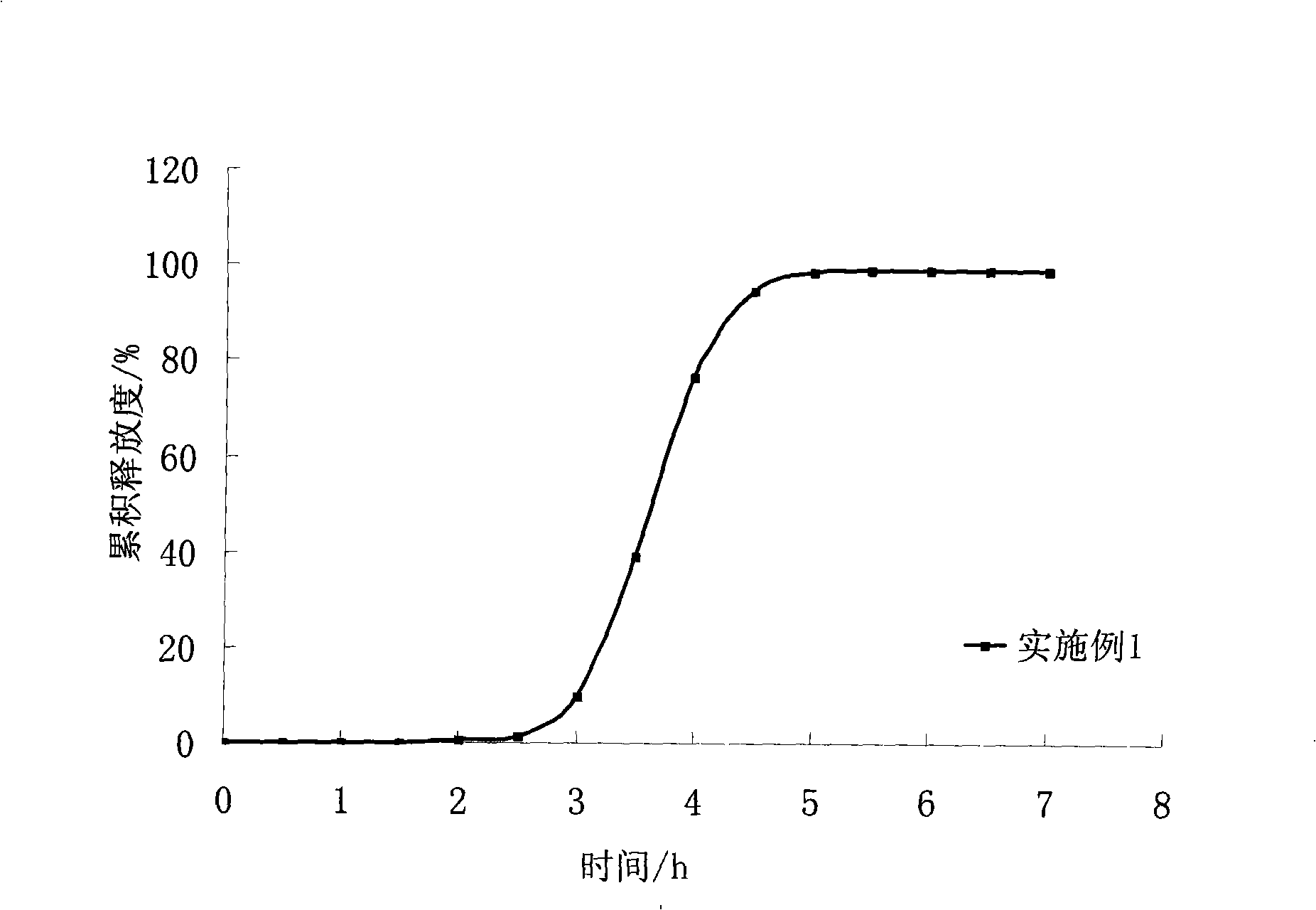
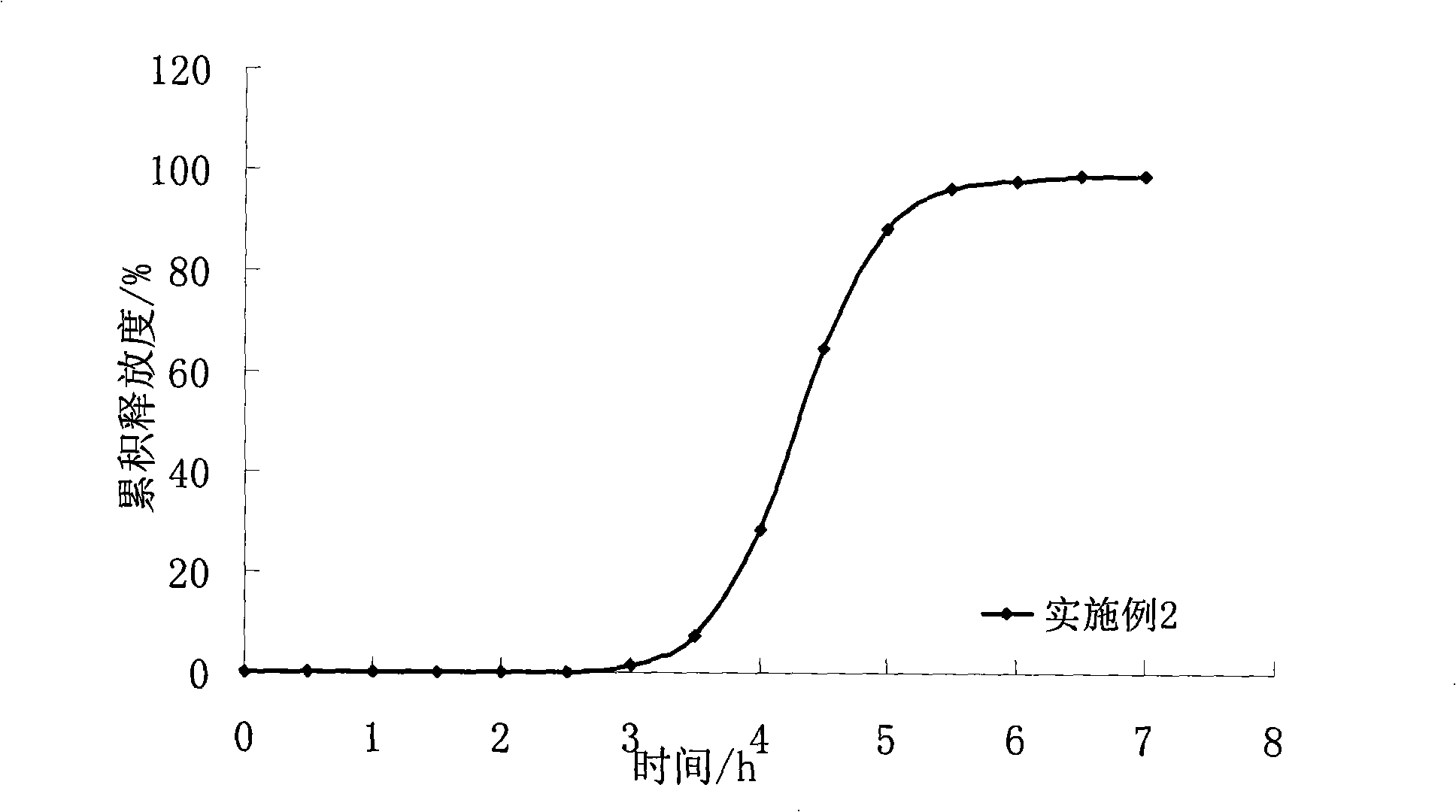
Abstract Oral preparation with controlled release A pharmaceutical pellet is provided, comprising a spherical core containing the active substance with a smooth surface and a coating on the core, which controls pH-independent release of the active substance. With a pellet of this kind, the release of the active substance may follow a profile with a lag-phase from 60 minutes to 840 minutes, where during the lag-phase a proportion of 5 wt. % or less of the active substance is released. Furthermore, the active substance may be released from the pellet with a profile such that, after the lag-phase, the release of the active substance is between and 25 wt. % per hour. The active substance is a metoprolol salt.
Owner:ADD ADVANCED DRUG DELIVERY TECH LTD
Synthesis and preparations of metoprolol and its salts
InactiveUS20090247642A1Speed up the processBiocideOrganic active ingredientsMetoprololMedicinal chemistry
Owner:MEDINCO
Injectable compositions for the controlled delivery of pharmacologically active compound
InactiveUS20050075296A1Low toxicitySmall investmentAntibacterial agentsBiocideRoxithromycinRelease time
Owner:IDEXX LABORATORIES
Novel composing prescription sustained-release preparation for treating high blood pressure and preparation method thereof
InactiveCN101214379APharmaceutical delivery mechanismOil/fats/waxes non-active ingredientsImmediate releaseFast release
The invention relates to a sustained-release preparation with novel composition for curing high blood pressure and a preparation method thereof. The novel composition consists of a cardiac selective Beta1 blocker metoprolol and an angiotensin II receptor antagonist (sartans). The sustained-release preparation consists of a sustained release section and a fast release section, wherein, the cardiac selective Beta1 blocker metoprolol is the sustained release section and the angiotensin II receptor antagonist (sartans) is the immediate-release section. The cardiac selective Beta1 blocker metoprolol releases 25 percent to 45 percent in the first hour, 40 percent to 75 percent in the fourth hour, and above 75 percent in the eighth hour; while the angiotensin II receptor antagonist (sartans) releases above 75 percent after 45 min. The invention has quick and long-lasting effect, and discloses the vitro release characteristics and preparation method of the sustained-release preparation.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com