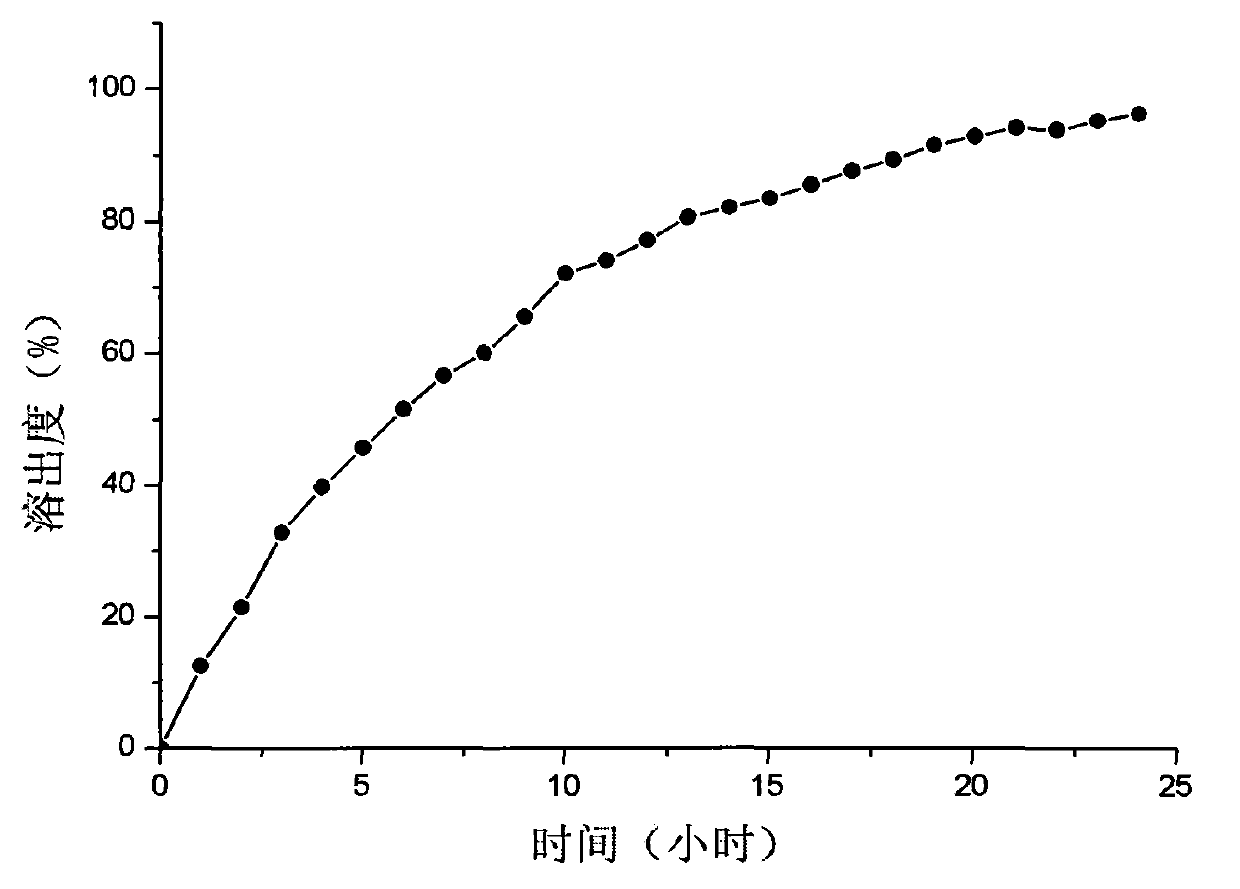
Metoprolol controlled release mixed matrix tablet and preparation method thereof
The technology of matrix tablet and skeleton is applied in the preparation of metoprolol sustained-release tablets, metoprolol controlled-release mixed matrix tablet and the field of preparation thereof, and can solve the problems of hard particles, uneven distribution, high tablet hardness and the like, To achieve the effect of simple production process, smooth tableting process and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The formula of metoprolol tartrate controlled release mixed matrix tablet is as shown in table 1:
[0036] Table 1 Metoprolol tartrate controlled-release mixed matrix tablet formula
[0037] Name of raw material
Prescription amount (mg)
percentage(%)
100
13.44
Hypromellose (Methocel K100M)
298
40.05
33
4.44
Ethyl cellulose (3-15μm)
236
31.72
Polyvinylpyrrolidone (K30)
60
8.06
15
2.02
8
1
95% ethanol
about 300
about 23
[0038] Tablet weight: about 750mg
[0039] Preparation:
[0040] 1. Processing of raw and auxiliary materials: Metoprolol tartrate, pulverized, and passed through a 60-mesh sieve. After the hydroxypropyl methylcellulose K100M passes through a 60-mesh sieve, put it in a rapid stirring granulator, an...
Embodiment 2
[0050] The formula of metoprolol succinate controlled-release mixed matrix tablet is shown in Table 2:
[0051] Table 2. Metoprolol succinate controlled-release mixed matrix tablet formula
[0052] Name of raw material
Prescription amount (mg)
percentage(%)
95
13.09
Hypromellose (Methocel K100M)
295
40.63
40
5.51
Ethyl cellulose (3-15μm)
226
31.13
Polyvinylpyrrolidone (K30)
55
7.58
13
1.79
10
1.3
95% ethanol
about 280
about 20
[0053] Tablet weight: about 735mg
[0054] The preparation method is as in Example 1.
Embodiment 3
[0056] The formula of metoprolol tartrate controlled-release mixed matrix tablet is shown in Table 3:
[0057] Table 3. Metoprolol tartrate controlled-release mixed matrix tablet formula
[0058] Name of raw material
Prescription amount (mg)
percentage(%)
50
10.75
Hypromellose (K15M)
168.3
36.18
51.2
11.01
Ethyl cellulose (3-15μm)
146.5
31.50
Polyvinylpyrrolidone (K30)
36.3
7.80
10.23
2.20
2.58
0.55
95% ethanol
about 177
about 13
[0059] Tablet weight: about 465mg
[0060] Preparation method is as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com