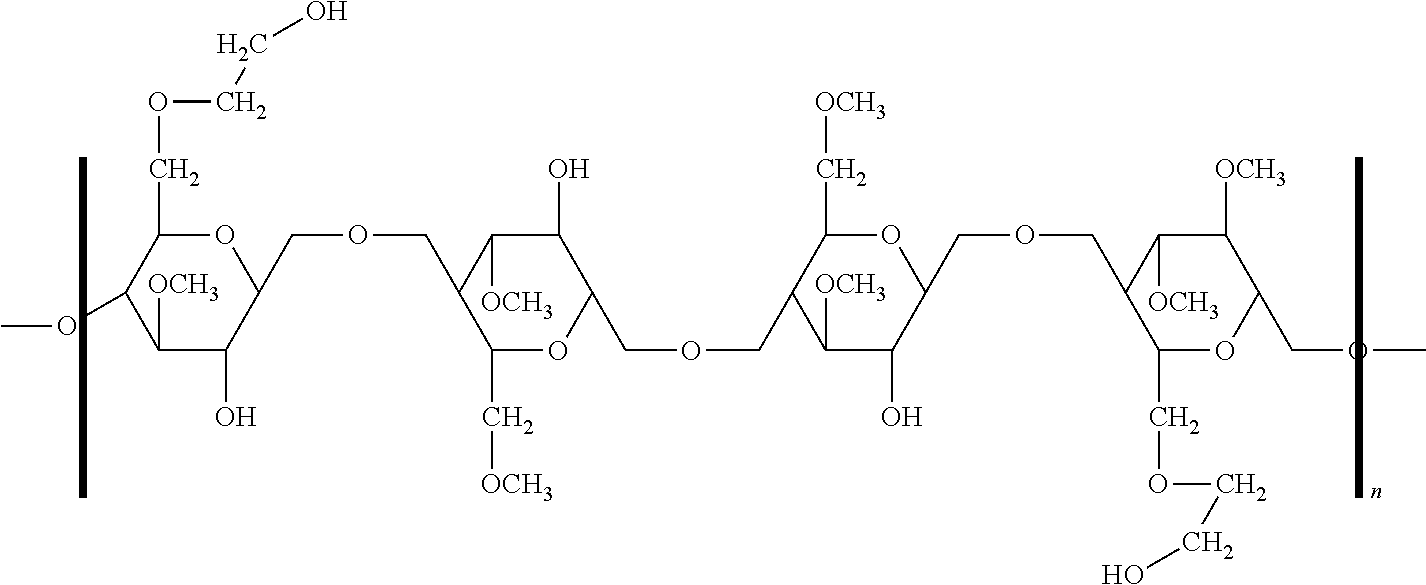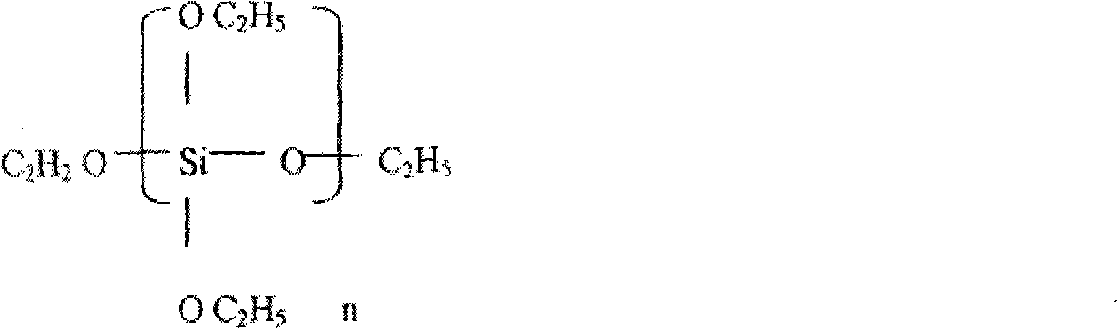Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3047 results about "Ethyl cellulose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
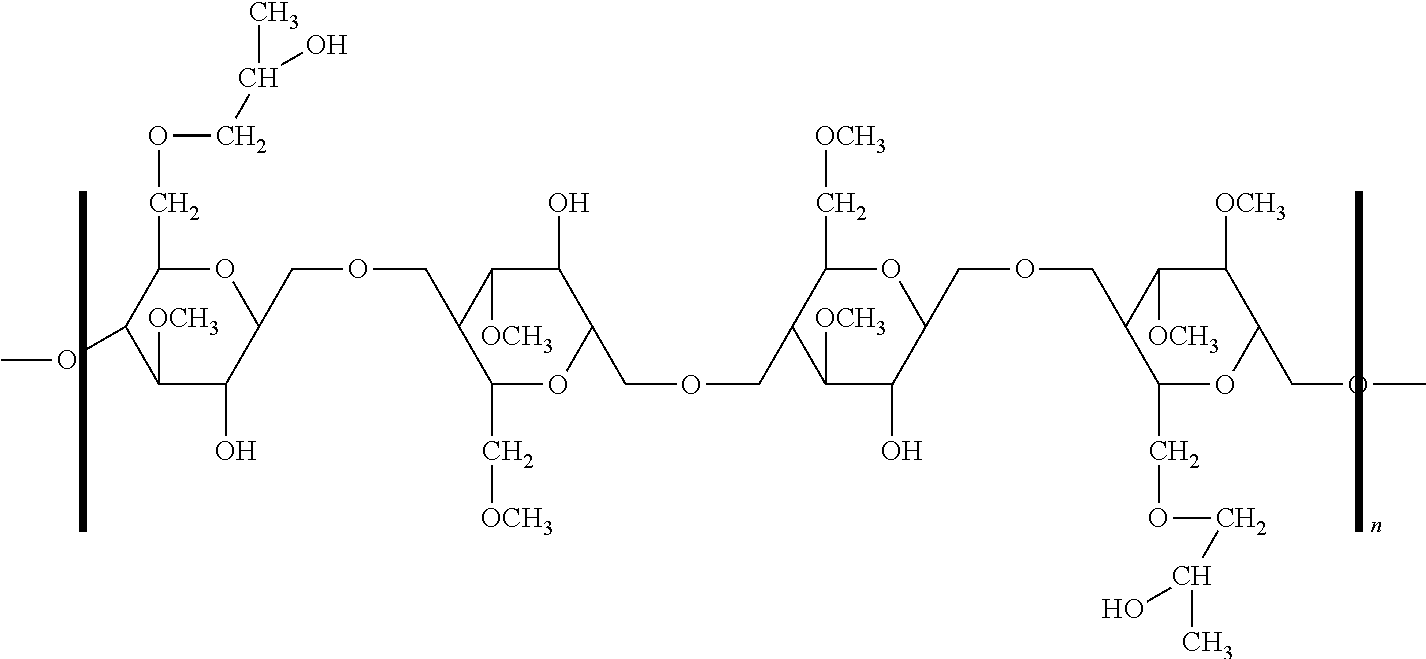
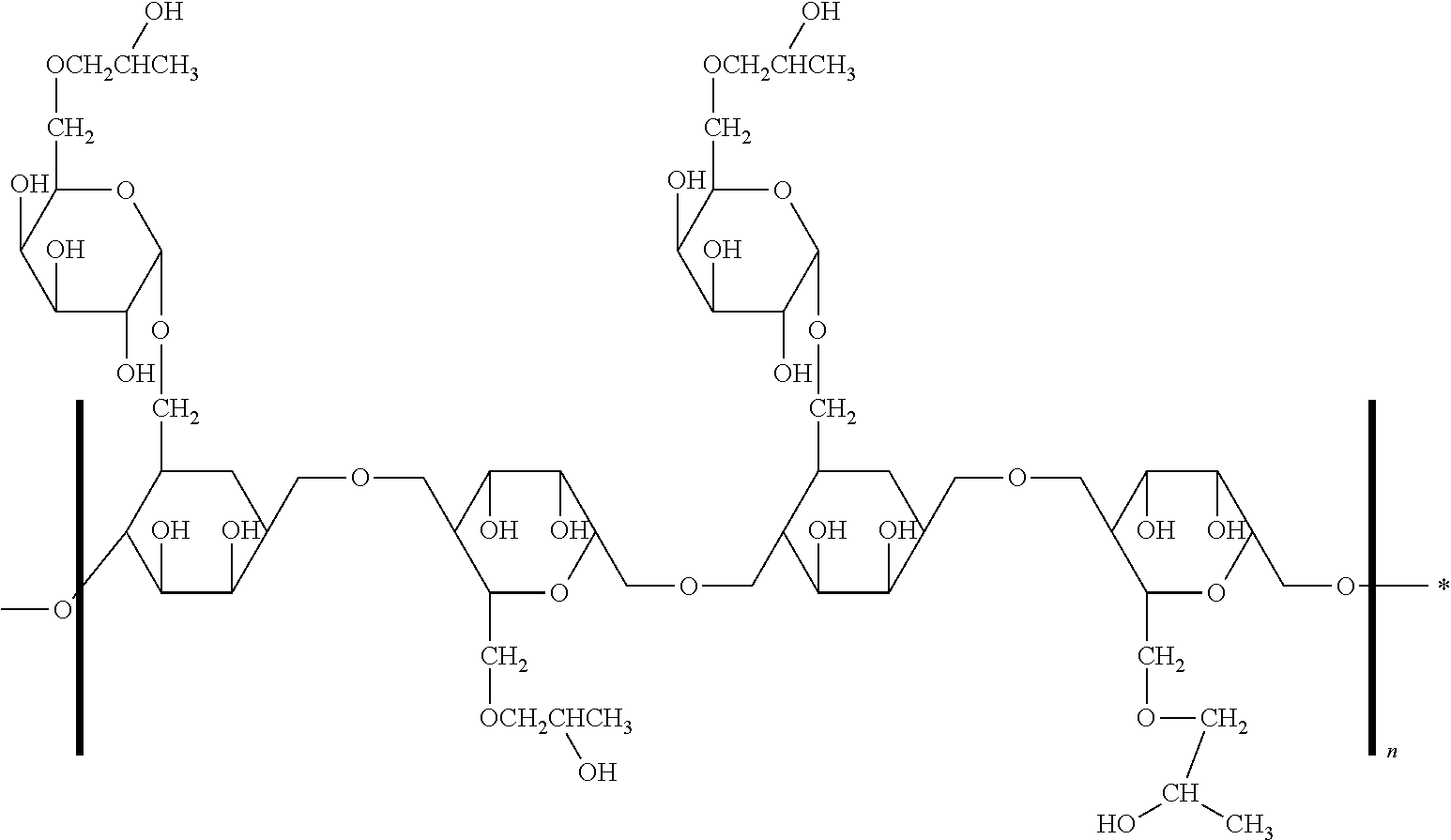
Ethyl cellulose (or ethylcellulose) is a derivative of cellulose in which some of the hydroxyl groups on the repeating glucose units are converted into ethyl ether groups. The number of ethyl groups can vary depending on the manufacturer.
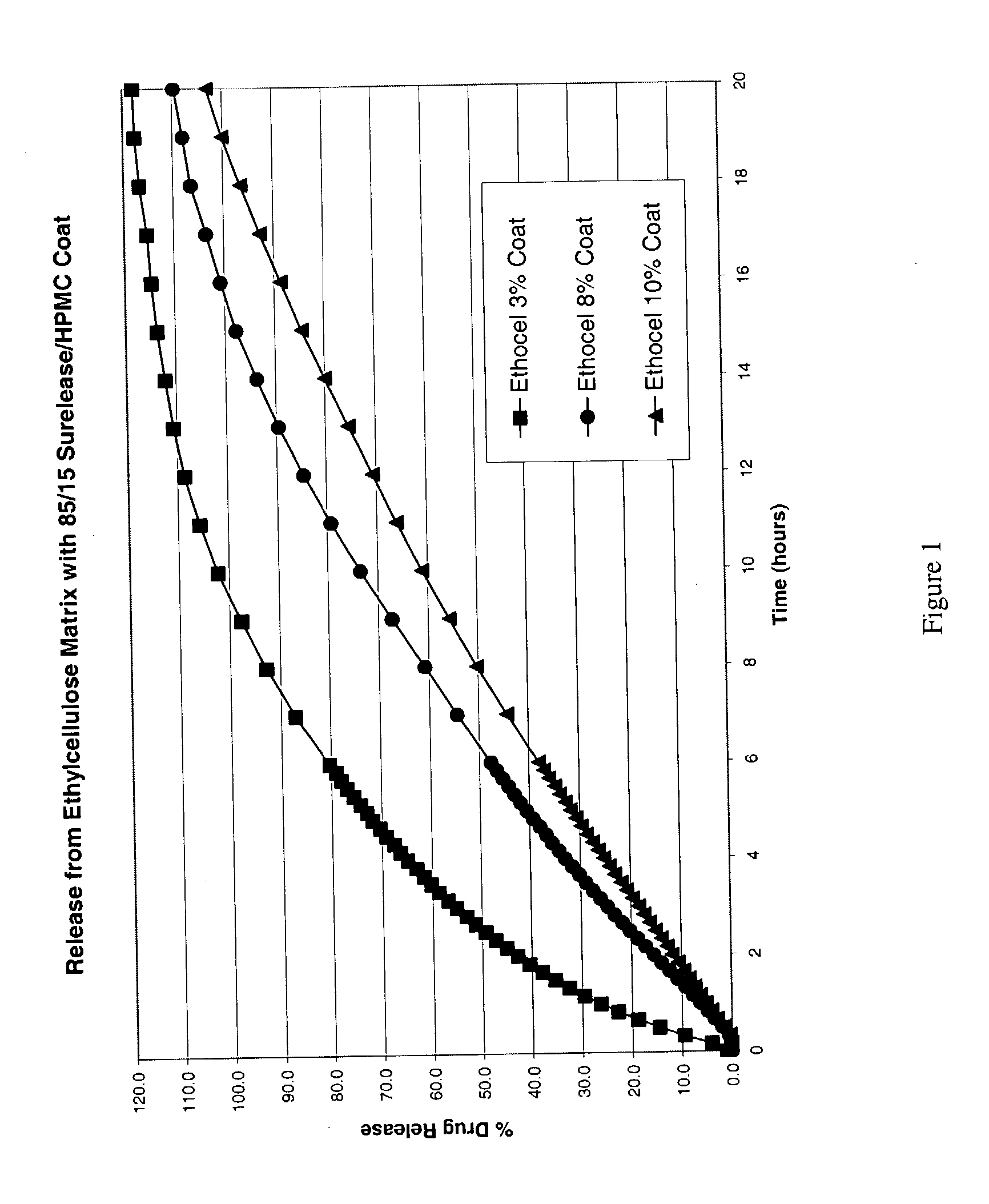
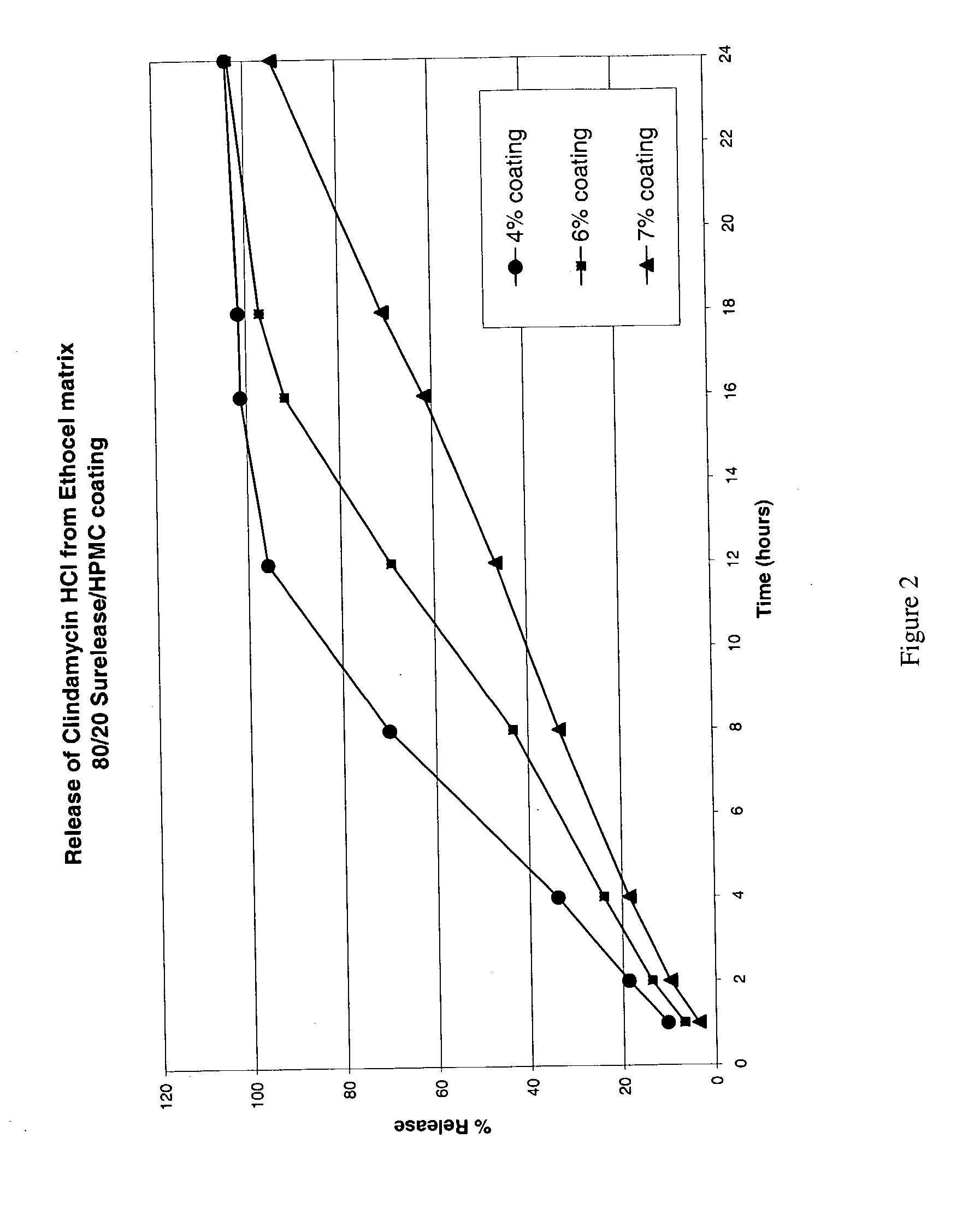
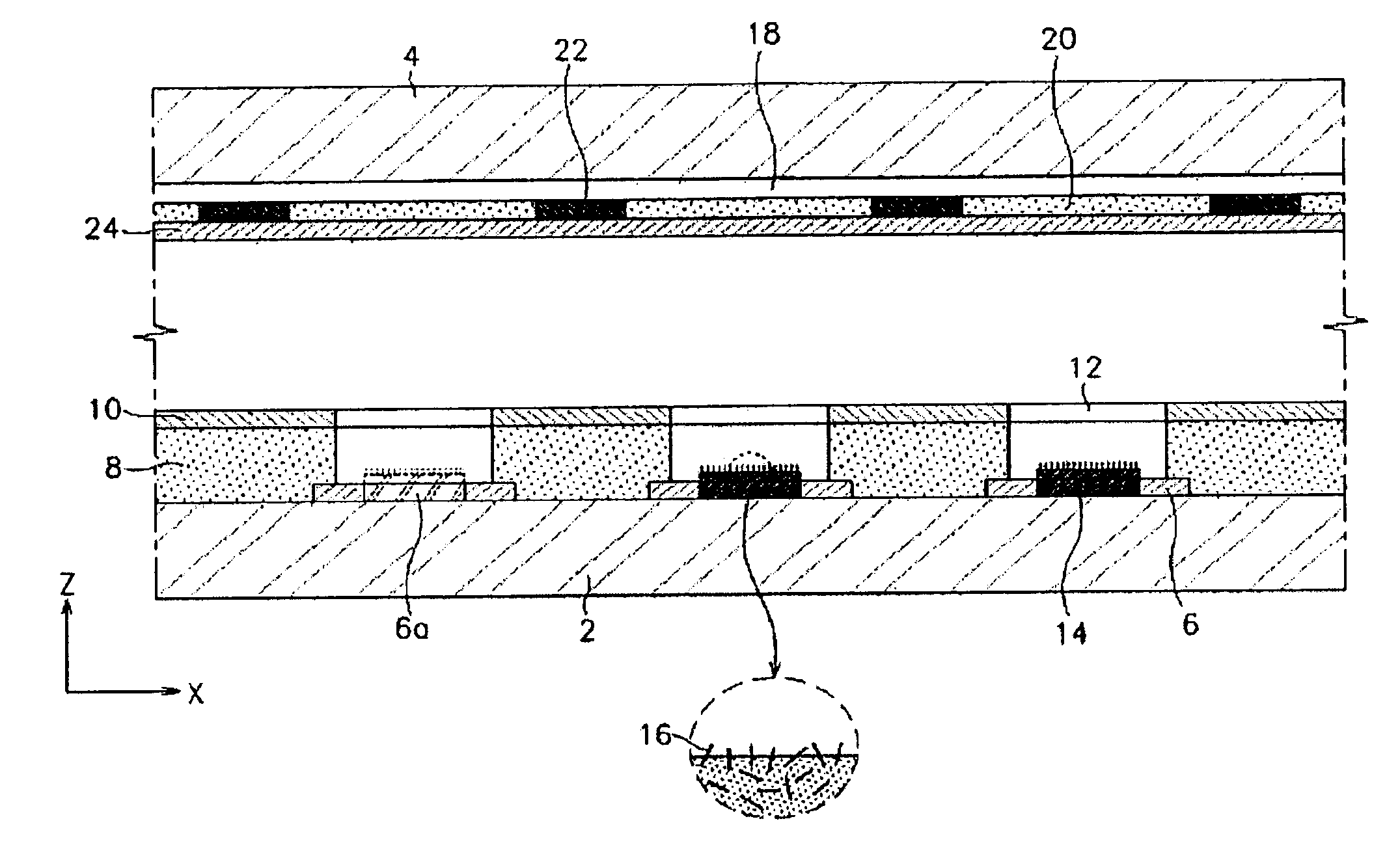
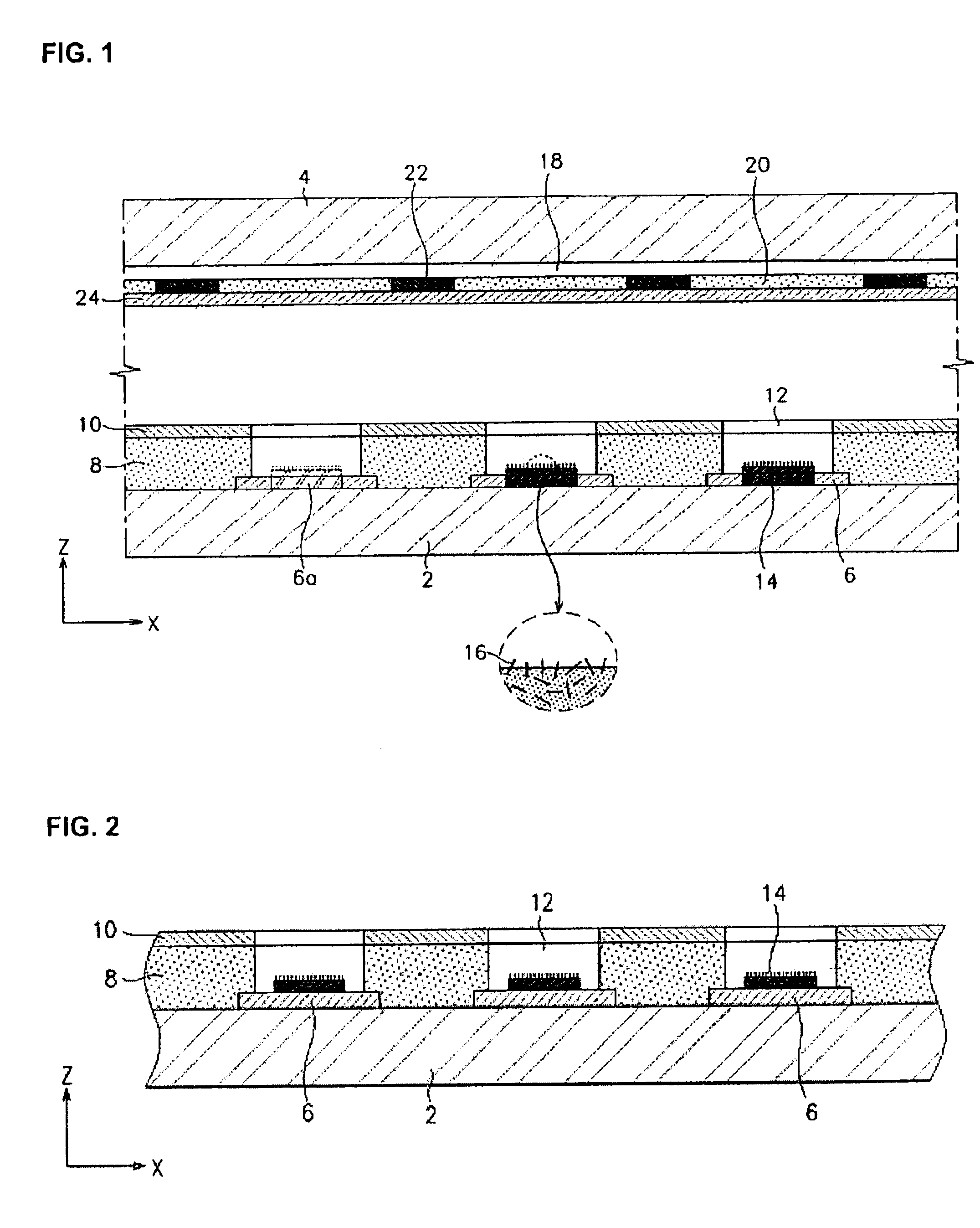
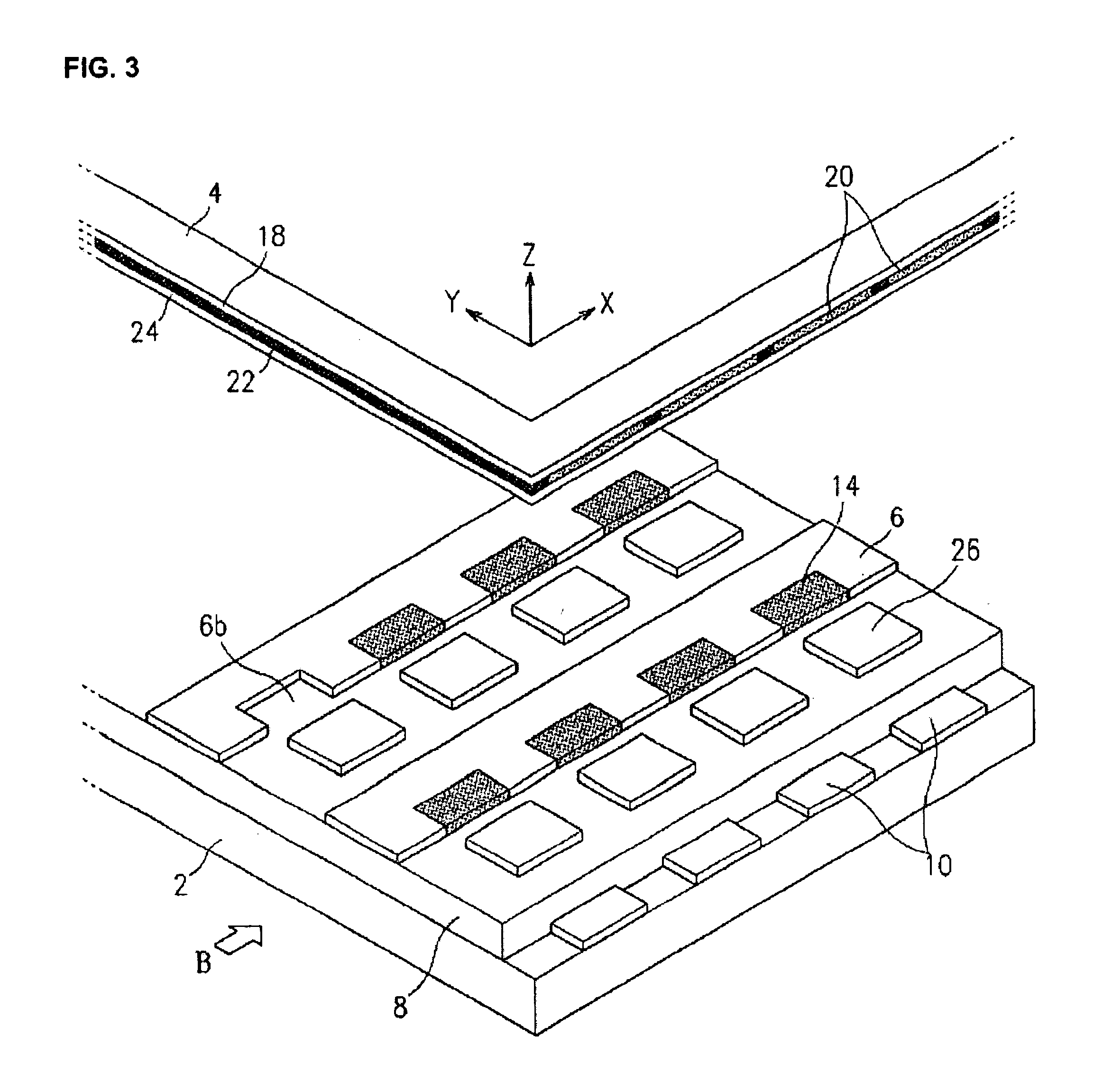
Stabilized controlled release substrate having a coating derived from an aqueous dispersion of hydrophobic polymer
InactiveUS6129933AReduce reunionLiquid surface applicatorsGranular deliveryHydrophobic polymerDissolution
A stabilized solid controlled release dosage form having a coating derived from an aqueous dispersion of ethylcellulose is obtained by overcoating a substrate including a therapeutically active with an aqueous dispersion of ethylcellulose and then curing the coated substrate at a temperature and relative humidity elevated to a suitable level above ambient conditions until the coated dosage form attains a stabilized dissolution profile substantially unaffected by exposure to storage conditions of elevated temperature and / or elevated relative humidity.
Owner:PURDUE PHARMA LP
Stabilized Glycosaminoglycan Preparations and Related Methods
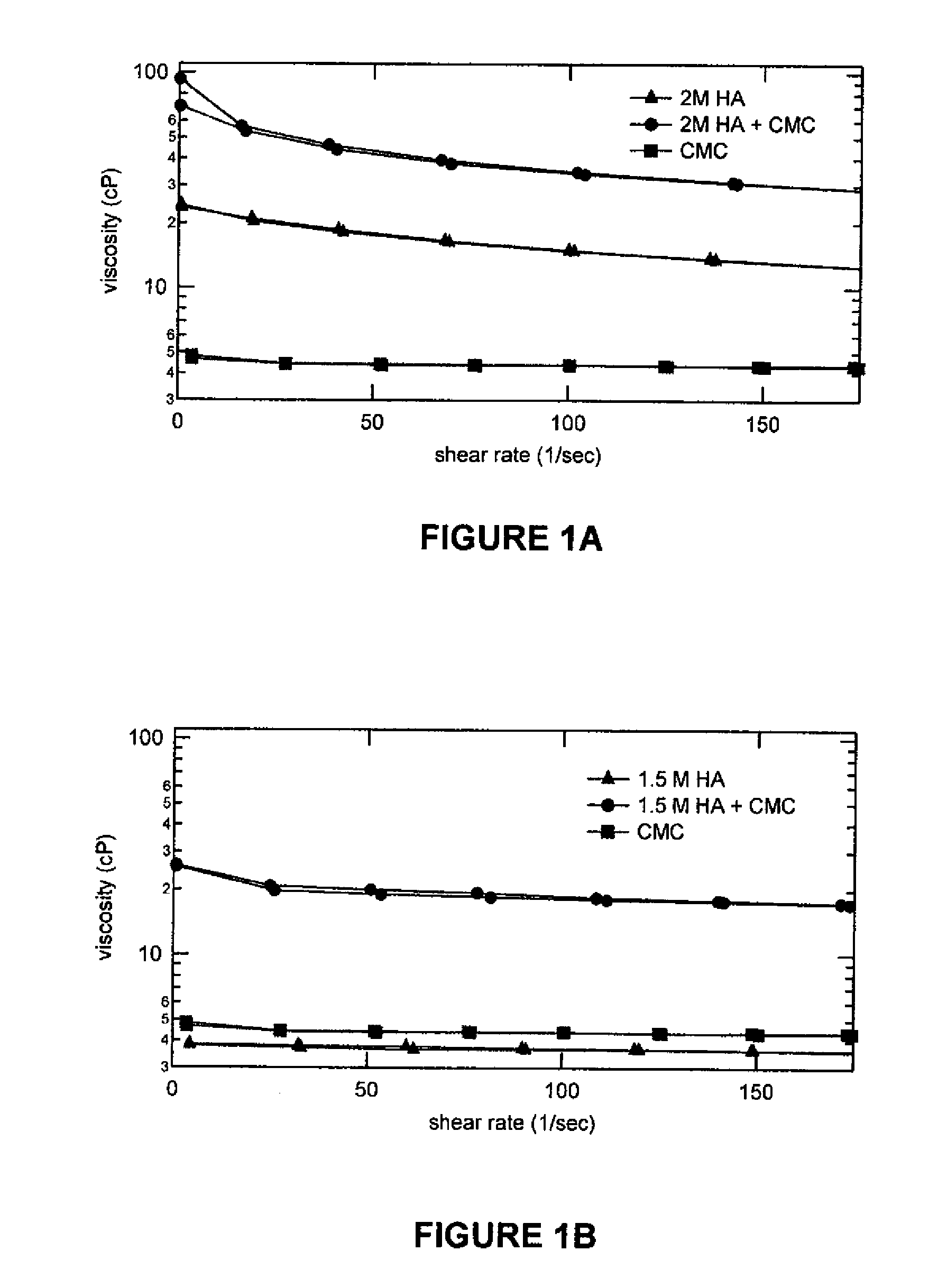
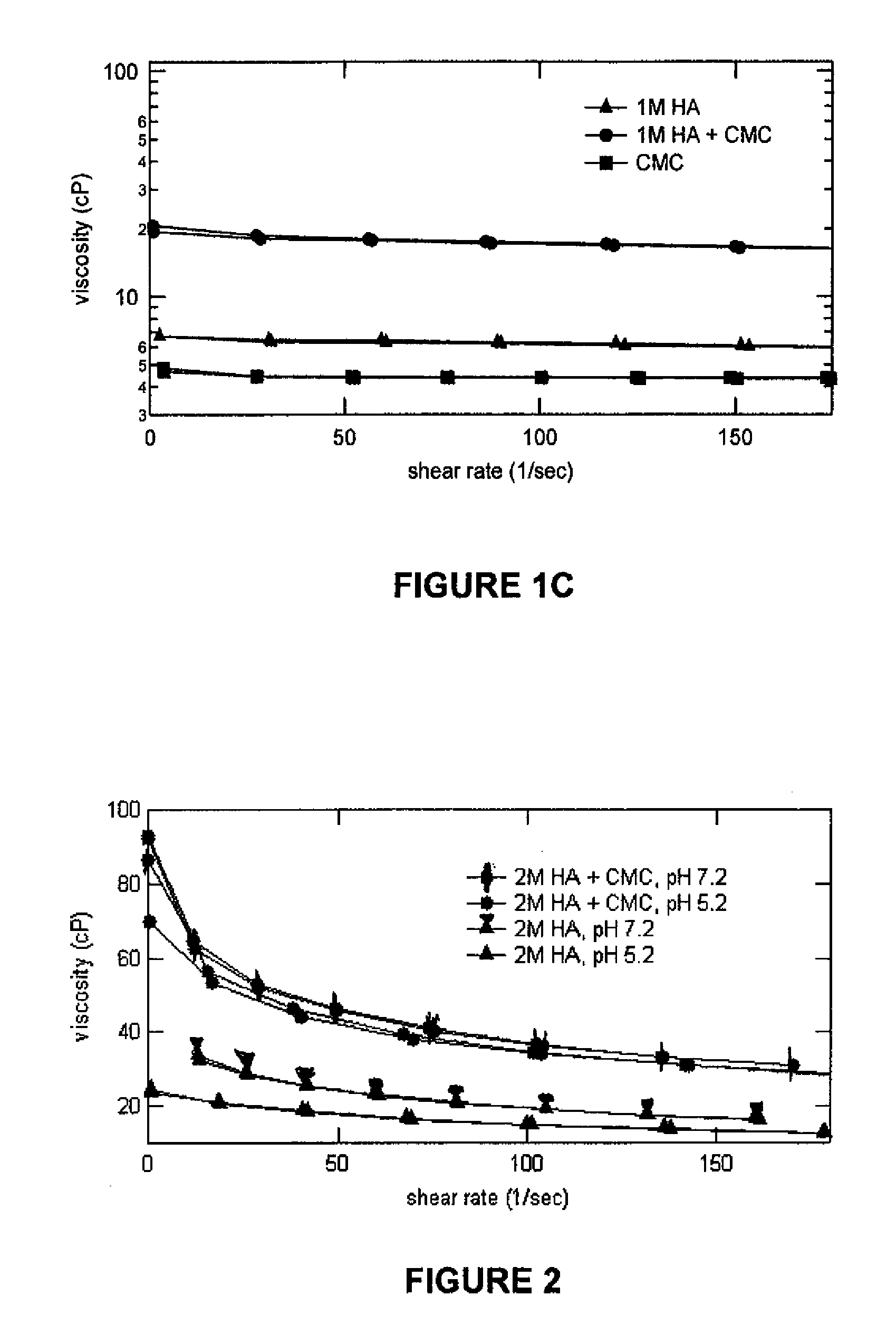
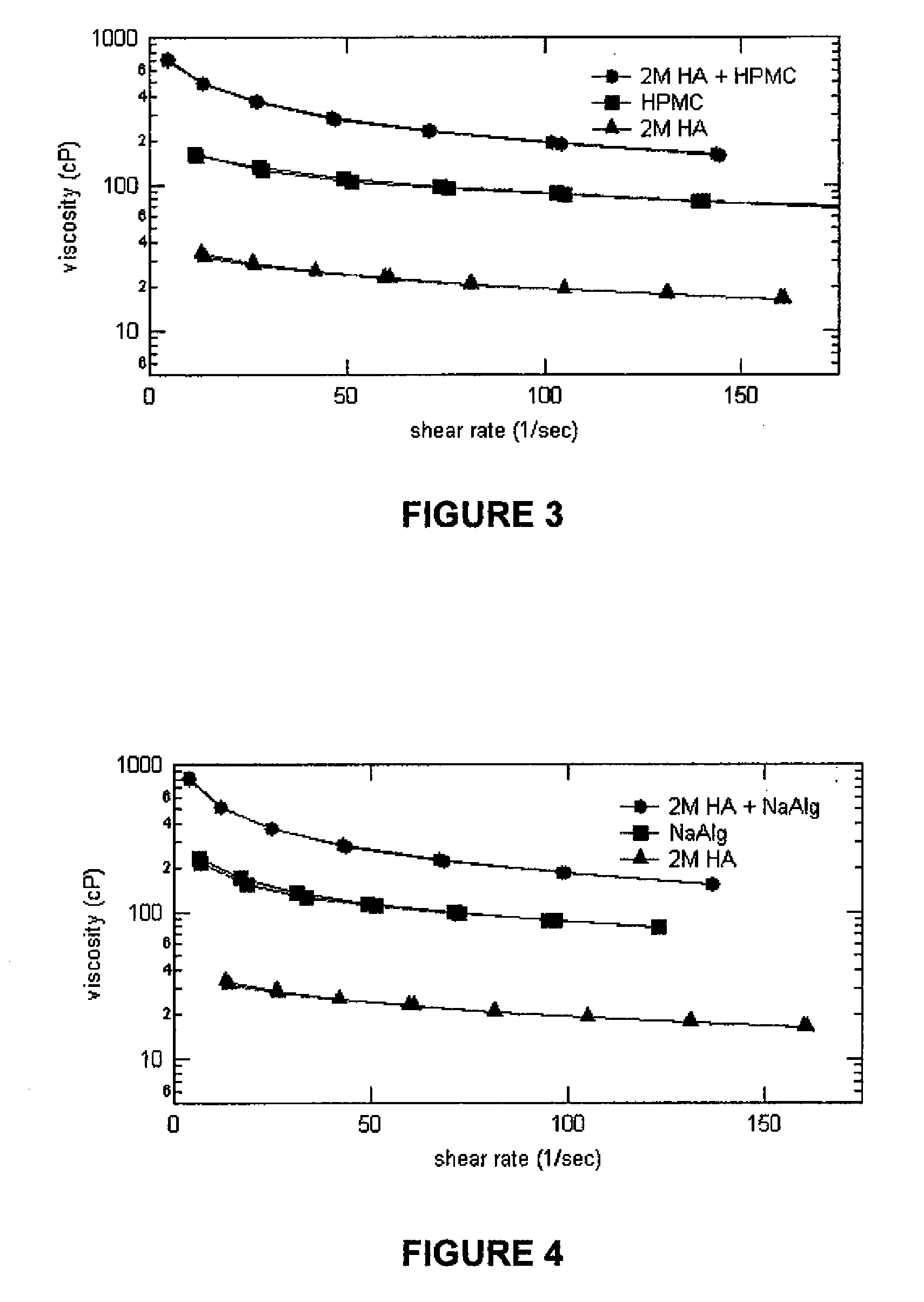
Compositions comprising a glycosaminoglycan (e.g., a hyaluronan, hyaluronic acid, hyaluronate, sodium hyaluronate, dermatan sulfate, karatan sulfate, chondroitin 6-sulfate, heparin, etc.) in combination with at least one component selected from; i) polyglycols (e.g., polyethylene glycol), ii) long chain hydroxy polyanionic polysaccharides (e.g., dextran, sodium alginate, alginic acid, propylene glycol alginate, carboxymethyl cellulose and carboxyethyl cellulose, hydroxyl ethyl starch, hydroxyl propyl methyl cellulose, hydroxy propyl ethyl cellulose, hydroxy propyl cellulose, methyl cellulose, polylysine, polyhistidine, polyhydroxy proline, poly ornithine, polyvinyl pyrolidone, polyvinyl alcohol, chitosan, etc.) and iii) long chain Nitrogen containing polymers (e.g., Polylysine, Polyvinylpyrrolidone, and polyvinyl alcohol). The invention also includes methods for using such compositions (e.g., as substance delivery materials, tissue fillers or bulking agents, as moistening or hydrating agents, etc.)
Owner:S K PHARMA INC
Zero-order sustained release dosage forms and method of making same
InactiveUS20030133982A1High drug loadingReduce releasePowder deliveryBiocideSustained release drugHydrophobic polymer
The present invention relates to zero-order sustained release solid dosage forms suitable for administration of a wide range of therapeutically active medicaments, especially those that are water-soluble, and to a process of making same. The solid dosage form comprises (a) a matrix core comprising ethylcellulose and the active agent and (b) a hydrophobic polymer coating encasing the entire matrix core.
Owner:PHARMACIA CORP
Sustained Release Dosage Forms For Delivery of Agents to an Oral Cavity of a User
Aspects of the invention include a sustained release dosage form that can be administered to an oral cavity, e.g., the mouth. In certain embodiments, the sustained release dosage form is formulated as a lozenge or gum that may be administered to an oral cavity of a user for the purpose of dissolving over a prolonged period of time and thereby delivering an essential oil component therein. In certain embodiments, the sustained release dosage form includes a beneficial agent and, therefore, not only provides for the prolonged delivery of an essential oil component to an oral cavity, but also provides for the sustained release of a beneficial agent thereto. In certain embodiments, the sustained release dosage form includes a biocompatible, water-insoluble polymer, e.g., ethylcellulose and an essential oil component, which are combined in such a manner so as to produce a dosage form that substantially dissolves over a prolonged period of time when positioned within an aqueous environment, such as an oral cavity of a user. In certain embodiments, the sustained release dosage form may include an additional water soluble agent, such as gum arabic, which may be included so as to further provide the dosage form with a desired dissolution characteristic. In certain embodiments, the dosage form may also include a beneficial agent to be delivered to the mouth. Methods of formulating such dosage forms and administering them to an oral cavity for the treatment of an adverse condition are also provided herein.
Owner:BENNES
Stabilized controlled release substrate having a coating derived from an aqueous dispersion of hydrophobic polymer
InactiveUS6316031B1Reduce reunionStable productionLiquid surface applicatorsGranular deliveryHydrophobic polymerDissolution
A stabilized solid controlled release dosage form having a coating derived from an aqueous dispersion of ethylcellulose is obtained by overcoating a substrate including a therapeutically active with an aqueous dispersion of ethylcellulose and then curing the coated substrate at a temperature and relative humidity elevated to a suitable level above ambient conditions until the coated dosage form attains a stabilized dissolution profile substantially unaffected by exposure to storage conditions of elevated temperature and / or elevated relative humidity.
Owner:PURDUE PHARMA LP
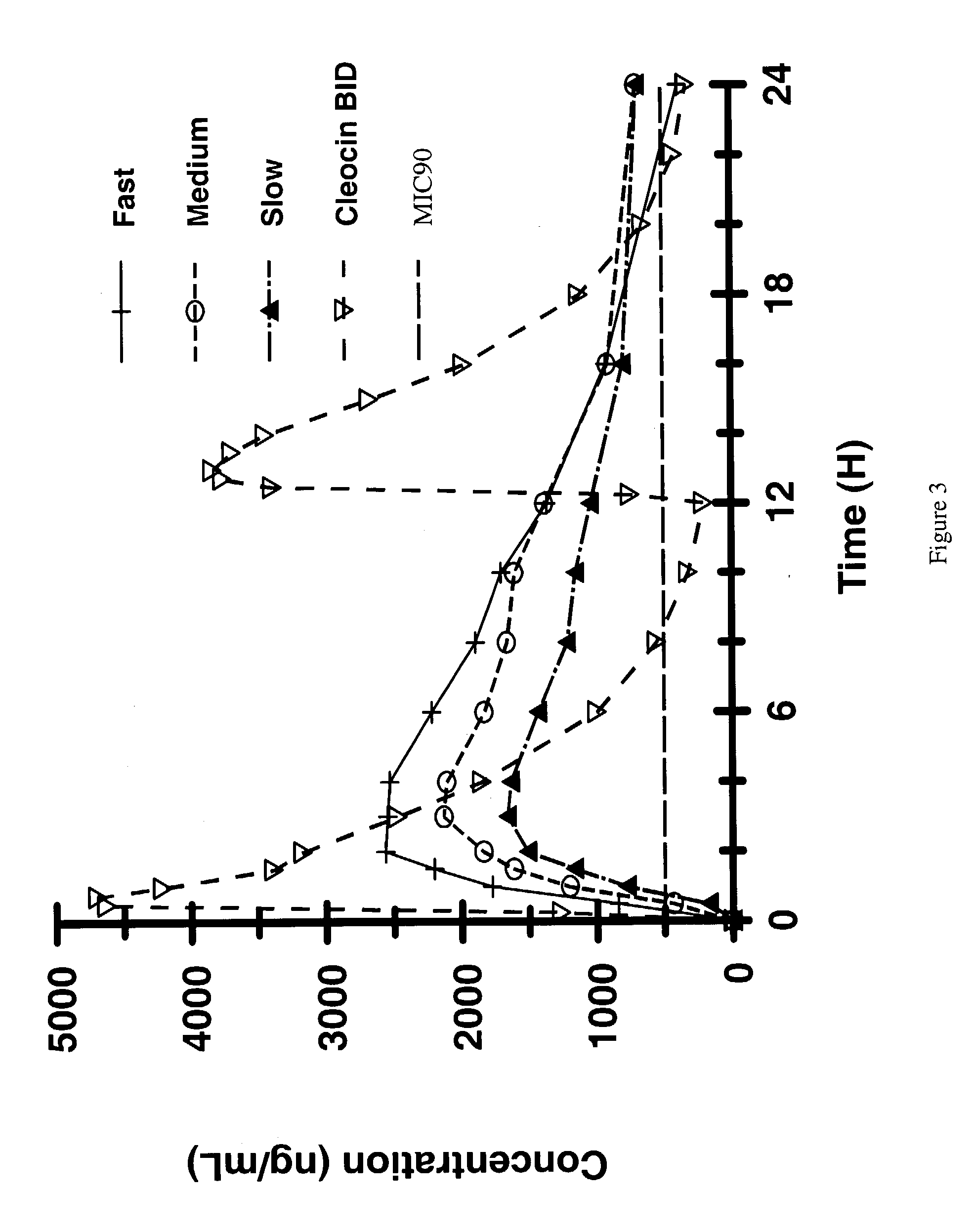
Controlled release formulations using intelligent polymers
InactiveUS6893661B1Promote absorptionMaintenance of therapeutically effective blood levelPowder deliveryOrganic active ingredientsSmart polymerWater contact
An extended release dosage composition of pharmaceutically active substances that have a water contact angle (θ) such that cos θ is between +0.9848 and −0.9848 presented as a matrix tablet containing the said pharmaceutically active substances, with / without suitable pharmaceutical excipients in intimate mixture with two groups of intelligent polymers having opposing wettability characteristics, one demonstrating a stronger tendency towards hydrophobicity and the other a stronger tendency towards hydrophilicity, the polymer combination being between the ratios of 1:50 and 50:1 amounts effective to control the release of said pharmaceutically active substances in a mathematically predictable manner, wherein the polymer demonstrating a stronger tendency towards hydrophobicity is not less than 5% wt / wt and preferably between 5-70% wt / wt of the final formulation composition. The intelligent polymers being ethylcellulose (EC) as a more strongly hydrophobic and hydroxyethylcellulose (HEC) and / or hydroxypropyl methylcellulose (HPMC) as more strongly hydrophilic (the ratio of HEC to HPMC being between 1:100 and 100:1). The matrix tablet is optionally coated with an enteric coat, 0-5%-15% wt / wt to prevent the initial burst effect seen in such systems and to impart gastrointestinal tract (GIT) “stealth” characteristics especially in the presence of food.
Owner:VALEANT INT BERMUDA
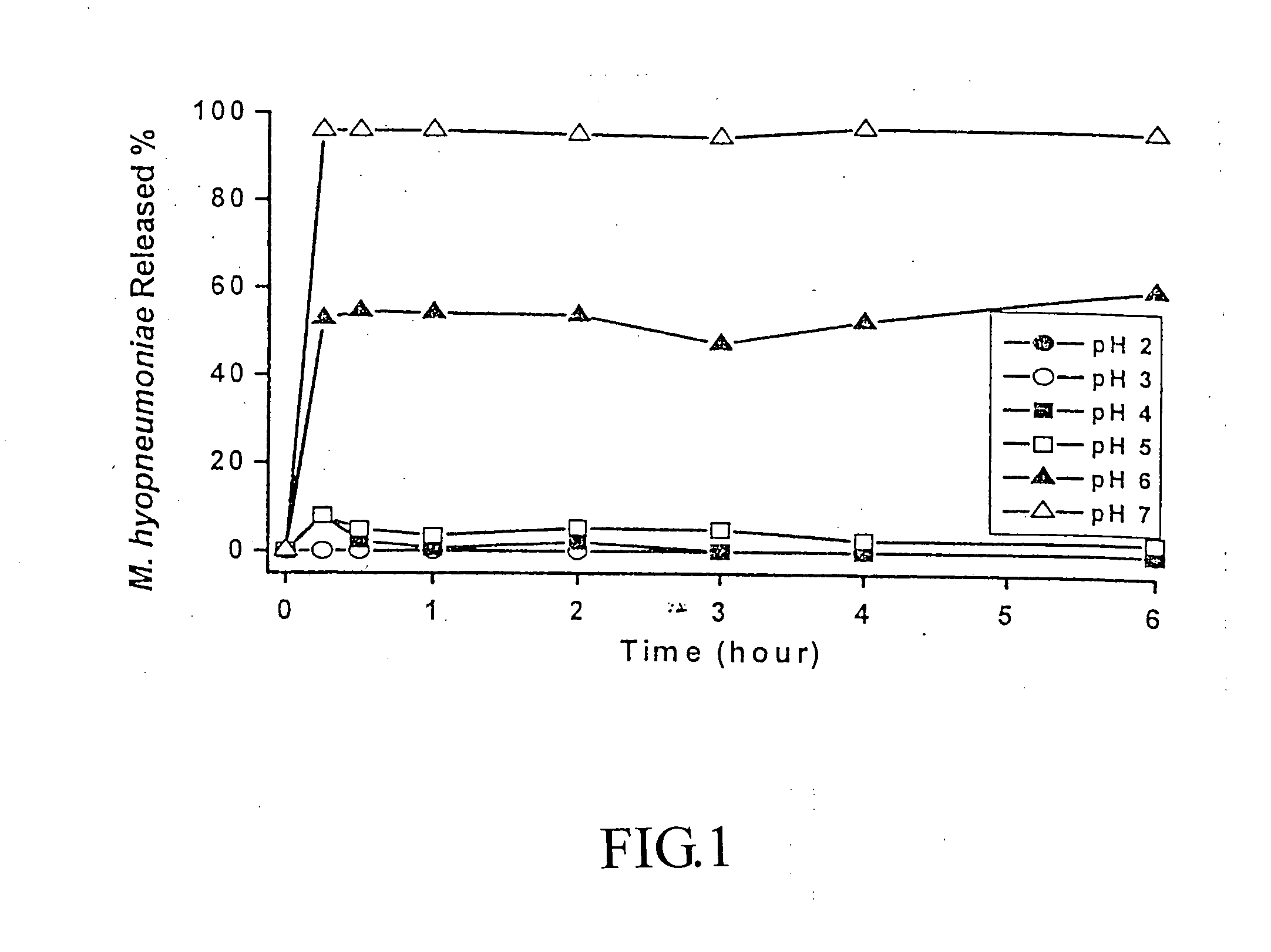
Probiotic composition having acid-resistant enteric coating
InactiveUS20070059296A1Improve digestibilityImproving resistance against diseaseOrganic active ingredientsBiocideBiotechnologyTalc
A probiotic composition essentially comprises 15 to 20 wt % of milk powder, 25 to 30 wt % of corn starch, 8 to 15 wt % of modified starch (capsul), 10 to 15 wt % of ethylcellulose, 5 to 15 wt % of bacterial broth, and 10 to 15 wt % of talc. The probiotic composition is microencapsulated to form a plurality of microencapsule coated with an acid-resistant enteric coating for improving the enteric acid-resistance, the probiotic survival rate, the antimicrobial property, the stability, the moisture-proof property, and the mobility of the probiotic composition preventing from coagulation in a moist environment and for being used as an additive applied to livestock feed.
Owner:BION TECH INC
Colorants for keratin fibers
InactiveUS20070006397A1Good conditionHigh viscosityCosmetic preparationsHair cosmeticsFiberCosmetic vehicle
The present patent application relates to an agent for the coloring of keratin fibers based on oxidative dye precursors and / or direct-penetrating dyes, that contains a combination of at least one cationic hydroxyethyl cellulose and at least one acrylate copolymer in a suitable cosmetic carrier as well as a multicomponent-kit for the coloring of keratin fibers.
Owner:THE PROCTER & GAMBLE COMPANY
Sustained release preparations
Disclosed are sustained release drug particles suitable for forming sustained release oral pharmaceutical compositions. The sustained release drug particles comprise a drug-ion exchange resin complex and a water-permeable, diffusion barrier surrounding at least a portion of the drug-ion exchange resin complex. The diffusion barrier comprises a film-forming polymer and is free or contains no substantial traces of organic solvent. Also disclosed are oral pharmaceutical compositions, for example, oral suspensions, comprising the sustained release drug particles, a method for the controlled administration of a drug to a patient, and a method for manufacturing the sustained release drug particles. The method of manufacturing involves the use of an aqueous coating composition comprising a water-permeable film-forming polymer such as ethylcellulose.
Owner:MALLINCKRODT INC
Quick disintegrating tablet in buccal cavity and manufacturing method thereof
InactiveUS6656492B2Good effectDisintegrates quicklyPowder deliveryLiquid surface applicatorsHigh concentrationLow speed
The present invention pertains to a quick disintegrating tablet in buccal cavity, characterized in that drug-containing particles with a mean particle diameter of approximately 50~approximately 250 mum and an apparent specific gravity of approximately 0.5~approximately 1.2 consisting of a bitter tasting drug and / or drug of inferior fluidity and a pharmaceutical preparation carrier and obtained by spray drying are added to a quick disintegrating tablet in buccal cavity comprising a drug and saccharide. Moreover, the present invention pertains to a method for manufacturing drug-containing particles having a specific mean particle diameter and specific apparent gravity by dissolving and suspending a bitter tasting drug and / or drug of inferior fluidity and a pharmaceutical preparation carrier (preferably containing water-insoluble polymer, particularly at least aqueous ethyl cellulose suspension (preferably containing plasticizer)) to a high concentration in terms of solid concentration in a solvent that is pharmaceutically acceptable and then spray drying this liquid using a rotating disk-type spray dryer, with the disk operating at low speed, and a method for manufacturing a quick disintegrating tablet in buccal cavity comprising said particles.
Owner:ASTELLAS PHARMA INC
Sustained-release preparation utilizing thermal change and process for the production thereof
A sustained-release preparation which can release a highly water-soluble medicinally active ingredient over a long time and a process for the production thereof are provided. The preparation has a sustained-releasing layer formed by heating and melting a layer composed of both an aqueous ethylcellulose latex containing a plasticizer and a wax to miscibilize them.
Owner:SHIONOGI & CO LTD
Slowly-released compound acidifier for poultry and livestock feed, preparation method thereof and feed
ActiveCN102578387AMatching scienceDefinitelyClimate change adaptationAnimal feeding stuffDiseaseFeed conversion ratio
The invention discloses a slowly-released compound acidifier for poultry and livestock feed, a preparation method thereof and feed containing the same. The compound acidifier comprises the following compositions in part by weight: 30 to 80 parts of complex organic acid, 15 to 50 parts of supplementary materials and 5 to 20 parts of coating agents. The complex organic acid is citric acid, fumaric acid, malic acid, lactic acid, linoleic acid, crataegolic acid, ursolic acid, chlorogenic acid, glycyrrhizic acid and oleanolic acid, and the supplementary materials are silicon dioxide, hydroxypropyl methylcellulose and ethyl cellulose. The acidifier is scientifically proportioned, has strong efficiency and is long-acting and slow-releasing, nutrients in the feed are free from being damaged, the acidifier has good fluidity and is easy to be uniformly mixed with the feed, the digestive absorption of nutrients can be promoted, the conversion rate of the feed can be improved, the productivity of poultry and livestock can be improved, the time for domestic animals for sale can be effectively shortened, the health of gastrointestinal mucosa of the animals can be protected, the immunologic function of the animals can be strengthened, and diseases can be prevented.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH +1
Leadless electronic slurry composition for solar silicon photovoltaic cell and preparation method thereof
InactiveCN101345263AHigh electrical conductivityRefined nanocrystalline structureFinal product manufactureSemiconductor devicesAdhesiveSlurry
The invention discloses a compounding and a preparation method for lead-free electronic slurry used for a solar silicon photocell; according to the mass percentage, the slurry is obtained by preparing 70 to 75 percent of aluminium powders, 20 to 25 percent of organic adhesive, 1 to 5 percent of inorganic glass powders and 1 to 5 percent of additives; the aluminium powder is ball-shaped aluminium powder with the surface covered by an aluminium nitride protection layer, with the purity not less than 99.9 percent and the average grain size of 2 to 6 microns; simultaneously, organic adhesives consisting of ethyl cellulose, resin, hexadecanol, diethyleneglycol monomethyl ether, diethyleneglycol monobutyl ether, terpineol and n-butyl alcohol are added into the aluminium powder; the inorganic glass powder is silicon dioxide, boracic acid, alumina, antimony oxide and zirconia; the additive consists of Span and pump oil. The product prepared by the method of the invention has no dust generation or aluminium peeling phenomenon and achieves the requirement of no lead and environmental protection.
Owner:NANTONG UNIVERSITY
Enteric coatings for orally ingestible substrates
Enteric film coating systems for orally ingestible substrates such as pharmaceutical tablets and dietary supplements are disclosed. In preferred aspects, the enteric film coatings include an ethylcellulose dispersion and a substantially gastro-insoluble pore former such as sodium alginate.
Owner:BPSI HLDG LLC
Enveloping acidifier for feed and preparation method thereof
ActiveCN102106461AHigh total acid contentImprove protectionAnimal feeding stuffAccessory food factorsMaterial consumptionMethyl cellulose
The invention relates to enveloping acidifier and the preparation method thereof. The enveloping acidifier comprises acidifier solid microparticles and an enveloping layer on the surface of the acidifier solid microparticles, wherein the enveloping layer comprises an ethyecellulose (EC) and hydroxypropyl methyl cellulose (HPMC) composite layer and an enteric-coated polyacrylic resin layer. In theinvention, sustained-release coating material with good film forming property and the coating process are adopted, so that excessive enveloping material consumption is avoided, the weight of the acidifier solid microparticles is increased slightly, the large total effective acid of the acidifier is kept, and the acidifier has good sustained-release effect.
Owner:安徽泰格生物技术股份有限公司
Lead-free aluminum paste for solar battery
InactiveCN101789456AMeet environmental protection requirementsStrong adhesionFinal product manufactureNon-conductive material with dispersed conductive materialAluminium powderSolar battery
The invention provides a lead-free aluminum paste for a solar battery, which is mainly used for producing a conductive electrode for a solar battery. The lead-free aluminum paste mainly comprises the following components by weight percent: 70 to 80 percent of aluminum powder, 19 to 30 percent of organic bond, 0.1 to 5 percent of inorganic glass powder and 0.1 to 2 percent of a first additive. The organic bond comprises the following components by weight percent: 40 to 60 percent of terpineol, 1 to 5 percent of ethylcellulose, 1 to 5 percent of lecithin and 20 to 50 percent of a second additive. The lead-free aluminum paste for the solar battery mainly solves the technical problems that the existing aluminum paste for the solar battery has poor electrical conductivity and low photoelectric conversion efficiency. The lead-free aluminum paste has the advantages of good electric conductivity, high photoelectric conversion efficiency and the like.
Owner:西安宏星电子浆料科技股份有限公司
Benefit agent delivery particles comprising non-ionic polysaccharides
InactiveUS20140206587A1High affinityCosmetic preparationsHair cosmeticsHydroxyethyl ethylcelluloseEmulsion polymerization
The invention provides a composition comprising a benefit agent delivery particle comprising at least one of hydroxylpropyl methyl cellulose, hydroxylethyl methyl cellulose, hydroxylpropyl guar, hydroxylethyl ethyl cellulose or methyl cellulose. The benefit agent delivery particle may further comprise a non-polysaccharide polymer, preferably an aminoplast polymer. The benefit agent delivery particle may comprise a perfume. The invention also provides a process for the manufacture of the particles in which perfume oil is encapsulated using emulsion polymerization to form core-shell particles, (in the alternative the perfume may be adsorbed later) and, a further polymer layer is formed on the outer surface of the core shell-particles in the presence of the delivery aid.
Owner:CONOPCO INC D B A UNILEVER
Electron emission source composition for field emission display device and field emission display device fabricated using same
Disclosed is an electron emission source composition for a field emission display device including 1 to 20% by weight of carbon nano tubes; glass frit; an organic binder resin comprising ethyl cellulose and acrylate resin and / or acryl resin; and an organic solvent, wherein the glass frit is present in an amount of 1 to 500 parts by weight with respect to 100 parts by weight of the carbon nano tubes.
Owner:SAMSUNG SDI CO LTD
Leadless solar battery silver paste and method for producing the same
InactiveCN101271929AImprove photoelectric conversion efficiencyStrong adhesionFinal product manufactureNon-conductive material with dispersed conductive materialAcrylic resinContact resistance
The invention discloses lead-free solar cell silver serum and a preparation method thereof, which consists of 70-85 percent of conducting silver powder, 5-10 percent of lead-free glass glue and 5.5-25 percent of organic carrier, wherein, the organic carrier consists of organic solvent and organic additive, the organic solvent is any kind of terpineol, terebinth and ethers or their combination; the organic additive is any kind of ethyl cellulose, dibutyl phthalate and acrylic resin or their combination; the particle size of the conducting silver powder is 0.2-2mum; and the lead-free glass glue belongs to bismuth-silicon- antimony glass system. The solar cell silver serum obtained by the method in the invention is lead-free, which complies with requirements of environmental protection, and a conducting electrode with strong adhesive force and low ohm contact resistance can be formed on the surface of the solar cell. The cell has high photoelectric conversion efficiency and is an ideal substituent of the lead silver serum.
Owner:EGING PHOTOVOLTAIC TECHNOLOGY CO LTD
Controlled Release Insect Repellent Materials
Generally, controlled release insect repellent materials include at least one polymer selected from the group consisting of an ethylene copolymer, an ethyl cellulose, a thermoplastic polyurethane, and any combination thereof and at least one insect repellent and may optionally include at least one insect repellent synergist, at least one additive, at least one additional polymer, and any combination thereof. Further, controlled release insect repellent materials may be used to form articles or products with long-term efficacy.
Owner:CELANESE EVA PERFORMANCE POLYMERS
Zinc metallic coating
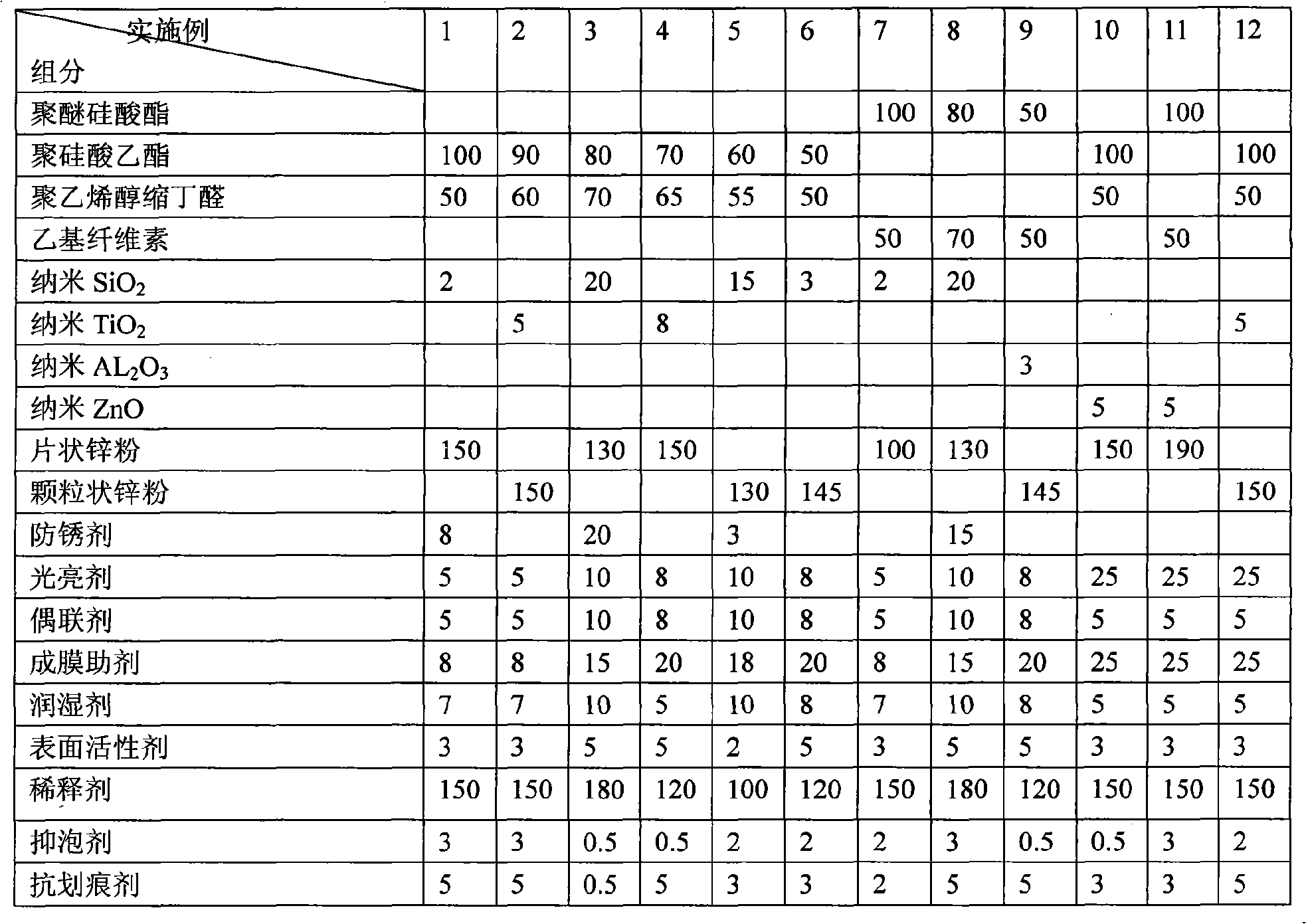
The invention discloses a zinc metal coating layer, the zinc metal coating is characterized in that when being calculated according to the parts by weight, the components of the the zinc metal coating are as follows: 50 parts to 100 parts of polyether silicate ester or polymerized silica acid ester, 50 parts to 100 parts of polyvinyl butyral or ethyl cellulose, 1 part to 20parts of one or a plurality of component (s) among nanometer silicon dioxide, nanometer titanium dioxide, nanometer molybdenum dioxide, or nanometer zinc oxide, 80 parts to 200 parts of zinc powder, 0 to 20 parts of rust preventing agent,, 5 parts to 100 parts of coupling agent, 5 parts to 25 parts of brightening agent, 8 parts to 250 parts of film forming auxiliary agent, 5 parts to 10 parts of wettinh agent, 1 part to 5 parts of surface activating agent, 100 parts to 200 parts of thinning agent, 0.5 part to 3 parts of anti-foaming agent and 0.5 part to 5 parts of anti-scratching agent. The bright metal zinc coating layer related by the invention can be taken as the anti-corrosive oxidation resistant zinc metal coating layer of metal product, particularly the black metal product, which can replace the commonly adopted galvanizing technology. The bright zinc metal coating layer of the invention can very conveniently and effectively paint a zinc metal coating layer the binding of which is firm on the metal product, particularly the black metal product.
Owner:胡仲寅
Water-insoluble medicine sustained-release pellet, sustained-release orally disintegrating tablet thereof and preparation method thereof
InactiveCN101862297ASmall particle sizeHigh strengthOrganic active ingredientsAntipyreticSustained release pelletsSide effect
The invention discloses a water-insoluble medicine sustained-release pellet, a sustained-release orally disintegrating tablet thereof and a preparation method thereof. The water-insoluble medicine sustained-release pellet comprises a hollow pellet core, a medicine layer, an insulation layer and a sustained-release layer, wherein the sustained-release layer comprises the following ingredients: 54 to 88 percent of sustained-release materials, 2 to 30 percent of antitackiness agents and 1 to 30 percent of pore-foaming agents, wherein the percentage is the mass percentage in the sustained-release layer, wherein the sustained-release materials are one kind or several kinds of materials selected from ethyl acrylate and methyl methacrylate copolymers, polyvinyl acetate and ethyl cellulose. Through regulating the coating combinations and filling auxiliary materials to be pressed into orally disintegrating tablets, the medicine can be slowly released for more than 8 to 13 hours, so the stable blood medicine concentration can be maintained, the side effect is reduced, the medicine taking times can be reduced, and the medicine taking is convenient. The invention conforms to zero-grade release, has the advantages of high final accumulated medicine release amount, high efficacy, strong selectivity, good mouth feeling, simple production steps and high efficiency, and can be applied to large-scale production.
Owner:SHANGHAI INST OF PHARMA IND +1
Essence microcapsule and preparation method and application thereof
ActiveCN108392635AReduce fragrance evaporationHigh strengthCosmetic preparationsTobacco preparationPolyvinyl alcoholMethyl cellulose
The invention relates to an essence microcapsule and a preparation method and an application thereof. The essence microcapsule comprises a capsule core, and a slow release layer and a rapid release layer coating the capsule core in order, the capsule core comprises the material of a first fragrance substance, the slow release layer comprises at least one of Arabic gum, hydroxypropyl methyl cellulose, chitosan, polyvinyl alcohol, polyethylene glycol, ethyl cellulose, konjac glucomannan, gelatin, and pullulan polysaccharide, the rapid release layer comprises a second fragrance substance, and theflavors of the second fragrance substance and the first fragrance substance are constant. The fragrance lasting time of the essence microcapsule is long, the fragrance intensity is strong, and the fragrance explosiveness is good.
Owner:DONGGUAN BOTON FLAVORS & FRAGRANCES
Controlled release composition
InactiveUS20080254124A1Easy for to follow prescribed regimenConstant of releasePowder deliverySolution deliveryEthyl(hydroxyethyl)celluloseCellulose acetate
A composition for controlled delivery of at least one active substance into an aqueous medium by erosion at a preprogrammed rate of at least one surface of the composition, comprising a matrix comprising the active substance, the matrix being erodible in the aqueous medium in which the composition is to be used, and a coating having at least one opening exposing at least one surface of said matrix, the coating comprising a first cellulose derivative which has thermoplastic properties and which is substantially insoluble in the aqueous medium in which the composition is to be used, and at least one of a second cellulose derivative which is soluble or dispersible in water, a plasticizer, and a filler. The coating is a coating which crumbles and / or erodes upon exposure to the aqueous medium such as a body fluid. The first cellulose derivative may be, e.g., ethylcellulose, cellulose acetate, cellulose propionate or cellulose nitrate, and the second cellulose derivative may be, e.g. methylcellulose, carboxymethylcellulose or salts thereof, cellulose acetate phthalate, microcrystalline cellulose, ethylhydroxyethylcellulose, ethylmethylcellulose, hydroxyethylcellulose, hydroxyethylmethylcellulose, hydroxypropylcellulose, hydroxypropylmethylcellulose, hydroxymethylcellulose or hydroxymethylpropylcellulose.
Owner:EGALET LTD
Degradable plastic packaging bag
InactiveCN104927318AHigh tensile strengthLow moisture permeabilityFlexible coversWrappersArray data structurePolyvinyl alcohol
The present invention belongs to the field of plastic products, and particularly relates to a biodegradable plastic packaging bag which is characterized by comprising the following components by weight: 5-15 parts of ethyl cellulose, 10-20 parts of polyethylene, 15-30 parts of modified starch, 15-30 parts of corn starch, 15-30 parts of sweet potato starch, 20-40 parts of polylactic acid, 2-10 parts of calcium carbonate powder, 1-5 parts of a lubricant, 1-5 parts of a plasticizer, 1-3 parts of a toughening agent, 1-10 parts of a degradation accelerator, 1-5 parts of polyvinyl alcohol, 2-5parts of chitosan, 1-5 parts of a compatibilizing agent and 2-6 parts of polycaprolactone. The biodegradable plastic packaging bag begins to thin, lose in weight, reduce in strength, then gradually splits into fragments after being exposed to the environment for two months, when these fragments are buried in garbage or soil, the degradation effect is very obvious; by addition of the plasticizer and the calcium carbonate powder, the added ingredients may enhance the tensile strength of the plastic packing tape, reduce the air and moisture permeability, and maintain the original characteristics of the plastic packaging bag.
Owner:TIANJIN YOUZHI TECH CO LTD
Cellulose acetate film for use in liquid crystal displays
A liquid crystal device having a plurality of pixel electrodes for transmitting light, a first panel having an activation portion for selectively activating the plurality of pixel electrodes, an orientation layer formed on the activation portion, a light shielding pattern formed on the orientation layer, a second panel having a second orientation layer, a liquid crystal formed between the first and second panels, a polarizing plate or a color filter and a protective film on at least one of the aforementioned surfaces wherein the protective layer includes a cellulose ester selected from the group consisting of cellulose acetate, cellulose formate, cellulose propionate, cellulose butyrate, ethyl cellulose, methyl cellulose and benzyl cellulose having an inherent viscosity of from about 1.0 to less than 2.0 dl / g.
Owner:EASTMAN CHEM CO
Method for preparation of electrode slurry
InactiveCN101207193AUniform and stable dispersionGood dispersionElectrode manufacturing processesActive material electrodesPolyethylene oxideInternal resistance
The invention relates to a preparation method of electrode pulp, which comprises the step that binding agent, conductive agent and dissolvent are mixed and agitated evenly. The invention is characterized in that the conductive agent is mixed with the binding agent, electrode active material and the dissolvent in the form of conductive agent dispersion, the conductive agent dispersion includes the conductive agent, conducting dispersing medium, dispersant and stabilizer, the dispersant is chosen from one or more of polyacrylamide, polyvinylpyrrolidone, hydroxy ethyl cellulose, polyacrylic ester, dodecyl polyethylene oxide ester, polycaprolactone and alkyl acid and the stabilizer is chosen from on or more of polyvinylidene fluoride, polyfluortetraethylene, polyacrylamide, polyvinylpyrrolidone, methylol cellulose, droxyethylcellulose and polyacrylic ester. The battery which is prepared by utilizing the method provided by the invention has the advantages that the battery capacity is high, the discharging platform is stable, the circulation is good, and the internal resistance change is little; and simultaneously, the invention overcomes the disadvantages that the using quantity of organic solvent is large, the cost is high and the pollution to the environment is large when the pulp is prepared in the prior art.
Owner:BYD CO LTD
Release control fertilizer and method for producing same
InactiveCN1666971AReduce degradationEasy to fixAgriculture gas emission reductionFertilizer mixturesAcrylic resinNitrification inhibitors
This invention relates to controlled release fertilizer, to be specific, it is a control efficiency fertilizer and its manufacturing technique. The control efficiency fertilizer is composed by capsule and fertilizer core, the core is nitrogen compound fertilizer with nitrification inhibitor, the capsule is acrylic resin or compound of acrylic resin and ethyl cellulose. The core is produced by blending comminution granulation; fluidized bed spray painting is used on capsule technique. Acrylic resin or compound of acrylic resin and ethyl cellulose is dissolved in ethanol solution. Inorganic membrane amendment and plasticizer are added to the solution, then fluidized state capsule surface is sprayed coated to form smoothing, continuous, uniform capsule. It can control nutrition release and transformation of fertilizer nitrogen efficiently, greatly evaluate the availability of azophoska, and the manufacturing cost is below high molecular polymer capsule compounding fertilizer which has equal fertilizer efficiency.
Owner:SHENYANG INST OF APPLIED ECOLOGY - CHINESE ACAD OF SCI
Tea polyphenol liposoluble microcapsules and preparation method thereof
InactiveCN103330213ASmall droplet sizeSmall particle sizeTripeptide ingredientsAntinoxious agentsPhenolic content in teaPolyphenol
The invention relates to microcapsules and a preparation method thereof, particularly to tea polyphenol liposoluble microcapsules and a preparation method thereof, wherein a core material of the tea polyphenol liposoluble microcapsules comprises tea polyphenol and reduced glutathione, a mass ratio of the tea polyphenol to the reduced glutathione is 95-99:1-5, a wall material of the tea polyphenol liposoluble microcapsules is one or a plurality of materials selected from arabic gum, dextrin, corn syrup, ethyl cellulose, methylcellulose, and hydroxypropyl methylcellulose phthalate, and a mass weight ratio of the core material to the wall material is 1:1-4. The tea polyphenol liposoluble microcapsule preparation method comprises the following steps: respectively preparing a core material emulsion liquid and a wall material solution, adding the core material emulsion liquid to the wall material solution, carrying out high speed stirring, homogenizing, and carrying out spray drying to obtain the tea polyphenol liposoluble microcapsules. The tea polyphenol liposoluble microcapsules have characteristics of fine average particle size and slow tea polyphenol release, such that anti-oxidation and absorption utilization efficiency of the tea polyphenol are substantially improved.
Owner:ZHEJIANG MINGHUANG NATURAL PRODS DEV
Disinfection sterilizing type medical supersonic couplant and method of preparing the same
ActiveCN101249268AIncrease concentrationImprove the bactericidal effectIn-vivo testing preparationsUltrasonographyChlorhexidine Acetate
The invention discloses a disinfection sterilization medical ultrasonic coupling medium with the bactericidal function and used in the ultrasonic detection and treatment of skin and cavitary mucosa and a preparation method of the coupling medium, wherein, the ultrasonic coupling medium comprises propylene glycol, de-ionized water and one viscosity modifier of the hydroxy ethyl cellulose, carbomer and hydroxy propyl cellulose. The coupling medium further comprises a fungicide of 0.05 to 0.15 percent and the constituents of the fungicide are any one of polyhexamethylene biguanide, benzalkonium chloride, benzalkonium bromide, chlorhexidine acetate, trichlorobenzene phenoxy diphenyl ether and nanometer silver or any combination of the olyhexamethylene biguanide, the benzalkonium chloride, the benzalkonium bromide, the chlorhexidine acetate, the trichlorobenzene phenoxy diphenyl etherand, and the nanometer silver; the preparation method used for preparing the ultrasonic coupling medium is as follows: dispersing the hydroxy ethyl cellulose in the propylene glycol, stirring and dispersing the hydroxy ethyl cellulose by adding enough water, heating to 75 to 80 DEG C, preserving the heat for 30 minutes to cool the hydroxy ethyl cellulose and the propylene glycol to 45 DEG C, adding the fungicide to stir and cool the fungicide, the hydroxy ethyl cellulose and the propylene glycol to obtain the filled coupling medium. Tests show that the products of the coupling medium have fast and long sterilization effect and meet the requirements of various related standards.
Owner:广州市一杰医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com