Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3593 results about "Polycaprolactone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
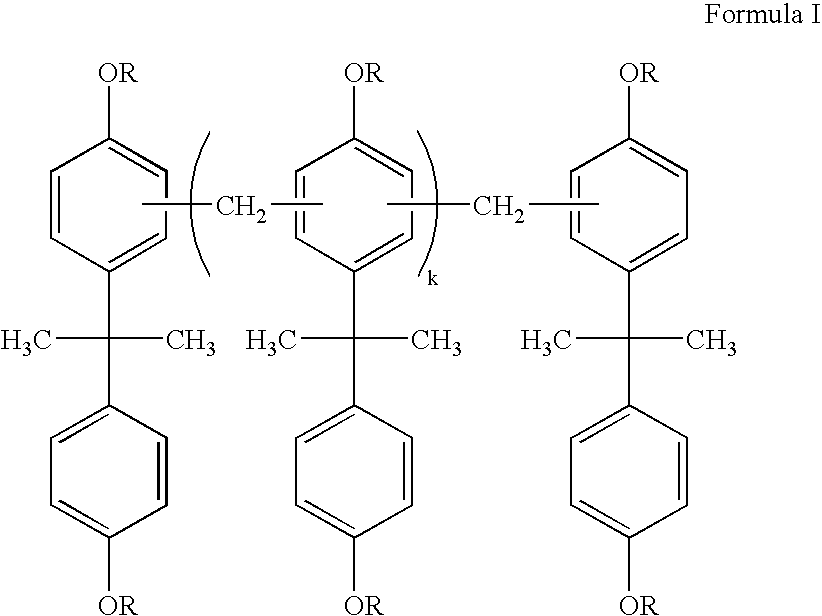
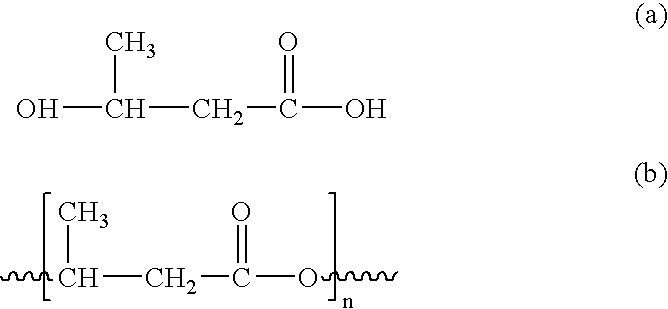
Polycaprolactone (PCL) is a biodegradable polyester with a low melting point of around 60 °C and a glass transition temperature of about −60 °C. The most common use of polycaprolactone is in the production of speciality polyurethanes. Polycaprolactones impart good resistance to water, oil, solvent and chlorine to the polyurethane produced.
Microparticles with adsorbent surfaces, methods of making same, and uses thereof
InactiveUS6884435B1Stimulate immune responseEasy to usePowder deliverySsRNA viruses positive-senseAntigenDisease
The present invention is directed to microparticles, to microparticle compositions containing the same, to methods of forming the same, and to uses for the same, including use for a vaccine, for raising an immune response, for treatment of a disease and for diagnosis of a disease. The microparticles comprise a biodegradable polymer, such as a poly(α-hydroxy acid), a polyhydroxy butyric acid, a polycaprolactone, a polyorthoester, a polyanhydride, or a polycyanoacrylate, and a detergent selected from a cationic detergent and an anionic detergent. The microparticles further comprise an antigen adsorbed on the surface of the microparticle.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Jettable compositions
ActiveUS20050171237A1Prevent long-term corrosionReaction is slowAdditive manufacturing apparatusLiquid surface applicatorsMeth-Oligomer
A fully curable jettable composition having a viscosity less than 30 cps at a temperature within the range of 15-180° C., more preferably at a temperature of 15-100° C., e.g. 60-80° C. the composition comprising: (A) at least one low viscosity reactive resin selected from the group consisting of compounds containing an oxetane ring, cycloaliphatic epoxy resins, tetrahydrofurans, hexahydropyrans and mono-functional (meth)acrylates, said resin having a molecular weight of not greater than 300 Daltons, e.g. 250 Daltons or less, and a viscosity at a temperature in the said range of less than 30 cps, e.g. 5 to 15 cps; (B) at least one higher viscosity resin selected from the group consisting of epoxy resins, compounds containing an oxetane ring and acrylates, which resin acts to thicken the low viscosity resin and strengthen a jetted deposit of the composition, the higher viscosity resin having: a viscosity greater than twice that of the low viscosity resin at the said temperature in the range stated above, and a functionality of greater than or equal to 2; (C) at least one curable toughener, preferably having a functionality of at least 2, such as hydroxy, epoxy, acrylic or other reactive functionalised polymer / oligomer (e.g. derived by functionalising poly(tetrahydrofuran), polycaprolactone, polycarbonate diol, or a dendrimeric polyol; (D) at least one initiator for the polymerisation of the resins, and (E) at least one stabiliser for delaying the curing of the resins of the composition; wherein the low viscosity resin is slower to react than the higher viscosity resin and acts to solvate the higher viscosity resin prior to curing and at least partly during curing and wherein at least 30% of the components A and B are cationically curable resins. The composition can be jetted from piezo electric printing heads under the control of a computer program to form a multi-layered article, e.g. a three dimensional article, in which the adjacent droplets merge and are cured homogeneously together.
Owner:3D SYST INC +1
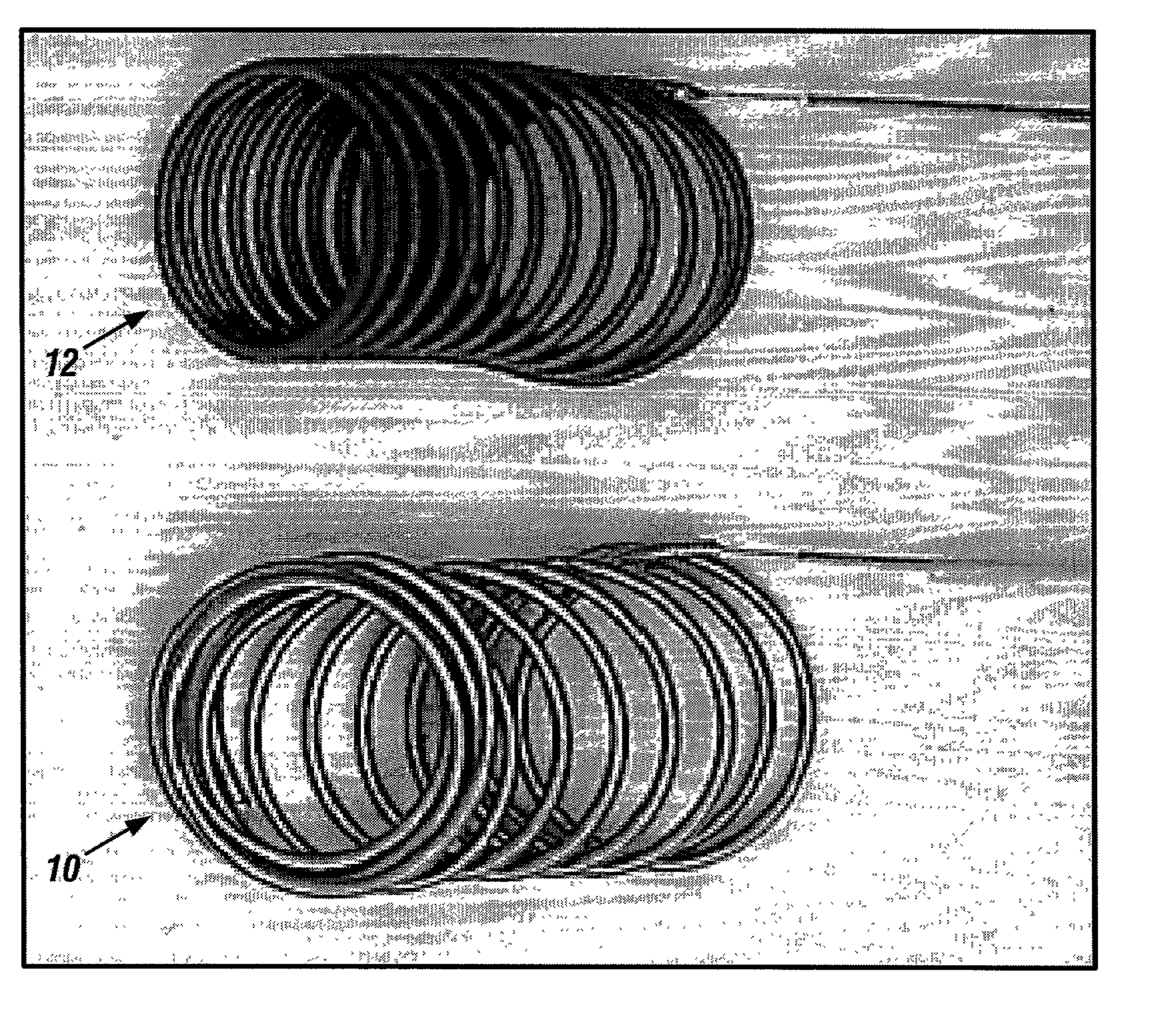
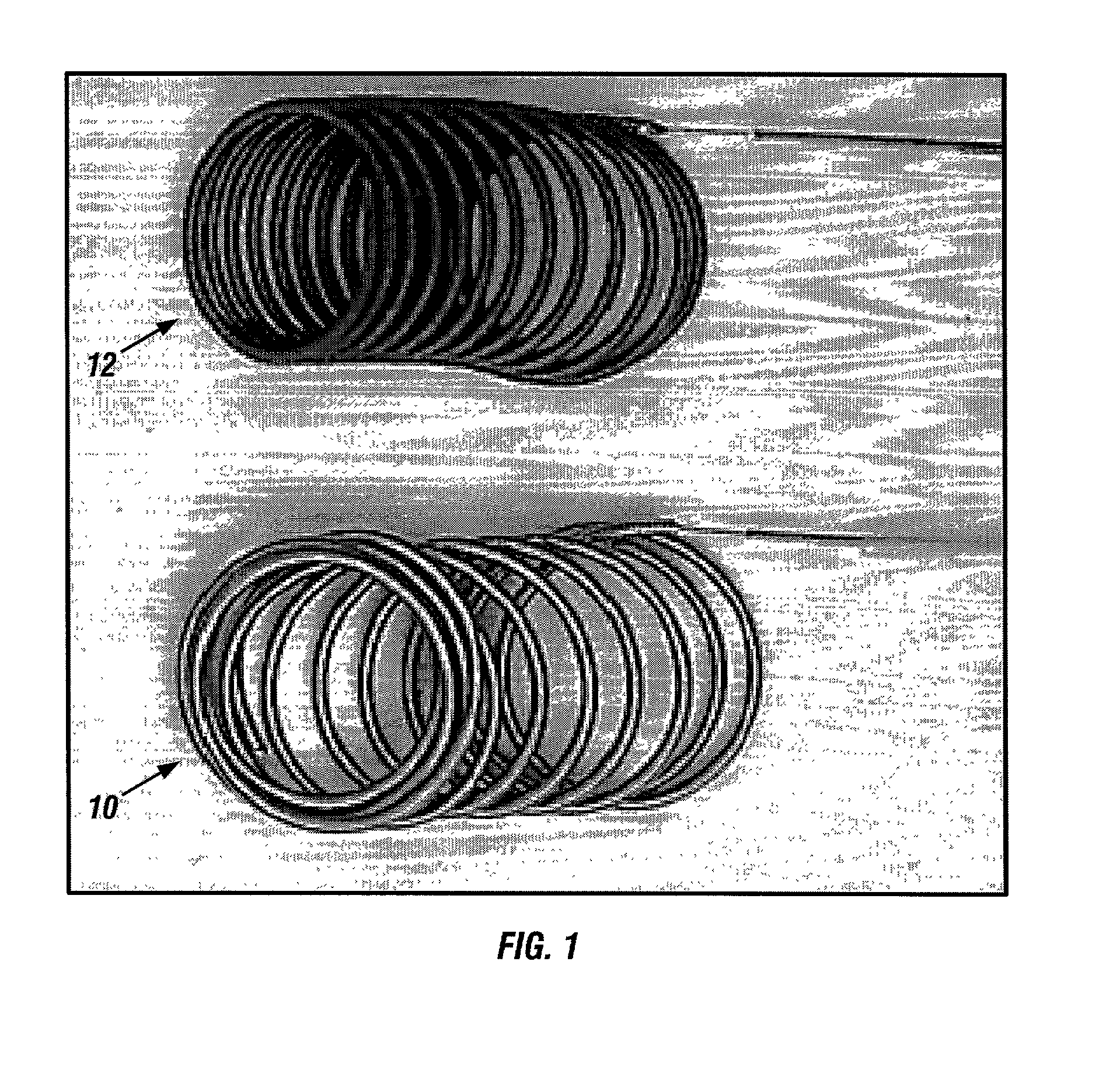
Biodegradable polymer coils for intraluminal implants
An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location of a separable coil comprised at least in part of at least one biocompatible and absorbable polymer or protein and growth factors. Typically a catheter associated with the separable coil is used to dispose the coil into a selected body lumen. The biocompatible and absorbable polymer or protein is thrombogenic. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and absorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides. The biocompatible and absorbable protein is at least one protein selected from the group consisting of collagen, fibrinogen, fibronectin, vitronectin, laminin, and gelatin. In one embodiment the coil is composed of the biocompatible and absorbable polymer or protein with a radio-opaque material is disposed thereon. Alternatively, the coil is composed of a radio-opaque material, and the biocompatible and absorbable polymer or protein is disposed thereon. This apparatus may be positioned within intracranial aneurysms or any aneurysm in the body as well as within other body cavities.
Owner:RGT UNIV OF CALIFORNIA
Environmentally friendly polylactide-based composite formulations
Polymeric materials and products, including sheet flooring materials prepared from the polymeric materials, and processes for preparing the polymeric materials, are disclosed. The polymeric materials include a polylactic acid-based polymer in combination with plasticizer and a compatibilizer, and optionally include a filler. The polymeric material can include between about 30 to about 50 percent by weight polyvinyl chloride, polyethylene glycol, polyglycolide, ethylene vinyl acetate, polycarbonate, polycaprolactone, polyhydroxyalkanoates, or polyolefins modified with polar groups, for example, ionomers. The plasticizer is typically an epoxidized vegetable oil or esterified and epoxidized vegetable oil and is typically present in an amount of between about 10 and about 50% by weight. In some embodiments, the compatibilizer is a polyolefin modified with one or more polar functional groups, and is typically present in an amount of between about 5 and about 10% by weight. The material can be used in decorative surface coverings, such as a floor coverings, particularly when it is in the form of a polymeric sheet. The polymeric material can be present in at least one layer of a floor covering, which floor covering can also include one or more additional layers such as wear layers, foamed or foamable layers, top coat layers and design layers. The additional layers can also include the polymeric material.
Owner:AFI LICENSING
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS20050065214A1Stimulate bone healing processLower potentialBiocidePowder deliveryBarium saltTG - Triglyceride
Two (or more), -component, body-implantable, absorbable, biocompatible, putty, and non-putty hemostatic tamponades for use in surgery. Component 1 is a finely powdered bulking material, preferably less than 50 microns, e.g. the calcium, magnesium, aluminum, or barium salts of saturated or unsaturated carboxylic acids containing about 6 to 22 carbon atoms, hydroxyapatite, DBM, polyglycolide, polylactide, poldioxinones, polycaprolactones, absorbable glasses, gelatin, collagens, mono, and polysaccharides starches. Component 2, a dispersing vehicle, may be esters of C8-C18 monohydric alcohols with C2-C6 aliphatic monocarboxylic acids; C2-C18 monohydric alcohols with polycarboxylic acids; C8-C30 monohydric alcohols; tocopherol and esters thereof with C2-C10 aliphatic monocarboxylic acids or polycarboxylic acids; absorbable 10-14C hydrocarbons; free carboxylic acids such as oleic, capric, and lauric; dialkyl ethers and ketones; alkyl aryl ethers and ketones, polyhydroxy compounds and esters and ethers thereof; (ethylene oxide / propylene oxide copolymers), oils e.g. olive oil, castor oil and triglycerides.
Owner:ABYRX
Composite fibre membrane with unidirectional water permeable performance and preparation method thereof
The invention discloses a composite fibre membrane with an unidirectional water permeable performance and a preparation method of the composite fibre membrane, belonging to the field of functional micron / nano composite fibre materials; the composite fibre membrane is composed of a two-layer structure; a hydrophile layer is a nano-scale fibre membrane composed of hydrophile polymers such as polyvinyl alcohol, cellulose acetate, polyacrylate and the like; fibre diameter is 100-800nm; thickness is 25-35 microns; a lyophobic layer is the micron-scale fibre membrane composed of lyophobic polymers such as polyurethane, polystyrene, polymethylmethacrylate or polycaprolactone and the like; thickness is 5-10 microns; and fibre diameter is1.0-3.0 microns. The composite fibre membrane can be controlled by regulating technical parameters and has hydrophile / lyophobic differences and an excellent directional water permeable performance; and the preparation method is characterized by simple operation, low energy consumption, high efficiency and is widely used for fields of perspiring-type waterproof dress materials, fuel cell electrolyte membranes, unidirectional liquid transmission and separation and the like.
Owner:BEIHANG UNIV
Glycosaminoglycan and Synthetic Polymer Material for Blood-Contacting Applications
ActiveUS20150196688A1Improve surface chemistryFeasible at commercial productionSuture equipmentsOrganic active ingredientsLow-density polyethyleneLinear low-density polyethylene
Provided herein is a composite, comprising: a polymer host selected from the group consisting of low-density polyethylene (LDPE), linear low-density polyethylene (LLDPE), polyethylene terephthalate (PET), polytetrafluoroethylene (PTFE), and polypropylene (PP), polyurethane, polycaprolactone (PCL), polydimethylsiloxane (PDMS), polymethylmethacrylate (PMMA), and polyoxymethylene (POM); and a guest molecule comprising hyaluronic acid; wherein the guest molecule is disposed within the polymer host, and wherein the guest molecule is covalently bonded to at least one other guest molecule. Also provided herein are methods for forming the composite, and blood-contracting devices made from the composite, such as heart valves and vascular grafts.
Owner:COLORADO STATE UNIVERSITY
Porous biocompatible implant material and method for its fabrication
a biocompatible and biodegradable implant for a cavity in a bone of a living organism is made of a biocompatible and biodegradable granules which are selected from the group including biopolymers, bioglasses, bioceramics preferably calcium sulfate, calcium phosphate such as monocalcium phosphate monohydrate, monocalcium phosphate anhydrous, dicalcium phosphate dihydrate, dicalcium phosphate anhydrous, tetracalcium phosphate, calcium orthophosphate phosphate, α-tricalcium phosphate, β-tricalcium phosphate, apatite such as hydroxyapatite, or a mixture thereof. The biocompatible and biodegradable granules are provided with a coating, which comprises at least one layer of a biocompatible and biodegradable polymer which is selected from the group including poly((α-hydroxyesters), poly(orthoesters), polyanhydrides, poly(phosphazenes), poly(propylene fumarate), poly(ester amides), poly(ethylene fumarate), polylactide, polyglycolide, polycaprolactone, poly(glycolide-co-trimethylene carbonate), polydioxanone, co-polymers thereof and blends of those polymers. The biocompatible and biodegradable implants are obtained by fusing together the polymer-coated granules through polymer-linkage of the polymer coatings of neighboring granules.
Owner:COLLAGEN MATRIX
Ternary mixture of biodegradable polyesters and products obtained therefrom
InactiveUS6841597B2RapidityImproves UV stabilitySynthetic resin layered productsPaper coatingPolymer scienceBiodegradable polyester
The invention relates to a mixture of biodegradable polyesters comprising: (A) a polyhydroxy acid of the poly-ε-caprolactone type and its copolymers, (B) aliphatic polyester, and (C) a polymer of polylactic acid, in which the concentration of (A) varies with respect to (A+B) in the range between 40 and 70% by weight, and the concentration of (C) with respects to (A+B+C) lies between 2 and 30%.
Owner:NOVAMONT SPA
Biodegradable card base
InactiveUS6350530B1High strengthReduce rigidityDecorative surface effectsDuplicating/marking methodsPolyesterPolymer science
The present invention provides a card base which is degradable by microbes in natural environment. The card base is excellent in properties necessary for card bases, such as tensile strength, impact strength, flex temperature, heat resistance, resistance to thermal expansion and contraction, blocking resistance and humidity resistance, and has rigidity, bending resistance and durability. The card base contains, as essential components, a 3-hydroxybutylate / 3-hydroxyvalerate copolymer and a lactic acid polymer, and, where necessary, a polycaprolactone or a high-molecular aliphatic polyester. The card base has a single-layer structure, or a sandwich structure further having overlay layers comprising a composition containing, as essential components, a lactic acid polymer and either or both of a polycaprolactone and a high-molecular alphatic polyester.
Owner:GUNZE LTD
Water Repellant Golf Balls Containing a Hydrophobic or Superhydrophobic Outer Layer or Coating
InactiveUS20080280699A1Improve performanceSynthetic resin layered productsPaper coatingPolymer sciencePolyethylene glycol
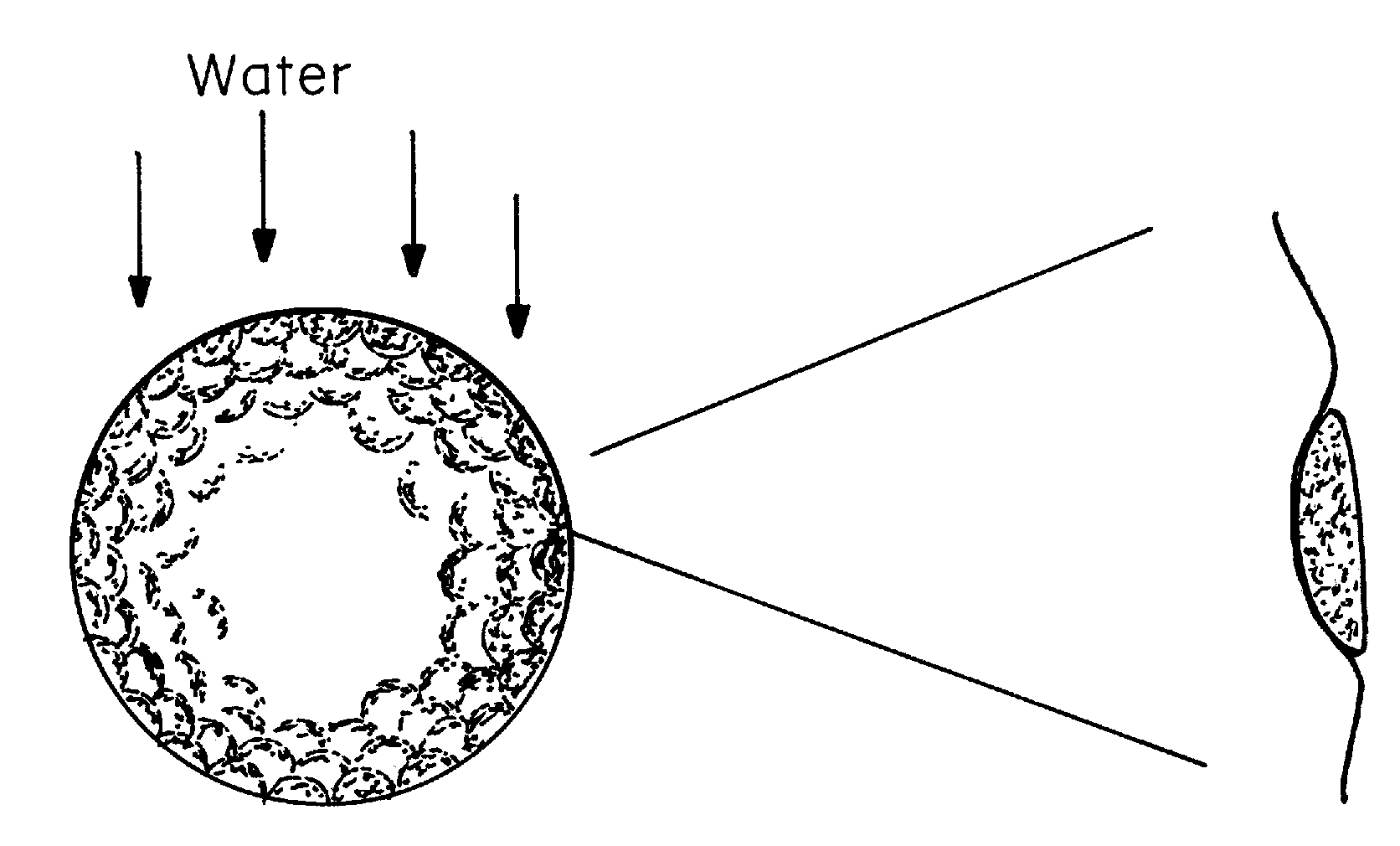
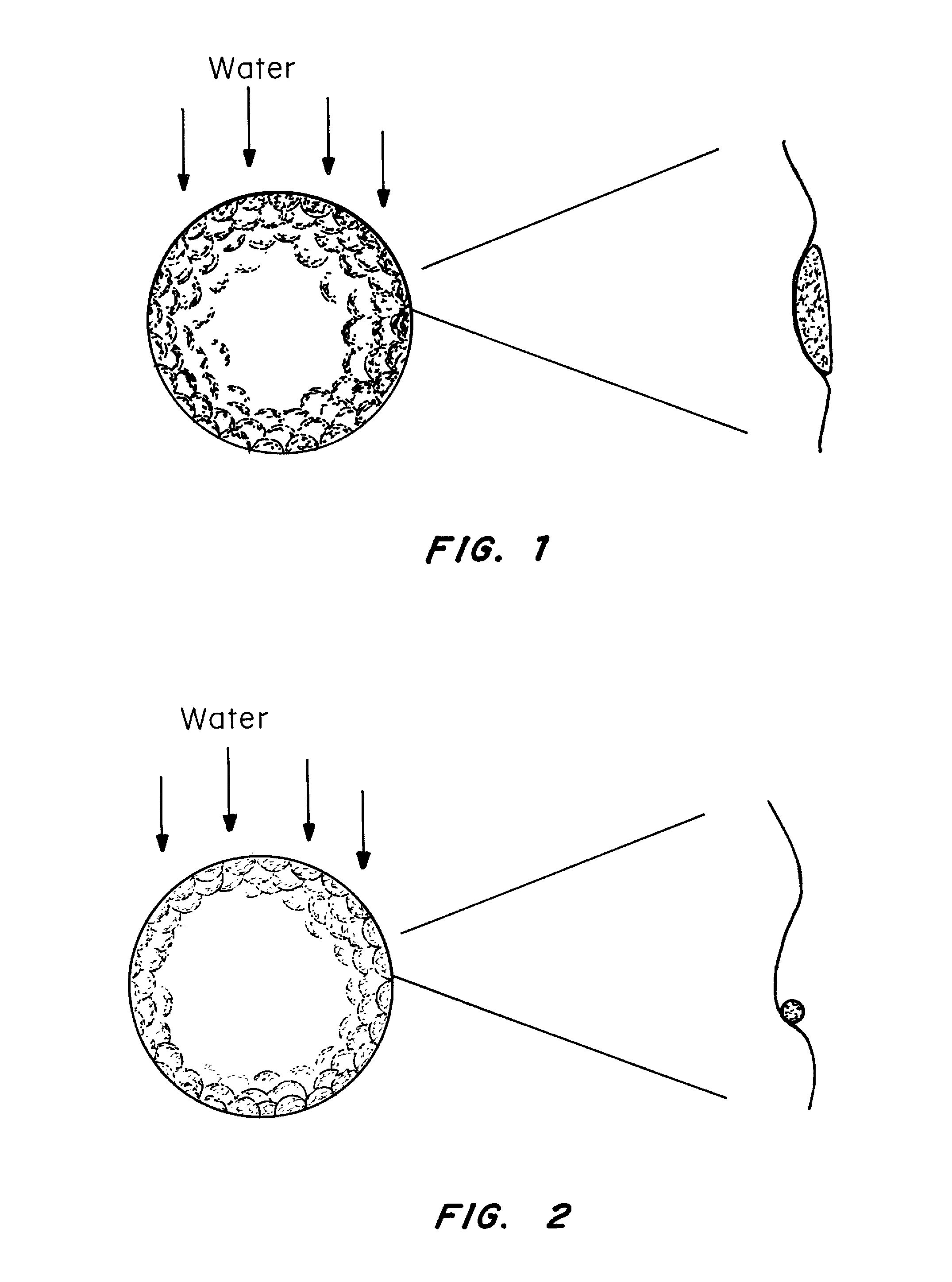
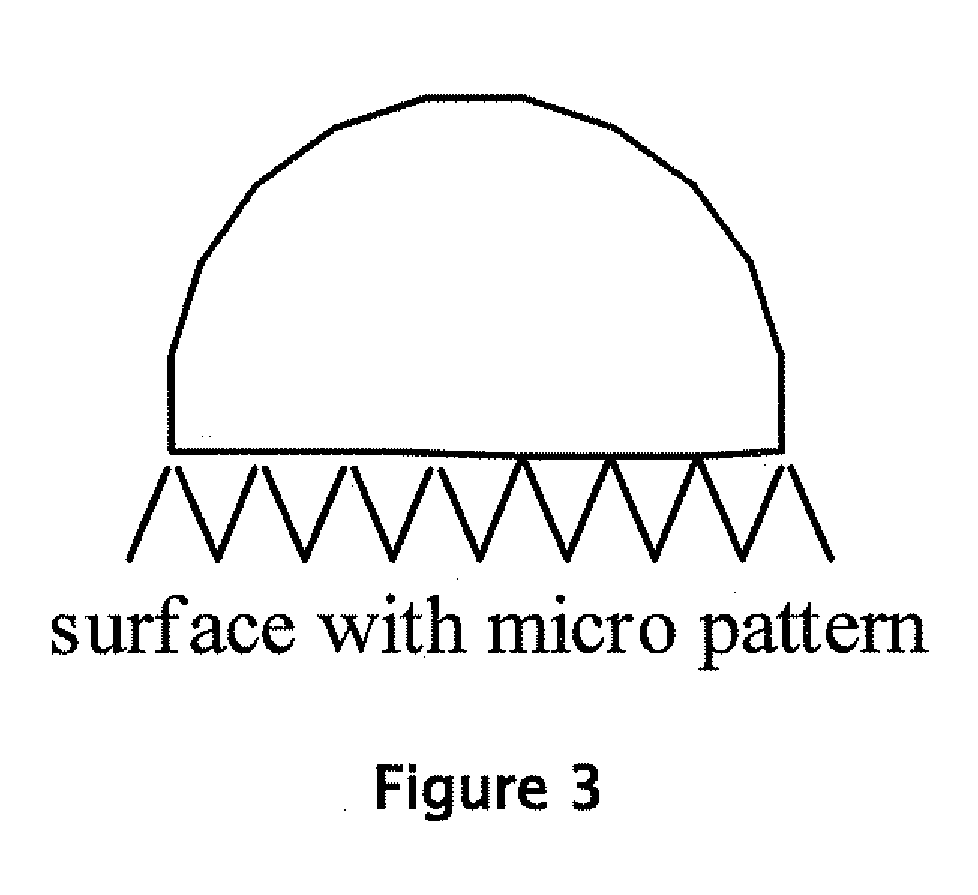
Water-repellant, self-cleaning coatings and methods of making and using thereof are described herein. In one embodiment, a hydrophobic or super hydrophobic coating is applied to the surface of a golf ball to make the golf-ball water-repellant and self-cleaning. Suitable coating materials include silicone compounds, silicone compounds, nanoparticles, silanes, fluorocarbon polymers, perfluoroalkyl ethyl methacrylate (PPFEMA) coated polycaprolactone, hydrocarbons, polymer mats made of polystyrene and poly[tetrafluoroethylene-co-(vinylidene fluoride)-co-propylene] (PTVFP); polyethylene glycol with glucose and sucrose in conjunction with a hydrophobic substance; combinations of nanoparticles with polyethylene or polypropylene; high density polyethylene, technical waxes; films of rough particles of metal oxides, polymer binder layers containing a plurality of porous protrusions, and combinations thereof. Suitable coating techniques include, but are not limited to, spraying, dipping, painting, brushing, or wiping (such as applying the coating from a towel or sponge). The coating material or the outer layer of the golf ball may be modified to create nano- or micro roughness or patterns on the surface of the golf ball, which can induce the lotus effect. This roughness or pattern can be created using a variety of techniques known in the art including, but not limited to, etching, top / down methodologies, bottom / up methodologies, or combinations thereof.
Owner:JARVHOLM ERIK JONAS
Method for augmenting, reducing, and repairing bone with thermoplastic materials
A method for augmenting a tissue including introducing into the tissue a first thermoplastic material at a first condition; treating the first thermoplastic material to achieve a second condition that includes an at least partially crystalline skin; and introducing a second material into the tissue whereby the first thermoplastic material and the second material are contained by the at least partially crystalline skin. Also a method of fracture reduction in a tissue including exposing to gamma radiation a mass of polycaprolactone characterized by a first shape; heating the mass of irradiated polycaprolactone above its melting temperature; introducing the heated mass of polycaprolactone into the tissue annulus to deform it from the first shape; allowing the material to return to the first shape.
Owner:DEPUY SYNTHES PROD INC
Photoimageable coating composition and composite article thereof
InactiveUS20050266335A1Photosensitive materialsPhotomechanical coating apparatusResistPhotoacid generator
A photoimagable composition suitable for use as a negative photoresist comprising: (A) at least one epoxidized polyfunctional bisphenol A formaldehyde novolak resin; (B) at least one polycaprolactone polyol reactive diluent, wherein the amount of component (A) is from about 95% to about 75% by weight of the sum of (A) and (B) and the amount of component (B) is from about 5% to about 25% by weight of the sum of (A) and (B); (C) at least one photoacid generator in an amount from about 2.5 to about 12.5 parts per hundred parts of resin and reactive diluent, which initiates polymerization upon exposure to actinic radiation; and (D) a sufficient amount of solvent to dissolve (A), (B) and (C); wherein the solvent comprises 2-pentanone, 3-pentanone, and 1,3-dioxolane and mixtures thereof.
Owner:MICROCHEM CORP +1
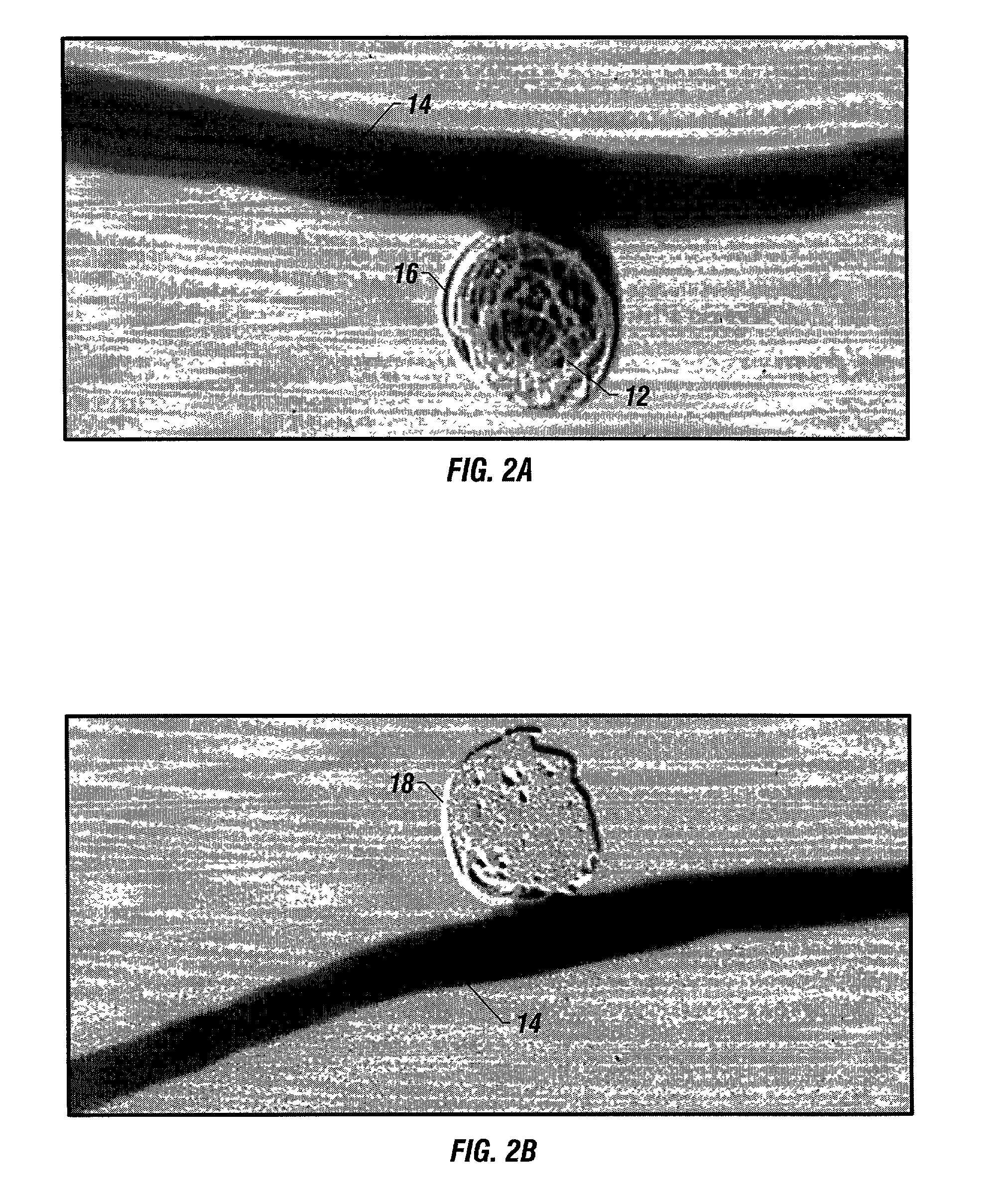
Bioabsorbable polymeric implants and a method of using the same to create occlusions
InactiveUS20020040239A1Peptide/protein ingredientsPharmaceutical containersPoly-L-lactideVascular compartment
A new embolic agent, bioabsorbable polymeric material (BPM) is incorporated to a Guglielmi detachable coil (GDC) to improve long-term anatomic results in the endovascular treatment of intracranial aneurysms. The embolic agent, comprised at least in part of at least one biocompatible and bioabsorbable polymer and growth factors, is carried by hybrid bioactive coils and is used to accelerate histopathologic transformation of unorganized clot into fibrous connective tissue in experimental aneurysms. An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location. Thrombogenicity of the biocompatible and bioabsorbable polymer is controlled by the composition of the polymer. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and bioabsorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid / poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides.
Owner:RGT UNIV OF CALIFORNIA
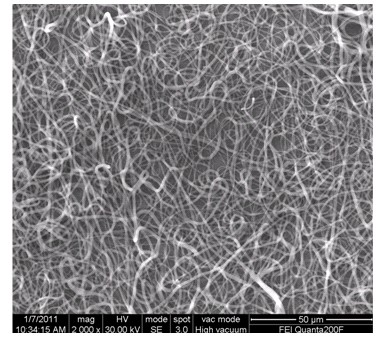
Preparation method and application of polycaprolactone/natural polymer composite porous scaffold
InactiveCN102277737AImprove hydrophilicityEasy to spreadLayered productsFibre typesFiberPolymer science
Preparation method and application of polycaprolactone / natural polymer composite porous scaffold, method: including preparation of polycaprolactone electrospun nanofiber membrane; (1) placing polycaprolactone electrospun nanofiber membrane in alkaline solution reaction, soaking and rinsing in deionized water; (2) putting the fiber membrane obtained in (1) into DMTMM or natural polymer solution of DTMMM to react, immersing and rinsing in deionized water, and then immersing in natural polymer for room temperature reaction, to remove Ionized water soaking and rinsing; (3) Evenly coating and casting the mixed solution of natural polymer materials on the fiber membrane obtained in (2), freeze-drying, and extracting the solvent; (4) Applying the double-layer scaffold obtained in (3) After soaking in the cross-linking room temperature reaction, soaking in deionized water, rinsing, and freeze-drying. The porous scaffold prepared by the invention is stable, has suitable pore diameter, good biocompatibility and fast degradation rate, and can be used for skin and clinical tissue and organ defect repair, reconstruction or wound dressing.
Owner:NANCHANG UNIV
Efficient oil and water separation composite fiber film and preparation method thereof
InactiveCN103866492ASelf-supportingRealize multiple reuseFilament/thread formingNon-woven fabricsFiberHydrophobic polymer
The invention discloses an efficiency oil and water separation composition fiber film and a preparation method thereof, and belongs to the field of functional nanometer fiber materials. Hydrophobic polymers such as polyurethane, polystyrene and polymethyl methacrylate or polycaprolactone are used as the main raw materials of the fiber film, the main raw materials are dissolved in an organic solvent to form a polymer solution, hydrophobic nanometer particles are added into the polymer solution in the preparation process, after the mixture is evenly mixed, electro-spinning is conducted on the mixed solution by means of the electrostatic spinning method, and then a fiber film material which is formed by micro-nanometer composite structures and is in the shape of non-woven fabric is obtained. The electro-spun fiber film of the micro-nanometer structure has super-hydrophobic / super-oleophylic property in the air and excellent oil adsorption performance, and the contact angle to oil is nearly zero. The efficient oil and water separation composite fiber film is simple in preparation method, low in energy consumption, high in efficiency, high in oil and water separation speed, and capable of being widely used in the fields such as oily water efficient purification.
Owner:BEIJING INSTITUTE OF CLOTHING TECHNOLOGY
Methods of making functional biodegradable polymers
There is provided a method to modify biodegradable polymers using the direct reaction of a biodegradable polymer in a vinyl monomer and may take place in the presence of initiators. The initiator may be free radical initiators, heat, ionic initiators, UV irradiation, ionizing irradiation and other high energy irradiation. Suitable biodegradable polymer include polycaprolactone, poly(lactic acid), poly(glycolic acid) and poly(lactic-co-glycolic acid). Suitable vinyl monomers include acrylates, methacrylates and the like.
Owner:KIMBERLY-CLARK WORLDWIDE INC
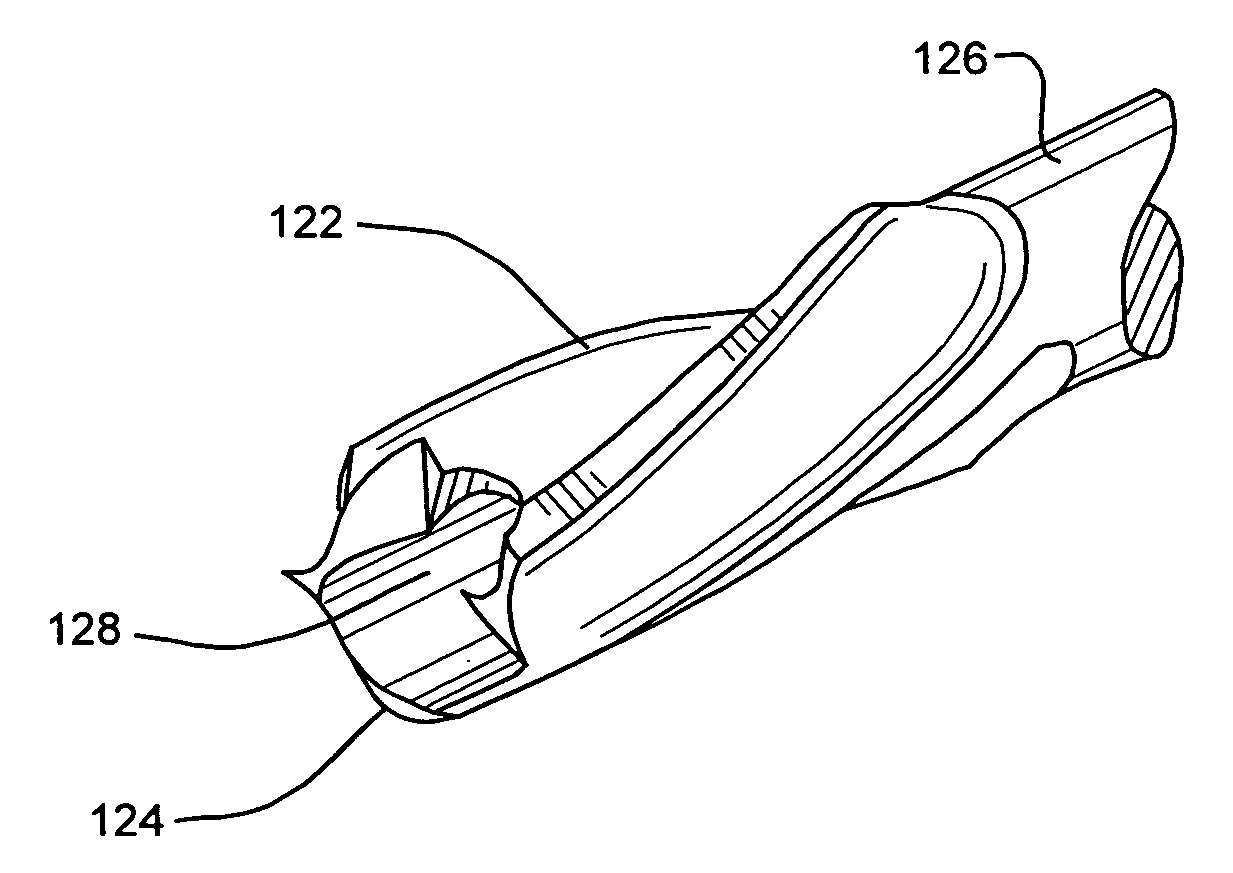
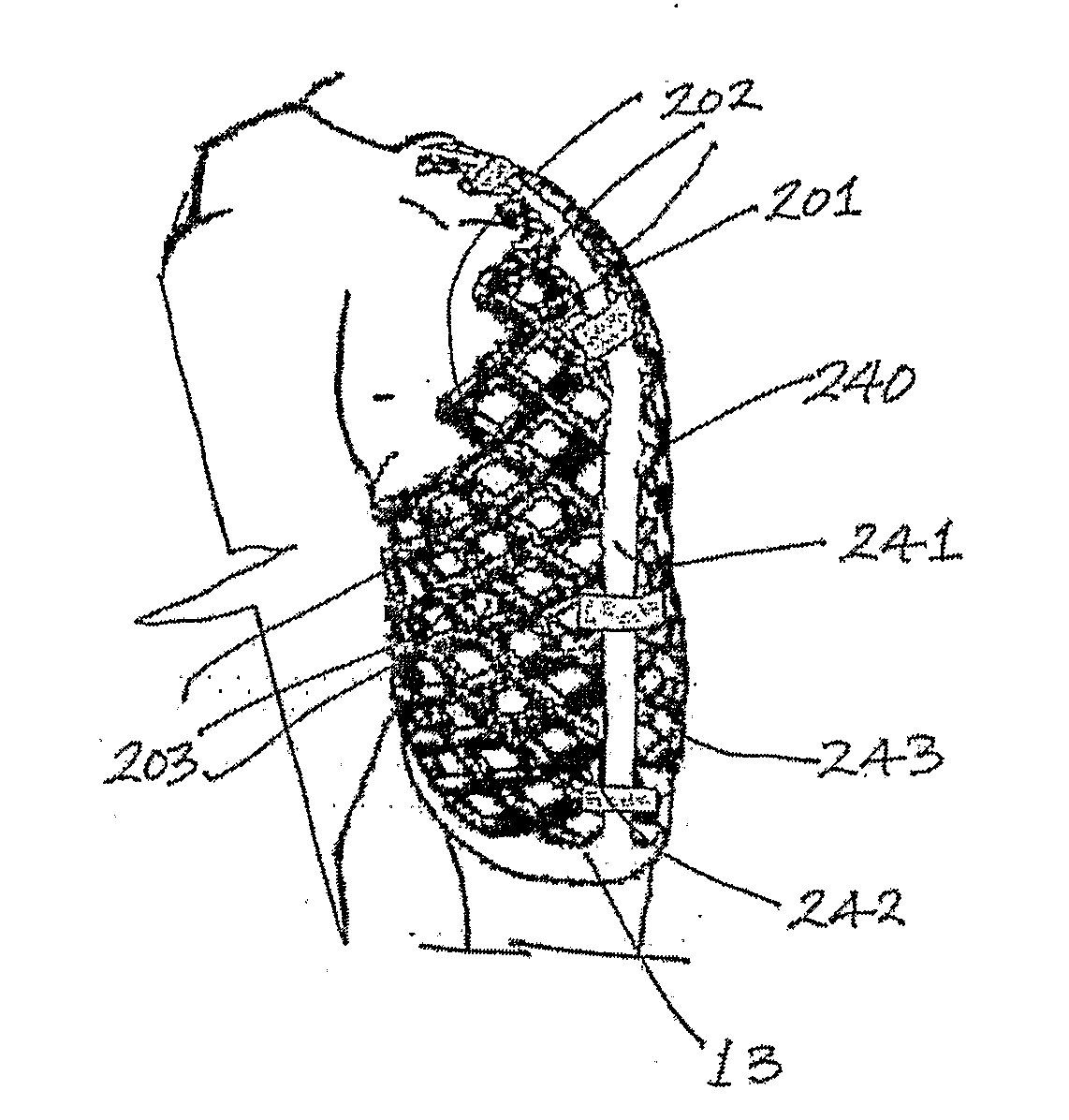
Geometrically Apertured Protective and/or Splint Device Comprising a Re-Mouldable Thermoplastic Material
ActiveUS20080154164A1Convenient ArrangementReduction in tackCosmetic preparationsImpression capsCelluloseMedicine
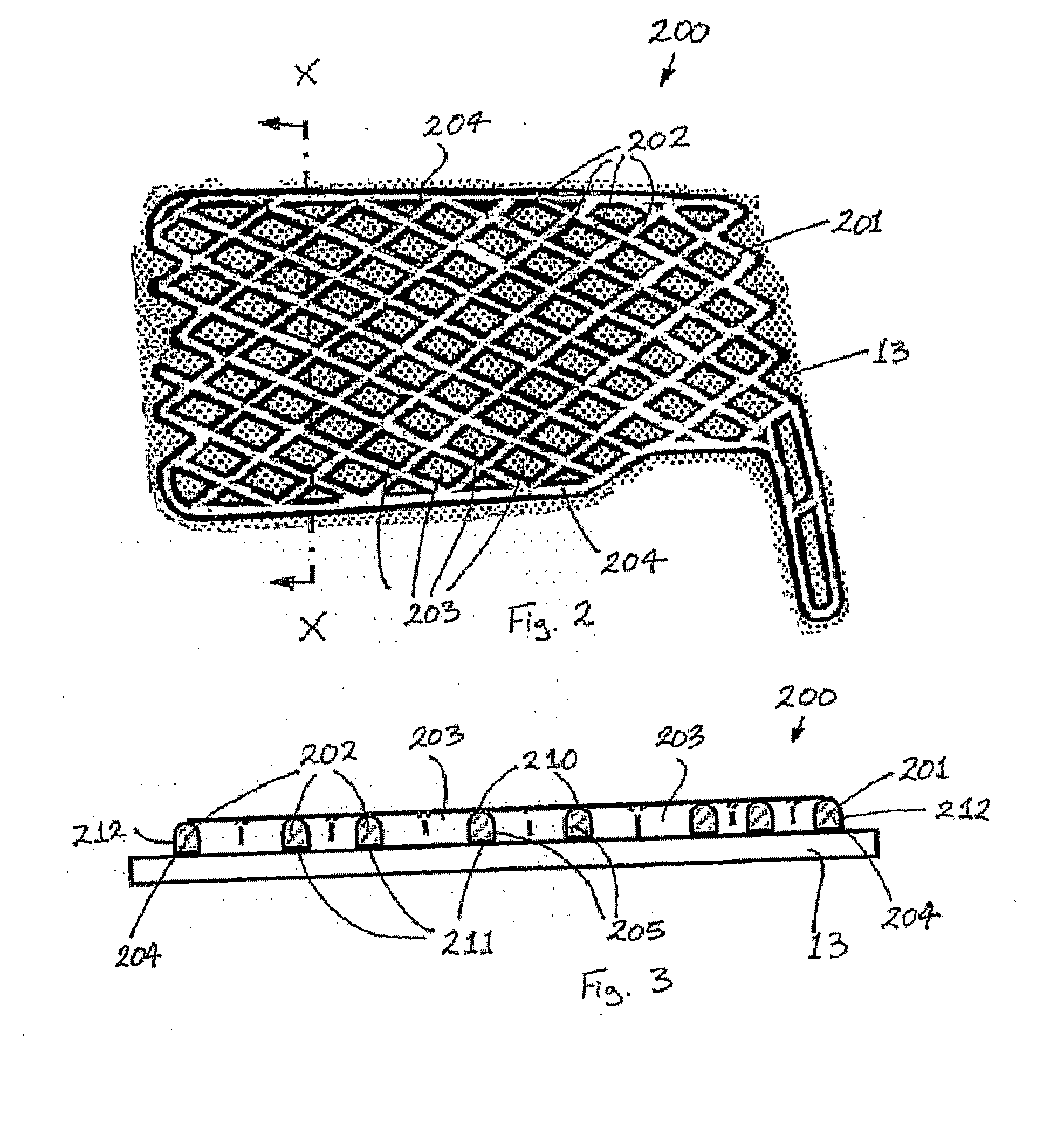
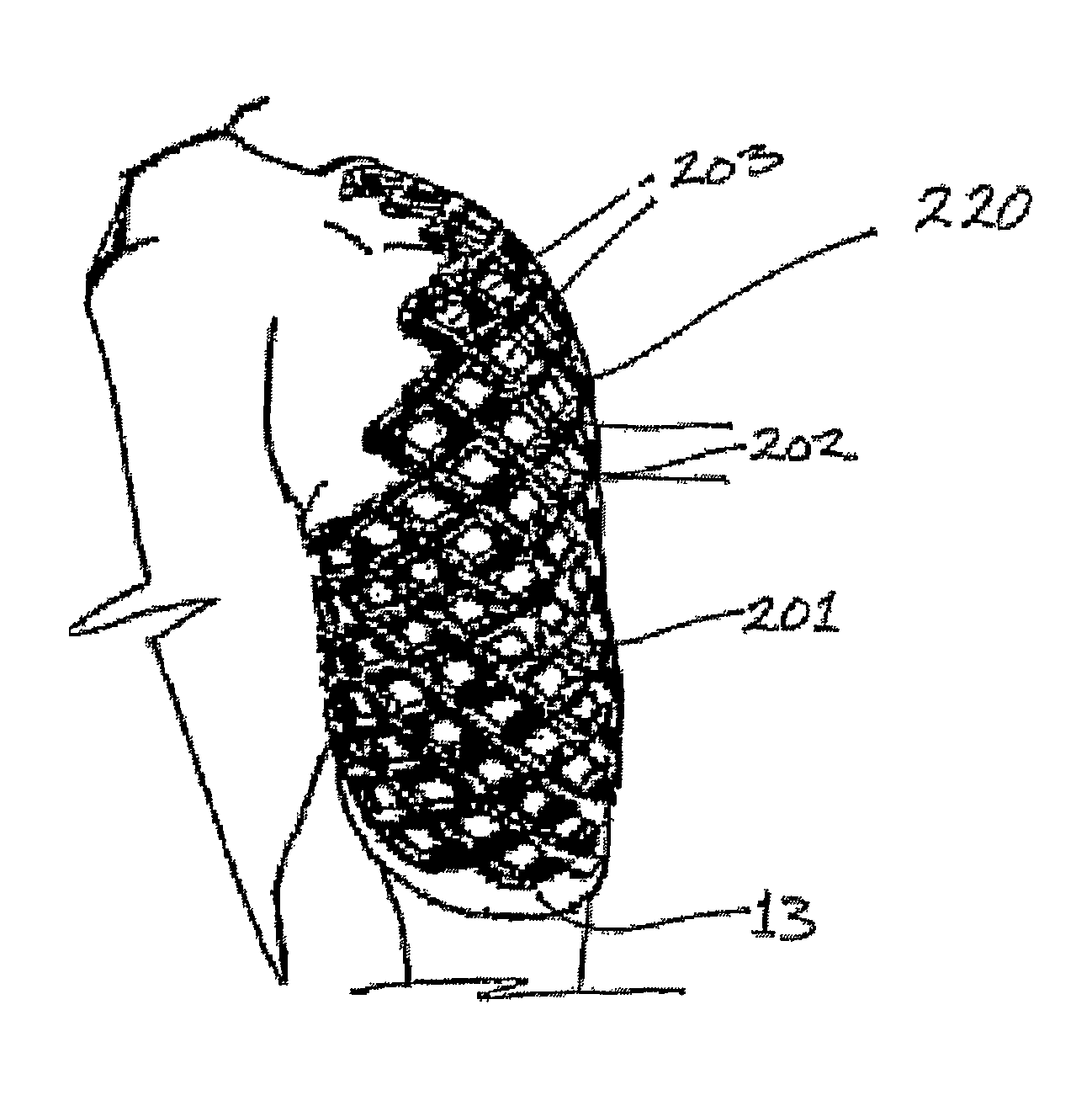
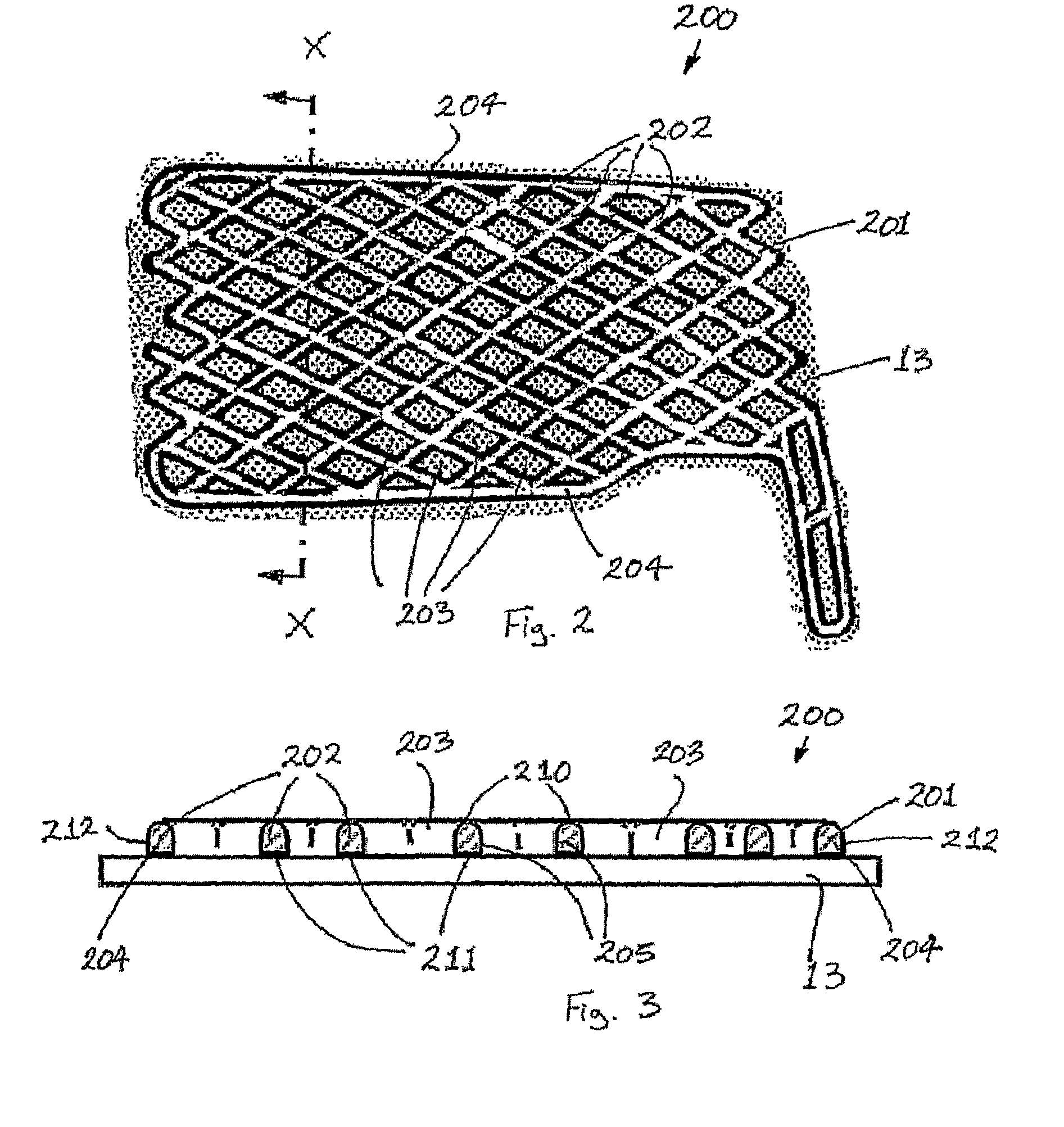
A protective and / or splint device, for example a distal radial splint device (200), comprises a protective and / or splint member (201) and a spacer member (13). The protective and / or splint member (201) comprises a composite protective and / or splint material and is formable at a forming temperature and is substantially rigid at ambient temperature. The material comprises a polycaprolactone and a ligno-cellulose additive material. The protective and / or splint member (201) comprises a mesh of elements (202), with a plurality of openings (203) through the protective and / or splint member (201). Away from the periphery of the splint member (201), the openings (203) are diamond-shaped. Two border elements (204) extend along the two sides of the periphery of the splint member (201). The three point bending strength to openness ratio of the member is greater than 0.1 and the unidirectional bending strength to openness ratio of the member is greater than 4. This results in a device (200) with sufficient strength, which is breathable with open surfaces, and the volume of material used is optimized. The strength of the member (201) parallel to the longitudinal direction of the arm is greater than the strength parallel too the circumferential direction. At the forming temperature the member (201) is stretchable. The member (201) is rounded between the outer surface of the member (201) and the edges around the openings (203).
Owner:FASTFORM RES
Geometrically apertured protective and/or splint device comprising a re-mouldable thermoplastic material
ActiveUS7985192B2Improve insulation performanceReduction in tackCosmetic preparationsImpression capsCelluloseMedicine
A protective and / or splint device, for example a distal radial splint device (200), comprises a protective and / or splint member (201) and a spacer member (13). The protective and / or splint member (201) comprises a composite protective and / or splint material and is formable at a forming temperature and is substantially rigid at ambient temperature. The material comprises a polycaprolactone and a ligno-cellulose additive material. The protective and / or splint member (201) comprises a mesh of elements (202), with a plurality of openings (203) through the protective and / or splint member (201). Away from the periphery of the splint member (201), the openings (203) are diamond-shaped. Two border elements (204) extend along the two sides of the periphery of the splint member (201). The three point bending strength to openness ratio of the member is greater than 0.1 and the unidirectional bending strength to openness ratio of the member is greater than 4. This results in a device (200) with sufficient strength, which is breathable with open surfaces, and the volume of material used is optimized. The strength of the member (201) parallel to the longitudinal direction of the arm is greater than the strength parallel too the circumferential direction. At the forming temperature the member (201) is stretchable. The member (201) is rounded between the outer surface of the member (201) and the edges around the openings (203).
Owner:FASTFORM RES
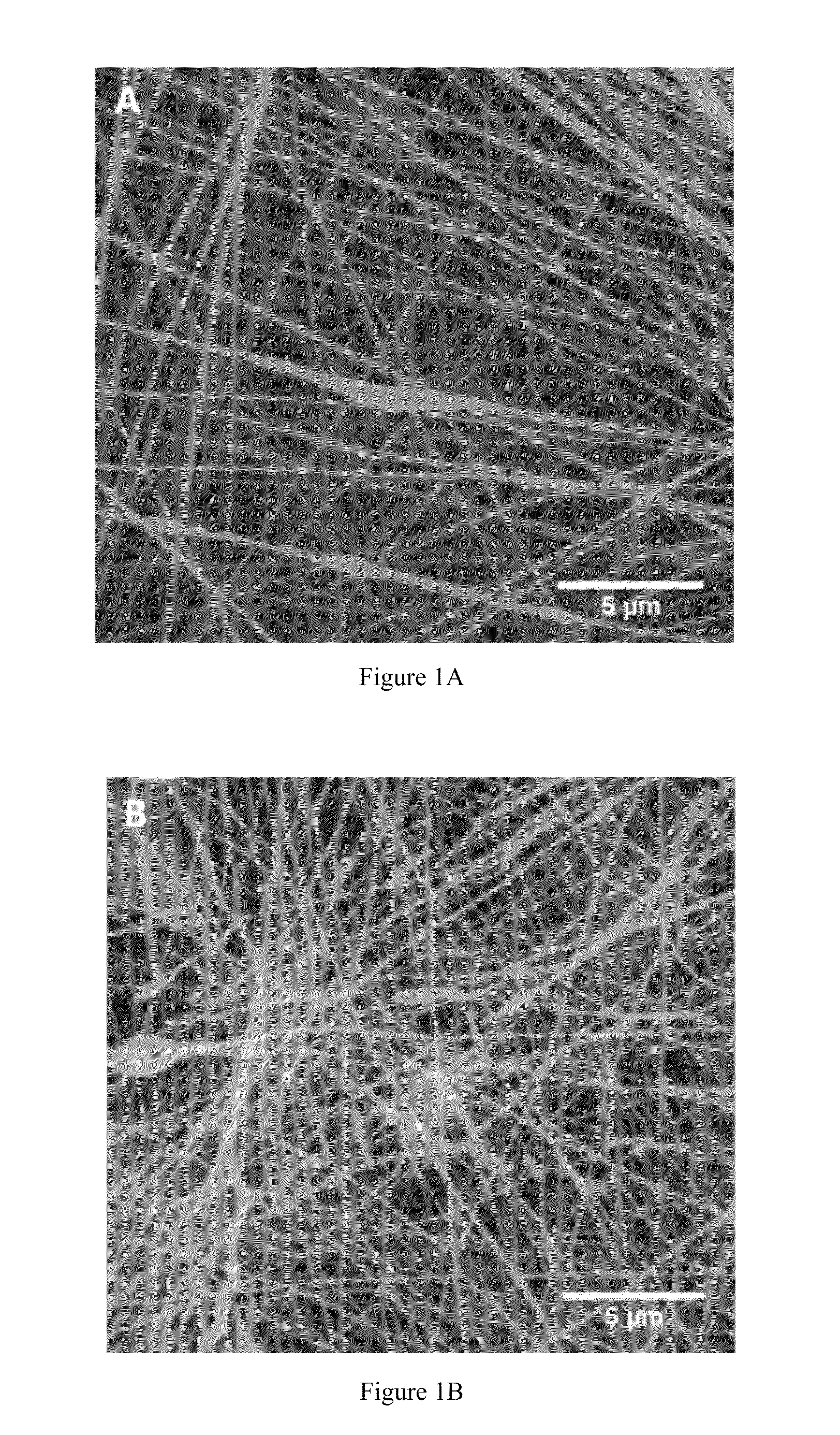
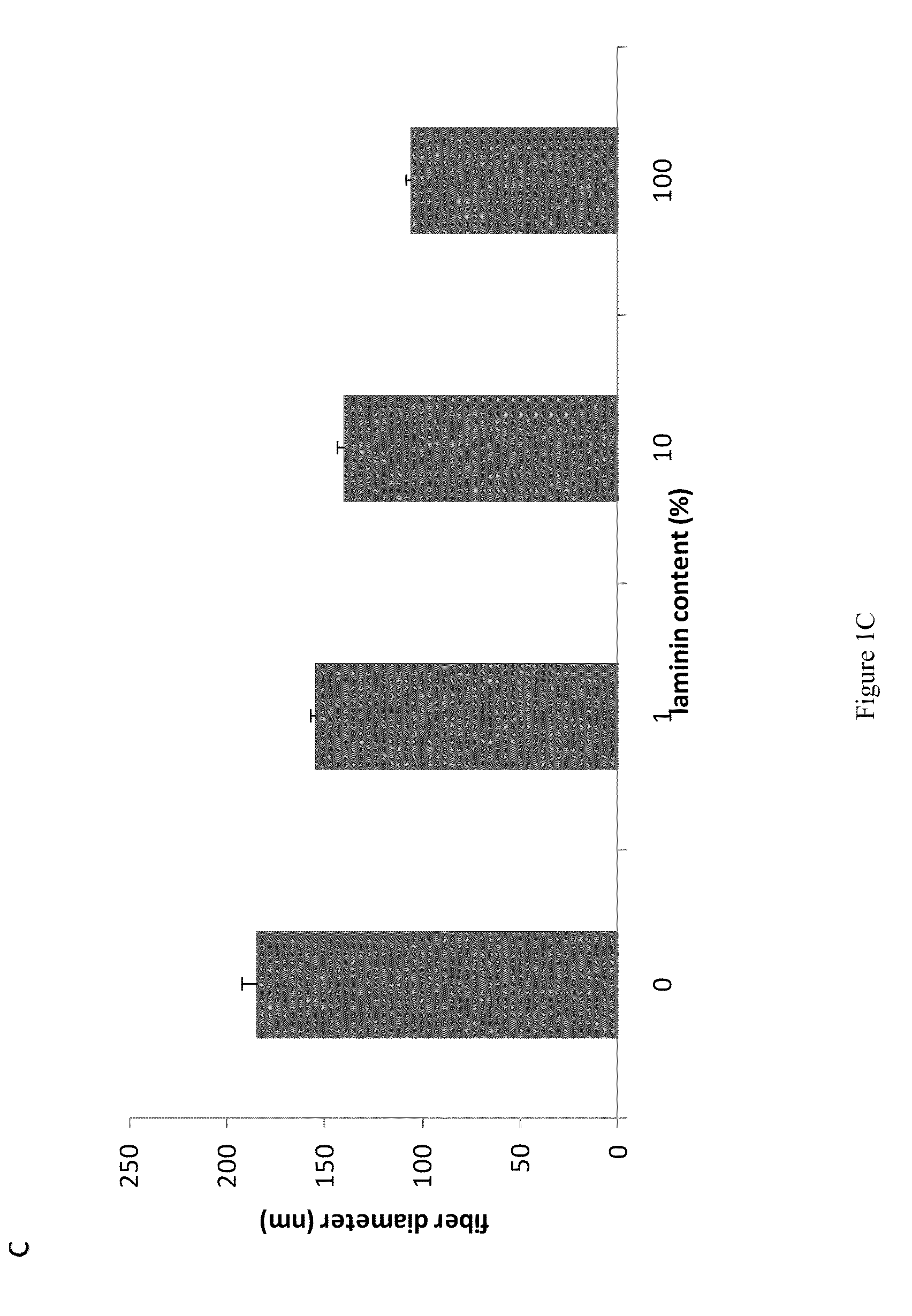
Compositions and methods for making and using laminin nanofibers
ActiveUS20110236974A1Low costHigh manufacturing requirementsElectric discharge heatingNanomedicineMicrosphereBiopolymer
The present invention provides methodologies and parameters for fabrication of the hybrid biomaterial by blending pure laminin or complex extracts of tissues containing laminin with biopolymers such as polycaprolactone (PCL), polylactic / polyglycolic acid copolymer (PLGA) or Polydioxanone (PDO) in fluoroalcohols (HFP, TFA), fabrication of substrates and scaffolds and devices from the hybrid biomaterial in forms such as films, nanofibers by electrospinning or microspheres, and the biological or biomedical use of the material or devices derived from it.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Ternary mixture of biodegradable polyesters and products obtained therefrom
InactiveUS20040092672A1RapidityMaintain transparencyFlexible coversWrappersPolymer scienceBiodegradable polyester
The invention relates to a mixture of biodegradable polyesters comprising: (A) a polyhydroxy acid of the poly-epsilon-caprolactone type and its copolymers, (B) aliphatic polyester, and (C) a polymer of polylactic acid, in which the concentration of (A) varies with respect to (A+B) in the range between 40 and 70% by weight, and the concentration of (C) with respects to (A+B+C) lies between 2 and 30%.
Owner:NOVAMONT SPA
Melt-processible polymer composition comprising fluoropolymer having long chain branches
A polymer melt additive that is suitable for use as a processing aid in the extrusion of a non-fluorinated polymer. The polymer melt additive composition includes a fluoropolymer that has a long chain branching index (LCBI) of at least 0.2 and a zero shear rate viscosity at 265° C. of not more than 107 Pa's. The polymer melt additive may additionally include other compounds such as, polyoxyalkylene polymer or polycaprolactone.
Owner:3M INNOVATIVE PROPERTIES CO
Three-dimensional bioresorbable scaffolds for tissue engineering applications
InactiveUS8071007B1Biocompatibility hardHard integrationAdditive manufacturing apparatusCeramic shaping apparatusBiocompatibility TestingHard tissue
The invention relates to the use of Fused Deposition Modeling to construct three-dimensional (3D) bioresorbable scaffolds from bioresorbable polymers such as polycaprolactone (PCL), or from composites of bioresorbable polymers and ceramics, such as polycaprolactone / hydroxyapatite (PCL / HA). Incorporation of a bioresorbable ceramic to produce a hybrid / composite material support provides the desired degradation and resorption kinetics. Such a composite material improves the biocompatibility and hard tissue integration and allows for increased initial flash spread of serum proteins. The basic resorption products of the composite also avoids the formation of an unfavorable environment for hard tissue cells due to a decreased pH. The scaffolds have applications in tissue engineering, e.g., in tissue engineering bone and cartilage.
Owner:OSTEOPORE INT PTE
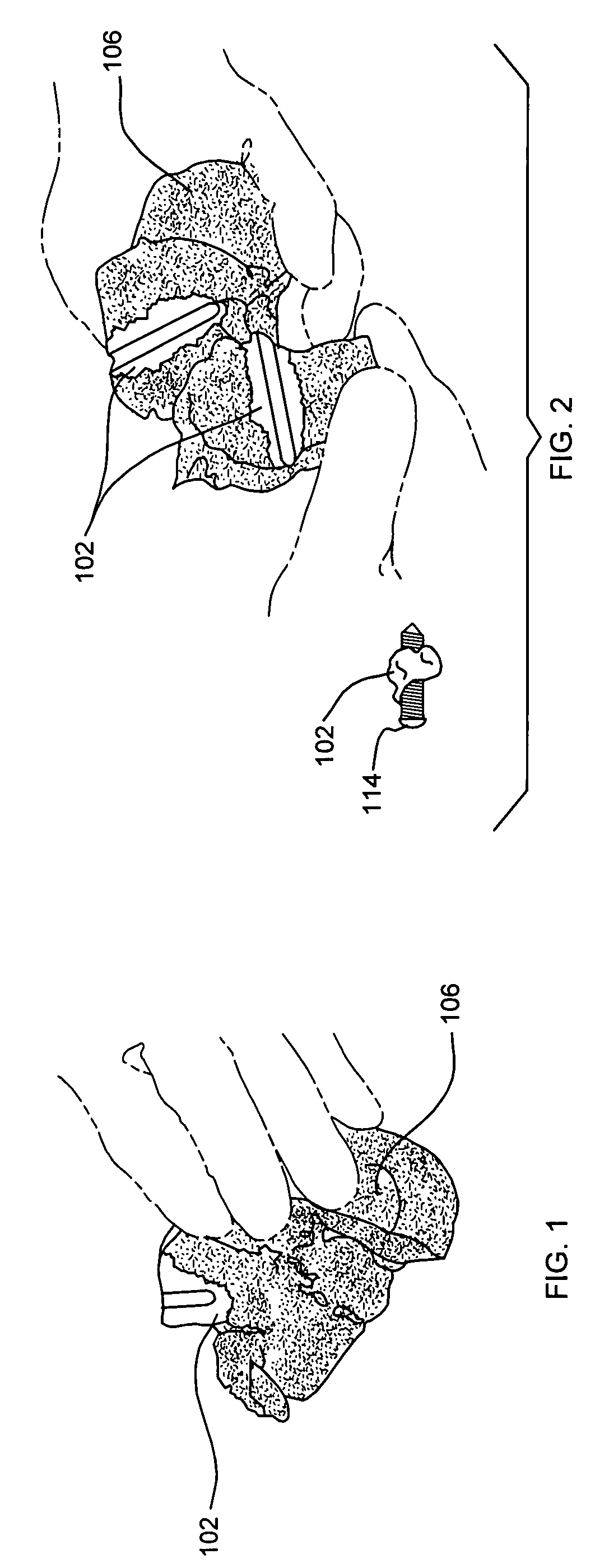
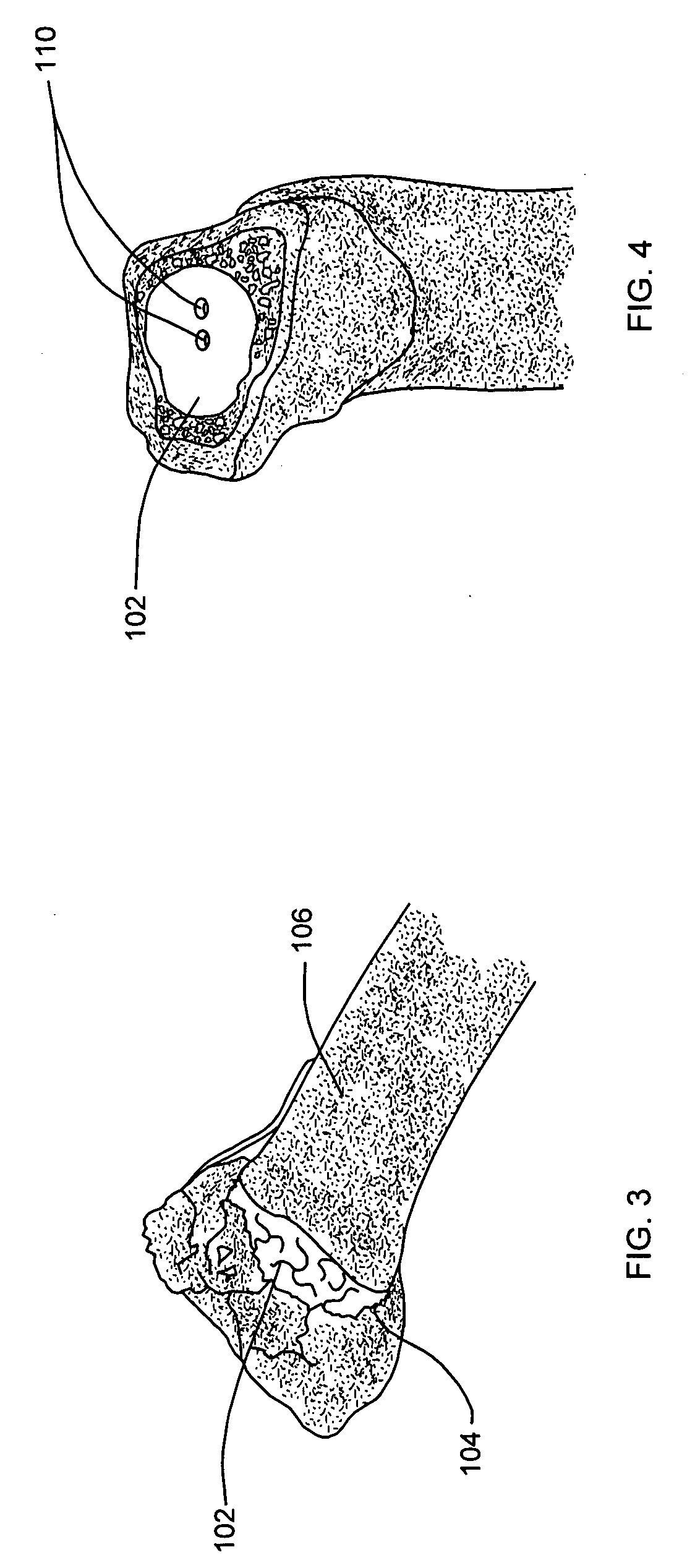
Degradable cage for bone fusion
InactiveUS20110282392A1Improve biological activityIncrease capacityInternal osteosythesisPeptide/protein ingredientsPolycaprolactoneBiomedical engineering
A cage for facilitating fusion of bones, such as vertebrae, or fusion of adjacent bone surfaces is disclosed. In one form, the cage includes a plurality of spaced apart walls comprising a biodegradable polymeric material (e.g., polycaprolactone); an osteoconductive mineral coating (e.g., a calcium compound) on at least a portion of the walls; and a bioactive agent (e.g., a bone morphogenetic protein) associated with the polymeric material and / or the coating. The bioactive agent is present in amount that induces ossification between the bones or adjacent bone surfaces. The cage may also include a fixation plate connected to at least one of the walls.
Owner:DEPUY SYNTHES PROD INC
Injection-moldable thermoplastic polyurethane elastomer
InactiveUS6521164B1Reduces intermolecular hydrogen bondingPromote sportsCoatingsThermoplastic polyurethaneDiol
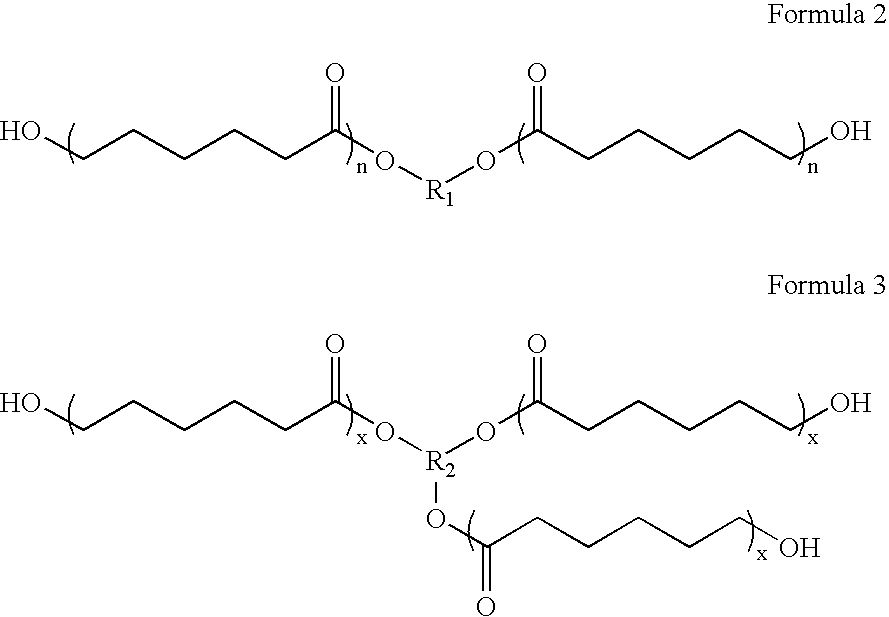
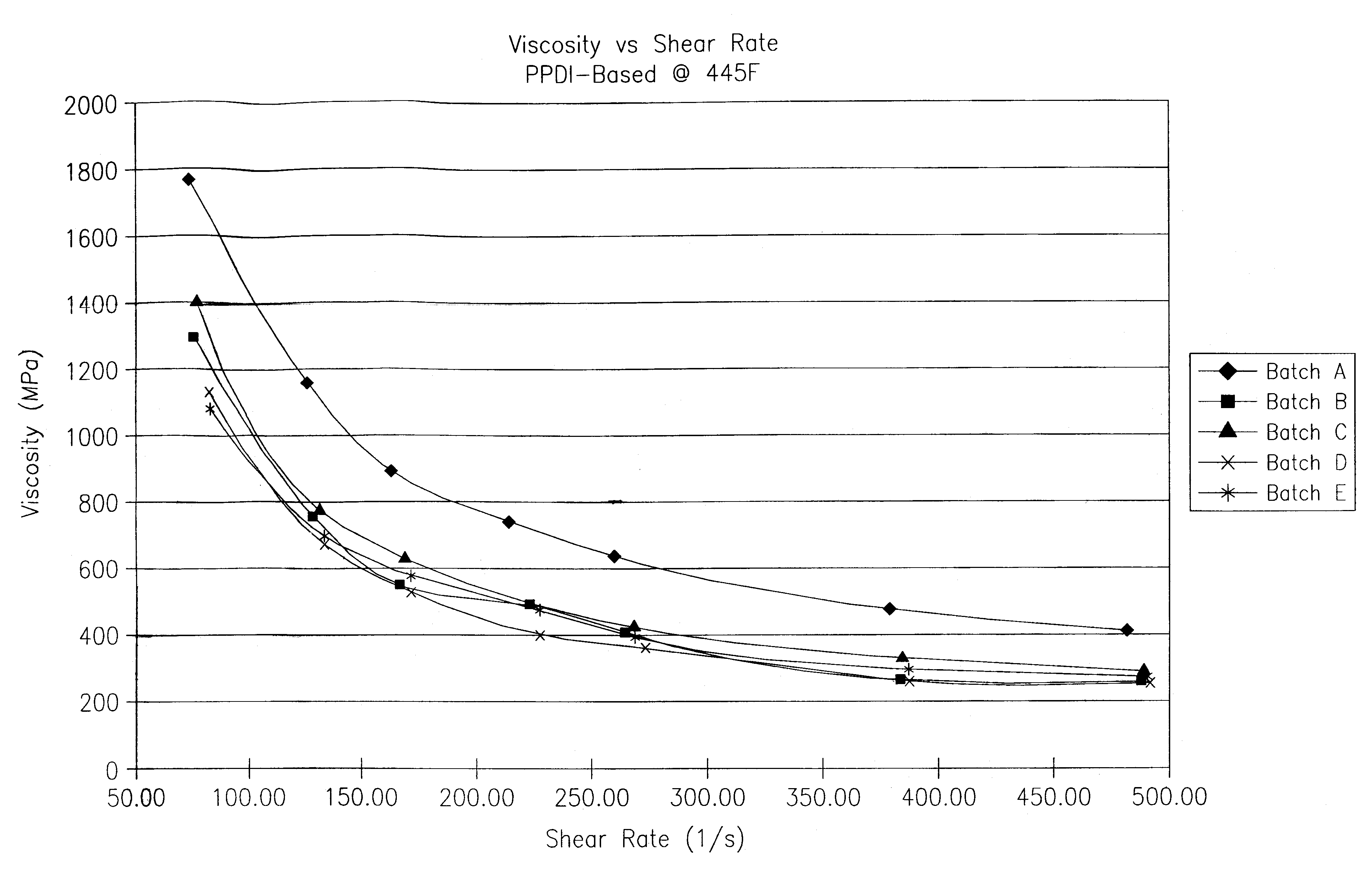
A thermoplastic polyurethane (TPU) elastomer and p-phenylene diisocyanate (PPDI) / polycaprolactone-based composition therefor having a unique chain extender combination for improved injection moldability. The elastomer is formed as the reaction product of: (A) from about 60 to 80% by weight of a hydroxyl-terminated poly(caprolactone) diol; (B) from about 17 to 22% by weight of a p-phenylene diisocyanate; (C) from about 3 to 10% by weight of a first hydroxyl-functional chain extender; and (D) less than about 2% by weight of a second hydroxyl-functional chain extender different from the first chain extender, the second chain extender being selected as effective to modify the crystallinity of the elastomer.
Owner:PARKER INTANGIBLES LLC
Balloon catheter comprising pressure sensitive microparticles
InactiveUS20120083734A1Improve brittlenessEffective treatmentSurgeryDilatorsPolyesterPoly(N-isopropylacrylamide)
The invention provides a solution to the above mentioned problem in that it provides a catheter balloon comprising a flexible coating on its outer surface wherein a plurality of microparticles are contained wherein said coating comprises a material selected from the group consisting of poly(N-vinyl-pirrolidone, poly(N-vinyl-pirrolidone-co-butylacrylate), poly(-vinyl pyridine), polyacrylamides, e.g. poly(N-isopropylacrylamide), poly(amido-amines), poly(ethylene imine), poly(ethylene oxide-block-propylene oxide), poly(ethylene oxide-block-propylene oxide-block-ethylene oxide), poly(styrene-block-isobutylene-block-styrene), poly(hydroxystyrene-block-isobutylene-block-hydroxystyrene), polydialkylsiloxanes, polysaccharides, polyacrylates and polyalkylmethacrylates, e.g. polymethylmethacrylate and poly(2-hydroxyethylmethacrylate) and wherein said microparticles comprise a material selected from the group consisting of polyesters, e.g. poly(lactic acid), poly(lactic-co-glycol acid), poly(glycolic acid), poly(3-hydroxybutyrate), poly(3-hydroxyvalerate), poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and polycaprolactone, polyamides, polysaccharides, polyurethanes, polyalkylmethacrylates and polyacrylates, e.g. polymethylmethacrylate and poly(2-hydroxyethylmethacrylate) and wherein the microparticles comprise a pharmaceutically active compound.
Owner:ENCAPSON
Custom-formable mouth guard and method of fabrication
ActiveUS20090038624A1Low softening pointEasy to customizeTeeth fillingSport apparatusMouthguardHealth professionals
A mouth guard composed of a polymer consisting essentially of polycaprolactone exhibits a low softening point, enabling it to be easily custom-fitted by the user or a health professional. The material is provided in the form of a generally U-shaped, unformed sheet of material, enabling the material to be heated with hot water or microwave energy and custom-fitted to the dentition of a user. The mouth guard may be perforated and may include a no-stick layer and / or a flavoring agent. A plurality of mouth guards may be provided in different sizes for children and adults and different shapes for different sports. In the preferred embodiments the mouth guard has a periphery including a plurality of lobes and cusps to enhance fitting. A method of fabricating an improved mouth guard according to the invention is also disclosed.
Owner:AKERVALL TECH
Environmentally degradable polymeric composition and process for obtaining an environmentally degradable polymeric composition
The present invention refers to a polymeric composition prepared from a biodegradable polymer defined by poly-hydroxybutyrate (PHB) or copolymers thereof, and at least one other biodegradable polymer, such as polycaprolactone (PCL) and poly (lactic acid) (PLA), so as to alter its structure, and further at least one additive of the type of natural filler and natural fibers, and, optionally, nucleant, thermal stabilizer, processing aid, with the object of preparing an environmentally degradable material. According to the production process described herein, the composition resulting from the mixture of the modified biodegradable polymer and additives can be utilized in the manufacture of injected packages for food products, injected packages for cosmetics, tubes, technical pieces and several injected products.
Owner:PHB IND
Medical device formed of polyester copolymer
InactiveUS6949112B1Improve performanceHigh flexibility and trackabilityStentsBalloon catheterPolyesterPercutaneous angioplasty
A medical device or component thereof, and particularly intracorporeal devices for therapeutic or diagnostic uses, formed at least in part of a copolymer having a hard block and a polylactone soft block. In a presently preferred embodiment, the hard block of the copolymer is a polyester, and more specifically, the copolymer comprises a di-block copolymer of poly(ethylene terephthalate) and polycaprolactone. The copolymer is suitable for forming a variety of medical devices or medical device components, and is preferably used to form a catheter balloon, such as a balloon for an angioplasty or stent delivery catheter. However, a variety of medical devices or medical device components can be formed of the copolymer, including stent covers, vascular grafts, and shaft components.
Owner:ADVANCED CARDIOVASCUAR SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com