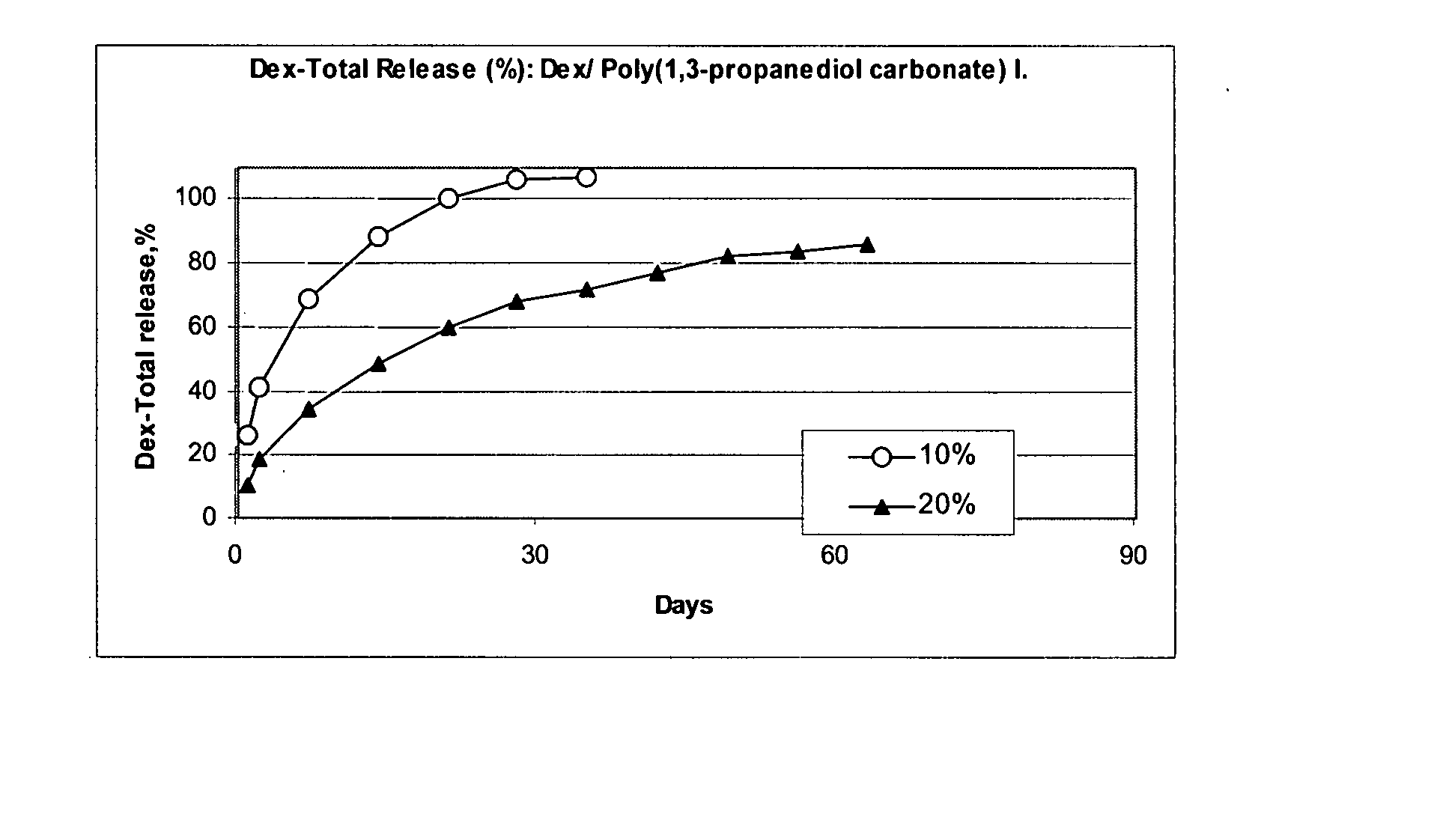
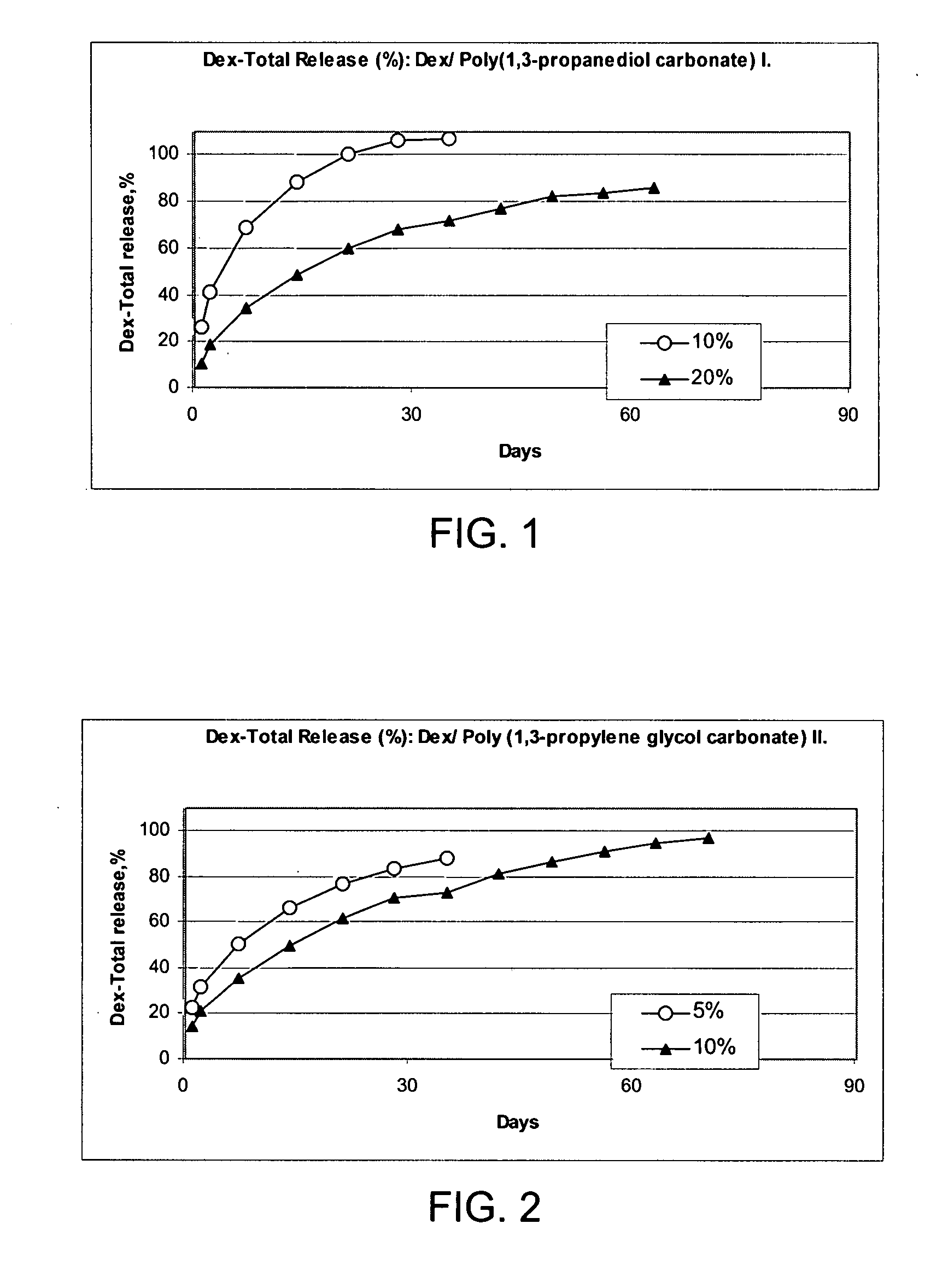
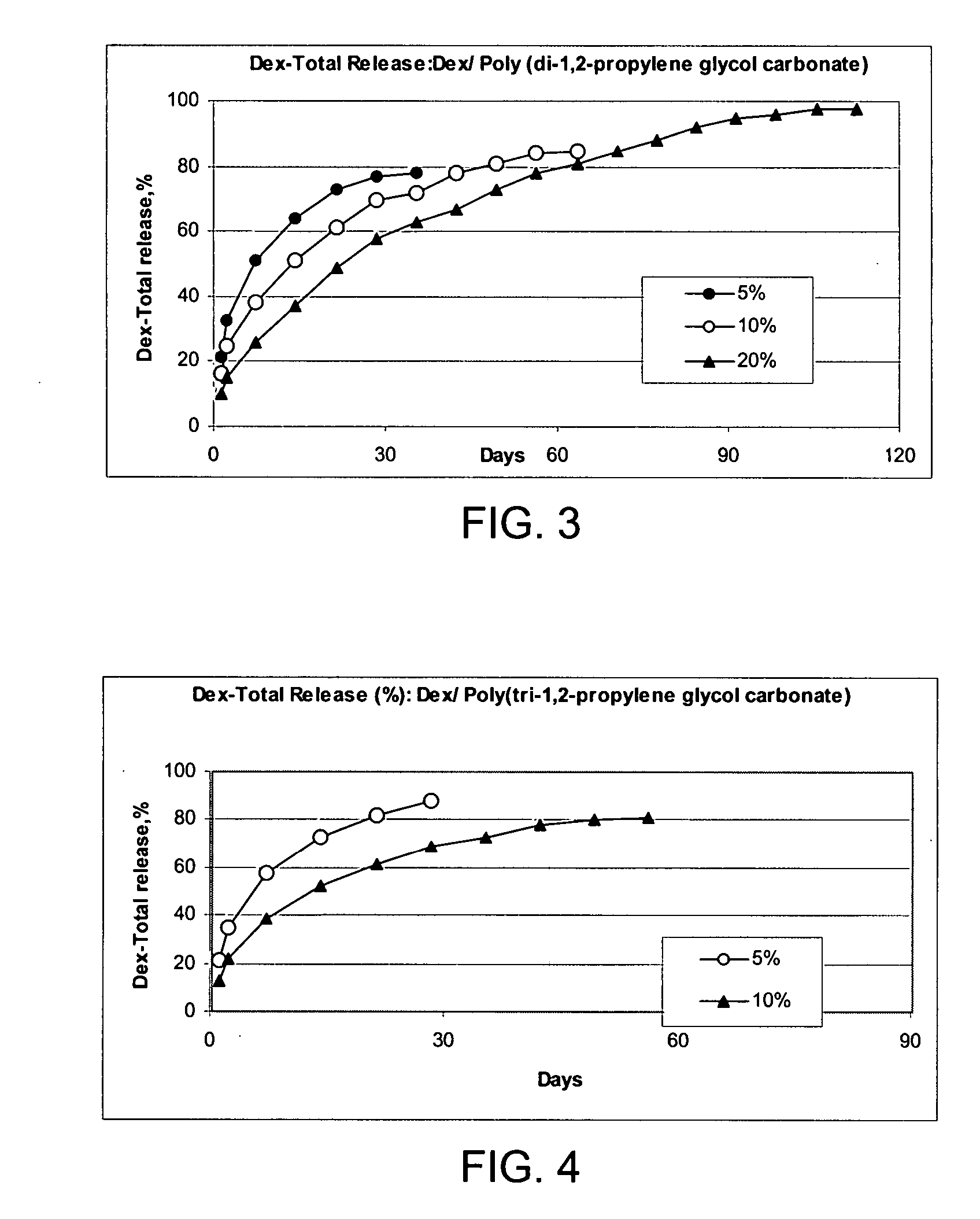
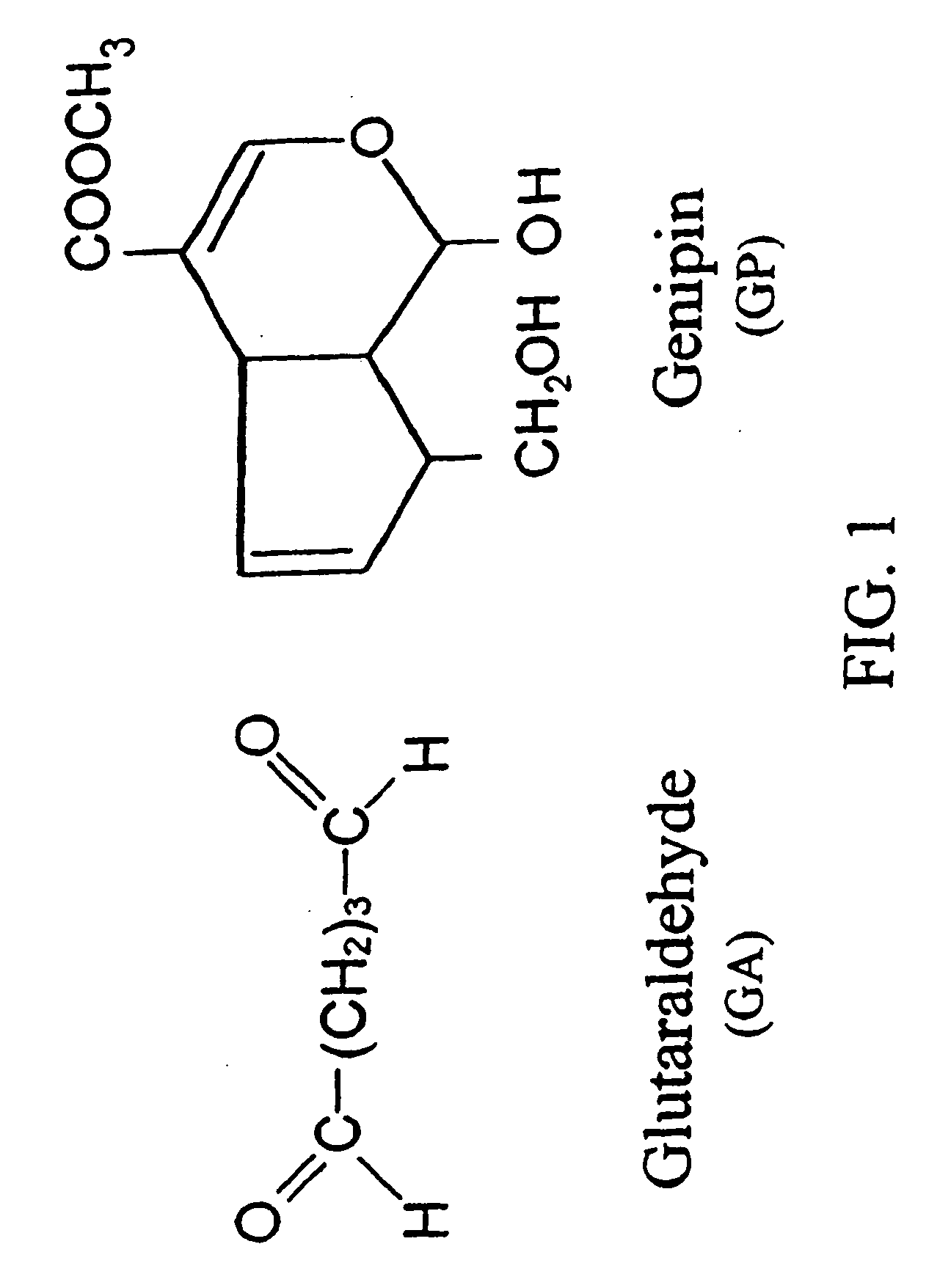
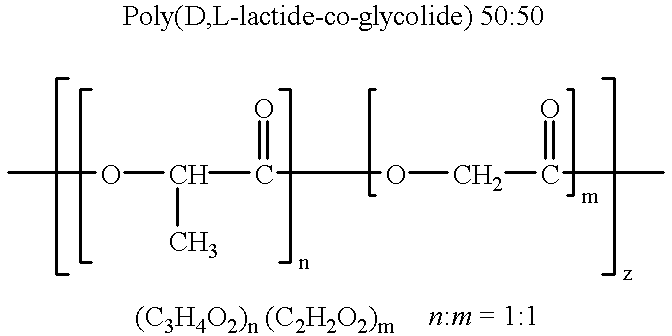Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
16738 results about "Biodegradation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
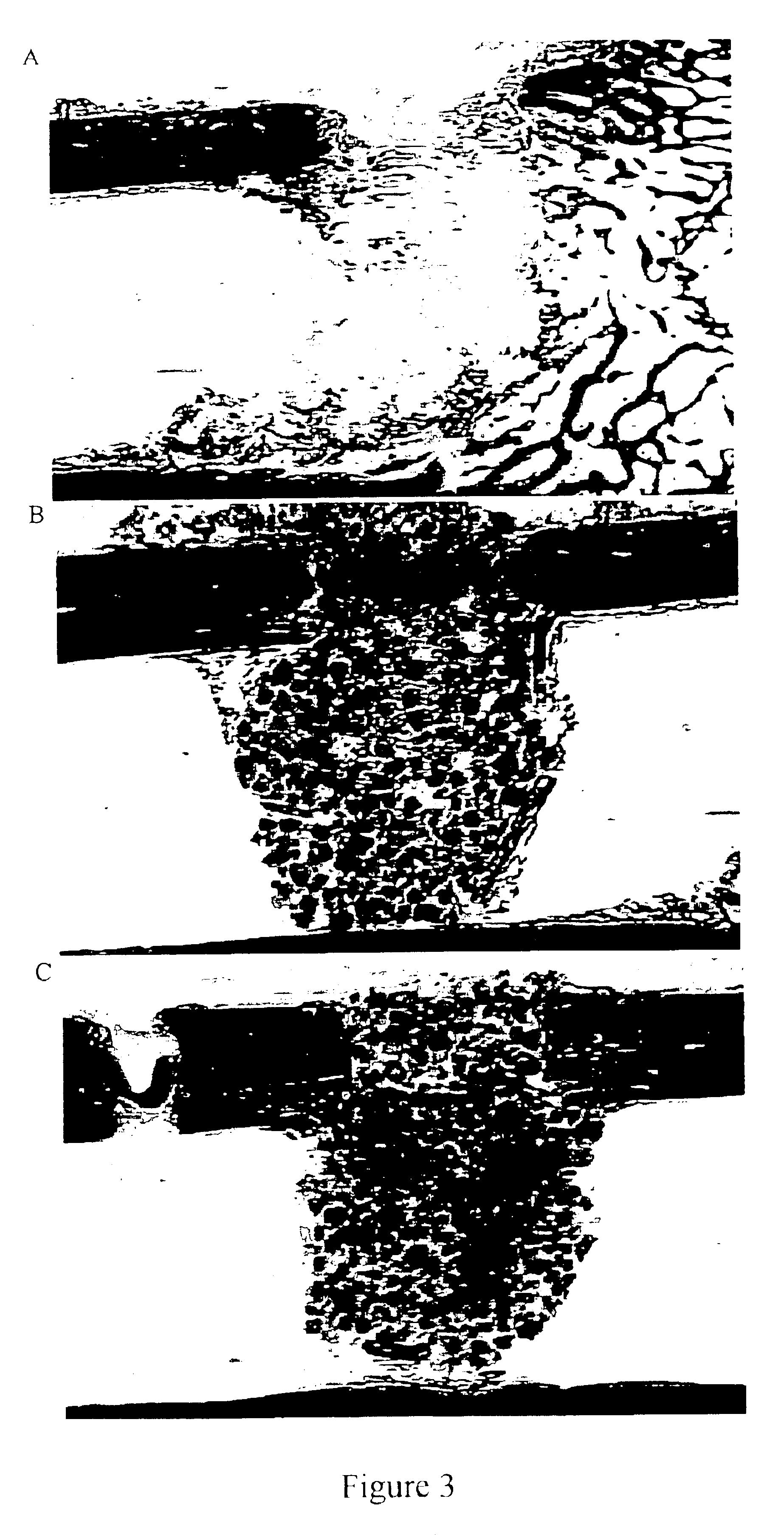
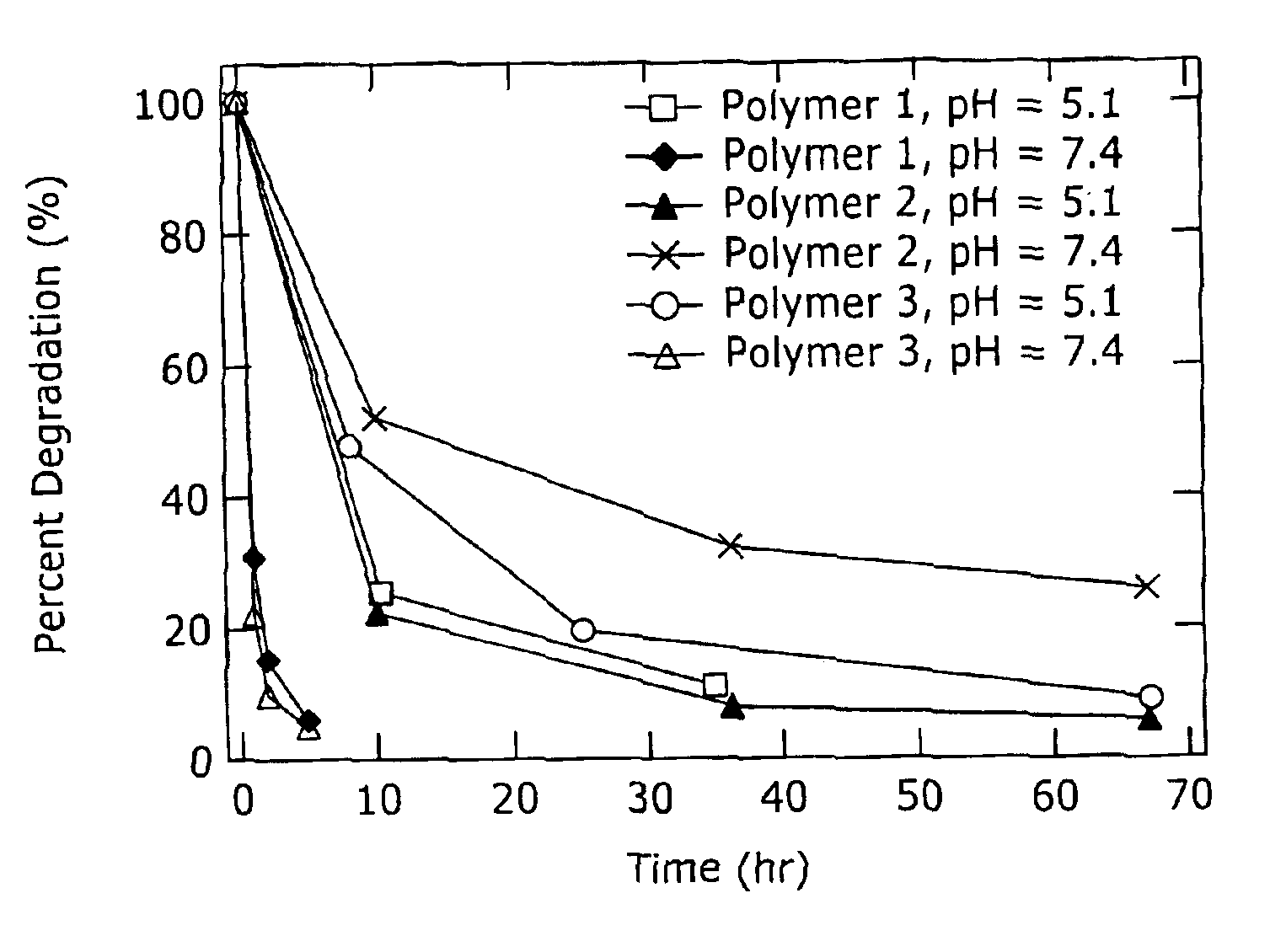
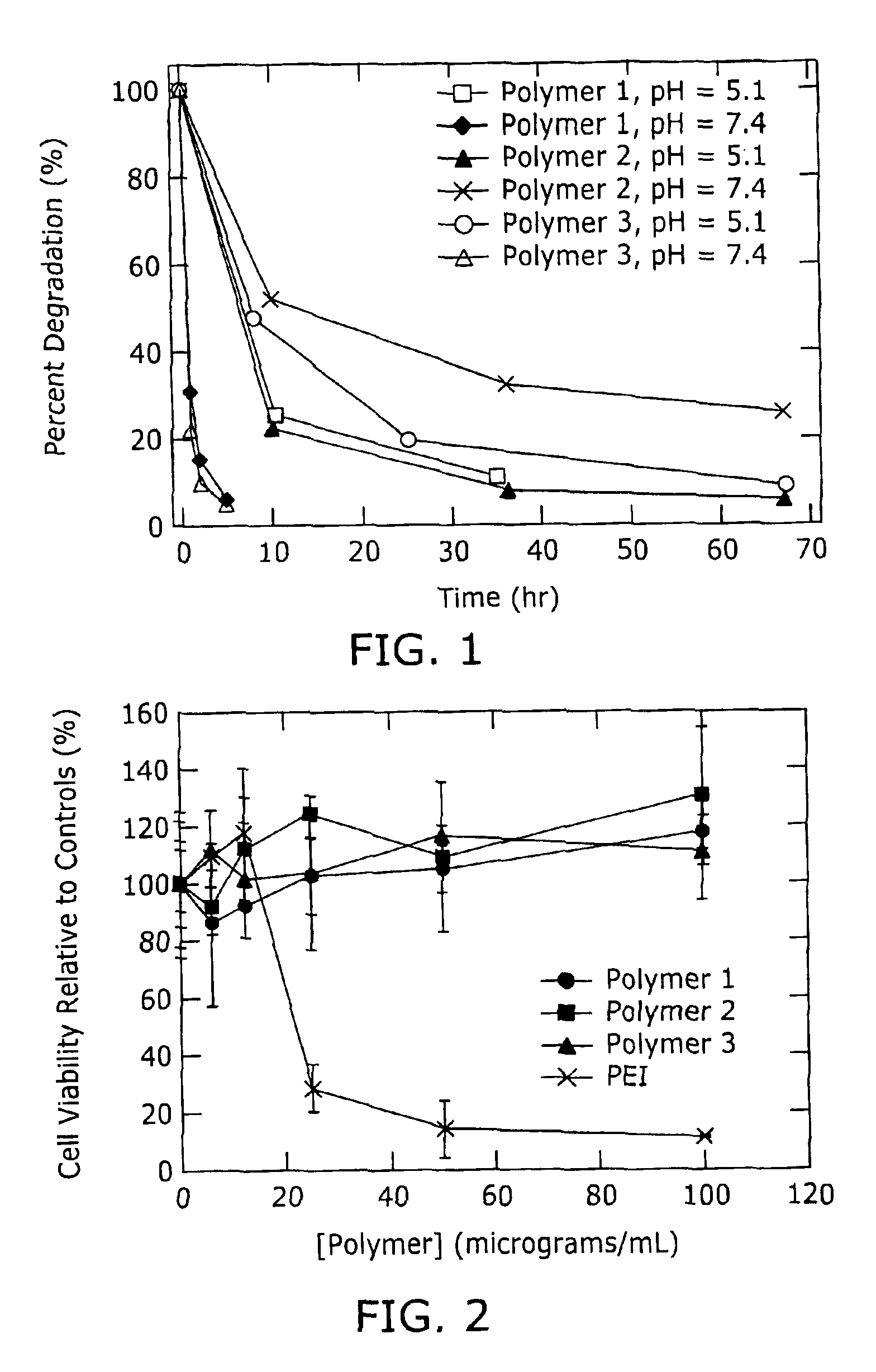
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi.
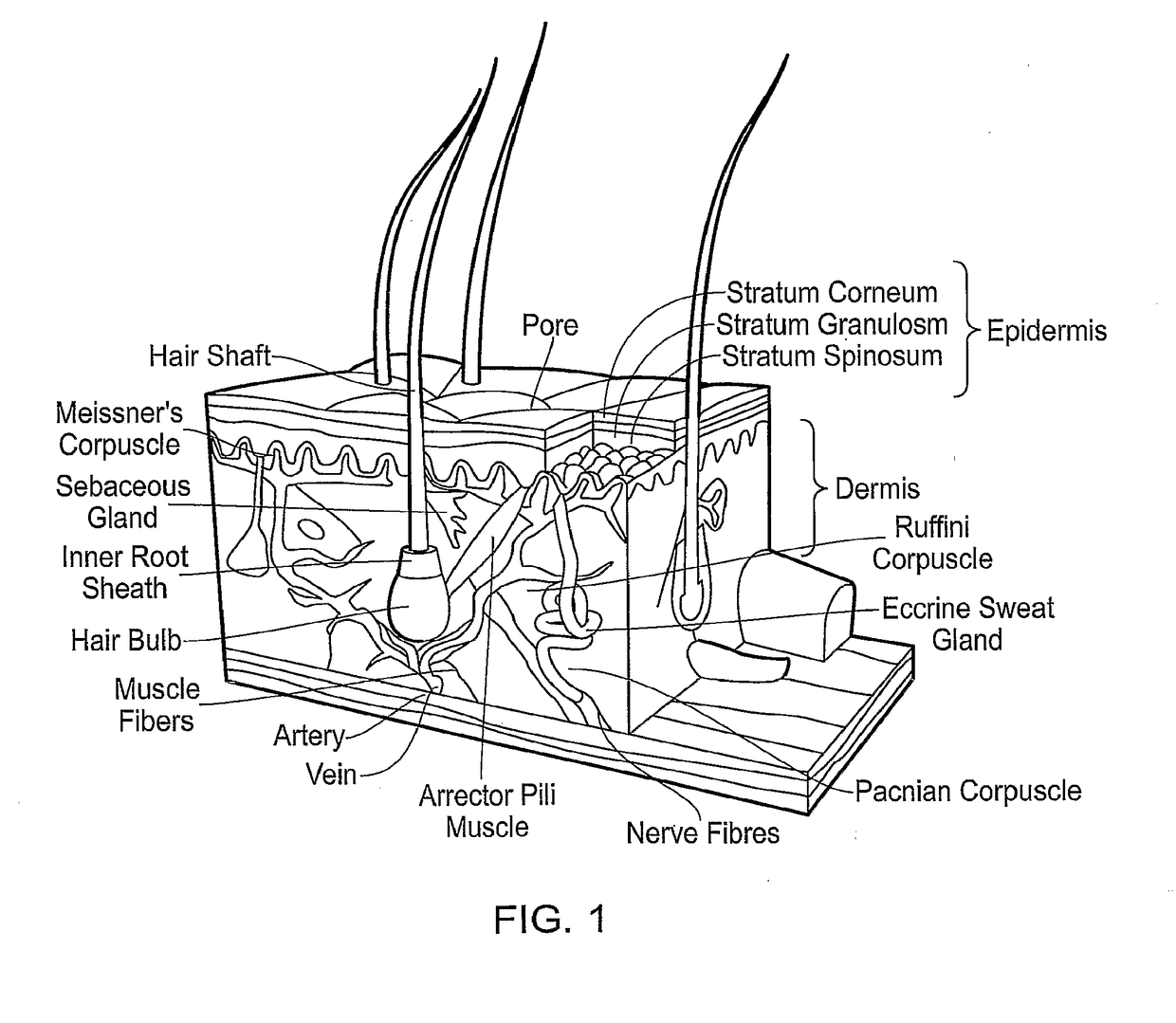
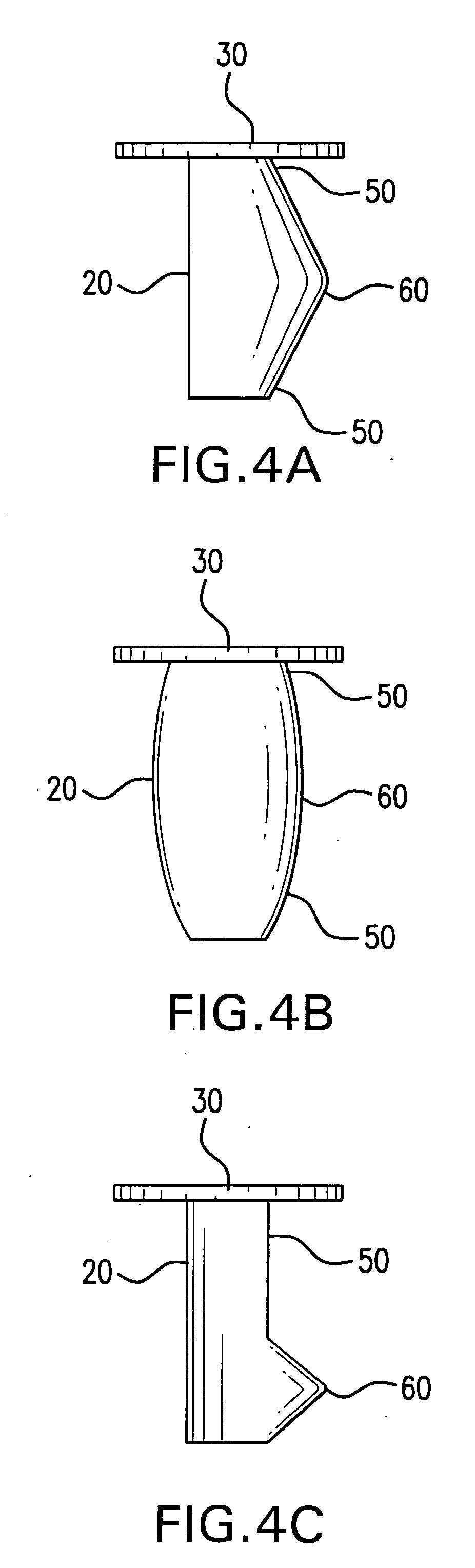
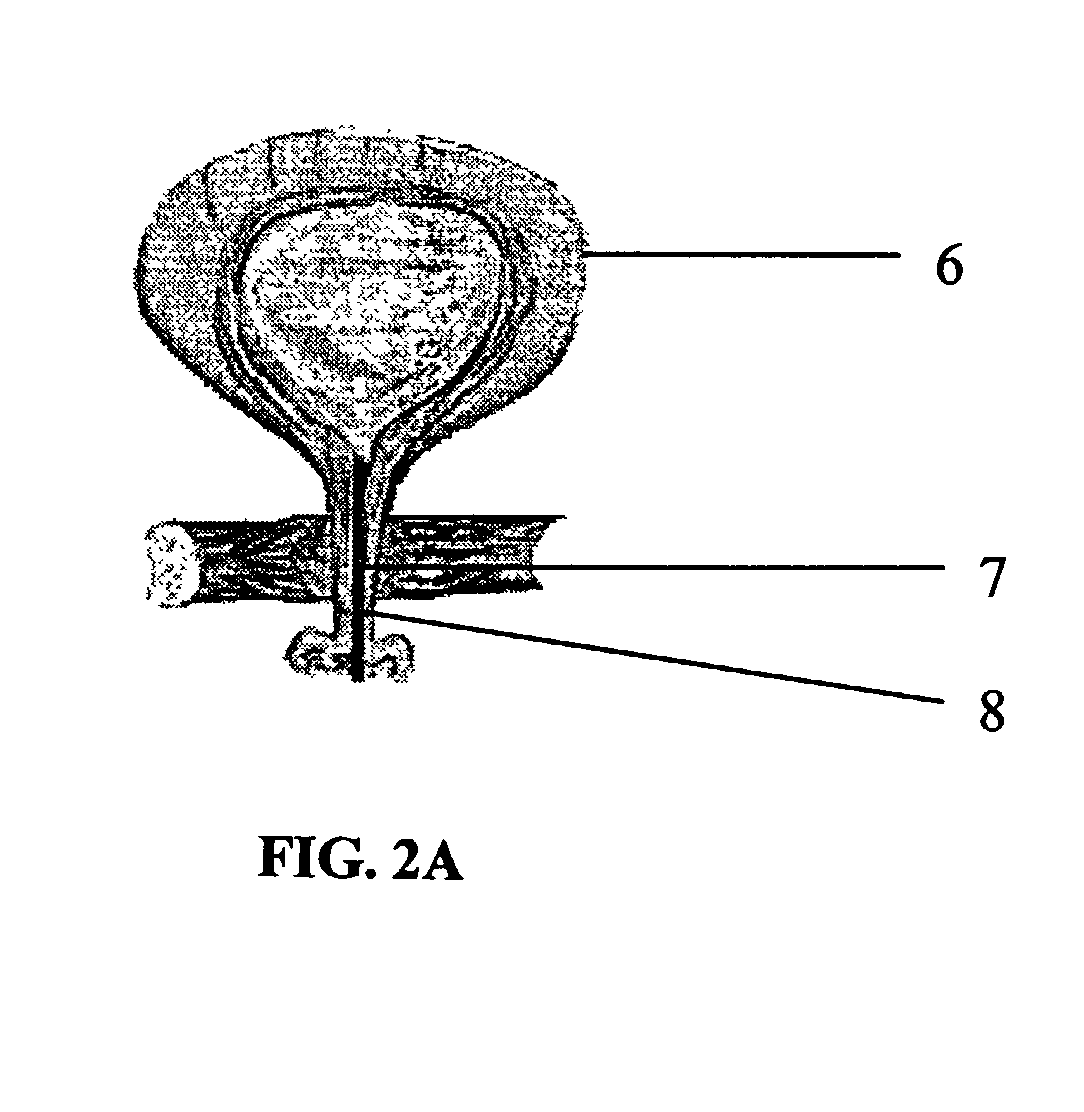
Medical film
InactiveUS7718556B2Good biocompatibilityHigh strengthSuture equipmentsBiocideGelatin filmThin membrane
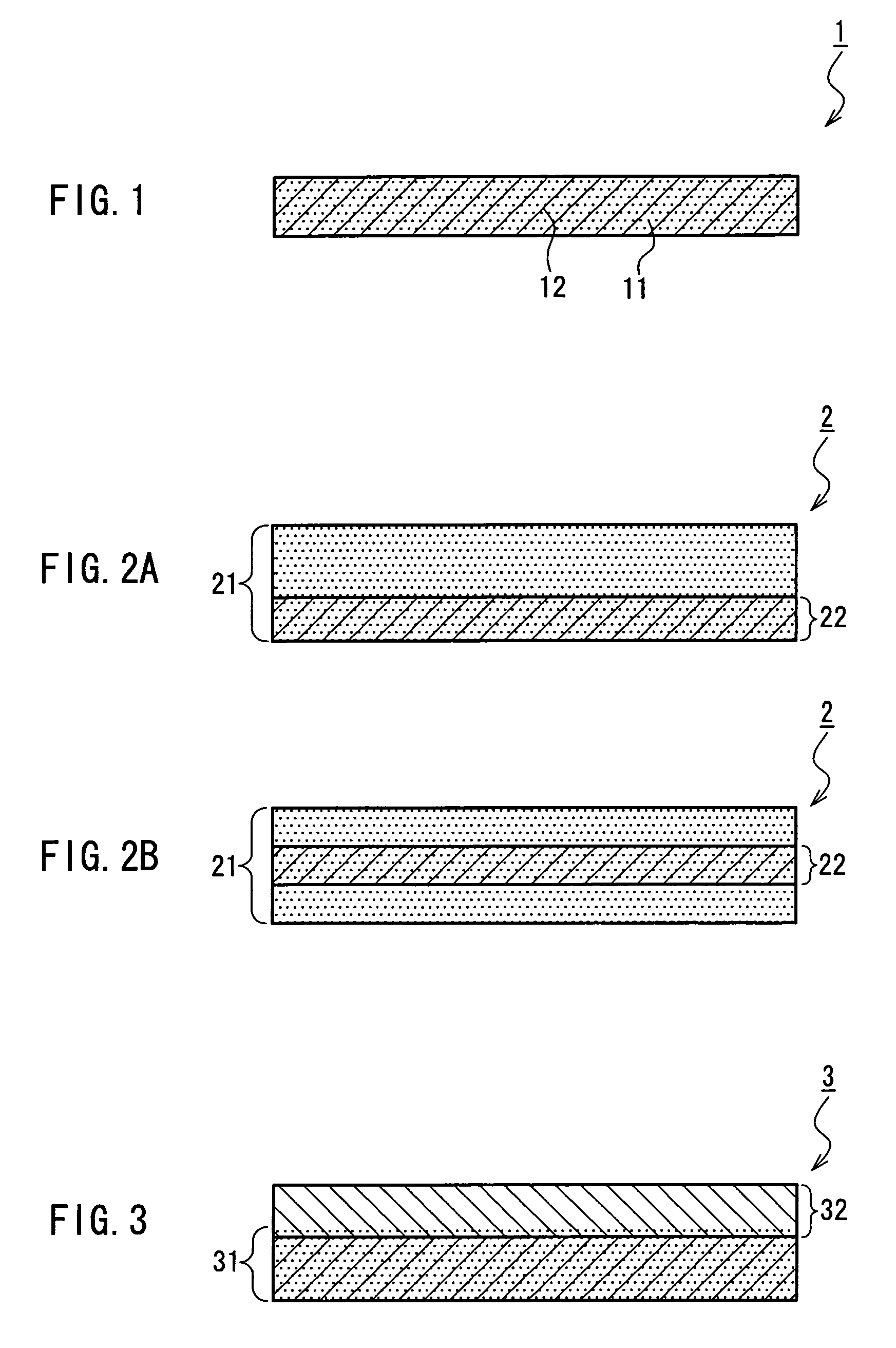
A medical film that is excellent in biocompatibility and bioabsorbability and has an excellent strength in suturing and bonding is provided. A reinforcing material 12 made of a biodegradable polymer is placed in a gelatin solution so as to allow the solution to infiltrate in the reinforcing material 12 and then the gelatin is dried. This allows the gelatin that has infiltrated entirely in an internal part of the reinforcing material 12 to gel, thereby forming a gelatin film 11. Thus, a medical film 1 in which the reinforcing material 12 and the gelatin film 11 are integrated is obtained. The gelatin film 11 preferably is a cross-linked gelatin film.
Owner:GUNZE LTD
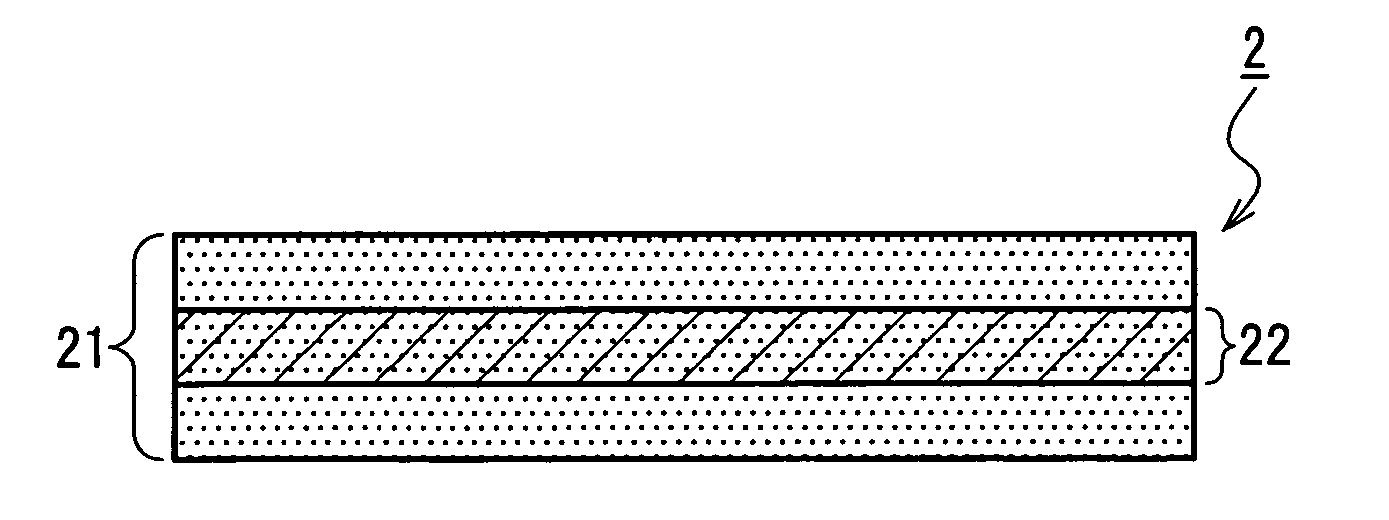
Multilayer scaffold
InactiveUS20170182211A1Avoid disease riskAvoids the potential ethical and religious barriers to the useSkin implantsTissue regenerationMedicinePlla scaffold
Owner:SMITH & NEPHEW PLC
Means to achieve sustained release of synergistic drugs by conjugation
A codrug composition of at least two drug compounds covalently linked to one another via a labile bond to form a single codrug composition, or ionically linked to one another to form a single workings composition, and methods of use of the codrug for the treatment of various medical conditions. The codrug may be administered by itself or in the form of a bioerodible or nonbioerodible substance.
Owner:UNIVERSITY OF KENTUCKY +1
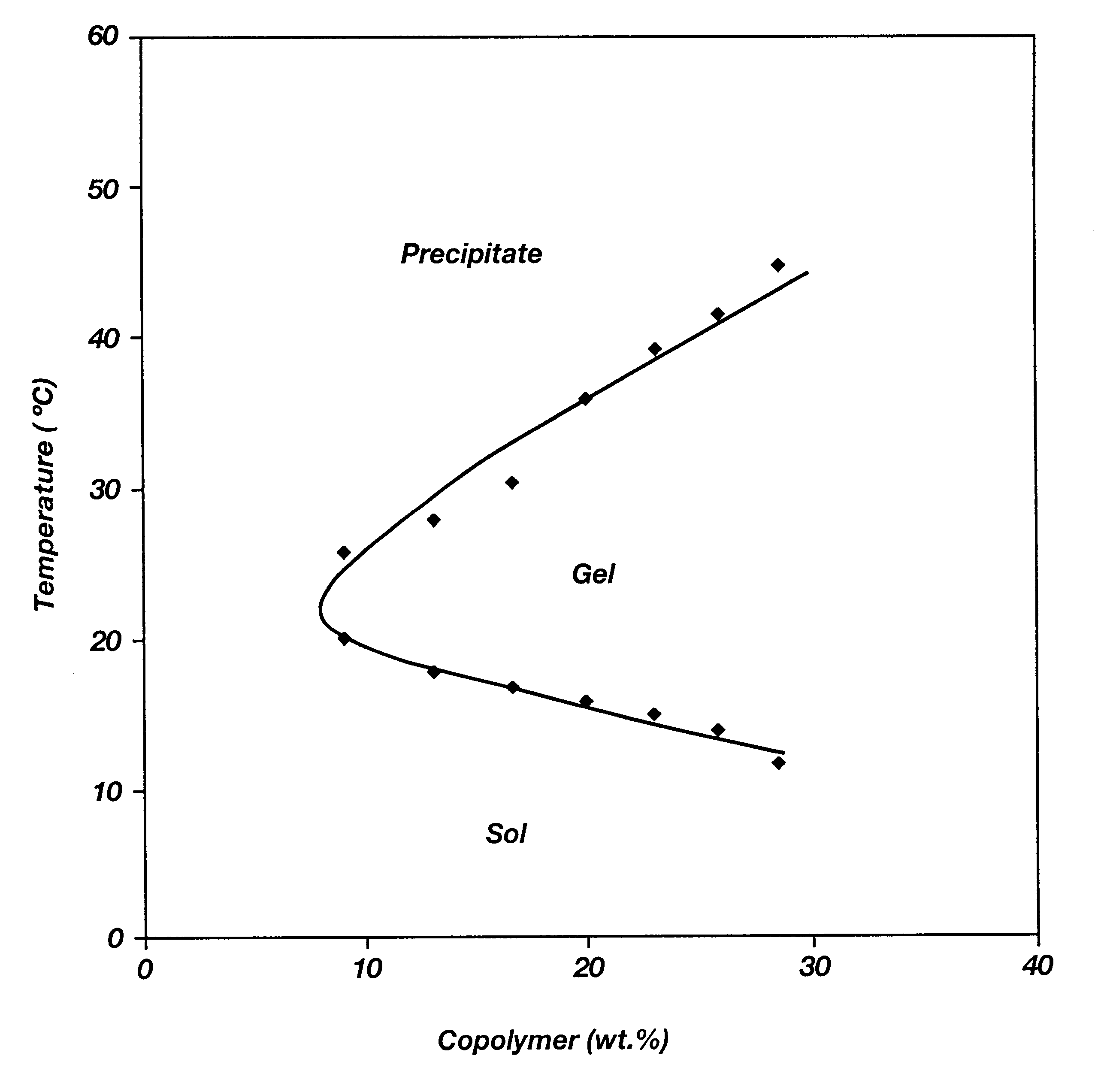
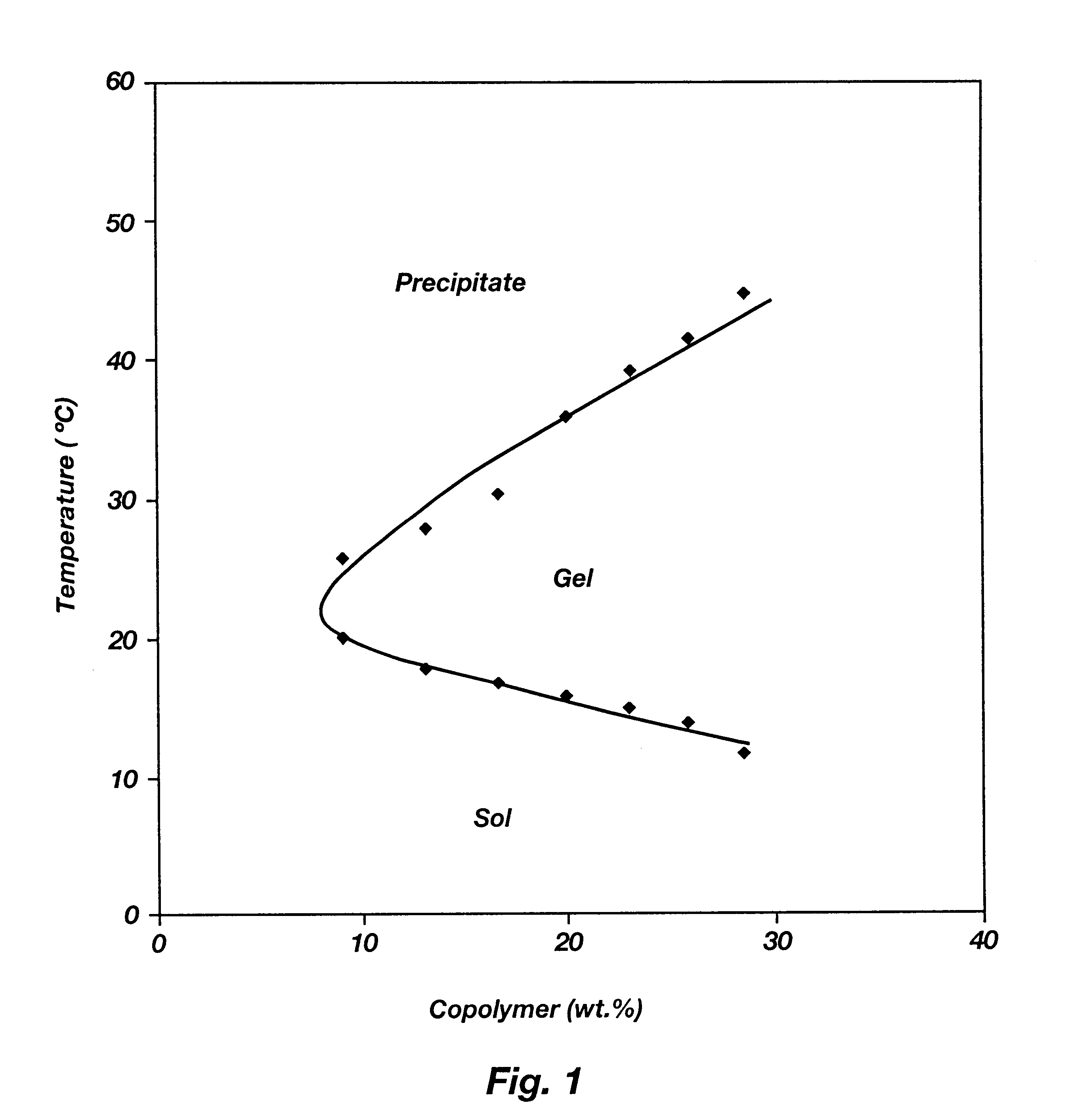
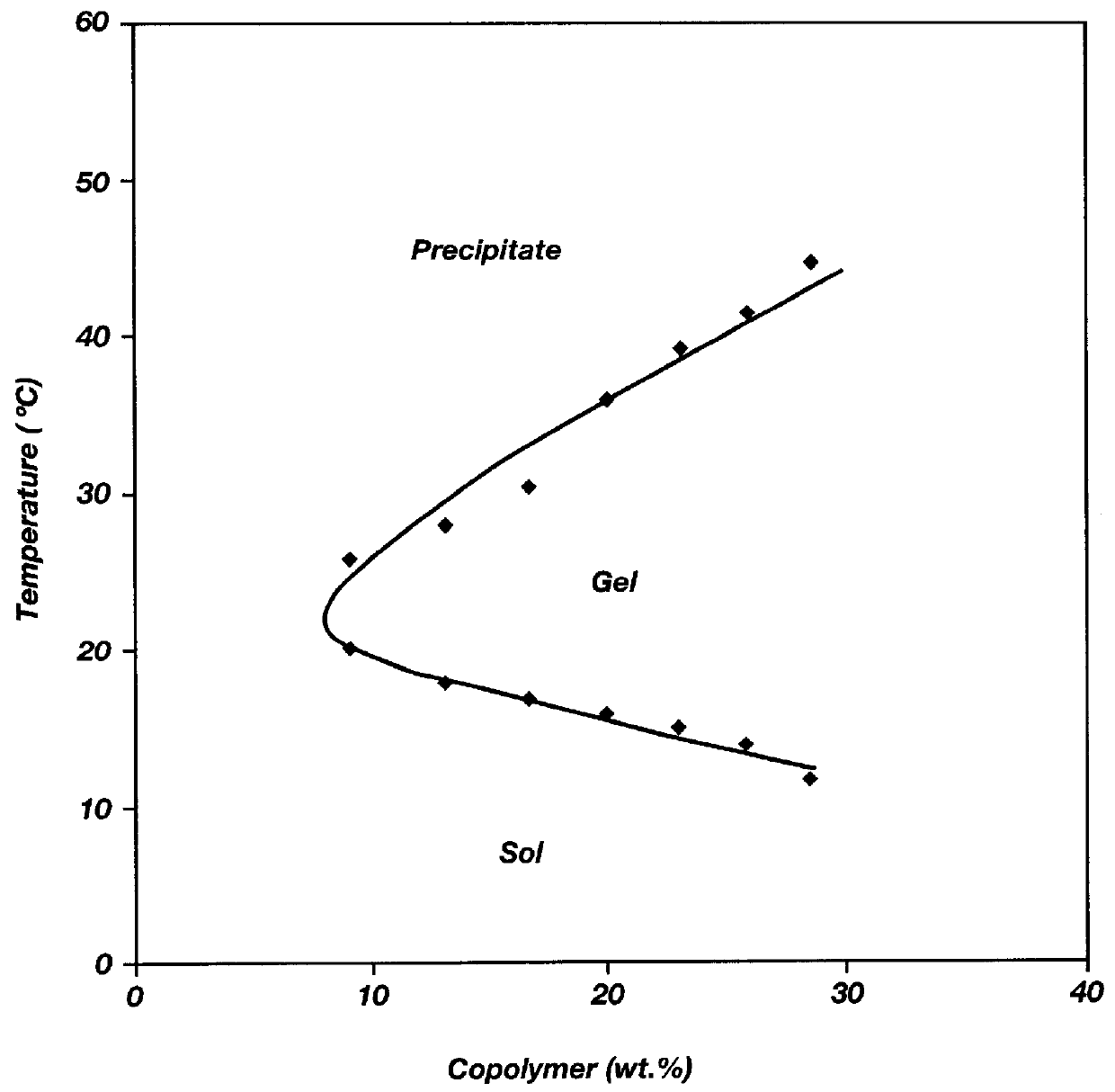
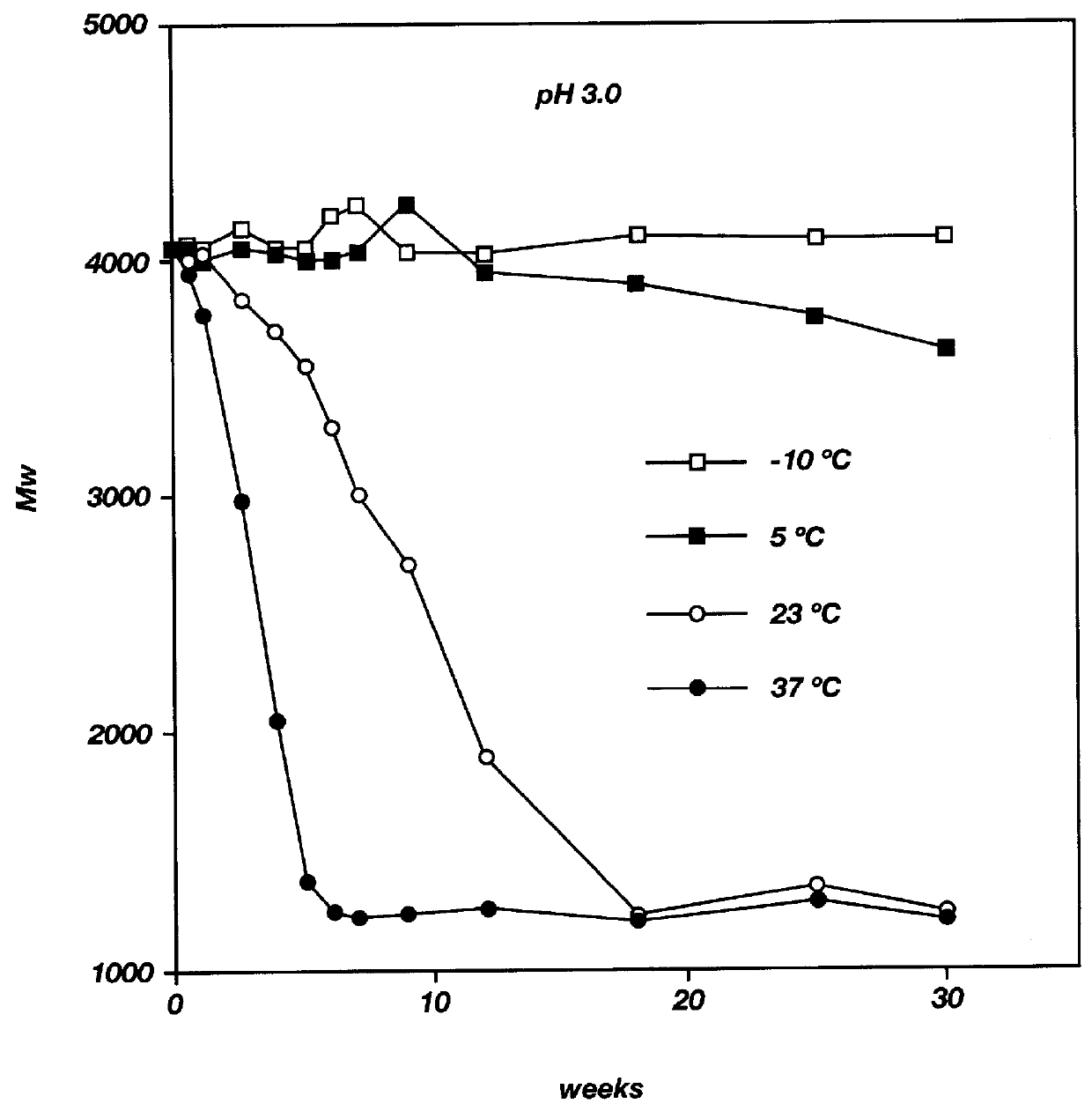
Biodegradable low molecular weight triblock poly(lactide-co- glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6201072B1Difficult to formulateDifficult to administerOrganic active ingredientsPowder deliverySolubilityPolymer science
A water soluble, biodegradable ABA- or BAB-type tri-block polymer is disclosed that is made up of a major amount of a hydrophobic A polymer block made of a biodegradable polyester and a minor amount of a hydrophilic polyethylene glycol(PEG) B polymer block, having an overall average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the tri-block polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the tri-block polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic component content, polymer concentration, molecular weight and polydispersity of the tri-block polymer. Because the tri-block polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:KIM PH D SUNG WAN +2
Controlled drug delivery system using the conjugation of drug to biodegradable polyester
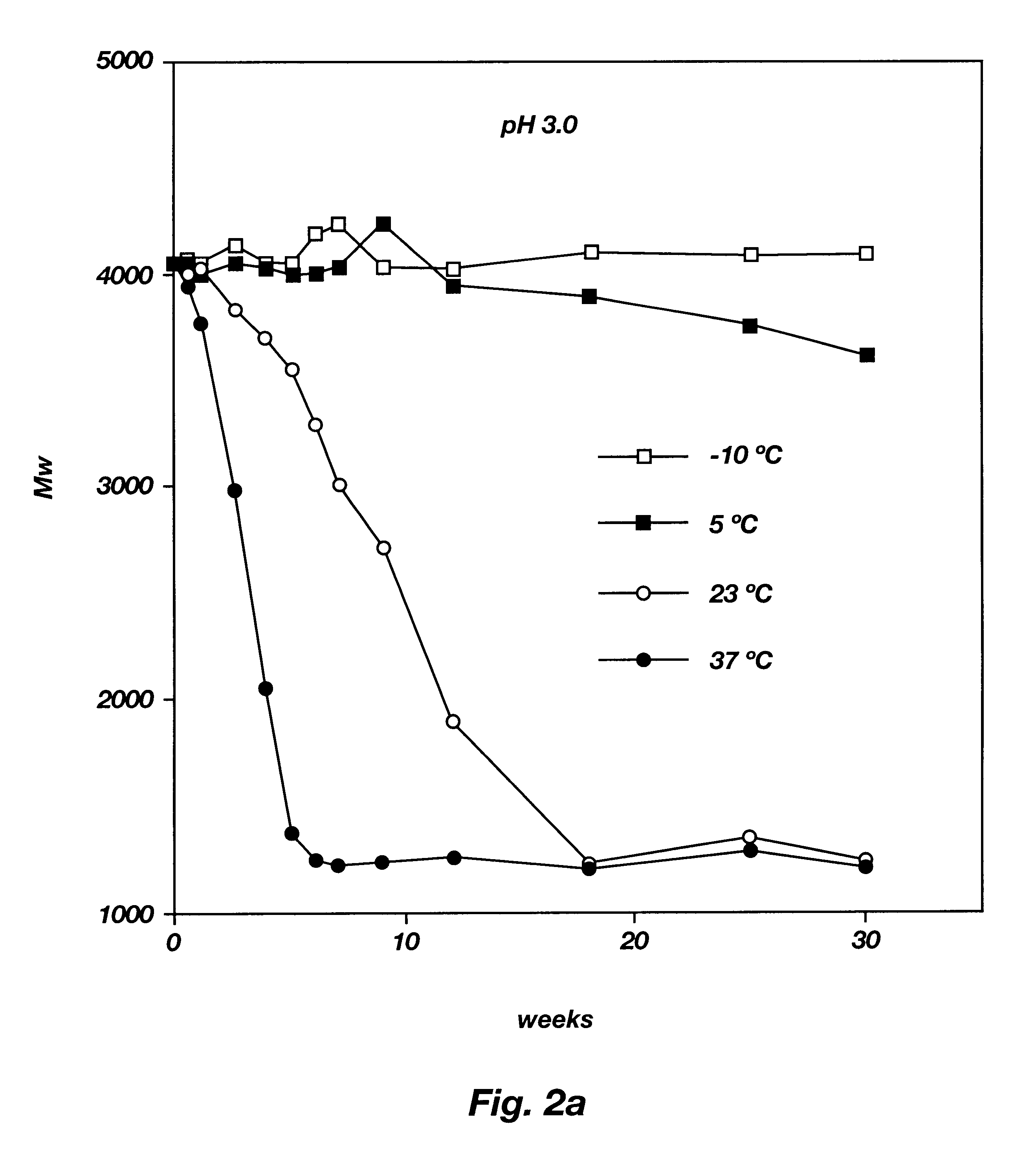
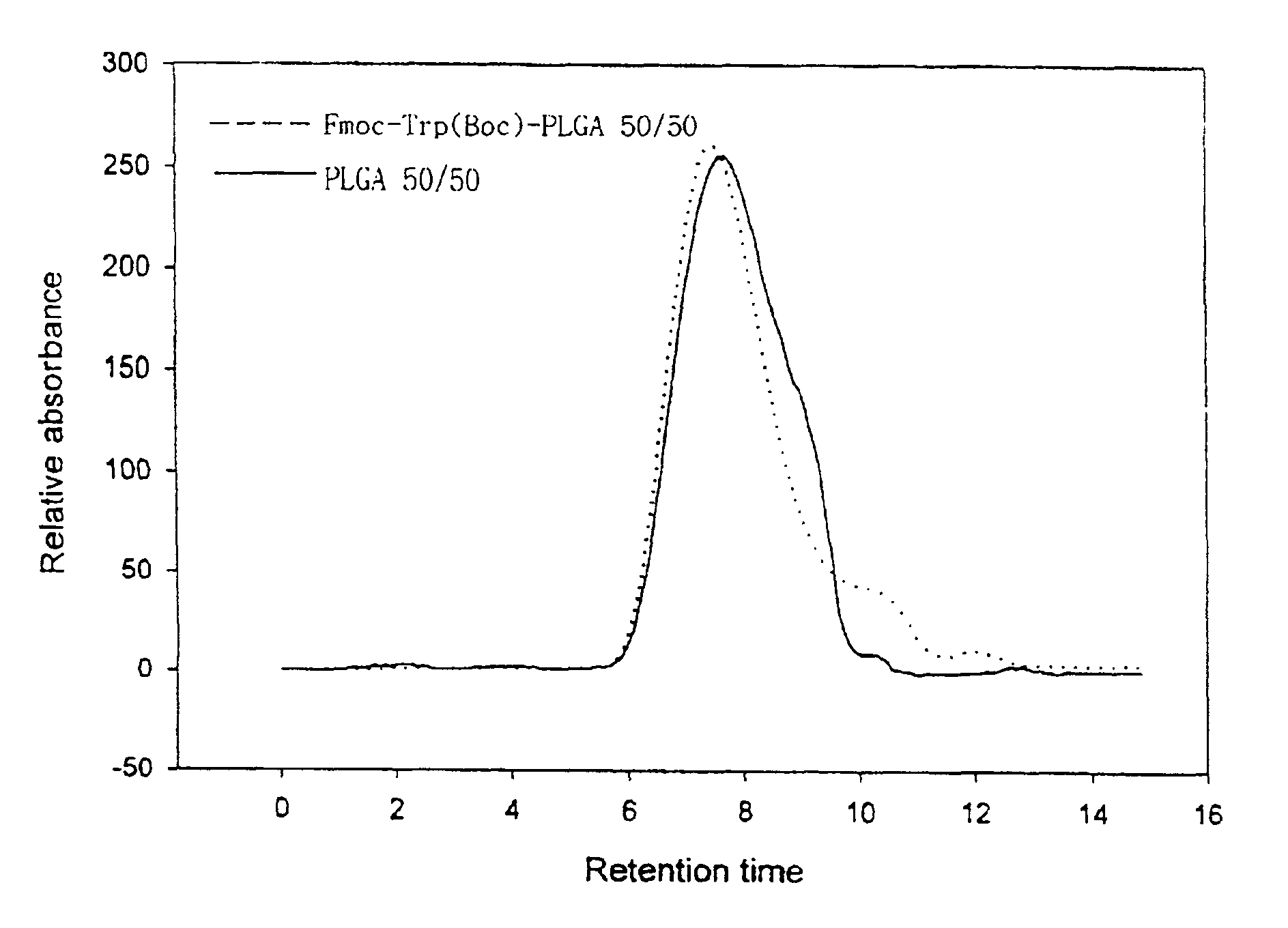
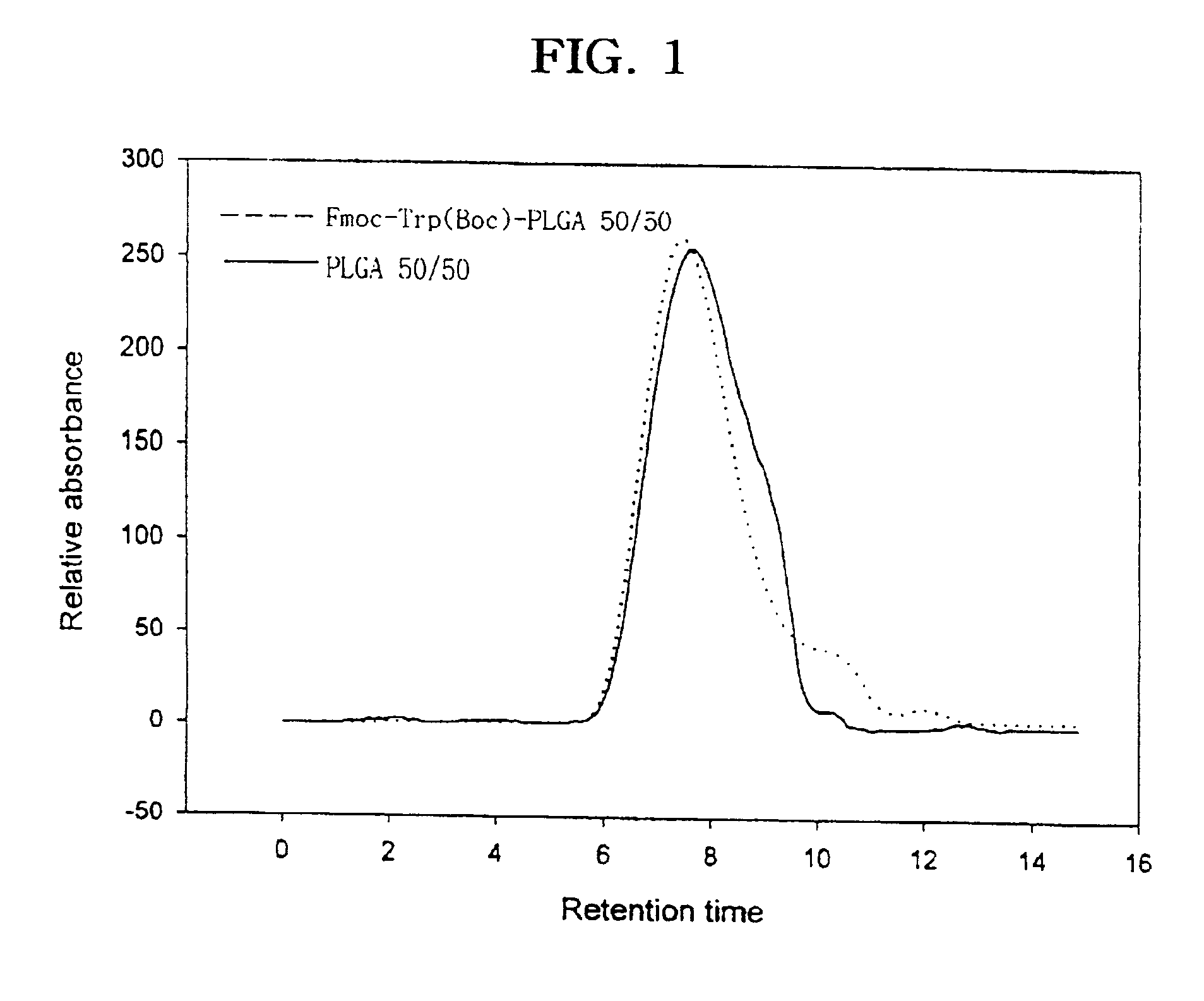
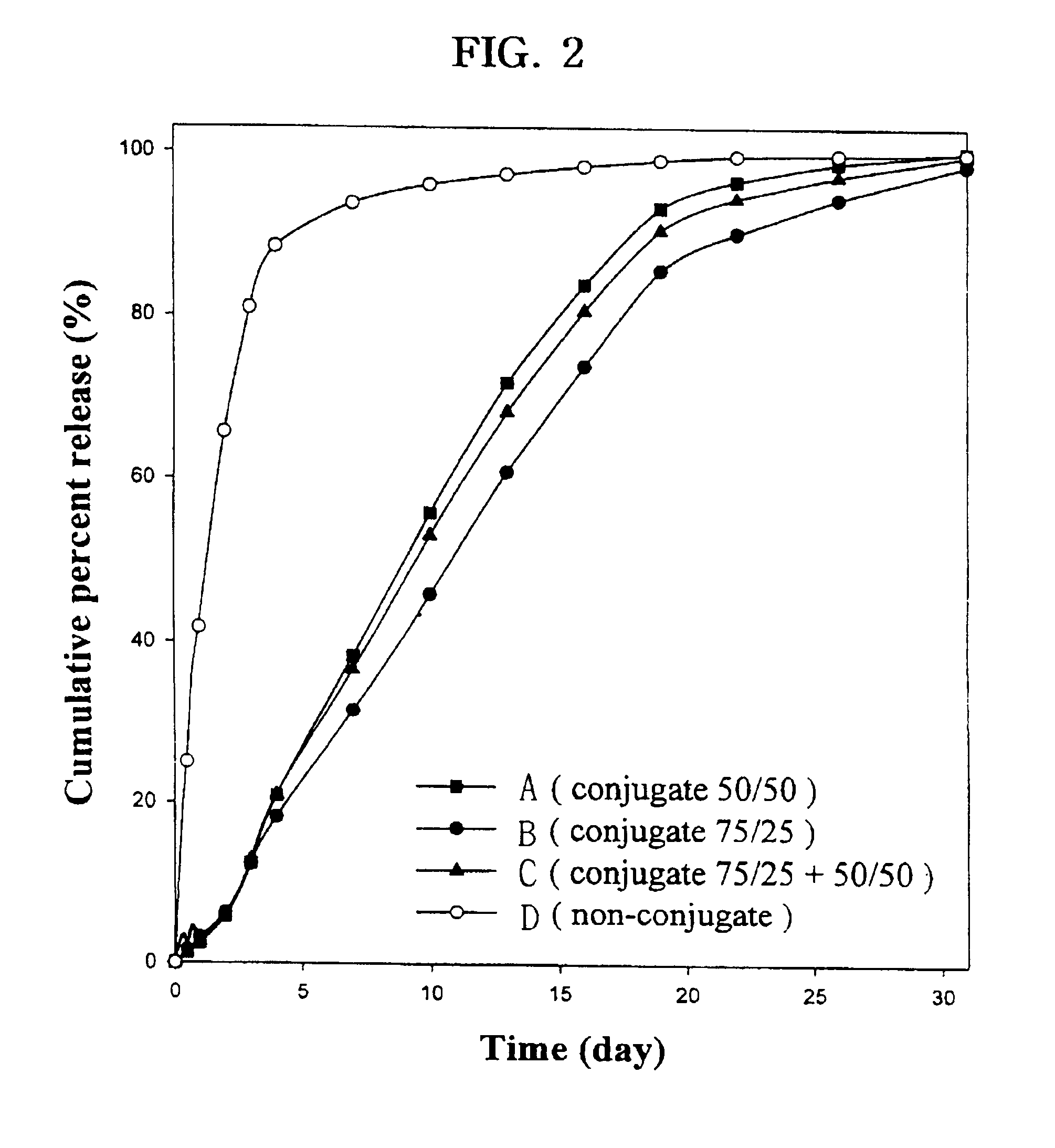
The present invention relates to a molecular sustained controlled release system constructed by the conjugation of molecules to be released with biodegradable polyester polymer via covalent bond and method for preparation thereof. In accordance with the present invention, the system may be formulated into microspheres, nanoparticles, or films. The molecular release rate from the above system can be regulated to be proportional to the chemical degradation rate of the biodegradable polyester polymers, resulting in near zero order kinetics profile of release without showing a burst effect, Moreover, a high loading efficiency of hydrophilic drugs can be achieved.
Owner:MOGAM BIOTECH RES INST +1
Thermoplastic starch compositions incorporating a particulate filler component
InactiveUS6231970B1Reduce molecular weightAvoid hydrolysisProtein adhesivesPaper coatingParticulatesCross-link
Thermoplastic starch compositions that include a particulate filler, e.g. an inorganic filler component, and optional fibrous component The compositions include a thermoplastic phase comprising a thermoplastic starch melt that contains, at a minimum, starch blended with an appropriate plasticizing agent under conditions in order for the starch to form a thermoplastic melt. The thermoplastic phase may also include one or more additional thermoplastic polymers and other optional reactants, liquids or cross-linking agents to improve the water-resistance, strength, and / or other mechanical properties of the thermoplastic melt, particularly upon solidification. The inorganic filler component may affect the mechanical properties but will mainly be added to reduce the cost of the thermoplastic starch compositions by displacing a significant portion of the more expensive starch or starch / polymer melt. Fibers may optionally be included in order to improve the mechanical properties of the thermoplastic starch compositions. The thermoplastic starch compositions may be shaped into a wide variety of useful articles, such as sheets, films, containers, and packaging materials. Because the thermoplastic starch compositions will typically include a thermoplastic phase that is biodegradable, and because the other components will either constitute a naturally occurring mineral and optionally a natural fiber, the overall composition will typically be more environmentally friendly compared to conventional thermoplastic materials.
Owner:BIO TEC BIOLOGISCHE NATURVERPACKUNGEN
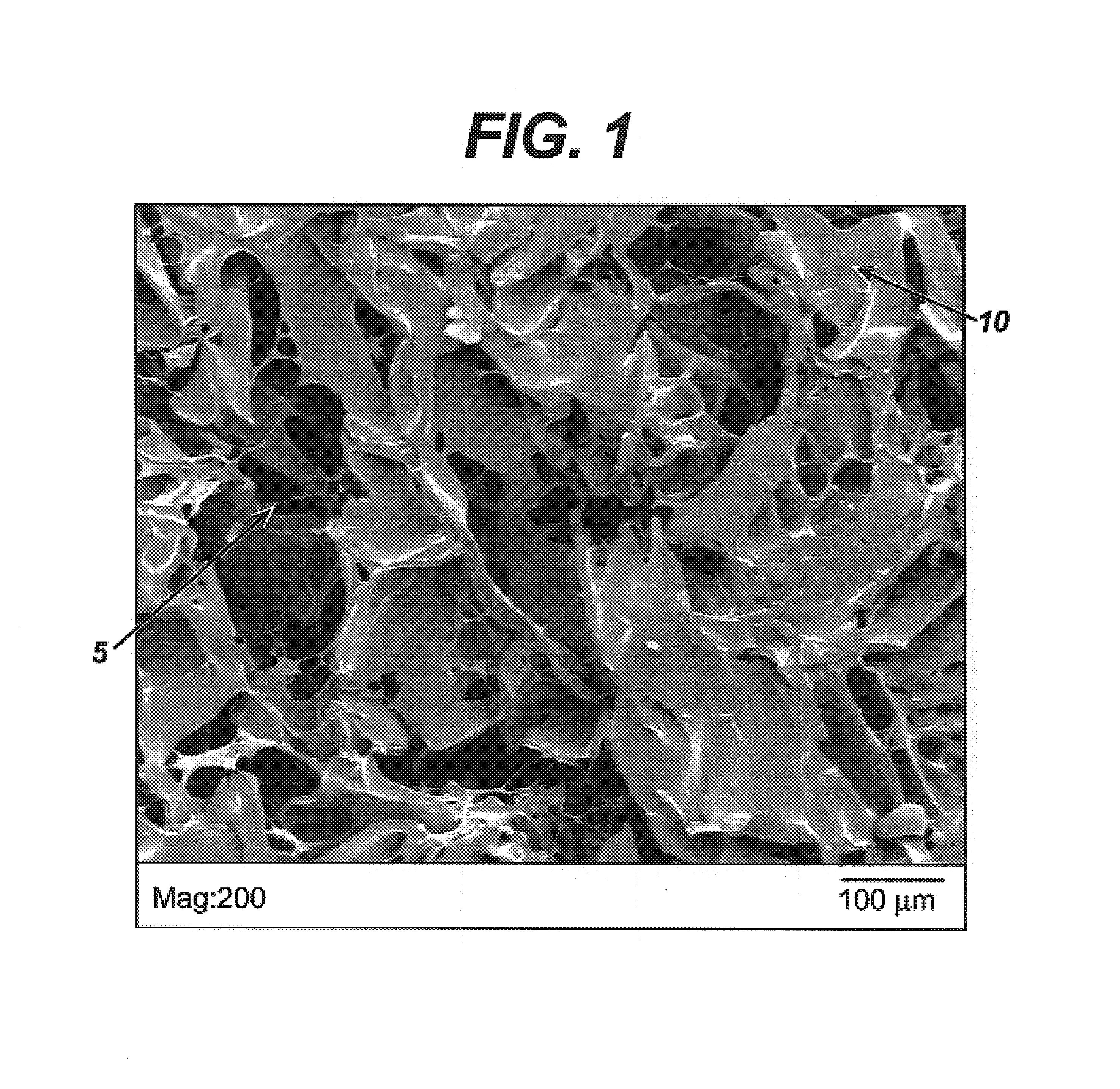
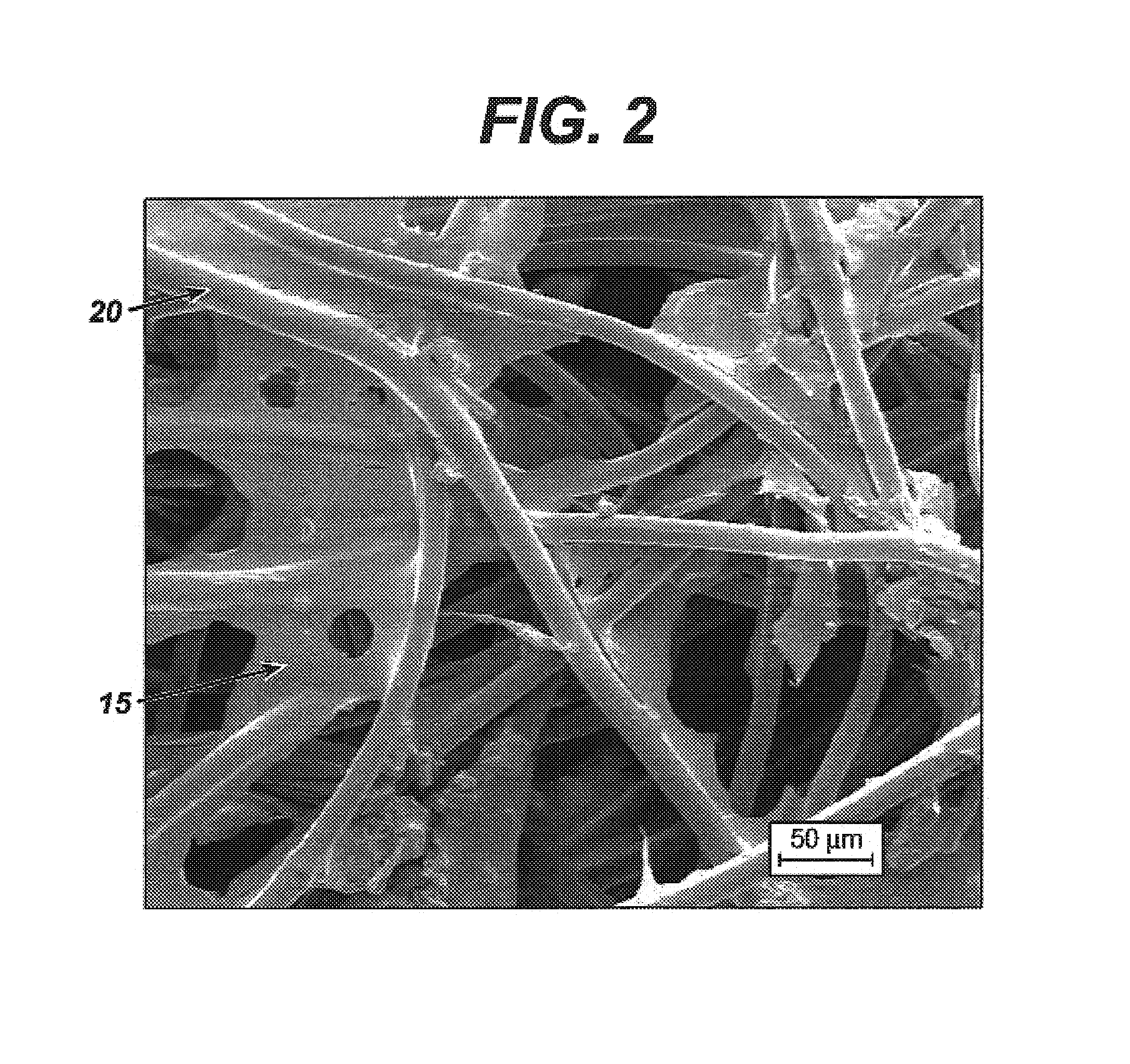
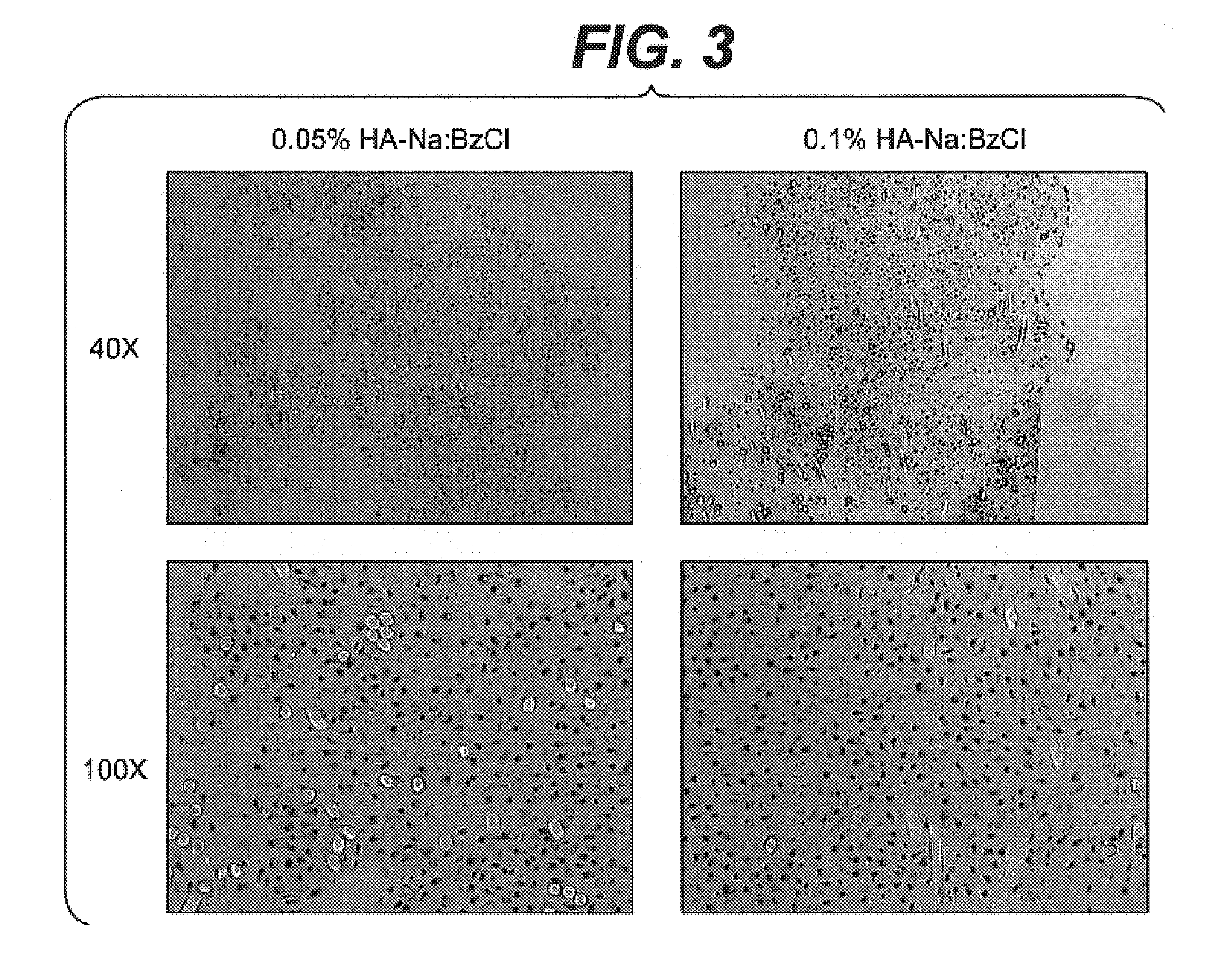
Modified hyaluronic acid for use in musculoskeletal tissue repair
The present invention includes hyaluronic acid complexes of a monovalent alkali metal salt of hyaluronic acid and a tetra alkyl ammonium halide that are suitable for incorporation with tissue scaffolds that are suitable for use in repair and / or regeneration of muscoloskeletal tissue and that include a biodegradable, porous substrate made from a biodegradable, hydrophobic polymer, where the hyaluronic acid complex is substantially insoluble in water at room temperature, yet soluble in mixtures of organic and aqueous solvents in which the selected hydrophobic polymer is soluble.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
Therapeutic treatment and prevention of infections with a bioactive materials encapsulated within a biodegradable-biocompatible polymeric matrix
InactiveUS6309669B1Sustained release of active agent over timeEfficient and effective usePowder deliveryPeptide/protein ingredientsAdjuvantEnd-group
Novel burst-free, sustained release biocompatible and biodegrable microcapsules which can be programmed to release their active core for variable durations ranging from 1-100 days in an aqueous physiological environment. The microcapsules are comprised of a core of polypeptide or other biologically active agent encapsulated in a matrix of poly(lactide / glycolide) copolymer, which may contain a pharmaceutically-acceptable adjuvant, as a blend of upcapped free carboxyl end group and end-capped forms ranging in ratios from 100 / 0 to 1 / 99.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
Bioactive agent delivering system comprised of microparticles within a biodegradable to improve release profiles
InactiveUS6589549B2Improve stabilityPowder deliveryPeptide/protein ingredientsActive agentEngineering
A composition and method for releasing a bio-active agent or a drug within a biological environment in a controlled manner is disclosed. The composition is a dual phase polymeric agent-delivery composition comprising a continuous biocompatible gel phase, a discontinuous particulate phase comprising defined microparticles and an agent to be delivered. A microparticle containing a bio-active agent is releasably entrained within a biocompatible polymeric gel matrix. The bioactive agent release may be contained in the microparticle phase alone or in both the microparticles and the gel matrix. The release of the agent is prolonged over a period of time, and the delivery may be modulated and / or controlled. In addition, a second agent may be loaded in some of the microparticles and / or the gel matrix.
Owner:BTG INT LTD
Biocompatible crosslinked polymers
InactiveUS7009034B2Improve performanceImprove visibilityUltrasonic/sonic/infrasonic diagnosticsPowder deliveryWound dressingPost operative
Biocompatible crosslinked polymers, and methods for their preparation and use, are disclosed in which the biocompatible crosslinked polymers are formed from water soluble precursors having electrophilic and nucleophilic functional groups capable of reacting and crosslinking in situ. Methods for making the resulting biocompatible crosslinked polymers biodegradable or not are provided, as are methods for controlling the rate of degradation. The crosslinking reactions may be carried out in situ on organs or tissues or outside the body. Applications for such biocompatible crosslinked polymers and their precursors include controlled delivery of drugs, prevention of post-operative adhesions, coating of medical devices such as vascular grafts, wound dressings and surgical sealants. Visualization agents may be included with the crosslinked polymers.
Owner:INCEPT LLC
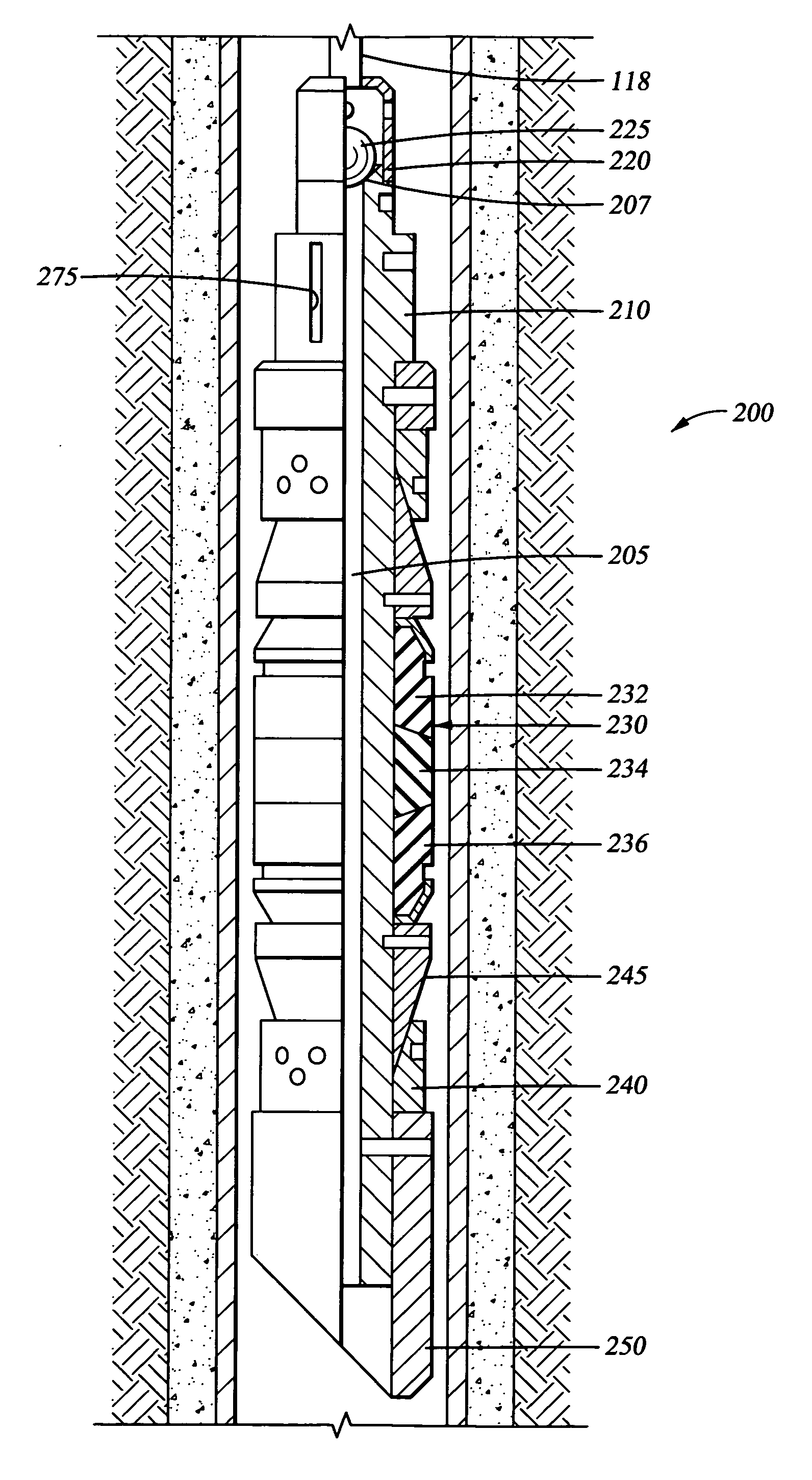
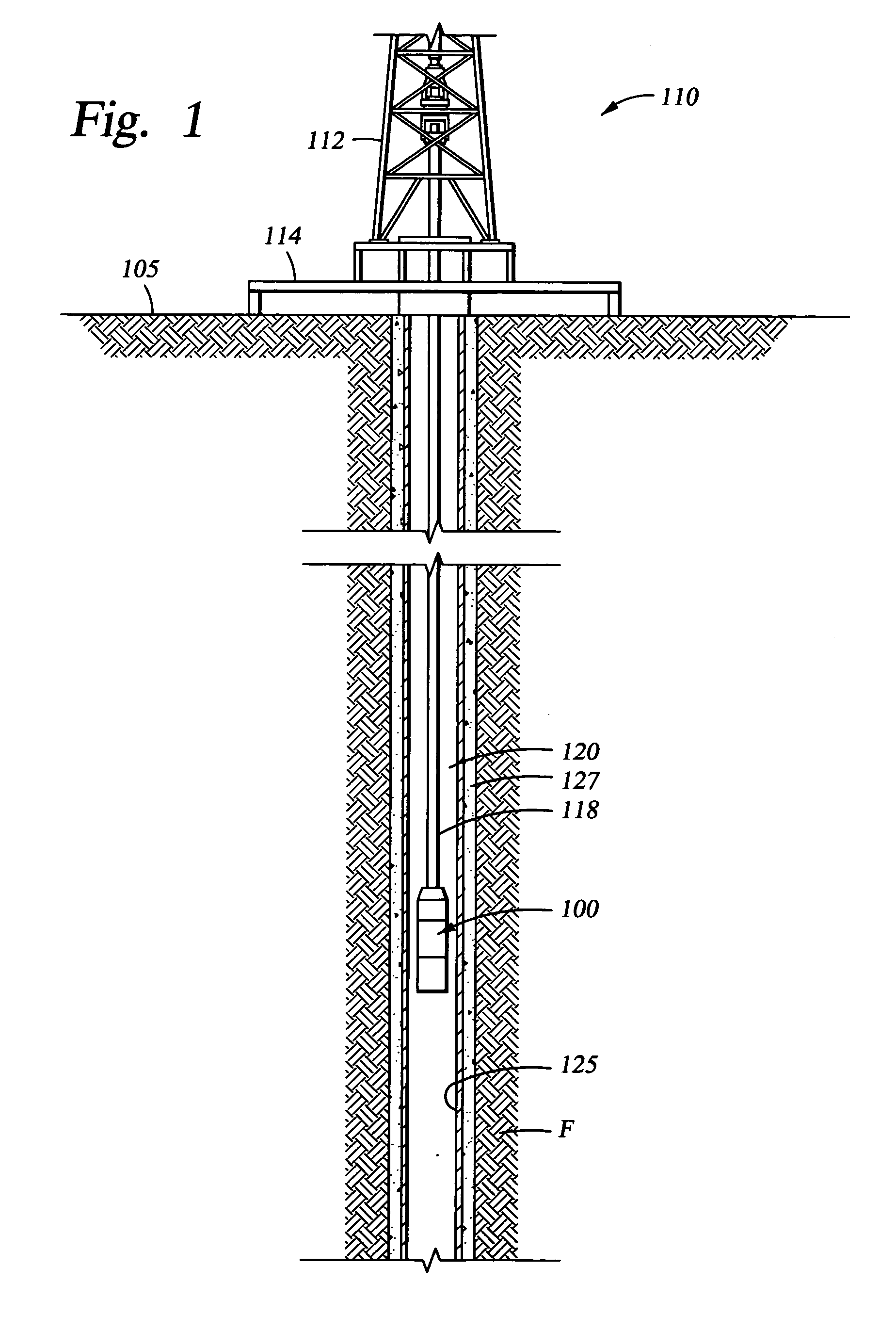
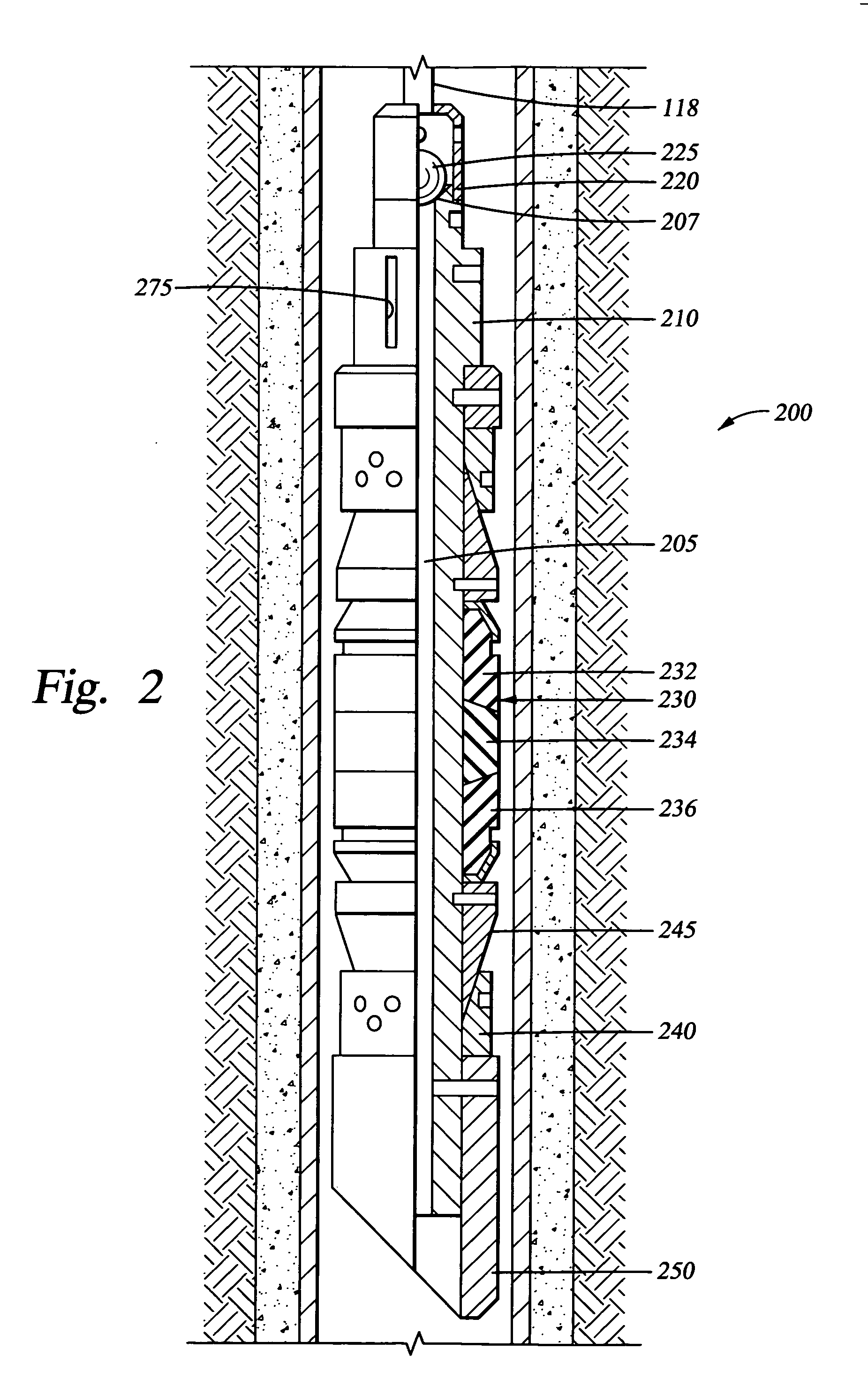
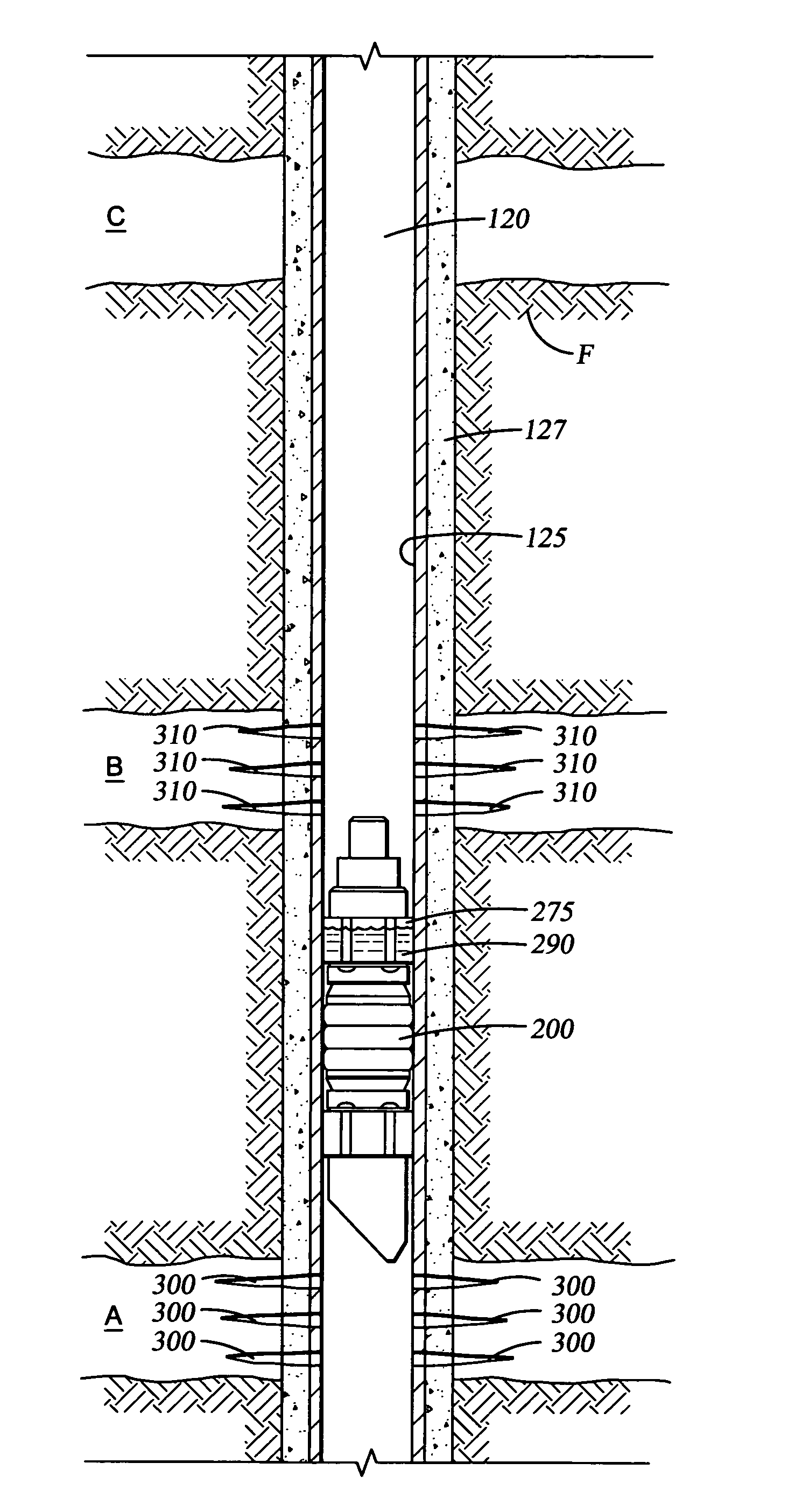
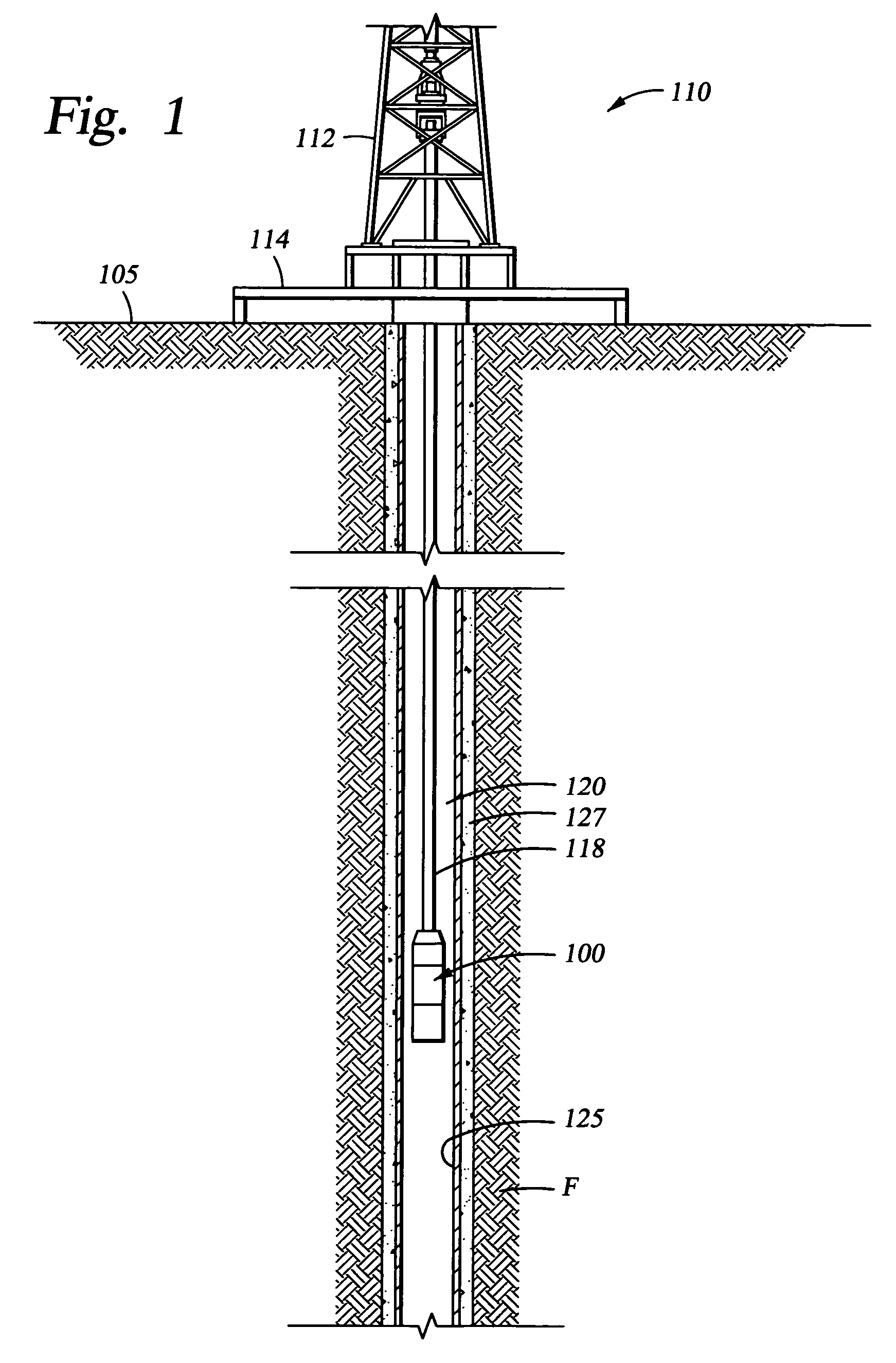
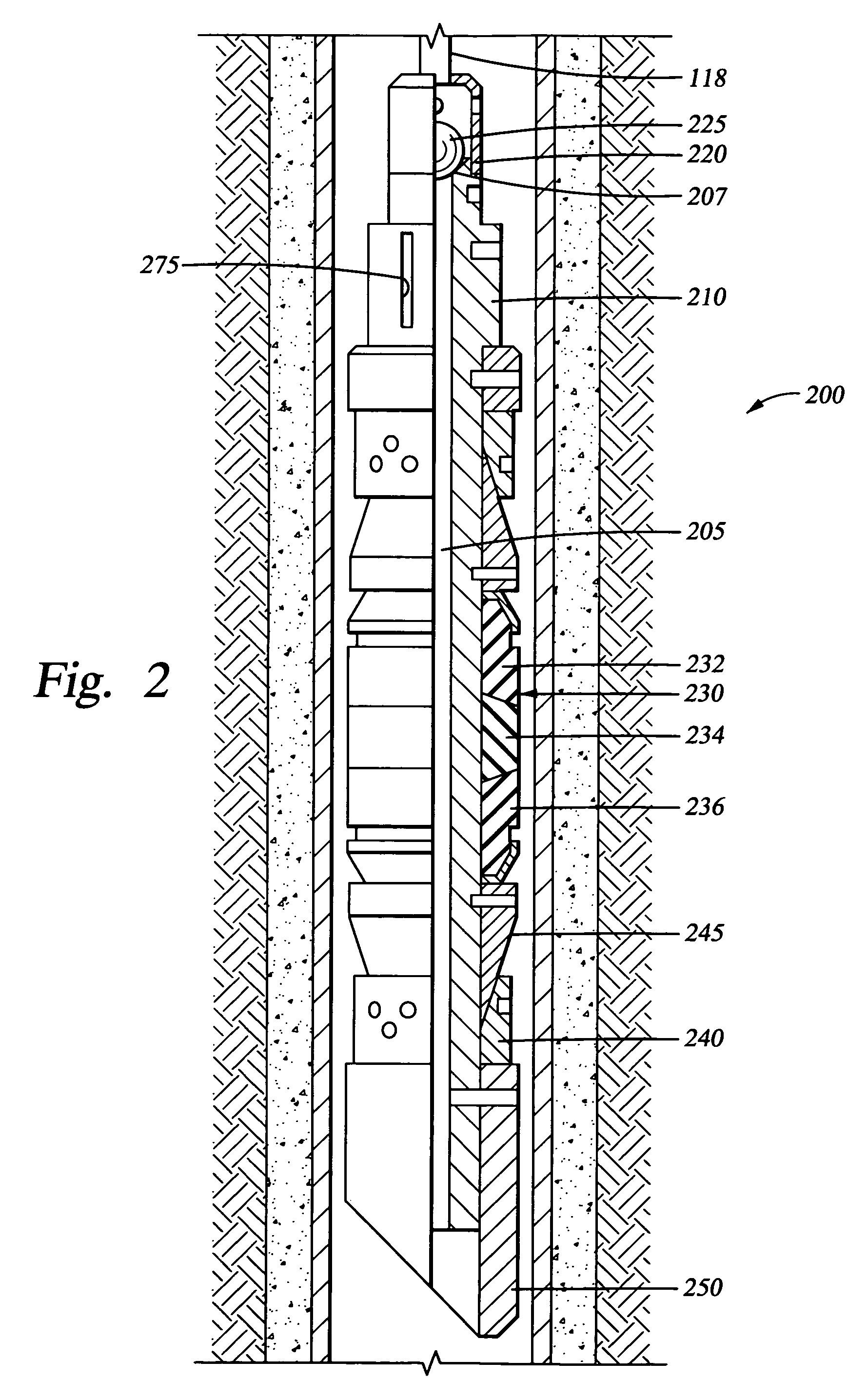
Biodegradable downhole tools
A disposable downhole tool or a component thereof comprises an effective amount of biodegradable material such that the tool or the component thereof desirably decomposes when exposed to a wellbore environment. In an embodiment, the biodegradable material comprises a degradable polymer. The biodegradable material may further comprise a hydrated organic or inorganic solid compound. The biodegradable material may also be selected to achieve a desired decomposition rate when the tool is exposed to the wellbore environment. In an embodiment, the disposable downhole tool further comprises an enclosure for storing a chemical solution that catalyzes decomposition. The tool may also comprise an activation mechanism for releasing the chemical solution from the enclosure. In various embodiments, the disposable downhole tool is a frac plug, a bridge plug, or a packer.
Owner:HALLIBURTON ENERGY SERVICES INC
Biodegradable low molecular weight triblock poly (lactide-co-glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6117949AReduce solubilityReduced stabilityPowder deliveryPeptide/protein ingredientsSolubilityPolymer science
A water soluble biodegradable ABA- or BAB-type triblock polymer is disclosed that is made up of a major amount of a hydrophobic polymer made of a poly(lactide-co-glycolide) copolymer or poly(lactide) polymer as the A-blocks and a minor amount of a hydrophilic polyethylene glycol polymer B-block, having an overall weight average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the triblock polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the triblock polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic componenet content, polymer concentration, molecular weight and polydispersity of the triblock polymer. Because the triblock polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:BTG INT LTD +2
Polyester polyether block copolymers
The present invention relates to novel bioabsorbable polymeric compositions based upon AB polyester polyether or related diblocks and triblocks. Compositions according to the present invention may be used in medical applications, for example, for reducing or preventing adhesion formation subsequent to medical procedures such as surgery, for producing surgical articles including stents and grafts, as coatings, sealants, lubricants, as transient barriers in the body, for materials which control the release of bioactive agents in the body, for wound and bum dressings and producing biodegradable articles, among numerous others.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Biodegradable downhole tools
A disposable downhole tool or a component thereof comprises an effective amount of biodegradable material such that the tool or the component thereof desirably decomposes when exposed to a wellbore environment. In an embodiment, the biodegradable material comprises a degradable polymer. The biodegradable material may further comprise a hydrated organic or inorganic solid compound. The biodegradable material may also be selected to achieve a desired decomposition rate when the tool is exposed to the wellbore environment. In an embodiment, the disposable downhole tool further comprises an enclosure for storing a chemical solution that catalyzes decomposition. The tool may also comprise an activation mechanism for releasing the chemical solution from the enclosure. In various embodiments, the disposable downhole tool is a frac plug, a bridge plug, or a packer.
Owner:HALLIBURTON ENERGY SERVICES INC
Biocompatible compositions and methods of using same
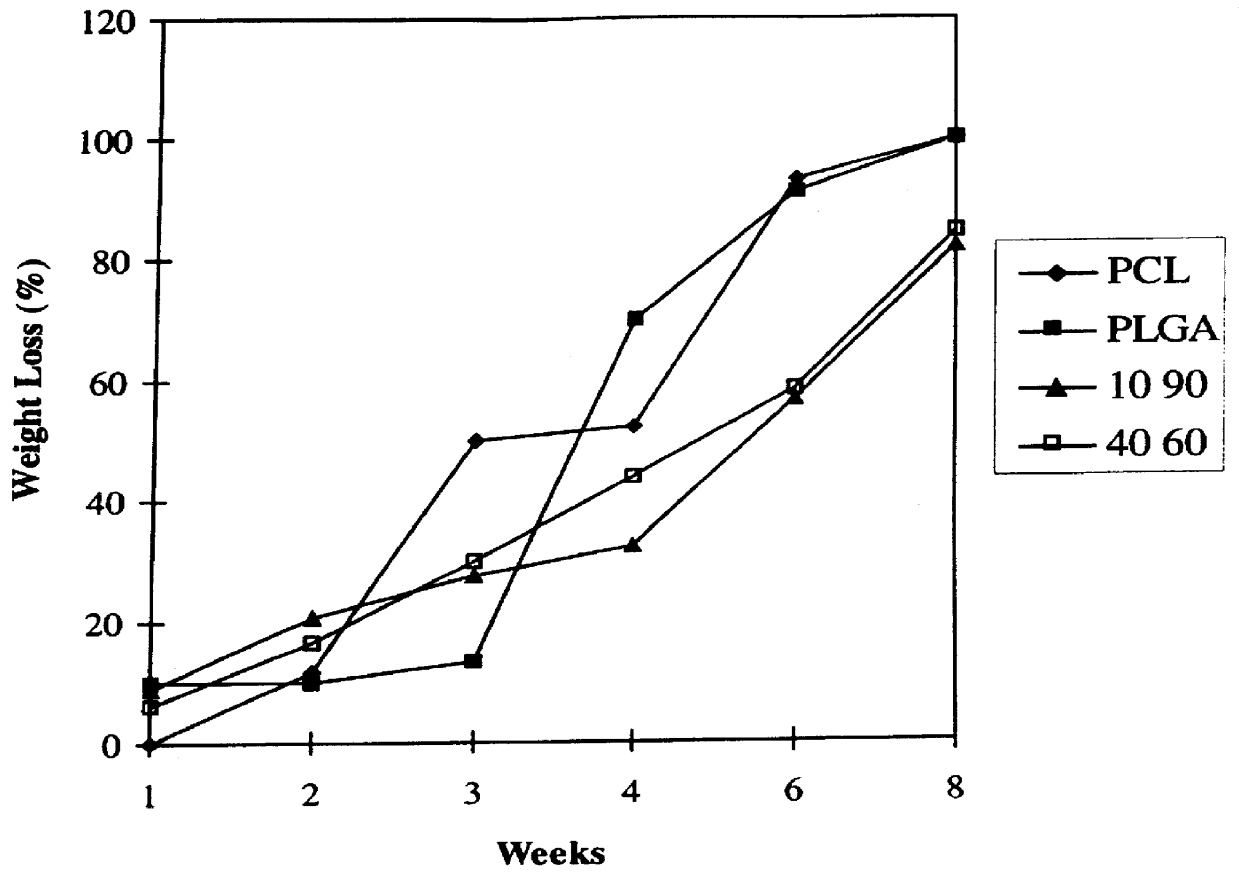
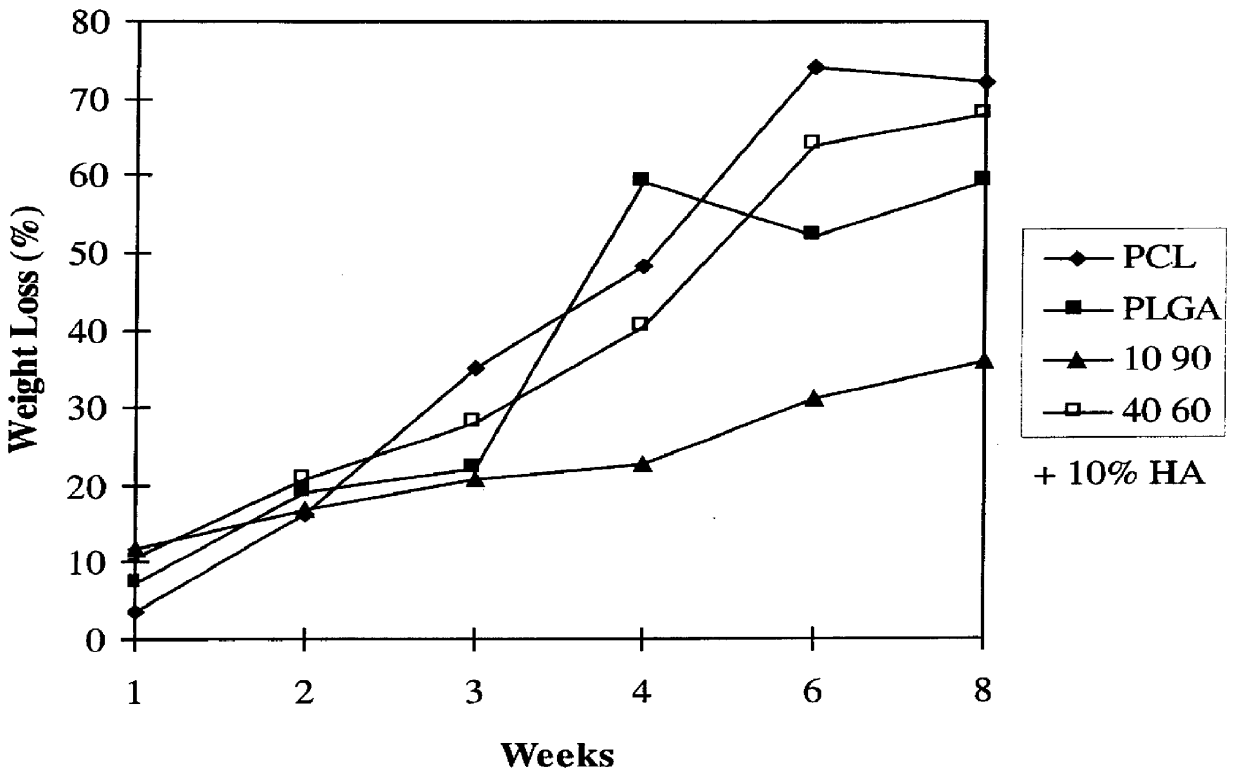
Blends of biodegradable polymers, preferably poly(caprolactone) and poly(D,L-lactic-co-glycolic) acid are discussed as well as their applications in the medical field, particularly with regard to bone tissue engineering. Preferably, hydroxyapatite ("HA") granules are incorporated into the blends and the resulting blends have desirable mechanical, physical, and biological characteristics. Even more preferably the compositions of the present invention are utilized to form osteoconductive composites that supported bone cell growth on the surface as well as throughout the scaffold.
Owner:CARNEGIE MELLON UNIV +1
Porous β-tricalcium phosphate granules for regeneration of bone tissue
InactiveUS6949251B2Improve regenerative abilitySurgical adhesivesSkeletal disorderActive agentBone tissue
A porous β-tricalcium phosphate material for bone implantation is provided. The multiple pores in the porous TCP body are separate discrete voids and are not interconnected. The pore size diameter is in the range of 20-500 μm, preferably 50-125 μm. The porous β-TCP material provides a carrier matrix for bioactive agents and can form a moldable putty composition upon the addition of a binder. Preferably, the bioactive agent is encapsulated in a biodegradable agent. The invention provides a kit and an implant device comprising the porous β-TCP, and a bioactive agent and a binder. The invention also provides an implantable prosthetic device comprising a prosthetic implant having a surface region, a porous β-TCP material disposed on the surface region and optionally comprising at least a bioactive agent or a binder. Methods of producing the porous β-TCP material and inducing bone formation are also provided.
Owner:STRYKER CORP
Biodegradable poly(β-amino esters) and uses thereof
Poly(β-amino esters) prepared from the conjugate addition of bis(secondary amines) or primary amines to a bis(acrylate ester) are described. Methods of preparing these polymers from commercially available starting materials are also provided. These tertiary amine-containing polymers are preferably biodegradable and biocompatible and may be used in a variety of drug delivery systems. Given the poly(amine) nature of these polymers, they are particularly suited for the delivery of polynucleotides. Nanoparticles containing polymer / polynucleotide complexes have been prepared. The inventive polymers may also be used to encapsulate other agents to be delivered. They are particularly useful in delivering labile agents given their ability to buffer the pH of their surroundings.
Owner:MASSACHUSETTS INST OF TECH
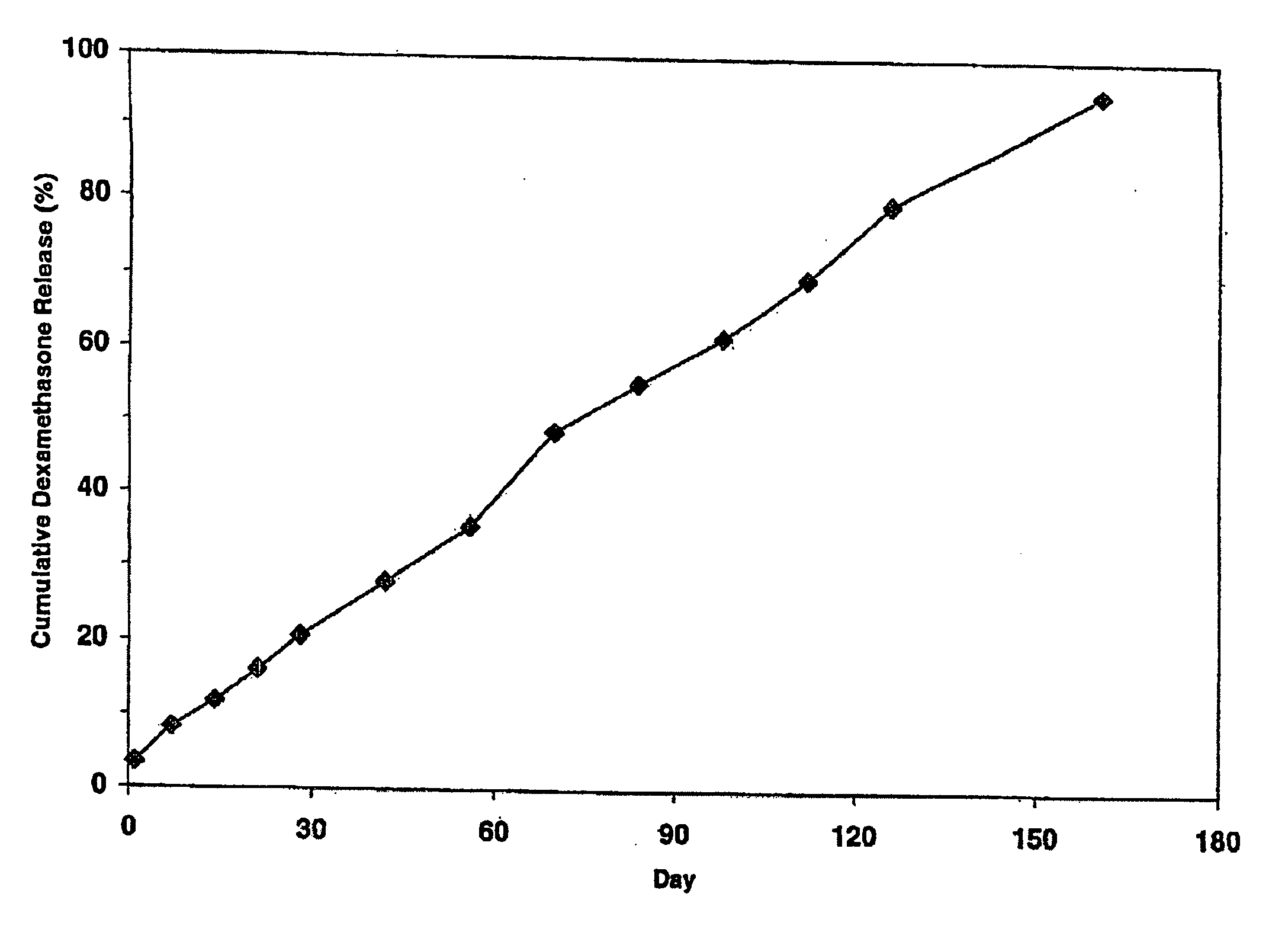
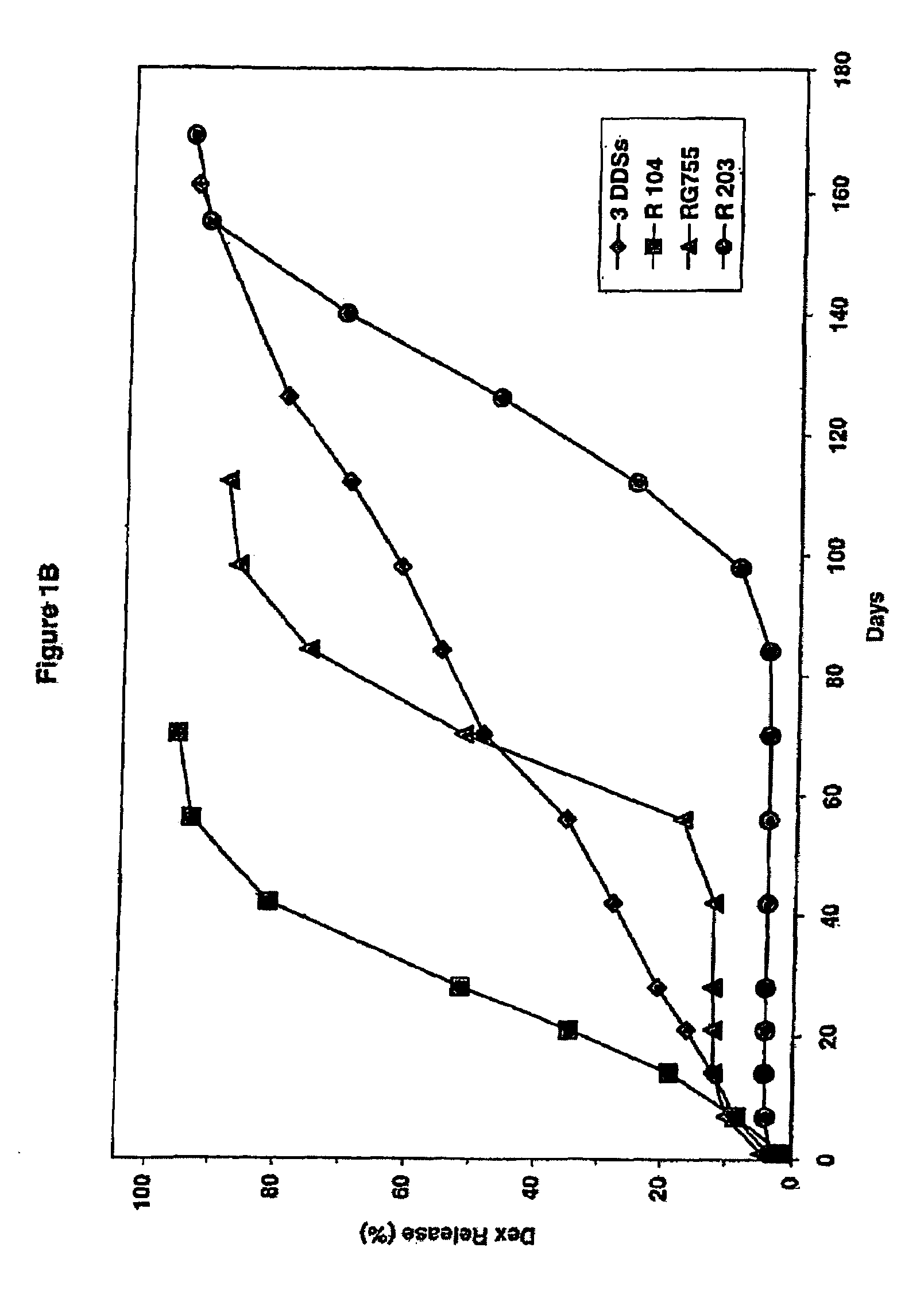
Extended release biodegradable ocular implants
Biodegradable implants sized and suitable for implantation in an ocular region or site and methods for treating ocular conditions. The implants provide an extended release of an active agent at a therapeutically effective amount for a period of time between 50 days and one year, or longer.
Owner:ALLERGAN INC
Microparticles with adsorbent surfaces, methods of making same, and uses thereof
InactiveUS6884435B1Stimulate immune responseEasy to usePowder deliverySsRNA viruses positive-senseAntigenDisease
The present invention is directed to microparticles, to microparticle compositions containing the same, to methods of forming the same, and to uses for the same, including use for a vaccine, for raising an immune response, for treatment of a disease and for diagnosis of a disease. The microparticles comprise a biodegradable polymer, such as a poly(α-hydroxy acid), a polyhydroxy butyric acid, a polycaprolactone, a polyorthoester, a polyanhydride, or a polycyanoacrylate, and a detergent selected from a cationic detergent and an anionic detergent. The microparticles further comprise an antigen adsorbed on the surface of the microparticle.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
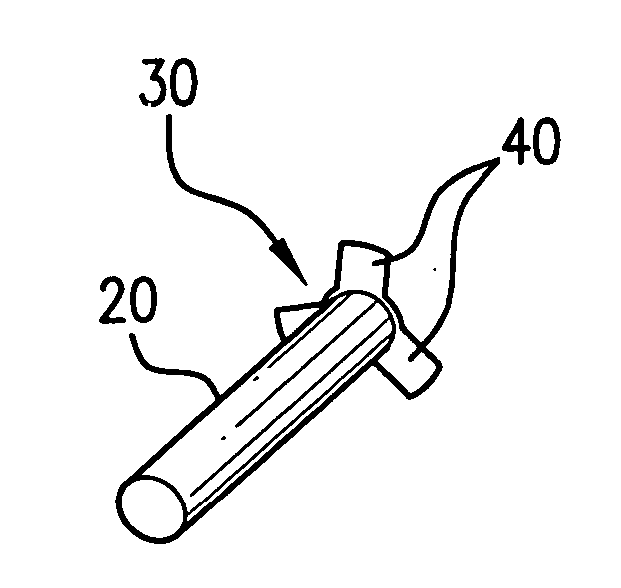
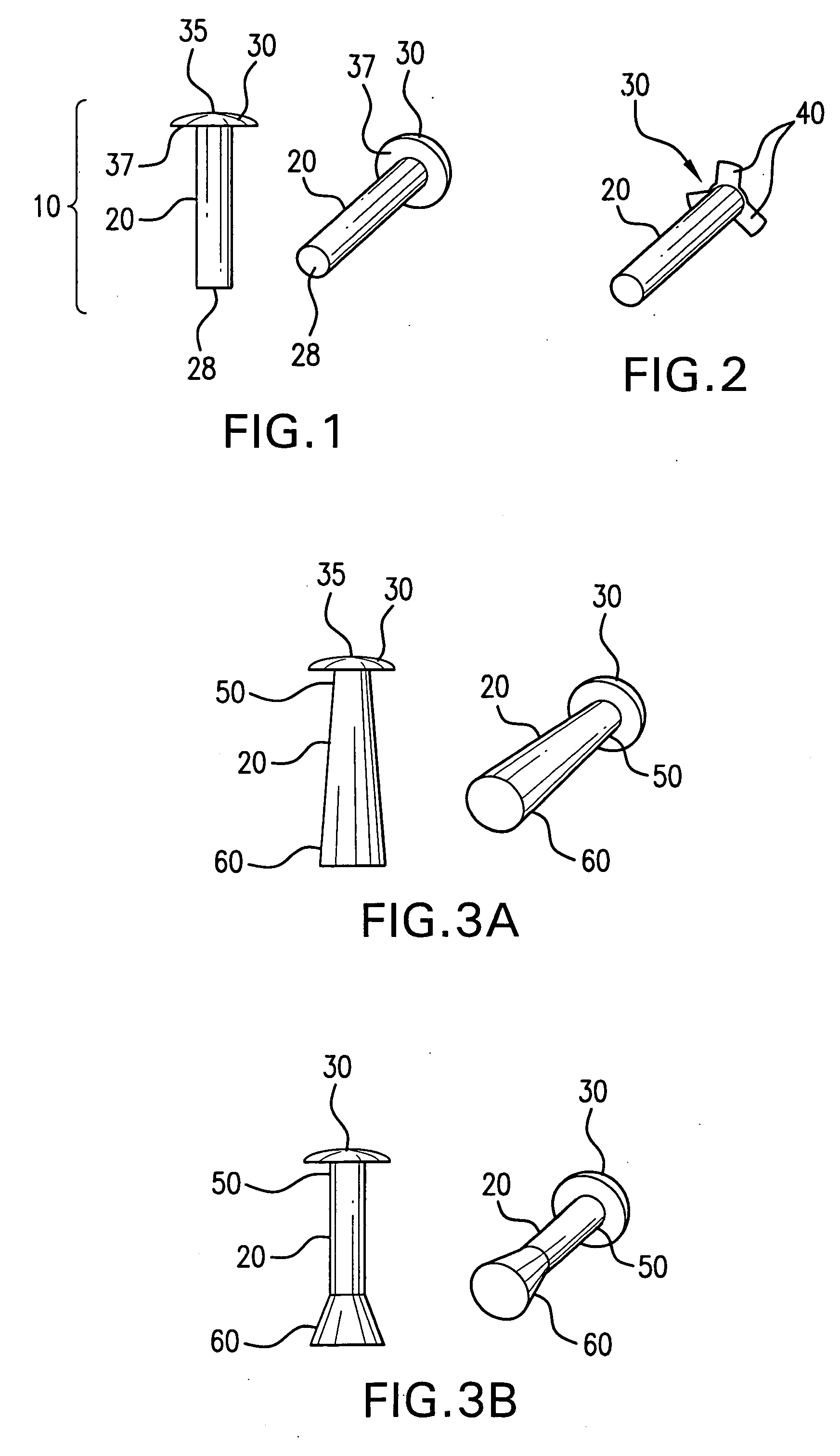
Ocular plug formed from placenta derived collagen biofabric
The present invention relates to ocular plugs formed from a biodegradable material. The plugs comprises a shaft and, optionally, a cap. The ocular plugs are intended to occlude, and to repair, discontinuities in the sclera, whether formed deliberately during injection or surgical foray into the eye, or accidentally. The method further provides methods of making the ocular plug. the invention also provides methods of using the ocular plugs to occlude and repair discontinuities in the sclera, or to deliver biologically active compounds to the sclera or the eye. Finally, the invention provides kits comprising one or more ocular plugs in a container.
Owner:LIU QING +1
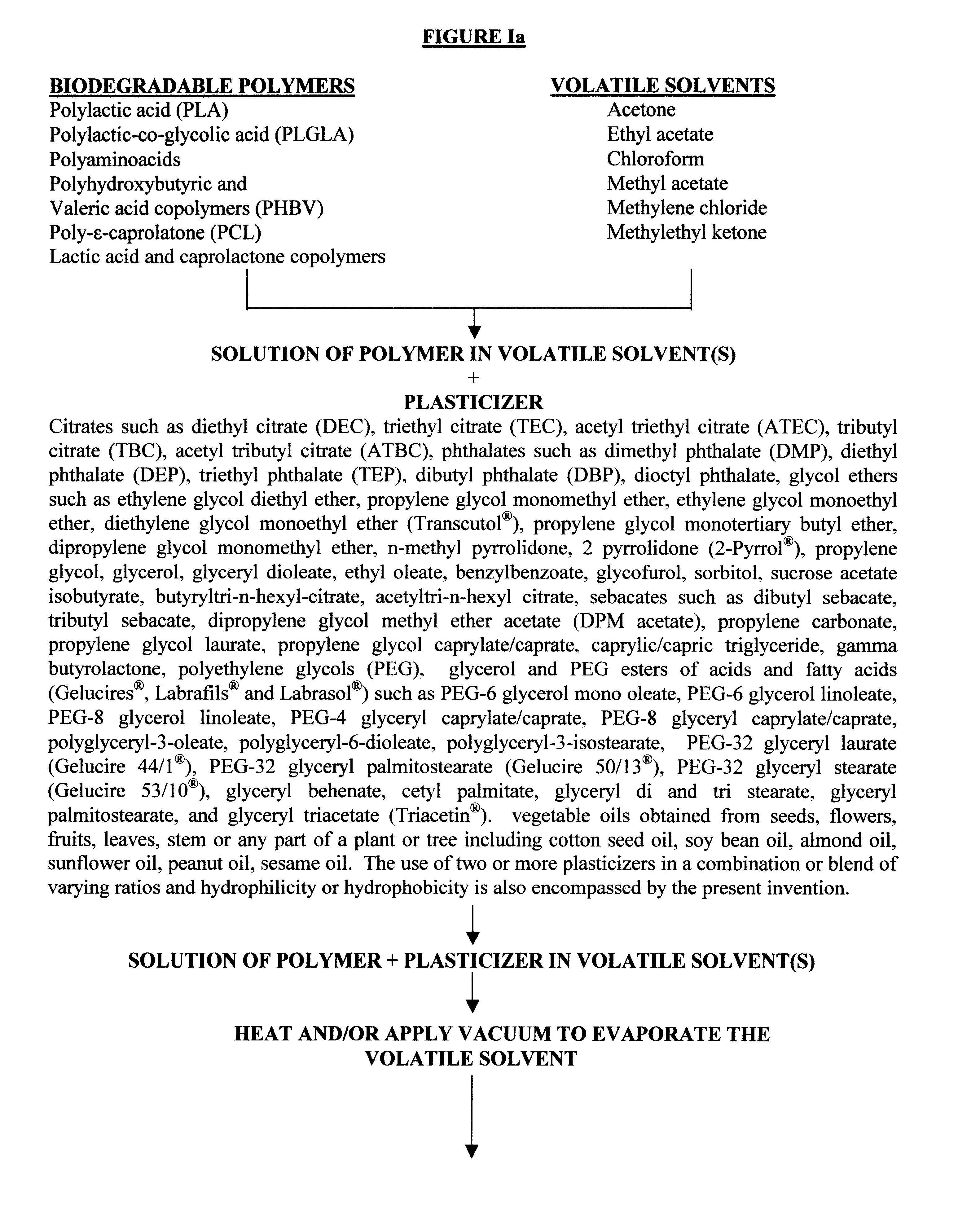
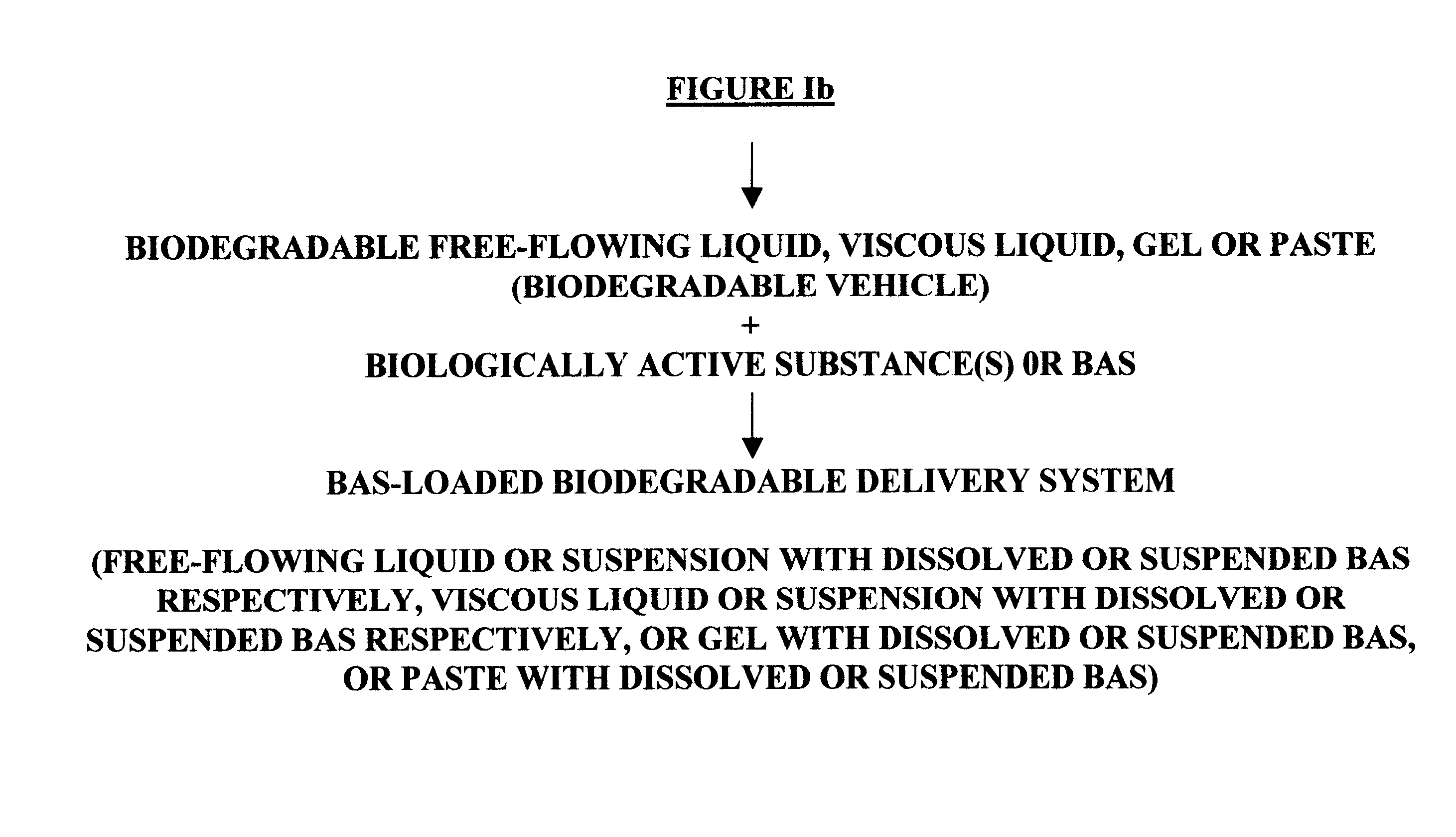
Biodegradable vehicle and filler
InactiveUS6432438B1Improve stabilityWell mixedOrganic active ingredientsPharmaceutical delivery mechanismPolymer sciencePlasticizer
A biodegradable vehicle and filler (referred to in this invention as biodegradable vehicle), which can be mixed with one or more biologically active substances (BAS), or can be used as a biodegradable filler to fill in cavities or body tissues in animals, birds and humans. The consistency and rheology, hydrophilicity and hydrophobicity, and in vivo degradation rates of the biodegradable vehicle is controlled by modulating the molecular weight of polymers and copolymers, concentration of plasticizers, ratios of two or more plasticizer in the blends, types of polymers and copolymers, copolymer ratios, and ratios of blends of polymers with different molecular weights or different copolymers. The biodegradable vehicle is mixed with one or more BAS (which is separately stored away from the biodegradable vehicle in an appropriate container) just prior to use. Mixing of the BAS with the biodegradable vehicle can be accomplished by simply stirring the mixture with a stirring device, or by triturating the mixture or employing an ointment mill or a suitable device or apparatus or equipment that can be used for blending / mixing. Alternatively, a device, which resembles two syringes, attached together with a removable partition or a valve assembly can also be used to uniformly mix the BAS with the biodegradable vehicle. The mixing is performed in order to dissolve or uniformly suspend the BAS particles in the biodegradable vehicle. Modulating the polymer to plasticizer ratio, polymer molecular weight, copolymer ratio, and hydrophobicity and hydrophilicity of the plasticizer controls the release of the BAS from the biodegradable vehicle.
Owner:SHUKLA ATUL J
Biodegradable injectable implants and related methods of manufacture and use
InactiveUS20030093157A1Broaden applicationSolution deliveryPharmaceutical non-active ingredientsGlycolic acidImplant
This invention is directed to the field of medical implants, and more specifically to biodegradable injectable implants and their methods of manufacture and use. The injectable implants disclosed herein comprise glycolic acid and bio-compatible / bio-absorbable polymeric particles containing a polymer of lactic acid. The particles are small enough to be injected through a needle but large enough to avoid engulfment by macrophages. The injectables of this invention may be in a pre-activated solid form or an activated form (e.g., injectable suspension or emulsion).
Owner:MEDGRAFT MICROTECH
Tissue engineering composite
InactiveUS6991652B2Facilitate formationNon-invasive methodBiocideCosmetic implantsTissue engineeringDamages tissue
The invention provides a biocompatible composite for use in a living subject for purposes of repairing damaged tissues and reconstructing a new tissue. The composite includes a biodegradable or absorbable three-dimensional support construct, a liquid or viscous fluid forming a gel matrix or viscous fluid when delivered to an area of interest in a living subject. The biodegradable construct provides an ideal surface for cell or cell extract attachment, while the gel matrix or viscous fluid acts as both a carrier material and a separator for maintaining the space between the constructs as well as the structural integrity of the developing issue.
Owner:CLEMSON UNIV RES FOUND +1
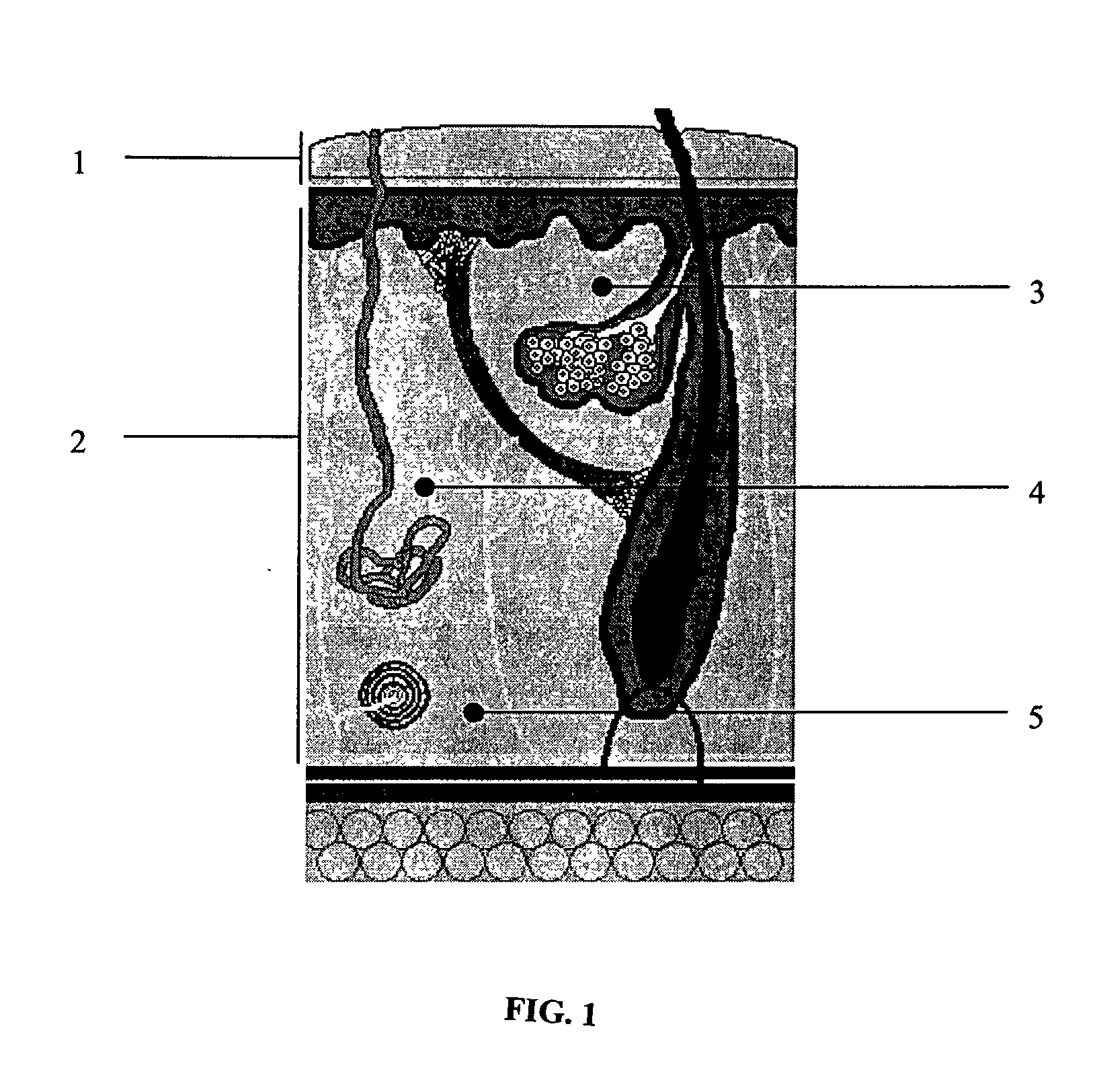
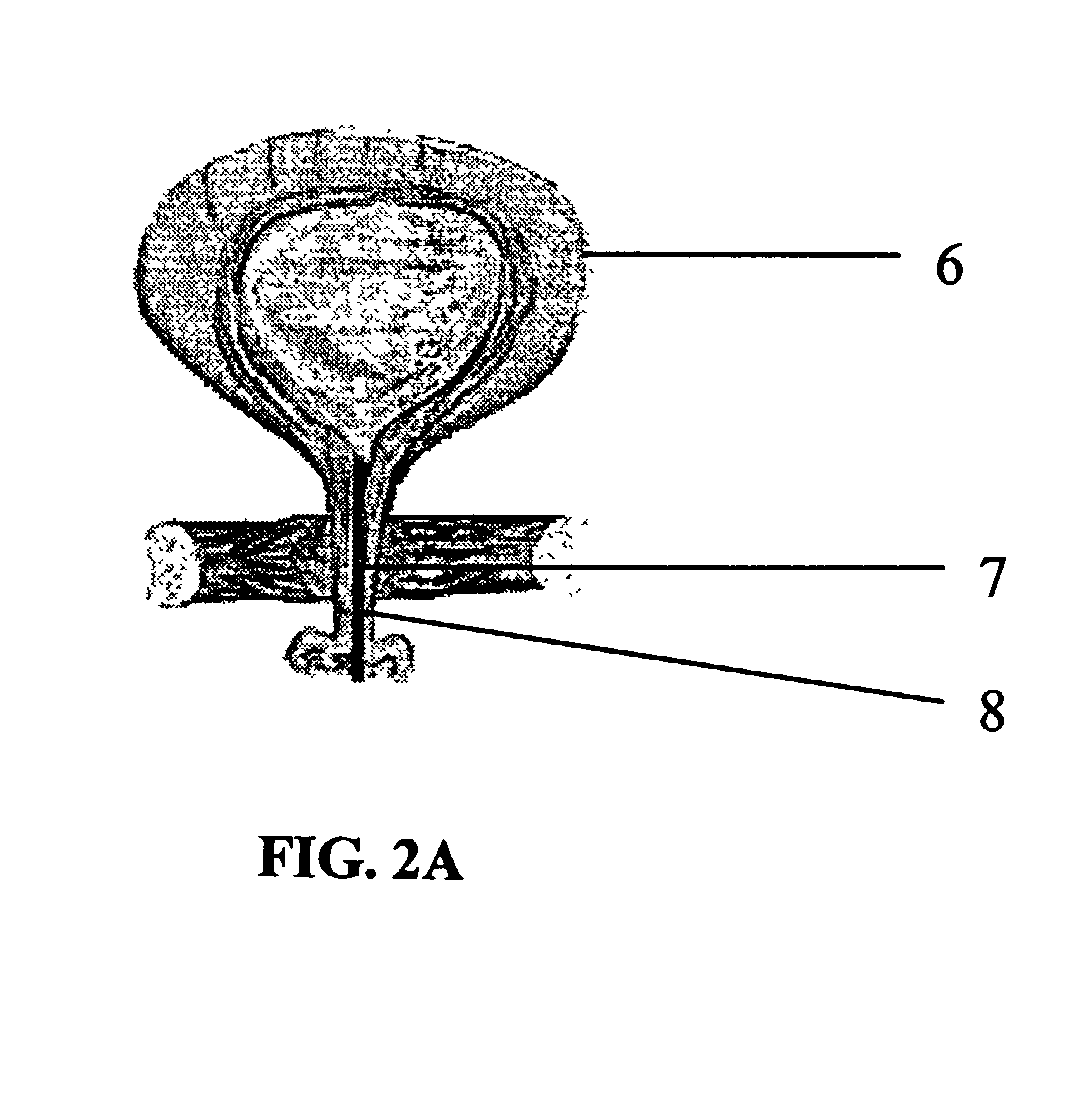
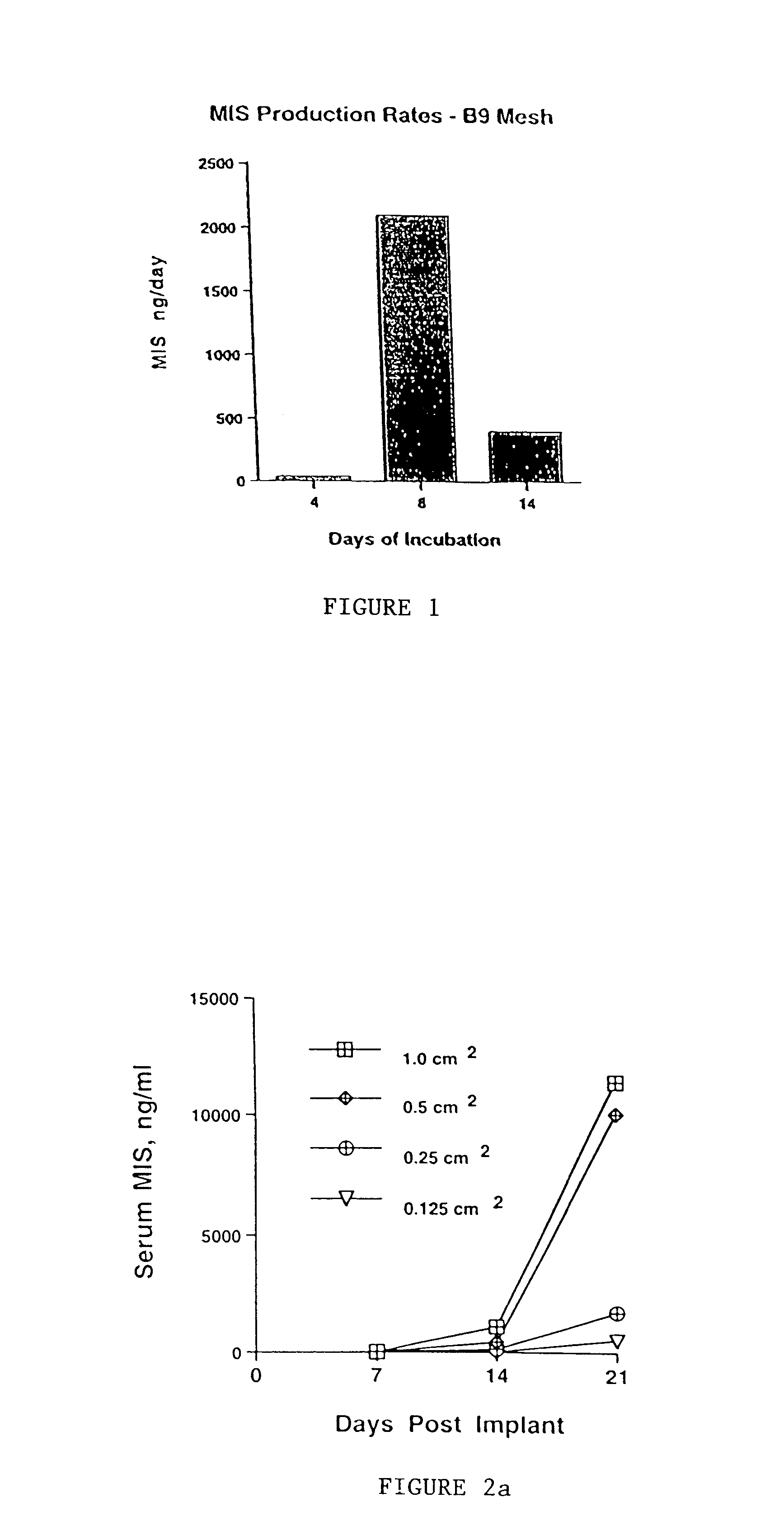
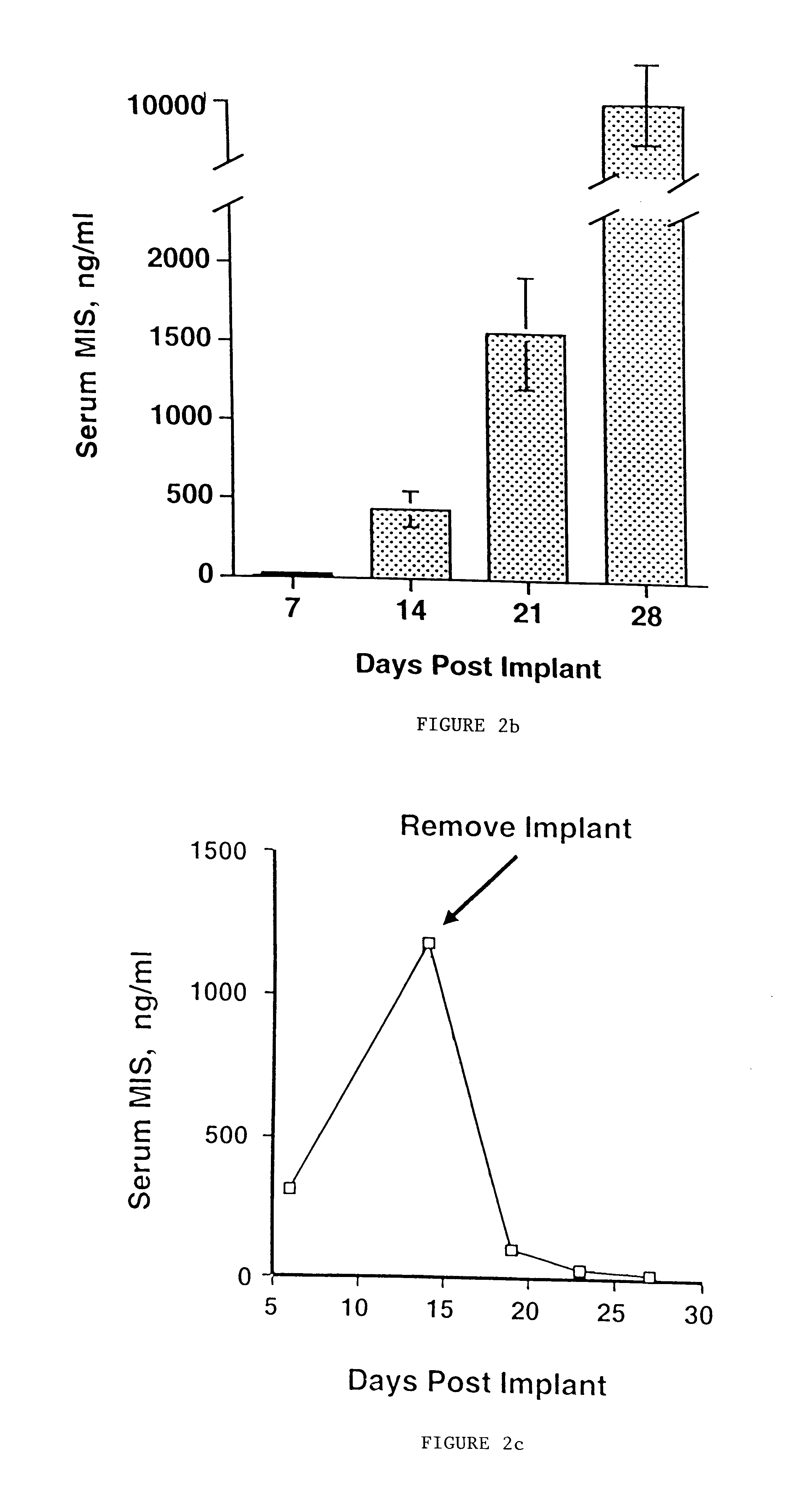
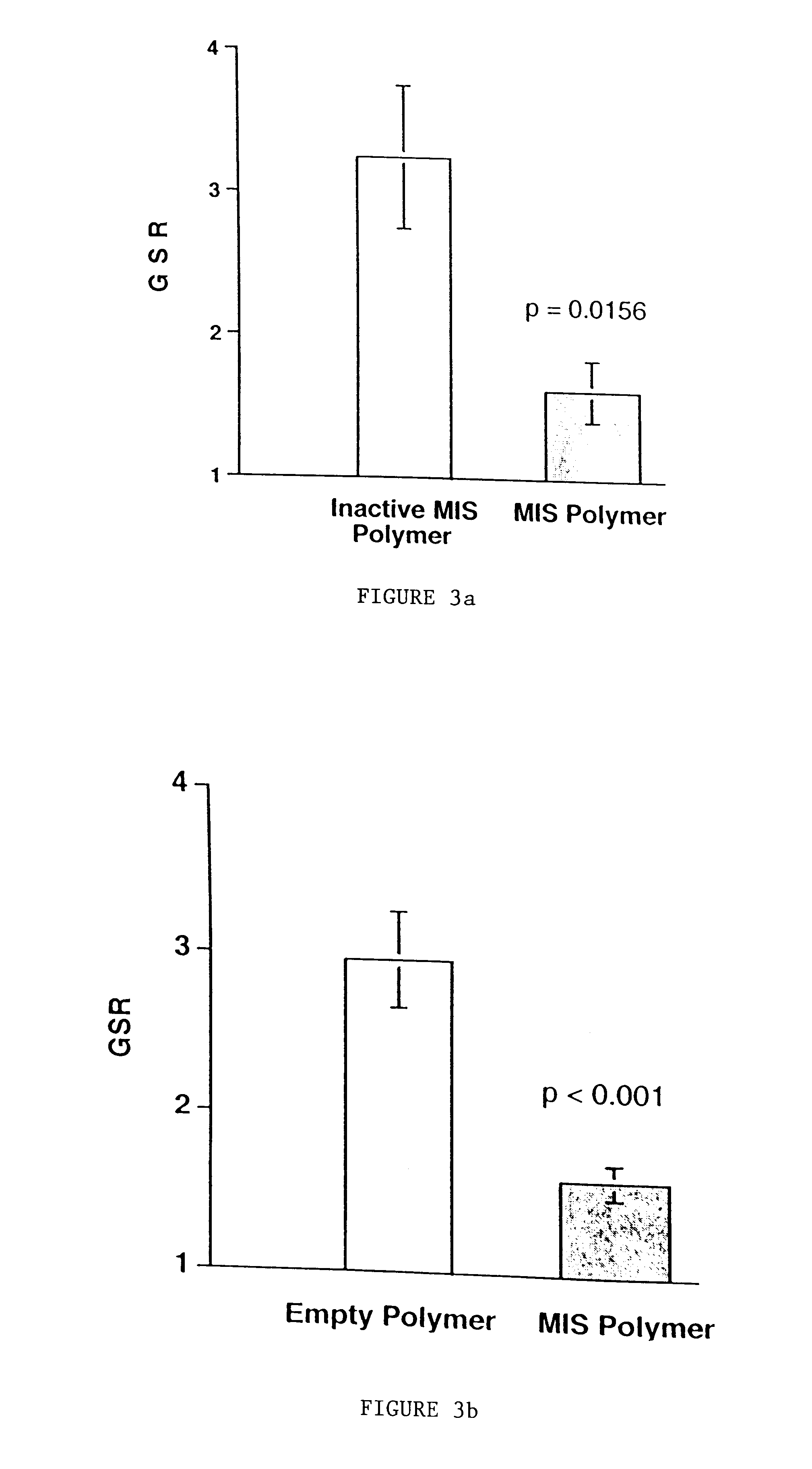
Delivery of therapeutic biologicals from implantable tissue matrices
Normal cells, such as fibroblasts or other tissue or organ cell types, are genetically engineered to express biologically active, therapeutic agents, such as proteins that are normally produced in small amounts, for example, MIS, or other members of the TGF-beta family Herceptin(TM), interferons, andanti-angiogenic factors. These cells are seeded into a matrix for implantation into the patient to be treated. Cells may also be engineered to include a lethal gene, so that implanted cells can be destroyed once treatment is completed. Cells can be implanted in a variety of different matrices. In a preferred embodiment, these matrices are implantable and biodegradable over a period of time equal to or less than the expected period of treatment, when cells engraft to form a functional tissue producing the desired biologically active agent. Implantation may be ectopic or in some cases orthotopic. Representative cell types include tissue specific cells, progenitor cells, and stem cells. Matrices can be formed of synthetic or natural materials, by chemical coupling at the time of implantation, using standard techniques for formation of fibrous matrices from polymeric fibers, and using micromachining or microfabrication techniques. These devices and strategies are used as delivery systems via standard or minimally invasive implantation techniques for any number of parenterally deliverable recombinant proteins, particularly those that are difficult to produce in large amounts and / or active forms using conventional methods of purification, for the treatment of a variety of conditions that produce abnormal growth, including treatment of malignant and benign neoplasias, vascular malformations (hemangiomas), inflammatory conditions, keloid formation, abdominal or plural adhesions, endometriosis, congenital or endocrine abnormalities, and other conditions that can produce abnormal growth such as infection. Efficacy of treatment with the therapeutic biologicals is detected by determining specific criteria, for example, cessation of cell proliferation, regression of abnormal tissue, or cell death, or expression of genes or proteins reflecting the above.
Owner:THE GENERAL HOSPITAL CORP
Biodegradable polyurethanes and use thereof
InactiveUS20050013793A1Improve responseIncrease ratingsCell culture supports/coatingSkeletal/connective tissue cellsPolymer scienceDrug biological activity
A biodegradable and biocompatible polyurethane composition synthesized by reacting isocyanate groups of at least one multifunctional isocyanate compound with at least one bioactive agent having at least one reactive group —X which is a hydroxyl group (—OH) or an amine group (—NH2). The polyurethane composition is biodegradable within a living organism to biocompatible degradation products including the bioactive agent. Preferably, the released bioactive agent affects at least one of biological activity or chemical activity in the host organism. A biodegradable polyurethane composition includes hard segments and soft segments. Each of the hard segments is preferably derived from a diurea diol or a diester diol and is preferably biodegradable into biomolecule degradation products or into biomolecule degradation products and a biocompatible diol. Another biodegradable polyurethane composition includes hard segments and soft segments. Each of the hard segments is derived from a diurethane diol and is biodegradable into biomolecule degradation products.
Owner:CARNEGIE MELLON UNIV +1
Conveniently implantable sustained release drug compositions
InactiveUS20080038316A1Economical and practical and efficientEasy to produceBiocideOrganic active ingredientsDiseaseSustained release drug
This invention provides for biocompatible and biodegradable syringeable liquid, implantable solid, and injectable gel pharmaceutical formulations useful for the treatment of systemic and local disease states.
Owner:RAMSCOR
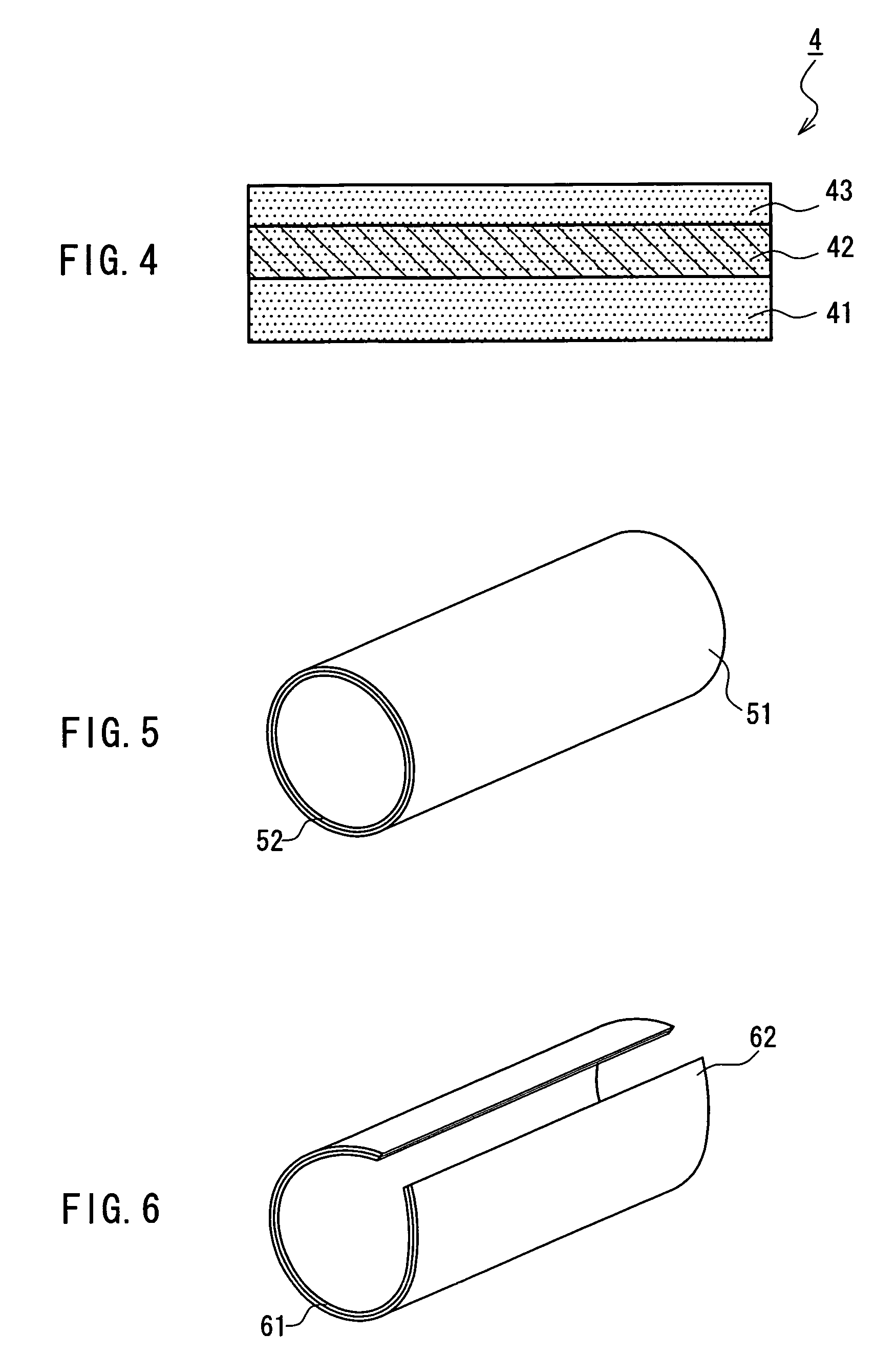
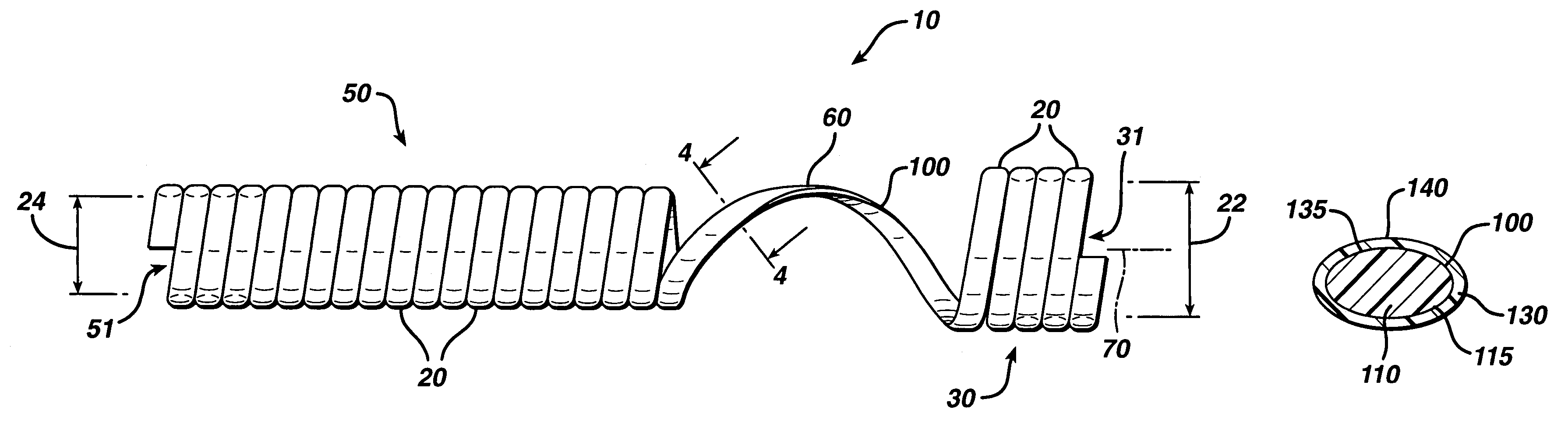
Drug-eluting biodegradable stent
The present invention relates to a drug-loaded biodegradable stent and methods for treating vulnerable plaques of a patient comprising a plurality of layers or zones, each layer or zone comprising its own specific biodegradation rate and its specific drug loading characteristics. In one embodiment, the layers and zones are configured and arranged, in combination, radially, circumferentially and longitudinally.
Owner:GP MEDICAL
Biodegradable injectable implants containing glycolic acid
InactiveUS7314636B2Non-migratoryEasy to moveSolution deliveryPharmaceutical non-active ingredientsEmulsionGlycolic acid
Owner:MEDGRAFT MICROTECH
Biodegradable stent
Owner:ETHICON INC
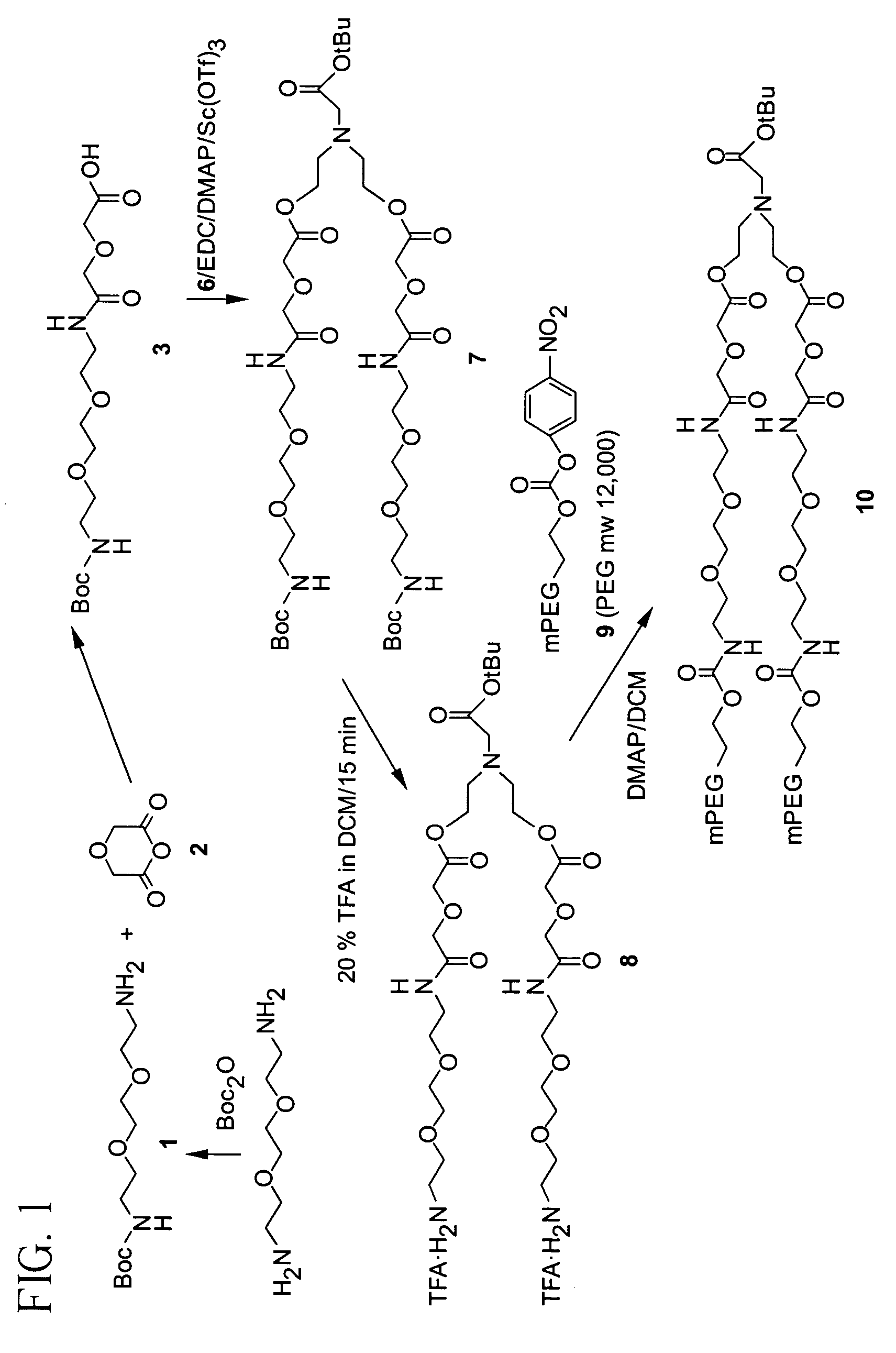
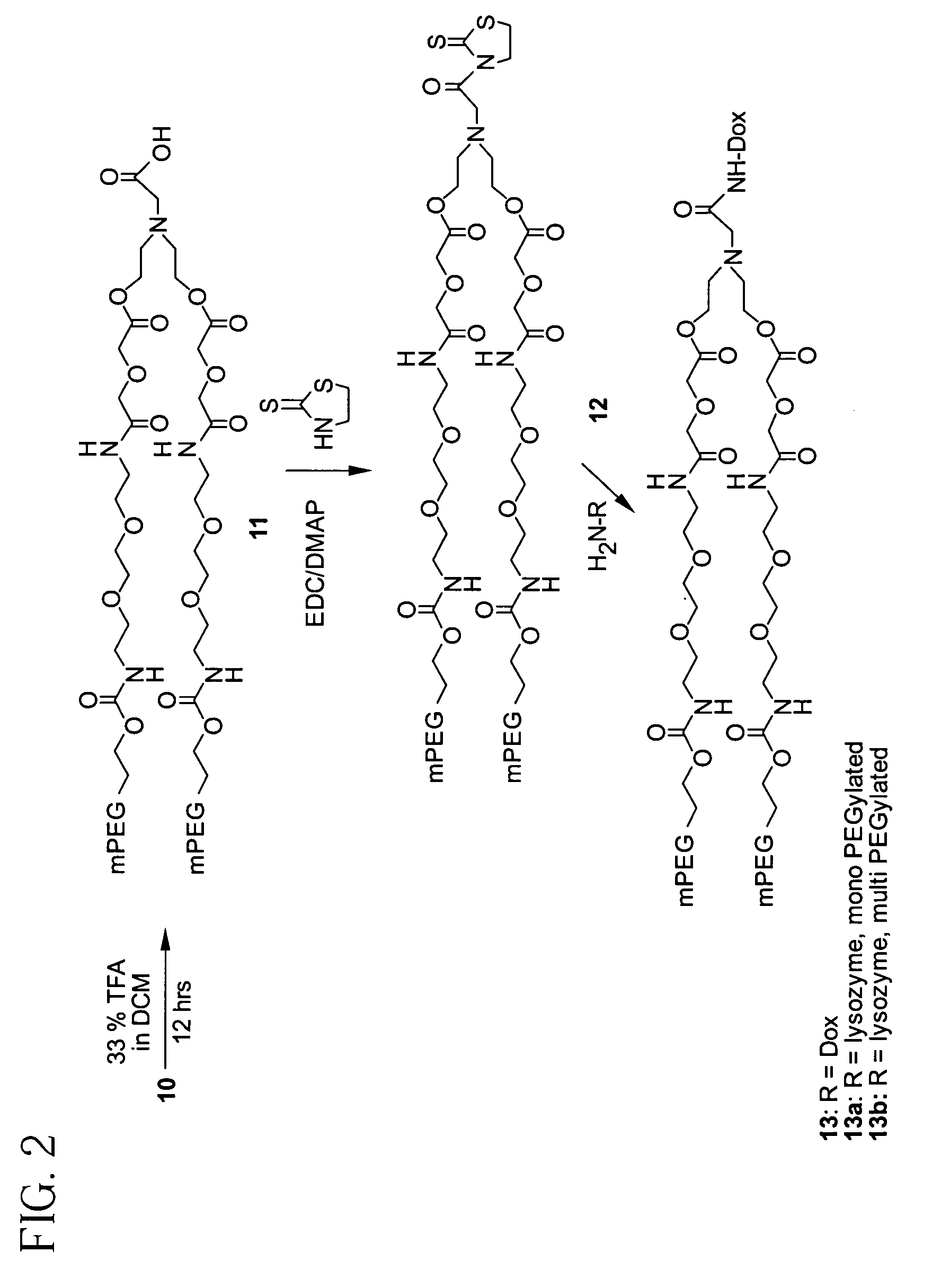
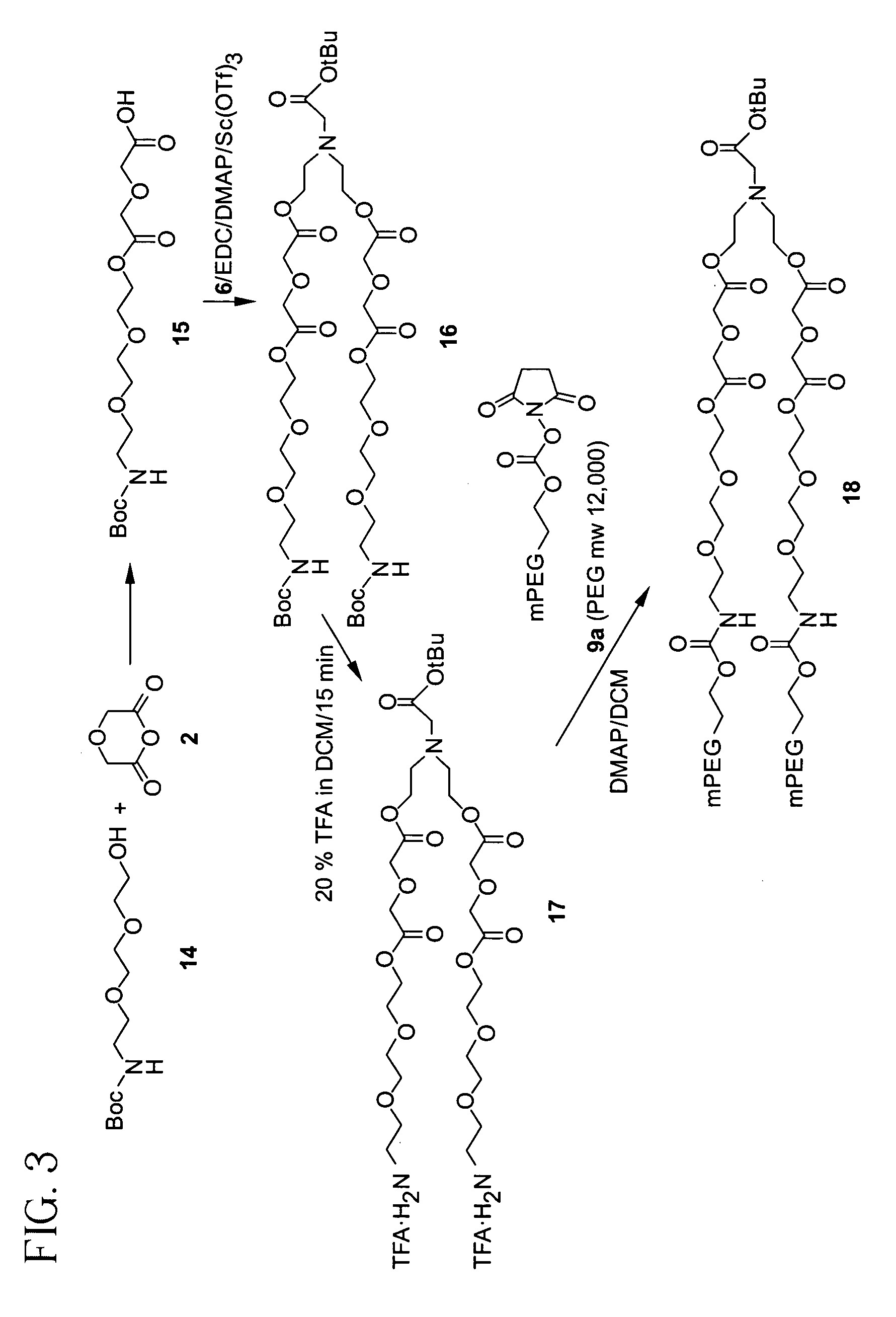
Releasable polymeric conjugates based on aliphatic biodegradable linkers
InactiveUS7087229B2Modulate the rate of hydrolysis of the prodrug and/or cellular uptakeGood water solubilityBiocideEther/acetal active ingredientsPolymerStereochemistry
Owner:BELROSE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com