Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
25884results about How to "Good biocompatibility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
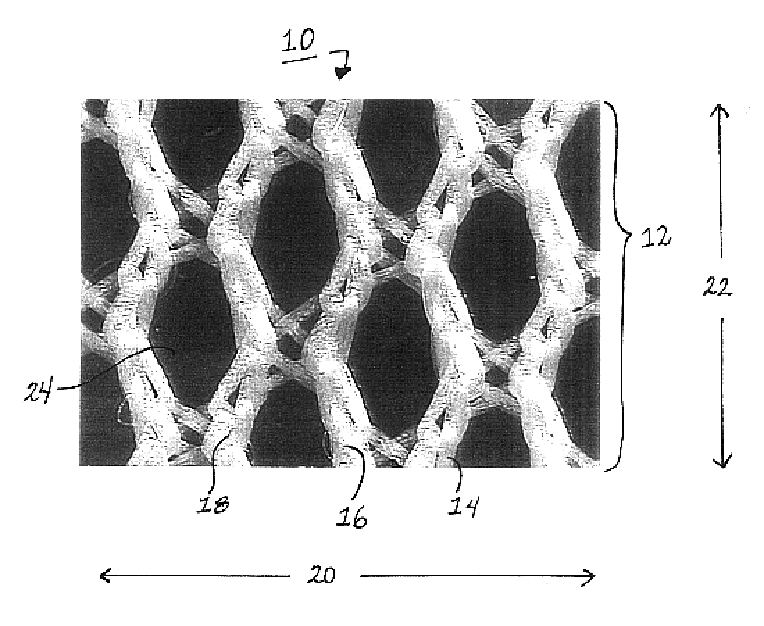
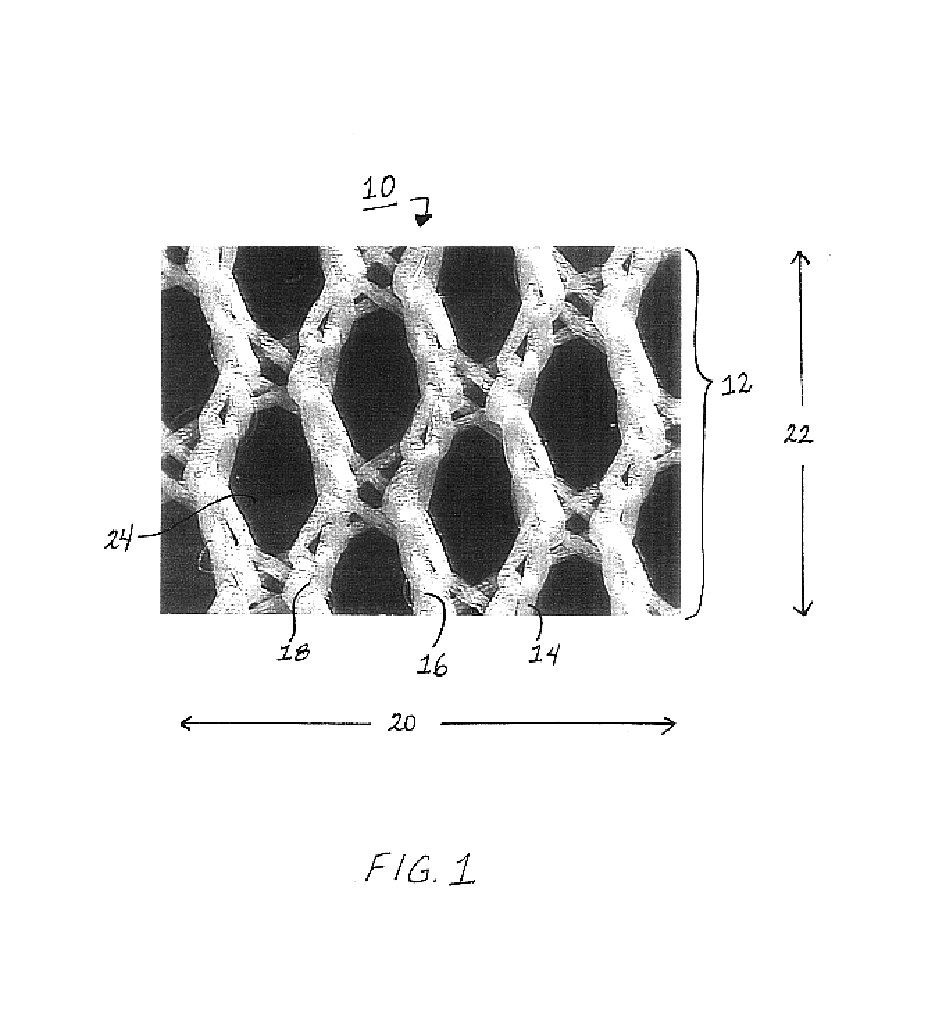
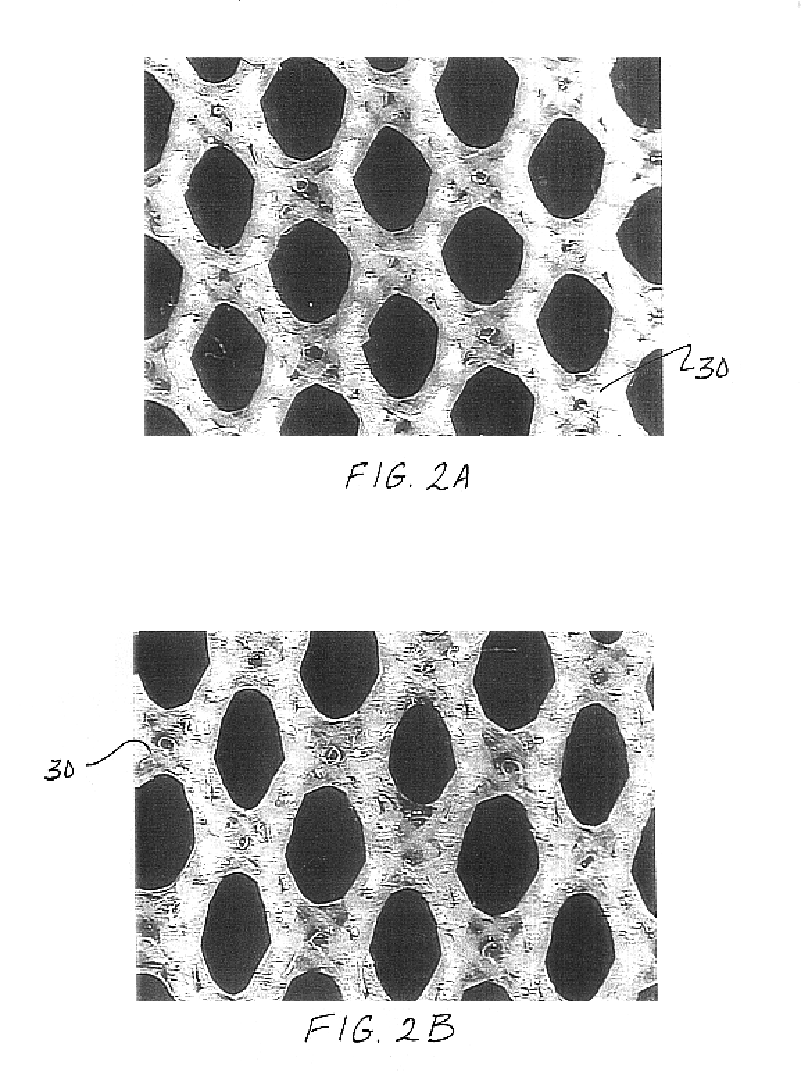
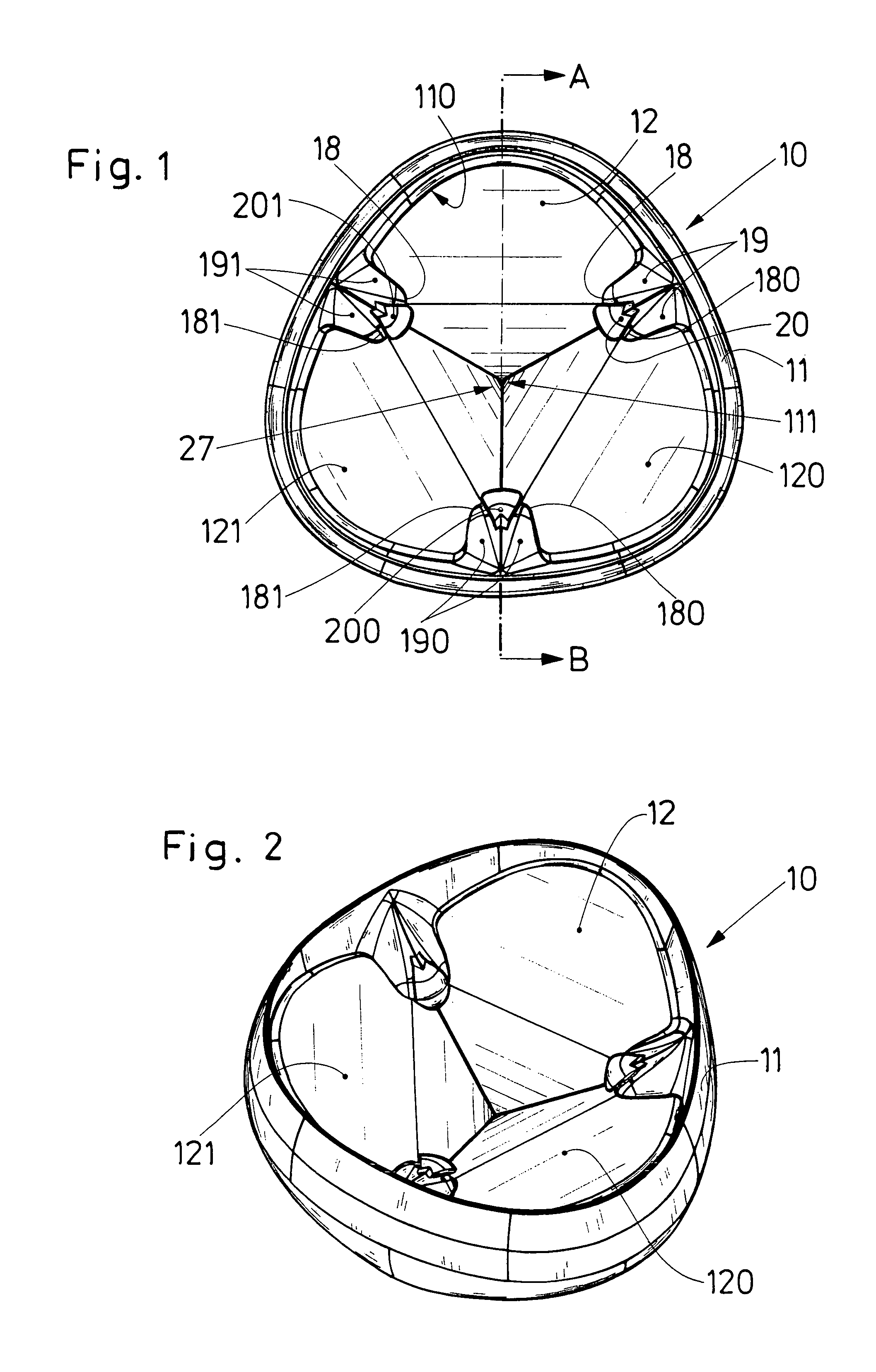
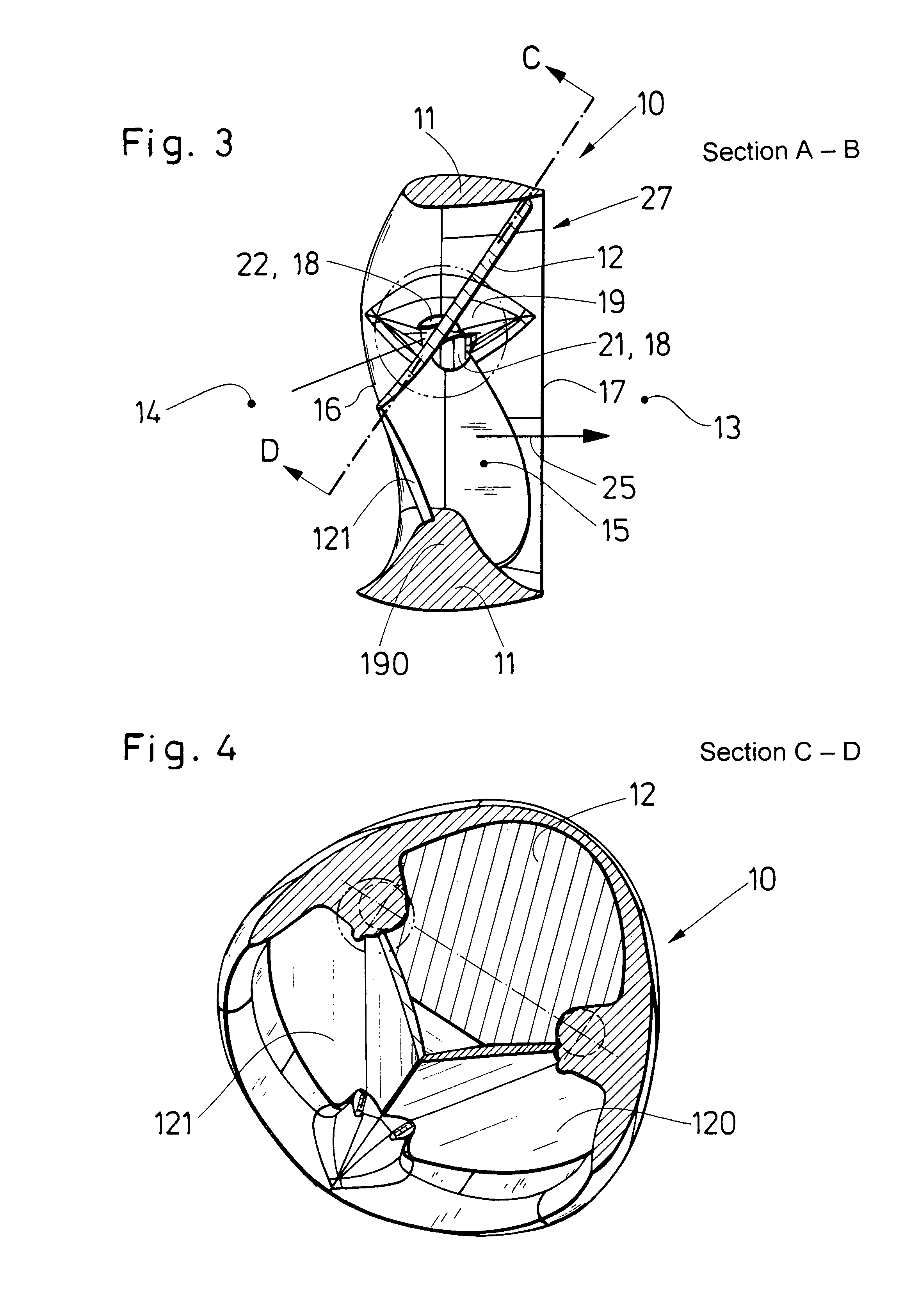
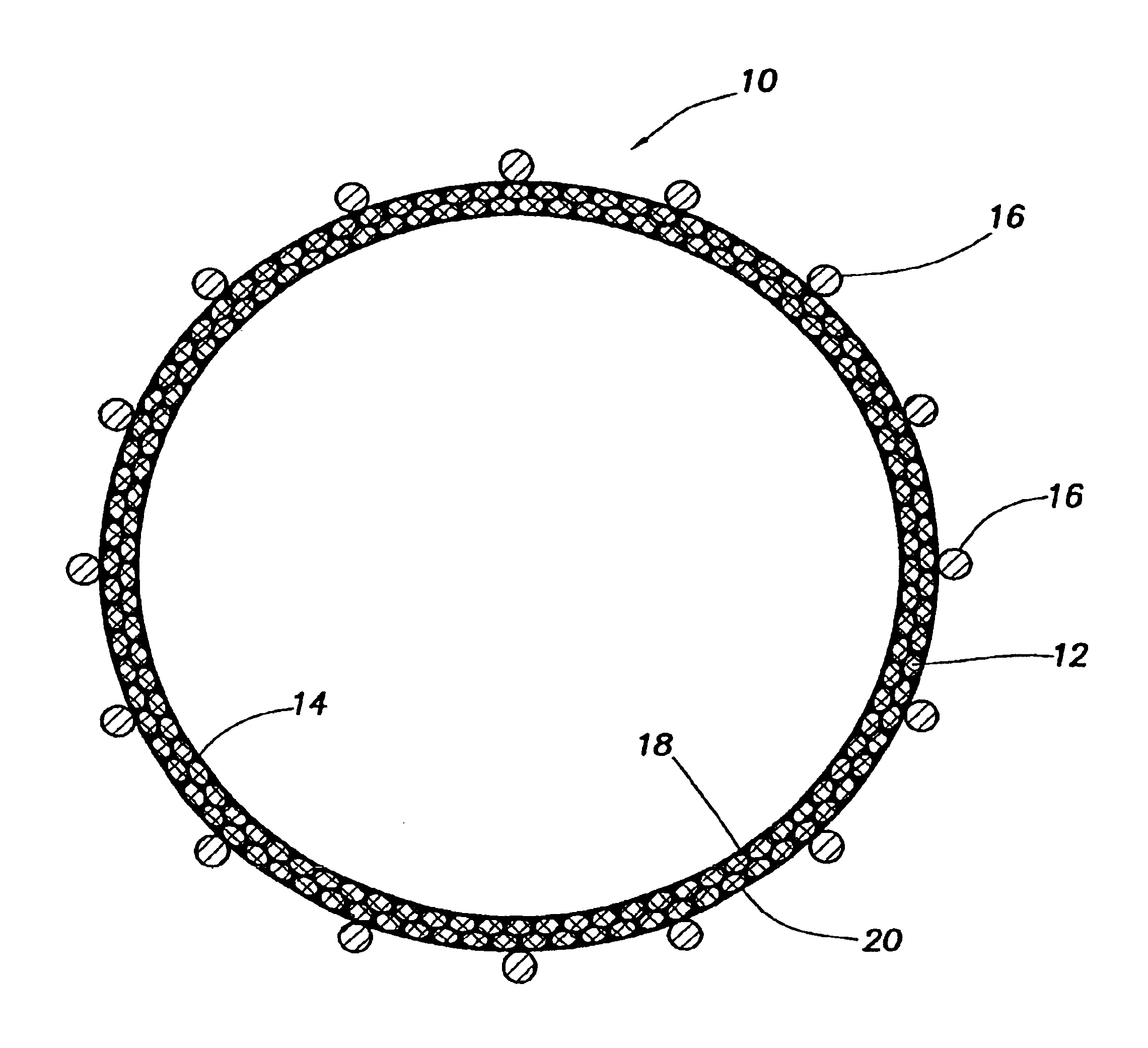
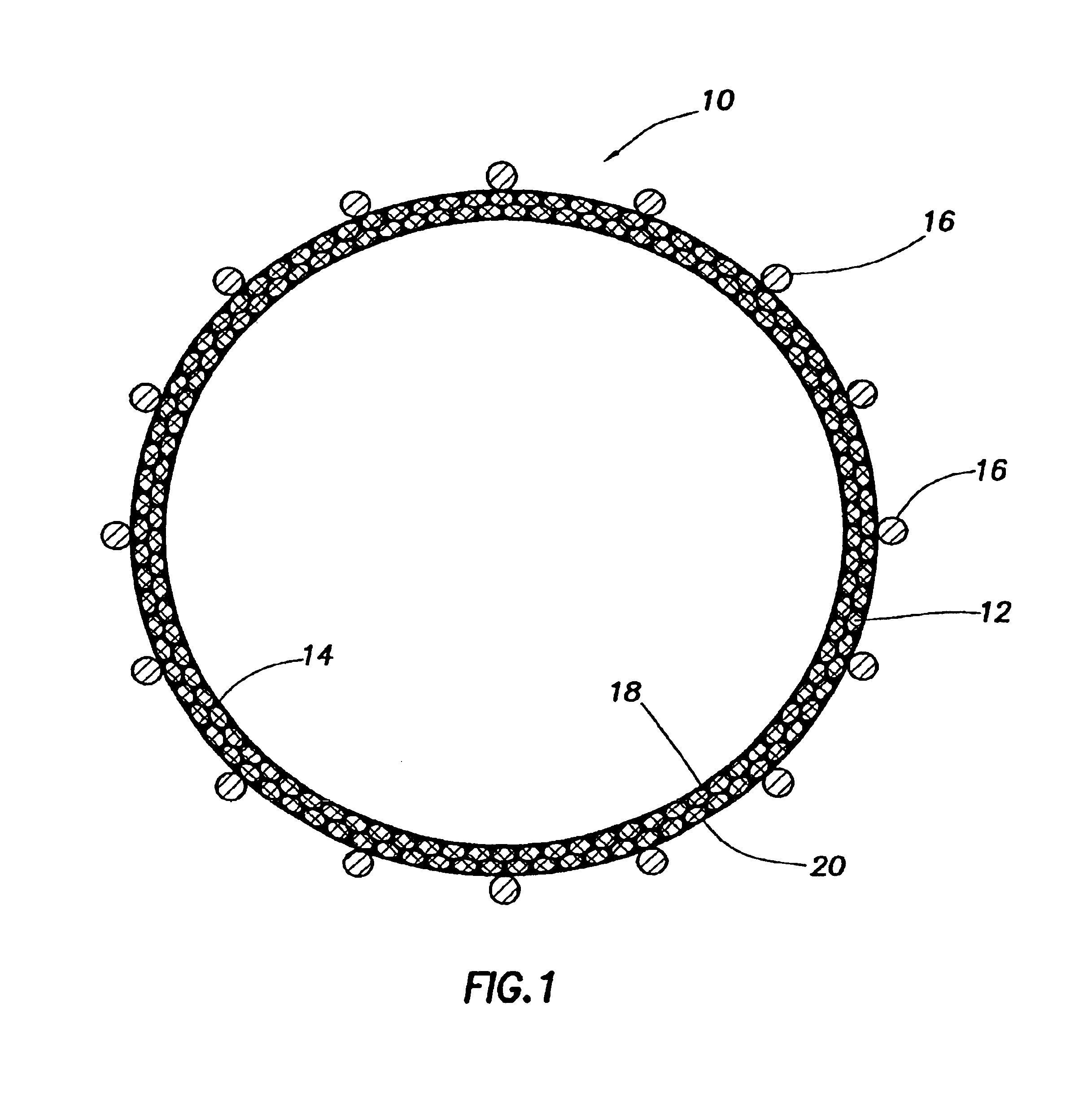
Medical film
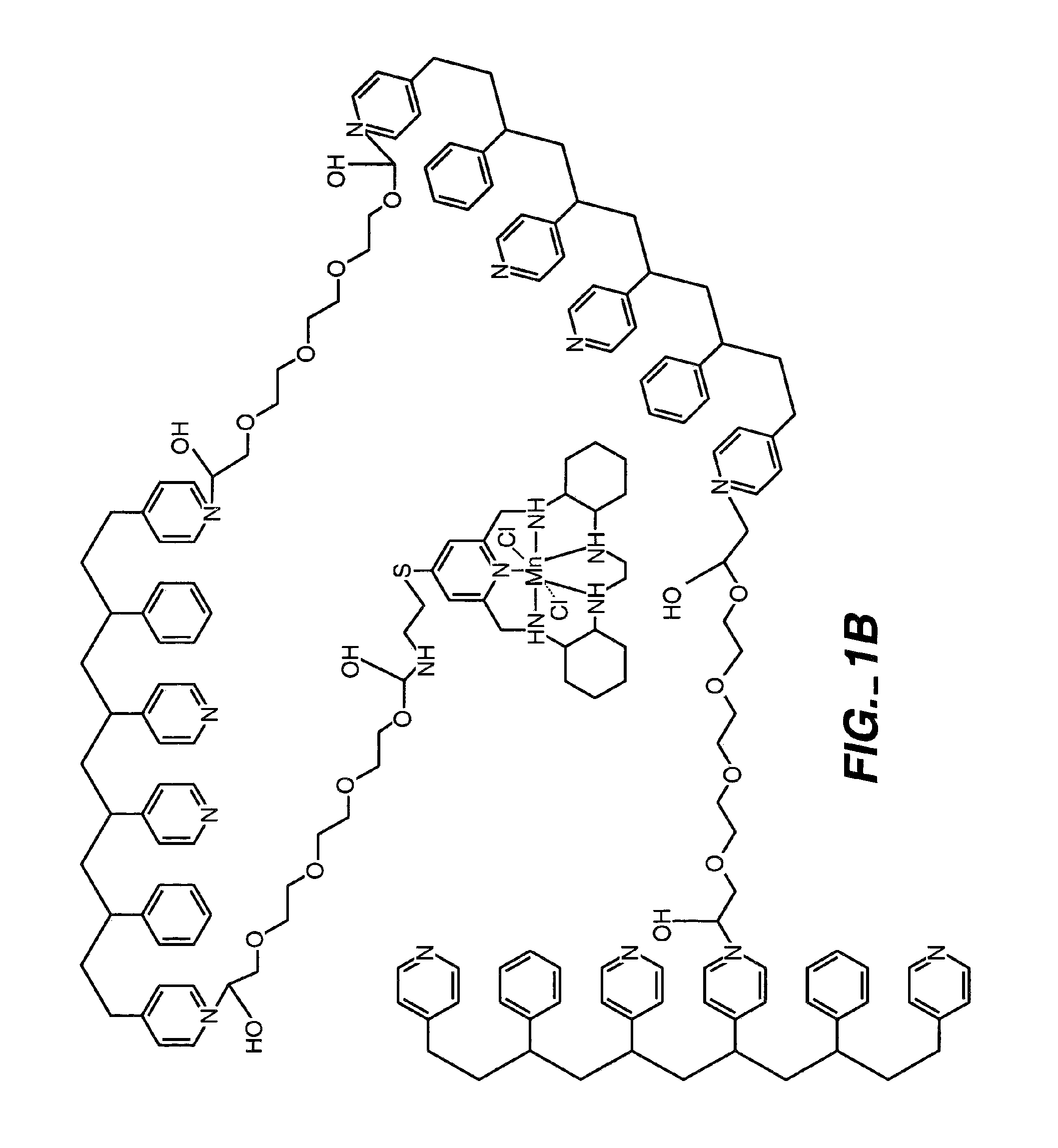
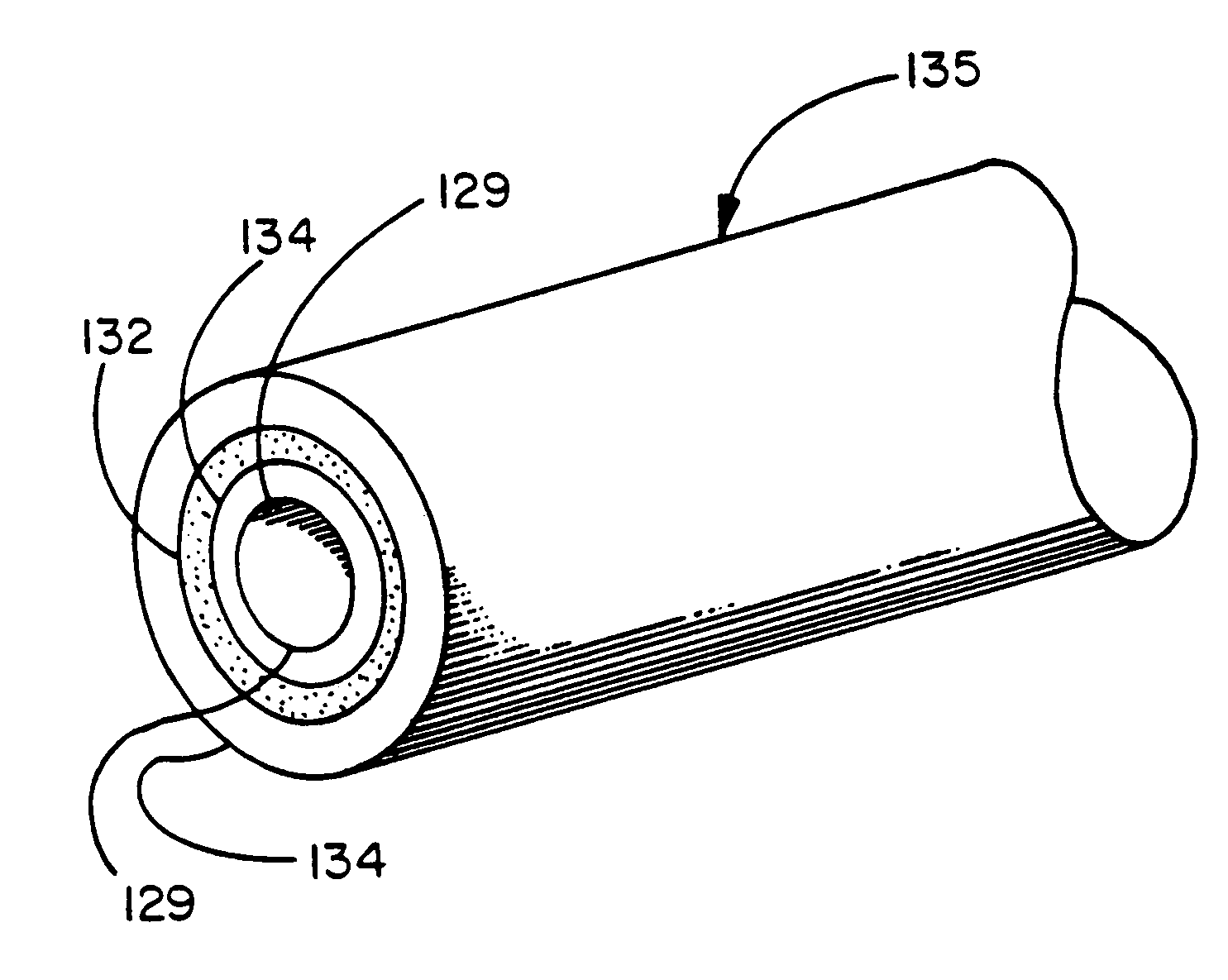
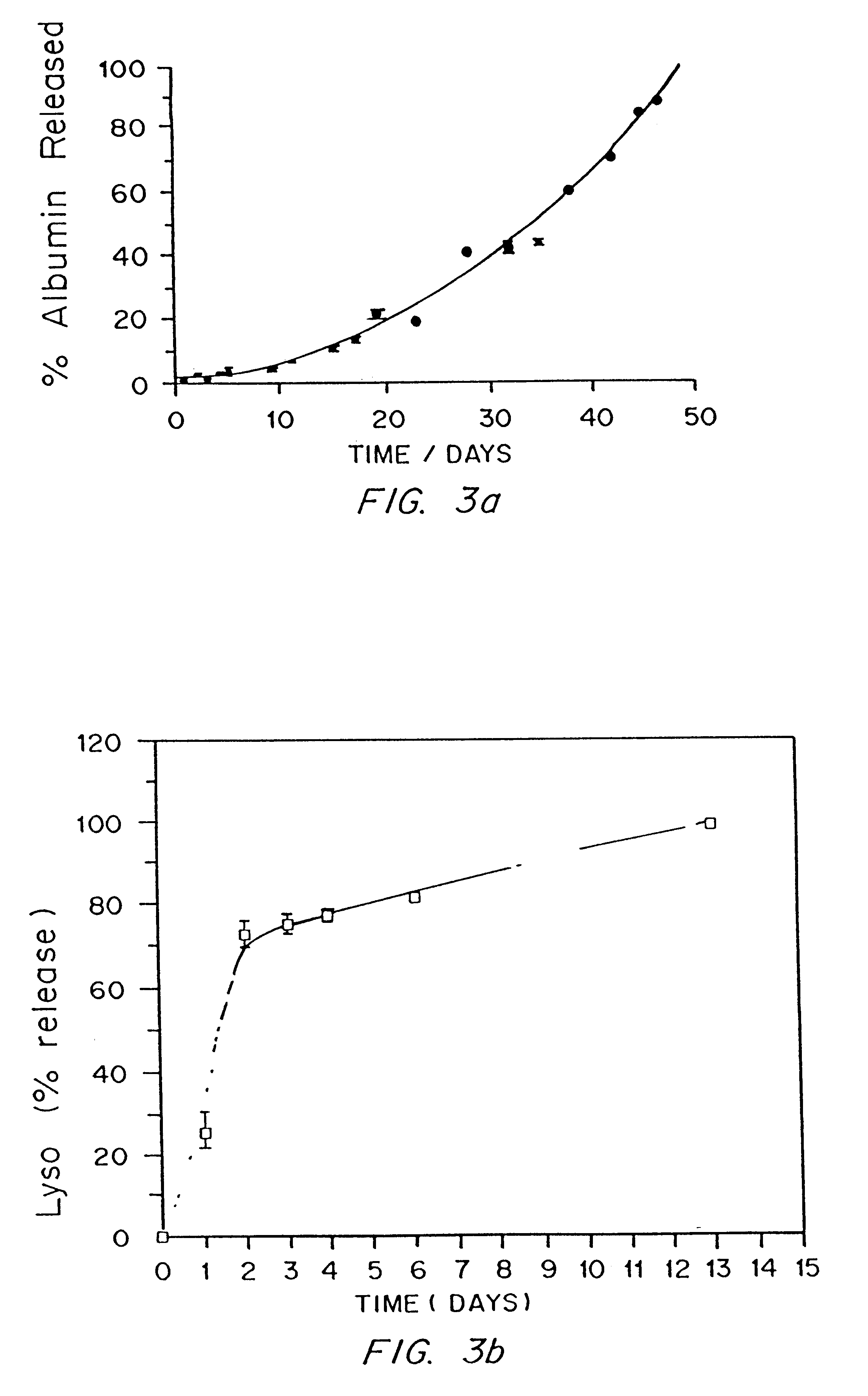
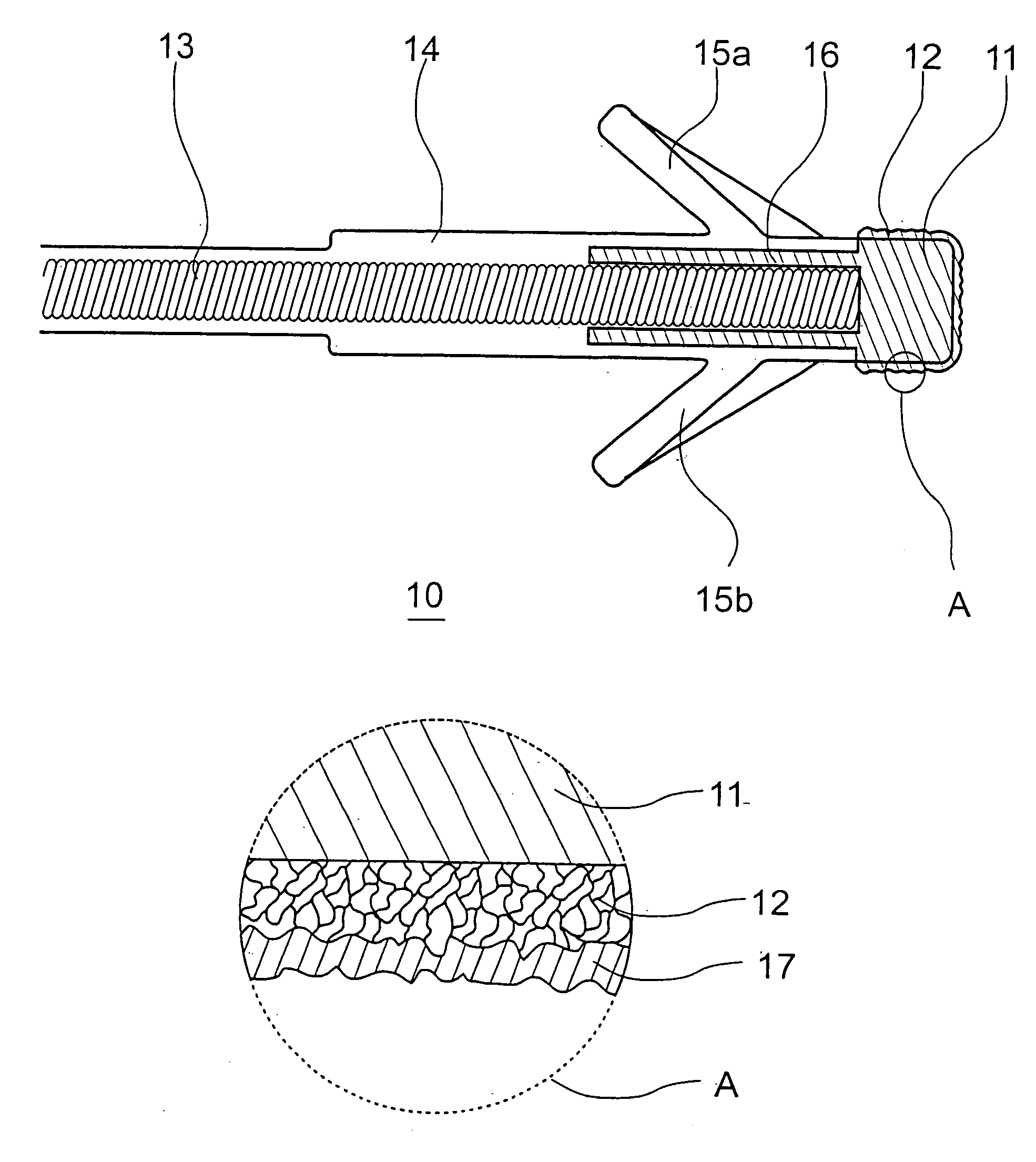
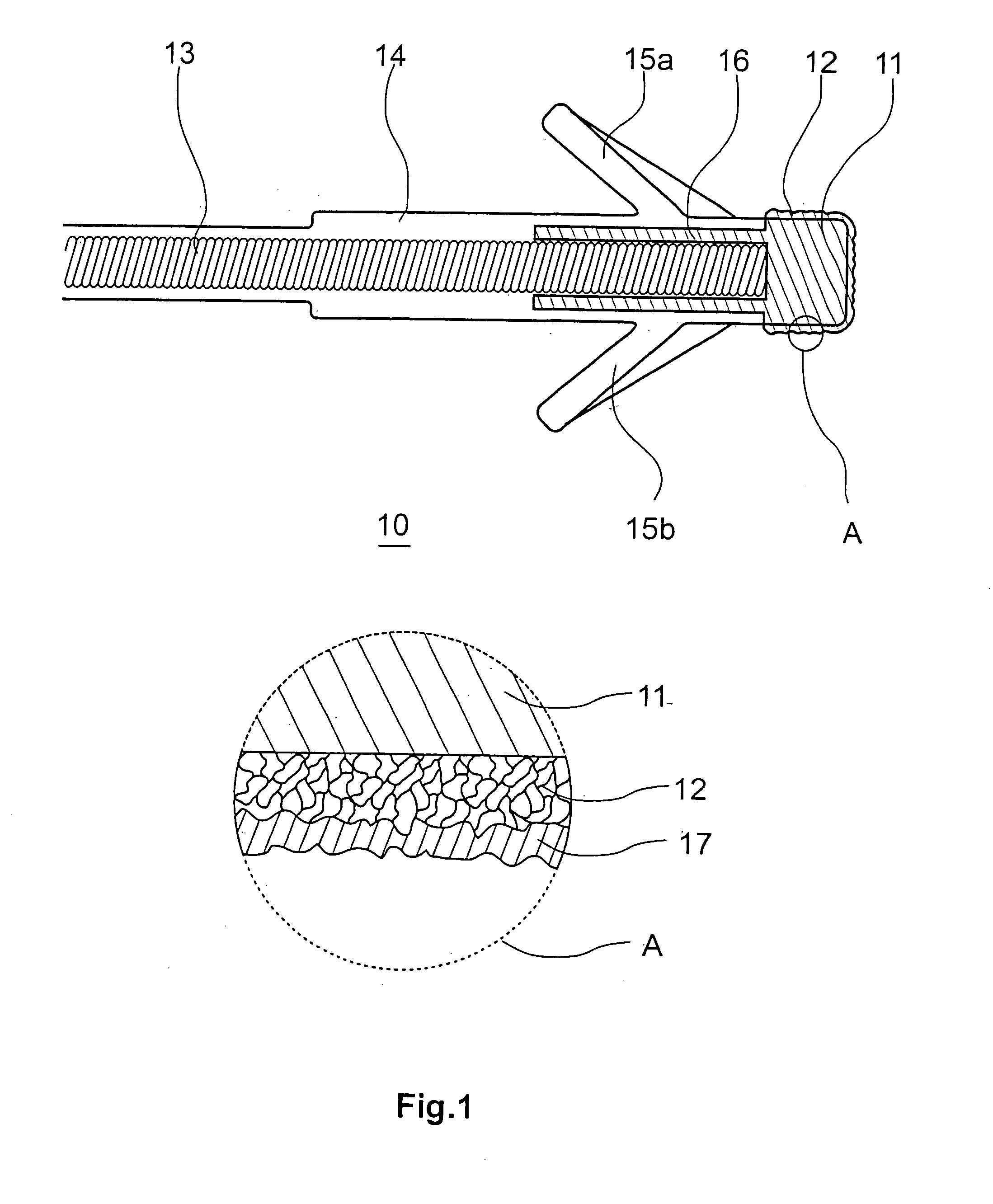
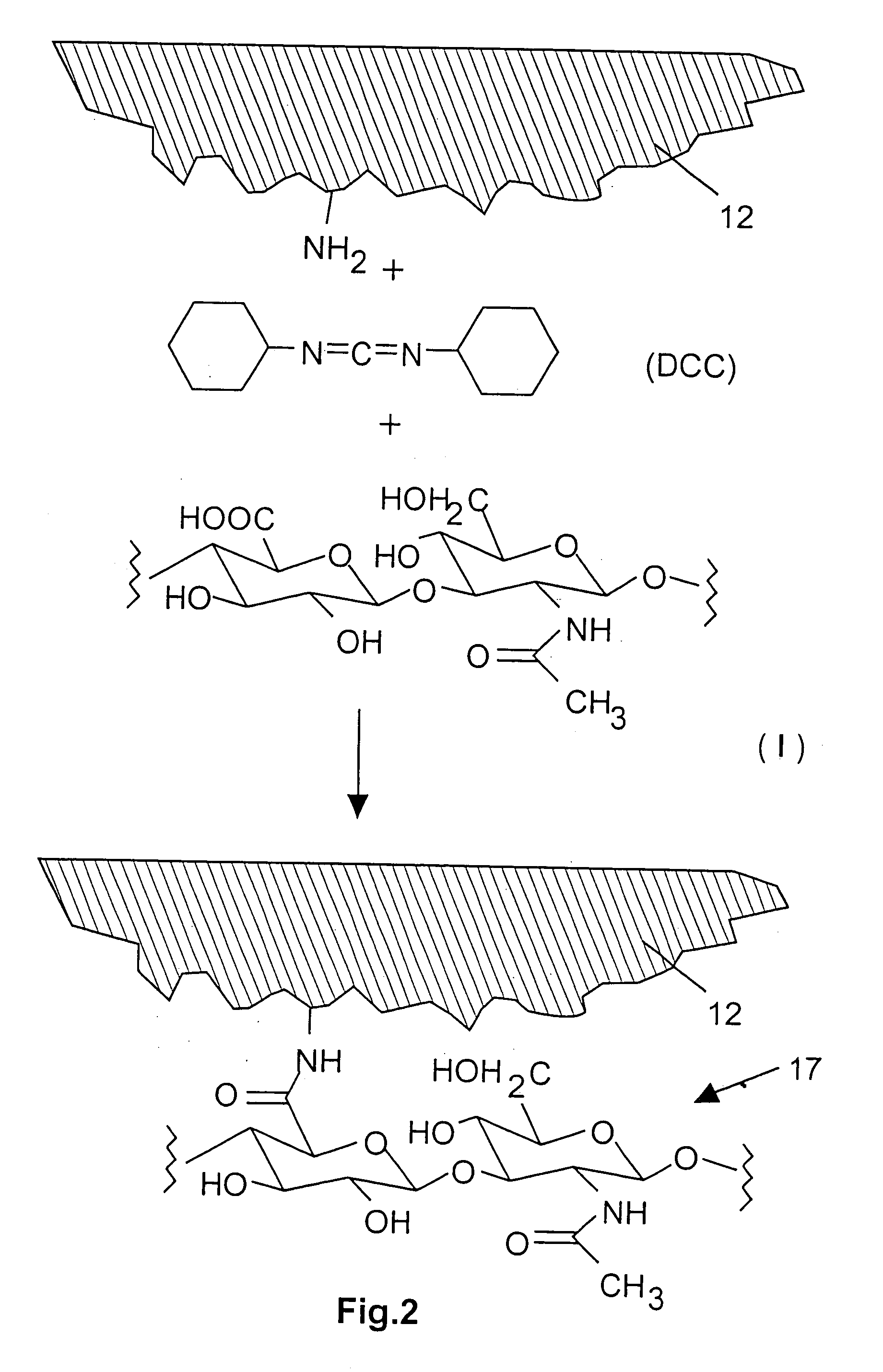
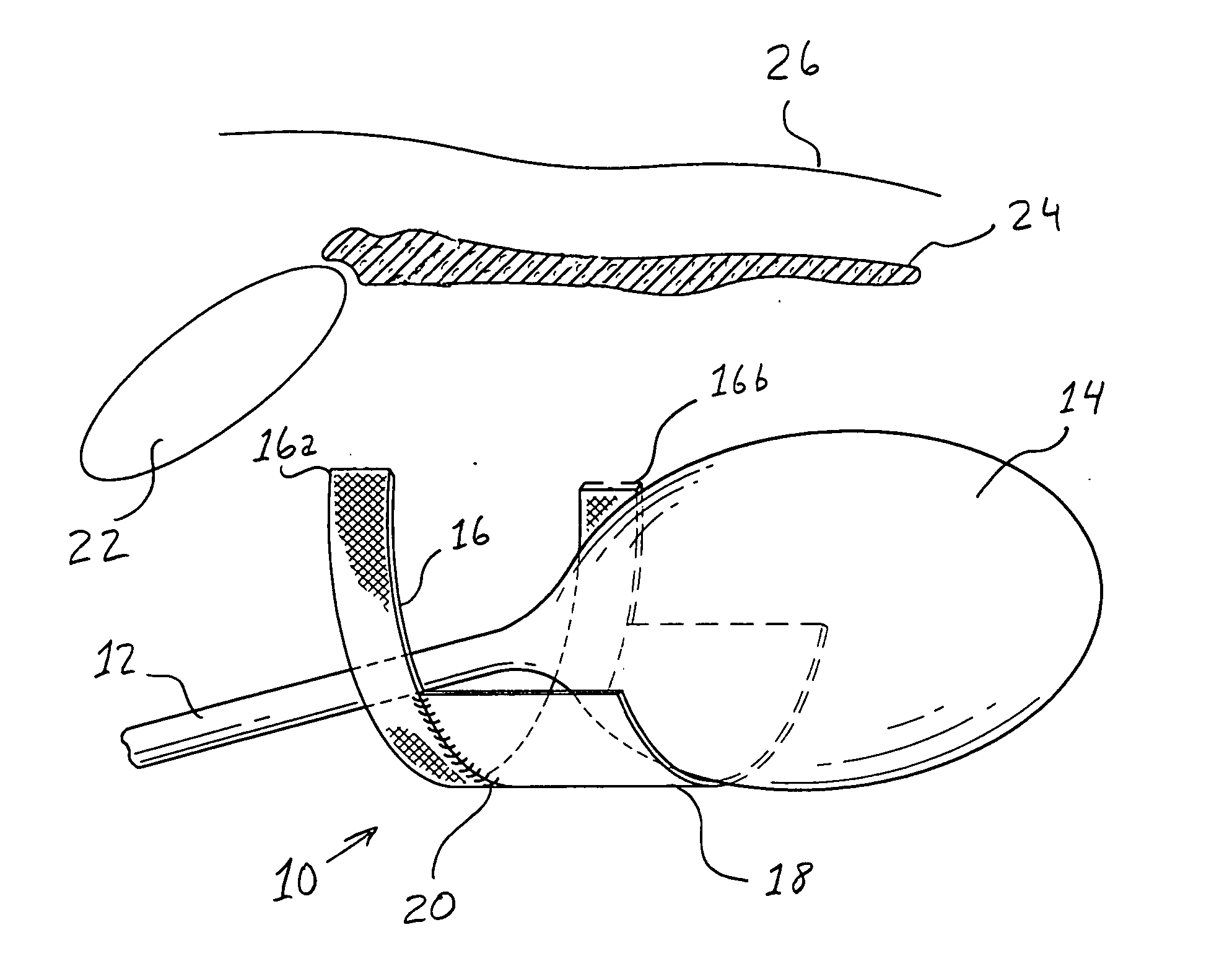
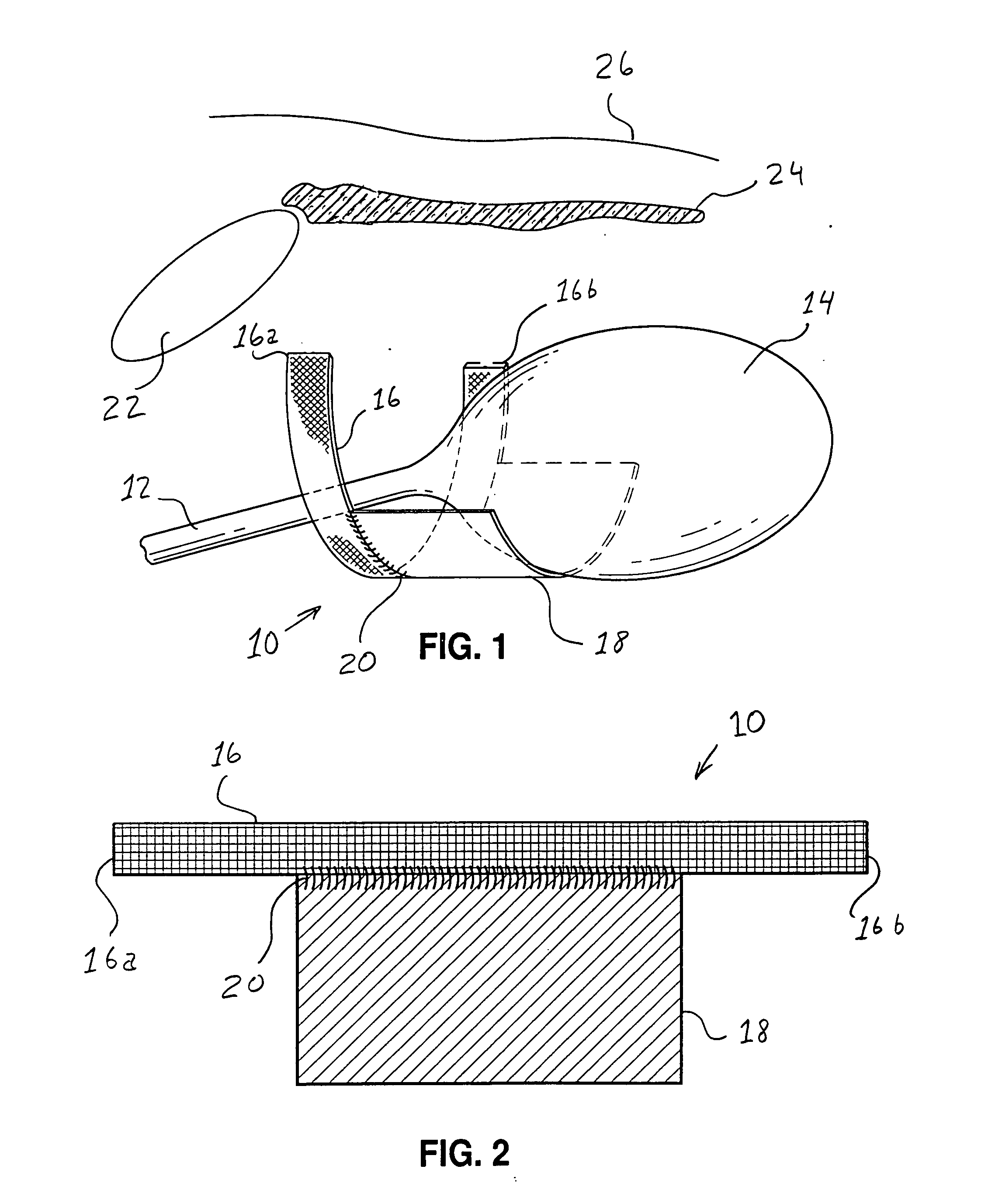
InactiveUS7718556B2Good biocompatibilityHigh strengthSuture equipmentsBiocideGelatin filmThin membrane
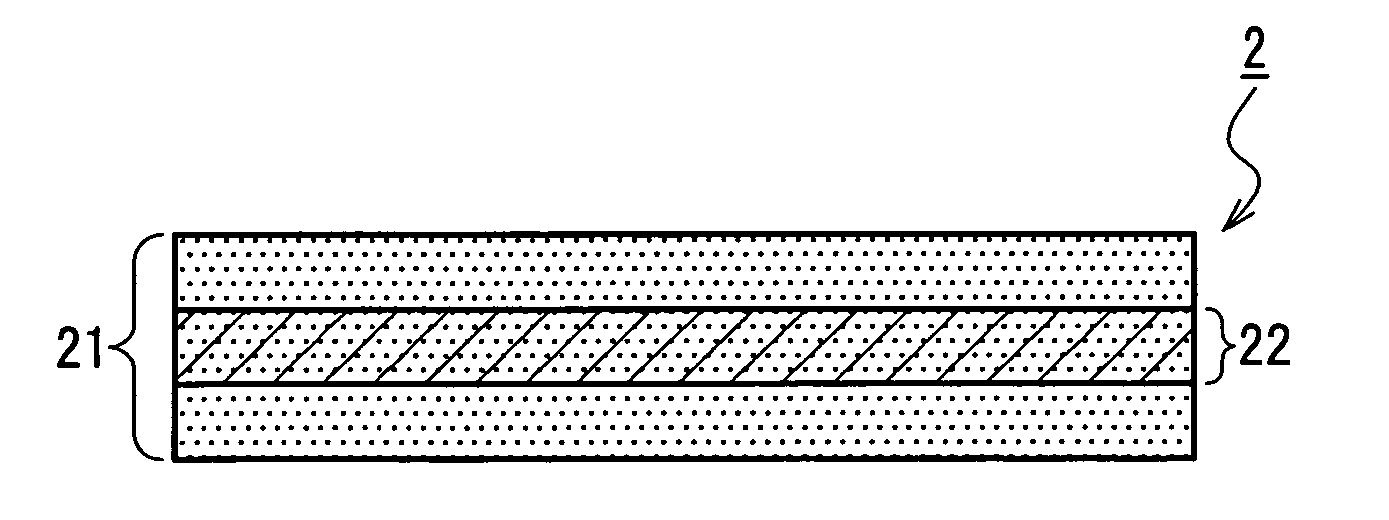
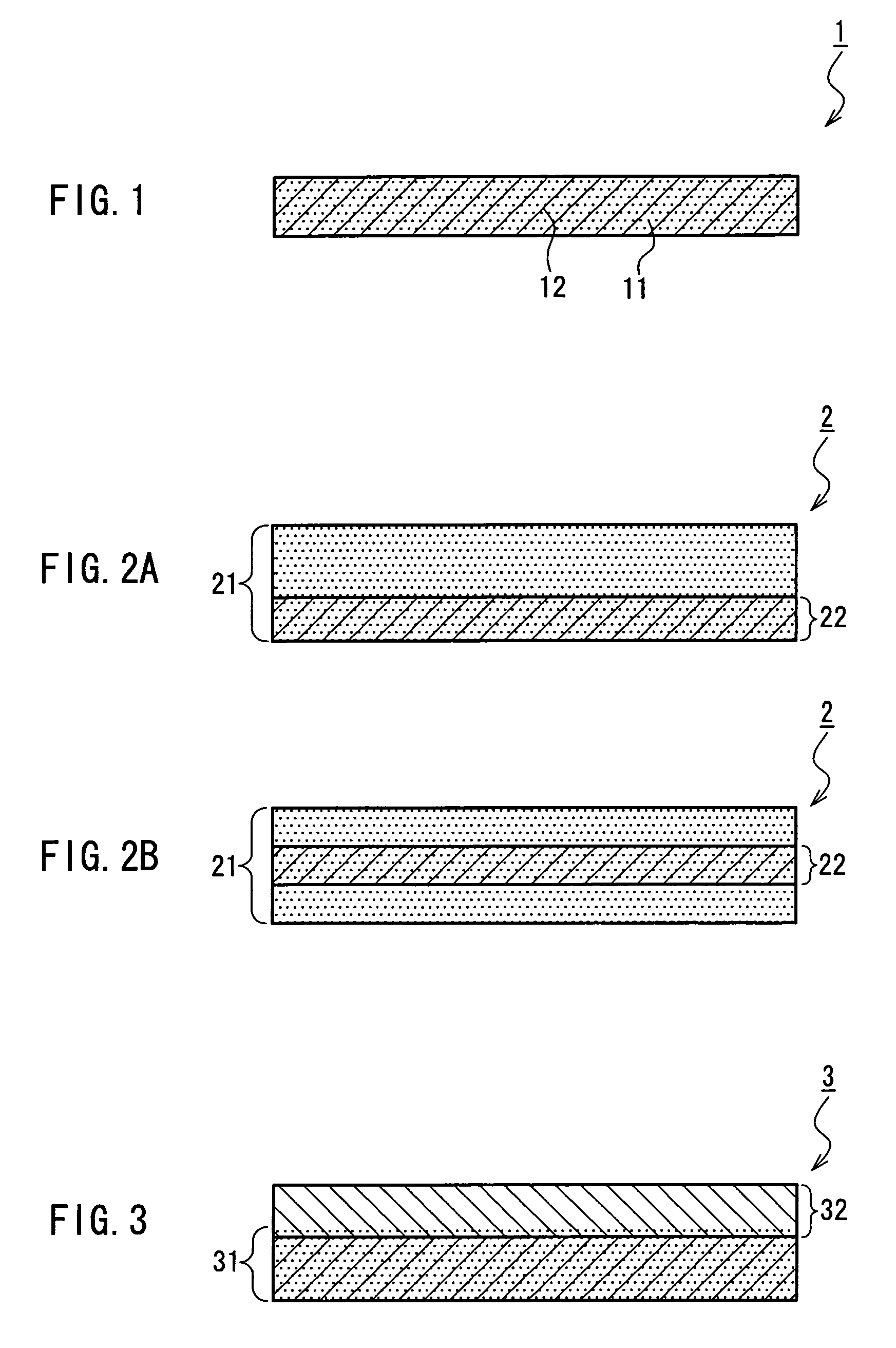
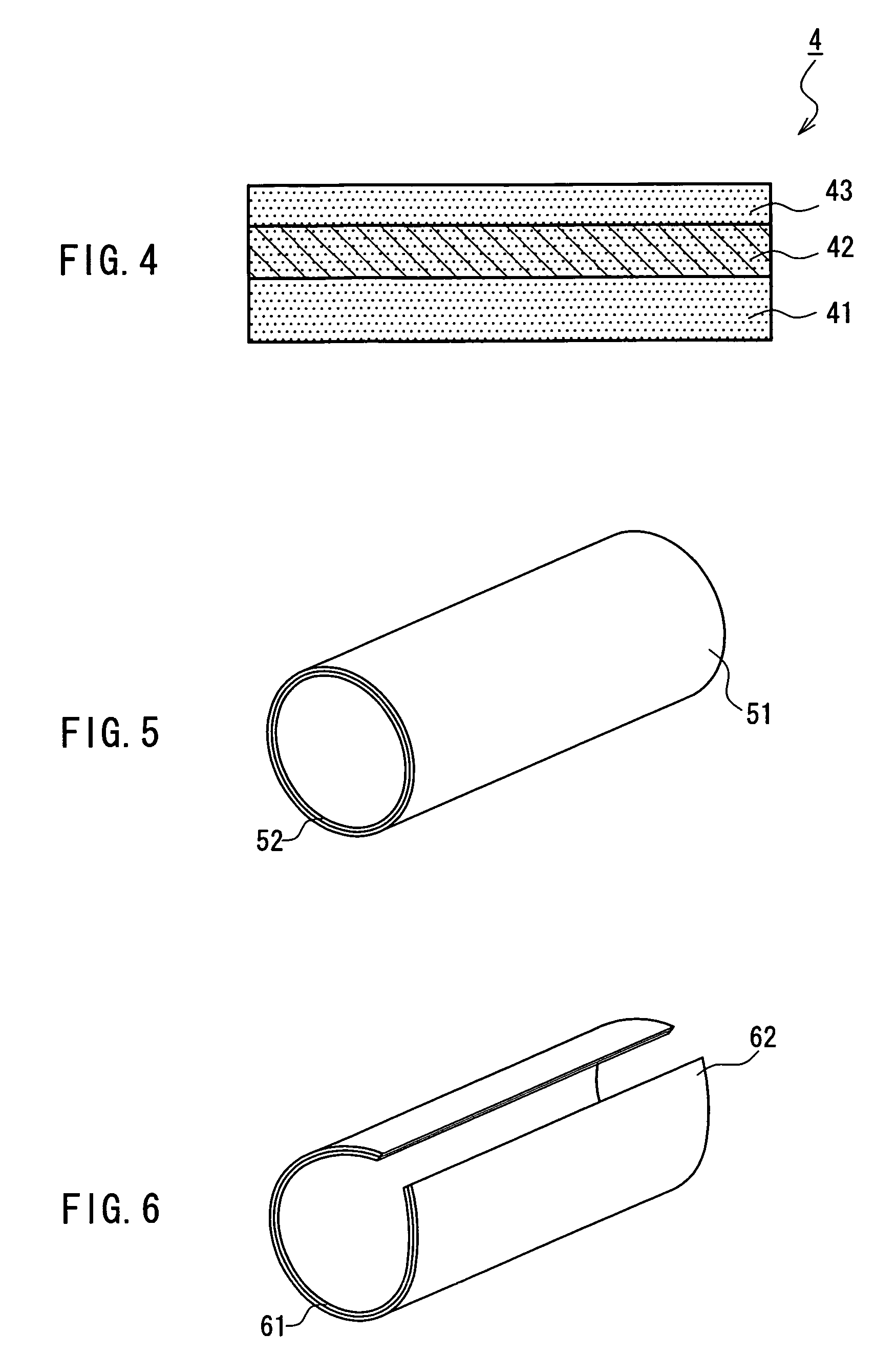
A medical film that is excellent in biocompatibility and bioabsorbability and has an excellent strength in suturing and bonding is provided. A reinforcing material 12 made of a biodegradable polymer is placed in a gelatin solution so as to allow the solution to infiltrate in the reinforcing material 12 and then the gelatin is dried. This allows the gelatin that has infiltrated entirely in an internal part of the reinforcing material 12 to gel, thereby forming a gelatin film 11. Thus, a medical film 1 in which the reinforcing material 12 and the gelatin film 11 are integrated is obtained. The gelatin film 11 preferably is a cross-linked gelatin film.
Owner:GUNZE LTD
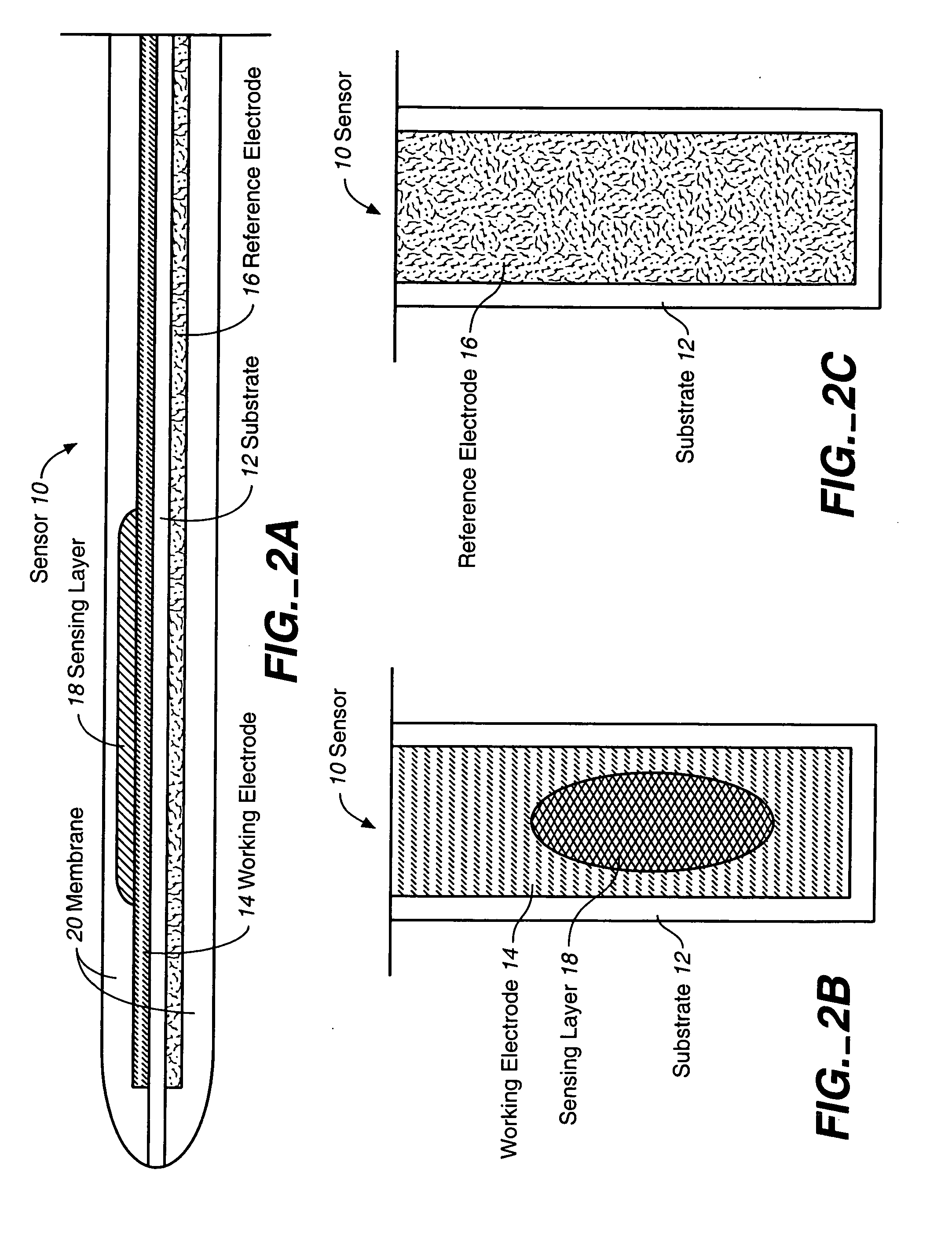
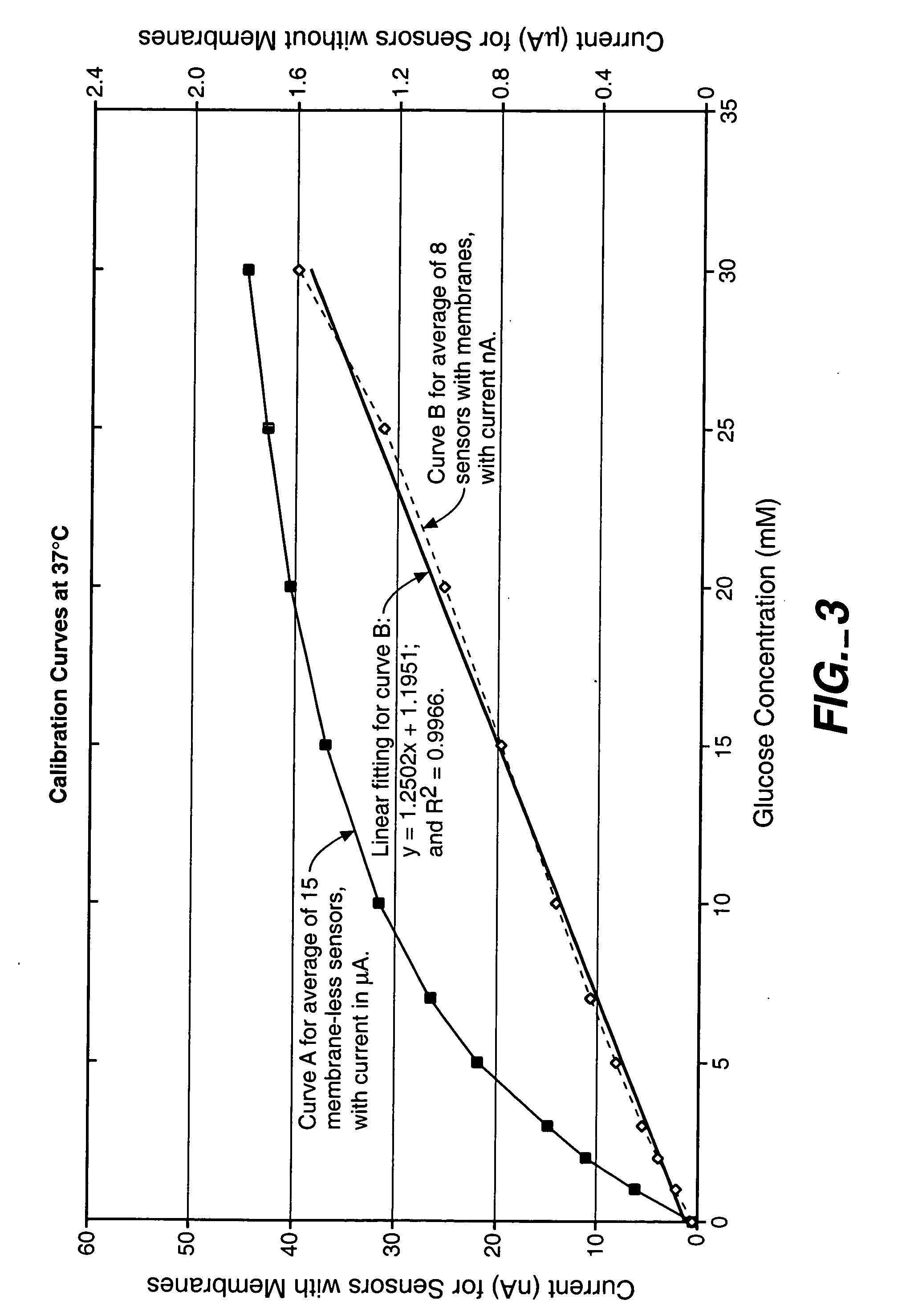
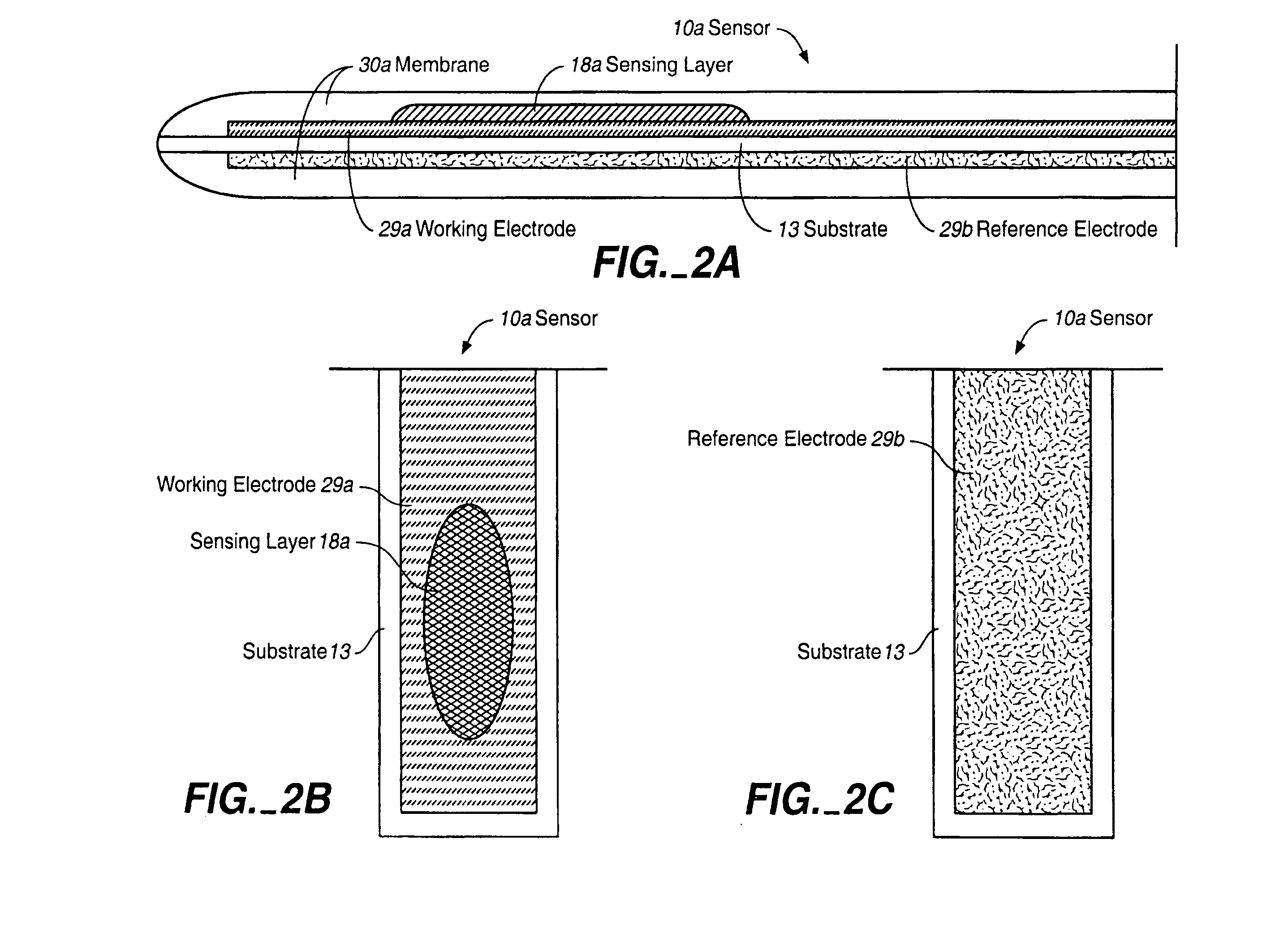
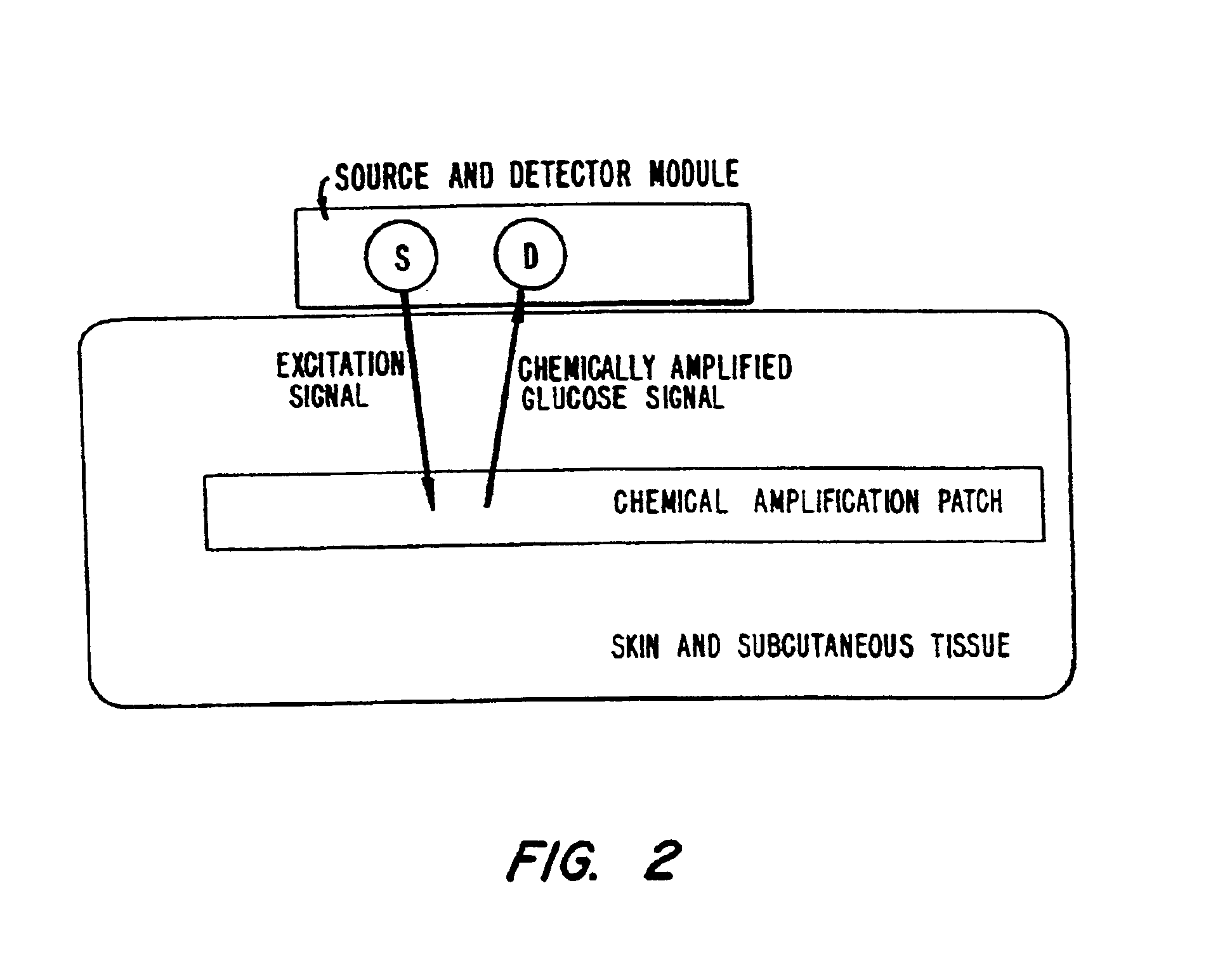
Membrane suitable for use in an analyte sensor, analyte sensor, and associated method
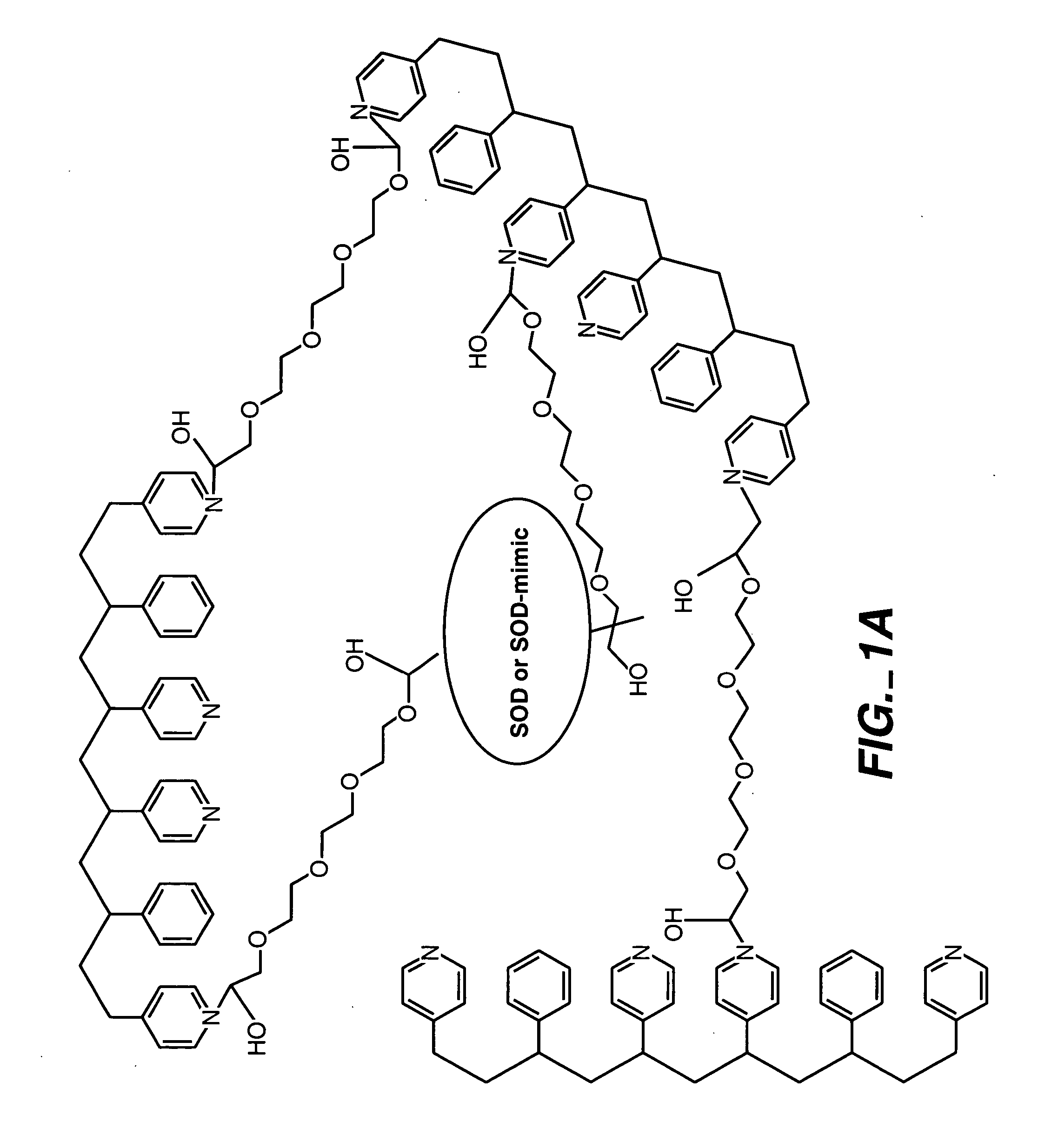
ActiveUS20050173245A1Facilitate linear responsivenessEasy CalibrationImmobilised enzymesBioreactor/fermenter combinationsMetaboliteSuperoxide
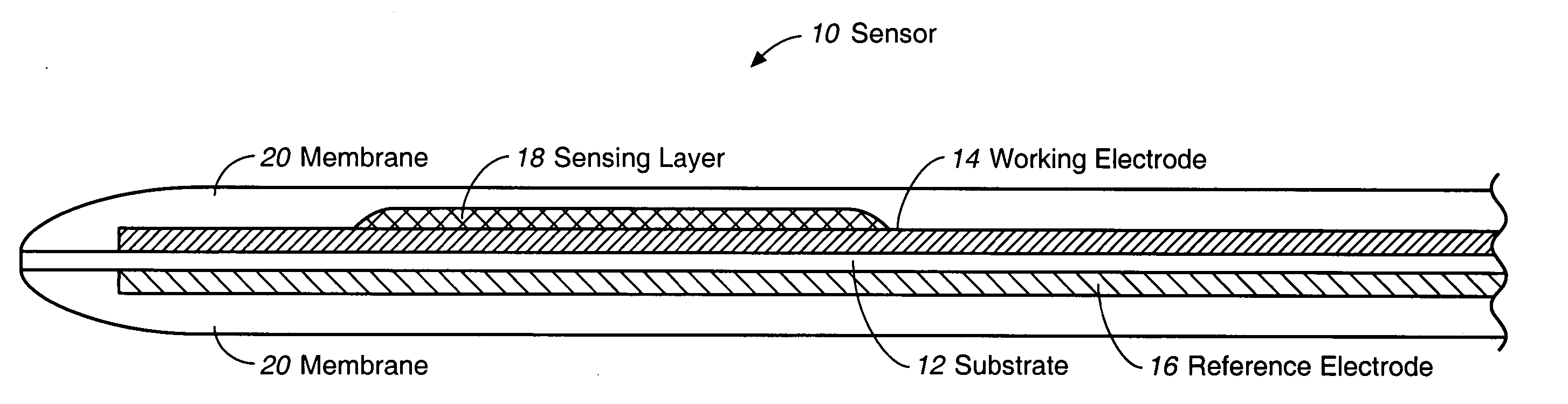
A multifunctional membrane is provided. The multifunctional membrane is suitable for use in an analyte sensor. In a particular application, the multifunctional membrane may be used in connection with an amperometric biosensor, such as a transcutaneous amperometric biosensor. Some functions of the membrane are associated with properties of membrane itself, which is comprised of crosslinked polymers containing heterocyclic nitrogen groups. For example, the membrane, by virtue of its polymeric composition, may regulate the flux of an analyte to a sensor. Such regulation generally improves the kinetic performance of the sensor over a broad range of analyte concentration. Other functions of the membrane are associated with functional components, such as a superoxide-dismutating / catalase catalyst, either in the form of an enzyme or an enzyme mimic, that can be bound to the scaffold provided by the membrane. The effect of any such enzyme or enzyme mimic is to lower the concentration of a metabolite, such as superoxide and / or hydrogen peroxide, in the immediate vicinity of the sensing layer of the biosensor. Lowering the concentrations of such metabolites, which are generally deleterious to the function of the sensor, generally protects or enhances biosensor integrity and performance. The membrane is thus an important tool for use in connection with analyte sensors, amperometric sensors, biosensors, and particularly, transcutaneous biosensors. A membrane-covered sensor and a method for making same are also provided.
Owner:ABBOTT DIABETES CARE INC
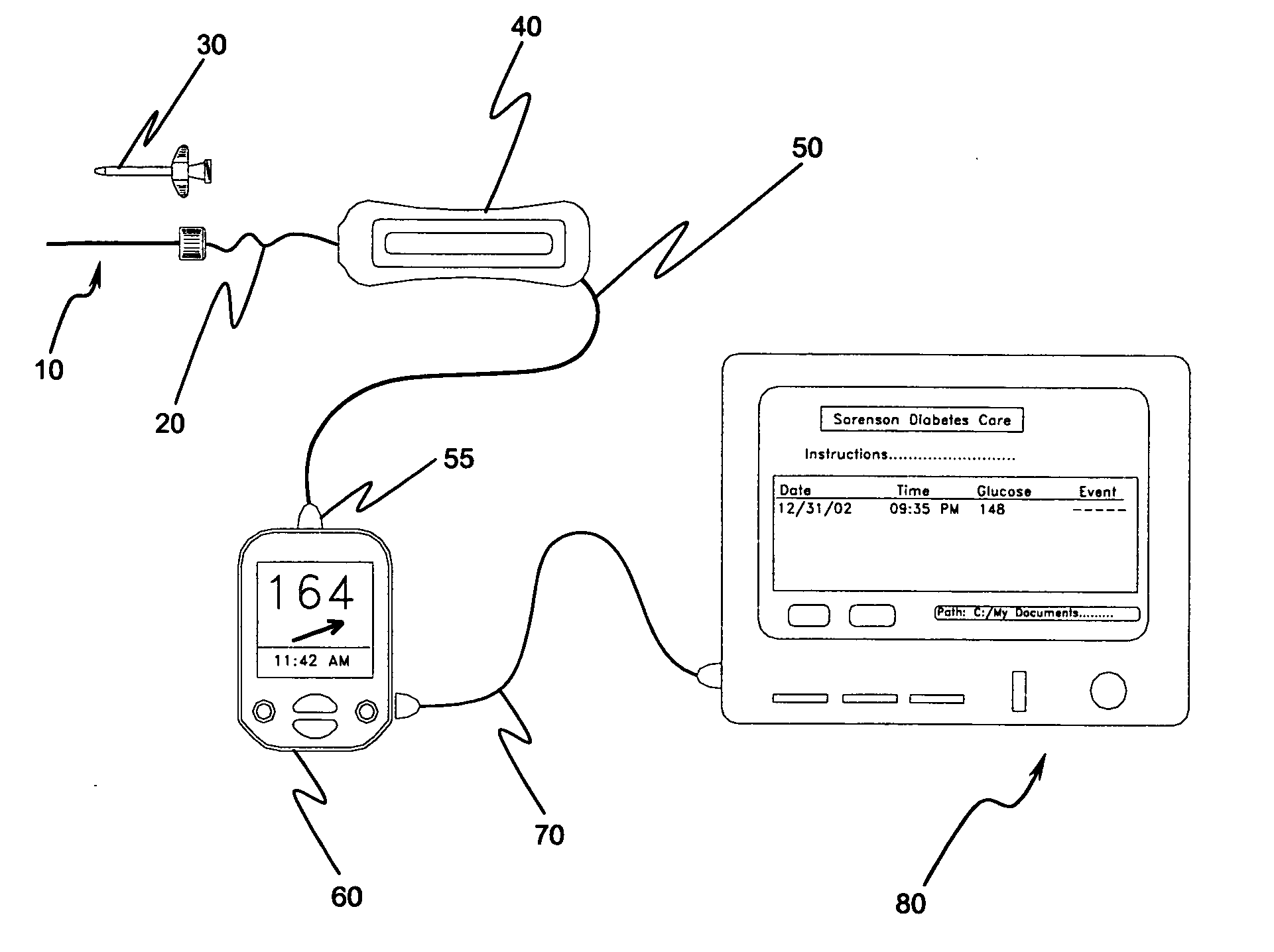
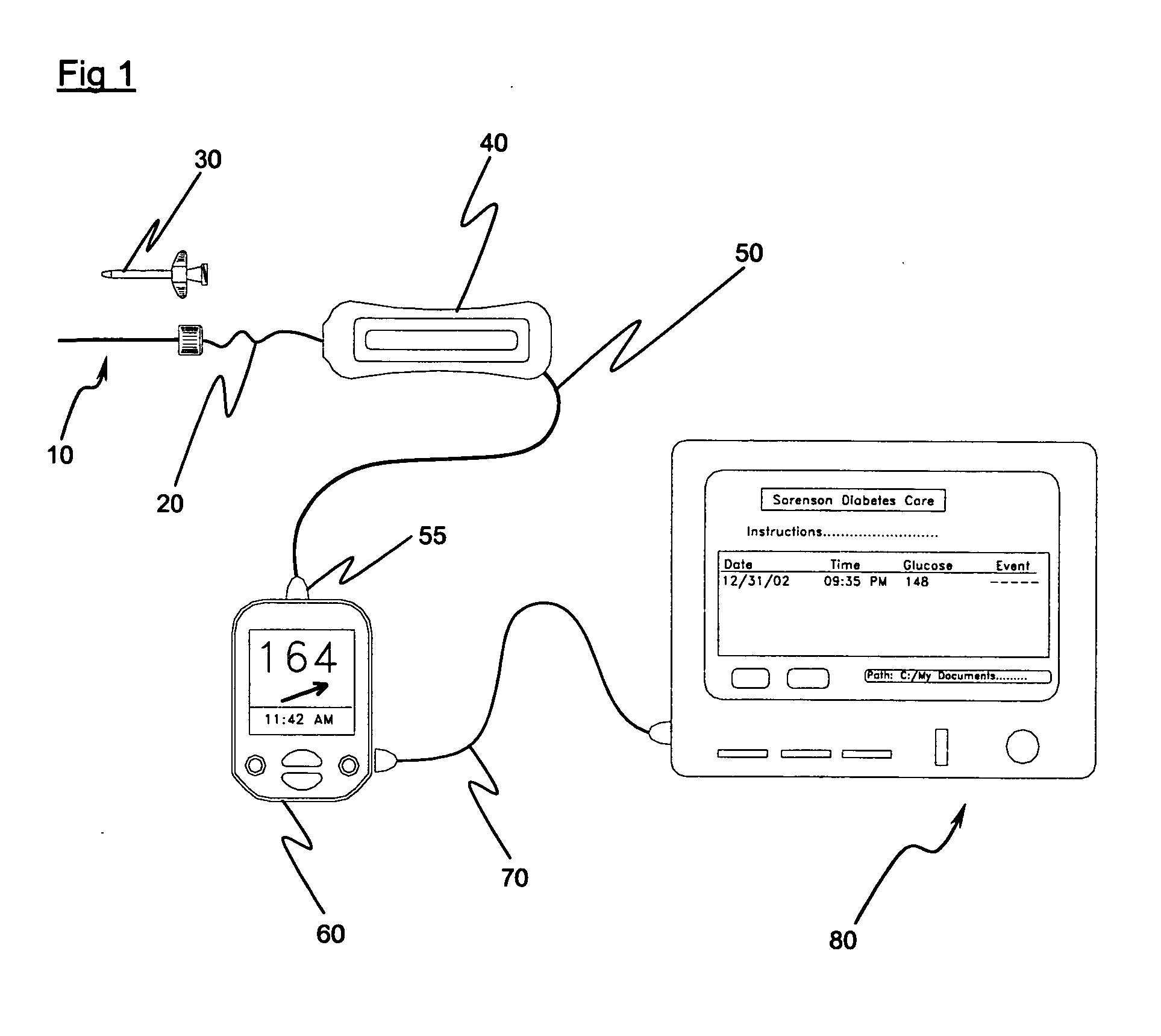
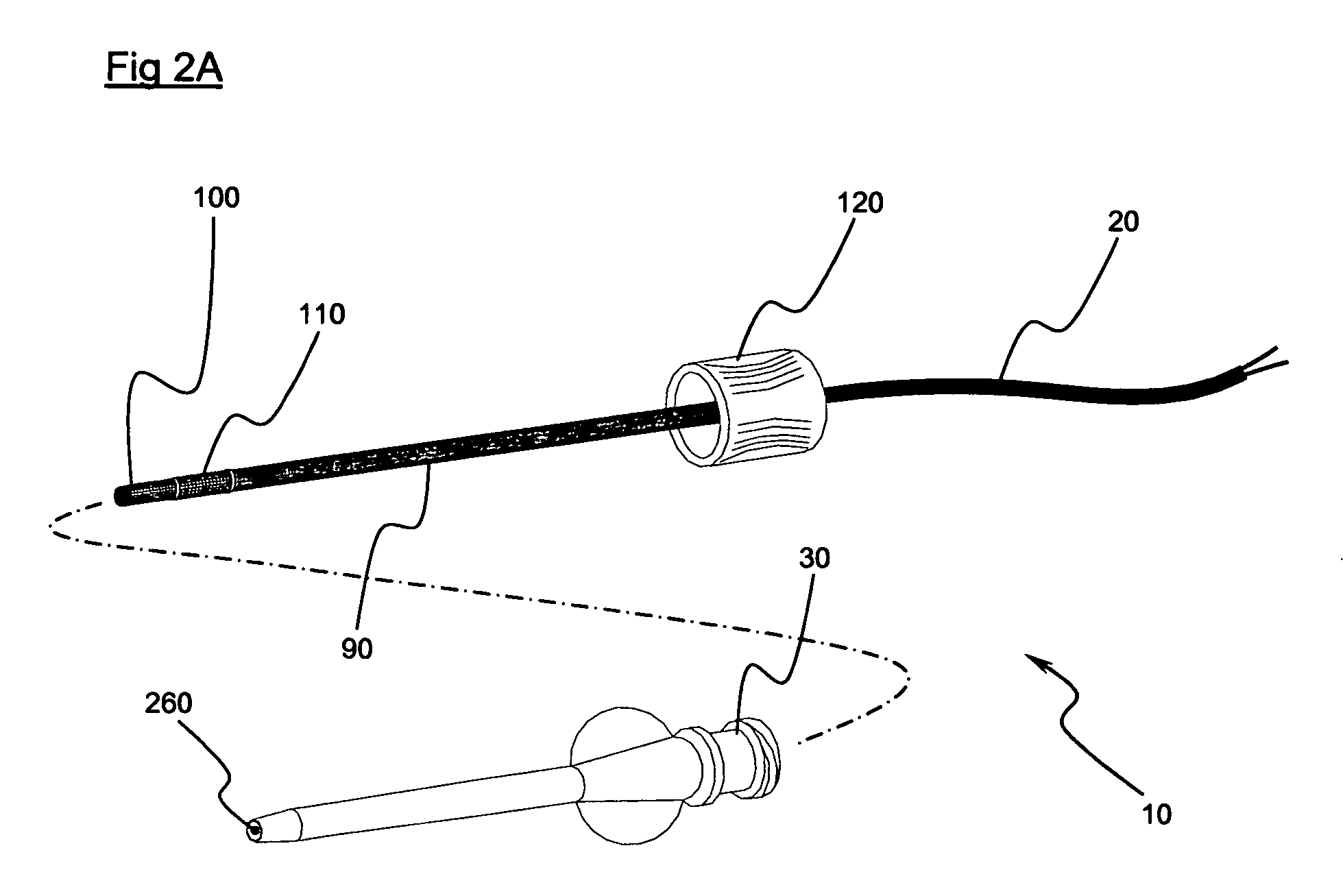
Implantable biosensor system, apparatus and method
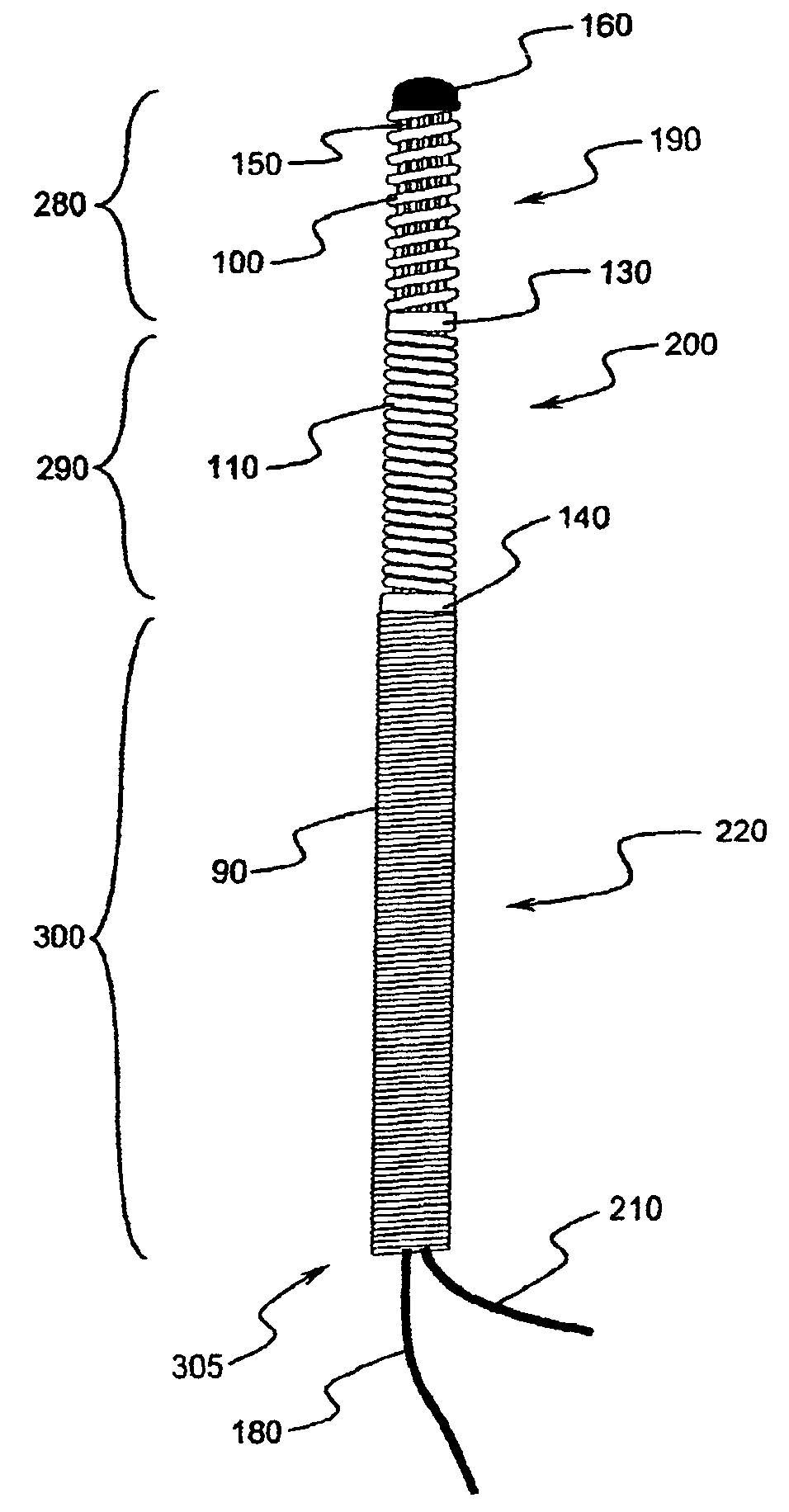
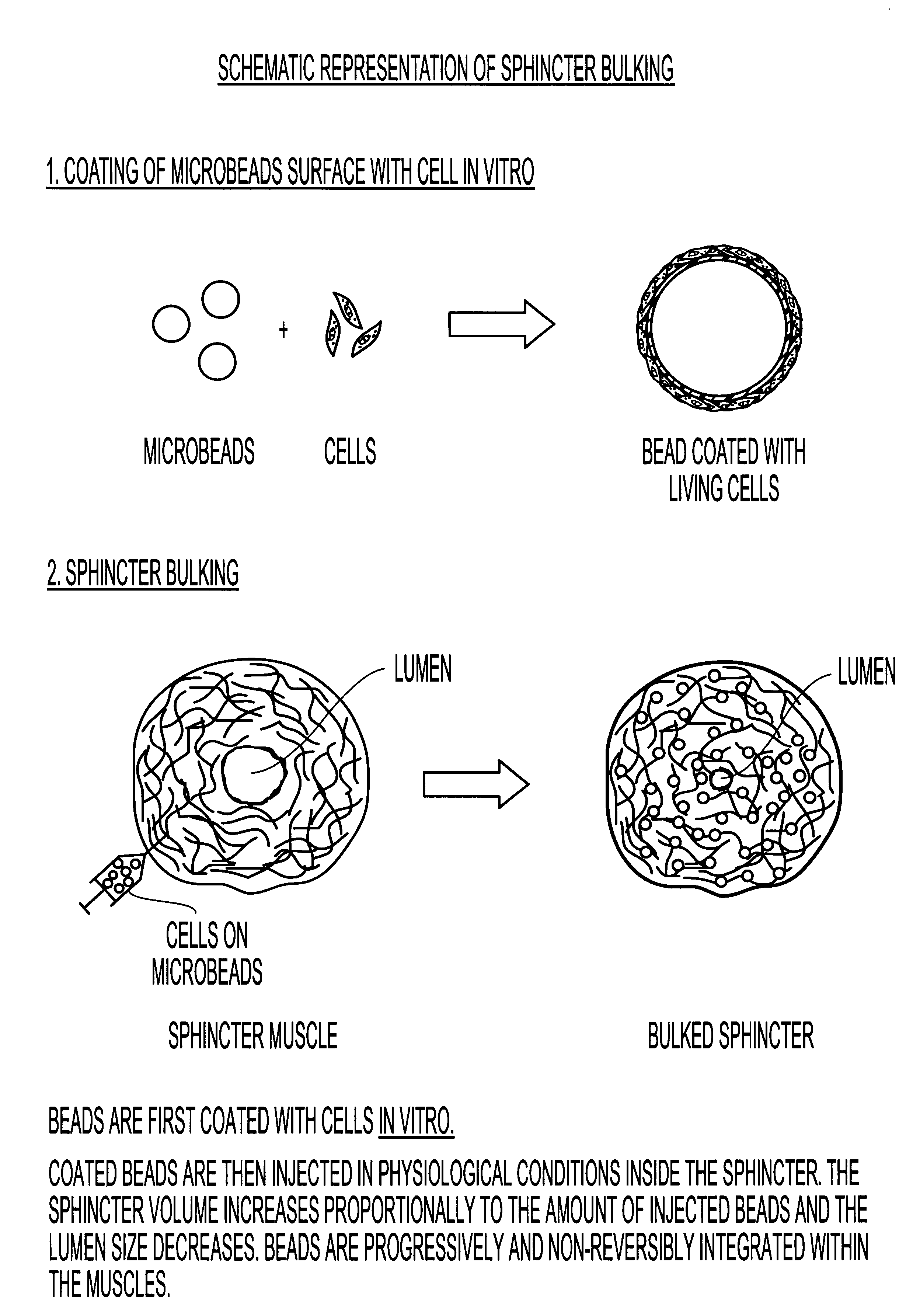
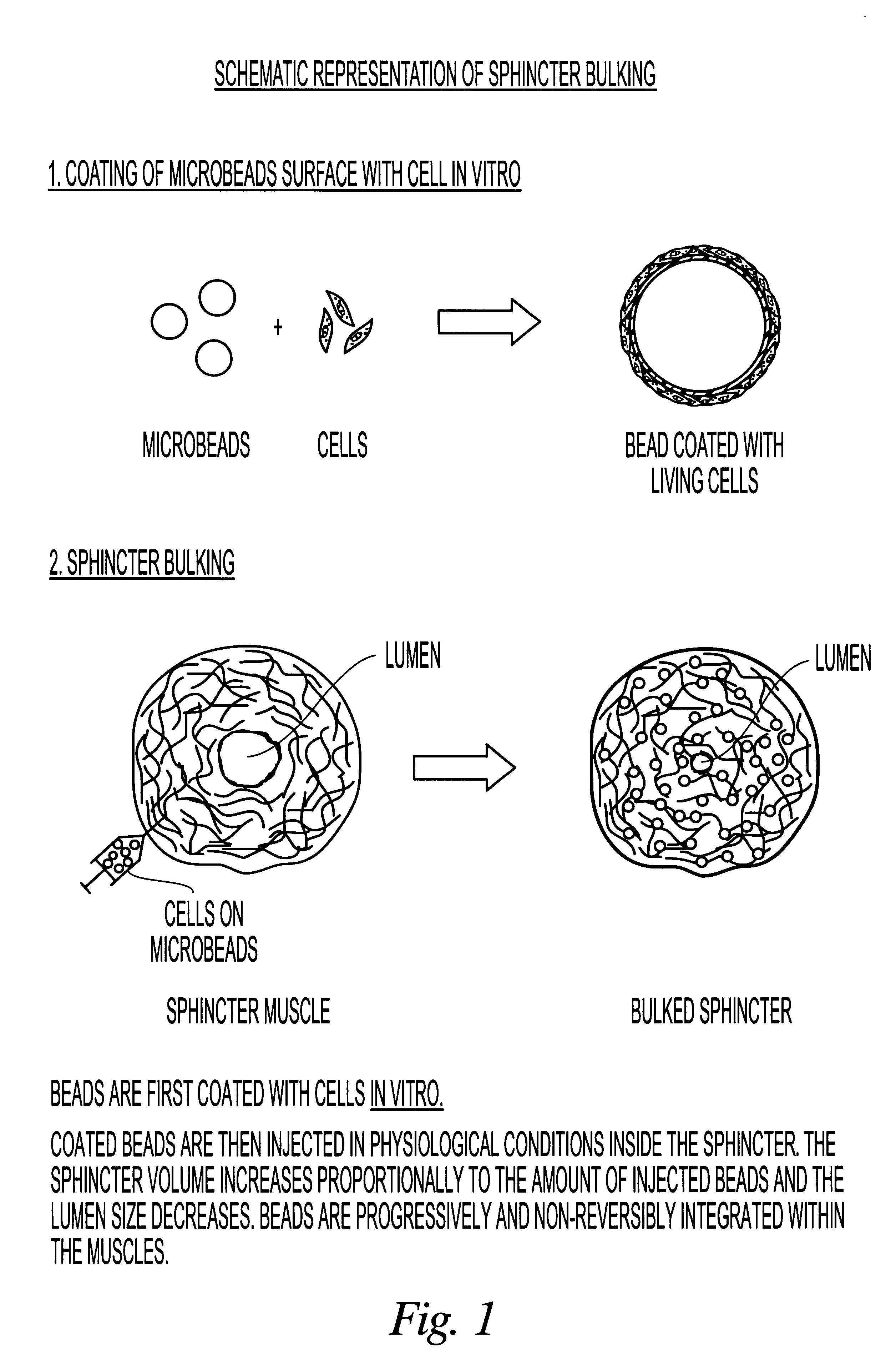
InactiveUS6965791B1Good biocompatibilityIncrease volumeImmobilised enzymesBioreactor/fermenter combinationsForeign matterInterstitial glucose
An implantable biosensor assembly and system includes an enzymatic sensor probe from which subcutaneous and interstitial glucose levels may be inferred. The assembly may be associated by direct percutaneous connection with electronics, such as for signal amplification, sensor polarization, and data download, manipulation, display, and storage. The biosensor comprises a miniature probe characterized by lateral flexibility and tensile strength and has large electrode surface area for increased sensitivity. Irritation of tissues surrounding the probe is minimized due to ease of flexibility and small cross section of the sensor. Foreign body reaction is diminished due to a microscopically rough porous probe surface.
Owner:SORENSON DEVMENT
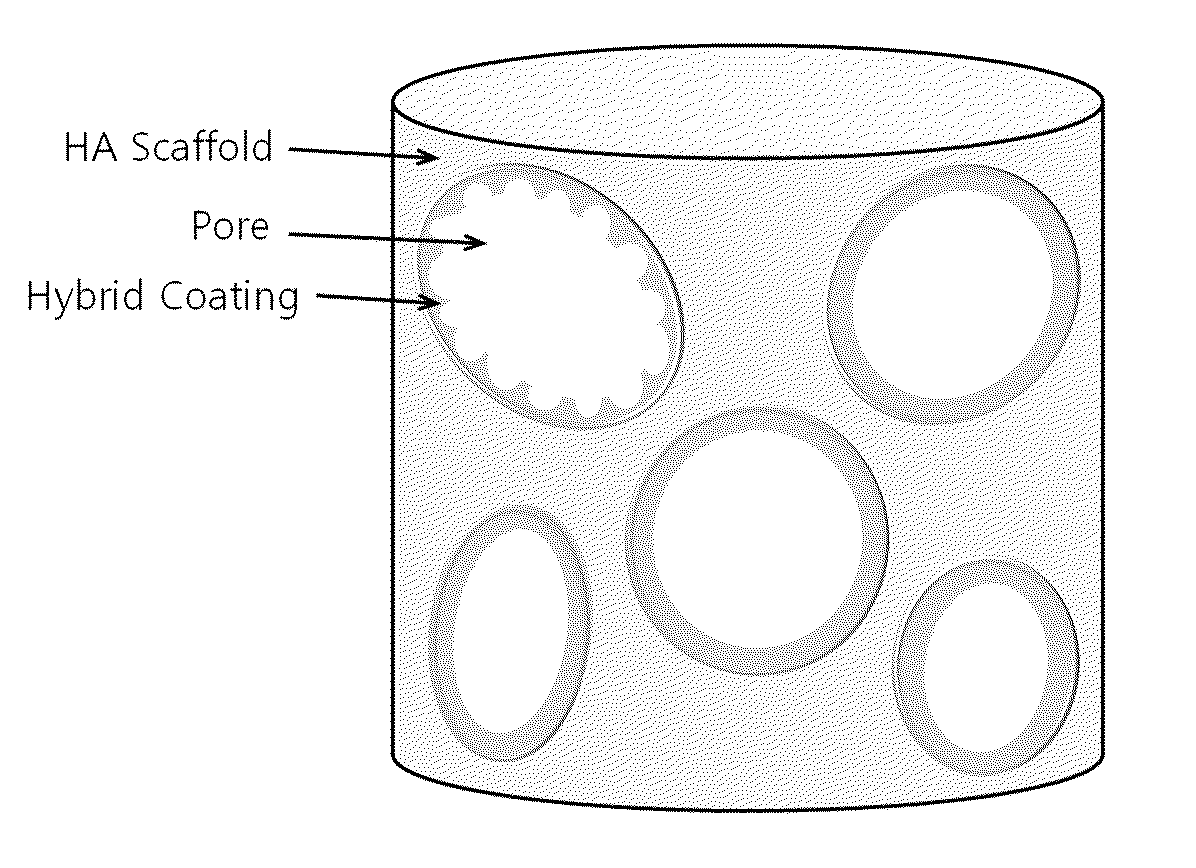
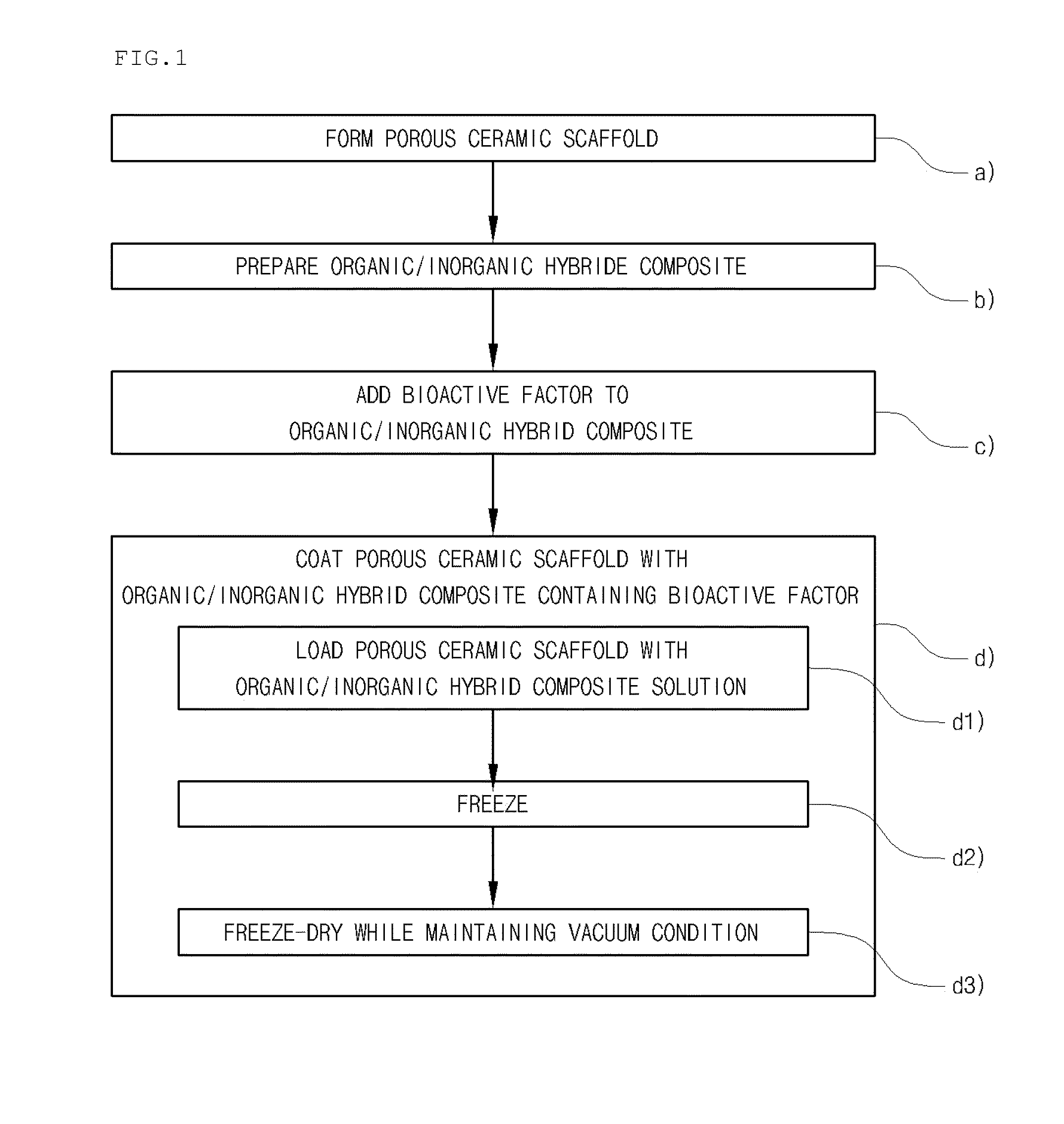
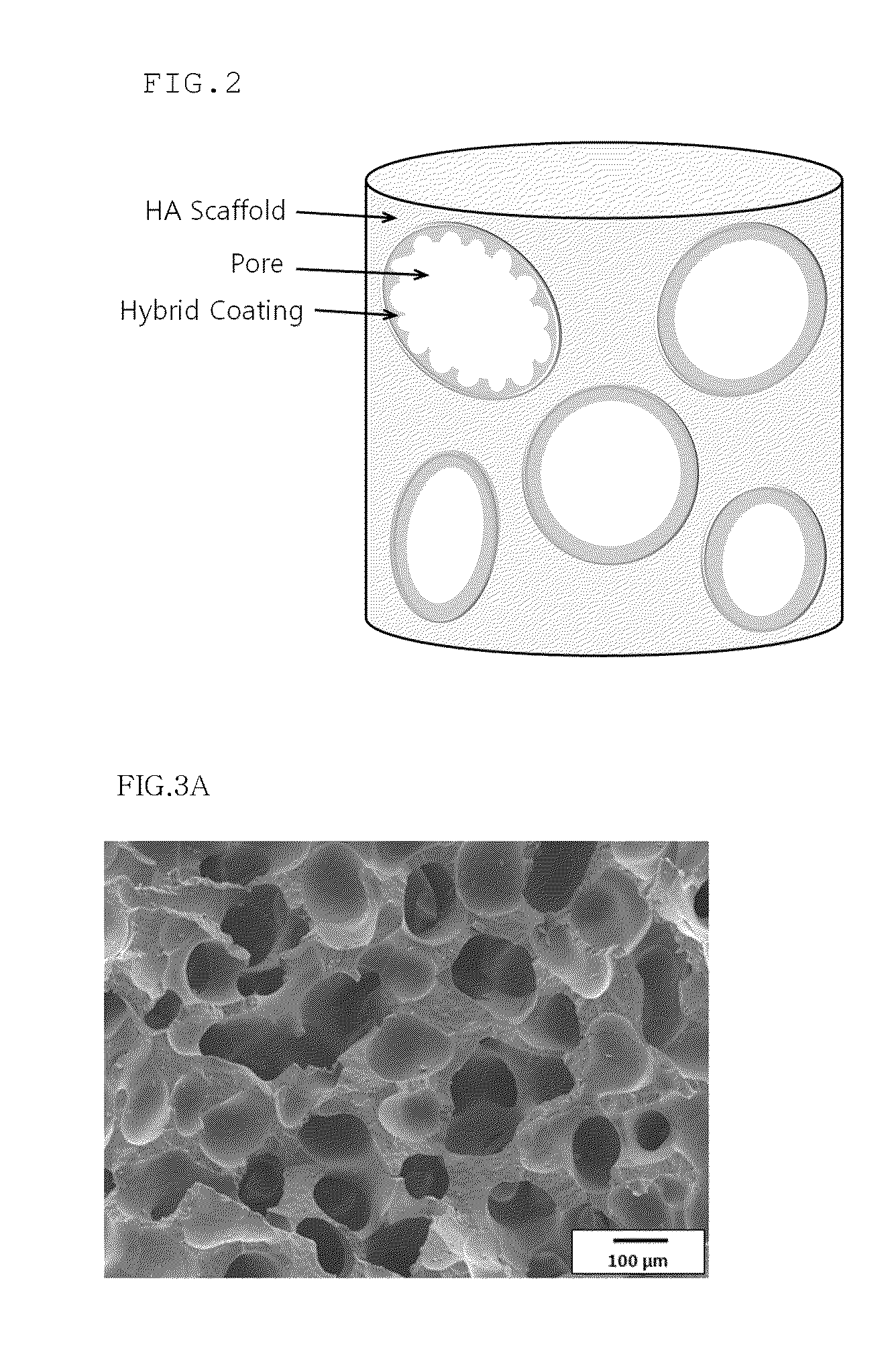
Method for manufacturing a porous ceramic scaffold having an organic/inorganic hybrid coating layer containing a bioactive factor
ActiveUS8734831B2Good biocompatibilityEasy to controlBiocideSurgical adhesivesOrganic matterPorous ceramics
A method for manufacturing a porous ceramic scaffold having an organic / inorganic hybrid coating layer containing a bioactive factor includes (a) forming a porous ceramic scaffold; (b) mixing a silica xerogel and a physiologically active organic substance in a volumetric ratio ranging from 30:70 to 90:10 and treating by a sol gel method to prepare an organic / inorganic hybrid composite solution; (c) adding a bioactive factor to the organic / inorganic hybrid composite solution and agitating until gelation occurs; and (d) coating the porous ceramic scaffold with the organic / inorganic composite containing the bioactive factor added thereto. In accordance with the method, the porous ceramic scaffold may be uniformly coated with the organic / inorganic hybrid composite while maintaining an open pore structure, and stably discharge the bioactive factor over a long period of time.
Owner:SEOUL NAT UNIV R&DB FOUND
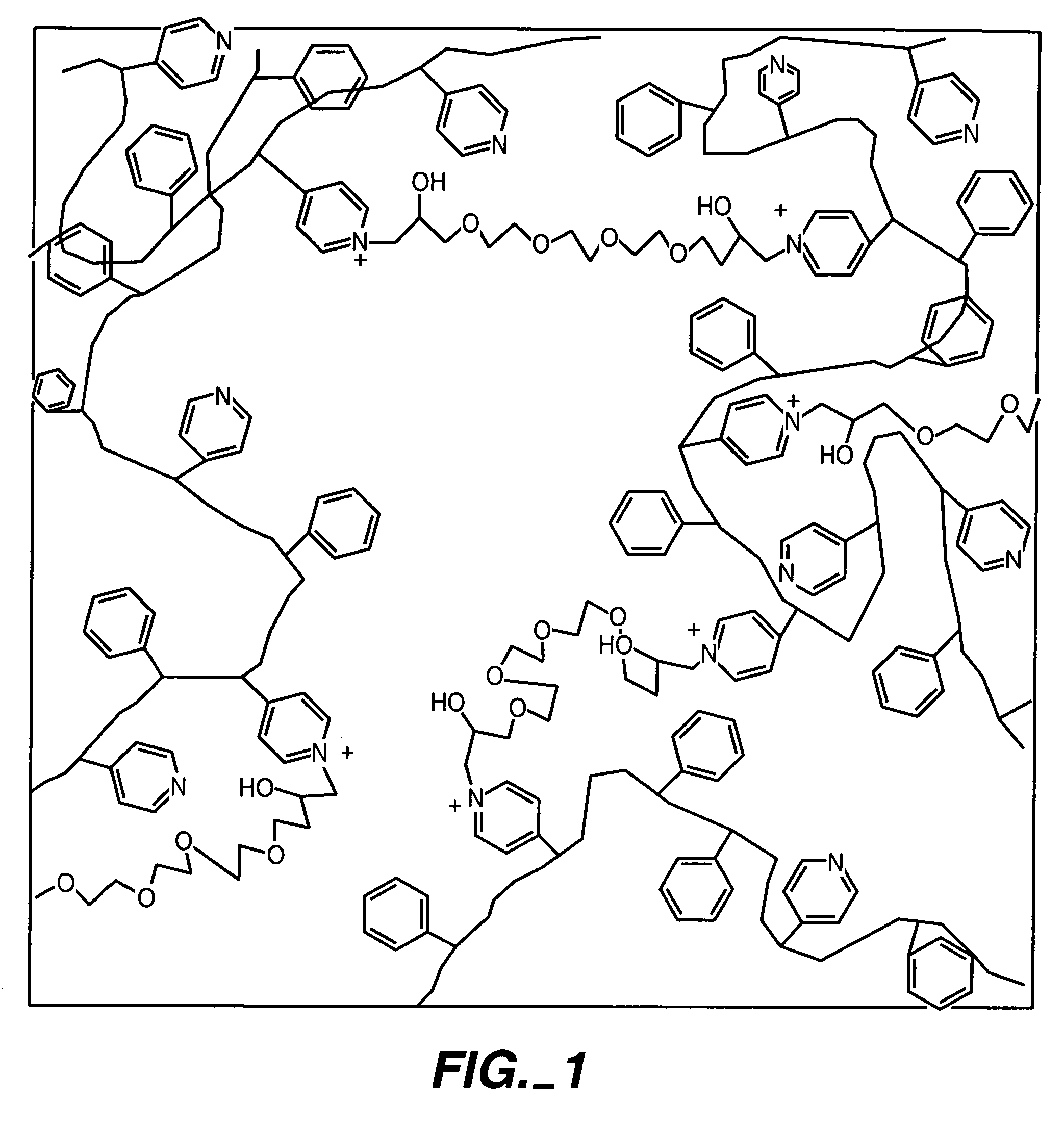
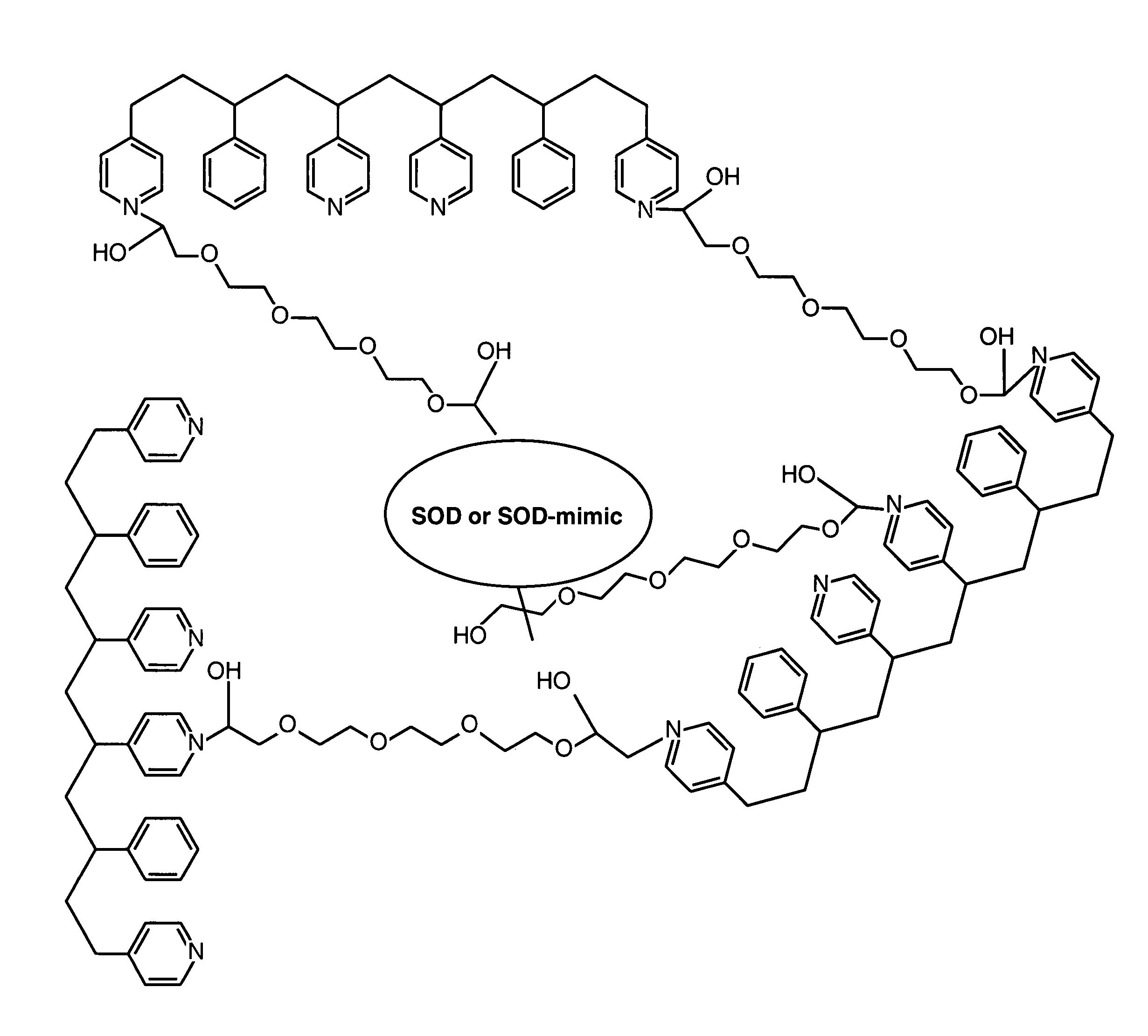
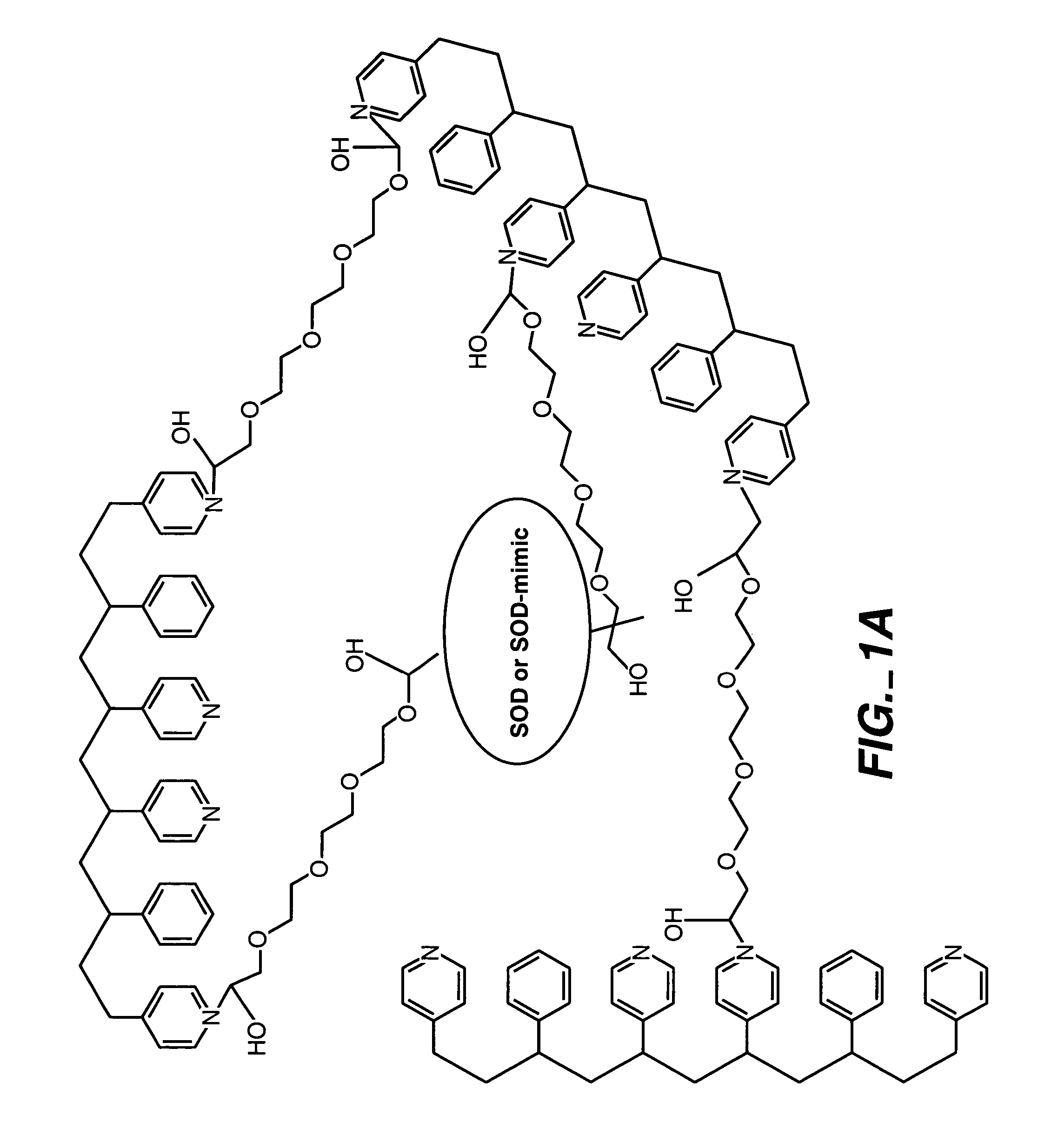
Biosensor membranes composed of polymers containing heterocyclic nitrogens
InactiveUS20050241957A1Easy CalibrationConsider sensitivityMicrobiological testing/measurementVolume/mass flow measurementAnalyteNitrogen
Owner:ABBOTT DIABETES CARE INC
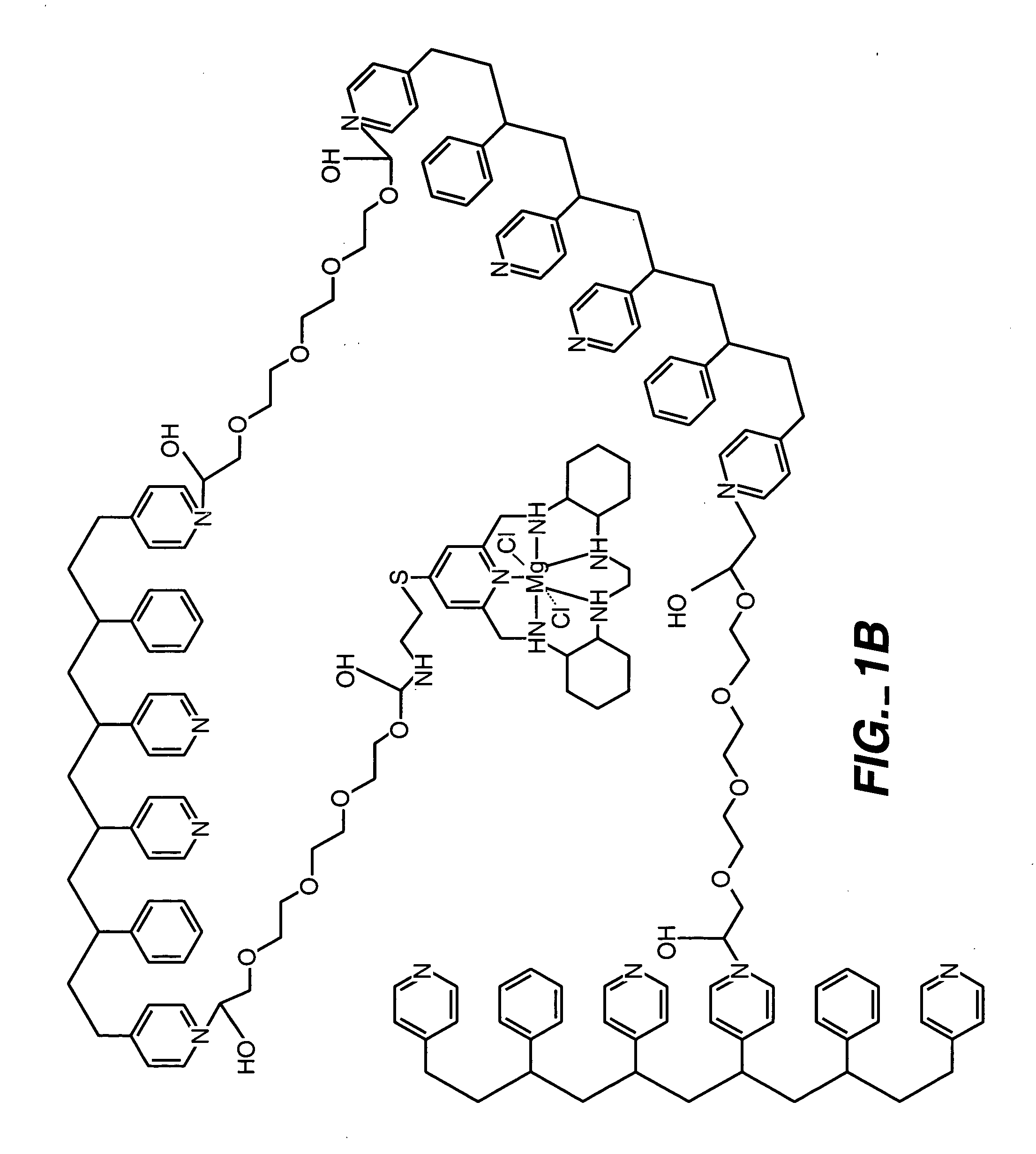
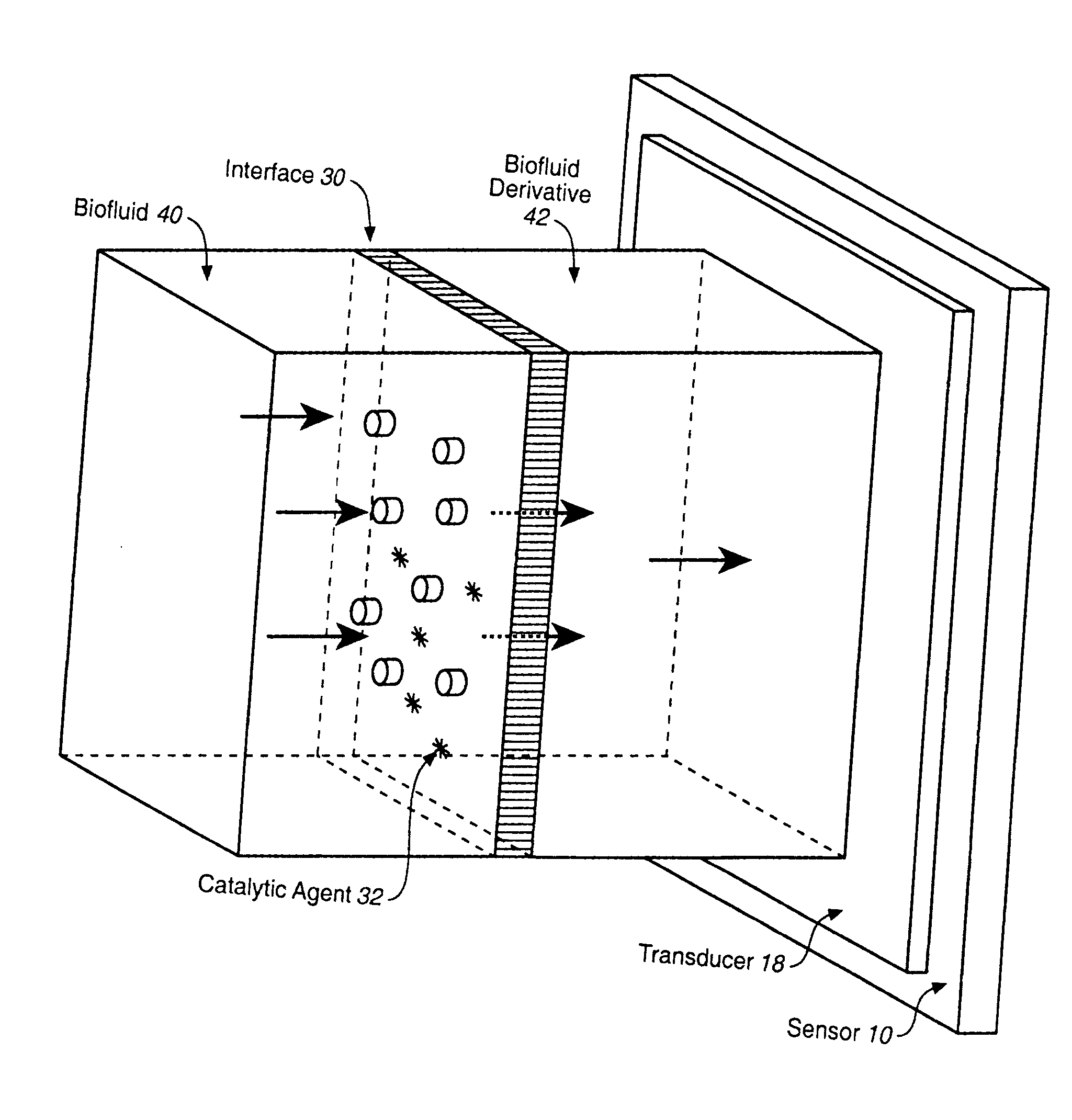
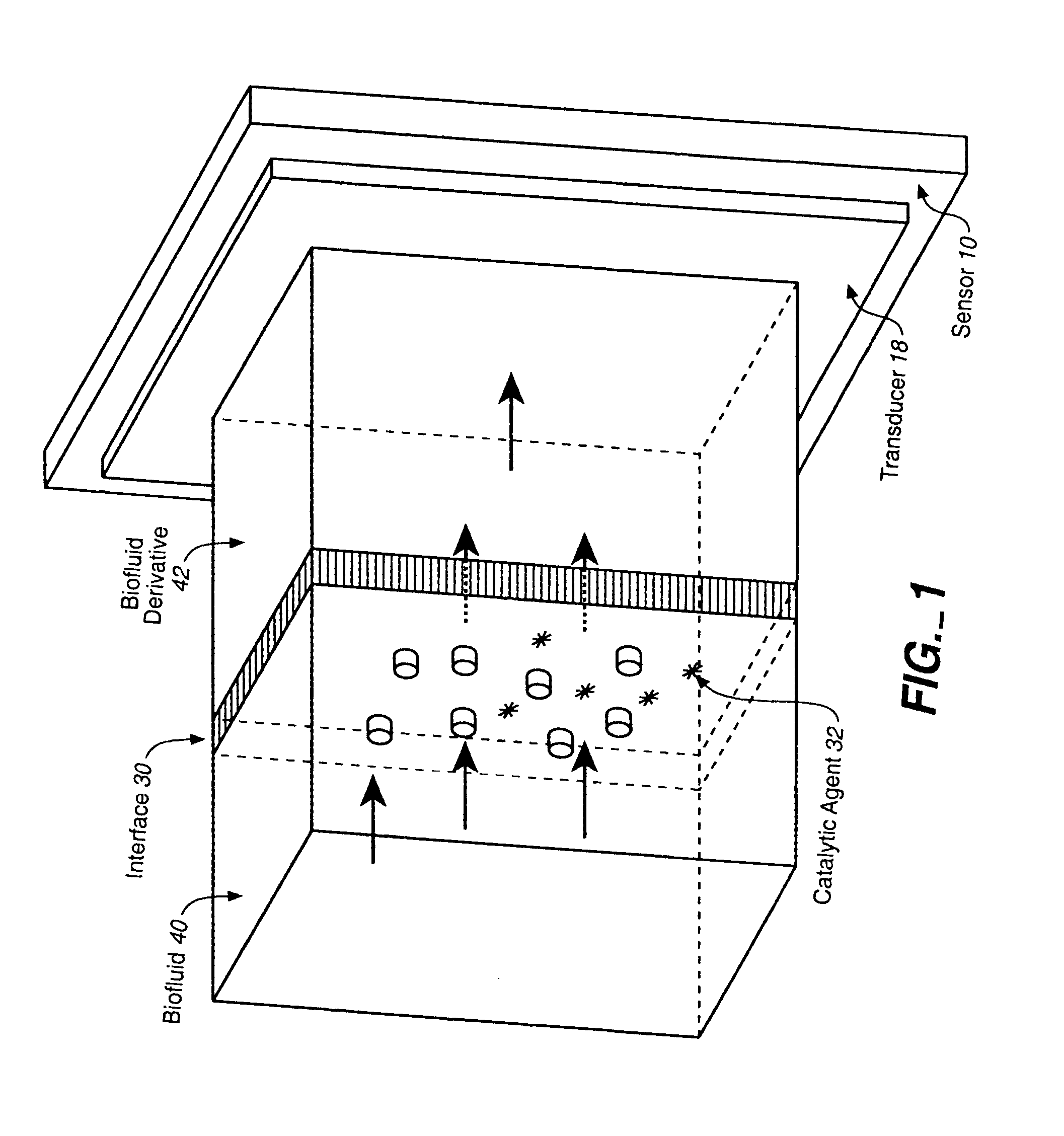
Analyte Sensor, and Associated System and Method Employing a Catalytic Agent
InactiveUS20070191701A1Good biocompatibilityExtend effective lifeImmobilised enzymesBioreactor/fermenter combinationsAnalyteTransducer
An analyte sensor for use in connection with a biofluid is described. The analyte sensor may comprise any suitable interface between the biofluid and a derivative of the biofluid and any suitable transducer of information concerning an analyte. At least one catalytic agent is provided in a locale or vicinity of the interface. The catalytic agent, such as a proteinaceous agent or a non-proteinaceous, organic-metal agent, is sufficient to catalyze the degradation of reactive oxygen and / or nitrogen species that may be present in the vicinity of the interface. An analyte-sensing kit and a method of sensing an analyte are also described.
Owner:ABBOTT DIABETES CARE INC
Biocompatibly coated medical implants
InactiveUS20050079200A1Easy to controlProperty variableStentsHeart valvesCarbon layerBiocompatible coating
Implantable medical devices with biocompatible coatings and processes for their production are described. The present invention relates in particular to medical implantable devices coated with a carbon-containing layer which devices are produced by at least partially coating the device with a polymer film and heating the polymer film in an atmosphere which is essentially free from oxygen to temperatures in the region of 200° C. to 2500° C., a carbon-containing layer being produced on the implantable medical device.
Owner:CINVENTION AG
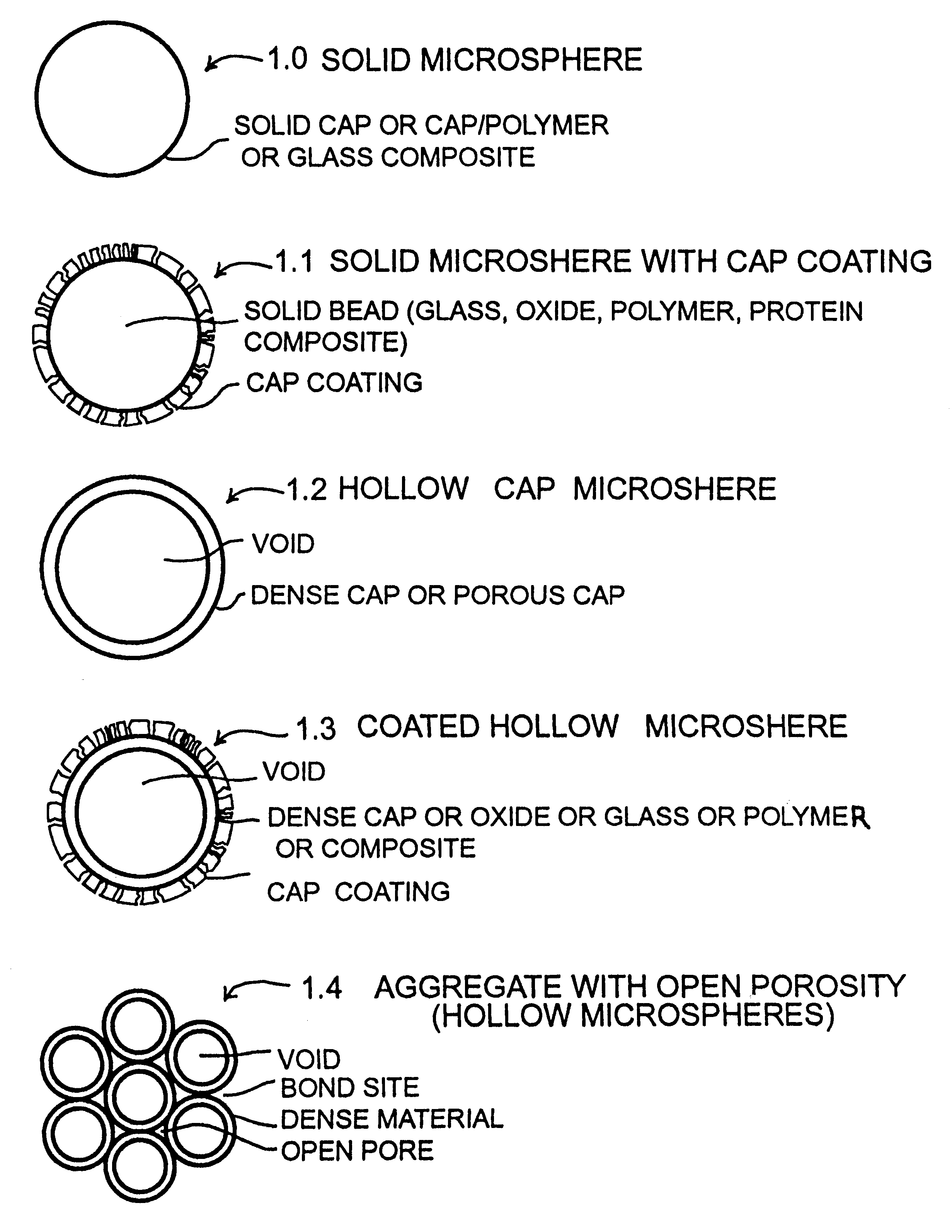
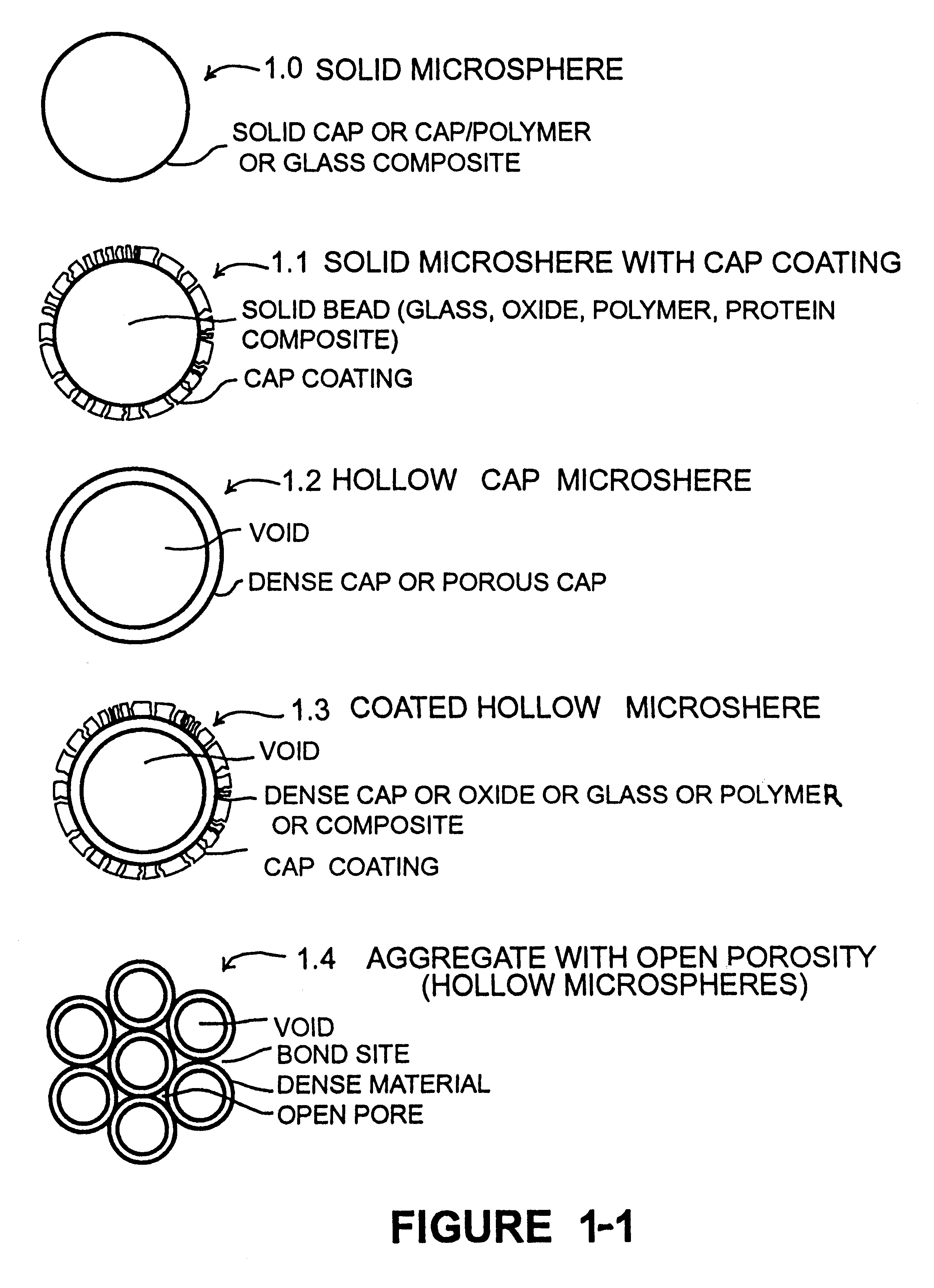
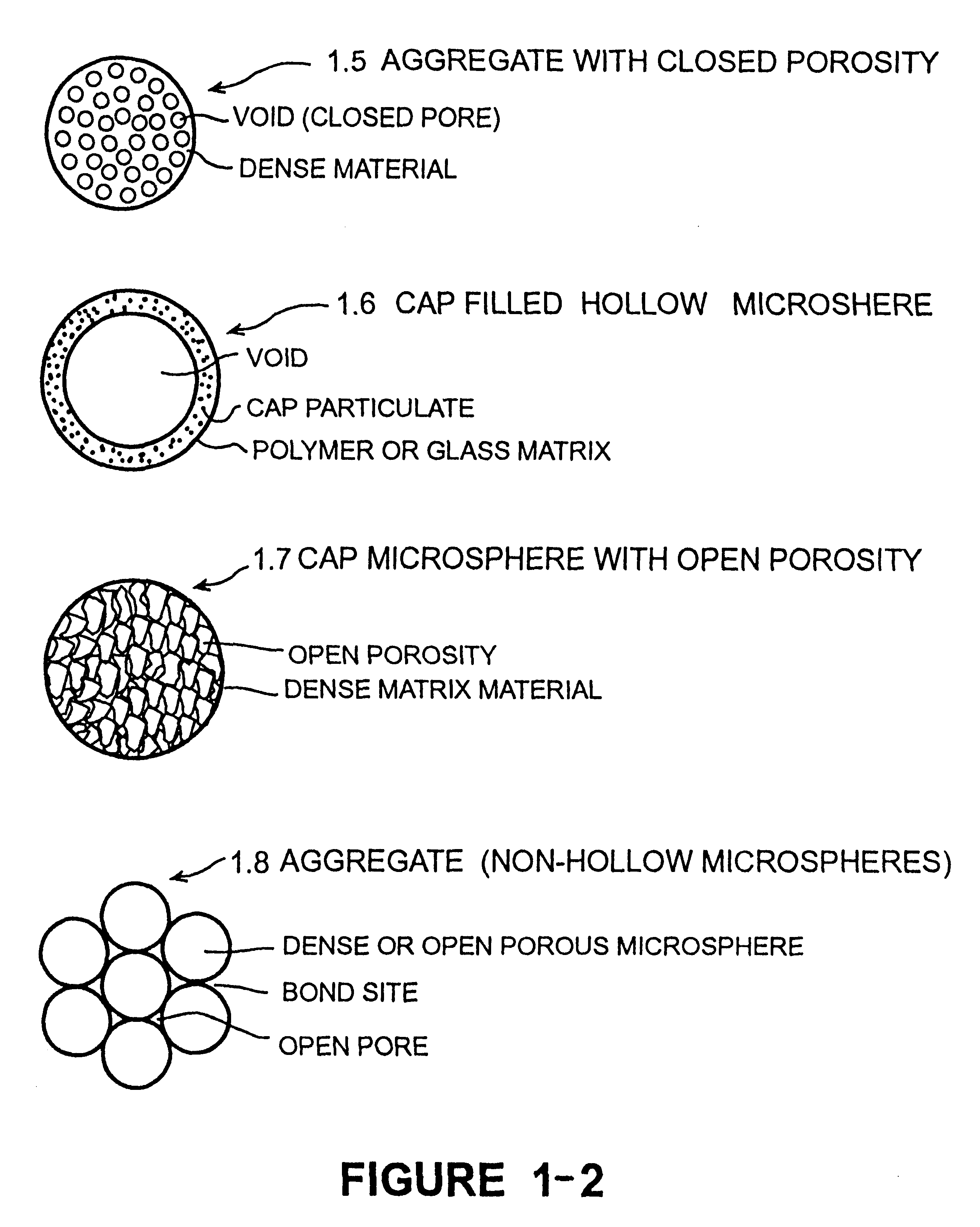
Calcium phosphate microcarriers and microspheres
InactiveUS6210715B1Increase resorbabilityCompressive strengthPowder deliveryBiocideChemistryCalcium biphosphate
The present invention provides calcium phosphate-based (CAP) microcarriers and microspheres and their use in cell culturing systems, chromatography and implantable biomedical materials.
Owner:CAP BIOTECH
Medical device
InactiveUS20050002981A1Reduce connective tissue hyperplasiaReduce restenosisStentsPeptide/protein ingredientsBiological propertyConnective tissue fiber
The present invention relates to the use of a gene transfer product to reduce hyperplastic connective tissue growth after tissue trauma or implantation of a medical device. The present invention also relates to a medical device with improved biological properties for an at least partial contact with blood, bodily fluids and / or tissues when introduced in a mammalian body, which device comprises a core and a nucleic acid, encoding a product capable of leading to production of extracellular superoxide dismutase present in a biologically compatible medium. Said nucleic acid encodes a translation or transcription product, which is capable of inhibiting hyperplastic connective tissue growth and promoting endothelialisation in vivo at least partially on a synthetic surface of said core. The present invention also relates to a method of producing a medical device according to the invention.
Owner:FIT BIOTECH OY PLC
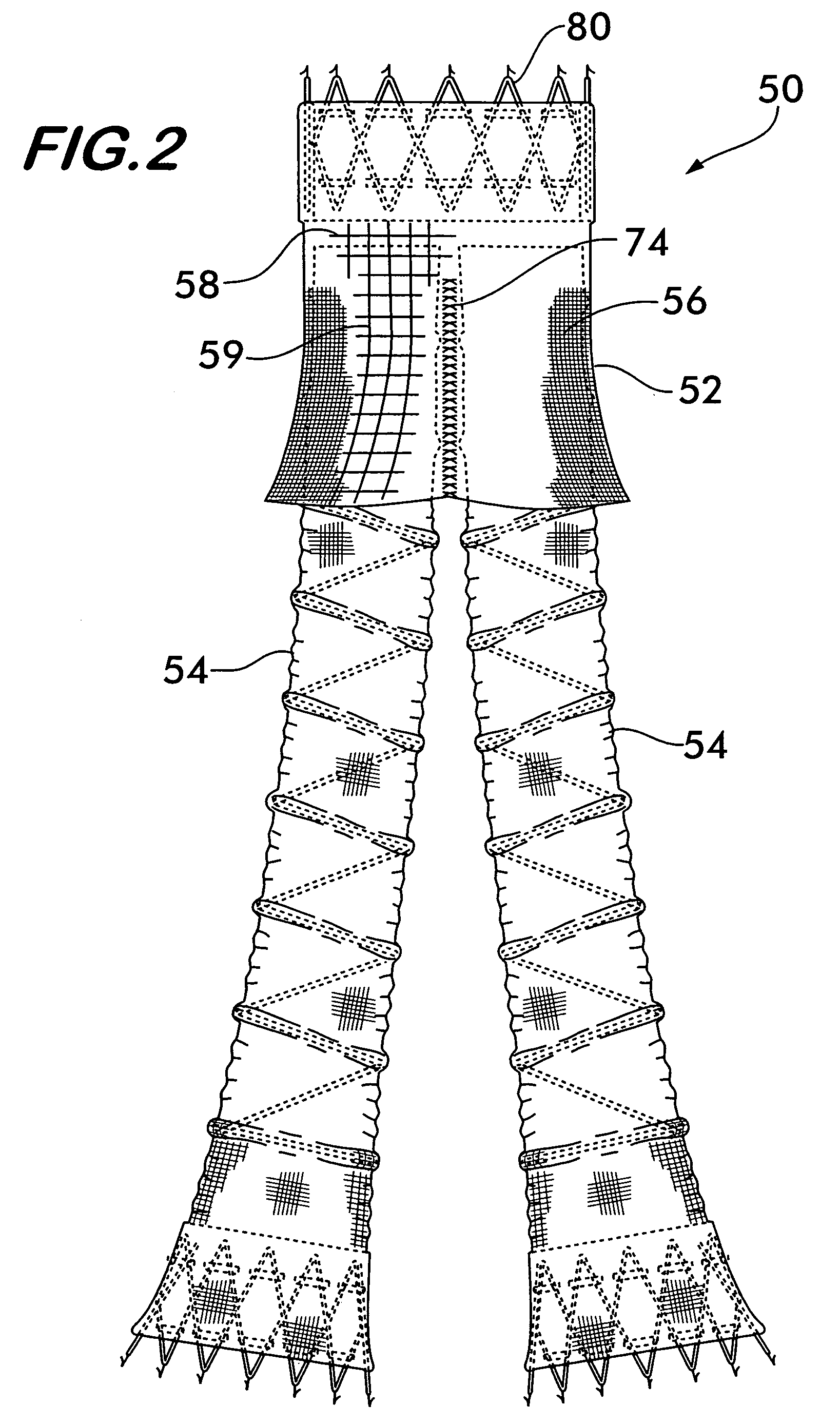
Coated sling material
InactiveUS7025063B2Minimize complexityGood biocompatibilitySuture equipmentsAnti-incontinence devicesUrethraShort urethra
The present invention relates to a sling, methods of making and using a sling, and kits comprising a sling for treating urinary incontinence. The sling has multiple elongation properties that serve to improve the support of the urethra. The sling may comprise a coated material adapted for urethral suspension. The coated sling has properties that appear to enhance the sling elongation characteristics. The coated sling further includes properties that reduce its susceptibility to bacterial infections. The sling further includes properties to enhance the proper tensioning of the sling.
Owner:BOSTON SCI SCIMED INC
Artificial heart valve
InactiveUS6991649B2Increase flow ratePrevent thrombosisHeart valvesJoint implantsMitral valve leafletPivot joint
Owner:SIEVERS HANS HINRICH
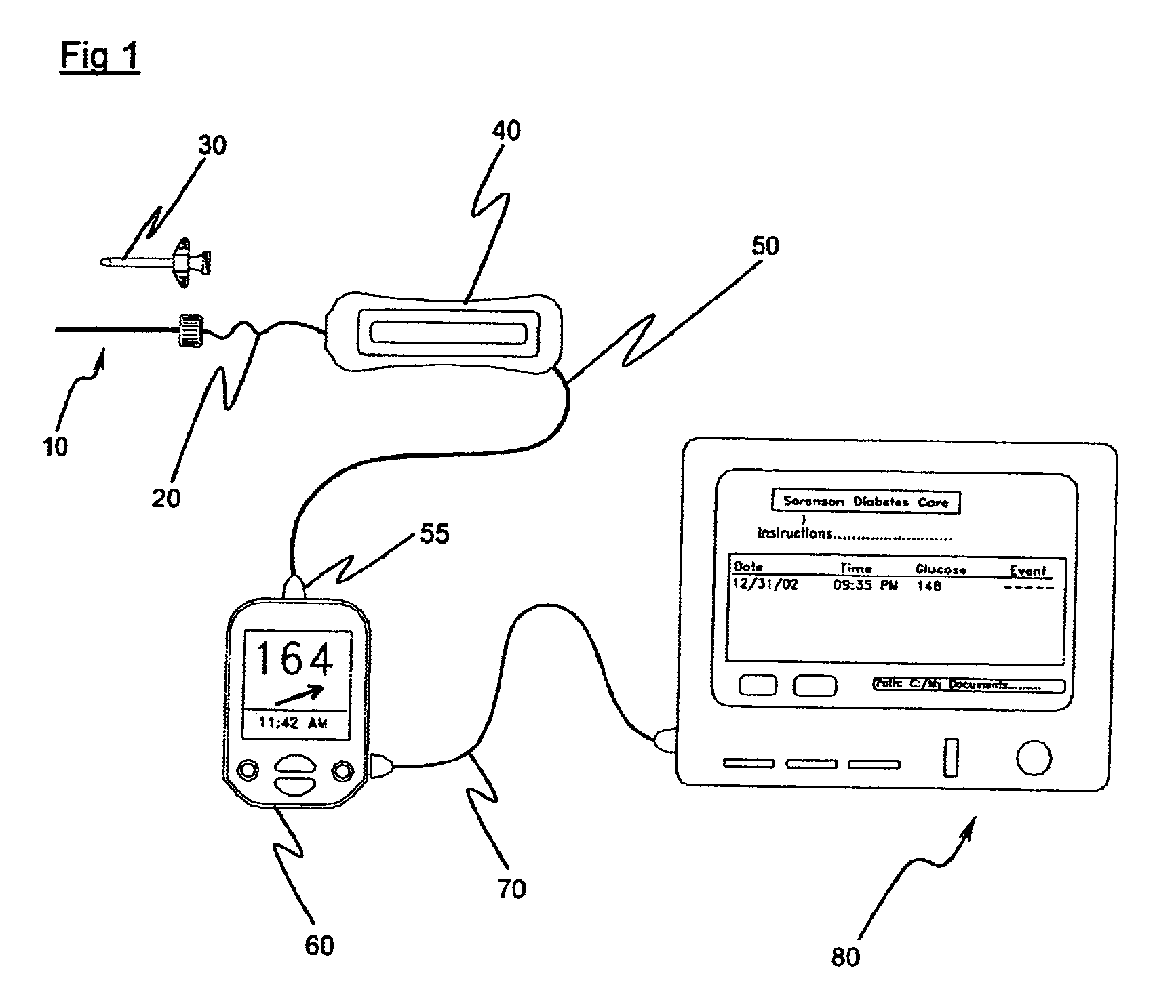
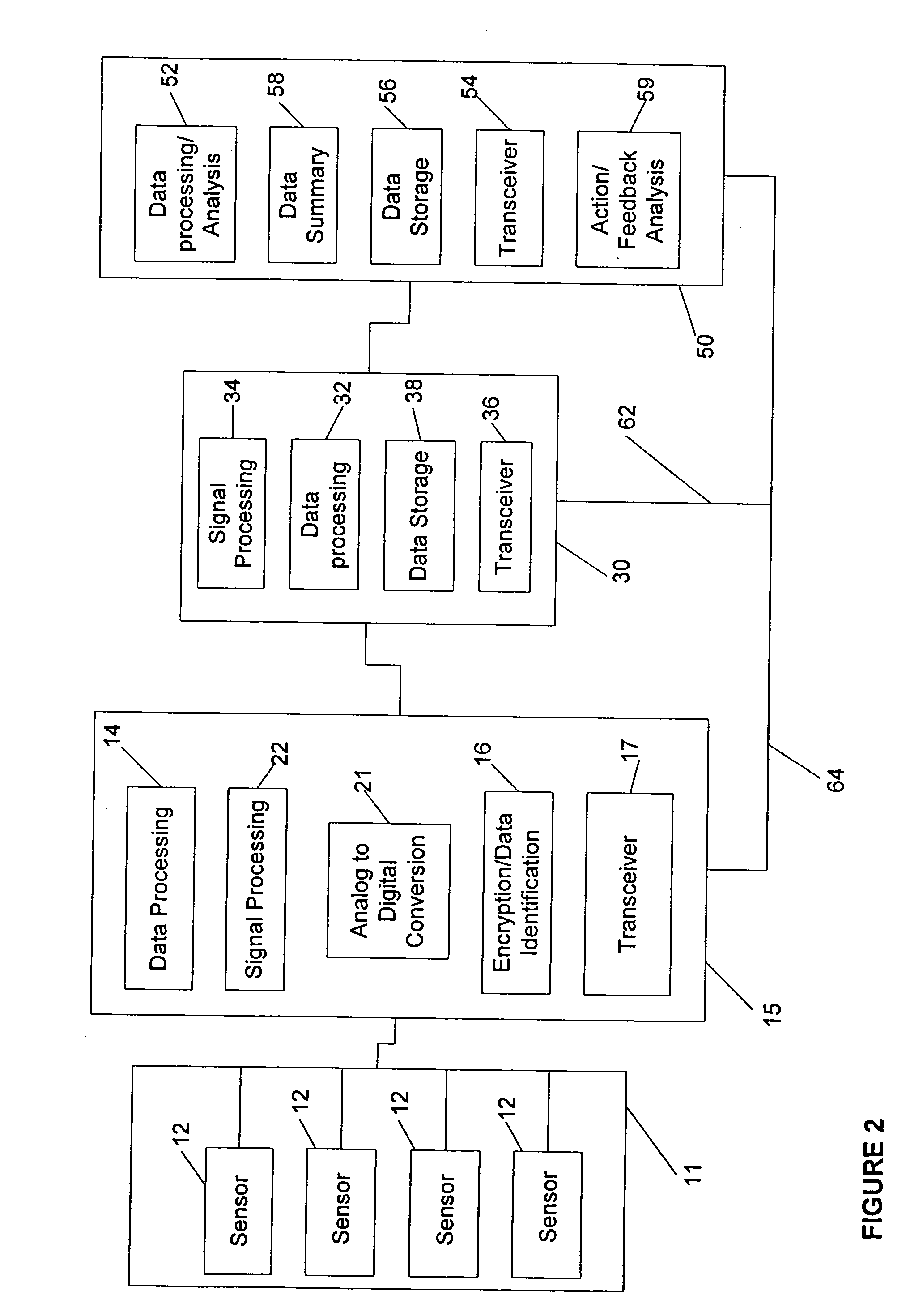
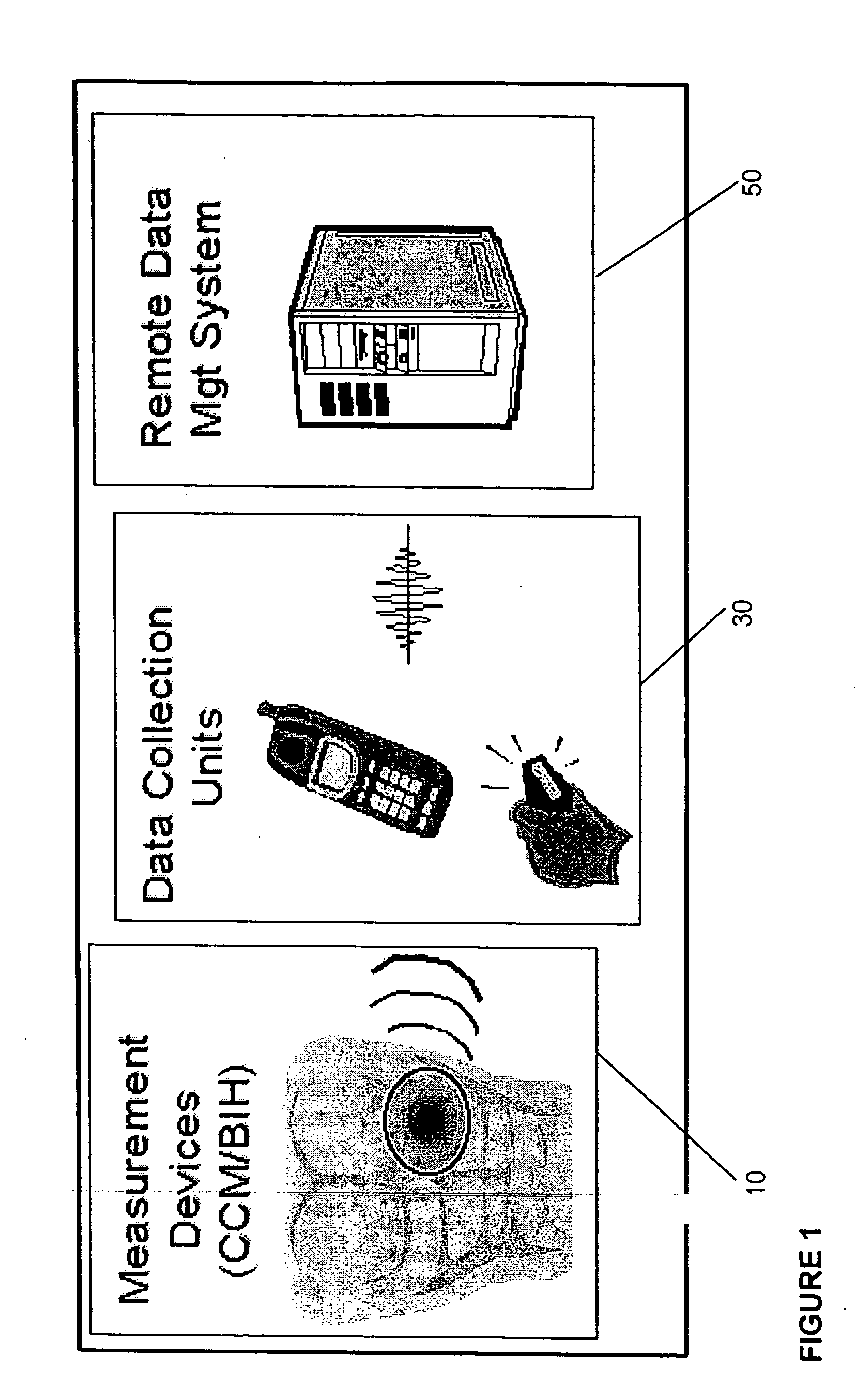
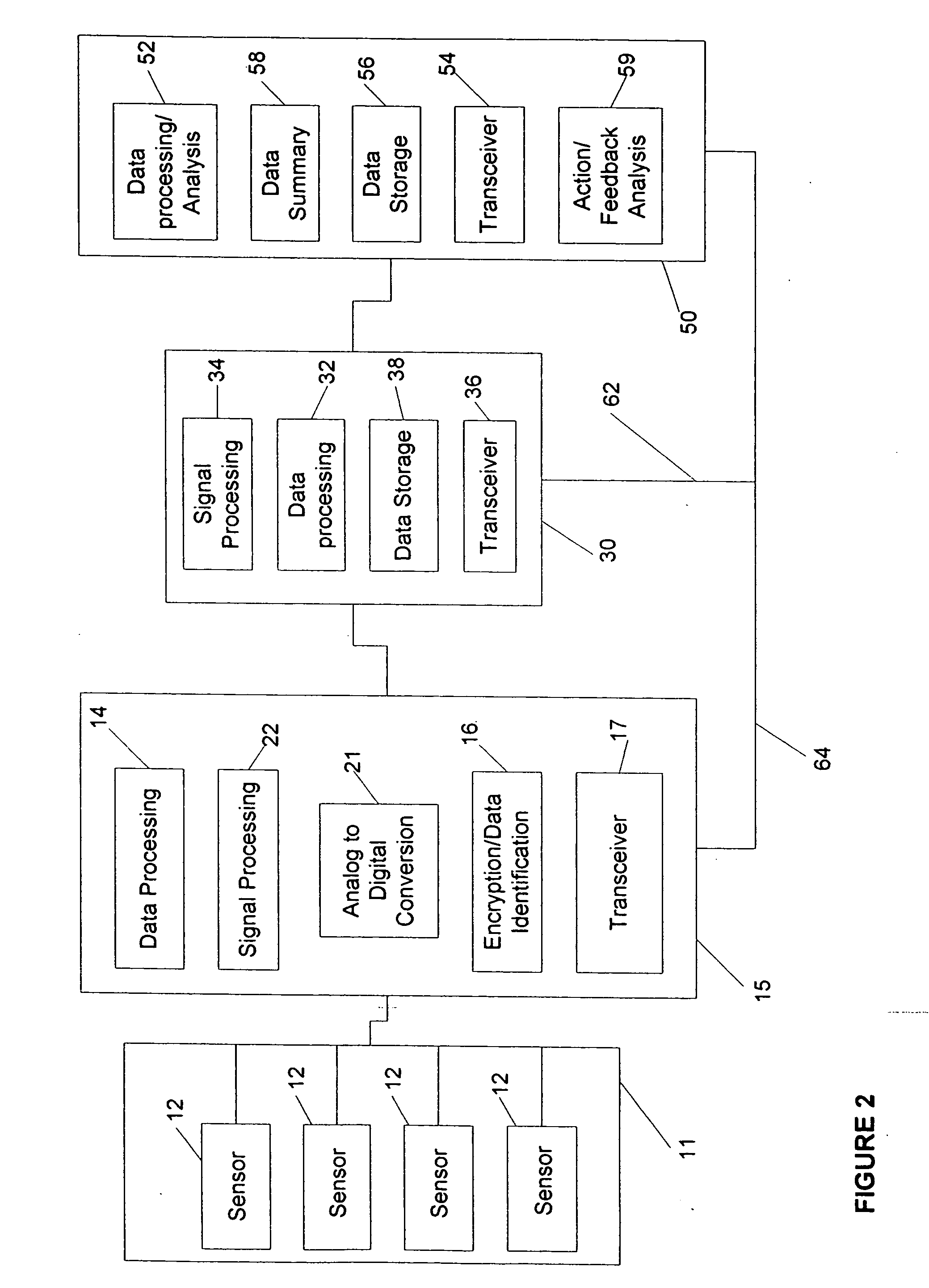
Gateway platform for biological monitoring and delivery of therapeutic compounds
InactiveUS20070060800A1Decrease adhesion and encapsulationImprove data qualityBioelectric signal measurementDrug and medicationsBiomonitoringComputer science
The invention relates to methods and devices for remote or distributed continuous monitoring of physiologically relevant states. The invention provides for methods to automatically detect deviations or other states in physiological parameters and automatically alert a measured subject, user or other authorized party. The device provides for a universal platform for sensors, and further provides for the automatic compensation or distribution of devices or bioactive agents at appropriate levels and / or intervals in response to deviations or other states sensed in various physiological parameters.
Owner:PHILOMETRON
Embolic Device Delivery Systems
InactiveUS20090287291A1Minimizing in-catheter/sheath forceImproves device trackabilityDiagnosticsDilatorsCompressibilityVolumetric Mass Density
Embolic implants, delivery systems and methods of manufacture and delivery are disclosed. The devices can be used for aneurysm treatment and / or parent vessel occlusion. Implant designs offer low profile compressibility for delivery to neurovasculature, while maintaining other necessary features such as density for occlusion purposes and desirable radial strength characteristics.
Owner:TYCO HEALTHCARE GRP LP
Knee joint prosthesis
InactiveUS20050043808A1Eliminate the effects ofImprove propertiesImpression capsAnkle jointsTissue repairProsthesis
A method, and related composition and apparatus for repairing a tissue site. The method involves the use of a curable polyurethane biomaterial composition having a plurality of parts adapted to be mixed at the time of use in order to provide a flowable composition and to initiate cure. The flowable composition can be delivered using minimally invasive means to a tissue site and there fully cured provide a permanent and biocompatible prosthesis for repair of the tissue site. Further provided are a mold apparatus, e.g., in the form of a balloon or tubular cavity, for receiving a biomaterial composition, and a method for delivering and filling the mold apparatus with a curable composition in situ to provide a prosthesis for tissue repair.
Owner:ADVANCED BIO SURFACE
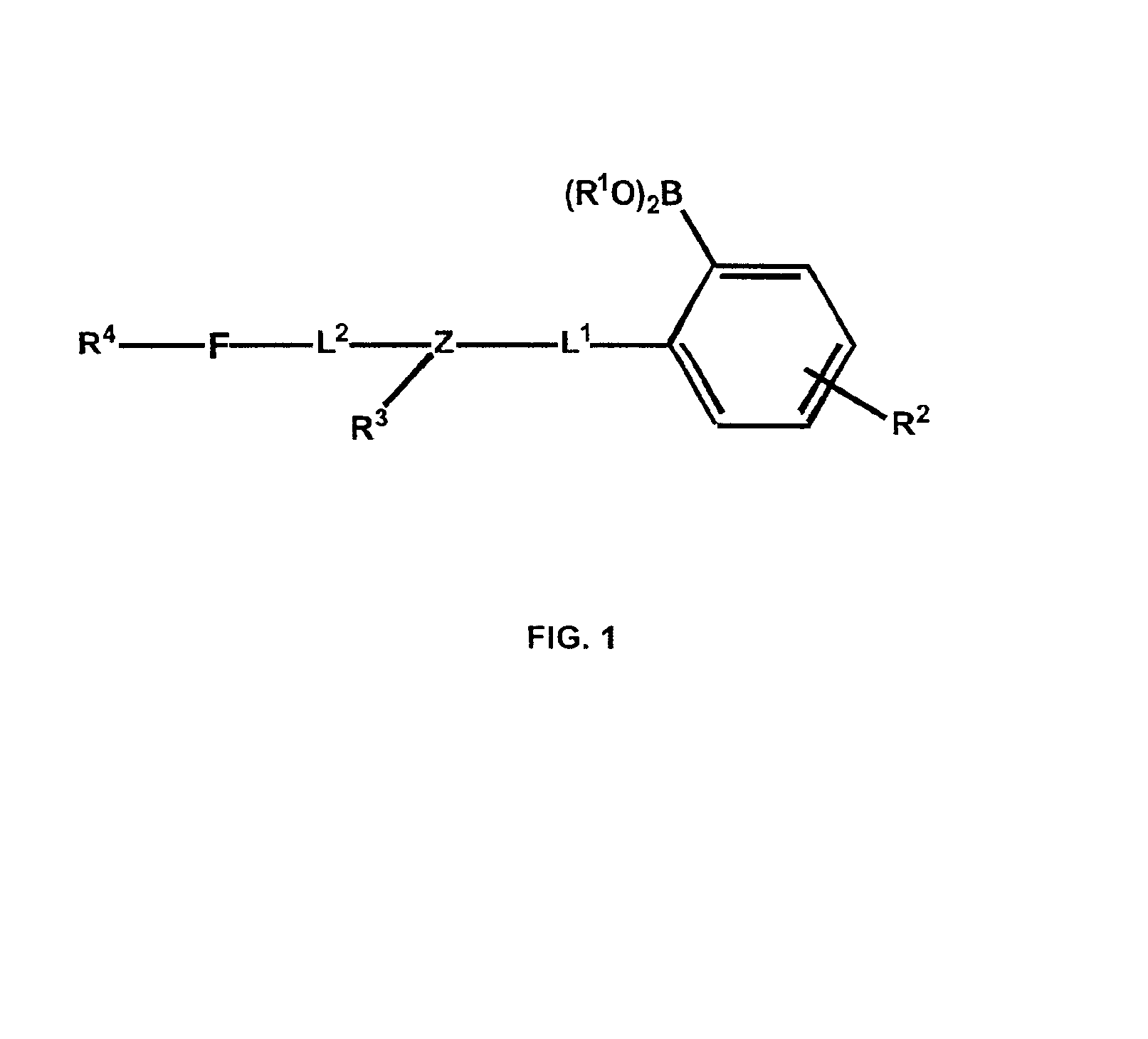
Polymers functionalized with fluorescent boronate motifs and methods for making them
InactiveUS6927246B2Enhance swellabilityGood biocompatibilityGroup 3/13 element organic compoundsBiological testingAnalyteConcentrations glucose
Improved polymer matrices which incorporate fluorescent biosensor molecules as well as methods of making and using these polymer matrices are described. Such matrices can be used in fluorescent biosensors and biosensor systems, including those which are used in the detection of polyhydroxylated analytes such as glucose. The properties of the polymer matrices of the invention renders biosensors utilizing such matrices particularly well-suited for detecting and measuring in-vivo glucose concentrations.
Owner:MEDTRONIC MIMIMED INC
Membrane suitable for use in an analyte sensor, analyte sensor, and associated method
ActiveUS7699964B2Facilitate linear responsiveness and calibration and stabilityPreserve and improve performance of sensorImmobilised enzymesBioreactor/fermenter combinationsMetaboliteAmperometric biosensor
A multifunctional membrane is provided. The multifunctional membrane is suitable for use in an analyte sensor. In a particular application, the multifunctional membrane may be used in connection with an amperometric biosensor, such as a transcutaneous amperometric biosensor. Some functions of the membrane are associated with properties of membrane itself, which is comprised of crosslinked polymers containing heterocyclic nitrogen groups. For example, the membrane, by virtue of its polymeric composition, may regulate the flux of an analyte to a sensor. Such regulation generally improves the kinetic performance of the sensor over a broad range of analyte concentration. Other functions of the membrane are associated with functional components, such as a superoxide-dismutating / catalase catalyst, either in the form of an enzyme or an enzyme mimic, that can be bound to the scaffold provided by the membrane. The effect of any such enzyme or enzyme mimic is to lower the concentration of a metabolite, such as superoxide and / or hydrogen peroxide, in the immediate vicinity of the sensing layer of the biosensor. Lowering the concentrations of such metabolites, which are generally deleterious to the function of the sensor, generally protects or enhances biosensor integrity and performance. The membrane is thus an important tool for use in connection with analyte sensors, amperometric sensors, biosensors, and particularly, transcutaneous biosensors. A membrane-covered sensor and a method for making same are also provided.
Owner:ABBOTT DIABETES CARE INC
Implantable biosensor system, apparatus and method
InactiveUS20050183954A1Good biocompatibilityIncrease volumeImmobilised enzymesBioreactor/fermenter combinationsForeign matterInterstitial glucose
An implantable biosensor assembly and system includes an enzymatic sensor probe from which subcutaneous and interstitial glucose levels may be inferred. The assembly may be associated by direct percutaneous connection with electronics, such as for signal amplification, sensor polarization, and data download, manipulation, display, and storage. The biosensor comprises a miniature probe characterized by lateral flexibility and tensile strength and has a large electrode surface area for increased sensitivity. Irritation of tissues surrounding the probe is minimized due to ease of flexibility and small cross section of the sensor. Foreign body reaction is diminished due to a microscopically rough porous probe surface.
Owner:SORENSON DEVMENT
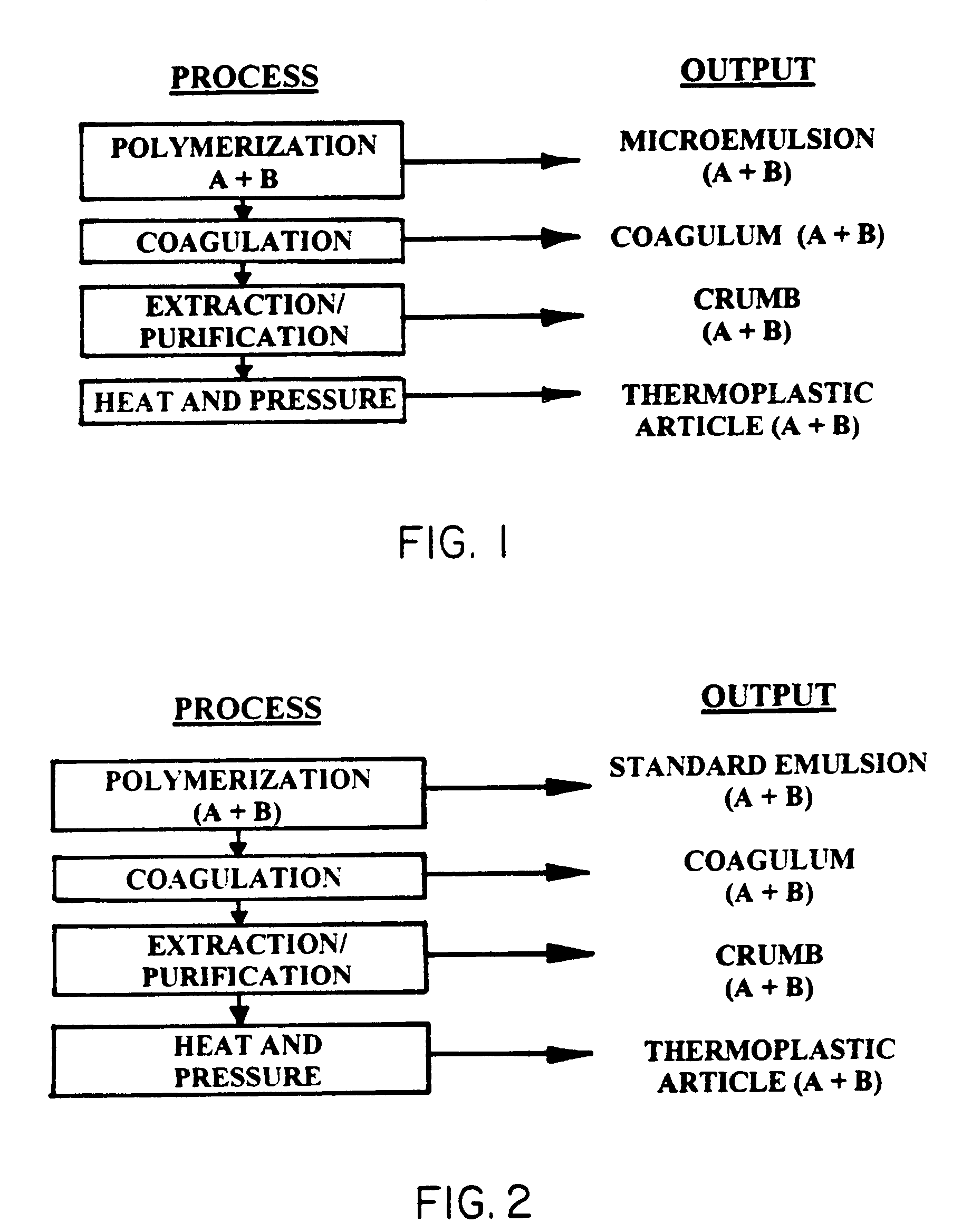
Thermoplastic copolymer of tetrafluoroethylene and perfluoromethyl vinyl ether and medical devices employing the copolymer
An improved elastomeric material is described comprising an essentially noncross-linkable amorphous copolymer of tetrafluoroethylene (TFE) and perfluoromethyl vinyl ether (PMVE) which is both a thermoplastic and exhibits exceptional mechanical properties. This material is particularly suitable for use in ultra-clean environments, and particularly for use in an implantable device, since it does not contain contaminants that previous thermoset TFE / PMVE copolymers have required. Among the improved properties of the present invention are excellent biocompatibility, high matrix tensile strength, high clarity, high abrasion resistance, high purity, adequate elasticity, and ease of processing due to the thermoplastic, and noncross-linkable structure of the copolymer. The material of the present invention is also a high strength bonding agent particularly suited for bonding porous PTFE to itself or to other porous substances at room or elevated temperatures.
Owner:WL GORE & ASSOC INC
Implant with composite coating
InactiveUS6261322B1High strengthCost effectiveImpression capsBone implantBiocompatible coatingBiocompatibility Testing
Systems and methods are described for implants with composite coatings to promote tissue in-growth and / or on-growth. An implant includes: a substrate; a structured surface formed on at least a portion of the substrate; and a biocompatible coating deposited on at least a fraction of the structured surface. The systems and methods provide advantages in that the implant has good biocompatibility while the biocompatible coating has good strength.
Owner:SHALBY ADVANCED TECH INC
Integral support stent graft assembly
ActiveUS7122052B2Promote formationGood biocompatibilityStentsBlood vesselsStent graftingInsertion stent
A stent graft is disclosed having a tubular graft formed from flexible filamentary members interwoven with a supporting monofilament defining radial corrugations around the graft. The ends of the graft are tapered from a smaller to a larger diameter and have stents attached thereto for engagement with a vascular vessel. The stents are attached to the graft by passing through two rows of slots cut in the graft and arranged in spaced relation to one another. The slots are positioned on a reverse fold of the graft to capture the stent. The stent graft may be modular and have limbs insertable into a base module. The base module has a main central space in communication with branch central spaces defined by a line of attachment between the branch central spaces. The terminus of the line of attachment forms a shoulder for retaining the limbs.
Owner:THE SECANT GRP
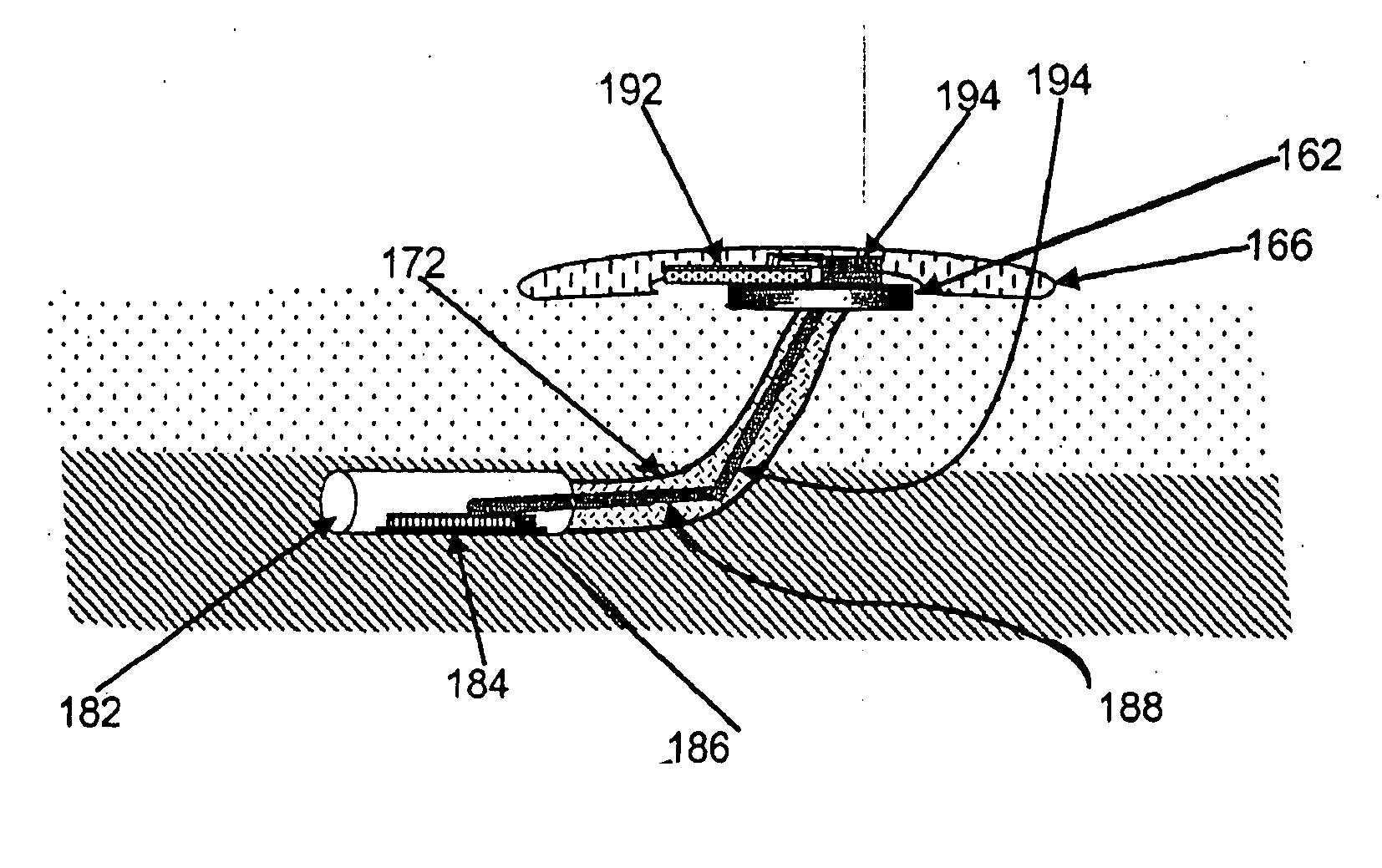
Implantable particles for urinary incontinence
InactiveUS6335028B1Less invasive effectGood biocompatibilityPowder deliveryAntipyreticCell adhesionWrinkle
Owner:BIOSPHERE MEDICAL INC
Gateway platform for biological monitoring and delivery of therapeutic compounds
InactiveUS20060253005A1Decrease adhesion and encapsulationImprove data qualityBioelectric signal measurementDrug and medicationsBiomonitoringComputer science
Owner:PHILOMETRON
Coated vascular grafts and methods of use
InactiveUS6939377B2Provide strengthProvide compliancePharmaceutical containersPretreated surfacesPorous coatingMedicine
A vascular graft, such as an AAA stent graft, includes a core zone of PET fabric with a non-porous coating comprising a polyurethane, such as Thoralon®, disposed on at least one surface. The coating provides a barrier to prevent short and long term leakage of fluids through the pores of the PET fabric core zone. A method for sealing the pores of a porous PET graft includes the step of coating at least one surface of said graft with at least one polyurethane, or mixtures and combinations thereof. Preferably, the coating is Thoralon®. A method for making a vascular prosthesis includes the steps of providing a core zone of PET fabric, and coating at least one surface of the core zone with at least one polyurethane, or mixtures and combinations thereof, such as Thoralon®. Finally, a method of repairing or treating a defective vessel includes the step of reinforcing or replacing the defective vessel with a graft according to the invention.
Owner:TC1 LLC
Methods of synthesis and use of chemospheres
InactiveUS20110104052A1Prevents immunoclearanceExtended half-lifePowder deliveryBiocideDiagnostic agentComputed tomography
The present invention provides, in general, compositions comprising a hydrogel and an agent, for example a therapeutic agent or an imaging agent, for locoregional delivery. In certain preferred embodiments of the invention, the hydrogel compositions are detectable by Magnetic Responance and CT Scan and are used for locoregional delivery of therapeutic agents, for example chemotherapeutic agents. The invention also features polymer matrix compositions comprising nanoparticles that can be loaded after polymerization with bioactive agents, for example a diagnostic agent or therapeutic agent.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
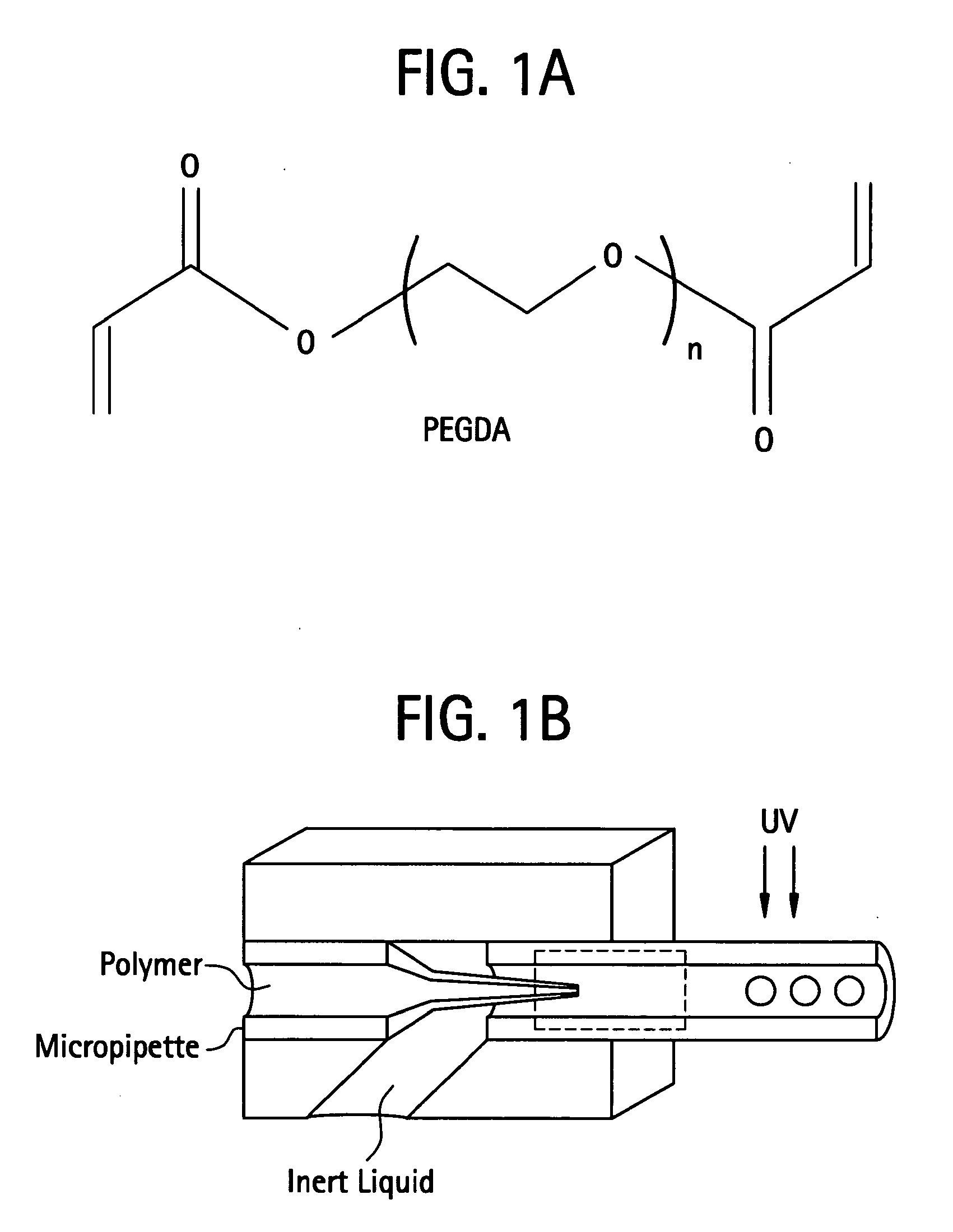
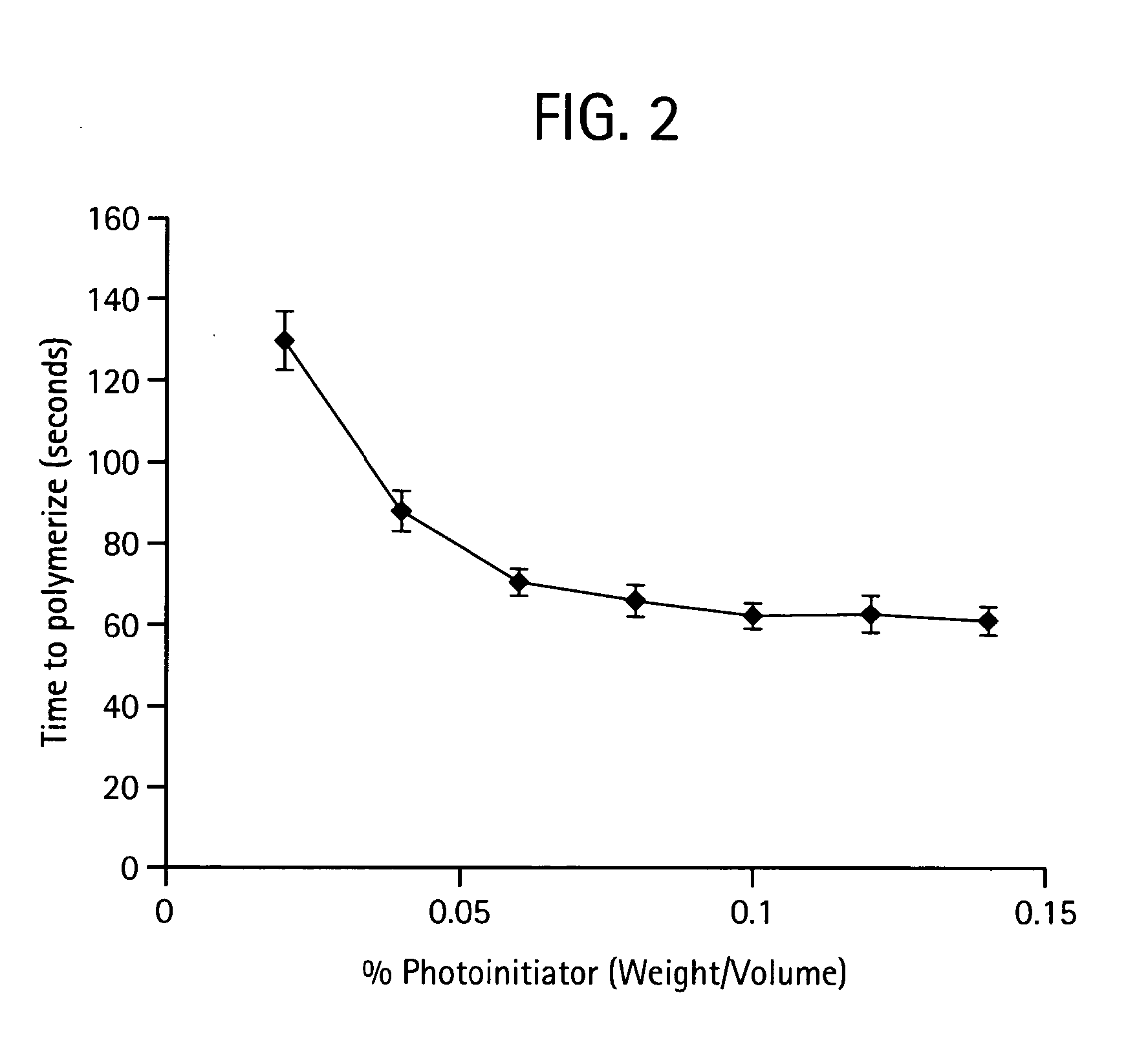
Photopolymerizable biodegradable hydrogels as tissue contacting materials and controlled-release carriers
InactiveUS6306922B1Fast gelationRapid polymerizationImmobilised enzymesPowder deliveryThermal energyUltraviolet lights
Hydrogels of polymerized and crosslinked macromers comprising hydrophilic oligomers having biodegradable monomeric or oligomeric extensions, which biodegradable extensions are terminated on free ends with end cap monomers or oligomers capable of polymerization and cross linking are described. The hydrophilic core itself may be degradable, thus combining the core and extension functions. Macromers are polymerized using free radical initiators under the influence of long wavelength ultraviolet light, visible light excitation or thermal energy. Biodegradation occurs at the linkages within the extension oligomers and results in fragments which are non-toxic and easily removed from the body. Preferred applications for the hydrogels include prevention of adhesion formation after surgical procedures, controlled release of drugs and other bioactive species, temporary protection or separation of tissue surfaces, adhering of sealing tissues together, and preventing the attachment of cells to tissue surfaces.
Owner:BOARD OF REGENTS
Implantable Stimulation Electrode with a Coating for Increasing Tissue Compatibility
InactiveUS20080234790A1Avoid tissue irritationGood biocompatibilitySurgeryInternal electrodesImplantable Stimulation ElectrodesIrritation
An implantable stimulation electrode for use with an implantable tissue stimulator, especially a pacemaker, a defibrillator, a bone stimulator or a neurostimulator includes a metal base body, optionally one or more intermediate layers disposed on the base body and a coating covering the base body and, optionally, intermediate layers in order to increase tissue compatibility. The coating should prevent tissue irritations after implantation and more particularly increase the stimulus threshold associated therewith, have very high biocompatibility and also has an anti-inflammatory effect. An increase in tissue compatibility is achieved by virtue of the fact that the coating has a polysaccharide layer made of hyaluronic acid and / or hyaluronic acid derivatives.
Owner:BIOTRONIK MESS UND THERAPIEGERAETE GMBH & CO
Natural plant extract composition and application thereof in cosmetics
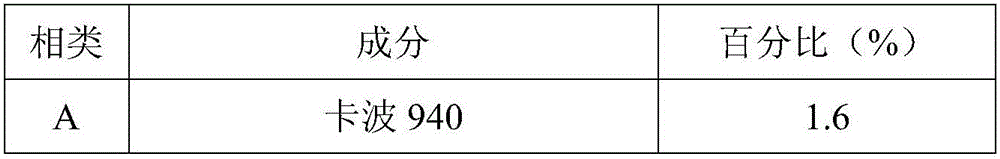
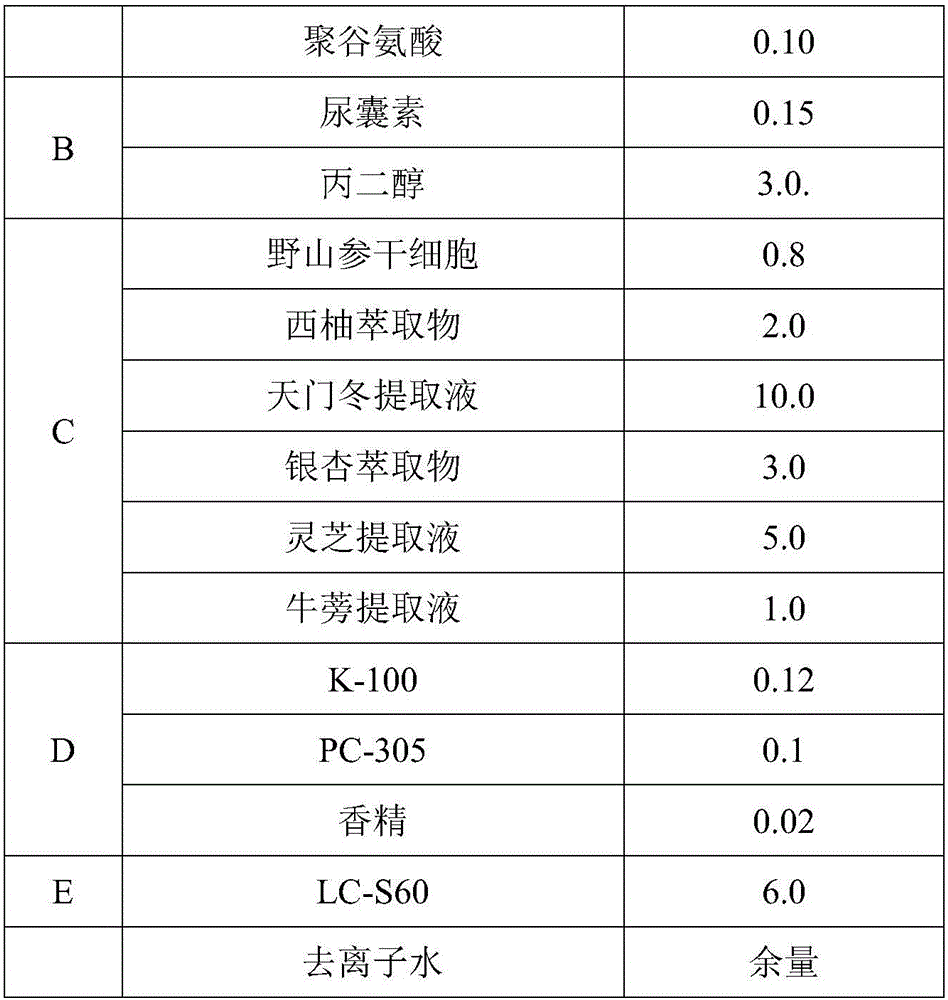
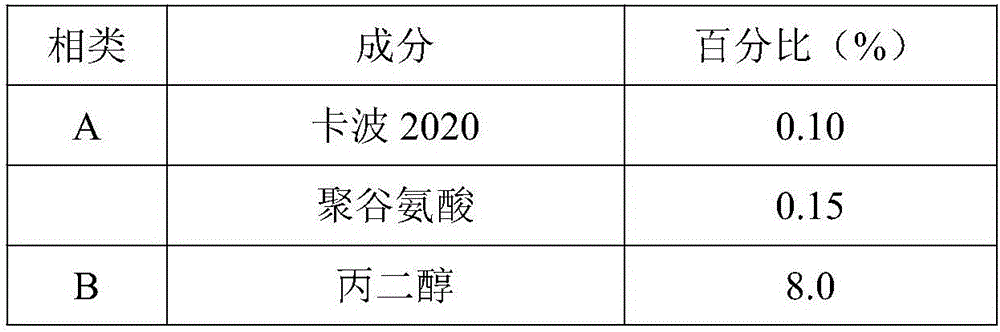
ActiveCN105748343ASpeed up the repair processIncrease elasticityCosmetic preparationsToilet preparationsBiologyInflammation
The invention relates to the technical field of cosmetics, in particular to a natural plant extract composition and application thereof in the cosmetics. The natural plant extract composition is prepared from the following raw materials in parts by weight: 0.1 to 10 parts of plant stem cells, 2 to 10 parts of grapefruit extracts, 1 to 10 parts of radix asparagi extracting solution, 1 to 10 parts of gingko extracts, 5 to 10 parts of lucid ganoderma extracting solution, and 1 to 5 parts of burdock extracting solution. The natural plant extract composition provided by the invention not only has remarkable moisturizing and anti-aging effect, but also has the effects in removing acnes, adjusting grease secretion, resisting inflammation and relieving, and can be used for preparing moisturizing, anti-aging or acne-removing cosmetics.
Owner:GUANGDONG COOWAY BIOTECH CO LTD
Implantable sling having bladder support
ActiveUS20050080317A1Sufficient degree of supportEliminate dependenciesAnti-incontinence devicesWound clampsUrethraCystocele repair
Surgical implants operative to simultaneously function as a pubovaginal sling for the treatment of incontinence and as a support member to effectuate cystocele repair. The implant comprises a first sling portion operative to be positioned beneath the urethra, per conventional pubovaginal sling surgery. The implant further includes a second bladder support portion extending from the sling support portion that is oriented to extend beneath and be surgically attached to a portion of the bladder to thus enable the same to be supported to a degree necessary to effectuate cystocele repair. The implant may be fabricated from a unitary piece of harvested tissue, synthetic material or combinations thereof. Preferably, the sling portion of the implant is fabricated from a synthetic material whereas the bladder support portion of the implant comprises a segment of harvested tissue sewn to the sling portion.
Owner:CALDERA MEDICAL
Implants with functionalized carbon surfaces
InactiveUS20050079201A1Potentiate effectAdd to effectSurgeryPharmaceutical containersActivated carbonBiomedical engineering
The invention relates to a method of producing medical implants having functionalized surfaces by providing a medical implant with at least one carbon-based layer on at least one part of the surface of the implant, activating the carbon-based layer by creating porosity and functionalizing the activated carbon-based layer. This invention also relates to functionalized implants obtained in by this method.
Owner:CINVENTION AG
Drug delivery compositions and medical devices containing block copolymer
InactiveUS6855770B2Good mechanical integrityGood biocompatibilityOrganic active ingredientsGenetic material ingredientsThermoplasticMethacrylate
A composition for delivery of a therapeutic agent is provided. The composition comprises: (a) a biocompatible block copolymer comprising one or more elastomeric blocks and one or more thermoplastic blocks and (b) a therapeutic agent, wherein the block copolymer is loaded with the therapeutic agent. The block copolymer is preferably of the formula X—(AB)n, where A is an elastomeric block, B is a thermoplastic block, n is a positive whole number and X is a seed molecule. The elastomeric blocks are preferably polyolefin blocks, and the thermoplastic blocks are preferably selected from vinyl aromatic blocks and methacrylate blocks. According to another aspect of the invention, a medical device is provided, at least a portion of which is insertable or implantable into the body of a patient. The medical device comprises (a) the above biocompatible block copolymer and (b) a therapeutic agent, wherein the block copolymer is loaded with the therapeutic agent. According to another aspect of the present invention, a method of treatment is provided in which the above device is implanted or inserted into a patient, resulting in the release of therapeutic agent in the patient over an extended period. According to yet another aspect of the invention, a coated medical device is provided which comprises: (a) an intravascular or intervascular medical device and (b) a coating over at least a portion of the intravascular or intervascular a medical device, wherein the coating comprises the above biocompatible block copolymer.
Owner:SCI MED LIFE SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com