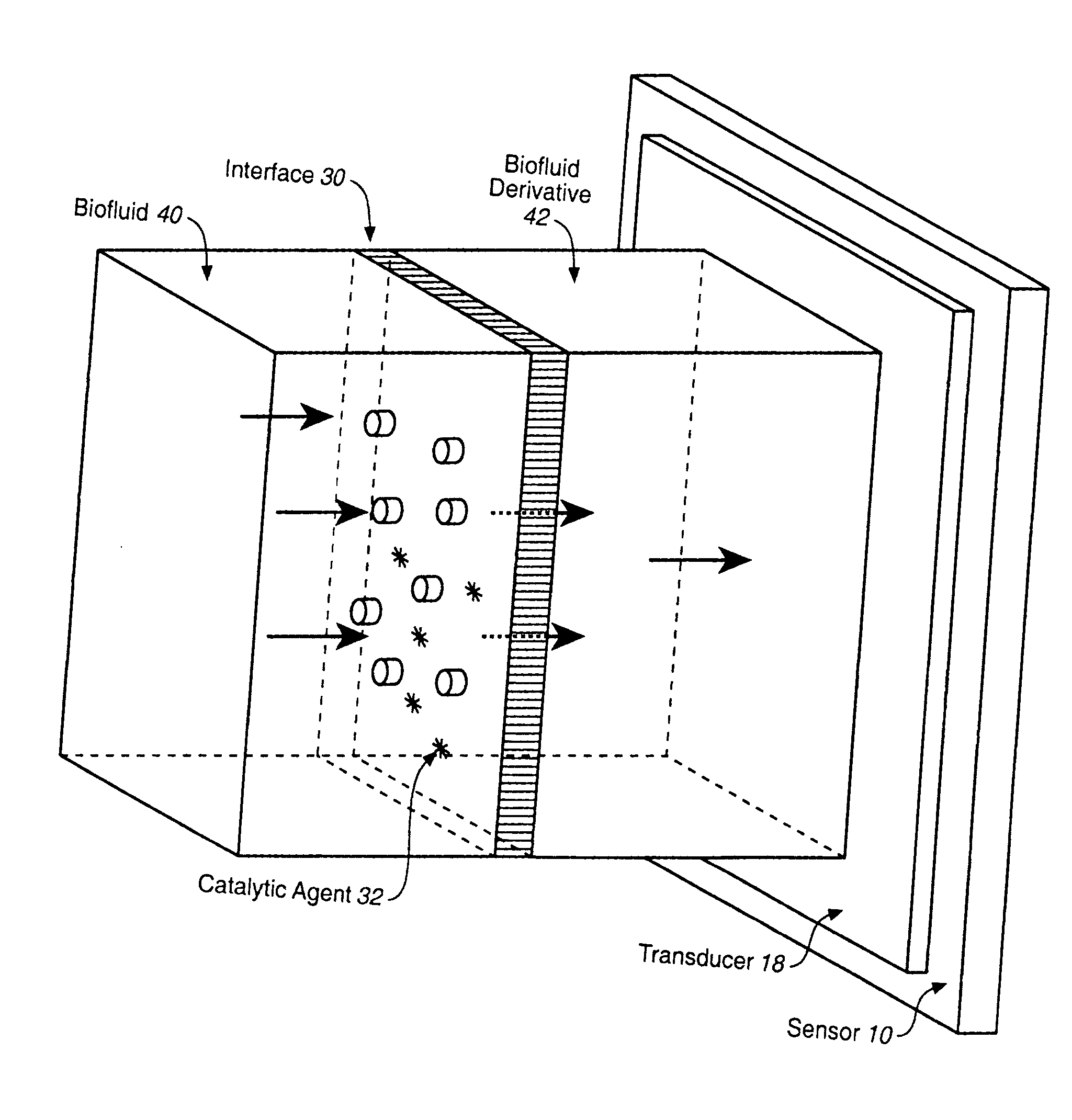
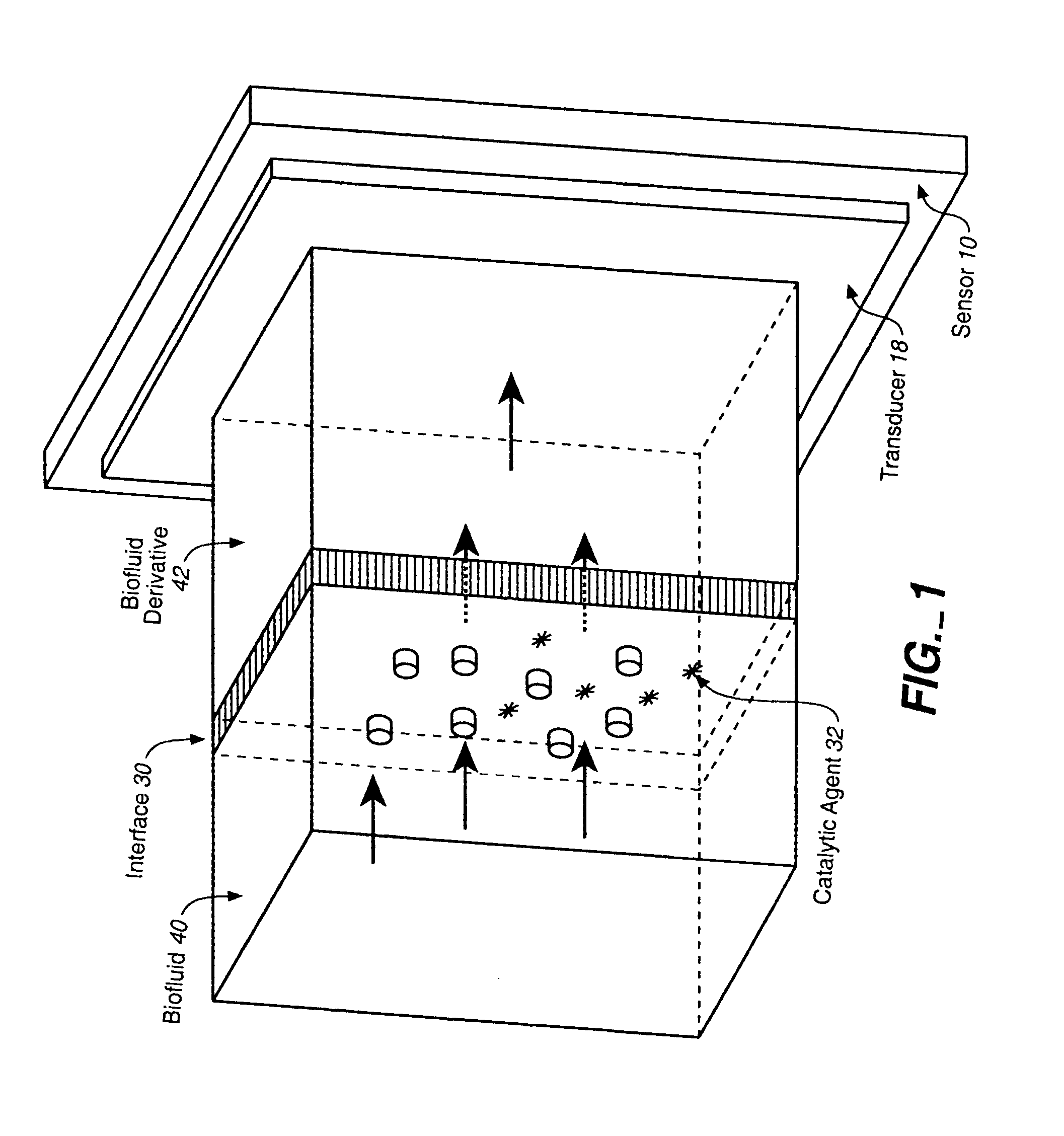
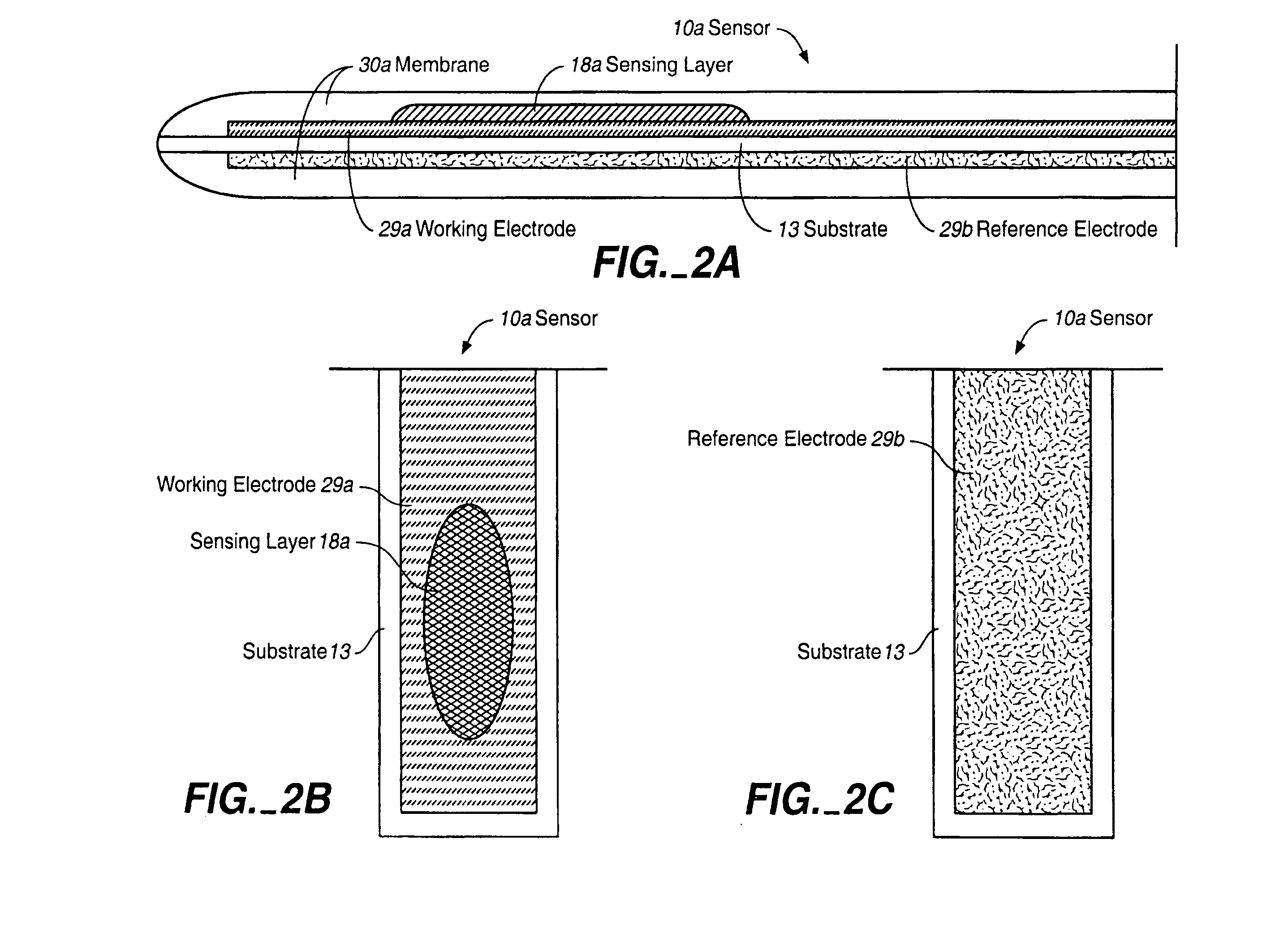
Analyte Sensor, and Associated System and Method Employing a Catalytic Agent
a technology of catalytic agent and analyte sensor, which is applied in the field of system and method employing catalytic agent, can solve the problems of non-linear relationship between the glucose concentration in the sample fluid and the response of the biosensor, complicated operation and performance of the enzyme-based biosensor, etc., and achieves the effects of enhancing the biocompatibility of the sensor, reducing the concentration of reactive species in the solution, and improving the performance of the sensor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Performance of Sensors With Catalyst-Enhanced Membranes in In Vitro Tests
[0097] A catalytic membrane solution that included a buffer solution and a membrane polymer preparation was prepared. The buffer solution comprised 4 parts of ethanol to 1 part of 10 mM HEPES, for a final concentration of 2 mM HEPES. The membrane polymer preparation comprised 116 mg / ml of a formulation called 10Q5, as depicted below (wherein x=0.85, y=0.1, z=0.05, n=9, m=1, and p=about 10), 8 mg / ml triglycidyl glycerol (the crosslinker), end 7.5 mg / ml manganese 5,10,15,20-tetra(4-pyridyl)-21H,23H-porphine chloride (MnTPyP), a compound possessing both superoxide dismutase and catalase activity.
[0098] A batch of sensors was prepared by dipping membrane-less sensors (which contained previously deposited, wired-enzyme sensing layers) three times, in succession, into the catalytic membrane solution. Each resulting sensor membrane contained approximately 13 micrograms of the catalyst, MnTPyP, or a load with respec...
example 2
Comparison of Performance of Sensors With a Conventional Membrane and Sensors With a Catalyst-Enhanced Membrane in Human Subjects
[0100] The performance of sensors with catalyst-enhanced membranes was tested in 22 volunteer, non-diabetic human subjects, and compared to the simultaneous performance of sensors with conventional membranes that have no catalyst enhancement. The human-subject study was approved by the Institutional Review Board of TheraSense, Inc. (now Abbott Diabetes Care, Alameda, Calif.). Subjects were informed of risks and consented to participate in view of possible risks, such as bruising, edema, erythema, and excessive bleeding. Subjects were free to discontinue the study at any time, and were limited to three sensor-attachment attempts over the course of the three-day study. Following the study and sensor removal, subjects were examined for any manifestation of the identified risks.
[0101] In this experiment, each volunteer subject was fitted simultaneously with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight cut | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com