Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
702 results about "Aneurysm" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
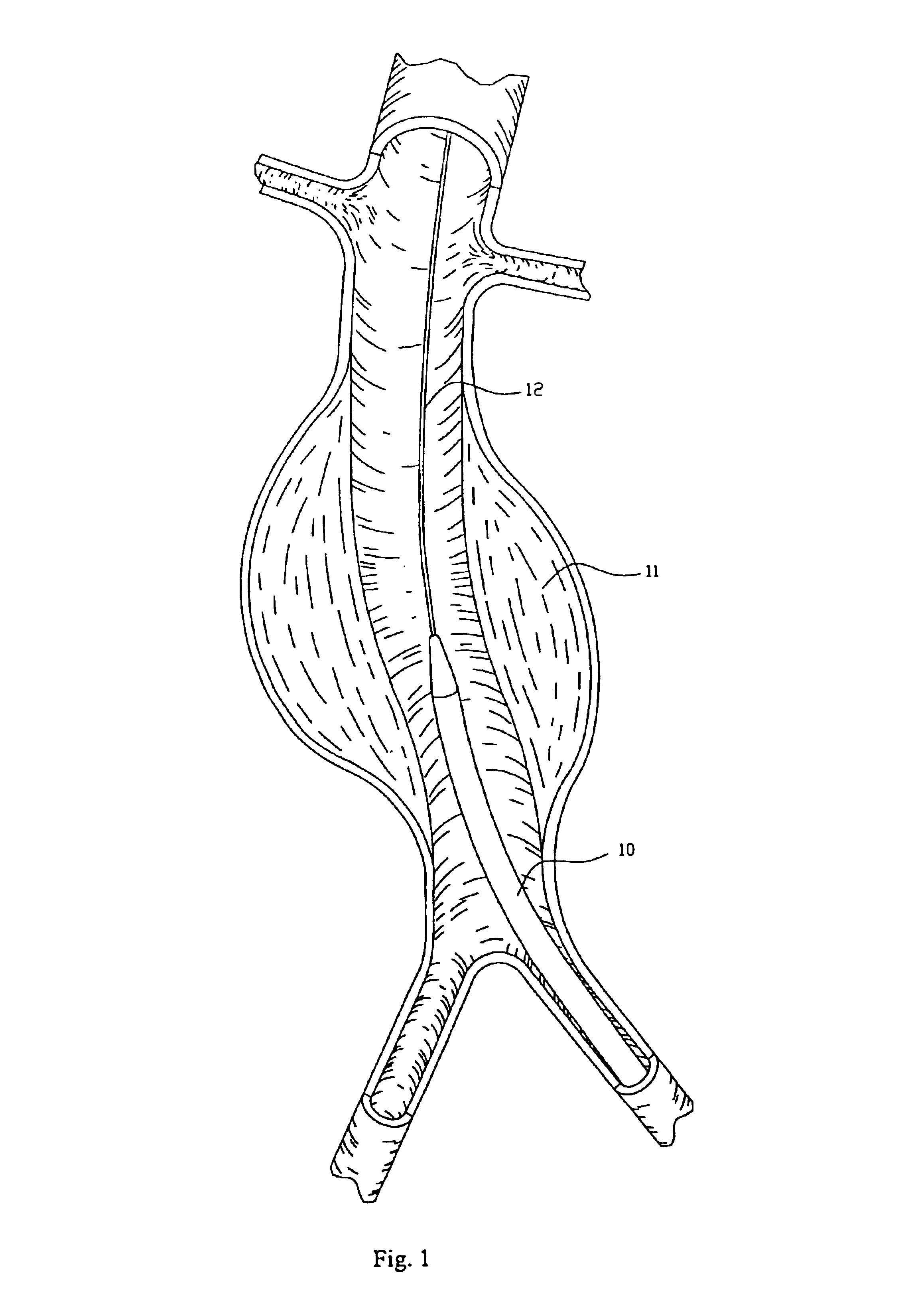
A dilatation in the wall of an artery supplying blood to a specific area.
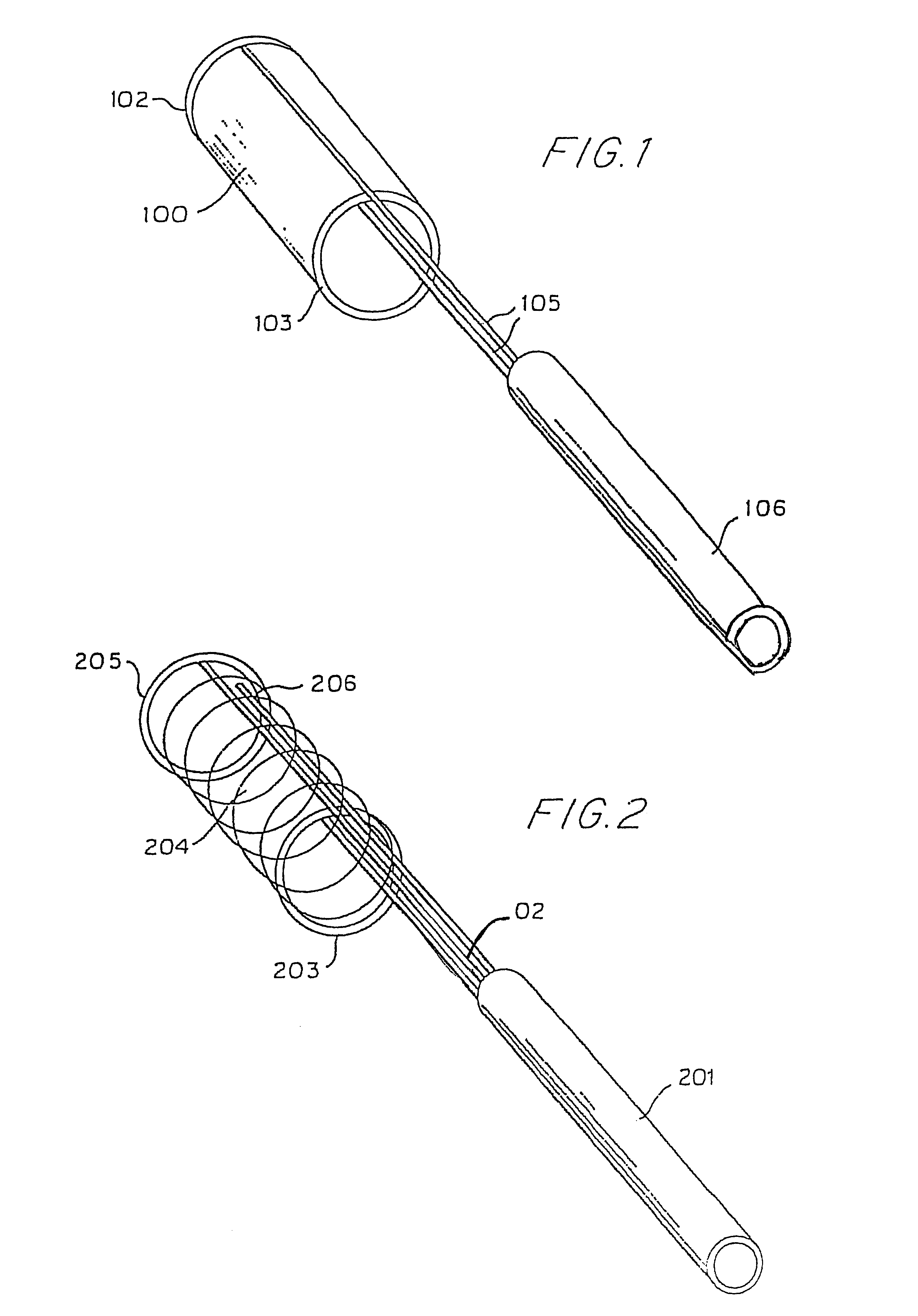
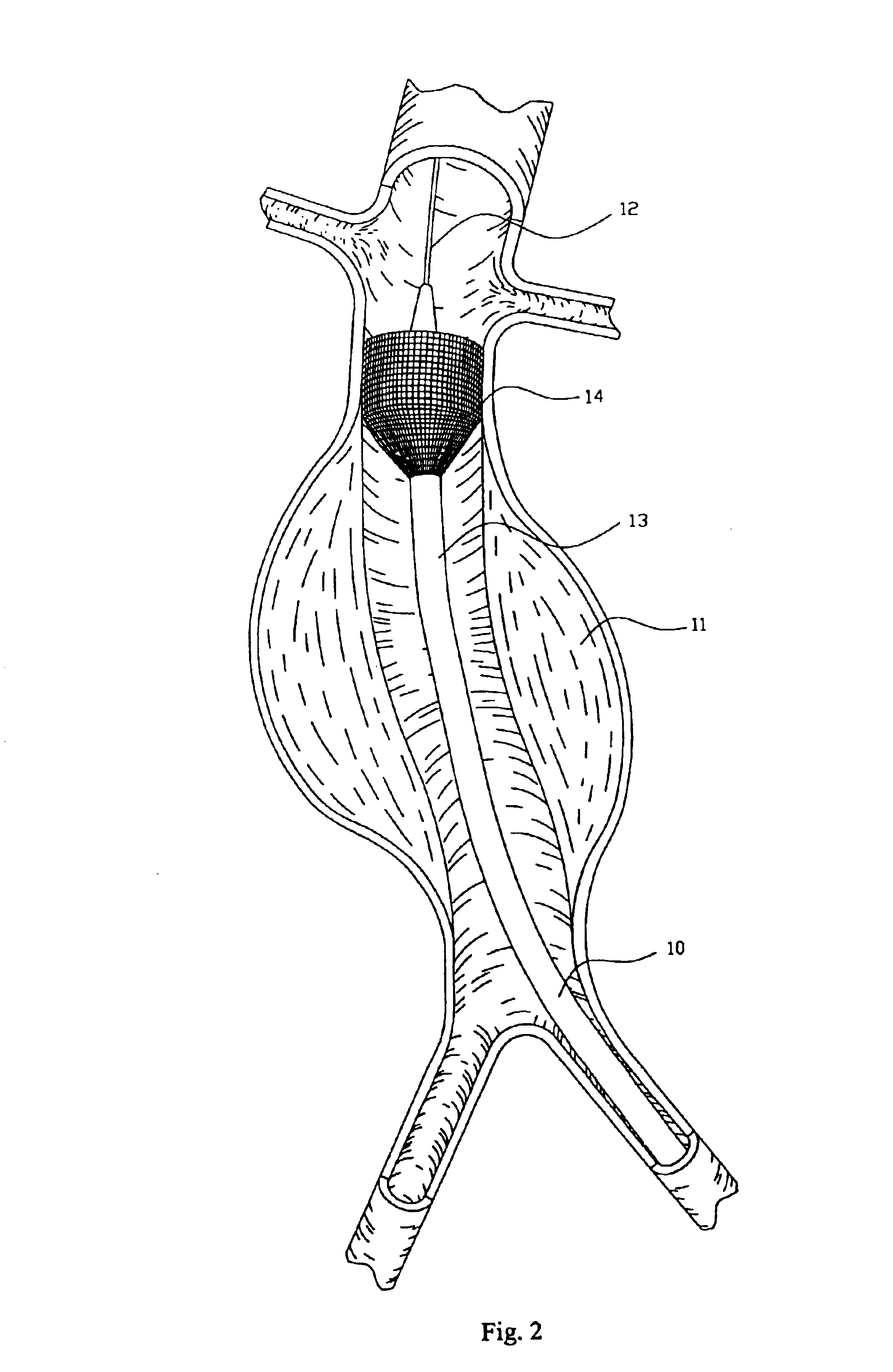
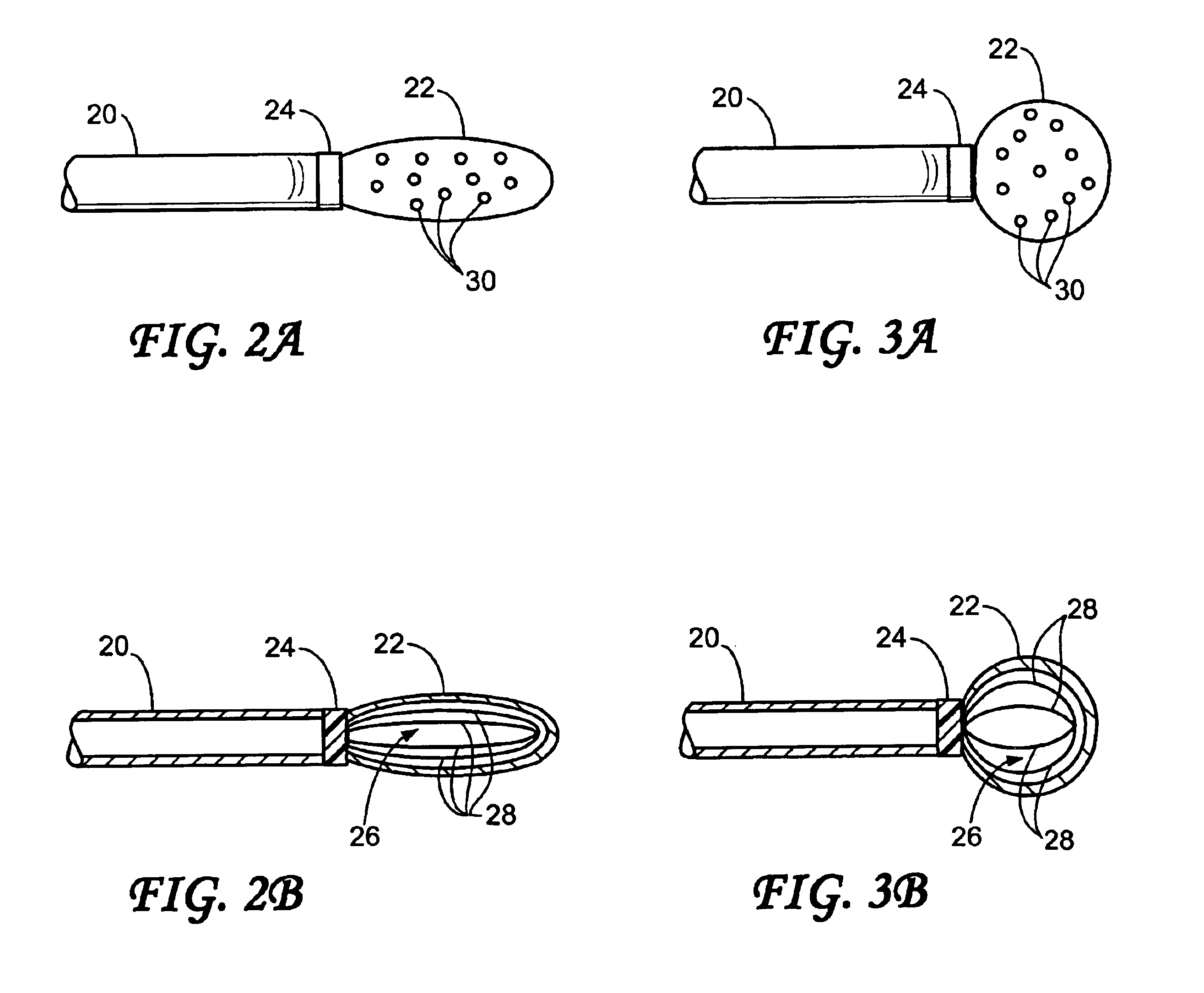
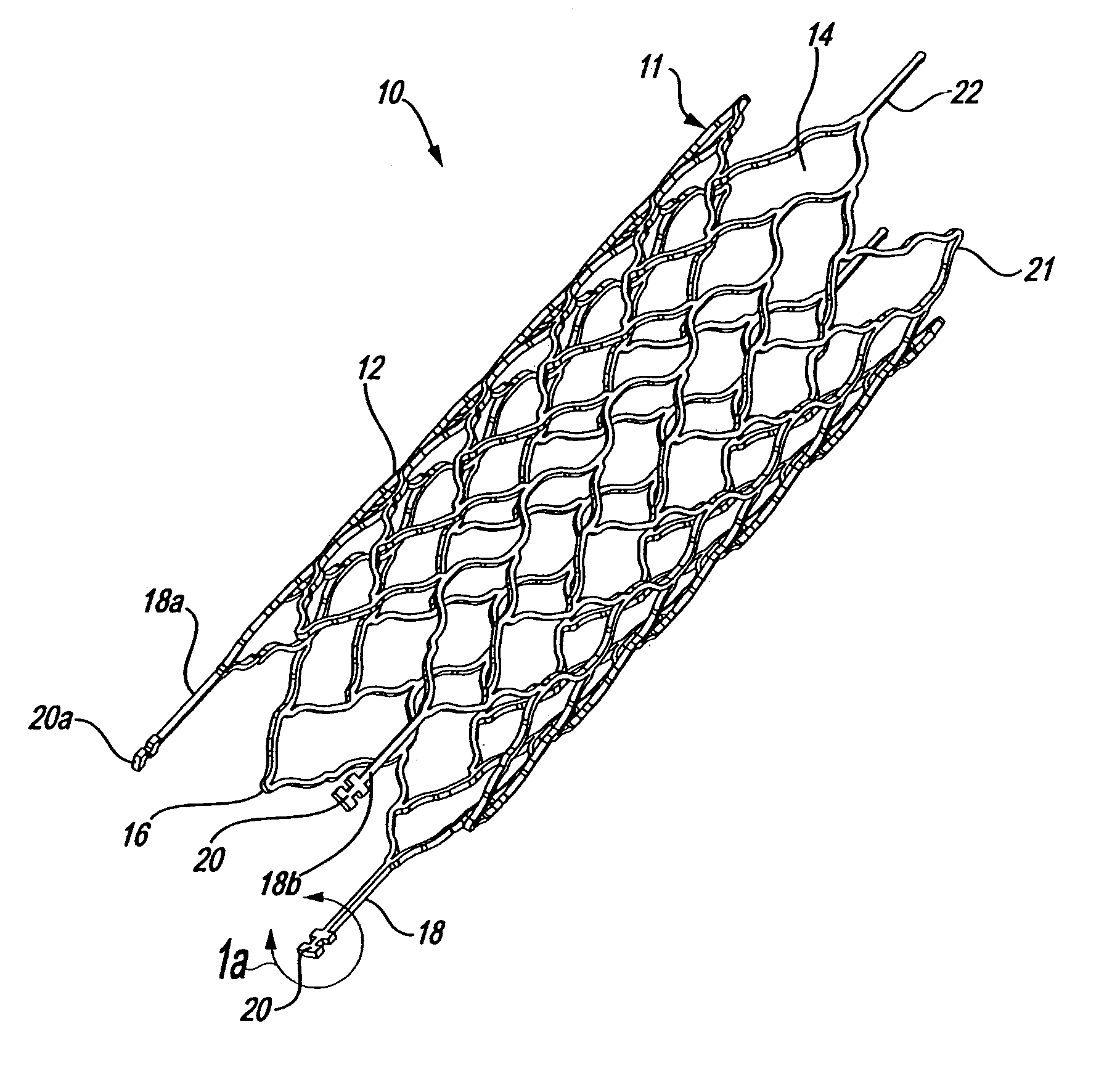
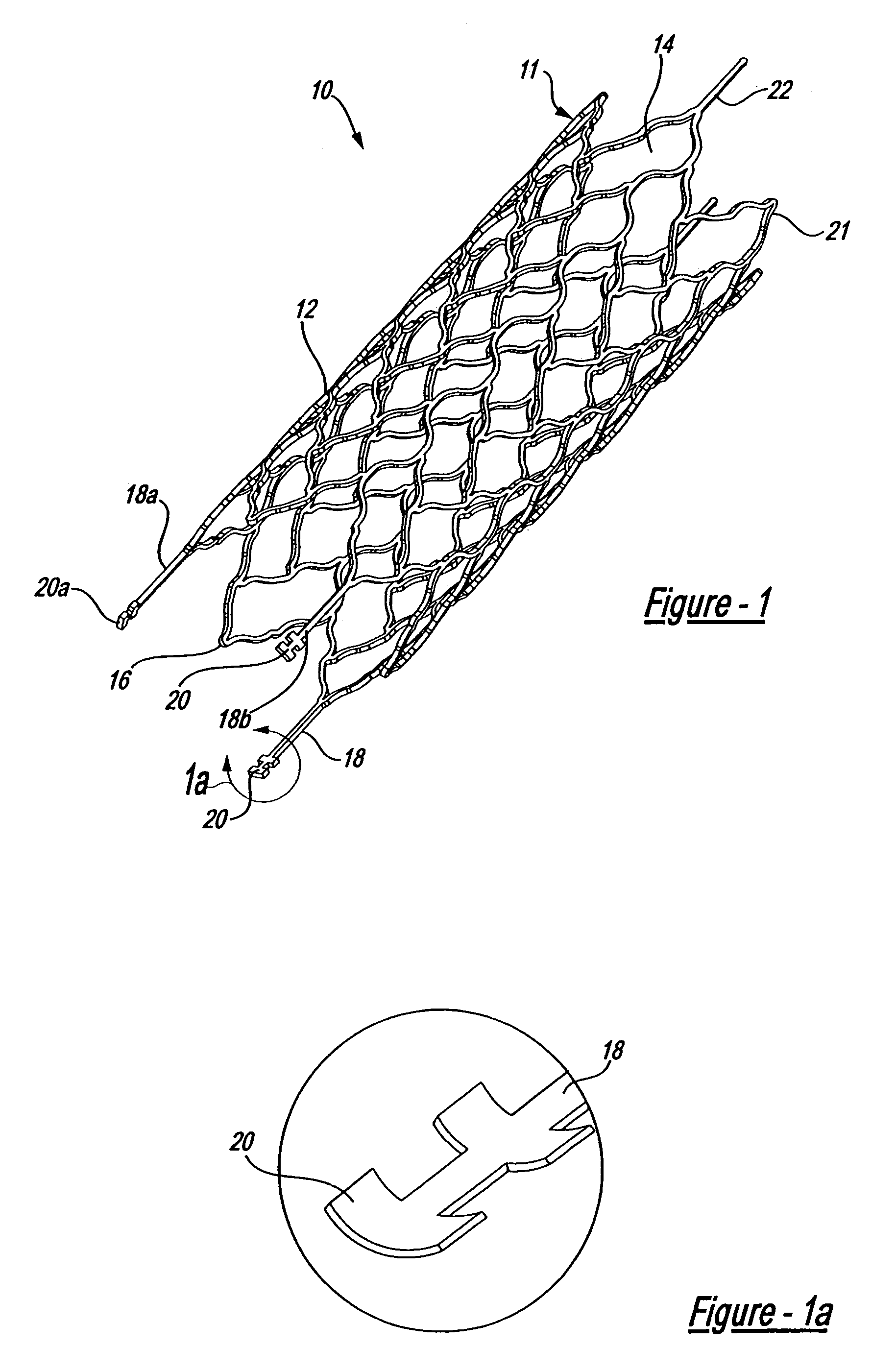
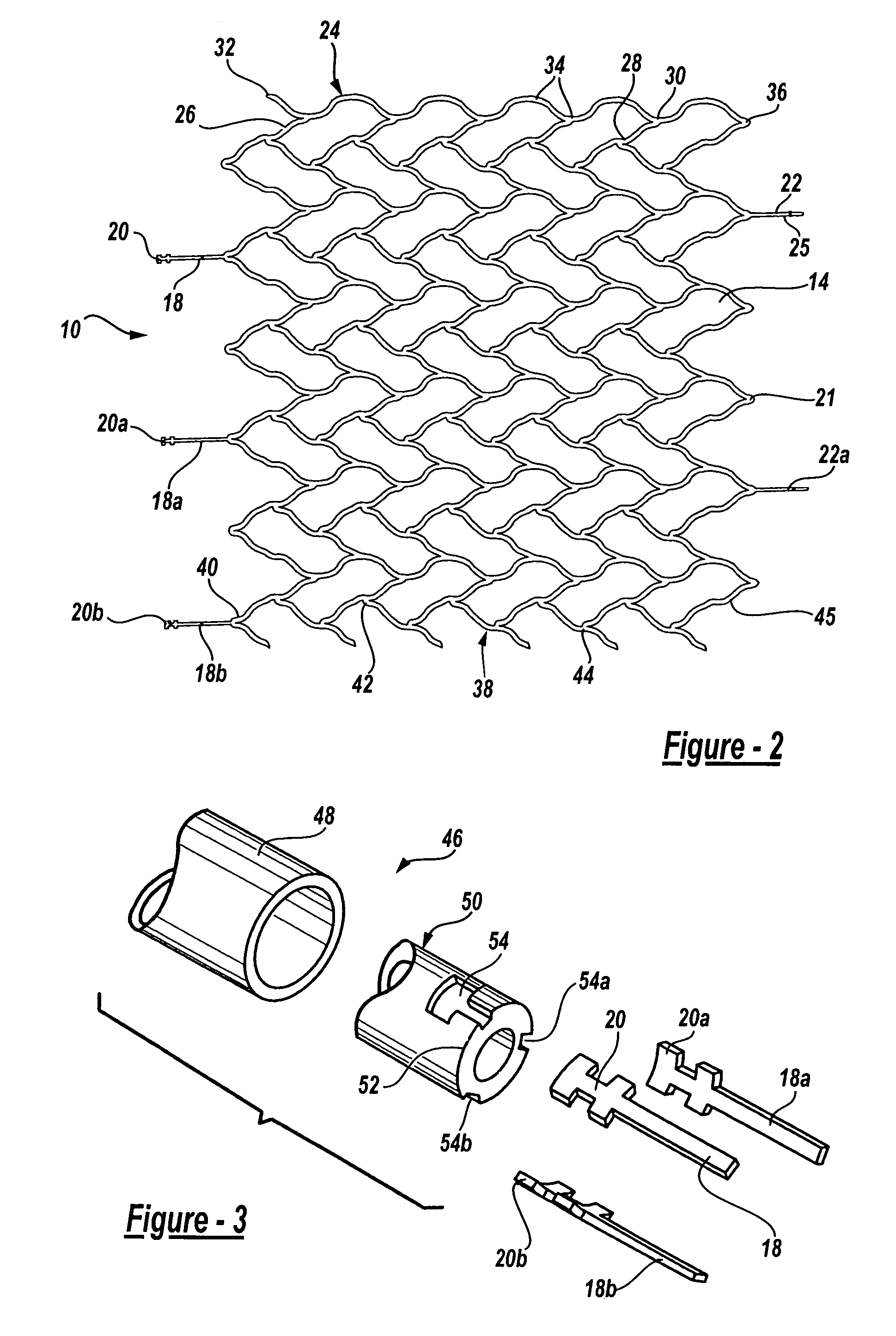
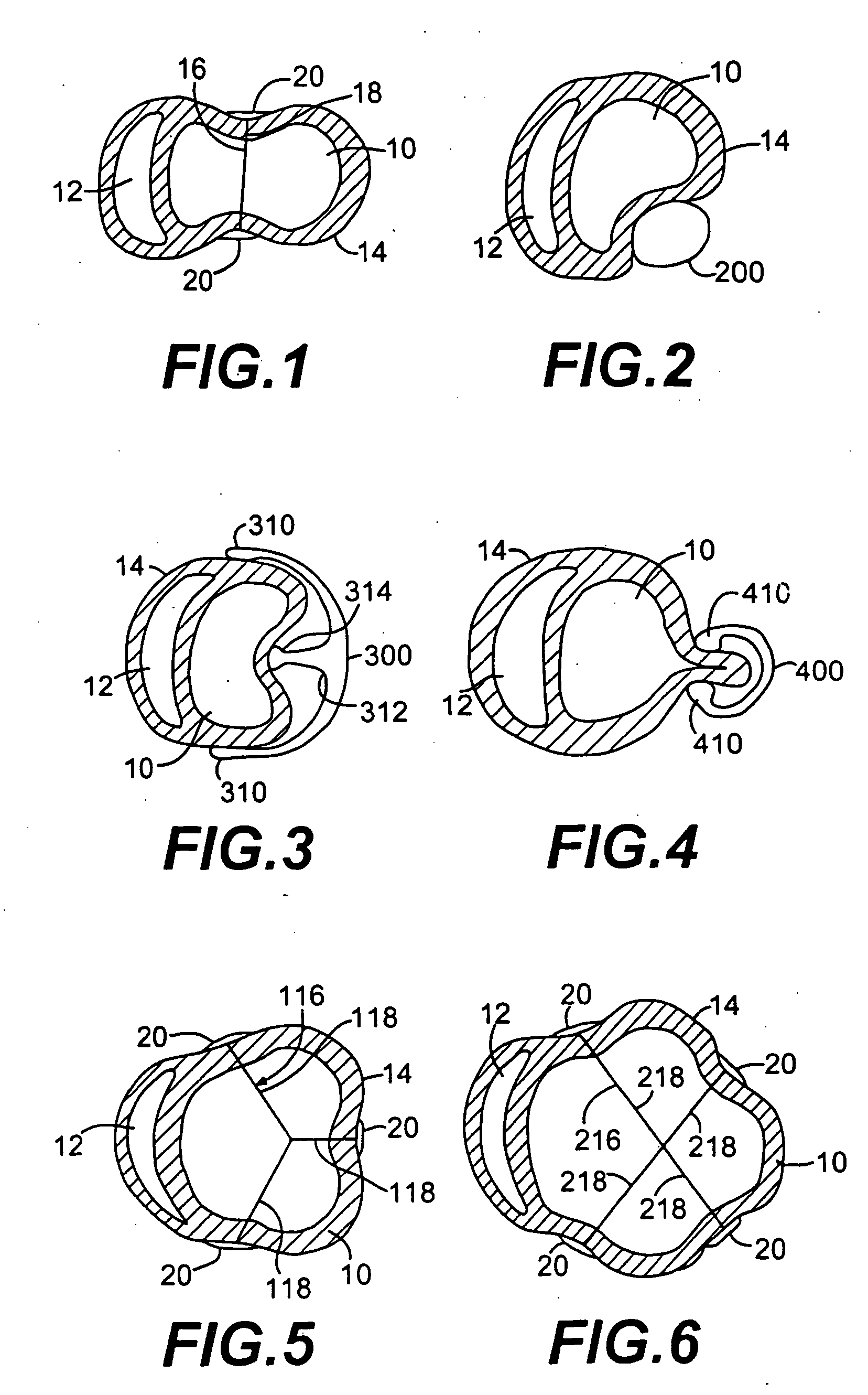
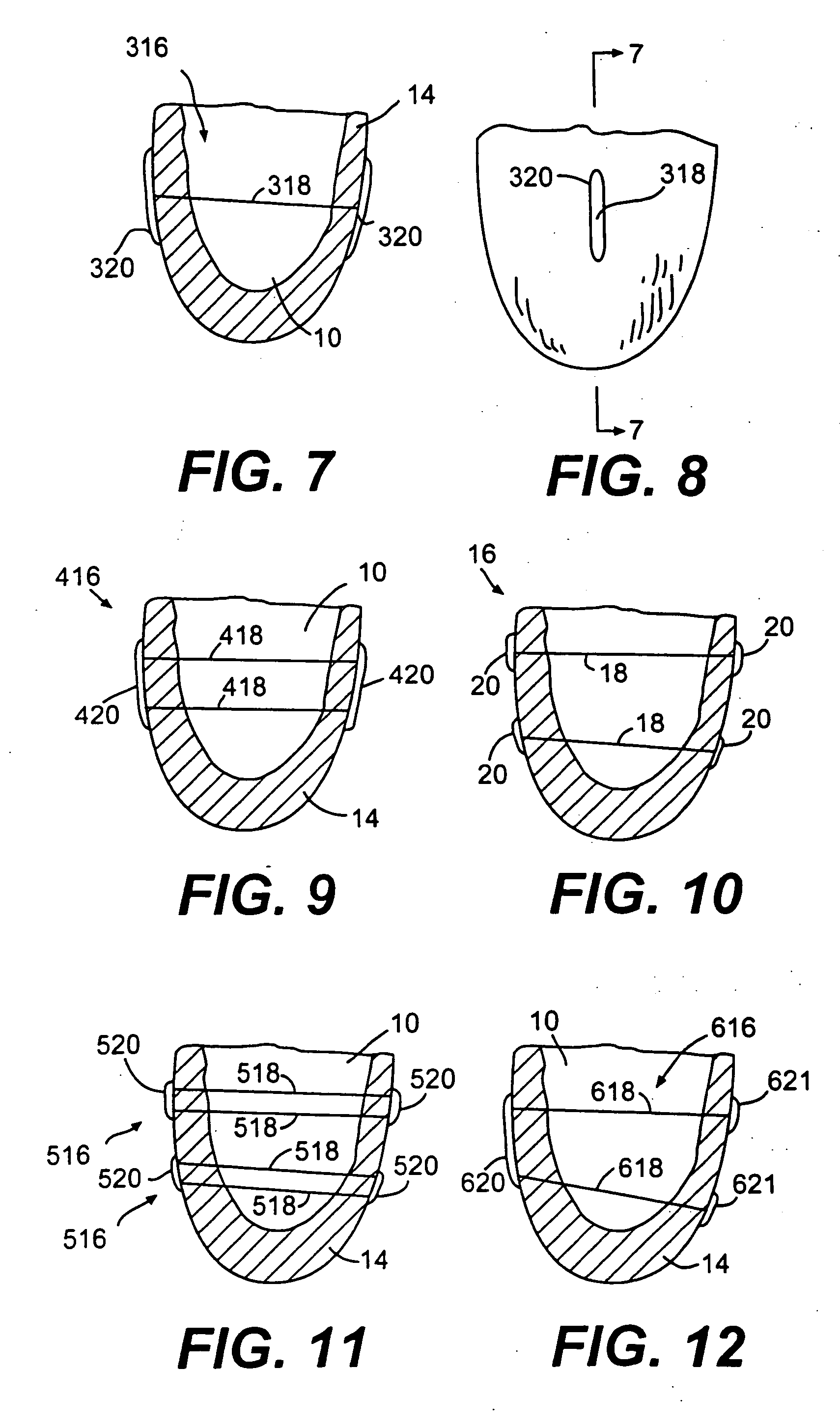
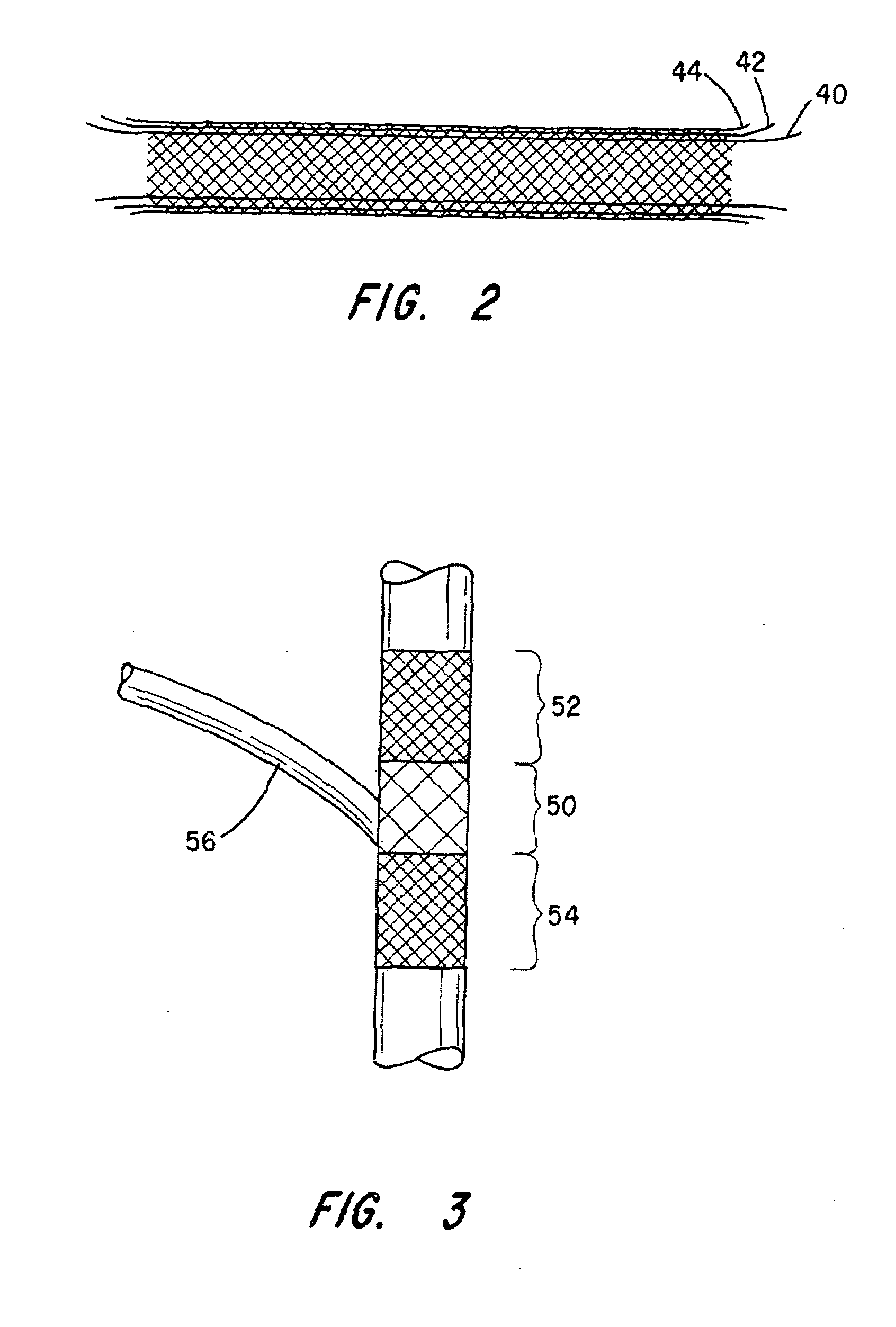
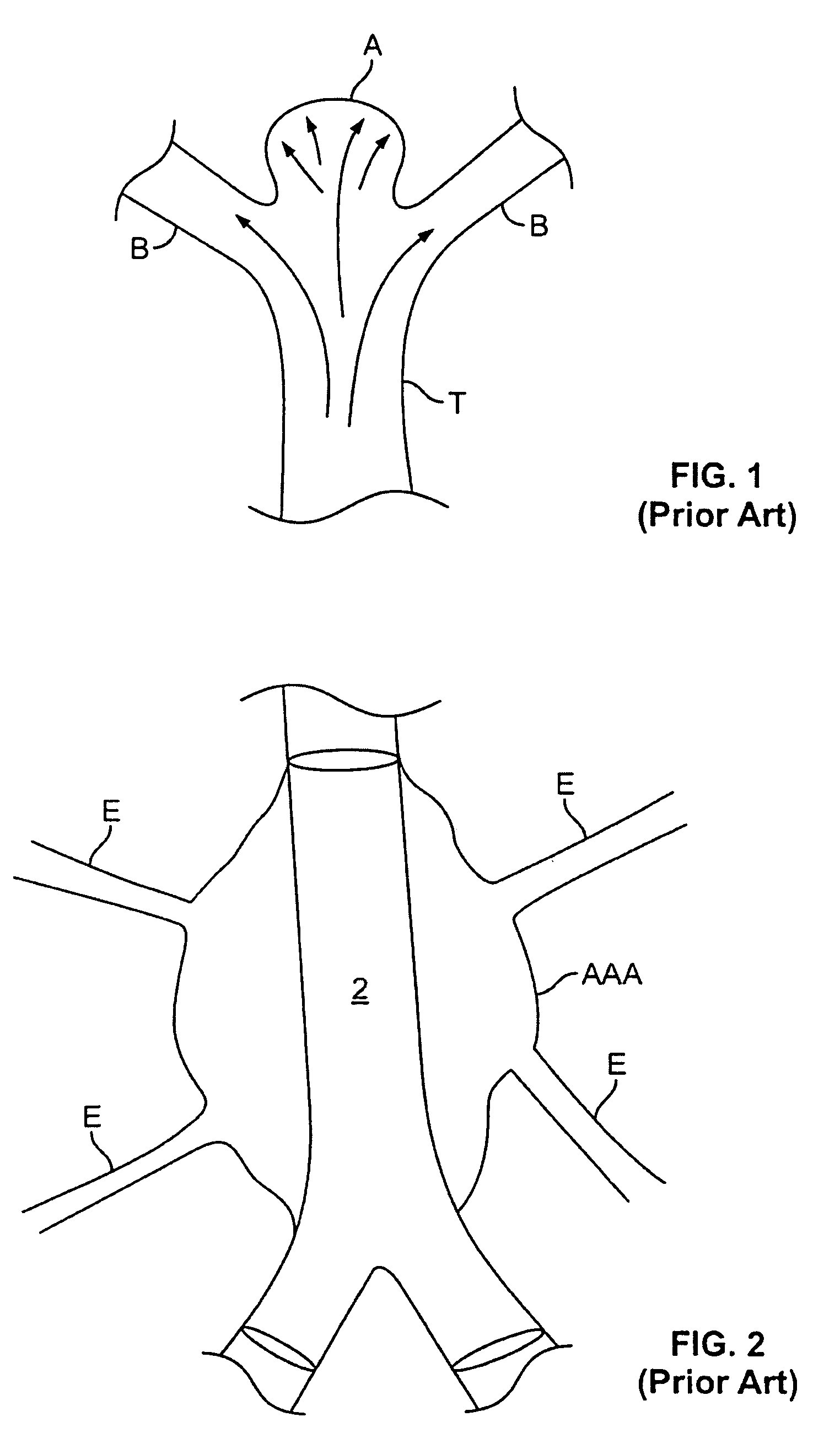
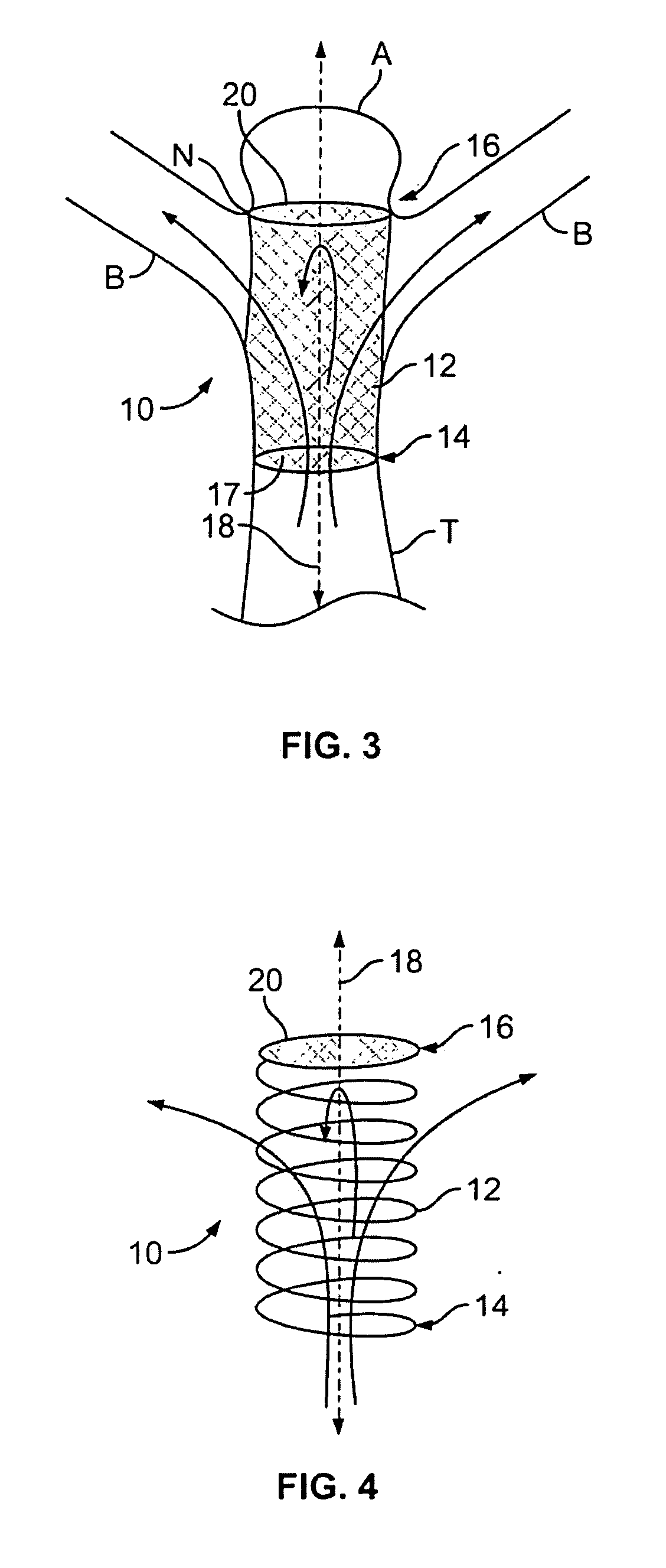
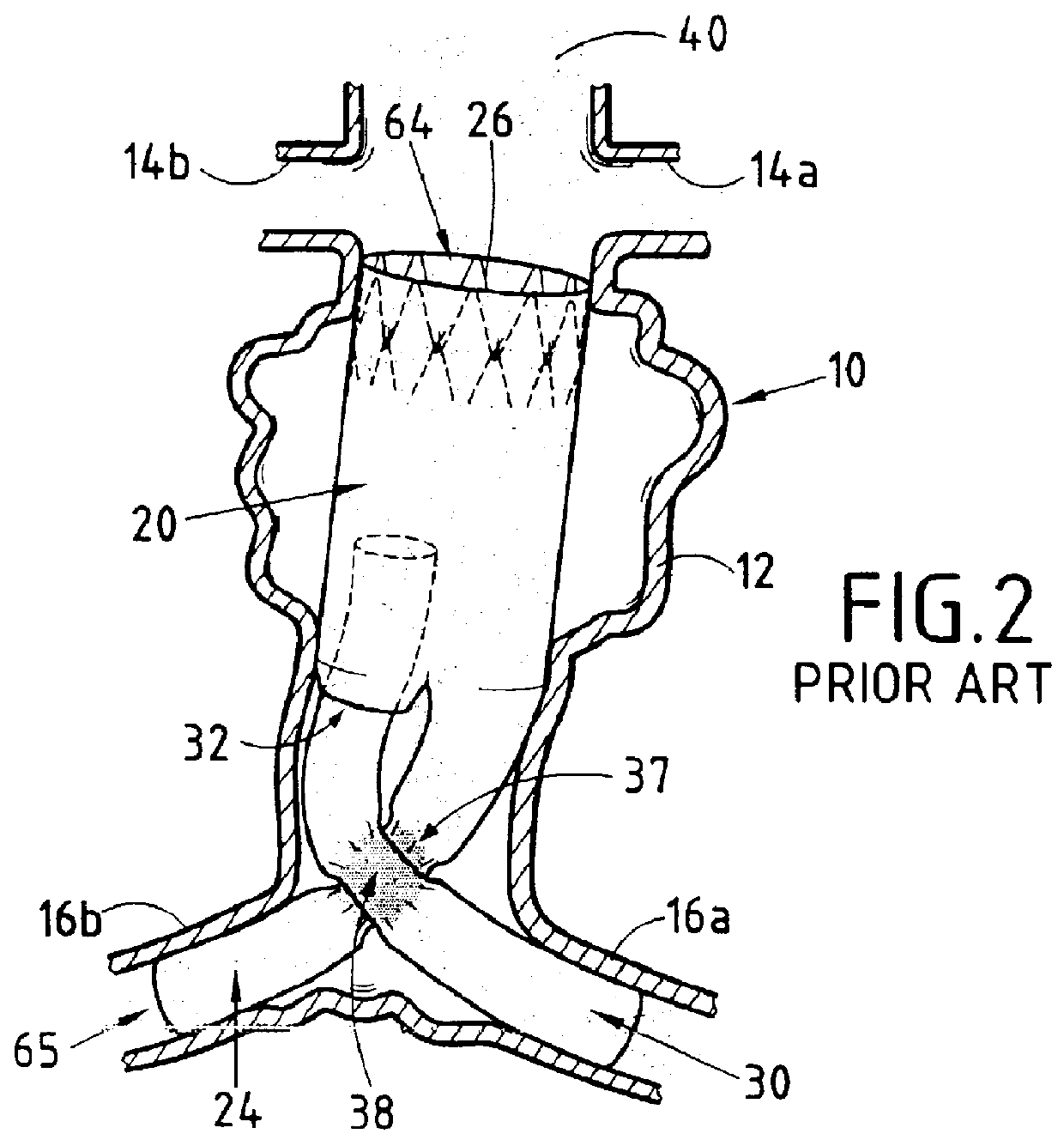
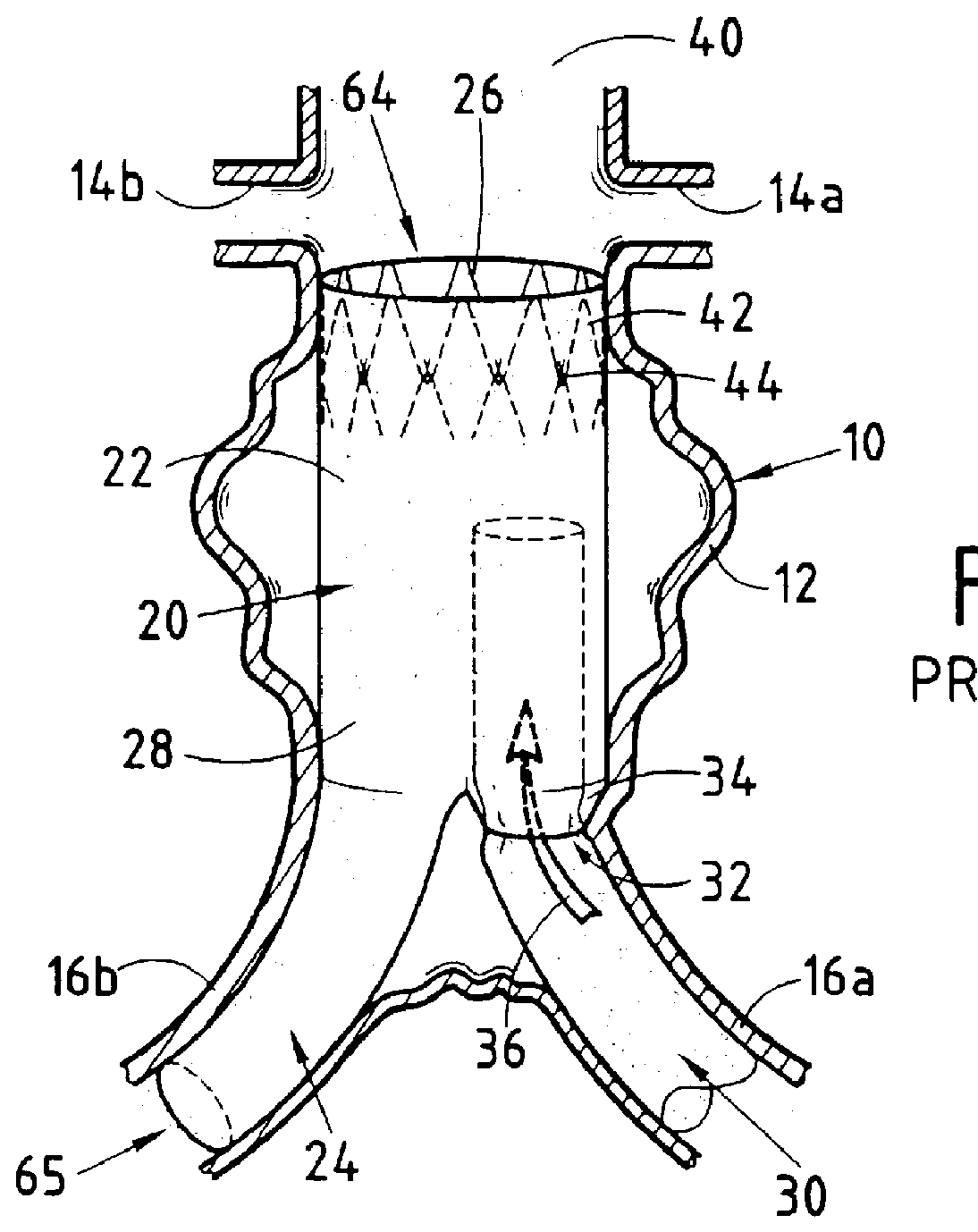
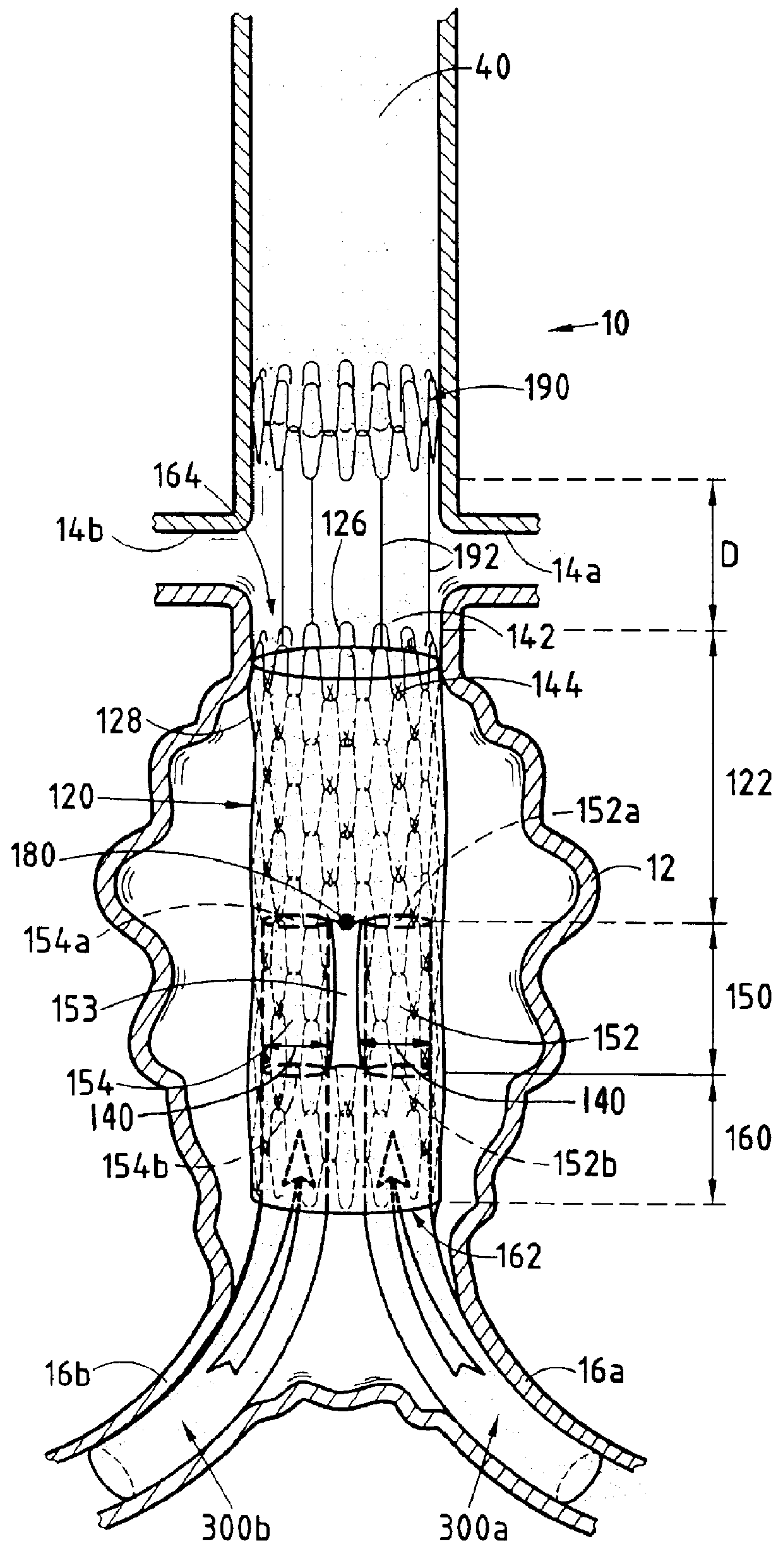
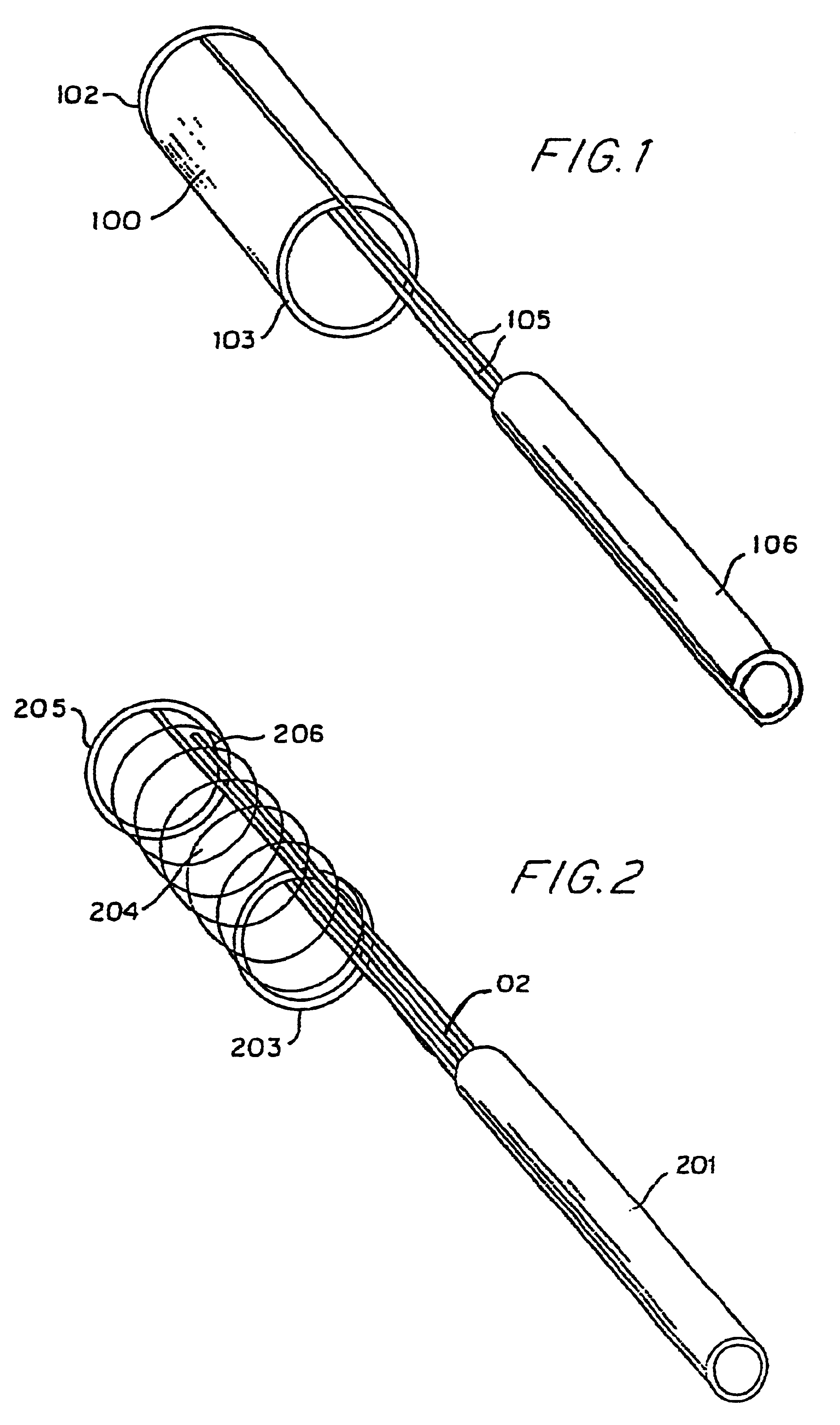
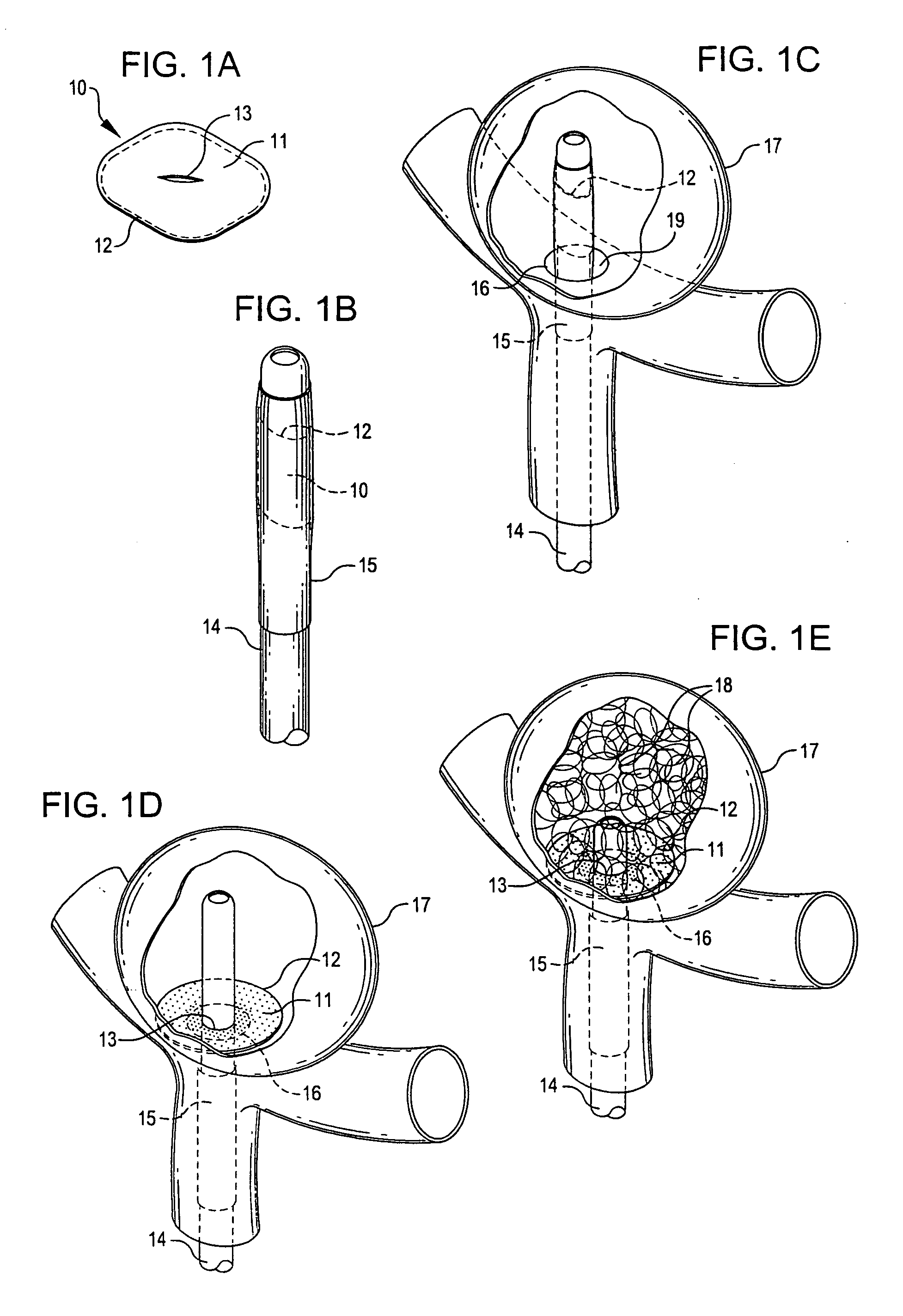
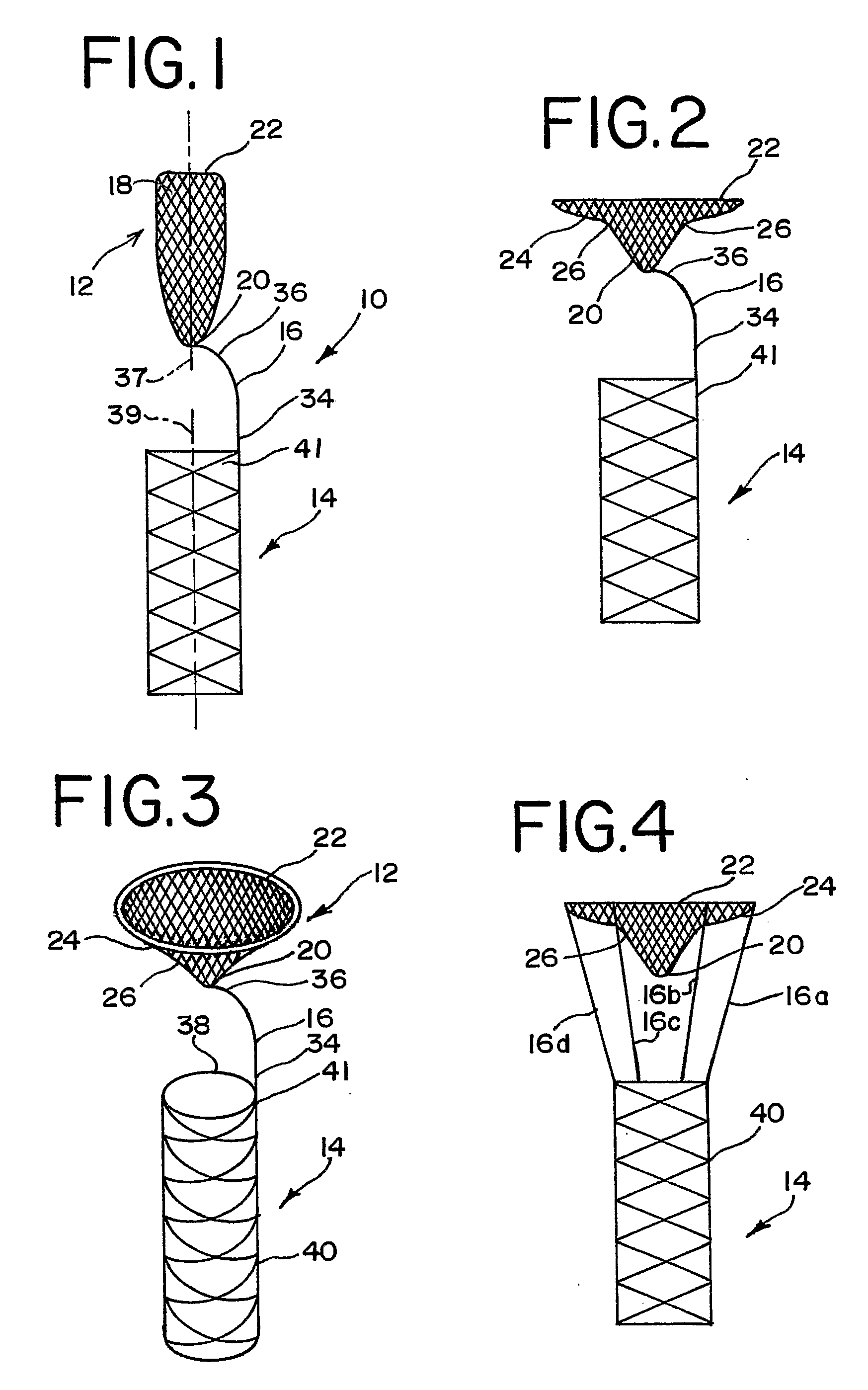
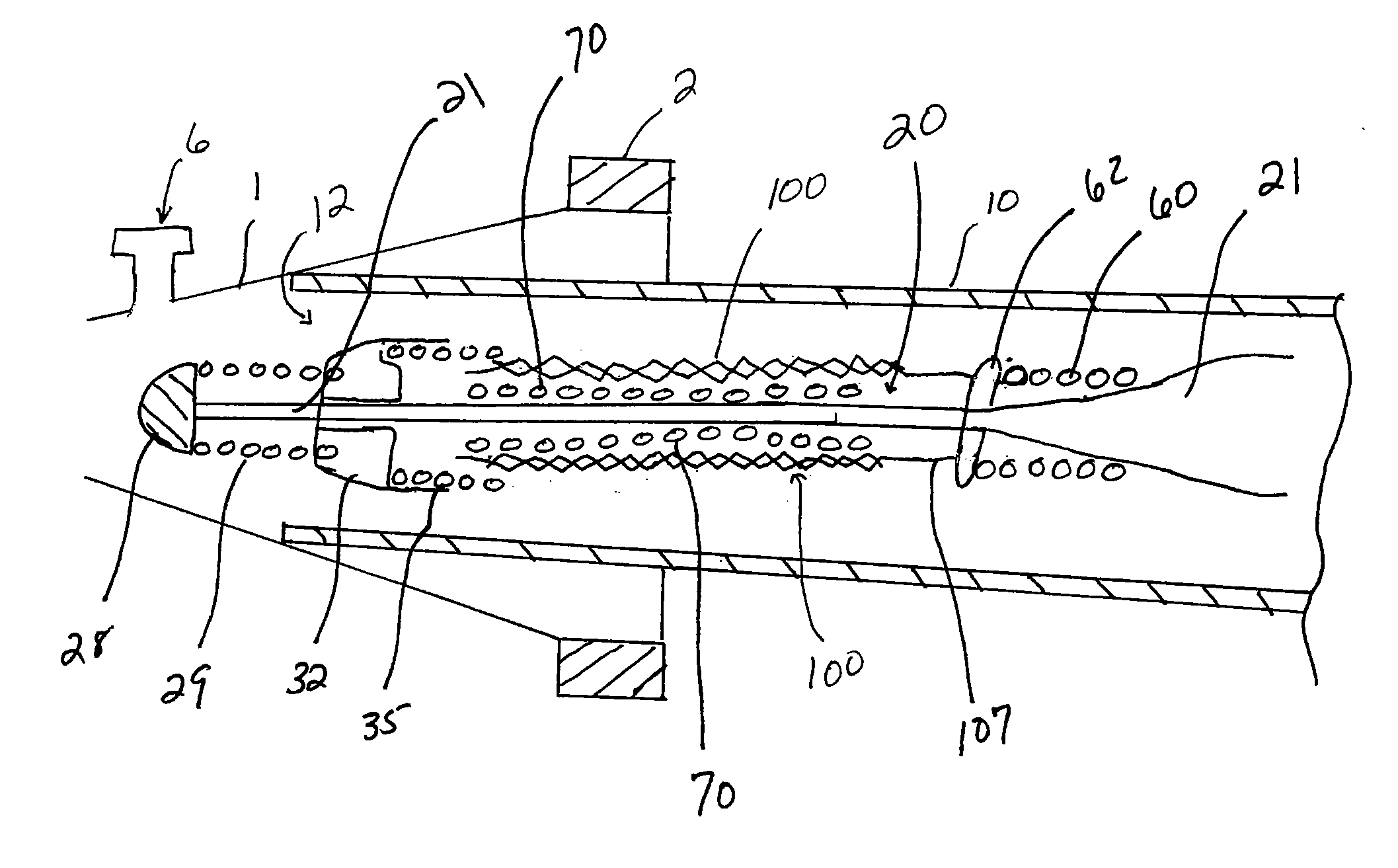
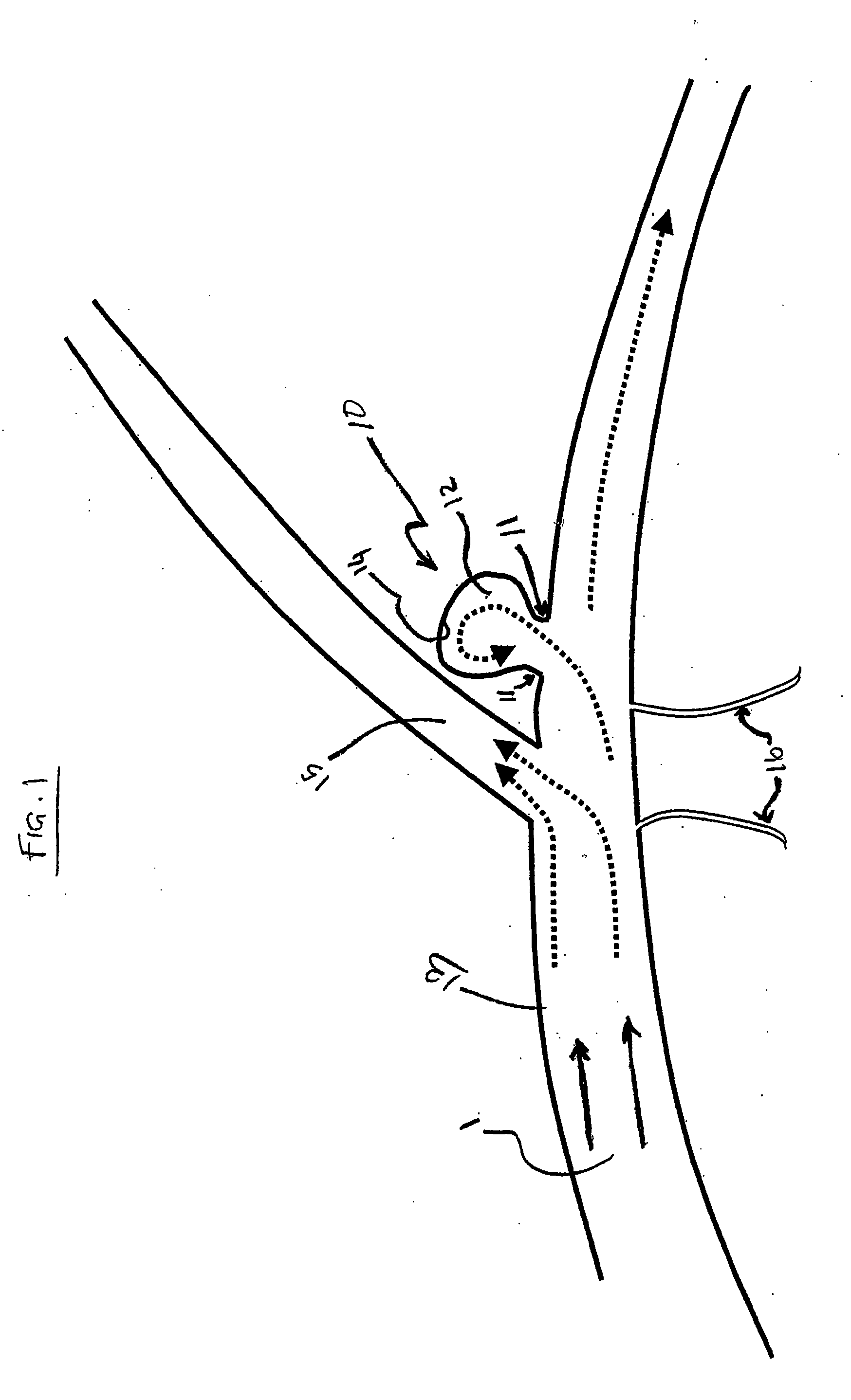
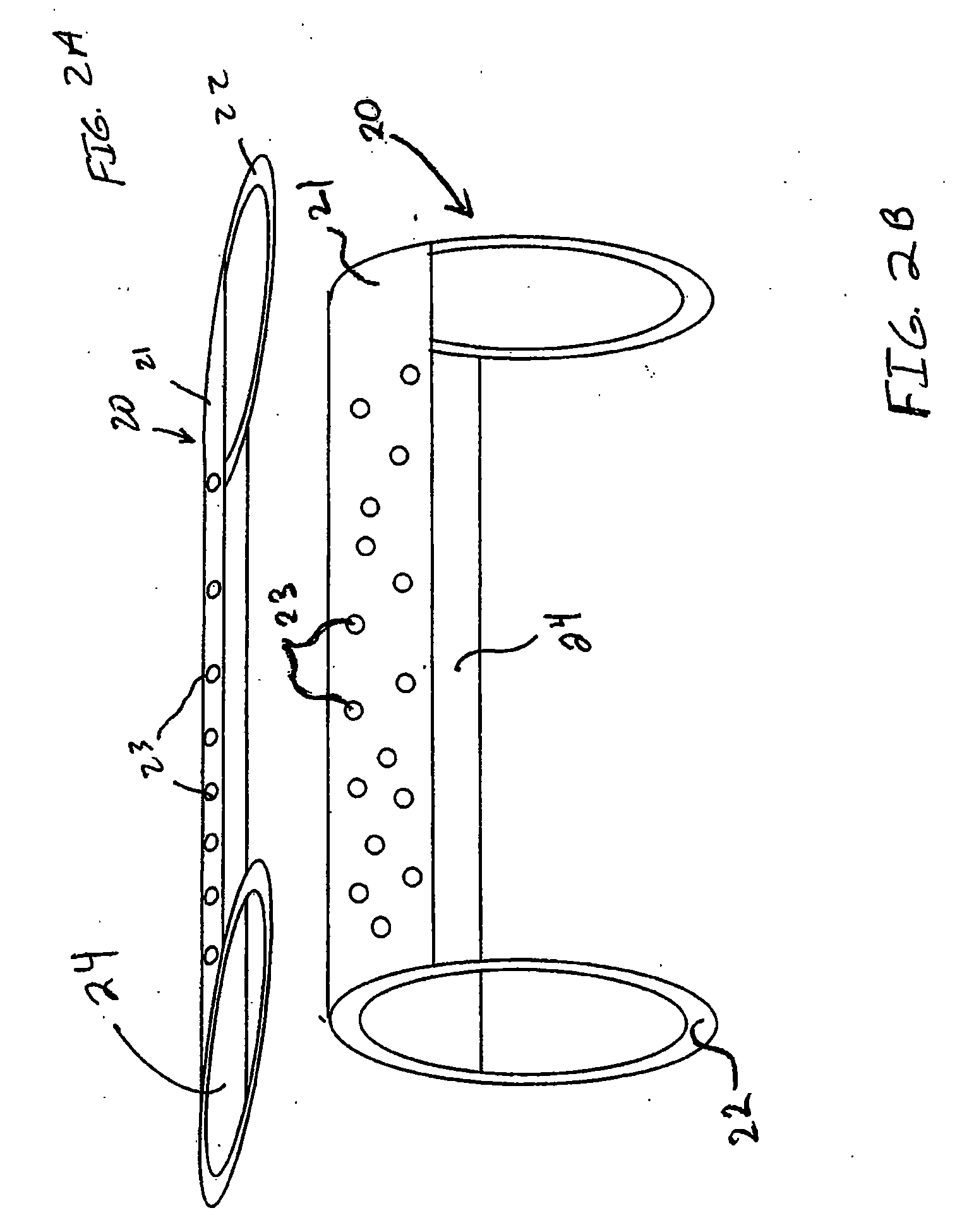
Endovascular aneurysm devices, systems, and methods
Devices, systems, and methods for implanting prostheses in the body lumens rely on tacking or anchoring the prostheses with separately introduced fasteners. After initial placement, a fastener applier system is introduced within the expanded prostheses to deploy a plurality of fasteners to at least one prosthesis end. The fasteners are usually helical fasteners which are releasably restrained on the fastener driver, and are delivered by rotation of the fastener driver. The fasteners may be applied singly, typically in circumferentially spaced-apart patterns about the interior of at least one end of the prosthesis. A lumen extension or lumens may be coupled to the prosthesis to extend the reach of the prosthesis within the implantation site. Fasteners may also be applied to the lumen extensions.
Owner:MEDTRONIC VASCULAR INC
Endovascular thin film devices and methods for treating and preventing stroke
InactiveUS6605111B2Treating and preventing ischemic and hemorrhagic strokeInhibit migrationStentsCatheterIn situ polymerizationProsthesis
Owner:NEW YORK UNIV
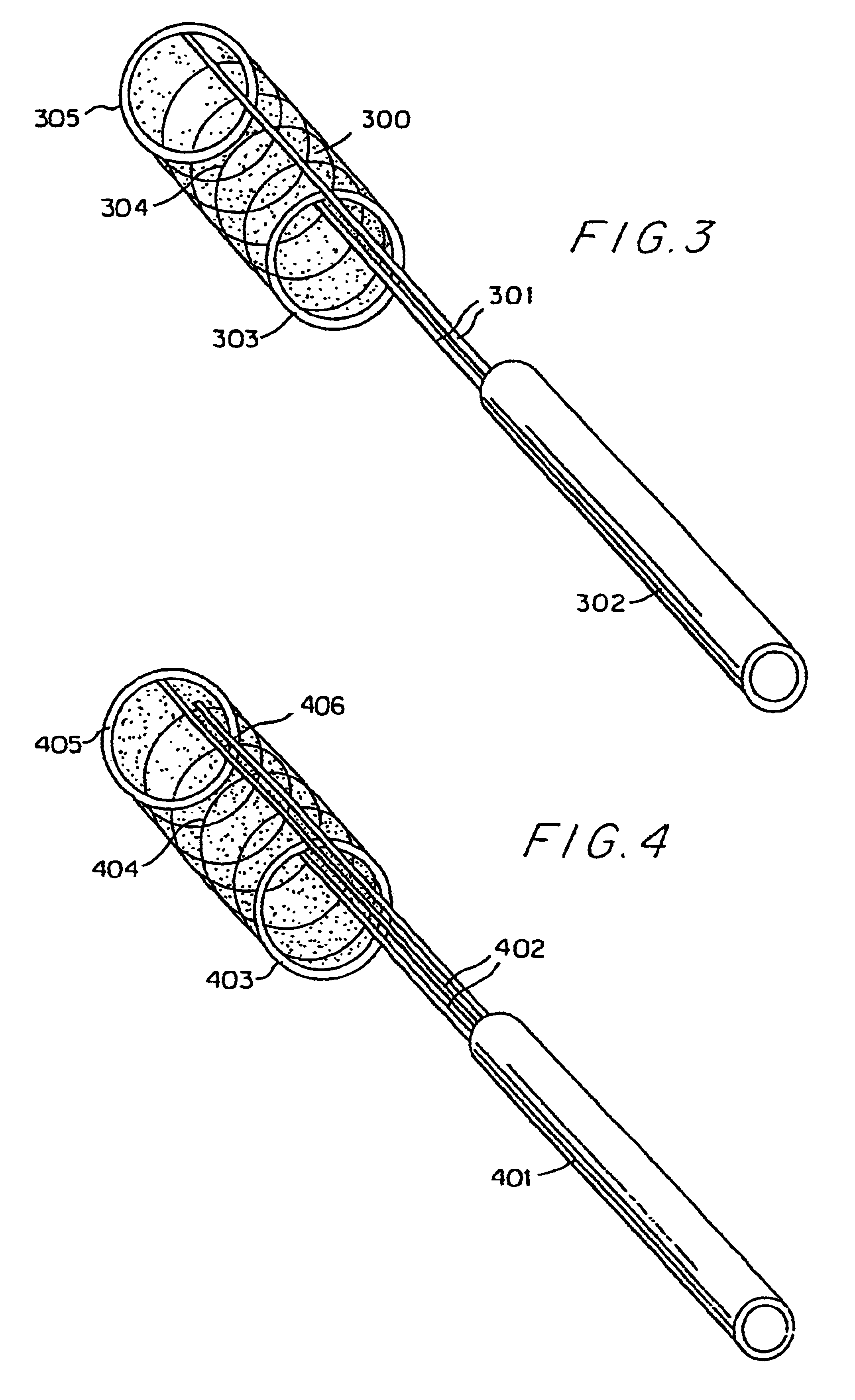
Endovascular aneurysm repair system
InactiveUS6960217B2Low profileEffective and less traumaticStentsEar treatmentEndovascular aneurysm repairBiomedical engineering
Method and apparatus for implanting radially expandable prostheses in the body lumens rely on tacking or anchoring of the prostheses with separately introduced fasteners. The prostheses may be self-expanding or balloon expandable. After initial placement, a fastener applier system is introduced within the expanded prostheses to deploy a plurality of fasteners at at least one prosthesis end, usually as each end of the prosthesis. The fasteners are usually helical fasteners which are delivered from a helical track in the fastener applier by rotation with a rotator wire. The fasteners will be applied singly, typically in circumferentially spaced-apart patterns about the interior of each end of the prosthesis.
Owner:MEDTRONIC VASCULAR INC
System for tissue cavity closure
InactiveUS20060004388A1Lower the volumeLess invasive treatmentSuture equipmentsSurgical staplesSurgical operationAnatomical structures
Surgical systems for less invasive access to and isolation of an atrial appendage are provided. A suturing grasper compresses soft tissue structures and deploys one or more sutures through complimentary pledget(s) carried by the grasper. The pledgets are reinforced, containing support members that define the profile of the stitch and distribute stresses applied by the stitch, once tightened, over a length of tissue. This hardware may be applicable to other surgical and catheter based applications as well including: compressing soft tissue structures lung resections and volume reductions; gastric procedures associated with reduction in volume, aneurysm repair, vessel ligation, or other procedure involving isolation of, resection of, and reduction of anatomic structures.
Owner:ATRICURE
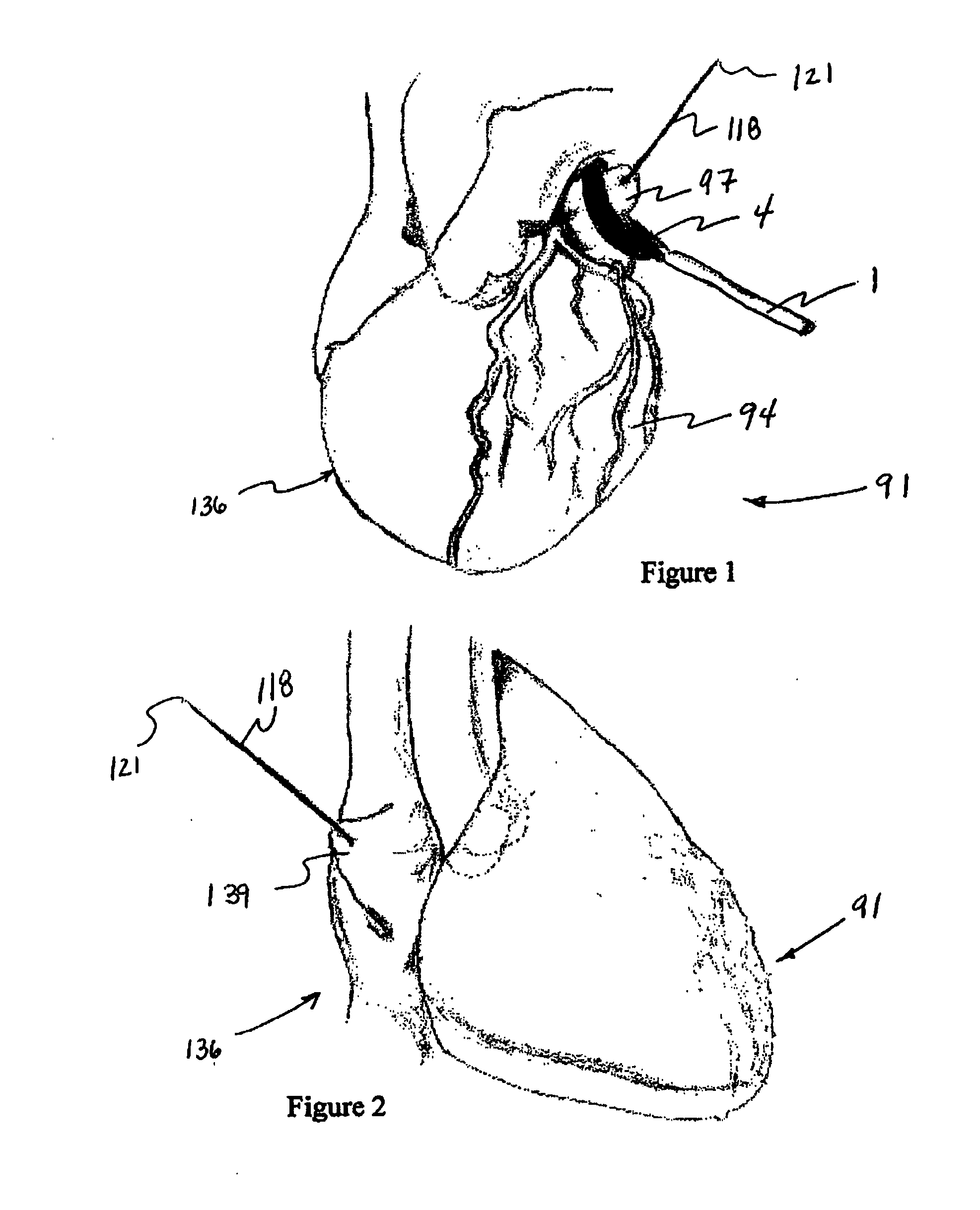
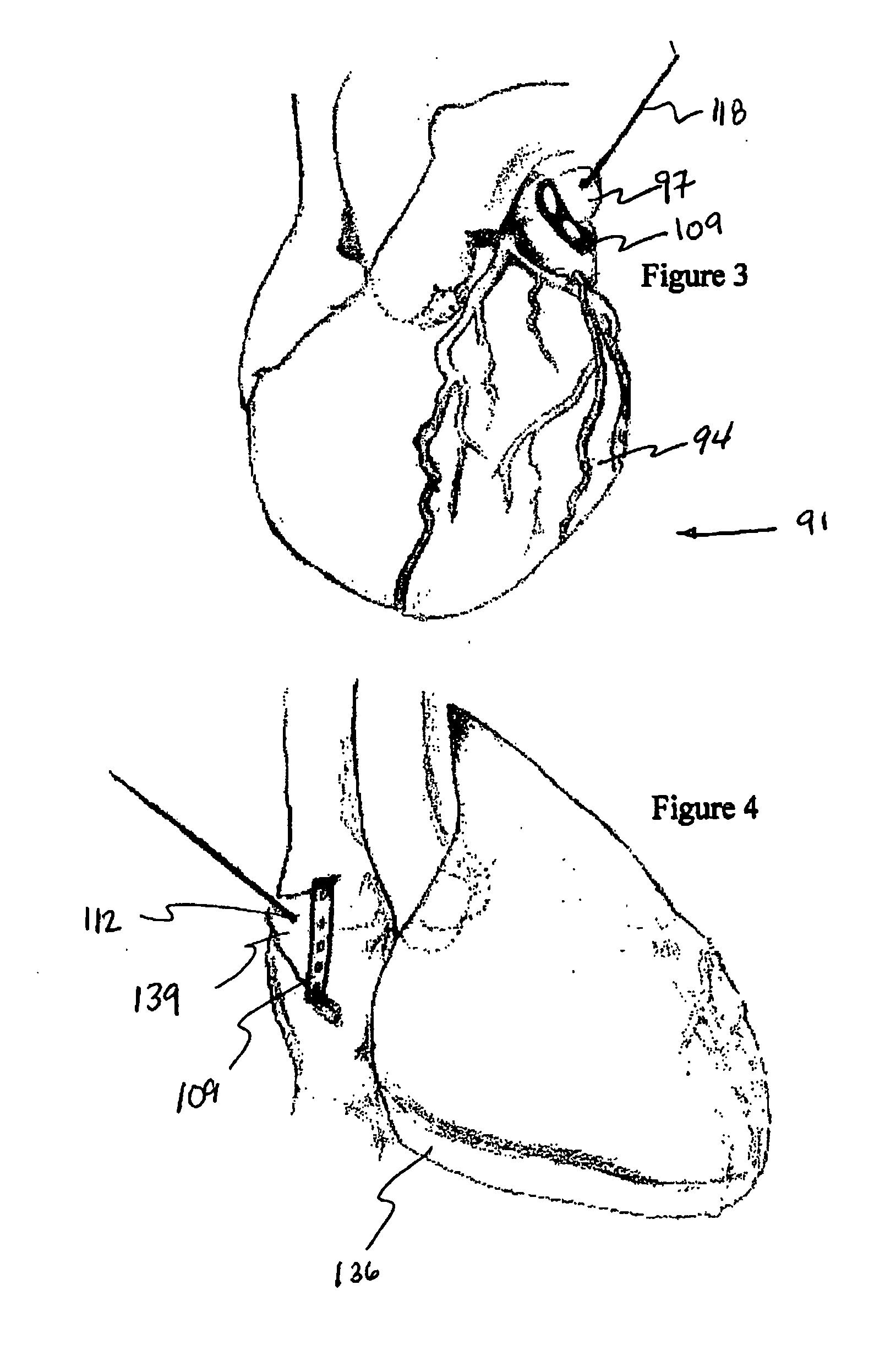
Methods and devices for improving cardiac function in hearts
InactiveUS7189199B2Reduce tensionReduce energy consumptionSuture equipmentsHeart valvesIliac AneurysmWall stress
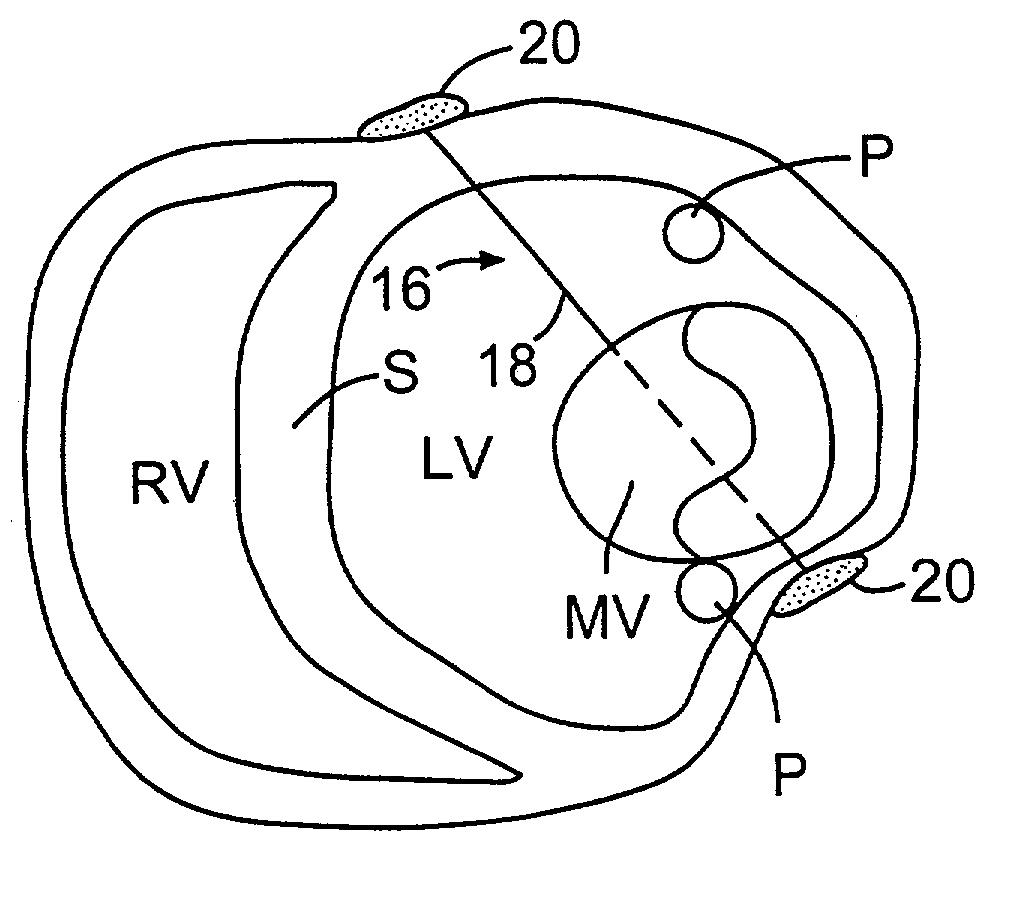
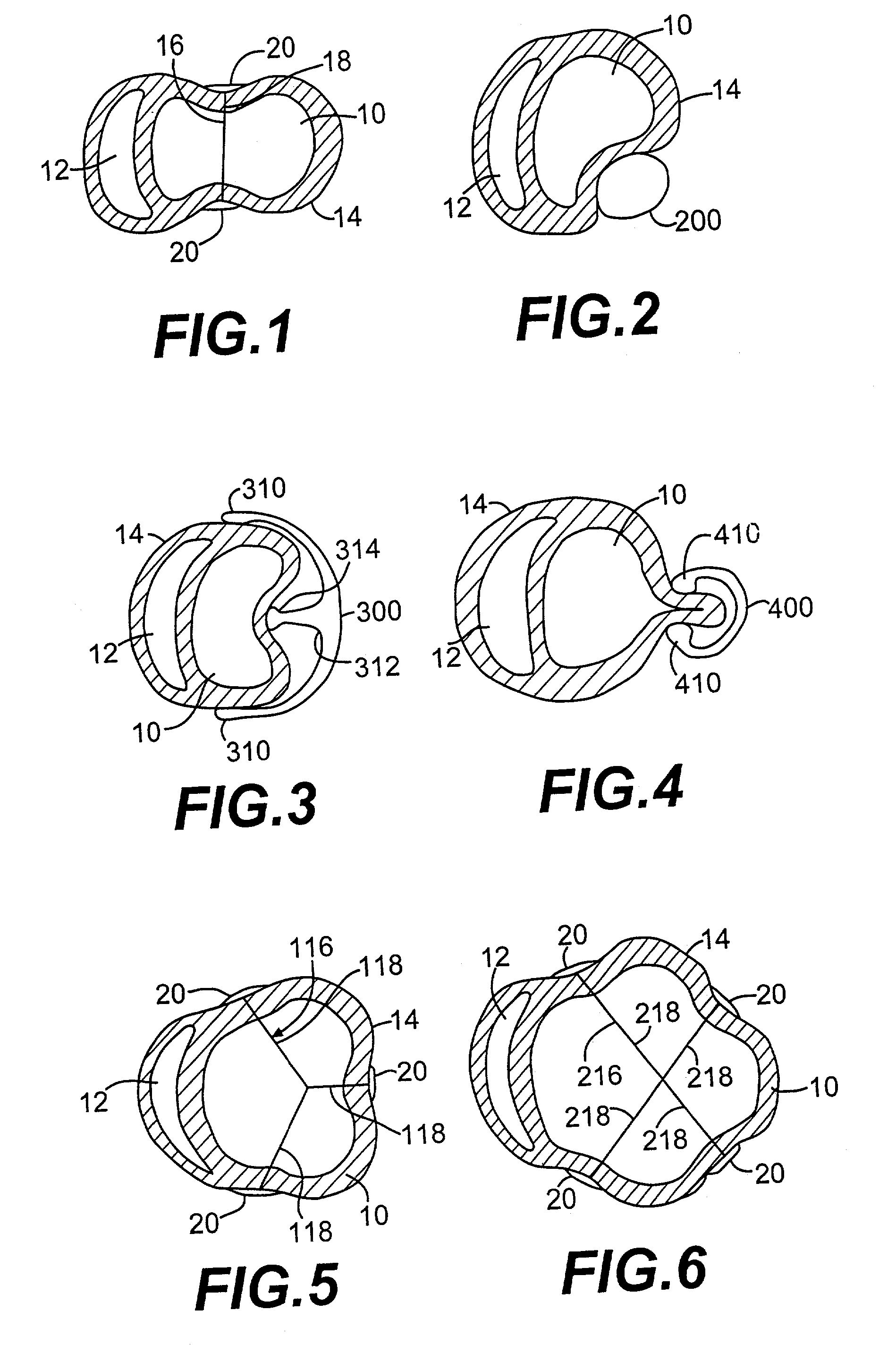
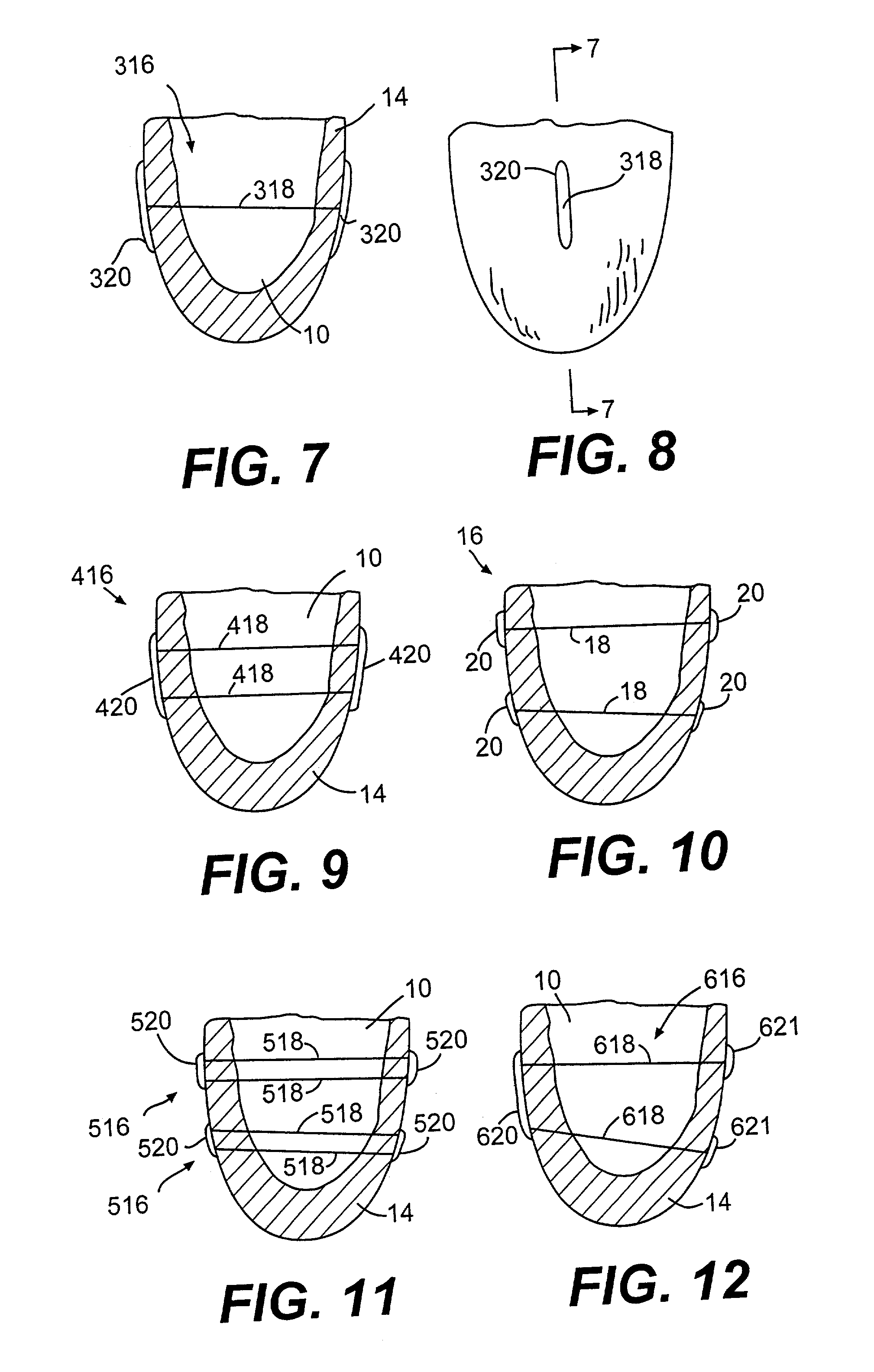
Various methods and devices are disclosed for improving cardiac function in hearts having zones of infarcted (akinetic) and aneurysmal (dyskinetic) tissue regions. The methods and devices reduce the radius of curvature in walls of the heart proximal infarcted and aneurysmal regions to reduce wall stress and improve pumping efficiency. The inventive methods and related devices include splinting of the chamber wall proximal the infarcted region and various other devices and methods including suture and patch techniques.
Owner:EDWARDS LIFESCIENCES LLC
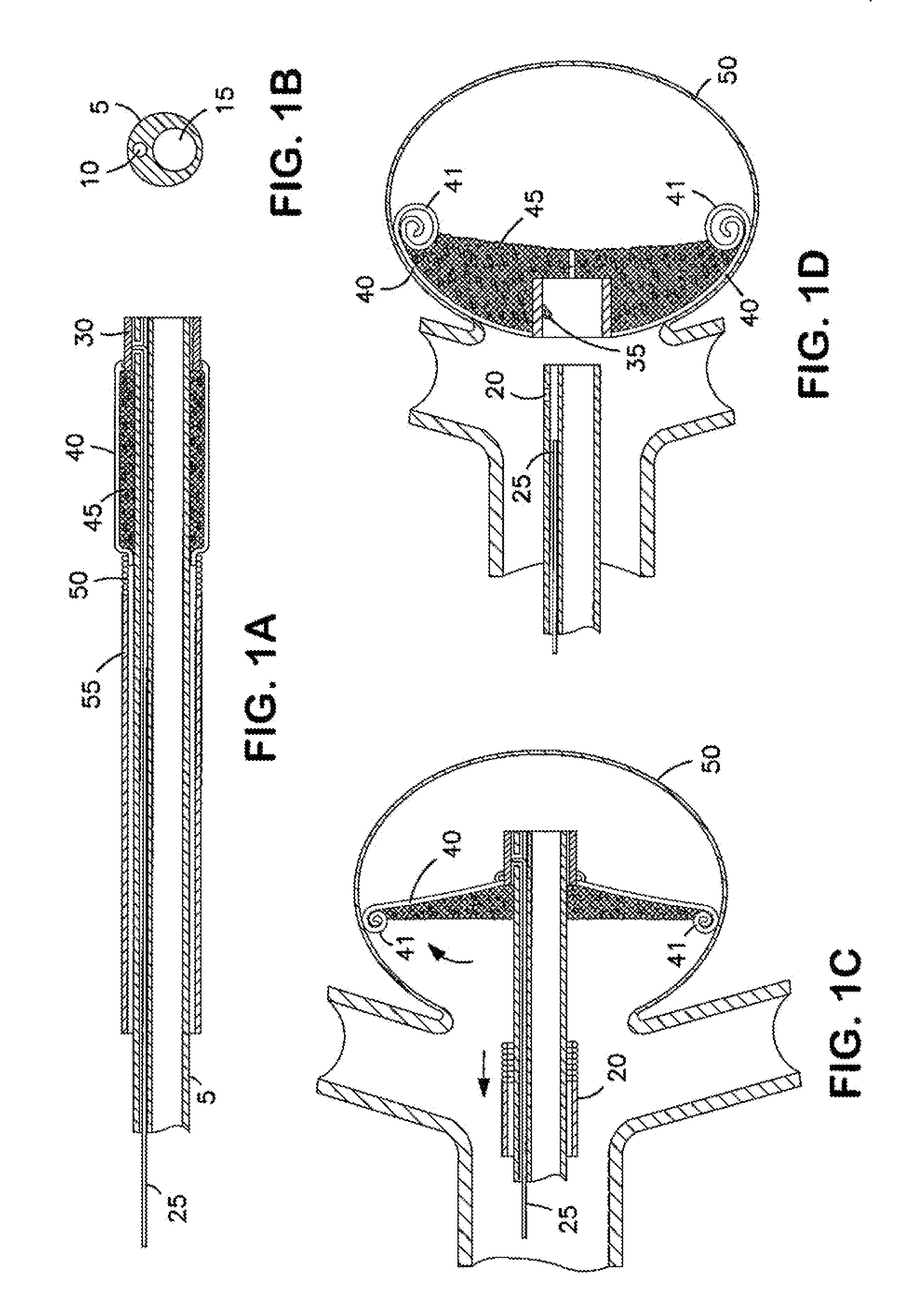
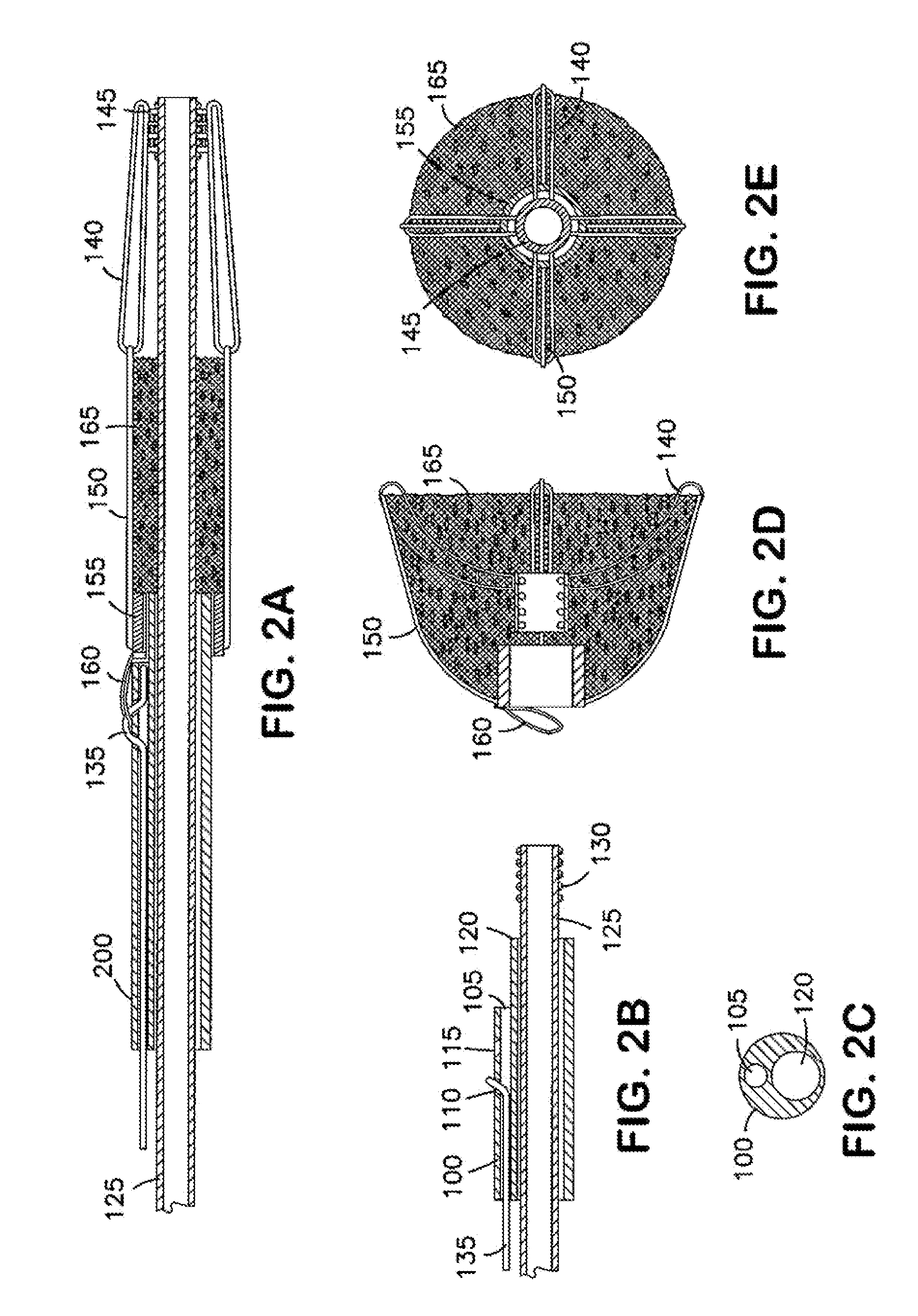
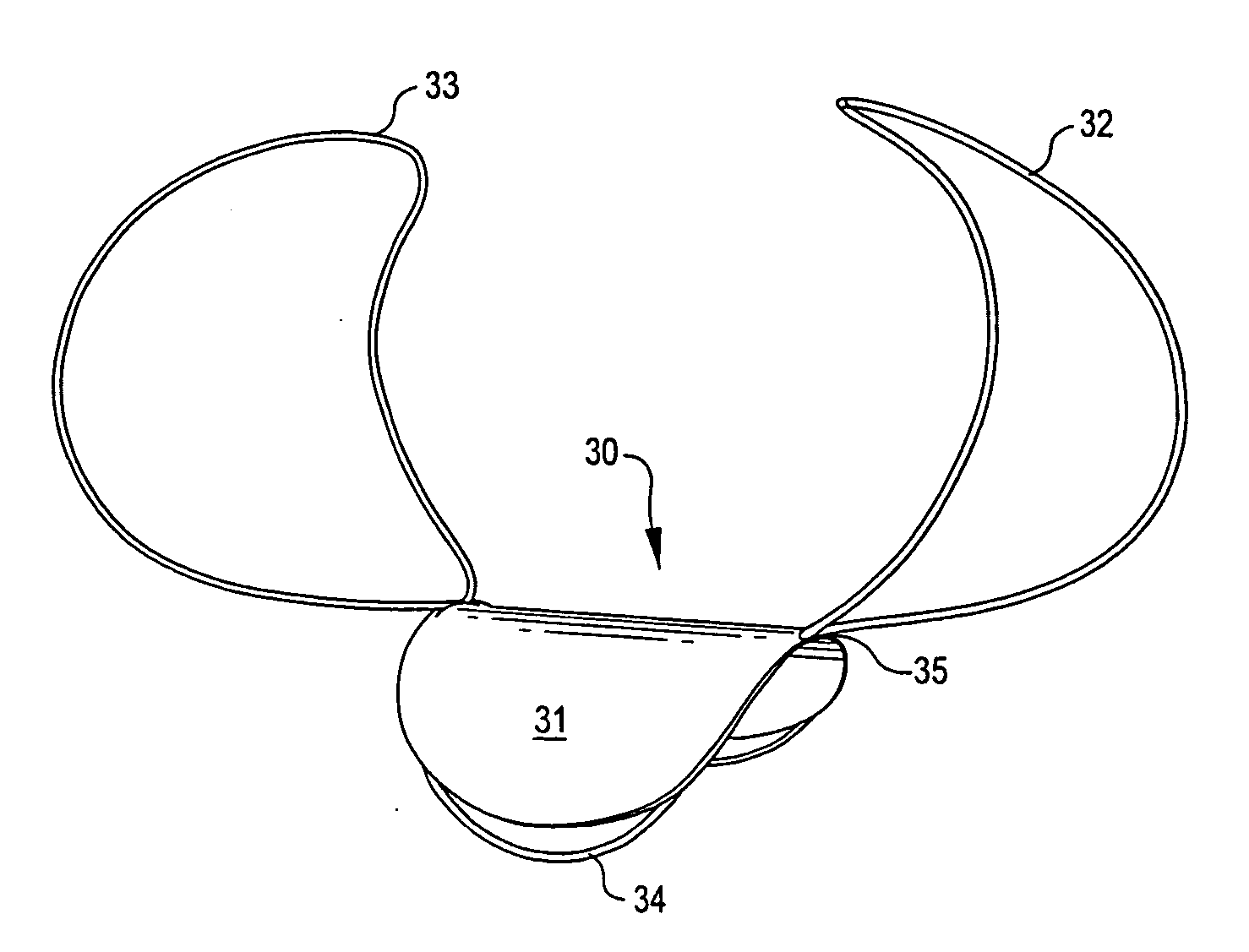
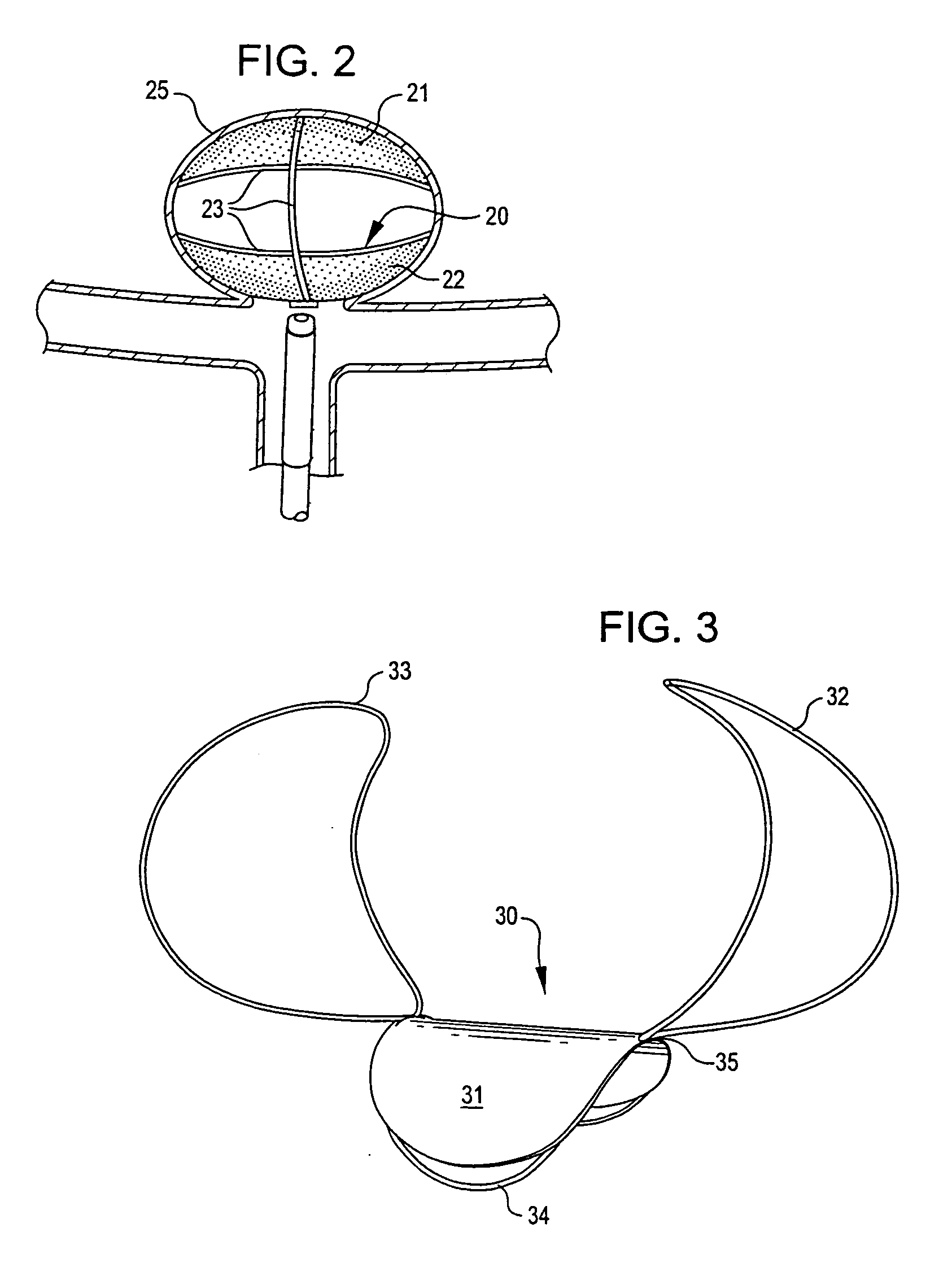
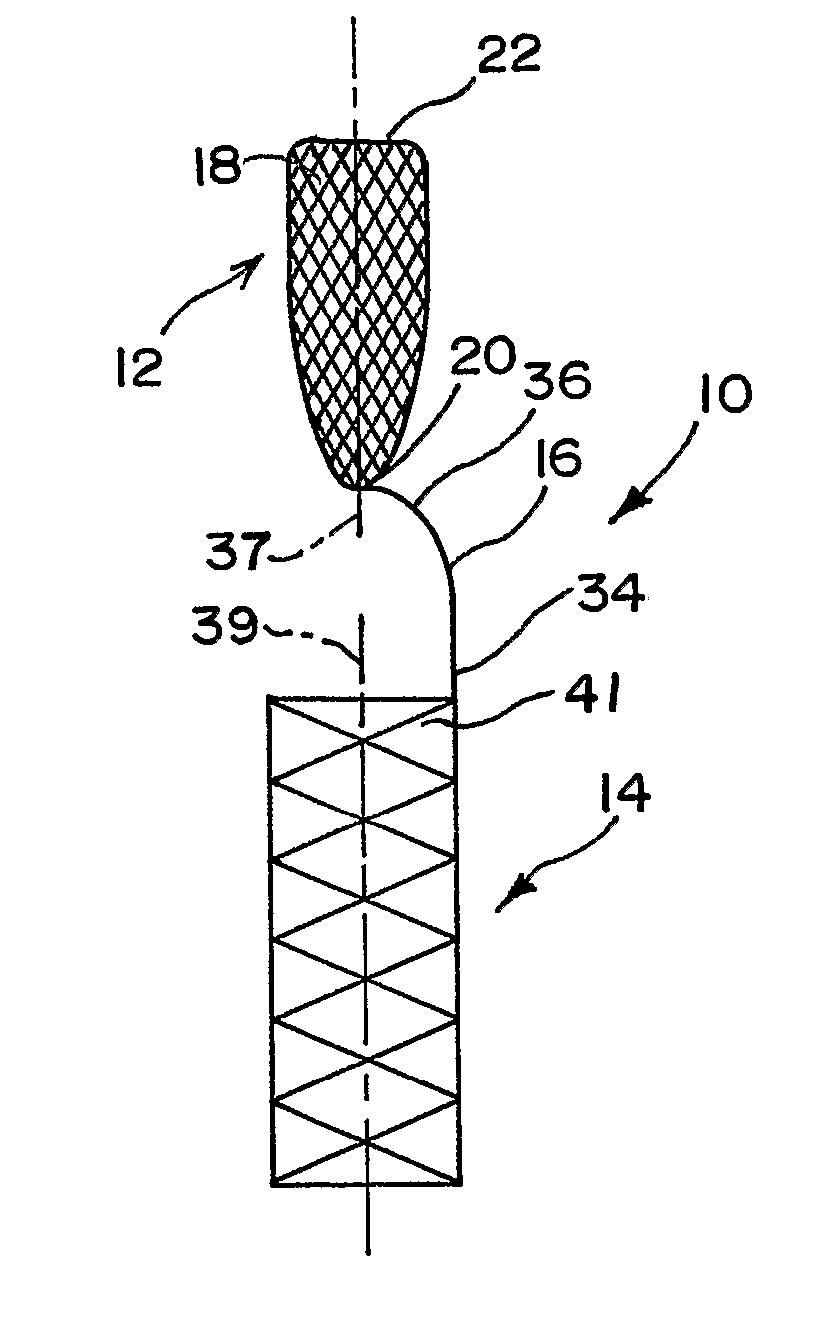
Aneurysm Occlusion Devices
InactiveUS20080281350A1Promote endothelial growthEliminate riskDilatorsCatheterMedicineImplanted device
An implantable occlusion device for bridging the neck of an aneurysm comprises a biocompatible matrix. The device is movable between a compressed position prior to implantation and a generally concave or cup-shaped position following implantation. The device may comprise a frame having a plurality of elements. The frame elements have a first pre-deployment position generally parallel to a major axis of the delivery lumen, and a second post-deployment position spread radially from the major axis of the delivery lumen. The biocompatible matrix and / or the frame elements may also form or be manipulated to form a generally concave or cupped shape. The matrix can be porous or semiporous, such as a foam or a reticulated matrix. The occlusion device can be folded, twisted and / or stretched to adopt a narrow profile for loading into a coaxial delivery device and expand in place as it adopts its original shape on release. The device may be released or manipulated to a desired shape to occlude an aneurysm. Methods of using the implantable device are also provided.
Owner:BIOMERIX CORP
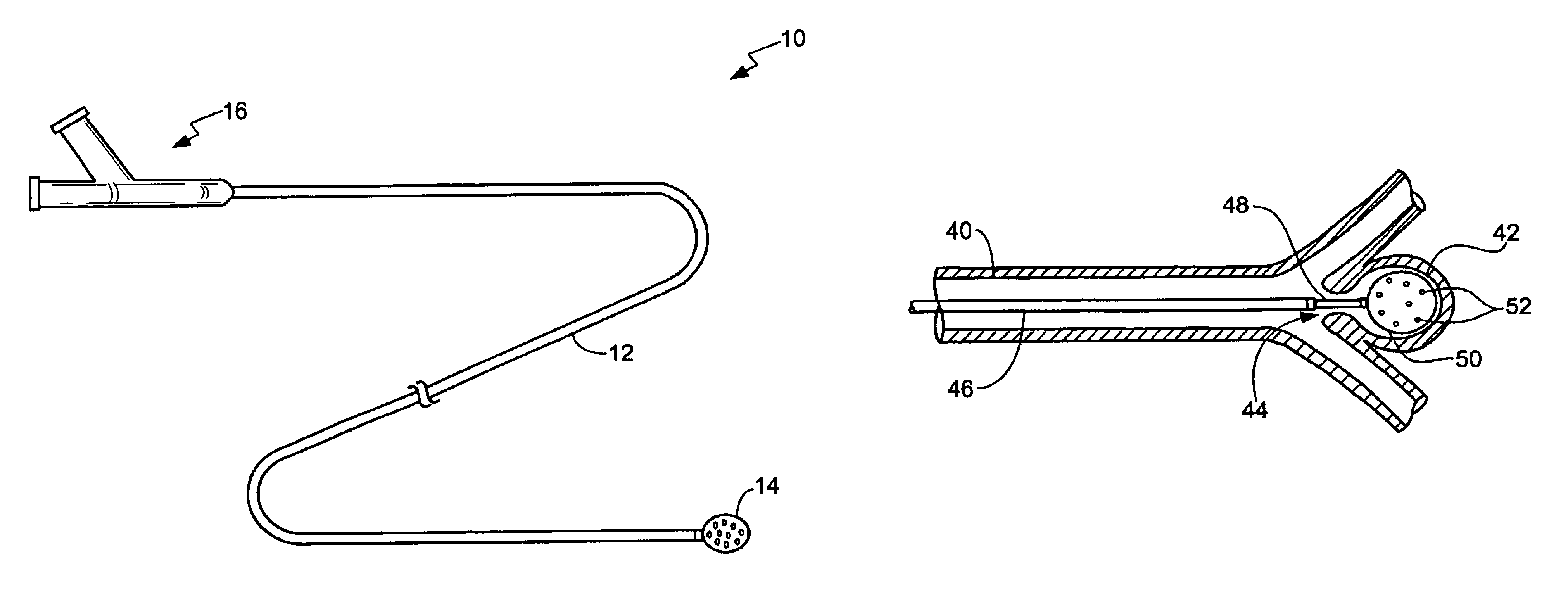
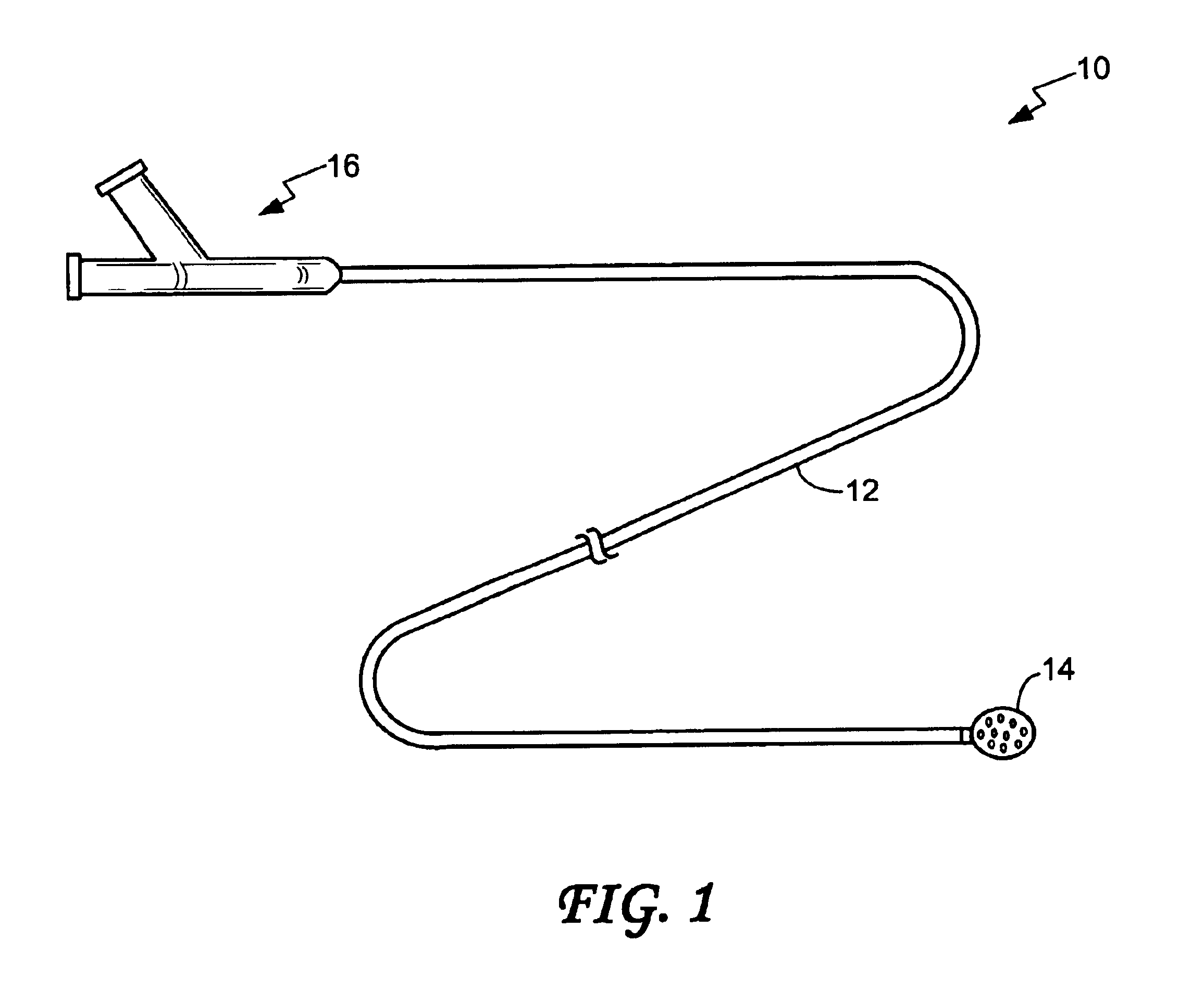
Embolic balloon
An embolic balloon assembly is disclosed herein. The assembly includes a detachable balloon system which expands while aspirating a quantity of surrounding blood to occlude a vessel or aneurysm. The balloon has a distensible membrane having a plurality of orifices throughout its surface. Within the distensible membrane is a plurality of expandable members made from a shape memory alloy, e.g., Ni—Ti alloy, which expand upon application of a stimulus. Alternatively, the expandable members or a single expandable wire may be inserted separately into the distensible membrane. Once the balloon begins to expand, internal pressure within a volume defined by the distensible membrane begins to drop, forcing the device to aspirate a quantity of surrounding blood inside the volume which then begins to coagulate by stasis or some stimulus. The balloon may be configured to automatically release into the aneurysm or vessel or it may be released by a detachable joint.
Owner:SAADAT VAHID
Intravascular stent device
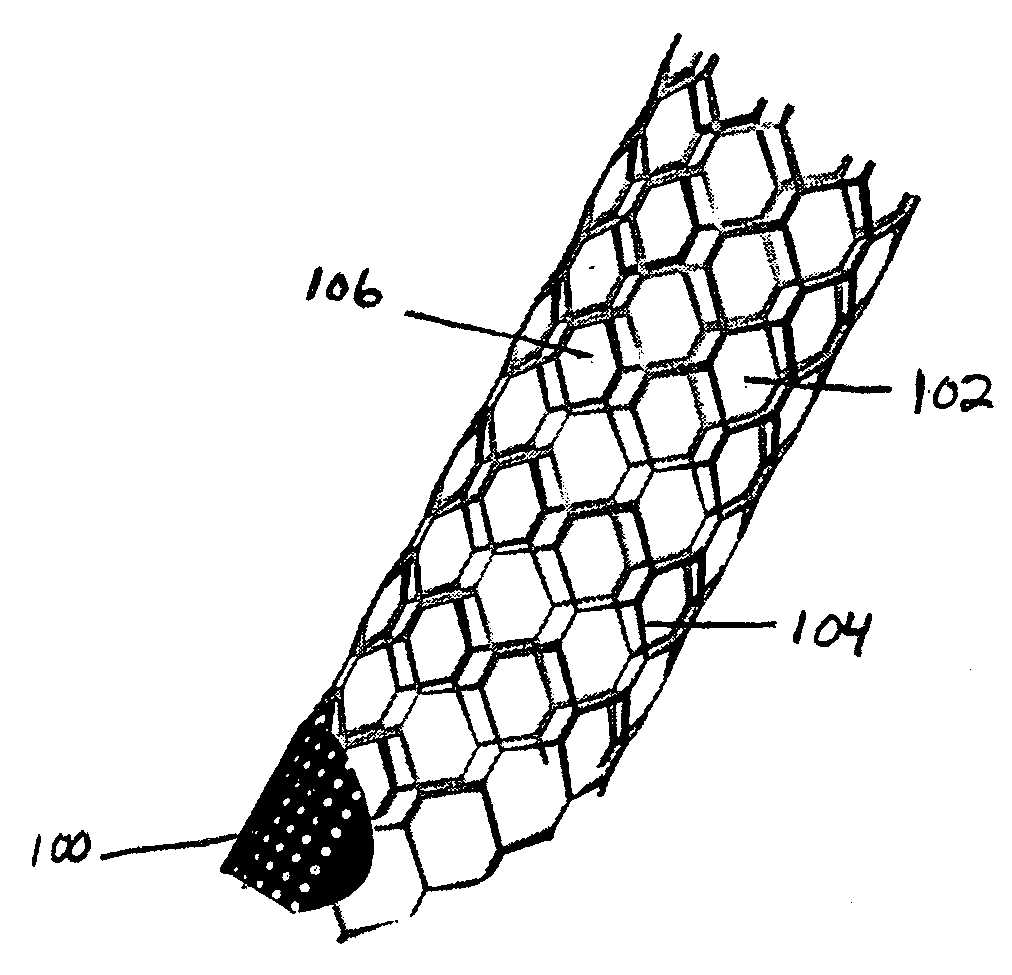
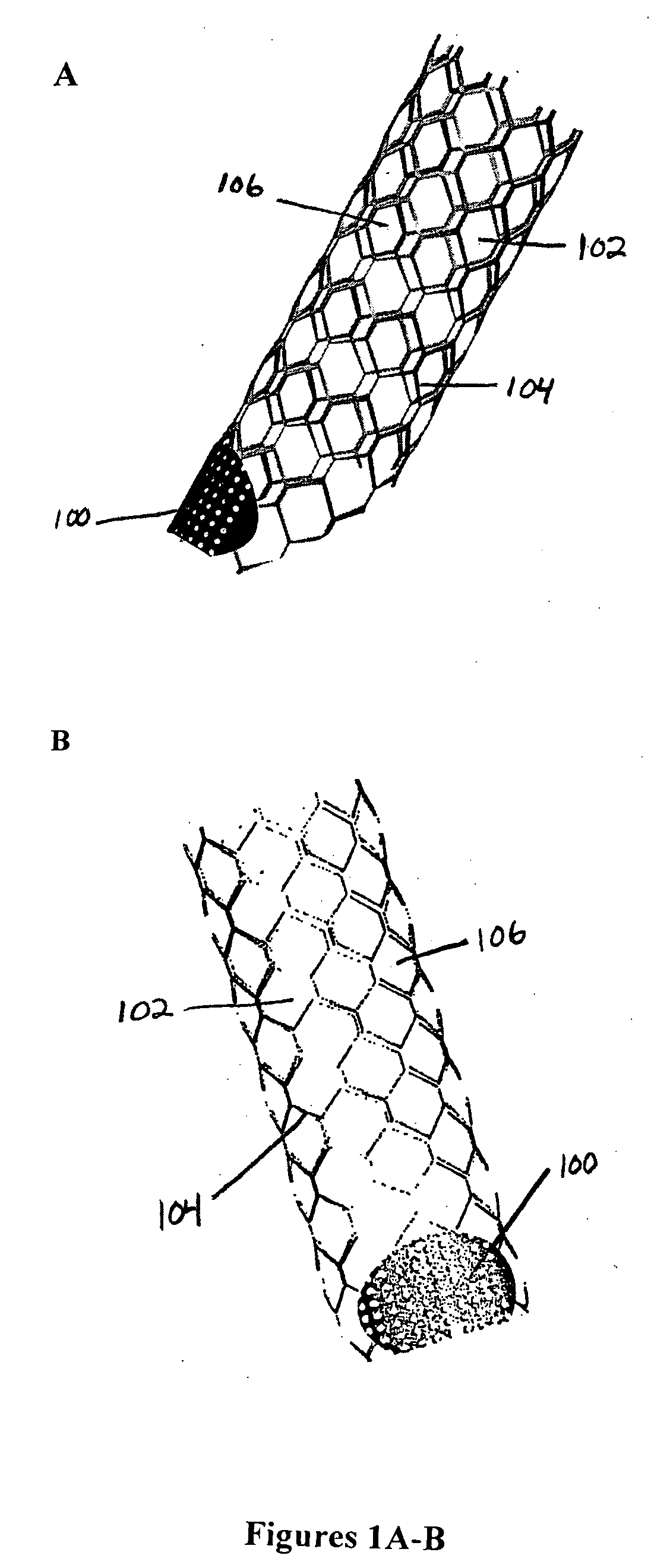
A very small diameter intravascular stent device which may be used to occlude or partially occlude an aneurysm in the human brain which is comprised of a thin-walled skeletal cylindrical tube formed of undulating or sinusoidal elements which, when compressed, nest tightly with each other.
Owner:CODMAN & SHURTLEFF INC
Shape memory tubular stent
A stent radially expandable from a radially contracted introduction state into a radially expanded position state, in which the final shape of the stent can be controlled by varying the amount and places energy is delivered onto the interior surfaces of the tubes from which the stent is fabricated, and means to seal the opening into the aneurysm, thereby causing the blood therein to clot and preventing the aneurysm from growing or rupturing.
Owner:UNSWORTH JOHN DUNCAN +1
Methods and devices for improving cardiac function in hearts
InactiveUS20060161040A1Reduce tensionReduce energy consumptionSuture equipmentsHeart valvesMedicineCardiac wall
Various methods and devices are disclosed for improving cardiac function in hearts having zones of infarcted (akinetic) and aneurysmal (dyskinetic) tissue regions. The methods and devices reduce the radius of curvature in walls of the heart proximal infarcted and aneurysmal regions to reduce wall stress and improve pumping efficiency. The inventive methods and related devices include splinting of the chamber wall proximal the infarcted region and various other devices and methods including suture and patch techniques.
Owner:EDWARDS LIFESCIENCES LLC
Intravascular deliverable stent for reinforcement of vascular abnormalities
InactiveUS20070168019A1Avoid interactionReduce overall outer diameterStentsCatheterVascular Skin TumorSaphenous veins
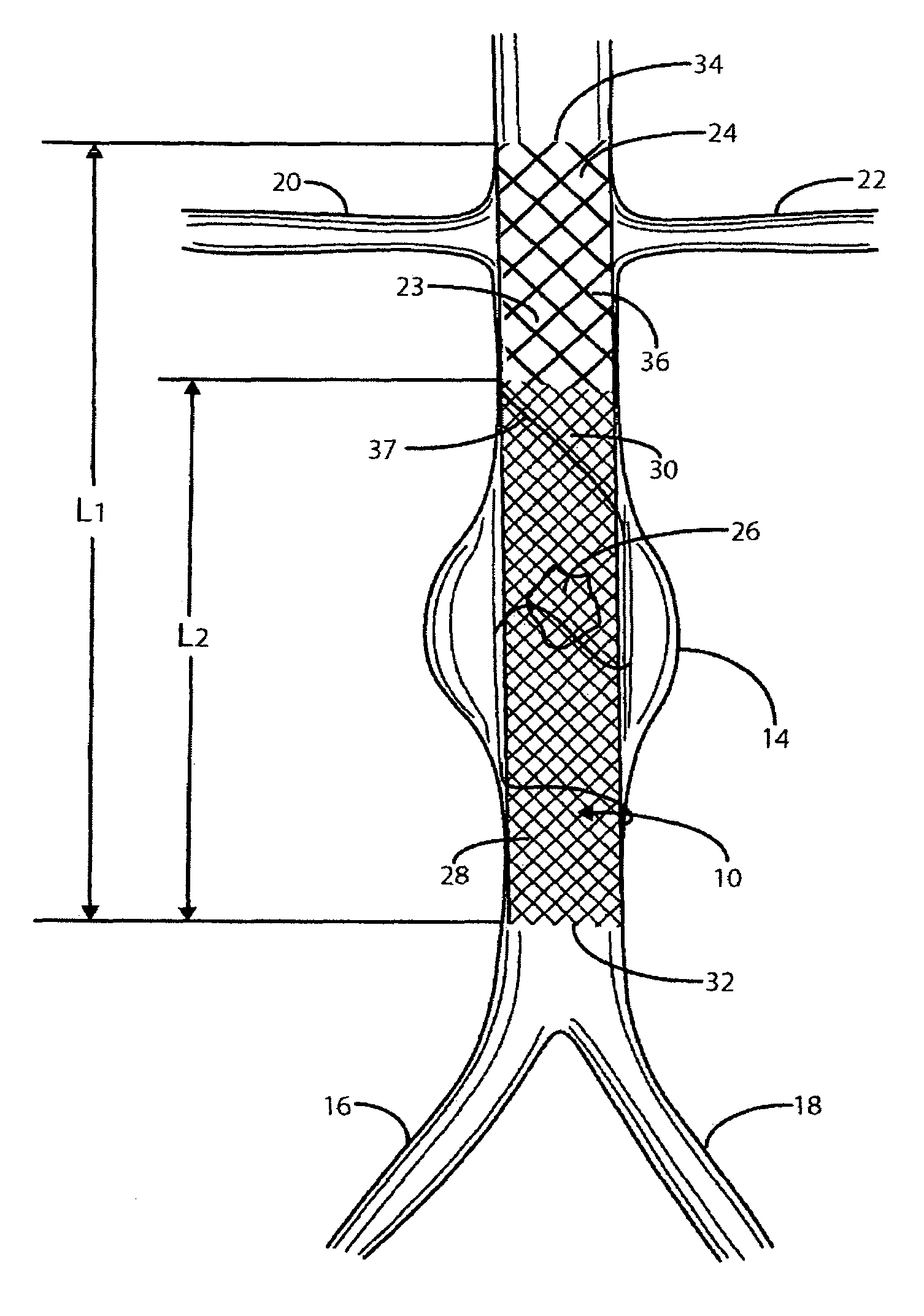
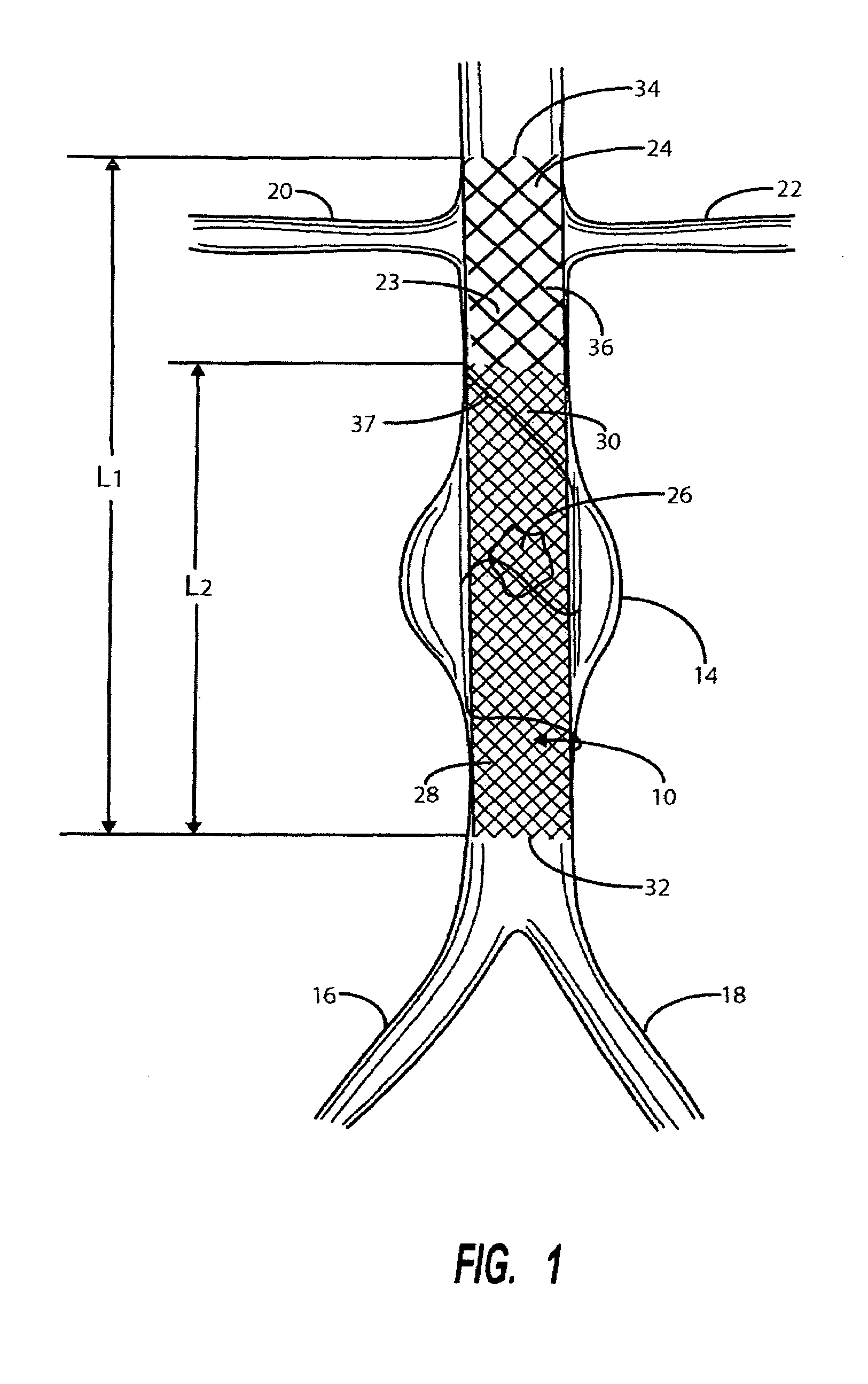
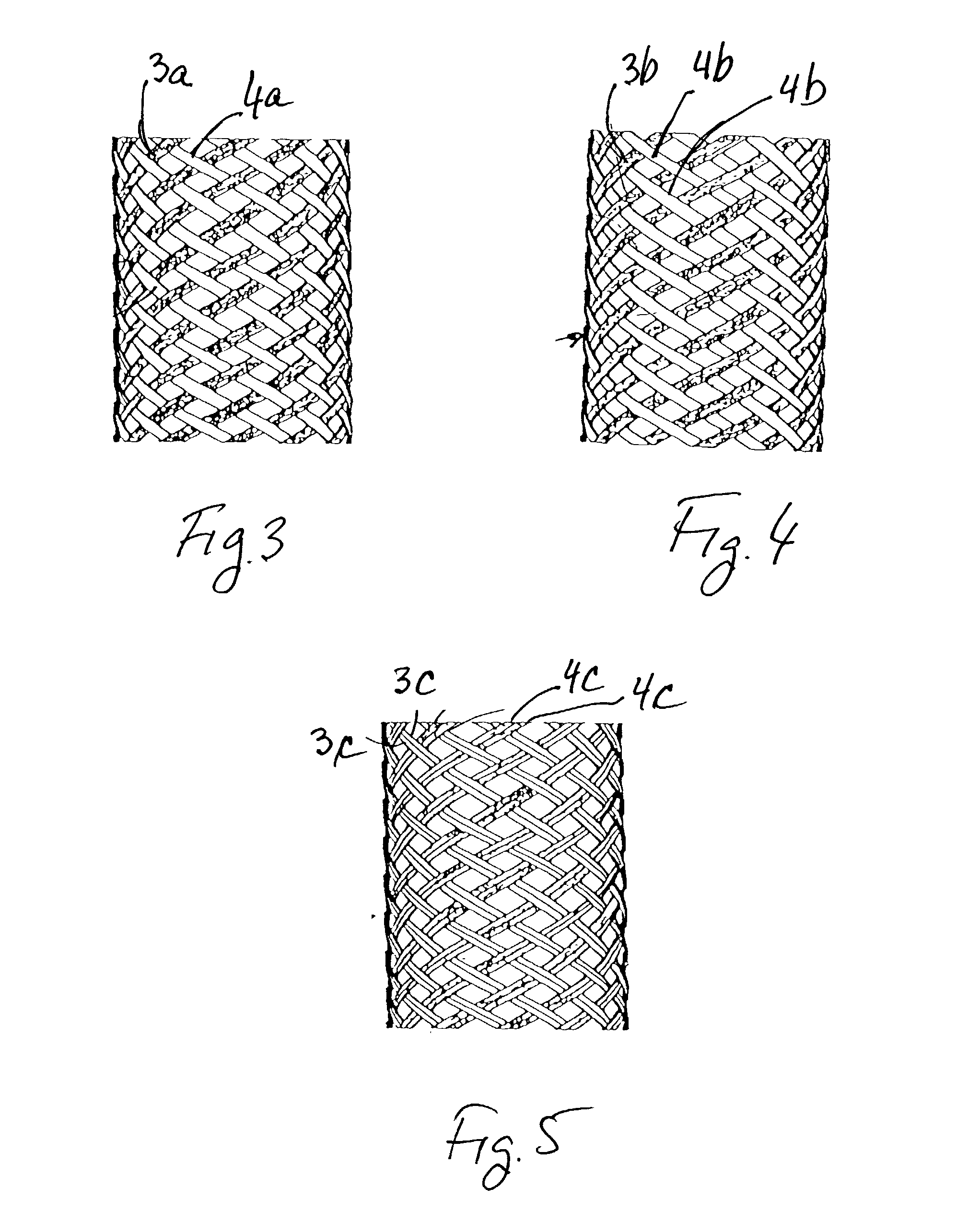
A catheter deliverable stent / graft especially designed to be used in a minimally invasive surgical procedure for treating a variety of vascular conditions such as aneurysms, stenotic lesions and saphenous vein grafts, comprises an innermost tubular structure and at least one further tubular member in coaxial arrangement. In one embodiment, the innermost tubular structure is of a length (L1) and is formed by braiding a relatively few strands of highly elastic metallic alloy. The pick and pitch of the braid are such as to provide relative large fenestrations in the tubular wall that permit blood flow through the wall and provide the primary radial support structure. A portion of the innermost tubular structure of a length L1 is surrounded by a further braided tubular structure having relatively many strands that substantially inhibit blood flow through the fenestrations of the innermost tubular structure. The composite structure can be stretched to reduce the outer diameter of the stent / graft, allowing it to be drawn into a lumen of a delivery catheter. The catheter can then be advanced through the vascular system to the site of treatment and then released, allowing it to self-expand against the vessel wall. Various optional embodiments are disclosed that allow one skilled in the art to tailor the design to the specific application.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
Endovascular thin film devices and methods for treating and preventing stroke
InactiveUS20030060782A1Safely and permanently excludingPreventing initial or recurrent aneurysmal subarachnoid hemorrhageStentsCatheterIn situ polymerizationProsthesis
Devices for excluding aneurysms and treating atherosclerotic disease, for intra-aneurysmal occlusion; and devices for preventing distal emboli. The devices are generally pliable and collapsible thin film devices which can be delivered via a microcatheter into the desired location where they are deployed and undergo either a shape memory phase transformation or in situ polymerization to assume the stable configuration of a permanent endoluminal prosthesis. Prior to being caused to assume their final shape, the devices remain soft, collapsible and pliable to ensure atraumatic delivery through the vascular system. Upon reaching the endoluminal defect in the vessel, the device is extruded from the microcatheter. Devices are also provided for retrieving clots.
Owner:NEW YORK UNIV
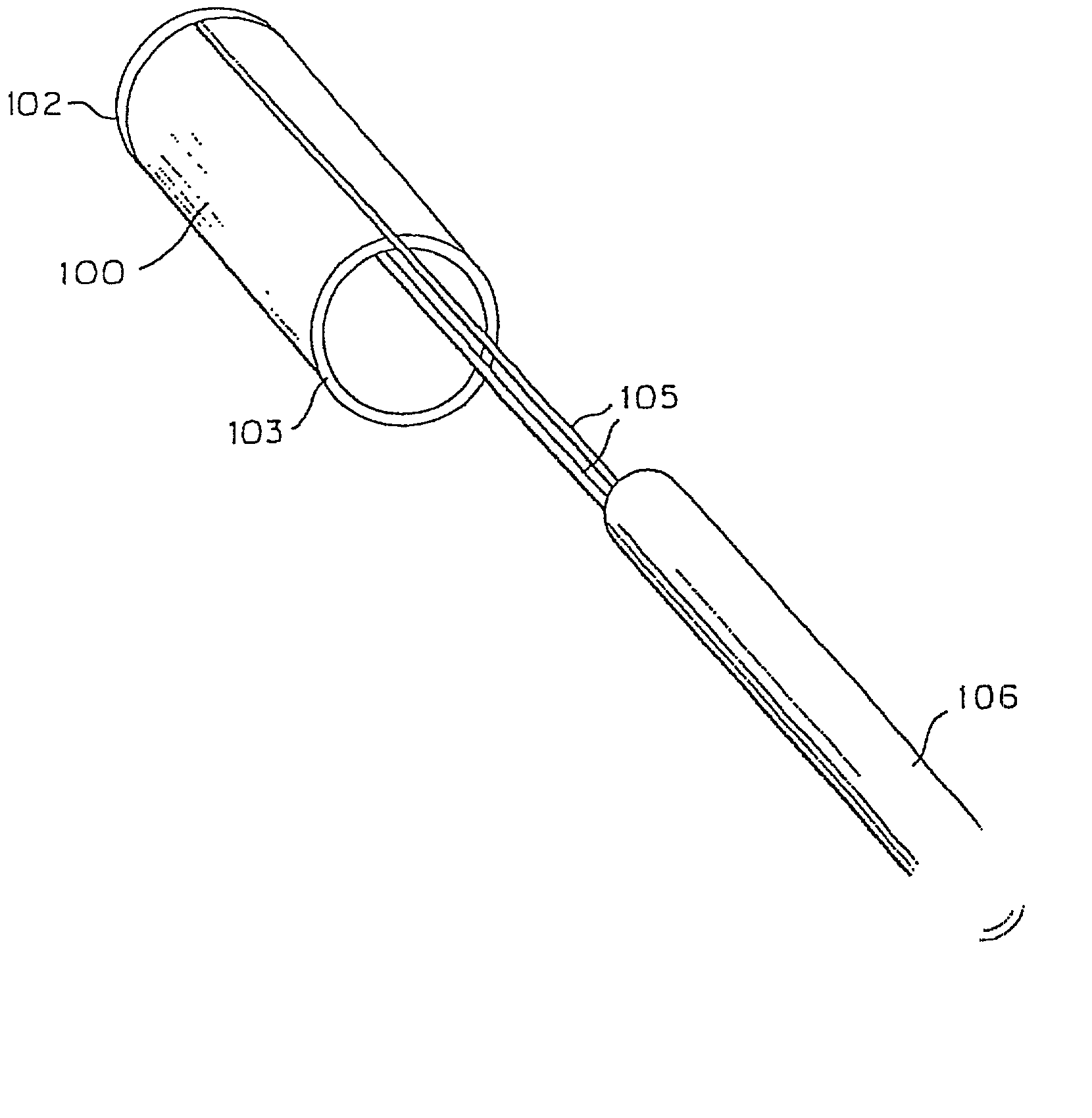
Stent vascular intervention device and methods for treating aneurysms
InactiveUS20070021816A1Shorten the construction periodGreat effect on aneurysmal blood flowStentsBlood vesselsPorosityInsertion stent
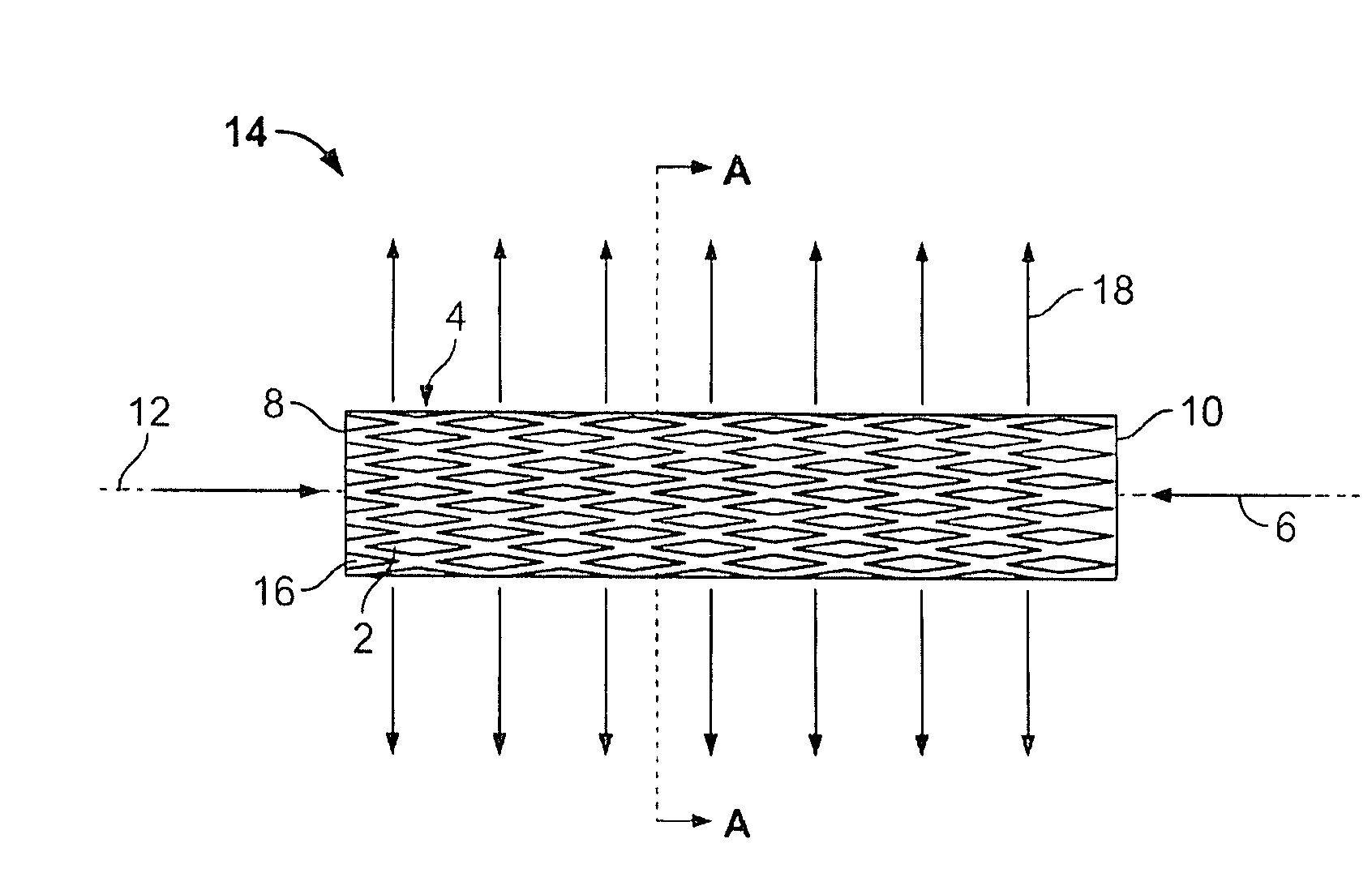
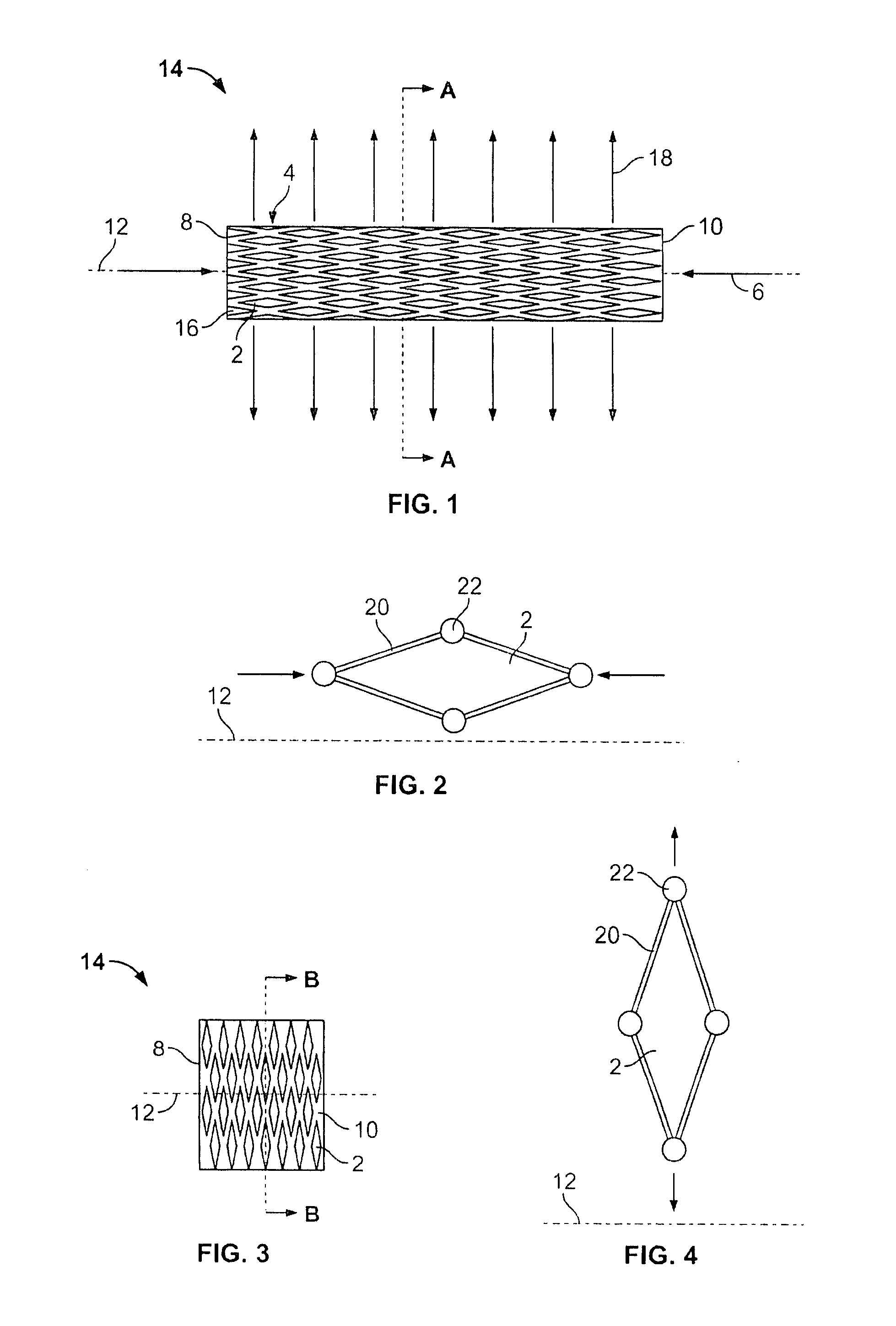
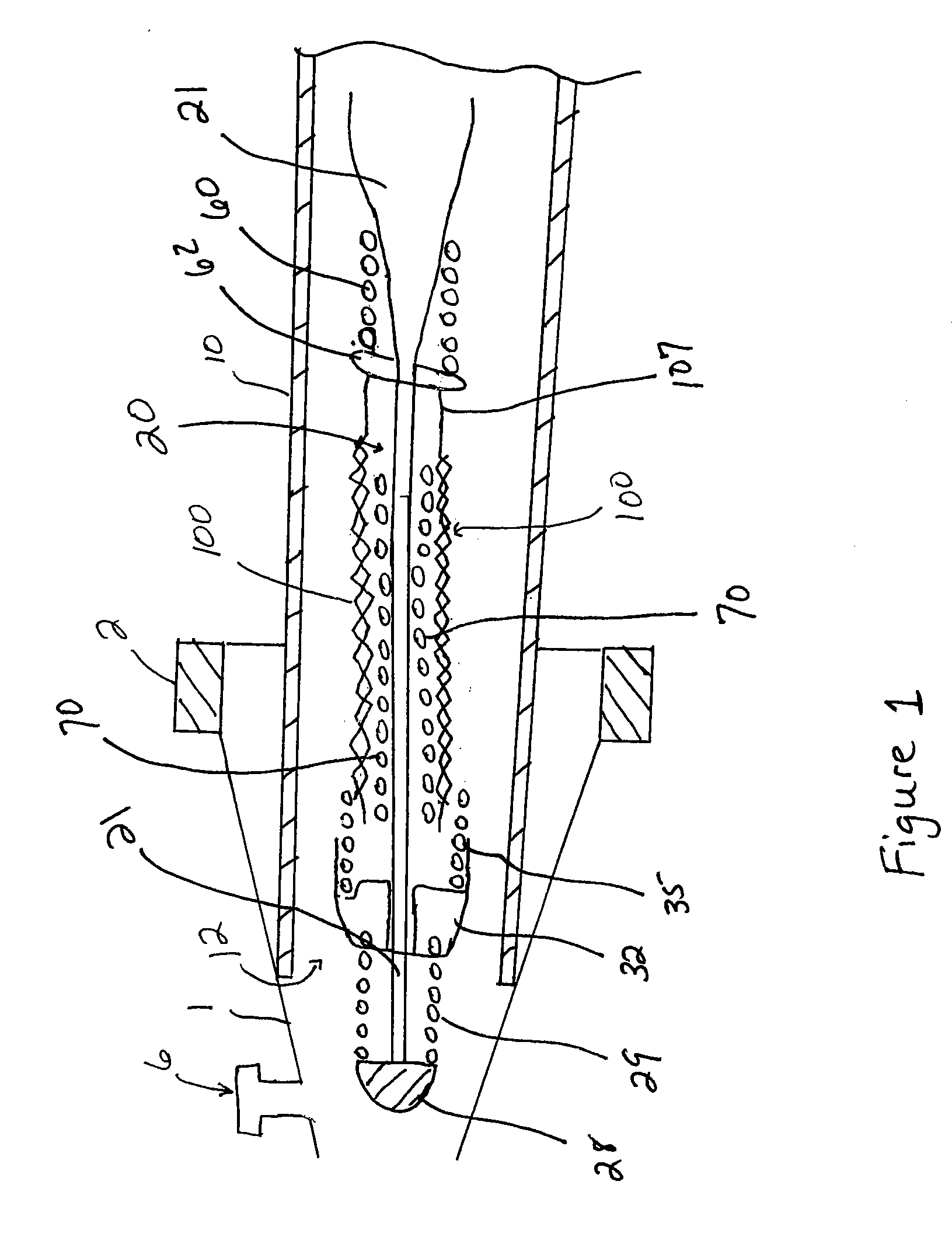
The present invention relates to a stent including a variable porosity, tubular structure having pores defined by structural surfaces. The tubular structure has a low porosity region in proximity to or at either end of the tubular structure, where the low porosity region is less porous than other regions located on the tubular structure and fully or partially obstructs passage of fluid. Any arcuate path that starts at one point within the low porosity region and goes around the perimeter of the tubular structure to stop at the same point within the low porosity region must have at least a portion that is outside of the low porosity region. Also disclosed is a method of modifying blood flow within and near an opening of an aneurysm in a blood vessel by deploying one or more stents of the present invention near an opening of the aneurysm in a blood vessel.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Isolation devices for the treatment of aneurysms
Device, systems and methods are provided to isolate aneurysms, particularly at bifurcations, while maintaining adequate blood flow through nearby vessels. These devices are deliverable to a desired target area and maintain position in a desired orientation so as to occlude flow in some aspect while allowing flow in others. In addition, devices, systems and methods are provided to occlude blood vessels, such as endoleaks, to improve the isolation of aneurysms.
Owner:TSUNAMI INNOVATIONS
Intrasaccular embolic device
An intrasaccular device particularly adapted for treating body lumens. The intrasaccular device includes structure that provides a strong framework as well as improved covering across an opening to an aneurysm sac. The intrasaccular device is intended to retain foreign bodies within the aneurysm sac and includes members for accomplishing this objective. The intrasaccular device is also provided with structure that facilitates the alignment of a plurality of devices deployed within the sac.
Owner:ABBOTT CARDIOVASCULAR
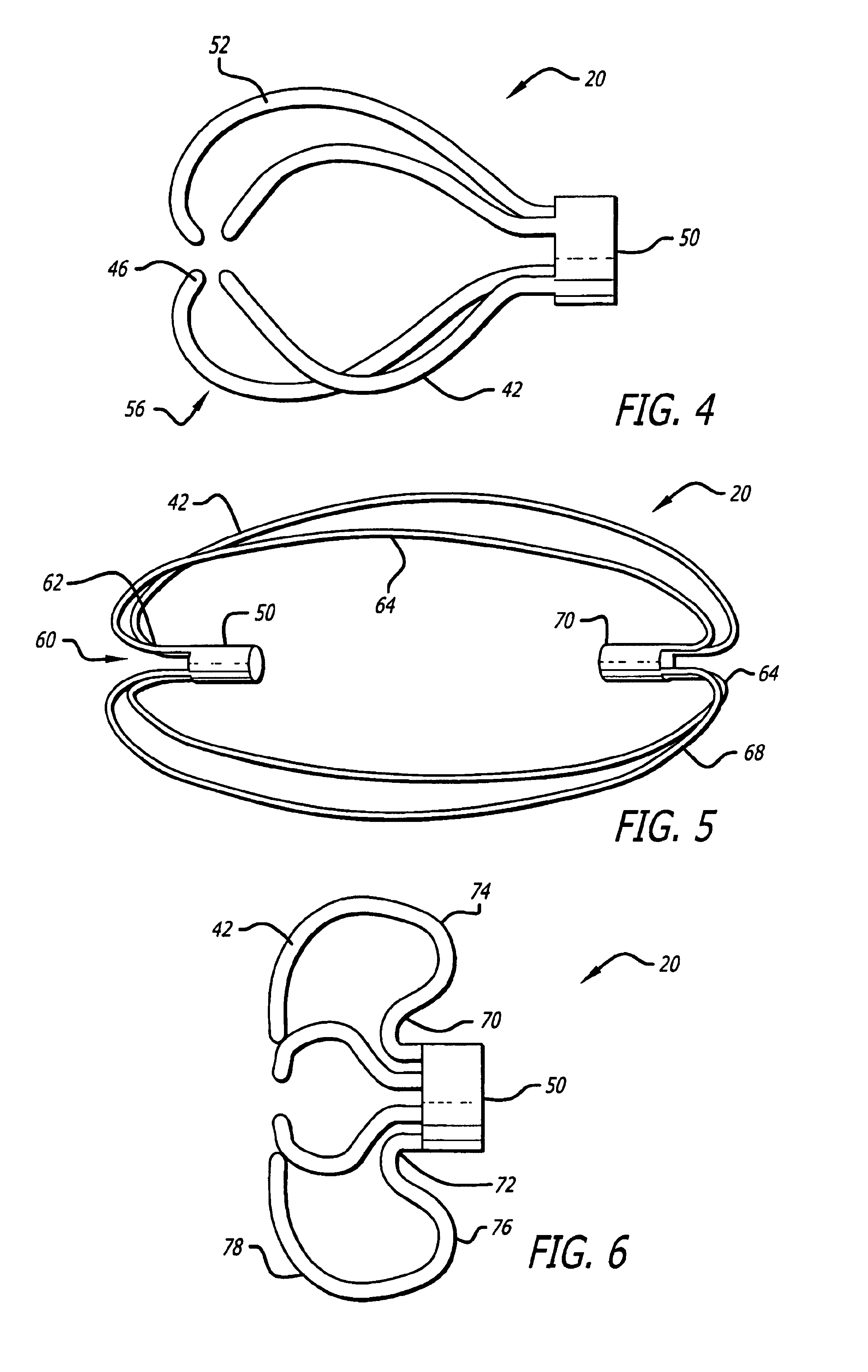
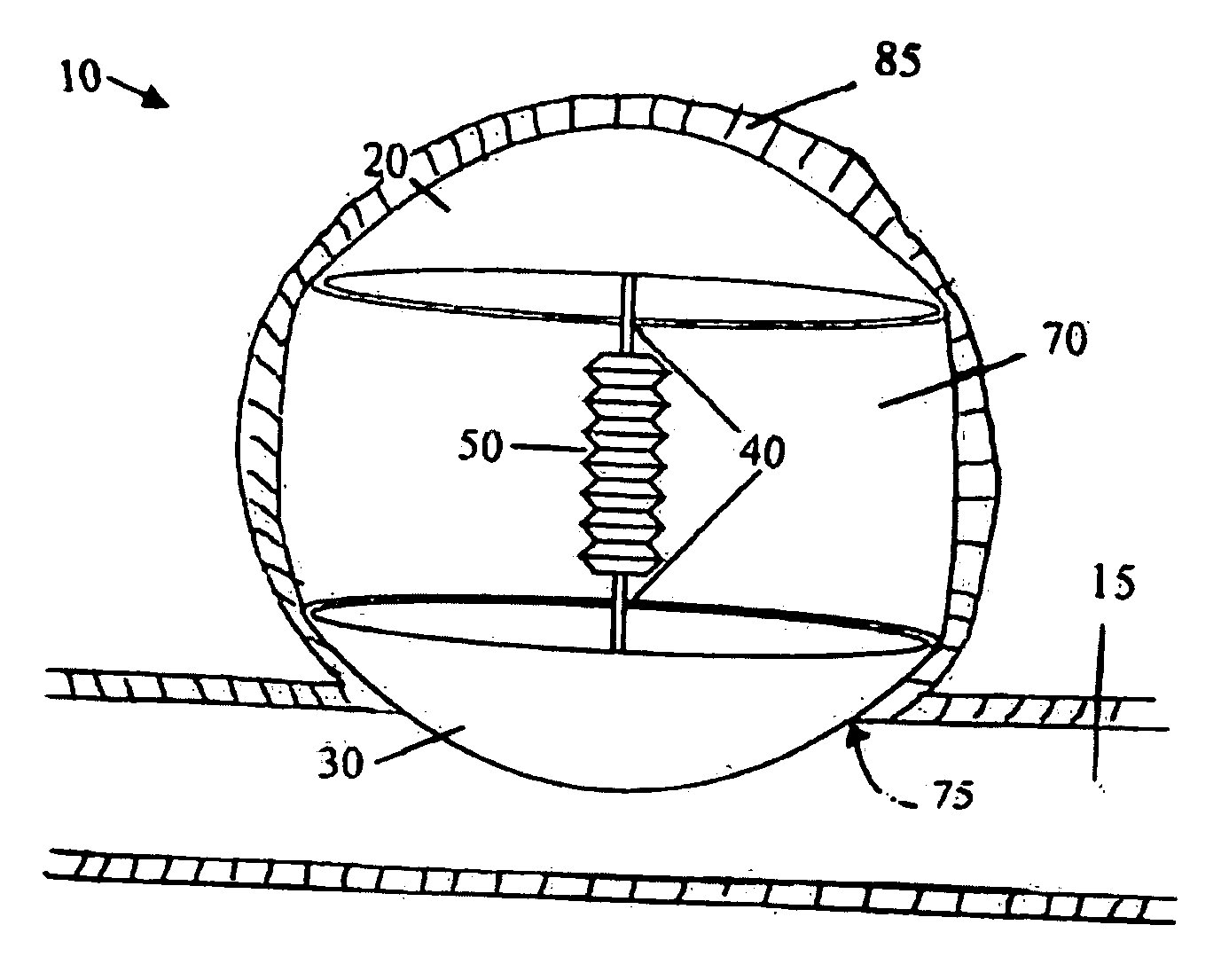
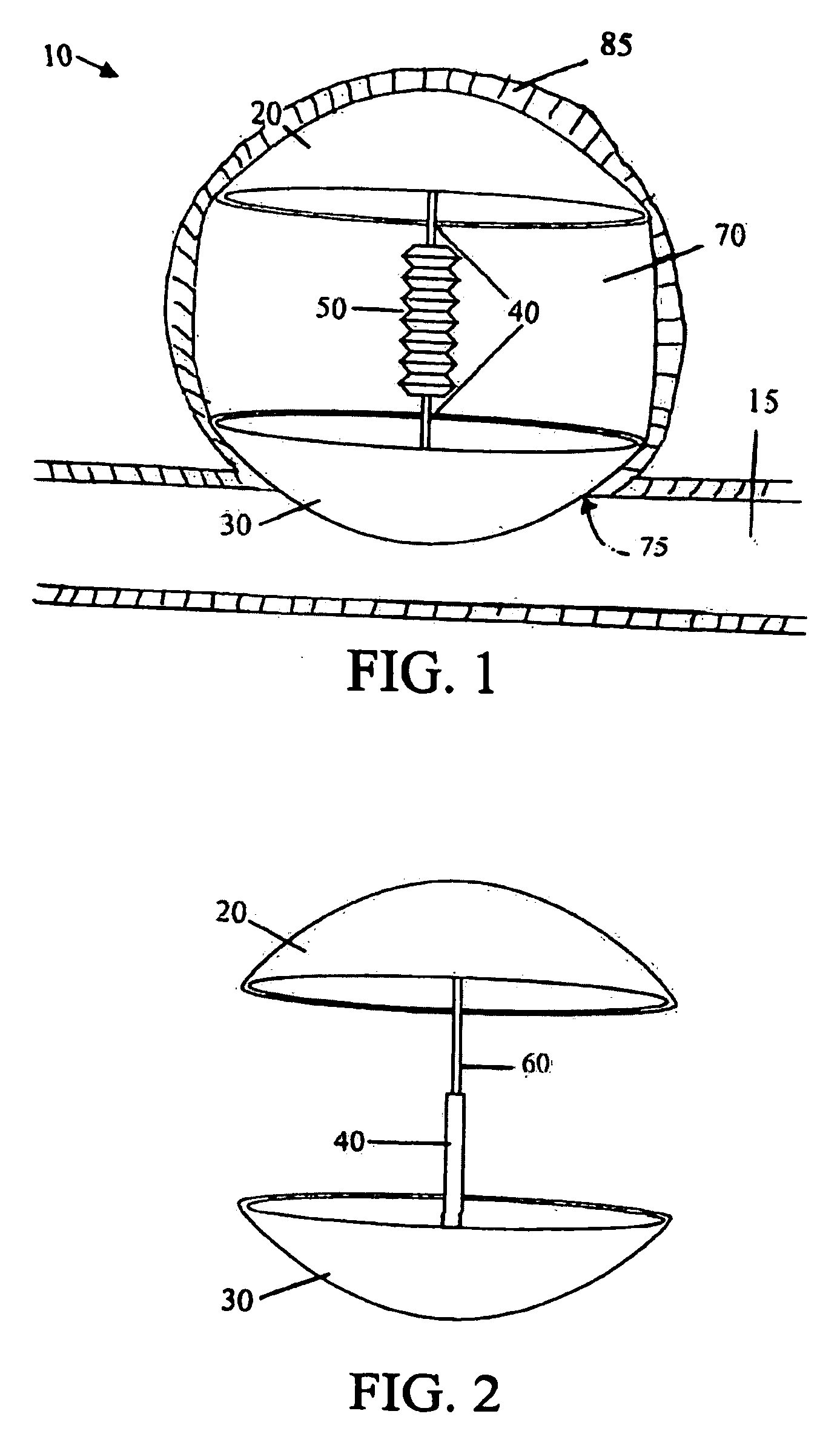
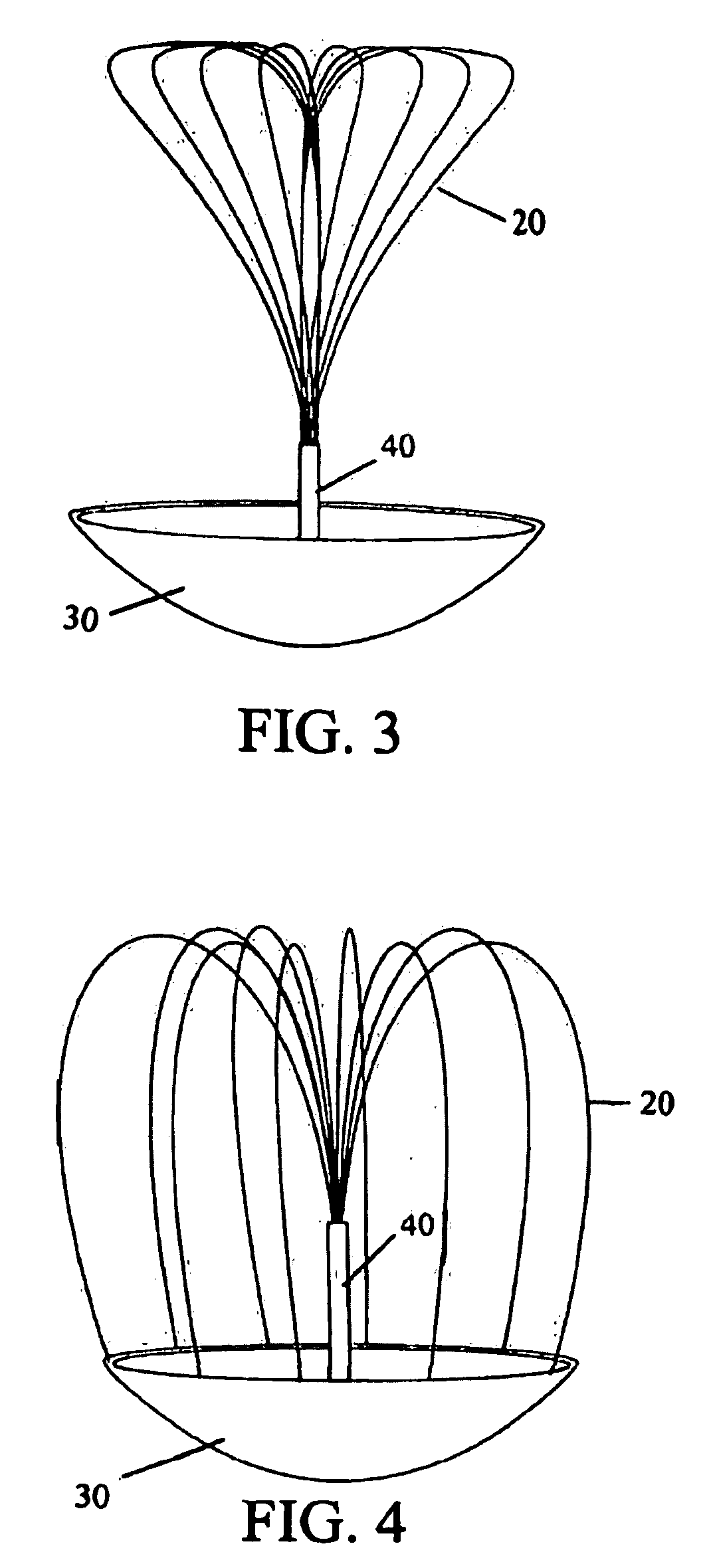
Intra-aneurysm devices
Devices for occluding an aneurysm are provided. In particular, the device include an upper member that sits against the dome of the aneurysm, a lower member that sits in the neck of the aneurysm, and a means of adjusting the overall dimensions of the device. Also provided are methods of making and using these devices.
Owner:STRYKER EURO OPERATIONS HLDG LLC +1
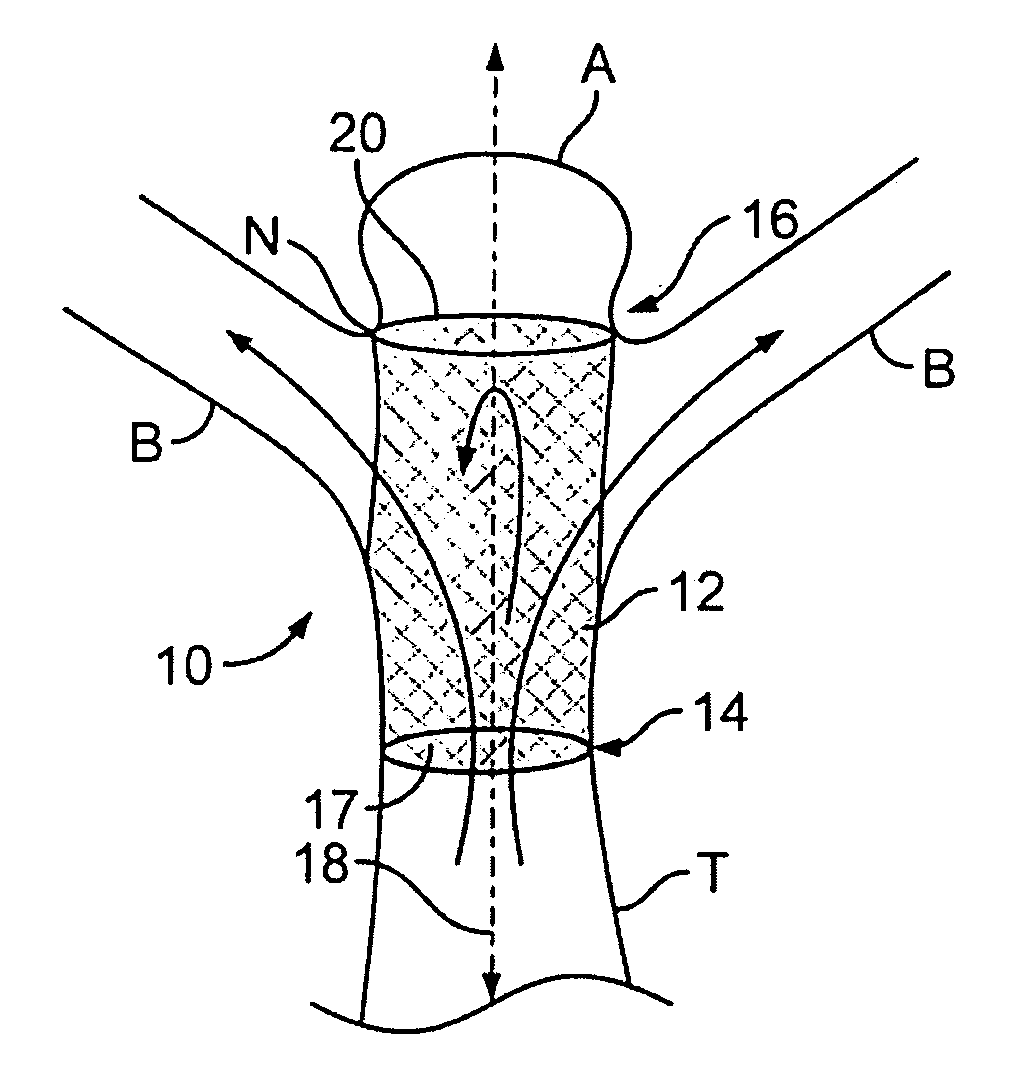
Device forming an endoluminal intracorporeal endoprosthesis, in particular for the abdominal aorta
The invention relates to a device forming an endoluminal endoprosthesis. The device comprises at least a first segment, and upstream from said segment, at a predetermined distance therefrom, it comprises a "fixing" upstream segment made of a biocompatible material so as to be deployable from a closed or non-deployed position for insertion purposes to a deployed working position, the deployed working position being designed to be located in a healthy zone of blood vessel and being separated from the first segment by a predetermined distance defined by links of predetermined length. The invention makes it easy to recatheterize a blood vessel such as the abdominal aorta suffering from an aneurysm.
Owner:LEGONA ANSTALT
Endovascular thin film devices and methods for treating and preventing stroke
InactiveUS6666882B1Treating and preventing ischemic and hemorrhagic strokeInhibit migrationStentsOcculdersIn situ polymerizationDistal embolization
Devices for excluding aneurysms and treating atherosclerotic disease, for intra-aneurysmal occlusion, and devices for preventing distal emboli. The devices are generally pliable and collapsible thin film devices which can be delivered via a microcatheter into the desired location where they are deployed and undergo either a shape memory phase transformation or in situ polymerization to assume the stable configuration of a permanent endoluminal prosthesis. Prior to being caused to assume their final shape, the devices remain soft, collapsible and pliable to ensure atraumatic delivery through the vascular system. Upon reaching the endoluminal defect in the vessel, the device is extruded from the microcatheter. Devices are also provided for retrieving clots.
Owner:NEW YORK UNIV
Expandable support device and methods of use
InactiveUS20080071356A1Uniform deploymentIncrease maximum radial and shear forceStentsSpinal implantsIntervertebral discBiomedical engineering
An expandable support device and methods of using the same are disclosed herein. The expandable support device can expand radially when compressed longitudinally. The expandable support device can be deployed in a bone, such as a vertebra, for example to repair a compression fraction. The expandable support device can be deployed into or in place of all or part of an intervertebral disc. The expandable support device can be deployed in a vessel, in an aneurysm, across a valve, or combinations thereof. The expandable support device can be deployed permanently and / or used as a removable tool to expand or clear a lumen and / or repair valve leaflets.
Owner:STOUT MEDICAL GROUP
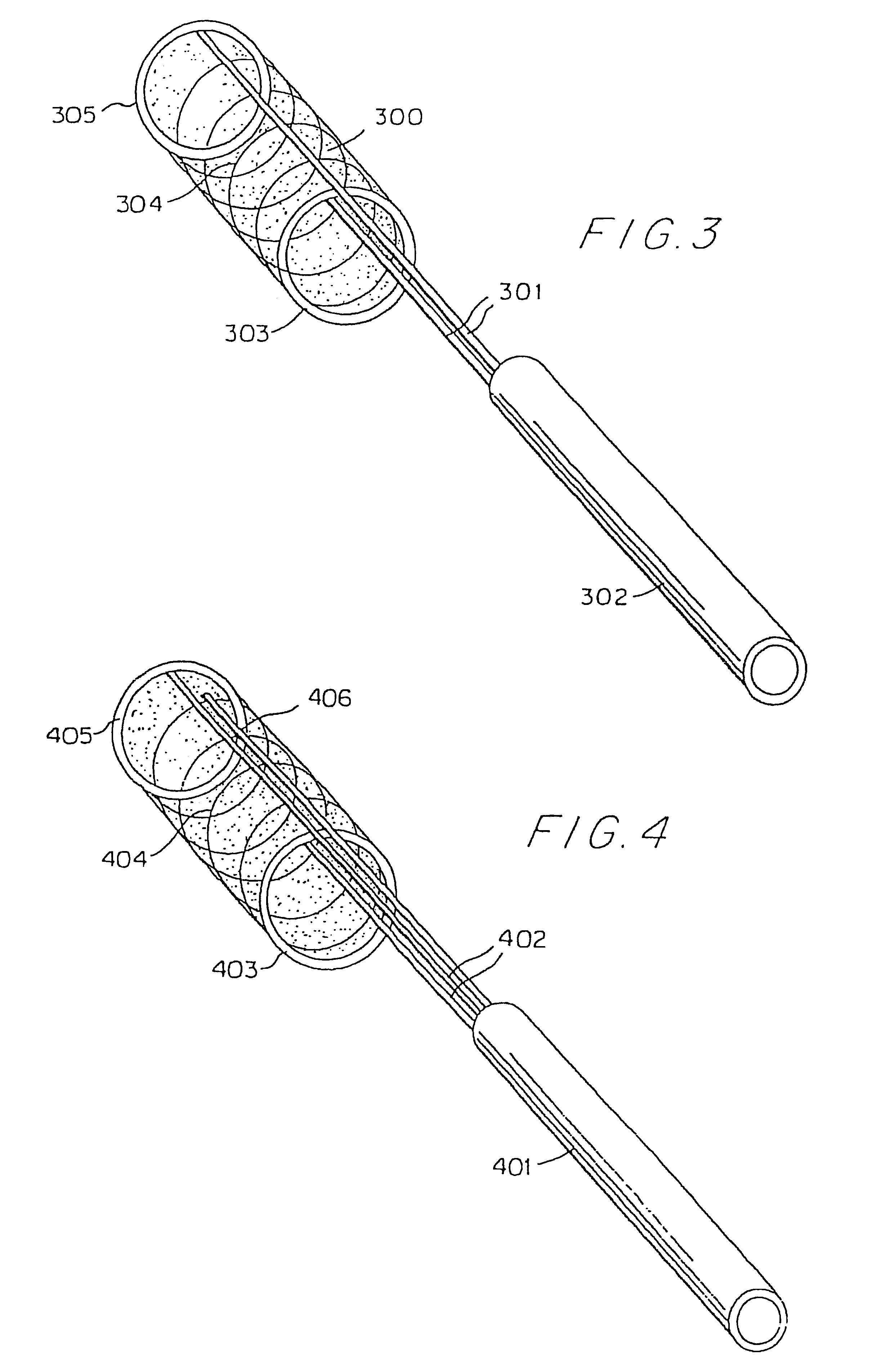
Self-expandable aneurysm filling device, system and method of placement
The self-expandable aneurysm filling device, system and method provide for placement of the stent into an aneurysm to at least partially fill and stabilize the aneurysm. The self-expandable aneurysm filling device has a compressed undeployed configuration and an expanded three-dimensional deployed configuration, and a severable deployment junction releasably connects the self-expandable aneurysm filling device to a pusher wire. The severable deployment junction can be mechanically, electrolytically, or thermally severed to separate the self-expandable aneurysm filling device from the pusher wire.
Owner:PENUMBRA
Implantable aneurysm closure systems and methods
An implantable closure structure is delivered using minimally invasive techniques and inhibits the migration of liquid and particulate matter from inside a physiological cavity or opening, such as an aneurysm, as well as inhibiting the flow of liquid and particulate matter, such as from an associated blood vessel, into a physiological cavity or opening. The device has a flexible patch supported by a framework structure that covers the neck or opening of a cavity such as an aneurysm and may have anchoring structures for supporting the flexible patch in place across the opening.
Owner:PULSAR VASCULAR
Thin Film Metallic Devices for Plugging Aneurysms or Vessels
InactiveUS20070270902A1Reduce riskFacilitates endoluminal deploymentStentsDilatorsBlood vesselEmbolization
Thin film metallic devices implantable within a human subject for occlusion of an aneurysm or blood vessel are provided. The devices include an embolization element that is moveable between a collapsed configuration for delivery to a deployed configuration within the body. The embolization device plugs the aneurysm or blood vessel preventing blood from flowing into or out of the aneurysm or other defective or diseased location of the blood vessel. The embolization element may be either self-supporting or supported by a strut structure. The occlusion device also includes an anchor element for anchoring the occlusion device and aiding in maintaining the embolization element in place. The anchor element is connected to the embolization device via a connector element.
Owner:DEPUY SYNTHES PROD INC
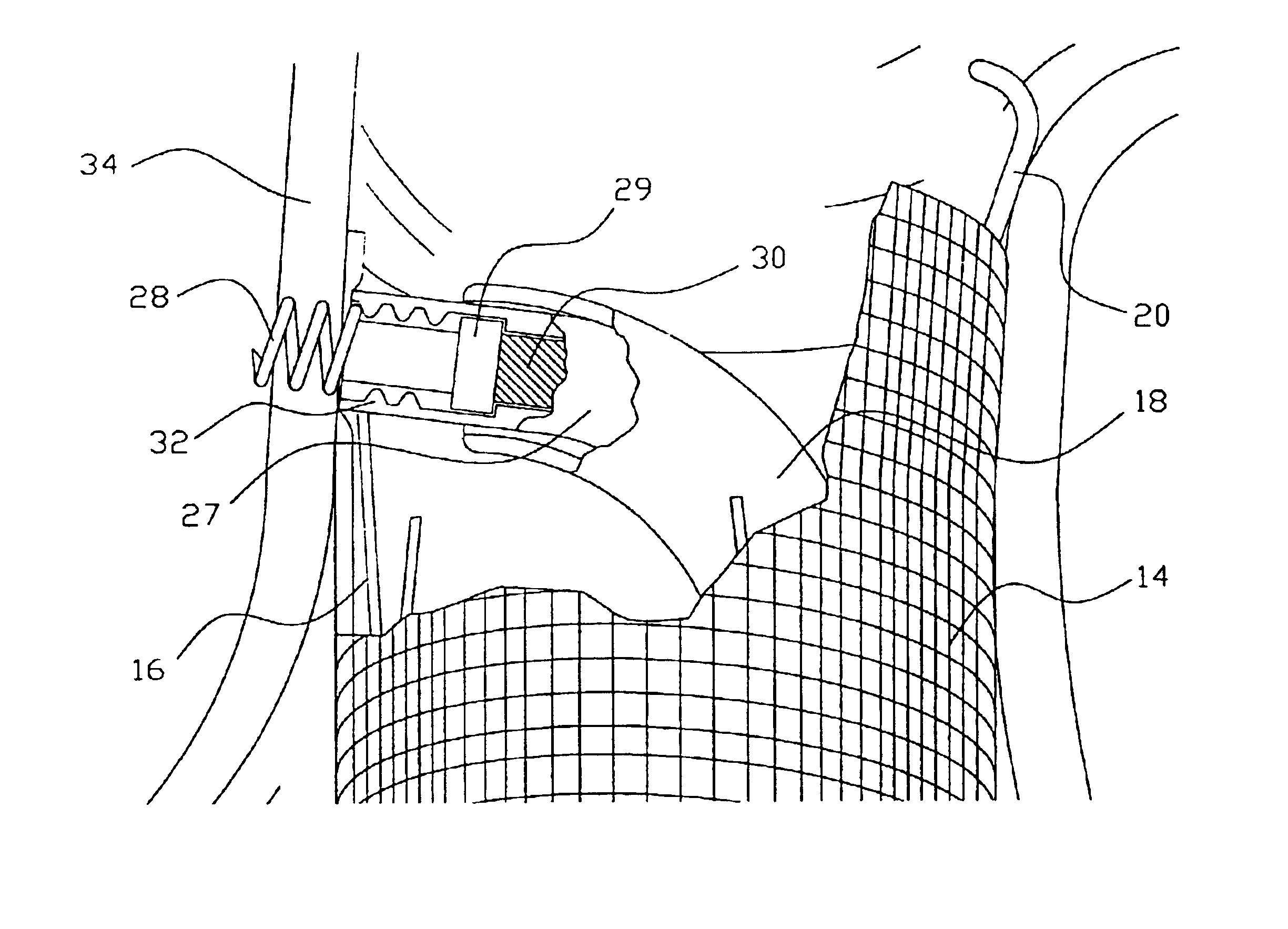
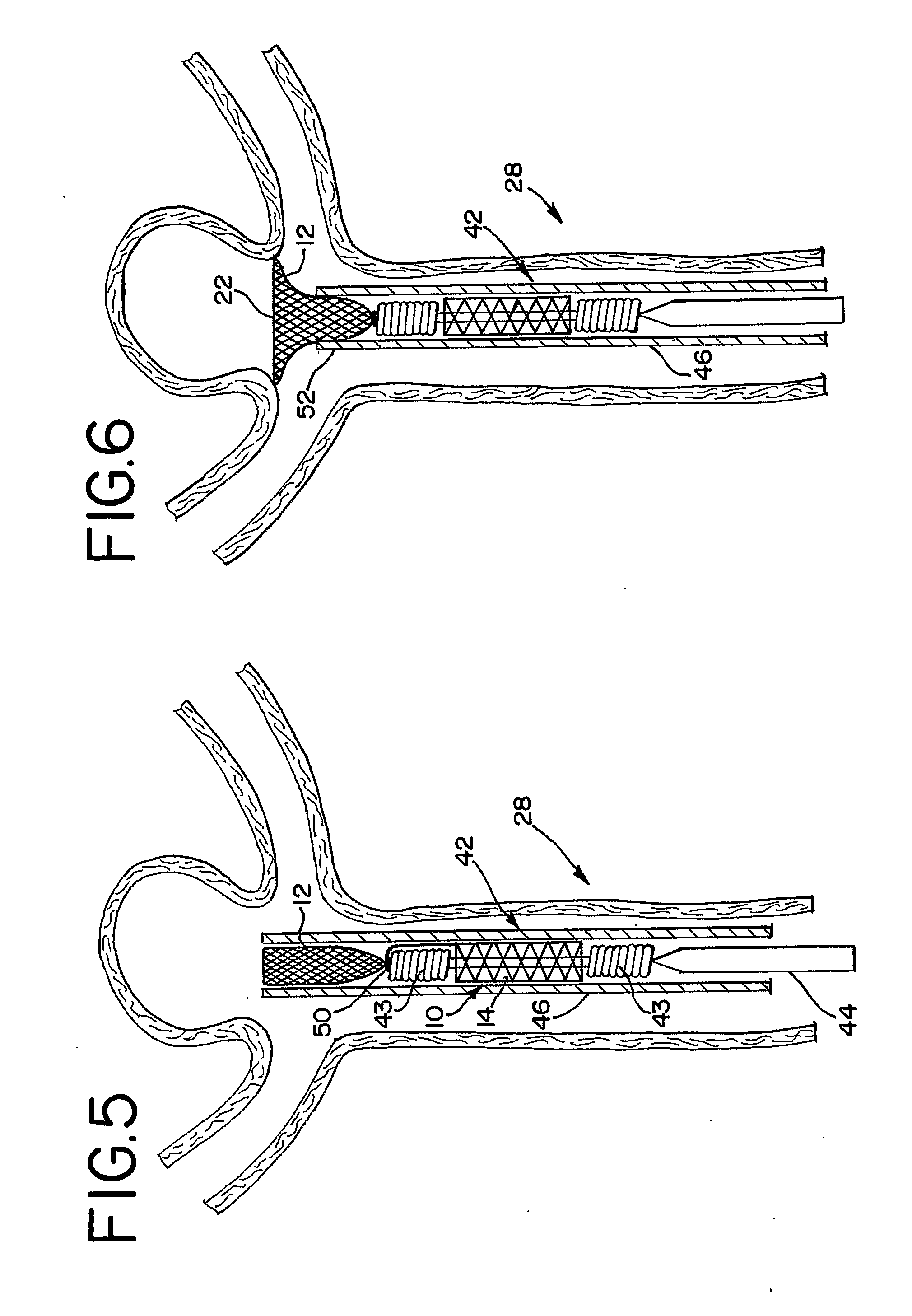
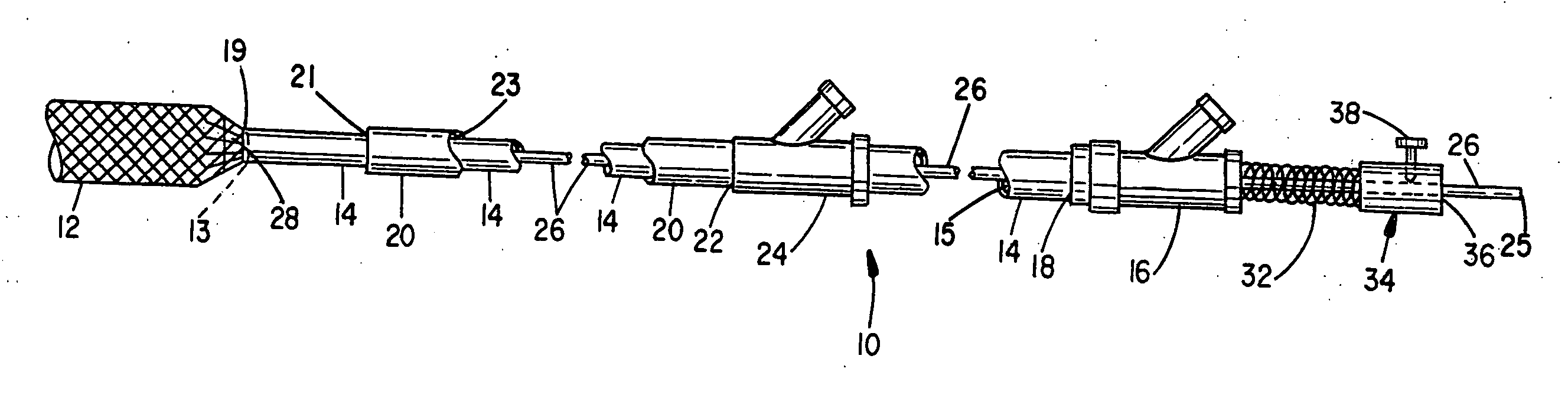
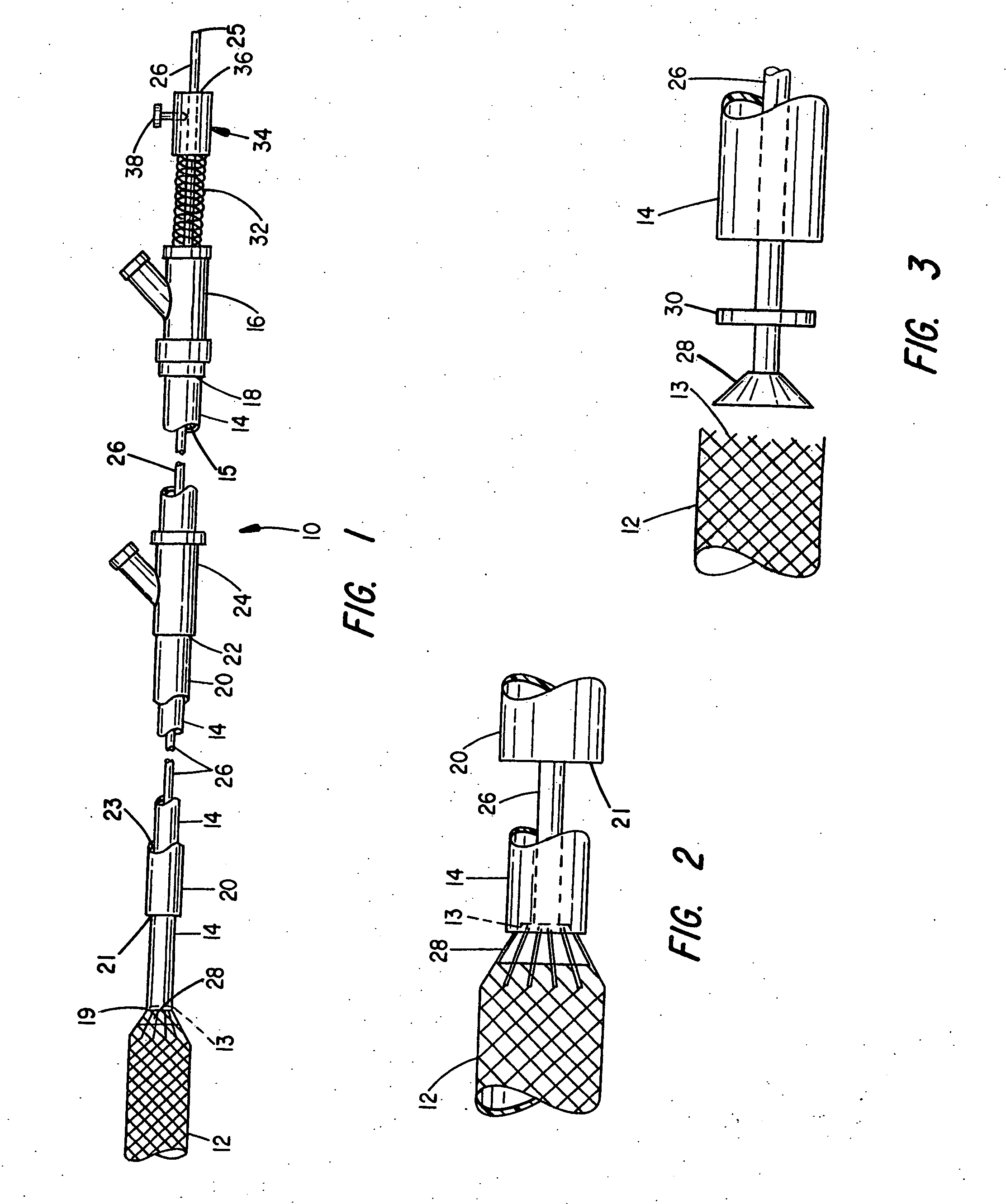
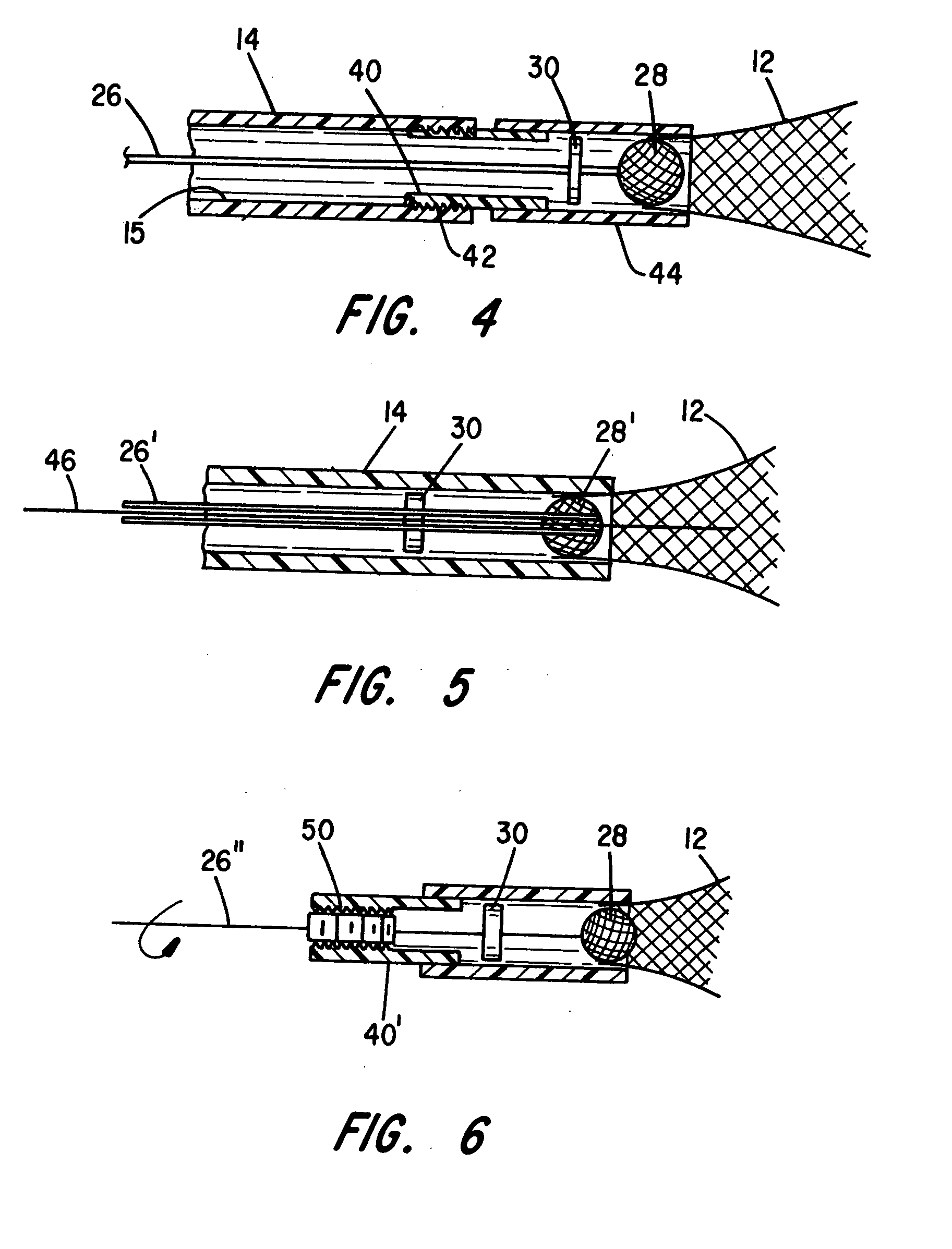
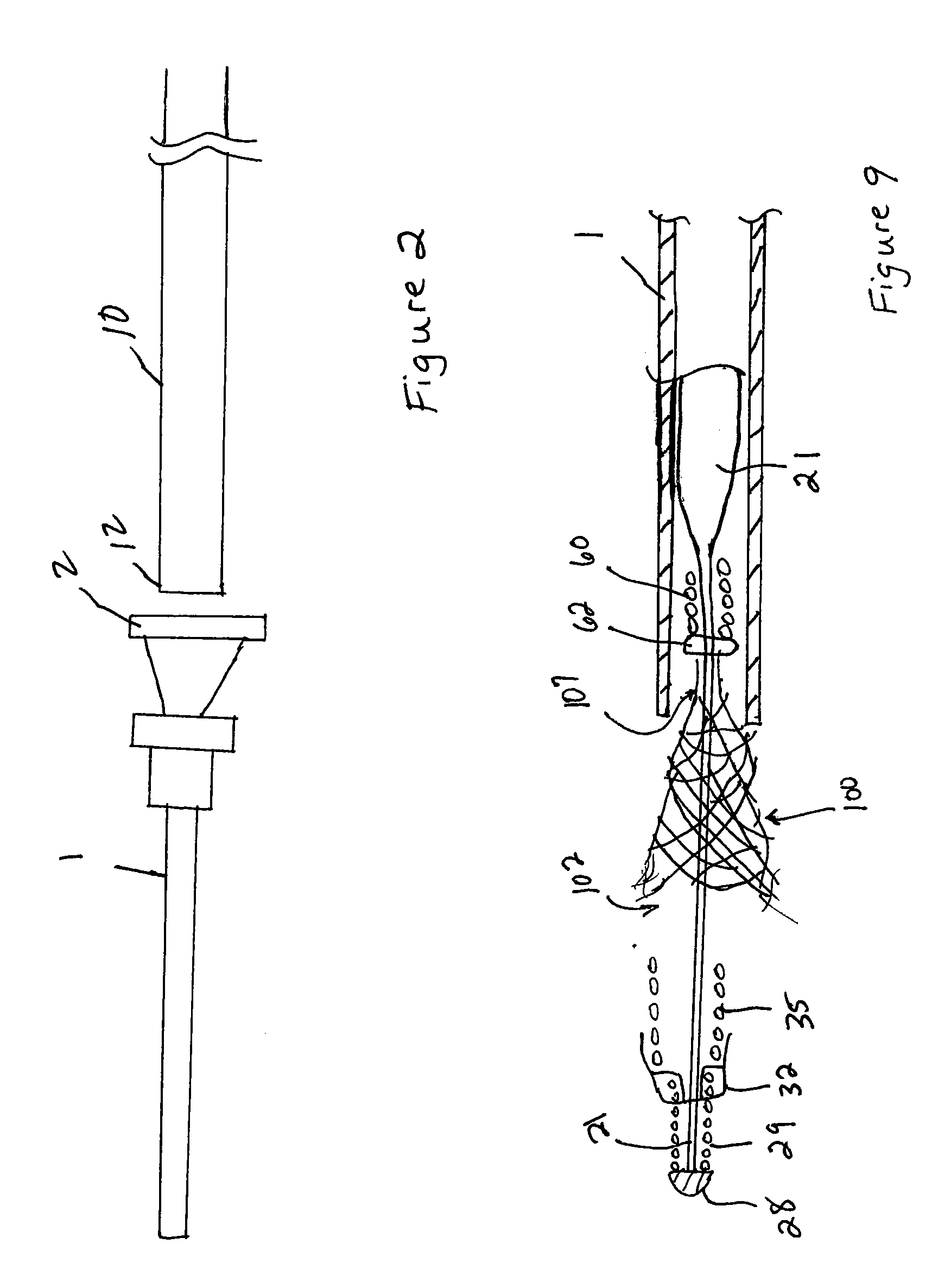
System for controlled delivery of stents and grafts
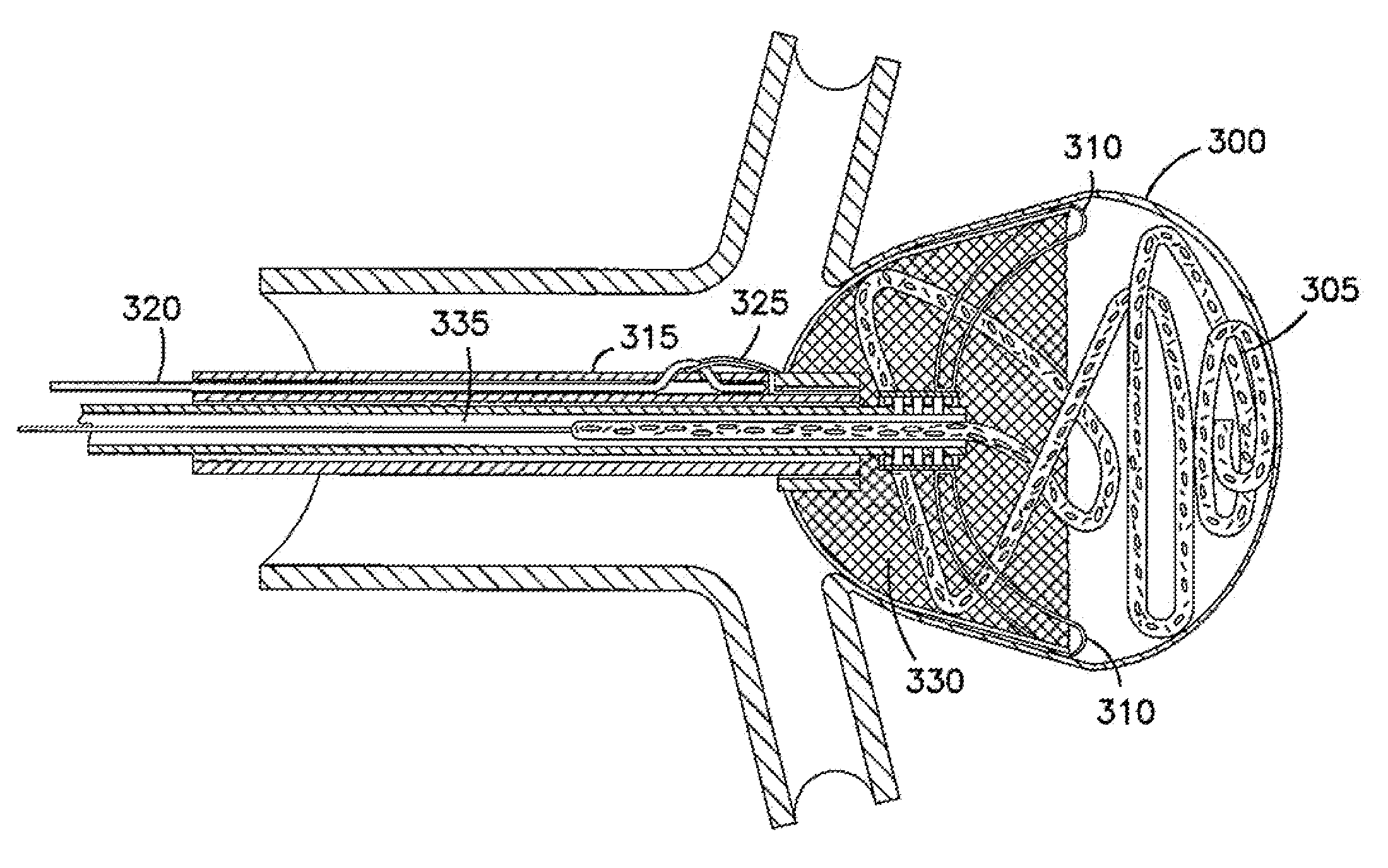
The present invention provides a delivery mechanism for percutaneously routing a stent or graft through the vascular system and procedures for addressing an aneurysm or an otherwise damaged vessel. The delivery system includes an outer tubular guide catheter 20, an inner tubular delivery (pusher) catheter 14 coaxially disposed and slidable relative to the outer guide catheter and an elongated flexible wire or cable 26 that is coaxially insertable through the lumen of the inner tubular catheter and that has a frusto-conical bead affixed at the distal end thereof which is sized to at least partially fit within the lumen of the inner pusher catheter when a proximally directed tension force is applied between the elongated flexible wire or cable 26 with respect to the pusher catheter 14. By inserting a compressed coil spring between a proximal end portion of the pusher catheter 14 and the proximal end portion of the cable 26, the requisite clamping force is maintained to secure the stent or graft to the distal end of the pusher catheter until the compression spring force is removed. With the stent or graft clamped to the distal end of the inner pusher catheter, it can be drawn within the lumen of the outer guide catheter for delivery therewith to the target site.
Owner:AGA MEDICAL CORP MS US
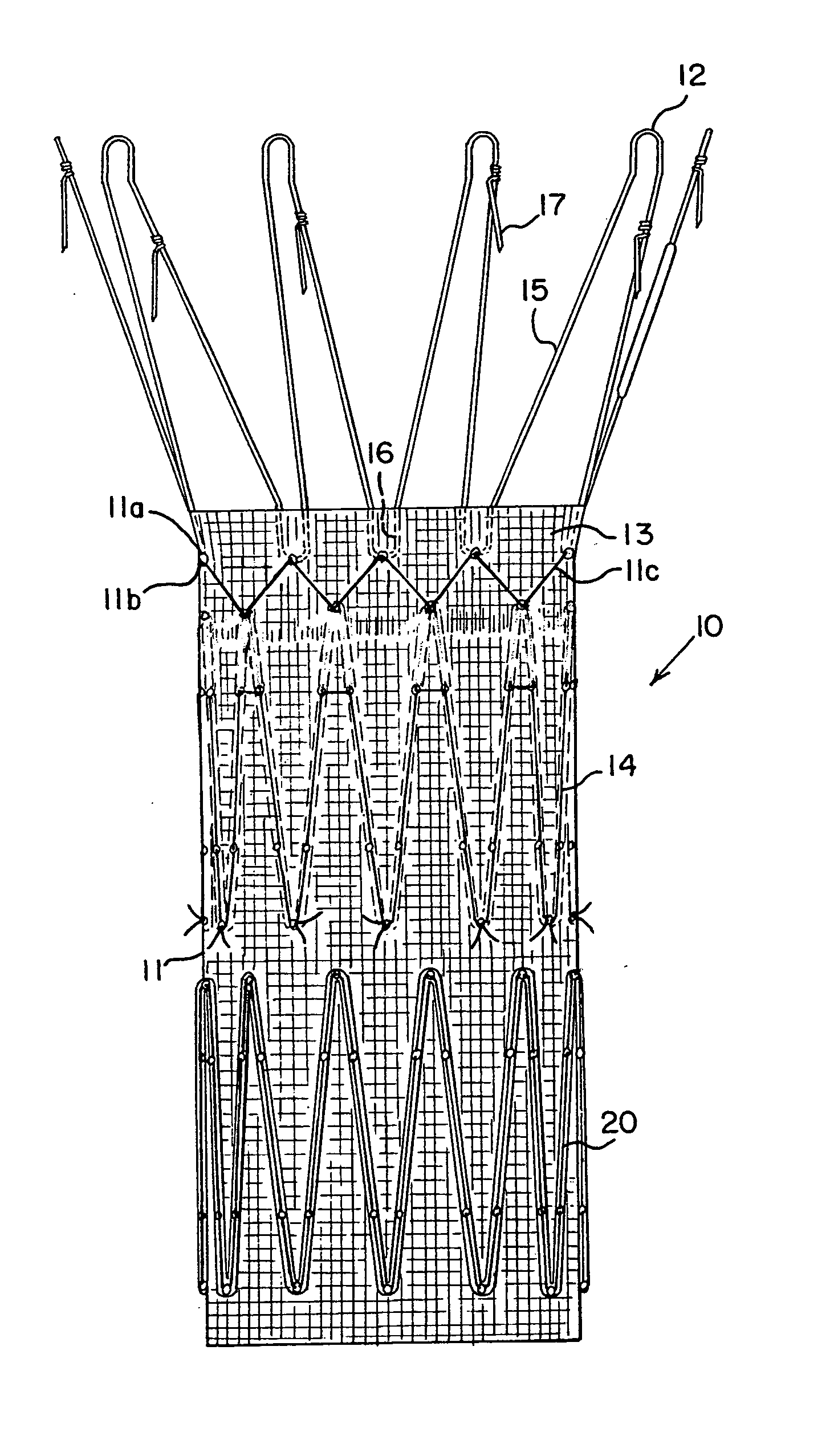
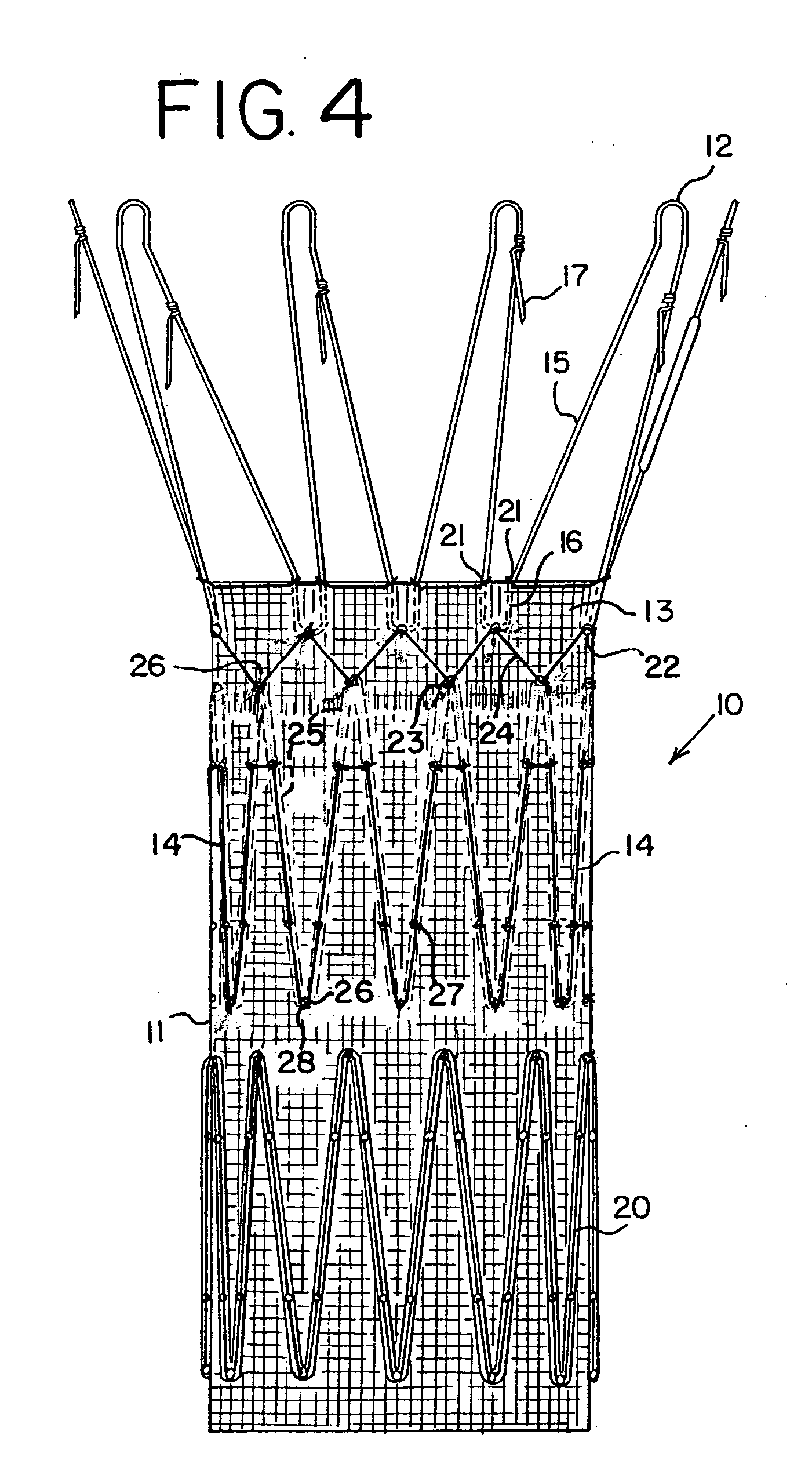
Endoluminal prosthetic device
An endoluminal prosthetic device for placement in a body lumen is formed by stitching stents to a graft. A first or anchoring stent is used for securing a graft made of biocompatible material that forms at least one lumen. There is also a second stent. The first and second stents each include a plurality of struts and apices between the struts. At least two of the apices in each of the first and second stents are secured to the graft by stitches. A running suture links at least one stitch of the first stent and one stitch of the second stent. The running suture linking the first stent and the second stent adds strength to the stitches and better secures the first stent to the device. The endoluminal prosthetic device may be used in an aortic vessel to treat stenoses or aneurysms.
Owner:COOK MEDICAL TECH LLC
Electrically actuated medical devices
The present invention relates to medical devices for implantation or insertion into body lumens, for example, catheters, guidewires, stents and aneurysm coils. The devices of the present invention comprise electrically actuated materials, such as electroactive polymers and piezoelectric and electrostrictive materials, which enhance or expand their functionality.
Owner:BOSTON SCI SCIMED INC
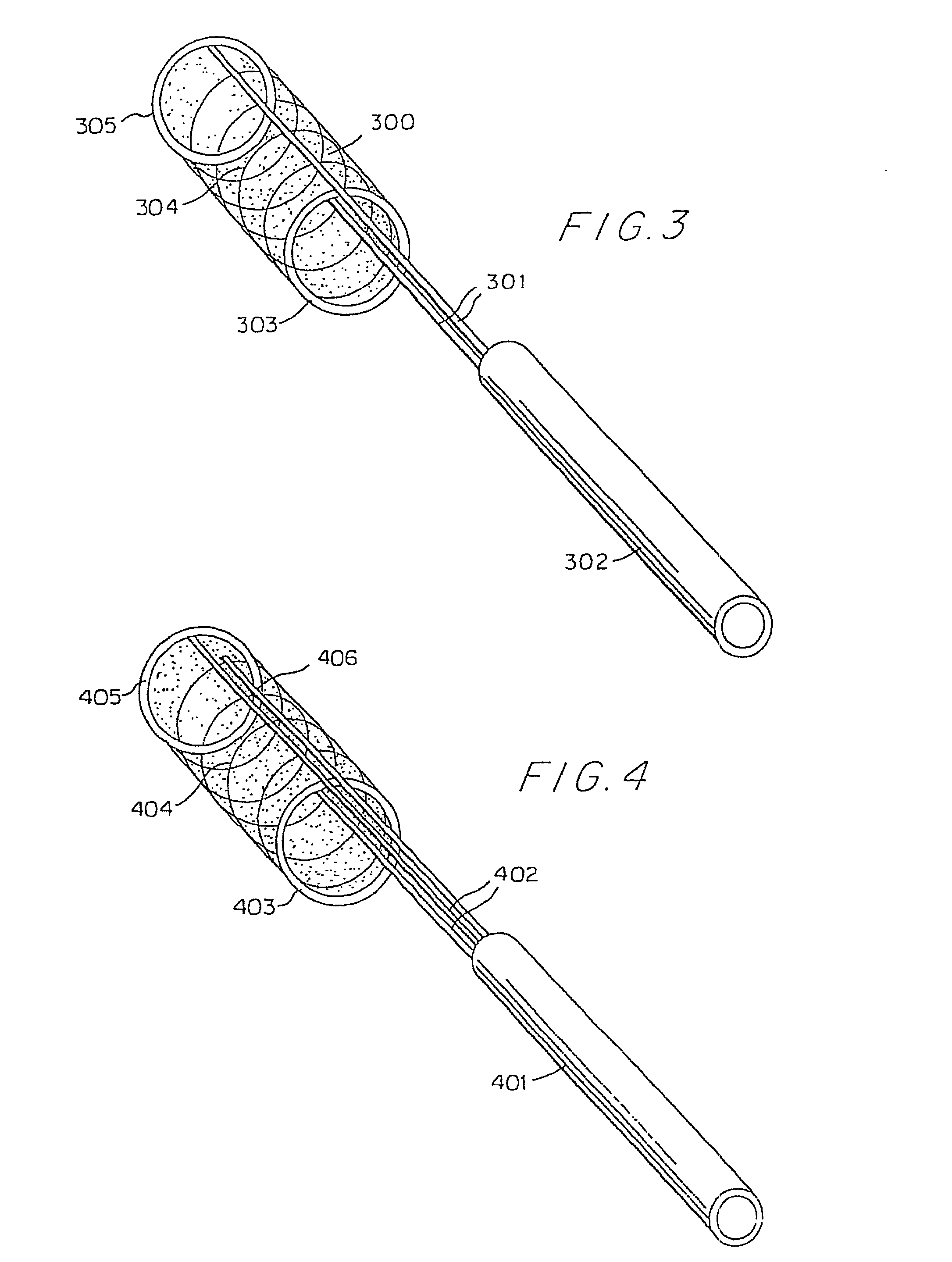
Implantable intraluminal device and method of using same in treating aneurysms
An intraluminal device implantable in a blood vessel having an aneurysm therein in the vicinity of a perforating vessel and / or of a bifurcation leading to a branch vessel. The intraluminal device includes a mesh-like tube of bio-compatible material having an expanded condition in which the tube diameter is slightly larger than the diameter of the blood vessel in which it is to be implanted, and the tube length is sufficient to straddle the aneurysm and to be anchored to the blood vessel on the opposite sides of the aneurysm. The mesh-like tube also has a contracted condition wherein it is sufficiently flexible so as to be easily manipulatable through the blood vessel to straddle the aneurysm. In its expanded condition, the mesh-like tube has a porosity index of 55%-80% such as to reduce the flow of blood through its wall to the aneurysm sufficiently to decrease the possibility of rupture of the aneurysm but not to unduly reduce the blood flow to a perforating or branch vessel to the degree likely to cause significant damage to tissues supplied with blood by such perforating or branch vessel.
Owner:STRYKER CORP
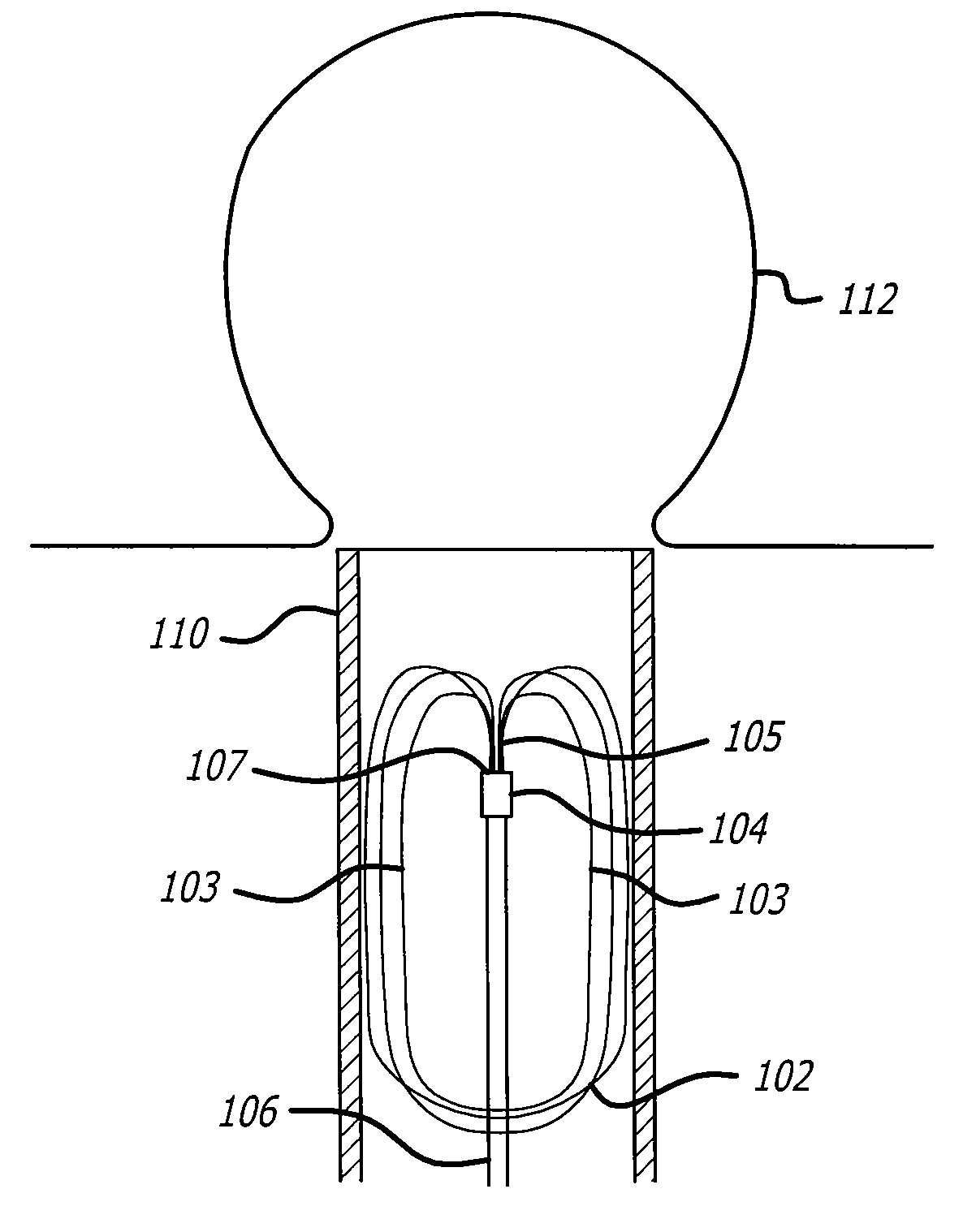
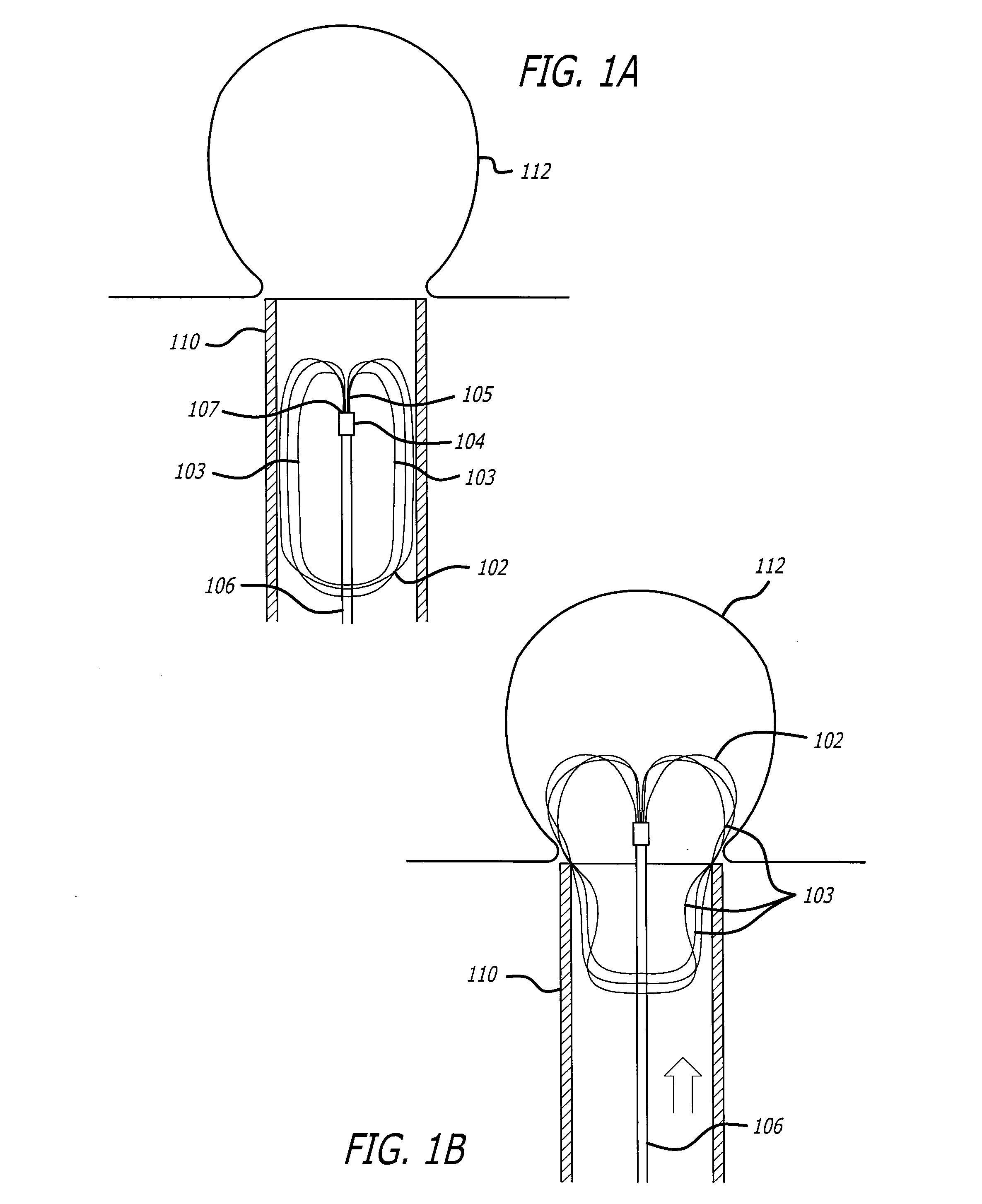
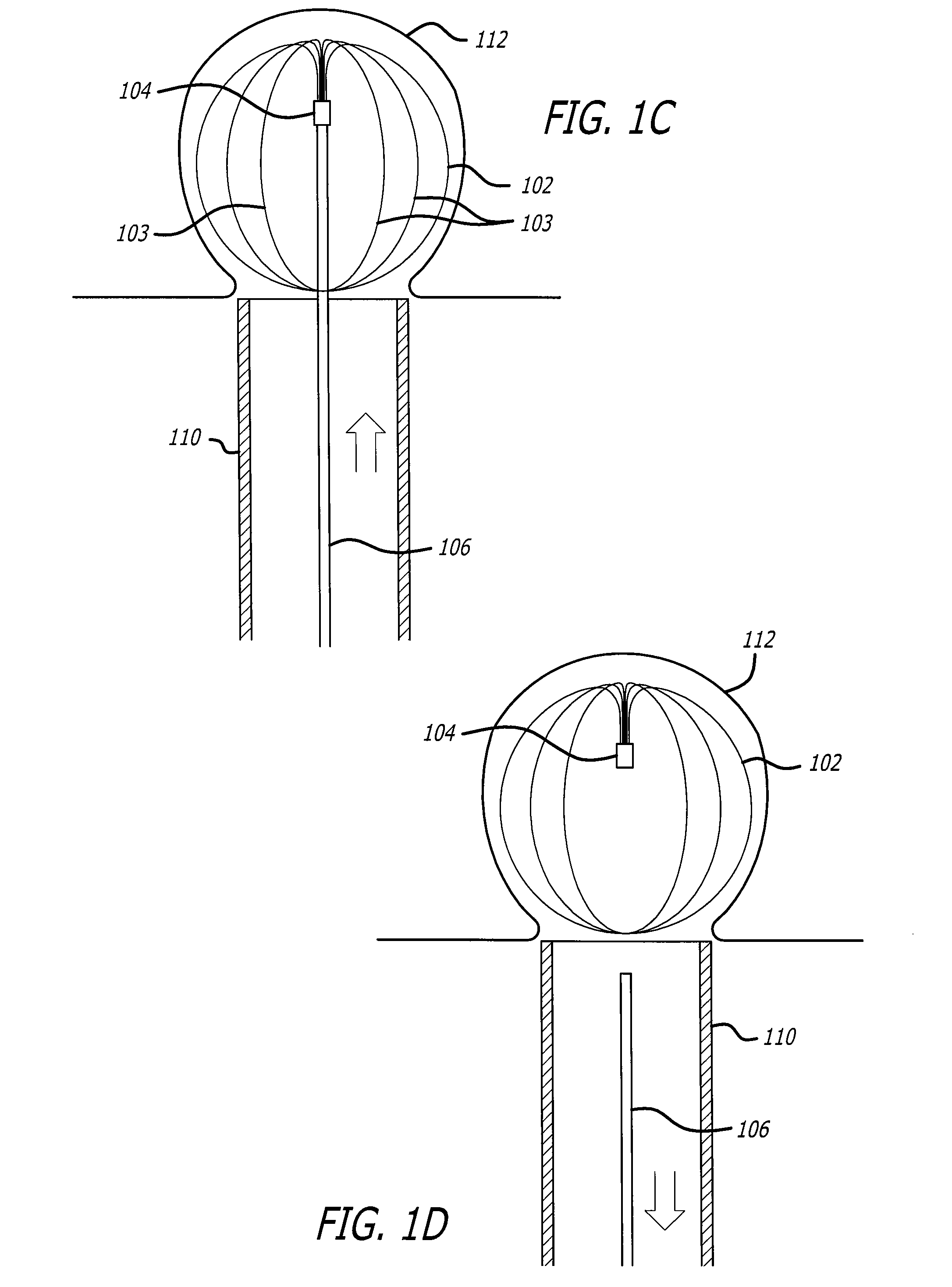
Implantable wireless sensor
InactiveUS20050187482A1Improve stabilityAvoid stickingEndoradiosondesCatheterLine sensorEndovascular aneurysm repair
The progress of a endovascular aneurysm repair can be monitored by inserting a pressure transducer sensor using a catheter into the sac during endovascular aneurysm repair and then using a small, hand-held read out device to measure pressure easily, safely, inexpensively and accurately. In one aspect a sensor is introduced into the body by the steps of loading the sensor into a catheter, and deploying into the aneurysm sac. This invention also has other applications for measuring physical properties in patients or in other sites.
Owner:CARDIOMEMS
Methods for embolizing blood vessels
InactiveUS6335384B1Reduce molecular weightEasy to adjustHeavy metal active ingredientsOrganic active ingredientsParticulatesMedicine
Disclosed are methods useful for treating vascular lesions wherein a non-particulate agent such as a metal coil is introduced into a vascular site (e.g., an aneurysm cavity) in conjunction with an embolizing composition comprising a biocompatible polymer and a biocompatible solvent.The biocompatible solvent is miscible or soluble in blood and also solubilizes the polymer during delivery. The biocompatible polymer is selected to be soluble in the biocompatible solvent but insoluble in blood. Upon contact with the blood, the biocompatible solvent dissipates from the embolic composition whereupon the biocompatible polymer precipitates. Precipitation of the polymer in the presence of the non-particular agent permits the agent to act as a structural lattice for the growing polymer precipitate.In another embodiment, the biocompatible polymer composition can be replaced with a biocompatible prepolymer composition containing a biocompatible prepolymer.
Owner:MICRO THEREPEUTICS INC
System and method for delivering and deploying an occluding device within a vessel
A system and method for deploying an occluding device that can be used to remodel an aneurysm within the vessel by, for example, neck reconstruction or balloon remodeling. The system comprises an introducer sheath and an assembly for carrying the occluding device. The assembly includes an elongated flexible member having an occluding device retaining member for receiving a first end of the occluding device, a proximally positioned retaining member for engaging a second end of the occluding device and a support surrounding a portion of the elongated flexible member over which the occluding device can be postioned.
Owner:TYCO HEALTHCARE GRP LP
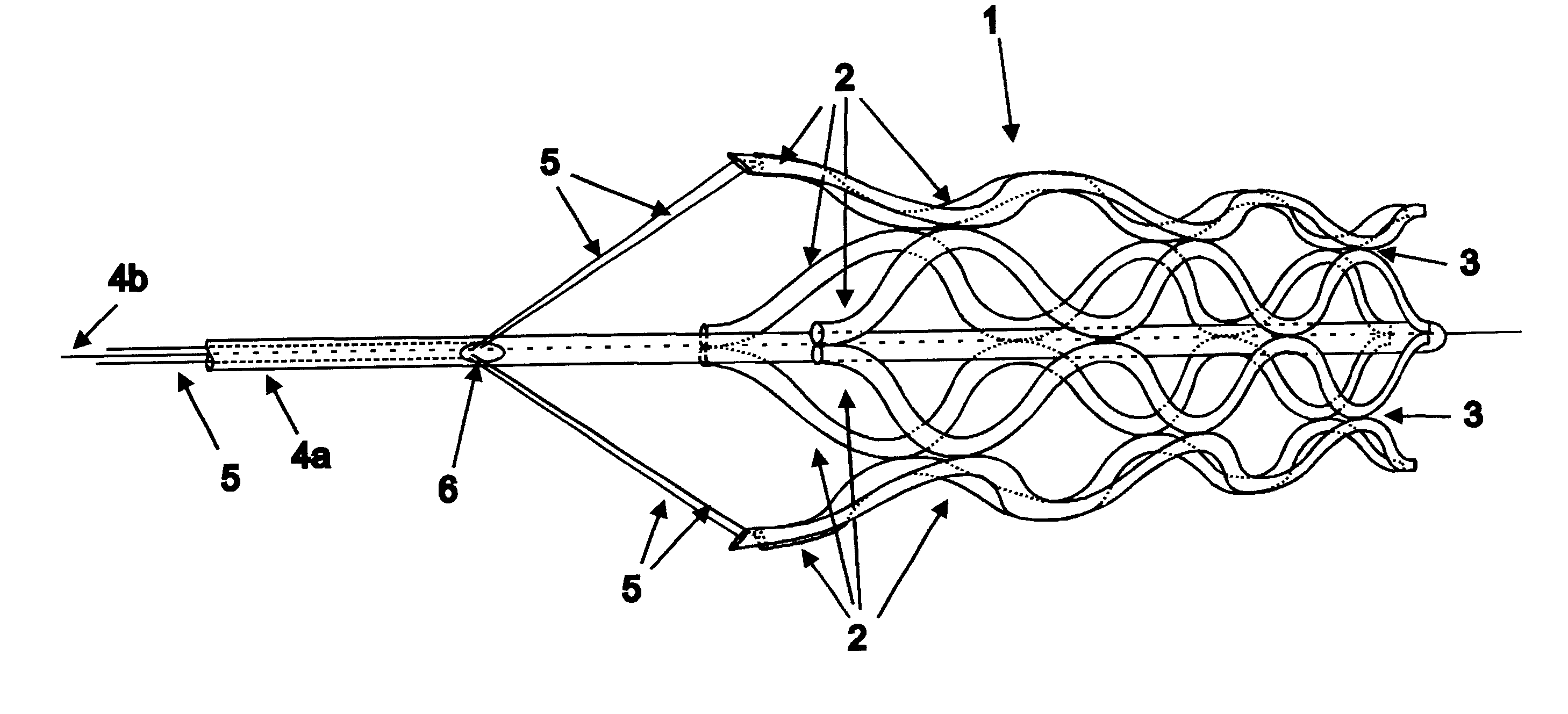
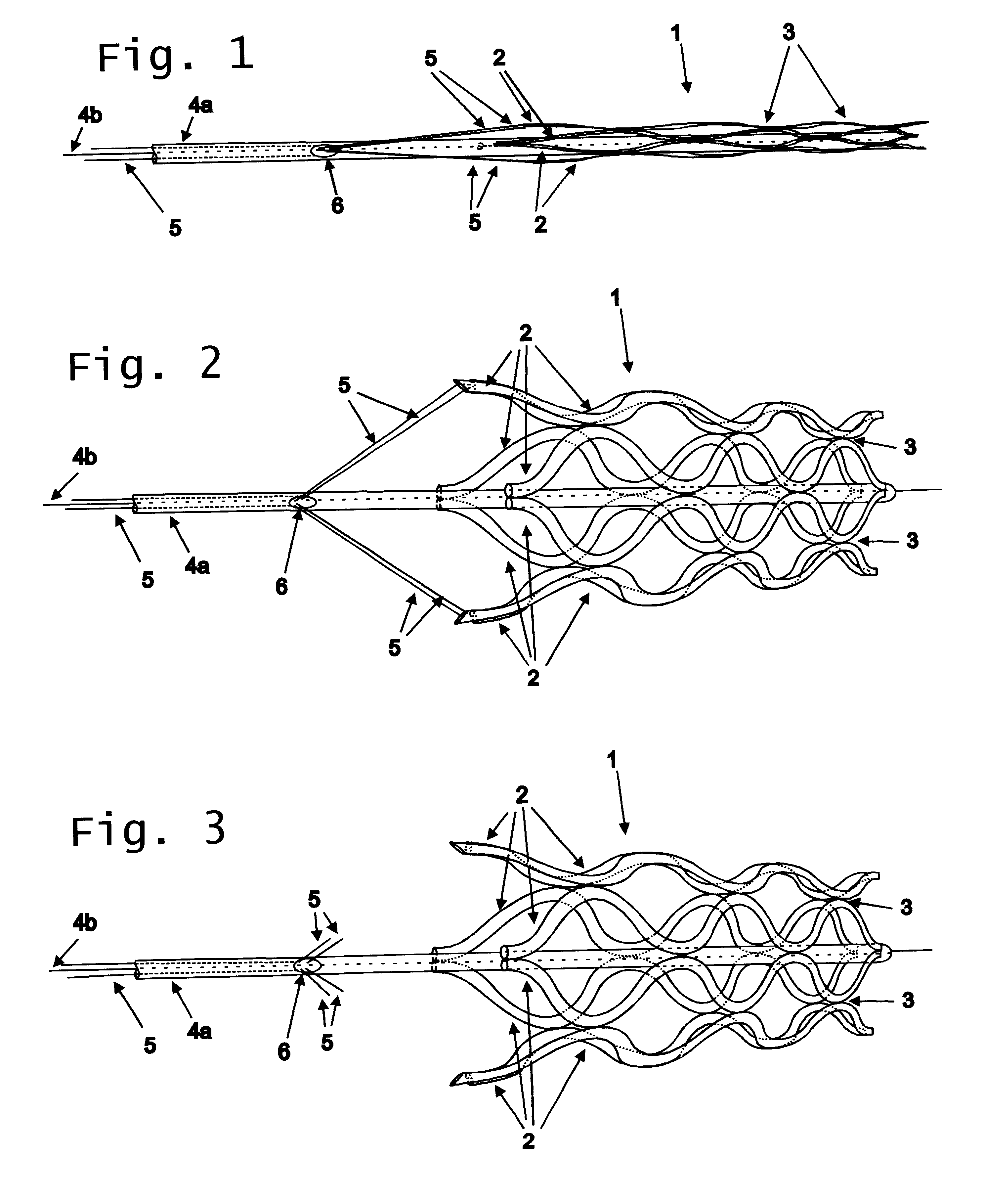
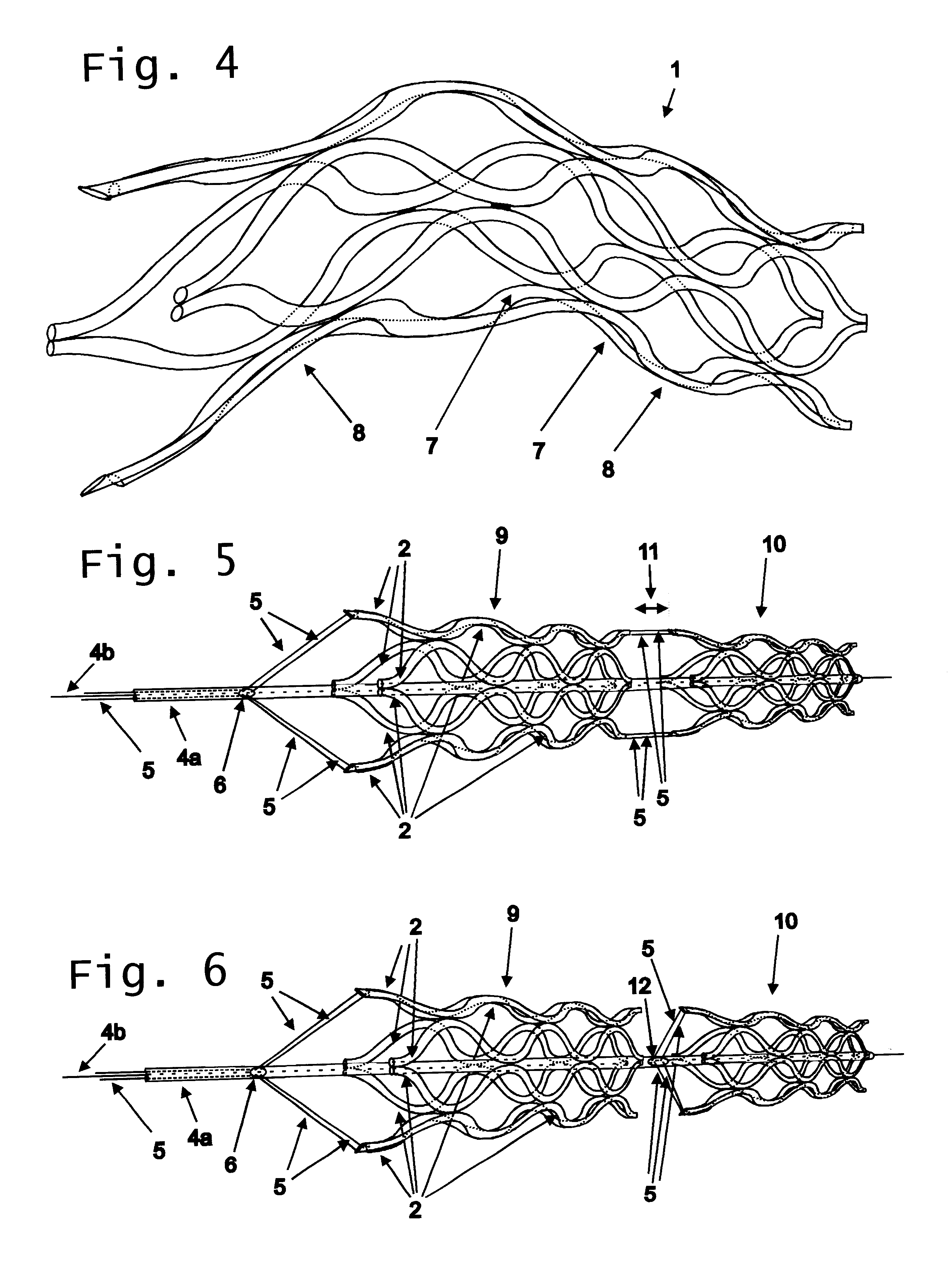
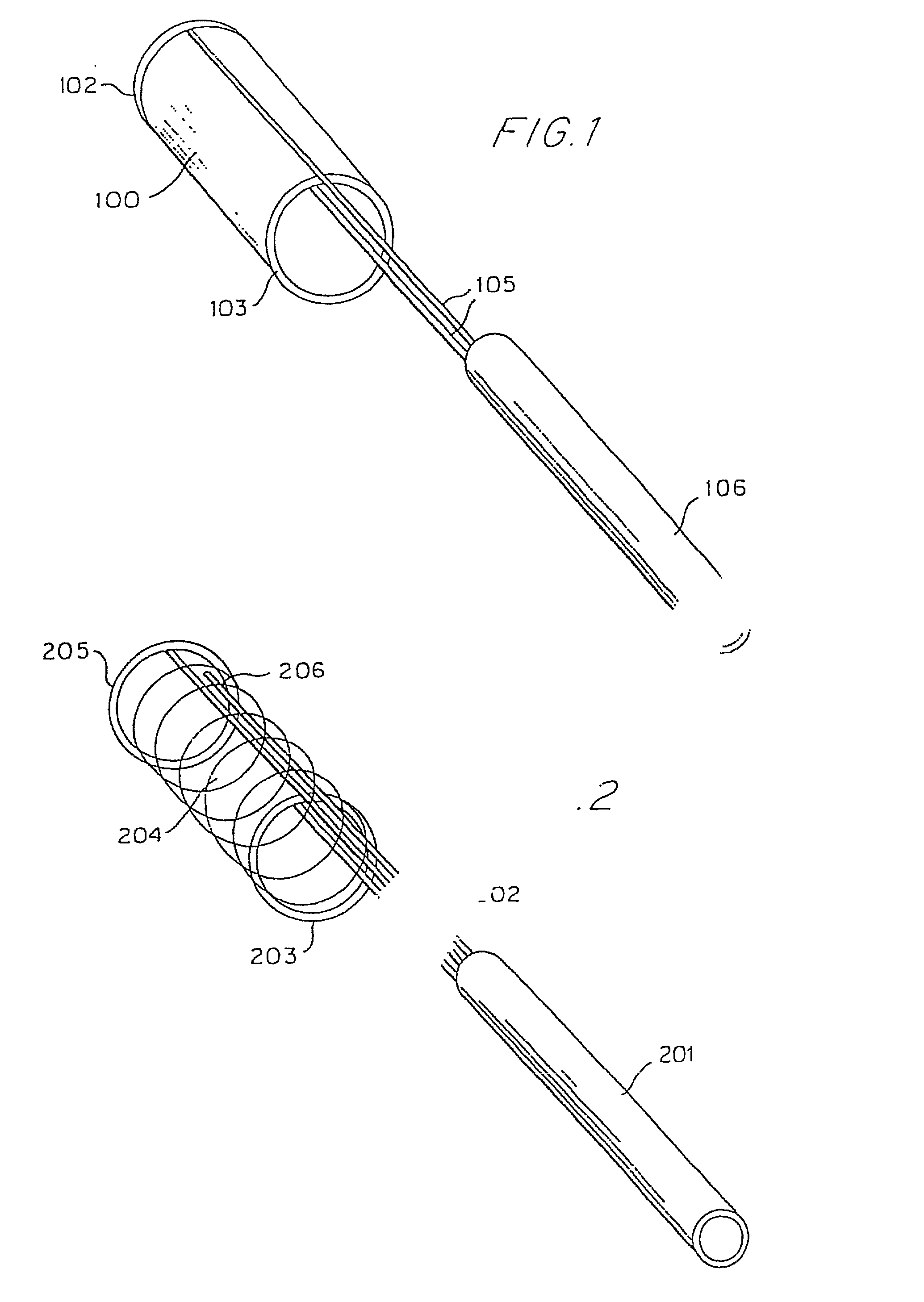
Flexible vascular occluding device
A vascular occluding device for modifying blood flow in a vessel, while maintaining blood flow to the surrounding tissue. The occluding device includes a flexible, easily compressible and bendable occluding device that is particularly suited for treating aneurysms in the brain. The neurovascular occluding device can be deployed using a micro-catheter. The occluding device can be formed by braiding wires in a helical fashion and can have varying lattice densities along the length of the occluding device. The occluding device could also have different lattice densities for surfaces on the same radial plane.
Owner:TYCO HEALTHCARE GRP LP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com