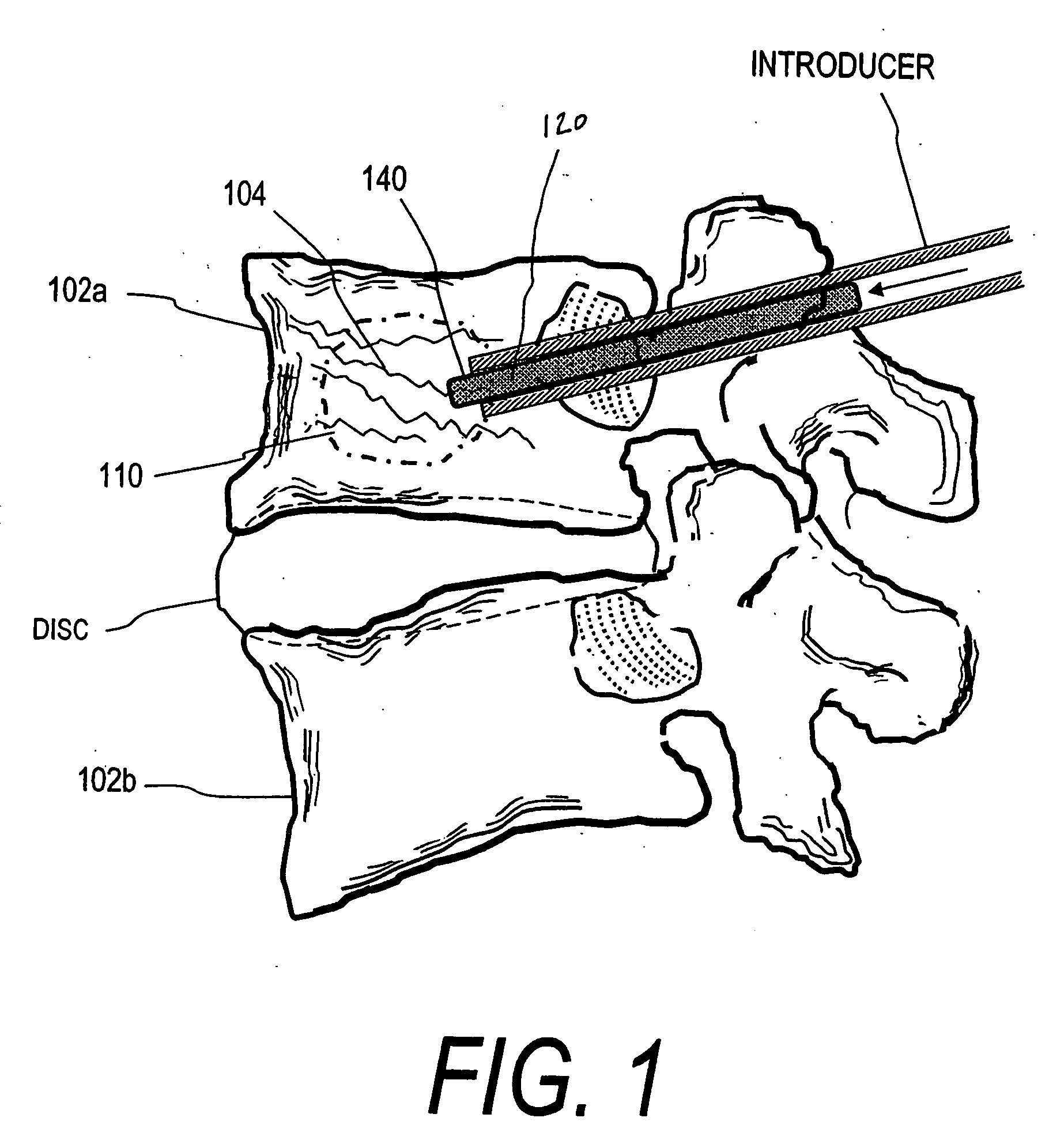
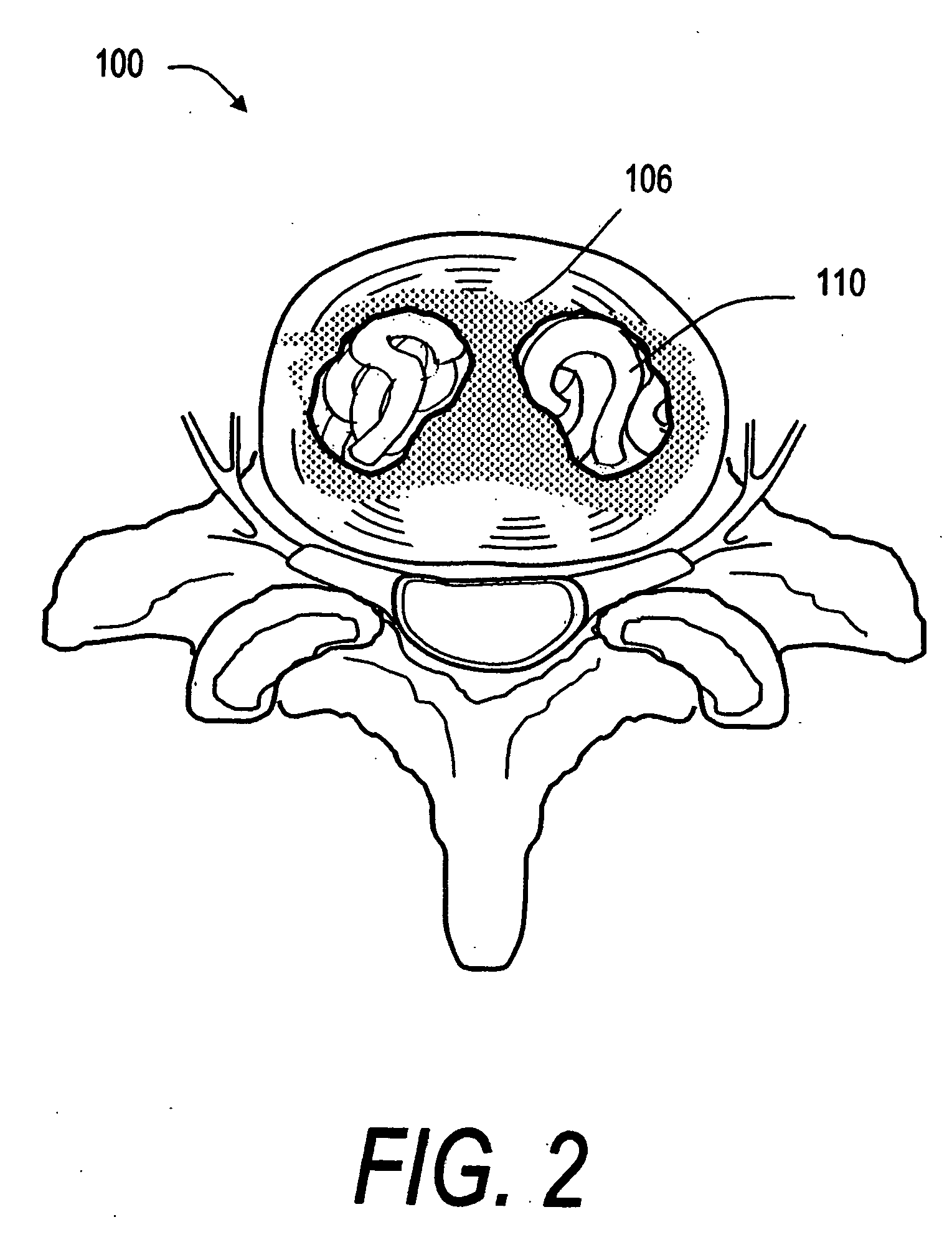
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
17932 results about "Porosity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
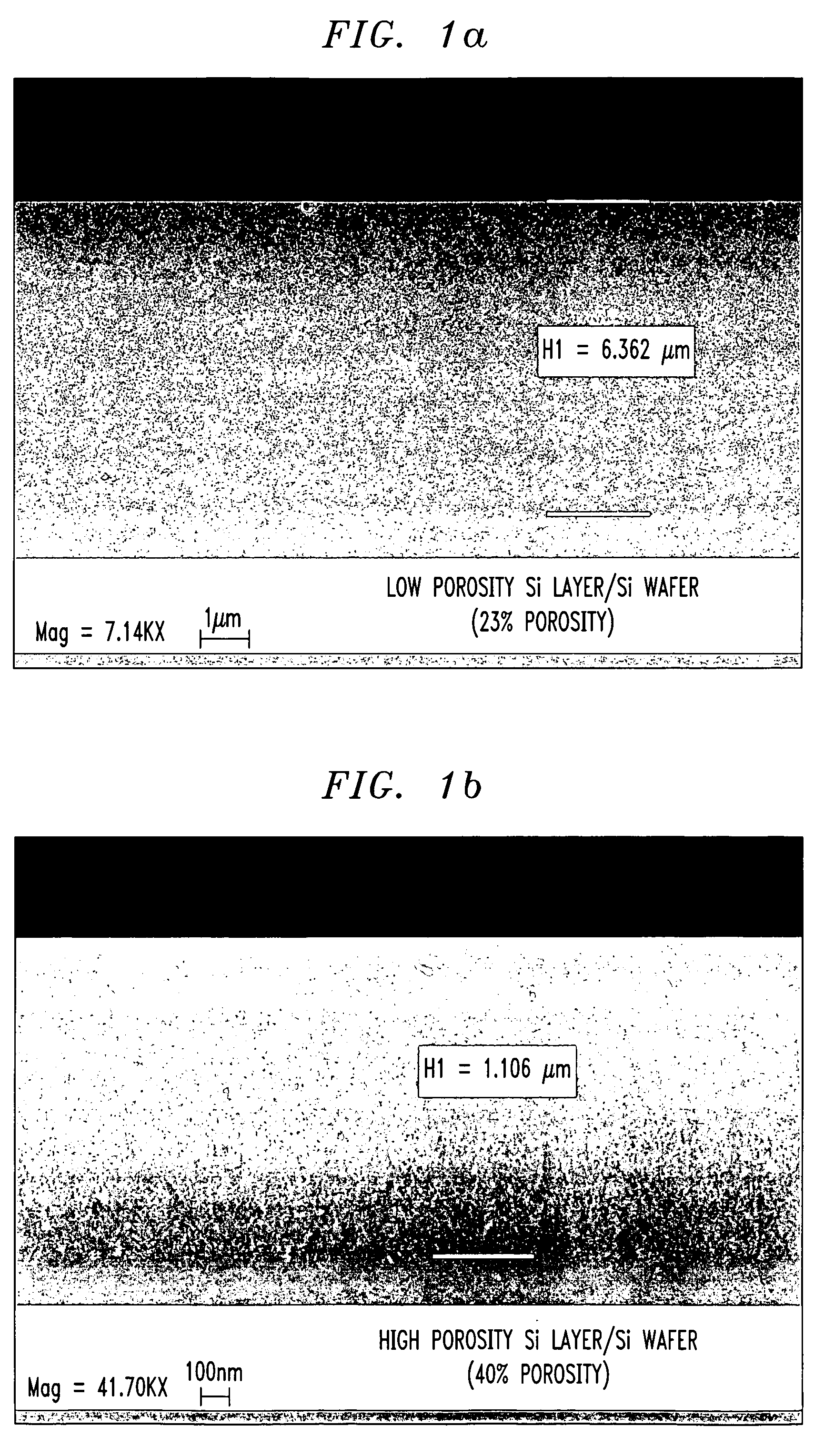
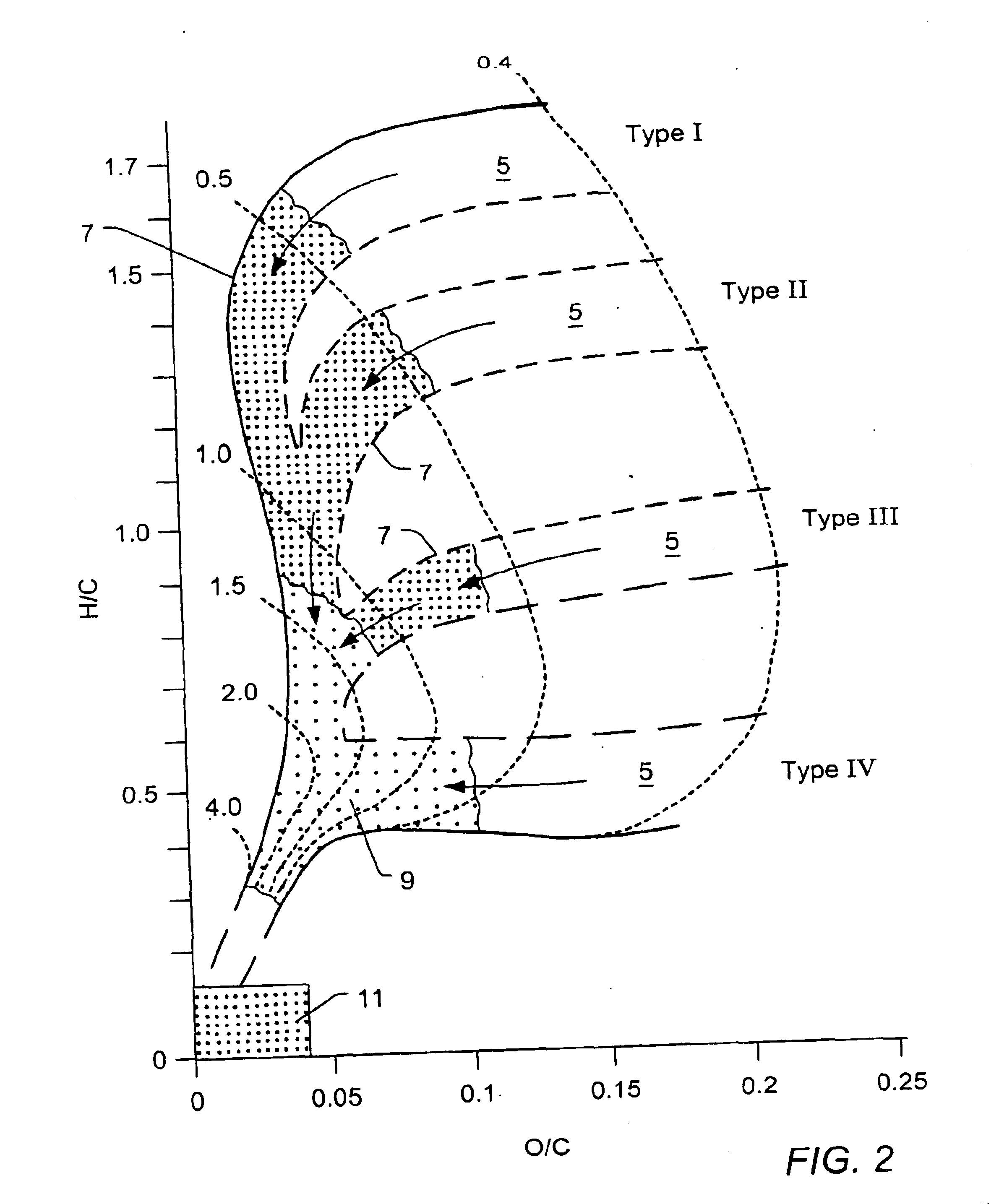
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measure the "accessible void", the total amount of void space accessible from the surface (cf. closed-cell foam). There are many ways to test porosity in a substance or part, such as industrial CT scanning. The term porosity is used in multiple fields including pharmaceutics, ceramics, metallurgy, materials, manufacturing, hydrology, earth sciences, soil mechanics and engineering.
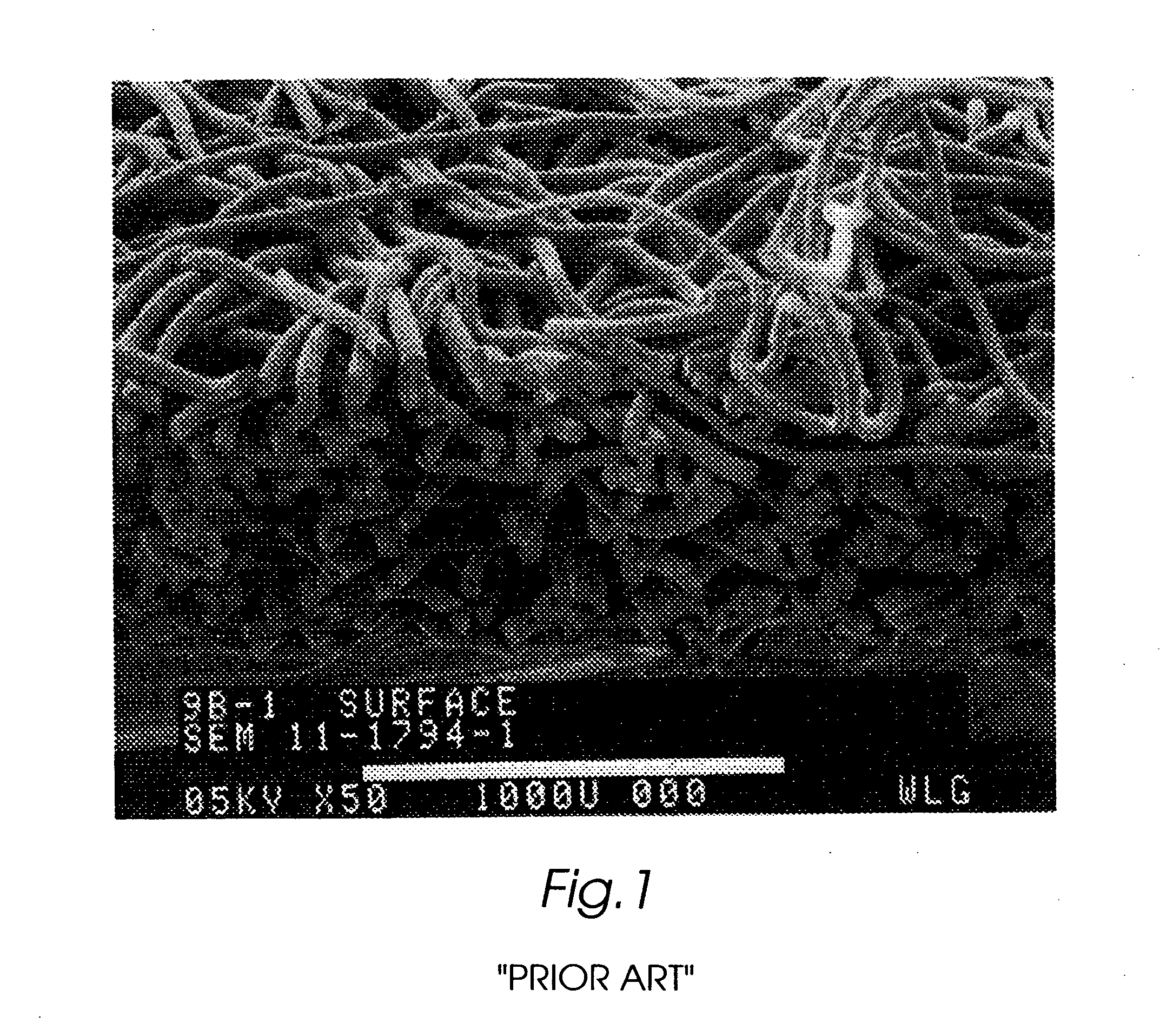
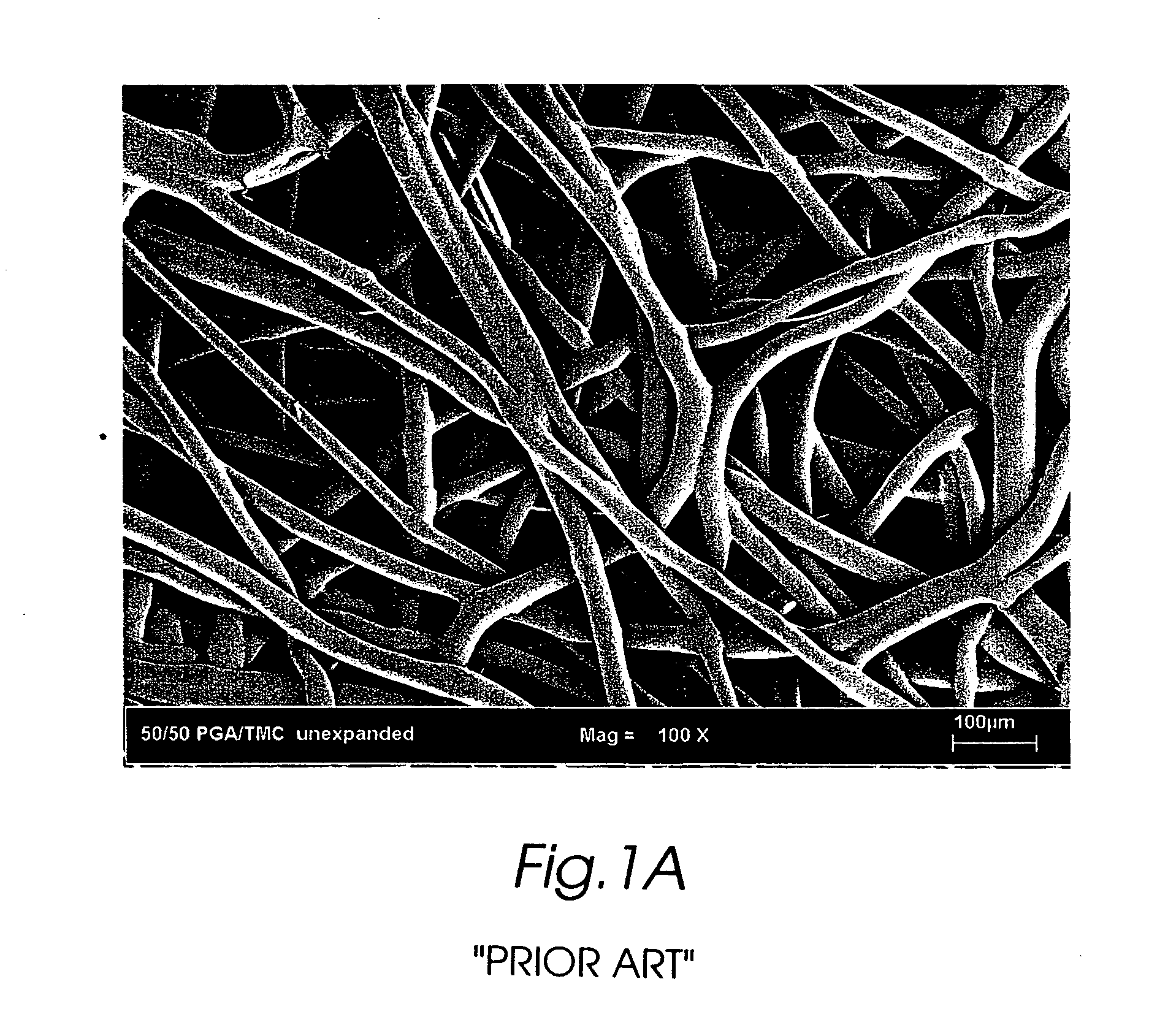
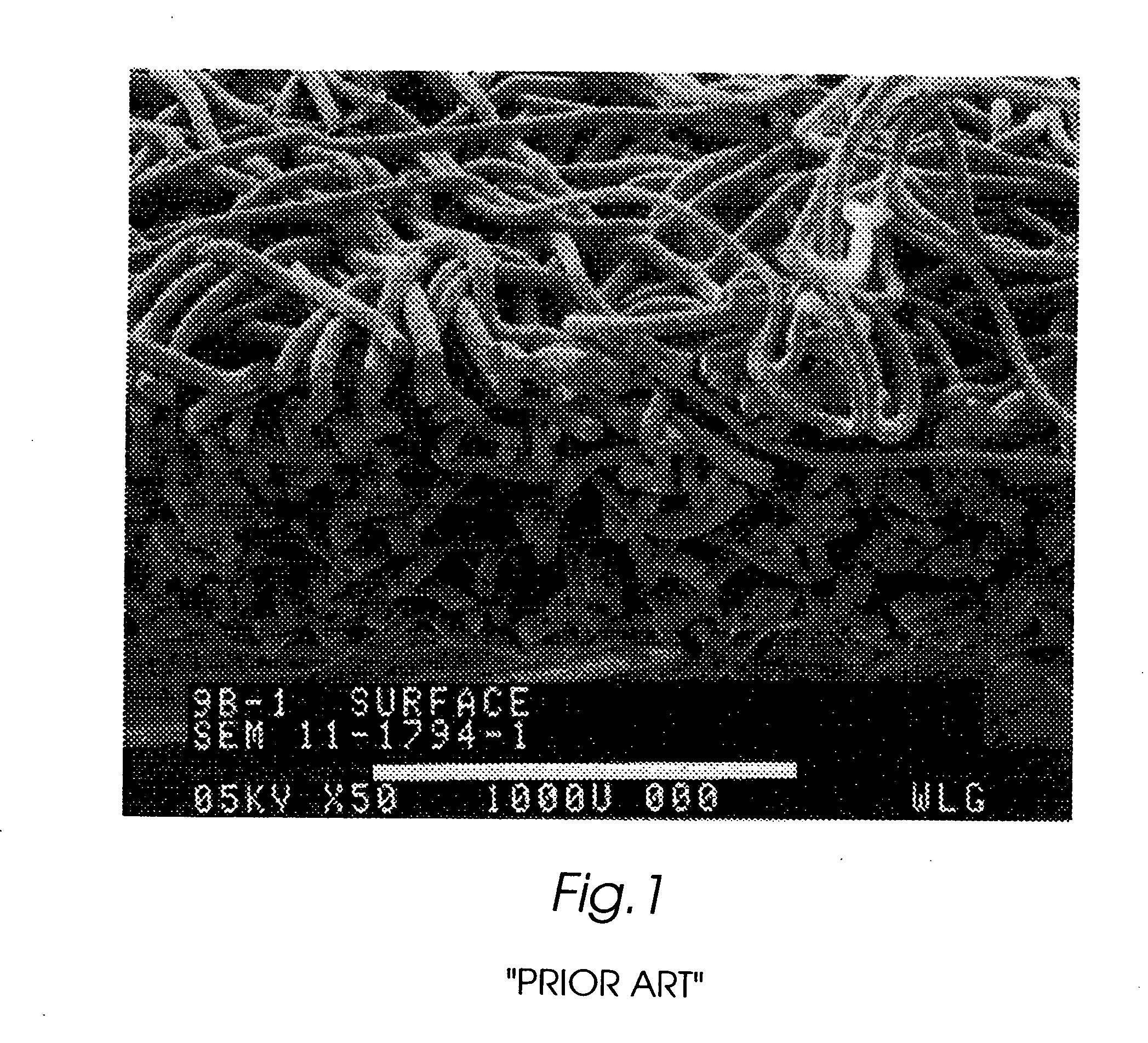
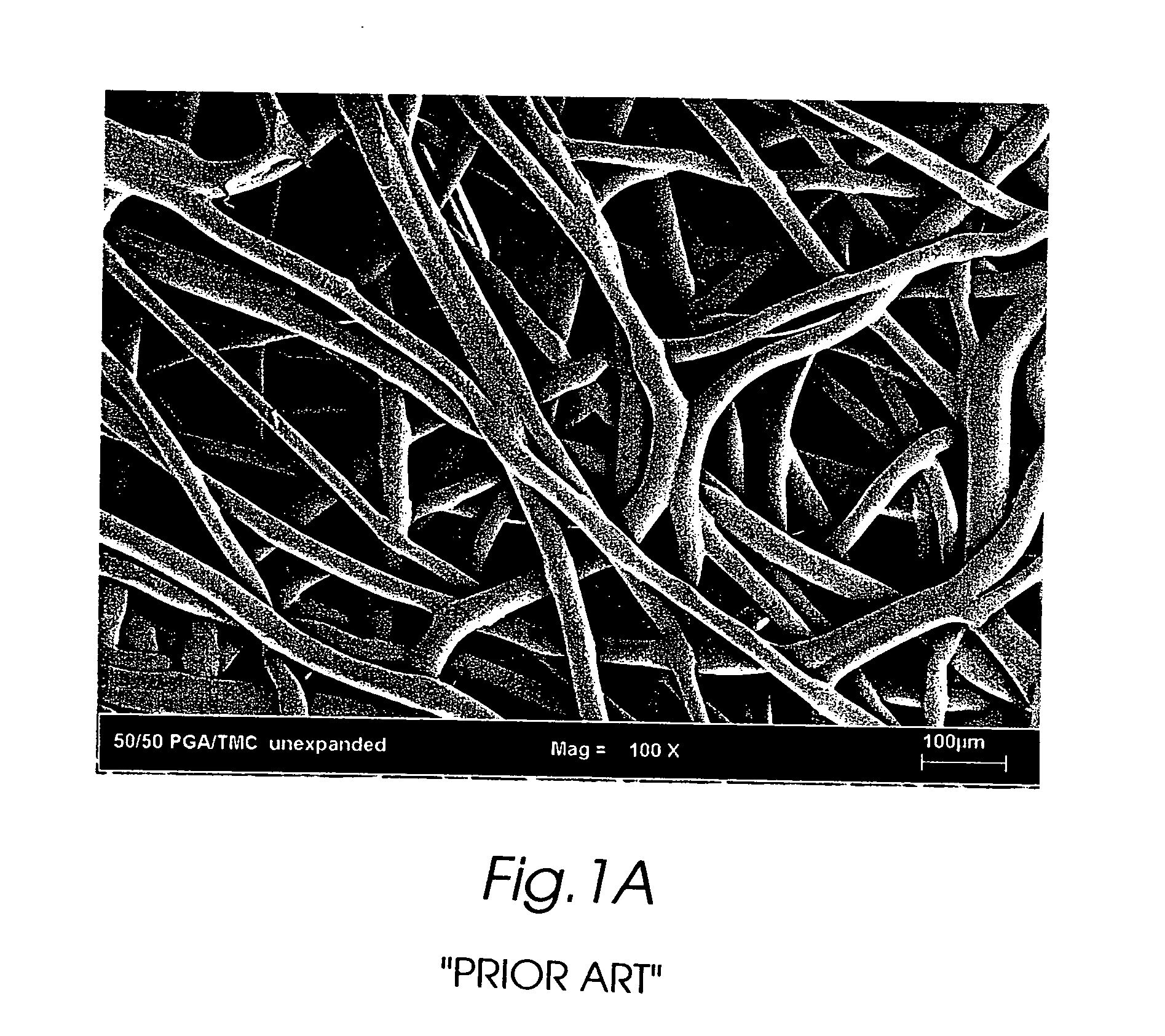
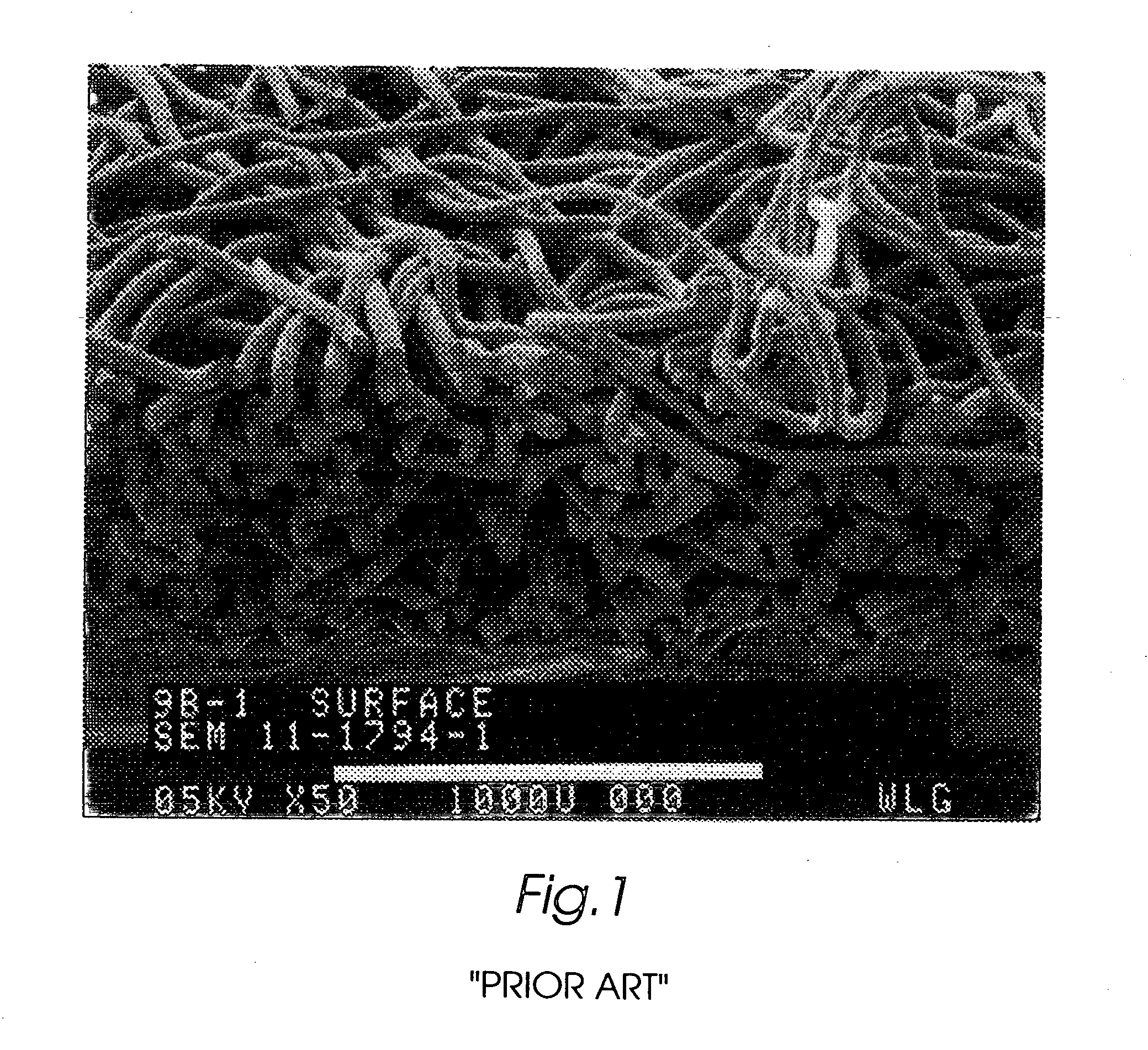
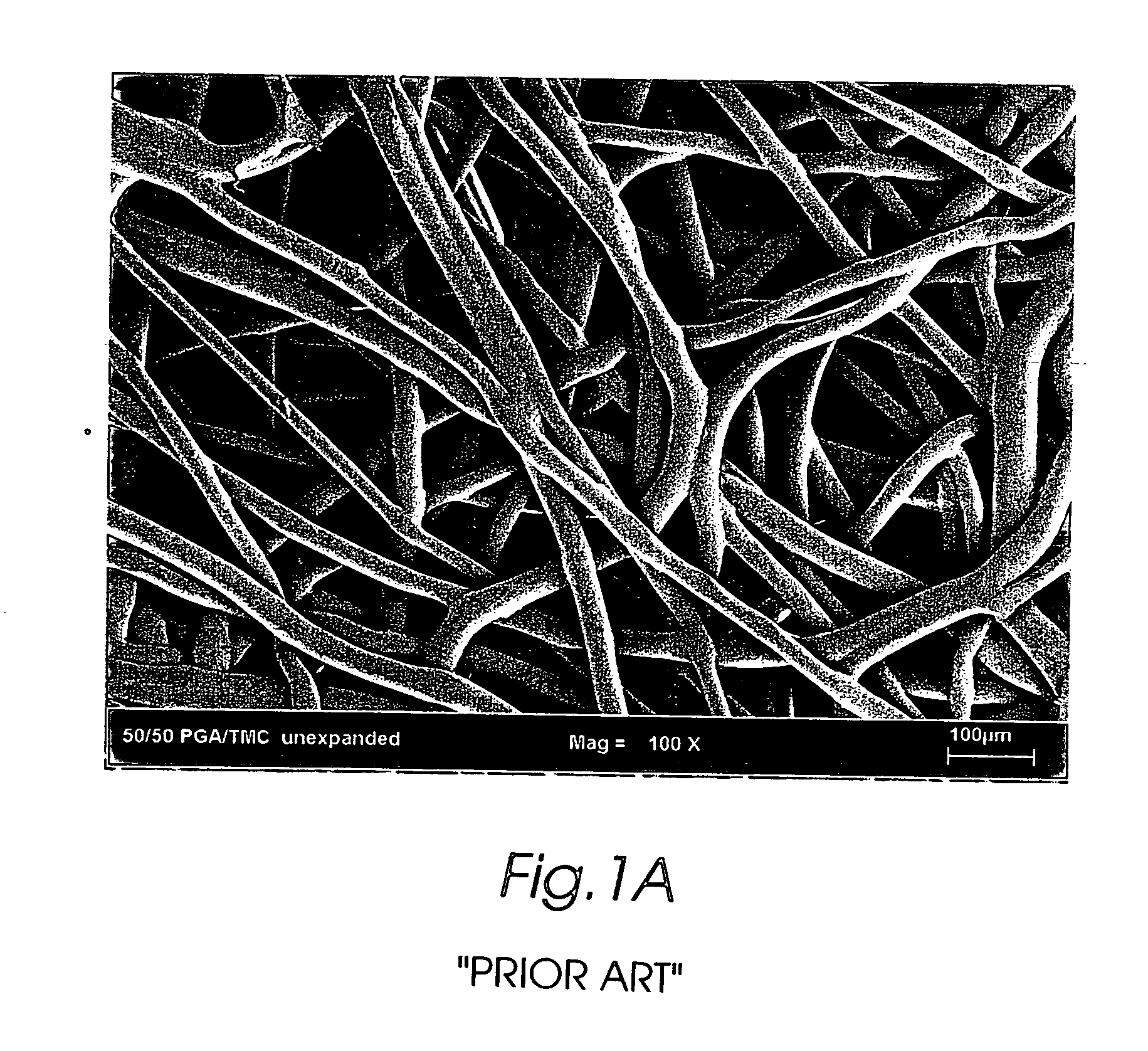
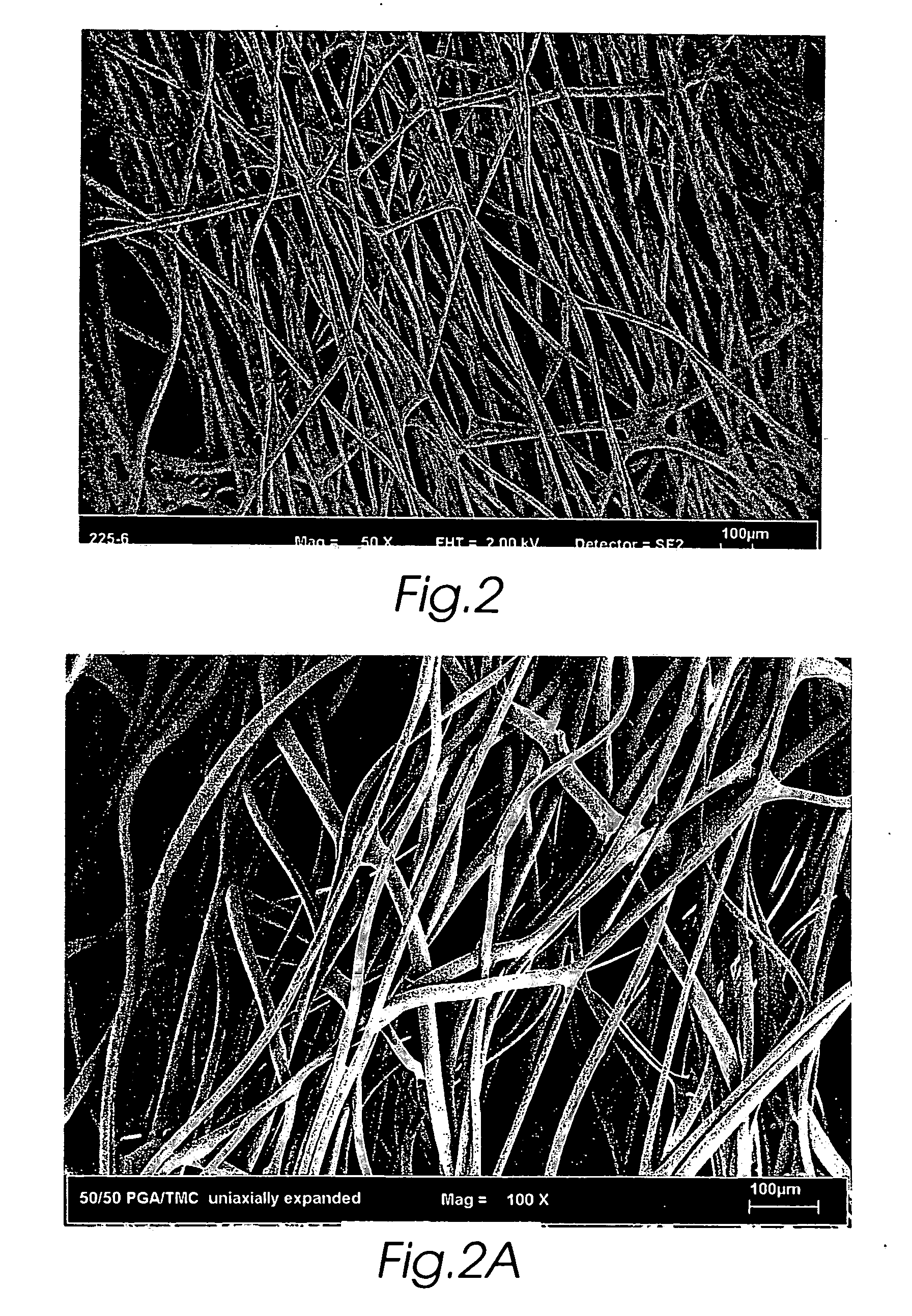
Composite self-cohered web materials
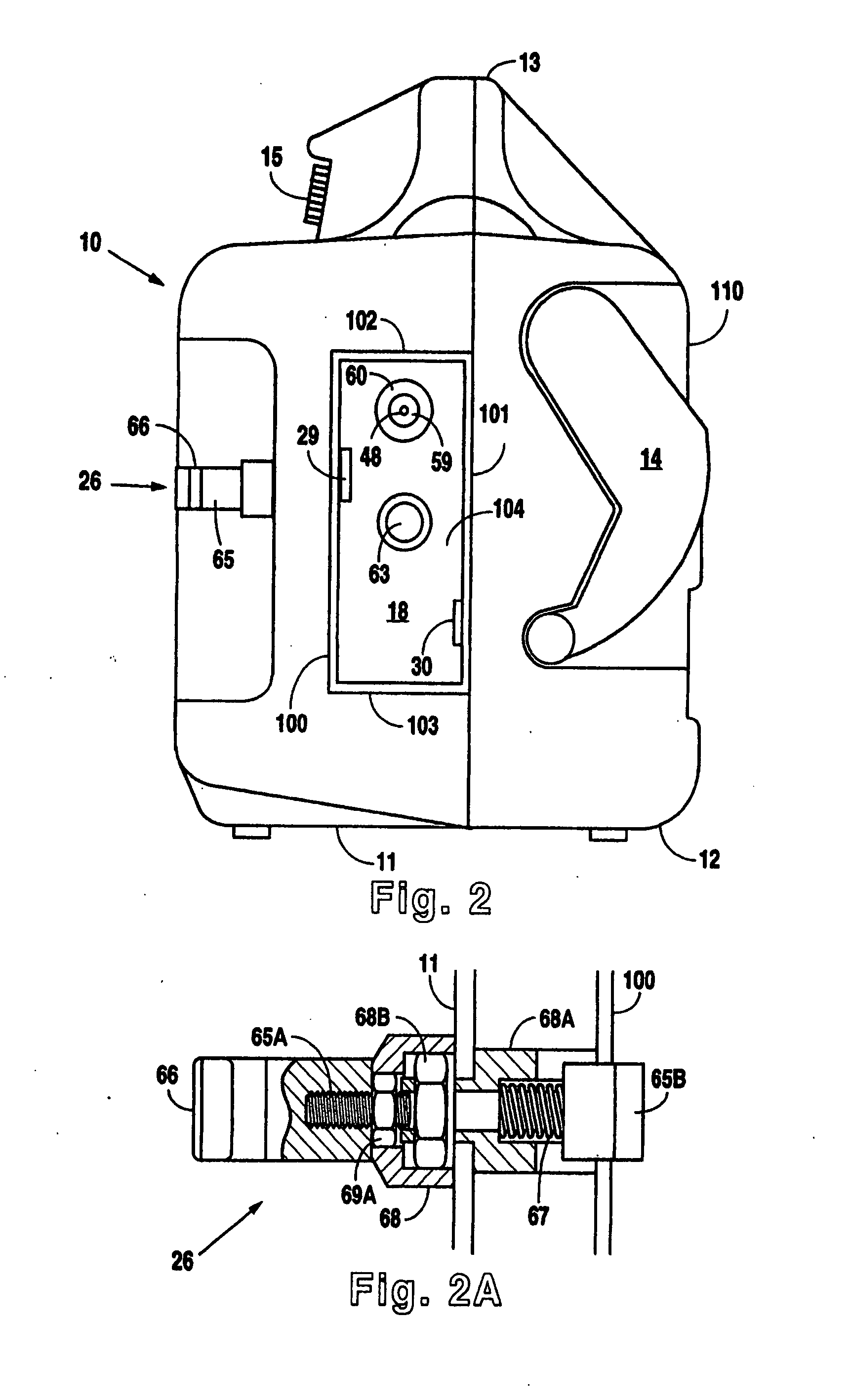
The present invention is directed to implantable bioabsorbable non-woven self-cohered web materials having a high degree of porosity. The web materials are very supple and soft, while exhibiting proportionally increased mechanical strength in one or more directions. The web materials often possess a high degree of loft. The web materials can be formed into a variety of shapes and forms suitable for use as implantable medical devices or components thereof.
Owner:WL GORE & ASSOC INC
Composite self-cohered web materials
InactiveUS20070026040A1High porositySuitable for useSurgical staplesProsthesisPorosityUltimate tensile strength
The present invention is directed to implantable bioabsorbable non-woven self-cohered web materials having a high degree of porosity. The web materials are very supple and soft, while exhibiting proportionally increased mechanical strength in one or more directions. The web materials often possess a high degree of loft. The web materials can be formed into a variety of shapes and forms suitable for use as implantable medical devices or components thereof.
Owner:WL GORE & ASSOC INC
Composite self-cohered web materials
InactiveUS20070026039A1High porositySuitable for useAntipyreticAnalgesicsBiomedical engineeringPorosity
The present invention is directed to implantable bioabsorbable non-woven self-cohered web materials having a high degree of porosity. The web materials are very supple and soft, while exhibiting proportionally increased mechanical strength in one or more directions. The web materials often possess a high degree of loft. The web materials can be formed into a variety of shapes and forms suitable for use as implantable medical devices or components thereof.
Owner:WL GORE & ASSOC INC
Removable wound closure
InactiveUS7381859B2Promote wound healingMinimizes adhesion formationNon-adhesive dressingsWound drainsElastomerPorosity
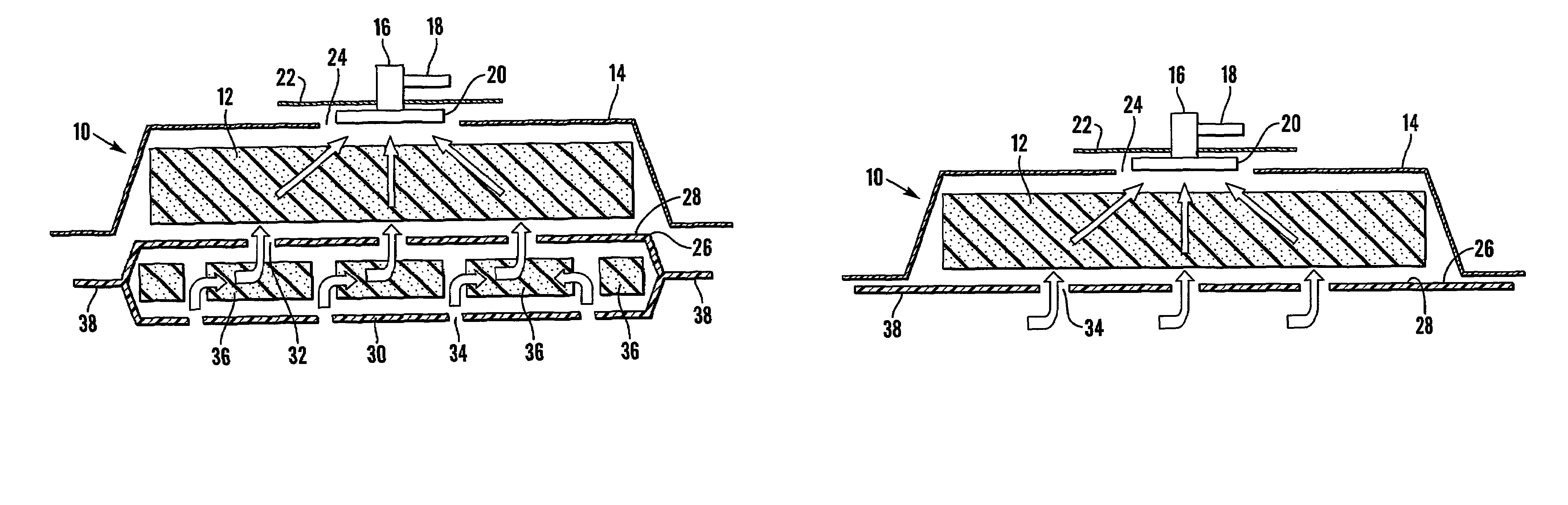
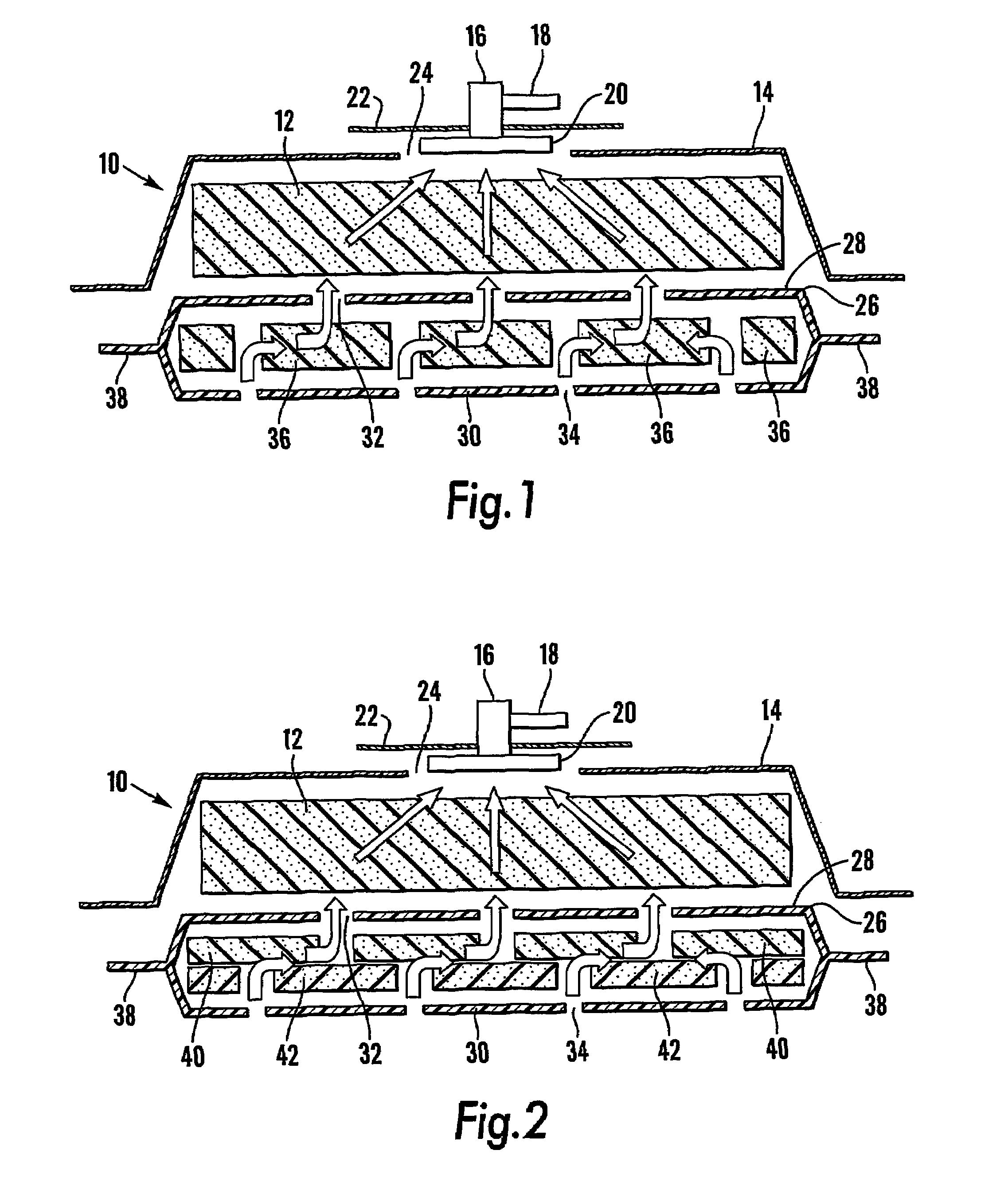
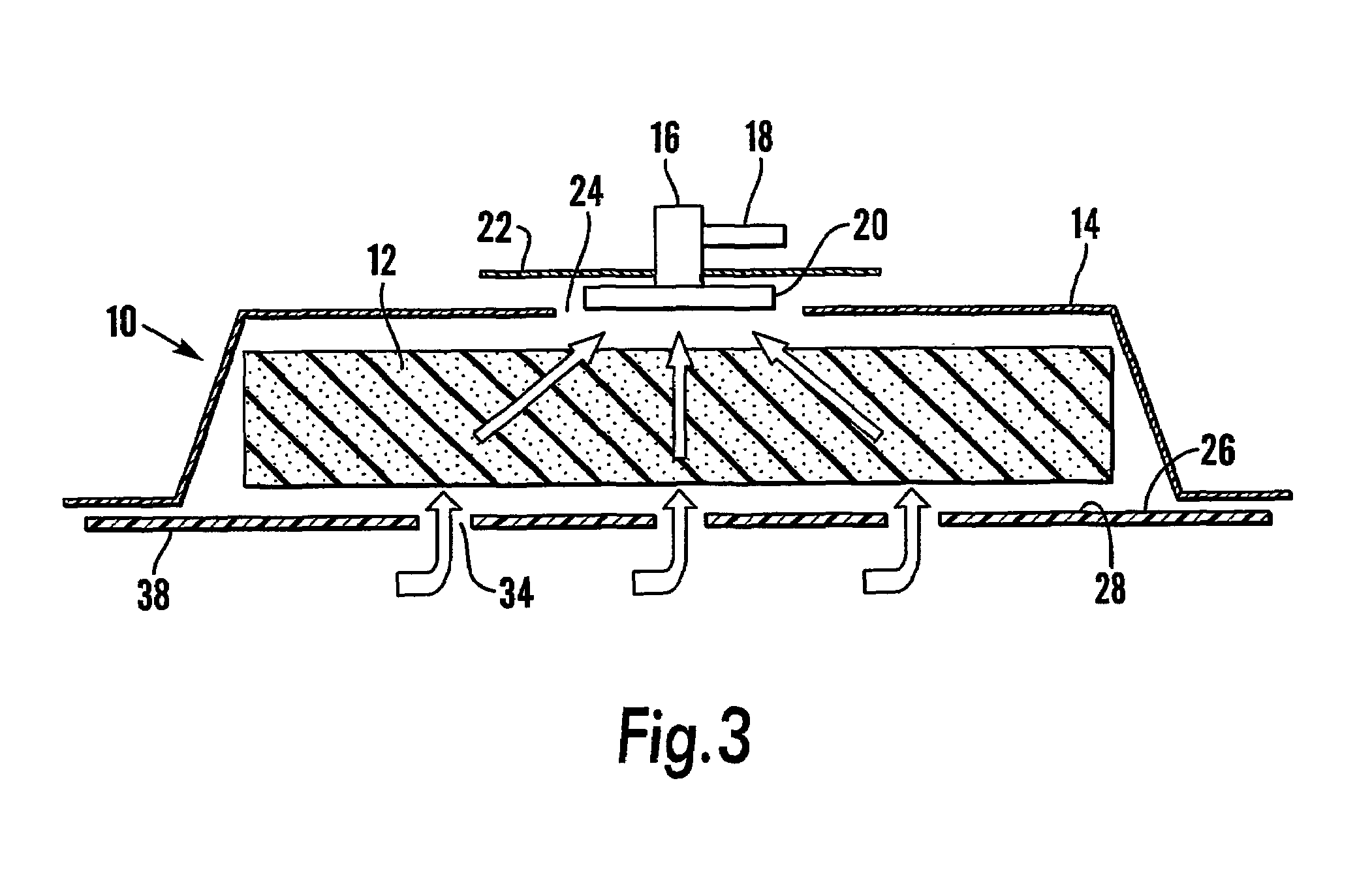
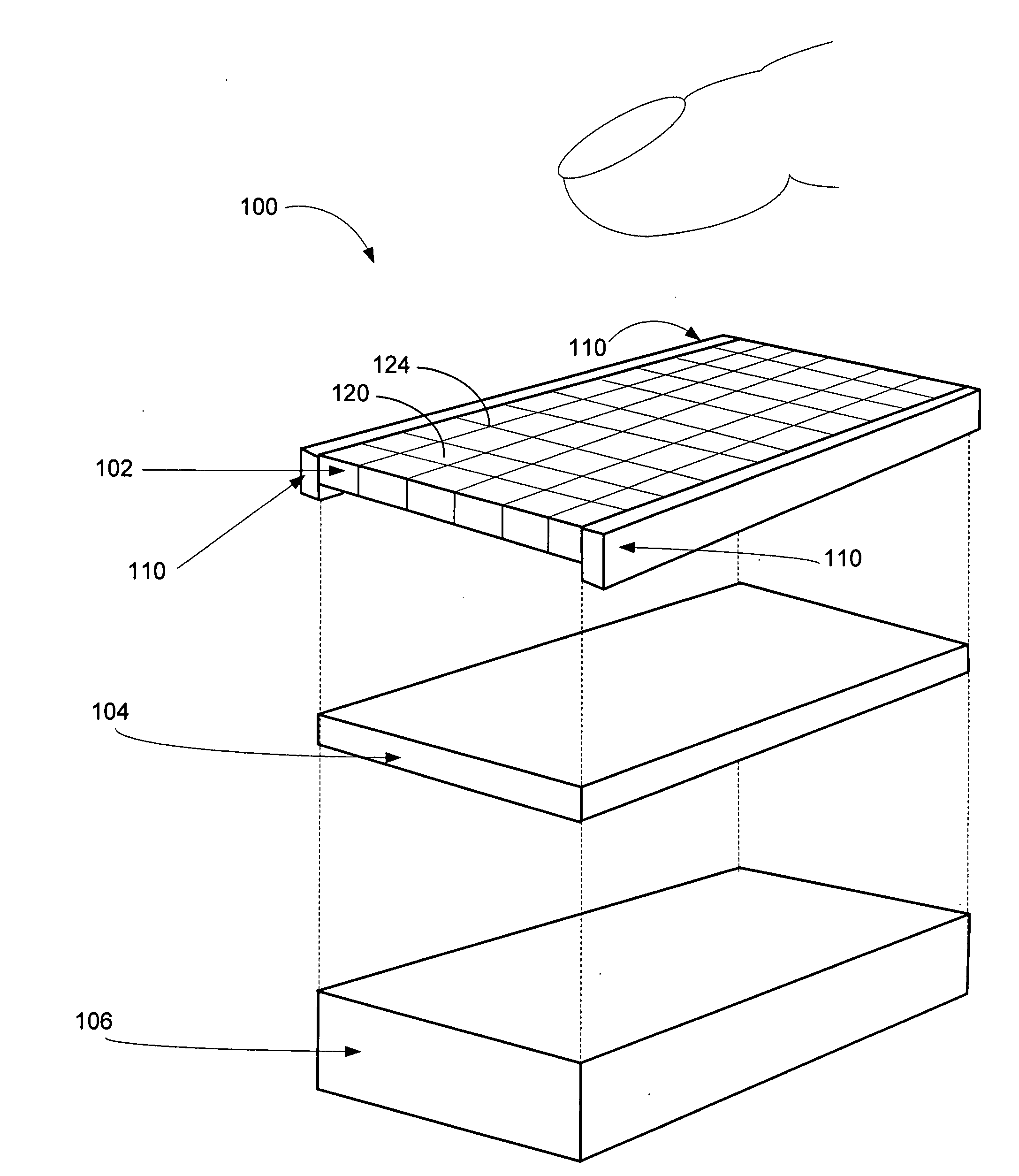
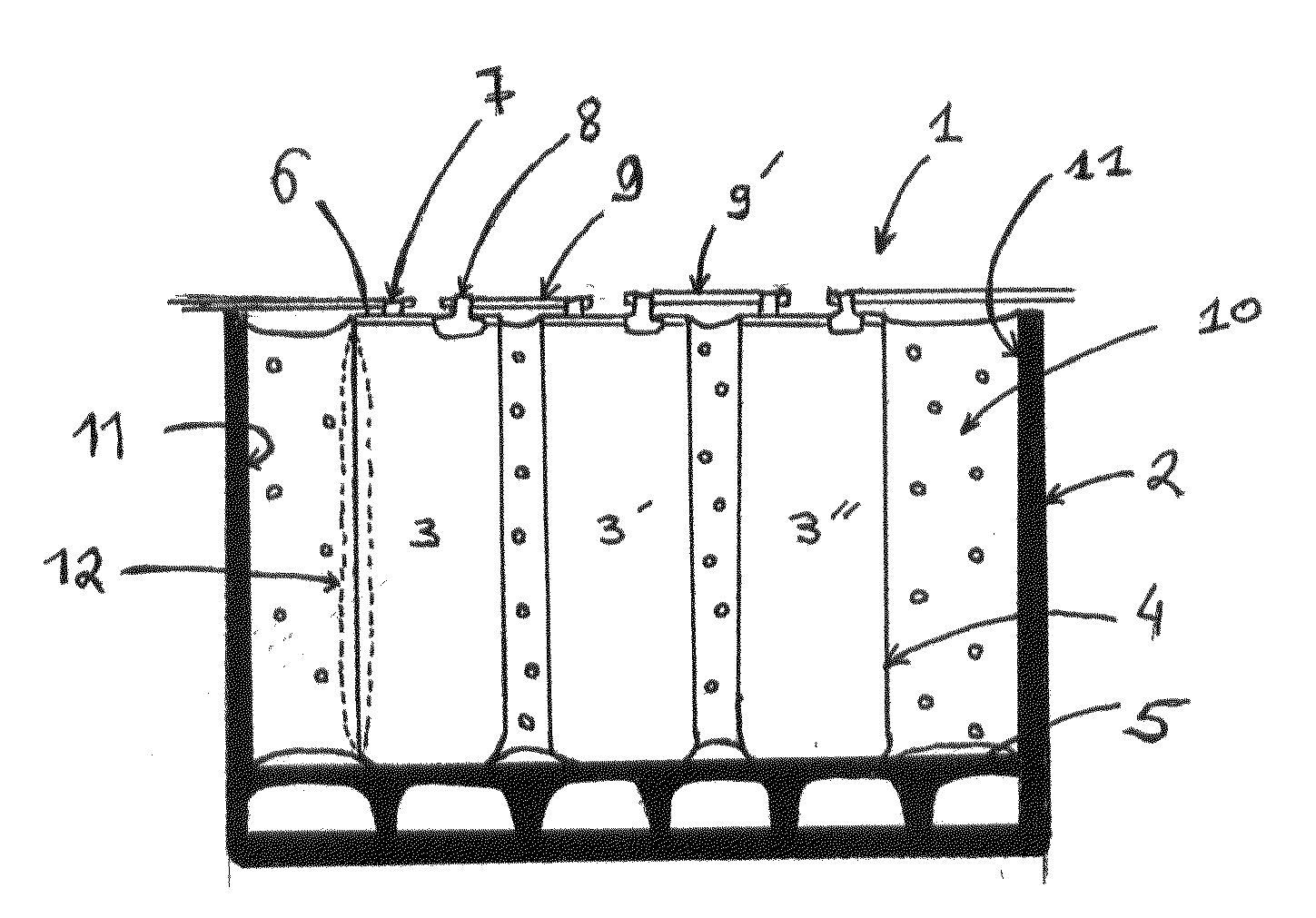
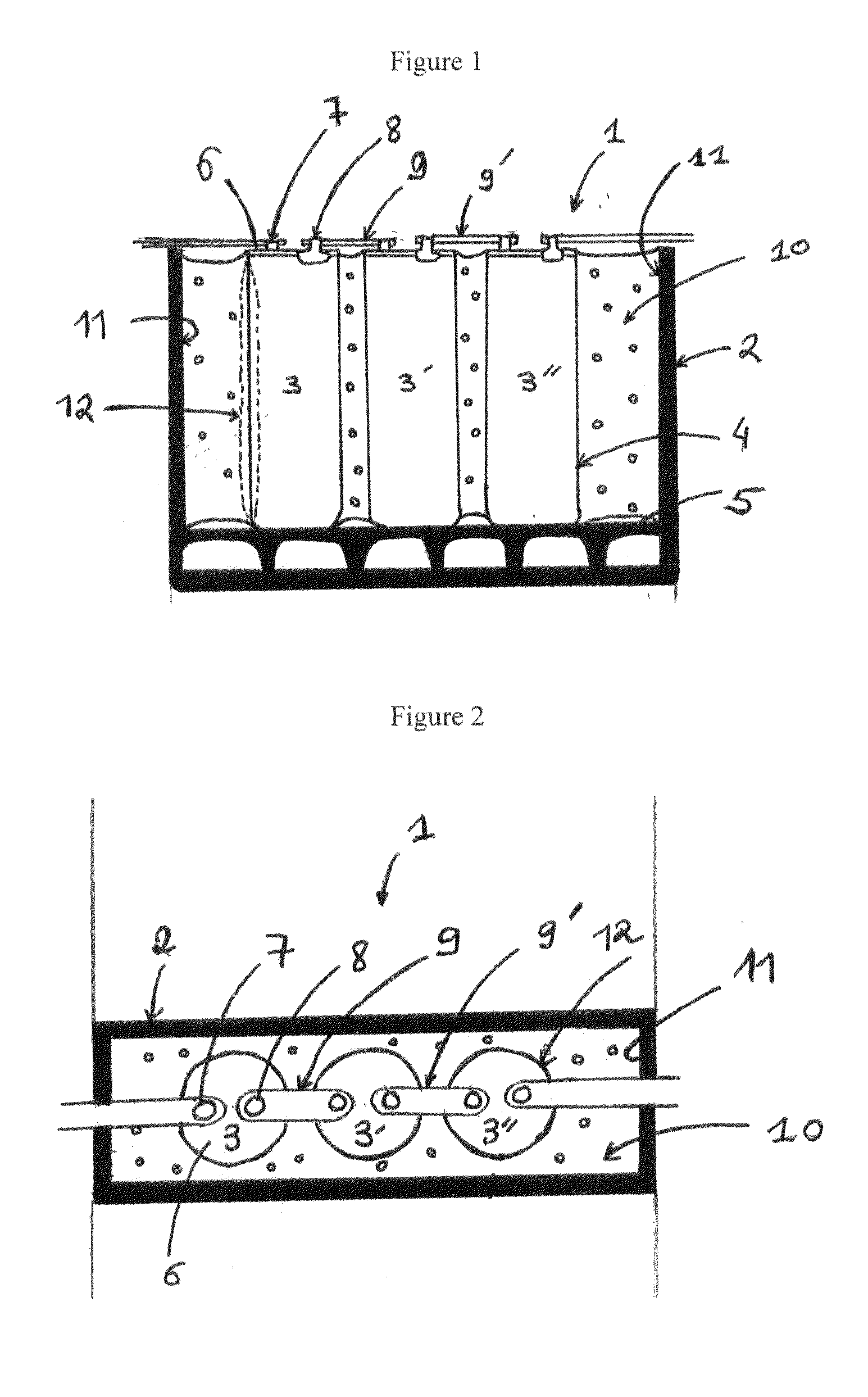
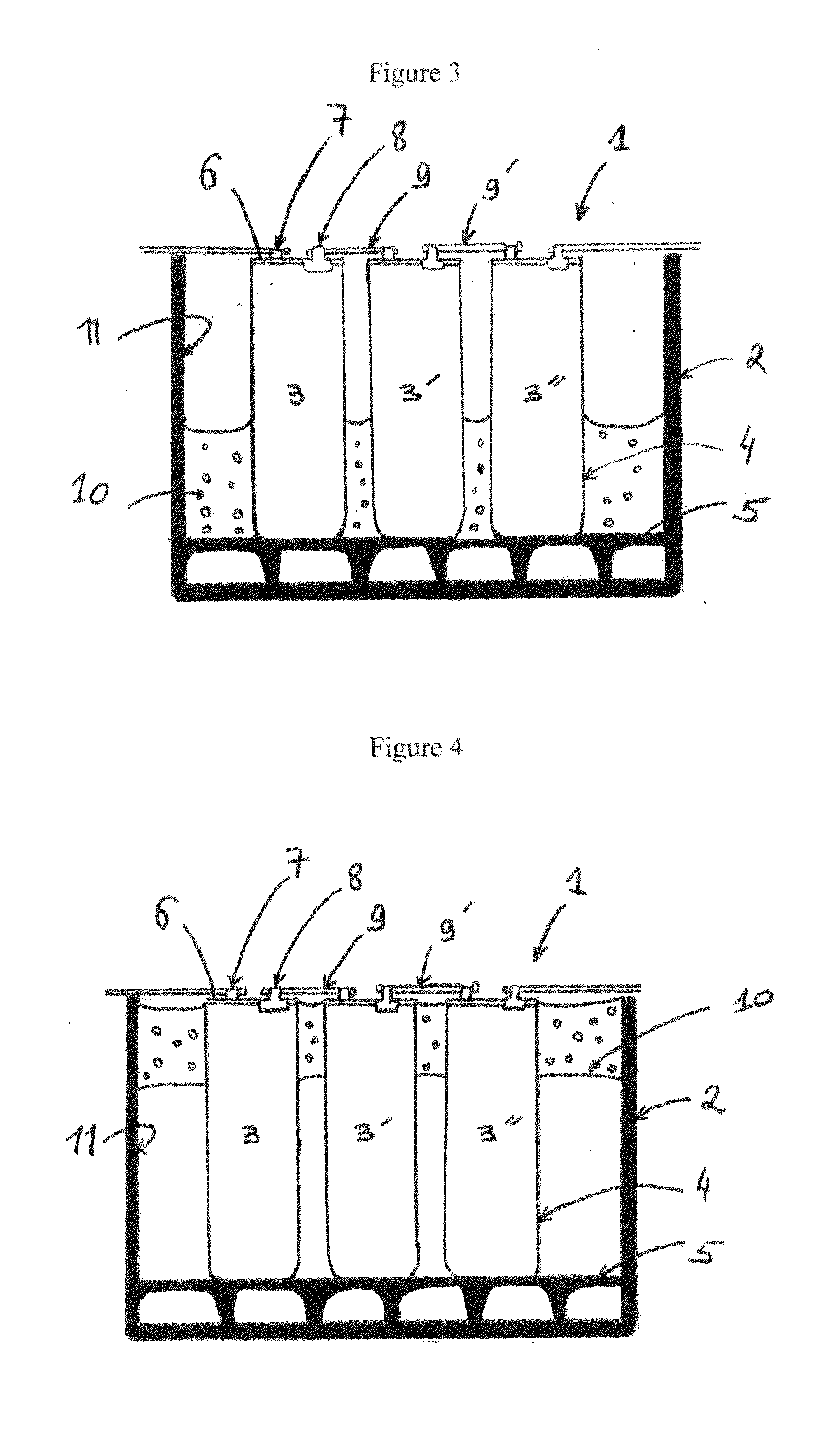
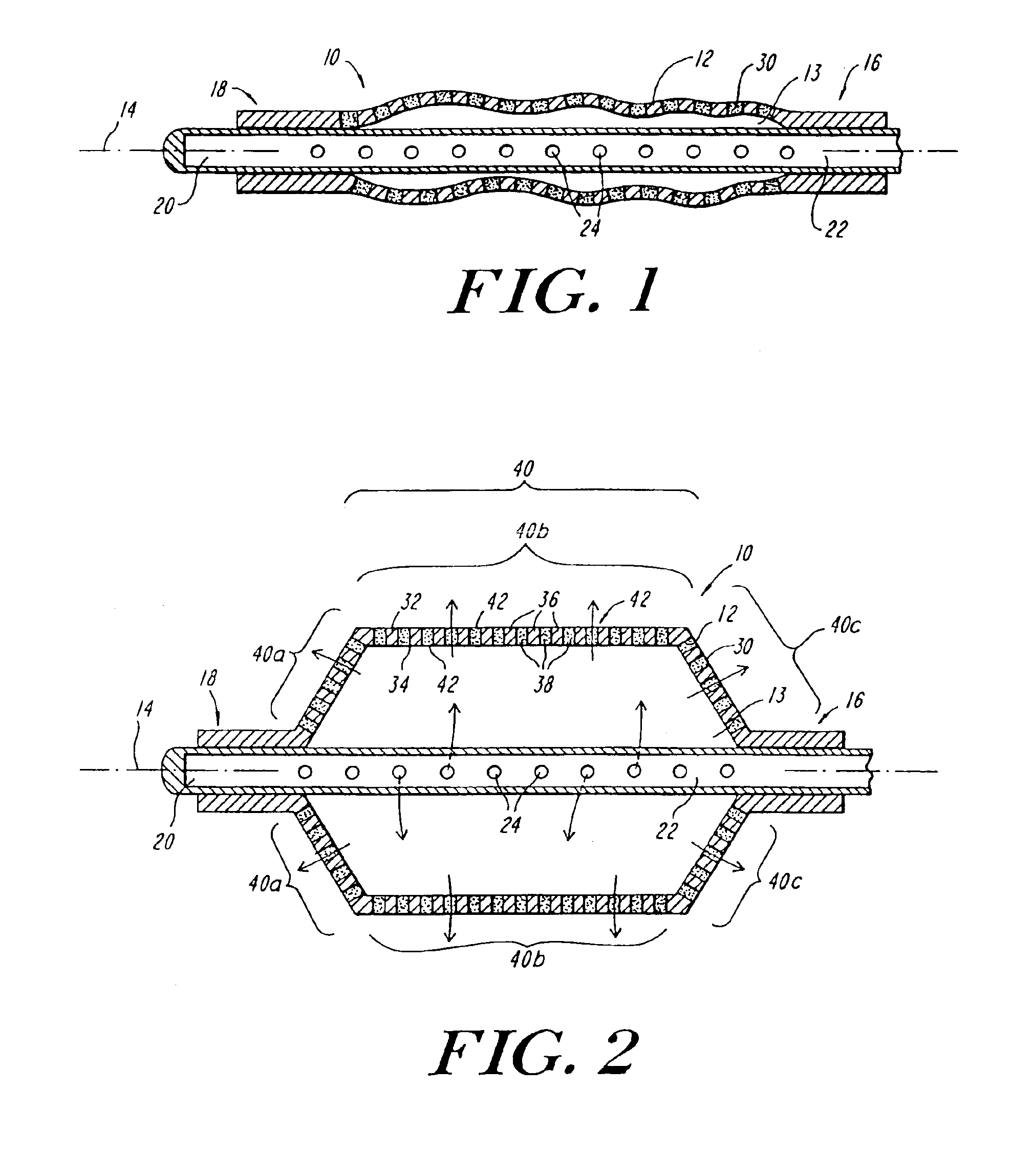
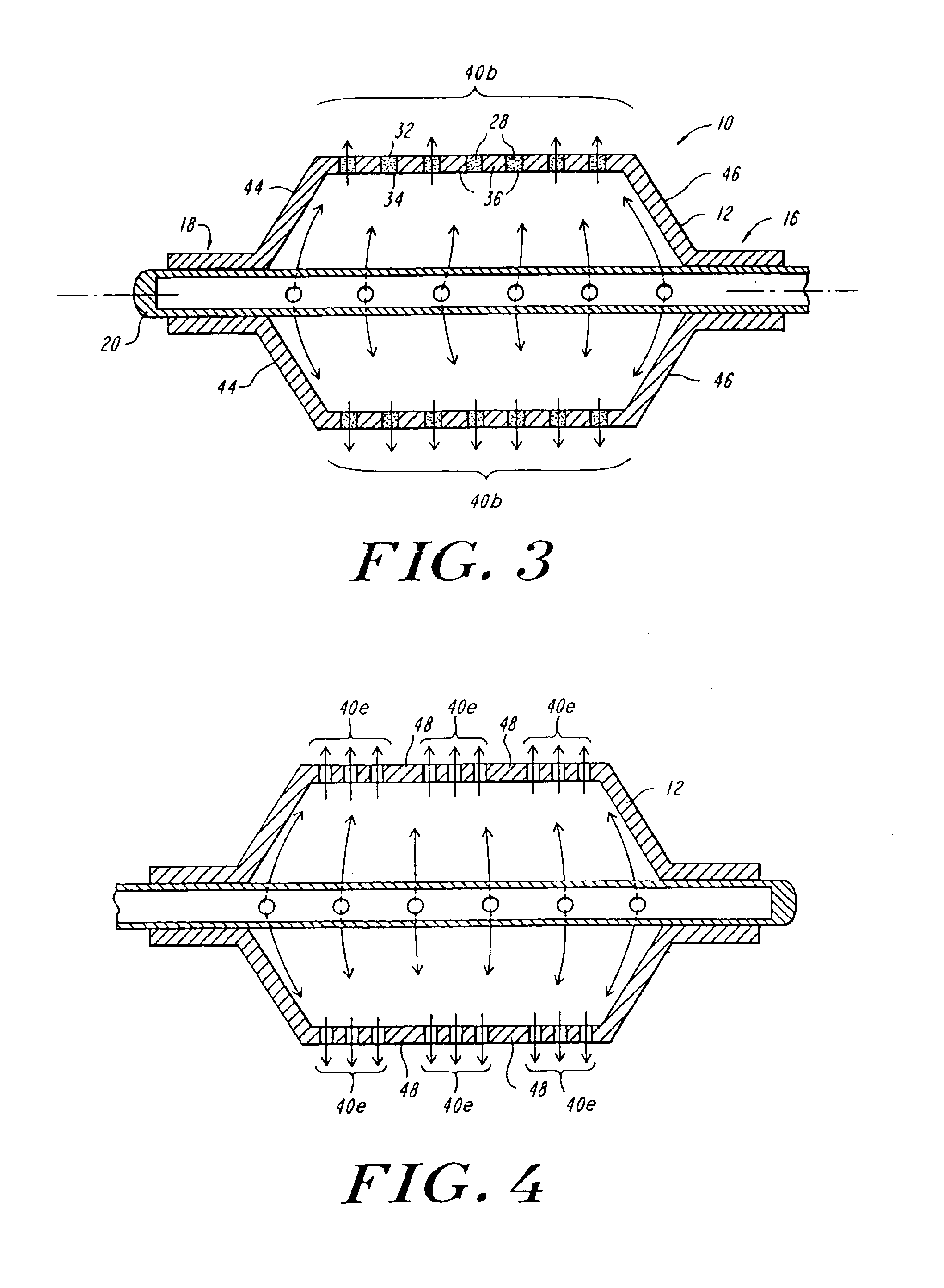
A system and method for the temporary closure of a wound, especially an abdominal wound, to facilitate re-entry, final closure, and long term healing of the wound. An abdominal wound dressing and methods of use are described that enable the application of negative pressure to the wound site in a site healing promoting manner while also limiting the formation of adhesions that would prevent the removal of the dressing. The dressing comprises a layer of porous foam material (36) enclosed by sheets of elastomeric material (38) punctuated by a number of appropriately placed holes (34). Multiple layers of porous foam may also be used. A suction tube connector (16) is provided on an upper surface of a layer of foam (12) for connection to a negative pressure source. At least one layer of foam is enclosed in elastomeric material and is placed in direct contact with the tissue within the open wound. Fluids are drawn by negative pressure through the holes positioned in the elastomeric envelope, and through the foam. If multiple foam layers are employed, the lower layer(s) of foam are of a finer porosity while the upper layer of foam is coarse. An adhesive elastomeric sheet (14) covers the entire wound dressing and seals the edges to the skin surrounding the wound. An appropriate vacuum device is attached to the suction tube connector.
Owner:KCI LICENSING INC
Method and apparatus for multi-touch tactile touch panel actuator mechanisms
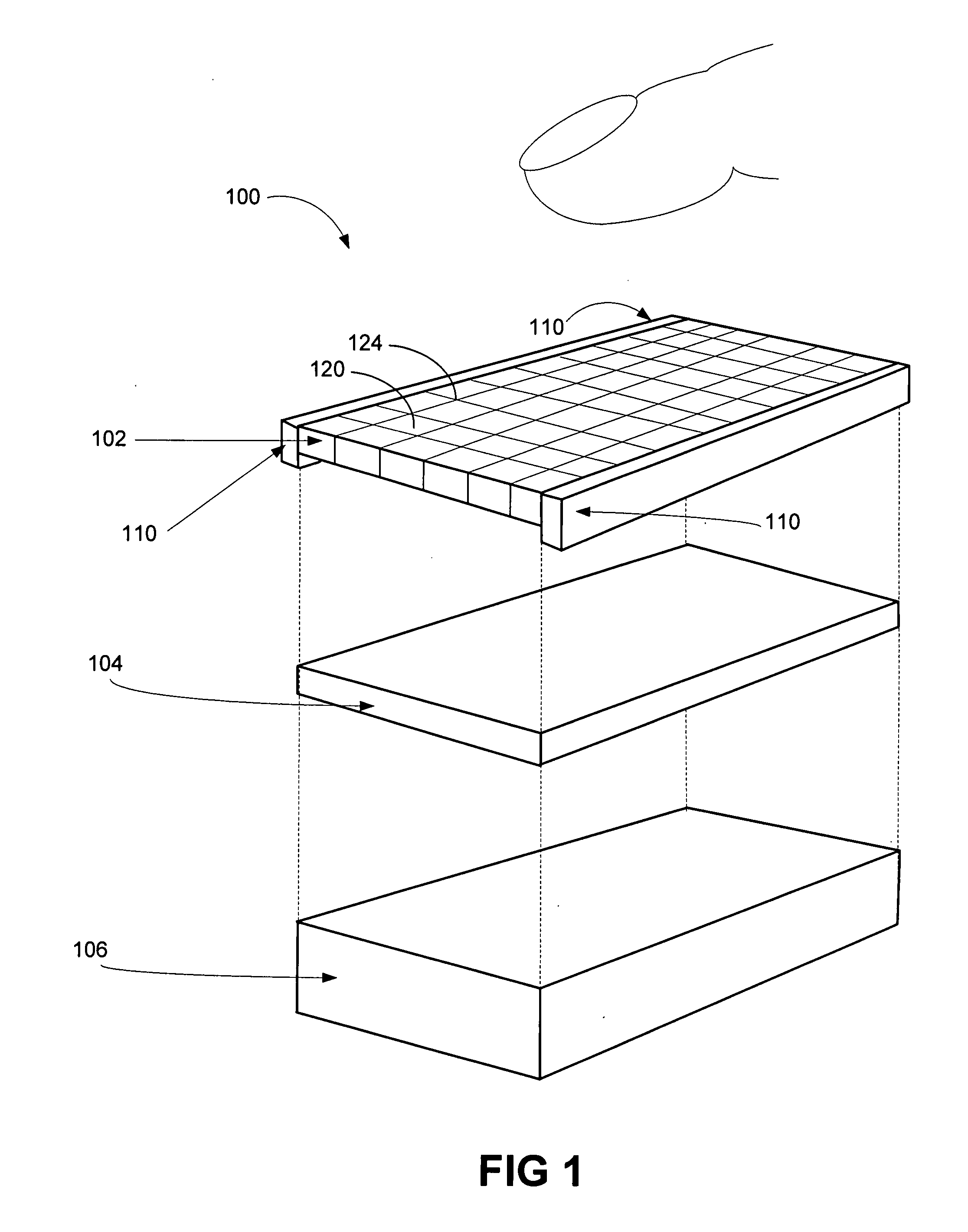
A method and apparatus of actuator mechanisms for a multi-touch tactile touch panel are disclosed. The tactile touch panel includes an electrical insulated layer and a tactile layer. The top surface of the electrical insulated layer is capable of receiving an input from a user. The tactile layer includes a grid or an array of haptic cells. The top surface of the haptic layer is situated adjacent to the bottom surface of the electrical insulated layer, while the bottom surface of the haptic layer is situated adjacent to a display. Each haptic cell further includes at least one piezoelectric material, Micro-Electro-Mechanical Systems (“MEMS”) element, thermal fluid pocket, MEMS pump, resonant device, variable porosity membrane, laminar flow modulation, or the like. Each haptic cell is configured to provide a haptic effect independent of other haptic cells in the tactile layer.
Owner:IMMERSION CORPORATION
Fabrication of porogen residues free and mechanically robust low-k materials
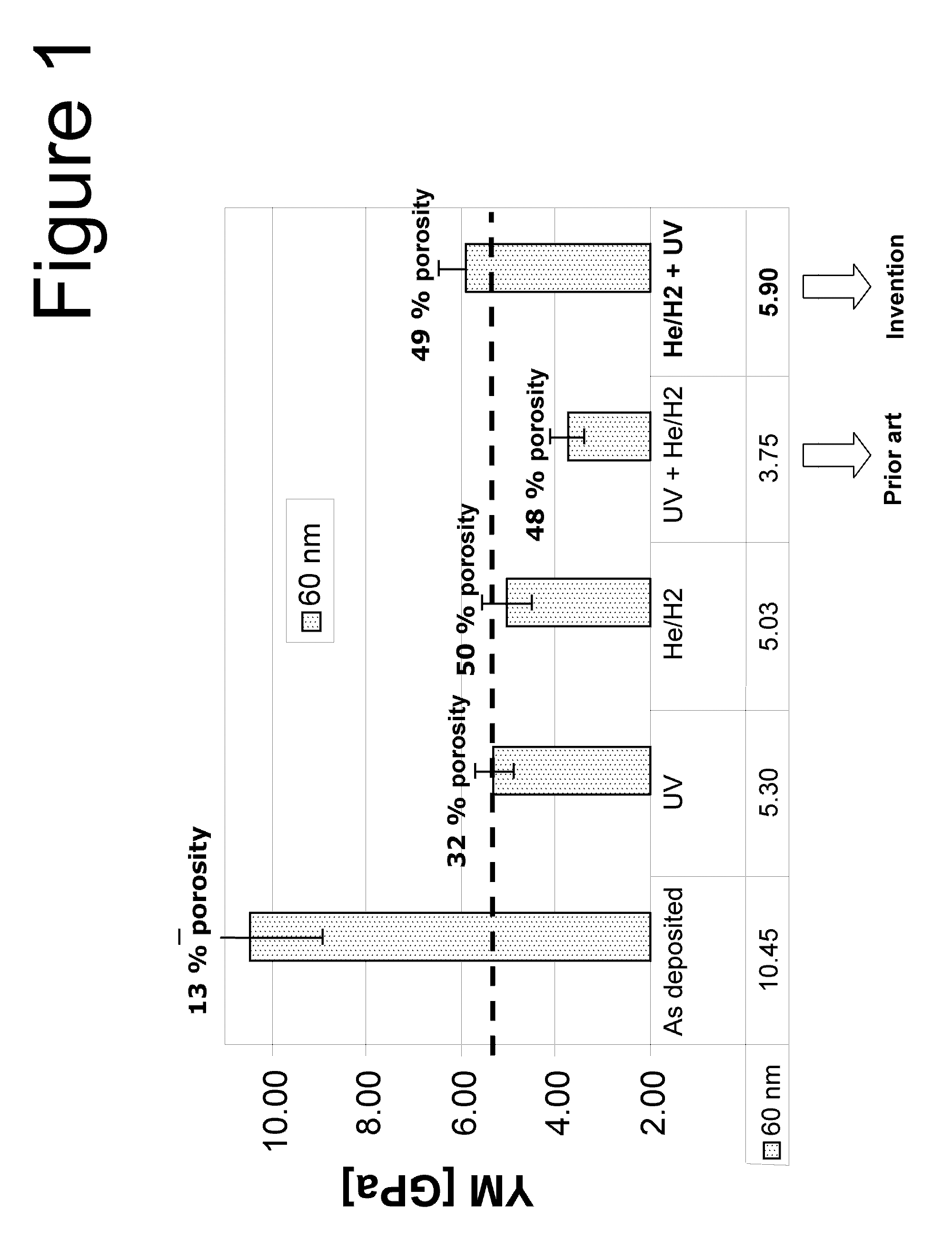
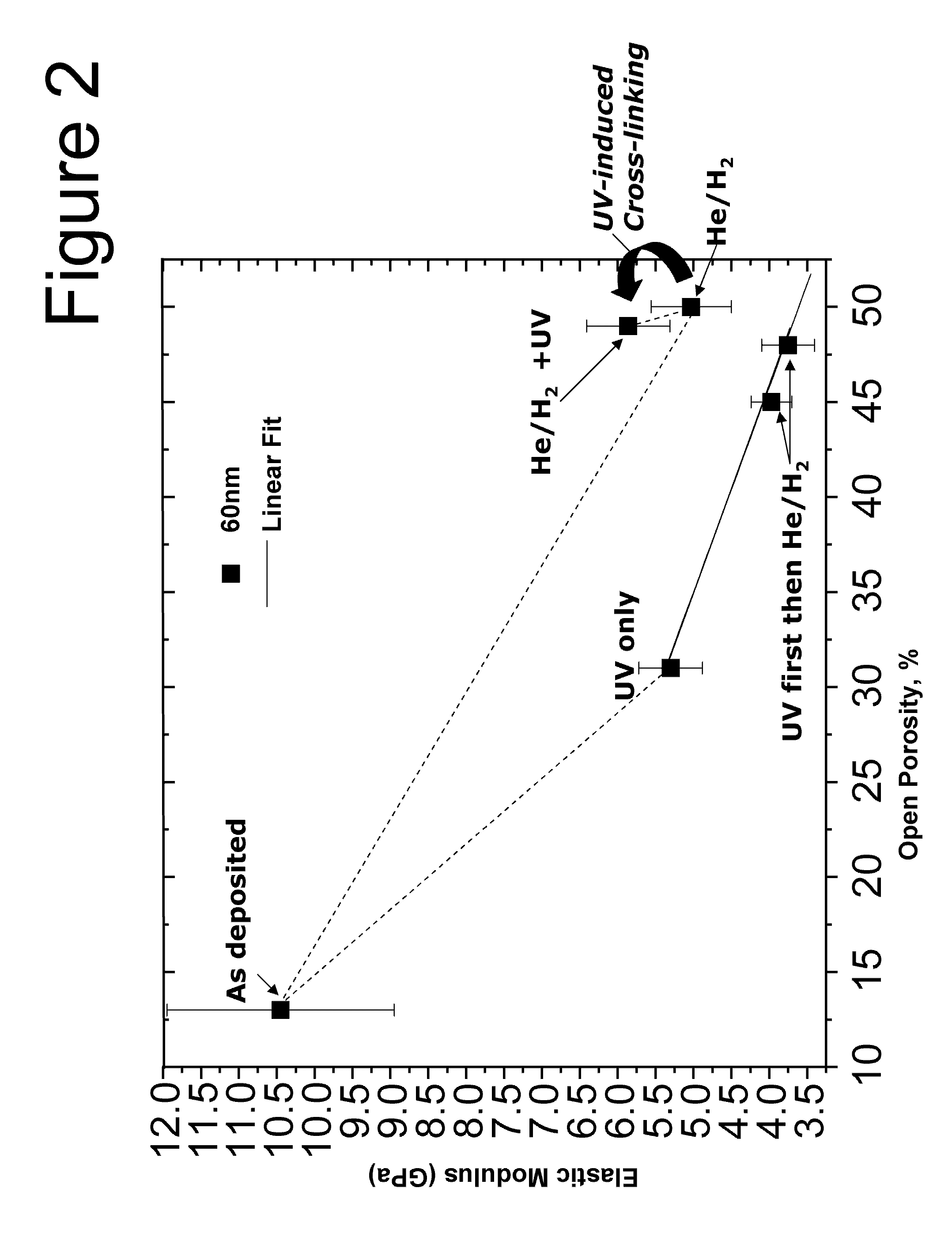
InactiveUS20110006406A1Improve H radical concentrationHigh elastic modulusSemiconductor/solid-state device detailsSolid-state devicesPorosityHydrogen treatment
A method is provided for producing a porogen-residue-free ultra low-k film with porosity higher than 50% and a high elastic modulus above 5 GPa. The method starts with depositing a SiCOH film using Plasma Enhanced Chemical Vapor Deposition (PE-CVD) or Chemical Vapor Deposition (CVD) onto a substrate and then first Performing an atomic hydrogen treatment at elevated wafer temperature in the range of 200° C. up to 350° C. to remove all the porogens and then performing a UV assisted thermal curing step.
Owner:INTERUNIVERSITAIR MICRO ELECTRONICS CENT (IMEC VZW) +1
Component for semicondutor processing apparatus and manufacturing method thereof
InactiveUS20090194233A1Increased durabilityLiquid surface applicatorsSemiconductor/solid-state device manufacturingTectorial membranePorosity
A component (10) for a semiconductor processing apparatus includes a matrix (10a) defining a shape of the component, and a protection film (10c) covering a predetermined surface of the matrix. The protection film (10c) consists essentially of an amorphous oxide of a first element selected from the group consisting of aluminum, silicon, hafnium, zirconium, and yttrium. The protection film (10c) has a porosity of less than 1% and a thickness of 1 nm to 10 μm.
Owner:TOKYO ELECTRON LTD
Composite solid electrolyte for protection of active metal anodes
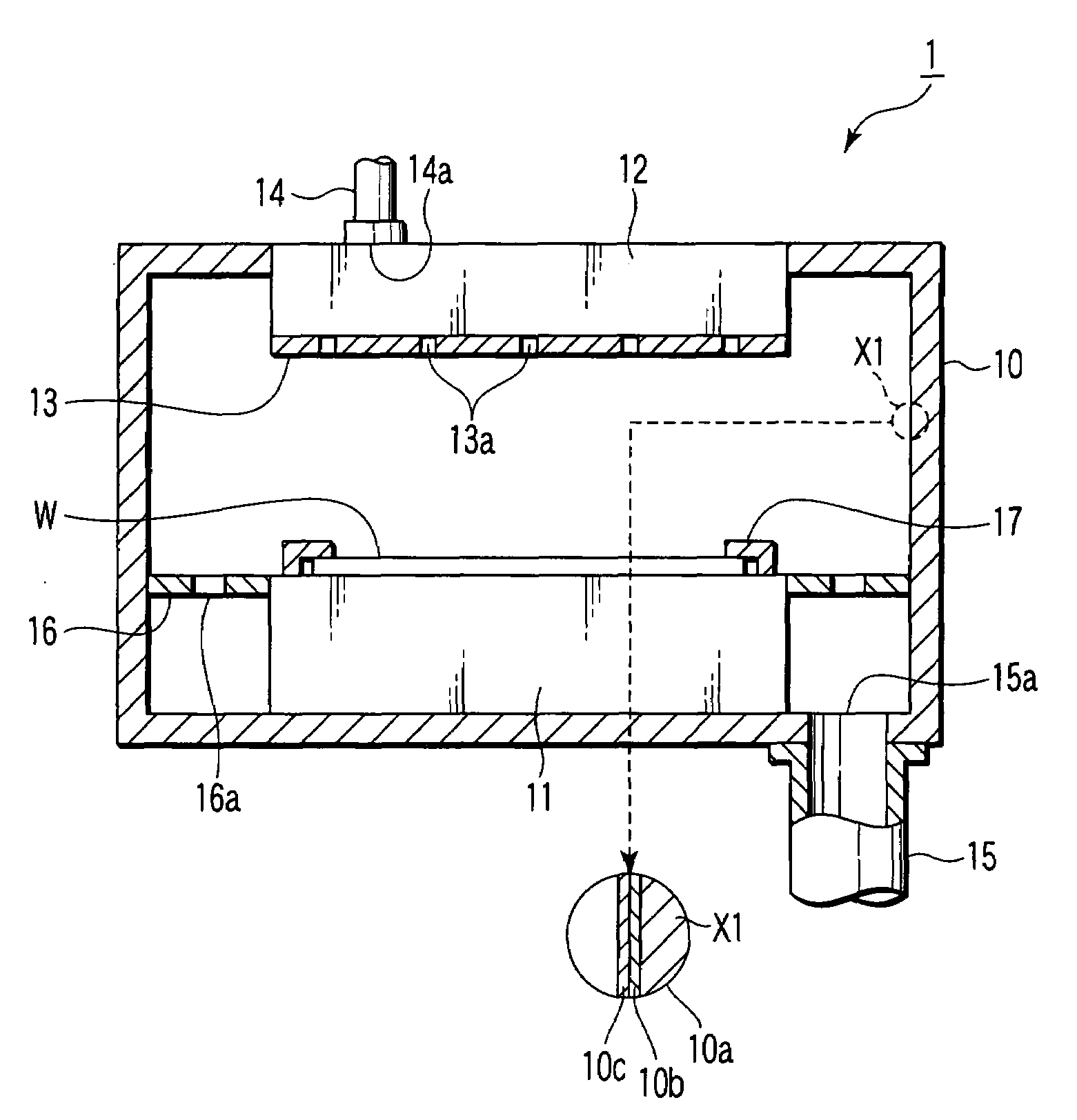
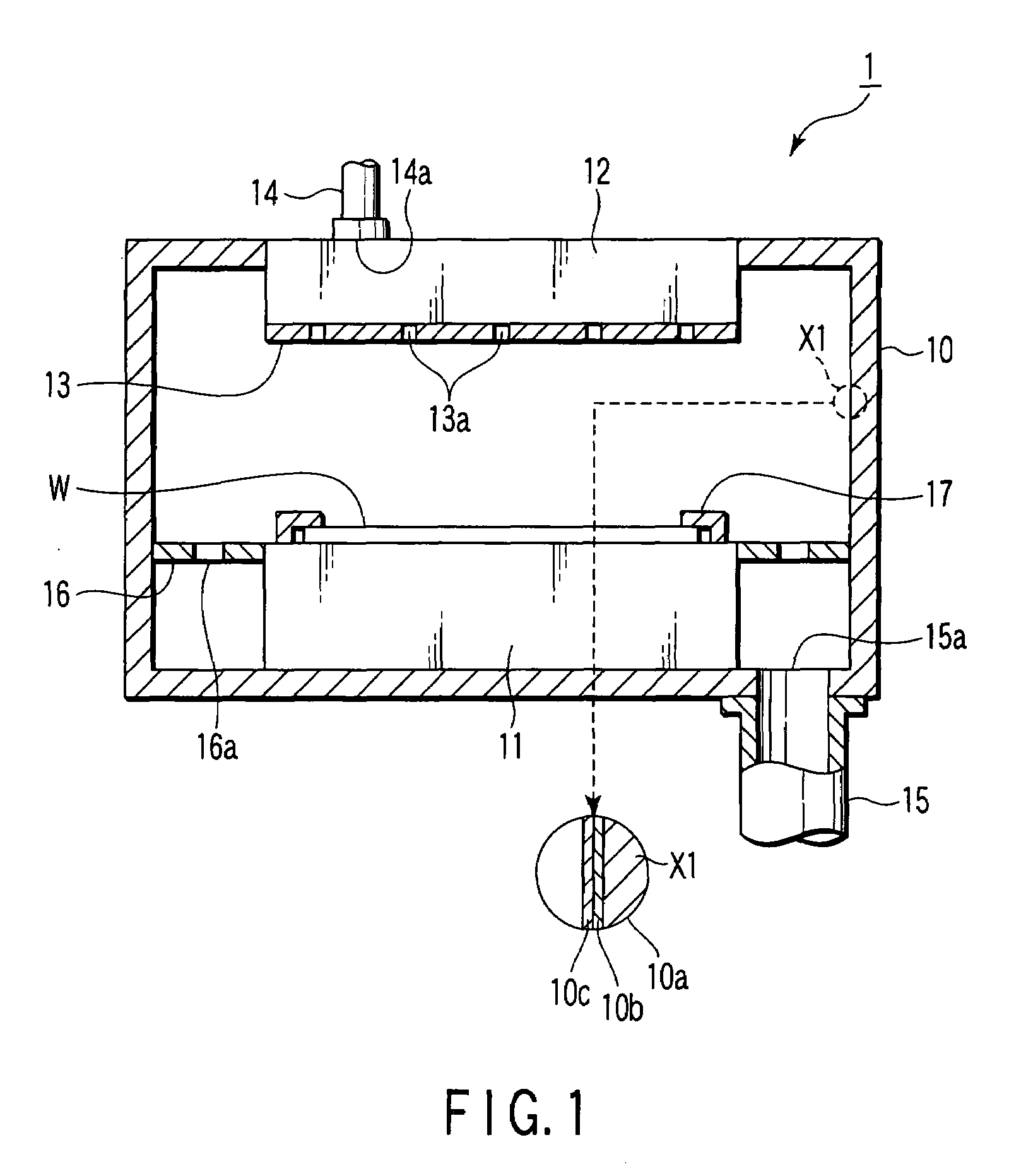
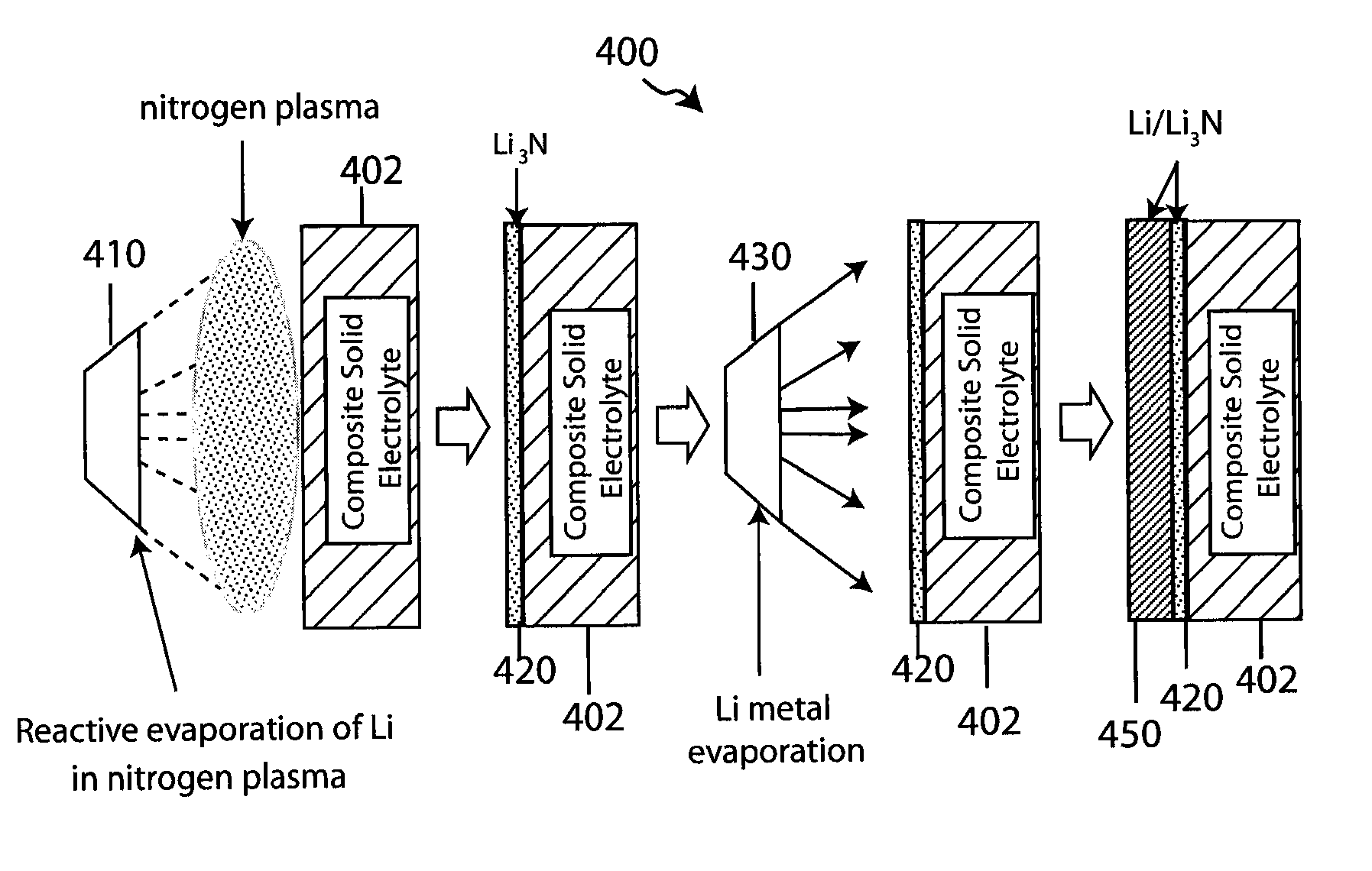
ActiveUS20070172739A1Eliminate through-porosityHigh metal ion conductivityCell electrodesPrimary cellsPorosityTectorial membrane
A composite solid electrolyte include a monolithic solid electrolyte base component that is a continuous matrix of an inorganic active metal ion conductor and a filler component used to eliminate through porosity in the solid electrolyte. In this way a solid electrolyte produced by any process that yields residual through porosity can be modified by the incorporation of a filler to form a substantially impervious composite solid electrolyte and eliminate through porosity in the base component. Methods of making the composites is also disclosed. The composites are generally useful in electrochemical cell structures such as battery cells and in particular protected active metal anodes, particularly lithium anodes, that are protected with a protective membrane architecture incorporating the composite solid electrolyte. The protective architecture prevents the active metal of the anode from deleterious reaction with the environment on the other (cathode) side of the architecture, which may include aqueous, air and organic liquid electrolytes and / or electrochemically active materials.
Owner:POLYPLUS BATTERY CO INC
Vapor deposition of silicon dioxide nanolaminates
ActiveUS20050112282A1Easy to produceUniform thicknessMaterial nanotechnologySemiconductor/solid-state device manufacturingPorosityElectrical conductor
This invention relates to materials and processes for thin film deposition on solid substrates. Silica / alumina nanolaminates were deposited on heated substrates by the reaction of an aluminum-containing compound with a silanol. The nanolaminates have very uniform thickness and excellent step coverage in holes with aspect ratios over 40:1. The films are transparent and good electrical insulators. This invention also relates to materials and processes for producing improved porous dielectric materials used in the insulation of electrical conductors in microelectronic devices, particularly through materials and processes for producing semi-porous dielectric materials wherein surface porosity is significantly reduced or removed while internal porosity is preserved to maintain a desired low-k value for the overall dielectric material. The invention can also be used to selectively fill narrow trenches with low-k dielectric material while at the same time avoiding deposition of any dielectric on the surface area outside of the trenches.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
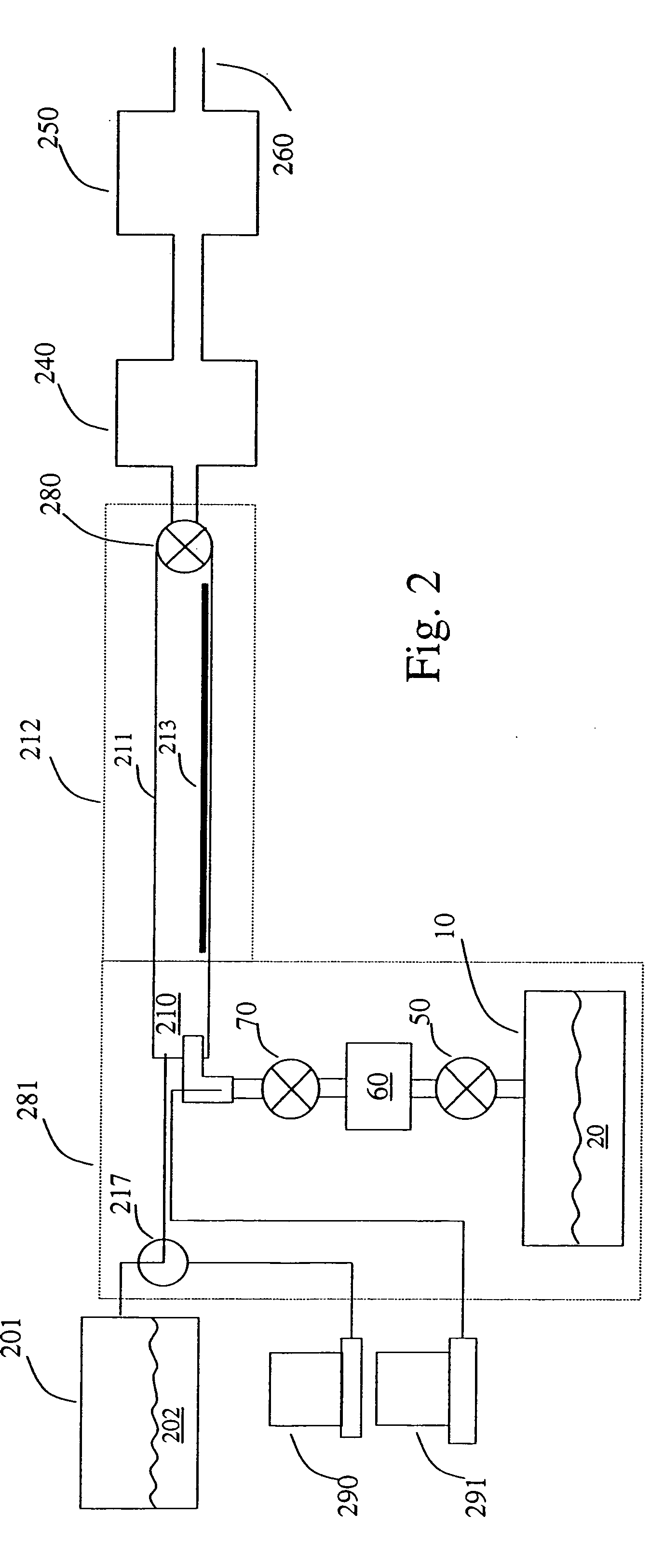
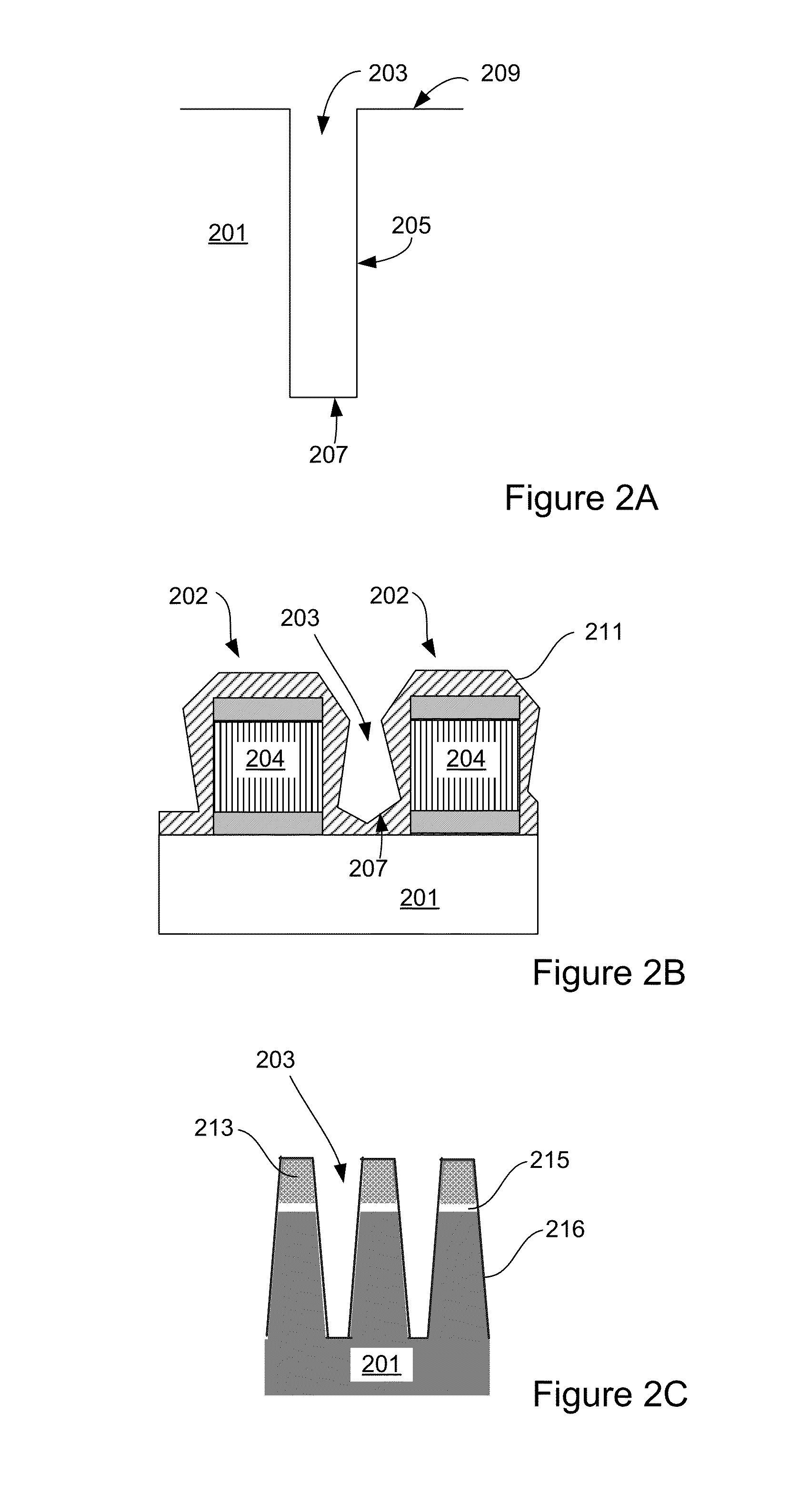
Methods and apparatus for forming flowable dielectric films having low porosity
InactiveUS20150118863A1Liquid surface applicatorsSemiconductor/solid-state device manufacturingPorosityNew materials
Provided herein are methods and apparatus for forming flowable dielectric films having low porosity. In some embodiments, the methods involve plasma post-treatments of flowable dielectric films. The treatments can involve exposing a flowable film to a plasma while the film is still in a flowable, reactive state but after deposition of new material has ceased.
Owner:LAM RES CORP
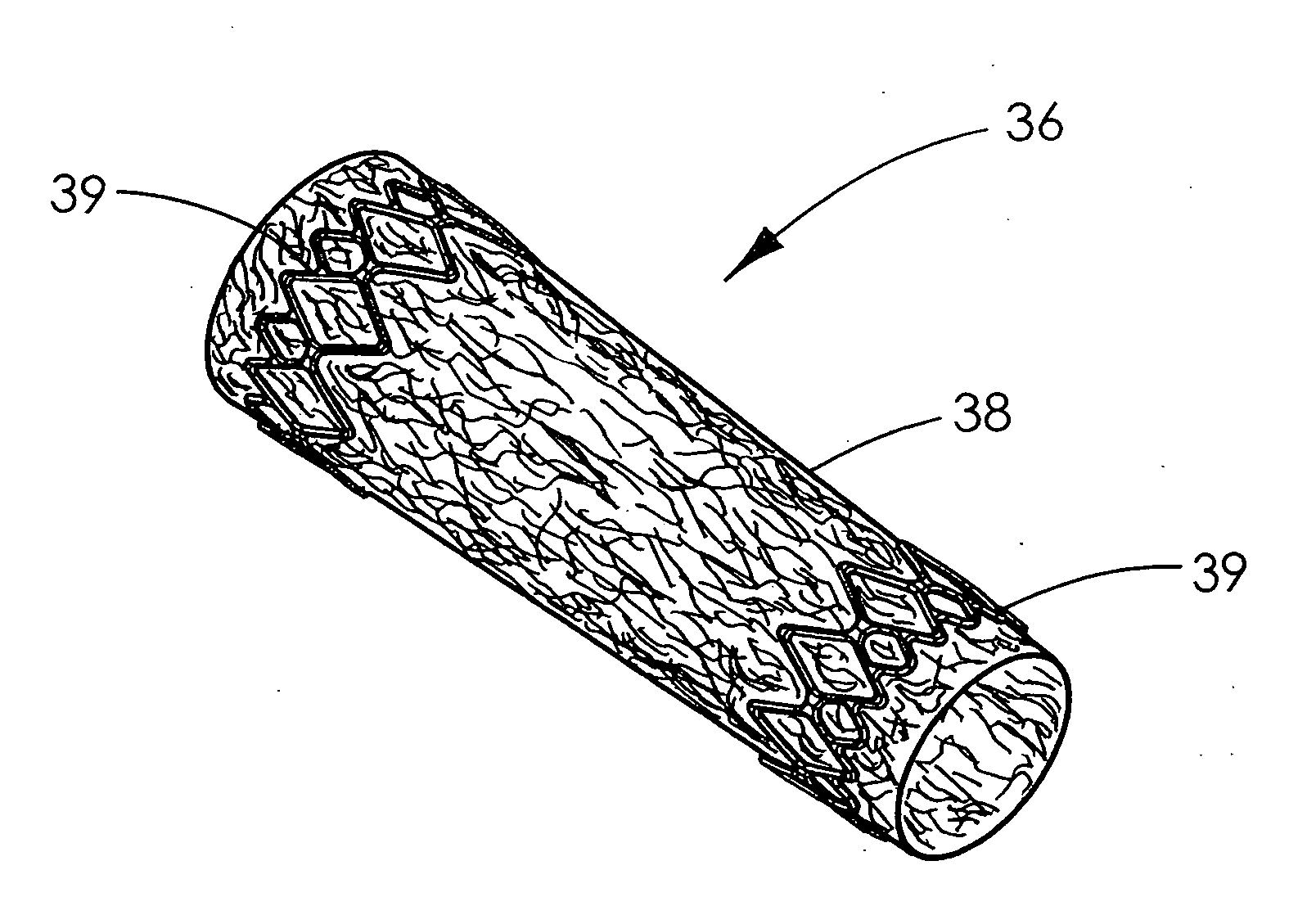
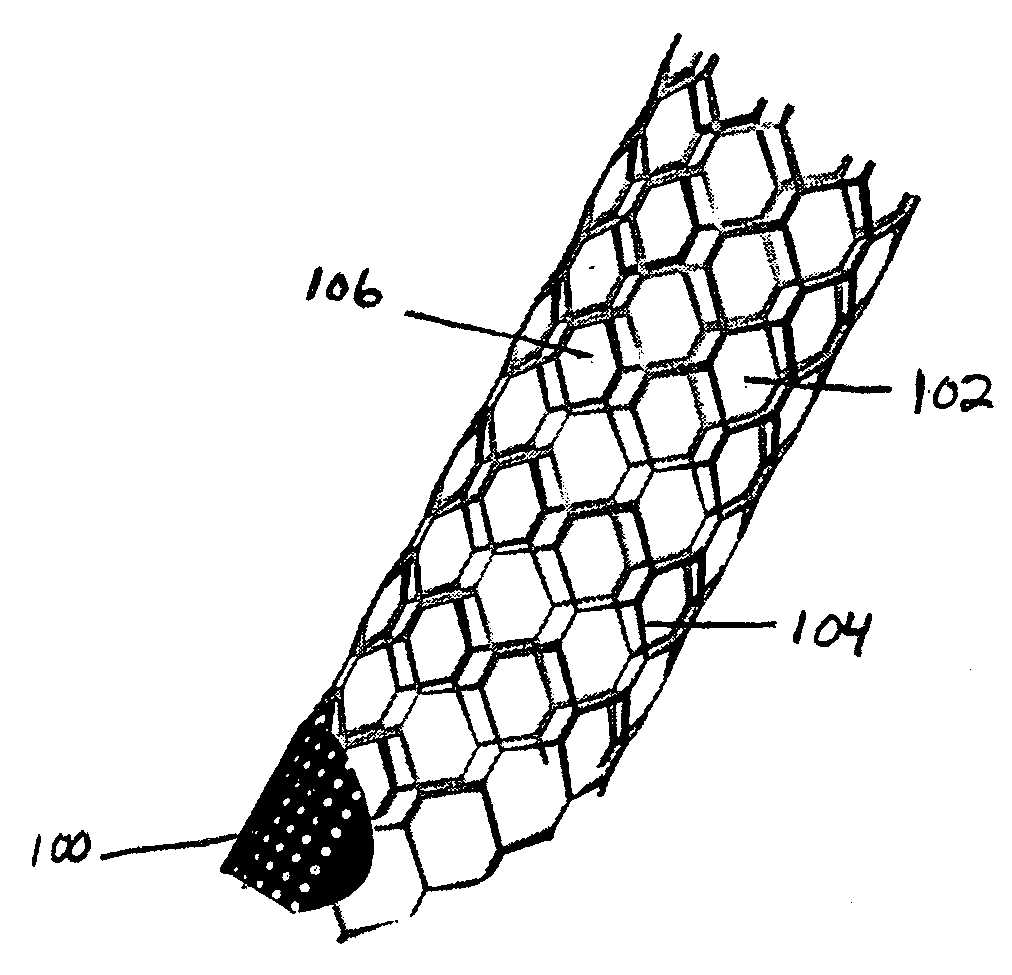
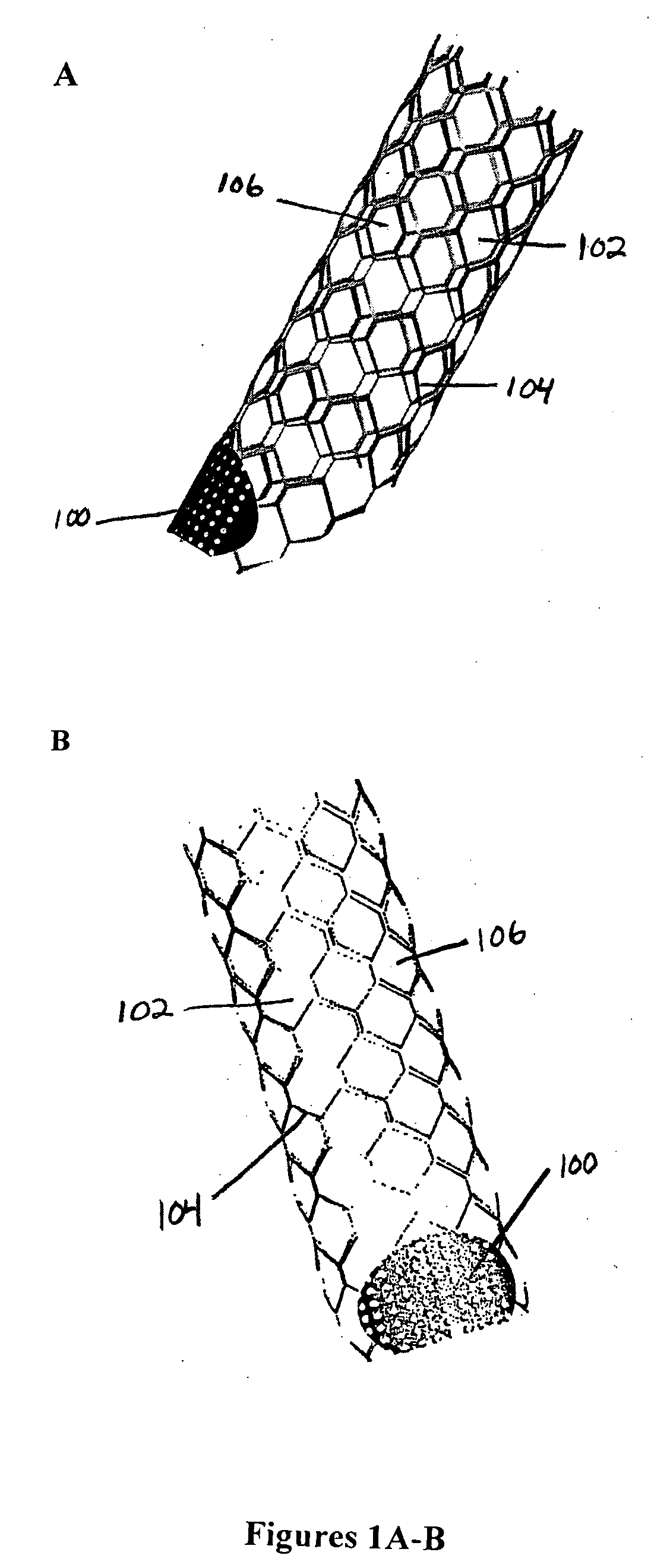
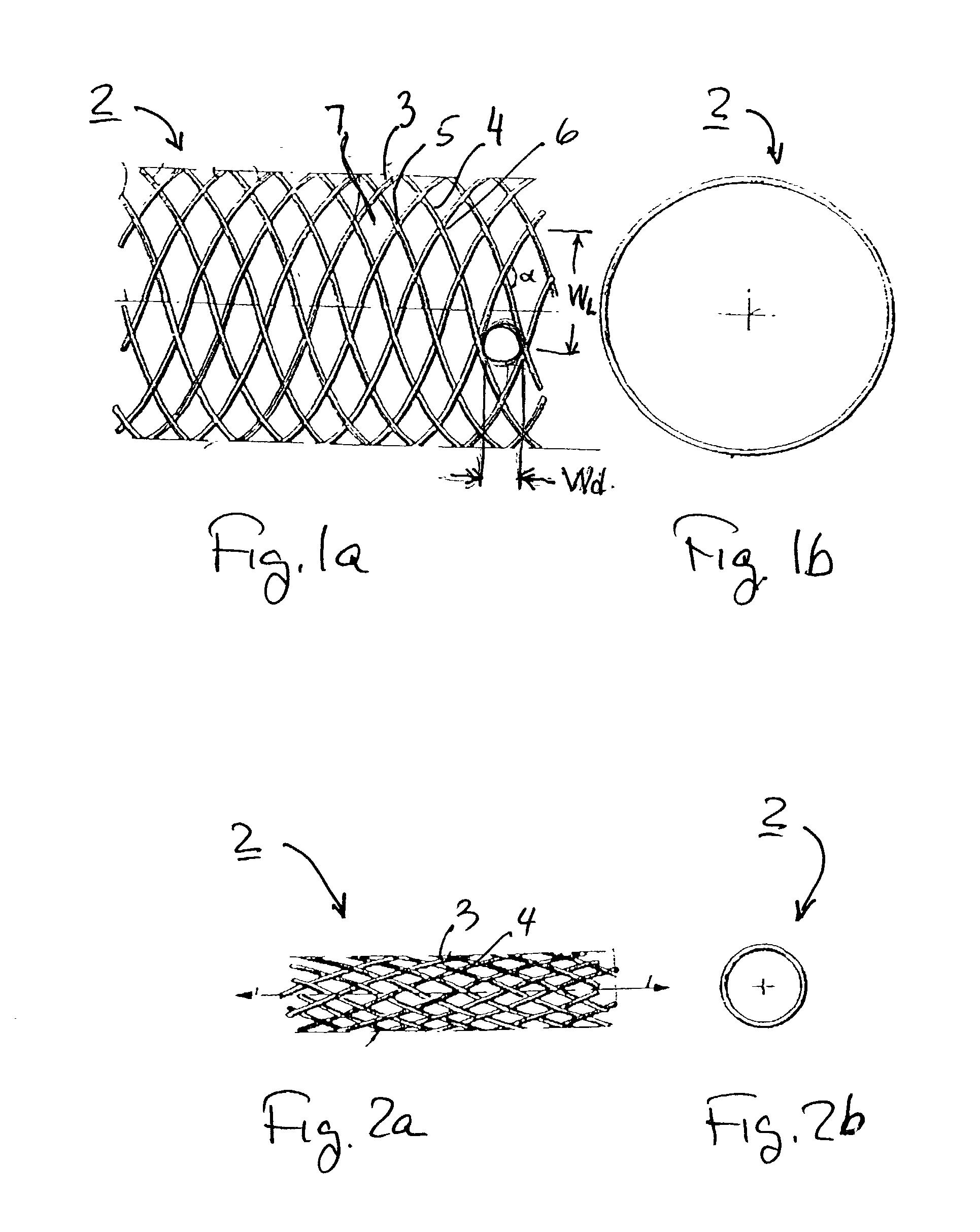
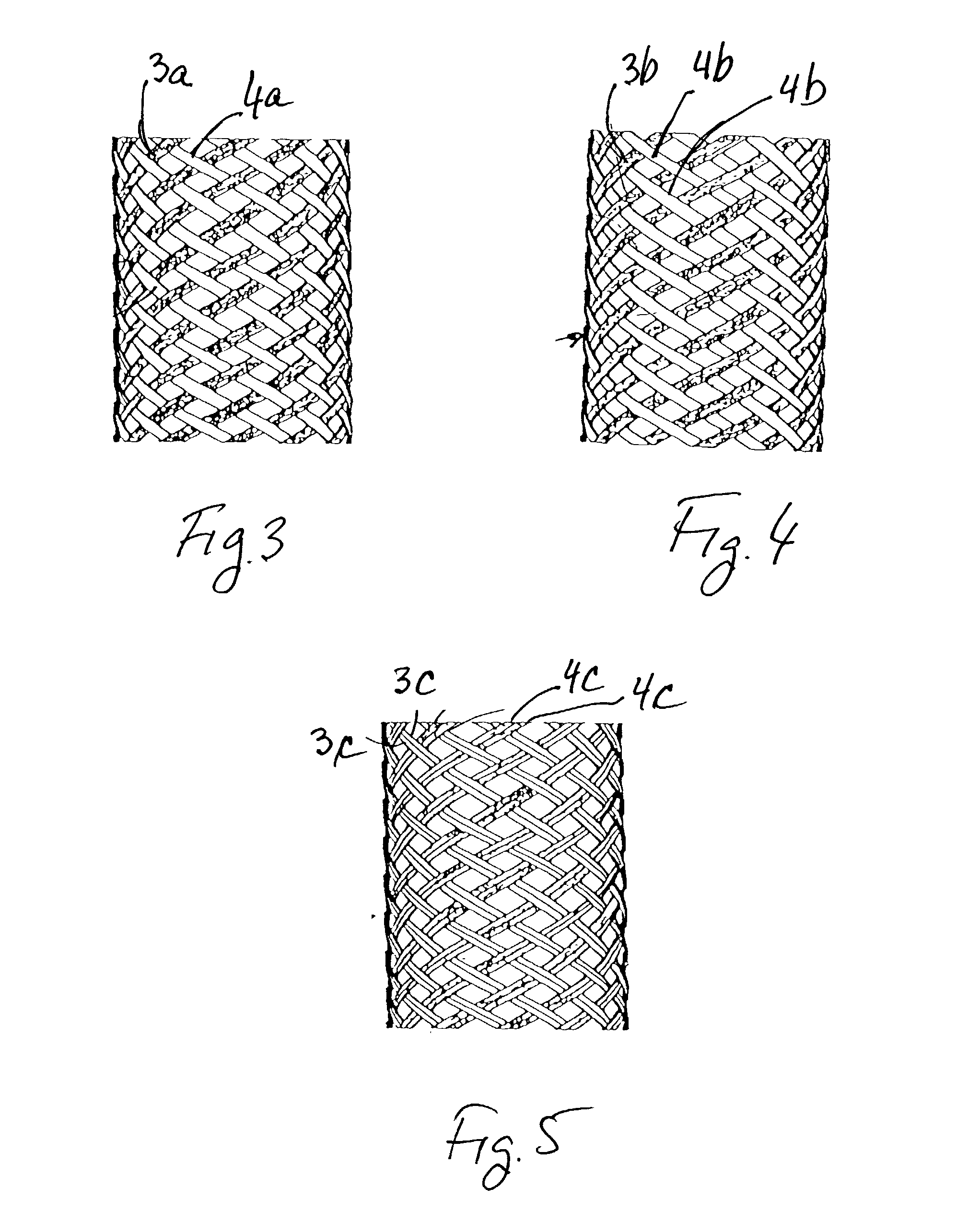
Stent vascular intervention device and methods for treating aneurysms
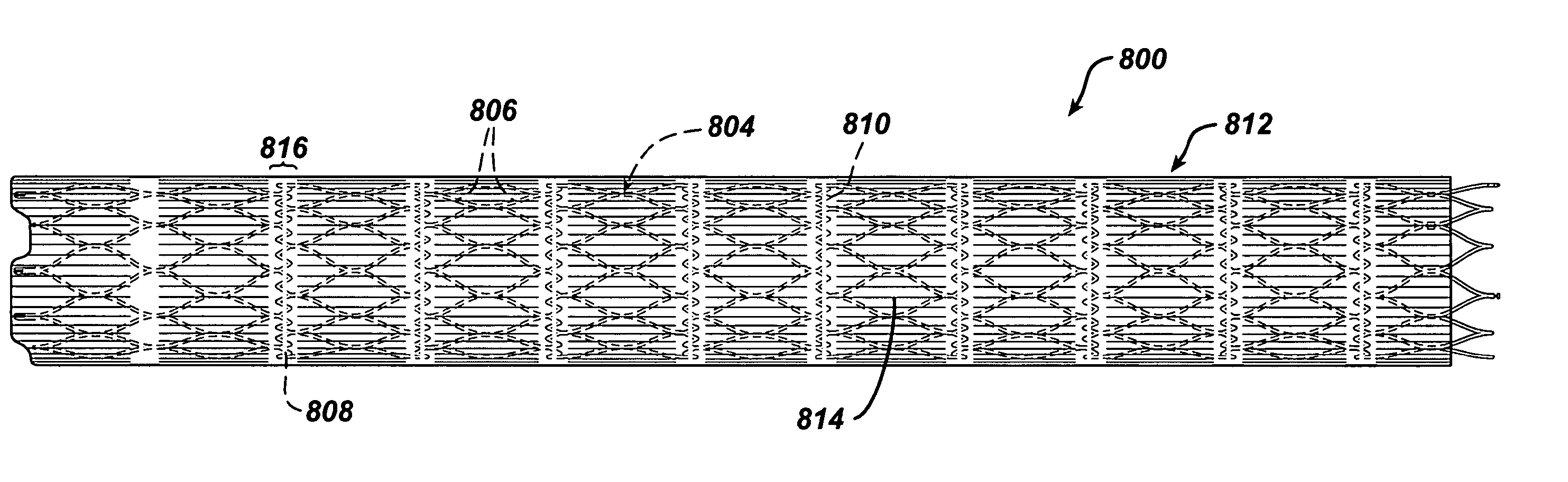
InactiveUS20070021816A1Shorten the construction periodGreat effect on aneurysmal blood flowStentsBlood vesselsPorosityInsertion stent
The present invention relates to a stent including a variable porosity, tubular structure having pores defined by structural surfaces. The tubular structure has a low porosity region in proximity to or at either end of the tubular structure, where the low porosity region is less porous than other regions located on the tubular structure and fully or partially obstructs passage of fluid. Any arcuate path that starts at one point within the low porosity region and goes around the perimeter of the tubular structure to stop at the same point within the low porosity region must have at least a portion that is outside of the low porosity region. Also disclosed is a method of modifying blood flow within and near an opening of an aneurysm in a blood vessel by deploying one or more stents of the present invention near an opening of the aneurysm in a blood vessel.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
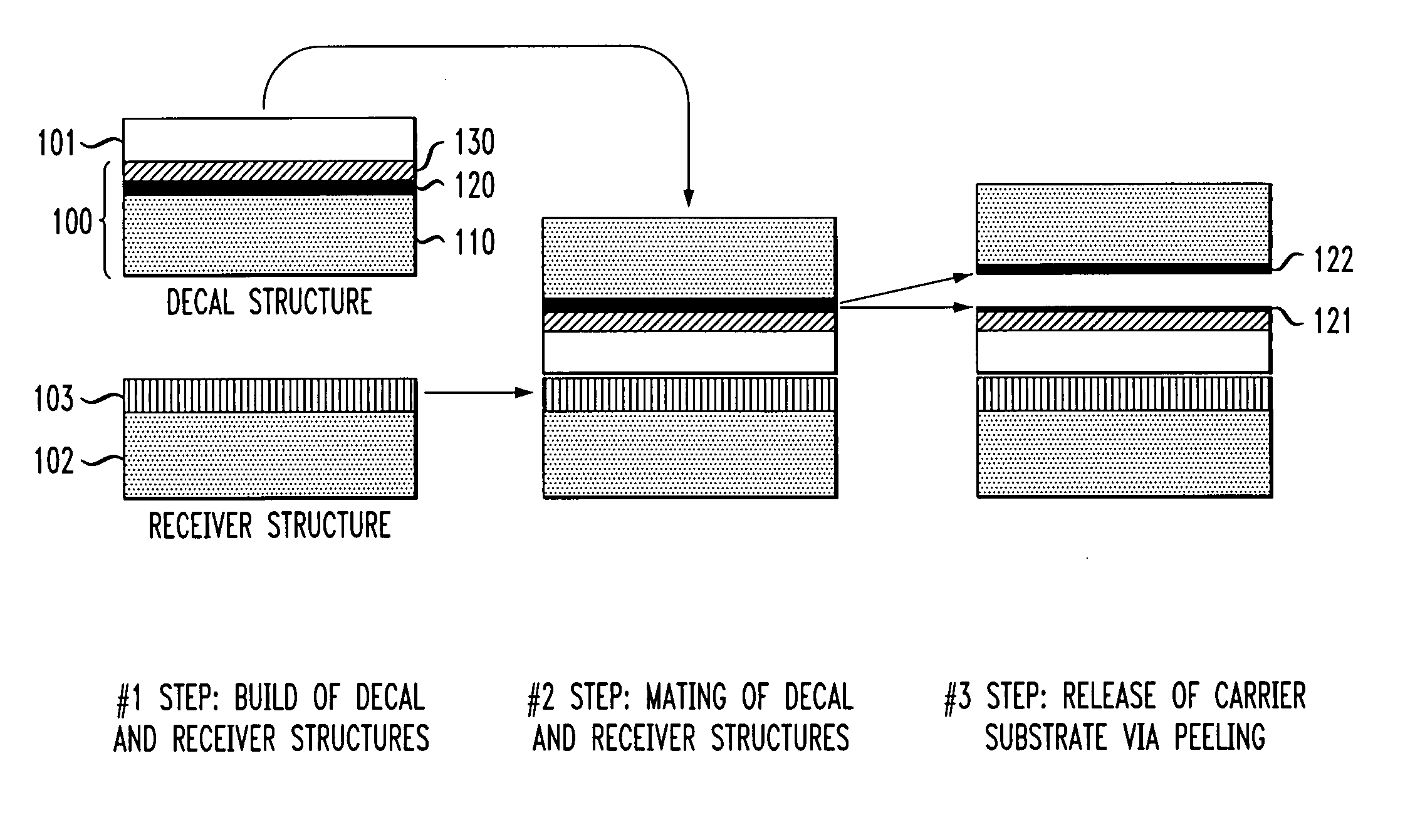
Techniques for layer transfer processing
InactiveUS20050082526A1Semiconductor/solid-state device manufacturingThin material handlingPorosityEngineering
Techniques for the fabrication of semiconductor devices are provided. In one aspect, a layer transfer structure is provided. The layer transfer structure comprises a carrier substrate having a porous region with a tuned porosity in combination with an implanted species defining a separation plane therein. In another aspect, a method of forming a layer transfer structure is provided. In yet another aspect, a method of forming a three dimensional integrated structure is provided.
Owner:GLOBALFOUNDRIES INC
Emboli diverting devices created by microfabricated means
A medical device for interposition between a first flow path and at least one second flow path is provided. The device includes a first surface facing toward the opening of at least one second flow path; and a second surface facing away from the opening of at least one second flow path. When the device is in the operative position, it extends less than the complete circumference of the first flow path and substantially covers the opening of at least one second flow path. The device contains one or more surface features to facilitate chronic implantation. The device further has one or more characteristic porosities. Different configurations are indicated depending on the pathophysiology being treated and dictate the characteristic porosity of the device. In some, cases blood is prevented from reaching the second flow path and in other cases, particulates traveling within the blood are prevented from reaching the second flow path. Methods of preventing emboli or blood flow into the second flow path are also provided. Methods and devices for delivery are also provided.
Owner:GERTNER MICHAEL
Ceramic material resistant to halogen plasma and member utilizing the same
InactiveUS6916559B2Improve the immunityRecord information storageLight beam reproducingPorosityHalogen
A member used within a plasma processing apparatus and exposed to a plasma of a halogen gas such as BCl3 or Cl2 is formed from a sintered body of metals of Group IIIa of Periodic Table such as Y, La, Ce, Nd and Dy, and Al and / or Si, for example, 3Y2O3.5Al2O3, 2Y2O3.Al2O3, Y2O3.Al2O3 or disilicate or monosilicate, and in particular, in this sintered body, the content of impurity metals of Group IIa of Periodic Table contained in the sintered body is controlled to be 0.15 wt % or more in total. Specifically, for this member, an yttrium-aluminum-garnet sintered body having a porosity of 3% or less and also having a surface roughness of 1 μm or less in center line average roughness Ra is utilized.
Owner:KYOCERA CORP
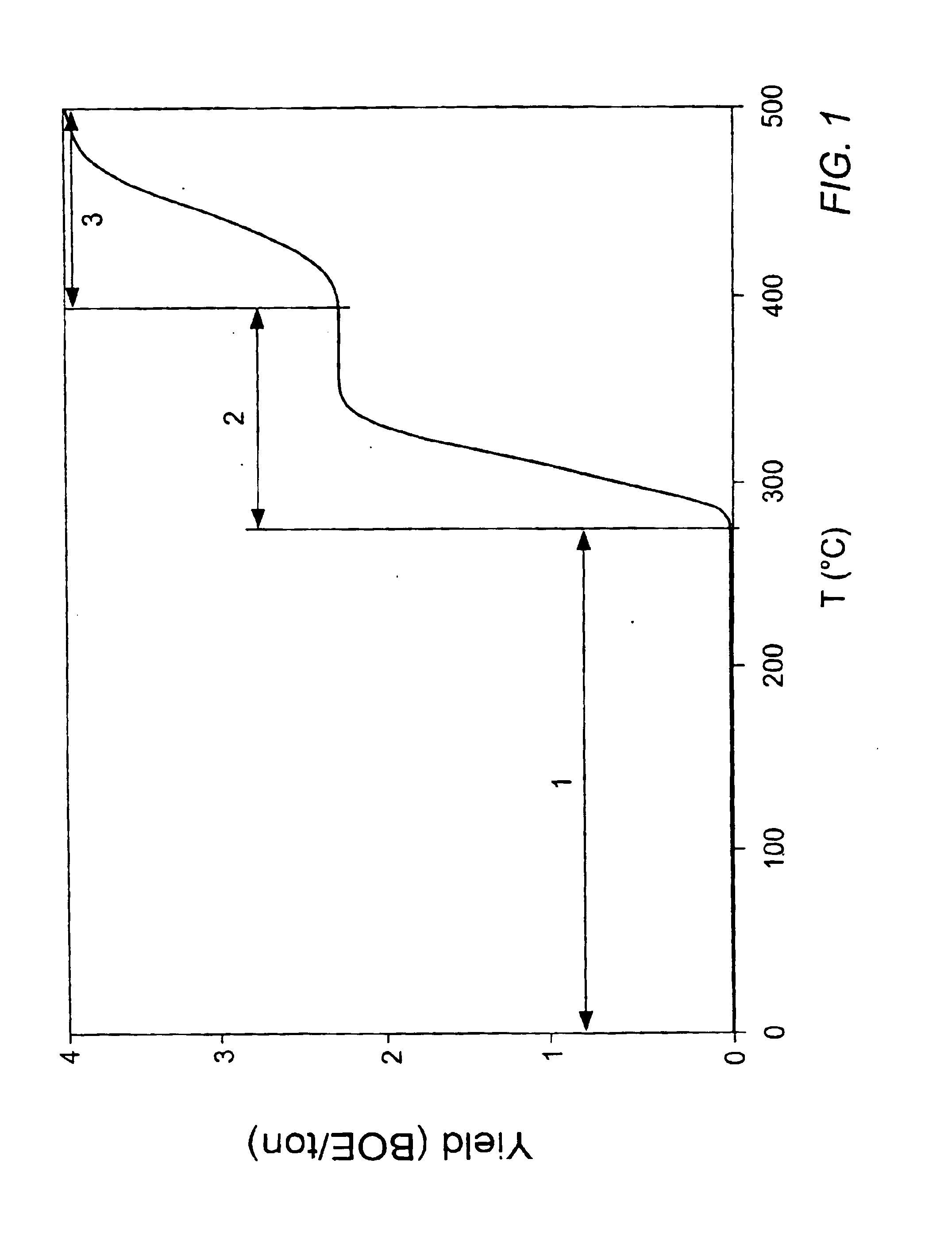
In situ thermal processing of a coal formation to increase a permeability/porosity of the formation
InactiveUS6866097B2Reduce the temperatureHigh strengthSurveyCombustion processPorosityInitial permeability
Owner:SHELL OIL CO
Implantable intraluminal device and method of using same in treating aneurysms
An intraluminal device implantable in a blood vessel having an aneurysm therein in the vicinity of a perforating vessel and / or of a bifurcation leading to a branch vessel. The intraluminal device includes a mesh-like tube of bio-compatible material having an expanded condition in which the tube diameter is slightly larger than the diameter of the blood vessel in which it is to be implanted, and the tube length is sufficient to straddle the aneurysm and to be anchored to the blood vessel on the opposite sides of the aneurysm. The mesh-like tube also has a contracted condition wherein it is sufficiently flexible so as to be easily manipulatable through the blood vessel to straddle the aneurysm. In its expanded condition, the mesh-like tube has a porosity index of 55%-80% such as to reduce the flow of blood through its wall to the aneurysm sufficiently to decrease the possibility of rupture of the aneurysm but not to unduly reduce the blood flow to a perforating or branch vessel to the degree likely to cause significant damage to tissues supplied with blood by such perforating or branch vessel.
Owner:STRYKER CORP
Radiolucent bone graft
An improved bone graft is provided for human implantation, bone graft includes a substrate block of high strength biocompatible material having a selected size and shape to fit the anatomical space, and a controlled porosity analogous to natural bone. The substrate block may be coated with a bio-active surface coating material such as hydroxyapatite or a calcium phosphate to promote bone ingrowth and enhanced bone fusion. Upon implantation, the bone graft provides a spacer element having a desired combination of mechanical strength together with osteoconductivity and osteoinductivity to promote bone ingrowth and fusion, as well as radiolucency for facilitated post-operative monitoring. The bone graft may additionally carry one or more natural or synthetic therapeutic agents for further promoting bone ingrowth and fusion.
Owner:AMEDICA A DELAWARE
Lithium ion conductive solid electrolyte and production process thereof
InactiveUS20070231704A1Increase battery capacitySimple and convenient manufactureSecondary cellsSolid electrolyte cellsPorosityLithium metal
A lithium ion conductive solid electrolyte formed by sintering a molding product containing an inorganic powder and having a porosity of 10 vol % or less, which is obtained by preparing a molding product comprising an inorganic powder as a main ingredient and sintering the molding product after pressing and / or sintering the same while pressing, the lithium ion conductive solid electrolyte providing a solid electrolyte having high battery capacity without using a liquid electrolyte, usable stably for a long time and simple and convenient in manufacture and handling also in industrial manufacture in the application use of secondary lithium ion battery or primary lithium battery, a solid electrolyte having good charge / discharge cyclic characteristic in the application use of the secondary lithium ion battery a solid electrolyte with less water permeation and being safe when used for lithium metal-air battery in the application use of primary lithium battery, a manufacturing method of the solid electrolyte, and a secondary lithium ion battery and a primary lithium battery using the solid electrolyte.
Owner:OHARA
Silk fibroin materials and use thereof
ActiveUS7842780B2Low densityIncreased proliferationPeptide/protein ingredientsMonocomponent fibroin artificial filamentPorosityBiology
The present invention provides processes for producing porous silk fibroin scaffold material. The porous silk fibroin scaffold can be used for tissue engineering. The porosity of the silk fibroin scaffolds described herein can be adjusted as to mimic the gradient of densities found in natural tissue. Accordingly, methods for engineering of 3-dimensional tissue, e.g. bone and cartilage, using the silk fibroin scaffold material are also provided.
Owner:TUFTS UNIV +1
Endovascular graft with differentiable porosity along its length
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device such as a stent-graft. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. A stent-graft fabricated from a thin-walled, high strength material provides for a more durable and lower profile endoprosthesis. The stent-graft comprises one or more stent segments covered with a fabric formed by the weaving, knitting or braiding of a biocompatible, high tensile strength, abrasion resistant, highly durable yarn such as ultra high molecular weight polyethylene. The one or more stent segments may be balloon expandable or self-expanding. The fabric may be attached to the stent segments utilizing any number of known materials and techniques. In addition, the pore size of the graft material may be varied.
Owner:CORDIS CORP
Reinforced, laminated, impregnated, and composite-like materials as crosslinked polyvinyl alcohol hydrogel structures
InactiveUS6855743B1High mechanical strengthIncrease modulusFibre treatmentSynthetic resin layered productsPorosityCross-link
Reinforced, laminated, impregnated, and materials with composite properties as cross linked polyvinyl alcohol hydrogel structures in bulk or cellular matrix forms that can take essentially any physical shape, or can have essentially any size, degree of porosity and surface texture. They have a wide range of physical properties, unusual and unique combinations of physical properties and unique responses to stress fields, which allows for their use in many end use applications.
Owner:HYDROMEDICA
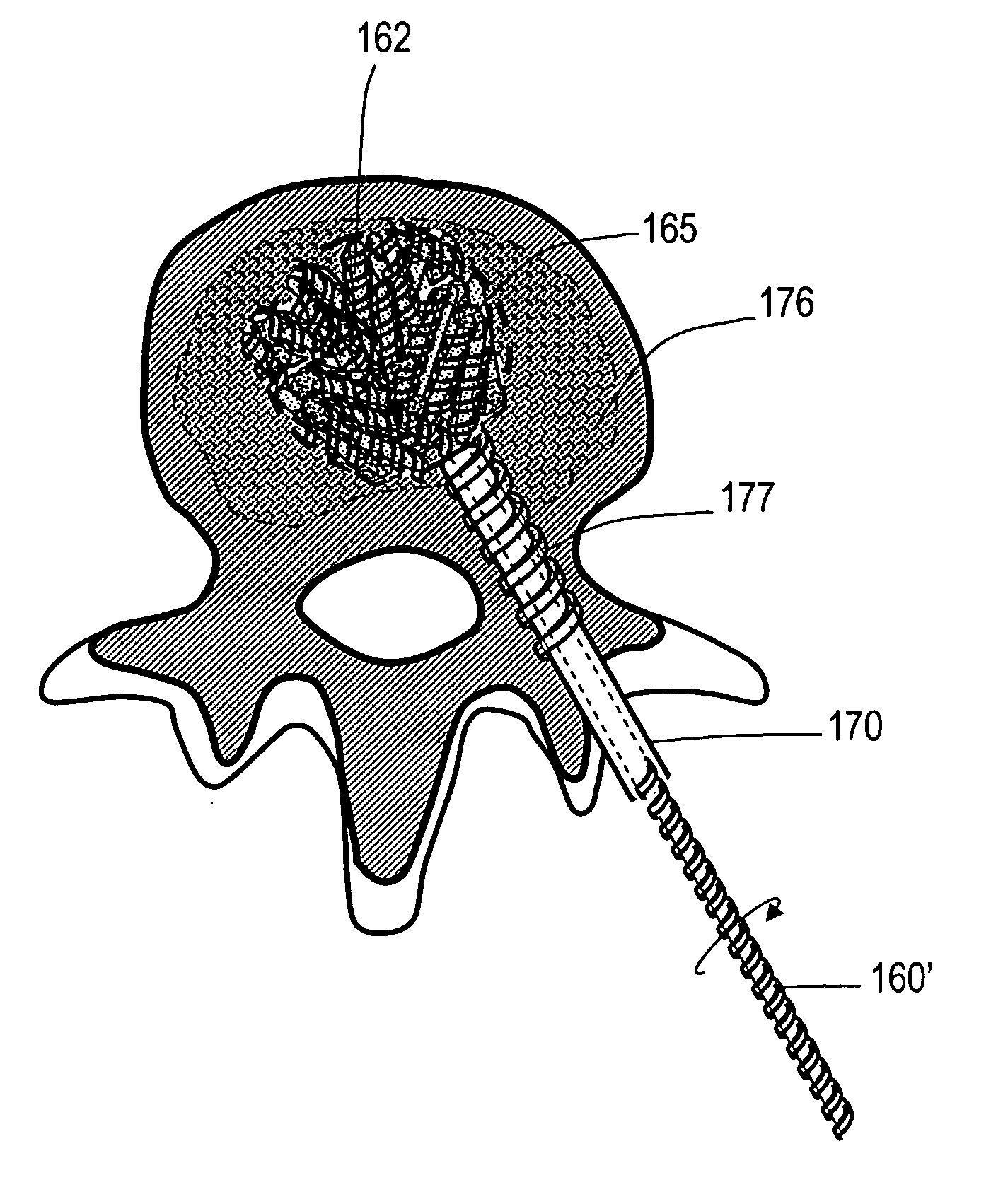
Implants and methods for treating bone
This invention relates to biomedical implants for filling, supporting or treating bone. In one embodiment, the implant comprises an electrospun polymer scaffold that is thereafter plated with a metal to provide a selected high modulus. Such an implant can be fabricated with a selected porosity for tissue ingrowth. The implant can be further provided with a varied modulus along the length of the implant body for inducing bending of the implant for packing in a bone. In another embodiment, the implant is fabricated in an elongated configuration for introducing into bone to treat a vertebral fracture. In another embodiment, the implant can be configured with helical threads for helically driving the implant into a bone.
Owner:DFINE INC
Composite self-cohered web materials
ActiveUS20070026031A1High porositySuitable for useWoven fabricsThin material handlingPorosityEngineering
The present invention is directed to implantable bioabsorbable non-woven self-cohered web materials having a high degree of porosity. The web materials are very supple and soft, while exhibiting proportionally increased mechanical strength in one or more directions. The web materials often possess a high degree of loft. The web materials can be formed into a variety of shapes and forms suitable for use as implantable medical devices or components thereof.
Owner:WL GORE & ASSOC INC
Bone fixation element
Owner:DEPUY SYNTHES PROD INC
Battery of electrochemical generators comprising a foam as inter-generator filler material
InactiveUS20120003508A1Improve securityFinal product manufactureSmall-sized cells cases/jacketsPorosityLithium
A battery of lithium electrochemical generators including a casing; a plurality of lithium electrochemical generators housed in the casing, each generator including a container; a rigid, flame-retardant foam with closed porosity formed of an electrically insulating material filling the space between the inner wall of the casing and the free surface of the side wall of the container of each electrochemical generator, the foam covering the free surface of the side wall of the container of each electrochemical generator over a length representing at least 25% of the height of the container.
Owner:SAFT GRP SA
Heat and corrosion resistant cast CF8C stainless steel with improved high temperature strength and ductility
A CF8C type stainless steel alloy and articles formed therefrom containing about 18.0 weight percent to about 22.0 weight percent chromium and 11.0 weight percent to about 14.0 weight percent nickel; from about 0.05 weight percent to about 0.15 weight percent carbon; from about 2.0 weight percent to about 10.0 weight percent manganese; and from about 0.3 weight percent to about 1.5 weight percent niobium. The present alloys further include less than 0.15 weight percent sulfur which provides high temperature strength both in the matrix and at the grain boundaries without reducing ductility due to cracking along boundaries with continuous or nearly-continuous carbides. The disclosed alloys also have increased nitrogen solubility thereby enhancing strength at all temperatures because nitride precipitates or nitrogen porosity during casting are not observed. The solubility of nitrogen is dramatically enhanced by the presence of manganese, which also retains or improves the solubility of carbon thereby providing additional solid solution strengthening due to the presence of manganese and nitrogen, and combined carbon.
Owner:UT BATTELLE LLC
Expandable fluoropolymer device for delivery of therapeutic agents and method of making
A radially expandable fluid delivery device for delivering a fluid to a treatment site within the body is disclosed. The fluid delivery device is constructed of a microporous, biocompatible fluoropolymer material having a microstructure that can provide a controlled, uniform, low-velocity fluid distribution through the walls of the fluid delivery device to effectively deliver fluid to the treatment site without damaging tissue proximate the walls of the device. The fluid delivery device includes a tubular member defined by a wall having a thickness transverse to the longitudinal axis of the tubular member and extending between an inner and an outer surface. The wall is characterized by a microstructure of nodes interconnected by fibrils. The tubular member is deployable from a first, reduced diameter configuration to a second, increased diameter configuration upon the introduction of a pressurized fluid to the lumen. The tubular member includes at least one microporous portion having a porosity sufficient for the pressurized fluid to permeate through the wall. Substantially all of the nodes within the microporous portion are oriented such that spaces between the nodes form micro-channels extending from the inner surface-to the outer surface of the wall.
Owner:ATRIUM MEDICAL
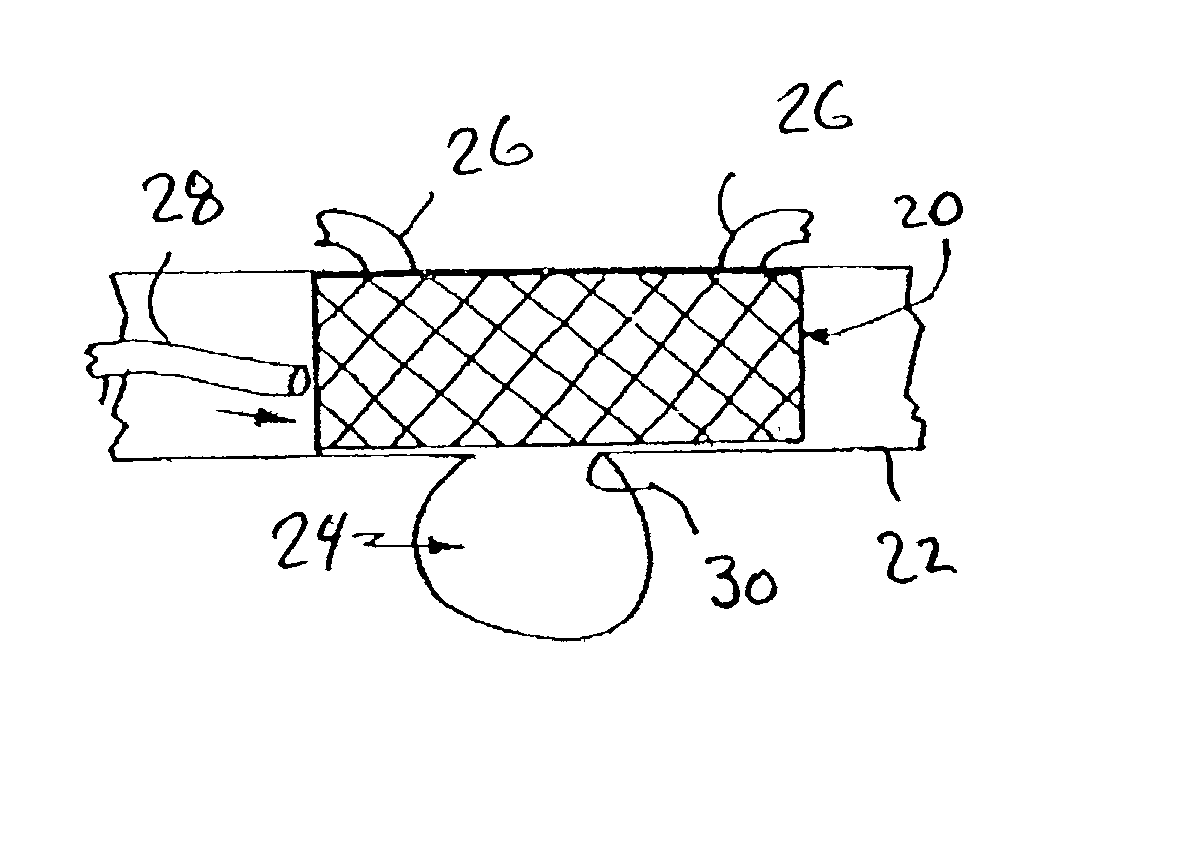
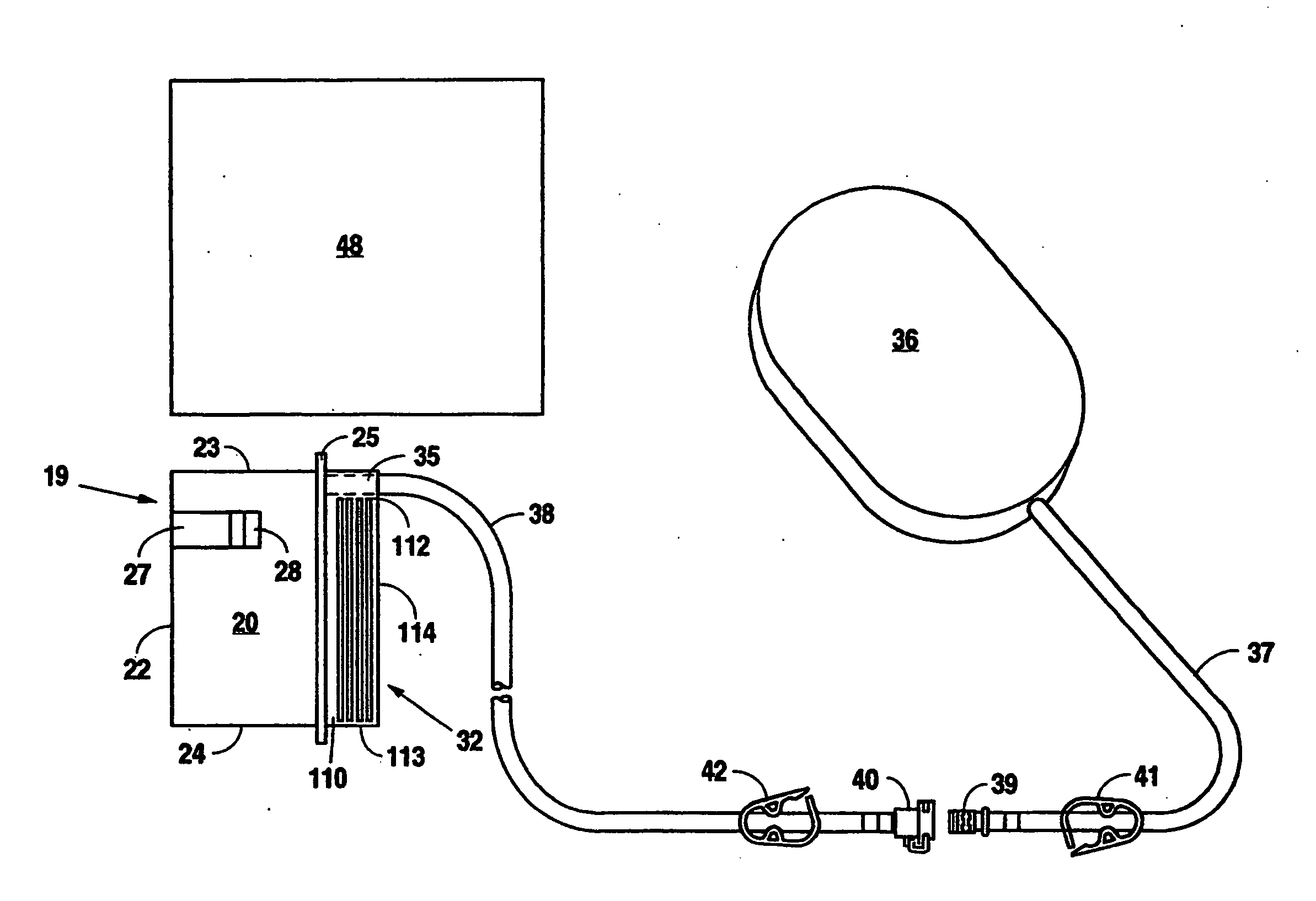
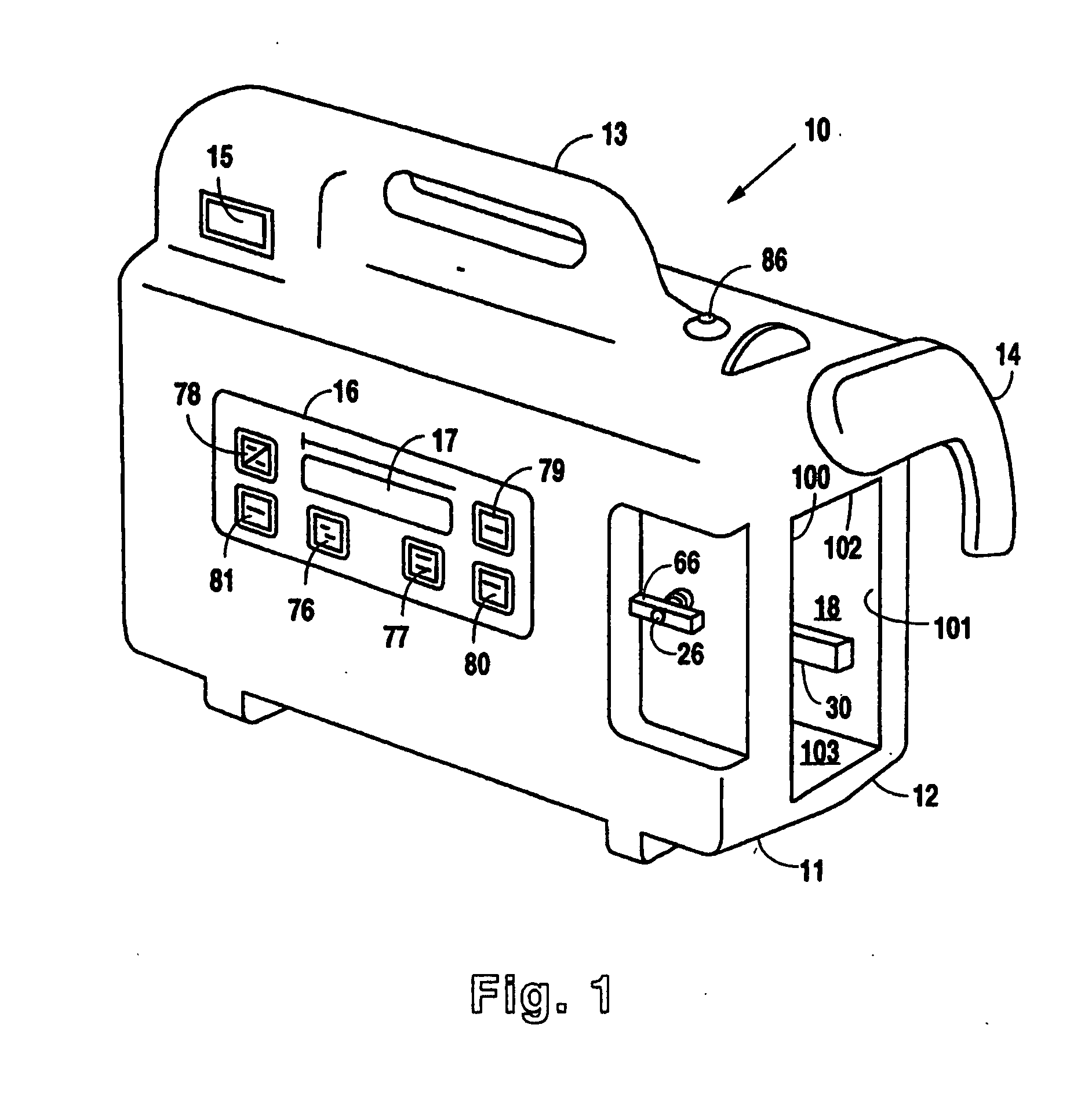
Reduced pressure treatment system having a dual porosity pad
A wound closure apparatus having a porous pad adapted to be fluidly connected to a vacuum pump. The pad is placed over or within a tissue site, and the vacuum pump may be activated to draw air and exudates from the tissue site. The pad contains multiple pore sizes to prevent granulation tissue from migrating into the pad as reduced pressure treatment is applied. The pad has an outer surface adjacent the wound with pore sizes of a diameter of approximately 100 microns or less to prevent tissue from growing into the pad and is treated for biocompatibility.
Owner:KCI LICENSING INC
Cordierite ceramic body and method
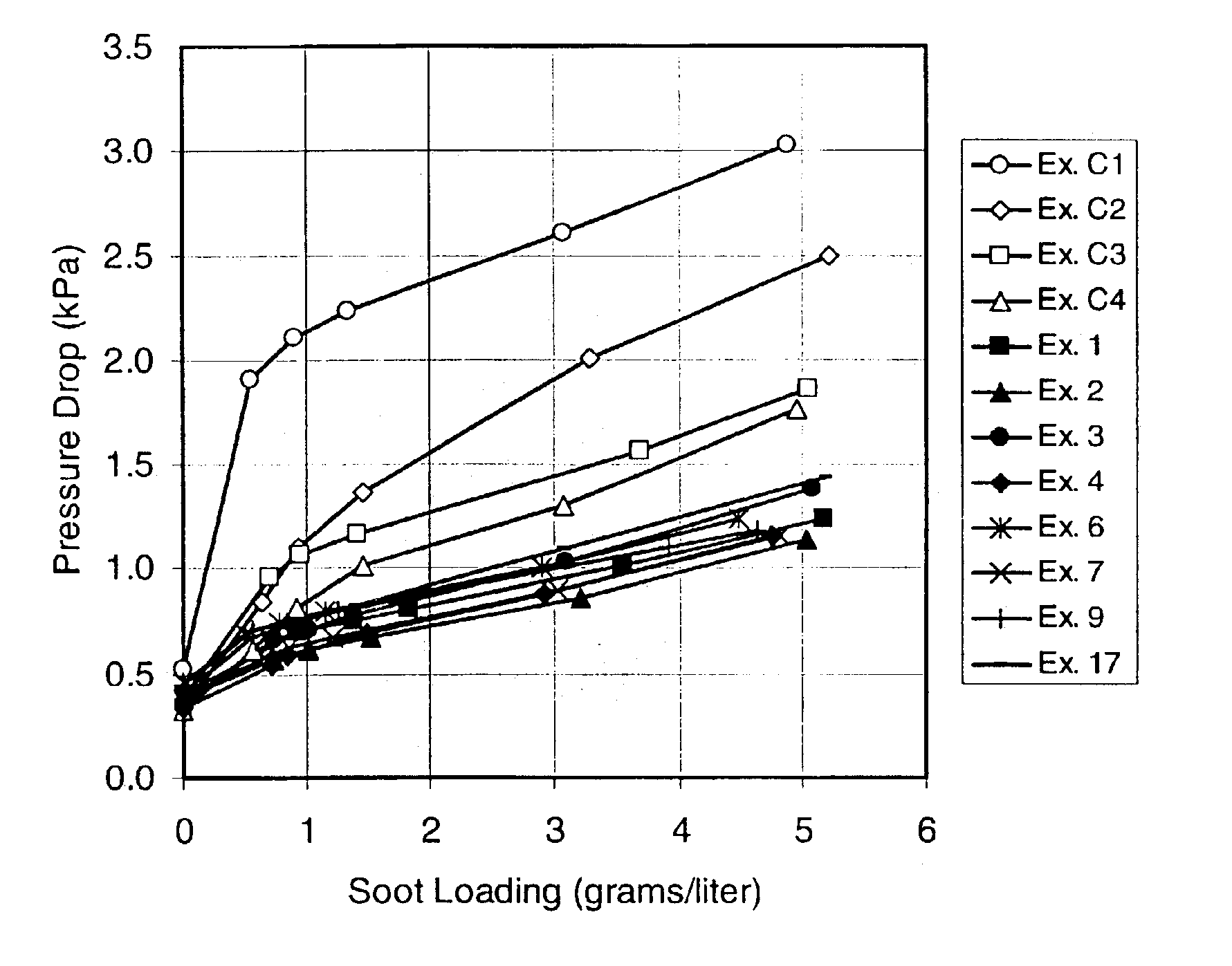
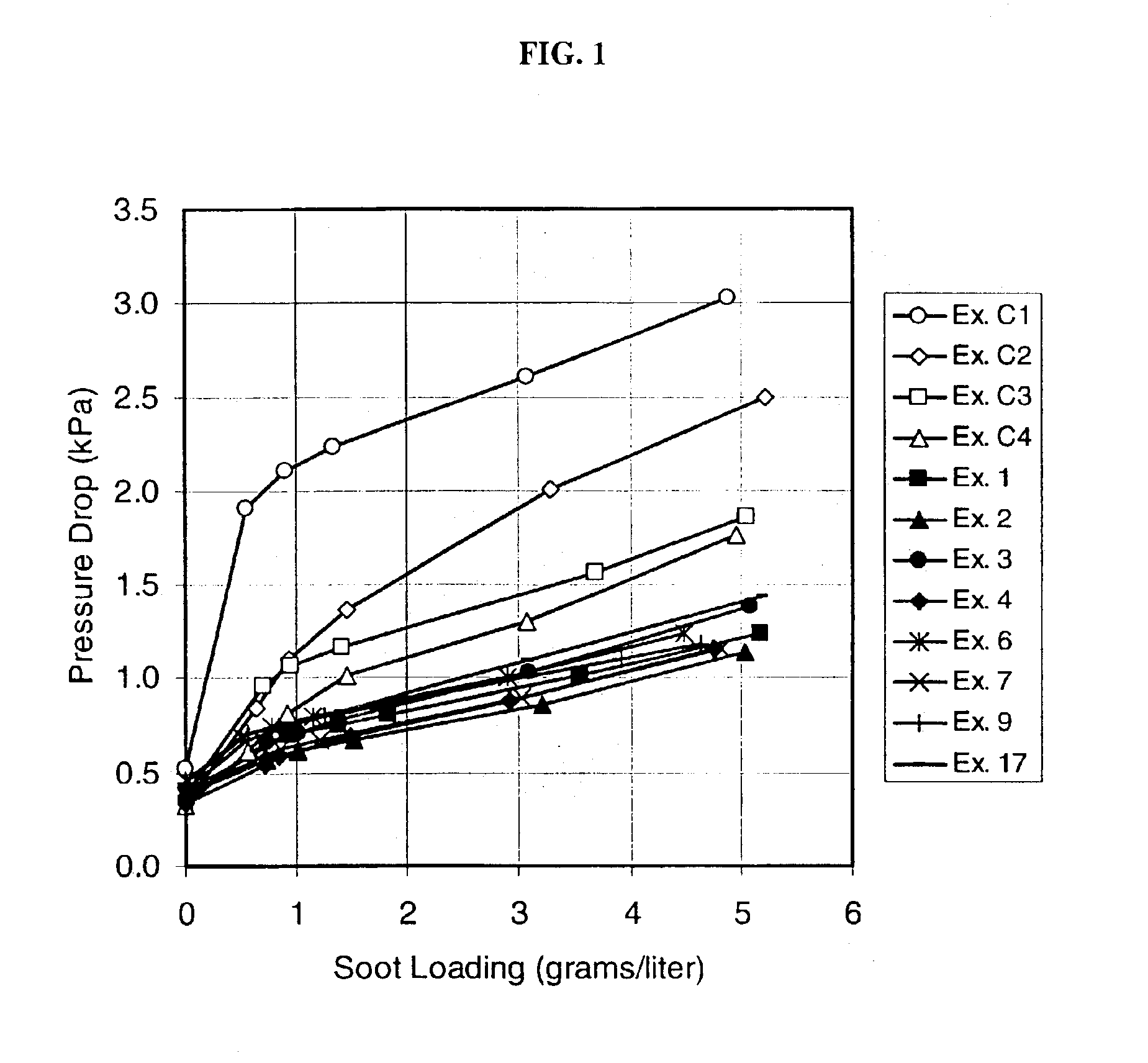
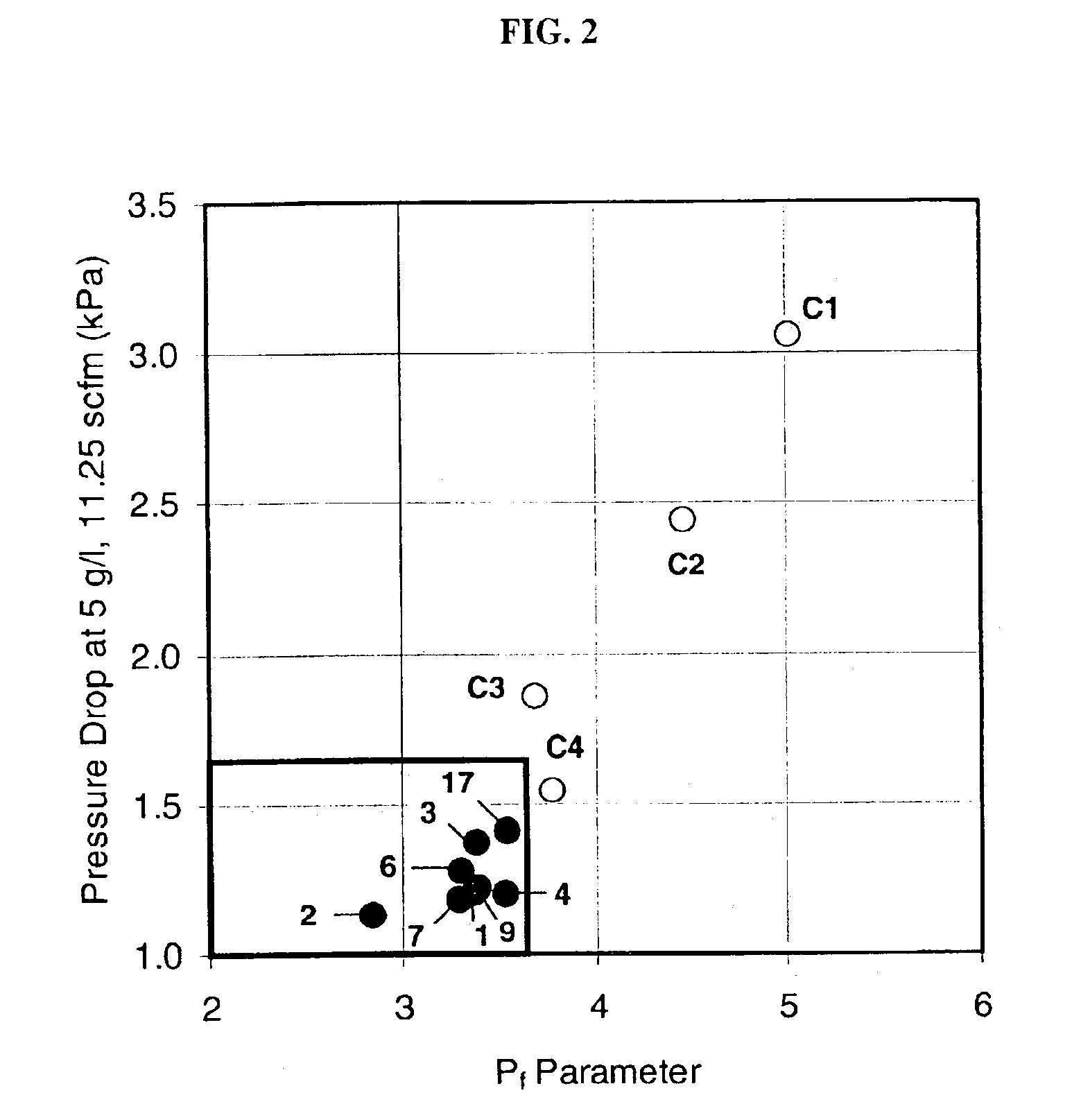
InactiveUS6864198B2Lower overall pressure dropHigh filtration efficiencyDispersed particle filtrationTransportation and packagingPorosityFiltration
Owner:CORNING INC
Cathode for lithium battery
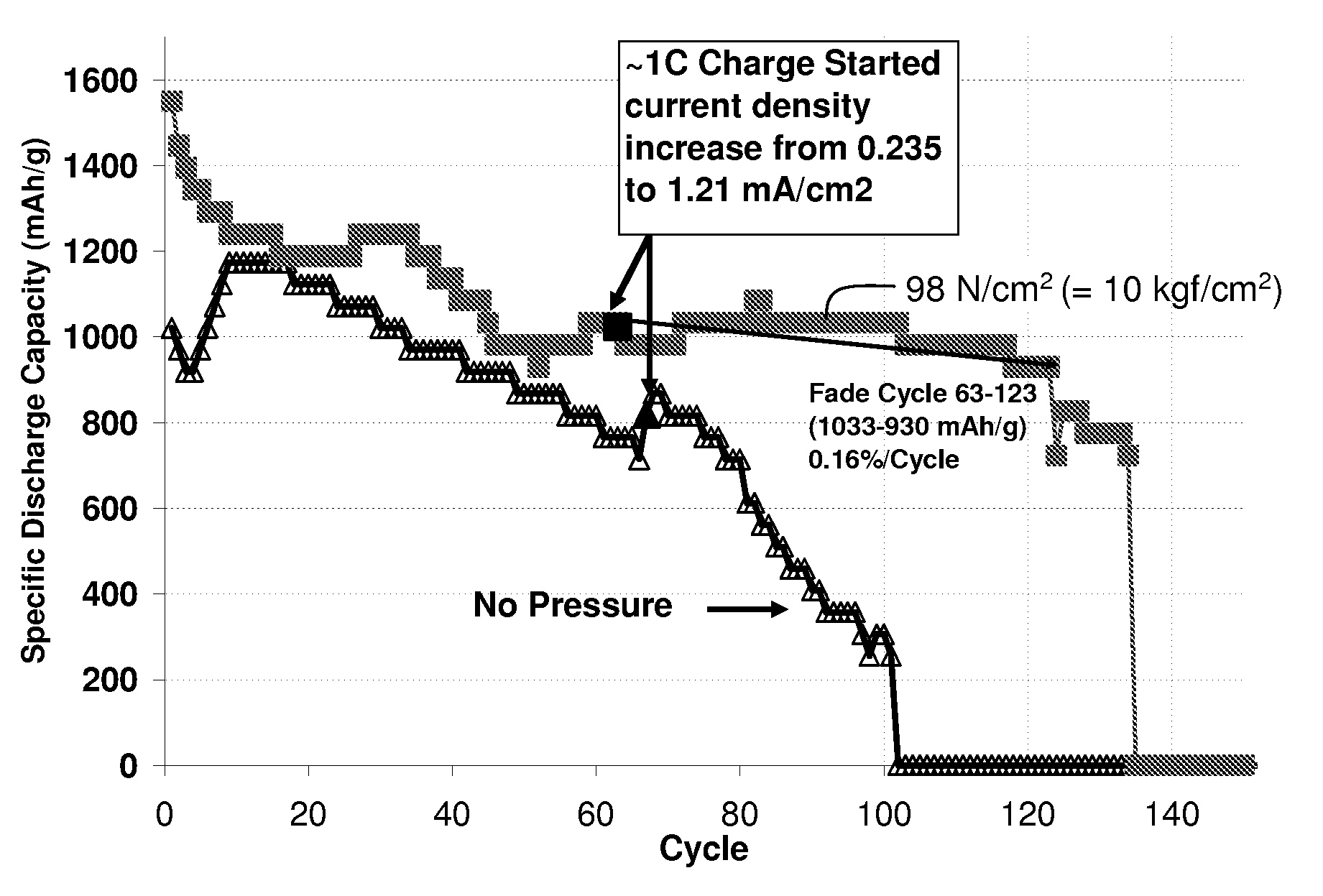
The present invention relates to cathodes used in electrochemical cells. A force, or forces, applied to portions of an electrochemical cell as described in this application can reduce irregularity or roughening of an electrode surface of the cell, improving performance. The cathodes described herein may possess enhanced properties that render them particularly suitable for use in electrochemical cells designed to be charged and / or discharged while a force is applied. In some embodiments, the cathode retains sufficient porosity to charge and discharge effectively when a force is applied to the cell. Cathodes described herein may also comprise relatively high electrolyte-accessible conductive material (e.g., carbon) areas. The cathode may comprise a relatively low ratio of the amount of binder and / or mass of electrolyte to cathode active material (e.g., sulfur) ratio in some instances. In some embodiments, electrochemical cells comprising the cathodes described herein may achieve relatively high specific capacities and / or relatively high discharge current densities. In addition, the cathode described herein may exhibit relatively high cathode active material (e.g., sulfur) utilization during charge and discharge. In still further cases, the electrical conductivity between conductive material in the cathode (e.g., carbon) may be enhanced during the application of the force.
Owner:SION POWER CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com