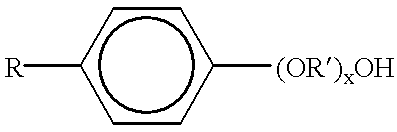
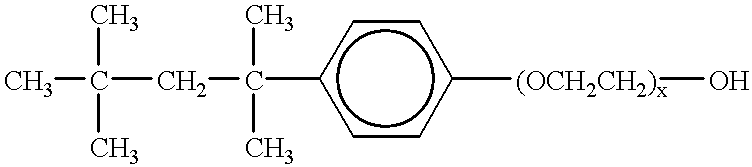
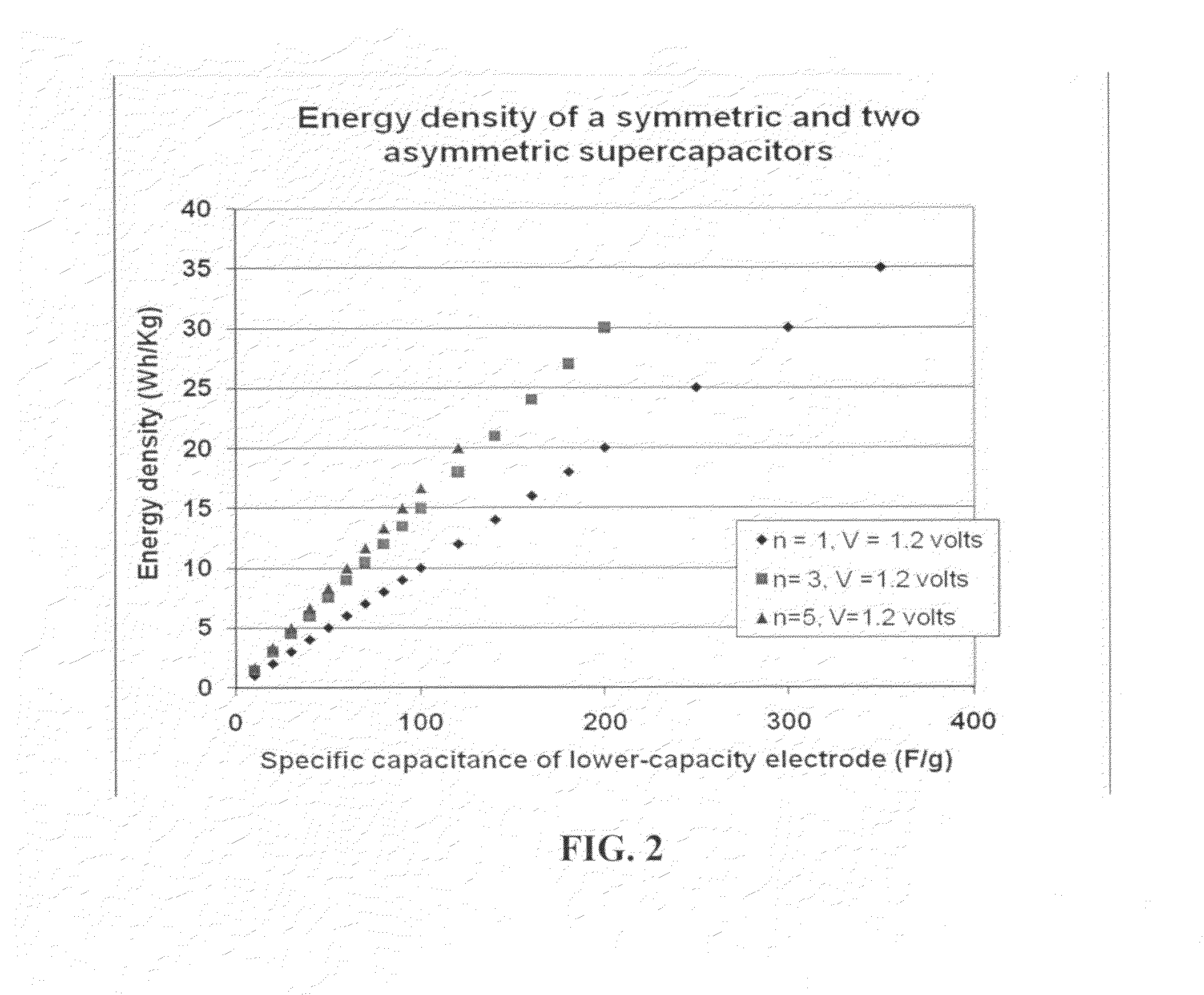
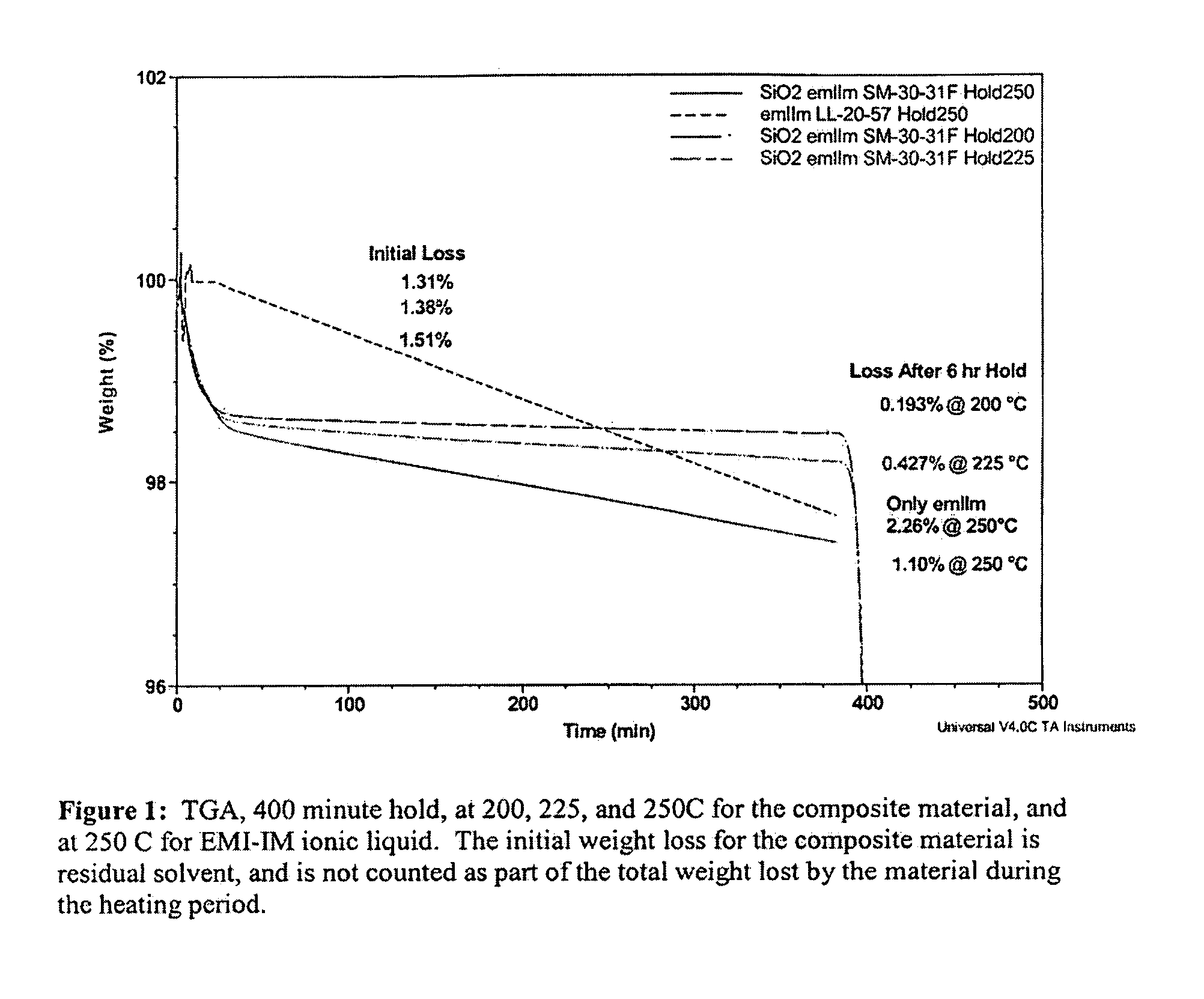
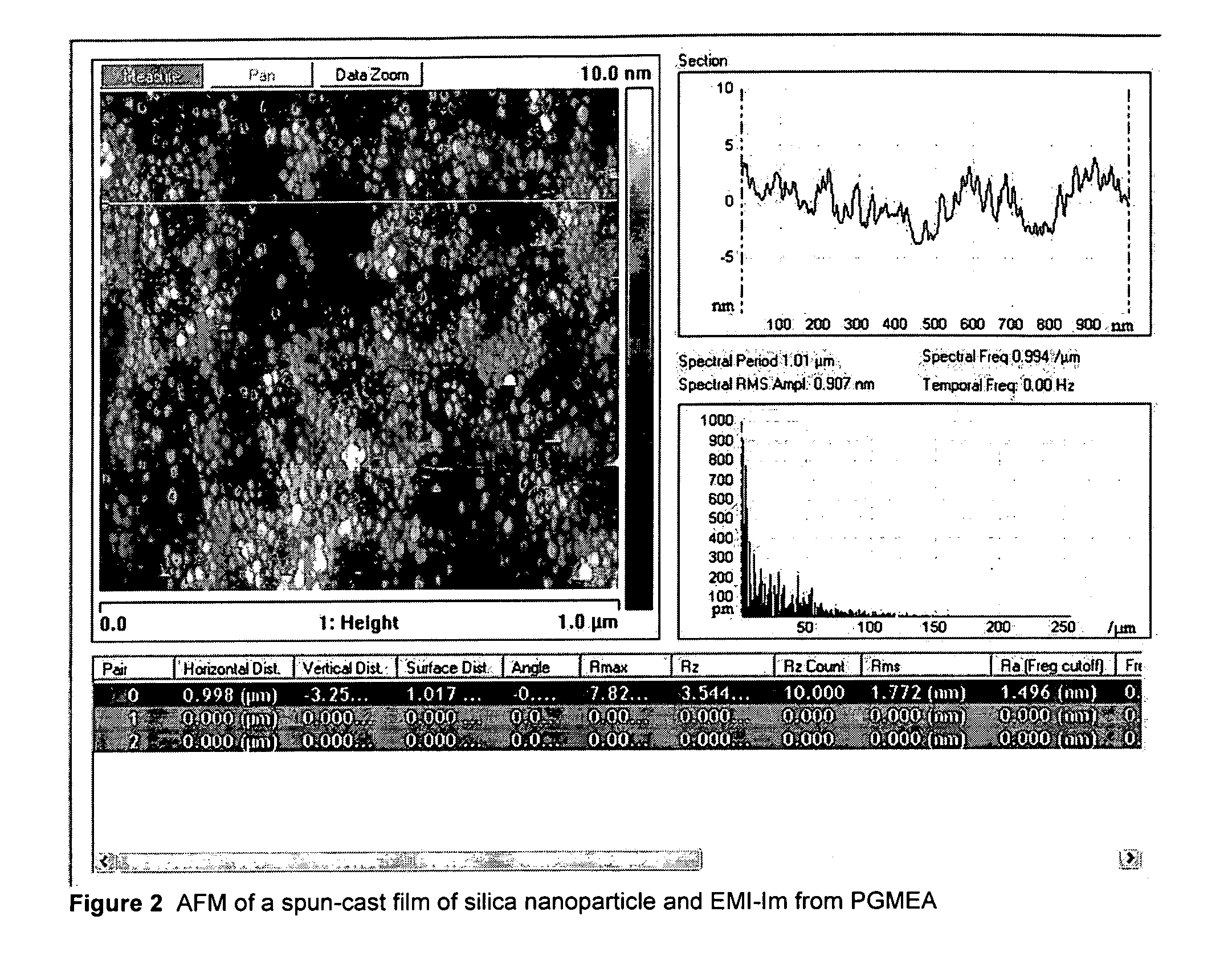
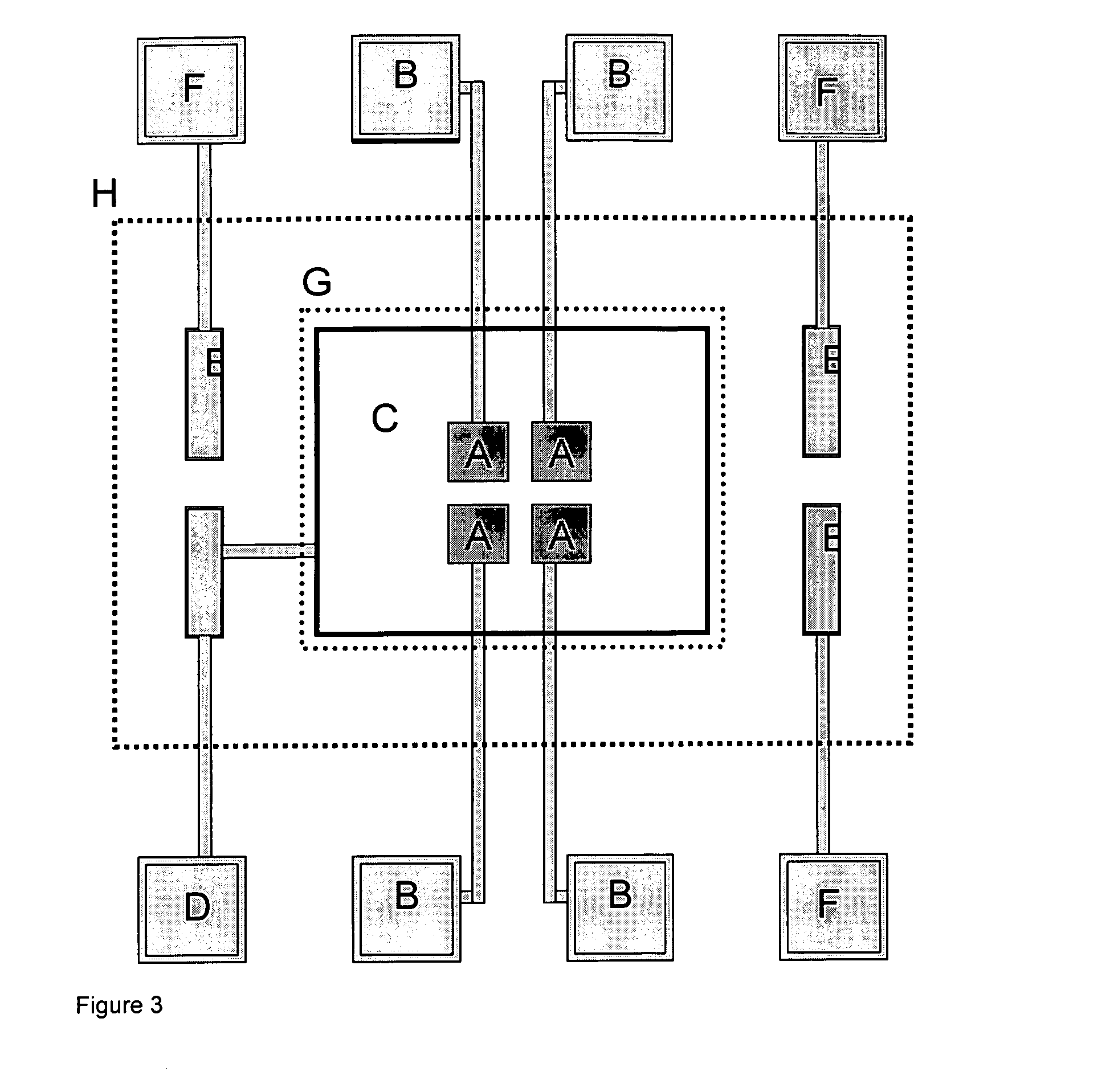
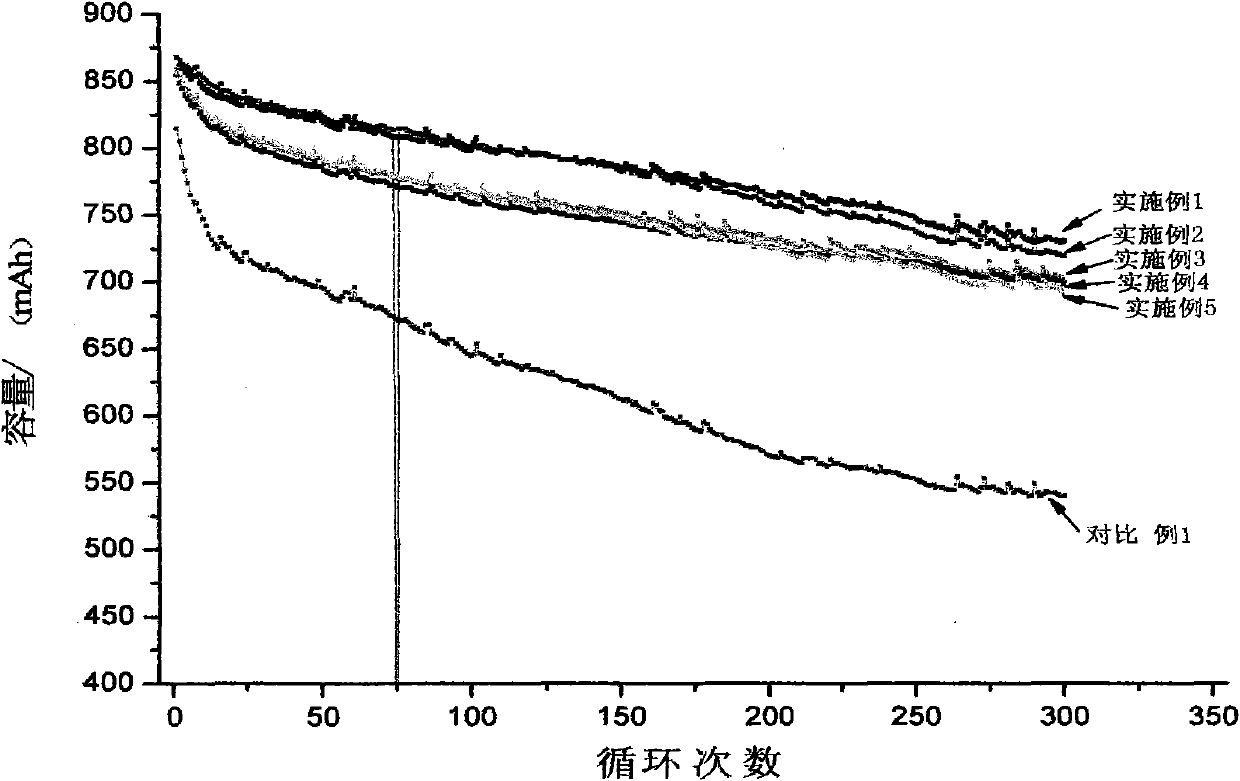
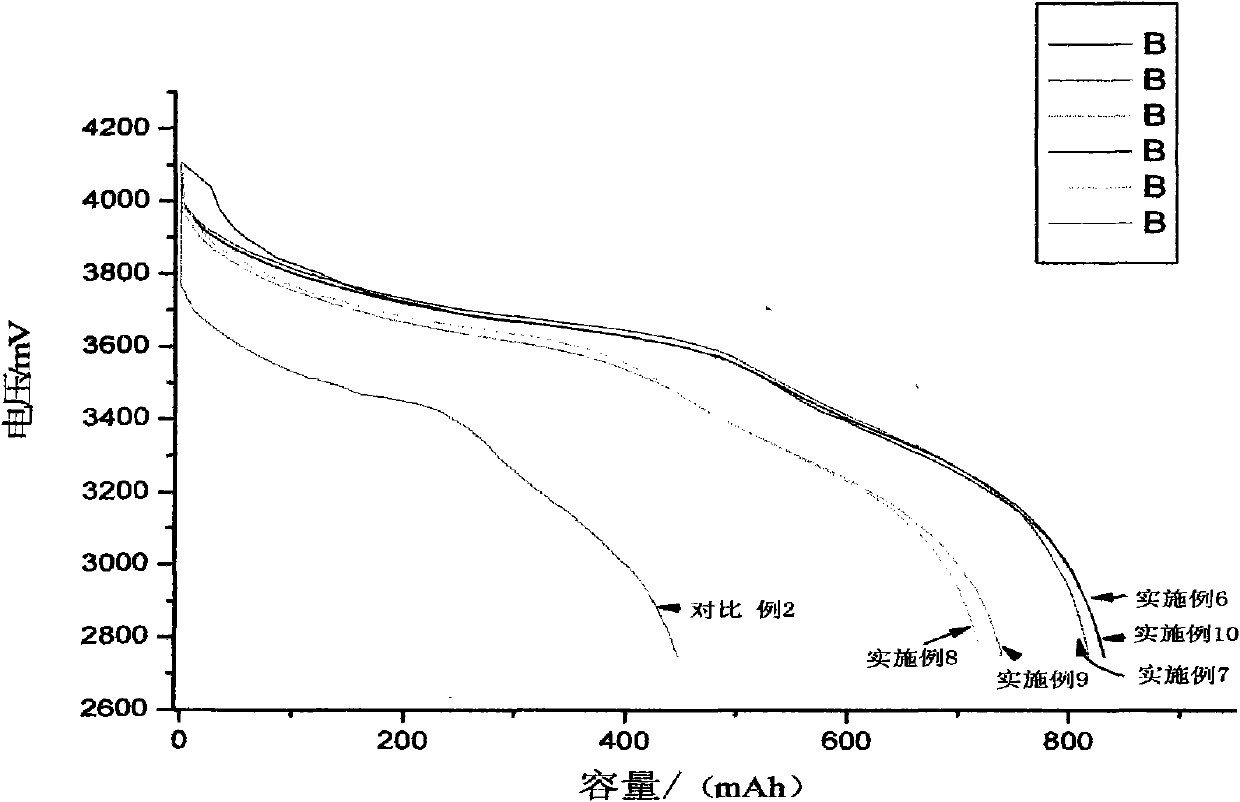
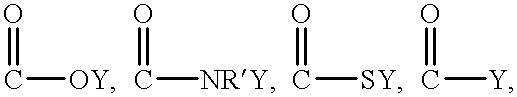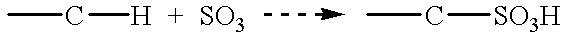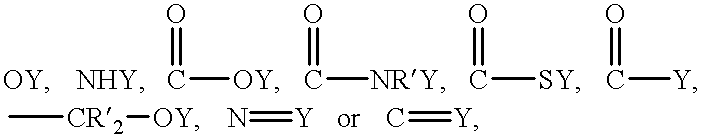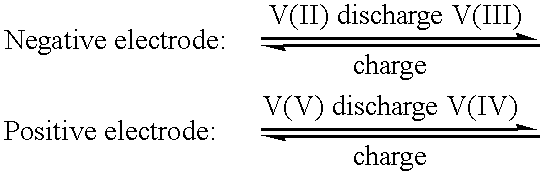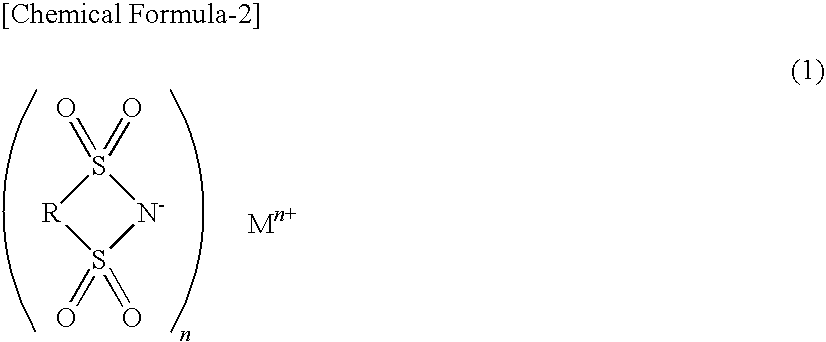Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
65195results about "Cell electrodes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
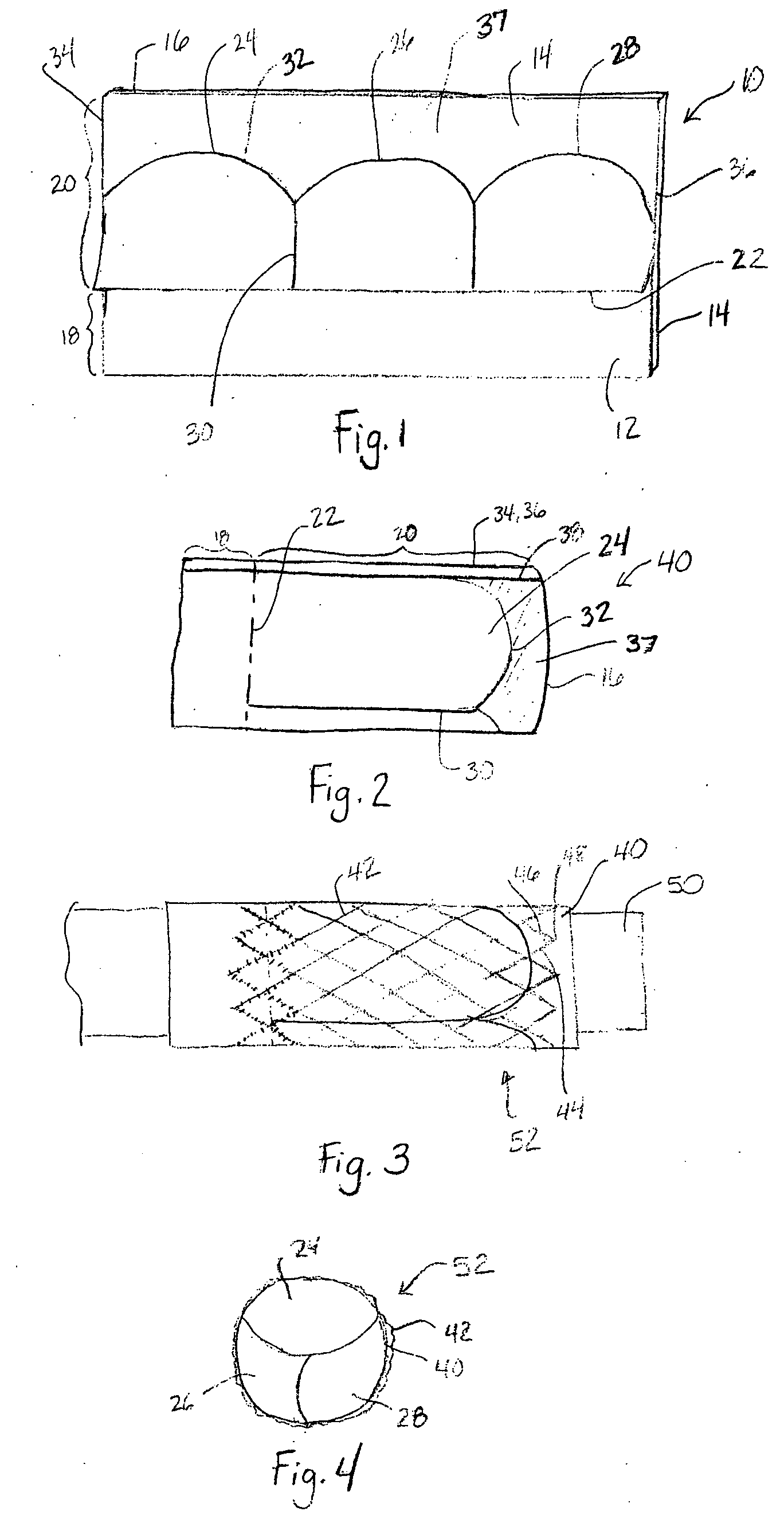
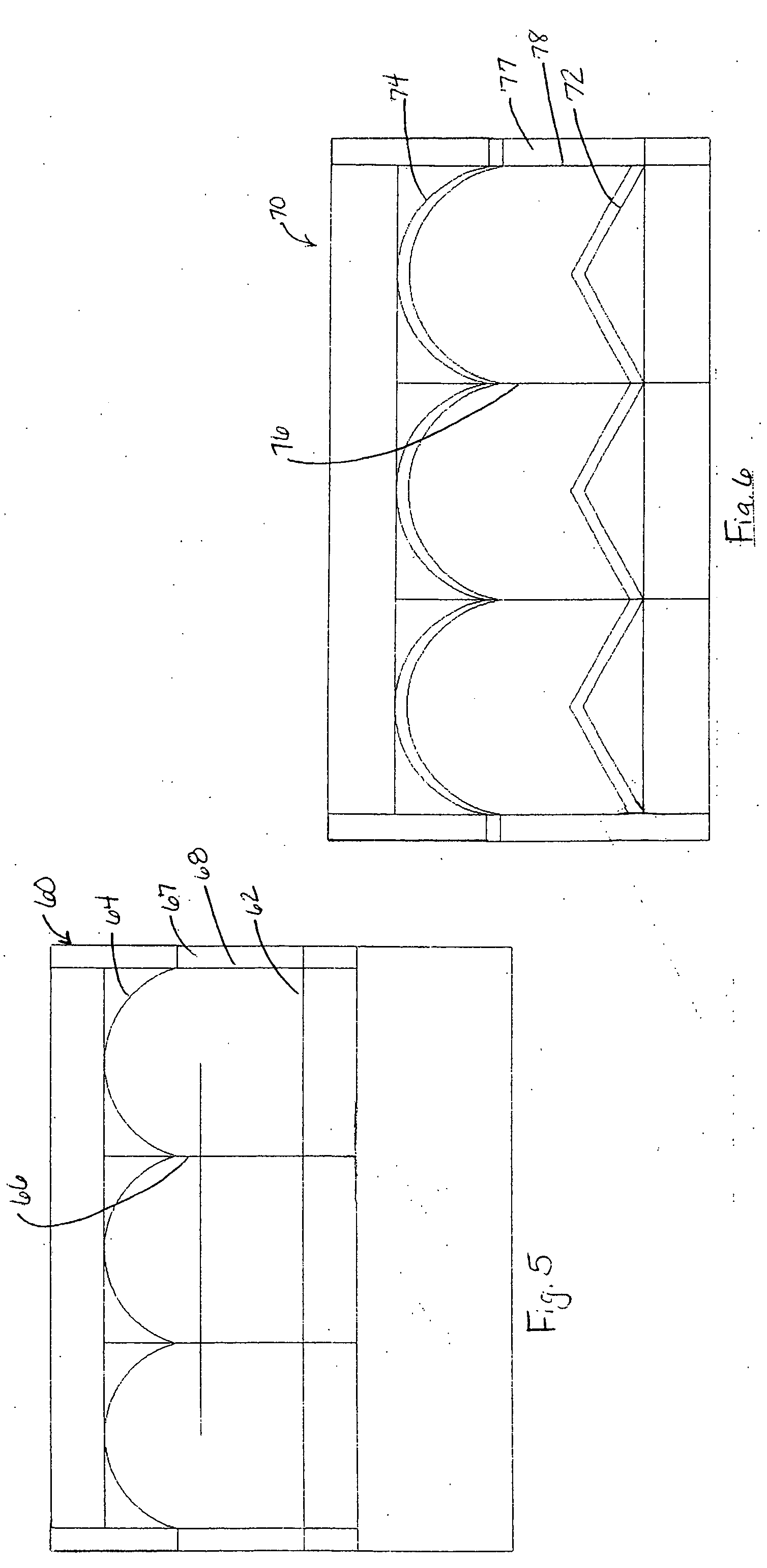
Aerogel metallic compositions
InactiveUS7071287B2Reduce nitrogen contentSuperior physical and electrical propertyMaterial nanotechnologyCell electrodesImidePolymer science
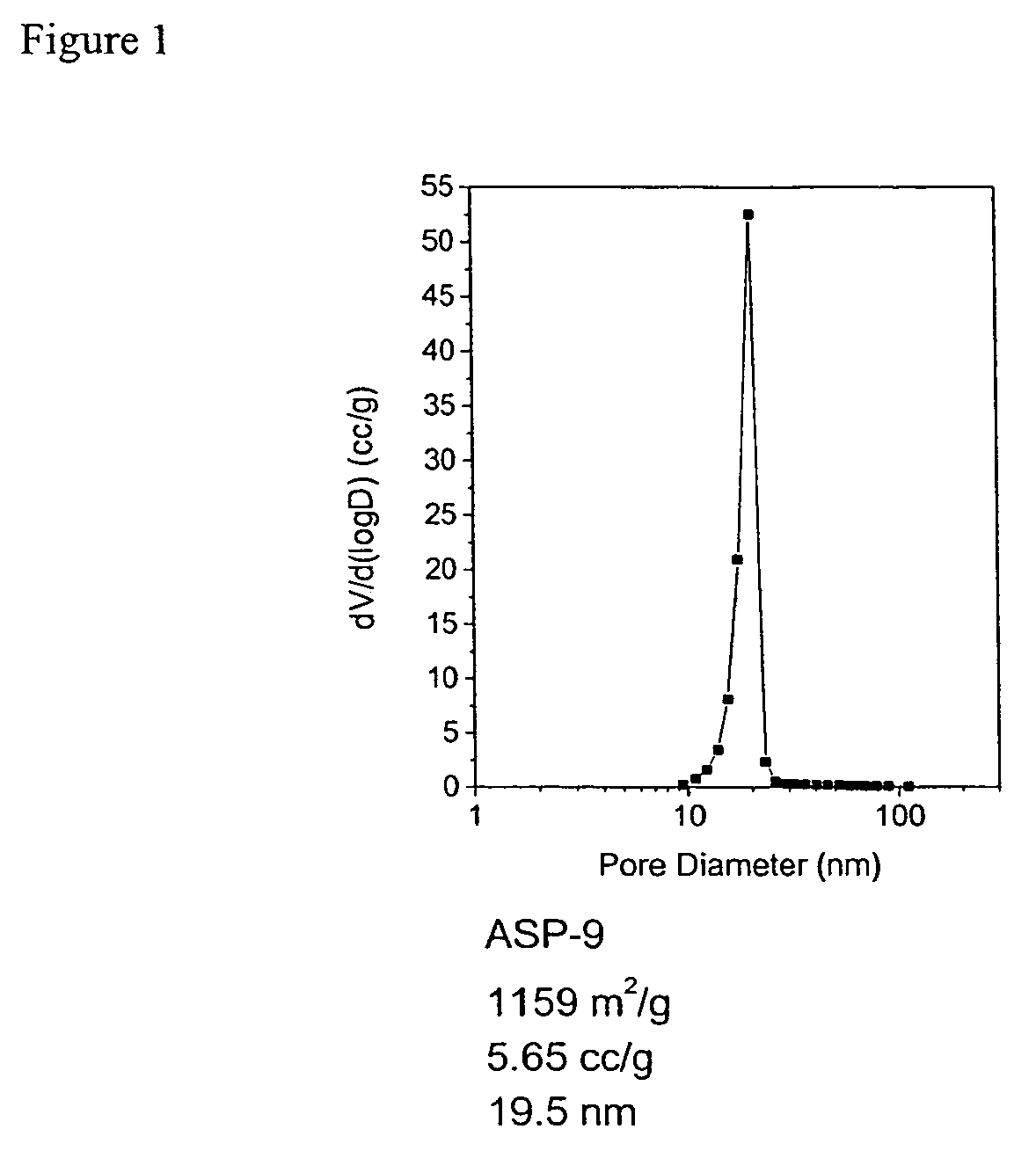
A preparation process of polyimide aerogels that composed of aromatic dianhydrides and aromatic diamines or a combined aromatic and aliphatic diamines is described. Also descried is a process to produce carbon aerogels derived from polyimide aerogel composed of a rigid aromatic diamine and an aromatic dianhydride. Finally, the processes to produce carbon aerogels or xerogel-aerogel hybrid, both of which impregnated with highly dispersed transition metal clusters, and metal carbide aerogels, deriving from the polyimide aerogels composed of a rigid aromatic diamine and an aromatic dianhydride, are described. The polyimide aerogels and the polyimide aerogel derivatives consist of interconnecting mesopores with average pore size at 10 to 30 nm and a mono-dispersed pore size distribution. The gel density could be as low as 0.008 g / cc and accessible surface area as high as 1300 m2 / g.
Owner:ASPEN AEROGELS INC
Prosthetic cardiac valve formed from pericardium material and methods of making same
ActiveUS20070233228A1Barrier to undesired abrasionPrevent and minimize valve leakageHeart valvesFinal product manufactureProsthetic valveProsthesis
Owner:MEDTRONIC INC
Method of making functionalized nanotubes
InactiveUS6203814B1Good dispersionImprove adhesionMaterial nanotechnologyCarbon compoundsChemical MoietyFiber
Owner:HYPERION CATALYSIS INT
Free-standing and aligned carbon nanotubes and synthesis thereof
One or more highly-oriented, multi-walled carbon nanotubes are grown on an outer surface of a substrate initially disposed with a catalyst film or catalyst nano-dot by plasma enhanced hot filament chemical vapor deposition of a carbon source gas and a catalyst gas at temperatures between 300° C. and 3000° C. The carbon nanotubes range from 4 to 500 nm in diameter and 0.1 to 50 μm in length depending on growth conditions. Carbon nanotube density can exceed 104 nanotubes / mm2. Acetylene is used as the carbon source gas, and ammonia is used as the catalyst gas. Plasma intensity, carbon source gas to catalyst gas ratio and their flow rates, catalyst film thickness, and temperature of chemical vapor deposition affect the lengths, diameters, density, and uniformity of the carbon nanotubes. The carbon nanotubes of the present invention are useful in electrochemical applications as well as in electron emission, structural composite, material storage, and microelectrode applications.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Graphitic nanofibers in electrochemical capacitors
Graphitic nanofibers, which include tubular fullerenes (commonly called "buckytubes"), nanotubes and fibrils, which are functionalized by chemical substitution, are used as electrodes in electrochemical capacitors. The graphitic nanofiber based electrode increases the performance of the electrochemical capacitors. Preferred nanofibers have a surface area greater than about 200 m2 / gm and are substantially free of micropores.
Owner:HYPERION CATALYSIS INT
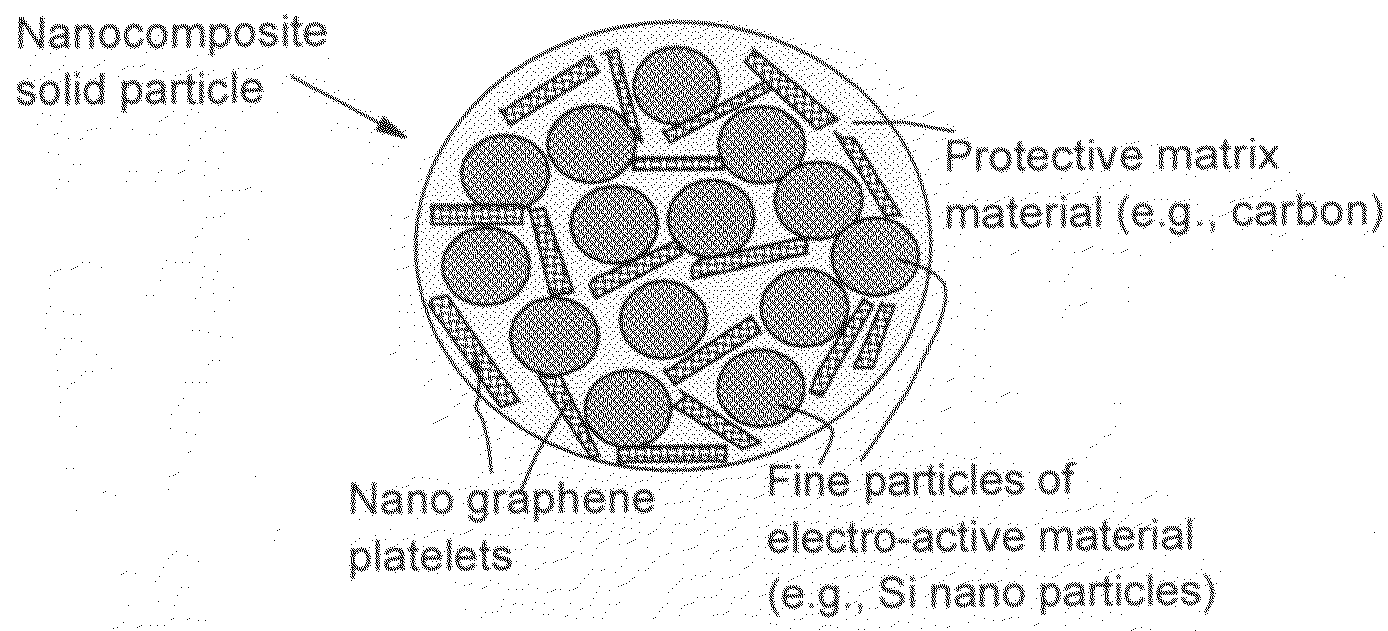
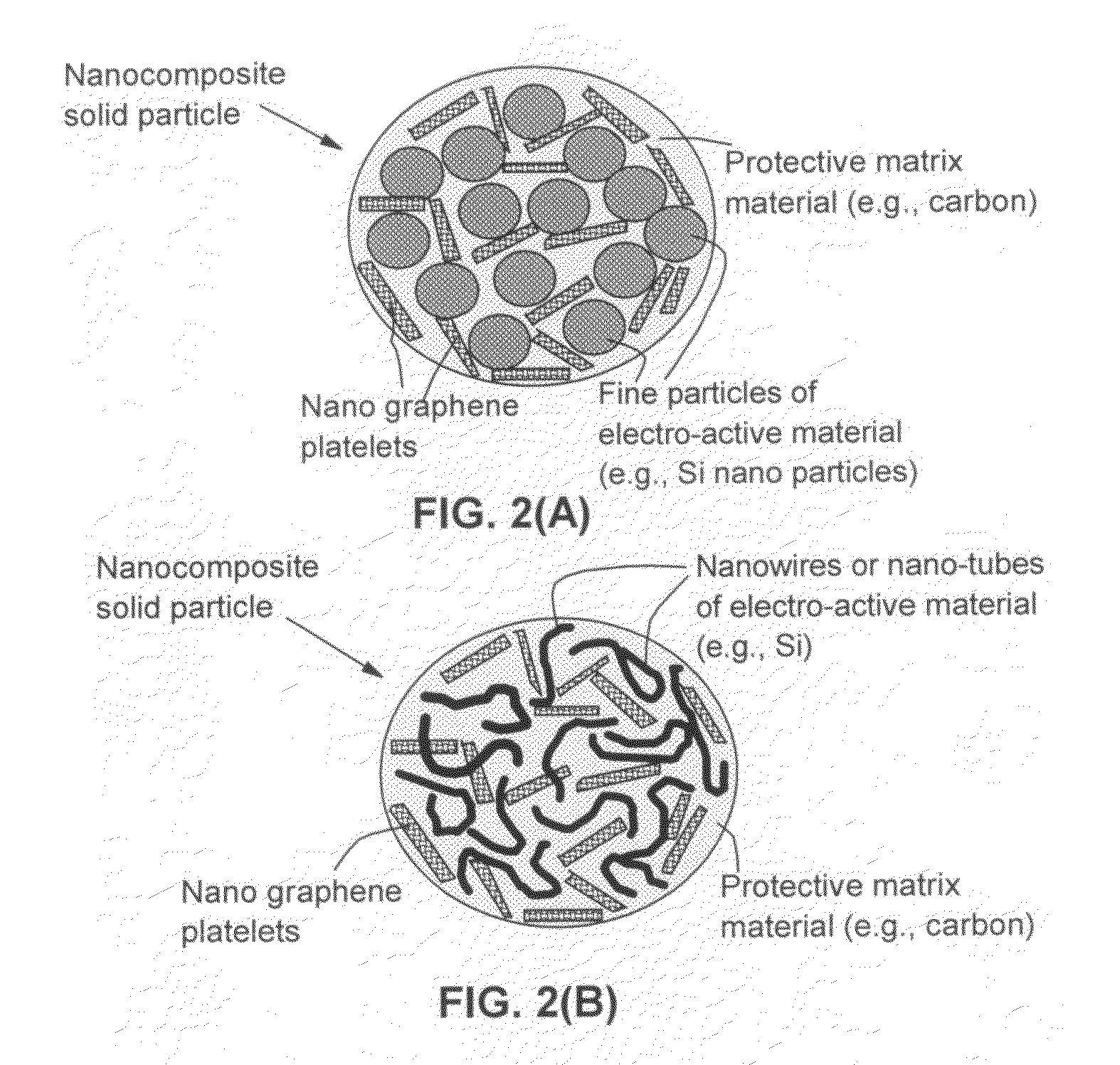
Process for producing nano graphene reinforced composite particles for lithium battery electrodes
ActiveUS20100176337A1Increase stiffnessHigh strengthCell electrodesLi-accumulatorsFiberLithium metal
Owner:SAMSUNG ELECTRONICS CO LTD
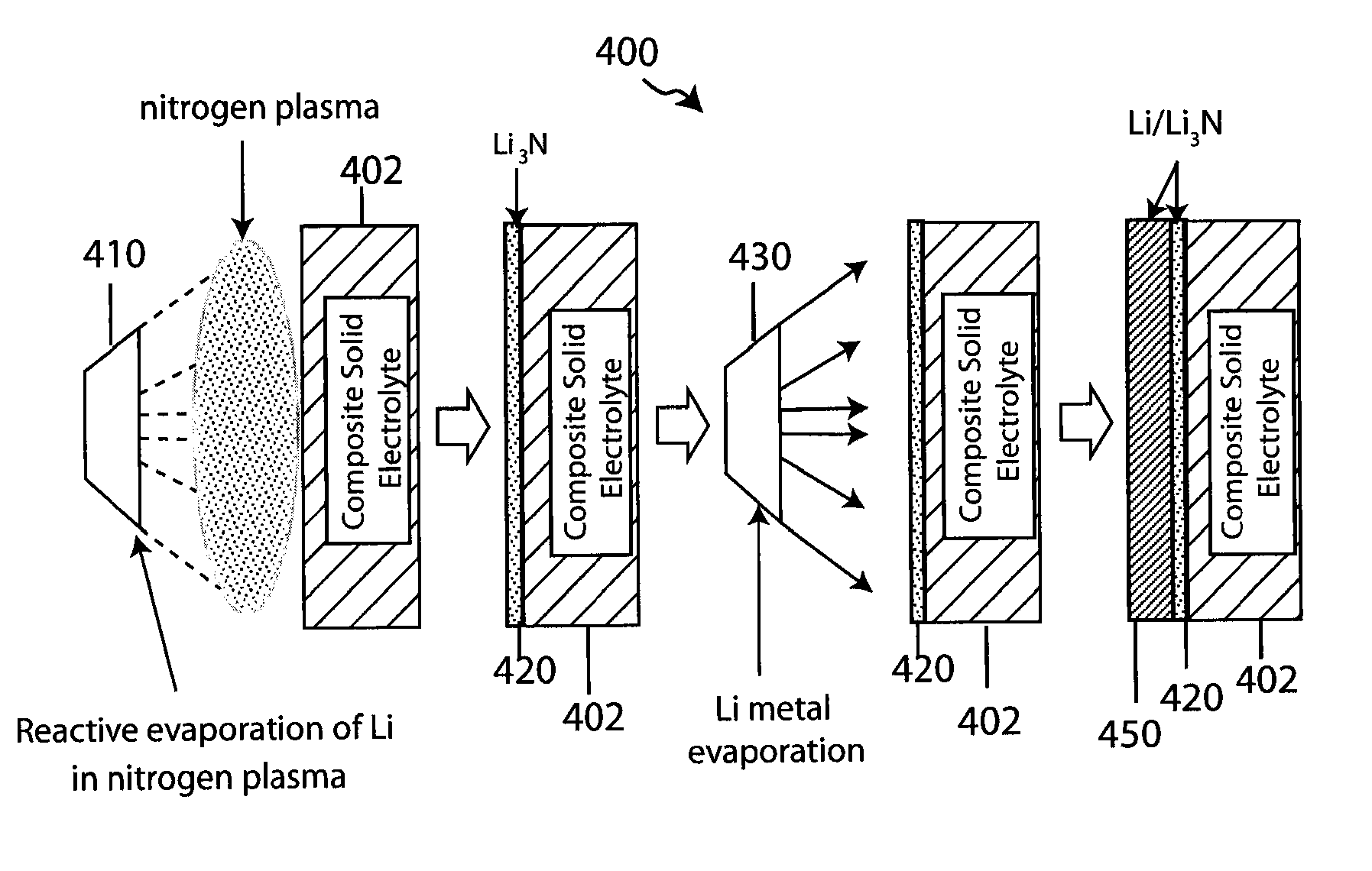
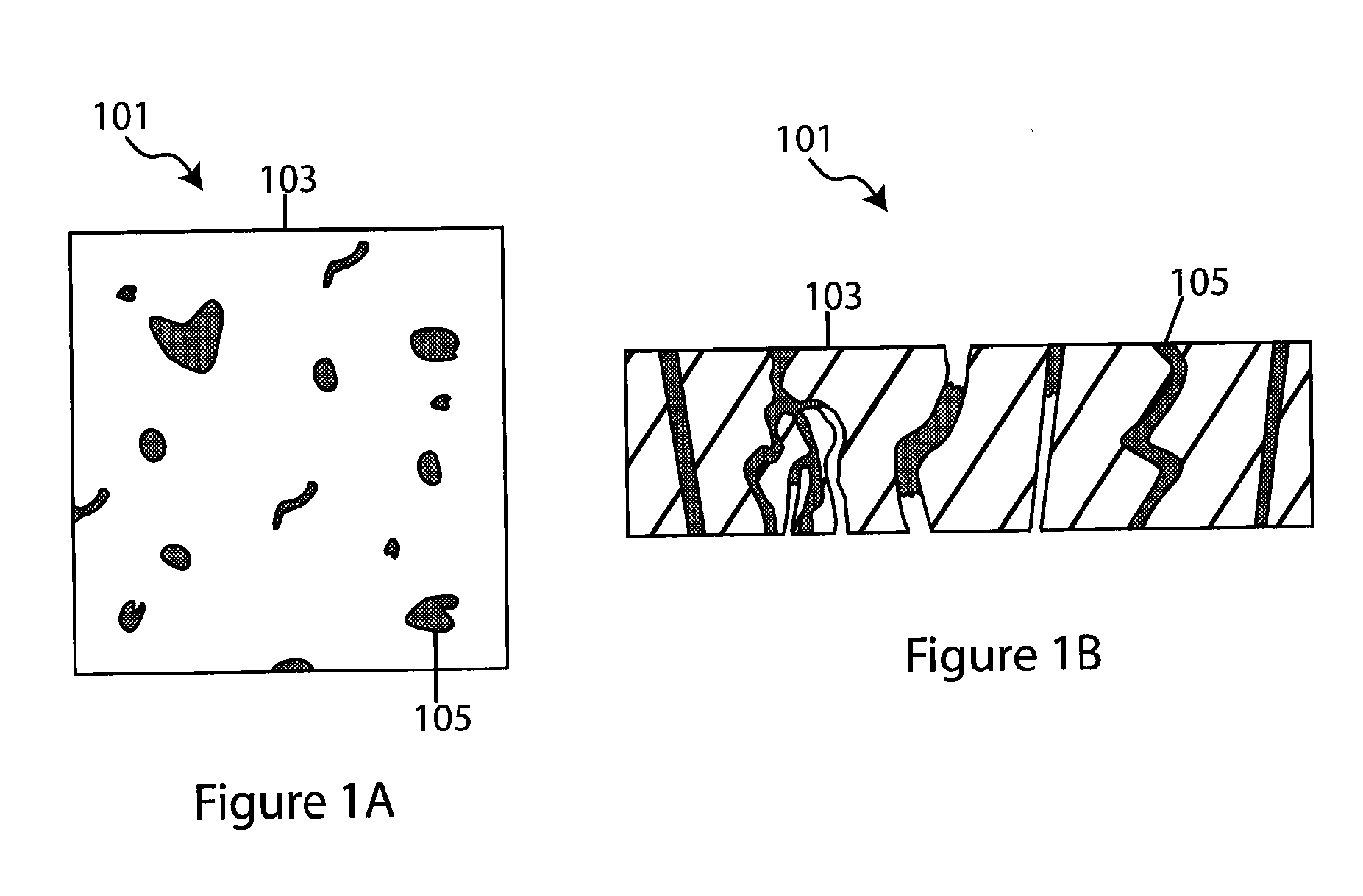
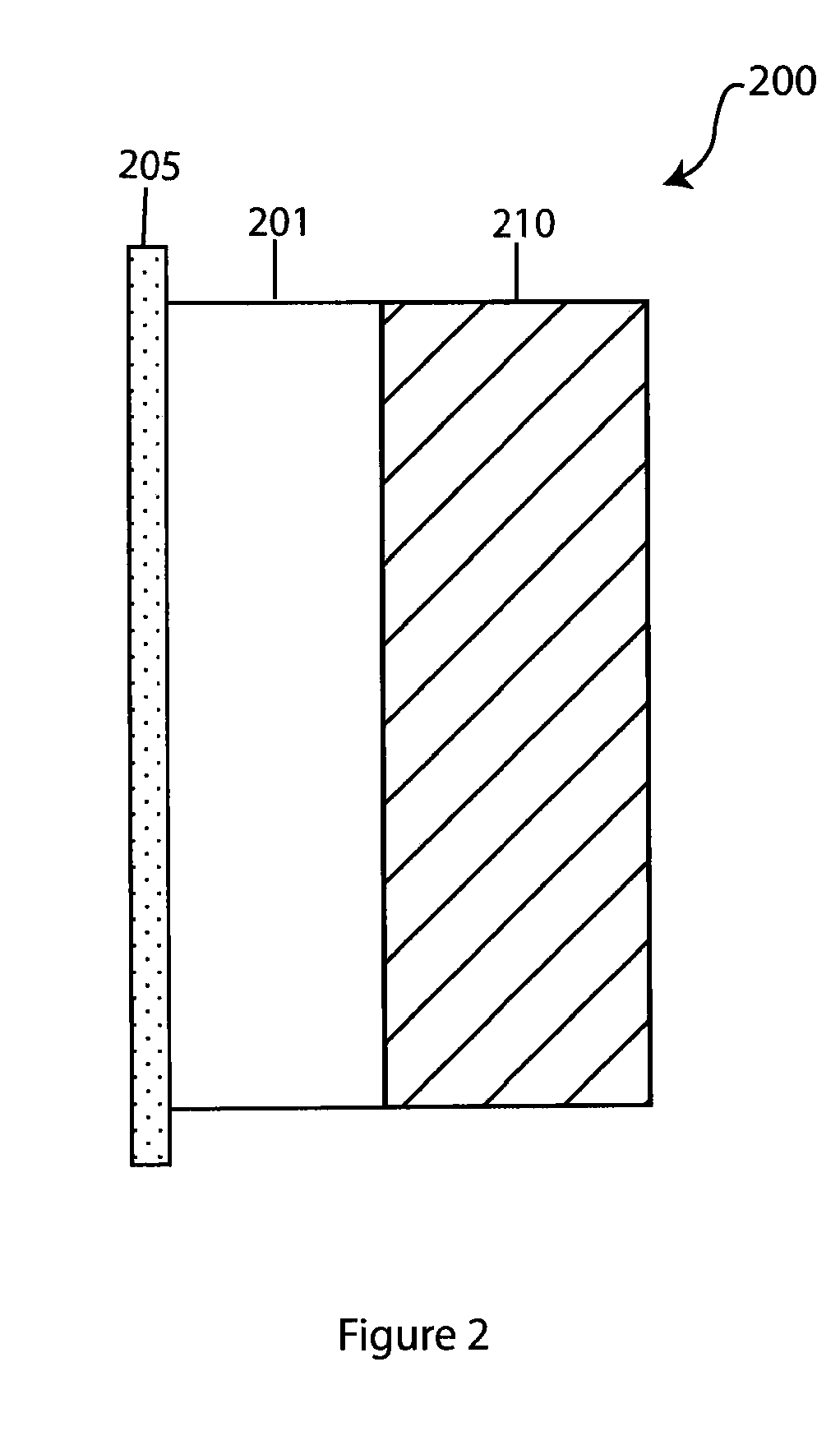
Composite solid electrolyte for protection of active metal anodes
ActiveUS20070172739A1Eliminate through-porosityHigh metal ion conductivityCell electrodesPrimary cellsPorosityTectorial membrane
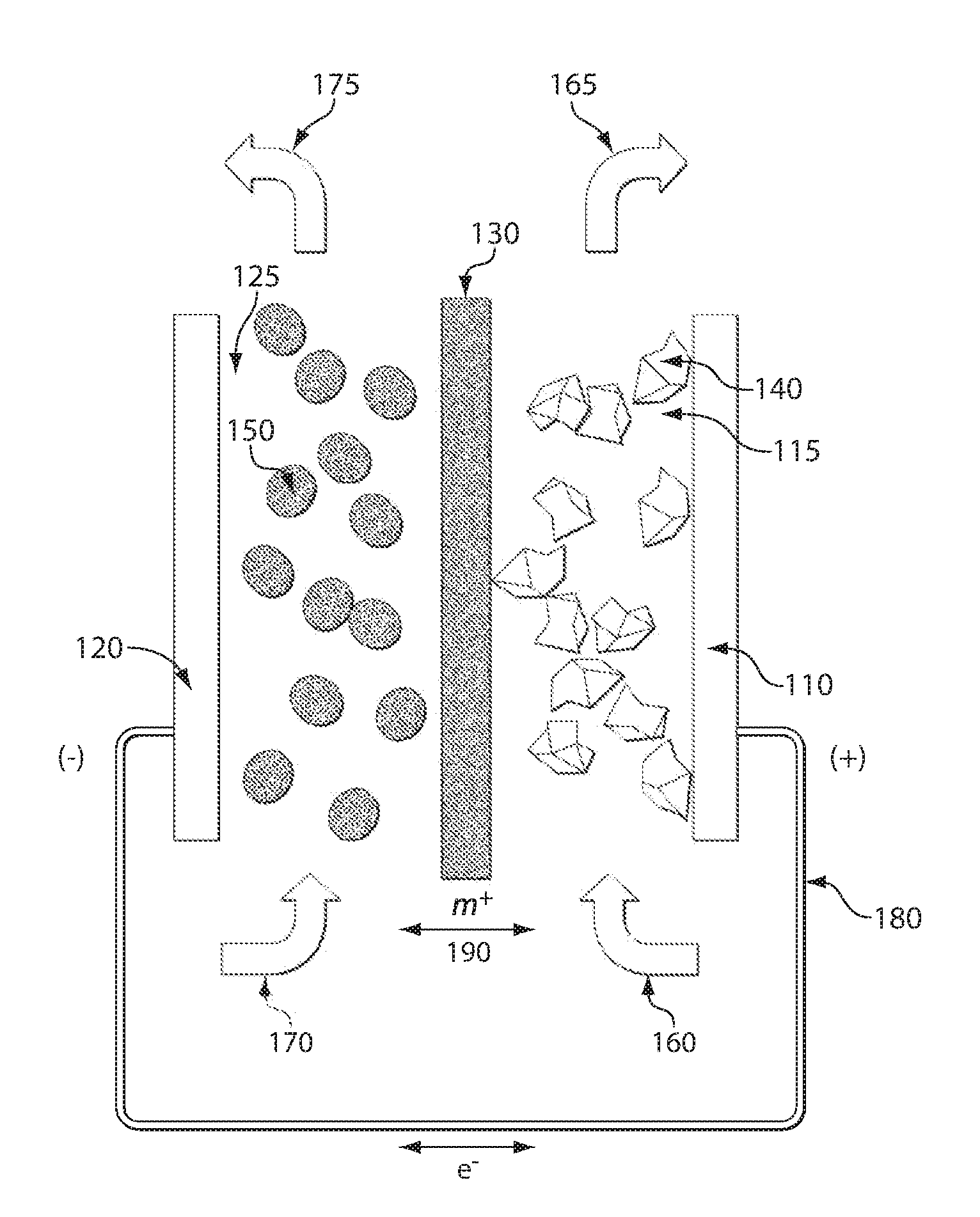
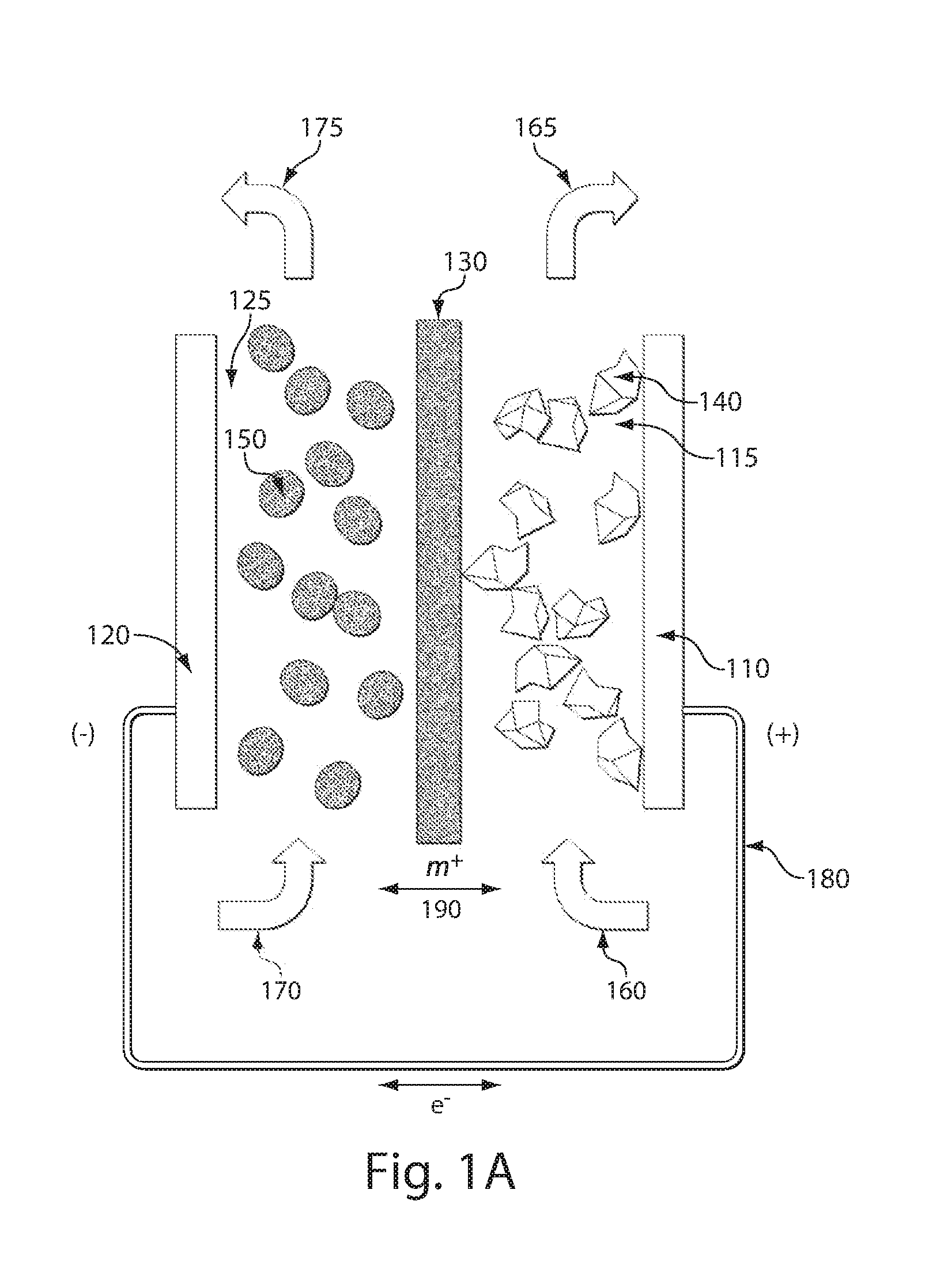
A composite solid electrolyte include a monolithic solid electrolyte base component that is a continuous matrix of an inorganic active metal ion conductor and a filler component used to eliminate through porosity in the solid electrolyte. In this way a solid electrolyte produced by any process that yields residual through porosity can be modified by the incorporation of a filler to form a substantially impervious composite solid electrolyte and eliminate through porosity in the base component. Methods of making the composites is also disclosed. The composites are generally useful in electrochemical cell structures such as battery cells and in particular protected active metal anodes, particularly lithium anodes, that are protected with a protective membrane architecture incorporating the composite solid electrolyte. The protective architecture prevents the active metal of the anode from deleterious reaction with the environment on the other (cathode) side of the architecture, which may include aqueous, air and organic liquid electrolytes and / or electrochemically active materials.
Owner:POLYPLUS BATTERY CO INC
Method and system for in situ formation of gas-phase compounds
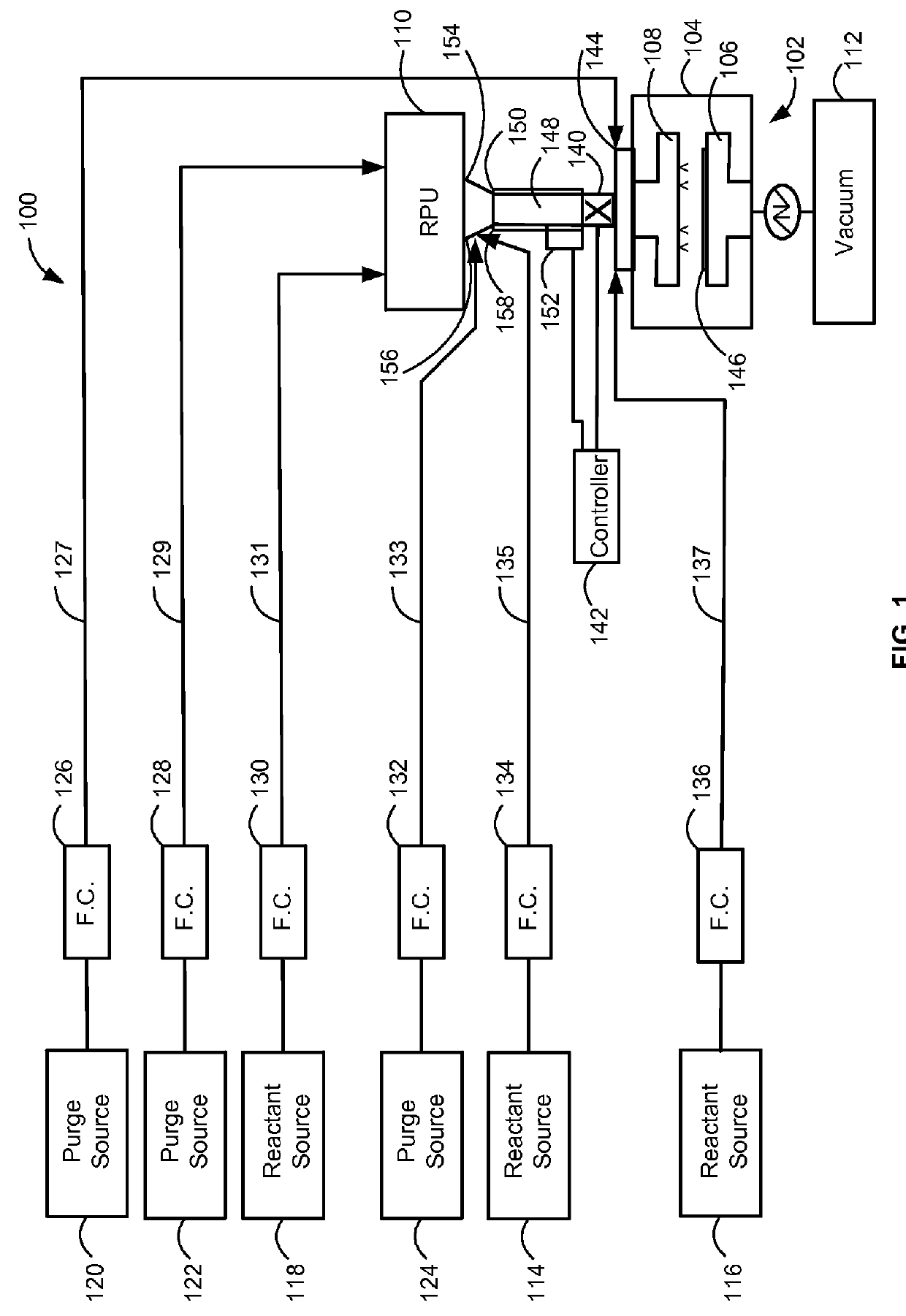
ActiveUS20160051964A1Process can be usedProcess control/regulationCell electrodesCompound aGas phase
A system and method for providing intermediate reactive species to a reaction chamber are disclosed. The system includes an intermediate reactive species formation chamber fluidly coupled to the reaction chamber to provide intermediate reactive species to the reaction chamber. A pressure control device can be used to control an operating pressure of the intermediate reactive species formation chamber, and a heater can be used to heat the intermediate reactive species formation chamber to a desired temperature.
Owner:ASM IP HLDG BV
Method of making, and, analyte sensor
Owner:ABBOTT DIABETES CARE INC
High energy density redox flow device
ActiveUS20110200848A1Avoid accumulationHigh enough specific energyOrganic chemistryFlow propertiesElectrochemical responseHigh energy
Redox flow devices are described in which at least one of the positive electrode or negative electrode-active materials is a semi-solid or is a condensed ion-storing electroactive material, and in which at least one of the electrode-active materials is transported to and from an assembly at which the electrochemical reaction occurs, producing electrical energy. The electronic conductivity of the semi-solid is increased by the addition of conductive particles to suspensions and / or via the surface modification of the solid in semi-solids (e.g., by coating the solid with a more electron conductive coating material to increase the power of the device). High energy density and high power redox flow devices are disclosed. The redox flow devices described herein can also include one or more inventive design features. In addition, inventive chemistries for use in redox flow devices are also described.
Owner:MASSACHUSETTS INST OF TECH +2
Graphitic nanotubes in luminescence assays
Graphitic nanotubes, which include tubular fullerenes (commonly called "buckytubes") and fibrils, which are functionalized by chemical substitution, are used as solid supports in electrogenerated chemiluminescence assays. The graphitic nanotubes are chemically modified with functional group biomolecules prior to use in an assay. Association of electrochemiluminescent ruthenium complexes with the functional group biomolecule-modified nanotubes permits detection of molecules including nucleic acids, antigens, enzymes, and enzyme substrates by multiple formats.
Owner:MESO SCALE TECH LLC
Li/air non-aqueous batteries
ActiveUS20070117007A1Improve battery performanceLarge capacityFuel and primary cellsFuel and secondary cellsLithiumOxygen
Non-aqueous alkali metal (e.g., Li) / oxygen battery cells constructed with a protected anode that minimizes anode degradation and maximizes cathode performance by enabling the use of cathode performance enhancing solvents in the catholyte have negligible self-discharge and high deliverable capacity. In particular, protected lithium-oxygen batteries with non-aqueous catholytes have this improved performance.
Owner:POLYPLUS BATTERY CO INC
Method and system for battery protection
ActiveUS20060091858A1Solution to short lifeSevere impactCharge equalisation circuitCell electrodesElectric powerBattery pack
A method of conducting an operation including a battery. The battery includes a cell having a voltage. Power is transferable between the cell and the electrical device. A controller is operable to control a function of the battery pack. The controller is also operable with a voltage at least one of equal to and greater than an operating voltage threshold. The cell is operable to selectively supply voltage to the controller. The method includes the act of enabling the controller to operate when the voltage supplied by the cell is below the operating voltage threshold.
Owner:MILWAUKEE ELECTRIC TOOL CORP
Cell controller, battery module and power supply system
ActiveUS20080050645A1Increase production capacityIncrease productivityBatteries circuit arrangementsCell electrodesProduction ratePotential difference
A cell controller having excellent productivity is provided. A cell-con 80 has 12 ICs IC-1 to IC-2 mounted on a substrate, and these ICs detect voltages of respective cells constituting a cell pack, perform capacity adjustment on the respective cells, and are mounted two by two on rectangular longer sides of a rectangular continuous straight line L-L′ defined on a substrate from the IC-1 on a highest potential side to the IC-12 on a lowest potential side continuously in order of potential differences of the corresponding cell packs. Distances between the rectangular shorter sides of the rectangular continuous straight line L-L′ are the same. On the cell-con 80, between the IC-1 to IC-12 having different ground voltages, each of the ICs has signal output terminals connected to signal input terminals of a lower order IC respectively in an electrically non-insulated state.
Owner:HITACHI ASTEMO LTD
Electrocatalyst powders, methods for producing powders and devices fabricated from same
Electrocatalyst powders and methods for producing electrocatalyst powders, such as carbon composite electrocatalyst powders. The powders have a well-controlled microstructure and morphology. The method includes forming the particles from an aerosol of precursors by heating the aerosol to a relatively low temperature, such as not greater than about 400° C.
Owner:CABOT CORP
Method for synthesis of carbon-coated redox materials with controlled size
ActiveUS20040033360A1Low costReduce the numberMaterial nanotechnologyHybrid capacitorsCross-linkRedox
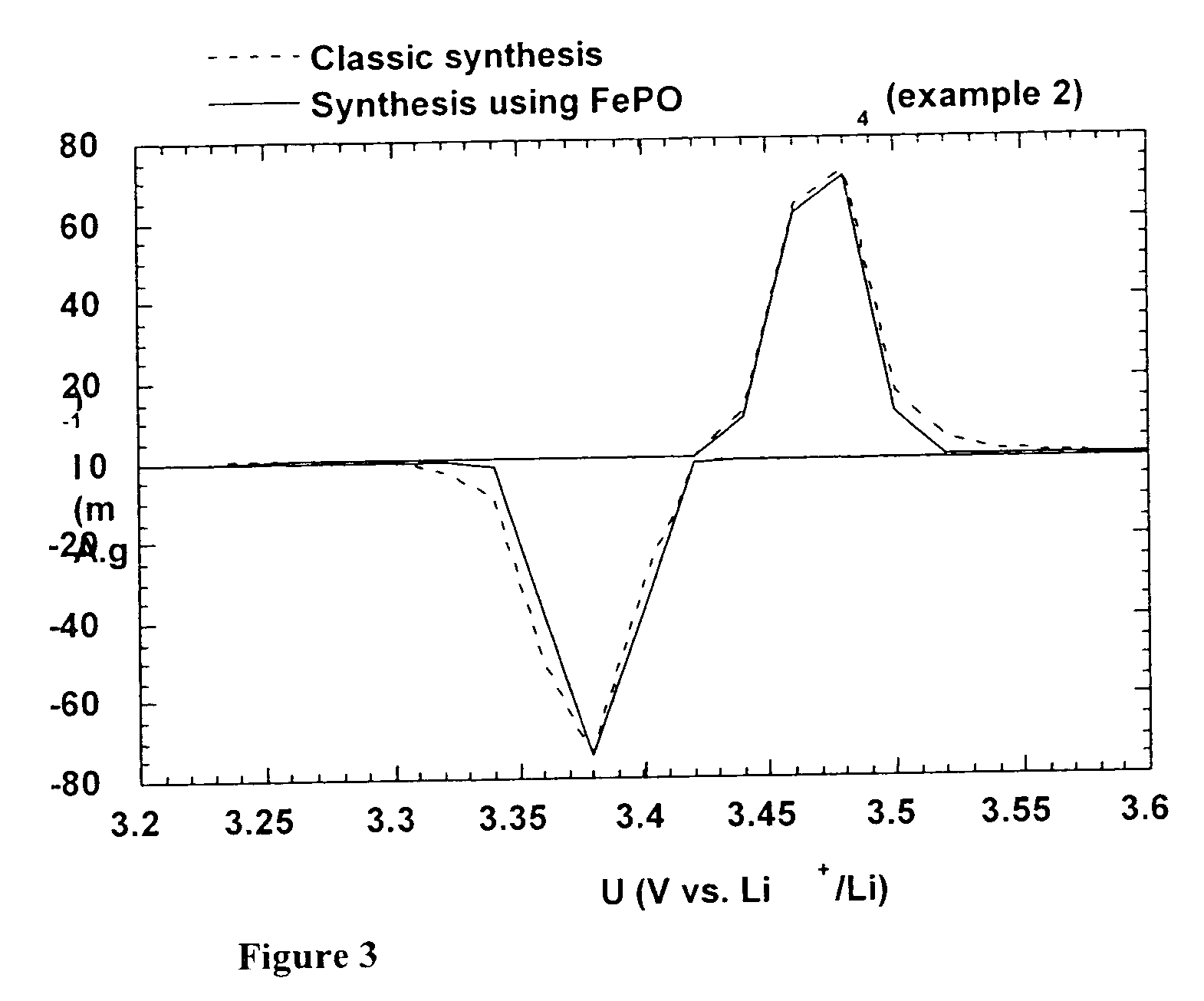
A method for the synthesis of compounds of the formula C-LixM1-yM'y(XO4)n, where C represents carbon cross-linked with the compound LixM1-yM'y(XO4)n, in which x, y and n are numbers such as 0<=x<=2, 0<=y<=0.6, and 1<=n<=1.5, M is a transition metal or a mixture of transition metals from the first period of the periodic table, M' is an element with fixed valency selected among Mg<2+>, Ca<2+>, Al<3+>, Zn<2+> or a combination of these same elements and X is chosen among S, P and Si, by bringing into equilibrium, in the required proportions, the mixture of precursors, with a gaseous atmosphere, the synthesis taking place by reaction and bringing into equilibrium, in the required proportions, the mixture of the precursors, the procedure comprising at least one pyrolysis step of the carbon source compound in such a way as to obtain a compound in which the electronic conductivity measured on a sample of powder compressed at a pressure of 3750 Kg.cm<-2 >is greater than 10<-8 >S.cm<-1>. The materials obtained have excellent electrical conductivity, as well a very improved chemical activity.
Owner:CENT NAT DE LA RECHERCHE SCI +2
Dendritic Polymers With Enhanced Amplification and Interior Functionality
ActiveUS20070298006A1Reduced responseSizePowder deliveryOrganic active ingredientsCross-linkScavenger
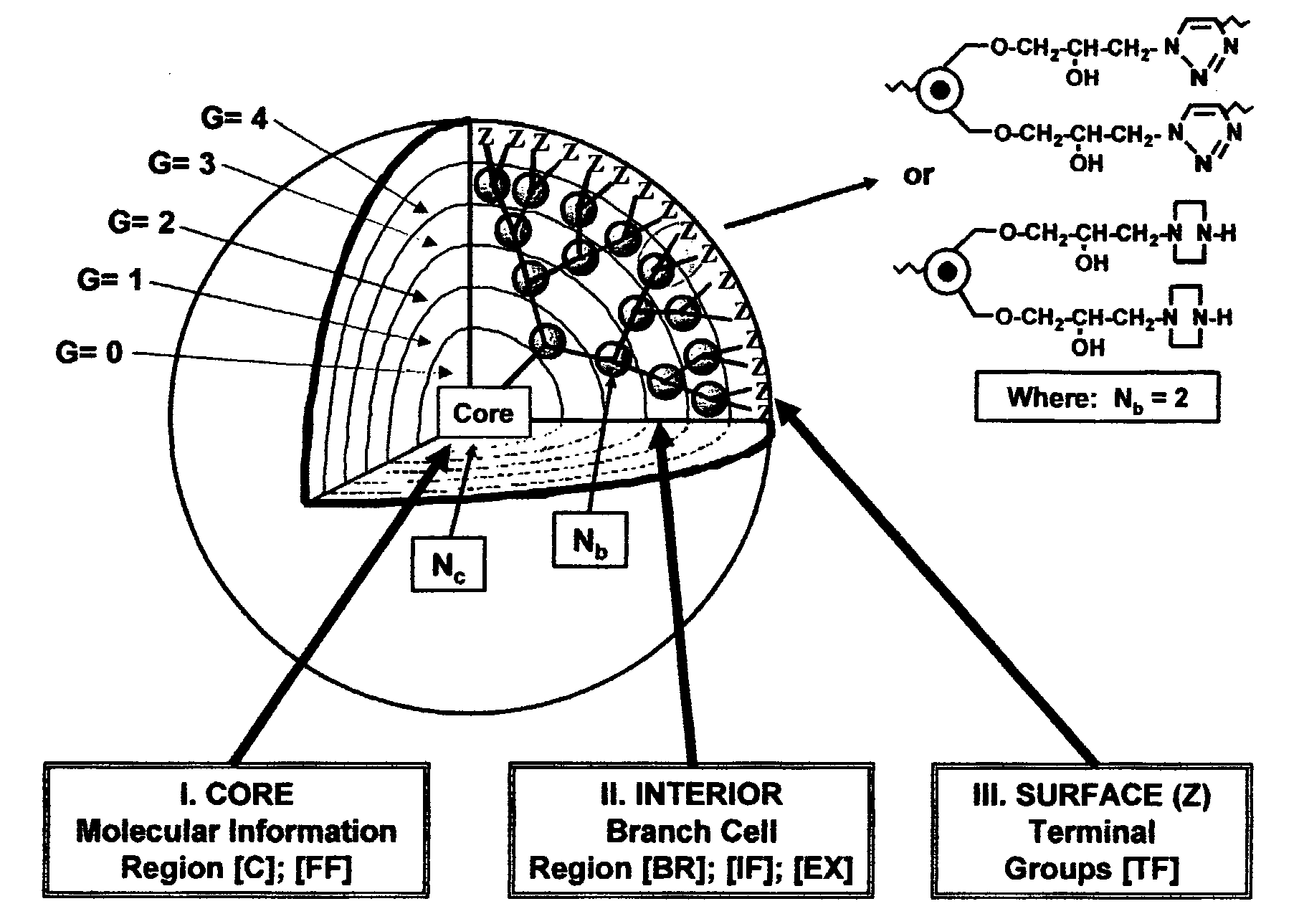
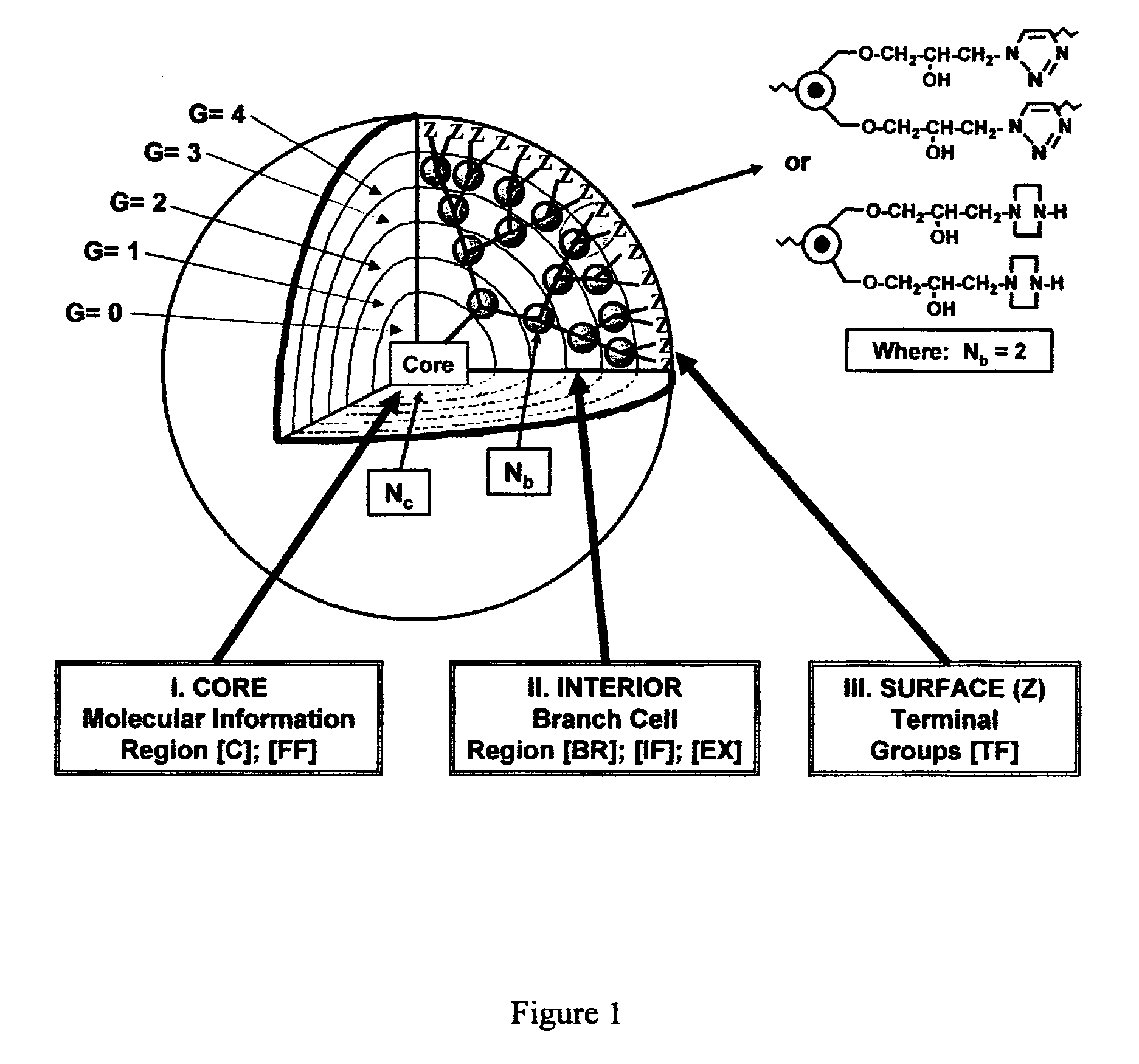
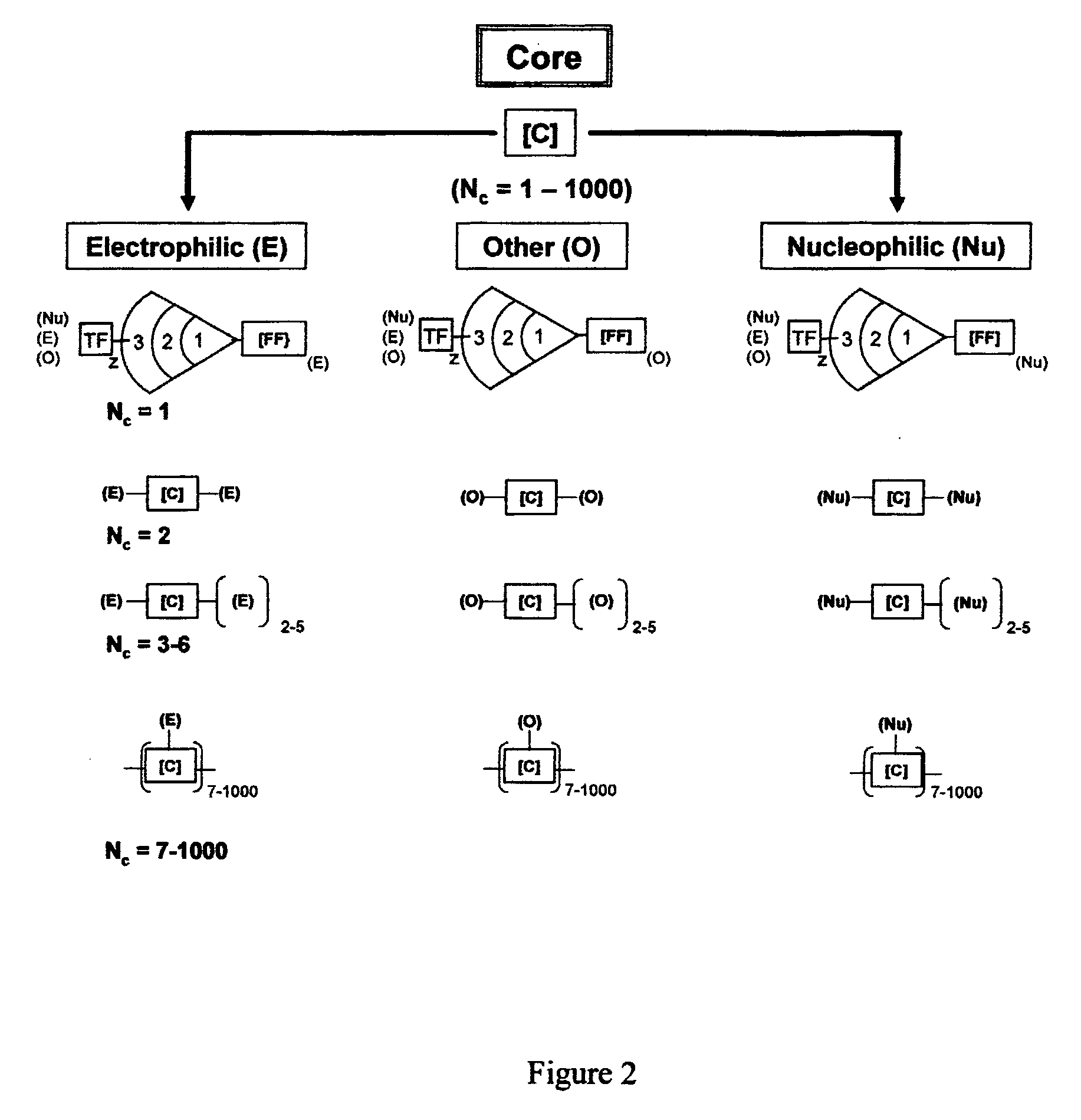
Dendritic polymers with enhanced amplification and interior functionality are disclosed. These dendritic polymers are made by use of fast, reactive ring-opening chemistry (or other fast reactions) combined with the use of branch cell reagents in a controlled way to rapidly and precisely build dendritic structures, generation by generation, with cleaner chemistry, often single products, lower excesses of reagents, lower levels of dilution, higher capacity method, more easily scaled to commercial dimensions, new ranges of materials, and lower cost. The dendritic compositions prepared have novel internal functionality, greater stability (e.g., thermal stability and less or no reverse Michael's reaction), and reach encapsulation surface densities at lower generations. Unexpectedly, these reactions of polyfunctional branch cell reagents with polyfunctional cores do not create cross-linked materials. Such dendritic polymers are useful as demulsifiers for oil / water emulsions, wet strength agents in the manufacture of paper, proton scavengers, polymers, nanoscale monomers, calibration standards for electron microscopy, making size selective membranes, and agents for modifying viscosity in aqueous formulations such as paint. When these dendritic polymers have a carried material associated with their surface and / or interior, then these dendritic polymers have additional properties for carrying materials due to the unique characteristics of the dendritic polymer, such as for drug delivery, transfection, and diagnostics.
Owner:DENDRITIC NANO TECH INC
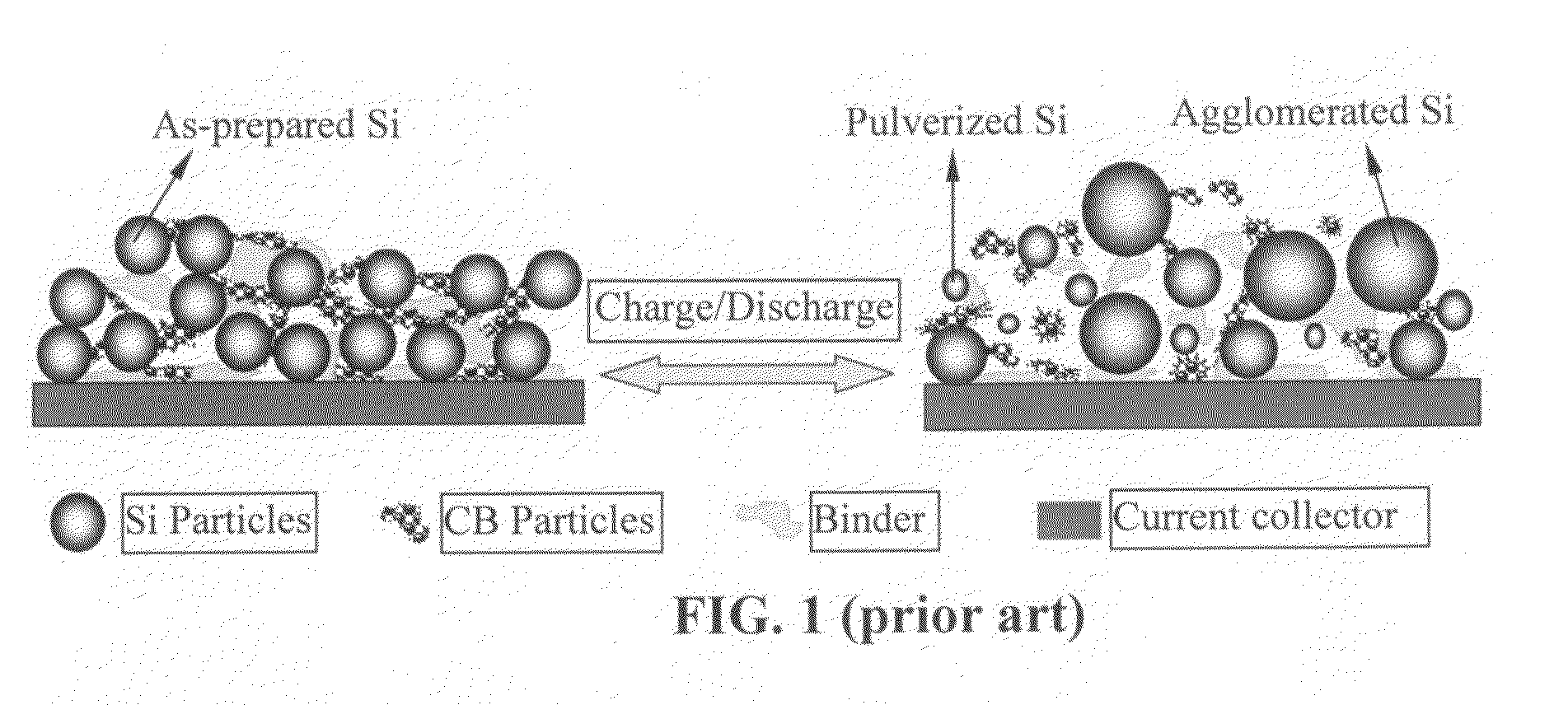
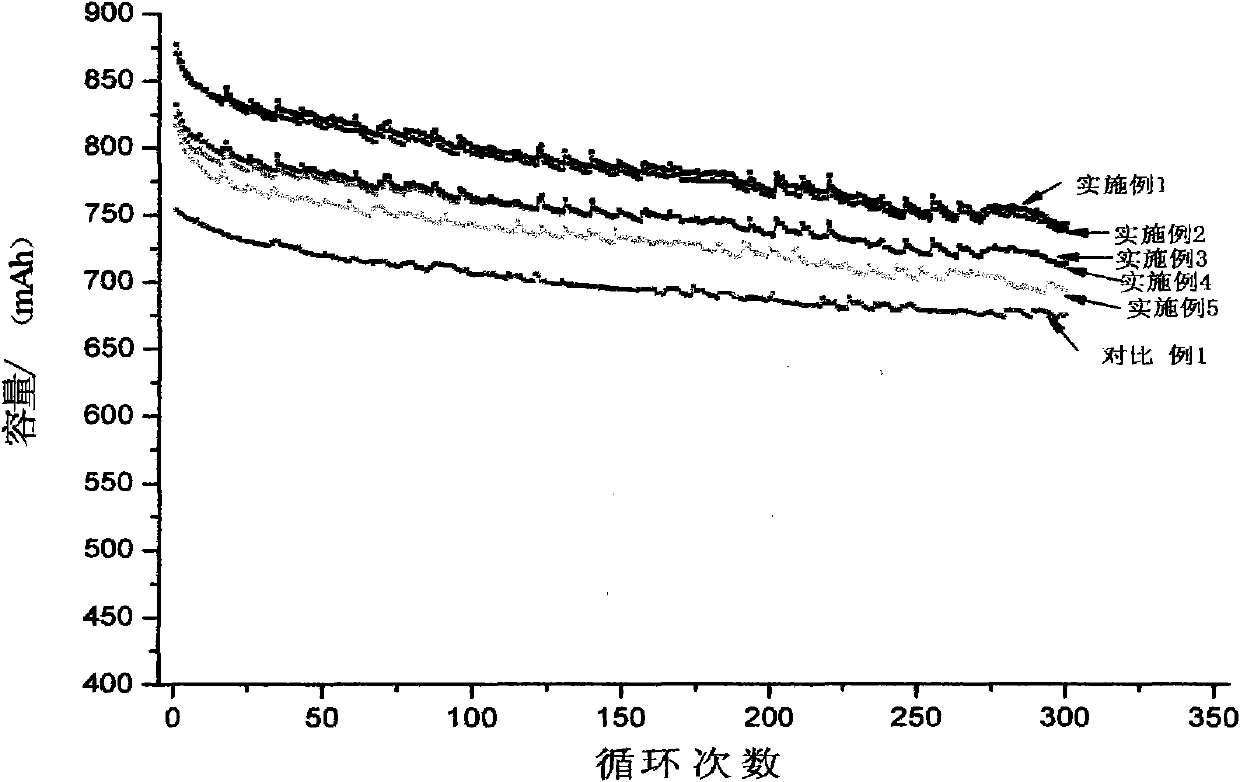
Carbon/silicon/carbon nano composite structure cathode material and preparation method thereof
The invention discloses a carbon / silicon / carbon nano composite structure cathode material and a preparation method thereof, belonging to the technical field of electrochemical power supply technologies. The cathode material consists of a carbon-based conductive substrate, nano silicon and a nano carbon coating layer, wherein the nano silicon is uniformly distributed on the carbon-based conductive substrate; the nano carbon coating layer is arranged on the surface of the nano silicon; the carbon-based conductive substrate is porous carbon, a carbon nanotube or graphene; the nano silicon exists in the state of nanoparticles or nano films; the weight percentage of the nano silicon in the cathode material is 10-90 percent; and the thickness of the nano carbon coating layer is 0.1-10 nanometers. The preparation method comprises the following steps of: depositing nano silicon on the carbon substrate in a reaction space in oxygen-free atmosphere by adopting a chemical vapor deposition process; and coating nano carbon on the surface of the nano silicon by adopting the chemical vapor deposition process. In the obtained carbon / silicon / carbon composite cathode material, the volume change of a silicon electrode material is controlled effectively in the charging and discharging processes, the electrode structure is kept complete, the circulation volume is large, the circulation service life is long, and the electrochemical performance is high.
Owner:TSINGHUA UNIV
High energy density vanadium electrolyte solutions, methods of preparation thereof and all-vanadium redox cells and batteries containing high energy vanadium electrolyte solutions
InactiveUS20010028977A1Effective amountEasy to modifyCharging stationsCell electrodesElectricityVanadium redox battery
Disclosed is a method for preparing a high energy density (HED) electrolyte solution for use in an all-vanadium redox cells, a high energy density electrolyte solution, in particular an all-vanadium high energy density electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the high energy density electrolyte solution, a redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the HED electrolyte solution, a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the HED electrolyte, a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell. A method for stabilising an electrolyte solution for use in a redox cell, in particular for stabilising an electrolyte solution for use in an all-vanadium redox cell, a stabilised electrolyte solution, in particular an all-vanadium stabilised electrolyte solution, a redox cell, in particular an all-vanadium redox cell, comprising the stabilised electrolyte solution, a redox battery, in particular an all-vanadium redox battery comprising the stabilised electrolyte solution, a process for recharging a discharged or partially discharged redox battery, in particular an all-vanadium redox battery, comprising the stabilised electrolyte solution, and a process for the production of electricity from a charged redox battery, and in particular a charged all-vanadium redox battery, comprising the stabilised electrolyte solution are disclosed. Also disclosed are a redox battery / fuel cell and a process for the production of electricity from a redox battery / fuel cell.
Owner:JD HLDG INC
Separator for Electrochemical Device, and Electrochemical Device
ActiveUS20070264577A1Improve securityCell electrodesSecondary cellsPhysical chemistryElectrochemistry
An electrochemical device having excellent safety at high temperature is provided by using a separator for an electrochemical device, which is made of a porous film comprising: a porous base (5) having a heat-resistant temperature of 150° C. or higher and including filler particles (3); at least one kind of shutdown resin (6) selected from the group consisting of resin A that has a melting point in a range of 80° C. to 130° C. and resin B that absorbs an electrolyte and swells due to heating, and the swelling degree is increased as the temperature rises; and a binder (4).
Owner:MAXELL HLDG LTD
Flexible asymmetric electrochemical cells using nano graphene platelet as an electrode material
InactiveUS20110183180A1Excellent specific capacitanceLarge specific surface areaElectrochemical generatorsHybrid capacitor separatorsPlateletGraphene
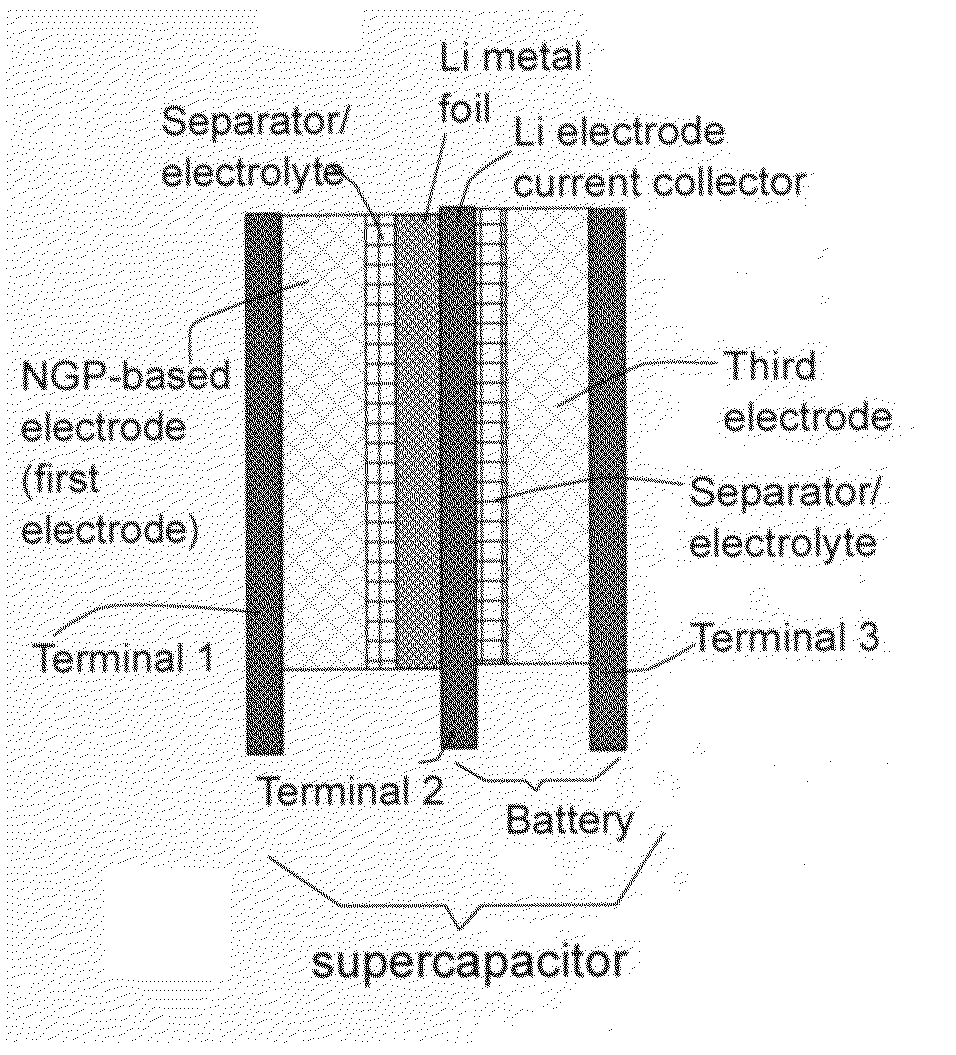
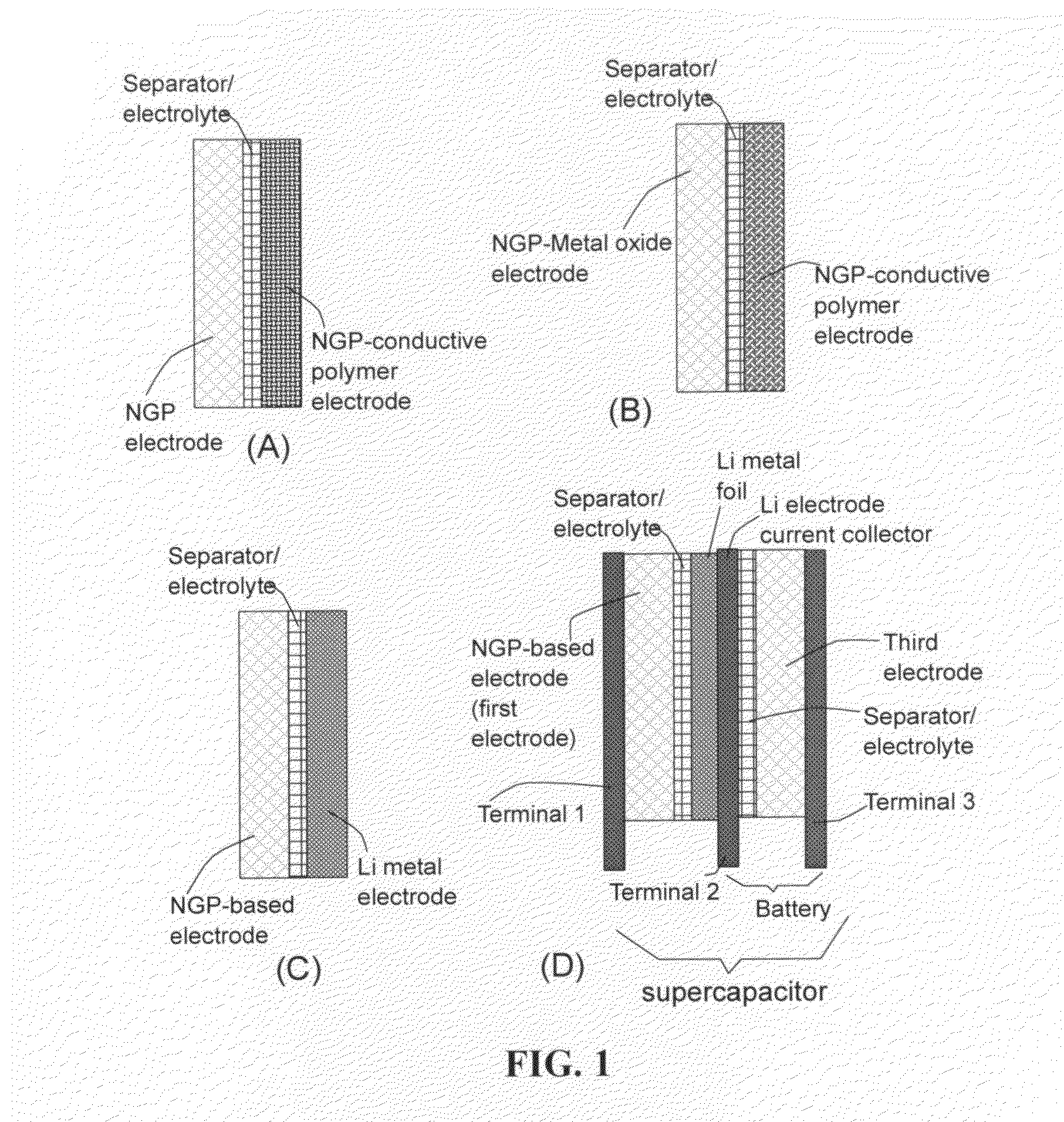
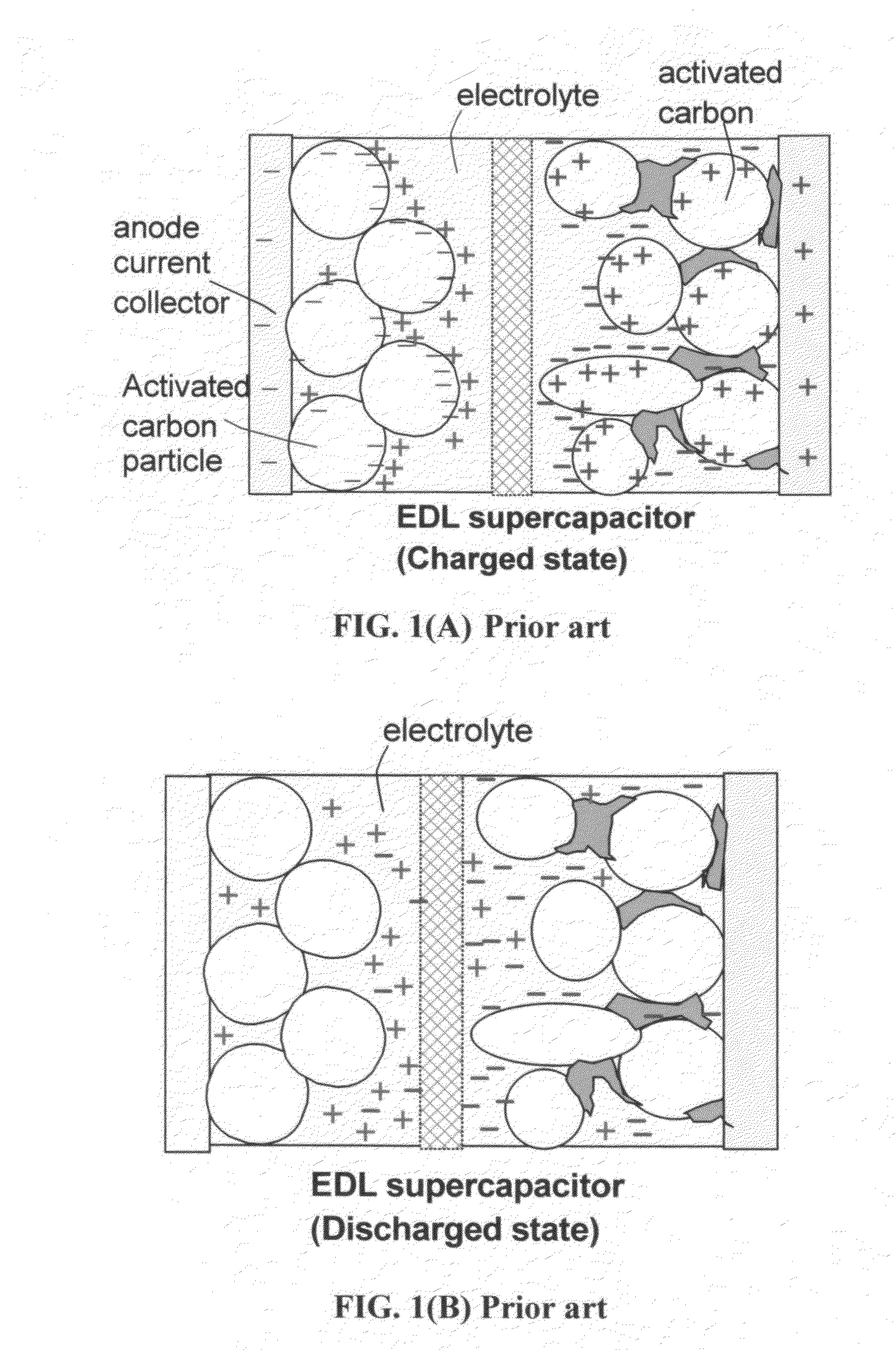
A flexible, asymmetric electrochemical cell comprising: (A) A sheet of graphene paper as first electrode comprising nano graphene platelets having a platelet thickness less than 1 nm, wherein the first electrode has electrolyte-accessible pores; (B) A thin-film or paper-like first separator and electrolyte; and (C) A thin-film or paper-like second electrode which is different in composition than the first electrode; wherein the separator is sandwiched between the first and second electrode to form a flexible laminate configuration. The asymmetric supercapacitor cells with different NGP-based electrodes exhibit an exceptionally high capacitance, specific energy, and stable and long cycle life.
Owner:NANOTEK INSTR GRP LLC
Ionically conductive composites for protection of active metal anodes
ActiveUS7282302B2Good chemical stabilityImprove ionic conductivitySolid electrolytesCell seperators/membranes/diaphragms/spacersChemical stabilityBattery cell
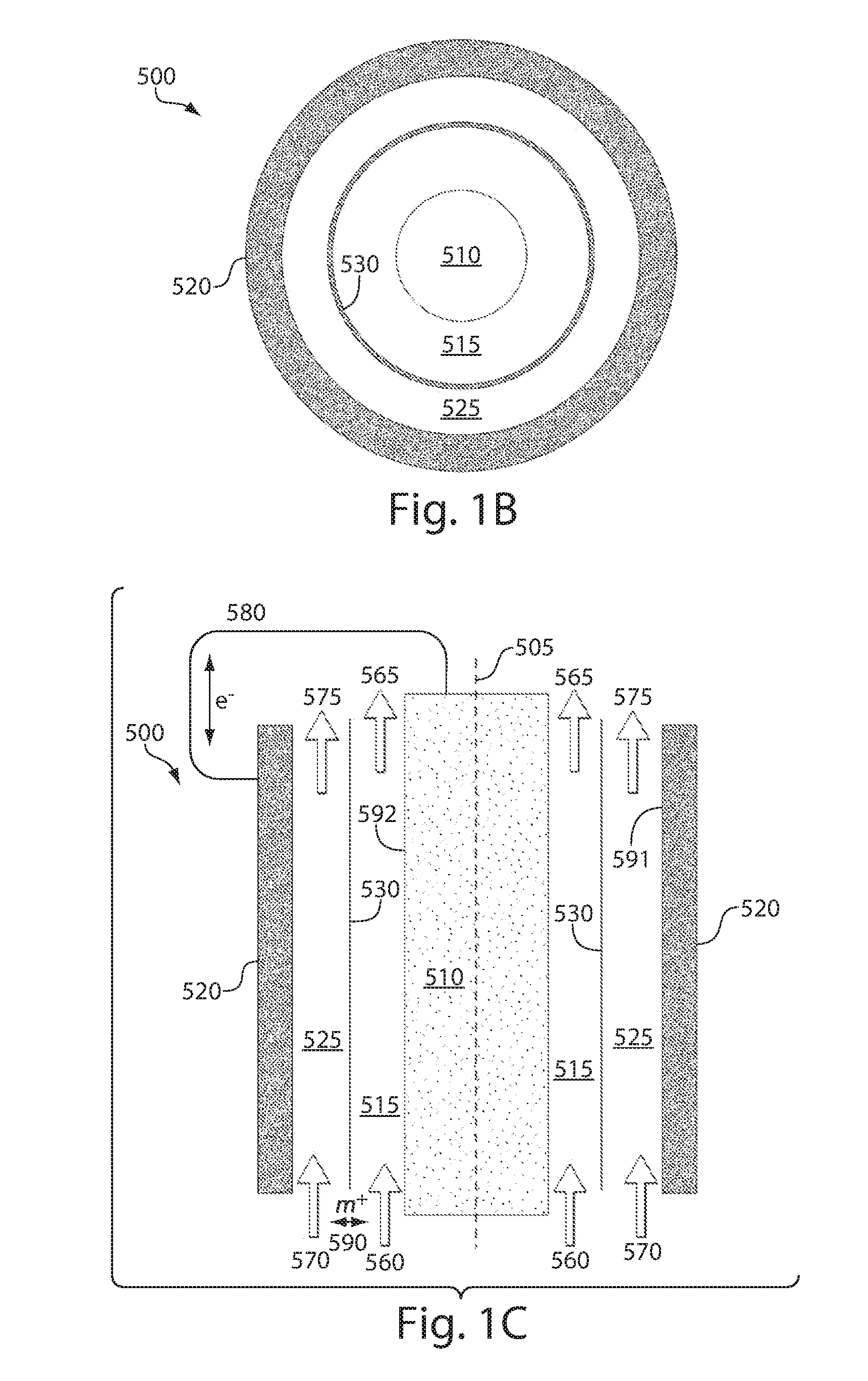
Disclosed are ionically conductive composites for protection of active metal anodes and methods for their fabrication. The composites may be incorporated in active metal negative electrode (anode) structures and battery cells. In accordance with the invention, the properties of different ionic conductors are combined in a composite material that has the desired properties of high overall ionic conductivity and chemical stability towards the anode, the cathode and ambient conditions encountered in battery manufacturing. The composite is capable of protecting an active metal anode from deleterious reaction with other battery components or ambient conditions while providing a high level of ionic conductivity to facilitate manufacture and / or enhance performance of a battery cell in which the composite is incorporated.
Owner:POLYPLUS BATTERY CO INC
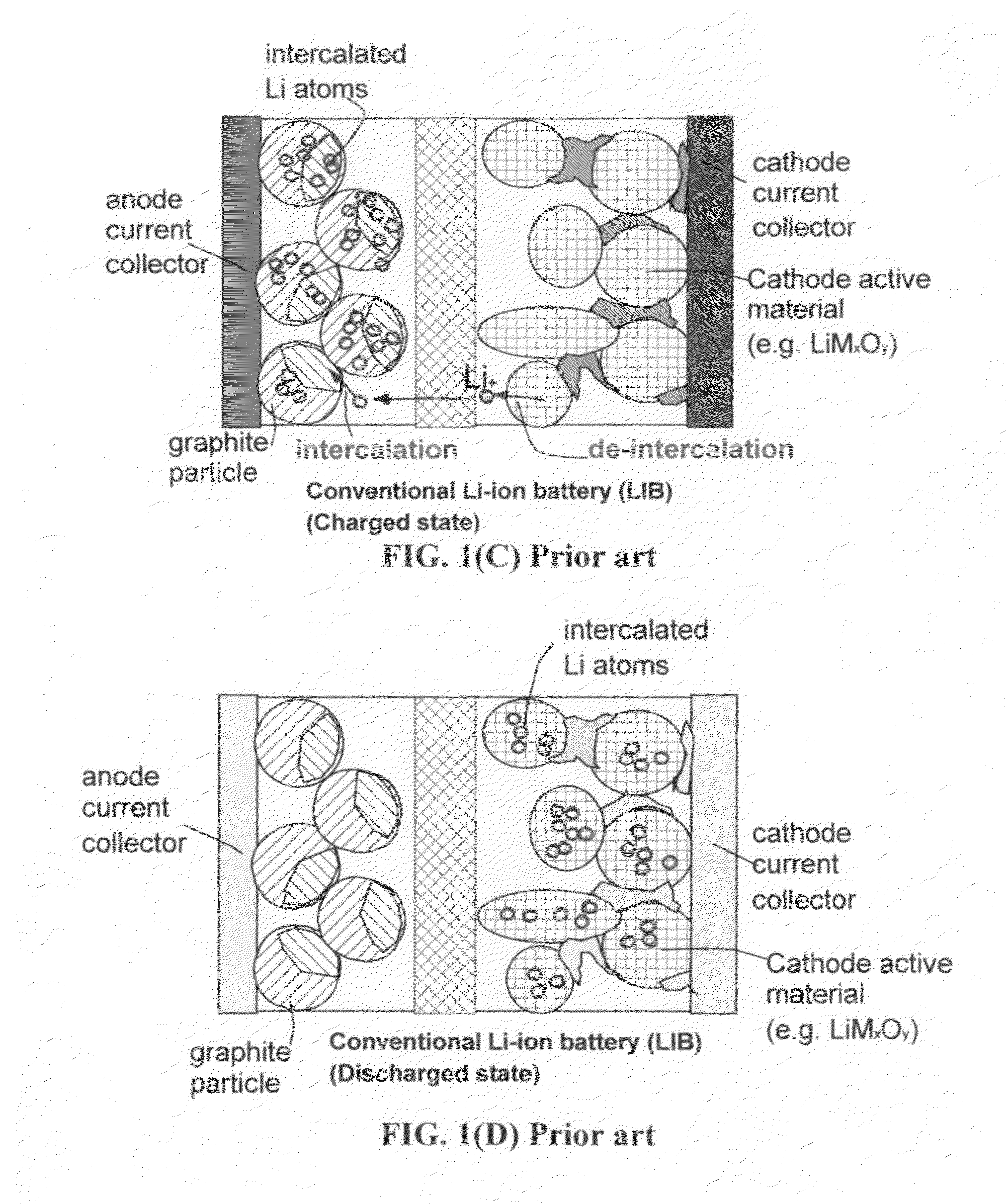
Lithium-ion cell having a high-capacity anode and a high-capacity cathode
ActiveUS20130224603A1Easy dischargeImprove power densityMaterial nanotechnologyHybrid capacitor electrodesLithiumHigh energy
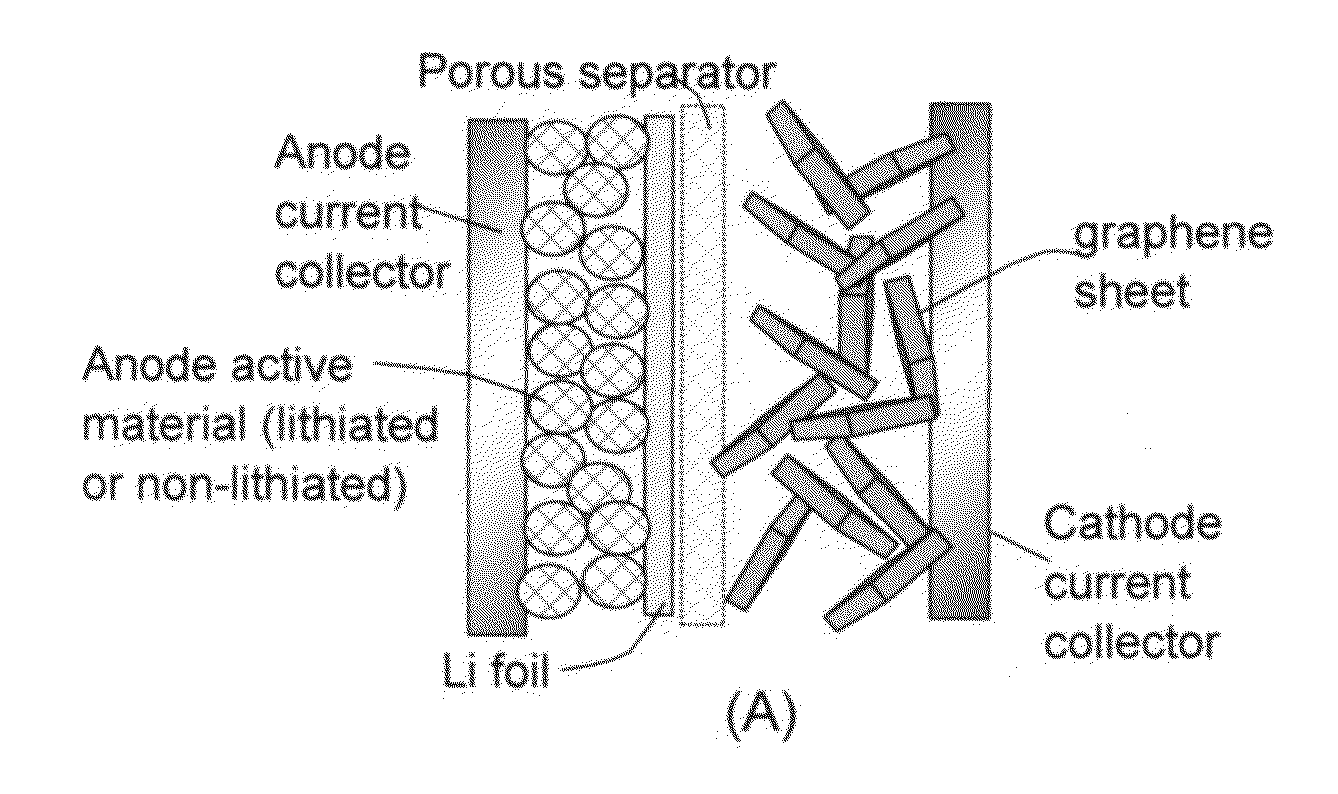
A lithium-ion cell comprising: (A) a cathode comprising graphene as the cathode active material having a surface area to capture and store lithium thereon and wherein said graphene cathode is meso-porous having a specific surface area greater than 100 m2 / g; (B) an anode comprising an anode active material for inserting and extracting lithium, wherein the anode active material is mixed with a conductive additive and / or a resin binder to form a porous electrode structure, or coated onto a current collector in a coating or thin film form; (C) a porous separator disposed between the anode and the cathode; (D) a lithium-containing electrolyte in physical contact with the two electrodes; and (E) a lithium source disposed in at least one of the two electrodes when the cell is made. This new Li-ion cell exhibits an unprecedentedly high energy density.
Owner:GLOBAL GRAPHENE GRP INC
Prelithiated current collector and secondary lithium cells containing same
ActiveUS20130045427A1Reduce capacityMaterial nanotechnologyHybrid capacitorsLithium metalLithium sulfur
The present invention provides a battery or supercapacitor current collector which is prelithiated. The prelithiated current collector comprises: (a) an electrically conductive substrate having two opposed primary surfaces, and (b) a mixture layer of carbon (and / or other stabilizing element, such as B, Al, Ga, In, C, Si, Ge, Sn, Pb, As, Sb, Bi, Te, or a combination thereof) and lithium or lithium alloy coated on at least one of the primary surfaces, wherein lithium element is present in an amount of 1% to 99% by weight of the mixture layer. This current collector serves as an effective and safe lithium source for a wide variety of electrochemical energy storage cells, including the rechargeable lithium cell (e.g. lithium-metal, lithium-ion, lithium-sulfur, lithium-air, lithium-graphene, lithium-carbon, and lithium-carbon nanotube cell) and the lithium ion based supercapacitor cell (e.g, symmetric ultracapacitor, asymmetric ultracapacitor, hybrid supercapacitor-battery, or lithium-ion capacitor).
Owner:GLOBAL GRAPHENE GRP INC
Metal-containing compounds
The invention relates to a novel solid state process for the preparation of metal-containing compounds comprising the steps i) forming a reaction mixture comprising one or more metal-containing precursor compounds and optionally one or more non-metal-containing reactants, and ii) using one or more hypophosphite-containing materials as a reducing agent; wherein one or more of the hypophosphite-containing materials is used as an agent to reduce one or more of the metal-containing precursor compounds; and further wherein the process is performed in the absence of an oxidizing atmosphere. Materials made by such a process are useful, for example, as electrode materials in alkali metal-ion battery applications.
Owner:LITHIUM WERKS TECH BV
Multi-port reconfigurable battery
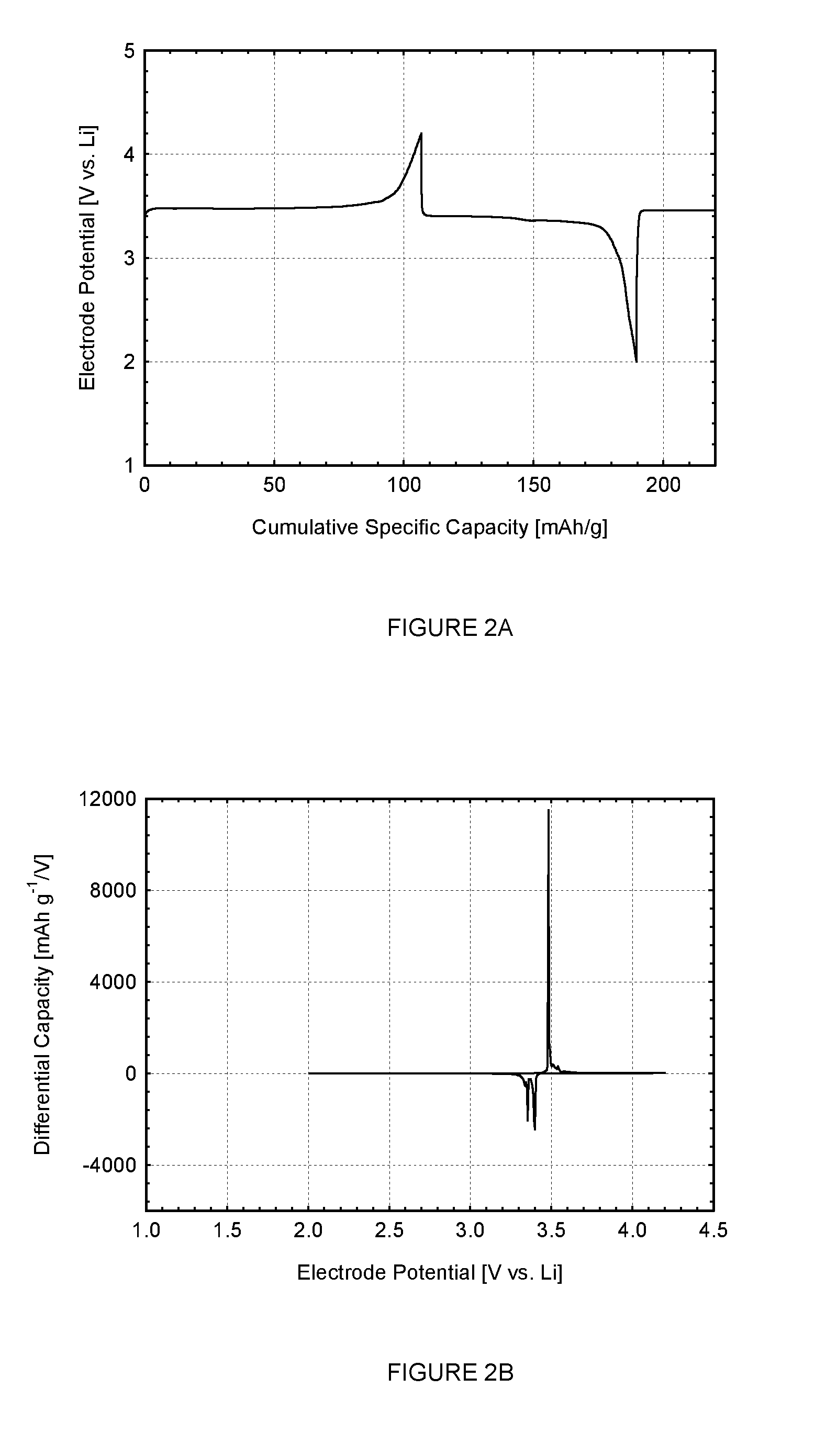
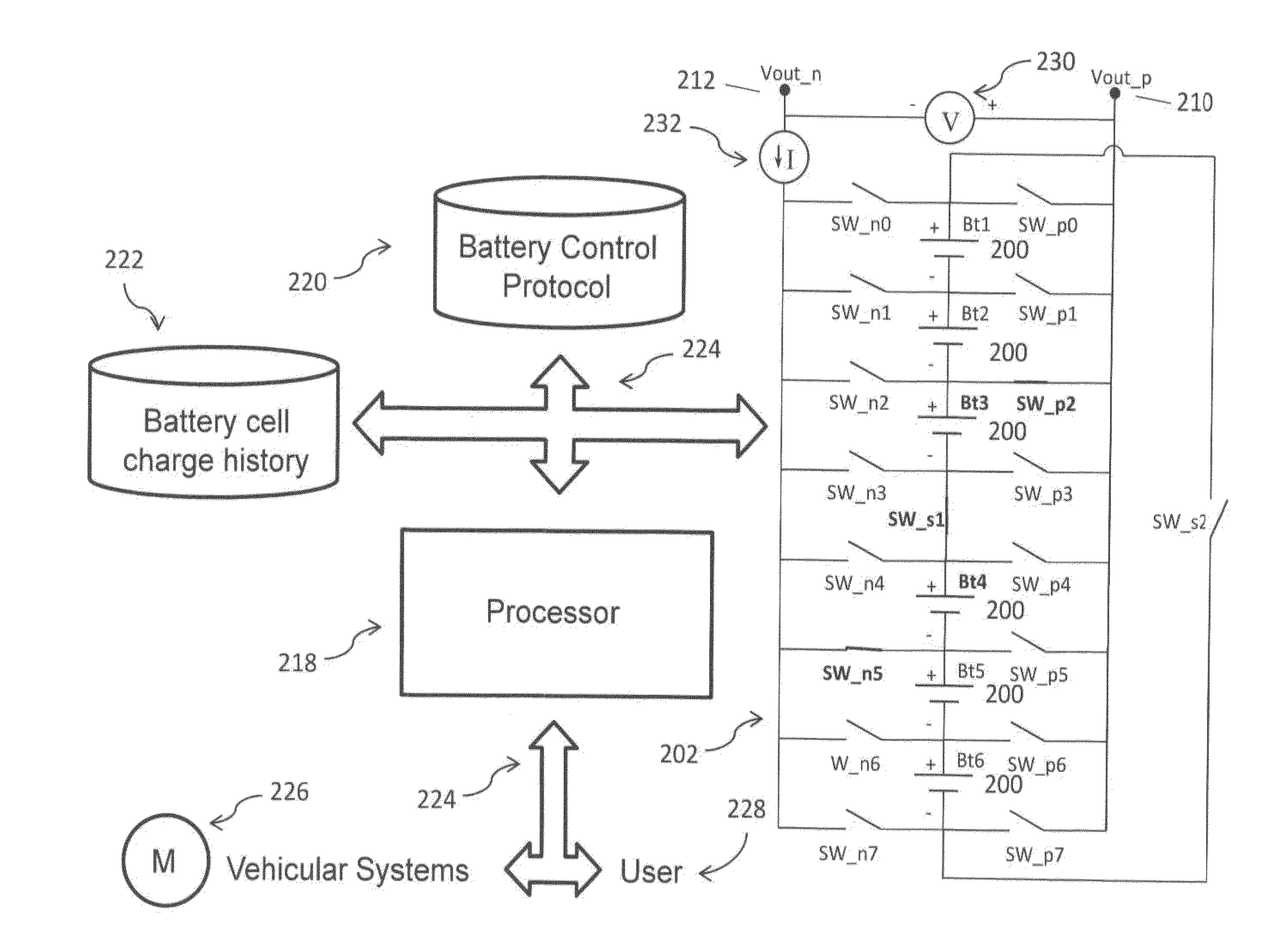
A multi-port reconfigurable battery has at least one bank of statically joined series connected battery cells, each including a positive and negative pole connected through switches to respective output connections on at least one port. Processor controlled switches reconfigure the cells to provide power for electrical loads on one or more ports and simultaneously provide charging on one or more other ports. An alternative configuration divides groups of series connected cells into separate battery banks that permit other configurations. Ports are configurable to share one electrically common connection with other ports providing a simplified configuration (multi-tap reconfigurable battery). Applications include selectable motor speed control and battery regeneration schemes matched to motor output, and single or multiphase AC power output at selectable frequencies for use as an Uninterruptible Power Supply. The battery is also described as a power source for a forced-air induction system (e.g. electric supercharger) for a combustion engine.
Owner:SOLSONA ENTERPRISE LLC
Liquid Composite Compositions Using Non-Volatile Liquids and Nanoparticles and Uses Thereof
InactiveUS20080209876A1Reduce CTEImprove performanceElectrolytic capacitorsCell electrodesOrganic solventNanoparticle
A solvent composition comprising an organic solvent; dispersed nanoparticles; and a non-volatile electrolyte.
Owner:ESIONIC
Nonaqueous electrolyte for secondary battery and nonaqueous-electrolyte secondary battery employing the same
ActiveUS20100119956A1Reduce impactLower performance requirementsCell electrodesOrganic electrolyte cellsHigh current densityDifluorophosphate
An object is to provide a nonaqueous electrolyte and a nonaqueous-electrolyte secondary battery which have excellent discharge load characteristics and are excellent in high-temperature storability, cycle characteristics, high capacity, continuous-charge characteristics, storability, gas evolution inhibition during continuous charge, high-current-density charge / discharge characteristics, discharge load characteristics, etc. The object has been accomplished with a nonaqueous electrolyte which comprises: a monofluorophosphate and / or a difluorophosphate; and further a compound having a specific chemical structure or specific properties.
Owner:MU IONIC SOLUTIONS CORP +1
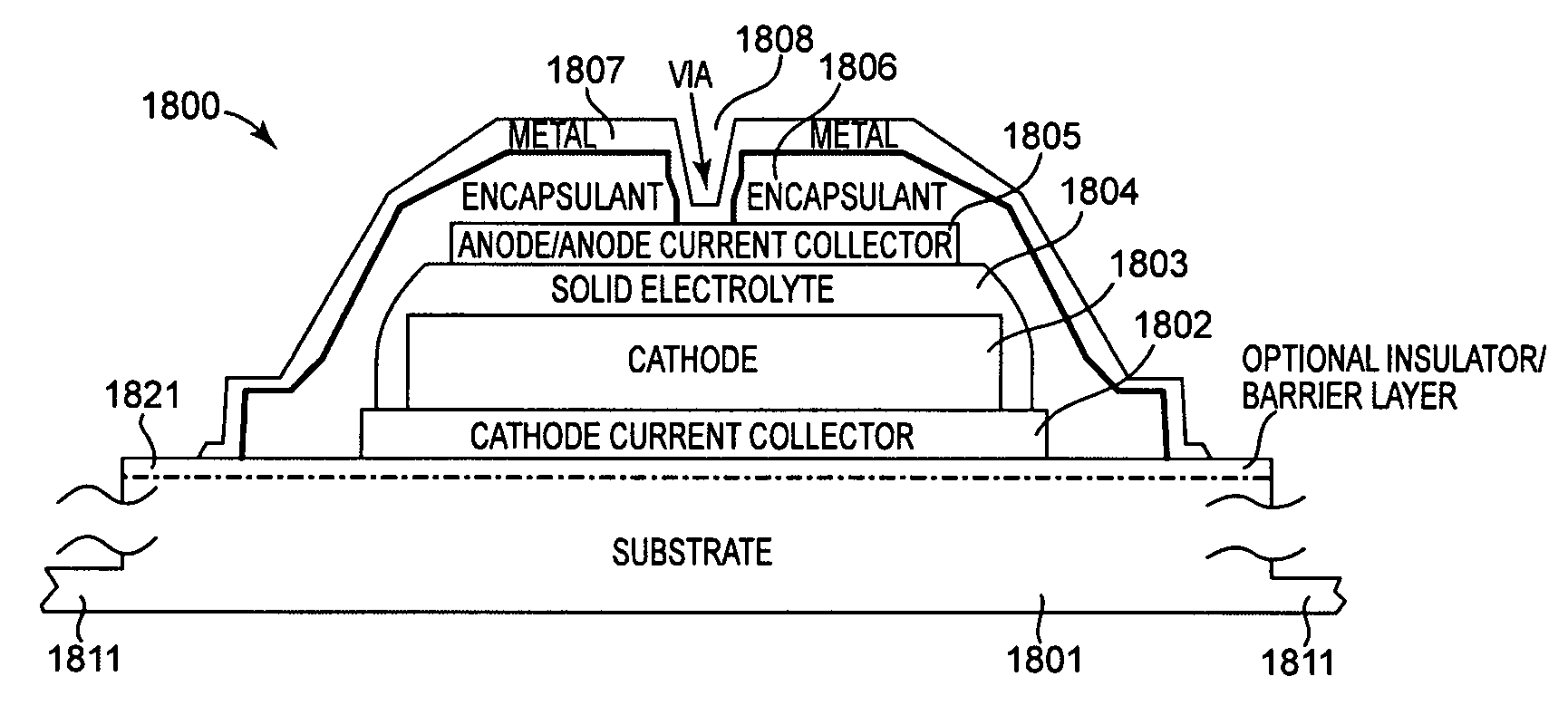
Method and apparatus for solid-state microbattery photolithographic manufacture, singulation and passivation
InactiveUS20080032236A1Efficient and economical manufactureReduced number of stepSolid electrolytesDecorative surface effectsChemical treatmentPhotoresist
A method for producing a thin film lithium battery is provided, comprising applying a cathode current collector, a cathode material, an anode current collector, and an electrolyte layer separating the cathode material from the anode current collector to a substrate, wherein at least one of the layers contains lithiated compounds that is patterned at least in part by a photolithography operation comprising removal of a photoresist material from the layer containing lithiated compounds by a process including a wet chemical treatment. Additionally, a method and apparatus for making lithium batteries by providing a first sheet that includes a substrate having a cathode material, an anode material, and a LiPON barrier / electrolyte layer separating the cathode material from the anode material; and removing a subset of first material to separate a plurality of cells from the first sheet. In some embodiments, the method further includes depositing second material on the sheet to cover the plurality of cells; and removing a subset of second material to separate a plurality of cells from the first sheet.
Owner:CYMBET CORP
High-capacity lithium-ion electrolyte, battery and preparation method of battery
ActiveCN101771167AIncrease capacityLow costFinal product manufactureCell electrodesElectrolytic agentPhysical chemistry
The invention relates to a high-capacity lithium-ion electrolyte, a battery and a preparation method of a battery, in particular to the high-capacity lithium-ion electrolyte and the battery using the high-capacity lithium-ion electrolyte and the preparation method of the battery. The electrolyte disclosed by the invention comprises lithium salt and non-aqueous organic solvent, and also consists of the following components in weight percent in terms of the total weight of the electrolyte: 0.5-7% of film-forming additive, 0-15% of flame-retardant additive, 2-10% of antiovefill additive, 0.01-2% of stabilizer and 0.01-1% of wetting agent; the electrolyte can enable the anode with high Ni content to work stably, and reduce the battery cost; the high-capacity lithium-ion battery can perform high ratio capacity and excellent safety and high temperature property and cyclic life fully due to the addition and synergetic functions of various functional additives.
Owner:JIUJIANG TINCI ADVANCED MATERIALS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com