Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2591results about "Fuel and secondary cells" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
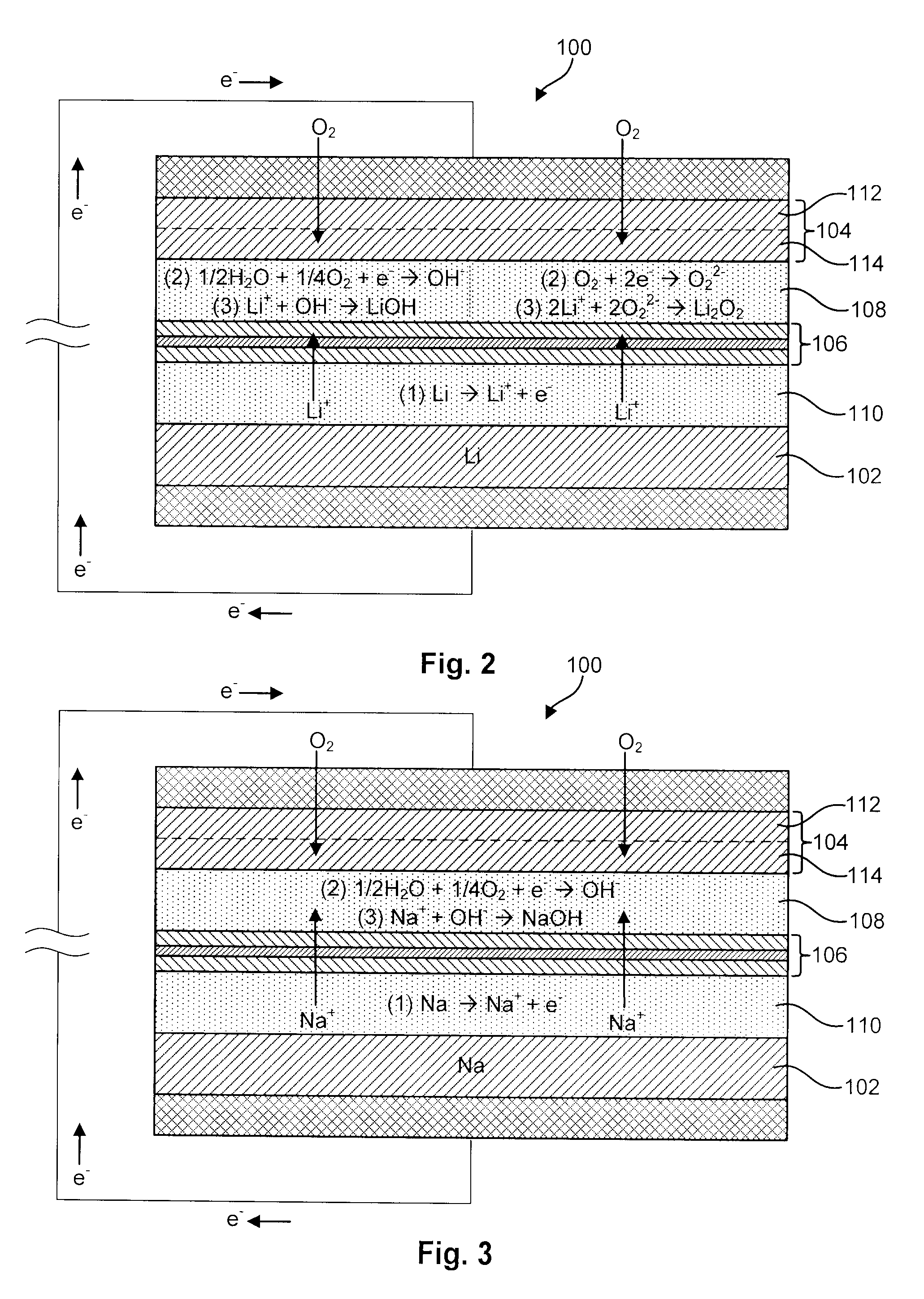
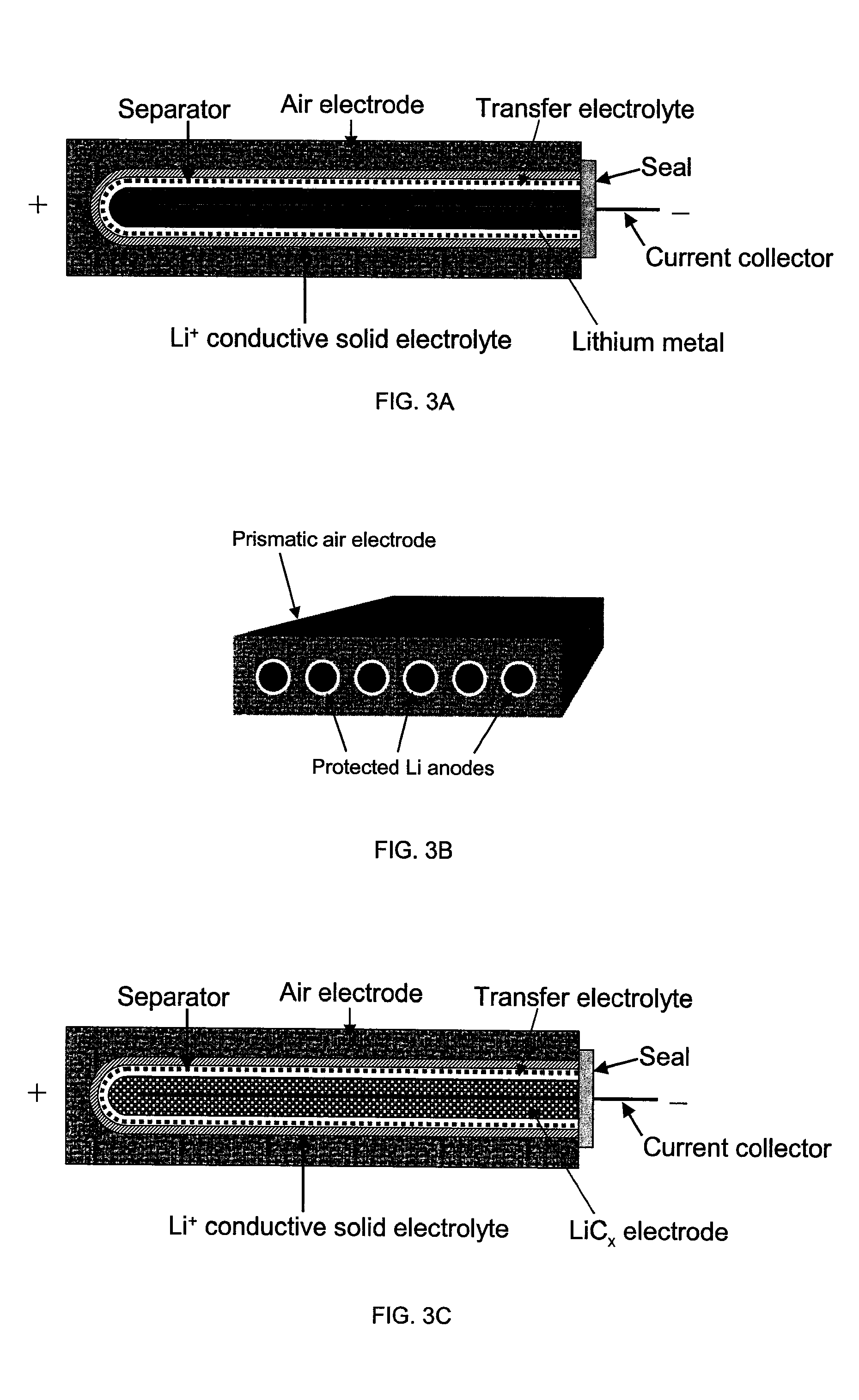
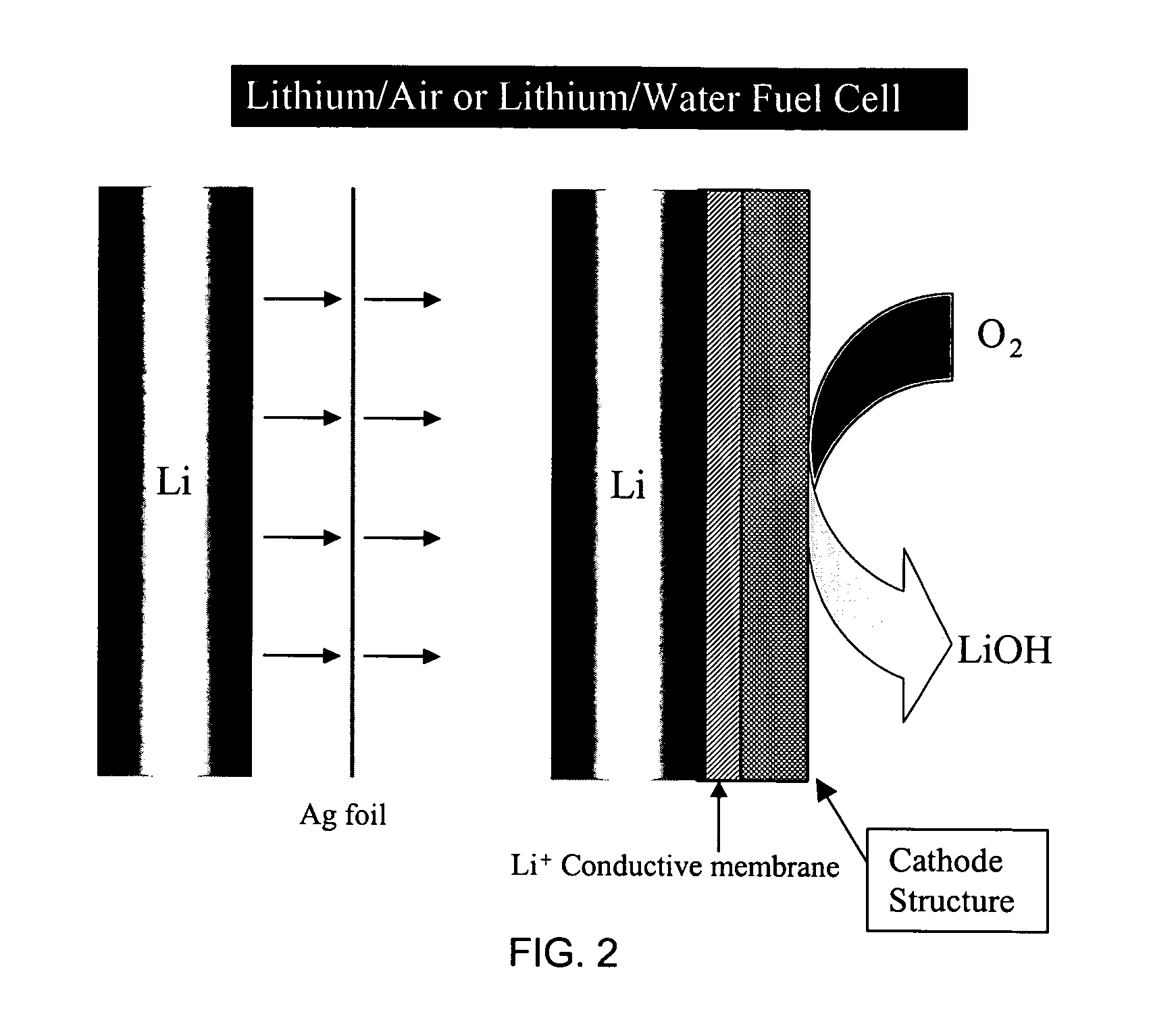
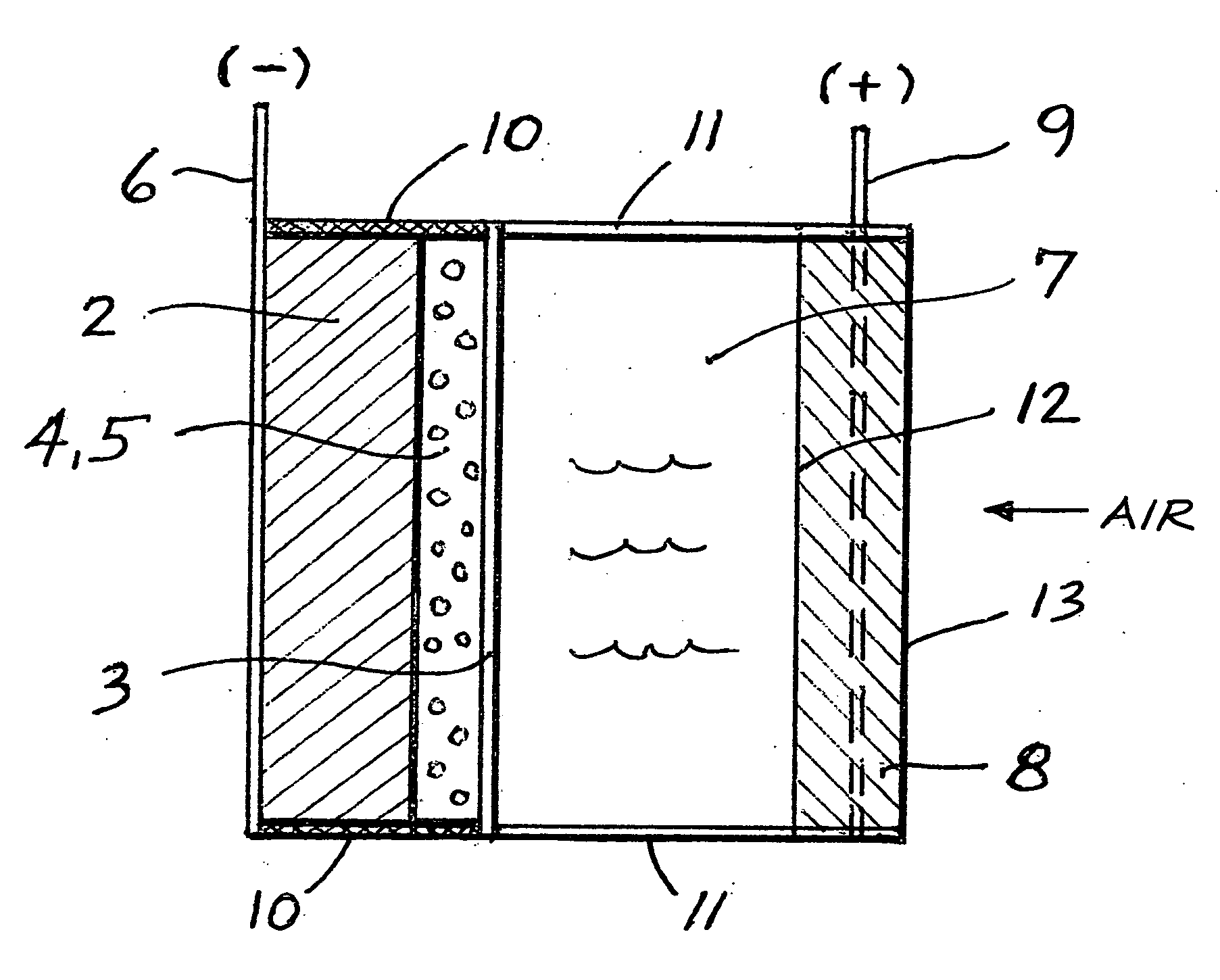
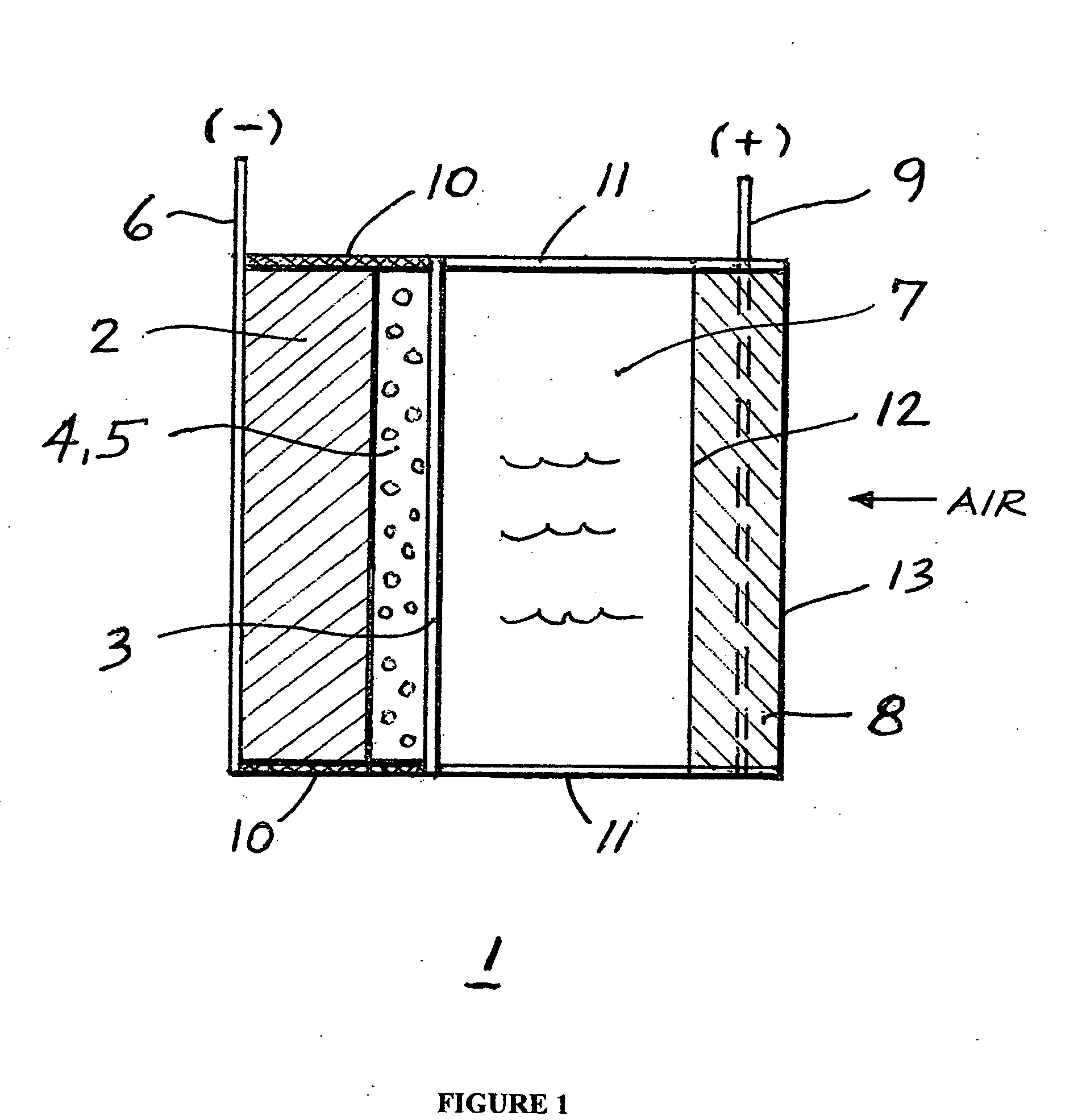
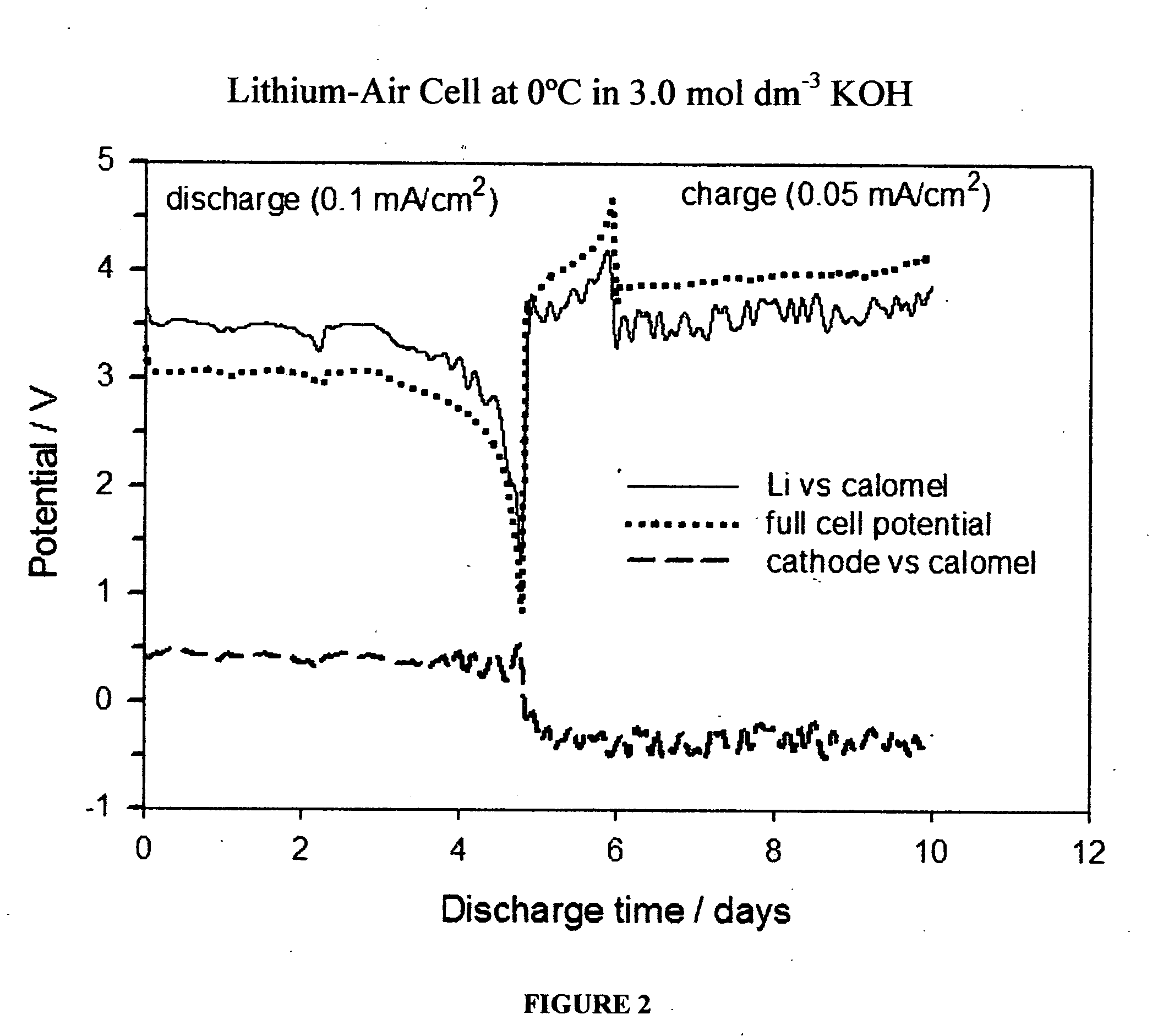
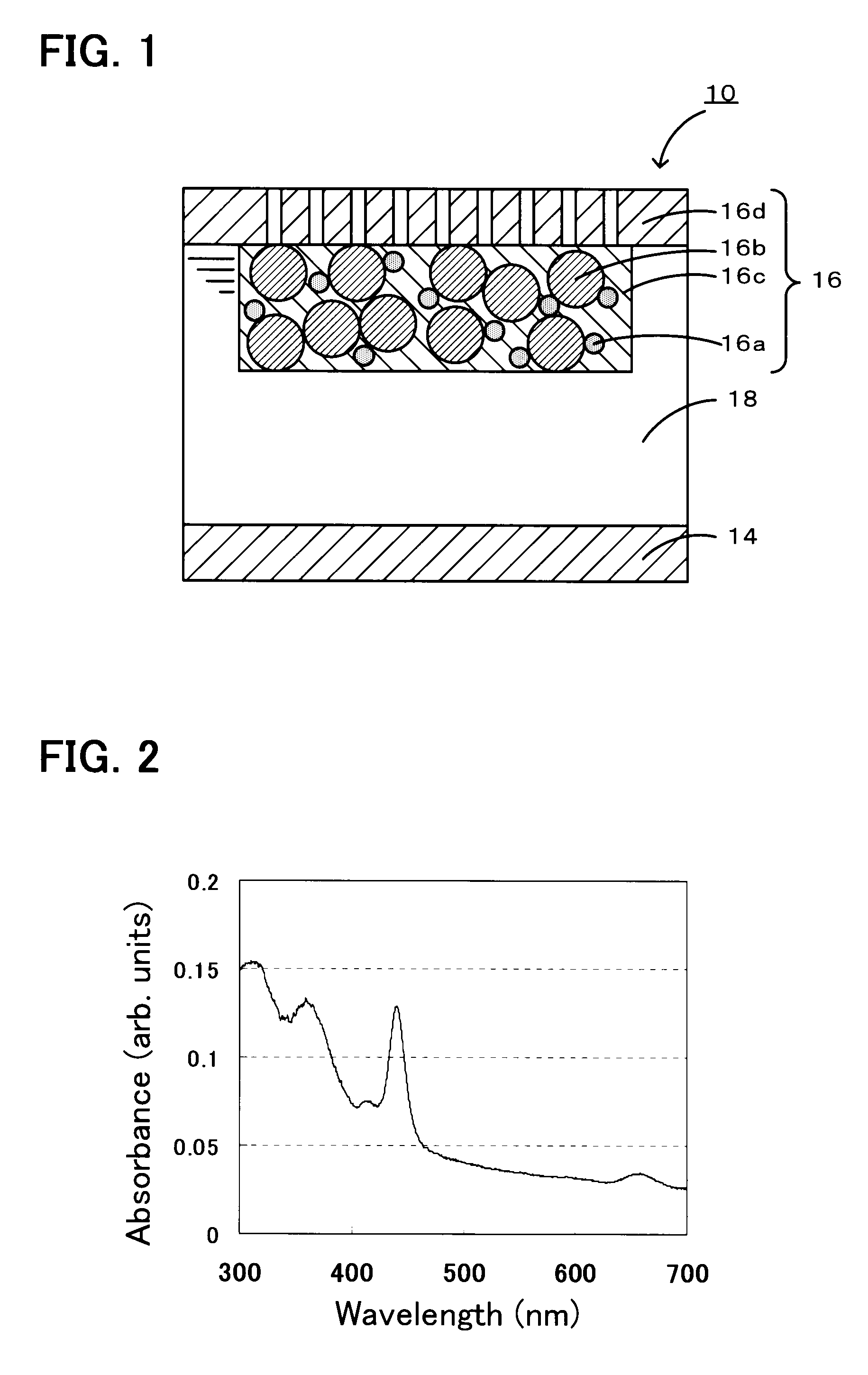
Li/air non-aqueous batteries
ActiveUS20070117007A1Improve battery performanceLarge capacityFuel and primary cellsFuel and secondary cellsLithiumOxygen
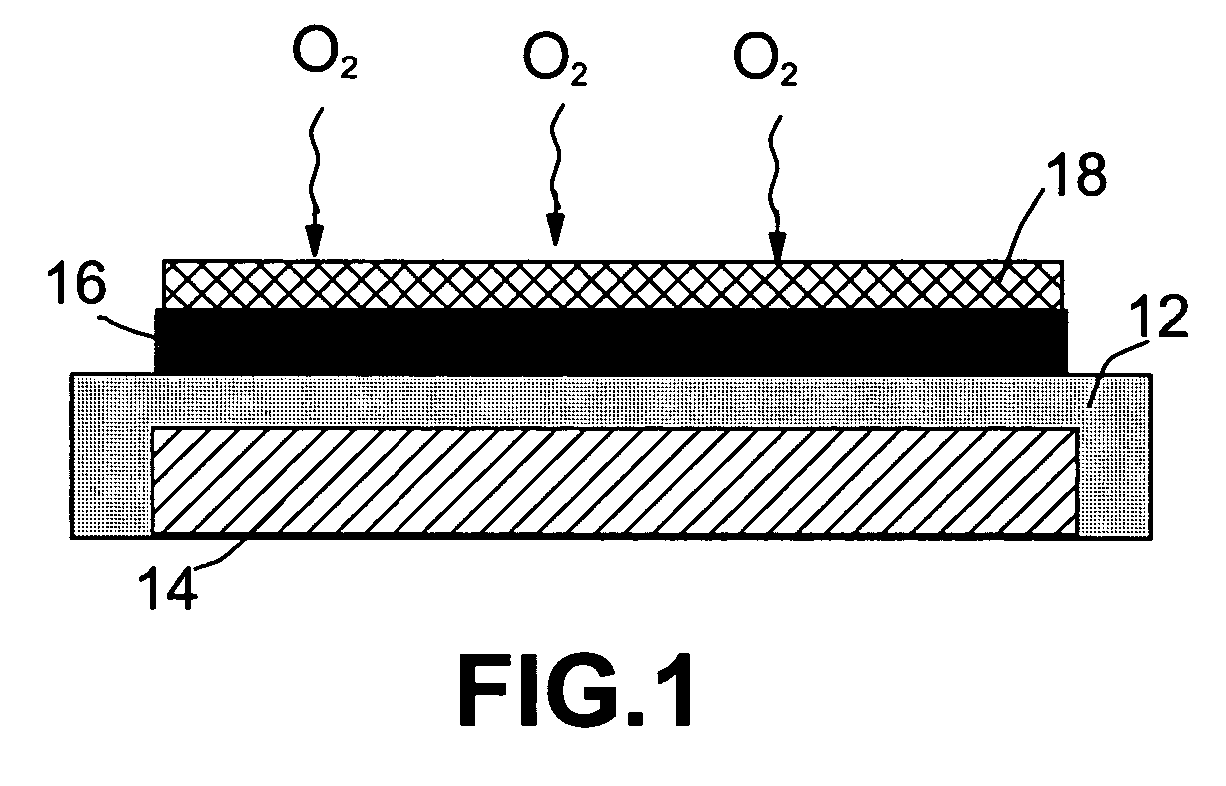
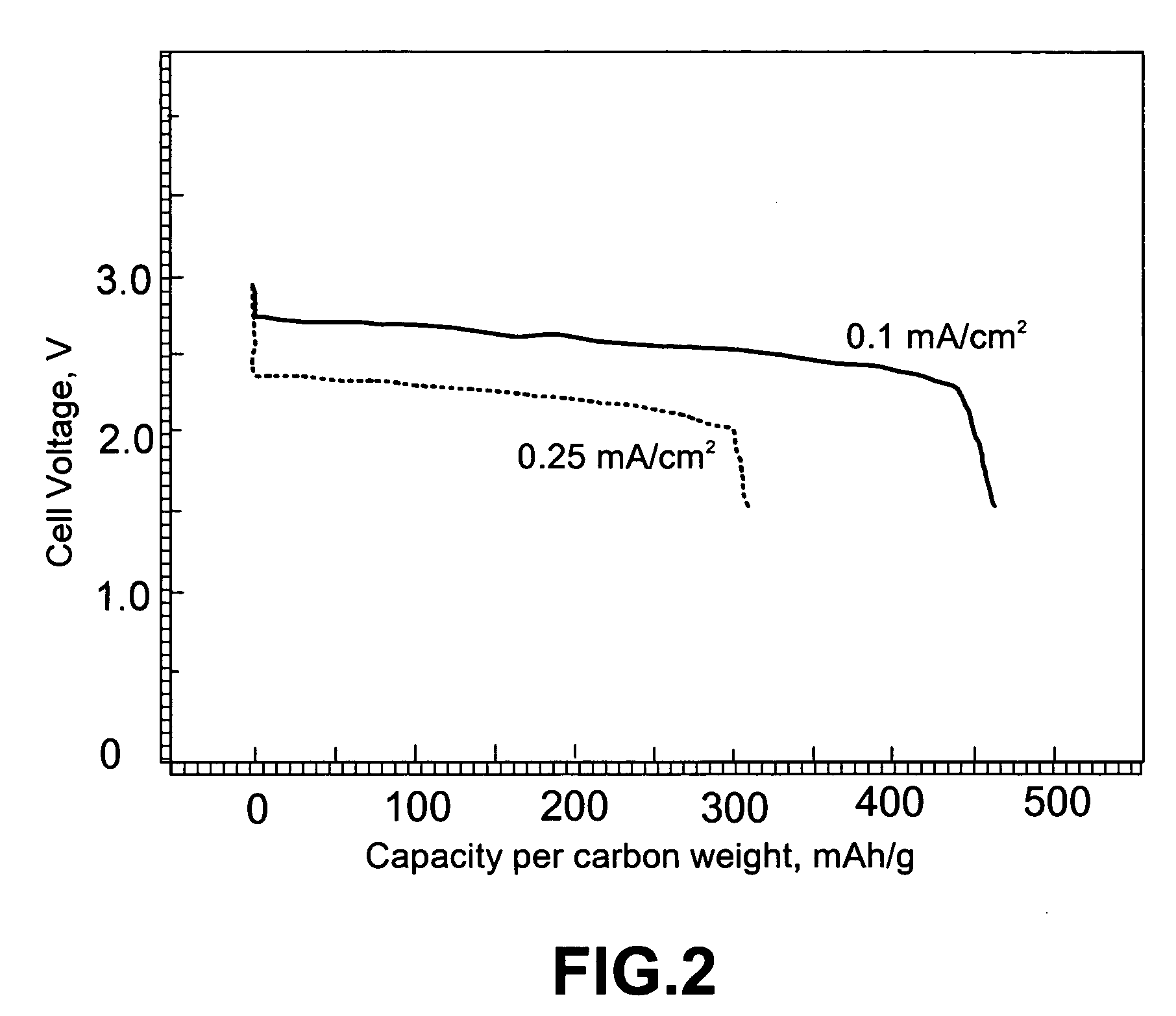
Non-aqueous alkali metal (e.g., Li) / oxygen battery cells constructed with a protected anode that minimizes anode degradation and maximizes cathode performance by enabling the use of cathode performance enhancing solvents in the catholyte have negligible self-discharge and high deliverable capacity. In particular, protected lithium-oxygen batteries with non-aqueous catholytes have this improved performance.
Owner:POLYPLUS BATTERY CO INC
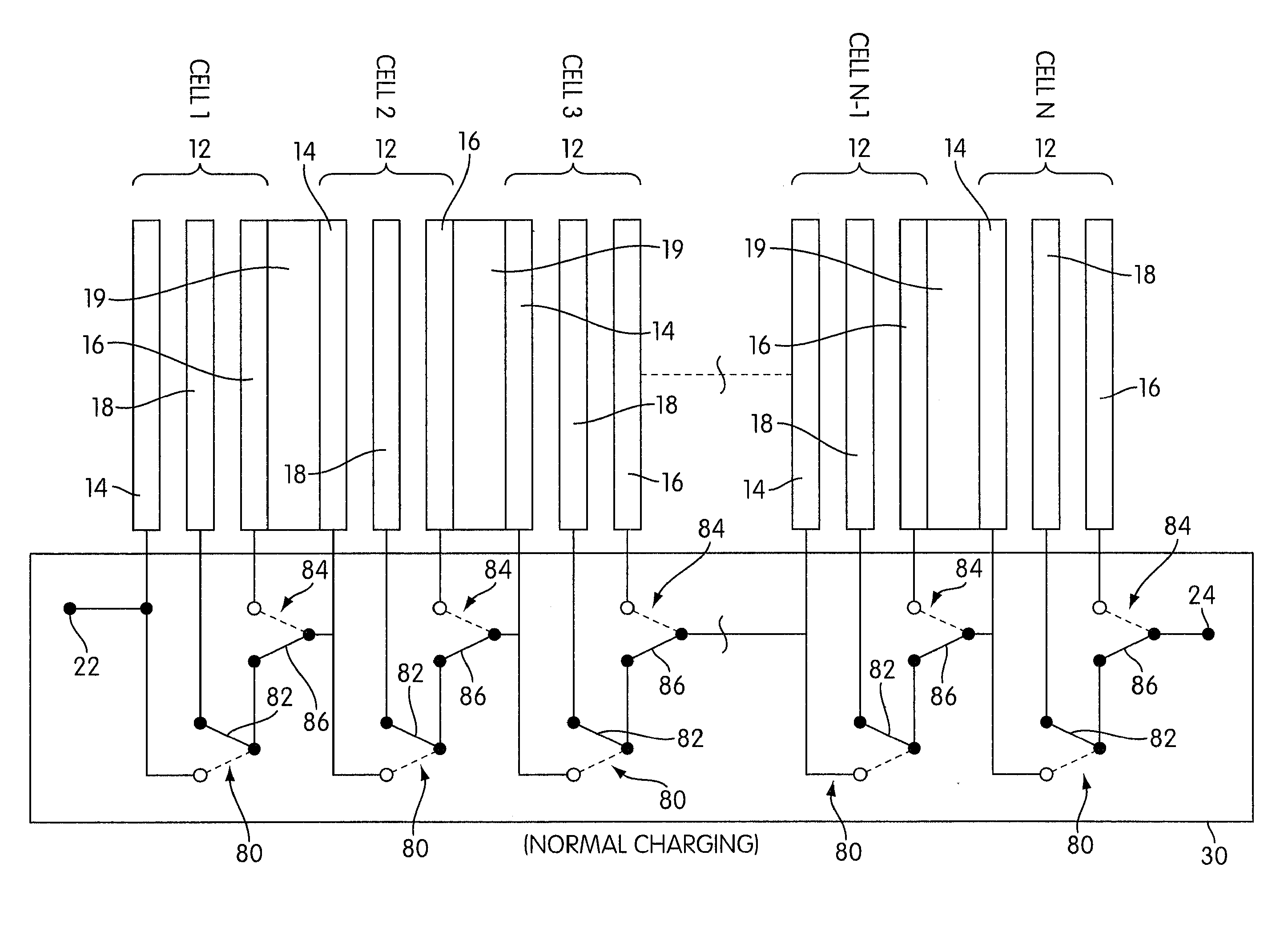
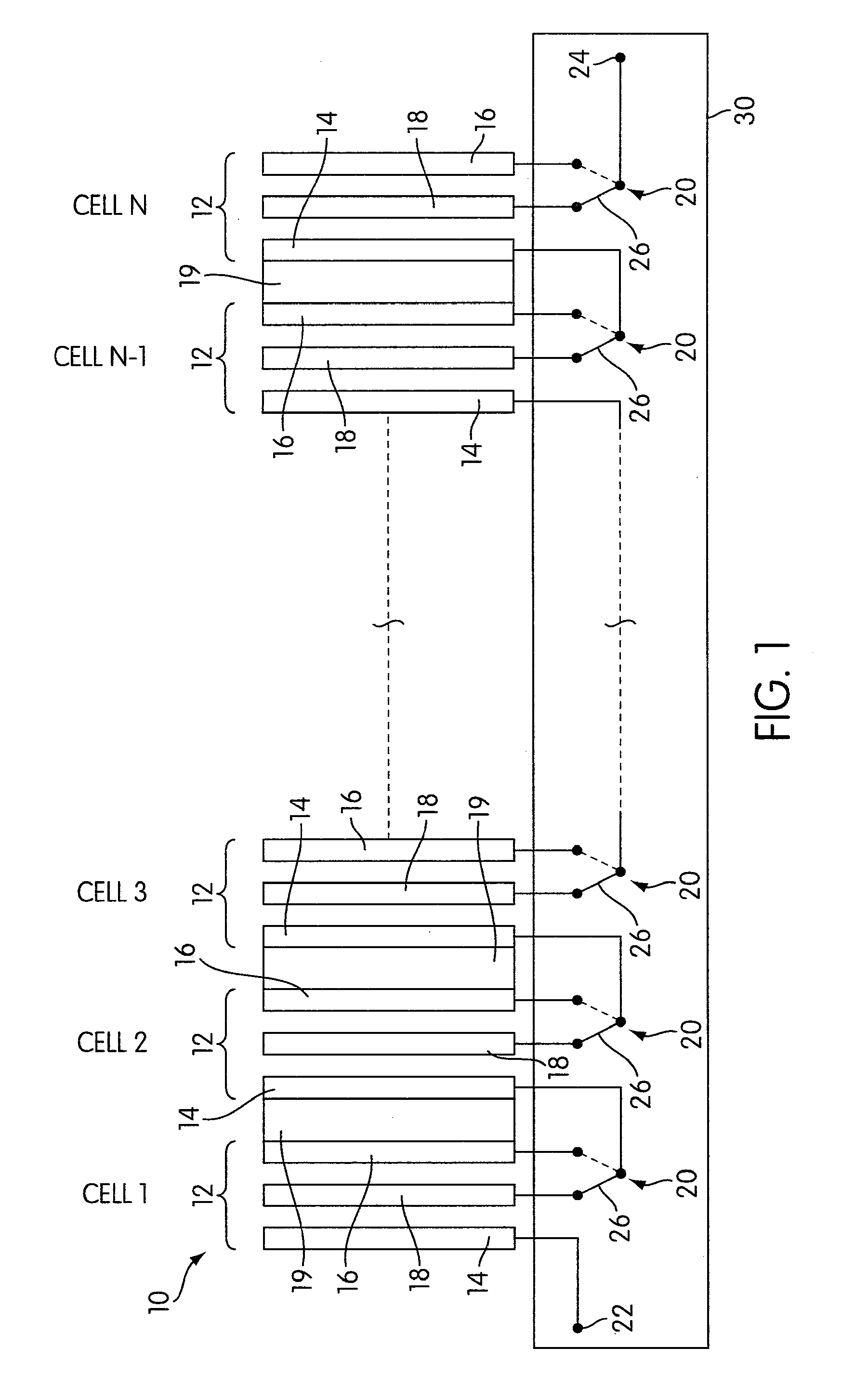
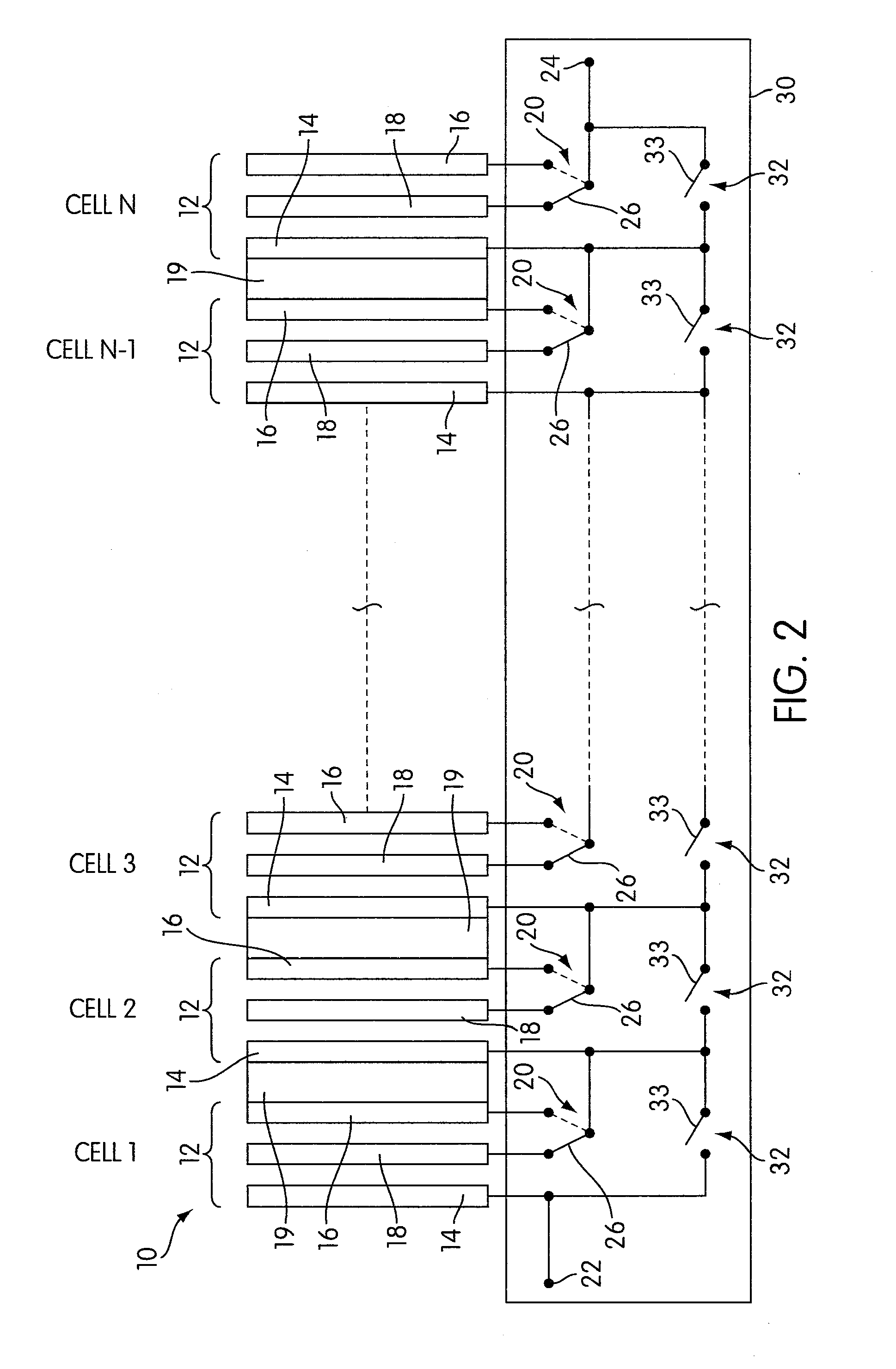
Rechargeable electrochemical cell system with a charging electrode charge/discharge mode switching in the cells
One aspect of the present invention provides a rechargeable electrochemical cell system for generating electrical current using a fuel and an oxidant. The cell system comprises N electrochemical cells each comprising a fuel electrode, an oxidant electrode, a charging electrode, and an ionically conductive medium communicating the electrodes, wherein N is an integer greater than or equal to two. Any number of cells may be used. The cell system includes a plurality of switches that are switcheable to a discharge mode coupling the oxidant electrode of each cell to the fuel electrode of the subsequent cell, a charge mode coupling the charging electrode of each cell to the fuel electrode of the subsequent cell, and a bypass mode coupling charging electrode or the oxidant electrode of a previous cell to the fuel electrode of a subsequent cell.
Owner:FLUIDIC
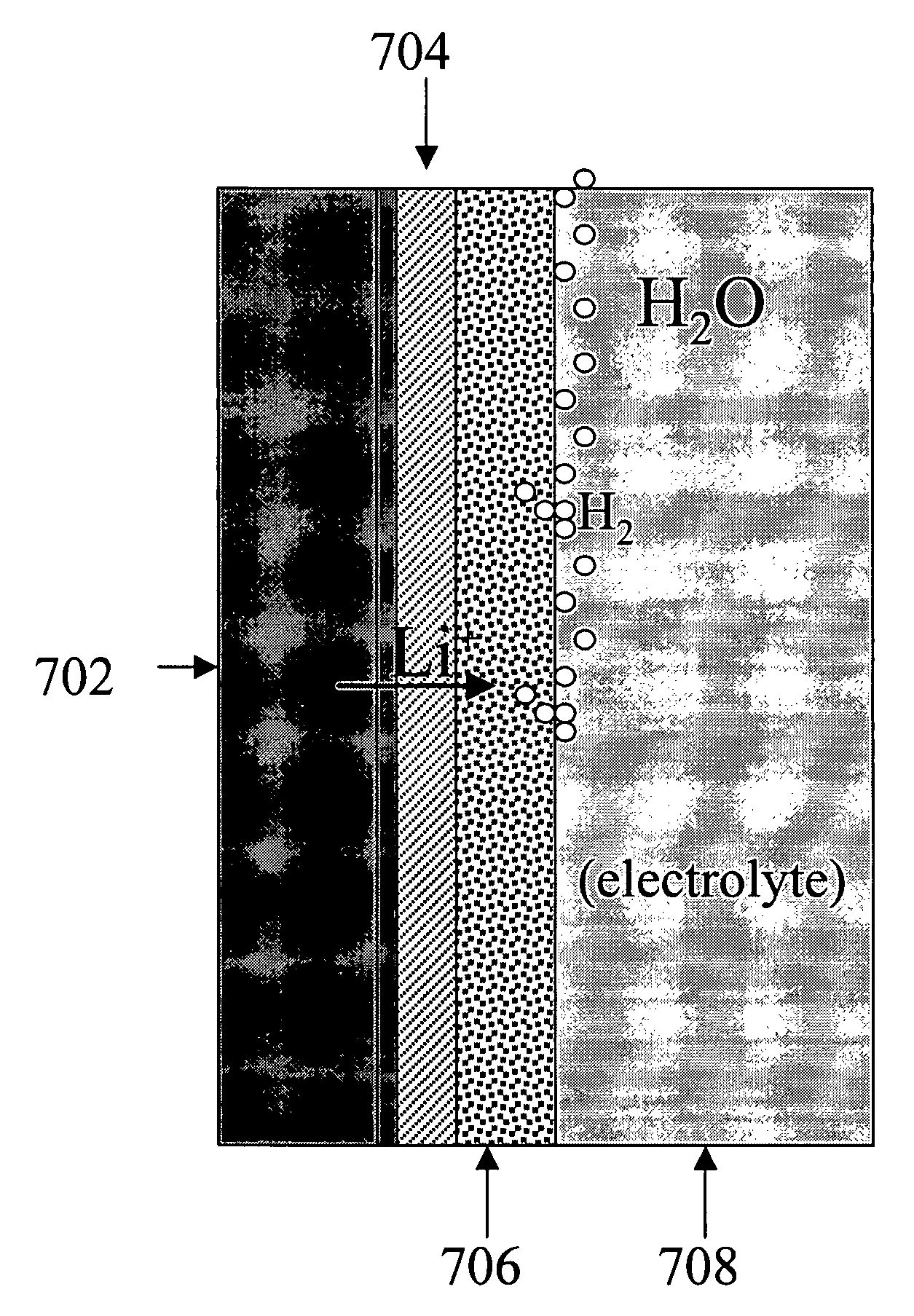
Advanced Metal-Air Battery Having a Ceramic Membrane Electrolyte Background of the Invention
ActiveUS20080268327A1Reduce oxygenReduce layeringFuel and primary cellsSolid electrolytesOxygenCeramic membrane
A metal-air battery is disclosed in one embodiment of the invention as including a cathode to reduce oxygen molecules and an alkali-metal-containing anode to oxidize the alkali metal (e.g., Li, Na, and K) contained therein to produce alkali-metal ions. An aqueous catholyte is placed in ionic communication with the cathode to store reaction products generated by reacting the alkali-metal ions with the oxygen containing anions. These reaction products are stored as solutes dissolved in the aqueous catholyte. An ion-selective membrane is interposed between the alkali-metal containing anode and the aqueous catholyte. The ion-selective membrane is designed to be conductive to the alkali-metal ions while being impermeable to the aqueous catholyte.
Owner:FIELD UPGRADING USA INC
Mesoporous carbon materials comprising bifunctional catalysts
InactiveUS20110223494A1Facilitate metal-air reactionHigh specific energyFuel and primary cellsFuel and secondary cellsLithium–air batteryElectrical devices
The present application is directed to mesoporous carbon materials comprising bi-functional catalysts. The mesoporous carbon materials find utility in any number of electrical devices, for example, in lithium-air batteries. Methods for making the disclosed carbon materials, and devices comprising the same, are also disclosed.
Owner:BASF AG
Metal-air electrochemical cell with high energy efficiency mode
ActiveUS20110250512A1Effective and efficient mannerFuel and primary cellsFuel and secondary cellsMetal–air electrochemical cellHigh energy
The present invention relates to a metal-air electrochemical cell with a high energy efficiency mode.
Owner:FORM ENERGY INC
Metal-air battery with ion-conducting inorganic glass electrolyte
InactiveUS20060063051A1Safe and reliable solid-stateIncrease energy densityFuel and primary cellsSolid electrolytesMetal–air electrochemical cellIonic conductivity
A solid-state metal-air electrochemical cell comprising: (A) a metal-containing electro-active anode; (B) an oxygen electro-active cathode; and (C) an ion-conducting glass electrolyte disposed between the metal-containing anode and the oxygen electro-active cathode. The cathode active material, which is oxygen gas, is not stored in the battery but rather fed from the environment. The oxygen cathode is preferably a composite carbon electrode which serves as the cathode current collector on which oxygen molecules are reduced during discharge of the battery to generate electric current. The glass electrolyte typically has an ion conductivity in the range of 5×10−5 to 2×10−3 S / cm. The electrolyte layer is preferably smaller than 10 μm in thickness and further preferably smaller than 1 μm. The anode metal is preferably lithium or lithium alloy, but may be selected from other elements such as sodium, magnesium, calcium, aluminum and zinc.
Owner:JANG BOR Z
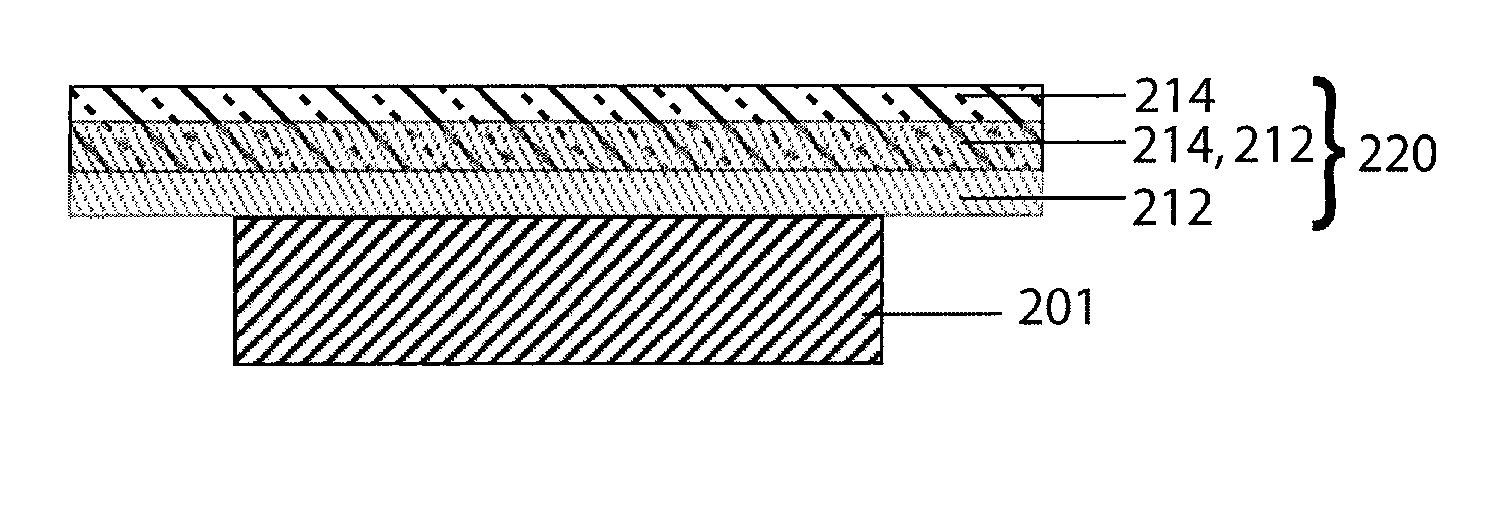
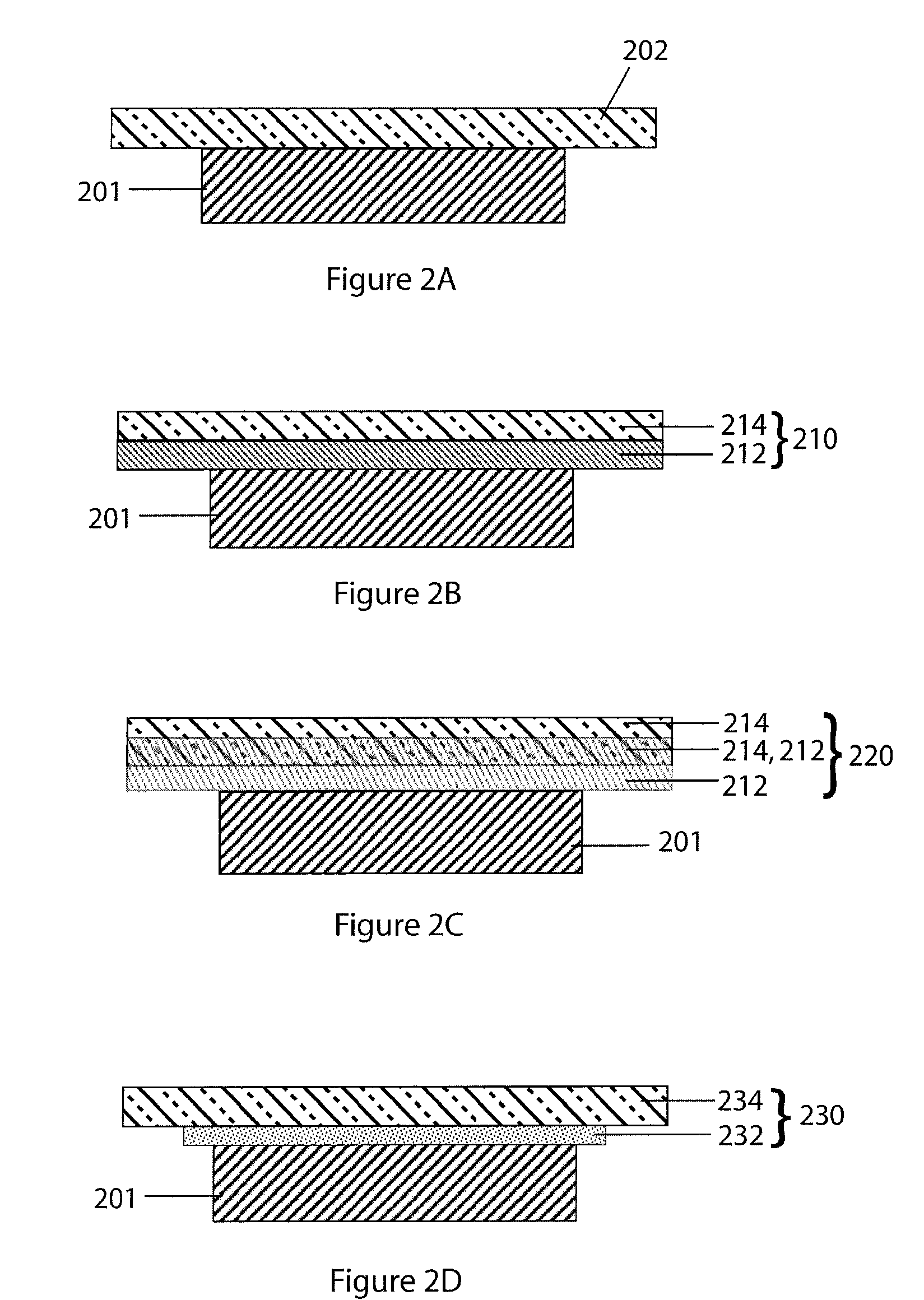
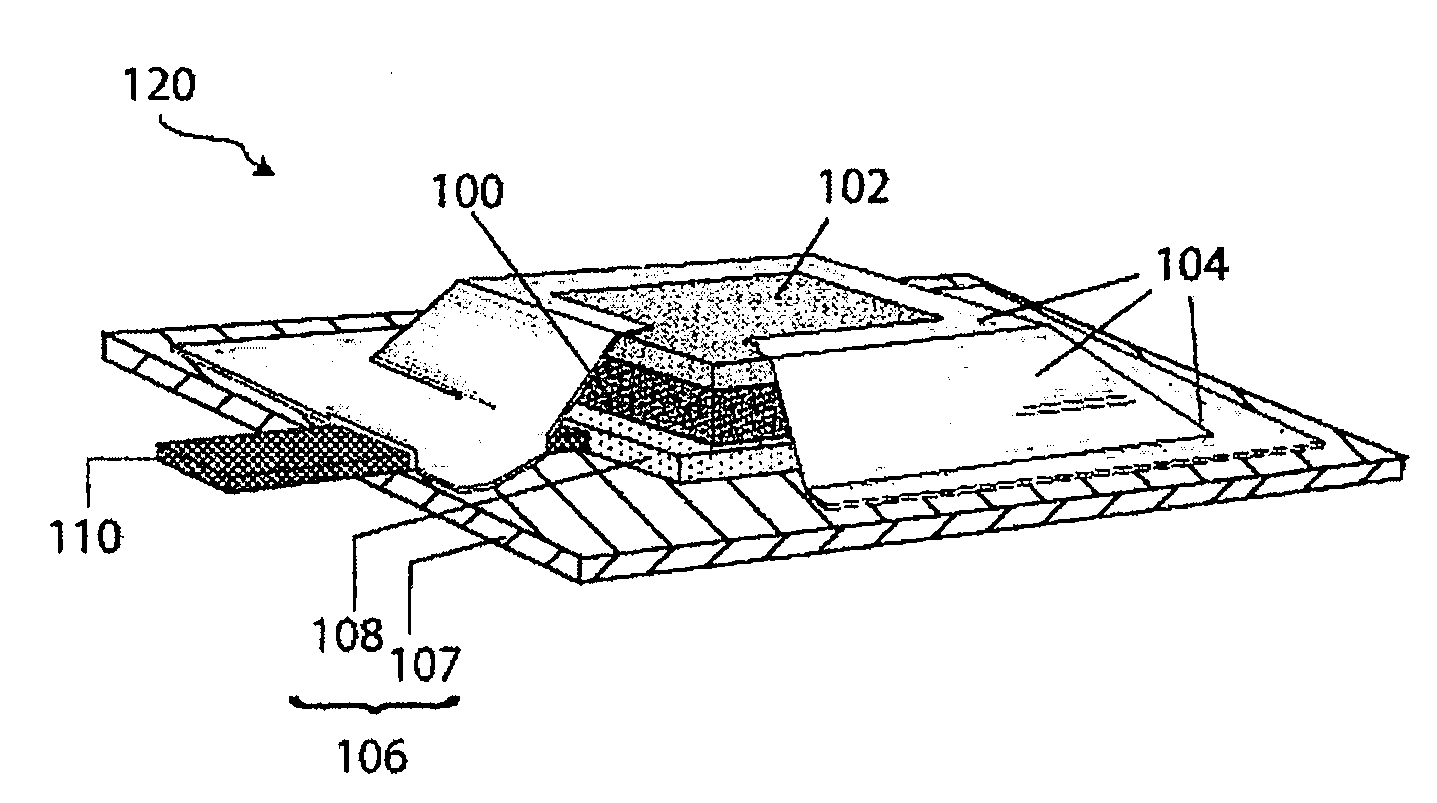
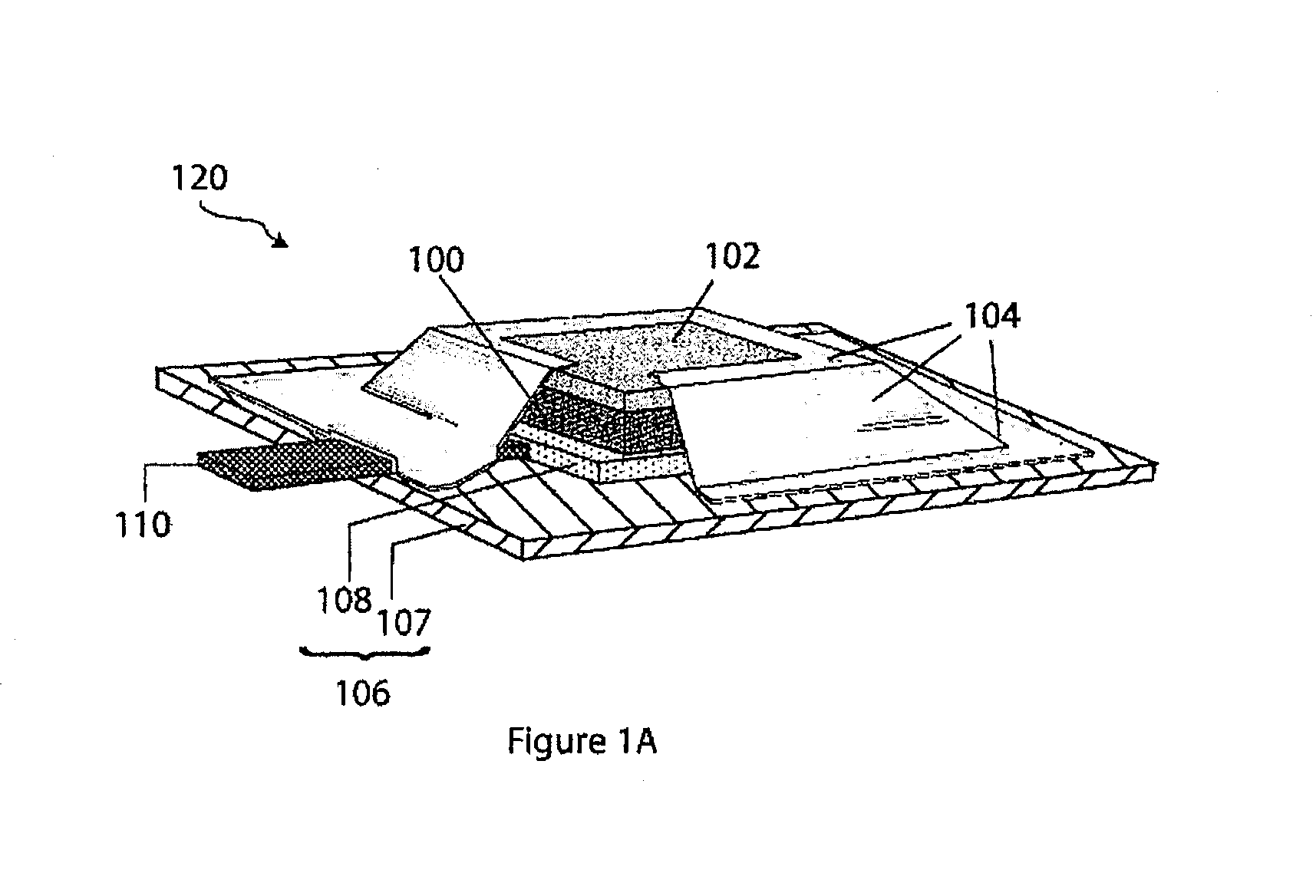
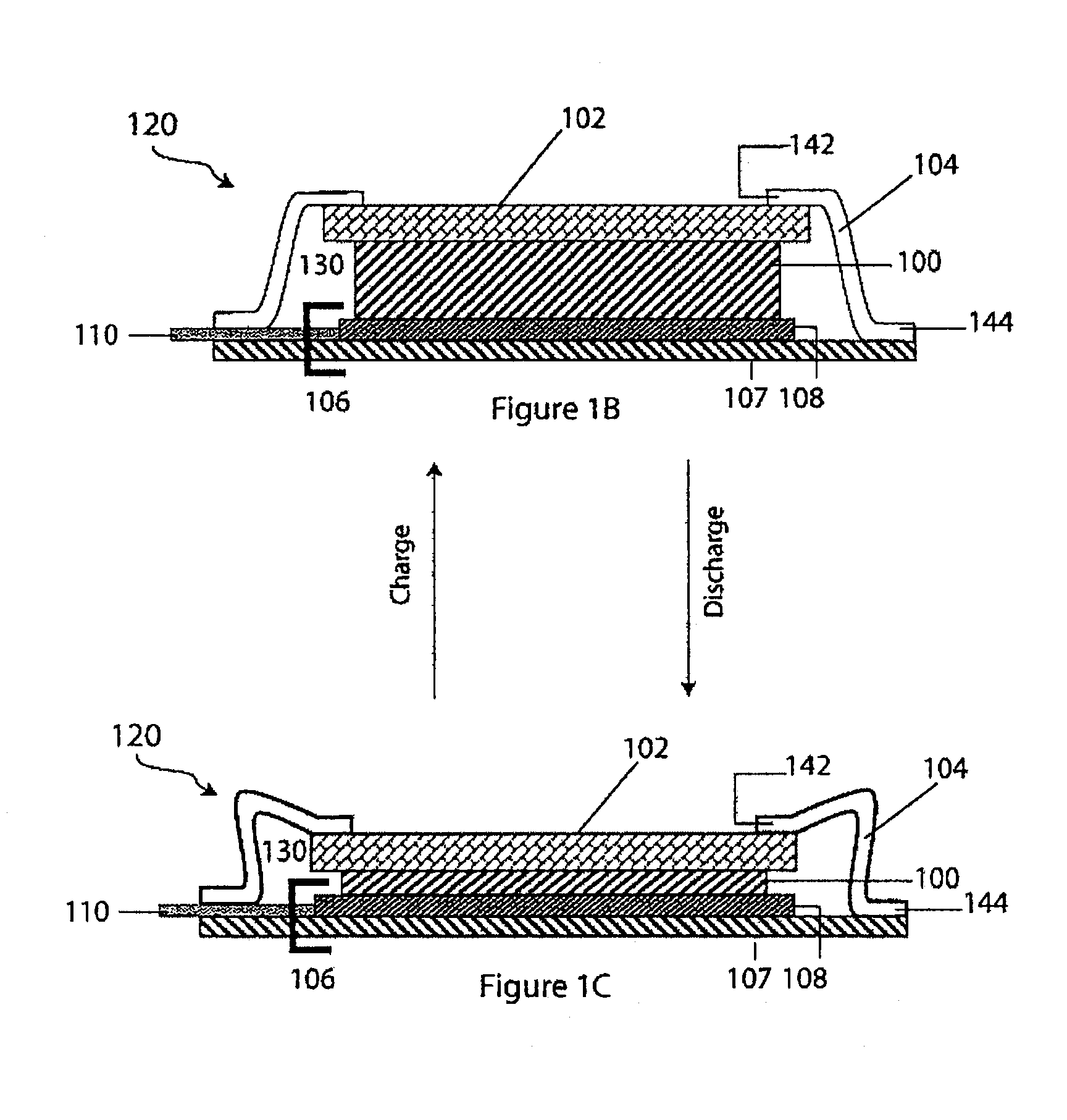
Compliant seal structures for protected active metal anodes
ActiveUS20100112454A1Avoid lostReduced ionic contact areaPrimary cell to battery groupingFuel and primary cellsOptoelectronicsAnodic protection
Protected anode architectures have ionically conductive protective membrane architectures that, in conjunction with compliant seal structures and anode backplanes, effectively enclose an active metal anode inside the interior of an anode compartment. This enclosure prevents the active metal from deleterious reaction with the environment external to the anode compartment, which may include aqueous, ambient moisture, and / or other materials corrosive to the active metal. The compliant seal structures are substantially impervious to anolytes, catholyes, dissolved species in electrolytes, and moisture and compliant to changes in anode volume such that physical continuity between the anode protective architecture and backplane are maintained. The protected anode architectures can be used in arrays of protected anode architectures and battery cells of various configurations incorporating the protected anode architectures or arrays.
Owner:POLYPLUS BATTERY CO INC
Lithium secondary batteries containing non-flammable quasi-solid electrolyte
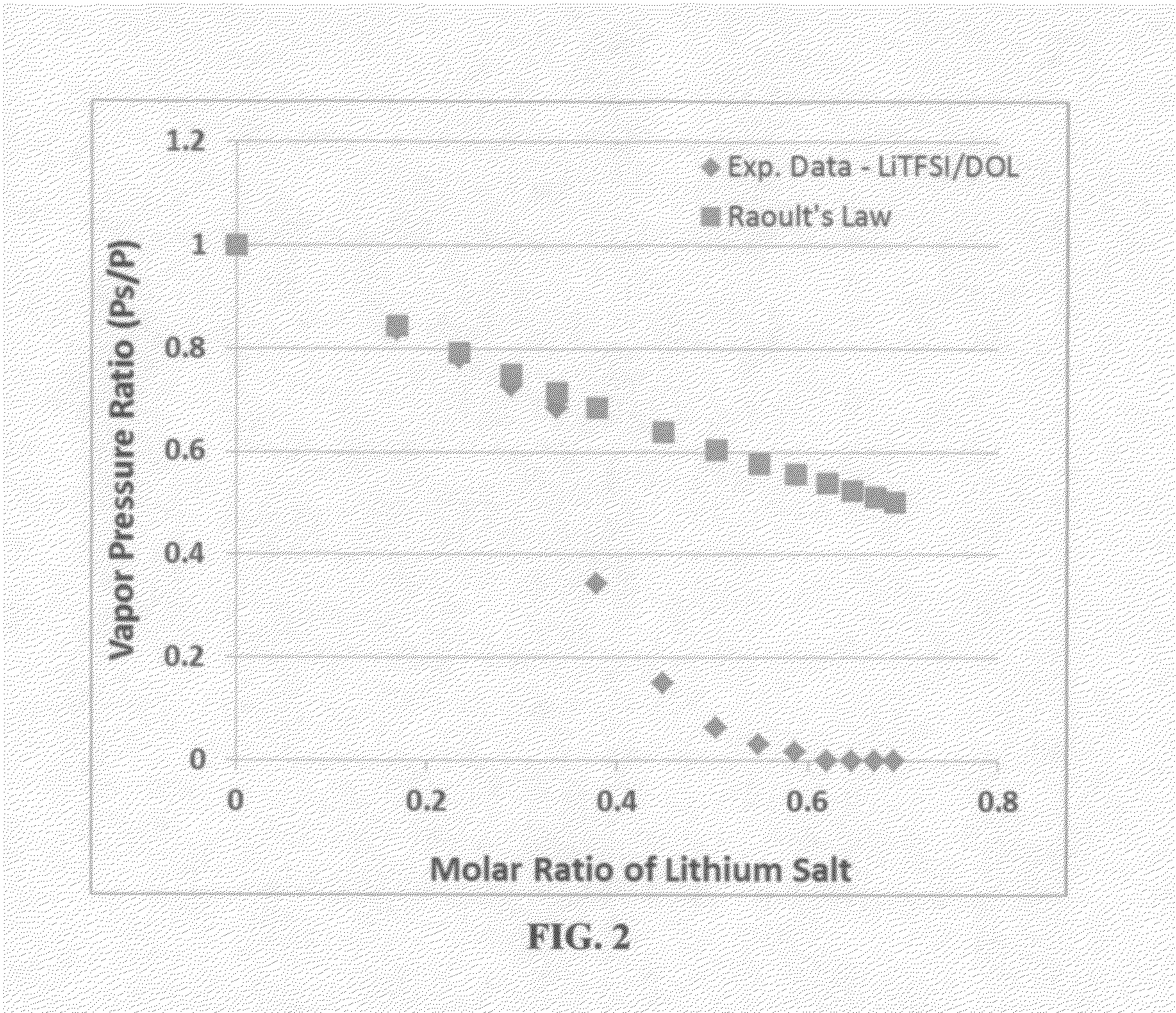
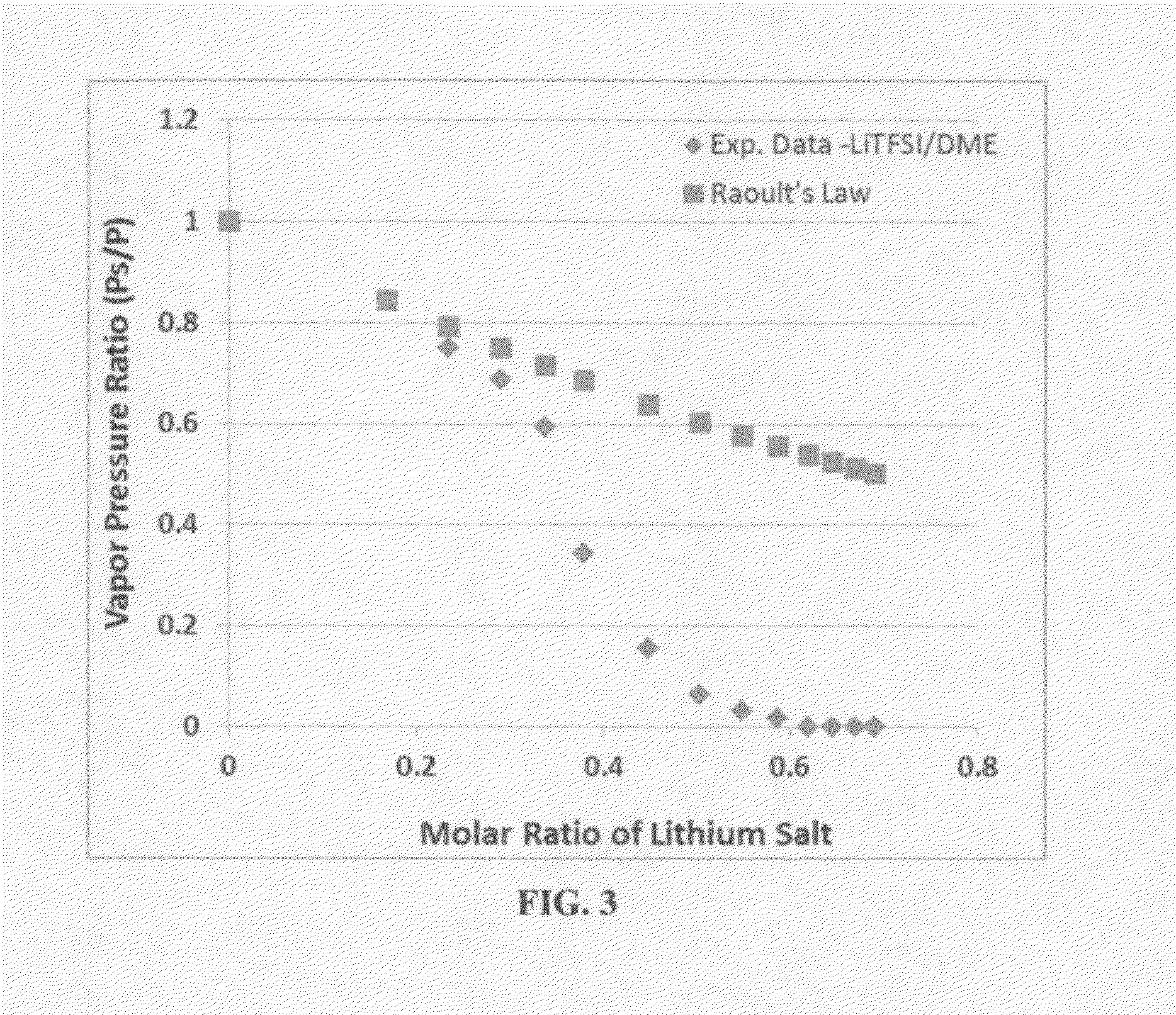
ActiveUS20140363746A1Reduce electrical conductivityLow ionic conductivityFuel and secondary cellsSolid electrolyte cellsSolventVapor pressure
A rechargeable lithium cell comprising a cathode having a cathode active material, an anode having an anode active material, a porous separator electronically separating the anode and the cathode, a non-flammable quasi-solid electrolyte in contact with the cathode and the anode, wherein the electrolyte contains a lithium salt dissolved in a first organic liquid solvent with a concentration sufficiently high so that the electrolyte exhibits a vapor pressure less than 0.01 kPa when measured at 20° C., a flash point at least 20 degrees Celsius higher than the flash point of the first organic liquid solvent alone, a flash point higher than 150° C., or no flash point. This battery cell is non-flammable and safe, has a long cycle life, high capacity, and high energy density.
Owner:GLOBAL GRAPHENE GRP INC
Anode compositions for lithium secondary batteries
ActiveUS20110165462A1High specific capacityHigh reversible capacitySolid electrolytesFuel and secondary cellsNano structuringElectrical battery
A lithium secondary battery comprising a cathode, an anode, and a separator-electrolyte assembly or electrolyte layer disposed between the cathode and the anode, wherein the anode comprises: (a) an integrated nano-structure of electrically conductive nanometer-scaled filaments that are interconnected to form a porous network of electron-conducting paths comprising interconnected pores, wherein the filaments have a transverse dimension less than 500 nm; and (b) a foil of lithium or lithium alloy as an anode active material. The battery exhibits an exceptionally high specific capacity, an excellent reversible capacity, and a long cycle life.
Owner:GLOBAL GRAPHENE GRP INC
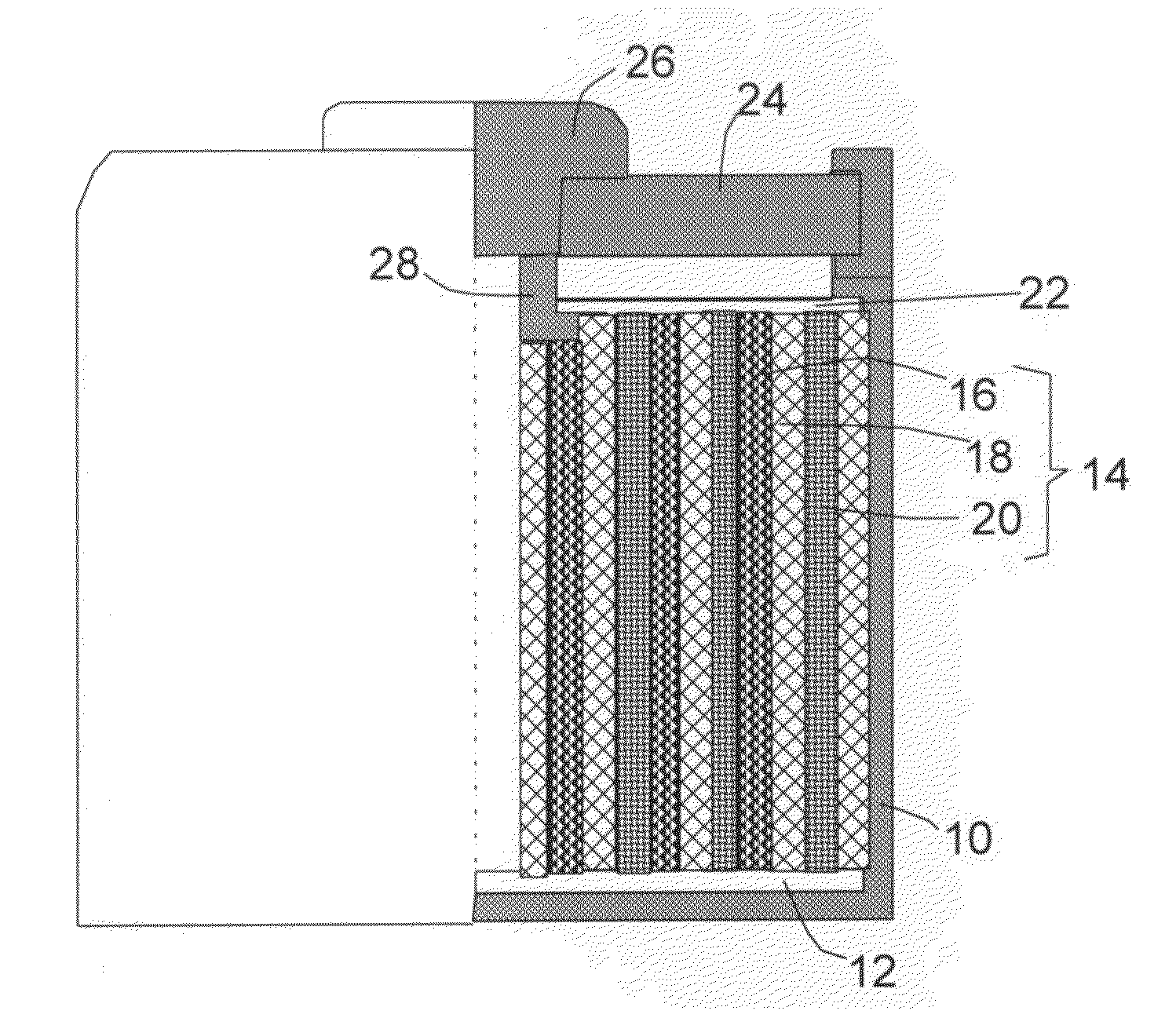
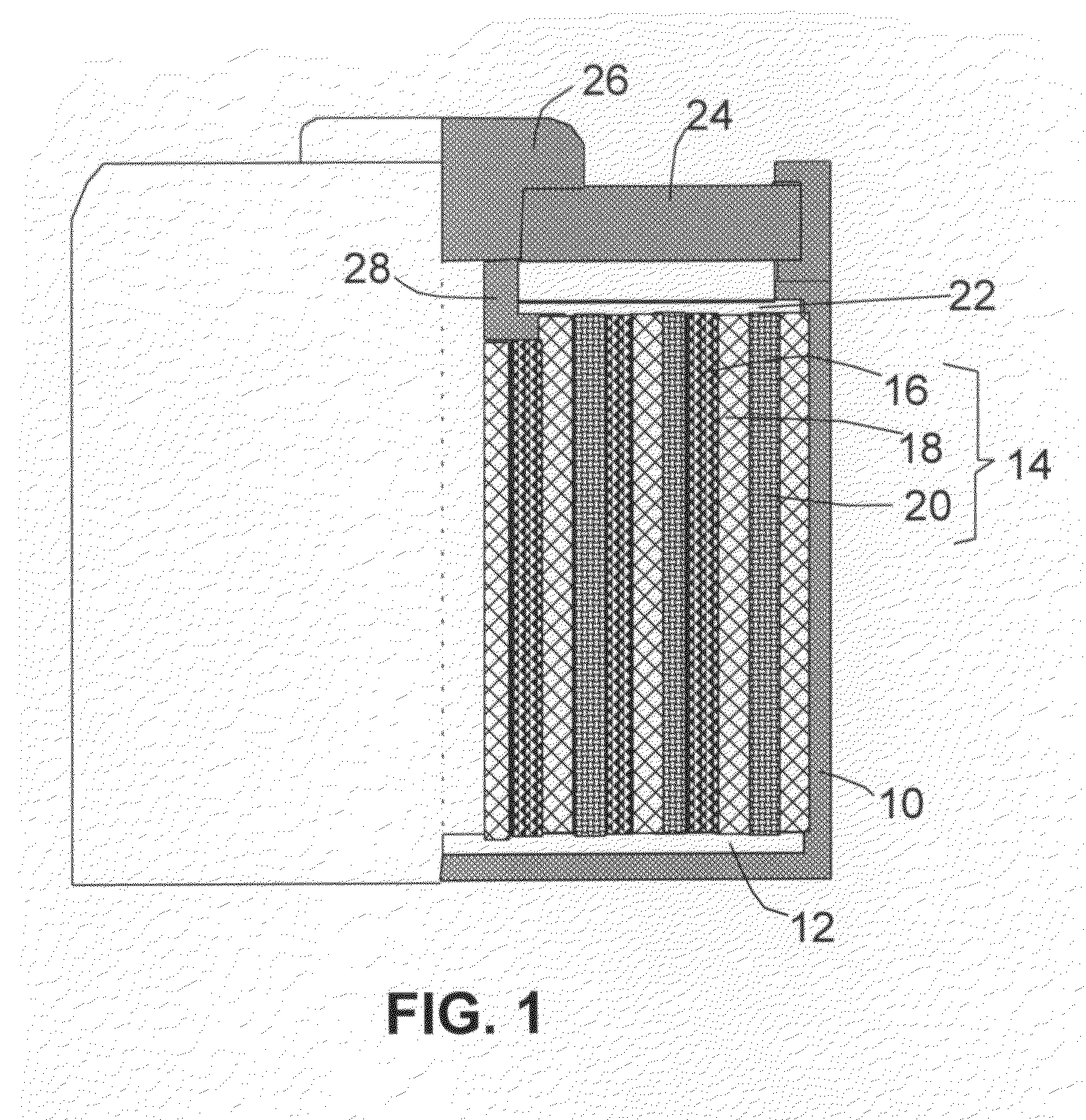
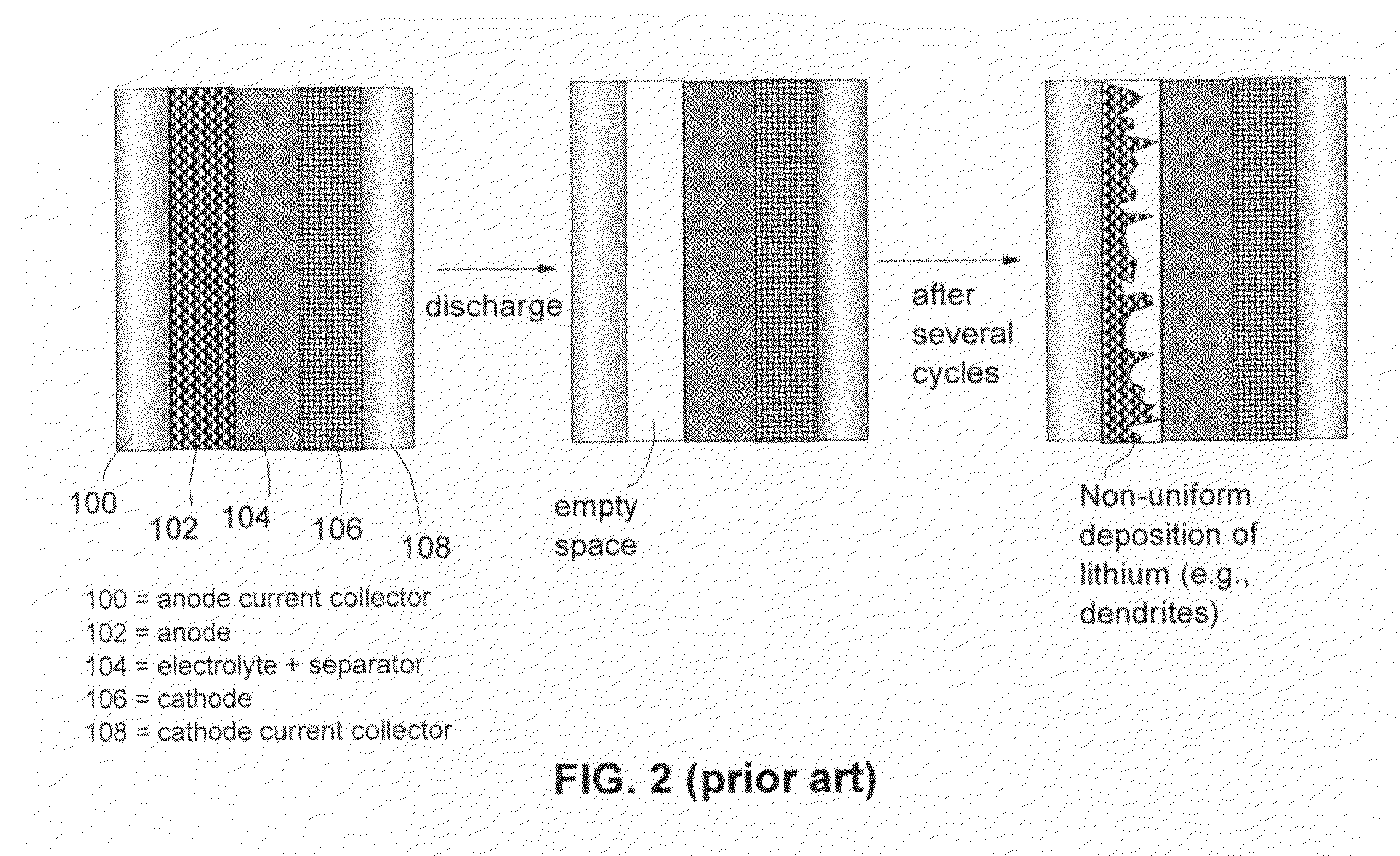
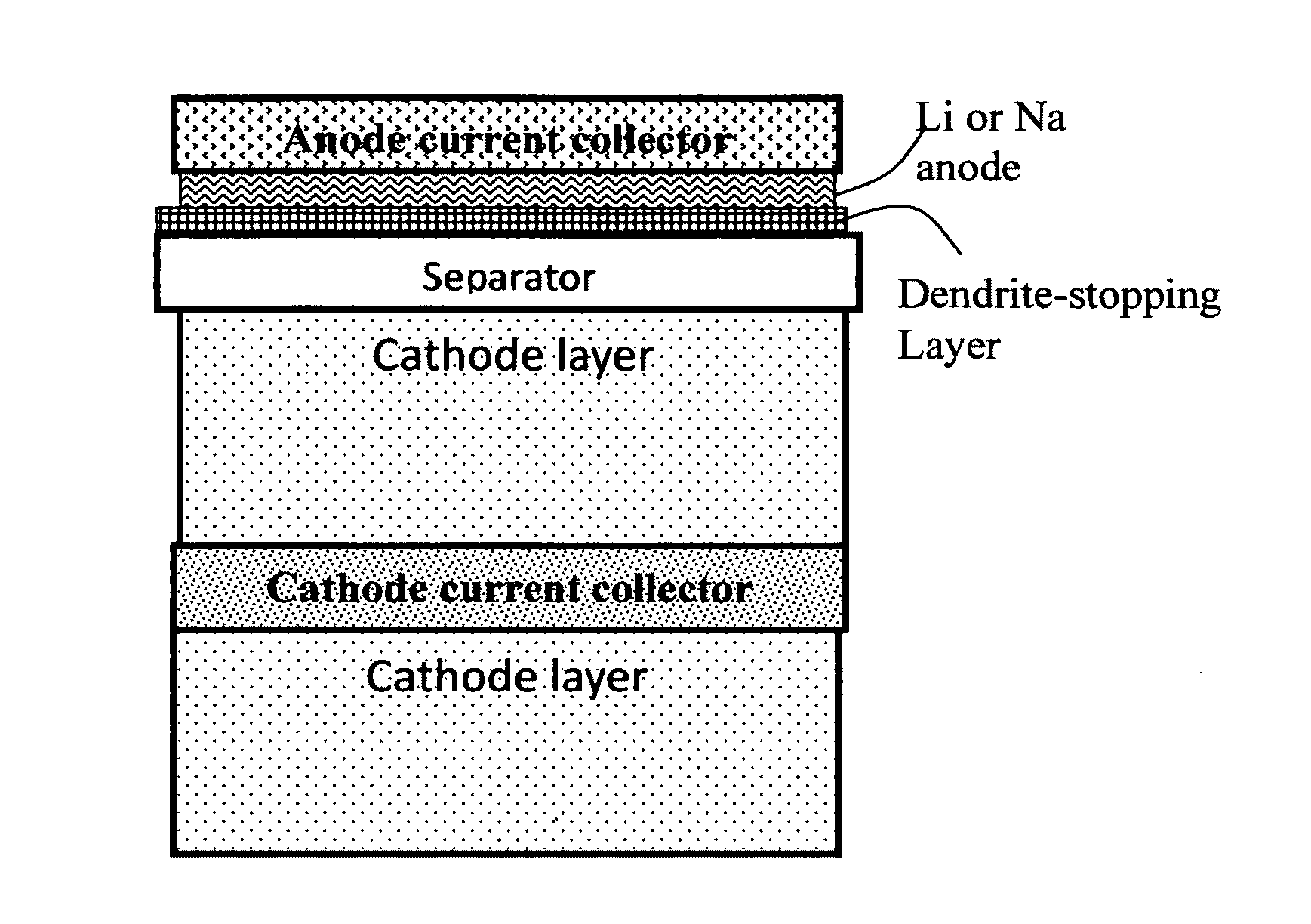
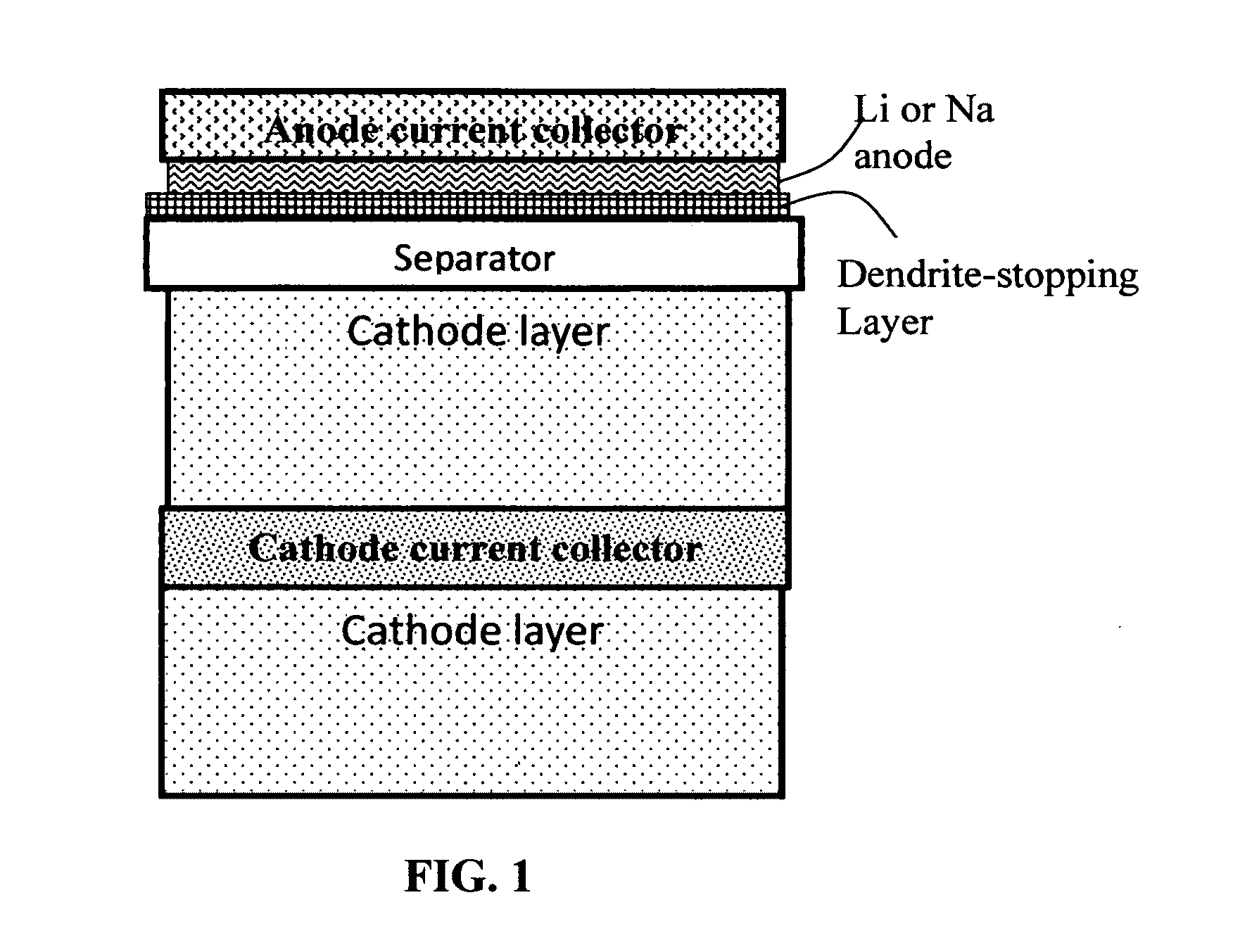
Alkali Metal Secondary Battery Containing a Carbon Matrix- or Carbon Matrix Composite-based Dendrite-Intercepting Layer
ActiveUS20160344035A1Increase energy densityReduce material costsFuel and secondary cellsPositive electrodesDendriteElectrolyte
A rechargeable alkali metal battery comprising: (a) an anode comprising an alkali metal layer and a dendrite penetration-resistant layer comprising an amorphous carbon or polymeric carbon matrix, an optional carbon or graphite reinforcement phase dispersed in this matrix, and a lithium- or sodium-containing species that are chemically bonded to the matrix and / or the optional carbon or graphite reinforcement to form an integral layer that prevents dendrite penetration, wherein the lithium- or sodium-containing species is selected from Li2CO3, Li2O, Li2C2O4, LiOH, LiX, ROCO2Li, HCOLi, ROLi, (ROCO2Li)2, (CH2OCO2Li)2, Li2S, LixSOy, Na2CO3, Na2O, Na2C2O4, NaOH, NaiX, ROCO2Na, HCONa, RONa, (ROCO2Na)2, (CH2OCO2Na)2, Na2S, NaxSOy, or a combination thereof, wherein X═F, Cl, I, or Br, R=a hydrocarbon group, x=0-1, y=1-4; (b) a cathode; and (c) a separator and electrolyte component; wherein the dendrite penetration-resistant layer is disposed between the alkali metal layer and the separator.
Owner:GLOBAL GRAPHENE GRP INC
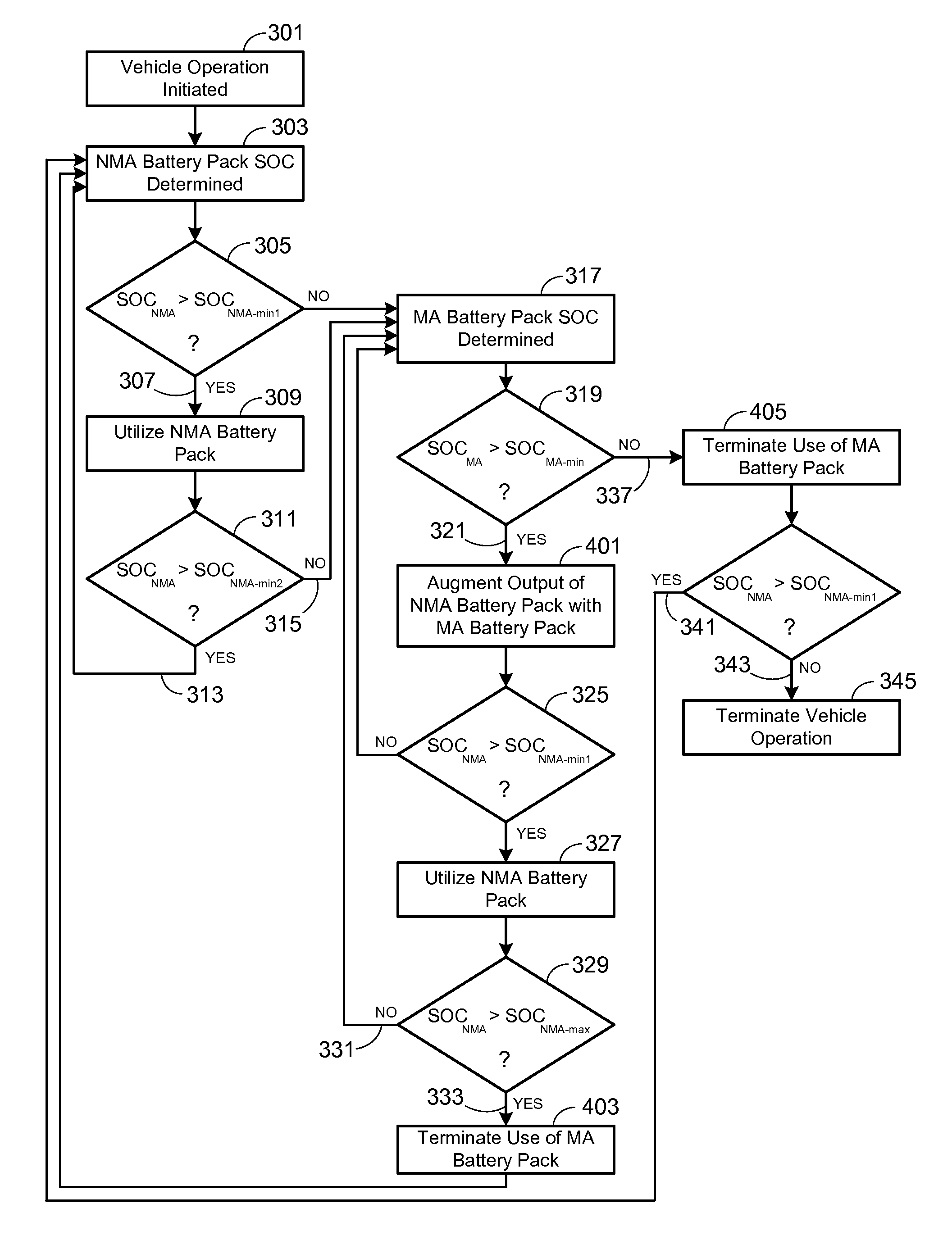
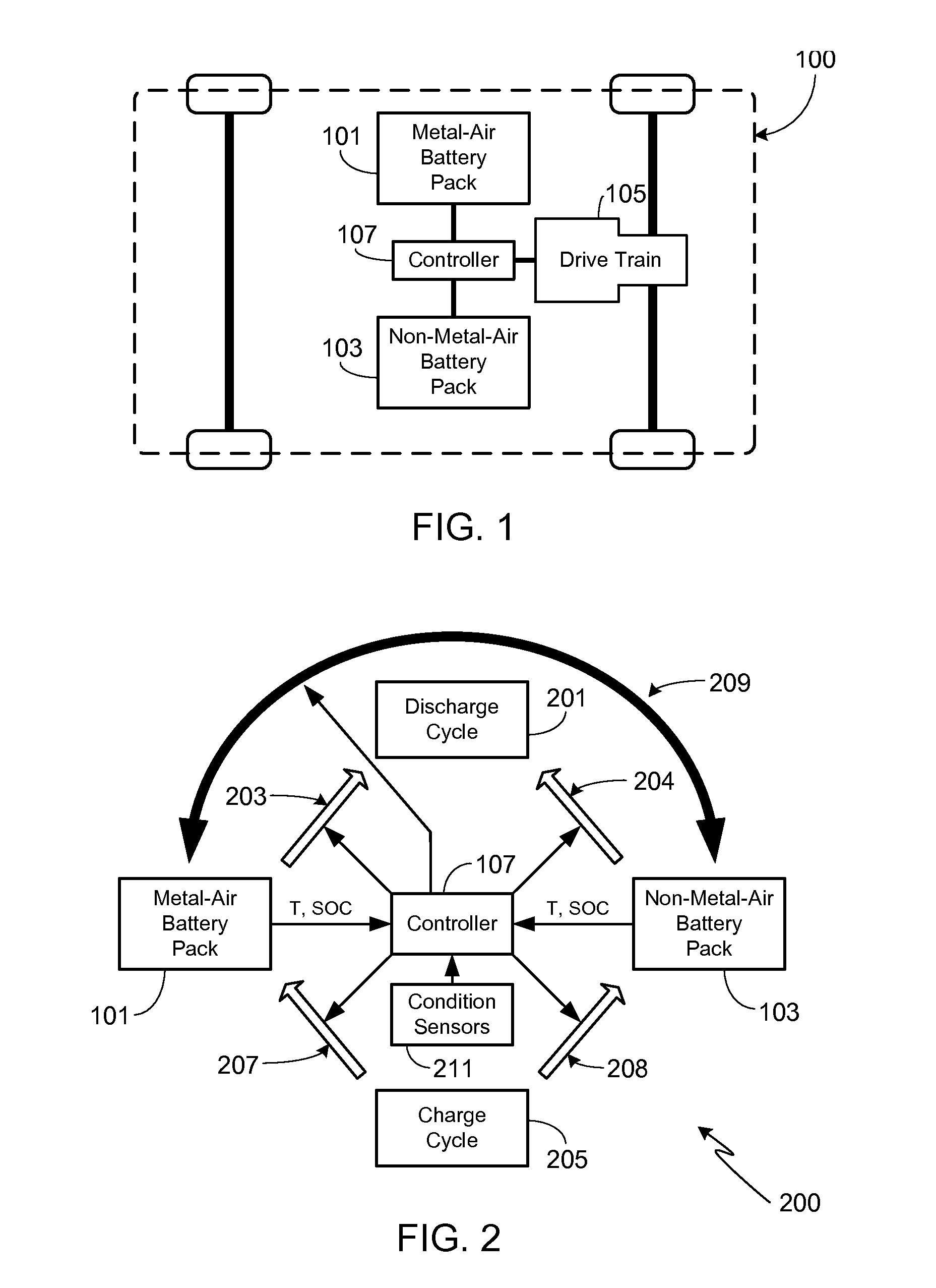
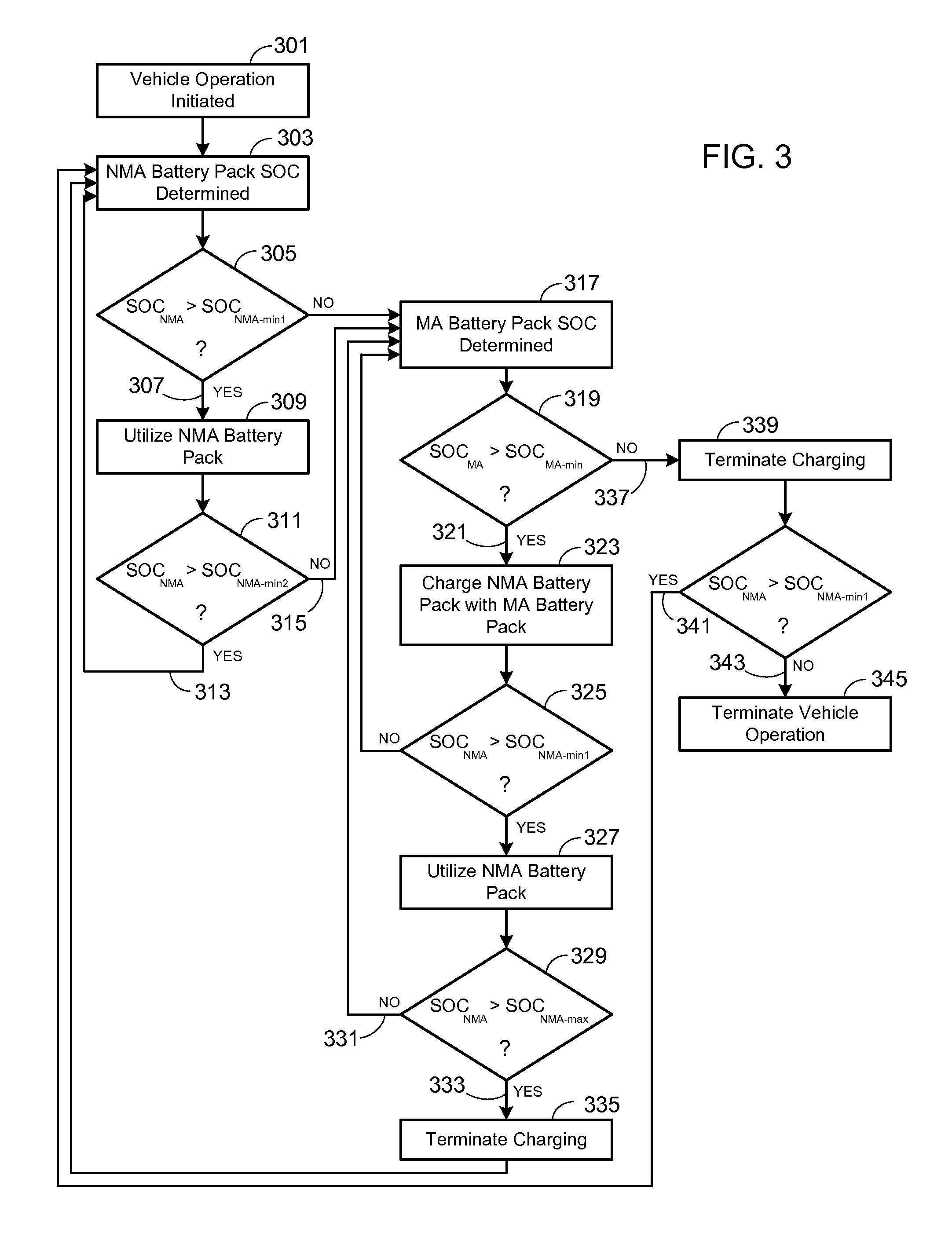
Electric Vehicle Extended Range Hybrid Battery Pack System
A power source comprised of a first battery pack (e.g., a non-metal-air battery pack) and a second battery pack (e.g., a metal-air battery pack) is provided, wherein the second battery pack is only used as required by the state-of-charge (SOC) of the first battery pack or as a result of the user selecting an extended range mode of operation. Minimizing use of the second battery pack prevents it from undergoing unnecessary, and potentially lifetime limiting, charge cycles. The second battery pack may be used to charge the first battery pack or used in combination with the first battery pack to supply operational power to the electric vehicle.
Owner:TESLA INC
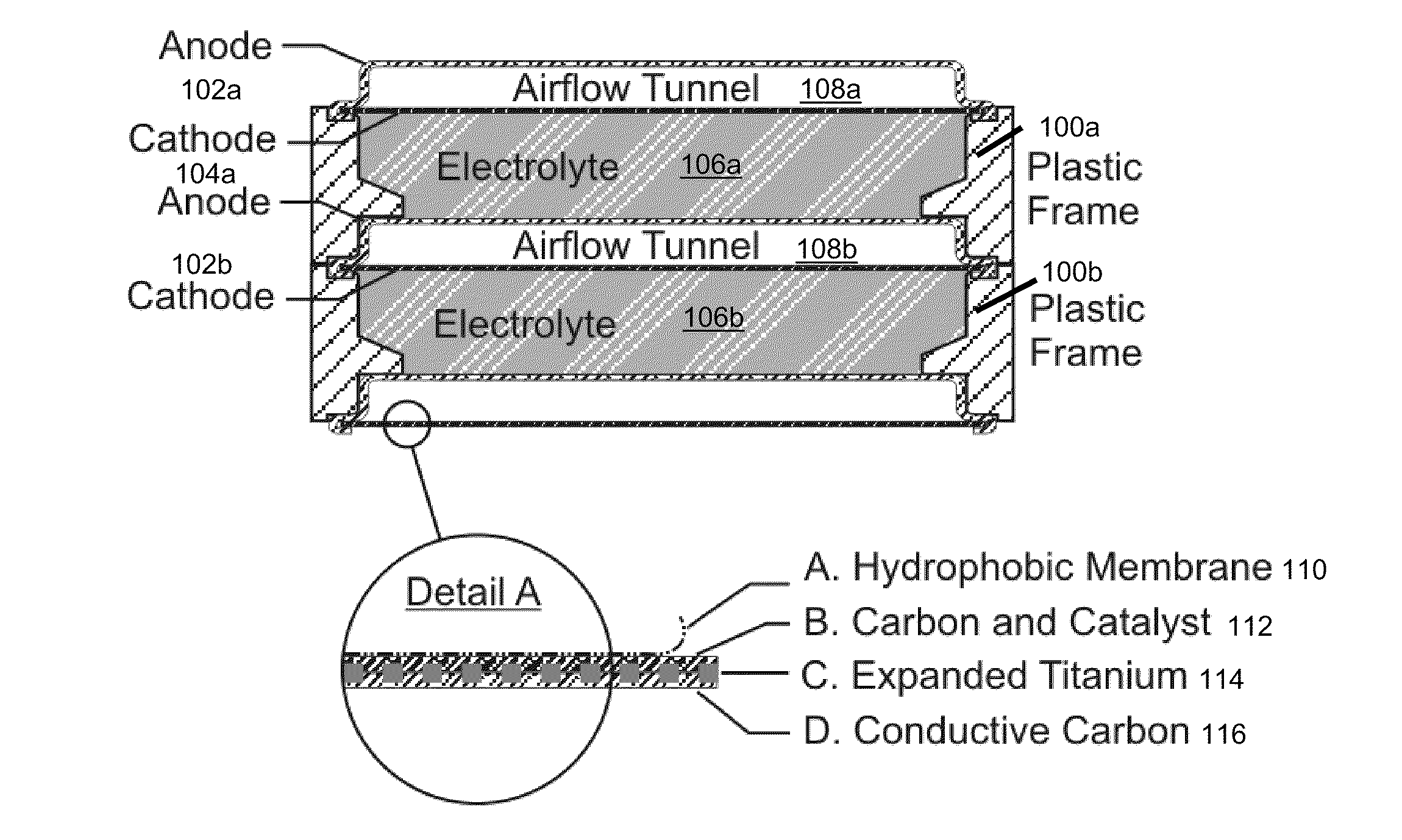
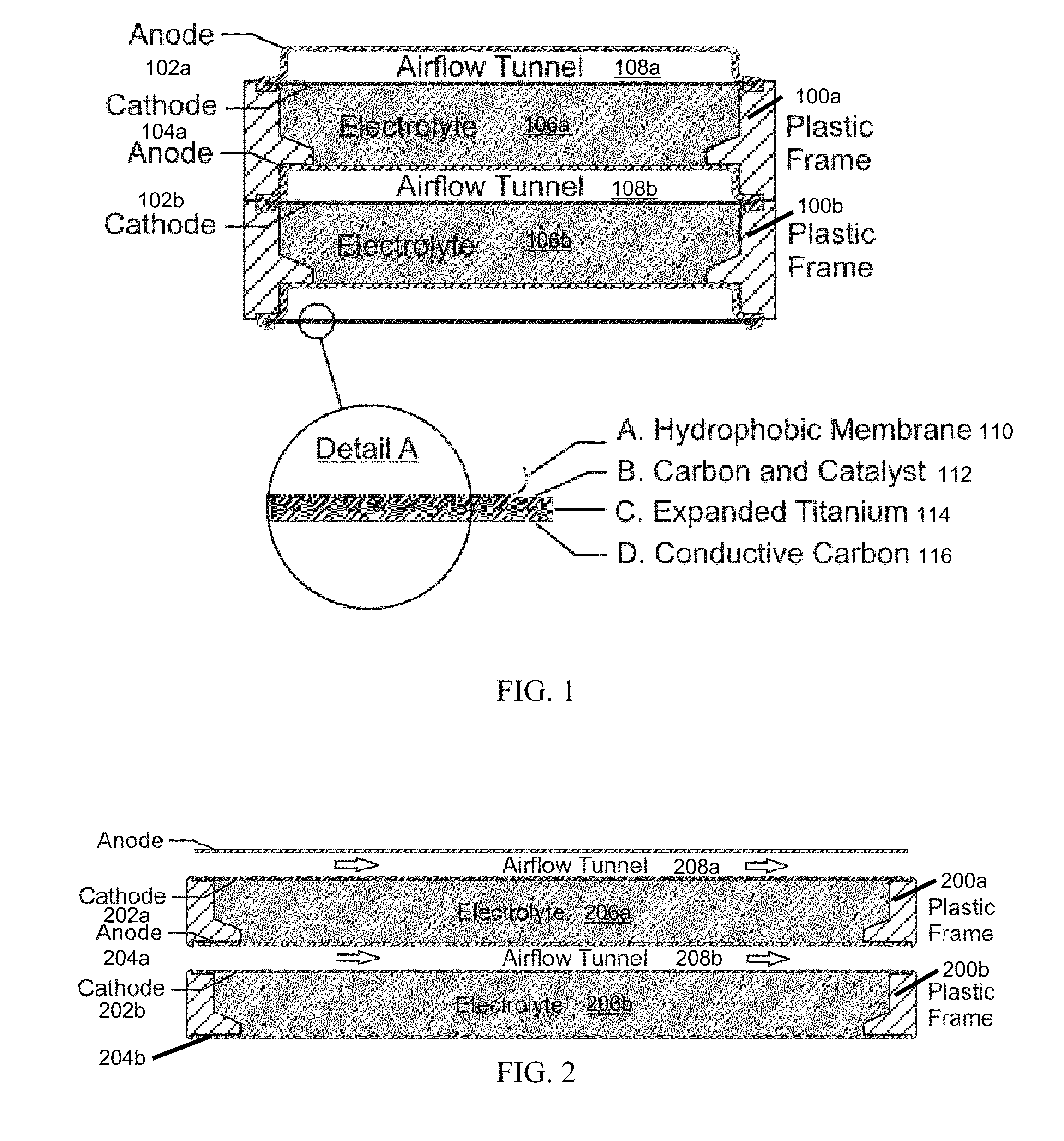
Electrically rechargeable, metal-air battery systems and methods
InactiveUS20120021303A1Solution to short lifeAffordable and practicalElectrolyte moving arrangementsFuel and secondary cellsElectricityElectrical battery
The invention provides for a fully electrically rechargeable metal-air battery systems and methods of achieving such systems. A rechargeable metal air battery cell may comprise a metal electrode an air electrode, and an aqueous electrolyte separating the metal electrode and the air electrode. In some embodiments, the metal electrode may directly contact the electrolyte and no separator or porous membrane need be provided between the air electrode and the electrolyte. Rechargeable metal air battery cells may be electrically connected to one another through a centrode connection between a metal electrode of a first battery cell and an air electrode of a second battery cell. Air tunnels may be provided between individual metal air battery cells. In some embodiments, an electrolyte flow management system may be provided.
Owner:EOS ENERGY STORAGE
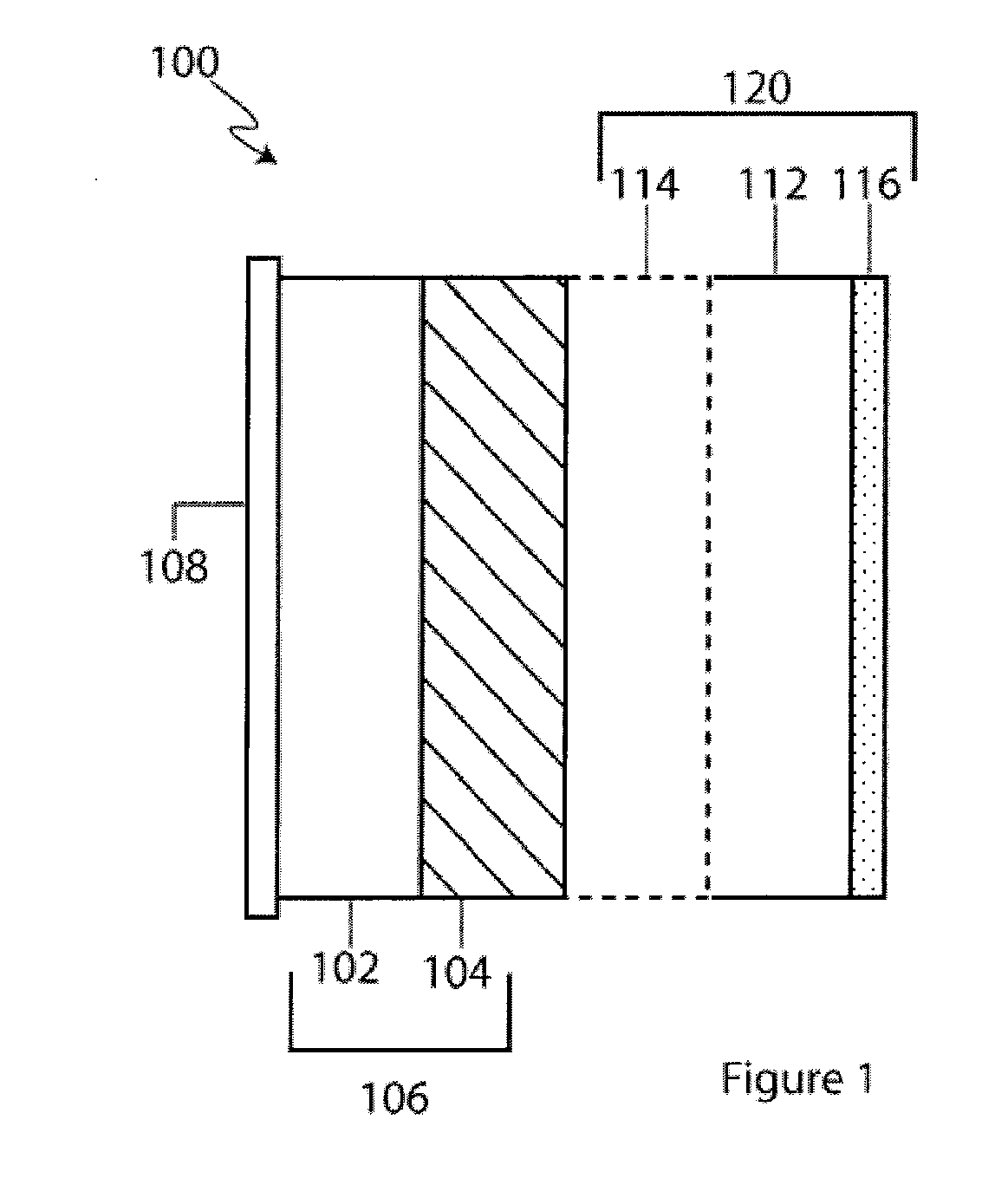
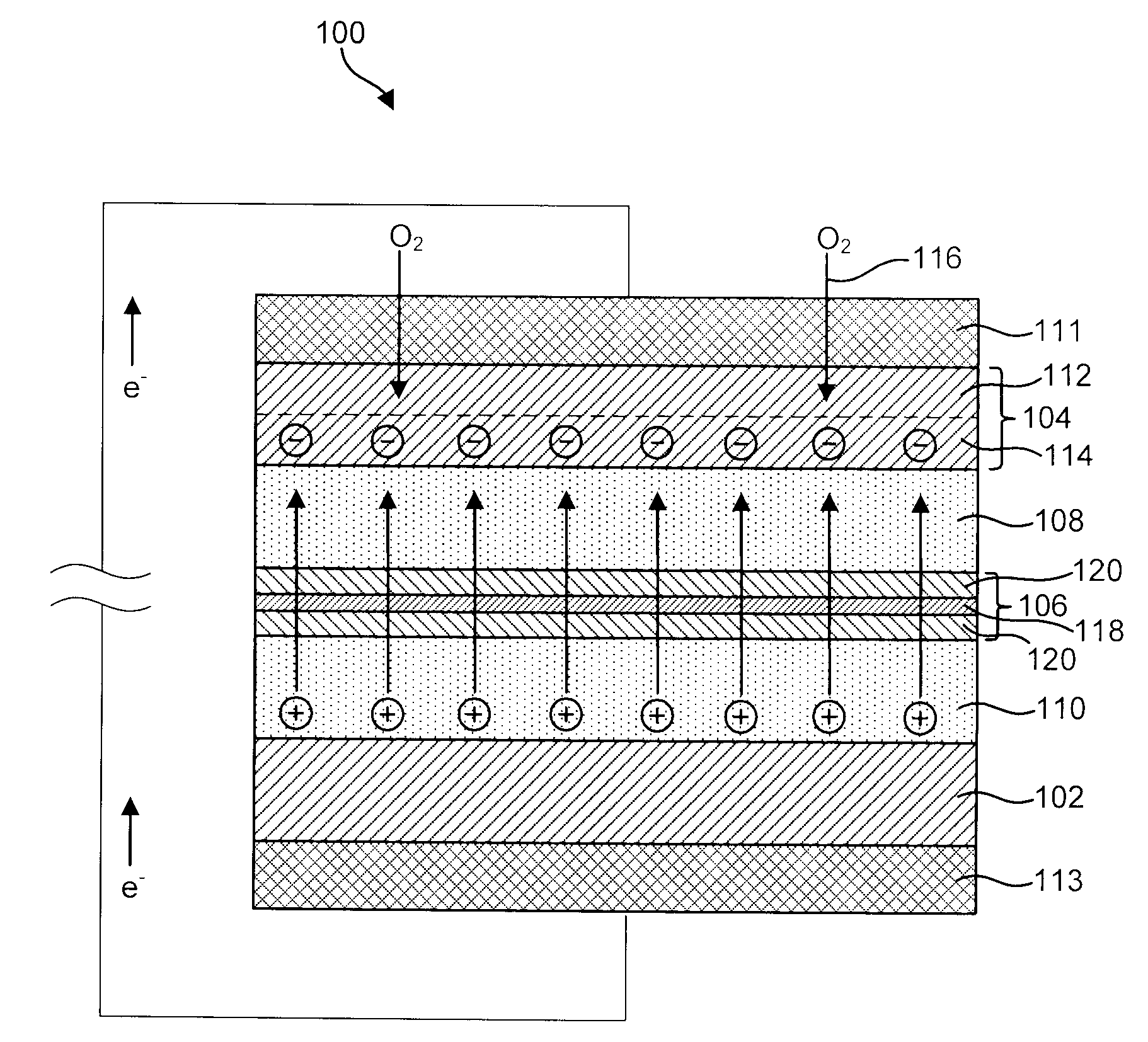
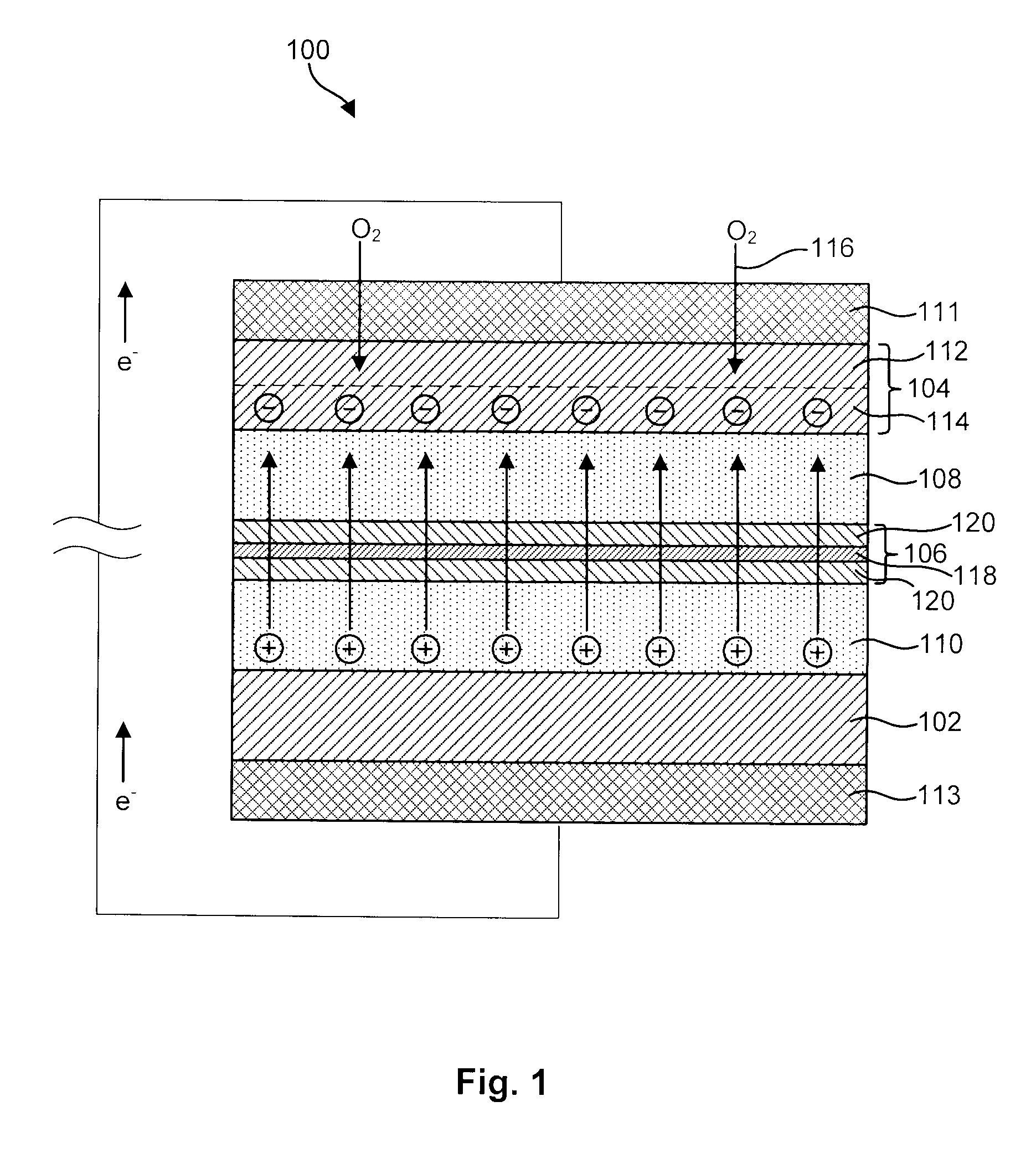
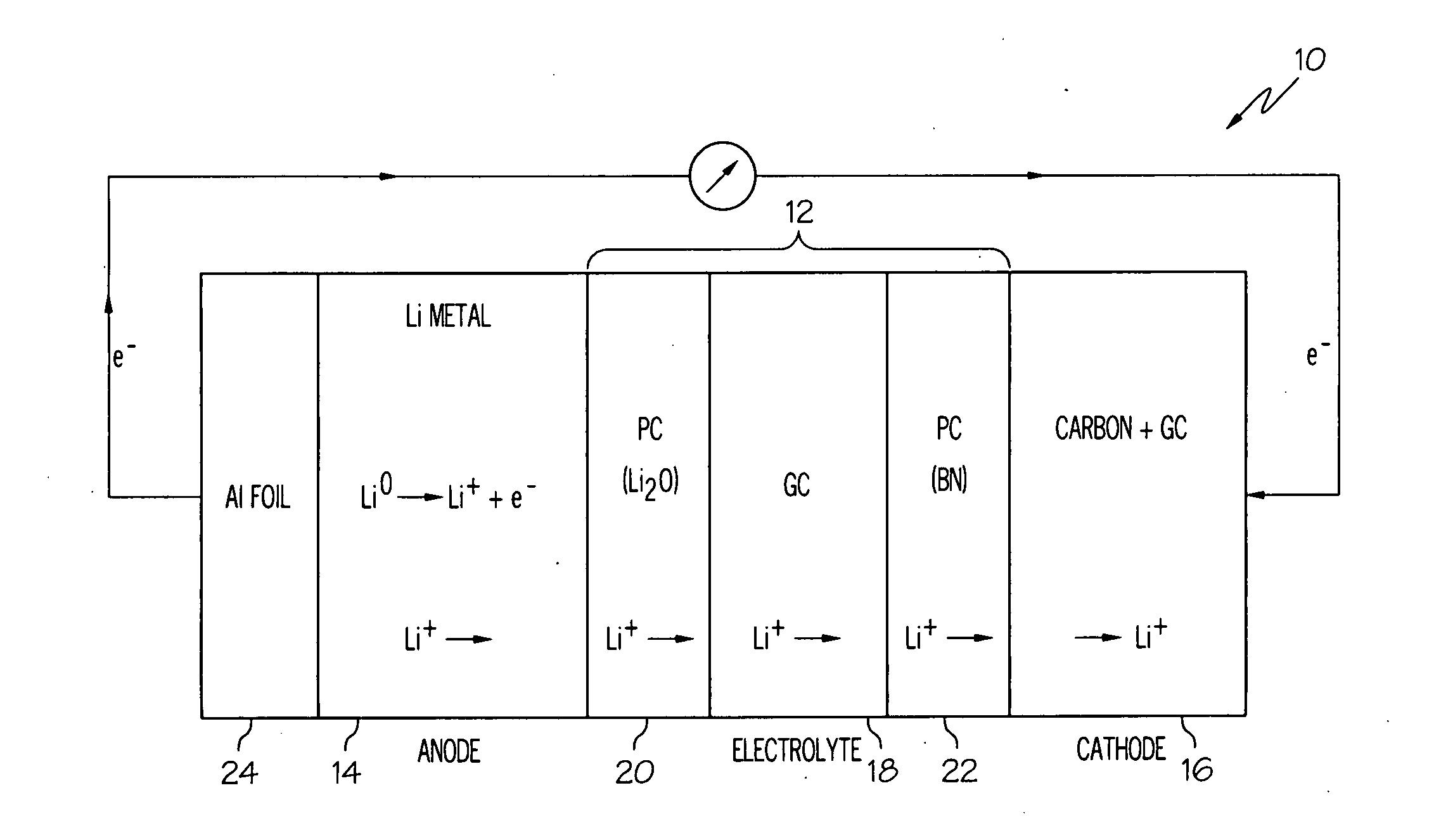
Active metal/aqueous electrochemical cells and systems
InactiveUS7645543B2Degree of flexibilityWithout performanceFuel and primary cellsAlkaline accumulatorsElectrochemical cellBattery cell
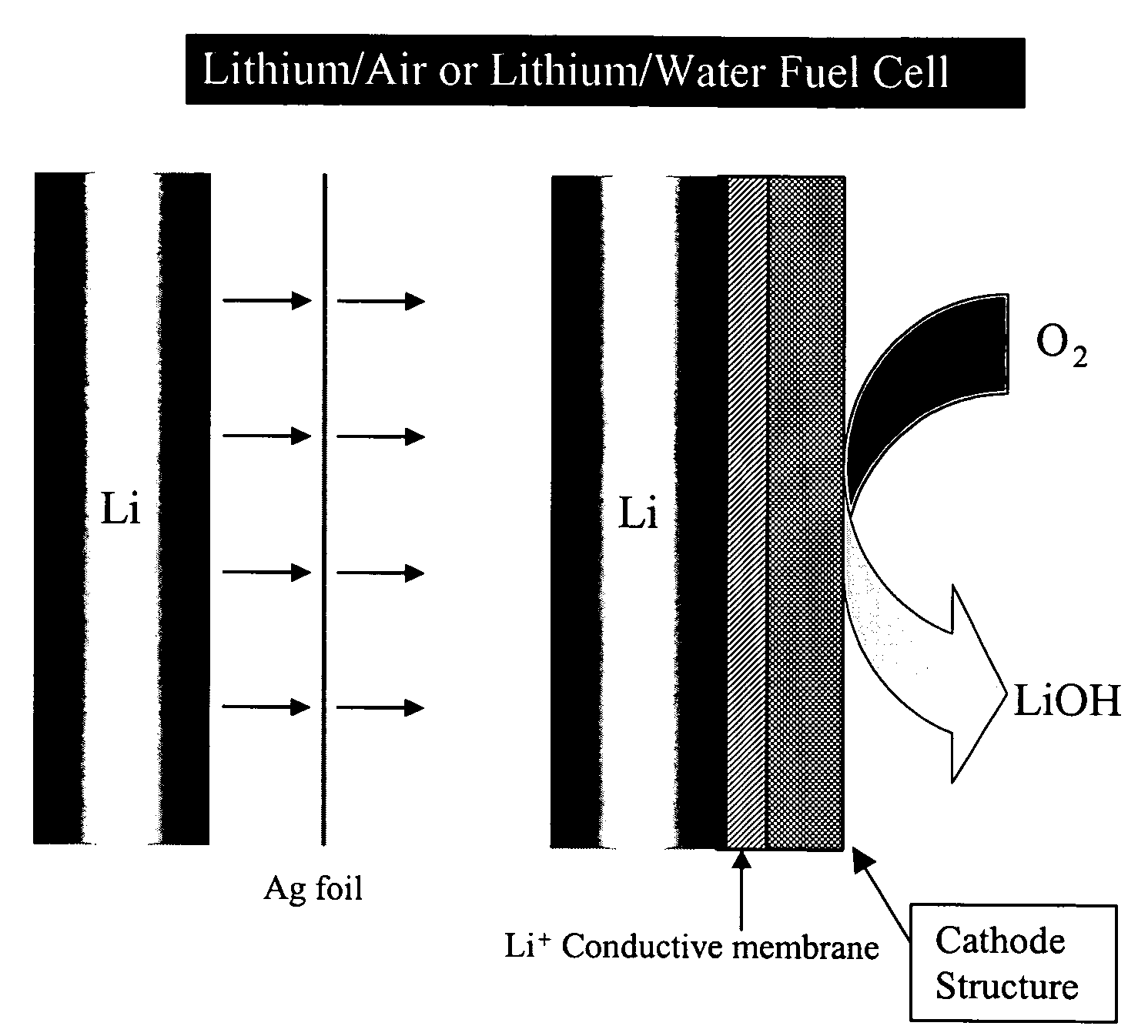
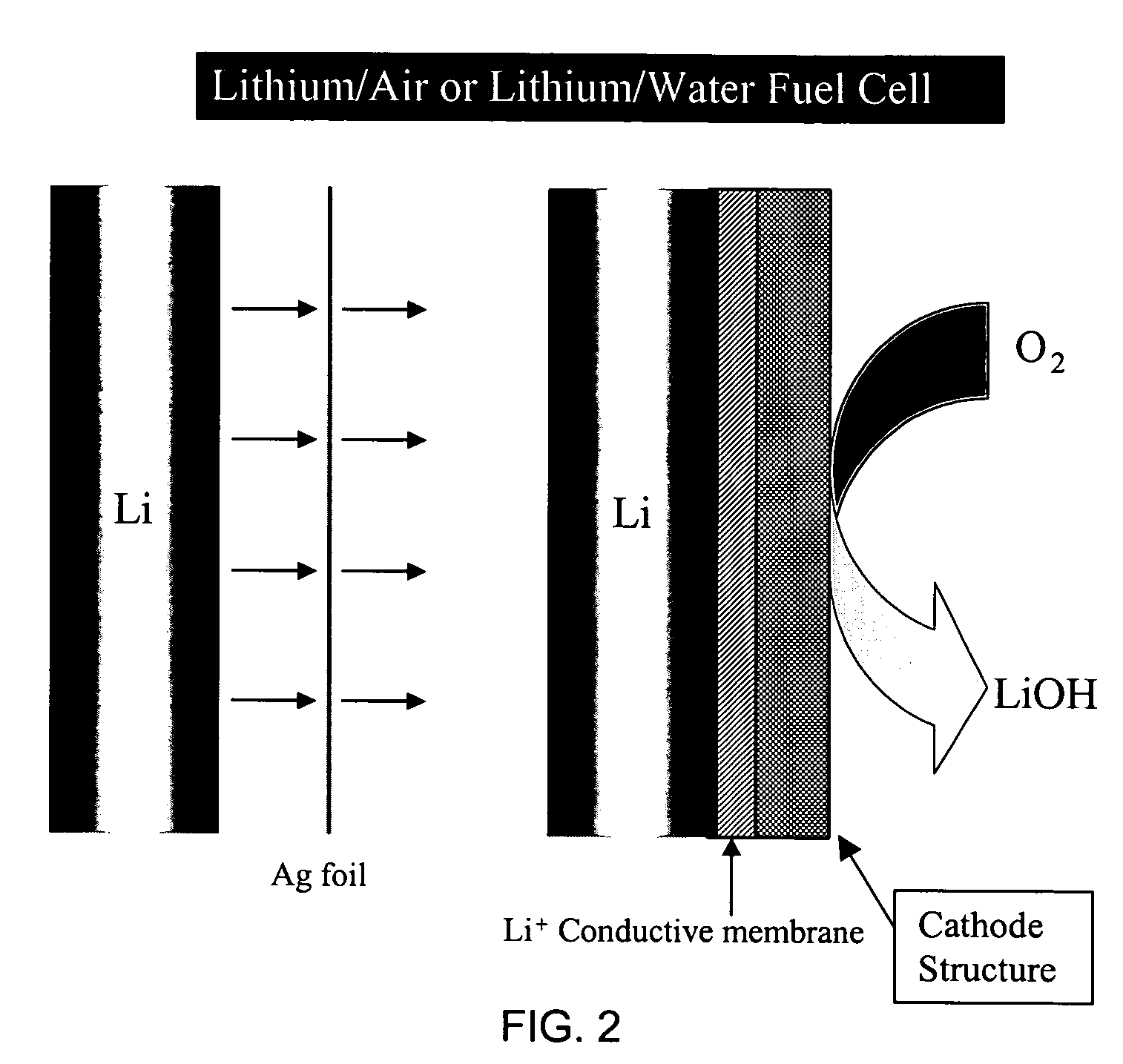
Alkali (or other active) metal battery and other electrochemical cells incorporating active metal anodes together with aqueous cathode / electrolyte systems. The battery cells have a highly ionically conductive protective membrane adjacent to the alkali metal anode that effectively isolates (de-couples) the alkali metal electrode from solvent, electrolyte processing and / or cathode environments, and at the same time allows ion transport in and out of these environments. Isolation of the anode from other components of a battery cell or other electrochemical cell in this way allows the use of virtually any solvent, electrolyte and / or cathode material in conjunction with the anode. Also, optimization of electrolytes or cathode-side solvent systems may be done without impacting anode stability or performance. In particular, Li / water, Li / air and Li / metal hydride cells, components, configurations and fabrication techniques are provided.
Owner:POLYPLUS BATTERY CO INC
Lithium-air cells incorporating solid electrolytes having enhanced ionic transport and catalytic activity
ActiveUS20090317724A1Enhanced ionic transportEasy to transportSolid electrolytesFuel and secondary cellsLithiumOxygen
Liquid-free lithium-air cells are provided which incorporate a solid electrolyte having enhanced ionic transport and catalytic activity. The solid electrolyte is positioned between a lithium anode and an oxygen cathode, and comprises a glass-ceramic and / or a polymer-ceramic electrolyte including a dielectric additive.
Owner:UNIV OF DAYTON
Protected lithium electrodes having a porous electrolyte interlayer and associated battery cells
ActiveUS20140170465A1Avoid harmful reactionsHybrid capacitor separatorsHybrid capacitor electrolytesBattery cellMetal
Active metal and active metal intercalation electrode structures and battery cells having ionically conductive protective architecture including an active metal (e.g., lithium) conductive impervious layer separated from the electrode (anode) by a porous separator impregnated with a non-aqueous electrolyte (anolyte). This protective architecture prevents the active metal from deleterious reaction with the environment on the other (cathode) side of the impervious layer, which may include aqueous or non-aqueous liquid electrolytes (catholytes) and / or a variety electrochemically active materials, including liquid, solid and gaseous oxidizers. Safety additives and designs that facilitate manufacture are also provided.
Owner:POLYPLUS BATTERY CO INC
Active metal fuel cells
InactiveUS7491458B2High densityImprove efficiencyFuel and primary cellsFuel and secondary cellsLithiumFuel cells
Active metal fuel cells are provided. An active metal fuel cell has a renewable active metal (e.g., lithium) anode and a cathode structure that includes an electronically conductive component (e.g., a porous metal or alloy), an ionically conductive component (e.g., an electrolyte), and a fluid oxidant (e.g., air, water or a peroxide or other aqueous solution). The pairing of an active metal anode with a cathode oxidant in a fuel cell is enabled by an ionically conductive protective membrane on the surface of the anode facing the cathode.
Owner:POLYPLUS BATTERY CO INC
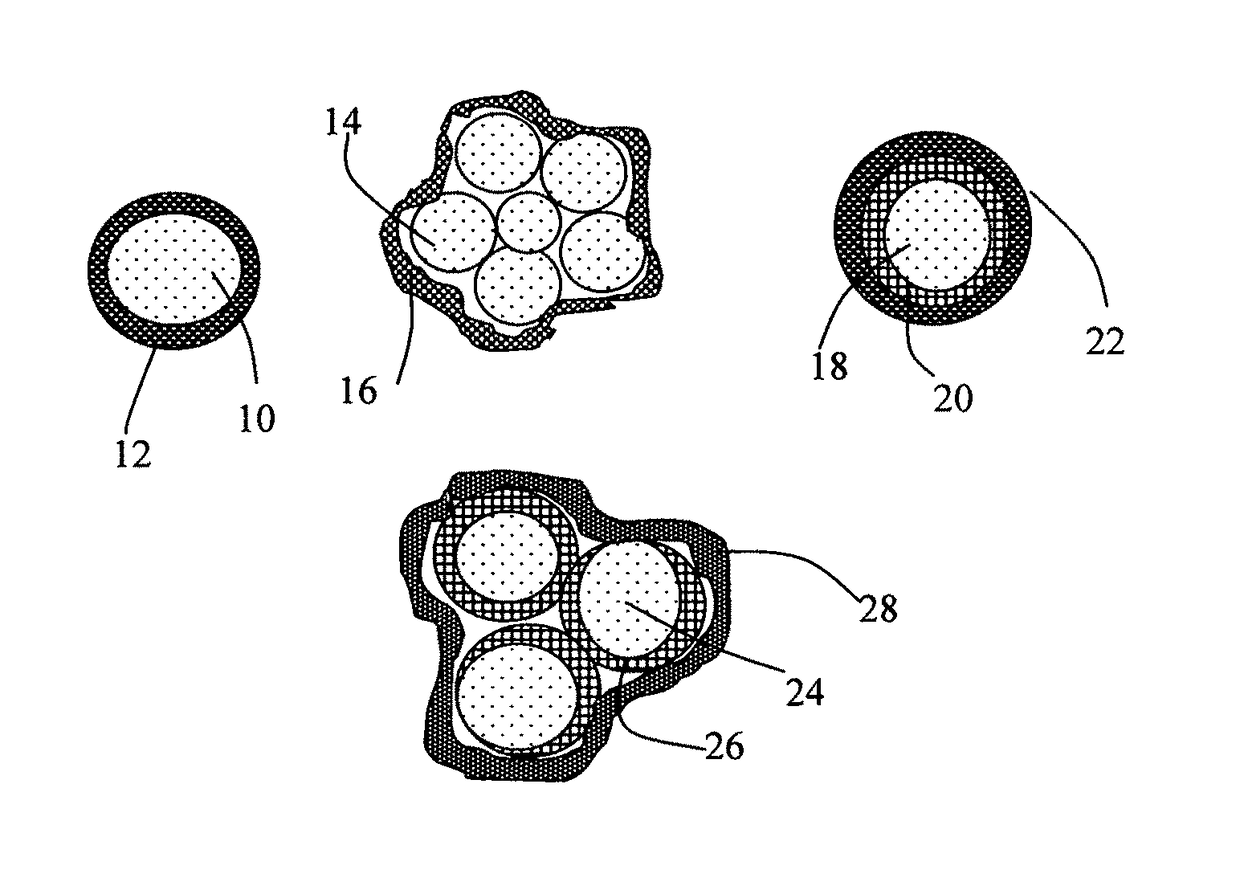
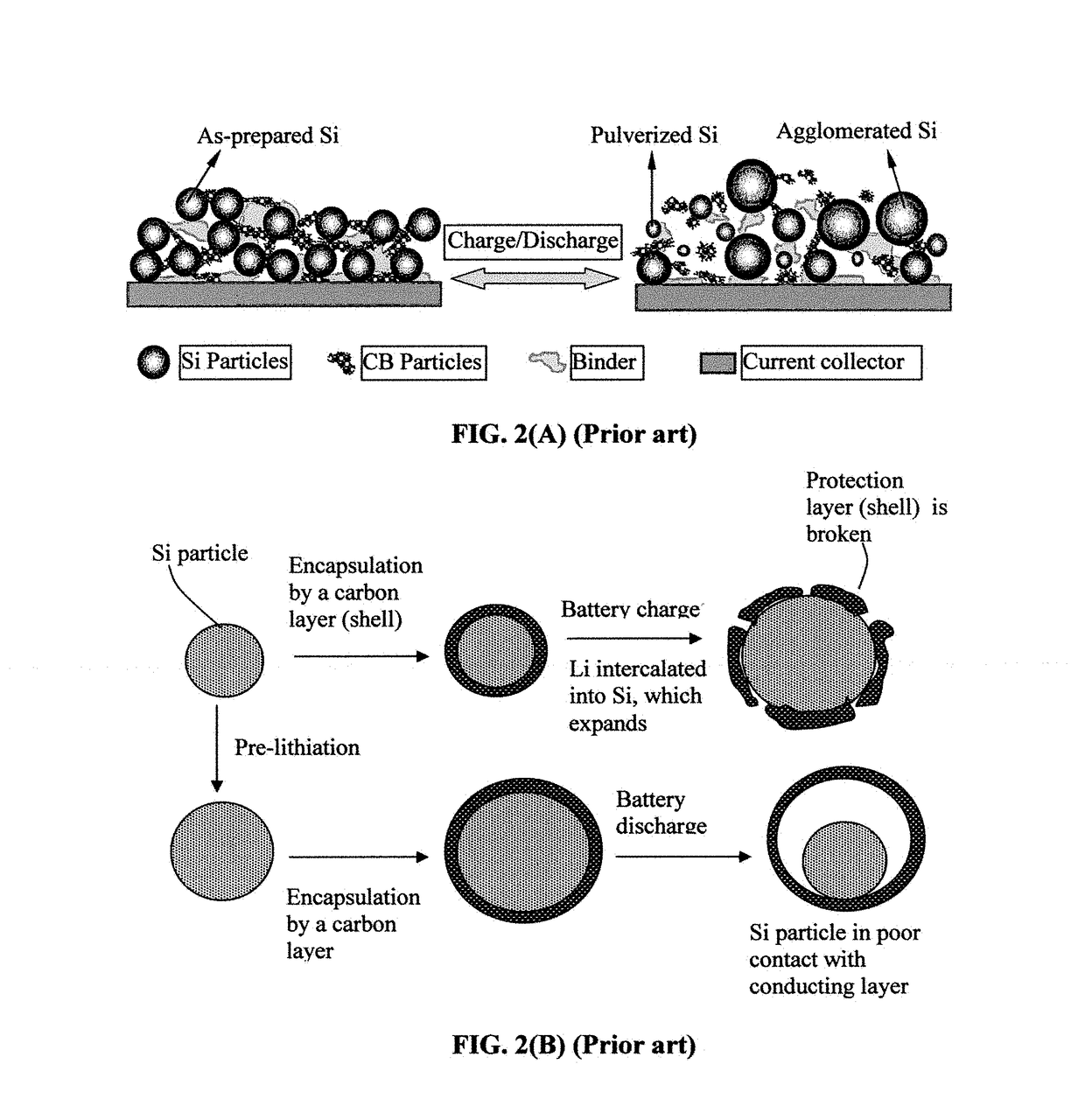
Elastomer-Encapsulated particles of high-capacity anode active materials for lithium batteries
ActiveUS20170288211A1Improve lithium ion conductivityFuel and secondary cellsElectrode thermal treatmentParticulatesElastomer
Provided is an anode active material layer for a lithium battery. This layer comprises multiple particulates of an anode active material, wherein at least a particulate is composed of one or a plurality of particles of a high-capacity anode active material being encapsulated by a thin layer of elastomeric material that has a lithium ion conductivity no less than 10−7 S / cm (preferably no less than 10−5 S / cm) at room temperature and an encapsulating shell thickness from 1 nm to 10 μm, and wherein the high-capacity anode active material (e.g. Si, Ge, Sn, SnO2, Co3O4, etc.) has a specific capacity of lithium storage greater than 372 mAh / g (the theoretical lithium storage limit of graphite).
Owner:GLOBAL GRAPHENE GRP INC
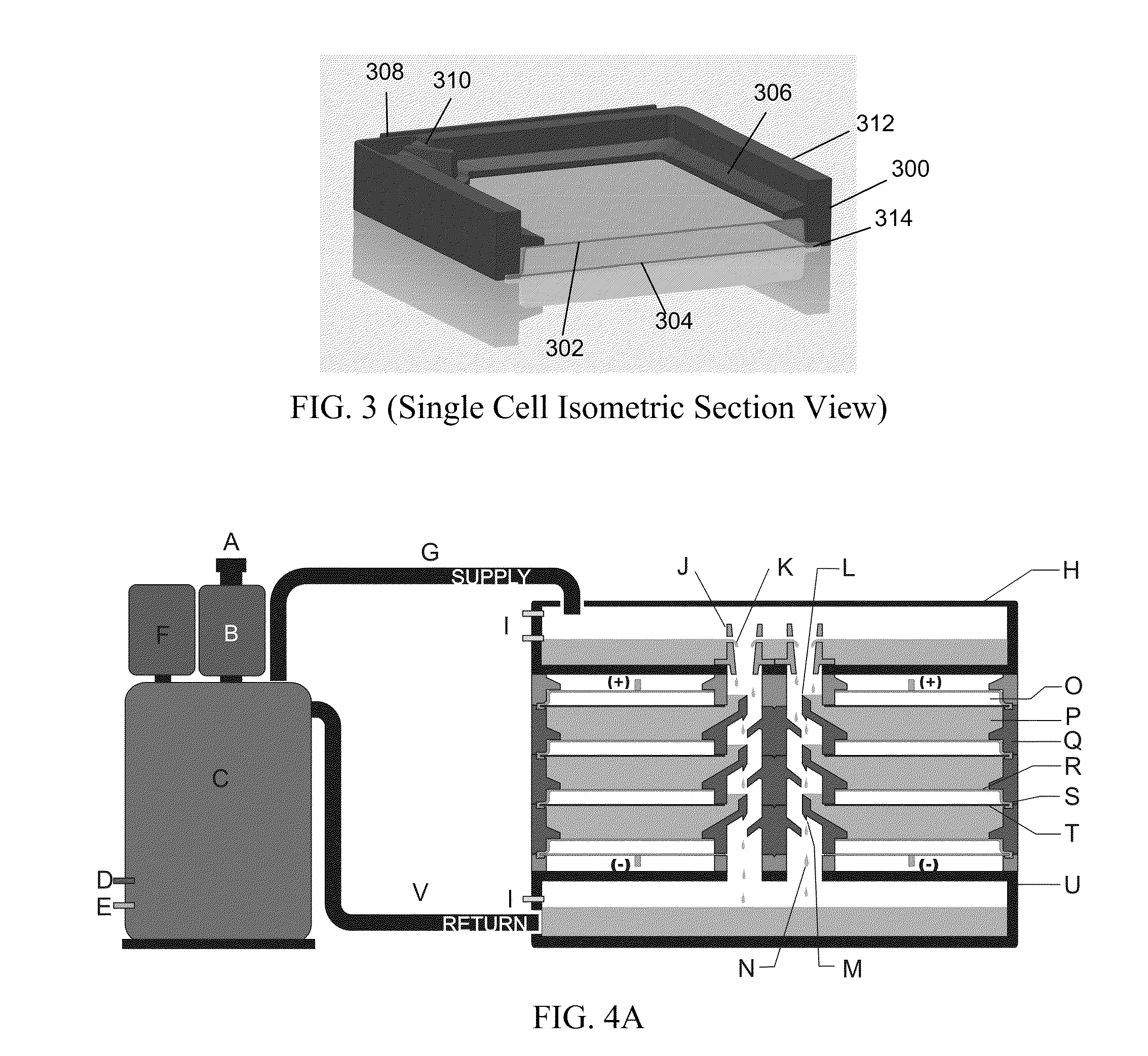
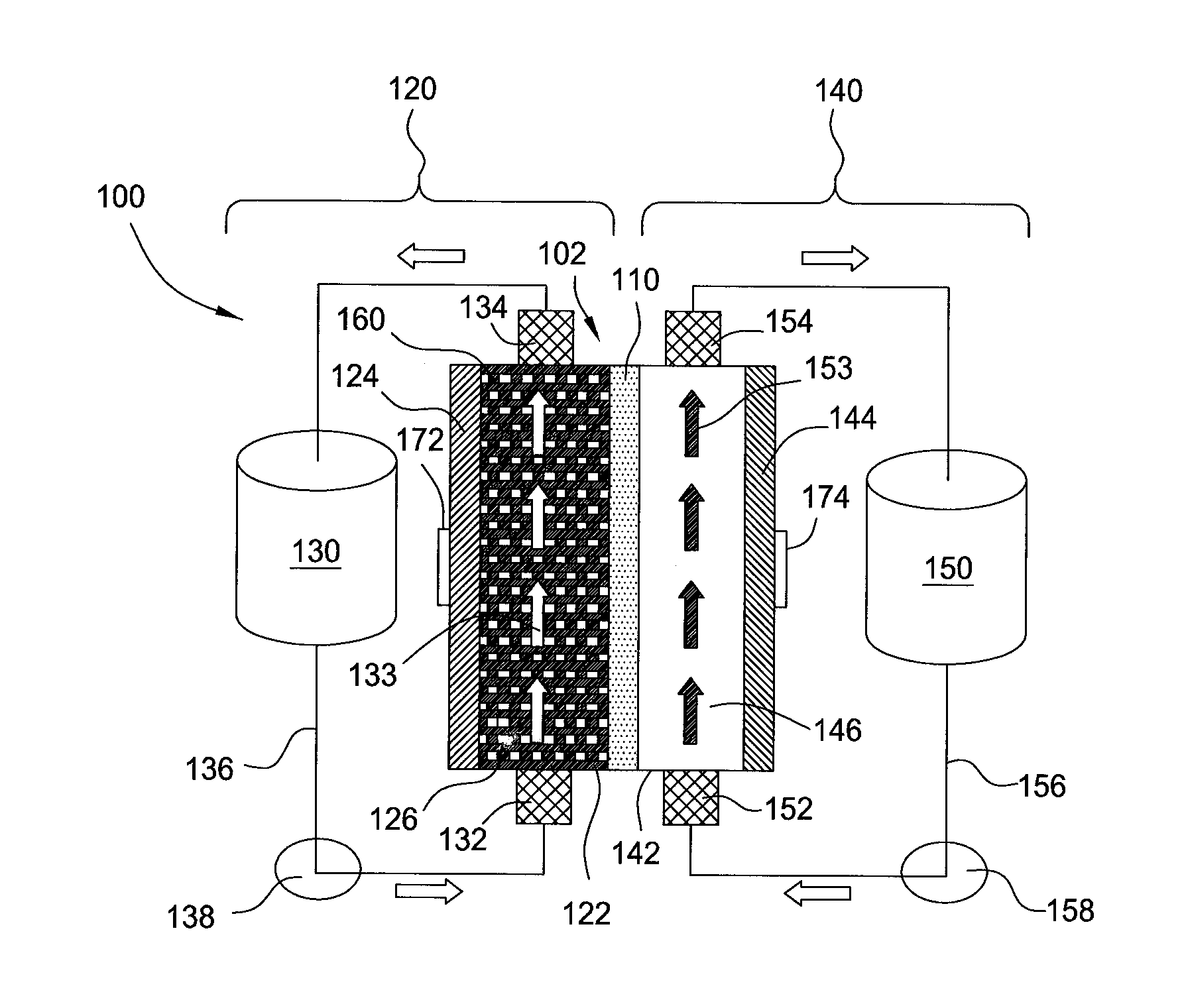
Flow battery systems
ActiveUS20120052347A1Uniform metal platingHigh cell current densityFuel and secondary cellsCell electrodesEngineeringMetal
Embodiments of the invention generally provide for flow battery cells and systems containing a plurality of flow battery cells, and methods for improving metal plating within the flow battery cell, such as by flowing and exposing the catholyte to various types of cathodes. In one embodiment, a flow battery cell is provided which includes a cathodic half cell and an anodic half cell separated by an electrolyte membrane, wherein the cathodic half cell contains a plurality of cathodic wires extending perpendicular or substantially perpendicular to and within the catholyte pathway and in contact with the catholyte, and each of the cathodic wires extends parallel or substantially parallel to each other. In some examples, the plurality of cathodic wires may have at least two arrays of cathodic wires, each array contains at least one row of cathodic wires, and each row extends along the catholyte pathway.
Owner:APPLIED MATERIALS INC
Lithium secondary batteries containing lithium salt-ionic liquid solvent electrolyte
ActiveUS20140342249A1Reduce electrical conductivityLow ionic conductivityFuel and secondary cellsCell electrodesNano structuringLithium metal
A rechargeable lithium metal or lithium-ion cell comprising a cathode having a cathode active material and / or a conductive supporting structure, an anode having an anode active material and / or a conductive supporting nano-structure, a porous separator electronically separating the anode and the cathode, a highly concentrated electrolyte in contact with the cathode active material and the anode active material, wherein the electrolyte contains a lithium salt dissolved in an ionic liquid solvent with a concentration greater than 3 M. The cell exhibits an exceptionally high specific energy, a relatively high power density, a long cycle life, and high safety with no flammability.
Owner:GLOBAL GRAPHENE GRP INC
Active metal fuel cells
InactiveUS20050100792A1Increase energy densityImprove fuel efficiencyFuel and primary cellsFuel and secondary cellsLithiumFuel cells
Owner:POLYPLUS BATTERY CO INC
Active metal/aqueous electrochemical cells and systems
InactiveUS7666233B2Degree of flexibilityWithout performanceFuel and primary cellsElectrode manufacturing processesManufacturing technologyElectrochemical cell
Owner:POLYPLUS BATTERY CO INC
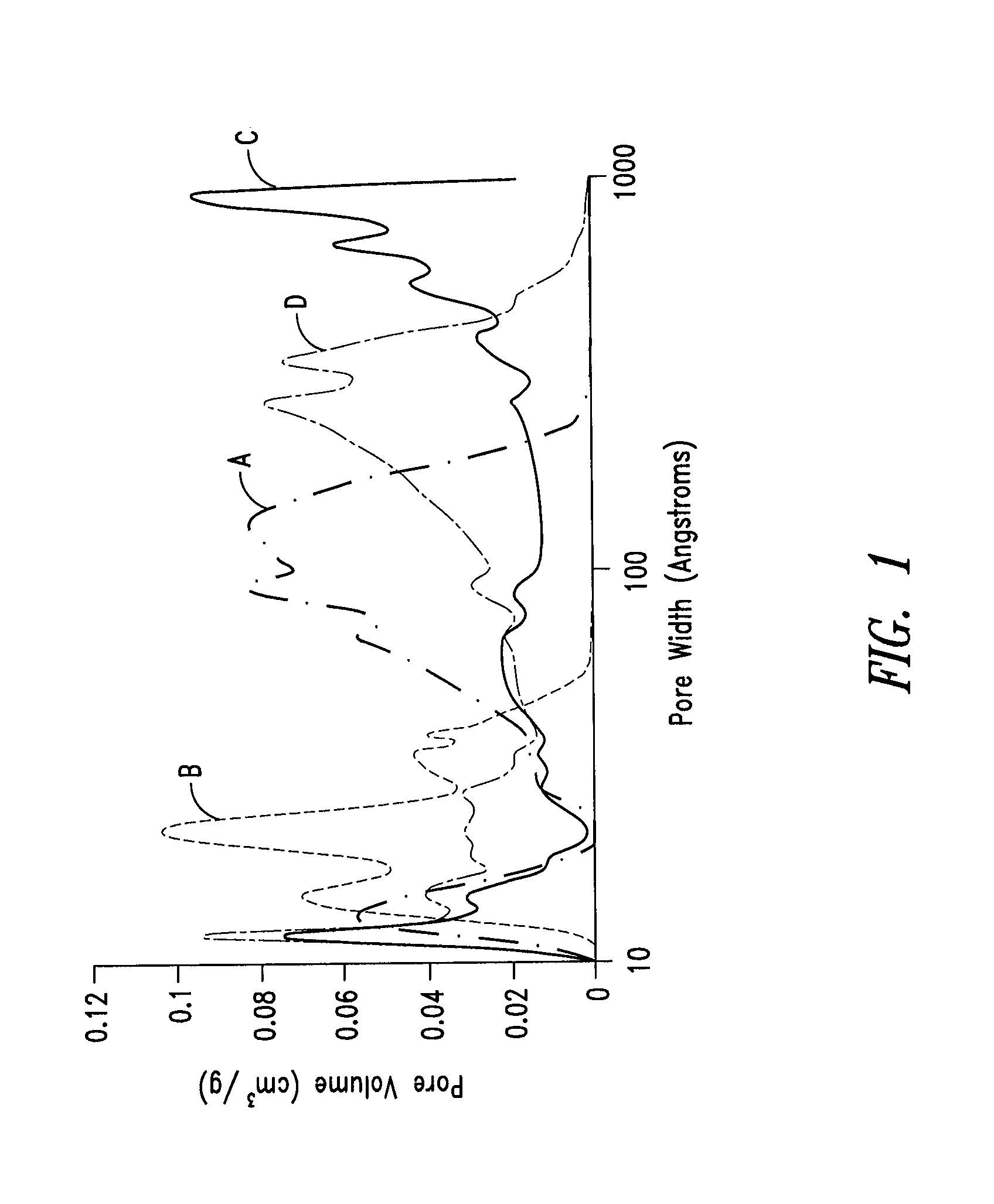
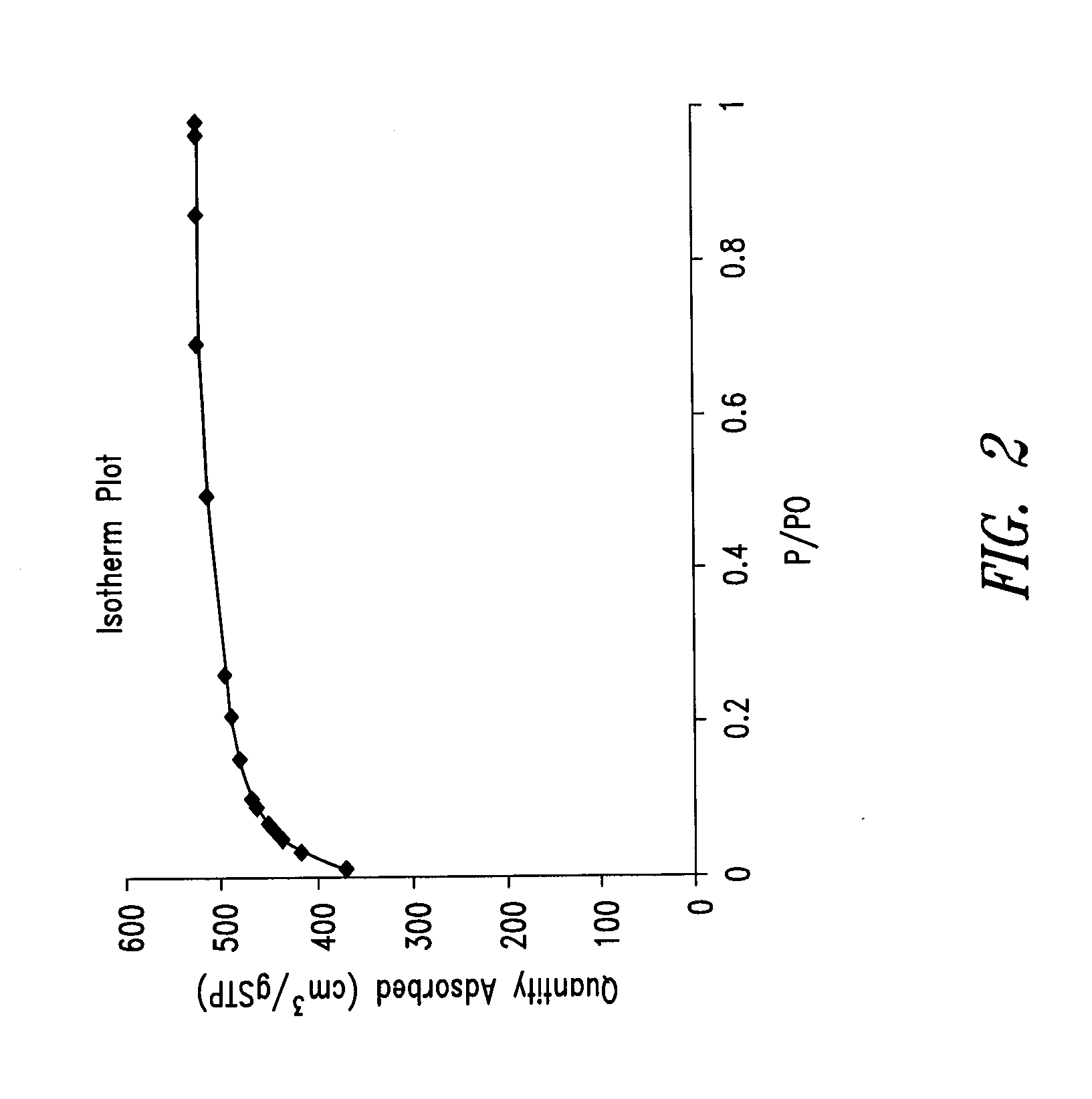
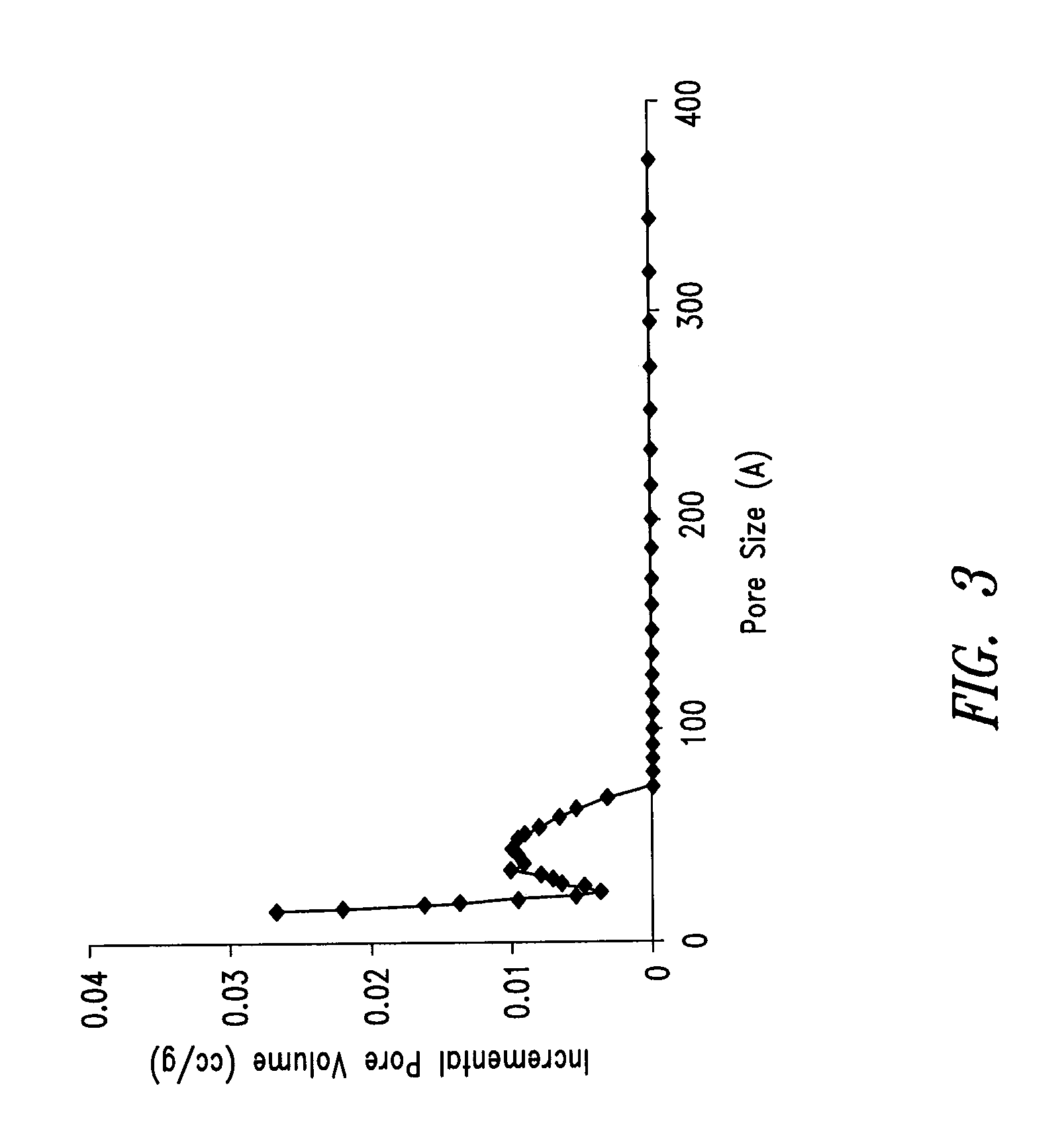
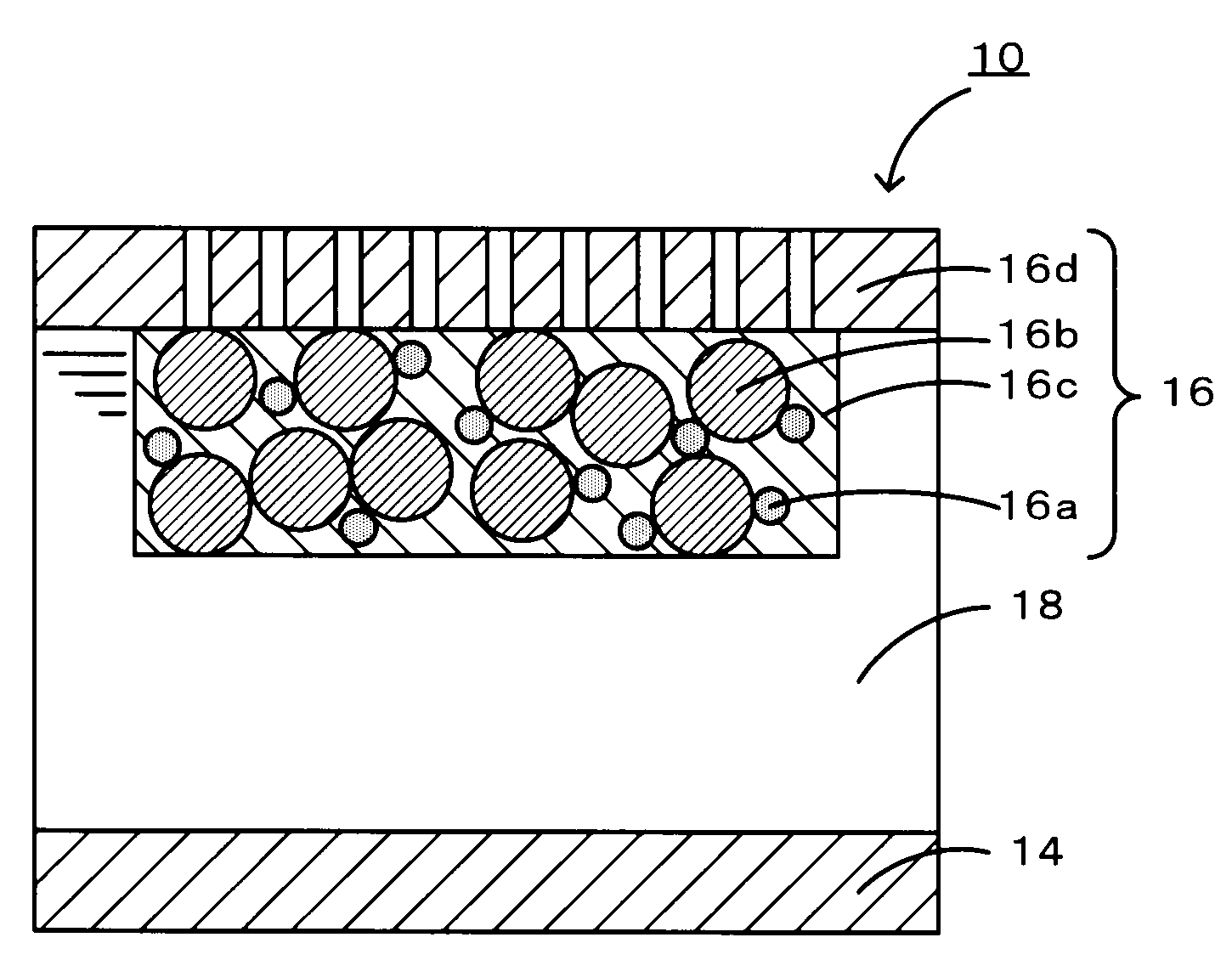
Nitrogen-doped porous carbon material for lithium-air battery positive electrode
ActiveCN103855366AHigh discharge specific capacityHigh voltage platformFuel and secondary cellsCell electrodesPorous carbonCharge discharge
The present invention relates to a nitrogen-doped porous carbon material for a lithium-air battery positive electrode, wherein the nitrogen-doped porous carbon material has an interconnected graded pore structure, N is uniformly doped in the C skeleton, N accounts for 0.2-15% of the carbon material atomic ratio, the graded pores comprise mass transfer pores and deposition holes, the deposition holes account for 40-95% of the total pore volume, and the mass transfer pores account for 4-55% of the total pore volume. According to the present invention, with application of the carbon material as the lithium-air battery electrode material, the space utilization rate of the carbon material during the charge-discharge process can be increased at a maximum, and the energy density and the power density of the lithium-air battery can be effectively increased; and the preparation process is simple, the material source is wide, the pore structure of the graded pore carbon material can be regulated, the regulation manner is diverse, and the nitrogen doping manner is easily achieved.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Metal-air cell with performance enhancing additive
ActiveUS20110281184A1Improves oxygen reduction thermodynamicsImproved kineticsFuel and primary cellsFuel and secondary cellsOxygenElectrochemical cell
Systems and methods drawn to an electrochemical cell comprising a low temperature ionic liquid comprising positive ions and negative ions and a performance enhancing additive added to the low temperature ionic liquid. The additive dissolves in the ionic liquid to form cations, which are coordinated with one or more negative ions forming ion complexes. The electrochemical cell also includes an air electrode configured to absorb and reduce oxygen. The ion complexes improve oxygen reduction thermodynamics and / or kinetics relative to the ionic liquid without the additive.
Owner:ARIZONA STATE UNIVERSITY
Preparation of polymeric resins and carbon materials
The present application is directed to methods for preparation of carbon materials. The carbon materials comprise enhanced electrochemical properties and find utility in any number of electrical devices, for example, as electrode material in ultracapacitors or batteries.
Owner:BASF AG
Lithium Metal Secondary Battery Containing an Anode-Protecting Polymer Layer and Manufacturing Method
ActiveUS20180294476A1Improve lithium ion conductivityReduce thicknessSolid electrolytesFuel and secondary cellsTensile strainLithium metal
Provided is lithium secondary battery comprising a cathode, an anode, and an electrolyte or separator-electrolyte assembly disposed between the cathode and the anode, wherein the anode comprises: (a) a foil or coating of lithium or lithium alloy as an anode active material; and (b) a thin layer of a high-elasticity polymer having a recoverable tensile strain no less than 5%, a lithium ion conductivity no less than 10−6 S / cm at room temperature, and a thickness from 1 nm to 10 μm, wherein the high-elasticity polymer contains an ultrahigh molecular weight polymer having a molecular weight from 0.5×106 to 9×106 g / mole and is disposed between the lithium or lithium alloy and the electrolyte or separator-electrolyte assembly.
Owner:GLOBAL GRAPHENE GRP INC
Mesoporous carbon materials comprising bifunctional catalysts
InactiveUS8916296B2Improve responseHigh specific energyFuel and primary cellsFuel and secondary cellsLithium–air batteryMesoporous carbon
Owner:BASF SE
Metal-air semi-fuel cell with an aqueous acid based cathode
InactiveUS20070259234A1Long time operationCell energy increasedFuel and primary cellsFuel and secondary cellsFuel cellsOxygen
A metal-air semi-fuel cell is provided, preferably based on lithium anode and a fuel cell type air / oxygen electrode immersed in an aqueous neutral, alkali or acid solution. The lithium anode is comprised of the active metal and one or more separators protecting the anode from reacting with an aqueous solution. The outermost layer on the lithium electrode is a solid-state lithium-ion conducting glass-ceramic which is impervious to and stable towards aqueous solutions. The cathode is comprised of an air or oxygen fuel cell type electrode in contact with the aqueous solution. The lithium anode of this invention also can be replaced by other electroactive metals which react with water and acids, bases and neutral solutions, such as metals from Groups 1 and 2 of the Periodic Table of Elements in addition to Zn, Mg, and Al.
Owner:CHUA DAVID +2
Non-aqueous air battery and catalyst therefor
InactiveUS20080299456A1Improve discharge capacityIncrease energy densityFuel and secondary cellsNon-aqueous electrolyte accumulator electrodesPorphyrinOxygen
A non-aqueous air battery of the present invention includes a negative electrode for which a material which absorbs and releases lithium ions is used as a negative electrode active material, a positive electrode for which oxygen is used as a positive electrode active material, and a non-aqueous electrolyte disposed between the negative electrode and the positive electrode. The positive electrode contains a donor-acceptor molecule in which an electron-donating donor (D) having a porphyrin ring is connected to an electron-accepting acceptor (A) composed of a fullerene derivative, with a conductive spacer therebetween. An example of the donor-acceptor molecule is triphenylporphyrinyl bithienyl N-methylpyrrolidino[60]fullerene.
Owner:TOYOTA CENT RES & DEV LAB INC
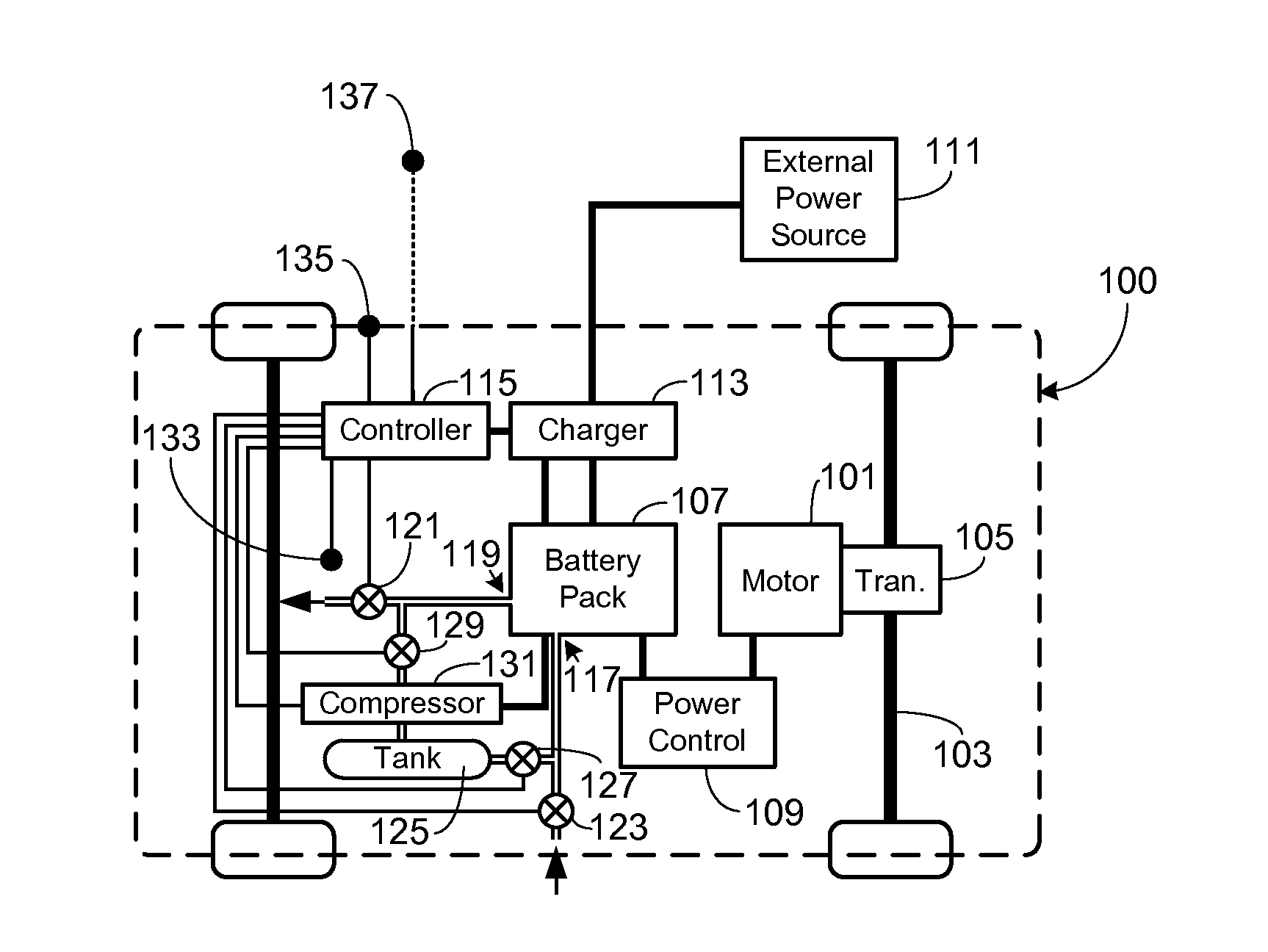
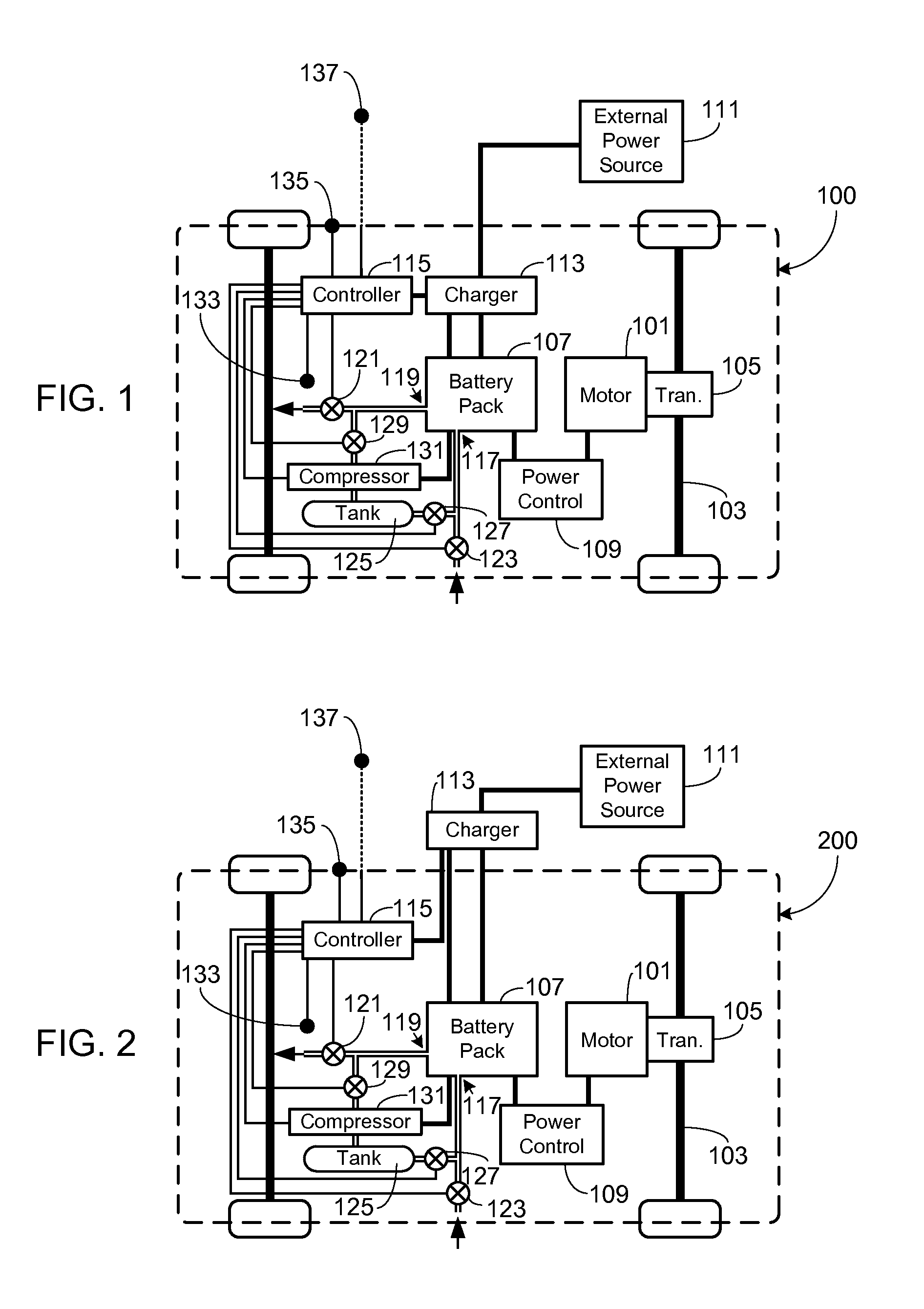
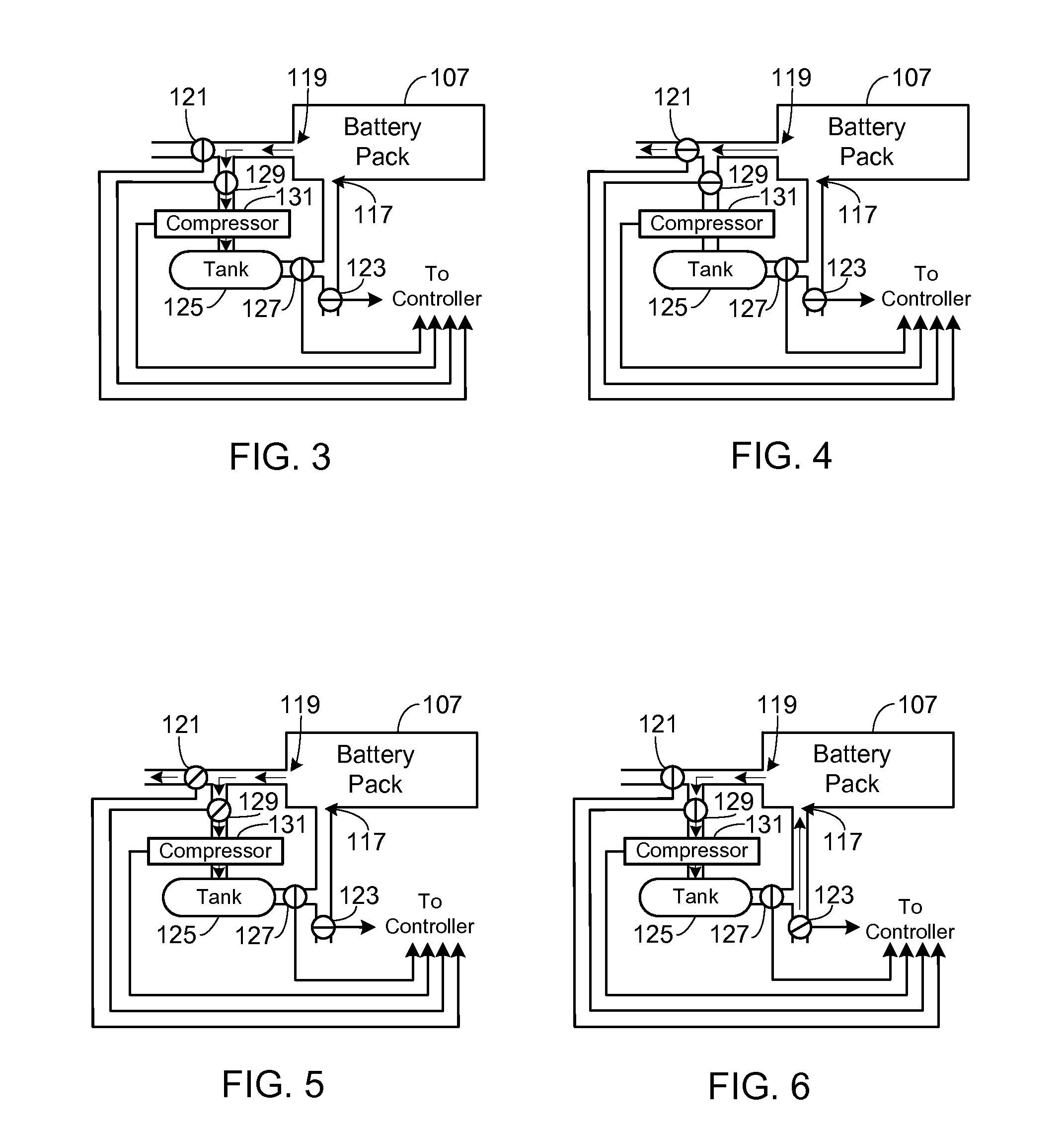
Control, Collection and Use of Metal-Air Battery Pack Effluent
A system and method for maintaining an ambient oxygen concentration below a preset concentration while charging a metal-air battery pack is provided, the system utilizing an on-board means for collecting and storing the oxygen-rich effluent generated during the charge cycle.
Owner:TESLA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com