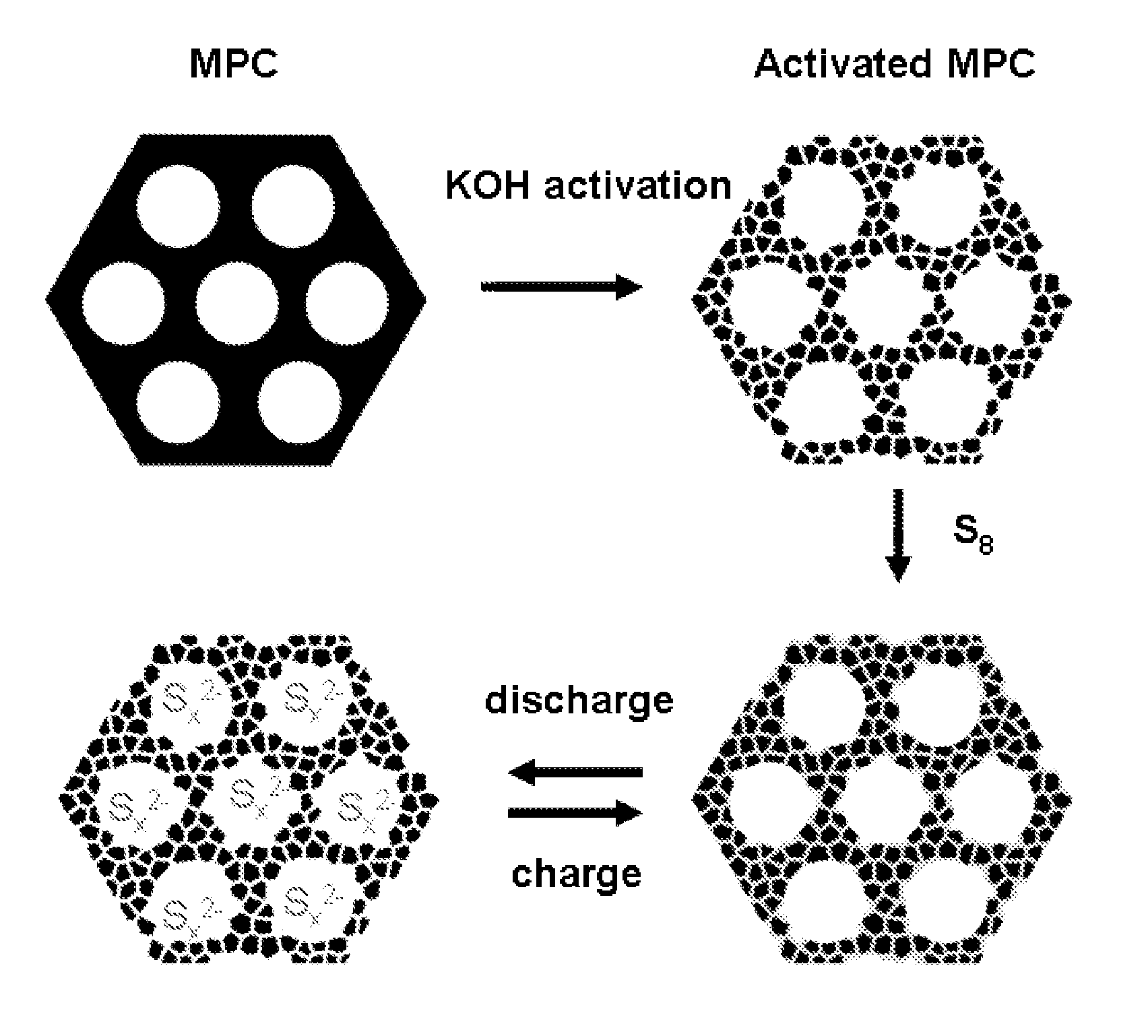
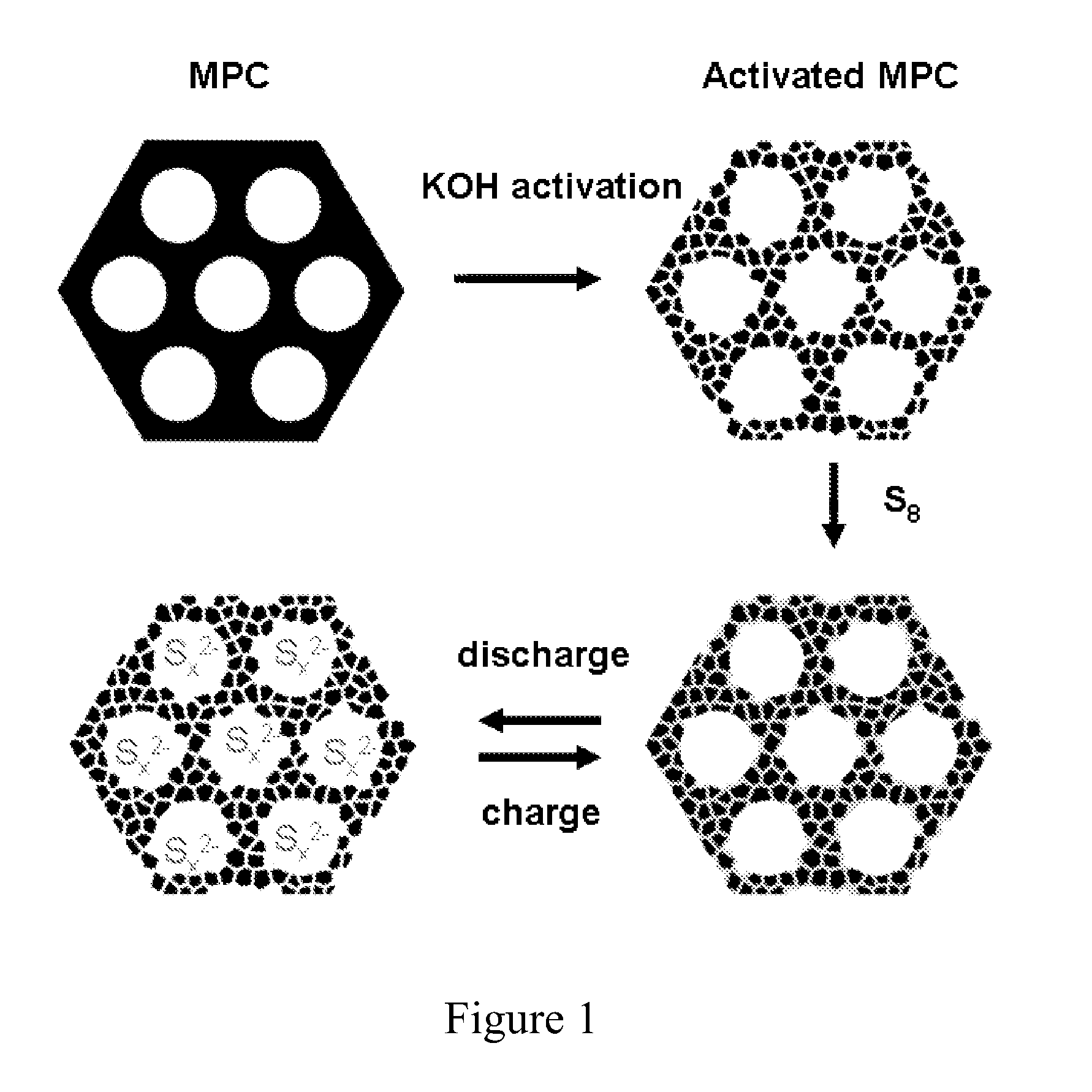
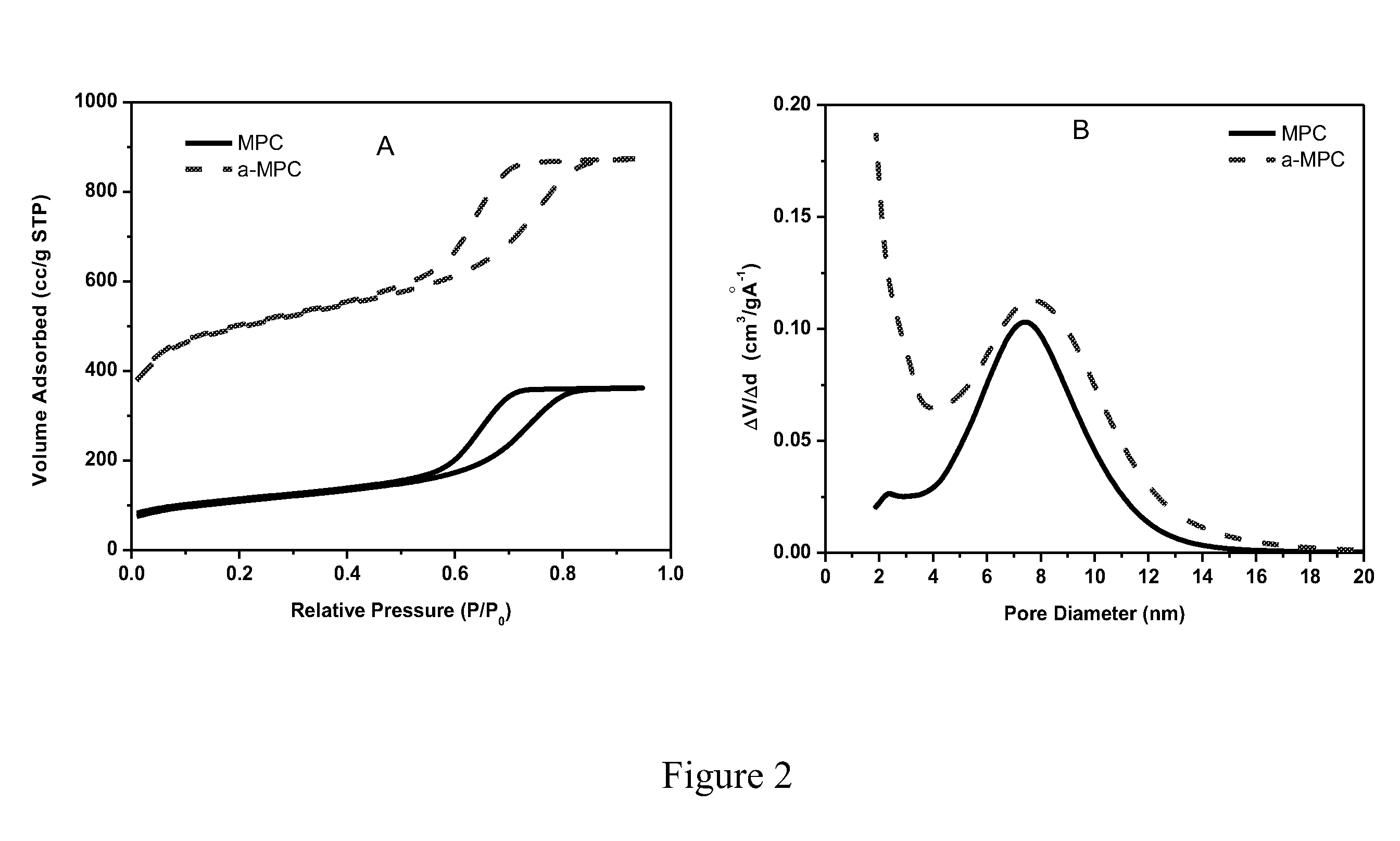
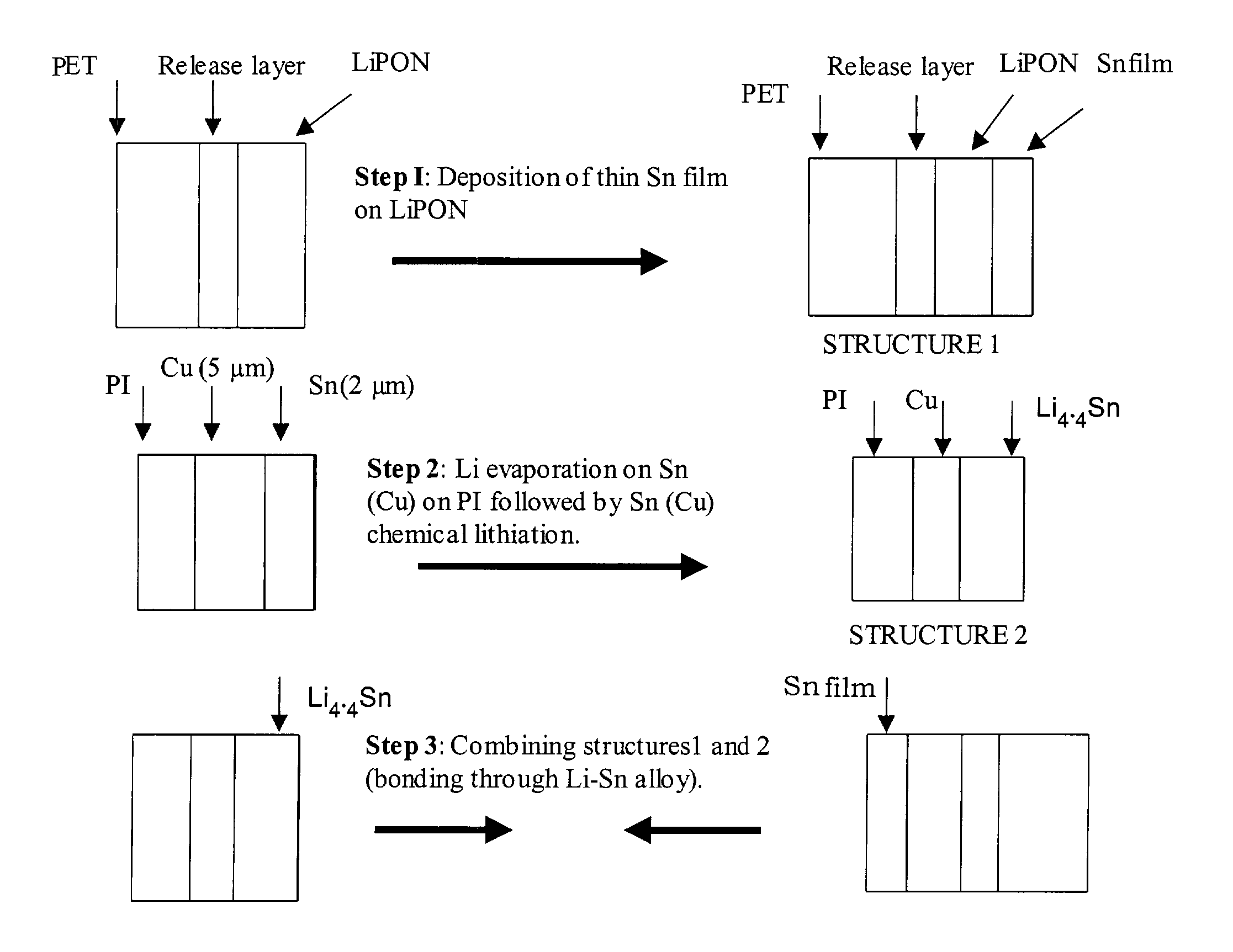
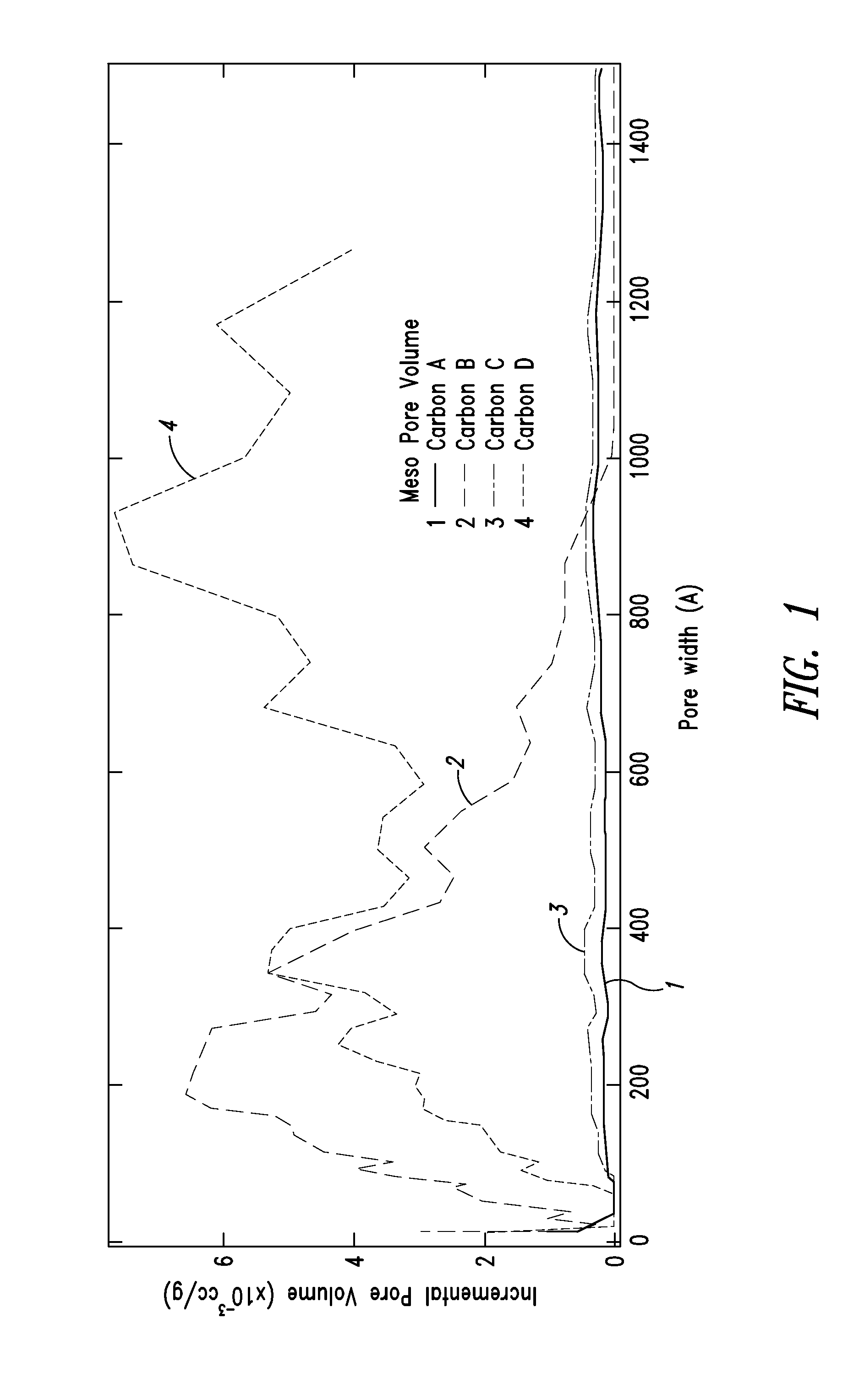
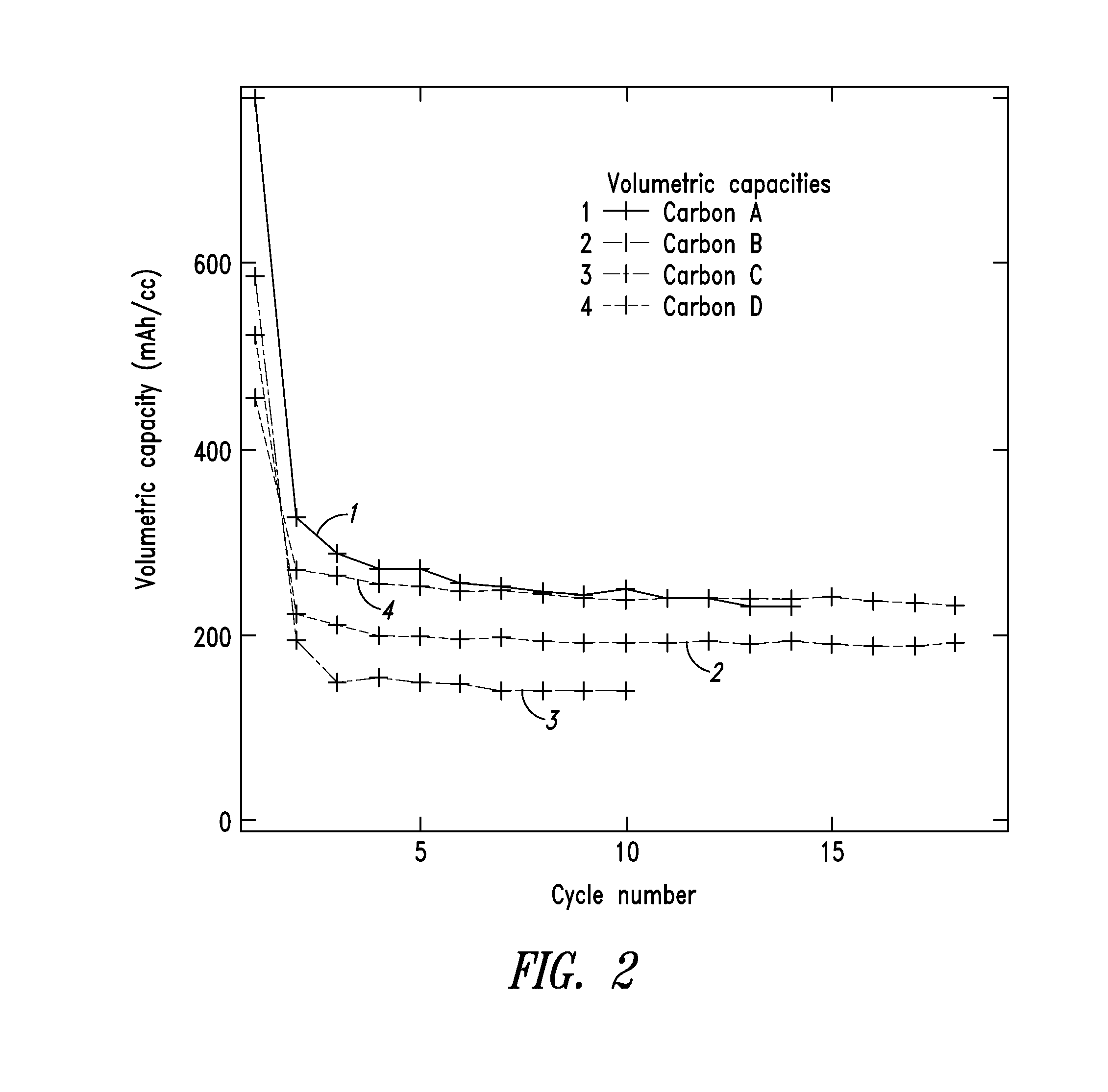
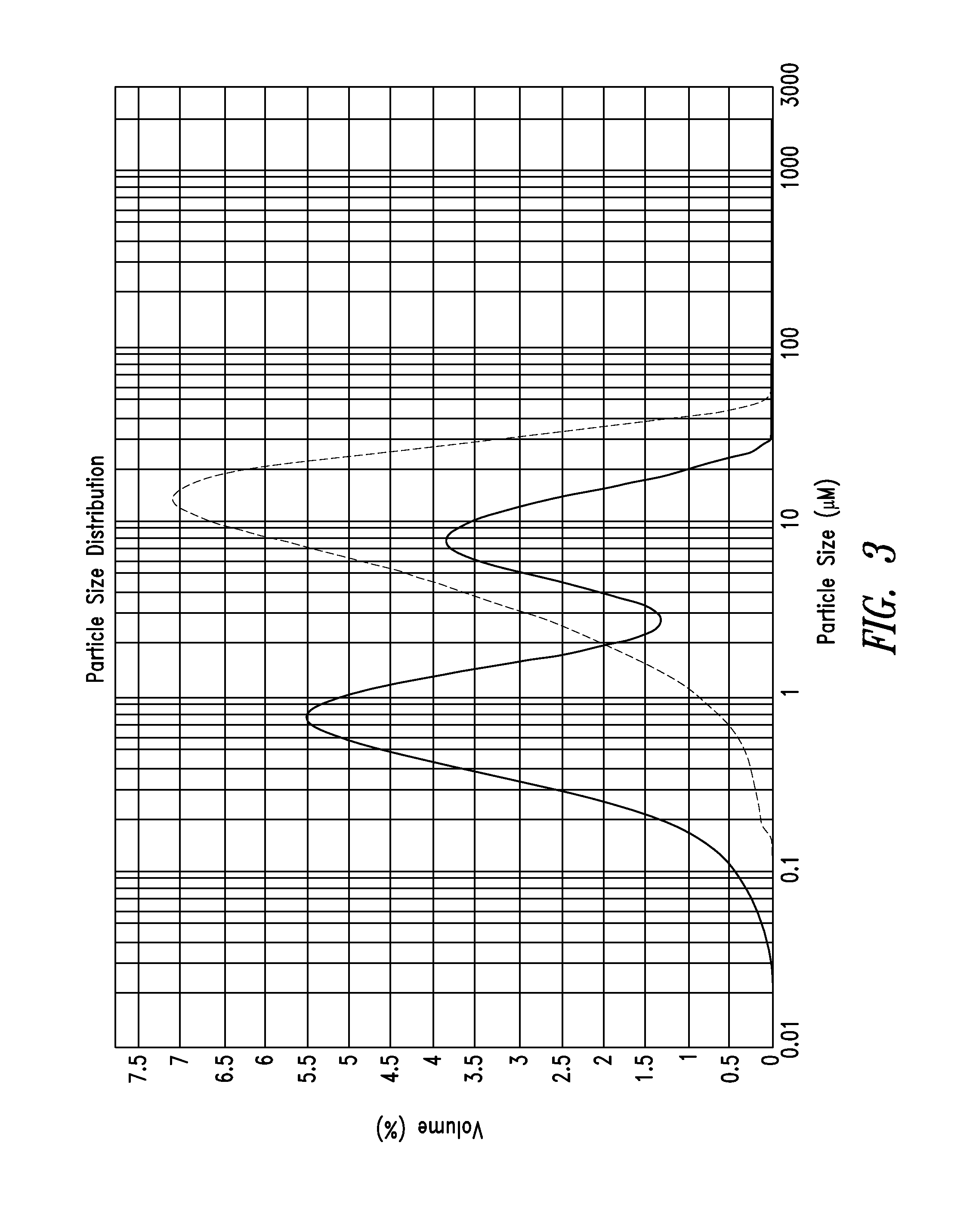
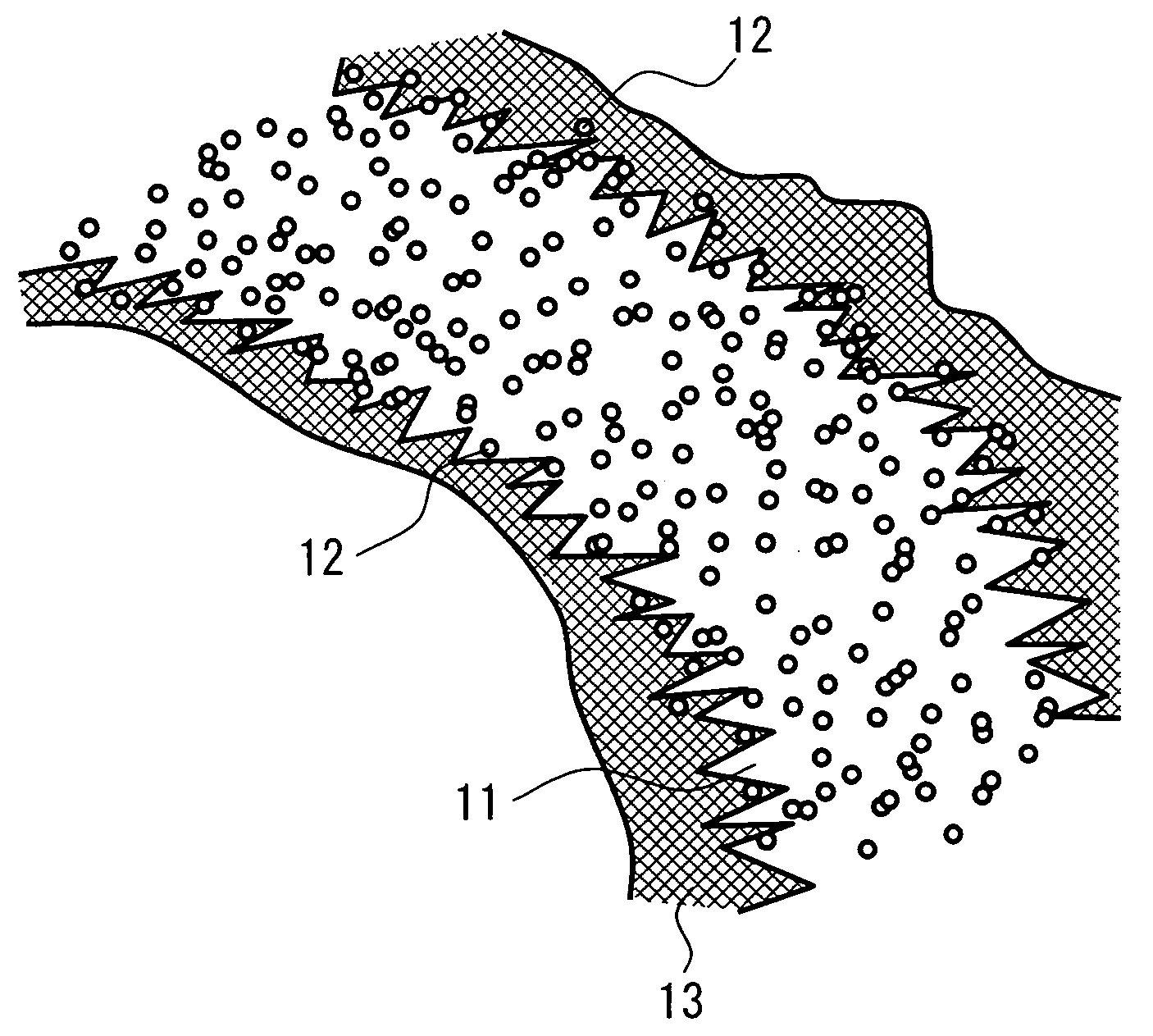
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4342results about "Electrode thermal treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
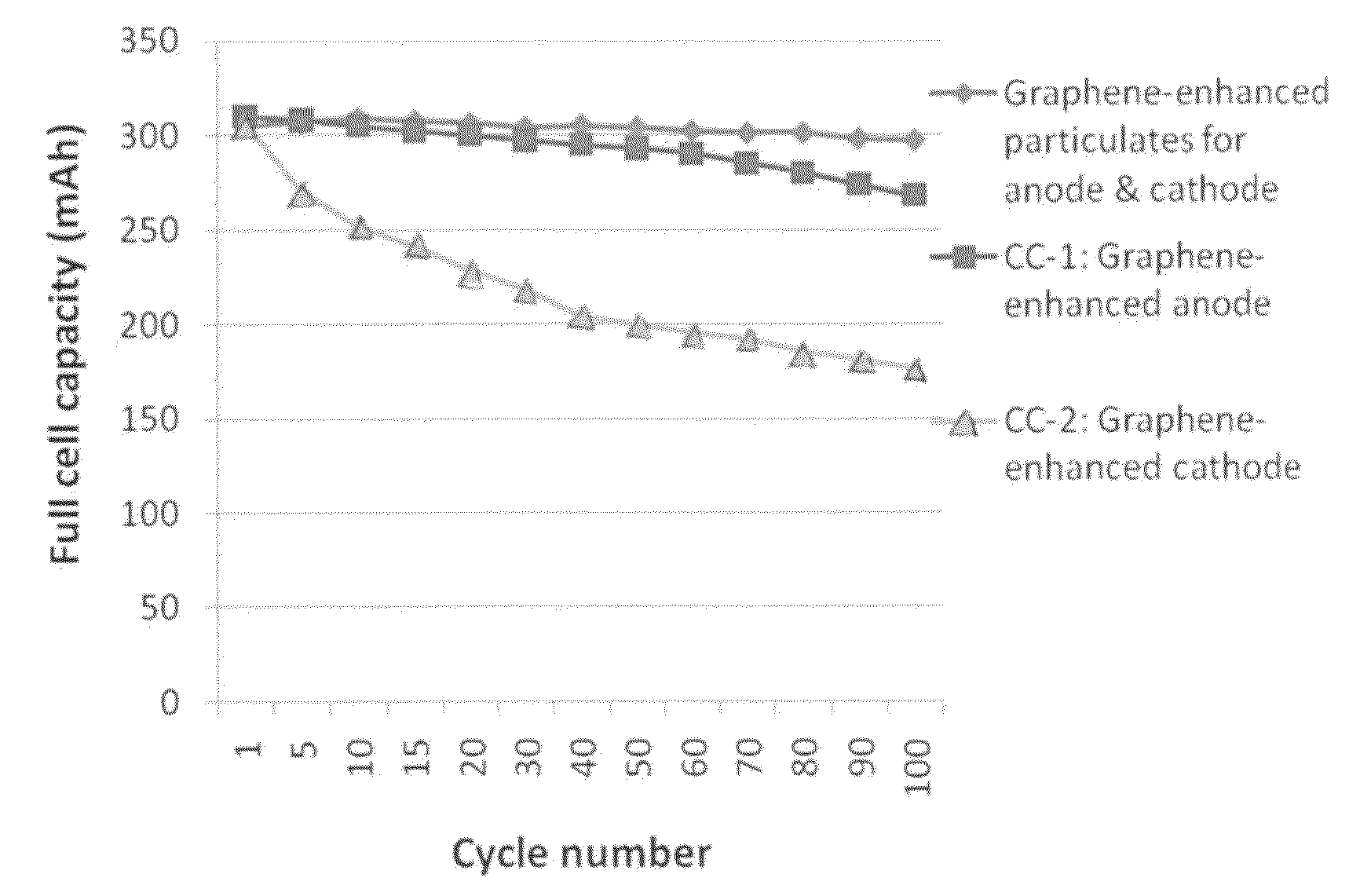
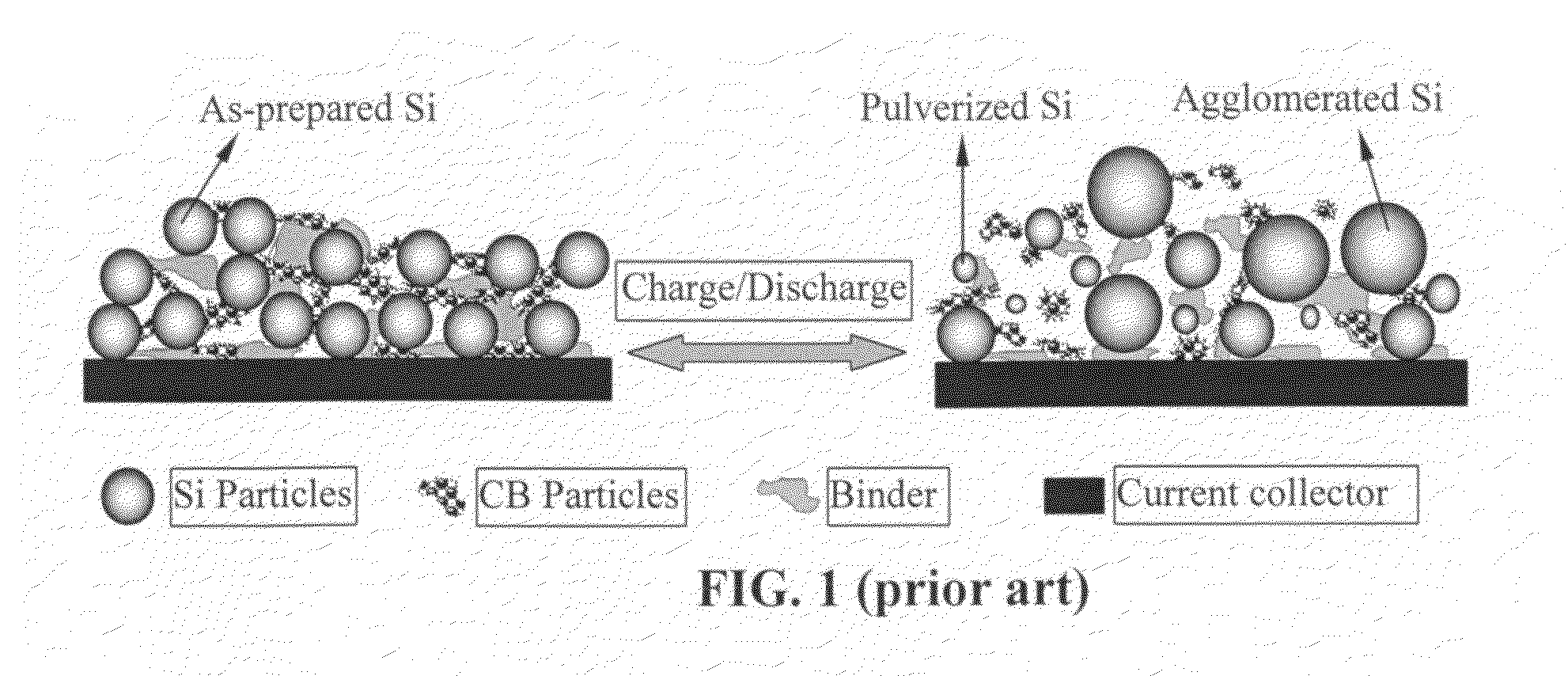
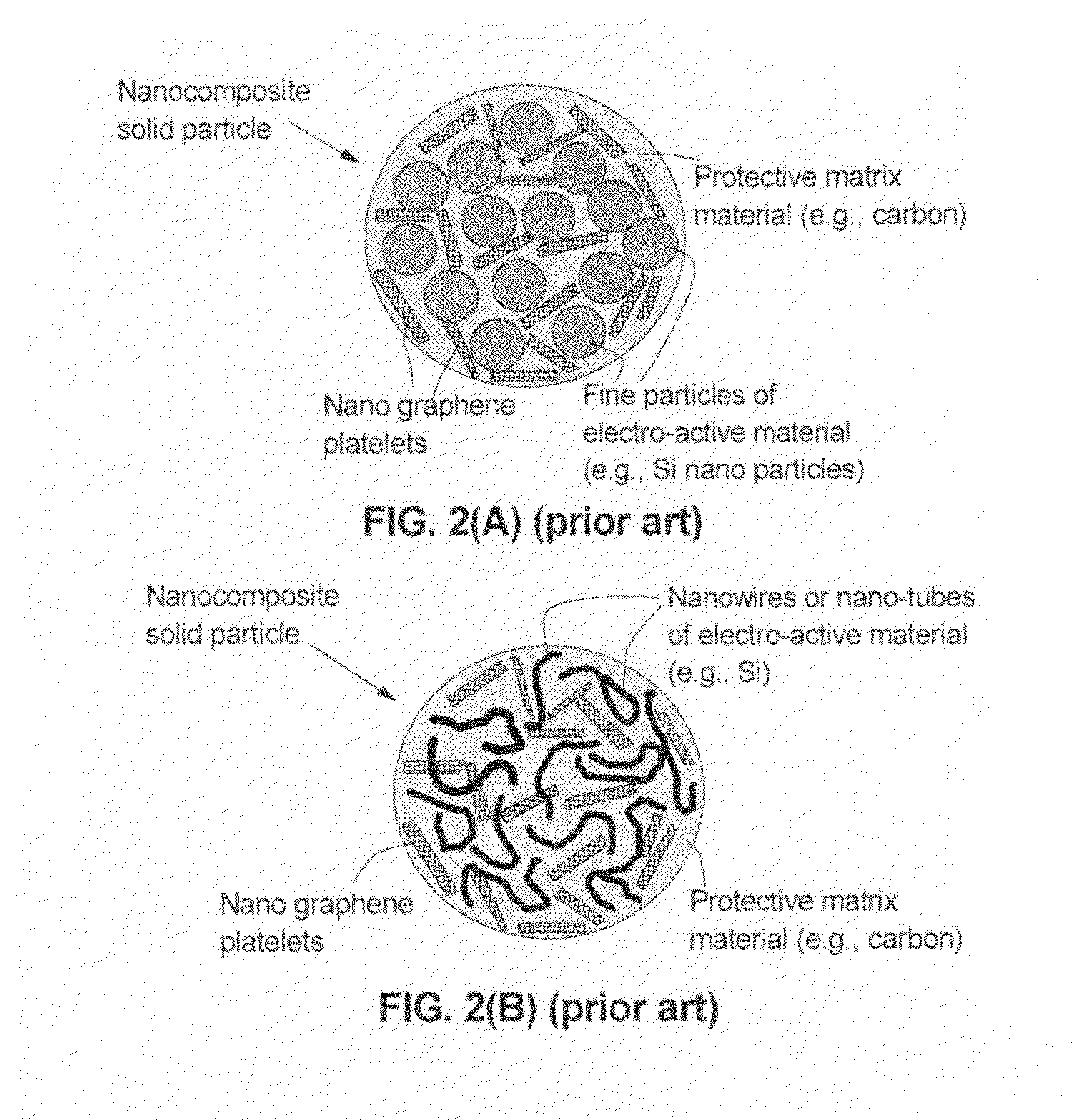
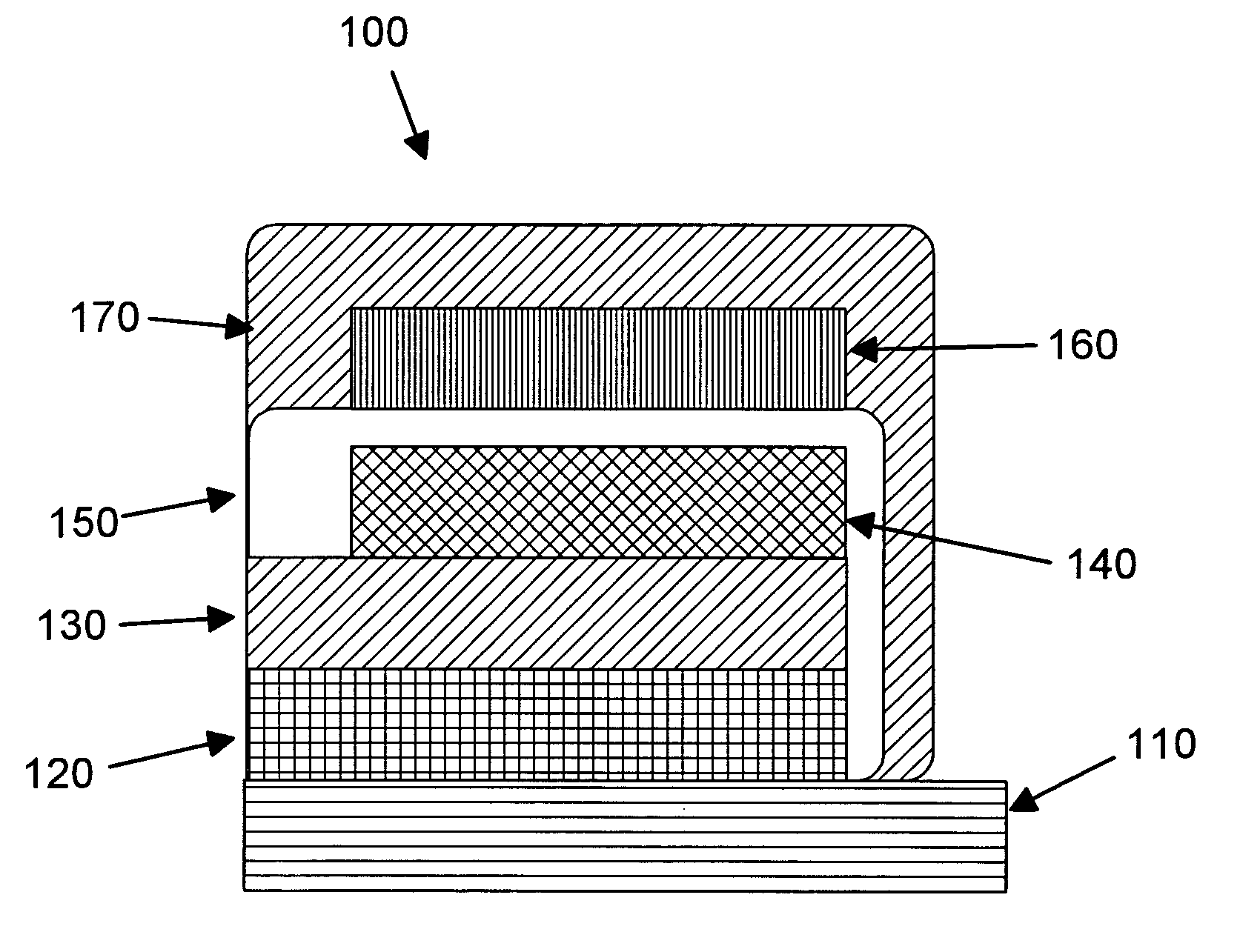
Graphene-enhanced anode particulates for lithium ion batteries
ActiveUS20120064409A1Enhanced Li-ion insertionIncrease capacityNon-metal conductorsMaterial nanotechnologyParticulatesMicroparticle
A nano graphene-enhanced particulate for use as a lithium-ion battery anode active material, wherein the particulate is formed of a single sheet of graphene or a plurality of graphene sheets and a plurality of fine anode active material particles with a size smaller than 10 μm. The graphene sheets and the particles are mutually bonded or agglomerated into the particulate with at least a graphene sheet embracing the anode active material particles. The amount of graphene is at least 0.01% by weight and the amount of the anode active material is at least 0.1% by weight, all based on the total weight of the particulate. A lithium-ion battery having an anode containing these graphene-enhanced particulates exhibits a stable charge and discharge cycling response, a high specific capacity per unit mass, a high first-cycle efficiency, a high capacity per electrode volume, and a long cycle life.
Owner:SAMSUNG ELECTRONICS CO LTD +1
Rechargeable thin film battery and method for making the same
InactiveUS6982132B1Improve lithium ion mobilityHigh voltageElectrode thermal treatmentFinal product manufactureElectrical batteryHigh energy
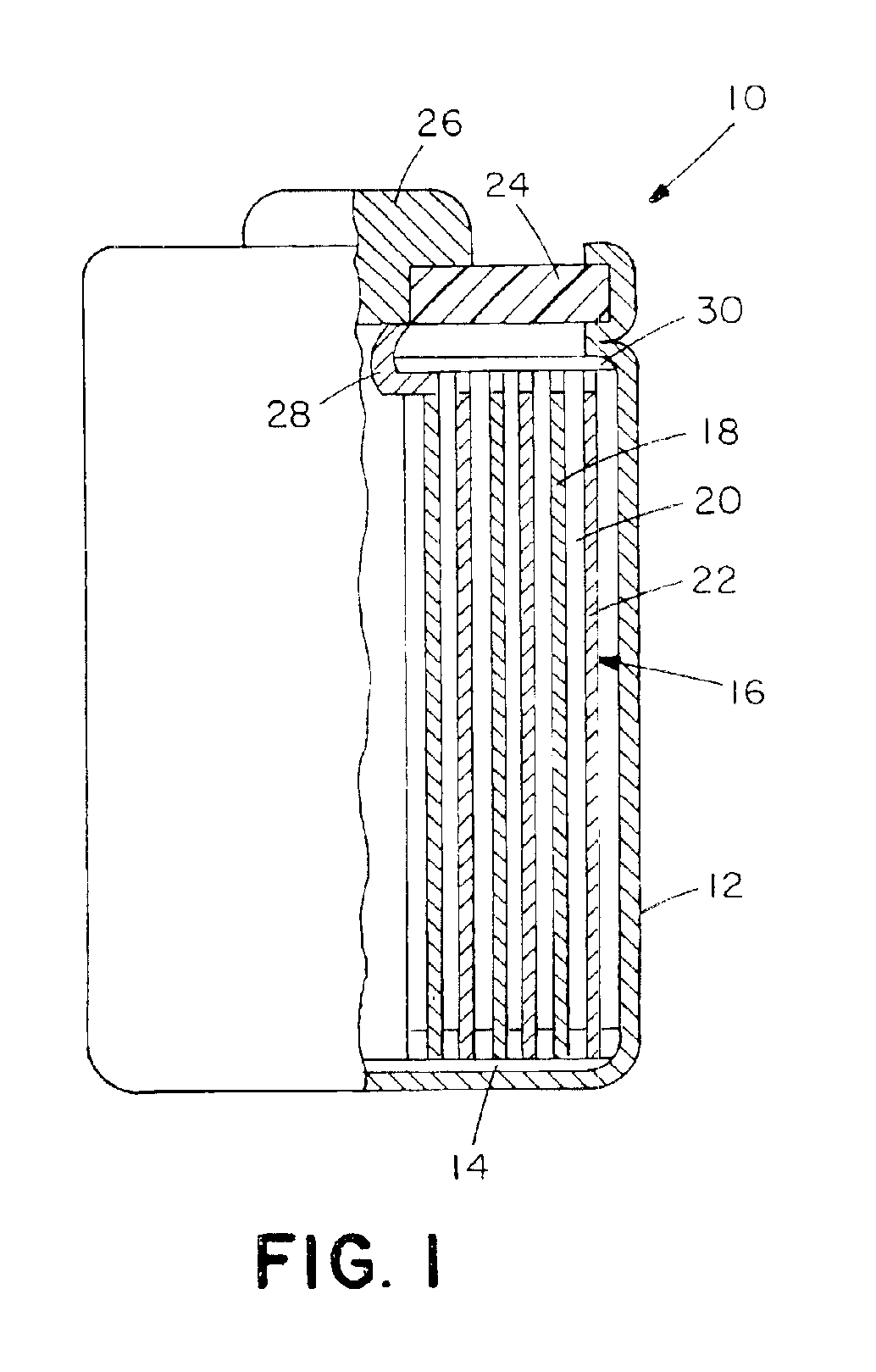
A rechargeable, stackable, thin film, solid-state lithium electrochemical cell, thin film lithium battery and method for making the same is disclosed. The cell and battery provide for a variety configurations, voltage and current capacities. An innovative low temperature ion beam assisted deposition method for fabricating thin film, solid-state anodes, cathodes and electrolytes is disclosed wherein a source of energetic ions and evaporants combine to form thin film cell components having preferred crystallinity, structure and orientation. The disclosed batteries are particularly useful as power sources for portable electronic devices and electric vehicle applications where high energy density, high reversible charge capacity, high discharge current and long battery lifetimes are required.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Carbon-coated silicon particle power as the anode material for lithium batteries and the method of making the same
InactiveUS20050136330A1Large capacityImprove efficiencyElectrode thermal treatmentElectrode carriers/collectorsCarbon compositesSilicon particle
A process for the production of coated silicon / carbon particles comprising: providing a carbon residue forming material; providing silicon particles; coating said silicon particles with said carbon residue forming material to form coated silicon particles; providing particles of a carbonaceous material; coating said particles of carbonaceous material with said carbon residue forming material to form coated carbonaceous particles; embedding said coated silicon particles onto said coated carbonaceous particles to form silicon / carbon composite particles; coating said silicon / carbon composite particles with said carbon residue forming material to form coated silicon / carbon composite particles; and stabilizing the coated composite particles by subjecting said coated composite particles to an oxidation reaction. The coated composite particles will have a substantially smooth coating. The particles may be coated with multiple layers of carbon residue forming material /
Owner:PYROTECK INC
Lithium metal dispersion in electrodes
InactiveUS20050130043A1High specific capacityPromote circulationElectrode rolling/calenderingElectrode thermal treatmentLithium metalHost material
Electrodes, such as anodes and cathodes, can include a host material that is prelithiated or undergoes lithiation upon electrolyte introduction into a battery. Lithiation of the host material can occur by the agitation of lithium metal and a host material, the agitation of a lithium metal powder and a host material at a temperature greater than room temperature, the application of pressure to a lithium metal and host material mixture, contact of the host material with molten lithium metal, the lamination of lithium foil or lithium mesh onto an electrode containing the host material, or by lamination of lithium metal or mesh onto an electrode at elevated temperatures.
Owner:AEA TECH BATTERY SYST +1
Anode active material, method of preparing the same, and anode and lithium battery containing the material
ActiveUS20060134516A1Improve capacity efficiencyImprove charge/discharge efficiencyElectrode thermal treatmentNon-aqueous electrolyte accumulatorsFiberGraphite
Owner:SAMSUNG SDI CO LTD
Hybrid nano-filament anode compositions for lithium ion batteries
ActiveUS20090169996A1Superior multiple-cycle behaviorImprove cycle lifeElectrochemical processing of electrodesElectrode thermal treatmentLithium-ion batteryNanometre
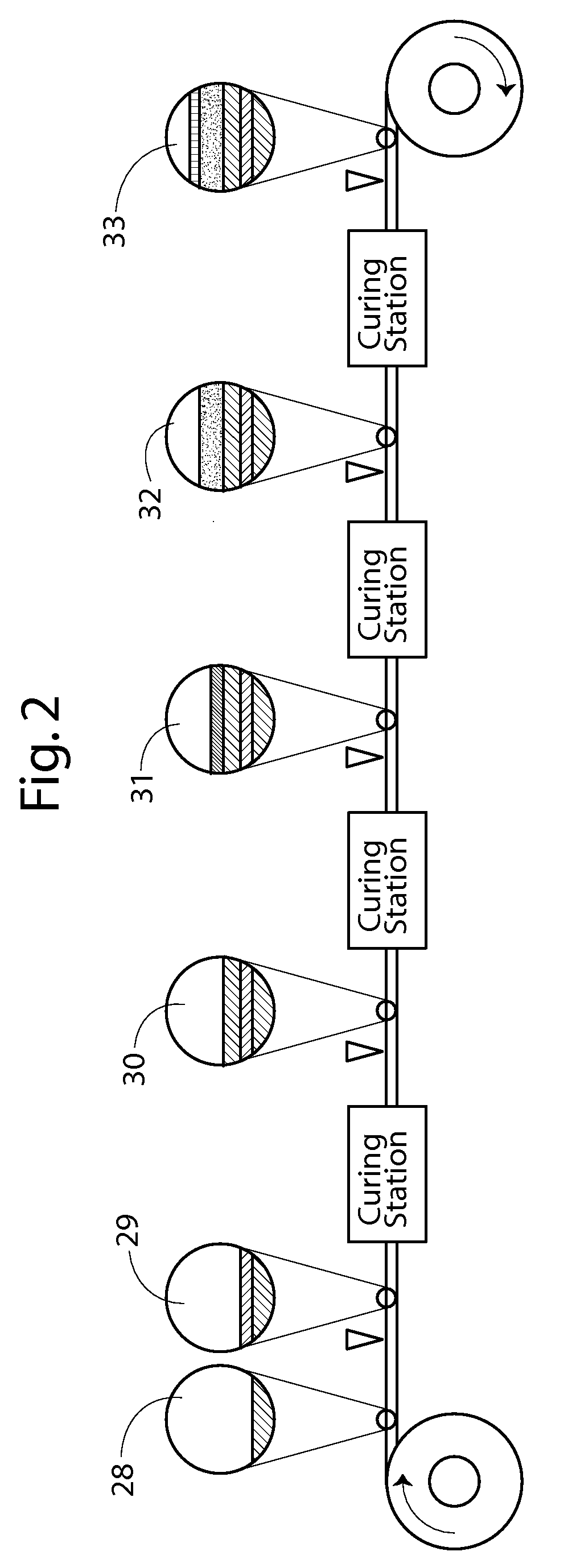
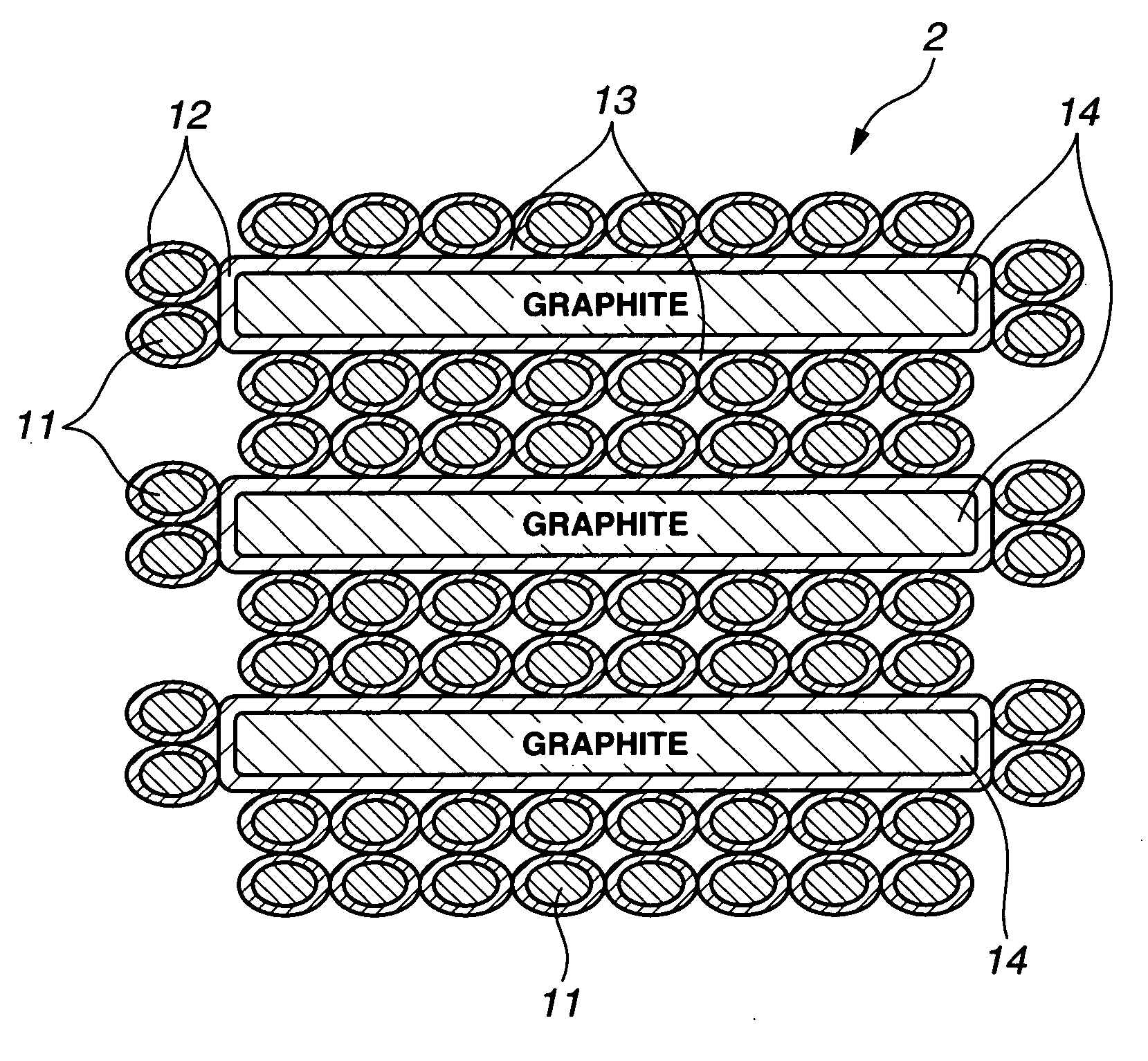
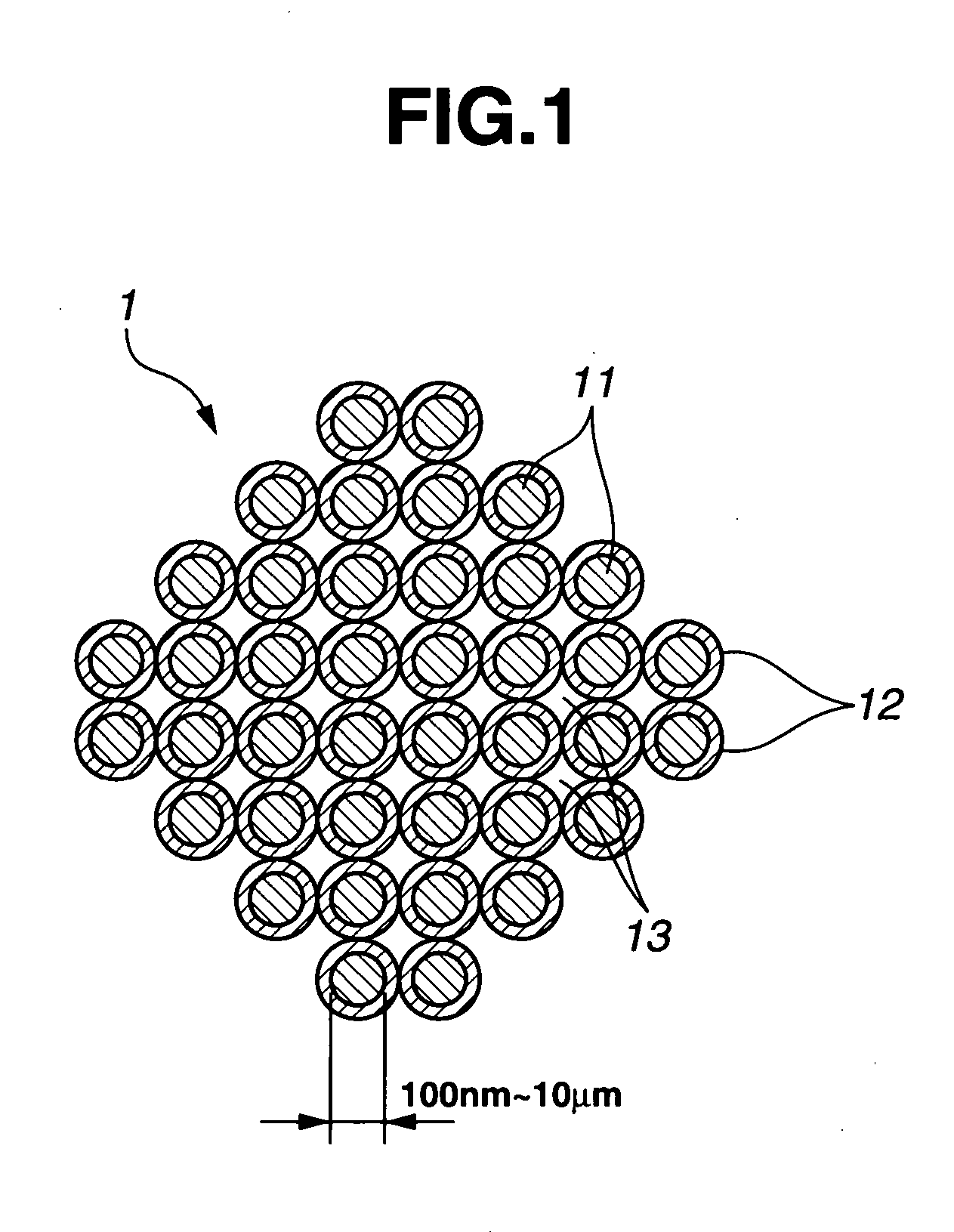
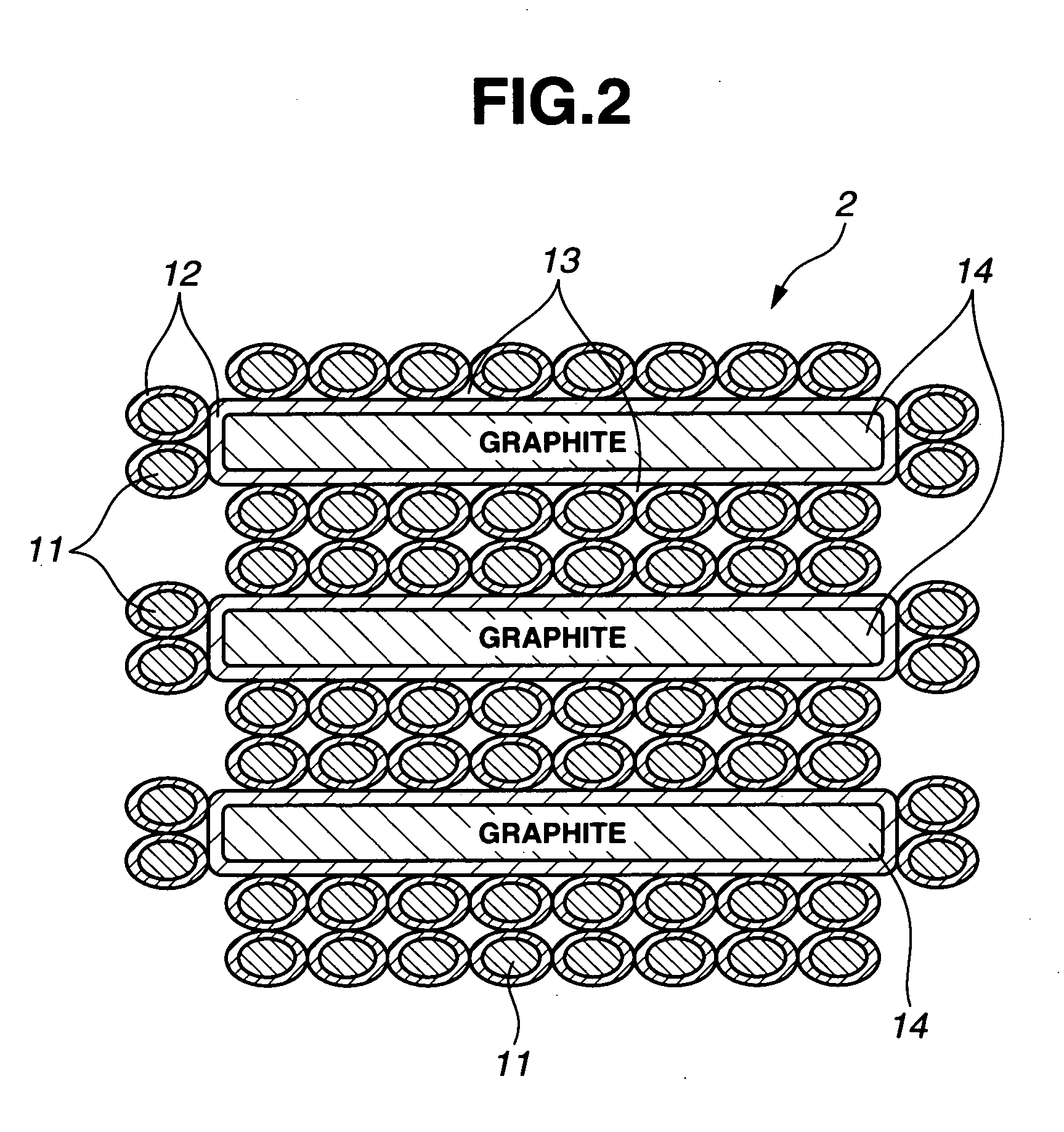
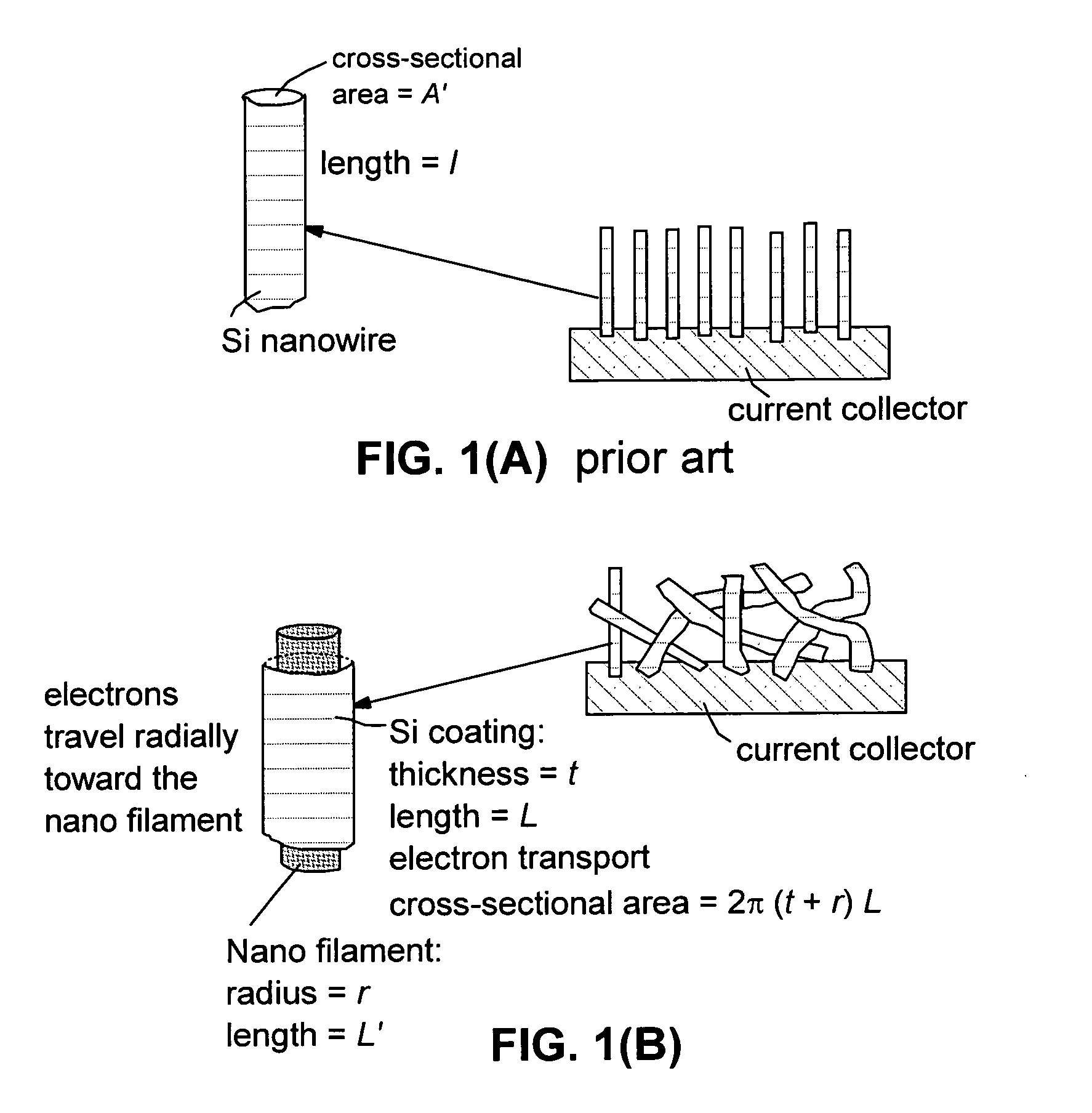
This invention provides a hybrid nano-filament composition for use as an electrochemical cell electrode. The composition comprises: (a) an aggregate of nanometer-scaled, electrically conductive filaments that are substantially interconnected, intersected, or percolated to form a porous, electrically conductive filament network comprising substantially interconnected pores, wherein the filaments have an elongate dimension and a first transverse dimension with the first transverse dimension being less than 500 nm (preferably less than 100 nm) and an aspect ratio of the elongate dimension to the first transverse dimension greater than 10; and (b) micron- or nanometer-scaled coating that is deposited on a surface of the filaments, wherein the coating comprises an anode active material capable of absorbing and desorbing lithium ions and the coating has a thickness less than 20 μm (preferably less than 1 μm). Also provided is a lithium ion battery comprising such an electrode as an anode. The battery exhibits an exceptionally high specific capacity, an excellent reversible capacity, and a long cycle life.
Owner:GLOBAL GRAPHENE GRP INC
Sulfur-carbon nanocomposites and their application as cathode materials in lithium-sulfur batteries
ActiveUS20110052998A1Minimize formationEasy to transportAlkaline accumulatorsElectrode thermal treatmentCarbon compositesPorous carbon
The invention is directed in a first aspect to a sulfur-carbon composite material comprising: (i) a bimodal porous carbon component containing therein a first mode of pores which are mesopores, and a second mode of pores which are micropores; and (ii) elemental sulfur contained in at least a portion of said micropores. The invention is also directed to the aforesaid sulfur-carbon composite as a layer on a current collector material; a lithium ion battery containing the sulfur-carbon composite in a cathode therein; as well as a method for preparing the sulfur-composite material.
Owner:UT BATTELLE LLC
Low Cost Solid State Rechargeable Battery and Method of Manufacturing Same
InactiveUS20090092903A1Solid electrolytesElectrode thermal treatmentOptoelectronicsConductive materials
A solid state Li battery and an all ceramic Li-ion battery are disclosed. The all ceramic battery has a solid state battery cathode comprised of a mixture of an active cathode material, an electronically conductive material, and a solid ionically conductive material. The cathode mixture is sintered. The battery also has a solid state battery anode comprised of a mixture of an active anode material, an electronically conductive material, and a solid ionically conductive material. The anode mixture is sintered. The battery also has a solid state separator positioned between said solid state battery cathode and said solid state battery anode. In the solid state Li battery the all ceramic anode is replaced with an evaporated thin film Li metal anode.
Owner:JOHNSON IP HLDG LLC
Silicon composite particles, preparation thereof, and negative electrode material for non-aqueous electrolyte secondary cell
ActiveUS20050214644A1Improve cycle performanceMinimize changesSilicaNitrogen compoundsSilicon alloyInorganic compound
Silicon composite particles are prepared by sintering primary fine particles of silicon, silicon alloy or silicon oxide together with an organosilicon compound. Sintering of the organosilicon compound results in a silicon-base inorganic compound which serves as a binder. Each particle has the structure that silicon or silicon alloy fine particles are dispersed in the silicon-base inorganic compound binder, and voids are present within the particle.
Owner:SHIN ETSU CHEM IND CO LTD
Garnet materials for li secondary batteries and methods of making and using garnet materials
Set forth herein are garnet material compositions, e.g., lithium-stuffed garnets and lithium-stuffed garnets doped with alumina, which are suitable for use as electrolytes and catholytes in solid state battery applications. Also set forth herein are lithium-stuffed garnet thin films having fine grains therein. Disclosed herein are novel and inventive methods of making and using lithium-stuffed garnets as catholytes, electrolytes and / or anolytes for all solid state lithium rechargeable batteries. Also disclosed herein are novel electrochemical devices which incorporate these garnet catholytes, electrolytes and / or anolytes. Also set forth herein are methods for preparing novel structures, including dense thin (<50 um) free standing membranes of an ionically conducting material for use as a catholyte, electrolyte, and, or, anolyte, in an electrochemical device, a battery component (positive or negative electrode materials), or a complete solid state electrochemical energy storage device. Also, the methods set forth herein disclose novel sintering techniques, e.g., for heating and / or field assisted (FAST) sintering, for solid state energy storage devices and the components thereof.
Owner:QUANTUMSCAPE BATTERY INC
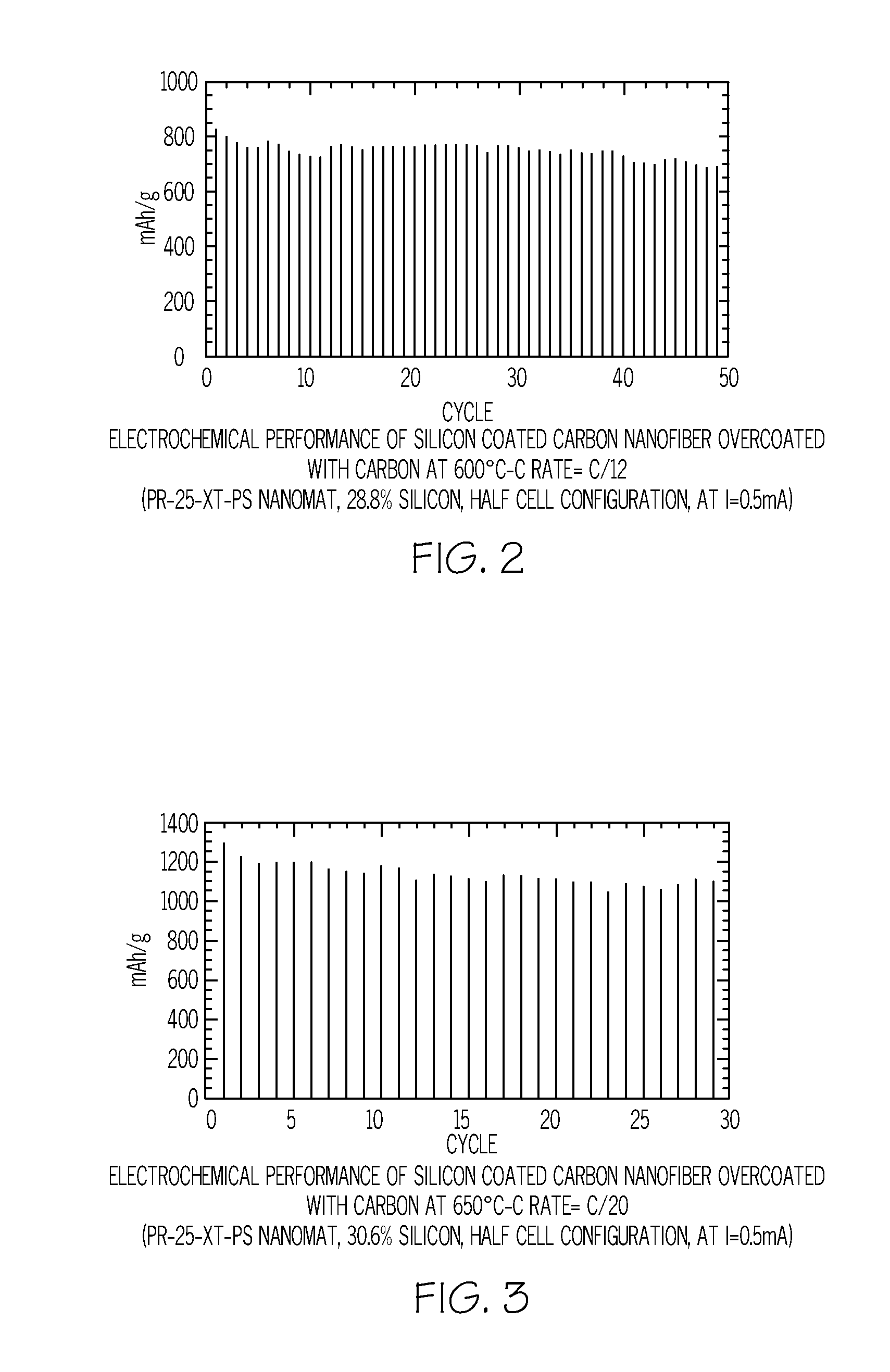
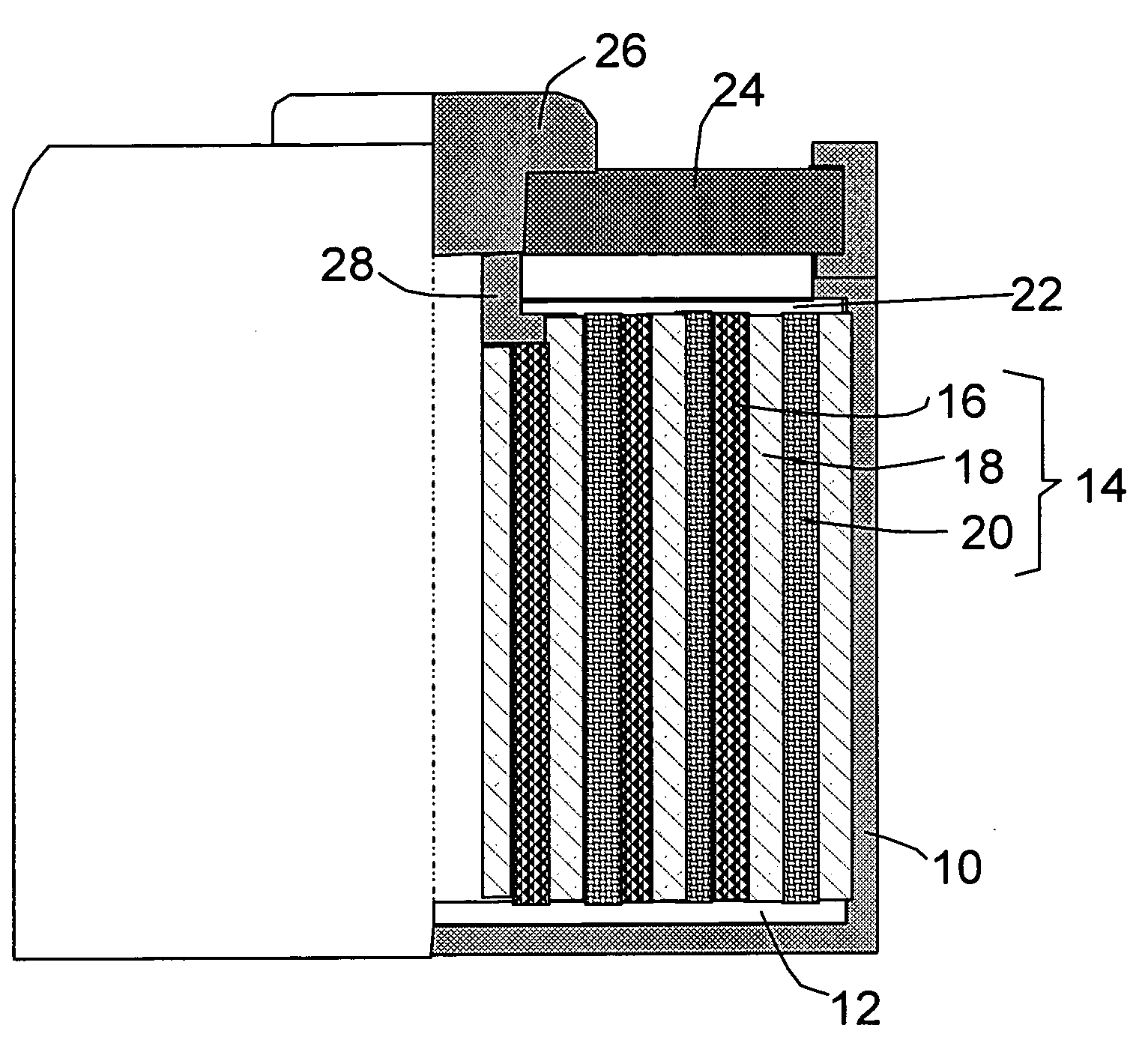
Method of depositing silicon on carbon nanomaterials
InactiveUS20120264020A1Increase surface areaHigh pore volumeMaterial nanotechnologyLiquid surface applicatorsCarbon coatingGas phase
A method of depositing silicon on carbon nanomaterials such as vapor grown carbon nanofibers, nanomats, or nanofiber powder is provided. The method includes flowing a silicon-containing precursor gas in contact with the carbon nanomaterial such that silicon is deposited on the exterior surface and within the hollow core of the carbon nanomaterials. A protective carbon coating may be deposited on the silicon-coated nanomaterials. The resulting nanocomposite materials may be used as anodes in lithium ion batteries.
Owner:APPLIED SCI
Active material for positive electrode used in lithium secondary battery and method of manufacturing same
InactiveUS6372385B1Easy to implementEvenly dispersedElectrode thermal treatmentActive material electrodesLithium-ion batteryMetal
Disclosed is active material for a positive electrode used in lithium secondary batteries of Formula 1 below and a method manufacturing the same, a surface of the active material being coated with metal oxide. The method includes the steps of producing a crystalline powder or a semi-crystalline powder of Formula 1; coating the crystalline powder or the semi-crystalline powder with metal alkoxide sol; and heat-treating the powder coated with the metal alkoxide sol.where 0<x<=0.3, 0<=y<=0.01, andA is an element selected from the group consisting of Ni, Co and Mn; B is an element selected from the group consisting of Ni, Co, Mn, B, Mg, Ca, Sr, Ba, Ti, V, Cr, Fe, Cu and Al; and C is an element selected from the group consisting of Ni, Co, Mn, B, Mg, Ca, Sr, Ba, Ti, V, Cr, Fe, Cu and Al.
Owner:SAMSUNG ELECTRONICS DEVICES CO LTD
Method of producing hybrid nano-filament electrodes for lithium metal or lithium ion batteries
ActiveUS20090169725A1High reversible capacityLower internal resistanceElectrochemical processing of electrodesElectrode thermal treatmentChemical LinkageFiber
Disclosed is a method of producing a hybrid nano-filament composition for use in a lithium battery electrode. The method comprises: (a) providing an aggregate of nanometer-scaled, electrically conductive filaments that are substantially interconnected, intersected, physically contacted, or chemically bonded to form a porous network of electrically conductive filaments, wherein the filaments comprise electro-spun nano-fibers that have a diameter less than 500 nm (preferably less than 100 nm); and (b) depositing micron- or nanometer-scaled coating onto a surface of the electro-spun nano-fibers, wherein the coating comprises an electro-active material capable of absorbing and desorbing lithium ions and the coating has a thickness less than 10 μm (preferably less than 1 μm). The same method can be followed to produce an anode or a cathode. The battery featuring an anode or cathode made with this method exhibits an exceptionally high specific capacity, an excellent reversible capacity, and a long cycle life.
Owner:GLOBAL GRAPHENE GRP INC
Battery, method of charging and discharging the battery and charge-discharge control device for the battery
InactiveUS20050214646A1Excellent cycle characteristicsSimple structureElectrode thermal treatmentElectrode carriers/collectorsOptoelectronicsAlloy
Provided is a battery capable of improving cycle characteristics through reducing a structural fracture according to charge and discharge of an anode and a reaction of the with an electrolyte. An anode active material layer includes at least one kind selected from the group consisting of a simple substance and alloys of Si capable of forming an alloy with Li. A cathode and an anode are formed so that the molar ratio Li / Si in the anode at the time of charge is 4.0 or the potential of the anode vs. Li is 0.04 V or more through adjusting, for example, a ratio between a cathode active material and an anode active material. Moreover, the cathode and the anode are formed so that the molar ratio Li / Si in the anode at the time of discharge is 0.4 or more, or the potential of the anode vs. Li is 1.4 V or less.
Owner:SONY CORP
Positive-electrode active material and nonaqueous-electrolyte secondary battery containing the same
InactiveUS20030170540A1Improve the immunityLarge ion permeabilityIron oxides/hydroxidesElectrode thermal treatmentCrystal structureOxygen
The present invention provides a high-capacity and low-cost non-aqueous electrolyte secondary battery, comprising: a negative electrode containing, as a negative electrode active material, a ssubstance capable of absorbing / desorbing lithium ions and / or metal lithium; a separator; a positive electrode; and an electrolyte, wherein the positive electrode active material contained in the positive electrode is composed of crystalline particles of an oxide containing two kinds of transition metal elements, the crystalline particles having a layered crystal structure, and oxygen atoms constituting the oxide forming a cubic closest packing structure.
Owner:PANASONIC CORP +1
Laminate Including Active Material Layer and Solid Electrolyte Layer, and All Solid Lithium Secondary Battery Using the Same
InactiveUS20070259271A1Improve life characteristicsSmall internal resistanceElectrode thermal treatmentFinal product manufactureLithiumX-ray
A laminate includes an active material layer and a solid electrolyte layer bonded to the active material layer by sintering. The active material layer includes a crystalline first substance capable of absorbing and desorbing lithium ions, and the solid electrolyte layer includes a crystalline second substance with lithium ion conductivity. An X-ray diffraction analysis of the laminate shows that there is no component other than constituent components of the active material layer and constituent components of the solid electrolyte layer. Also, an all solid lithium secondary battery includes such a laminate and a negative electrode active material layer.
Owner:PANASONIC CORP
Wet chemistry method for preparing lithium iron phosphate
InactiveCN1431147AEasy to controlEvenly distributedElectrode thermal treatmentLi-accumulatorsChemical compositionLithium iron phosphate
A wet chemical process for preparing iron lithium phosphate includes mixing the solution or suspensions of Li source compound, Fe source compound, P source compound, doping element compound or electric conducting agent, and precipitant, reaction at 5-120 deg.C for 0.5-24 hr while stirring, filtering, washing, baking to obtain nano precursor, quickly heating to 500-800 deg.C in non-air or non-oxidizing atmosphere, calcining for 5-48 hr, and cooling. Its advantages are easy control, high uniformity and electric conductivity.
Owner:郑绵平
Composite carbon materials comprising lithium alloying electrochemical modifiers
InactiveUS20140272592A1Electrode thermal treatmentHybrid capacitor electrodesElectrical devicesLithium-ion battery
The present application is generally directed to composites comprising a hard carbon material and an electrochemical modifier. The composite materials find utility in any number of electrical devices, for example, in lithium ion batteries. Methods for making the disclosed composite materials are also disclosed.
Owner:GRP 14 TECH INC
Manufacturing methods of catalysts for carbon fiber composition and carbon material compound, manufacturing methods of carbon fiber and catalyst material for fuel cell, and catalyst material for fuel cell
The carbon fibers of this invention is characterized in that irreducible inorganic material particles in a mean primary particle size below 500 nm and reducible inorganic material particles in a mean primary particle size below 500 nm were mixed by pulverizing and then, the mixture was heat treated under the reducing atmosphere and metal particles in a mean particle size below 1 μm were obtained, and the mixed powder of the thus obtained metal particles with the irreducible inorganic material particles are included in the carbon fibers.
Owner:KK TOSHIBA
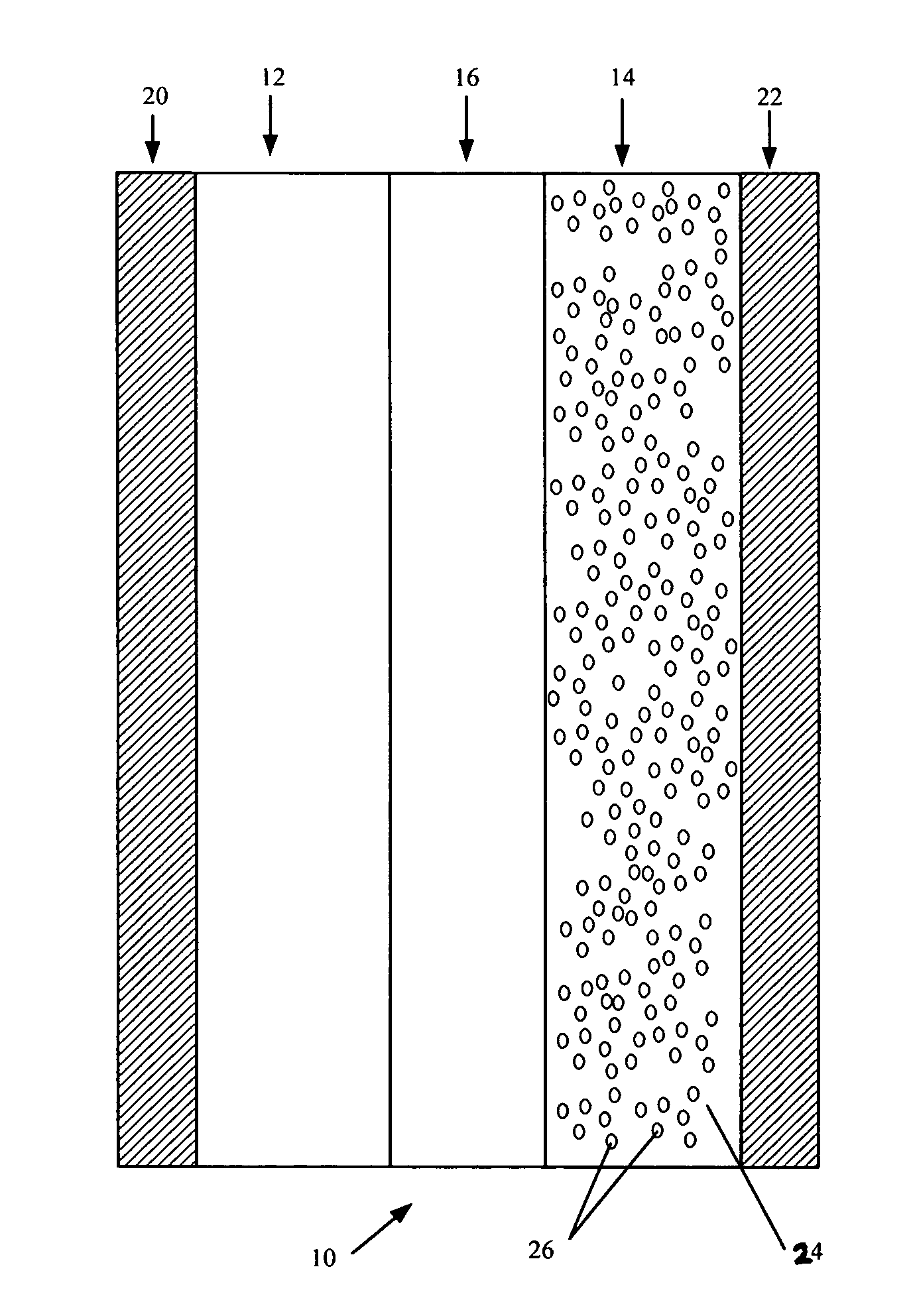
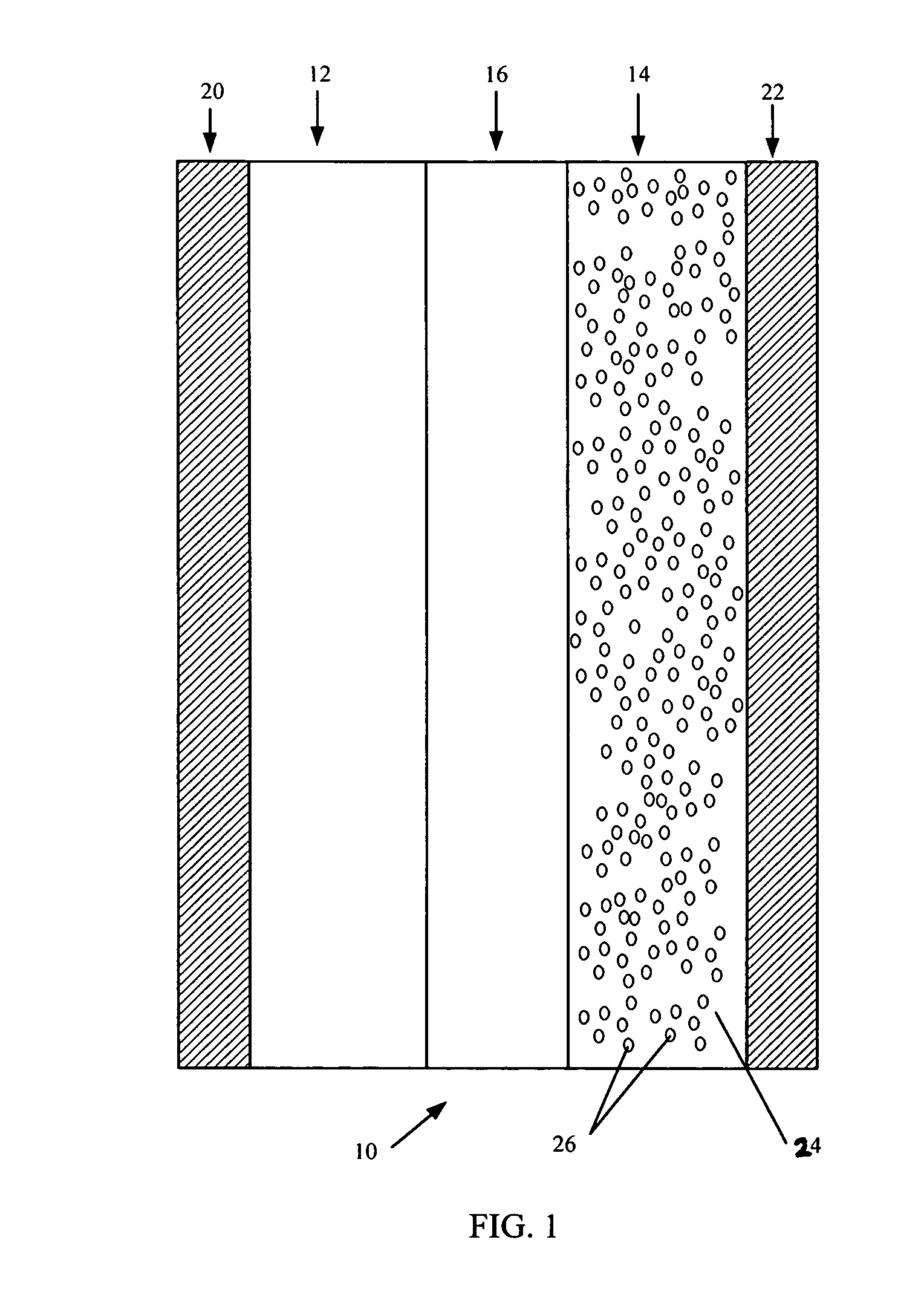
Reticulated and controlled porosity battery structures
InactiveUS7553584B2Boost energyHigh porosityElectrode thermal treatmentElectrolytic capacitorsEngineeringTortuous retinal vessels
The effective ionic conductivity in a composite structure is believed to decrease rapidly with volume fraction. A system, such as a bipolar device or energy storage device, has structures or components in which the diffusion length or path that electrodes or ions must traverse is minimized and the interfacial area exposed to the ions or electrons is maximized. The device includes components that can be reticulated or has a reticulated interface so that an interface area can be increased. The increased interfacial perimeter increases the available sites for reaction of ionic species. Many different reticulation patterns can be used. The aspect ratio of the reticulated features can be varied. Such bipolar devices can be fabricated by a variety of methods or procedures. A bipolar device having structures of reticulated interface can be tailored for the purposes of controlling and optimizing charge and discharge kinetics. A bipolar device having graded porosity structures can have improved transport properties because the diffusion controlling reaction kinetics can be modified. Graded porosity electrodes can be linearly or nonlinearly graded. A bipolar device having perforated structures also provides improved transport properties by removing tortuosity and reducing diffusion distance.
Owner:MASSACHUSETTS INST OF TECH
Elastomer-Encapsulated particles of high-capacity anode active materials for lithium batteries
ActiveUS20170288211A1Improve lithium ion conductivityFuel and secondary cellsElectrode thermal treatmentParticulatesElastomer
Provided is an anode active material layer for a lithium battery. This layer comprises multiple particulates of an anode active material, wherein at least a particulate is composed of one or a plurality of particles of a high-capacity anode active material being encapsulated by a thin layer of elastomeric material that has a lithium ion conductivity no less than 10−7 S / cm (preferably no less than 10−5 S / cm) at room temperature and an encapsulating shell thickness from 1 nm to 10 μm, and wherein the high-capacity anode active material (e.g. Si, Ge, Sn, SnO2, Co3O4, etc.) has a specific capacity of lithium storage greater than 372 mAh / g (the theoretical lithium storage limit of graphite).
Owner:GLOBAL GRAPHENE GRP INC
Cathode intercalation compositions, production methods and rechargeable lithium batteries containing the same
InactiveUS6248477B1Easy to adaptOxygen/ozone/oxide/hydroxideElectrode thermal treatmentMetalSpinel group
Intercalation compositions having spinel structures with crystallites of metal oxides (M2O3) dispersed throughout the structure are provided having the general formula Li1+xMyMn2-x-yO4. Methods of producing the intercalation compositions and rechargeable lithium batteries containing the compositions are also provided.
Owner:EMD ACQUISITION LLC
Lithium metal oxide electrodes for lithium batteries
ActiveUS20050026040A1Increase capacityImprove cycle stabilityCellsElectrode thermal treatmentLithium metalOxidation state
An uncycled electrode for a non-aqueous lithium electrochemical cell including a lithium metal oxide having the formula Li(2+2x) / (2+x)M′2x / (2+x)M(2-2x) / (2+x)O2-δ, in which 0≦x<1 and δ is less than 0.2, and in which M is a non-lithium metal ion with an average trivalent oxidation state selected from two or more of the first row transition metals or lighter metal elements in the periodic table, and M′ is one or more ions with an average tetravalent oxidation state selected from the first and second row transition metal elements and Sn. Methods of preconditioning the electrodes are disclosed as are electrochemical cells and batteries containing the electrodes.
Owner:UCHICAGO ARGONNE LLC
Materials for positive electrodes of lithium ion batteries and their methods of fabrication
InactiveUS20050130042A1Cycle wellEasy dischargeElectrode thermal treatmentSecondary cellsCeriumSolvent
This invention discloses materials for positive electrodes of secondary batteries and their methods of fabrication. Said materials comprise of granules of an active material for positive electrodes coated with an oxide layer. The active material is one or more of the following: oxides of lithium cobalt, oxides of lithium nickel cobalt, oxides of lithium nickel cobalt manganese, oxides of lithium manganese, LiCoO2, LiNi1-xCoxO2, LiNi1 / 3Co1 / 3Mn1 / 3O2, and LiMn2O4. The non-oxygen component in the oxide layer is one or more of the following: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B. Said non-oxygen component of the granules is between 0.01 wt. % to 10 wt. % of said granules of active material. The methods of fabrication for said materials includes the steps of mixing an additive and an active material for positive electrodes uniformly in water or solvent, evaporating said solvent or water, and heat treating the remaining mixture at 300° C. to 900° C. for between 1 hour to 20 hours. The additive is a compound of one or more of the following elements: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B where the element is between 0.01 wt. % to 10 wt. % of said active material. Using the materials of positive electrodes disclosed above or materials for positive electrodes fabricated in the methods disclosed above in batteries produces batteries with excellent cycling and high temperature properties.
Owner:BYD AMERICA CORP
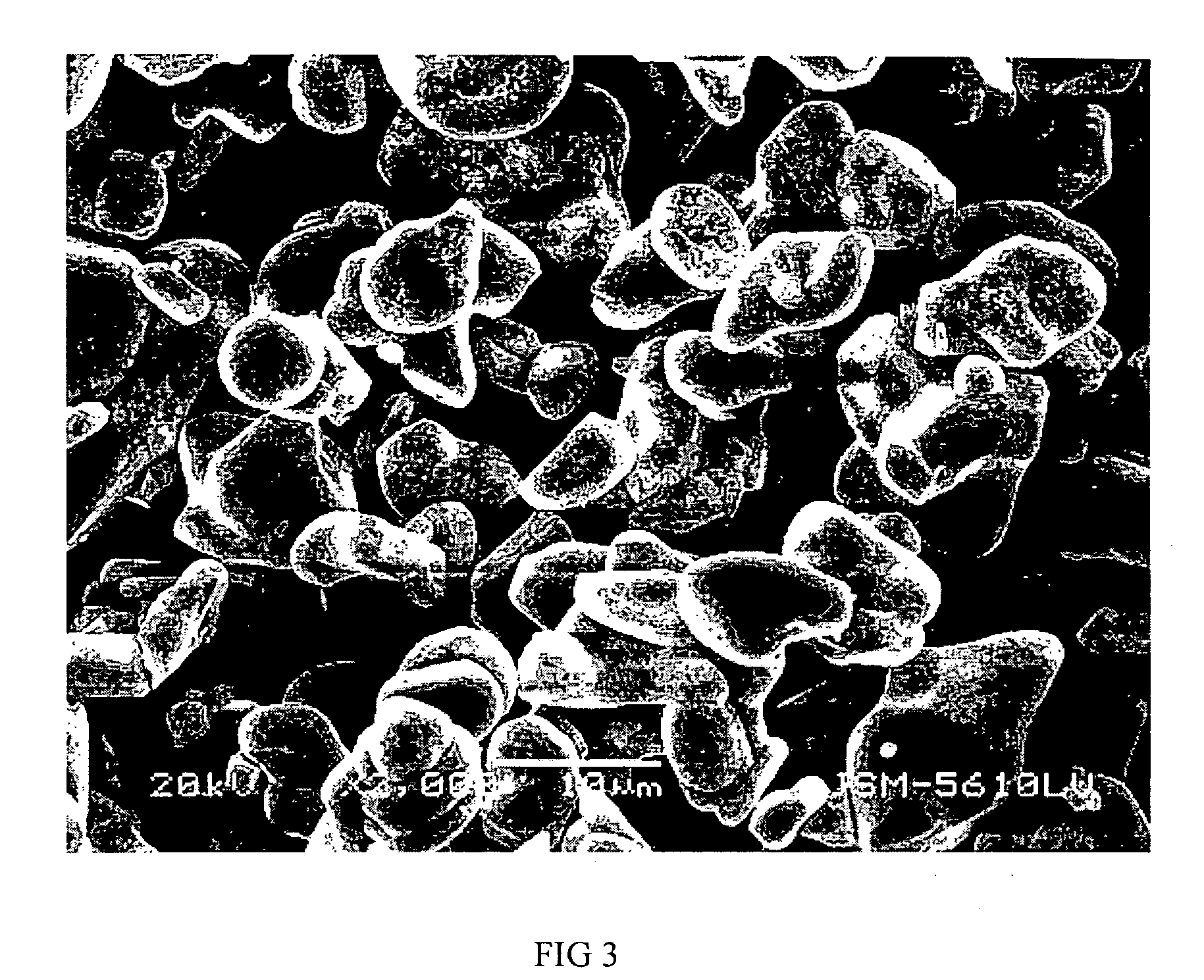
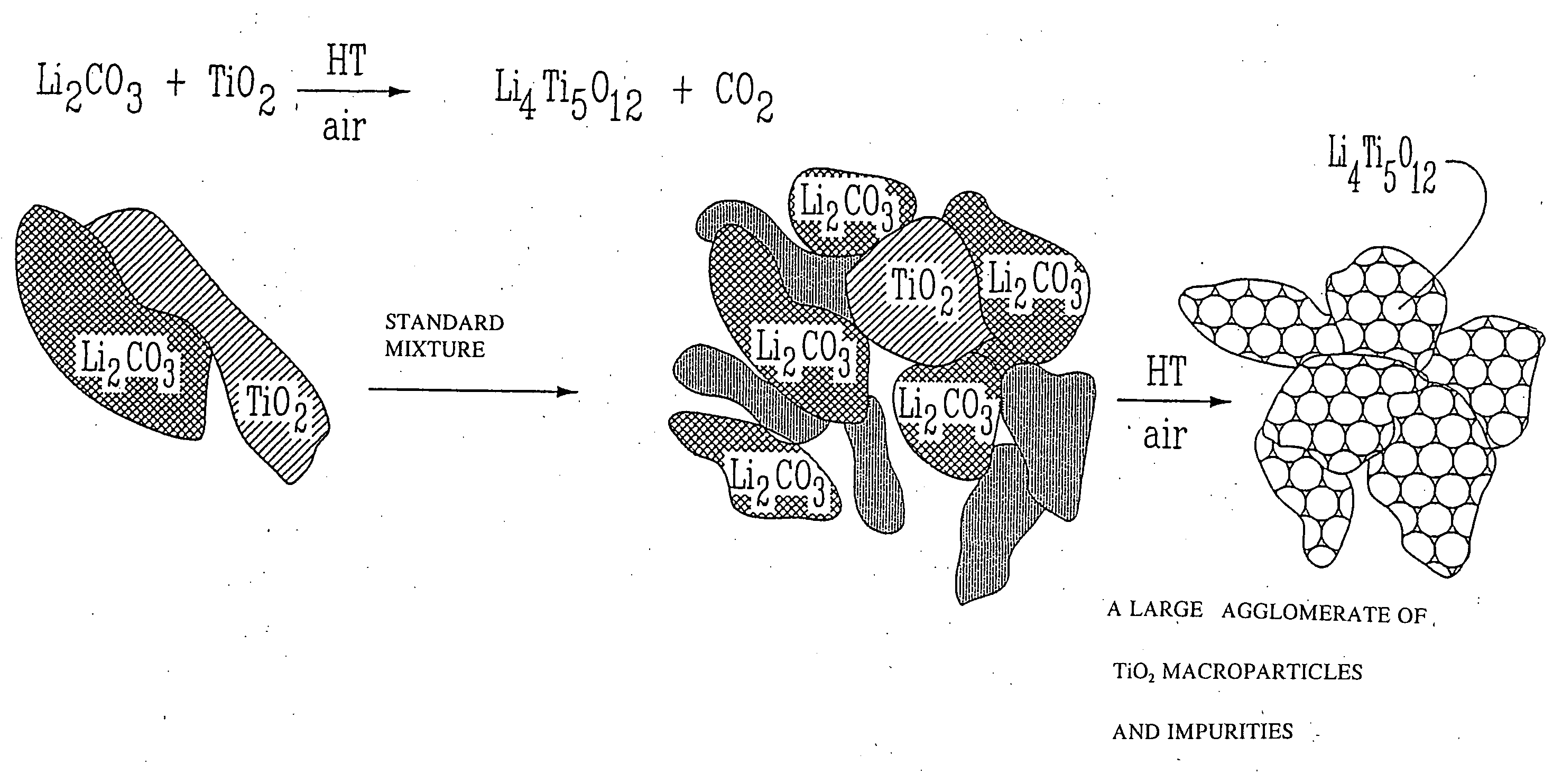
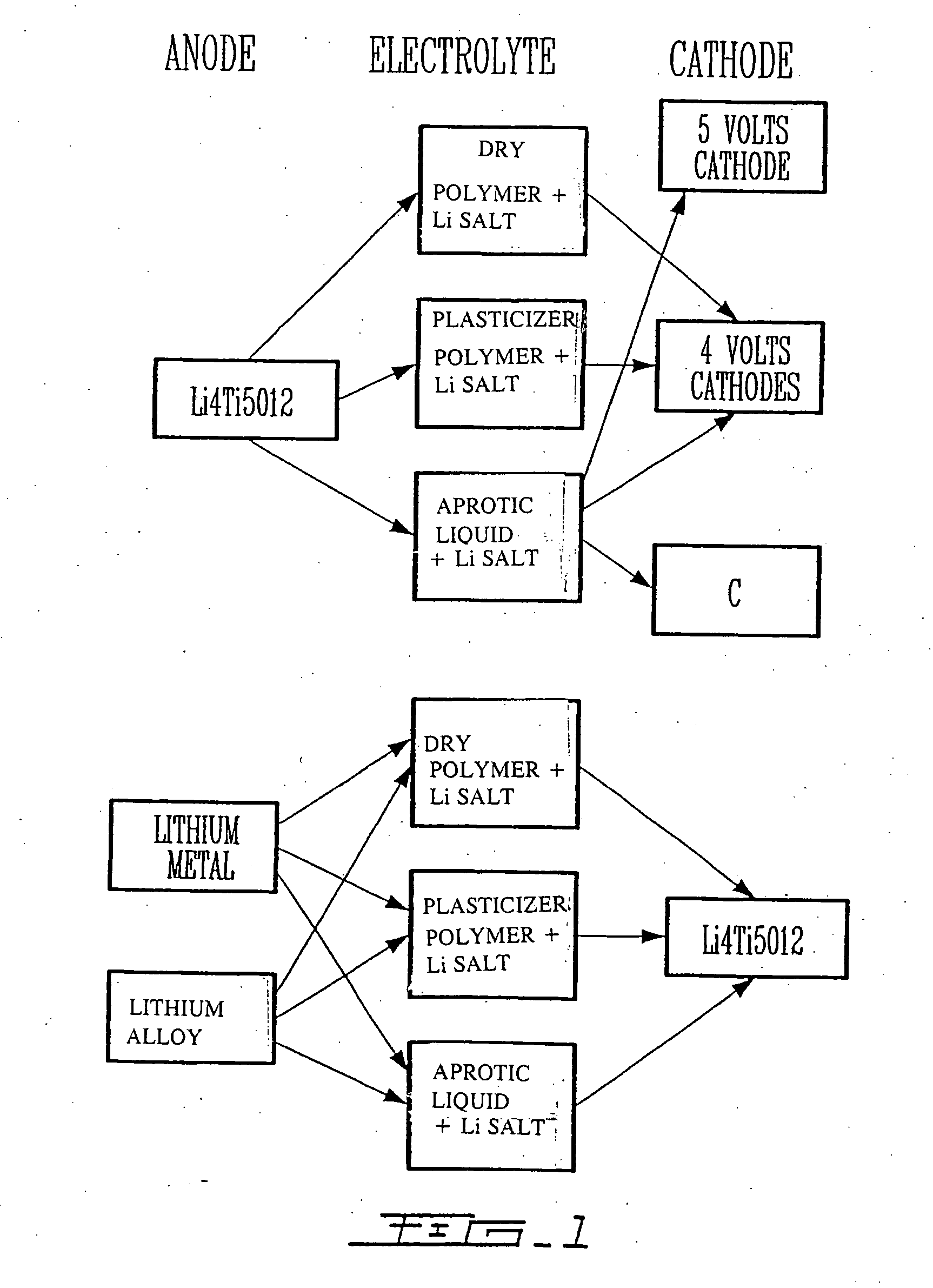
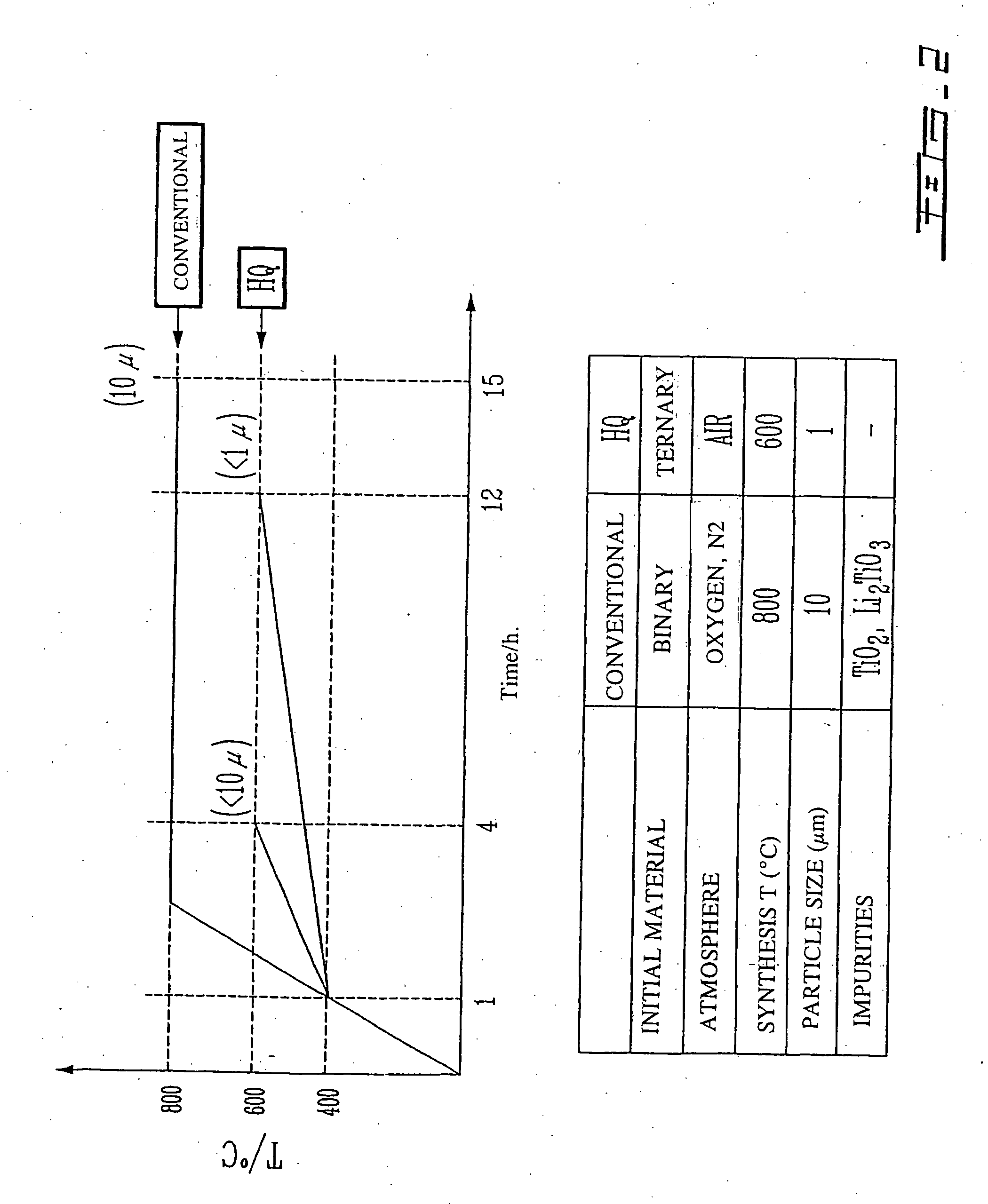
Li4Ti5O12, Li(4-alpha)Zalpha Ti5O12 or Li4ZbetaTi(5-beta)O12 particles, processes for obtaining same and use as electrochemical generators
Synthesis process for new particles of Li4Ti5O12, Li(4-alpha)ZalphaTi5O12 or Li4ZbetaTi(5-beta)O12, preferably having a spinel structure, wherein beta is greater than 0 and less than or equal to 0.5 (preferably having a spinel structure), alpha representing a number greater than zero and less than or equal to 0.33, Z representing a source of at least one metal, preferably chosen from the group made up of Mg, Nb, Al, Zr, Ni, Co. These particles coated with a layer of carbon notably exhibit electrochemical properties that are particularly interesting as components of anodes and / or cathodes in electrochemical generators.
Owner:HYDRO QUEBEC CORP
Anode active material, method of manufacturing the same, and lithium battery using the same
ActiveUS20070077490A1Large capacityGood capacity retentionNon-aqueous electrolyte accumulatorsElectrode thermal treatmentCarbon layerMetal alloy
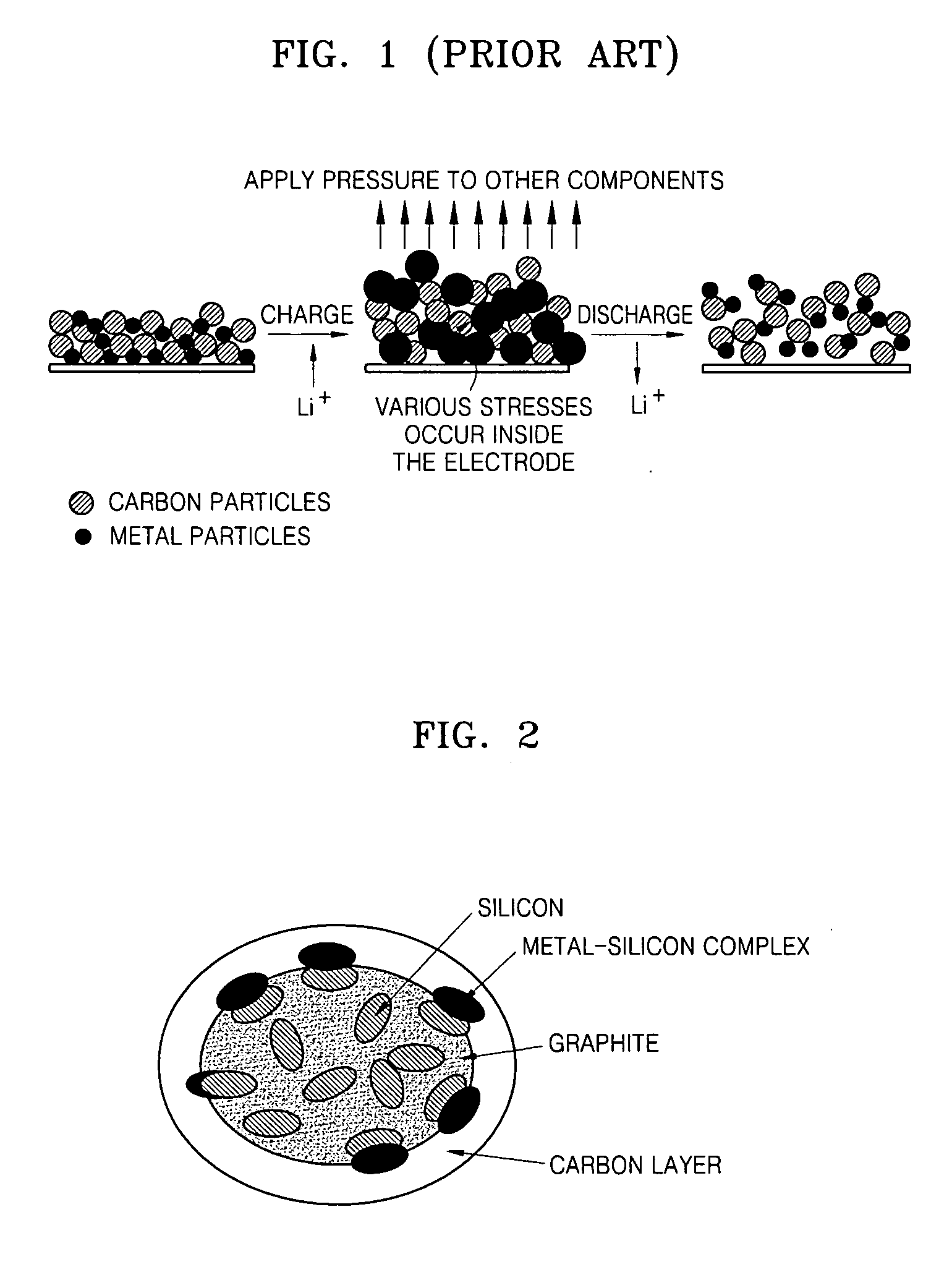
Anode active materials, methods of producing the same and lithium batteries using the same are provided. More particularly, an anode active material having high capacity and excellent capacity retention, a method of producing the same and a lithium battery having a long lifespan using the same are provided. The anode active material comprises complex material particles comprising silicon and graphite, a carbon layer covering the surface of the complex material particles, and a silicon-metal alloy formed between the complex material particles and the amorphous carbon layer.
Owner:SAMSUNG SDI CO LTD
Lithium secondary battery and method for producing same
ActiveUS20060003226A1High charge and discharge capacityExcellent cycle characteristicsElectrode thermal treatmentFinal product manufactureSilicon alloyMetal foil
A rechargeable lithium battery including a negative electrode made by sintering, on a surface of a conductive metal foil as a current collector, a layer of a mixture of active material particles containing silicon and / or a silicon alloy and a binder, a positive electrode and a nonaqueous electrolyte, characterized in that the nonaqueous electrolyte contains carbon dioxide dissolved therein.
Owner:SANYO ELECTRIC CO LTD
Method for manufacturing positive electrode active material for energy storage device and energy storage device
ActiveUS20120088156A1Reduce loadLow costNon-metal conductorsCarbon compoundsCarbon coatingConductive materials
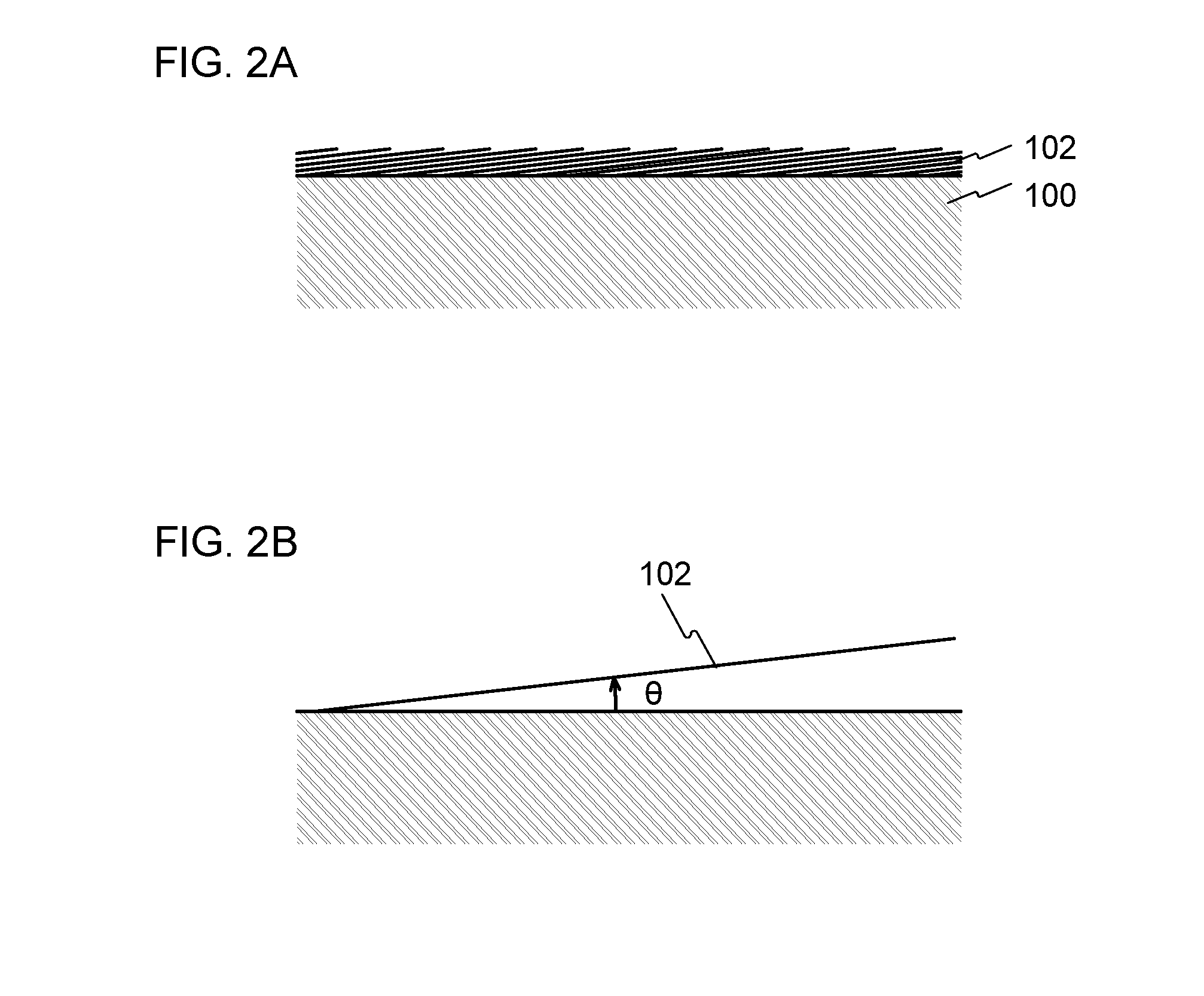
An energy storage device having high capacity per weight or volume and a positive electrode active material for the energy storage device are manufactured. A surface of a main material included in the positive electrode active material for the energy storage device is coated with two-dimensional carbon. The main material included in the positive electrode active material is coated with a highly conductive material which has a structure expanding two-dimensionally and whose thickness is ignorable, whereby the amount of carbon coating can be reduced and an energy storage device having capacity close to theoretical capacity can be obtained even when a conduction auxiliary agent is not used or the amount of the conduction auxiliary agent is extremely small. Accordingly, the amount of carbon coating in a positive electrode and the volume of the conduction auxiliary agent can be reduced; consequently, the volume of the positive electrode can be reduced.
Owner:SEMICON ENERGY LAB CO LTD
Gradient cathode material for lithium rechargeable batteries
InactiveUS6921609B2Increase capacityImproved cyclabilityAluminium compoundsAlkaline accumulatorsManganesePotassium
A composition suitable for use as a cathode material of a lithium battery includes a core material having an empirical formula LixM′zNi1−yM″yO2. “x” is equal to or greater than about 0.1 and equal to or less than about 1.3. “y” is greater than about 0.0 and equal to or less than about 0.5. “z” is greater than about 0.0 and equal to or less than about 0.2. M′ is at least one member of the group consisting of sodium, potassium, nickel, calcium, magnesium and strontium. M″ is at least one member of the group consisting of cobalt, iron, manganese, chromium, vanadium, titanium, magnesium, silicon, boron, aluminum and gallium. A coating on the core has a greater ratio of cobalt to nickel than the core. The coating and, optionally, the core can be a material having an empirical formula Lix1Ax2Ni1−y1−z1Coy1Bz1Oa. “x1” is greater than about 0.1 a equal to or less than about 1.3. “x2,”“y1” and “z1” each is greater than about 0.0 and equal to or less than about 0.2. “a” is greater than 1.5 and less than about 2.1. “A” is at least one element selected from the group consisting of barium, magnesium, calcium and strontium. “B” is at least one element selected from the group consisting of boron, aluminum, gallium, manganese, titanium, vanadium and zirconium.
Owner:TIAX LLC
Popular searches
Electric discharge heating Conductive material Non-conductive material with dispersed conductive material Non-aqueous electrolyte accumulator electrodes Carbon-silicon compound conductors Vacuum evaporation coating Solid electrolyte cells Light protection screens Sputtering coating Vehicular energy storage
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com