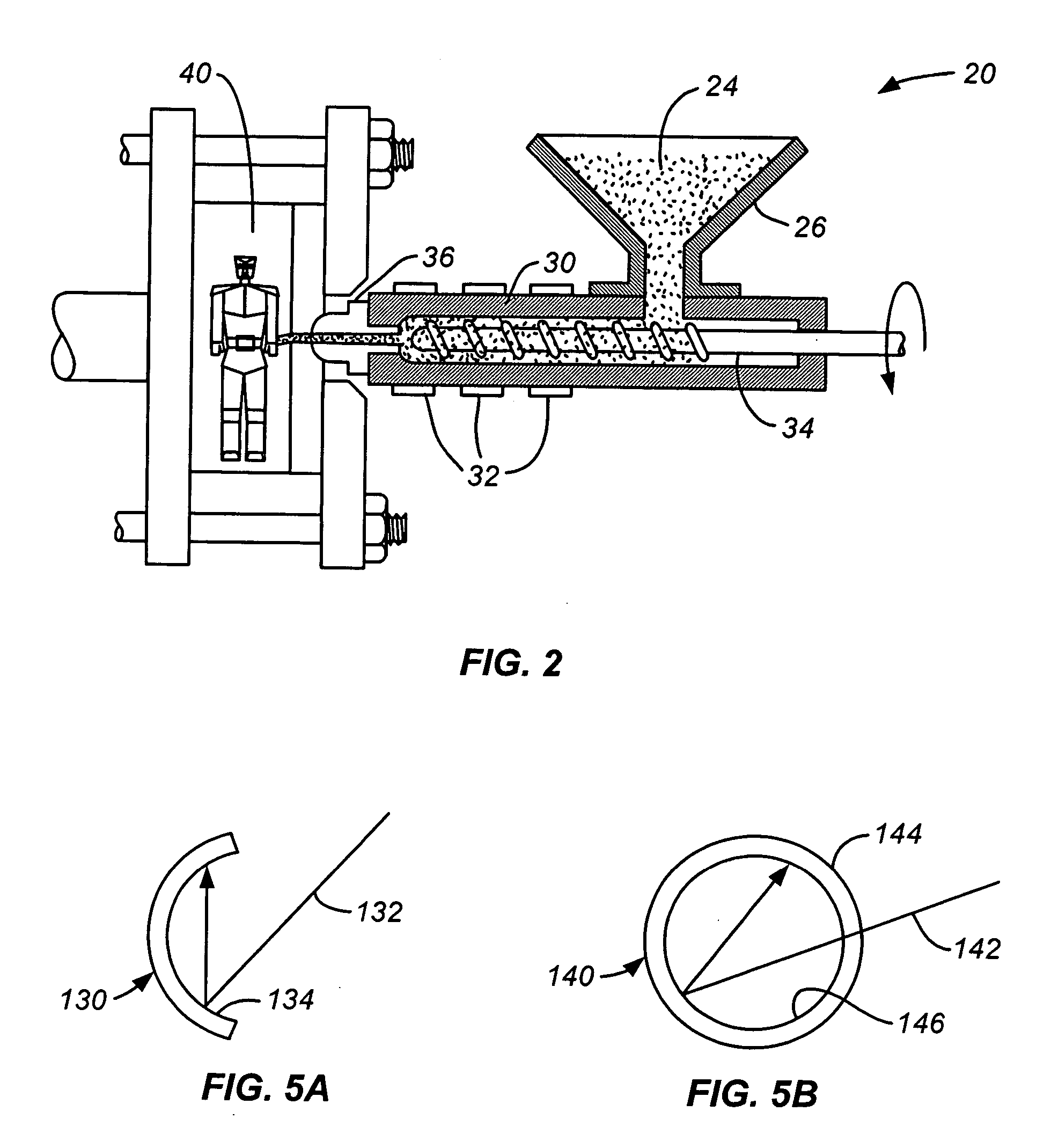
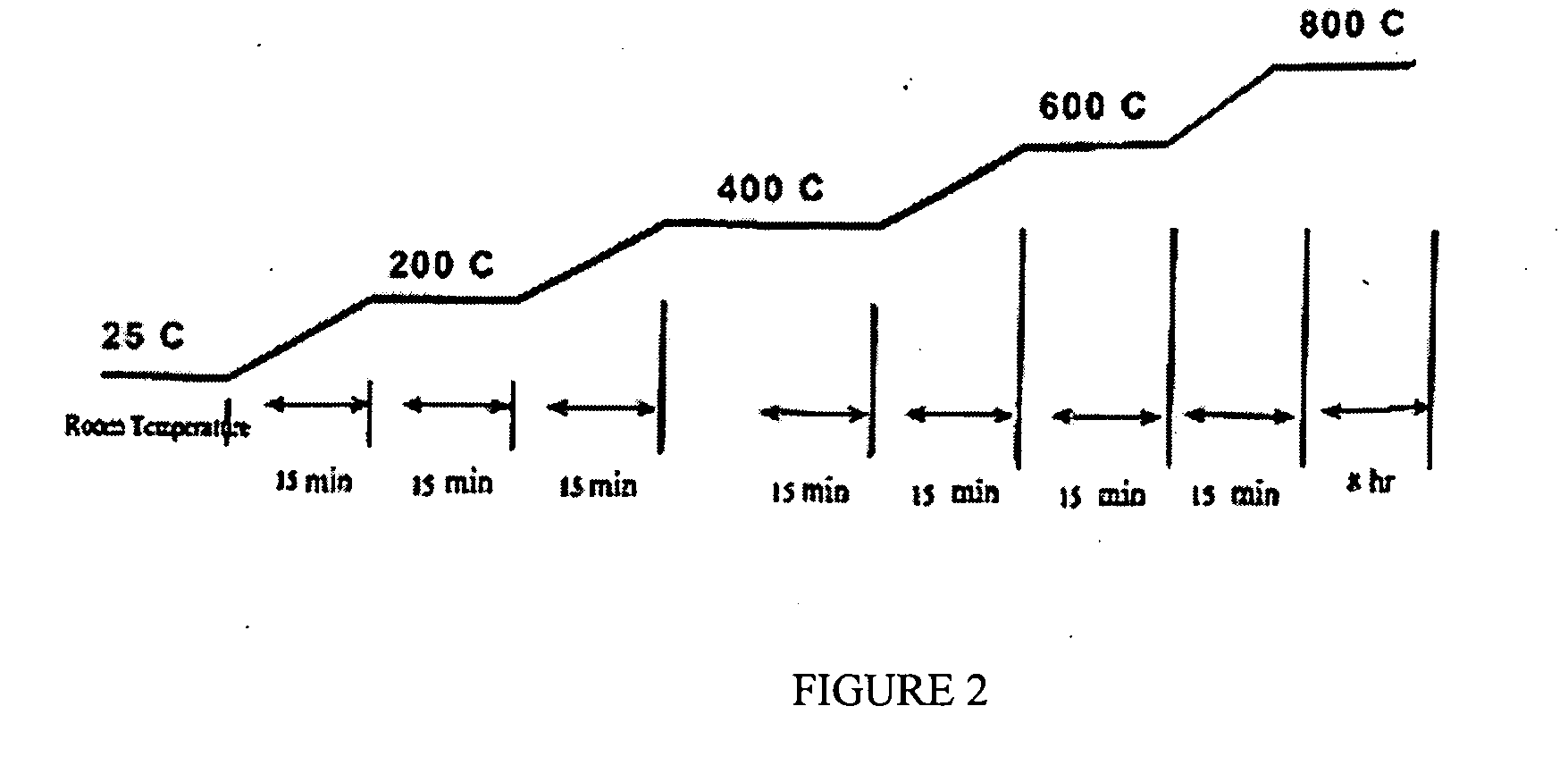
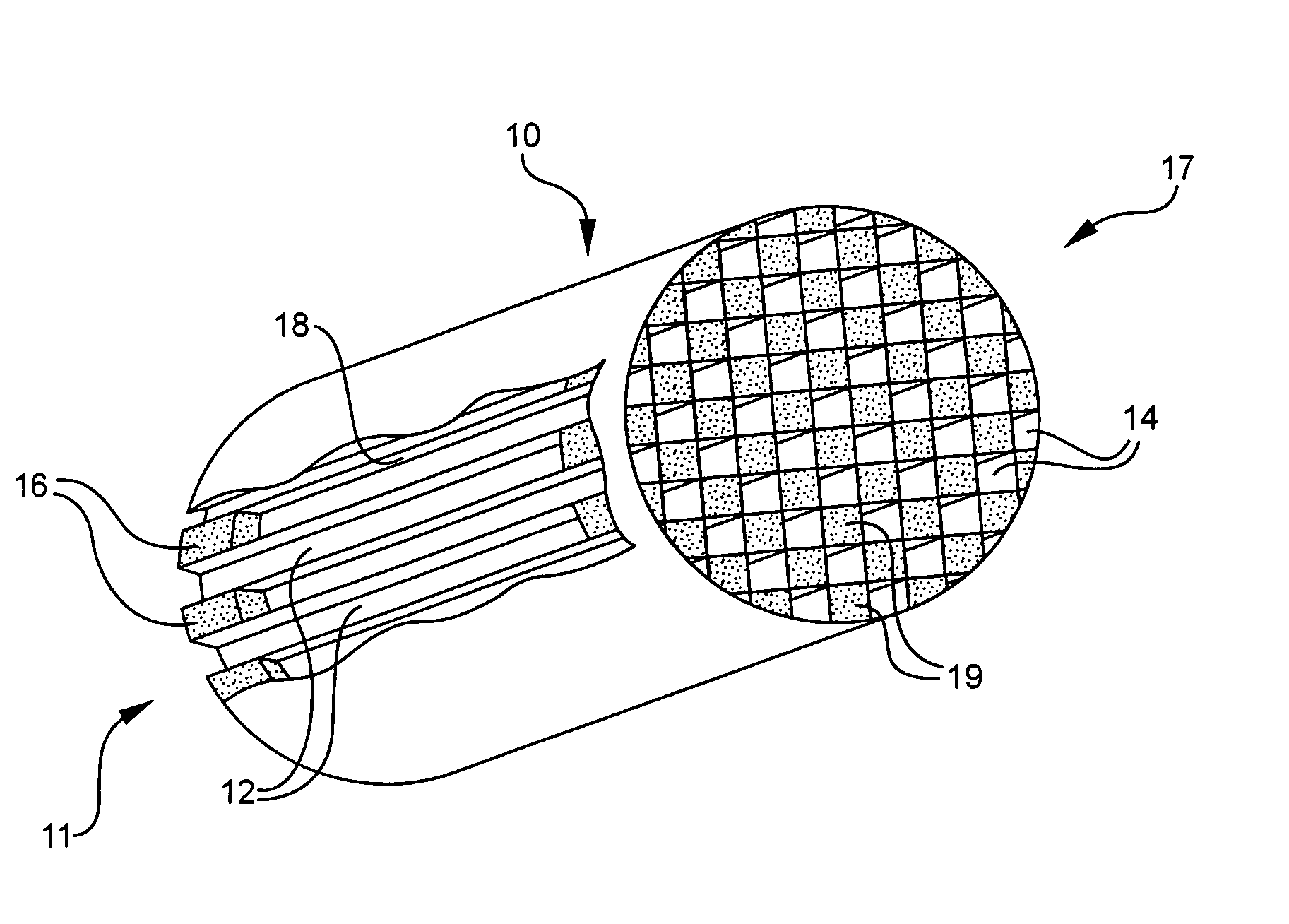
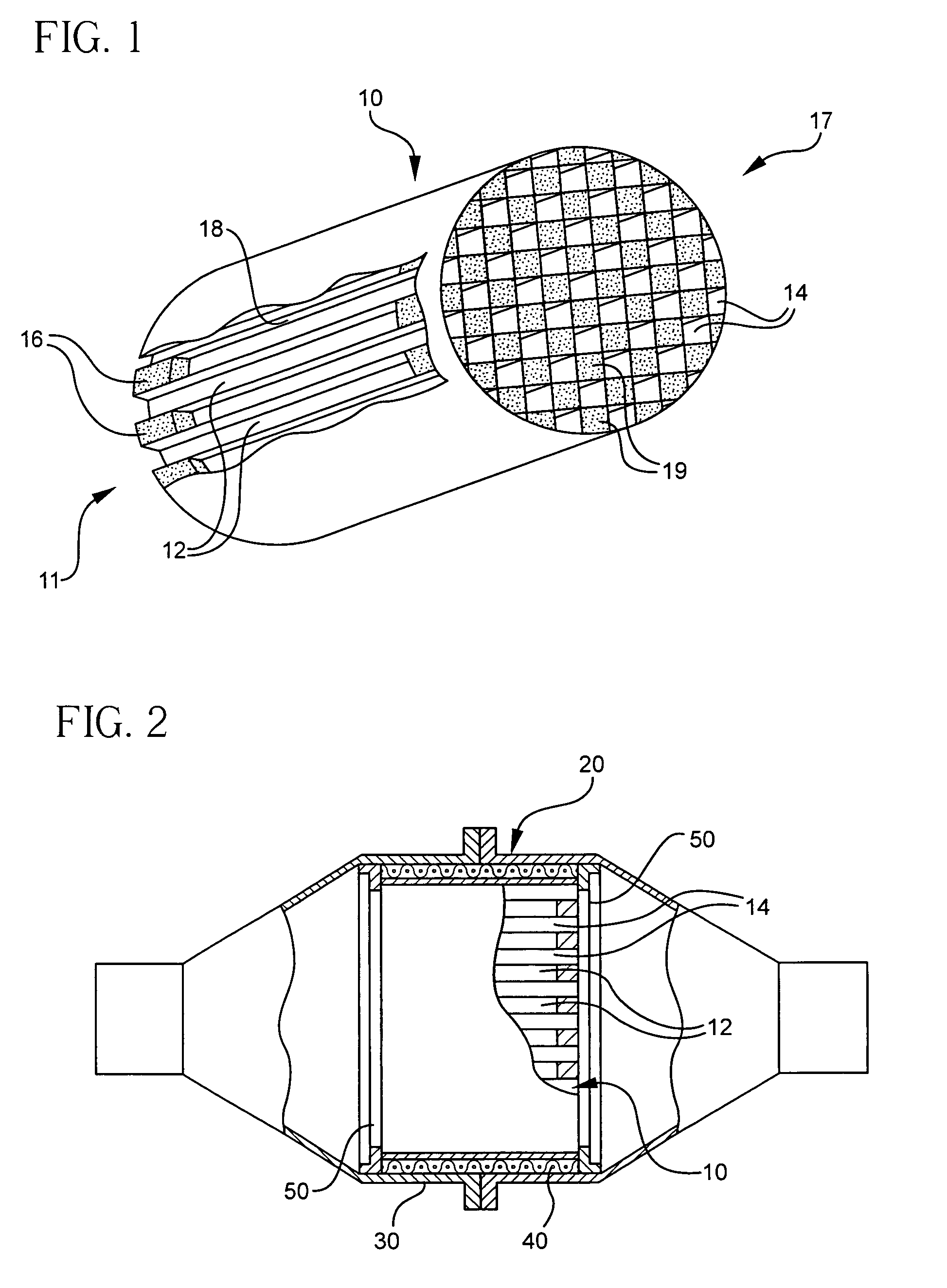
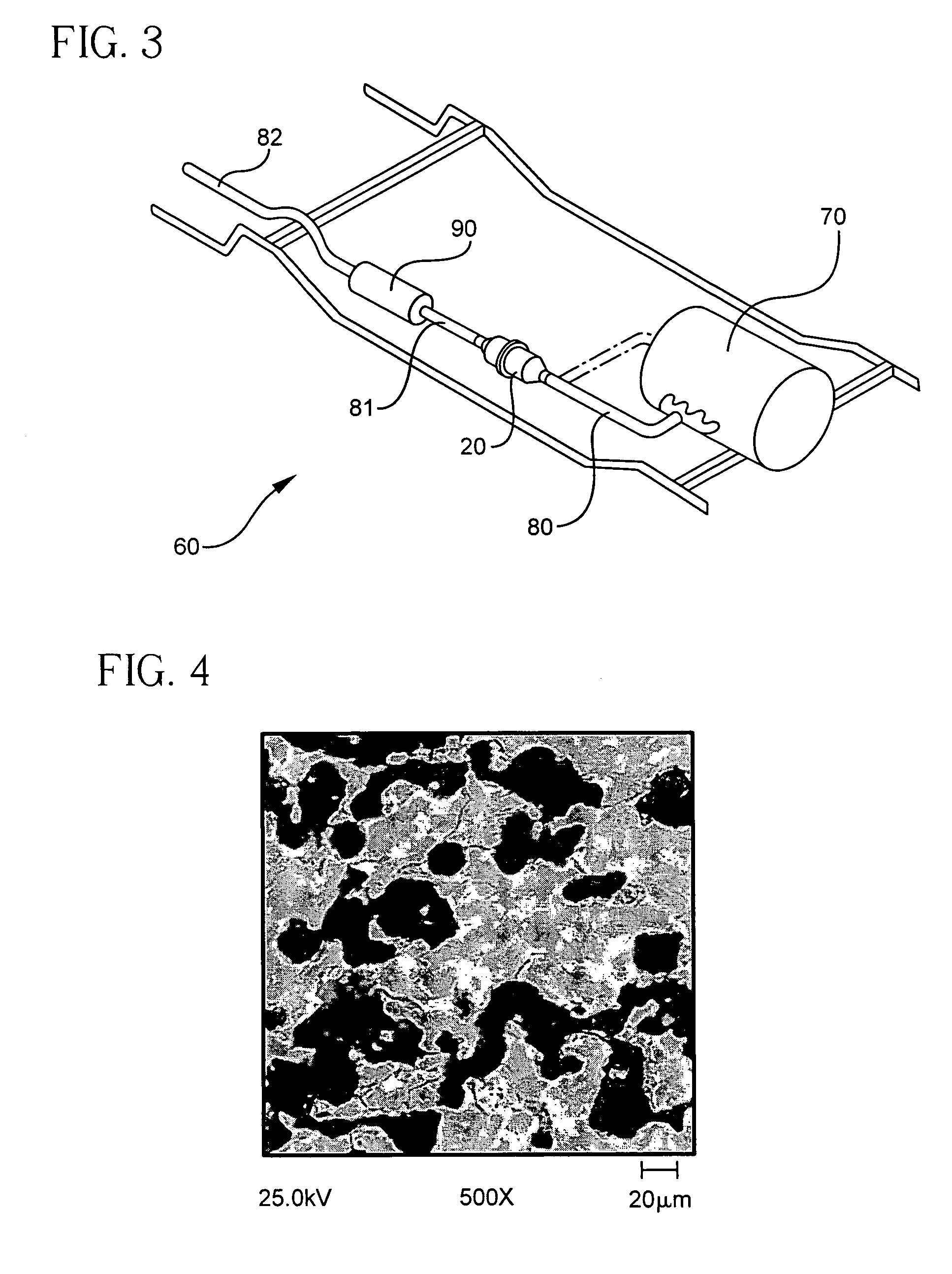
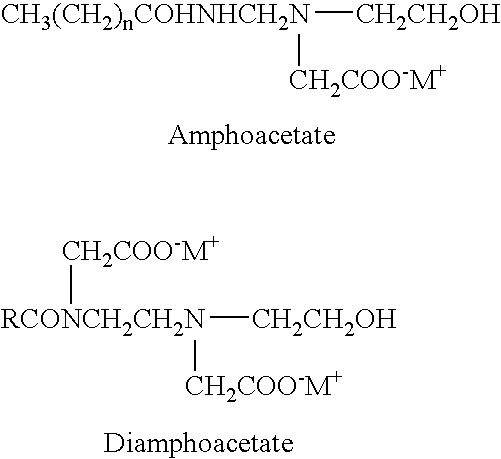Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
6346 results about "Barium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element. Its hydroxide, known in pre-modern times as baryta, does not occur as a mineral, but can be prepared by heating barium carbonate.
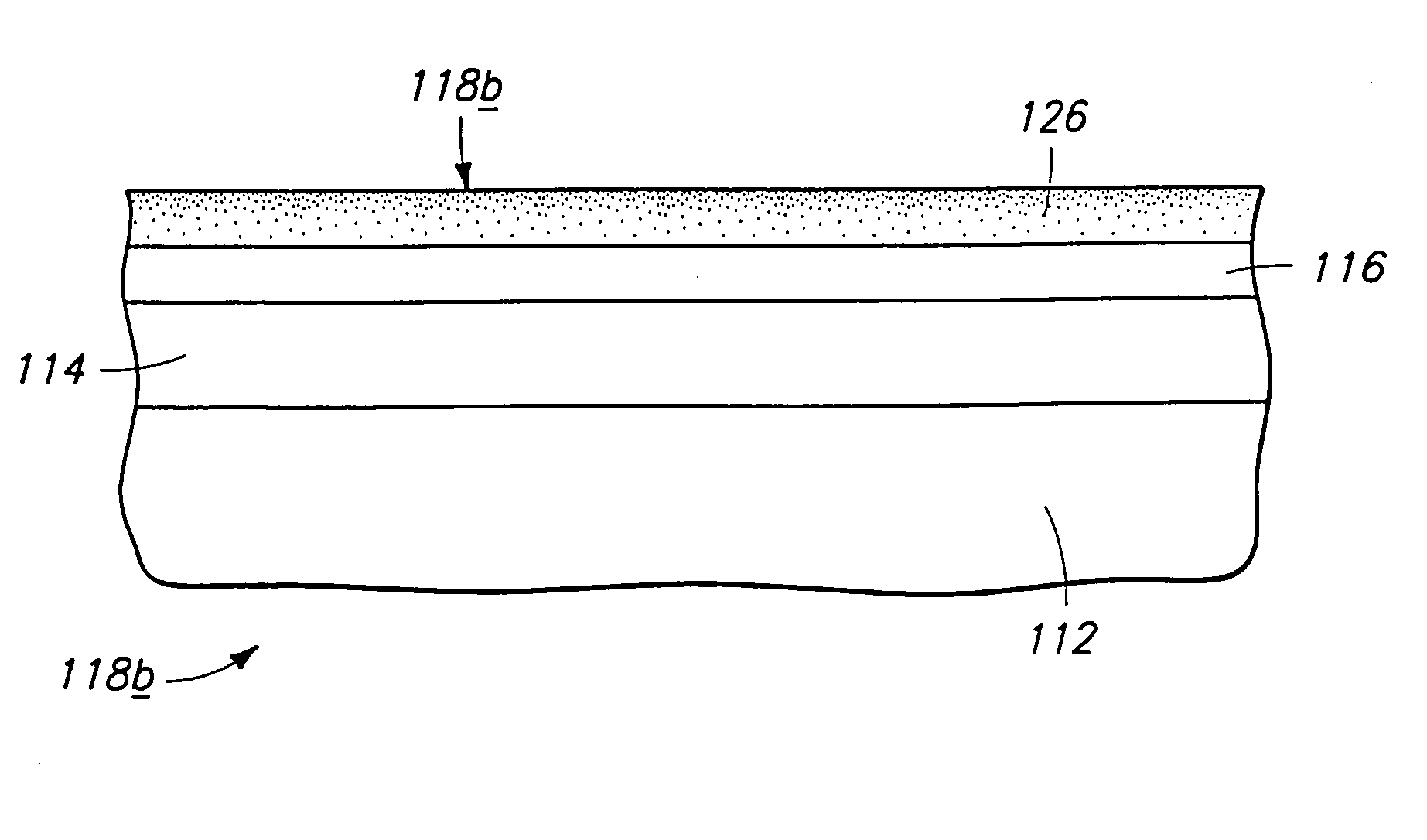
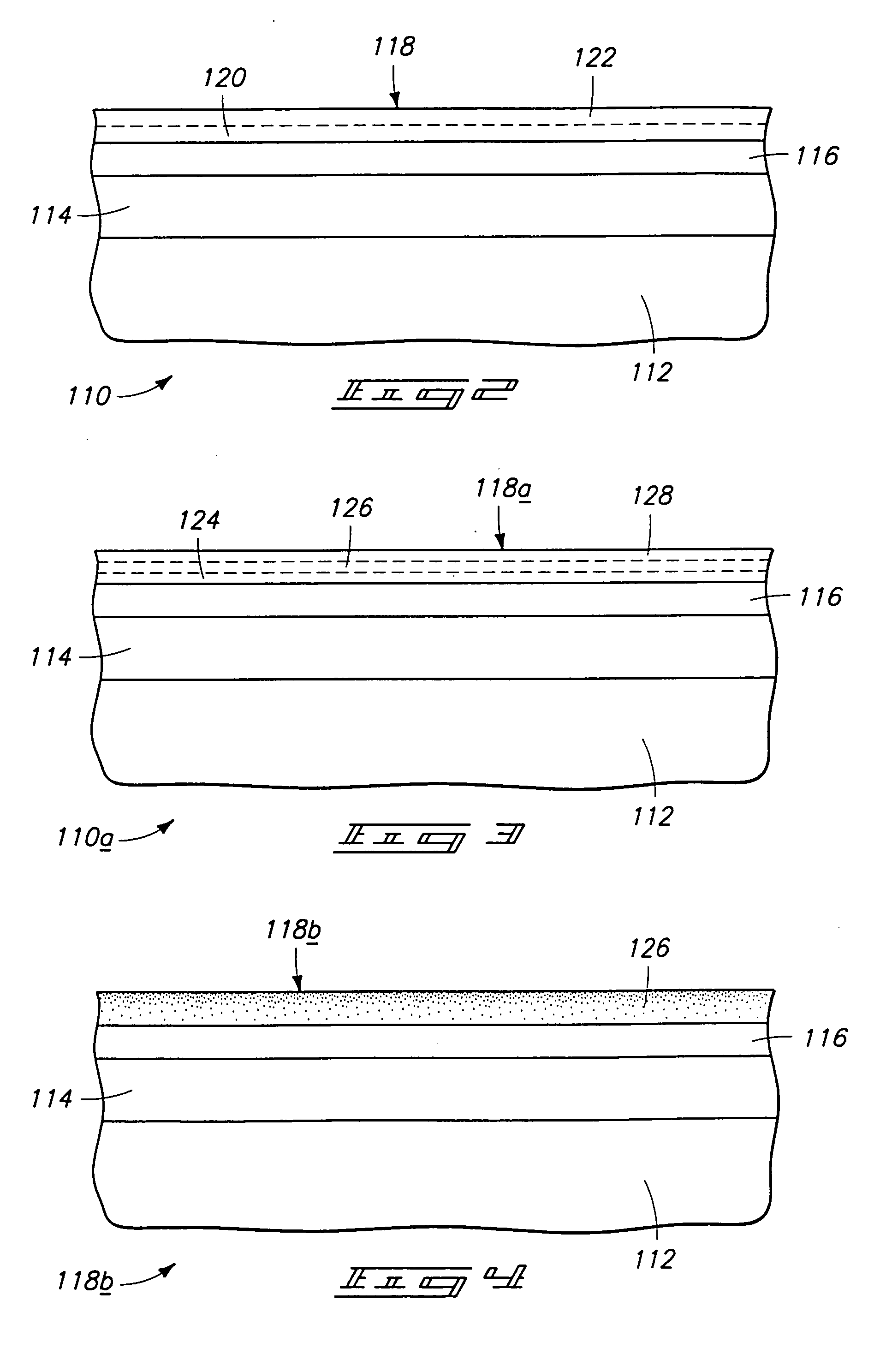
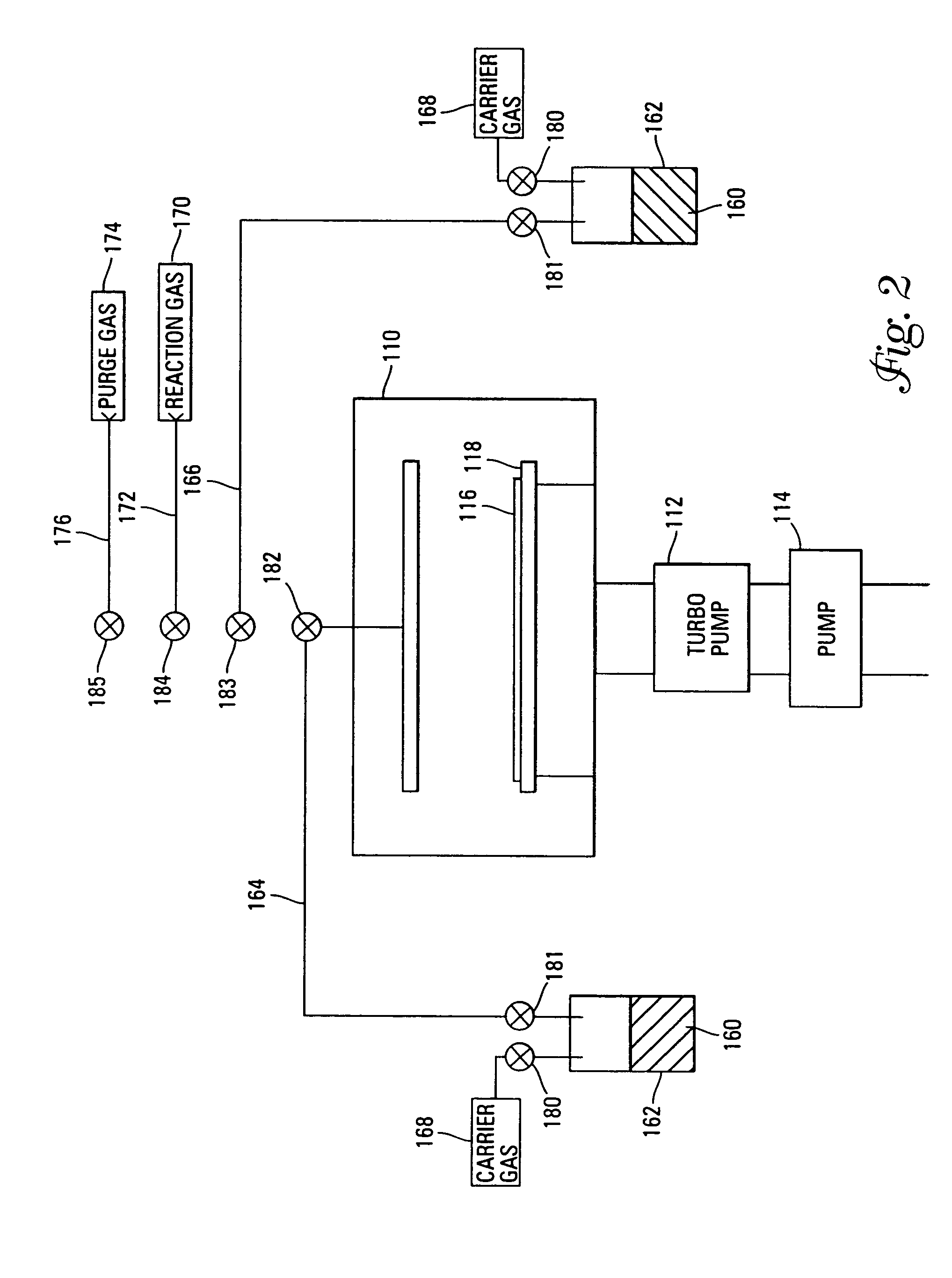
Chemical vapor deposition methods of forming barium strontium titanate comprising dielectric layers, including such layers having a varied concentration of barium and strontium within the layer
The invention includes a chemical vapor deposition method of forming a barium strontium titanate comprising dielectric layer having a varied concentration of barium and strontium, and / or titanium, within the layer. A substrate is positioned within a chemical vapor deposition reactor. Barium and strontium are provided within the reactor by flowing at least one metal organic precursor to the reactor. Titanium is provided within the reactor. One or more oxidizers are flowed to the reactor. In one aspect, conditions are provided within the reactor to be effective to deposit a barium strontium titanate comprising dielectric layer on the substrate from the reactants.
Owner:MICRON TECH INC
Temperature controlled chamber liner
InactiveUS6099651APrevent unwanted condensationPrevent decomposition and condensationSemiconductor/solid-state device manufacturingChemical vapor deposition coatingEngineeringTitanium oxide
The invention relates to an apparatus and process for the vaporization of liquid precursors and deposition of a film on a suitable substrate. Particularly contemplated is an apparatus and process for the deposition of a metal-oxide film, such as a barium, strontium, titanium oxide (BST) film, on a silicon wafer to make integrated circuit capacitors useful in high capacity dynamic memory modules.
Owner:APPLIED MATERIALS INC
Tunable dielectric compositions including low loss glass
InactiveUS6905989B2Lower sintering temperatureIncrease varietyFixed capacitor dielectricCeramic layered productsBreakdown strengthStrontium titanate
Tunable dielectric materials including an electronically tunable dielectric ceramic and a low loss glass additive are disclosed. The tunable dielectric may comprise a ferroelectric perskovite material such as barium strontium titanate. The glass additive may comprise boron, barium, calcium, lithium, manganese, silicon, zinc and / or aluminum-containing glasses having dielectric losses of less than 0.003 at 2 GHz. The materials may further include other additives such as non-tunable metal oxides and silicates. The low loss glass additive enables the materials to be sintered at relatively low temperatures while providing improved properties such as low microwave losses and high breakdown strengths.
Owner:NXP USA INC
Single phosphor for creating white light with high luminosity and high CRI in a UV LED device
InactiveUS6853131B2Avoids and reduces problemGas-filled discharge tubesDischarge tube luminescnet screensX-rayUltraviolet
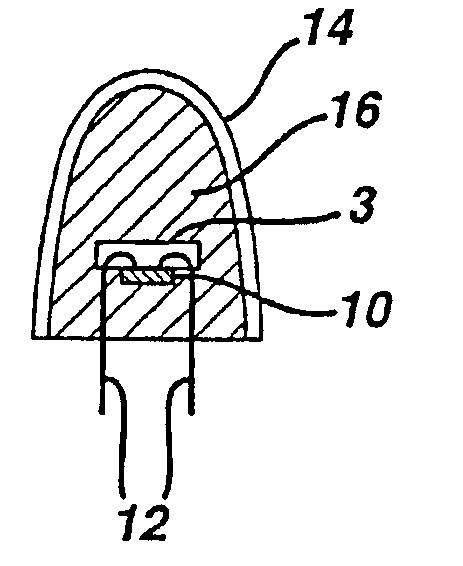
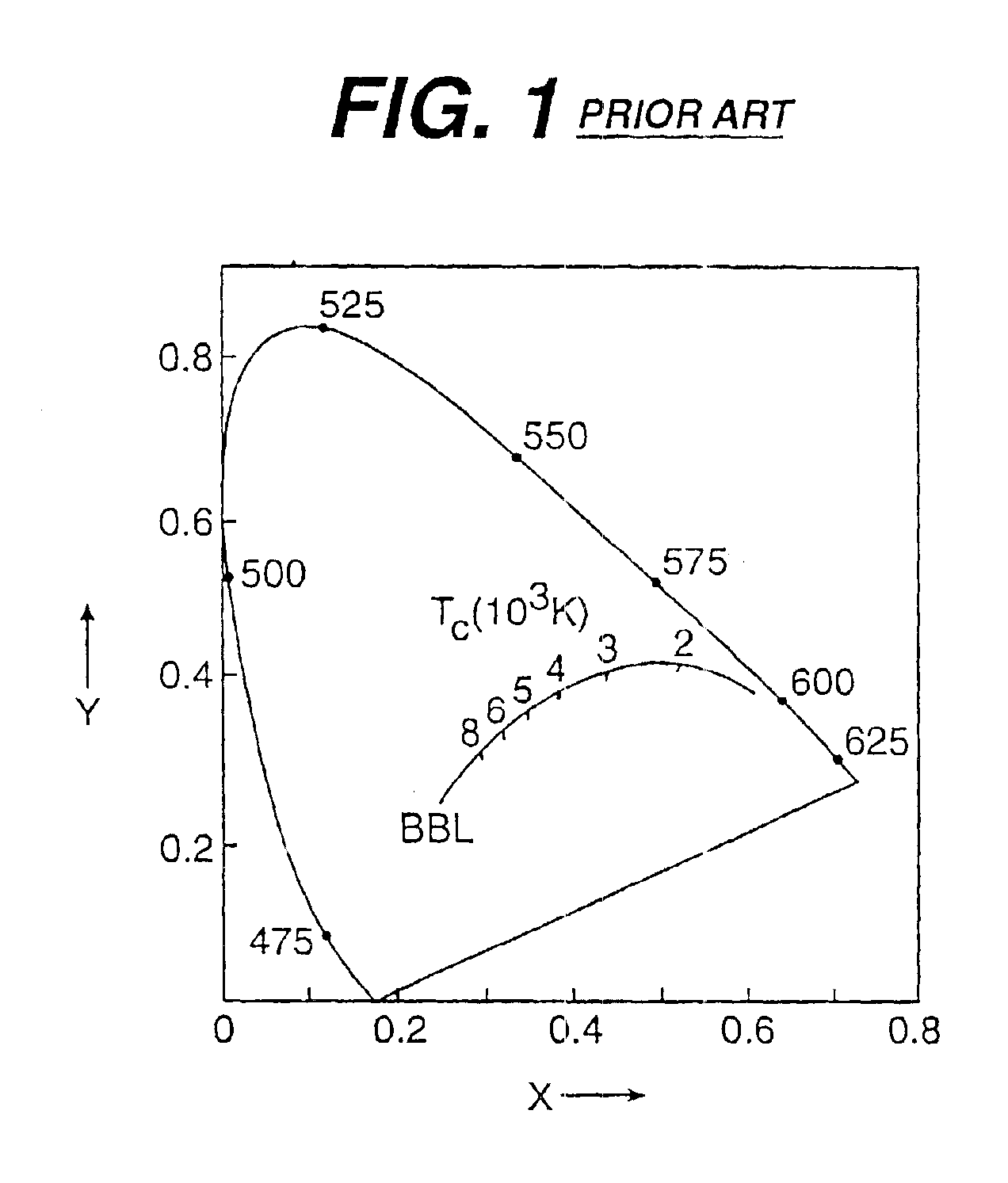
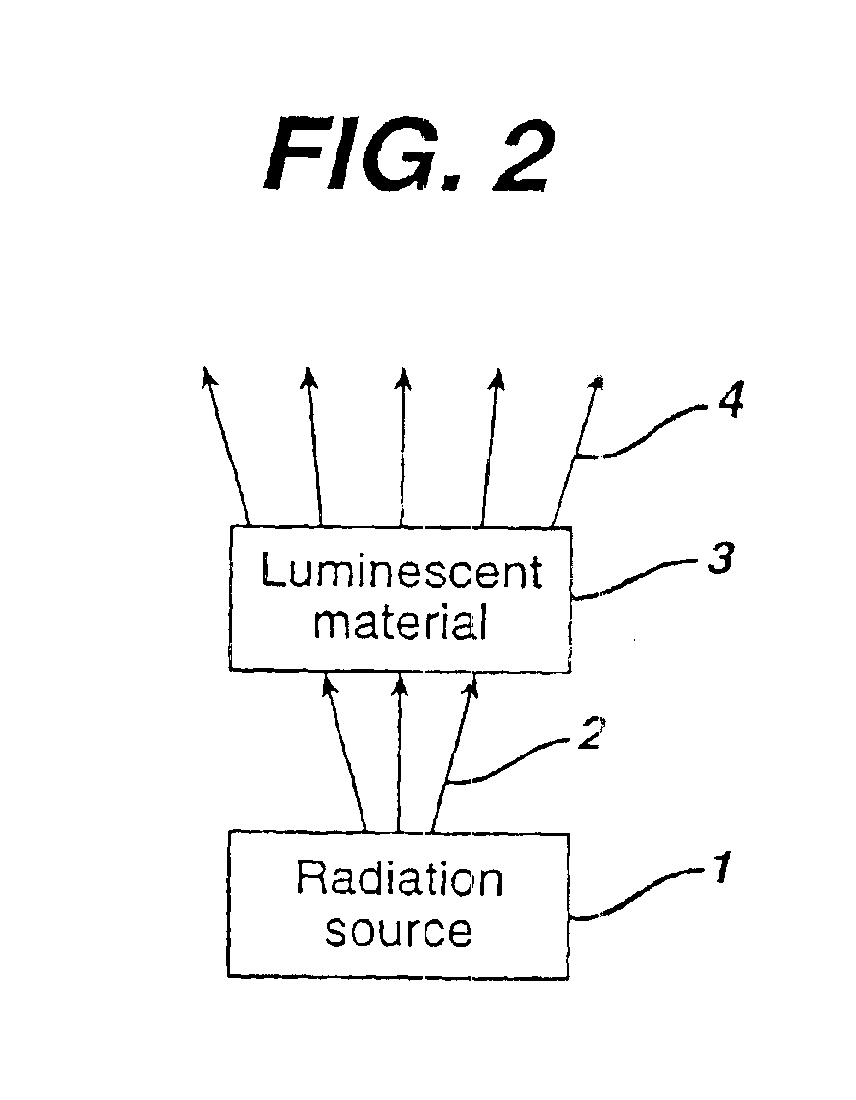
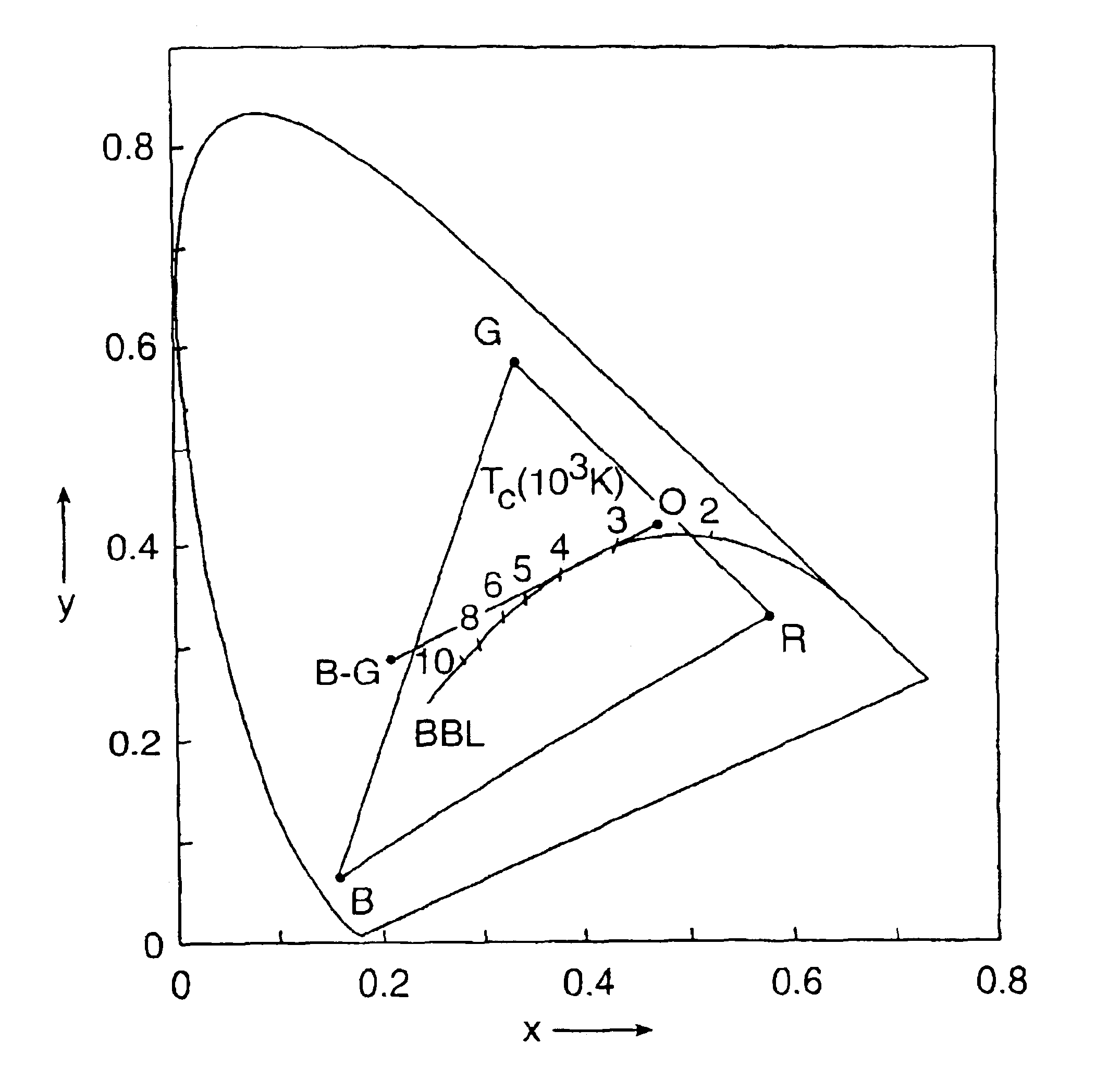
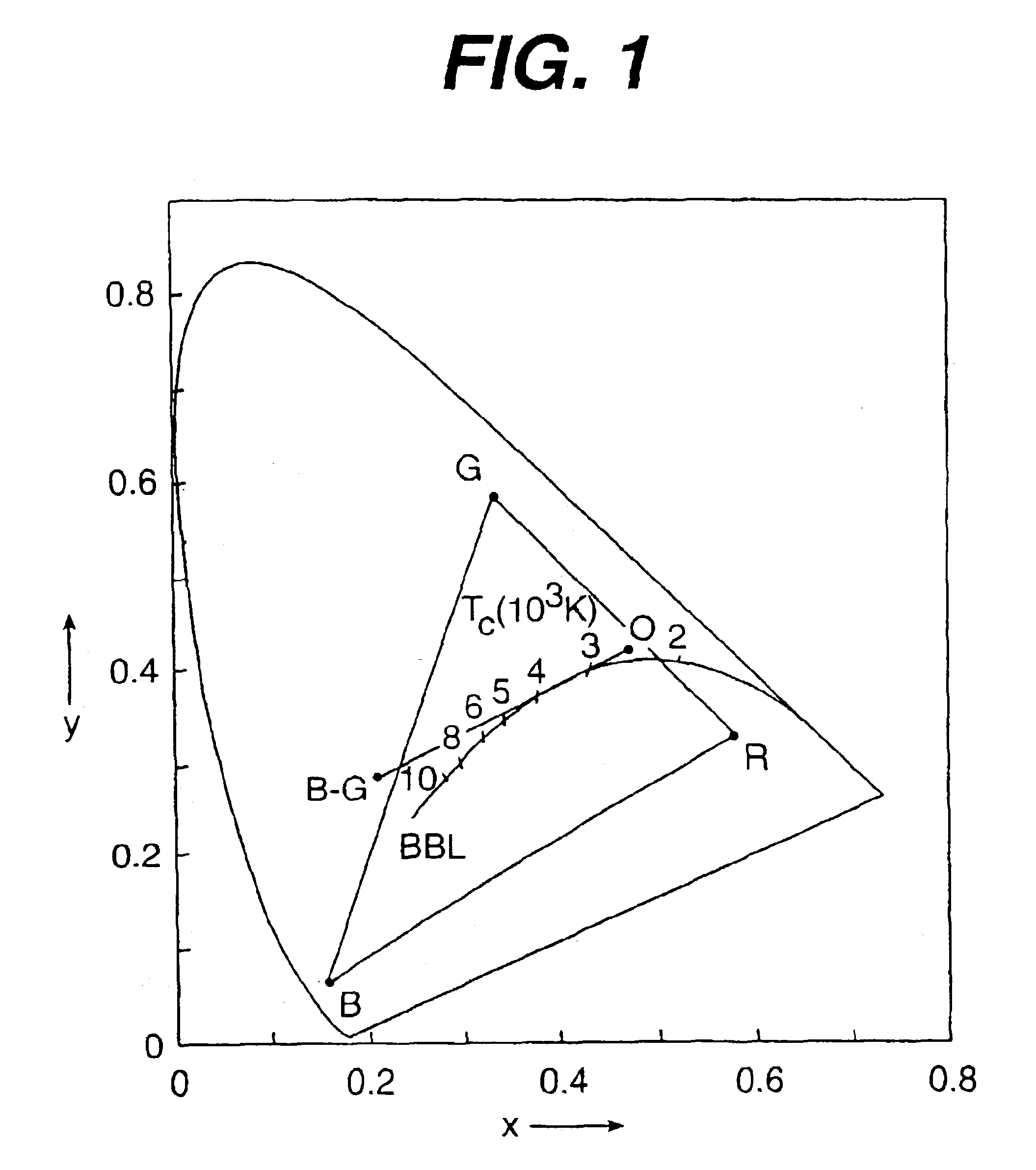
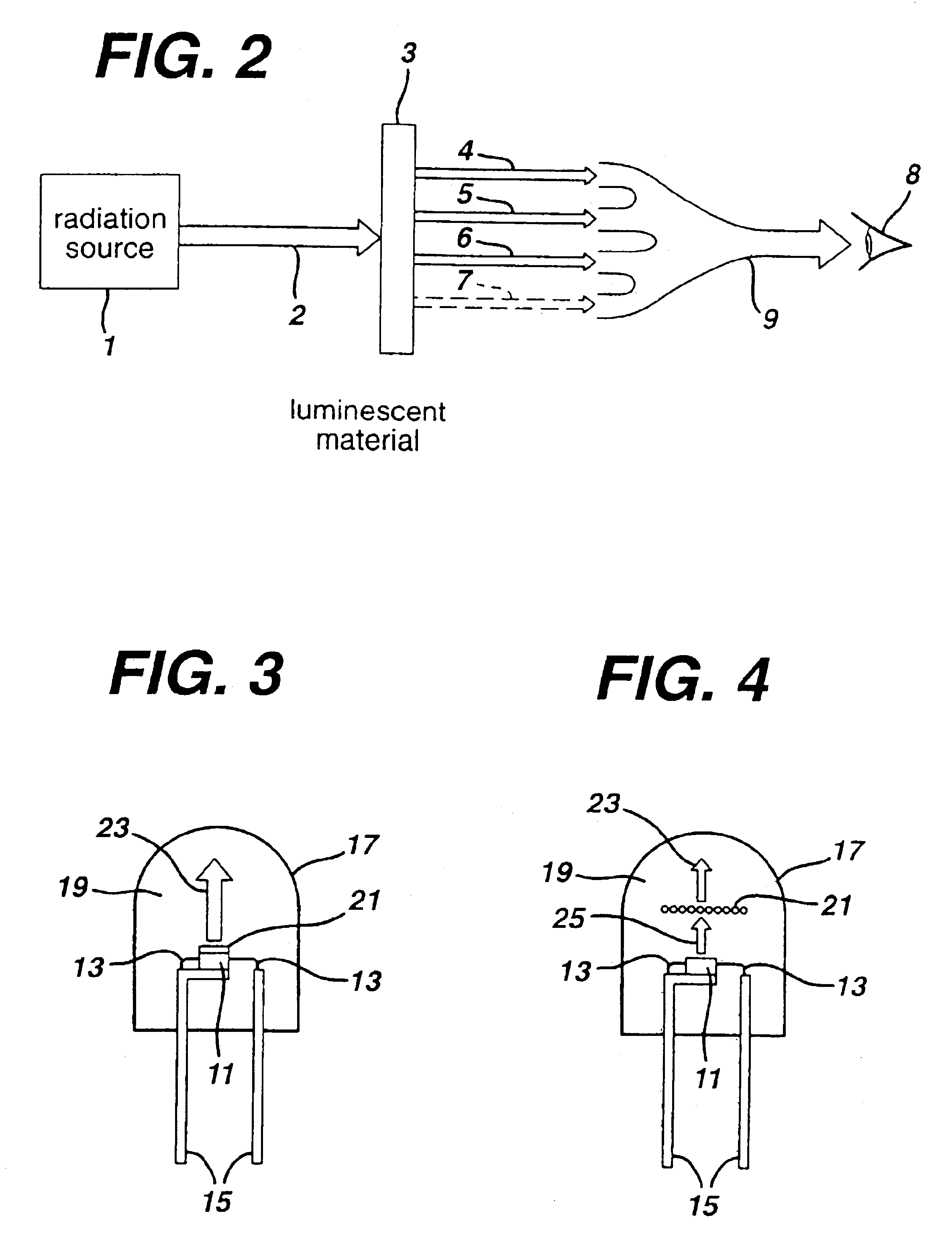
There is provided a white light illumination system. The illumination system includes a radiation source which emits either ultra-violet (UV) or x-ray radiation. The illumination system also includes a luminescent material which absorbs the UV or x-ray radiation and emits the white light. The luminescent material has composition A2−2xNa1+xExD2V3O12. A may be calcium, barium, strontium, or combinations of these three elements. E may be europium, dysprosium, samarium, thulium, or erbium, or combinations thereof. D may be magnesium or zinc, or combinations thereof. The value of x ranges from 0.01 to 0.3, inclusive.
Owner:GENERAL ELECTRIC CO
White light emitting phosphor blends for LED devices
There is provided white light illumination system including a radiation source, a first luminescent material having a peak emission wavelength of about 575 to about 620 nm, a second luminescent material having a peak emission wavelength of about 495 to about 550 nm, which is different from the first luminescent material and a third luminescent material having a peak emission wavelength of about 420 to about 480 nm, which is different from the first and second luminescent materials. The LED may be a UV LED and the luminescent materials may be a blend of three or four phosphors. The first phosphor may be an orange emitting Eu2+, Mn2+ activated strontium pyrophosphate, Sr2P2O7:Eu2+, Mn2+. The second phosphor may be a blue-green emitting Eu2+ activated barium silicate, (Ba,Sr,Ca)2SiO4:Eu2+. The third phosphor may be a blue emitting SECA phosphor, (Sr,Ba,Ca)5(PO4)3Cl:Eu2+. Optionally, the fourth phosphor may be a red emitting Mn4+ activated magnesium fluorogermanate, 3.5MgO*0.5MgF2*GeO2:Mn4+. A human observer perceives the combination of the orange, blue-green, blue and / or red phosphor emissions as white light.
Owner:GE LIGHTING SOLUTIONS LLC
Radiation detectable and protective articles
InactiveUS20050211930A1Attribute be easilyPresence be easilyNuclear engineering problemsNuclear engineering solutionsEmulsionCompound (substance)
Compositions and processes for forming radiopaque polymeric articles are disclosed. In one embodiment, radiation inspection apparatuses and methods are then used to determine the presence and attributes of such radiopaque polymeric articles. A radiopaque polymeric article of the present invention can be created by mixing a radiopaque material, such as barium, bismuth, tungsten or their compounds, with a powdered polymer, pelletized polymer or liquid solution, emulsion or suspension of a polymer in solvent or water. In addition to creating radiation detectable objects, the radiopaque polymeric materials of the present invention can be used to create radiation protective articles, such as radiation protective garments and bomb containment vessels. Enhanced radiation protection can also be achieved through the use of nano-materials. The principals of the present invention can be used to provide protection against other types of hazards, including fire, chemical, biological and projectile hazards.
Owner:MERIDIAN RES & DEV
Polymer nanocomposite implants with enhanced transparency and mechanical properties for administration within humans or animals
Polymer nanocomposite implants with nanofillers and additives are described. The nanofillers described can be any composition with the preferred composition being those composing barium, bismuth, cerium, dysprosium, europium, gadolinium, hafnium, indium, lanthanum, neodymium, niobium, praseodymium, strontium, tantalum, tin, tungsten, ytterbium, yttrium, zinc, and zirconium. The additives can be of any composition with the preferred form being inorganic nanopowders comprising aluminum, calcium, gallium, iron, lithium, magnesium, silicon, sodium, strontium, titanium. Such nanocomposites are particularly useful as materials for biological use in applications such as drug delivery, biomed devices, bone or dental implants.
Owner:PPG IND OHIO INC
Catalyst for preparing alcohol by acetic ester hydrogenation as well as preparation method and application thereof
InactiveCN101934228AHigh reactivityLow investment costOrganic compound preparationHydroxy compound preparationManganeseCopper oxide
The invention discloses a catalyst for preparing alcohol by acetic ester hydrogenation as well as a preparation method and an application thereof, which is characterized in that the main catalyst of the catalyst is copper or copper oxide or a mixture of the copper and the copper oxide, and the cocatalyst can be also added, wherein the cocatalyst is one or more of oxides of zinc, manganese, chromium, calcium, barium, iron, nickel and magnesium; and the carrier is alumina or silica sol. The catalyst has high activity and high selectivity under the condition of low temperature and low pressure, thus greatly reducing the investment cost of permanent plants, lowering production energy consumption, being extremely beneficial for the industrial production, and having good stability and long service life. The catalyst of the invention is used to cause percent conversion of a reaction of converting the acetic ester into the alcohol is more than or equal to 80% and the selectivity of the alcohol is more than or equal to 95%.
Owner:江苏丹化煤制化学品工程技术有限公司
Catalyst for purifying exhaust gas
InactiveUS6066587AImprove responseImprove purification effectMolecular sieve catalystsInternal combustion piston enginesPlatinumPartial oxidation
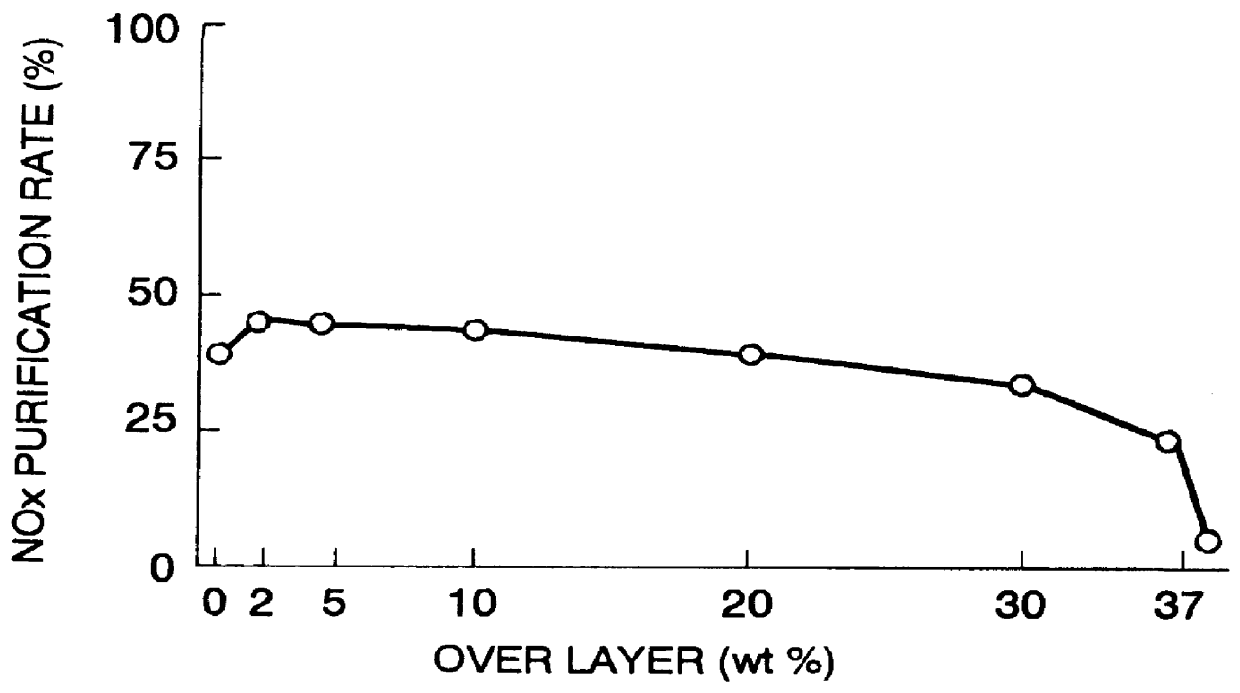
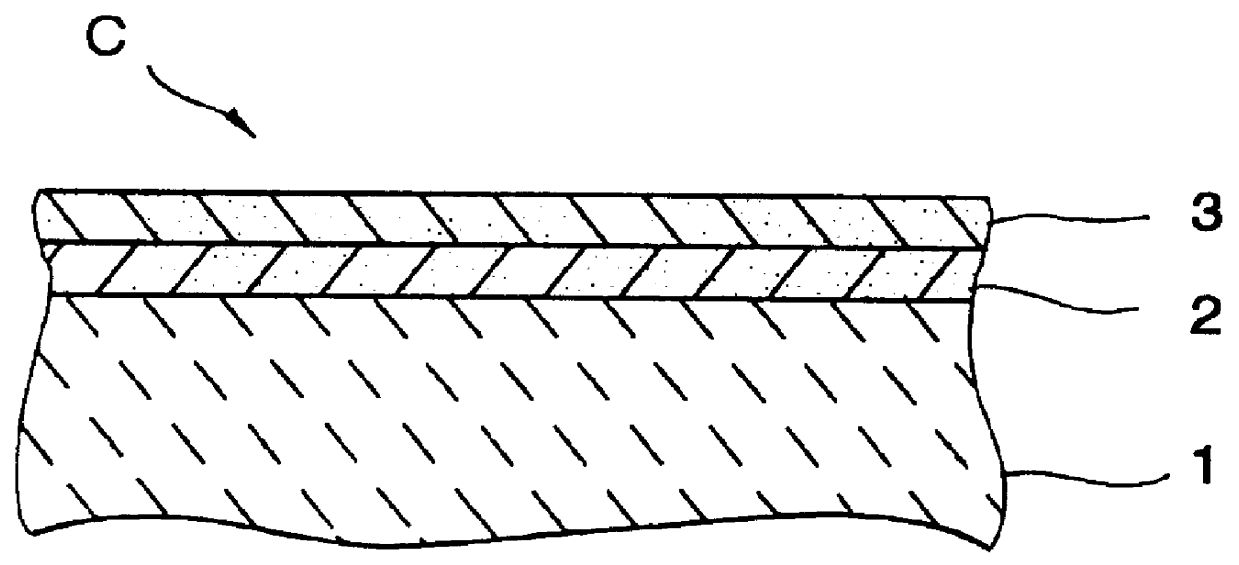
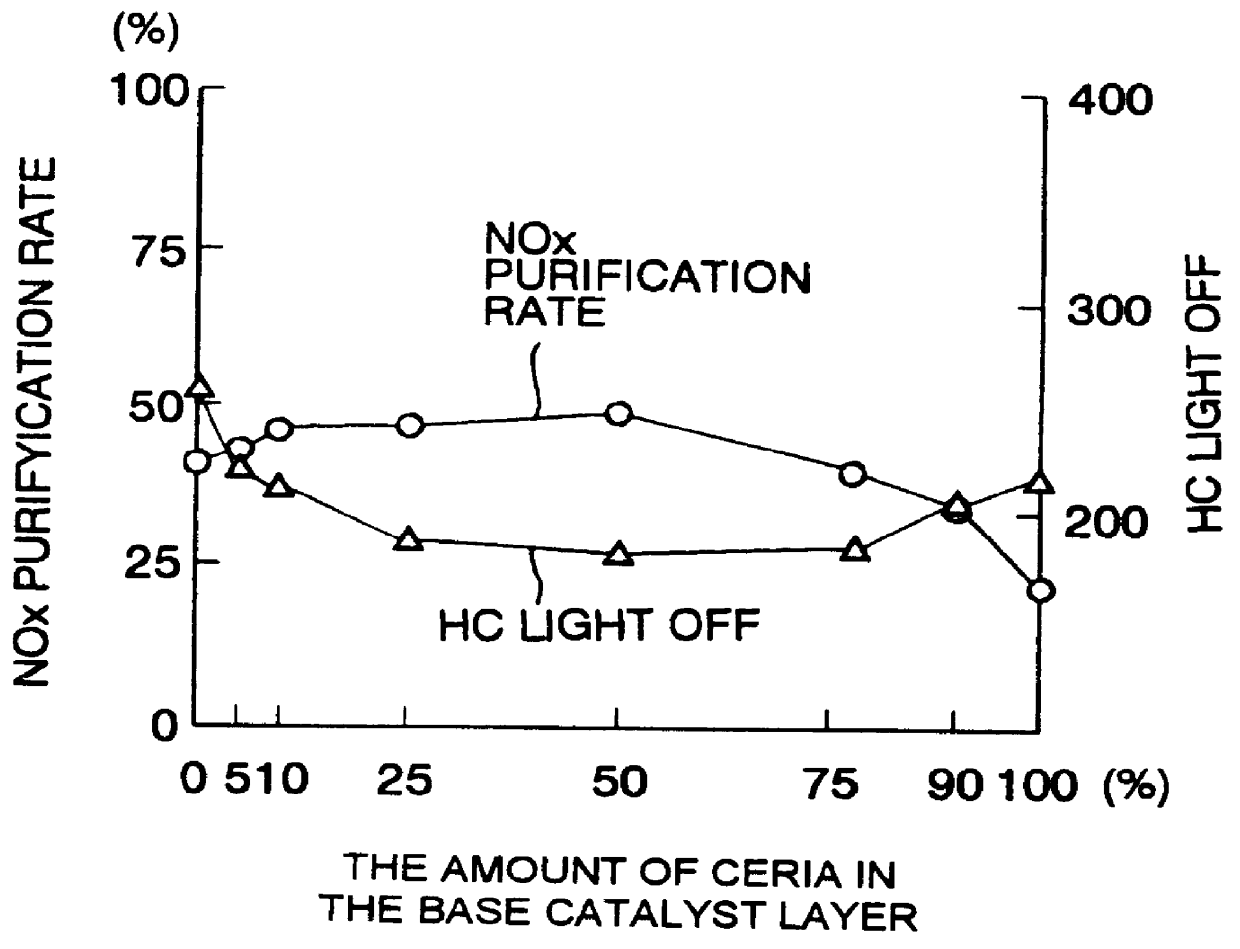
A catalyst has a base catalyst layer containing platinum and barium as precious metal supported by alumina and an over catalyst layer containing platinum and rhodium as precious metal supported by zeolitr. The platinum and rhodium in the over catalyst layer activate NOx and HC so as to make them more reactive in terms of energy, and the barium in the base catalyst layer makes the platinum be more dispersive in the base catalyst layer. Under the existence of dispersive platinum, NOx in exhaust gas is decomposed and purified by reaction with reactive NO2 and partially oxidized HC generated in the over catalyst layer.
Owner:MAZDA MOTOR CORP
Systems and methods for forming strontium- and/or barium-containing layers
InactiveUS7115166B2Easy to controlMinimizing detrimental gas phase reactionPolycrystalline material growthSolid-state devicesStrontium titanateBarium strontium titanate
Owner:MICRON TECH INC
Inorganic dopants, inks and related nanotechnology
InactiveUS6849109B2Facilitated DiffusionLower transition temperatureSelenium/tellurium compundsCell electrodesIndiumCerium
Ink compositions with modified properties result from using a powder size below 100 nanometers. Colored inks are illustrated. Nanoscale coated, uncoated, whisker inorganic fillers are included. The pigment nanopowders taught comprise one or more elements from the group actinium, aluminum, antimony, arsenic, barium, beryllium, bismuth, cadmuim, calcium, cerium, cesium, chalcogenide, cobalt, copper, dysprosium, erbium, europium, gadolinium, gallium, gold, hafnium, hydrogen, indium, iridium, iron, lanthanum, lithium, magnesium, manganese, mendelevium, mercury, molybdenum, neodymium, neptunium, nickel, niobium, nitrogen, oxygen, osmium, palladium, platinum, potassium, praseodymium, promethium, protactinium, rhenium, rubidium, scandium, silver, sodium, strontium, tantalum, terbium, thallium, thorium, tin, titanium, tungsten, vanadium, ytterbium, yttrium, zinc, and zirconium.
Owner:PPG IND OHIO INC
Phase shifters deposited en masse for an electronically scanned antenna
ActiveUS7324043B2Increase processing costPatterning of backsideSimultaneous aerial operationsRadiating elements structural formsDielectricEngineering
A system and method for an electronically scanned antenna is provided in which phase shifters are deposited en masse along with other electronically scanned antenna components on a wafer scale substrate using a thin film process. Alternative wafer scale sizes may be utilized to furnish a required antenna aperture area. Significant processing costs for radar and communication systems are saved utilizing the present invention as compared with contemporary discrete phase shifters that are individually mounted on an antenna. In an aspect, the phase shifter is made up of a base electrode, a barium strontanate titanate (BST) ferroelectric varactor and a top electrode. The BST ferroelectric material is a voltage variable dielectric, which generates a radiation phase. The radiation phase is regulated by a phase shifter control. The radiation phase generates an electromagnetic field about a radiating element and electromagnetic radio waves are radiated from the radiating element.
Owner:APTIV TECH LTD
Integrated oxygen generation and carbon dioxide absorption method apparatus and systems
InactiveUS20050031522A1Improvement in mission durationLower acquisition costsFuel cell auxillariesMachines/enginesChemical speciesCo2 absorption
A method for producing oxygen and absorbing carbon dioxide in a single operation using a solution that contains an oxygen source and a redox partner that can react to form oxygen and a chemical species that can form an insoluble carbonate to precipitate and chemically store carbon dioxide. Carbon dioxide is introduced into the solution and the carbonate precipitates as the oxygen is generated. In particular, the invention uses an aqueous solution of permanganate and hydrogen peroxide that react in the presence of a catalyst to produce oxygen and manganese (II) ions. Carbon dioxide gas introduced into the solution reacts with the manganese (II) ions to precipitate manganese carbonate. Other cations capable of reacting with carbon dioxide to form an insoluble carbonate, for example calcium, barium and magnesium, may also be added to the solution to precipitate carbonate salts. Calcium permanganate may used as a source of both calcium and permanganate.
Owner:CAPITAL MANAGEMENT +1
Aluminum porous body and fabrication method of same
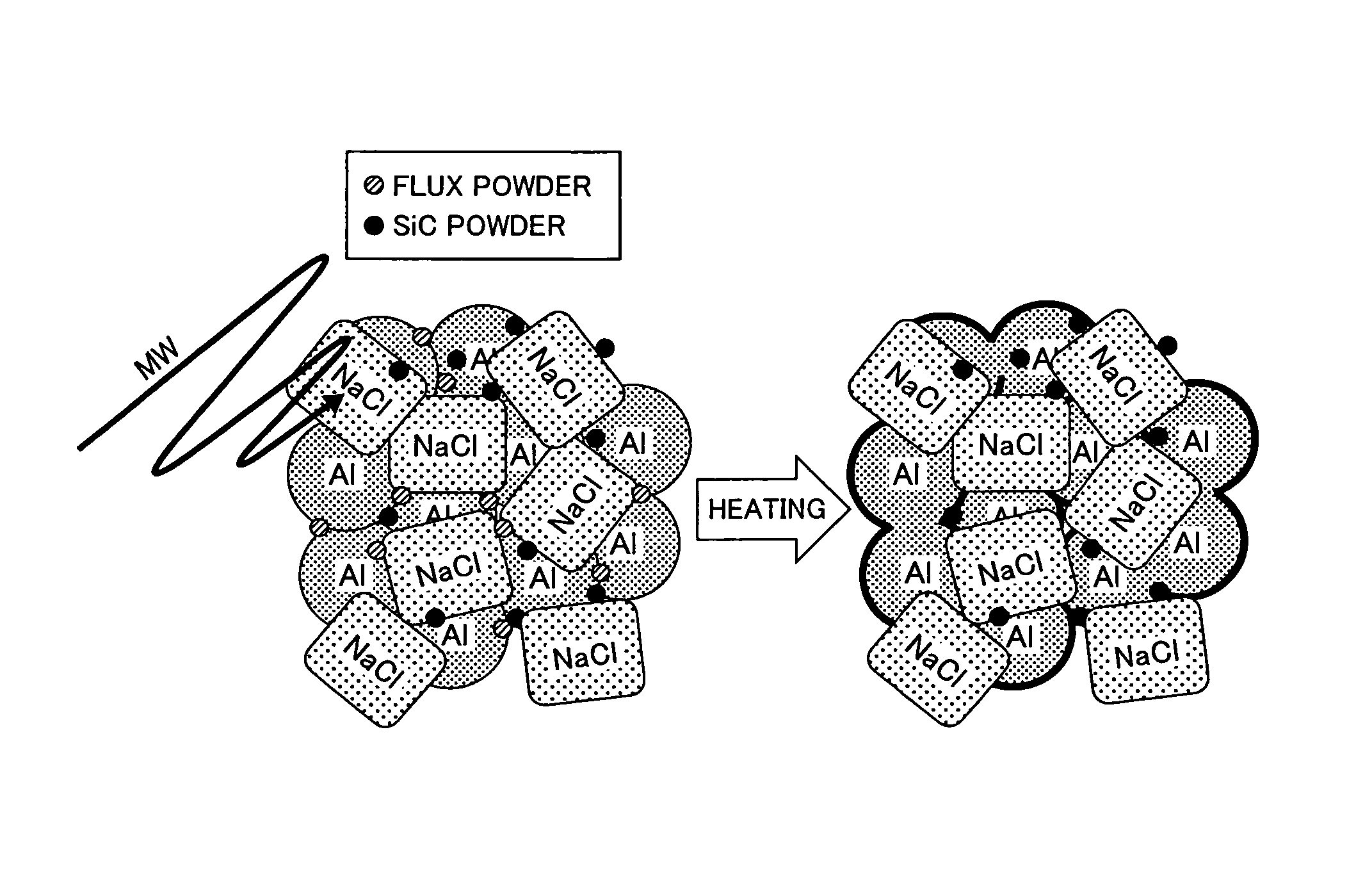
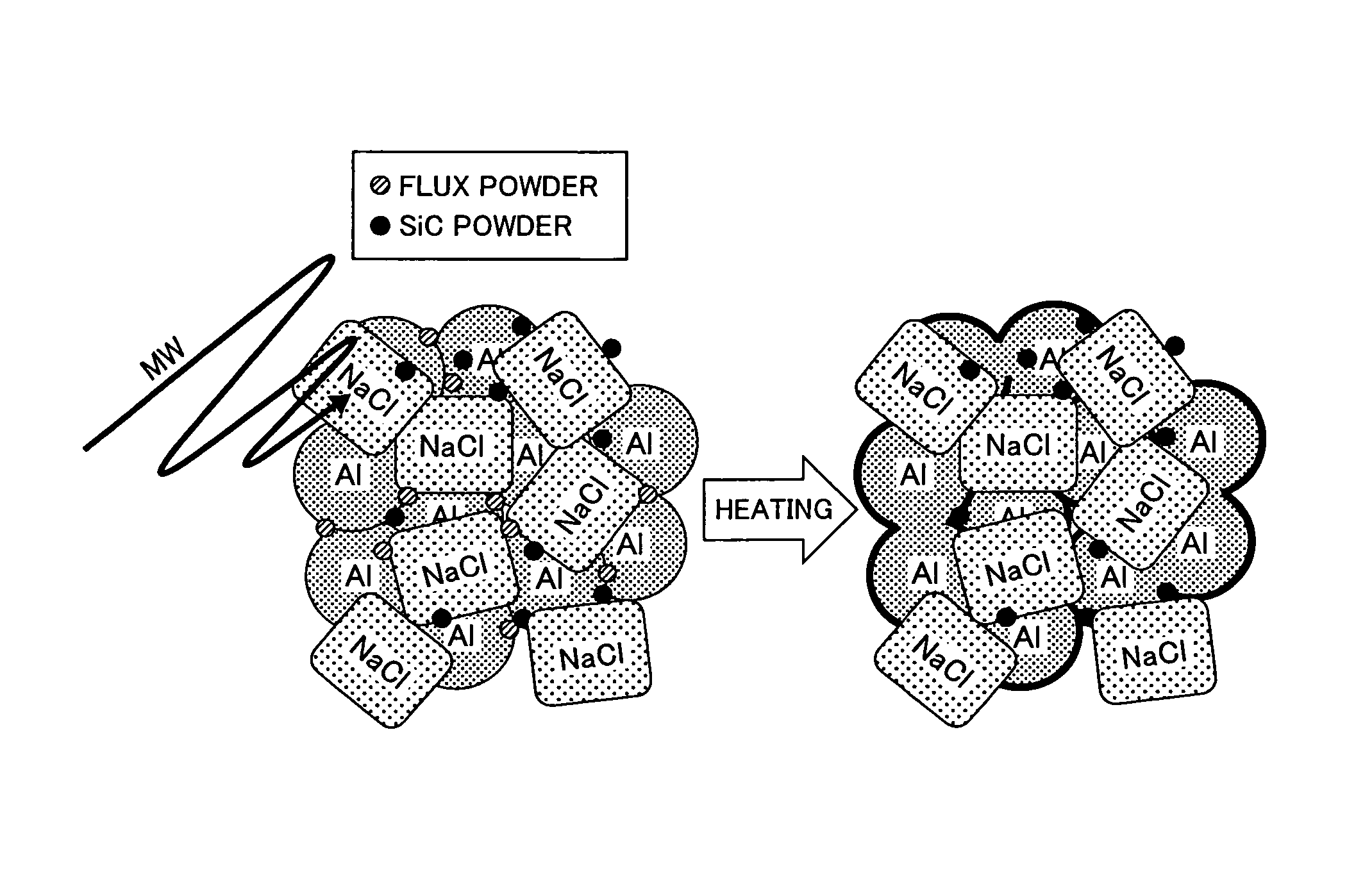
It is an objective of the present invention to provide an aluminum porous body which is formed of a pure aluminum and / or aluminum alloy base material and has excellent sinterability and high dimensional accuracy without employing metal stamping. There is provided an aluminum porous body having a relative density of from 5 to 80% with respect to the theoretical density of pure aluminum, in which the aluminum porous body contains 50 mass % or more of aluminum (Al) and from 0.001 to 5 mass % of at least one selected from chlorine (Cl), sodium (Na), potassium (K), fluorine (F), and barium (Ba). It is preferred that the aluminum porous body further contains from 0.1 to 20 mass % of at least one selected from carbon (C), silicon carbide (SiC), iron (II) oxide (FeO), iron (III) oxide (Fe2O3), and iron (II,III) oxide (Fe3O4).
Owner:HITACHI LTD
Stabilized boehmite-derived catalyst supports, catalysts, methods of making and using
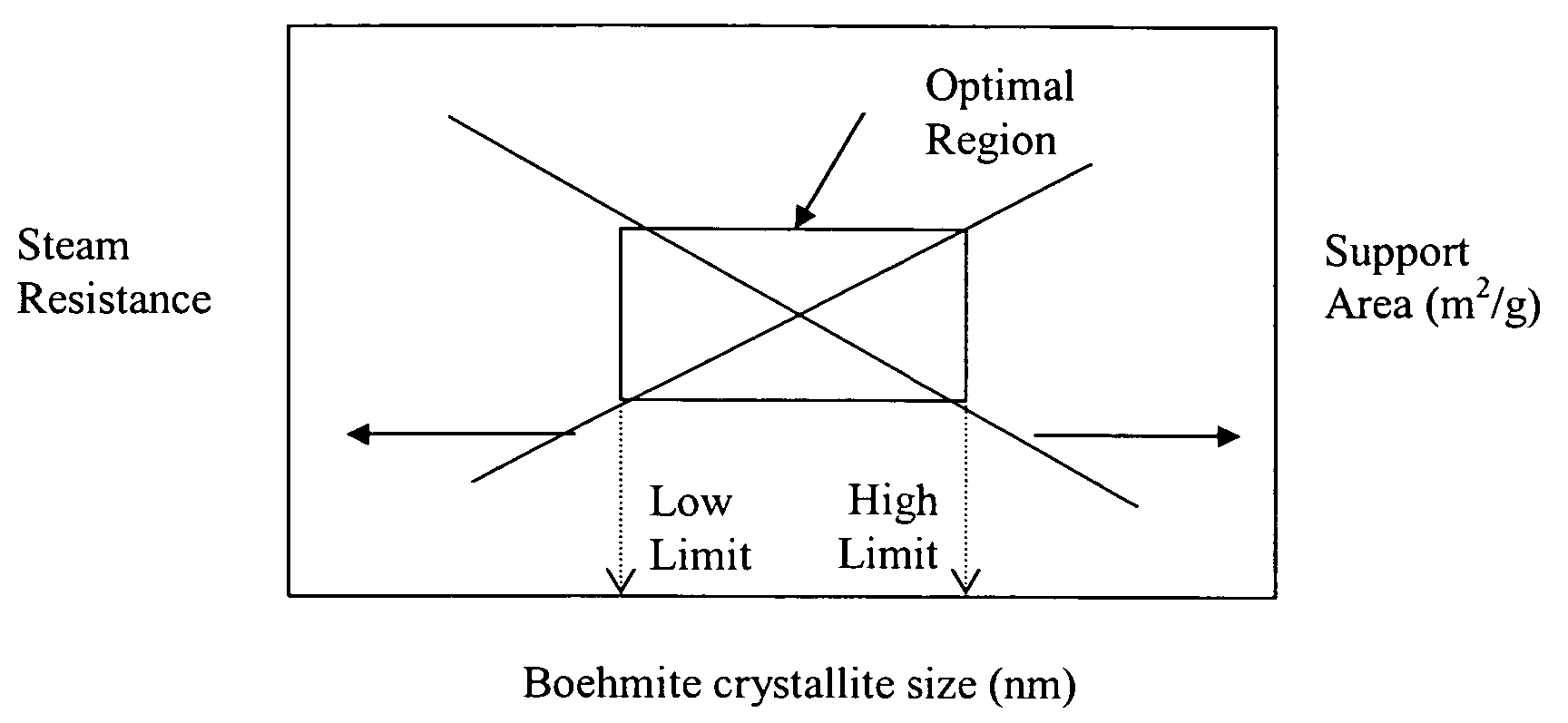
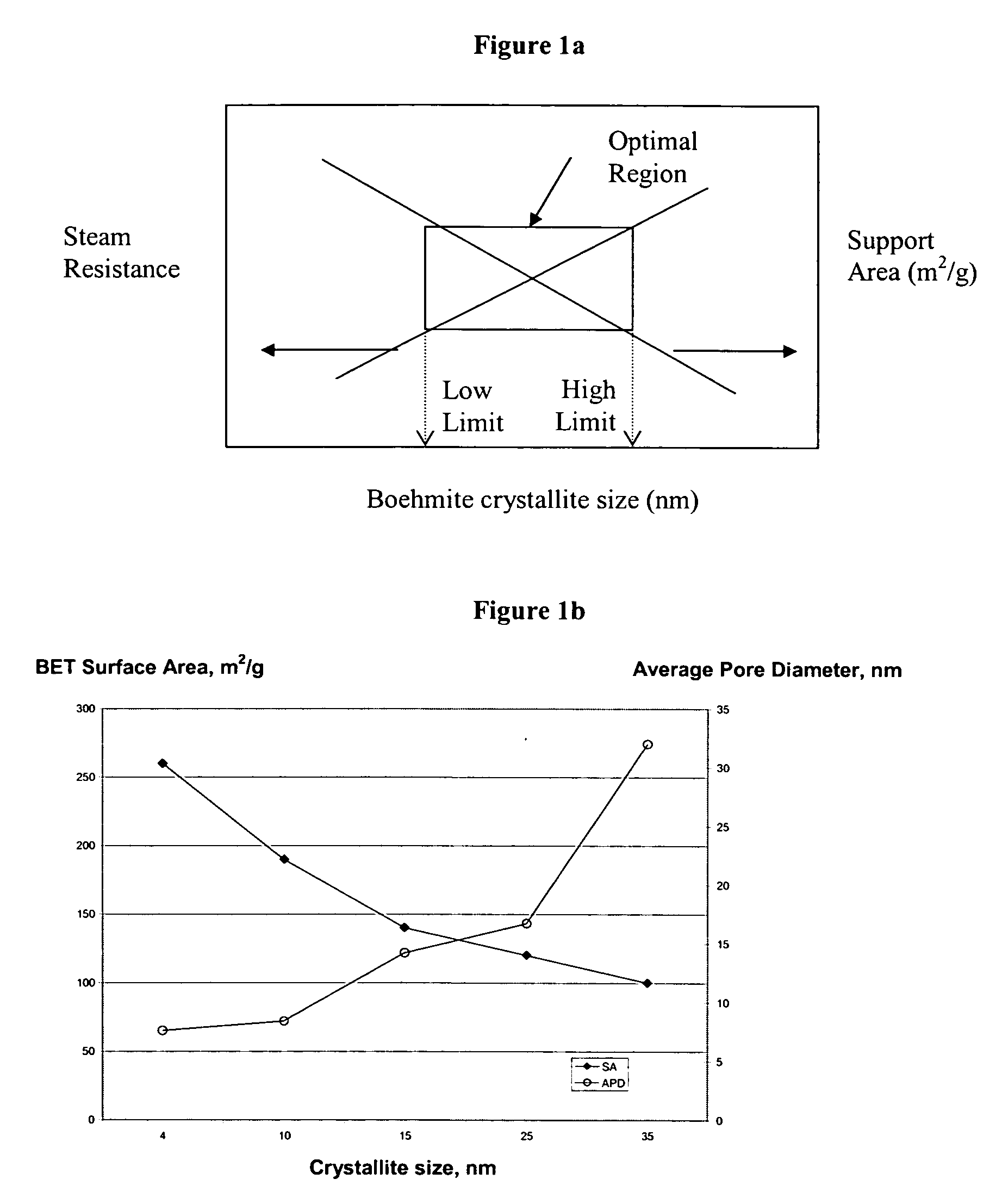
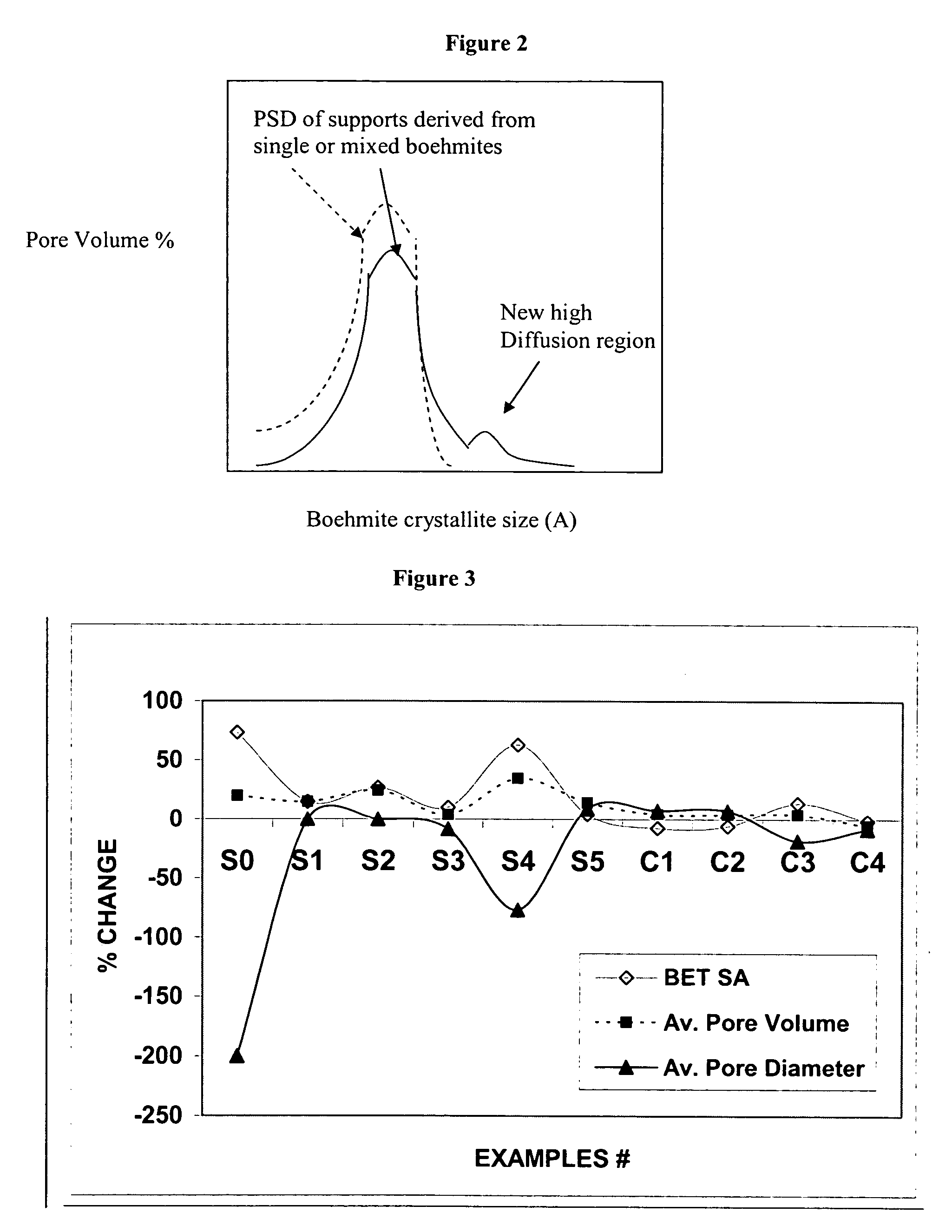
ActiveUS20050234137A1Good hydrothermal stabilityImprove stabilityMaterial nanotechnologyCatalyst carriersPorosityAluminium hydroxide
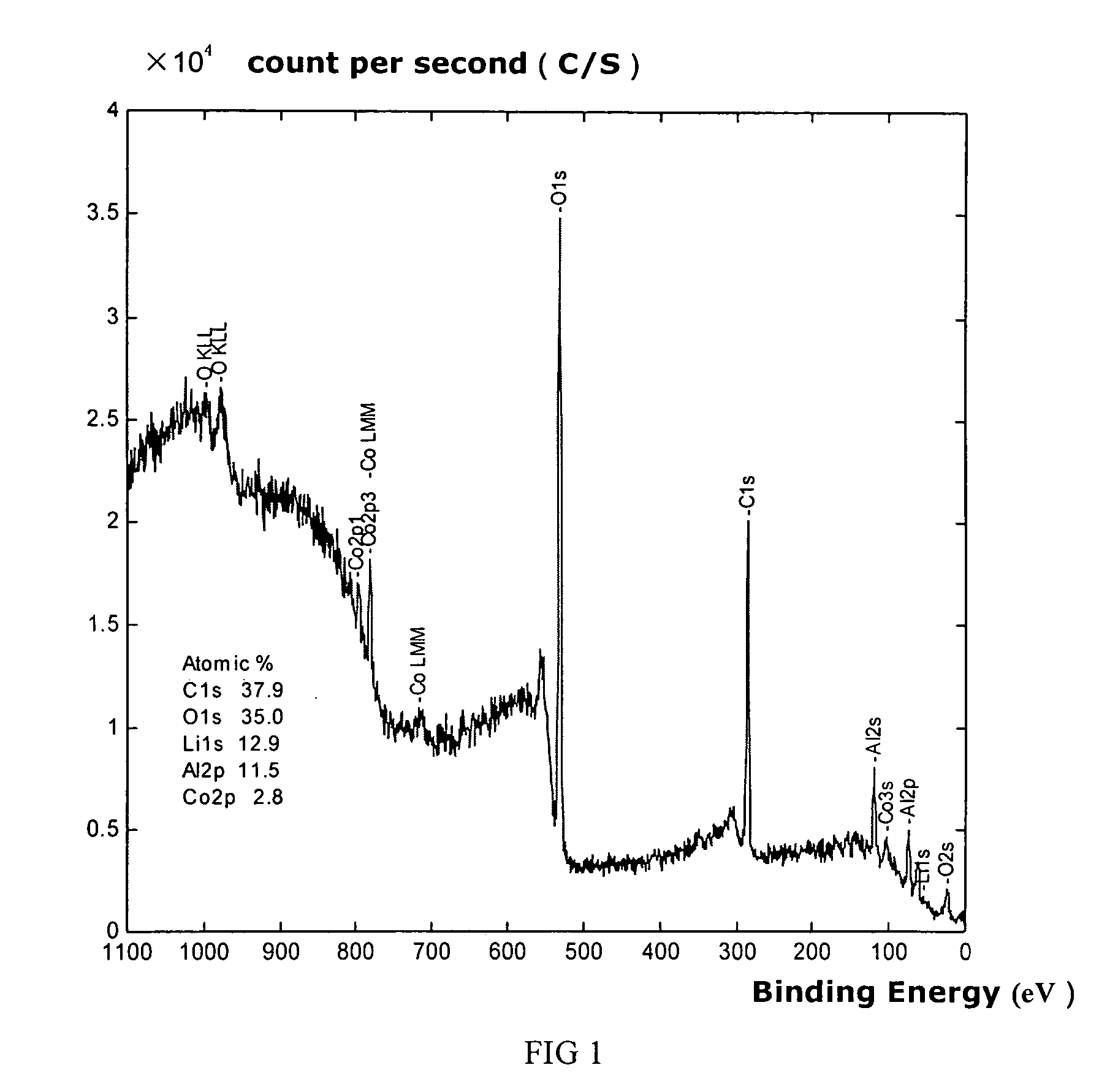
A stabilized catalyst support having improved hydrothermal stability, catalyst made therefrom, and method for producing hydrocarbons from synthesis gas using said catalyst. The stabilized support is made by a method comprising treating a crystalline hydrous alumina precursor in contact with at least one structural stabilizer or compound thereof. The crystalline hydrous alumina precursor preferably includes an average crystallite size selected from an optimum range delimited by desired hydrothermal resistance and desired porosity. The crystalline hydrous alumina precursor preferably includes an alumina hydroxide, such as crystalline boehmite, crystalline bayerite, or a plurality thereof differing in average crystallite sizes by at least about 1 nm. The crystalline hydrous alumina precursor may be shaped before or after contact with the structural stabilizer or compound thereof. The treating includes calcining at 450° C. or more. Preferred structural stabilizers can include cobalt, magnesium, manganese, manganese, zirconium, boron, aluminum, barium, silicon, lanthanum, oxides thereof, or combinations thereof.
Owner:CLARIANT INT LTD
Materials for positive electrodes of lithium ion batteries and their methods of fabrication
InactiveUS20050130042A1Cycle wellEasy dischargeElectrode thermal treatmentSecondary cellsCeriumSolvent
This invention discloses materials for positive electrodes of secondary batteries and their methods of fabrication. Said materials comprise of granules of an active material for positive electrodes coated with an oxide layer. The active material is one or more of the following: oxides of lithium cobalt, oxides of lithium nickel cobalt, oxides of lithium nickel cobalt manganese, oxides of lithium manganese, LiCoO2, LiNi1-xCoxO2, LiNi1 / 3Co1 / 3Mn1 / 3O2, and LiMn2O4. The non-oxygen component in the oxide layer is one or more of the following: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B. Said non-oxygen component of the granules is between 0.01 wt. % to 10 wt. % of said granules of active material. The methods of fabrication for said materials includes the steps of mixing an additive and an active material for positive electrodes uniformly in water or solvent, evaporating said solvent or water, and heat treating the remaining mixture at 300° C. to 900° C. for between 1 hour to 20 hours. The additive is a compound of one or more of the following elements: aluminum, magnesium, zinc, calcium, barium, strontium, lanthanum, cerium, vanadium, titanium, tin, silicon, boron, Al, Mg, Zn, Ca, Ba, Sr, La, Ce, V, Ti, Sn, Si, and B where the element is between 0.01 wt. % to 10 wt. % of said active material. Using the materials of positive electrodes disclosed above or materials for positive electrodes fabricated in the methods disclosed above in batteries produces batteries with excellent cycling and high temperature properties.
Owner:BYD AMERICA CORP
Gradient cathode material for lithium rechargeable batteries
InactiveUS6921609B2Increase capacityImproved cyclabilityAluminium compoundsAlkaline accumulatorsManganesePotassium
A composition suitable for use as a cathode material of a lithium battery includes a core material having an empirical formula LixM′zNi1−yM″yO2. “x” is equal to or greater than about 0.1 and equal to or less than about 1.3. “y” is greater than about 0.0 and equal to or less than about 0.5. “z” is greater than about 0.0 and equal to or less than about 0.2. M′ is at least one member of the group consisting of sodium, potassium, nickel, calcium, magnesium and strontium. M″ is at least one member of the group consisting of cobalt, iron, manganese, chromium, vanadium, titanium, magnesium, silicon, boron, aluminum and gallium. A coating on the core has a greater ratio of cobalt to nickel than the core. The coating and, optionally, the core can be a material having an empirical formula Lix1Ax2Ni1−y1−z1Coy1Bz1Oa. “x1” is greater than about 0.1 a equal to or less than about 1.3. “x2,”“y1” and “z1” each is greater than about 0.0 and equal to or less than about 0.2. “a” is greater than 1.5 and less than about 2.1. “A” is at least one element selected from the group consisting of barium, magnesium, calcium and strontium. “B” is at least one element selected from the group consisting of boron, aluminum, gallium, manganese, titanium, vanadium and zirconium.
Owner:TIAX LLC
Alkali-free glasses containing iron and tin as fining agents
ActiveUS7534734B2Glass drawing apparatusGlass forming apparatusActive-matrix liquid-crystal displayAlkali free
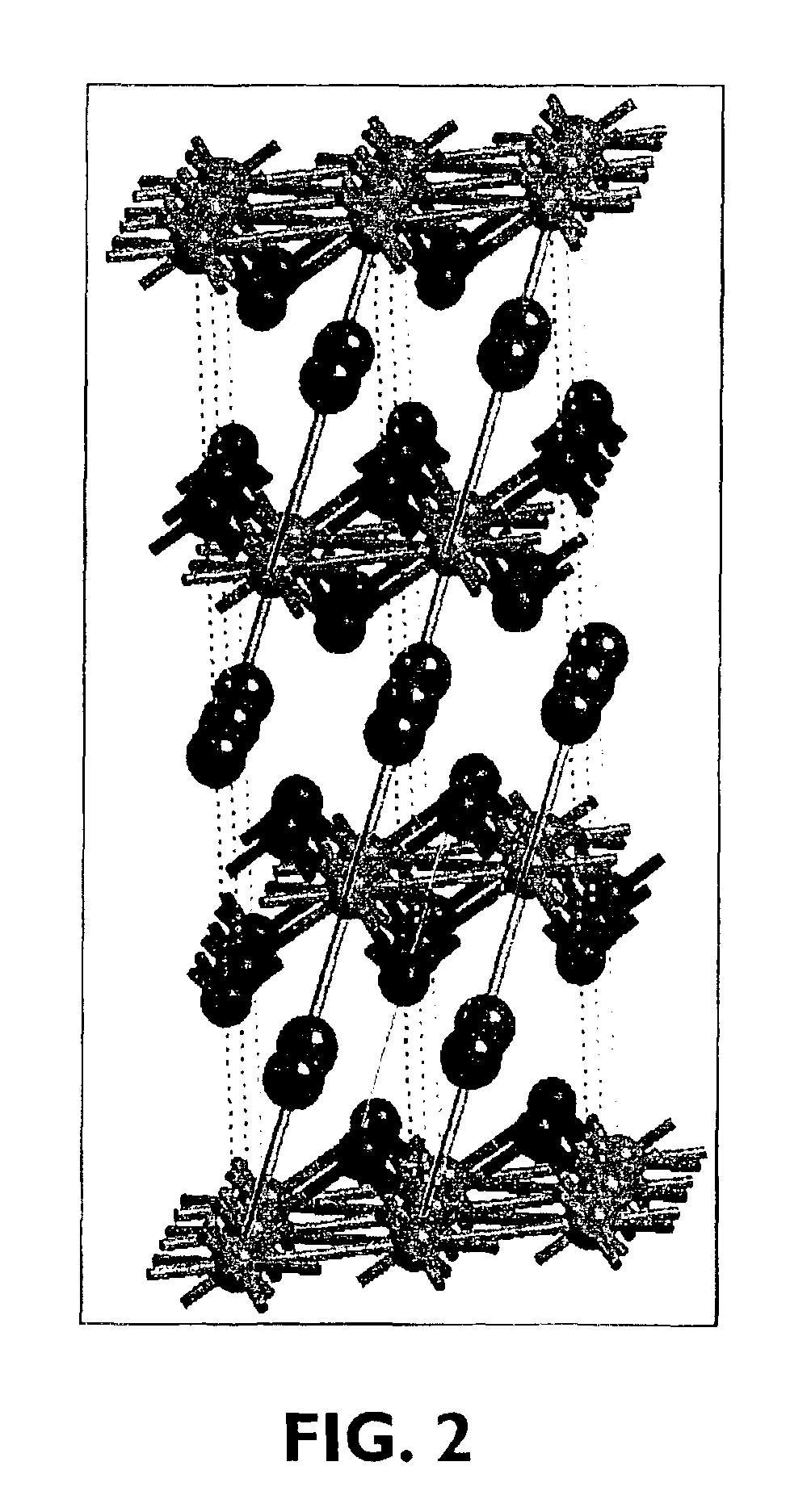
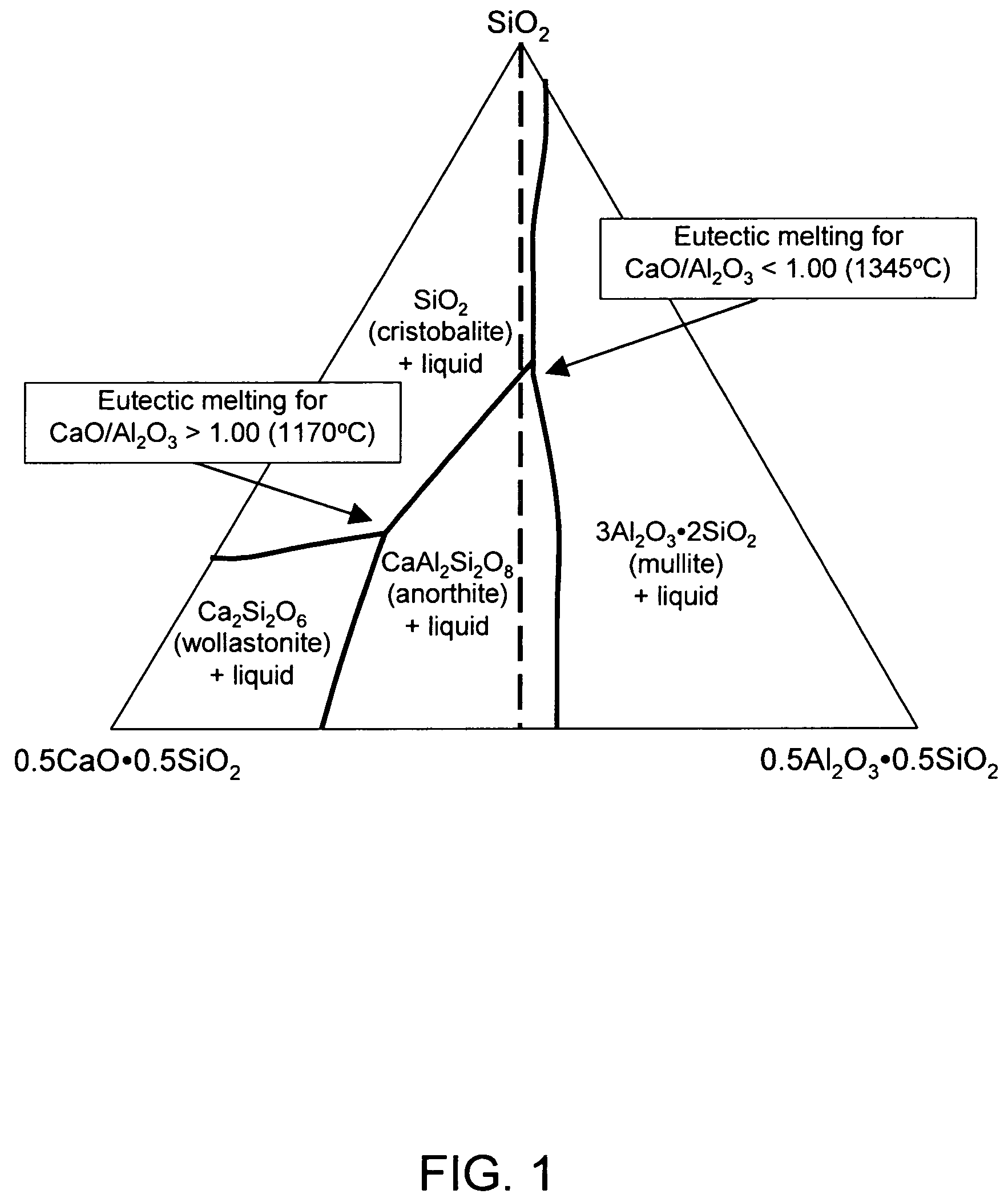
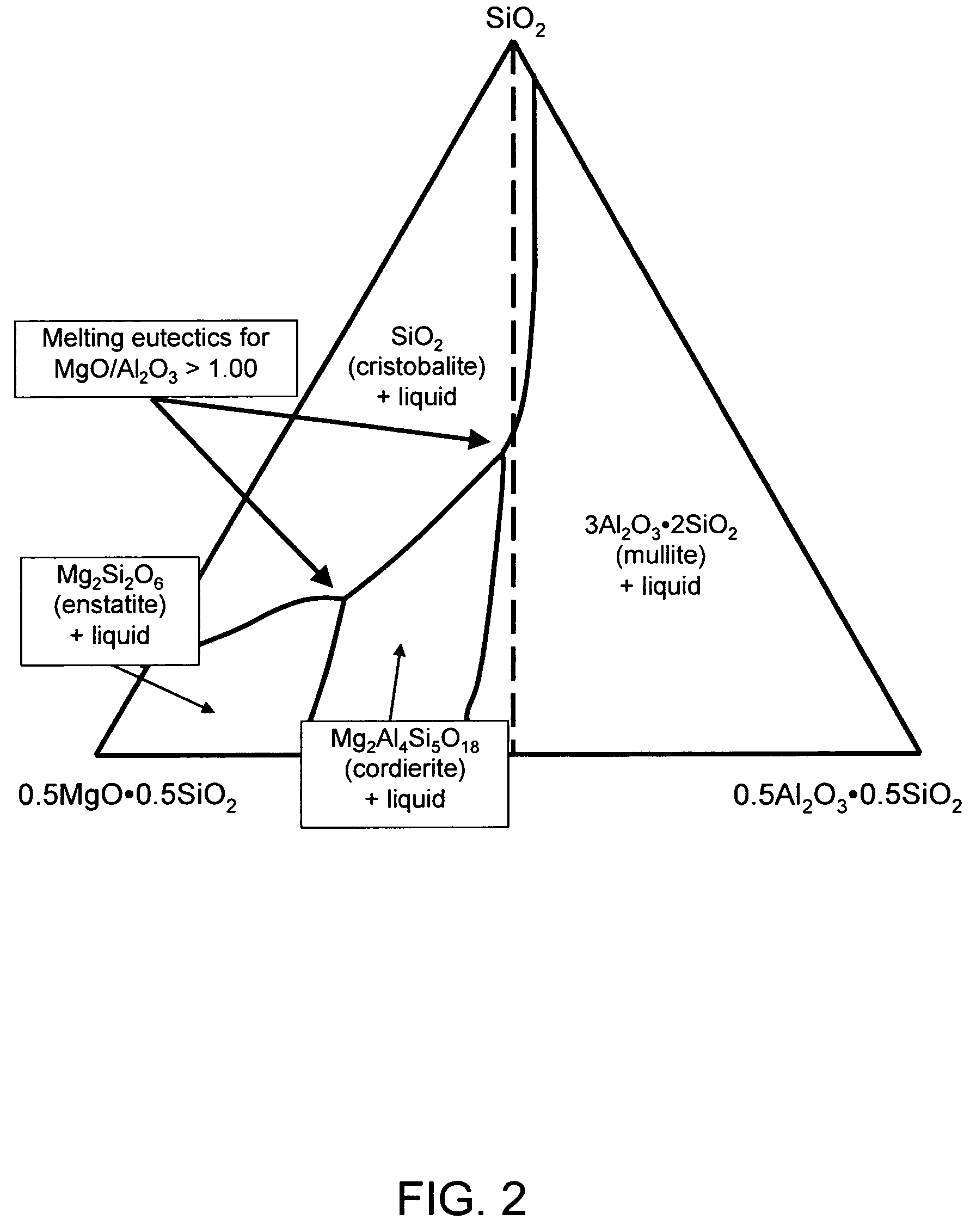
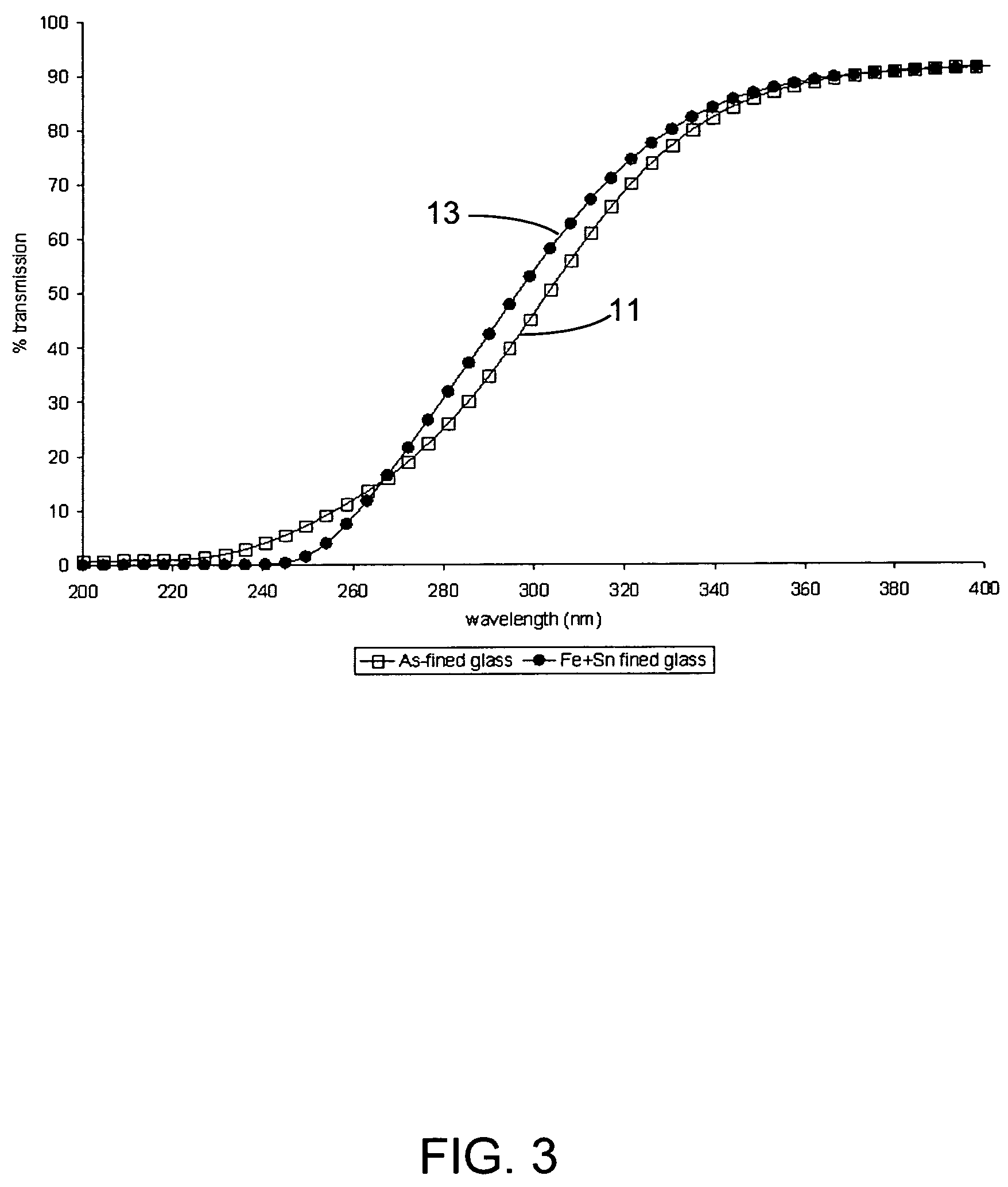
Alkali-free glasses are disclosed which can be used to produce substrates for flat panel display devices, e.g., active matrix liquid crystal displays (AMLCDs). The glasses contain iron and tin as fining agents, and preferably are substantially free of arsenic and antimony. In certain embodiments, the glasses are also substantially free of barium. Methods for producing alkali-free glass sheets using a downdraw process (e.g., a fusion process) are also disclosed.
Owner:CORNING INC
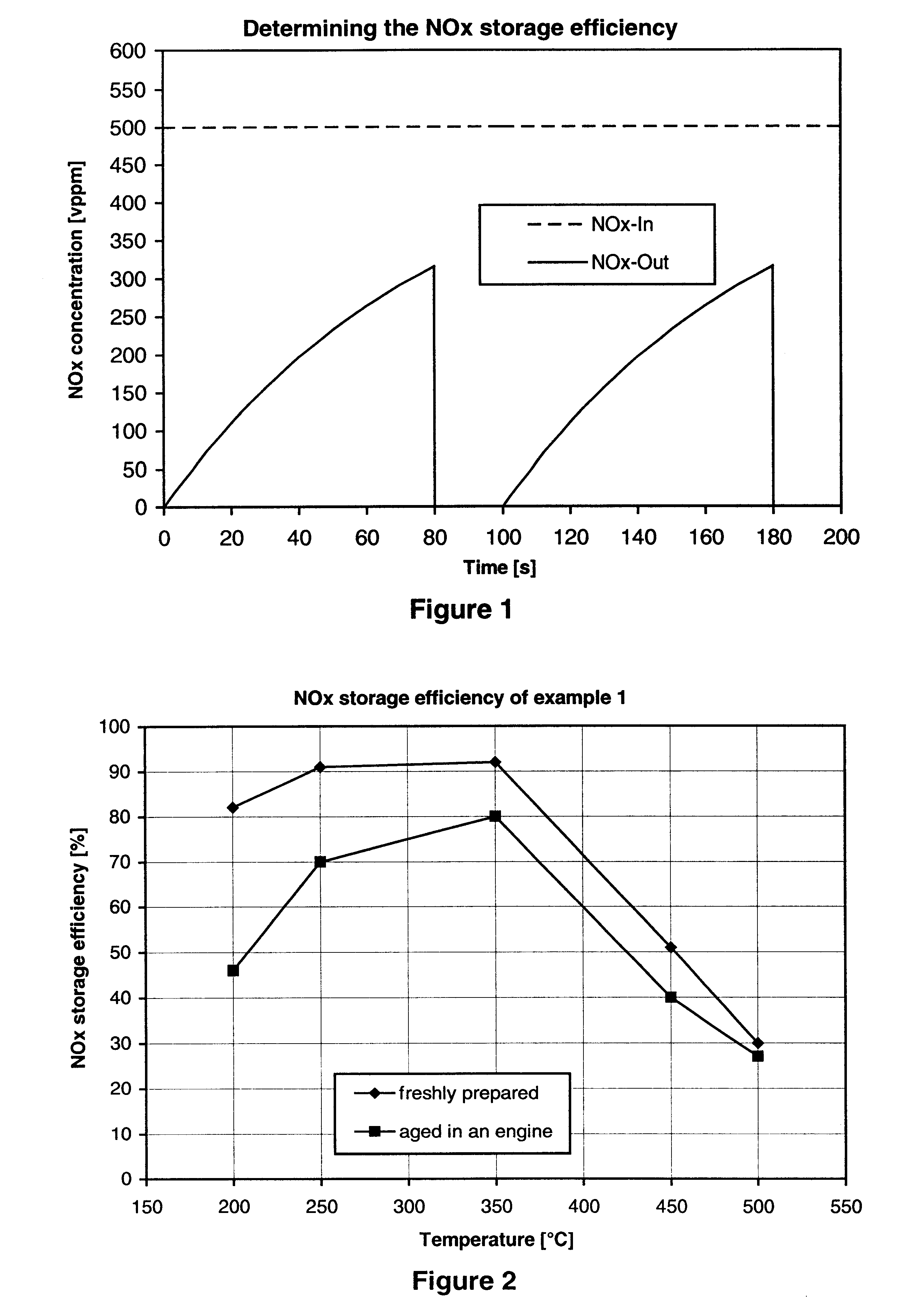
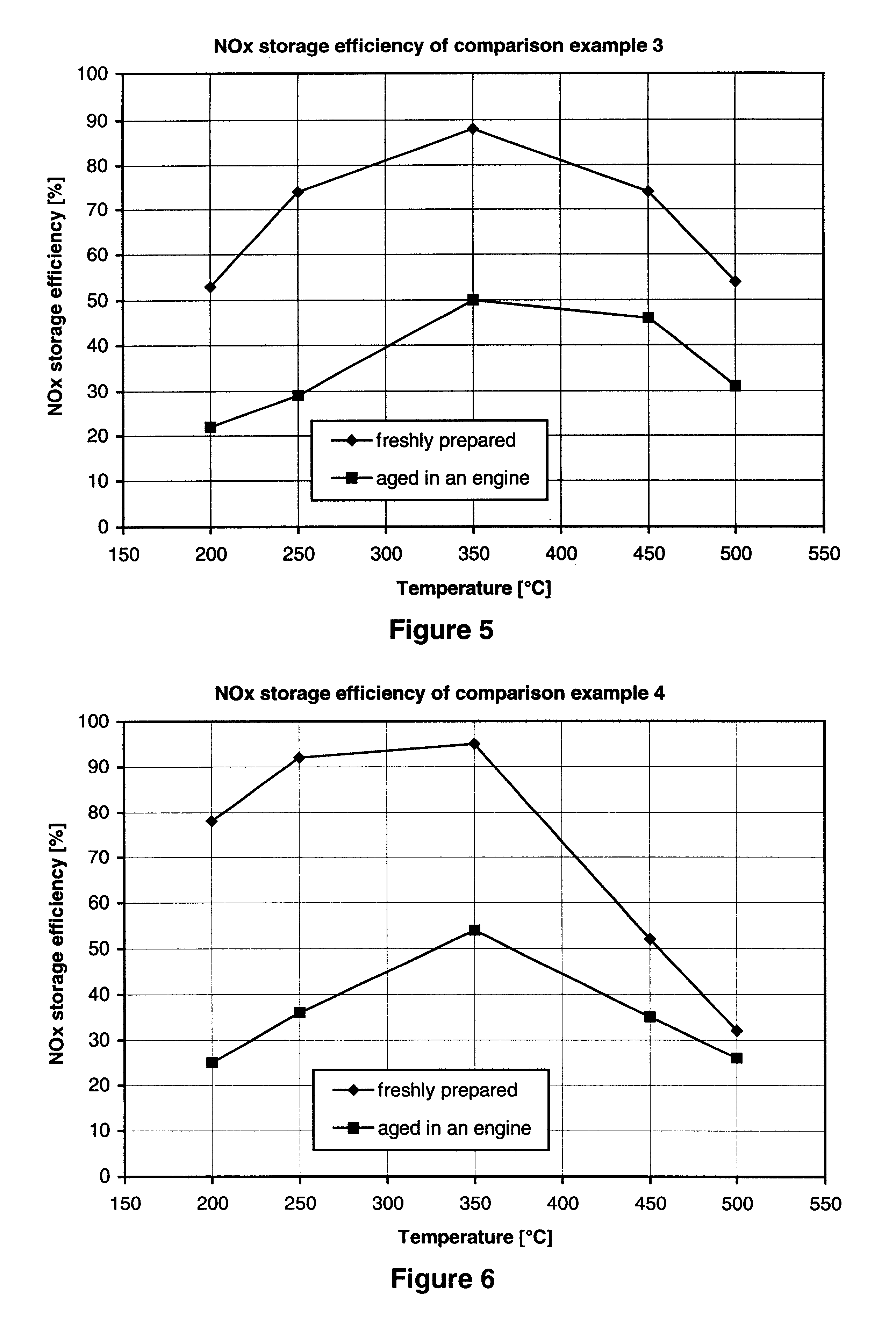
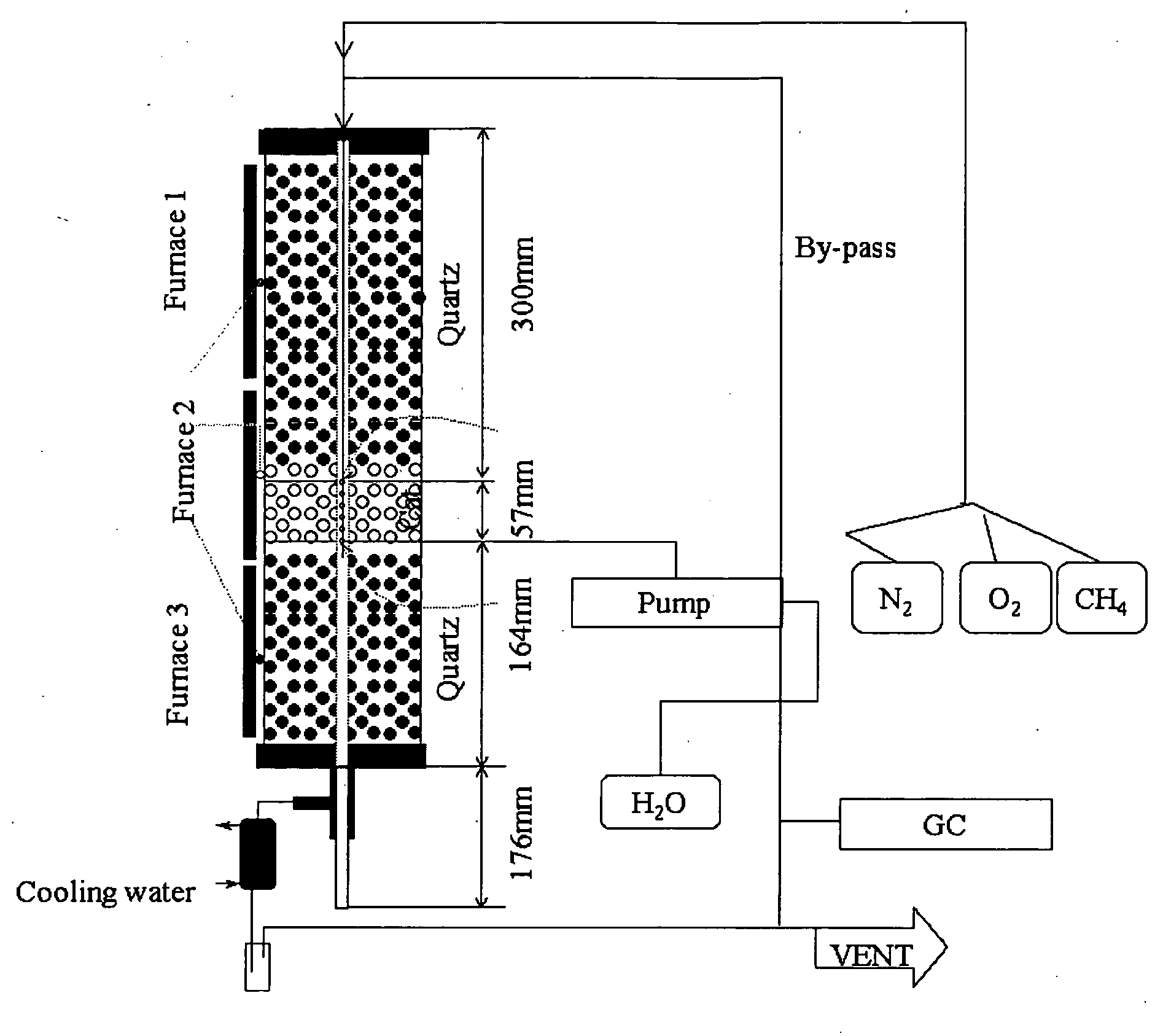
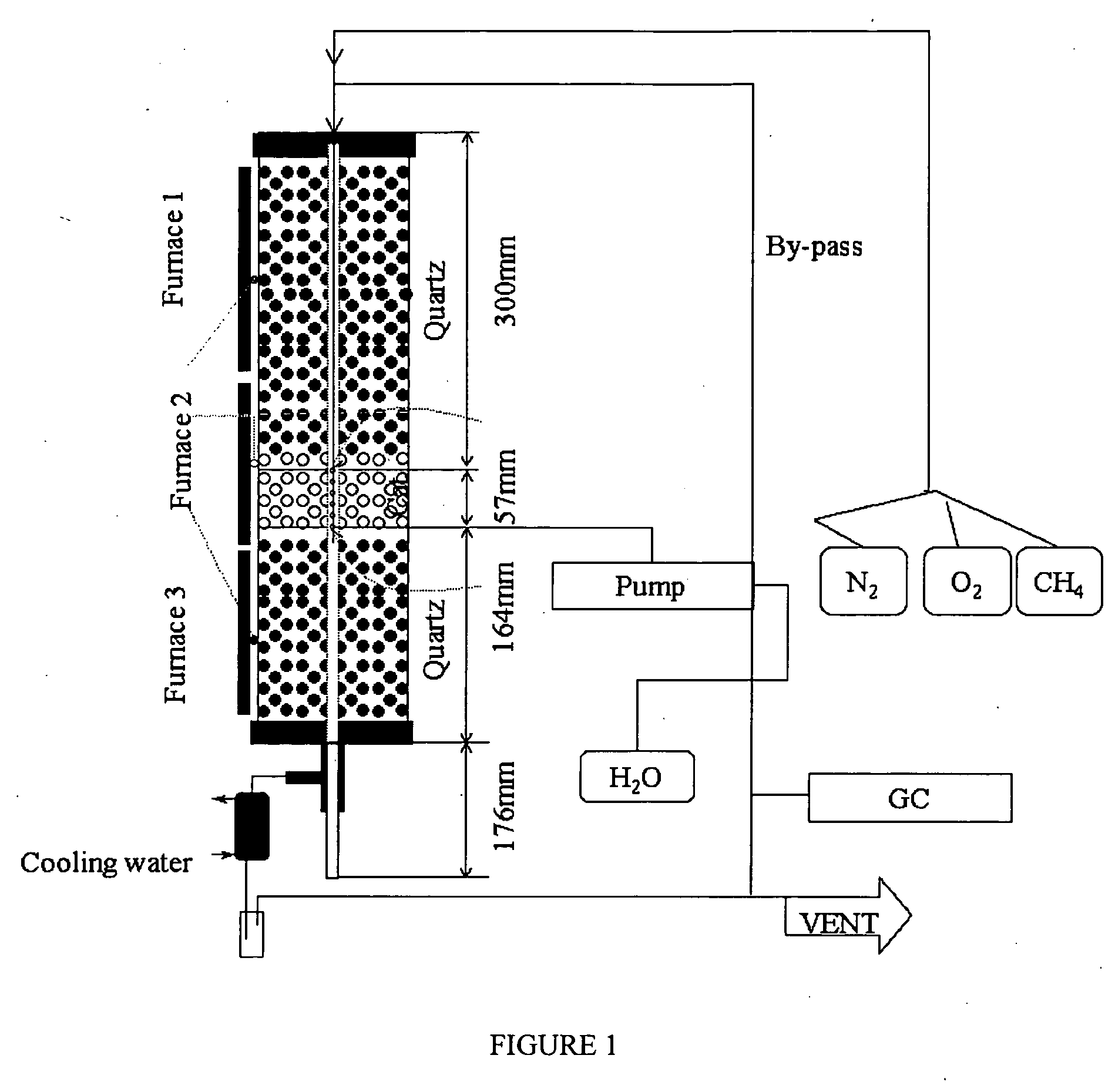
Nitrogen oxide storage material and nitrogen oxide storing catalyst prepared therefrom
InactiveUS6350421B1Determine efficiencyNitrogen compoundsExhaust apparatusAlkaline earth metalCuprate
A nitrogen oxide storage material is disclosed which contains at least one storage component for nitrogen oxides in the form of an oxide, mixed oxide, carbonate or hydroxide of the alkaline earth metals magnesium, calcium, strontium and barium and the alkali metals potassium and caesium on a high surface area support material. The support material can be doped cerium oxide, cerium / zirconium mixed oxide, calcium titanate, strontium titanate, barium titanate, barium stannate, barium zirconate, magnesium oxide, lanthanum oxide, praseodymium oxide, samarium oxide, neodymium oxide, yttrium oxide, zirconium silicate, yttrium barium cuprate, lead titanate, tin titanate, bismuth titanate, lanthanum cobaltate, lanthanum manganate and barium cuprate or mixtures thereof.
Owner:DMC2 DEGUSSA METALS +1
Catalyst and method for converting low molecular weight paraffinic hydrocarbons into alkenes and organic compounds with carbon numbers of 2 or more
InactiveUS20070083073A1Reduce the amount requiredPromotes oxidative couplingHydrogenHeterogenous catalyst chemical elementsCarbon numberOxygen
A catalyst and process for formation of hydrocarbons having carbon numbers of two or greater, the result of both oxidative coupling of methane (“OCM”), and other reforming reactions of OCM end products. An OCM catalyst has a structure represented by formula ABTiO3, wherein A is samarium or tin, B is barium; the reforming catalysts a composition represented by formula XYZ, wherein X is a metal from Group IA, Group IIA or Group VIIIA, or not present, Y a metal from Group VA, Group VIA, Group VIIA or Group VIIIA, Z chosen from oxygen, silica, silicalite and alumina. The inventive catalyst comprises an OCM catalyst and a reforming catalyst blended together; when used in a reactor effects an increased yield of hydrocarbons having a carbon number greater than 2 (in excess of 27%-30%, first pass rate of methane conversion about 50%) than occurs under OCM conditions alone.
Owner:HRD CORP
High performance lithium ion battery anode material lithium manganate and preparation method thereof
The invention provides a high performance lithium ion battery anode material lithium manganate and a preparation method of the material. The lithium manganate is a doped lithium manganate LiMn2-yXy04 which is doped with one kind or a plurality of other metal elements X, wherein X element is at least one kind selected form the group of aluminium, lithium, fluorine, silver, copper, chromium, zinc, titanium, bismuth, germanium, gallium, zirconium, stannum, silicon, cobalt, nickel, vanadium, magnesium, calcium, strontium, barium and rare earth elements lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium and lutetium, and y is larger than 0 but less than or equal to 0.11. The lithium ion battery anode material lithium manganate provided in the invention has extraordinary charge and discharge cycle performance both in the environments of normal temperature and high temperature. According to the invention, the preparation method of the material is a solid phase method, the operation is simple and controllable and the cost is low so that it is easy to realize large-scale productions.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Aluminum titanate ceramic articles and methods of making same
ActiveUS7259120B2Reduced strengthLower firing temperatureInternal combustion piston enginesSilencing apparatusAlkaline earth metalRare earth
An aluminum titanate ceramic article having a predominant crystal phase of aluminum titanate and a material composition including aluminum, titanium, silica, an alkaline earth metal (e.g., at least one selected from the group of strontium, calcium, barium, or combinations), and a rare earth metal (e.g., at least one selected from the group consisting of yttrium, lanthanum, and combinations) and methods of making such aluminum titanate bodies are described. An oxide of yttrium metal or lanthanide metals is preferably used as a sintering aid in combination with the other compositional components to enable firing of the resulting green body at a lower heating temperature of less than 1500° C., and more preferably between 1400°-1450° C., with a preferable hold time of less than 8 hours, more preferably of 6 to 8 hours.
Owner:CORNING INC
Piezoelectric ceramic composition and method of production of same, piezoelectric element, and dielectric element
InactiveUS20040058797A1Excellent piezoelectric propertiesIncrease temperaturePiezoelectric/electrostrictive device manufacture/assemblyPiezoelectric/electrostrictive device material selectionRheniumIridium
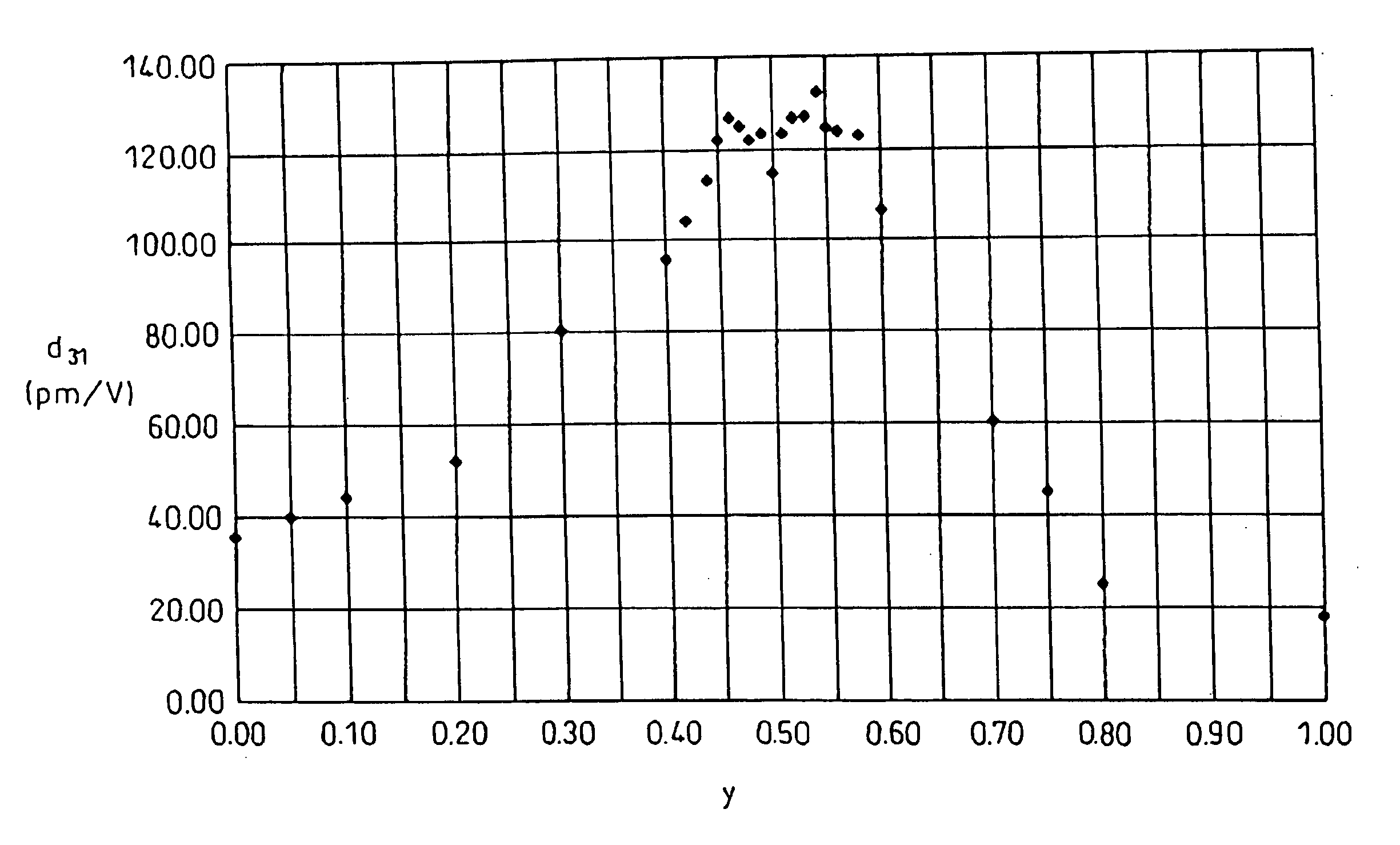
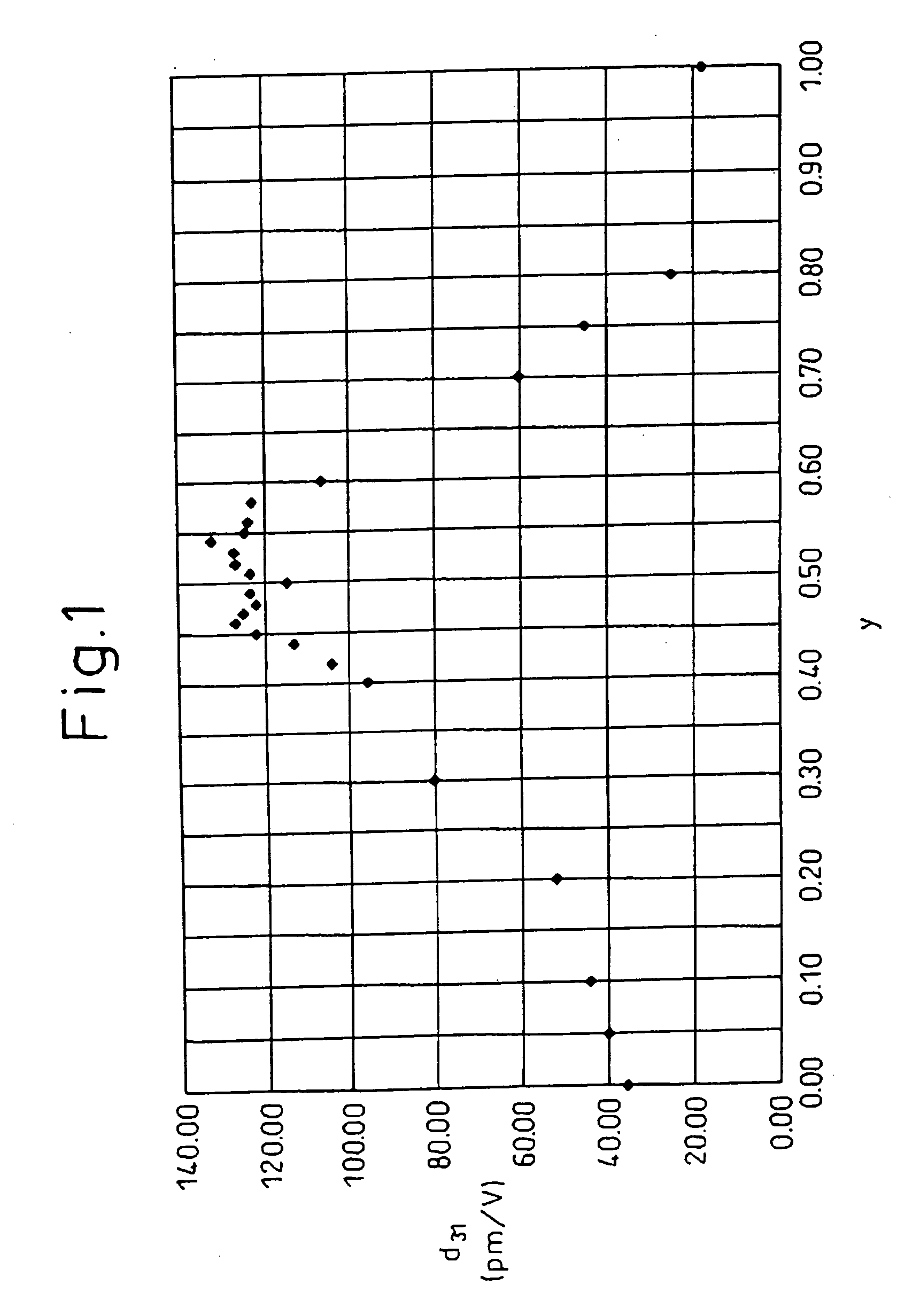
A piezoelectric ceramic composition not containing lead, able to be sintered at ordinary pressure, and superior to the past in at least one of the properties unique to piezoelectric ceramic compositions such as the piezoelectric d31 constant, that is, a piezoelectric ceramic composition having a compound of a general formula {Lix(K1-yNay)1-x}(Nb1-z-wTazSbw)O3 where x, y, z, and w are in the ranges of 0<=x<=0.2, 0<=y<=1, 0<z<=0.4, and 0<w<=0.2 as a main ingredient, where the piezoelectric ceramic composition contains at least one metal element selected from (1) palladium, silver, gold, ruthenium, rhodium, rhenium, osmium, iridium, and platinum, (2) nickel, iron, manganese, copper, and zinc, or (3) magnesium, calcium, strontium, and barium as an added element, and a method of production of the same and a piezoelectric element and dielectric element utilizing that piezoelectric ceramic composition.
Owner:DENSO CORP +1
Use of alkaline earth metal containing small pore non-zeolitic molecular sieve catalysts in oxygenate conversion
A method for converting starting material to olefins comprising contacting the starting material with a small pore non-zeolitic molecular sieve catalyst under effective conditions to produce olefins, wherein the non-zeolitic molecular sieve has been prepared in-situ or modified after synthesis by incorporation using an alkaline earth metal compound, wherein the alkaline earth metal ion is selected from the group consisting of strontium, calcium, barium, and mixtures thereof.
Owner:EXXON CHEM PAT INC
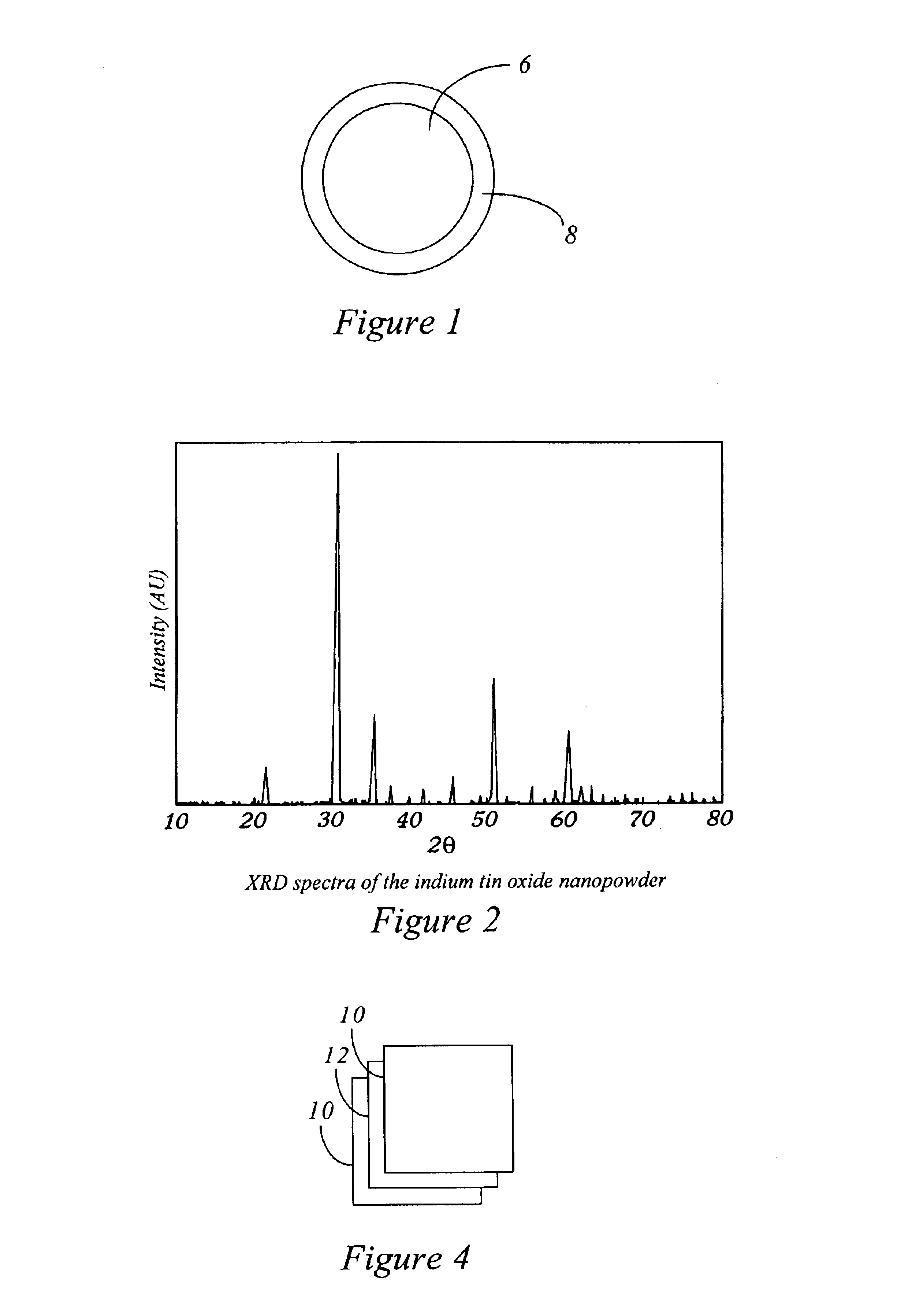
Production Of Barium Titanate Compounds
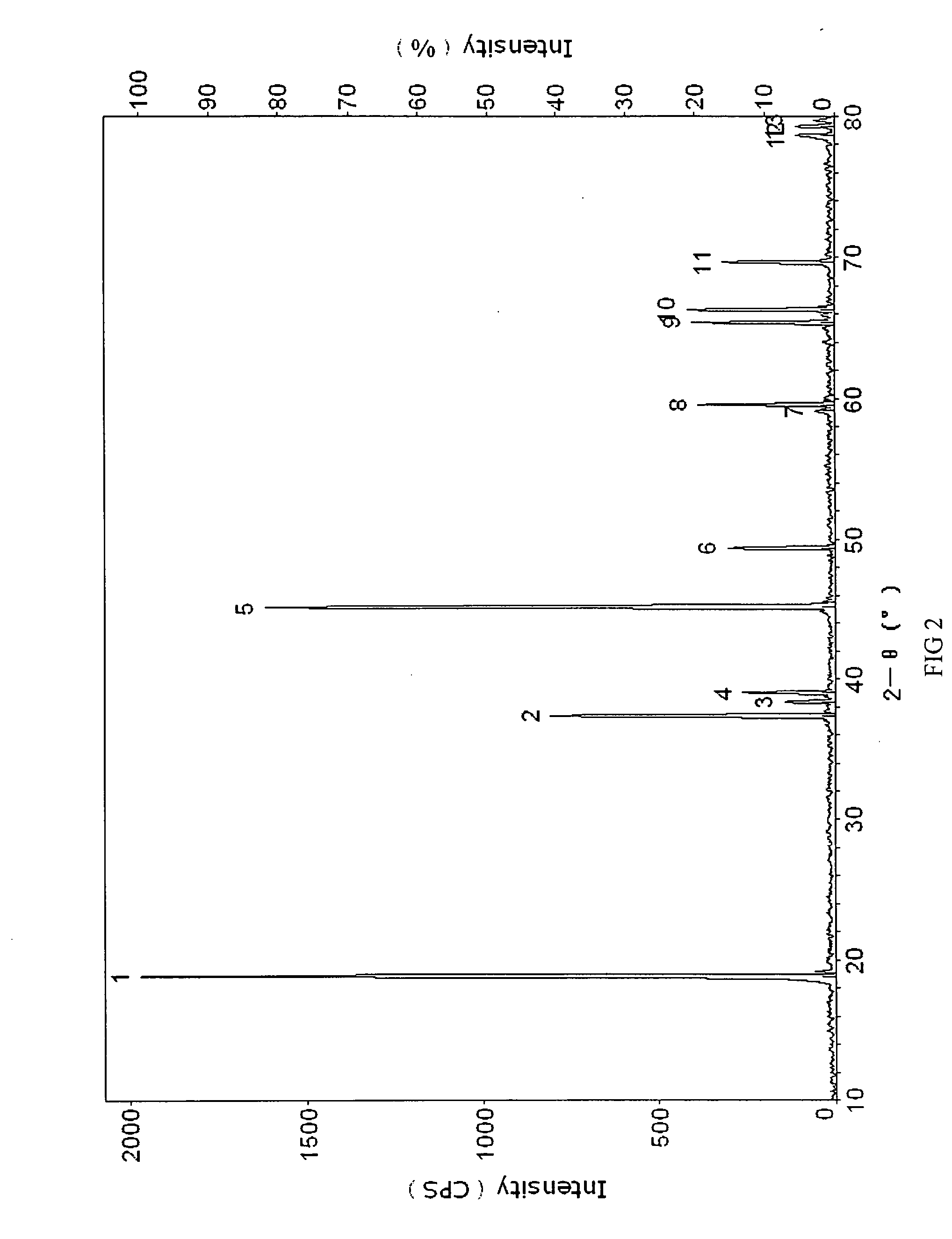
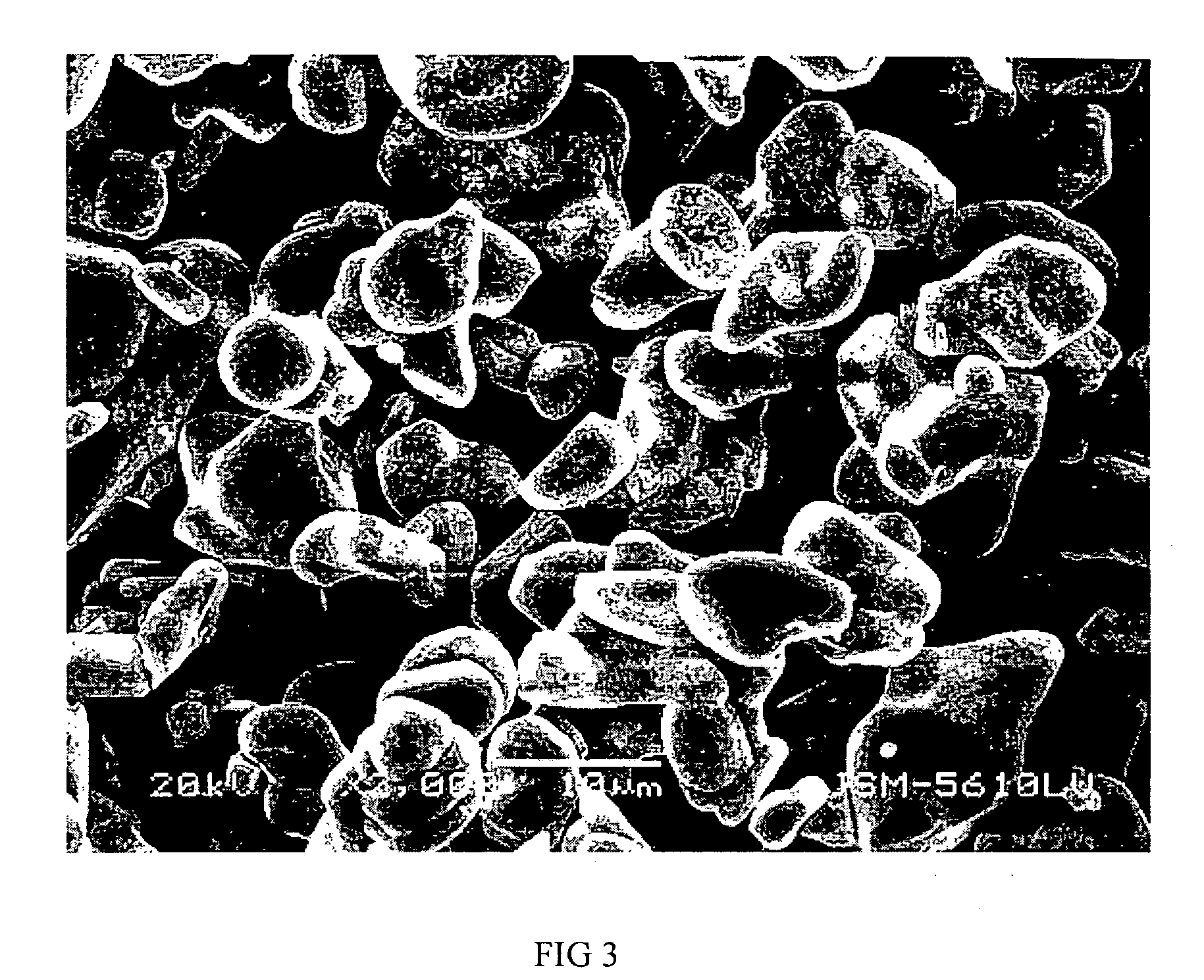
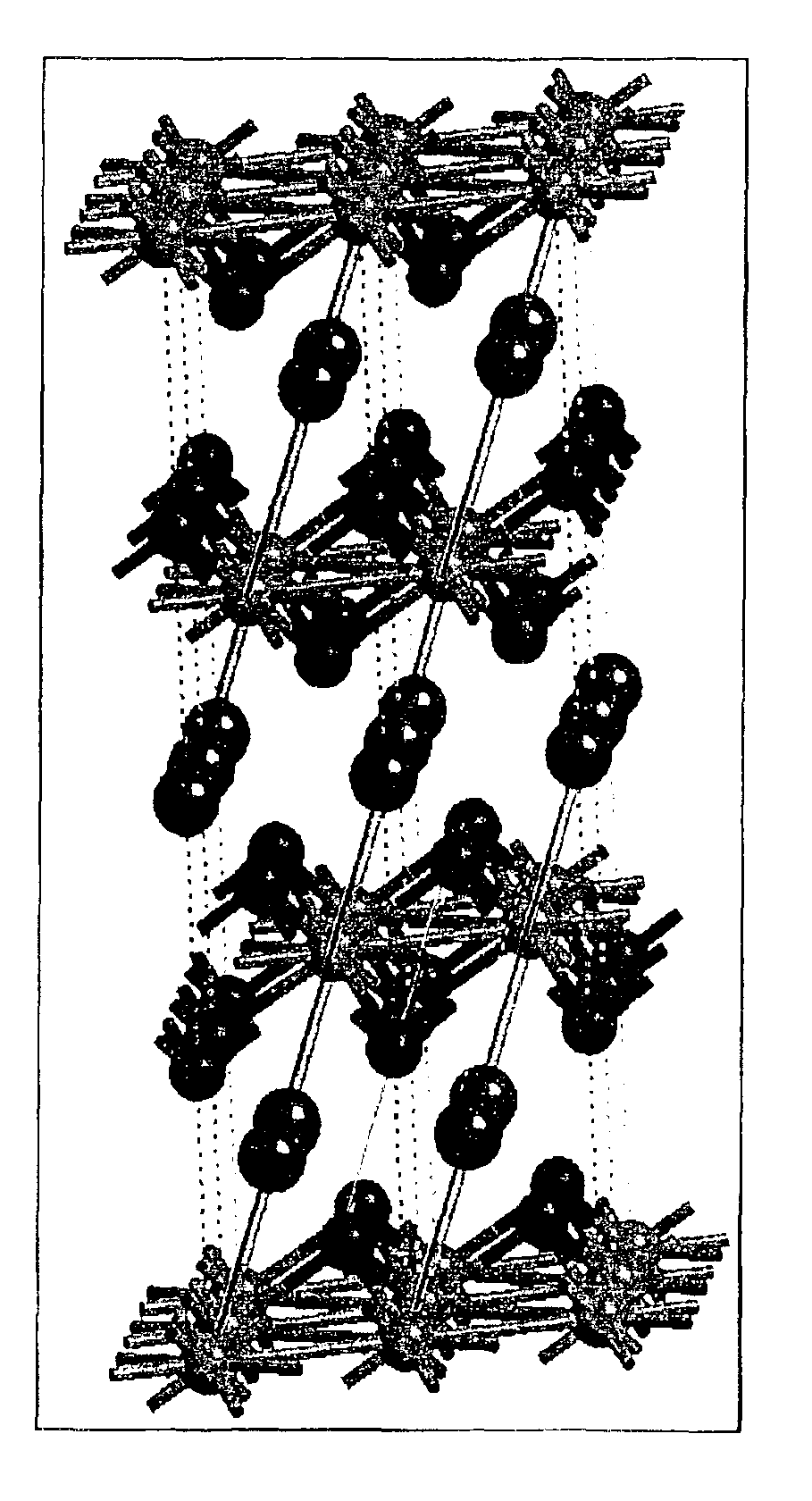
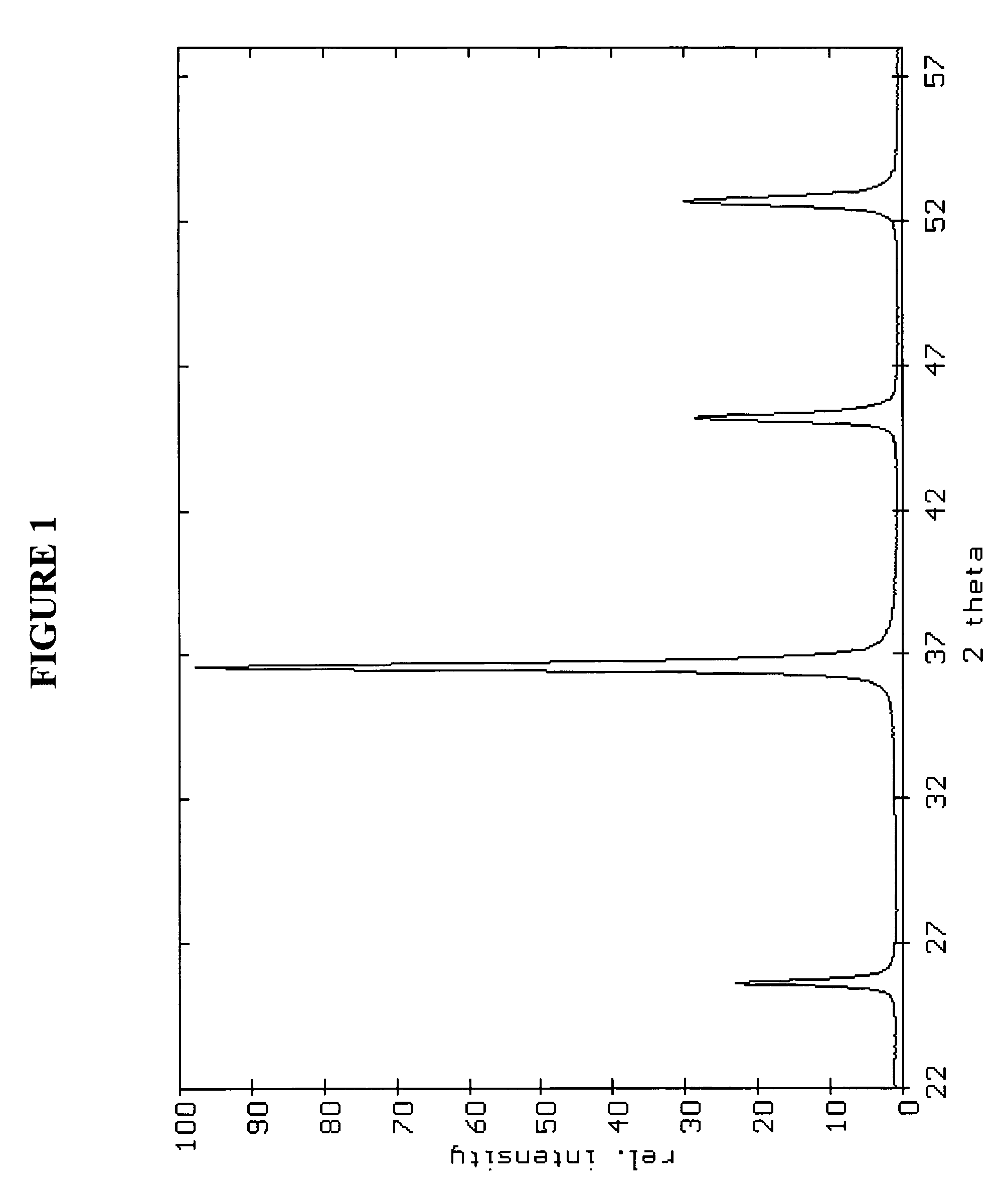
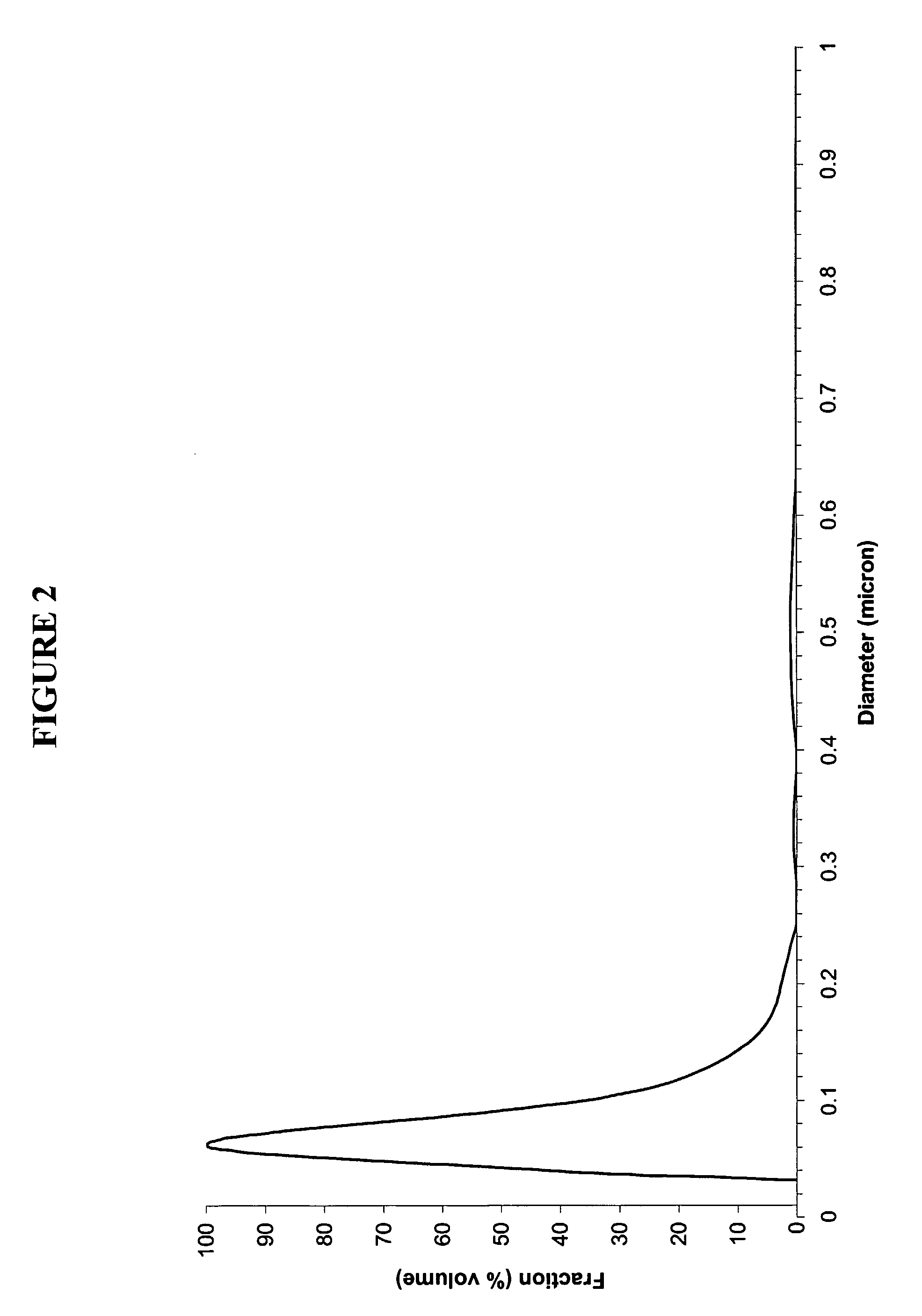
InactiveUS20070202036A1High purityLow costMaterial nanotechnologyAlkaline earth titanatesBarium titanateSpherical shaped
An ultrafine powder of barium titanate including solid solutions and doped compounds that meets up to specific characteristics is produced by method comprising two main steps. The first step is a reaction, typically in a Segmented Flow Tubular Reactor, between reactants to produce cubic-structure barium titanate composed of non-agglomerated ultrafine particles having a shape of given aspect ratio, usually a generally spherical shape, of low density corresponding at most to 90% of the intrinsic density, all particles being smaller than 1 micron and having a narrow particle size distribution and wherein the ratio of Ba:Ti including substitutents and dopants is very close to the ideal stoichiometry. This is followed by subjecting the powder produced in the first step to a second stage solvothermal post treatment typically in an autoclave at temperature less than 400° C. to convert the cubic-structure particles of low density to ultrafine tetragonal particles of increased density corresponding to at least 90% of the intrinsic density while maintaining the same aspect ratio, and maintaining the size of all particles below 1 micron, the narrow particle size distribution span, and the given ideal stoichiometry. The produced particles can have a non-spherical facetted shape such as cube-like.
Owner:JONGEN NATHALIE +1
Multiple hazard protection articles and methods for making them
InactiveUS6841791B2Improve cooling effectInorganic/elemental detergent compounding agentsChemical protectionTectorial membraneSolvent
Articles, including fabrics and film layers, are disclosed which can protect against multiple hazards, including radiation, chemical, biological agents, metal projectiles and fire hazards. In some embodiments, the fabrics and films of the present invention are used to produce garments having protection against multiple hazards and superior heat dissipating properties. A radiation protective compound is preferably created by mixing a radiopaque material, such as barium, bismuth, tungsten or their compounds, with powdered polymer, pelletized polymer or a liquid solution, emulsion or suspension of a polymer in solvent or water. This radiation protective mixture can then be laminated or otherwise adhered to other types of protective films or fabric, such as the protective polymer films or fabrics used for chemical protective garments, biological protective garments, bullet proof vests or fire retardant garments. The principles of this invention can also be applied to a broad range of other articles including surgical hoods, hospital gowns, gloves, patient drapes, partitions, coverings, jumpsuits, ponchos, uniforms, fatigues, tents, probes, envelopes, pouches, wallpaper, liners, drywall, house sidings, house foundations, house roofings etc.
Owner:MERIDIAN RES & DEV
High temperature ceramic dielectric composition and capacitors made from the composition
ActiveUS8076257B1Highly desirable propertyExcellent dielectric constant/voltage characteristicFixed capacitor dielectricStacked capacitorsCapacitanceDielectric
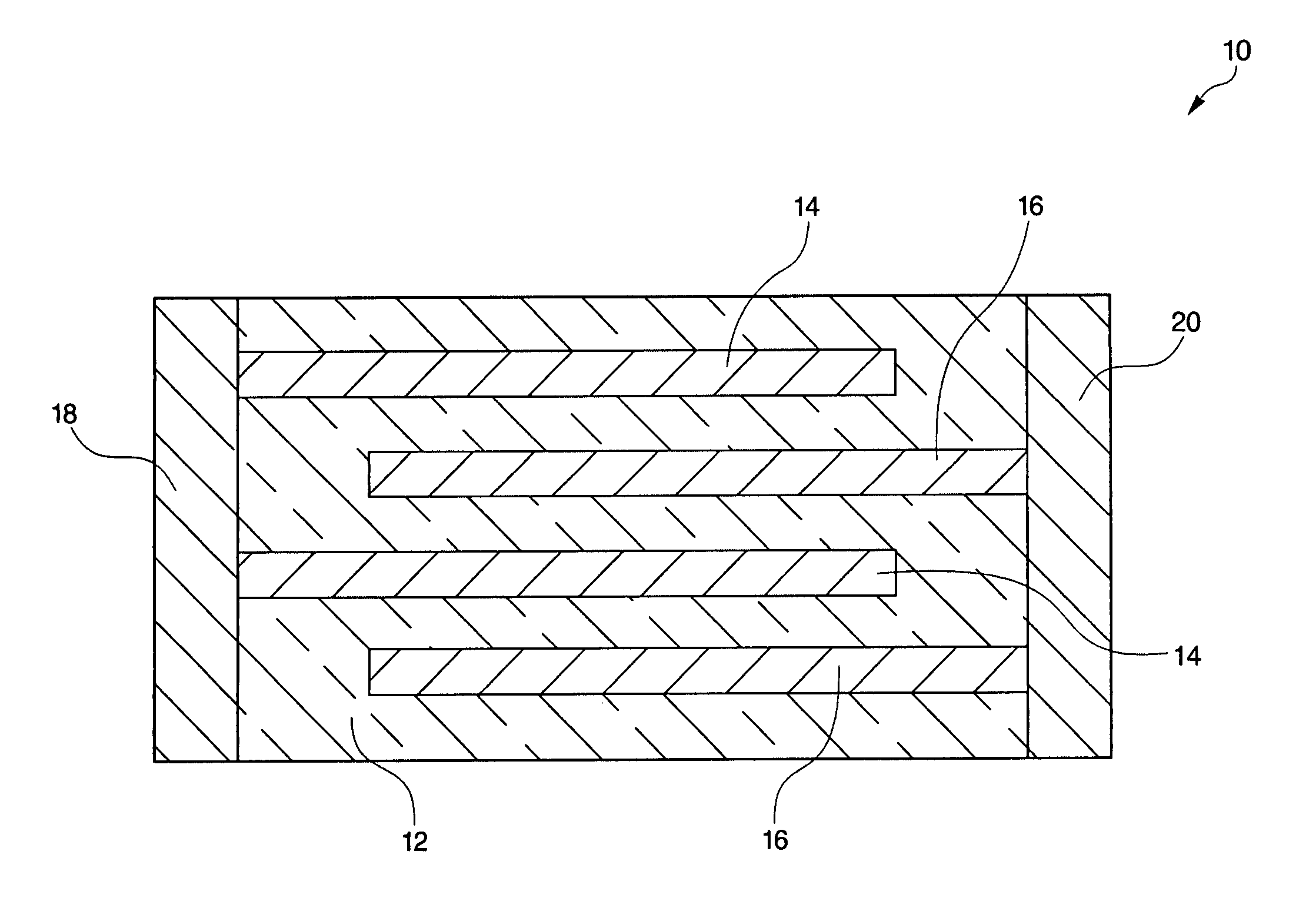
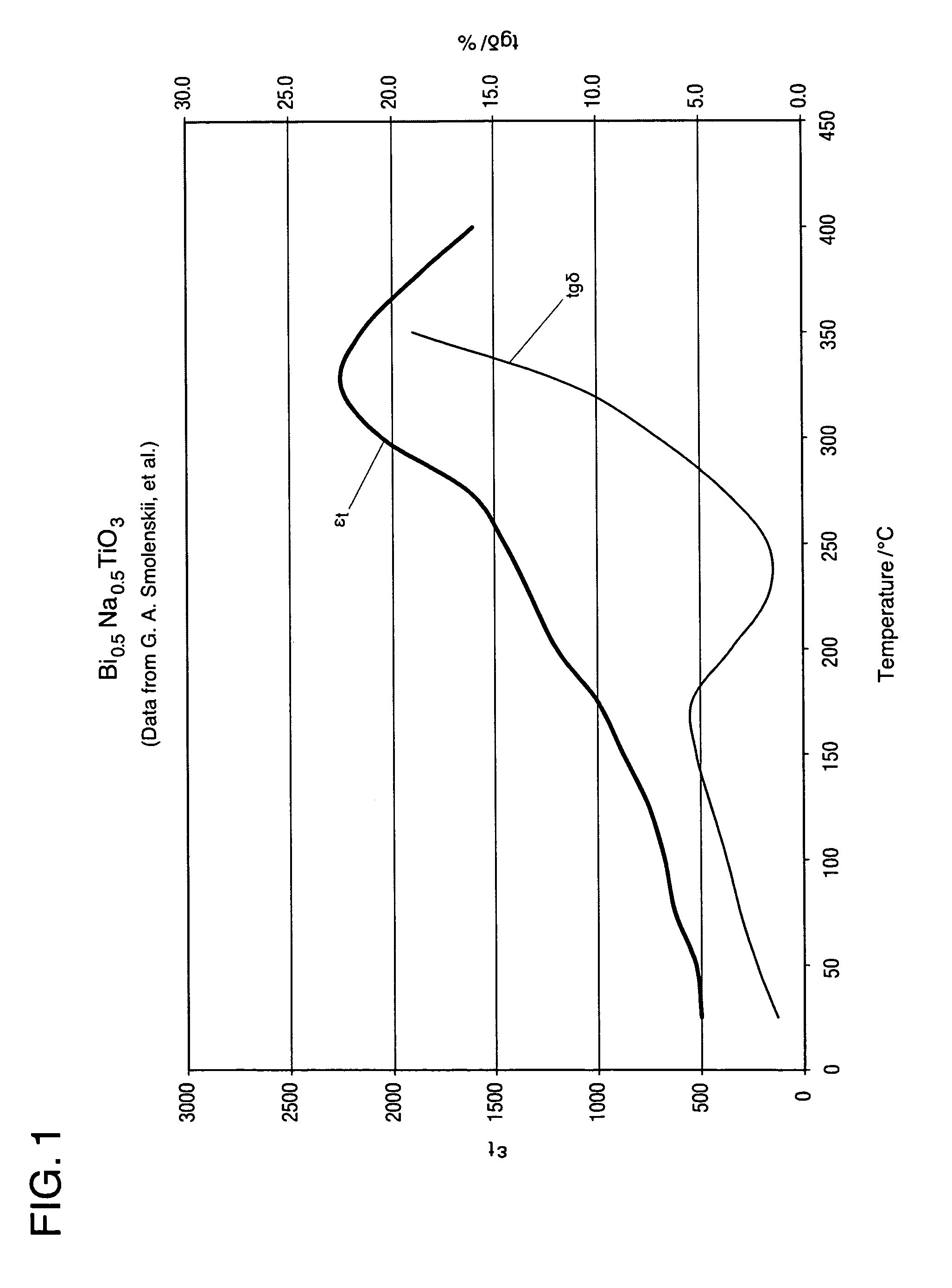
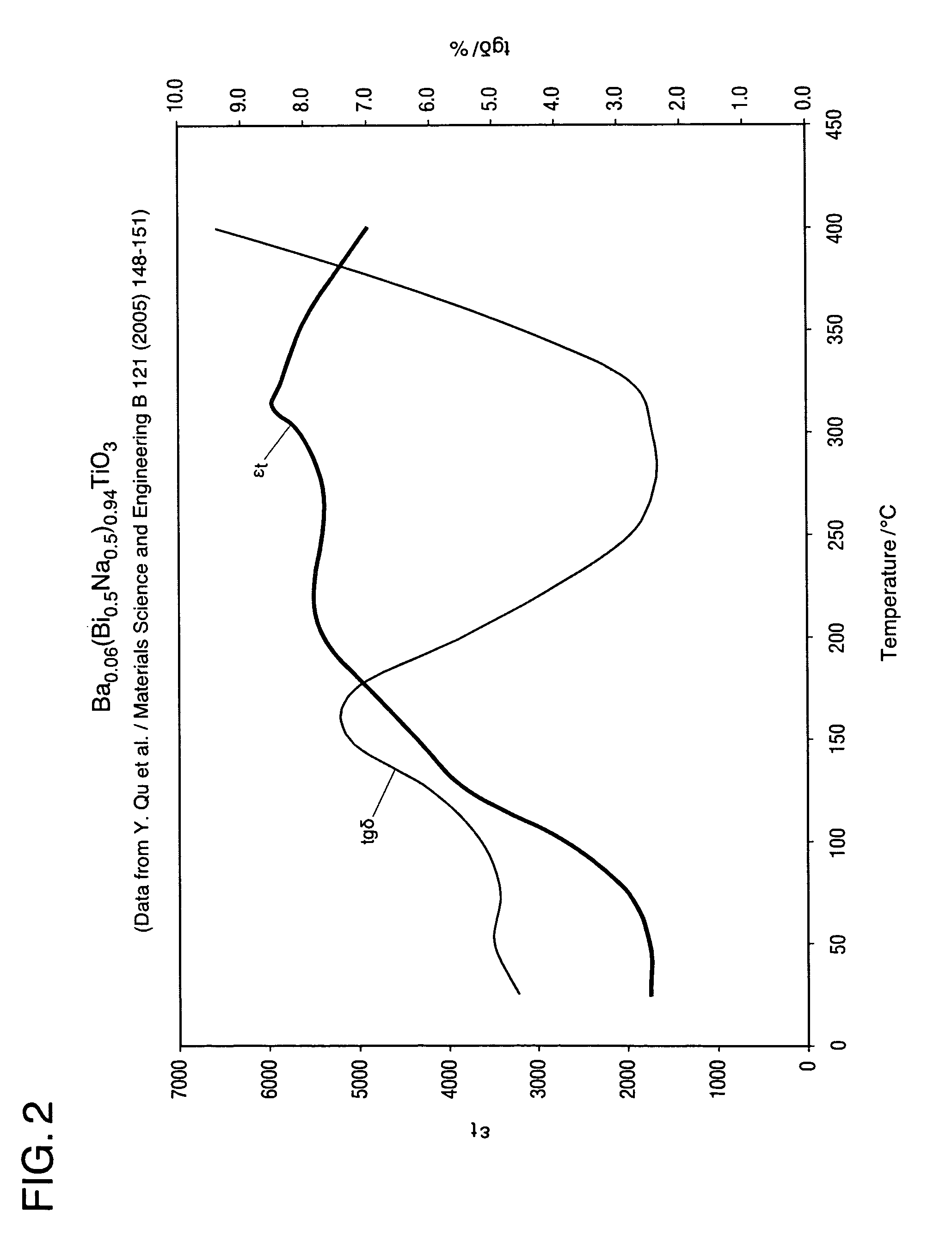
A bismuth sodium titanate (Bi0.5Na0.5TiO3) base material is modified by the partial substitution of aliovalent A-site cations such as barium (as BaO) or strontium (as SrO), as well as certain b-site donor / acceptor dopants and sintering aids to form a multi-phase system, much like known “core / shell” X7R dielectrics based solely on BaTiO3. The resulting ceramic dielectric composition is particularly suitable for producing a multilayer ceramic capacitor (10) that maintains high dielectric constant (and thus the capability of maintaining high capacitance) over a broad temperature range of from about 150° C. to about 300° C. Such capacitors (10) are appropriate for high temperature power electronics applications in fields such as down-hole oil and gas well drilling.
Owner:FERRO CORP
Multi-phased personal care composition
The present invention is a multi-phased personal care composition that contains at least two visually distinct phases. At least one visually distinct phase contains a cleansing phase and at least one visually distinct phase contains a colorant. The colorant is substantially free of Barium and / or Aluminum. The phases are packaged in physical contact with one another
Owner:THE PROCTER & GAMBLE COMPANY
Composite lithium-base grease and method for making same
The present invention provides composite lithium-based lubricating grease and a preparation method thereof. The composite lithium-based lubricating grease consists of thickener, base oil and additive. The thickener consists of 12-hydroxy stearic acid lithium dibasic acid and / or lithium borate, and the mol ratio is 1 ®U 0.1 to 1 ®U 0.1to 1. The base oil is mineral oil or poly-alpha-olefin synthesis oil. The additive consists of organic amine compound antioxidant, benzotriazole and barium petroleum sulfonate or barium dinonylnaphthalene sulfonate anti-corrosion additive and rust protection agent, dialkyl dithiocarbamate or dibenzyl disulfide and sulfurized olefin cottonseed oil extreme pressure antiwear additive and nanometer copper powder repairing additive. The contents of the thickener and the base oil are respectively 6 percent to 18 percent and 82 percent to 94 percent according to the weight percentage. The lubricating grease has the multi-effect performances of high dripping point, good high-temperature performance and low-temperature performance, chemical invariability, colloid invariability, antiwear extreme pressure performance etc., and especially has the repairing function towards a damaged bearing.
Owner:BC P INC CHINA NAT PETROLEUM CORP +1
Oxide solid electrolyte material, and preparation method and application thereof
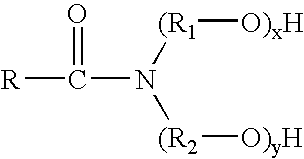
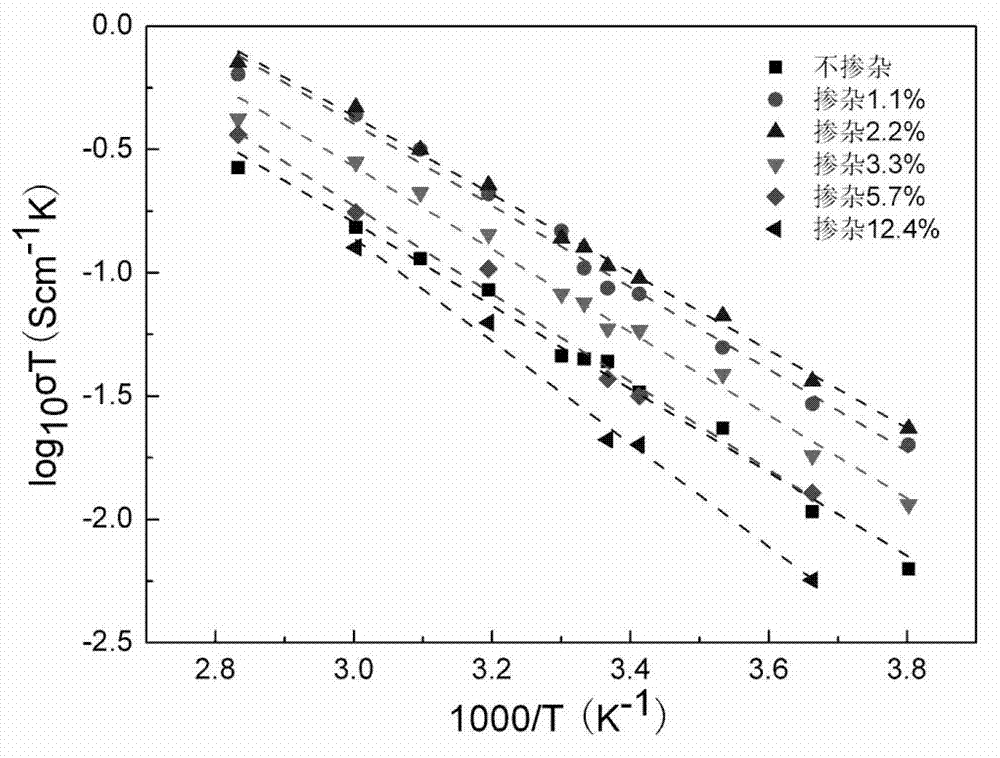
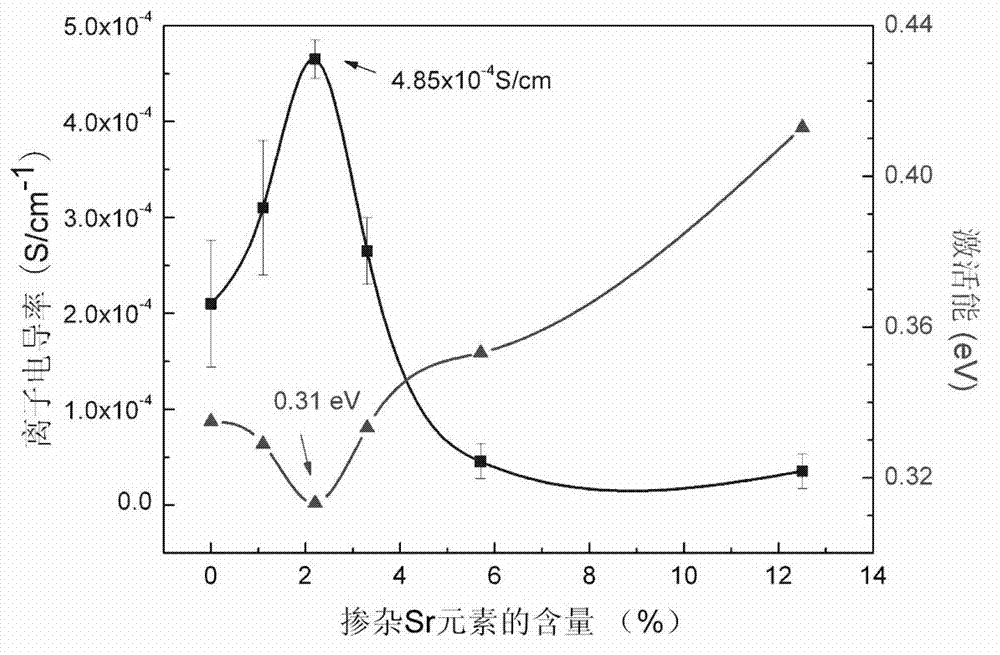
The invention discloses a lithium-lanthanum-zirconium-oxygen-base oxide solid electrolyte material and a preparation method thereof. The solid electrolyte material is composed of a base material and a doping element, wherein the base material is a lithium-lanthanum-zirconium-oxygen solid electrolyte of which the chemical formula is Li7La3Zr2O12, the doping element is selected from at least one of calcium, strontium, barium and germanium, and the weight of the doping element does not exceed 15% of that of the base material. The preparation method comprises the following steps: a lithium source compound, a lanthanum source compound, a zirconium source compound and a doping element compound are evenly mixed, calcined and sintered to obtain the lithium-lanthanum-zirconium-oxygen-base oxide solid electrolyte material. The lithium-lanthanum-zirconium-oxygen-base oxide solid electrolyte material can be prepared under the conditions of wide sources of doping element, lower sintering temperature and shorter sintering time, and the total room-temperature ionic conductivity is greater than 1*10<-4>S / cm; and thus, the material has important application value.
Owner:TSINGHUA UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com