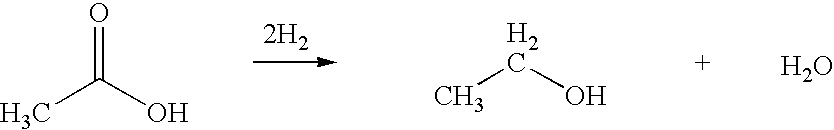Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
8569 results about "Palladium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs). These have similar chemical properties, but palladium has the lowest melting point and is the least dense of them.
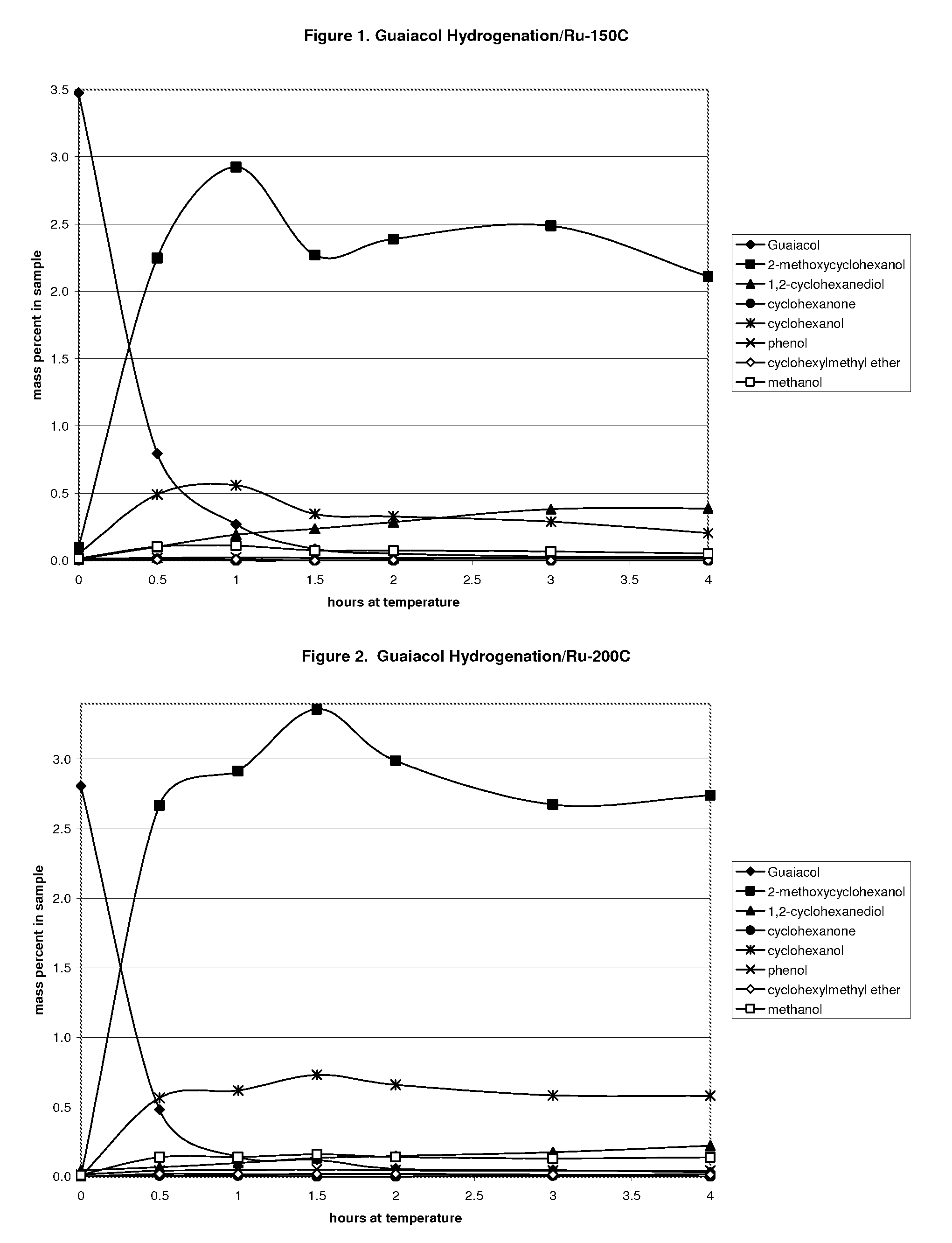
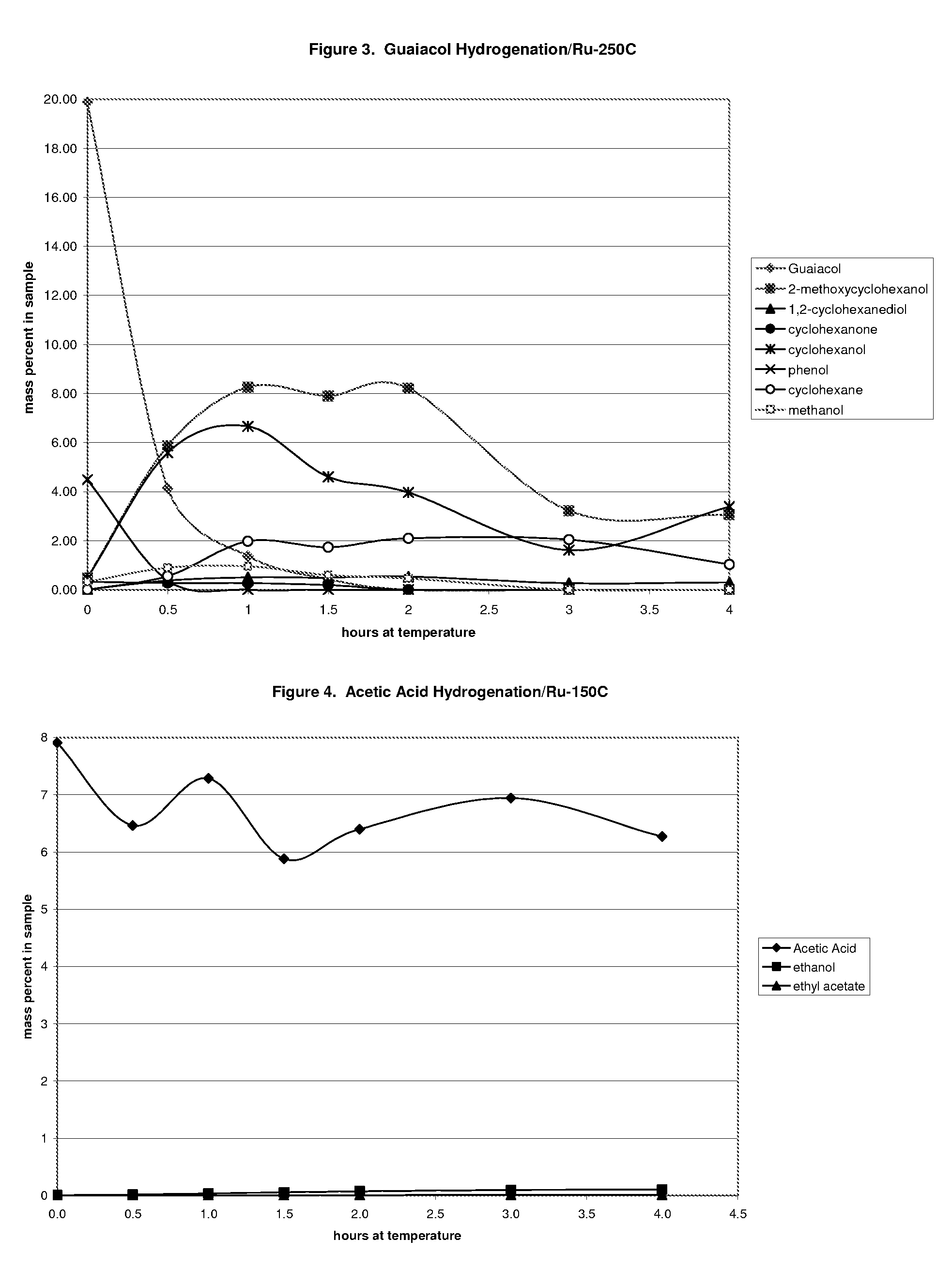
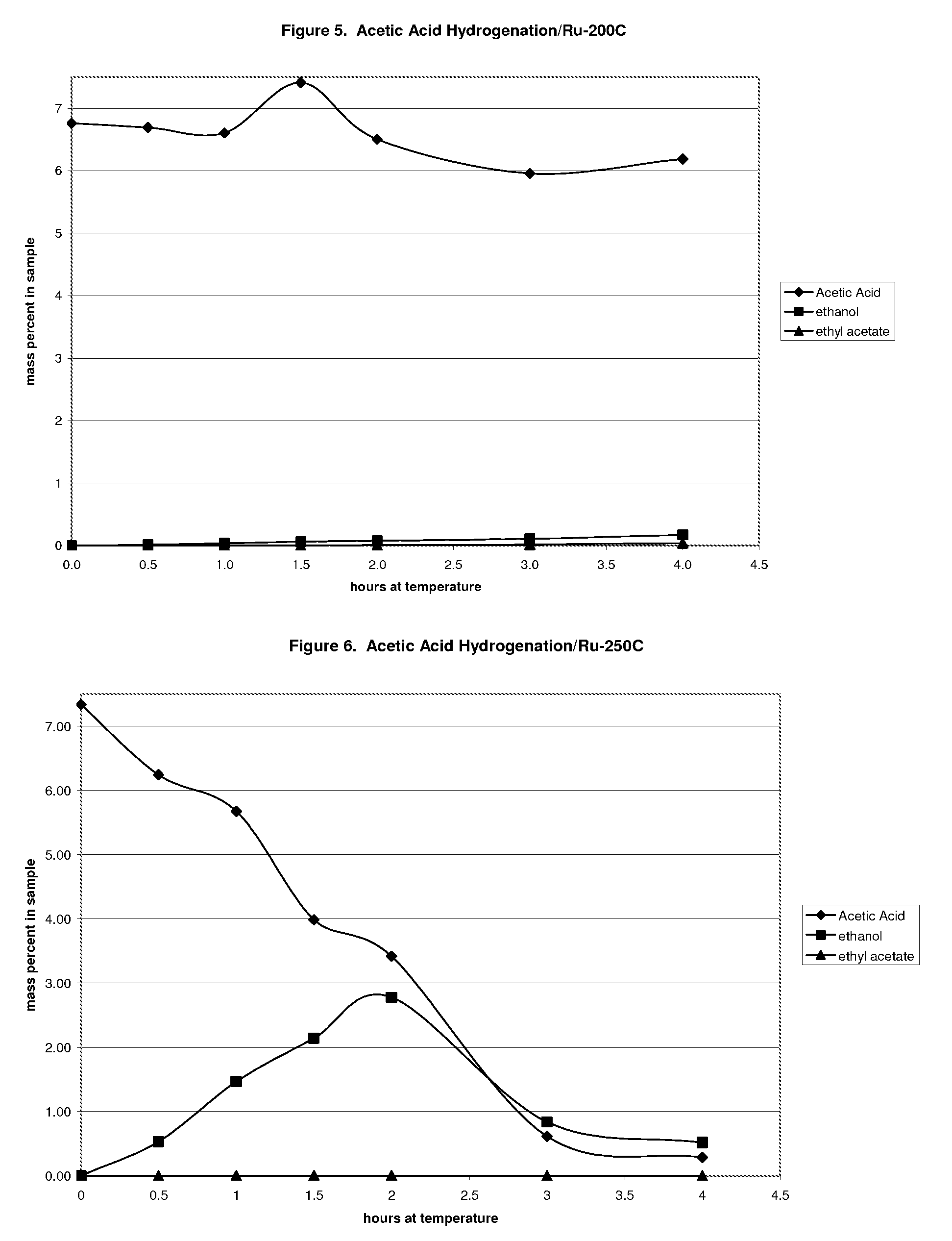
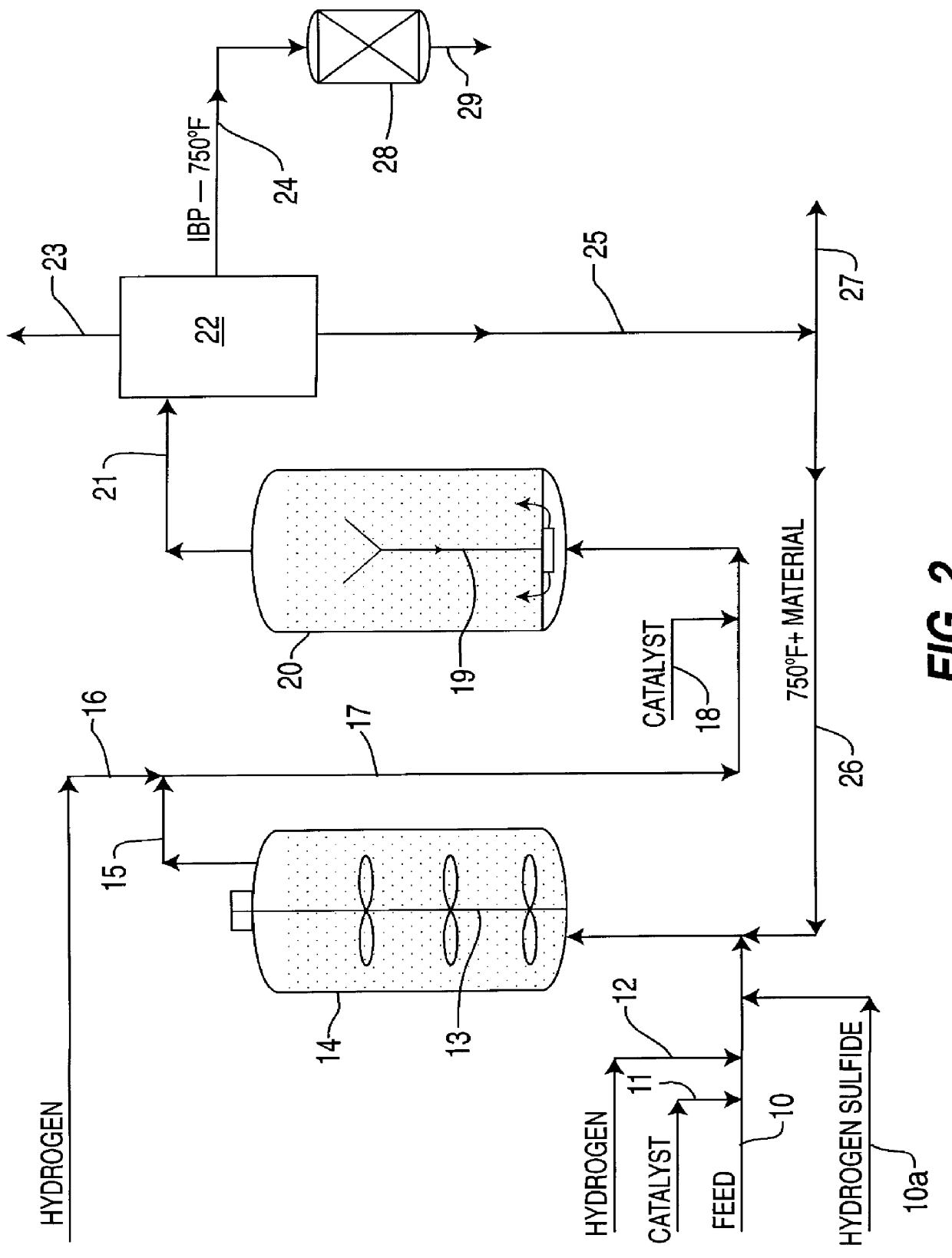
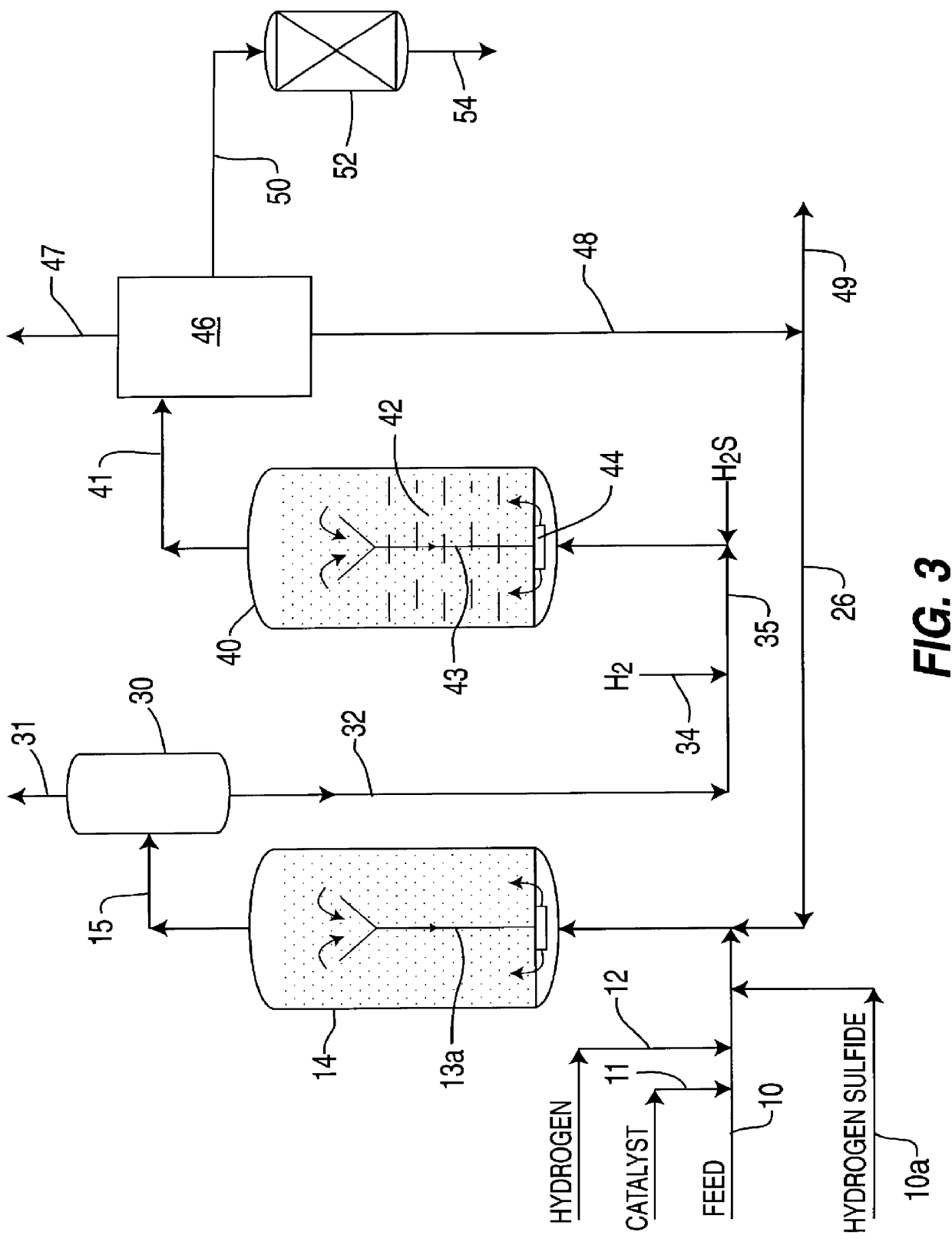
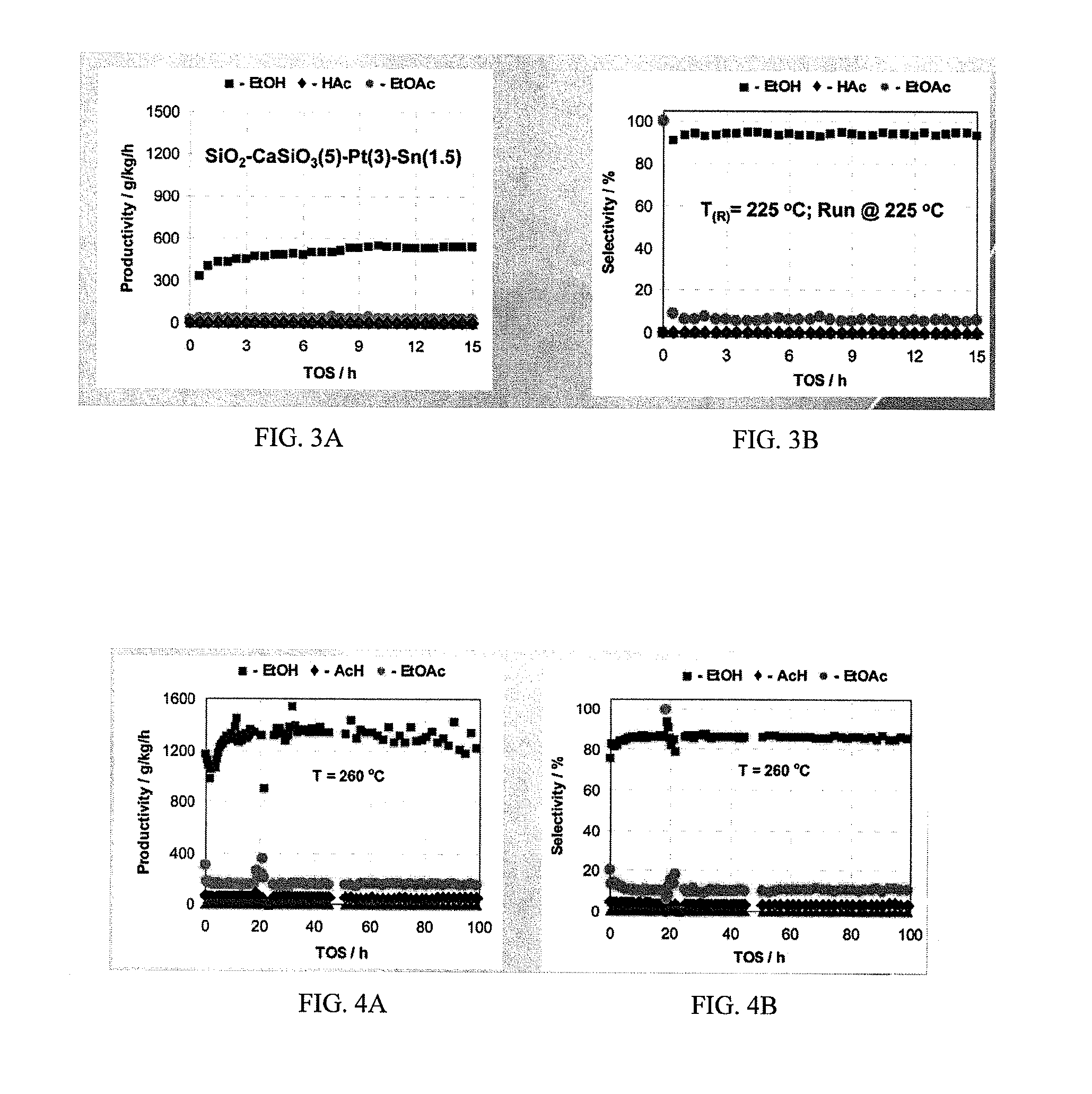
Ethanol production from acetic acid utilizing a cobalt catalyst
InactiveUS7608744B1High selectivityHigh yieldOrganic compound preparationOxygen compounds preparation by reductionAcetic acidPlatinum
A process for the selective production of ethanol by vapor phase reaction of acetic acid over a hydrogenating catalyst composition to form ethanol is disclosed and claimed. In an embodiment of this invention reaction of acetic acid and hydrogen over either cobalt and palladium supported on graphite or cobalt and platinum supported on silica selectively produces ethanol in a vapor phase at a temperature of about 250° C.
Owner:CELANESE INT CORP
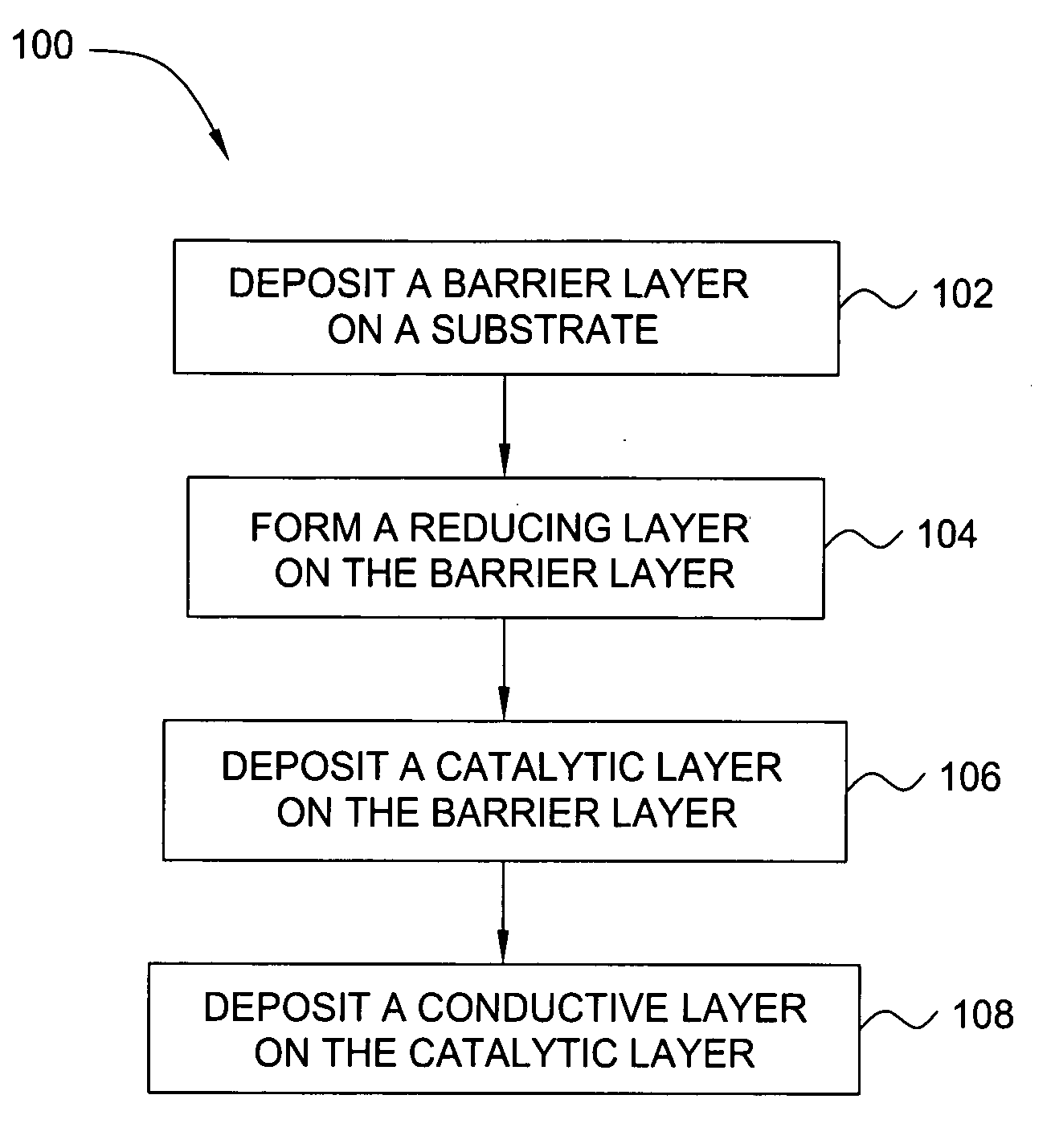
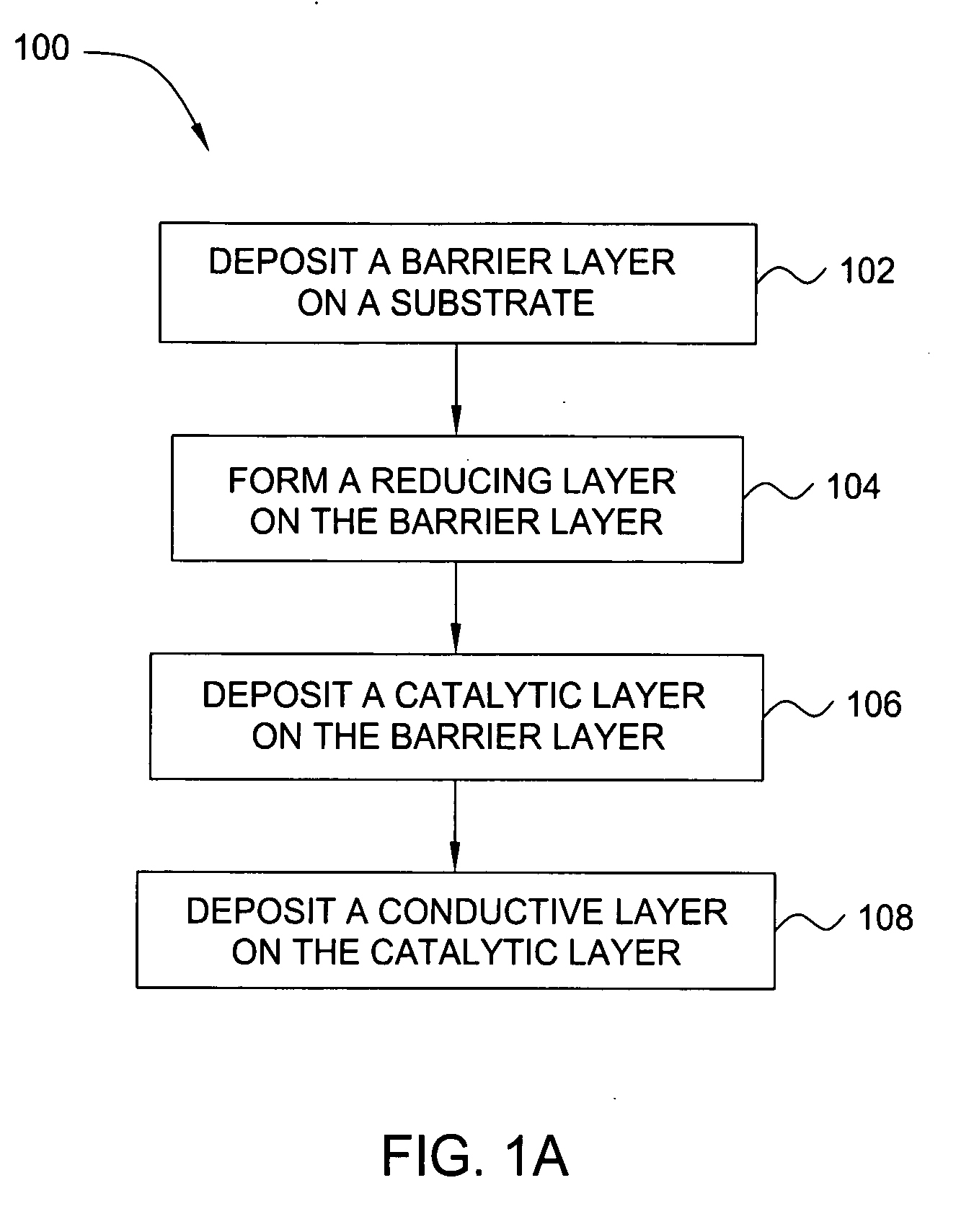
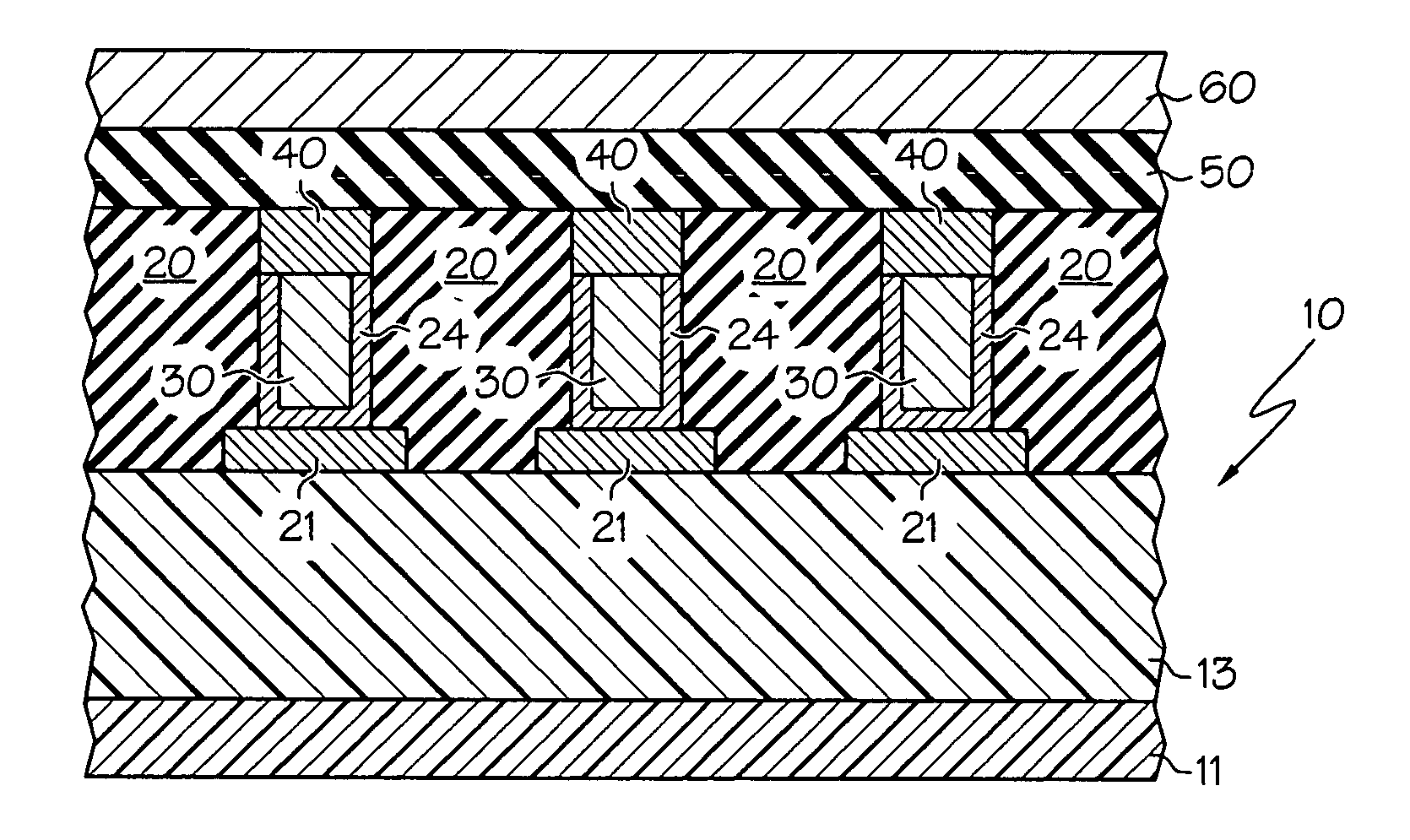
Deposition of an intermediate catalytic layer on a barrier layer for copper metallization
InactiveUS20060240187A1Solid-state devicesSemiconductor/solid-state device manufacturingIridiumSilanes
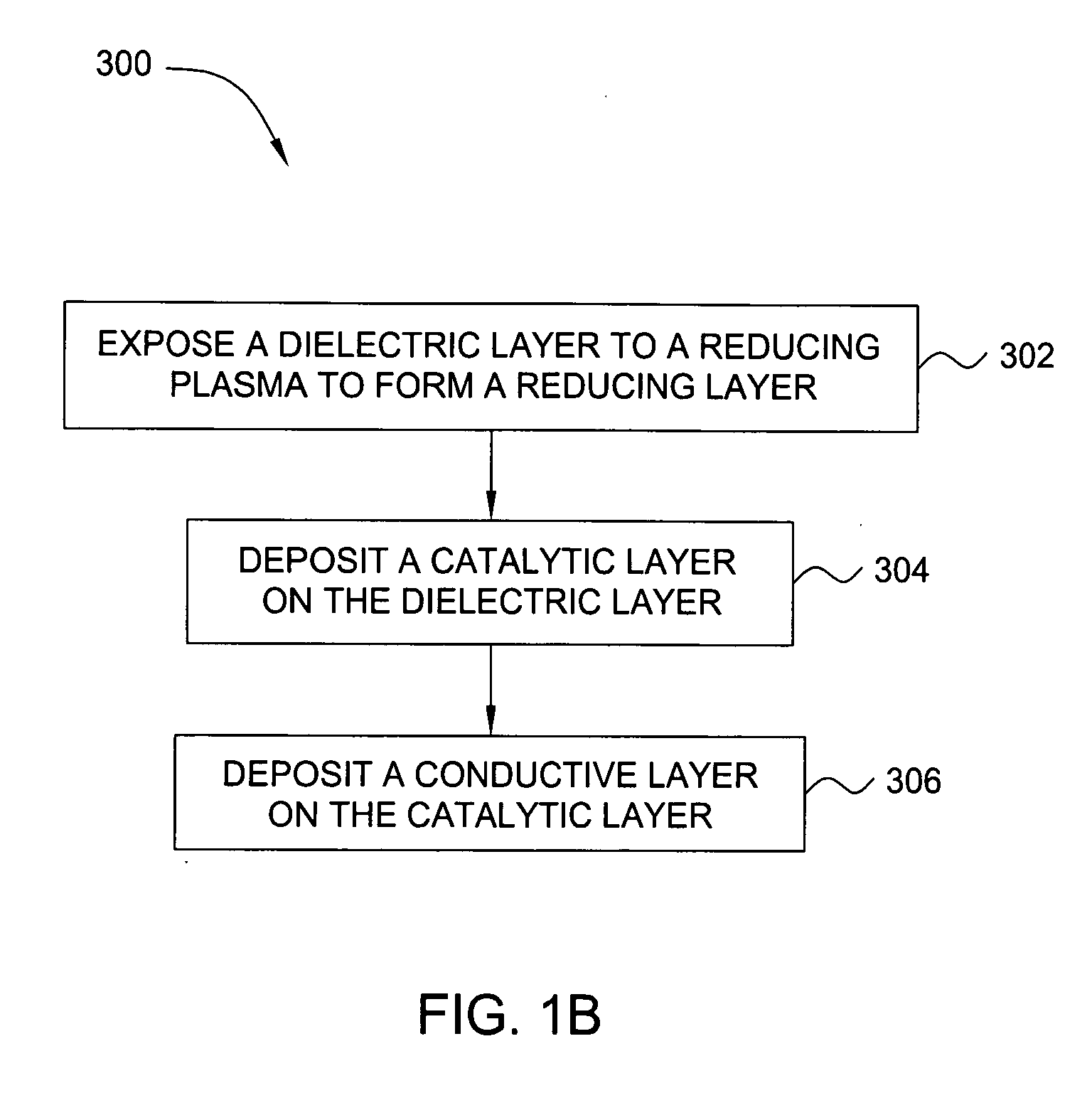
In one embodiment, a method for depositing a conductive material on a substrate is provided which includes exposing a substrate containing a barrier layer to a volatile reducing precursor to form a reducing layer during a soak process, exposing the reducing layer to a catalytic-metal precursor to deposit a catalytic metal-containing layer on the barrier layer, and depositing a conductive layer (e.g., copper) on the catalytic metal-containing layer. The volatile reducing precursor may include phosphine, diborane, silane, a plasma thereof, or a combination thereof and be exposed to the substrate for a time period within a range from about 1 second to about 30 seconds during the soak process. The catalytic metal-containing layer may contain ruthenium, cobalt, rhodium, iridium, nickel, palladium, platinum, silver, or copper. In one example, the catalytic metal-containing layer is deposited by a vapor deposition process utilizing ruthenium tetroxide formed by an in situ process.
Owner:APPLIED MATERIALS INC
Method of electroless plating copper on nitride barrier
InactiveUS6436816B1Insulating substrate metal adhesion improvementSolid-state devicesCopper platingElectroless deposition
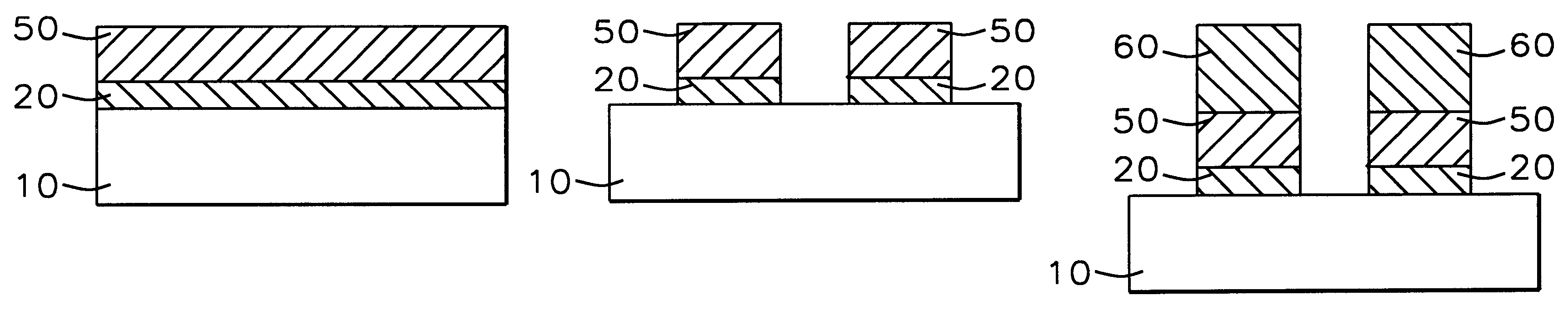
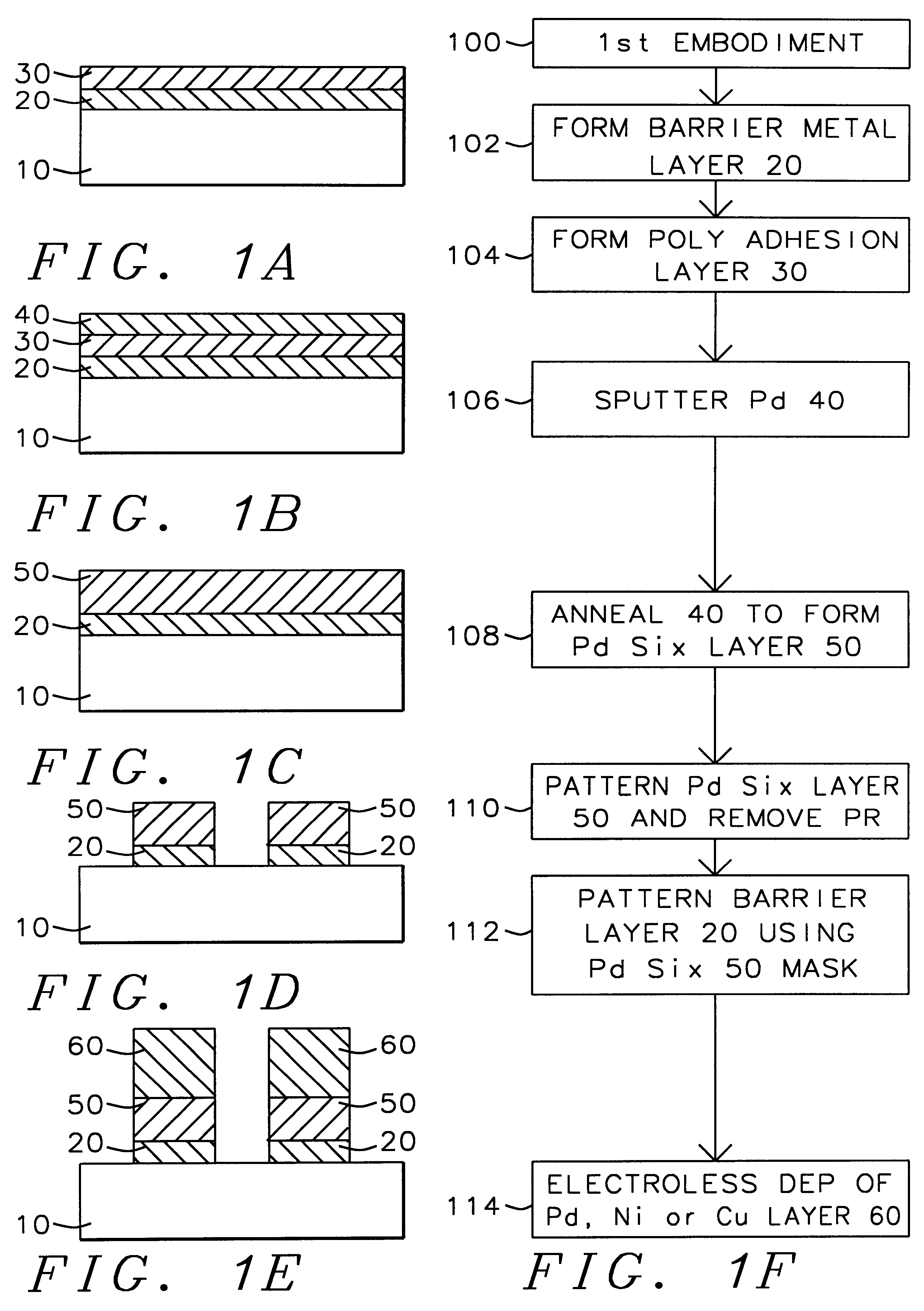
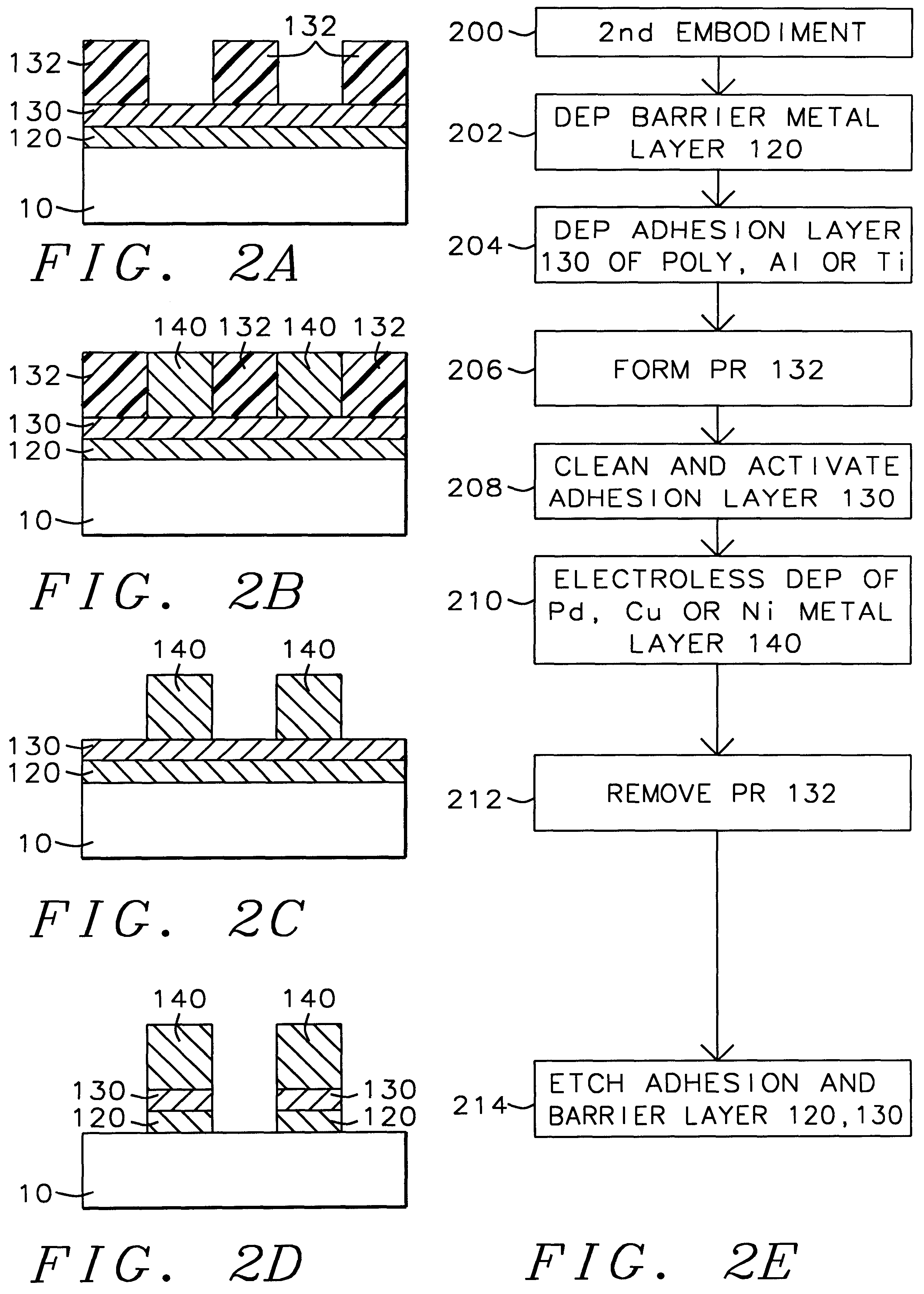
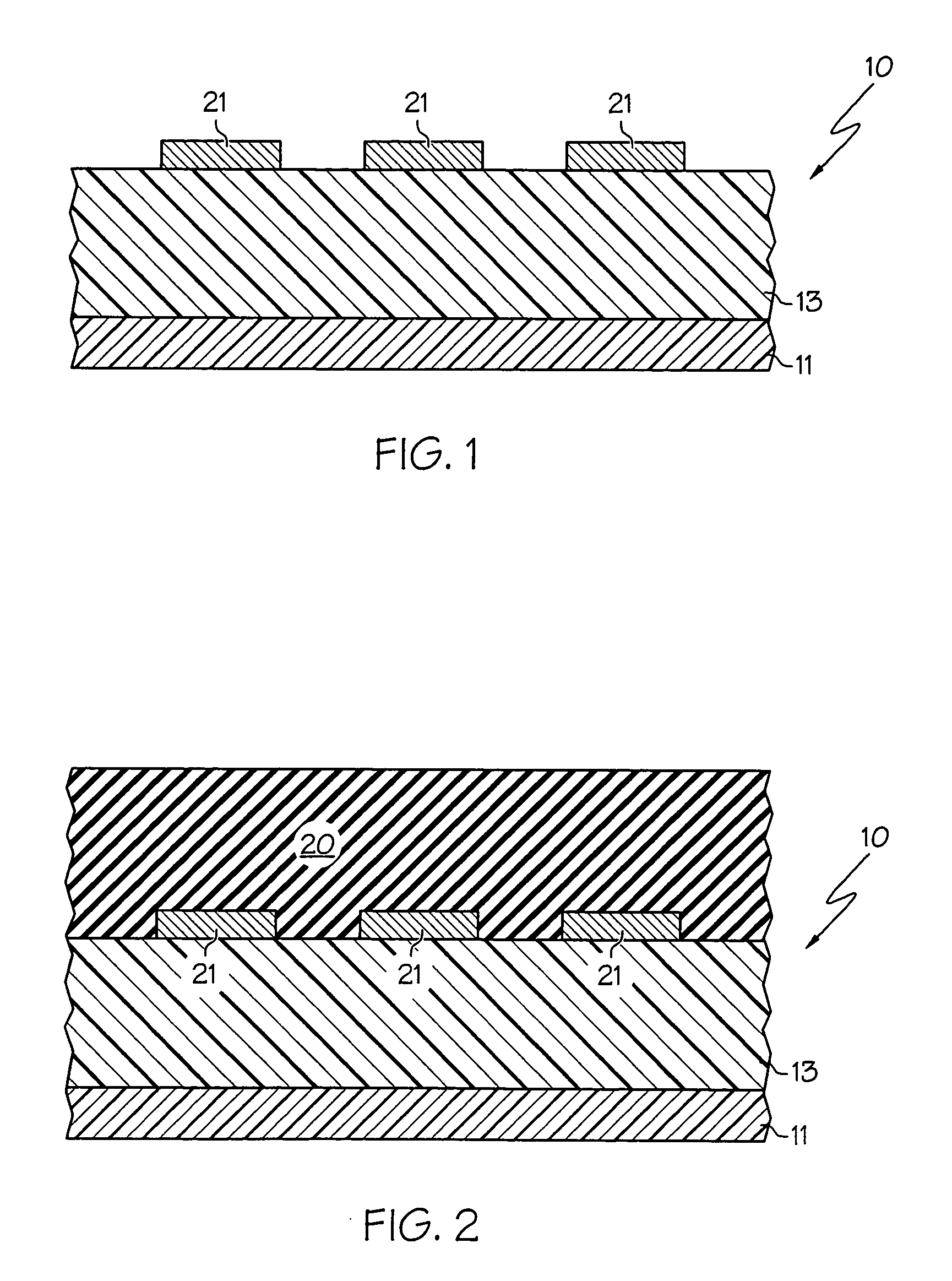
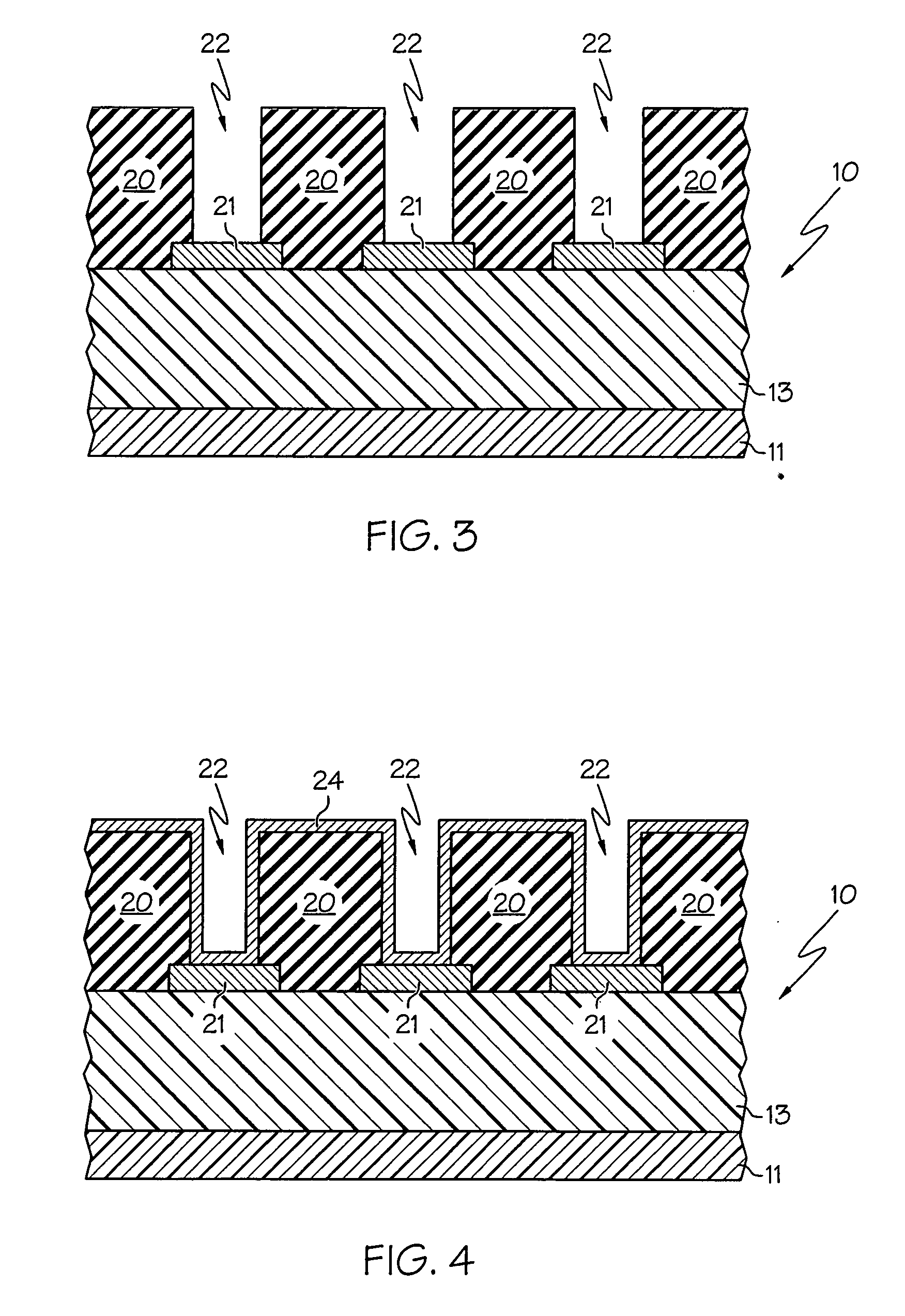
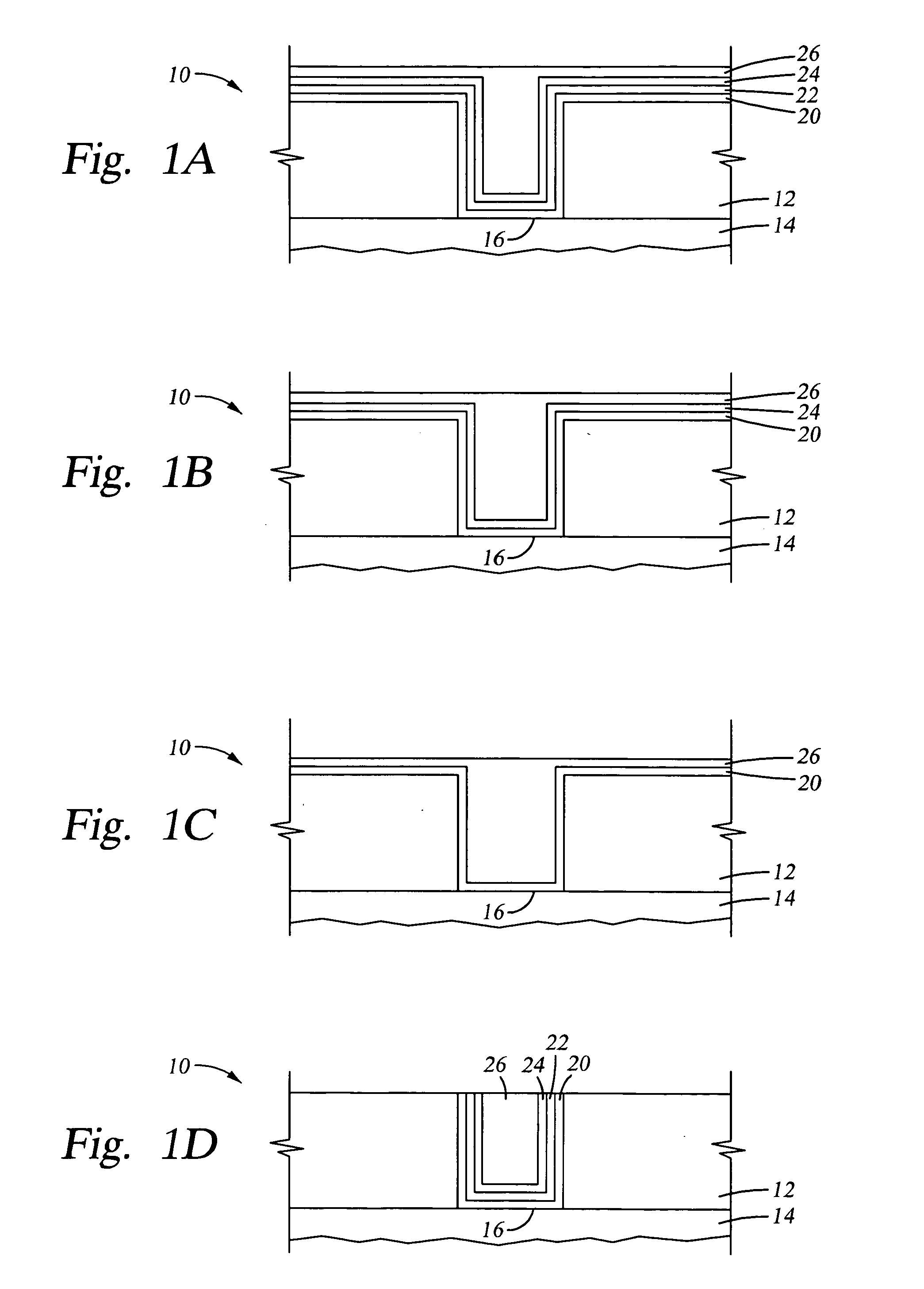
A method with three embodiments of manufacturing metal lines and solder bumps using electroless deposition techniques. The first embodiment uses a PdSix seed layer 50 for electroless deposition. The PdSix layer 50 does not require activation. A metal line is formed on a barrier layer 20 and an adhesion layer 30. A Palladium silicide seed layer 50 is then formed and patterned. Ni, Pd or Cu is electroless deposited over the Palladium silicide layer 50 to form a metal line. The second embodiment selectively electrolessly deposits metal 140 over an Adhesion layer 130 composed of Poly Si, Al, or Ti. A photoresist pattern 132 is formed over the adhesion layer. A metal layer 140 of Cu or Ni is electrolessly deposited over the adhesion layer. The photoresist layer 132 is removed and the exposed portion of the adhesion layer 130 and the underlying barrier metal layer 120 are etched thereby forming a metal line. The third embodiment electroless deposits metal over a metal barrier layer that is roughen by chemical mechanical polishing. A solder bump is formed using an electroless deposition of Cu or Ni by: depositing an Al layer 220 and a barrier metal layer 230 over a substrate 10. The barrier layer 230 is polished and activated. Next, the aluminum layer 220 and the barrier metal layer 230 are patterned. A metal layer 240 is electroless deposited. Next a solder bump 250 is formed over the electroless metal layer 240.
Owner:TAIWAN SEMICON MFG CO LTD
Electroless plating of metal caps for chalcogenide-based memory devices
InactiveUS20060094236A1Good electrical contactSemiconductor/solid-state device manufacturingPlatinumSulfur
A method of forming a metal cap over a conductive interconnect in a chalcogenide-based memory device is provided and includes, forming a layer of a first conductive material over a substrate, depositing an insulating layer over the first conductive material and the substrate, forming an opening in the insulating layer to expose at least a portion of the first conductive material, depositing a second conductive material over the insulating layer and within the opening, removing portions of the second conductive material to form a conductive area within the opening, recessing the conductive area within the opening to a level below an upper surface of the insulating layer, forming a cap of a third conductive material over the recessed conductive area within the opening, the third conductive material selected from the group consisting of cobalt, silver, gold, copper, nickel, palladium, platinum, and alloys thereof, depositing a stack of a chalcogenide based memory cell material over the cap, and depositing a conductive material over the chalcogenide stack.
Owner:MICRON TECH INC
Electroless deposition processes and compositions for forming interconnects
InactiveUS20060252252A1Material nanotechnologySemiconductor/solid-state device detailsHydrogen fluorideTungstate
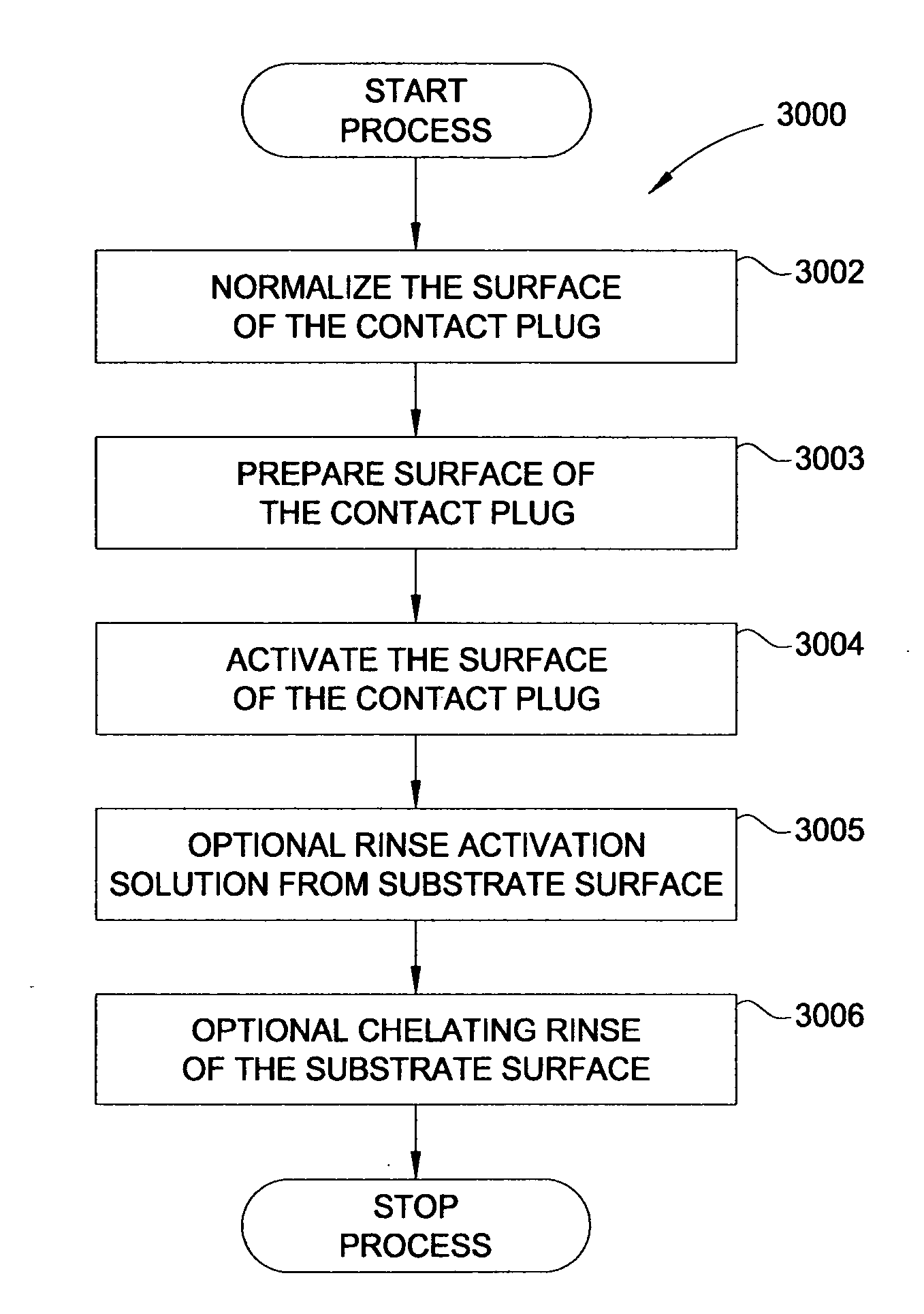
In one embodiment, a method for depositing a material on a substrate is provided which includes positioning a substrate containing a contact within a process chamber, exposing the substrate to at least one pretreatment step and depositing a fill the contact vias by an electroless deposition process. The pretreatment step contains multiple processes for exposing the substrate to a wet-clean solution, a hydrogen fluoride solution, a tungstate solution, a palladium activation solution, an acidic rinse solution, a complexing agent solution or combinations thereof. Generally, the HARC via contains a tungsten oxide surface and the shallow contact via may contain a tungsten silicide surface. In some example, the substrate is pretreated such that both vias are filled at substantially the same time by a nickel-containing material through an electroless deposition process.
Owner:APPLIED MATERIALS INC
Electroless deposition apparatus
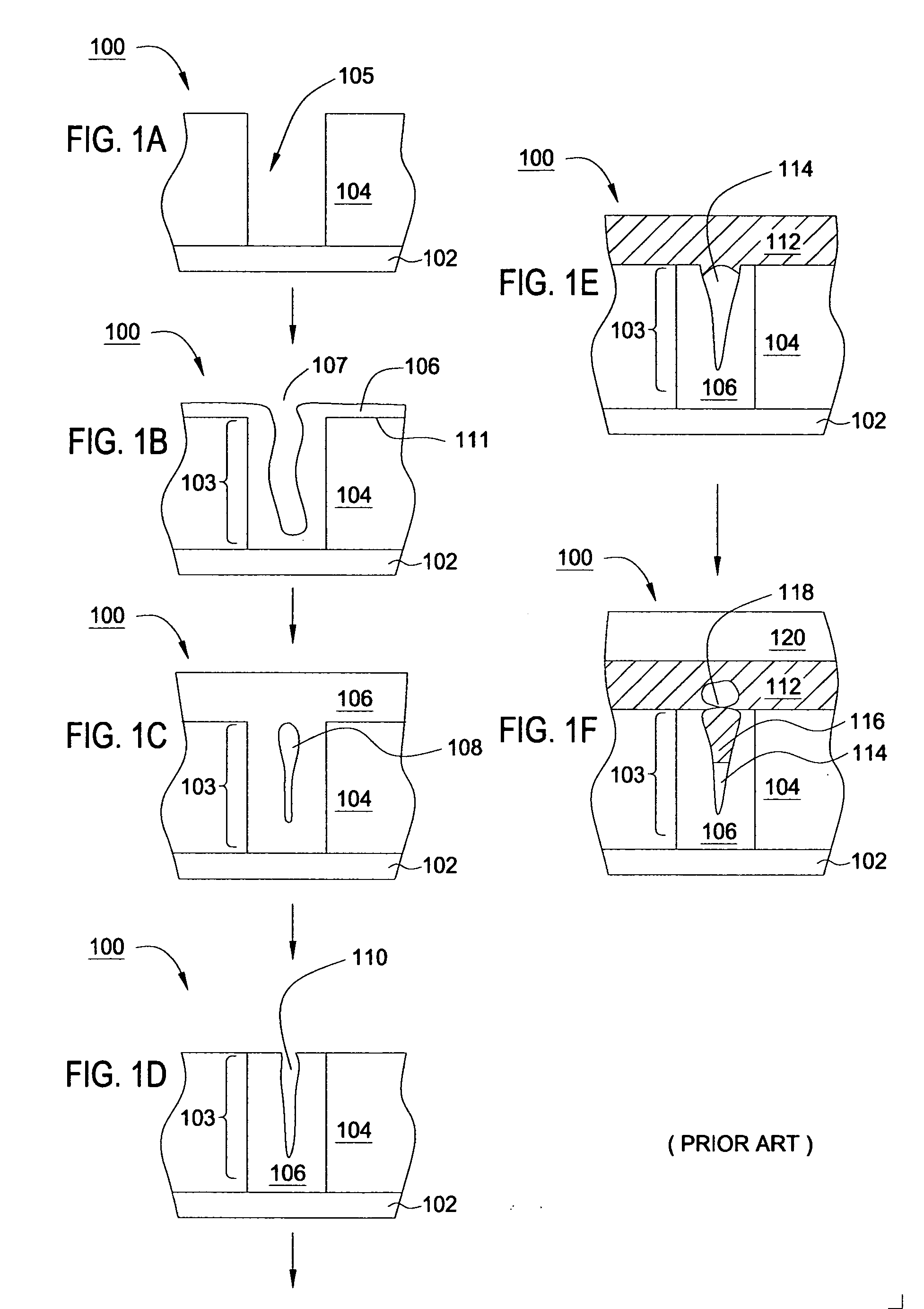
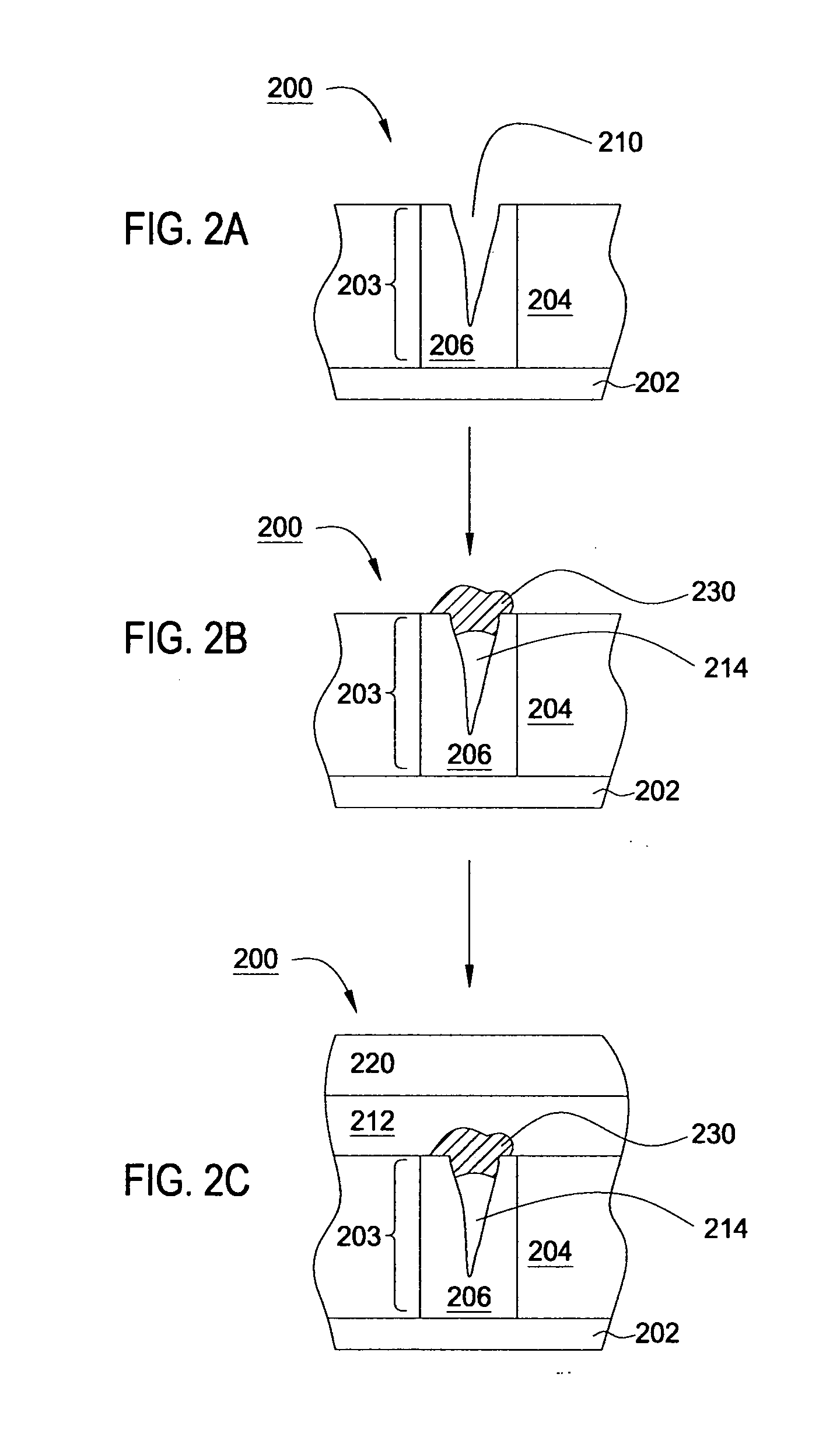
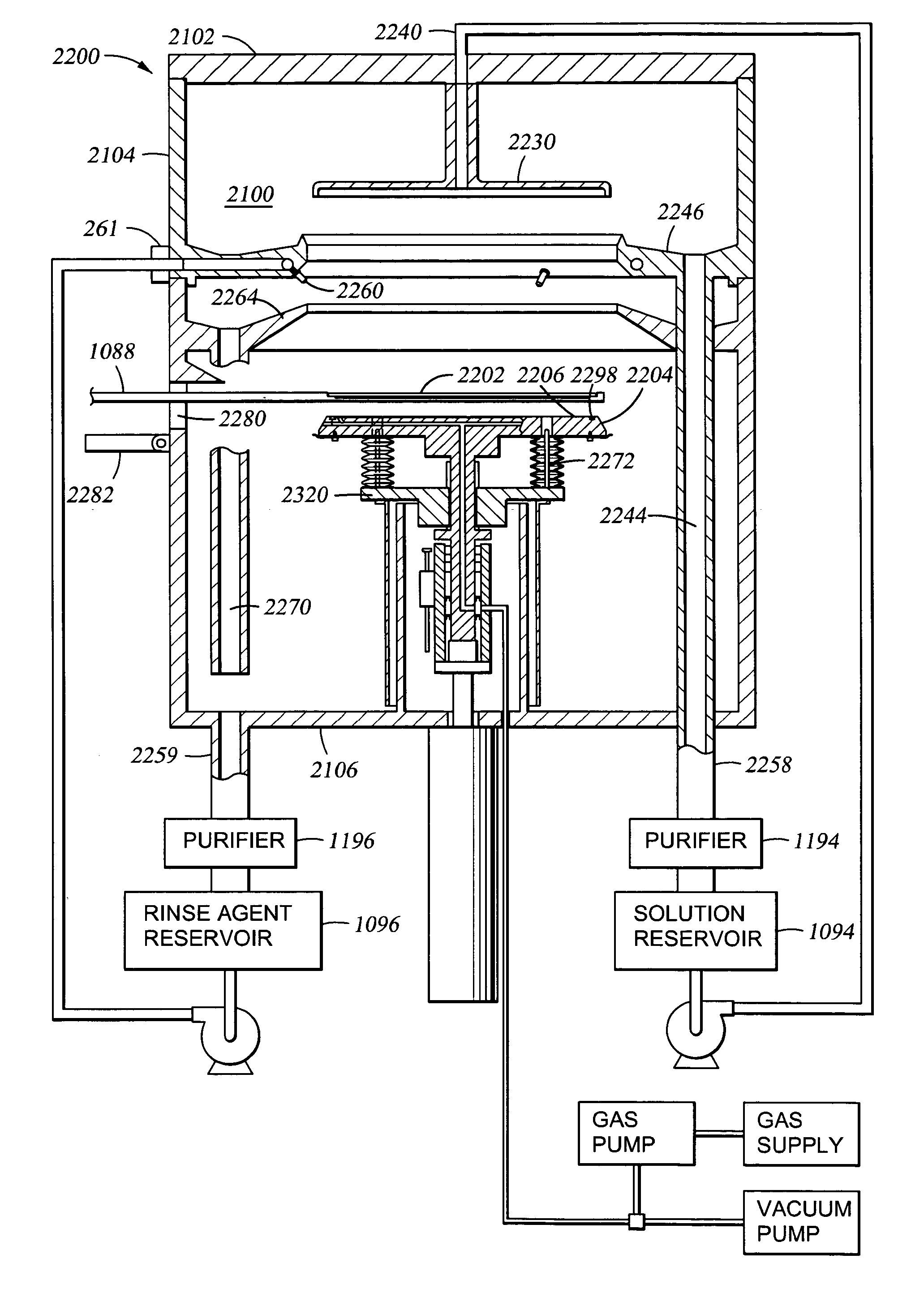
An apparatus and a method of depositing a catalytic layer comprising at least one metal selected from the group consisting of noble metals, semi-noble metals, alloys thereof, and combinations thereof in sub-micron features formed on a substrate. Examples of noble metals include palladium and platinum. Examples of semi-noble metals include cobalt, nickel, and tungsten. The catalytic layer may be deposited by electroless deposition, electroplating, or chemical vapor deposition. In one embodiment, the catalytic layer may be deposited in the feature to act as a barrier layer to a subsequently deposited conductive material. In another embodiment, the catalytic layer may be deposited over a barrier layer. In yet another embodiment, the catalytic layer may be deposited over a seed layer deposited over the barrier layer to act as a “patch” of any discontinuities in the seed layer. Once the catalytic layer has been deposited, a conductive material, such as copper, may be deposited over the catalytic layer. In one embodiment, the conductive material is deposited over the catalytic layer by electroless deposition. In another embodiment, the conductive material is deposited over the catalytic layer by electroless deposition followed by electroplating or followed by chemical vapor deposition. In still another embodiment, the conductive material is deposited over the catalytic layer by electroplating or by chemical vapor deposition.
Owner:APPLIED MATERIALS INC
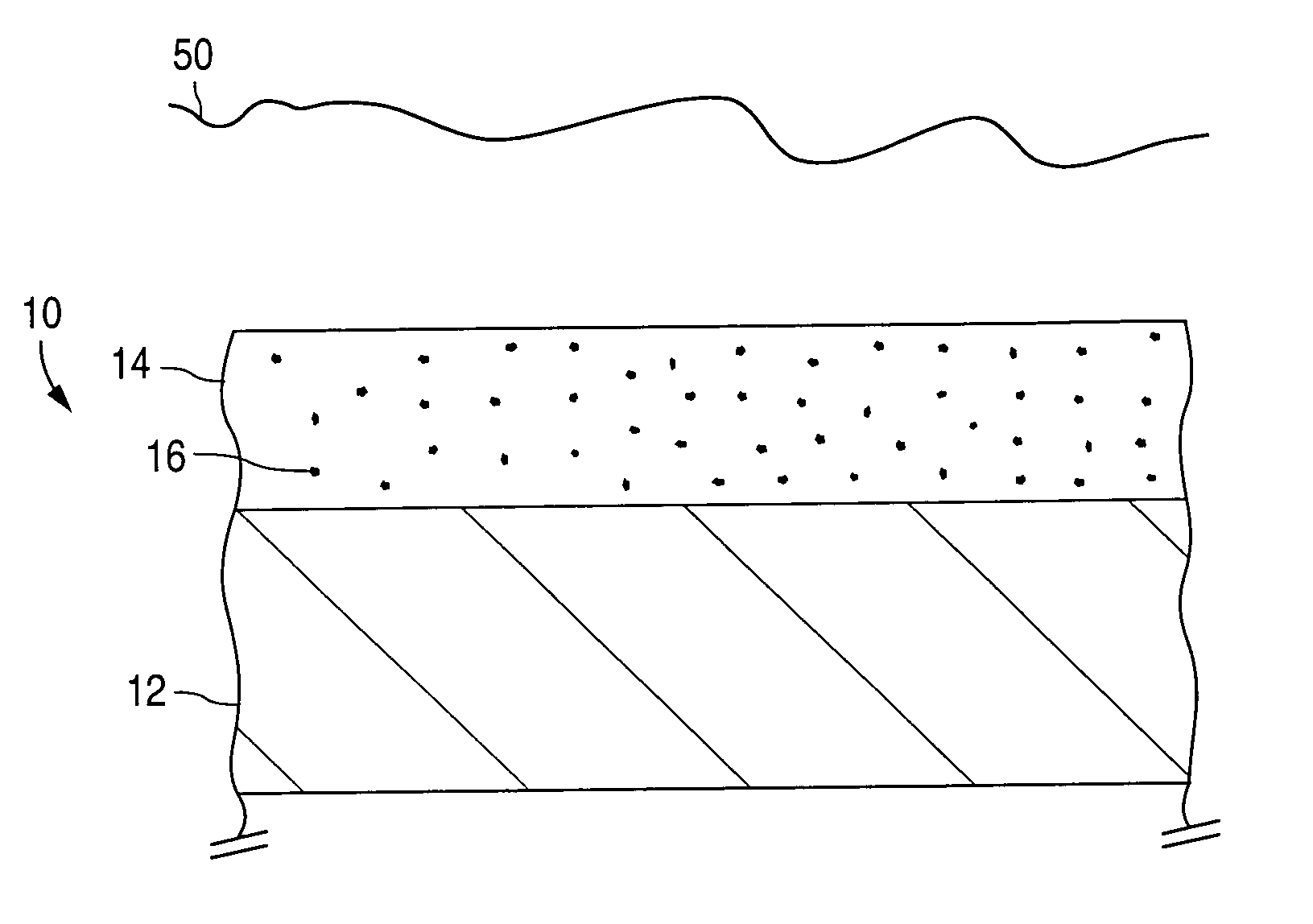
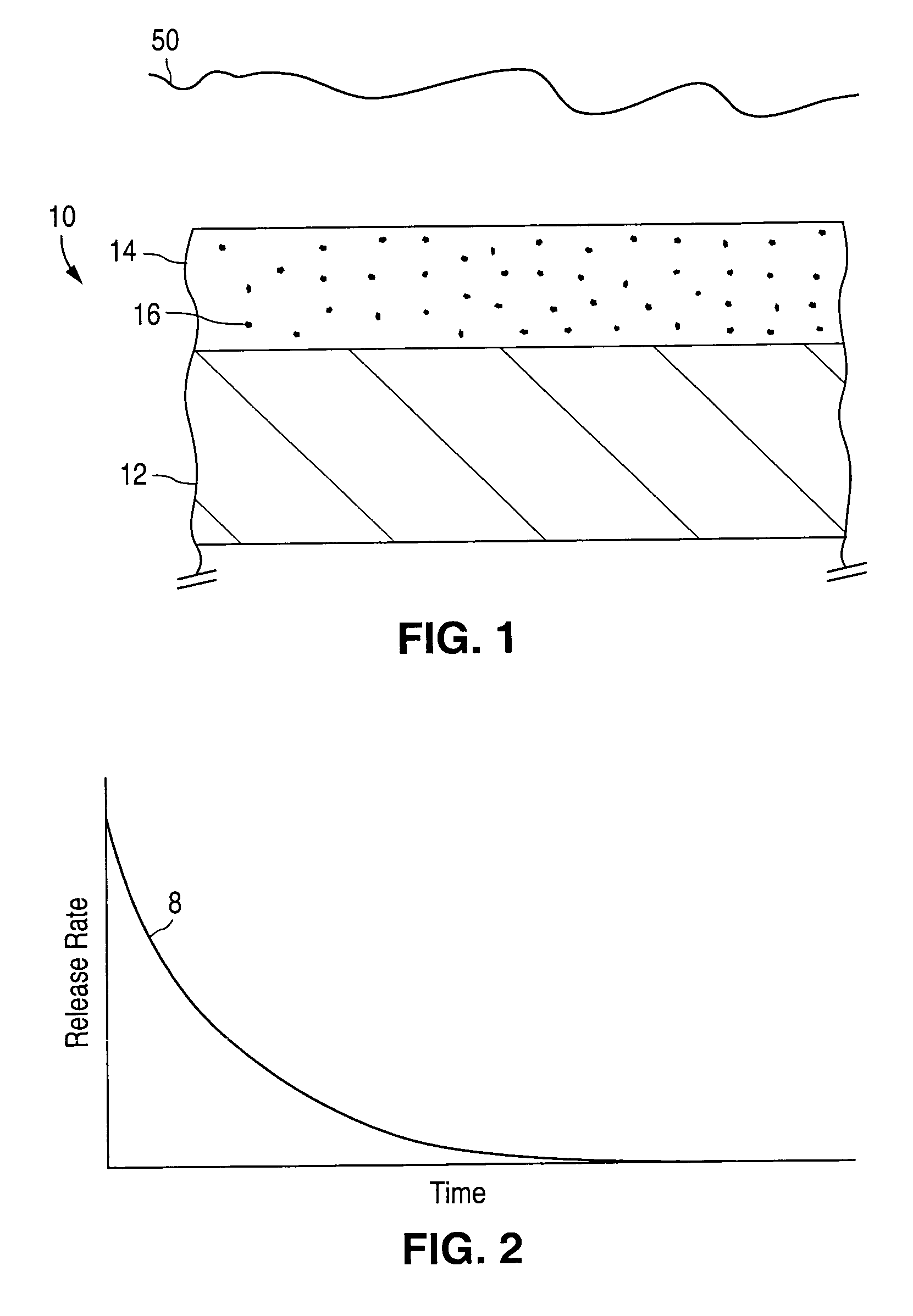
Bioabsorbable brachytherapy device
InactiveUS6575888B2Controlled release rateMinimally shieldsRadioactive preparation formsX-ray/gamma-ray/particle-irradiation therapyBrachytherapy deviceRadiopaque medium
A bioabsorbable brachytherapy device includes a tubular housing with sealed ends and an enclosed radioactive material. The radioactive material includes a radioisotope, such as palladium-103 or iodine-125. The tubular housing is made from a biocompatible and bioabsorbable polymeric material, and is sealed by means such as heat welding or solvent fixing. The device may further include a radiopaque medium and one or more therapeutic drugs.
Owner:FERRING BV
Method for producing alcohols by hydrogenation of carbonyl compounds
InactiveUS6486366B1Less amountHigh strengthSugar derivativesOrganic compound preparationCobaltPt element
A method for preparation of alcohols by catalytic hydrogenation of carbonyl compounds with hydrogen or hydrogen-containing gases in the presence of a hydrogenation catalyst of Raney type, where the catalyst is used in the form of hollow bodies, Preferred as catalytically active components are nickel, cobalt, copper, iron, platinum, palladium or ruthenium.
Owner:DEGUSSA AG
Radiopaque nitinol alloys for medical devices
Owner:ABBOTT CARDIOVASCULAR
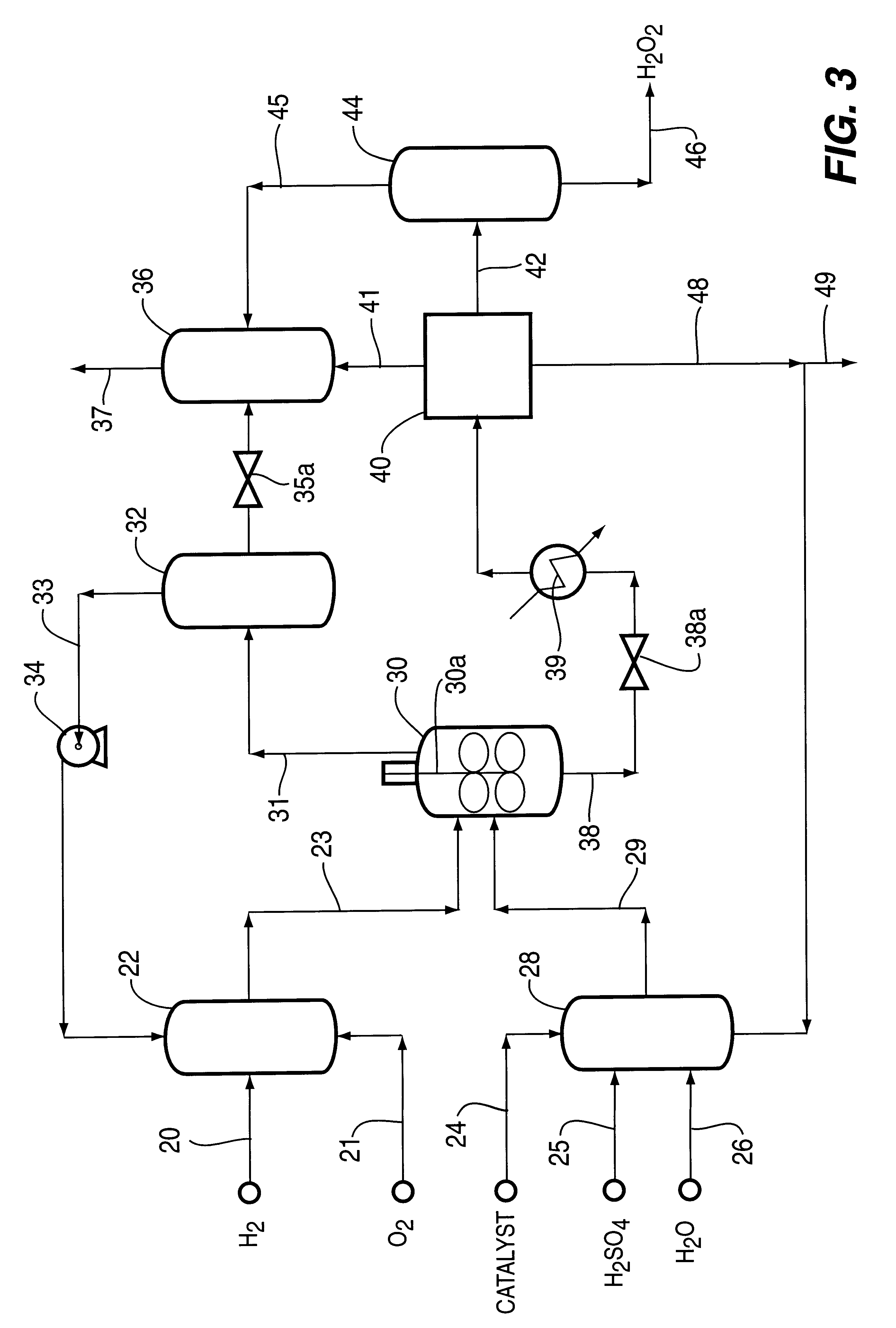
Catalyst and process for direct catalytic production of hydrogen peroxide, (H2O2)
InactiveUS6168775B1High catalytic activityHigh activityHydrogen peroxideCatalyst activation/preparationParticulatesHydrogen
A particulate supported noble metal phase-controlled catalyst material having 5-1000 mum surface area of 50-500 m2 / gm is provided for use in direct catalytic production of hydrogen peroxide (H2O2) product from hydrogen and oxygen-containing feedstreams. The catalyst is made by depositing phase controlled crystals of a noble metal such as palladium on a suitable particulate support material such as carbon black, by utilizing a precursor solution of the metal and a suitable control ionic polymer having molecular weight of 300-8000 such as sodium polyacrylate in a selected metal to polymer molar ratio of 1:0.1 to 1:10, which procedure provides desired phase control of the noble metal atoms to form widely dispersed minute noble metal crystals on the support material. The invention includes methods for making the catalyst, and also a process for utilizing the catalyst to directly produce high yields of hydrogen peroxide (H2O2) product from hydrogen and oxygen-containing gaseous feedstreams.
Owner:BORAL IP HLDG
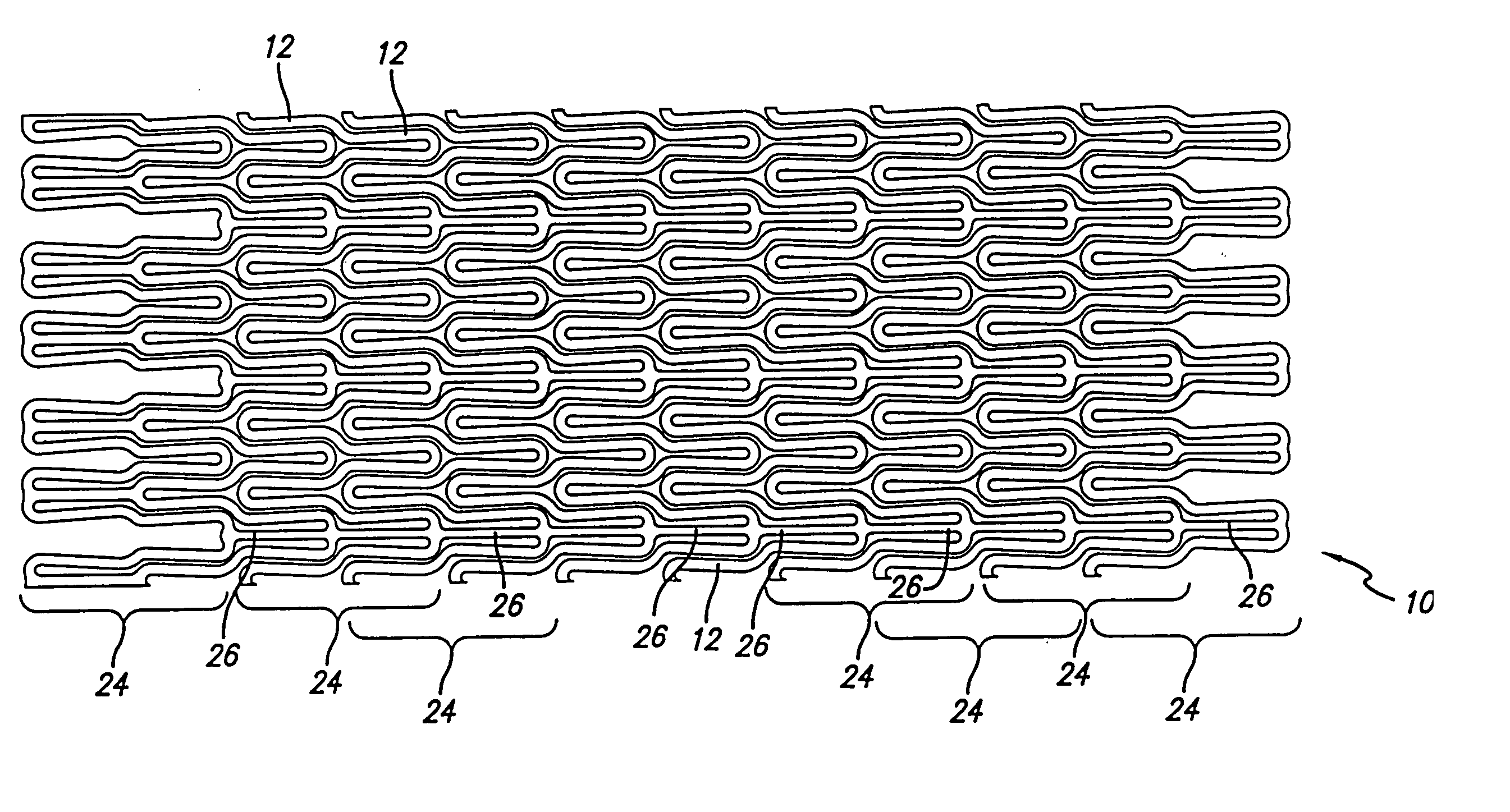
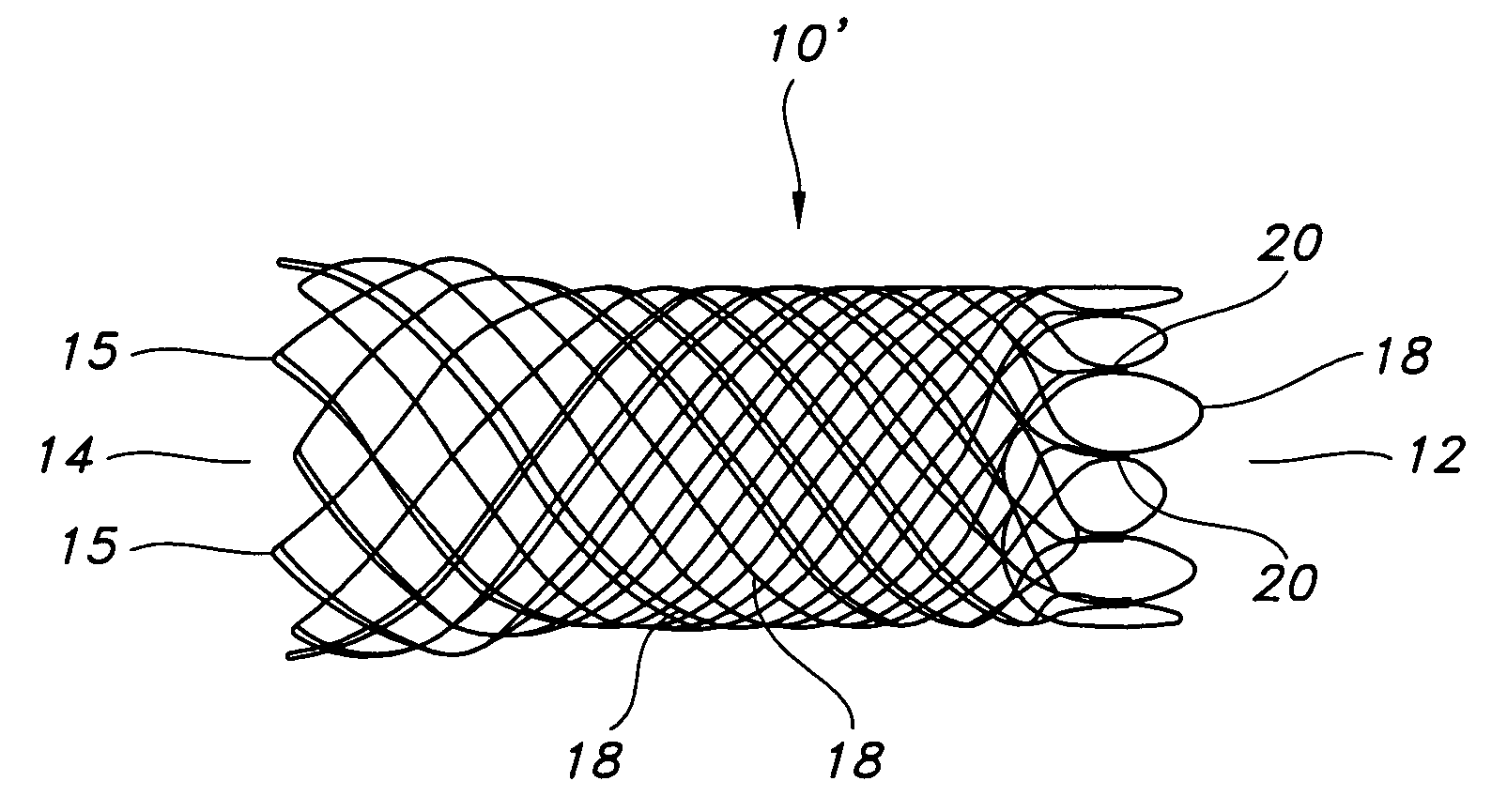
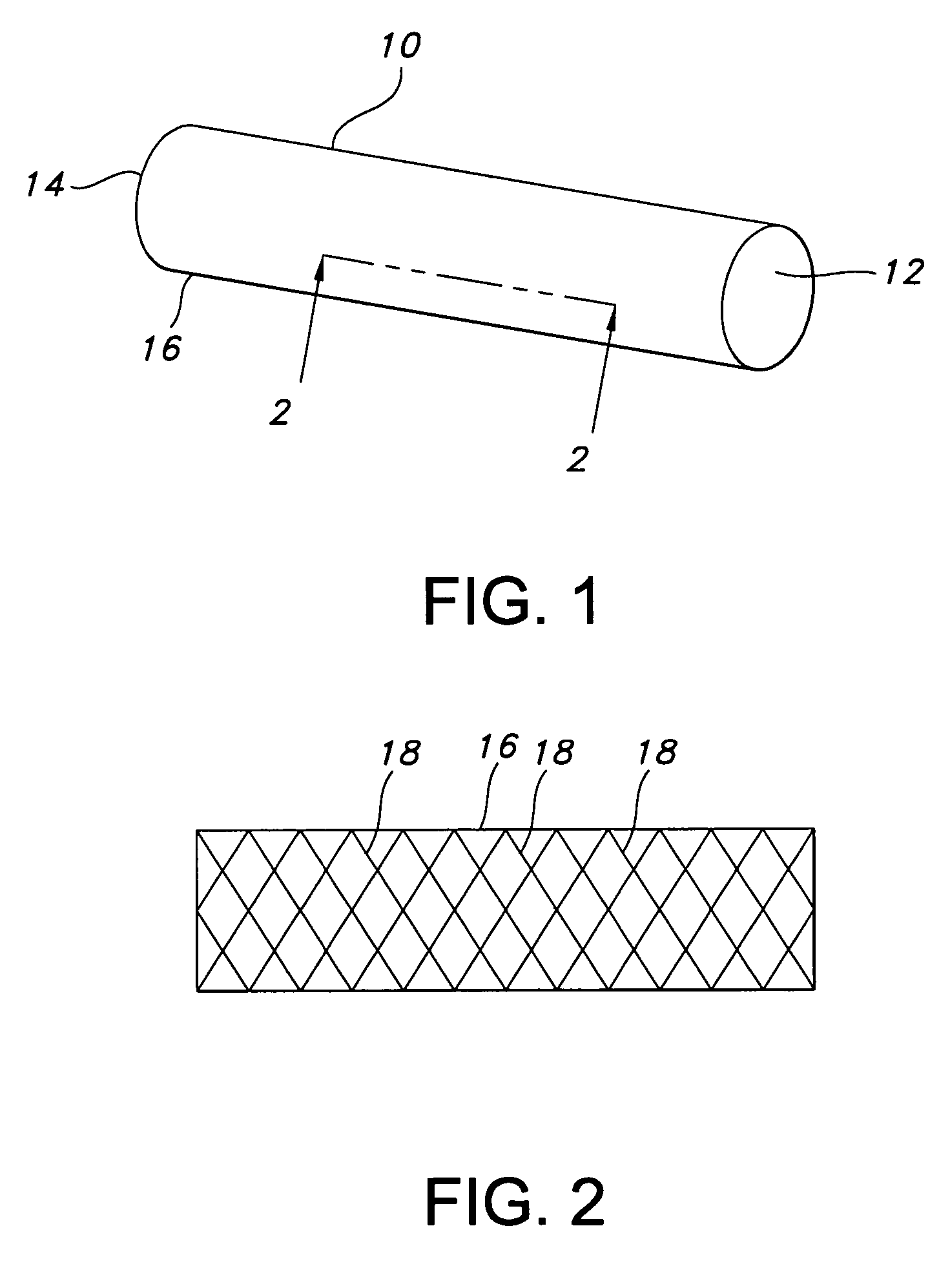
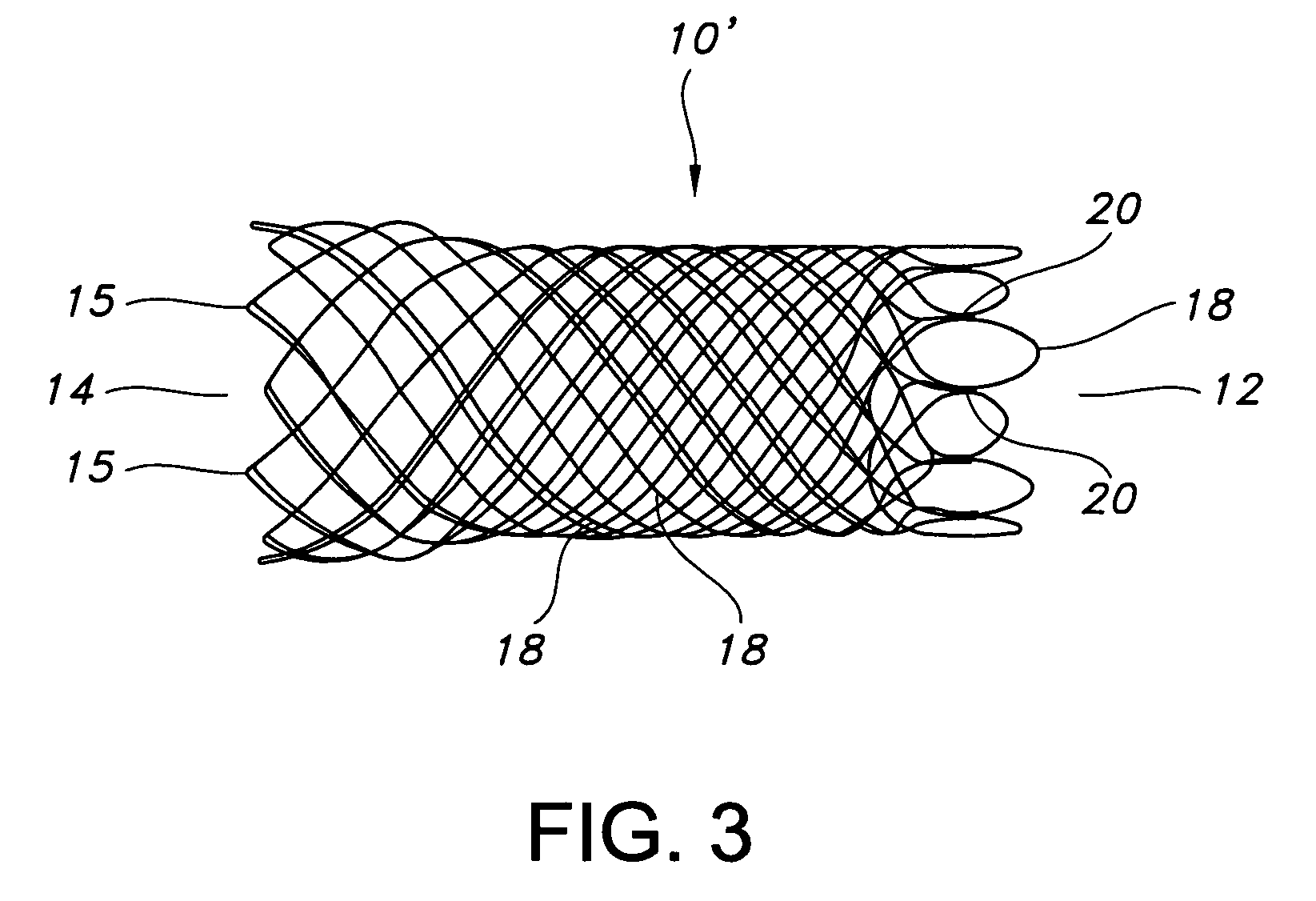
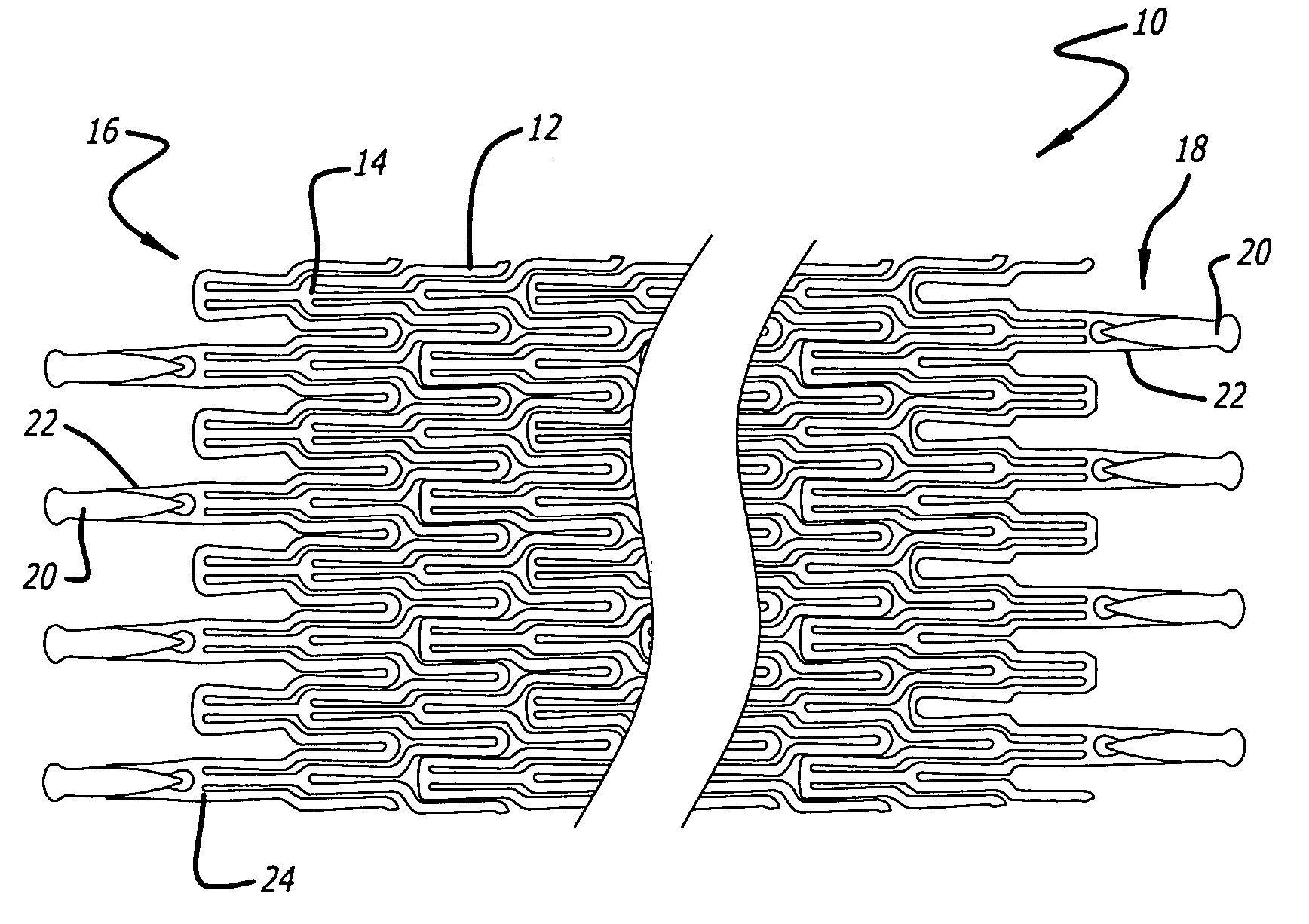
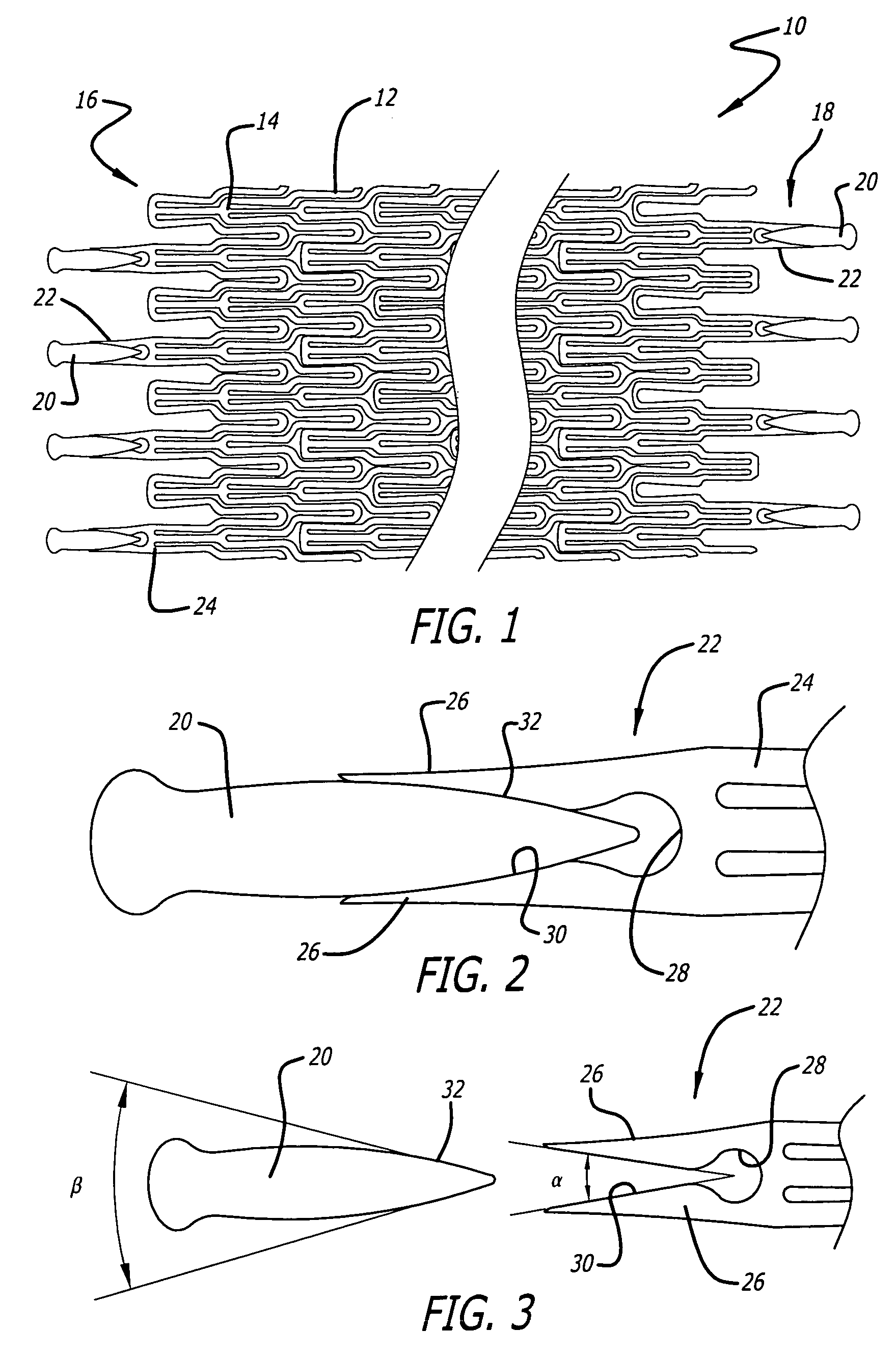
Atraumatic stent with reduced deployment force, method for making the same and method and apparatus for deploying and positioning the stent
An implantable stent includes a plurality of elongate wires braided to form a hollow tubular structure having a tubular wall to define an interior surface and an exterior surface and having opposed open first and second ends, wherein the opposed open first and second ends are atraumatic ends The atraumatic ends of the stent are desirably free of any loose wire ends. The wires include composite wires to enhance visibility of the wires to provide improved external imaging of the wires in the body. The elongate composite wires of the stent may be metallic wires having an outer metallic portion including a first metal, such as nitinol, and an inner metallic core portion including a second metal, which is a radiopaque material, such as gold, barium sulfate, ferritic particles, platinum, platinum-tungsten, palladium, platinum-iridium, rhodium, tantalum or combinations thereof.
Owner:BOSTON SCI SCIMED INC
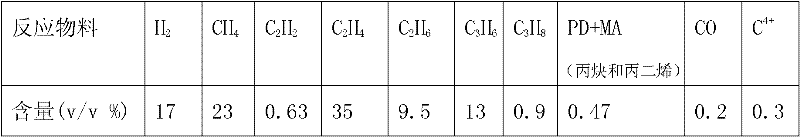
Catalyst for preparing propylene by propane dehydrogenation and its prepn.
InactiveCN101015802AHigh selectivityImprove reaction stabilityCatalyst carriersHydrocarbonsAdhesiveDehydrogenation
The invention relates to a catalyst for dehydrogenating propane to prepare propone, which uses thermal-resistant oxide as carrier, uses palladium-group metal as main catalyst, uses IV A group metal and rare-earth metal as agents, uses halogen as modifier, and uses inorganic oxide with high temperature resistance as adhesive. The inventive catalyst under high temperature and low pressure has higher propane transfer rate, propone selectivity and reaction stability. And the preparation comprises that at 60-100Deg. C, immerging thermal-resistant oxide with rare-earth metal water solution for 2-10h, at 60-180Deg. C baking for 2-10h, at 400-600Deg. C baking for 3-10h, immerging said carrier and the water solution which contains palladium metal and IVA metal at 60-100Deg. C for 2-10h, and baking for 2-10h at 60-180Deg. C, adding adhesive, protruding agent and acid gel solvent, protruding and shaping, baking for 2-10h at 60-180Deg. C, activating for 3-10h at 400-600Deg. C, and reducing for 2-10h in hydrogen flow at 400-600Deg. C.
Owner:SOUTHEAST UNIV
Radiopaque markers for medical devices
InactiveUS20050060025A1High level of radiopacitySufficient radiopacityStentsBlood vesselsIridiumRhenium
An implantable medical device includes a structural body made from a superelastic material and includes one or more marker holders integrally formed on the structural body. Each marker holder is designed to hold a radiopaque marker which has a level of radiopacity greater than the superelastic material. The radiopaque marker can be made from a nickel-titanium alloy which includes a ternary element. The ternary element can be selected from the group of elements consisting of iridium, platinum, gold, rhenium, tungsten, palladium, rhodium, tantalum, silver, ruthenium, and hafnium. In one form, the marker holder includes a pair of projecting fingers connected together at a notched region to cooperatively create a particular-shaped opening. This opening, in turn, is adapted to receive a similarly shaped portion formed on the radiopaque marker. In one form, the radiopaque marker includes an inner core which is partially, or completely, encased by an outer layer. This inner core can be made from a highly radiopaque material while the outer layer is formed from a material that is easier to weld to the marker.
Owner:ABBOTT VASCULAR SOLUTIONS
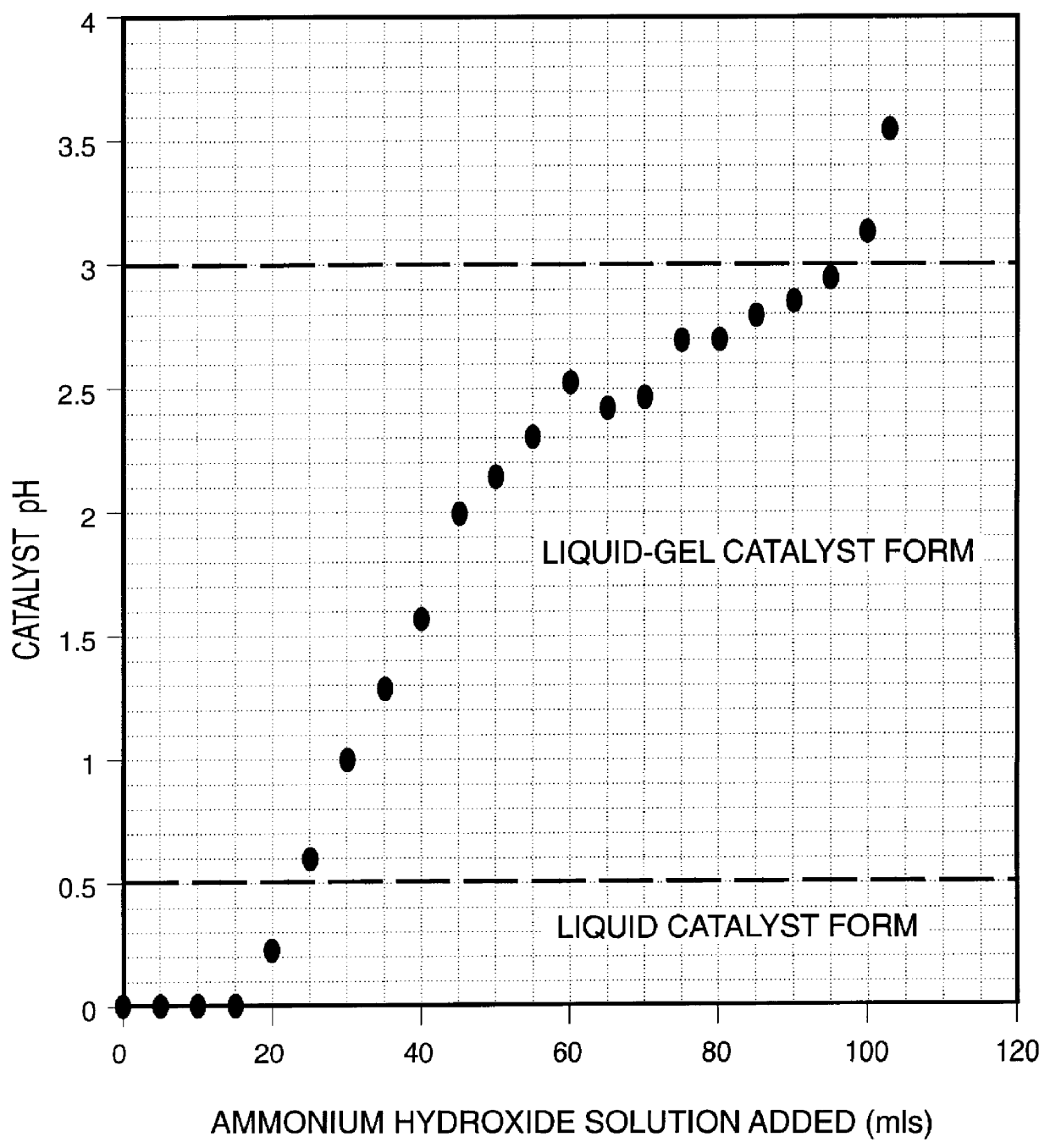
Iron-based ionic liquid catalysts for hydroprocessing carbonaceous feeds
InactiveUS6139723AIncrease hydrocracking ability of catalystIndirect and direct heating destructive distillationCatalyst activation/preparationLiquid productIron salts
A highly dispersed iron-based ionic liquid or liquid-gel catalyst which may be anion-modified and metals-promoted has high catalytic activity, and is useful for hydrocracking / hydrogenation reactions for carbonaceous feed materials. The catalyst is produced by aqueous precipitation from saturated iron salt solutions such as ferric sulfate and ferric alum, and may be modified during preparation with anionic sulfate (SO42-) and promoted with small percentages of at least one active metal such as cobalt, molybdenum, palladium, platinum, nickel, or tungsten or mixtures thereof. The resulting catalyst may be used in a preferred ionic liquid form or in a liquid-gel form, and either fluidic form can be easily mixed and reacted with carbonaceous feed materials such as coal, heavy petroleum fractions, mixed plastic waste, or mixtures thereof. The invention includes methods for making the ionic liquid or liquid-gel catalyst, and processes for using the fluidic catalysts for hydroprocessing the carbonaceous feed materials to produce desirable low-boiling hydrocarbon liquid products.
Owner:HEADWATERS CTL
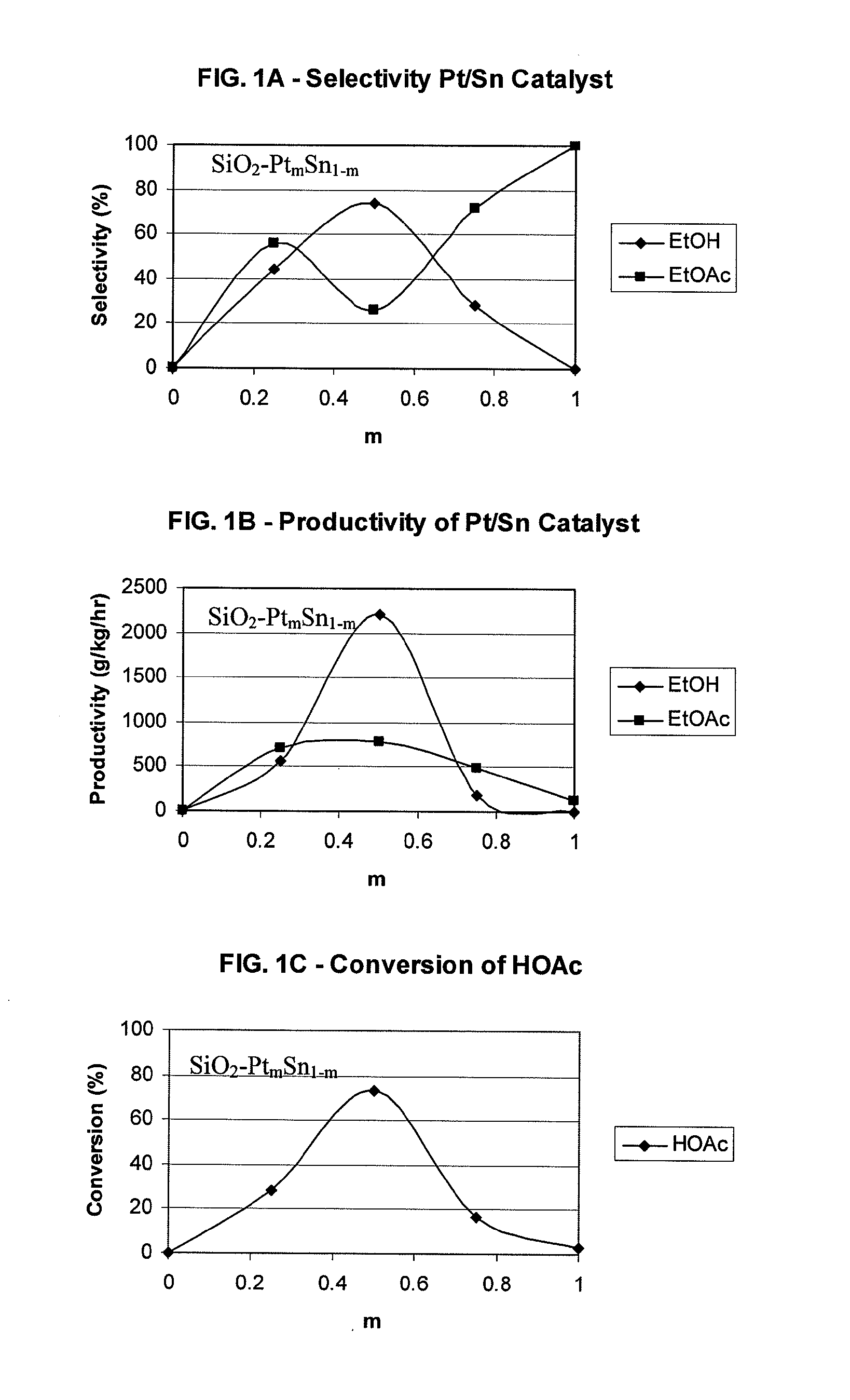
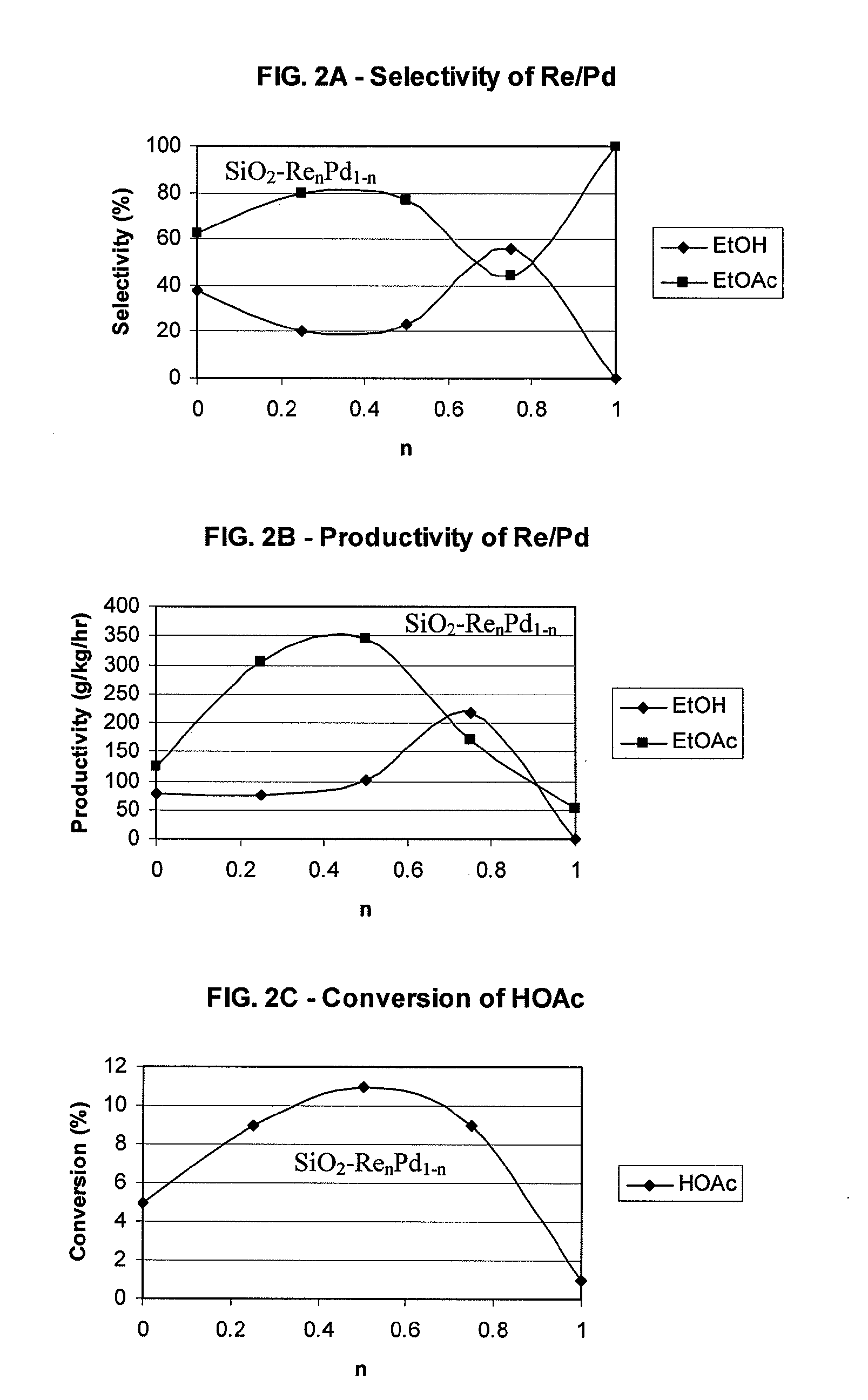
Processes for making ethanol from acetic acid
InactiveUS20100197985A1High selectivityPreparation by oxo-reaction and reductionEthylene productionCeriumCobalt
A process for selective formation of ethanol from acetic acid by hydrogenating acetic acid in the presence of first metal, a silicaceous support, and at least one support modifier. Preferably, the first metal is selected from the group consisting of copper, iron, cobalt, nickel, ruthenium, rhodium, palladium, osmium, iridium, platinum, titanium, zinc, chromium, rhenium, molybdenum, and tungsten. In addition the catalyst may comprise a second metal preferably selected from the group consisting of copper, molybdenum, tin, chromium, iron, cobalt, vanadium, tungsten, palladium, platinum, lanthanum, cerium, manganese, ruthenium, rhenium, gold, and nickel.
Owner:CELANESE INT CORP
High surface area sol-gel route prepared hydrogenation catalysts
InactiveUS6686310B1Good dispersionImprove uniformityCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsRheniumSilicon oxide
This invention concerns novel compositions, useful as hydrogenation catalyst, said compositions comprising metals and metal ions such as ruthenium (Ru) or palladium (Pd) dispersed in and distributed throughout a matrix comprising an inorganic or silicon oxide network. The catalyst may be prepared by the sol-gel method; a solution of at least one catalytic metal compound is added to a solution of at least one metal alkoxide selected from Al, Ti, Nb, Zr, Ta, Si and other inorganic alkoxides, and then gelling the mixture. Promotors such as rhenium (Re), molybdenum (Mo) and tin (Sn) may be added. The catalyst may be used in the reduction of metallic acid or gamma-butyrolactone to tetrahydrofuran (THF) and 1,4-butanediol (BDO).
Owner:EI DU PONT DE NEMOURS & CO
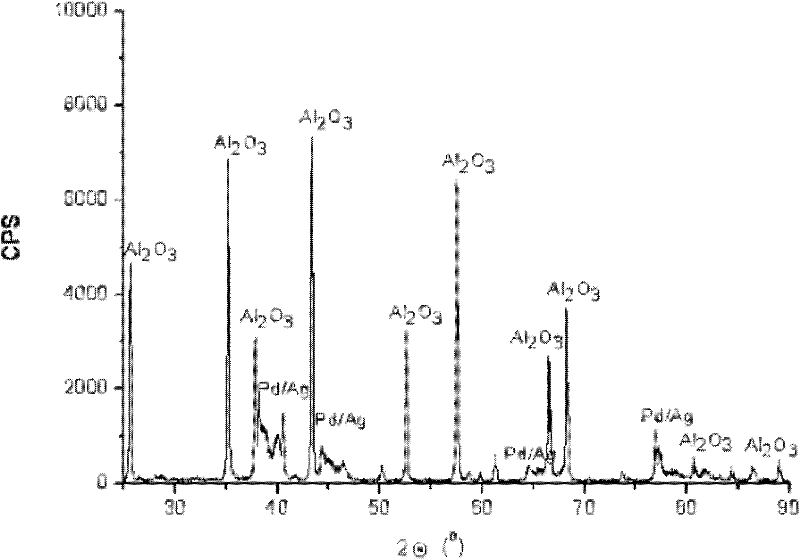
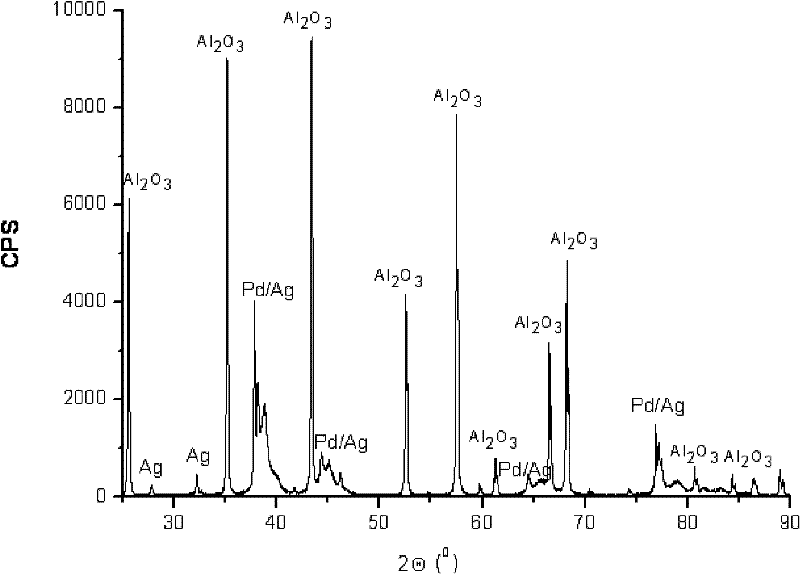
Palladium-silver bimetallic hydrogenation catalyst
ActiveCN102205243AGood choiceImprove hydrogenation activityHydrocarbon purification/separationHydrocarbonsHydrogenation processSolvation
The invention relates to a palladium-silver bimetallic hydrogenation catalyst, a carrier of which mainly contains Al2O3 and which is characterized by comprising, based on 100% of the catalyst weight, 0.01-0.4% of Pd, 0.02-0.2% of Ag, and has a specific surface area of 1-100m<2> / g. The catalyst is obtained through three steps of: first, preparing a functionalized-high-molecular / Al2O3 precursor, then preparing a Pd-Ag-high-molecular / Al2O3 precursor, and lastly roasting the precursors at 380-550 DEG C for 2-6h. The preparation method overcomes the influences of dipping liquid surface tensionand solvation effect on palladium-silver disperseness, and the prepared catalyst has excellent selectivity. The catalyst of the invention can be applied to selective hydrogenation process of fractions such as C2 and C3, and has a good hydrogenation activity, excellent selectivity and good hydrogenation stability.
Owner:PETROCHINA CO LTD
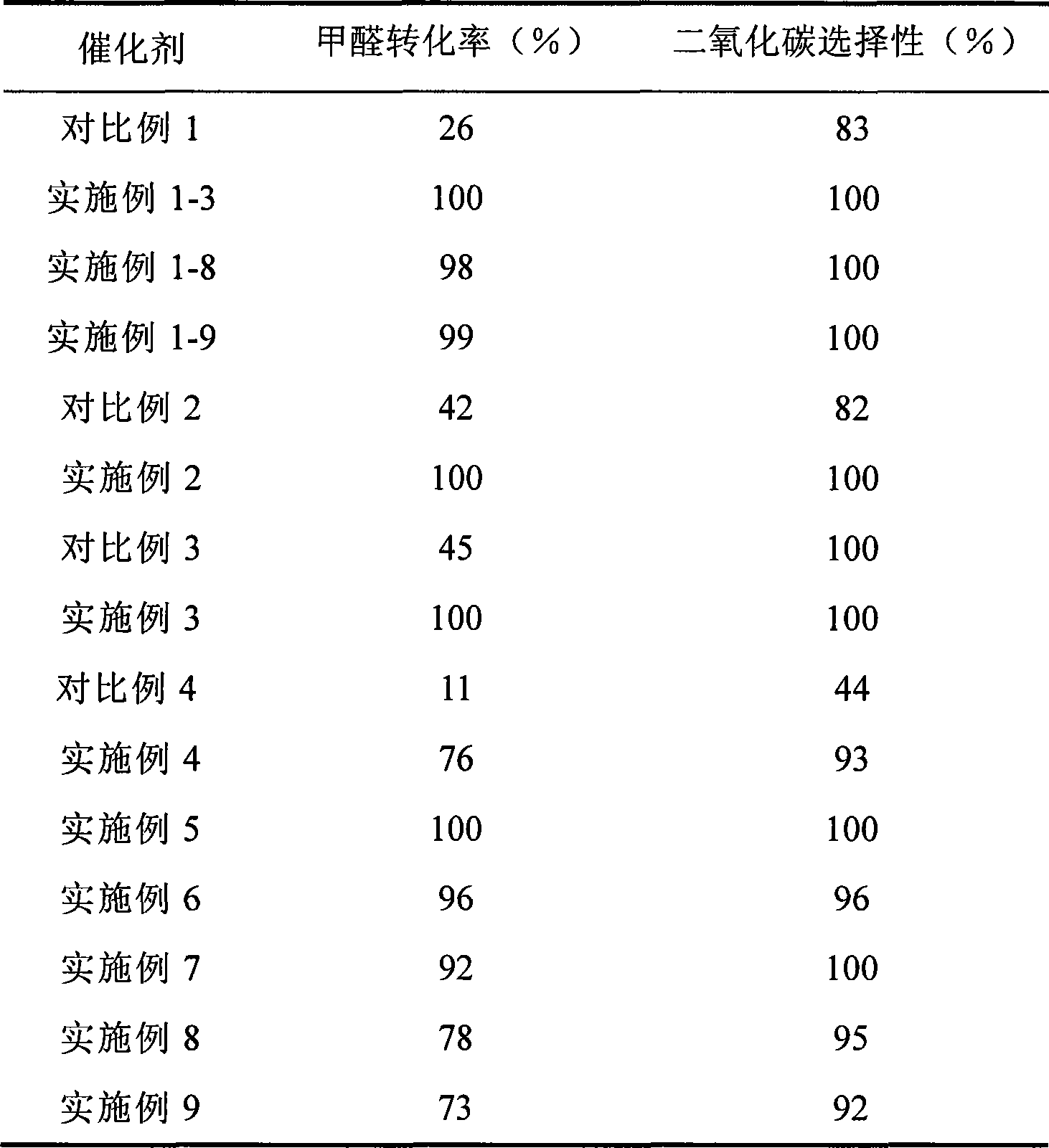
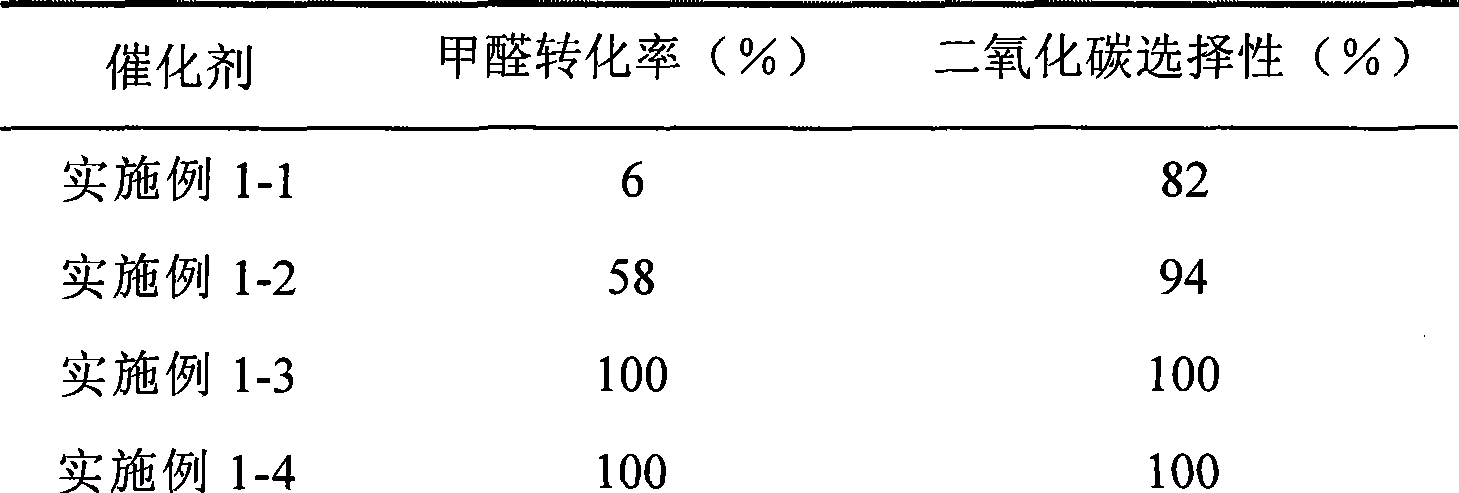
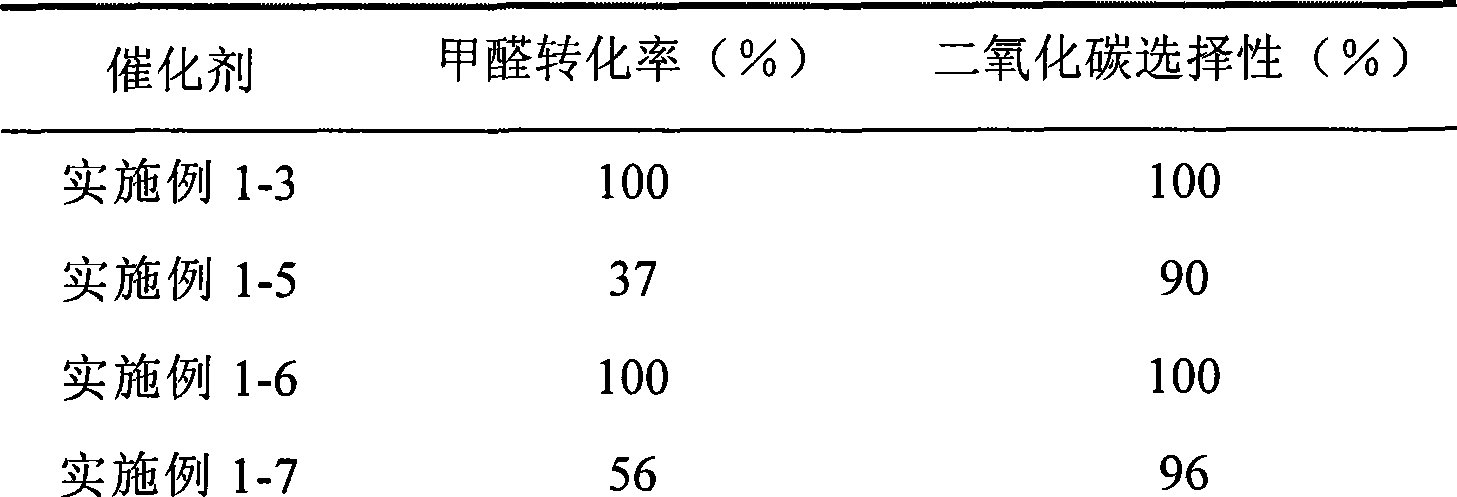
Catalyst for complete oxidation of formaldehyde at room temperature
ActiveCN101380574AEasy to makeEasy to operateDeodrantsMetal/metal-oxides/metal-hydroxide catalystsPorous carbonPt element
The invention provides a high selectivity catalyst used for catalyzing and completely oxidizing formaldehyde with low concentration at room temperature. The catalyst can catalyze formaldehyde completely so as to lead the formaldehyde to be converted into carbon dioxide and water at room temperature. In addition, the conversion rate of formaldehyde remains 100% within a long period of time, without complex auxiliary facilities such as light source, a heating oven and the like, and external conditions. The catalyst comprises three parts which are inorganic oxide carrier, noble metal component and auxiliary ingredient. Porous inorganic oxide carrier is one of cerium dioxide, zirconium dioxide, titanium dioxide, aluminium sesquioxide, tin dioxide, silicon dioxide, lanthanum sesquioxide, magnesium oxide and zinc oxide or the mixture thereof or composite oxide thereof, zeolite, sepiolite and porous carbon materials. The noble metal component of the catalyst is at least one of platinum, rhodium, palladium, gold and silver. The auxiliary ingredient is at least one of the alkali metals of lithium, sodium, kalium, rubidium and cesium. The loading of the noble metal component used in the catalyst of the invention is 0.1 to 10% according to weight converter of metal elements and the selective preference is 0.3 to 2%. The loading of the auxiliary ingredient is 0.2 to 30% according to weight converter of metal elements and the selective preference is 1 to 10%. When the loading of the auxiliary ingredient is lower than 0.2% or higher than 30%, the activity of the catalyst for catalyzing and oxidizing formaldehyde at room temperature is decreased remarkably.
Owner:广东顺德中科鸿图环境材料有限公司
Supported palladium-gold catalysts and preparation of vinyl acetate therewith
InactiveUS20100121100A1High activity selectivityImproves oxygen selectivityOrganic compound preparationCarboxylic acid esters preparationTungsten trioxidePalladium
Disclosed is a catalyst. The catalyst comprises palladium, gold, and a support comprising titanium dioxide and tungsten trioxide. The support preferably comprises from 75 wt % to 99 wt % of titanium dioxide and from 1 wt % to 25 wt % of tungsten trioxide. A method for preparing the catalyst is also disclosed. The method comprises impregnating the support with a palladium compound and a gold compound, calcining the impregnated support, and then reducing the calcined support. Further disclosed is a method for preparing vinyl acetate with the catalyst. The catalyst exhibits improved catalytic activity and selectivity.
Owner:LYONDELLBASELL ACETYLS
Corrosion-Resistant Downhole Transmission System
An apparatus in accordance with the invention may include a downhole tool and a data transmission path incorporated into the downhole tool. The data transmission path may include one or more contact surfaces providing electrical continuity to the data transmission path. To protect the contact surfaces from corrosion while maintaining electrical conductivity, a coating may be attached to one or more of the contact surfaces. The coating may include any of various materials that increase the corrosion-resistance of the underlying base metal, including but not limited to cobalt, nickel, tin, tin-lead, platinum, palladium, gold, silver, zinc, or combinations thereof.
Owner:INTELLISERV
Catalyst for manufacturing hydrogen or synthesis gas and manufacturing method of hydrogen or synthesis gas
InactiveUS6361757B1Produce hydrogenEfficient productionIron compoundsCobalt compoundsIridiumForming gas
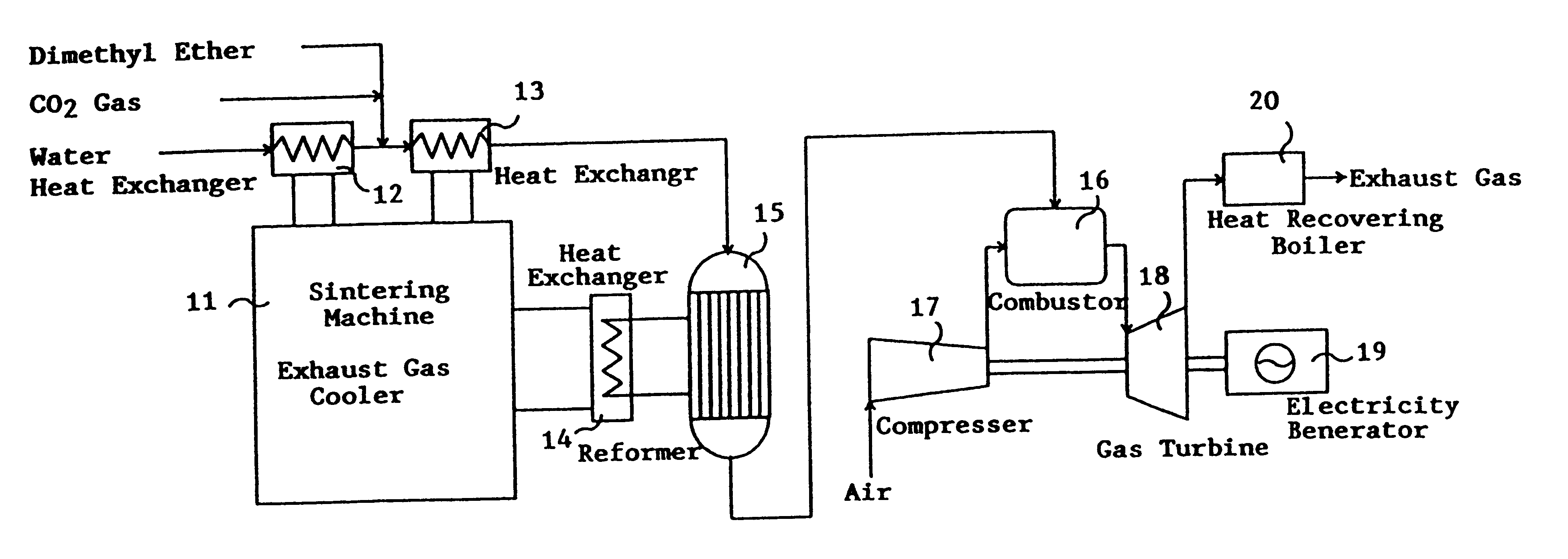
This invention provides a catalyst for producing hydrogen gas from a mixed gas comprising dimethyl ether and water vapor or carbon dioxide gas, which comprises copper, iron, cobalt, palladium, iridium, platinum, rhodium, or nickel as an active component, and a method of producing synthesis gas or hydrogen gas in a high yield at a low temperature. By using the catalyst, a fuel cell, electricity generation, reduction of iron ore and the like can be carried out.
Owner:NIPPON KOKAN KK
Noble metal support
InactiveUS6228800B1Efficient use ofLong catalyst lifeOrganic compound preparationCatalyst activation/preparationPalladiumChemistry
A noble metal-supported article which comprises a carrier and a palladium-containing metal component supported on the carrier, which article has (A) a layer in which substantially no palladium is supported in the interior of the carrier and (B) a layer in which palladium is supported in the region from the outer surface to a depth of less than 100 mum of the carrier.
Owner:ASAHI KASEI KK
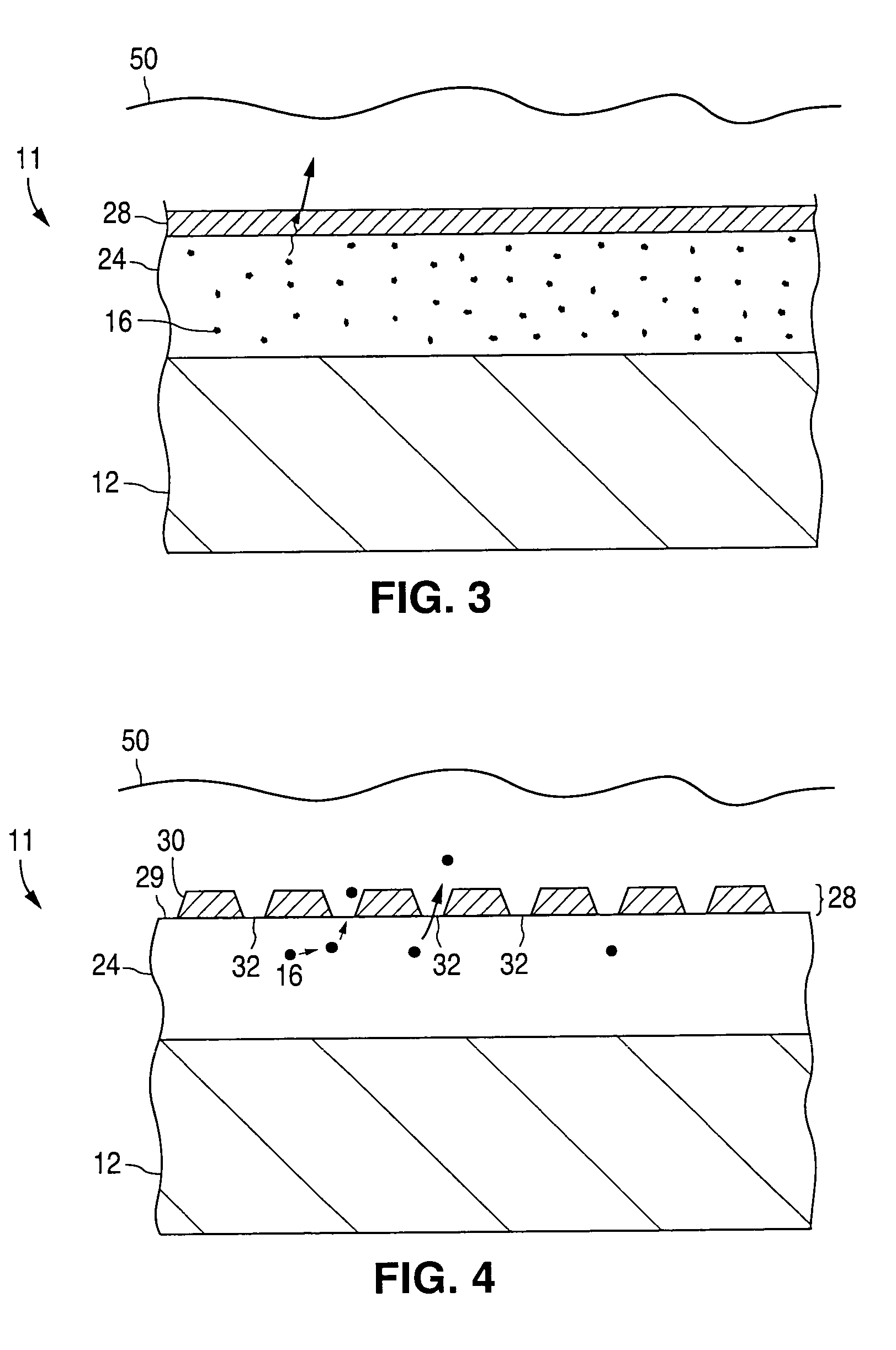
Barriers for polymer-coated implantable medical devices and methods for making the same
InactiveUS6953560B1Reduce and prevent and inflammationReduce and prevent proliferationStentsSurgeryHafniumPt element
An implantable medical device and methods for making the implantable medical device are disclosed. The implantable medical device includes a substrate. At least a portion of the substrate is coated with a first layer including a polymer containing a drug. A barrier overlies the first layer. The barrier significantly reduces the rate of release of the drug from the polymer, thereby sustaining release of the drug from the medical device for a longer time.The barrier may be a homogeneous layer overlying the first layer, or a number of discrete deposits over the first layer. Alternatively, the barrier may be intermixed with an outer portion of the first layer. The barrier material is biocompatible, and typically has a thickness ranging from about 50 angstroms to about 20,000 microns. Suitable materials for the barrier include, but are not limited to, inorganic compounds, such as inorganic silicides, oxides, nitrides, carbides, as well as pure metals such as aluminum, chromium, gold, hafnium, iridium, niobium, palladium, platinum, tantalum, titanium, tungsten, zirconium, and alloys of these metals. The barriers disclosed may be applied to the first layer by several techniques, depending on the material being applied. Exemplary deposition techniques include physical vapor deposition, alkoxide hydrolysis, and electroless plating.The implantable device may be a stent or a graft, among other possibilities.
Owner:ABBOTT CARDIOVASCULAR
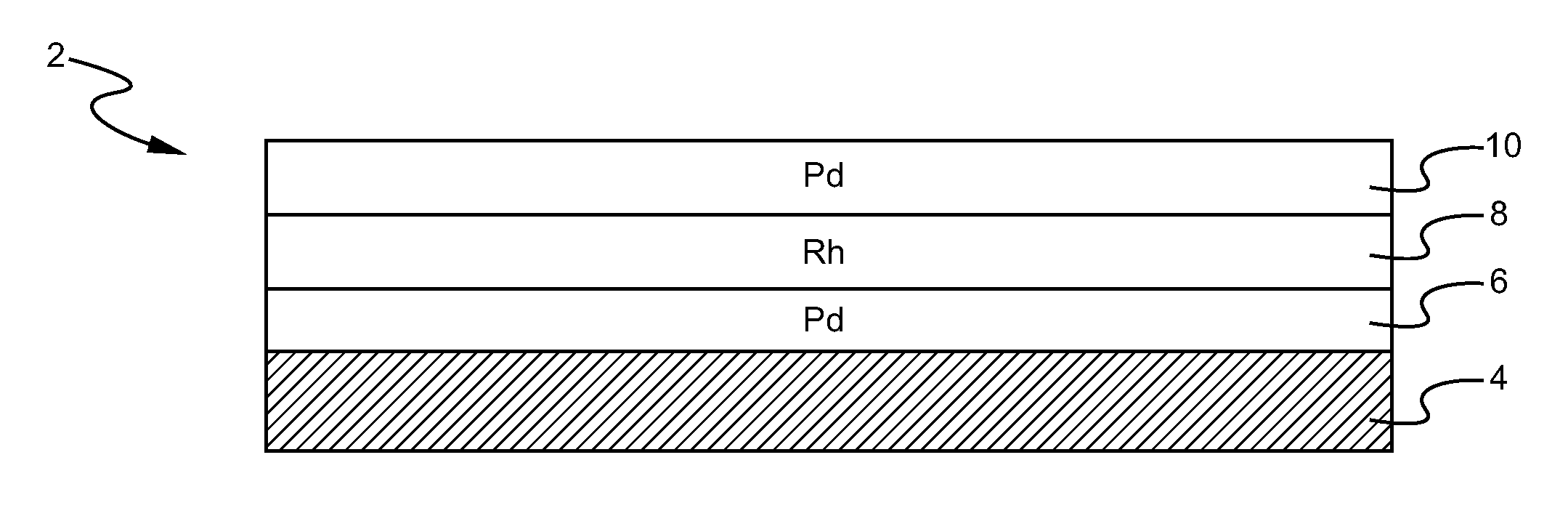
Layered catalyst composite
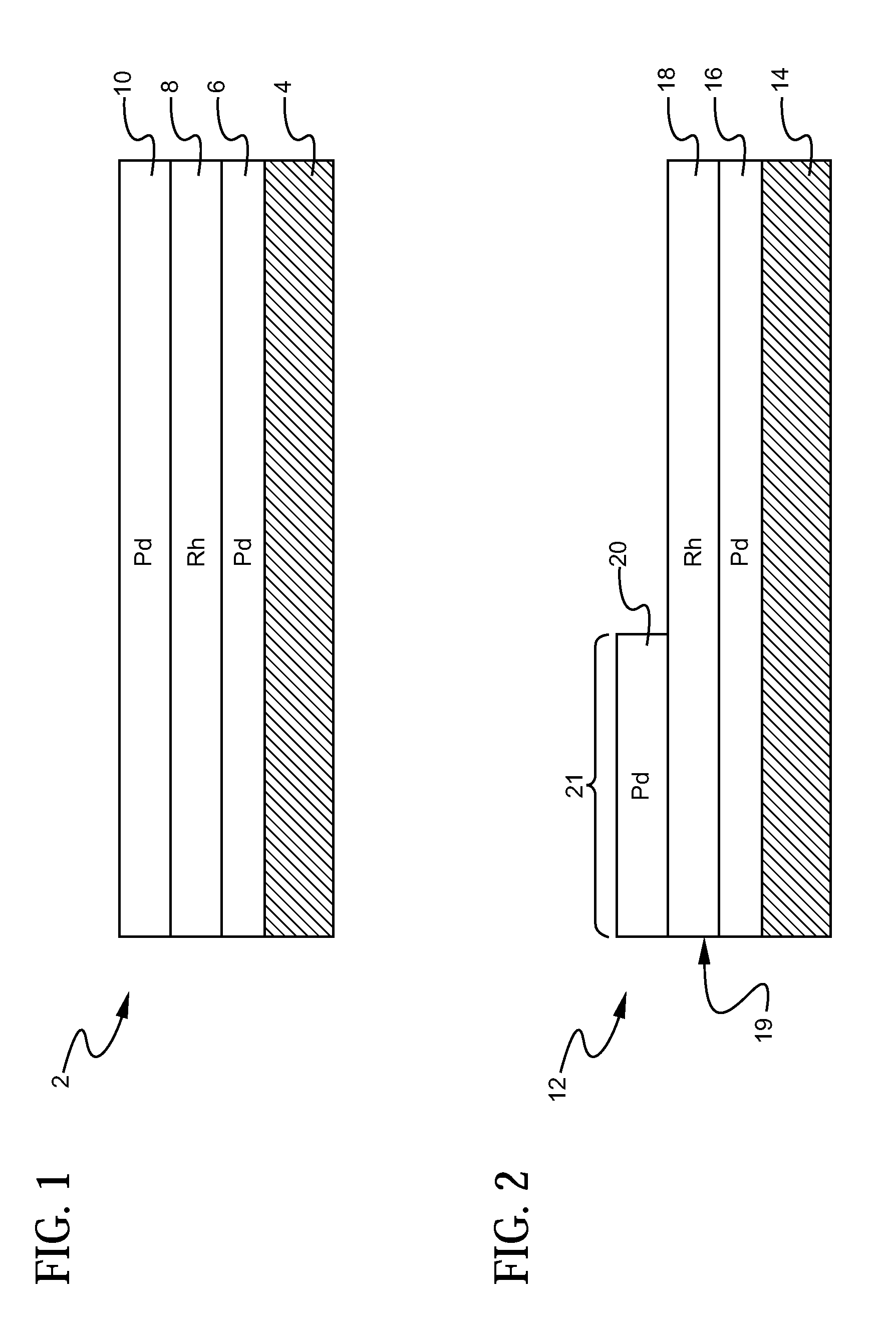
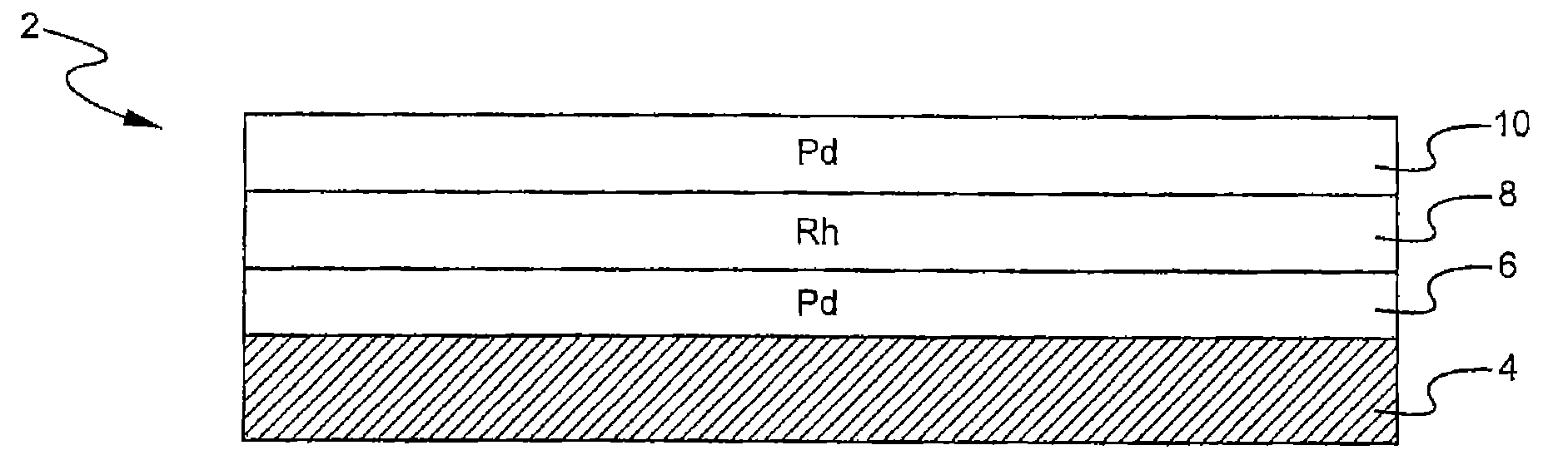
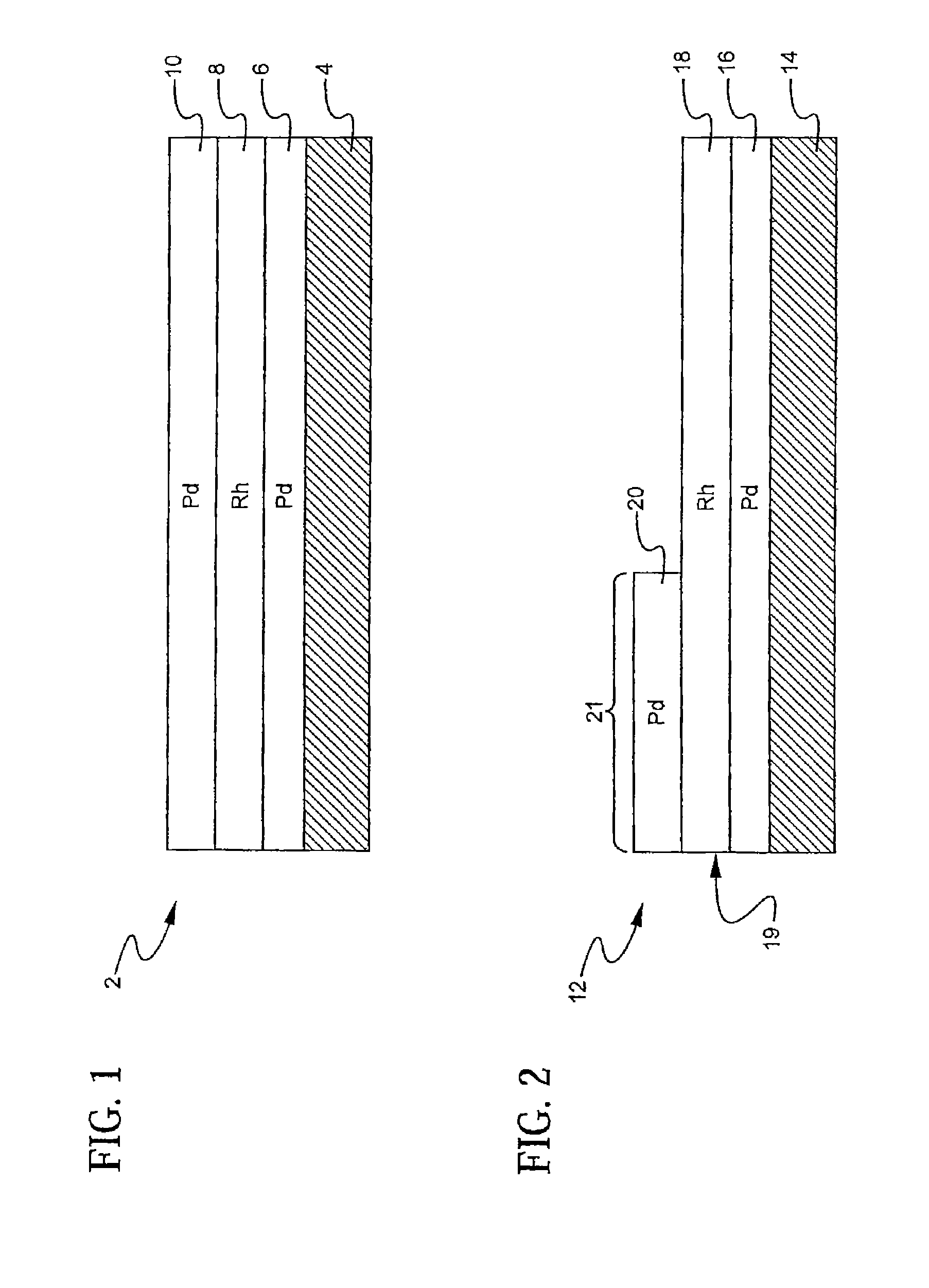
A layered, three-way conversion catalyst having the capability of simultaneously catalyzing the oxidation of hydrocarbons and carbon monoxide and the reduction of nitrogen oxides is disclosed. In one or more embodiments, the catalyst comprises three layers in conjunction with a carrier: a first layer deposited on the carrier and comprising palladium deposited on a refractory metal oxide and an oxygen storage component; a second layer deposited on the first layer and comprising rhodium deposited on a refractory metal oxide and an oxygen storage component; and a third layer deposited on the second layer and comprising palladium deposited on a refractory metal oxide.
Owner:BASF CATALYSTS LLC
Layered catalyst composite
A layered, three-way conversion catalyst having the capability of simultaneously catalyzing the oxidation of hydrocarbons and carbon monoxide and the reduction of nitrogen oxides is disclosed. In one or more embodiments, the catalyst comprises three layers in conjunction with a carrier: a first layer deposited on the carrier and comprising palladium deposited on a refractory metal oxide and an oxygen storage component; a second layer deposited on the first layer and comprising rhodium deposited on a refractory metal oxide and an oxygen storage component; and a third layer deposited on the second layer and comprising palladium deposited on a refractory metal oxide.
Owner:BASF CATALYSTS LLC
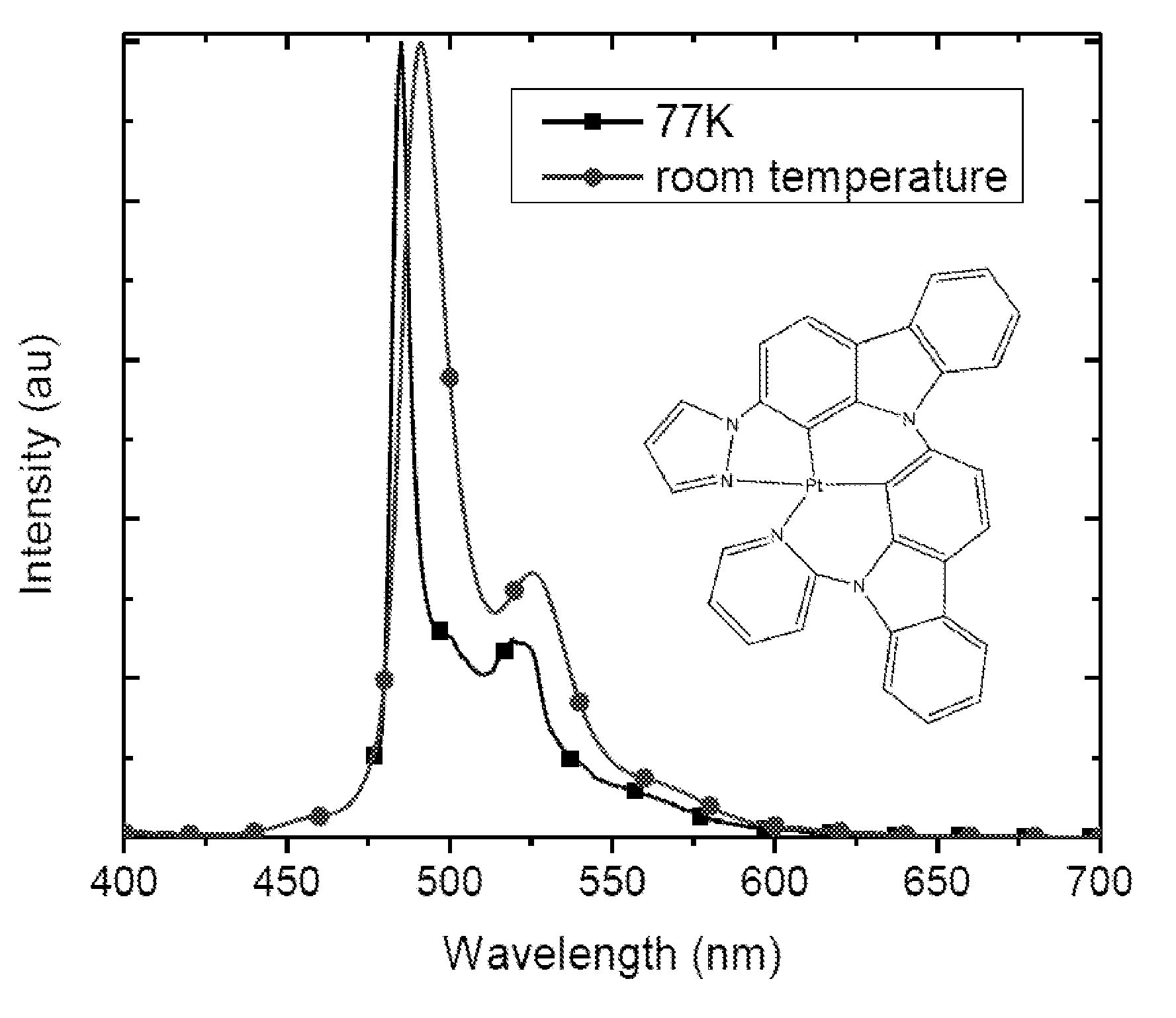
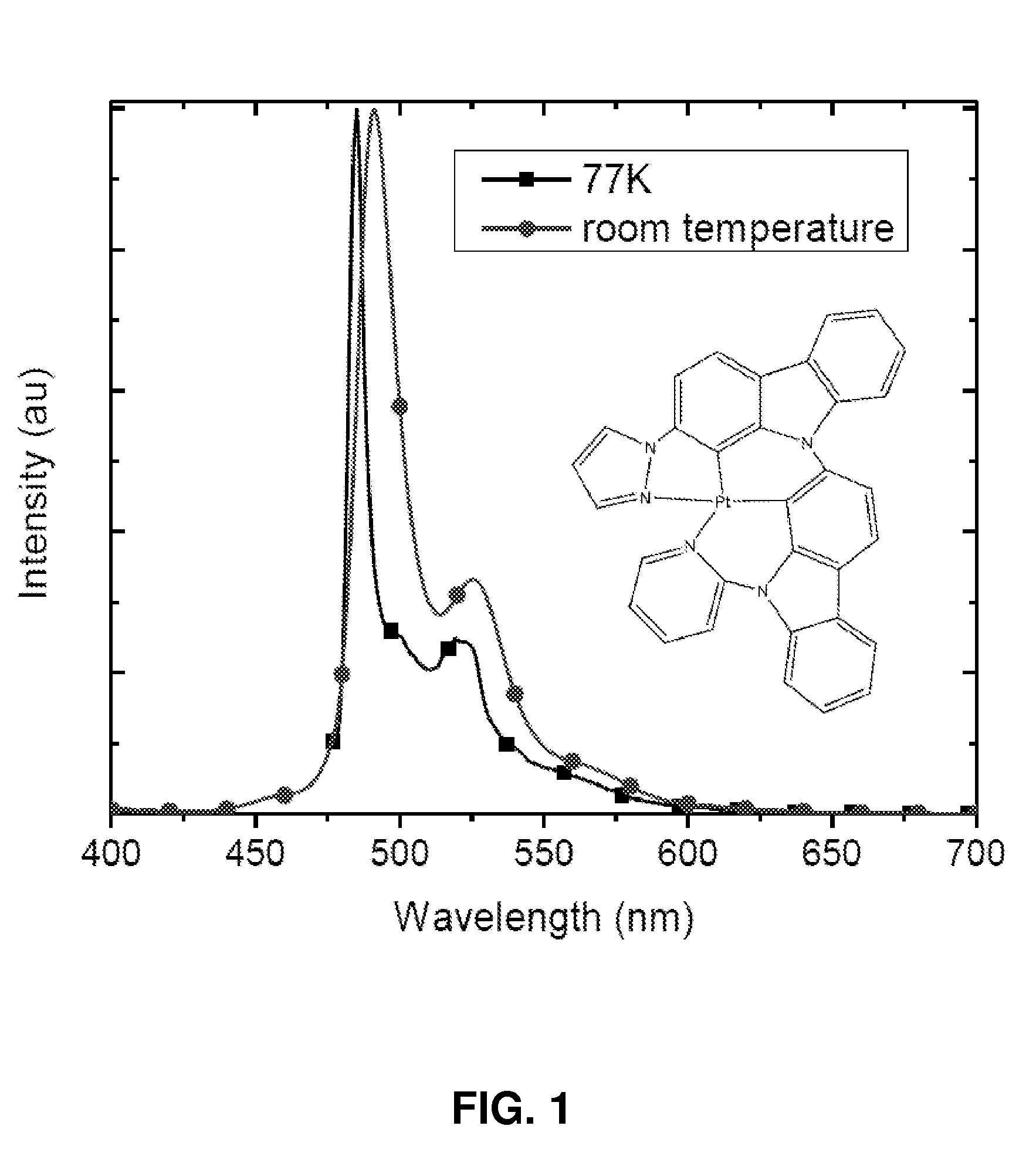
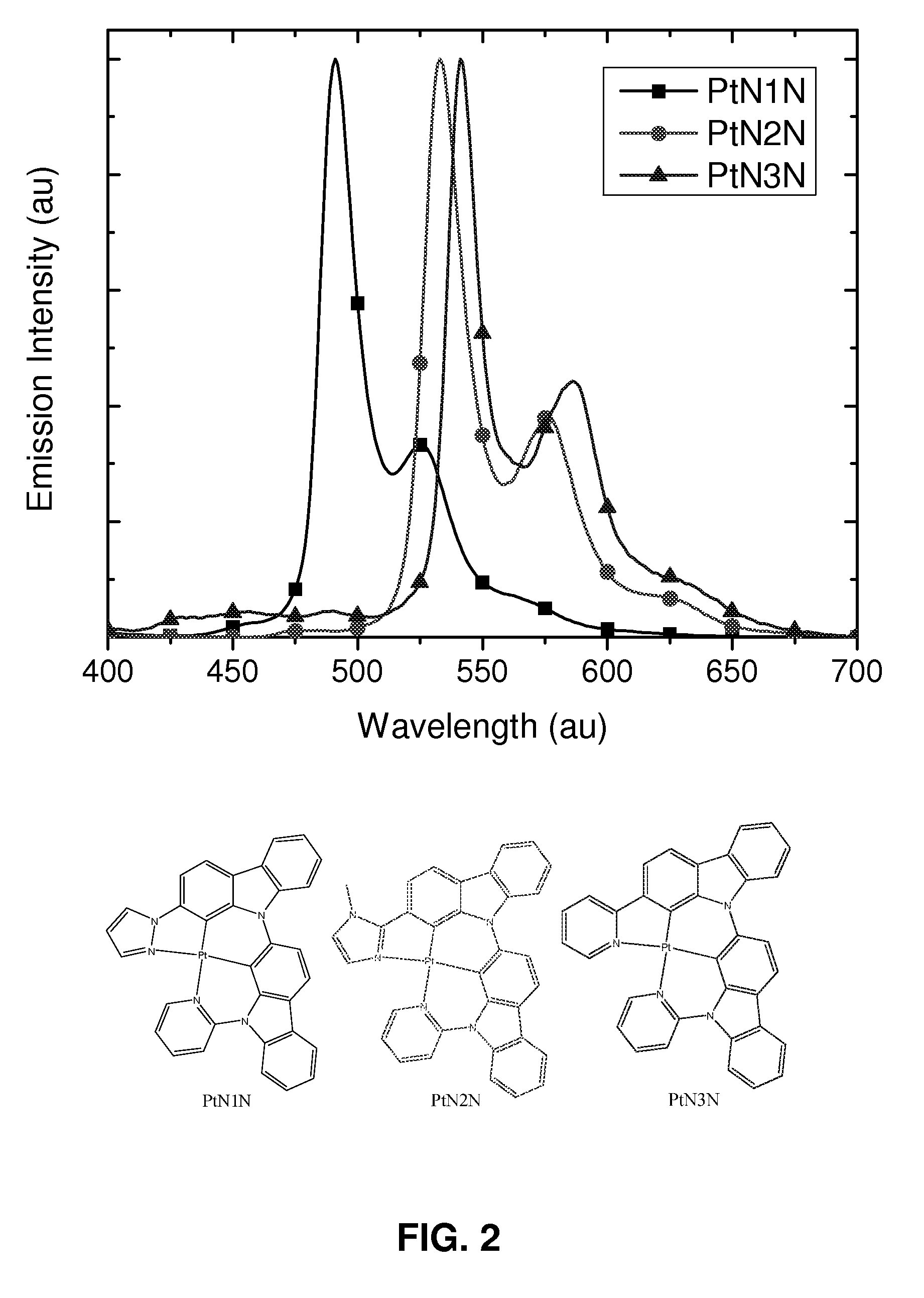
Synthesis of platinum and palladium complexes as narrow-band phosphorescent emitters for full color displays
Platinum and palladium complexes are disclosed that can be useful as narrow band phosphorescent emitters. Also disclosed are methods for preparing and using the platinum and palladium complexes
Owner:ARIZONA STATE UNIVERSITY
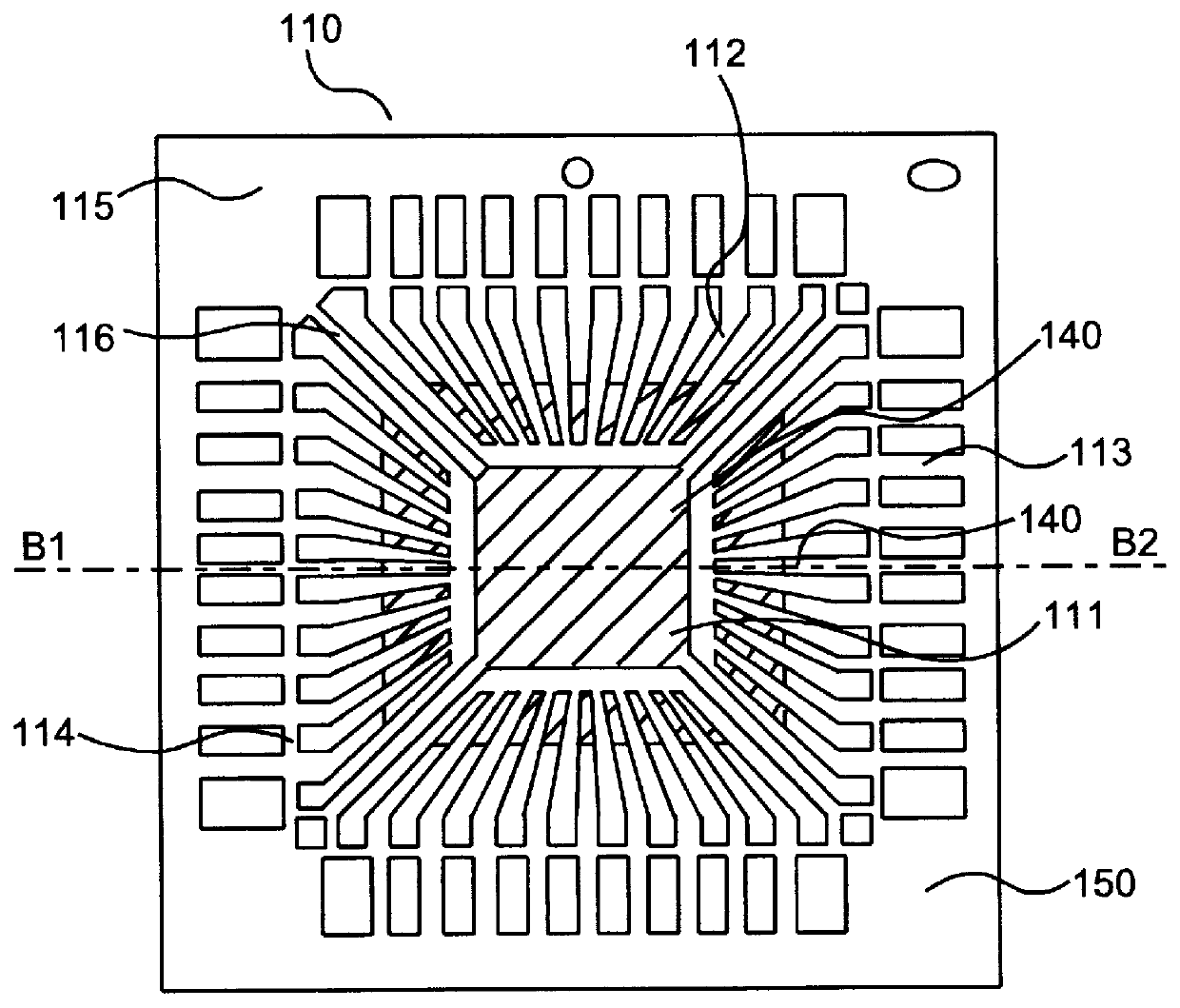
Lead frame, method for partial noble plating of said lead frame and semiconductor device having said lead frame
InactiveUS6034422ASemiconductor/solid-state device detailsSolid-state devicesCopper platingPalladium
A lead frame for a semiconductor device, made of a copper alloy, capable of preventing the creation of delamination between encapsuling resin and attributable to a lead frame without sacrificing the wire bondability and, a process for producing the lead frame and a semiconductor device using the lead frame. According to the present invention, (1) there is provided a lead frame for a plastic molded type semiconductor device, made of a copper alloy material partially plated with at least one noble metal, for wire bonding or die bonding purposes, selected from silver, gold, and palladium, wherein the whole area or a predetermined area of the surface of the copper at least on its side to be contacted with a encapsuling resin has a thin noble metal plating of at least one member selected from silver, gold, platinum, and palladium. (2) A copper strike plating is provided as a primer plating for the partial noble plating, a copper plating is provided on the thin noble metal plating, and the partial noble metal plating is provided on the copper plating in its predetermined area. (3) A die pad for mounting a semiconductor chip is provided, a partial silver plating is provided, and a zinc flash plating and a copper strike plating are provided in that order at least one on the surface of copper in the back surface of the die pad remote from the surface on which the semiconductor chip is mounted.
Owner:DAI NIPPON PRINTING CO LTD
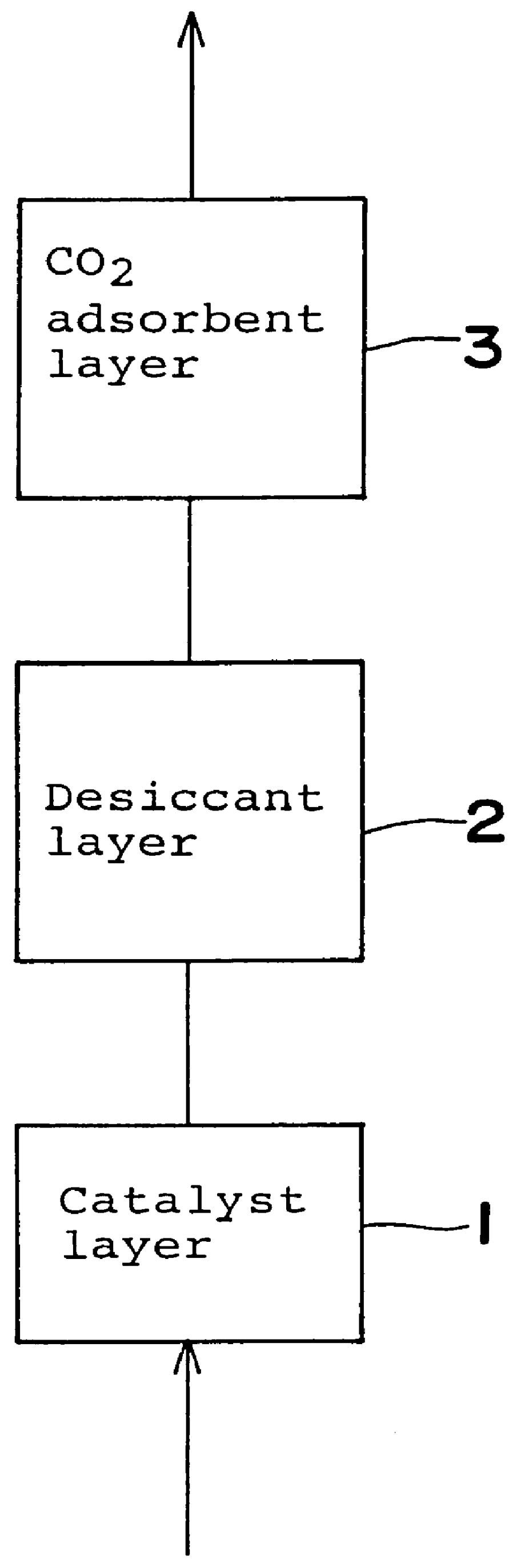
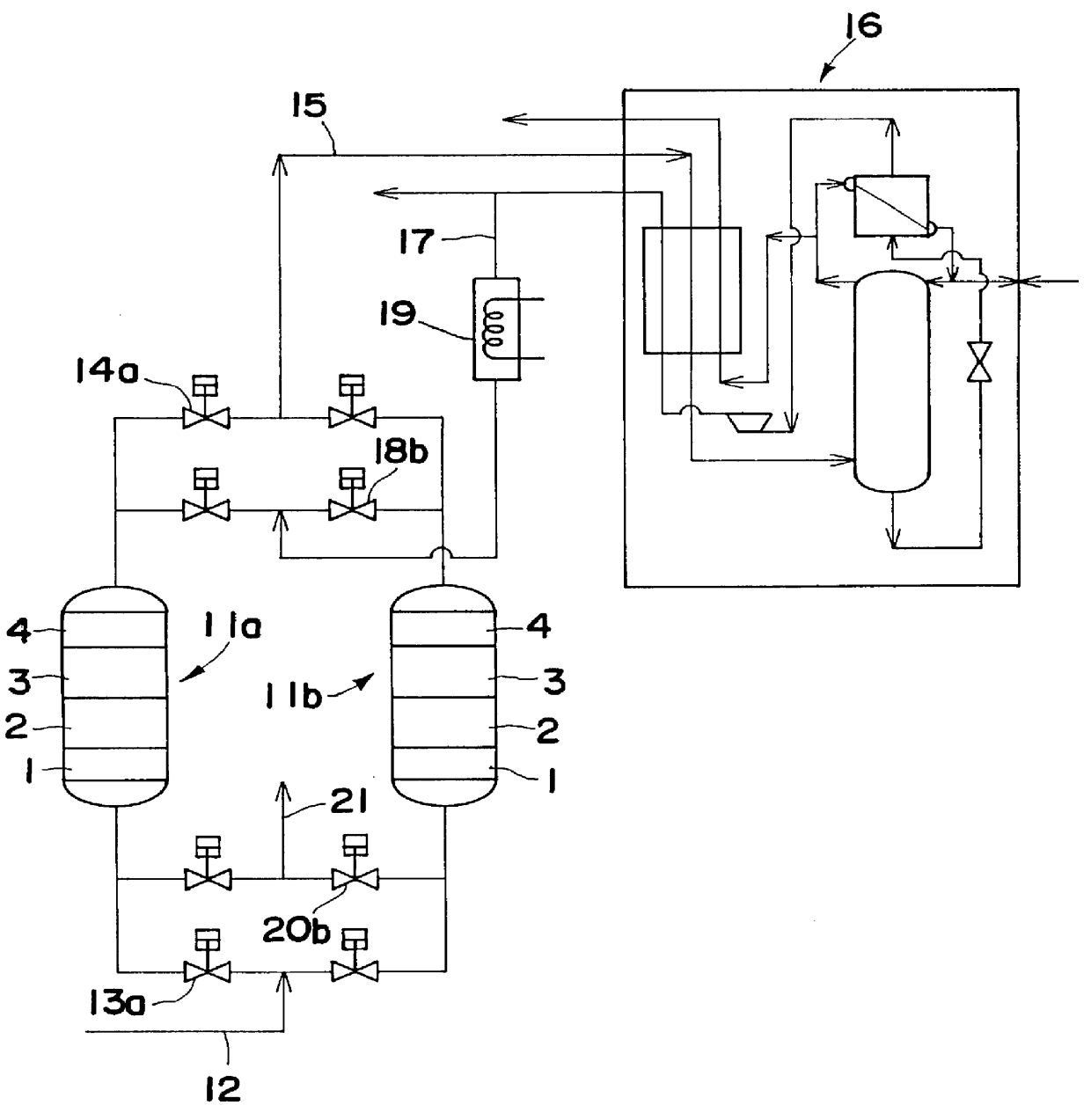
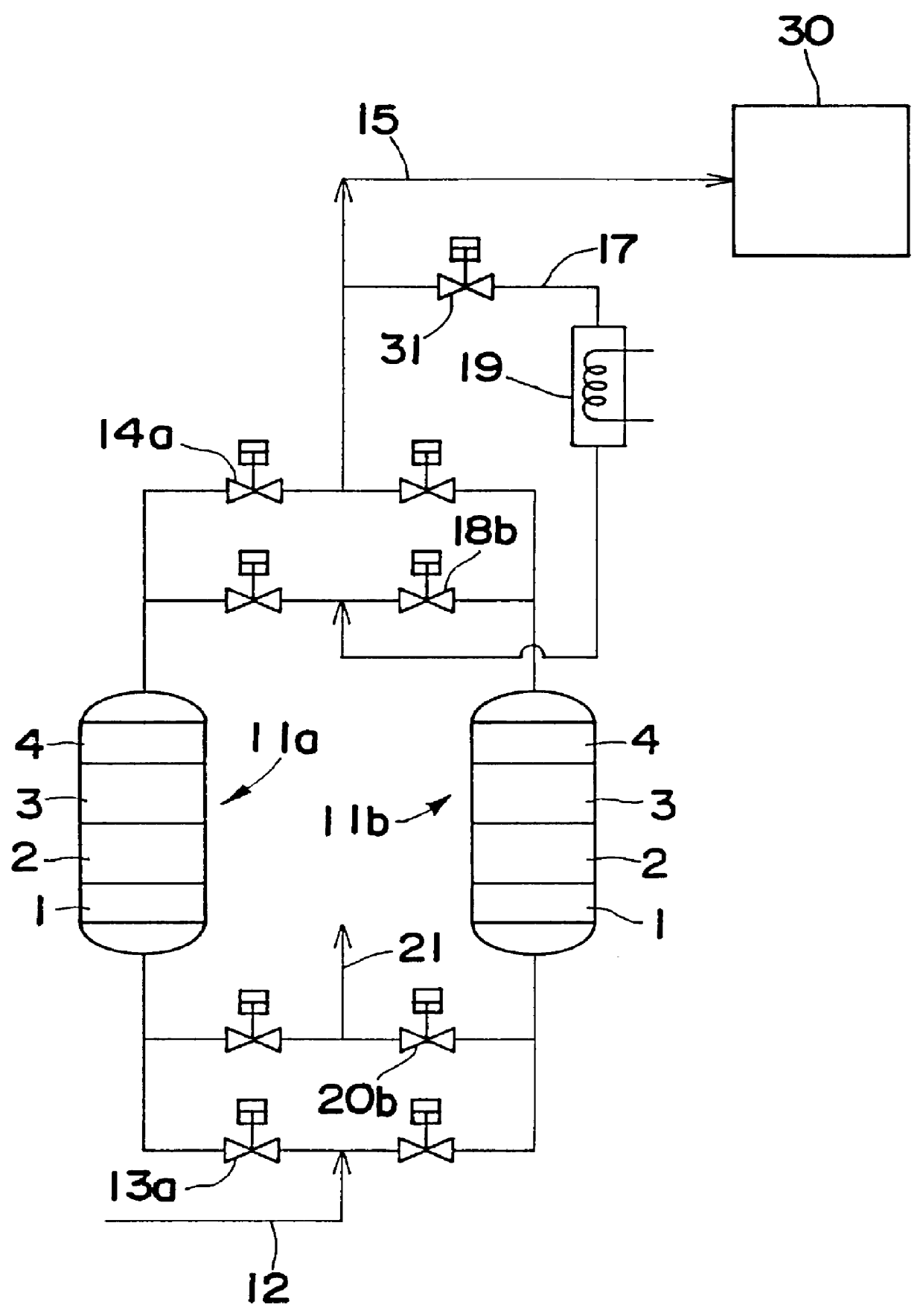
Gas purifying process and gas purifying apparatus
A method is provided for removing water, carbon monoxide and carbon dioxide out of a gas, such as air, by passing the gas through a packed column so that the gas sequentially contacts a catalyst consisting of platinum or palladium and at least one member selected from the group consisting of iron, cobalt, nickel, manganese, copper, chromium, tin, lead and cerium wherein the catalyst is supported on alumina containing substantially no pores having pore diameters of 110 Angstroms or less under conditions which oxidize the carbon monoxide in the gas into carbon dioxide; an adsorbent selected from the group consisting of silica gel, activated alumina, zeolite and combinations thereof under conditions in which water is adsorbed and removed from the gas and an adsorbent selected from the group consisting of calcium ion exchanged A zeolite; calcium ion exchanged X zeolite; sodium ion exchanged X zeolite and mixtures thereof under conditions which carbon dioxide is adsorbed and removed from the gas. The gas may also be subjected to a catalyst / adsorbent in the packed column to effect oxidation and removal of hydrogen in the gas.
Owner:NIPPON SANSO CORP
Non-chrome plating on plastic
InactiveUS20050199587A1Insulating substrate metal adhesion improvementDecorative surface effectsPERMANGANATE IONBiological activation
The invention comprises a process of preparing a non-conductive substrate for subsequent metalization. The process replaces the traditional chromic acid etching step with an etching solution comprising a permanganate and a mineral acid. The process also includes a novel activation solution comprising a palladium salt and an amine complexor. The new process of the invention is more environmentally friendly than the traditional chromic acid etching solutions but achieves a comparable result on most non-conductive substrates.
Owner:MACDERMID INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com