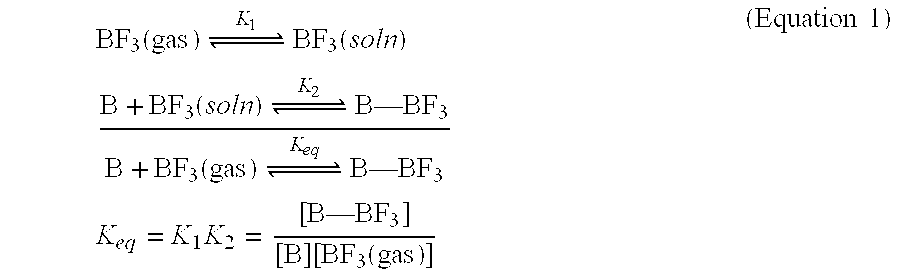Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
164 results about "Diborane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
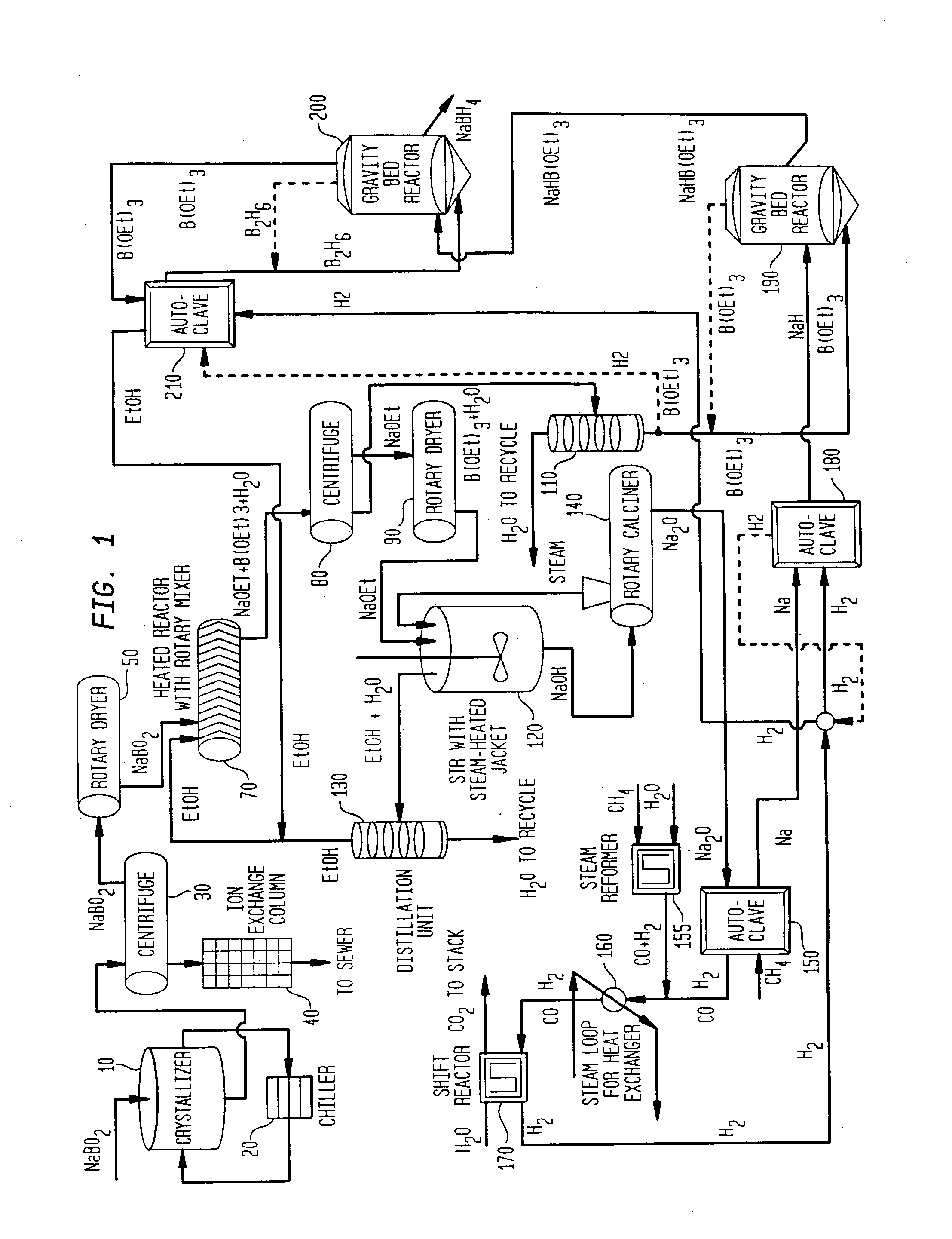
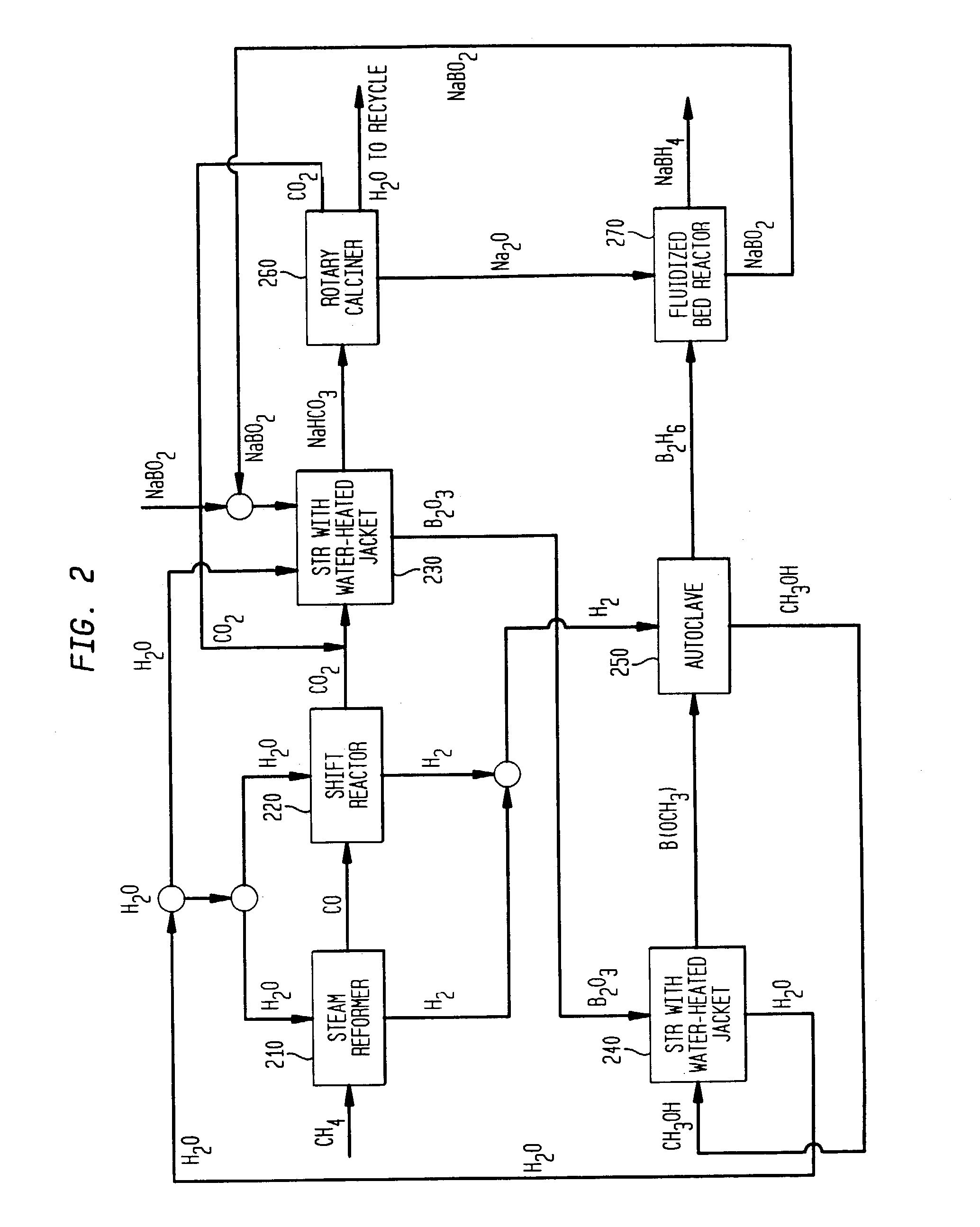
Diborane(6), generally known as diborane is the chemical compound consisting of boron and hydrogen with the formula B₂H₆. It is a colorless, pyrophoric gas with a repulsively sweet odor. Synonyms include boroethane, boron hydride, and diboron hexahydride. Diborane is a key boron compound with a variety of applications. It has attracted wide attention for its electronic structure. Its derivatives are useful reagents.
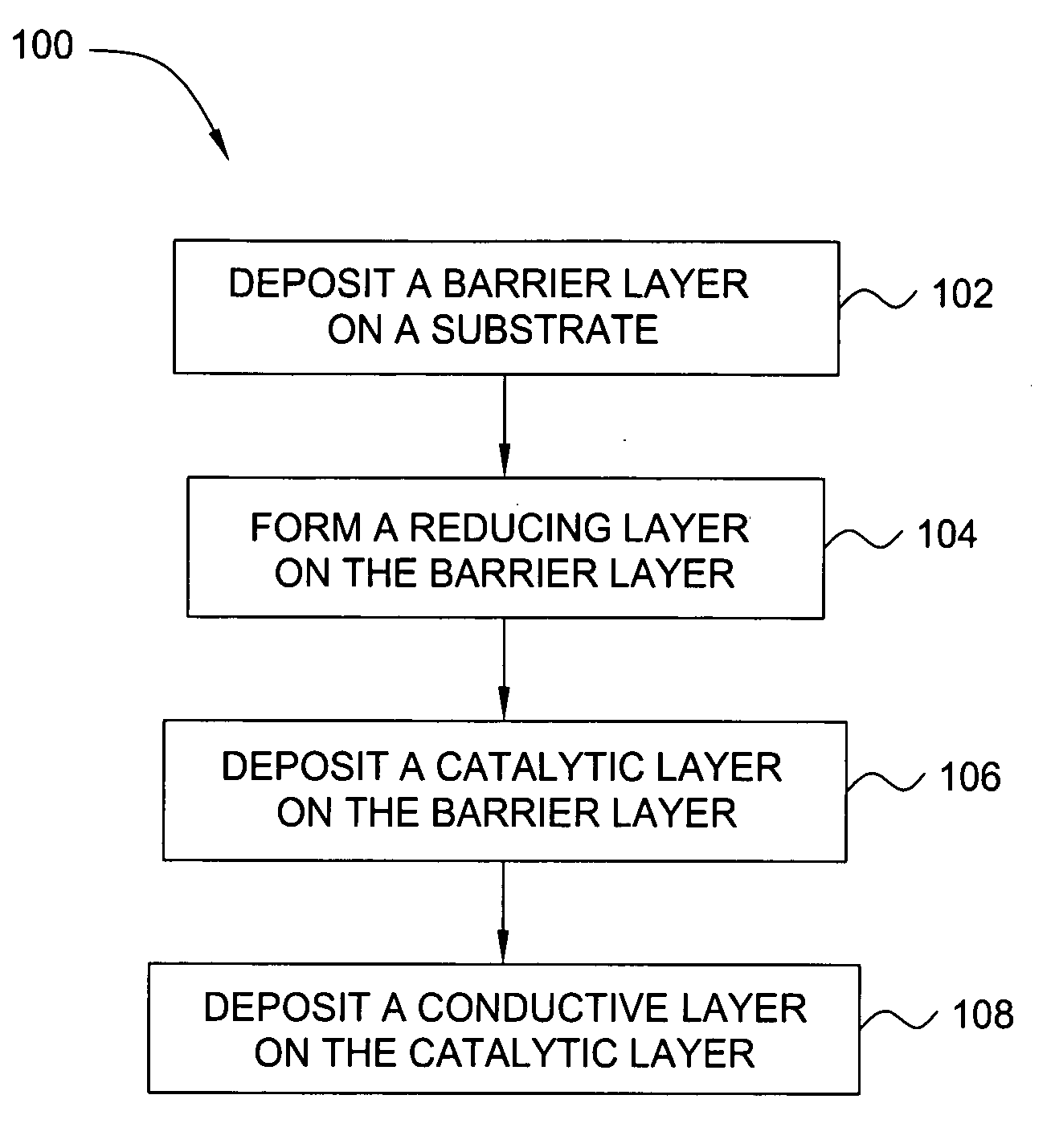
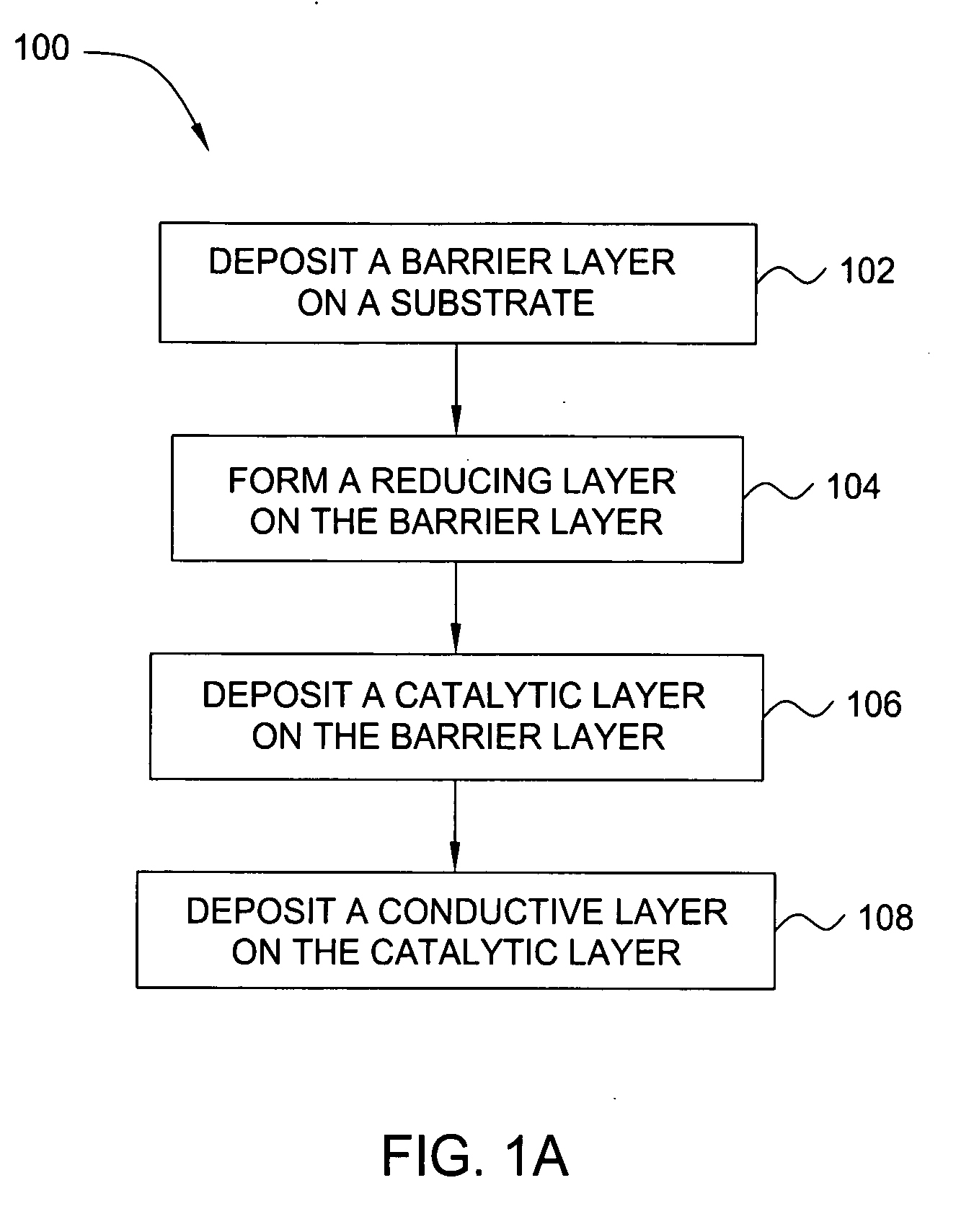
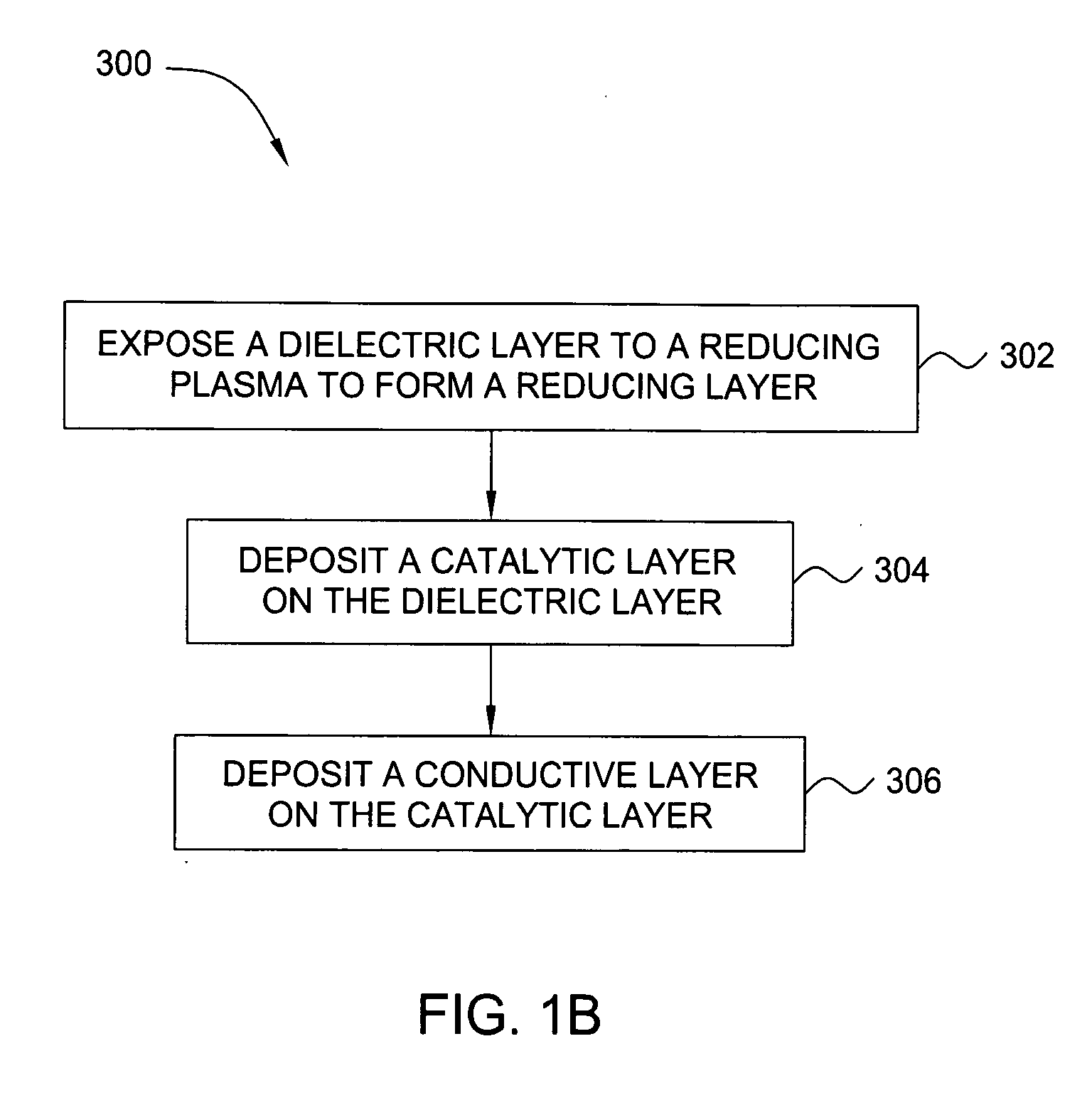
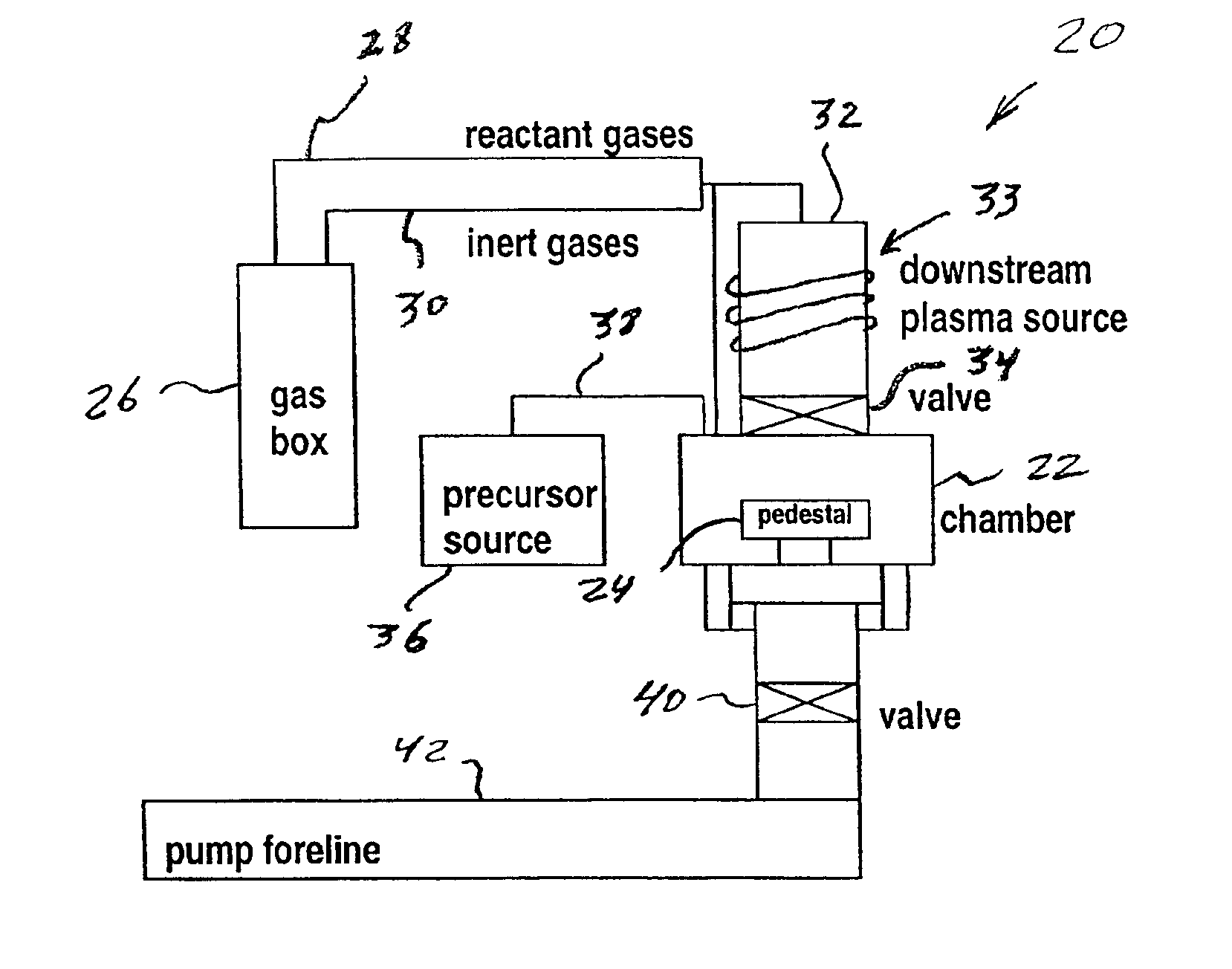
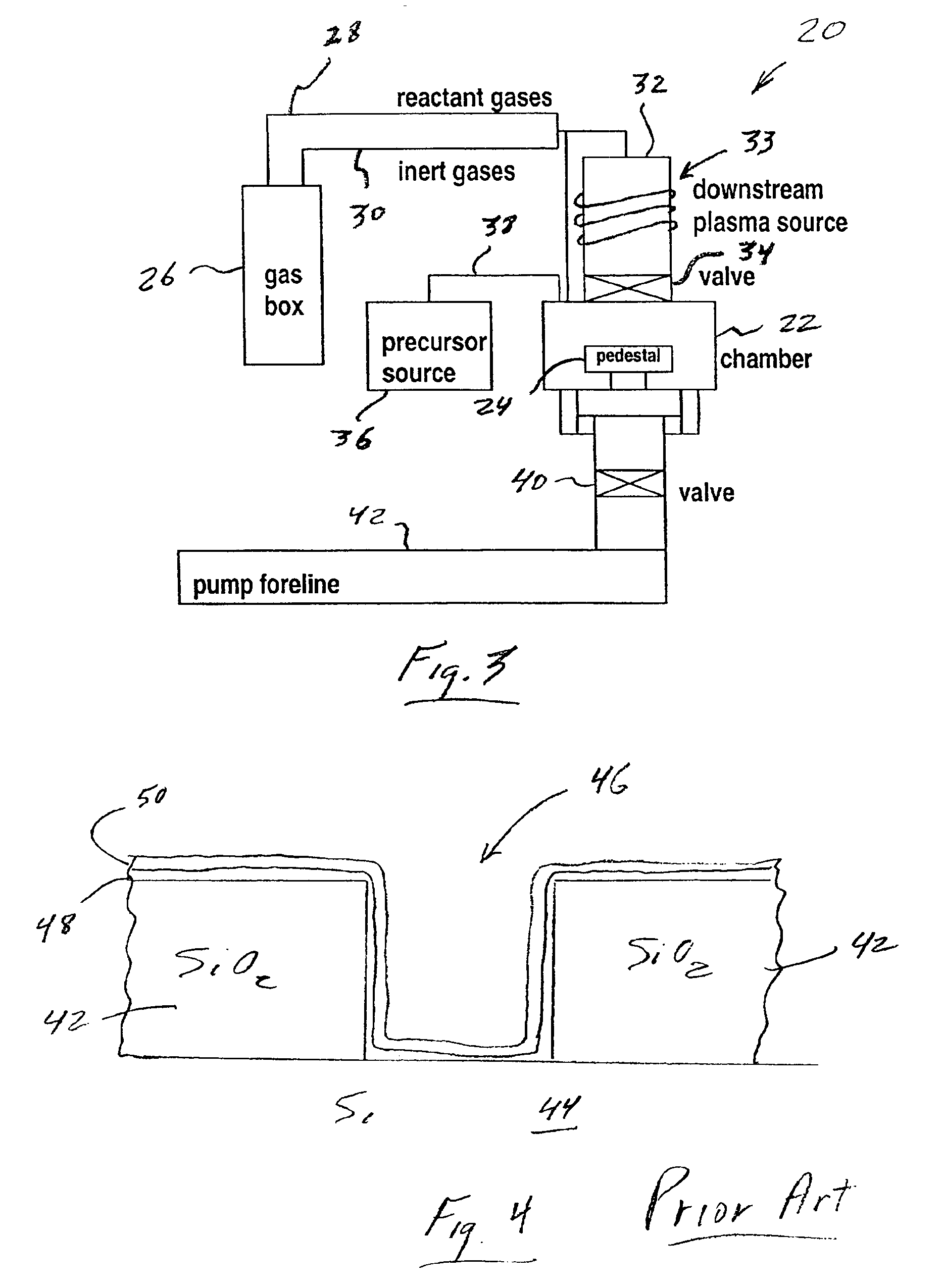
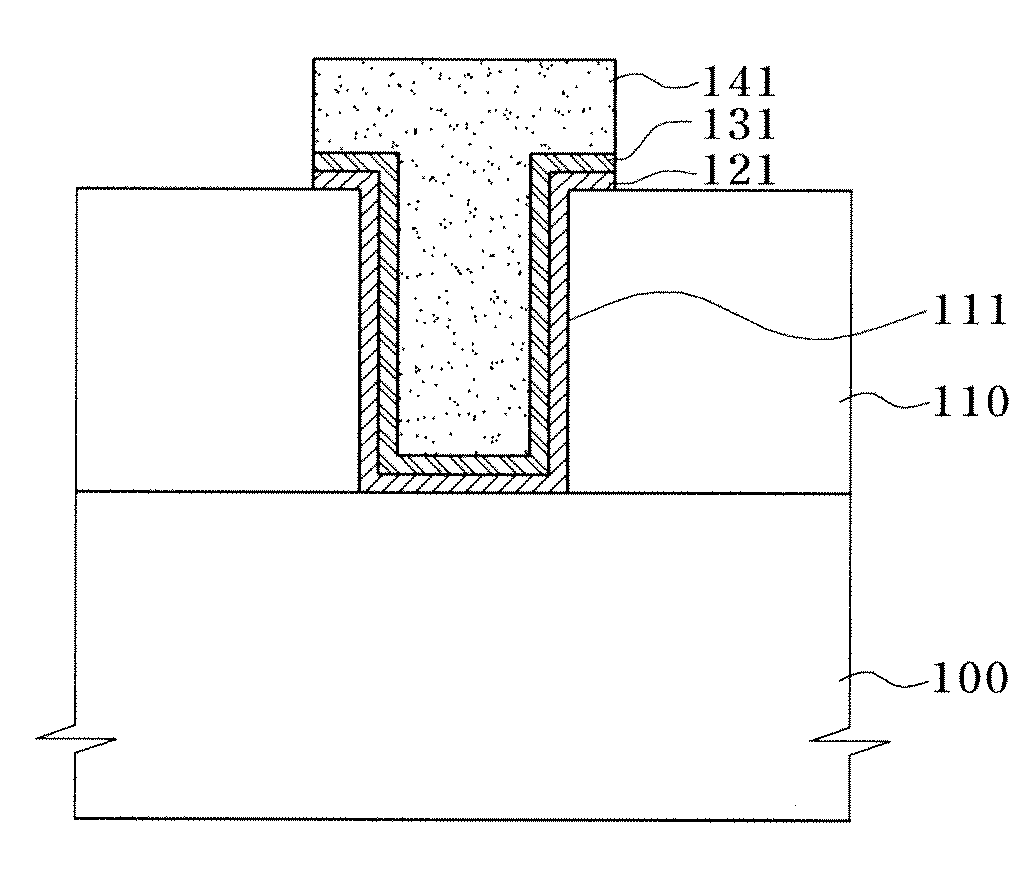
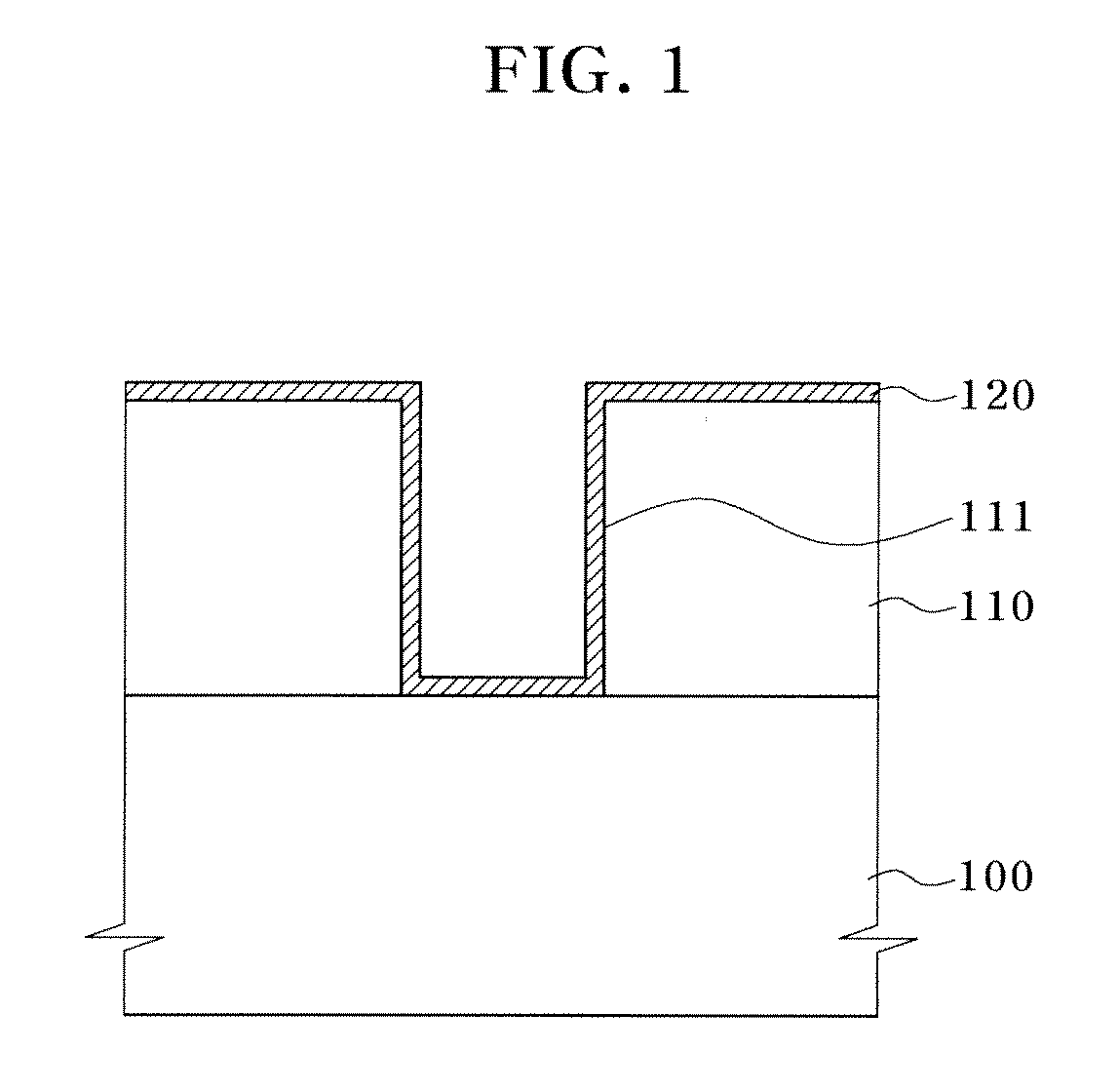
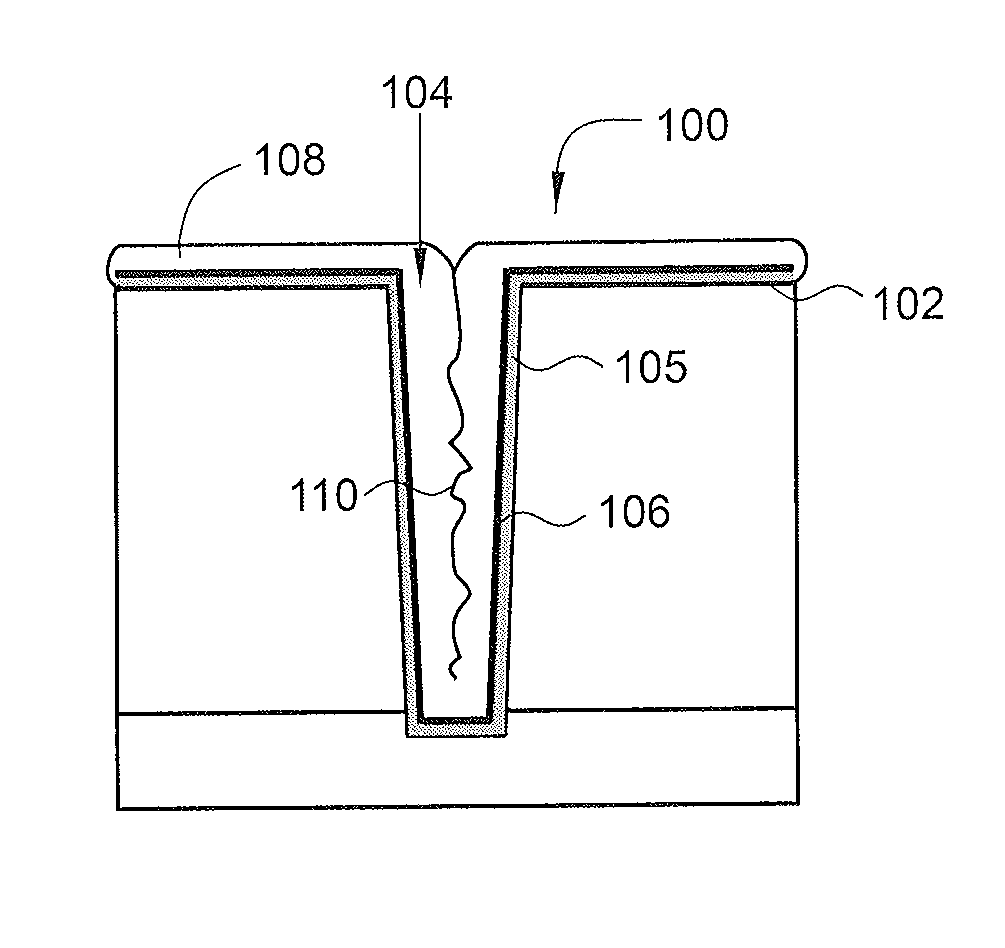
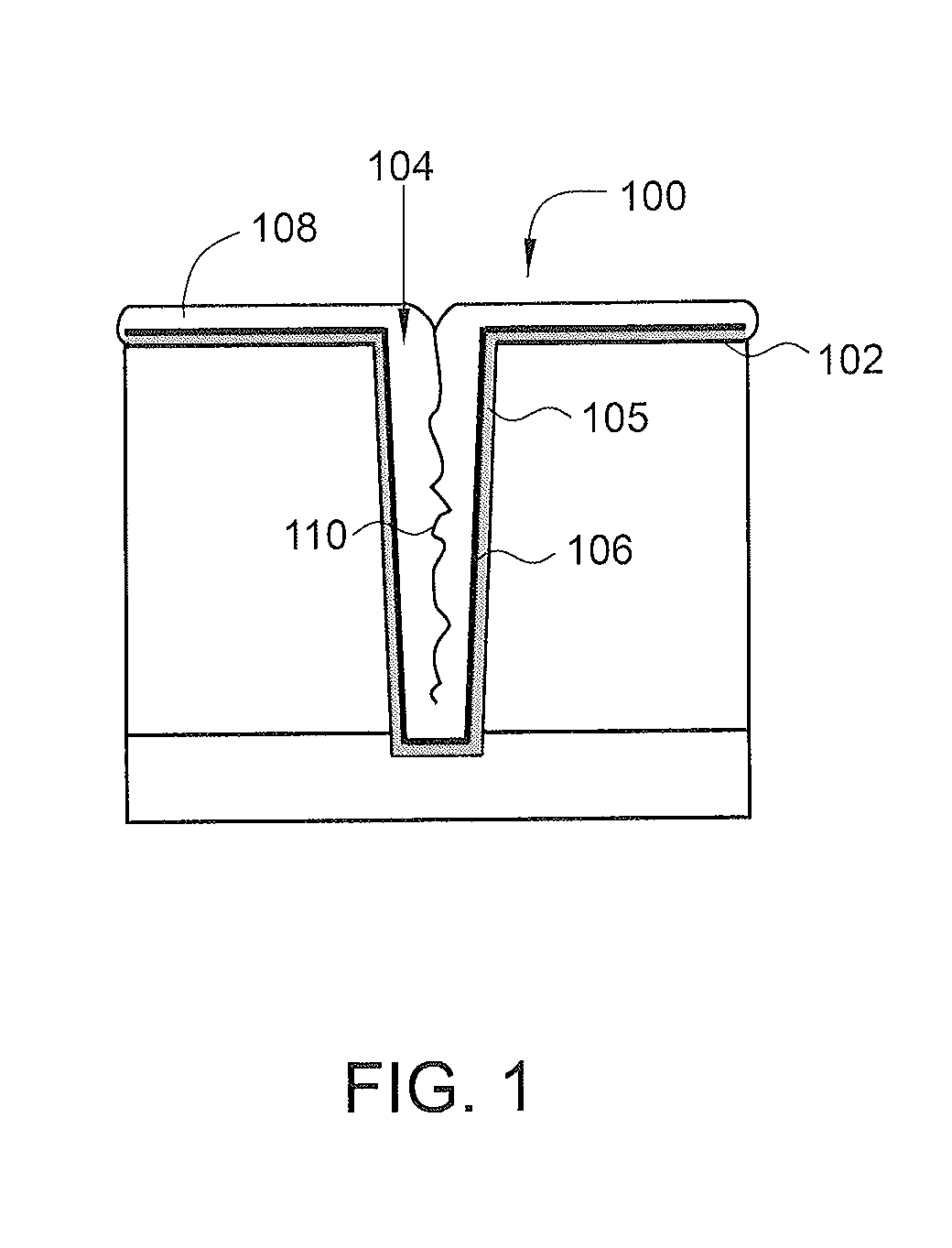
Deposition of an intermediate catalytic layer on a barrier layer for copper metallization
InactiveUS20060240187A1Solid-state devicesSemiconductor/solid-state device manufacturingIridiumSilanes
In one embodiment, a method for depositing a conductive material on a substrate is provided which includes exposing a substrate containing a barrier layer to a volatile reducing precursor to form a reducing layer during a soak process, exposing the reducing layer to a catalytic-metal precursor to deposit a catalytic metal-containing layer on the barrier layer, and depositing a conductive layer (e.g., copper) on the catalytic metal-containing layer. The volatile reducing precursor may include phosphine, diborane, silane, a plasma thereof, or a combination thereof and be exposed to the substrate for a time period within a range from about 1 second to about 30 seconds during the soak process. The catalytic metal-containing layer may contain ruthenium, cobalt, rhodium, iridium, nickel, palladium, platinum, silver, or copper. In one example, the catalytic metal-containing layer is deposited by a vapor deposition process utilizing ruthenium tetroxide formed by an in situ process.
Owner:APPLIED MATERIALS INC
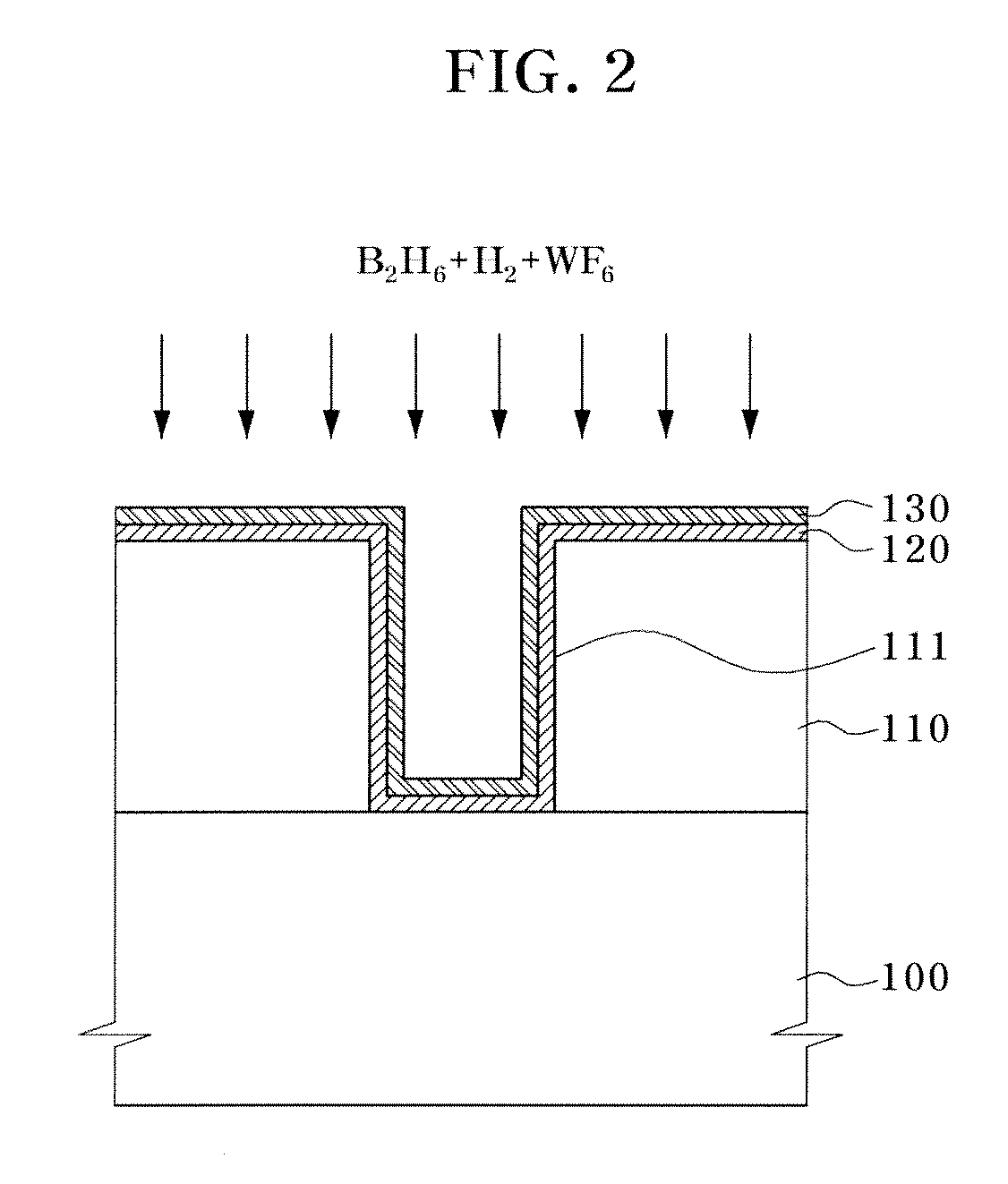
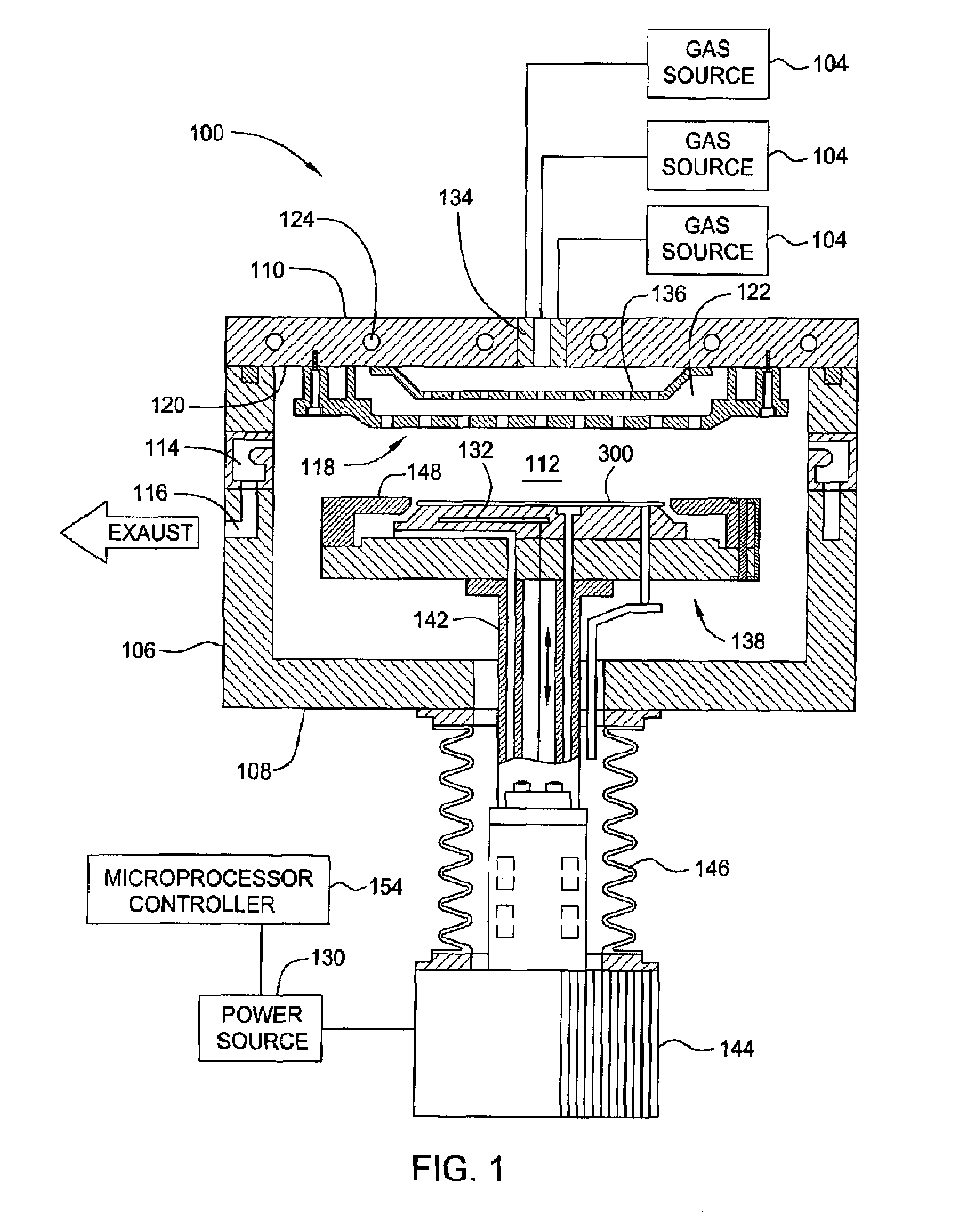
Atomic layer deposition of tungsten material
InactiveCN101308794ASemiconductor/solid-state device manufacturingChemical vapor deposition coatingGas phaseNucleation
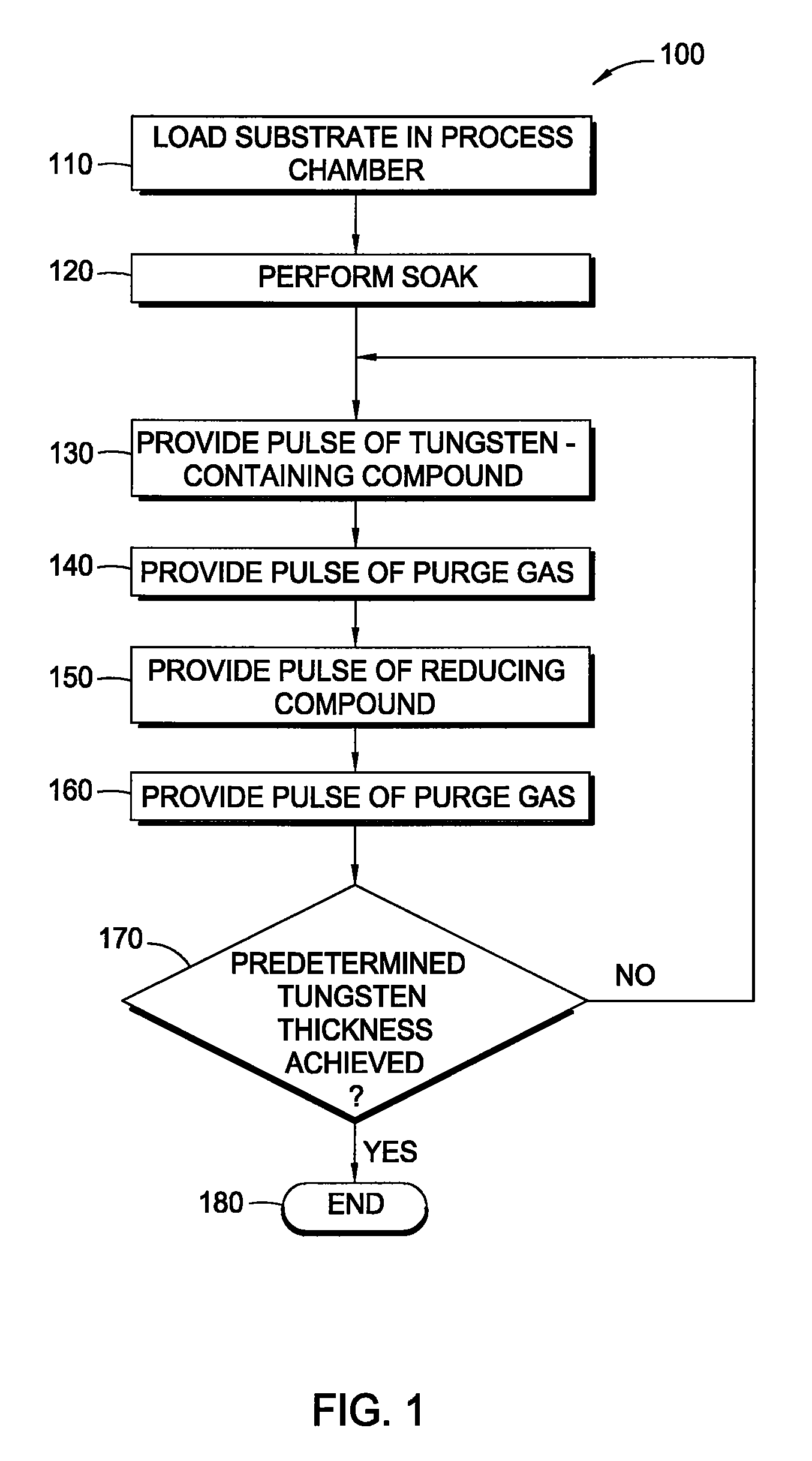
An implementing mode of the invention provides an improved technology for depositing materials containing tungsten. The technology utilizes an infusion technology and a gaseous phase deposition technology, such as atomic layer deposition (ALD), to provide tungsten-containing materials with obviously improved surface evenness and yield. In one implementing mode, a method for forming tungsten-containing materials on a substrate is provided. The method comprises deposing a substrate, which contains a bottom coating deposited thereon, in a technological chamber; exposing the substrate orderly in a precursor of tungsten and reducing gases so as to deposit a tungsten nucleation layer on the bottom coating, during the ALD technology; and depositing a tungsten block layer on the tungsten nucleation layer. The invention is characterized in that the reducing gases comprise a hydrogen gas / hydride flow ratio of 40:1, 100:1, 500:1, 800: 1, 1000:1 or more, and comprise hydride such as diborane, silicane or silicoethane.
Owner:APPLIED MATERIALS INC
Atomic layer deposition of tungsten materials
ActiveUS7964505B2Improve conductivityImprove throughputSolid-state devicesSemiconductor/solid-state device manufacturingHydrogenSilanes
Embodiments of the invention provide an improved process for depositing tungsten-containing materials. The process utilizes soak processes and vapor deposition processes, such as atomic layer deposition (ALD) to provide tungsten films having significantly improved surface uniformity and production level throughput. In one embodiment, a method for forming a tungsten-containing material on a substrate is provided which includes positioning a substrate within a process chamber, wherein the substrate contains an underlayer disposed thereon, exposing the substrate sequentially to a tungsten precursor and a reducing gas to deposit a tungsten nucleation layer on the underlayer during an ALD process, wherein the reducing gas contains a hydrogen / hydride flow rate ratio of about 40:1, 100:1, 500:1, 800:1, 1,000:1, or greater, and depositing a tungsten bulk layer on the tungsten nucleation layer. The reducing gas contains a hydride compound, such as diborane, silane, or disilane.
Owner:APPLIED MATERIALS INC
Methods for depositing tungsten layers employing atomic layer deposition techniques
InactiveUS7405158B2Solid-state devicesSemiconductor/solid-state device manufacturingDisilaneGas phase
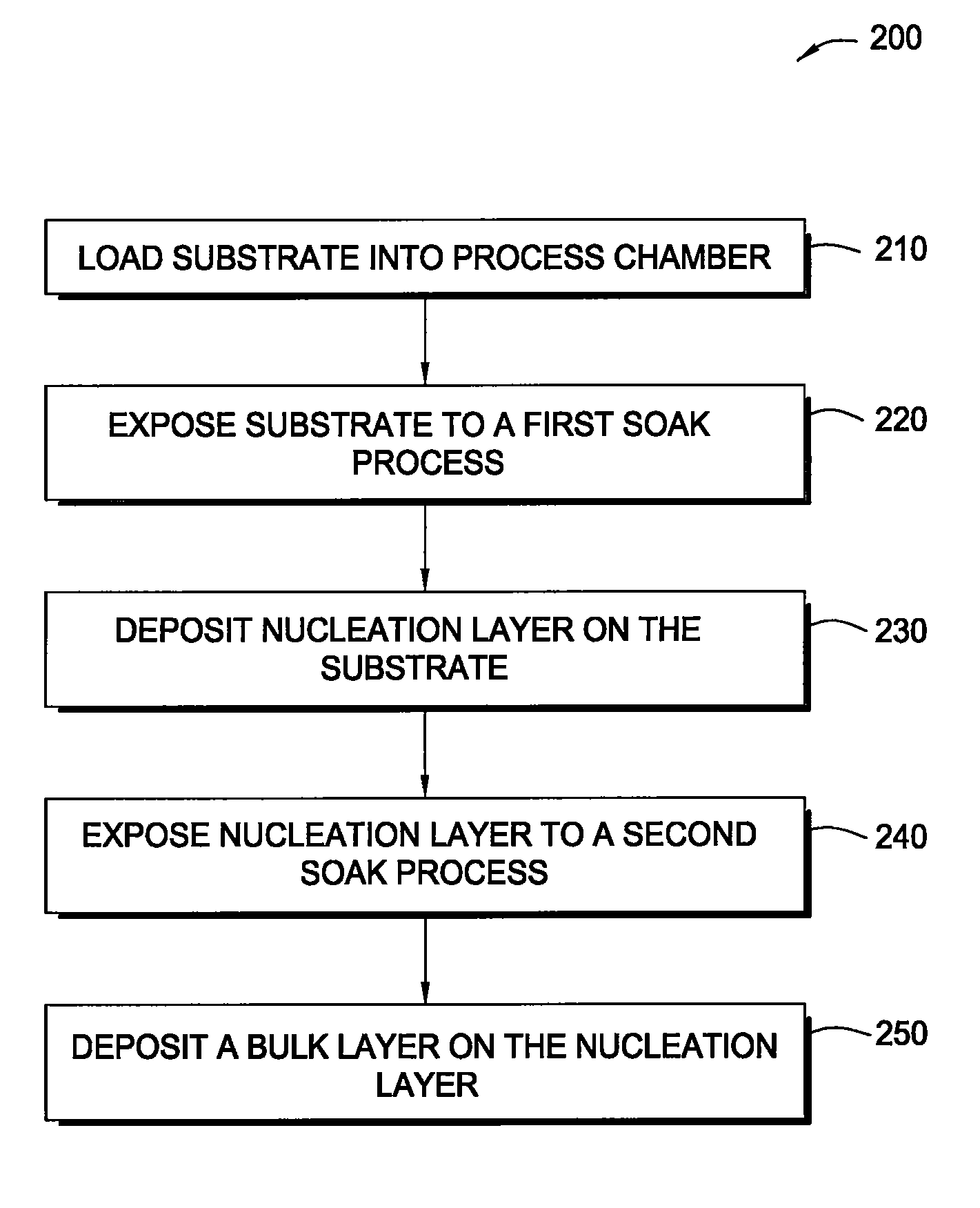
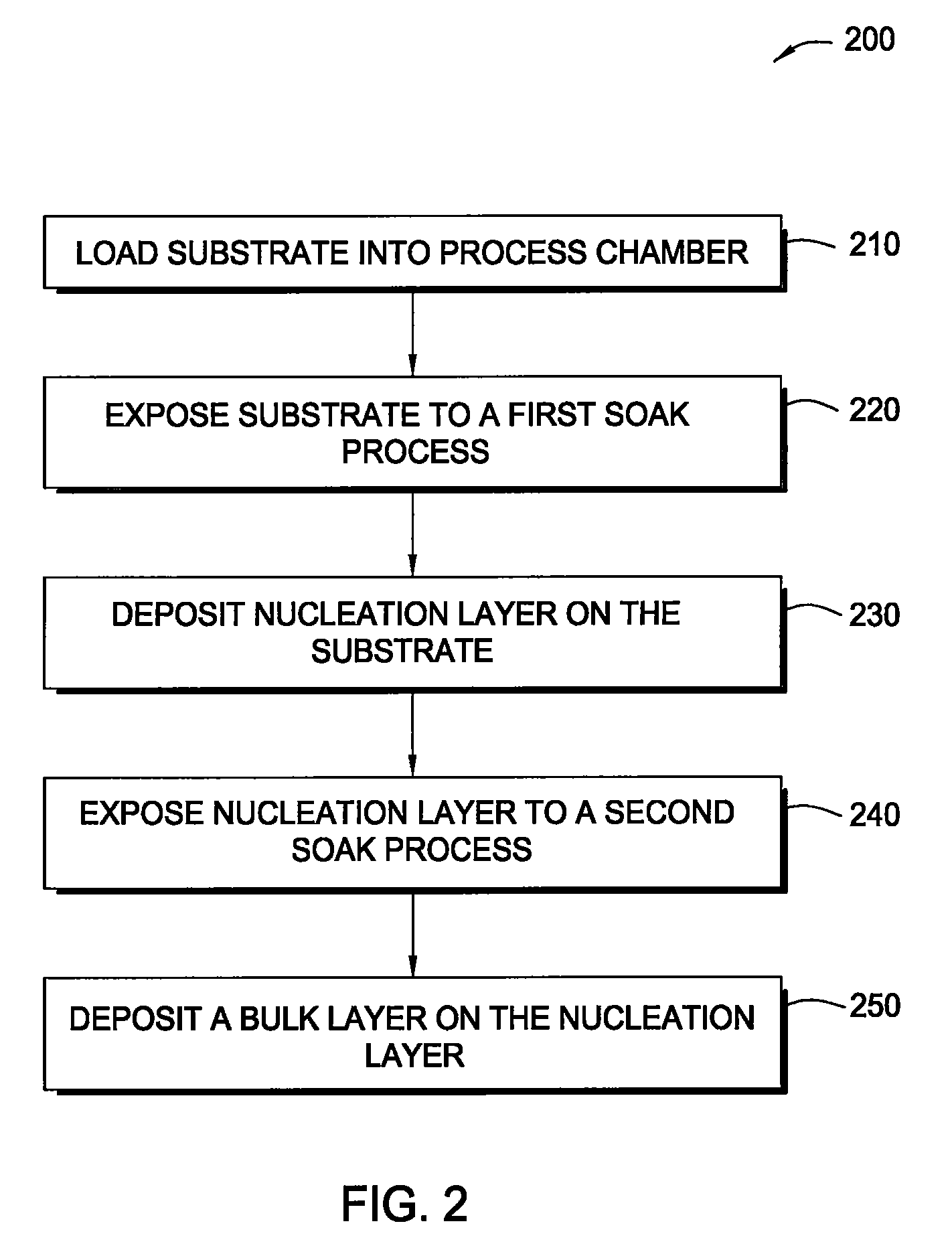
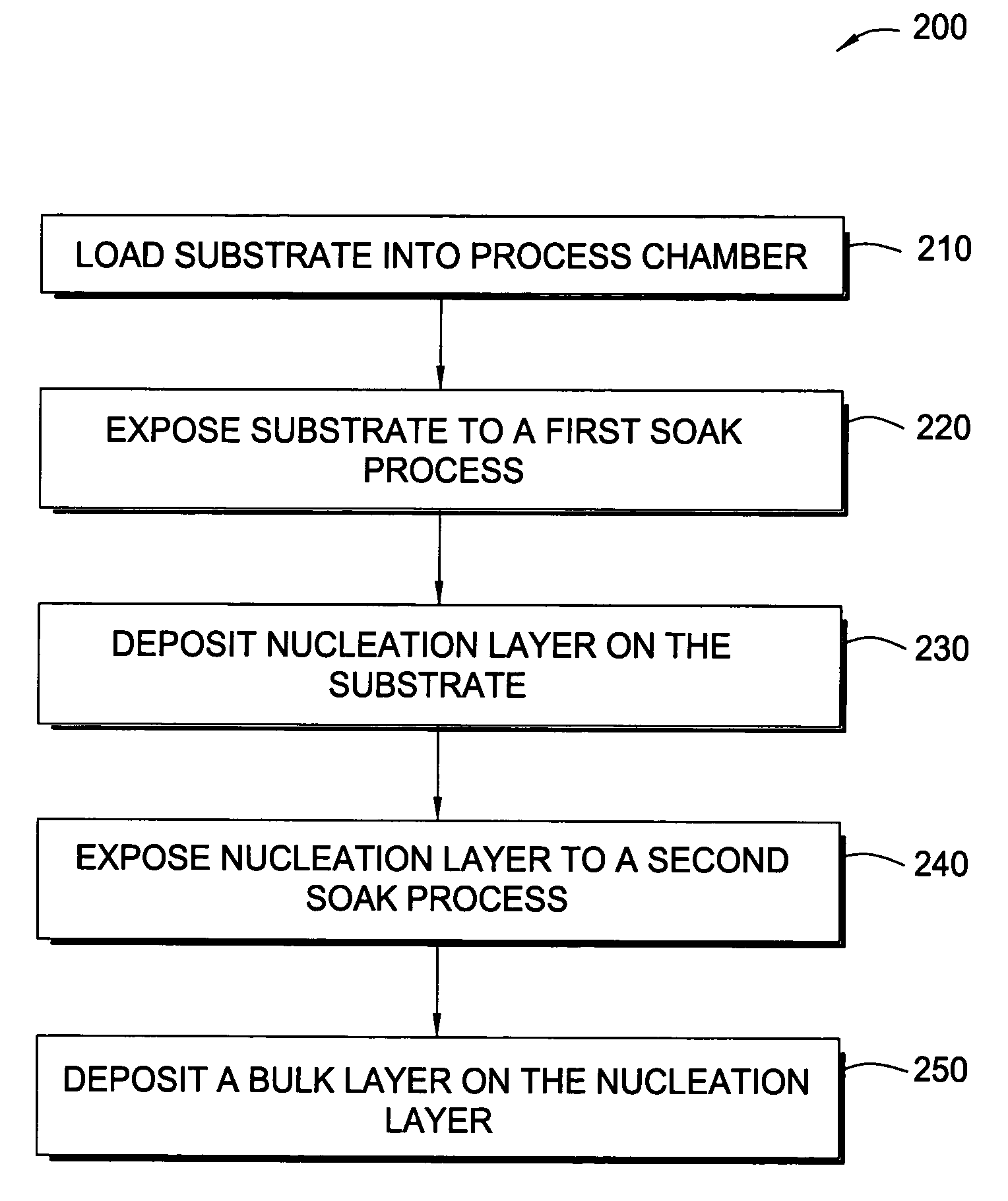
In one embodiment of the invention, a method for forming a tungsten-containing layer on a substrate is provided which includes positioning a substrate containing a barrier layer disposed thereon in a process chamber, exposing the substrate to a first soak process for a first time period and depositing a nucleation layer on the barrier layer by flowing a tungsten-containing precursor and a reductant into the process chamber. The method further includes exposing the nucleation layer to a second soak process for a second time period and depositing a bulk layer on the nucleation layer. In one example, the barrier layer contains titanium nitride, the first and second soak processes independently comprise at least one reducing gas selected from the group consisting of hydrogen, silane, disilane, dichlorosilane, borane, diborane, derivatives thereof and combinations thereof and the nucleation layer may be deposited by an atomic layer deposition process or a pulsed chemical vapor deposition process while the bulk layer may be deposited by a chemical vapor deposition process or a physical vapor deposition process.
Owner:APPLIED MATERIALS INC
Methods for depositing tungsten layers employing atomic layer deposition techniques
InactiveUS20060009034A1Solid-state devicesSemiconductor/solid-state device manufacturingDisilaneGas phase
In one embodiment of the invention, a method for forming a tungsten-containing layer on a substrate is provided which includes positioning a substrate containing a barrier layer disposed thereon in a process chamber, exposing the substrate to a first soak process for a first time period and depositing a nucleation layer on the barrier layer by flowing a tungsten-containing precursor and a reductant into the process chamber. The method further includes exposing the nucleation layer to a second soak process for a second time period and depositing a bulk layer on the nucleation layer. In one example, the barrier layer contains titanium nitride, the first and second soak processes independently comprise at least one reducing gas selected from the group consisting of hydrogen, silane, disilane, dichlorosilane, borane, diborane, derivatives thereof and combinations thereof and the nucleation layer may be deposited by an atomic layer deposition process or a pulsed chemical vapor deposition process while the bulk layer may be deposited by a chemical vapor deposition process or a physical vapor deposition process.
Owner:APPLIED MATERIALS INC
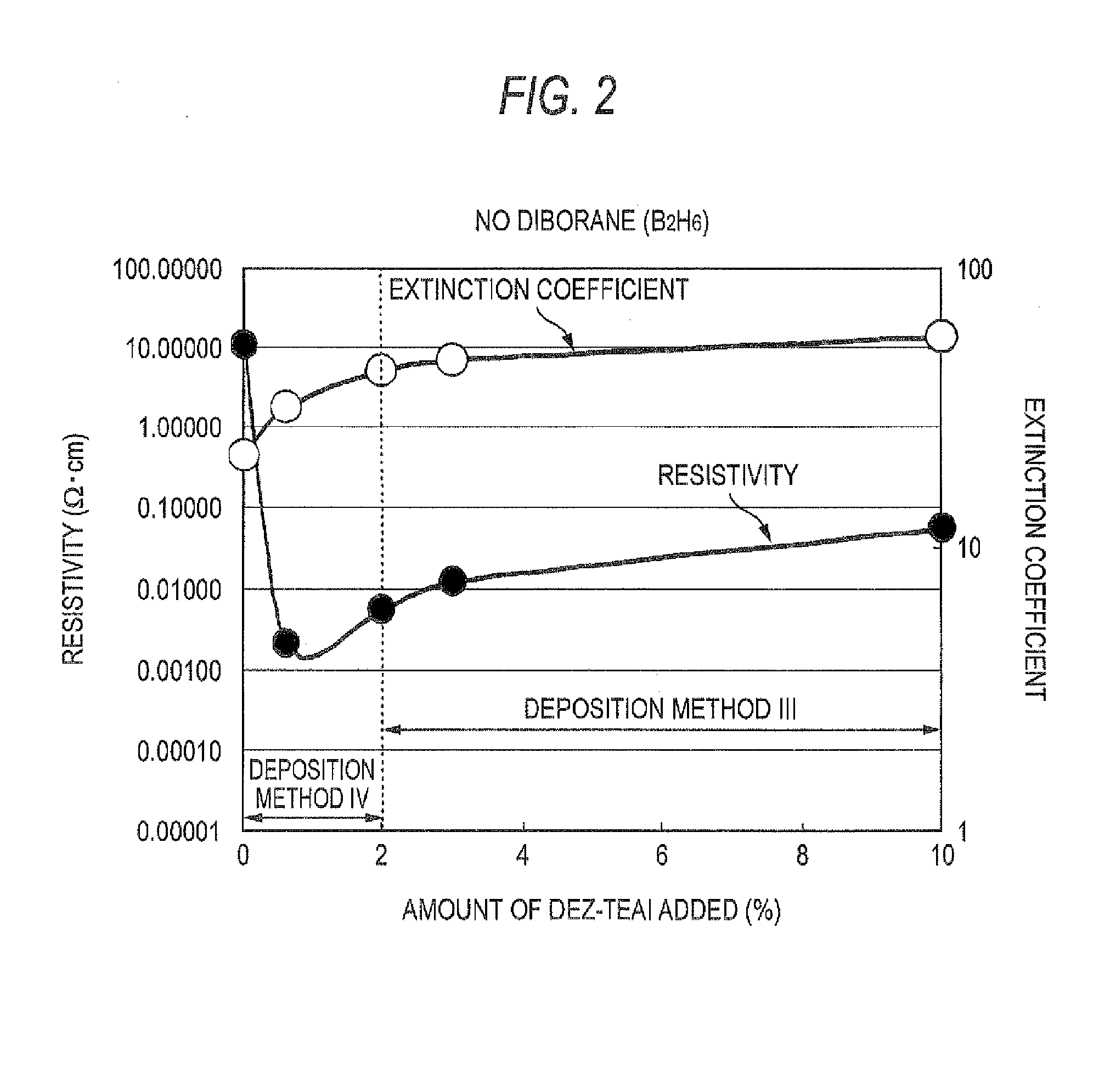
Process For Producing Zno Transparent Conductive Film By Mocvd (Metal-Organic Chemical Vapor Deposition) Method
InactiveUS20080032044A1Low costEliminate useChemical vapor deposition coatingPhotovoltaic energy generationWater vaporGas phase
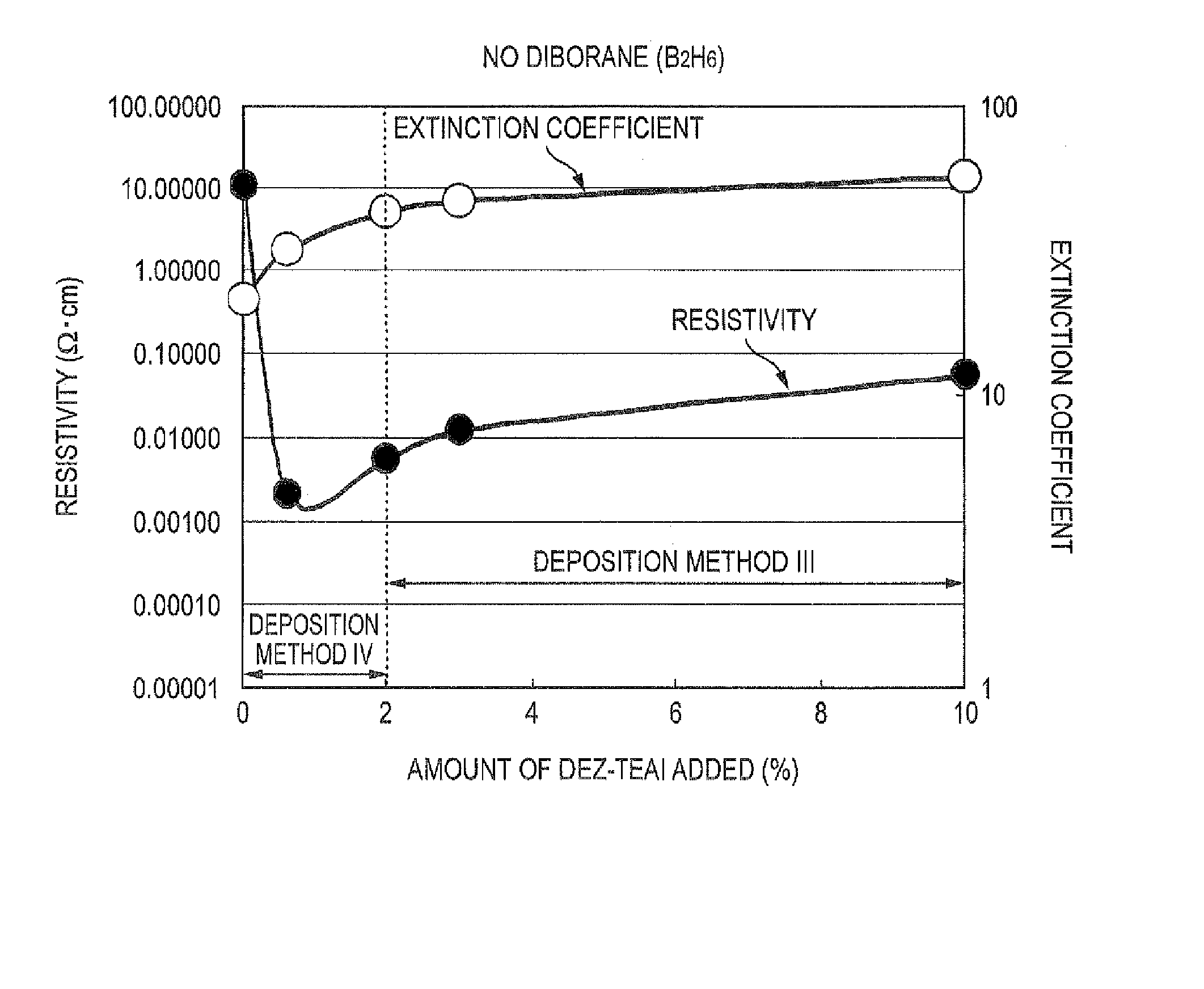
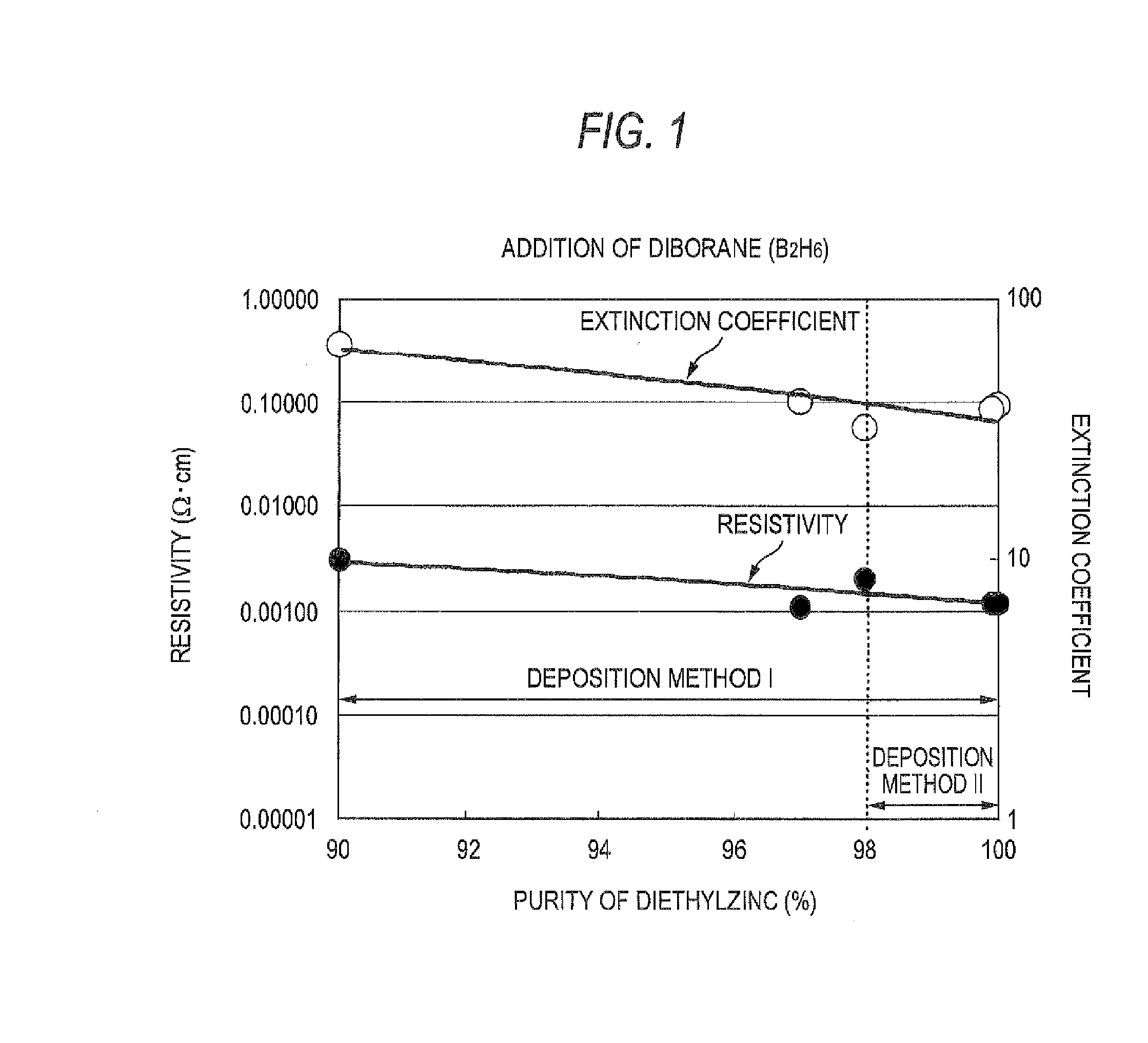
The triethylaluminum contained as an impurity in low-purity raw-material diethylzinc, which is inexpensive, is utilized as an additive to reduce the cost of film formation. Diethylzinc having a low purity (99.99-98% or 99.99-90%) is used as a raw material to produce a ZnO transparent conductive film by the MOCVD (metal-organic chemical vapor deposition) method. Water vapor (H2O) is used as an oxidizing agent and the triethylaluminum contained as an impurity in the raw material is utilized as an additive (diborane is further added as an additive) to cause the diethylzinc, the water vapor (H2O), and the triethylaluminum (and the diborane) to undergo a vapor-phase reaction to produce a ZnO transparent conductive film.
Owner:SHOWA SHELL SEKIYU KK
Processes for synthesizing alkali metal borohydride compounds
Processes for synthesizing borohydride compounds with reduced energy requirements and high efficiency are disclosed. The processes include the reaction of a base with a borane complex or diborane to produce a borohydride compound of formula YBH4, where Y is a monovalent cationic moiety.
Owner:MILLENNIUM CELL
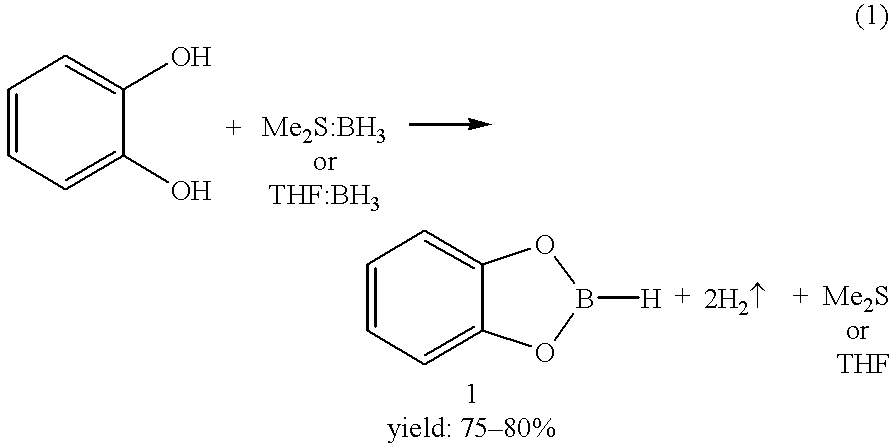
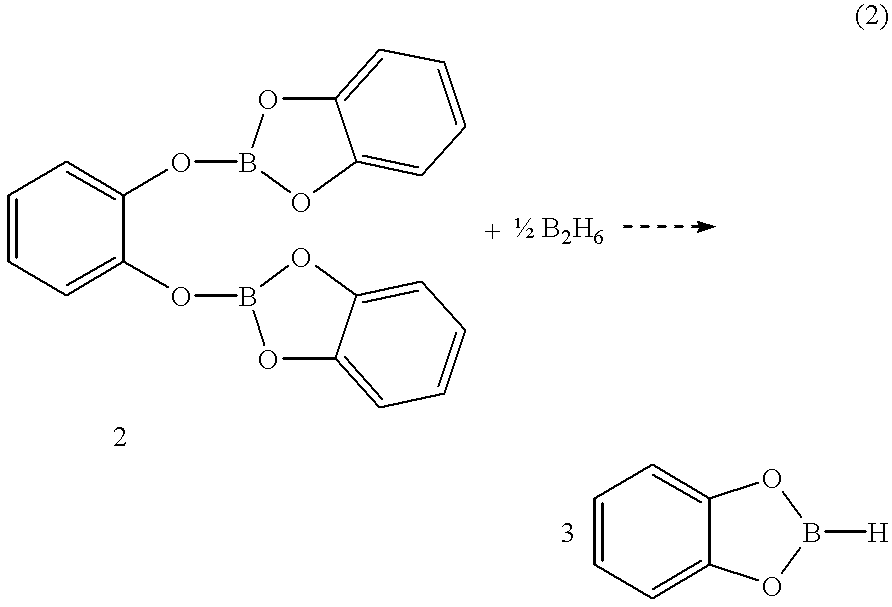
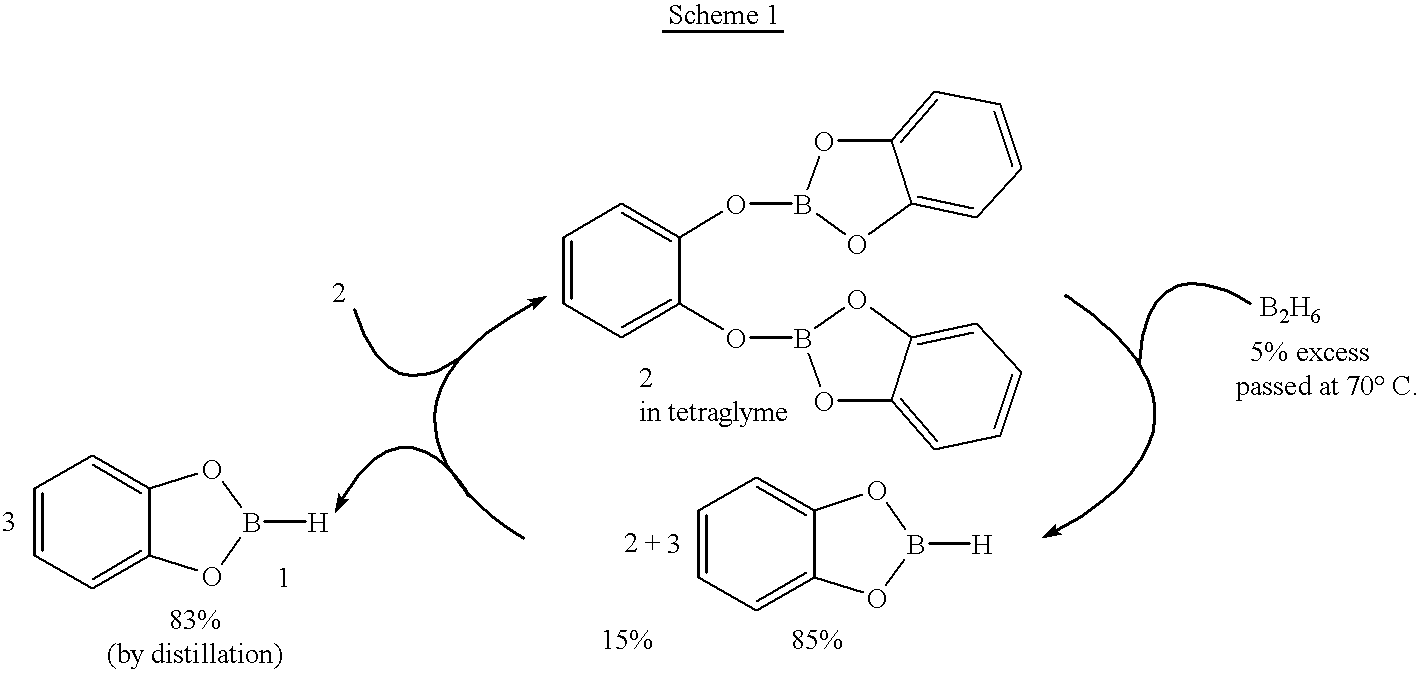
Economical and convenient procedures for the synthesis of catecholborane
New, economical and convenient procedures for the preparation of catecholborane in high chemically pure form using tri-O-phenylene bis borate readily prepared from the reaction of catechol with boric acid, and diborane or borane-Lewis base complexes.
Owner:ALDRICH CHEM
Methods of forming tungsten nucleation layer
InactiveUS6905543B1Eliminate delaysHighly active andPolycrystalline material growthFrom chemically reactive gasesTitanium nitrideNucleation
The nucleation delay in the formation of a tungsten layer on a substrate is reduced or eliminated by alternative processes. In one process the substrate is exposed to atomic hydrogen before the tungsten nucleation layer is formed. In the other process the substrate is exposed to a boron hydride such as diborane (B2H6) before the nucleation layer is formed. The process works effectively to reduce or eliminate the tungsten nucleation delay on a variety of surfaces, including silicon, silicon dioxide, silicon nitride and titanium nitride.
Owner:NOVELLUS SYSTEMS
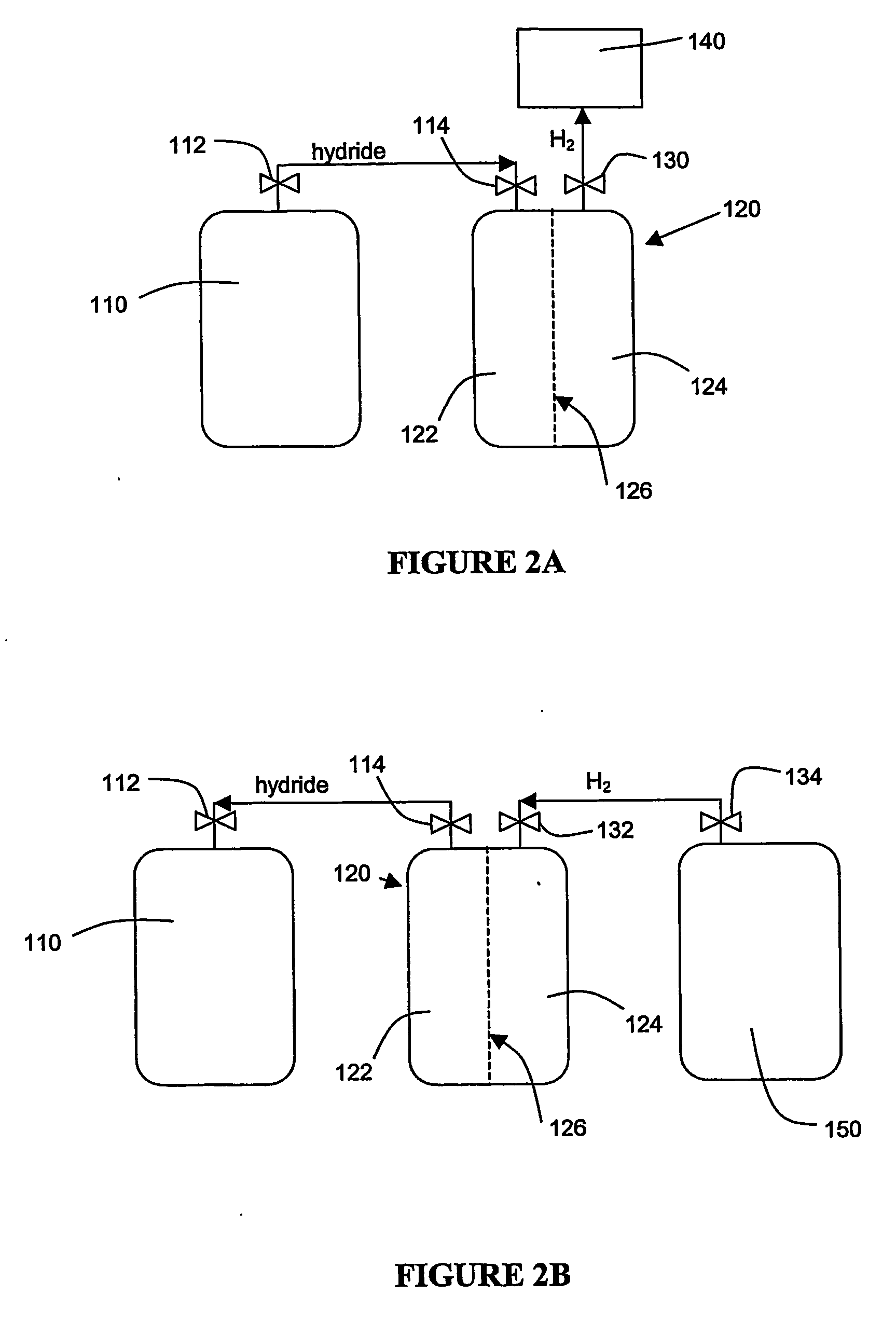
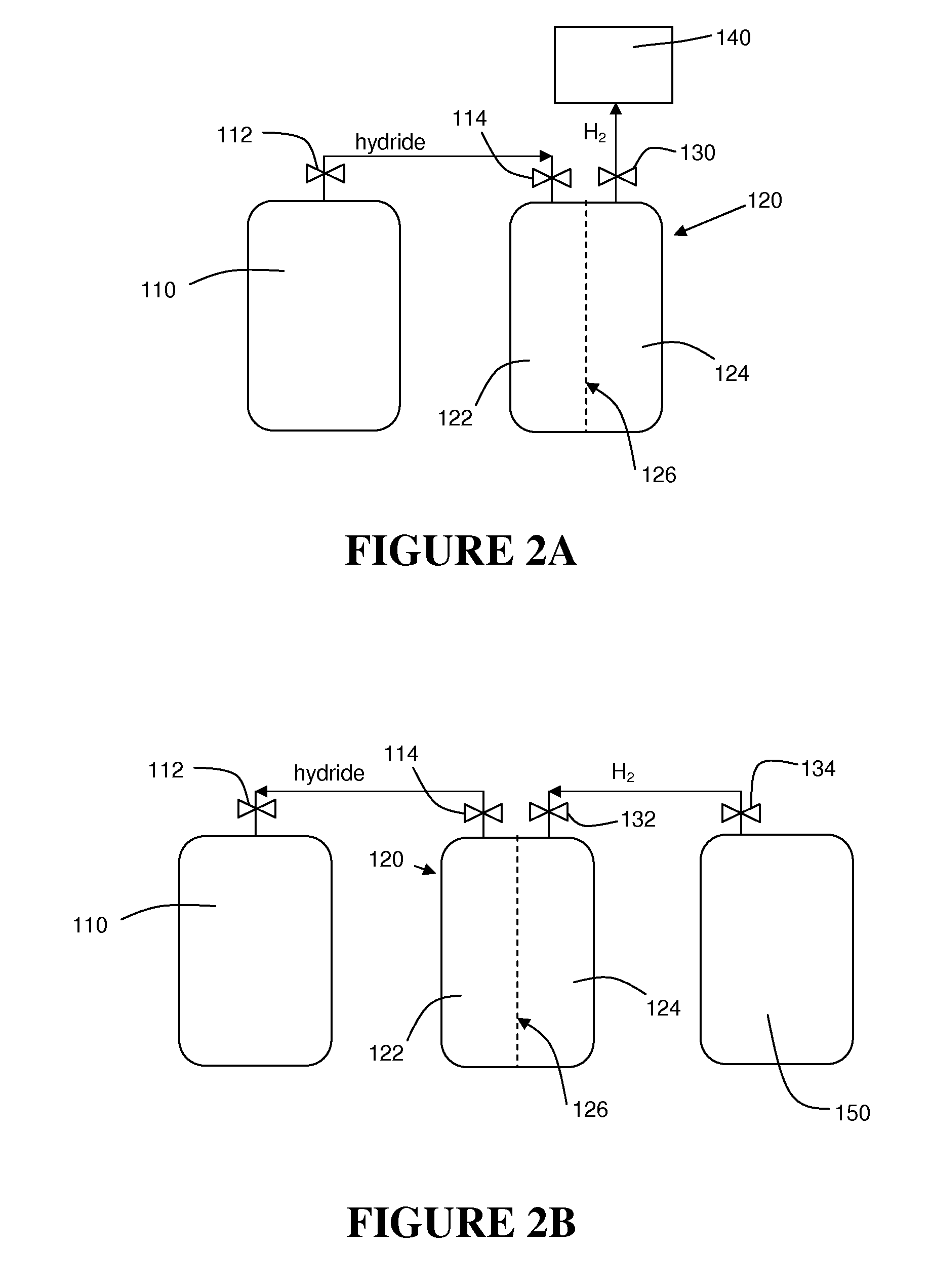
Hydrogen generation
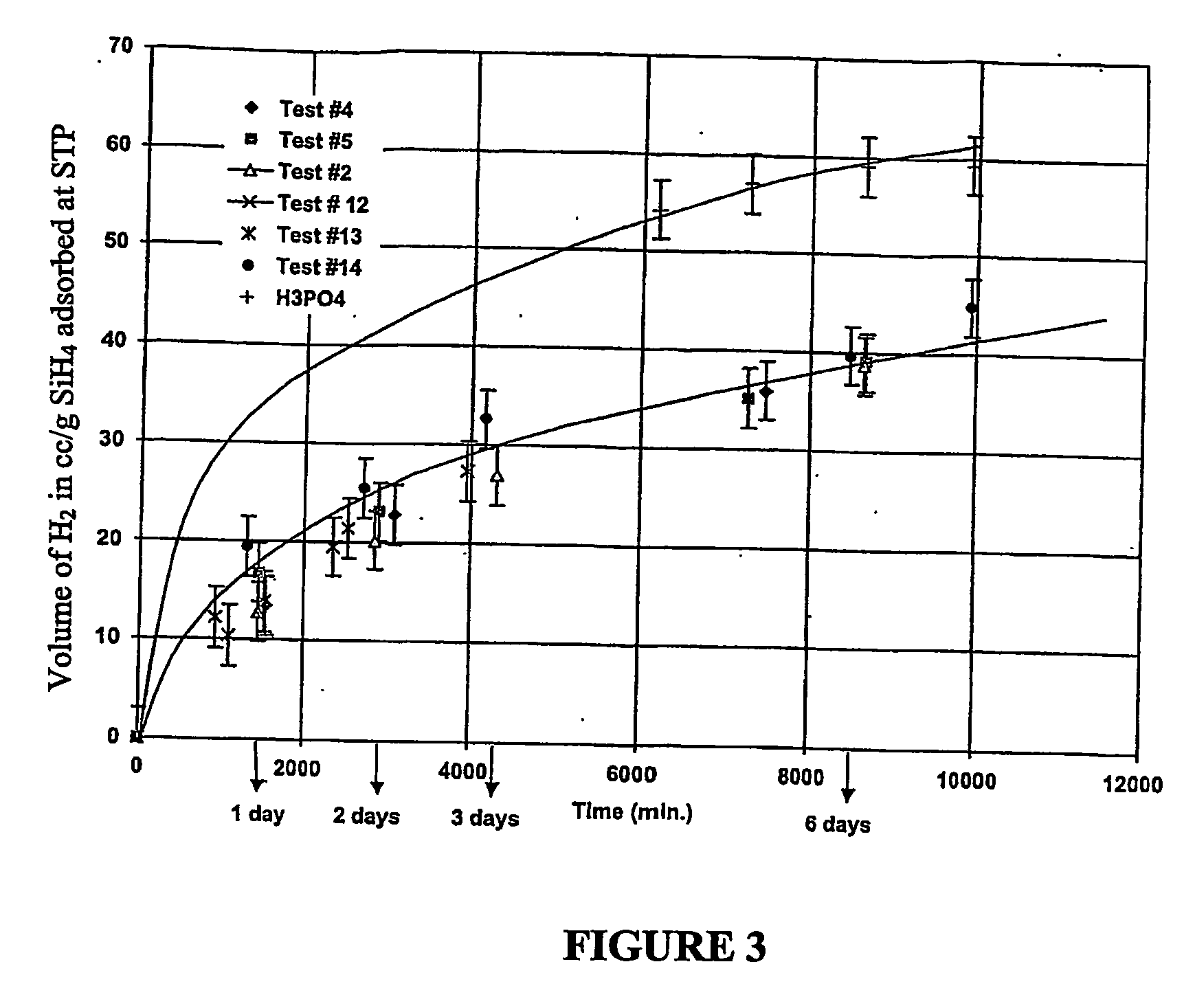
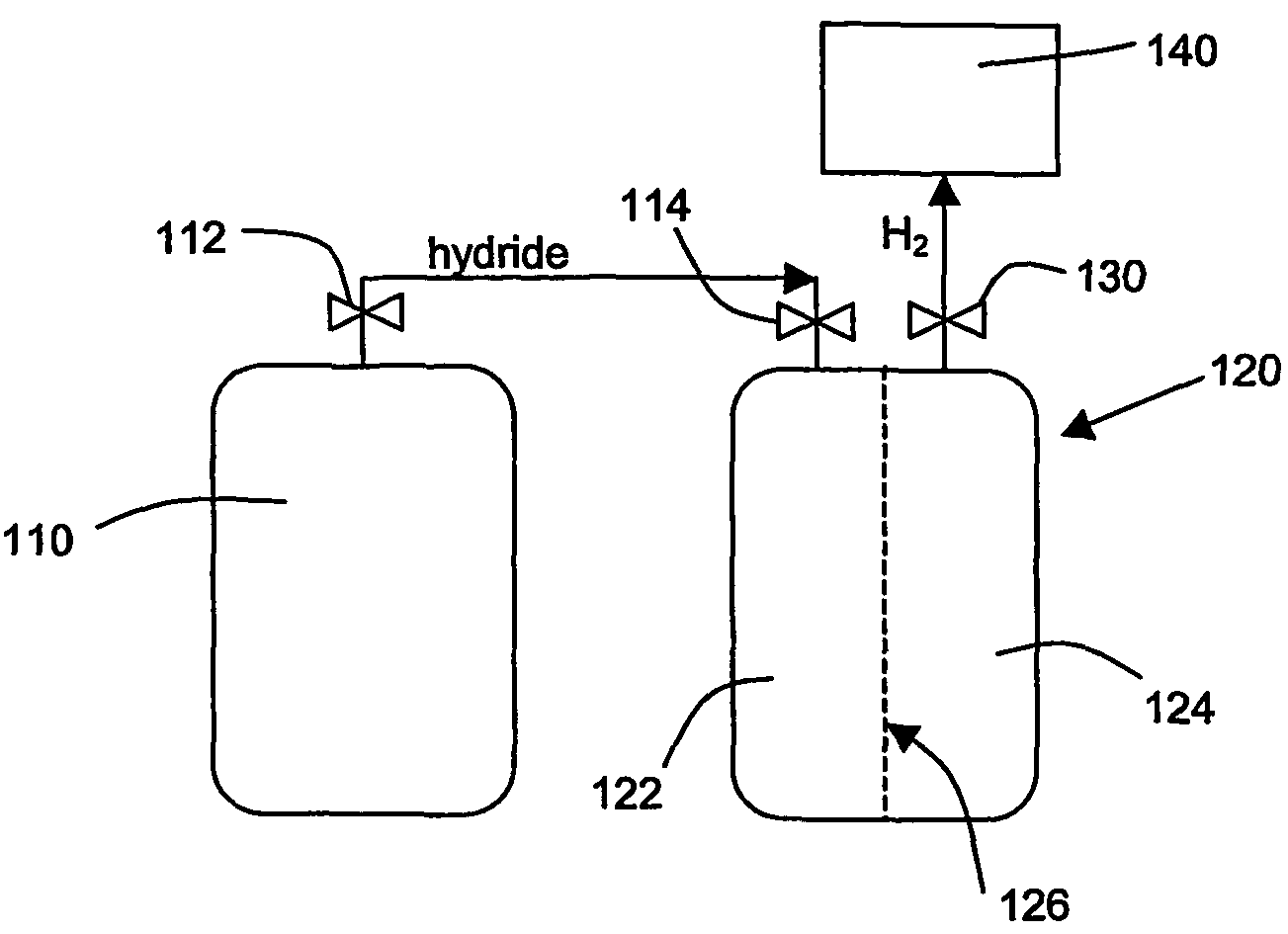
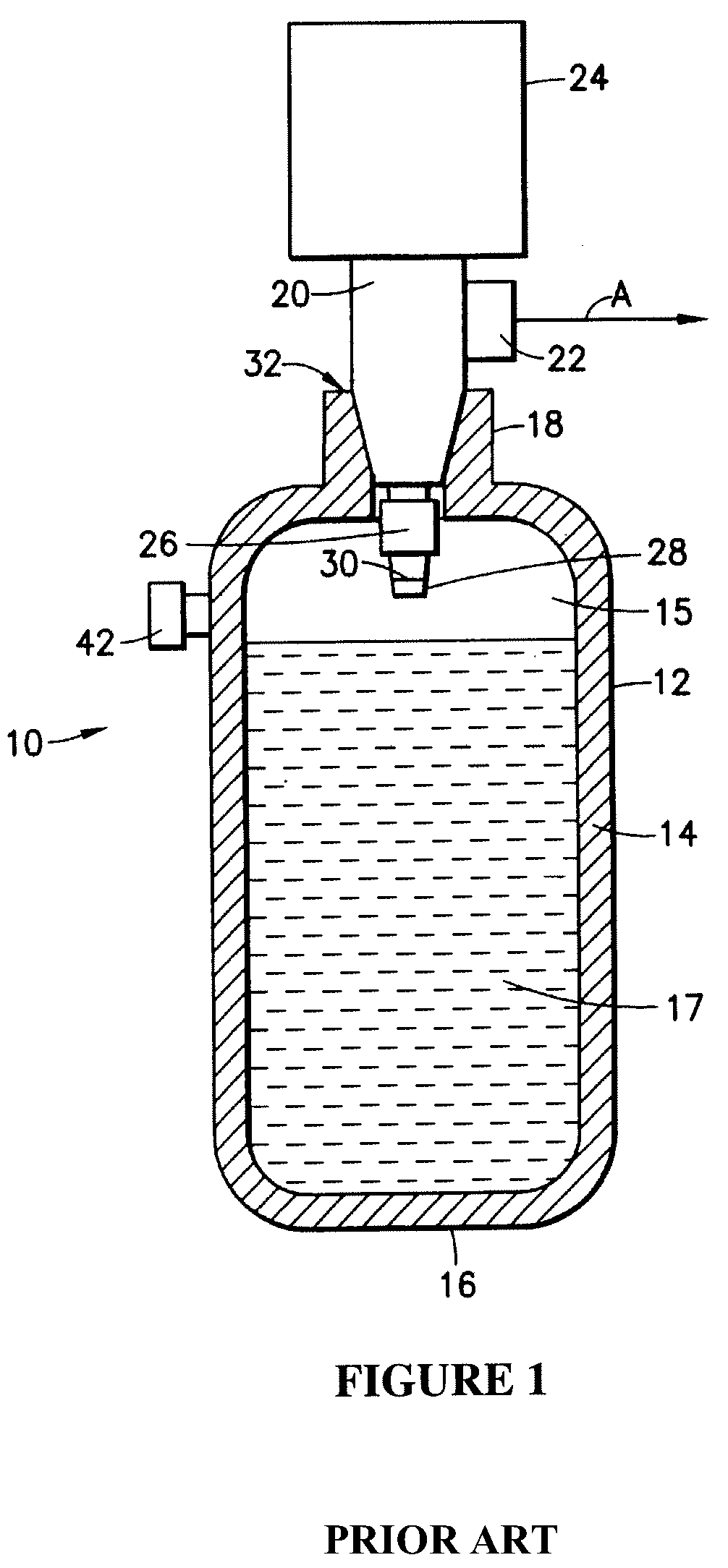
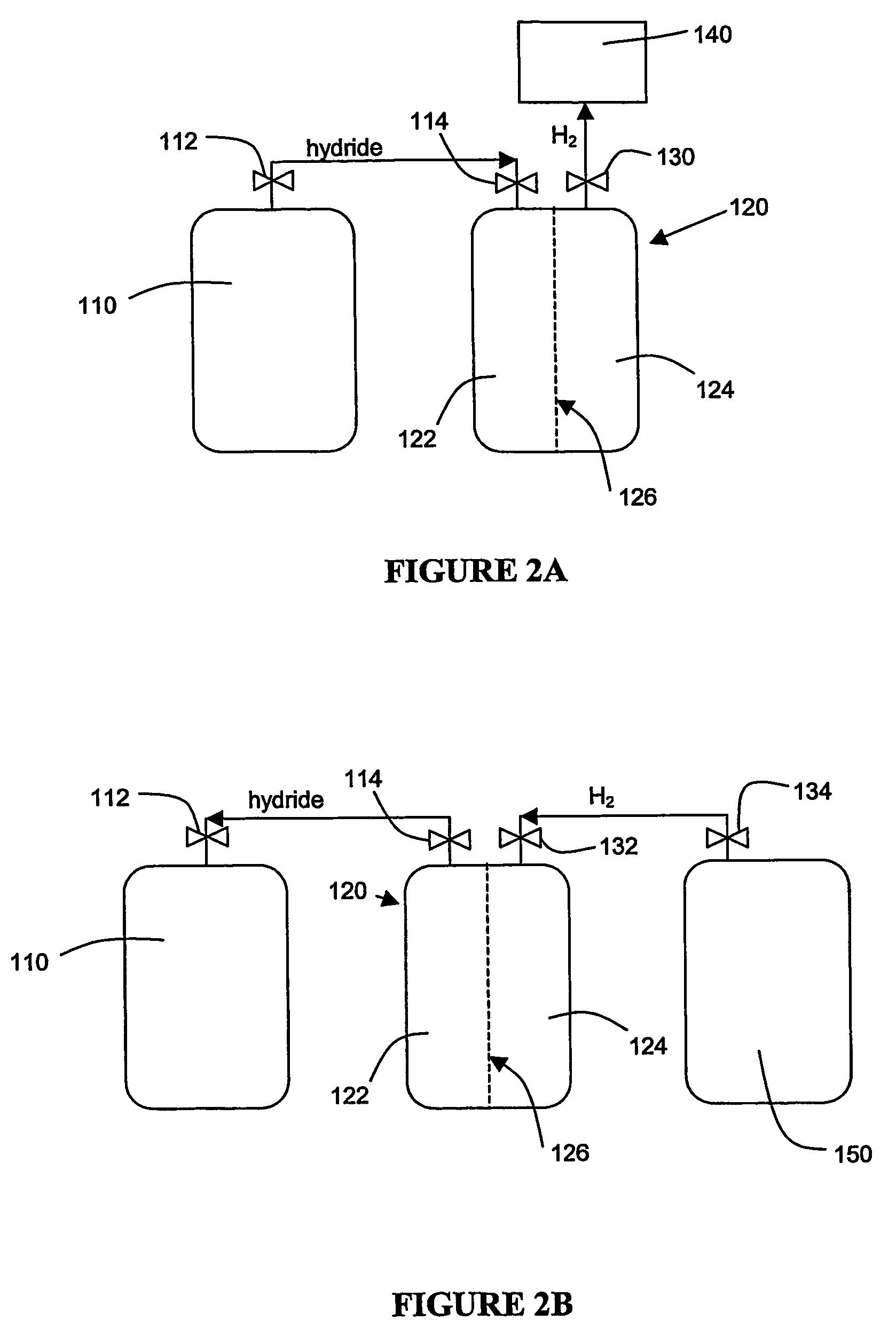
An apparatus and method including storage and dispensing vessels to safely store and dispense gaseous hydrides, where the storage and dispensing vessels contain a solid-phase physical sorbent medium having a physically sorptive affinity for gaseous hydrides, and wherein the gaseous hydride is decomposed in the apparatus to generate hydrogen gas. The gaseous hydrides include, but are not limited to, silane, germane, stibine and diborane. The gaseous hydrides decompose spontaneously and / or decomposition is enhanced using surface modified adsorbents. The hydrogen generated by the apparatus may be used in a fuel cell or other hydrogen gas consuming unit.
Owner:ADVANCED TECH MATERIALS INC
Triborohydride salts as hydrogen storage materials and preparation thereof
InactiveUS20050135996A1Physical/chemical process catalystsAlkali/alkaline-earth/beryllium/magnesium hydridesFuel cellsSlurry
The present invention relates to the use of triborohydride salts as hydrogen storage materials. The present invention also relates to a system of using triborohydride salts to generate hydrogen gas for use in a fuel cell or other hydrogen-consuming device. A novel method of preparing triborohydride salts is also disclosed, wherein gaseous diborane is reacted with a carbonate suspended in a non-aqueous solvent in a suitable vessel with agitation. The process is typically carried out utilizing sodium carbonate to form sodium triborohydride. Other triborohydride salts can then be formed by cationic exchange. Hydrogen generating fuels according to the present invention include aqueous or hydroalcoholic solutions or slurries of a triborohydride salt, which may additionally contain a borohydride salt to provide operation over a broader temperature range.
Owner:MILLENNIUM CELL
Process for forming a low carbon, low resistance metal film during the manufacture of a semiconductor device and systems including same
InactiveUS20060261441A1Reduce pollutionImprove conductivitySemiconductor/solid-state device detailsSolid-state devicesDevice materialLow resistance
A method for forming a conductive feature comprises forming a metal film such as ruthenium then annealing the film in an atmosphere comprising a hydrogen-rich gas such as ammonia, hydrogen, borane, or diborane, or in another gas such as carbon monoxide. The anneal may decrease the carbon content of the film and results in a metal layer having a lower resistance than the preannealed metal film.
Owner:MICRON TECH INC
Processes for synthesizing borohydride compounds
InactiveUS20030092877A1HydrogenMonoborane/diborane hydridesAlkaline earth metalCombinatorial chemistry
The present invention relates to processes for producing borohydride compounds. In particular, the present invention provides efficient processes and compositions for the large-scale production of borohydride compounds of the formula YBH.sub.4 by the reaction of a boron-containing compound represented by the formula BX.sub.3 with hydrogen or an aldehyde to obtain diborane and HX, and reacting the diborane with a Y-containing base selected from those represented by the formula Y.sub.2O, YOH and Y.sub.2CO.sub.3 to obtain YBH.sub.4 and YBO.sub.2. Y is selected from the group consisting of the alkali metals, pseudo-alkali metals, alkaline earth metals, an ammonium ion, and quaternary amines of the formula NR.sub.4.sup.+, wherein each R is independently selected from hydrogen and a straight- or branched-chain C.sub.1-4 alkyl group, and X is selected from the group consisting of halide ions, --OH, --R' and --OR' groups, chalcogens, and chalcogenides, wherein R' is a straight- or branched-chain C.sub.1-4 alkyl group.
Owner:MILLENNIUM CELL
Apparatus and method for hydrogen generation from gaseous hydride
An apparatus and method including storage and dispensing vessels to safely store and dispense gaseous hydrides, where the storage and dispensing vessels contain a solid-phase physical sorbent medium having a physically sorptive affinity for gaseous hydrides, and wherein the gaseous hydride is decomposed in the apparatus to generate hydrogen gas. The gaseous hydrides include, but are not limited to, silane, germane, stibine and diborane. The gaseous hydrides decompose spontaneously and / or decomposition is enhanced using surface modified adsorbents. The hydrogen generated by the apparatus may be used in a fuel cell or other hydrogen gas consuming unit.
Owner:ADVANCED TECH MATERIALS INC
Triborohydride salts as hydrogen storage materials and preparation thereof
The present invention relates to the use of triborohydride salts as hydrogen storage materials. The present invention also relates to a system of using triborohydride salts to generate hydrogen gas for use in a fuel cell or other hydrogen-consuming device. A novel method of preparing triborohydride salts is also disclosed, wherein gaseous diborane is reacted with a carbonate suspended in a non-aqueous solvent in a suitable vessel with agitation. The process is typically carried out utilizing sodium carbonate to form sodium triborohydride. Other triborohydride salts can then be formed by cationic exchange. Hydrogen generating fuels according to the present invention include aqueous or hydroalcoholic solutions or slurries of a triborohydride salt, which may additionally contain a borohydride salt to provide operation over a broader temperature range.
Owner:MILLENNIUM CELL
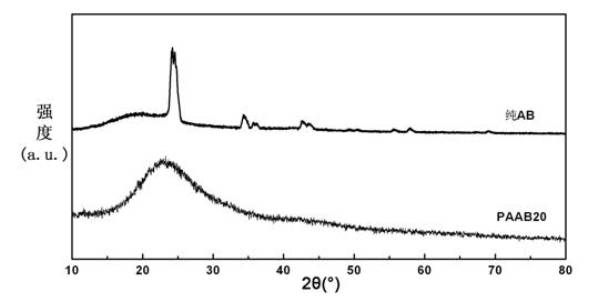
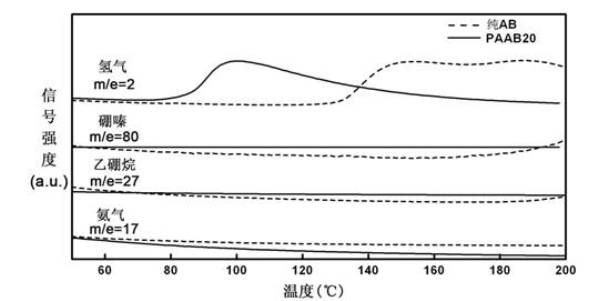
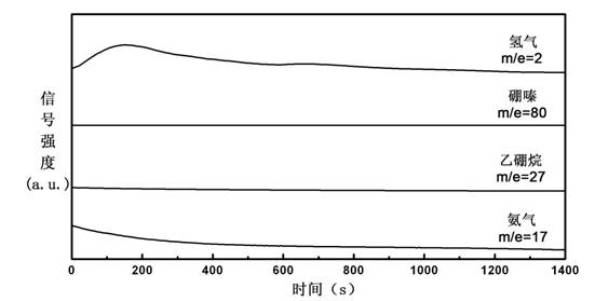
Organic matter and ammonia borane compounded hydrogen storage material and preparation method thereof
ActiveCN102030313ALowering the temperature of thermally liberated hydrogenInhibitionMonoborane/diborane hydridesPolyethylene oxideSolvent
The invention relates to an organic matter and ammonia borane compounded hydrogen storage material. The hydrogen storage material is prepared by compounding the organic matter and the ammonia borane, wherein the organic matter is phthalic anhydride, polyethylene oxide, dextrose, mannitol or mannitol hexaacetic ester. The preparation method comprises the following steps: 1) adding the organic matter to the purified acetonitrile solvent, and stirring for dissolving; 2) dissolving the ammonia borane into the mixing solvent comprising acetonitrile and methanol, and stirring at the temperature of 20 to 70 DEG C to obtain a uniform solution; and 3) carrying out vacuum drying, and removing the solvent, thus obtaining the hydrogen storage material. The invention has the advantages that the ammonia borane and the organic matter are taken as raw materials to prepare the hydrogen storage material at the lower hydrogen discharge temperature; the thermal decomposition and hydrogen discharge temperature of the ammonia borane can be effectively reduced; the generation of harmful gas impurities of borazole, diborane, ammonia and the like is effectively inhibited; the hydrogen storage material has quicker hydrogen discharge kinetics; in addition, the heat discharge amount is less in the hydrogen discharge course; and the enthalpy change of a decomposition reaction approaches to thermal neutrality; and the hydrogen storage material is beneficial to realizing the regeneration of reaction products through a solid-gas reaction or a chemical process under the relatively mild condition.
Owner:NANKAI UNIV
Method for fabricating interconnection in semiconductor device
A method for fabricating an interconnection in a semiconductor device includes forming a hydrogenated tungsten nucleation layer on a semiconductor substrate, and forming a bulk tungsten layer on the tungsten nucleation layer. Boron ions react with a hydrogen gas supplied together with a diborane gas to be restored to a diborane again, thereby preventing a boron layer from being formed on an interface of the tungsten nucleation layer.
Owner:SK HYNIX INC
Processes for synthesizing alkali metal borohydride compounds
Processes for synthesizing borohydride compounds with reduced energy requirements and high efficiency are disclosed. The processes include the reaction of a base with a borane complex or diborane to produce a borohydride compound of formula YBH4, where Y is a monovalent cationic moiety.
Owner:MILLENNIUM CELL
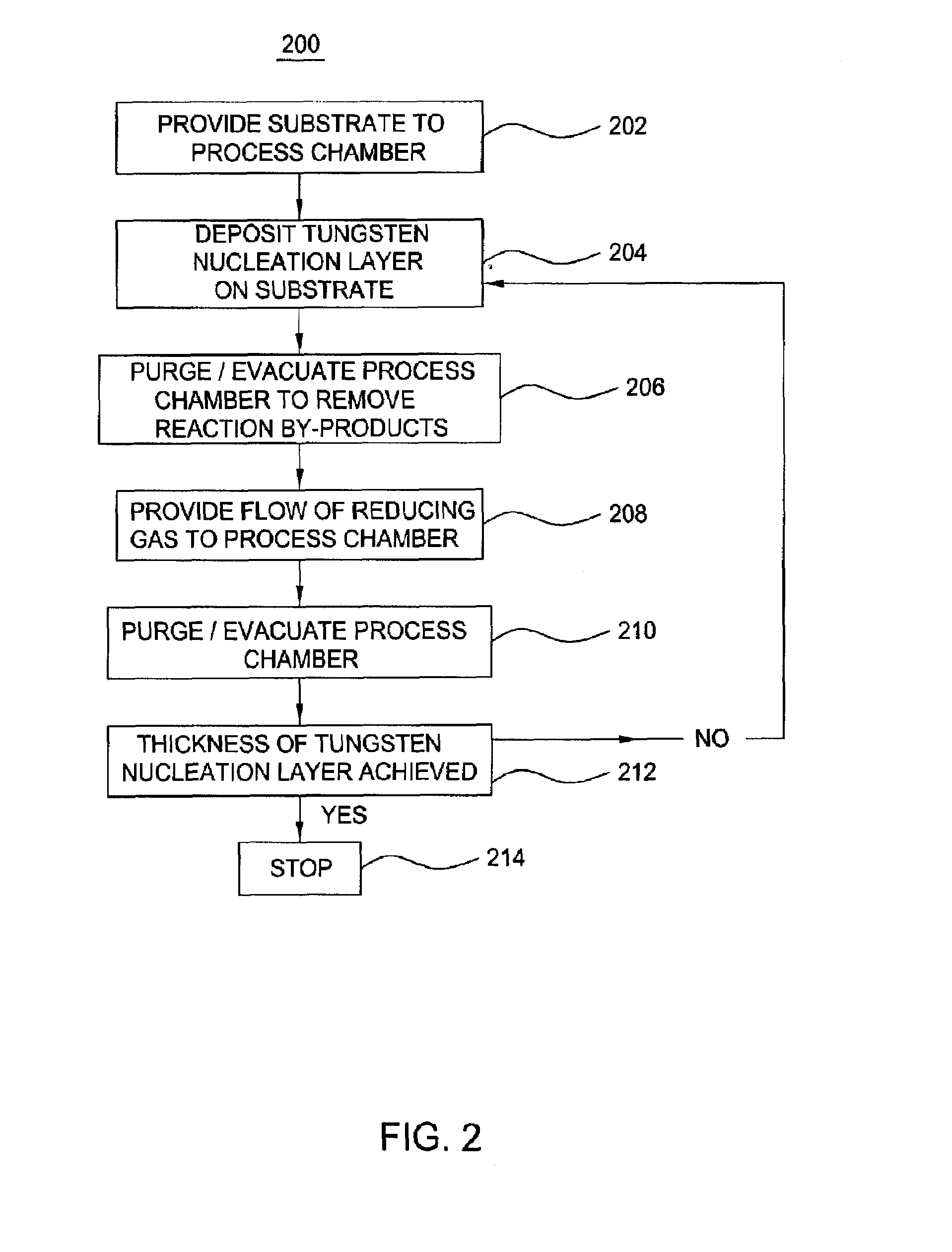
Pulsed deposition process for tungsten nucleation
InactiveUS20080317954A1Good step coveragePolycrystalline material growthSemiconductor/solid-state device manufacturingSilanesProduct gas
In one embodiment, a method for depositing a tungsten material on a substrate within a process chamber is provided which includes exposing the substrate to a gaseous mixture containing a tungsten precursor and a reducing gas to deposit a tungsten nucleation layer on the substrate during a tungsten deposition process. The process further includes removing reaction by-products generated during the tungsten deposition process from the process chamber, exposing the substrate to the reducing gas to react with residual tungsten precursor within the process chamber during a soak process, removing reaction by-products generated during the soak process from the process chamber, and repeating the tungsten deposition process and the soak process during a cyclic deposition process. In the examples, the reducing gas may contain diborane or silane.
Owner:APPLIED MATERIALS INC
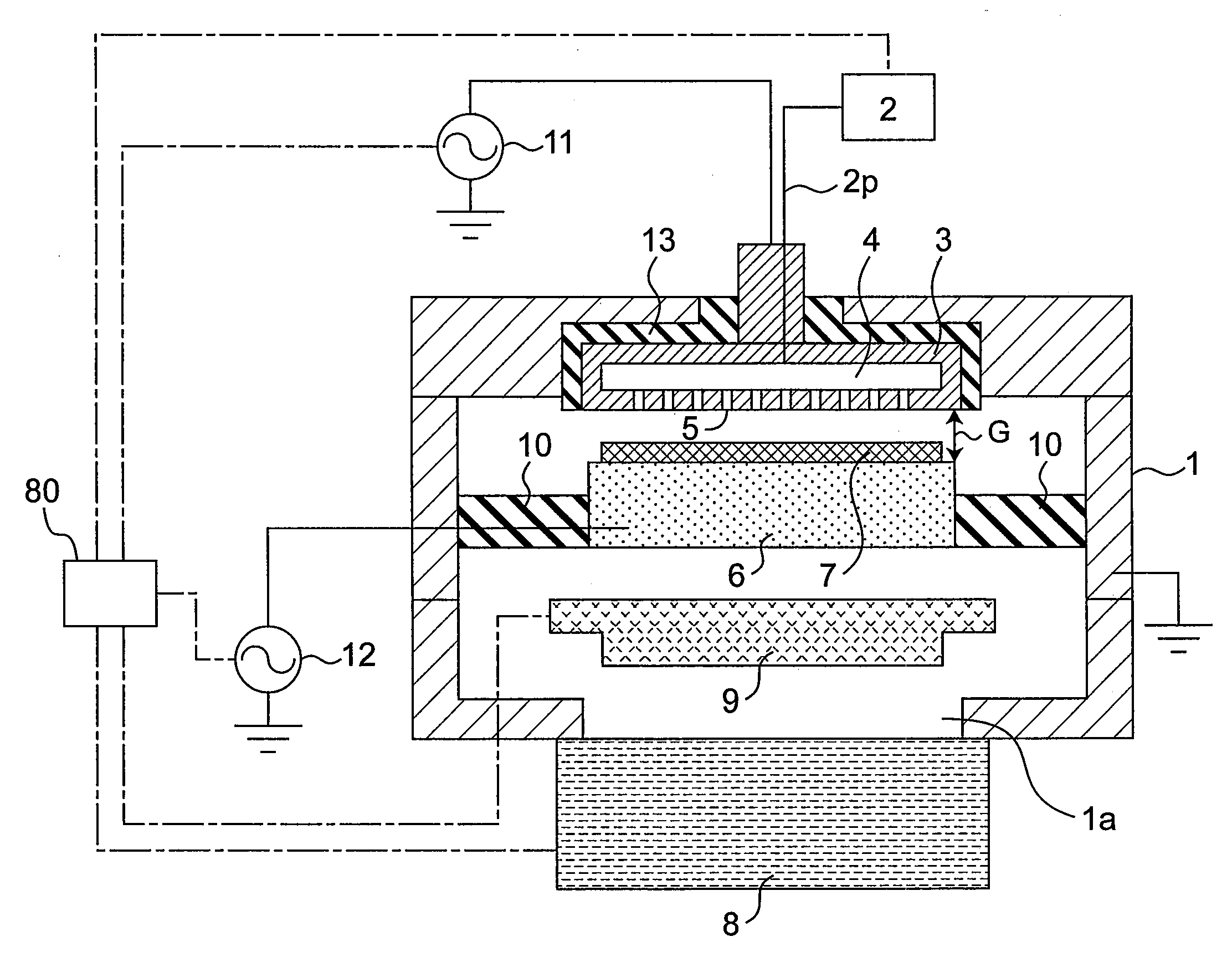
Plasma doping method and apparatus
InactiveUS20080233723A1Prevent implantationGood reproducibilityElectric discharge tubesSemiconductor/solid-state device manufacturingCapacitanceEngineering
There are provided a plasma doping method and an apparatus which have excellent reproducibility of the concentration of impurities implanted into the surfaces of samples. In a vacuum container, in a state where gas is ejected toward a substrate placed on a sample electrode through gas ejection holes provided in a counter electrode, gas is exhausted from the vacuum container through a turbo molecular pump as an exhaust device, and the inside of the vacuum container is maintained at a predetermined pressure through a pressure adjustment valve, the distance between the counter electrode and the sample electrode is set to be sufficiently small with respect to the area of the counter electrode to prevent plasma from being diffused outward, and capacitive-coupled plasma is generated between the counter electrode and the sample electrode to perform plasma doping. The gas used herein is a gas with a low concentration which contains impurities such as diborane or phosphine.
Owner:PANASONIC CORP
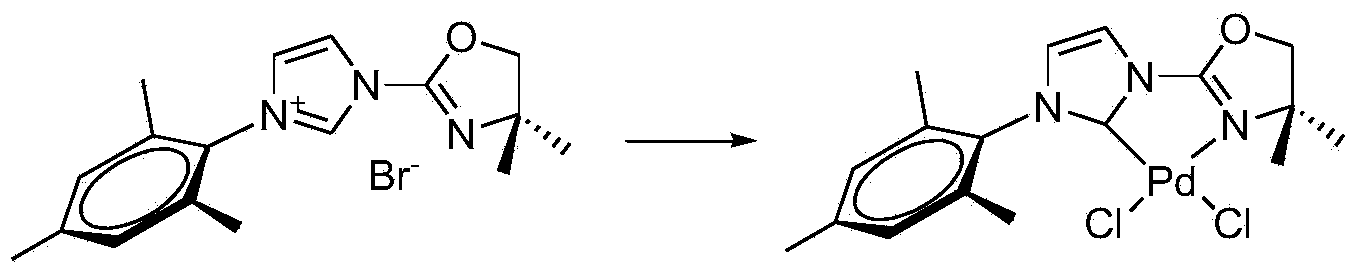
Chiral hexahydroxy n-heterocyclic carbine precursor compound as well as preparation method and application thereof
InactiveCN103772297AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsOrganic synthesisKetone
The invention relates to the field of organic synthesis, and particularly relates to a preparation method and an application of a chiral hexahydroxy n-heterocyclic carbine precursor compound. The compound has a structure as shown in a formula (V) in descriptions. The chiral hexahydroxy n-heterocyclic carbine precursor compound can be used for catalyzing multiple chiral reactions such as an addition reaction of unsaturated esters, alpha,beta-unsaturated imine and diborane pinacol borate, or a condensation reaction of aldehyde and boric acid compounds or a reduction reaction of ketone and has relatively good catalytic efficiency and enantioselectivity. (imgfile='DDA0000462319780000011.T'IF wi='392 he='496' / ).
Owner:SHANGHAI UNIV OF ENG SCI
Method for growing monocrystalline diamonds
ActiveUS20110014112A1Free from defectImprove suppression propertiesPolycrystalline material growthUltra-high pressure processesHydrogenGas phase
A method of forming mono-crystalline diamond by chemical vapour deposition, the method comprising the steps of: (a) providing at least one diamond seed; (b) exposing the seed to conditions for growing diamond by chemical vapour deposition, including supplying reaction gases that include a carbon-containing gas and hydrogen for growing diamond and include a nitrogen-containing gas; and (c) controlling the quantity of nitrogen-containing gas relative to other gases in the reaction gases such that diamond is caused to grow by step-growth with defect free steps without inclusions. The nitrogen is present in the range of 0.0001 to 0.02 vol %. Diborane can also be present in a range of from 0.00002 to 0.002 vol %. The carbon-containing gas can be methane.
Owner:IIA TECH +1
Apparatus and method for hydrogen generation from gaseous hydride
An apparatus and method including storage and dispensing vessels to safely store and dispense gaseous hydrides, where the storage and dispensing vessels contain a solid-phase physical sorbent medium having a physically sorptive affinity for gaseous hydrides, and wherein the gaseous hydride is decomposed in the apparatus to generate hydrogen gas. The gaseous hydrides include, but are not limited to, silane, germane, stibine and diborane. The gaseous hydrides decompose spontaneously and / or decomposition is enhanced using surface modified adsorbents. The hydrogen generated by the apparatus may be used in a fuel cell or other hydrogen gas consuming unit.
Owner:ADVANCED TECH MATERIALS INC
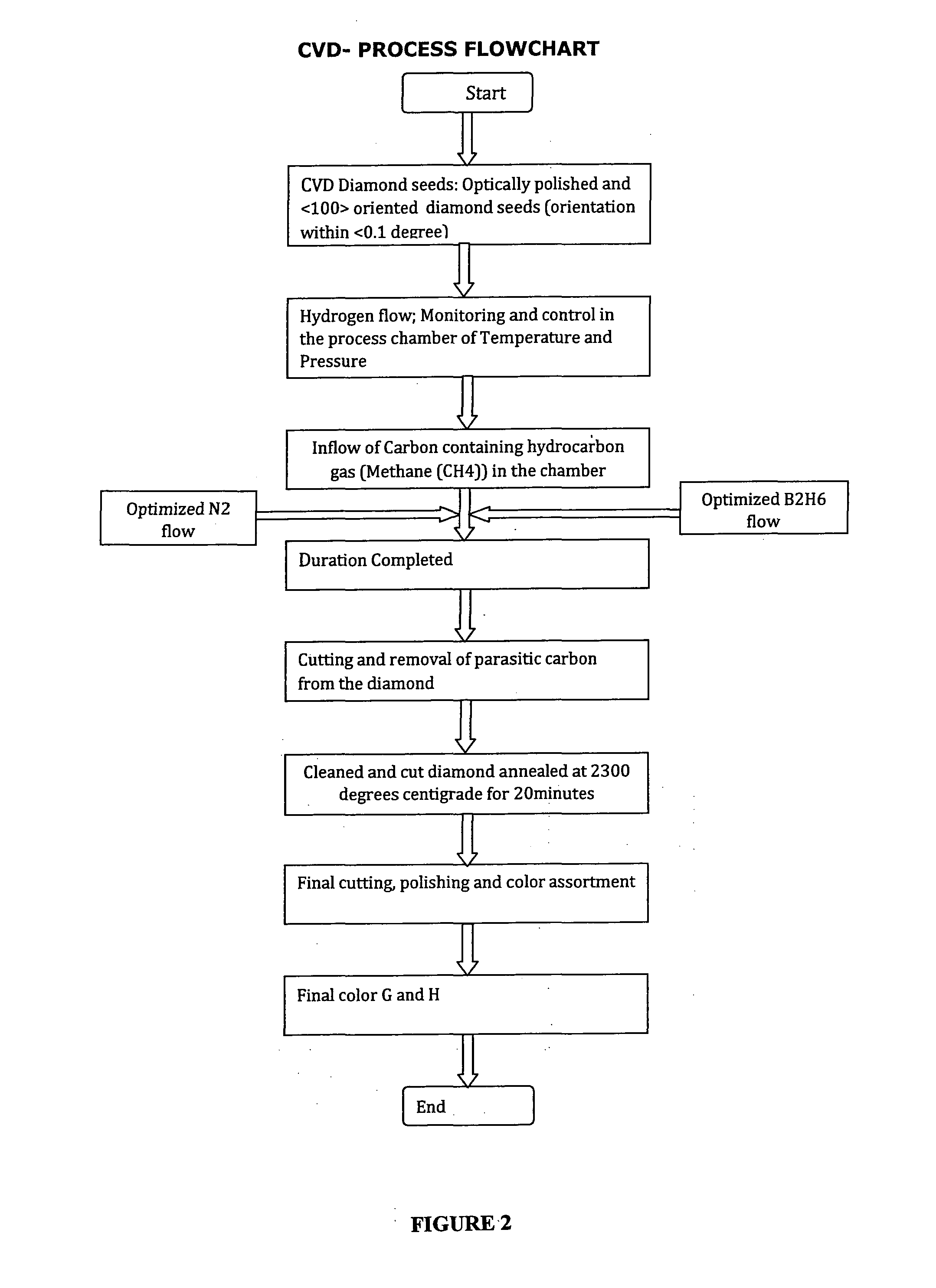
Method for growing white color diamonds by using diborane and nitrogen in combination in a microwave plasma chemical vapor deposition system
InactiveUS20130239615A1Color defectImprove clarityPolycrystalline material growthAfter-treatment detailsGas phaseNitrogen gas
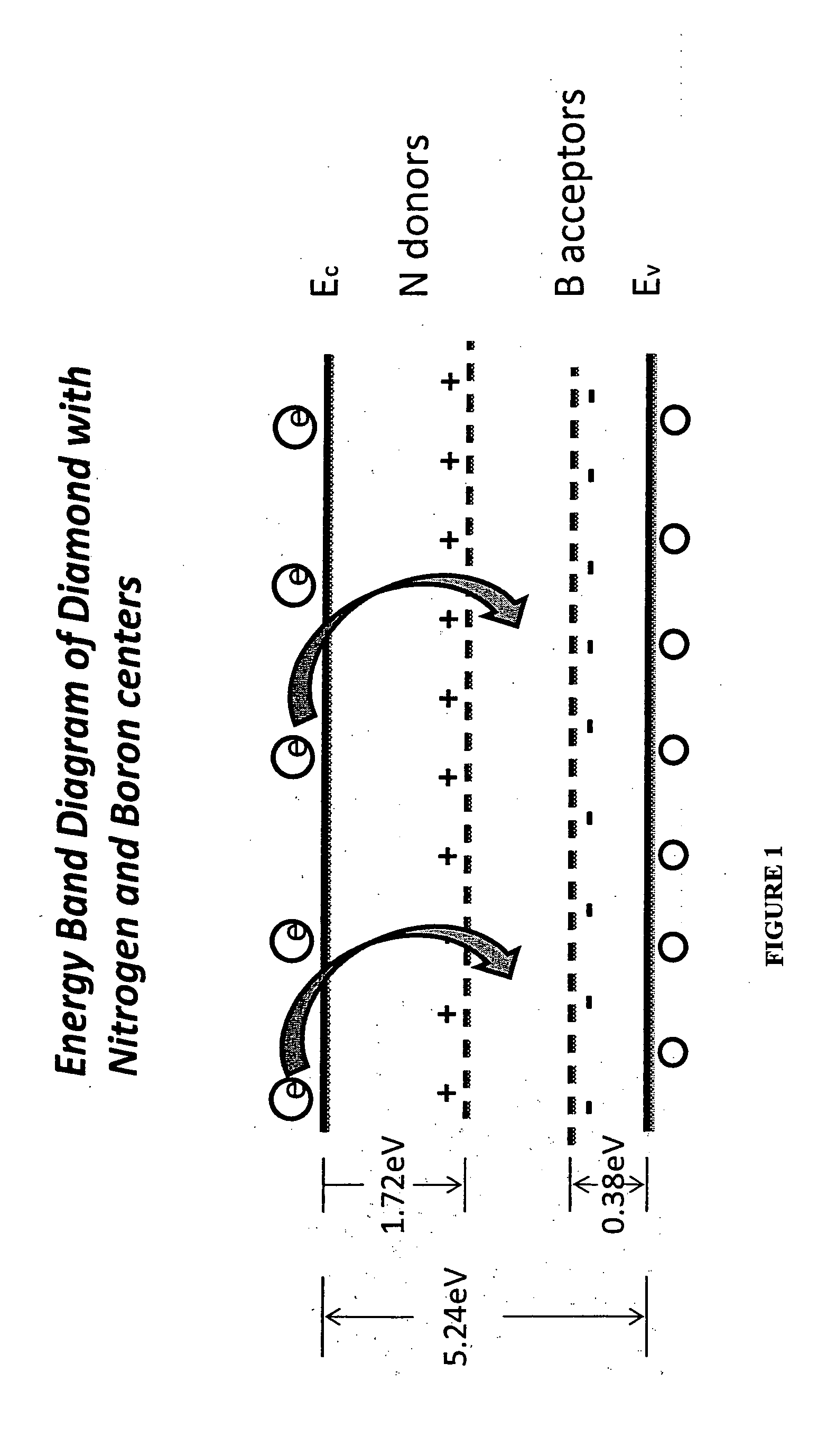
The present application discloses the details of a microwave plasma chemical vapor deposition process that uses Nitrogen and Diborane simultaneously in combination along with the Methane and Hydrogen gases to grow white color diamonds. The invention embodies using nitrogen to avoid inclusions and impurities in the CVD diamond samples and Diborane for the color enhancement during the growth of diamond. It is also found that heating of the so grown diamonds to 2000 C results in significant color enhancement due to the compensation of Nitrogen and Boron centers in the samples. The origin of the various colors in diamond is explained on the basis of the band diagram of CVD diamond.
Owner:IIA TECH
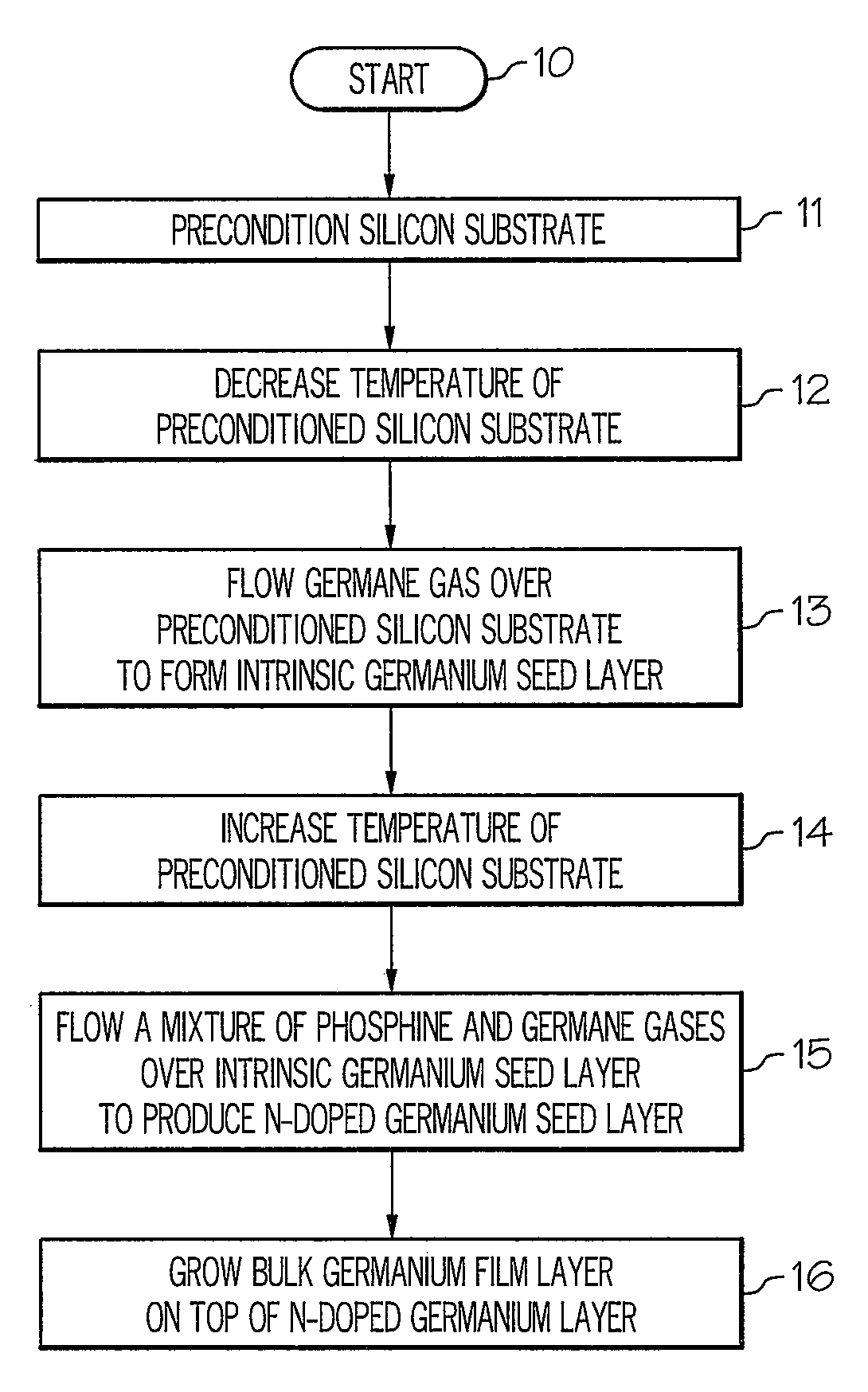
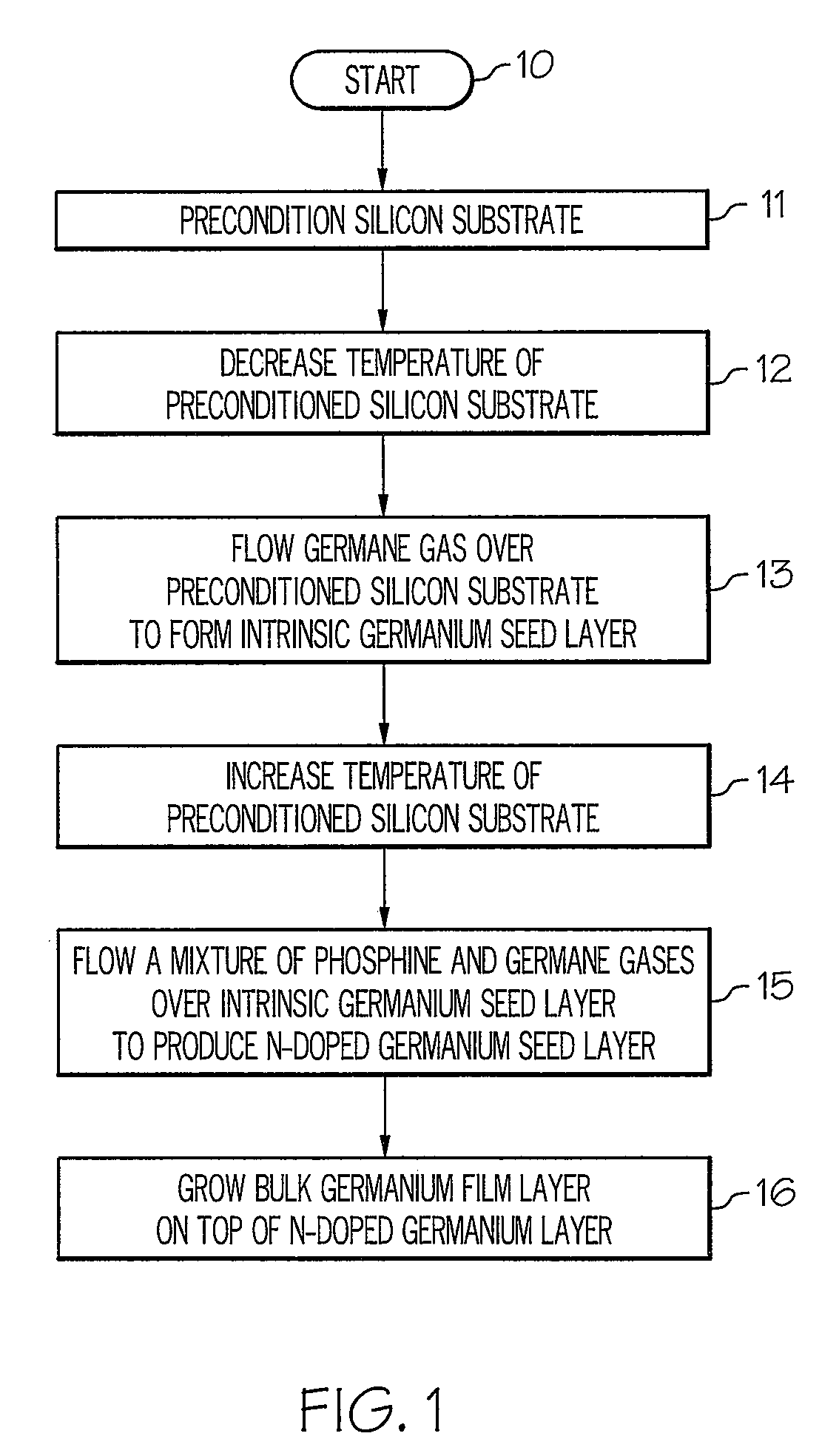
Method for growing germanium epitaxial films
InactiveUS20110036289A1Polycrystalline material growthSemiconductor/solid-state device manufacturingHydrogenPhosphine
A method for growing germanium epitaxial films is disclosed. Initially, a silicon substrate is preconditioned with hydrogen gas. The temperature of the preconditioned silicon substrate is then decreased, and germane gas is flowed over the preconditioned silicon substrate to form an intrinsic germanium seed layer. Next, a mixture of germane and phosphine gases can be flowed over the intrinsic germanium seed layer to produce an n-doped germanium seed layer. Otherwise, a mixture of diborane and germane gases can be flowed over the intrinsic germanium seed layer to produce a p-doped germanium seed layer. At this point, a bulk germanium layer can be grown on top of the doped germanium seed layer.
Owner:BAE SYST INFORMATION & ELECTRONICS SYST INTERGRATION INC
Lanthanum hexaboride nanowire and method for preparing same
InactiveCN102616799AThe experiment process is simpleEasy to operateMetal boridesNanowireField electron emission
The invention discloses a method for preparing a lanthanum hexaboride nanowire on a copper substrate. The method comprises the following steps of: (1) cleaning the copper substrate, and removing oil stain from the copper substrate; (2) placing a lanthanum source in the middle of a quartz tube of an electric tube furnace, placing the copper substrate in the down gas flow direction of a precursor, sealing the quartz tube, filling protective and reduction gas, and vacuumizing the quartz tube and raising temperature; (3) heating the quartz tube to 900 to 1,020 DEG C, introducing diborane gas and reacting at constant temperature for 10 to 30 minutes; and (4) stopping introducing diborane, and reducing temperature at vacuum atmosphere. The method for preparing the lanthanum hexaboride nanowire on the copper substrate is simple, and any catalyst is not needed; and the prepared LaB6 nanowire can be used as a cold cathode electron source, and is applied to field electron emission flat-panel displays, cold cathode fluorescent tubes, field emission pressure sensors and the like, and has good application prospect in the aspect of field electron emission display materials.
Owner:XINYANG NORMAL UNIVERSITY
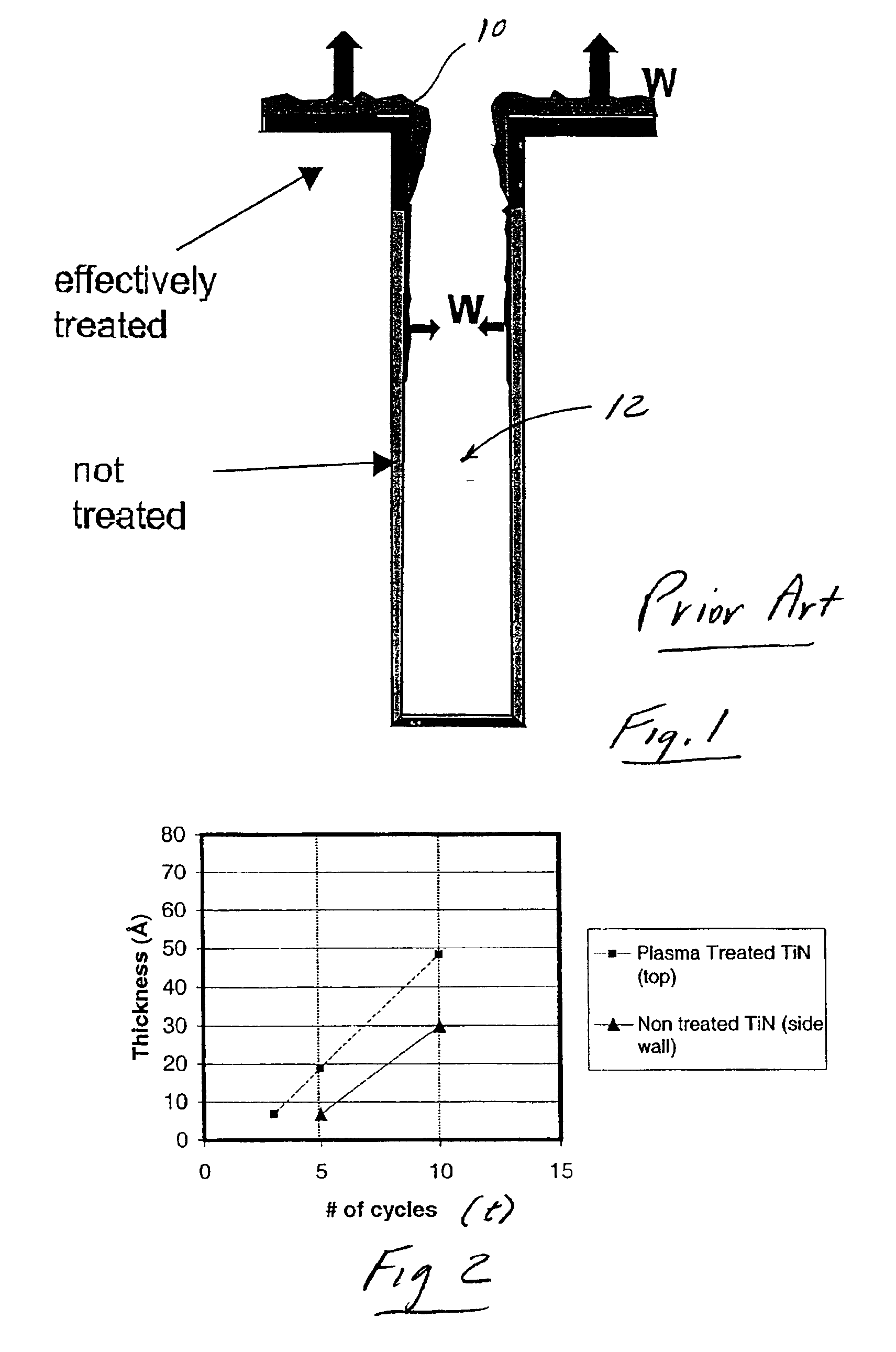
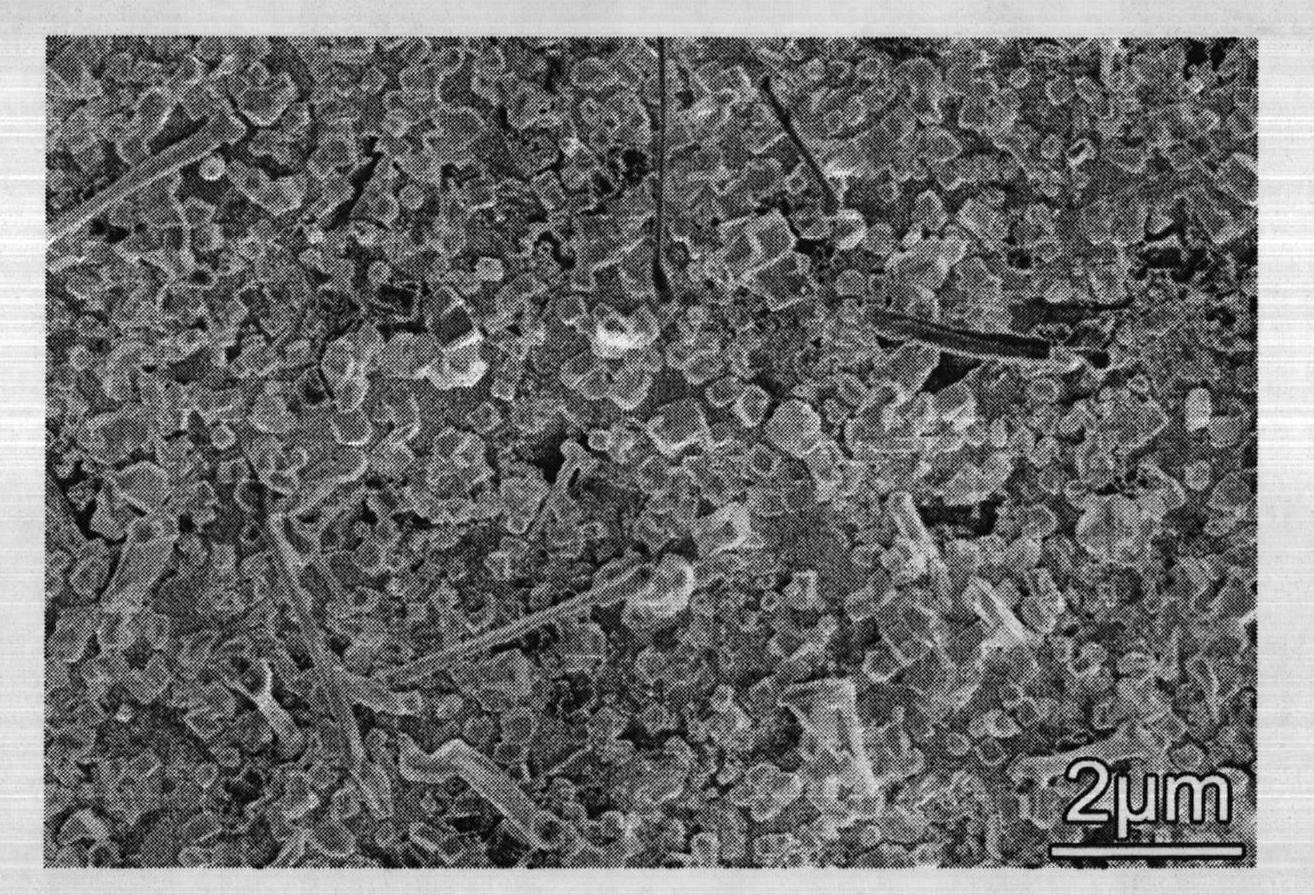
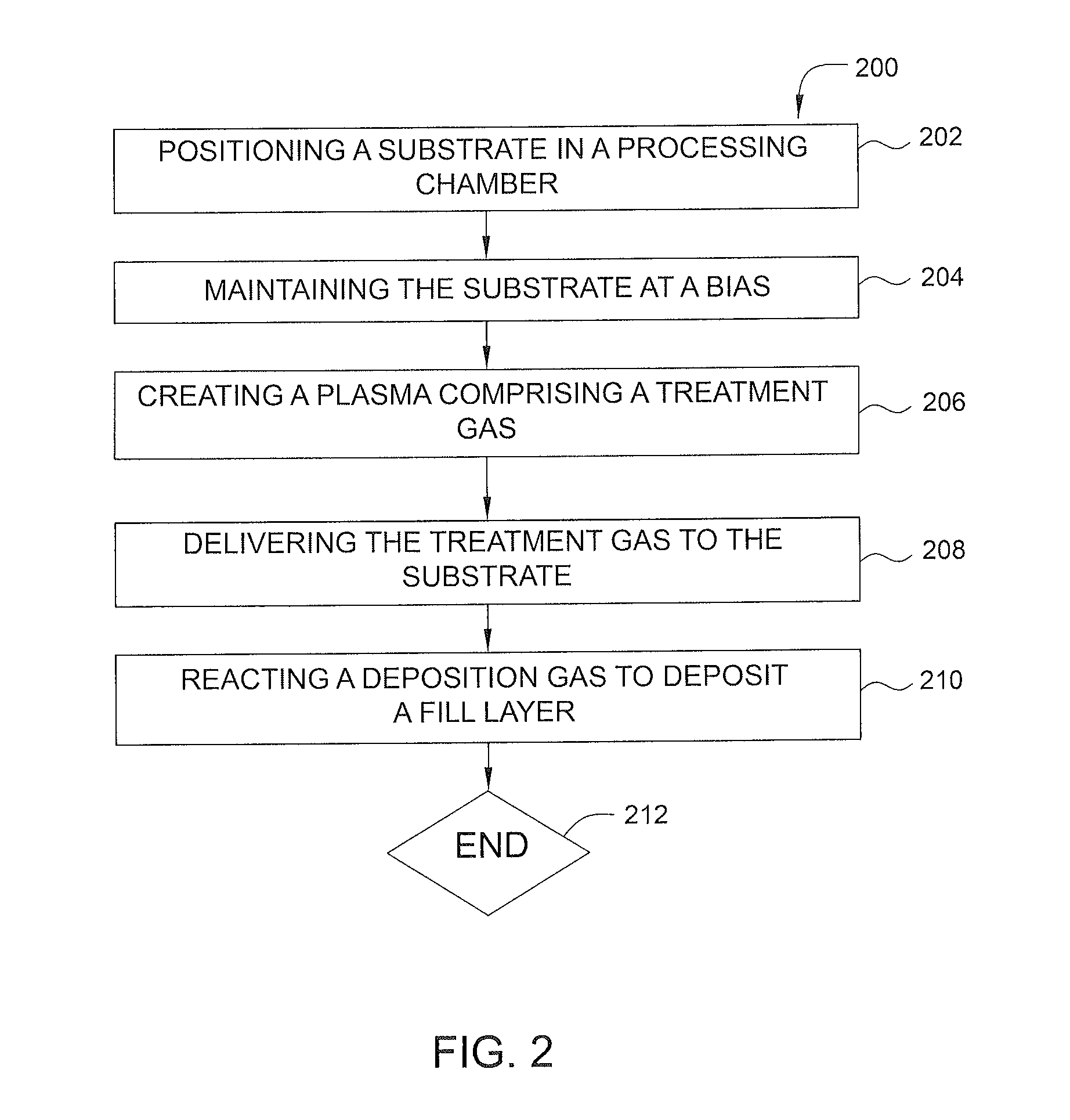
Plasma treatment of film for impurity removal
ActiveUS20140120700A1Reduce resistanceUndesired effectSemiconductor/solid-state device manufacturingChemical vapor deposition coatingHydrogenNucleation
Methods for plasma treatment of films to remove impurities are disclosed herein. Methods for removing impurities can include positioning a substrate with a barrier layer in a processing chamber, the barrier layer comprising a barrier metal and one or more impurities, maintaining the substrate at a bias, creating a plasma comprising a treatment gas, the treatment gas comprising an inert gas, delivering the treatment gas to the substrate to reduce the ratio of one or more impurities in the barrier layer, and reacting a deposition gas comprising a metal halide and hydrogen-containing gas to deposit a bulk metal layer on the barrier layer. The methods can further include the use of diborane to create selective nucleation in features over surface regions of the substrate.
Owner:APPLIED MATERIALS INC
Liquid media containing Lewis basic reactive compounds for storage and delivery of Lewis acidic gases
ActiveUS20060081482A1Reliable sourceEasy to operateLiquid degasificationFluid handledLiquid mediumCompound (substance)
This invention relates to an improvement in a low-pressure storage and delivery system for gases having Lewis acidity, particularly hazardous specialty gases such as BF3 and diborane, which are utilized in the electronics industry. The improvement resides in storing the gases in a liquid incorporating a reactive compound having Lewis basicity capable of effecting a reversible reaction between a gas having Lewis acidity. The reactive compound comprises a reactive species that is dissolved, suspended, dispersed, or otherwise mixed with a nonvolatile liquid.
Owner:VERSUM MATERIALS US LLC
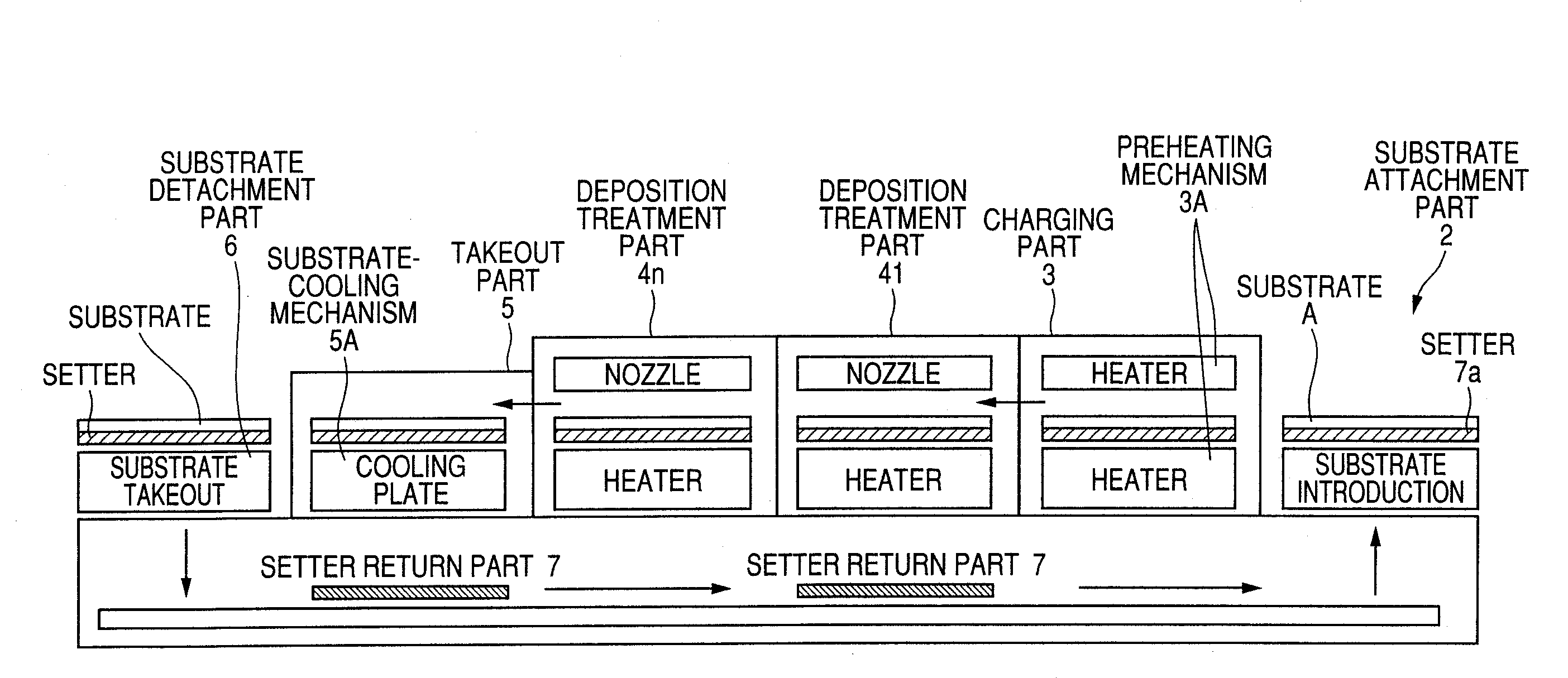
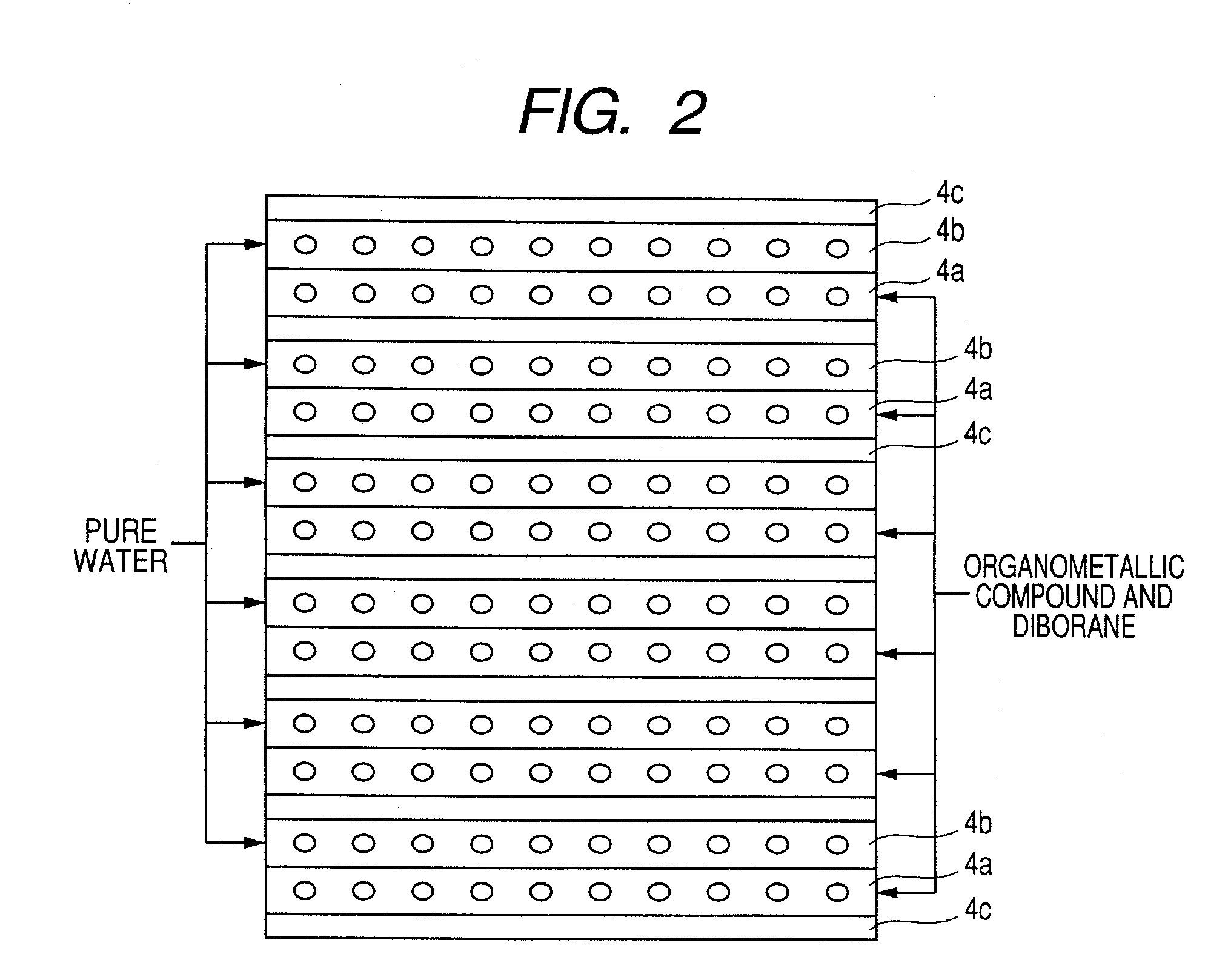
Transparent conductive film deposition apparatus, film deposition apparatus for continuous formation of multilayered transparent conductive film, and method of forming the film
InactiveUS20090203194A1Reduce necessityImprove utilization efficiencySemiconductor/solid-state device manufacturingChemical vapor deposition coatingGas phaseTransparent conducting film
Raw materials are economized and a film deposition rate is improved while maintaining film evenness and high film quality.A film deposition apparatus for the continuous formation of a multilayered transparent conductive film is provided which comprises a substrate attachment part, a charging part where evacuation is conducted, a multilayer deposition treatment part comprising two or more deposition treatment parts for forming a transparent conductive film on a substrate by the MOCVD method by reacting an organometallic compound (diethylzinc), diborane, and water in a vapor phase, a substrate takeout part, a substrate detachment part, and a setter return part where the substrate setter is returned to the substrate attachment part. Film deposition is successively conducted while moving a substrate sequentially through the parts to form a multilayered transparent conductive film on the substrate. Each deposition treatment part is equipped with nozzles for spraying the organometallic compound, diborane, and water and with a cooling mechanism for cooling the nozzles.
Owner:SHOWA SHELL SEKIYU KK
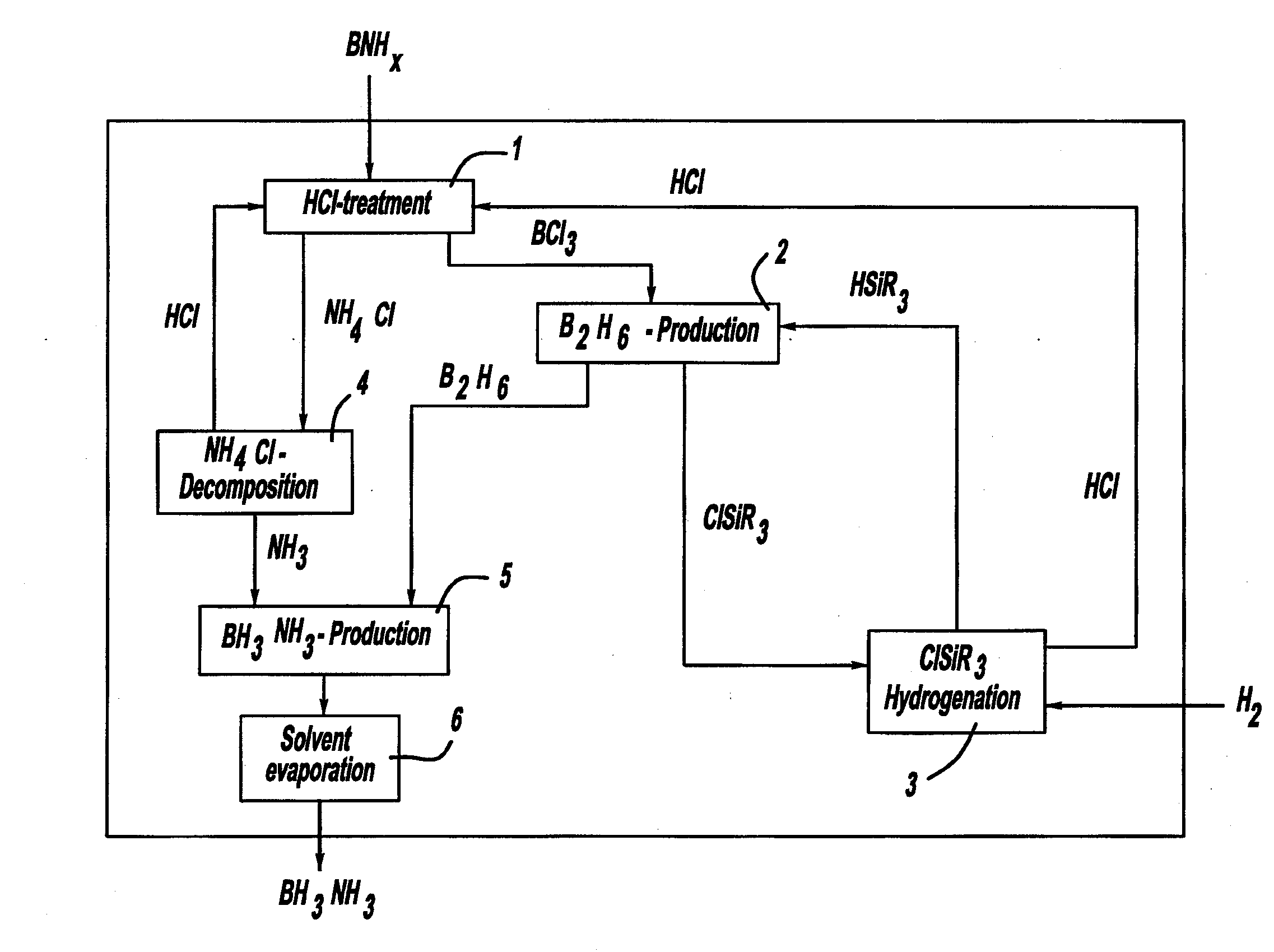
Procedure for the hydrogenation of bnh-containing compounds
InactiveUS20080193356A1Improve energy efficiencyNitrogen compoundsOther chemical processesHydrogen halideCompound a
A process for producing borazane from boron-nitrogen and boron-nitrogen-hydrogen containing BNH-waste products. The process includes reacting the BNH-waste products with a hydrogen halide, having the formula HX, wherein X is selected from the group consisting of F, Cl, Br, I, and combinations thereof, to form any of the following: a boron trihalide, having the formula BX3, an ammonium halide, having the formula NH4X, and hydrogen. The boron trihalide is then reacted with the hydrogen to form diborane, having the formula B2H6, and hydrogen halide. The ammonium halide is then converted to ammonia, having the formula NH3, and hydrogen halide. The diborane is then reacted with the ammonia to form borazane, having the formula BH3NH3.
Owner:GM GLOBAL TECH OPERATIONS LLC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com