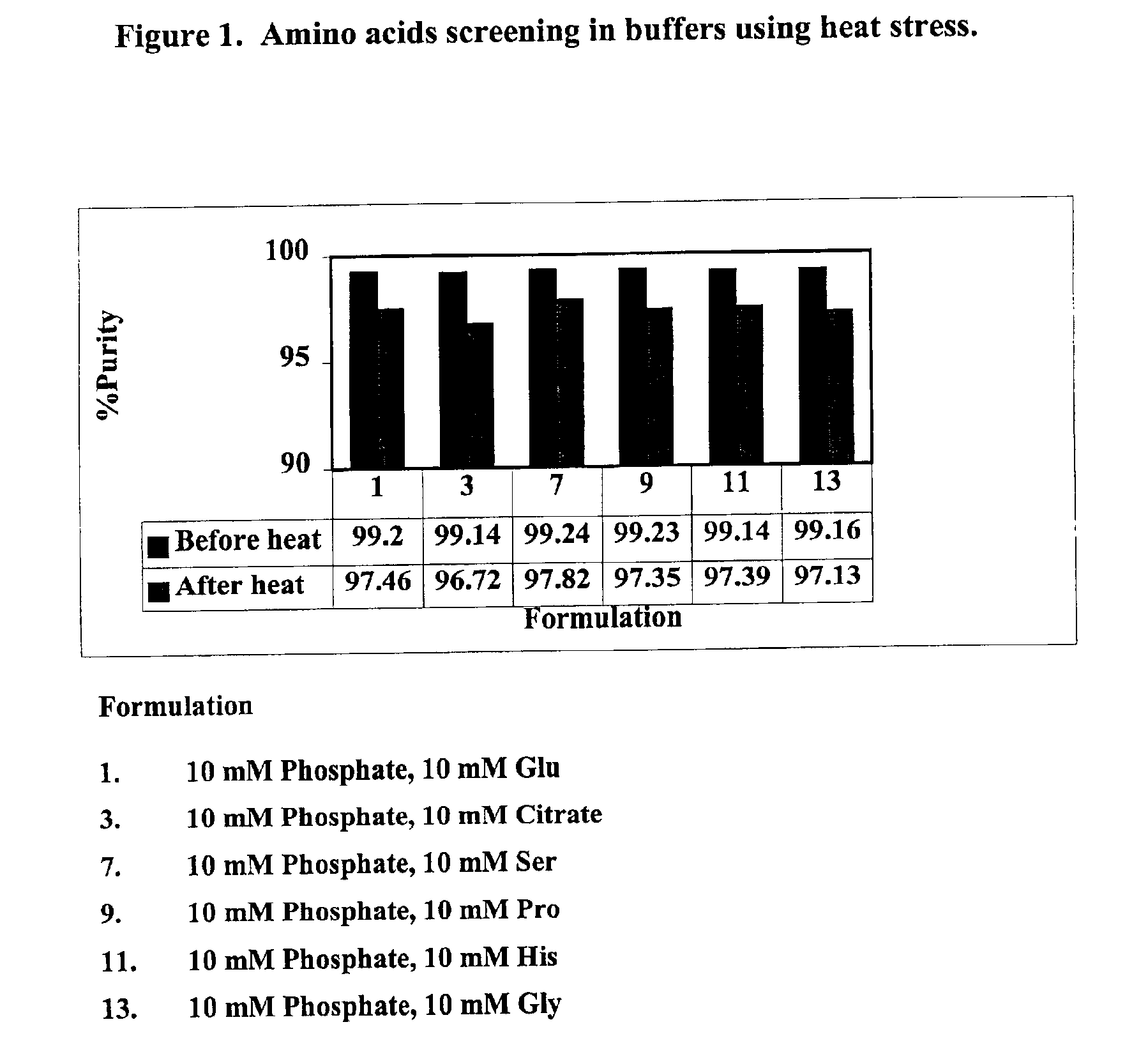
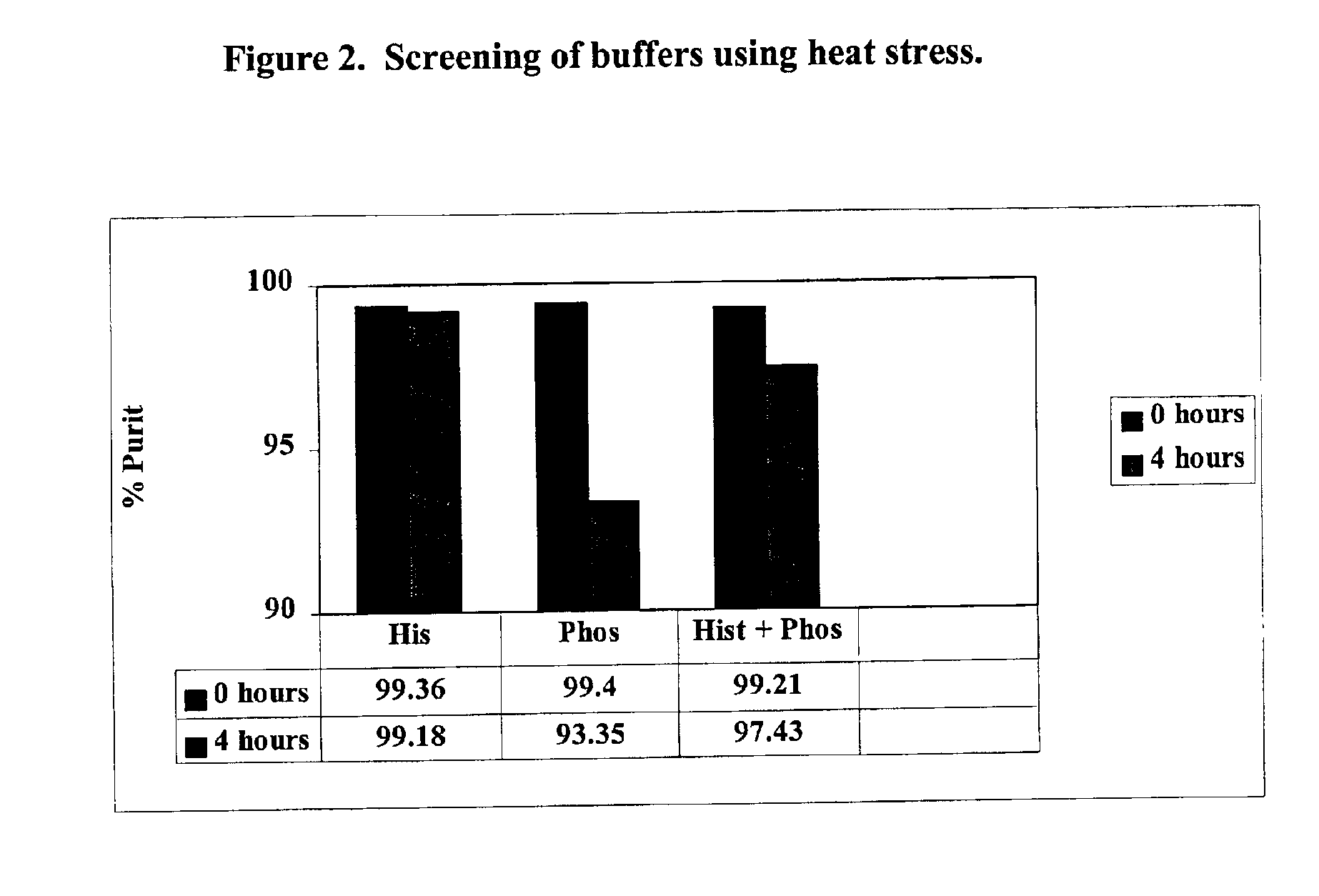
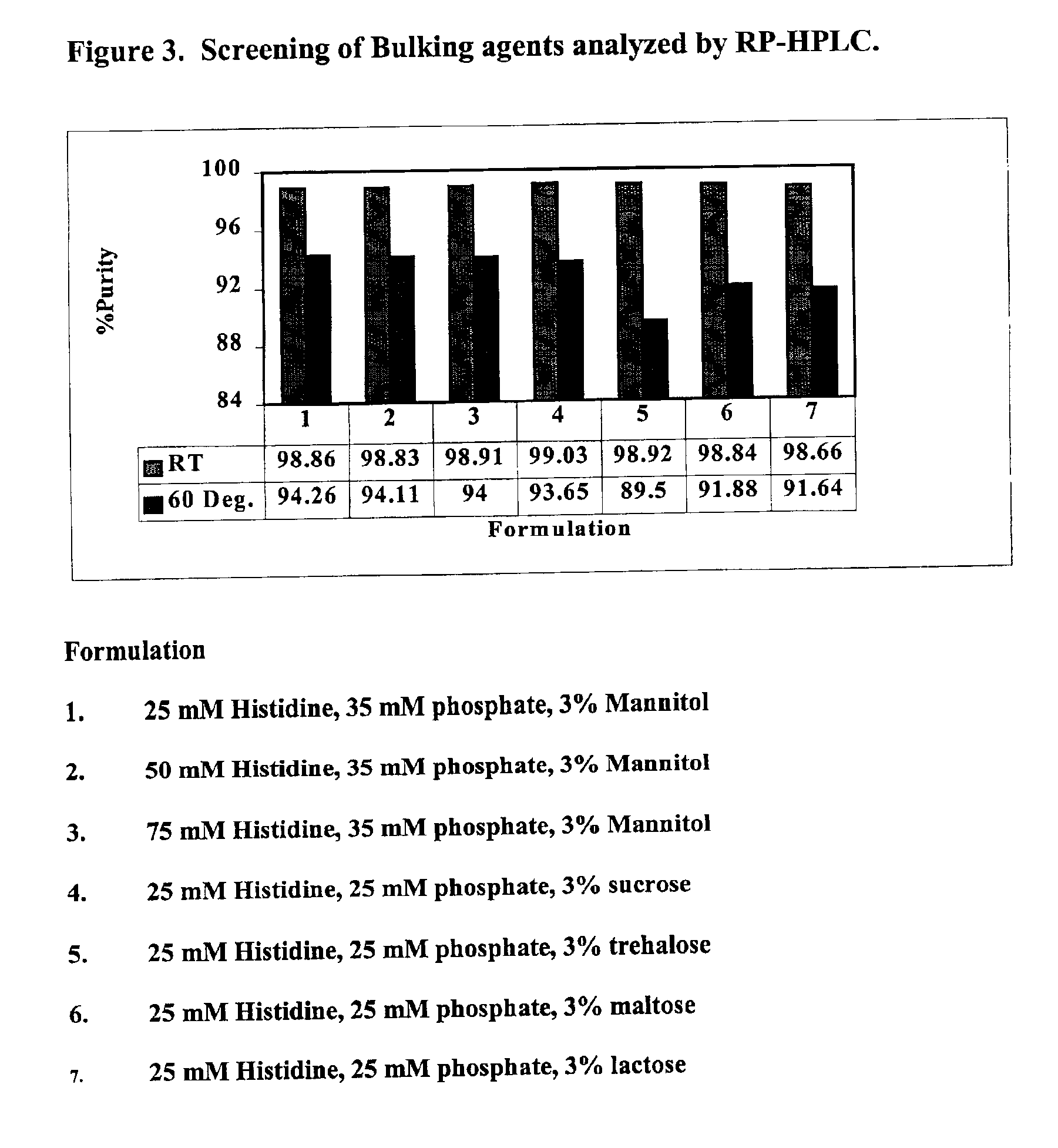
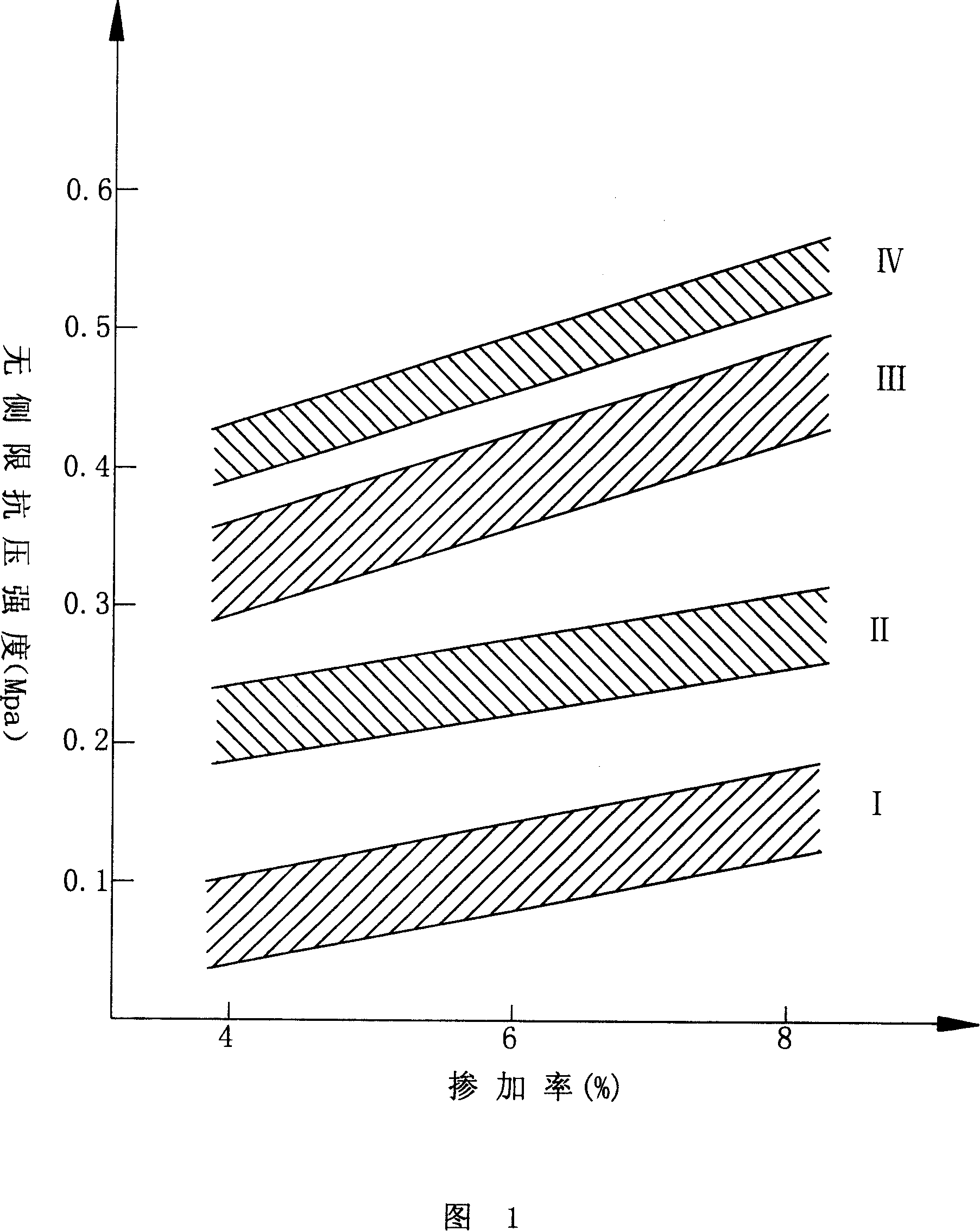
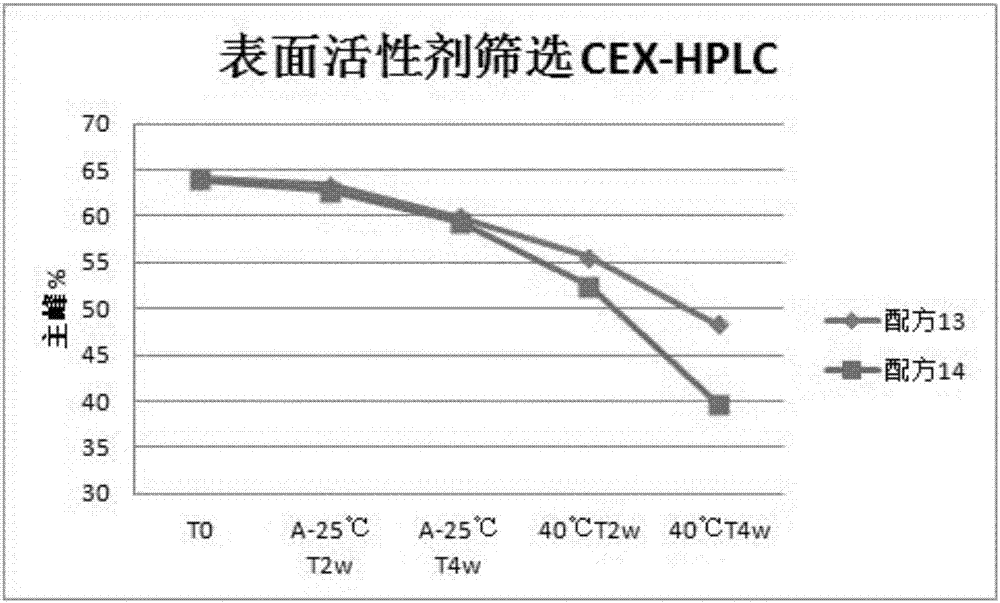
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3296 results about "Mannitol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
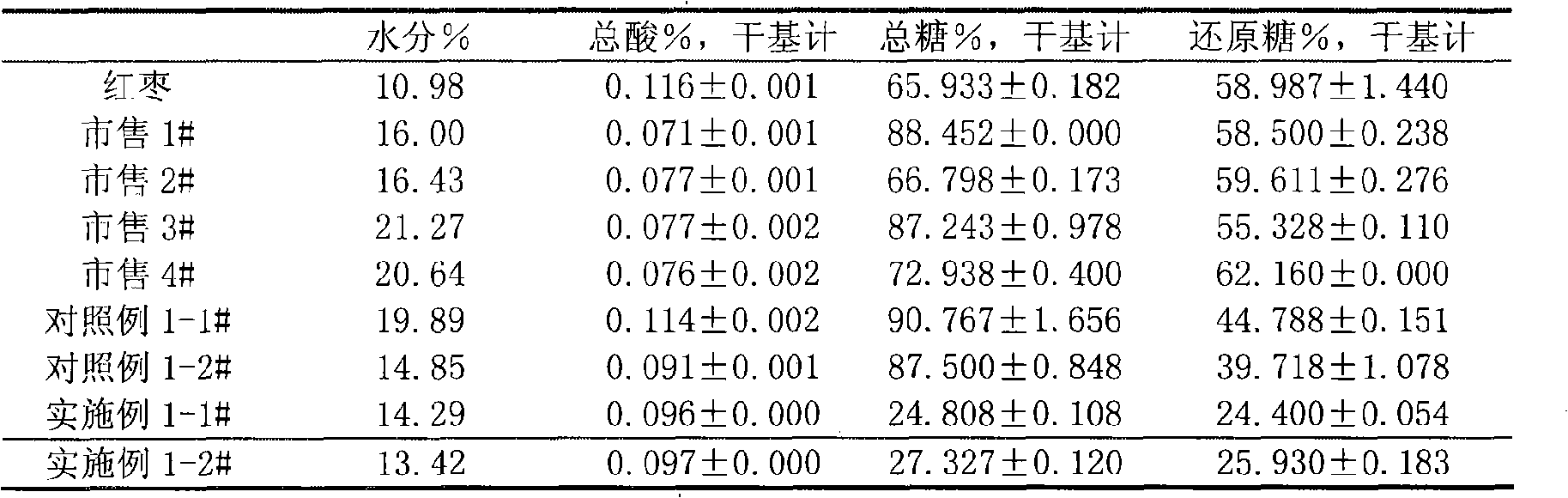
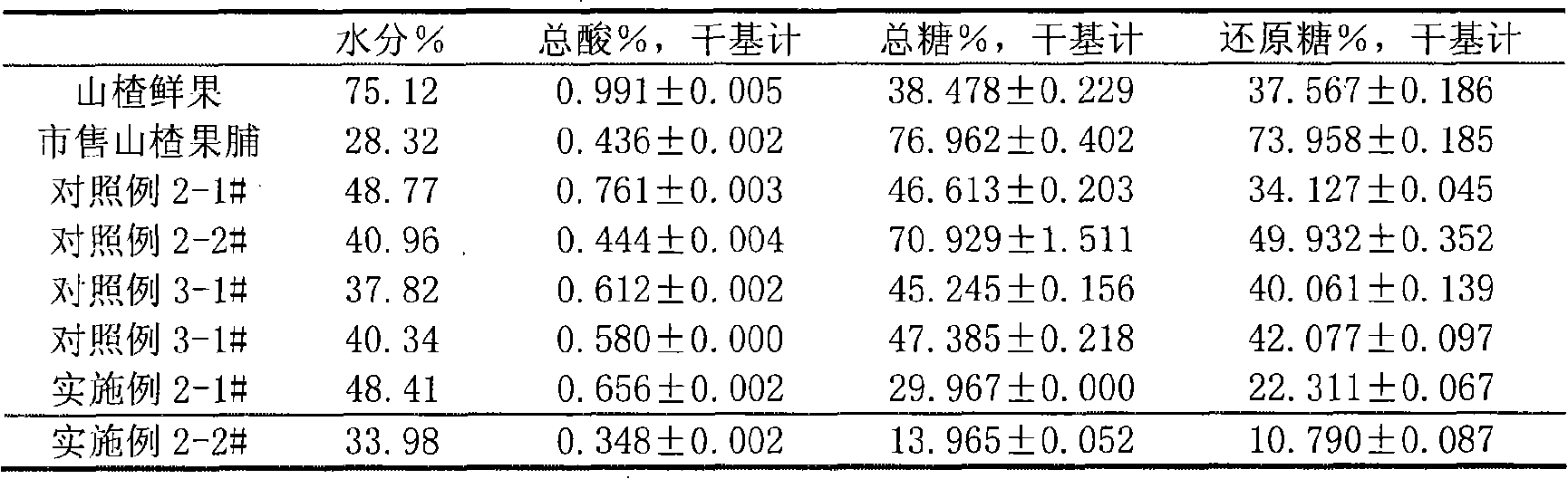
Mannitol is a type of sugar alcohol used as a sweetener and medication. As a sweetener it is used in diabetic food as it is poorly absorbed from the intestines. As a medication, it is used to decrease pressure in the eyes, as in glaucoma, and to lower increased intracranial pressure. Medically, it is given by injection. Effects typically begin within 15 minutes and last up to 8 hours.
Oral cavity disintegrating tablet and method of producing the same
ActiveUS20100278930A1Easy to produceDisintegrates quicklyBiocideOrganic active ingredientsSucroseOrally disintegrating tablet
The invention provides an orally disintegrating tablet containing (a) one or more saccharides or sugar alcohols selected from the group consisting of mannitol, lactose, xylitol, sucrose, erythritol and glucose and (b) low substituted hydroxypropylcellulose and substantially free of a starch disintegrant, which tablet is produced by steps of granulating a composition containing the above-mentioned components (a) and (b) by an agitation granulation method, and compression-molding the obtained granulation product. The invention also provides a method of producing an orally disintegrating tablet substantially free of a starch disintegrant, including steps of granulating a composition containing the above-mentioned components by an agitation granulation method, and compression-molding the obtained granulation product.
Owner:SAWAI PHARMA
Sludge curing agent and application thereof
ActiveCN101081718AGood boardIncreased durabilitySludge treatment by de-watering/drying/thickeningSolid waste managementSludgeSlag
The present invention is sludge curing agent and its application, and belongs to the field of soil treating chemicals technology. The sludge curing agent includes powdered components and liquid components, the powdered components include cement clinker 30-60 weight portions, slag 30-60 weight portions, lime 3-8 weight portions, gypsum 1-7 weight portions and other sulfates 1-7 weight portions; and the liquid components include polyacrylamide 5-30 weight portions, polyaluminum chloride 0-20 weight portions, mannitol 0-30 weight portions, lignosulfonate 20-80 weight portions, lignosulfonate-iron or chromium ion complex 0-30 weight portions, alkylphenol ethoxylate 0.2-2 weight portions, tannin 0-10 weight portions, humate 0-10 weight portions, and alpa-olefin sulfonate 0.2-2.5 weight portions. The sludge curing agent has low cost, small consumption, high cumulate strength and high cumulate water tolerance, and may be applied widely.
Owner:BEIJING ZHONGYONGJI FIRMING AGENT TECH DEV
Albumin-free factor VIII formulations
InactiveUS7087723B2Lower temperatureFactor VIIPowder deliveryPharmaceutical preservativesBuffering agent
A Factor VIII composition formulated without albumin, comprising the following formulation excipients in addition to Factor VIII: 4% to 10% of a bulking agent selected from the group consisting of mannitol, glycine and alanine; 1% to 4% of a stabilizing agent selected from the group consisting of sucrose, trehalose, raffinose, and arginine; 1 mM to 5 mM calcium salt; 100 mM to 300 mM NaCl; and a buffering agent for maintaining a pH of approximately between 6 and 8. Alternatively, the formulation can comprise 2% to 6% hydroxyethyl starch; 1% to 4% of a stabilizing agent selected from the group consisting of sucrose, trehalose, raffinose, and arginine; 1 mM to 5 mM calcium salt; 100 mM to 300 mM NaCl; and a buffering agent for maintaining a pH of approximately between 6 and 8. In a further embodiment, the formulation can comprise: 300 mM to 500 mM NaCl; 1% to 4% of a stabilizing agent selected from the group consisting of sucrose, trehalose, raffinose, and arginine; 1 mM to 5 mM calcium salt; and a buffering agent.
Owner:UNIV OF CONNECTICUT +1
Solvent for electronic cigarette liquid and an electronic cigarette liquid
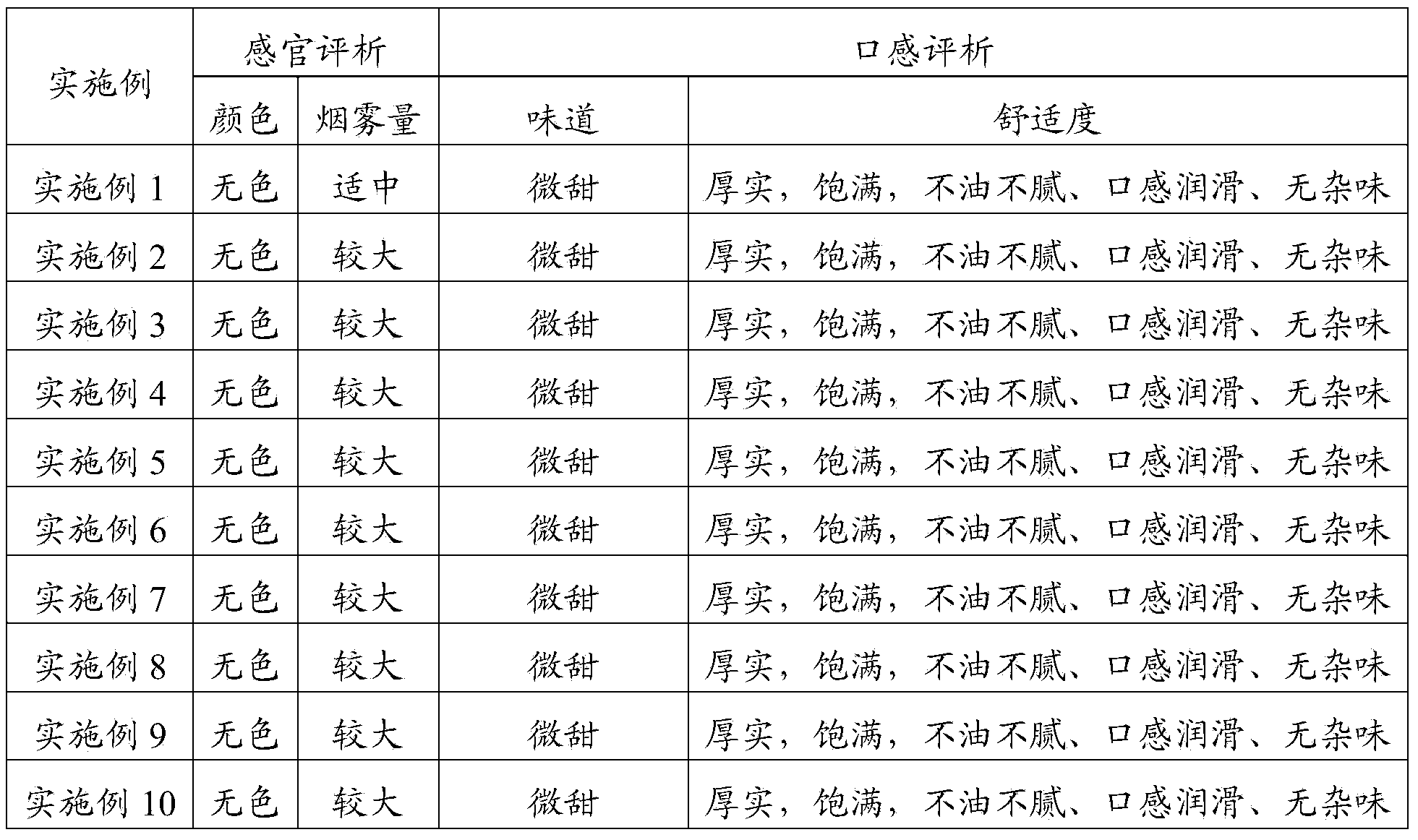
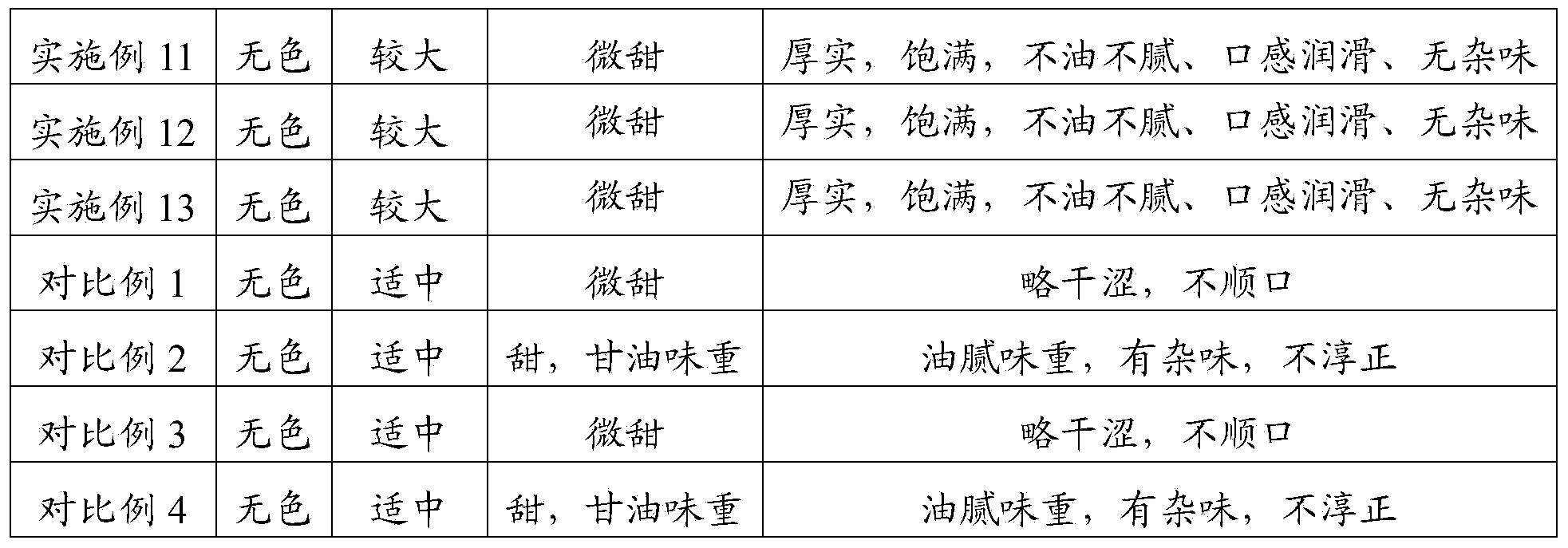
The present invention provides a solvent for electronic cigarette liquid, comprising sorbitol. In the present invention, sorbitol is used as the solvent for electronic cigarette liquid. The electronic cigarette liquid which is prepared with the solvent for electronic cigarette liquid provided by the present invention is not greasy and free of miscellaneous taste, and therefore provides relatively higher comfort level during smoking. Further, the electronic cigarette liquid provided by the present invention may further comprise one or more of propylene glycol, glycerol, and mannitol. Due to the effects of propylene glycol, glycerol and mannitol, the sorbitol makes the electronic cigarette liquid which is prepared with the solvent for electronic cigarette liquid provided by the present invention generate a relatively greater amount of smoke, improve the experience of the smoker, and provide a thick and full experience, a smooth mouthfeel without any miscellaneous taste, and a relatively higher comfort level during smoking. Furthermore, all the components in the solvent for electronic cigarette liquid provided by the present invention are of food grade, and are harmless to the body of the smoker.
Owner:KIMREE HI TECH
Tablet Quickly Disintegrating in Oral Cavity
ActiveUS20070275058A1Sufficient hardnessOral disintegration time is shortenedPowder deliveryBiocidePharmaceutical preservativesHardness
The present invention provides rapid disintegrating tablets in oral cavity having a shortened disintegration time in oral cavity as well as a sufficient hardness compared to rapid disintegrating tablets of the prior art. The above objective is solved by a composition in which the inorganic excipient and the disintegrating agent are dispersed in the complex particles consisting of mannitol and other saccharide(s) in a specific ratio, and rapid disintegrating tablets in oral cavity obtained by direct compression of the composition.
Owner:FUJI CHEM IND CO LTD
Barrier film
InactiveUS7854994B2Improve the level ofImprove homogeneityFibre treatmentBottlesPolyethylene terephthalate glycolPolyethylene oxide
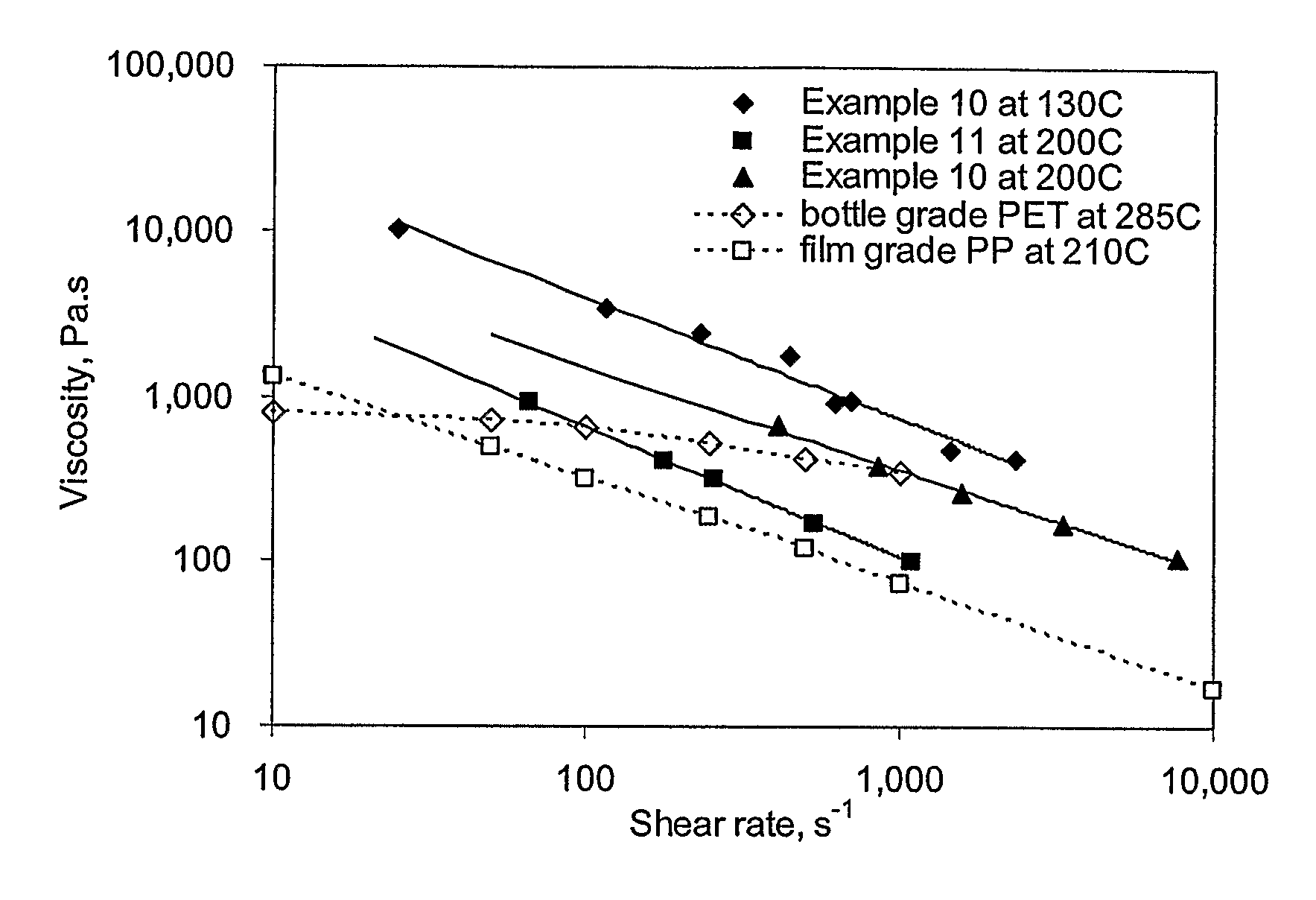
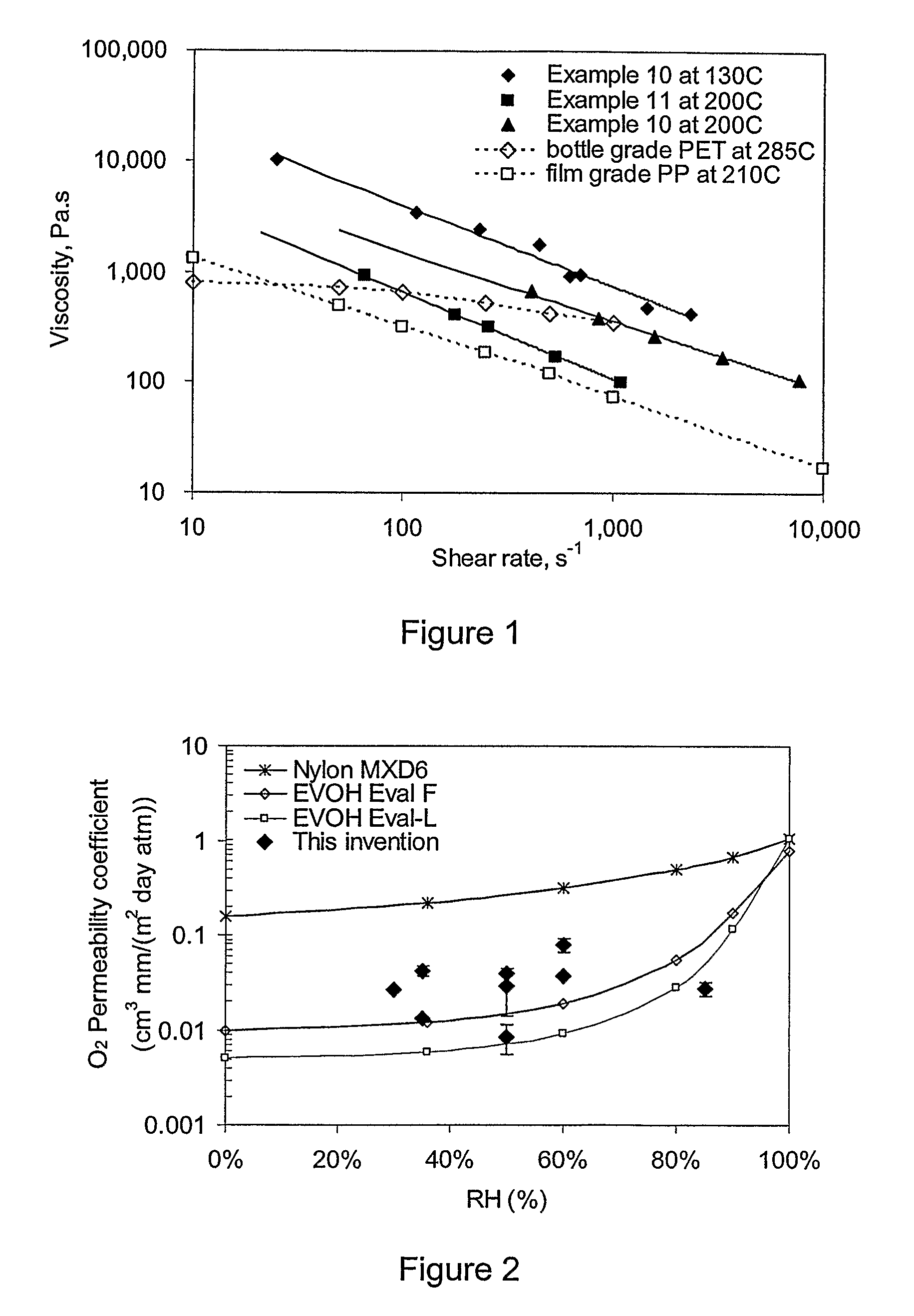
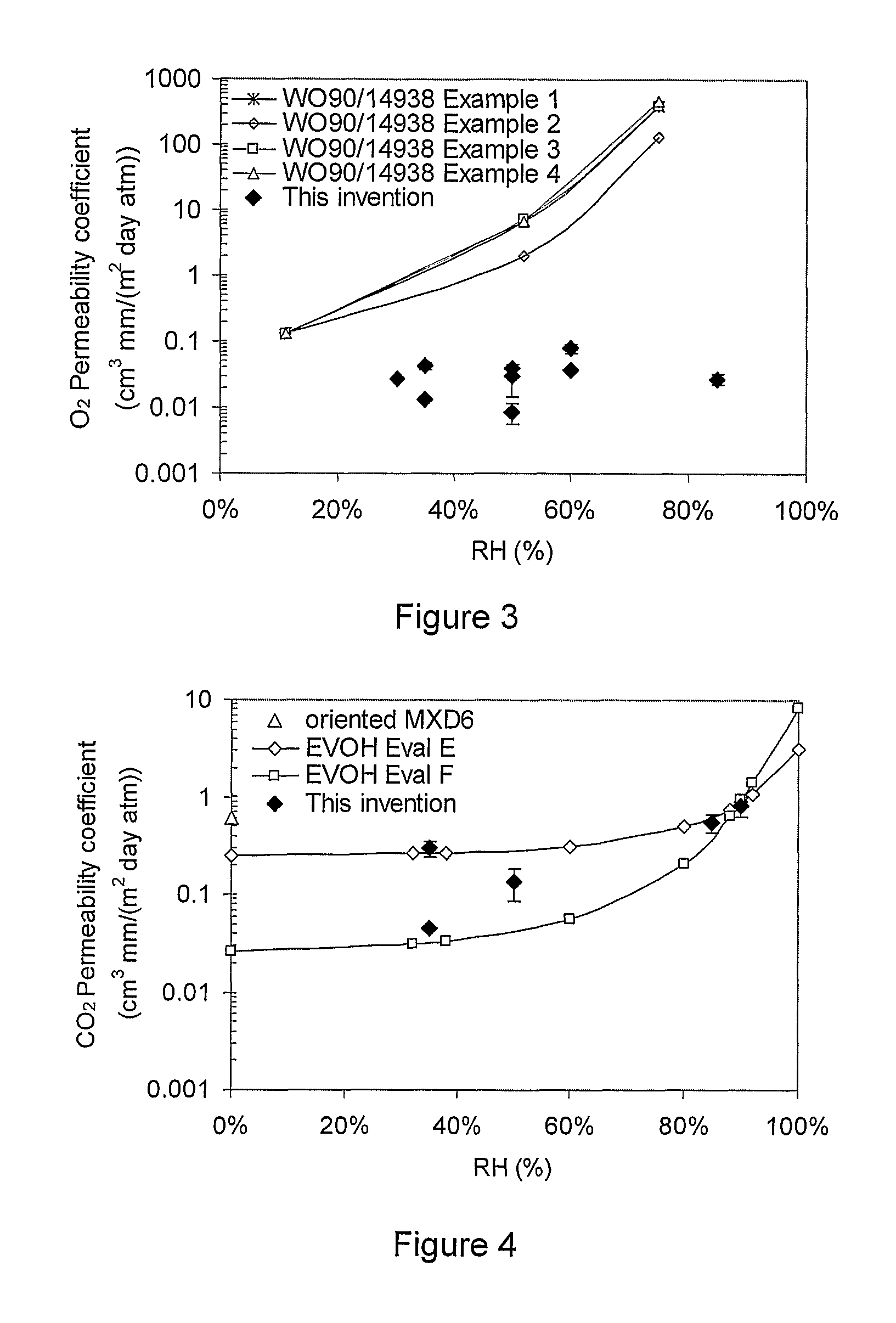
A barrier composition which is injection mouldable and able to be made into a transparent film or incorporated (by co-extrusion and / or lamination) into multi-layer film products, the composition on dry basis: a) from 45 to 90% by weight of a starch and / or a modified starch selected from starches modified by reaction with a hydroxyl alkyl group, an acetate or a dicarboxylic acid anhydride or a grafting polymer; b) from 4 to 12% by weight of a water soluble polymer selected from polyvinyl alcohol, polyvinylacetate, and copolymers of ethylene and vinylalcohol which have a melting point compatible with the molten state of the starch components c) from 5 to 45% by weight of a non-crystallising mixture of sorbitol and at least one other plasticizer selected from glycerol, maltitol, xylitol, mannitol, glycerol trioleate, epoxidised linseed or soybean oil, tributyl citrate, acetyl tri-ethyl citrate, glyceryl triacetate, 2,2,4-trimethyl-1,3-pentanediol diisobutyrate; polyethylene oxide or polyethylene glycol; d) from 0.3 to 2.5% by weight of a C12-22 fatty acid or salt; e) from 0.25% to 3% of an emulsifier system having a hydrophilic lipophilic balance value between 2 and 10. The barrier film may be co-injection moulded with polyethylene terephthalate (PET) or polylactic acid (PLA) for blow moulding into beverage bottles, with polyethylene (PE) or polypropylene (PP) or biodegradable polymers for high gas-barrier containers or closures, or may be co-extruded with polyethylene, polypropylene or polylactic acid for thin film packaging applications or for blow-moulded containers.
Owner:PLANTIC TECH
Probiotic microcapsules as well as preparation method and application thereof
ActiveCN105310080AImprove the situation of low freeze-drying survival rateImprove stabilityFood freezingFood shapingFreeze-dryingK carrageenan
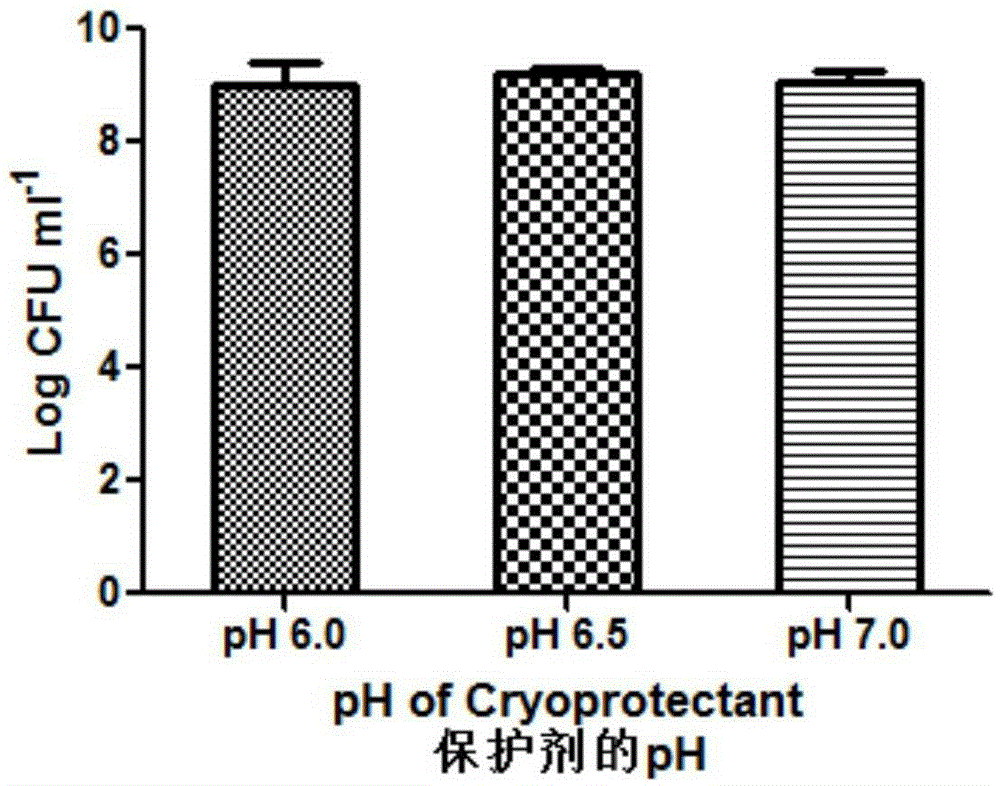
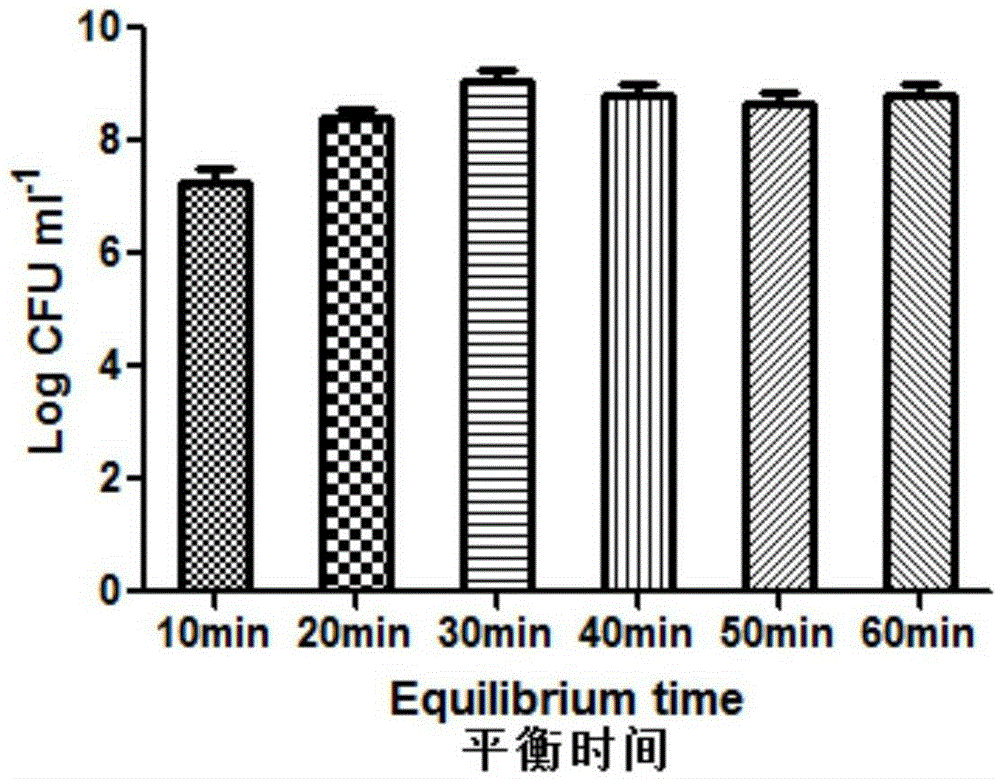
The invention relates to probiotic microcapsules as well as a preparation method and application thereof. The probiotic microcapsules comprise a core material and a wall material, wherein the core material is probiotics; the outer layer of the wall material is coated with chitosan; the wall material is prepared from an aqueous solution containing a natural polymer material and a freeze-drying protection agent; the freeze-drying protection agent comprises one or more of glucose, fructose, sucrose, lactose, trehalose, soluble starch, glycerin, mannitol, Arabic gum, dextran 40 and skim milk; the natural polymer material comprises one or more of gellan gum, xanthan gum, k-carrageenan, sodium alginate, cellulose acetate phthalate or gelatin; in the aqueous solution, the volume fraction of the freeze-drying protection agent is 4.0%-20.0% and the volume fraction of the polymer material is 0.5%-5.0%. The probiotic microcapsules can keep excellent acid resistance and storage stability before and after being freeze-dried.
Owner:SUN YAT SEN UNIV
Microparticles which controllably release olfactorily active substances, methods of using same and processes for preparing same
InactiveUS6235274B1Increase the fragranceAugmenting and enhancing and imparting aroma and tasteCosmetic preparationsPowder deliveryParticulatesFlavor
Described are flavor composition, flavor component, perfume composition and perfume component-containing microparticles which are particulate matrices composed of:(a) an olfactorily active component (e.g., perfume component);(b) silica; and(c) a saccharide composition which is a mixture of mannitol and maltose.The microparticles are useful in augmenting, enhancing and / or imparting aroma and / or taste (over relatively long periods of time in a controllably releasable manner) to perfume compositions, perfumed articles (e.g., deodorancy and antiperspirant sticks), foodstuffs, chewing gums, beverages and the like. Also described is a process for preparing the above-mentioned microparticles using, in sequence, (1) adsorption of the olfactorily active material onto silica followed by (2) a blending / extrusion step followed by (3) at least one particularization step.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
Pet food compositions
Disclosed herein are pet food compositions. In one embodiment, pet food compositions which are described comprise a component selected from 2-deoxy-D-glucose; 5-thio-D-glucose; 3-O-methylglucose; 1,5-anhydro-D-glucitol; 2,5-anhydro-D-mannitol; mannoheptulose; and mixtures thereof. In yet another embodiment, pet food compositions which are described comprise an extract of plant matter selected from avocado, alfalfa, fig, primrose, and mixtures thereof. The pet food compositions may be prepared by any of a variety of processes including, but not limited to, optional processes described herein.
Owner:THE PROCTER & GAMBLE COMPANY +1
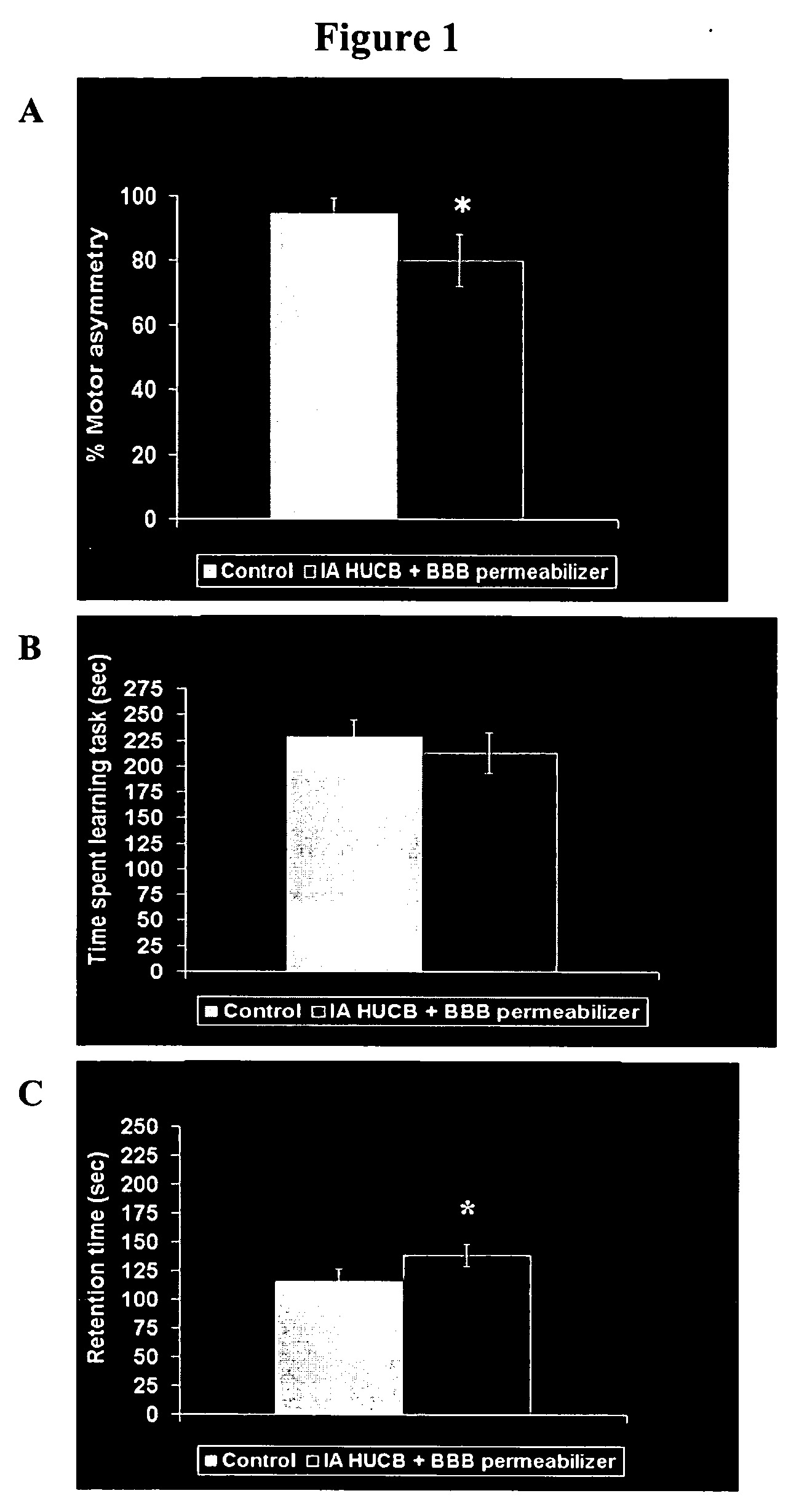
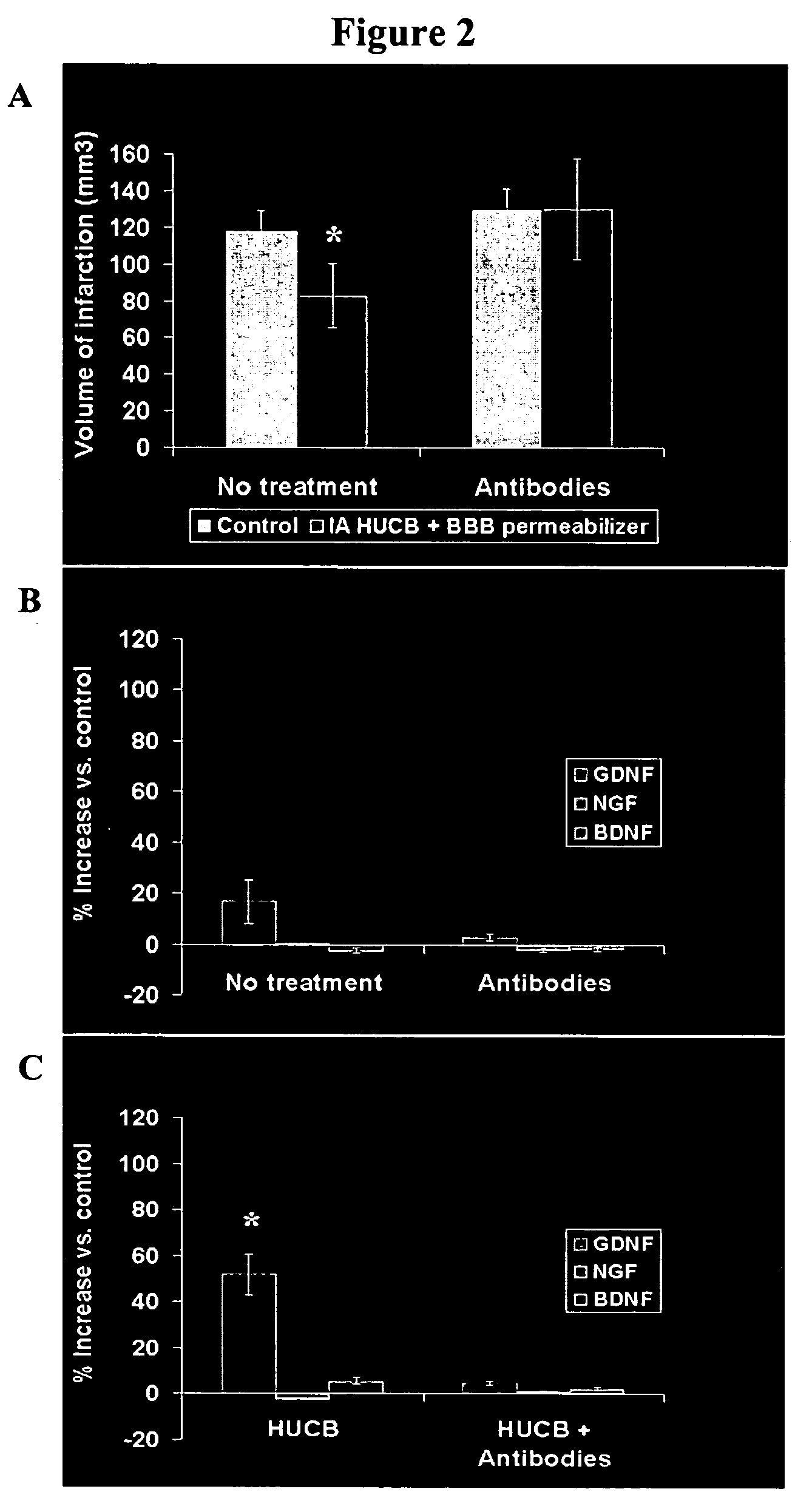
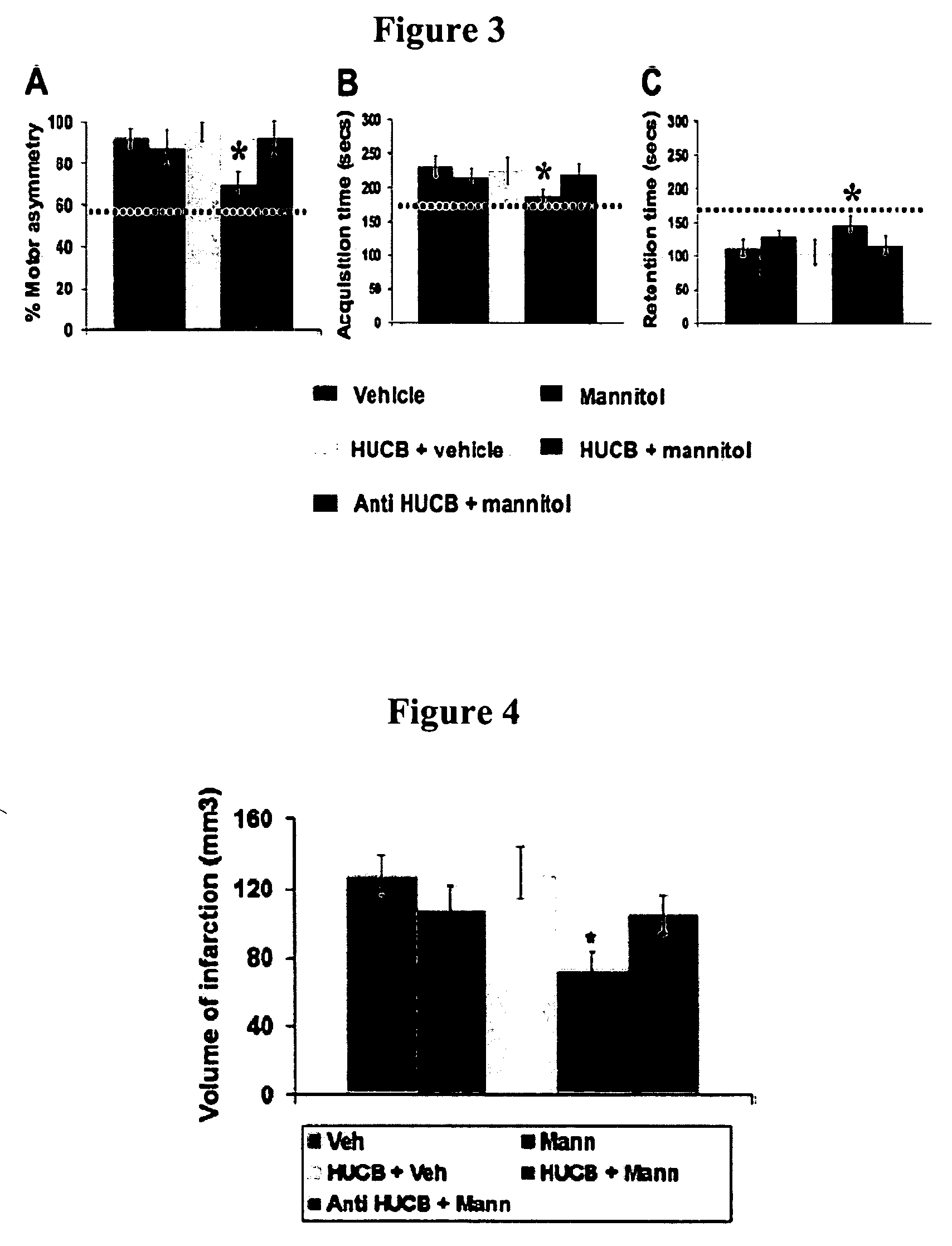
Compositions and methods for enhancing neuroprotection via administration of stem cells and blood brain barrier permeabilizers
ActiveUS20050169902A1Significant neuroprotective effectGood effectBiocidePharmaceutical delivery mechanismMedicineMannitol
The present invention provides compositions and methods for enhancing the neuroprotective effect of umbilical cord blood cells. More particularly, the present invention provides methods of treating neurodegenerative disorders by administering umbilical cord blood cells and a substance capable of permeabilizing the blood brain barrier. In one embodiment, the blood brain barrier permeabilizer is mannitol. In another embodiment, the blood brain barrier permeabilizer is Cereport.
Owner:UNIV OF SOUTH FLORIDA
Orally disintegrating tablets and process for obtaining them
InactiveUS20060165781A1High dissolution rateImprove compression performancePill deliveryPharmaceutical non-active ingredientsMANNITOL/SORBITOLOrally disintegrating tablet
The tablets comprise: at least 59.5% spray-dried mannitol; active ingredient below or equal to 10%, where at least 90% in weight of the active ingredient has a particle size below 100 μm; microcrystalline cellulose 10-18%, with an average particle size of 50 μm and where at least 99% in weight of microcrystalline cellulose has a particle size below 250 μm; sodium croscarmellose 14%; and a lubricant agent 0.5-2%; where, unless specified otherwise, the percentages are expressed in weight of the total weight of the tablet. And also a process comprising: sieving and mixing of components except for the lubricant agent; mixing of all components; and direct compression of the final mixture. The tablets of the invention give lower disintegration times as well as good perception on the tongue after disintegration, and overcome the problem of insufficient mechanical resistance for packaging and transport operations.
Owner:WARNER CHILCOTT IBERIA
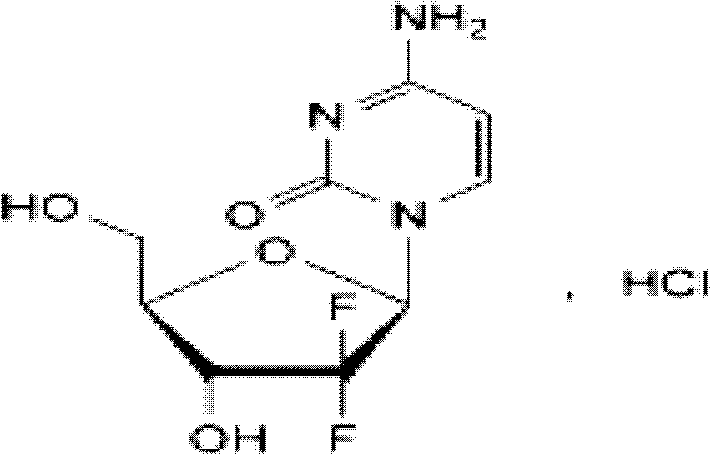
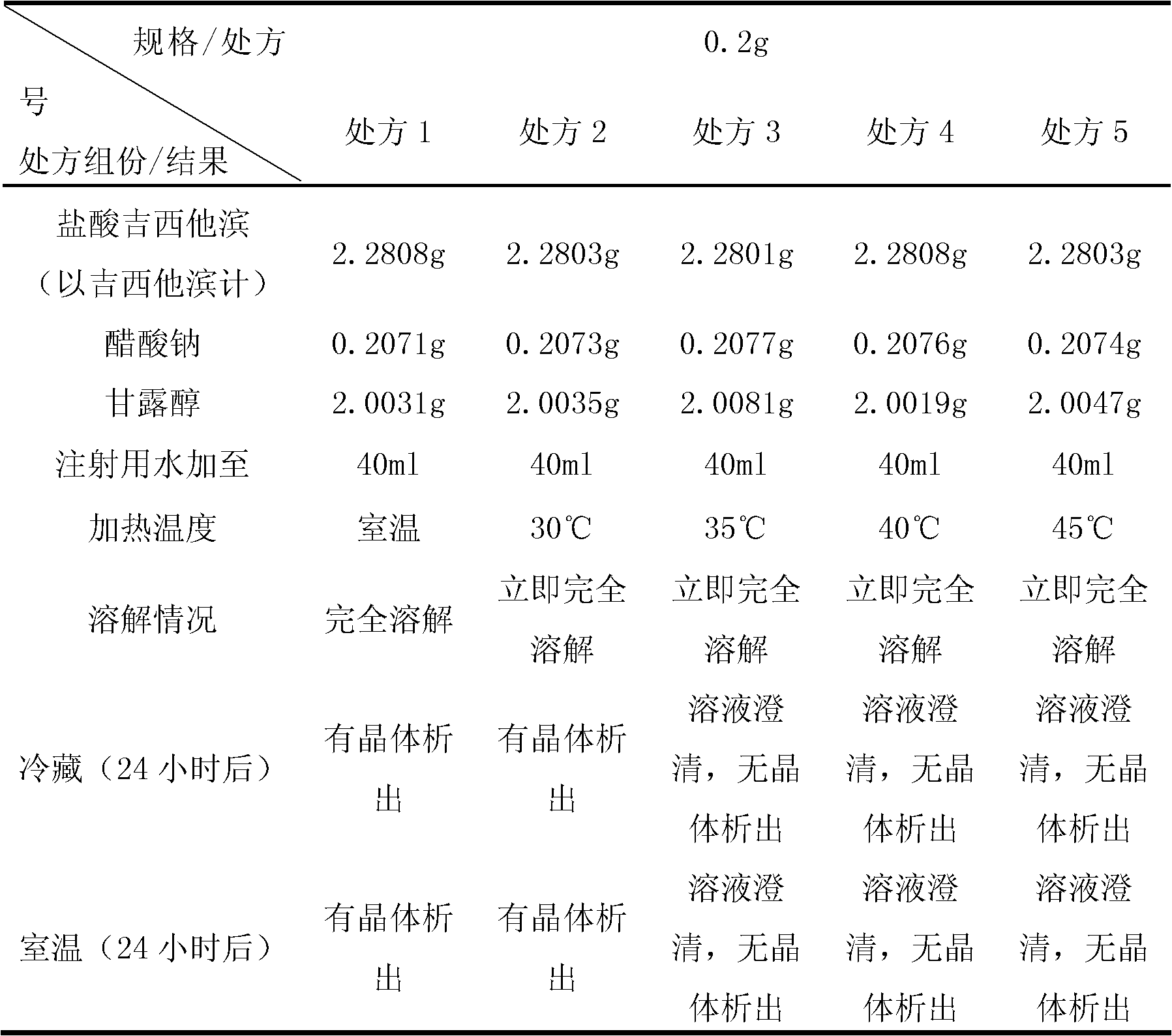
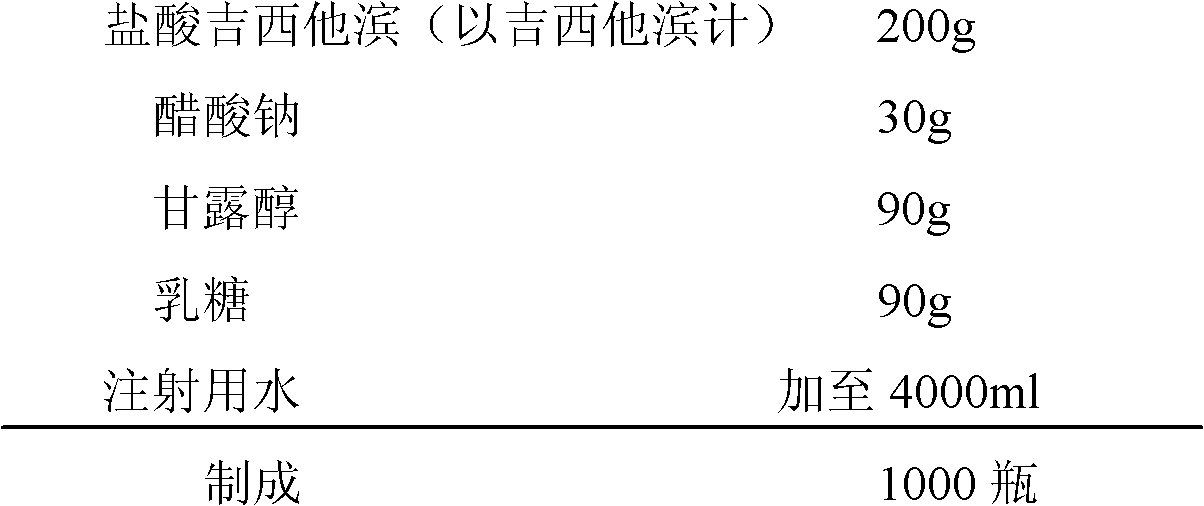
Gemcitabine hydrochloride lyophilized powder injection
ActiveCN101564381AImprove stabilityLow content of related substancesPowder deliveryOrganic active ingredientsPancreas CarcinomaDrug
The invention relates to a gemcitabine hydrochloride lyophilized powder injection and a preparation method thereof. The gemcitabine hydrochloride lyophilized powder injection prepared by the method can be used as a therapeutic medicament for treating middle and late non-small cell lung cancer, pancreatic cancer and the like. The gemcitabine hydrochloride lyophilized powder injection is characterized by consisting of gemcitabine hydrochloride, mannitol and sodium acetate, wherein the weight ratio of the gemcitabine hydrochloride to the mannitol is 1:0.5-5, and the weight ratio of the gemcitabine hydrochloride to the sodium acetate is 1:0.01-0.1. The preparation method comprises the following steps: taking the mannitol and the sodium acetate; dissolving the mannitol and the sodium acetate by adding injection water; adding the gemcitabine hydrochloride to the mixture, stirring and dissolving the mixture, and adjusting the pH to between 2.7 and 3.3; fixing the volume; filtering the product by a 0.22 mu m microporous membrane; filling, dishing up, lyophilizing, and compressing; taking the product out of a box, and tying the product with an aluminum-plastic composite cover; and inspecting the quality, and packaging the product after passing the quality inspection to obtain the gemcitabine hydrochloride lyophilized powder injection.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Pemetrexed disodium freeze-dried powder injection and preparation method thereof
ActiveCN102106833AReduce adverse effectsSimple prescriptionOrganic active ingredientsPowder deliveryActivated carbonFreeze-drying
The invention belongs to the technical field of medication, and in particular relates to a pemetrexed disodium freeze-dried powder injection and a preparation method thereof. The pemetrexed disodium freeze-dried powder injection consists of pemetrexed disodium and mannitol, wherein the mass ratio of the mannitol to the pemetrexed disodium is (0.6-2.0):1. The preparation method comprises the following steps: adding injecting water into a liquid preparation tank; adding the pemetrexed disodium weighted according to the formula; stirring until the pemetrexed disodium completely dissolved; adding the mannitol; regulating the pH by utilizing a hydrochloric acid solution or a sodium hydroxide solution; adding activated carbon for decoloration; filtering to remove the carbon; finely filtering with a filter membrane; subpackaging; and freezing and drying. The pemetrexed disodium freeze-dried powder injection has excellent moldability; the appearance of the solution before freezing is clear; the frozen and dry product has good re-dissolubility; and the re-dissolved product has the advantages of good clarity, low impurity content, low moisture content, good stability and controllable quality.
Owner:HAINAN JINRUI PHARMA
Electronic cigarette tobacco juice solvent and electronic cigarette tobacco juice
InactiveCN103622161ATasting experience is not greasyStrong tasting experienceTobacco treatmentTobacco devicesFlavorLiquid smoke
The invention provides an electronic cigarette tobacco juice solvent comprising sorbitol. The sorbitol is used as the electronic cigarette tobacco juice solvent. As for electronic cigarette tobacco juice made of the electronic cigarette tobacco juice solvent, smoking experience is not greasy, foreign flavor is avoided, and higher smoking comfort is achieved. Furthermore, the electronic cigarette tobacco juice comprises one or more of propylene glycol, glycerin and mannitol, the sorbitol is reacted with the propylene glycol, the glycerin and the mannitol so that a large amount of smoke can be generated when the electronic cigarette tobacco juice made of the electronic cigarette tobacco juice solvent is smoked, smoking experience of a smoker is enhanced, smoking experience is dense and flush, mouth feel is smooth and free of the foreign flavor, and the higher smoking comfort is achieved. In addition, the electronic cigarette tobacco juice solvent is composed of food solvents and has no harm to the smoker.
Owner:HUIZHOU KIMREE TECH
Cassia obtusifolia tea bag with functions of weight reduction, hypotension and hypoglycemic effect and its production process
The invention provides a cassia obtusifolia tea bag with functions of weight reduction, hypotension and hypoglycemic effect, which is characterized in that the production process comprises the following steps: washing, cleaning and drying the cassia obtusifolia stem, leaf and grain, crushing by a pulverizer to 280 meshes, uniformly stirring, packing, disinfecting to obtain the cassia obtusifolia tea bag with functions of lipid lowering, hypotension and hypoglycemic effect. The optimal composition and the weight part ratio of the tea bag are: 40-60 parts of dried cassia obtusifolia stem, leaf and grain, 10 parts of dried lotus leaf, 5 parts of haw, 5 parts of dried balsam pear, 5 parts of Chinese wolfberry, 10 parts of Pu'er tea, 10 parts of cassia seed, 5 parts of mulberry leaf, 5 parts of folium apocyni veneti, 5 parts of Chinese yam as medicine, 3 parts of rhubarb, 2 parts of chrysanthemum, 2 parts of honeysuckle flower, 1 part of boat-fruited sterculia, 5 parts of cassia seed, 5 parts of kudzu root and 10 parts of additive. The additive is composed of fatty acid sucrose ester, xylitol, mannitol crystal, natural menthol, citric acid, trisodium phosphate, sodium saccharin and steviosid; the above components are carried out the processes of crushing, mixing, uniformly stirring, packing and disinfecting to obtain the tea bag.
Owner:肖梅芬
Chinese herbal medicine feed additive capable of improving immunity, preparation method thereof and feed
InactiveCN103109980AEnhance physical fitnessImprove immunityAnimal feeding stuffSide effectVitamin C
The invention provides a Chinese herbal medicine feed additive capable of improving the immunity. The Chinese herbal medicine feed additive is characterized by comprising the following raw materials: willow herb, red-backed vegetable, Chinese yam, herba epimedii, jiaoshen yeast, caulis spatholobi, puerarin, cimicifugae foetidae, Chinese herbaceous peony, jian yeast, rheum officinale, mangnolia officinalis, cyrtomium fortunei, liquorice, radix scutellariae, honeysuckle stem, gynostemma pentaphylla, fuling, soy isoflavone, soyasaponin and additional components, wherein the additional components comprise vitamin A, vitamin B, vitamin C, chromium trichloride, huanyuan gel, xylitol and mannitol crystals according to the proportion of 0.2:0.2:0.5:0.3:1:1:1. The Chinese herbal medicine feed additive can replace antibiotics and medicines and has no toxic and side effects; the immunity of animals such as pigs, chicken and ducks can be obviously improved; relatively high economical benefits can be achieved with relatively small investment; antibiotic and chemical medicine residues are avoided; and an effective way is supplied to reducing the antibiotic residues in animal products.
Owner:LIAOCHENG UNIV
Preserved fruits without sugar additives
The invention provides a preserved fruit without additive sugar which is prepared by the steps of selecting fruits, gum dipping, boiling in sugar, sugar dipping and baking, etc., and has the advantage that cane sugar and syrup in traditional technique are replaces by xylitol, sorbierite, maltitol or mannite, etc. Not only the sugar content of the product obtained by the invention is low and is lower than 30 percent but also the product has the advantages of abundant nourishment, unique flavor and good taste, which is suitable for wide crowd and especially for the special crowd of diabetics, etc.
Owner:SHANXI UNIV
Liquid preparation of recombinant anti-PD-L1 whole-human monoclonal antibody
InactiveCN107198773AImprove stabilityEasy to administerAntibody ingredientsImmunoglobulinsMonoclonal antibodyPD-L1
The invention discloses a stable liquid preparation of a recombinant anti-PD-L1 whole-human monoclonal antibody. The liquid preparation is prepared from a recombinant anti-PD-L1 whole-human monoclonal antibody, a buffer solution, a stabilizing agent, an osmotic pressure regulator and a surfactant, wherein the buffer solution is a histidine-histidine hydrochloride buffer solution; the stabilizing agent is mannitol; the osmotic pressure regulator is sodium chloride; the surfactant is polysorbate 80. The liquid preparation of the recombinant anti-PD-L1 whole-human monoclonal antibody has superior stability, and can be stored stably at the temperature of 5+ / -3 DEG C for at least 24 months.
Owner:CSTONE PHARM (SUZHOU) CO LTD +1
Concentrated Protein Lyophilates, Methods, and Uses
InactiveUS20080213215A1Reduce moisture contentSpeed up the flowRadiation pyrometryPeptide/protein ingredientsRaman imagingReal time analysis
The invention provides, among other things, lyophilized compositions of high surface area that comprise a protein and that reconstitute quickly and efficiently to solution of high protein concentration with minimal formation, if any, of foam, effervescence, bubbles, turbidity, or particulates that might be deleterious. The invention also provides, among other things, methods for making the lyophilized compositions. The invention in additional aspects also provides Raman Imaging Spectrographic methods for real time analyses of polymorphs in a sample using PLS algorithms. By way of particular example, the use of the method for the analysis of mannitol polymorphs is described, and the use of the analysis to determine optimum compositions and lyophilization methods for producing lyophilates of pharmaceutical proteins having a predefined distribution of mannitol polymorphs and having the aforementioned reconstitution properties is also described.
Owner:AMGEN INC
Natural deodorant composition
The present invention relates to a natural deodorant system and a natural system for topical and systemic delivery of active ingredients, both systems being primarily free of, preferably substantially free of, more preferably essentially free of, and most preferably completely free of ethoxylates or other petrochemical derivatives, and comprising: (a) at least one of (1) glycerine (preferably of plant origin), (2) a polyol selected from the group consisting of galactitol, erythritol, inositol, ribitol, dithioerythritol, dithiothreitol, (3) a sugar alcohol, selected from the group consisting of mannitol, sorbitol, xylitol and maltitol, (4) a hydrogenated starch hydrosylates of at least one of berries, apples or plums, and (5) mixtures thereof; (b) water or a lower monohydric alcohol, selected from the group of methanol, ethanol, propanol and isoproponal, or mixtures thereof, present at a combined concentration of at least 20%; (c) one or more carrageenans (preferably of plant origin) or alginates, or mixtures thereof, present in combined concentrations of less than about 2%; and (d) optionally, one or more thickeners or gums selected from the group consisting of tara, guar, xanthan, Arabic, tragacanth, agar, locust bean gum, ghatti and microcrystalline celluloses.
Owner:TERRA FA NATUALS
Taxine kind anti-cancer slow release injection
InactiveCN1923189AOrganic active ingredientsPharmaceutical delivery mechanismCelluloseAcetic acid ethenyl ester
The invention relates to a slow-release injection of taxine anti-cancer drug, which comprises anti-cancer drug, slow-release finding, suspension and / or solvent. Wherein, said anti-cancer drug is taxine, 2'-hydroxy Paclitaxel, etc; the slow-release finding is polymer of hydroxyl, glycollic acid and glycolic acid, one of acetic acid ethyenyl ester polymer and polyphony; the suspension is polyphenyl (sodium), and mannite; the solvent is distilled water, injection water, absolute ethyl alcohol, etc. The invention can be injected to reduce the toxicity effect of drug, and improve the density locally to strengthen the treatment effect of chemotherapy and radiation therapy.
Owner:孔庆忠
Well Treatment Composition Crosslinkers and Uses Thereof
This invention relates to compositions used in treating subterranean formations, which include a hydrated polymer, and a dry blended multi-functional component. The hydrated polymer and dry blended multi-functional component are mixed at the ground surface of a wellsite, and subsequently injected into the formation providing controlled delay in crosslinking to achieve targeted fluid viscosity properties. The hydrated polymer may be a guar, hydroxypropyl guar, carboxymethyl guar, carboxymethylhydroxypropyl guar, synthetic polymers, and guar-containing compounds. The dry blended multi-functional component may include a crosslinker and a chelating agent, and the well treatment fluid may further include an activator mixed with the hydratable polymer. The chelating agent may be a polyols, gluconate, sorbitol, mannitol, carbonate, or any mixtures thereof. The crosslinker may be any source of boron, alkaline earth metal borates, alkali metal borates, zirconium compounds, titanium compounds, or any combination thereof, while the activator may be a caustic soda or magnesium oxide compound. The invention further provides methods for producing a well treatment composition including providing a hydrated polymer, and providing a dry blended multi-functional component. Also, methods of hydraulically fracturing a subterranean formation, as well as cleanup operations and gravel packing a wellbore are provided as well.
Owner:DESSINGES MARIE NOELLE +1
Preparing method and application of human mesenchymal stem cell-sourced exosome beautifying preparation
InactiveCN108721200APrecise regulation of immune statusRepair the traces of timeCosmetic preparationsToilet preparationsCuticleFreeze-drying
The invention discloses a preparing method and application of a human mesenchymal stem cell-sourced exosome beautifying preparation. The beautifying preparation comprises 92-108 parts of human umbilical cord mesenchymal stem cell exosome, 8-13 parts of mannitol, 0.42-0.55 part of oligopeptide-32, 1.3-2.5 parts of tetrapeptide-4, 0.7-1.3 parts of palmitoyl tripeptide-1 and 0.8-1.2 parts of palmitoyl pentapeptide-4. The preparation method includes: evenly mixing and stirring the human mesenchymal stem cell exosome, the mannitol, the oligopeptide-32, the tetrapeptide-4, the palmitoyl tripeptide-1and the palmitoyl pentapeptide-4 according to the weight, filtering through a filter membrane, performing split charging, and performing freeze drying to obtain the human mesenchymal stem cell-sourced exosome beautifying preparation. The exosome beautifying preparation can be externally applied to the epidermal layer or guided into an epidermis deep layer and the corium layer through a microneedle, a rolling needle or a hydro-lifting needle. The ImmuReg technology is utilized to bring the optimal repairing effect of freeze-dried powder into play, the skin immune state can be precisely adjusted, and accordingly aged cells can be removed, and skin cells can be rejuvenated.
Owner:成都赛伊泰生物科技有限公司
Liraglutide sustained-release microsphere preparation and preparation method thereof
InactiveCN104382860AUniform particle sizeLow burst ratePeptide/protein ingredientsMetabolism disorderSucroseMicrosphere
The invention relates to a liraglutide sustained-release micro sphere preparation and a preparation method thereof. The liraglutide sustained-release microsphere preparation comprises 5mg to 100mg of liraglutide, 0.5mg to 10mg of a protective agent and 50mg to 1000mg of a polylactic acid-glycolic acid copolymer, wherein the protective agent is one or a mixture of a plurality of sucrose, mycose, gelatin, mannitol, glycine, lysine and human serum albumin; the molecular weight of the polylactic acid-glycolic acid copolymer is 5000-20000 Daltons, and the ratio of polylactic acid to glycolic acid in the polylactic acid-glycolic acid copolymer is 1:3 to 3:1. According to the liraglutide sustained-release microsphere preparation disclosed by the invention, regular microspheres and medicines uniformly distributed in the microspheres can be obtained by just emulsifying and volatizing an organic solvent; processing procedures are simple; operation is simple; good repeatability is realized in preparation; the prepared liraglutide sustained-release microsphere preparations in batches have no remarkable difference; the obtained microspheres are uniform in grain size, narrow in distribution, controllable in grain size, round and orderly in surfaces and low in burst release rate.
Owner:浙江美华鼎昌医药科技有限公司 +1
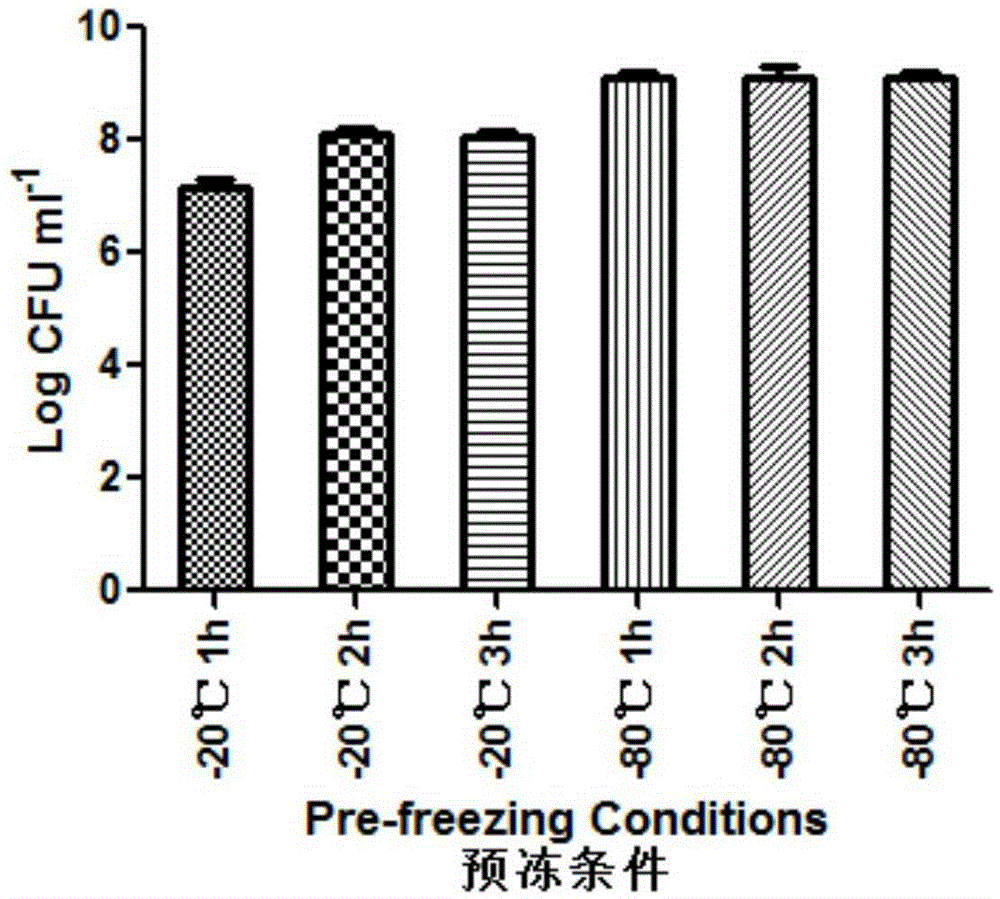
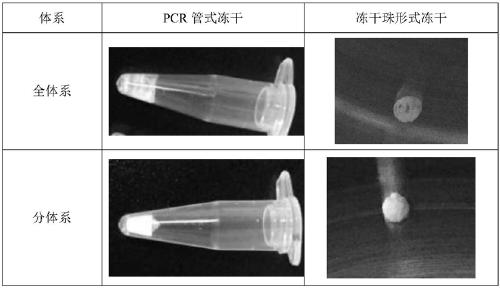
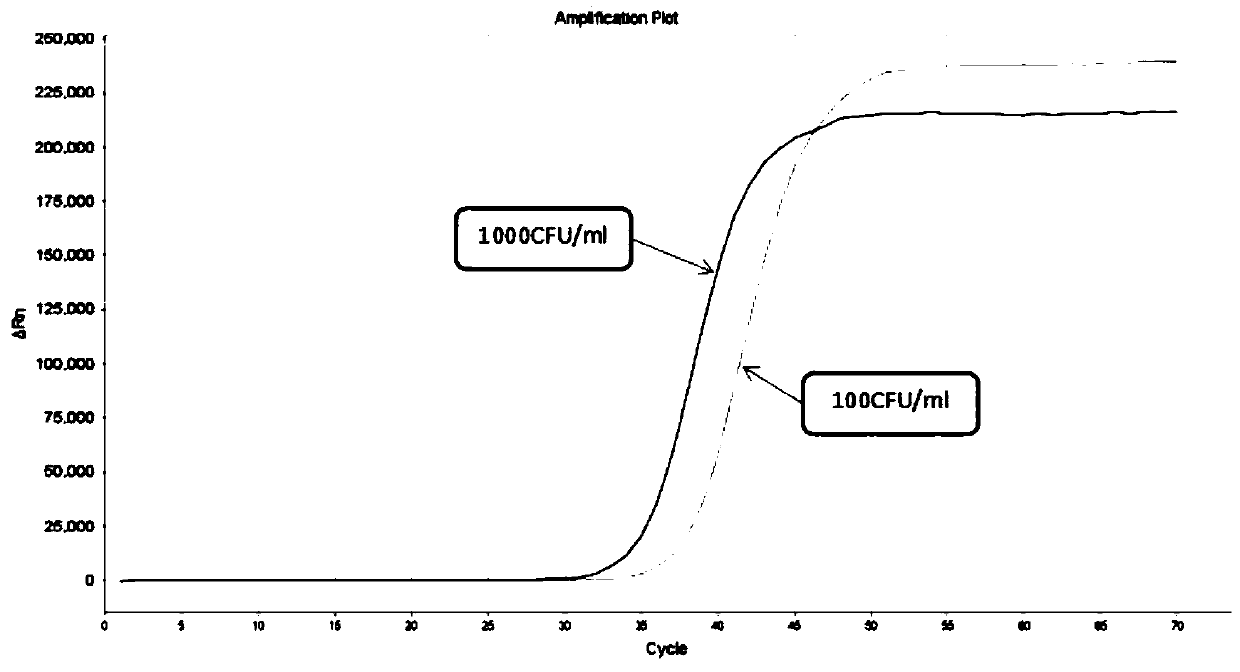
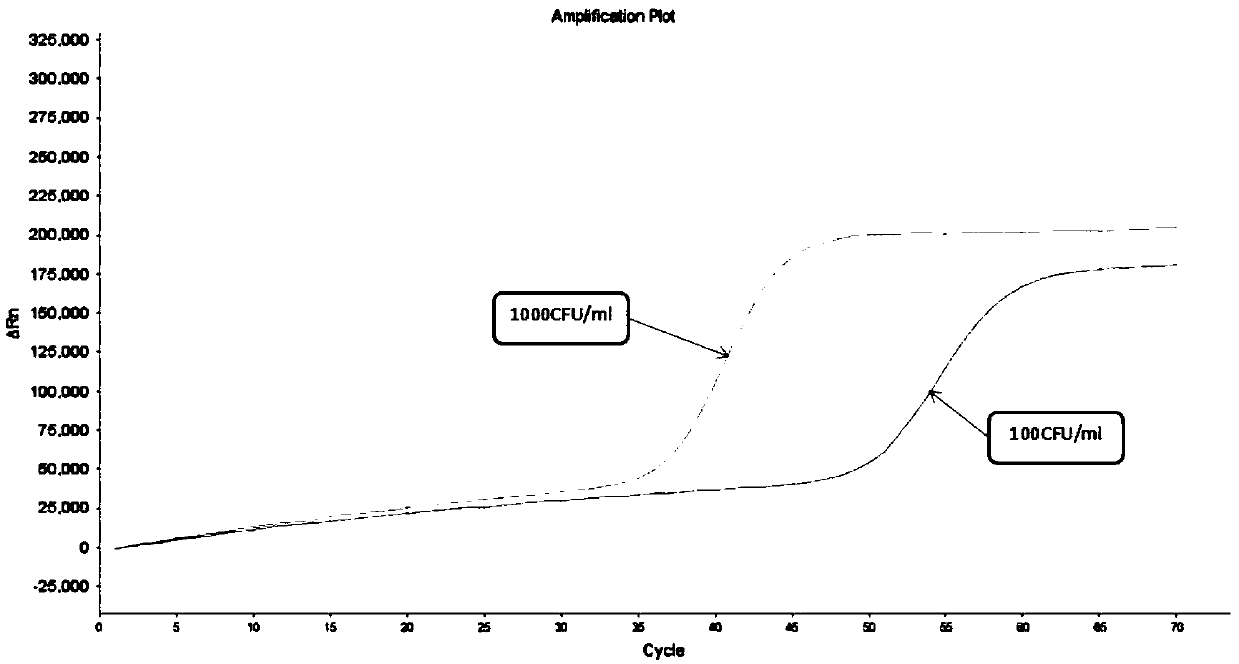
Freeze-drying protection system required for nucleic acid amplification reagent and preparation method of freeze-drying protection system
InactiveCN109593834AKeep aliveNo biohazardMicrobiological testing/measurementFreeze thawingFreeze-drying
The invention belongs to the technical field of medicine preparation, and particularly relates to a freeze-drying protection system required for a nucleic acid amplification reagent and a preparationmethod of the freeze-drying protection system. The freeze-drying protection system comprises the nucleic acid amplification reagent and a freeze-drying protection additive, wherein the nucleic acid amplification reagent is a reagent used for LAMP reaction amplification, the freeze-drying protection additive is one or a compound of the following reagents: polyethylene glycol, mannitol, polyvinylpyrrolidone, glucan, trehalose, sucrose, bovine serum albumin, collagen, threonine and glycine, and the concentration of the weight-to-volume ratio of the freeze-drying protection additive and the amplification reaction reagent is 3% to 25%. The freeze-drying protection additive used in the invention has the advantages of small volume, short freeze-drying time, high efficiency and low energy consumption, and the freeze-drying protection system can be directly used for gene chip experiments, does not cause repeated freeze-thaw and waste of reagents, and can effectively ensure the activity of active substances in the freeze-drying process.
Owner:百康芯(天津)生物科技有限公司
Medicament formula for relieving pain caused by infusion medicament and preventing phlebitis
InactiveCN102579458ARelief the painRelieve and prevent phlebitisAntipyreticAnalgesicsFormularySodium phosphates
The invention discloses a medicament formula for relieving pain caused by infusion medicament and preventing phlebitis. The medicament formula can be used for relieving pain caused by intravenous supplementing potassium, infusion mannitol, azithromycin, fructose diphosphate sodium and chemotherapeutic medicaments, and can be used for effectively preventing and treating extravasation phlebitis caused by long-term use of a remaining needle, infusion of vein high-nutrition medicaments, infusion of chemotherapeutic medicaments, infusion extravasation and the like. According to the medicament formula, 20-35ng of anisodamine, 6-13ml of lidocaine, 15-26mg of dexamethasone and 4-6ml of glycerin are arranged in a sterile kidney basin, sterile gauze is coated above a puncture part after being uniformly wetted, and an external preservative film is wound on the gauze. The medicinal formula has the advantages of continually and efficiently relieving local pain caused by infusion medicaments, and effectively preventing and treating local inflammation, swelling pain and stripped cable change caused by infusion.
Owner:GENERAL HOSPITAL OF JIZHONG ENERGY FENGFENG GRPCO
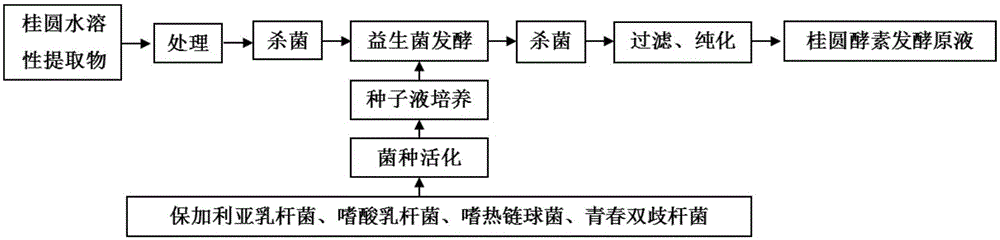
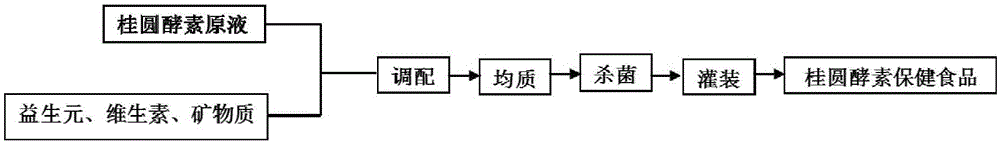
Method for preparing fruit/vegetable or cereal or medicine-food homologous ferment health-care foods by using probiotics fermentation
The invention relates to a method for preparing fruit / vegetable or cereal or medicine-food homologous ferment health-care foods by using probiotics fermentation. According to the method, inventors disclose strains, which are applicable to the production of ferments by taking fruits / vegetables or cereals or medicine-food homologous food materials as fermentation raw materials, and optimize a fermentation process, ferment fermented stock solutions of the fruits / vegetables or cereals or medicine-food homologous food materials or the like are obtained, and various ferment products are obtained through subsequent processing. Obtained ferments are rich in functional components, i.e., exopolysaccharides, mannitol and SOD accumulated during fermentation, thereby being ideal health-care foods.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
Gemcitabine hydrochloride lyophilized powder injection and preparation method thereof
ActiveCN102144981AReduce dosageImprove stabilityPowder deliveryOrganic active ingredientsSodium acetateAdjuvant
The invention relates to a gemcitabine hydrochloride lyophilized powder injection and a preparation method thereof. The lyophilized powder injection comprises the following components in parts by weight: 20-30 parts of gemcitabine hydrochloride, 5-9 parts of mannitol, and 3-10 parts of sodium acetate. The freeze-drying step includes the following three stages: a pre-freezing stage, a primary drying stage and a secondary drying stage, and the entire freeze-drying time is lower than 20 hours. The gemcitabine hydrochloride lyophilized powder injection provided by the invention has the advantages of less types and amounts of adjuvants, easily-controlled technological parameters, simple process route, short freeze-drying time, convenience in operation, good repeatability, low contents of related substances, and controllable quality; and the redissolved lyophilized powder injection has good clarity and forming performance. The lyophilized powder injection has stable and controllable quality, is easy to realize industrial production, and can generate considerable economic and social benefits.
Owner:HAINAN JINRUI PHARMA CO LTD
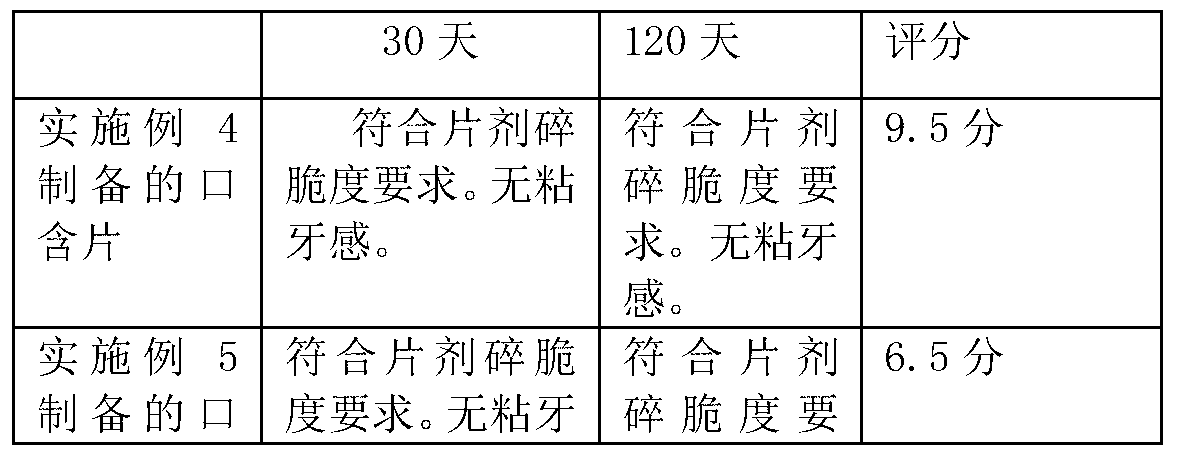
Dendrobium buccal tablets and preparation method for same
ActiveCN103285242ASimple processEasy to operatePill deliveryRespiratory disorderLoss rateSlice thickness
The invention belongs to the field of traditional Chinese medicines or healthcare foods, relates to a dendrobium preparation, and in particular relates to dendrobium buccal tablets. The formula of the dendrobium buccal tablets is composed of dendrobium ultra-fine powder, mannitol, white granulated sugar powder, magnesium stearate, aerosol, citric acid, borneol / menthol, mint oil and mint essence, wherein under the formula aforementioned, wet granulation is performed, and then tabletting is performed to prepare the buccal tablets; and the dendrobium buccal tablets prepared by the formula are high in bioavailability and good in taste. Additionally, the invention further discloses a preparation process for dendrobium ultra-fine powder. The preparation process specifically comprises the following steps of: peeling the cleaned fresh dendrobium bars; drying and slicing to achieve a thickness of not greater than 1 mm; and performing drying treatment at 50-60 DEG C to cause the water content to be 2-3%, and crushing for 10-30 minutes by an ultra-fine crusher, so as to obtain the dendrobium ultra-fine powder. According to the process, the loss rate of the precious medicine of dendrobium is further reduced by optimizing the slice thickness and omitting the step of processing crude powder.
Owner:CHONGQING ACAD OF CHINESE MATERIA MEDICA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com