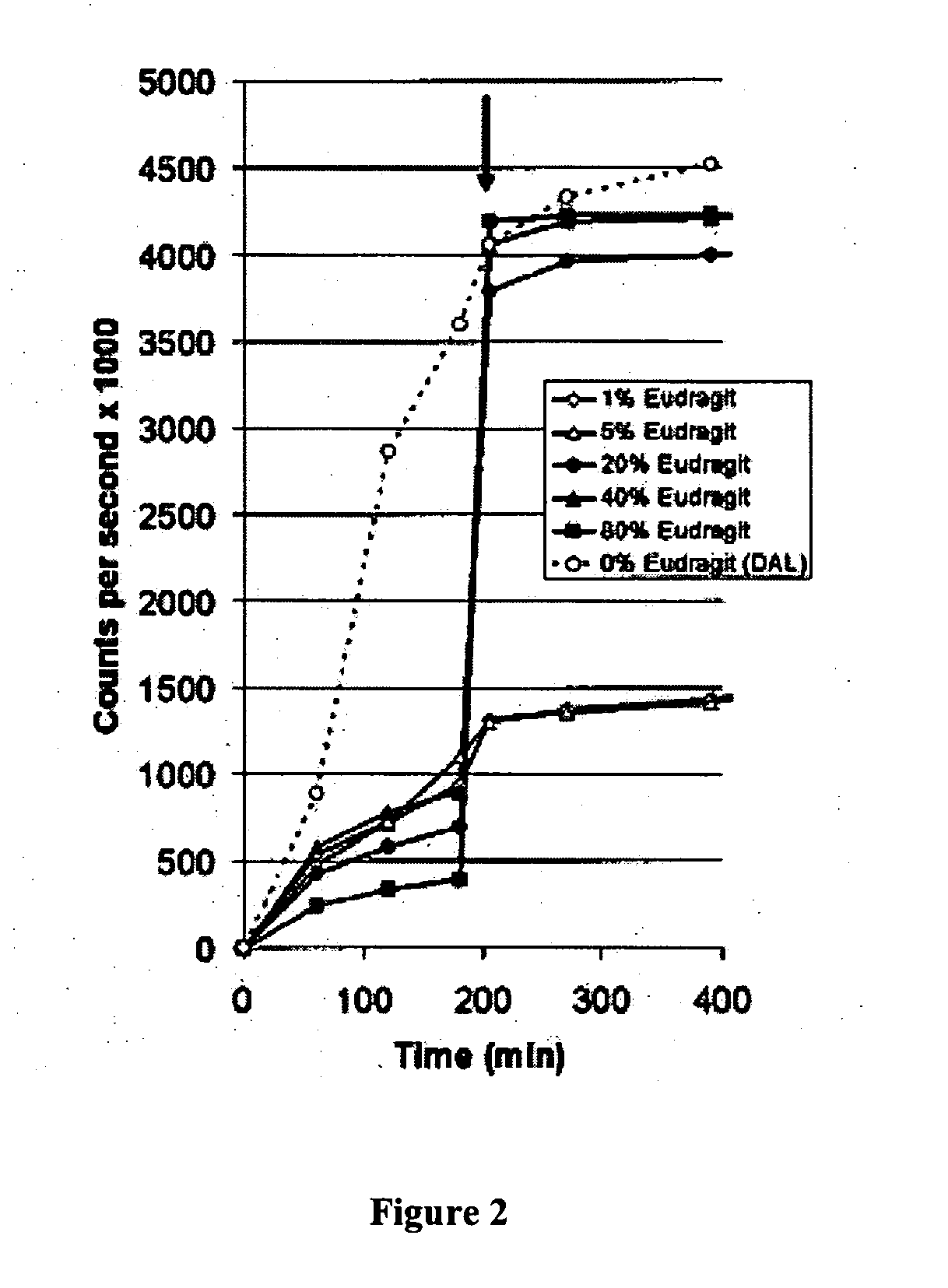
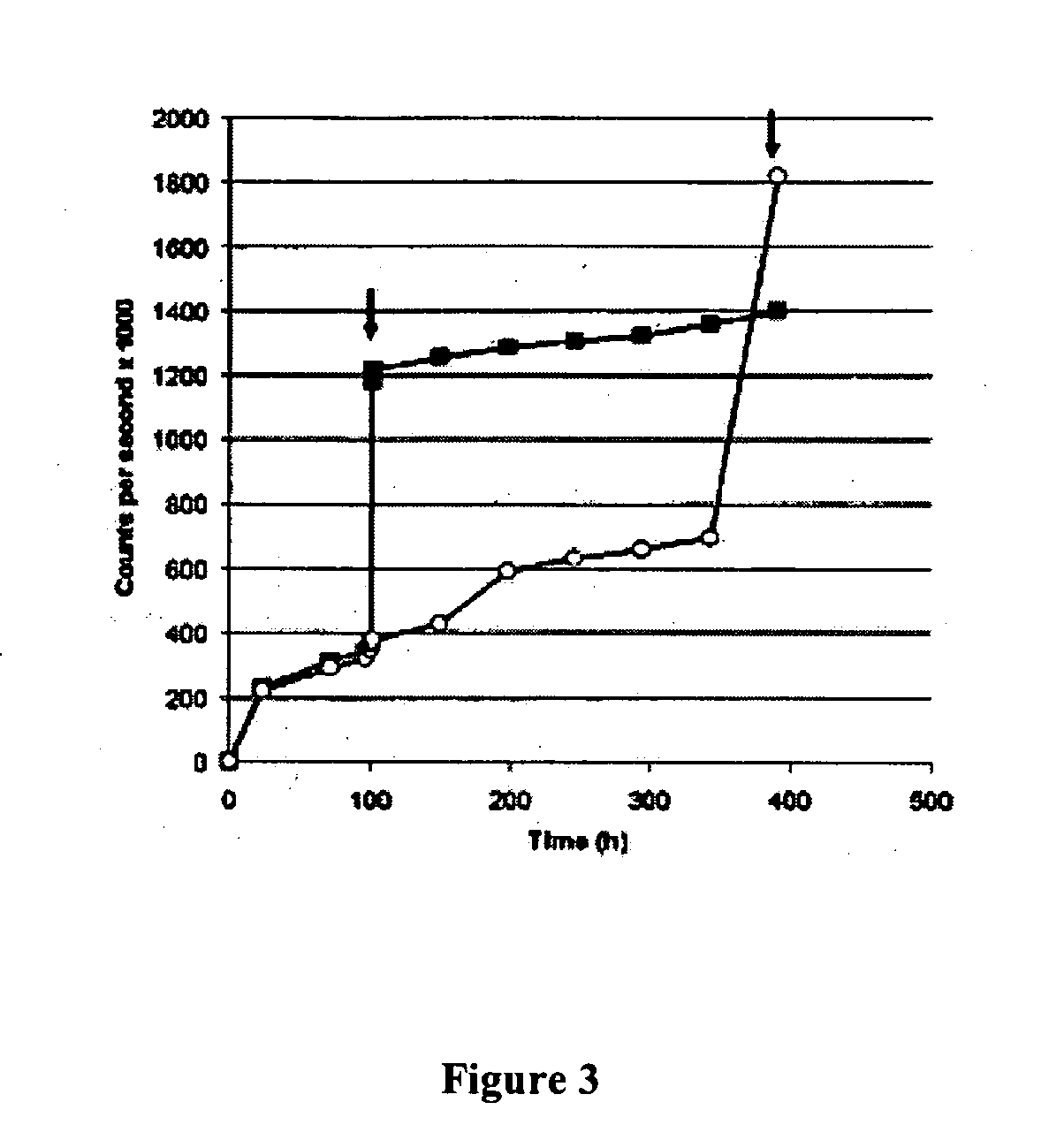
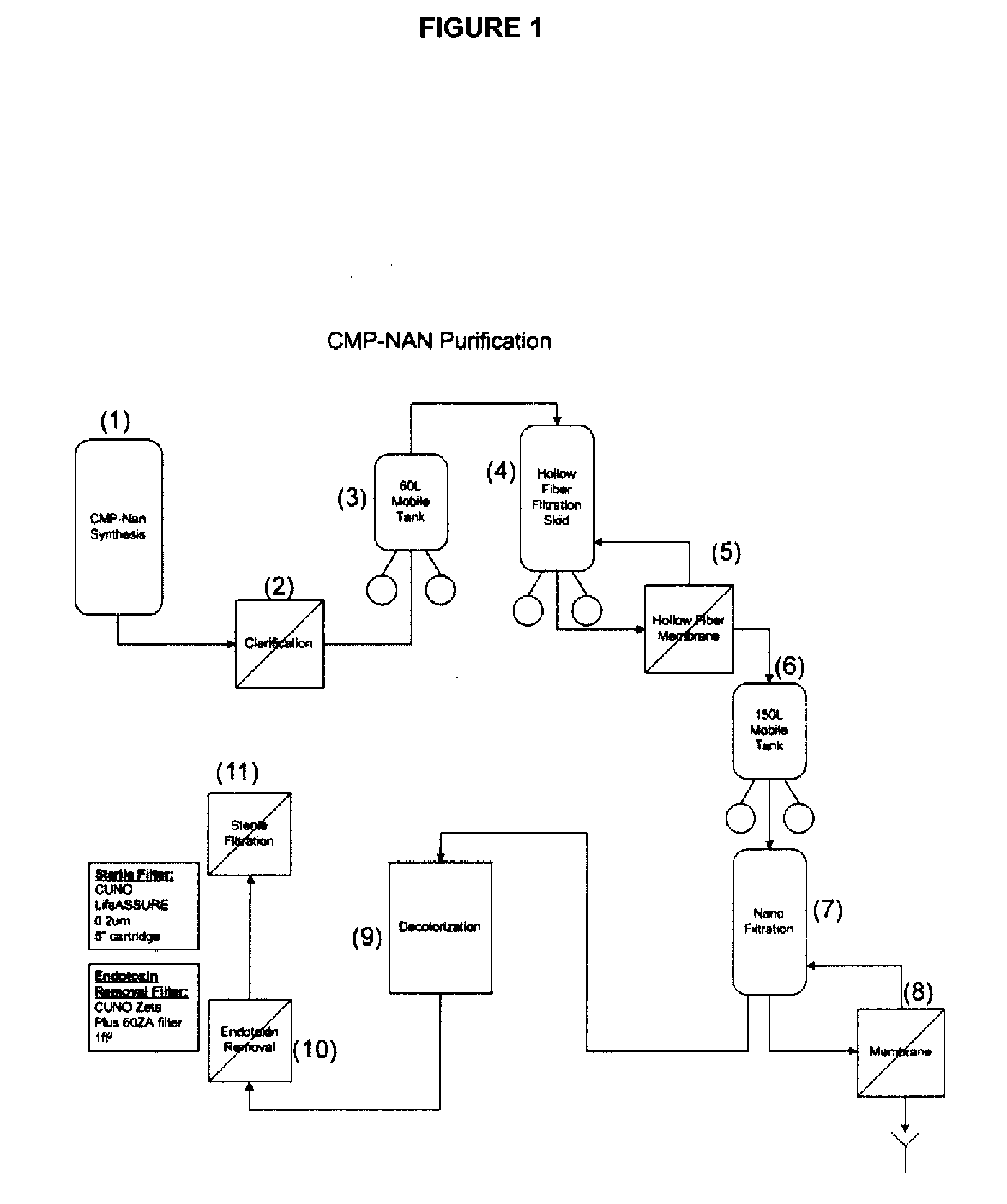
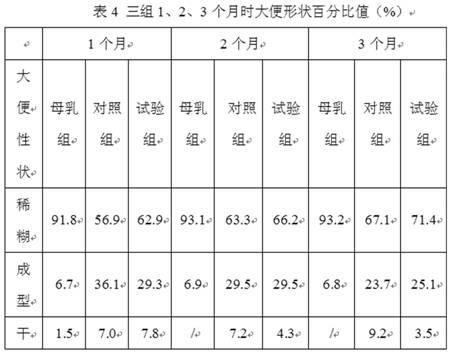
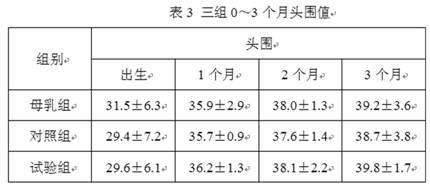
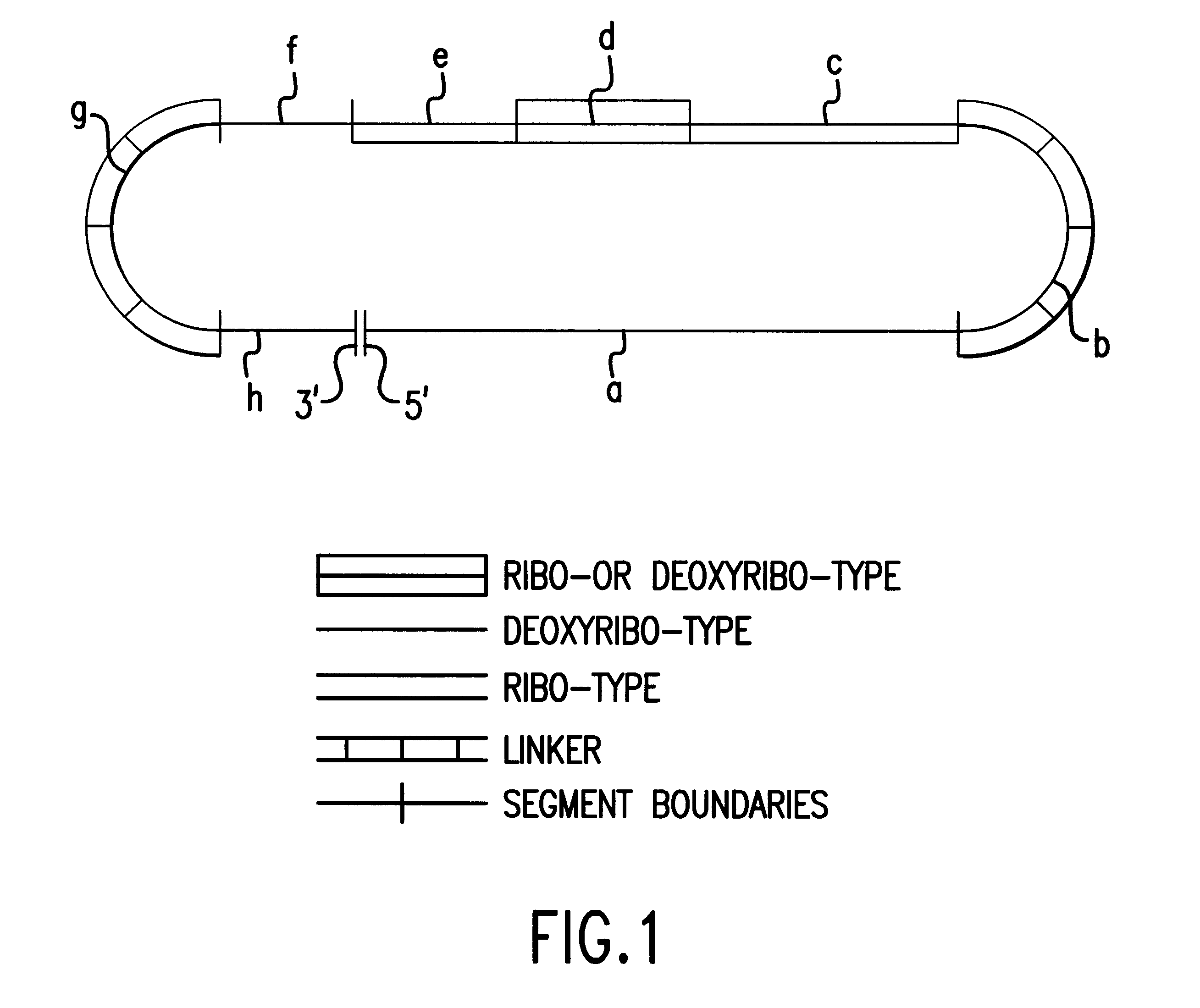
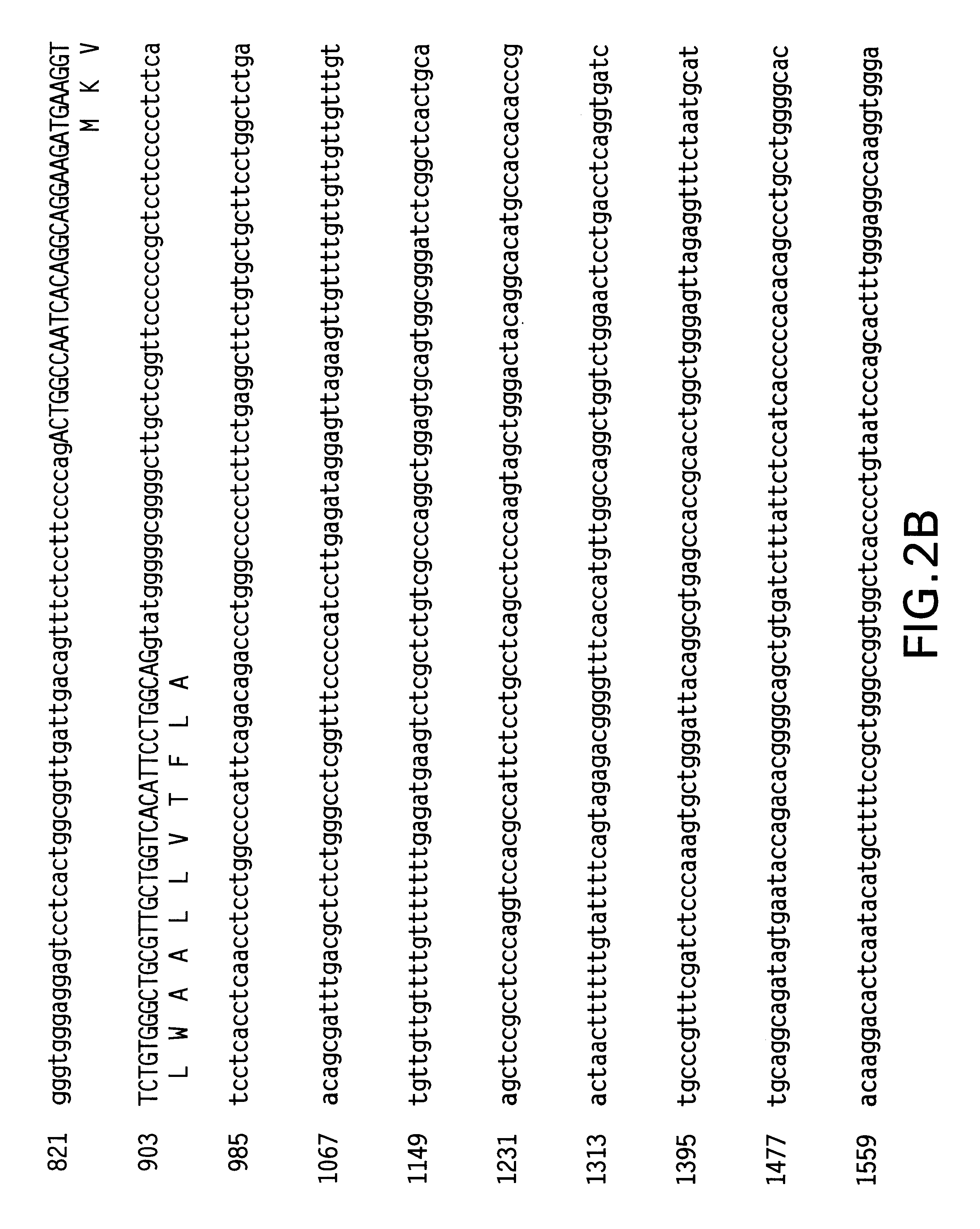
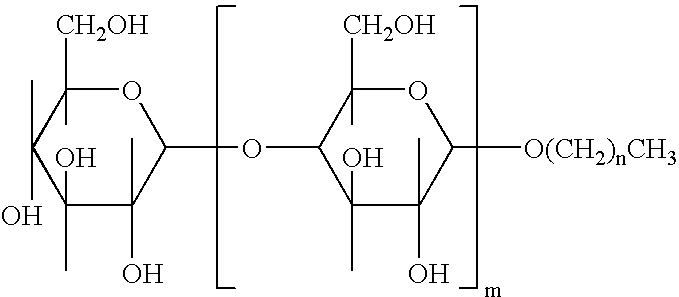
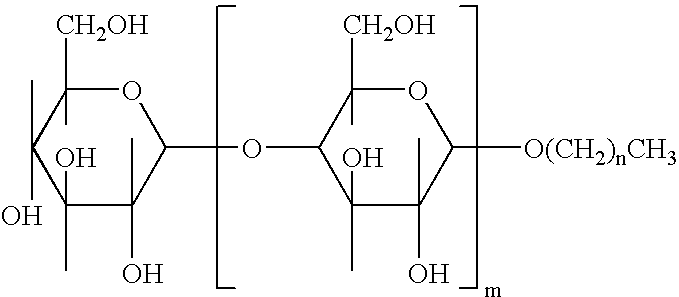
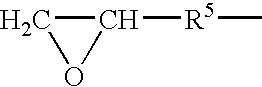Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
6612 results about "Lactose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lactose is a disaccharide. It is a sugar composed of galactose and glucose subunits and has the molecular formula C₁₂H₂₂O₁₁. Lactose makes up around 2–8% of milk (by weight). The name comes from lac (gen. lactis), the Latin word for milk, plus the suffix -ose used to name sugars. The compound is a white, water-soluble, non-hygroscopic solid with a mildly sweet taste. It is used in the food industry.
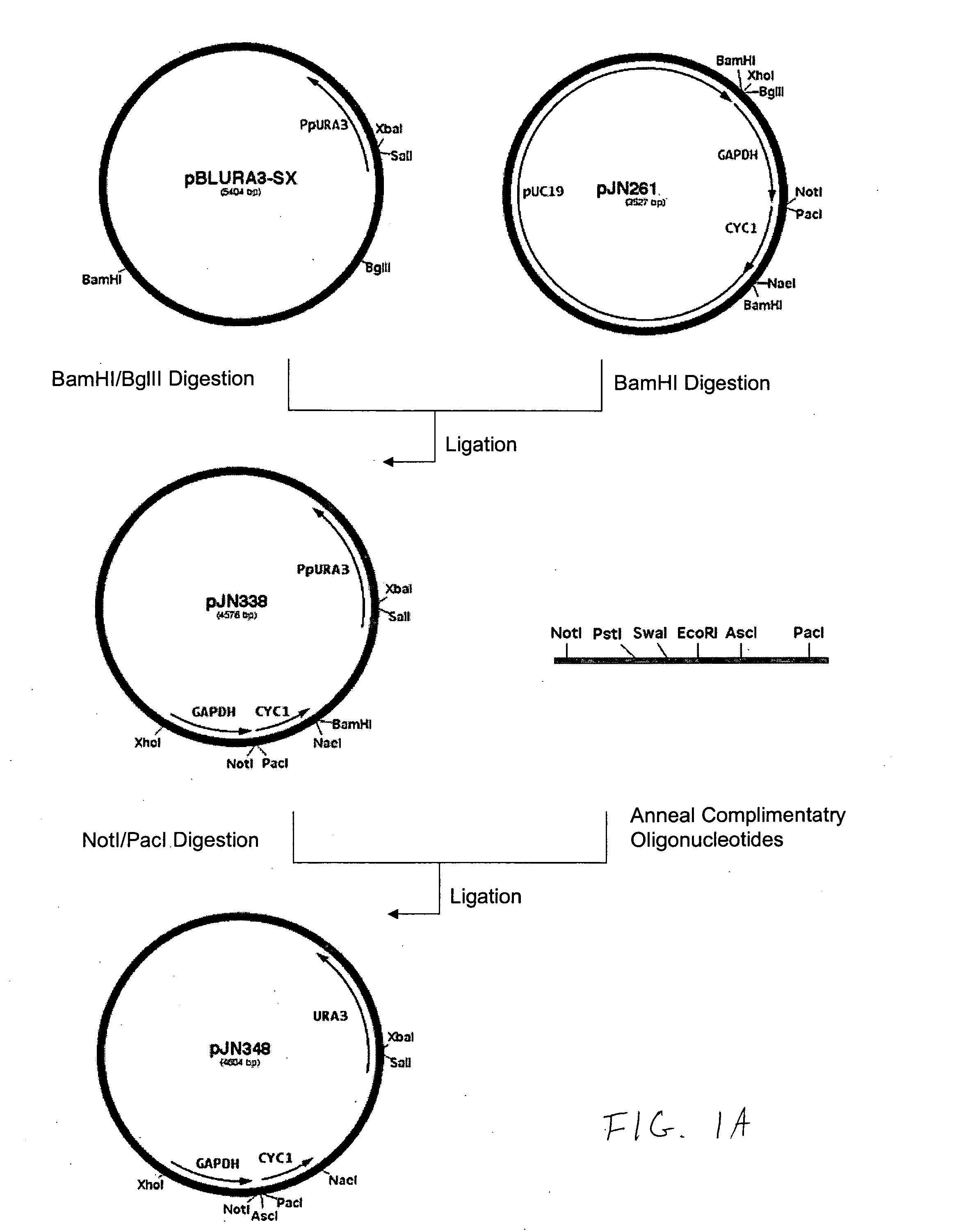
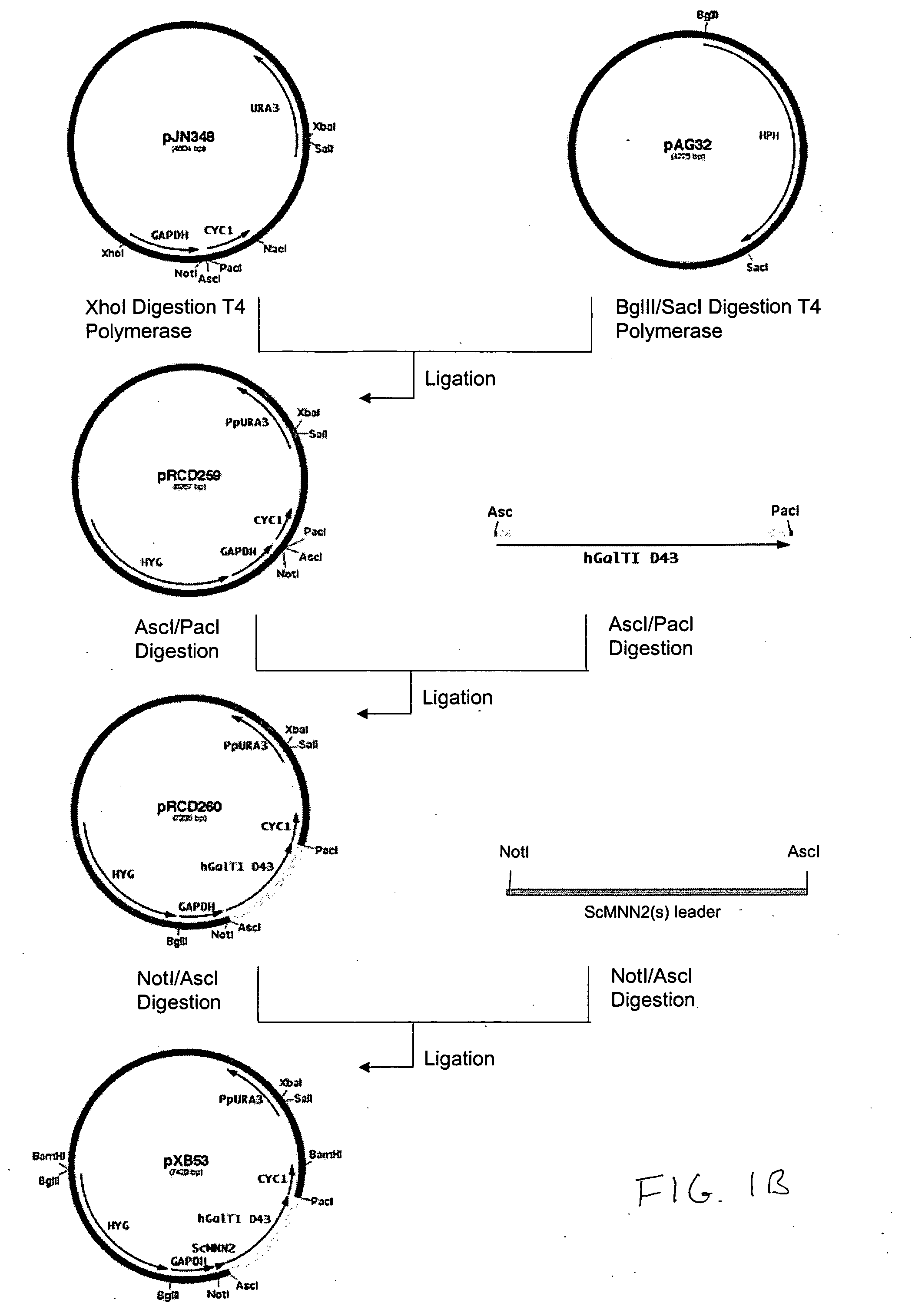
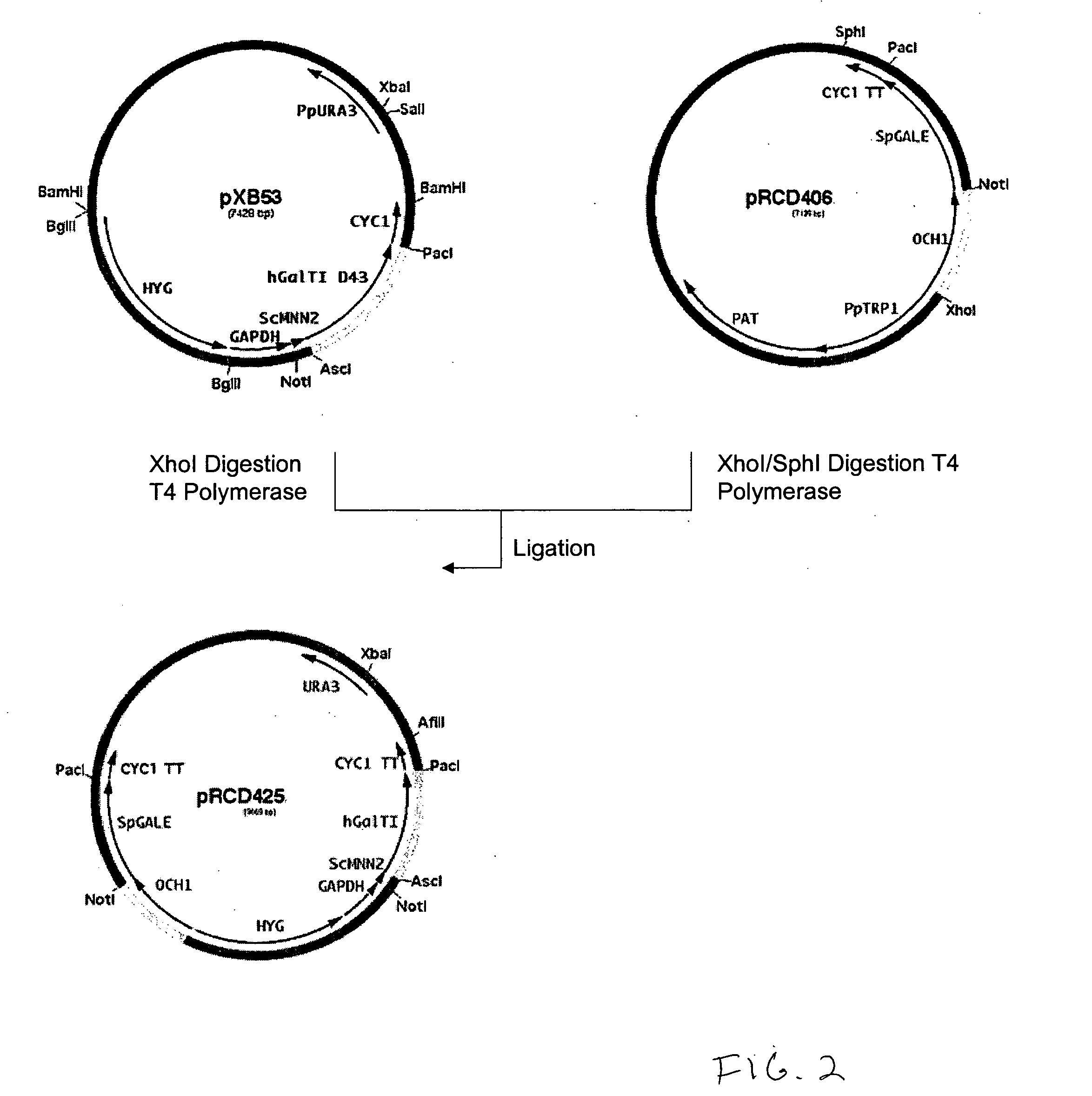
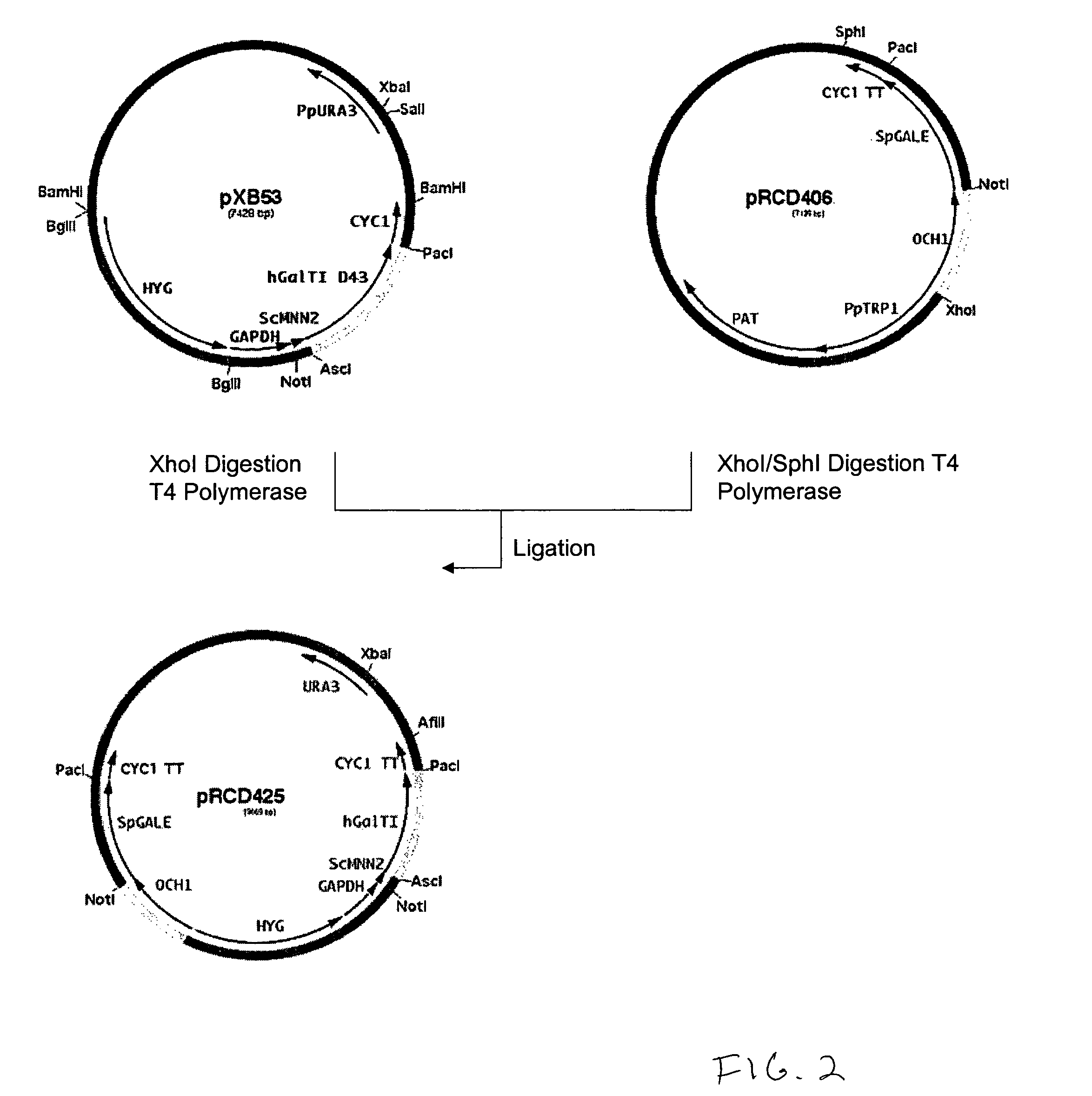
Production of galactosylated glycoproteins in lower eukaryotes
InactiveUS20060040353A1Reducing and eliminating activityEfficient workFungiSugar derivativesUDP galactoseSialic acid
The present invention provides a novel lower eukaryotic host cell producing human-like glycoproteins characterized as having a terminal β-galactose residue and essentially lacking fucose and sialic acid residues. The present invention also provides a method for catalyzing the transfer of a galactose residue from UDP-galactose onto an acceptor substrate in a recombinant lower eukaryotic host cell, which can be used as a therapeutic glycoprotein.
Owner:GLYCOFI
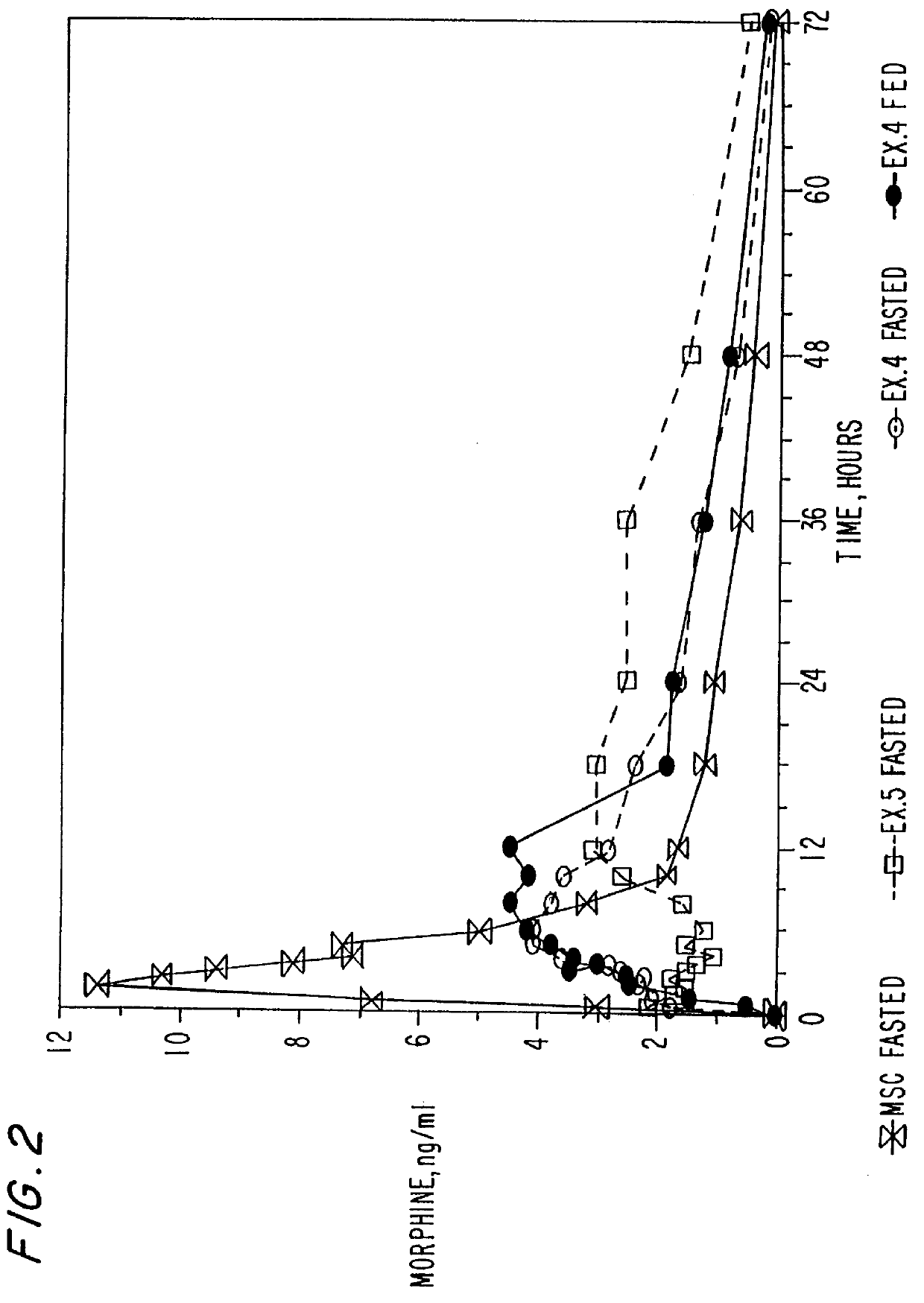
Powder-layered oral dosage forms
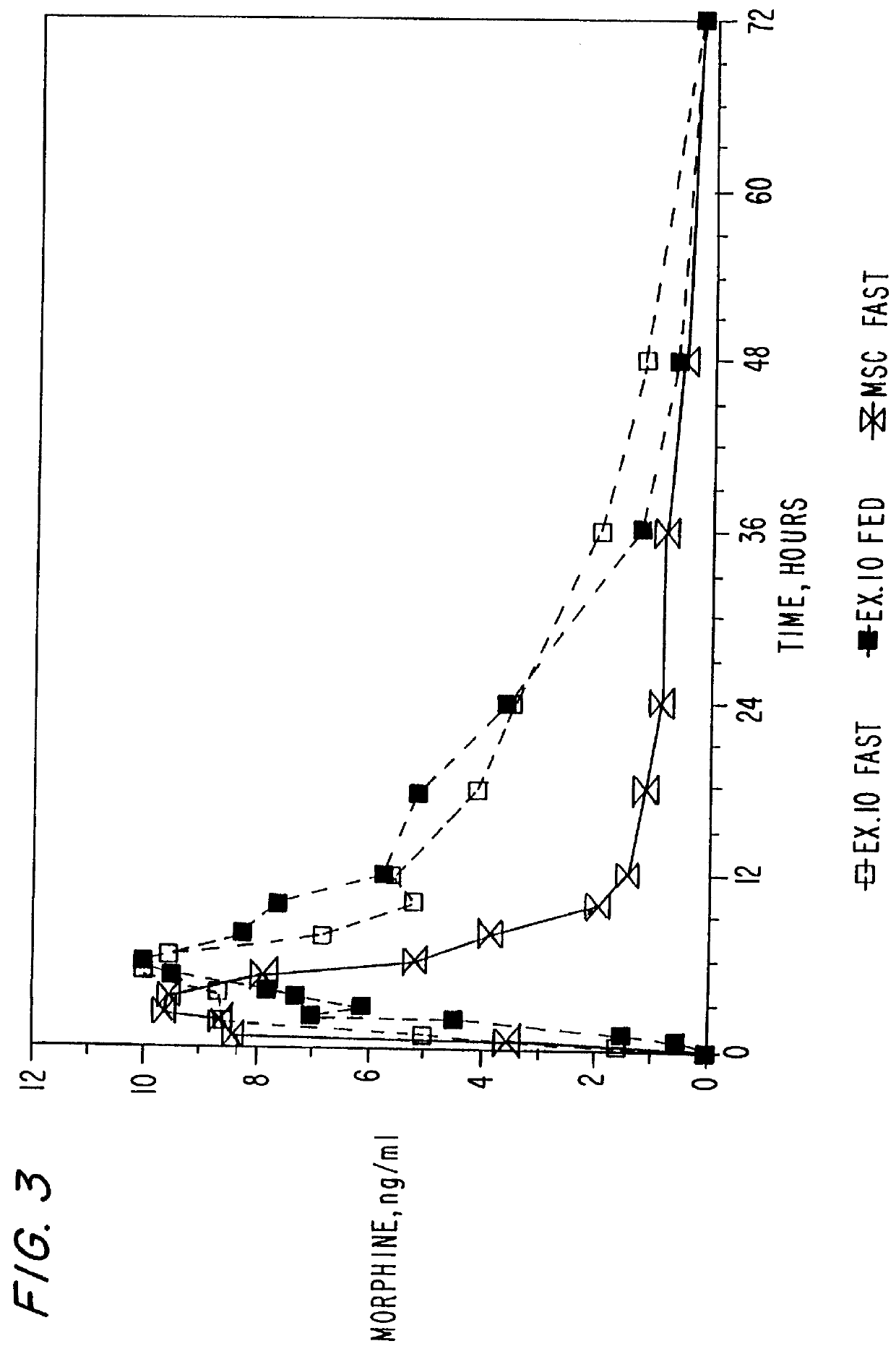
InactiveUS6077533AFacilitated releaseMinimize impactPretreated surfacesGranular deliveryImmediate releaseLactose
An oral dosage form of morphine is formulated by powder-layering an homogeneous mixture of morphine sulfate and hydrous lactose impalpable onto inert beads to obtain a multiparticulate product. A plurality of the powder-layered beads may be administered either in immediate release form or in an extended release form by coating with a hydrophobic material. In addition, multi-particulate oral dosage forms containing therapeutically effective agents containing a plurality of pharmaceutically acceptable inert beads powder-layered with homogeneous mixture of a therapeutically effective agent and hydrous lactose impalpable are also disclosed. A method of preparing the dosage forms as well as a method preparing spheroids containing the homogeneous mixture of therapeutically effective agent and hydrous lactose impalpable are also disclosed.
Owner:PURDUE PHARMA LP
Product quality enhancement in mammalian cell culture processes for protein production
ActiveUS7332303B2Low production costQuality improvementAnimal cellsCell receptors/surface-antigens/surface-determinantsHigh cellBiotechnology
Owner:BRISTOL MYERS SQUIBB CO
Liver-targeting agents and their synthesis
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
pH-triggered microparticles
InactiveUS20050123596A1Efficient deliveryGood biocompatibilityPowder deliveryMicroencapsulation basedPolyesterLipid formation
Microparticles that are designed to release their payload when exposed to acidic conditions are provided as a vehicle for drug delivery. Any therapeutic, diagnostic, or prophylatic agent may be encapsulated in a lipid-protein-sugar or polymeric matrix including a pH triggering agent to form pH triggerable microparticles. Preferably the diameter of the pH triggered microparticles ranges from 50 nm to 10 micrometers. The matrix of the particles may be prepared using any known lipid (e.g., DPPC), protein (e.g., albumin), or sugar (e.g., lactose). The matrix of the particles may also be prepared using any synthetic polymers such as polyesters. Methods of preparing and administering the particles are provided. Methods of immunization, transfection, and gene therapy are also provided by administering pH triggerable microparticles.
Owner:DANA FARBER CANCER INST INC +2
Anti liver disease drug R-YEEE and method of synthesizing branched galactose-terminal glycoproteins
The present invention provides a novel method of synthesizing branched galactose-terminal glycoproteins. A number of these glycoproteins have binding affinity to the asialoglycoprotein receptor. The present invention also provides novel conjugates having branched galactose-terminal glycoproteins that is complexed to a therapeutically effective agent, such as an isolated protein, polysacharides, lipids and radioactive isotope. These conjugates may be used to deliver the therapeutically effective agent to mammalian cells generally, and to hepatocytes specifically.
Owner:ACAD SINIC
Conjugates of galactose-binding lectins and clostridial neurotoxins as analgesics
InactiveUS7052702B1Pain reliefReduce and preferably prevent transmissionNervous disorderBacteriaGalactose binding lectinProtein translocation
A class of novel agents that are able to modify nociceptive afferent function is provided. The agents may inhibit the release of neurotransmitters from discrete populations of neurones and thereby reduce or preferably prevent the transmission of afferent pain signals from peripheral to central pain fibers. They comprise a galactose-binding lectin linked to a derivative of a clostridial neurotoxin. The derivative of the clostridial neurotoxin comprises the L-chain, or a fragment thereof, which includes the active proteolytic enzyme domain of the light (L) chain, linked to a molecule or domain with membrane translocating activity. The agents may be used in or as pharmaceuticals for the treatment of pain, particularly chronic pain.
Owner:HEALTH PROTECTION AGENCY +1
Prevention of opportunistic infections in immune-compromised subjects
ActiveUS20100260720A1Reduce the risk of infectionAvoid stickingAntibacterial agentsBiocideFucosylationImmune compromised
This invention relates to a composition suitable for use in the prevention of opportunistic infections in immune-compromised individuals comprising a probiotic Bifidobacterium lactis, Bifidobacterium infantis, Bifidobacterium breve or Bifidobacterium longum and a fucosylated oligosaccharide selected from the group comprising 2′-fucosyllactose, 3′fucosyllactose, difucosyllactose, lacto-N-fucopentaose, lacto-N-fucohexaose, fucosyllacto-N-hexaose and fucosyllacto-N-neohexaose. The invention further extends to the use of such a composition in the prevention of opportunistic infections in immune-compromised individuals.
Owner:SOC DES PROD NESTLE SA
Process aid for preparing a flowable slurry
A process for preparing a flowable slurry comprising mixing 25-70 wt. % water; an alkaline material selected from the group consisting of chlorosilicon manufacturing byproducts, direct process residue gels, cement kiln dust, and mixtures thereof; and a process aid selected from the group consisting of sucrose, raffinose, lignin, methylglucopyranoside, lactose, fructose, sodium polyphosphate, trehalose and mixtures thereof to form a flowable slurry. This slurry is especially useful in the manufacture of cement.
Owner:DOW CORNING CORP
Oral cavity disintegrating tablet and method of producing the same
ActiveUS20100278930A1Easy to produceDisintegrates quicklyBiocideOrganic active ingredientsSucroseOrally disintegrating tablet
The invention provides an orally disintegrating tablet containing (a) one or more saccharides or sugar alcohols selected from the group consisting of mannitol, lactose, xylitol, sucrose, erythritol and glucose and (b) low substituted hydroxypropylcellulose and substantially free of a starch disintegrant, which tablet is produced by steps of granulating a composition containing the above-mentioned components (a) and (b) by an agitation granulation method, and compression-molding the obtained granulation product. The invention also provides a method of producing an orally disintegrating tablet substantially free of a starch disintegrant, including steps of granulating a composition containing the above-mentioned components by an agitation granulation method, and compression-molding the obtained granulation product.
Owner:SAWAI PHARMA
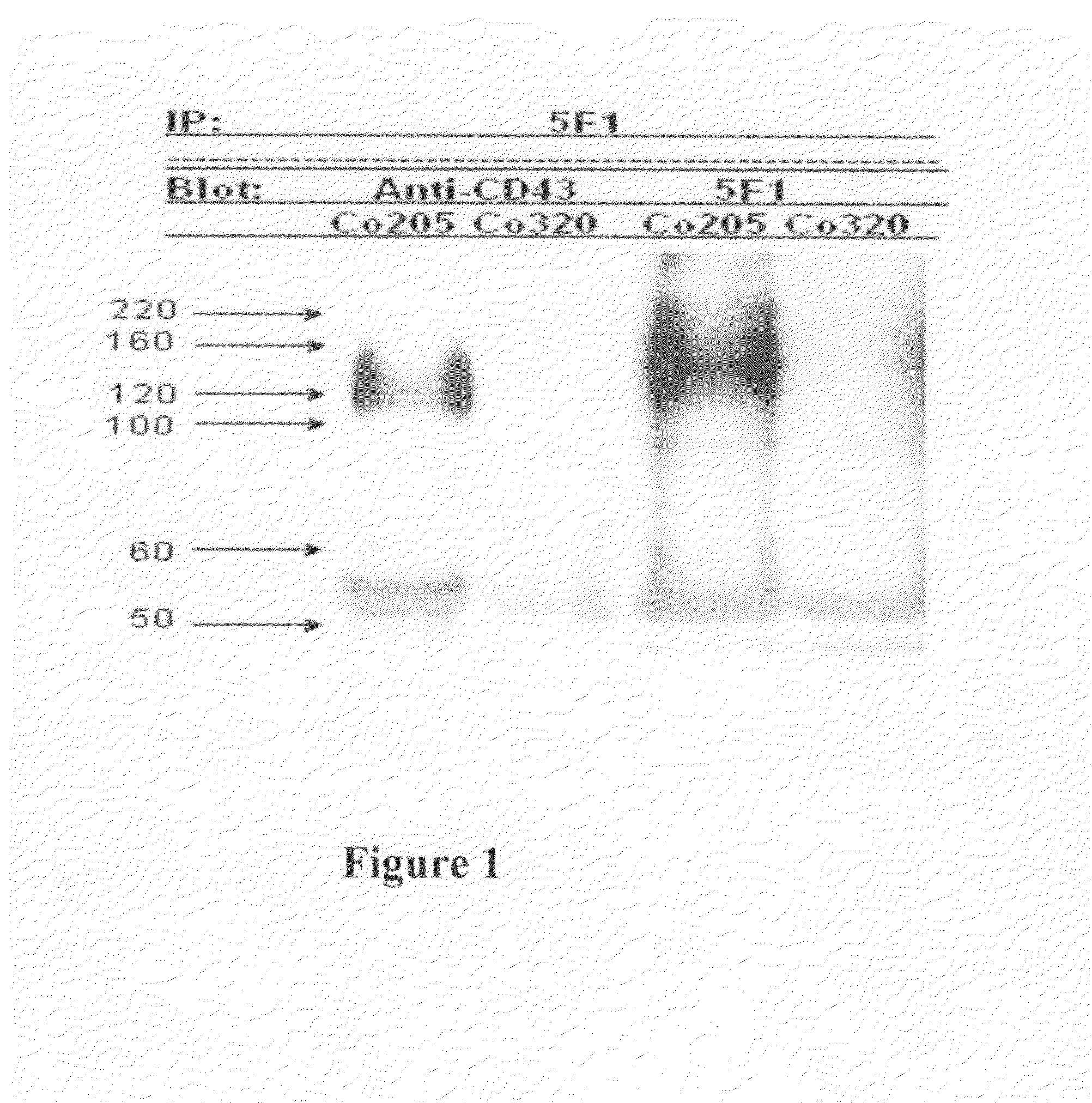
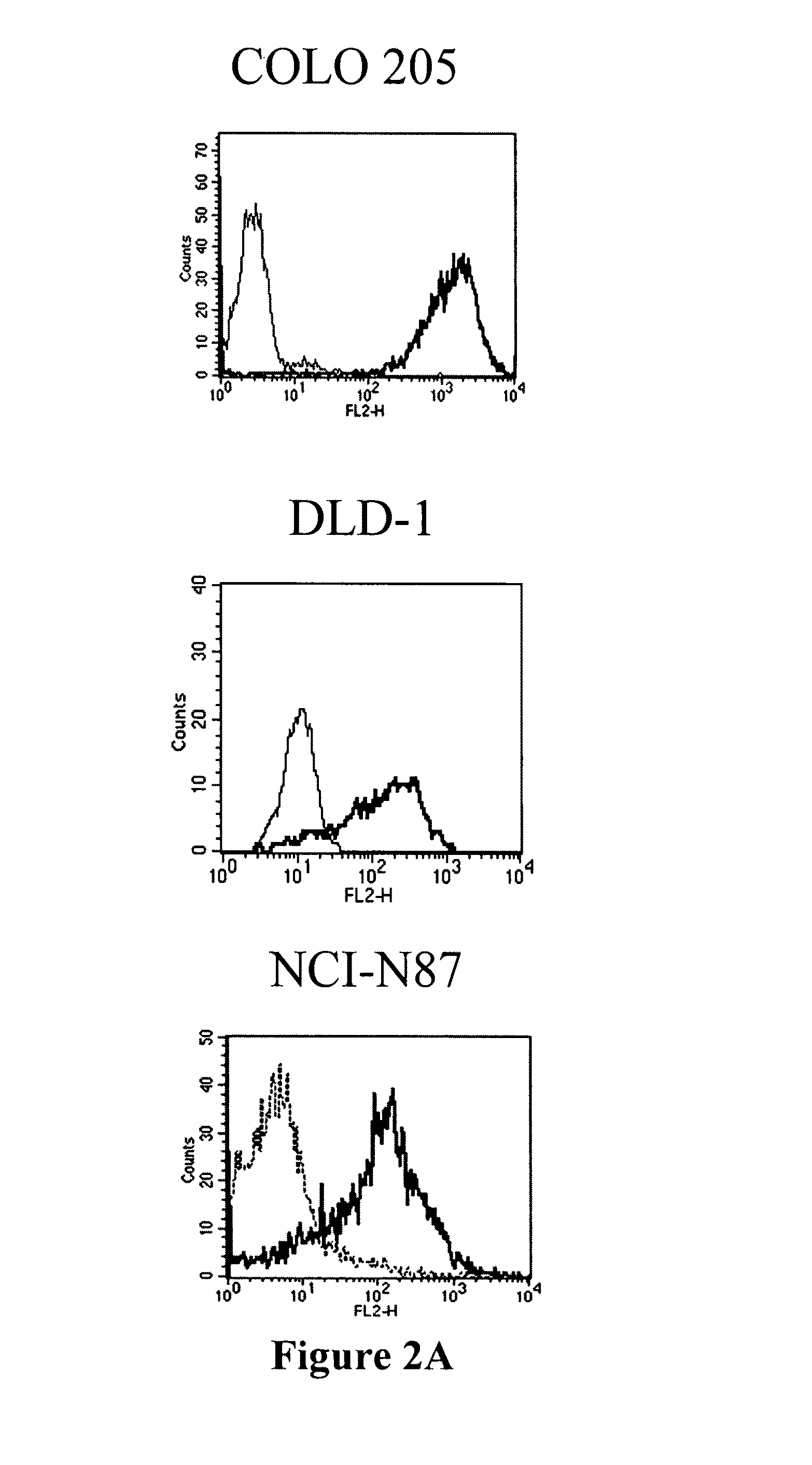
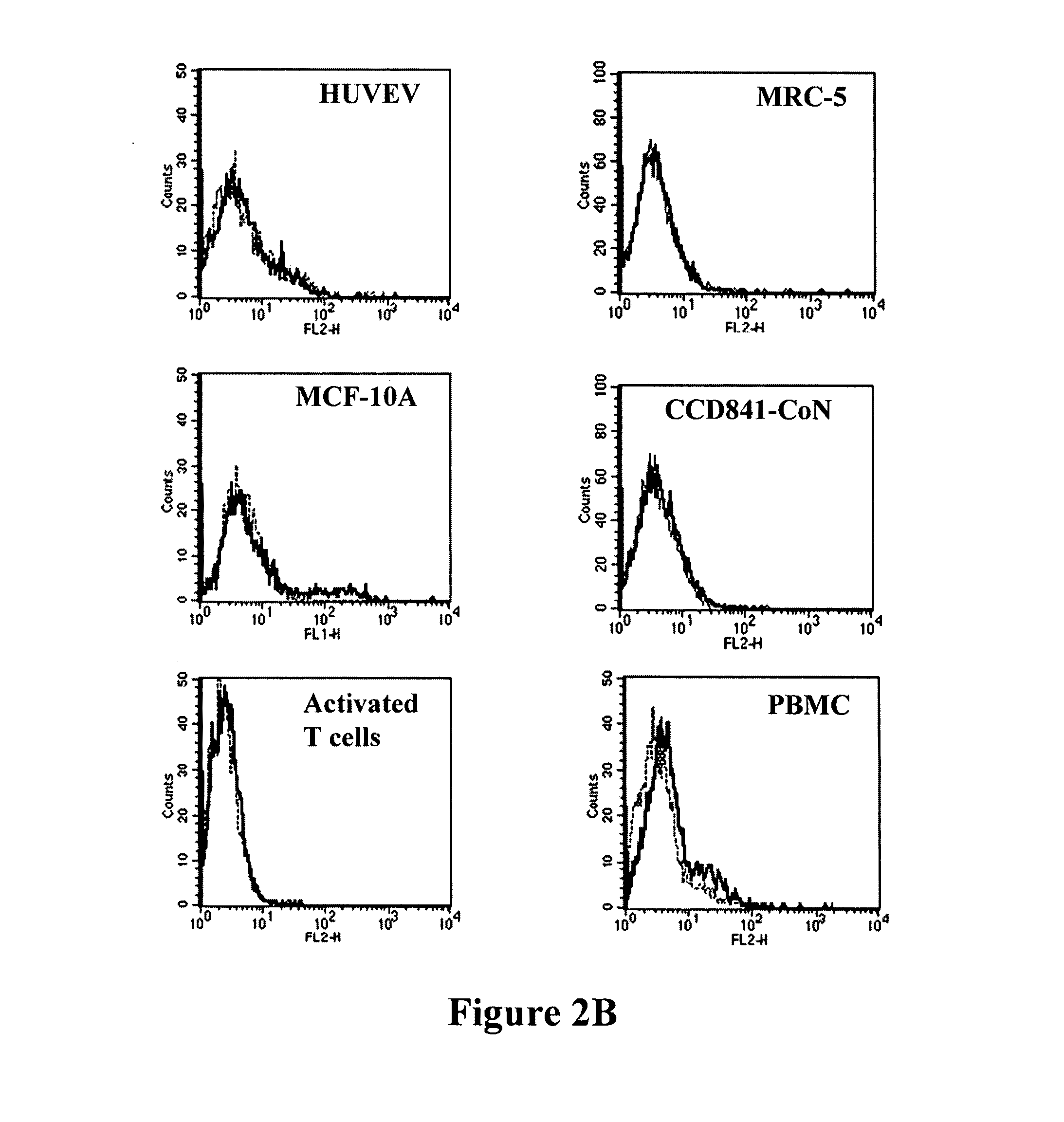
Antibodies recognizing a carbohydrate containing epitope on CD-43 and CEA expressed on cancer cells and methods using same
ActiveUS7674605B2Reduce in quantityInhibit growth and proliferationImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationHematopoietic cellCancer cell
The present invention provides novel antibodies specifically bind to an epitope on CD43 and CEA expressed on nonhematopoietic cancer cells, but do not specifically bind to a CD43 expressed by a leukocyte or by a Jurkat cell, and is capable of inducing apoptosis of the nonhematopoietic cancer cell after binding to the epitope on cell surface of the nonhematopoietic cancer cell in the absence of cytotoxin conjugation and immune effector function, wherein the epitope comprises a carbohydrate structure and the binding of the antibody to the epitope is inhibited by a carbohydrate comprising a Lea structure, a Lea-lactose structure, a LNDFH II structure, or a LNT structure. In addition, the present invention also provides use of the antibodies described herein for diagnostic and therapeutic purposes.
Owner:ALTRUBIO INC
Nucleotide Sugar Purification Using Membranes
InactiveUS20090048440A1Raise the ratioReduce concentrationSugar derivativesChemical recyclingNucleotidePhosphate
The invention provides methods of removing contaminants from a mixture of a desired product and contaminants by pH adjustments and molecular weight cut-offs. The contaminants include phosphate groups, magnesium sulfate, sodium pyruvate and tetrasodium pyrophosphate groups. The desired product includes nucleotide sugars, glycolipids, LnNT, sialyl lactose, and salts.
Owner:NOVO NORDISK AS
A kind of infant formula milk powder that does not get angry and its preparation process
ActiveCN102283289ANot ediblePromote digestion and absorptionMilk preparationVegetable oilFructooligosaccharide
The invention relates to an anti-inflaming infant formula milk powder and a preparation process thereof. The anti-inflaming infant formula milk powder comprises the following components in percentage by weight: 22%-50% of lactose, 10%-20% of goat milk whey protein concentrate, 9.8%-20% of structural grease 1,3-dioleoyl 2-palmitoyl triglyceride, 10%-13.5% of non-fat goat milk powder, 8%-10% of vegetable oil, 2%-8% of beta-casein, 0.8%-1.6% of fructooligosaccharides, 0.5%-1.5% of mineral premix, 0.3%-1% of galactooligosaccharide, 0.2%-1% of immunoglobulin G, 0.2%-0.5% of lactulose, 0.10%-0.45% of arachidonic acid, 0.1%-0.6% of docosahexaenoic acid, 0.06%-0.12% of vitamin premix, 0.04%-0.06% of lactoferrin, and 0.01%-0.06% of nucleotide. The invention also includes the preparation process ofthe infant formula milk powder. The nutrition constituents and the functions of the infant formula milk powder are close to those of breast milk, the infant formula milk powder is easy to assimilate,and infants do not get inflamed after eating the milk powder.
Owner:AUSNUTRIA DAIRY CHINA
Shampoo containing a gel network and a non-guar galactomannan polymer derivative
Shampoo compositions comprise (a) from about 5% to about 50% of one or more detersive surfactants; (b) a dispersed gel network phase comprising: (i) at least about 0.05% of one or more fatty amphiphiles; (ii) at least about 0.01% of one or more secondary surfactants; and (iii) water; (c) at least about 0.05% of a galactomannan polymer derivative with a net positive charge and having a mannose to galactose ratio of greater than 2:1 on a monomer to monomer basis, wherein the galactomannan polymer derivative has: (i) a molecular weight from about 1,000 to about 10,000,000; and (ii) a cationic charge density from about 0.7 meq / g to about 7 meq / g; and (d) at least about 20% of an aqueous carrier; all by weight of the shampoo composition.
Owner:THE PROCTER & GAMBLE COMPANY
Product quality enhancement in mammalian cell culture processes for protein production
ActiveUS20050084933A1Increased and enhanced sialic acid contentLow production costAnimal cellsCell receptors/surface-antigens/surface-determinantsBiotechnologyHigh cell
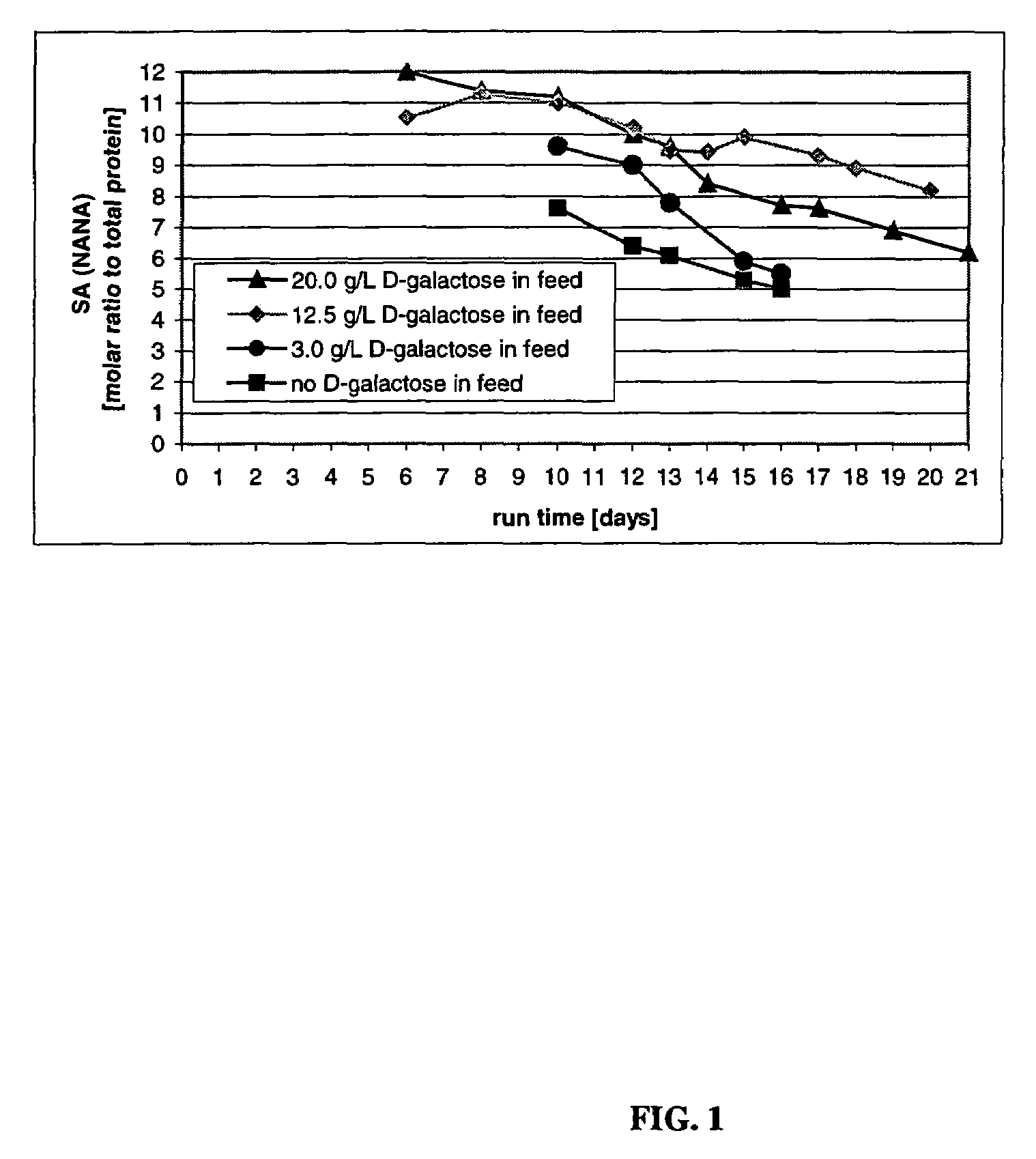
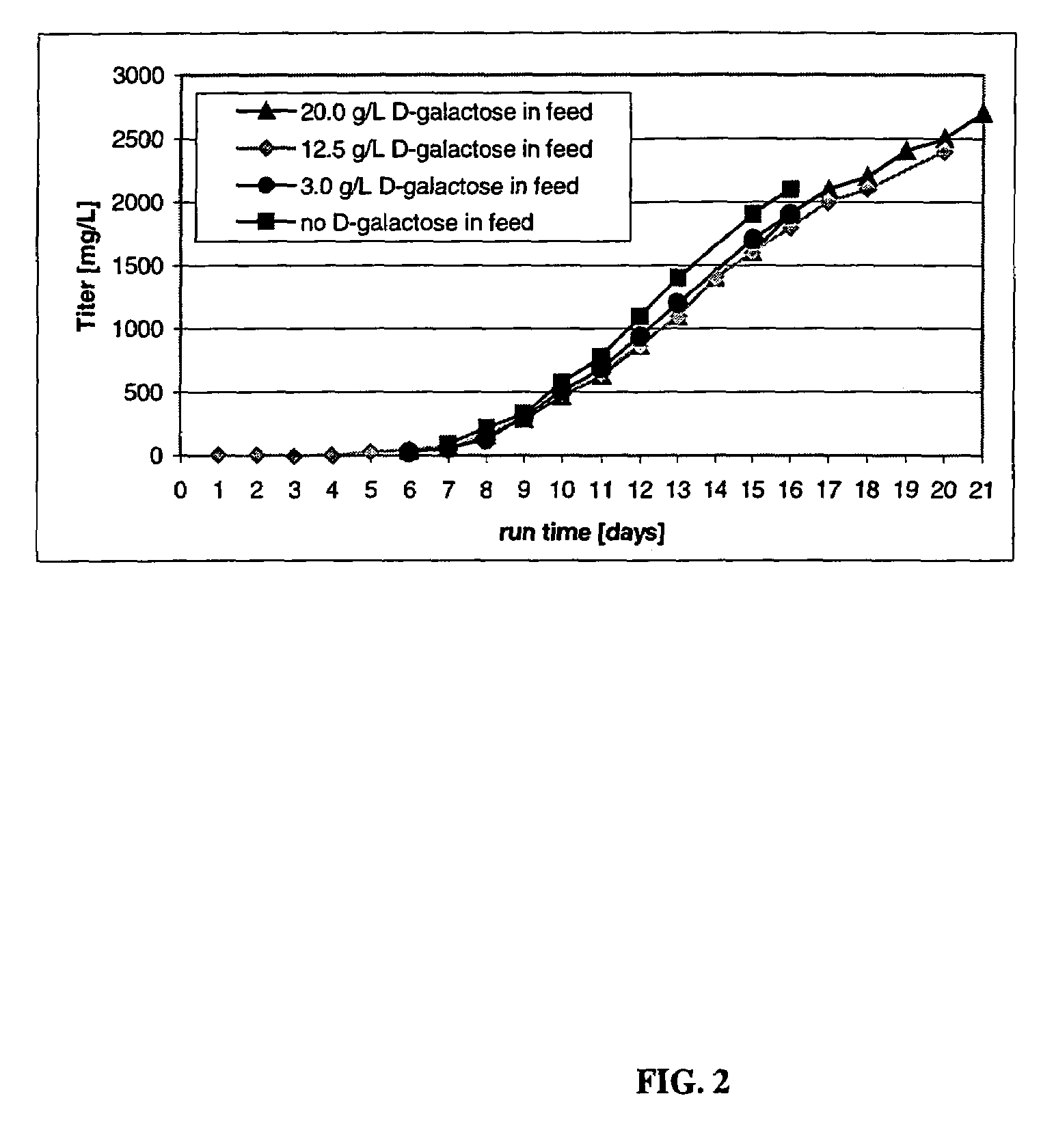
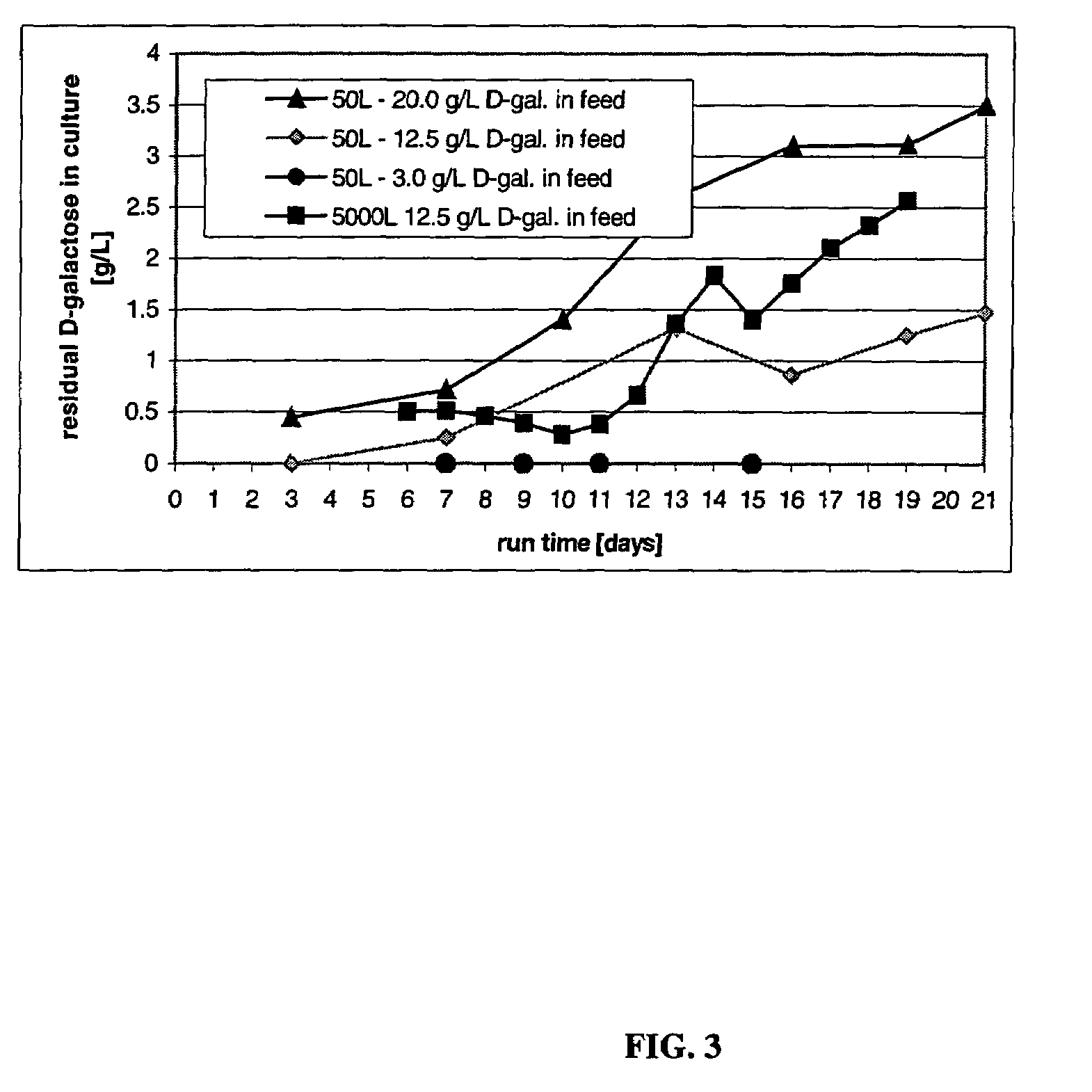
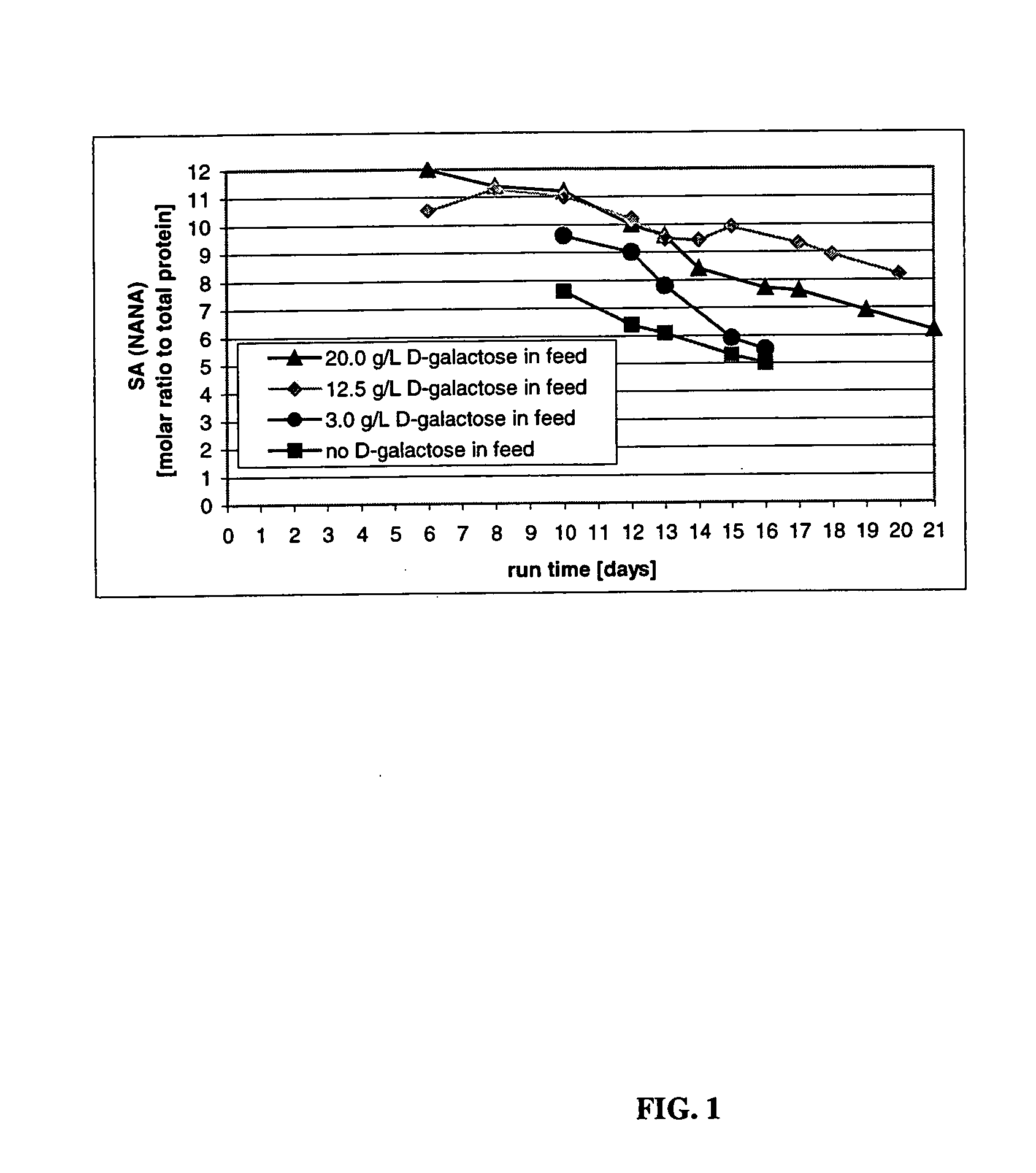
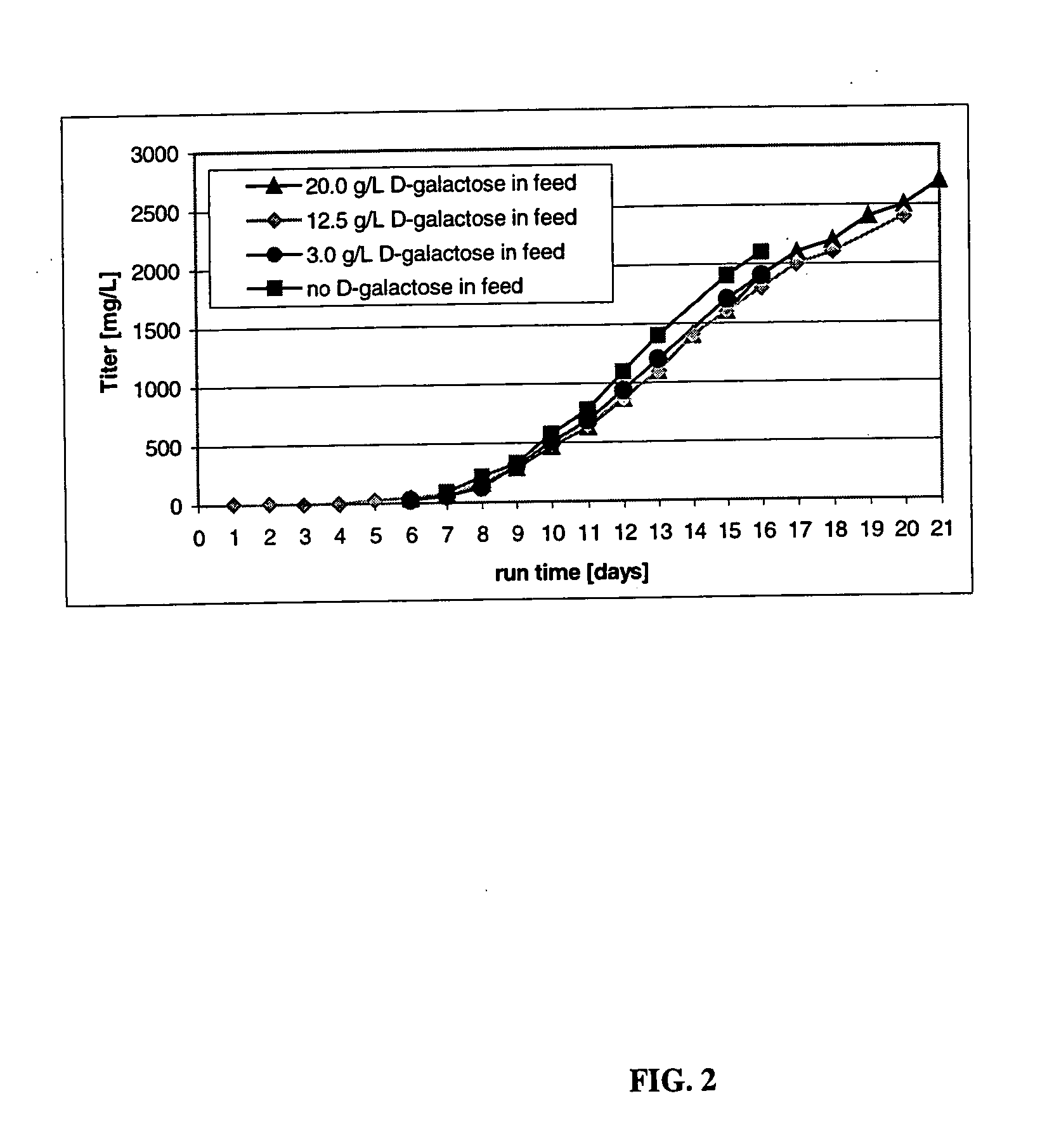
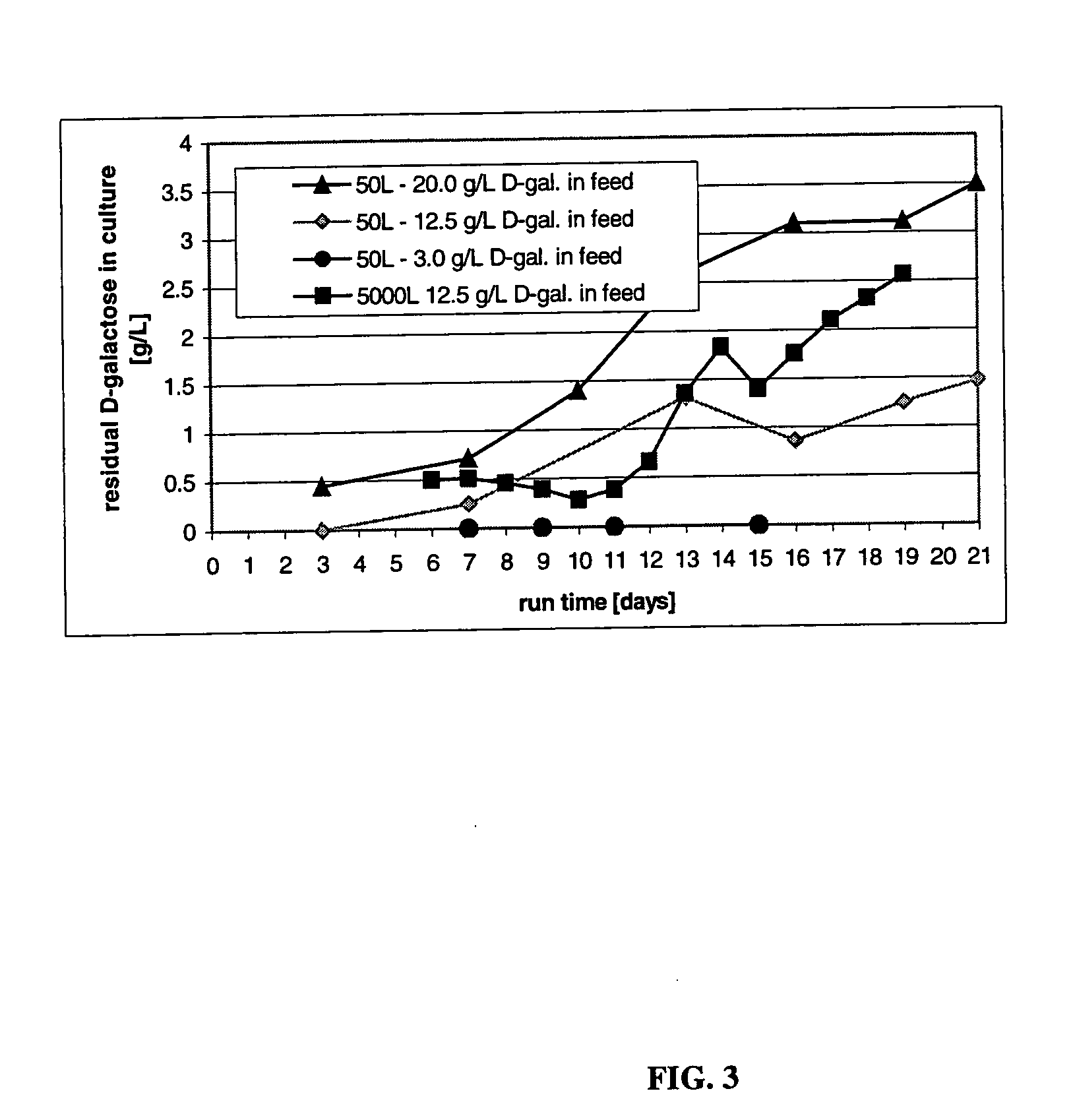
The present invention describes methods and processes for the production of proteins, particularly glycoproteins, by animal cell or mammalian cell culture, illustratively, but not limited to, fed-batch cell cultures. The methods comprise feeding the cells with D-galactose, preferably with feed medium containing D-galactose, preferably daily, to sustain a sialylation effective level of D-galactose in the culture for its duration, thus increasing sialylation of the produced proteins. The methods can also comprise at least two temperature shifts performed during the culturing period, in which the temperature is lower at the end of the culturing period than at the time of initial cell culture. The cell culture processes of the invention involving two or more temperature shifts sustain a high cell viability, and can allow for an extended protein production phase. The methods can also comprise the delayed addition of polyanionic compound at a time after innoculation. Supplementation of the cultures with D-galactose, preferably in a feed medium, to sustain galactose at sialylation effective levels in the cultures until the end of a culture run reverses a decline in sialylation that accompanies culture scale up, and is advantageous for large scale culturing processes.
Owner:BRISTOL MYERS SQUIBB CO
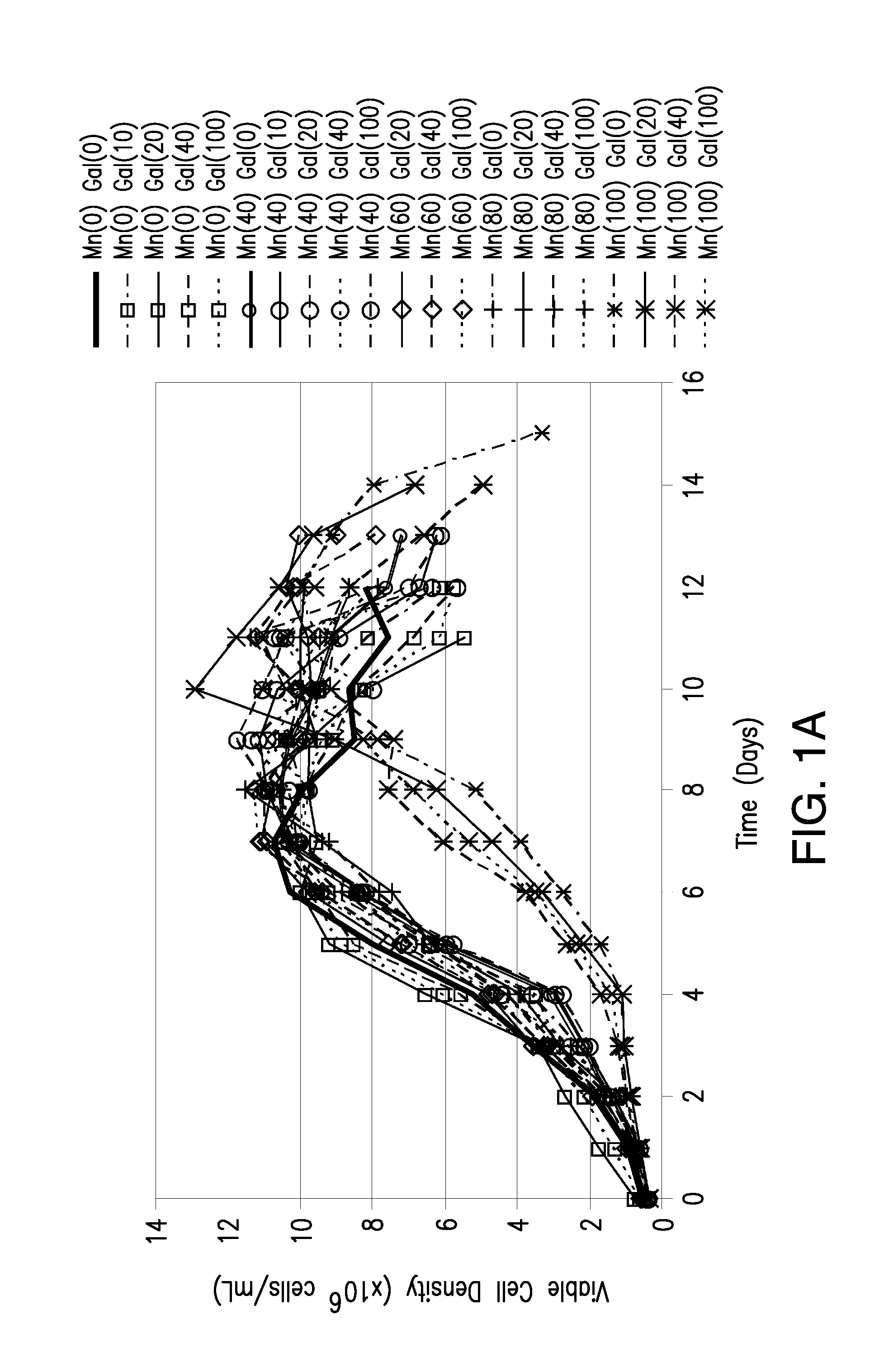
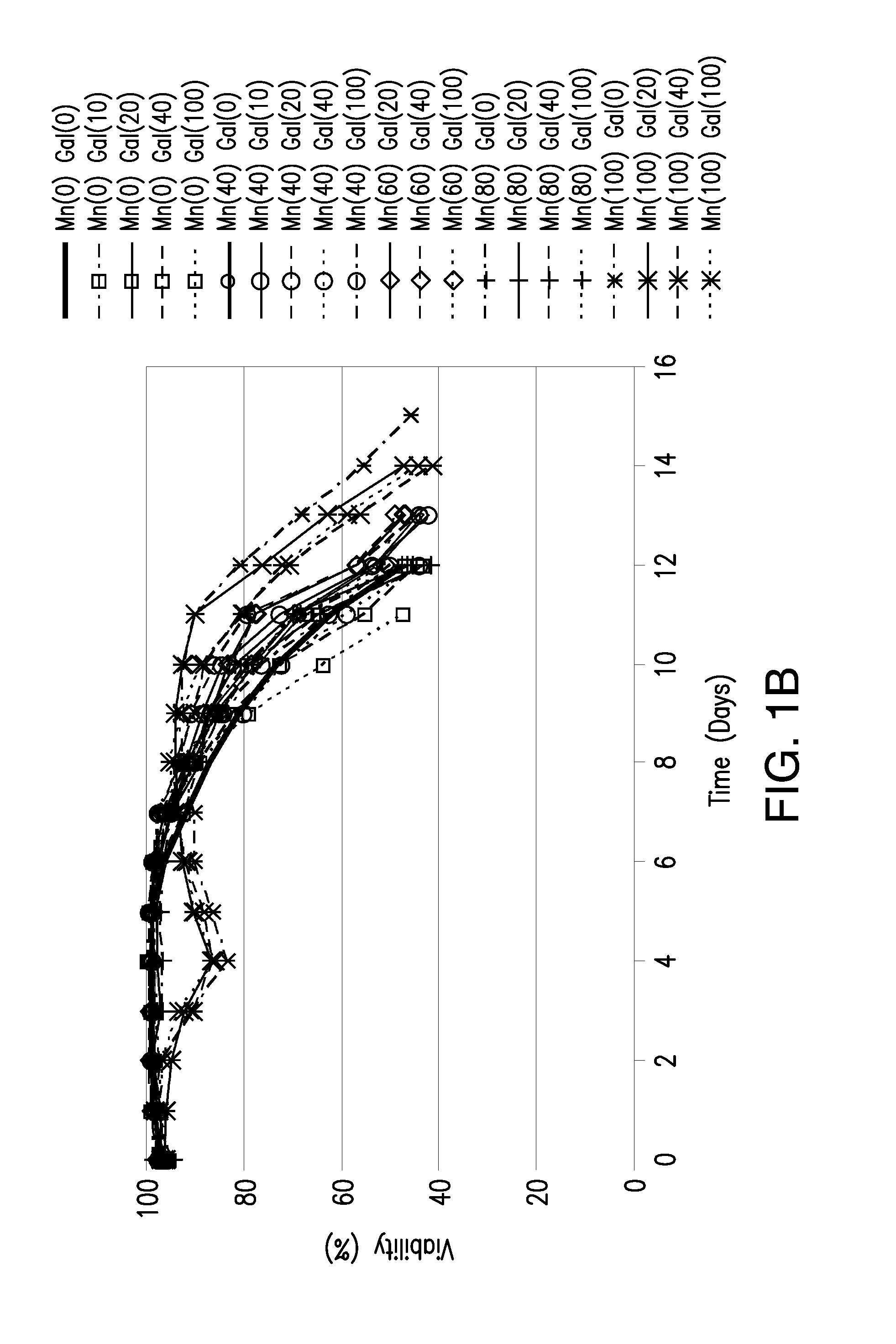
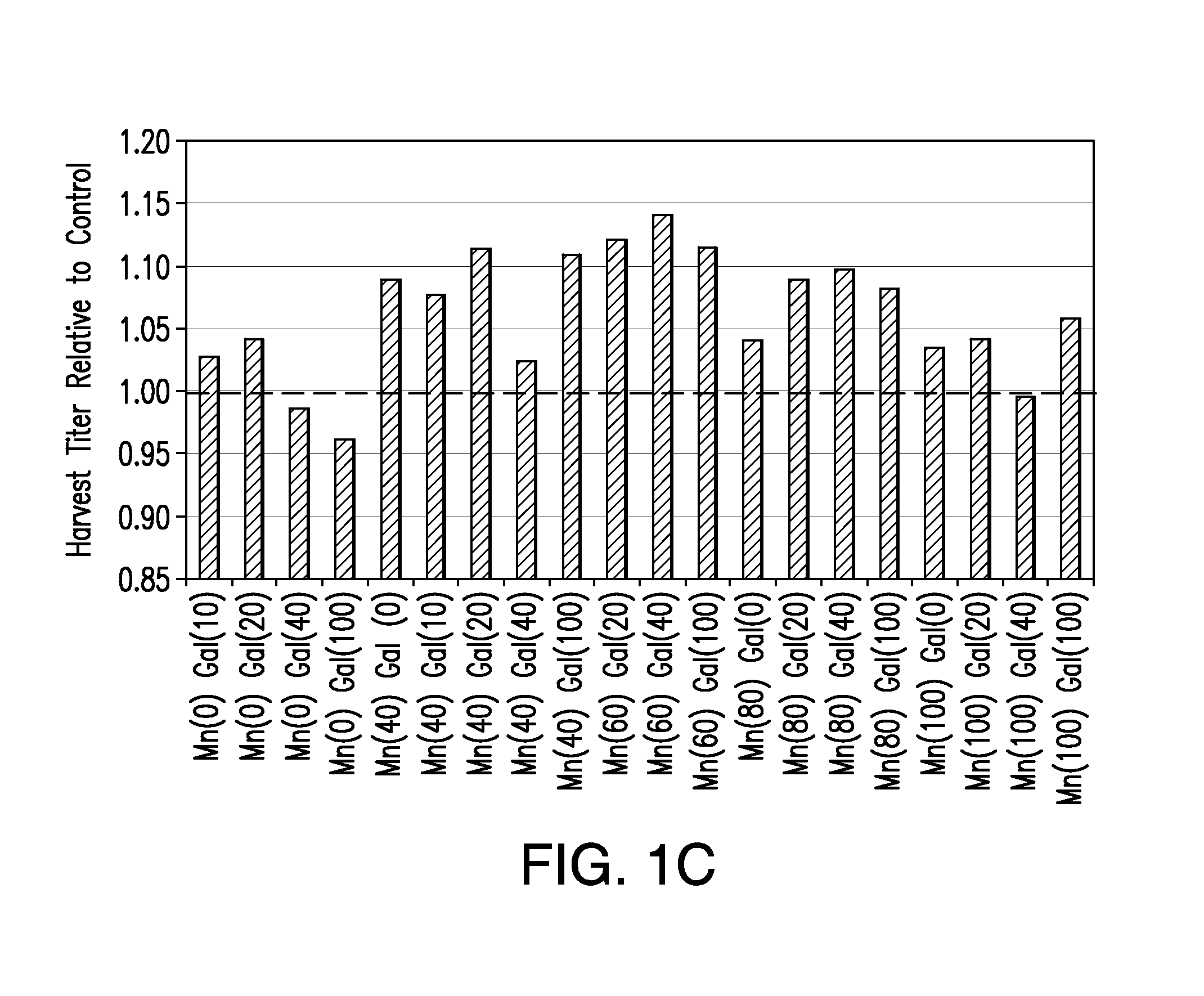
Methods for controlling the galactosylation profile of recombinantly-expressed proteins
The present invention relates to methods for modulating the glycosylation profile of recombinantly-expressed proteins. In particular, the present invention relates to methods of controlling the galactosylation profile of recombinantly-expressed proteins by supplementing production medium, e.g., a hydrolysate-based or a chemically defined medium, with manganese and / or D-galactose.
Owner:ABBVIE INC
Formulation of powder containing nanoparticles for aerosol delivery to the lungs
Respirable particles carrying active principles or diagnostics in nanoparticle form are created by mixing the nanoparticles with liquid carrier, then forming the resultant mixture into respirable particles. Spray-drying, freeze spray drying and drying followed by comminution may be used to create the respirable particles, which may be delivered to the lung via a dry powder inhaler. In one example, lactose was used as the excipient and spray-dried with two different types of nanoparticle: gelatin and poly butylcyanoacrylate nanoparticles. The incorporation of nanoparticles did not affect the respirable fraction of the carrier powders.
Owner:FINLAY WARREN HUGH +2
Conjugates of galactose-binding lectins and clostridial neurotoxins as analgesics
InactiveUS7452543B2Pain reliefReduce and preferably prevent transmissionNervous disorderBacteriaGalactose binding lectinProtein translocation
A class of novel agents that are able to modify nociceptive afferent function is provided. The agents may inhibit the release of neurotransmitters from discrete populations of neurones and thereby reduce or preferably prevent the transmission of afferent pain signals from peripheral to central pain fibers. They comprise a galactose-binding lectin linked to a derivative of a clostridial neurotoxin. The derivative of the clostridial neurotoxin comprises the L-chain, or a fragment thereof, which includes the active proteolytic enzyme domain of the light (L) chain, linked to a molecule or domain with membrane translocating activity. The agents may be used in or as pharmaceuticals for the treatment of pain, particular chronic pain.
Owner:HEALTH PROTECTION AGENCY
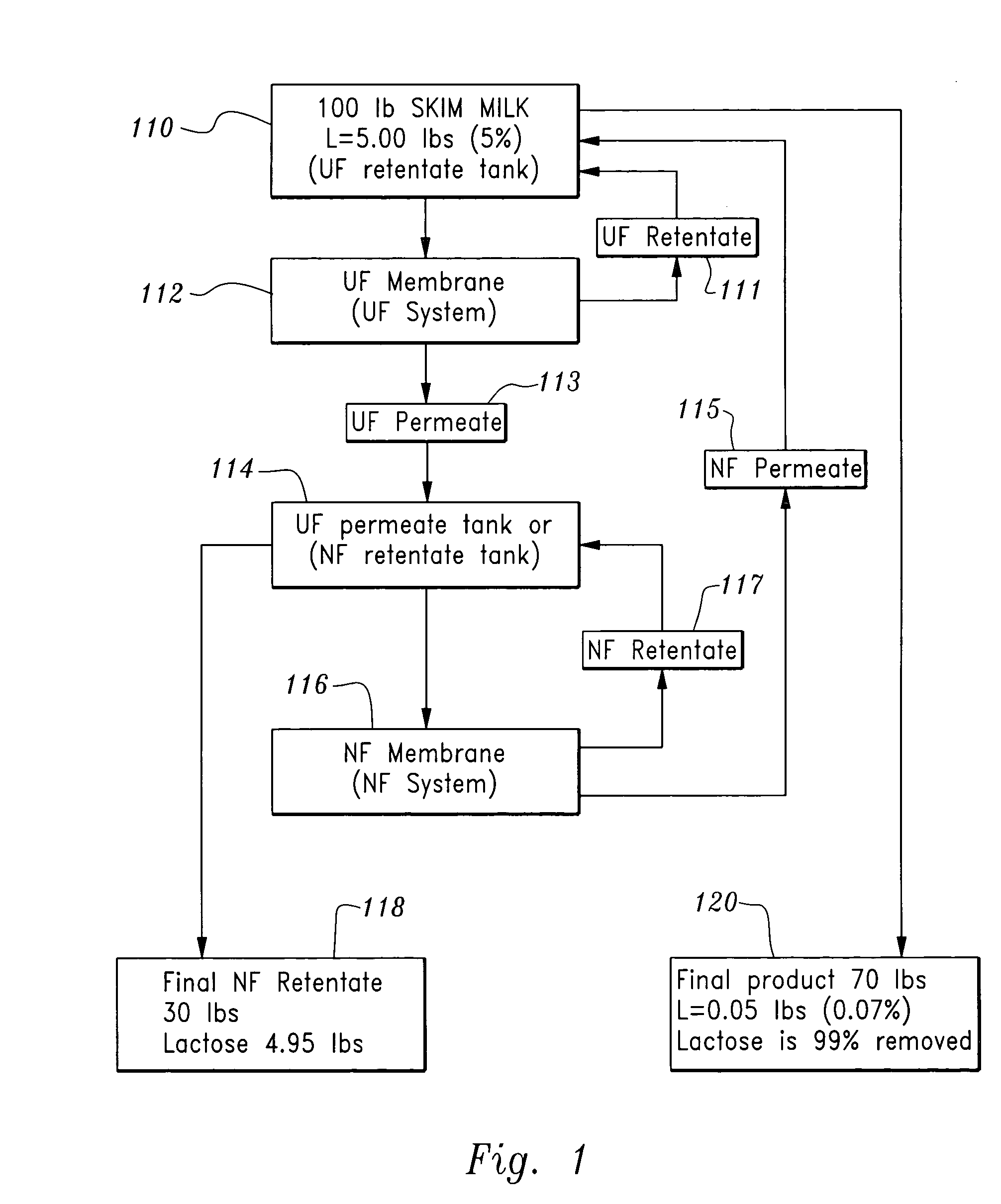
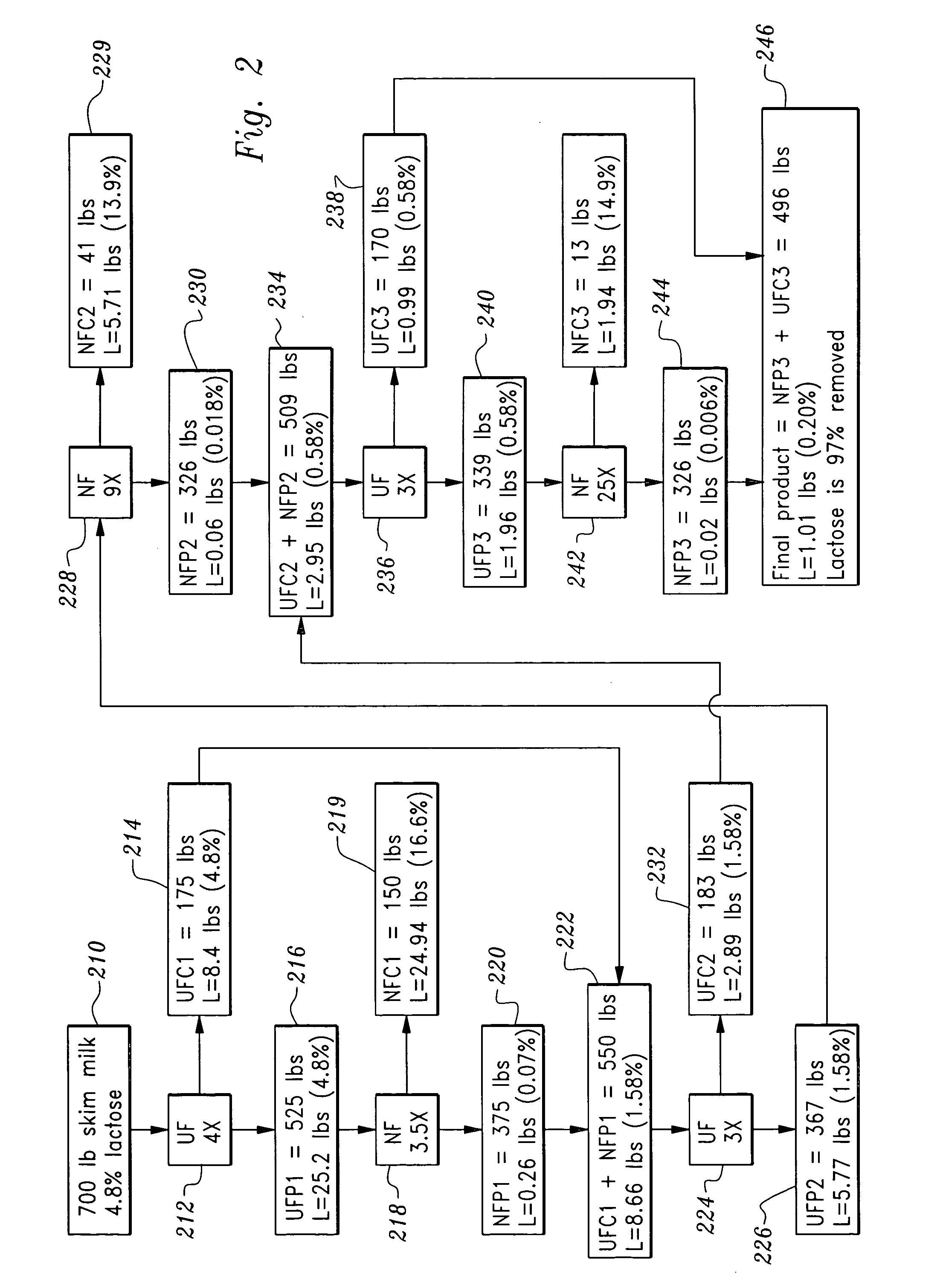
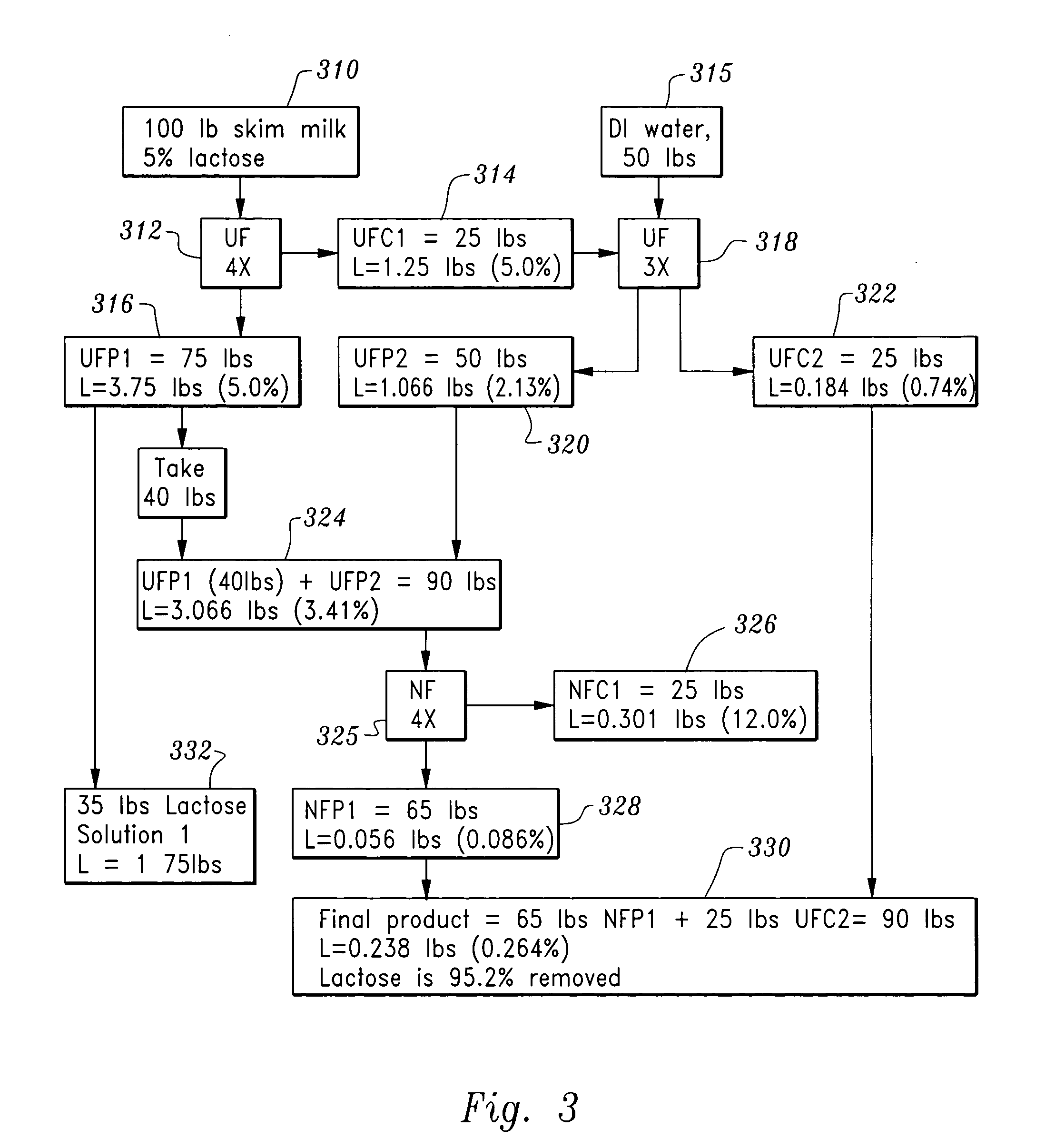
Lactose-removed milk product and process for the preparation thereof
InactiveUS20050196508A1Large separationAdd flavorMilk preparationOther dairy technologyFiltrationLactose
A sequential filtration process has been developed to reduce the content of carbohydrate, such as lactose, in milk feed stocks such as whole, low fat, skim milk, and milk powder. The milk product produced with the inventive process can be classified as a lactose-removed milk product.
Owner:WANG JOSEPH
Formula milk powder for promoting absorption of fatty acid and calcium and preparation method thereof
The invention discloses a formula milk powder for promoting the absorption of fatty acid and calcium and a preparation method thereof. Raw cow milk, lactose, 1,3-Dioleoyl 2-palmitoyl triglyceride and demineralized whey powder as main materials are added with concentrated whey albumen powder, Alpha-lactalbumin powder, oligosaccharide, walnut oil, casein phosphopeptide, docosahexaenoic acid, arachidonic acid, nucleotide, lutein, inositol, carnitine and the like as well as vitamins, mineral substances and other nutrients needed for strengthening infants, and fat humanization, protein humanization and carbohydrate humanization are realized. The powdery product is produced by the processes of blending, homogenization, concentration, spray-drying, packaging and the like. According to the physiological characteristics and nutritional demand of the infants, the invention reinforces the calcium, the 1,3-Dioleoyl 2-palmitoyl triglyceride, other nutrient ingredients and the like, and aiming at the oversea clinical test conclusion of the 1,3-Dioleoyl 2-palmitoyl triglyceride, the final test conclusion of comparison with breast milk and infant formula milk powder sold on the market in the process of a clinical feeding test is that the feeding result of the designed formula approximates the feeding result of the breast milk and is better than the feeding result of an infant formula milk powder group sold on the market.
Owner:HEILONGJIANG FEIHE DAIRY
Hepatocellular chimeraplasty
InactiveUS6524613B1Decrease in levelReduce riskOrganic active ingredientsBiocideGenetic ChangeFhit gene
The present invention concerns compositions and methods for the introduction of specific genetic changes in endogenous genes of the cells of an animal. The genetic changes are effected by oligonucleotides or oligonucleotide derivatives and analogs, which are generally less than about 100 nucleotides in length. The invention provides for macromolecular carriers, optionally incorporating ligands for clathrin coated pit receptors. In one embodiment the ligand is a lactose or galactose and the genetic changes are made in hepatocytes. By means of the invention up to 40% of the copies of a target gene have been changed in vitro. Repair of mutant genes having a Crigler-Najjar like phenotype and Hemophilia B phenotype were observed.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV +1
Low lactose, low moisture shelf-stable, bakeable savory cheese product and process for preparing it
InactiveUS20020155198A1Good cheese flavorSuitable cheeseBaking mixturesConfectionerySmall dropletAdditive ingredient
A savory, smooth-textured, bakeable and shelf-stable product is prepared as a three-phase formulation, including an aqueous liquid phase, a dispersed fat phase and a solids phase, preferably containing cheese in significant proportion. The liquid phase is present in sufficient quantity to suspend and disperse the fat and solids phases. The dispersed fat must have sufficiently small droplet size raise the viscosity for this phase sufficiently to result in a creamy texture for the final product. The savory flavor ingredients are present as undissolved solids of sufficiently small particle size to provide the proper flavor release for the flavor and a texture consistent with the savory flavor. Preferred cheese products will have a lubricous, slippery, smooth mouthfeel and a flavor release that endures until the palate is essentially clean. The product can be applied to unbaked doughs prior to baking and retain their desired properties after baking. The product can also be packaged for use as is with any number of complimentary foods.
Owner:INTERCONTINENTAL GREAT BRANDS LLC
Purified galactomannan as an improved pharmaceutical excipient
InactiveUS6063402AExcellent hardness propertiesOrganic active ingredientsBiocideProtein materialsImpurity
Disclosed is a substantially anhydrous, powdered, galactomannan composition consisting essentially of a galactomannan hydrocolloid exhibiting about 50% to about 90% by weight of anhydromannose residues and about 10% to about 50% by weight anhydrogalactose residues; less than about 1% by weight of protein material and less than about 3% of other nonaqueous impurities. This material is useful for preparing pharmaceutical compositions both in the substantially anhydrous form but preferably in an anhydrated form which includes about 5-15% by weight water. The pharmaceutical compositions comprise a therapeutically effective amount of a drug, the hydrated powered gallactomannan composition and optionally other pharmaceutically-acceptable excipients. When the hydrated powdered purified glactomannan of the invention is used to form a tablet, one sees improved hardness in the tablet formed. The pharmaceutical composition of the invention is particularly valuable for delivering a therapeutically effective drug to the colon without significant release of the drug in the upper GI tract after oral administration of the composition. Unique means to prepare the purified galactomannan in large quantities is provided.
Owner:AMARIN DEV
Low-lactose partially hydrolyzed infant formula
ActiveUS20060286252A1Lesser priming effectGreat tasteMilk preparationVitamin food ingredientsWhey proteinLactose
The present invention relates to a low-lactose, partially hydrolyzed infant formula. The carbohydrate component of the infant formula comprises between 0% and 60% lactose and the protein component of the infant formula comprises partially hydrolyzed whey protein and casein, the protein component having a particular molecular weight.
Owner:MEAD JOHNSON NUTRITION
Nutritional Composition Comprising Indigestible Oligosaccharides
ActiveUS20080124323A1Reduce generationRelieve pressureOrganic active ingredientsBiocideDiseaseLactose
The present invention provides a method and composition for the treatment and / or prevention of respiratory tract infection and / or respiratory tract infection disease, said method comprising orally administering a composition to a mammal, said composition comprising a galactose containing indigestible oligosaccharide and at least 5 wt. % digestible galactose saccharide.
Owner:NV NUTRICIA
Fucosyllactose as breast milk identical non-digestible oligosaccharide with new functional benefit
InactiveUS20120177691A1Simple structureIncrease NK cell activityBiocideOrganic active ingredientsNutritional compositionPhysiology
Owner:NUTRICIA
Probiotic microcapsules as well as preparation method and application thereof
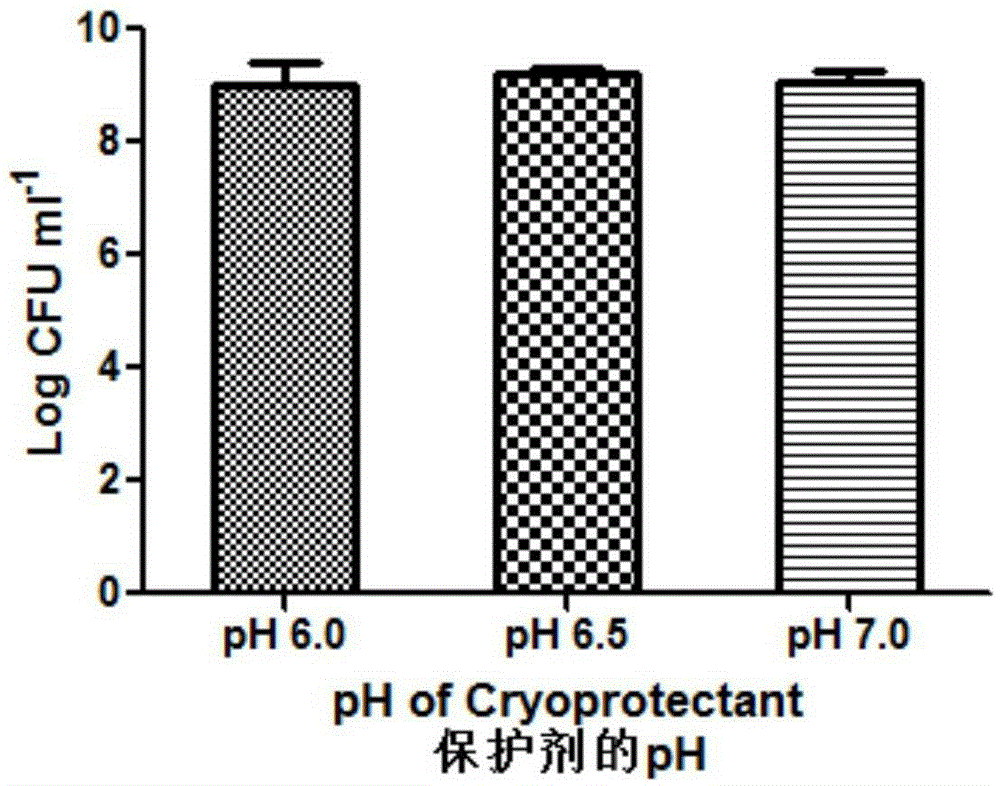
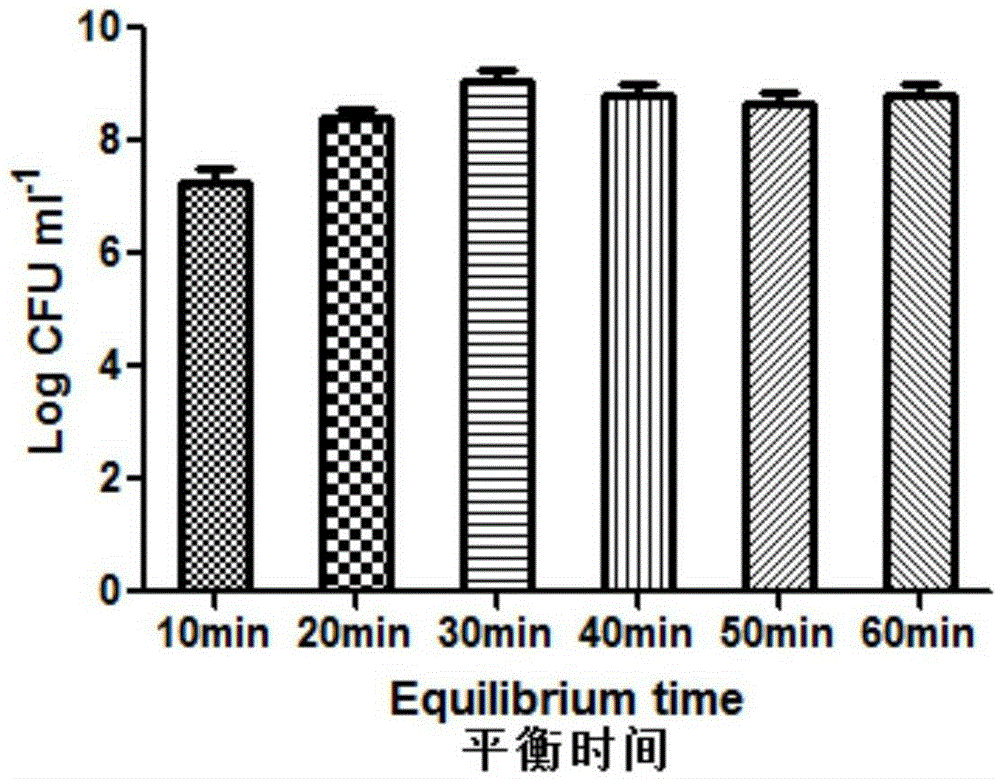
ActiveCN105310080AImprove the situation of low freeze-drying survival rateImprove stabilityFood freezingFood shapingFreeze-dryingK carrageenan
The invention relates to probiotic microcapsules as well as a preparation method and application thereof. The probiotic microcapsules comprise a core material and a wall material, wherein the core material is probiotics; the outer layer of the wall material is coated with chitosan; the wall material is prepared from an aqueous solution containing a natural polymer material and a freeze-drying protection agent; the freeze-drying protection agent comprises one or more of glucose, fructose, sucrose, lactose, trehalose, soluble starch, glycerin, mannitol, Arabic gum, dextran 40 and skim milk; the natural polymer material comprises one or more of gellan gum, xanthan gum, k-carrageenan, sodium alginate, cellulose acetate phthalate or gelatin; in the aqueous solution, the volume fraction of the freeze-drying protection agent is 4.0%-20.0% and the volume fraction of the polymer material is 0.5%-5.0%. The probiotic microcapsules can keep excellent acid resistance and storage stability before and after being freeze-dried.
Owner:SUN YAT SEN UNIV
Production of galactosylated glycoproteins in lower eukaryotes
InactiveUS7795002B2Reducing and eliminating activityEfficient workSugar derivativesPeptide/protein ingredientsUDP galactoseSialic acid
The present invention provides a novel lower eukaryotic host cell producing human-like glycoproteins characterized as having a terminal β-galactose residue and essentially lacking fucose and sialic acid residues. The present invention also provides a method for catalyzing the transfer of a galactose residue from UDP-galactose onto an acceptor substrate in a recombinant lower eukaryotic host cell, which can be used as a therapeutic glycoprotein.
Owner:GLYCOFI
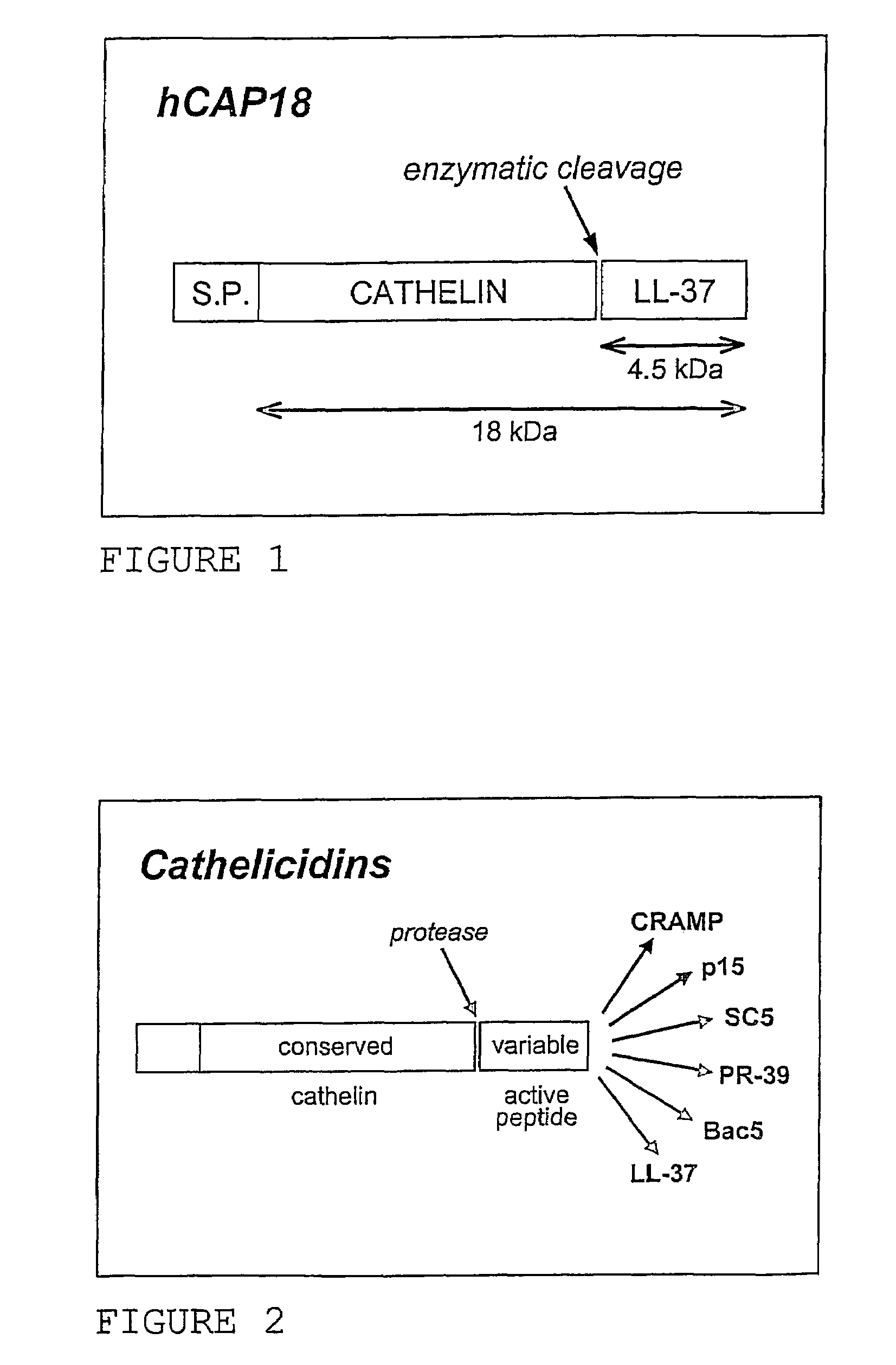
Use of the cathelicidin LL-37 and derivatives thereof for wound healing
ActiveUS7452864B2Promote regenerationWound be enhancedAntibacterial agentsPeptide/protein ingredientsCytotoxicityCell culture media
Use of the antimicrobial cathelicidin peptide ll-37, N-terminal fragments of LL-37 or extended sequences of LL-37 having 1-3 amino acids in the C-terminal end, for stimulating proliferation of epithalial and stromal cells and thereby healing of wounds, such as chronic ulcers. The cytotoxic effect of LL-37 may be reduced by including a bilayer-forming polar lipid, especially a digalactosyldiacylglycerol, in pharmaceutical compositions and growth media comprising LL-37.
Owner:PROMORE PHARMA AB
Alkylpolyglucosides containing disinfectant compositions active against pseudomonas microorganism
An antiseptic cleansing composition comprising an antimicrobial agent, an effective amount of an alkylpolysaccharide surfactant, at least one alkyl alcohol and at least one aryl alcohol. Suitable surfactant alkylpolysaccharides may contain one or more sugar units selected from the group consisting of maltose, arabinose, xylose, mannose, galactose, gulose, idose, talose, allose, altrose, sucrose, fructose, sorbose, levulose, lactose, allulose, tagatose, alloheptulose, sedoheptulose, glucoheptulose, mannoheptulose, guloheptulose, idoheptulose, galactoheptulose, taloheptulose and derivatives thereof. Suitable antimicrobial agents include chlorhexidine, chlorhexidine salt, chlorophenol derivative, octenidindihydrochloride (CH3—(CH2)7—NHON—(CH2)10—NO—NH(CH2)7—CH2 or any other salt thereof, and quaternary ammonium compounds.
Owner:NOVAPHARM RES AUSTRALIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com