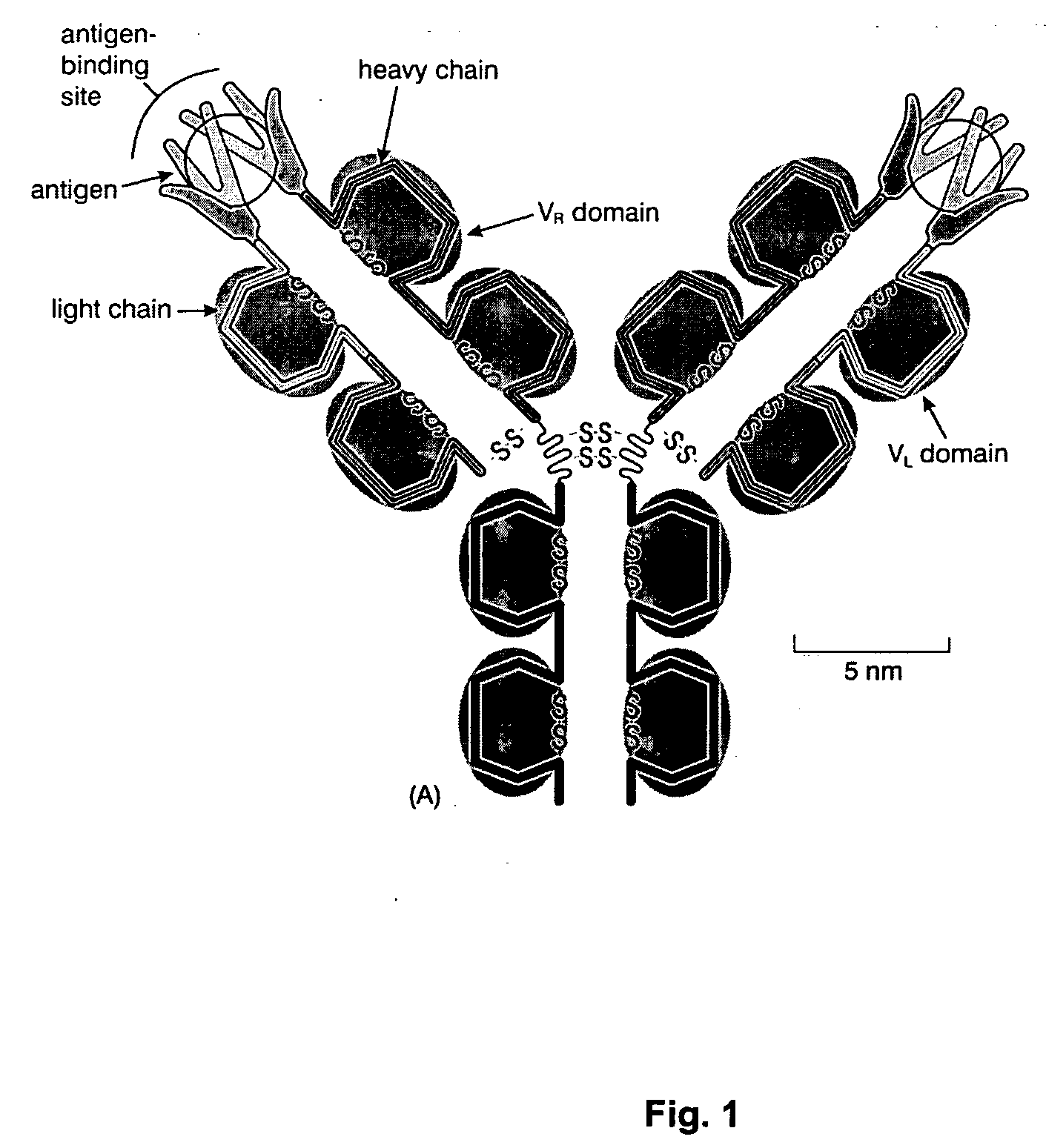
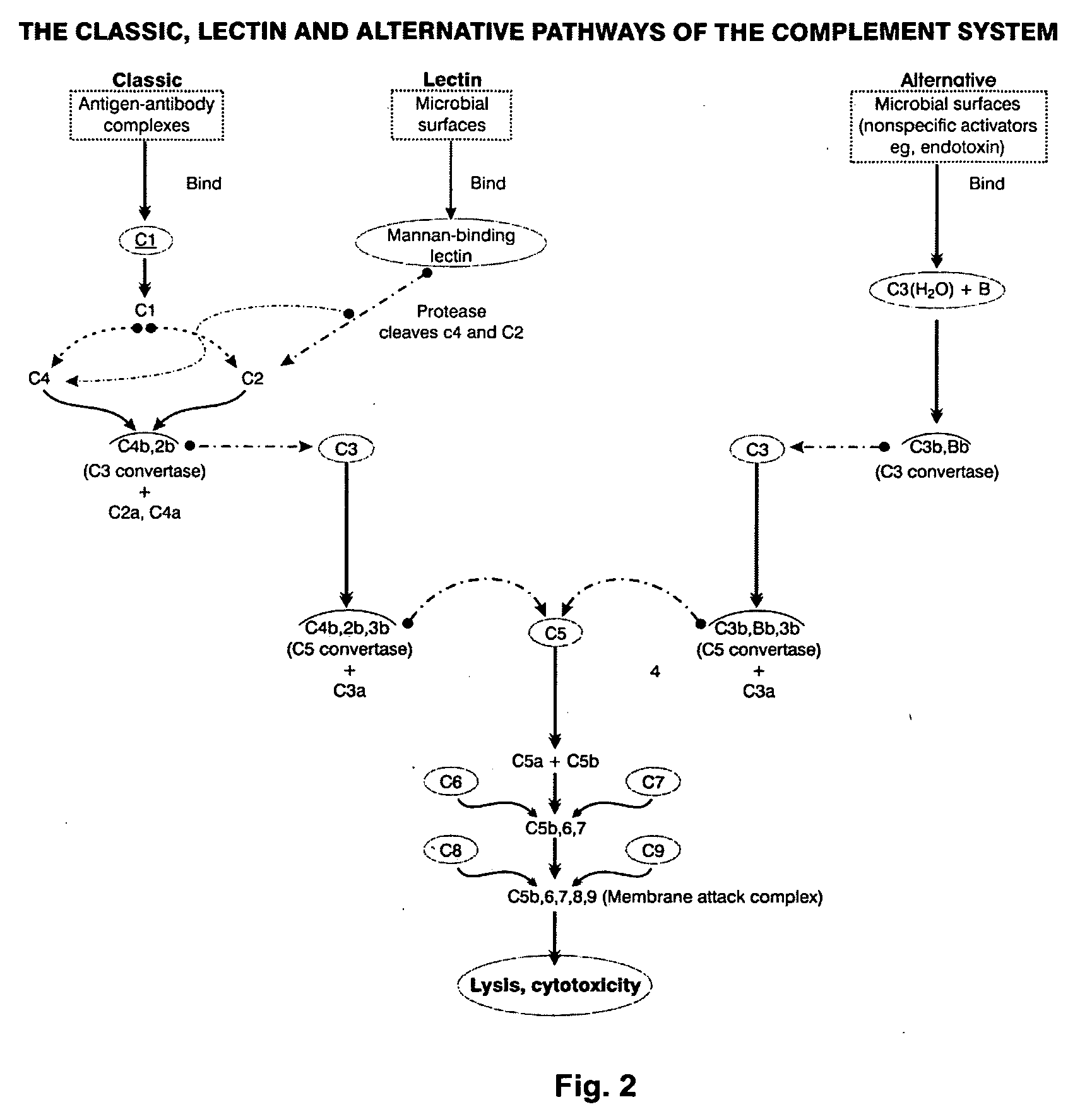
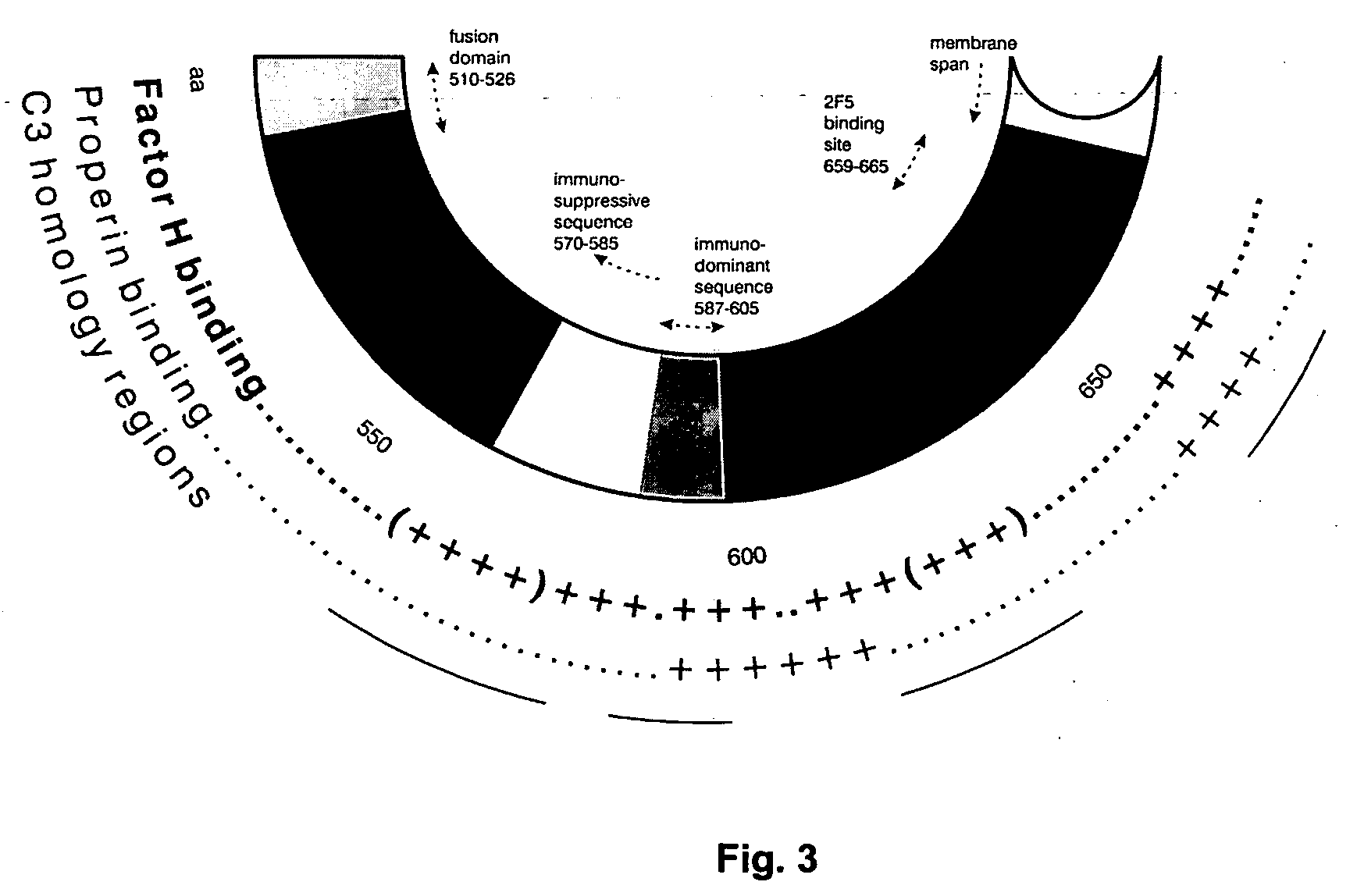
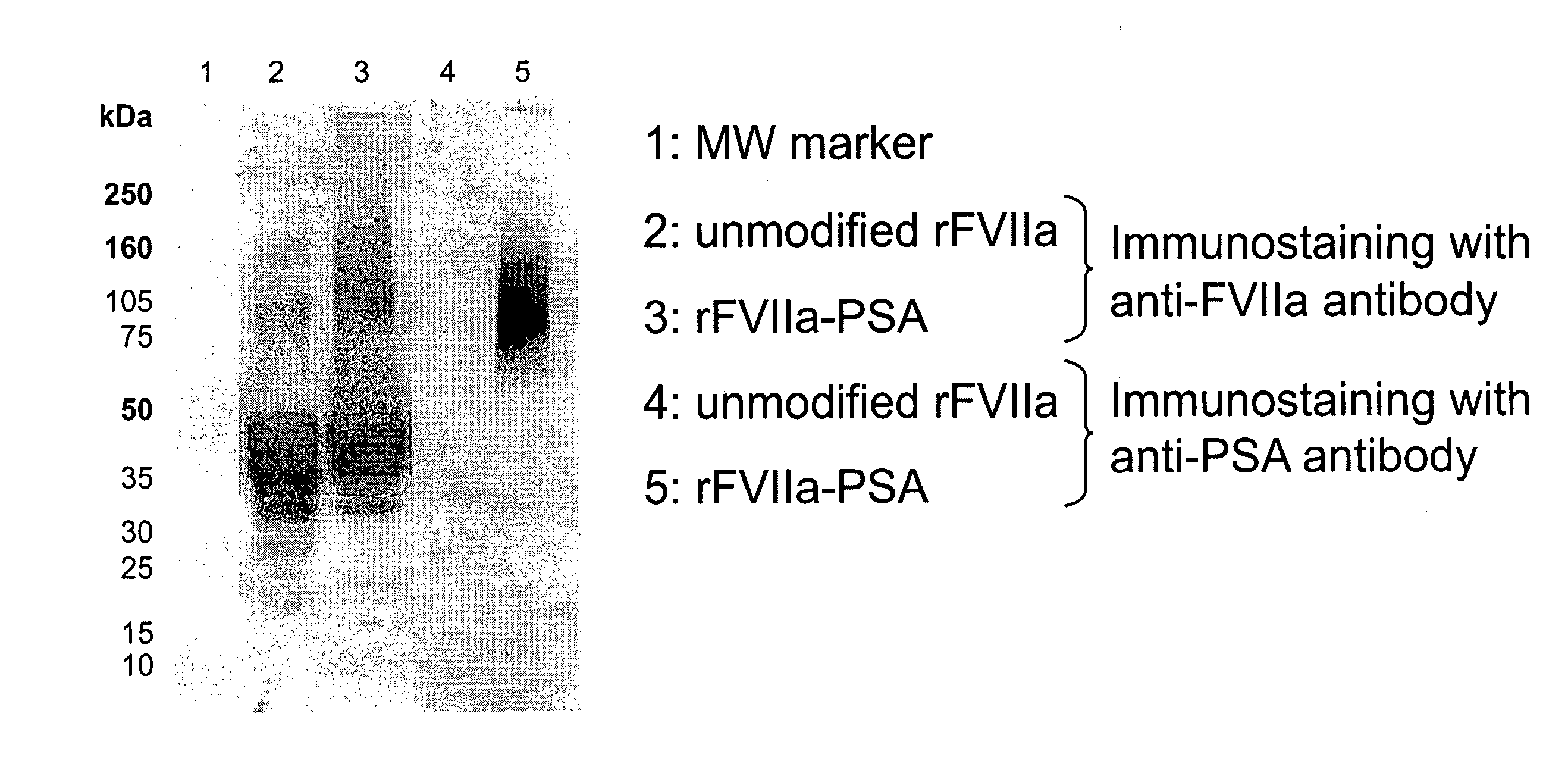
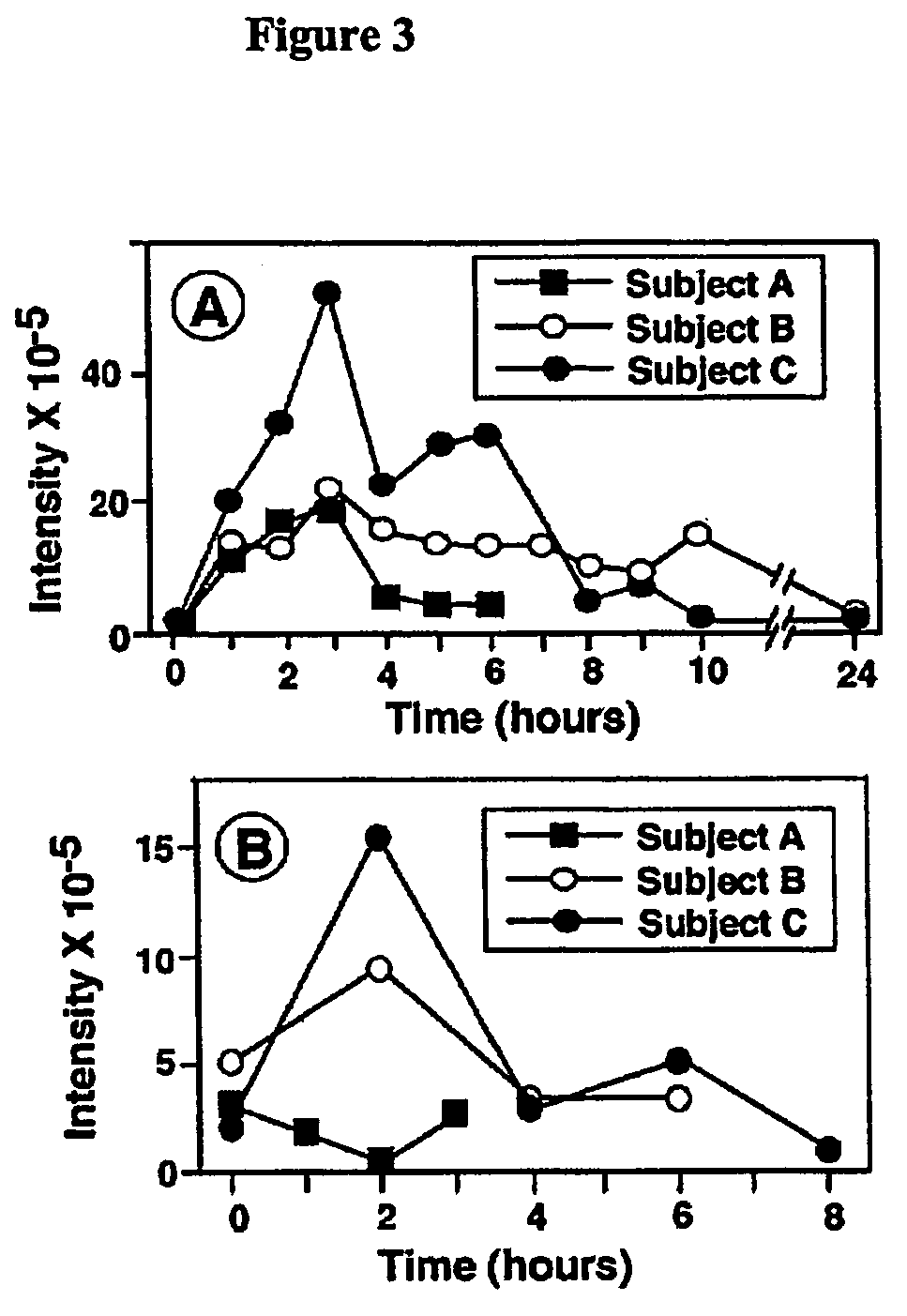
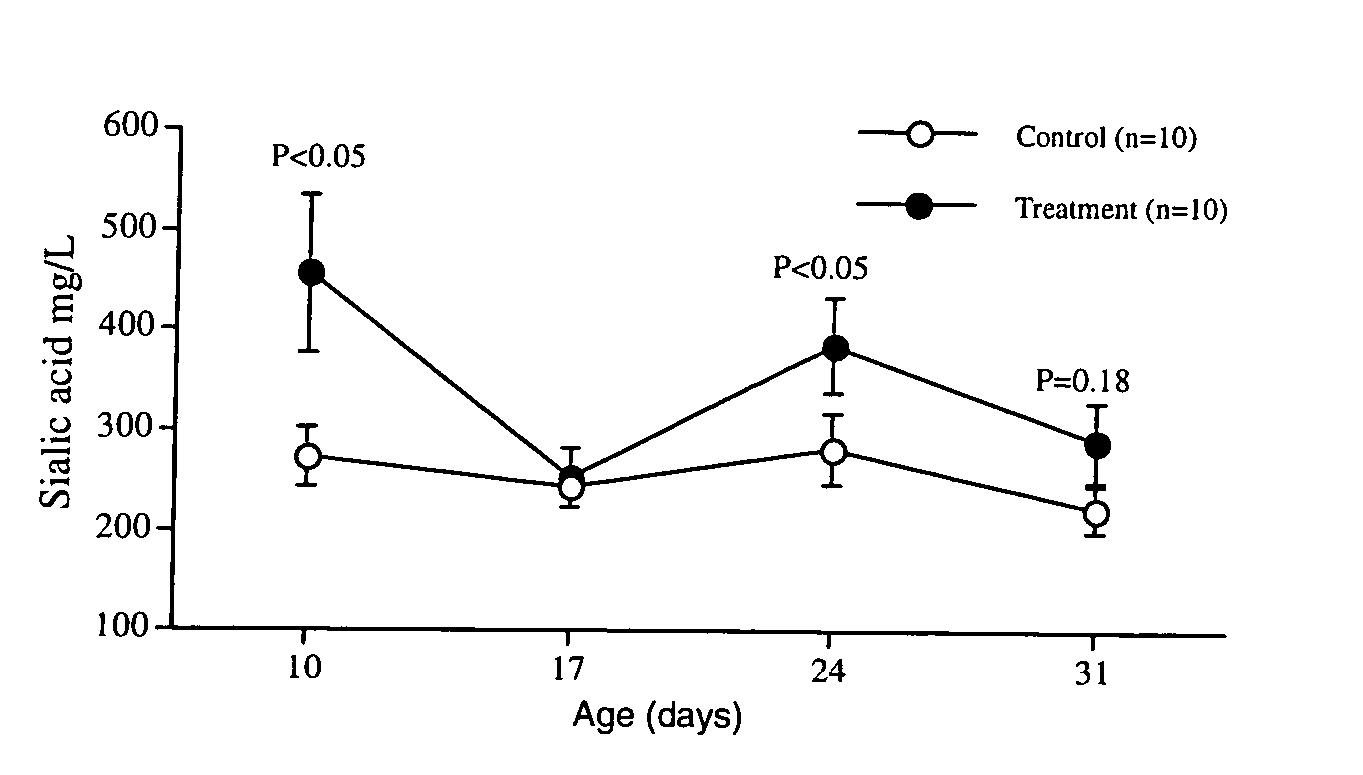
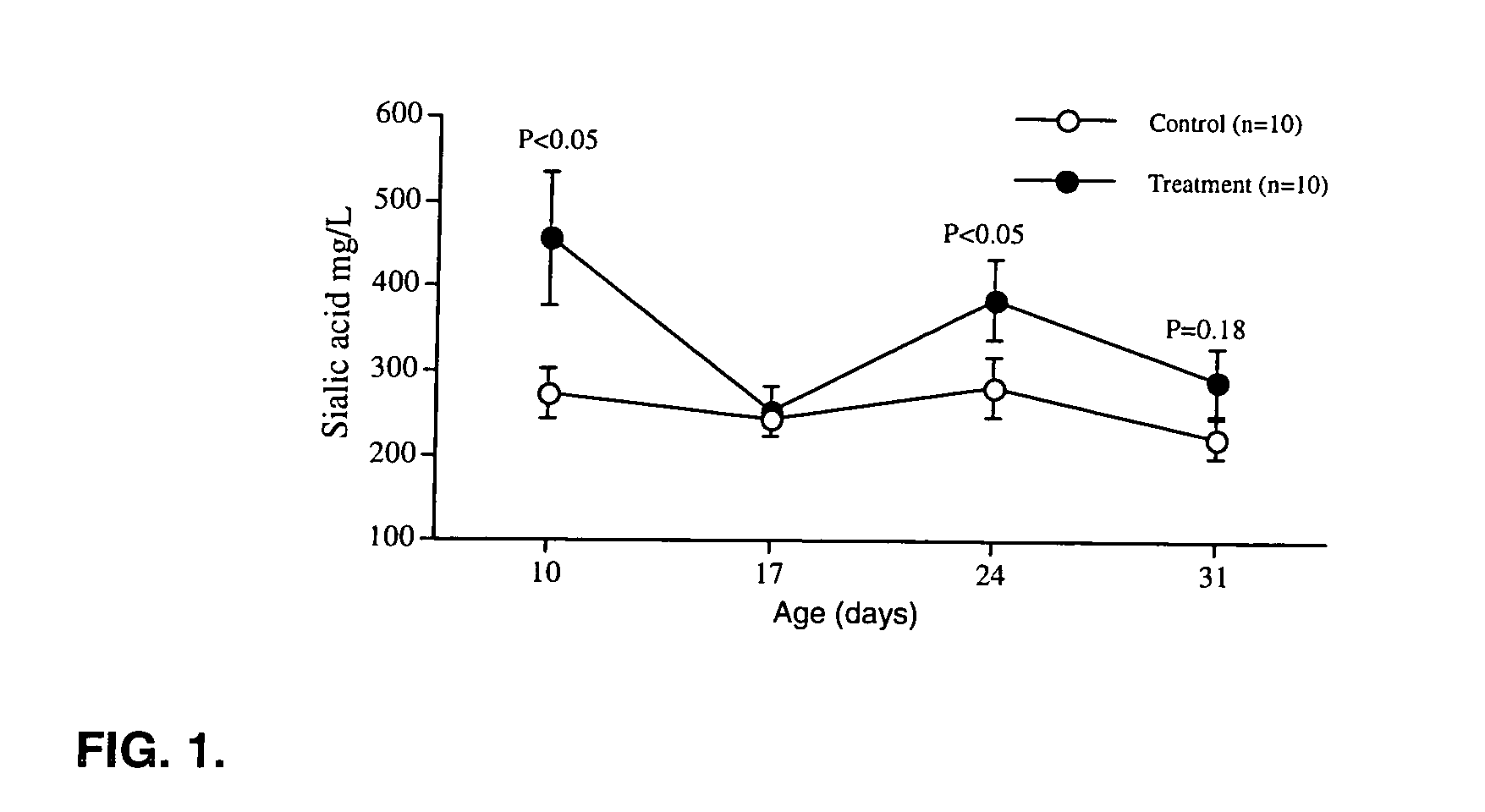
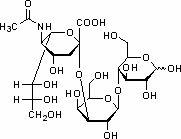
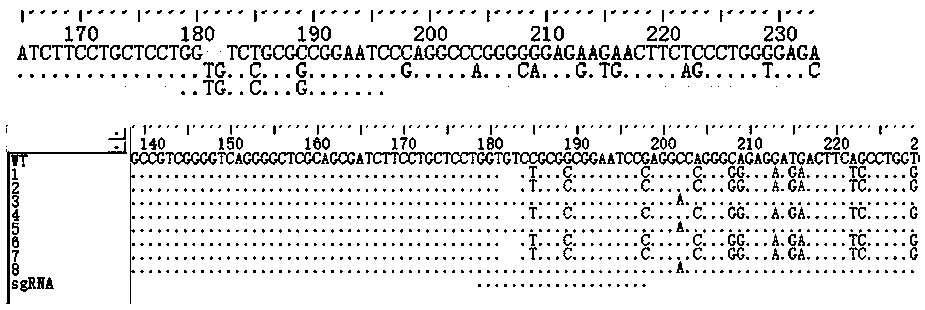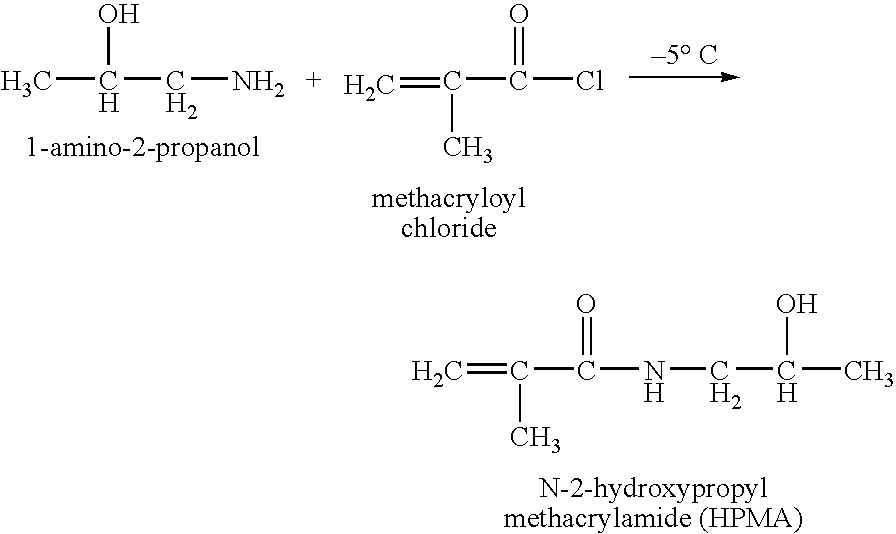Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
895 results about "Sialic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sialic acid is a generic term for a family of derivatives of neuraminic acid, an acidic sugar with a nine-carbon backbone. It is also the name for the most common member of this group, N-acetylneuraminic acid (Neu5Ac or NANA).
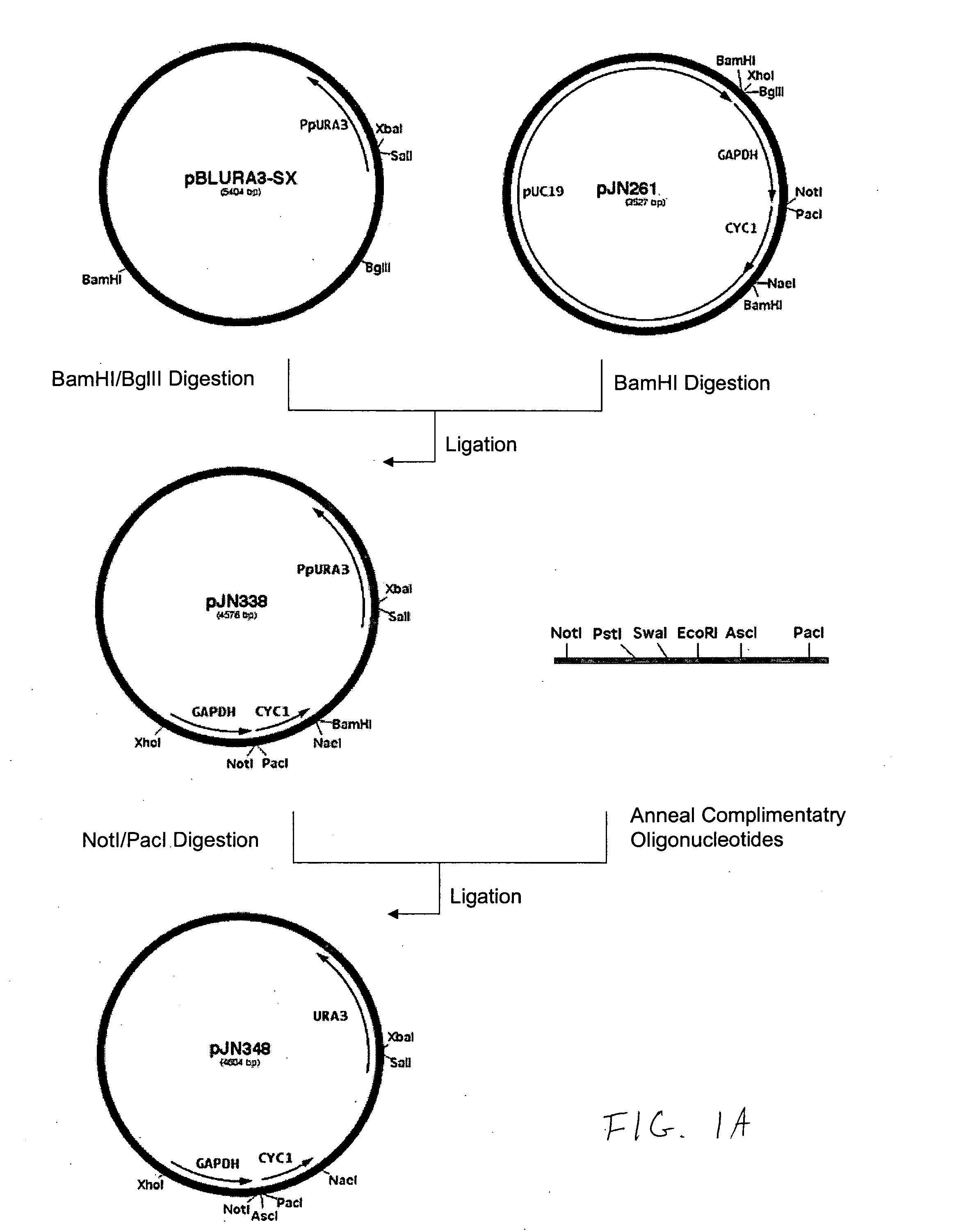
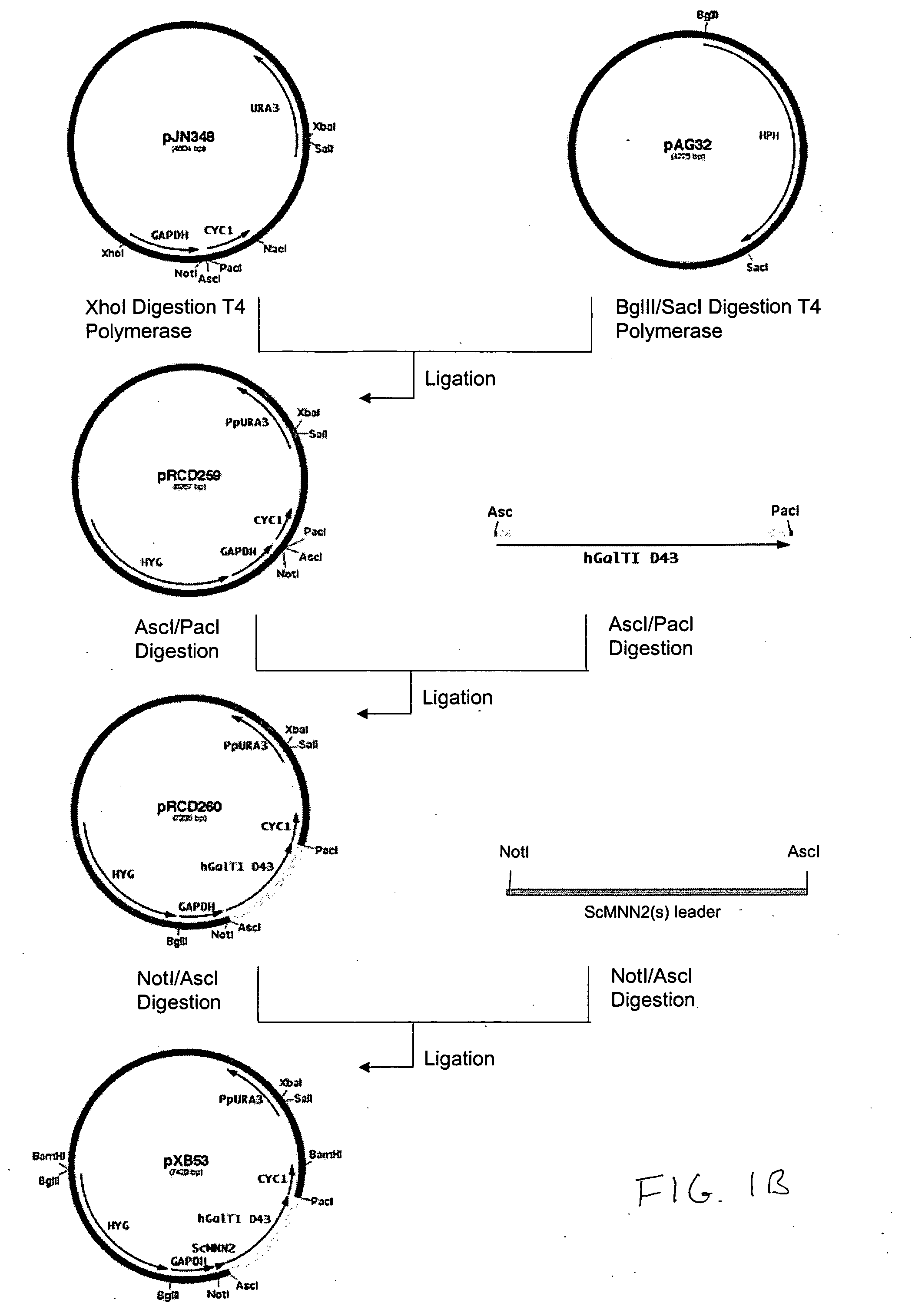
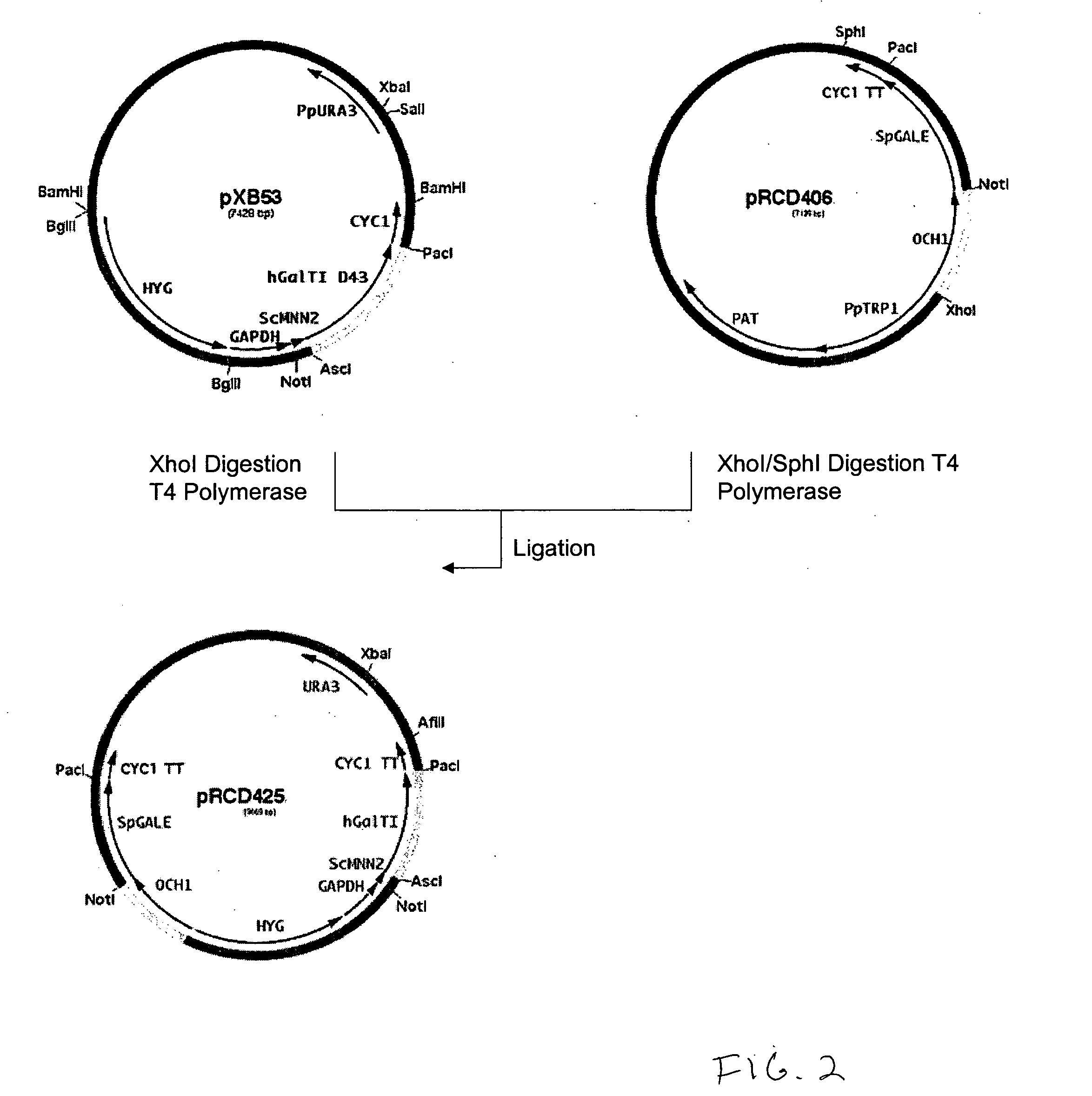
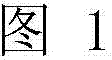
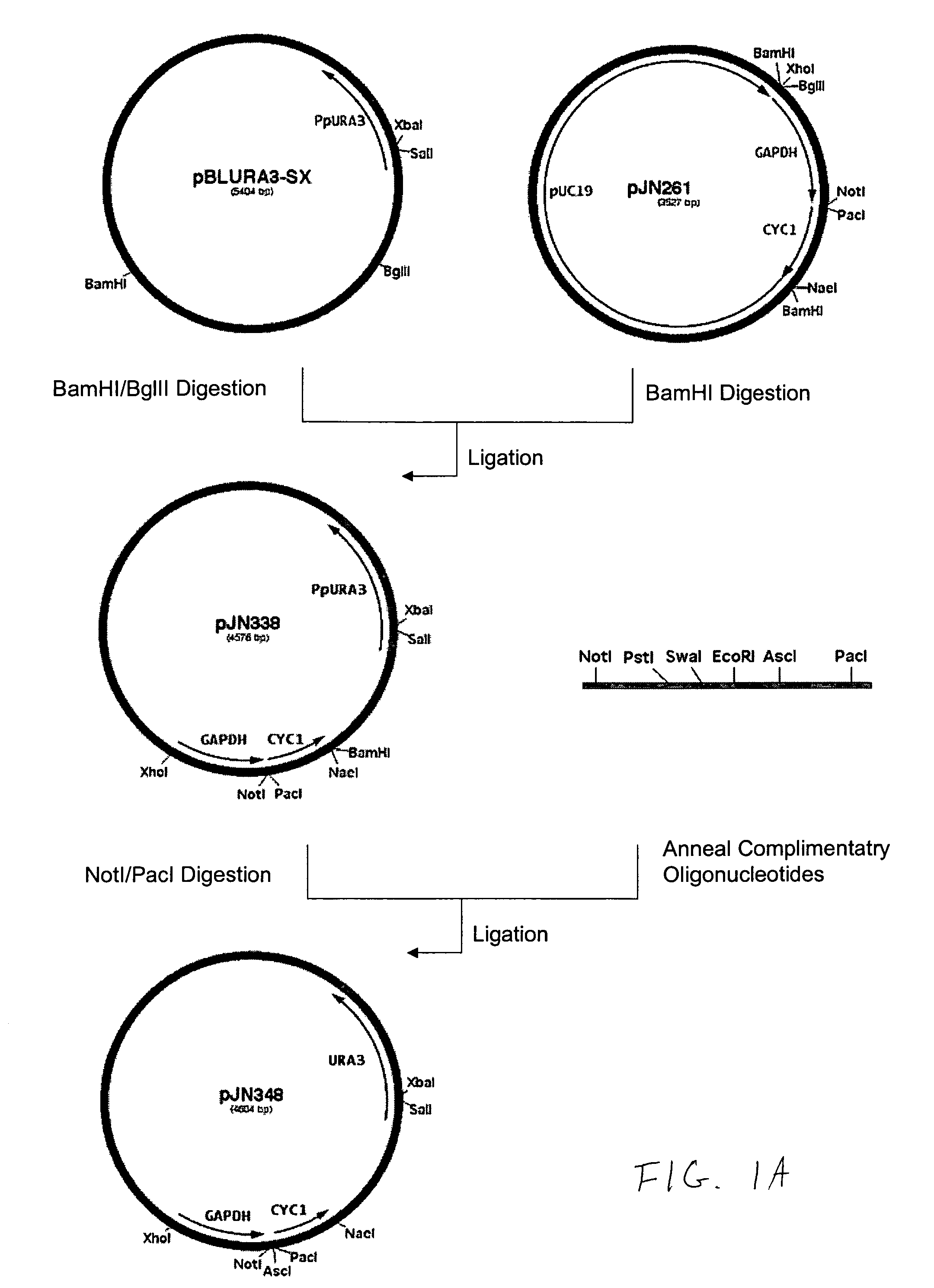
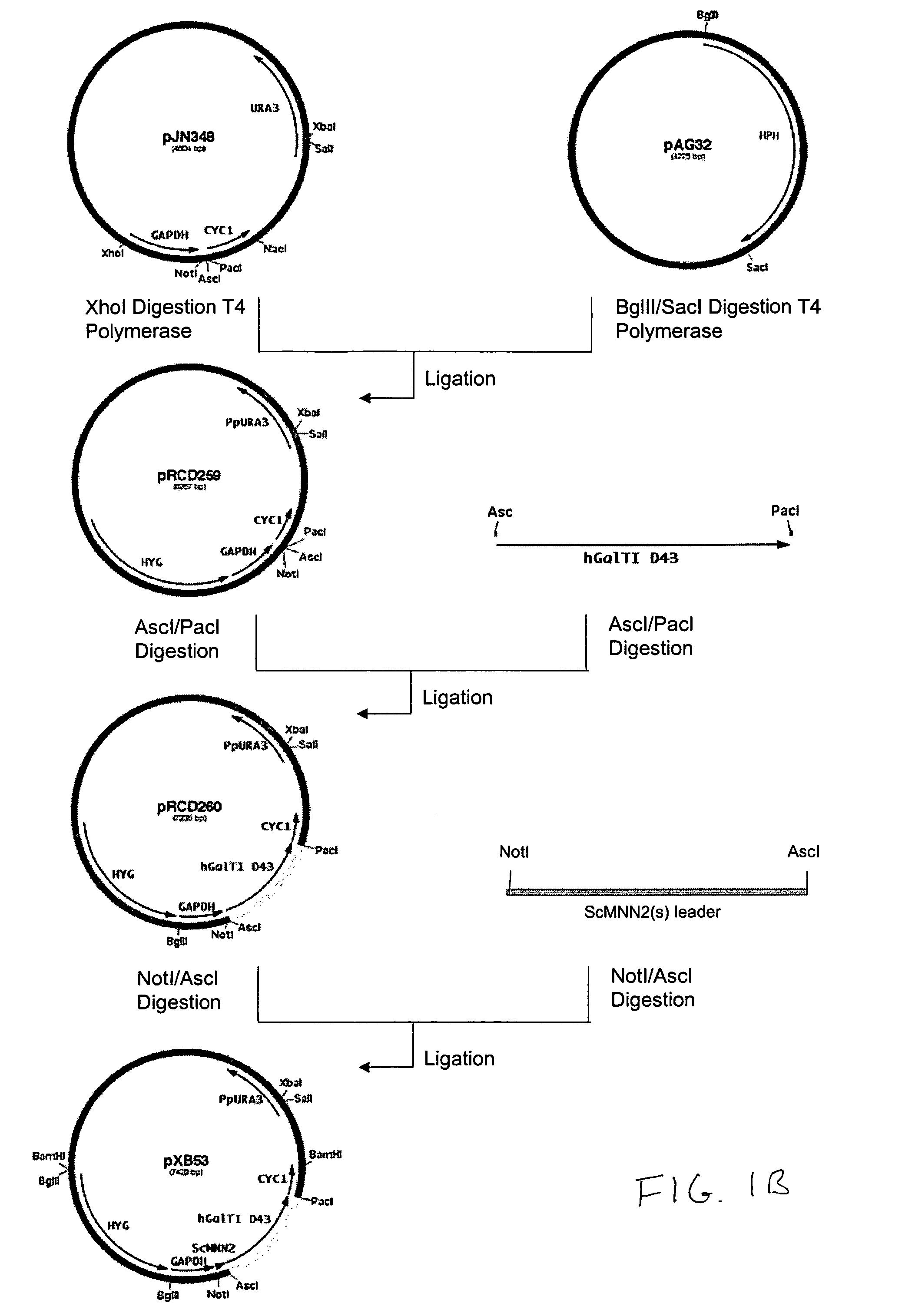
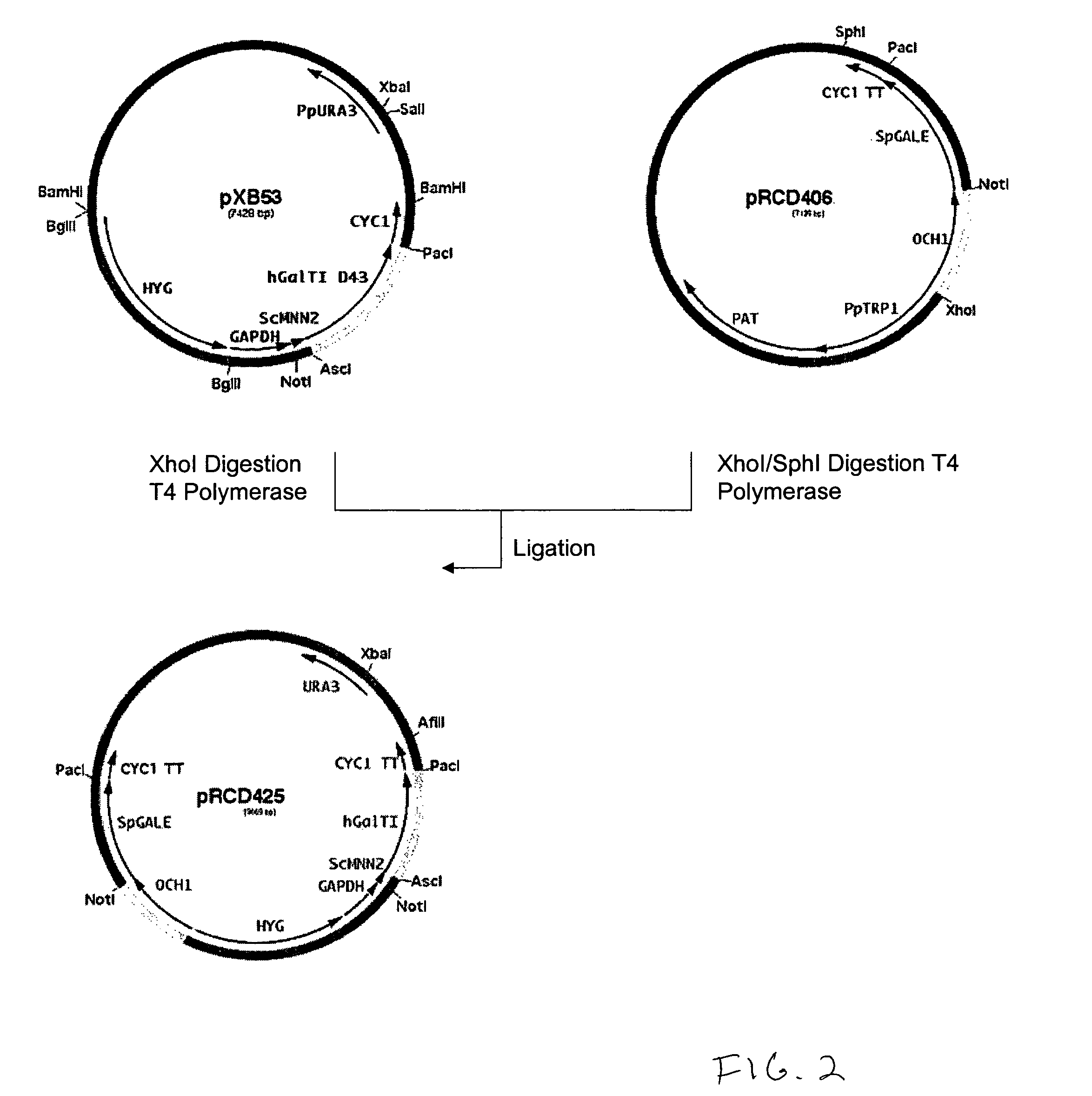
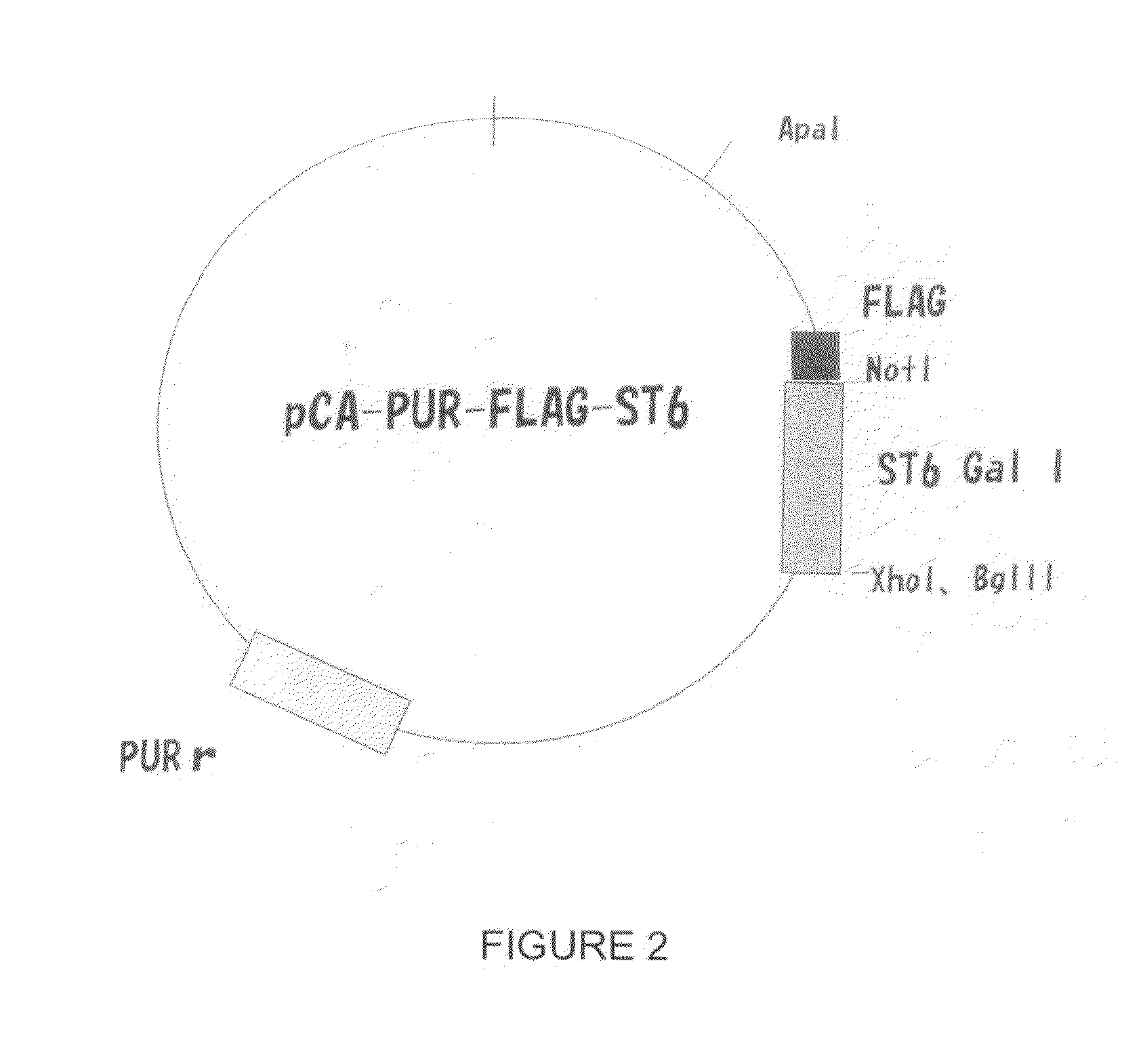
Production of galactosylated glycoproteins in lower eukaryotes
InactiveUS20060040353A1Reducing and eliminating activityEfficient workFungiSugar derivativesUDP galactoseSialic acid
The present invention provides a novel lower eukaryotic host cell producing human-like glycoproteins characterized as having a terminal β-galactose residue and essentially lacking fucose and sialic acid residues. The present invention also provides a method for catalyzing the transfer of a galactose residue from UDP-galactose onto an acceptor substrate in a recombinant lower eukaryotic host cell, which can be used as a therapeutic glycoprotein.
Owner:GLYCOFI
Infant formulas for early brain development
Disclosed are infant formulas comprising fat, protein, carbohydrate, vitamins, and minerals, including on an as-fed basis, at least about 5 mg / L of gangliosides, at least about 150 mg / L of phospholipids, at least about 70 mg / L of total sialic acid with at least about 2.5% as lipid-bound sialic acid, at least about 0.13% docosahexaenoic acid by weight of total fatty acids, and at least about 0.25% arachidonic acid by weight of total fatty acids. Also disclosed are methods of accelerating brain development, neural migration, and cognitive development in an infant by administering the infant formulas during the first 2-4 months of life, preferably as a sole source of nutrition.
Owner:ABBOTT LAB INC
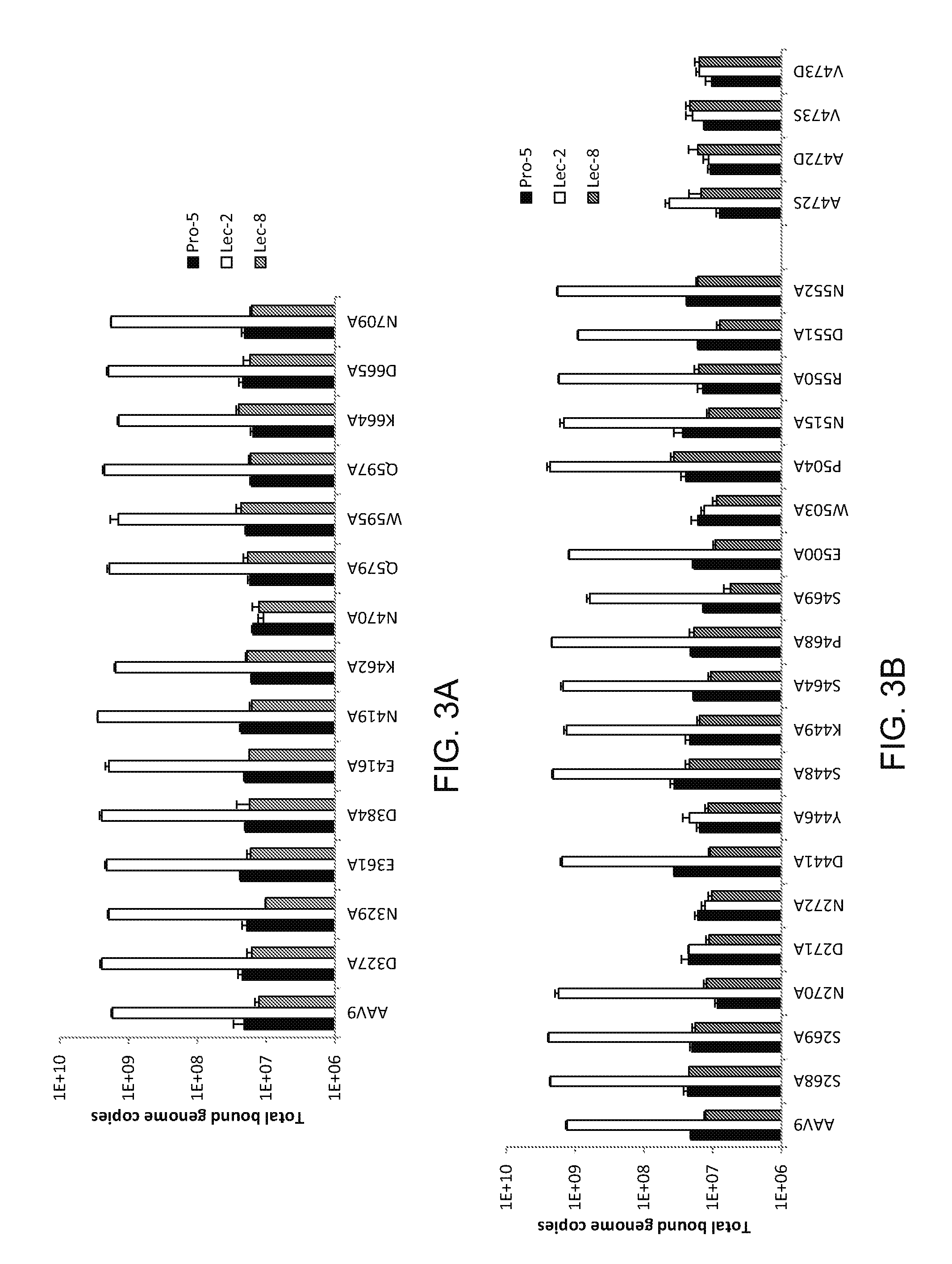
Compositions and Methods for Altering Tissue Specificity and Improving AAV9-Mediated Gene Transfer
ActiveUS20130323226A1Improve efficiencyImprove usabilityOrganic active ingredientsVectorsViral vectorFhit gene
A method of altering the targeting and / or cellular uptake efficiency of an adeno-associated virus (AAV) viral vector having a capsid containing an AAV9 cell surface binding domain is described. The method involves modifying a clade F cell surface receptor which comprises a glycan having a terminal sialic acid residue and a penultimate β-galactose residue. The modification may involve retargeting the vector by temporarily functionally ablate AAV9 binding in a subset of cells, thereby redirecting the vector to another subset of cells. Alternatively, the modification may involve increasing cellular update efficiency by treating the cells with a neuraminidase to expose cell surface β-galactose. Also provided are compositions containing the AAV9 vector and a neuraminidase. Also provided is a method for purifying AAV9 using β-galactose linked to solid support. Also provided are mutant vectors which have been modified to alter their targeting specificity, including mutant AAV9 in which the galactose binding domain is mutated and AAV in which an AAV9 galactose binding domain is engineered.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Nutritional supplement
A nutritional supplement comprising an infant milk formula having long chain poly unsaturated fatty acids, sialic acids, and cholesterol.
Owner:UNIV KANSAS MEDICAL CENT
Glycoconjugation of polypeptides using oligosaccharyltransferases
The current invention provides polypeptides and polypeptide conjugates that include an exogenous N-linked glycosylation sequence. The N-linked glycosylation sequence is preferably a substrate for an oligosaccharyltransferase (e.g., bacterial PgIB), which can catalyze the transfer of a glycosyl moiety from a lipid-bound glycosyl donor molecule (e.g., a lipid-pyrophosphate-linked glycosyl moiety) to an asparagine (N) residue of the glycosylation sequence. In one example, the asparagine residue is part of an exogenous N-linked glycosylation sequence of the invention. The invention further provides methods of making the polypeptide conjugates that include contacting a polypeptide having an N-linked glycosylation sequence of the invention and a lipid-pyrophosphate-linked glycosyl moiety (or phospholipid-linked glycosyl moiety) in the presence of an oligosaccharyltransferase under conditions sufficient for the enzyme to transfer the glycosyl moiety to an asparagine residue of the N-linked glycosylation sequence. Exemplary glycosyl moieties that can be conjugated to the glycosylation sequence include GlcNAc, GlcNH, bacillosamine, 6-hydroybacillosamine, GalNAc, GaINH, GlcNAc-GlcNAc, GlcNAc-GlcNH, GlcNAc-Gal, GlcNAc-GlcNAc-Gal-Sia, GlcNAc-Gal-Sia, GlcNAc-GlcNAc-Man, and GlcNAc-GlcNAc-Man(Man)2. The transferred glycosyl moiety is optionally modified with a modifying group, such as a polymer (e.g., PEG). In one example, the modified glycosyl moiety is a GIcNAc or a sialic acid moiety.
Owner:RATIOPHARM GMBH +1
Enriched infant formulas
InactiveUS20080003329A1Reduce riskShorten the construction periodFood preparationSialic acidPhospholipid
Disclosed are infant formulas comprising fat, protein, carbohydrate, vitamins, and minerals, including on an as-fed basis (A) at least about 5 mg / L of gangliosides, (B) at least about 150 mg / L of phospholipids, and (C) lactoferrin, and (D) at least about 70 mg / L of sialic acid, with at least about 2.5% by weight of the sialic acid as lipid-bound sialic acid. Also disclosed are methods of using the formula to reduce the risk of diarrhea infants, and to produce a gut microflora profile similar to that of breast-fed infants.
Owner:ABBOTT LAB INC
Mammalian cell culture processes for protein production
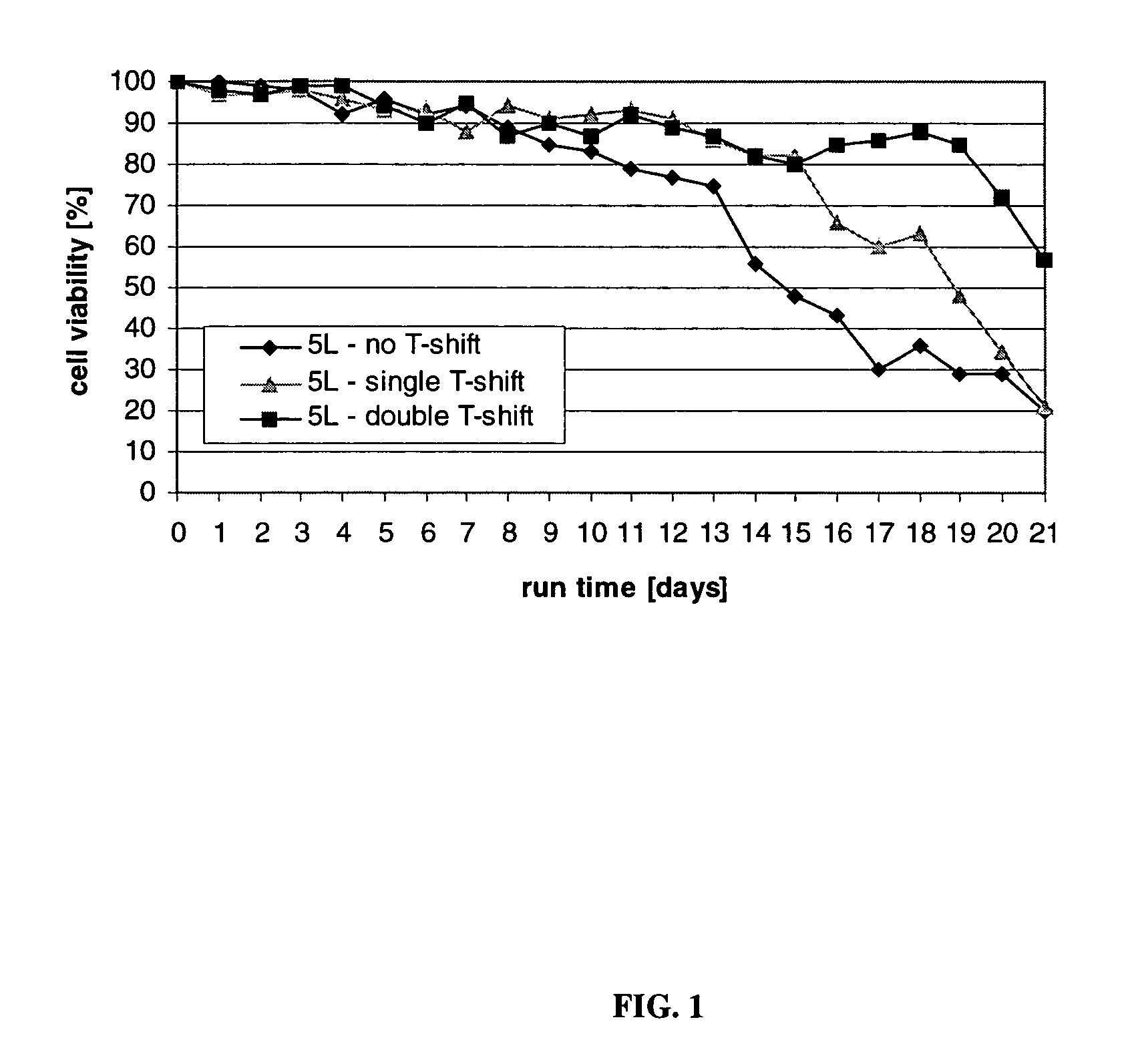
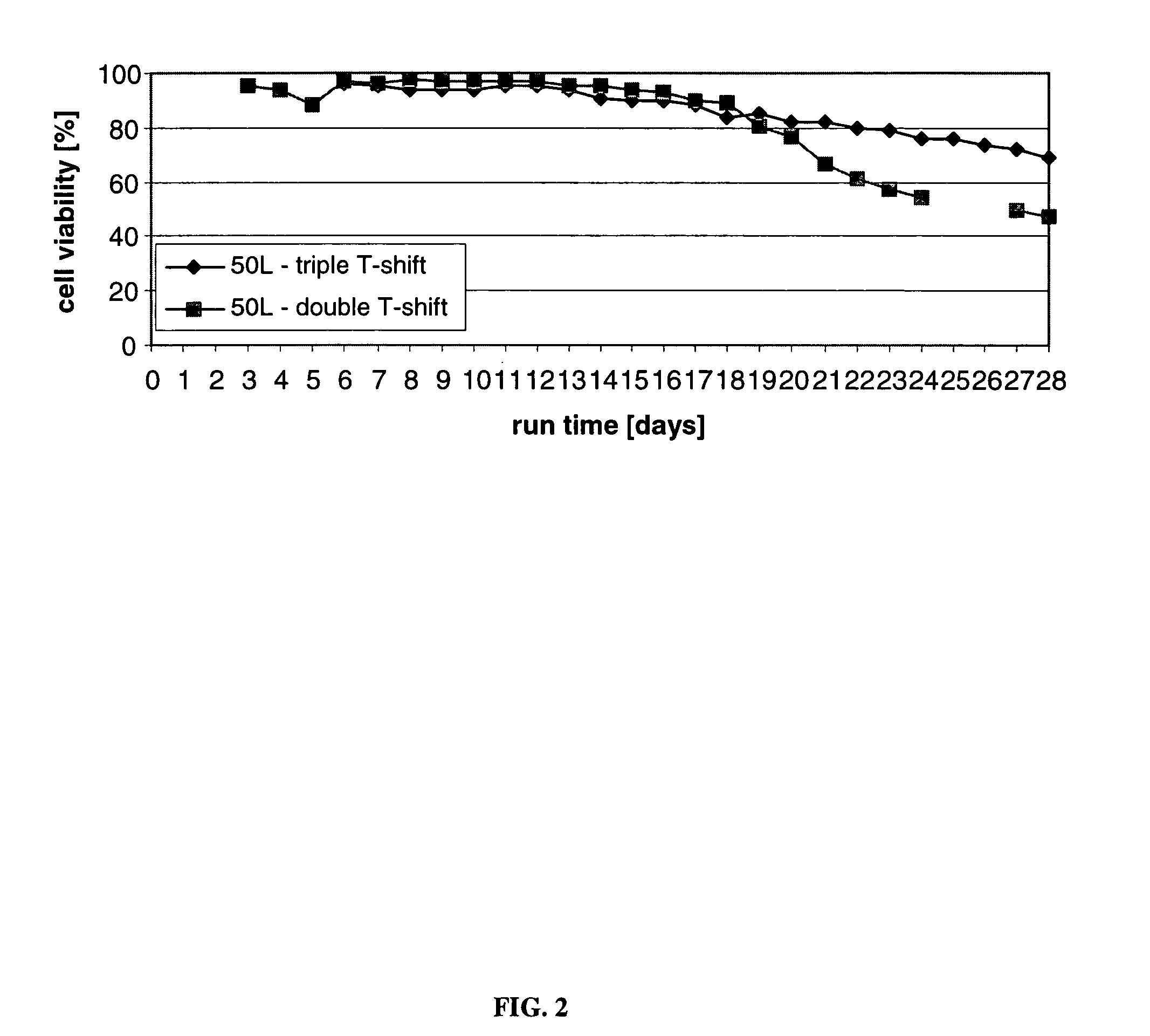
ActiveUS7541164B2Enhance cell viabilityPeptide/protein ingredientsImmunoglobulinsGrowth phaseTwo temperature
Owner:BRISTOL MYERS SQUIBB CO
Human tumor necrosis factor-immunoglobulin(TNFR1-IgG1) chimera composition
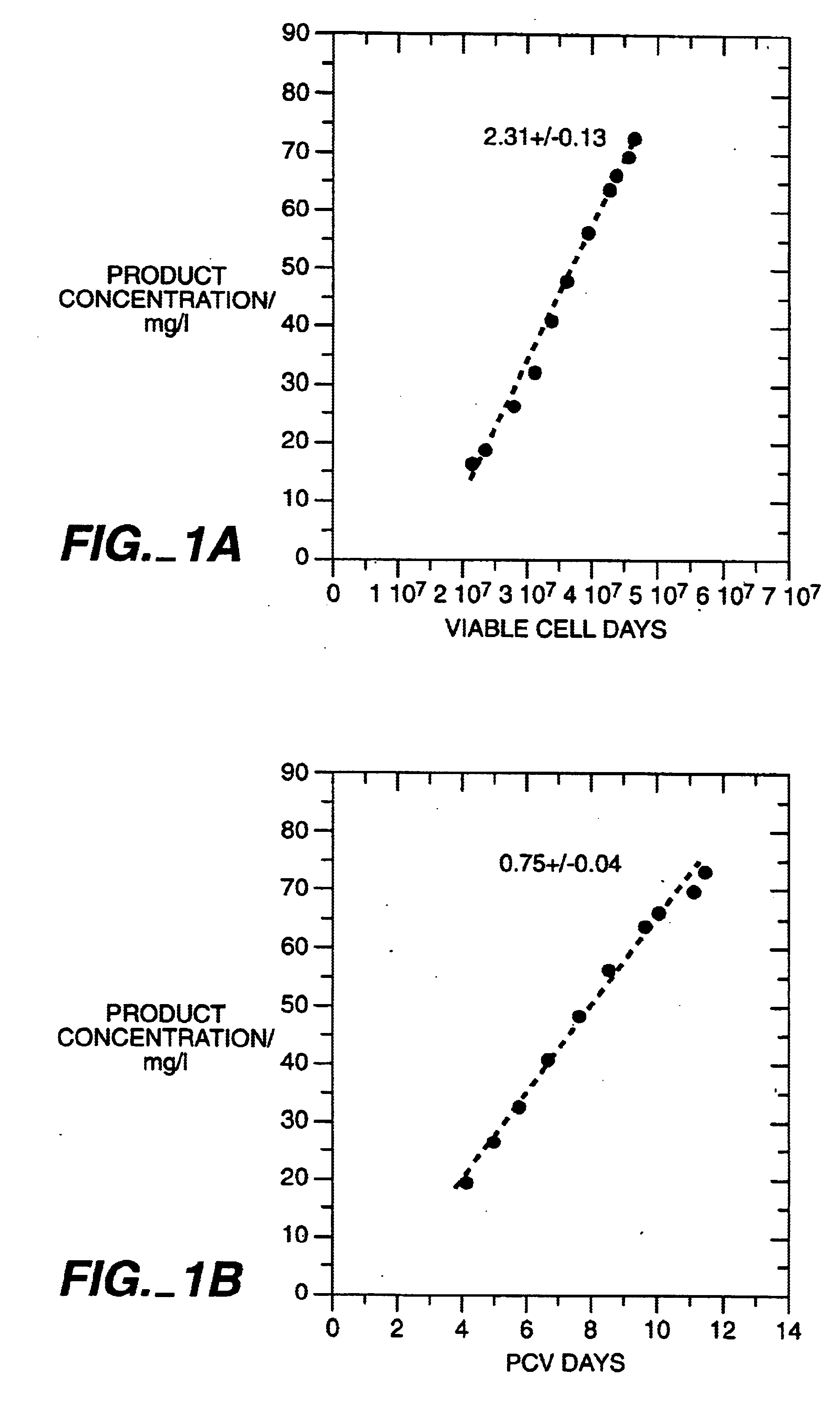
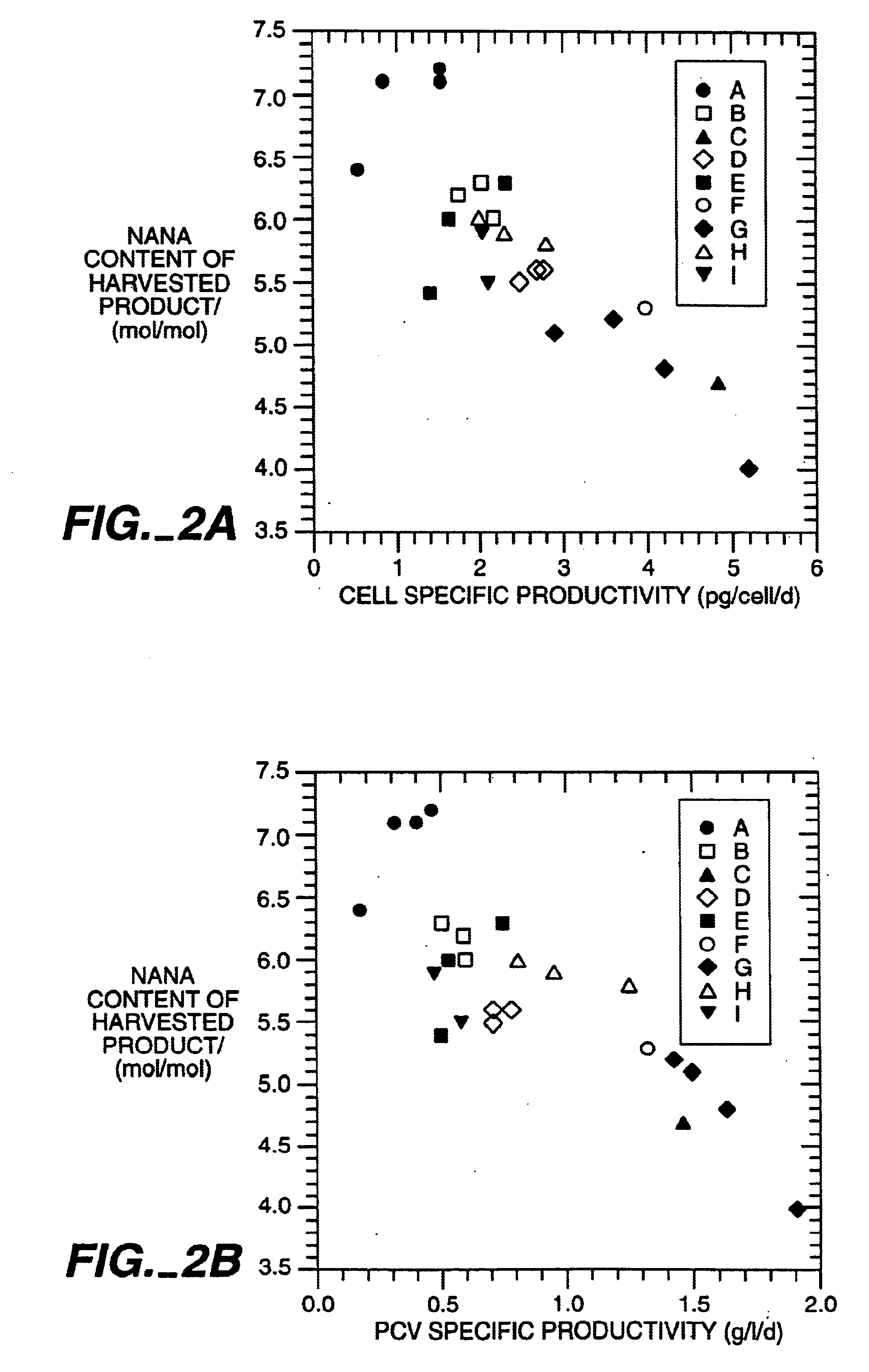
The present invention relates to novel process for the preparation of glycoproteins by mammalian cell culture wherein the sialic acid content of the glycoprotein produced is controlled over a broad range of values by manipulating the cell culture environment. The invention provides for processes in which the sialic acid content of the glycoprotein is modified by changes in cell culture parameters which affect cell specific productivity. Preferred embodiments of the invention include cell culture processes in the osmolality of the cell culture is controlled as well as the concentration of a transcription enhancer during the production phase of the cell culture. The invention further provides for novel preparations of soluble type 1 tumor necrosis factor immunoglobulin G1 and their uses in the treatment of inflammatory or immune related disorders.
Owner:GENENTECH INC
Mammalian cell culture processes for protein production
ActiveUS20110081679A1High densityReduce aggregationPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsBiotechnologyGlucocorticoid
The present invention describes methods and processes for the production of proteins, particularly glycoproteins, by animal cell or mammalian cell culture, preferably, but not limited to, fed-batch cell cultures. In one aspect, the methods comprise the addition of glucocorticoid compound during the culturing period. The addition of glucocorticoid compound sustain a high viability of the cultured cells, and can yield an increased end titer of protein product, and a high quality of protein product, as determined, e.g., by sialic acid content of the produced protein.
Owner:BRISTOL MYERS SQUIBB CO
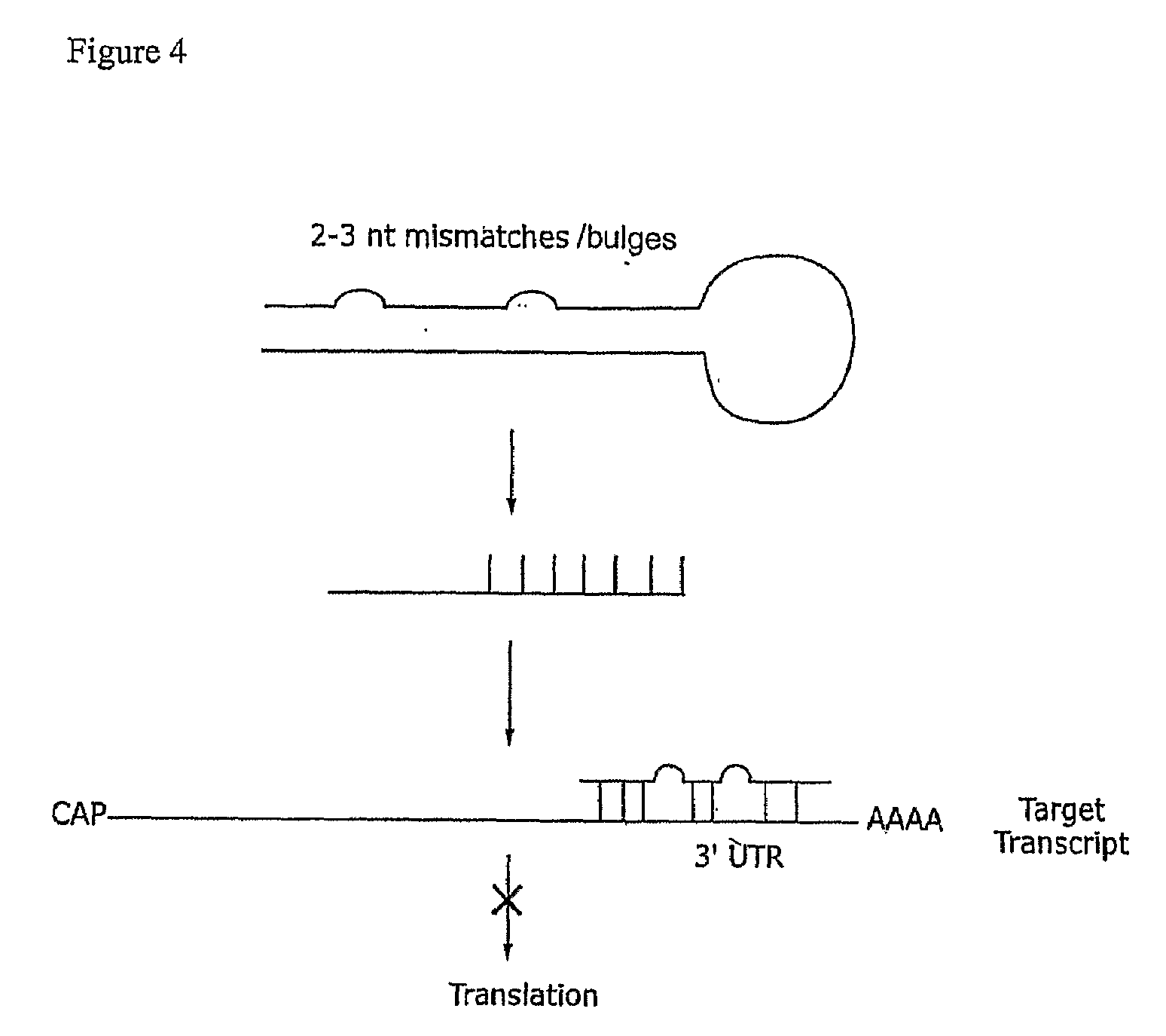
Compositions and methods for RNA interference with sialidase expression and uses thereof
InactiveUS20090203055A1Reducing sialidase activityReduce decreaseSugar derivativesMicrobiological testing/measurementSialic acidControl cell
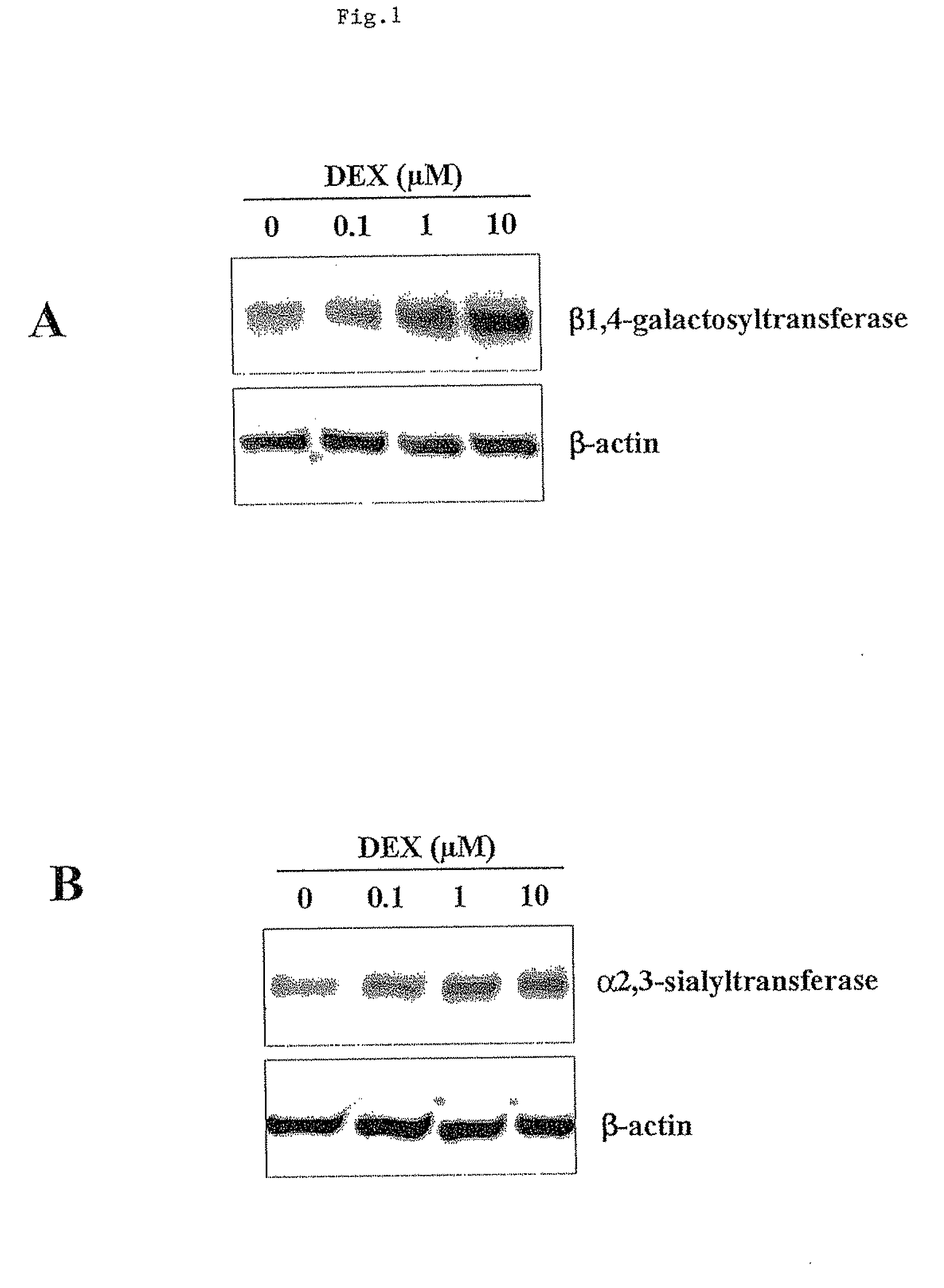
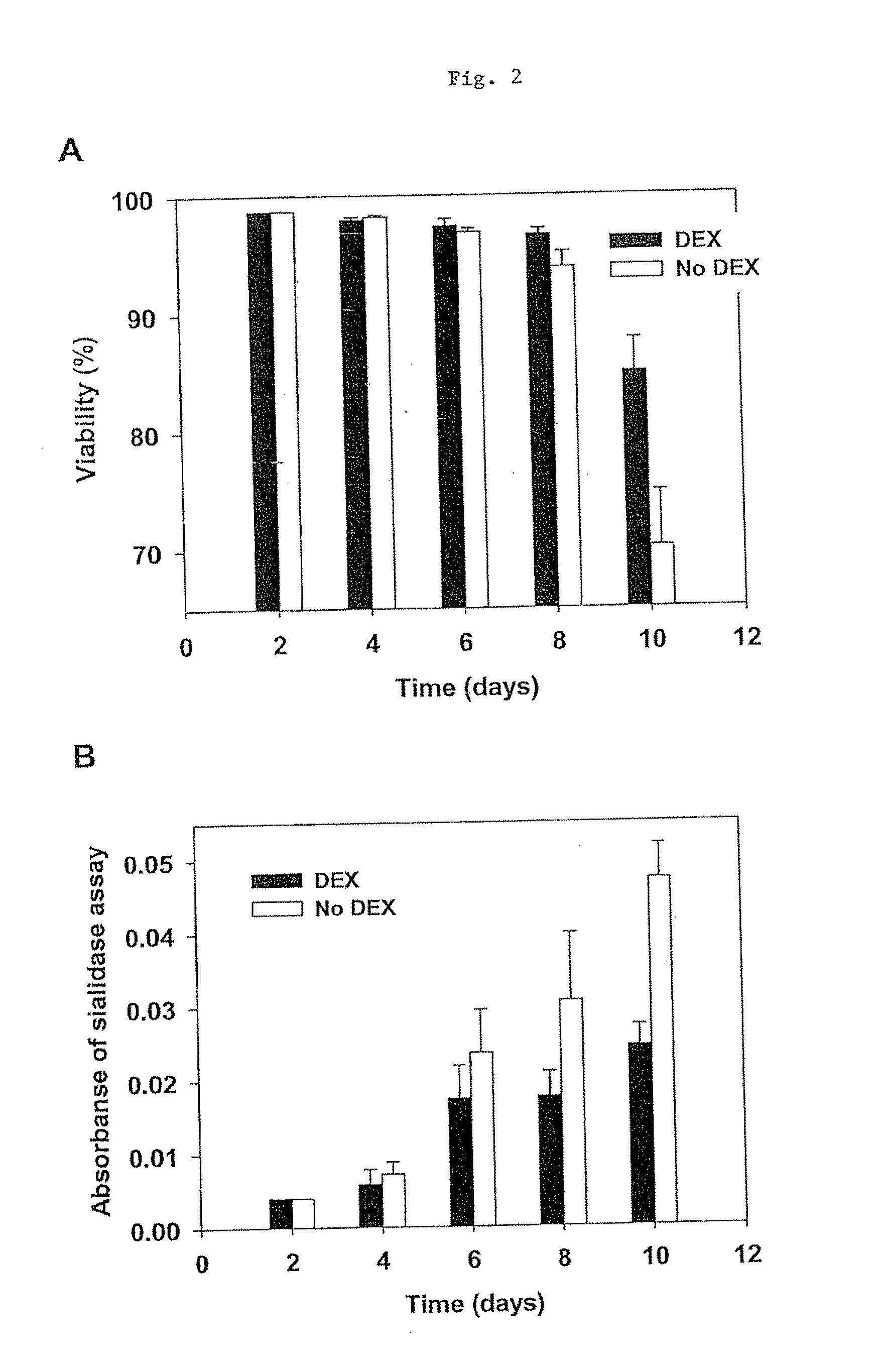
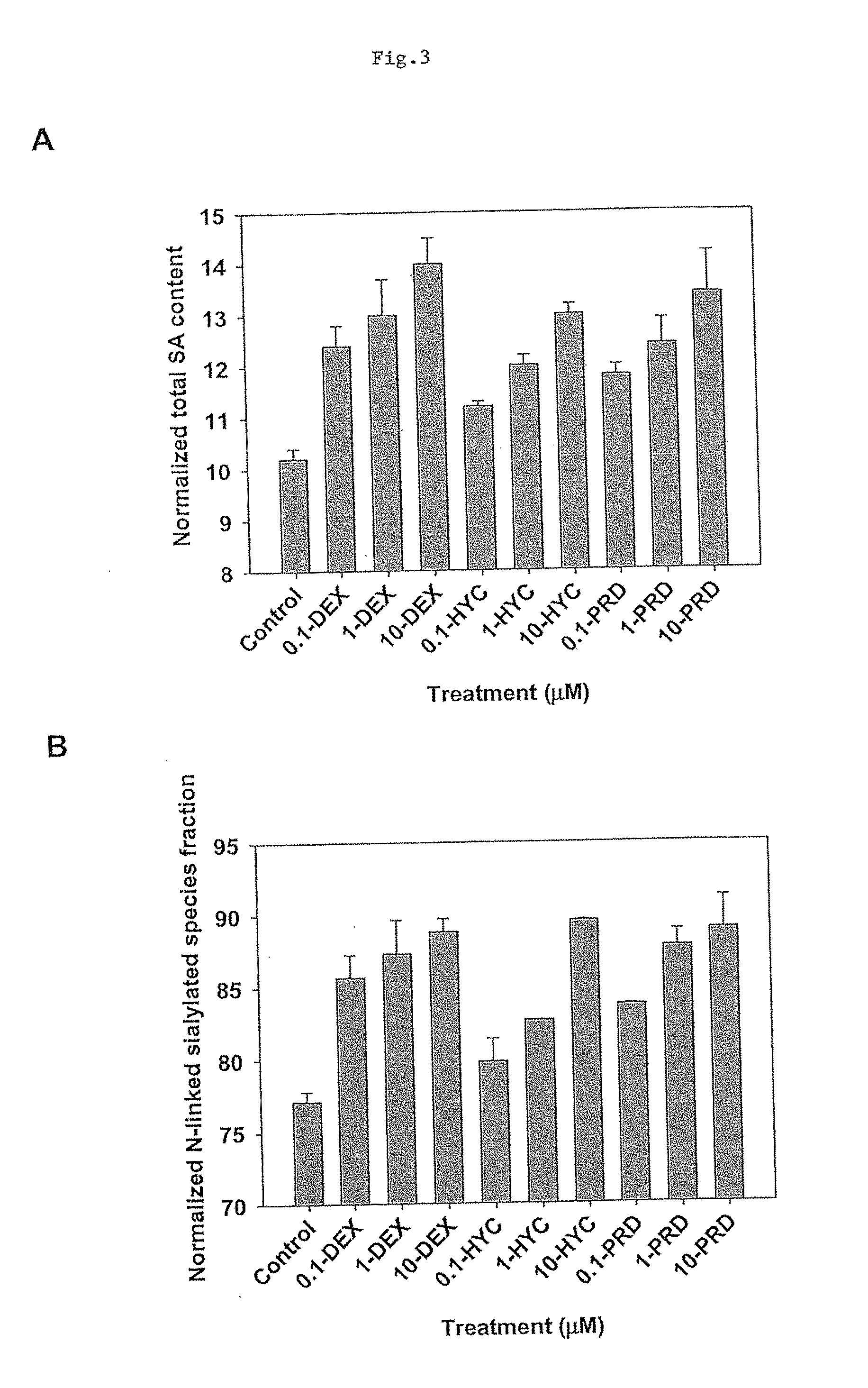
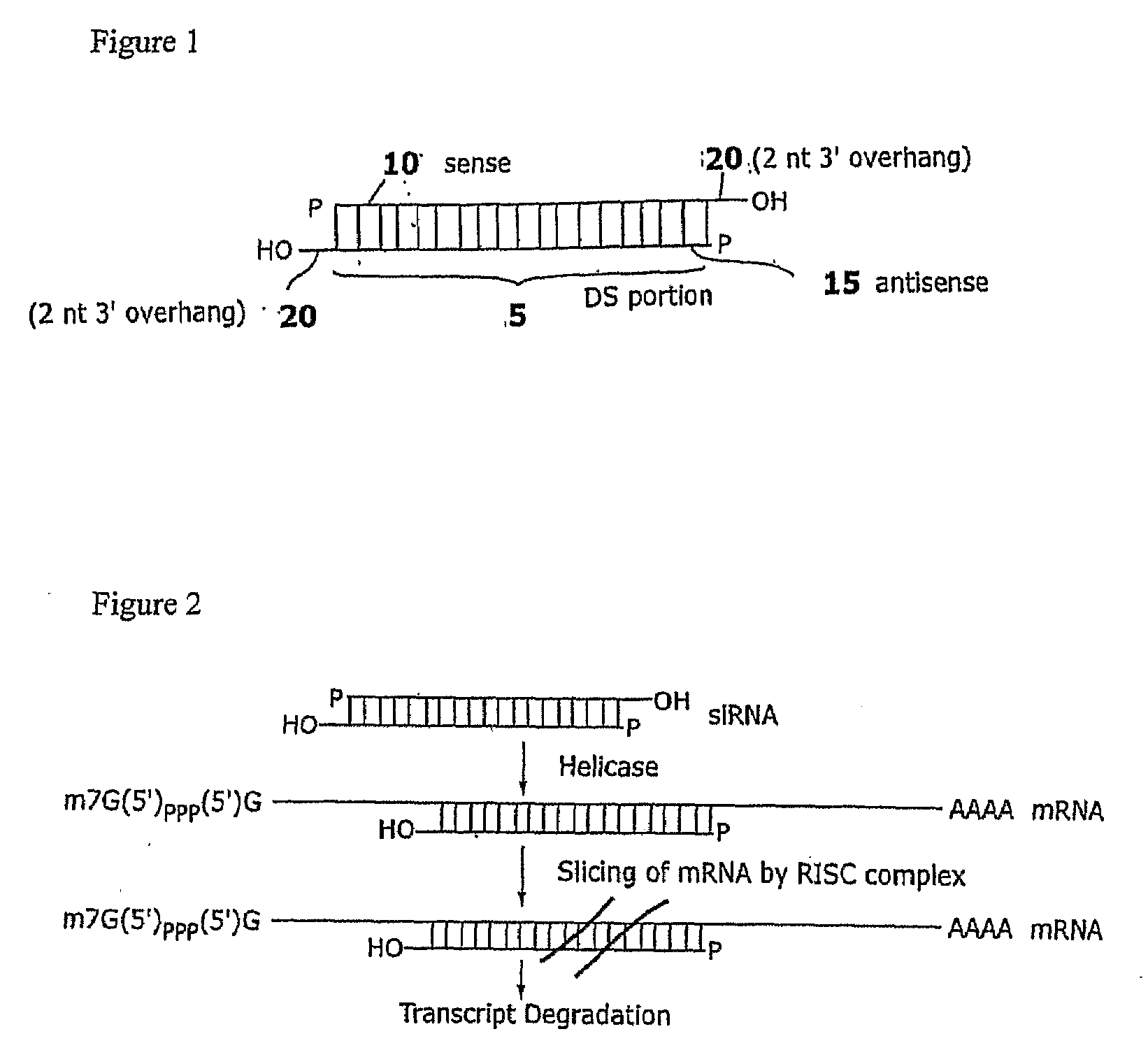
The present invention provides RNAi agents targeted to sialidase. The RNAi agents include siRNA, shRNA, and expression vectors that comprise a template for transcription of an siRNA or shRNA. The invention further provides cells and cell lines that comprise an RNAi agent targeted to sialidase. The cells and cell lines exhibit reduced sialidase activity relative to control cells that do not comprise an RNAi agent targeted to sialidase. Certain of the cell lines stably express the RNAi agent. The invention further provides methods of producing the cells and cell lines. The invention further provides methods for producing a glycoprotein in cells that comprise an RNAi agent targeted to sialidase. The glycoproteins exhibit an improved sialic acid profile relative to glycoproteins produced by cells that do not comprise an RNAi agent targeted to sialidase. The invention further provides glycoproteins, e.g., therapeutic glycoproteins, produced in the cells.
Owner:MASSACHUSETTS INST OF TECH
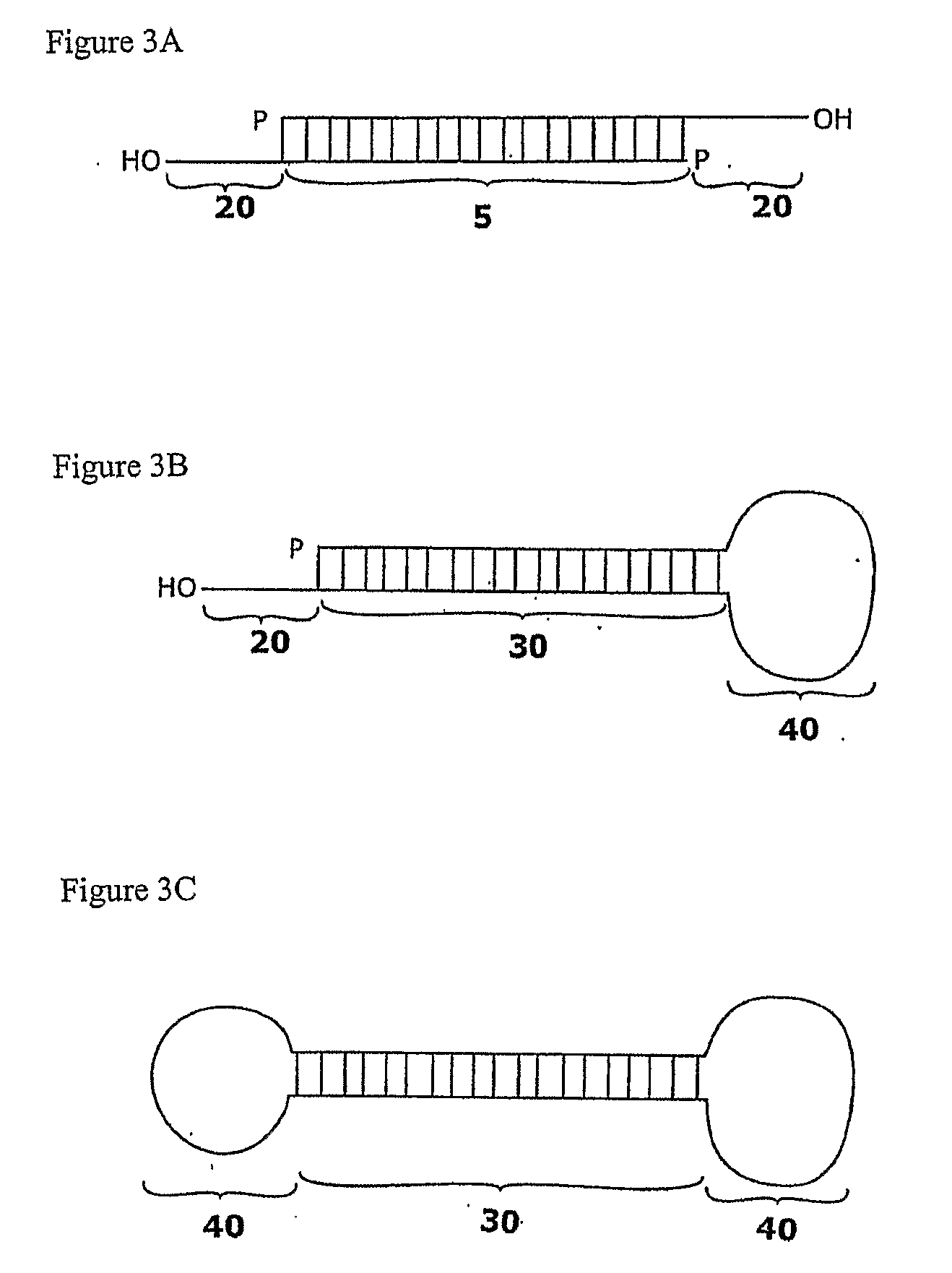
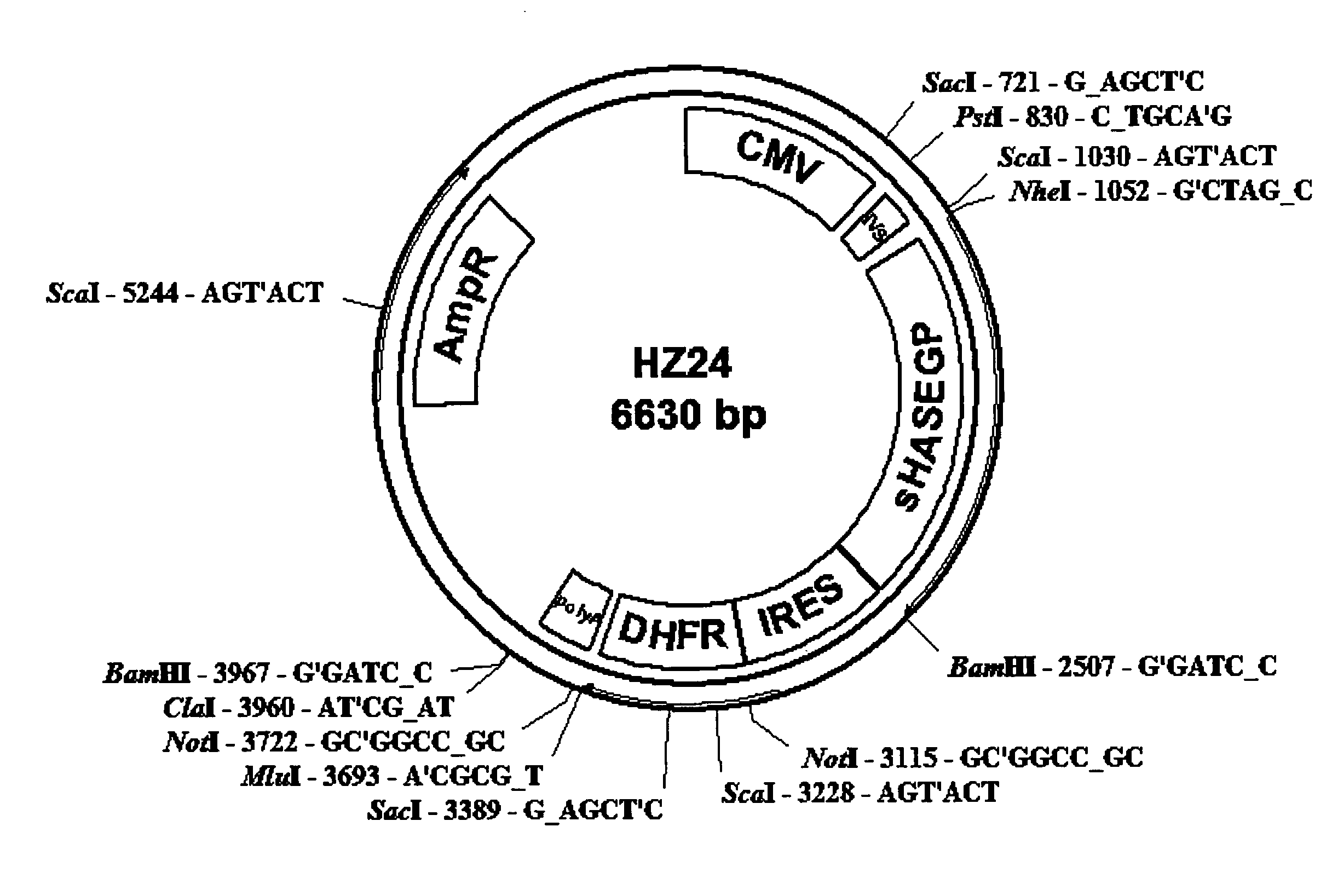
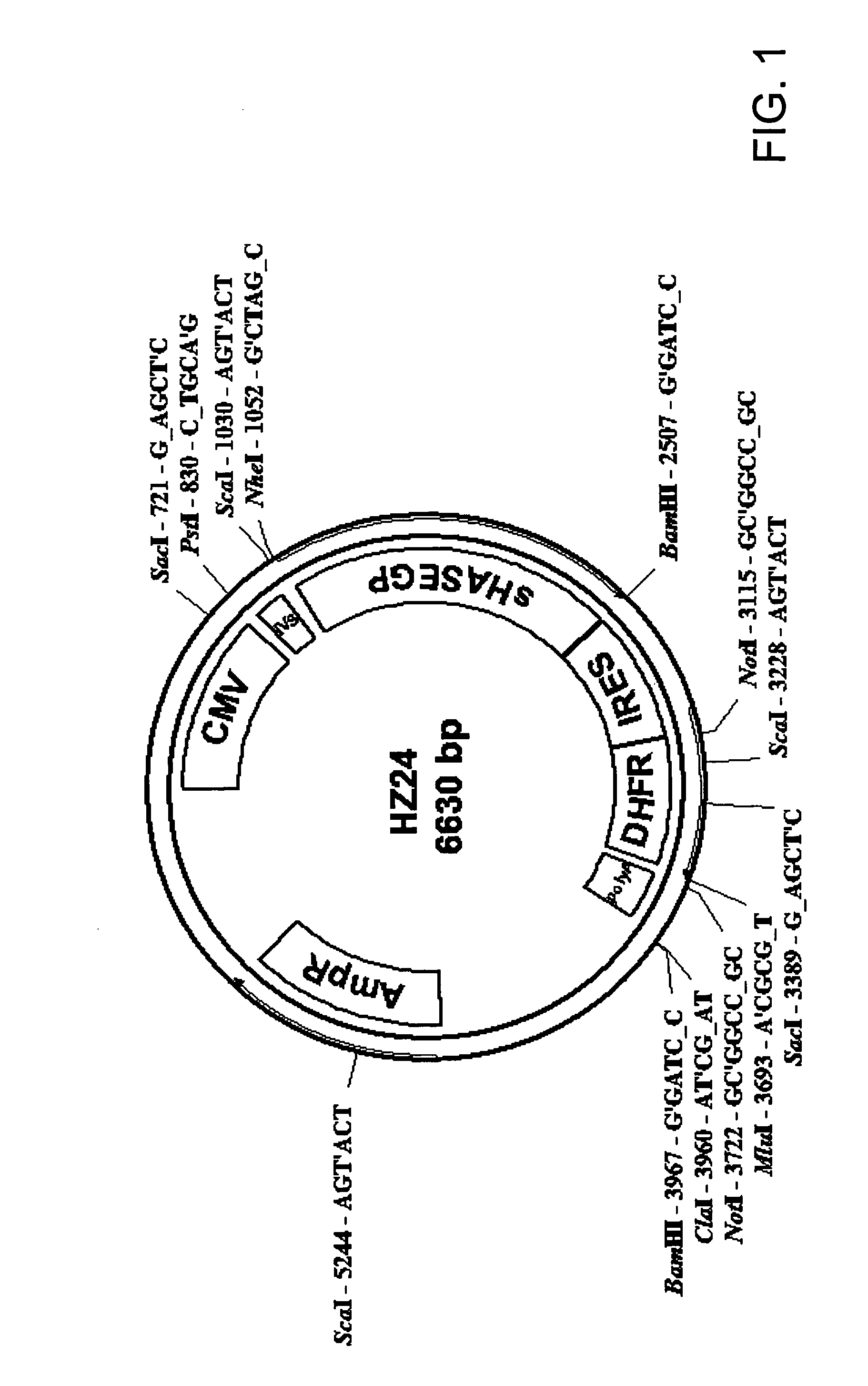
Soluble hyaluronidase glycoprotein (sHASEGP), process for preparing the same, uses and pharmaceutical compositions comprising thereof
ActiveUS20090181013A1Reduce deliveryImprove usabilityAntibacterial agentsOrganic active ingredientsHyaluronidasePathology diagnosis
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGP's), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Synbiotic mixture
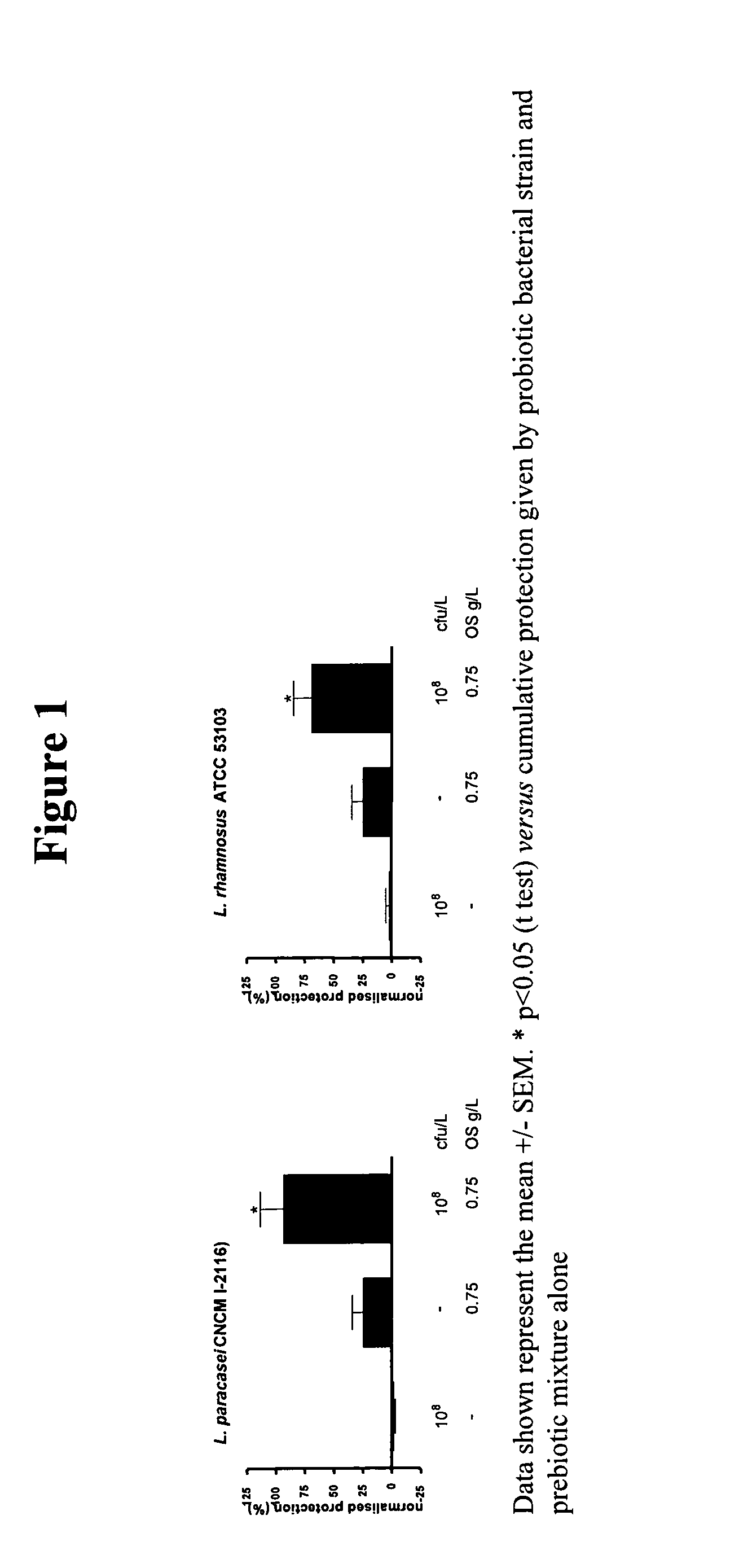
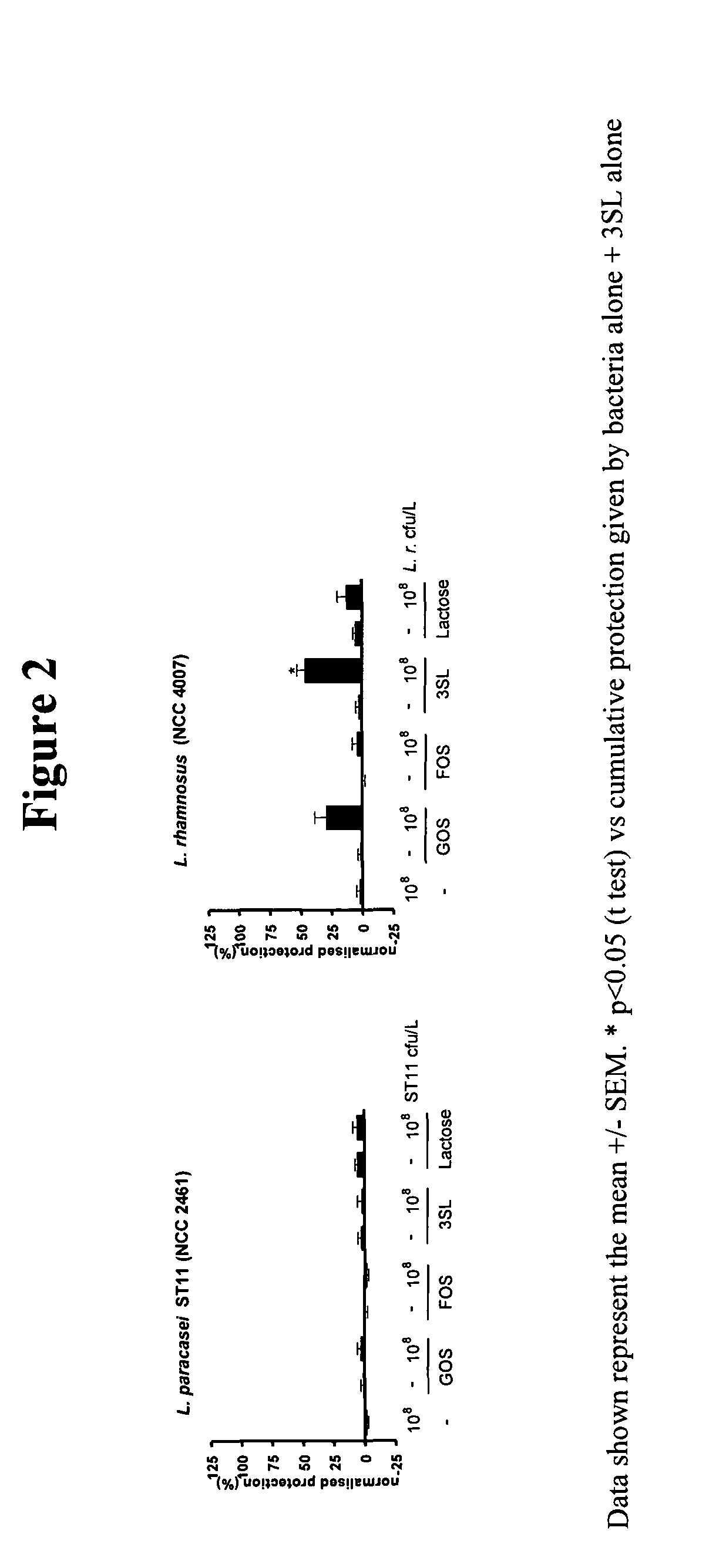
This invention relates to a preparation comprising a probiotic bacterial strain and a prebiotic mixture comprising 5-70 wt % of at least one N-acetylated oligosaccharide selected from the group comprising Ga1NAcα1,3Ga1β1,4G1c and Ga1β1,6Ga1NAcα1,3Ga1β1,4G1c, 20-95 wt % of at least one neutral oligosaccharide selected from the group comprising Ga1β1,6Ga1, Ga1β1,6Ga1β1,4G1c Ga1β1,6Ga1β1,6G1c, Ga1β1,3Ga1β1,3G1c, Ga1β1,3Ga1β1,4G1c, Ga1β 1,6Ga1β 1,6Ga1β 1,4G1c, Ga1β 1,6Ga1β 1,3Ga1β 1,4GIc Ga1β 1,3Ga1β 1,6Ga1β 1,4GIc and Ga1β1,3Ga1β1,3Ga1β1,4G1c and 2-50 wt % of at least one sialylated oligosaccharide selected from the group comprising NeuAcα2,3Ga1β1,4G1c and NeuAcα2,6Ga1β1,4G1c. The invention extends to food products comprising said preparation and to the use of the preparation in the prevention and treatment of infections.
Owner:SOC DES PROD NESTLE SA
Mammallian cell culture process for protein production
InactiveUS20120015438A1Enhance cell viabilityEnhanced final titer and concentrationAnimal cellsCell receptors/surface-antigens/surface-determinantsGrowth phaseSialic acid
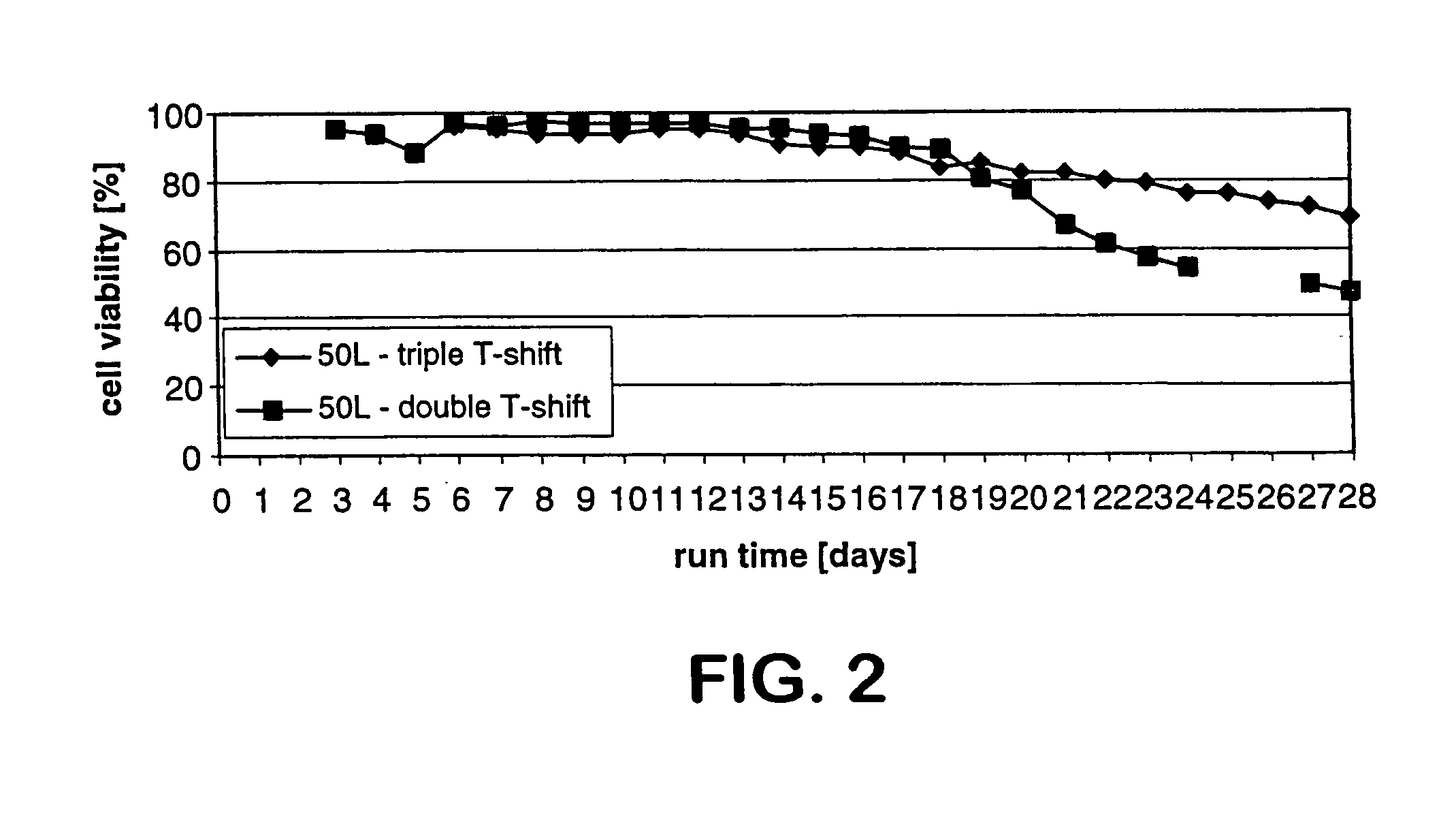
The present invention describes methods and processes for the production of proteins, particularly glycoproteins, by animal cell or mammalian cell culture, preferably, but not limited to, fed-batch cell cultures. In one aspect, the methods comprise at least two temperature shifts performed during the culturing period, in which the temperature is lower at the end of the culturing period than at the time of initial cell culture. Throughout their duration, the culturing processes of the invention involving two or more downward shifts in temperature sustain a high viability of the cultured cells, and can yield an increased end titer of protein product, and a high quality of protein product, as determined, e.g., by sialic acid content of the produced protein. In another aspect, the methods comprise the delayed addition of polyanionic compound during the culturing period. The delayed addition of polyanionic compound sustains a high viability of the cultured cells, and can extend the growth phase, delay the onset of the death phase, and arrest the death phase.
Owner:BRISTOL MYERS SQUIBB CO
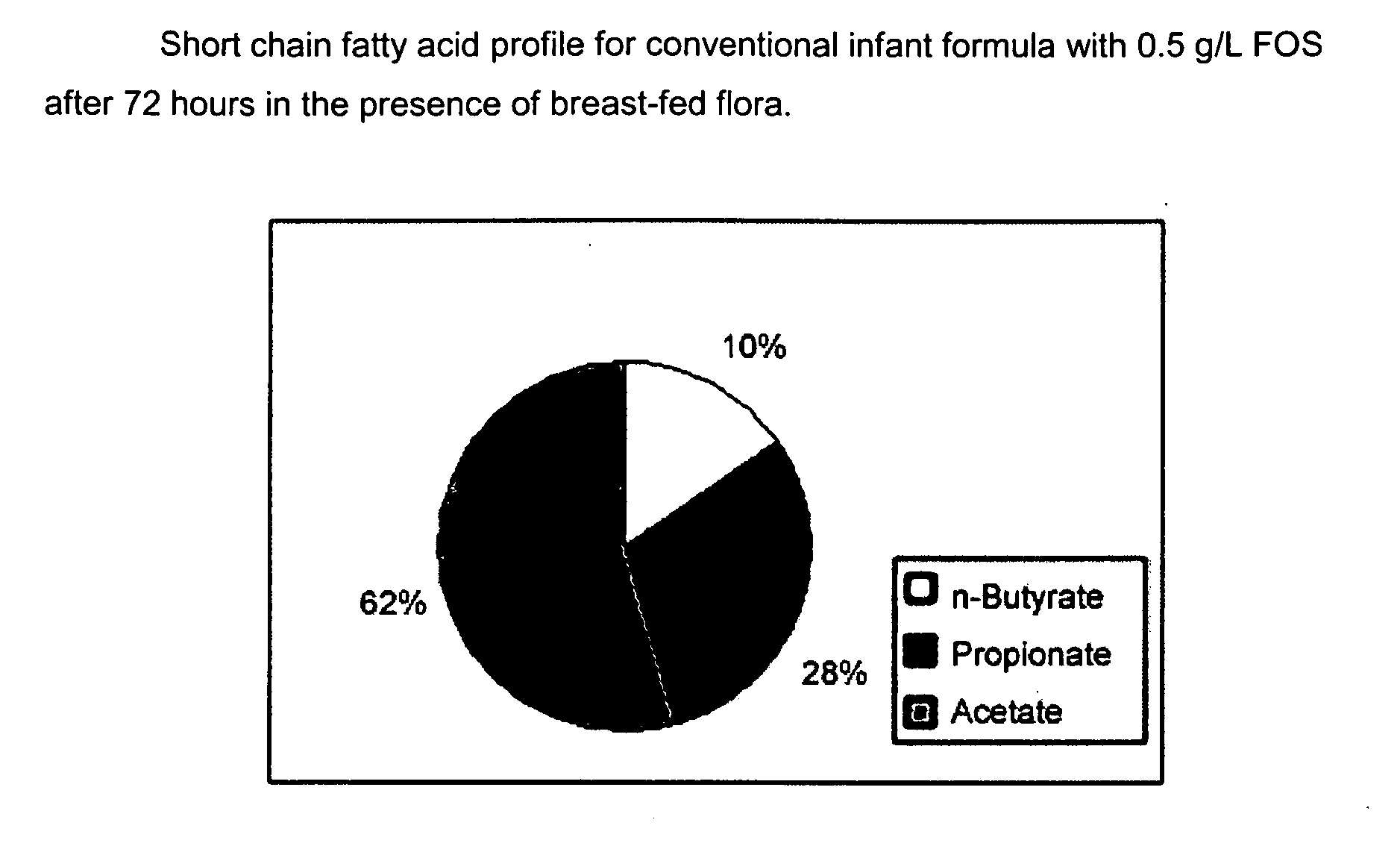
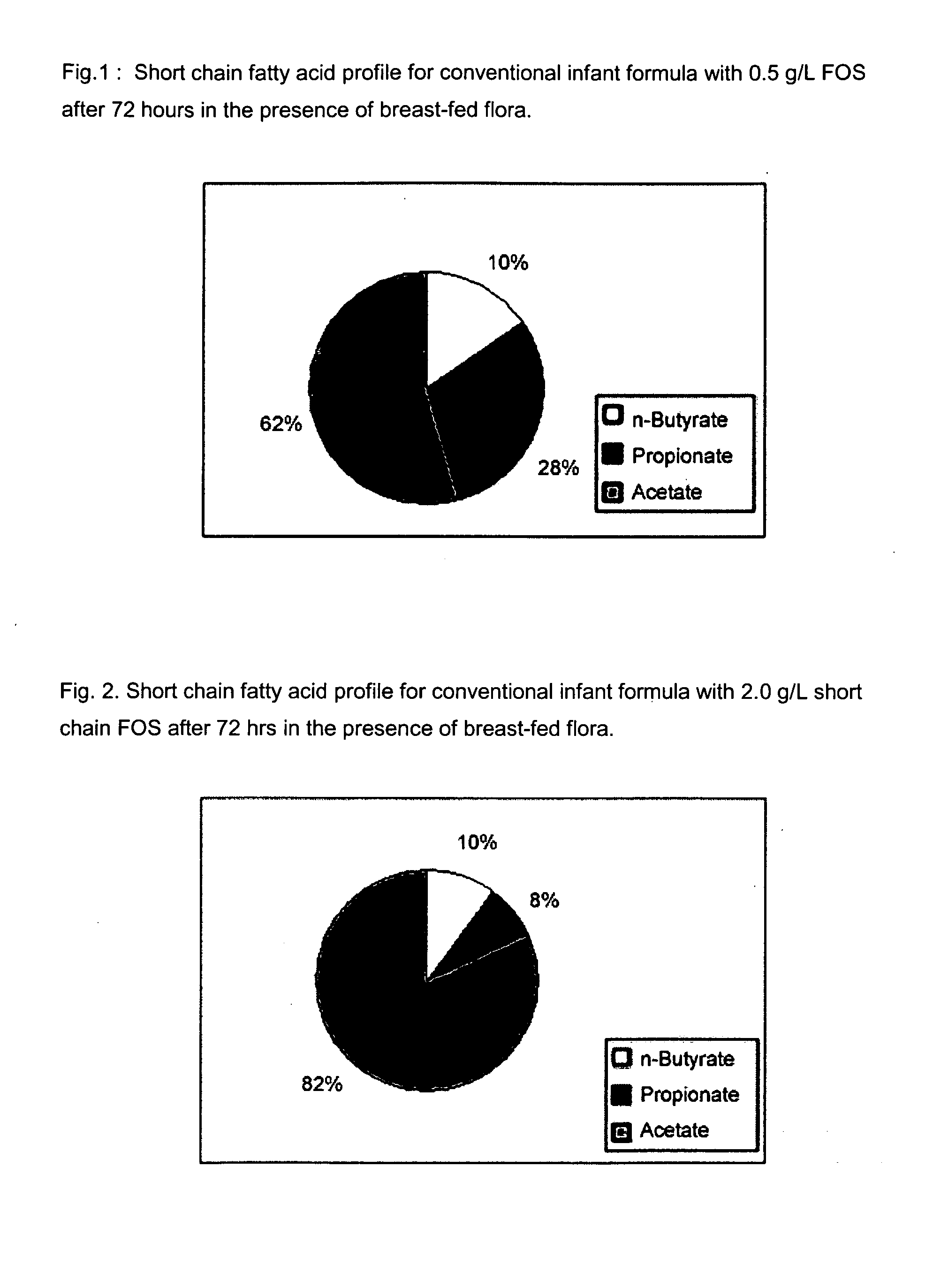
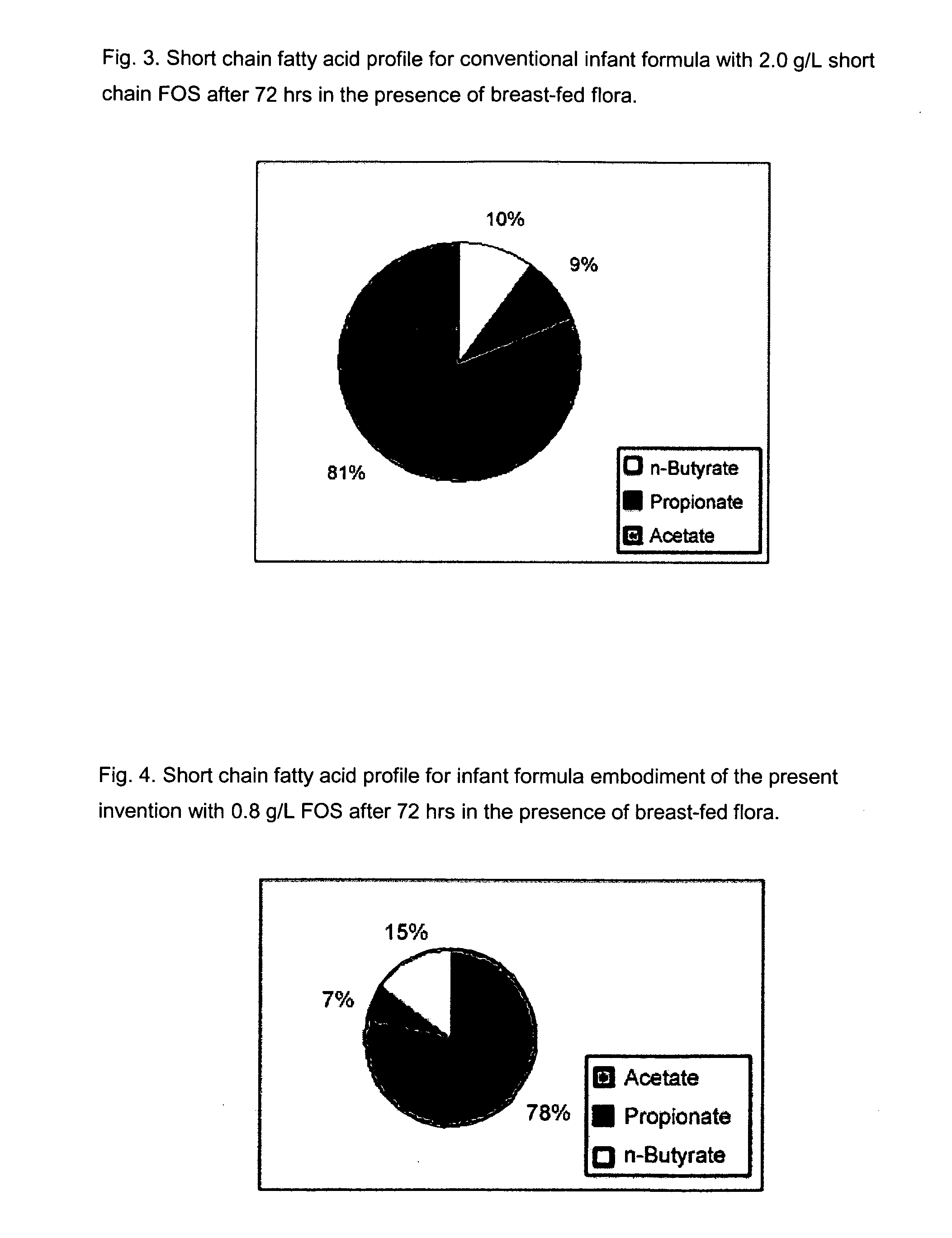
Compositions and methods of formulation for enteral formulas containing sialic acid
A nutritionally complete infant formula containing sialic acid derived from one or a number of nutritionally appropriate sources is described.
Owner:MEAD JOHNSON NUTRITION
Bird's nest oligopeptide composition and preparation method and use thereof
ActiveCN102102119AImprove immunityUnique biological functionCosmetic preparationsPeptide/protein ingredientsBULK ACTIVE INGREDIENTOligopeptide
The invention provides a method for extracting small molecule oligopeptides from bird's nests by hydrolysis, which is to obtain a small molecular nano oligopeptide containing composition by hydrolyzing the bird's nests through the combination of enzymolysis and acid hydrolysis. The bird's nest oligopeptide composition is characterized in that: the molecular weight of the nano oligopeptides mainlyranges from 180 to 1,000D; preferably, the percentage of Gaussian distribution peak area of the nano oligopeptides with a molecular weight ranging from 180 to 1,000D in the composition is over 40 percent. The Cordyceps militarisoligopeptide extract provided by the invention retain other active ingredients such as polysaccharide, bird's nest sialic acid, micro elements and the like. The invention also relates to the use of the nano oligopeptide containing composition extracted from bird's nests in the preparation of medicines, health-care products and cosmetics.
Owner:BEIJING INST OF OLIGO PEPTIDE BIOLOGY
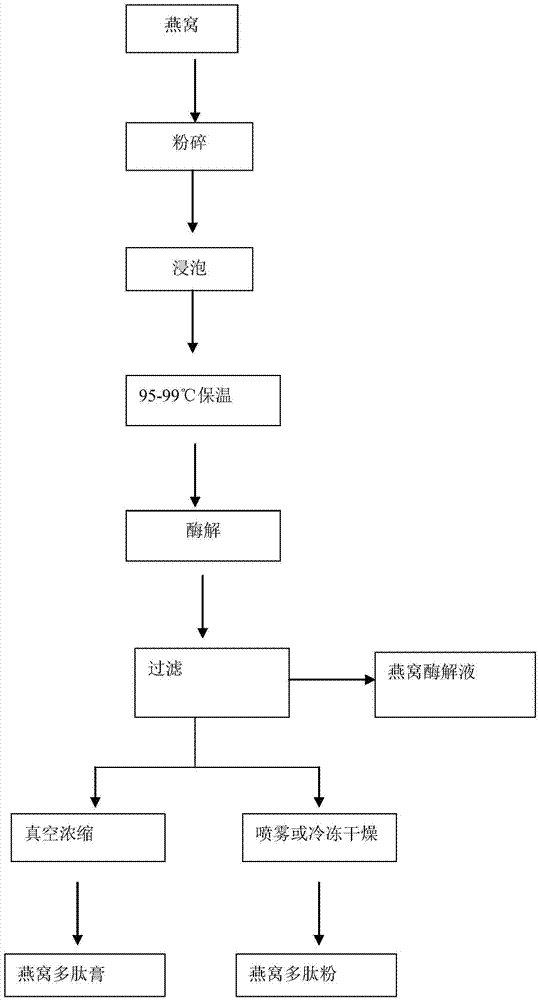
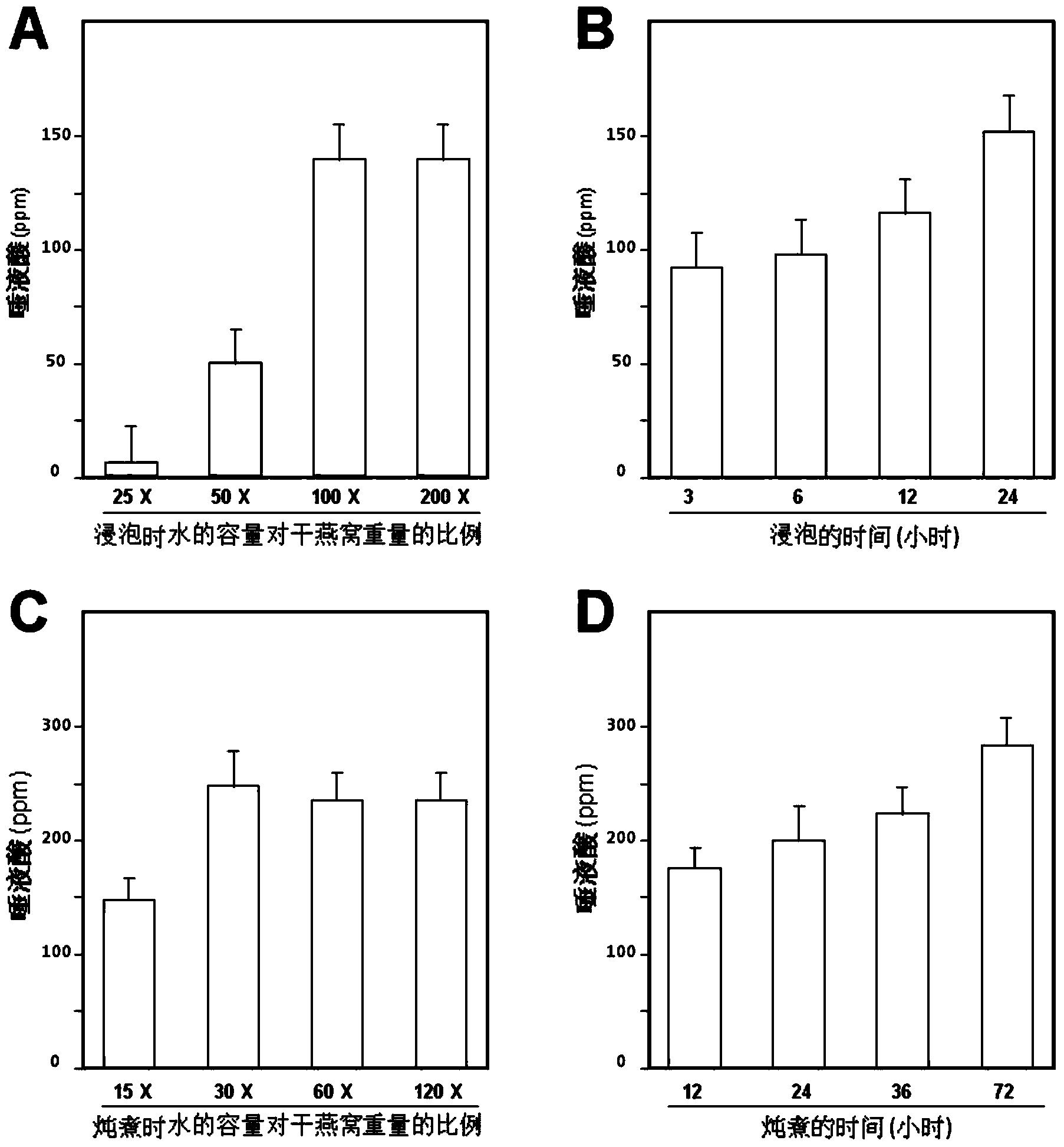
Composite enzymolysis process for cubilose
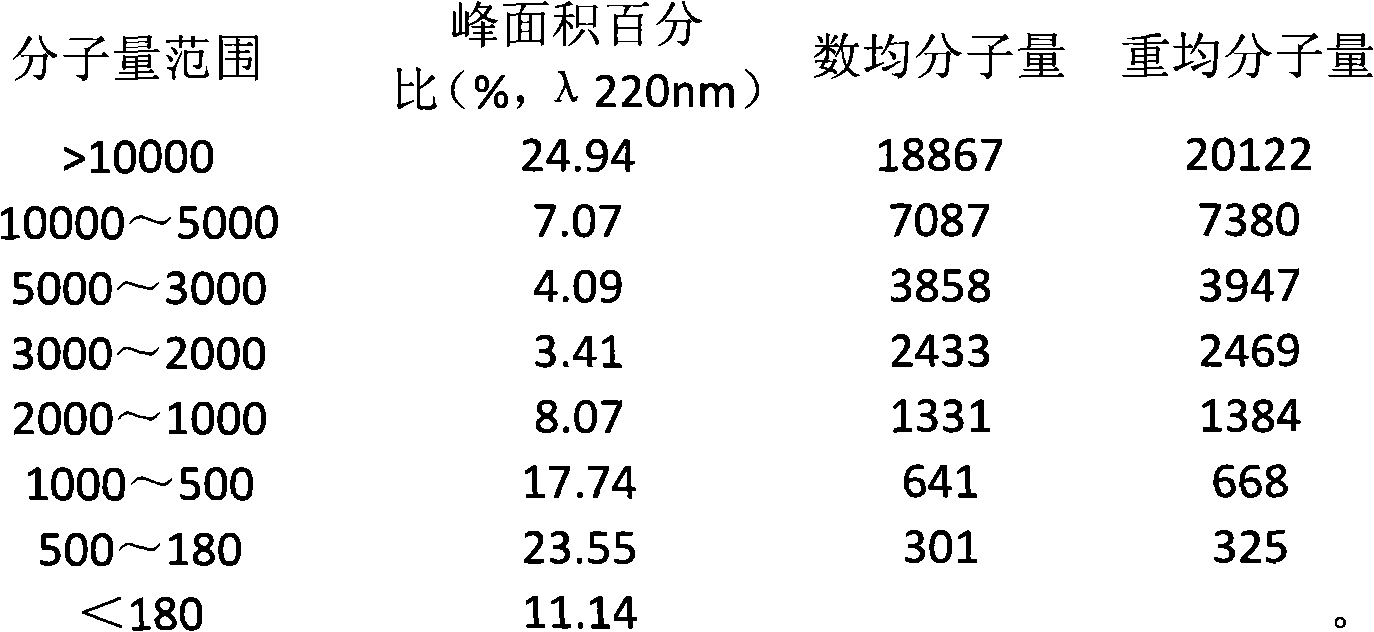
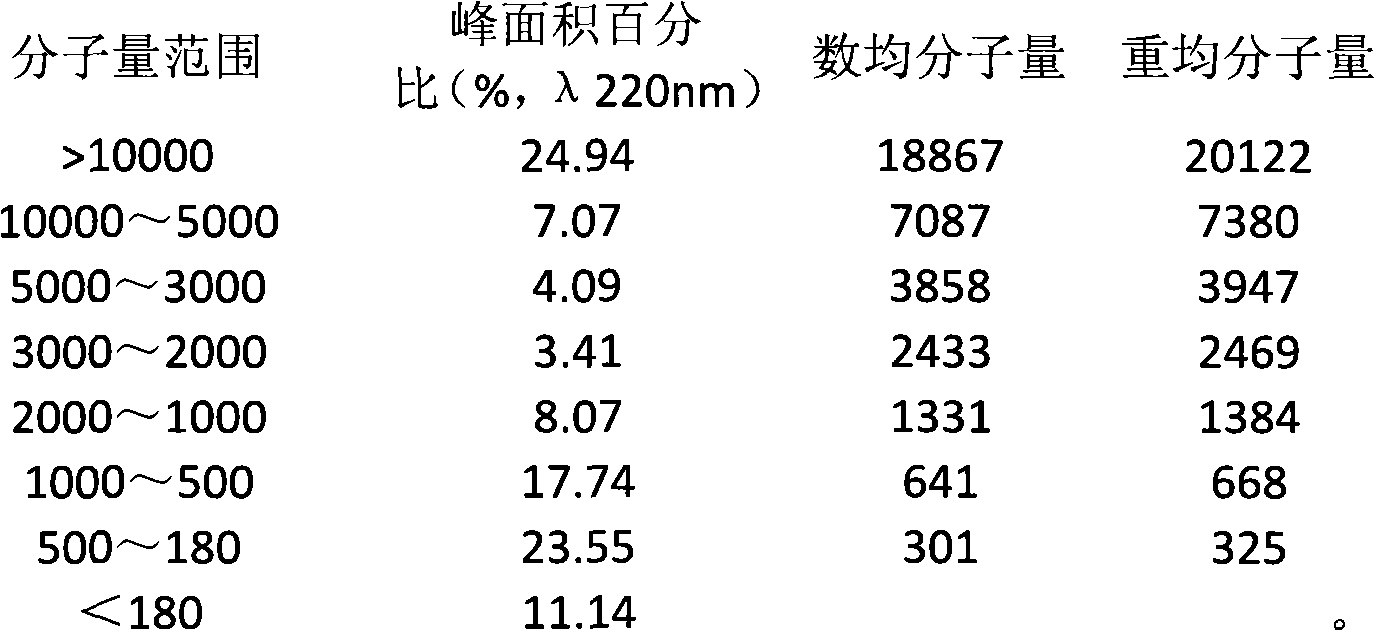
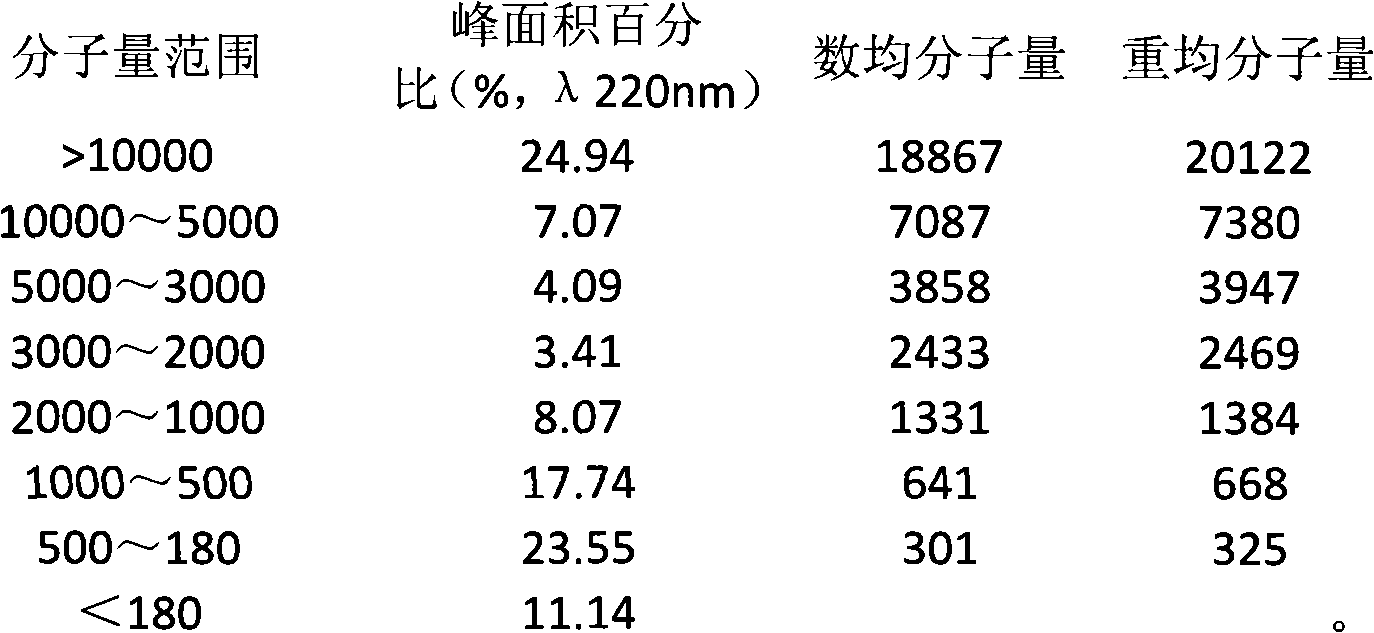
The invention discloses a composite enzymolysis process for cubilose, and relates to the technical field of cubilose processing. The process comprises the following steps: 1 drying cubilose with hot air, crushing, sieving, adding water which is 25 times of dry weight of the cubilose, and soaking for 4 hours; 2 under a mechanical stirring condition, controlling the temperature to be 95-99 DEG C and carrying out heat preservation for 60 minutes; 3 cooling to 50-60 DEG C, adding 0.3% of animal hydrolytic enzyme, 0.3% of neutral protease and 0.15% of flavor enzyme on the basis of the dry weight of the cubilose, naturally adjusting the pH value, and carrying out enzymolysis for 6 hours; 4 heating to 85-90 DEG C again, keeping for 20-30 minutes, and carrying out enzyme inactivation; and 5 filtering and removing insoluble impurities, and carrying out vacuum concentration or spray or freeze drying. The composite enzymolysis process is simple; the yield is about 80%; the characteristic index, namely sialic acid content of the cubilose is 10.5%; the biological activity is reserved; the total bacterial count is 460cfu / g, and accords with the hygienic requirements of food and cosmetics; and the cubilose is good in water solubility, has a protein fragrance, and is free of a bitter taste.
Owner:燕之初健康美(厦门)食品有限公司
Production of galactosylated glycoproteins in lower eukaryotes
InactiveUS7795002B2Reducing and eliminating activityEfficient workSugar derivativesPeptide/protein ingredientsUDP galactoseSialic acid
The present invention provides a novel lower eukaryotic host cell producing human-like glycoproteins characterized as having a terminal β-galactose residue and essentially lacking fucose and sialic acid residues. The present invention also provides a method for catalyzing the transfer of a galactose residue from UDP-galactose onto an acceptor substrate in a recombinant lower eukaryotic host cell, which can be used as a therapeutic glycoprotein.
Owner:GLYCOFI
Immunogenic composition and method of developing a vaccine based on factor H binding sites
InactiveUS20050112139A1Rapid decrease in viremiaEasy to copyVirus peptidesPharmaceutical delivery mechanismBinding siteAutoimmune responses
An immunogenic composition able to provide an immune response to Human Complement Factor H binding on one or more HIV glycoproteins is disclosed, which is characterized by at least one binding site epitope of the glycoproteins. Such immunogenic composition wherein the glycoprotein comprises gp120, gp41, or both glycoproteins gp120 and gp41 is hereby disclosed. Sialic acid is removed to enhance immune recognition of the composition and to impair Factor H binding. A medication having an inhibitive action on autoimmune response by specific inhibition of the cleavage of C3b by Factor H into inactive cell fragments.
Owner:KARP NELSON M
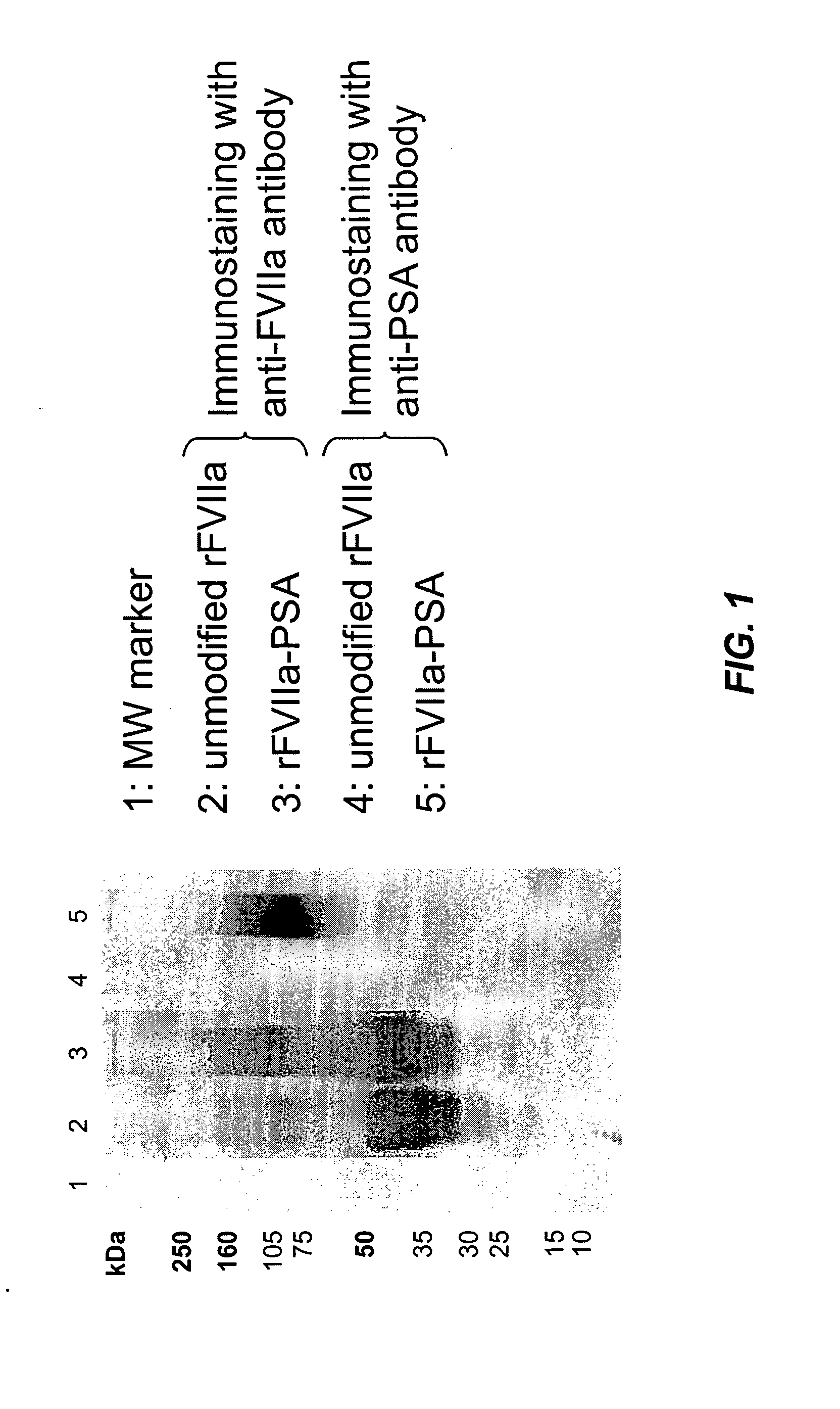
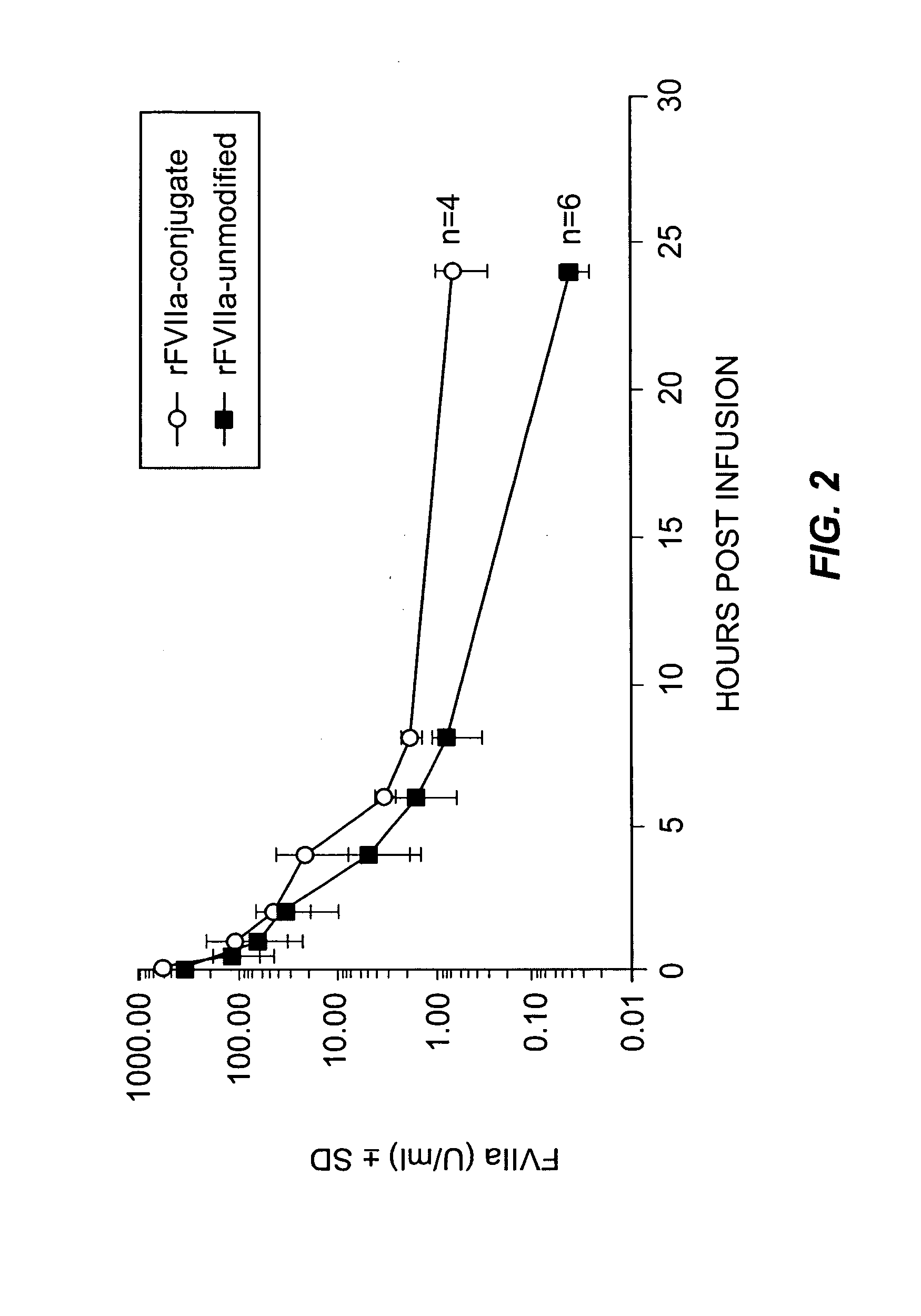
Factor VIIa-Polysialic Acid Conjugate Having Prolonged In Vivo Half-Life
ActiveUS20080221032A1Control bleedingProlong half-life in vivoPeptide/protein ingredientsPeptide preparation methodsFactor VIIaHalf-life
The present invention relates to a proteinaceous construct comprising plasmatic or recombinant factor VIIa (FVIIa) or biologically active derivatives thereof, which are bound to a carbohydrate moiety comprising 1-4 sialic acid units, wherein the in vivo half-life of the proteinaceous construct is substantially prolonged in the blood of a mammal, as compared to the in vivo half-life of a FVIIa molecule not bound to a carbohydrate moiety. The invention also provides a method for controlling bleeding in a mammal having a bleeding disorder due to functional defects or deficiencies of FVIIa, FVIII, or FIX. The invention also provides a method for controlling bleeding in a mammal during surgery or trauma.
Owner:TAKEDA PHARMA CO LTD
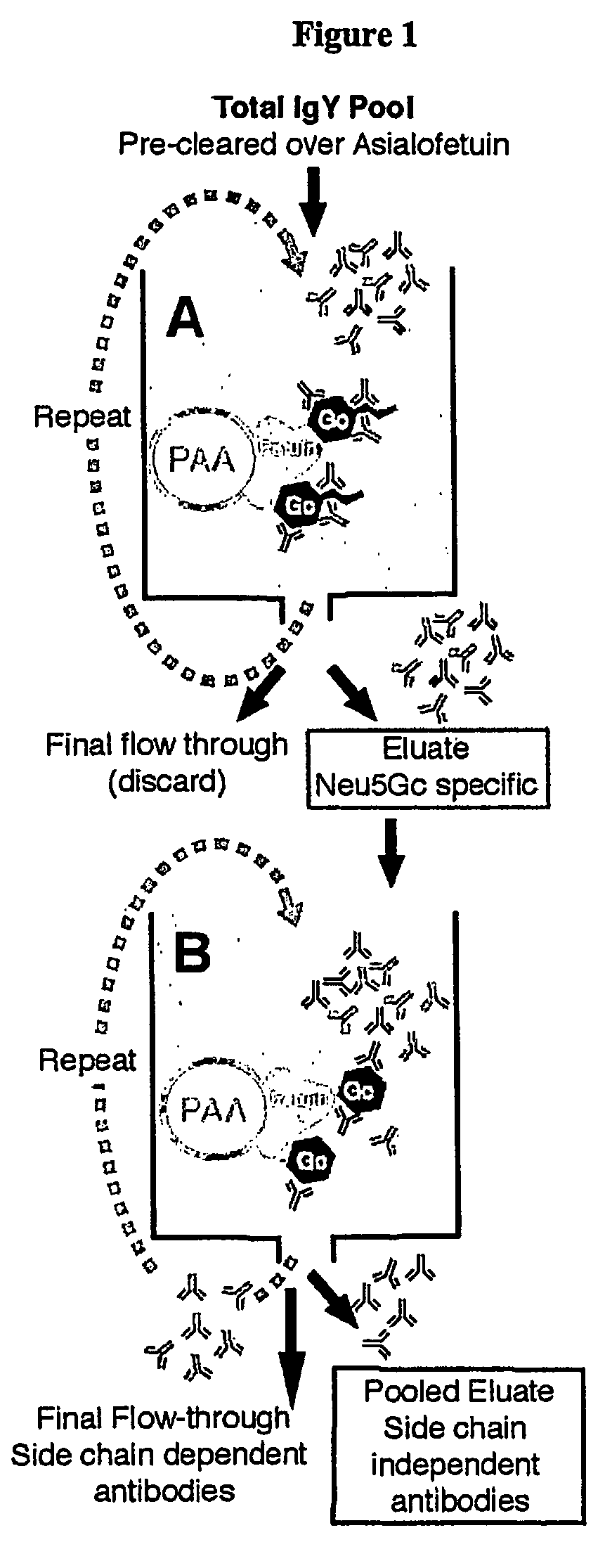
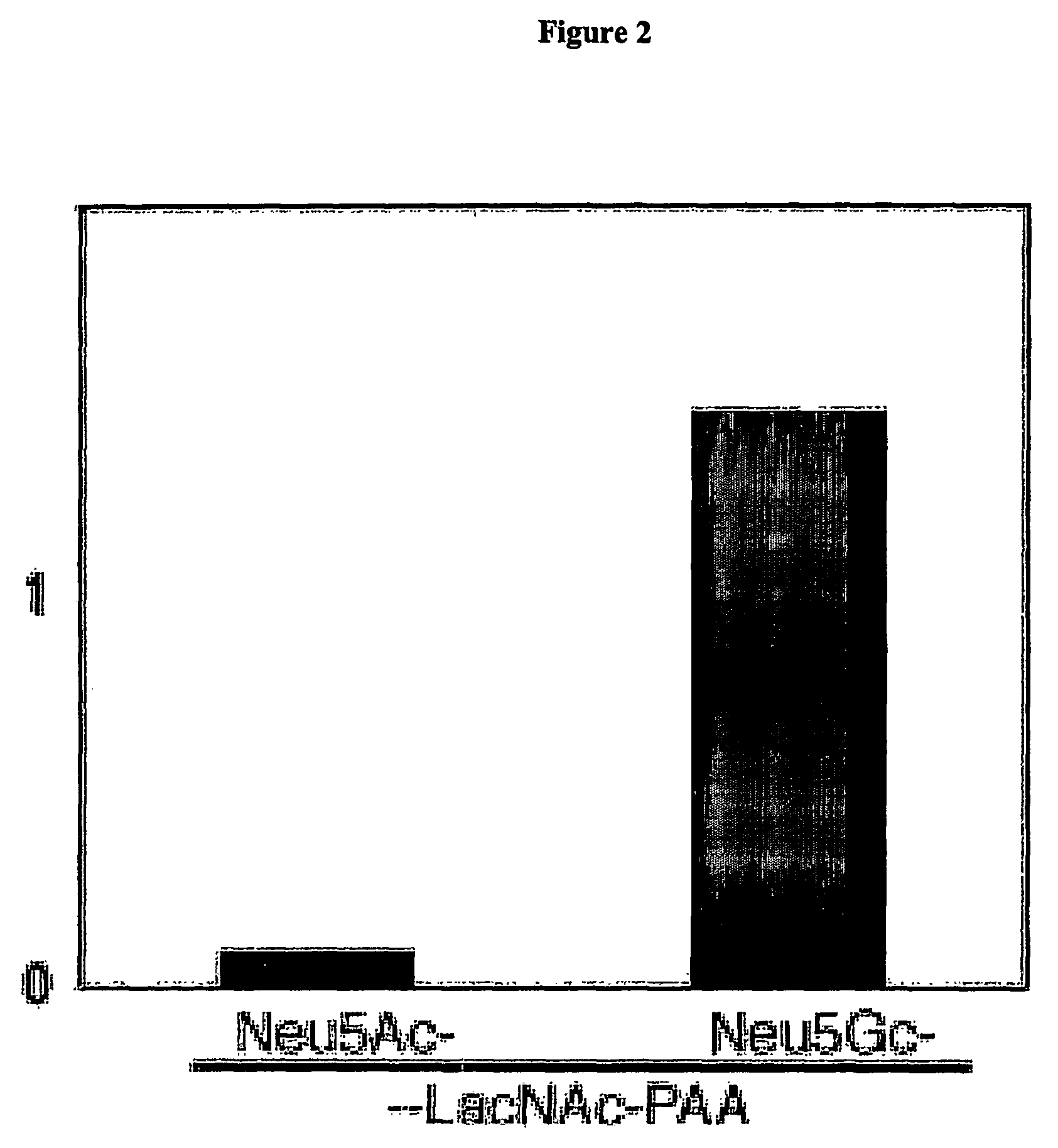
Methods for detecting and analyzing N-glycolylneuraminic acid (Neu5Gc) in biological materials
InactiveUS7682794B2Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesSialic acid
The present application is in the field of sialic acid chemistry, metabolism and antigenicity. More particularly, the present invention relates to the detection and analysis of the non-human sialic acid, N-glycolylneuraminic acid (Neu5Gc) in bio-logical materials, such as food and clinical specimens. Such detection and analysis is facilitated by the use of Neu5Gc specific antibodies. The present invention also relates to the detection of anti Neu5Gc antibodies in clinical samples, as well as the production of anti-Neu5Gc specific antibodies.
Owner:RGT UNIV OF CALIFORNIA
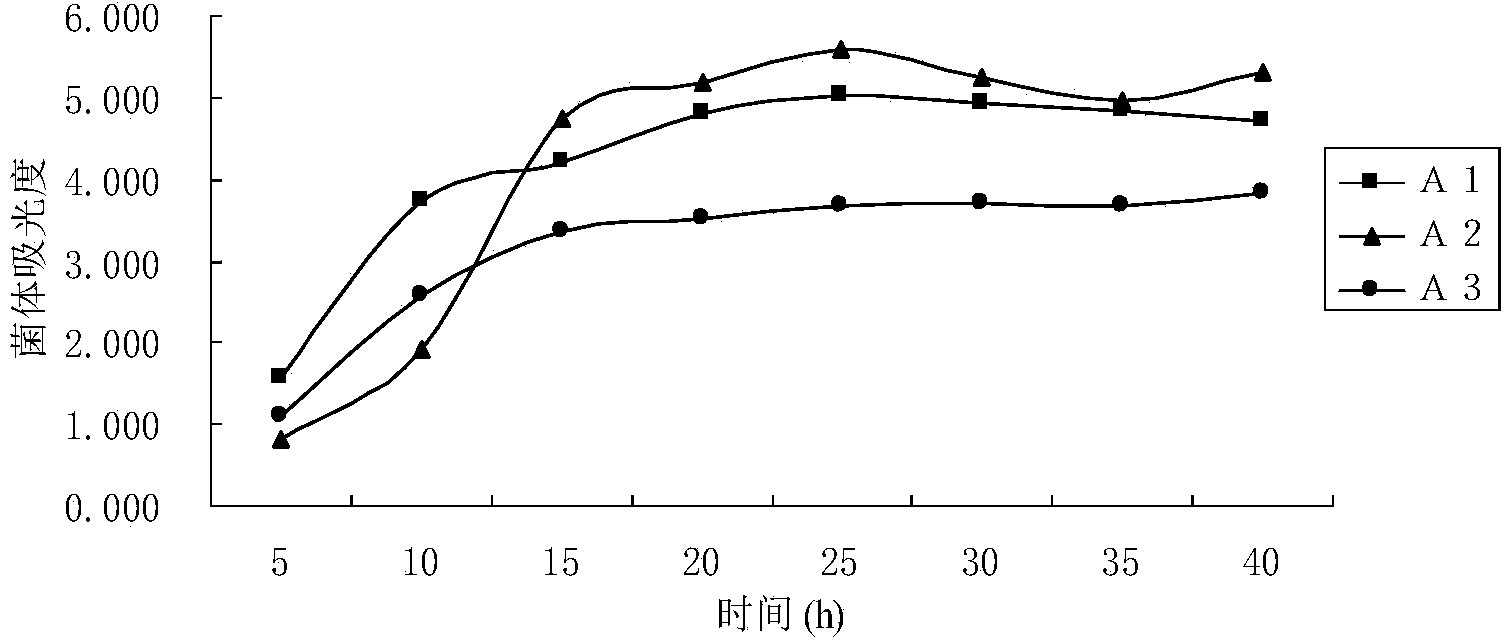
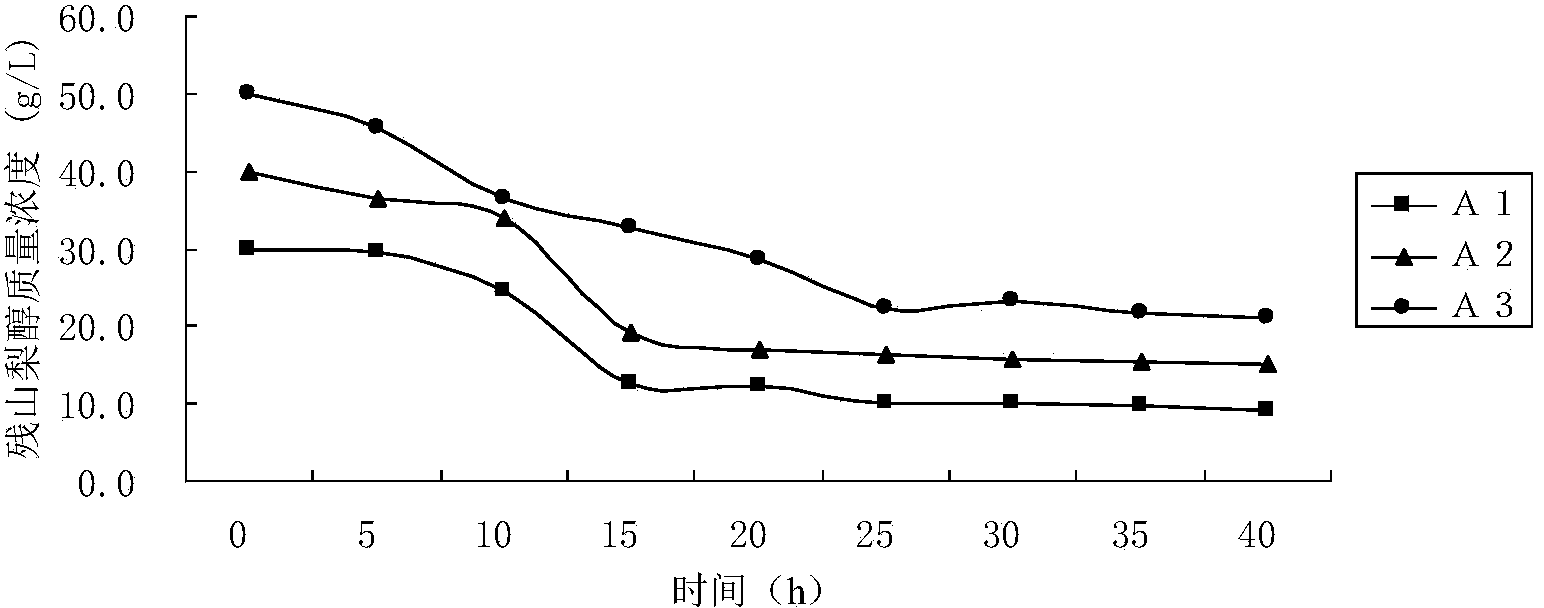
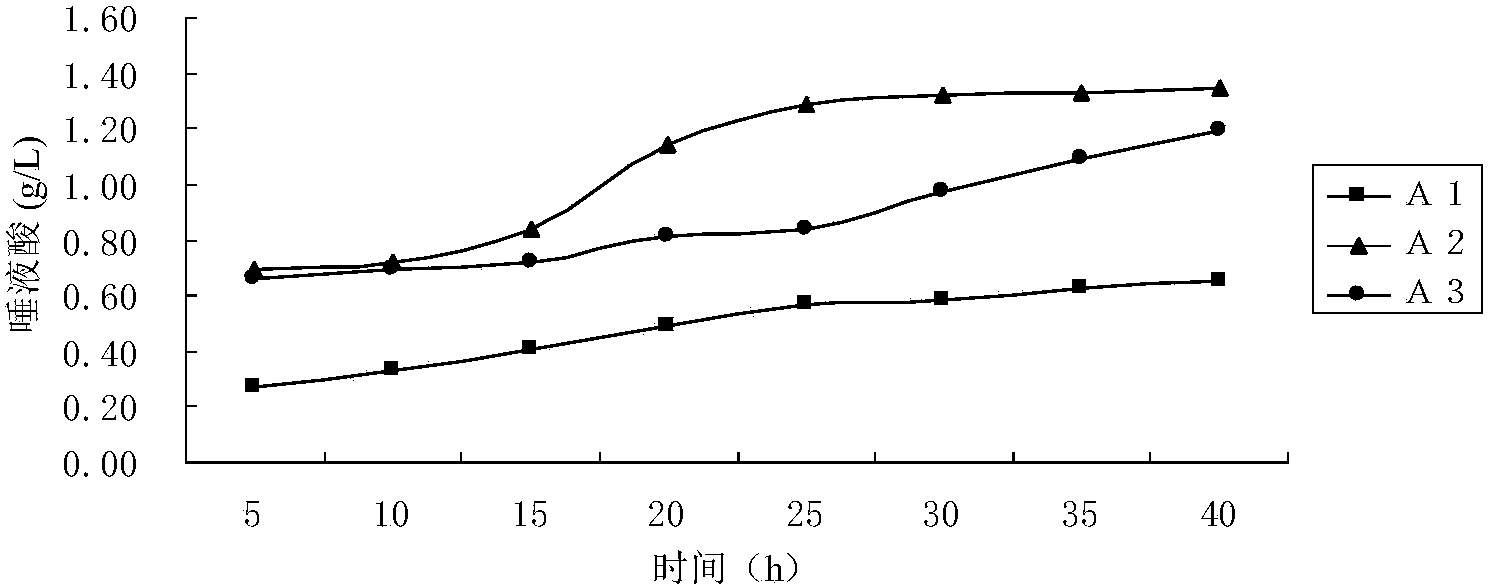
Method of increasing the salivary sialic acid content in a mammal
InactiveUS20060246146A1Increasing salivary sialic acid contentIncrease in sialic acid contentBiocideVitamin food ingredientsSialic acidMammal
A method of increasing the salivary sialic acid content of a mammal is described which involves administering to the mammal casein glycomacropeptide in an amount sufficient to increase the salivary sialic acid content of the mammal.
Owner:BRISTOL MYERS SQUIBB CO +1
Preparation method of 3'-sialic acid lactose
InactiveCN102154163AChange the status quo of less ingredientsEfficient additionBacteriaMicroorganism based processesEscherichia coliSialic acid
The invention provides a preparation method of 3'-sialic acid lactose, belonging to the technical field of biological engineering. The preparation method comprises the following steps of: taking colibacillus CCTCC (colibacillus China center for type culture collection) NO: M208088 as a starting strain, adding a fermentation medium into a fermentation tank, fermenting for 2-6h, then adding a fed batch culture medium, and adding lactose in a flowing way at the mid-later period of the fermentation; and centrifuging the fermentation liquid to obtain liquid supernatant 1 and a thallus, diluting the centrifuged thallus by adding water and splitting in a heating way, recentrifuging to obtain liquid supernatant 2, merging the liquid supernatant 1 and the liquid supernatant 2, and absorbing, desorbing and vacuum drying by ion exchange resin to obtain the 3'-sialic acid lactose. A mass of the 3'-sialic acid lactose can be prepared by the CCTCC NO: M208088. The 3'-sialic acid lactose can change the current situation that the sialyloligosaccharide component is less in exogenous adding substances in conventional baby milk, and can provide the guarantee to add more efficient sialic acid trophic factors into the baby milk.
Owner:朱莉
Composition for the prevention and the treatment of helicobacter pylori infection
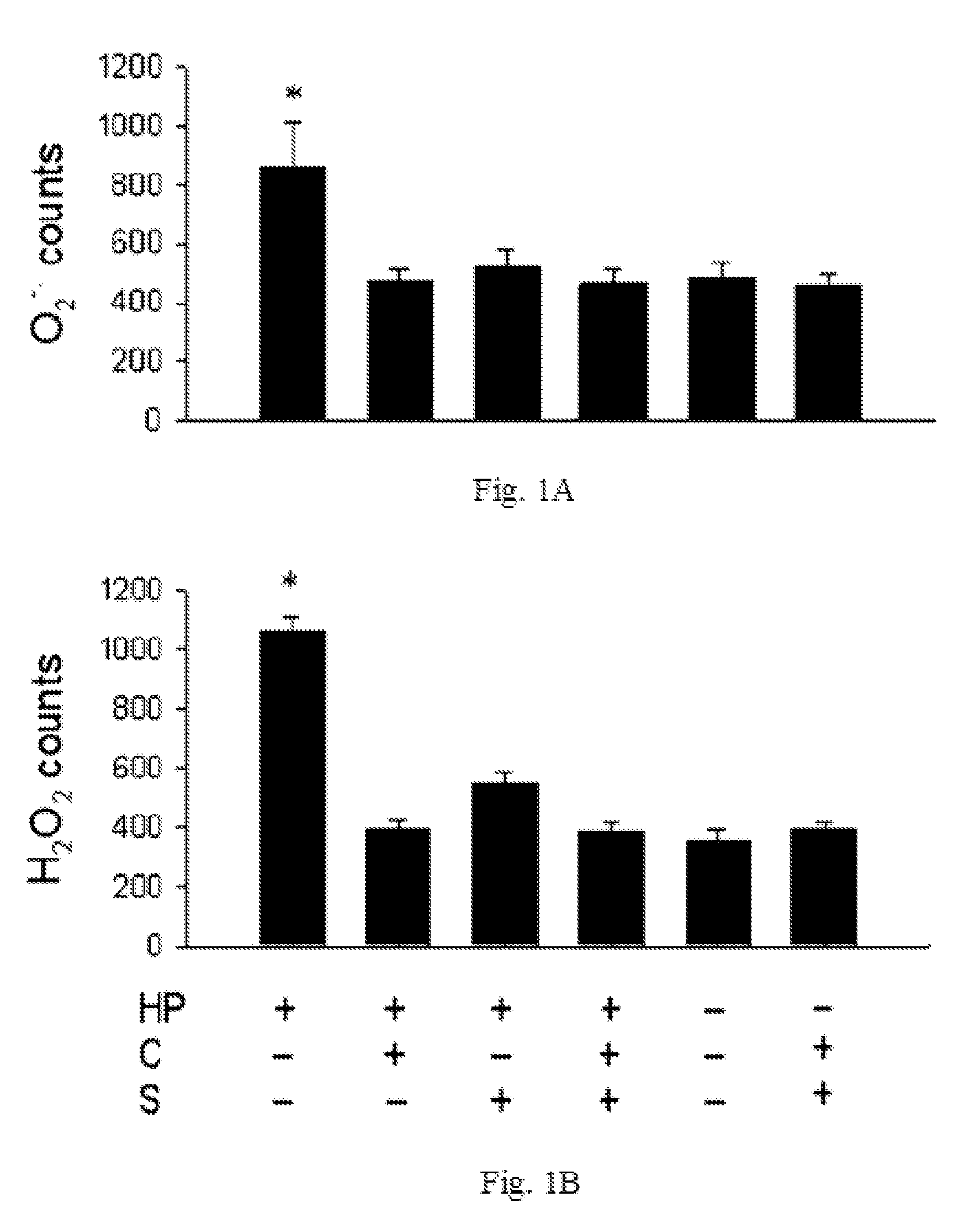
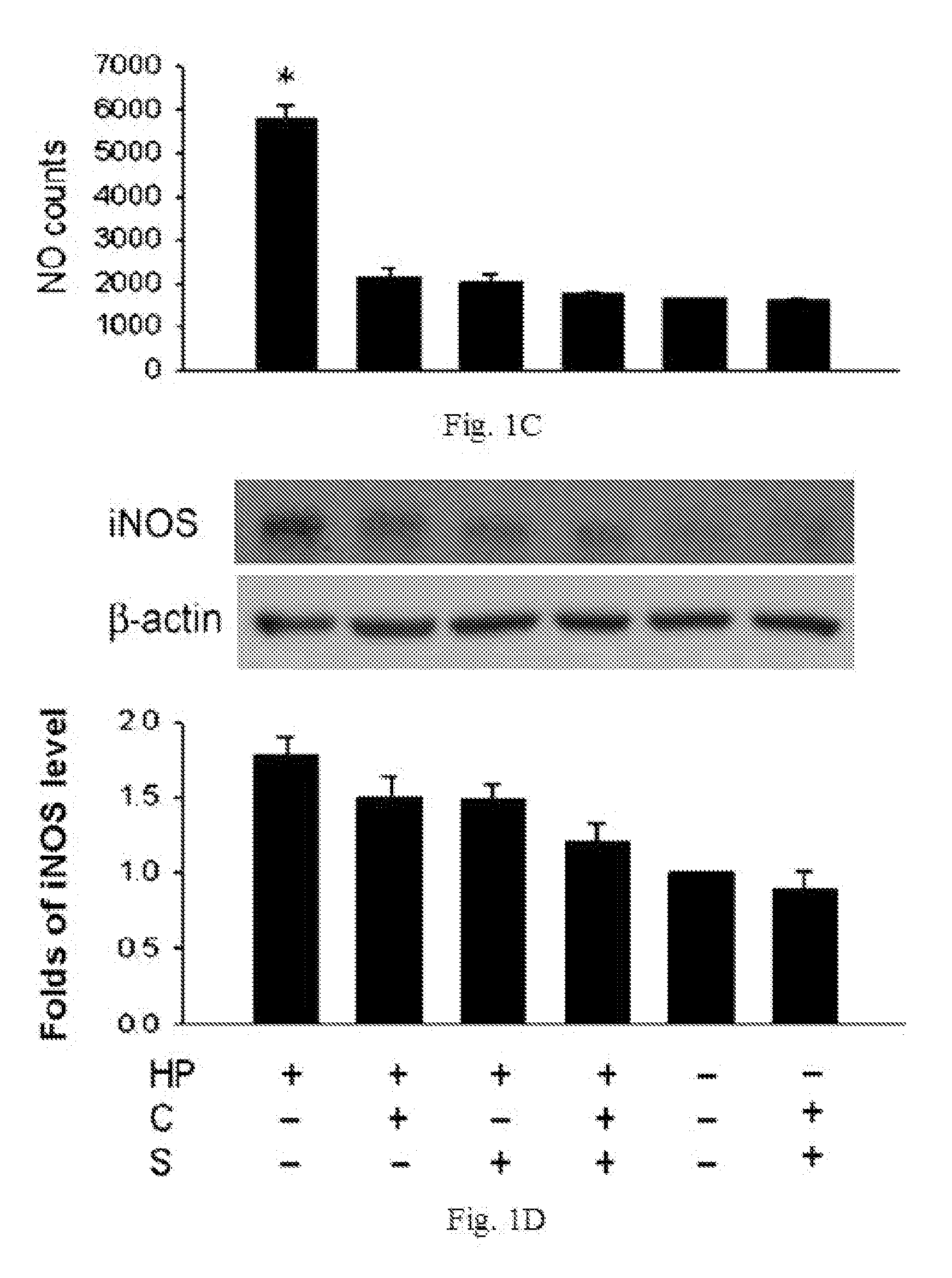
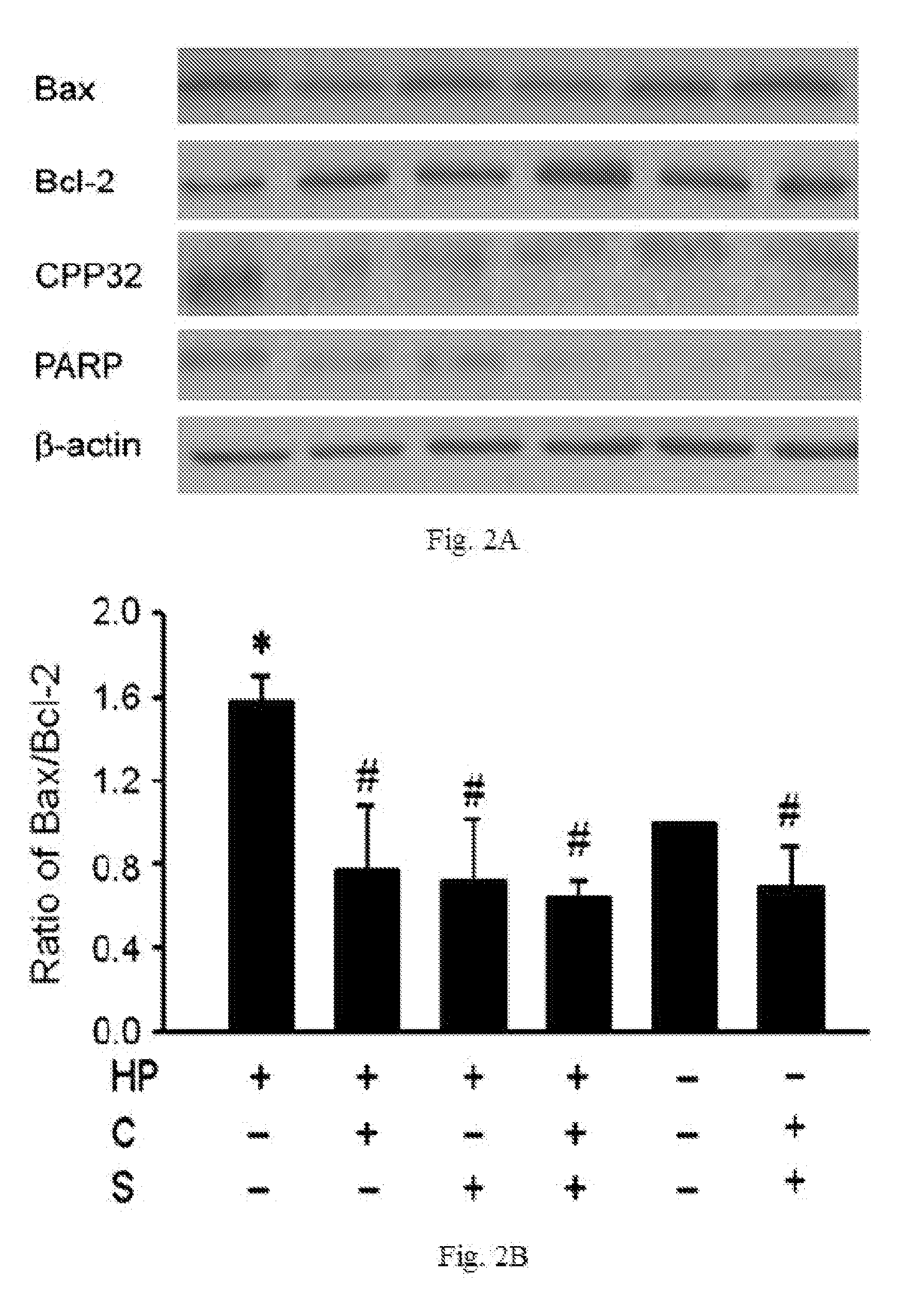
InactiveUS20100298244A1Decrease apoptotic damageReduce productionAntibacterial agentsBiocideSialic acidMedicine
The disclosed item is a composition comprising catechins and sialic acid to provide an effective prevention and treatment for Helicobacter Pylori infection. This composition inhibits growth of Helicobacter pylori, and can increase the antioxidant activity of gastric mucosal cells through reducing the production of O2.−, H2O2 and NO, and decreasing the expression of inducible nitric oxide synthase (iNOS) in cells. The apoptotic damage of stomach caused by Helicobacter pylori was decreased through up-regulation of autophagy to enhance the defensive ability of gastric epithelial cells toward Helicobacter pylori. Therefore the composition can be used in the treatment or prevention on damages caused by Helicobacter pylori infection.
Owner:NAT TAIWAN UNIV
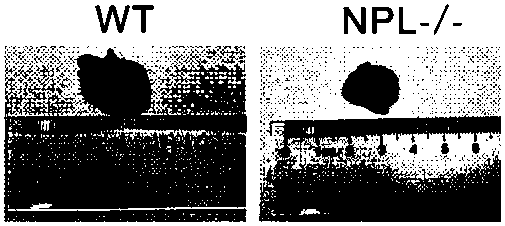
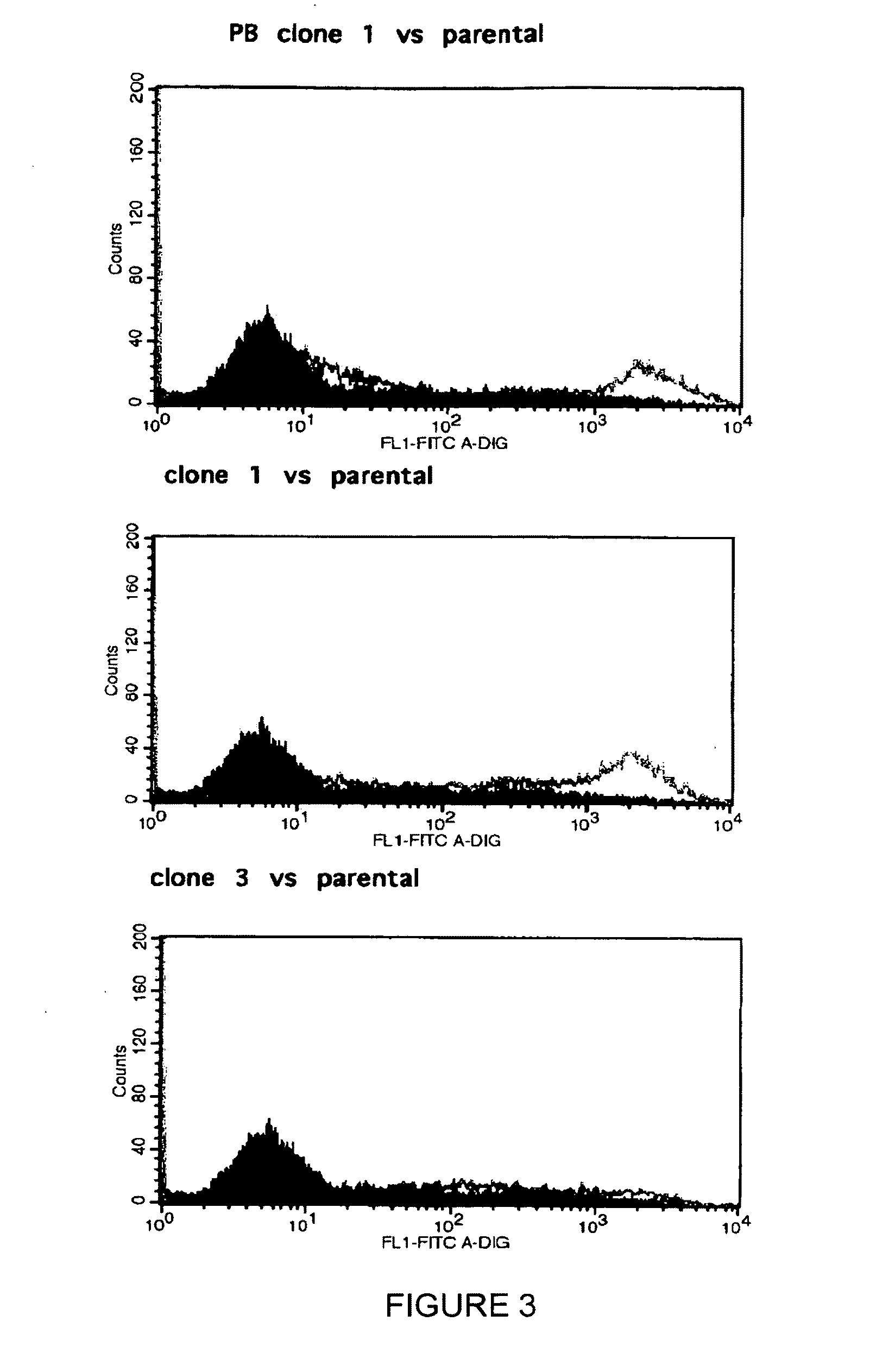
Construction method for sialidase gene knockout mouse model and application thereof
InactiveCN108486154AShort experiment cycleReduce experiment costVector-based foreign material introductionGlycosylasesCancer cellWilms' tumor
The invention relates to the field of biotechnologies, and provides a construction method for a sialidase gene knockout mouse model and application thereof. An in vitro and vivo targeted mouse genomeNPL is used for genetically recombining a CRISPR knockout plasmid vector, and a diploid double-knockout NPL mouse knockout model is successfully constructed through an oosperm microinjection technology. The NPL has the important function in a pathophysiological process of a body. Vicious transformation of a cancer cell is always along with overexpression of sialic acid. Now, the overexpression ofthe sialic acid has become one of important markers of the malignant transformation and transferring of a tumor. The model is capable of constructing a new research platform for researching a processof cell canceration, beneficial to a key step of researching generation and transferring of the tumor, and providing a foundation for the research and development of a novel anti-tumor drug.
Owner:FUZHOU UNIV
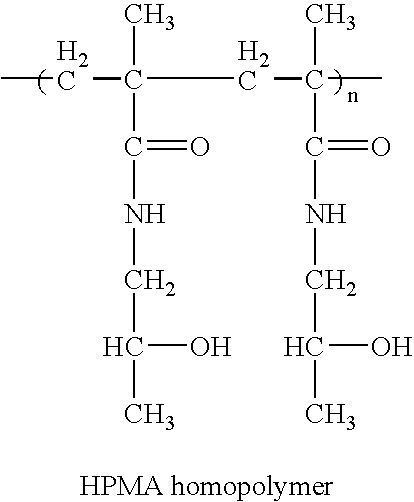
Hpma-polyamine conjugates and uses therefore
InactiveUS20060014695A1Prevent proliferationStimulate immune responsePeptide/protein ingredientsGenetic material ingredientsSomatostatin analogTreatment effect
The inventions provide compositions and methods for nucleic acid delivery comprising IIPMA conjugated to a polyamine. These compositions have the benefit of the steric hindrance of HPMA and the nucleic acid binding capability of a polyamine. Useful polyamines for this purpose include spermine, spermidine and their analogues, and DFMO. These polyamines have the ability not only to bind nucleic acids, but also have anti-cancer effects themselves. The compounds provided can also include ligand binding domains, such as vascular endothelial growth factors, somatostatin and somatostatin analogs, transferring, melanotropin, ApoE and ApoE peptides, von Willebrand's factor and von Willebrand's factor peptides, adenoviral fiber protein and adenoviral fiber protein peptides, PD 1 and PD 1 peptides, EGF and EGF peptides, RGD peptides, CCK peptides, antibody and antibody fragments, folate, pyridoxyl and sialyl-LewisX and chemical analogs. Methods for using these compositions to achieve a therapeutic effect, including for vaccination, are also provided.
Owner:UNIV OF MARYLAND
Fermentation production method of sialic acid
The invention discloses a fermentation production method of sialic acid and belongs to the technical field of bioengineering. The method comprises the following steps: adding a seed liquid into a fermentation tank containing a sterilizing fermentation culture medium; continuously fermenting to produce fermentation liquid containing polysialic acid in a way of replenishing feeding liquid and a hydrogen dioxide solution; then carrying out centrifugal separation, ethanol precipitation and filtration to obtain refined polysialic acid; then, carrying out acidolysis and crystallization on the polysialic acid; and finally, washing and freeze-drying to obtain sialic acid powder. The output of the polysialic acid produced by the method provided by the invention can reach 12.96g / L, the already reported maximum content of the polysialic acid is improved by 87.28%, the hydrolysis rate of polysialic acid is 95%, the content of purified sialic acid reaches 10.01g / L and the purity reaches 94.80%. The method is suitable for industrial promotion and application.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Method for identifying quality of bird's nest
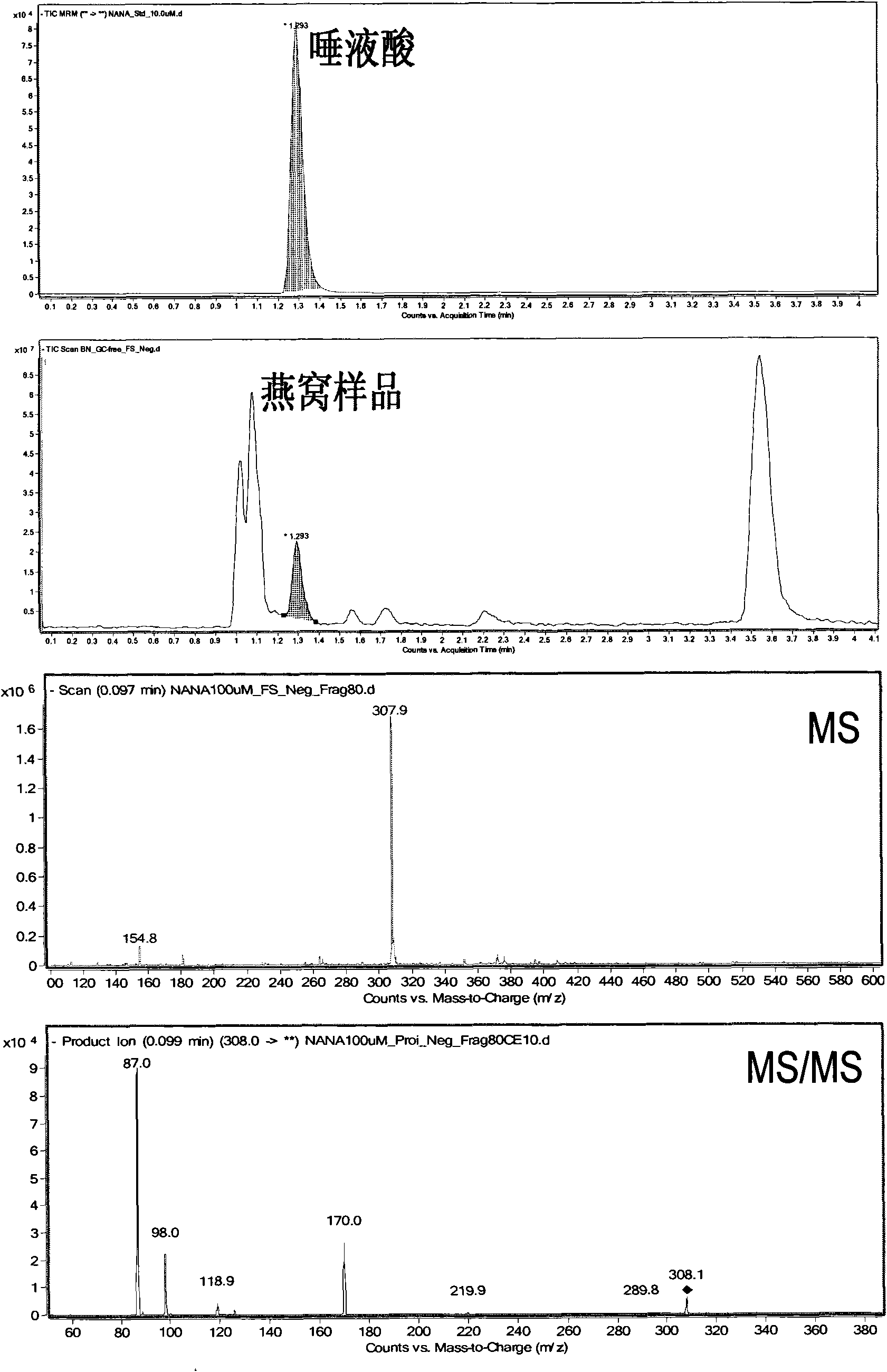
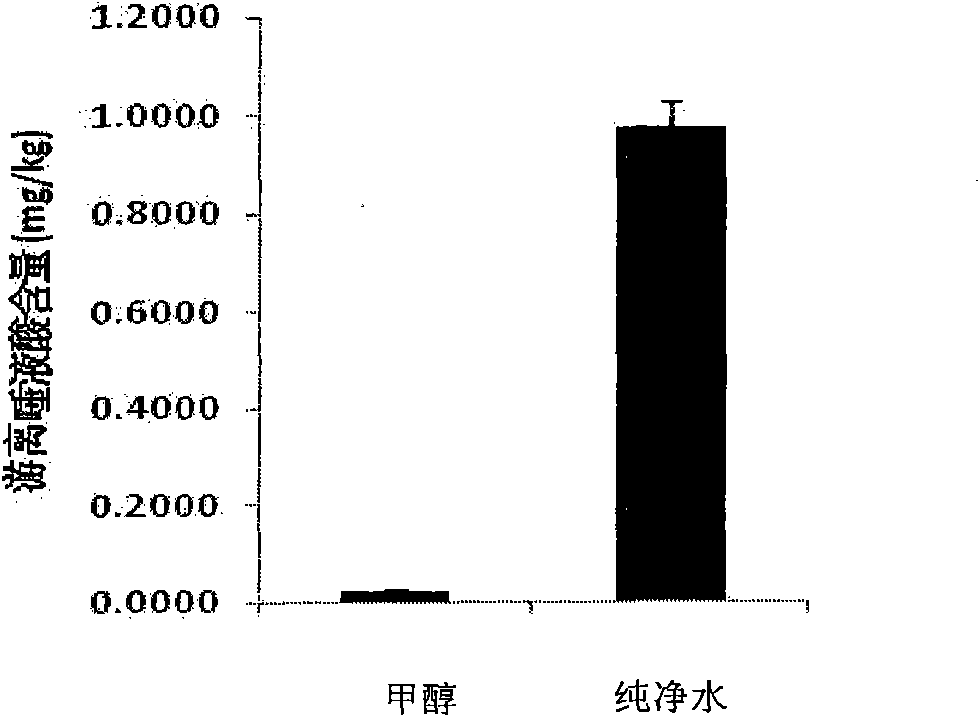
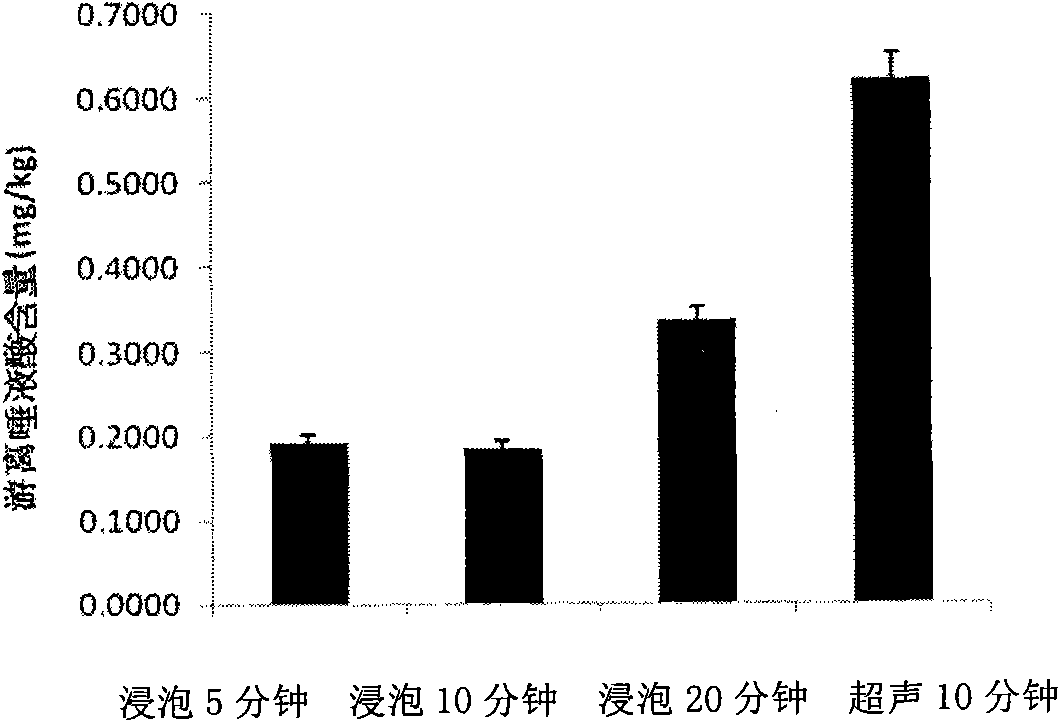
ActiveCN101806788AQuick resolutionHigh sensitivityComponent separationSialic acidMass Spectrometry-Mass Spectrometry
The invention discloses a method for identifying the quality of a bird's nest, which identifies the quality of the bird's nest by using a high performance liquid chromatography triple tandem quadrupole mass spectrometry (HPLC-QQQ) to determine the dissociative sialic acid content of the bird's nest, and comprises the steps of sample treatment, high performance liquid chromatography separation and mass spectrometric detection. The method has the advantages of high speed, accuracy and high sensitivity, thereby being a good method for identifying whether the bird's nest is true or not and whether the quality is good or not. Compared with the traditional identifying method based on appearance and properties, the method of the invention is more objective and more scientific.
Owner:SHENZHEN MANNAY COSMETIC
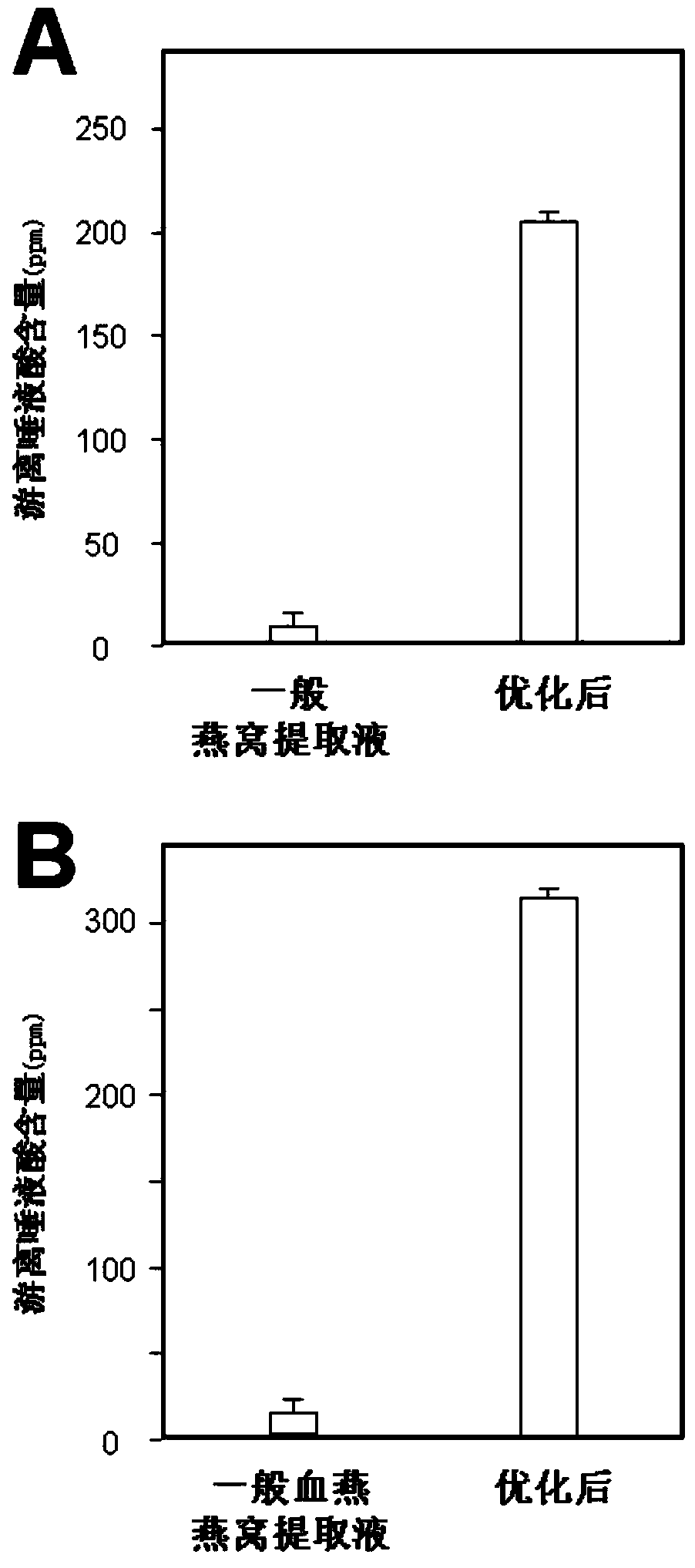
Cubilose extracting solution as well as preparation method and application thereof
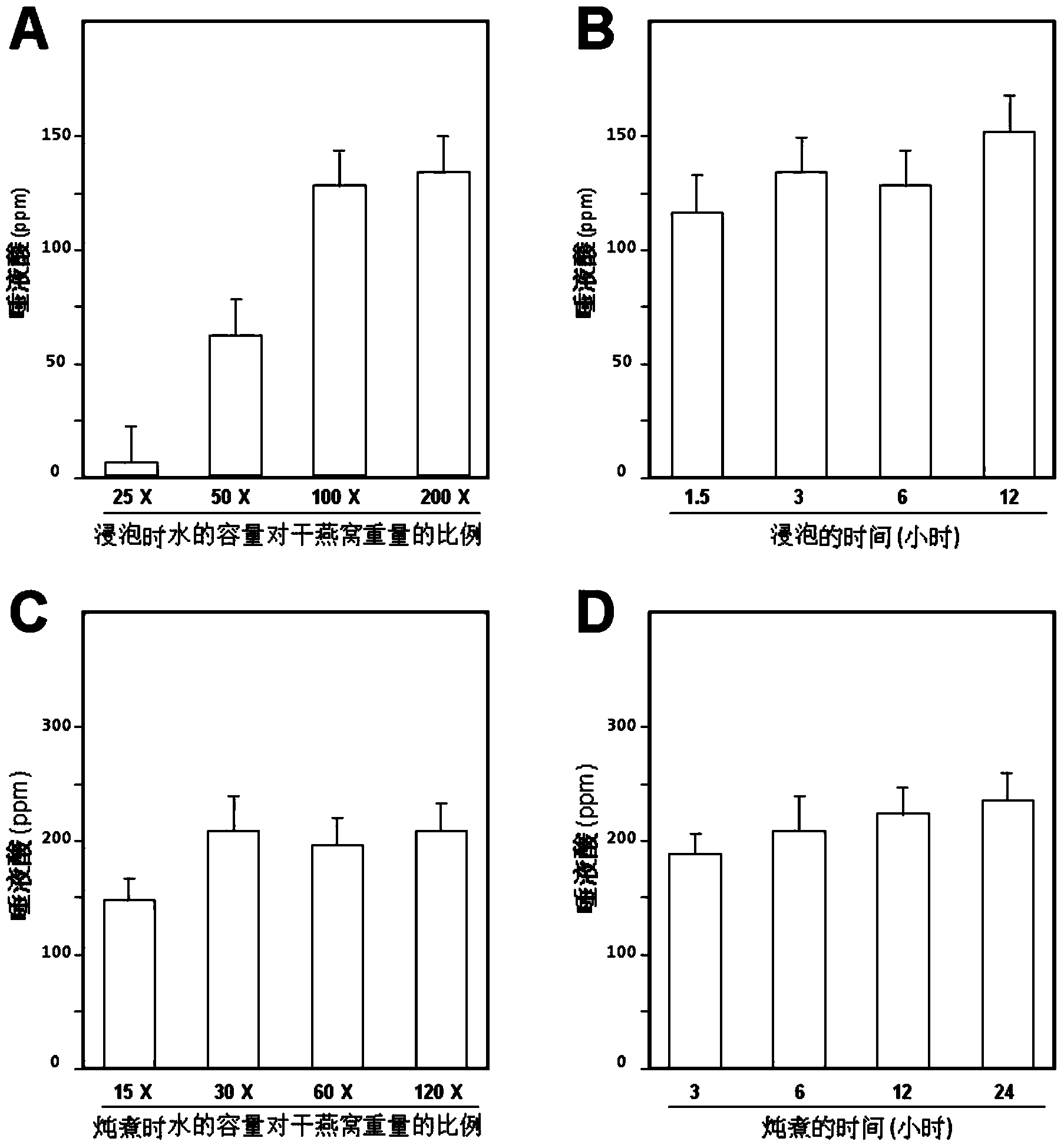
ActiveCN103479670AIncrease contentEasy to operateCosmetic preparationsToilet preparationsBiotechnologyEnzymatic hydrolysis
The invention discloses a cubilose extracting solution as well as a preparation method thereof. The method comprises the following steps: soaking cubilose by water which is 30-100 times of weight of the cubilose, soaking white cubilose and yellow cubilose for 1.5-12 hours and red cubilose for 12-26 hours, thereby obtaining cubilose residue; stewing the residue by water which is 30-100 times of weight of the cubilose; stewing the white cubilose and the yellow cubilose for 3-24 hours and the red cubilose for 15-74 hours, and then extracting the cubilose extracting solution. Compared with generally similar methods, the technological process of the method is greatly simplified; enzymatic hydrolysis is not needed; the production cost is reduced; the product yield is improved. The cubilose extracting solution prepared according to the method contains a lot of cosmetic active ingredients (such as sialic acid) in the cubilose; the concentration of the sialic acid is much higher than that of the cubilose extracting solution prepared by the general preparation method; kinds of obtained cubilose extracting solutions can be processed into kinds of products, so that precious natural supplements of the cubilose are relatively low in material costs and product prices.
Owner:SHENZHEN MANNAY COSMETIC
Cell-based systems for producing influenza vaccines
ActiveUS20100021499A1Minimize and prevent virus infectionAvoid componentsSsRNA viruses negative-senseAnimal cellsHemagglutininBinding site
The present invention relates to a cell-based method for producing influenza virus vaccines by enriching the population of surface-bound α2,6-sialic acid receptors on a cell surface, such as on a Chinese Hamster Ovary (CHO) cell surface. The host cell therefore presents numerous binding sites to which an influenza virus can bind via its hemagglutinin spike protein and infect the host cell. In contrast to wild-type CHO cells, the surface of the mutated CHO cells of the present invention contains an enriched population of α2,6-sialic acid receptors which makes the inventive CHO cells highly susceptible to viral infection, and therefore safe, effective, and highly efficient cells for rapidly producing influenza vaccines.
Owner:FLUGEN
Monoclonal antibody which recognizes the oligosaccharide N-glycolylated-galactose-glucose sialic acid in malignant tumors, and composition containing it
InactiveUS6429295B1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseMelanoma
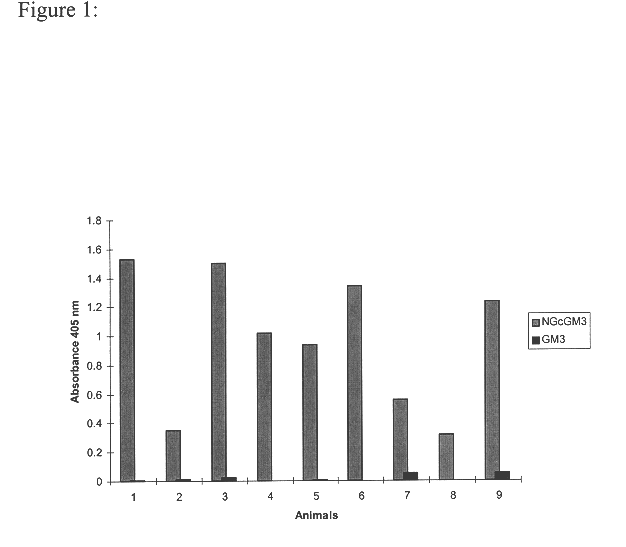
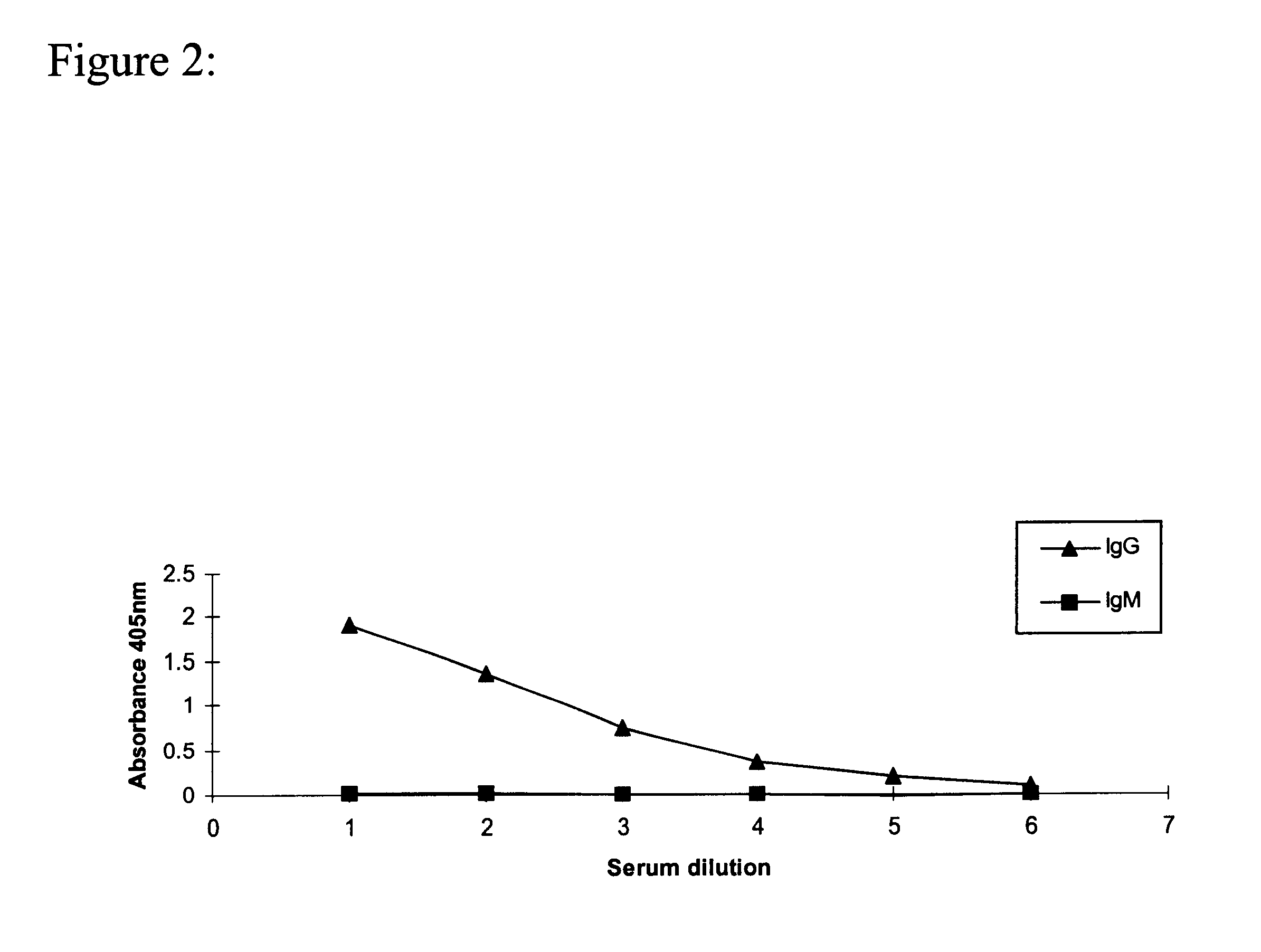
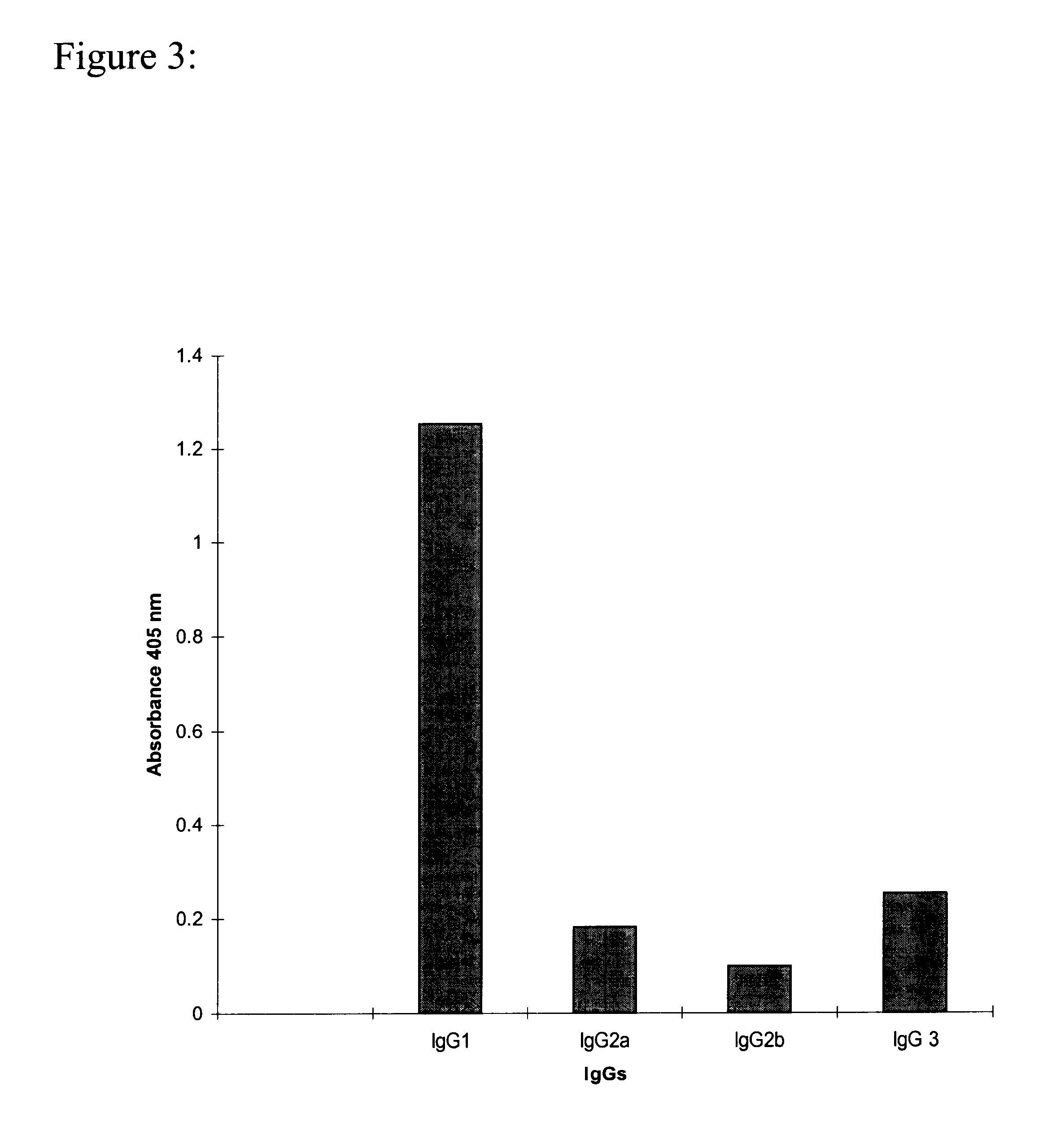
The present invention is related with the field of immunology and human medicine, particularly with the generation and selection of a monoclonal antibody (Mab) that recognizes the N-glycolylated-galactose-glucose sialic acid olygosaccharide sequence presents in malignant tumors.One of the objectives of this invention is to provide a Mab of the IgG1 type that has the characteristic of recognizing with high specificity N-glycolylated-galactose-glucose sialic acid olygosaccharide sequence presents in malignant tissues of breast, melanomas and tumors of the liver, stomach, colon, rectum and kidneys. It also has the capacity of producing direct cytolysis of the tumoral cells bearing the N-glycolylated-galactose-glucose sialic acid olygosaccharide sequence, thus can be used for the diagnosis and treatment of certain neoplasic diseases.Another objective of the present invention is to provide the hybridoma producing the referred Mab as well as the pharmaceutical composition containing it, for the treatment of neoplasic diseases.
Owner:CENT DE INMUNOLOGIA MOLECULAR CENT DE INMUNOLO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com