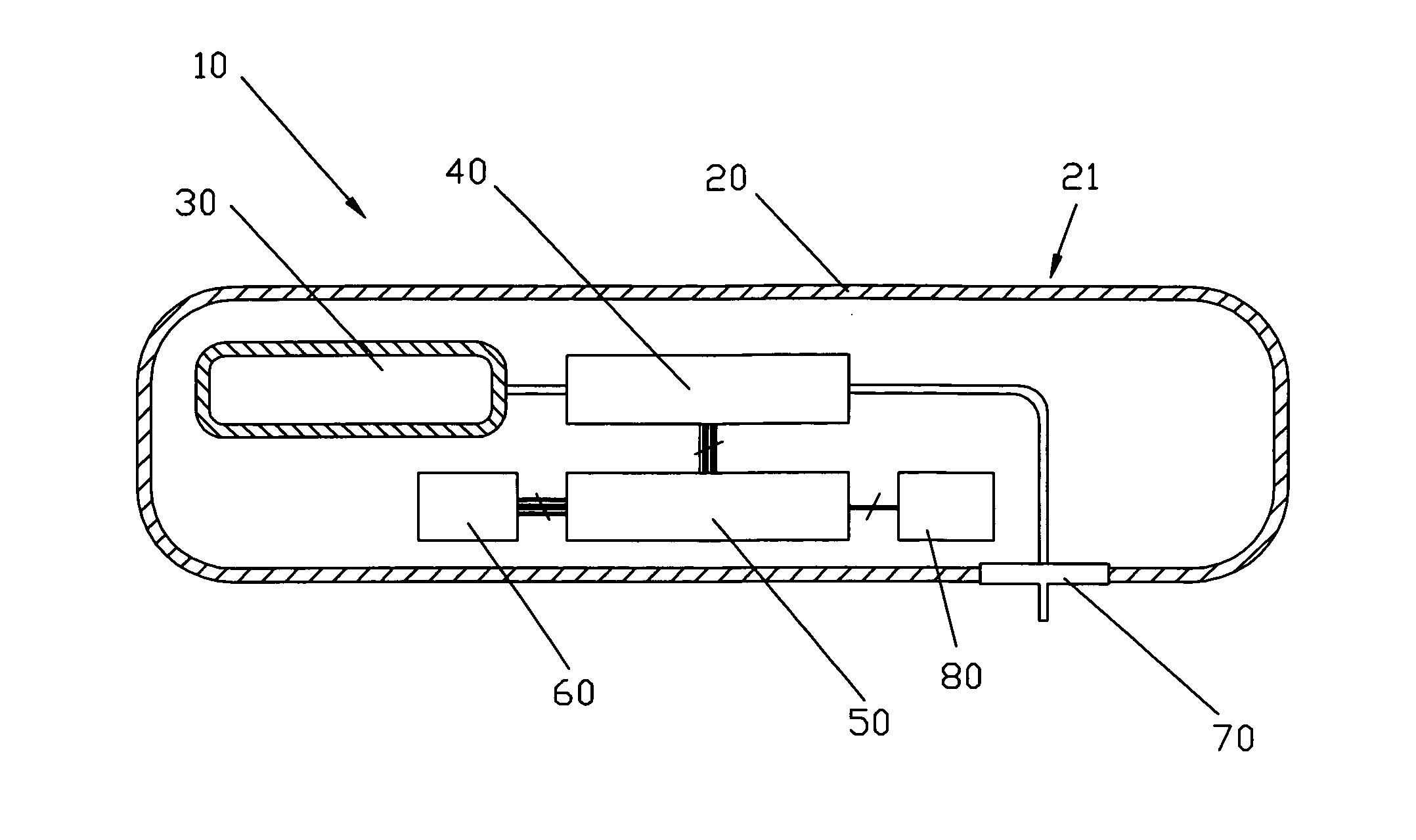
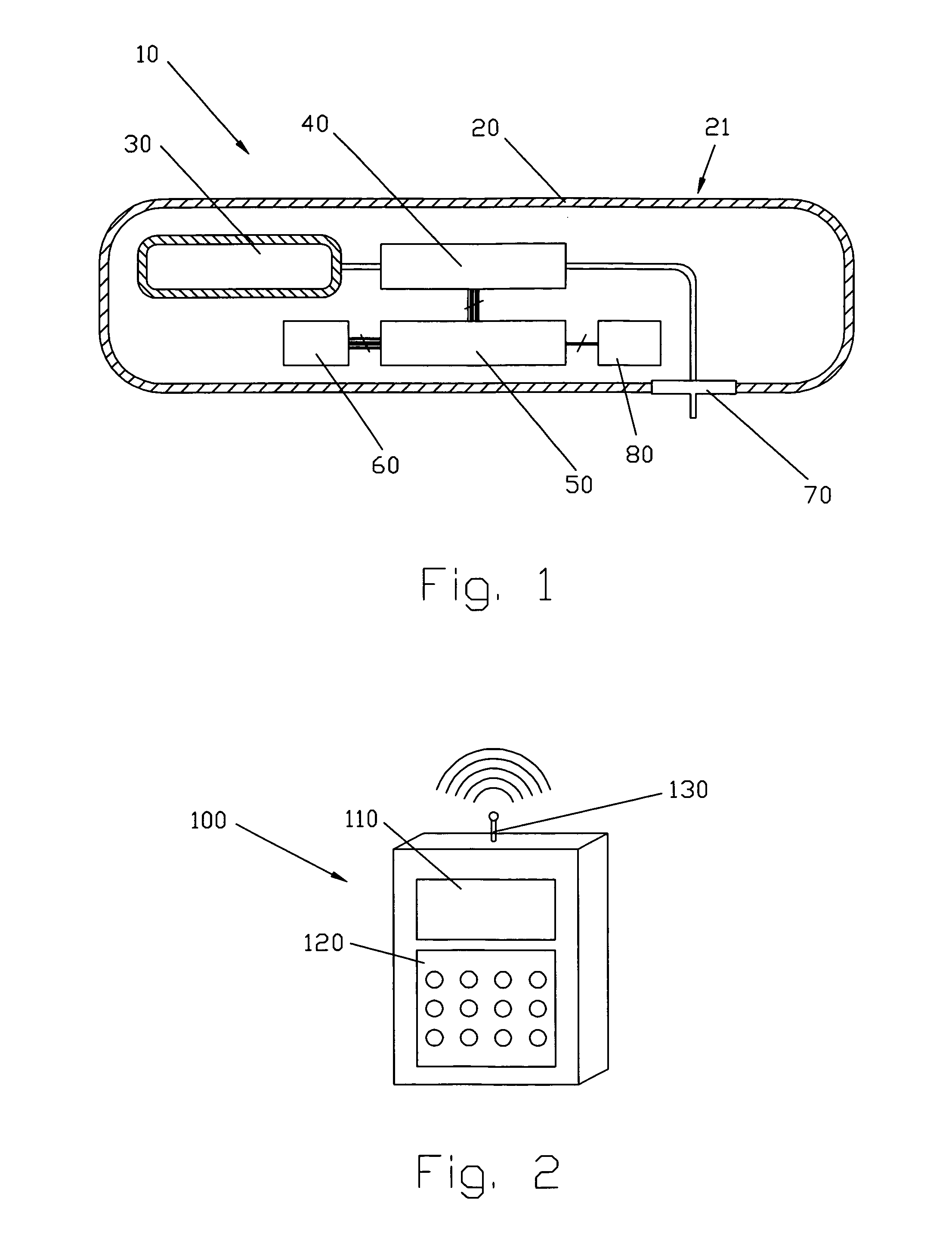
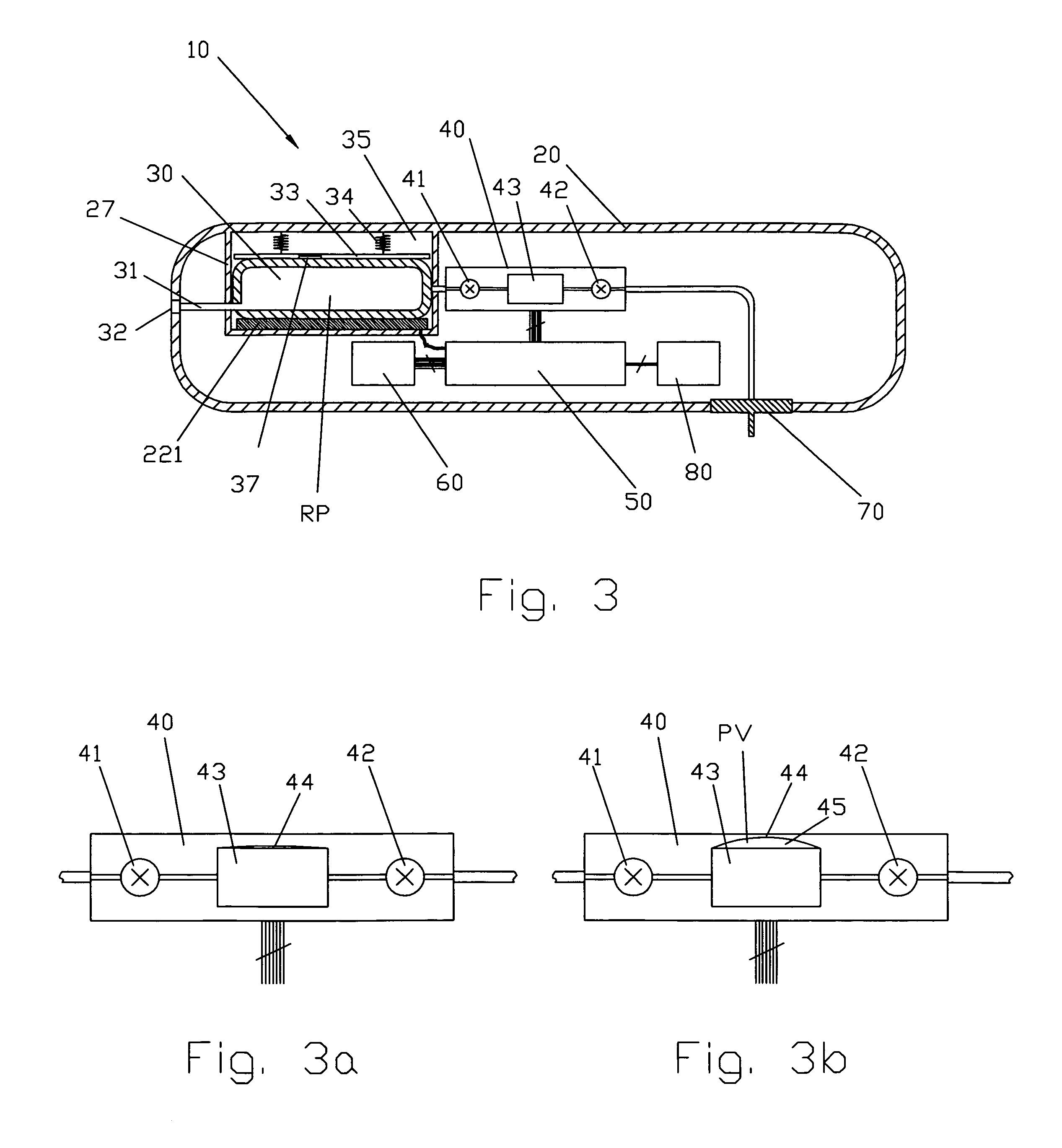
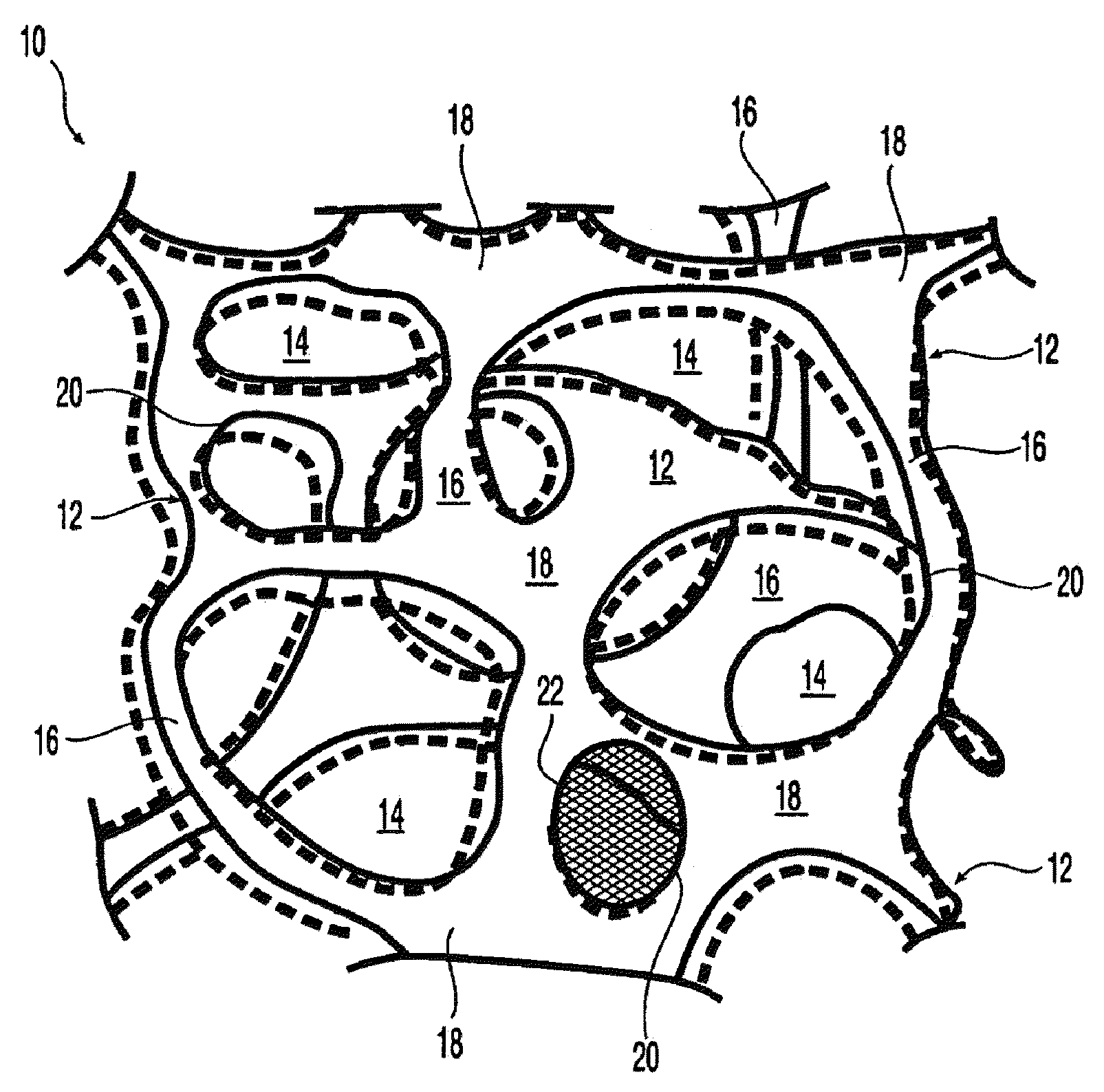
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
59330results about "Pharmaceutical delivery mechanism" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Flameless electronic atomizing cigarette
InactiveUS20060196518A1Reduce cancer riskTobacco preparationBatteries circuit arrangementsEngineeringElectric control
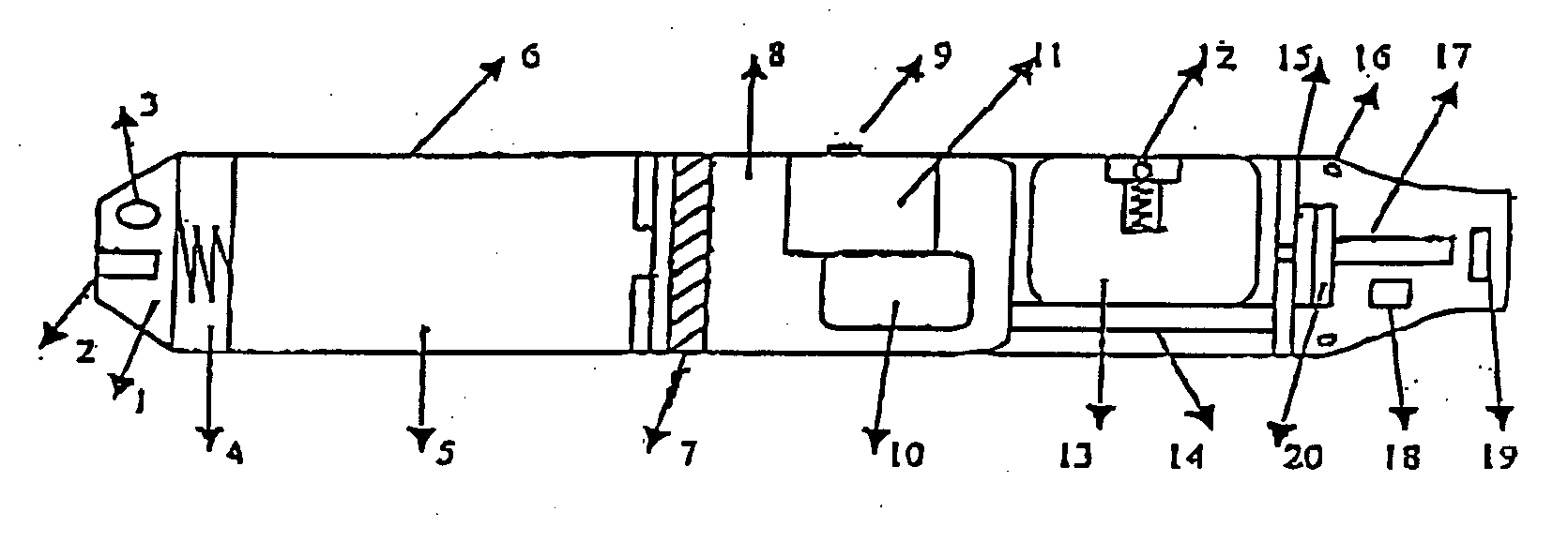
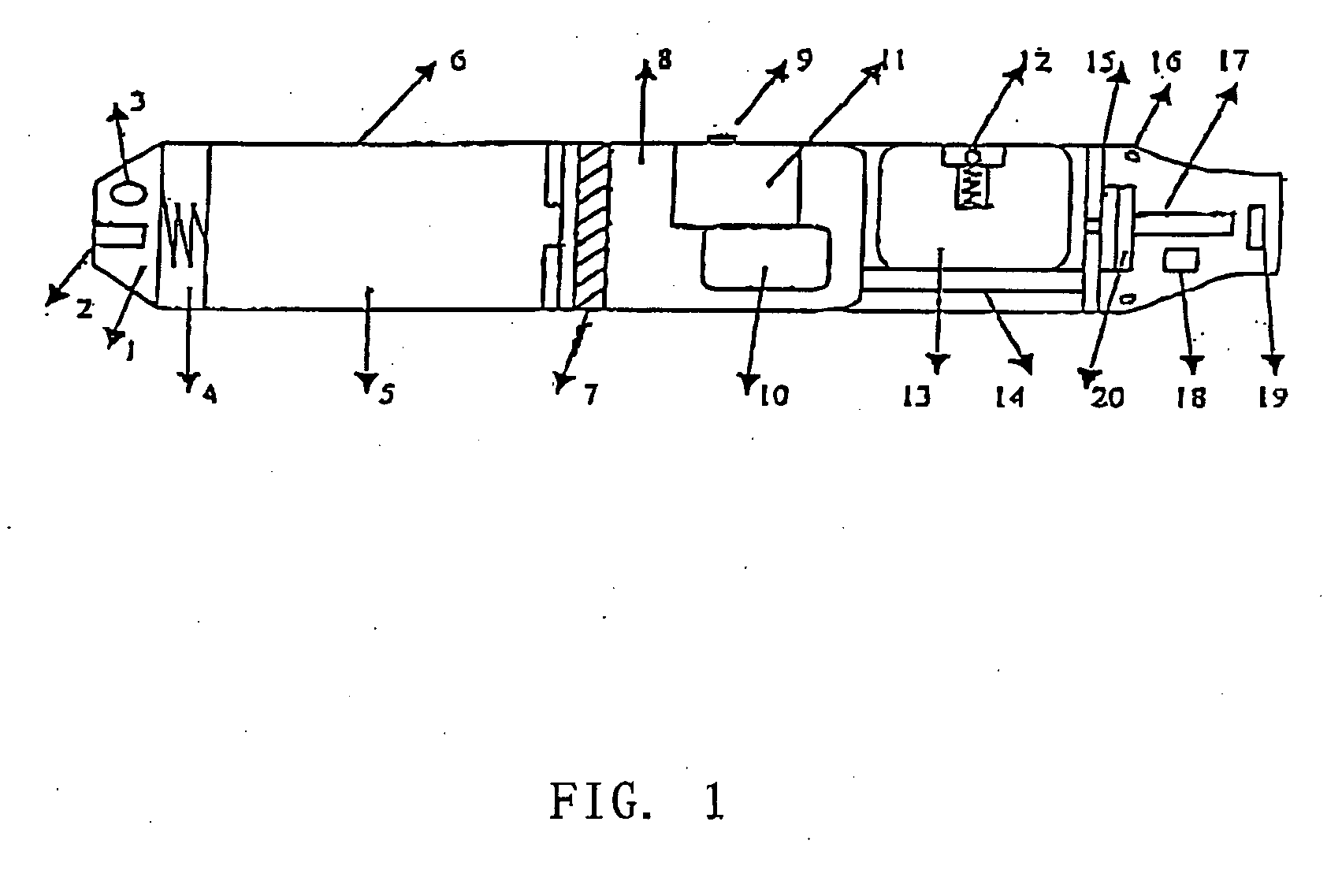
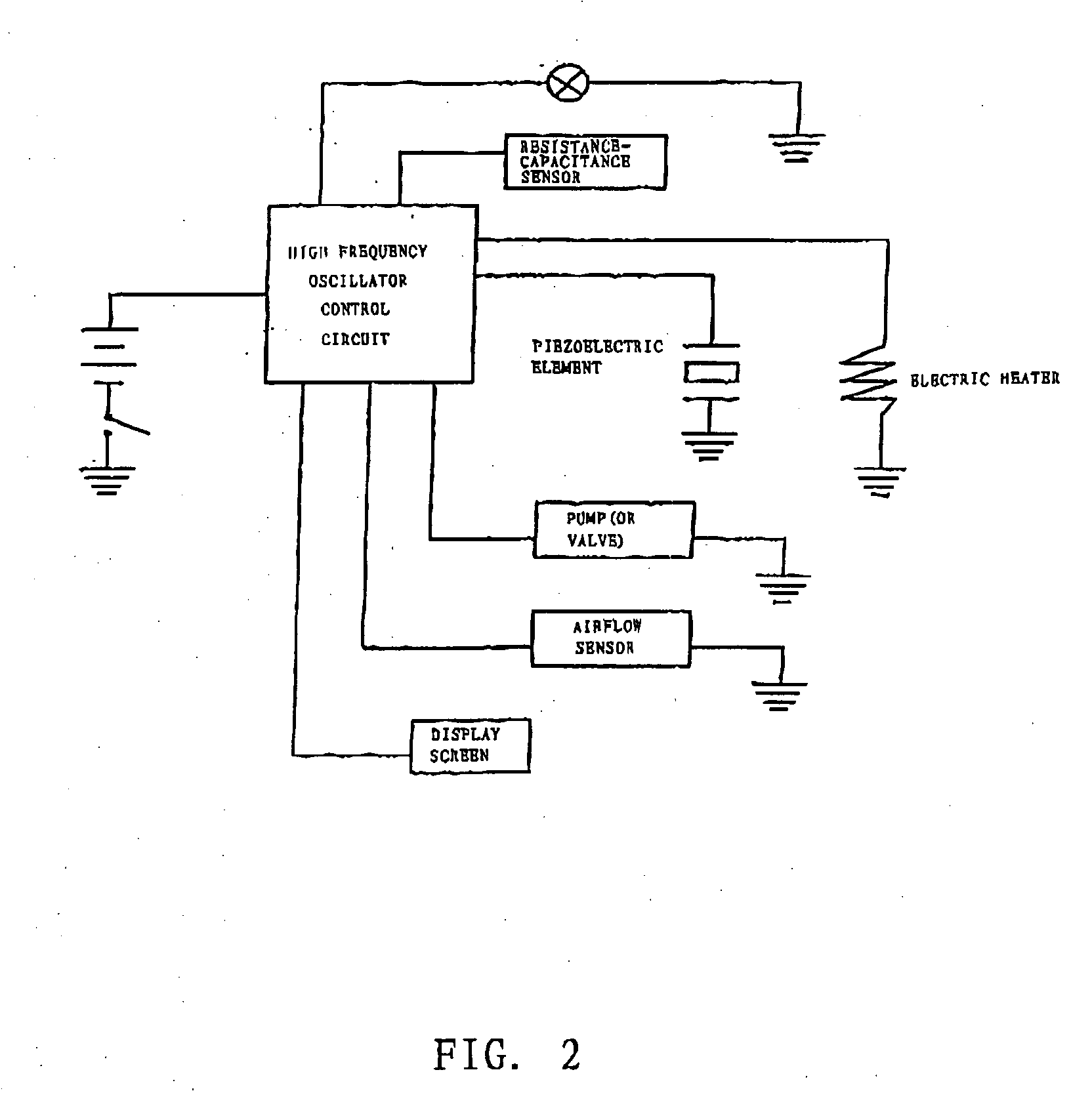
The invention relates to a non-smokable electronic spray cigarette which only comprises nicotine without harmful tar. The cigarette includes a smoke mouth integer comprised with a shell, a cell, a high frequency ionzer, nicotine solution storage and its container, control circuit, a display screen, a human contact sensor, a piezoelectric supersound atomizer, a high temperature vaporization nozzle and attachments, an electro-thermal vaporization nozzle installed in the air suction end of the shell goes through an electric control pump or a valve with a measuring chamber and a liquid storage container which contains nicotine solution and is connected to the electric control pump or a valve with a one-way flow valve, the control circuit plate has four export ends individually connected with the high frequency ionizer, electric heater, pump or valve and the display screen, a human resistence sensor and an air flow sensor are connected to the input end of the control circuit. The advantages of the present invention are smoking without tar, reducing the cancerogenic risk, the user still feel smoking and experiercing the excitement, the cigarette is no need to be lit and is no fire danger.
Owner:FONTEM HLDG 1
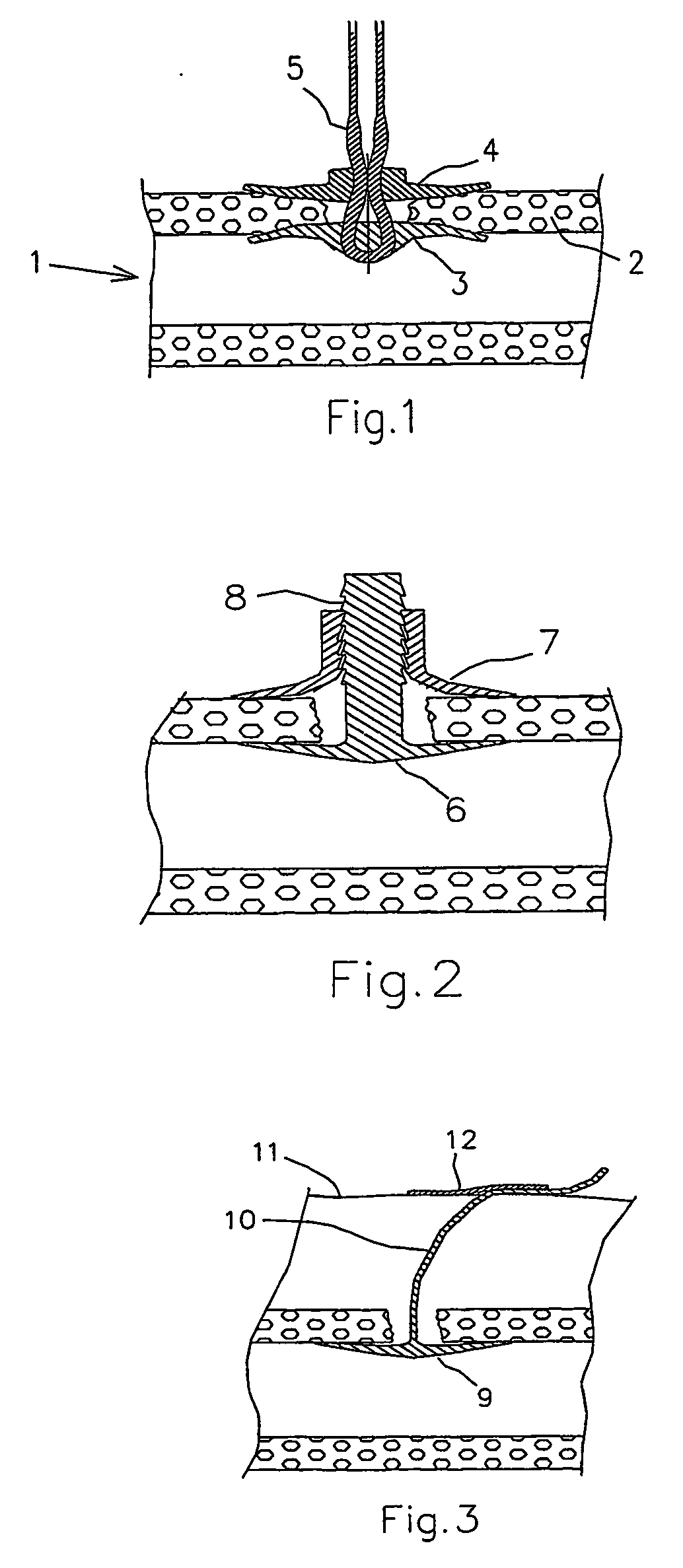
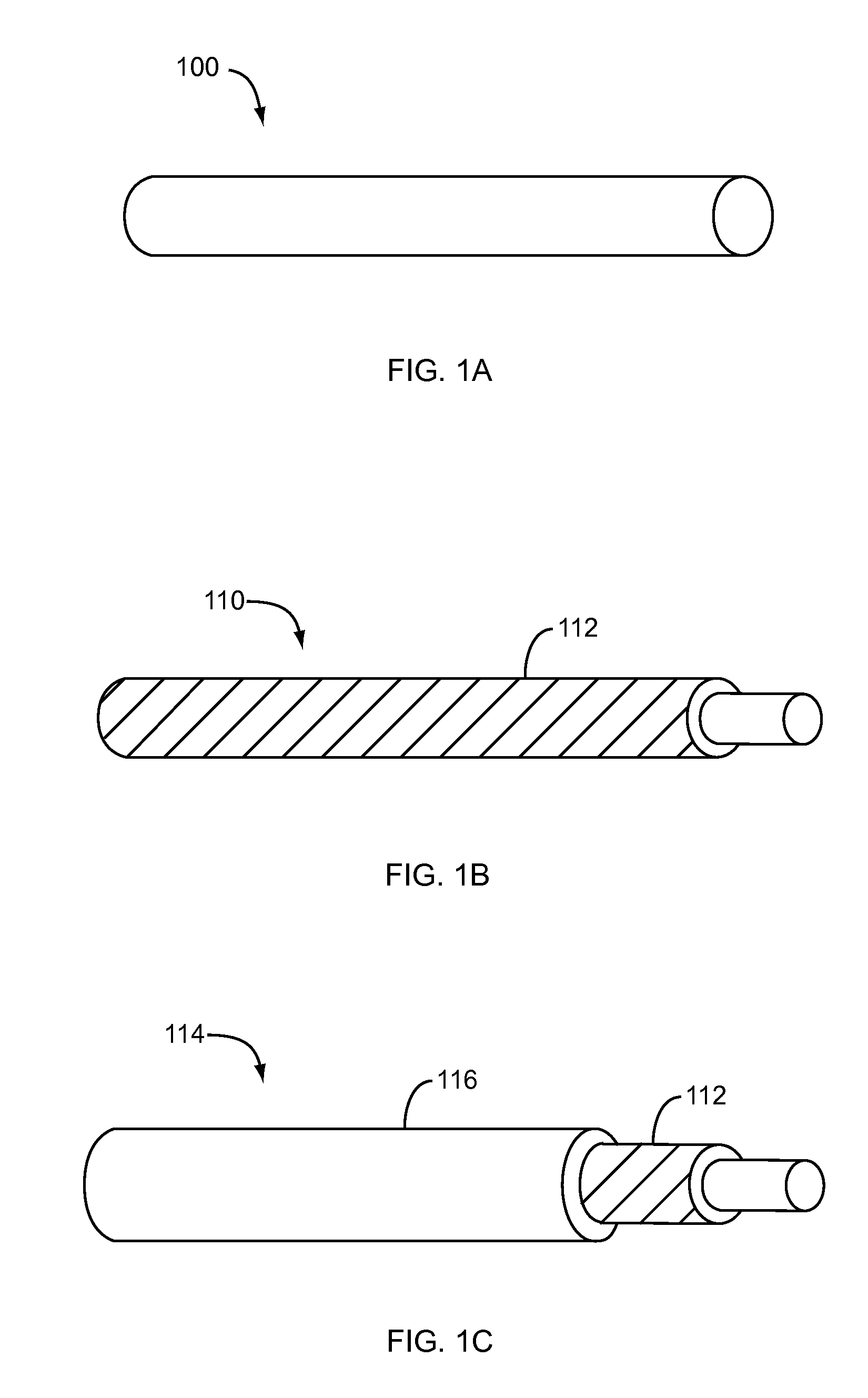
Dissolvable medical sealing device
ActiveUS20050169974A1Shorten the timeSuture equipmentsPharmaceutical delivery mechanismOrganismal ProcessPolyvinyl alcohol
The present invention provides a dissolvable medical sealing device (3, 4; 6, 7; 9) for closing a wound in vessel. A sealing device (3, 4, 6, 7, 9) according to the invention is made of a material that dissolves by means of physical processes, rather than by means of chemical or biological processes. Such a sealing device (3, 4; 6, 7; 9) can be made of polyethylene glycol, polypropylene glycol, copolymers containing ethylene glycol and propylene glycol, polyvinyl alcohol or polyvinyl pyrolidone, or any combinations thereof.
Owner:TERUMO MEDICAL CORP
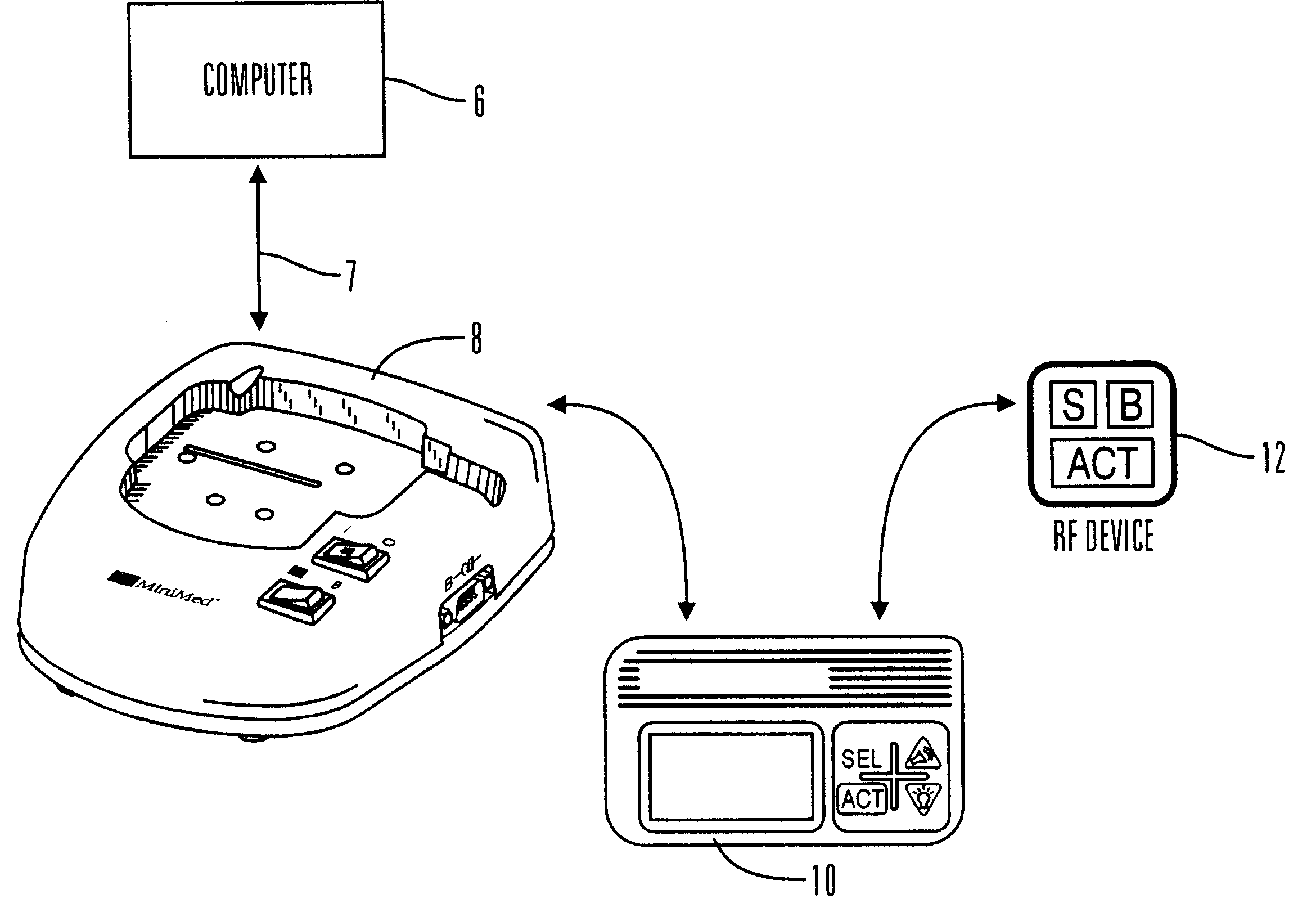
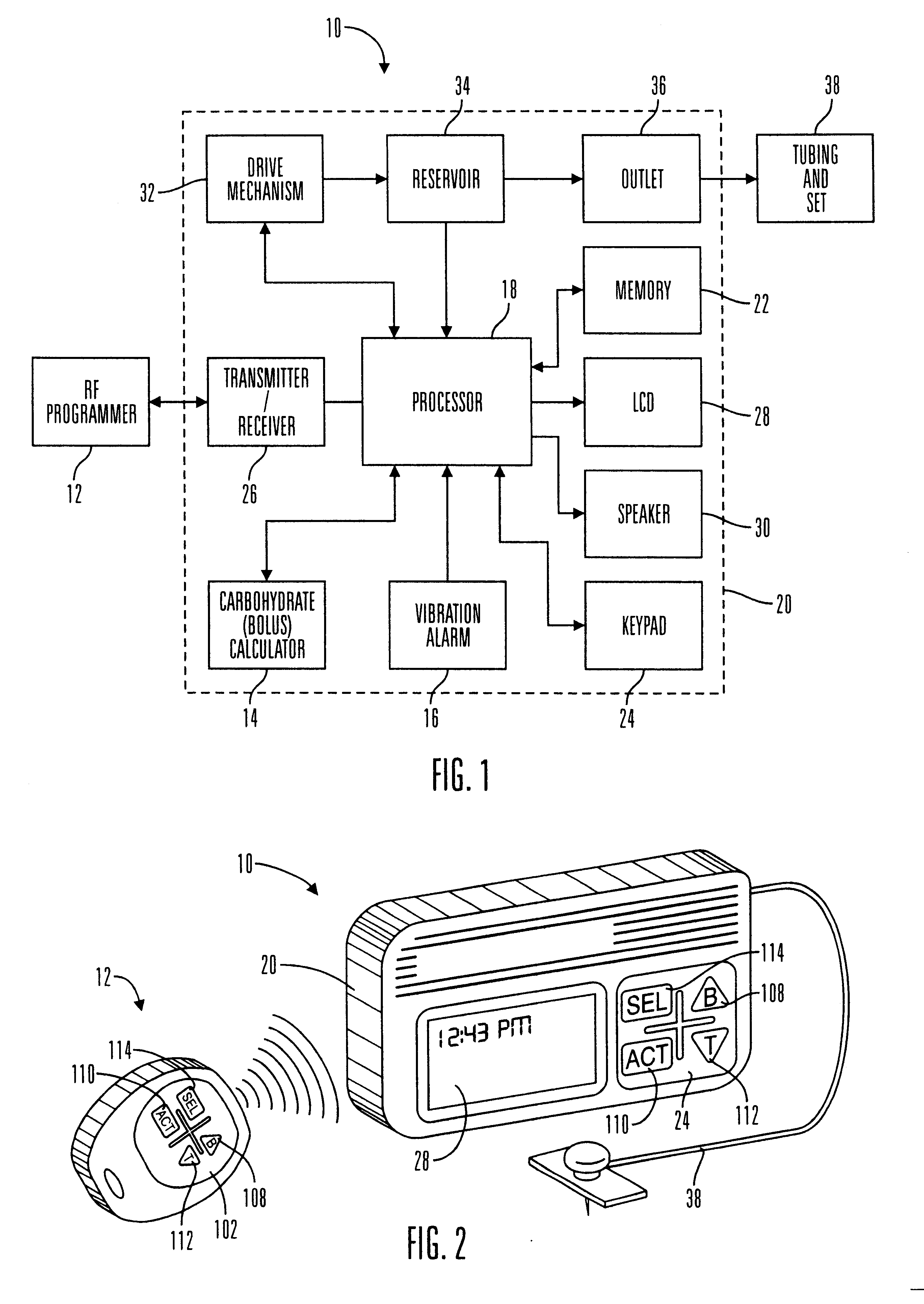
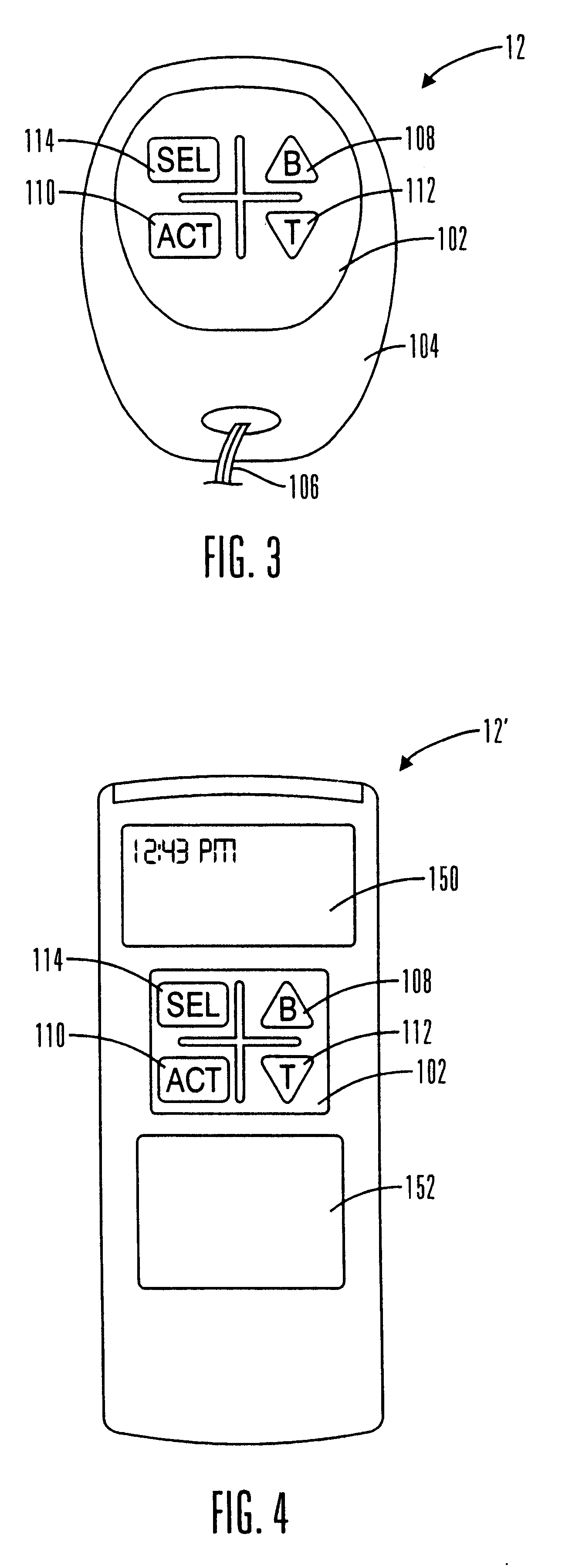
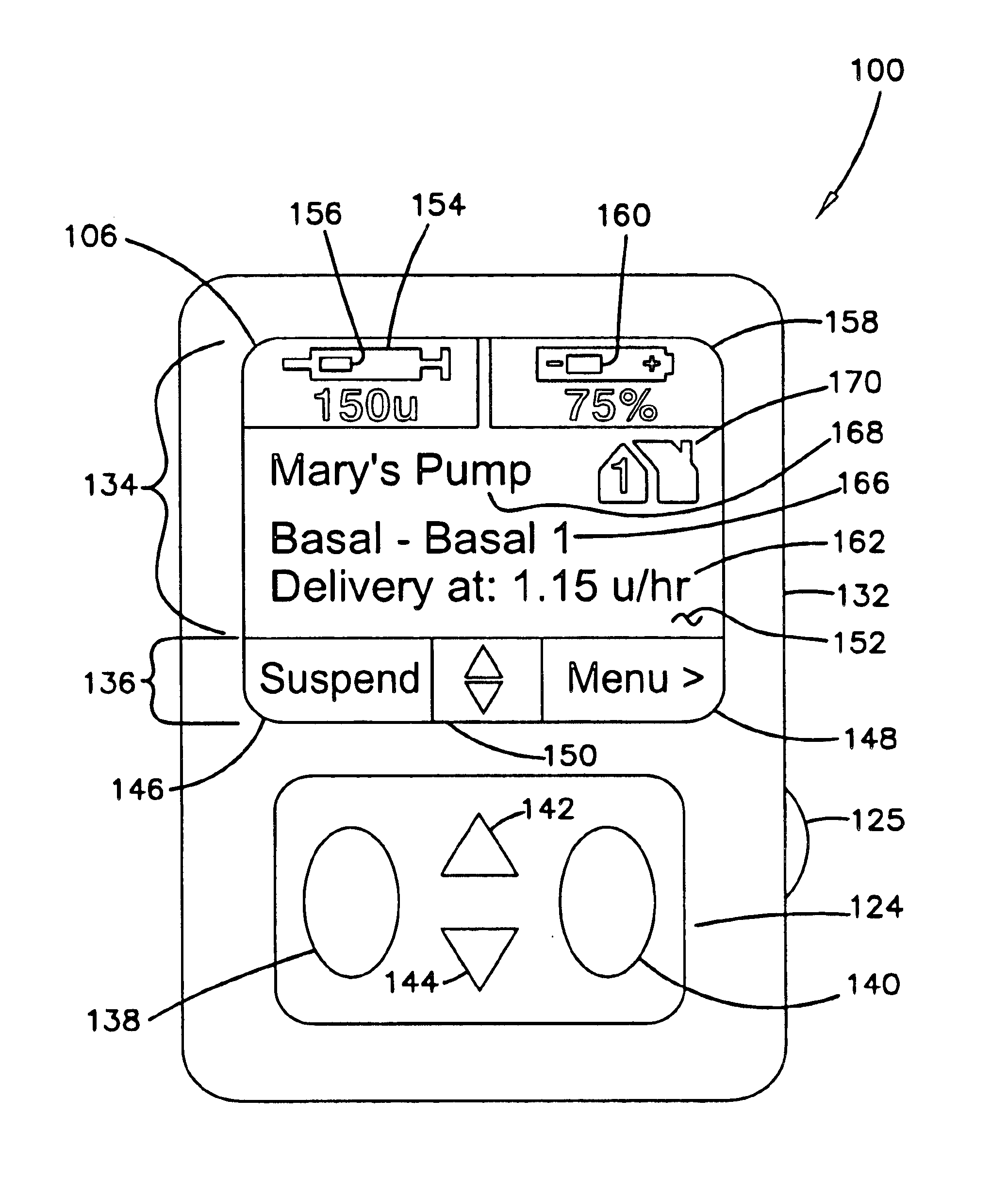
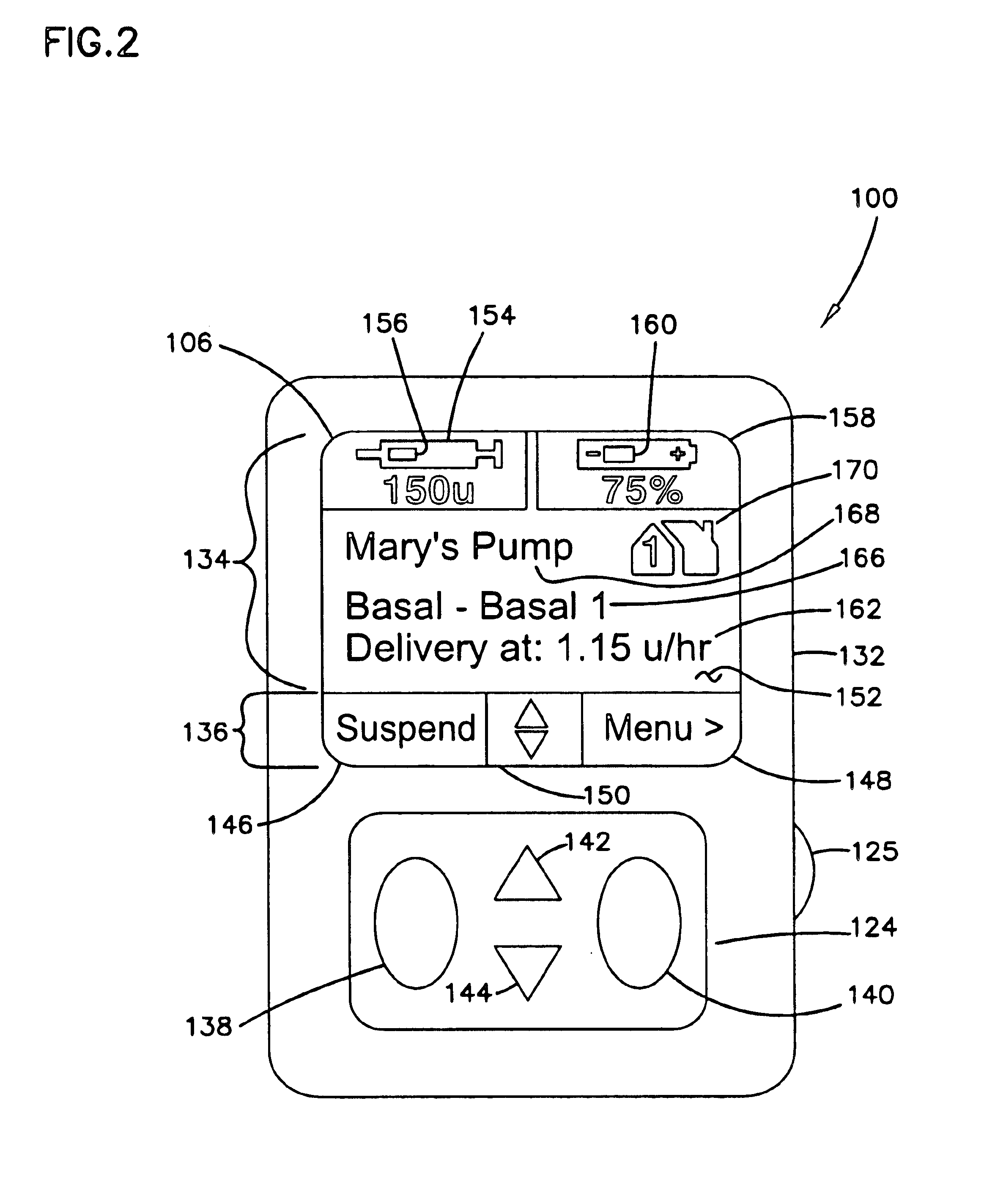
External infusion device with remote programming bolus estimator and/or vibration alarm capabilities
InactiveUS6551276B1Short and long period of timeTime indicationAutomatic syringesIntensive care medicineTransmitter
An infusion system for infusing a liquid into a body includes an external infusion device and a remote commander. The external infusion device includes a housing, a receiver, a processor and an indication device. The receiver is coupled to the housing and for receiving remotely generated commands. The processor is coupled to the housing and the receiver to receive remotely generated commands and to control the external infusion device in accordance with the commands. The indication device indicates when a command has been received and indicates when the command is being utilized to control the external infusion device so that the external infusion device is capable of being concealed from view when being remotely commanded. The remote commander includes a commander housing, a keypad for transmitting commands, and a transmitter for transmitting commands to the receiver of the external infusion device.
Owner:MEDTRONIC MIMIMED INC
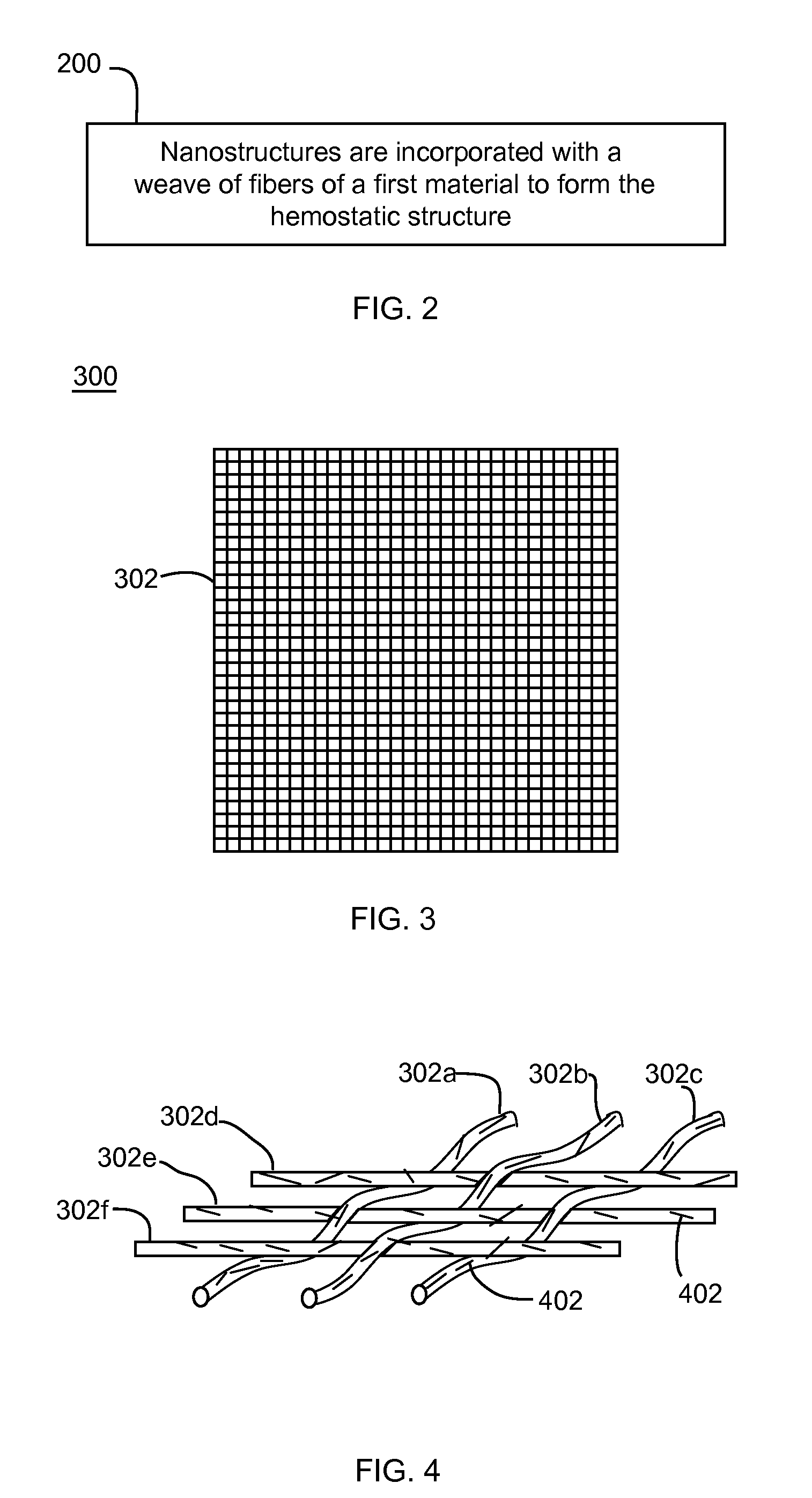
Nanostructure-enhanced platelet binding and hemostatic structures
InactiveUS8319002B2Enhancing overall rate and strengthInduce platelet binding and efficient hemostasisBiocideSurgical adhesivesPlateletNanofiber
Methods, systems, and apparatuses for nanomaterial-enhanced platelet binding and hemostatic medical devices are provided. Hemostatic materials and structures are provided that induce platelet binding, including platelet binding and the coagulation of blood at a wound / opening caused by trauma, a surgical procedure, ulceration, or other cause. Example embodiments include platelet binding devices, hemostatic bandages, hemostatic plugs, and hemostatic formulations. The hemostatic materials and structures may incorporate nanostructures and / or further hemostatic elements such as polymers, silicon nanofibers, silicon dioxide nanofibers, and / or glass beads into a highly absorbent, gelling scaffold. The hemostatic materials and structures may be resorbable.
Owner:NANOSYS INC
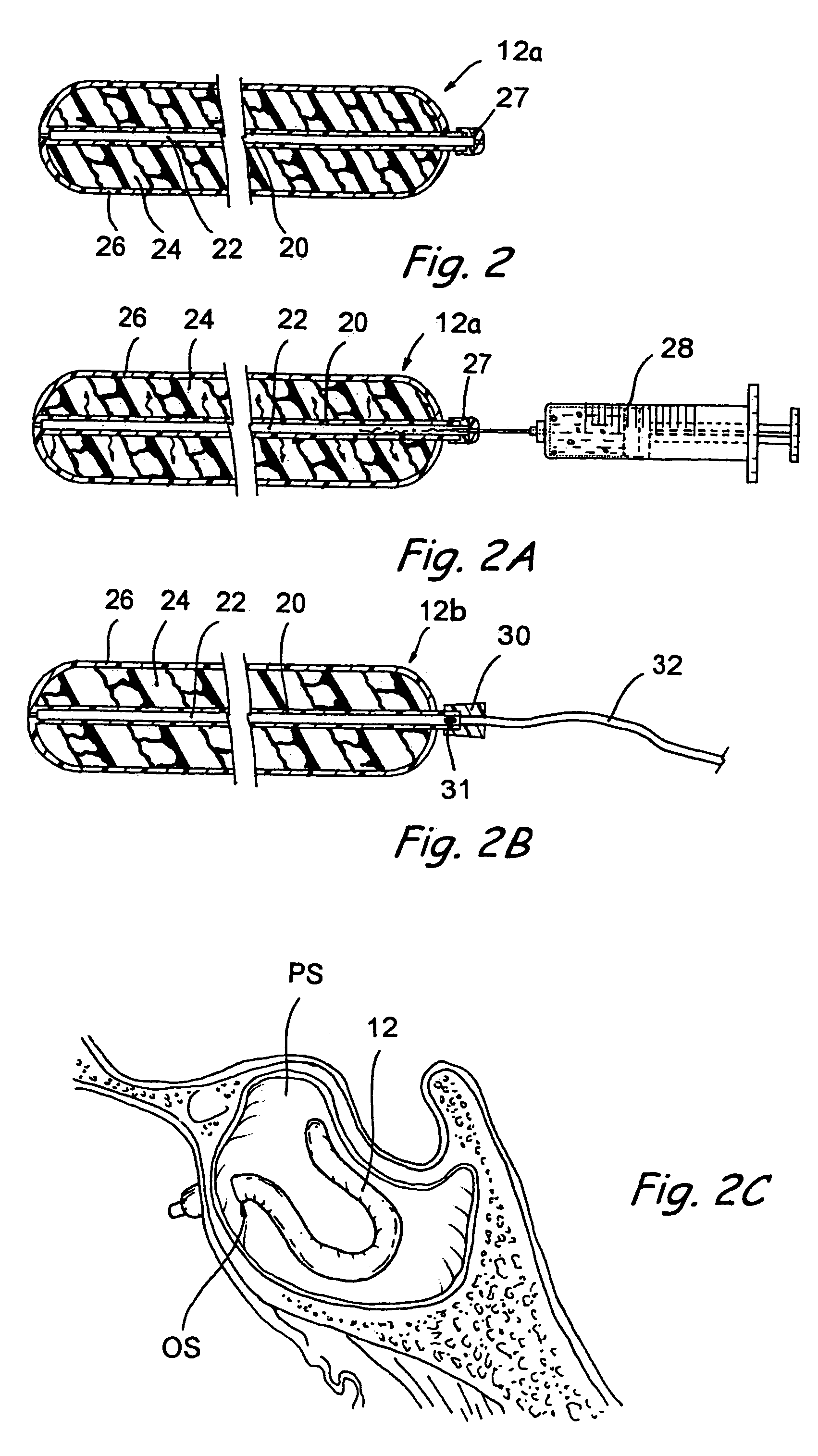
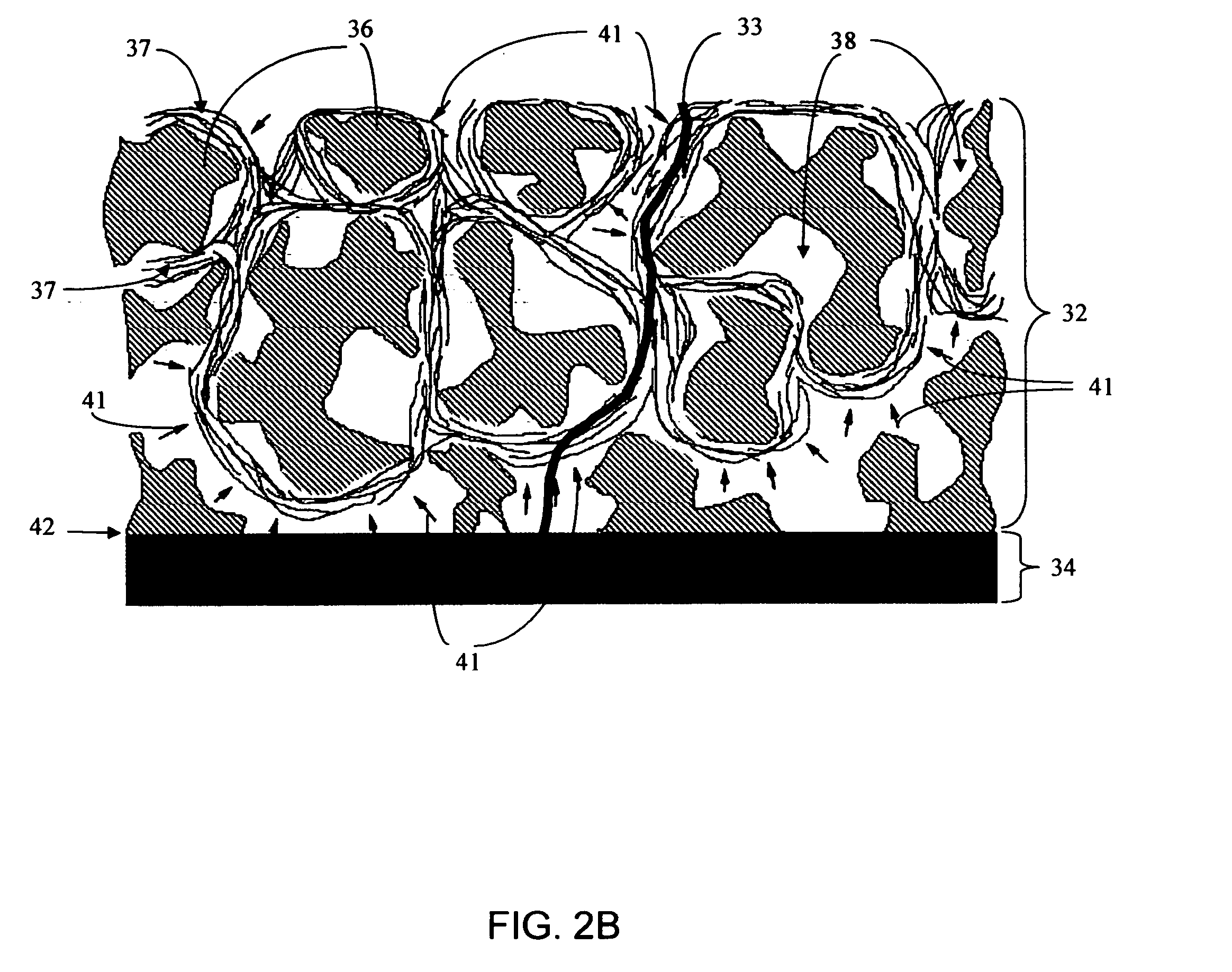
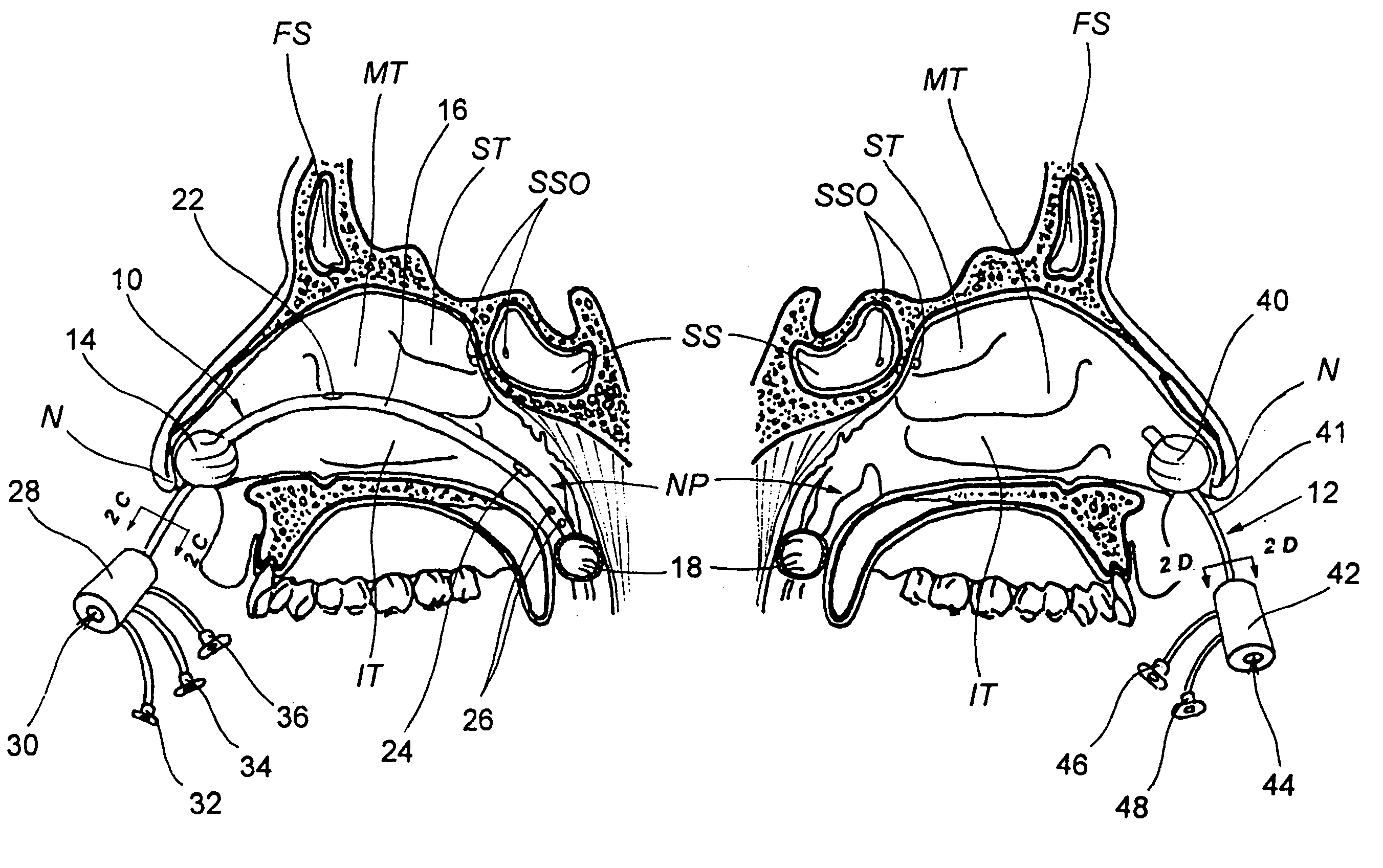
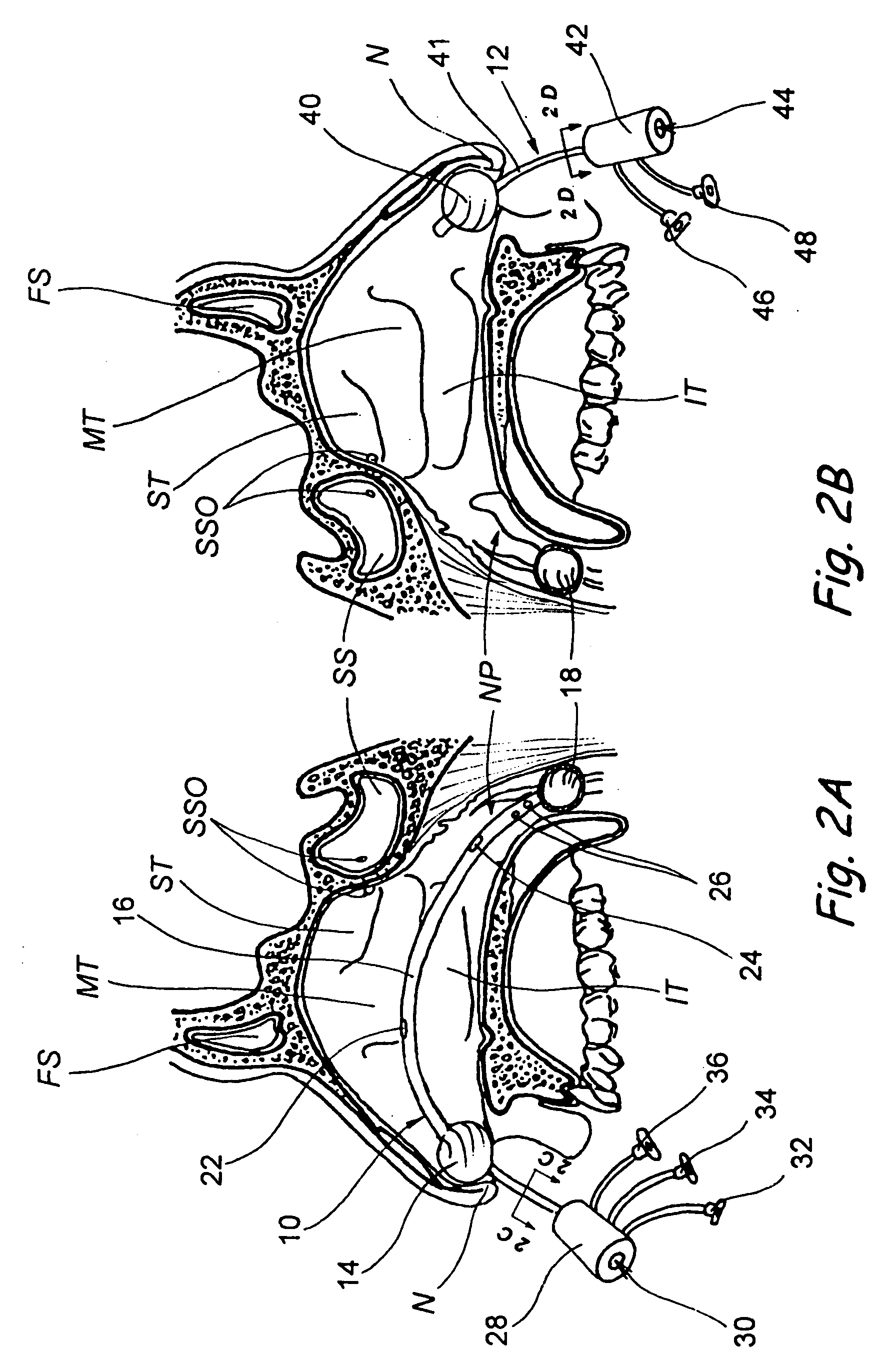
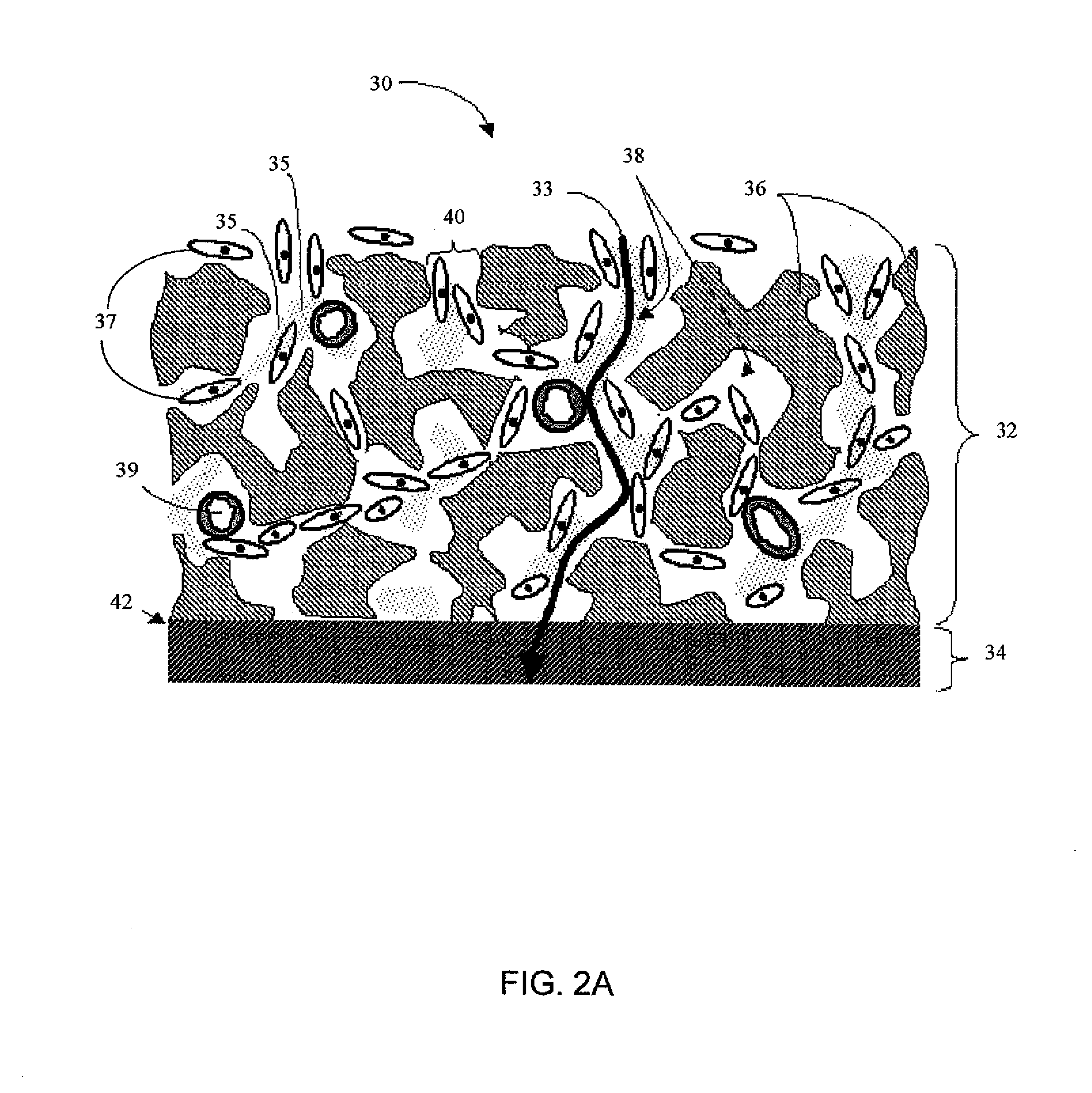
Implantable device and methods for delivering drugs and other substances to treat sinusitis and other disorders
Implantable devices and methods for delivering drugs and other substances to locations within the body of a human or animal subject to treat or diagnose sinusitis and a variety of other disorders. The invention includes implantable substance delivery devices that comprise reservoirs and barriers that control the rate at which substances pass out of the reservoirs. The delivery devices may be advanced into the body using guidewires, catheters, ports, introducers and other access apparatus. In some embodiments the delivery devices may be loaded with one or more desired substance before their introduction into the body. In other embodiments the delivery devices are loaded and / or reloaded with a desired substance after the delivery device has been introduced into the body.
Owner:ACCLARENT INC
Water-swellable copolymers and articles and coatings made therefrom
Owner:TYCO HEALTHCARE GRP LP
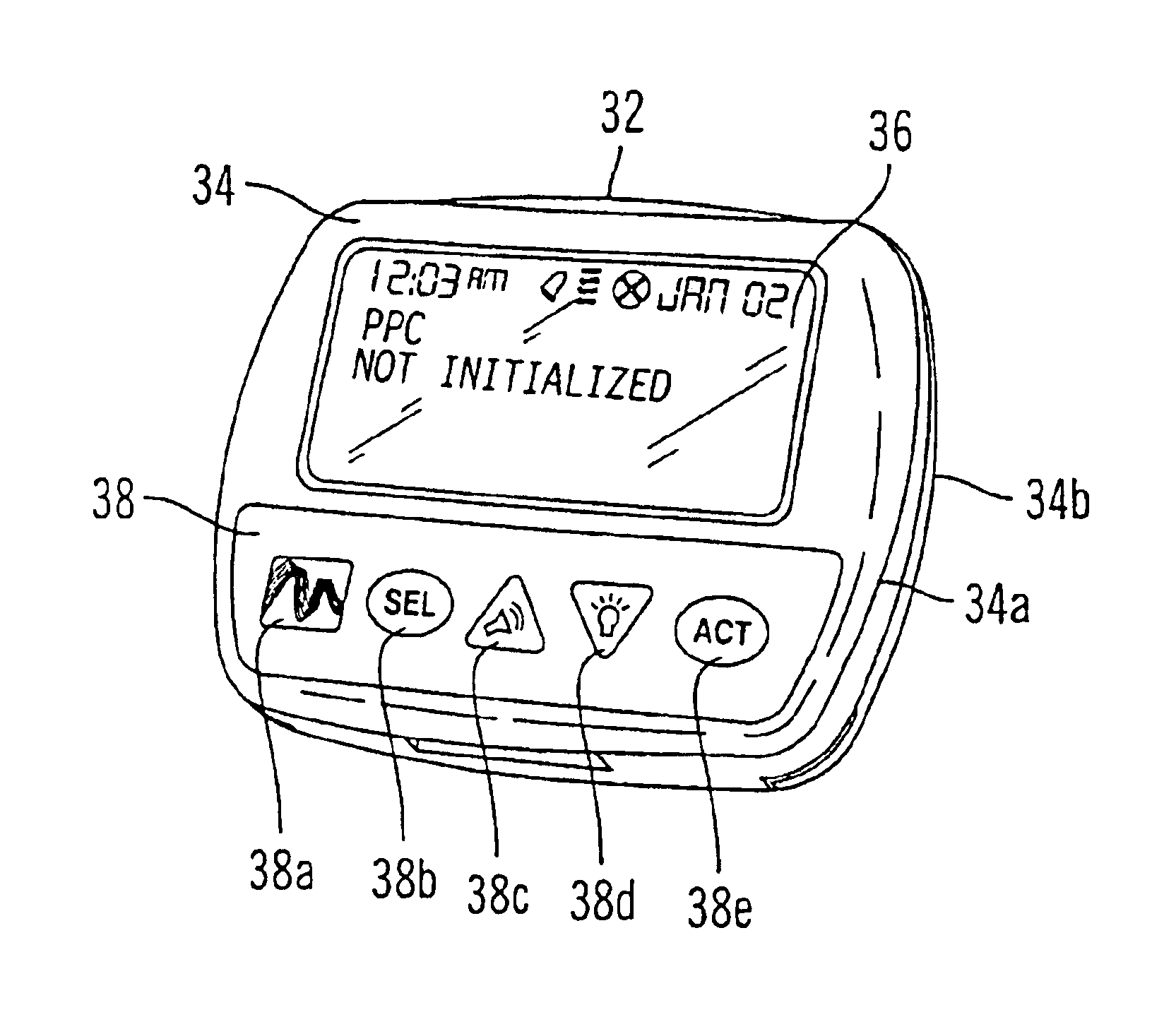
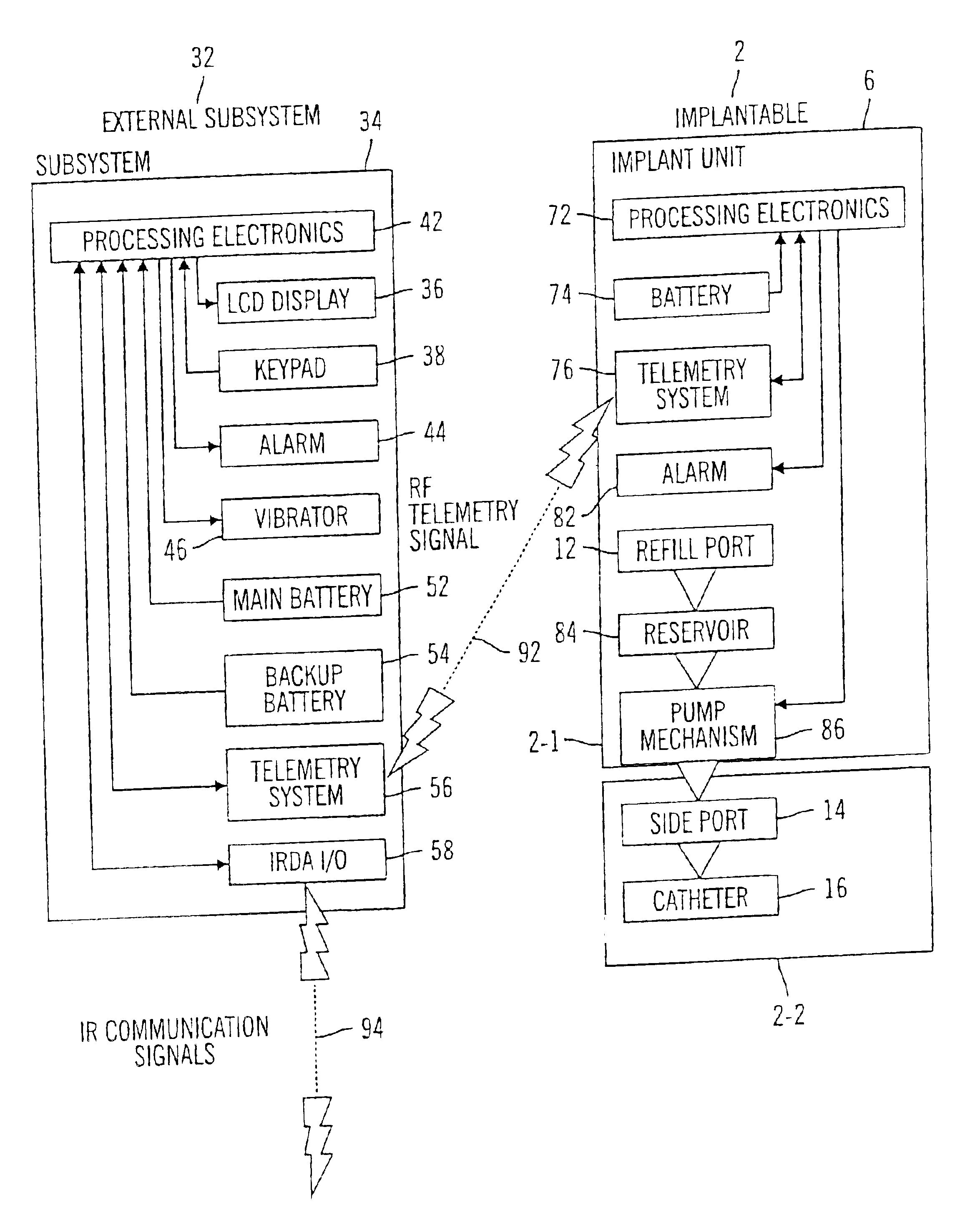
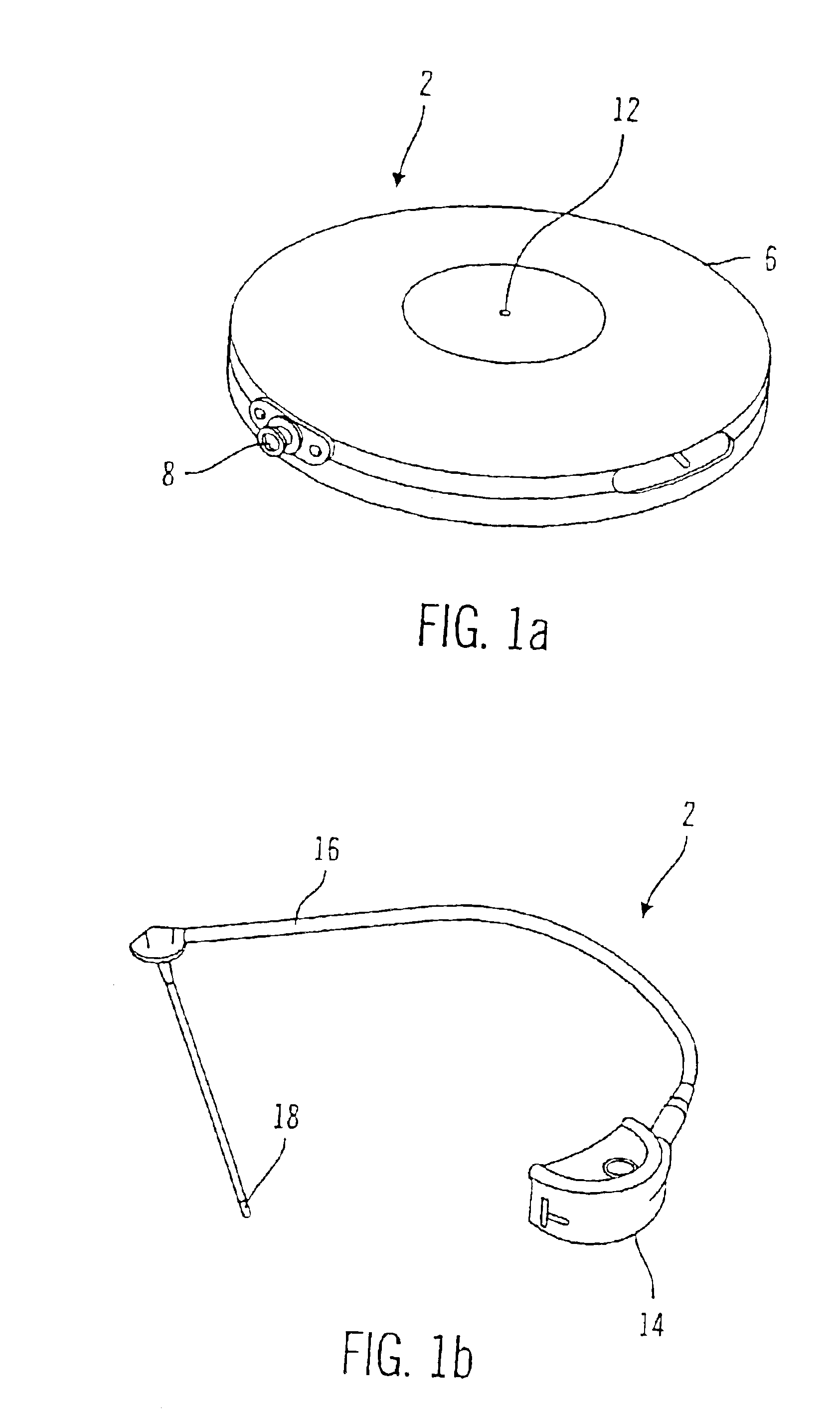
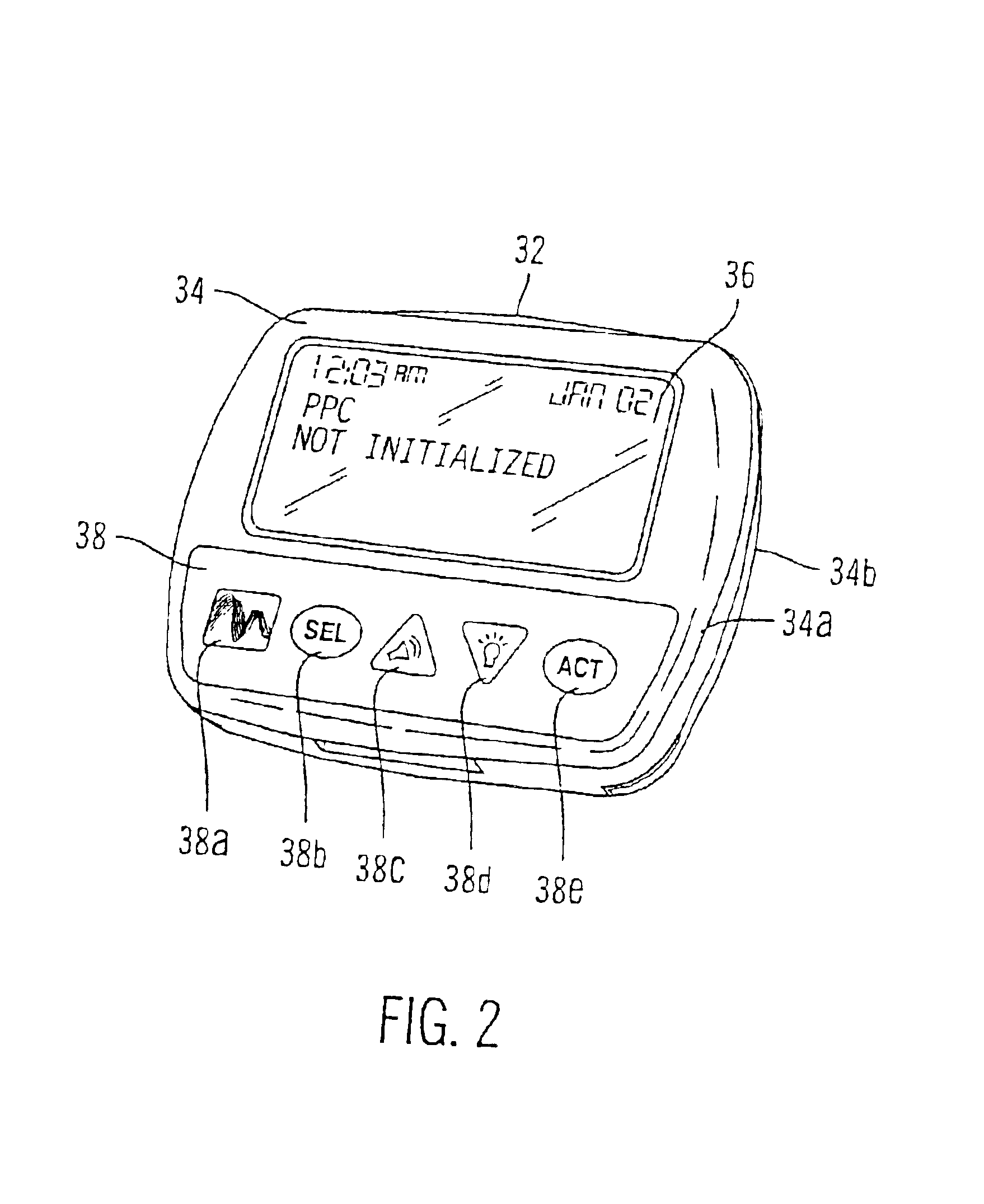
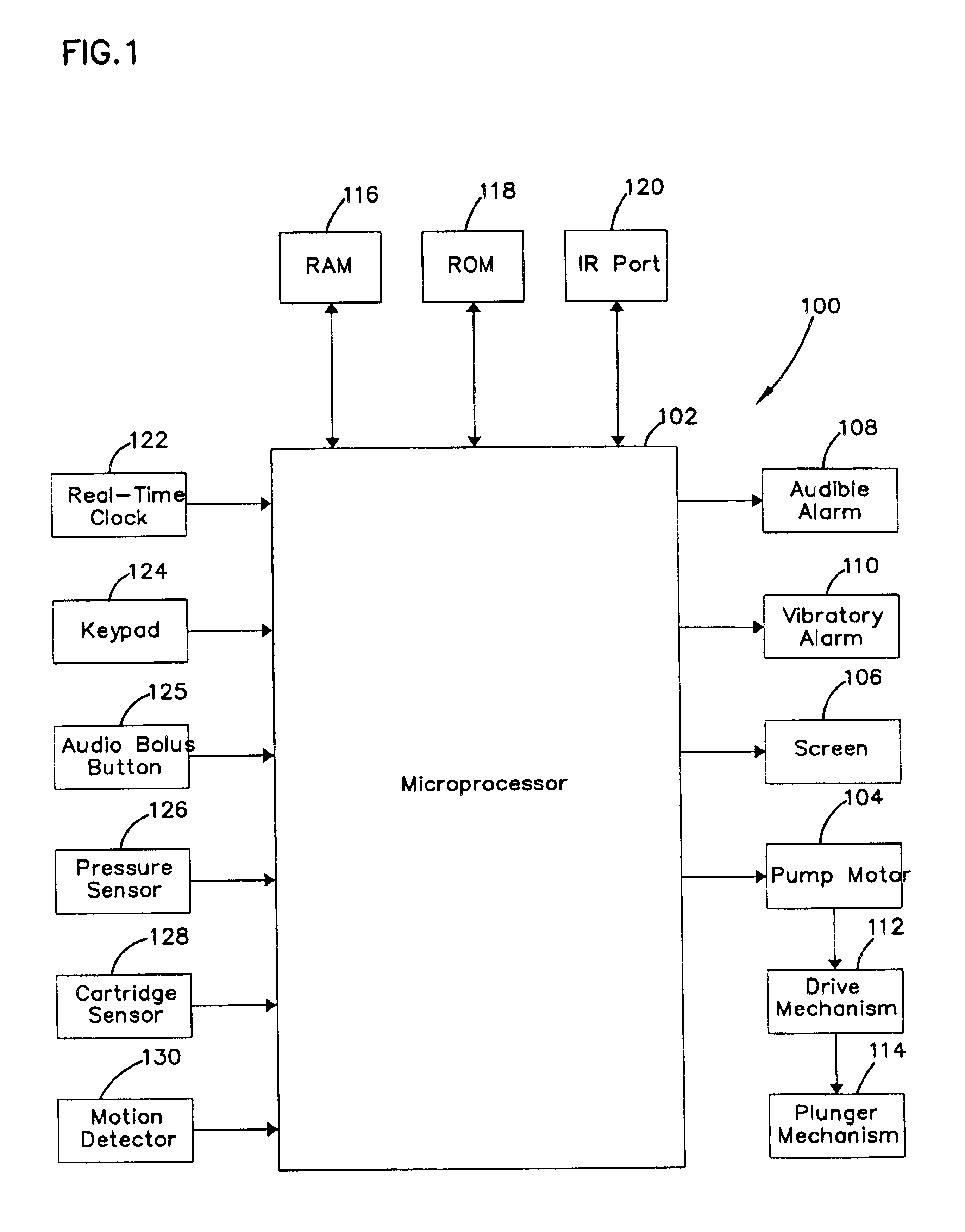
Microprocessor controlled ambulatory medical apparatus with hand held communication device
InactiveUS6873268B2Enhance user interfaceReduce system sizeEnergy efficient ICTElectrotherapyDrugs infusionHand held
An implantable infusion pump possesses operational functionality that is, at least in part, controlled by software operating in two processor ICs which are configured to perform some different and some duplicate functions. The pump exchanges messages with an external device via telemetry. Each processor controls a different part of the drug infusion mechanism such that both processors must agree on the appropriateness of drug delivery for infusion to occur. Delivery accumulators are incremented and decremented with delivery requests and with deliveries made. When accumulated amounts reach or exceed, quantized deliverable amounts, infusion is made to occur. The accumulators are capable of being incremented by two or more independent types of delivery requests. Operational modes of the infusion device are changed automatically in view of various system errors that are trapped, various system alarm conditions that are detected, and when excess periods of time lapse between pump and external device interactions.
Owner:MEDTRONIC MIMIMED INC
Analyte measuring device
InactiveUS20050033132A1Additive manufacturing apparatusMicrobiological testing/measurementAnalyteCell layer
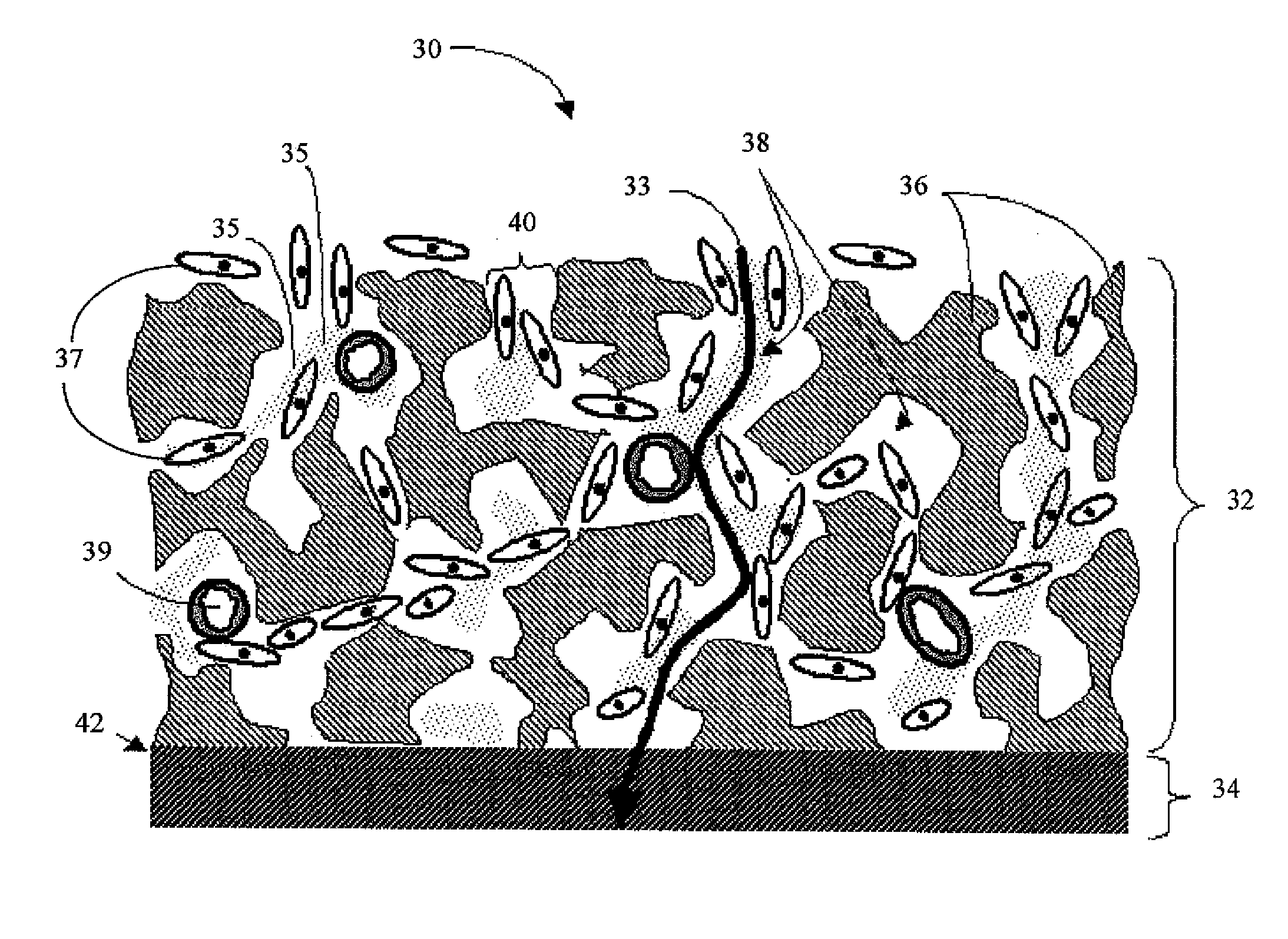
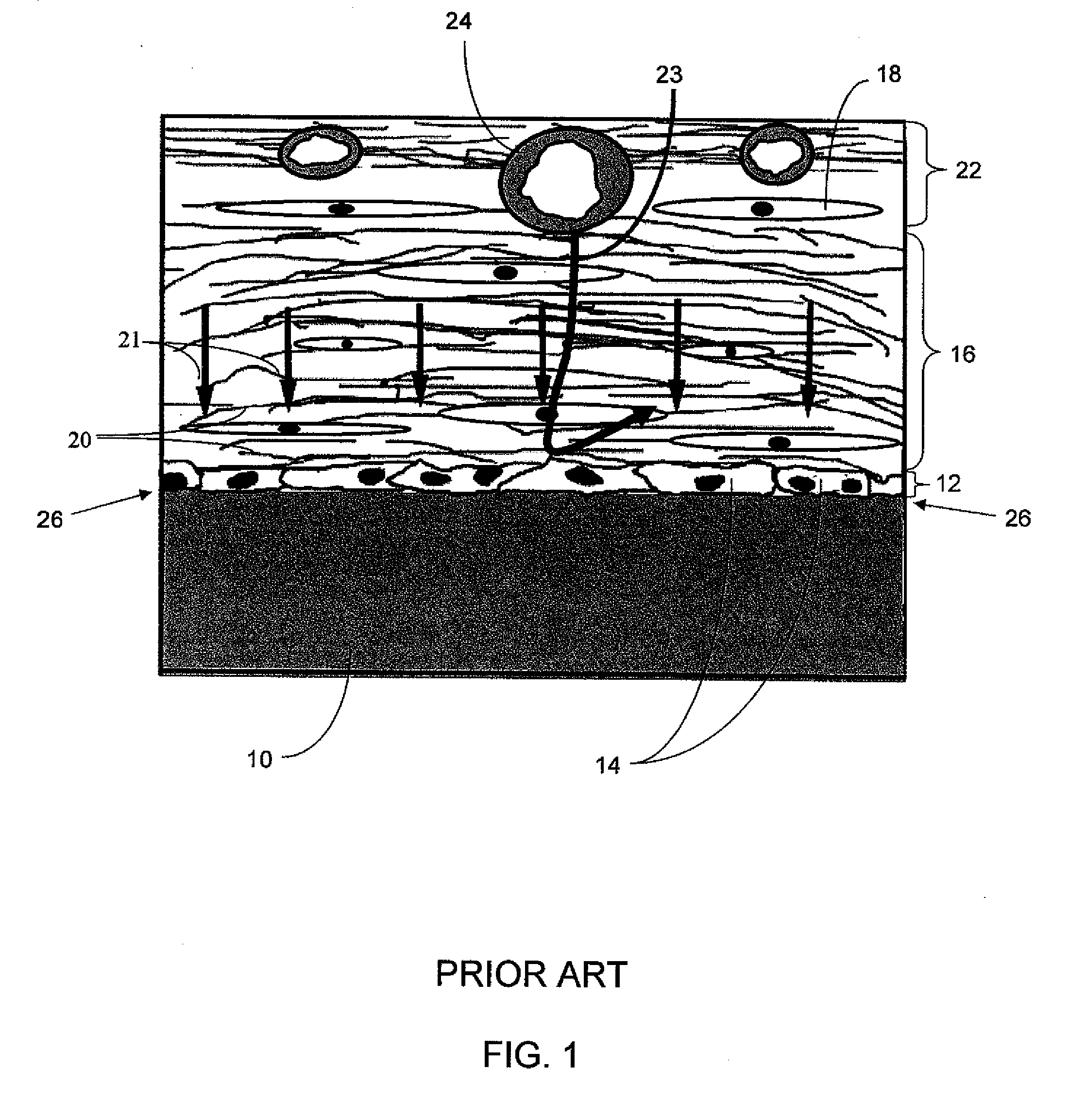
An implantable analyte-measuring device including a membrane adapted to promote vascularization and / or interfere with barrier cell layer formation. The membrane includes any combination of materials, architecture, and bioactive agents that facilitate analyte transport to provide long-term in vivo performance of the implantable analyte-measuring device.
Owner:DEXCOM
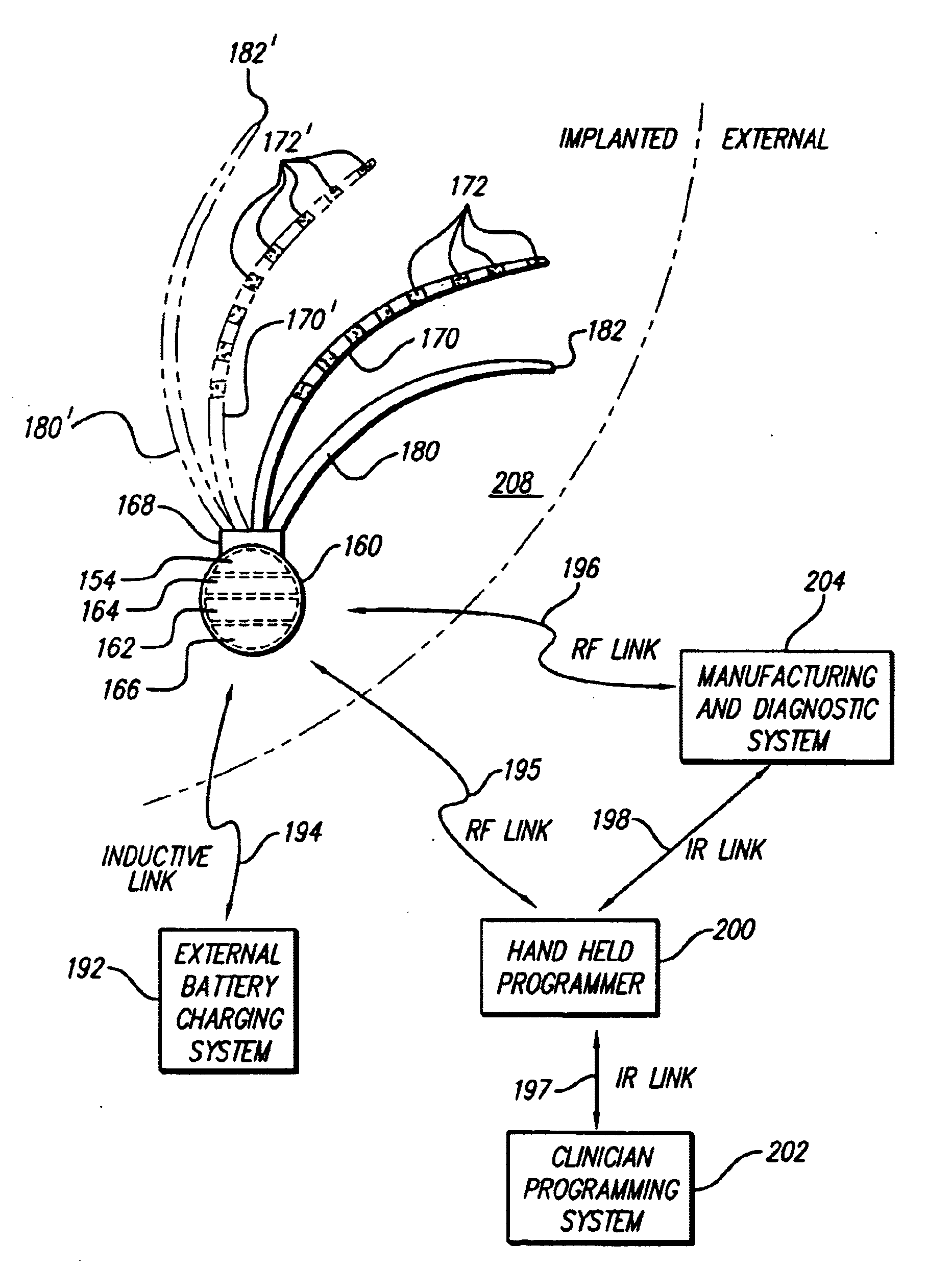
Systems and methods for modulation of circulatory perfusion by electrical and/or drug stimulation
InactiveUS6845267B2Minimal discomfortAvoid the needElectrotherapyPharmaceutical delivery mechanismElectrical batteryClosed loop
One or more implantable system control units (SCU) apply one or more stimulating drugs and / or electrical pulses to one or more predetermined areas affecting circulatory perfusion. The SCU preferably includes a programmable memory for storing data and / or control parameters, and preferably uses a power source / storage device, such as a rechargeable battery. If necessary, periodic recharging of such a power source / storage device is accomplished, for example, by inductive coupling with an external appliance. The SCU provides a means of stimulating a nerve(s) or other tissue with electrical and / or infusion pulses when desired, without the need for external appliances during the stimulation session. When necessary, external appliances are used for the transmission of data to and / or from the SCU(s) and / or for the transmission of power. In a preferred embodiment, the system is capable of open- and closed-loop operation. In closed-loop operation, at least one SCU includes a sensor, and the sensed condition is used to adjust electrical and / or drug stimulation parameters.
Owner:BOSTON SCI NEUROMODULATION CORP
Ambulatory medical apparatus and method using a telemetry system with predefined reception listening
InactiveUS6950708B2Reduce power consumptionConsuming and burdensomeEnergy efficient ICTElectrotherapyAmbulatoryStart time
An implanted medical device (e.g. infusion pump) and an external device communicate with one another via telemetry messages that are receivable only during windows or listening periods. Each listening period is open for a prescribed period of time and is spaced from successive listening periods by an interval. The prescribed period of time is typically kept small to minimize power consumption. To increase likelihood of successful communication, the window may be forced to an open state, by use of an attention signal, in anticipation of an incoming message. To further minimize power consumption, it is desirable to minimize use of extended attention signals, which is accomplished by the transmitter maintaining an estimate of listening period start times and attempting to send messages only during listening periods. In the communication device, the estimate is updated as a result of information obtained with the reception of each message from the medical device.
Owner:MEDTRONIC MIMIMED INC
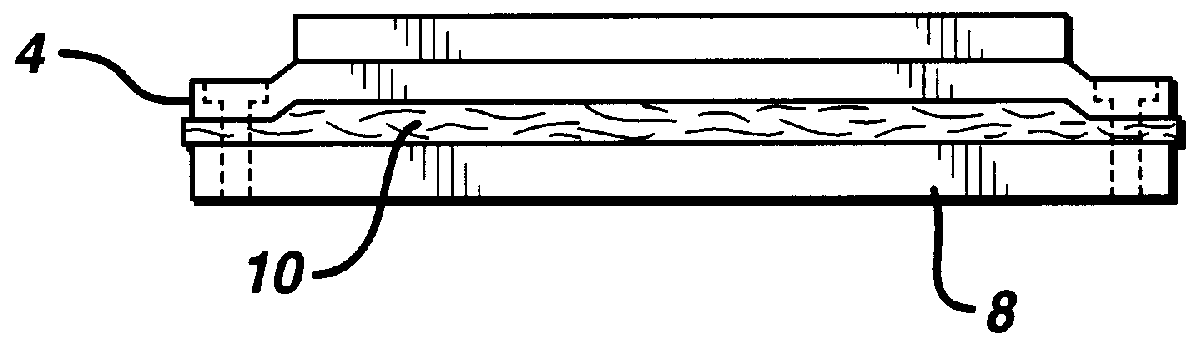
Fabrication of biocompatible polymeric composites
InactiveUS6147135ASure easyImprove performanceSuture equipmentsCosmetic preparationsFiberPolymer science
Composite materials formed from biocompatible polymer fibers and biodegradable polymers are disclosed. The heat treatment conditions for the reinforcing fibers are described so that the mechanical properties of the fibers can be retained during composite consolidation process. The processing conditions and set-ups to consolidations are constrained to the temperatures lower than fiber heat treatment temperatures. The reinforcing fibers are restrained under tension so that the minimum relaxation occurs during consolidation process.
Owner:ETHICON INC
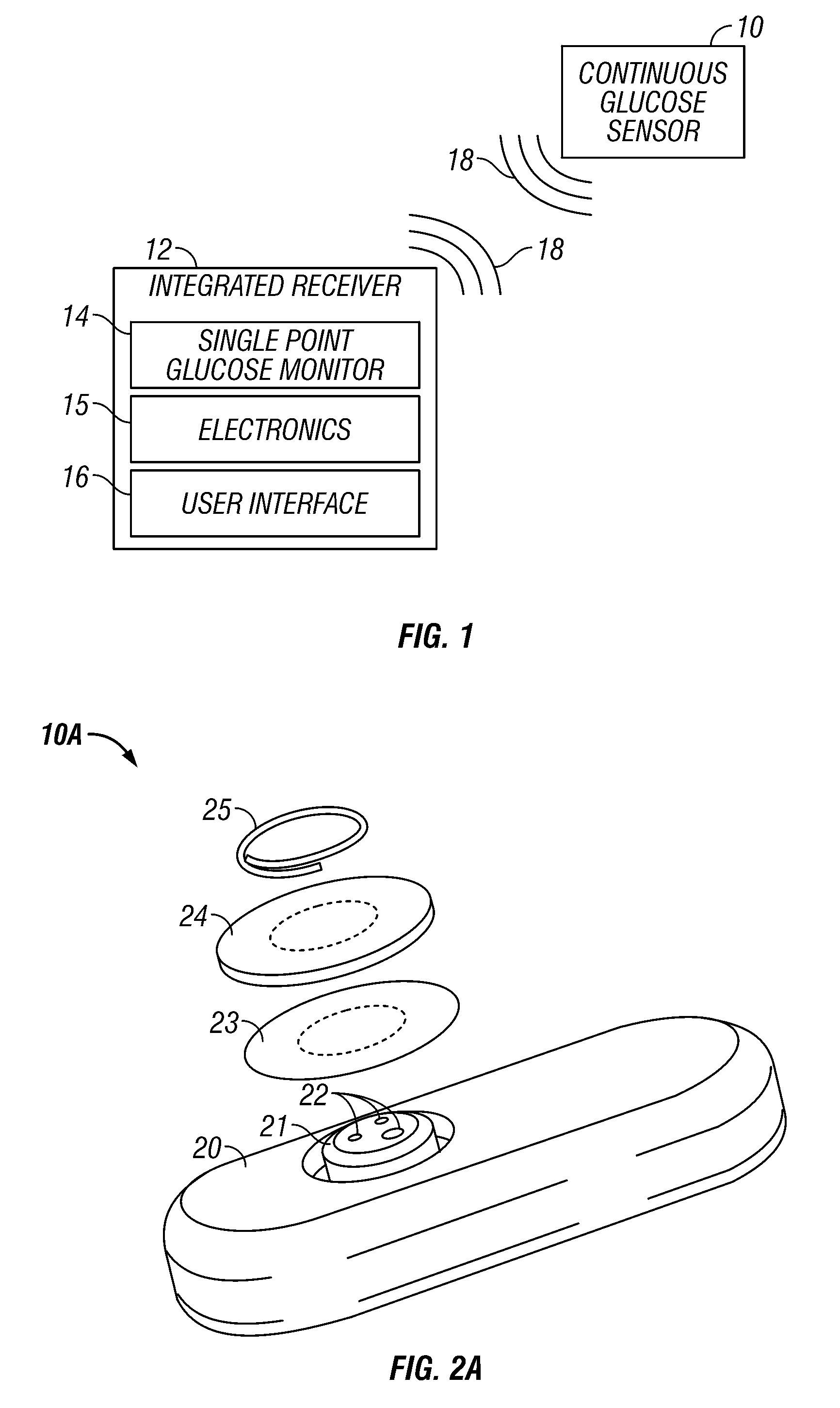
Integrated receiver for continuous analyte sensor
InactiveUS20080287764A1Simpler and few componentReduce errorsPharmaceutical delivery mechanismEndoradiosondesGlucose sensorsData stream
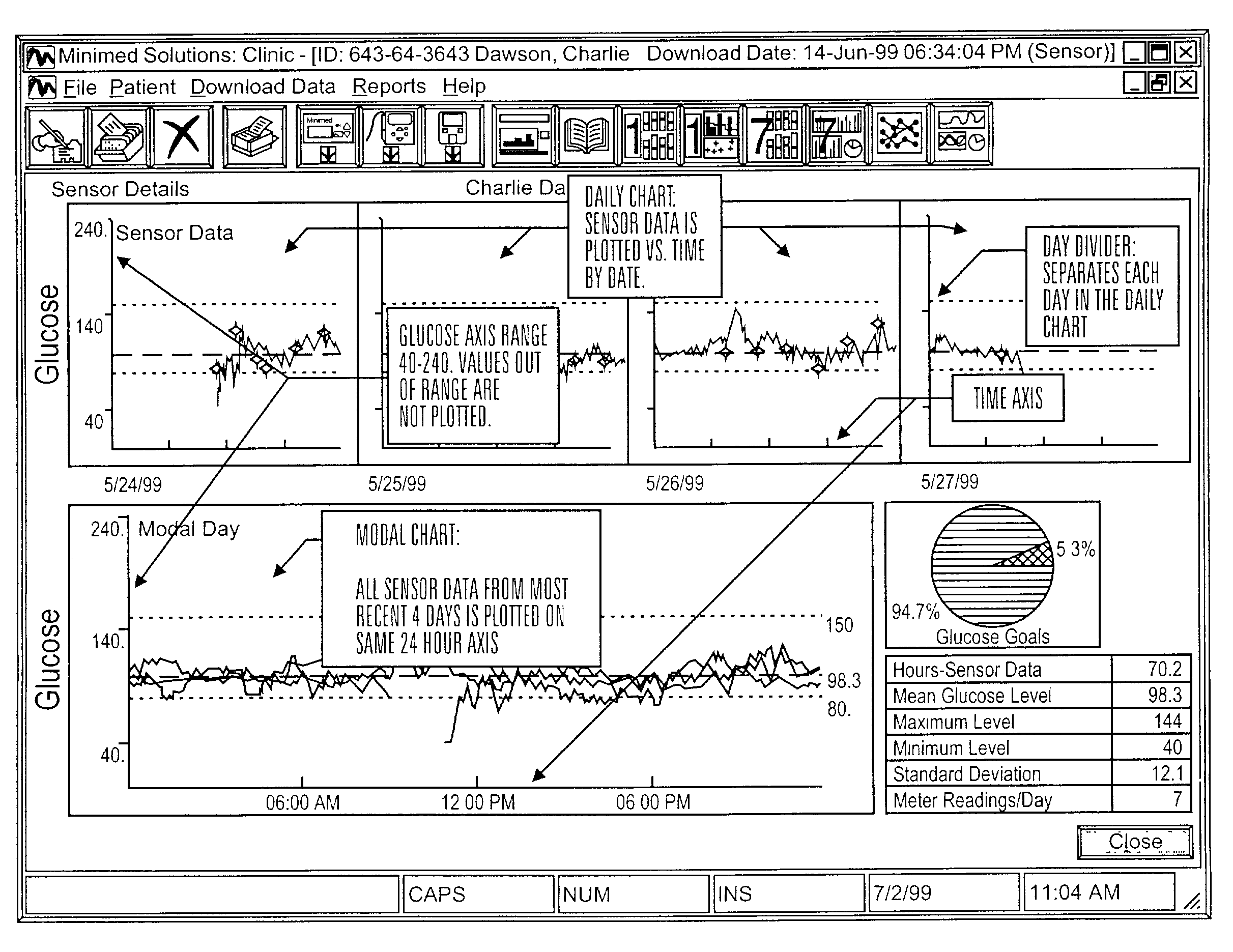
A system is provided for monitoring glucose in a host, including a continuous glucose sensor that produces a data stream indicative of a host's glucose concentration and an integrated receiver that receives the data stream from the continuous glucose sensor and calibrates the data stream using a single point glucose monitor that is integral with the integrated receiver. The integrated receiver obtains a glucose value from the single point glucose monitor, calibrates the sensor data stream received from the continuous glucose sensor, and displays one or both of the single point glucose measurement values and the calibrated continuous glucose sensor values on the user interface.
Owner:DEXCOM INC
Human antibodies that bind human IL-12 and methods for producing
InactiveUS6914128B1Avoid interferencePreservationNervous disorderPeptide/protein ingredientsAntigen bindingIn vivo
Human antibodies, preferably recombinant human antibodies, that specifically bind to human interleukin-12 (hIL-12) are disclosed. Preferred antibodies have high affinity for hIL-12 and neutralize hIL-12 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. The antibodies, or antibody portions, of the invention are useful for detecting hIL-12 and for inhibiting hIL-12 activity, e.g., in a human subject suffering from a disorder in which hIL-12 activity is detrimental. Nucleic acids, vectors and host cells for expressing the recombinant human antibodies of the invention, and methods of synthesizing the recombinant human antibodies, are also encompassed by the invention.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Method of preparation of bioabsorbable porous reinforced tissue implants and implants thereof
Owner:DEPUY SYNTHES PROD INC
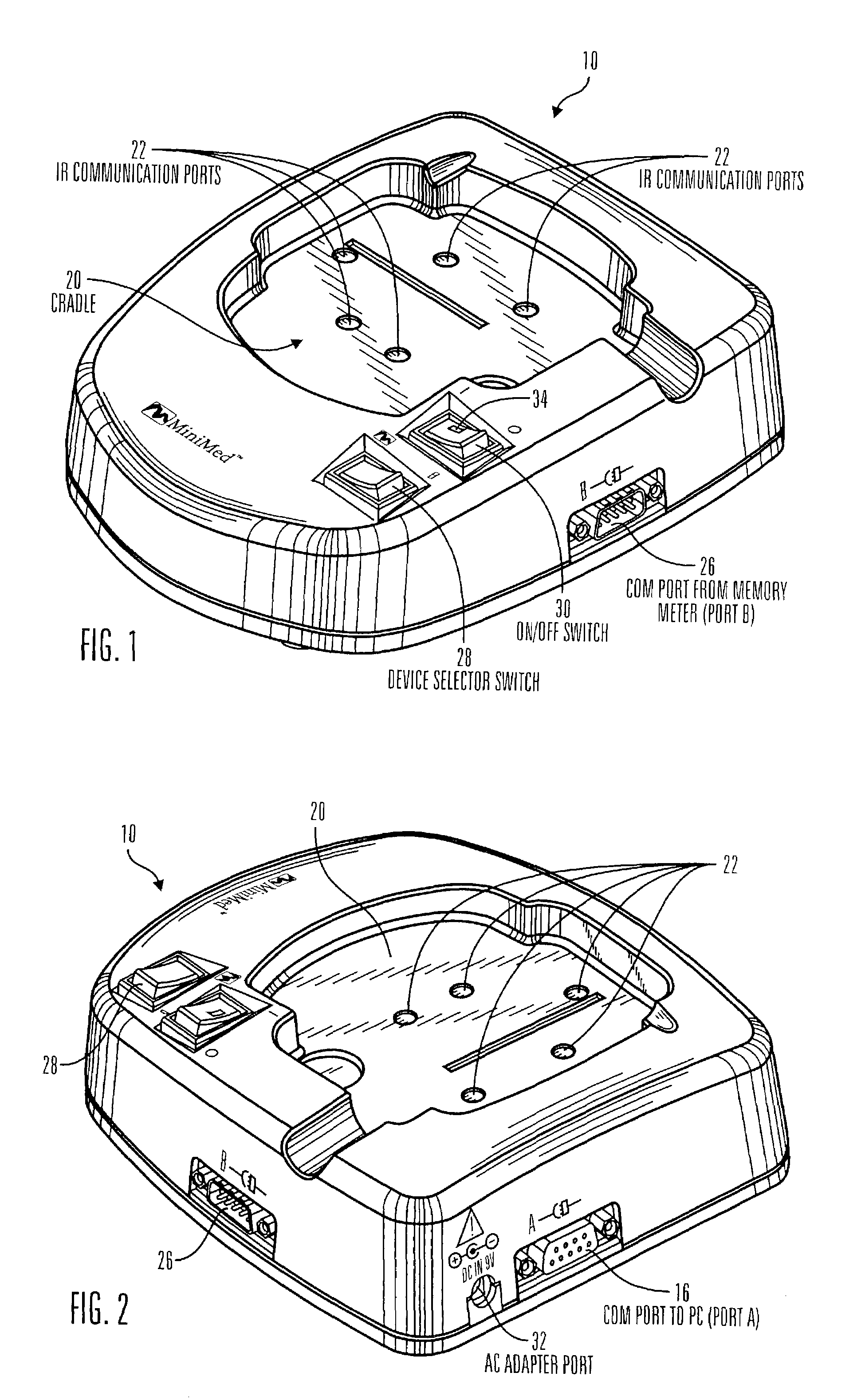
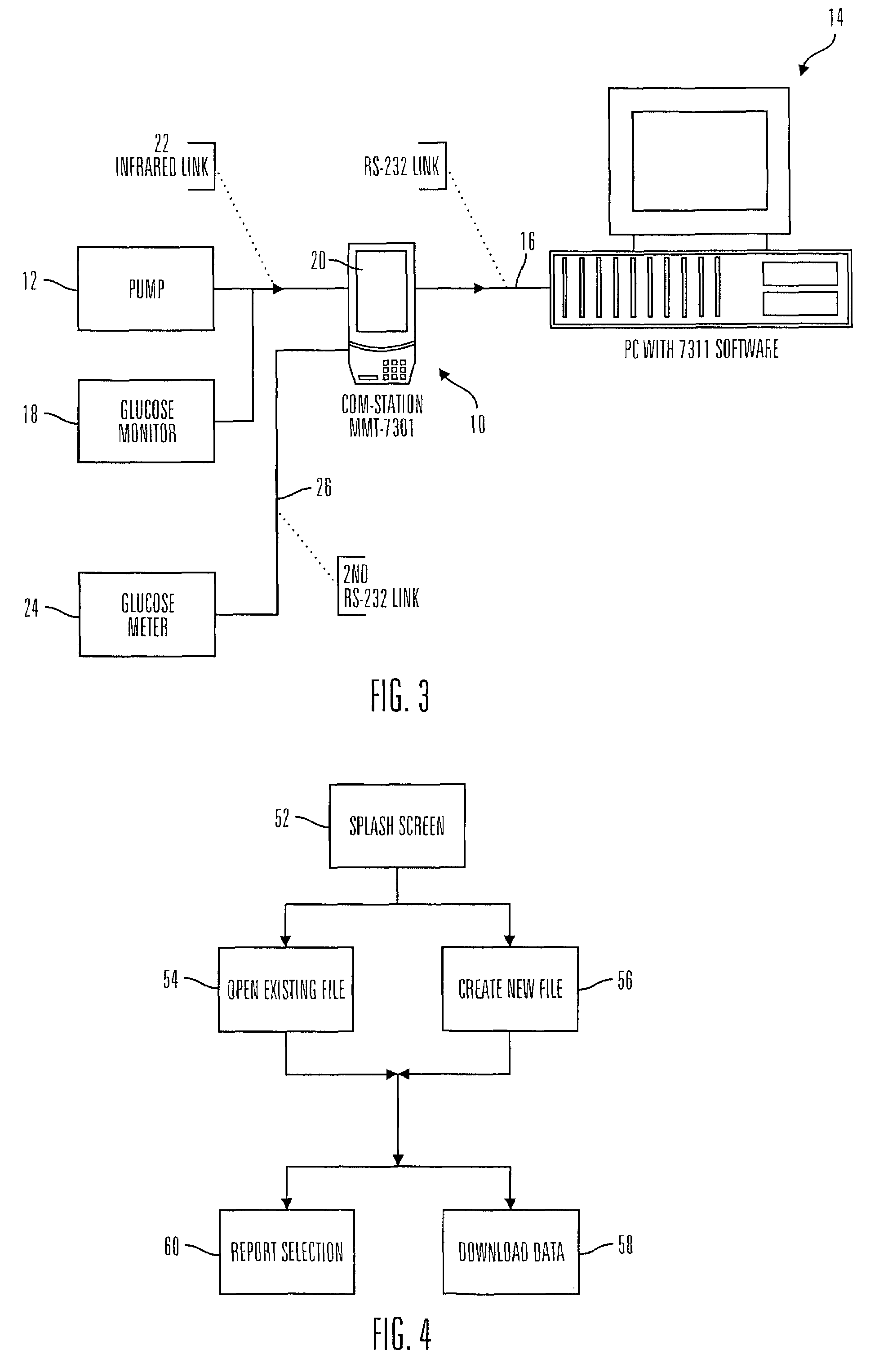
Communication station and software for interfacing with an infusion pump, analyte monitor, analyte meter, or the like
InactiveUS7647237B2Peptide/protein ingredientsDigital data processing detailsAnalyteSystem combination
A communication station is for use with a medical device (such as an infusion pump) and a processing device (such as a computer). The communication station includes a housing, a medical device interface coupled to the housing, a processing device interface coupled to the housing and a processor coupled to the housing. The device interface interfaces with the medical device, and the processing device interface interfaces with the processing device. The processor provides a communication path between the medical device and the processing device such that programming and instructions may be communicated from the processing device to the medical device and data may be transferred from the medical device to the processing device. The communication station may be combined with a system that is capable of generating reports either locally or remotely. In addition, the medical device interface may be a cradle that is configurable to attach to different shaped medical devices.
Owner:MINIMED
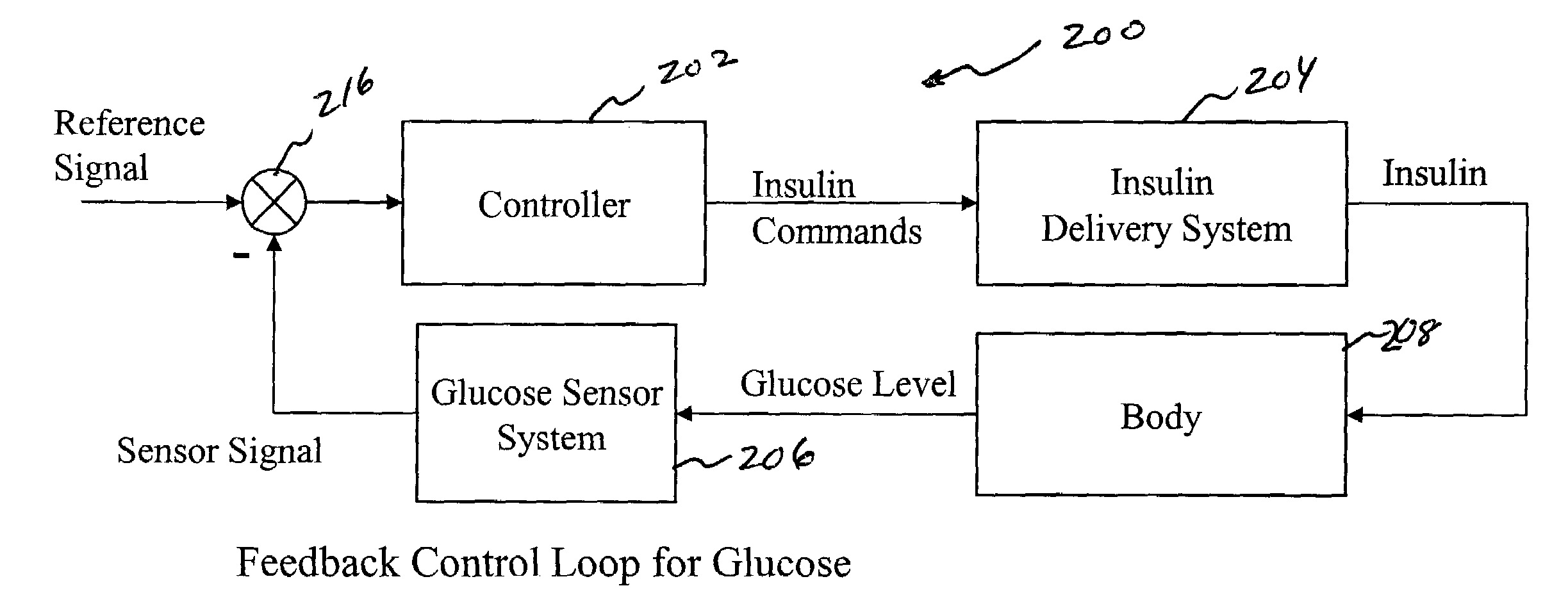
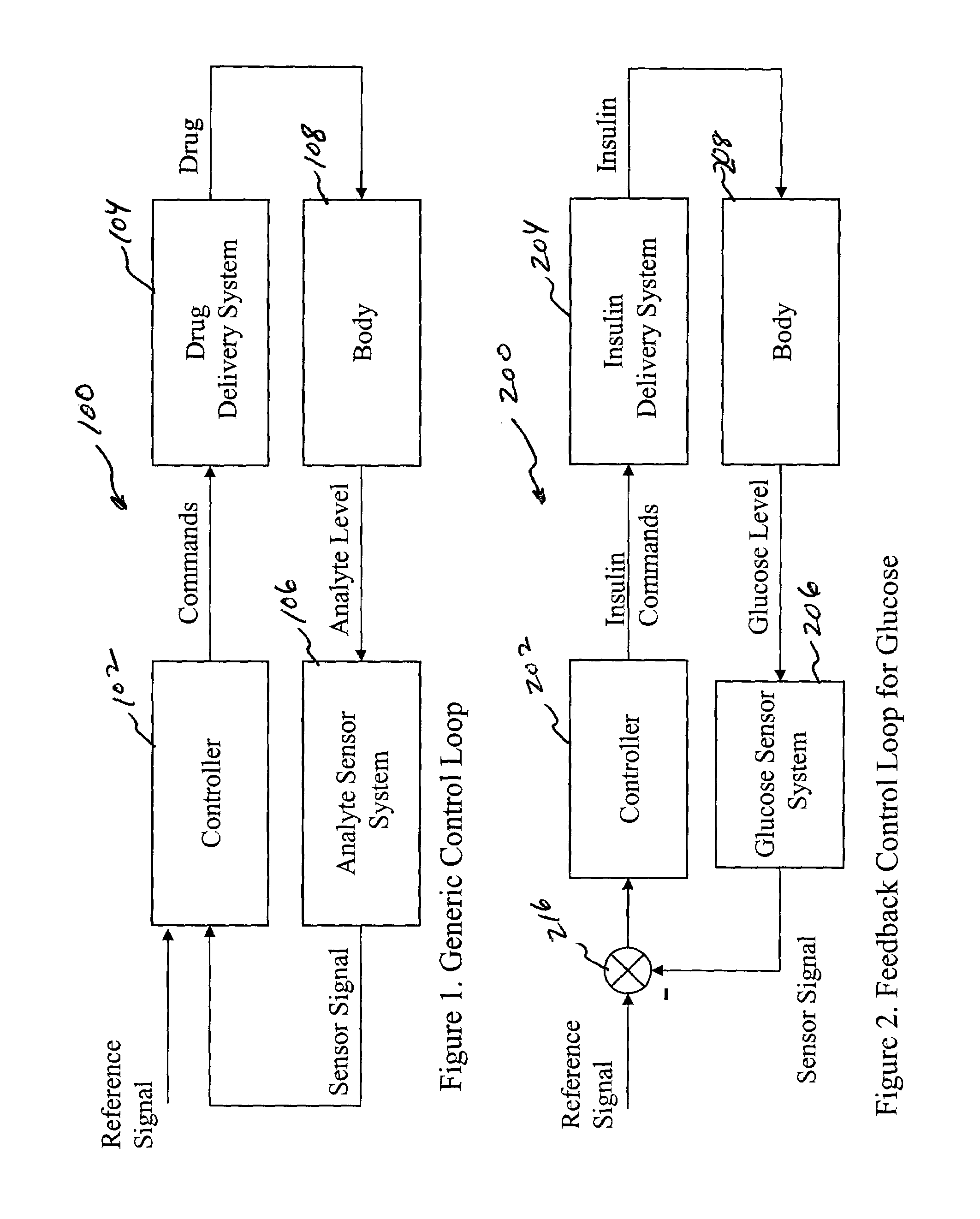
System and method for initiating and maintaining continuous, long-term control of a concentration of a substance in a patient using a feedback or model-based controller coupled to a single-needle or multi-needle intradermal (ID) delivery device
ActiveUS7060059B2Maintaining continuous, long-term control of the blood glucose concentrationsImprove performancePeptide/protein ingredientsDrug and medicationsInsulin infusionClosed loop
A closed loop therapy system for controlling a concentration of a substance, such as blood glucose concentration, in the body of a user. The system and method employ a sensor system that measures a glucose level in the body, a controller that uses the measured glucose levels to generate an output that can be used to automatically or manually control an intradermal insulin infusion system to set a constant or time-varying profile of target blood glucose concentrations in a user, and then infuse an appropriate amount of insulin into the body of the user so as to reach and maintain the target values of the blood glucose concentration.
Owner:BECTON DICKINSON & CO
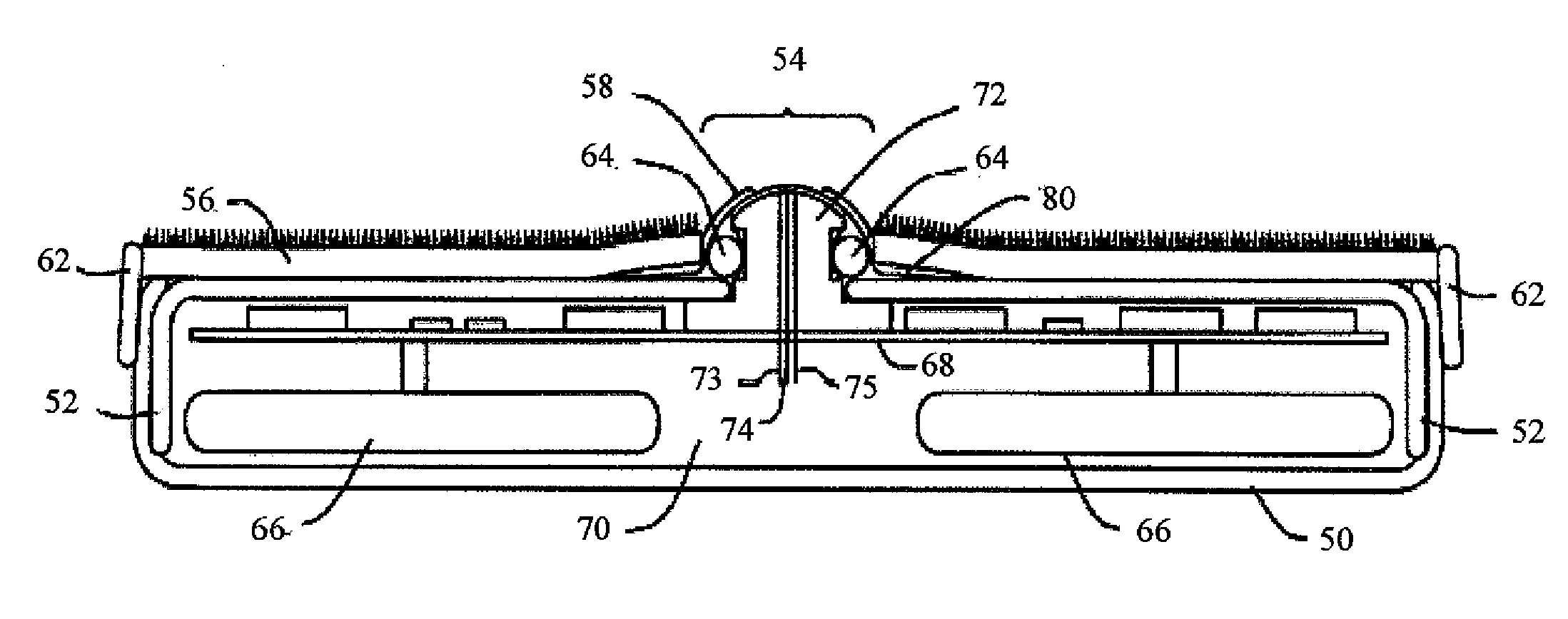
Wound dressing and method for controlling severe, life-threatening bleeding
ActiveUS7371403B2Stanching flowProhibiting flow of bloodBiocideSuspensory bandagesHydrophilic polymersWound dressing
This invention is directed to advanced hemorrhage control wound dressings, and methods of using and producing same. The subject wound dressing is constructed from a non-mammalian material for control of severe bleeding. The wound dressing for controlling severe bleeding is formed of a biomaterial comprising chitosan, a hydrophilic polymer, a polyacrylic polymer or a combination thereof. The kind of severe, life-threatening bleeding contemplated by this invention is typically of the type not capable of being stanched when a conventional gauze wound dressing is applied with conventional pressure to the subject wound. The wound dressing being capable of substantially stanching the flow of the severe life-threatening bleeding from the wound by adhering to the wound site, to seal the wound, to accelerate blood clot formation at the wound site, to reinforce clot formation at the wound site and prevent bleed out from the wound site, and to substantially prohibit the flow of blood out of the wound site.
Owner:PROVIDENCE HEALTH SYST OREGONDBA ST VINCENTMEDICAL CENT
Programmable insulin pump
Owner:TANDEM DIABETES CARE INC
Analyte measuring device
InactiveUS20080228054A1Additive manufacturing apparatusMicrobiological testing/measurementAnalyteCell layer
An implantable analyte-measuring device including a membrane adapted to promote vascularization and / or interfere with barrier cell layer formation. The membrane includes any combination of materials, architecture, and bioactive agents that facilitate analyte transport to provide long-term in vivo performance of the implantable analyte-measuring device.
Owner:DEXCOM INC
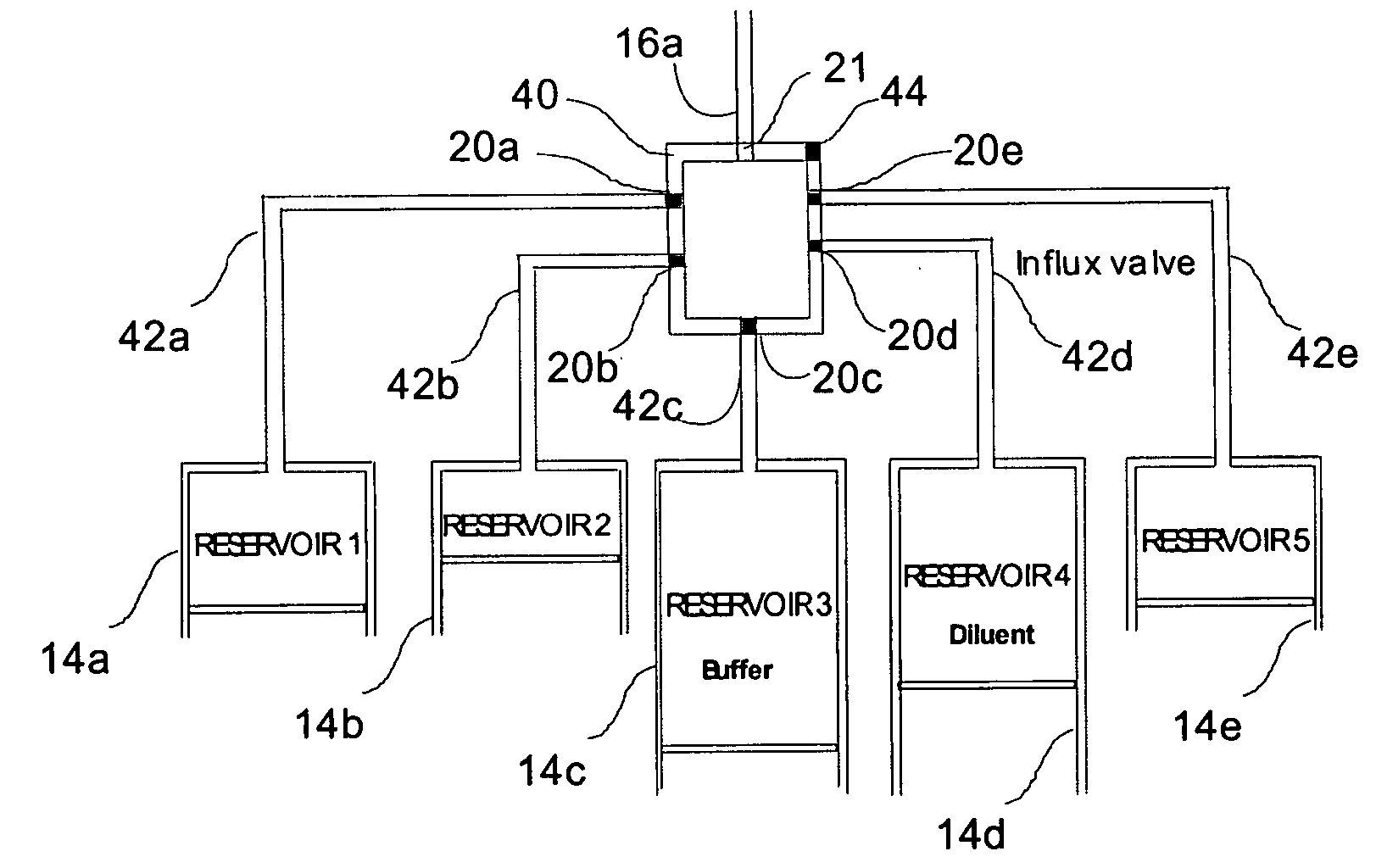
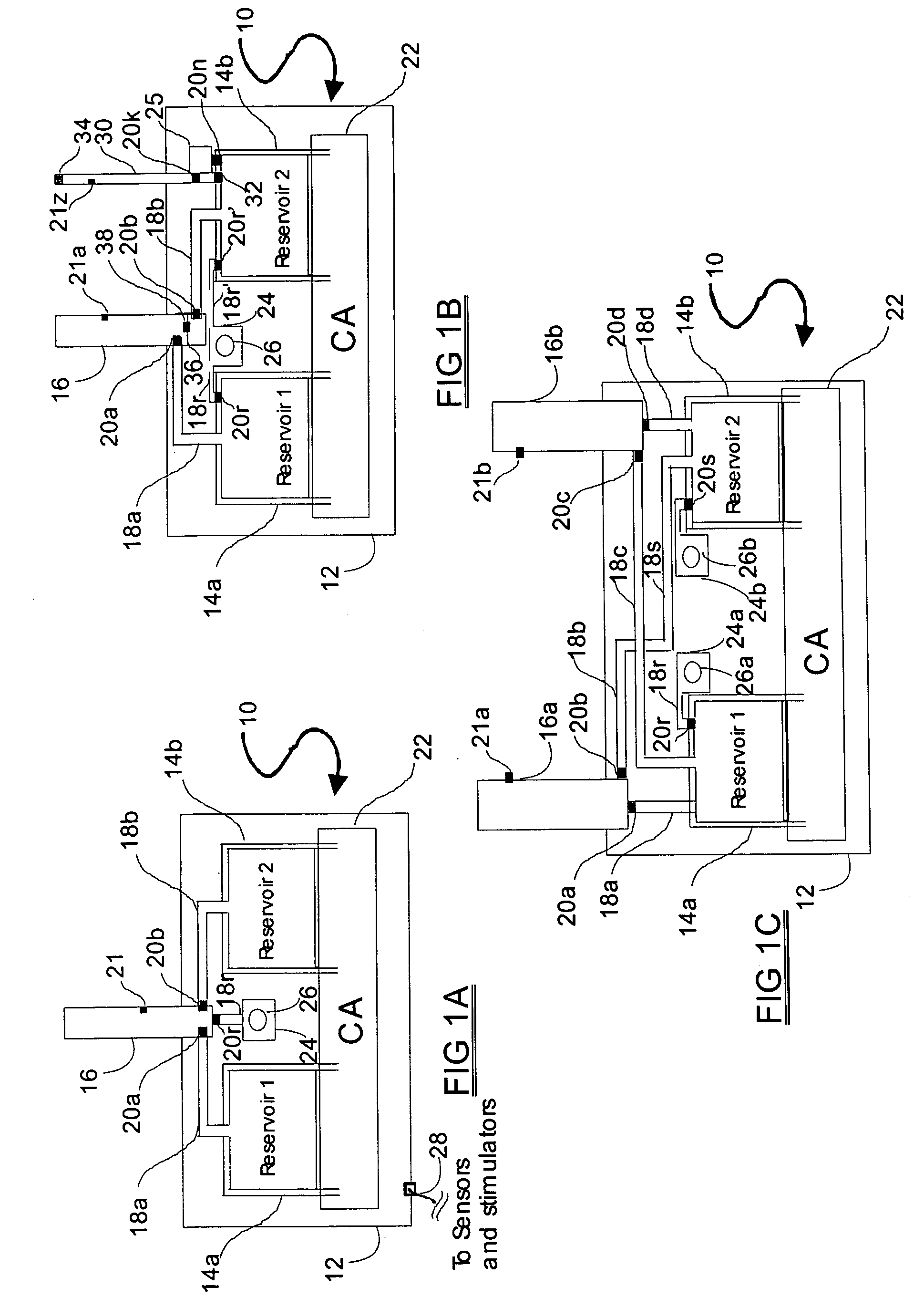
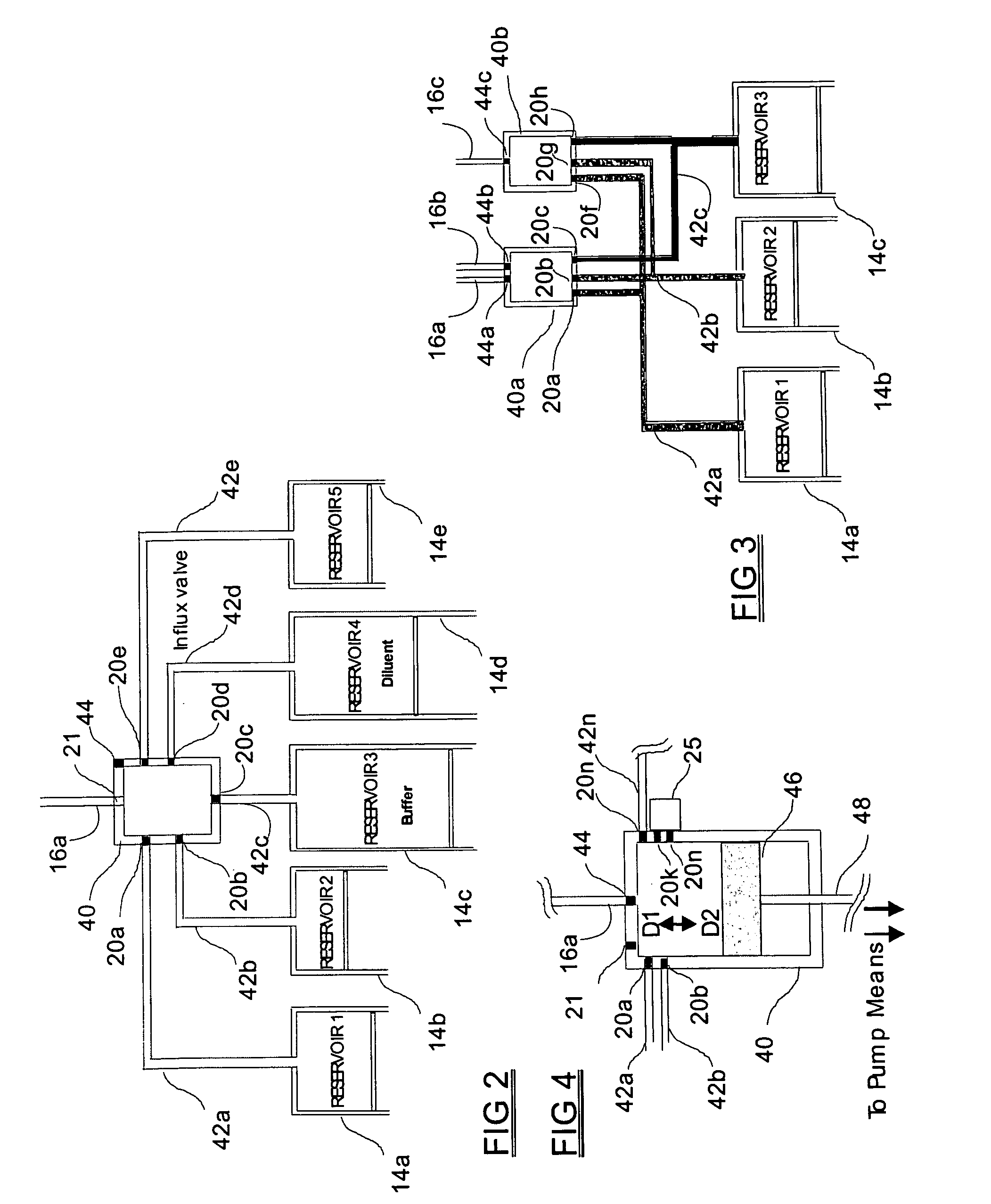
Programmable medical drug delivery systems and methods for delivery of multiple fluids and concentrations
InactiveUS20050277912A1Extension of timeEasy to fillMulti-lumen catheterDrug and medicationsControl mannerDelivery system
A drug delivery system provides for mixing various drugs in an optimally controlled manner, for using flow controllers to guide multiple drugs into a single or into multiple catheters, for enabling a single lumen catheter to treat a specific region with several drugs, for allowing for dilution of a concentrated drug in order to both increase the time between refilling and also for providing any concentration of a drug that might be desired, for using a buffer fluid to deliver exact amounts of several drugs from the same catheter or to separate several drugs within a single catheter, for using external fluid present in the human body either as a diluent or buffer fluid, and for providing for a drug testing / filler apparatus to be used prior to implant to ensure proper function and easy means of filling multiple reservoirs with different fluids, and also after implant for refilling operations. The drug delivery system (DDS) can perform both bolus and continuous delivery of substances, and enable the measured delivery of any one of several drugs to one or more distal locations at independently programmable rates. New types of catheter systems and uses therefore are also described. Catheter hub assemblies allowing for easy replacement of drug delivery systems offer advantages when replacing drug delivery systems. New methods for using the DDS in the promotion of healthy pregnancy and treatment of a developing fetus are also possible.
Owner:JOHN SASHA
Fast curing compositions
Fast curing surgical adhesives and sealants contain an NCO-terminated hydrophilic urethane prepolymer derived from an aromatic diisocyanate and a polyol. Substantially all the aromatic diisocyanate used to prepare the NCO-terminated hydrophilic urethane prepolymer is in the para form. Optionally, the aromatic diisocyanate is substituted with at least one electron withdrawing group, such as, for example, a fluorine group.
Owner:TYCO HEALTHCARE GRP LP
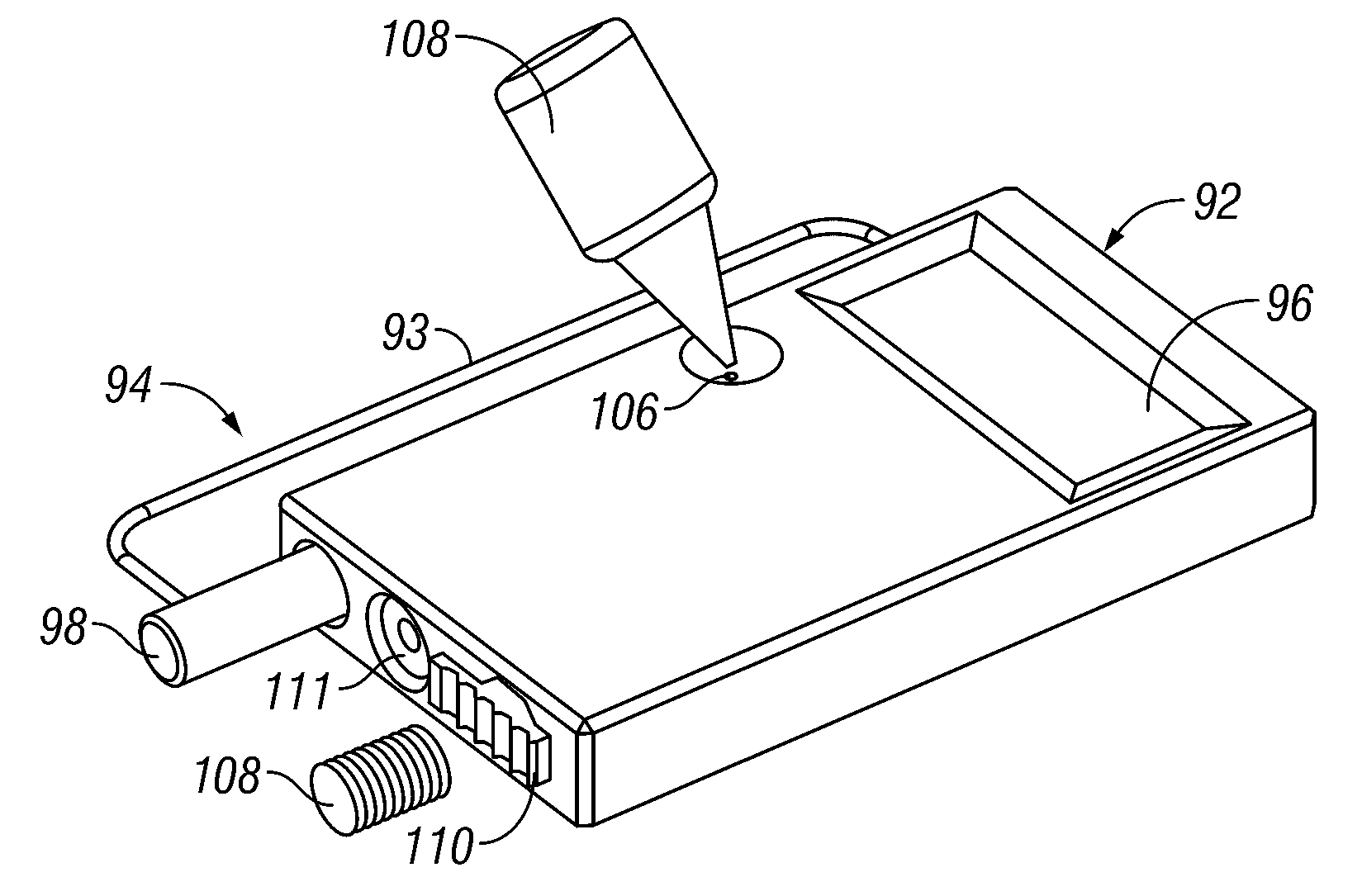
Devices, systems and methods for diagnosing and treating sinusitus and other disorders of the ears, nose and/or throat
Sinusitis, enlarged nasal turbinates, tumors, infections, hearing disorders, allergic conditions, facial fractures and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies, endoscopic studies and transillumination studies. Access and occluder devices may be used to establish fluid tight seals in the anterior or posterior nasal cavities / nasopharynx and to facilitate insertion of working devices (e.g., scopes, guidewires, catheters, tissue cutting or remodeling devices, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting devices, substance delivery implants, etc.
Owner:ACCLARENT INC
Devices, systems and methods for patient infusion
InactiveUS7029455B2Easy to carryReduce financial burdenDrug and medicationsPharmaceutical delivery mechanismUser inputRemote control
A device for delivering a fluid to a patient, including an exit port, a dispenser for causing fluid from a reservoir to flow to the exit port, a local processor programmed to cause a flow of fluid to the exit port based on flow instructions from a separate, remote control device, and a wireless receiver connected to the local processor for receiving the flow instructions. The device also includes a housing free of user input components for providing flow instructions to the local processor, in order to reduce the complexity and costs of the device so that the device lends itself to being disposable in nature. A system and a kit are also described that include the fluid delivery device, a separate, remote control device, and accessories for transcutaneous delivery of fluid medications. Methods of utilizing the fluid delivery device to infuse fluid medications are additionally disclosed.
Owner:INSULET CORP
At least partially resorbable reticulated elastomeric matrix elements and methods of making same
ActiveUS9050176B2Small volumeIncrease in sizePharmaceutical delivery mechanismPharmaceutical non-active ingredientsElastomerBiomedical engineering
The present disclosure relates to reticulated elastomeric matrices, and more particularly to at least partially degradable elastomeric elements that are compressible and exhibit resilience in their recovery and that can be employed in diverse applications including, without limitation, biological implantation, especially in humans.
Owner:DSM IP ASSETS BV
Means to achieve sustained release of synergistic drugs by conjugation
A codrug composition of at least two drug compounds covalently linked to one another via a labile bond to form a single codrug composition, or ionically linked to one another to form a single workings composition, and methods of use of the codrug for the treatment of various medical conditions. The codrug may be administered by itself or in the form of a bioerodible or nonbioerodible substance.
Owner:UNIVERSITY OF KENTUCKY +1
Analyte measuring device
InactiveUS20080228051A1Additive manufacturing apparatusMicrobiological testing/measurementAnalyteCell layer
An implantable analyte-measuring device including a membrane adapted to promote vascularization and / or interfere with barrier cell layer formation. The membrane includes any combination of materials, architecture, and bioactive agents that facilitate analyte transport to provide long-term in vivo performance of the implantable analyte-measuring device.
Owner:DEXCOM
Adhesive-containing wound closure device and method
InactiveUS20050182443A1Enlarging woundShorten operation timeSurgical adhesivesPharmaceutical delivery mechanismBiomedical engineeringWound closure
Owner:ETHICON INC
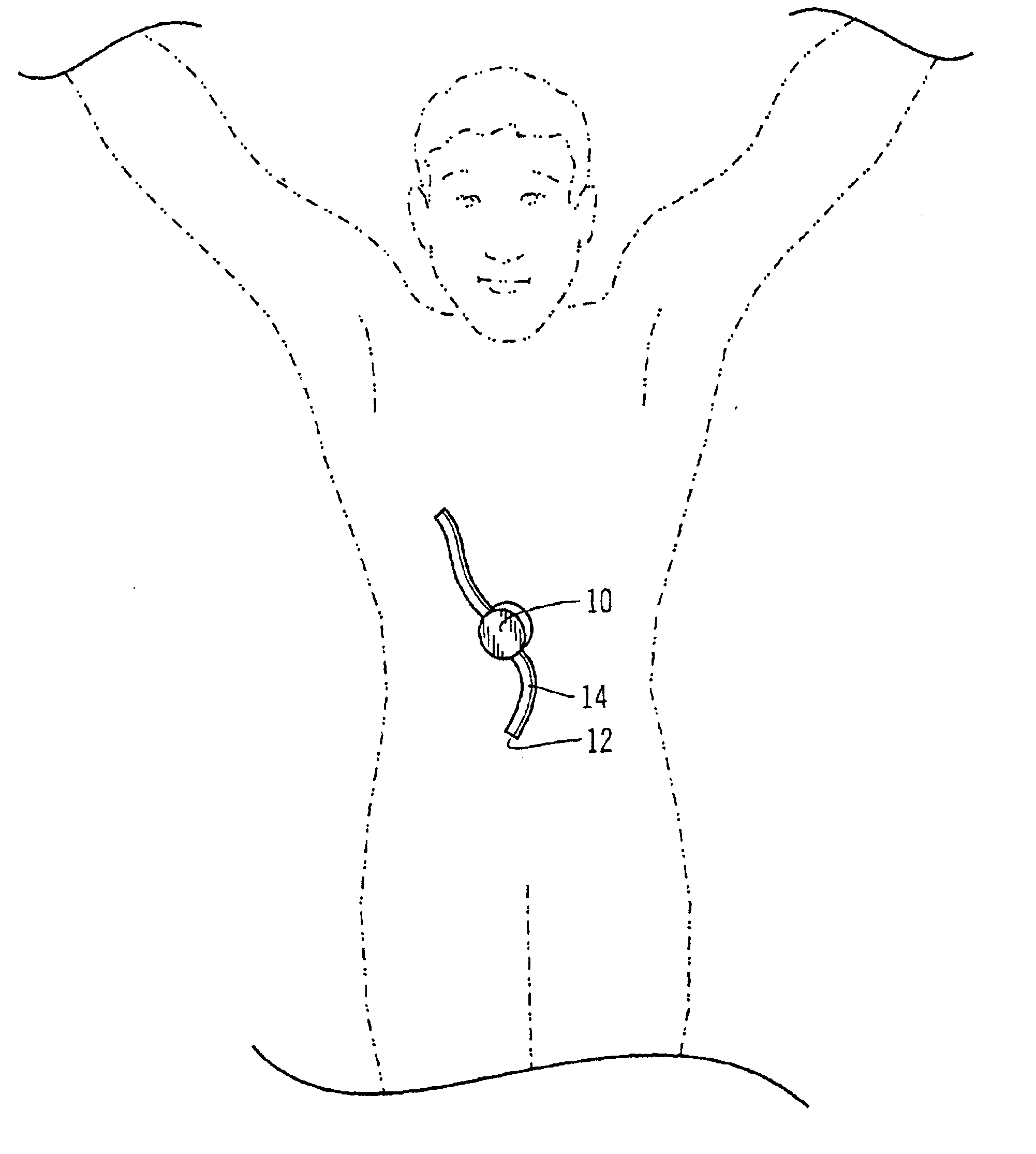
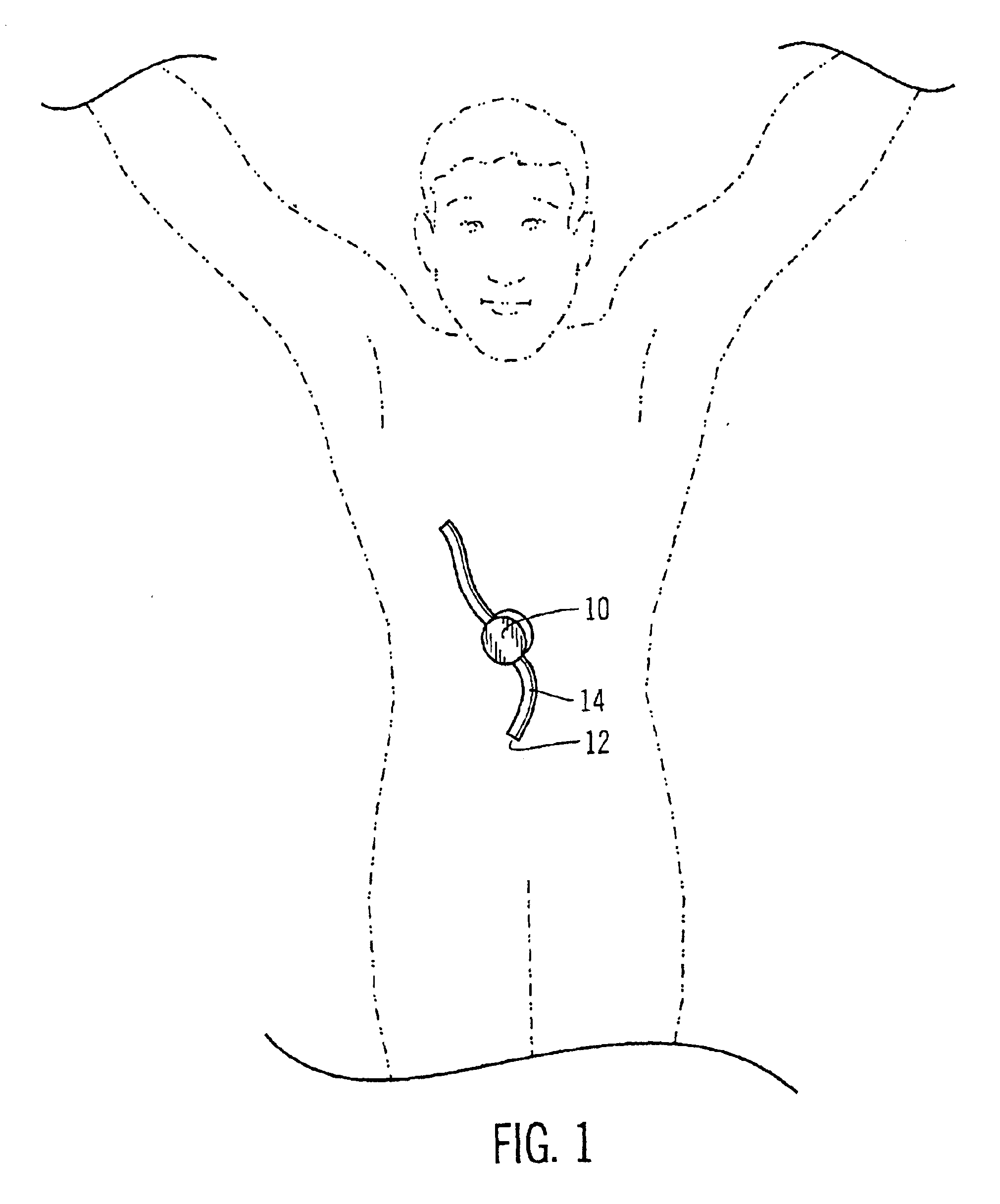
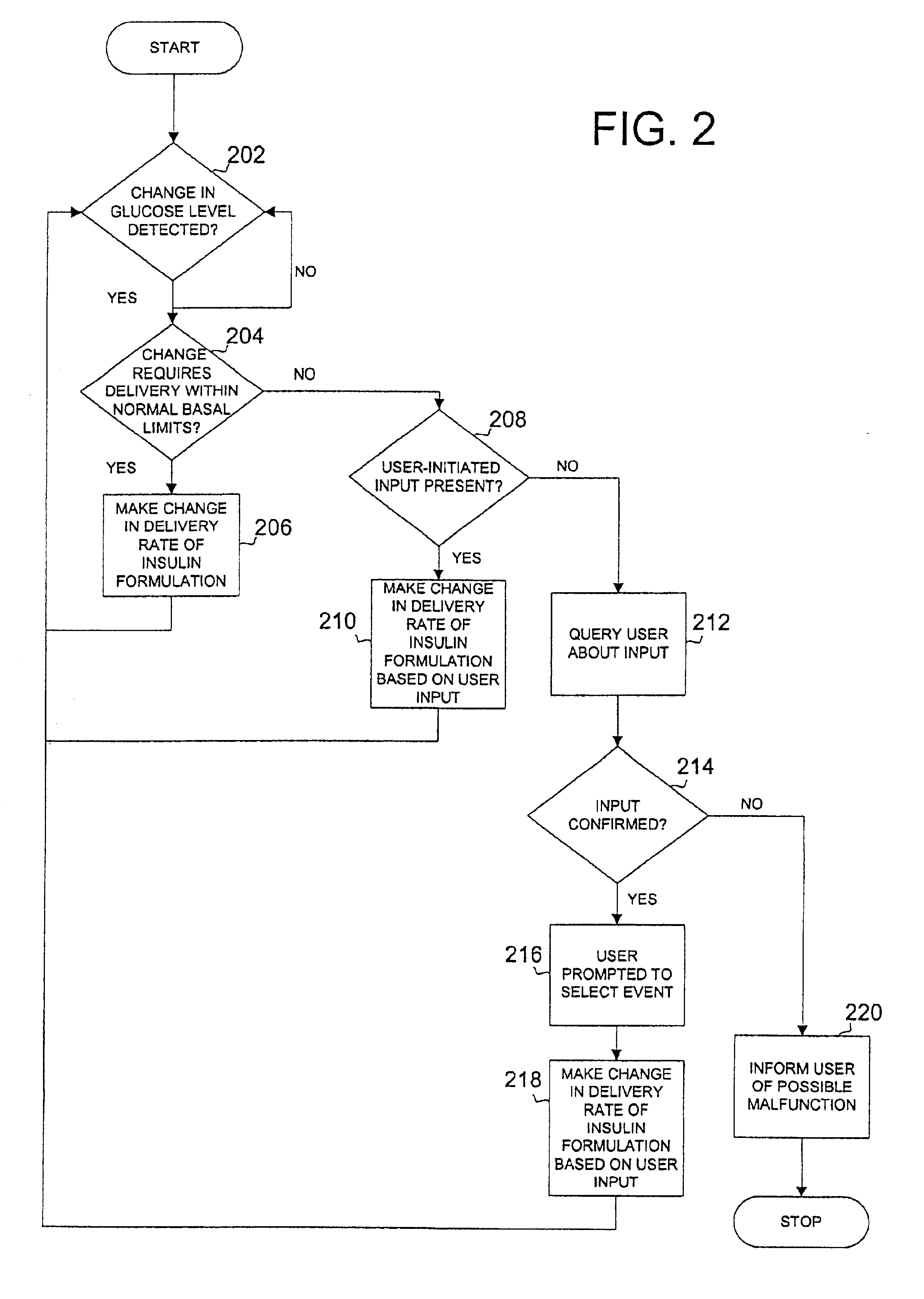
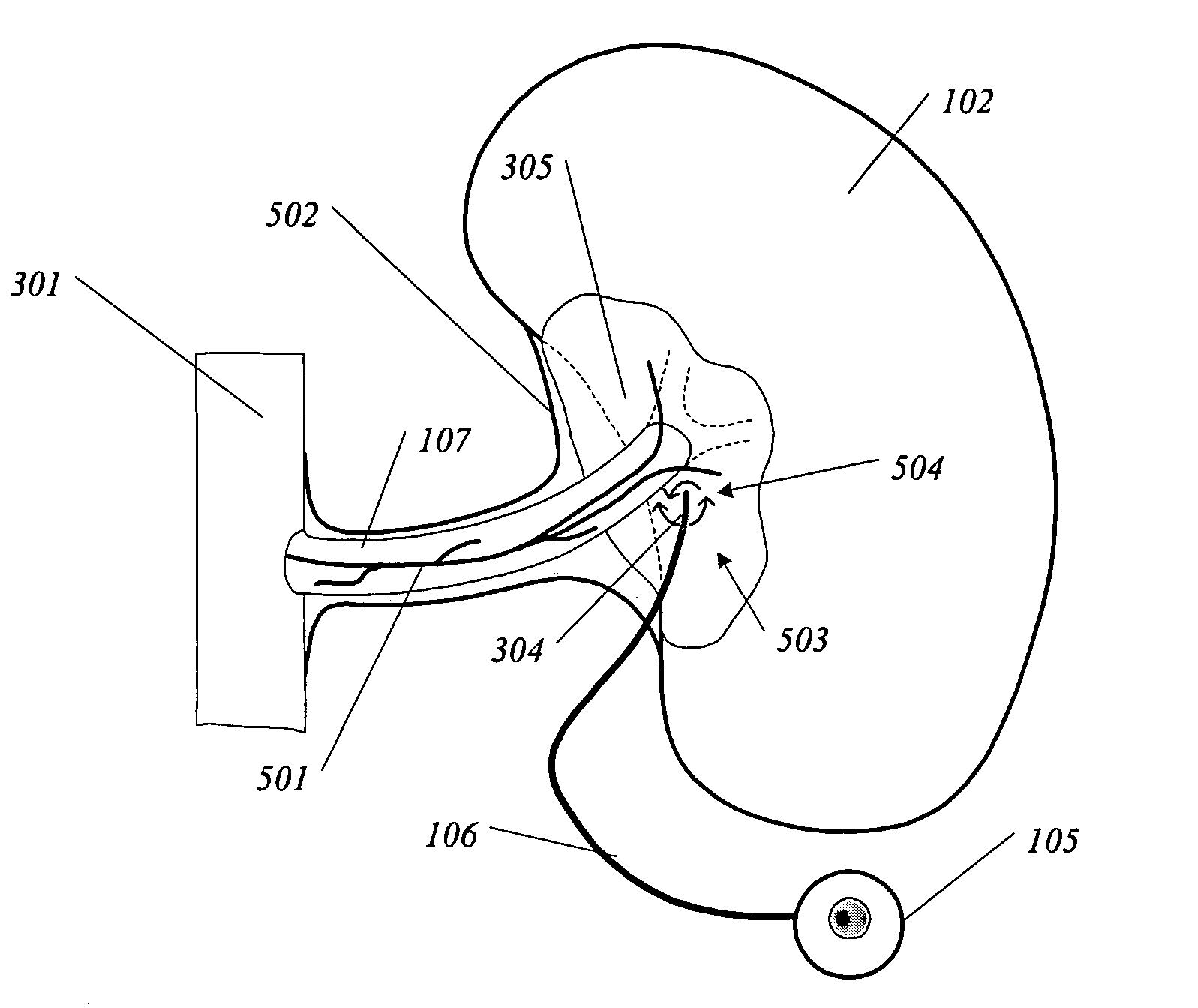
Safety limits for closed-loop infusion pump control
A system and process for providing safety limits on the delivery of an infusion formulation by an infusion pump system in response to a sensed biological state. The safety limits may comprise user-initiated event signals corresponding to events that may significantly affect the biological state. The safety limits may further comprise user-initiated event ranking signals for respective events which specify a degree, quantity, or measure for the respective event. The user-initiated event and event ranking signals may be communicated to a computing element associated with the infusion pump by an associated communication device having a user interface which comprises a plurality of user-selectable operators for entering information about the events and event rankings.
Owner:MEDTRONIC MIMIMED INC
Methods and devices for renal nerve blocking
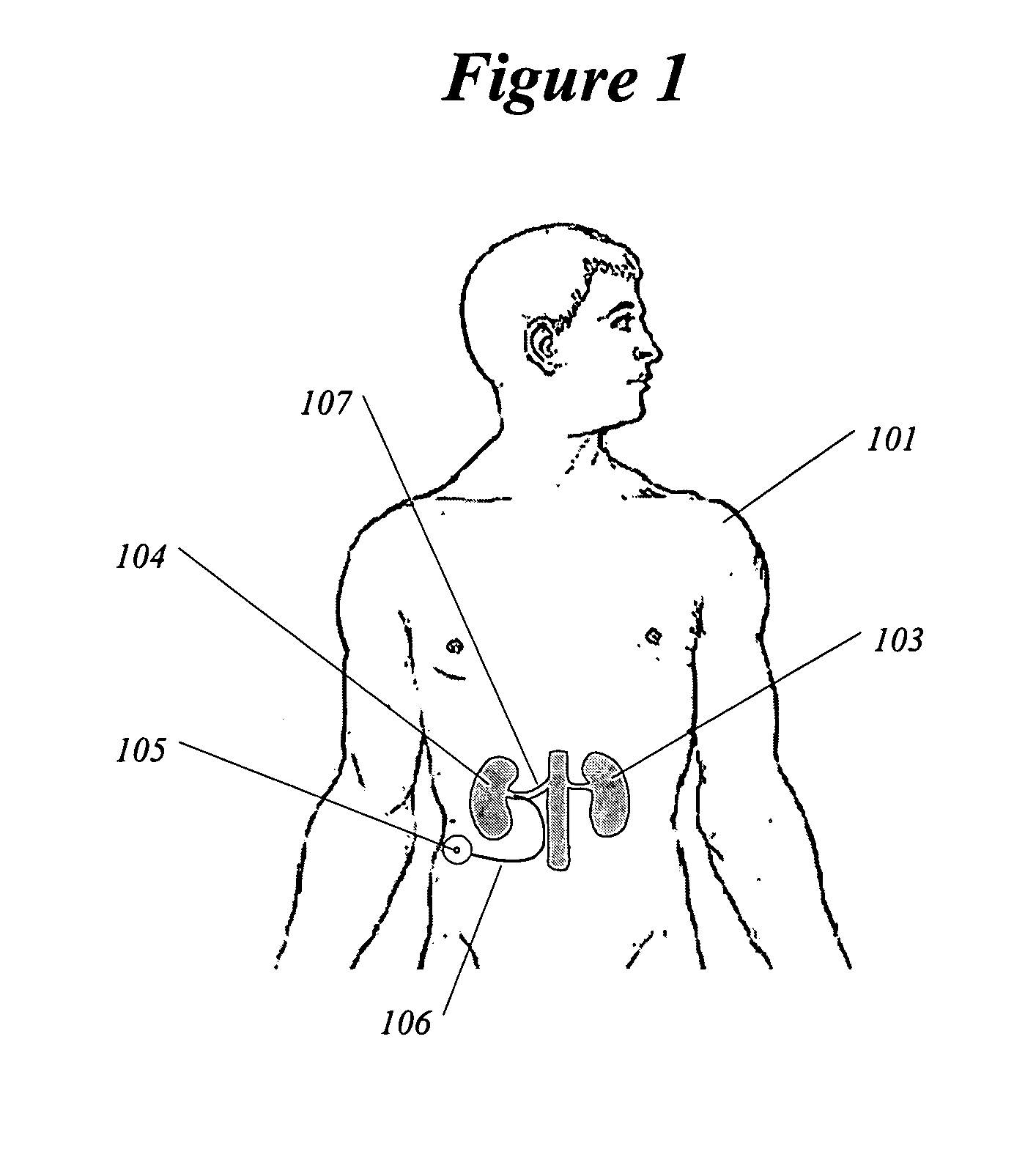
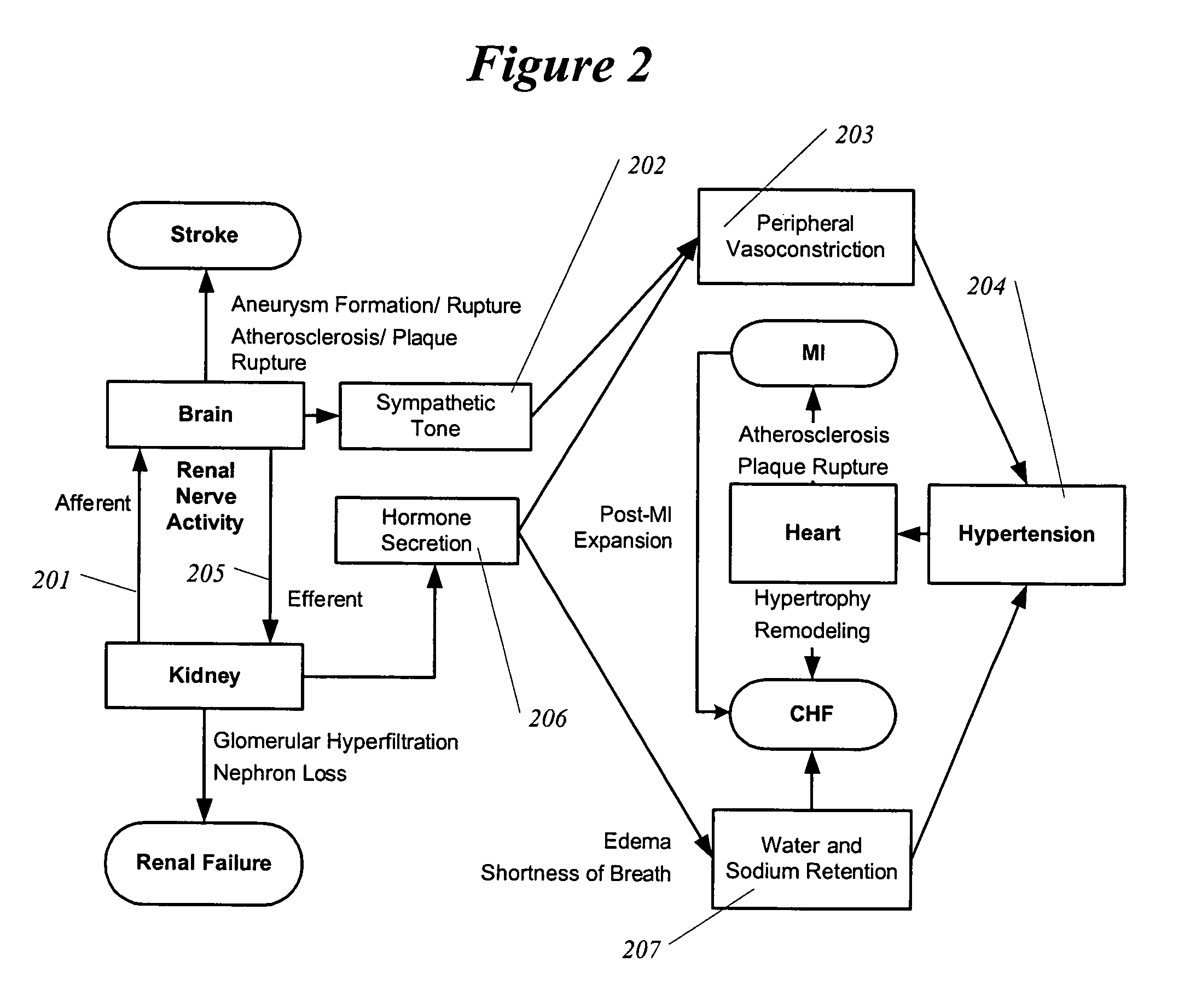
InactiveUS6978174B2Shorten the progressResolution of overloadPharmaceutical delivery mechanismImplantable neurostimulatorsDiseaseNephropathy
A method and apparatus for treatment of cardiac and renal diseases associated with the elevated sympathetic renal nerve activity by implanting a device to block the renal nerve signals to and from the kidney. The device can be a drug pump eluting implant for targeted delivery of a nerve-blocking agent to the periarterial space of the renal artery.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
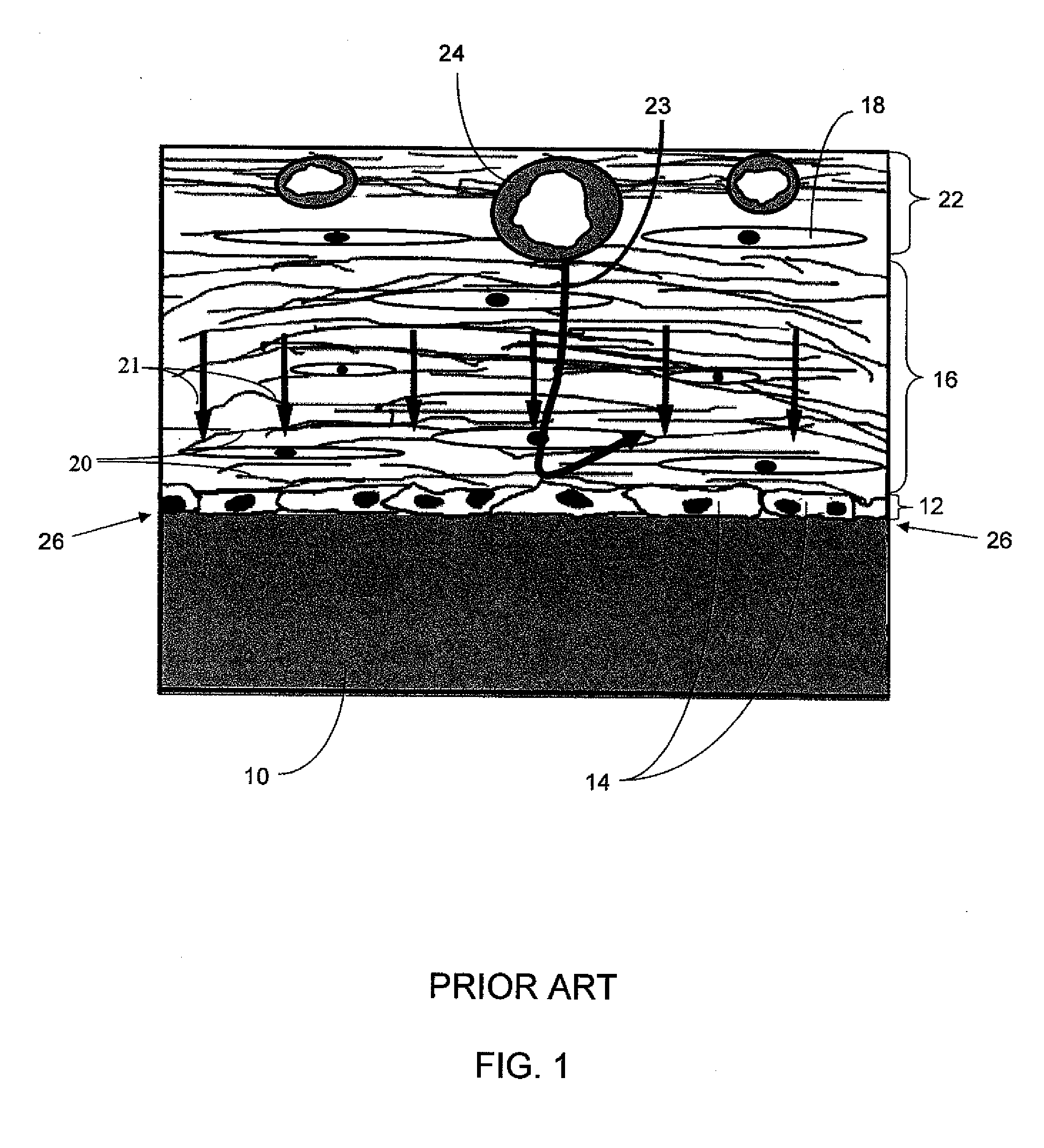
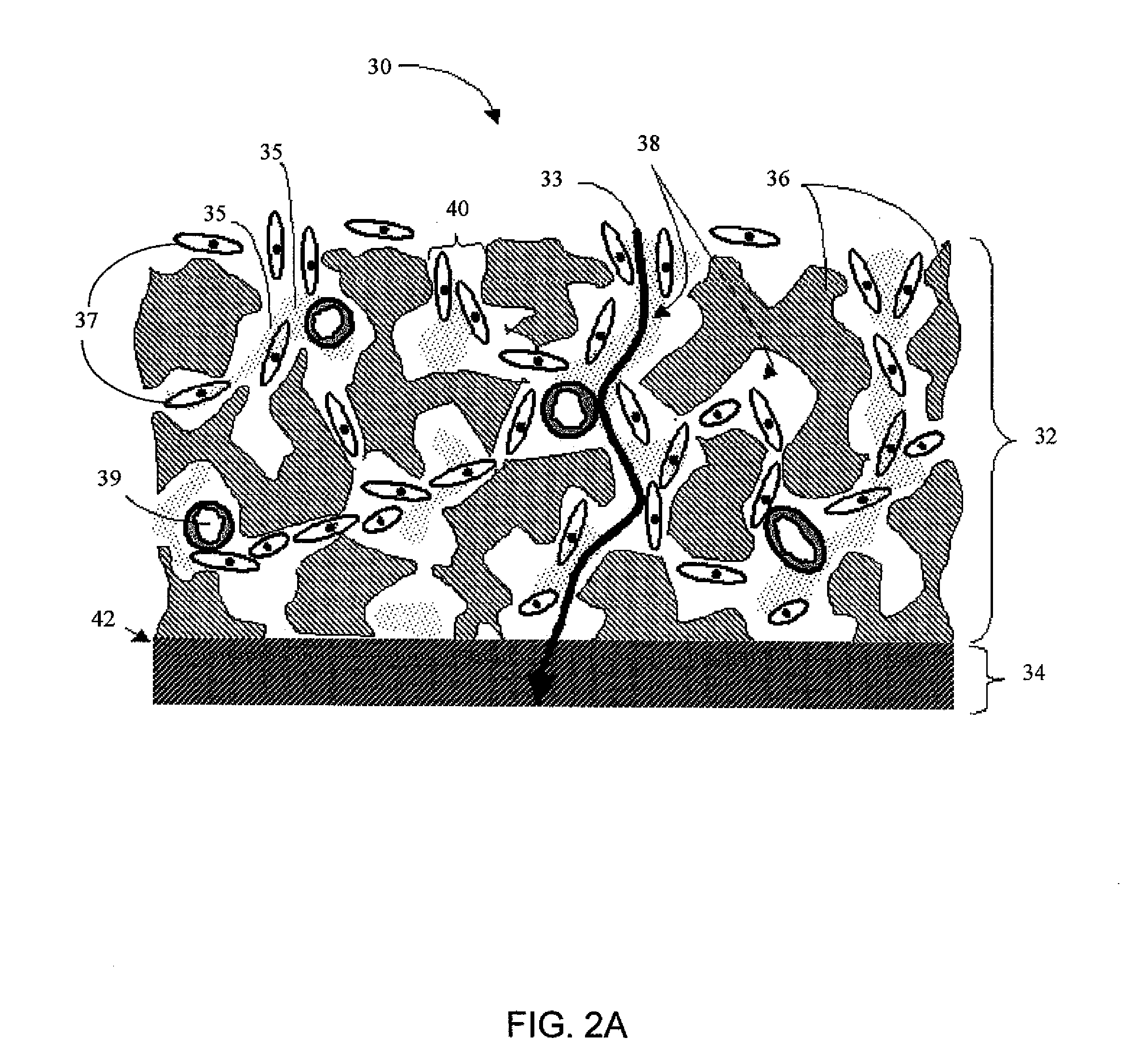
Biointerface with macro- and micro-architecture
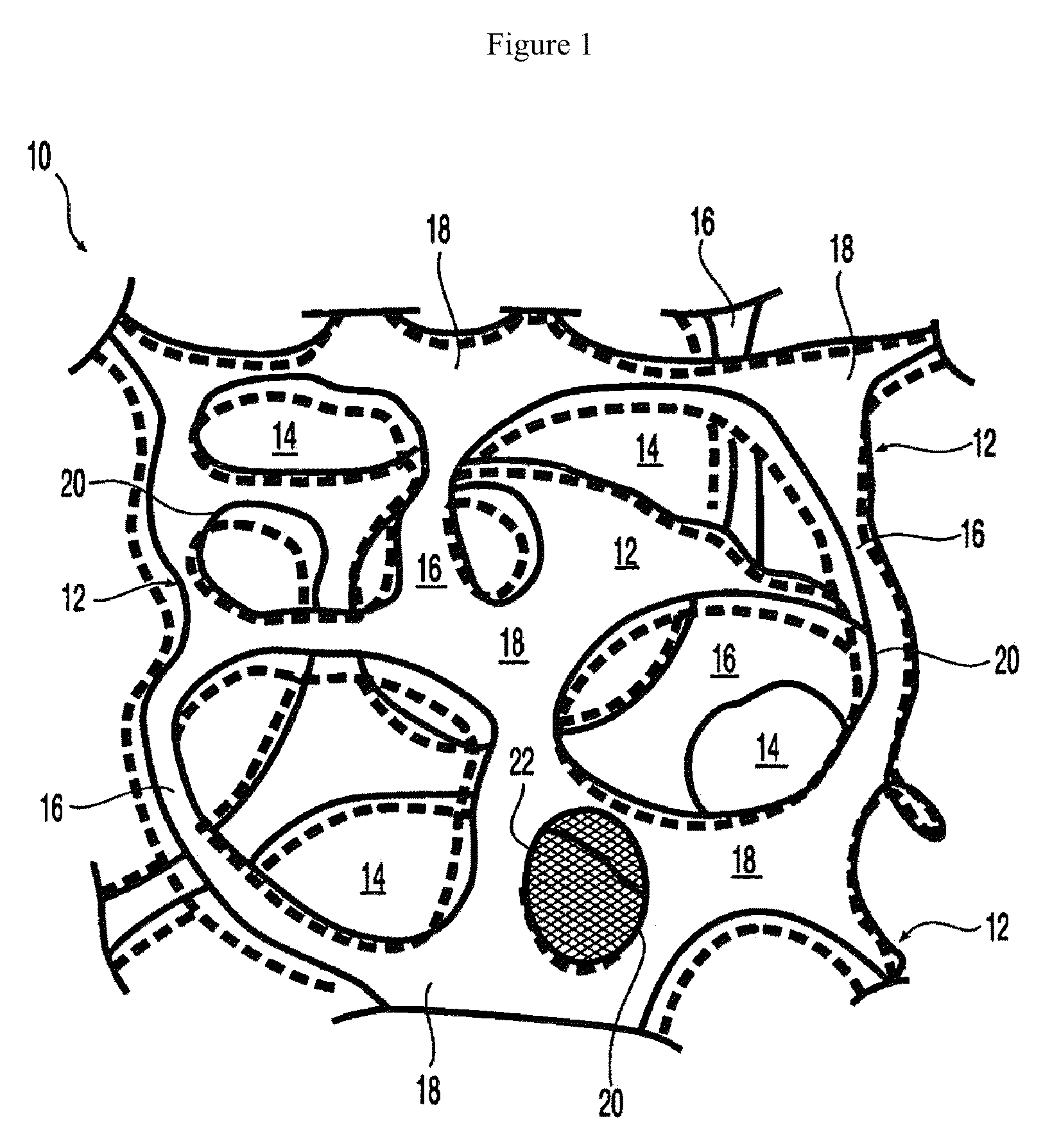
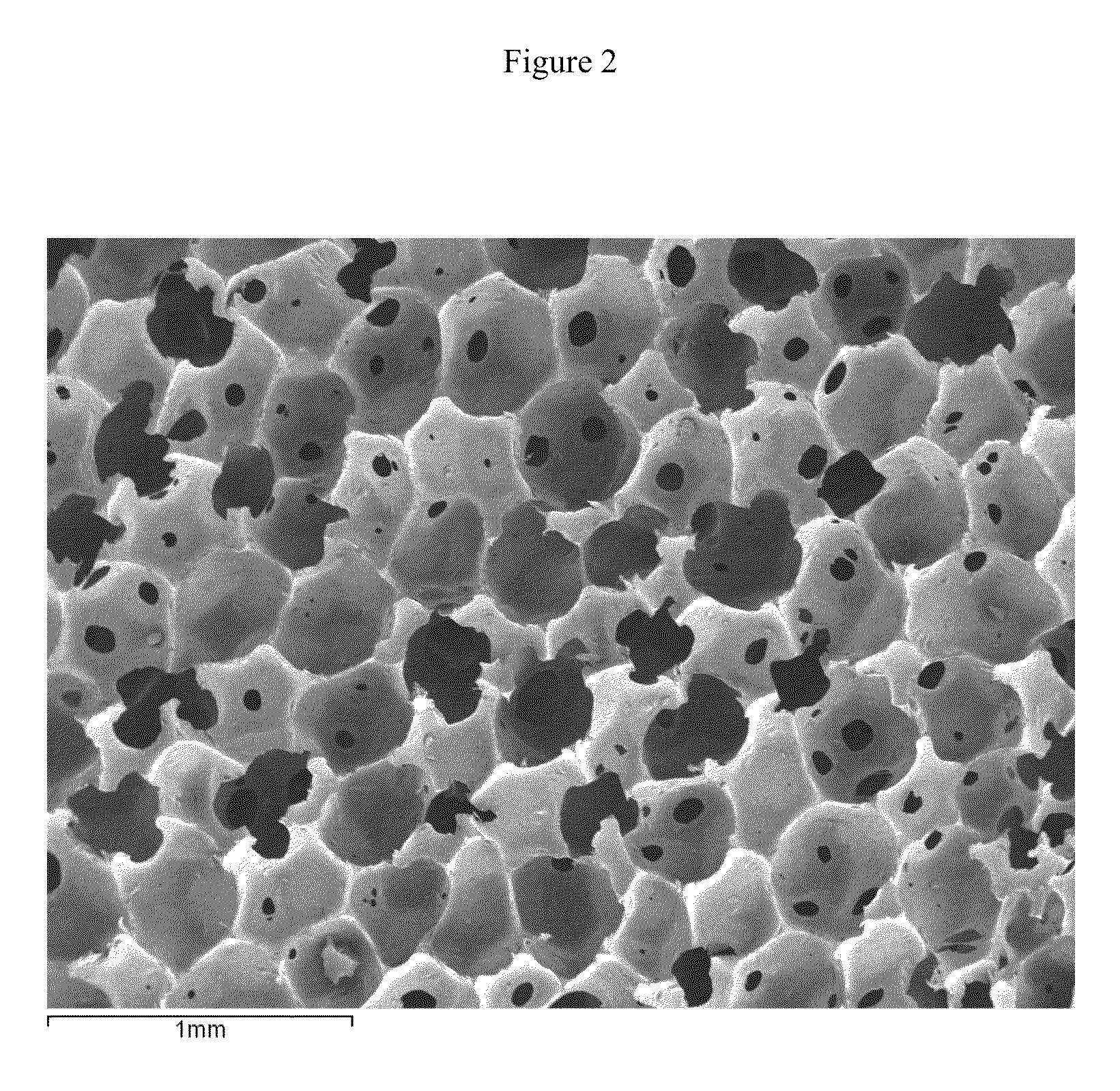
Owner:DEXCOM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com