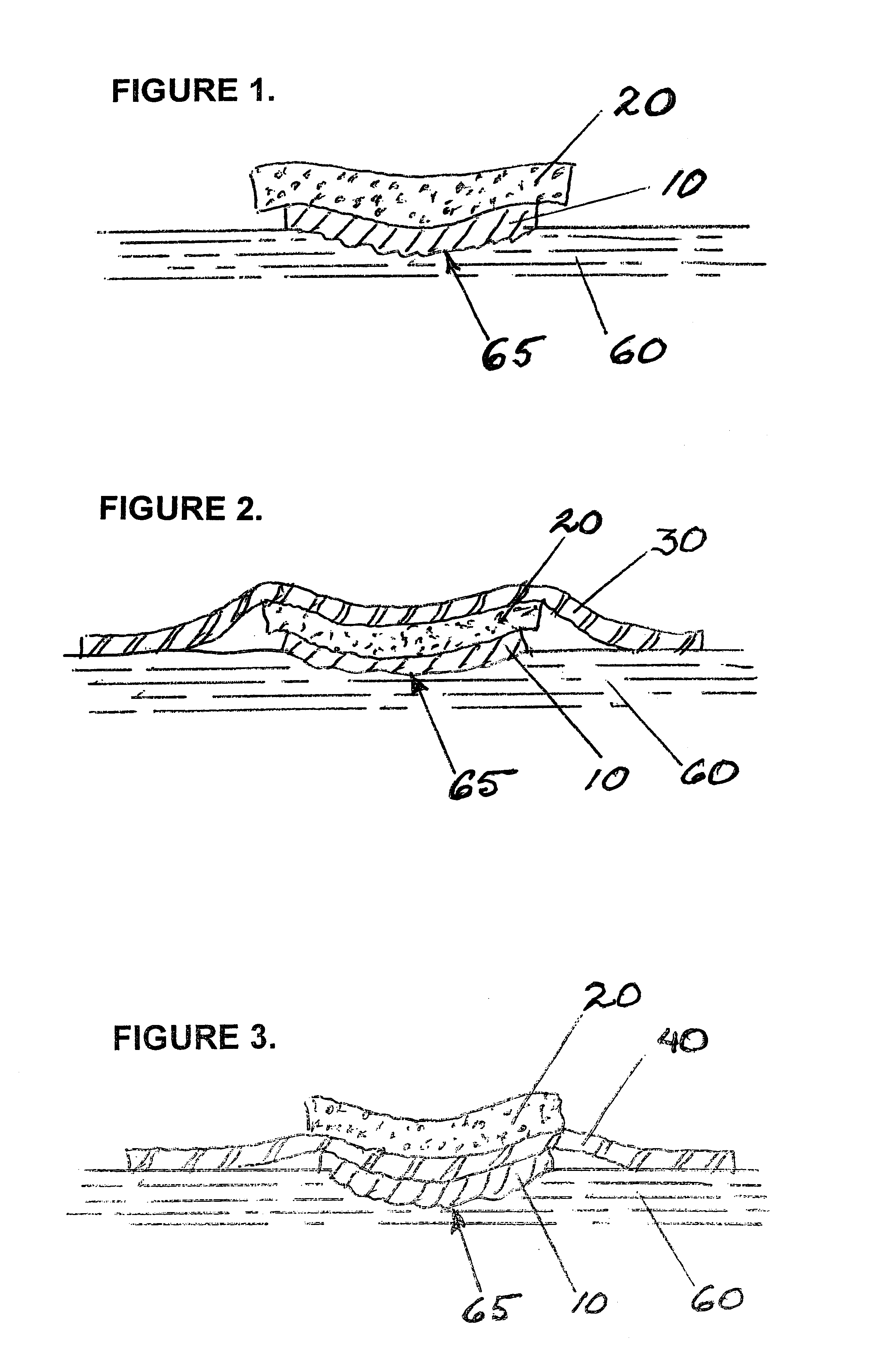
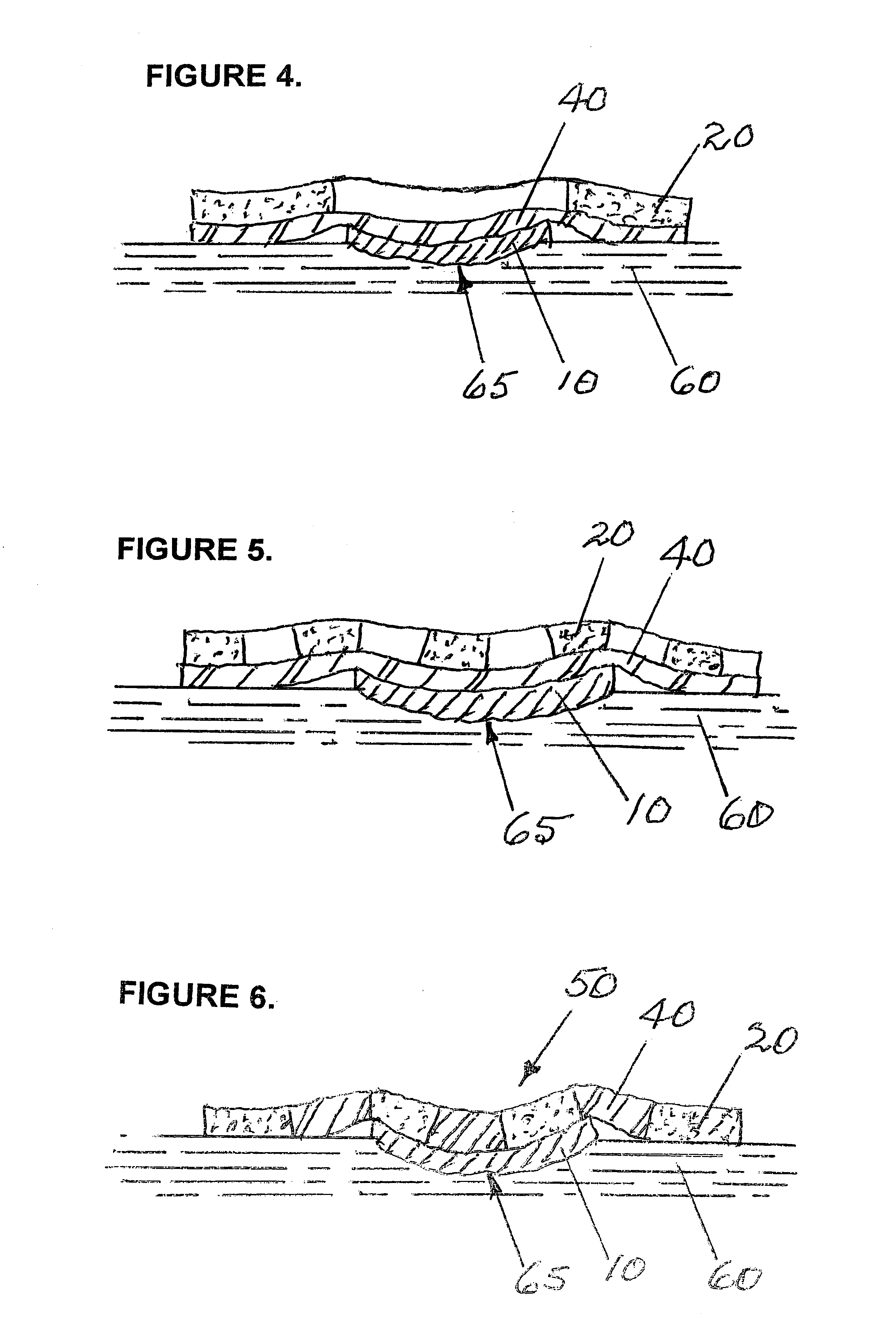
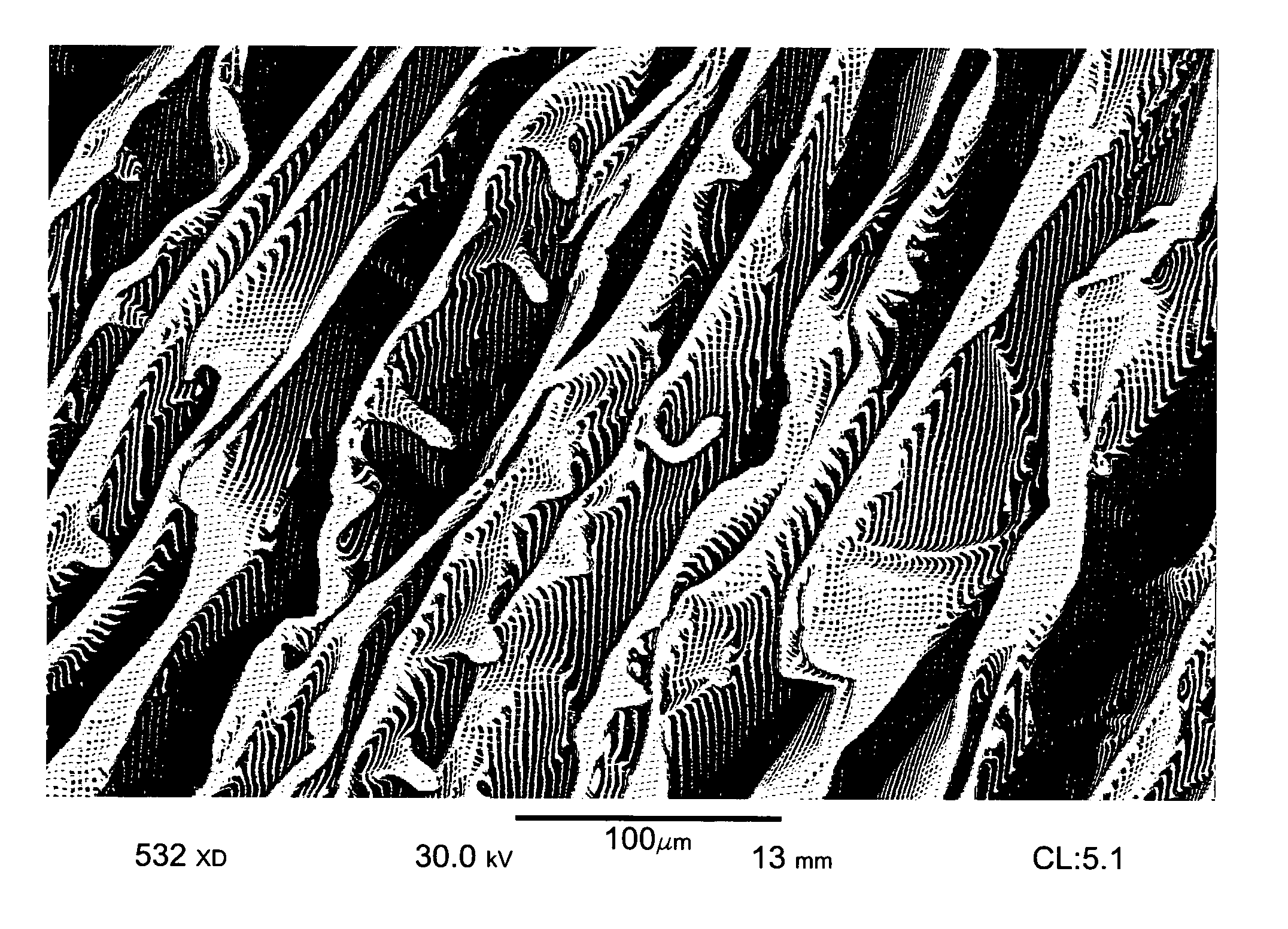
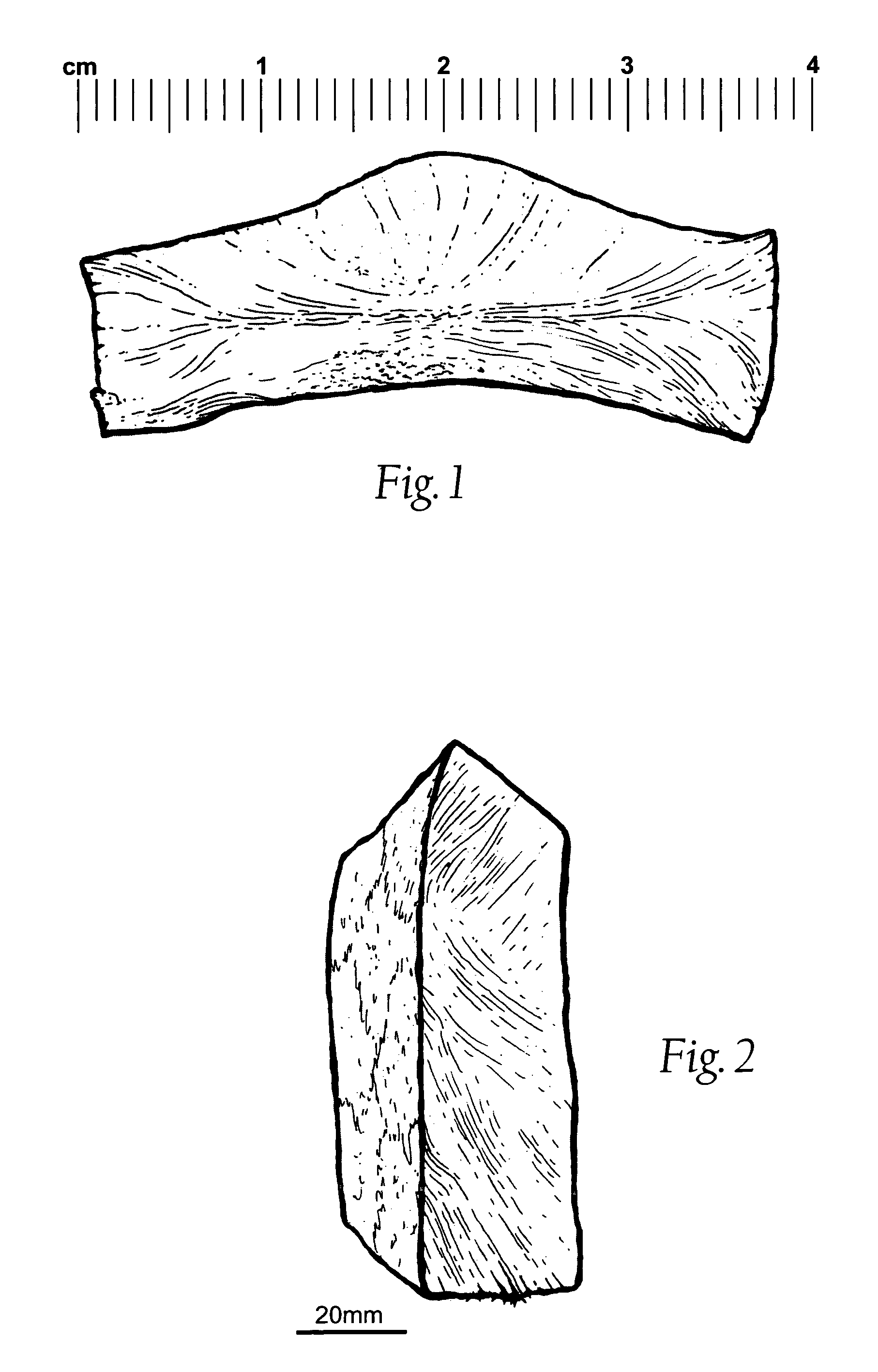
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2252 results about "Wound dressing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
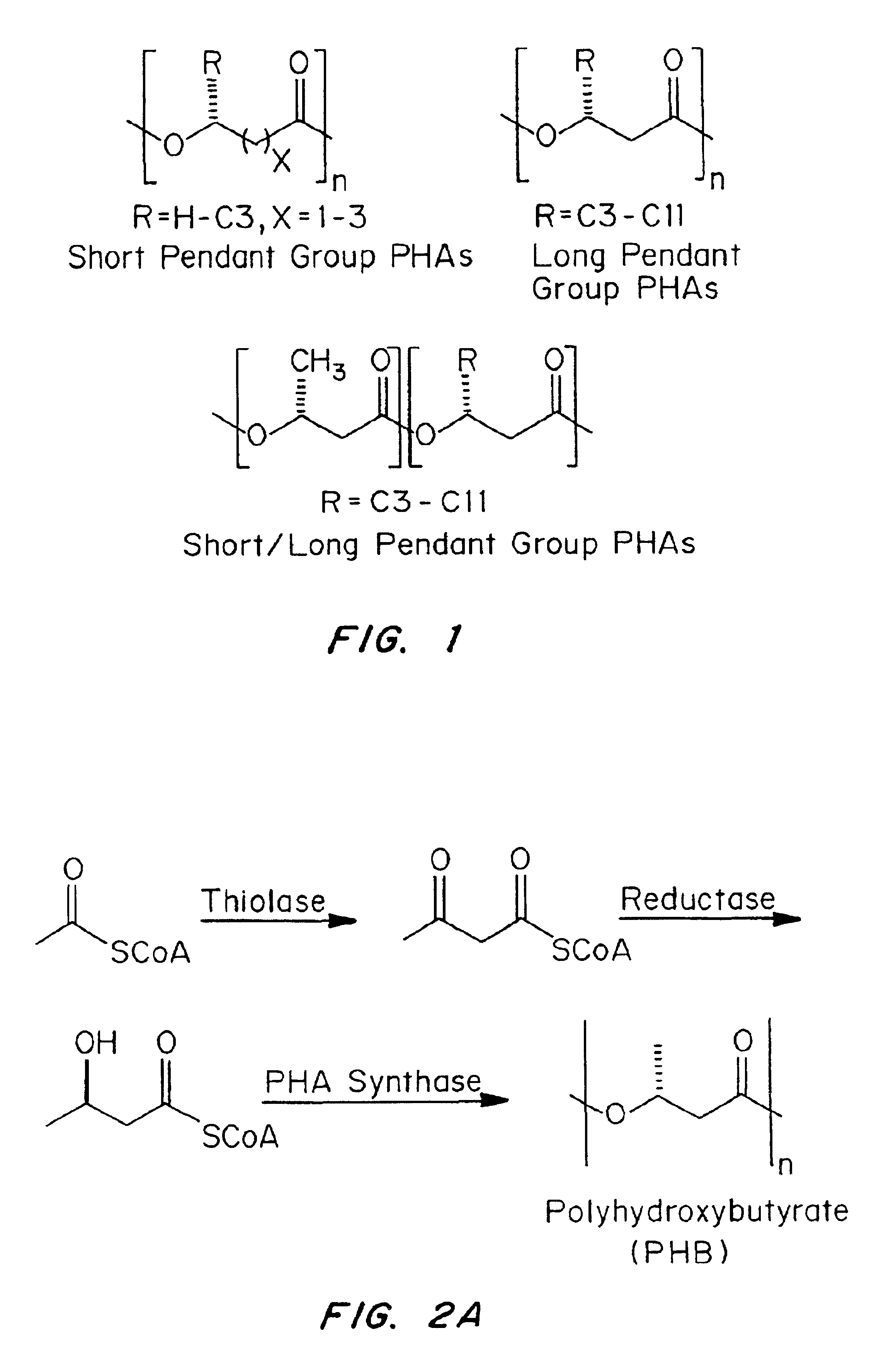
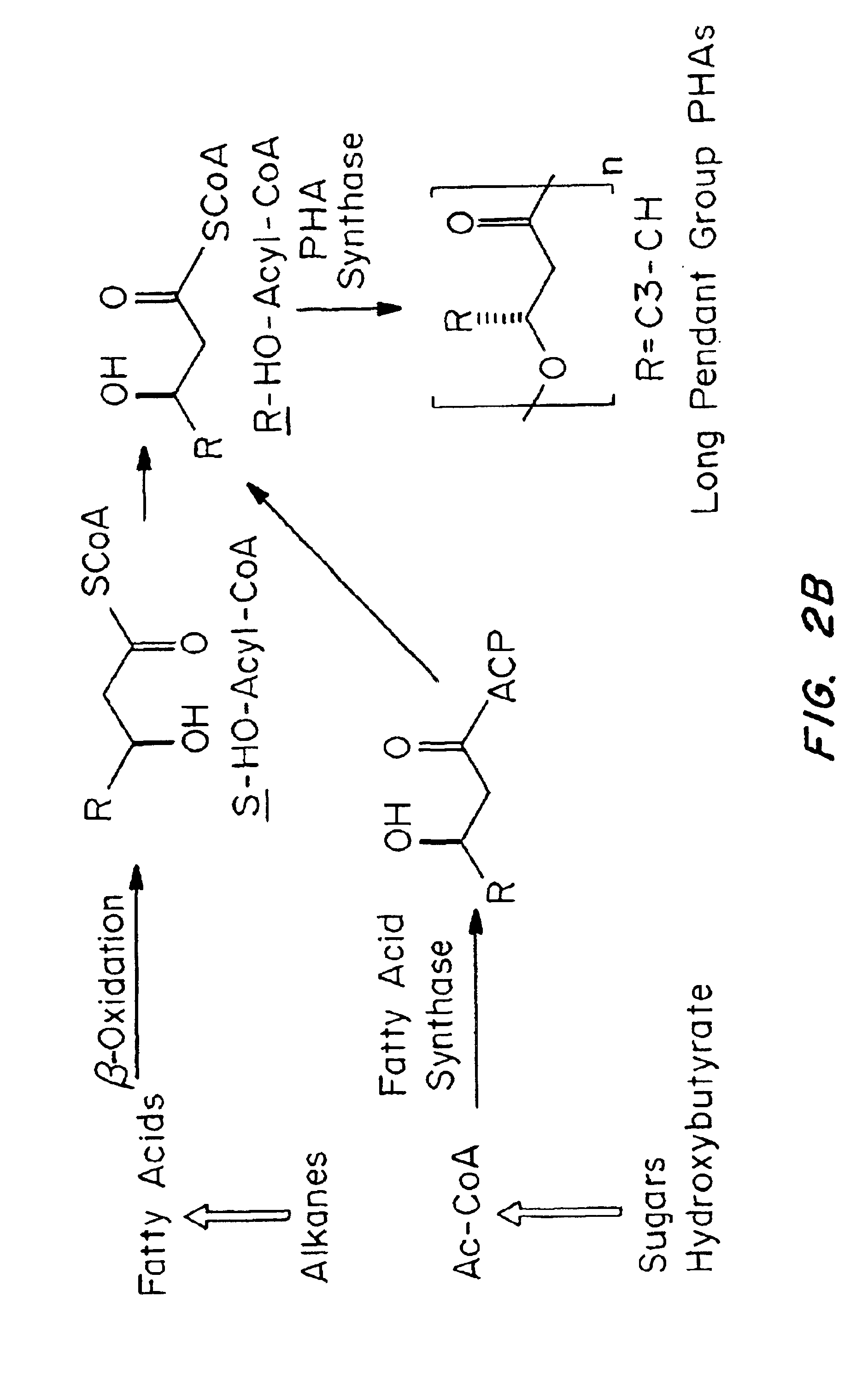
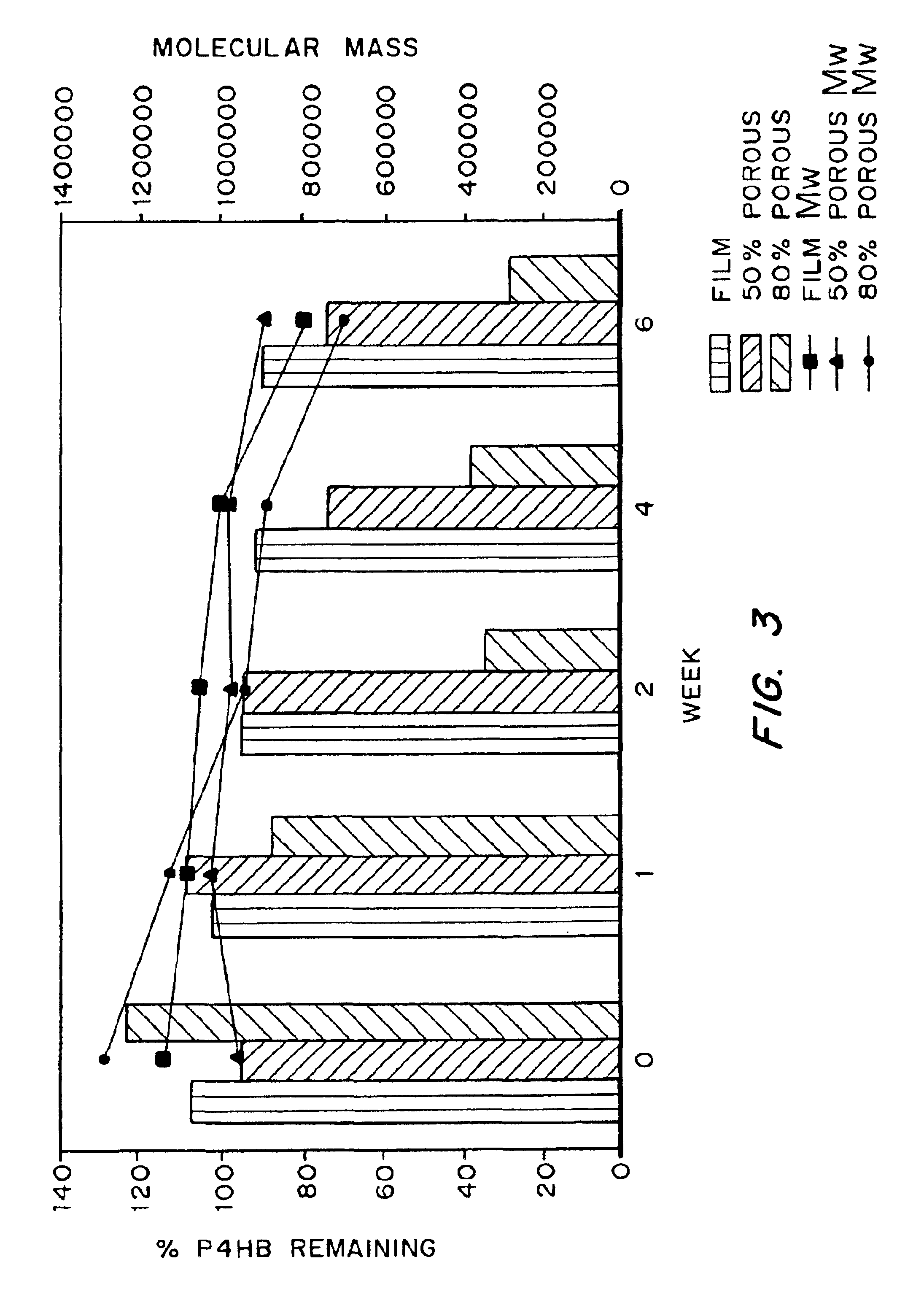
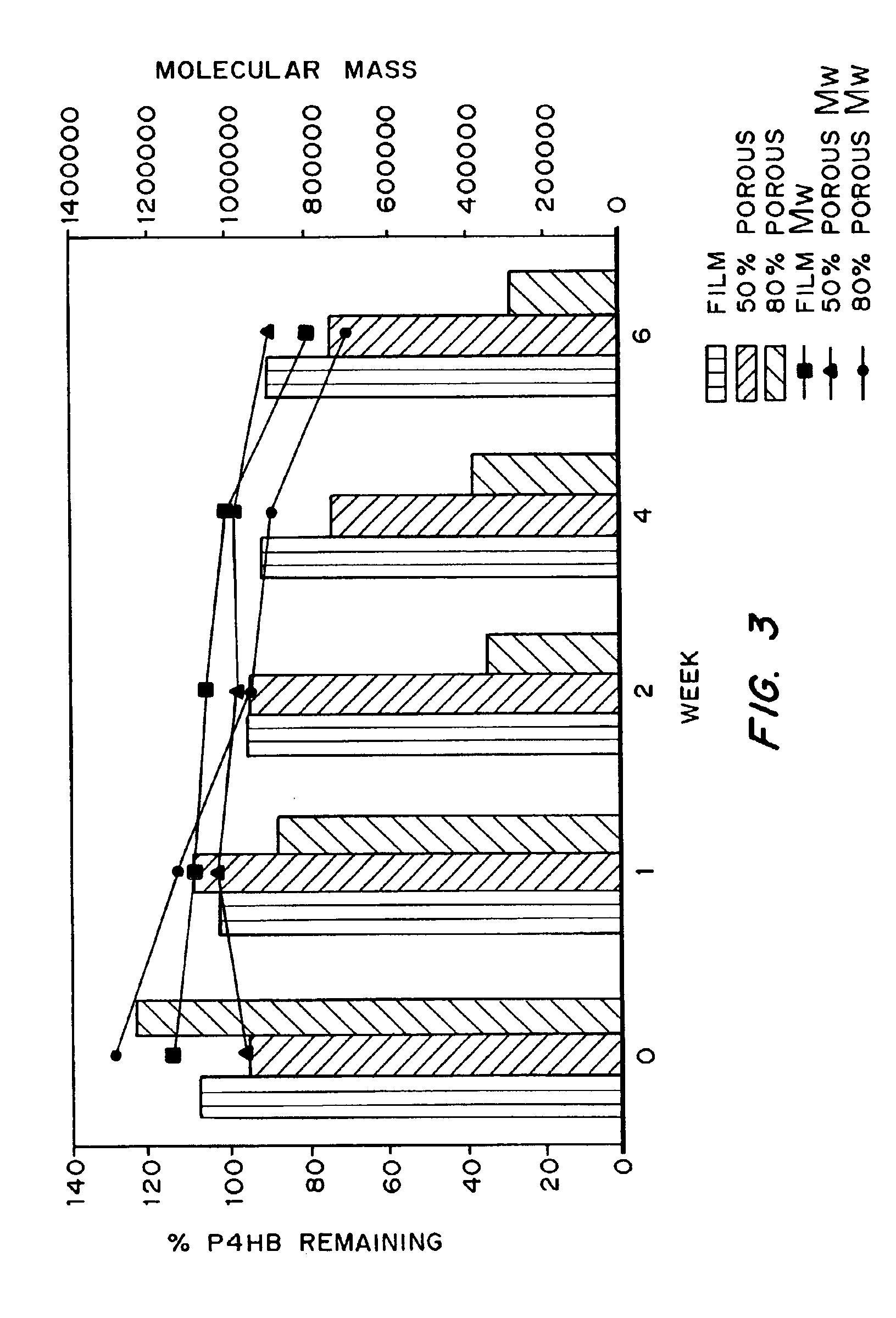
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6838493B2High porosityReduce probabilitySuture equipmentsOrganic active ingredientsTissue repairBiocompatibility Testing
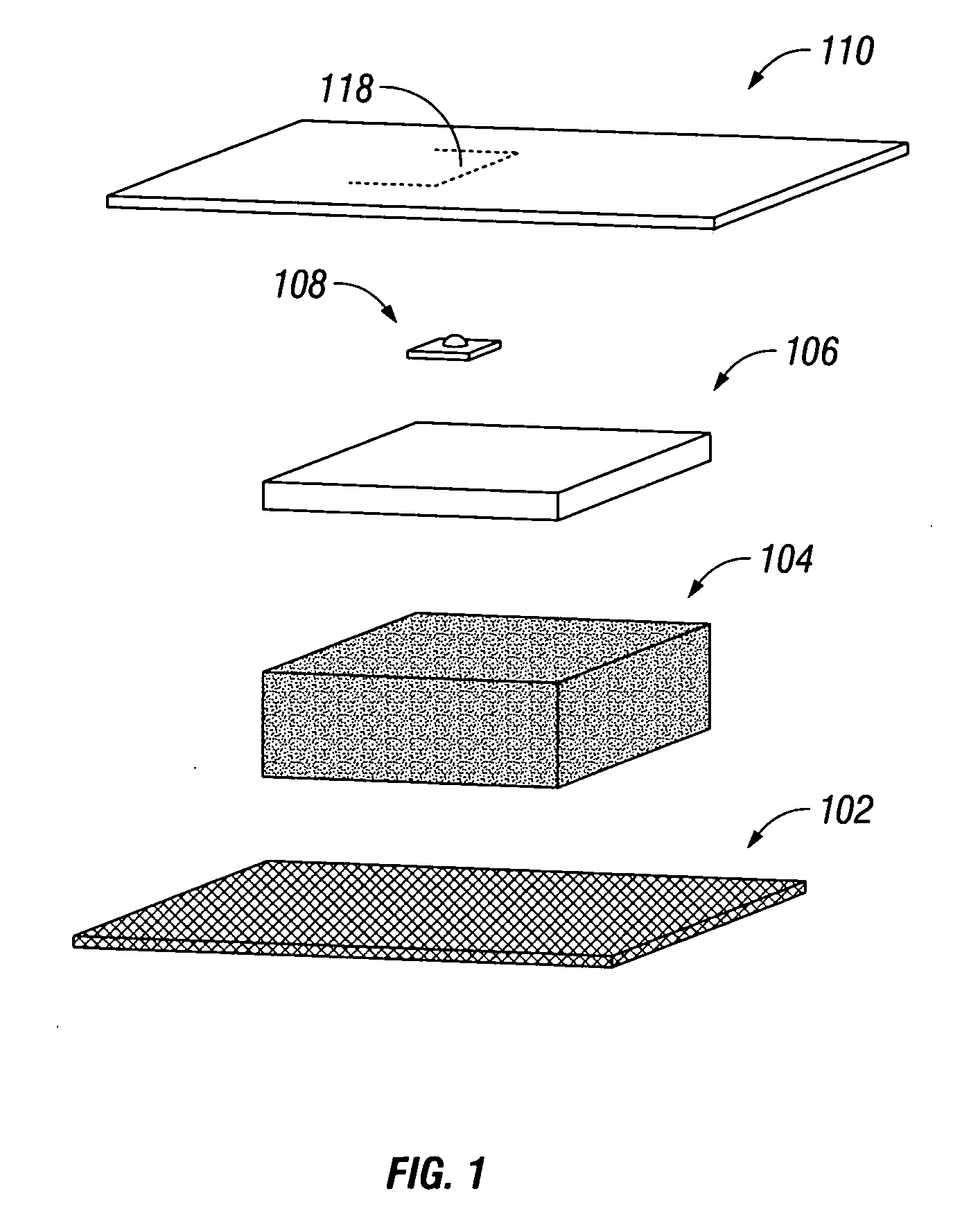
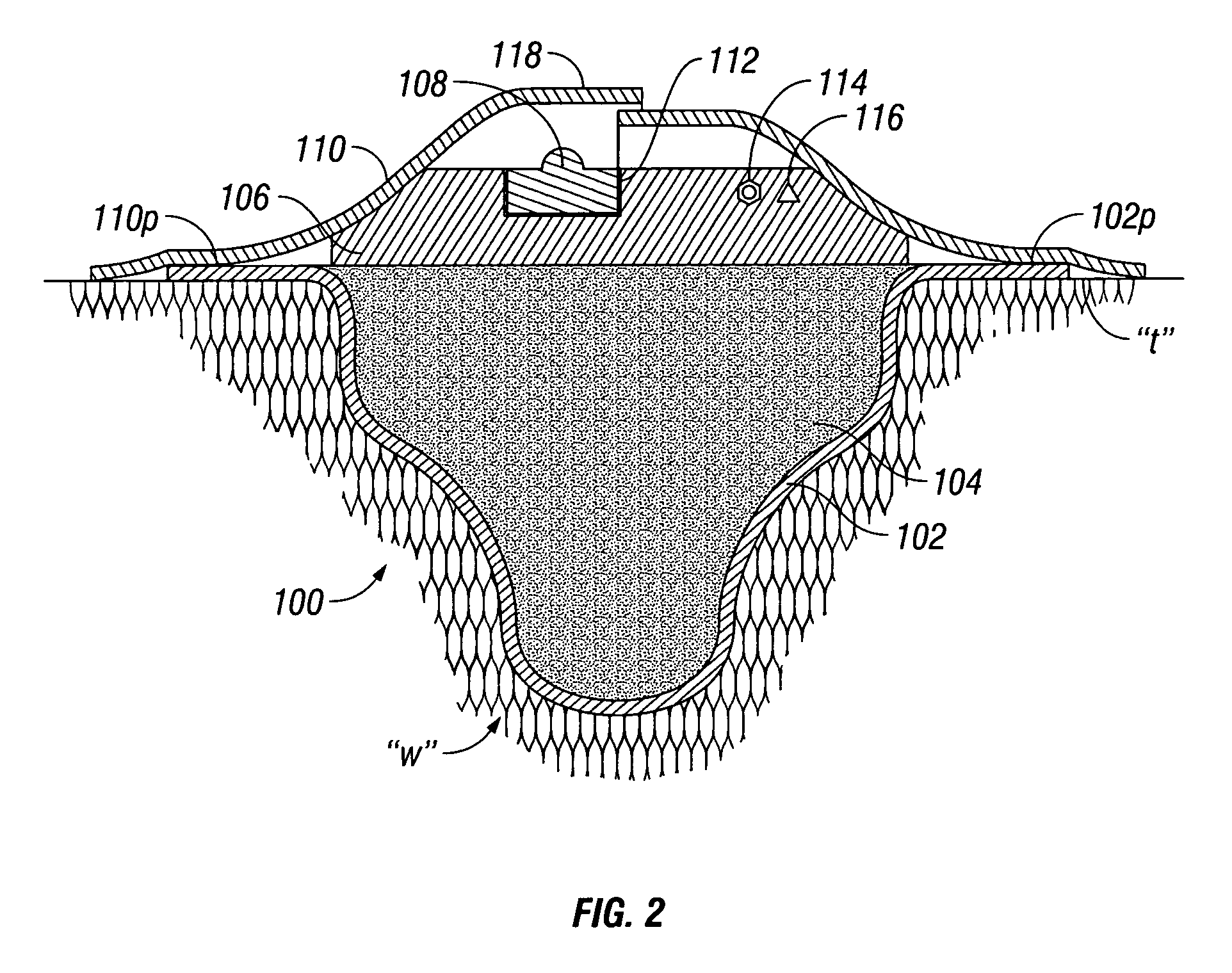
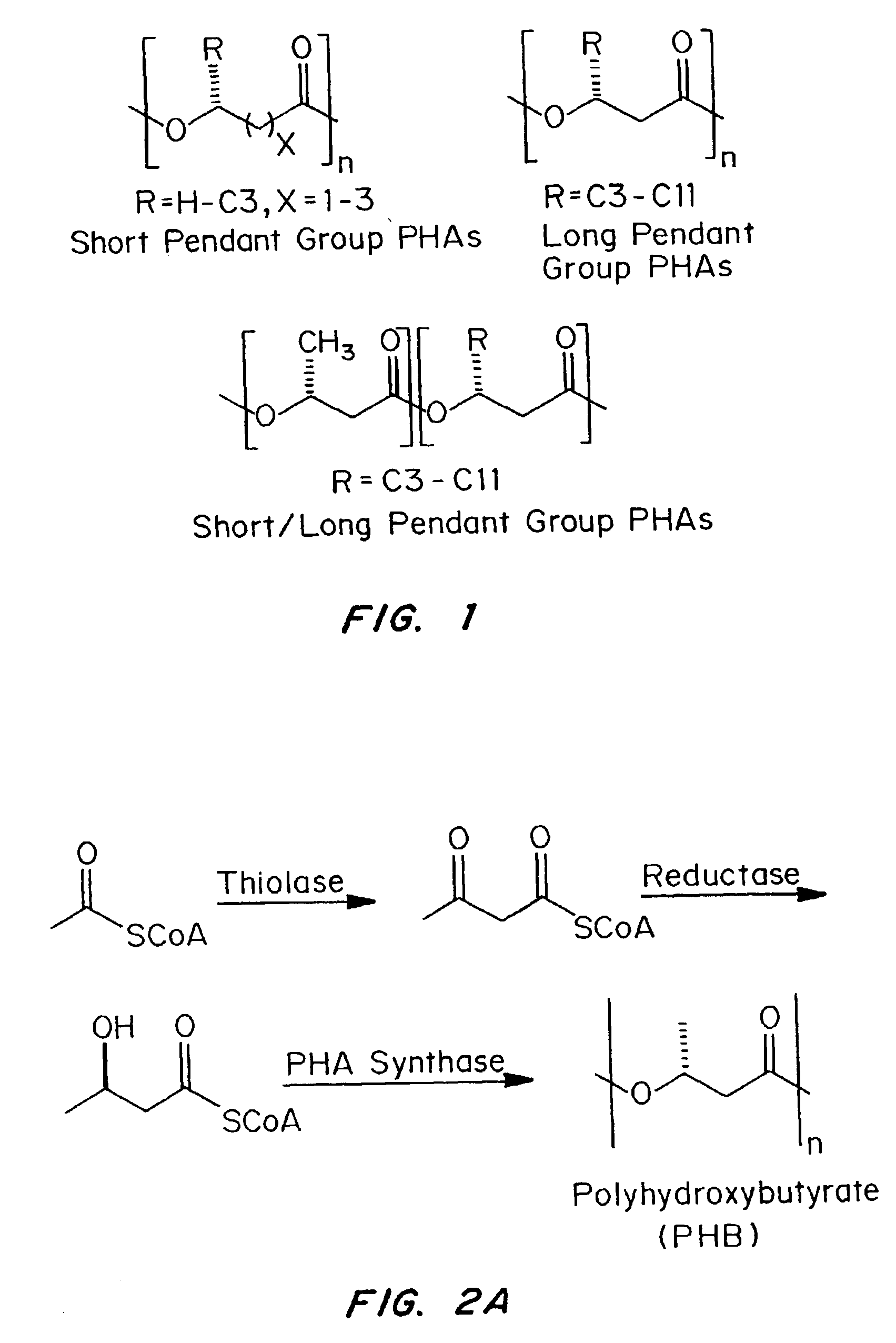
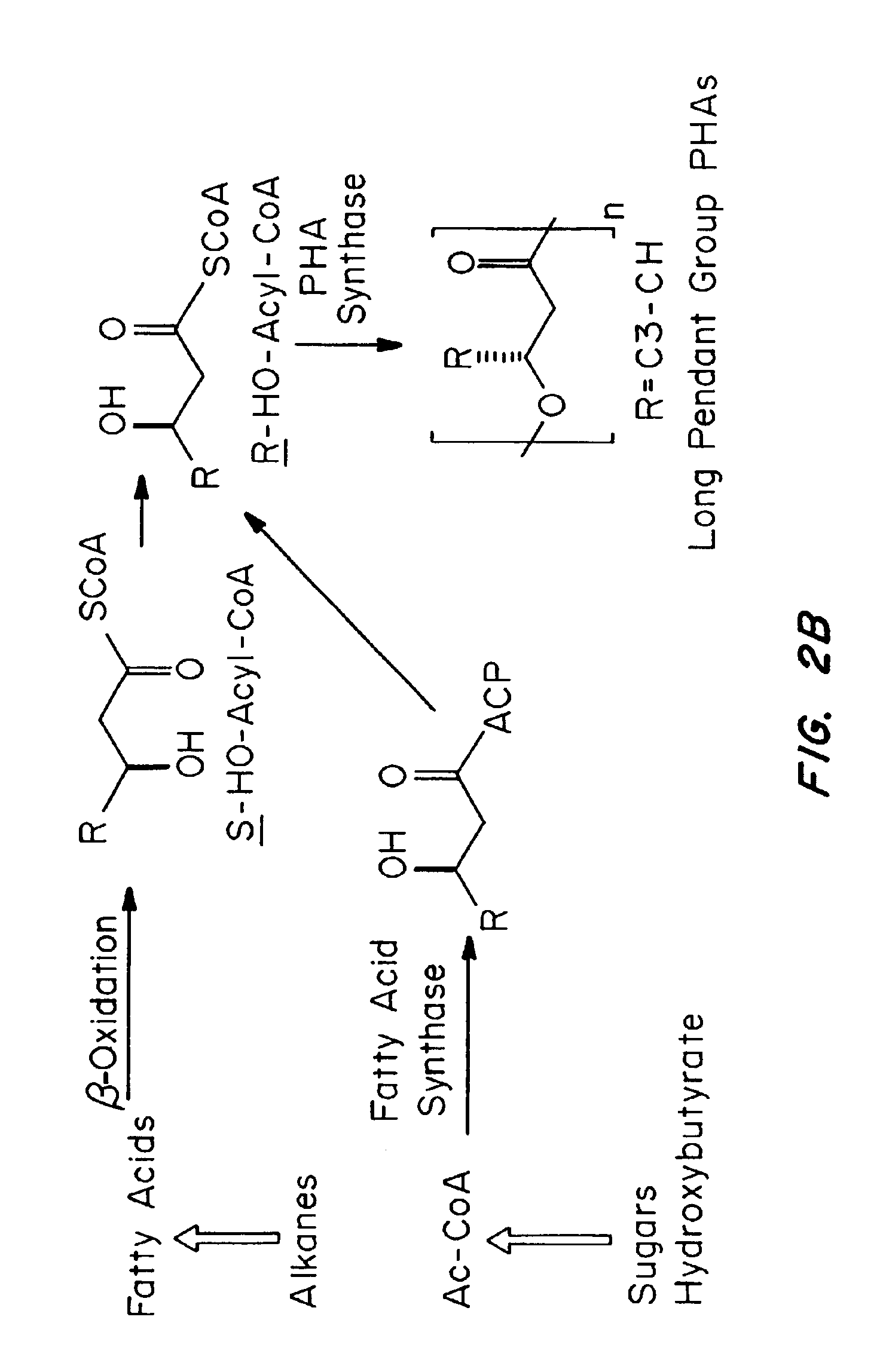
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
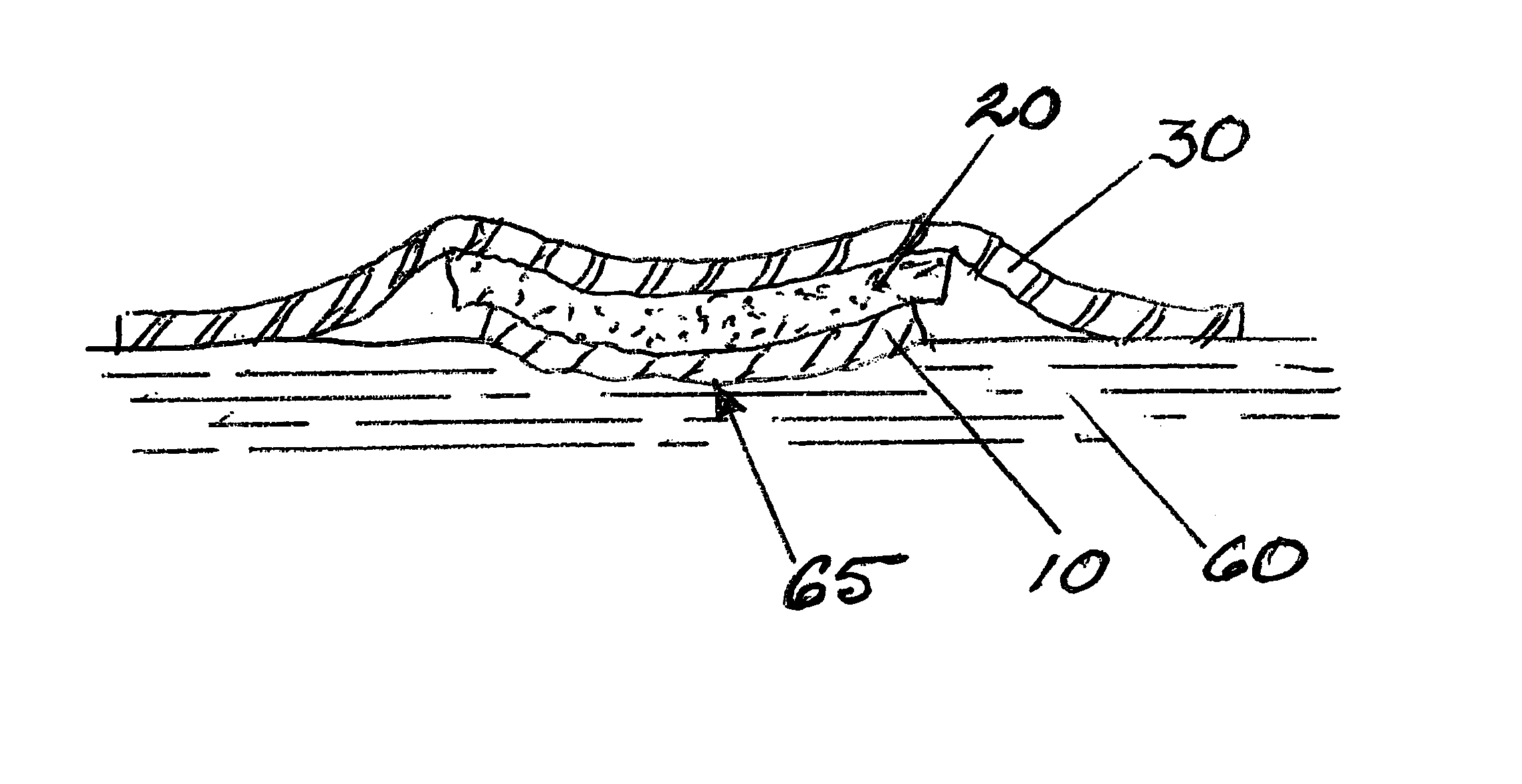
Bioabsorbable wound dressing
ActiveUS7041868B2Promote wound healingEasy to useAdhesive dressingsNon-surgical orthopedic devicesCell adhesionAdhesion process
A wound dressing includes a first layer located adjacent the wound and which comprises a material that is bioabsorbable, porous and adapted for serving as a scaffold for cell attachment and proliferation; and a second layer which is in contact with the first layer and which comprises an absorbent, gel forming material adapted for serving as a barrier to cell adhesion and penetration. A method of treating a wound with the dressing is also disclosed.
Owner:AVENT INC
Hemostatic compositions for arresting blood flow from an open wound or surgical site
A hemostatic composition for stopping or decreasing blood flow from an open wound or medical or surgical procedure. Compositions of the invention comprise a mixture of a cationic polymer and a cation exchange material. In one embodiment, the composition comprises a mixture: (1) a high molecular weight copolymer of diallyl dimethyl ammonium chloride (DADMAC) and acrylamide [DADMAC copolymer], and (2) the hydrogen form of a crosslinked, sulfonated polystyrene (hydrogen resin). In an exemplified embodiment, a composition of the invention comprises the mixture of DADMAC copolymer and hydrogen resin provided in a dry powdered form. The compositions of the invention may be applied directly to a wound or treatment site, or they may be incorporated into a wound dressing, such as a bandage. The seal formed at a wound or treatment site treated with the present invention is adhesive and exhibits considerable toughness.
Owner:BIOLIFE
Wound dressing and method for controlling severe, life-threatening bleeding
ActiveUS7371403B2Stanching flowProhibiting flow of bloodBiocideSuspensory bandagesHydrophilic polymersWound dressing
This invention is directed to advanced hemorrhage control wound dressings, and methods of using and producing same. The subject wound dressing is constructed from a non-mammalian material for control of severe bleeding. The wound dressing for controlling severe bleeding is formed of a biomaterial comprising chitosan, a hydrophilic polymer, a polyacrylic polymer or a combination thereof. The kind of severe, life-threatening bleeding contemplated by this invention is typically of the type not capable of being stanched when a conventional gauze wound dressing is applied with conventional pressure to the subject wound. The wound dressing being capable of substantially stanching the flow of the severe life-threatening bleeding from the wound by adhering to the wound site, to seal the wound, to accelerate blood clot formation at the wound site, to reinforce clot formation at the wound site and prevent bleed out from the wound site, and to substantially prohibit the flow of blood out of the wound site.
Owner:PROVIDENCE HEALTH SYST OREGONDBA ST VINCENTMEDICAL CENT
Biocompatible wound dressing
InactiveUS7070584B2Promote cell growthPrevent vacuum leakageWound drainsMedical applicatorsWound dressingWound site
A biocompatible wound dressing comprised of a pad for insertion substantially into a wound site and a wound drape for sealing enclosure of the foam pad at the wound site. The pad, comprised of a foam or other like material having relatively few open cells in contact with the areas upon which cell growth is to be encouraged so as to avoid unwanted adhesions, but having sufficiently numerous open cells so that drainage and negative pressure therapy may continue unimpaired, is placed in fluid communication with a vacuum source for promotion of fluid drainage, as known in the art. The pad is further comprised of an ultra-low density fused-fibrous ceramic, or a bioabsorbable branched polymer, or cell growth enhancing matrix or scaffolding.
Owner:KCI LICENSING INC
Collagen biofabric and methods of preparation and use therefor
InactiveUS20040048796A1Improved biophysical propertyImprove featuresSenses disorderPeptide/protein ingredientsSurgical GraftWound dressing
The present invention relates to collagenous membranes produced from amnion, herein referred to as a collagen biofabric. The collagen biofabric of the invention has the structural integrity of the native non-treated amniotic membrane, i.e., the native tertiary and quaternary structure. The present invention provides a method for preparing a collagen biofabric from a placental membrane, preferably a human placental membrane having a chorionic and amniotic membrane, by decellularizing the amniotic membrane. In a preferred embodiment, the amniotic membrane is completely decellularized. The collagen biofabric of the invention has numerous utilities in the medical and surgical field including for example, blood vessel repair, construction and replacement of a blood vessel, tendon and ligament replacement, wound-dressing, surgical grafts, ophthalmic uses, sutures, and others. The benefits of the biofabric are, in part, due to its physical properties such as biomechanical strength, flexibility, suturability, and low immunogenicity, particularly when derived from human placenta.
Owner:CELLULAR THERAPEUTICS DIV OF CELGENE +1
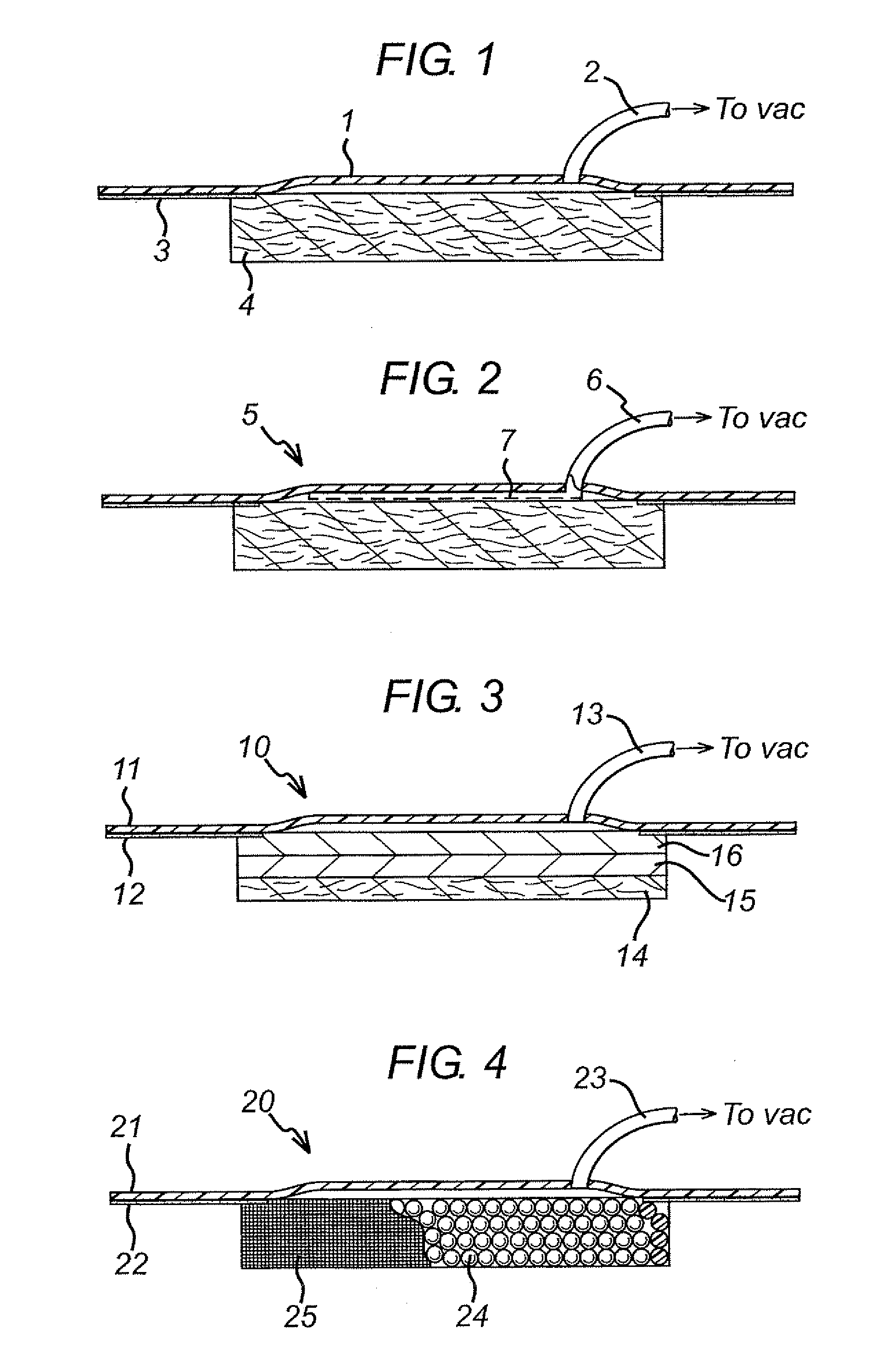
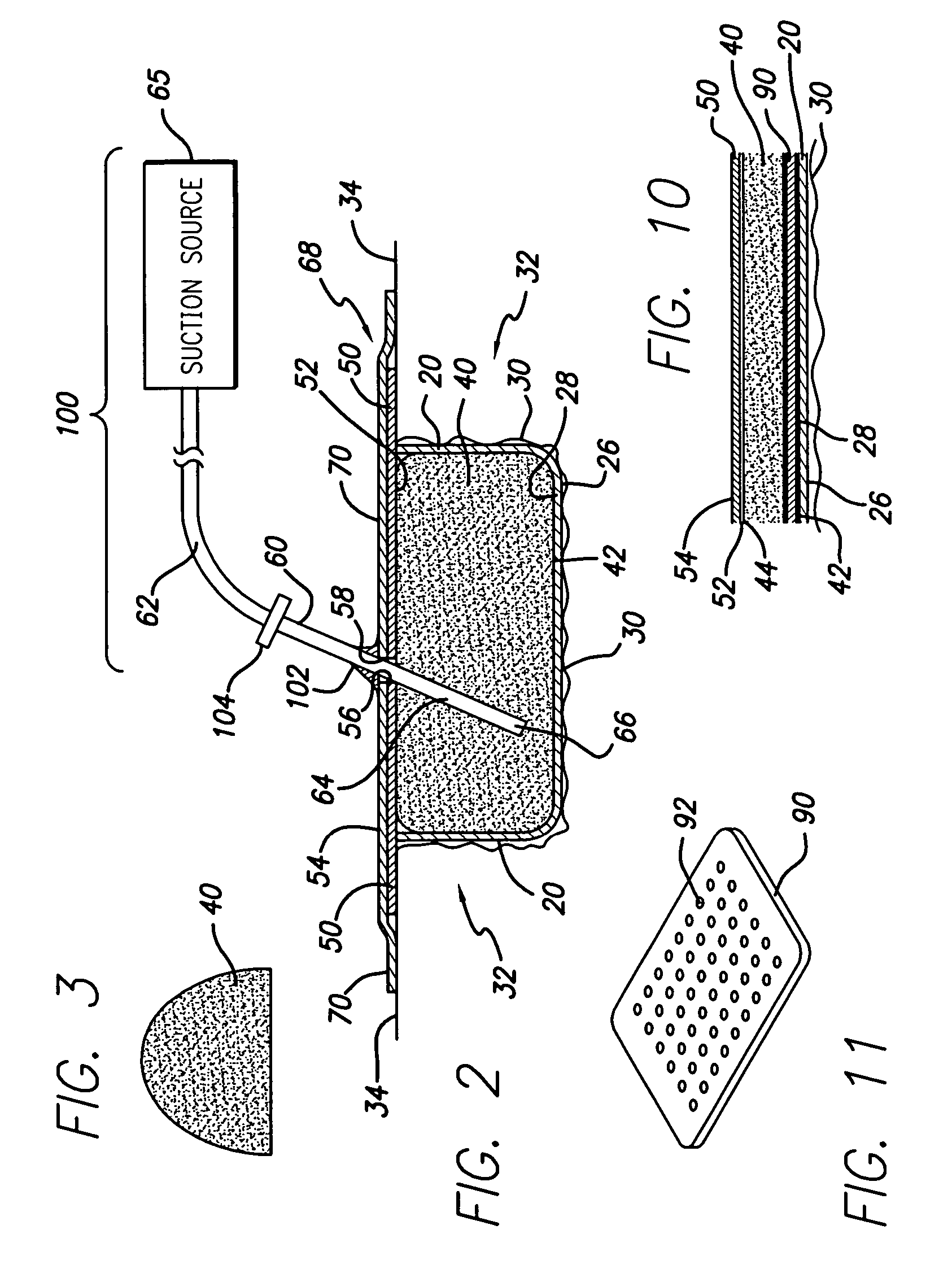
Removable wound closure
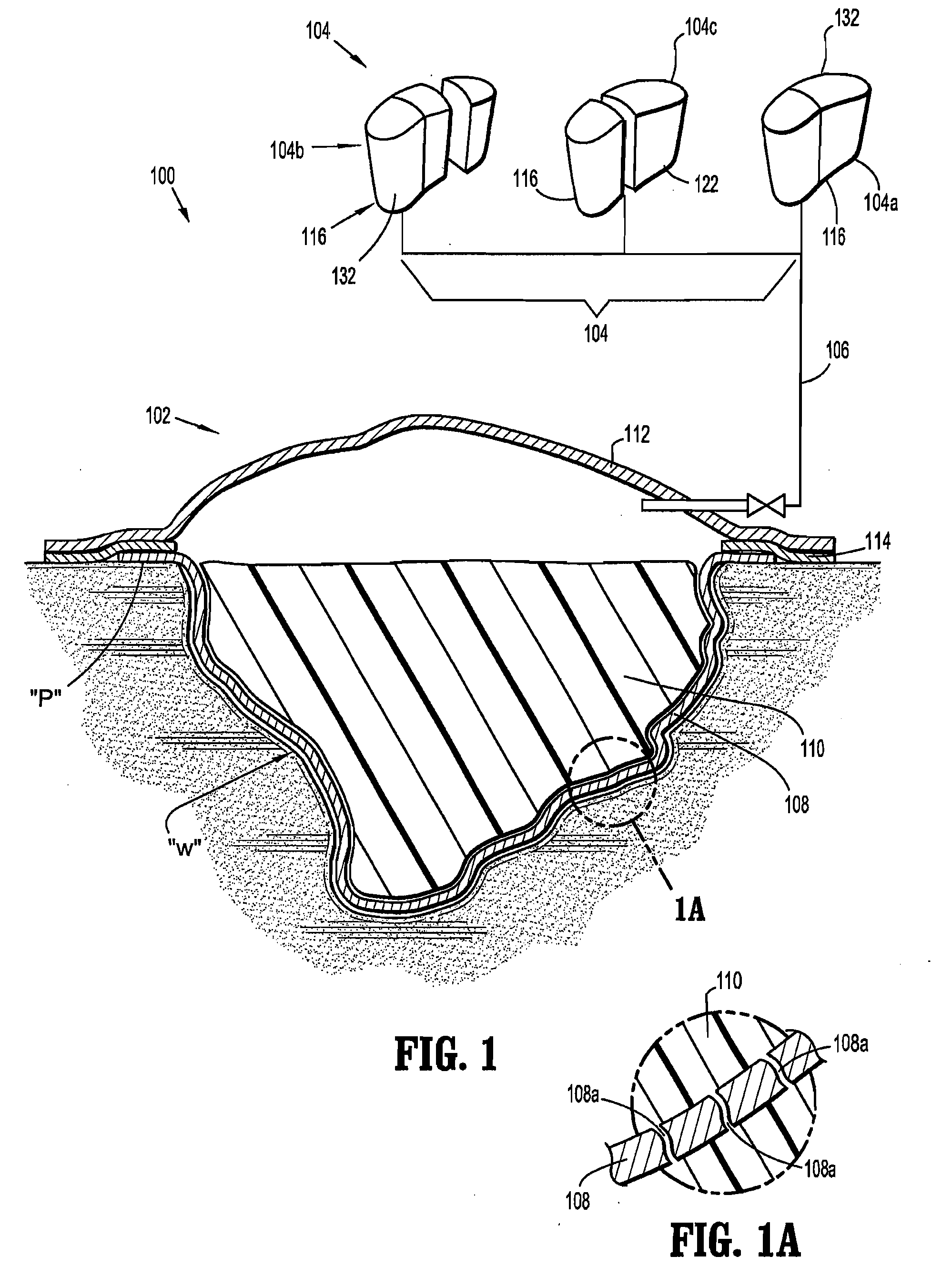
InactiveUS7381859B2Promote wound healingMinimizes adhesion formationNon-adhesive dressingsWound drainsElastomerPorosity
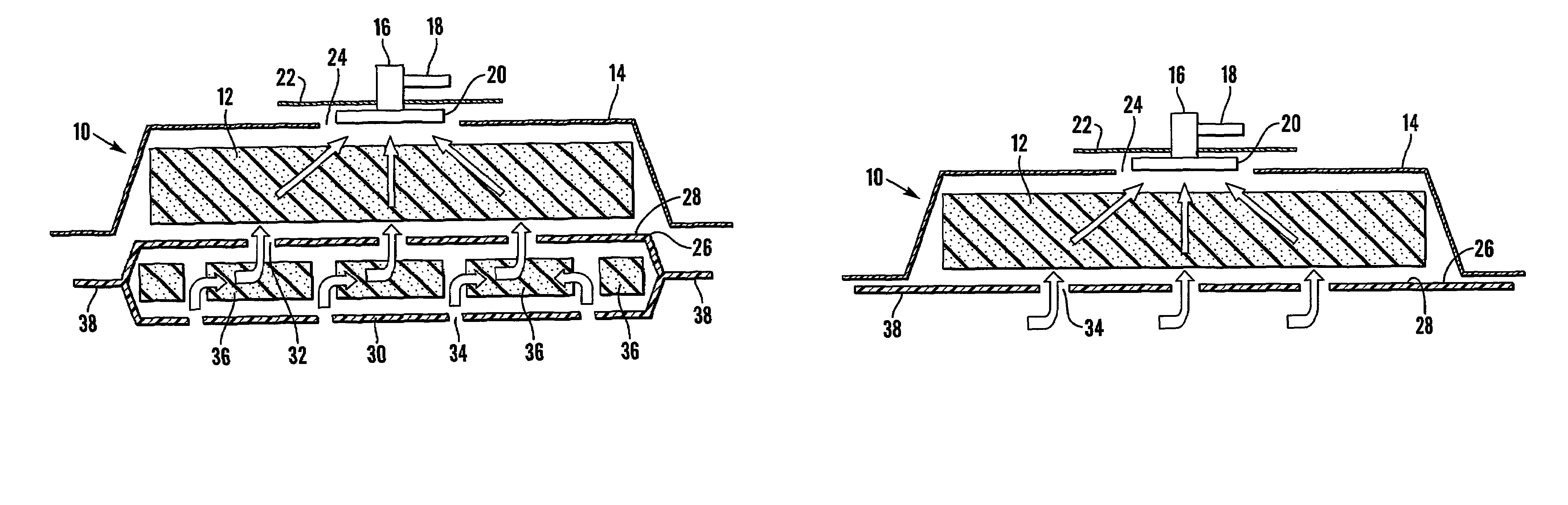
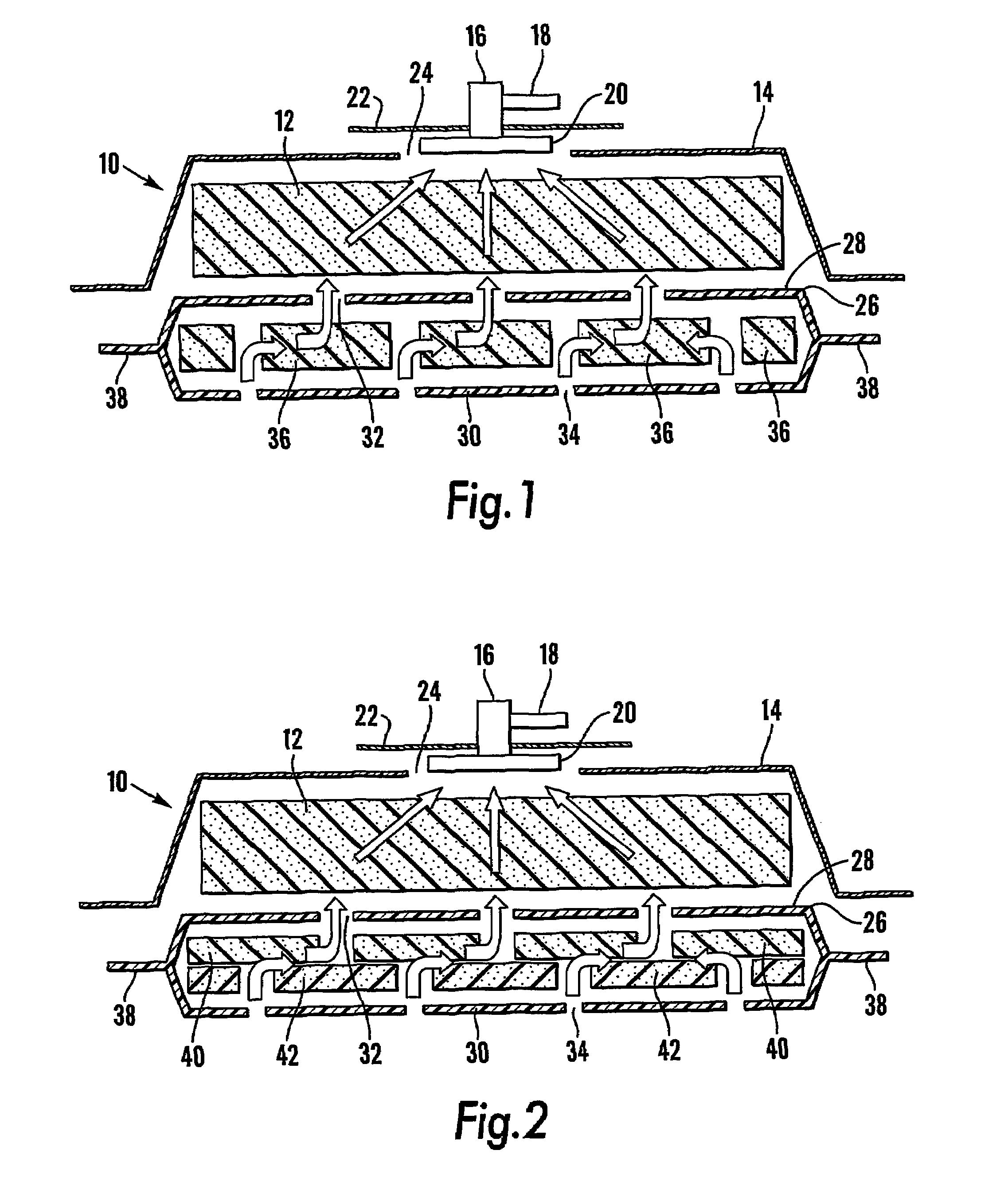
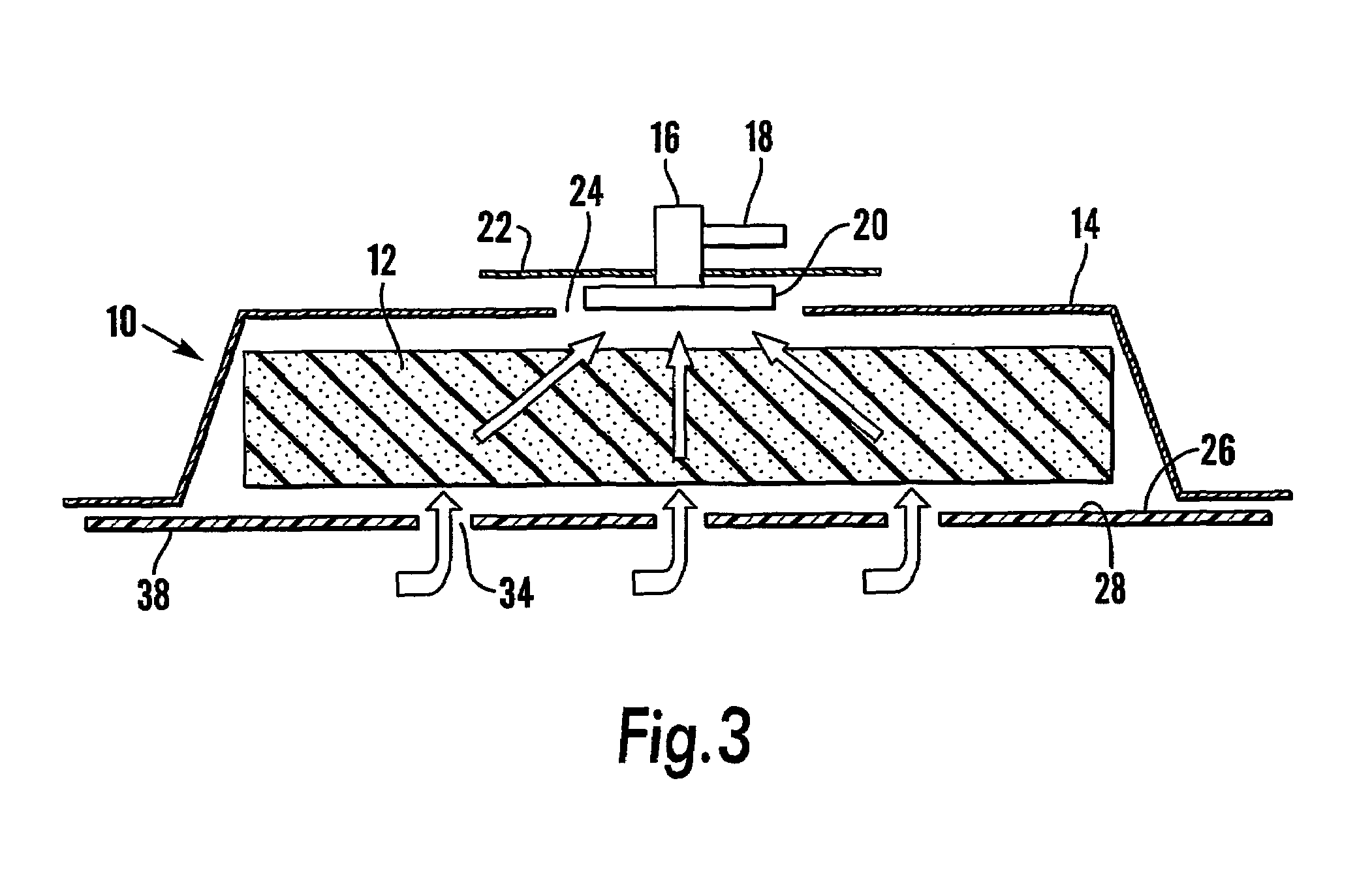
A system and method for the temporary closure of a wound, especially an abdominal wound, to facilitate re-entry, final closure, and long term healing of the wound. An abdominal wound dressing and methods of use are described that enable the application of negative pressure to the wound site in a site healing promoting manner while also limiting the formation of adhesions that would prevent the removal of the dressing. The dressing comprises a layer of porous foam material (36) enclosed by sheets of elastomeric material (38) punctuated by a number of appropriately placed holes (34). Multiple layers of porous foam may also be used. A suction tube connector (16) is provided on an upper surface of a layer of foam (12) for connection to a negative pressure source. At least one layer of foam is enclosed in elastomeric material and is placed in direct contact with the tissue within the open wound. Fluids are drawn by negative pressure through the holes positioned in the elastomeric envelope, and through the foam. If multiple foam layers are employed, the lower layer(s) of foam are of a finer porosity while the upper layer of foam is coarse. An adhesive elastomeric sheet (14) covers the entire wound dressing and seals the edges to the skin surrounding the wound. An appropriate vacuum device is attached to the suction tube connector.
Owner:KCI LICENSING INC
Biocompatible wound dressing
InactiveUS20070185426A1Reduce pressureReduced tissue treatmentNon-adhesive dressingsAdhesive dressingsWound dressingContact layer
A multi-layer reduced pressure delivery apparatus is provided for applying reduced pressure tissue treatment to a tissue site. The multi-layer apparatus includes a tissue contact layer, a release layer, and a manifold layer. The tissue contact layer includes a scaffold adapted to contact the tissue site, the release layer includes a hydrogel-forming material and a plurality of flow channels, and the manifold layer includes a distribution manifold. The release layer is positioned between the tissue contact layer and the manifold layer to allow easy release of the manifold layer from the tissue contact layer following the administration of reduced pressure tissue treatment.
Owner:KCI LICENSING INC
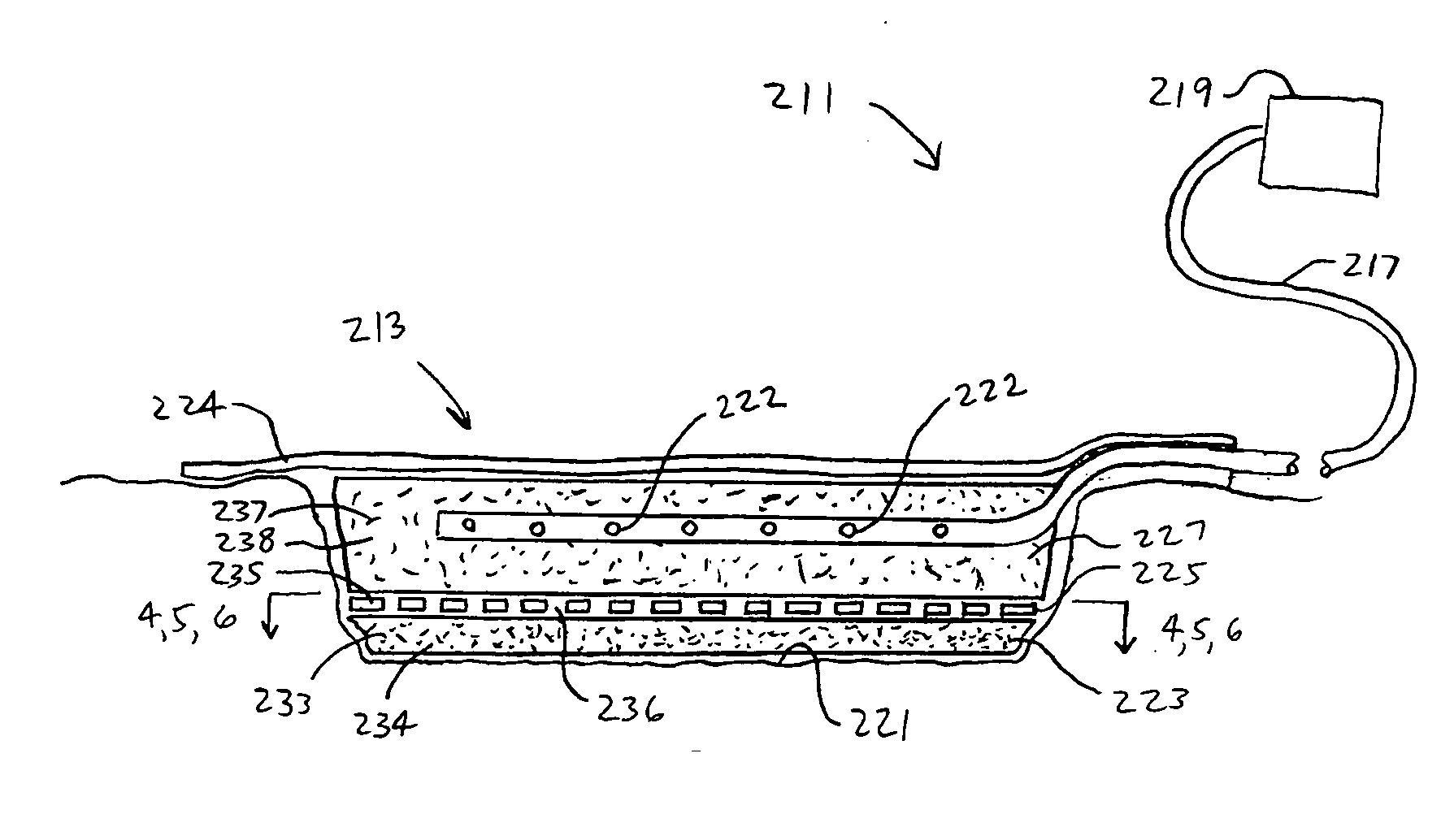
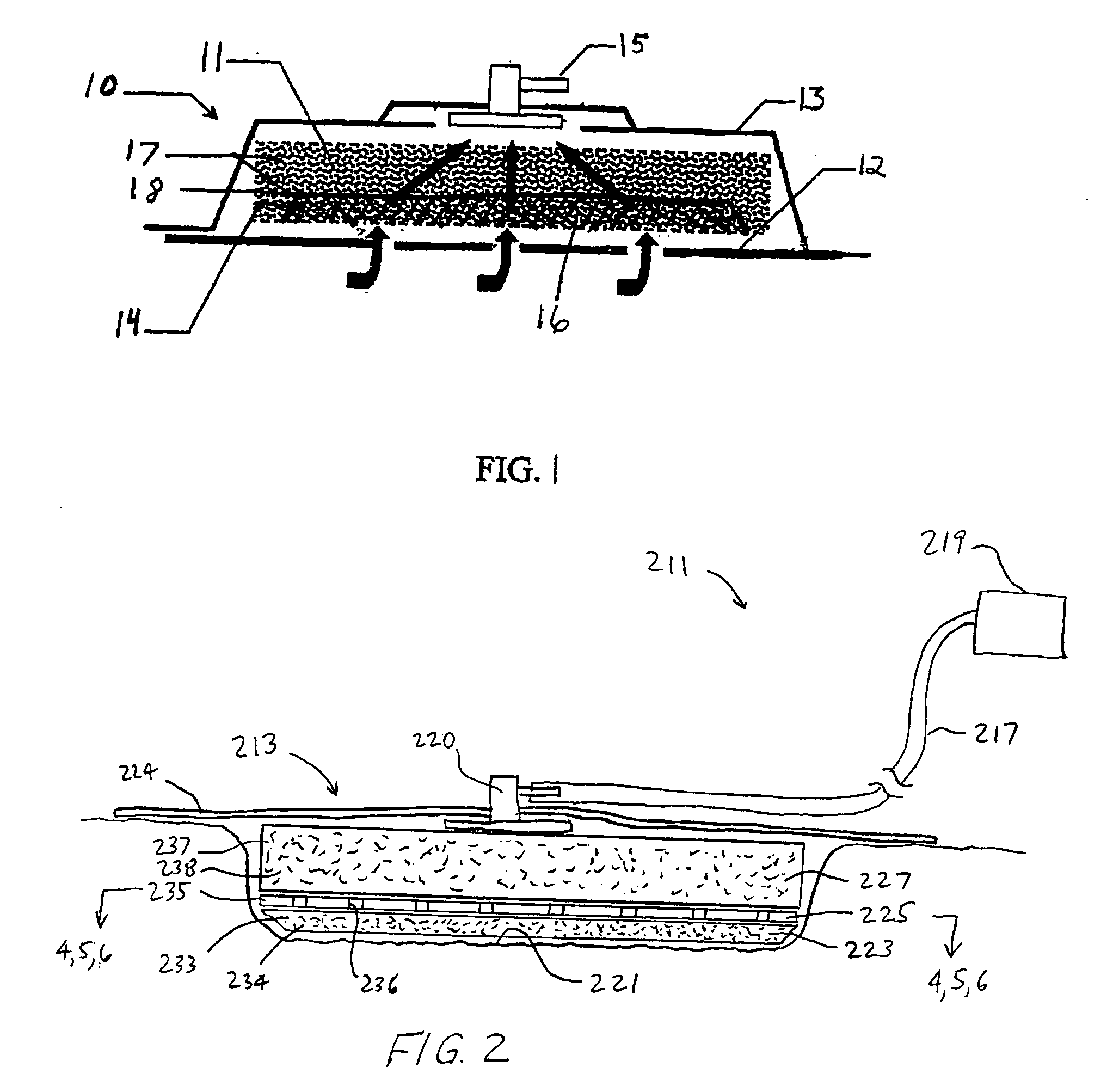
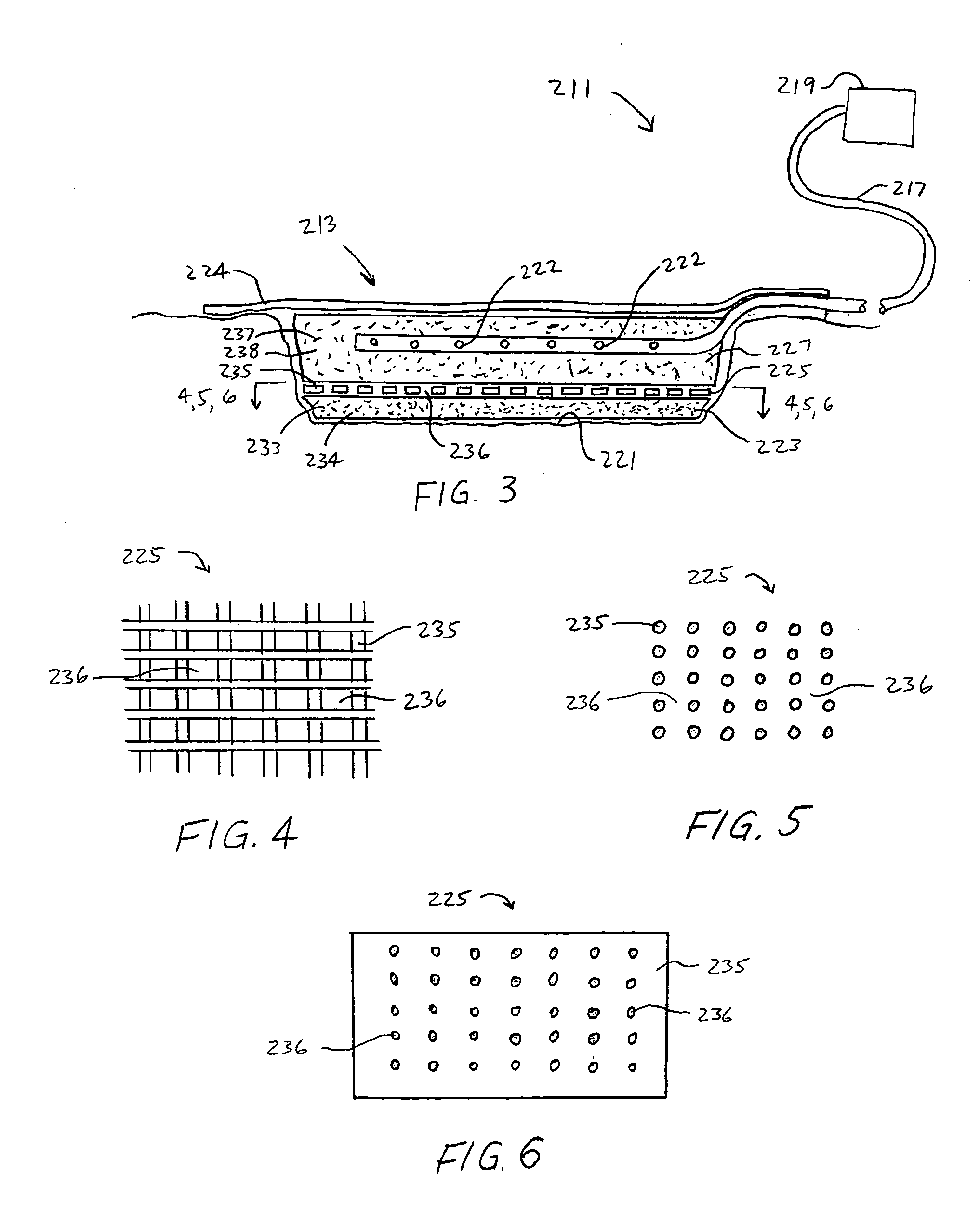
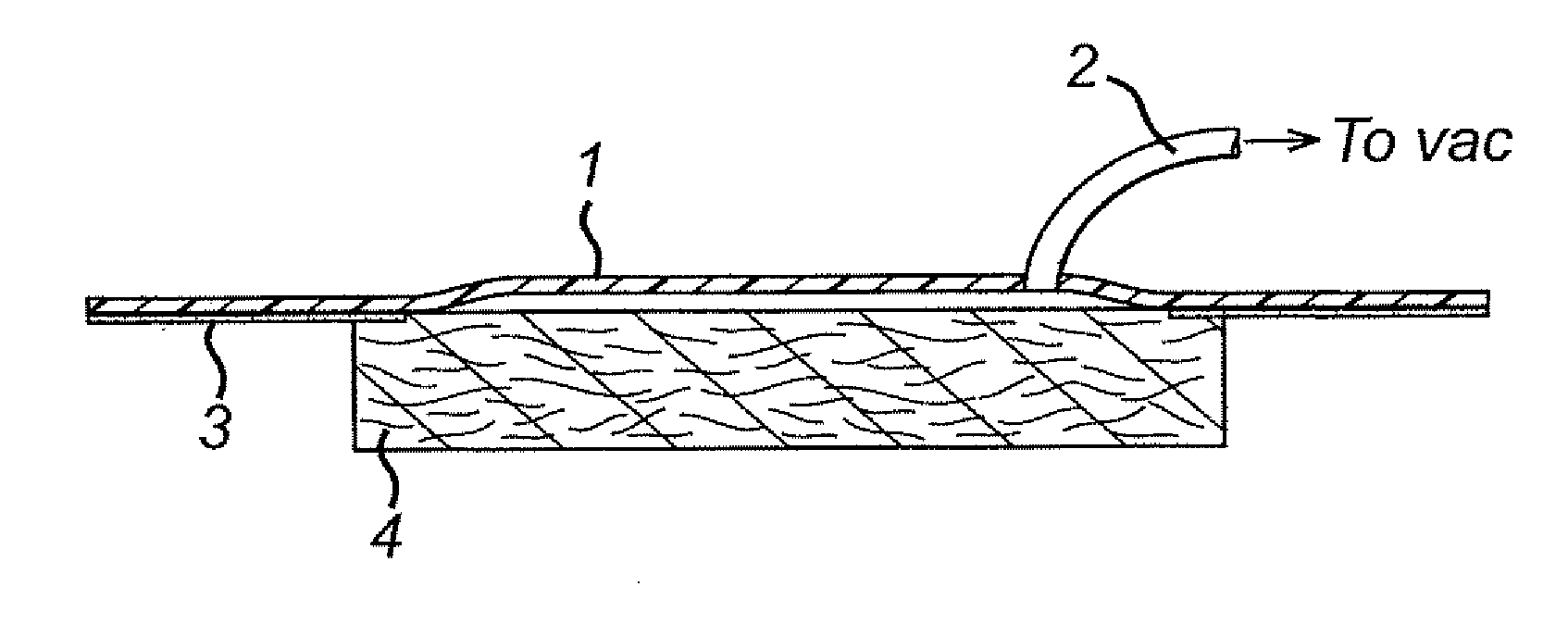
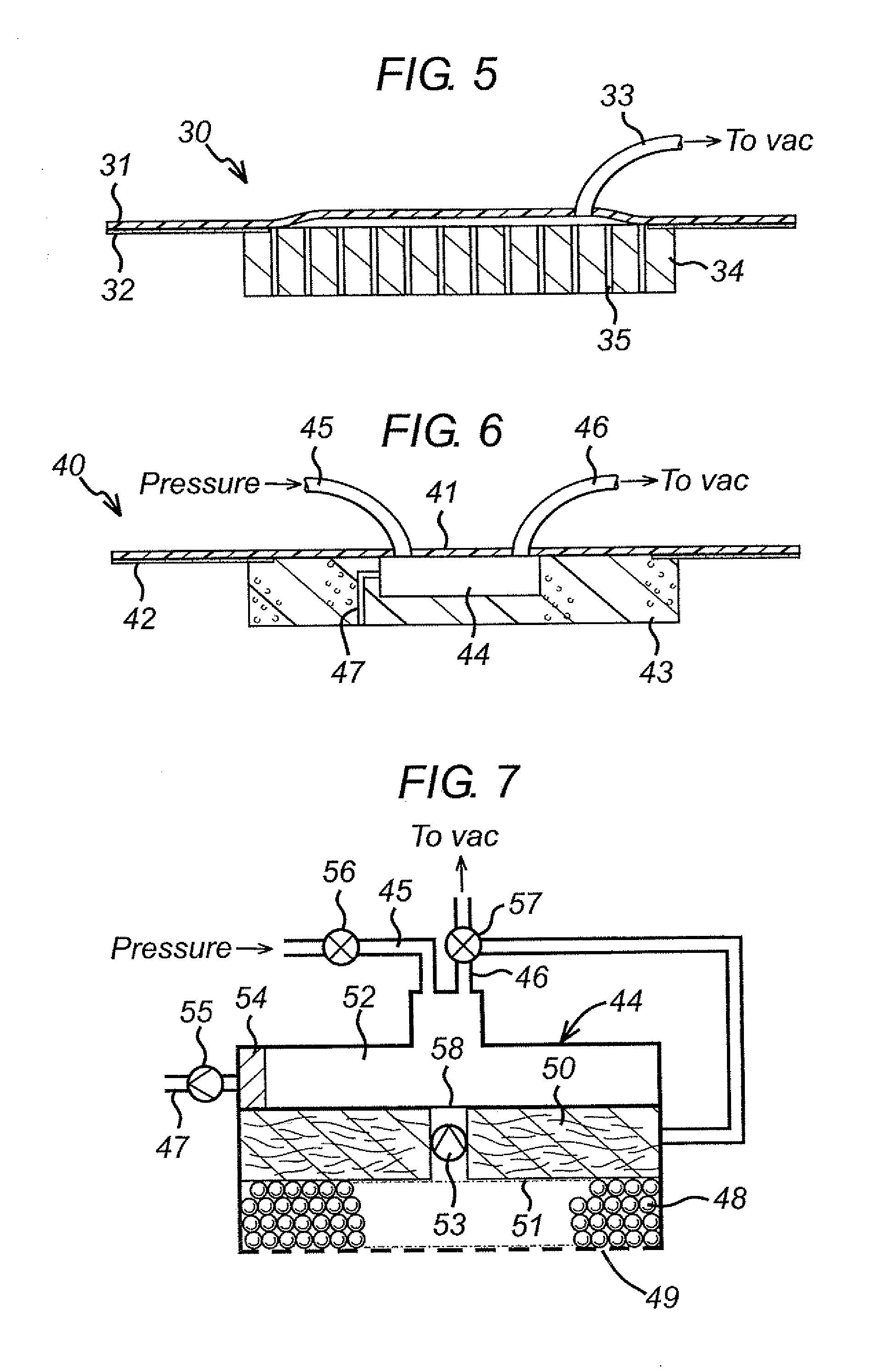
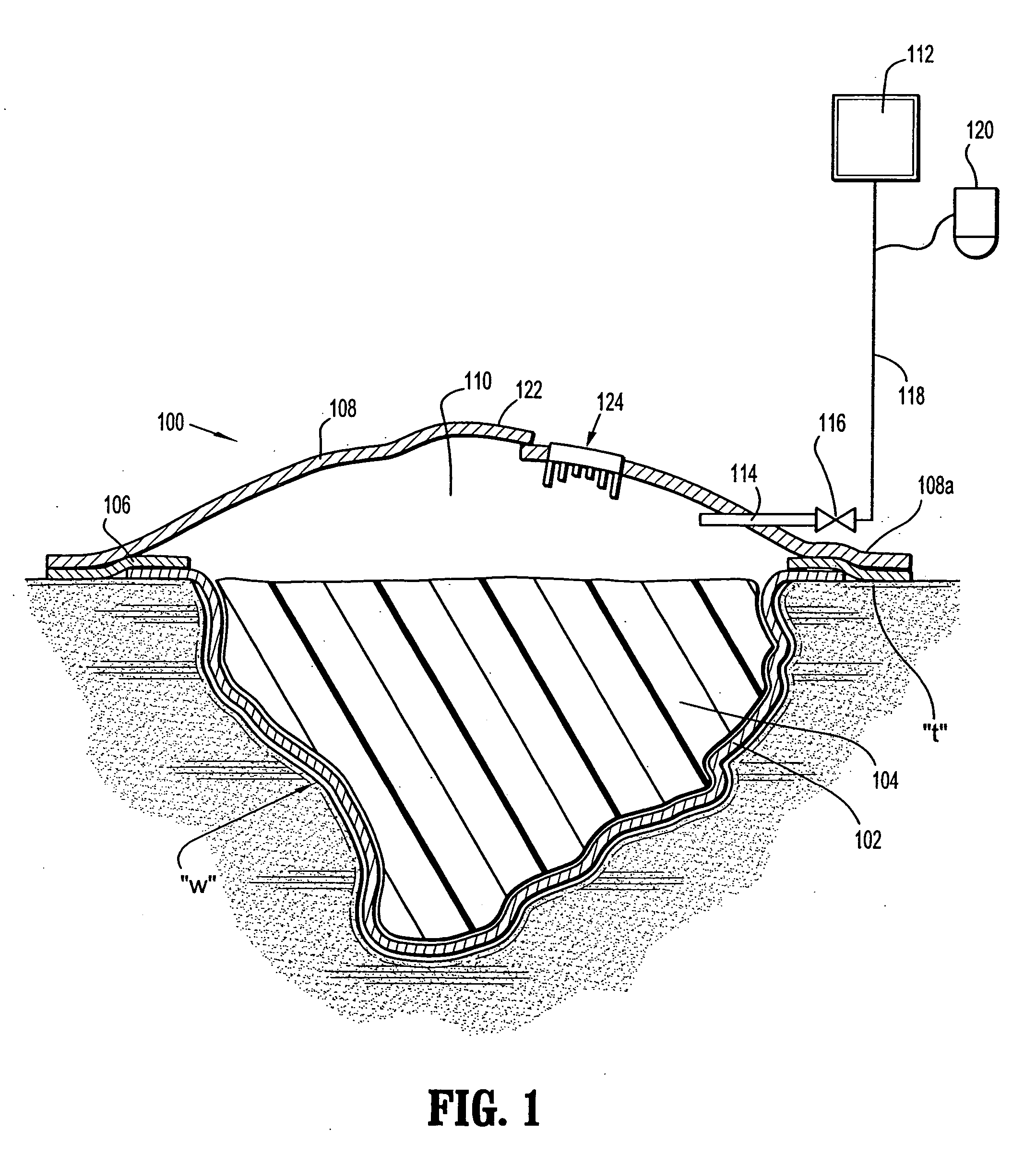
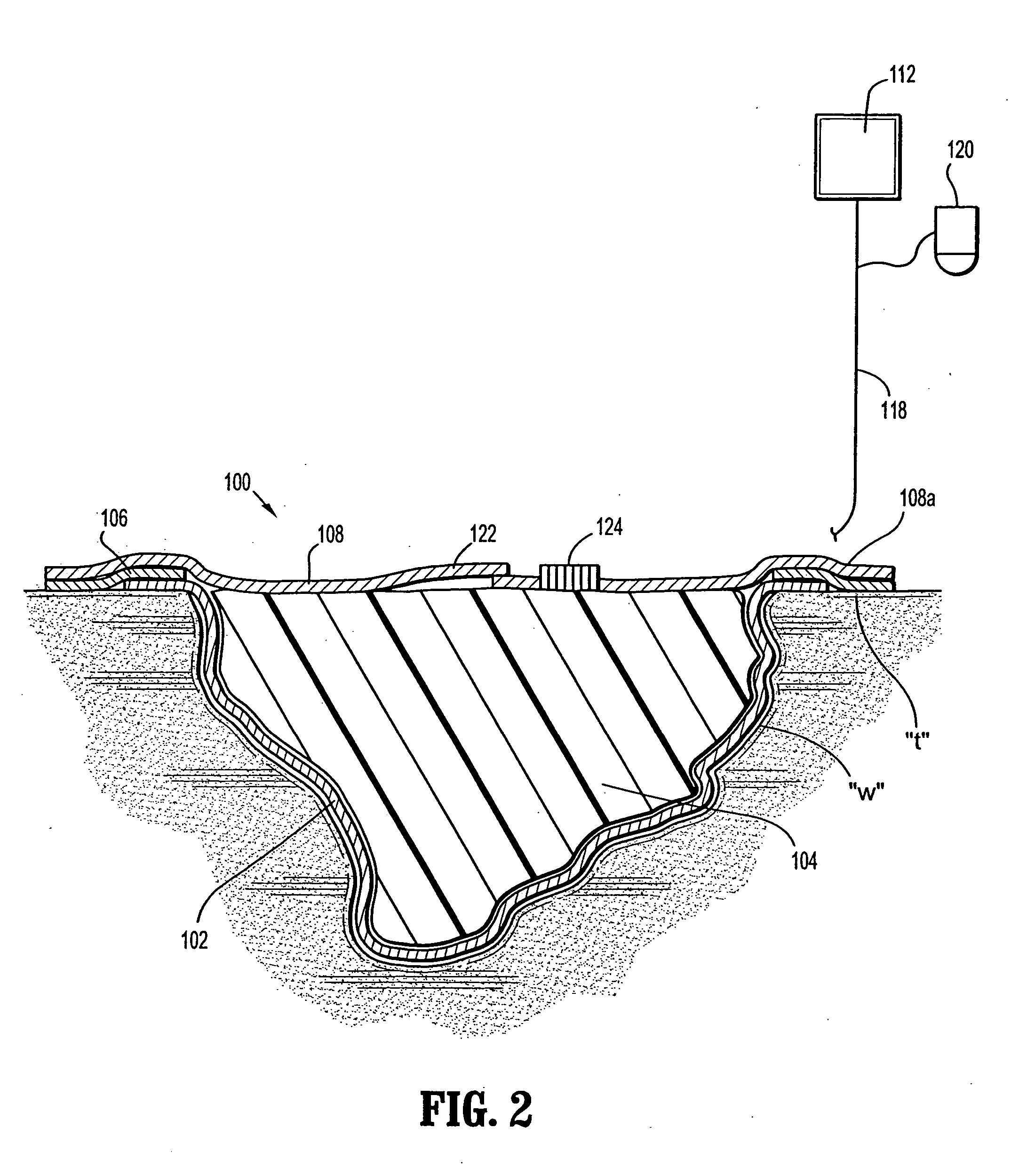
Wound dressings for vacuum therapy
A wound dressing for vacuum therapy comprising: a cover configured for placement over the wound to maintain a reduced pressure over the wound and adapted for communication with a source of vacuum, and a screen structure for placement between the cover and the wound, wherein the screen structure is adapted to remove or inactivate undesirable components from the wound environment and / or to concentrate desirable components present in the wound environment. Also provided are kits for the assembly of such wound dressings, and systems comprising the wound dressings in combination with a source of vacuum.
Owner:KCI USA
Laminar construction negative pressure wound dressing including bioabsorbable material
InactiveUS20070027414A1Safer and efficient and less painfulWound drainsAdhesive dressingsWound dressingDressing change
A laminated negative pressure wound dressing system and method is described. The wound dressing is disposed in the wound in layers including at least one bioabsorbable layer that contacts the wound bed, a bioabsorbable fluid communicating layer, an atmospheric barrier layer, and a tube for applying a negative pressure to the wound bed. Ingrowth of granulation tissue into the bioabsorbable wound bed layer does not need to be inhibited as the bioabsorbable material need not be removed during dressing changes. A kit containing the components of the wound dressing system is also disclosed as well as a method for applying the dressing.
Owner:INTEGRA LIFESCI
Self contained wound dressing with micropump
A composite wound dressing apparatus promotes healing of a wound via the use of a micropump system housed within or above a wound dressing member. The micropump system includes a miniature pump that applies a subatmospheric pressure to the wound to effectively draw wound fluid or exudate away from the wound bed without the need for a cumbersome external vacuum source. Hence, the wound dressing and micropump system is portable which allows the patient mobility that is unavailable when an external vacuum source is used. The patient does not need to be constrained for any period of time while exudate is being removed from the wound.
Owner:SMITH & NEPHEW INC
Biocompatible crosslinked polymers
InactiveUS7009034B2Improve performanceImprove visibilityUltrasonic/sonic/infrasonic diagnosticsPowder deliveryWound dressingPost operative
Biocompatible crosslinked polymers, and methods for their preparation and use, are disclosed in which the biocompatible crosslinked polymers are formed from water soluble precursors having electrophilic and nucleophilic functional groups capable of reacting and crosslinking in situ. Methods for making the resulting biocompatible crosslinked polymers biodegradable or not are provided, as are methods for controlling the rate of degradation. The crosslinking reactions may be carried out in situ on organs or tissues or outside the body. Applications for such biocompatible crosslinked polymers and their precursors include controlled delivery of drugs, prevention of post-operative adhesions, coating of medical devices such as vascular grafts, wound dressings and surgical sealants. Visualization agents may be included with the crosslinked polymers.
Owner:INCEPT LLC
Targeted and high density drug loaded polymeric materials
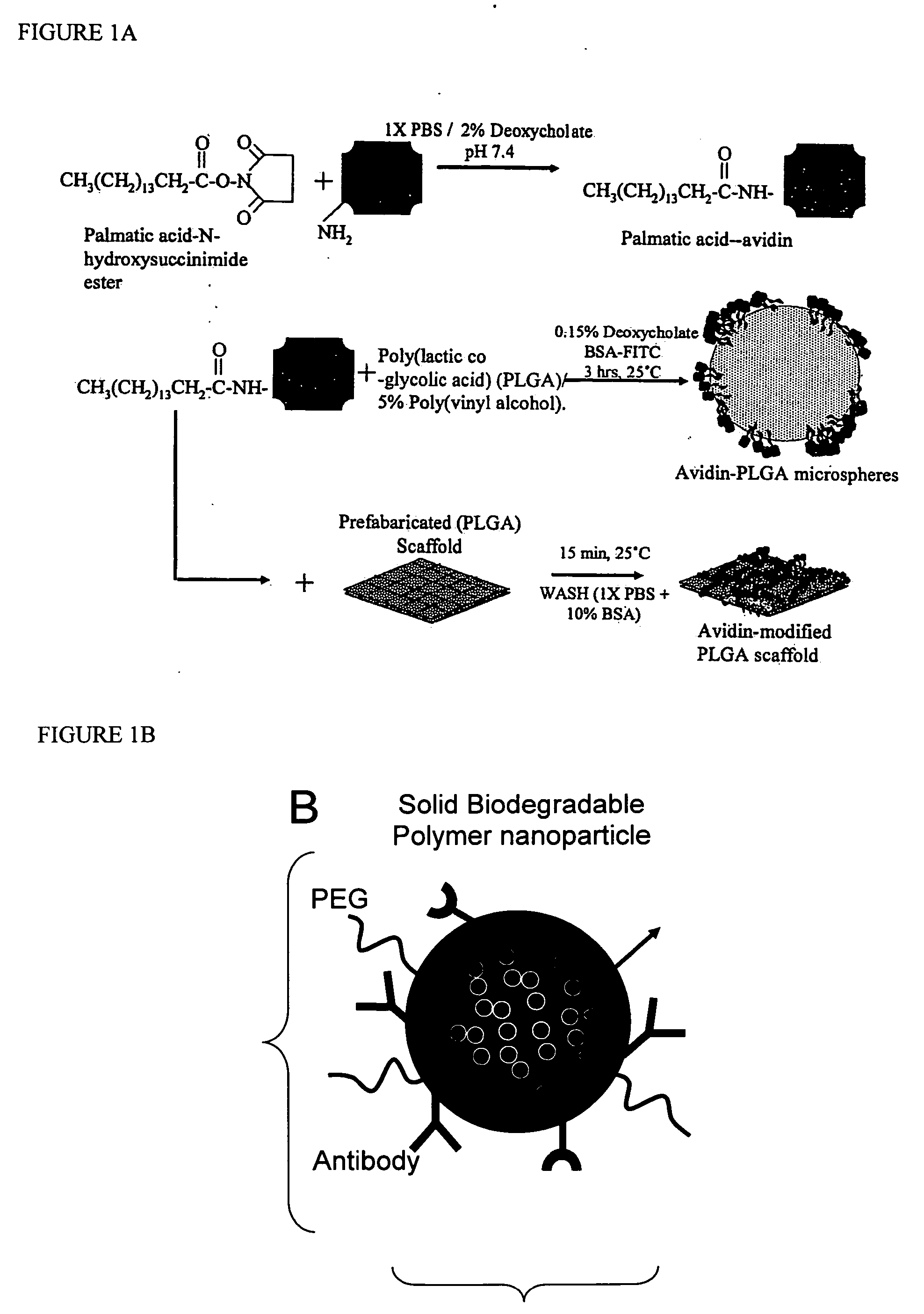
ActiveUS20060002852A1Increase molecular densityHigh densityPowder deliveryBiocideAntigenWound dressing
Polymeric delivery devices have been developed which combine high loading / high density of molecules to be delivered with the option of targeting. As used herein, “high density” refers to microparticles having a high density of ligands or coupling agents, which is in the range of 1000-10,000,000, more preferably between 10,000 and 1,000,000 ligands per square micron of microparticle surface area. A general method for incorporating molecules into the surface of biocompatible polymers using materials with an HLB of less than 10, more preferably less than 5, such as fatty acids, has been developed. Because of its ease, generality and flexibility, this method has widespread utility in modifying the surface of polymeric materials for applications in drug delivery and tissue engineering, as well other other fields. Targeted polymeric microparticles have also been developed which encapsulate therapeutic compounds such as drugs, cellular materials or components, and antigens, and have targeting ligands directly bound to the microparticle surface. Preferred applications include use in tissue engineering matrices, wound dressings, bone repair or regeneration materials, and other applications where the microparticles are retained at the site of application or implantation. Another preferred application is in the use of microparticles to deliver anti-proliferative agents to the lining of blood vessels following angioplasty, transplantation or bypass surgery to prevent or decrease restenosis, and in cancer therapy. In still another application, the microparticles are used to treat or prevent macular degeneration when administered to the eye, where agents such as complement inhibitors are administered.
Owner:YALE UNIV
Self contained wound dressing apparatus
InactiveUS20070055209A1Easy to disassemblePromote healingAntibacterial agentsSurgical needlesPeristaltic pumpVacuum pressure
The composite wound dressing apparatus promotes healing of a wound via the use of an external peristaltic vacuum pump. The external peristaltic pump applies a vacuum pressure to the wound to effectively draw wound fluid or exudate away from the wound bed. The external peristaltic pump is tethered to the wound dressing and is portable, preferably, carried by the patient in a support bag, which permits patient mobility. Moreover, the patient does not need to be constrained for any period of time while exudate is being removed from the wound.
Owner:TYCO HEALTHCARE GRP LP
Therapeutic apparatus for treating ulcers
InactiveUS7214202B1Pneumatic massageVibration massageWound dressingIntermittent pneumatic compression therapy
A method and apparatus for providing concurrent applications of intermittent pneumatic compression therapy and vacuum assisted closure therapy generally comprises a wound dressing for introduction of a negative pressure into a wound on a patient's foot and a foot wrap for application of positive, compressive forces to substantially all of the patients foot. A suction pump, having an associated vacuum sensor and first feedback mechanism, supplies negative pressure to the wound dressing. A ventable source of pressurized gas, having an associated pressure transducer and second feedback mechanism, supplies positive force to the foot wrap. At least one control system is operably associated with the suction pump and ventable source of pressurized gas for controlling the negative and positive applications of pressure to the patient's foot. Controlled modes for operation include continuous or intermittent application of one or both therapies and simultaneous or cycled application of the therapies.
Owner:KCI LICENSING INC
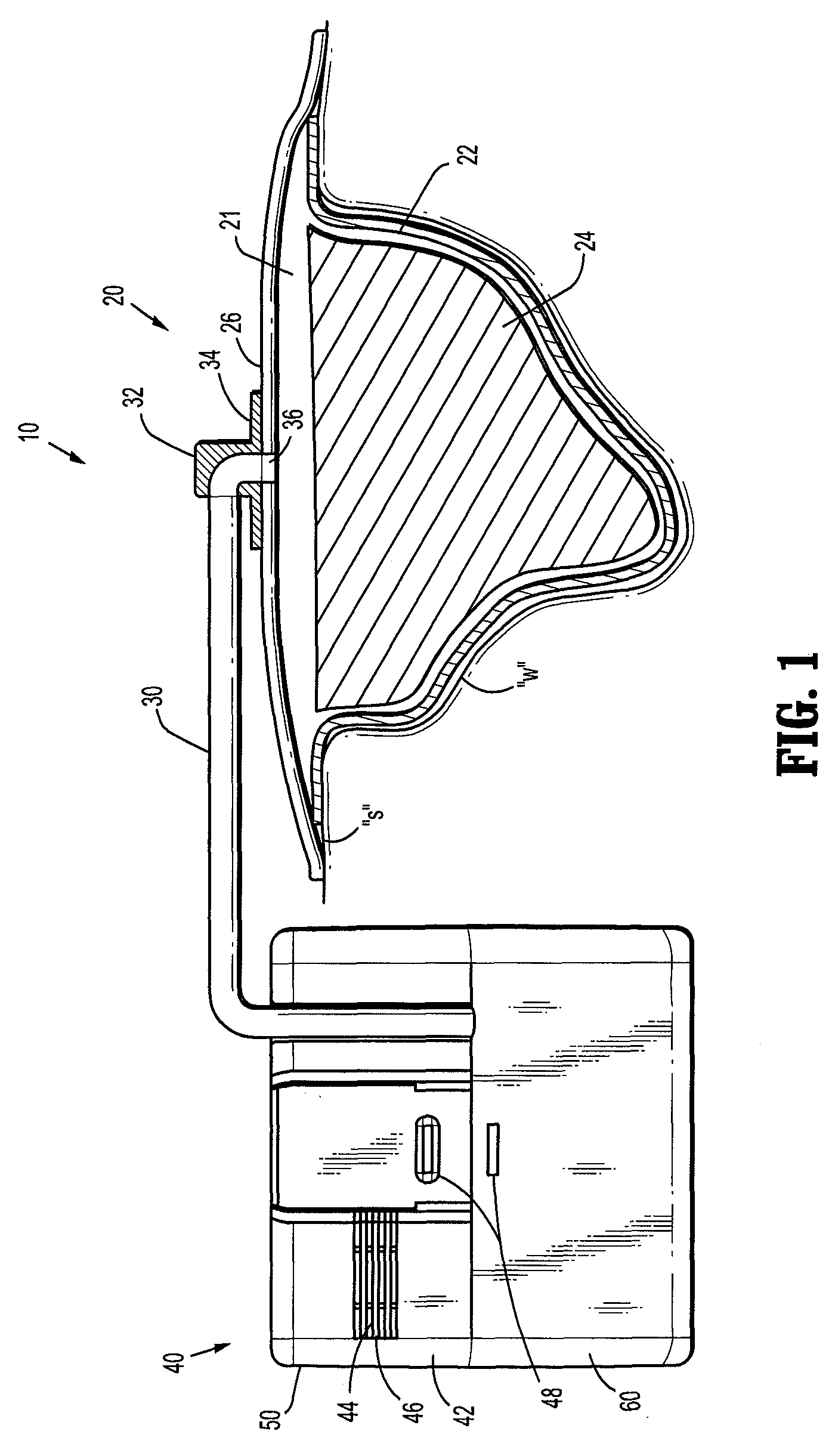
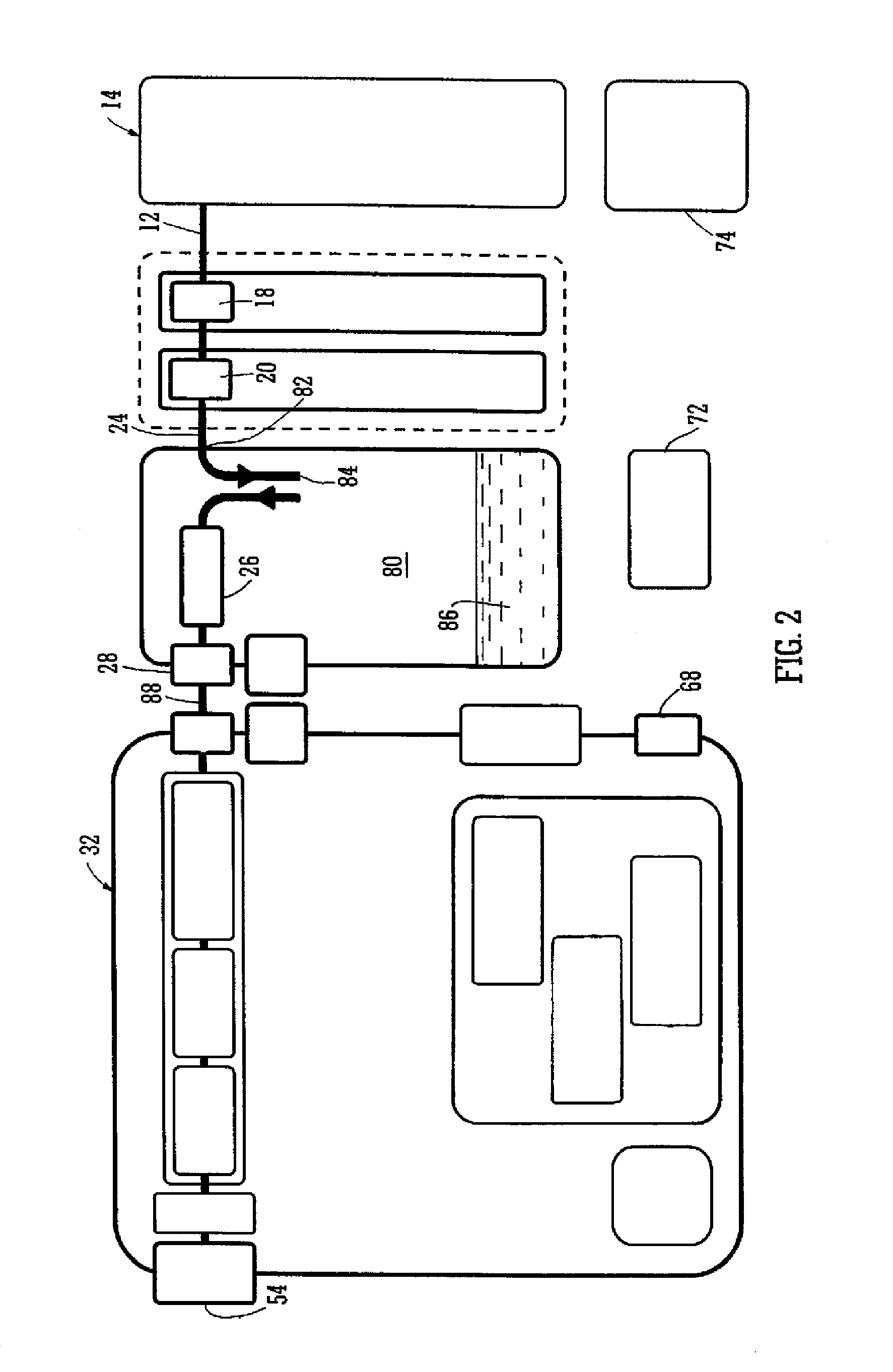
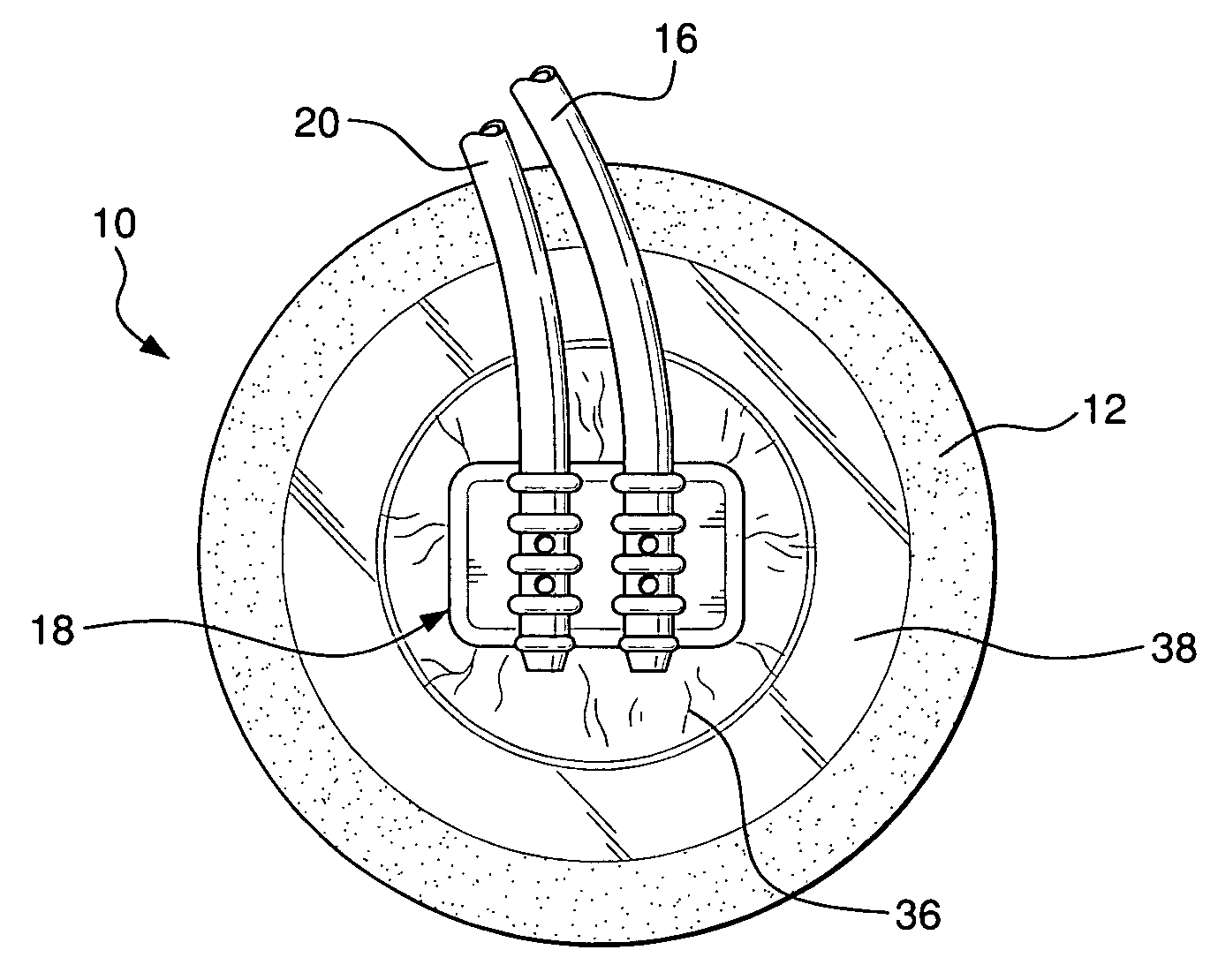
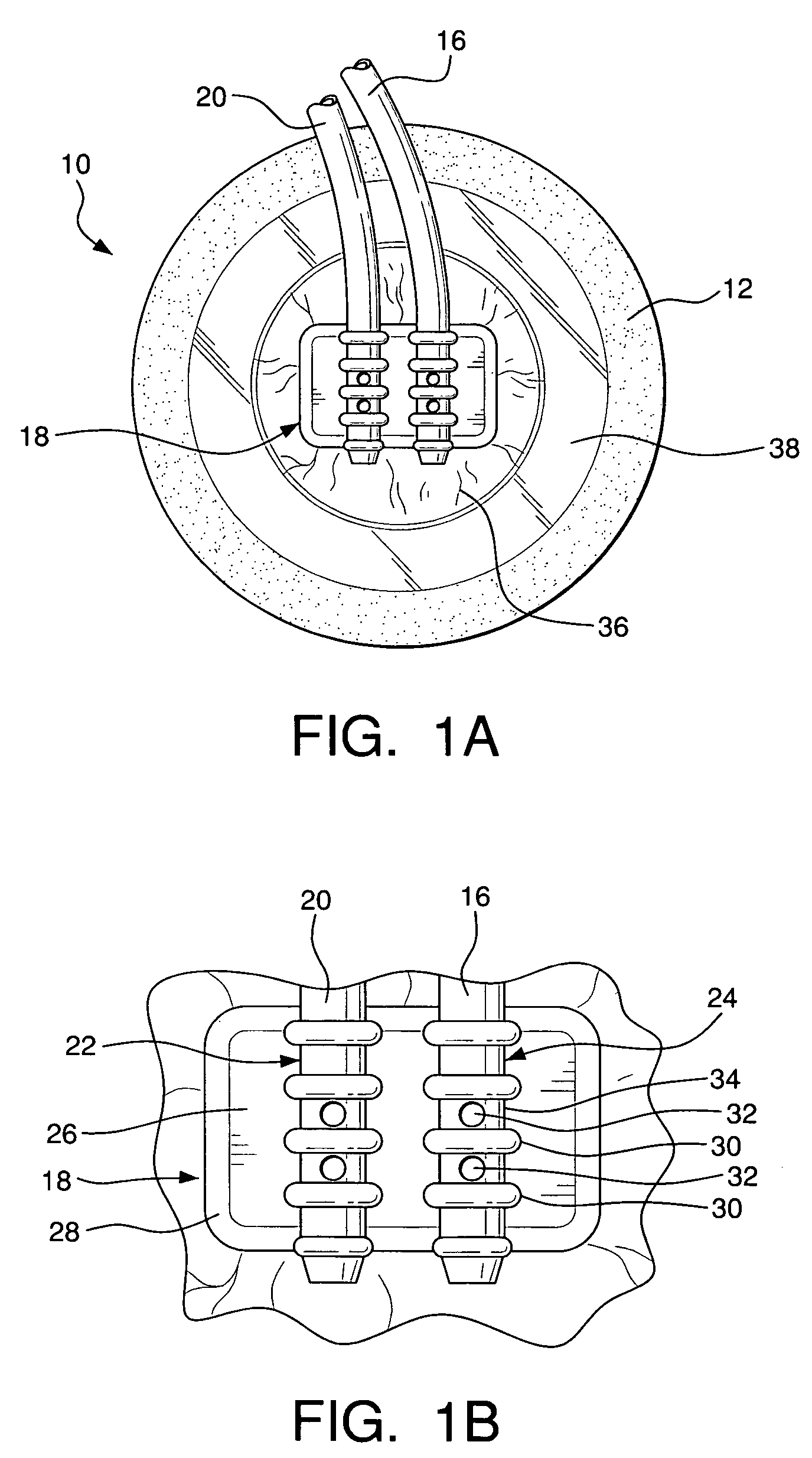
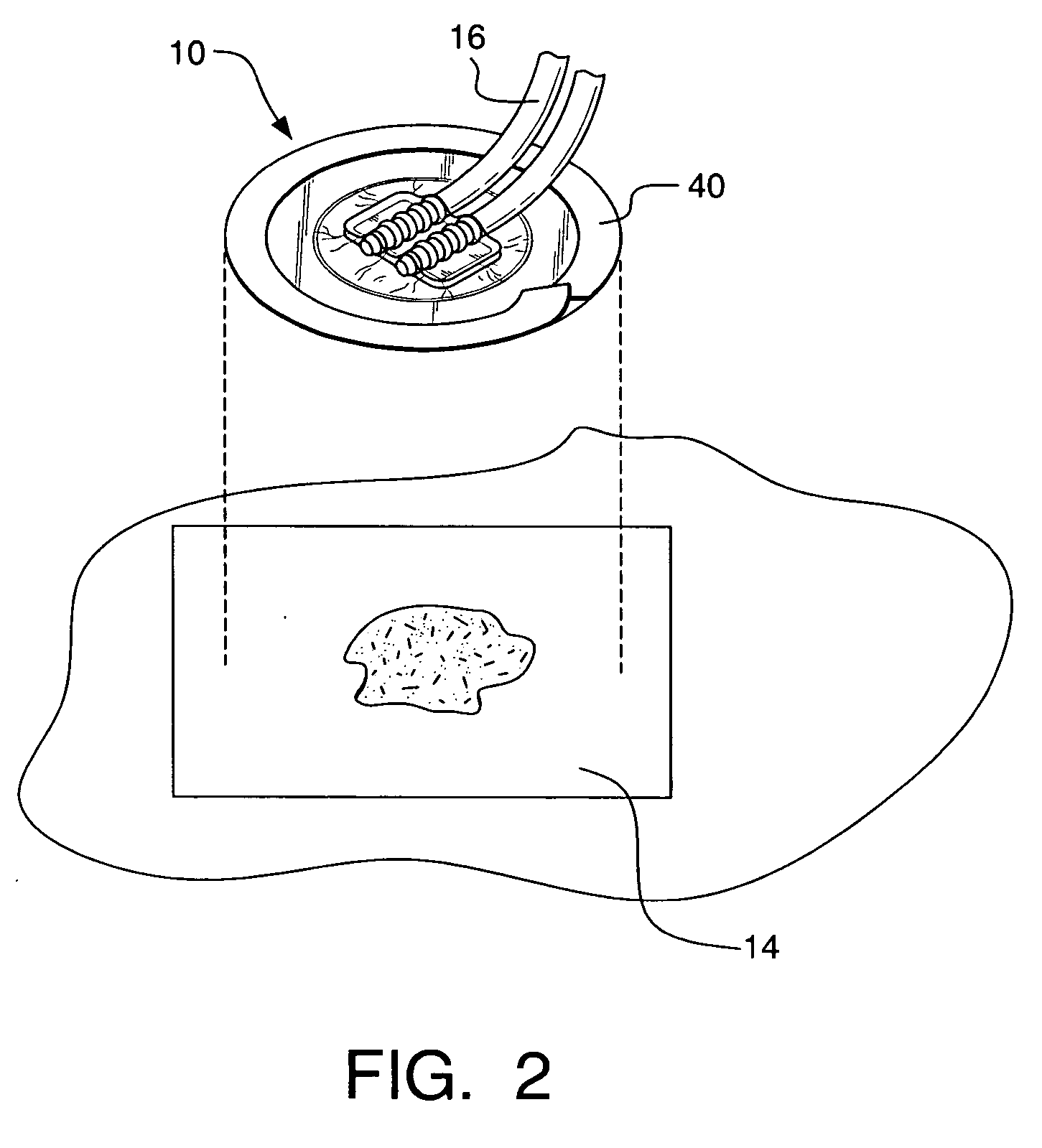
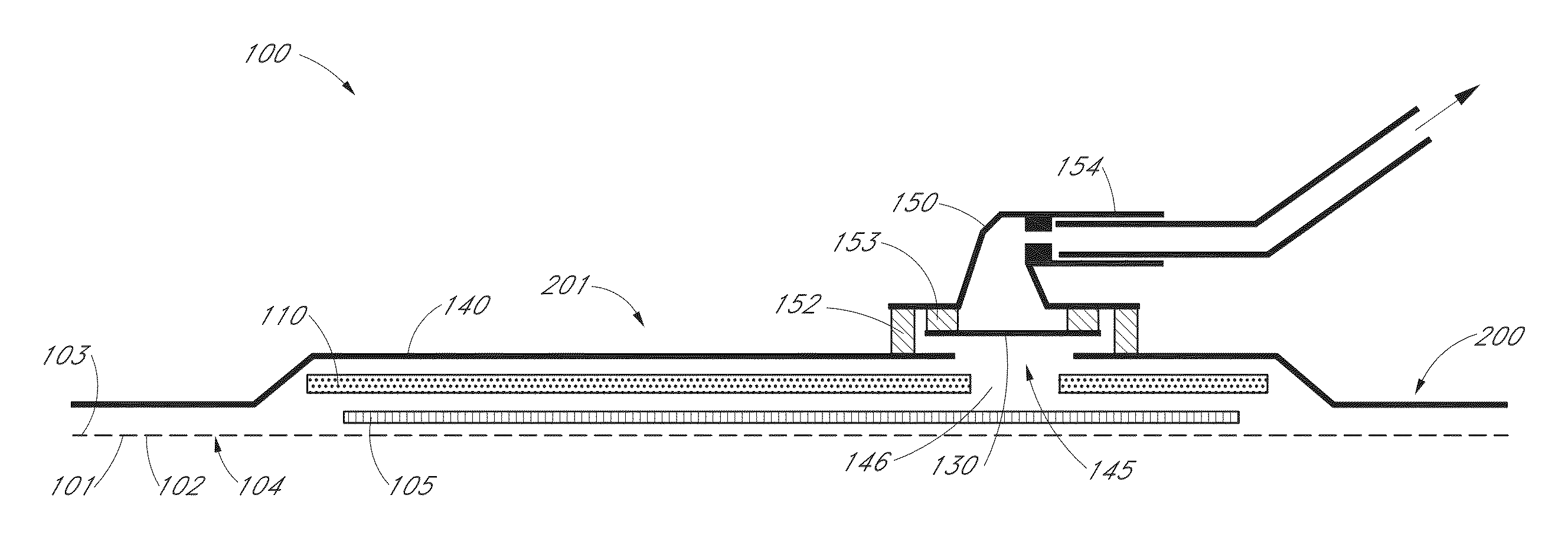
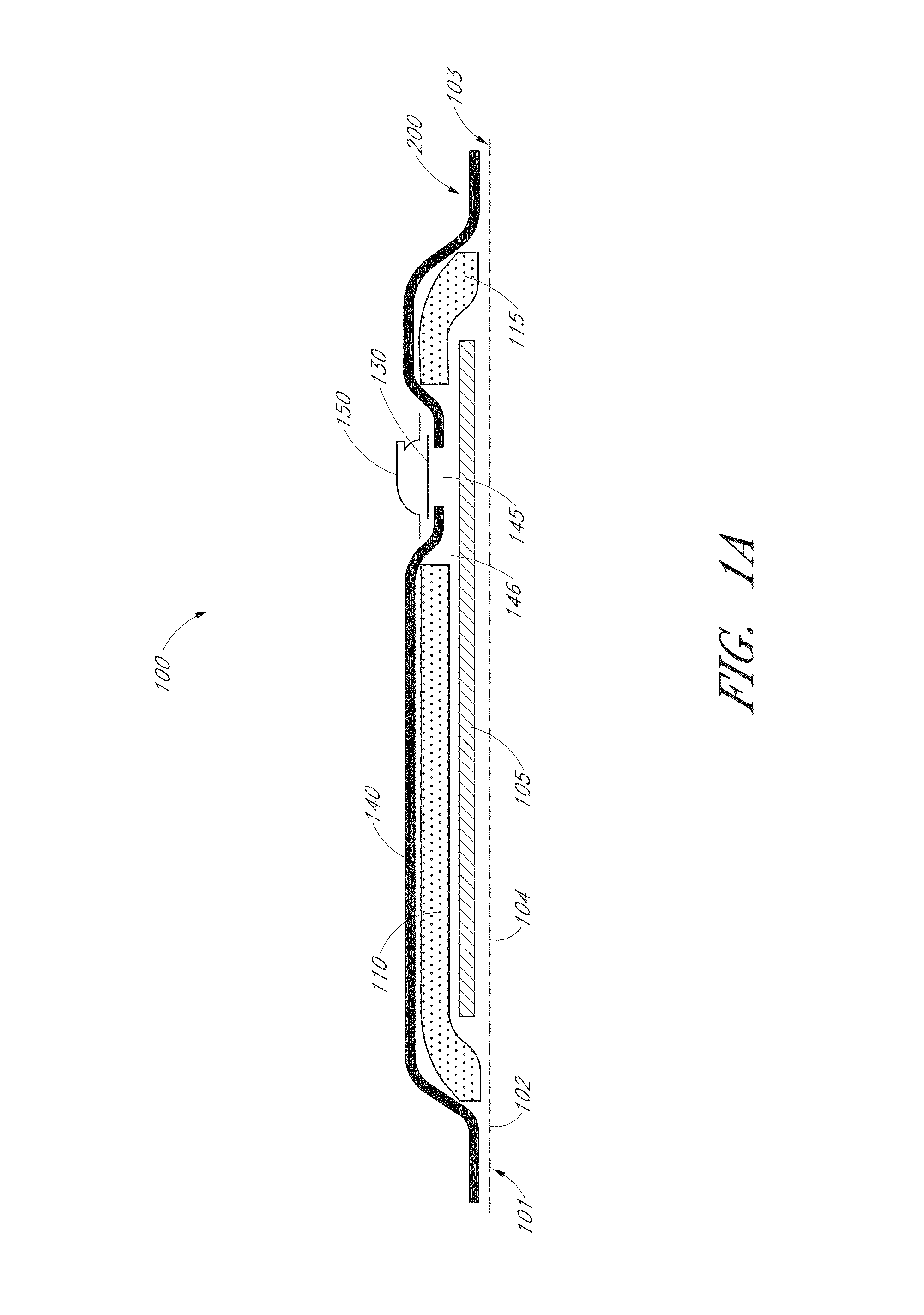
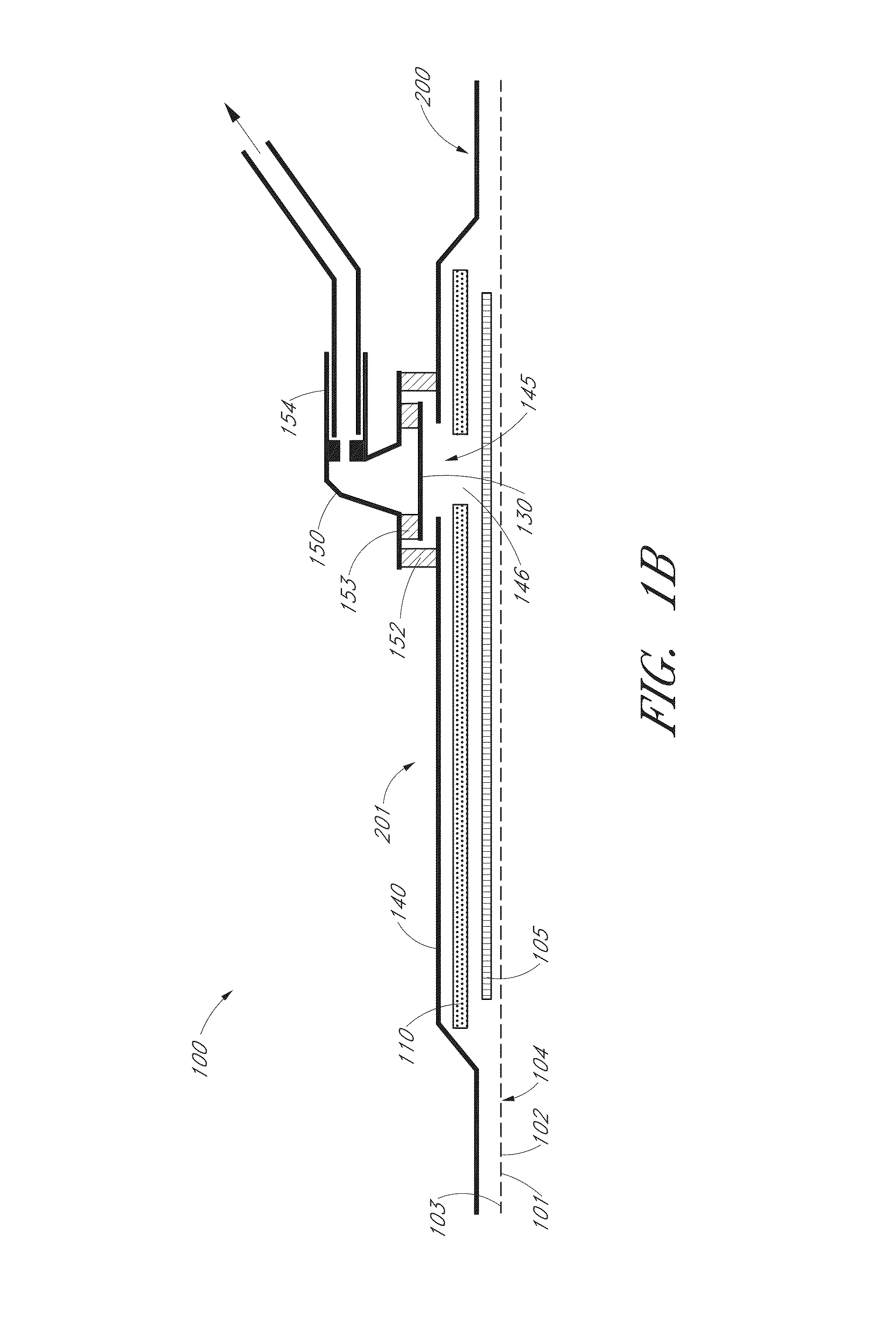
Wound dressing
A method and apparatus are disclosed for dressing a wound. The apparatus comprises an absorbent layer for absorbing wound exudate, a liquid impermeable, gas permeable filter layer over the absorbent layer, a cover layer comprising at least one orifice and a first liquid and gas permeable transmission layer underlying the absorbent layer. The transmission layer is in fluid communication with the filter layer.
Owner:SMITH & NEPHEW INC
Self contained wound dressing with micropump
A composite wound dressing apparatus promotes healing of a wound via the use of a micropump system housed within or above a wound dressing member. The micropump system includes a miniature pump that applies a subatmospheric pressure to the wound to effectively draw wound fluid or exudate away from the wound bed without the need for a cumbersome external vacuum source. Hence, the wound dressing and micropump system is portable which allows the patient mobility that is unavailable when an external vacuum source is used. The patient does not need to be constrained for any period of time while exudate is being removed from the wound.
Owner:SMITH & NEPHEW INC
Medical devices and applications of polyhydroxyalkanoate polymers
InactiveUS6867247B2Reduce probabilityHigh porositySuture equipmentsStentsTissue repairBiocompatibility Testing
Devices formed of or including biocompatible polyhydroxyalkanoates are provided with controlled degradation rates, preferably less than one year under physiological conditions. Preferred devices include sutures, suture fasteners, meniscus repair devices, rivets, tacks, staples, screws (including interference screws), bone plates and bone plating systems, surgical mesh, repair patches, slings, cardiovascular patches, orthopedic pins (including bone filling augmentation material), adhesion barriers, stents, guided tissue repair / regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, atrial septal defect repair devices, pericardial patches, bulking and filling agents, vein valves, bone marrow scaffolds, meniscus regeneration devices, ligament and tendon grafts, ocular cell implants, spinal fusion cages, skin substitutes, dural substitutes, bone graft substitutes, bone dowels, wound dressings, and hemostats. The polyhydroxyalkanoates can contain additives, be formed of mixtures of monomers or include pendant groups or modifications in their backbones, or can be chemically modified, all to alter the degradation rates. The polyhydroxyalkanoate compositions also provide favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under physiological conditions.
Owner:TEPHA INC
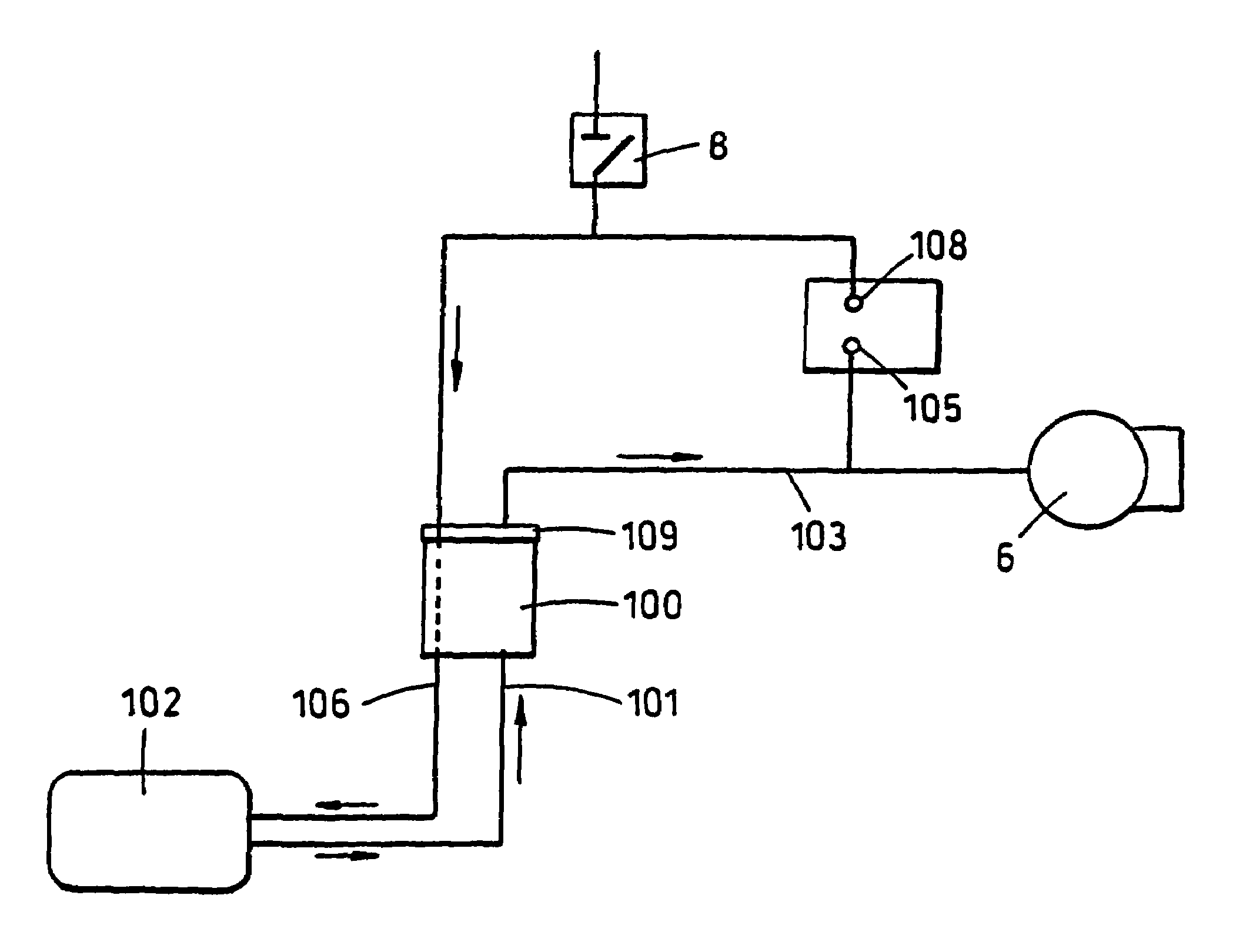
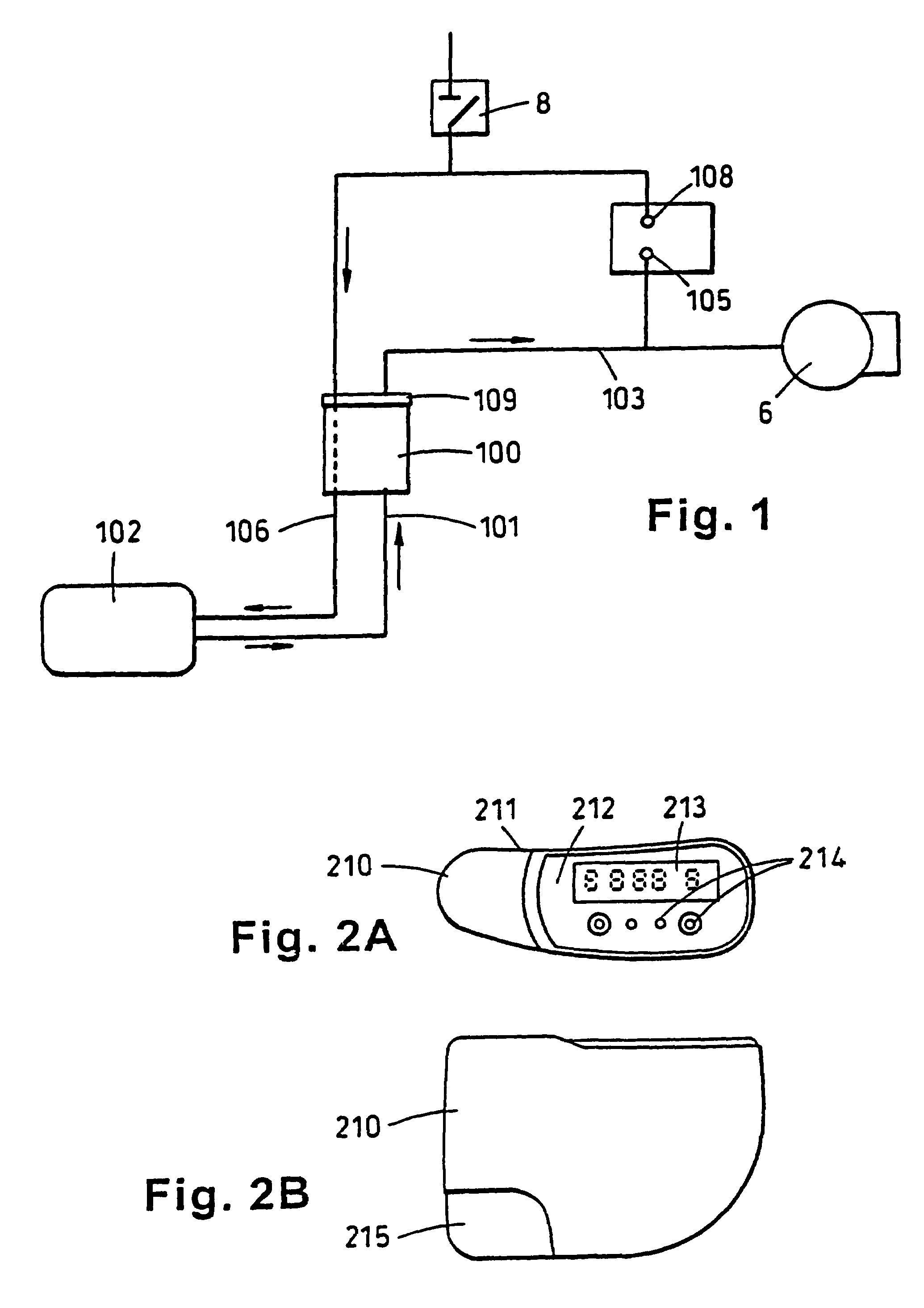
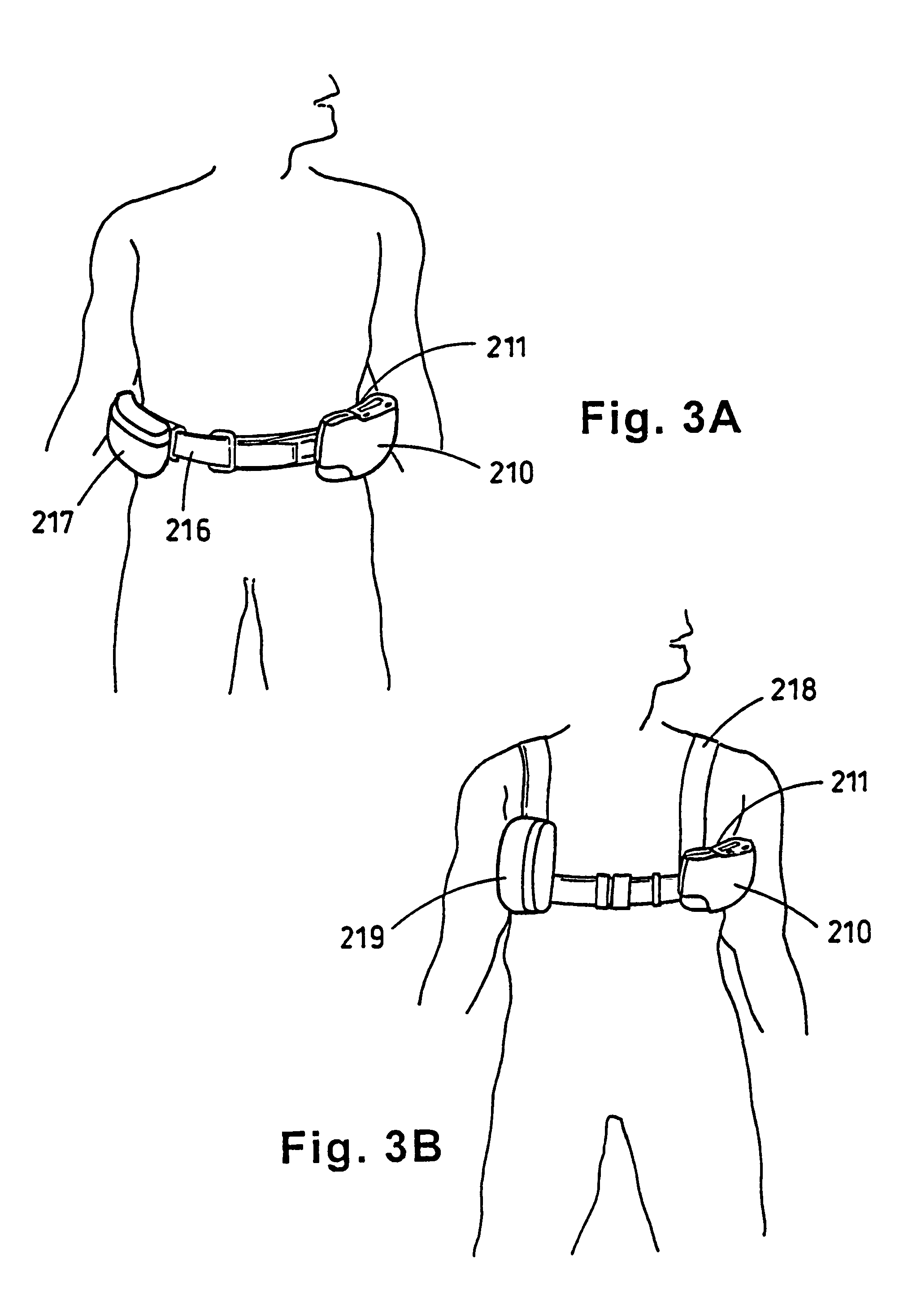
Portable wound treatment apparatus
A portable wound treatment apparatus, for stimulating the healing of superficial wounds, comprises a housing containing a suction pump and a canister for containing fluids drawn from the wound. The housing is supported on a harness or belt worn by the patient. The canister is connected to a porous wound dressing at the wound site via a plurality of tubes, a multi-lumen tube or a combination thereof. A rechargeable battery pack may be incorporated within the housing or externally thereto. The external battery pack may be shaped to balance the housing on the harness or belt. Pressure transducers are provided to monitor and report pressures at the wound site or internal to the canister. Monitored pressures may also be utilized to determine the filled state of the canister and, thereafter, either report this state to the operator or automatically discontinue suction from the wound, or both.
Owner:KCI LICENSING INC
Canister for receiving wound exudate in a negative pressure therapy system
ActiveUS8216198B2Promote healingIncrease surface areaMedical devicesIntravenous devicesWound dressingNegative-pressure wound therapy
A system to promote the healing of an exuding wound includes a wound dressing, a subatmospheric pressure mechanism, and a collection canister. The wound dressing is dimensioned for positioning relative to a wound bed of a subject. The subatmospheric pressure mechanism includes a control unit disposed within a housing. The control unit includes a vacuum source associated with a vacuum port. The collection canister has an interior wall which defines an internal chamber. The internal chamber is in fluid communication with the vacuum source of the subatmospheric pressure mechanism through the vacuum port and with the wound dressing for collecting exudates removed for the wound bed under influence of the vacuum source. The canister including a baffle mechanism disposed within the internal chamber for dampening the movement of the collected exudates.
Owner:SMITH & NEPHEW INC
Portable wound therapy system
A portable system for subatmospheric pressure therapy in connection with healing a surgical wound, includes a wound dressing dimensioned for positioning relative to a wound bed of a subject, a portable subatmospheric pressure mechanism dimensioned to be carried or worn by the subject and a container for collecting exudates from the wound bed removed under the subatmospheric pressure supplied by the subatmospheric pressure mechanism. The portable subatmospheric pressure mechanism includes a housing, a subatmospheric pressure source disposed within the housing and in fluid communication with the wound dressing to supply subatmospheric pressure to the wound dressing and a power source mounted to or within the housing for supplying power to actuate the subatmospheric pressure source.
Owner:SMITH & NEPHEW INC
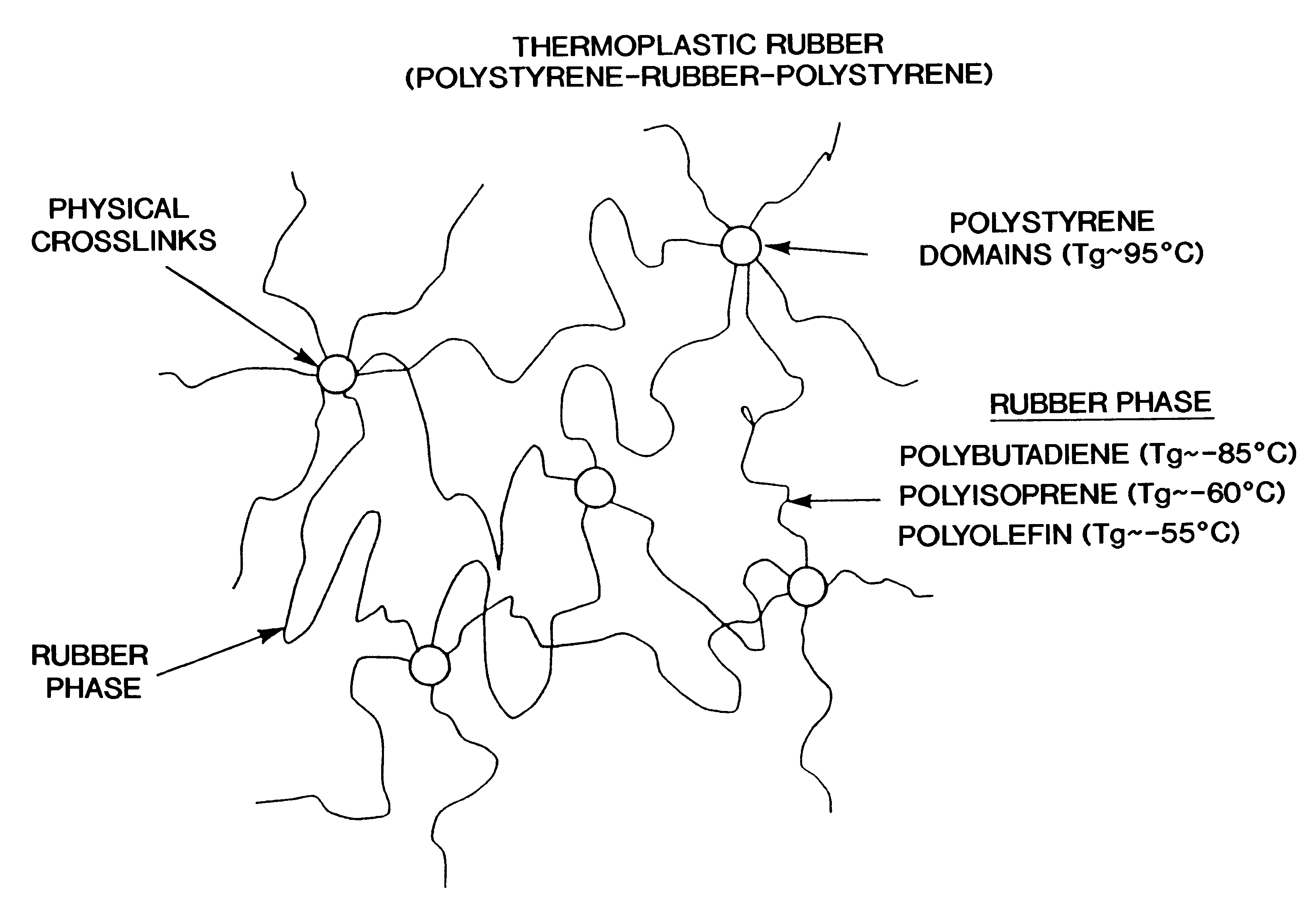
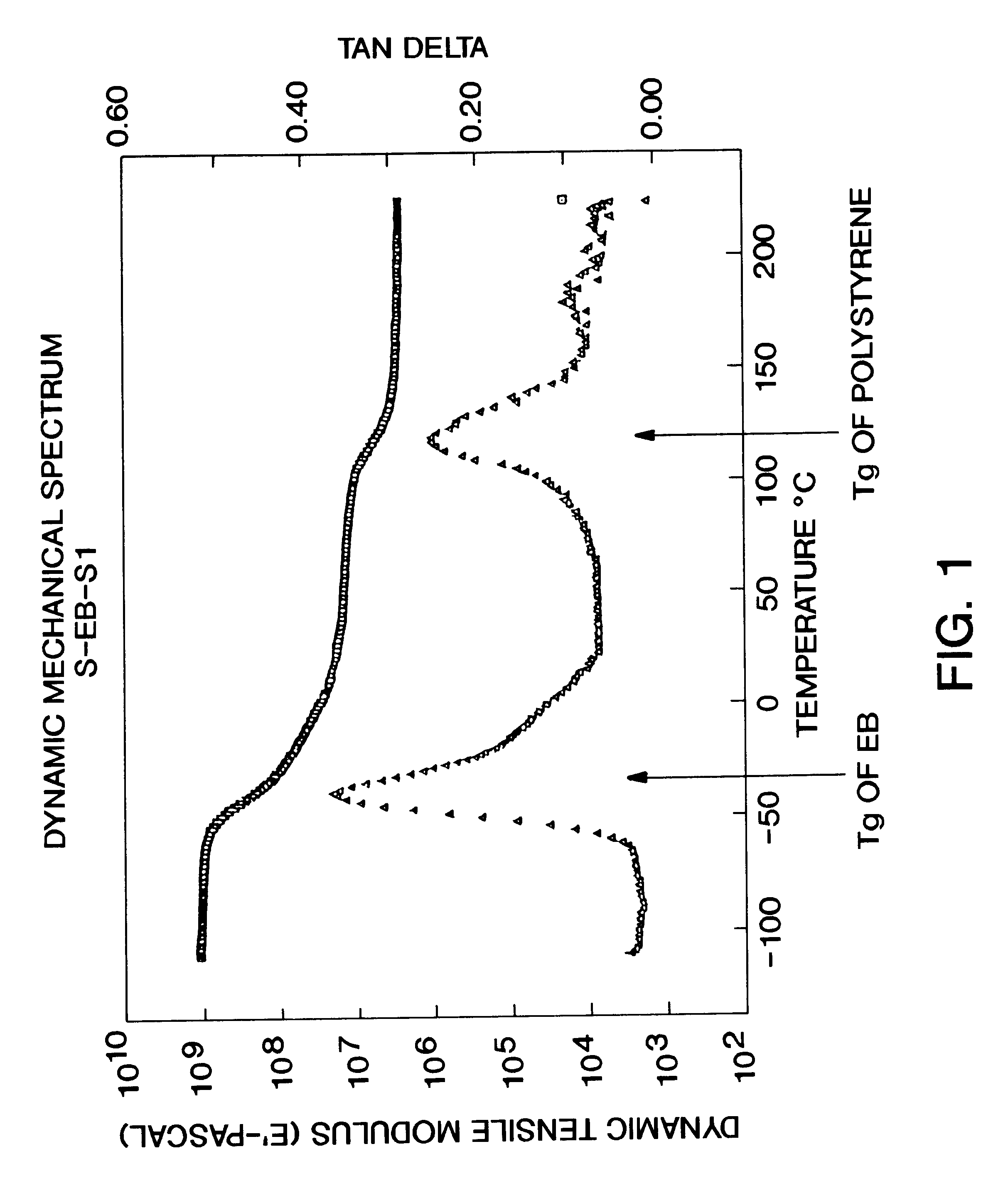
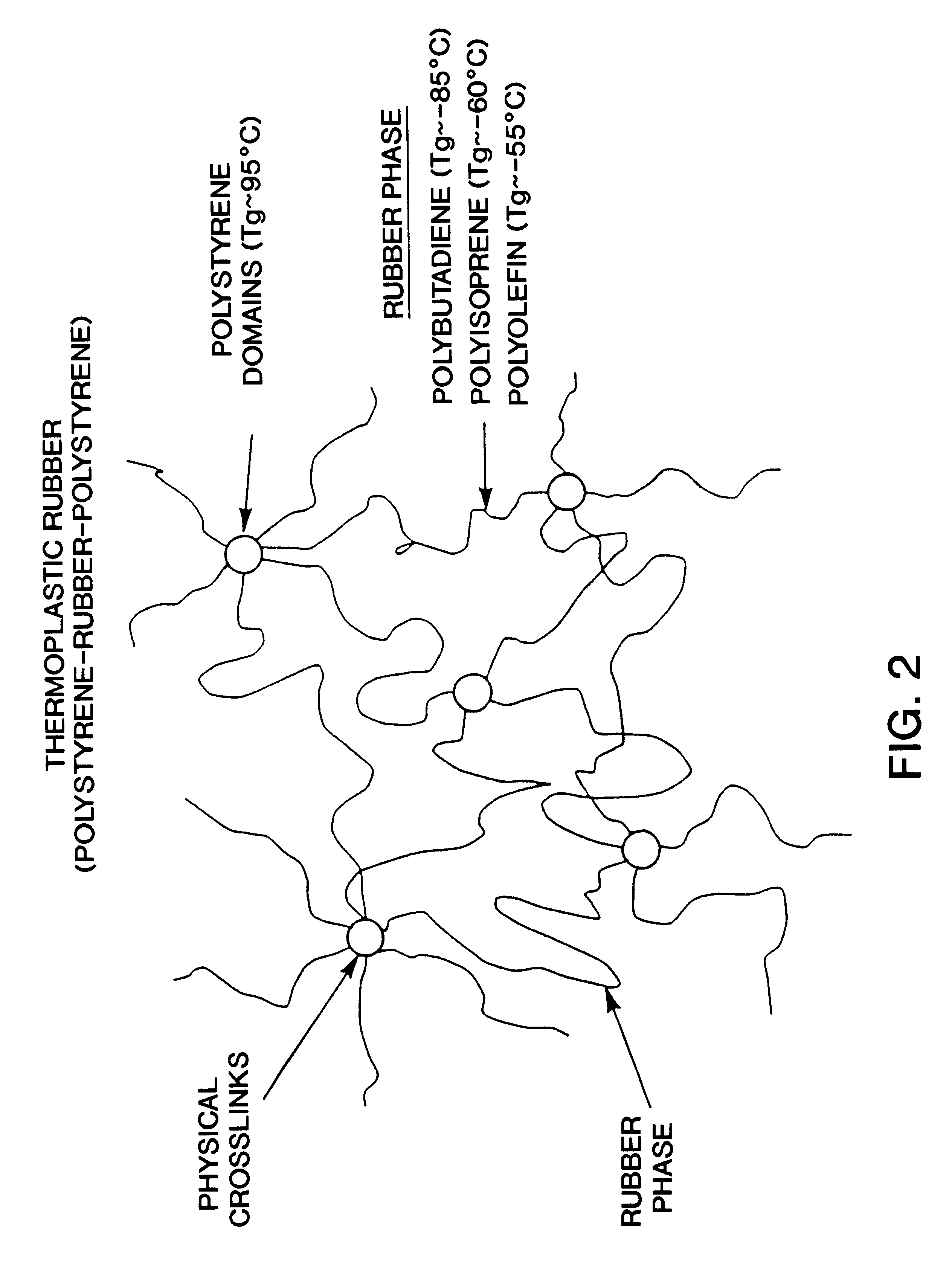
Dimensionally stable, breathable, stretch-thinned, elastic films
A method for producing a stretch-thinned elastic article having dimensional stability over time and at elevated temperatures in which a thermoplastic block copolymer is melt-processed into a stretchable article such as a film or fiber. The article is then conditioned at an elevated temperature greater than or equal to a glass transition temperature (Tg) of a hard phase of the thermoplastic block copolymer. The article is stretch-thinned at the elevated temperature to a desired percentage stretch, forming a stretch-thinned article, after which it is cooled to a temperature below the glass transition temperature of the hard phase of the thermoplastic block copolymer. Films produced by this method are suitable for use in durable and disposable articles including personal care articles such as diapers, incontinence wear, training pants, and feminine care articles, as well as wound dressings, wipes, towels, napkins, and protective apparel.
Owner:KIMBERLY-CLARK WORLDWIDE INC
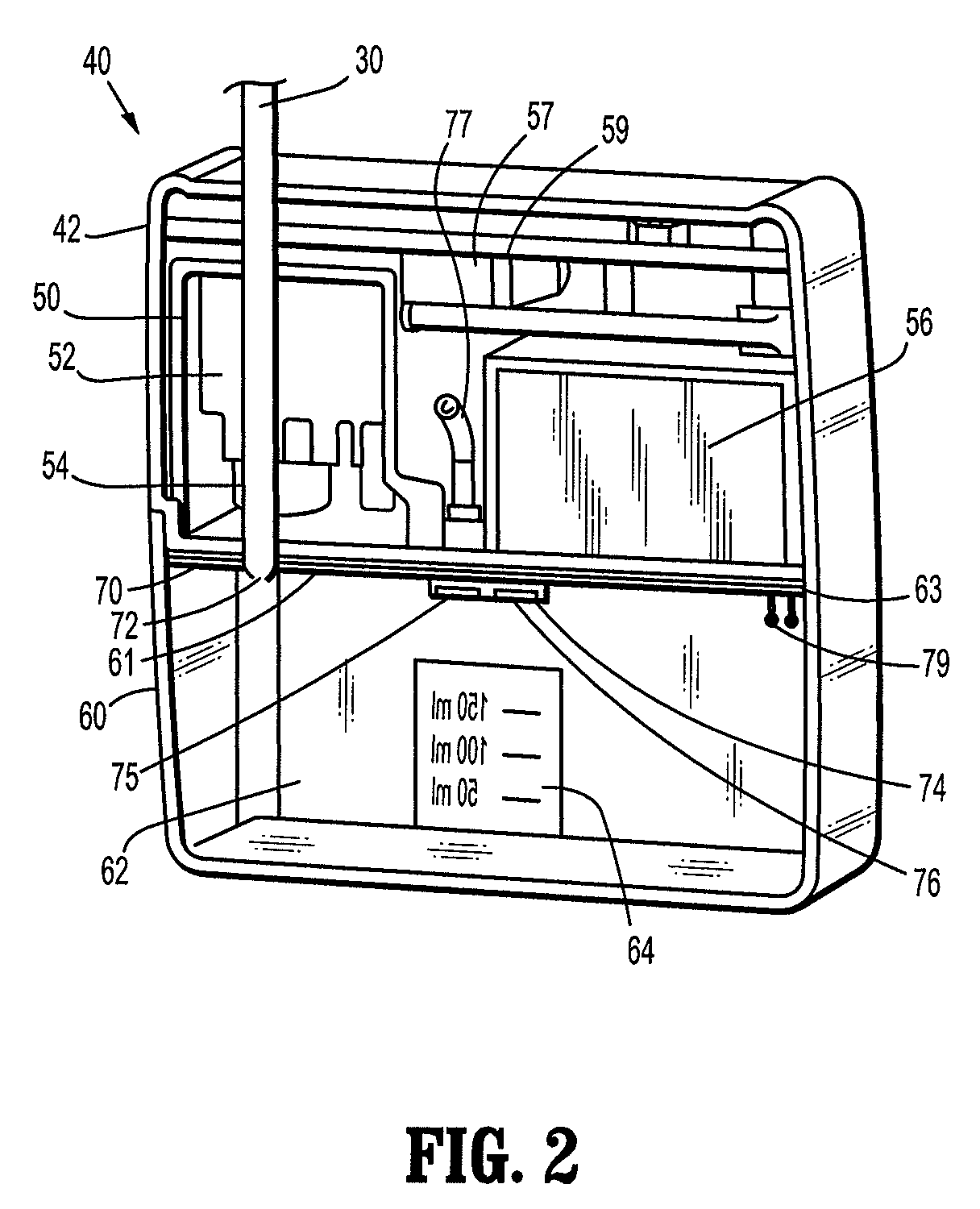
Wound dressing with vacuum reservoir
A wound dressing apparatus includes a wound dressing member dimensioned for positioning relative to a wound bed. The wound dressing member including an internal vacuum reservoir and has a port in communication with the vacuum reservoir for applying subatmospheric pressure to the vacuum reservoir to facilitate removal of fluid from the wound bed. The wound dressing member includes a visual pressure indicator associated therewith for indicating a level of pressure within the vacuum reservoir. The visual pressure indicator includes color indicia having a plurality of colors corresponding to a condition of the pressure within the vacuum reservoir. The wound dressing member includes a lower absorbent member positionable adjacent the wound bed and an upper member which at least partially defines the vacuum reservoir. At least one of the top member and the lower absorbent member has the visual pressure indicator mounted thereto. The preferred visual pressure indicator includes an electronic position sensor. The visual pressure indicator further includes circuit means and visible alarm means. The circuit means is adapted to actuate the visible alarm means when the position sensor detects a relative positioning of the top member of the wound dressing member to provide a visual indication of the condition of the subatmospheric pressure within the vacuum reservoir.
Owner:SMITH & NEPHEW INC
Biocompatible wound dressing
InactiveUS7700819B2Reduce pressureReduced tissue treatmentNon-adhesive dressingsAdhesive dressingsWound dressingContact layer
A multi-layer reduced pressure delivery apparatus is provided for applying reduced pressure tissue treatment to a tissue site. The multi-layer apparatus includes a tissue contact layer, a release layer, and a manifold layer. The tissue contact layer includes a scaffold adapted to contact the tissue site, the release layer includes a hydrogel-forming material and a plurality of flow channels, and the manifold layer includes a distribution manifold. The release layer is positioned between the tissue contact layer and the manifold layer to allow easy release of the manifold layer from the tissue contact layer following the administration of reduced pressure tissue treatment.
Owner:3M INNOVATIVE PROPERTIES CO
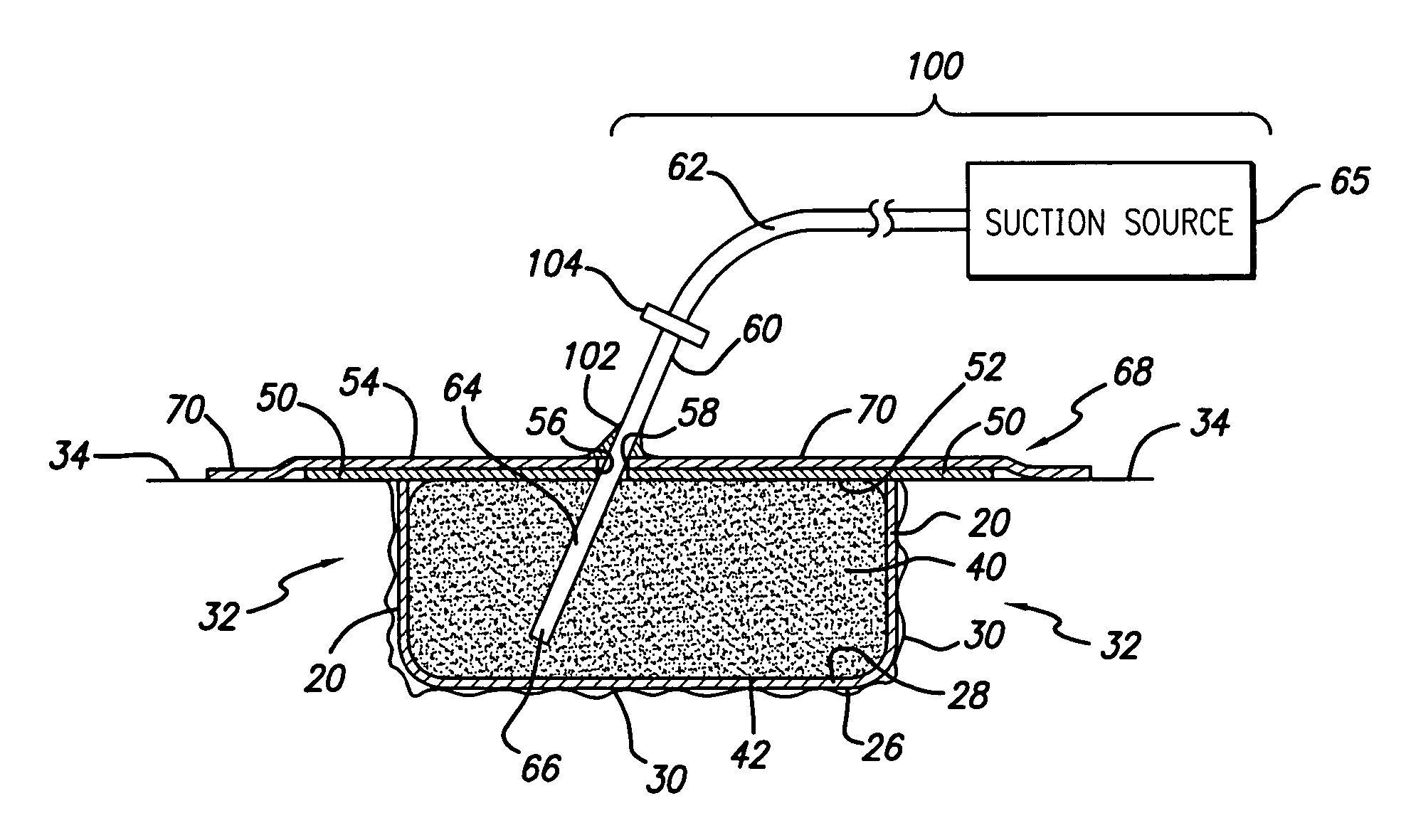
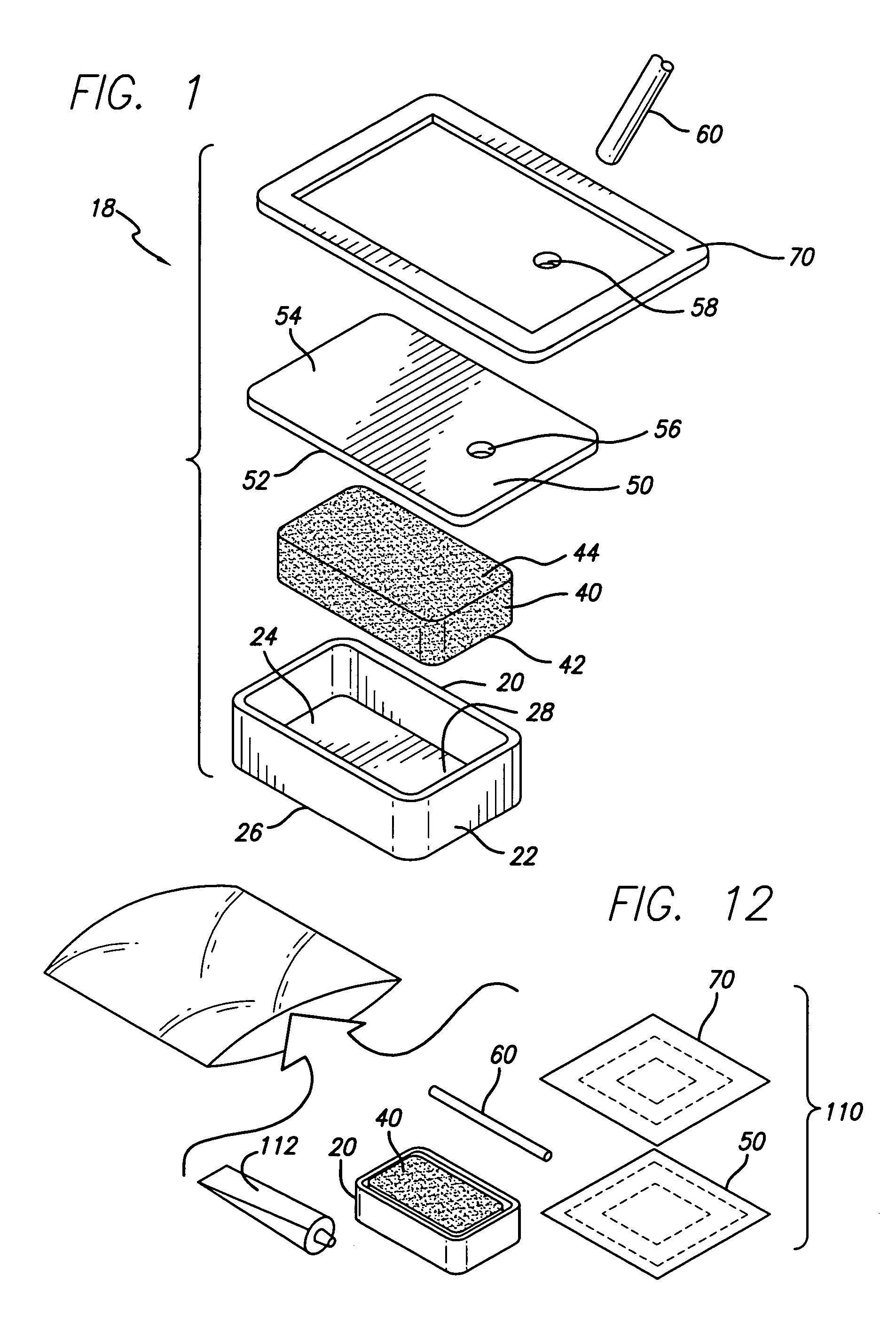
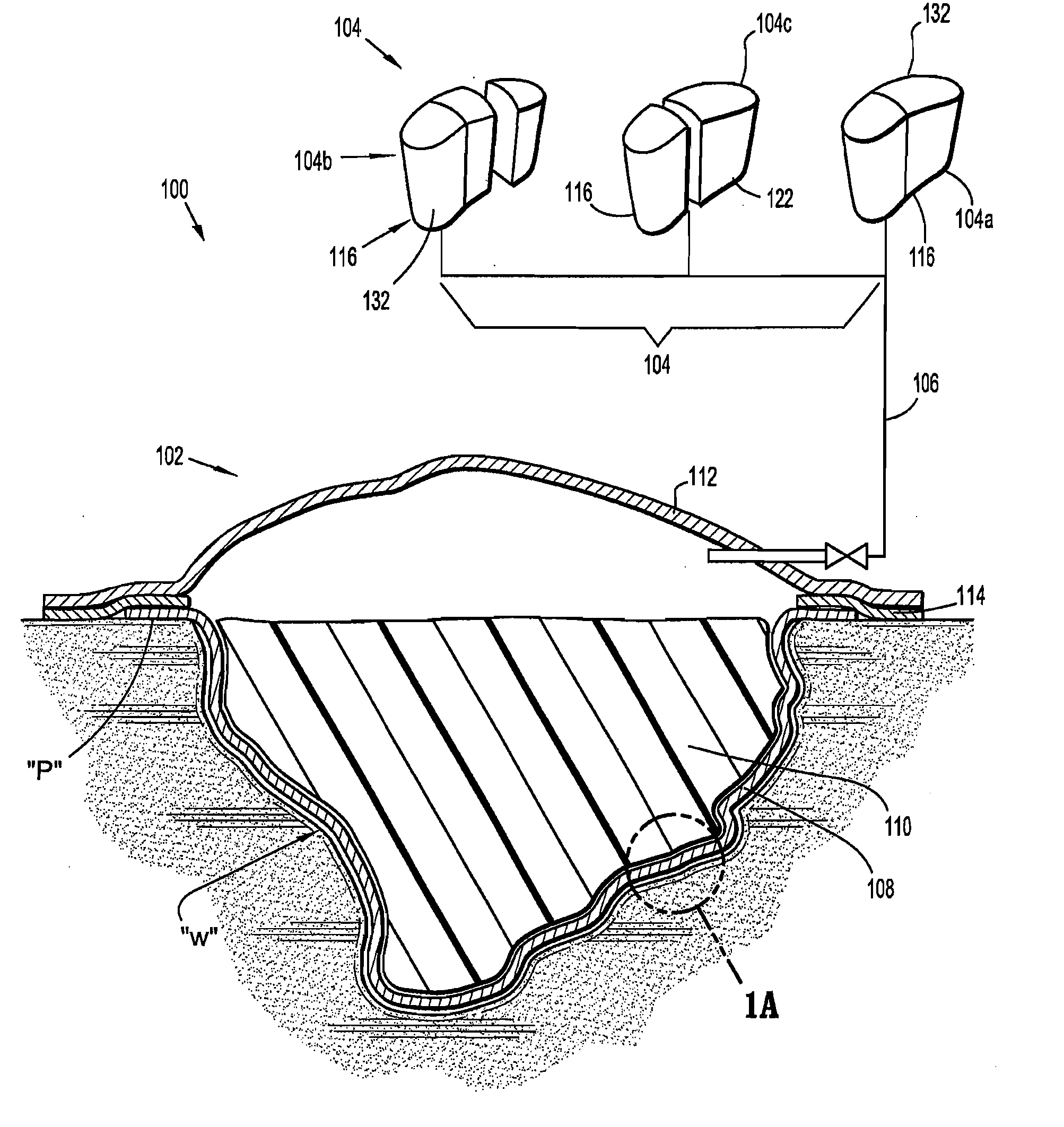
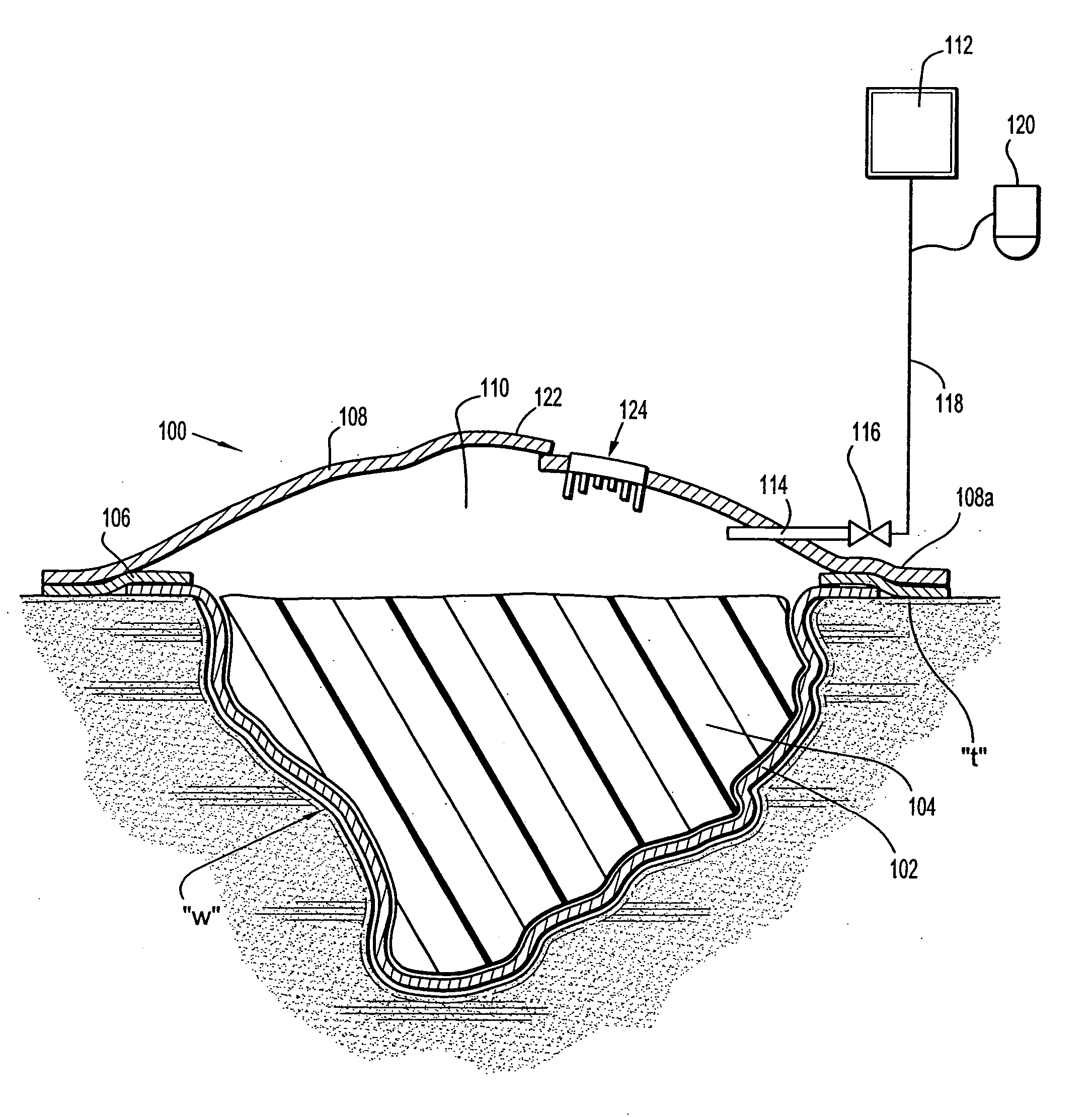
Surgical wound dressing
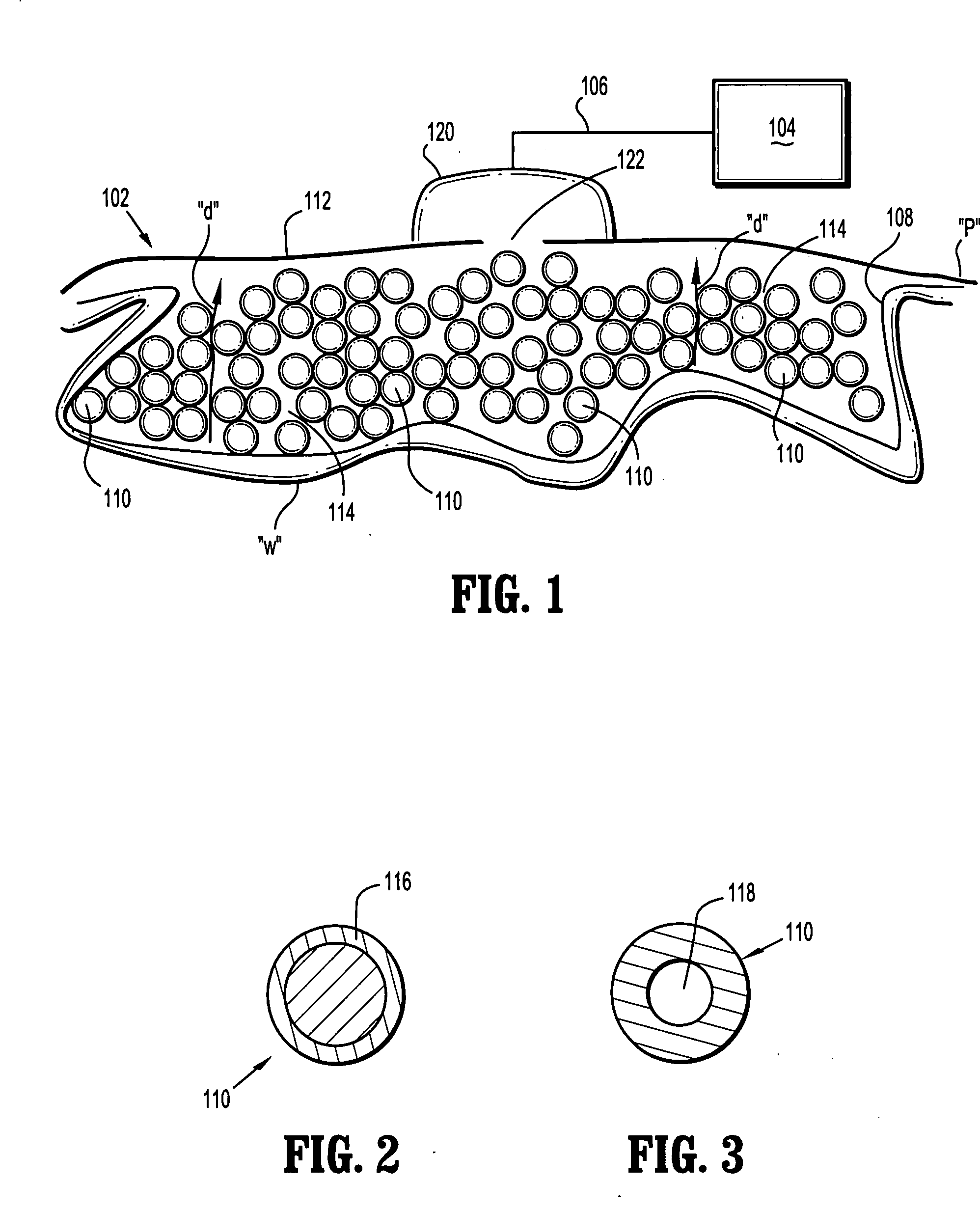
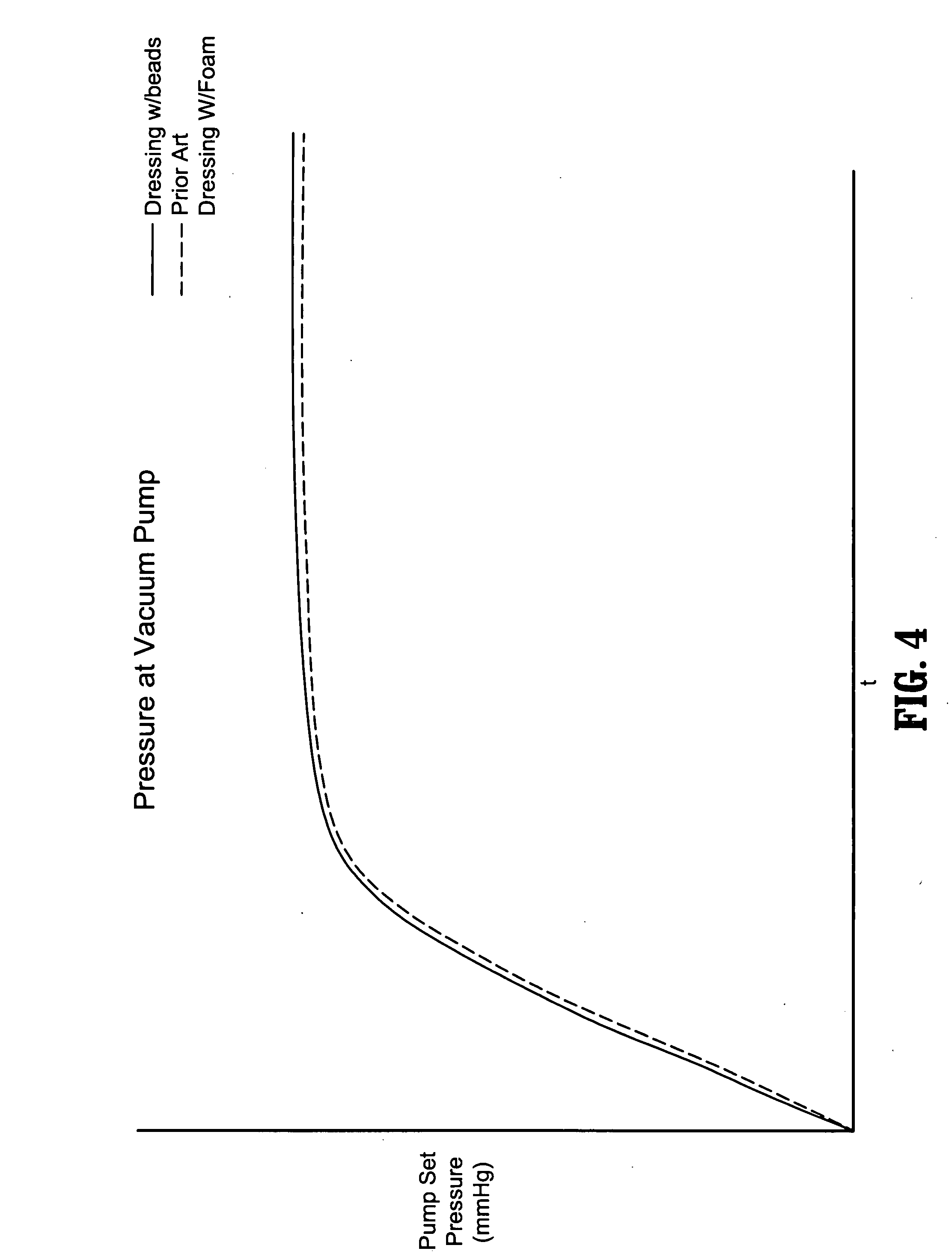
A wound dressing system includes a fluid permeable support member for positioning within a wound and adapted to generally conform to a topography of a wound, a plurality of beads supported by the support member, an outer member for positioning over the wound to substantially enclose the beads and a conduit for supplying reduced pressure to the wound. The support member is adapted to permit exudates from the wound to pass therethrough. The beads are characterized by having sufficient rigidity to substantially maintain respective shapes thereof to thereby facilitate passage of the exudates through spaces or passages defined between adjacent beads. The beads may comprise glass, an acrylic or a polymeric material. The support member may comprise a polymeric or fabric material. The support member may be an enclosure member or pouch which houses the beads. Multiple pouches are also envisioned.
Owner:SMITH & NEPHEW INC
Apparatus and method for applying topical negative pressure
A method and apparatus for alerting a user of topical negative pressure therapy apparatus to a full waste canister condition are described, the apparatus comprising a device having vacuum pump means and a waste canister connected to the device and the waste canister operably connected to a wound dressing by aspiration conduit means for aspirating fluid from the wound, the aspiration conduit means, the waste canister and the device providing a fluid flow path therethrough and the vacuum pump means providing fluid flow through the apparatus, the apparatus further comprising fluid flow restriction means in the fluid flow path of said vacuum pump and fluid pressure sensing means upstream and downstream of said fluid flow restriction means.
Owner:SMITH & NEPHEW INC
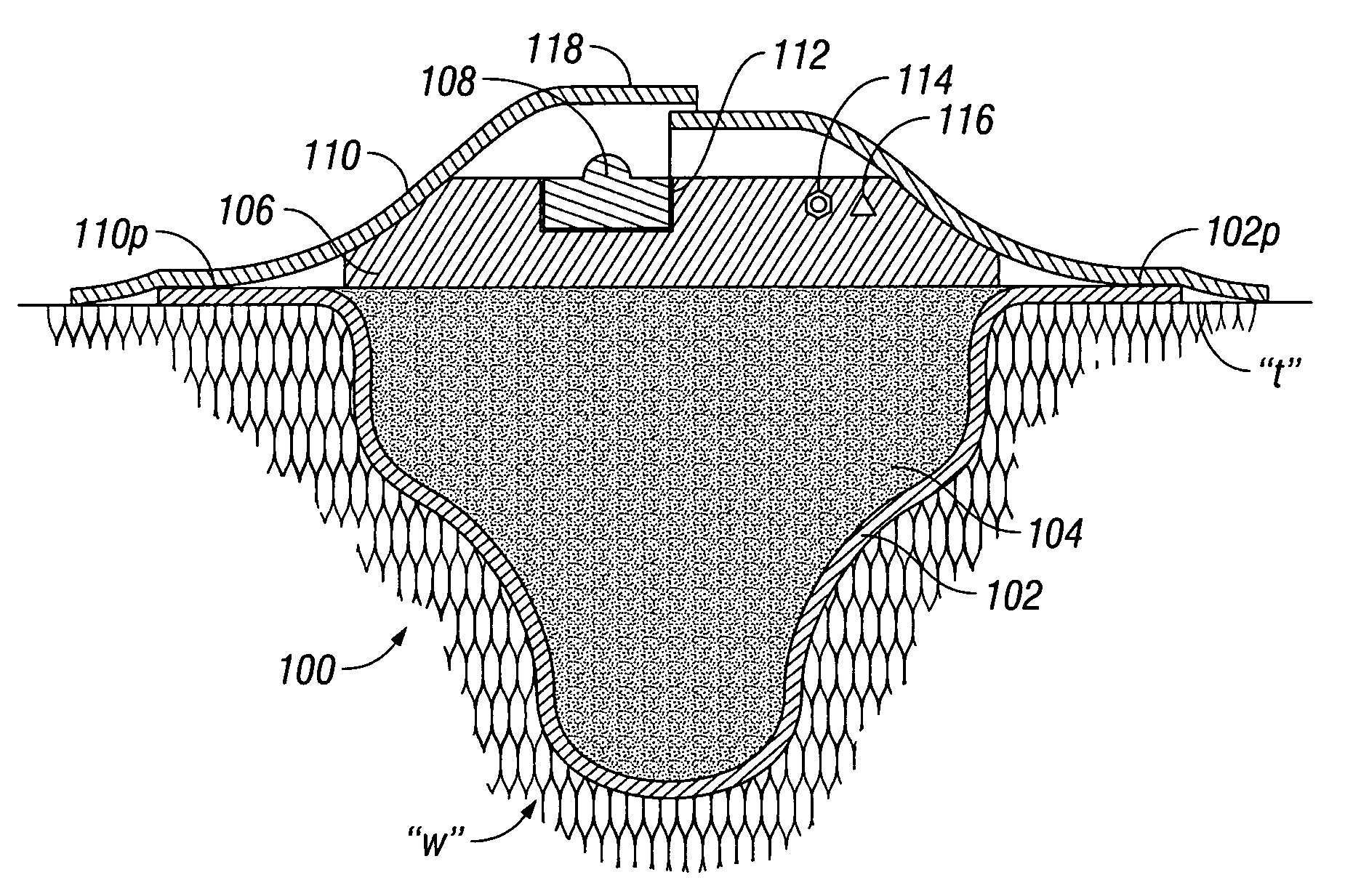
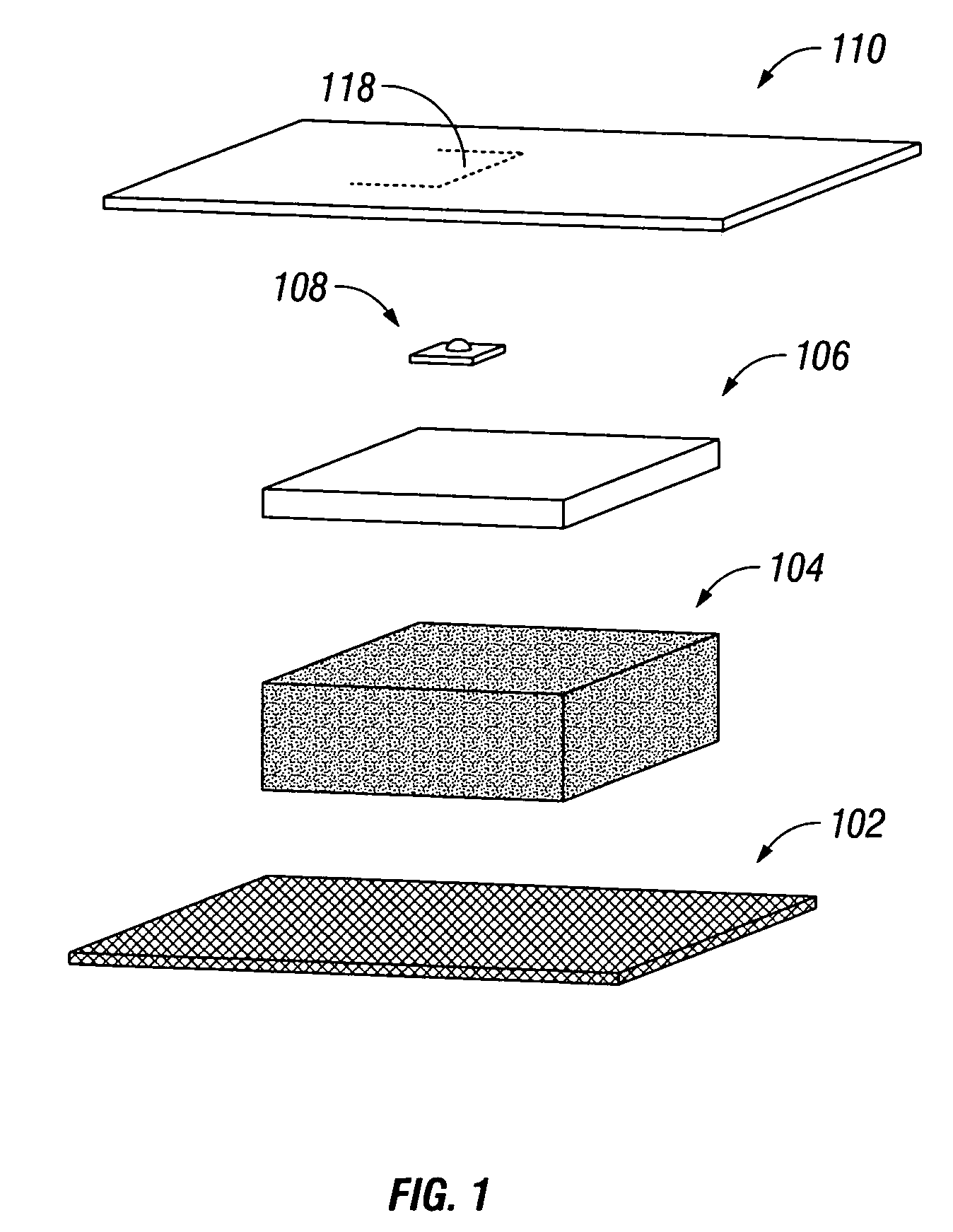
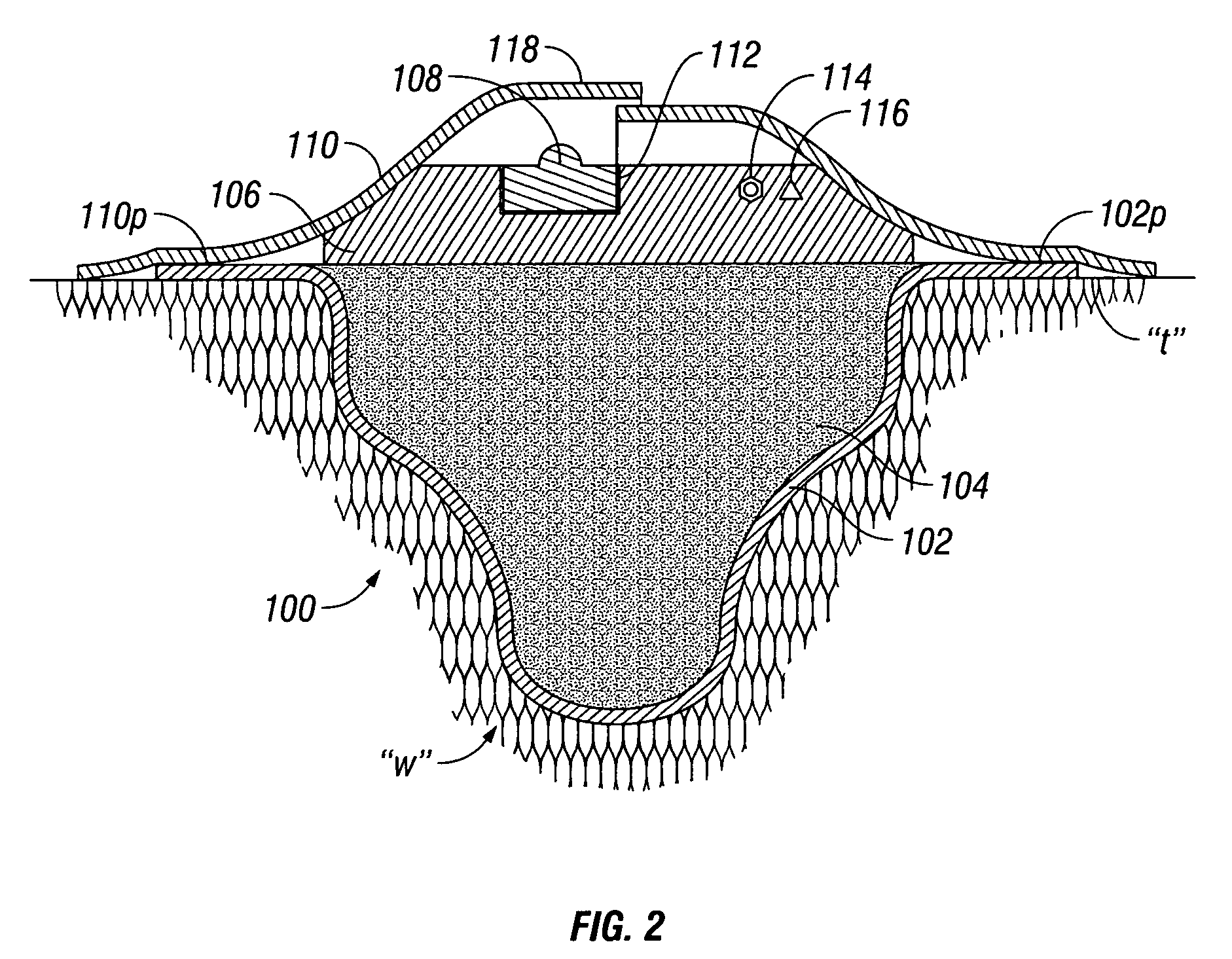
Tube attachment device for wound treatment
The invention provides a vacuum tube attachment device for vacuum assisted wound dressings. The device is in the form of a patch that can be attached to the primary wound cover. The patch forms a substantially air-tight seal to the primary wound cover, and a vacuum tube is fixed to the patch such that the patch can be oriented on the wound cover to locate the tube near an opening in the cover to allow vacuum pressure to be communicated to the wound. The patch has an adhesive area around its perimeter for attaching the patch in a substantially air-tight seal to the wound cover at any convenient location on the cover. Several embodiments of the patch are described.
Owner:PAUL HARTMANN AG
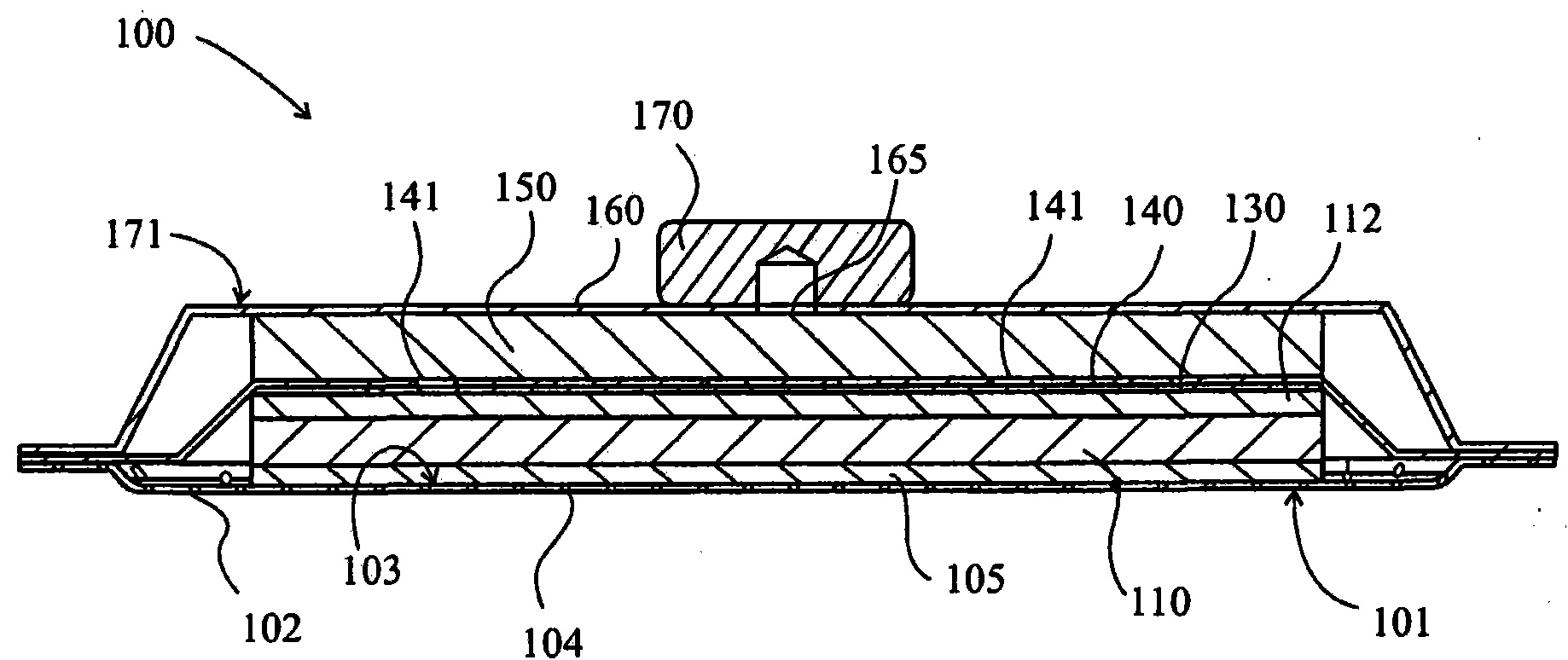
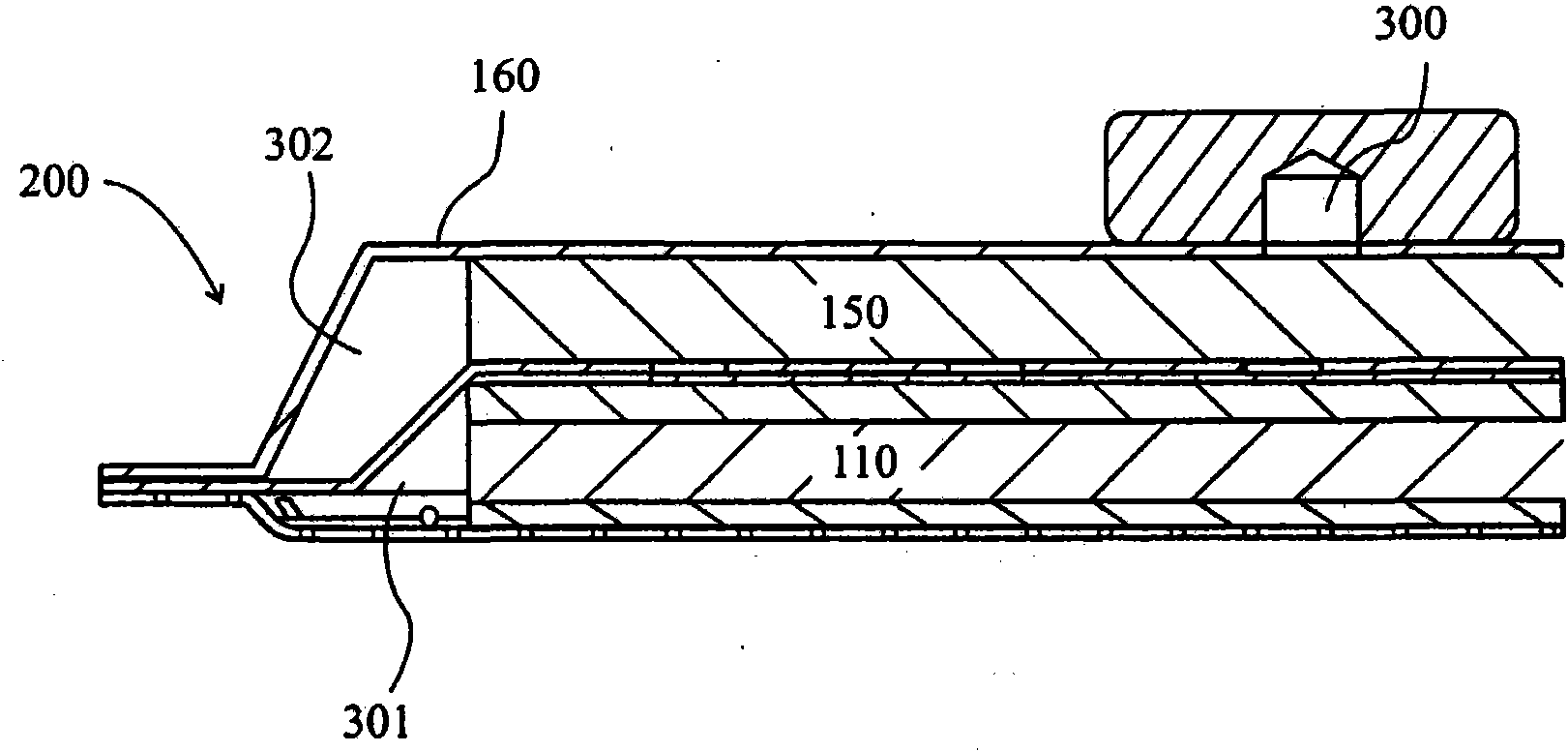
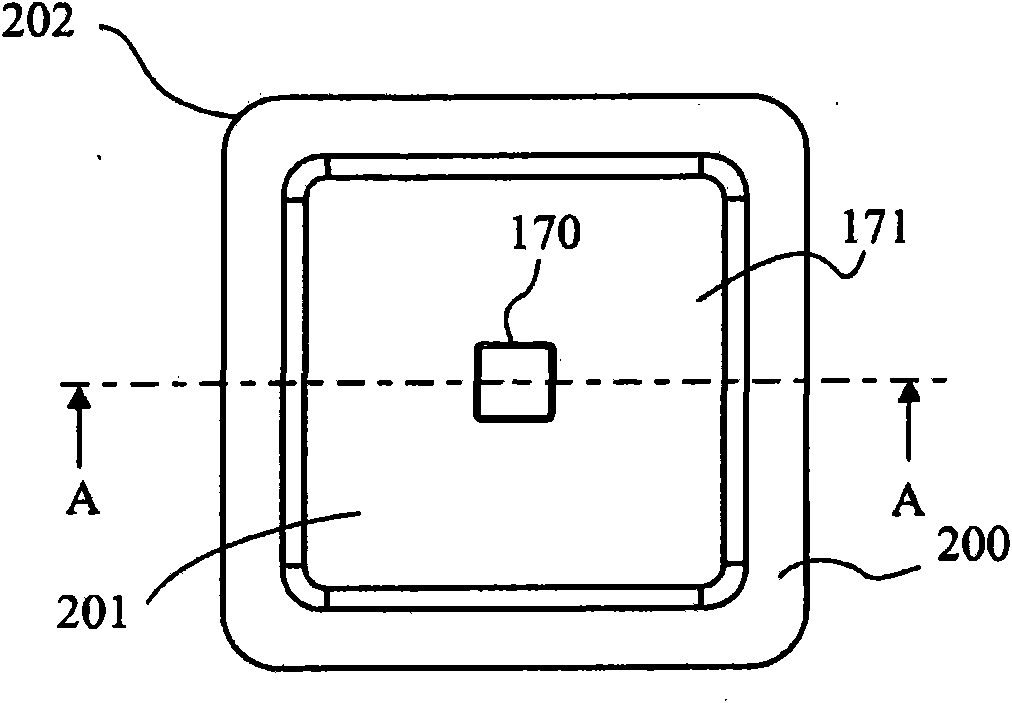
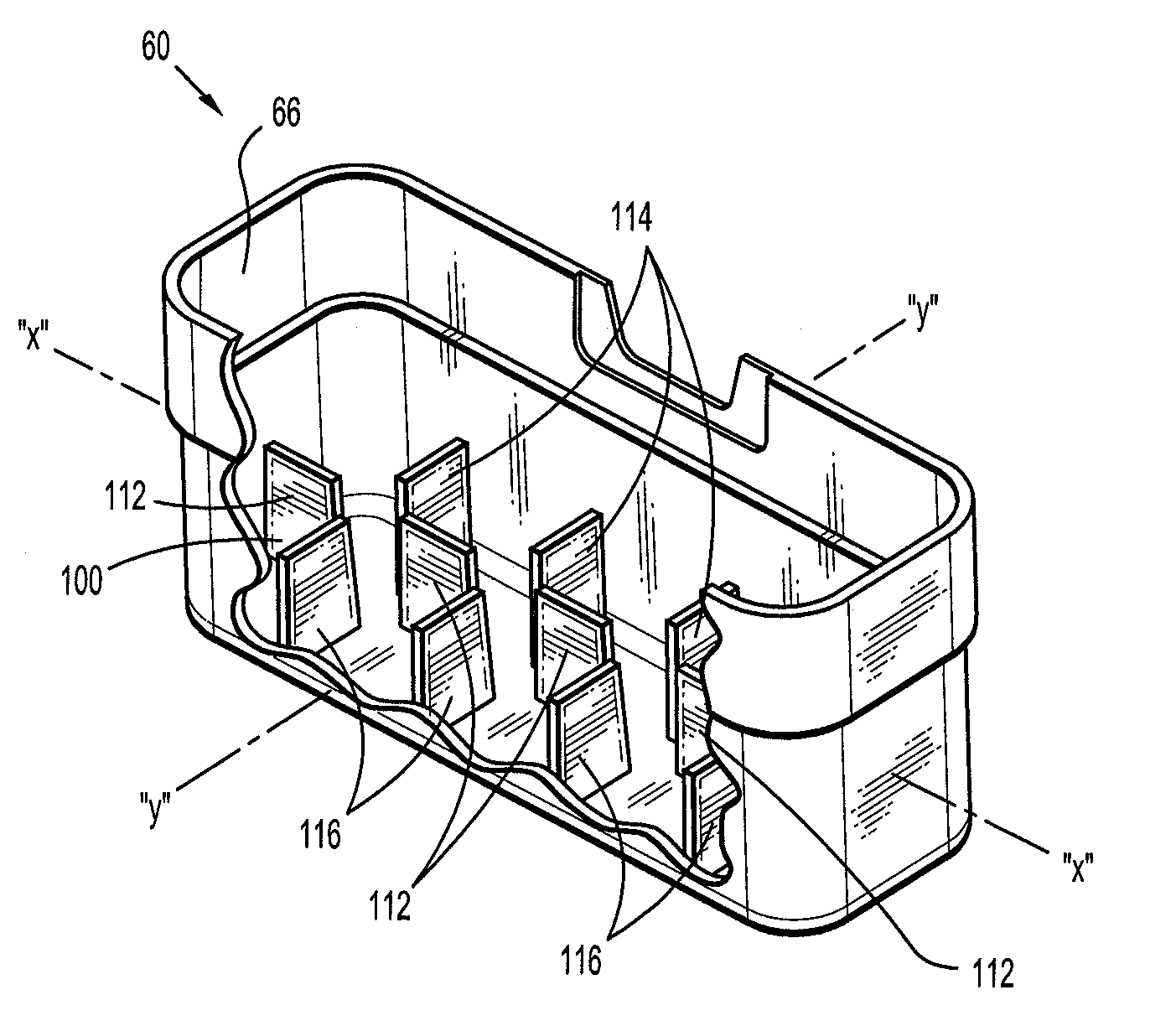
Wound dressing and method of use
A system, method, and apparatus are disclosed for dressing a wound. The apparatus comprises a liquid and gas permeable transmission layer, an absorbent layer for absorbing wound exudate, the absorbent layer overlying the transmission layer, a gas impermeable cover layer overlying the absorbent layer and comprising a first orifice, wherein the cover layer is moisture vapor permeable.
Owner:SMITH & NEPHEW PLC
Wound dressing with vacuum reservoir
InactiveUS20070066946A1Easy to disassemblePromote wound healingWound drainsMedical devicesWound dressingPosition sensor
A wound dressing apparatus includes a wound dressing member dimensioned for positioning relative to a wound bed. The wound dressing member including an internal vacuum reservoir and has a port in communication with the vacuum reservoir for applying subatmospheric pressure to the vacuum reservoir to facilitate removal of fluid from the wound bed. The wound dressing member includes a visual pressure indicator associated therewith for indicating a level of pressure within the vacuum reservoir. The visual pressure indicator includes color indicia having a plurality of colors corresponding to a condition of the pressure within the vacuum reservoir. The wound dressing member includes a lower absorbent member positionable adjacent the wound bed and an upper member which at least partially defines the vacuum reservoir. At least one of the top member and the lower absorbent member has the visual pressure indicator mounted thereto. The preferred visual pressure indicator includes an electronic position sensor. The visual pressure indicator further includes circuit means and visible alarm means. The circuit means is adapted to actuate the visible alarm means when the position sensor detects a relative positioning of the top member of the wound dressing member to provide a visual indication of the condition of the subatmospheric pressure within the vacuum reservoir.
Owner:SMITH & NEPHEW INC
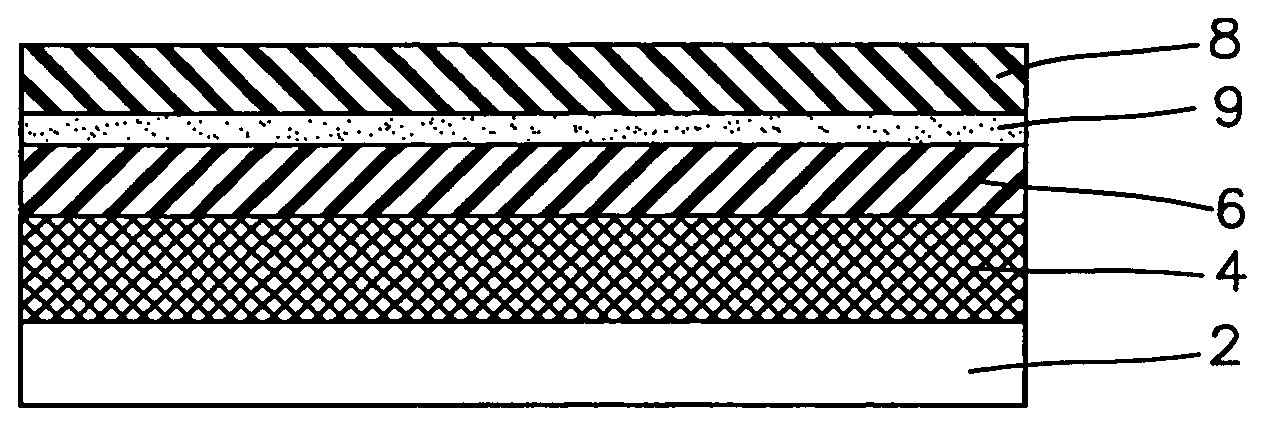
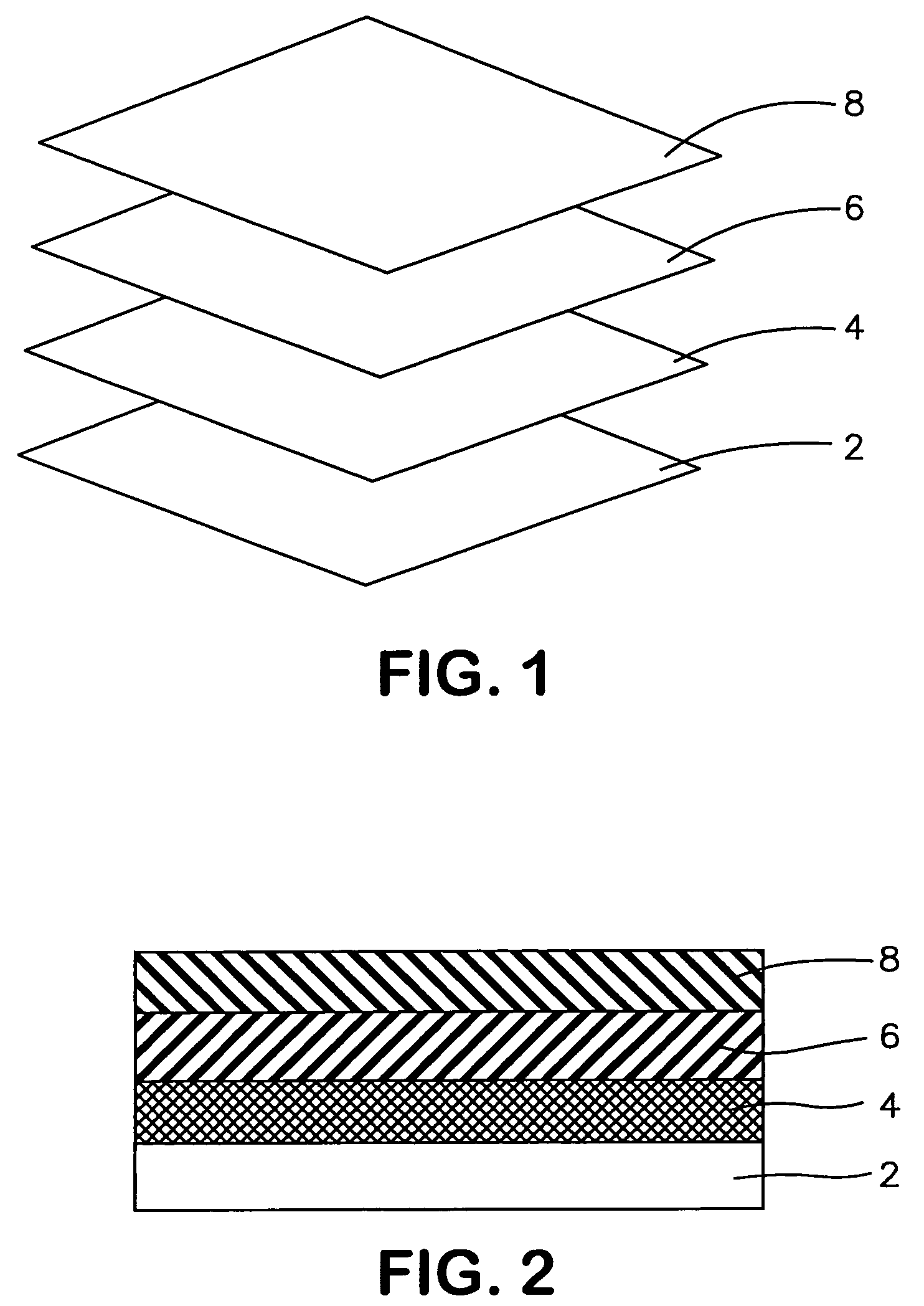
Multi layered wound dressing
ActiveUS7759537B2Allowing versatilityReduce usagePlastersAdhesive dressingsWound dressingContact layer
A multi layered wound dressing for use on wounds producing high levels of exudate, the dressing comprising a transmission layer having a high MVTR; an absorbent core capable of absorbing and retaining exudates; and a wound contacting layer which transmits exudate to the absorbent core, the absorbent core and wound contacting layer limiting the lateral spread of exudate in the dressing to the region of the wound.
Owner:CONVATEC TECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com