Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
48 results about "Surgical Graft" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
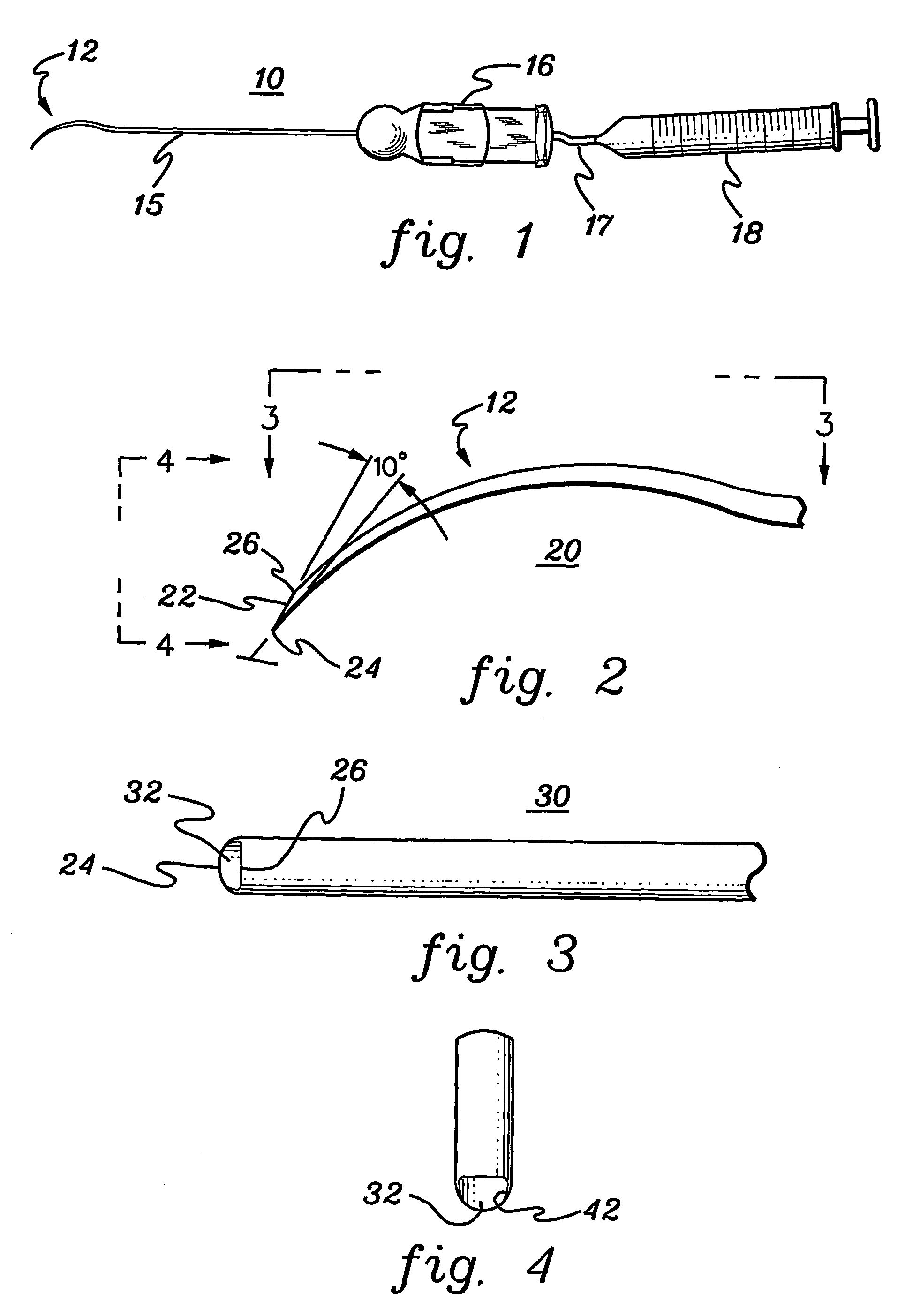
Collagen biofabric and methods of preparation and use therefor
InactiveUS20040048796A1Improved biophysical propertyImprove featuresSenses disorderPeptide/protein ingredientsSurgical GraftWound dressing
The present invention relates to collagenous membranes produced from amnion, herein referred to as a collagen biofabric. The collagen biofabric of the invention has the structural integrity of the native non-treated amniotic membrane, i.e., the native tertiary and quaternary structure. The present invention provides a method for preparing a collagen biofabric from a placental membrane, preferably a human placental membrane having a chorionic and amniotic membrane, by decellularizing the amniotic membrane. In a preferred embodiment, the amniotic membrane is completely decellularized. The collagen biofabric of the invention has numerous utilities in the medical and surgical field including for example, blood vessel repair, construction and replacement of a blood vessel, tendon and ligament replacement, wound-dressing, surgical grafts, ophthalmic uses, sutures, and others. The benefits of the biofabric are, in part, due to its physical properties such as biomechanical strength, flexibility, suturability, and low immunogenicity, particularly when derived from human placenta.
Owner:CELLULAR THERAPEUTICS DIV OF CELGENE +1
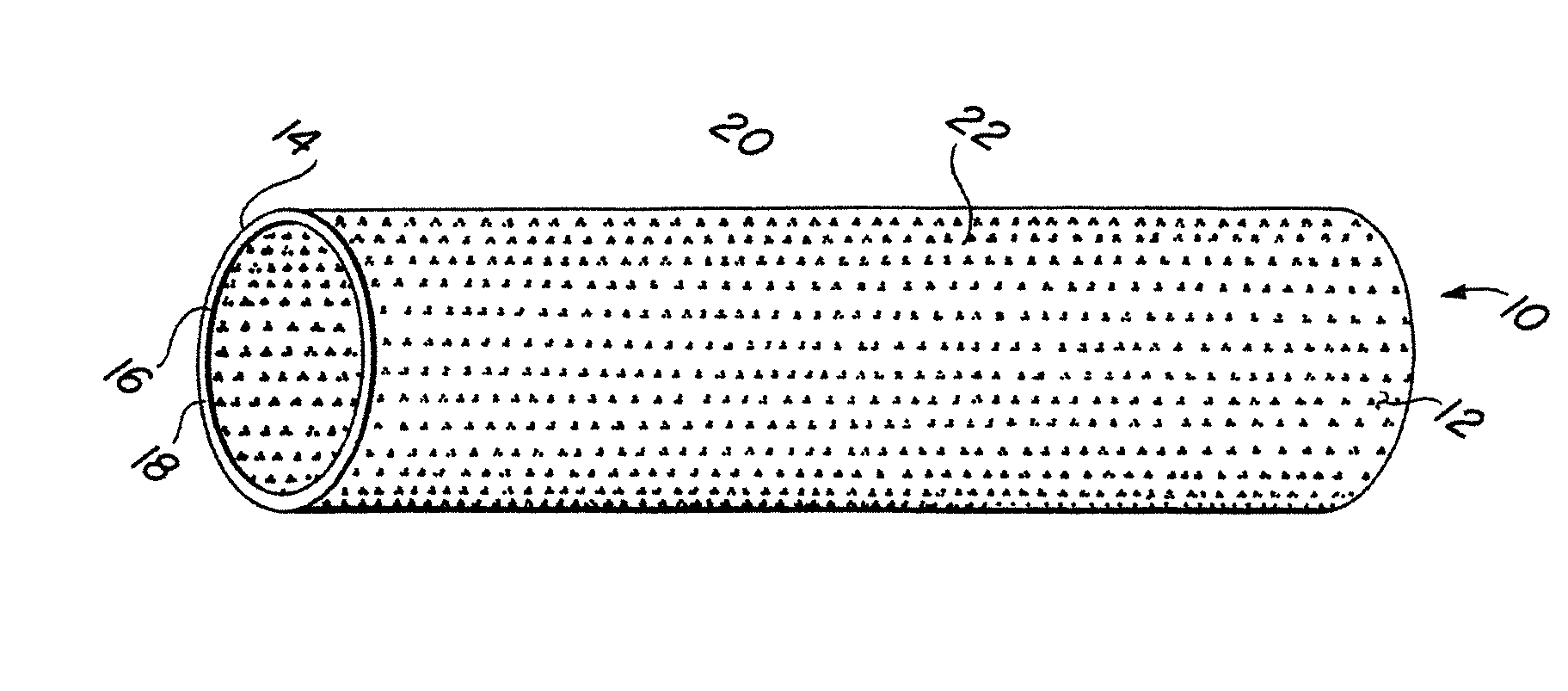
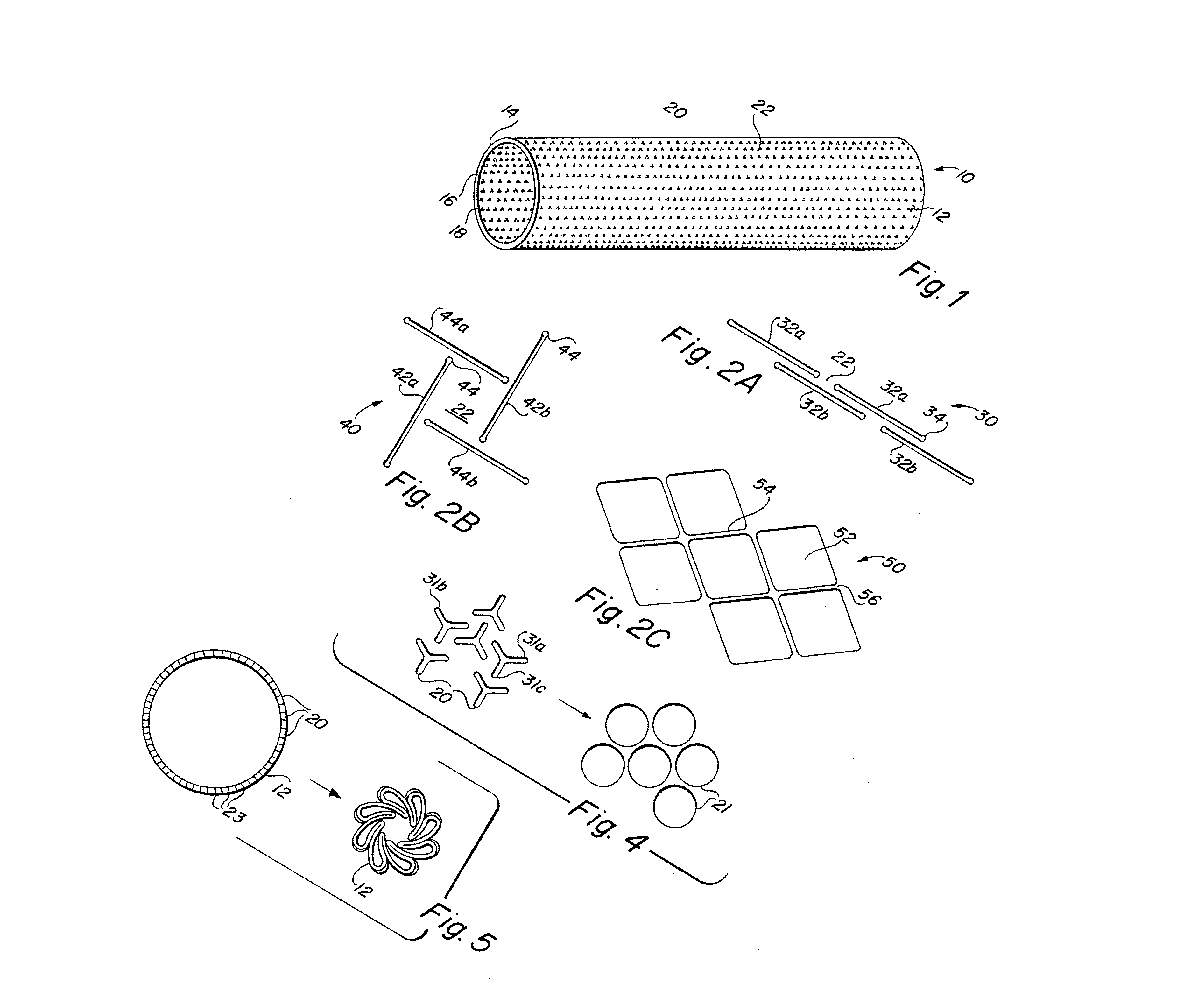
Complaint implantable medical devices and methods of making same
InactiveUS6936066B2Give flexibilityFacilitating transmural endothelializationStentsHeart valvesSurgical GraftMetallic materials
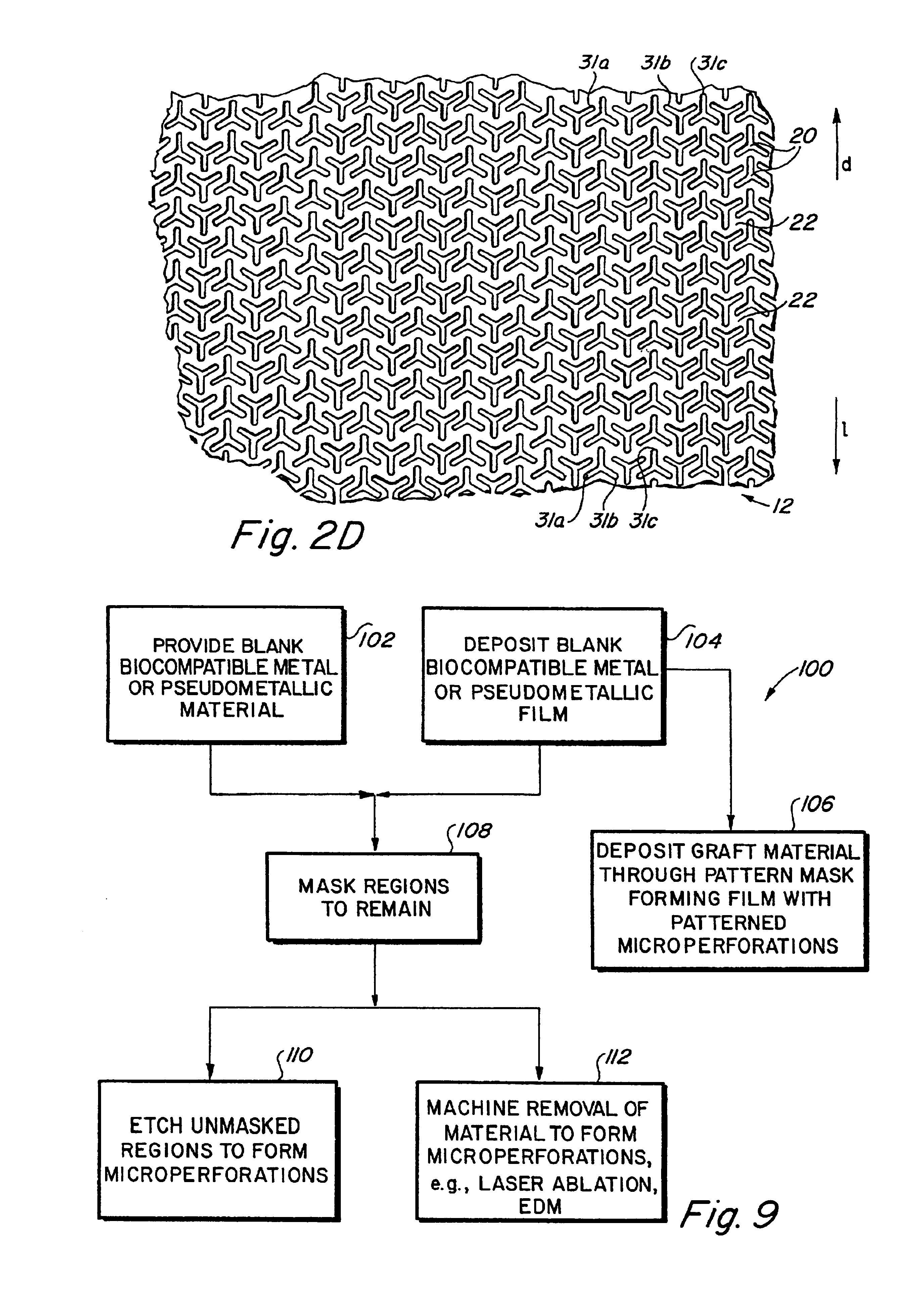
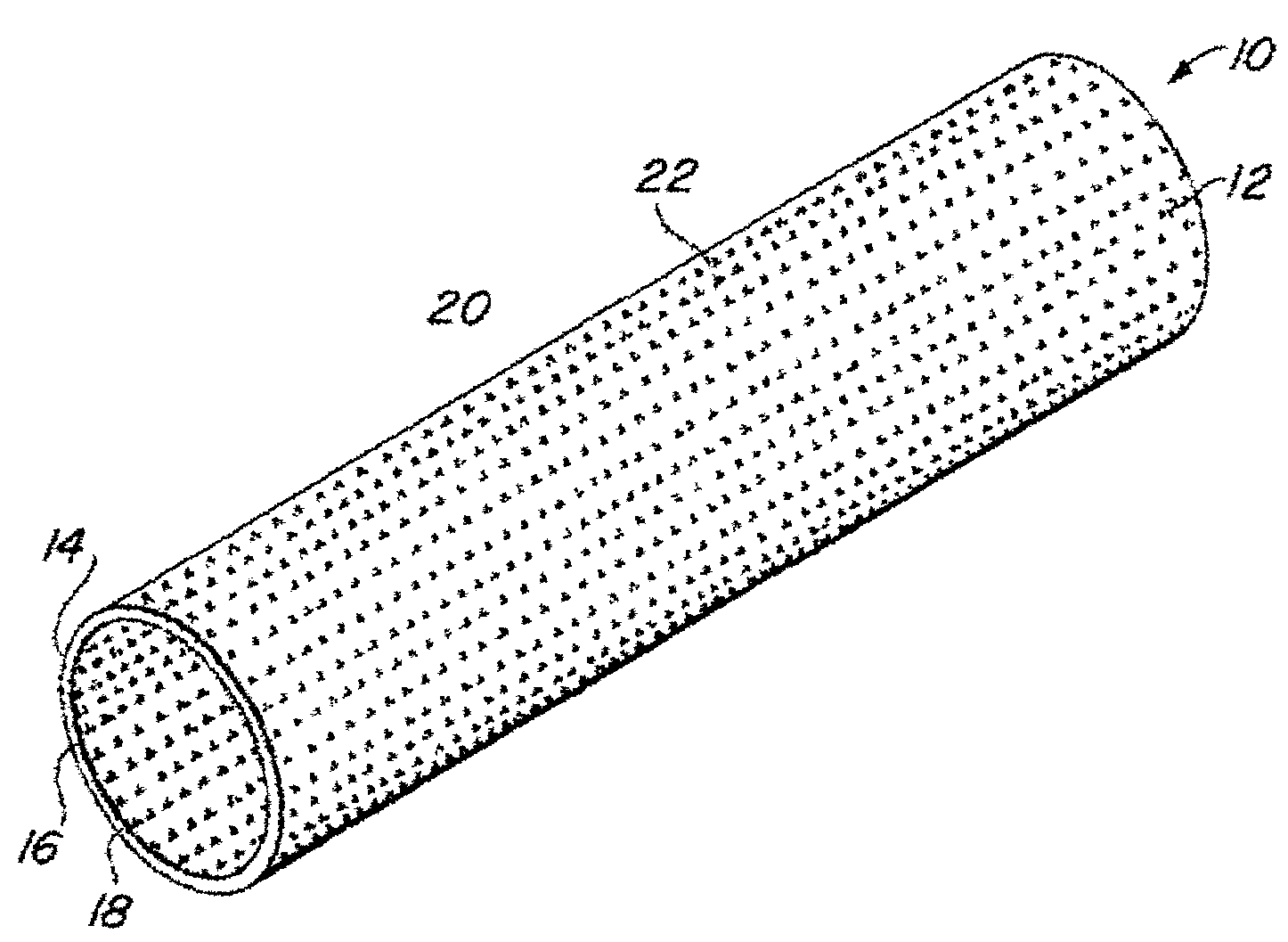
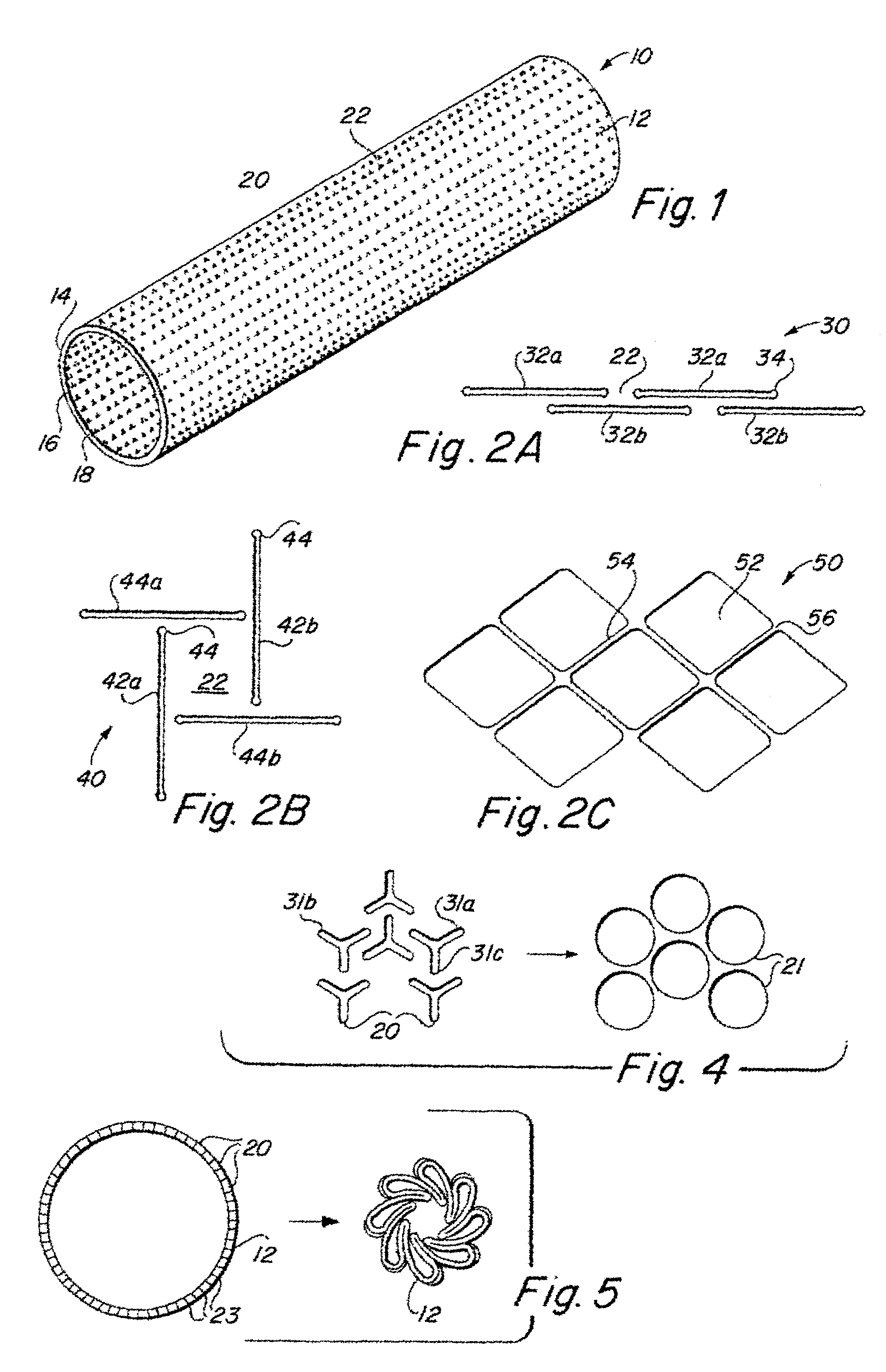
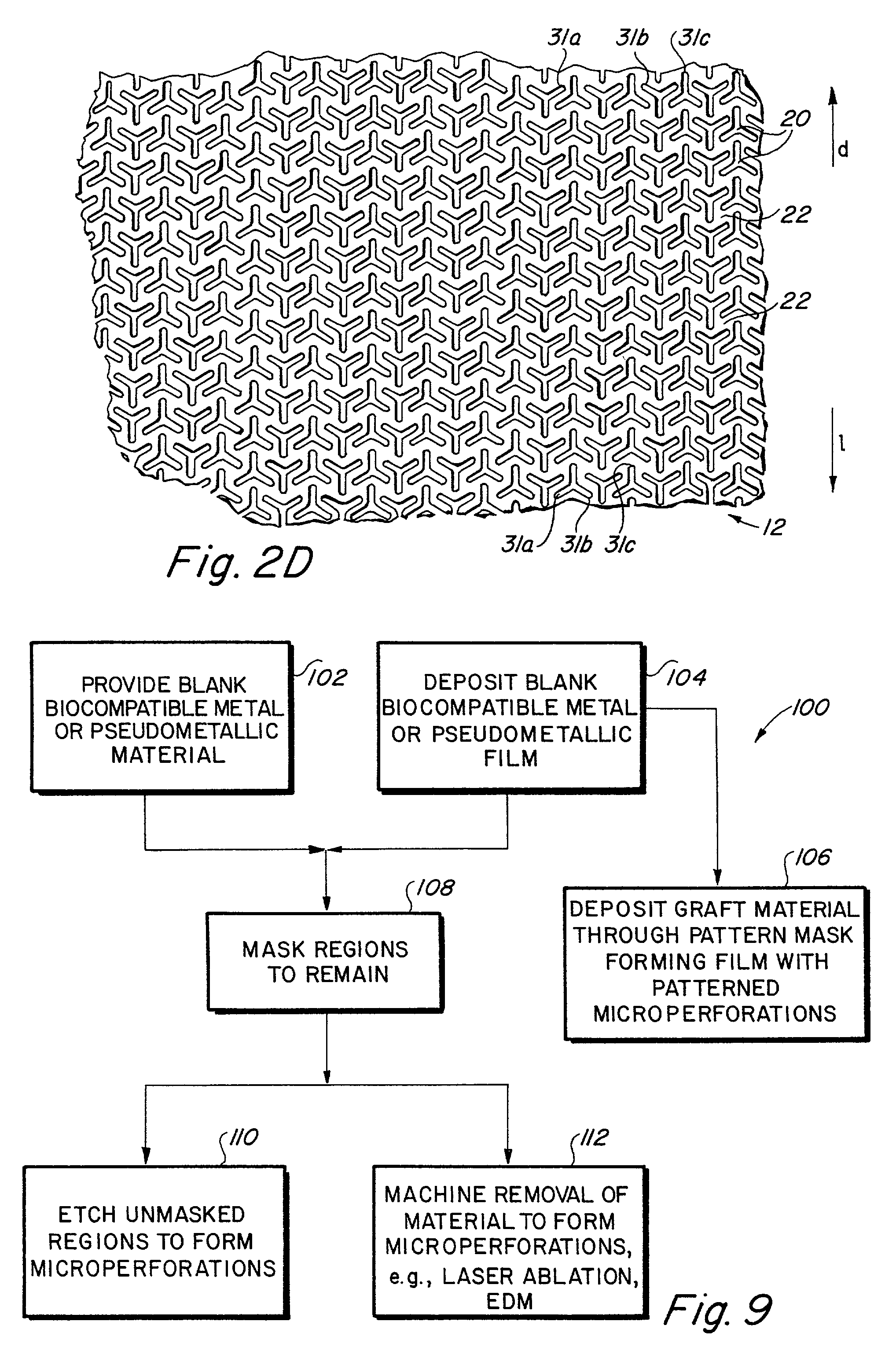
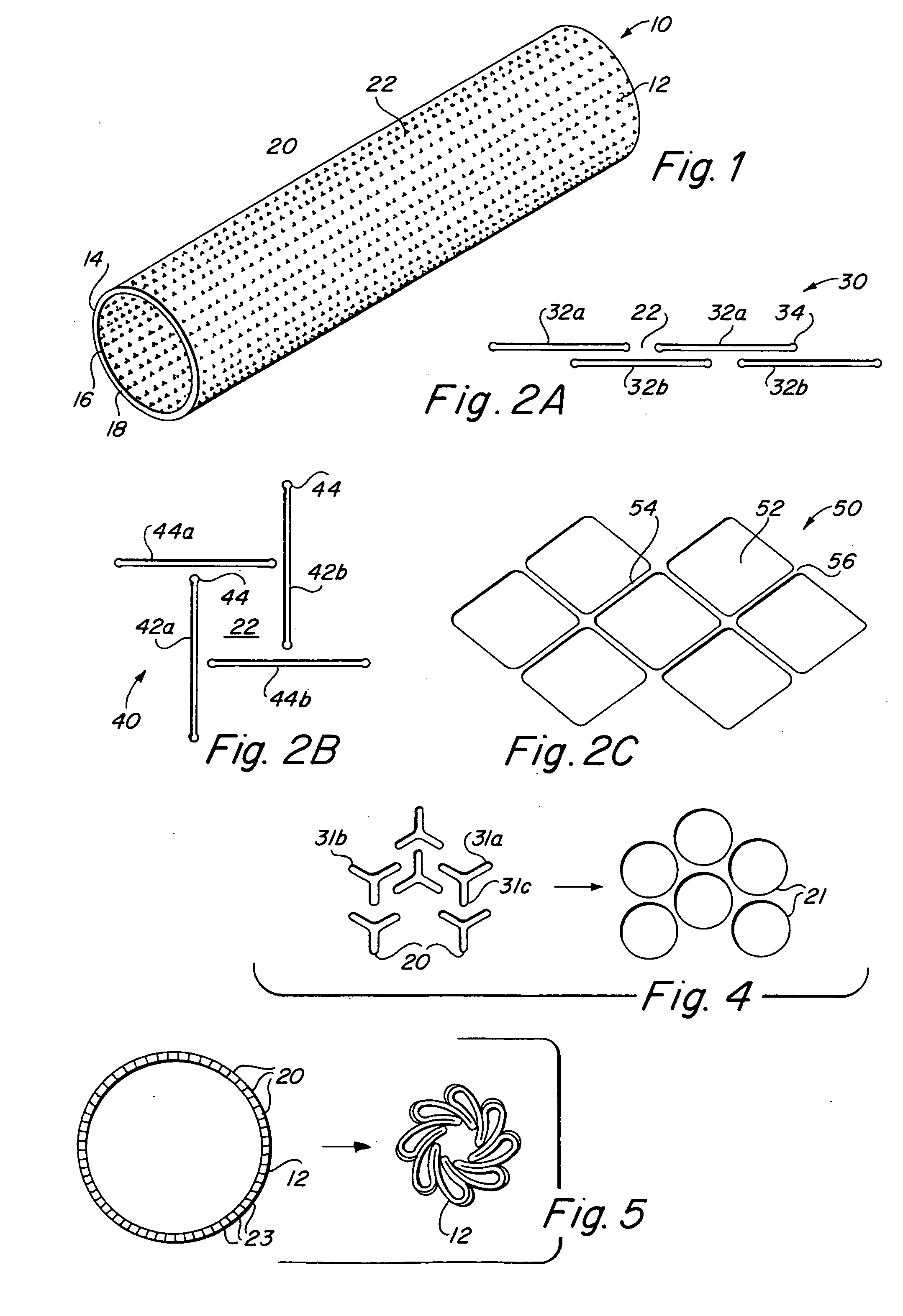
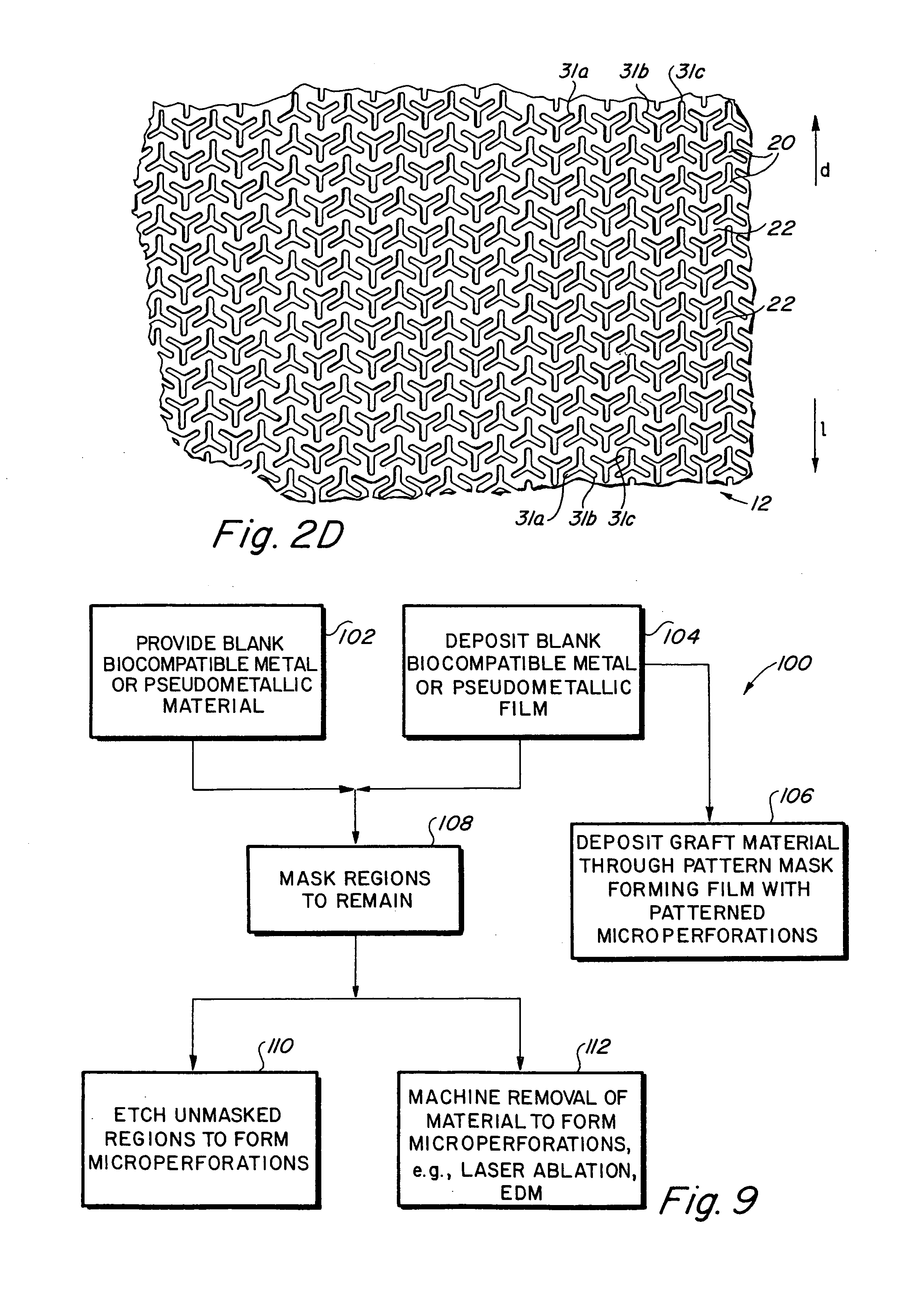
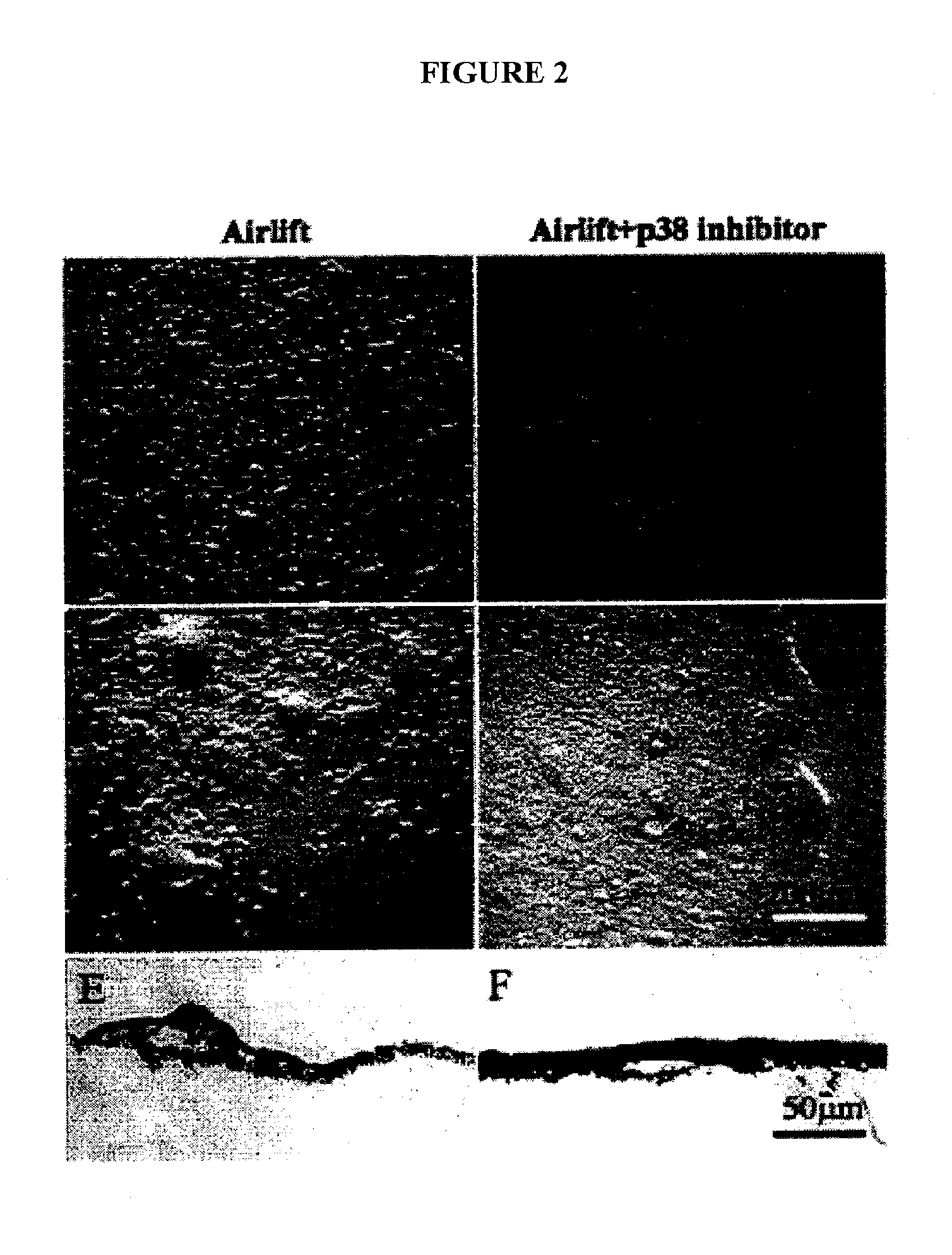
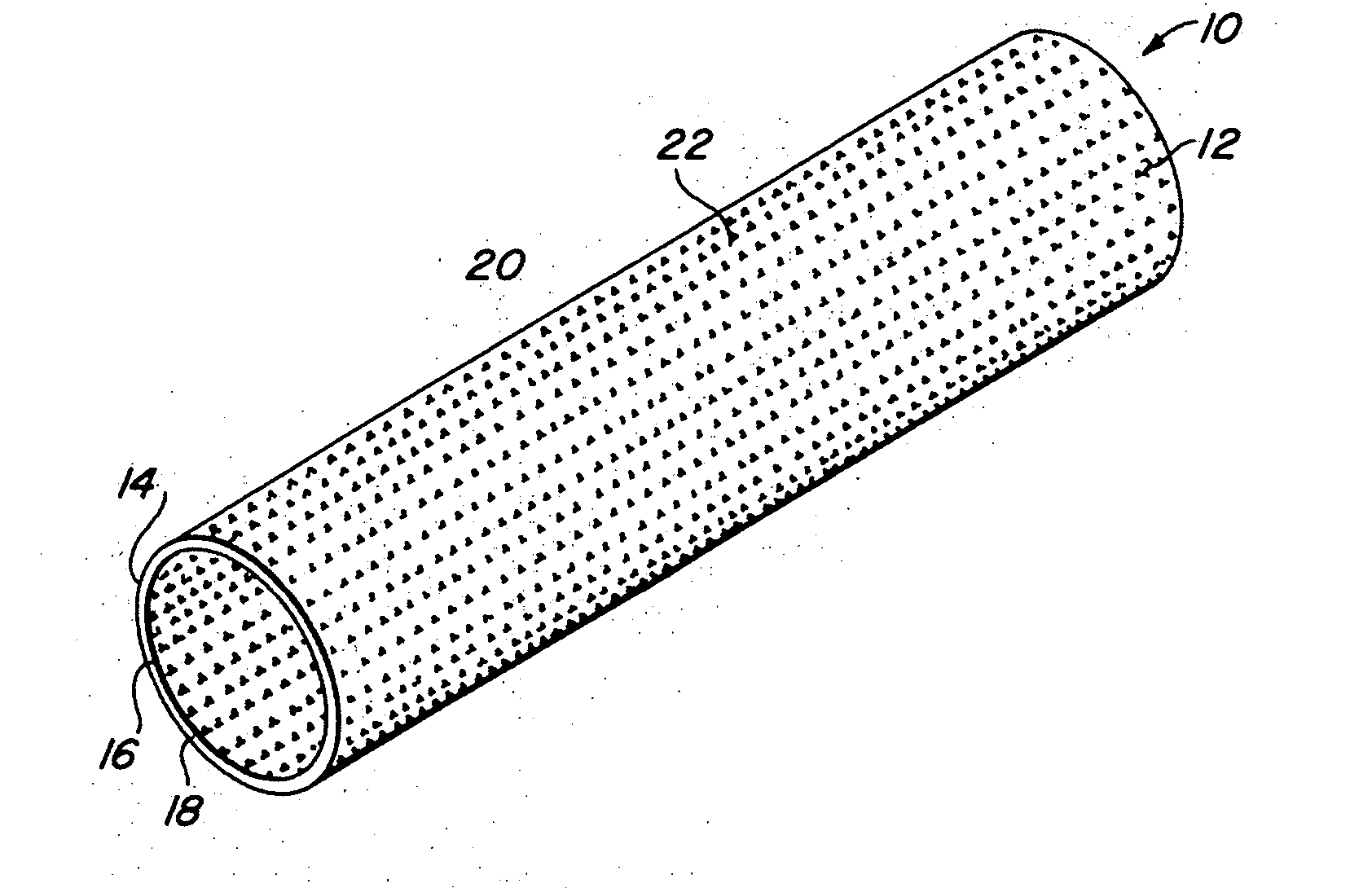
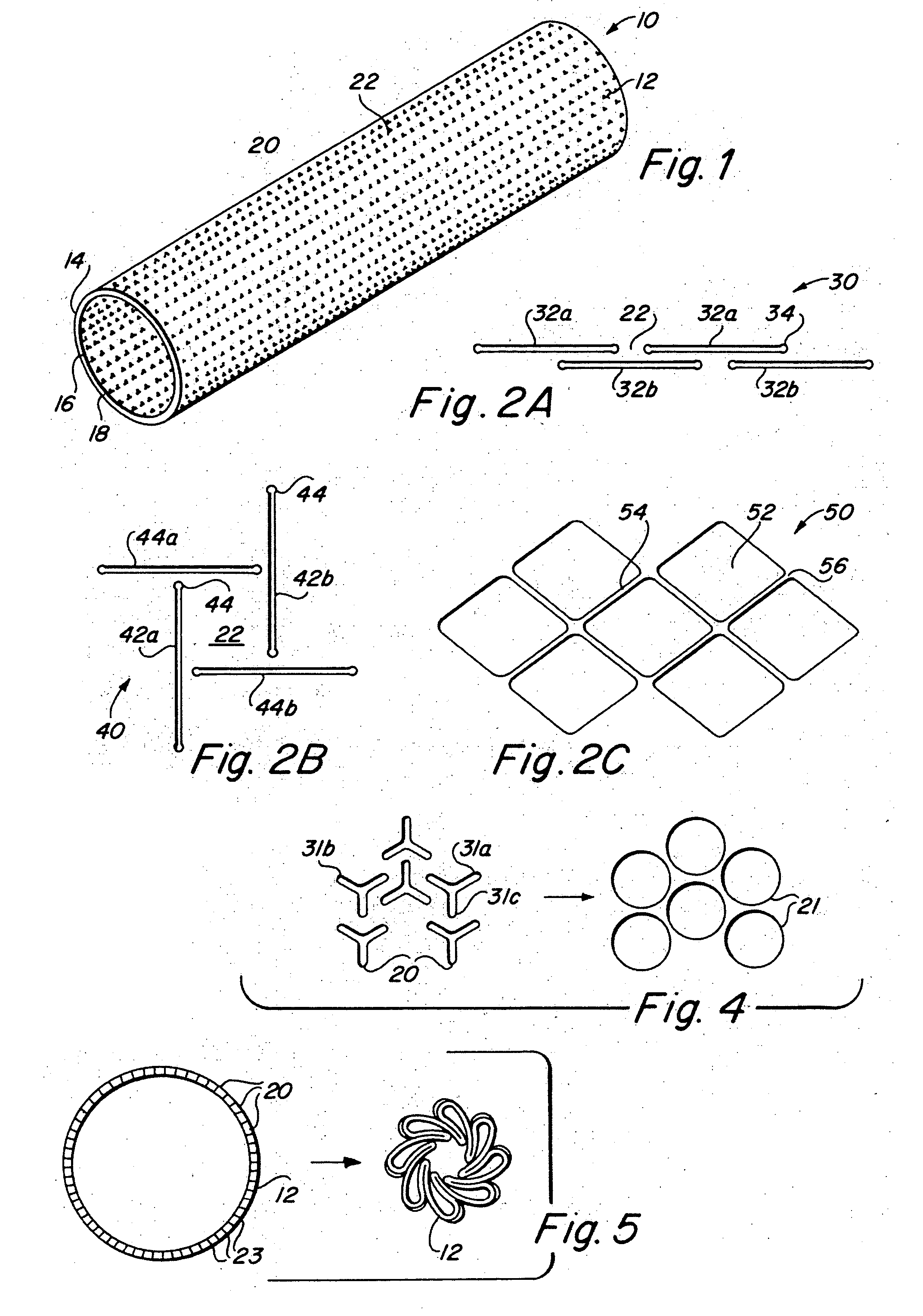
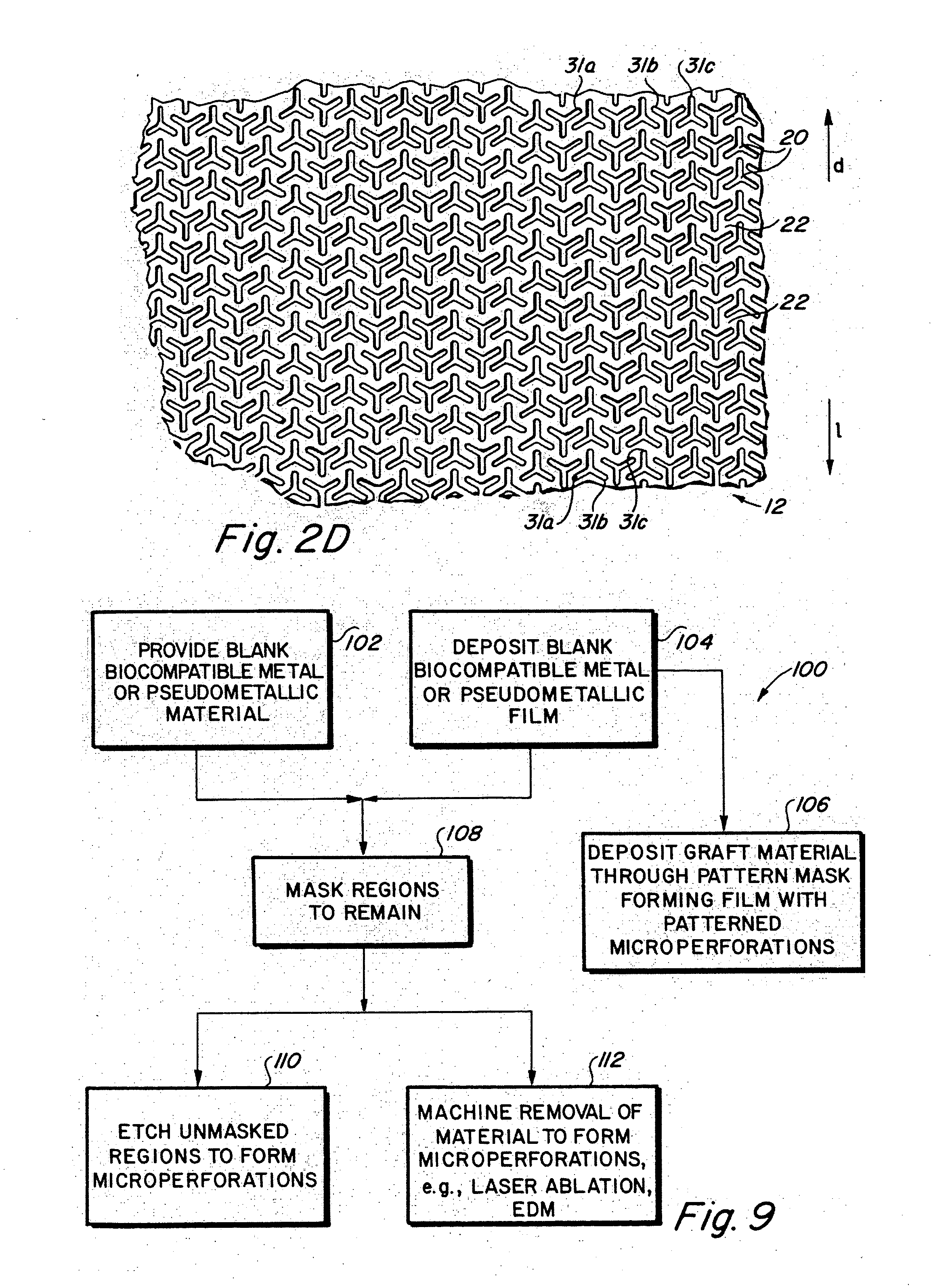
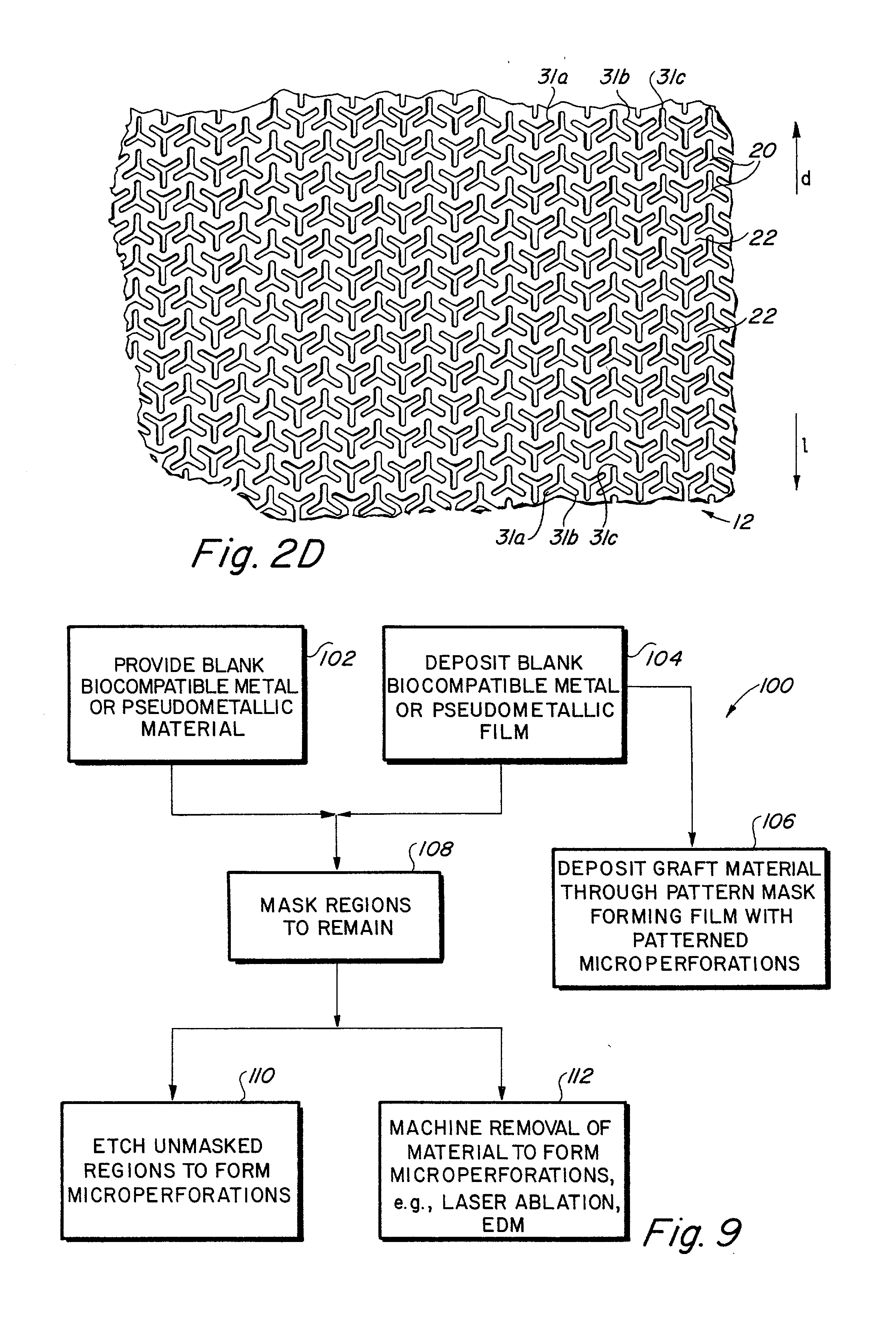
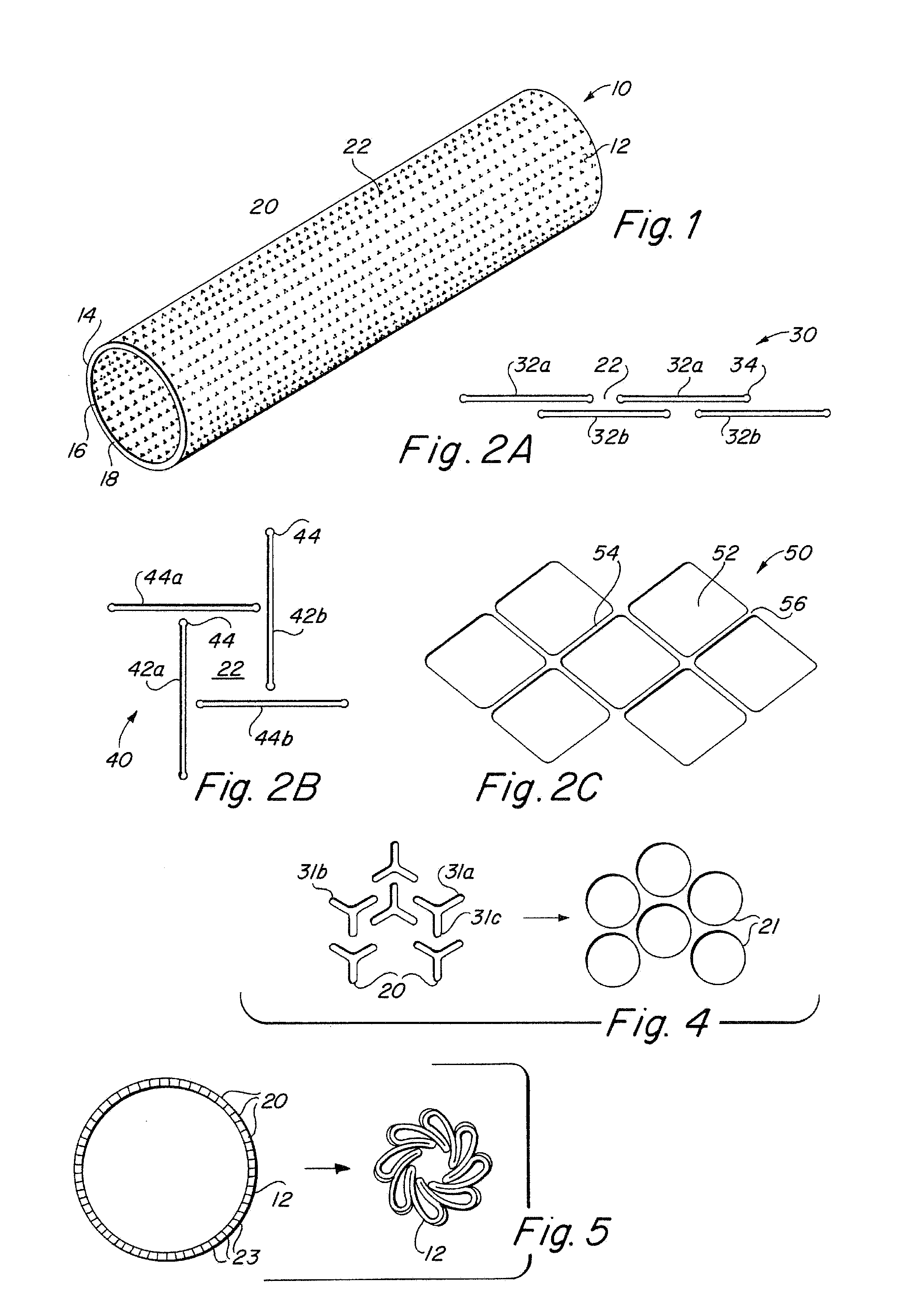
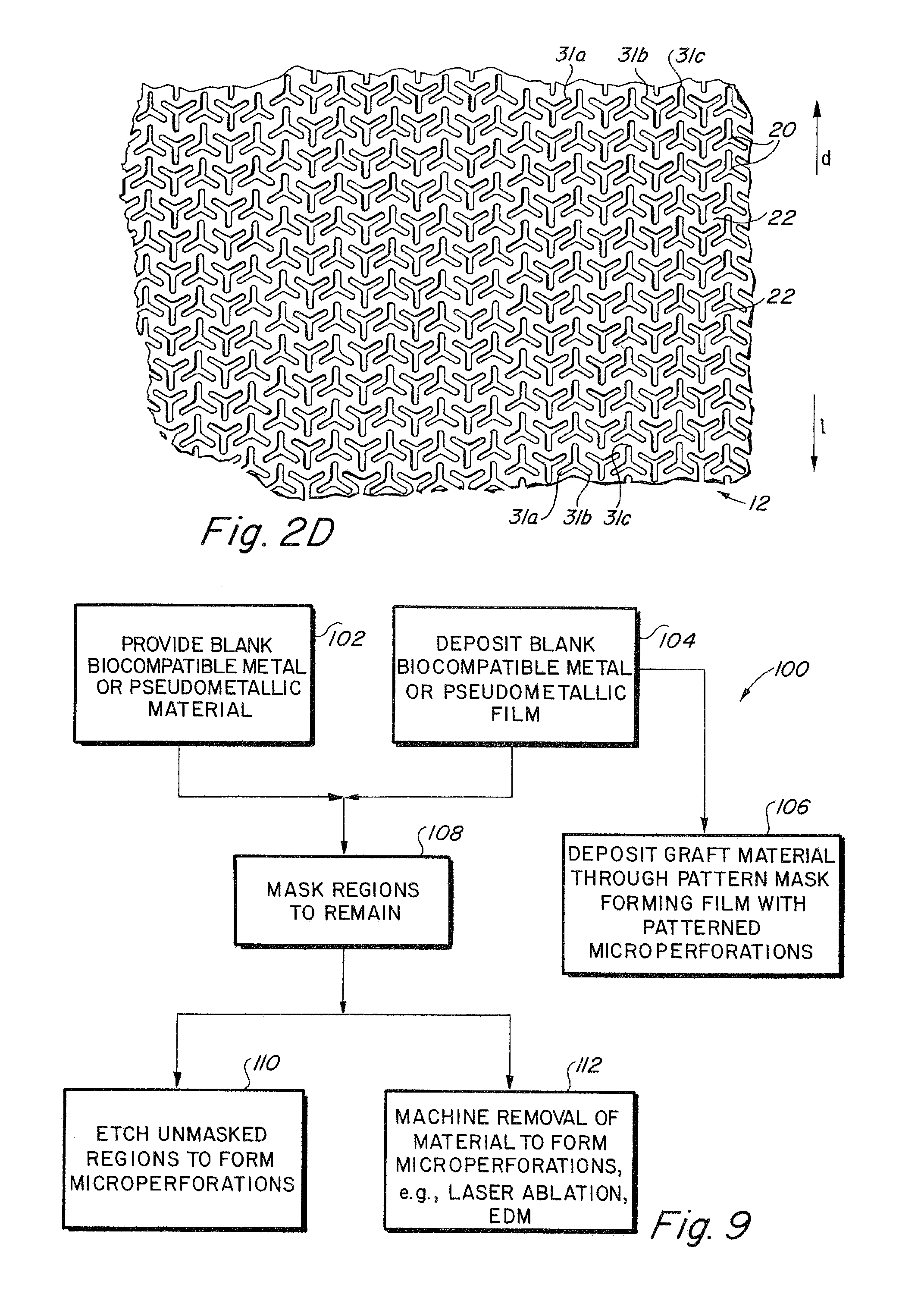
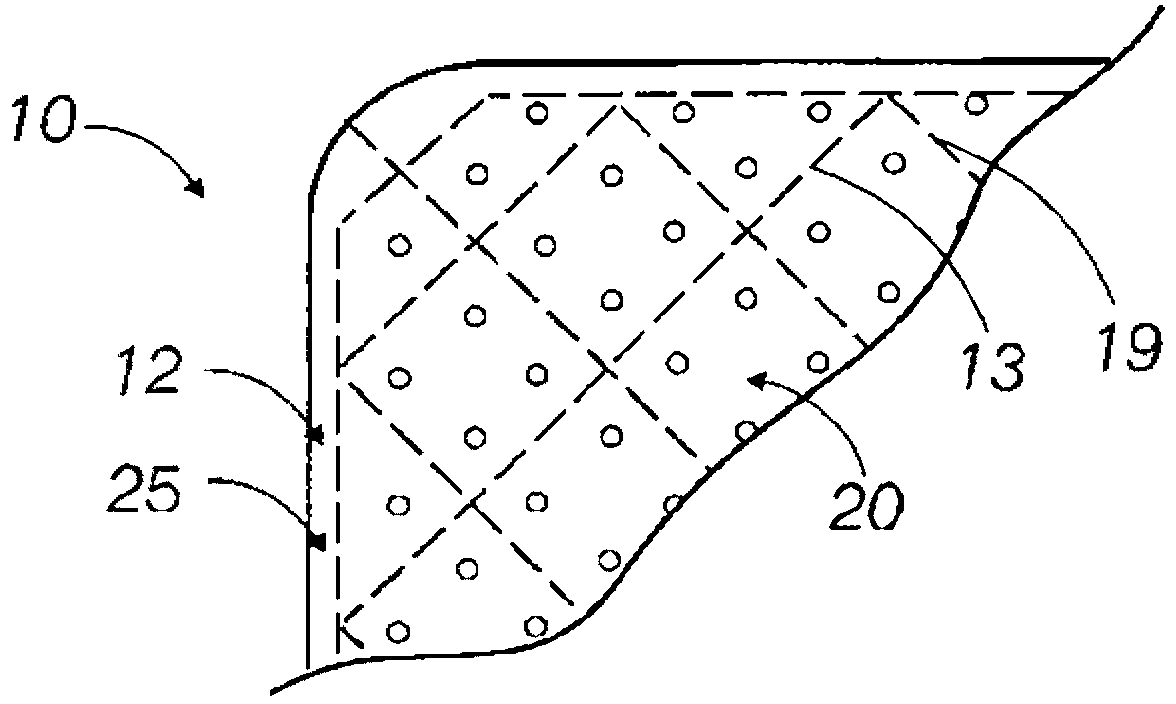
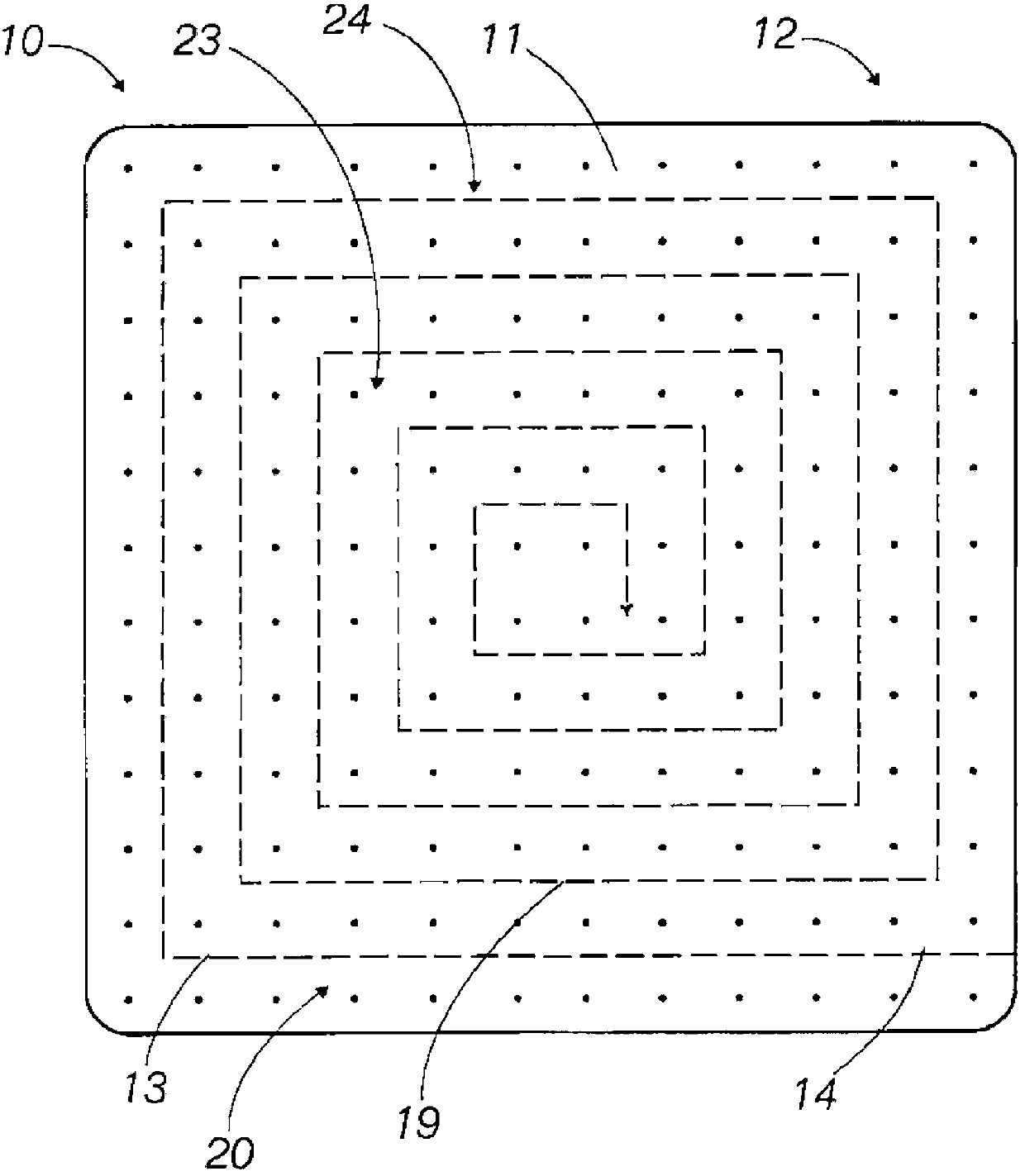
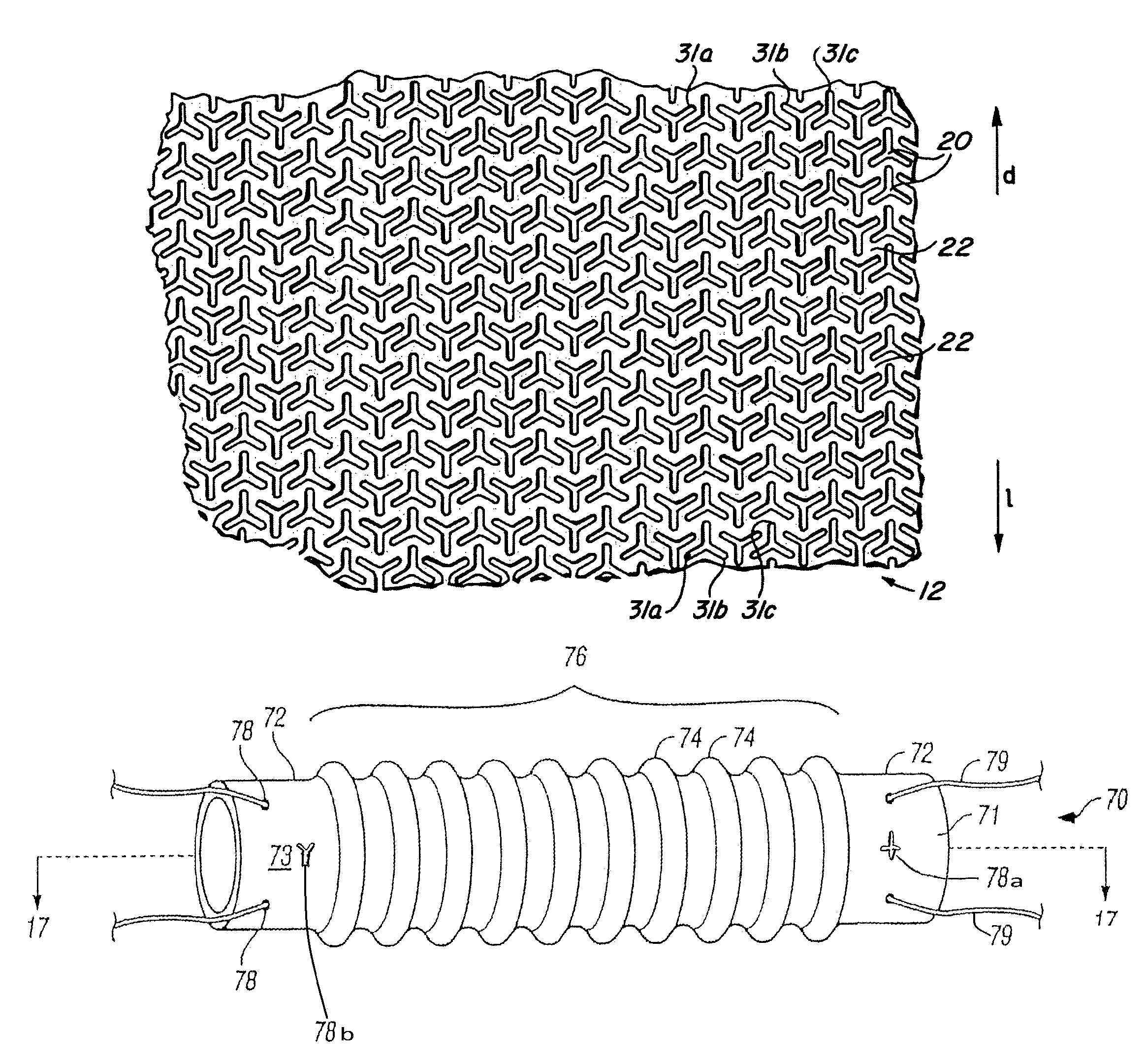
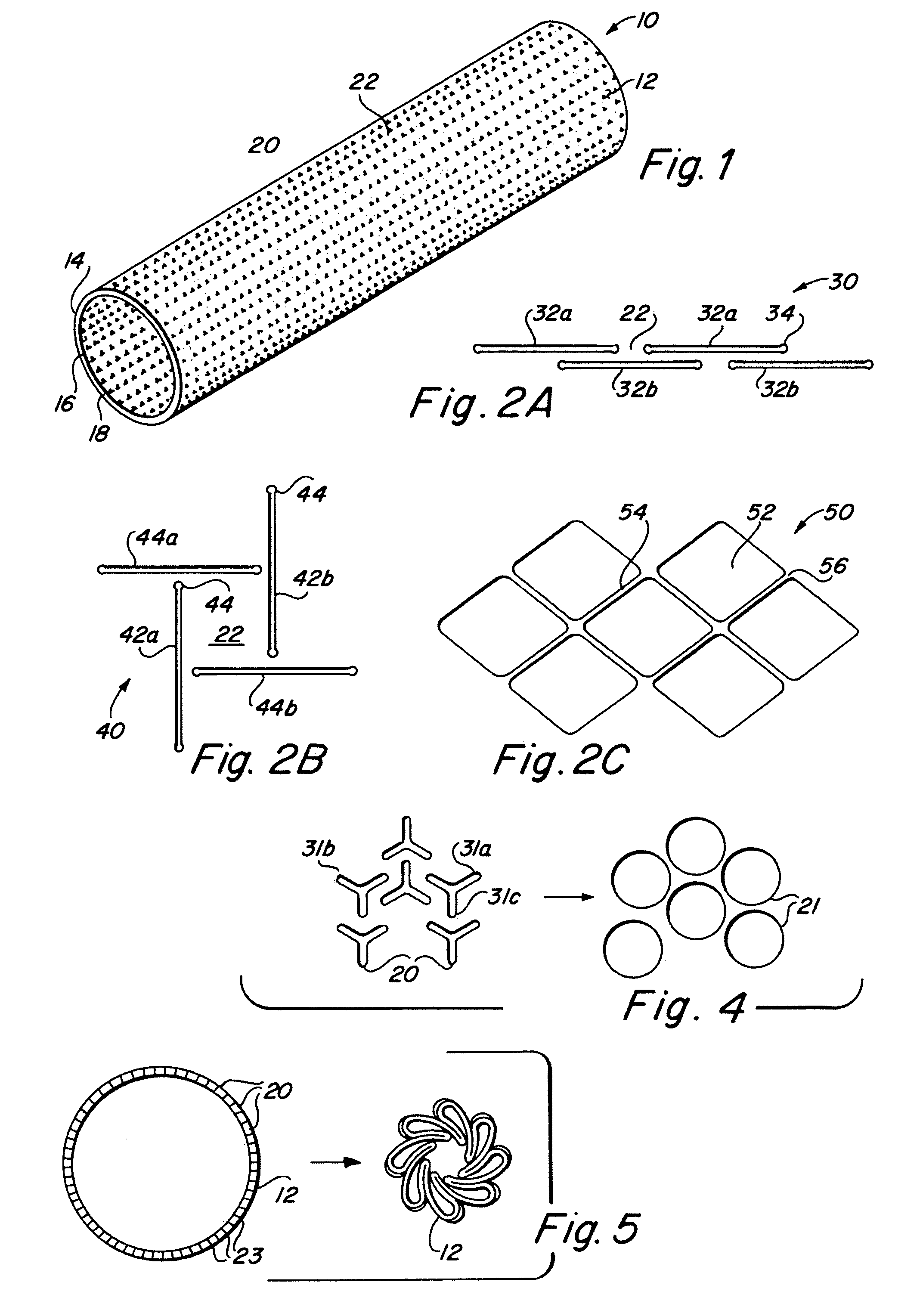
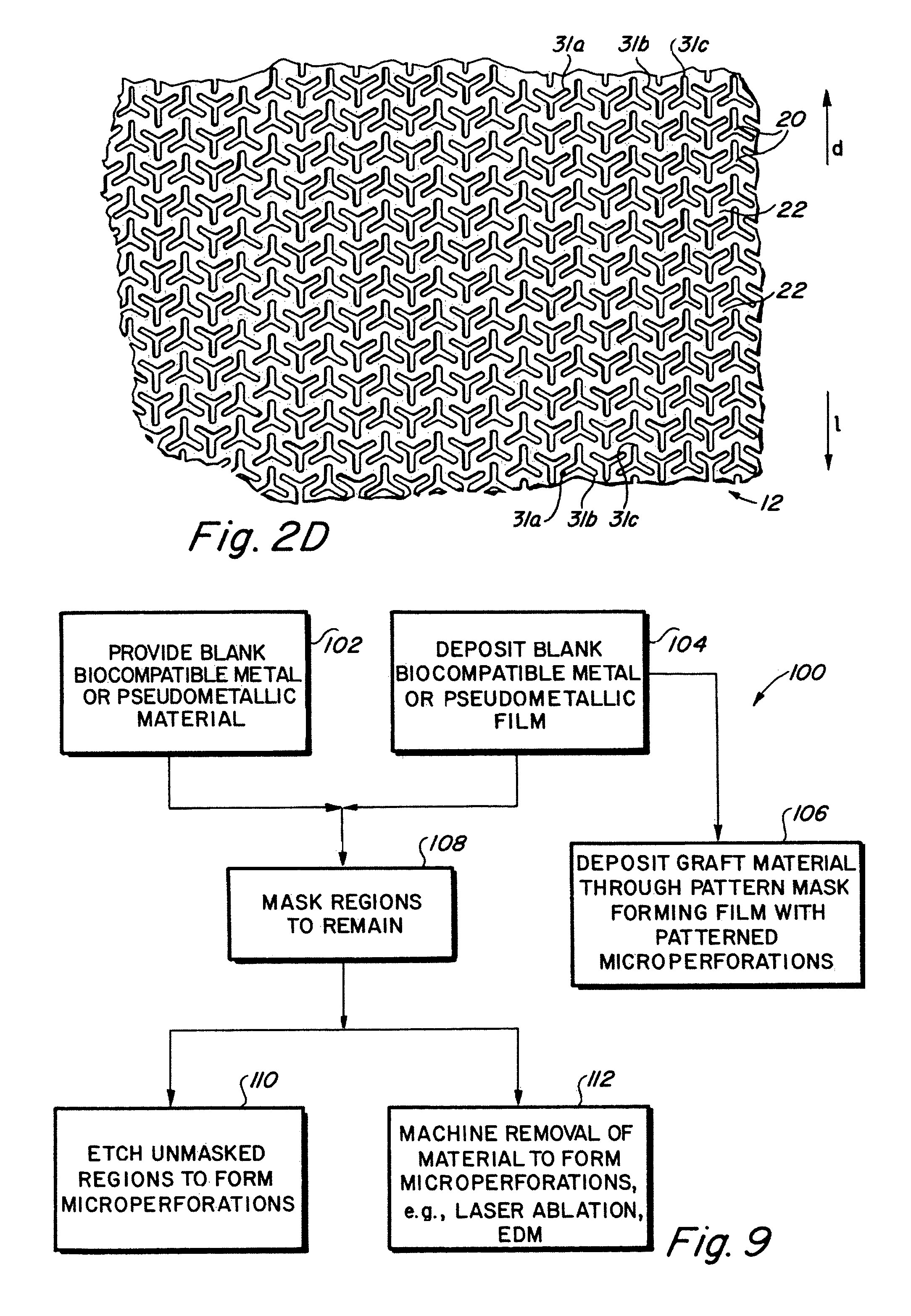
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
Self-supporting metallic implantable grafts, compliant implantable medical devices and methods of making same
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
Surgical Grafts
InactiveUS20080125869A1Anti-incontinence devicesSurgical staplesAnterior vaginal wall prolapseSurgical Graft
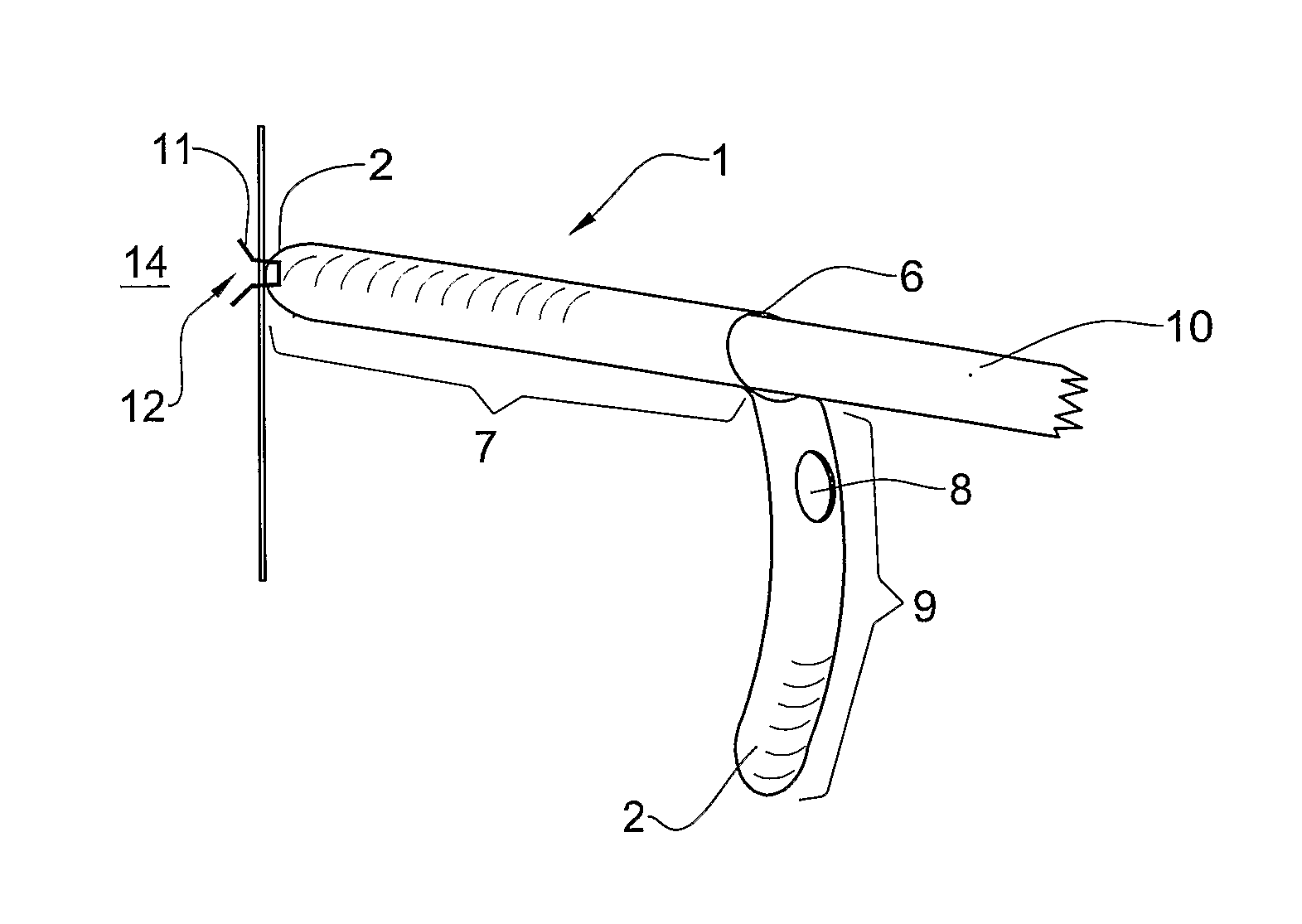
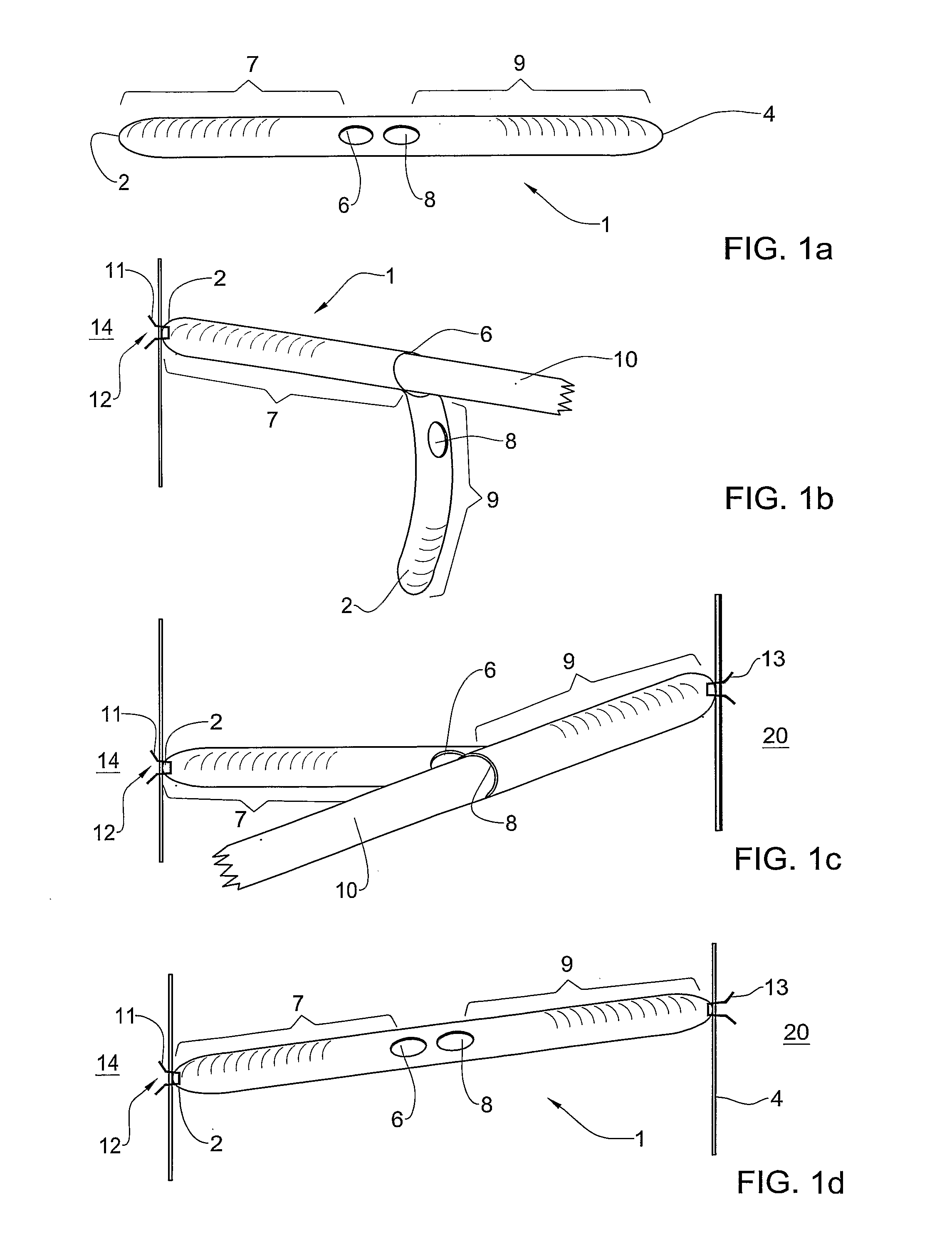
Provided is a surgical graft having one or more pockets adapted to receive a shaft tip of a surgical fastening device. The graft of the invention may be made from a biodegradable material, a biological material, a non-biodegradable material, biodegradable material, or a combination of any of these. The graft may be of a mesh construction, and may have an elongated shape, or may be shaped as a patch. The graft may be used, for example, in a method for treating urinary incontinence, vaginal vault repair, posterior vaginal wall prolapse, anterior vaginal wall prolapse and inguinal hernia. The invention also provides a system including a surgical graft of the invention, one or more surgical fasteners, and a surgical fastening device having a shaft and configured to eject at least one of the surgical fasteners from the tip of the shaft.
Owner:ENDOGUN MEDICAL SYST
Compliant implantable medical devices and methods of making same
Owner:VACTRONIX SCI LLC
Retinal pigment epithelial cell cultures on amniotic membrane and transplantation
InactiveUS20060002900A1Promote growthPromotes differentiationBiocideSenses disorderAntigenSurgical Graft
The present invention relates to a composition for implantation in the subretinal space of an eye, the composition including amniotic membrane, which may be cryopreserved human amniotic membrane, and a plurality of retinal pigment epithelial (RPE) cells or RPE equivalent cells present at the amniotic membrane. The amniotic membrane may be intact, epithelially denuded, or otherwise treated. The invention includes the use of amniotic membrane for the culturing of RPE cells thereon, forming a surgical graft for replacement of Bruch's membrane as a substrate, and for the transplanting of RPE cells to the subretinal space. The composition does not elicit immunological reactions to alloantigens or to RPE specific autoantigens; and exerts anti-inflammatory, anti angiogentic, and anti-scarring effects. The invention includes methods and kits for making or using composites including amniotic membrane and RPE cells. Also disclosed is a device for harvesting RPE cells.
Owner:TISSUETECH INC
Isolation and expansion of animal cells in cell cultures
Described are methods for isolating / purifying and expanding animal stem cells and stem-cell-like cells. Isolation methods include conditions comprising preferentially digesting non-stem cells and non-stem-cell-like cells in a population and preferentially adhering stem cells and stem-cell-like cells in a population. Expansion methods include culturing such cells under conditions comprising modulation of TGF-β signaling, inhibition of cell signaling mediated by p38 MAP kinase using small molecular weight inhibitors, expansion of the cells on human amniotic epithelial cells as feeder layers, control of cell seeding density, control of levels of Ca2+ in the culture media, rapid adhesion on a substrate or by a combination of such conditions. More particularly, what is disclosed relates to methods and systems for expanding animal cells in ex vivo cell cultures, while preventing cellular differentiation, and selectively enriching stem cells. The embodiments also disclose a culture system for ex vivo expansion of limbal epithelial cells or mesenchymal cells, as well as surgical grafts made there from.
Owner:TISSUETECH INC
Metallic implantable grafts and method of making same
ActiveUS20050033418A1Promote endothelializationGive flexibilityStentsSurgerySurgical GraftLigament structure
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
Use of equine amniotic membrane in ophthalmic surgeries in veterinary medicine
InactiveUS20080102135A1SaveMinimal amountMammal material medical ingredientsSurgical GraftOphthalmology department
A method for making, storing and using a surgical graft from equine amniotic membrane in veterinary ophthalmology. The amniotic membrane is obtained from equine placenta, from which the chorion has been separated. Sheets of the amniotic membrane are cut to size and mounted on filter paper. The cells of the amniotic membrane are killed, preferably while being frozen and thawed in the storage solution. The equine amniotic membrane can be used in a variety of ocular surgeries in horses but also other species such as food animals, dogs and cats. It represents a strong biomaterial that will give a good physical support to the ocular tissues while inducing a minimal amount of scarring which is primordial in ocular surgeries in order to obtain the best visual outcome.
Owner:OLLIVIER FRANCK JEAN
Retinal pigment epithelial cell cultures on amniotic membrane and transplantation
InactiveUS7824671B2Promotes growth and differentiationMaintenance of morphological appearanceBiocideSenses disorderSurgical GraftRetinal pigment epithelial cell
The present invention relates to a composition for implantation in the subretinal space of an eye, the composition including amniotic membrane, which may be cryopreserved human amniotic membrane, and a plurality of retinal pigment epithelial (RPE) cells or RPE equivalent cells present at the amniotic membrane. The amniotic membrane may be intact, epithelially denuded, or otherwise treated. The invention includes the use of amniotic membrane for the culturing of RPE cells thereon, forming a surgical graft for replacement of Bruch's membrane as a substrate, and for the transplanting of RPE cells to the subretinal space. The composition does not elicit immunological reactions to alloantigens or to RPE specific autoantigens; and exerts anti-inflammatory, and angiogentic, and anti-scarring effects. The invention includes methods and kits for making or using composites including amniotic membrane and RPE cells. Also disclosed is a device for harvesting RPE cells.
Owner:TISSUETECH INC
Self-supporting metallic implantable grafts, compliant implantable medical devices and methods of making same
InactiveUS20070250156A1Give flexibilityFacilitating transmural endothelializationStentsBlood vesselsSurgical GraftMetallic materials
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
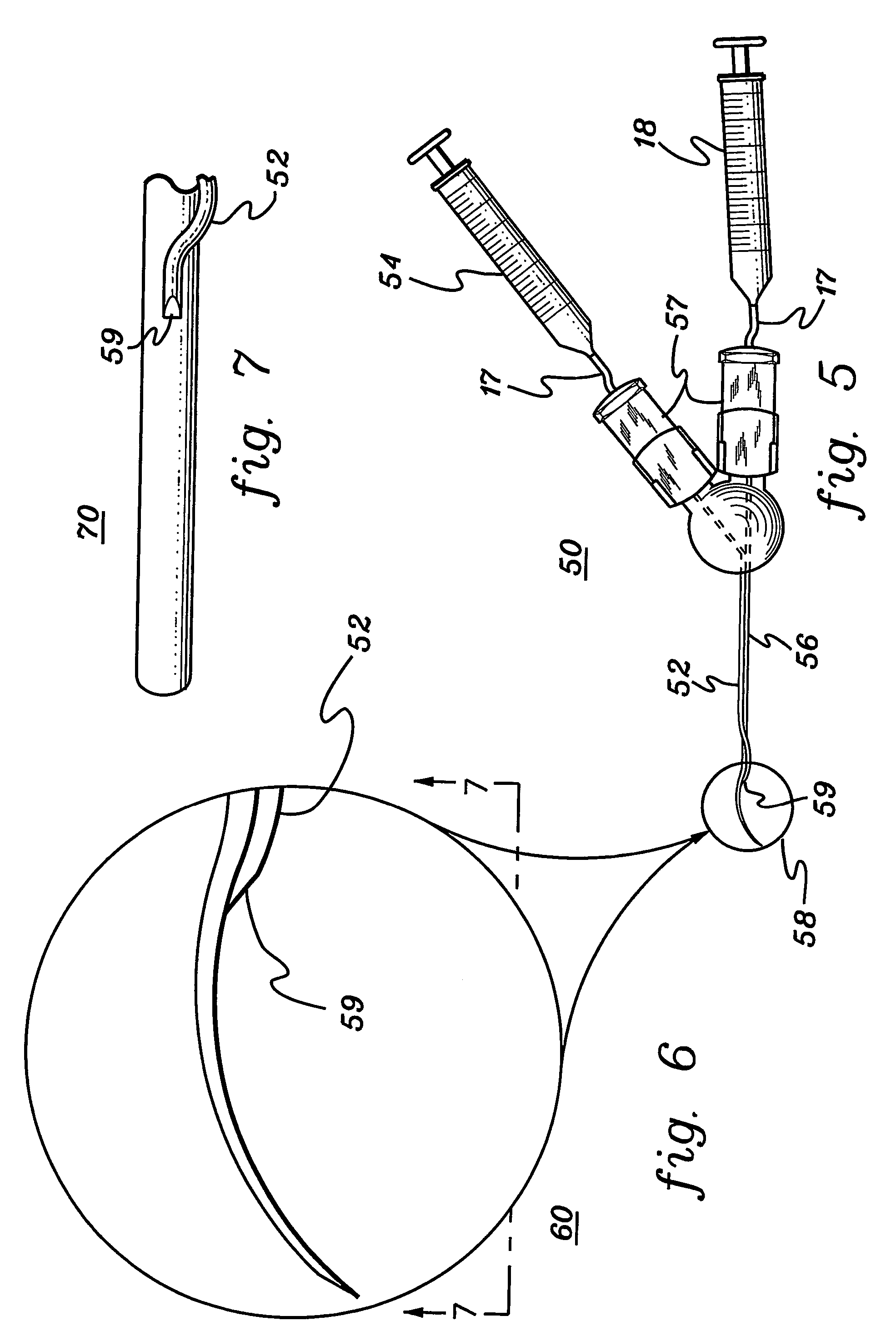
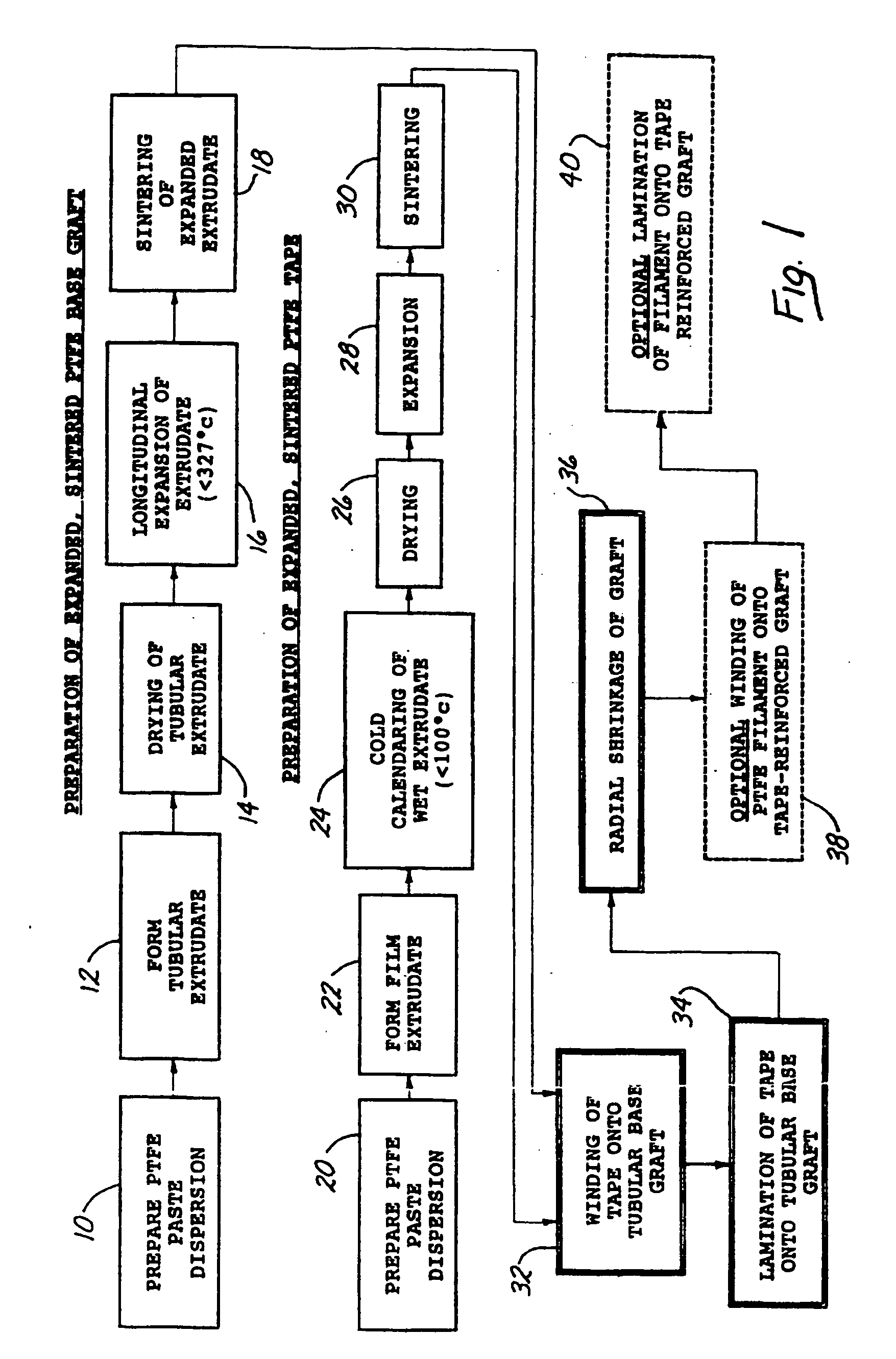
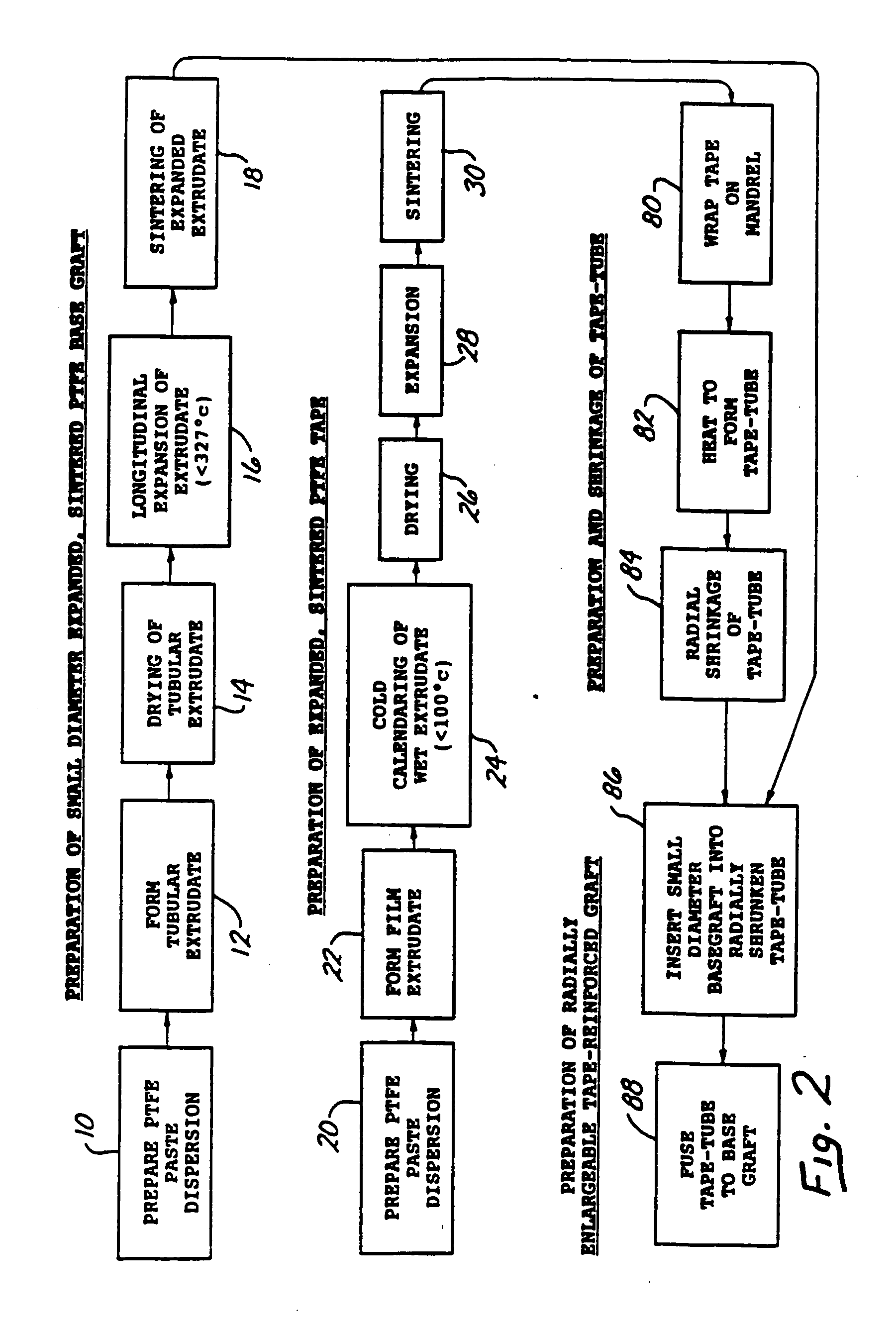
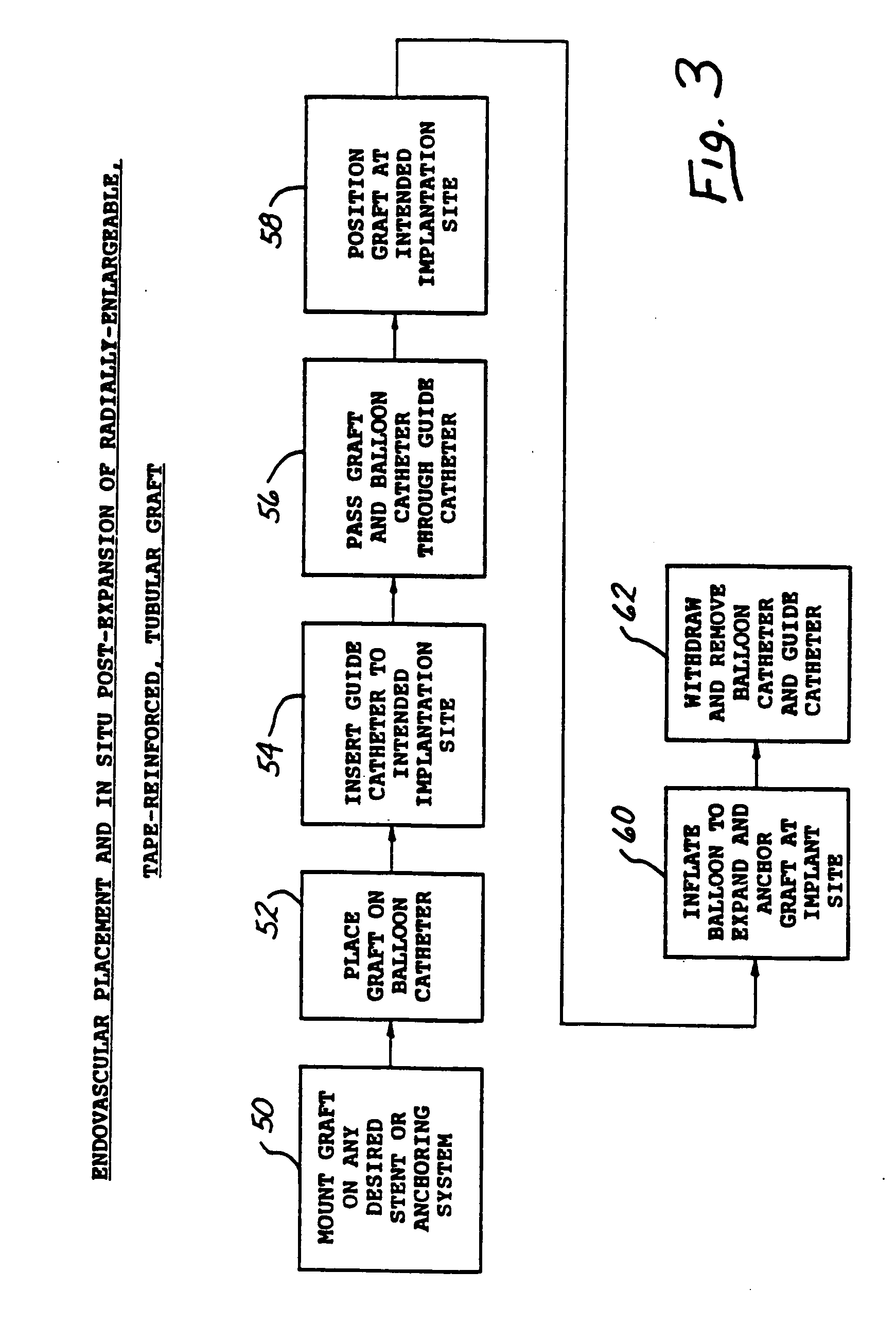
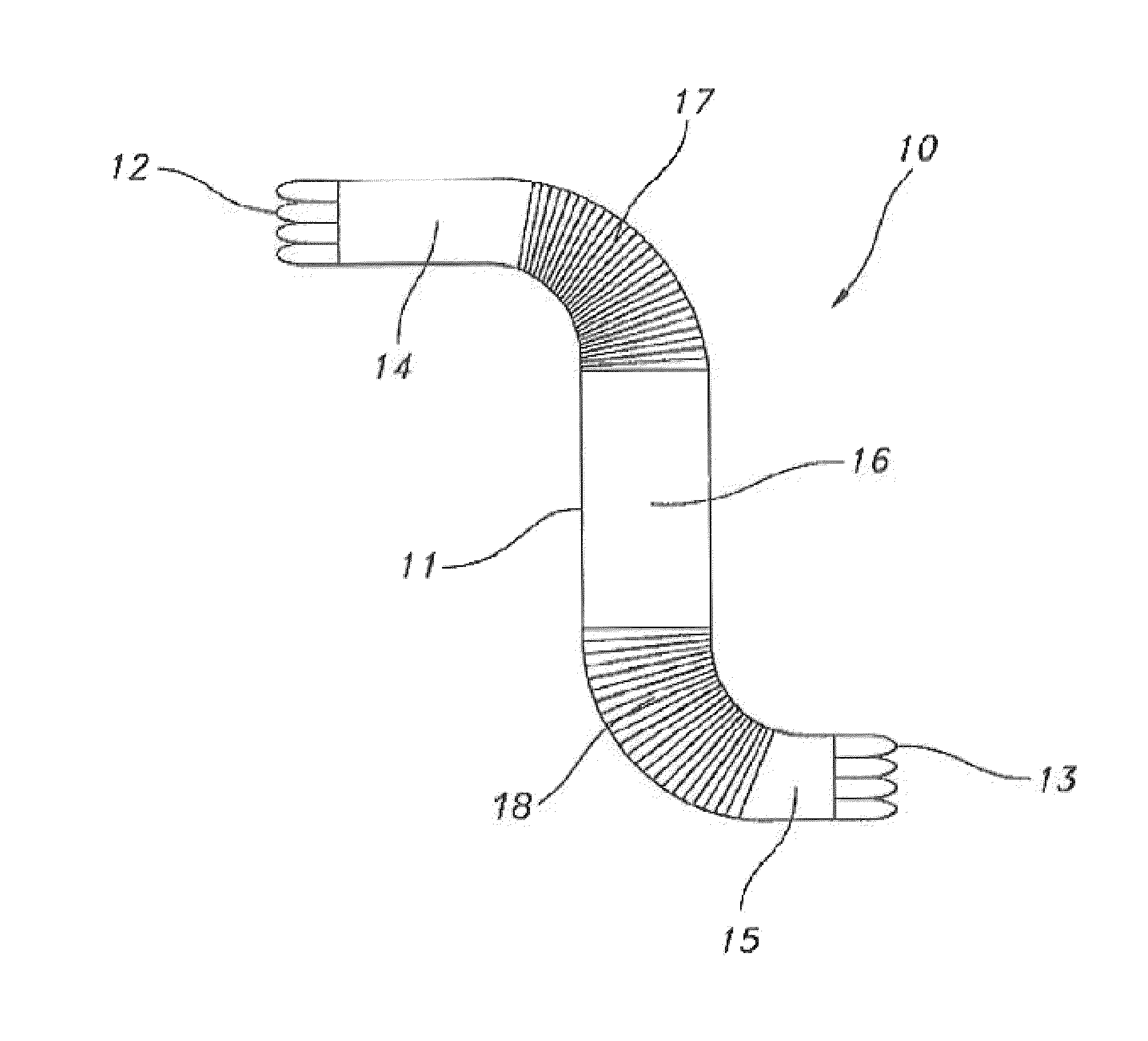
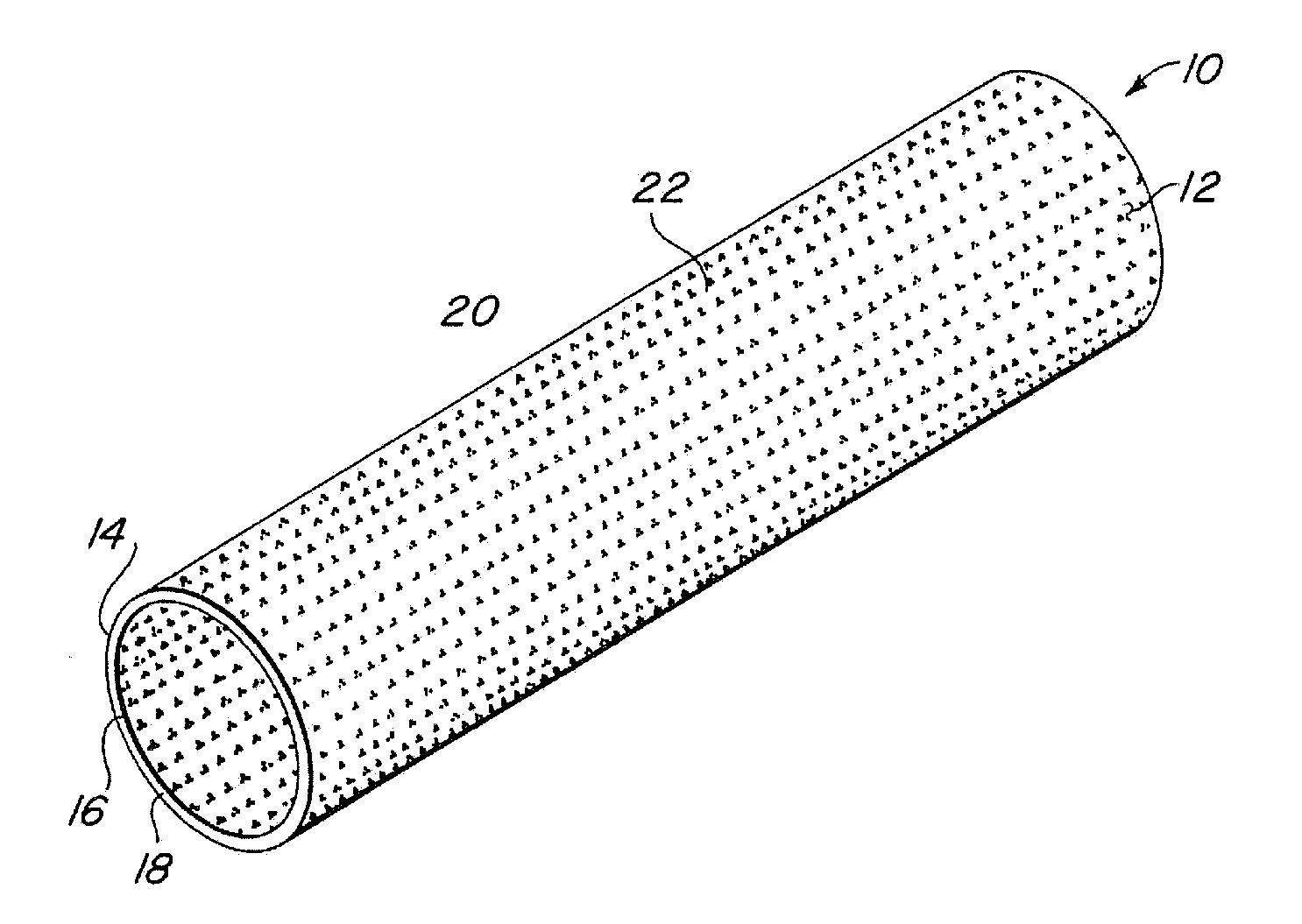
Radially expandable tape-reinforced vascular grafts
InactiveUS6863686B2Increasing and improving abilityReduce radial sizeWrappers shrinkageSurgerySurgical GraftInsertion stent
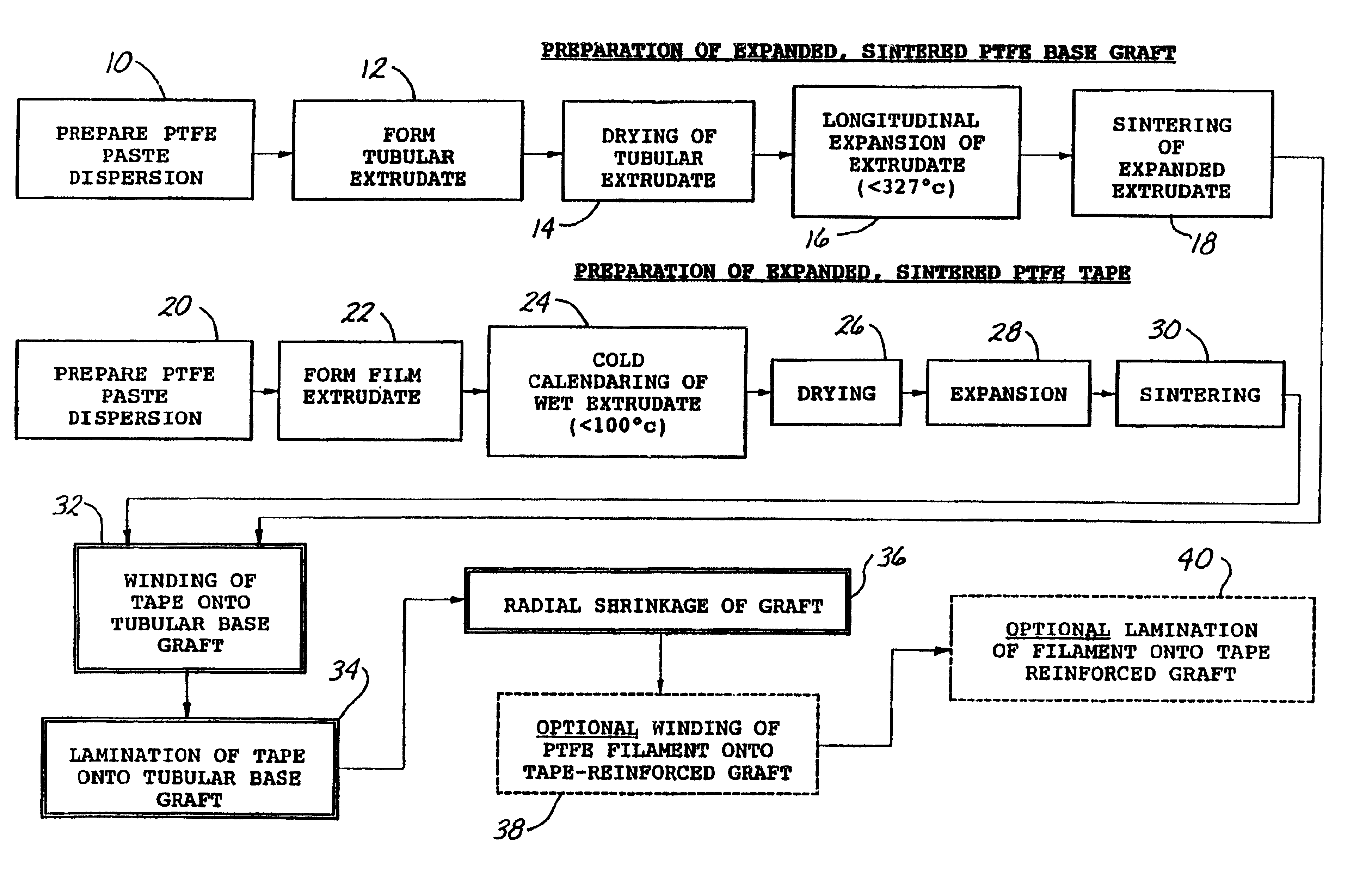
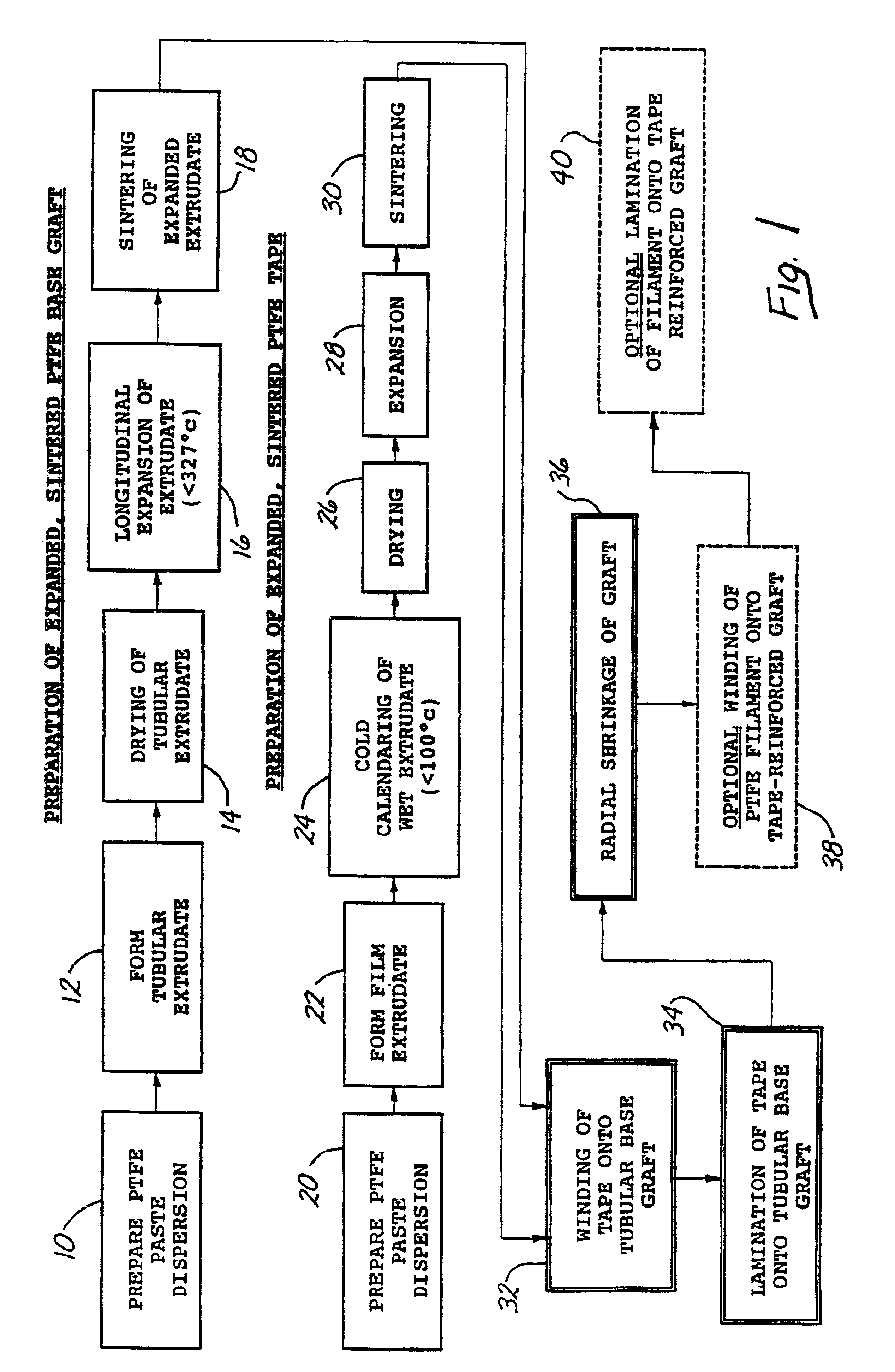
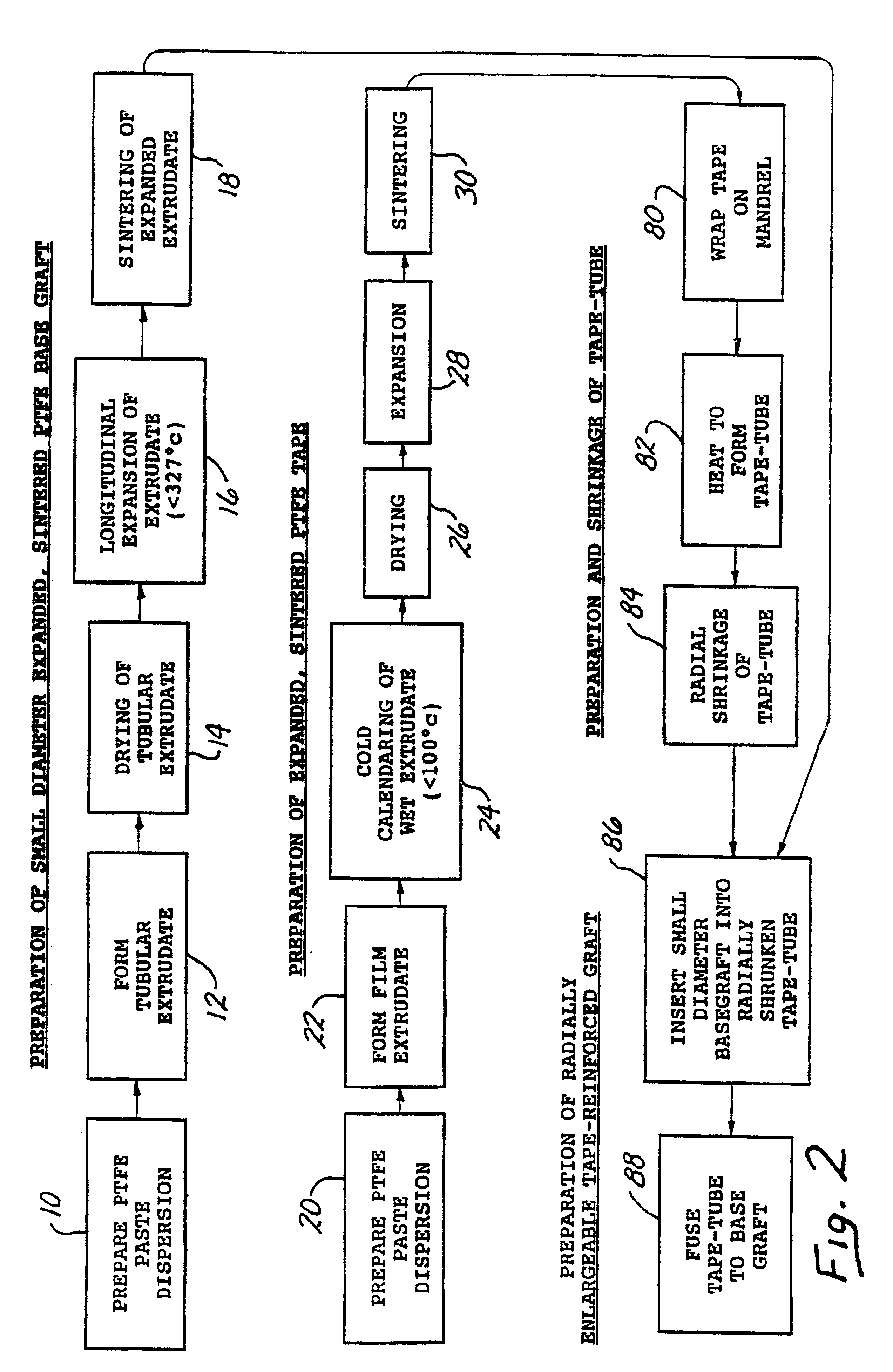
A tape-reinforced tubular vascular graft formed of sintered fluoropolymer(s), such as expanded, sintered PTFE. The graft includes a base graft and a reinforcing tape applied thereto. The tape may be spirally wrapped about the graft or spirally wrapped into a tube about a cylindrical mandrel and then applied to the exterior of The graft. Radial shrinkage of the combined base graft and tape, or of the reinforcing tape tube, renders the vascular graft subsequently radially enlargeable by more than 5%, without tearing or breaking of the reinforcement tape layer of the graft. Radially enlargeable grafts of the present invention may be combined with various types of stents or anchoring systems, to form endovascular graft devices which are transluminally insertable and implantable within the lumen of a host blood vessel. Alternatively, radially enlargeable grafts of the present invention may be implanted by way of traditional surgical graft implantation techniques, without any radial enlargement of the graft at the time of implantation, so as to take advantage of the improved strength properties and suture-holding properties of the radially-shrunken tape-reinforced grafts of the present invention.
Owner:EDWARDS LIFESCIENCES CORP
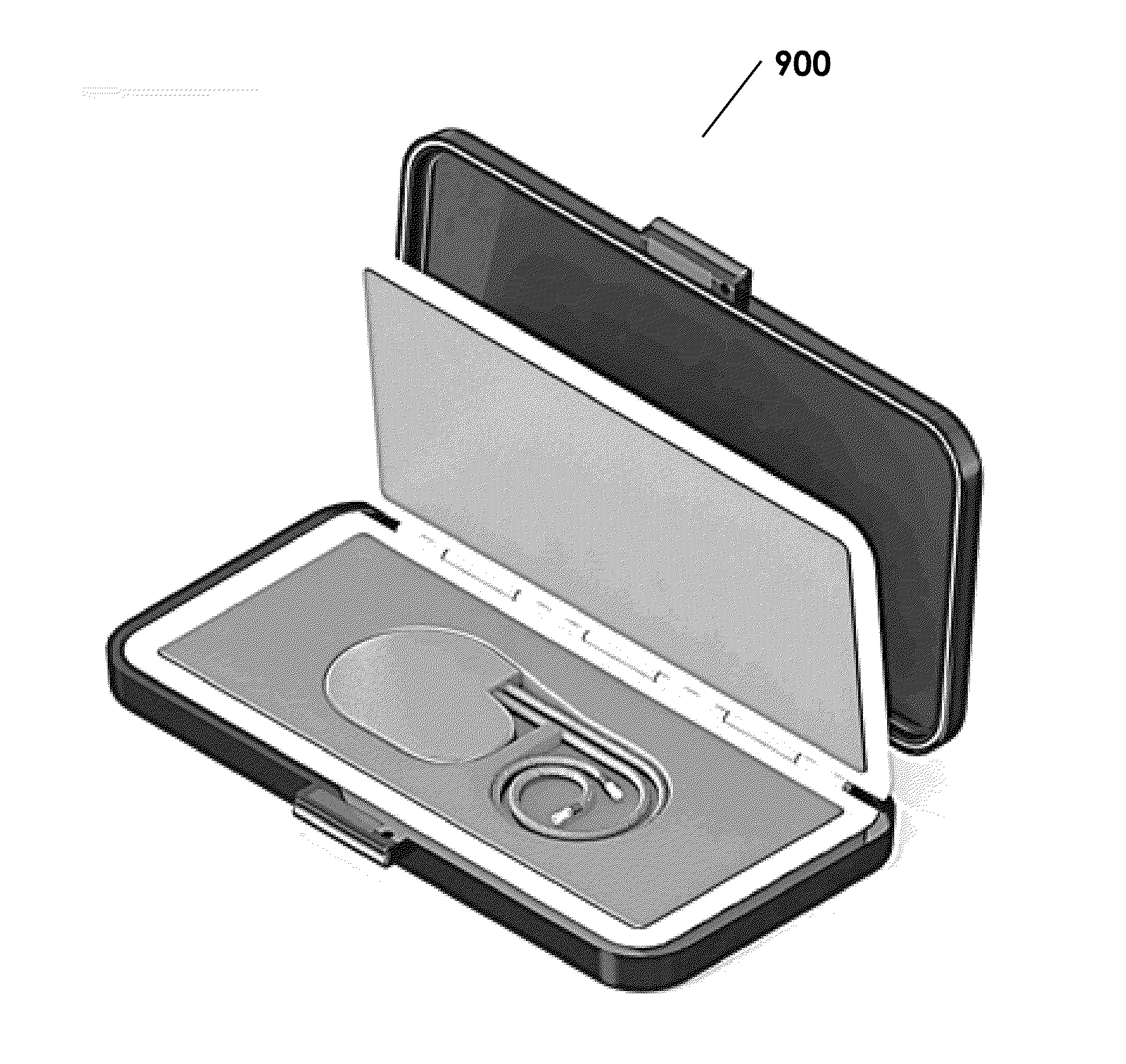
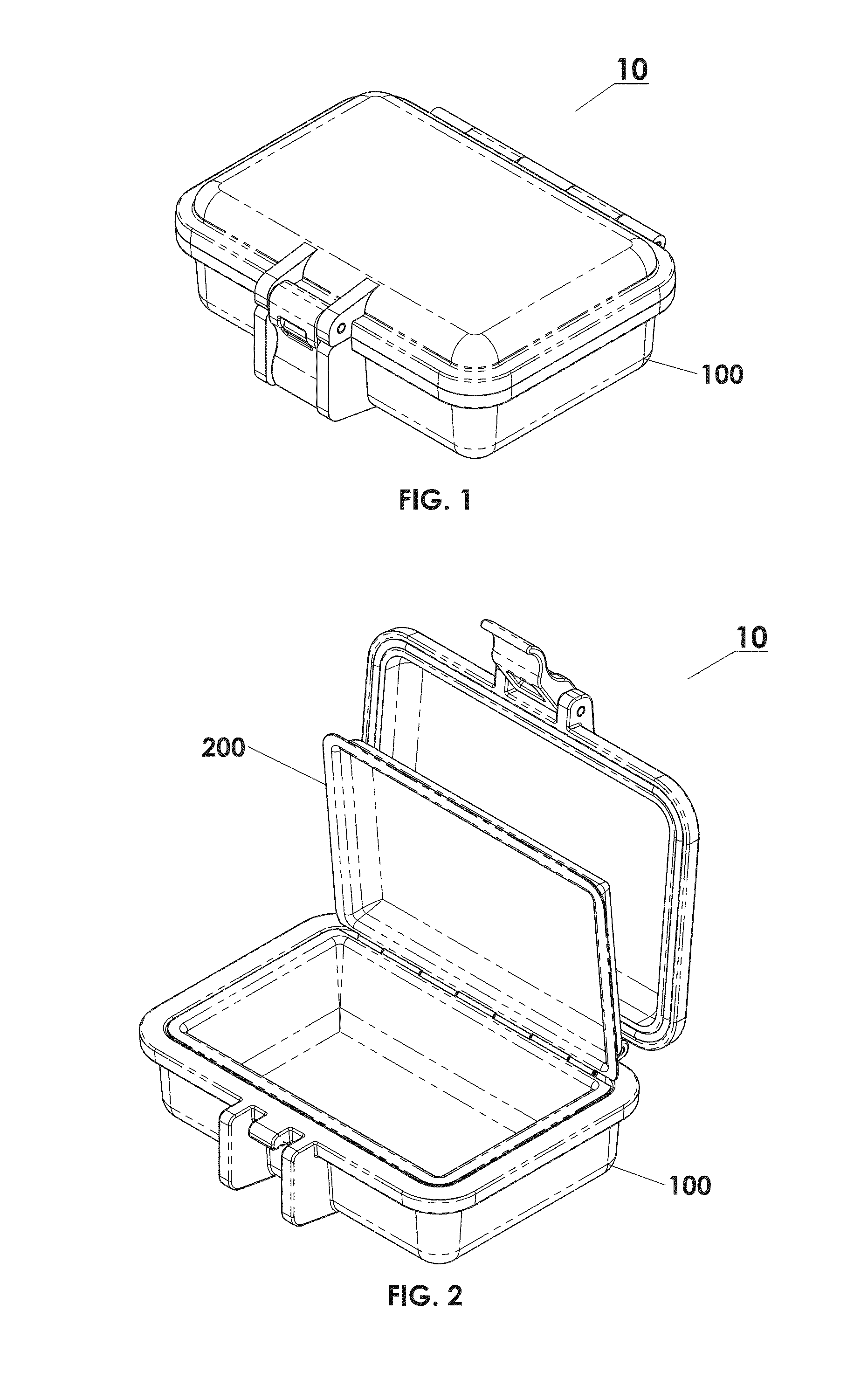
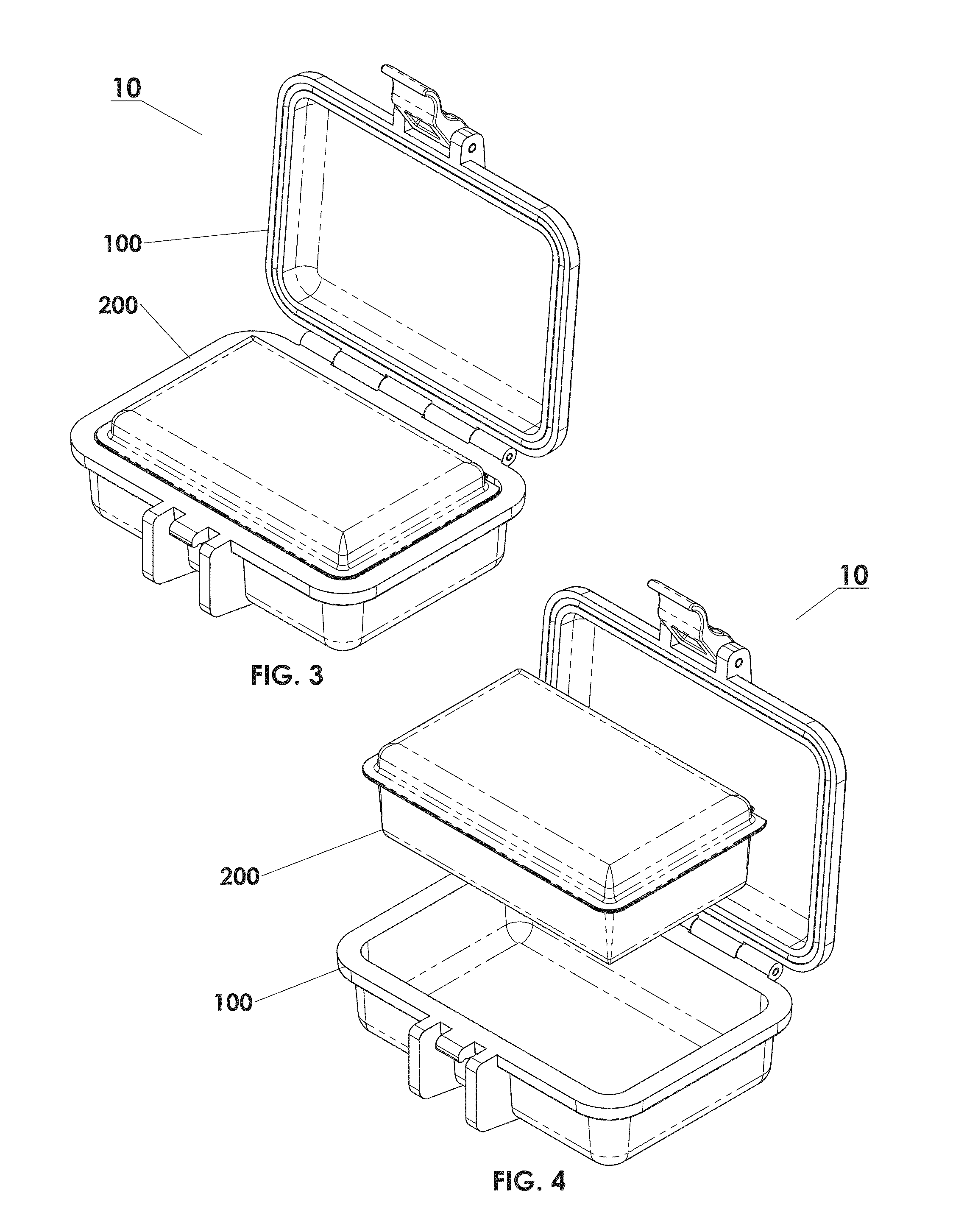
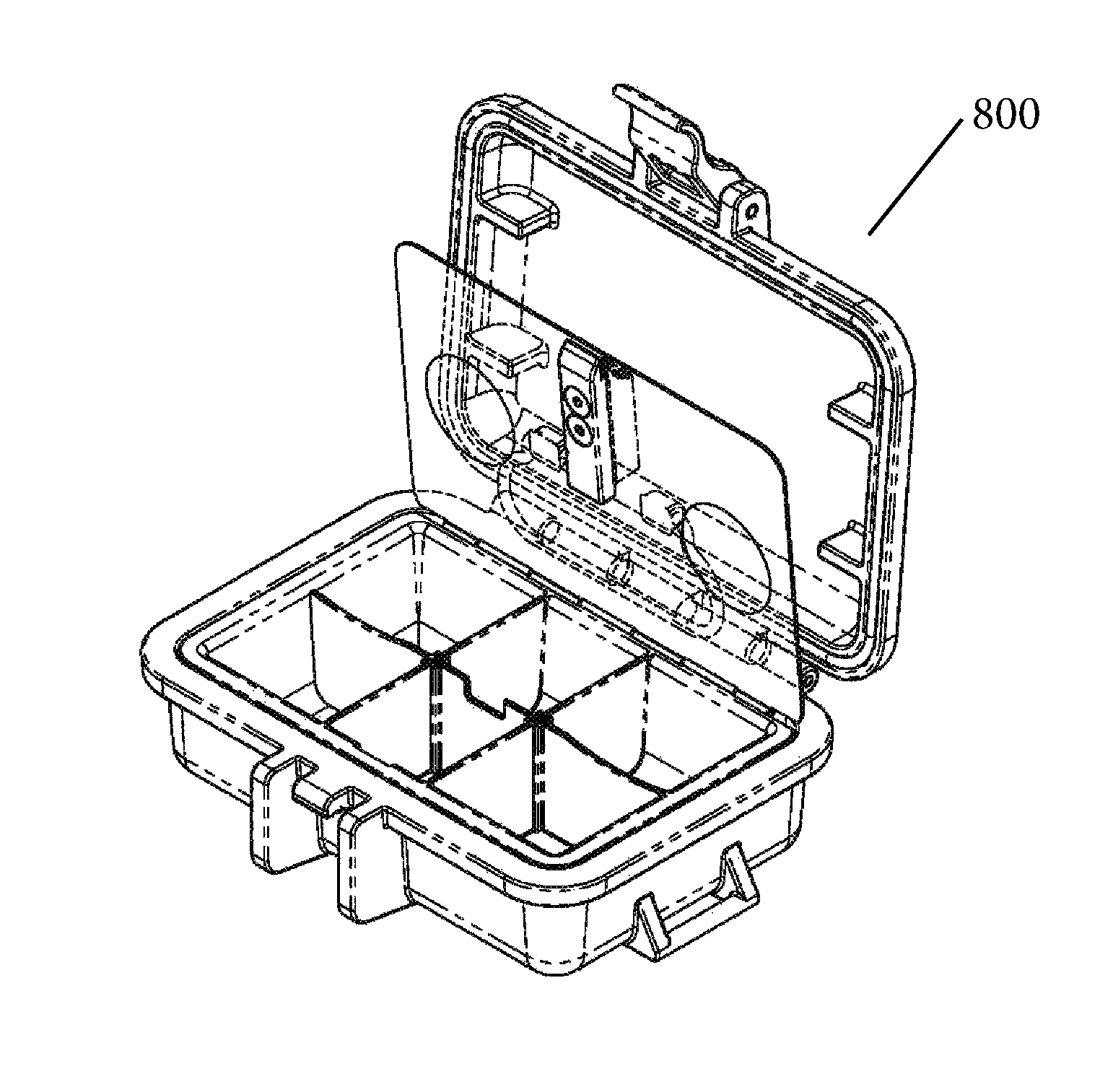
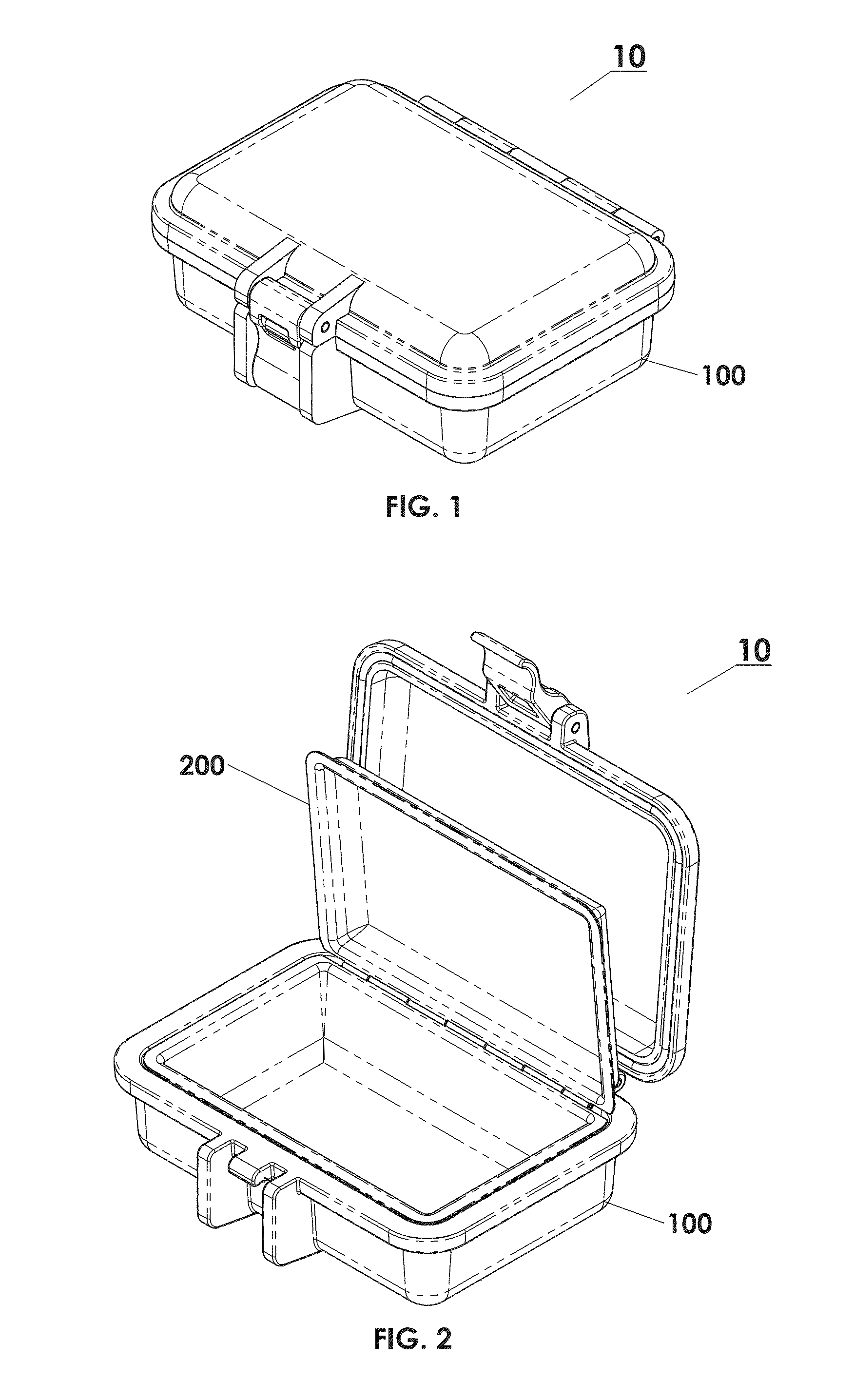
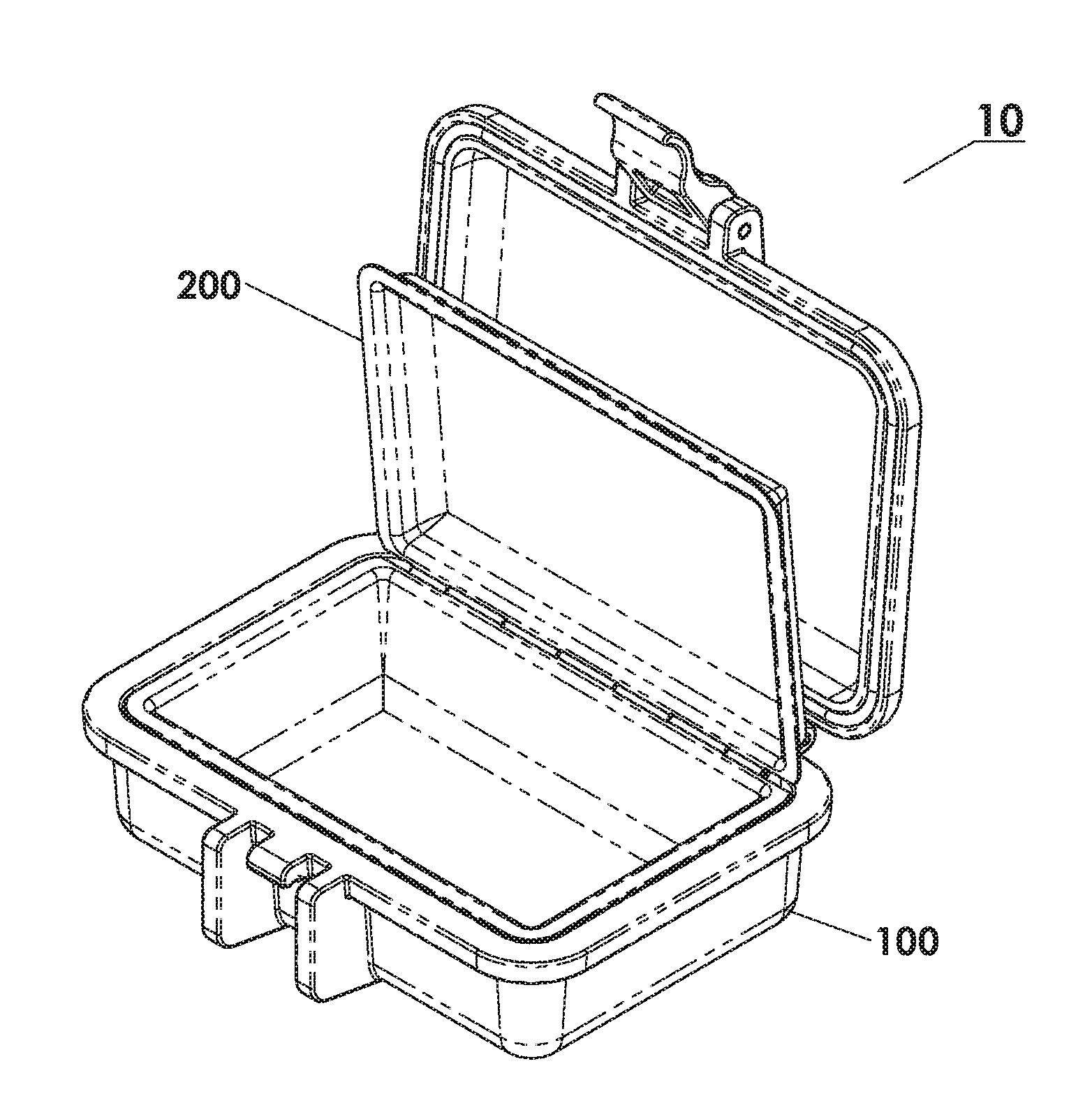
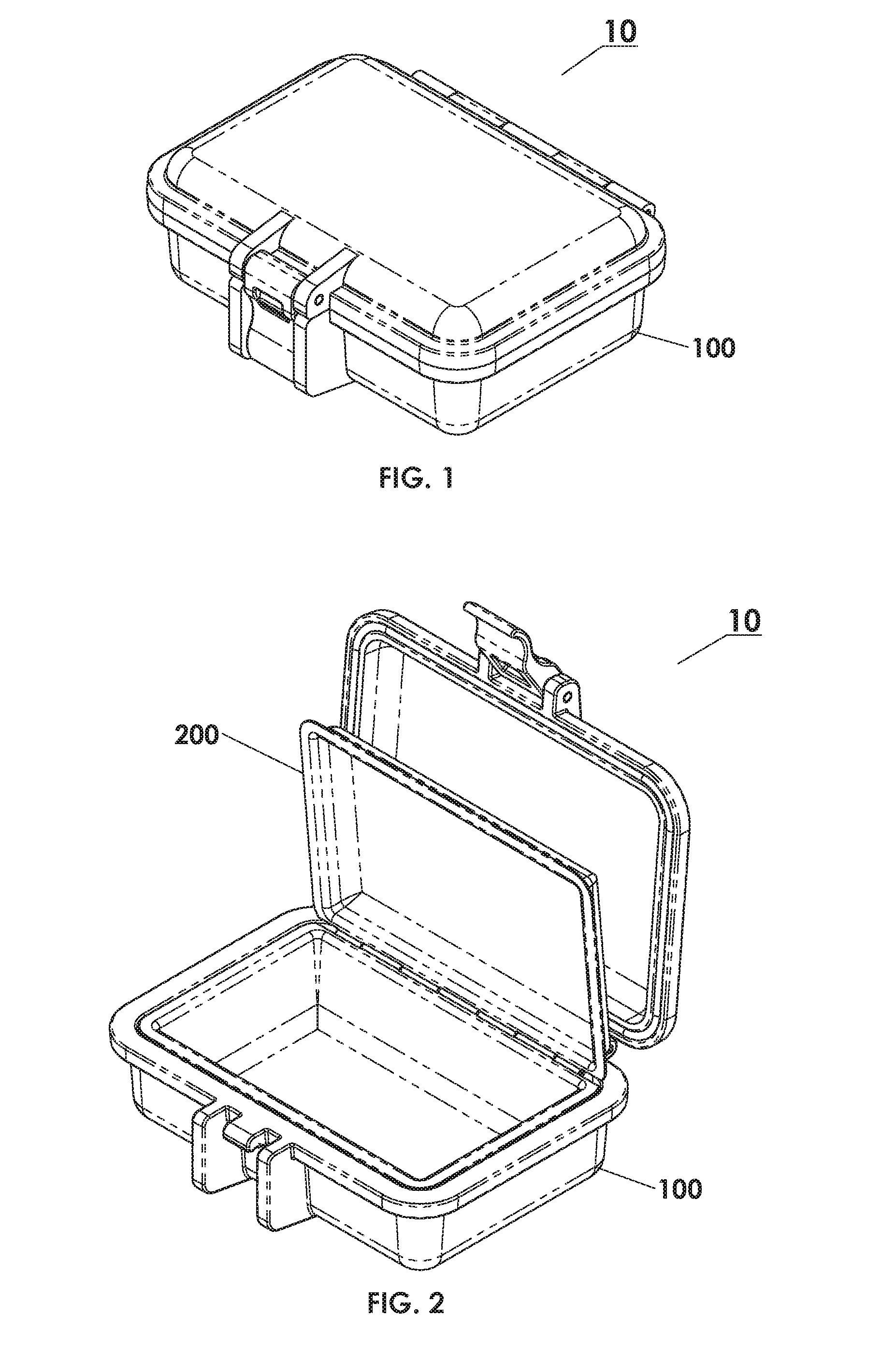
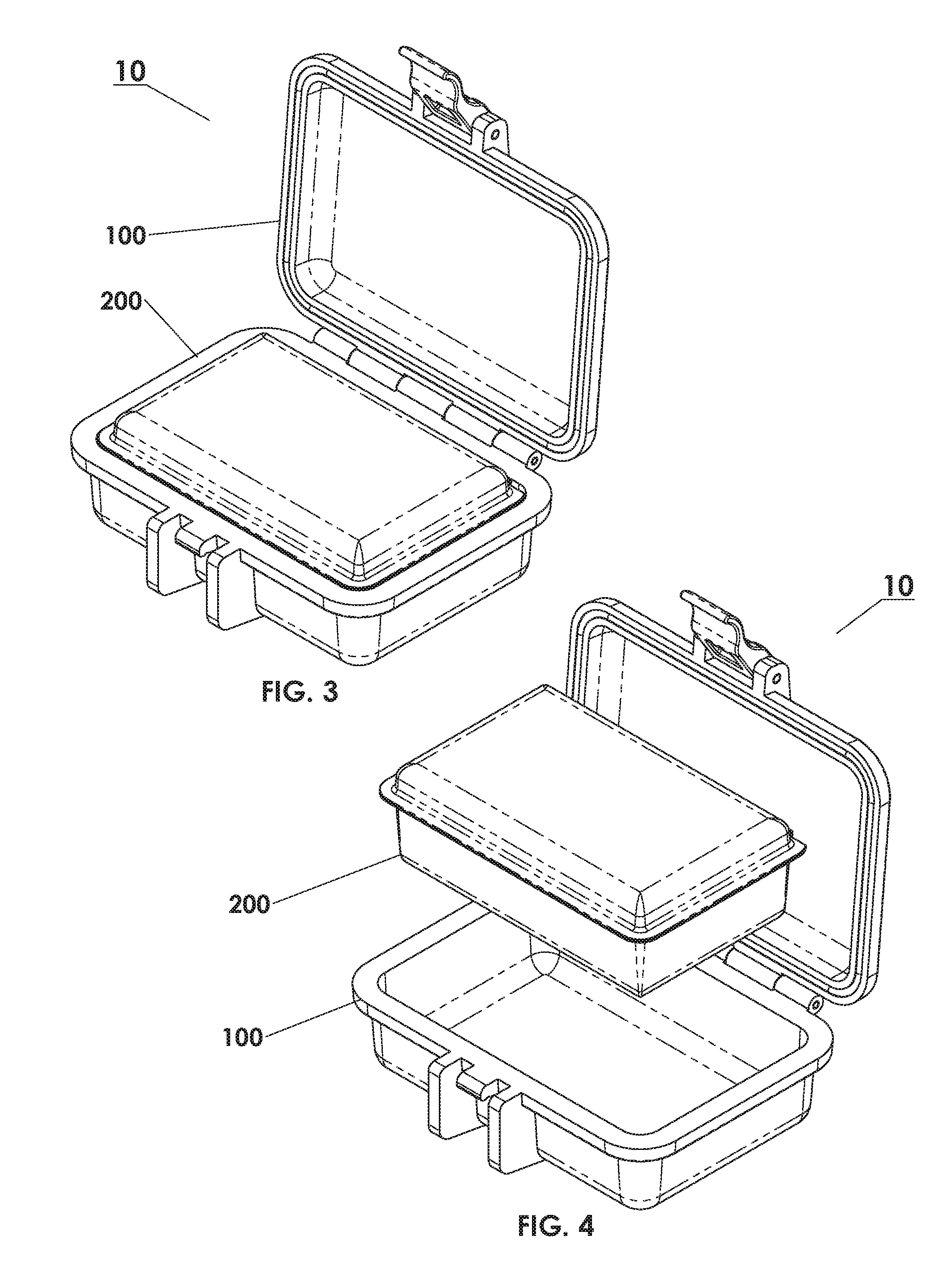
Protective Box for Surgery
ActiveUS20140346072A1Readily availableContamination damageSurgical furnitureDiagnosticsSurgical GraftSTERILE FIELD
The present invention is directed to providing a sterile container protecting various surgical grafts, implants and devices from incidental contamination and damage during operative procedures. The design includes an inner box to contain the items, and an outer box to protect the exterior of the inner box from contamination in the event of fall or other contamination, such as from airborne particles or liquids, fall from the sterile field, or by non-sterile handling. In the event of fall or contamination of the outer box, the surgical staff will be able to recover the inner box and its contents in sterile and unspoiled condition without difficulty.
Owner:JACOBSON DANIEL R
Method for expansion of epithelial stem cells
InactiveUS7347876B2Expand the populationEye implantsTissue cultureComposite Tissue AllograftTissue biopsy
Transplantation of epithelial stem cells, cultured ex vivo on specifically treated amniotic membrane, yields, with that amniotic membrane, a surgical graft having expanded epithelial stem cells. The method of creating this graft and the graft itself are simple and effective to reconstruct damaged tissue, a preferred example being corneal tissue. The source of the epithelial stem cells can be a very small explant from healthy autologous and allogeneic tissue biopsy. The amniotic membrane is treated such that its extracellular matrix is maintained, but its cells are killed.
Owner:TSAI RAY JUI FANG
Radially-expandable PTFE tape-reinforced vascular grafts
InactiveUS20050096737A1Increasing and improving abilityReduce radial sizeLaminationLamination apparatusSurgical GraftInsertion stent
A tape-reinforced tubular vascular graft formed of sintered fluoropolymer(s), such as expanded, sintered PTFE. The graft includes a base graft and a reinforcing tape applied thereto. The tape may be spirally wrapped about the graft or spirally wrapped into a tube about a cylindrical mandrel and then applied to the exterior of the graft. Radial shrinkage of the combined base graft and tape, or of the reinforcing tape tube, renders the vascular graft subsequently radially enlargeable by more than 5%, without tearing or breaking of the reinforcement tape layer of the graft. Radially enlargeable grafts of the present invention may be combined with various types of stents or anchoring systems, to form endovascular graft devices which are transluminally insertable and implantable within the lumen of a host blood vessel. Alternatively, radially enlargeable grafts of the present invention may be implanted by way of traditional surgical graft implantation techniques, without any radial enlargement of the graft at the time of implantation, so as to take advantage of the improved strength properties and suture-holding properties of the radially-shrunken tape-reinforced grafts of the present invention.
Owner:EDWARDS LIFESCIENCES CORP
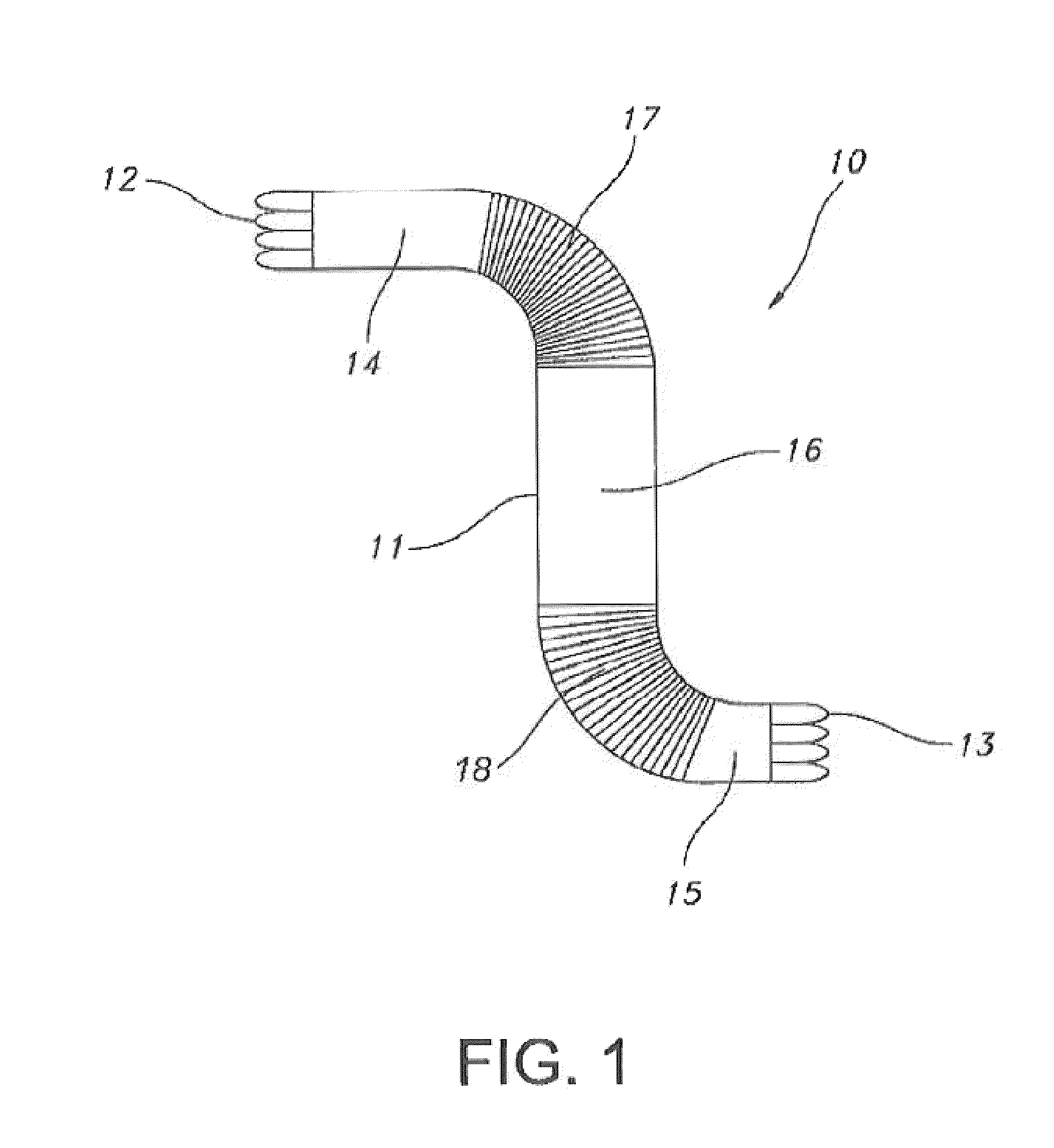
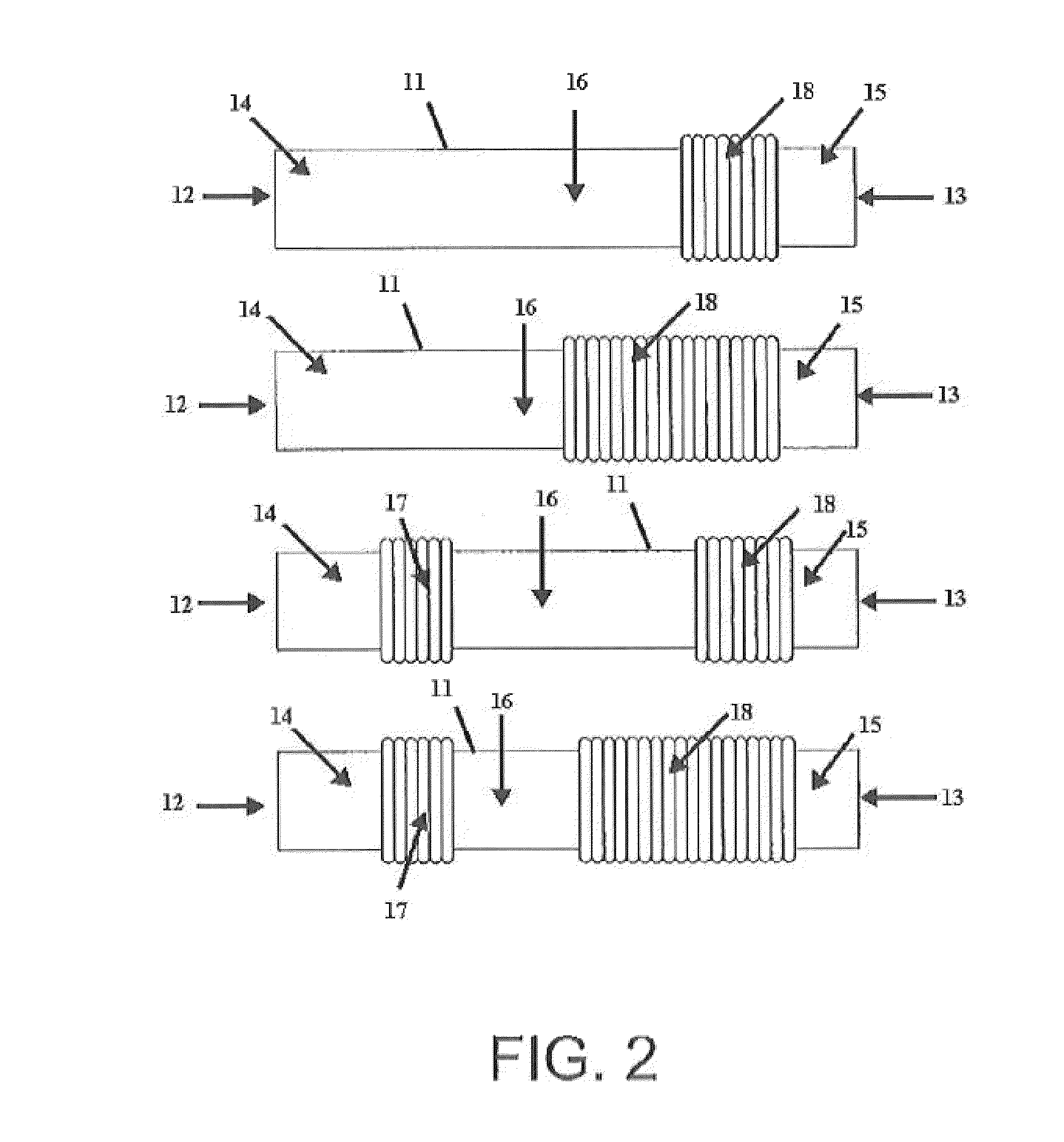
Sectional crimped graft
InactiveUS20070198079A1Limits overall longitudinal extensionEasy to deployStentsBlood vesselsSurgical GraftStent grafting
The present invention provides a sectional crimped graft that allows graft flexibility where required and limits the overall longitudinal extension. The present invention overcomes the disadvantage of fully crimped grafts by controlling the number of crimps per unit length, crimp height, crimp geometry and their location along the graft wall, dependent on the particular end-use application. In so doing, flexibility and elongation can be controllably tailored only in areas where significant anatomical angulation is present. It may also be useful in applications other than stent grafts such as surgical grafts for aortic and peripheral areas. Limiting the overall graft longitudinal extension also facilitates the deployment of the stent-graft into the blood vessel.
Owner:LIFESHIELD SCI
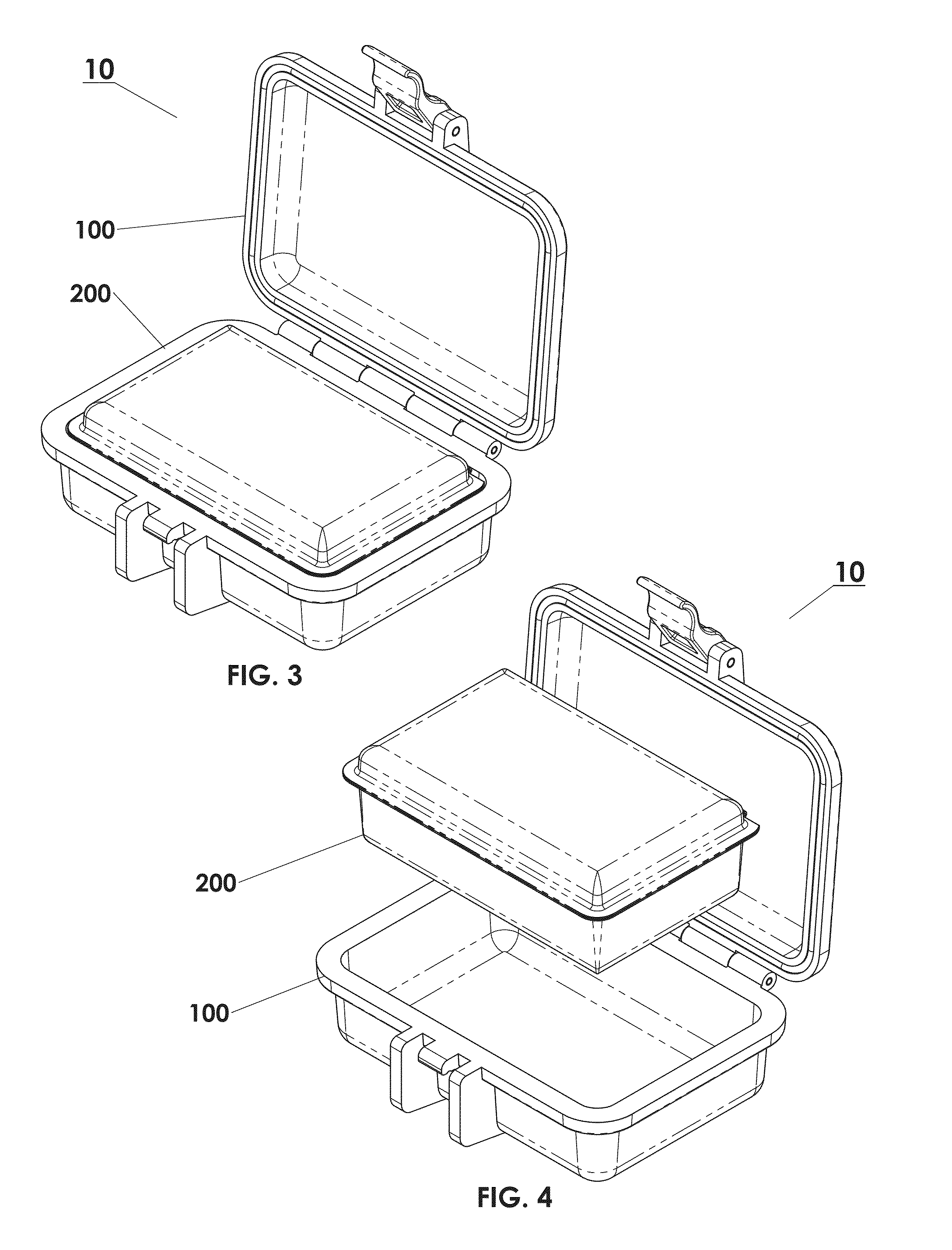
Protective box for surgery
ActiveUS9414893B2Contamination damageDamage to materialSurgical furniturePackaging protectionSurgical GraftSTERILE FIELD
The present invention is directed to providing a sterile container protecting various surgical grafts, implants and devices from incidental contamination and damage during operative procedures. The design includes an inner box to contain the items, and an outer box to protect the exterior of the inner box from contamination in the event of fall or other contamination, such as from airborne particles or liquids, fall from the sterile field, or by non-sterile handling. In the event of fall or contamination of the outer box, the surgical staff will be able to recover the inner box and its contents in sterile and unspoiled condition without difficulty.
Owner:JACOBSON DANIEL R
Method for expansion of epithelial stem cells
InactiveUS20030208266A1Promote epithelializationReduce inflammationEye implantsTissue biopsyComposite Tissue Allograft
Transplantation of epithelial stem cells, cultured ex vivo on specifically treated amniotic membrane, yields, with that amniotic membrane, a surgical graft having expanded epithelial stem cells. The method of creating this graft and the graft itself provide simple and effective means to reconstruct damaged tissue, a preferred example being corneal tissue. The source of the epithelial stem cells can be a very small explant from healthy autologous and allogeneic tissue biopsy. The amniotic membrane is treated such that its extracellular matrix is maintained, but its cells are killed.
Owner:TSAI RAY JUI FANG
Surgical grafts for repairing chondral defects
An implant containing a collagen matrix embedded with chondrocyte-like cells, its use in repairing a chondral defect, and a method of preparing the implant.
Owner:KARTIGEN BIOMEDICINE INC
RNAi Methods and Compositions for Stimulating Proliferation of Cells with Adherent Functions
ActiveUS20100003299A1Promotes HCEC adhesionReduce thicknessBiocideOrganic active ingredientsSurgical GraftIn vivo
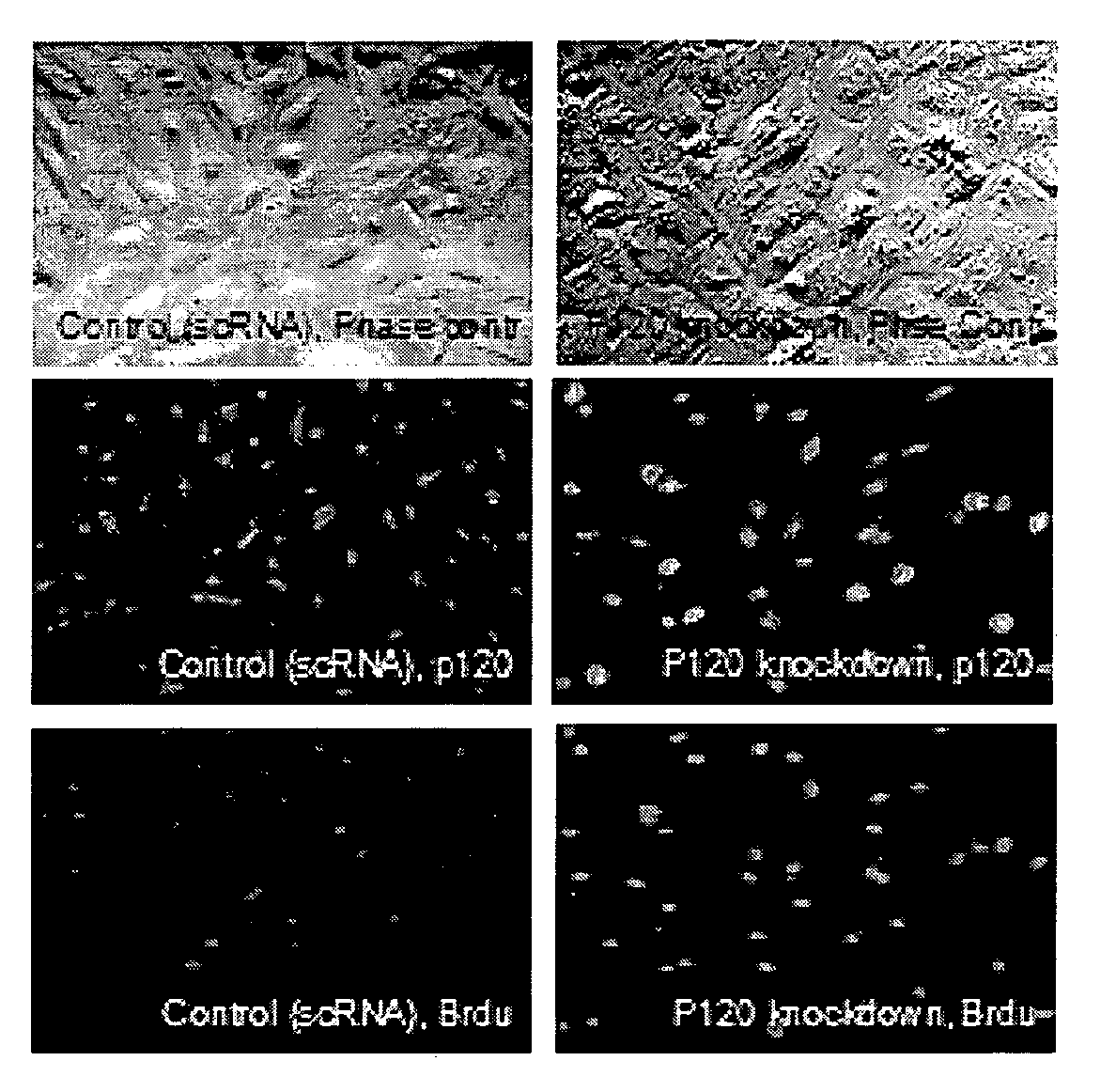
Described herein are methods and compositions for stimulating proliferation of cells that express adherent junctions and cease proliferation, for example, human corneal endothelial cells, by downregulation of certain cell-cell junctions. In one embodiment, downregulation is achieved using RNA interference, and contacting the cells with mitogenic growth factors and an agent that elevates intracytoplasmic cAMP. Furthermore, described herein are methods of isolating human corneal endothelial cells from keratocytes, and methods of preserving and maintaining viability of human corneal endothelial cell aggregates. Also described are surgical grafts comprising human corneal endothelial cells that have been isolated, optionally stored, and transiently contacted with an agent that downregulates expression of p 120, and a biocompatible support. The methods and compositions described herein can be used in novel therapies to help expand human corneal endothelial cells during in vitro tissue engineering and for in vivo treatment of corneal endothelial dysfunction.
Owner:TISSUETECH INC
Sectional crimped graft
InactiveUS8579961B2Easy to deployLimits the overall longitudinal extensionStentsBlood vesselsSurgical GraftStent grafting
The present invention provides a sectional crimped graft that allows graft flexibility where required and limits the overall longitudinal extension. The present invention overcomes the disadvantage of fully crimped grafts by controlling the number of crimps per unit length, crimp height, crimp geometry and their location along the graft wall, dependent on the particular end-use application. In so doing, flexibility and elongation can be controllably tailored only in areas where significant anatomical angulation is present. It may also be useful in applications other than stent grafts such as surgical grafts for aortic and peripheral areas. Limiting the overall graft longitudinal extension also facilitates the deployment of the stent-graft into the blood vessel.
Owner:LIFESHIELD SCI
Method for expansion of human corneal endothelial cells
A method for expanding human corneal endothelial cells includes: (a) providing an amniotic membrane with or without amniotic cells, wherein the amniotic membrane has an extracellular matrix; (b) placing onto the amniotic membrane, a sheet of endothelial layer, or a cell suspension including human corneal endothelial stem cells; and (c) culturing the corneal endothelial cells on the amniotic membrane for a duration sufficient for the corneal endothelial stem cells to expand to an appropriate area. The invention also relates to a method for creating a surgical graft for a recipient site of a patient using the method for expanding human corneal endothelial cells, and the surgical graft prepared therefrom.
Owner:TSAI RAY JUI FANG +1
Compliant implantable medical devices and methods of making same
InactiveUS20100154197A1Promote migrationStrict controlVacuum evaporation coatingSputtering coatingSurgical GraftMetallic materials
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
Surgical grafts for repairing chondral defects
An implant containing a collagen matrix embedded with chondrocyte-like cells, its use in repairing a chondral defect, and a method of preparing the implant.
Owner:KARTIGEN BIOMEDICINE INC
Quilted implantable graft
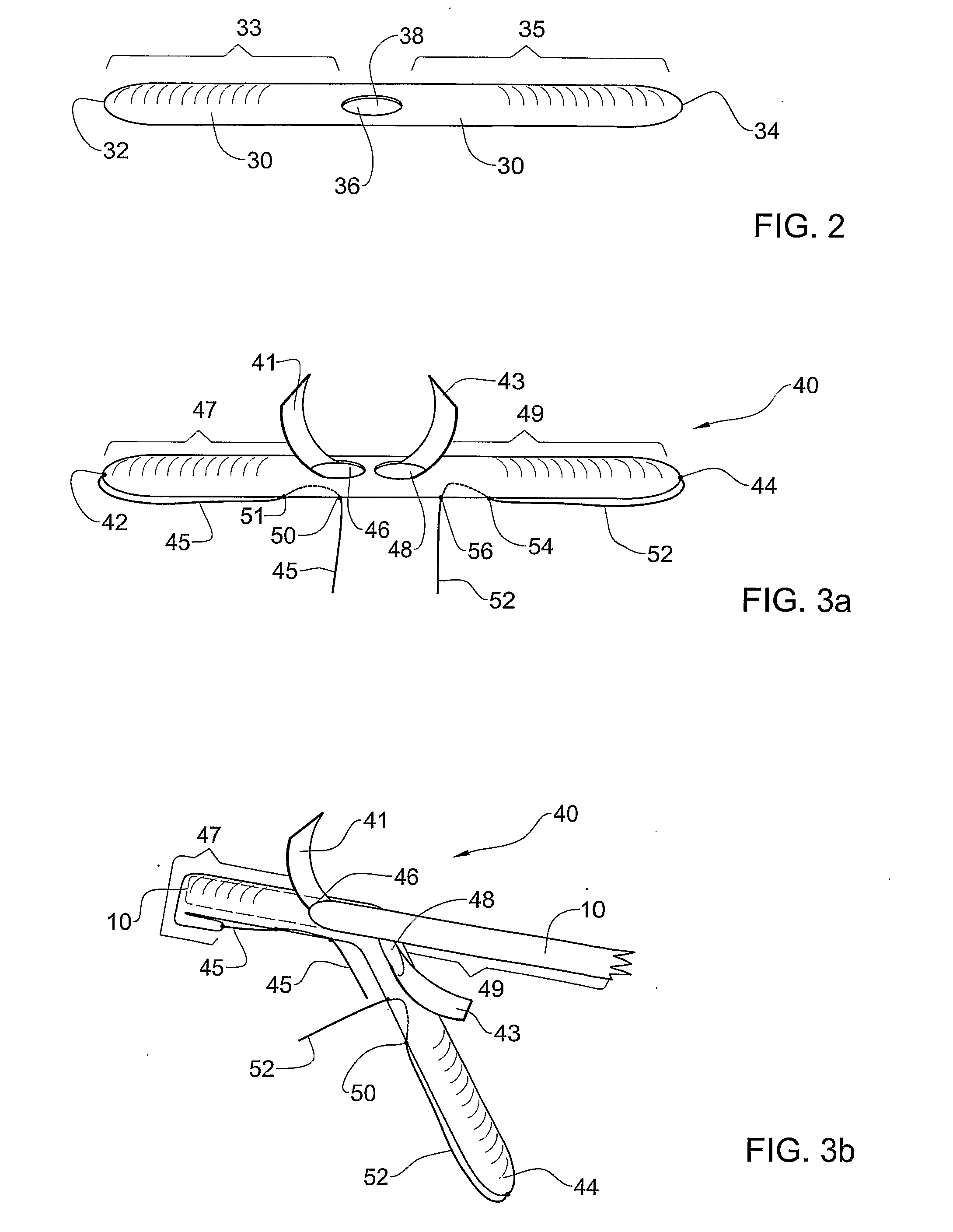
Described are embodiments of a multilaminate or multiple layer implantable surgical graft comprising remodelable collagenous sheet material, the graft including one or more interweaving members to stitch together the graft to help prevent the layers from delaminating or separating during handling and the initial stages of remodeling. The interweaving members may comprise lines of suture, thread, individual stitches, strips of material, etc. that are woven through the layers of biomaterial in a desired pattern. In one embodiment, the interweaving members comprise a pharmacologically active substance, such as a drug, growth factors, etc. to elicit a desired biological response in the host tissue. In another embodiment, the graft further comprises a reinforcing material, such as a synthetic mesh, within the layers of remodelable biomaterial and stitched together by one or more interweaving members.
Owner:COOK BIOTECH
Quilted implantable graft
Described is an embodiment of a multilaminate or multiple layers of implantable surgical graft. The graft of the embodiment comprises a remodelable collagenous sheet material, and one or more interweaving members to stitch together the graft to help prevent the layers from delaminating or separating during handling and the initial stages of remodeling. The interweaving members may comprise lines of suture, thread, individual stitches, strips of material, etc. that are woven through the layers of biomaterial in a desired pattern. In one embodiment, the interweaving members comprise a pharmacologically active substance, such as a drug, growth factors, etc. to elicit a desired biological response in the host tissue. In another embodiment, the graft further comprises a reinforcing material, such as a synthetic mesh, within the layers of remodelable biomaterial and stitched together by one or more interweaving members.
Owner:COOK BIOTECH
Metallic implantable grafts and method of making same
ActiveUS8313523B2Promote endothelializationGive flexibilityStentsSurgerySurgical GraftMetallic materials
Implantable medical grafts fabricated of metallic or pseudometallic films of biocompatible materials having a plurality of microperforations passing through the film in a pattern that imparts fabric-like qualities to the graft or permits the geometric deformation of the graft. The implantable graft is preferably fabricated by vacuum deposition of metallic and / or pseudometallic materials into either single or multi-layered structures with the plurality of microperforations either being formed during deposition or after deposition by selective removal of sections of the deposited film. The implantable medical grafts are suitable for use as endoluminal or surgical grafts and may be used as vascular grafts, stent-grafts, skin grafts, shunts, bone grafts, surgical patches, non-vascular conduits, valvular leaflets, filters, occlusion membranes, artificial sphincters, tendons and ligaments.
Owner:VACTRONIX SCI LLC
Targeted delivery of therapeutic agents with lyophilized matrices
InactiveUS20120093801A1Healing time constantPromote healingBiocideOrganic active ingredientsSurgical GraftTissue repair
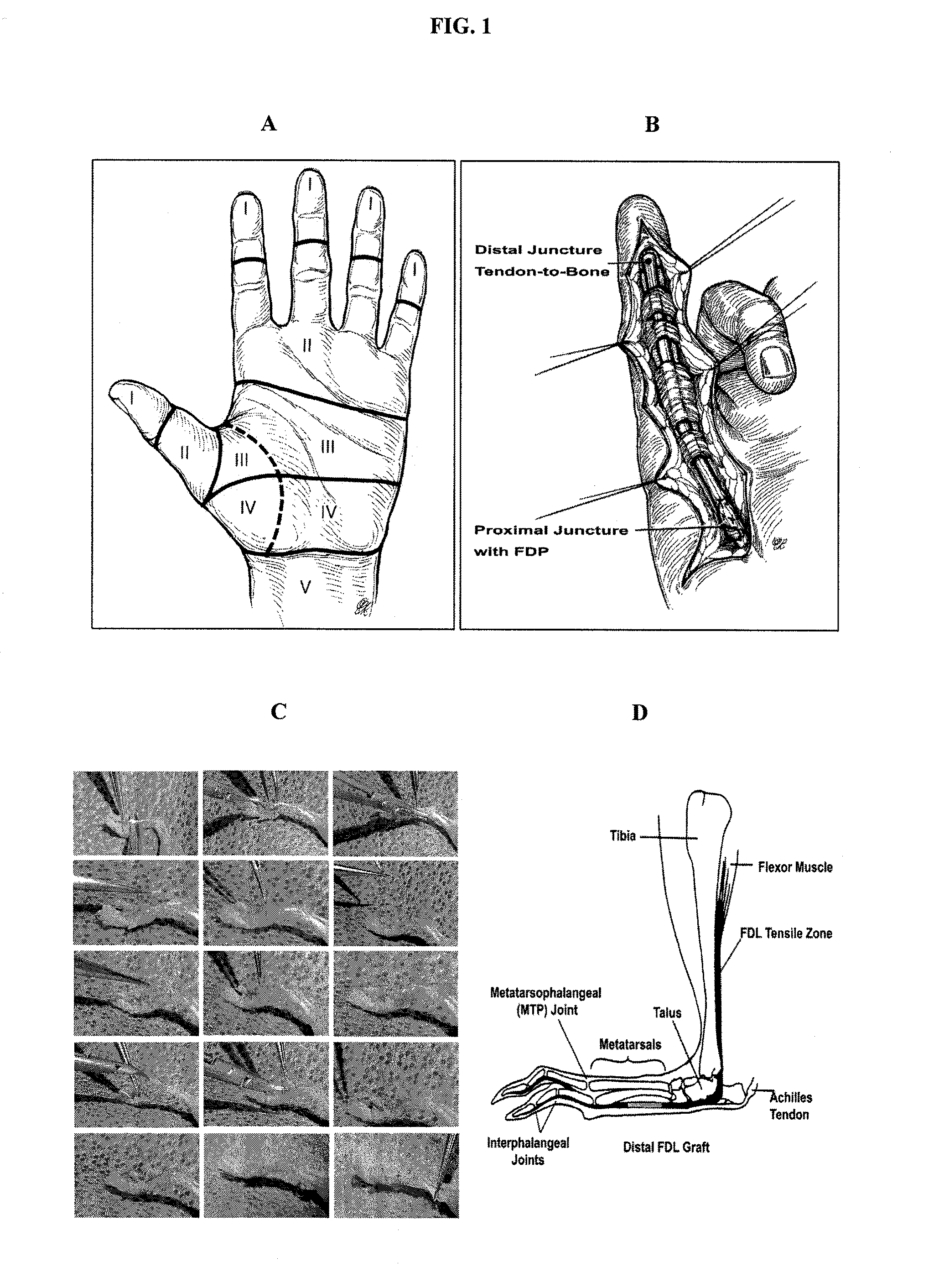
Embodiments of surgical grafts, and methods, for the delivery of therapeutic agents to a target tissue via acellular matrices, are described. In some embodiments, nonviable matrices are successful in preventing or lessening adhesion formation by guiding tissue repair and remodeling, while also providing the target tissue with therapeutic agents that can act as repair and remodeling factors. An exemplary method to modulate flexor tendon healing and provide elimination or reduction of fibrotic adhesions involves loading a freeze-dried flexor digitorum longus allograft with recombinant adeno-associated viral (rAAV) vectors for the targeted and transient expression of growth / differentiation factor 5 (GDF5).
Owner:UNIVERSITY OF ROCHESTER
Protective Box for Surgery
InactiveUS20160346055A1Contamination damageDamage to materialSurgical furniturePackaging protectionSurgical GraftSTERILE FIELD
The present invention is directed to providing a sterile container protecting various surgical grafts, implants and devices from incidental contamination and damage during operative procedures. The design includes an inner box to contain the items, and an outer box to protect the exterior of the inner box from contamination in the event of fall or other contamination, such as from airborne particles or liquids, fall from the sterile field, or by non-sterile handling. In the event of fall or contamination of the outer box, the surgical staff will be able to recover the inner box and its contents in sterile and unspoiled condition without difficulty.
Owner:JACOBSON DANIEL R
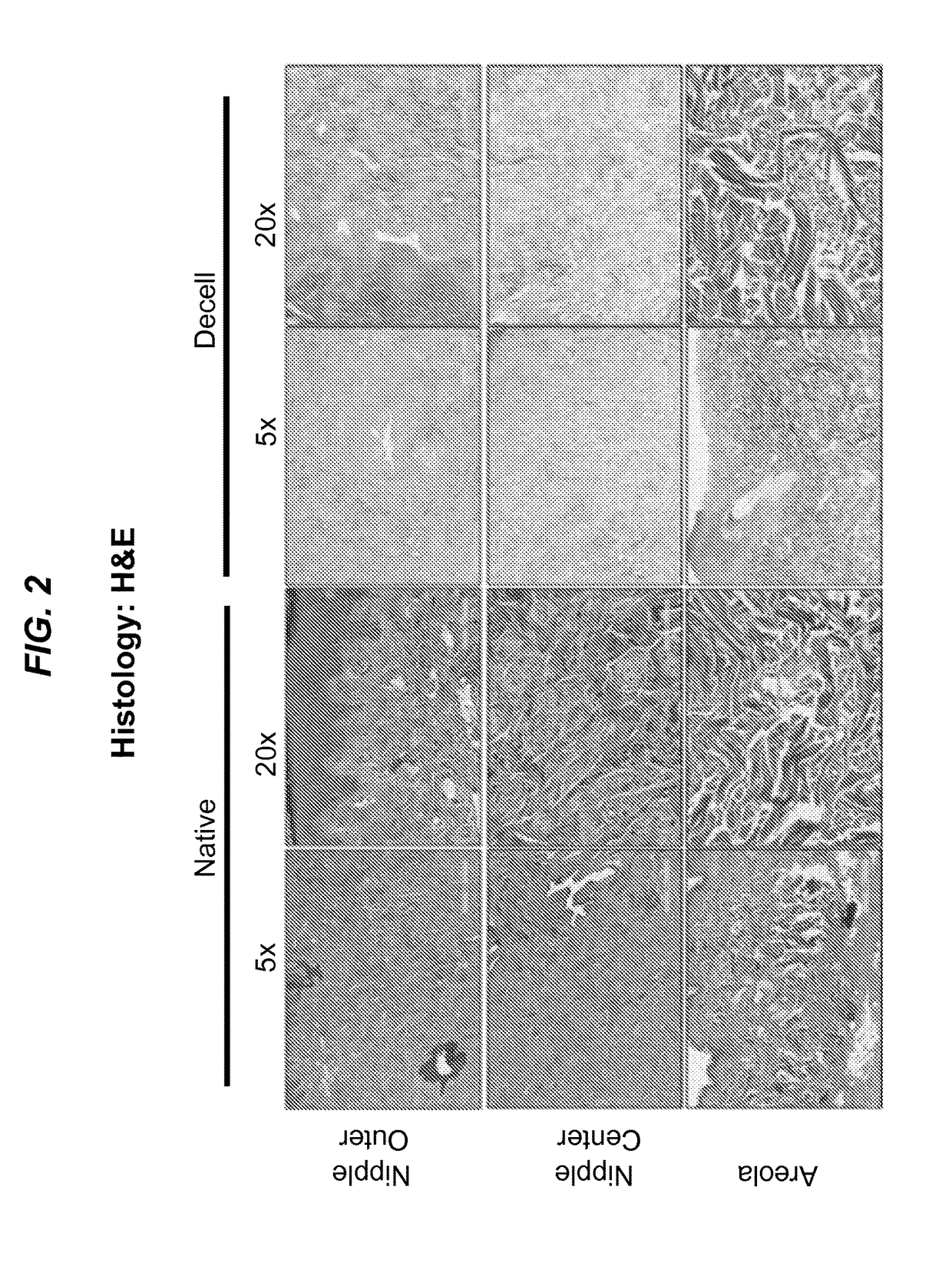
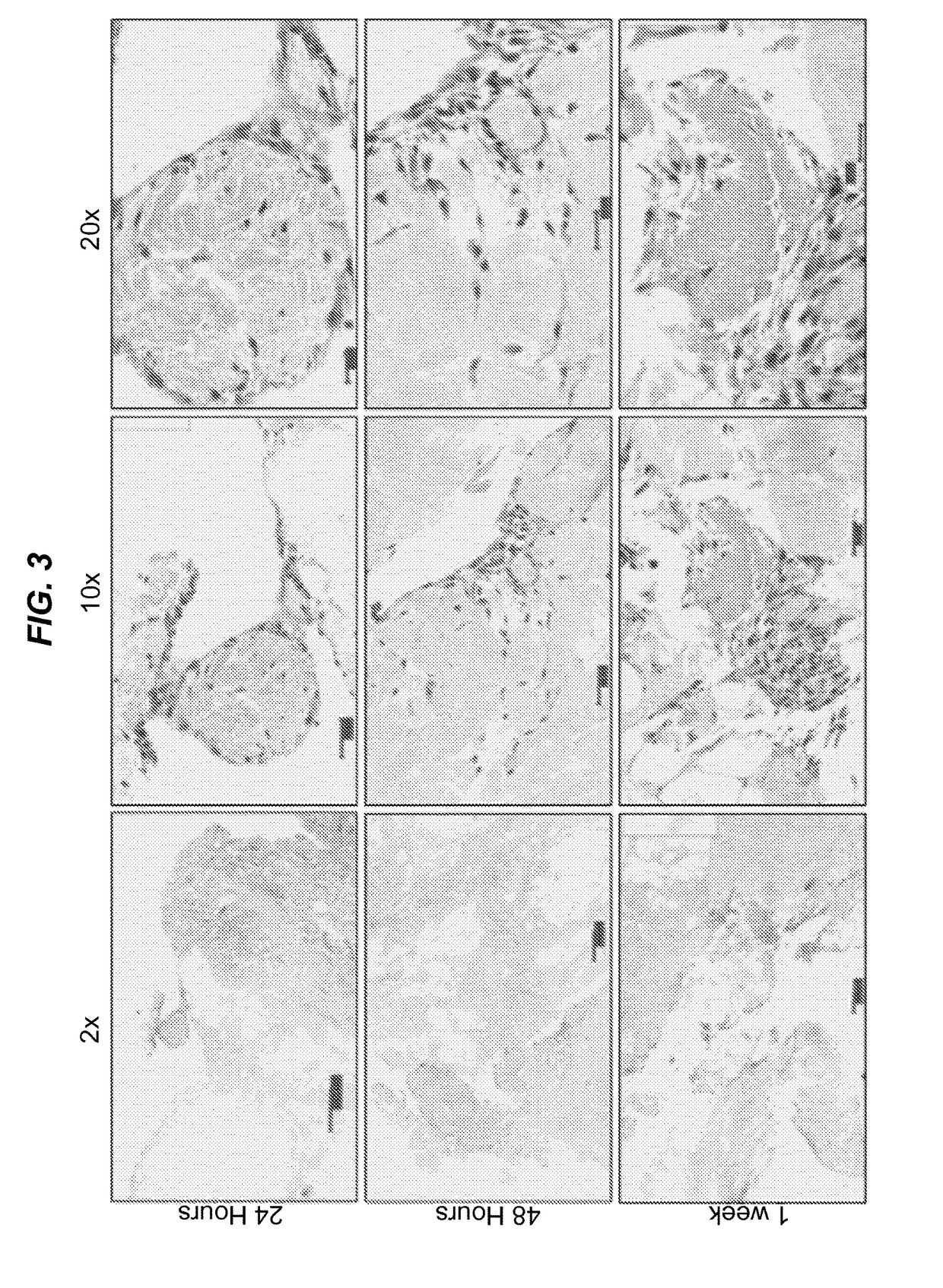
Surgical grafts for replacing the nipple and areola or damaged epidermis
ActiveUS20180015204A1Decellularizing epidermisDecellularize epidermisMammary implantsCosmetic implantsSurgical GraftInjury mouth
The present disclosure relates to surgical grafts for replacing nipples and areolas lost to disease or trauma with surgical grafts of decellularized donor nipples and areolas and to placing and recellularizing such grafts. The disclosure further provides methods for decellularizing epidermis. The decellularized epidermis can be used as a protective cover for skin wounds.
Owner:TULANE EDUCATIONAL FUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com