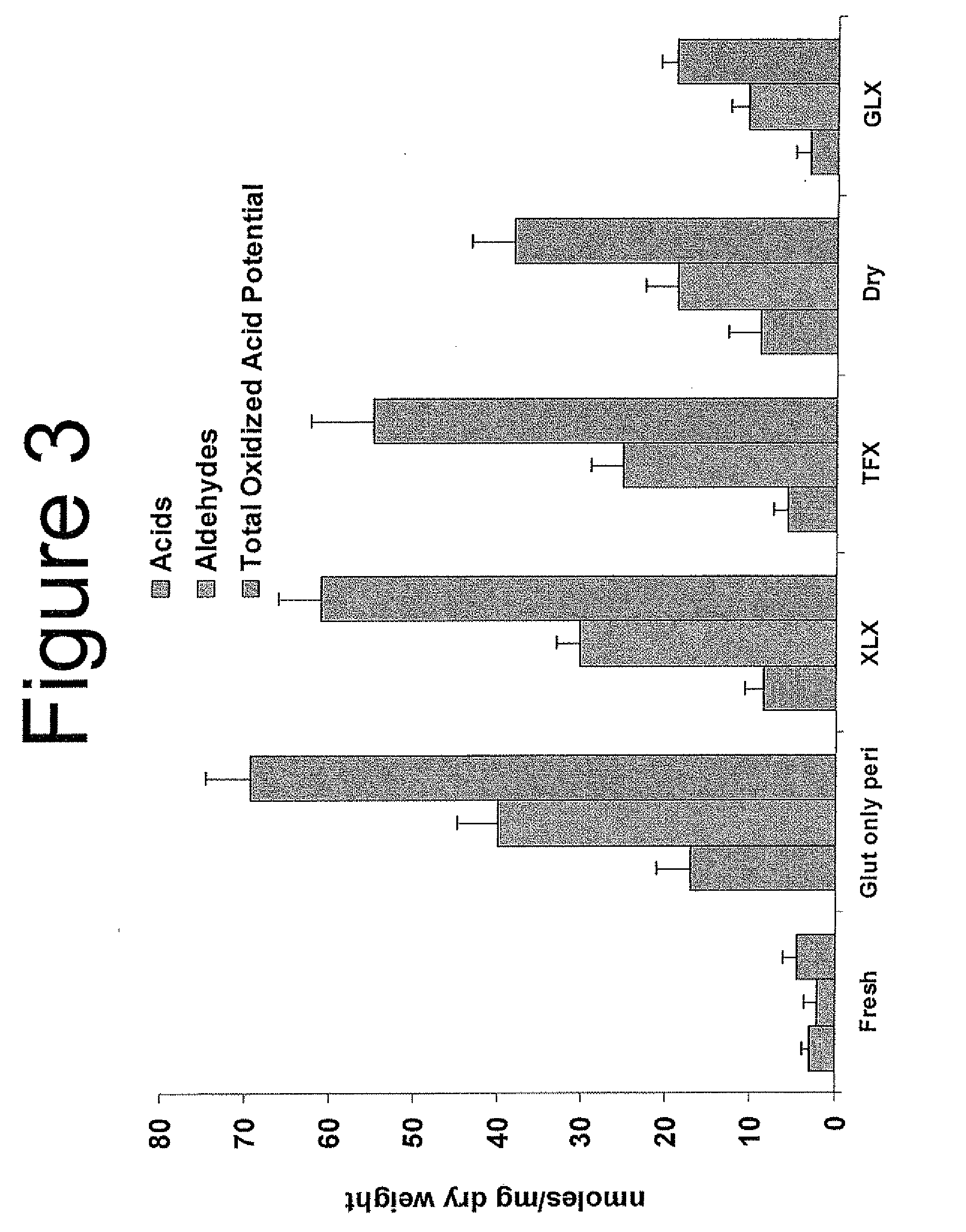
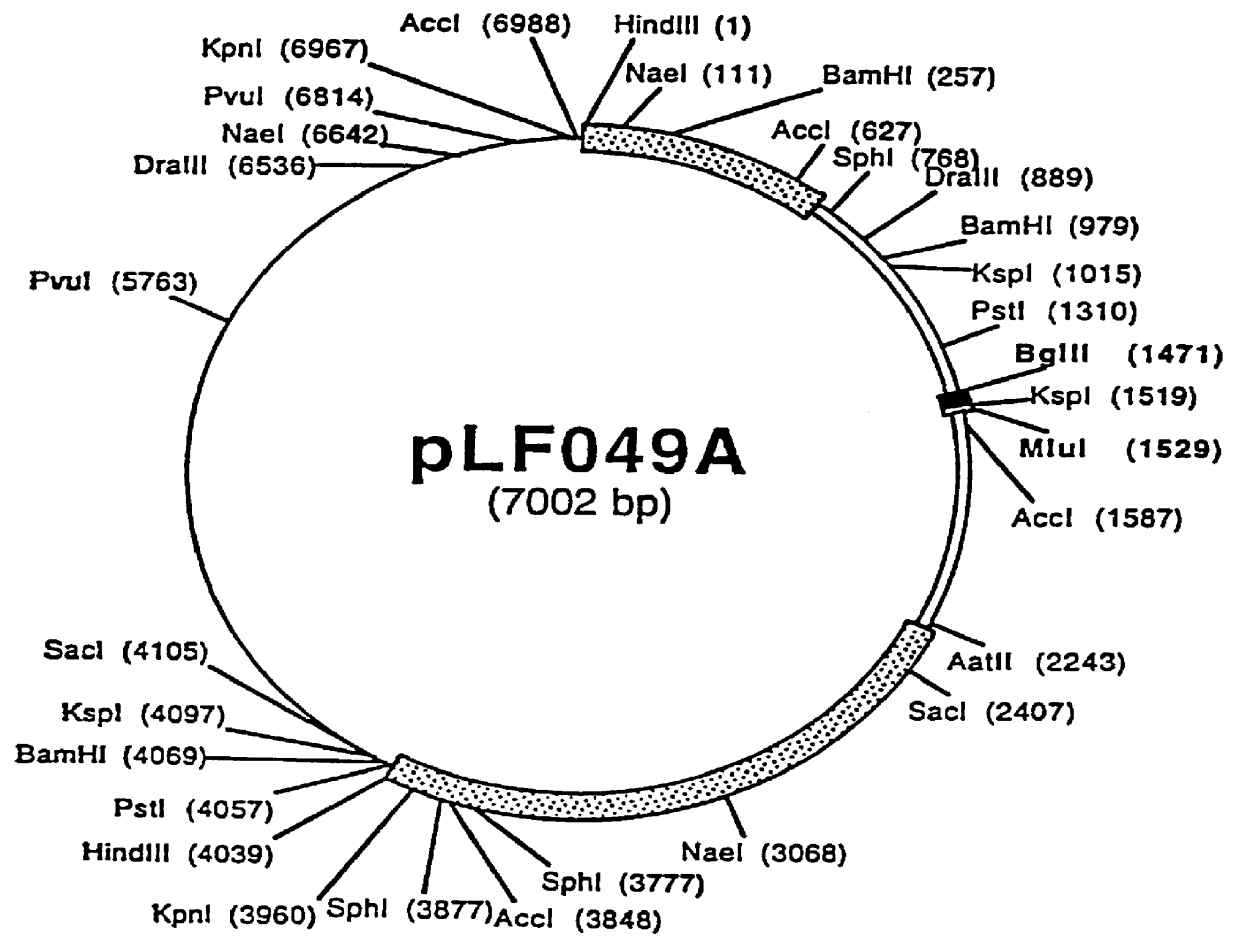
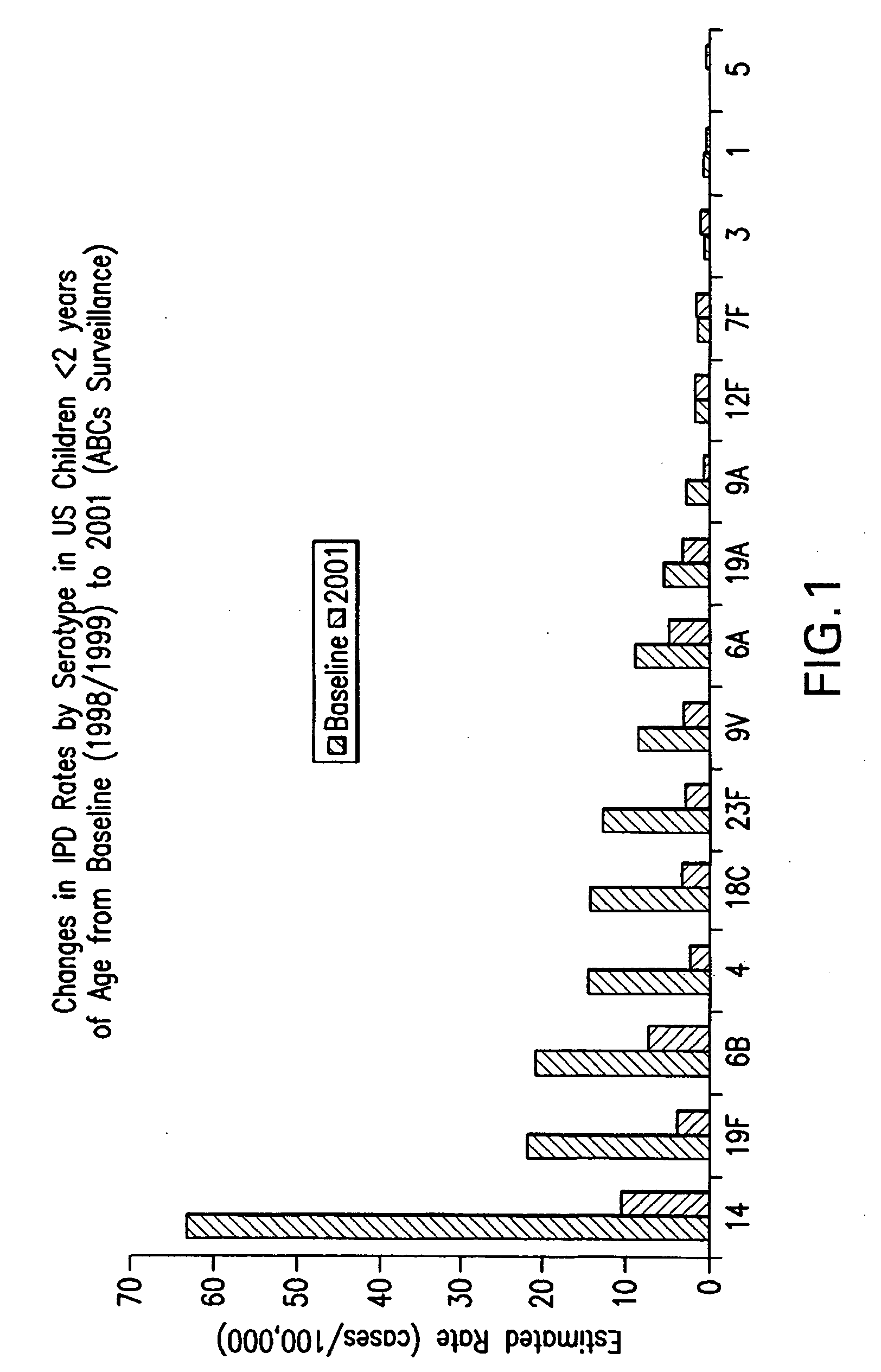
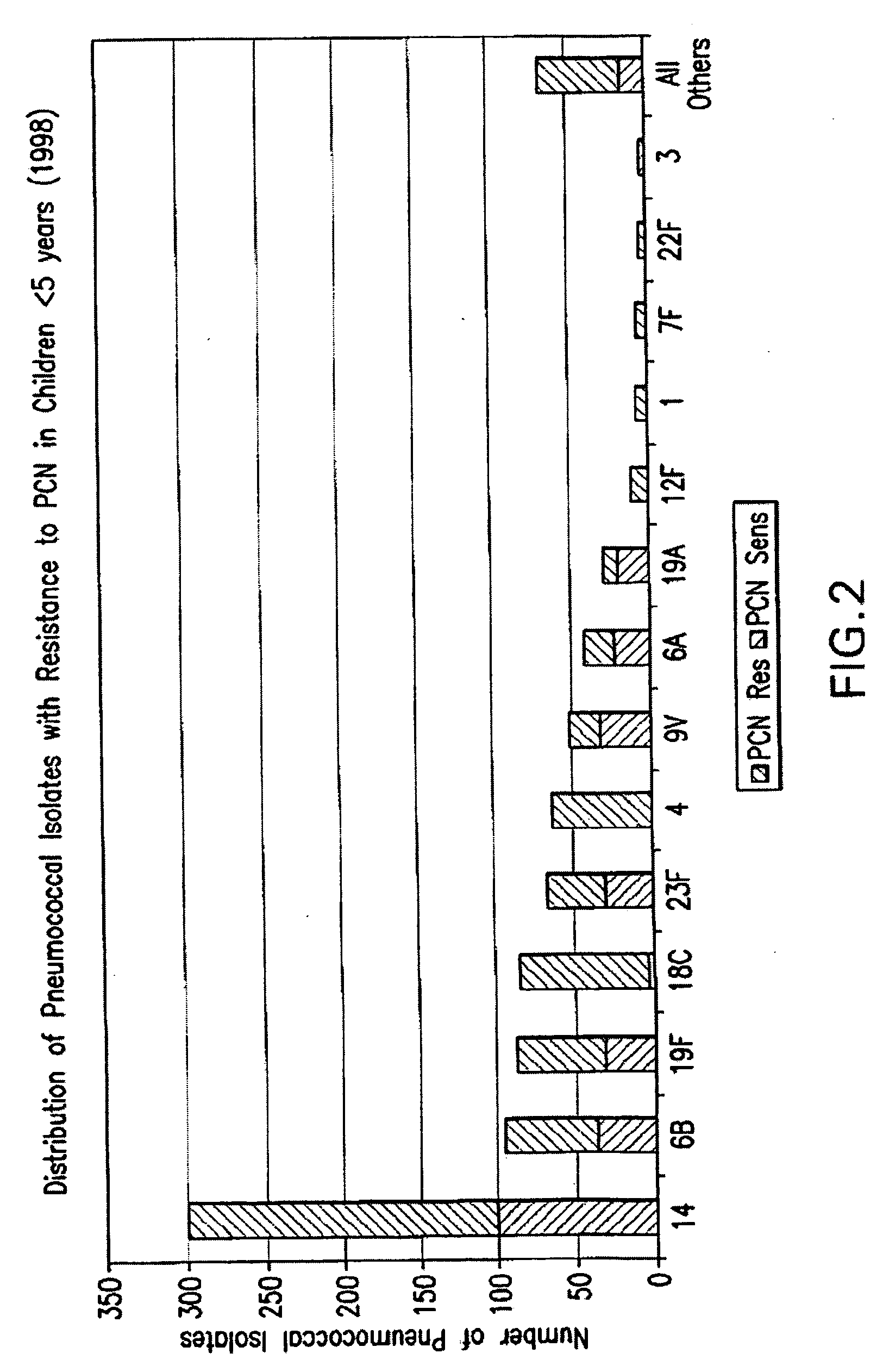
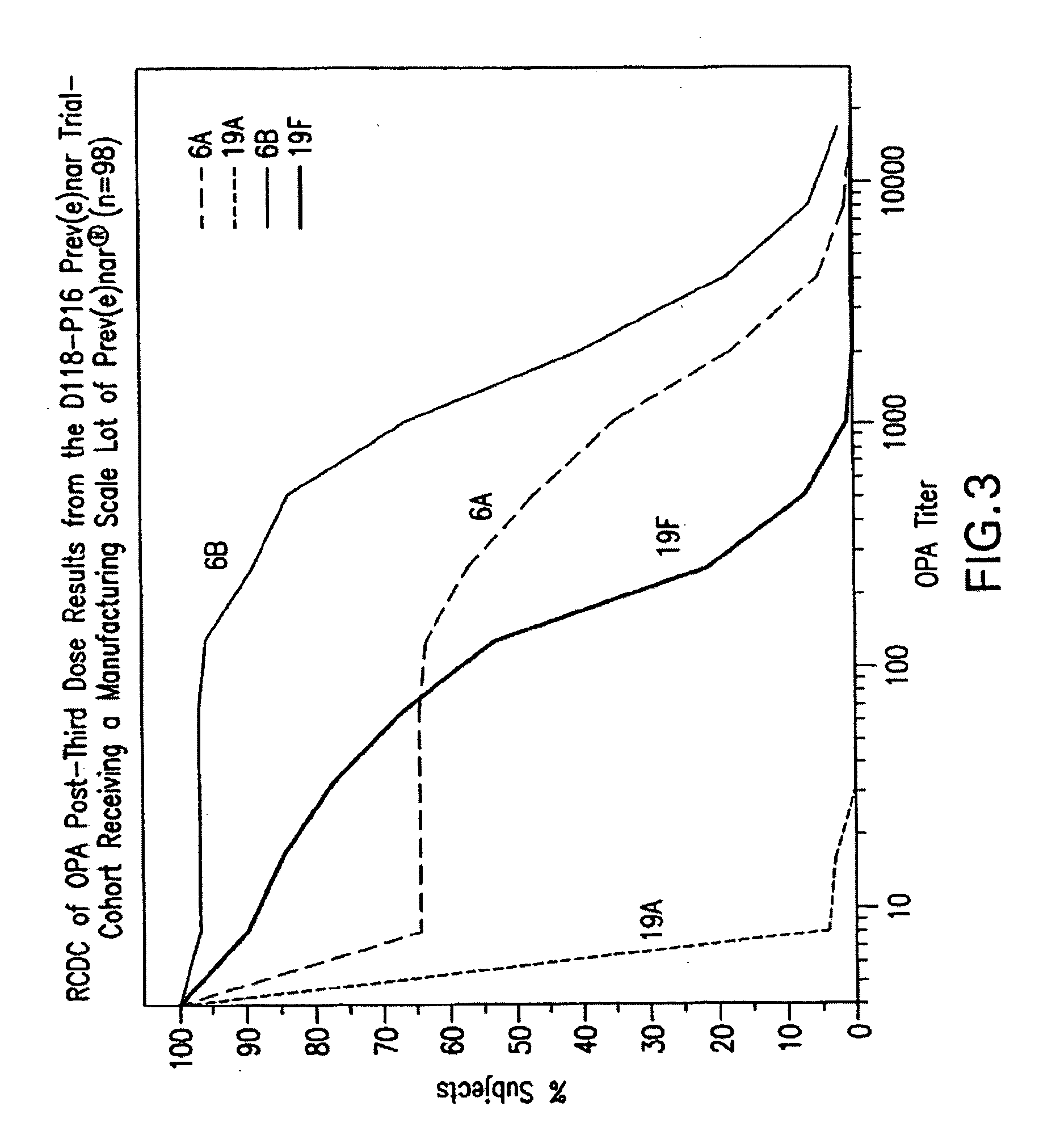
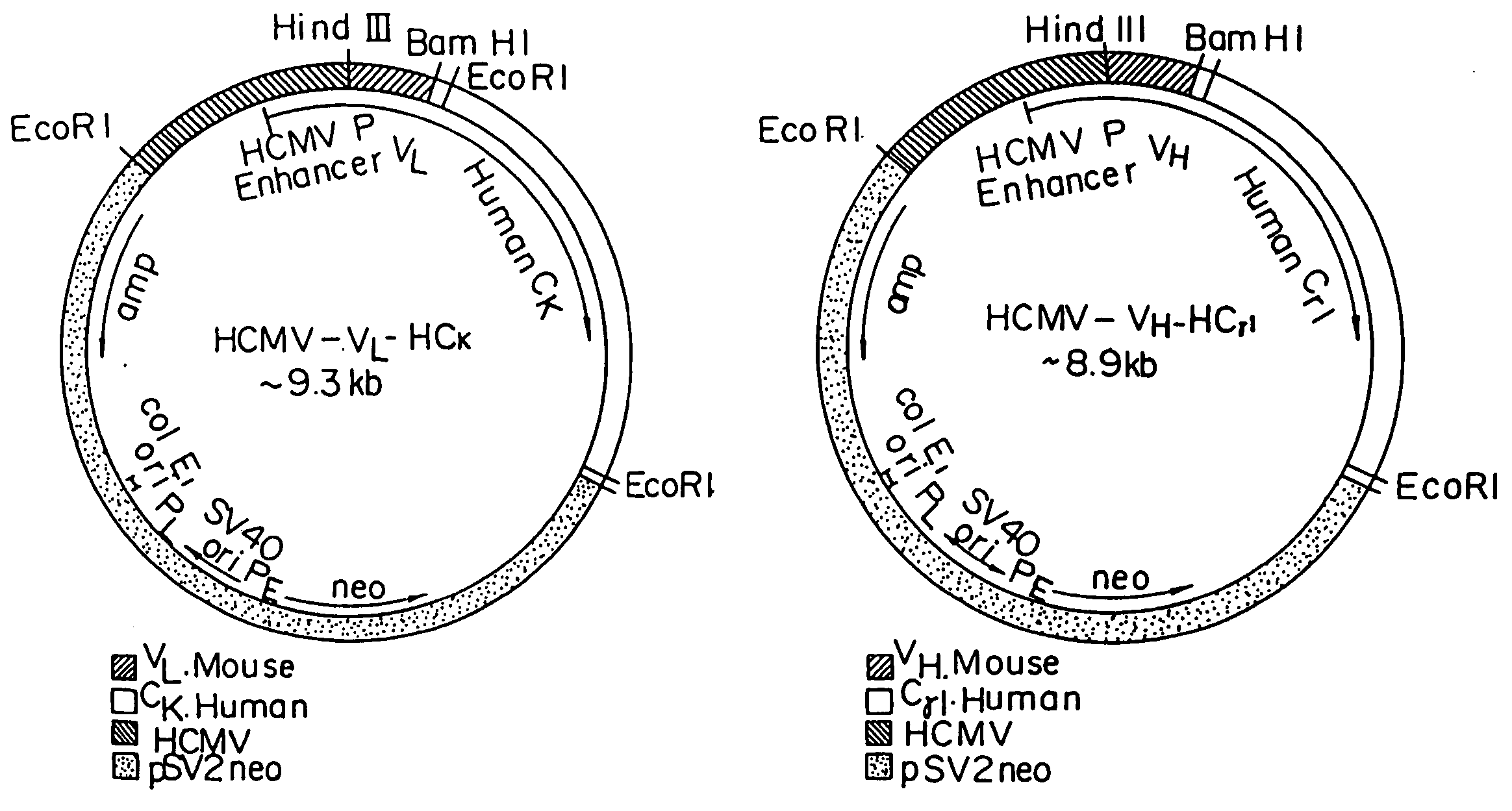
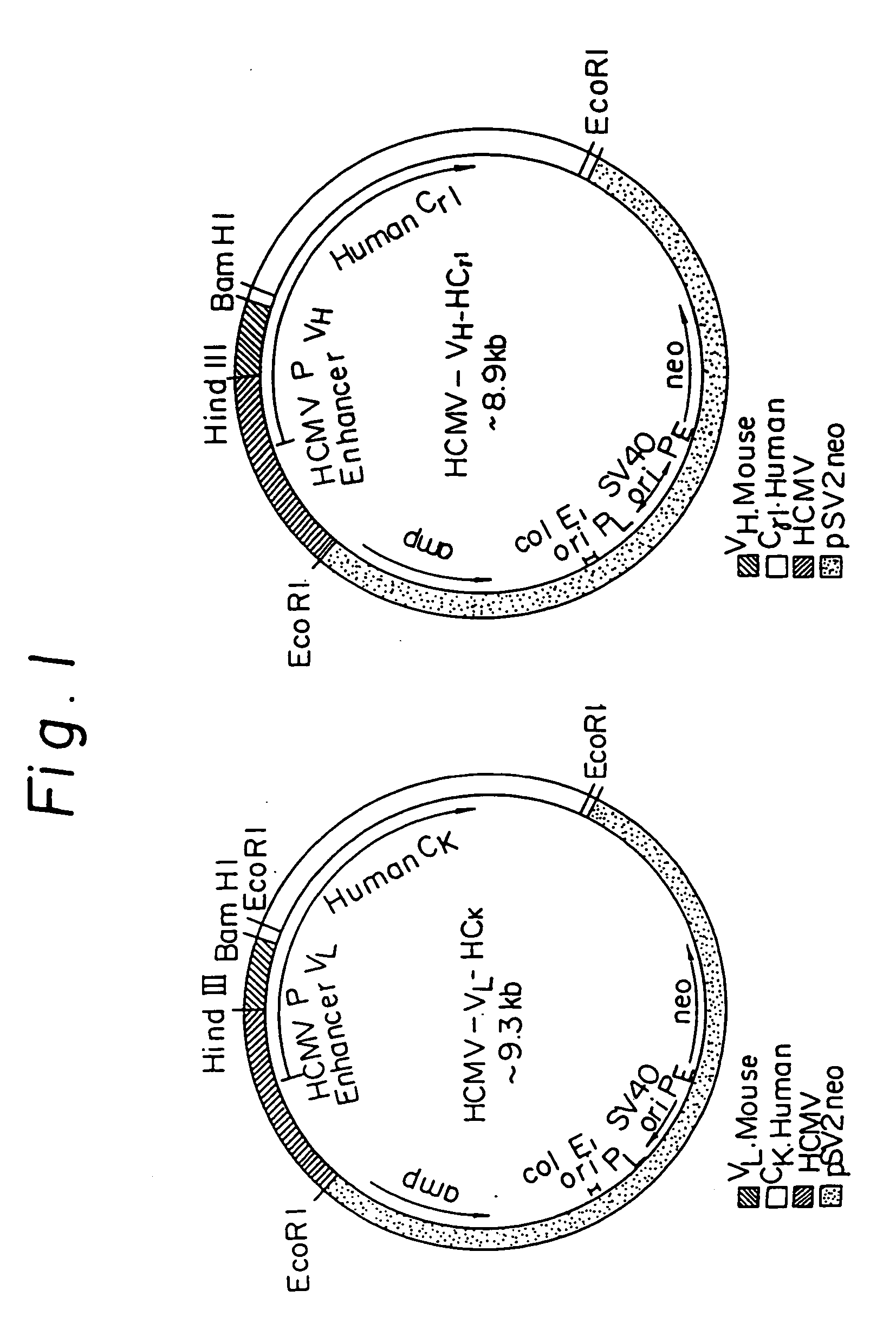
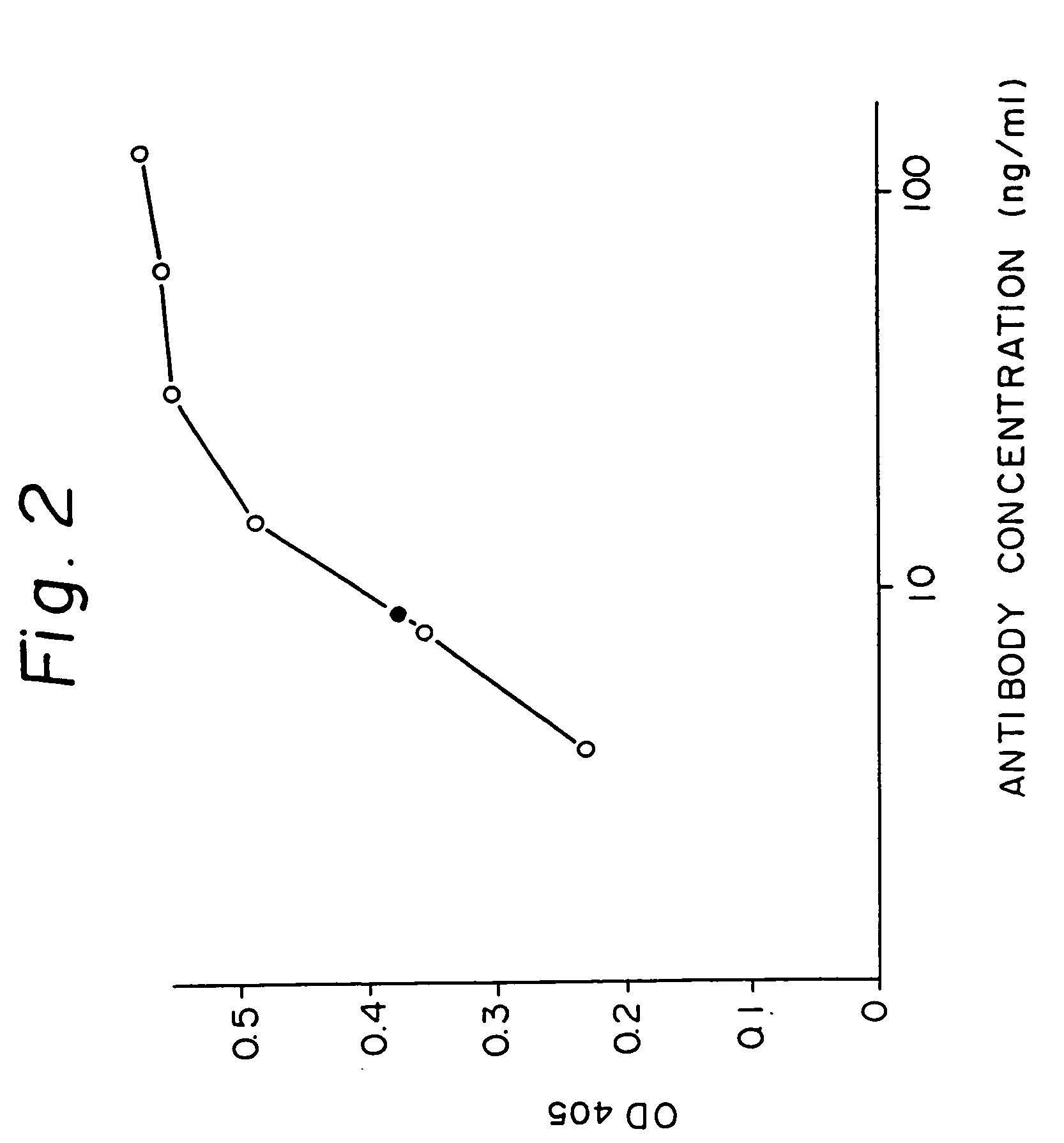
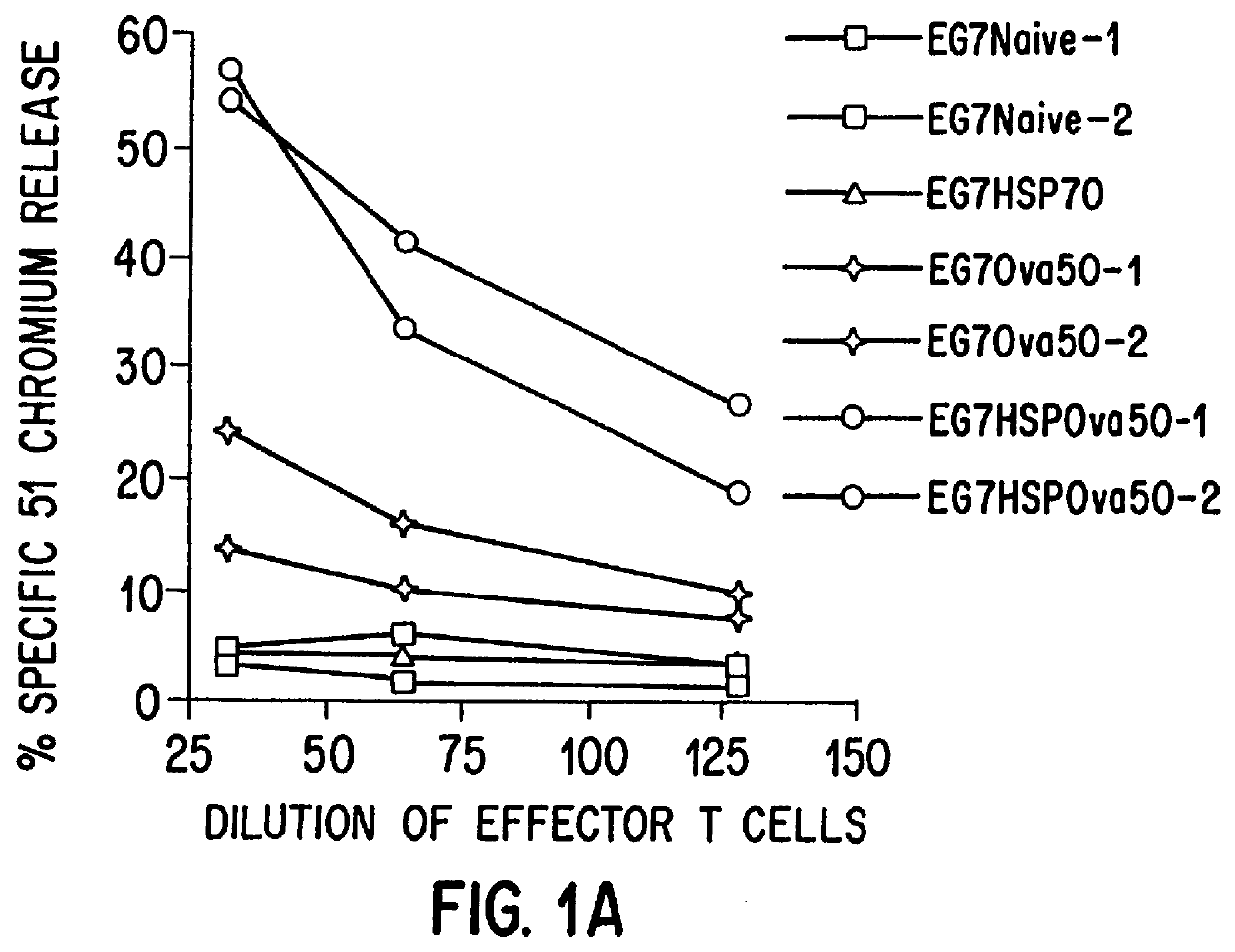
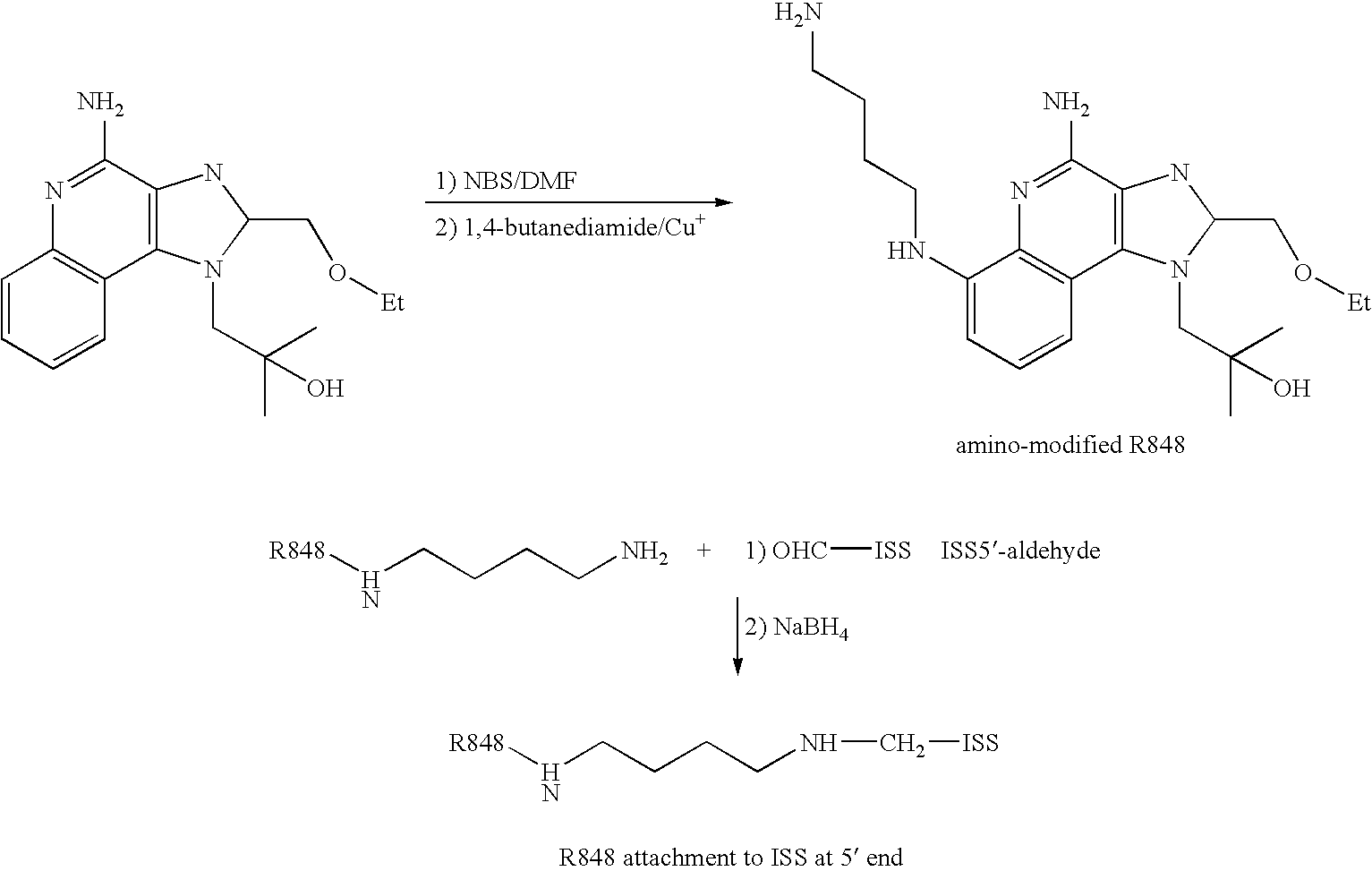
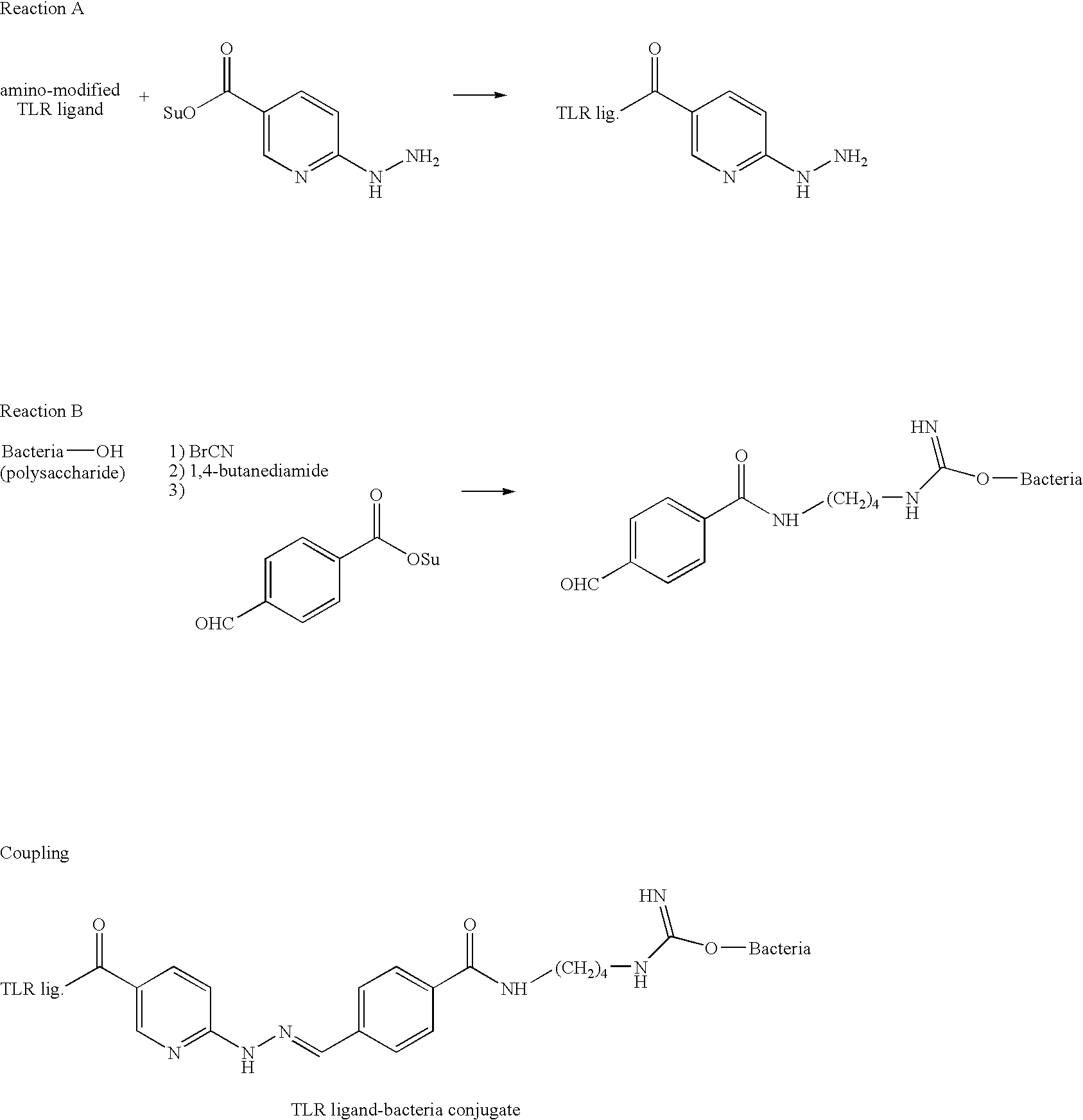
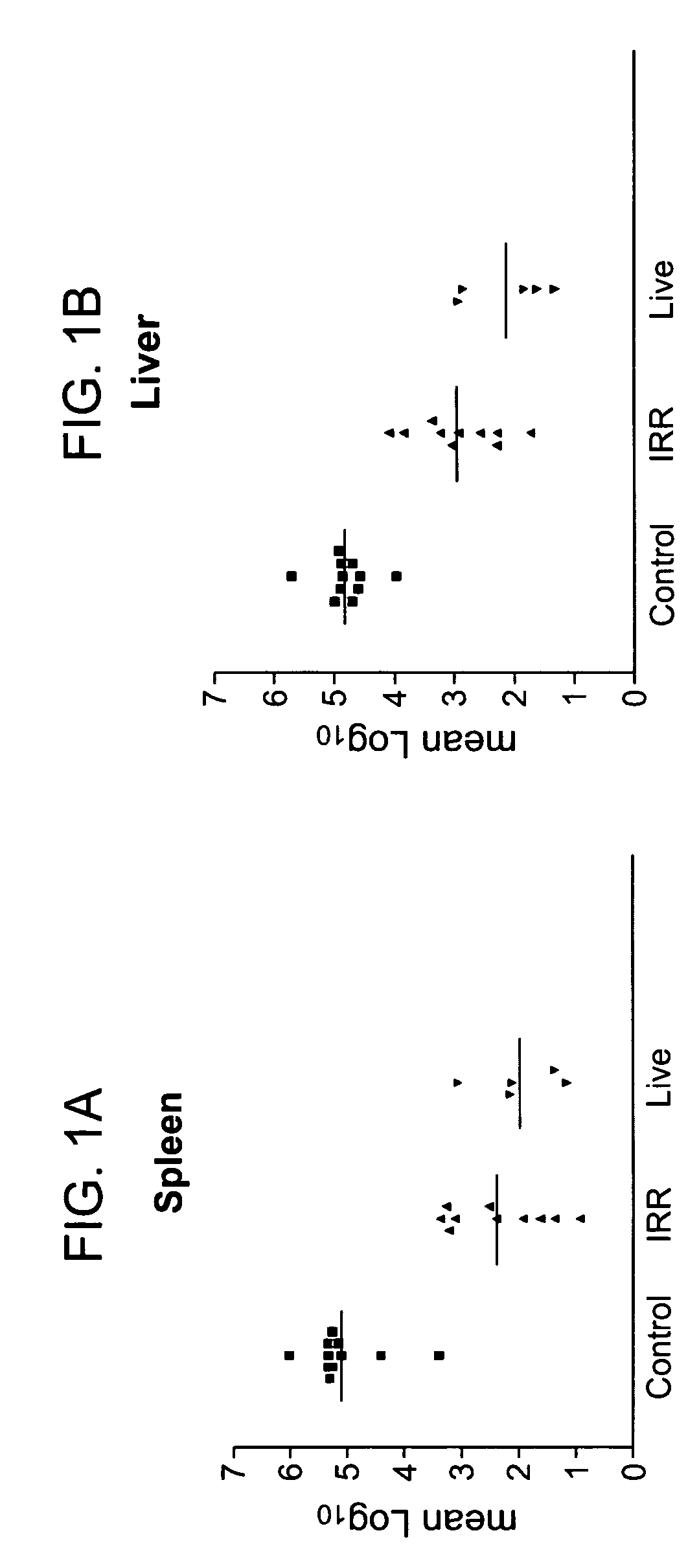
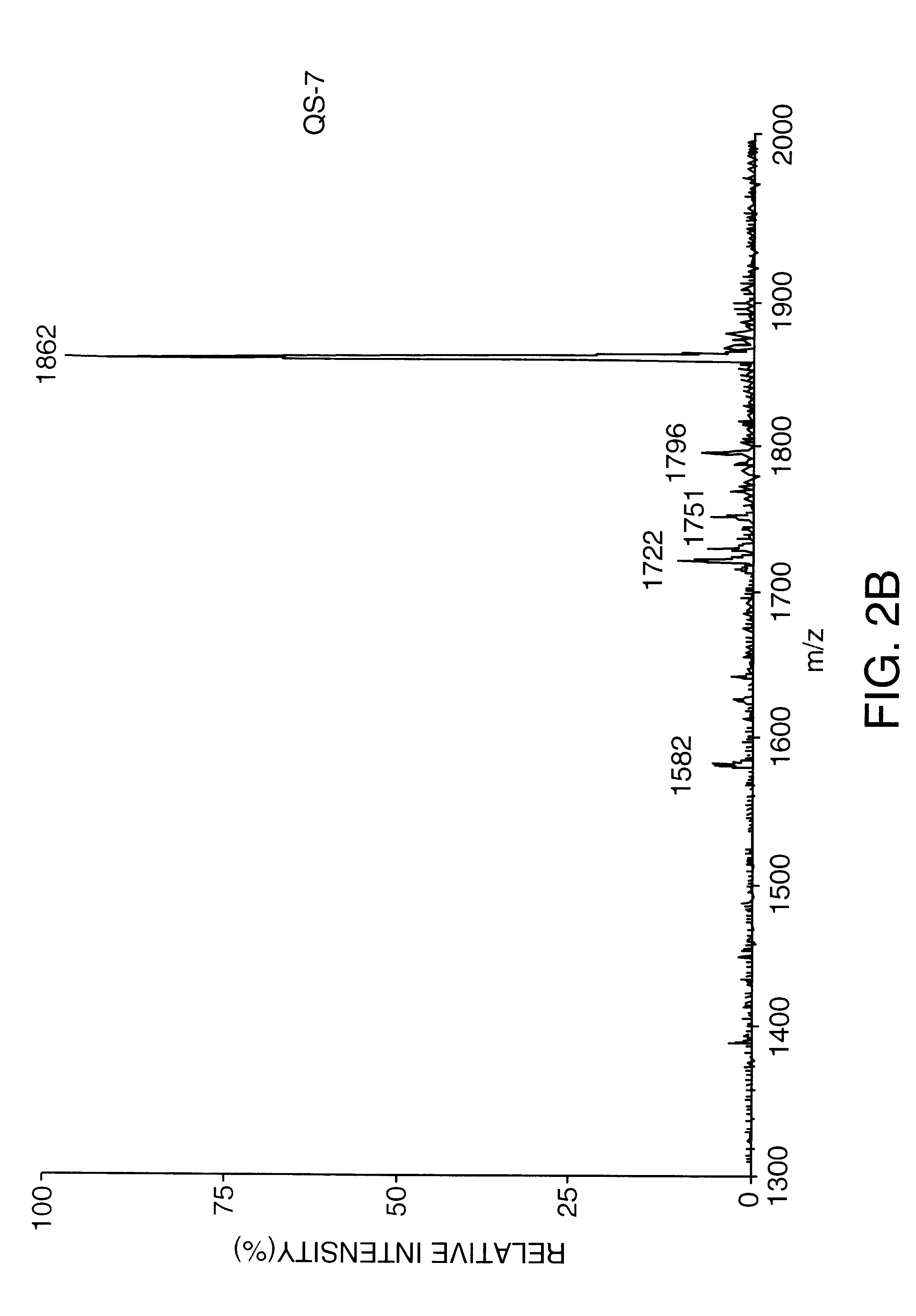
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
7501 results about "Immunogenicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunogenicity is the ability of a particular substance, such as an antigen or epitope, to provoke an immune response in the body of a human and other animal. In other words, immunogenicity is the ability to induce a humoral and/or cell-mediated immune responses.
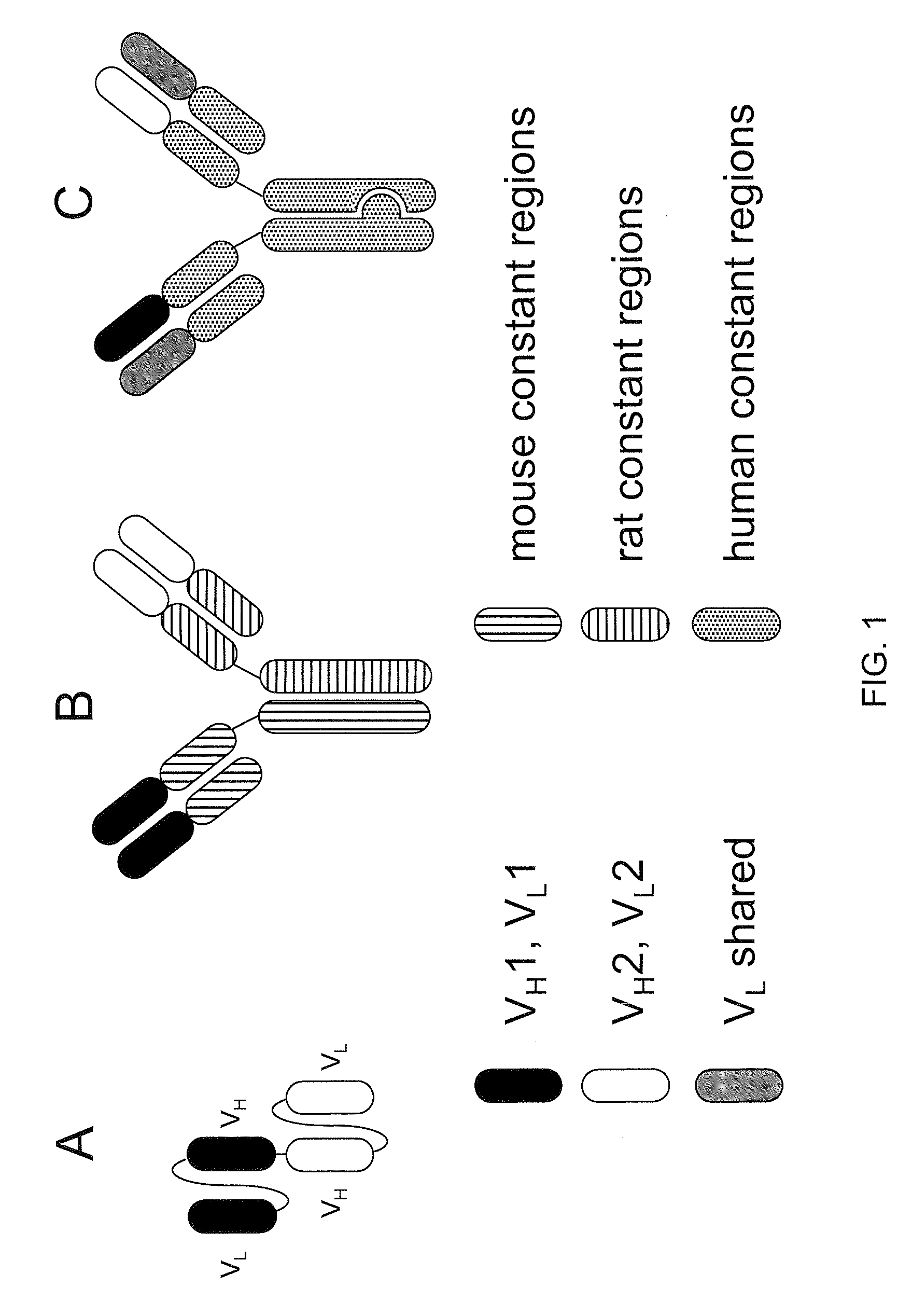
Readily Isolated Bispecific Antibodies with Native Immunoglobulin Format
ActiveUS20100331527A1Improve abilitiesReduces and eliminates bindingHybrid immunoglobulinsSerum immunoglobulinsImmunoglobulin heavy chainHeavy chain
A bispecific antibody format providing ease of isolation is provided, comprising immunoglobulin heavy chain variable domains that are differentially modified in the CH3 domain, wherein the differential modifications are non-immunogenic or substantially non-immunogenic with respect to the CH3 modifications, and at least one of the modifications results in a differential affinity for the bispecific antibody for an affinity reagent such as Protein A, and the bispecific antibody is isolable from a disrupted cell, from medium, or from a mixture of antibodies based on its affinity for Protein A.
Owner:REGENERON PHARM INC
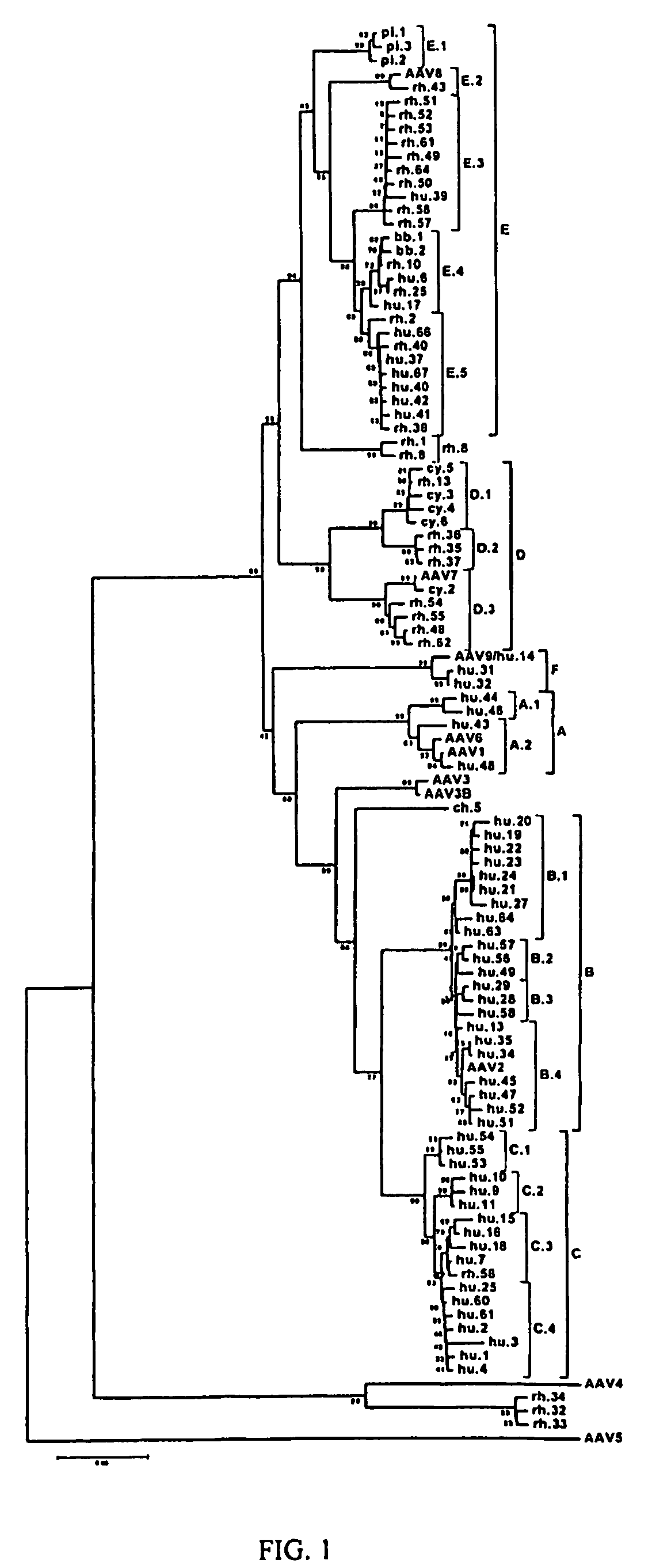
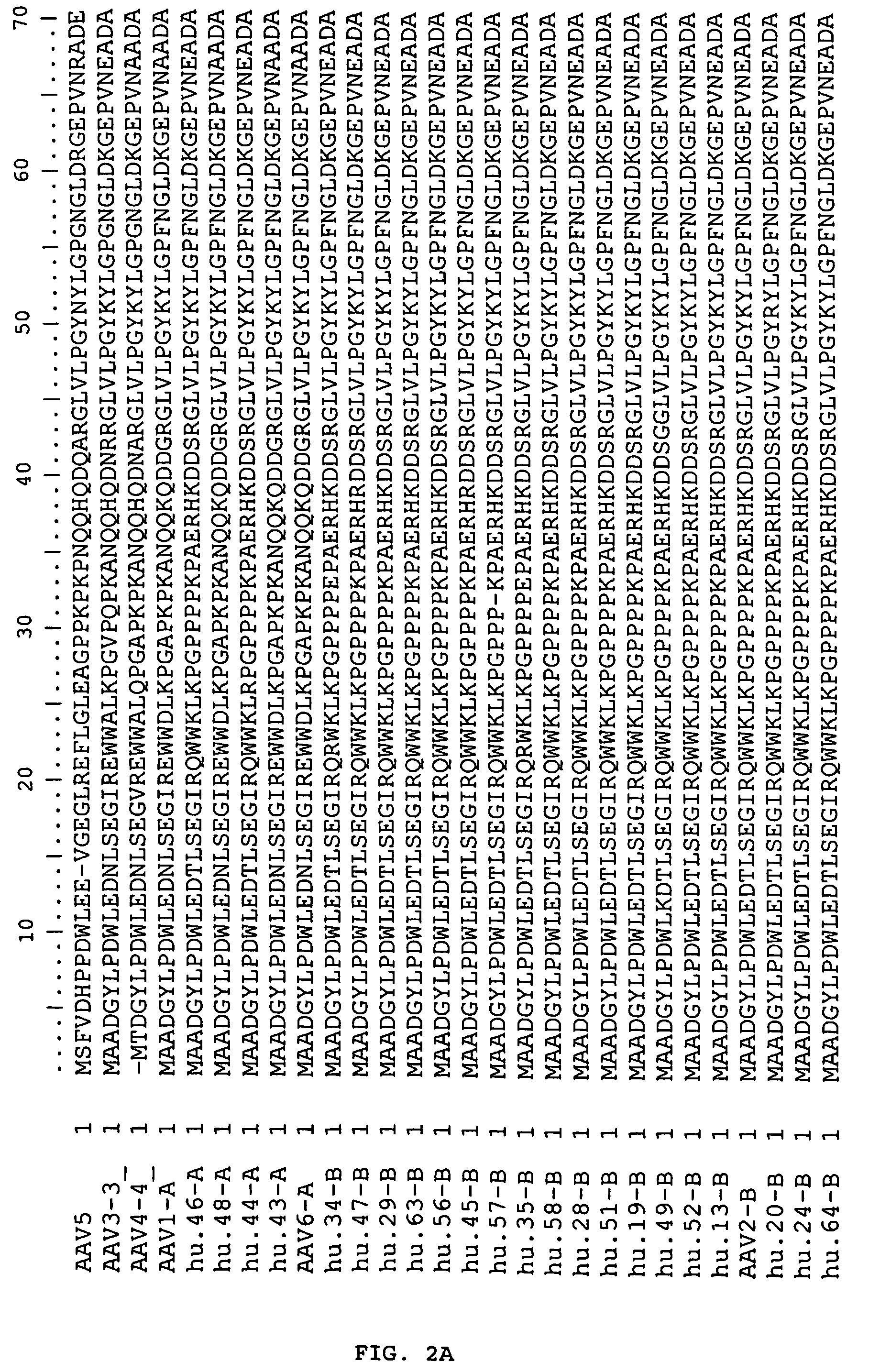
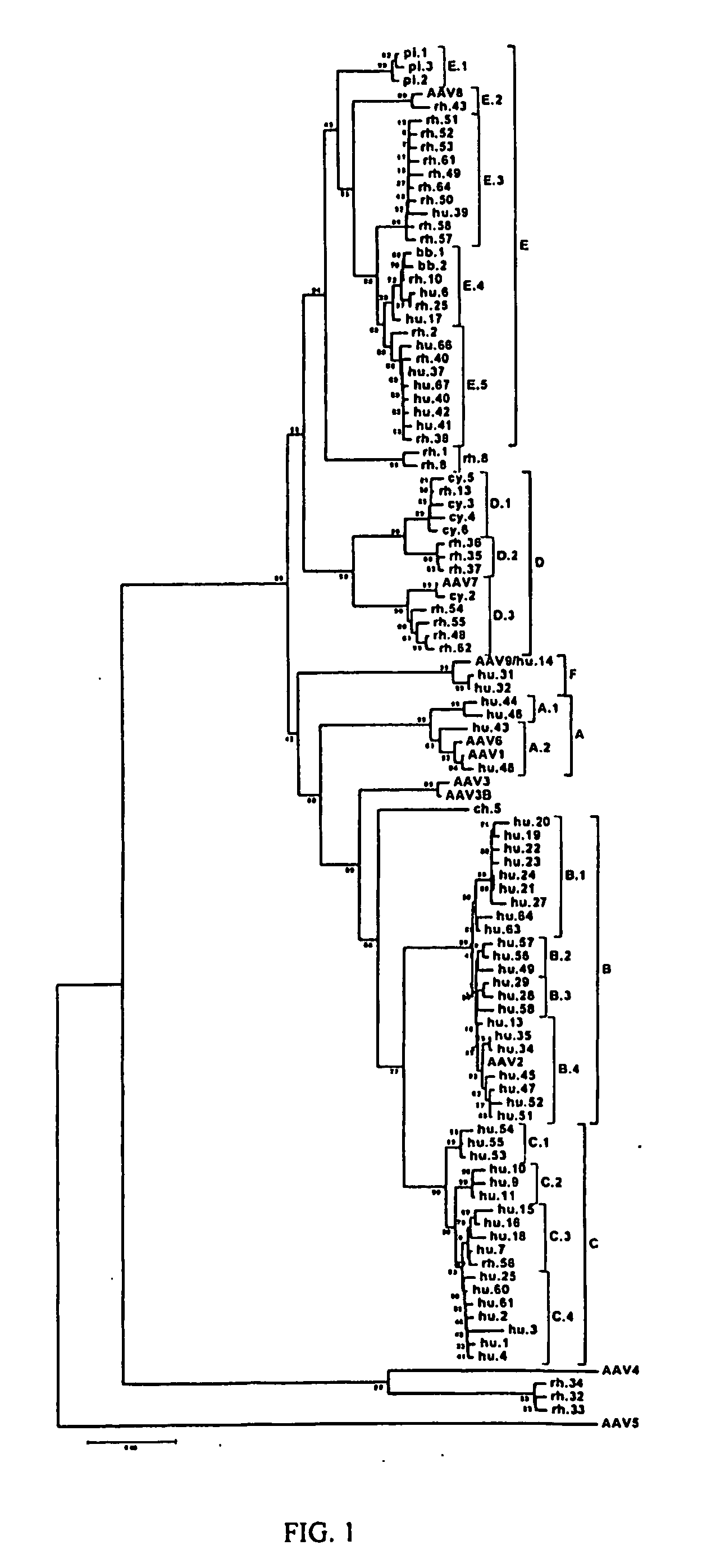
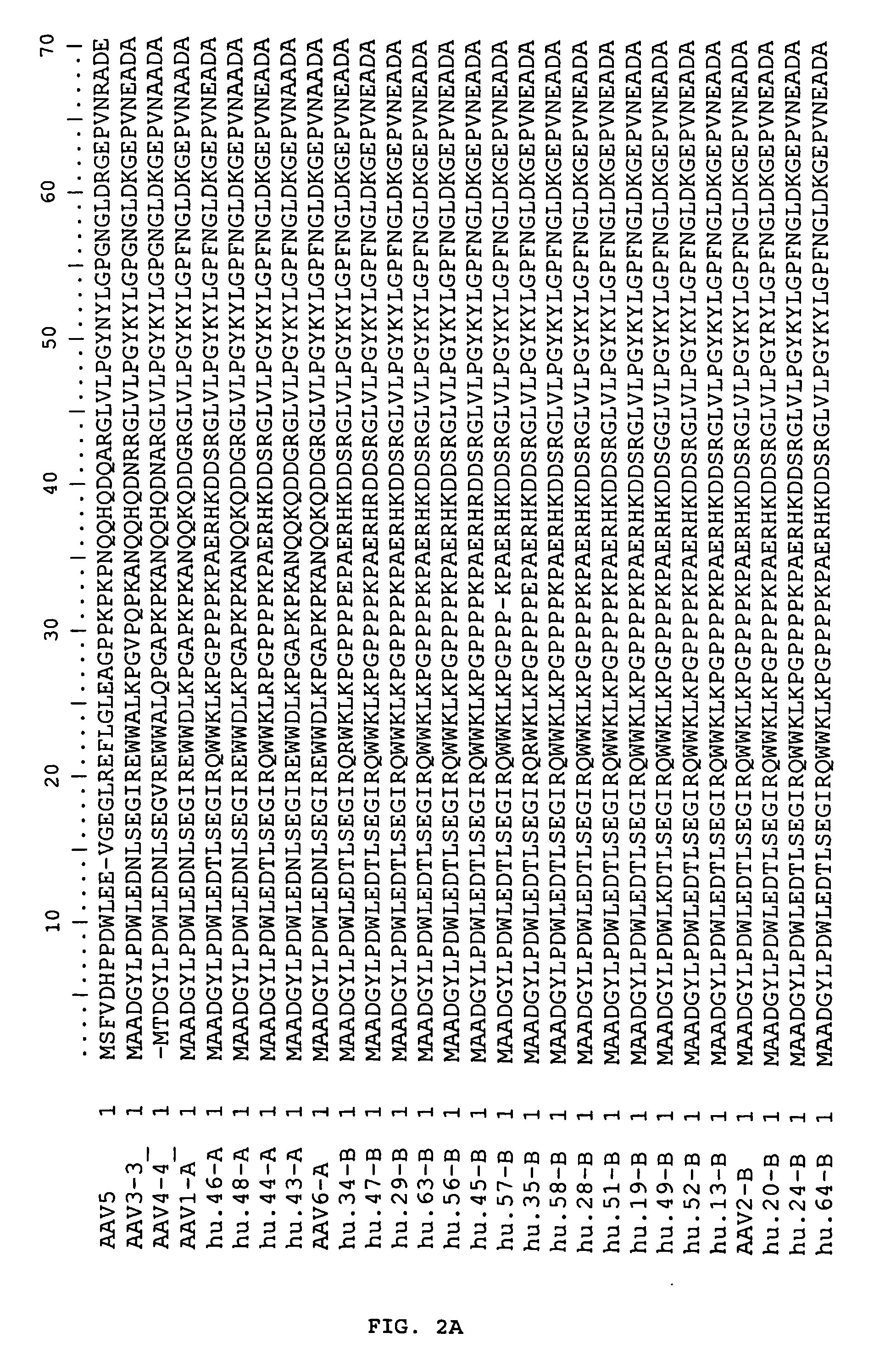
Adeno-associated virus (AAV) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
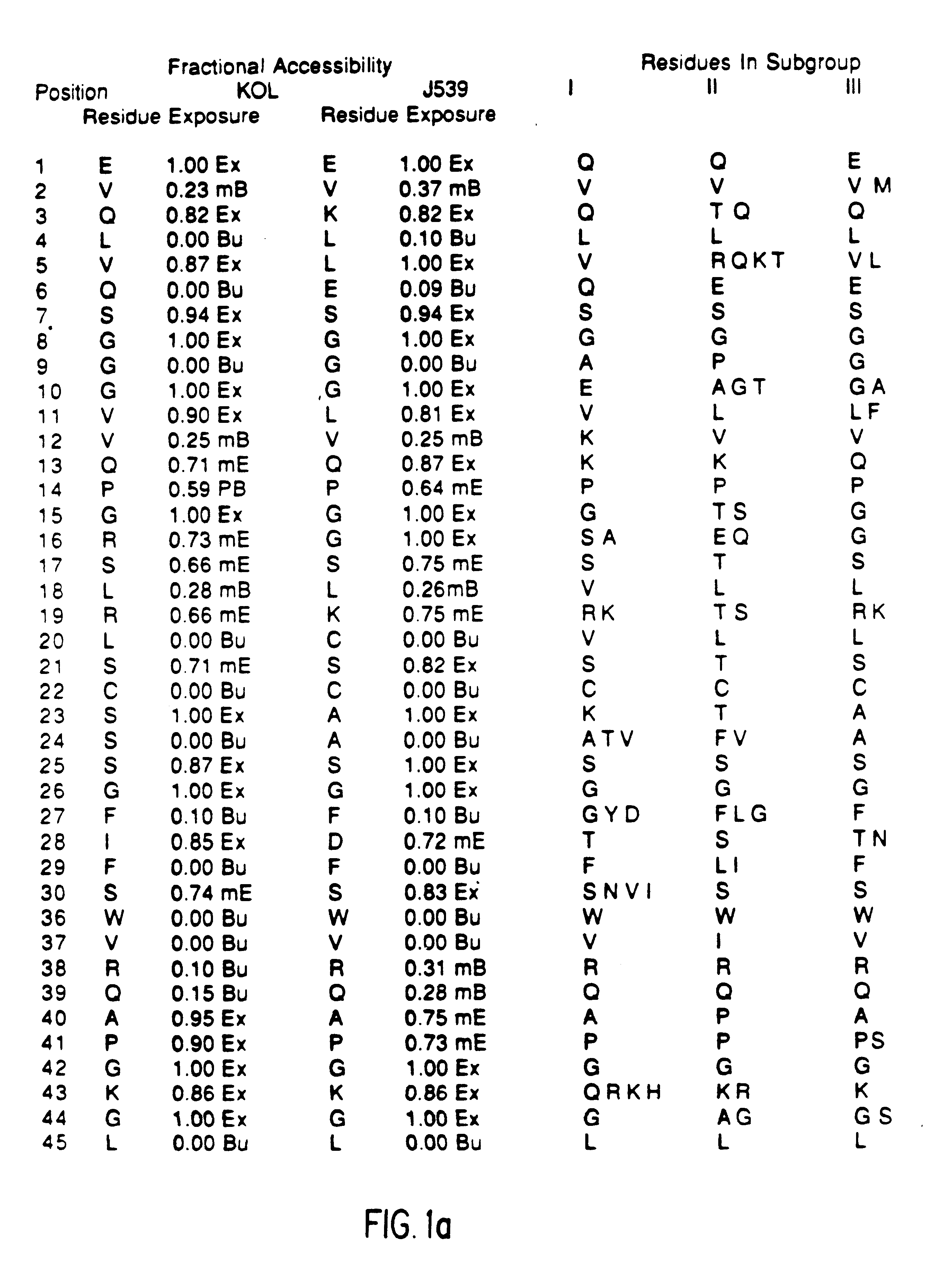
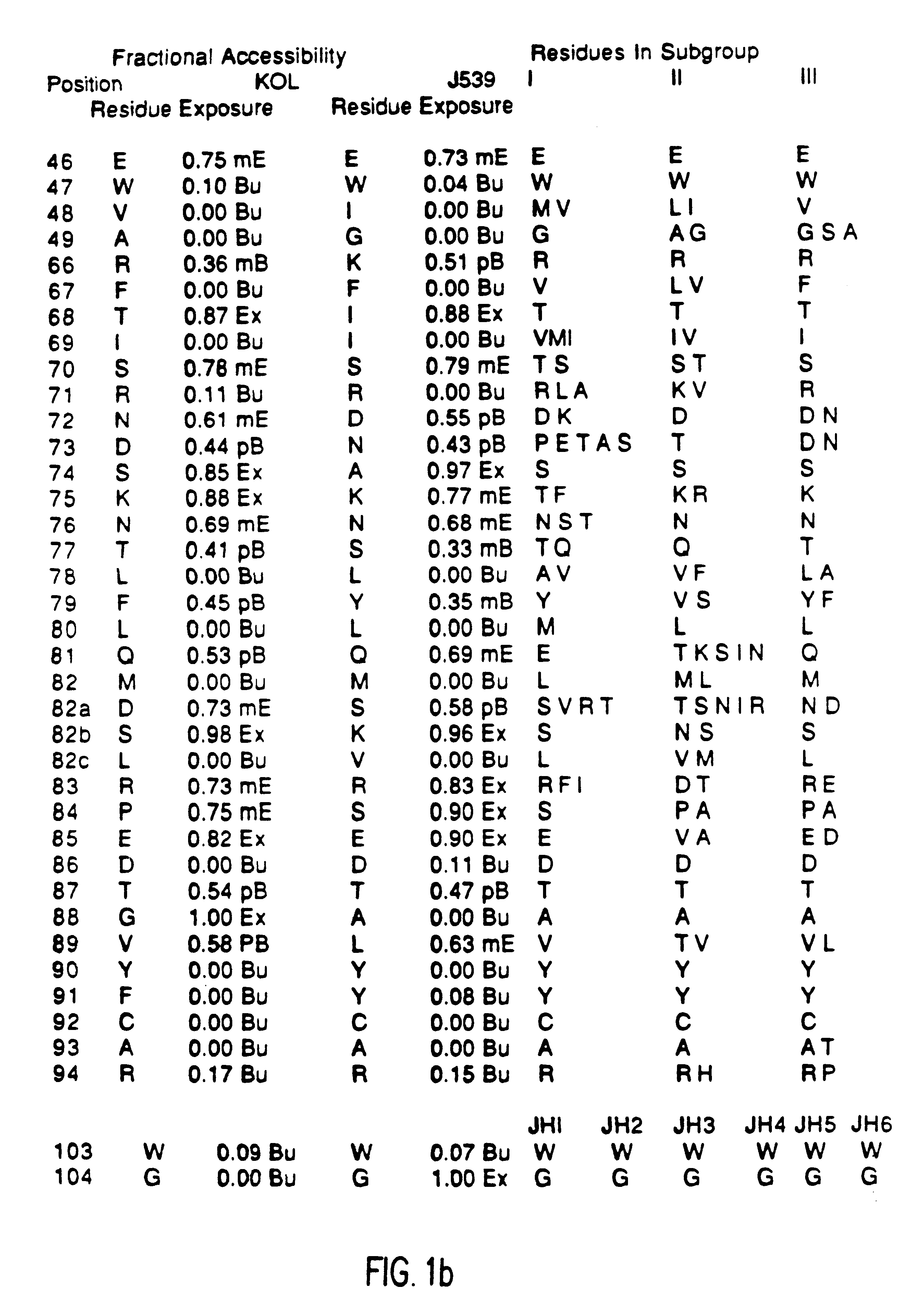
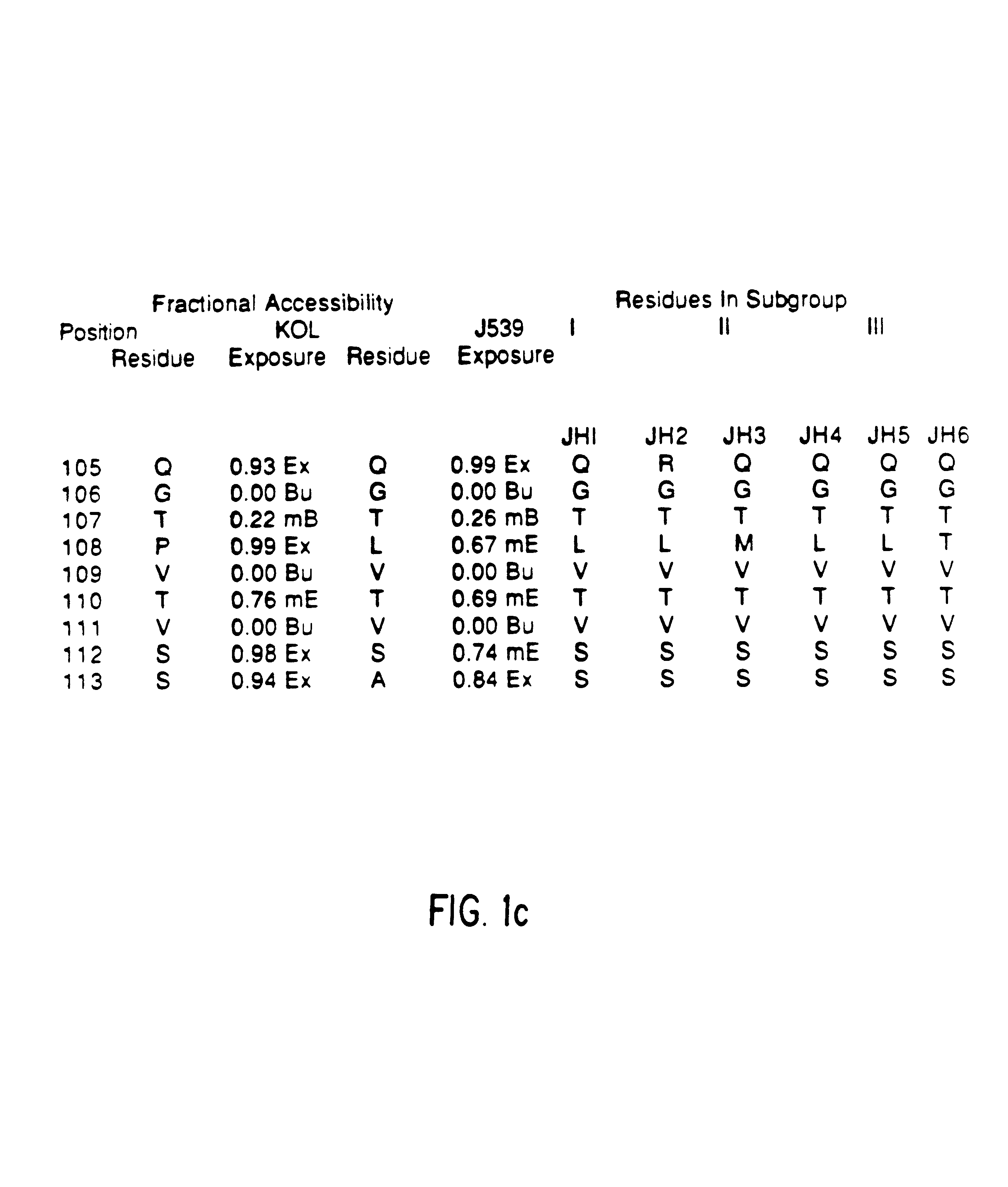
Method for reducing the immunogenicity of antibody variable domains
A unique method is disclosed for identifying and replacing immunoglobulin surface amino acid residues which converts the antigenicity of a first mammalian species to that of a second mammalian species. The method will simultaneously change immunogenicity and strictly preserve ligind binding properties. The judicious replacement of exterior amino acid residues has no effect on the ligind binding properties but greatly alters immunogenicity.
Owner:DEPT OF HEALTH & HUMAN SERVICES US SEC THE +1
Adeno-associated virus (aav) clades, sequences, vectors containing same, and uses therefor
Sequences of novel adeno-associated virus capsids and vectors and host cells containing these sequences are provided. Also described are methods of using such host cells and vectors in production of rAAV particles. AAV-mediated delivery of therapeutic and immunogenic genes using the vectors of the invention is also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
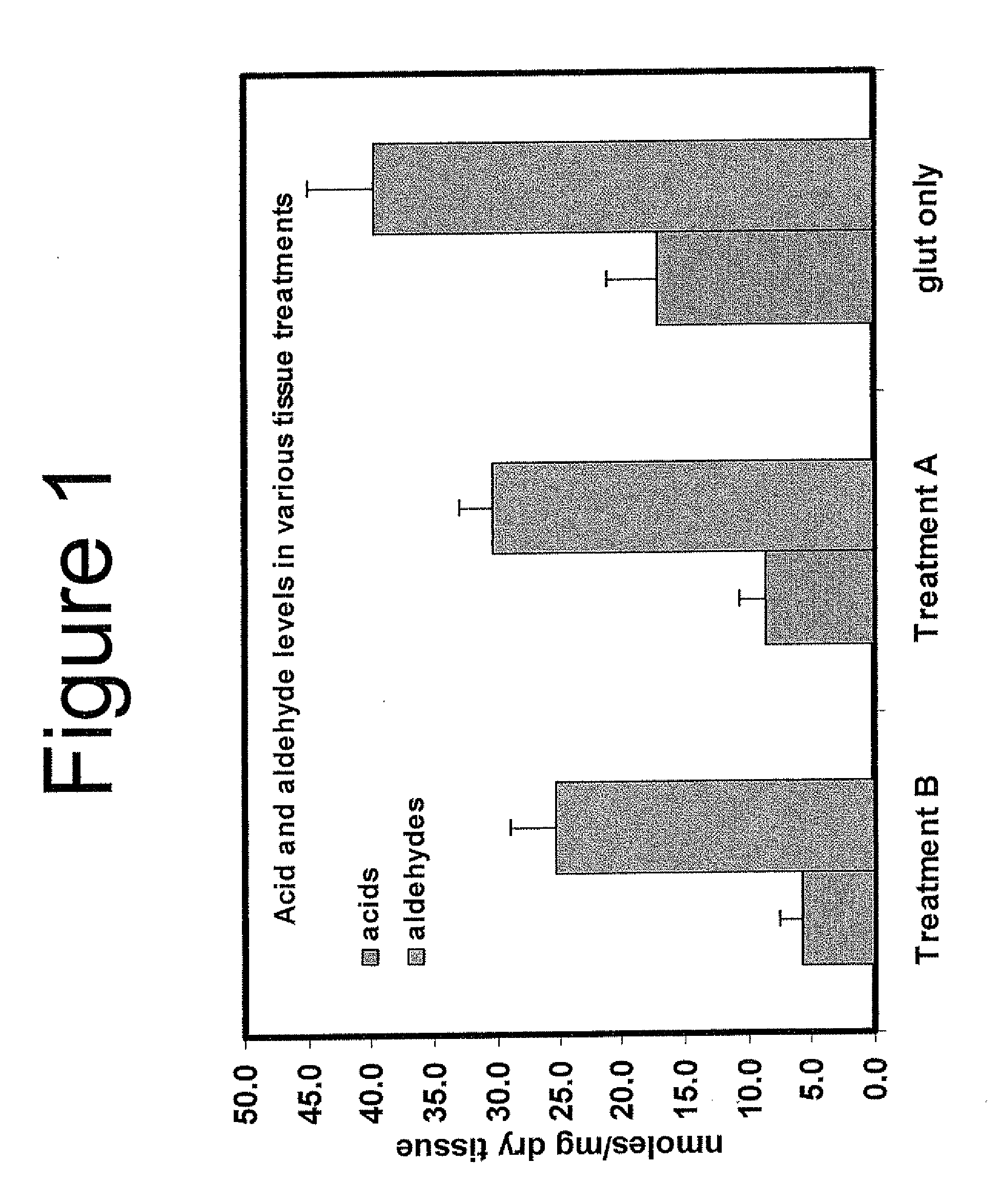
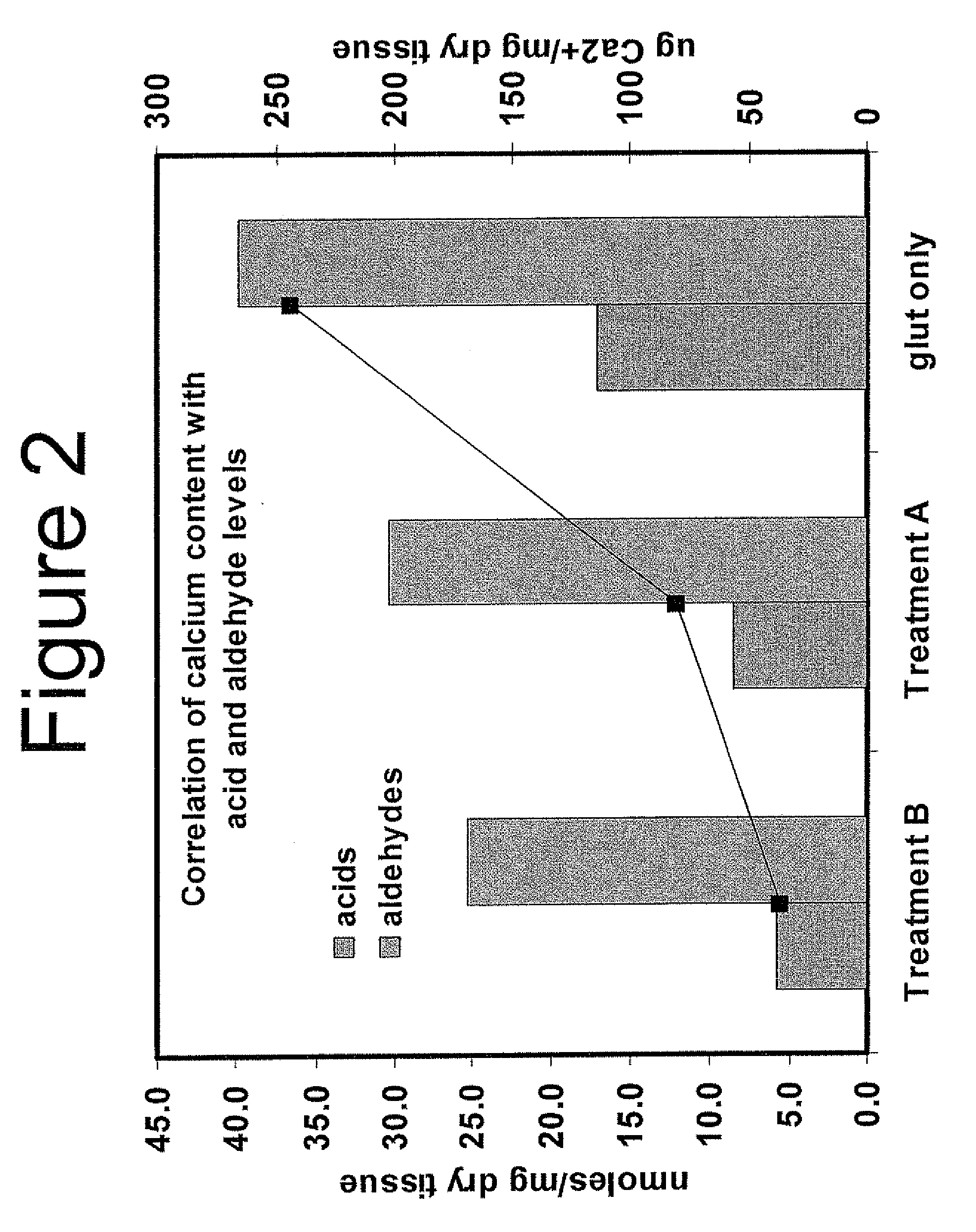
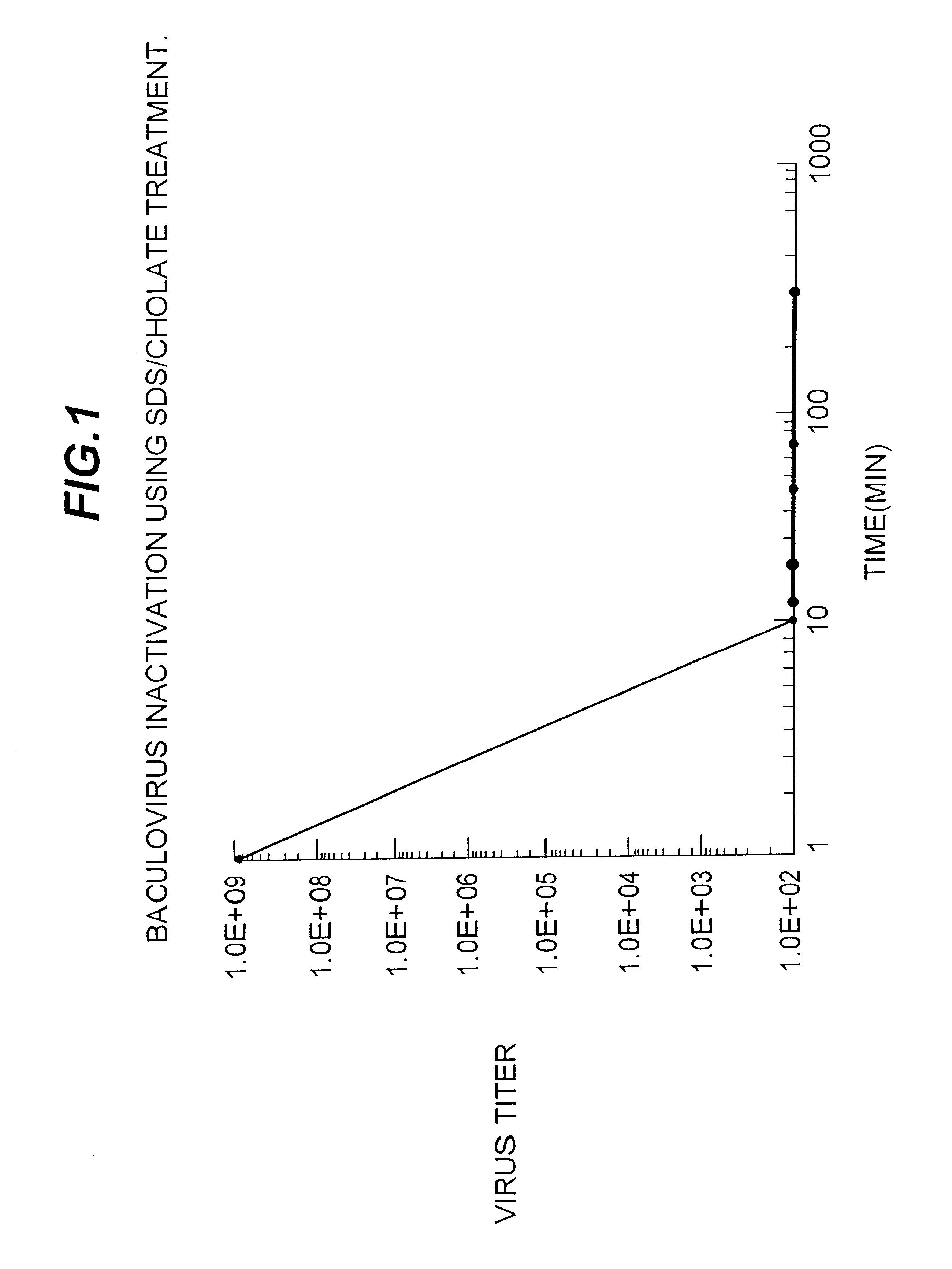
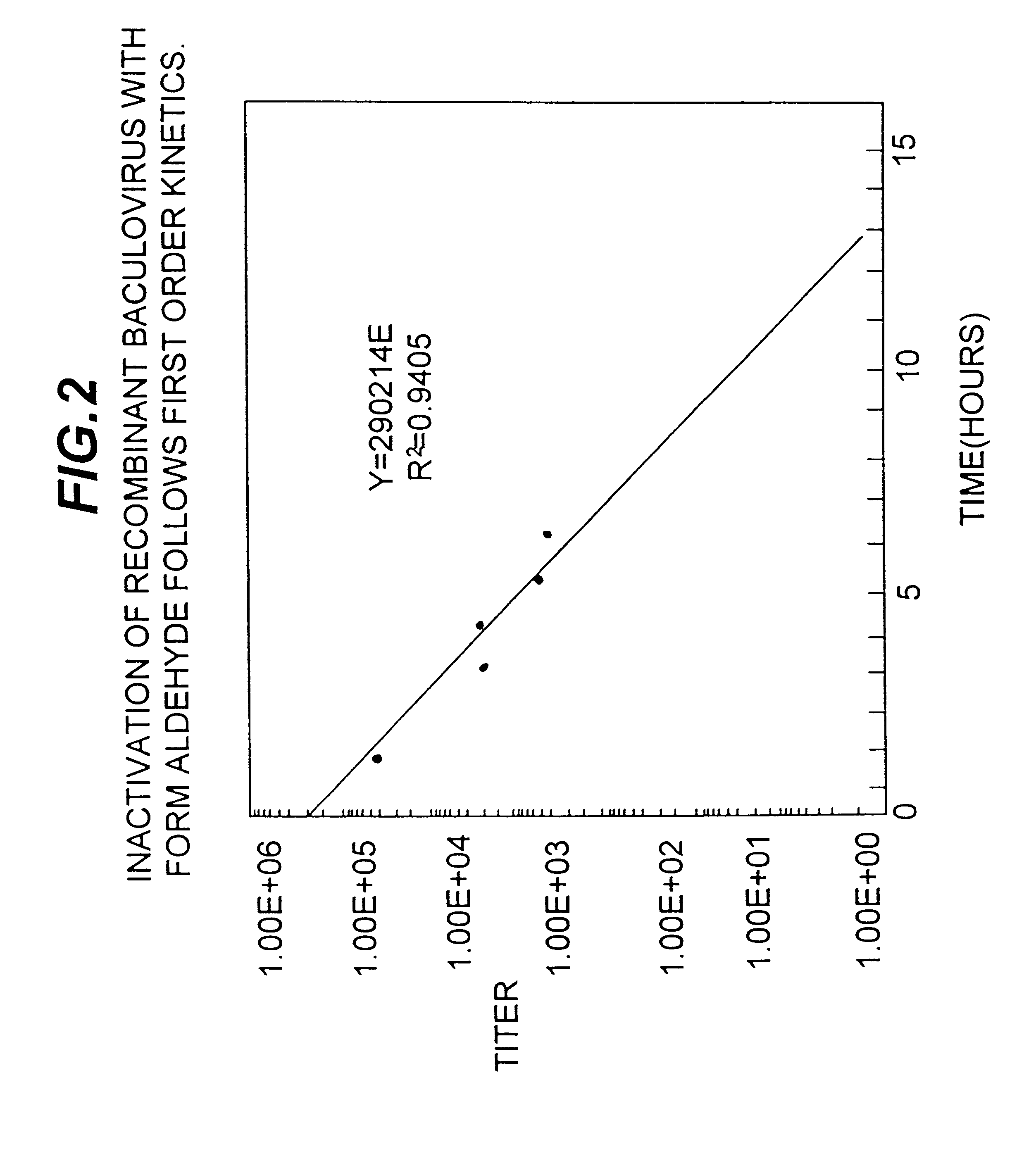
Capping Bioprosthetic Tissue to Reduce Calcification
A treatment for bioprosthetic tissue used in implants or for assembled bioprosthetic heart valves to reduce in vivo calcification. The method includes applying a calcification mitigant such as a capping agent or an antioxidant to the tissue to specifically inhibit oxidation in tissue. Also, the method can be used to inhibit oxidation in dehydrated tissue. The capping agent suppresses the formation of binding sites in the tissue that are exposed or generated by the oxidation and otherwise would, upon implant, attract calcium, phosphate, immunogenic factors, or other precursors to calcification. In one method, tissue leaflets in assembled bioprosthetic heart valves are pretreated with an aldehyde capping agent prior to dehydration and sterilization.
Owner:EDWARDS LIFESCIENCES CORP
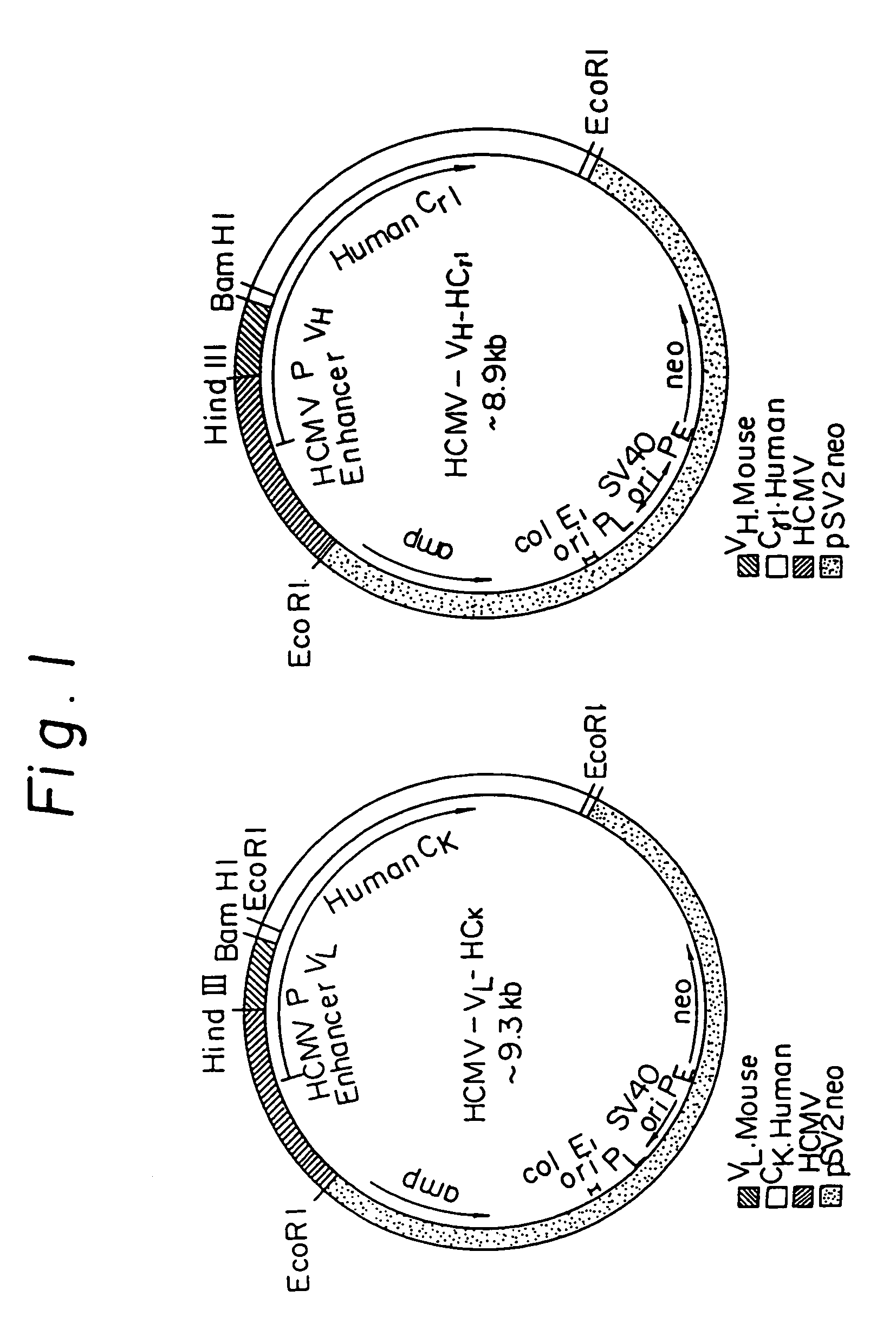
Humanized immunoglobulins
Novel methods for producing, and compositions of, humanized immunoglobulins having one or more complementarity determining regions (CDR's) and possible additional amino acids from a donor immunoglobulin and a framework region from an accepting human immunoglobulin are provided. Each humanized immunoglobulin chain will usually comprise, in addition to the CDR's, amino acids from the donor immunoglobulin framework that are, e.g., capable of interacting with the CDR's to effect binding affinity, such as one or more amino acids which are immediately adjacent to a CDR in the donor immunoglobulin or those within about about 3Å as predicted by molecular modeling. The heavy and light chains may each be designed by using any one or all of various position criteria. When combined into an intact antibody, the humanized immunoglobulins of the present invention will be substantially non-immunogenic in humans and retain substantially the same affinity as the donor immunoglobulin to the antigen, such as a protein or other compound containing an epitope.
Owner:PDL BIOPHARMA INCORPORATED
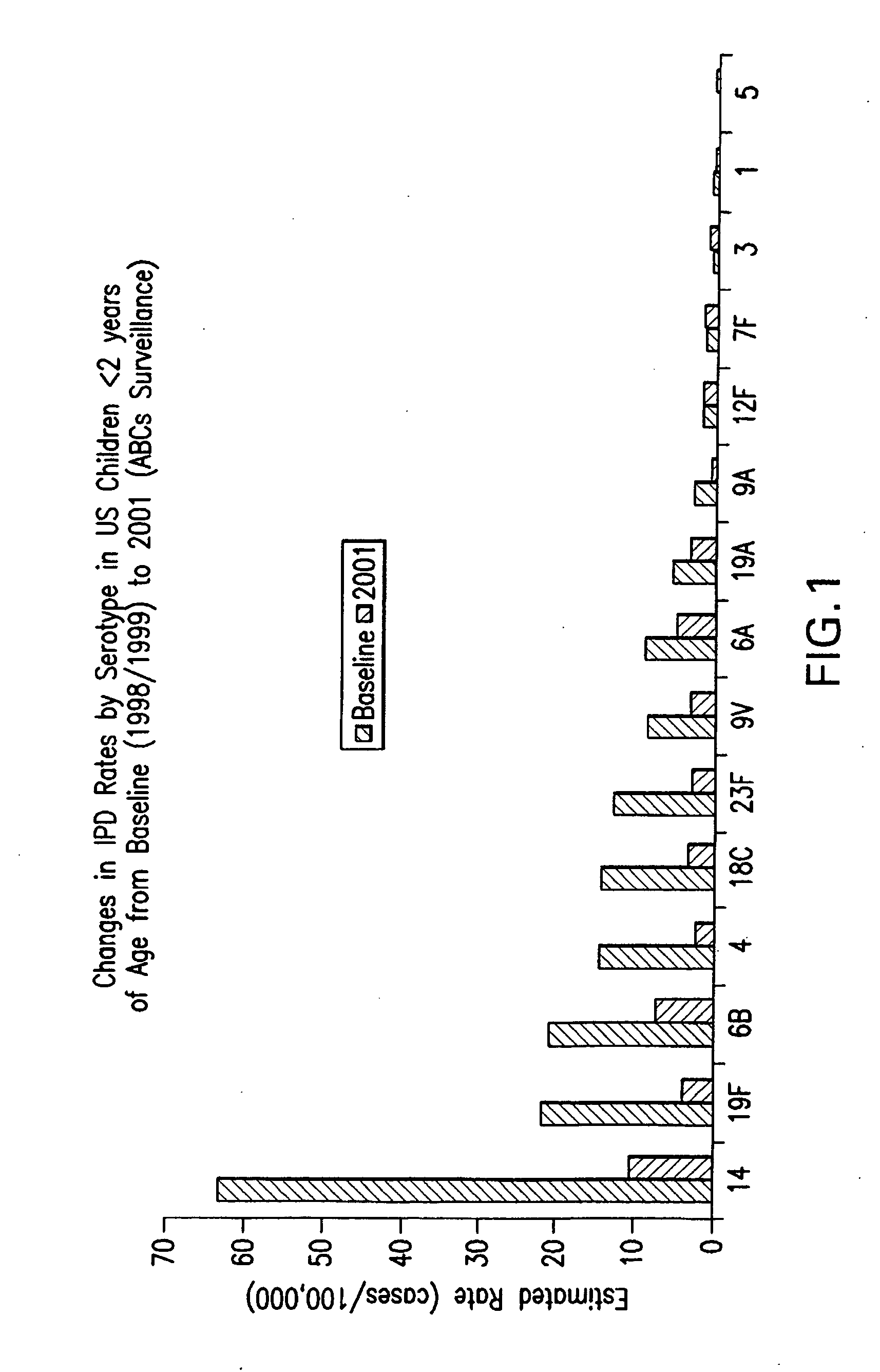
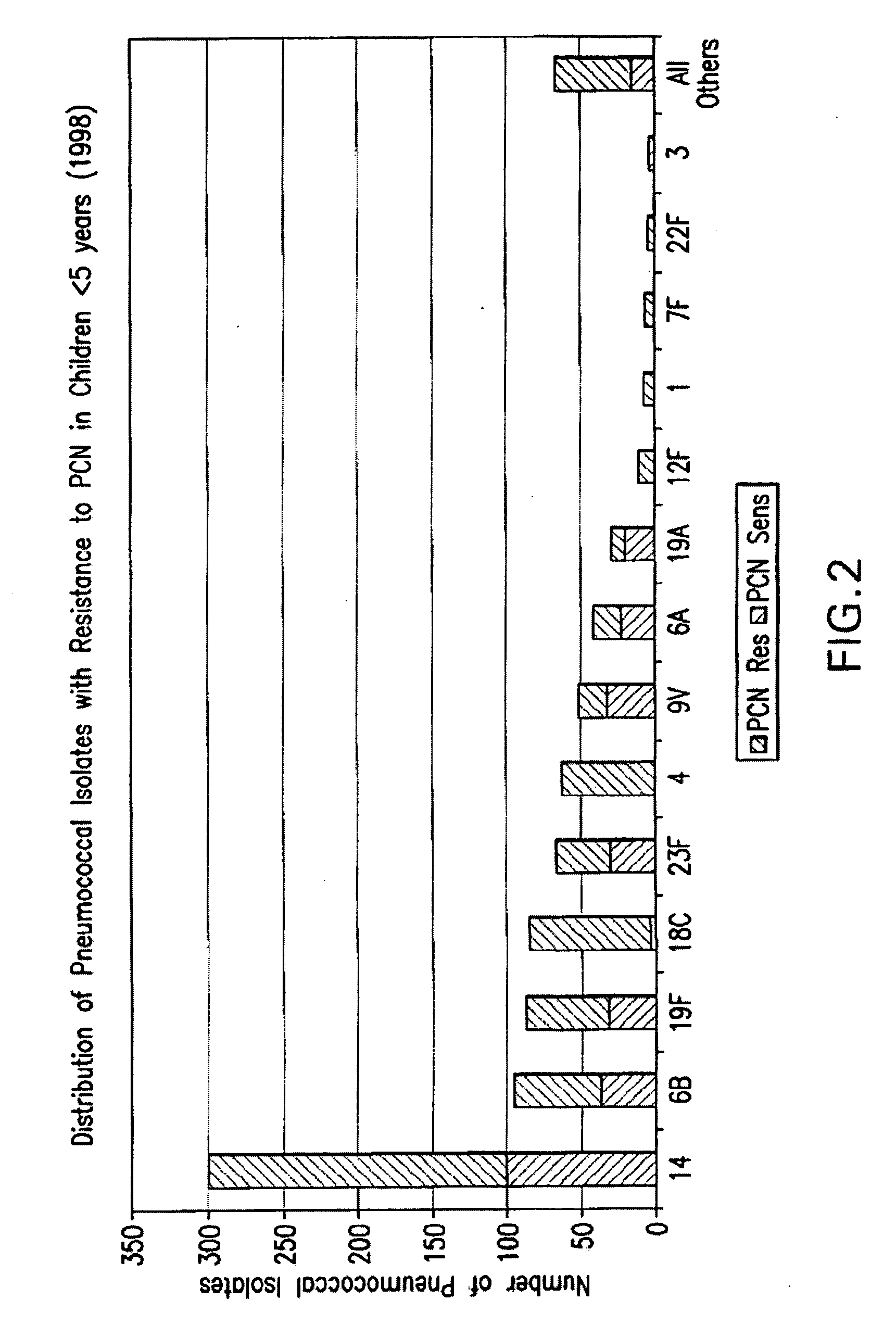
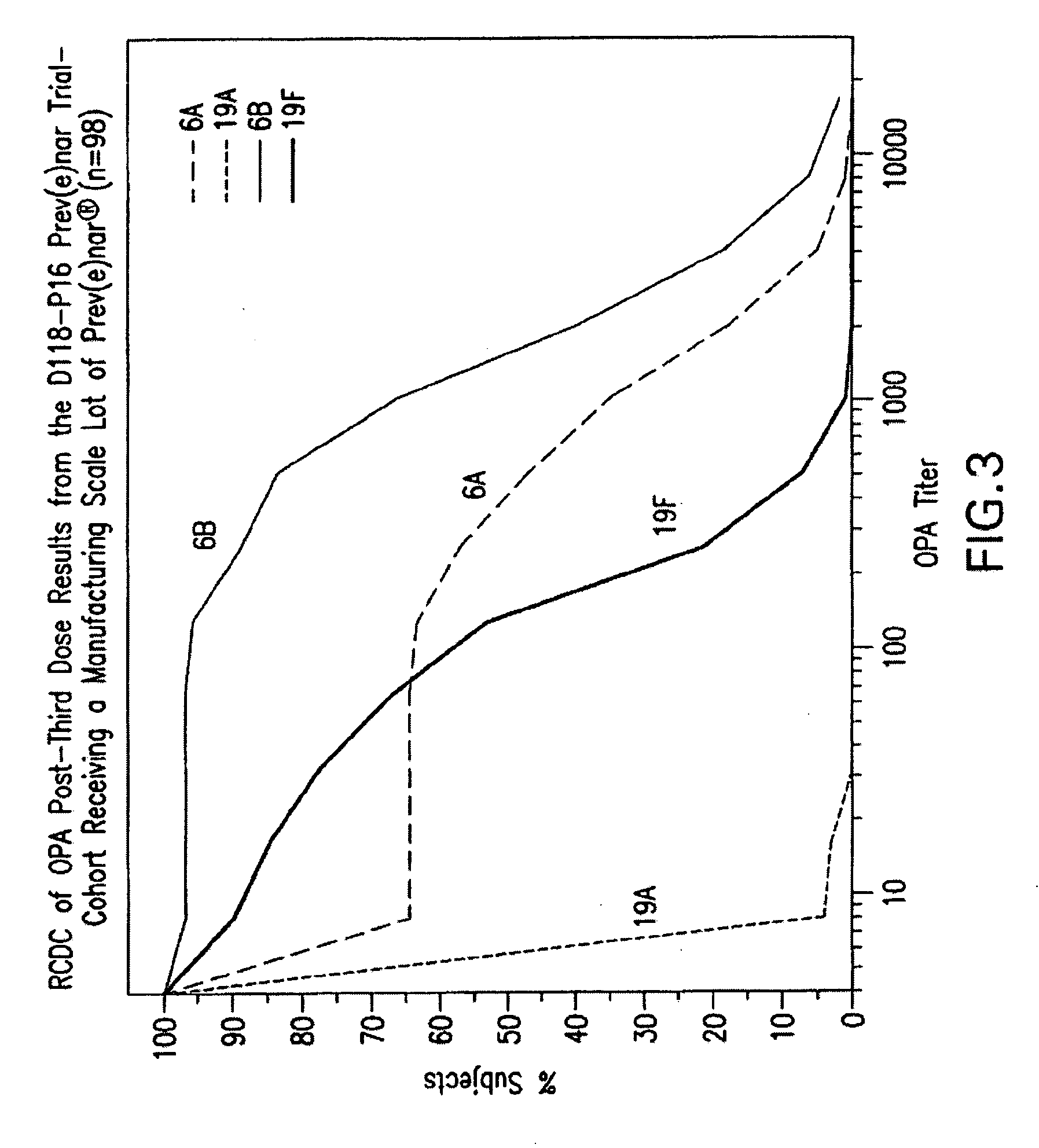
Multivalent pneumococcal polysaccharide-protein conjugate composition
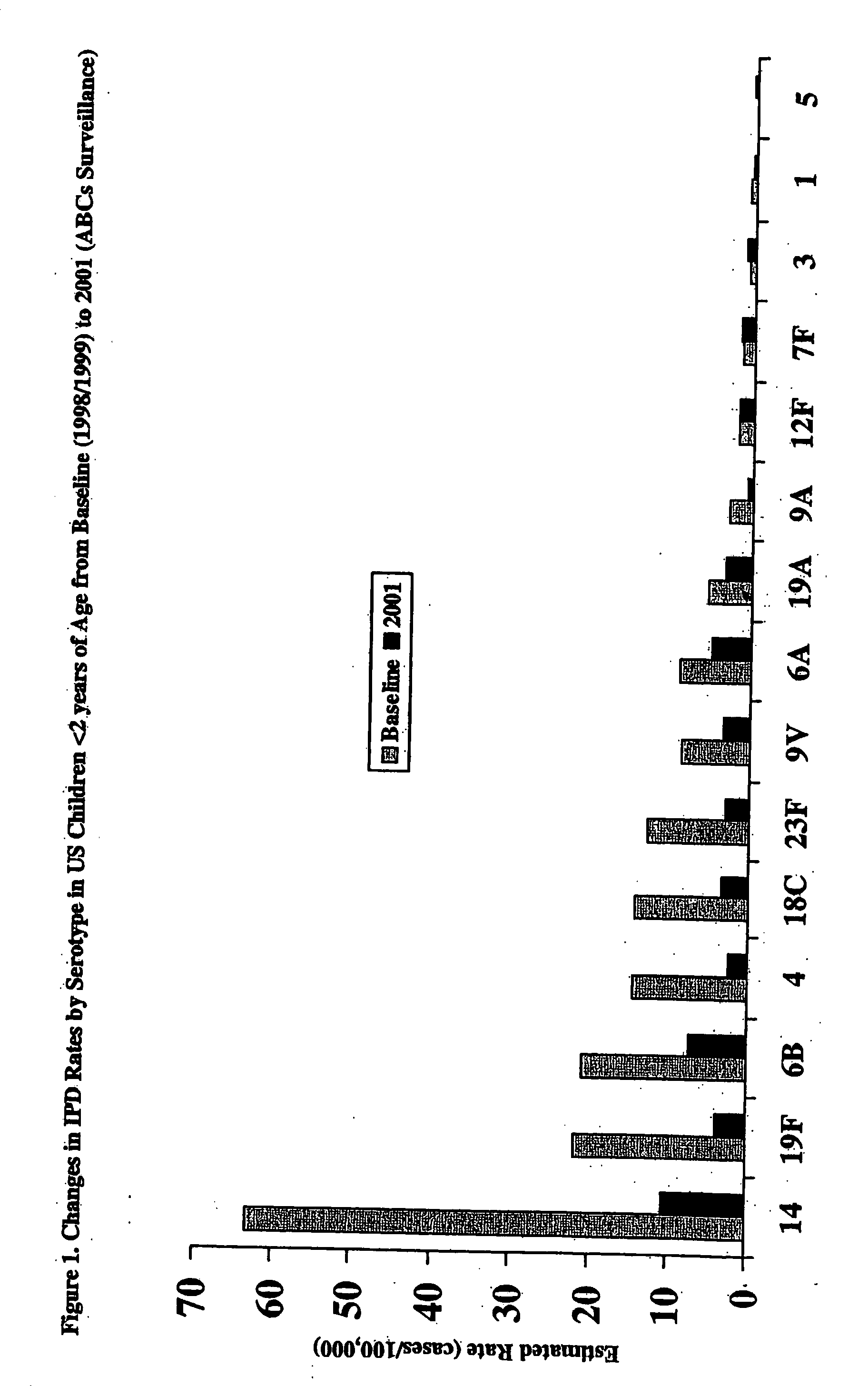
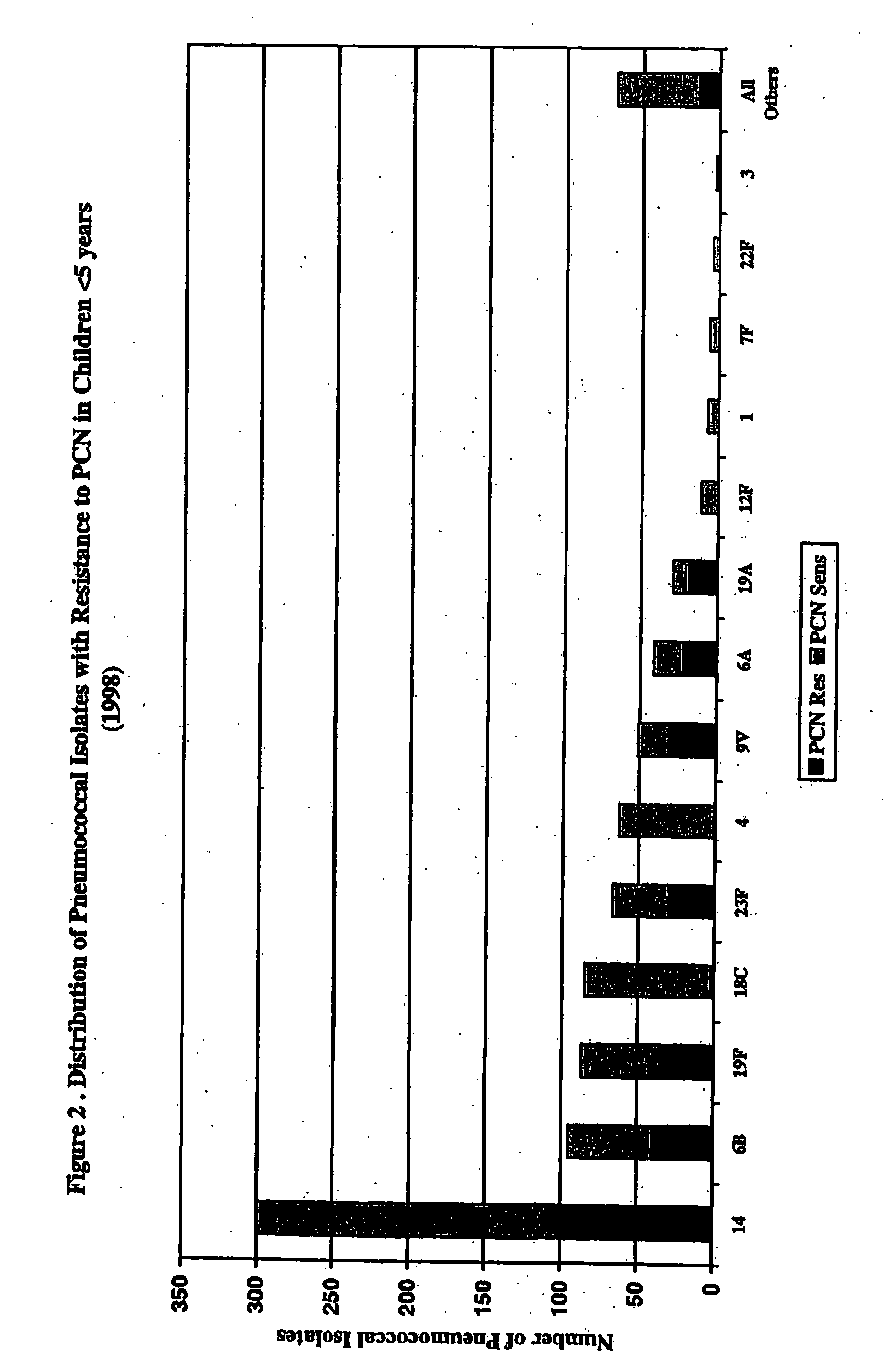
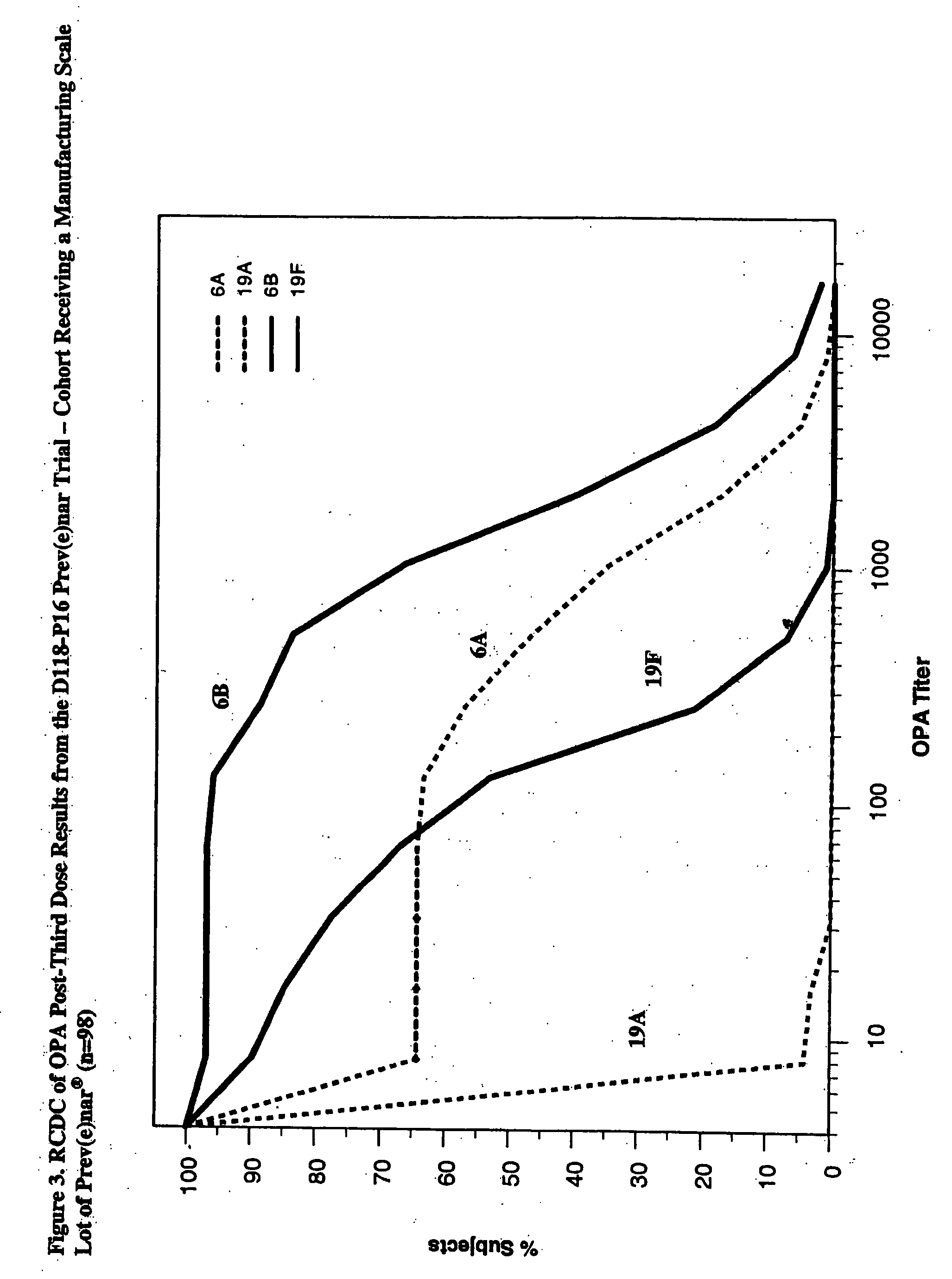
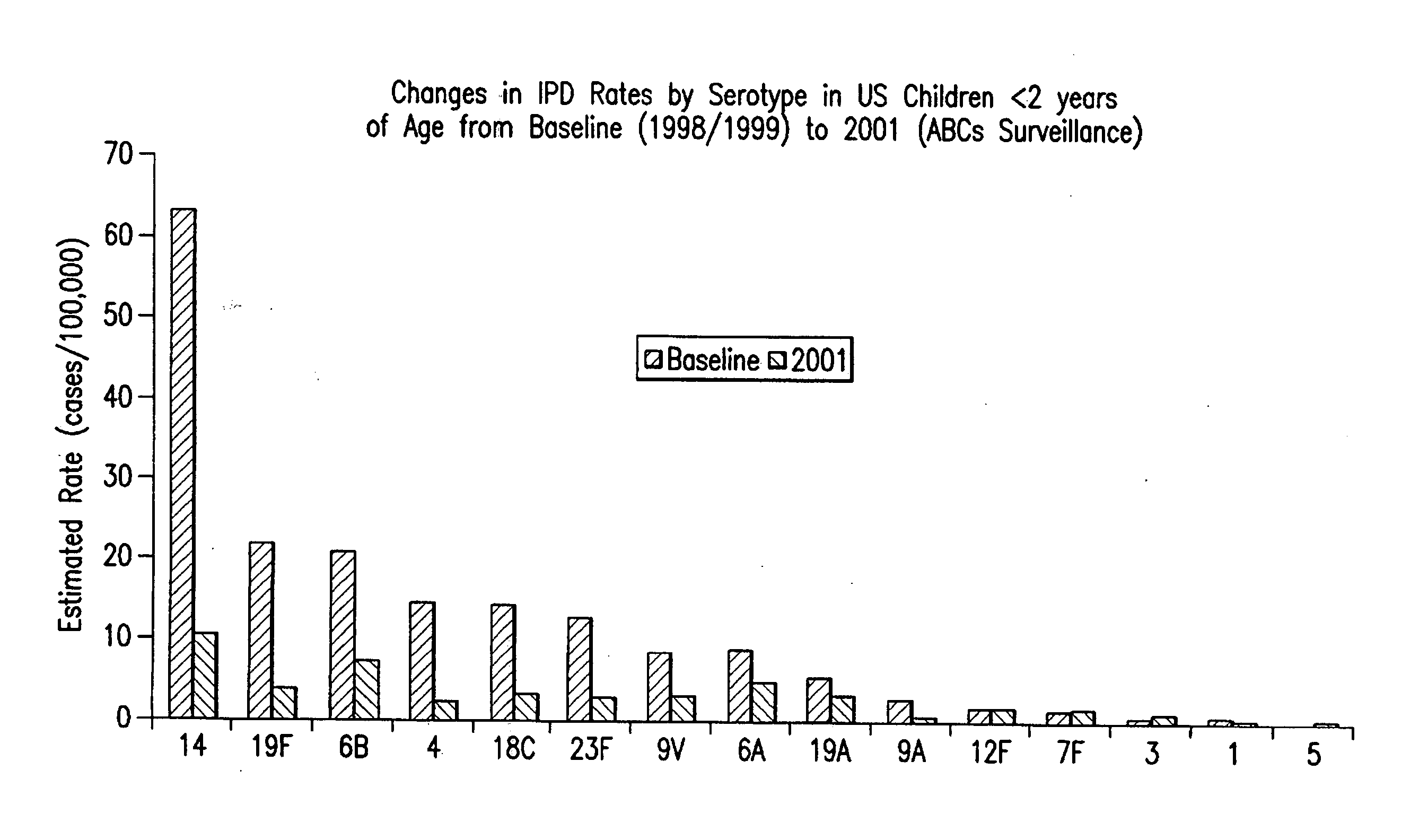
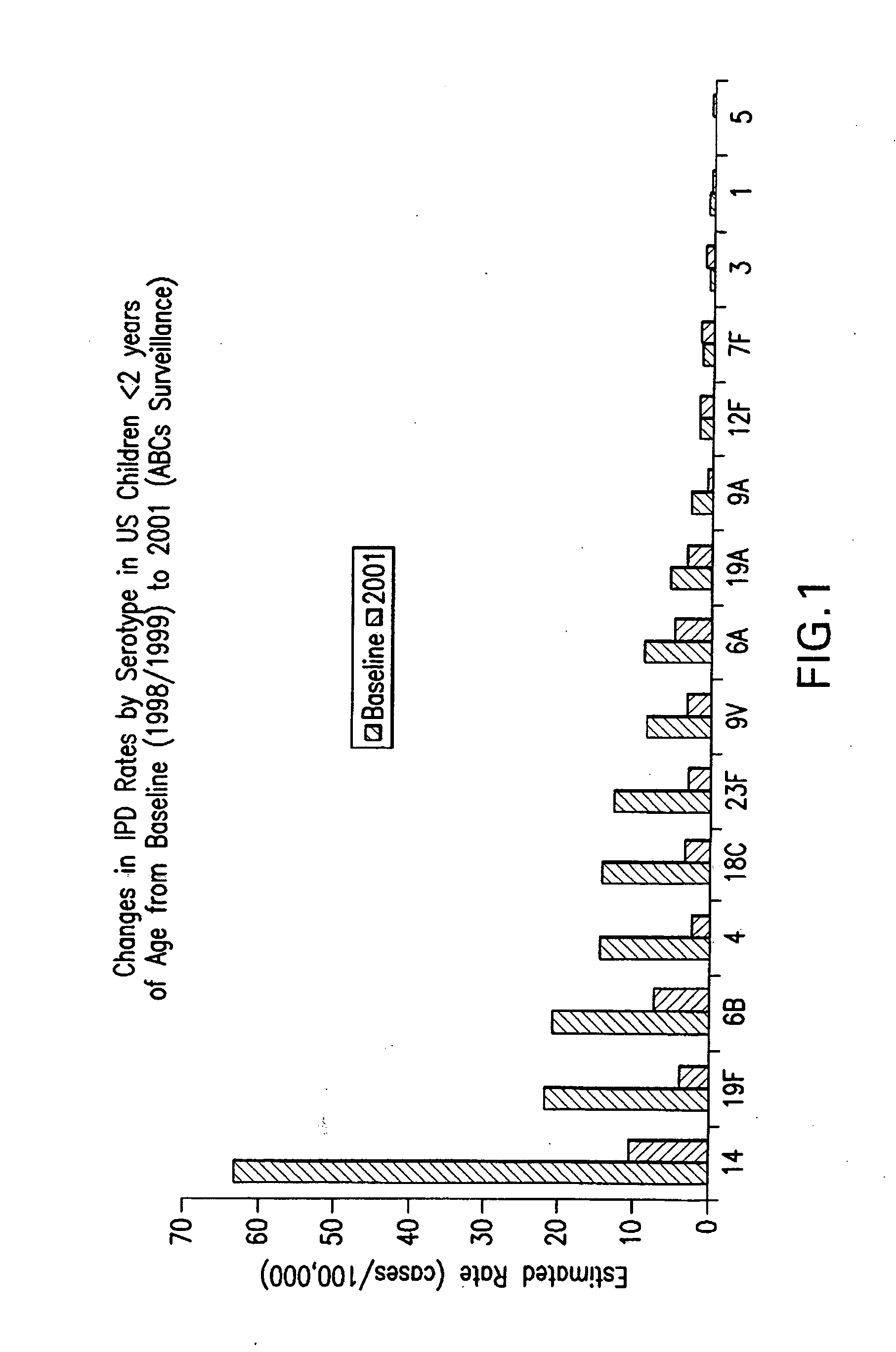
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection.
Owner:WYETH LLC
Reshaped human antibody to human interleukin-6 receptor
InactiveUS7479543B2Peptide/protein ingredientsAntibody mimetics/scaffoldsComplementarity determining regionV region
A reshaped human antibody to the human IL-6R, comprising:(A) an L chain comprising,(1) a human L chain C region, and(2) an L chain V region comprising human L chain framework regions (FRs), and mouse L chain complementary determination regions (CDRs) of a momoclonal antibody to the IL-6 receptor (IL-6R); and(B) an H chain comprising,(1) a human H chain C region, and(2) an H chain V region comprising human H chain FRS, and mouse H chain CDRs of a monoclonal antibody to the IL-6R.Since major portion of the reshaped human antibody is derived from a human antibody and the mouse CDRs which are less immunogenic, the present reshaped human antibody is less immunogenic to human, and therefor is promised for therapeutic uses.
Owner:CHUGAI PHARMA CO LTD
Production of humanized antibodies in transgenic animals
InactiveUS20030017534A1Low immunogenicityUseful in therapyImmunoglobulins against bacteriaImmunoglobulins against virusesHuman animalGene conversion
This invention relates to humanized antibodies and antibody preparations produced from transgenic non-human animals. The non-human animals are genetically engineered to contain one or more humanized immunoglobulin loci which are capable of undergoing gene rearrangement and gene conversion in the transgenic non-human animals to produce diversified humanized immunoglobulins. The present invention further relates to novel sequences, recombination vectors and transgenic vectors useful for making these transgenic animals. The humanized antibodies of the present invention have minimal immunogenicity to humans and are appropriate for use in the therapeutic treatment of human subjects.
Owner:THERAPEUTIC HUMAN POLYCLONALS
Recombinant canine adenoviruses, method for making and uses thereof
InactiveUS6090393AProvide securityIncrease capacitySsRNA viruses negative-senseVectorsBiotechnologyImmunogenicity
Disclosed and claimed are recombinant adenoviruses, methods of making them, uses for them (including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector), expression products from them, and uses for the expression products. More particularly, disclosed and claimed are recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses. Additionally, disclosed and claimed are truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes.
Owner:VIROGENETICS +1
Truncated transcriptionally active cytomegalovirus promoters
InactiveUS6156567AProvide securityIncrease capacitySsRNA viruses negative-senseGenetic material ingredientsBiotechnologyEukaryotic plasmids
Recombinant adenoviruses, methods of making them, uses for them, including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector, expression products from them, and uses for the expression products are provided. More particularly, recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses are provided. Additionally, truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes are provided.
Owner:VIROGENETICS
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Methods for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 19A polysaccharide are also provided in which the serotype 19A polysaccharide is co-lyophilized with a carrier protein and conjugation is carried out in dimethyl sulfoxide (DMSO) via a reductive amination mechanism.
Owner:WYETH LLC
Reshaped human antibody to human interleukin-6 receptor
InactiveUS20050142635A1Less immunogenicPeptide/protein ingredientsHydrolasesComplementarity determining regionV region
A reshaped human antibody to the human IL-6R, comprising: (A) an L chain comprising, (1) a human L chain C region, and (2) an L chain V region comprising human L chain framework regions (FRs), and mouse L chain complementary determination regions (CDRs) of a momoclonal antibody to the IL-6 receptor (IL-6R); and (B) an H chain comprising, (1) a human H chain C region, and (2) an H chain V region comprising human H chain FRS, and mouse H chain CDRs of a monoclonal antibody to the IL-6R. Since major portion of the reshaped human antibody is derived from a human antibody and the mouse CDRs which are less immunogenic, the present reshaped human antibody is less immunogenic to human, and therefor is promised for therapeutic uses.
Owner:CHUGAI PHARMA CO LTD
Therapeutic and prophylactic methods using heat shock proteins
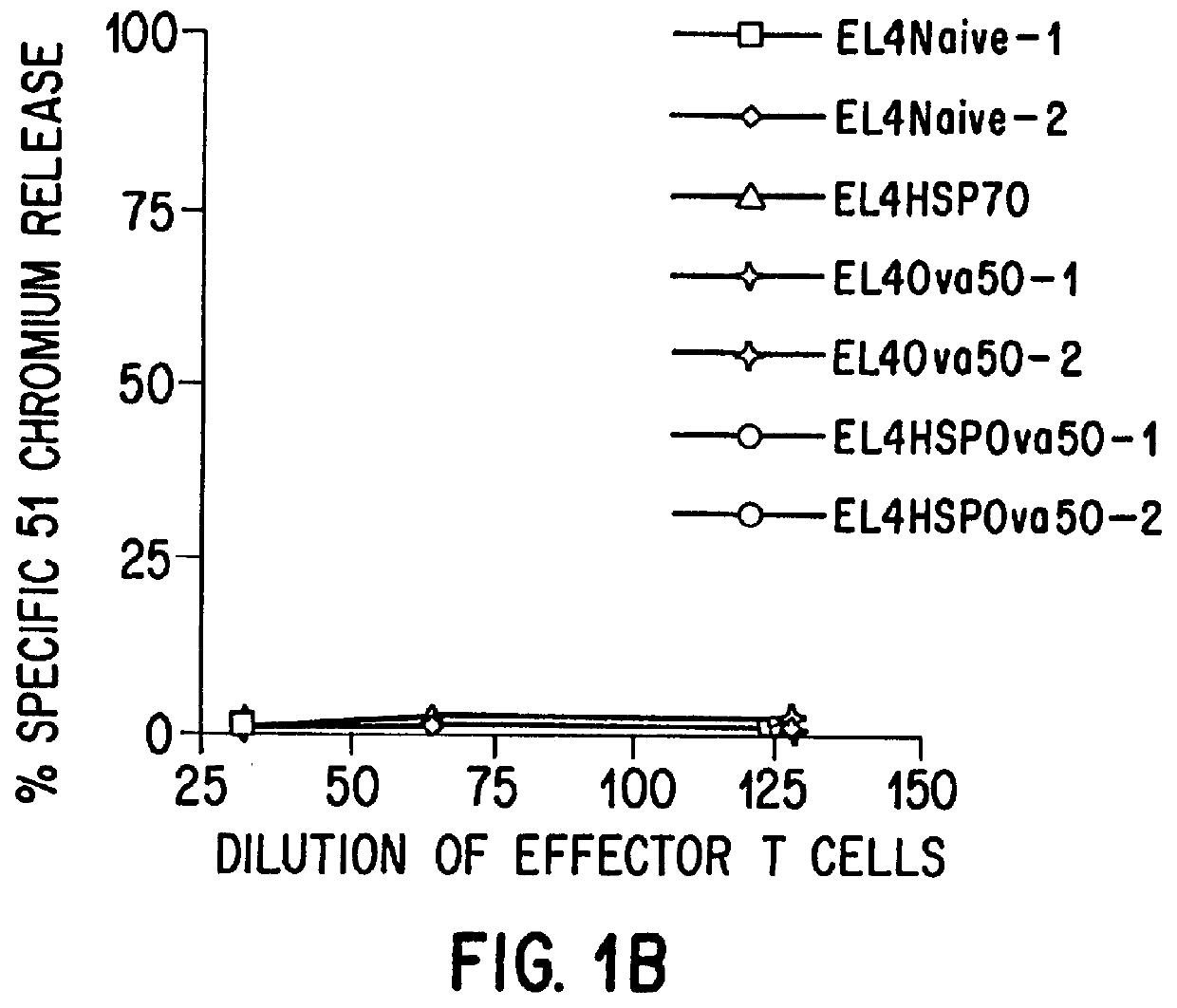
The present invention relates to immunogenic complexes of heat shock proteins (hsp) noncovalently bound to exogenous antigenic molecules which when administered to an individual elicit specific immunological responses in the host. Methods of prevention and treatment of cancer and infectious disease are provided.
Owner:FORDHAM UNIVERSITY
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 1 polysaccharide covalently linked to a carrier protein, the method including partial de-O-acetylation of the polysaccharide by mild hydrolysis in an alkaline pH buffer.
Owner:WYETH LLC
Multivalent pneumococcal polysaccharide-protein conjugate composition
An immunogenic composition having 13 distinct polysaccharide-protein conjugates and optionally, an aluminum-based adjuvant, is described. Each conjugate contains a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) conjugated to a carrier protein. The immunogenic composition, formulated as a vaccine, increases coverage against pneumococcal disease in infants and young children globally, and provides coverage for serotypes 6A and 19A that is not dependent on the limitations of serogroup cross-protection. Also described is a method for making an immunogenic conjugate comprising Streptococcus pneumoniae serotype 3 polysaccharide covalently linked to a carrier protein, the method including periodic acid oxidation of the polysaccharide in the presence of bivalent cations.
Owner:WYETH LLC
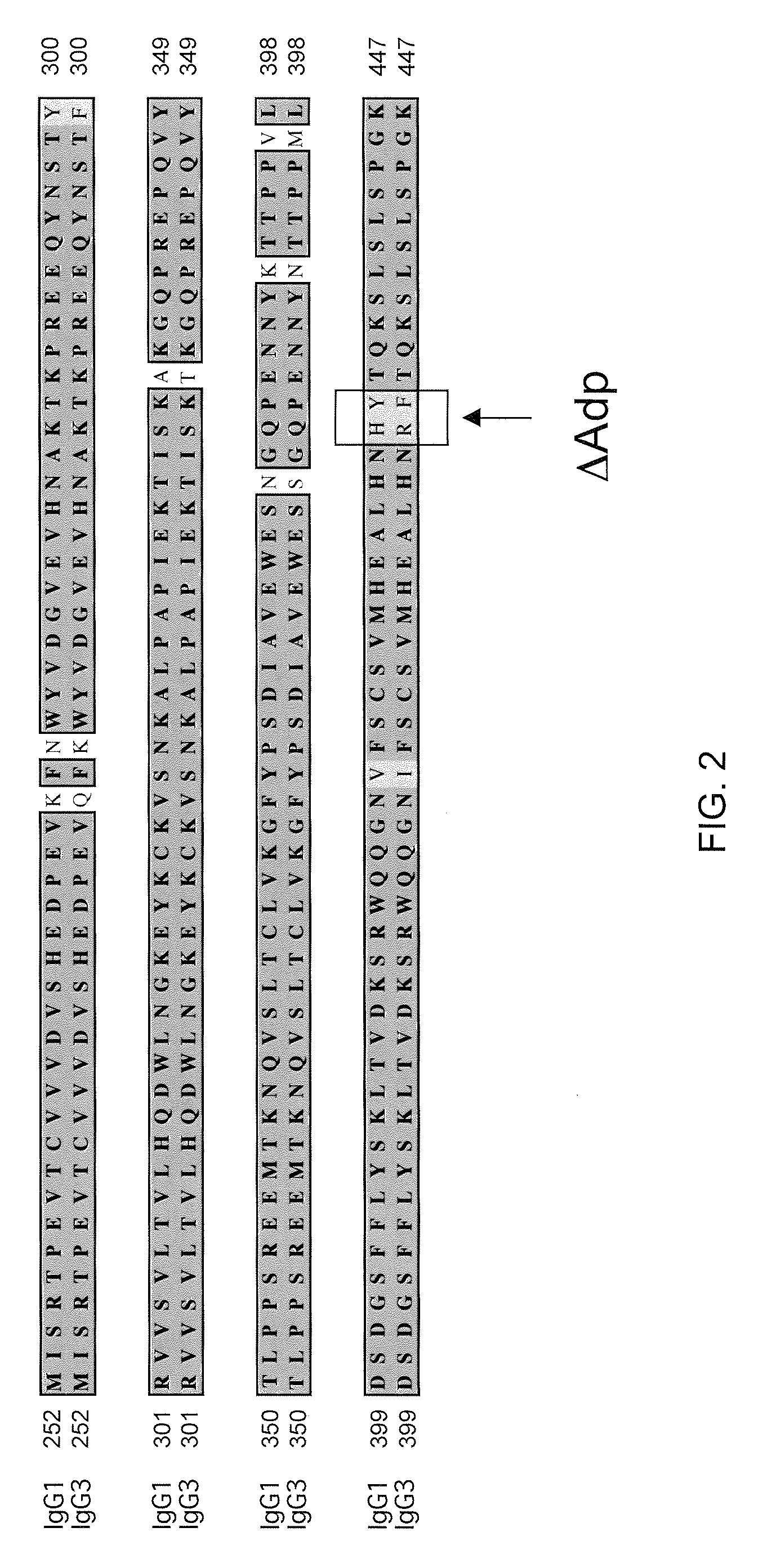
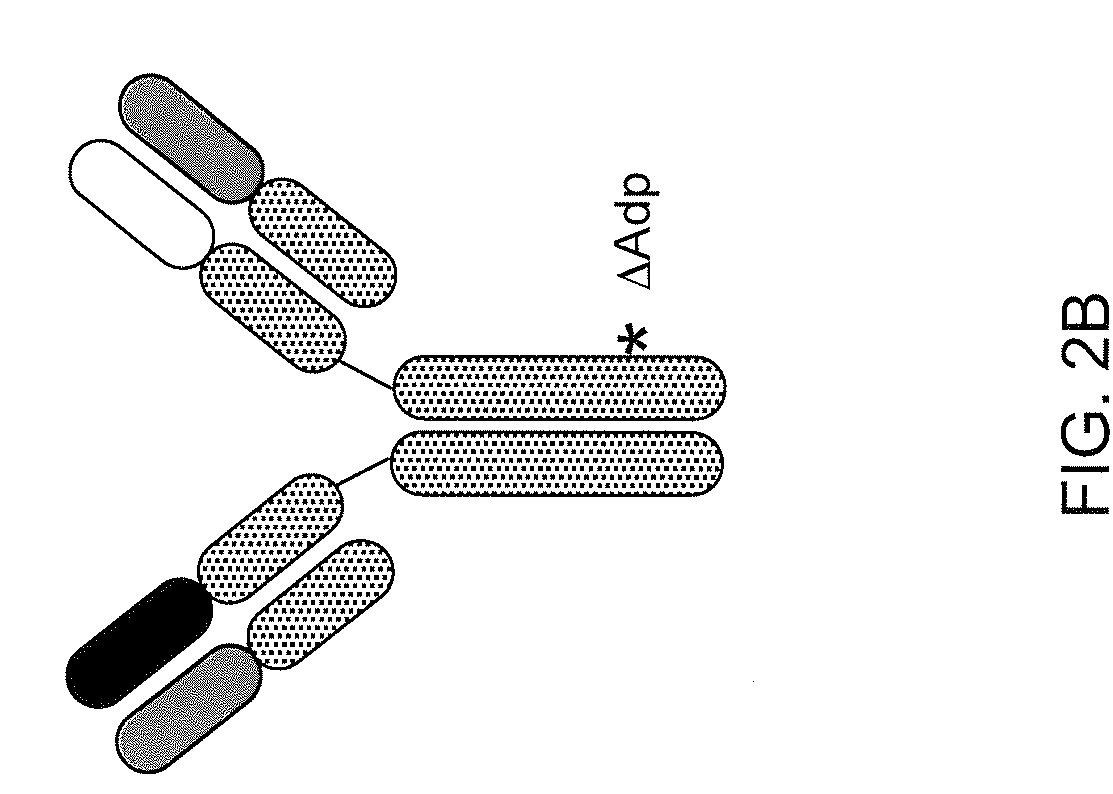
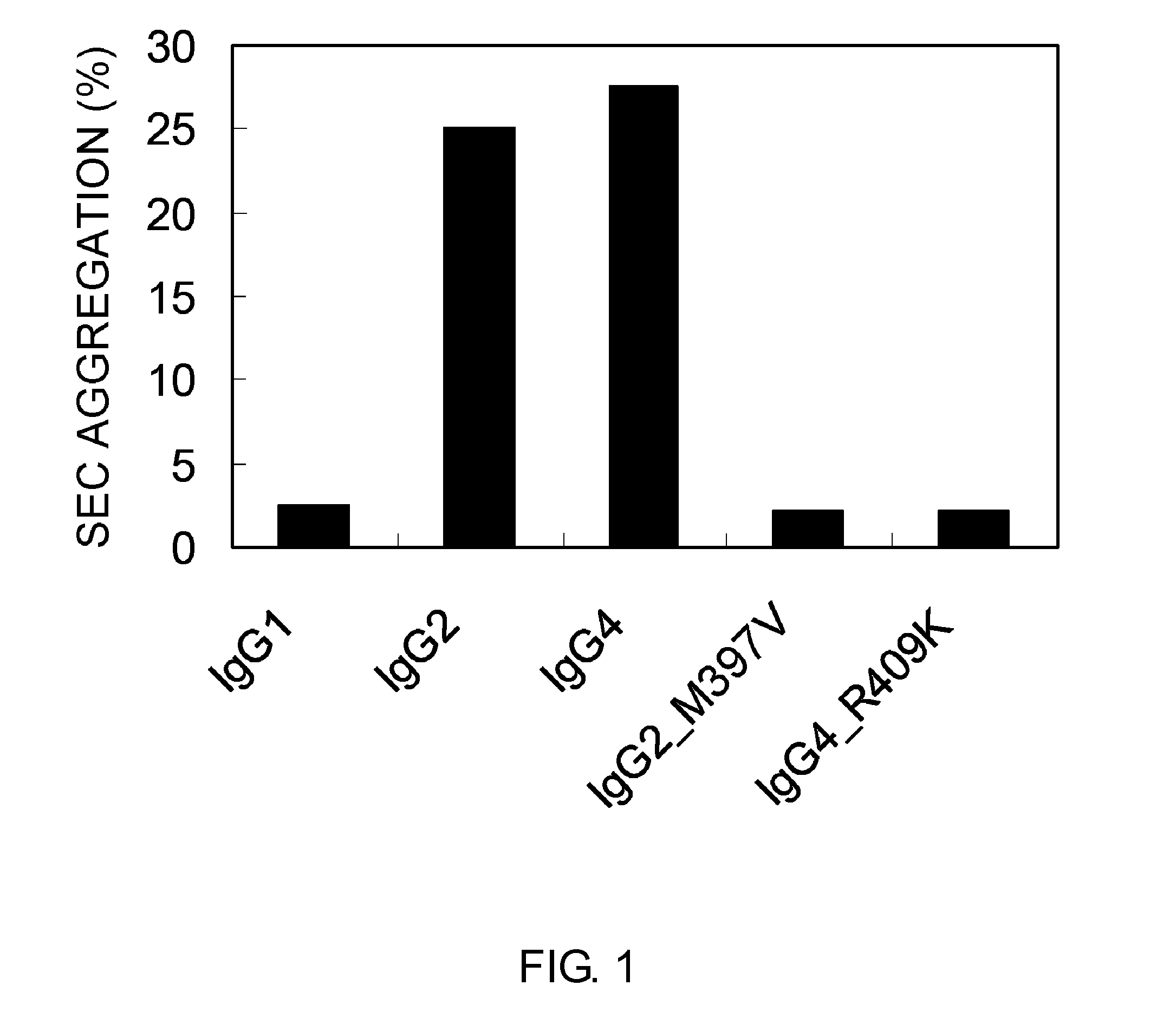
Modified Antibody Constant Region
ActiveUS20100298542A1Improving immunogenicityImprove propertiesAntipyreticAnalgesicsHigh concentrationHinge region
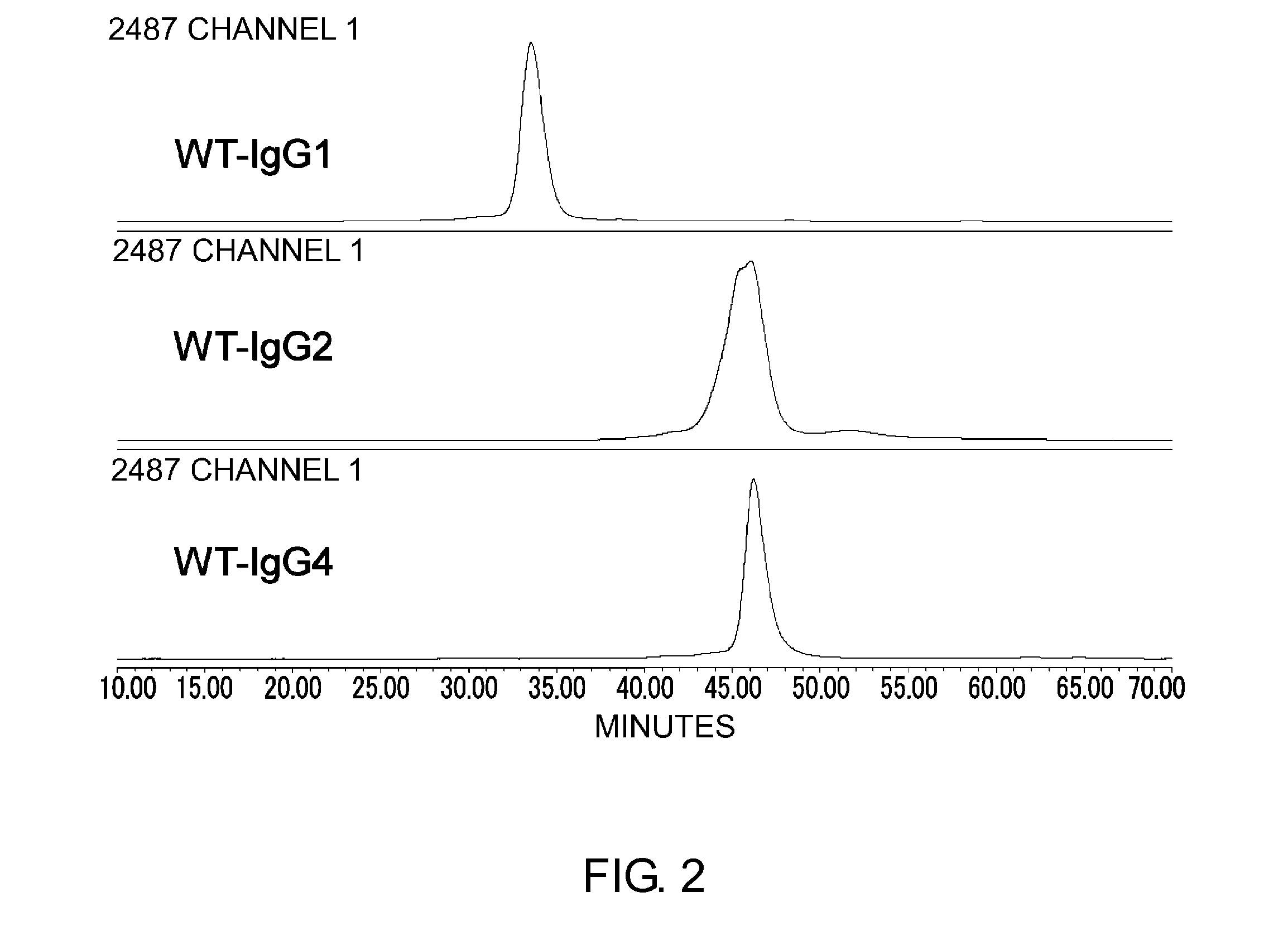
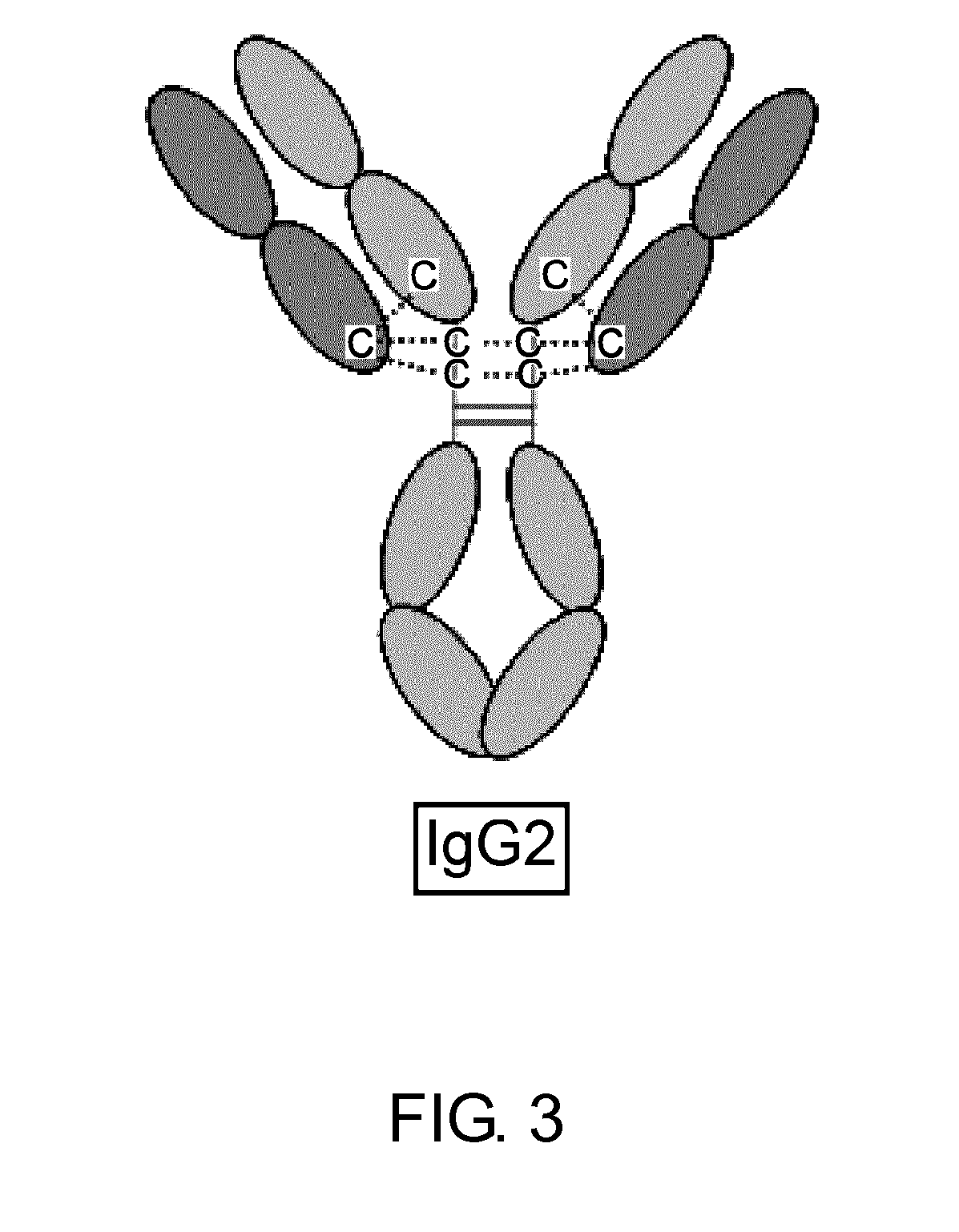
The present inventors succeeded in improving the antibody constant region to have increased stability under acid conditions, reduced heterogeneity originated from disulfide bonds in the hinge region, reduced heterogeneity originated from the H chain C terminus, and increased stability at high concentrations as well as in discovering novel constant region sequences having reduced Fcγ receptor-binding, while minimizing the generation of novel T-cell epitope peptides. As a result, the present inventors successfully discovered antibody constant regions with improved physicochemical properties (stability and homogeneity), immunogenicity, safety, and pharmacokinetics.
Owner:CHUGAI PHARMA CO LTD
Structure-based selection and affinity maturation of antibody library
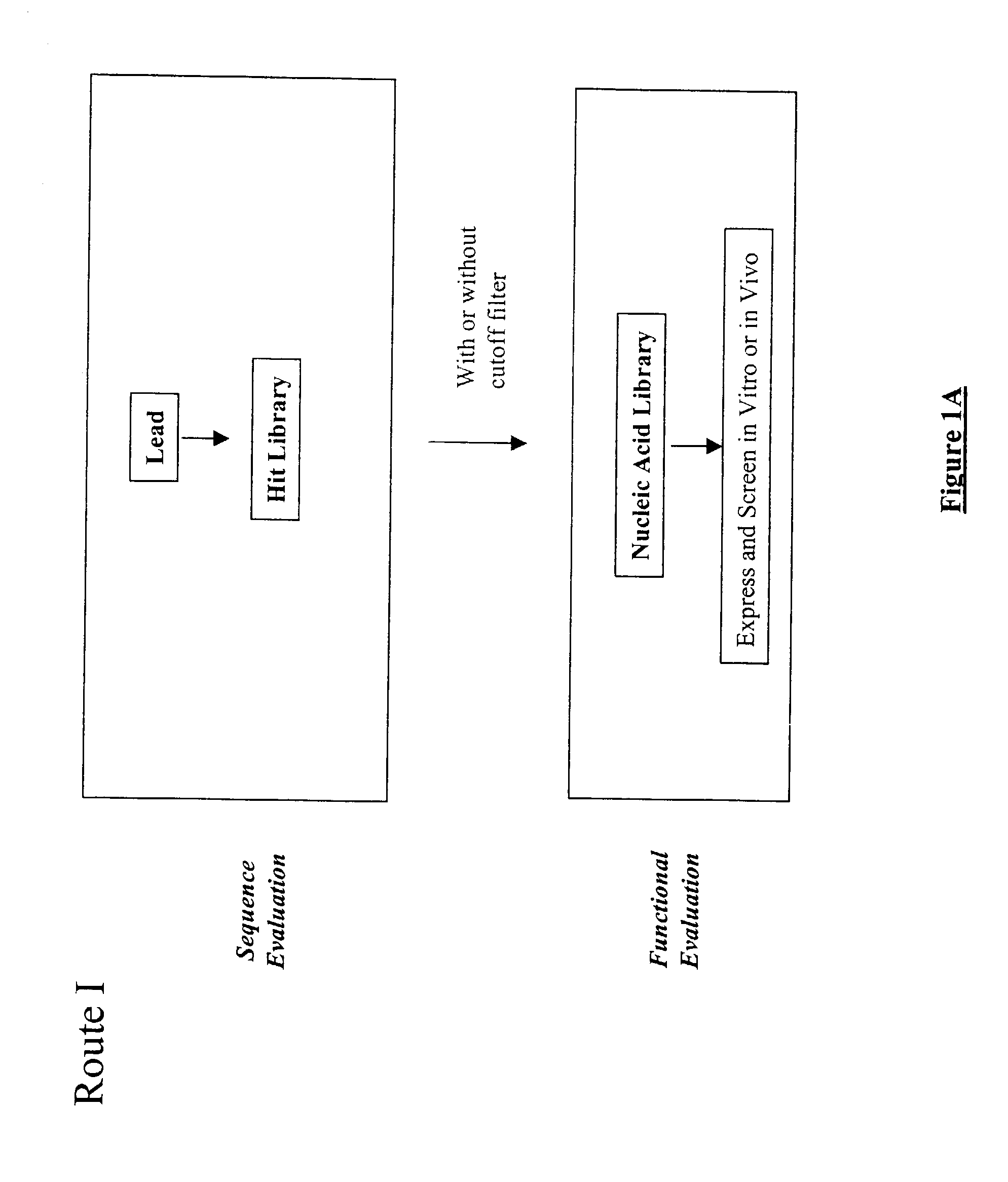
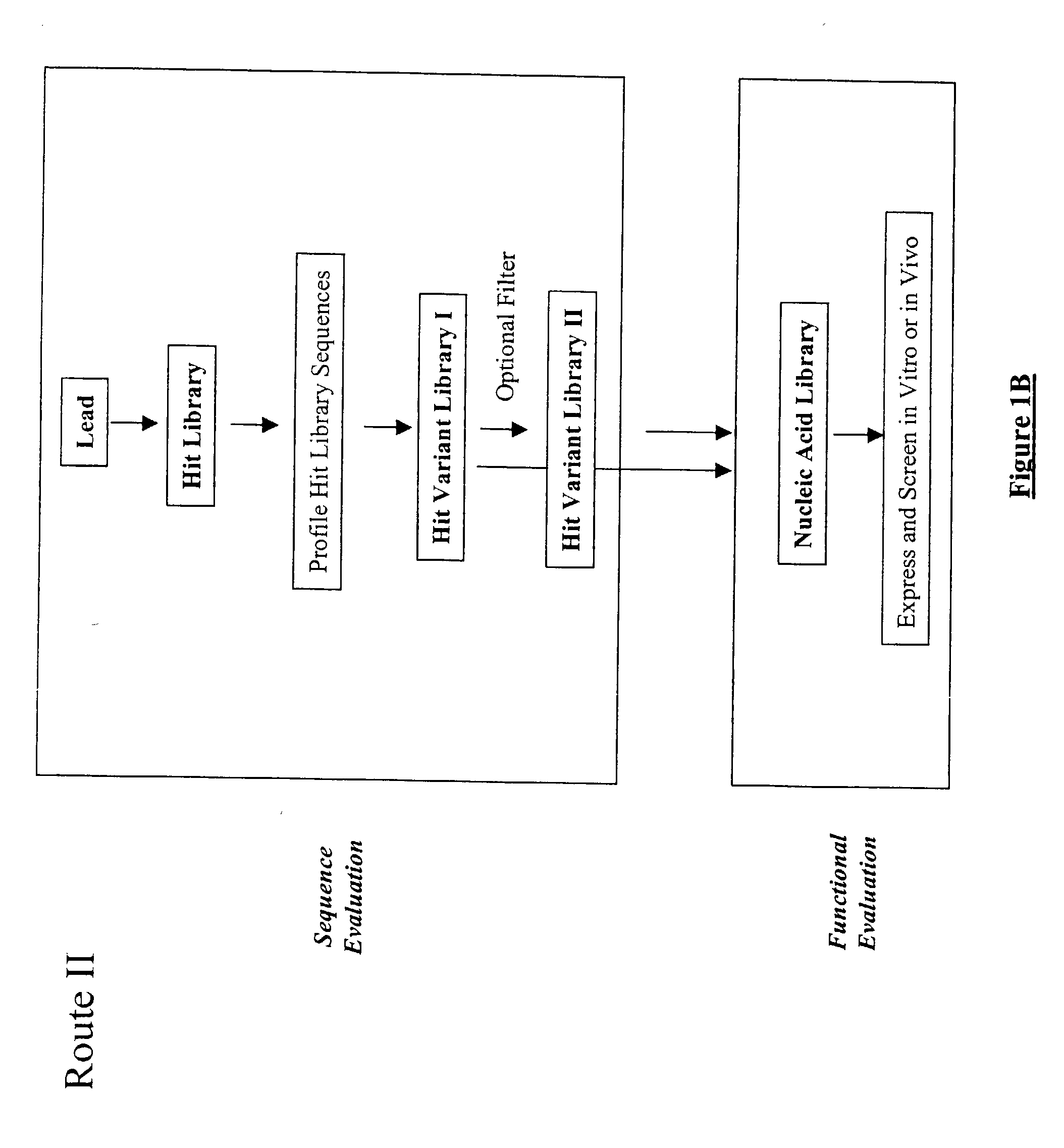
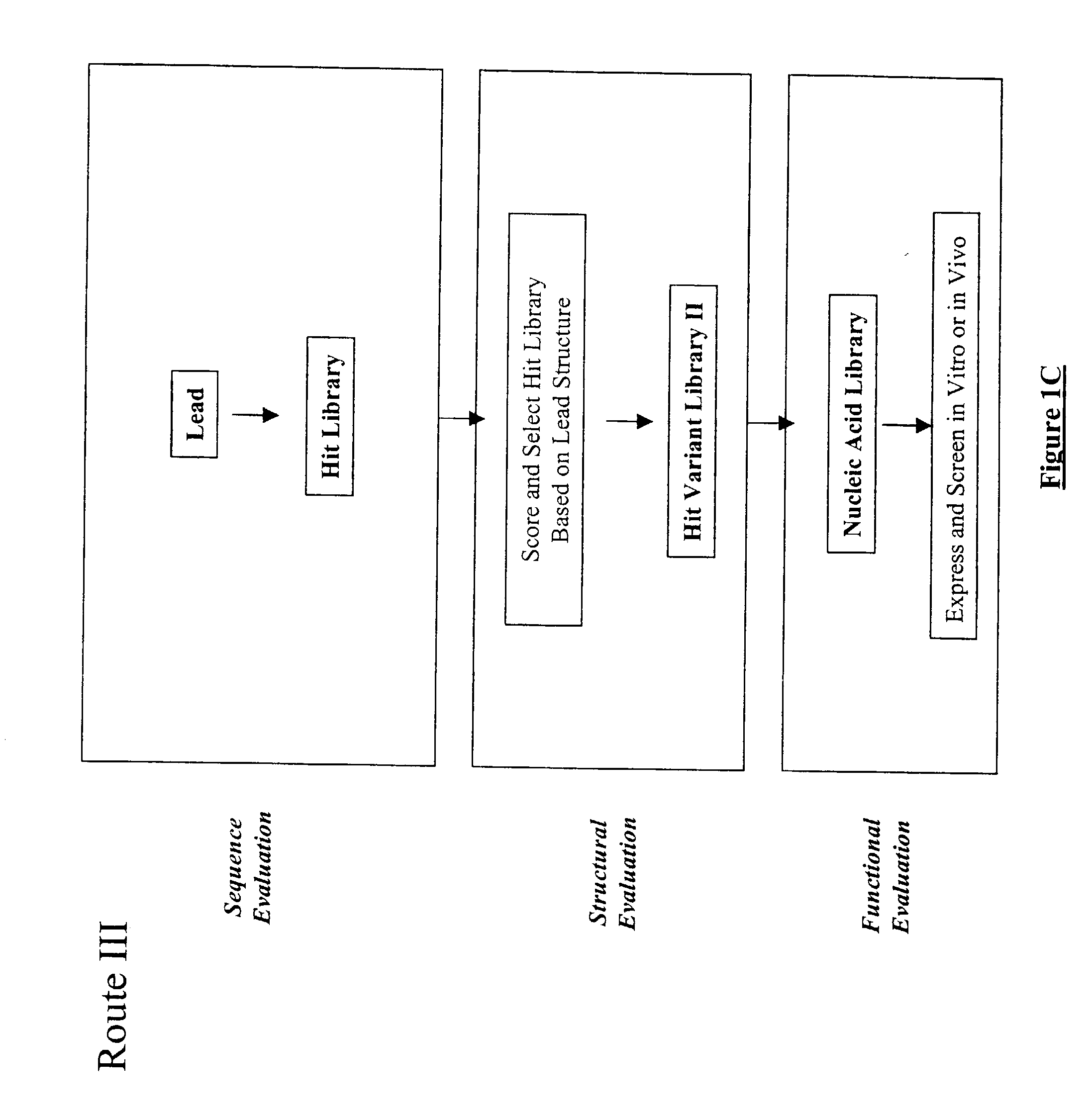
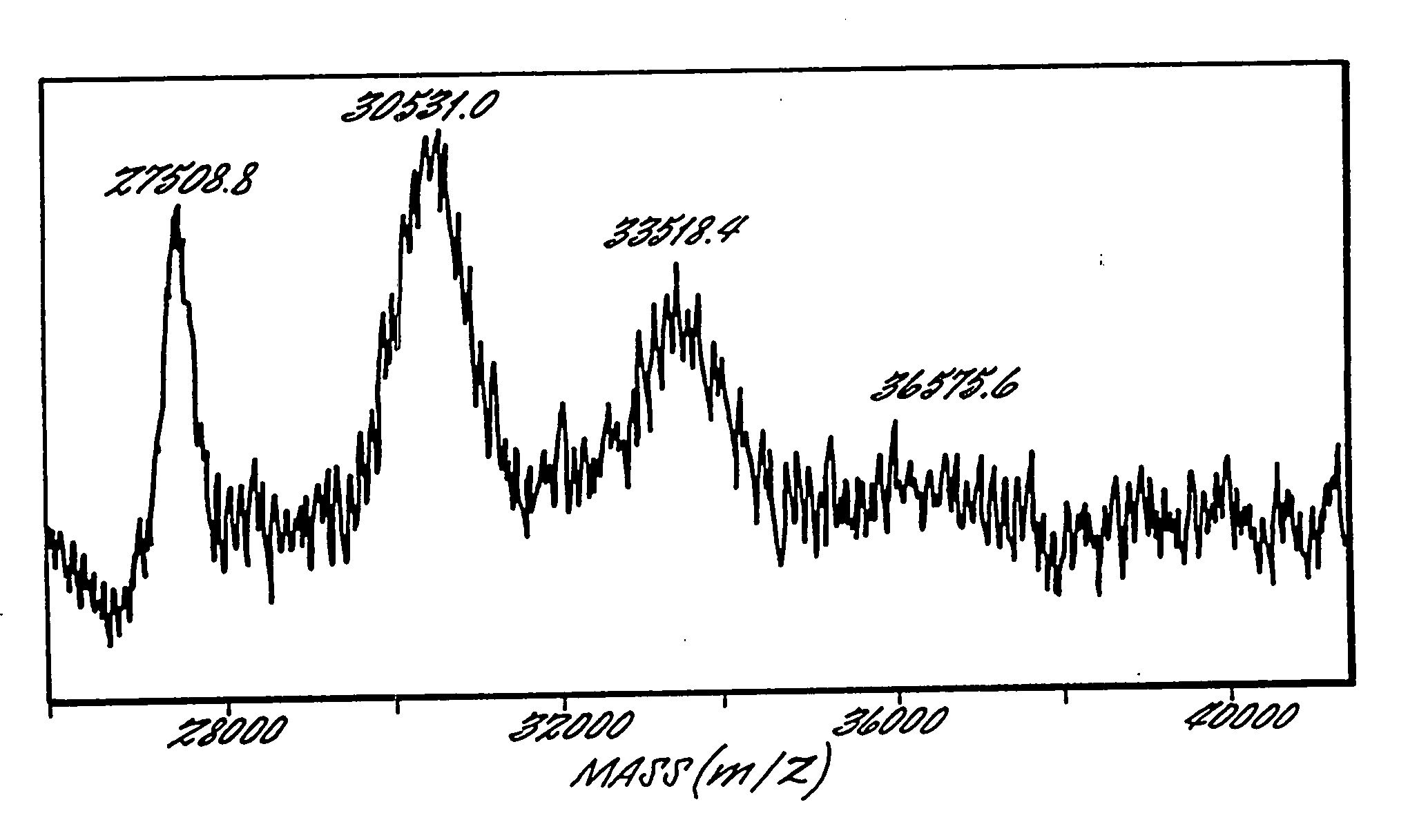
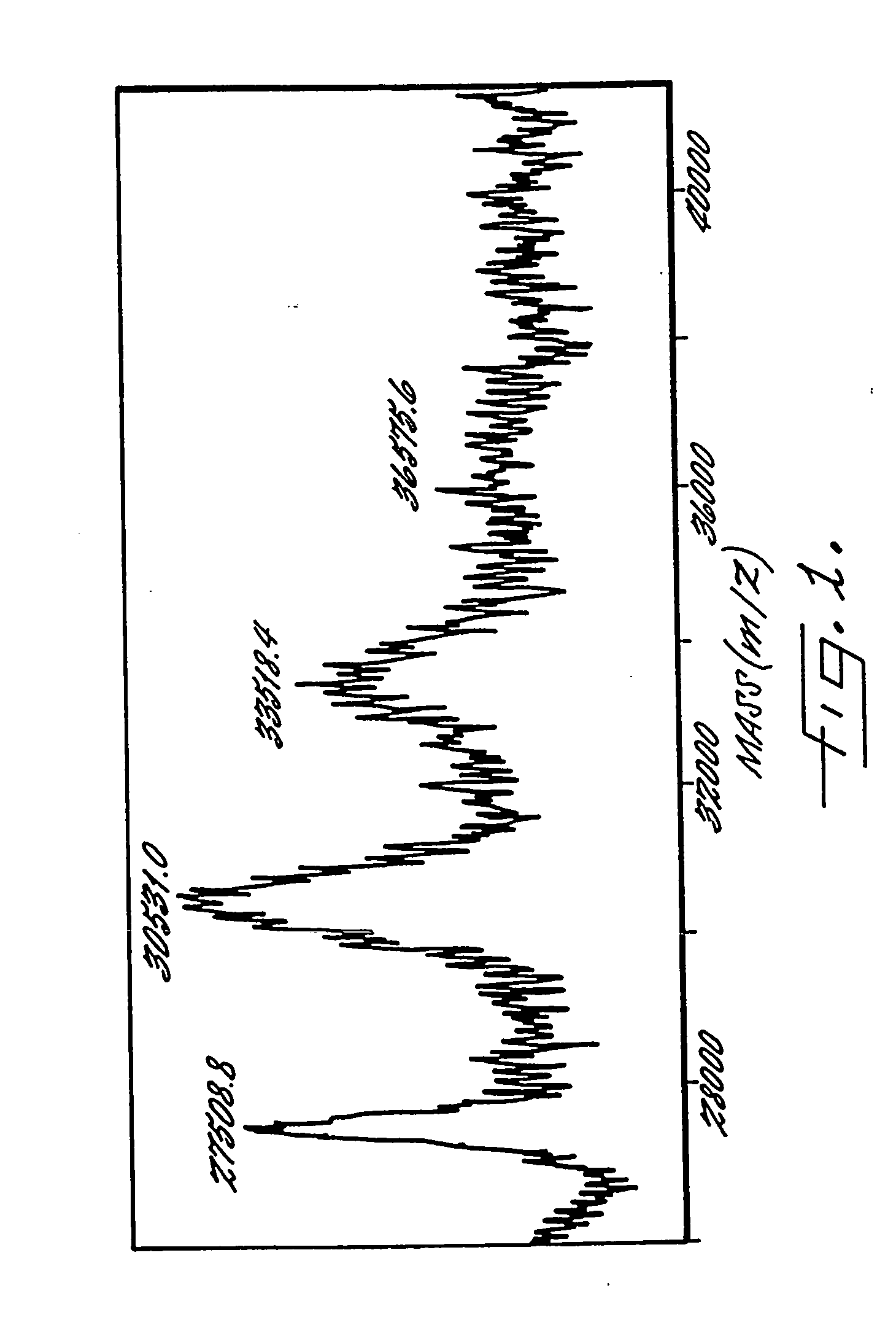
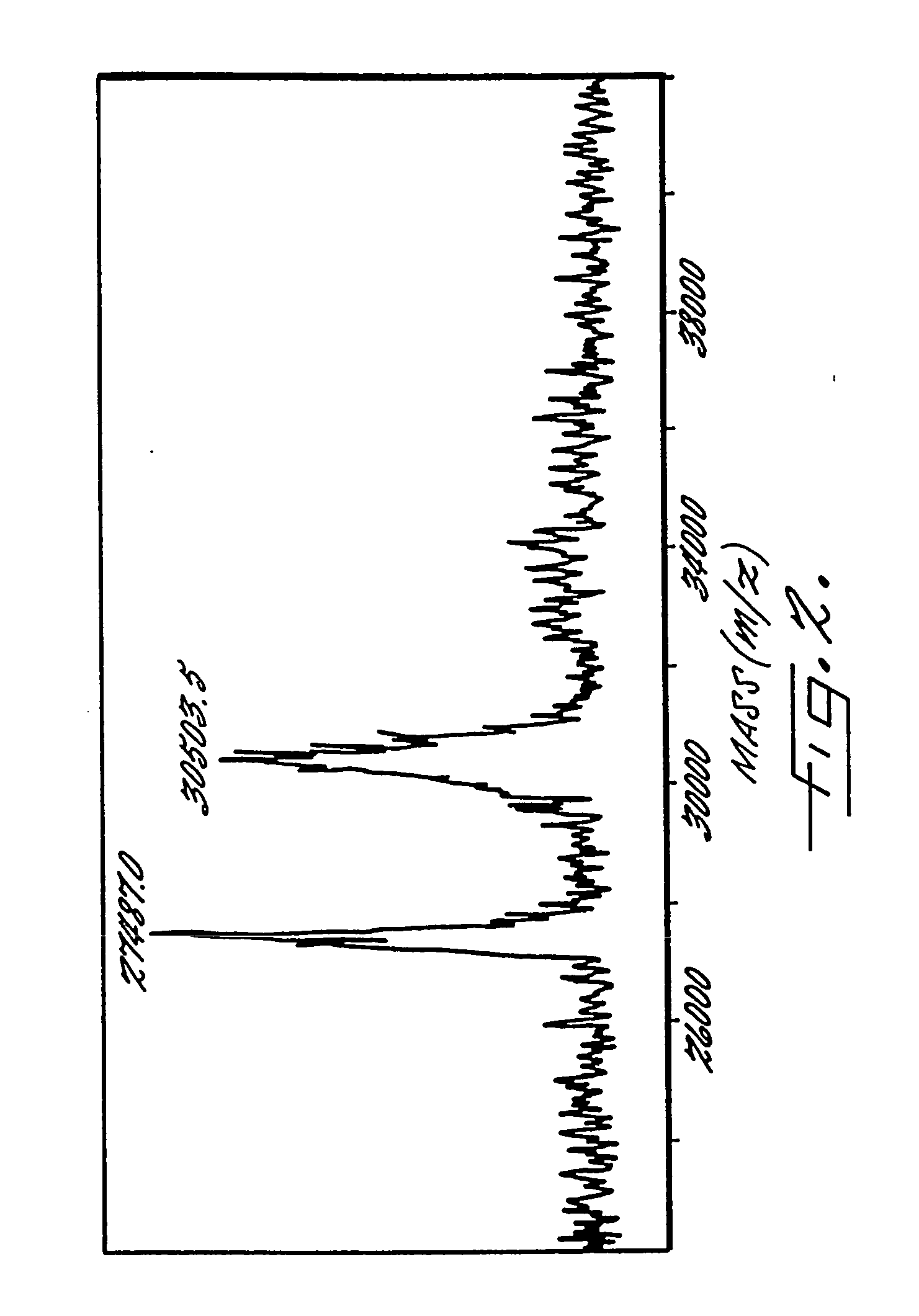
The present invention provides a structure-based methodology for efficiently generating and screening protein libraries for optimized proteins with desirable biological functions, such as antibodies with high binding affinity and low immunogenicity in humans. In one embodiment, a method is provided for constructing a library of antibody sequences based on a three dimensional structure of a lead antibody. The method comprises: providing an amino acid sequence of the variable region of the heavy chain (VH) or light chain (VL) of a lead antibody, the lead antibody having a known three dimensional structure which is defined as a lead structural template; identifying the amino acid sequences in the CDRs of the lead antibody; selecting one of the CDRs in the VH or VL region of the lead antibody; providing an amino acid sequence that comprises at least 3 consecutive amino acid residues in the selected CDR, the selected amino acid sequence being a lead sequence; comparing the lead sequence profile with a plurality of tester protein sequences; selecting from the plurality of tester protein sequences at least two peptide segments that have at least 10% sequence identity with lead sequence, the selected peptide segments forming a hit library; determining if a member of the hit library is structurally compatible with the lead structural template using a scoring function; and selecting the members of the hit library that score equal to or better than or equal to the lead sequence. The selected members of the hit library can be expressed in vitro or in vivo to produce a library of recombinant antibodies that can be screened for novel or improved function(s) over the lead antibody.
Owner:ABMAXIS
Soluble, degradable poly(ethylene glycol) derivatives for conrollable release of bound molecules into solution
InactiveUS20050171328A1Reduce rateEnhance solubility and circulation lifetimePharmaceutical non-active ingredientsSynthetic polymeric active ingredientsSolubilityIncreased sizes
PEG and related polymer derivatives having weak, hydrolytically unstable linkages near the reactive end of the polymer are provided for conjugation to drugs, including proteins, enzymes, small molecules, and others. These derivatives provide a sufficient circulation period for a drug-PEG conjugate, followed by hydrolytic breakdown of the conjugate and release of the bound molecule. In some cases, drugs that demonstrate reduced activity when permanently coupled to PEG maintain a therapeutically suitable activity when coupled to a degradable PEG in accordance with the invention. The PEG derivatives of the invention can be used to impart improved water solubility, increased size, a slower rate of kidney clearance, and reduced immunogenicity to a conjugate formed by attachment thereto. Controlled hydrolytic release of the bound molecule into an aqueous environment can then enhance the drug's delivery profile by providing a delivery system which employs such polymers and utilizes the teachings provided herein.
Owner:NEKTAR THERAPEUTICS INC
Production of humanized antibodies in transgenic animals
InactiveUS7129084B2Low immunogenicityUseful in therapyImmunoglobulins against bacteriaImmunoglobulins against virusesHuman animalGene conversion
This invention relates to humanized antibodies and antibody preparations produced from transgenic non-human animals. The non-human animals are genetically engineered to contain one or more humanized immunoglobulin loci which are capable of undergoing gene rearrangement and gene conversion in the transgenic non-human animals to produce diversified humanized immunoglobulins. The present invention further relates to novel sequences, recombination vectors and transgenic vectors useful for making these transgenic animals. The humanized antibodies of the present invention have minimal immunogenicity to humans and are appropriate for use in the therapeutic treatment of human subjects.
Owner:THERAPEUTIC HUMAN POLYCLONALS
2'-f modified RNA interference agents
InactiveUS20110269814A1Inhibit expressionSuppress gene expressionOrganic active ingredientsSugar derivativesNucleotideSense strand
This invention relates to a method of modulating the expression of a target gene in an organism comprising administering an iRNA agent, wherein the iRNA comprises at least one 2′-deoxy-2′-fluoro (2′-F) nucleotide in the antisense strand and at least one modified nucleotide in the sense strand. The invention also relates to compositions comprising a single-stranded oligonucleotide that contains at least one 2′-deoxy-2′-fluoro (2′-F) nucleotide. siRNA molecule containing these oligonucleotides have decreased immunogenicity.
Owner:ALNYLAM PHARM INC
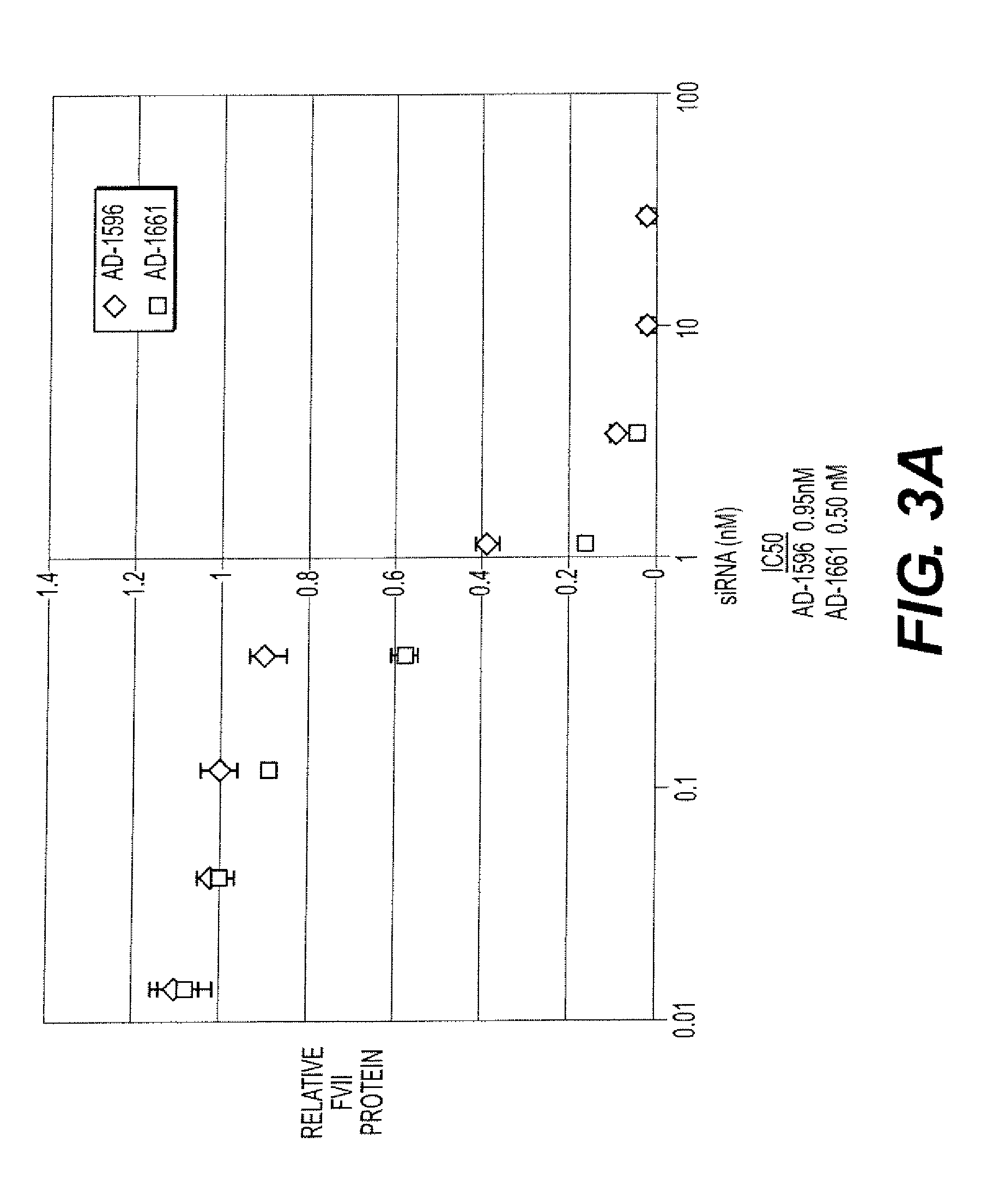
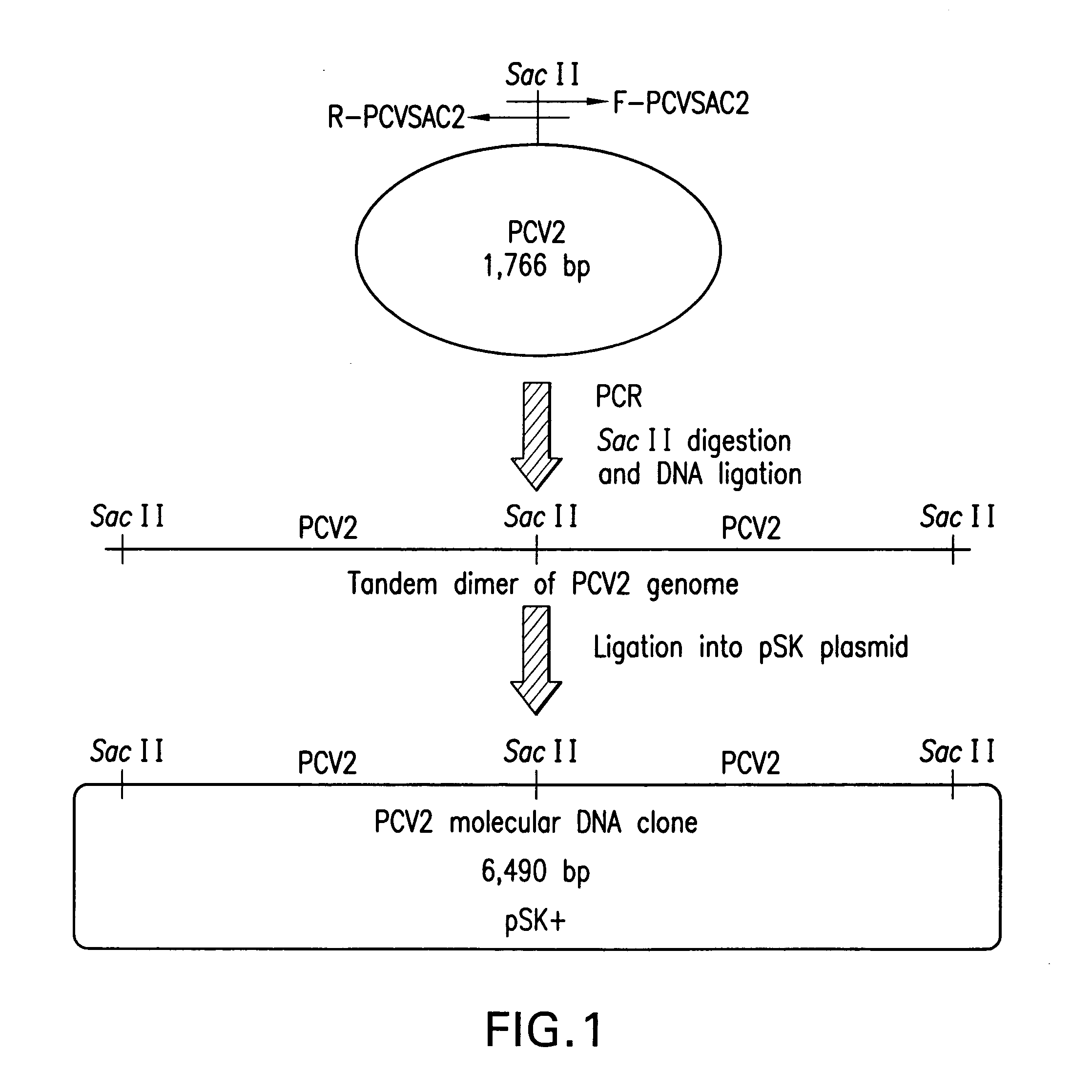
Chimeric infectious DNA clones, chimeric porcine circoviruses and uses thereof
InactiveUS7279166B2Facilitate cell culture growthEnsure vaccine safetyFungiBacteriaSpecific immunityADAMTS Proteins
The present invention relates to infectious DNA clones, infectious chimeric DNA clones of porcine circovirus (PCV), vaccines and means of protecting pigs against viral infection or postweaning multisystemic wasting syndrome (PMWS) caused by PCV2. The new chimeric infectious DNA clone and its derived, avirulent chimeric virus are constructed from the nonpathogenic PCV1 in which the immunogenic ORF gene of the pathogenic PCV2 replaces a gene of the nonpathogenic PCV1, preferably in the same position. The chimeric virus advantageously retains the nonpathogenic phenotype of PCV1 but elicits specific immune responses against the pathogenic PCV2. The invention further embraces the immunogenic polypeptide expression products. In addition, the invention encompasses two mutations in the PCV2 immunogenic capsid gene and protein, and the introduction of the ORF2 mutations in the chimeric clones.
Owner:IOWA STATE UNIV RES FOUND +1
Immunogenic compositions and methods of use thereof
The present invention provides an immunogenic composition comprising lethally irradiated bacteria formulated for mucosal delivery. The present invention further provides methods of preparing a subject immunogenic composition. The present invention further provides a method of inducing an immune response in an individual to an antigen, the method generally involving administering a subject immunogenic composition to a mucosal tissue of the individual.
Owner:RGT UNIV OF CALIFORNIA
Multi plasmid system for the production of influenza virus
InactiveUS20050266026A1Easy to copyEnhanced ability to replicateSsRNA viruses negative-senseVectorsEmbryonated chicken eggCold adapted
Vectors and methods for the production of influenza viruses suitable as recombinant influenza vaccines in cell culture are provided. Bi-directional expression vectors for use in a multi-plasmid influenza virus expression system are provided. Additionally, the invention provides methods of producing influenza viruses with enhanced ability to replicate in embryonated chicken eggs and / or cells (e.g., Vero and / or MDCK) and further provides influenza viruses with enhanced replication characteristics. A method of producing a cold adapted (ca) influenza virus that replicates efficiently at, e.g., 25° C. (and immunogenic compositions comprising the same) is also provided.
Owner:MEDIMMUNE LLC
Reshaped human antibody to human interleukin-6
InactiveUS6121423AUseful purposeAntibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsDiseaseV region
A reshaped antibody comprising: (A) L chains comprising: (1) a human C region, and (2) an L chain V region comprising human L chain FRs and L chain CDRs of a mouse monoclonal antibody; and (B) H chains comprising: (1) a human H chain C region, and (2) an H chain V region comprising human H chain FRs, and H chain CDRs of a mouse monoclonal antibody to human IL-6. Since the major portions of the reshaped human antibody are derived from human, and the mouse CDRs are less immunogenic, then the present reshaped human antibody is less immunogenic, and therefore inhibits information transfer by IL-6, and is promising as a therapeutic agent for diseases caused by IL-6.
Owner:CHUGAI PHARMA CO LTD
Composite gel microparticles as active principle carriers
The invention relates to vectors for delivering medicinal, nutritional, plant-protection or cosmetic active principles, these delivery particles being of small, controllable and adjustable particle size, which protect the active principle, and being biocompatible, biodegradable, non-immunogenic, stable and free of solvent. The particles do not denature the active principle and allow the active principle to be released. The microparticles of the invention are of a cohesive structure made of a physicochemically stable and integral composite gel which includes an oil such as coconut oil, an aqueous phase and a linear, non-crosslinked copolyamino acid of Leu / Glu type (random or diblock). The microparticles have a controllable and adjustable size of between 0.05 and 500 mum.
Owner:FLAMEL TECHNOLOGIES
Insect cells or fractions as adjuvant for antigens
Disclosed and claimed is an adjuvant for immunogenic, immunological, antigenic or vaccine compositions. The adjuvant is composed of insect cells or fractions thereof. Disclosed and claimed are also methods for preparing and using the adjuvant and compositions containing the adjuvant. Advantageously, a recombinant baculovirus containing DNA encoding and expressing an epitope of interest or antigen can be infected into insect cells such as insect cells derived from a Lepidopteran species such as S. frugiperda for expression, and the infected insect cells or a fraction thereof can be used with the expressed epitope of interest or antigen as an inventive antigen or in an inventive immunological, antigen or vaccine composition.
Owner:MERIAL LTD
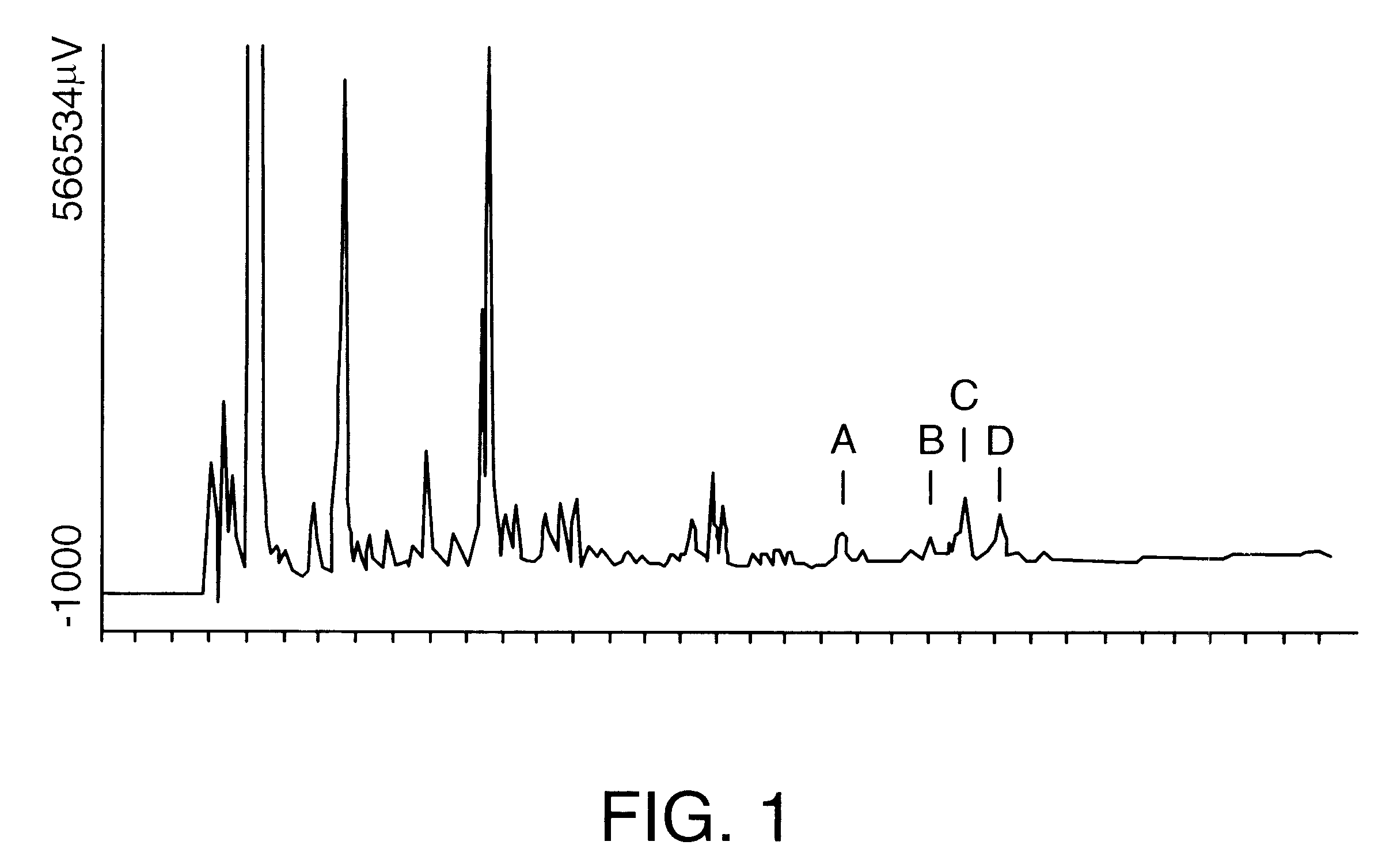
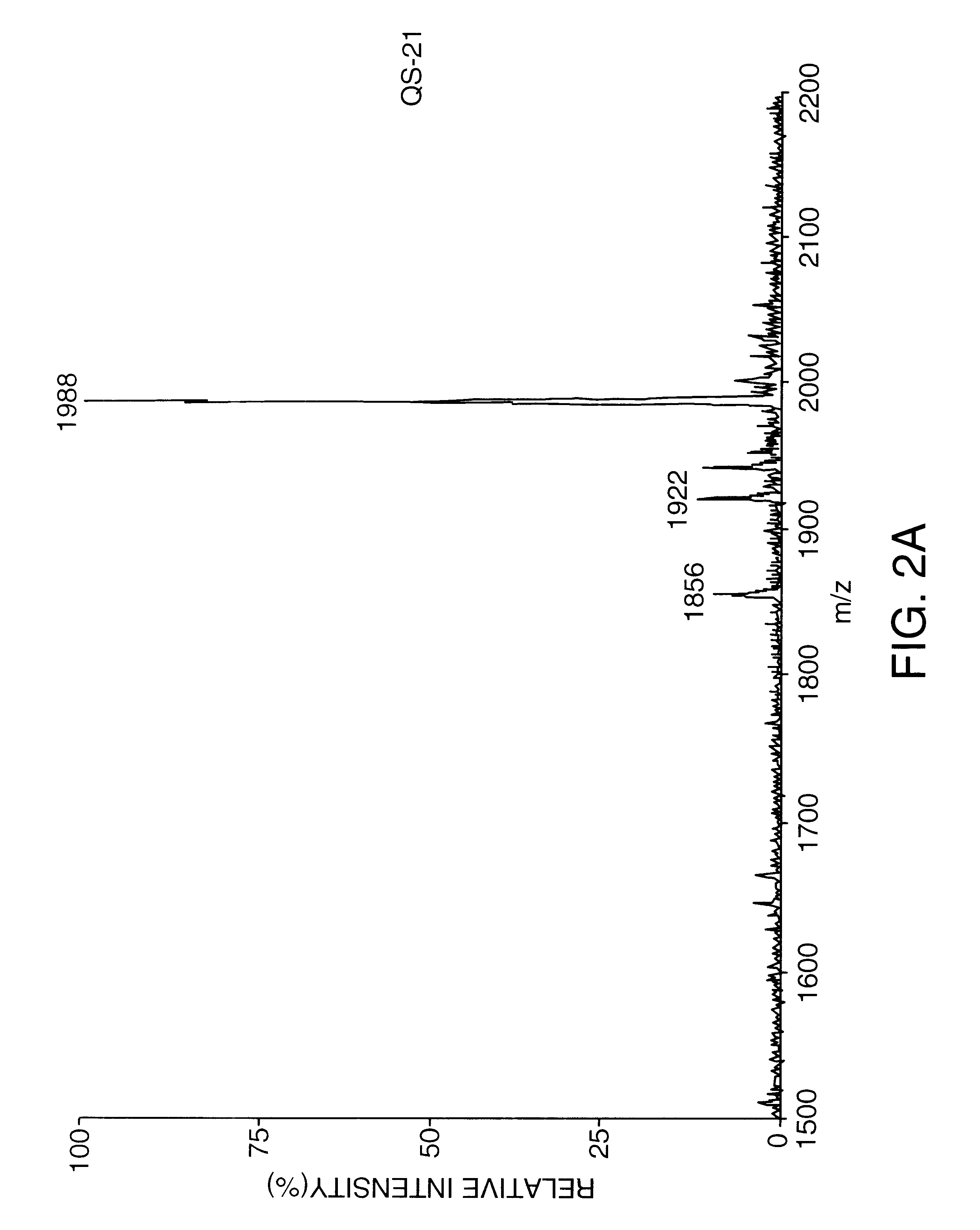
Saponin compositions and uses thereof
Owner:ANTIGENICS
Prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases with heat shock/stress protein-peptide complexes
InactiveUS6017540AEnhancing host 's immunocompetenceHigh activityBiocidePeptide/protein ingredientsStress ProteinsIn vivo
The present invention relates to methods and compositions for eliciting an immune response and the prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases. The methods of the invention comprise administering a composition comprising an effective amount of a complex, in which the complex consists essentially of a heat shock protein (hsp) noncovalently bound to an antigenic molecule. Optionally, the methods further comprise administering antigen presenting cells sensitized with complexes of hsps noncovalently bound to an antigenic molecule. "Antigenic molecule" as used herein refers to the peptides with which the hsps are endogenously associated in vivo as well as exogenous antigens / immunogens (i.e., with which the hsps are not complexed in vivo) or antigenic / immunogenic fragments and derivatives thereof. In a preferred embodiment, the complex is autologous to the individual. In a specific embodiment, the effective amounts of the complex are in the range of 0.1 to 9.0 micrograms for complexes comprising hsp70, 5 to 49 micrograms for hsp90, and 0.1 to 9.0 micrograms for gp96.
Owner:FORDHAM UNIVERSITY
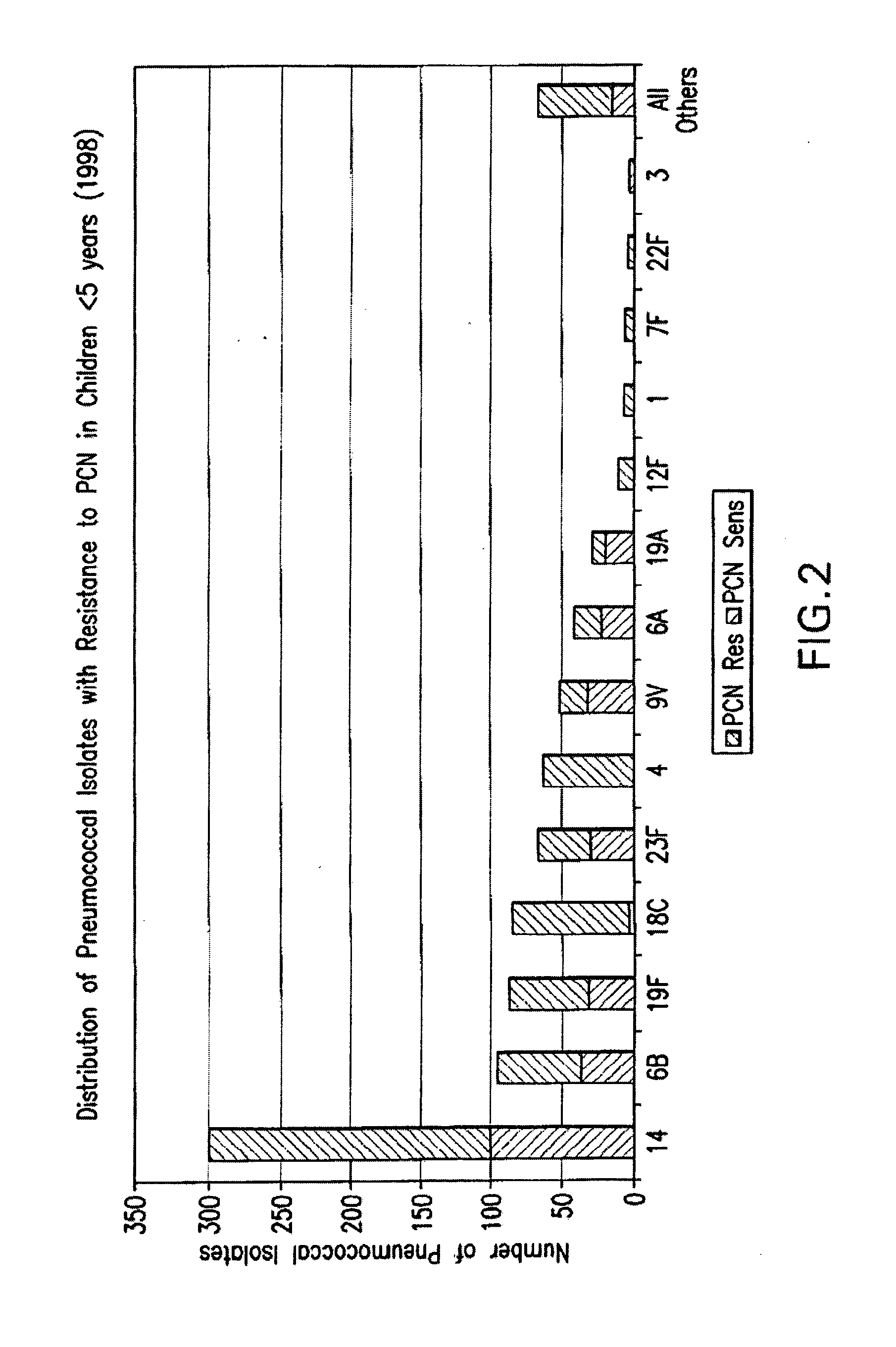
Streptococcus pneumoniae 37-kDa surface adhesin a protein
The invention provides a nucleic acid encoding the 37-kDa protein from Streptococcus pneumoniae. Also provided are isolated nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. The invention also provides purified polypeptides encoded by the nucleic acid encoding the 37-kDa protein from and the nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. Also provided are antibodies which selectively binds the polypeptides encoded by the nucleic acid encoding the 37-kDa protein and the nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. Also provided are vaccines comprising immunogenic polypeptides encoded by the nucleic acid encoding the 37-kDa protein and the nucleic acids comprising a unique fragment of at least 10 nucleotides of the 37-kDa protein. Further provided is a method of detecting the presence of Streptococcus pneumoniae in a sample comprising the steps of contacting a sample suspected of containing Streptococcus pneumoniae with nucleic acid primers capable of hybridizing to a nucleic acid comprising a portion of the nucleic acid encoding the 37-kDa protein, amplifying the nucleic acid and detecting the presence of an amplification product, the presence of the amplification product indicating the presence of Streptococcus pneumoniae in the sample. Further provided are methods of detecting the presence of Streptococcus pneumoniae in a sample using antibodies or antigens, methods of preventing and treating Streptococcus pneumoniae infection in a subject.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com