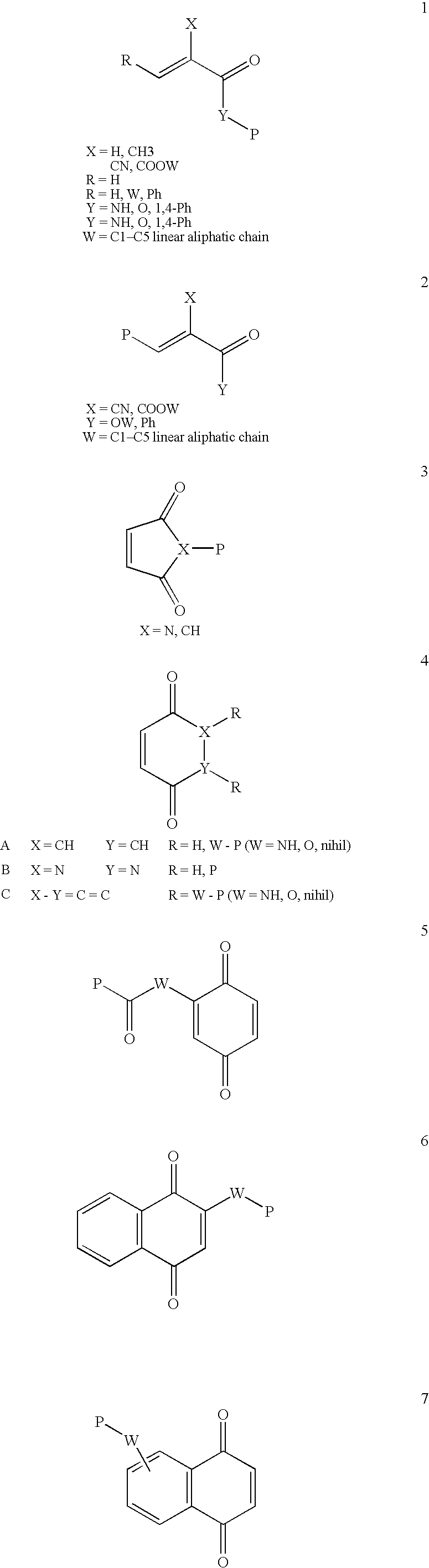
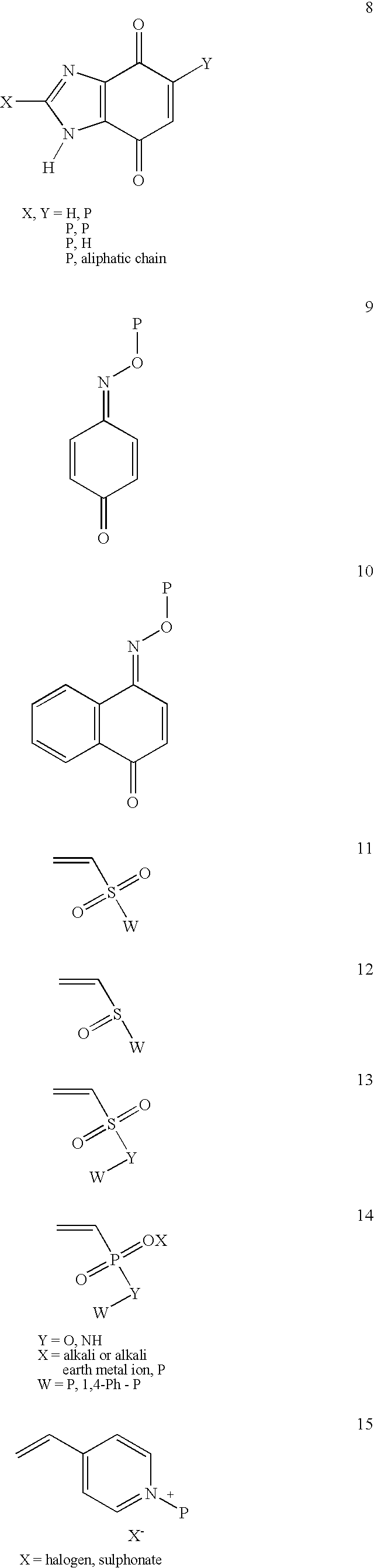
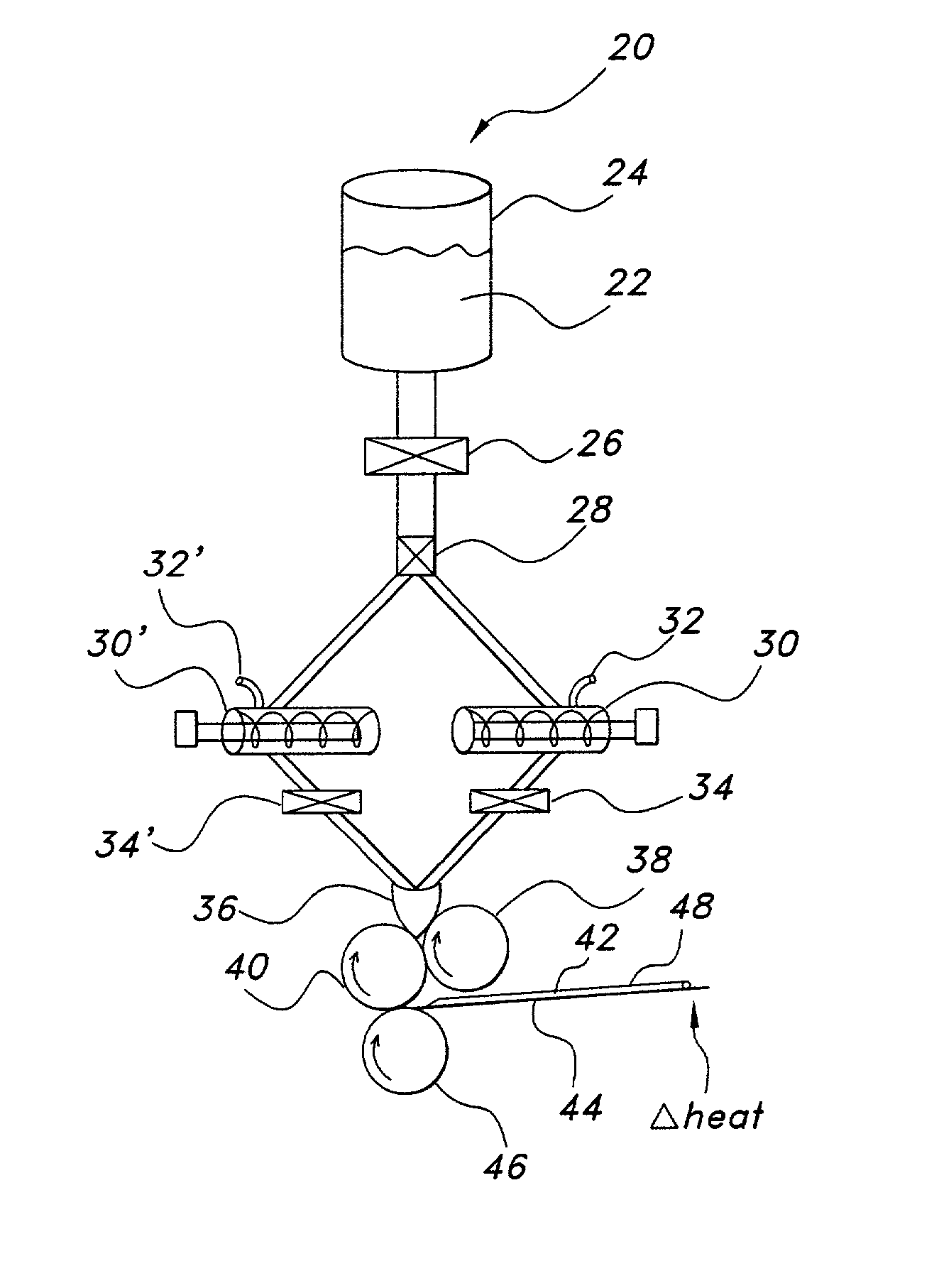
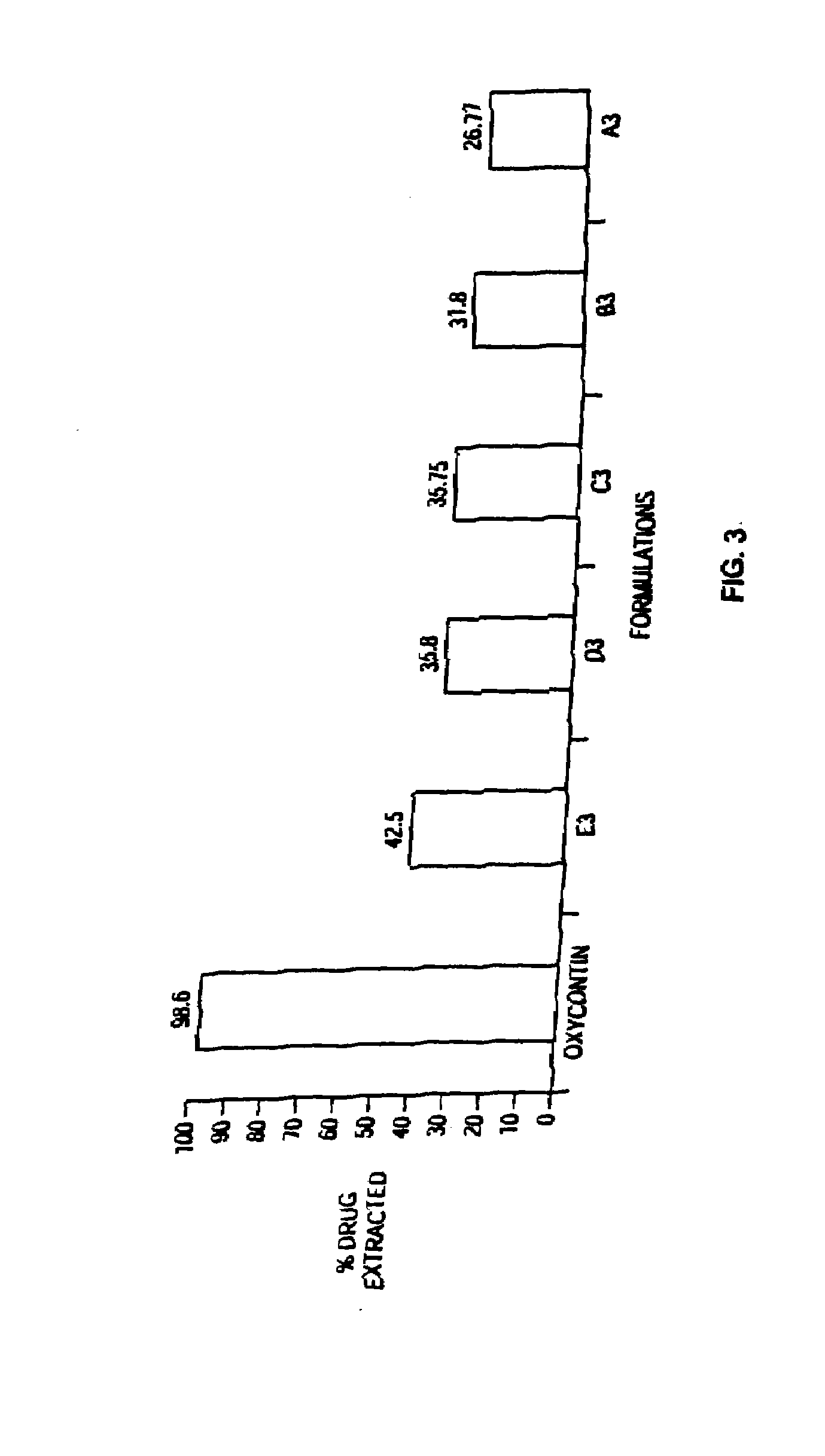
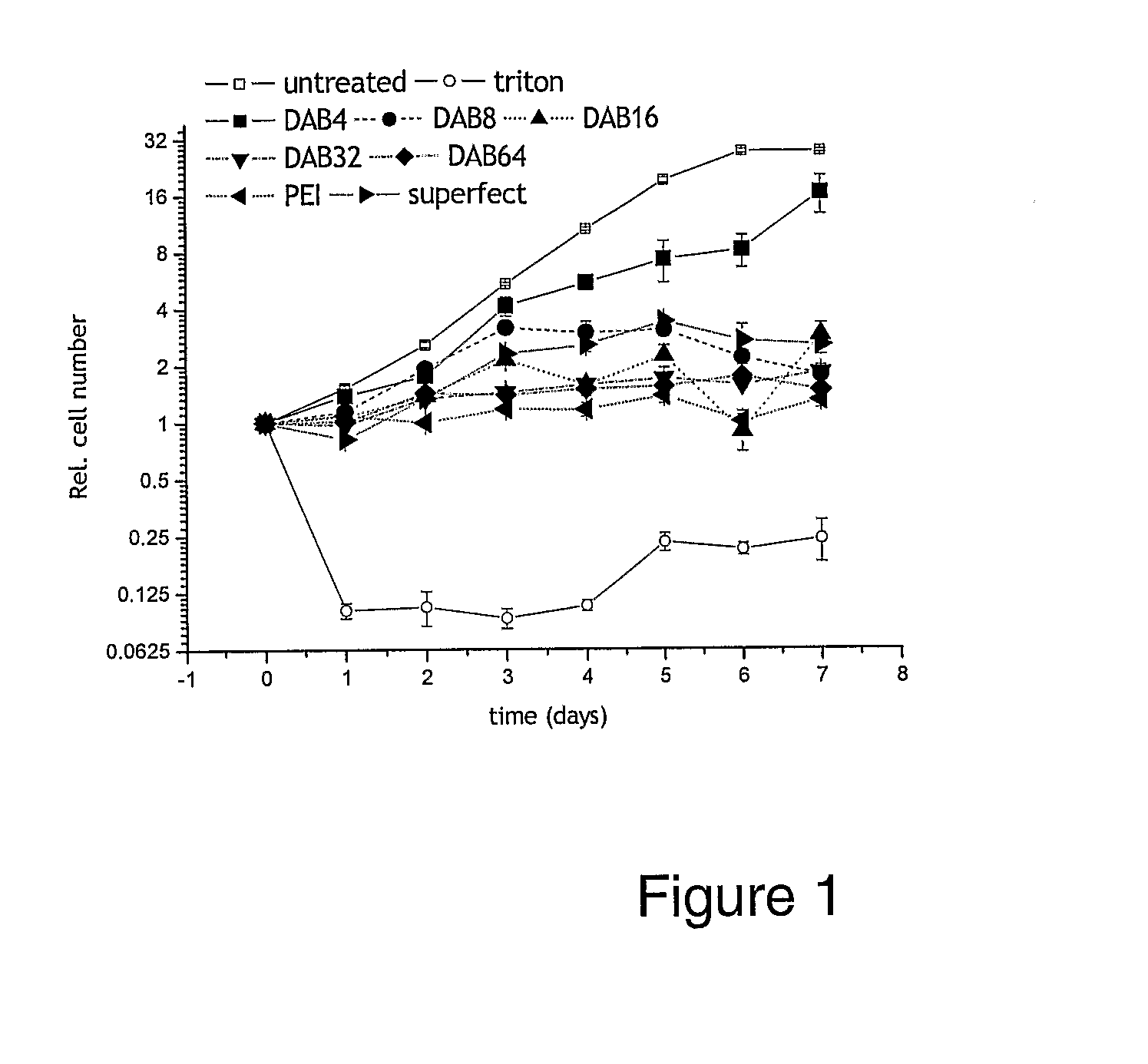
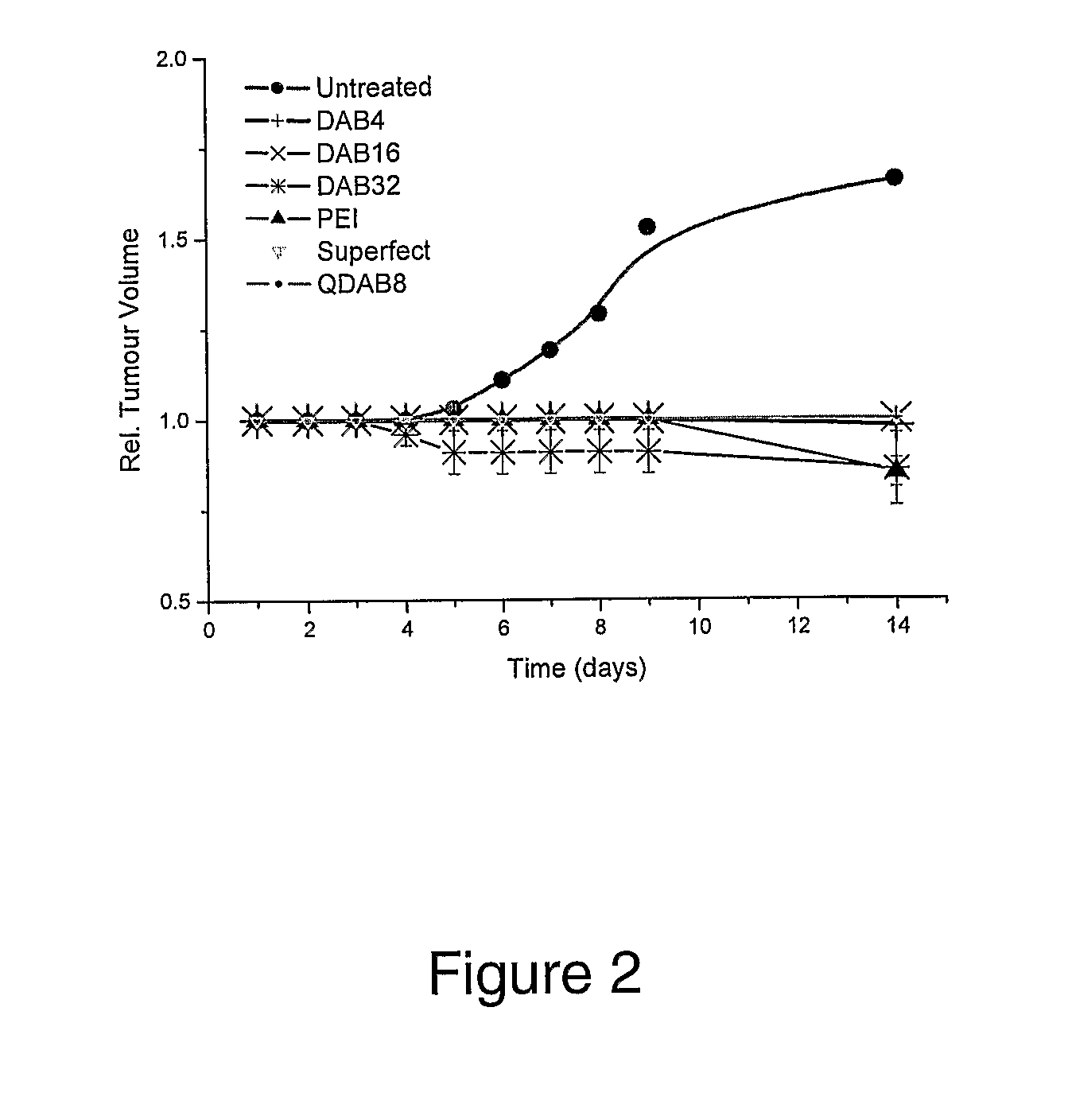
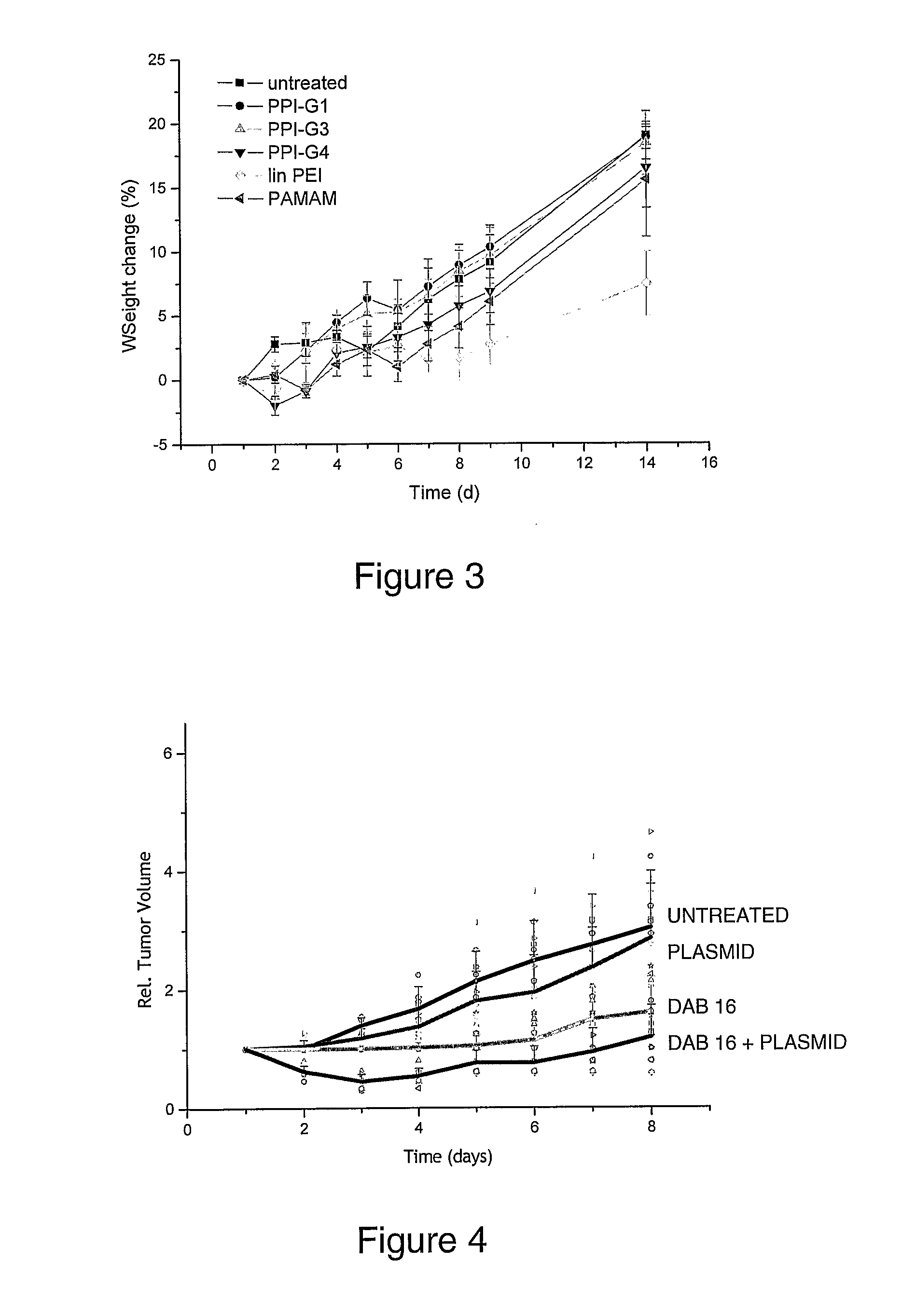
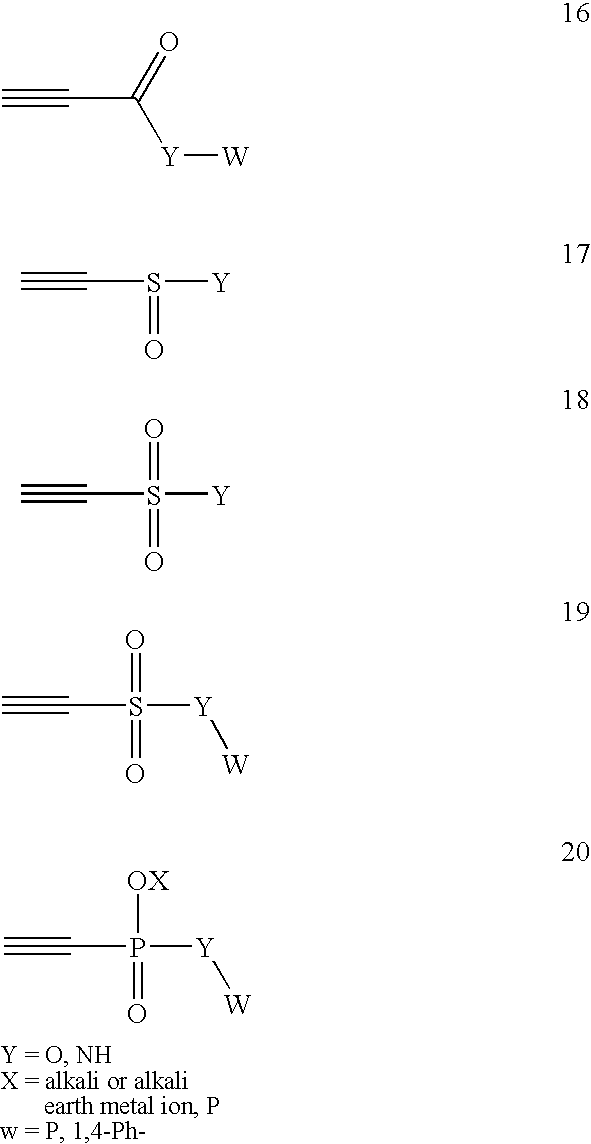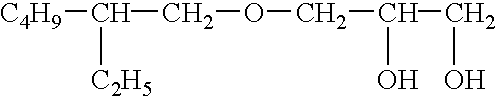Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4135results about "Synthetic polymeric active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Adhesive formulations
ActiveUS8349987B2Organic non-macromolecular adhesiveSynthetic polymeric active ingredientsArylPolymer science
The disclosure relates to biocompatible components useful for forming compositions for use as medical / surgical synthetic adhesives and sealants. Biocompatible components of the present disclosure may include a polymeric polyol core, which may be treated with a nitroaryl compound to form a nitro ester. The resulting nitro ester groups may be reduced to form amino groups which, in turn, may be treated to form isocyanate groups. The resulting isocyanate may then be reacted with a second component to form adhesive and / or sealant compositions.
Owner:COVIDIEN LP
Methods and compositions for reducing or eliminating post-surgical adhesion formation
The present invention relates to a method for reducing adhesions associated with post-operative surgery. The present method comprises administering or affixing a polymeric composition preferably comprising chain extended, coupled or crosslinked polyester / poly(oxyalkylene) ABA triblocks or AB diblocks having favorable EO / LA ratios to a site in the body which has been subjected to trauma, e.g. by surgery, excision or inflammatory disease. In the present invention, the polymeric material provides a barrier to prevent or reduce the extent of adhesions forming.
Owner:YISSUM RES DEV CO OF THE HEBREW UNIV OF JERUSALEM LTD
High molecular wegiht polymers, devices and method for making and using same
Anhydride polymers that release active or activatable agent(s) have pre-selected properties such as molecular weight, flexibility, hardness, adhesiveness, and other valuable properties. The polymers are suitable for use in compositions, formulations, coatings, devices, and the like that benefit from the controlled release of an agent(s) over a period of time. The polymers are prepared by a process involving various alternative and sequential steps that allow the design a priori of products with specific characteristics. The polymers are suitable as delivery systems, either by themselves, as compositions, formulations or devices.
Owner:RUTGERS THE STATE UNIV
Articles having bioactive surfaces and solvent-free methods of preparation thereof
Methods for preparing articles having a bioactive surface comprising treating a substrate to form free reactive groups, depositing a monomer onto the treated substrate, and covalently immobilizing a biologically functional molecule onto the deposited monomer. Additional embodiments include methods for the deposition of the monomer onto the treated substrate in a solvent-free environment. Further embodiments include articles having surfaces prepared using the methods described herein. Additional embodiments include articles prepared using the methods described herein.
Owner:BECTON DICKINSON & CO
Breathable polyurethanes, blends, and articles
InactiveUS6897281B2Improve breathabilityImprove moisture vapor transmission rateSynthetic resin layered productsPolyurea/polyurethane coatingsGramSide chain
A breathable polyurethane having an upright moisture vapor transmission rate (MVTR) of more than about 500 gms / m2 / 24 hr comprises:(a) poly(alkylene oxide) side-chain units in an amount comprising about 12 wt. % to about 80 wt. % of said polyurethane, wherein (i) alkylene oxide groups in said poly(alkylene oxide) side-chain units have from 2 to 10 carbon atoms and are unsubstituted, substituted, or both unsubstituted and substituted, (ii) at least about 50 wt. % of said alkylene oxide groups are ethylene oxide, and (iii) said amount of said side-chain units is (i) at least about 30 wt. % when the molecular weight of said side-chain units is less than about 600 grams / mole, (ii) at least about 15 wt. % when the molecular weight of said side-chain units is from about 600 to about 1,000 grams / mole, and at least about 12 wt. % when the molecular weight of said side-chain units is more than about 1,000 grams / mole, and(b) poly(ethylene oxide) main-chain units in an amount comprising less than about 25 wt. % of said polyurethane.Coatings and films for textiles and other articles and applications using such polyurethanes have excellent breathability, i.e., high moisture vapor transmission rates (MVTR).
Owner:LUBRIZOL ADVANCED MATERIALS INC
Methods and topical formulations comprising colloidal metal for treating or preventing skin conditions
In preferred embodiments, the present invention relates to compositions comprising colloidal metals and / or metals for the treatment and prevention of skin conditions and / or diseases. More specifically, the disclosed metal containing compositions are useful as antioxidants, anti-aging agents, anti-wrinkle agents, anti-peroxidation agents, antimicrobial agents, anti-inflammatory agents, pain-relieving agents, wound recovery agents, sun-screens, sunblocks, and integument and skin-supporting agents when applied to the skin / integument, or administered generally to an animal or human body.
Owner:MARGULIES JOEL +1
Synovial fluid barrier
InactiveUS20060178743A1Improve regenerative healingImprove responseSuture equipmentsPeptide/protein ingredientsSynovial jointsSacroiliac joint
A composite tissue formed in situ is provided. The composite tissue includes a synovial joint tissue; and a barrier material adhered thereto for sealing the synovial joint tissue against synovial fluid. Also provided is a method for regenerating synovial joint tissue in situ by excluding synovial fluid therefrom. The method includes providing a synovial joint tissue having a defect; and placing a barrier material in intimate contact with the defect for sealing the defect against synovial fluid. The barrier material includes a curable protein copolymer. The method further includes curing the protein copolymer in situ. The barrier material can include a crosslinked network, or a self gelled network of repeating elastin-like and fibroin-like polymer chains.
Owner:SPINEWAVE
Conjugate addition reactions for the controlled delivery of pharmaceutically active compounds
InactiveUS6958212B1Reducing and delaying onsetGood water solubilitySugar derivativesPeptide/protein ingredientsBiological materialsPolymer
The invention features polymeric biomaterials formed by nucleophilic addition reactions to conjugated unsaturated groups. These biomaterials may be used for medical treatments.
Owner:ETH ZZURICH +1
Thin film with non-self-aggregating uniform heterogeneity and drug delivery systems made therefrom
The invention relates to the film products and methods of their preparation that demonstrate a non-self-aggregating uniform heterogeneity. Desirably the films disintegrate in water and may be formed by a controlled drying process, or other process that maintains the required uniformity of the film.
Owner:AQUESTIVE THERAPEUTICS INC
Personal Care Compositions Containing At Least Two Cationic Polymers and an Anionic Surfactant
A personal cleansing composition comprising:a. from about 5% to about 50% by weight of an anionic detersive surfactant;b. from about 0.025% to about 5% by weight of a first cationic polymer having a cationic charge density of less than about 4 meq / gm, wherein said first cationic polymer forms an isotropic coacervate;c. from about 0.025% to about 5% by weight of a second cationic polymer having a cationic charge density of greater than or equal to about 4 meq / gm, wherein said second cationic polymer forms a lyotropic liquid crystal coacervate; andd. from about 20% to about 94% by weight of water.
Owner:THE PROCTER & GAMBLE COMPANY
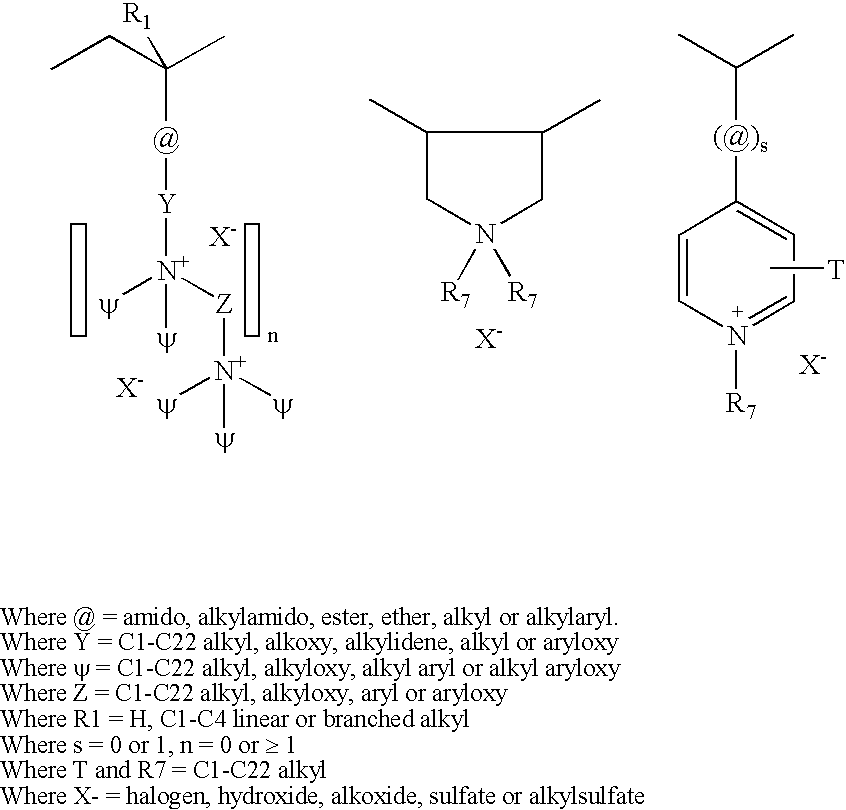
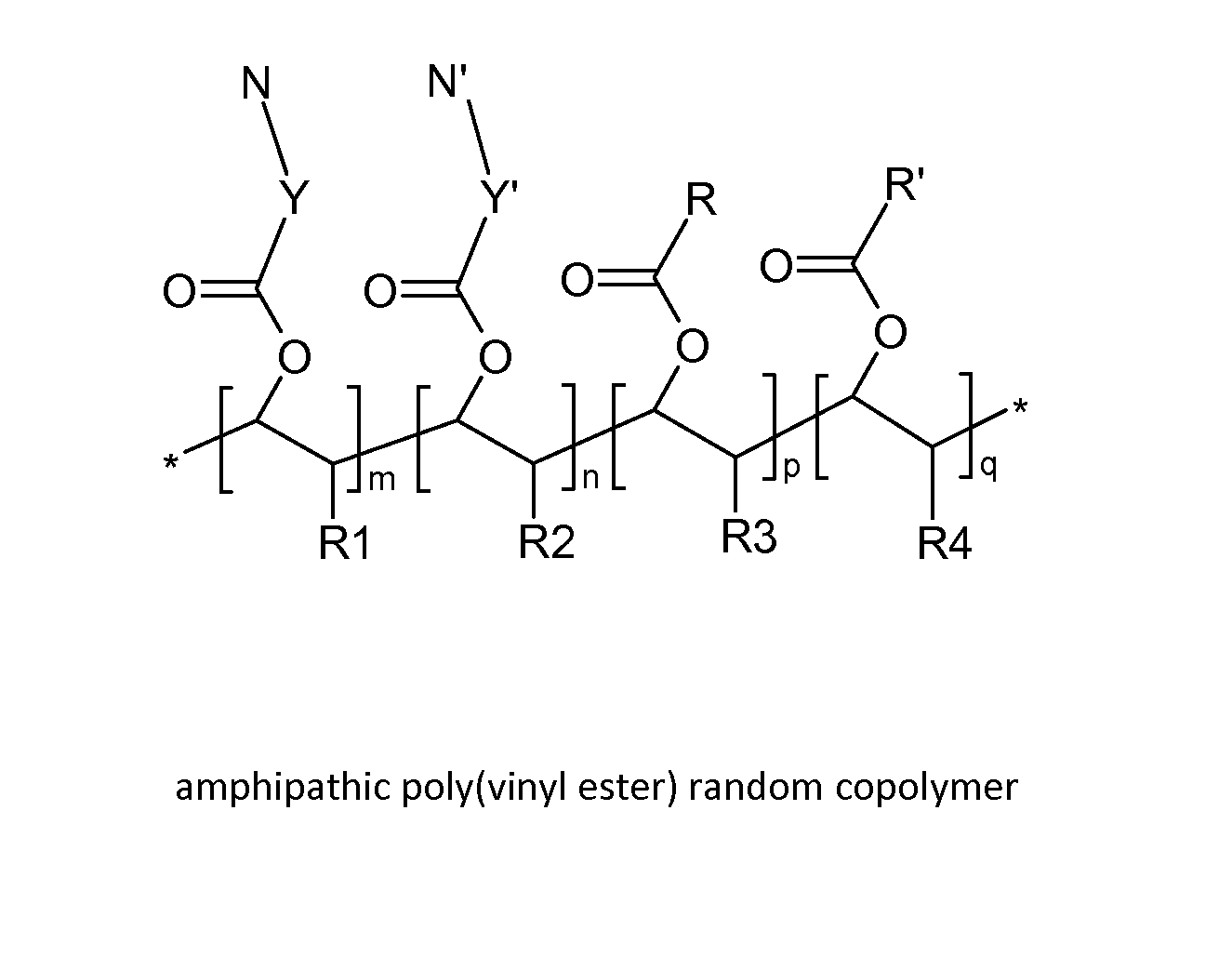
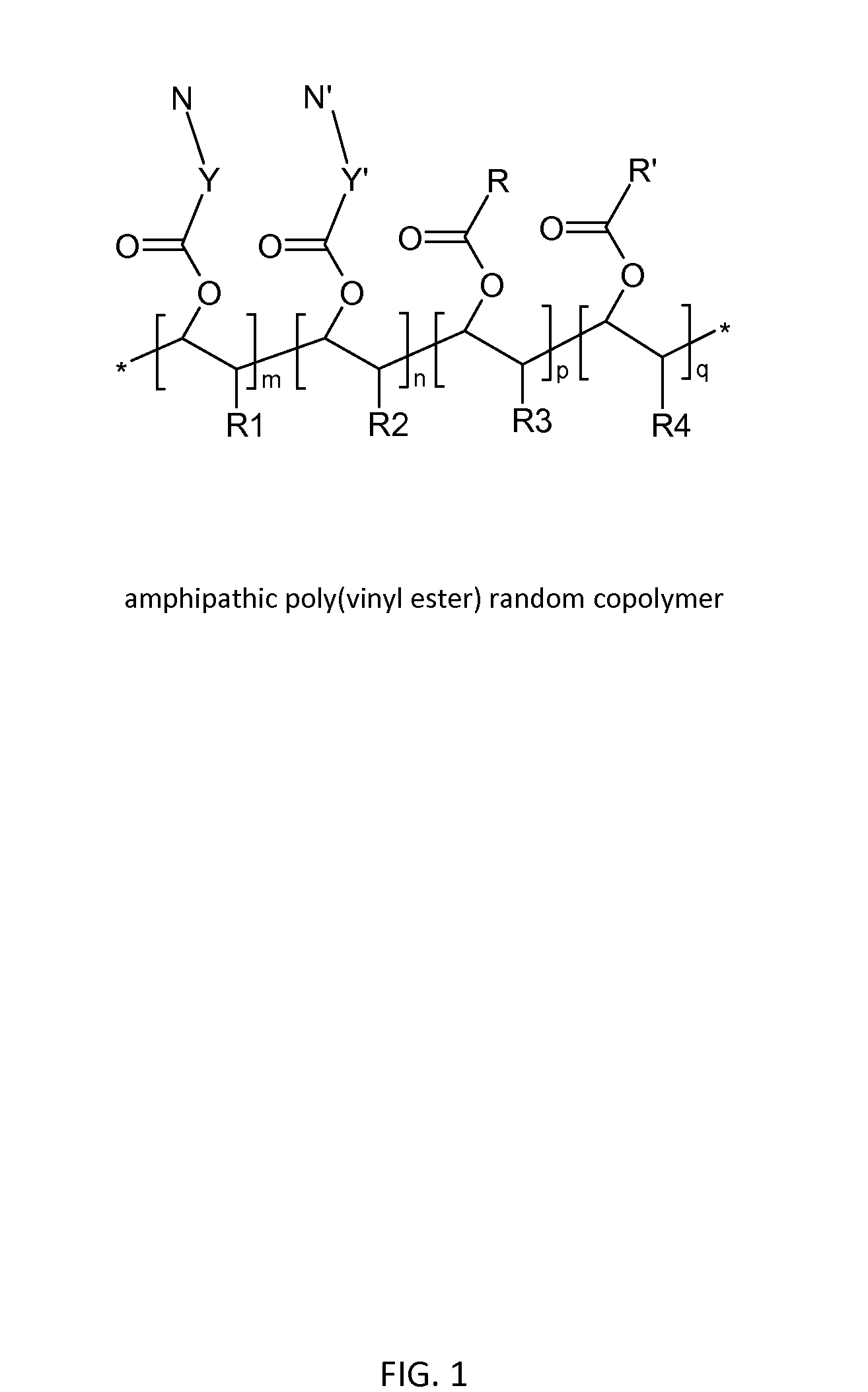
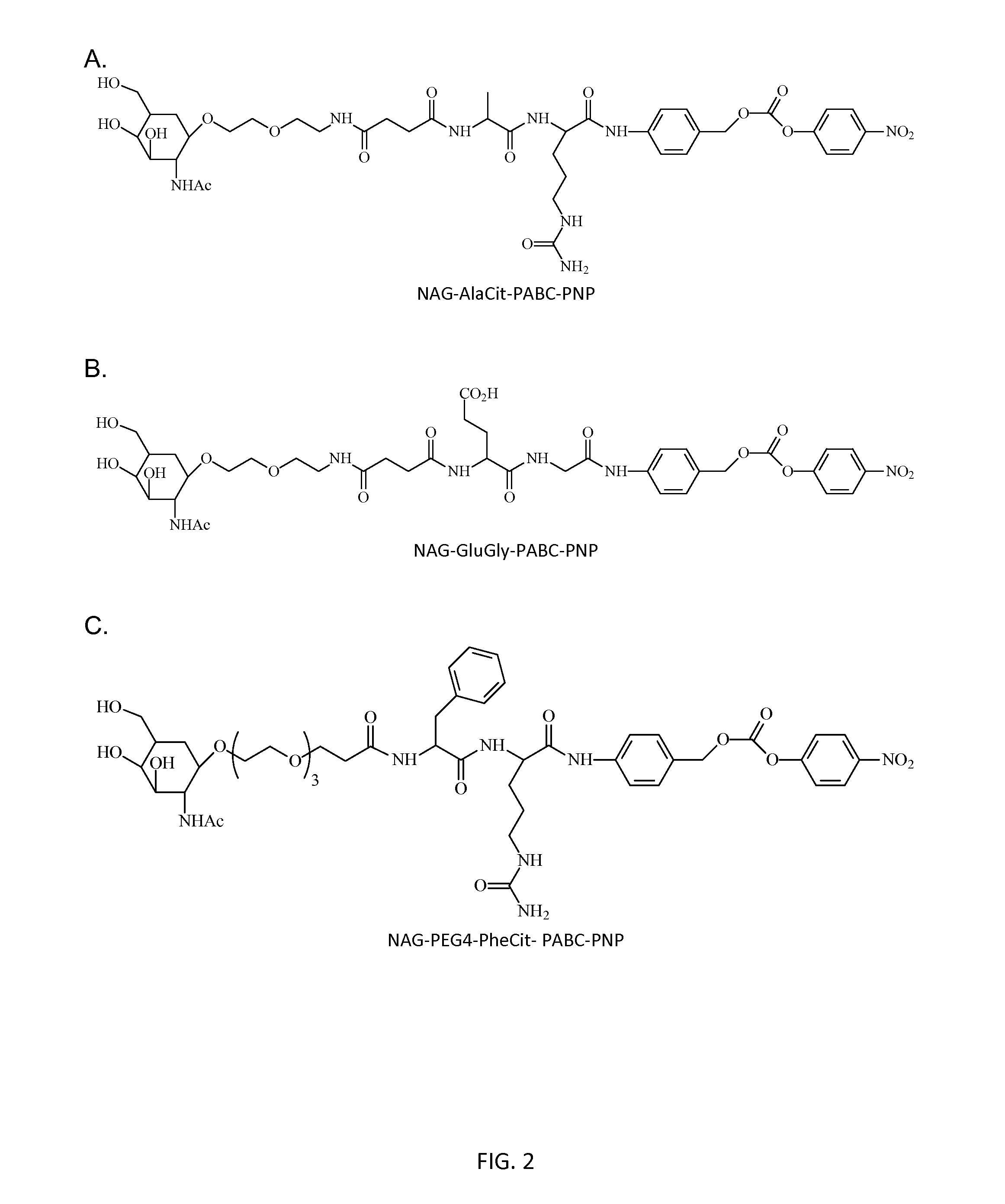
Poly(vinyl ester) Polymers for In Vivo Nucleic Acid Delivery
ActiveUS20130121954A1Suppression problemBeneficial level of expressionPharmaceutical non-active ingredientsDrug compositionsMembrane activityVinyl ester
The present invention is directed membrane active poly(vinyl ester) polymers and compositions for targeted delivery of RNA interference (RNAi) polynucleotides to cells in vivo. RNAi polynucleotides are conjugated to the poly(vinyl ester) polymers and the polymers are reversibly modified to enable in vivo targeted delivery. Membrane activity of the poly(vinyl ester) provides for movement of the RNAi polynucleotides from outside the cell to inside the cell. Reversible modification provides physiological responsiveness.
Owner:ARROWHEAD MADISON
Biodegradable injectable implants and related methods of manufacture and use
InactiveUS20030093157A1Broaden applicationSolution deliveryPharmaceutical non-active ingredientsGlycolic acidImplant
This invention is directed to the field of medical implants, and more specifically to biodegradable injectable implants and their methods of manufacture and use. The injectable implants disclosed herein comprise glycolic acid and bio-compatible / bio-absorbable polymeric particles containing a polymer of lactic acid. The particles are small enough to be injected through a needle but large enough to avoid engulfment by macrophages. The injectables of this invention may be in a pre-activated solid form or an activated form (e.g., injectable suspension or emulsion).
Owner:MEDGRAFT MICROTECH
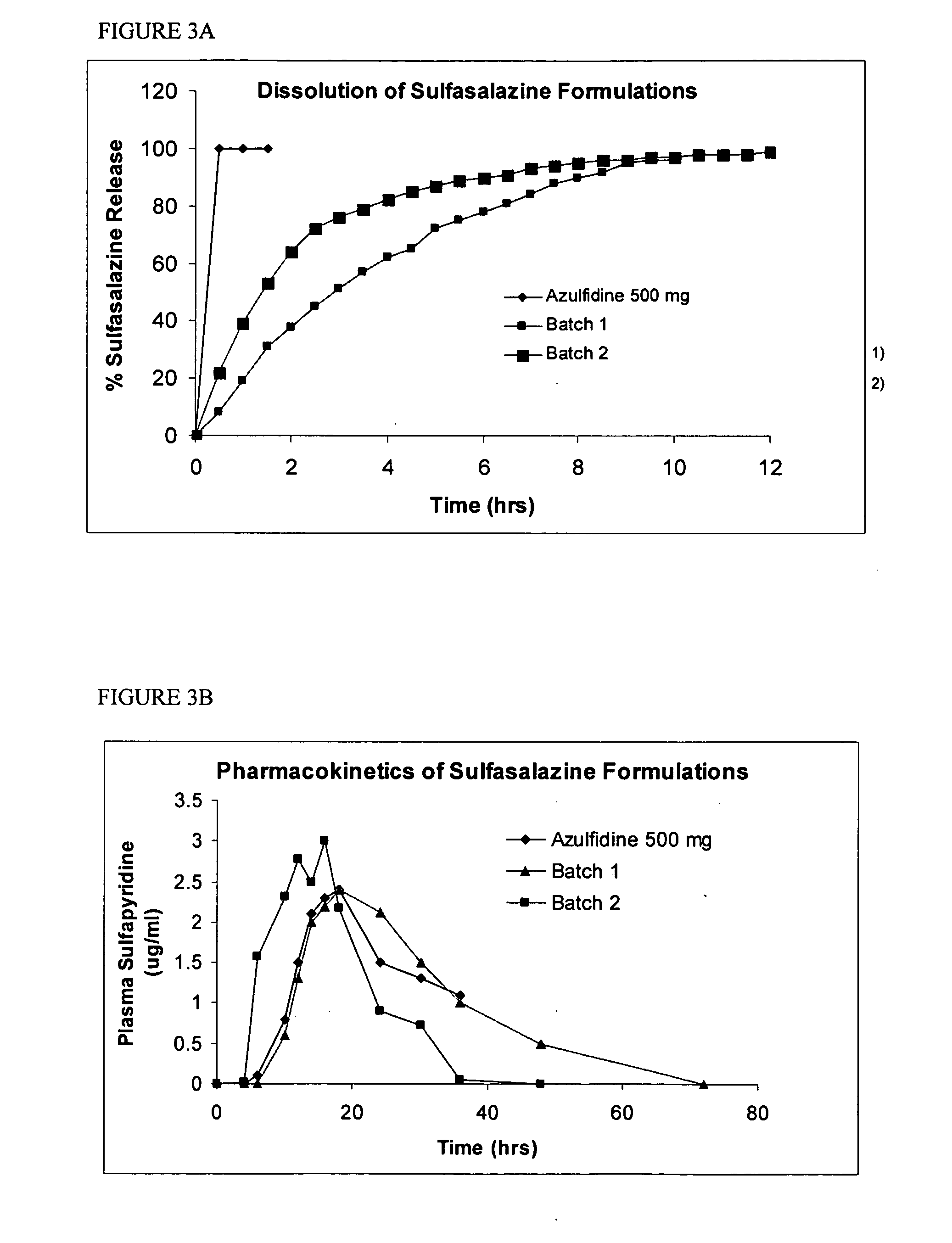
Controlled regional oral delivery
InactiveUS20060045865A1Significant variabilityLow variabilityPill deliveryGranular deliverySolubilityGabapentin
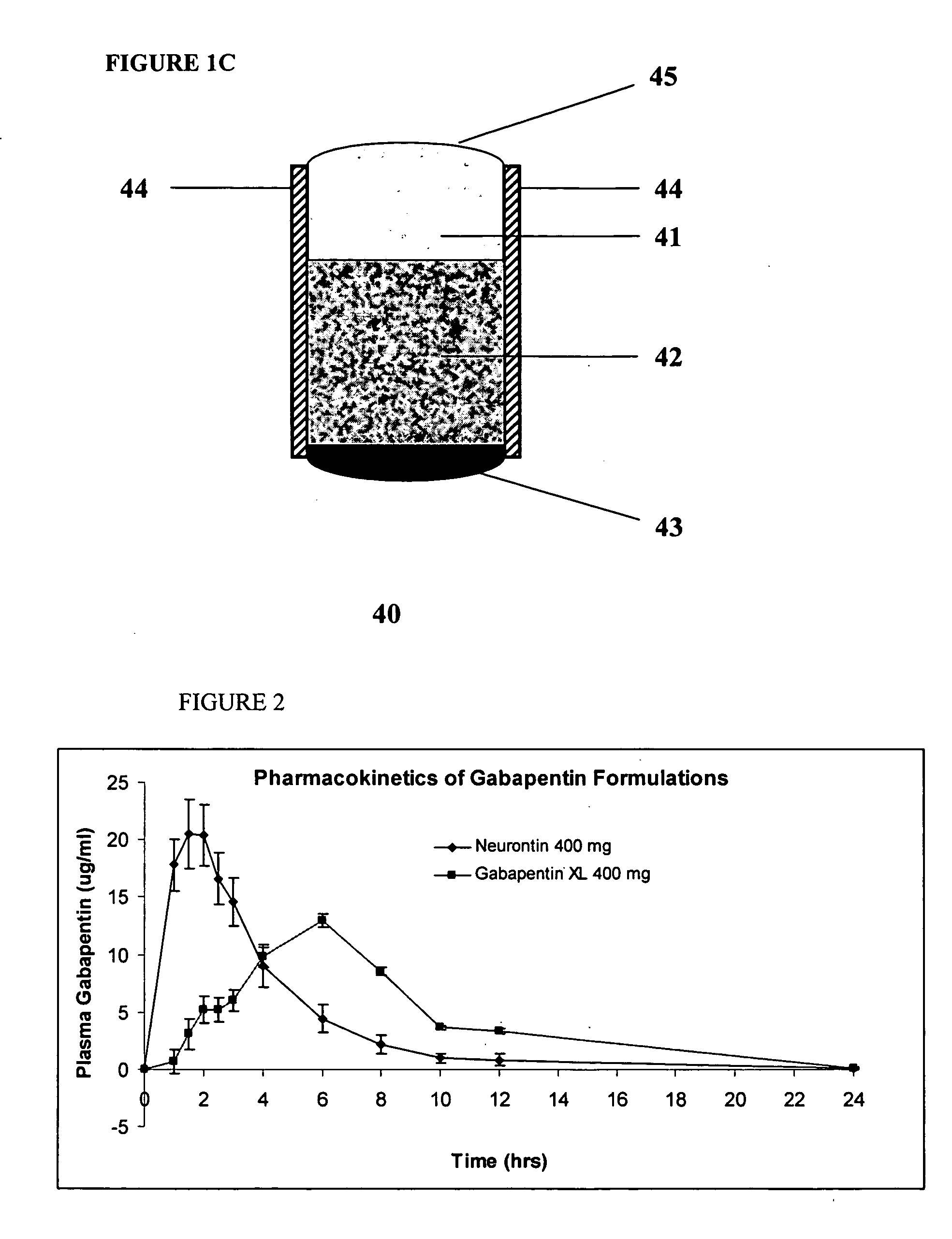
A composite formulation has been developed for selective, high efficacy delivery to specific regions of the mouth and gastrointestinal tract. The formulation is typically in the form of a tablet or capsule, which may include microparticles or beads. The formulation uses bioadhesive and controlled release elements to direct release to specific regions, where the drug is absorbed in enhanced amounts relative to the formulation in the absence of the bioadhesive and / or controlled release elements. This is demonstrated by an example showing delivery of gabapentin with a greater area under the curve (“AUC”) relative to the FDA reference immediate release drug, i.e., the AUC of the composite bioadhesive formulation is greater than 100% of the AUC of the immediate release drug. In the preferred embodiments, the formulation includes drug to be delivered, controlled release elements, and one or more bioadhesive elements. The bioadhesive polymer may be either dispersed in the matrix of the tablet or applied as a direct compressed coating to the solid oral dosage form. The controlled release elements are selected to determine the site of release. The bioadhesive components are selected to provide retention of the formulation at the desired site of uptake and administration. By selecting for both release and retention at a specific site, typically based on time of transit through the gastrointestinal tract, one obtains enhanced efficacy of uptake of the drug. This is particularly useful for drugs with narrow windows of absorption, and drugs with poor solubility such as the BCE class III and class IV drugs.
Owner:VAUNNEX
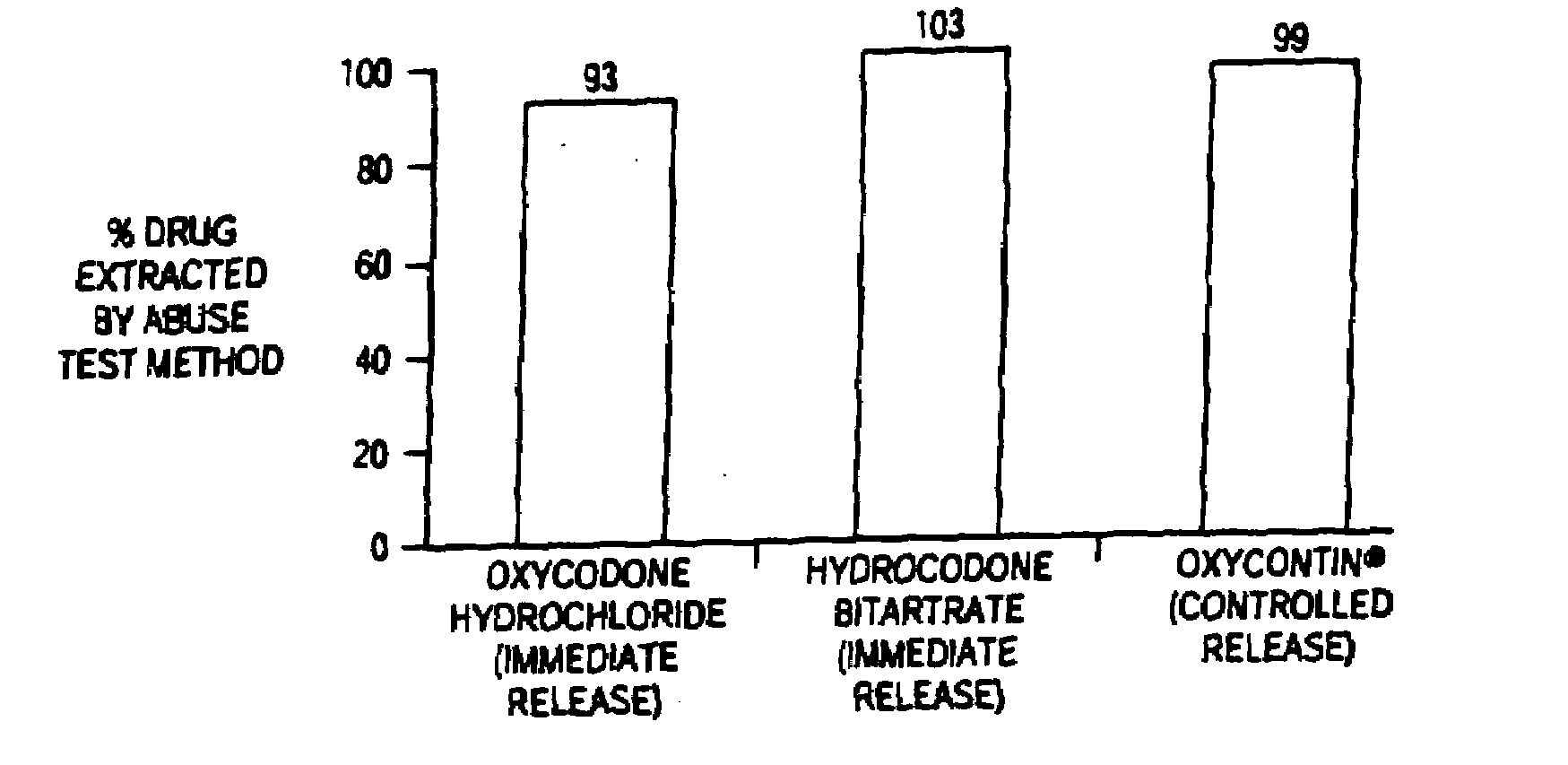
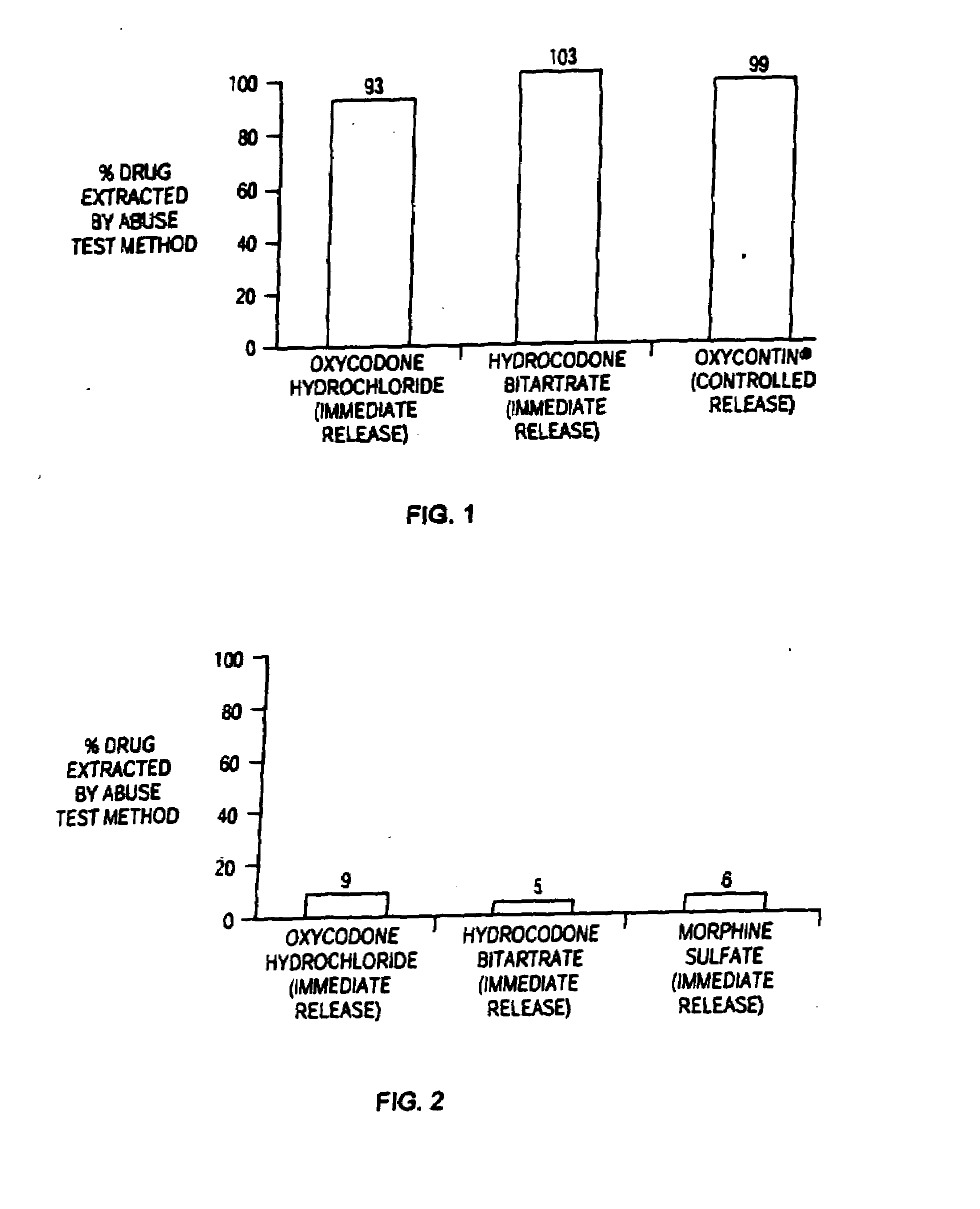
Methods and compositions for deterring abuse of orally administered pharmaceutical products
This invention relates to an abuse deterrent formulation of an oral dosage form of a therapeutically effective amount of any active drug substance that can be subject to abuse combined with a gel forming polymer, a nasal mucosal irritating surfactant and a flushing agent. Such a dosage form is intended to deter abuse of the active drug substance via injection, nasal inhalation or consumption of quantities of the dosage unit exceeding the usual therapeutically effective dose.
Owner:ACURA PHARMA
Bioactive Polymers
InactiveUS20080267903A1Strong specificityEnhanced interactionSynthetic polymeric active ingredientsAntineoplastic agentsDelivery vehicleActive agent
Various polymers, including cationic polyamine polymers and dendrimeric polymers, are shown to possess anti-proliferative activity, and may therefore be useful for treatment of disorders characterised by undesirable cellular proliferation such as neoplasms and tumours, inflammatory disorders (including autoimmune disorders), psoriasis and atherosclerosis. The polymers may be used alone as active agents, or as delivery vehicles for other therapeutic agents, such as drug molecules or nucleic acids for gene therapy. In such cases, the polymers' own intrinsic anti-tumour activity may complement the activity of the agent to be delivered.
Owner:UNIV COLLEGE OF LONDON
Dosage forms using drug-loaded ion exchange resins
InactiveUS20050181050A1Avoid breakingPharmaceutical non-active ingredientsPill deliveryOral medicationImmediate release
A multiparticulate, modified release composition for oral administration has been developed. The formulation is made by complexing a drug with an ion-exchange resin in the form of small particles, typically less than 150 microns. The present invention provides novel extended release coated ion exchange particles comprising drug-resin complexes, produced by binding the salt form of the drug, that do not require impregnating agents to insure the integrity of the extended release coat. To prepare a modified release formulation, one or more of the following types of particles are formulated into a final dosage form: (a) Immediate release particles, (b) Enteric coated particles, (c) Extended release particles, (d) Enteric coated-extended release particles; and (e) Delayed release particles. The various drug-containing particles described above can be further formulated into a number of different easy-to-swallow final dosage forms including, but not limited to, a liquid suspension, gel, chewable tablet, crushable tablet, rapidly dissolving tablet, or unit of use sachet or capsule for reconstitution
Owner:COLLEGIUM PHARMA INC
Bone substitute compositions and method of use
The present invention relates to novel bone substitute compositions and methods of use. It further encompasses the use of these novel bone substitute compositions for bone augmentation and the treatment of disease conditions. The invention also contemplates a kit including bone substitute compositions and a percutaneous delivery device.
Owner:KYPHON
Biodegradable polyurethanes and use thereof
InactiveUS20050013793A1Improve responseIncrease ratingsCell culture supports/coatingSkeletal/connective tissue cellsPolymer scienceDrug biological activity
A biodegradable and biocompatible polyurethane composition synthesized by reacting isocyanate groups of at least one multifunctional isocyanate compound with at least one bioactive agent having at least one reactive group —X which is a hydroxyl group (—OH) or an amine group (—NH2). The polyurethane composition is biodegradable within a living organism to biocompatible degradation products including the bioactive agent. Preferably, the released bioactive agent affects at least one of biological activity or chemical activity in the host organism. A biodegradable polyurethane composition includes hard segments and soft segments. Each of the hard segments is preferably derived from a diurea diol or a diester diol and is preferably biodegradable into biomolecule degradation products or into biomolecule degradation products and a biocompatible diol. Another biodegradable polyurethane composition includes hard segments and soft segments. Each of the hard segments is derived from a diurethane diol and is biodegradable into biomolecule degradation products.
Owner:CARNEGIE MELLON UNIV +1
Biodegradable injectable implants containing glycolic acid
InactiveUS7314636B2Non-migratoryEasy to moveSolution deliveryPharmaceutical non-active ingredientsEmulsionGlycolic acid
Owner:MEDGRAFT MICROTECH
Spot-on formulations for combating parasites
InactiveUS6998131B2Effective and lasting destructionProphylaxis of parasite infestationsBiocideDead animal preservationAntiparasiticMammal
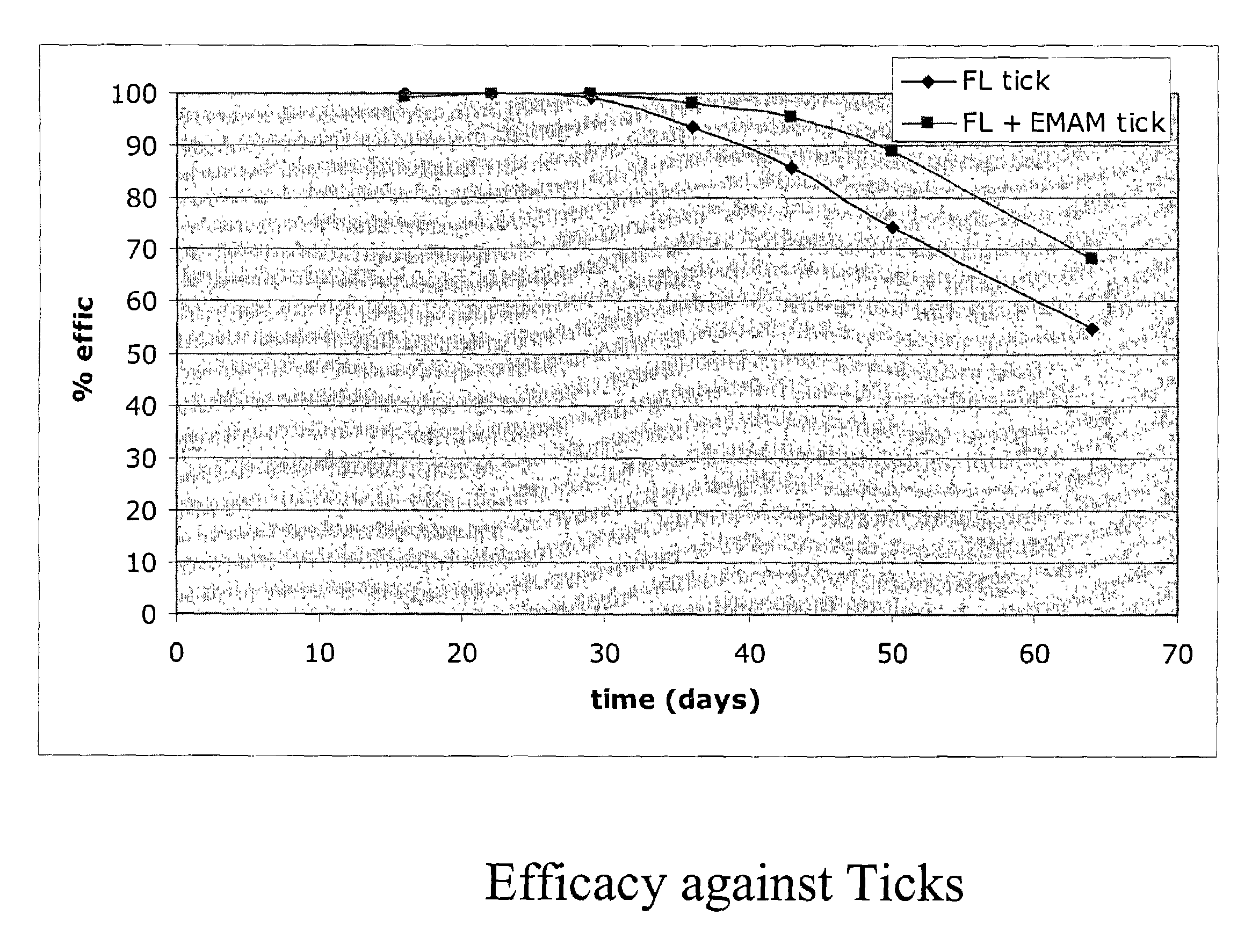
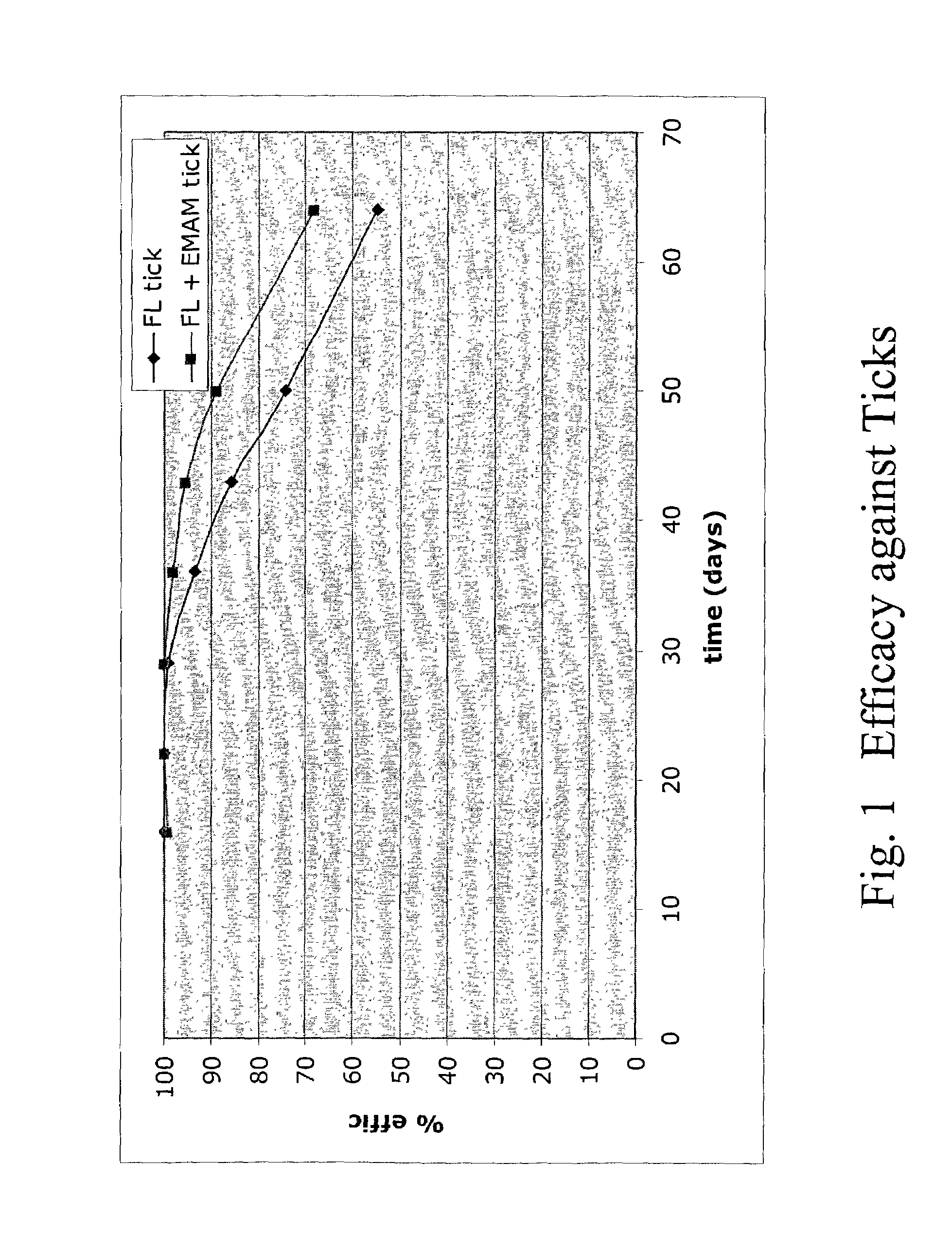
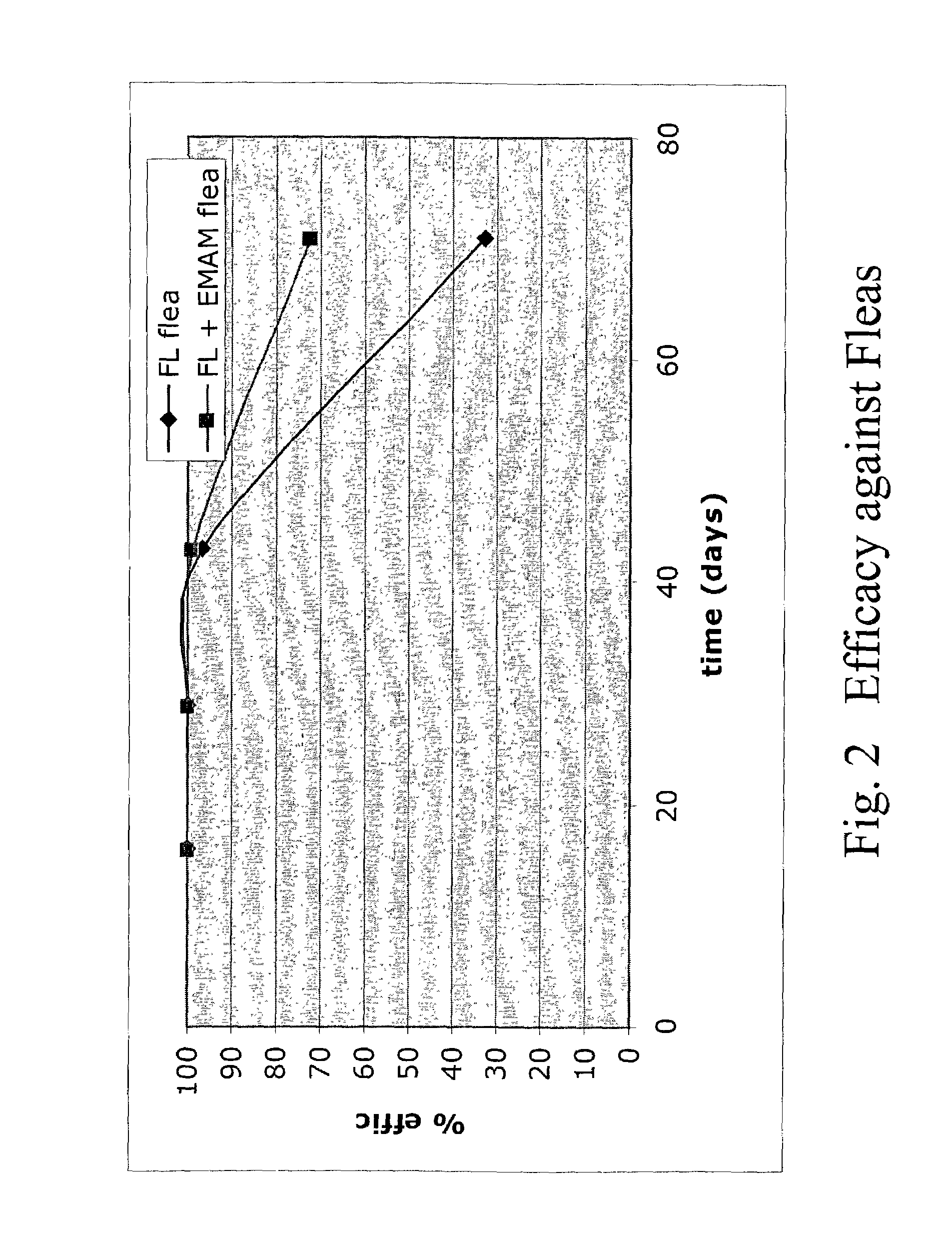
In particular this invention provides for spot-on compositions for the treatment or prophylaxis of parasite infestations in mammals or birds which comprise:(1) a composition comprising(A) an effective amount of a 1-phenylpyrazole derivative; and(B) an effective amount of emamectin;(2) an acceptable liquid carrier vehicle; and(3) optionally, a crystallization inhibitor.The invention also provides for a method of treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal treating parasitic infestations or for the prophylaxis of parasite infestations in mammals or birds which comprises topically applying to said mammal or bird an effective amount of a composition according to the present invention.
Owner:MERIAL LTD
Gentle-acting skin disinfectants
InactiveUS6846846B2Minimize skin irritationUnexpected antimicrobial effectivenessCosmetic preparationsBiocideOctoxyglycerinMedicine
Antimicrobial compositions having synergistic combinations of octoxyglycerin and at least one other antimicrobial agent in formulations which are more effective than prior art compositions without causing increased irritation to the skin of the average user. In certain embodiments, skin irritation may be minimized by low concentrations of antimicrobials and / or the presence of soothing compounds such as zinc. Preferred embodiments include combinations of octoxyglycerin, a quaternary compound, and at least one other antimicrobial agent. Without being bound to any particular theory, it is hypothesized that the unexpected antimicrobial effectiveness of combinations of octoxyglycerin may result from an enhancement of the permeability of microbes to antimicrobials caused by octoxyglycerin.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Apparatus and formulations for suprachoroidal drug delivery
InactiveUS20070202186A1Avoid traumaMinimally-invasive deliveryBiocidePowder deliveryPosterior regionPharmaceutical formulation
Drug formulations, devices and methods are provided to deliver biologically active substances to the eye. The formulations are delivered into scleral tissues adjacent to or into the suprachoroidal space without damage to the underlying choroid. One class of formulations is provided wherein the formulation is localized in the suprachoroidal space near the region into which it is administered. Another class of formulations is provided wherein the formulation can migrate to another region of the suprachoroidal space, thus allowing an injection in the anterior region of the eye in order to treat the posterior region.
Owner:CLEARSIDE BIOMEDICAL
Inherently radiopaque polymeric products for embolotherapy
ActiveUS20050106119A1X-ray constrast preparationsSynthetic polymeric active ingredientsMedicineBiomedical engineering
Owner:RUTGERS THE STATE UNIV
Surgical adhesive and uses therefore
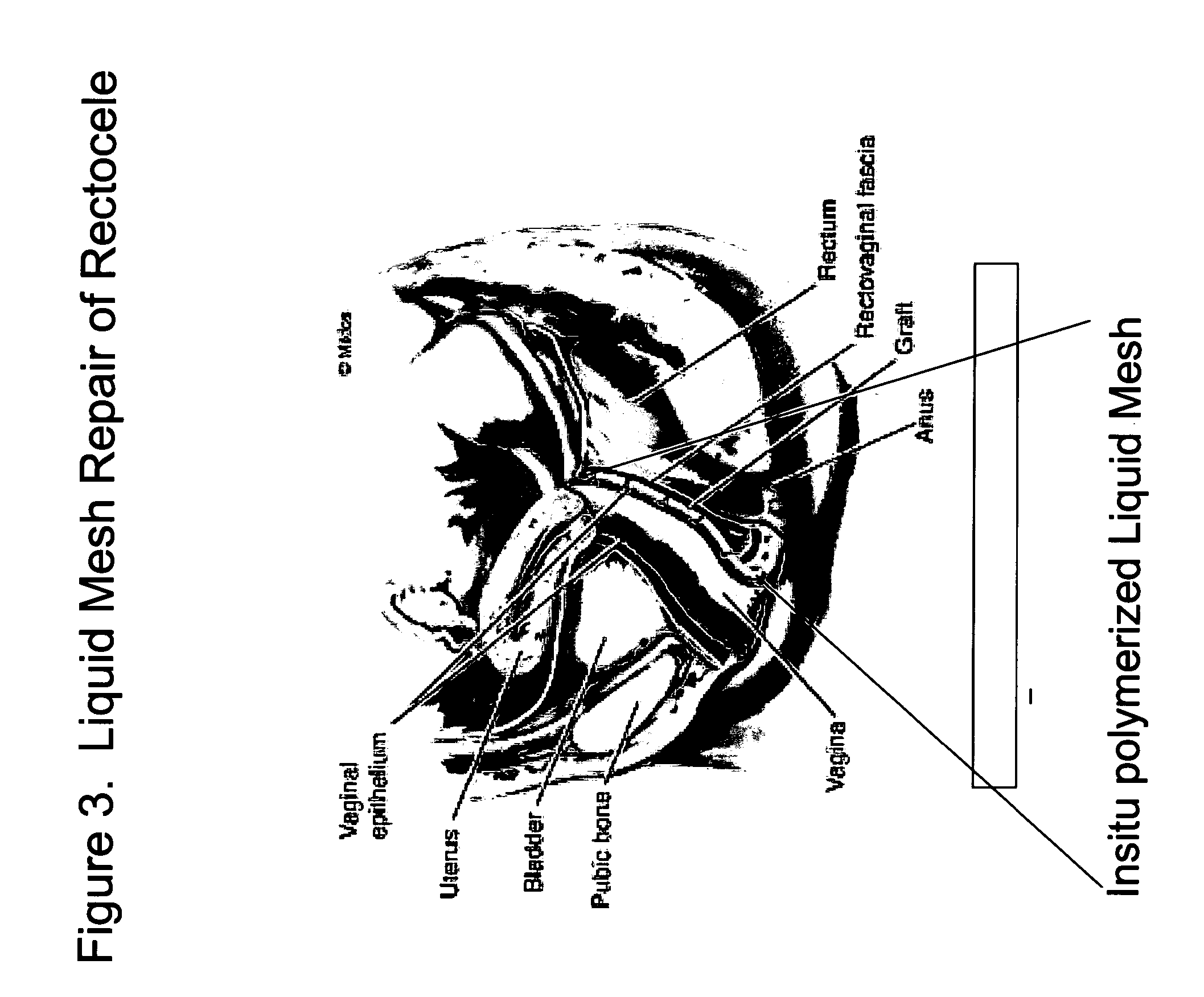
InactiveUS20050129733A1Controlled strengthLess erosiveSurgical adhesivesPharmaceutical delivery mechanismIn situ polymerizationEnteroceles
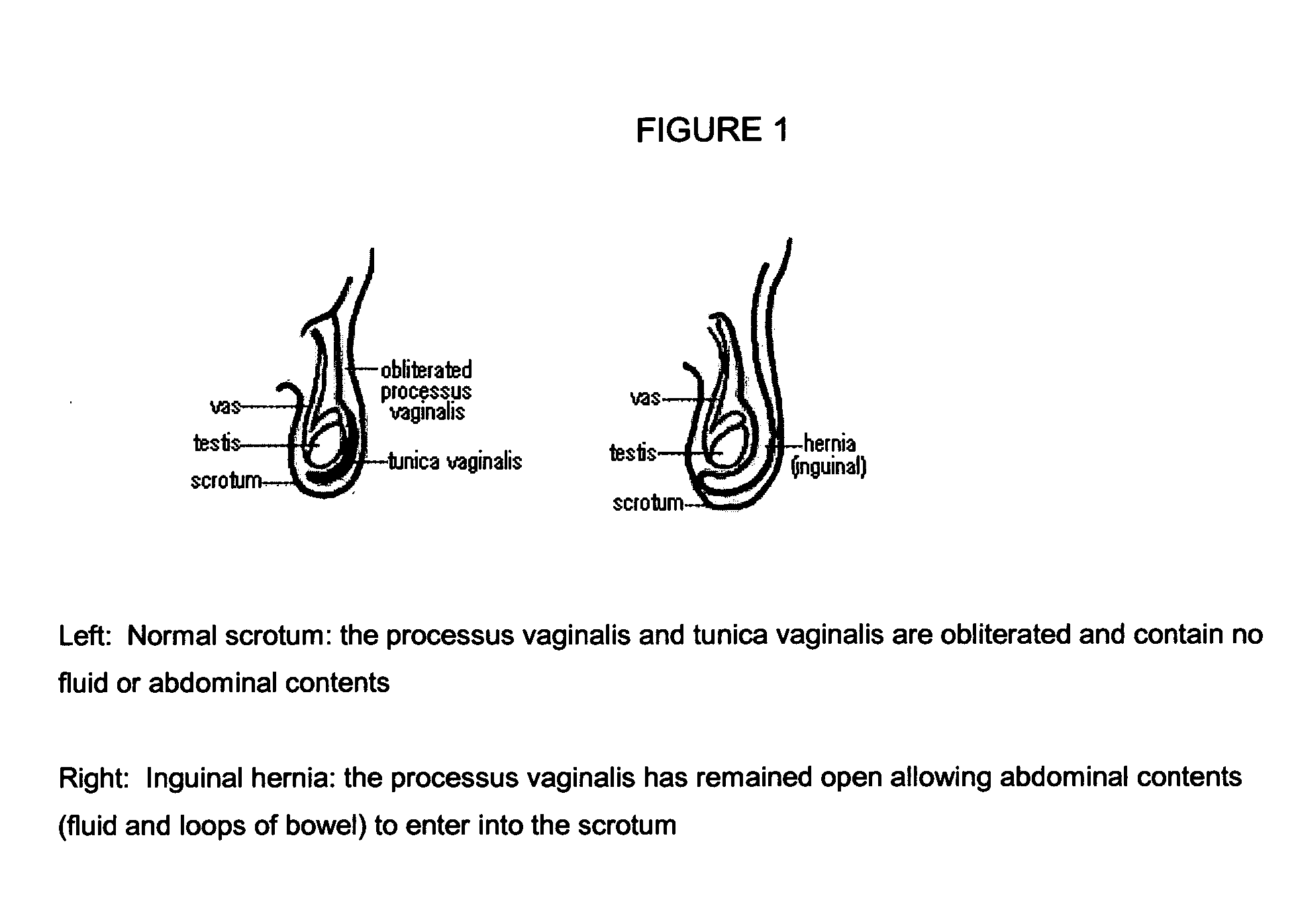
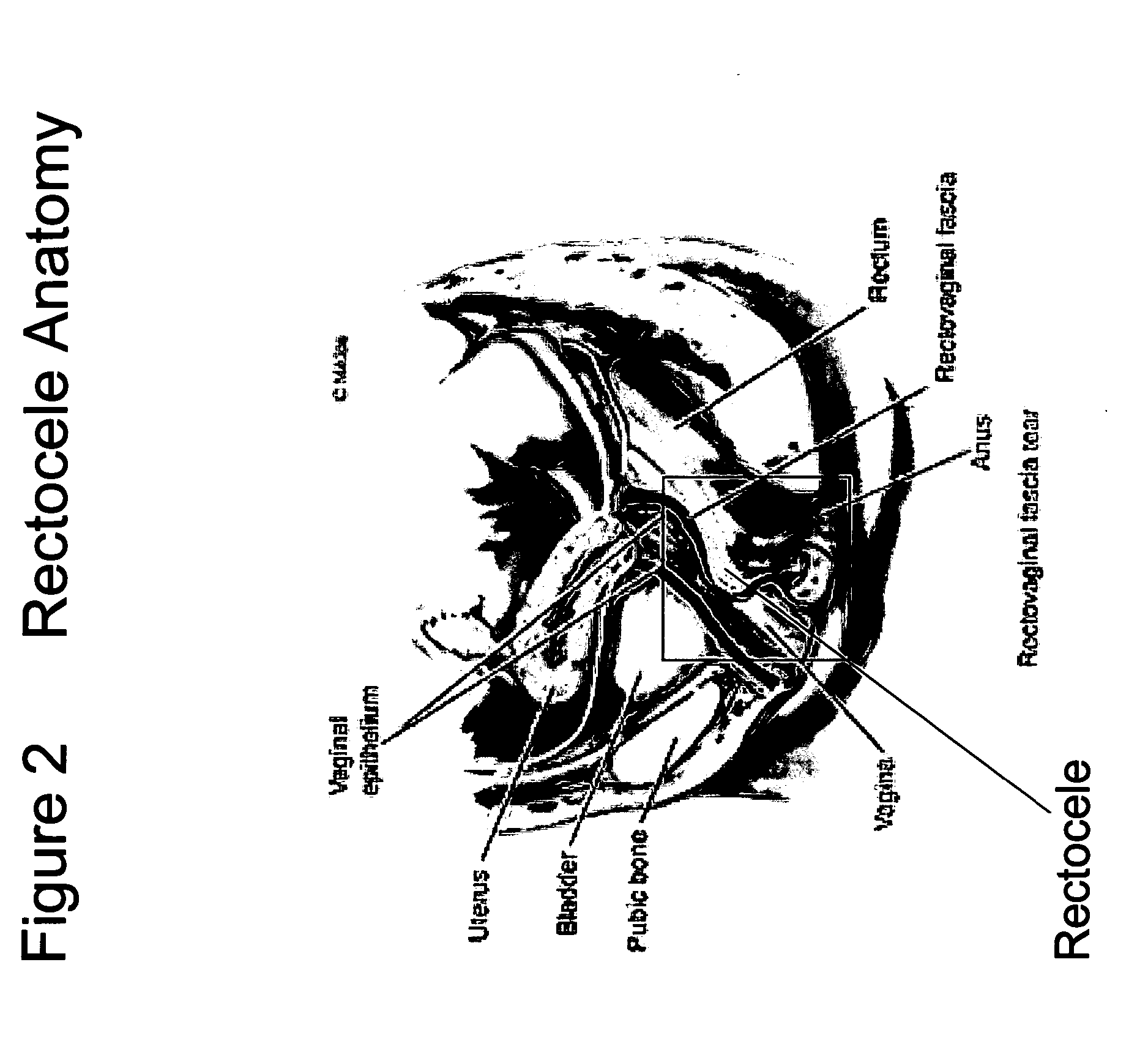
The present invention provides a liquid polymer composition which can be implanted into a living mammal and which forms a solid hydrogel by in situ polymerization upon contact with body fluid and tissue. The composition also can be used as a coating on a medical device, or for the formation of a medical device. Formation of a solid implant or coating involves crosslinking of the adhesive with itself and with surrounding tissue. The liquid implant, by itself or in conjunction with various prostheses, can be used for many purposed, including fixation of the urethra for providing treatment for incontinence, and repair of herniations in the abdominal cavity, including rectocele, cystocele, enterocele, and inguinal hernia. The adhesive may be used to establish adhesion prevention during such repairs, in part by coating or being the material of a repair mesh.
Owner:PROMETHEAN SURGICAL DEVICES
Methacrylate copolymers for medical devices
A polymer of hydrophobic monomers and hydrophilic monomers is provided. It is also provided a polymer blend that contains the polymer and another biocompatible polymer. The polymer or polymer blend and optionally a biobeneficial material and / or a bioactive agent can form a coating on an implantable device such as a drug delivery stent. The implantable device can be used for treating or preventing a disorder such as atherosclerosis, thrombosis, restenosis, hemorrhage, vascular dissection or perforation, vascular aneurysm, vulnerable plaque, chronic total occlusion, patent foramen ovale, claudication, anastomotic proliferation for vein and artificial grafts, bile duct obstruction, ureter obstruction, tumor obstruction, or combinations thereof.
Owner:ABBOTT CARDIOVASCULAR
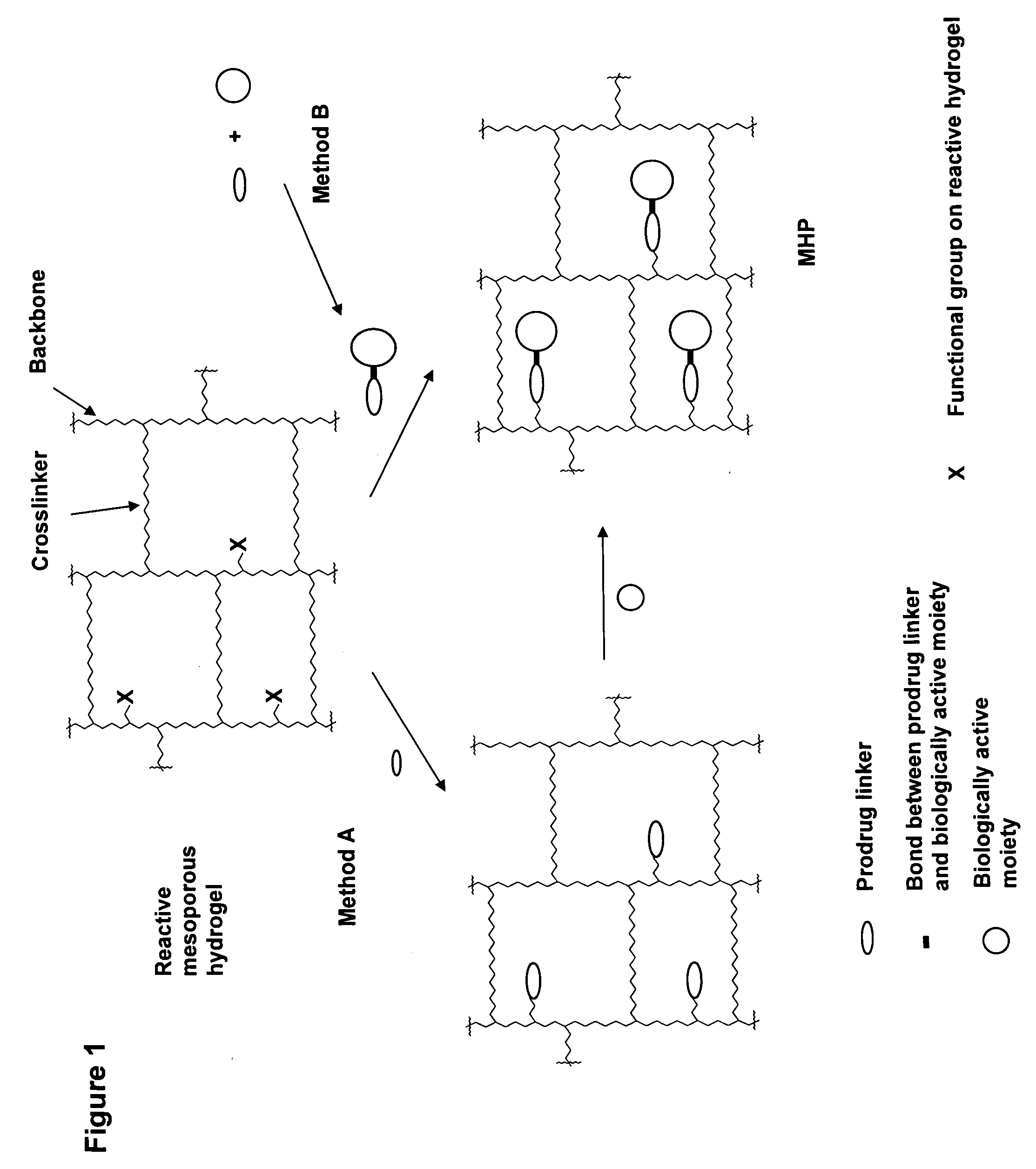
Hydrogel formulations
ActiveUS20060002890A1Reduce deliveryIncreased susceptibilityBiocidePeptide/protein ingredientsPolymeric prodrugBiological activity
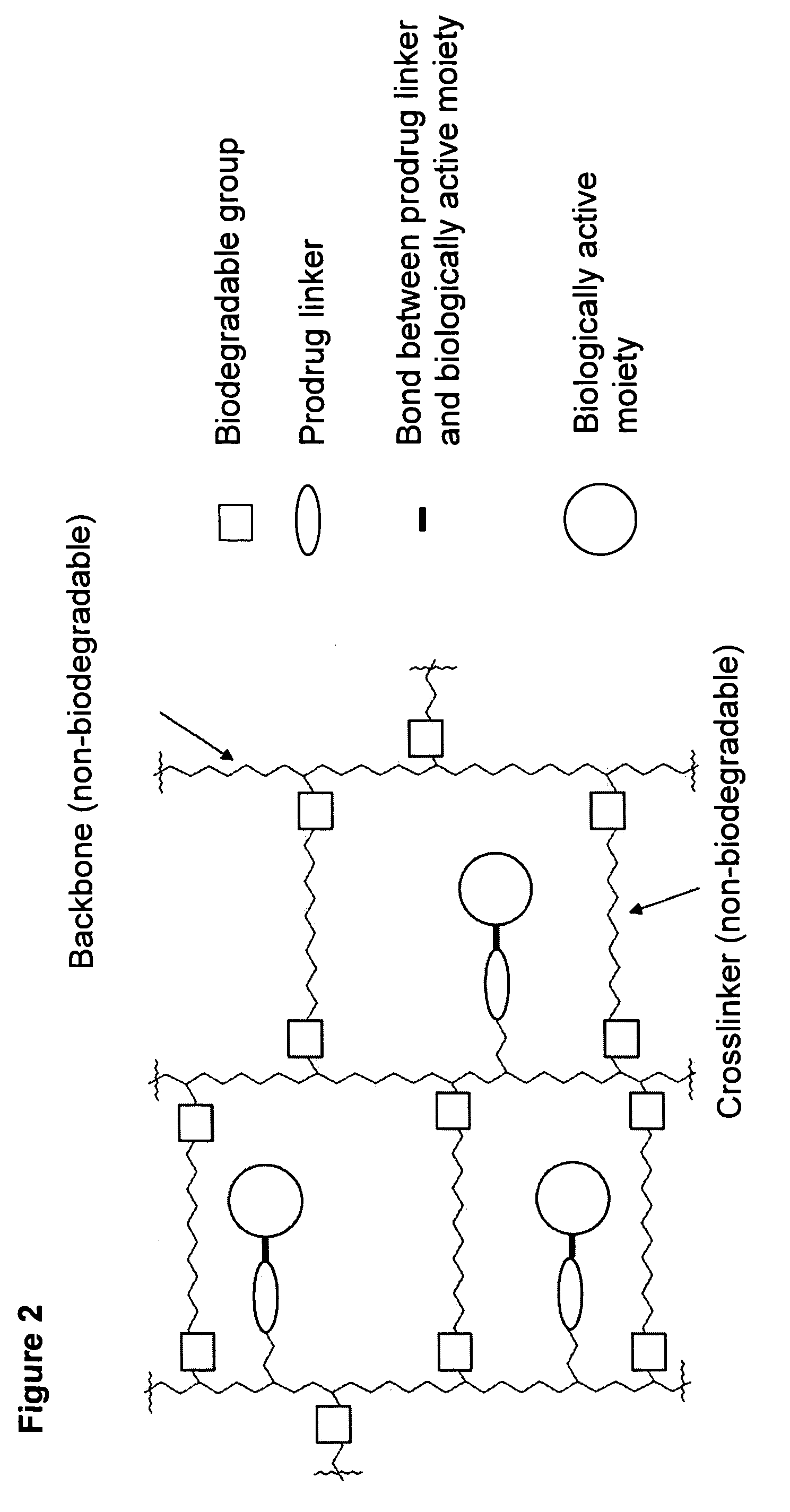
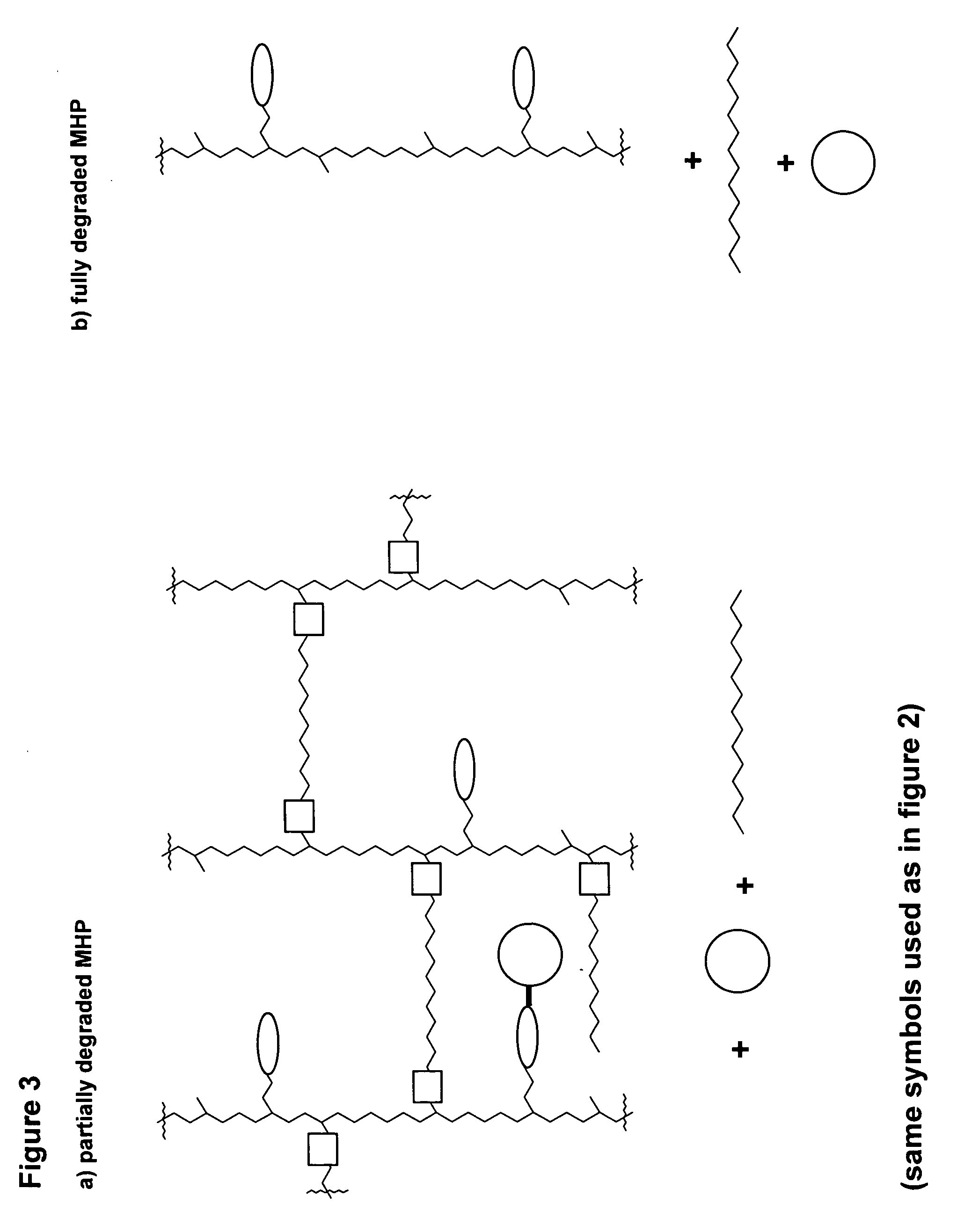
A polymeric prodrug composition including a hydrogel, a biologically active moiety and a reversible prodrug linker. The prodrug linker covalently links the hydrogel and the biologically active moiety at a position and the hydrogel has a plurality of pores with openings on its surface. The diameter of the pores is larger than that of the biologically active moiety at least at all points of the pore between at least one of the openings and the position of the biologically active moiety.
Owner:ASCENDIS PHARM AS
Biodegradable resin compositions
InactiveUS6669771B2Increase resistanceHigh strengthCosmetic preparationsAntifouling/underwater paintsBiodegradable productDigestion
Owner:NAT INST OF ADVANCED IND SCI & TECH +2
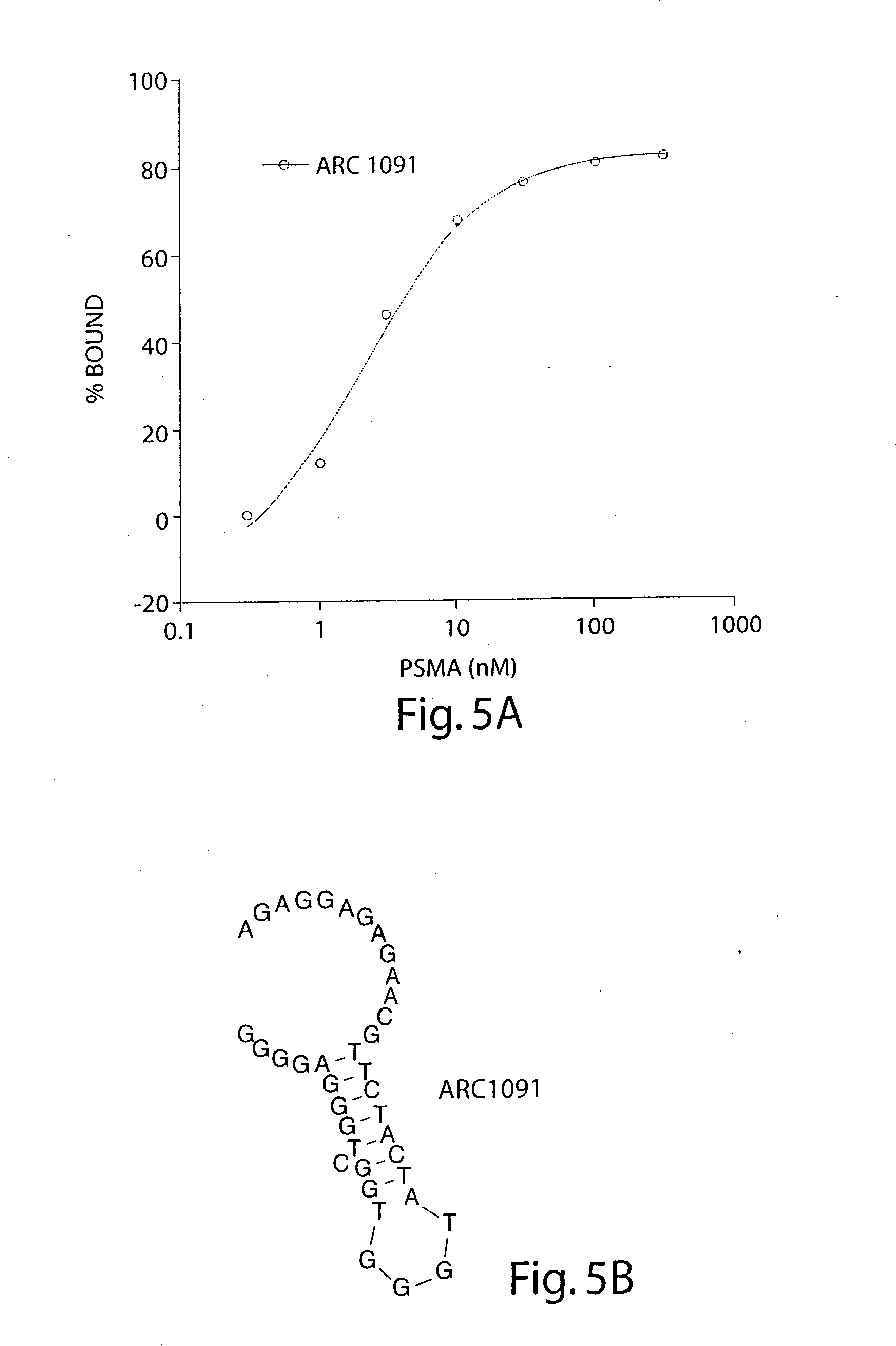
Stabilized aptamers to PSMA and their use as prostate cancer therapeutics
The present invention provides stabilized, high affinity nucleic acid ligands to PSMA. Methods for the identification and preparation of novel, stable, high affinity ligands to PSMA using the SELEX™ method with 2′-O-methyl substituted nucleic acids, and cell surface SELEX™ are described herein. Also included are methods and compositions for the treatment and diagnosis of disease characterized by PSMA expression, using the described nucleic acid ligands.
Owner:ARCHEMIX CORP
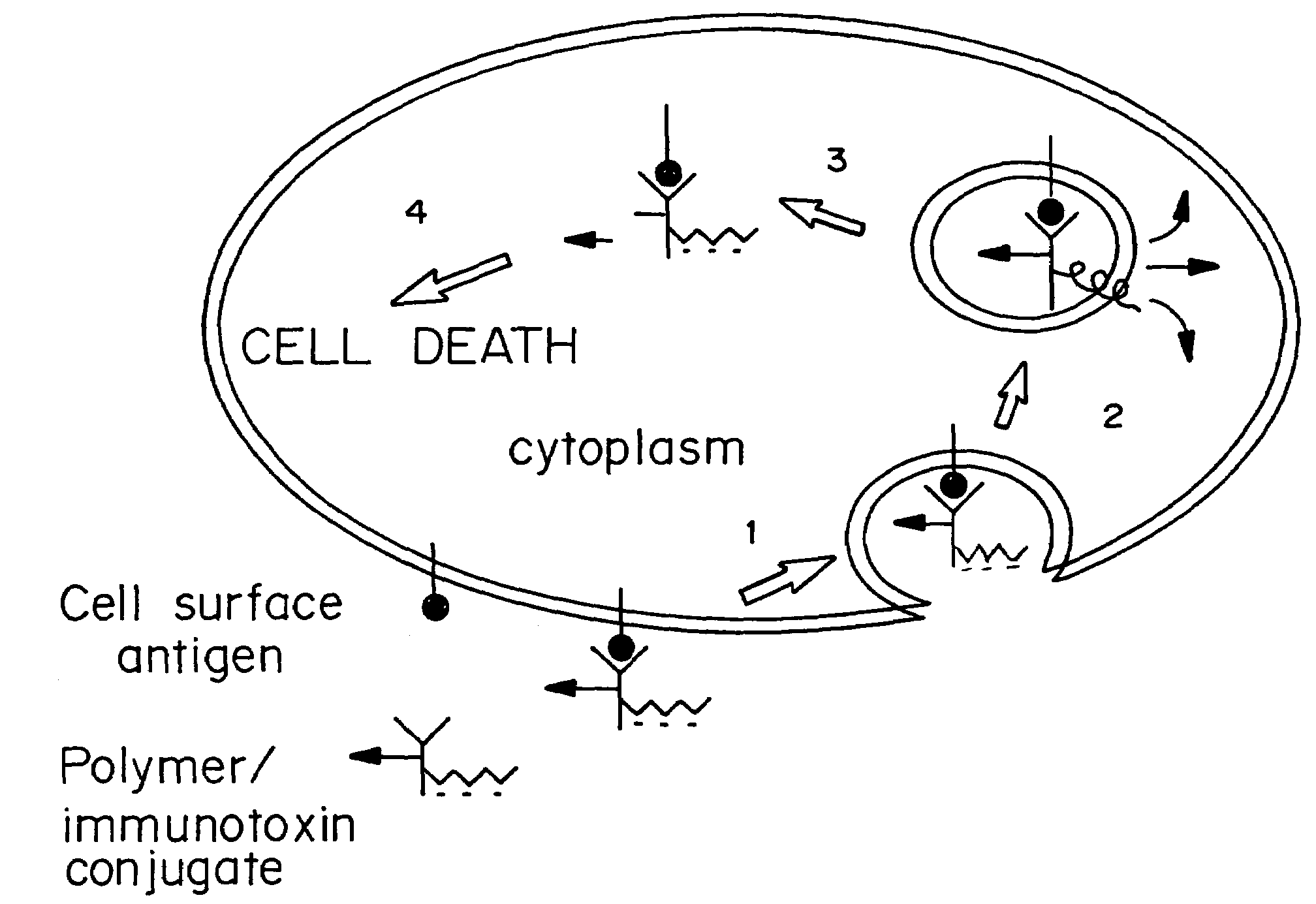
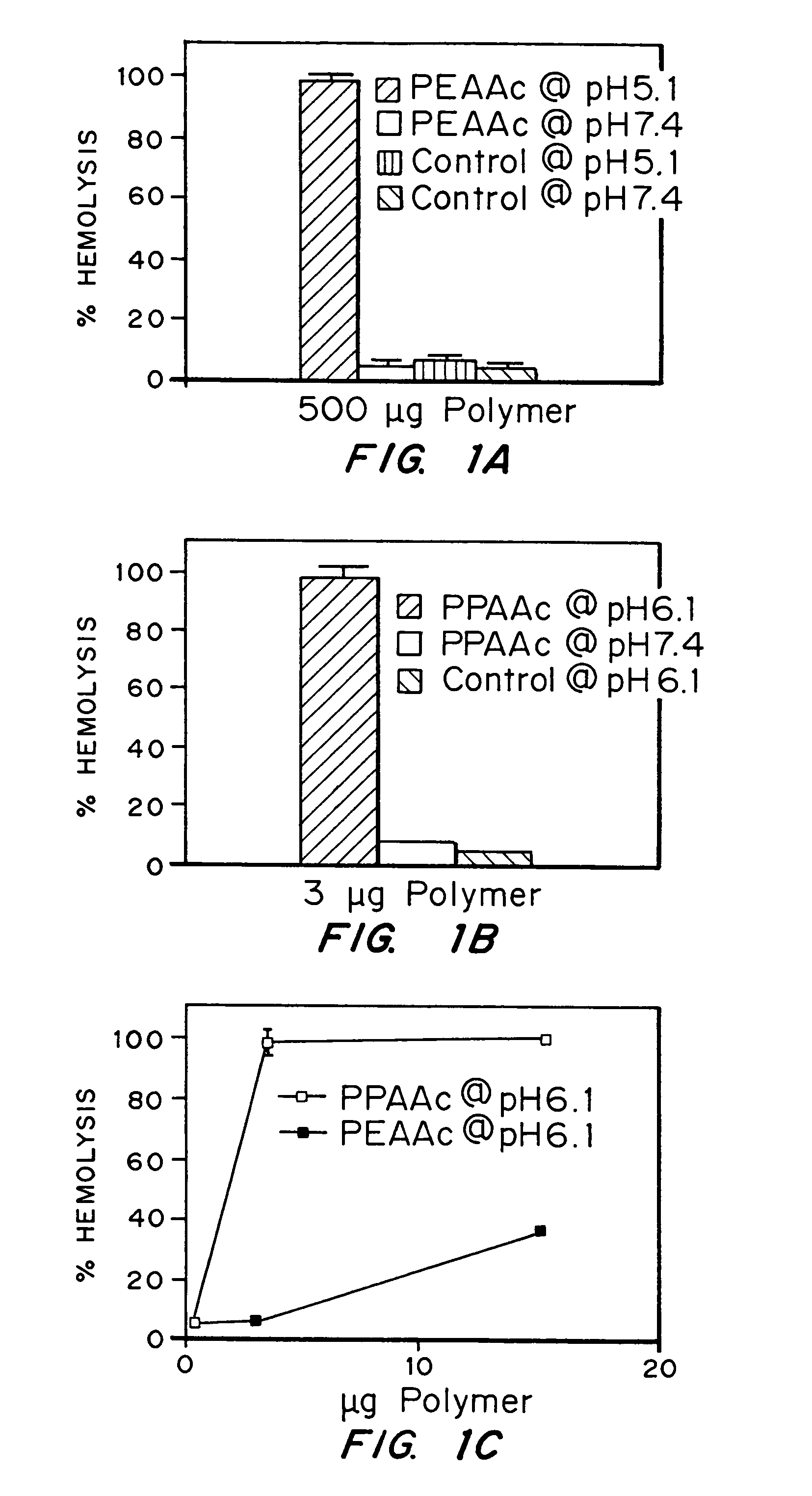
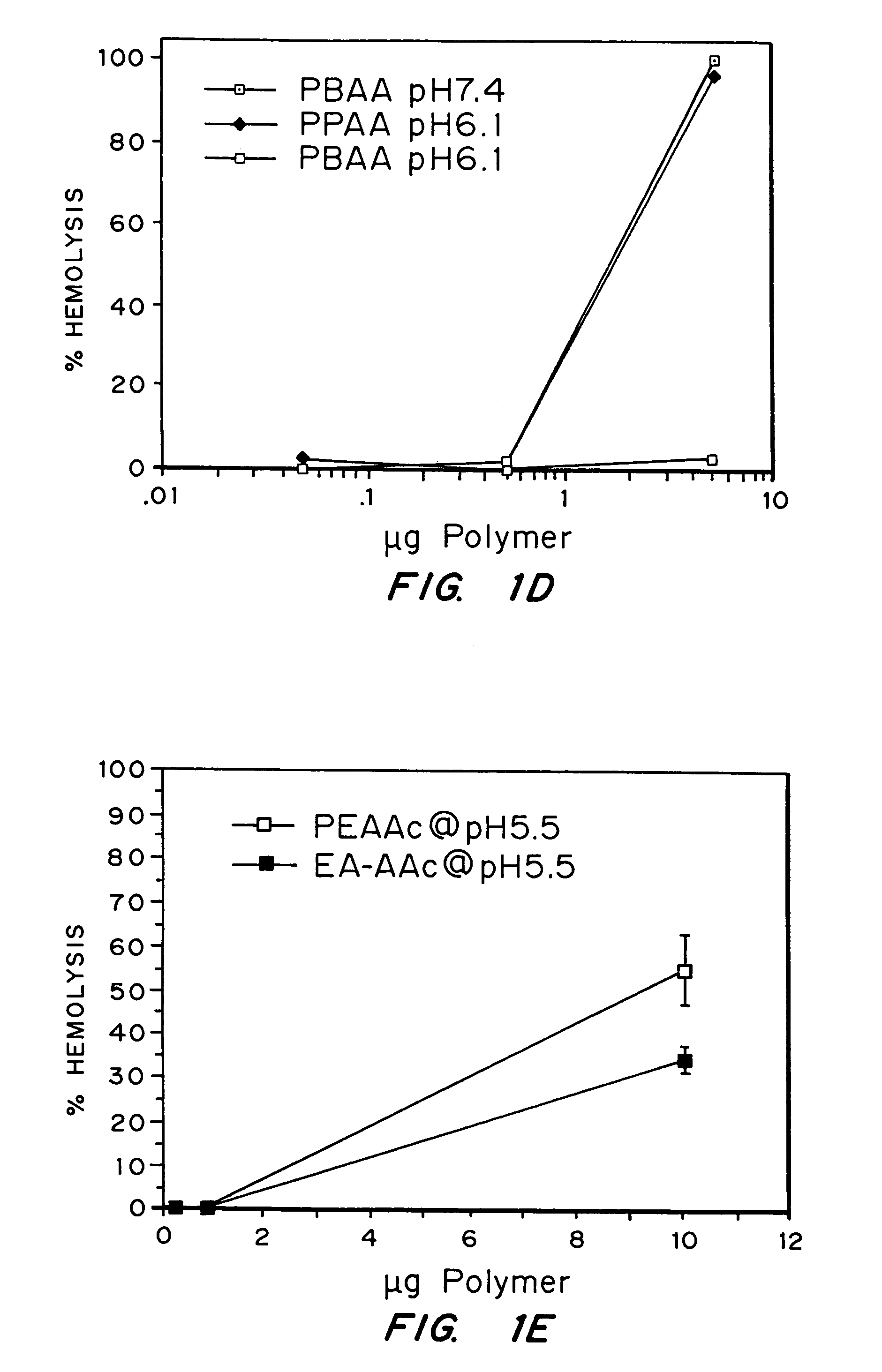
Enhanced transport using membrane disruptive agents
Owner:UNIV OF WASHINGTON +1
Topical nitric oxide donor compositions
InactiveUS6287601B1Reduced and failing organ functionPoor appetitePowder deliveryBiocideLipid formationEquine Species
Compositions and methods of the topical treatment of equine laminitis are disclosed. In particular, combinations of a fast acting nitric oxide (NO) donor, a sustained acting NO donor and an NSAID mixed in a lipid-based carrier are described. The application of such combinations to the affected areas, e.g., the hoofs and surrounding tissues, of an equine afflicted with laminitis provides relief from the debilitating effects of this painful, often life-threatening condition.
Owner:STREHKEHN INT LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com