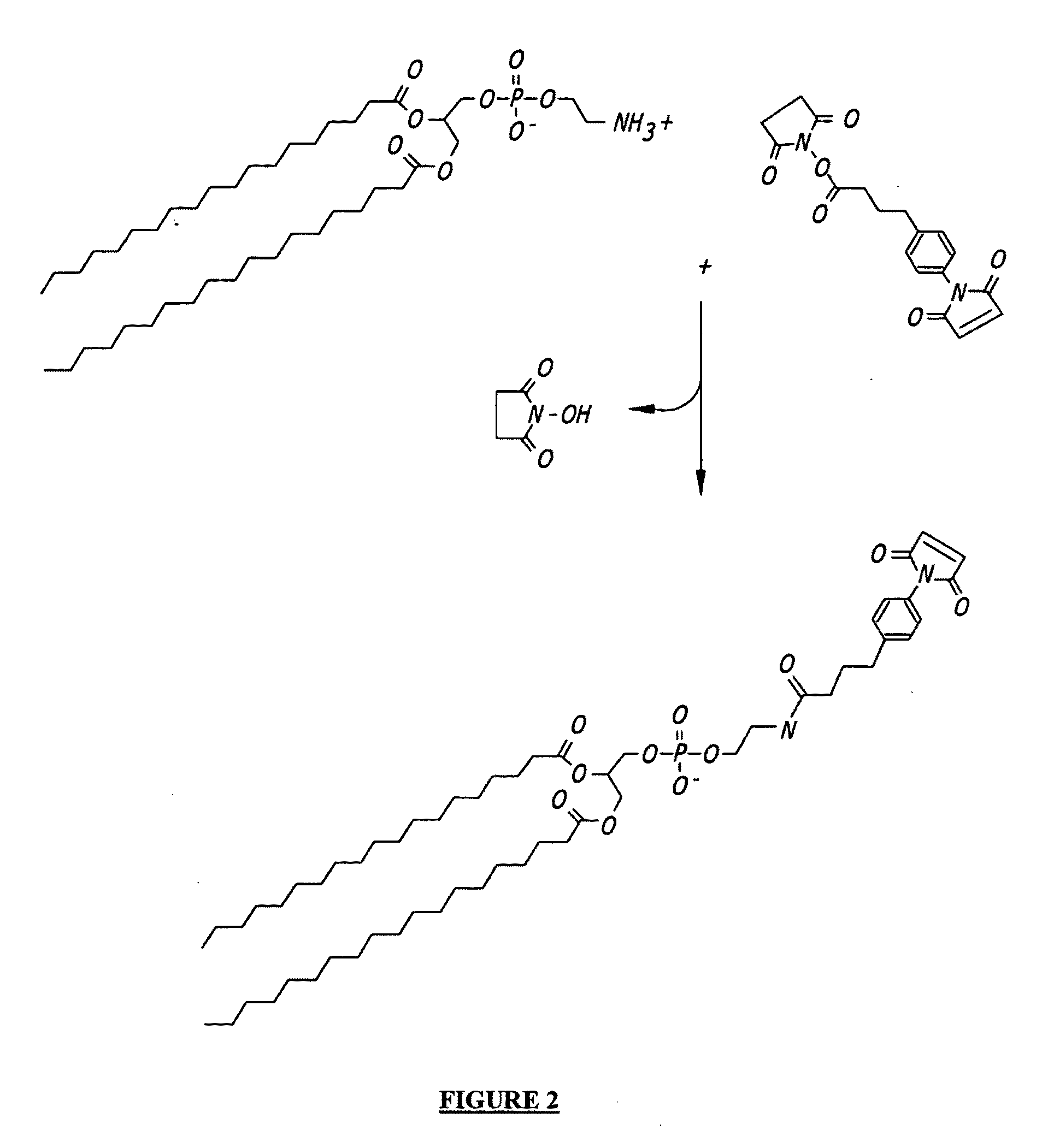
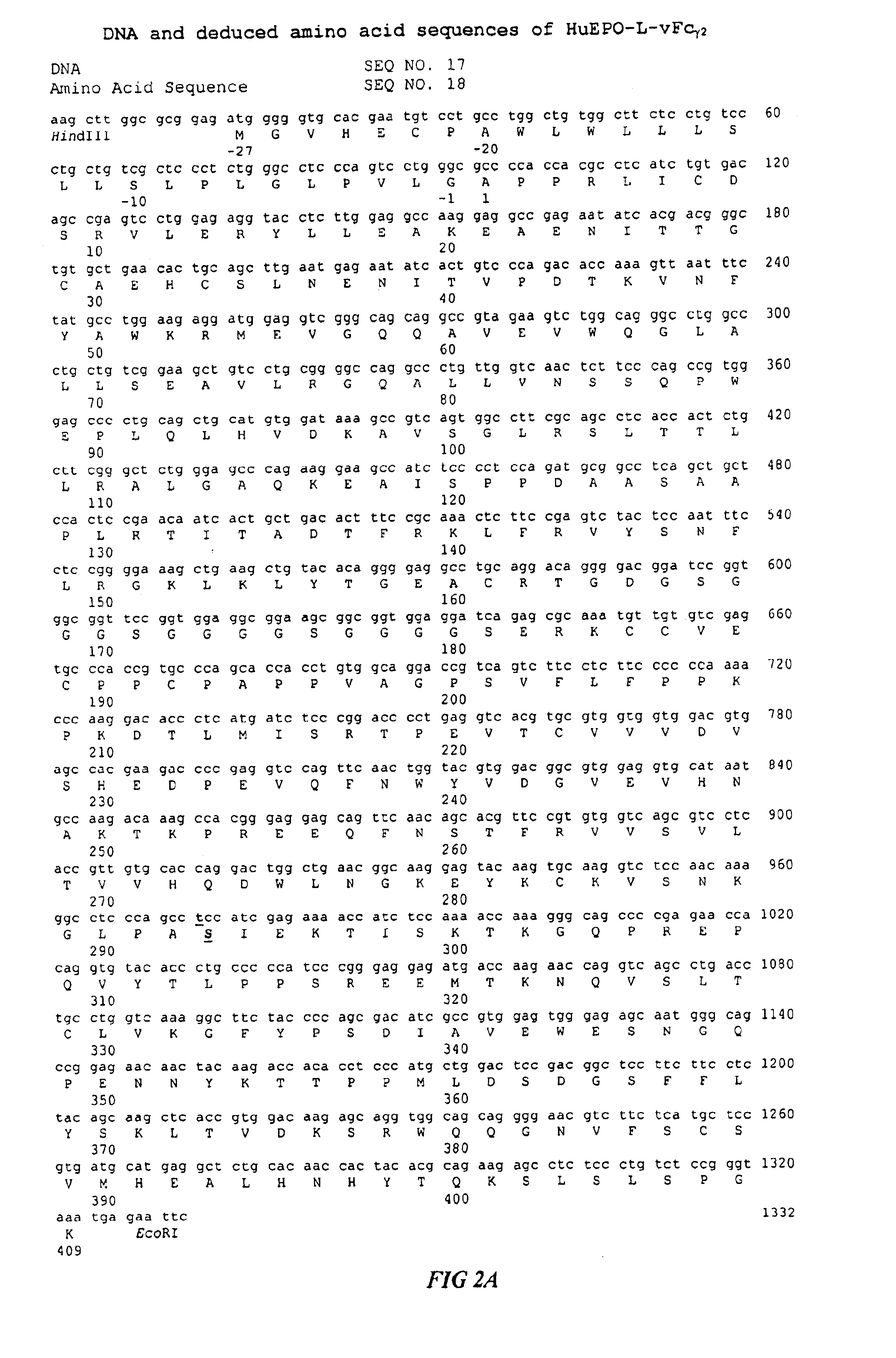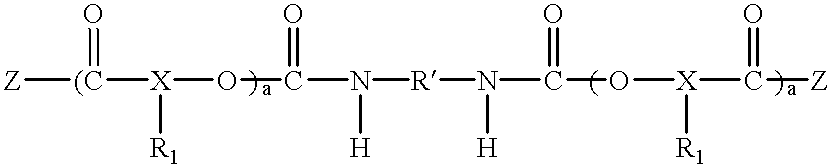Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
12226 results about "Drug biological activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In pharmacology, biological activity or pharmacological activity describes the beneficial or adverse effects of a drug on living matter. When a drug is a complex chemical mixture, this activity is exerted by the substance's active ingredient or pharmacophore but can be modified by the other constituents.
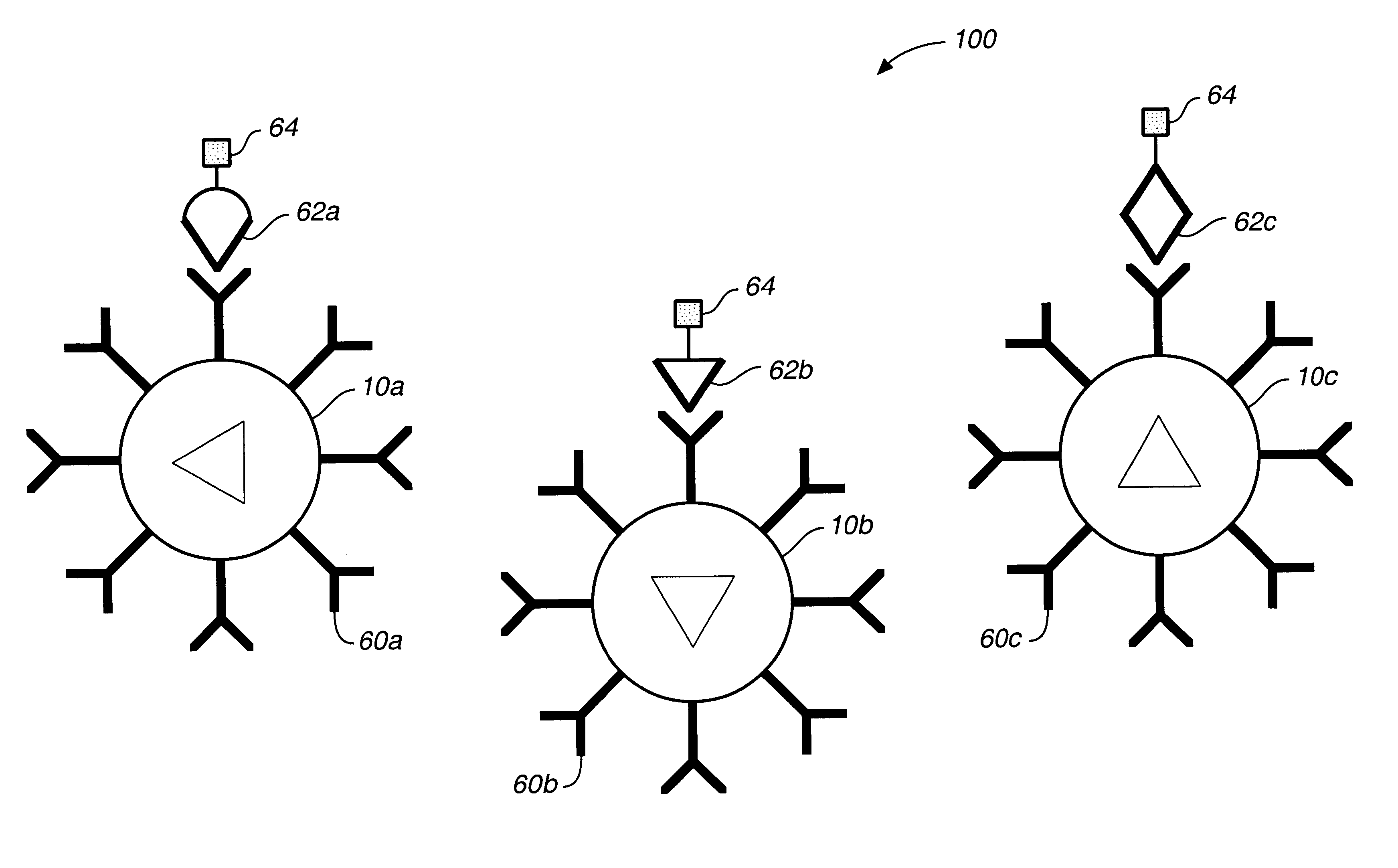
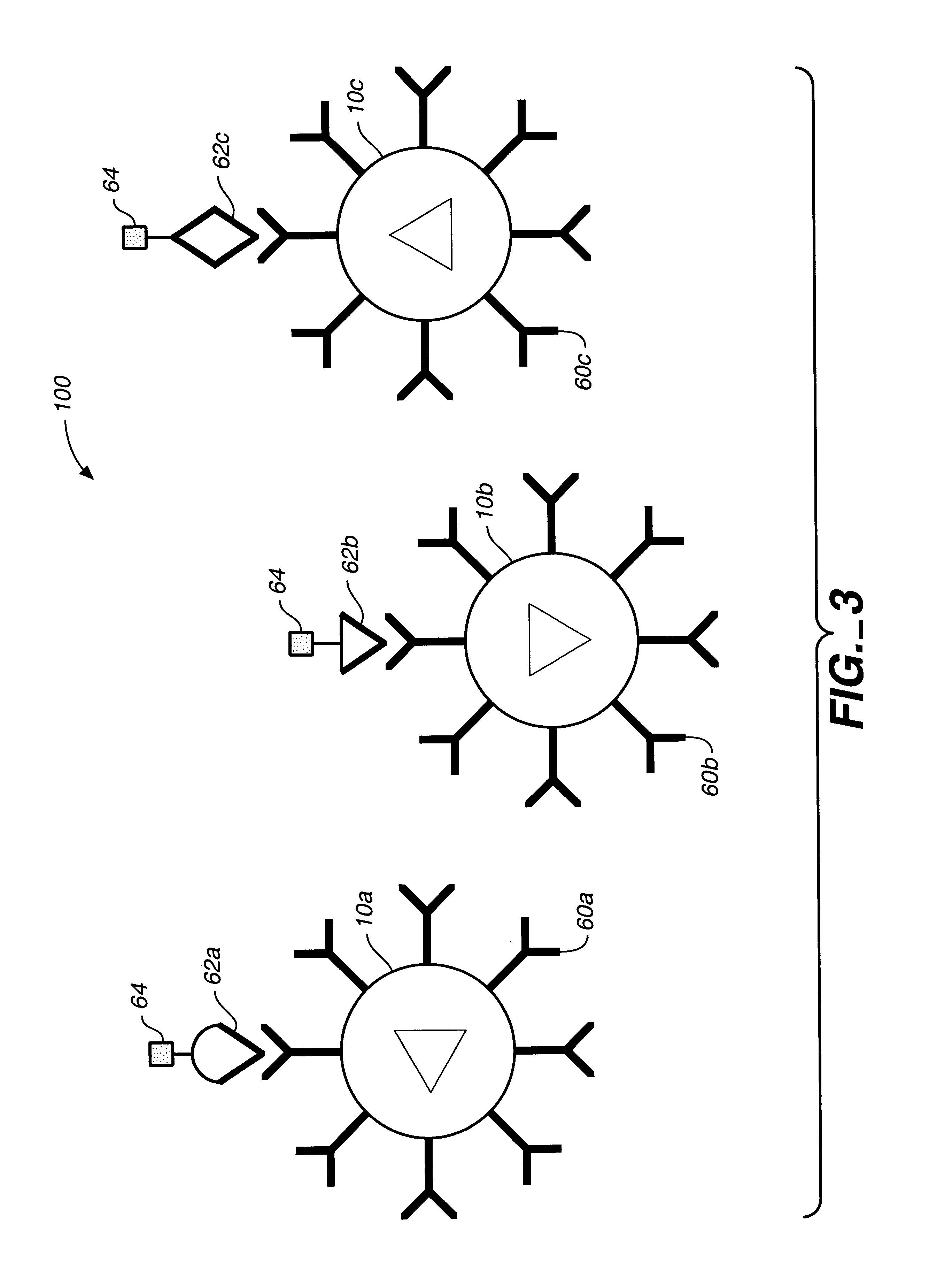
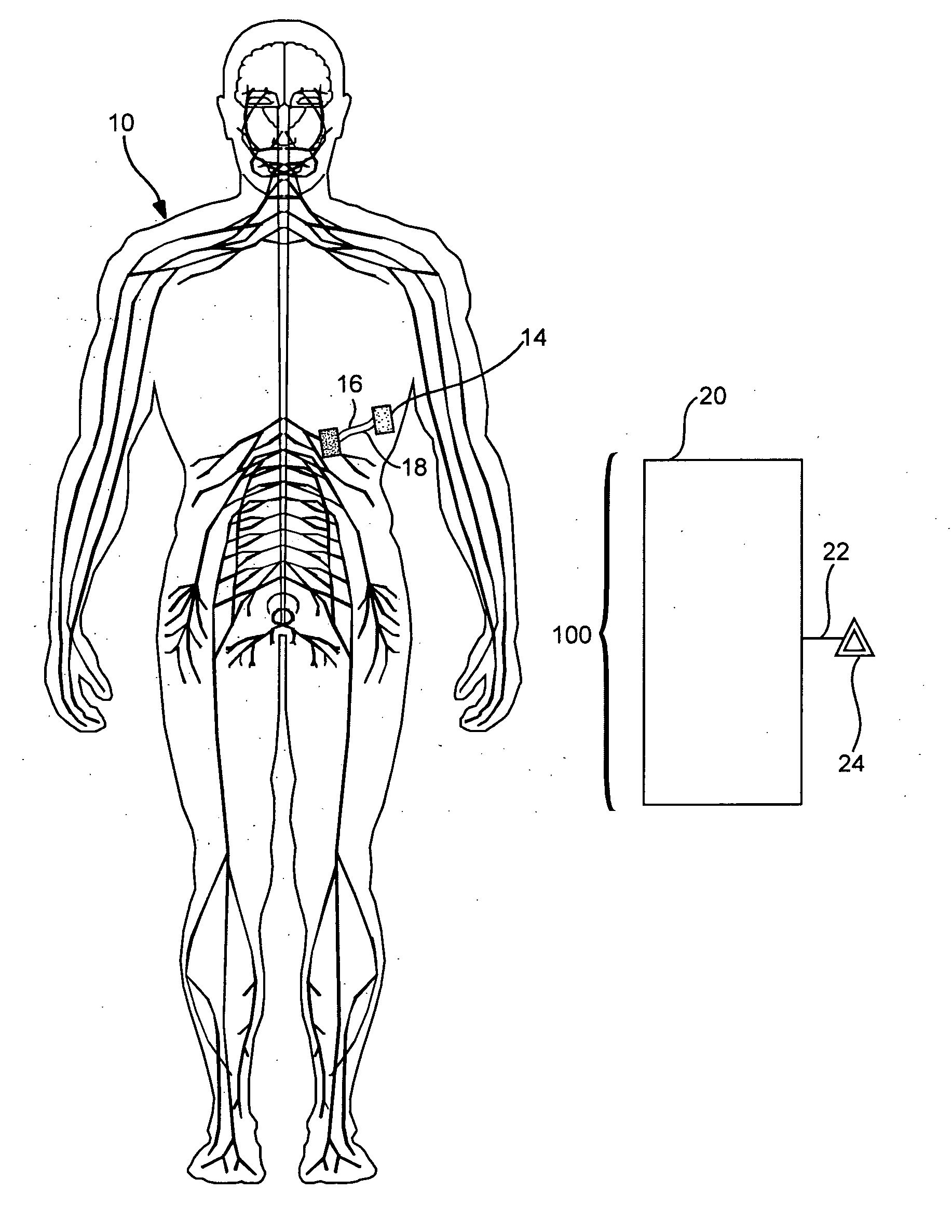
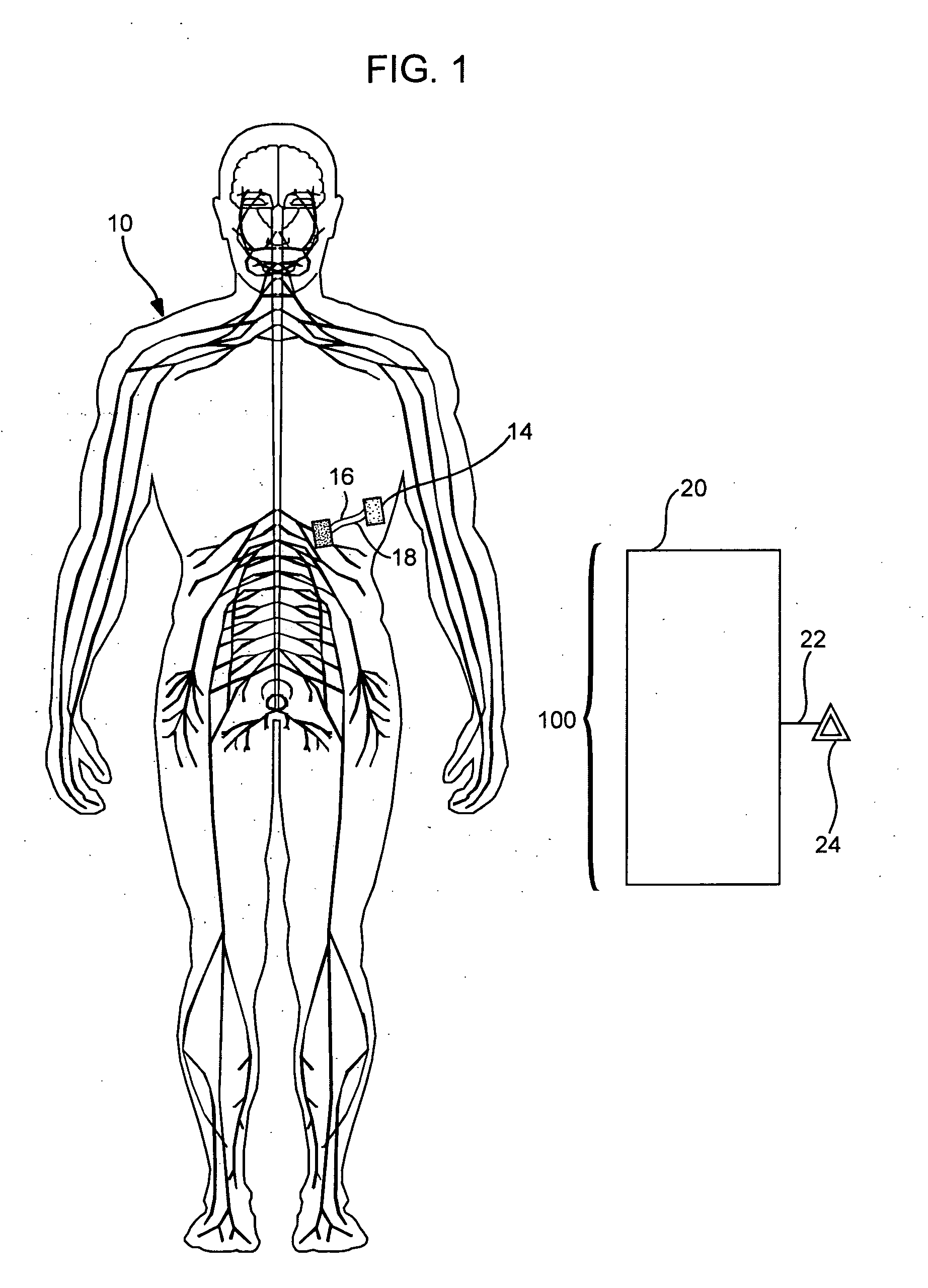
Target analyte sensors utilizing microspheres
A microsphere-based analytic chemistry system and method for making the same is disclosed in which microspheres or particles carrying bioactive agents may be combined randomly or in ordered fashion and dispersed on a substrate to form an array while maintaining the ability to identify the location of bioactive agents and particles within the array using an optically interrogatable, optical signature encoding scheme. A wide variety of modified substrates may be employed which provide either discrete or non-discrete sites for accommodating the microspheres in either random or patterned distributions. The substrates may be constructed from a variety of materials to form either two-dimensional or three-dimensional configurations. In a preferred embodiment, a modified fiber optic bundle or array is employed as a substrate to produce a high density array. The disclosed system and method have utility for detecting target analytes and screening large libraries of bioactive agents.
Owner:TRUSTEES OF TUFTS COLLEGETHE
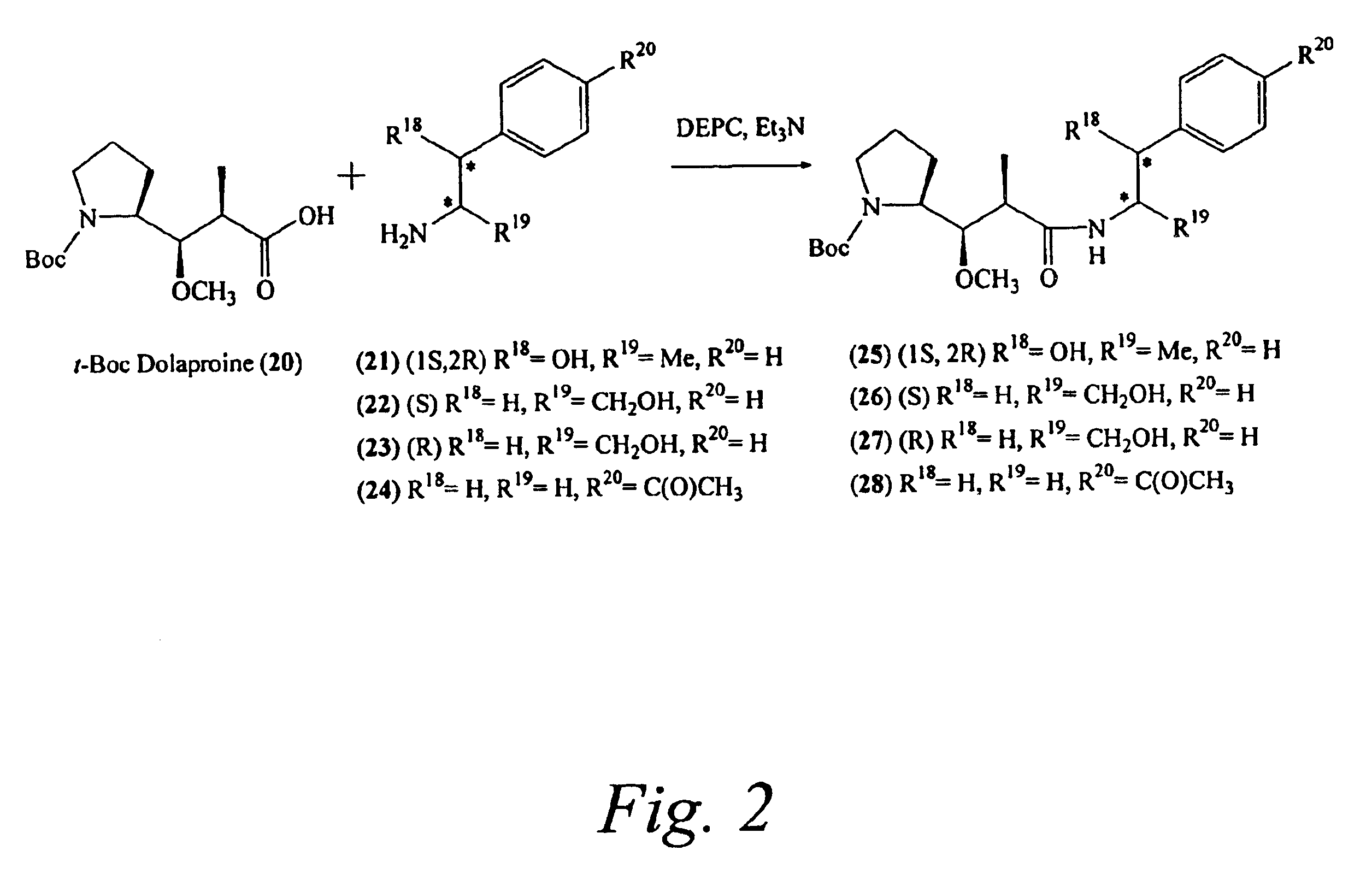
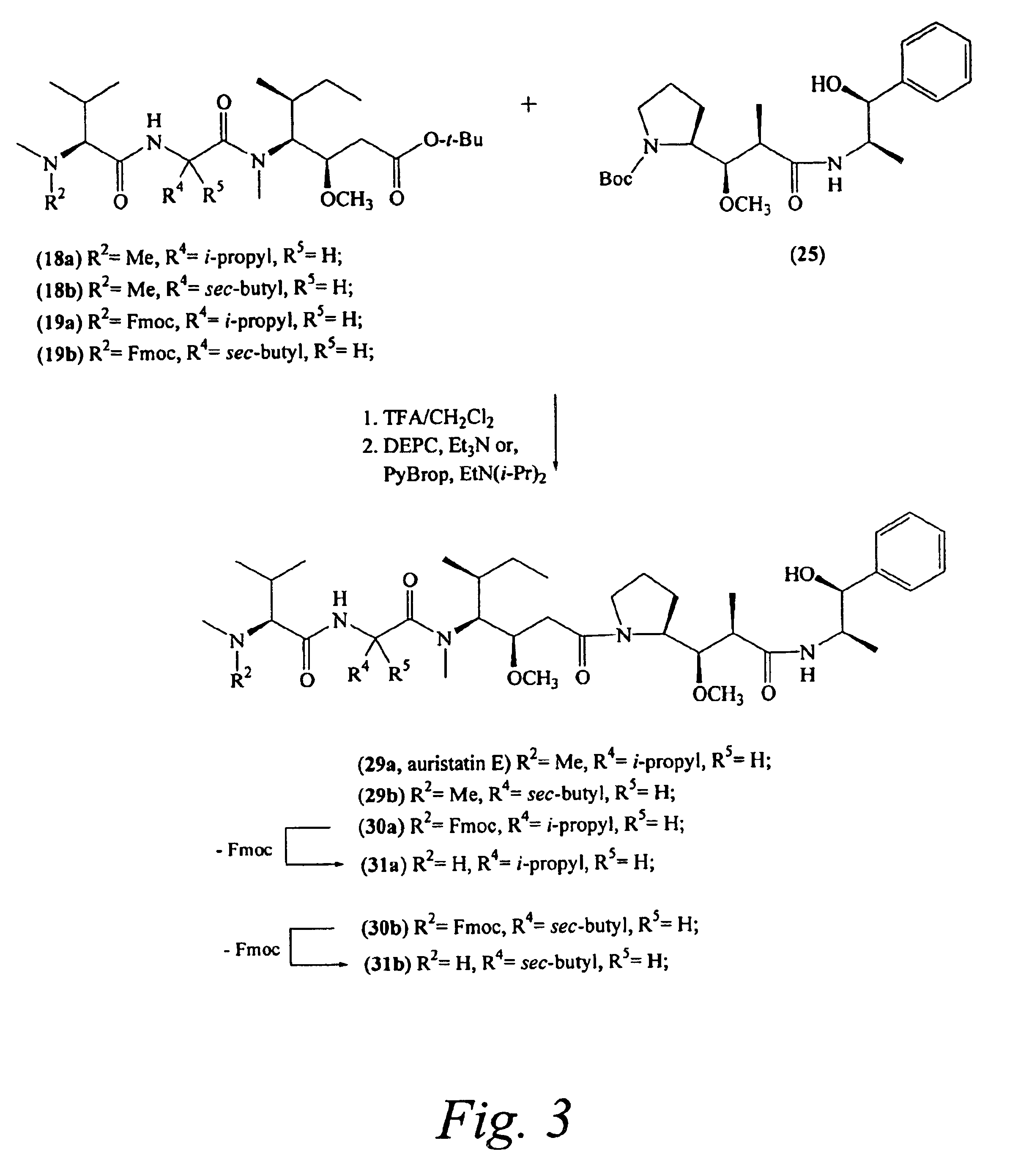
Pentapeptide compounds and uses related thereto
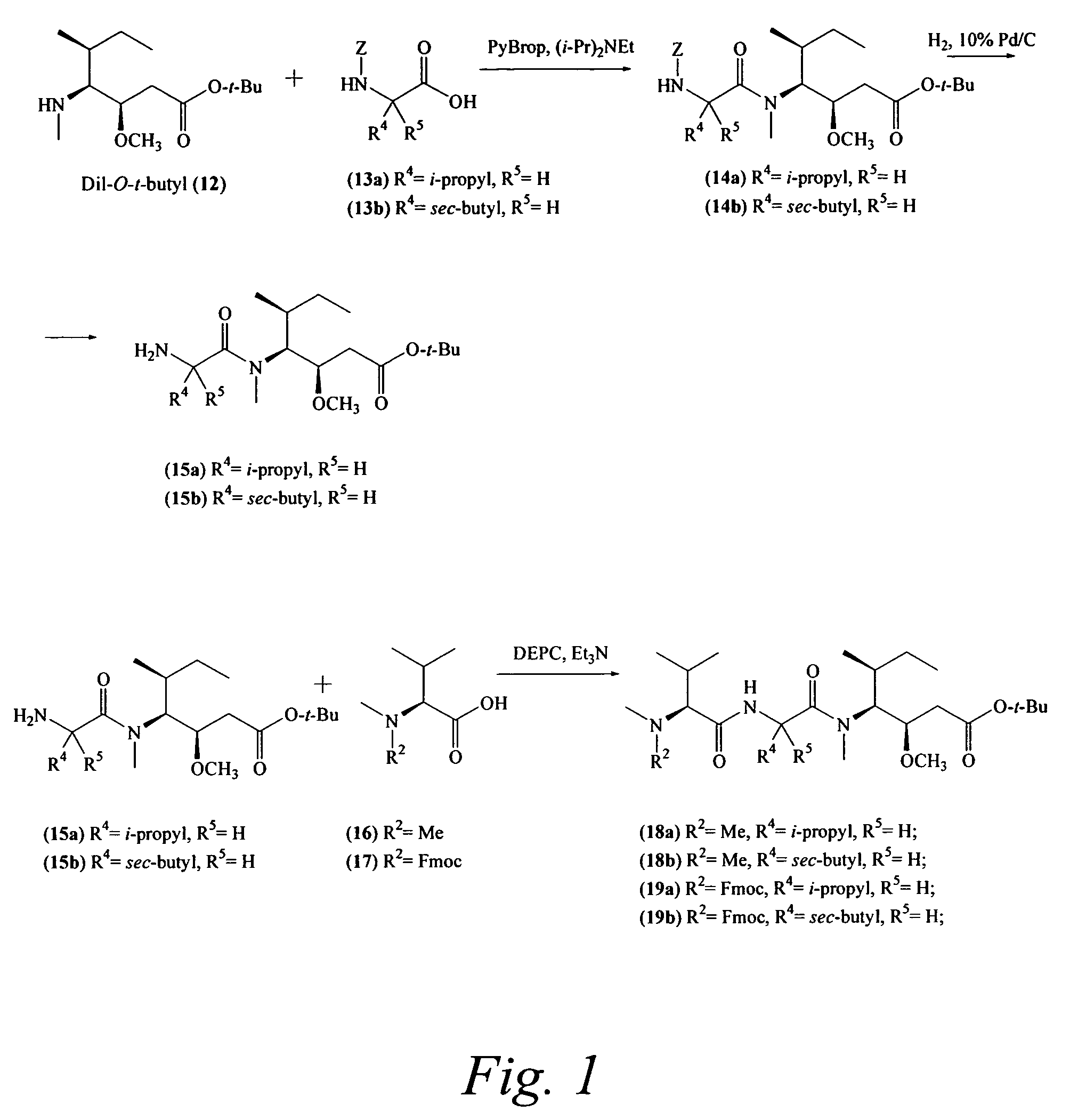
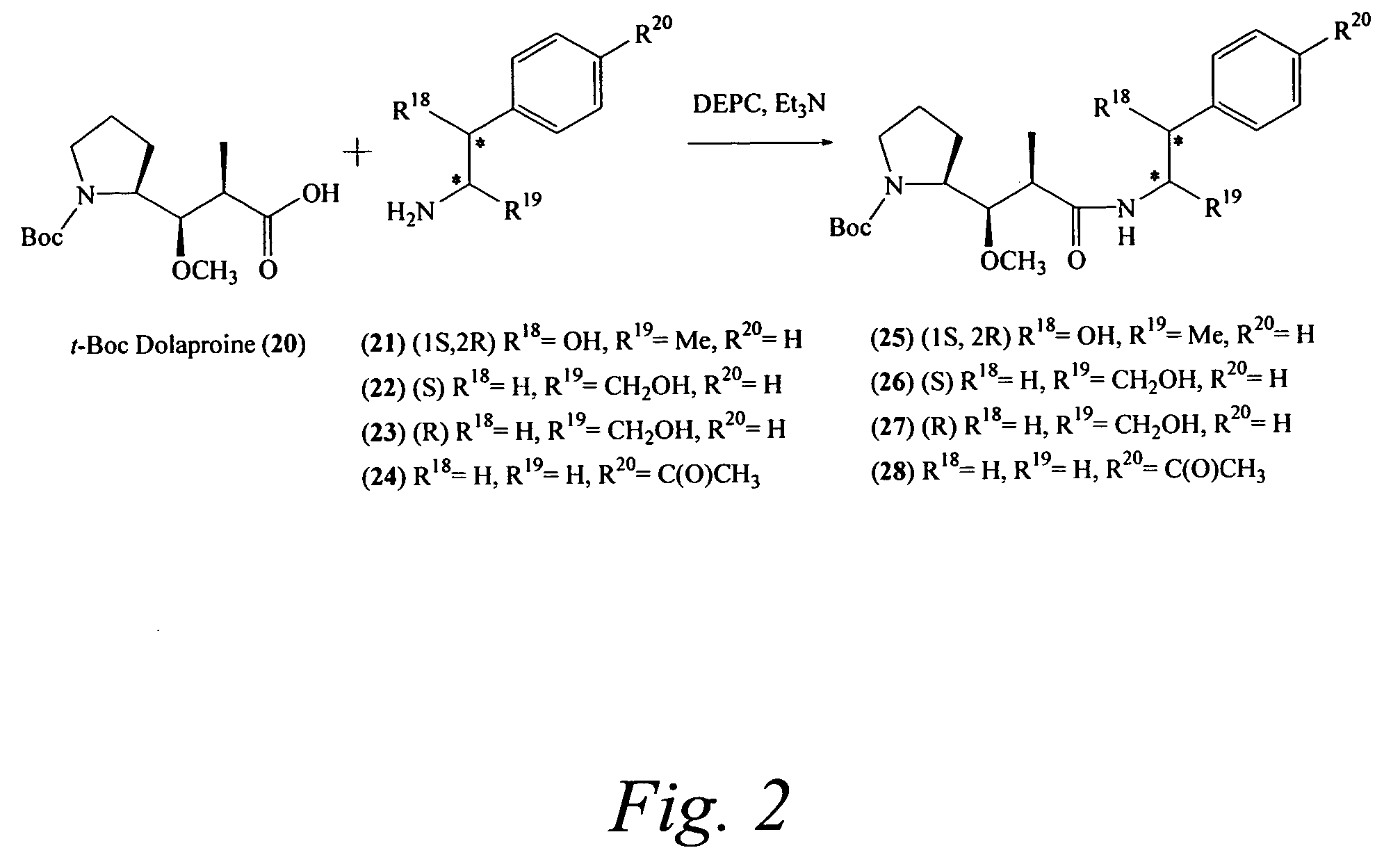
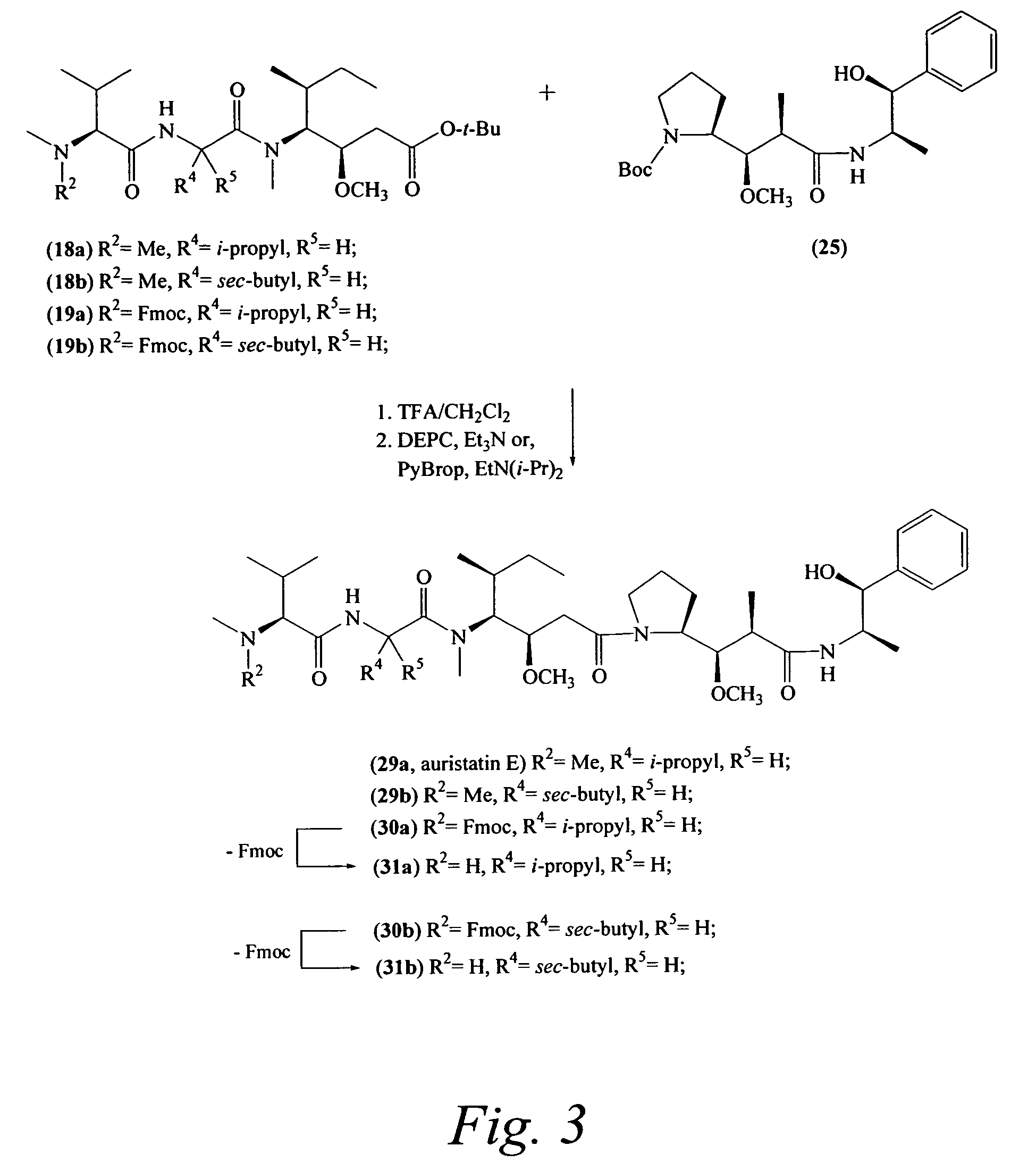
Pentapeptide compounds are disclosed. The compounds have biological activity, e.g., cytotoxicity. Prodrugs having targeting groups and pentapeptide moieities, as well as precursors thereof are also disclosed. For example, precursors having a reactive linker that can serve as a reaction site for joining to a targeting agent, e.g., an antibody, as disclosed.
Owner:SEAGEN INC
Targeted delivery of active/bioactive and perfuming compositions
Described are controlled, time-release microparticulate active and bioactive compositions (including perfuming compositions) for targeted delivery to surfaces such as skin, hair and fabric and the environment proximate thereto, where the active and bioactive materials have a calculated log10P values of between 1 and 8 (P being the n-octanol-water partition coefficient). Such compositions include the active or bioactive material in single phase, solid solution in a wax or polymer matrix also having coated thereon and / or containing a compatible surfactant. Also described are processes and apparatus for preparing such compositions and processes for using same. Furthermore, certain component(s) of the aforementioned compositions in combination with one another are novel, and other components have novel uses in increasing fragrance substantivity, particularly in hair care preparations such as hair gels and shampoos.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
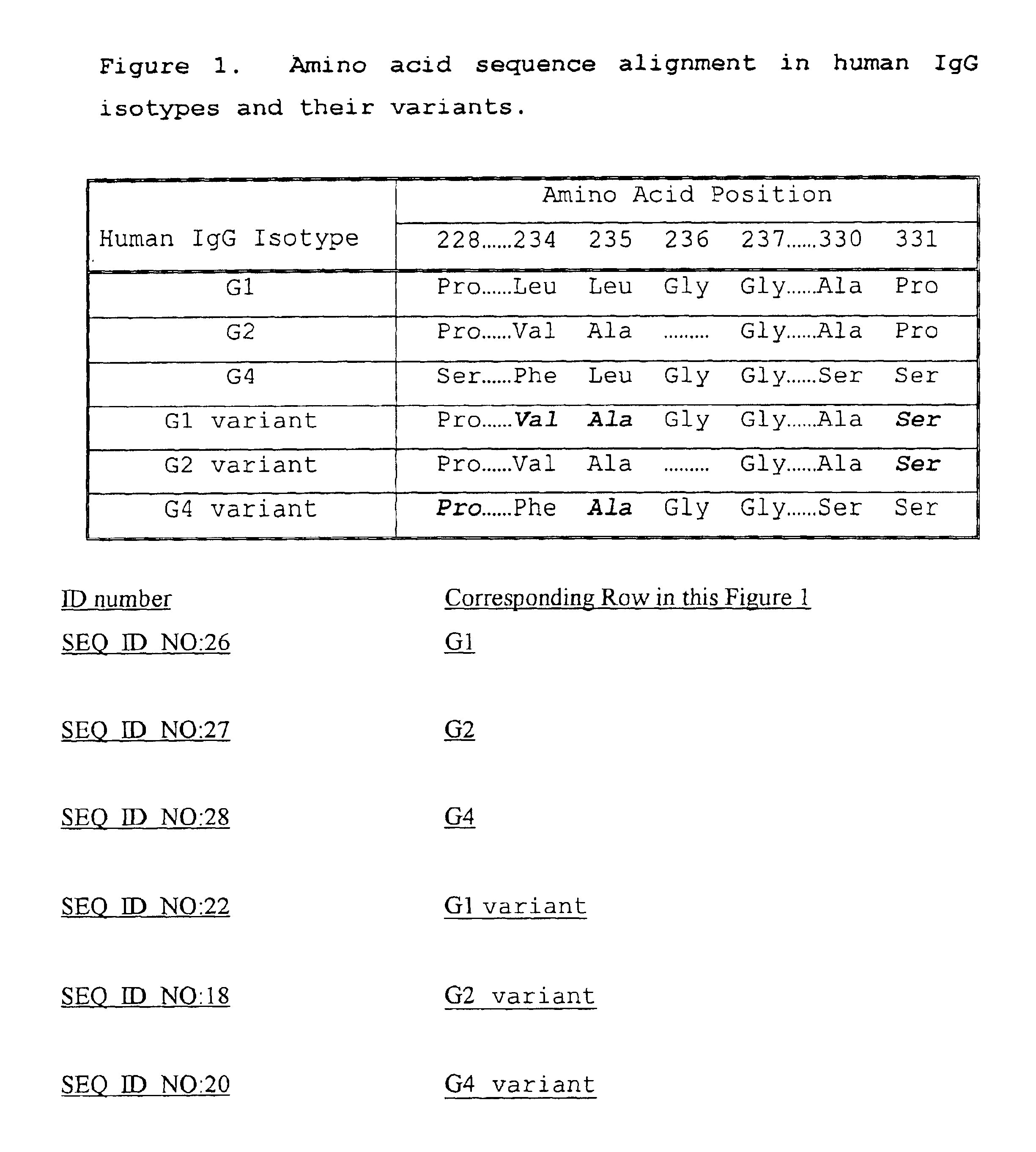
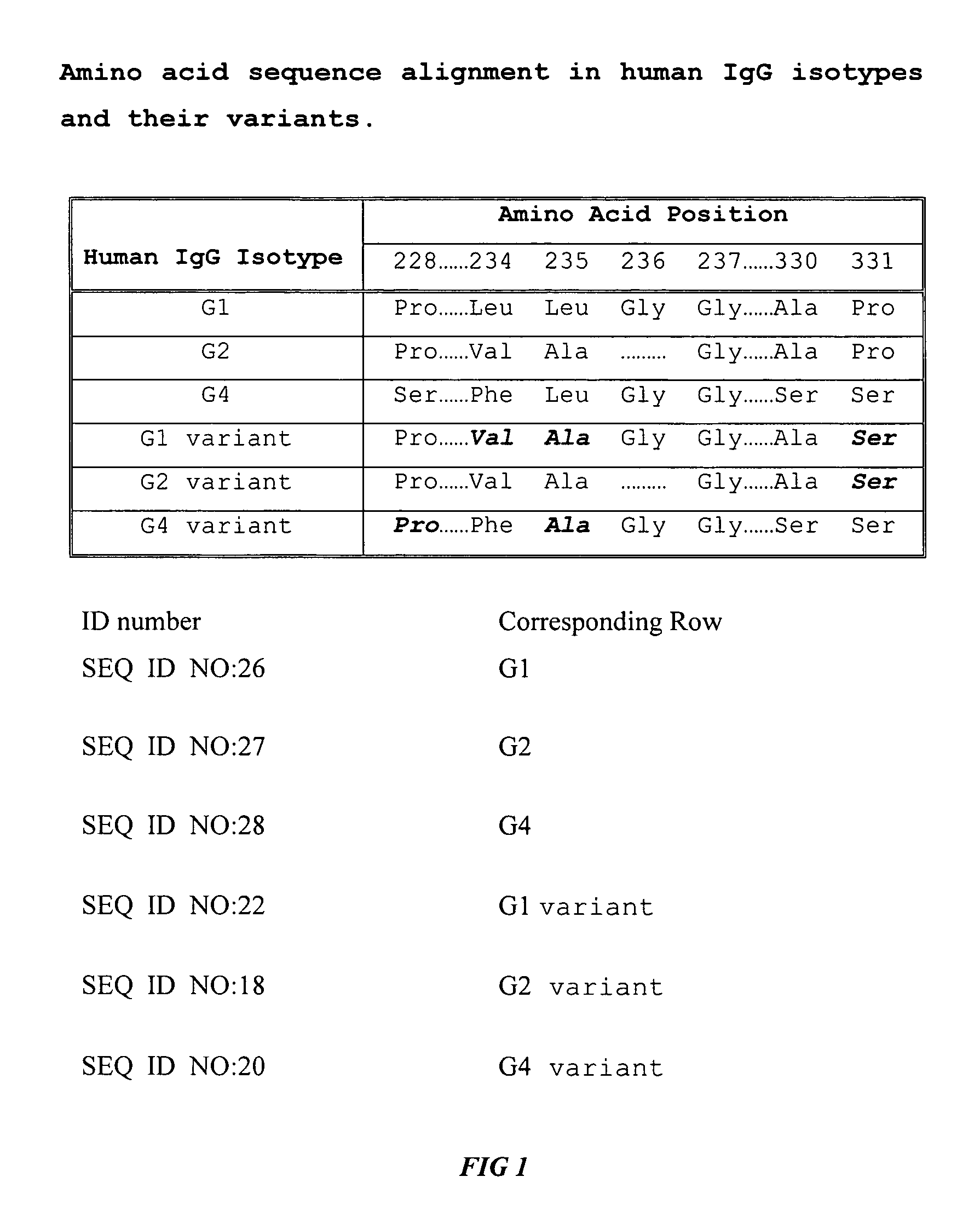
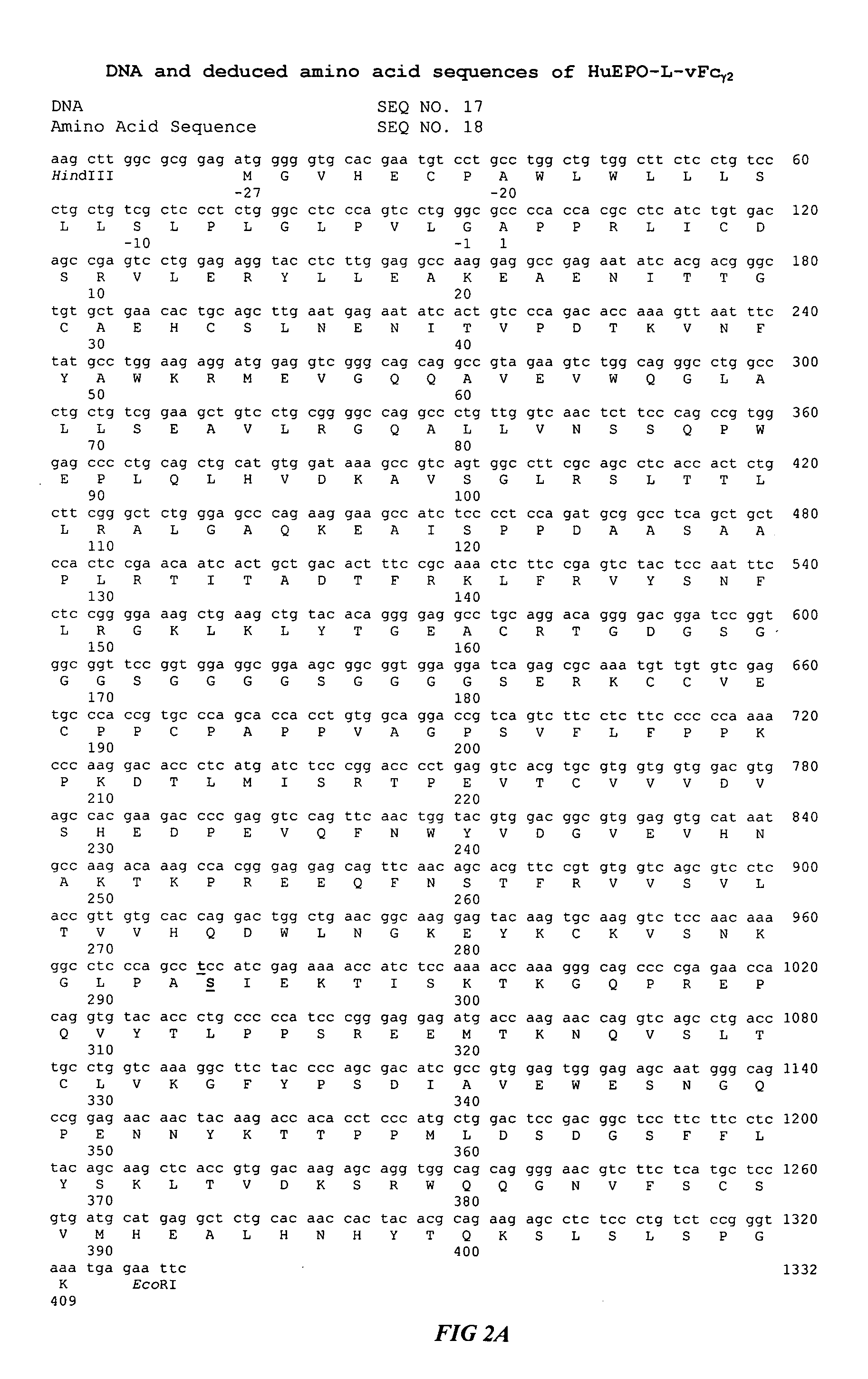
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS6900292B2Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:LONGBIO PHARM (SUZHOU) CO LTD
Pharmaceutical and cosmetic carrier or composition for topical application
A pharmaceutical or cosmetic carrier or composition for topical application characterized by rheological properties which render the carrier or composition semi-solid at rest and a liquid upon application of shear forces thereto. The composition or carrier are prepared by mixing 1-25 percent of a solidifying agent and 75-99 percent of a hydrophobic solvent, by weight, wherein at least one of them has therapeutic or cosmetic benefits, in the presence or absence of a biologically active substance.
Owner:VYNE PHARMA LTD
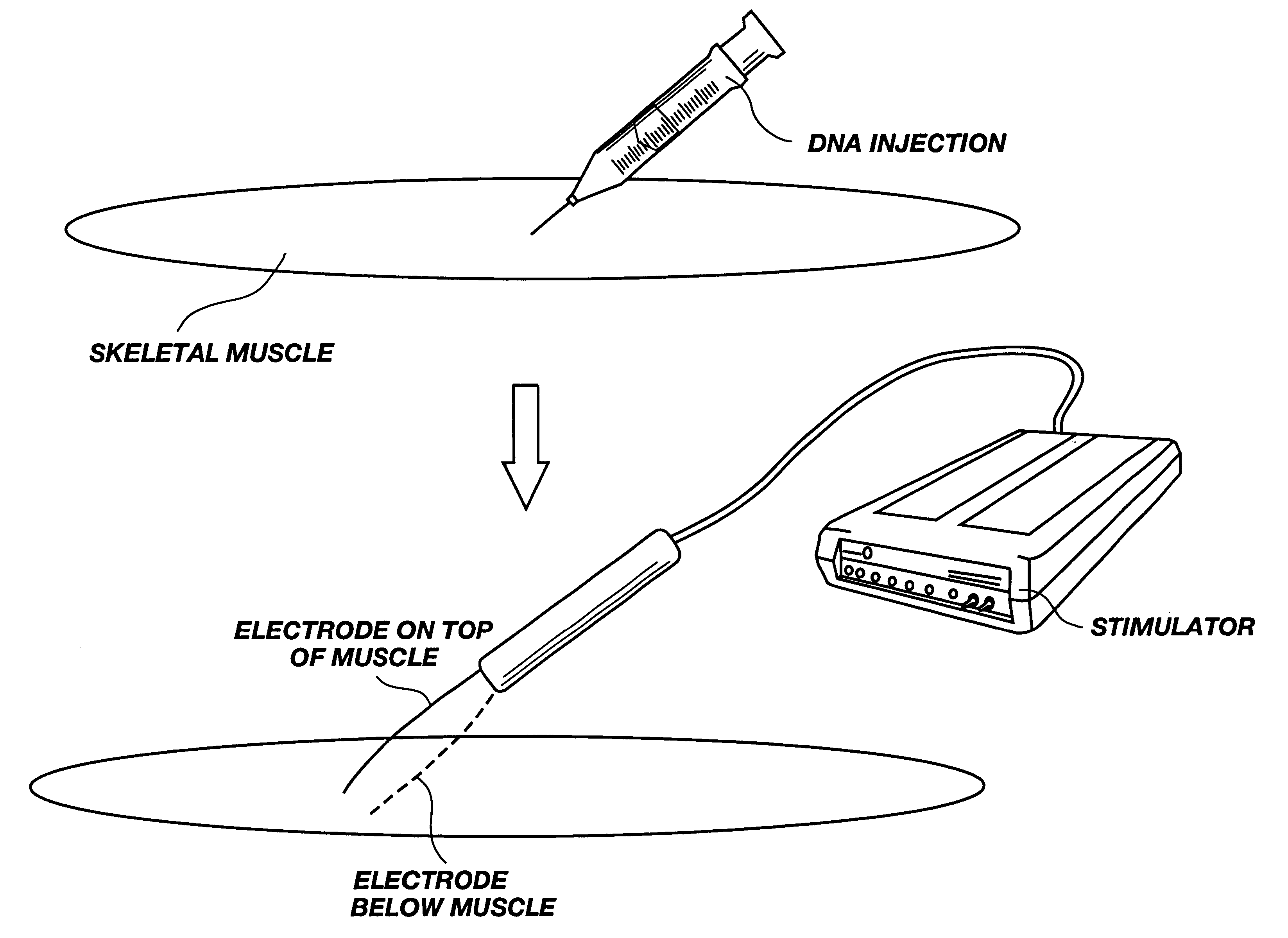
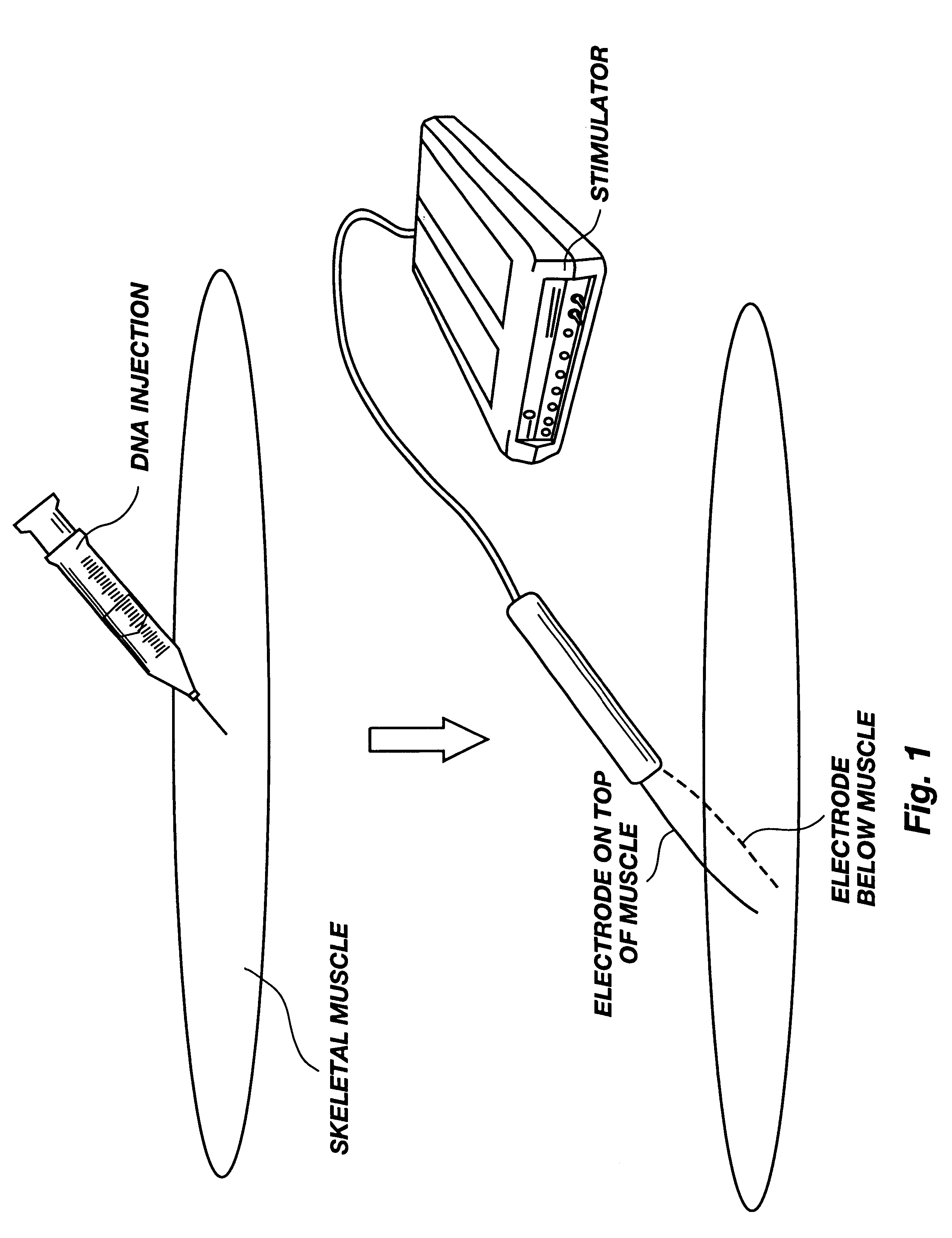
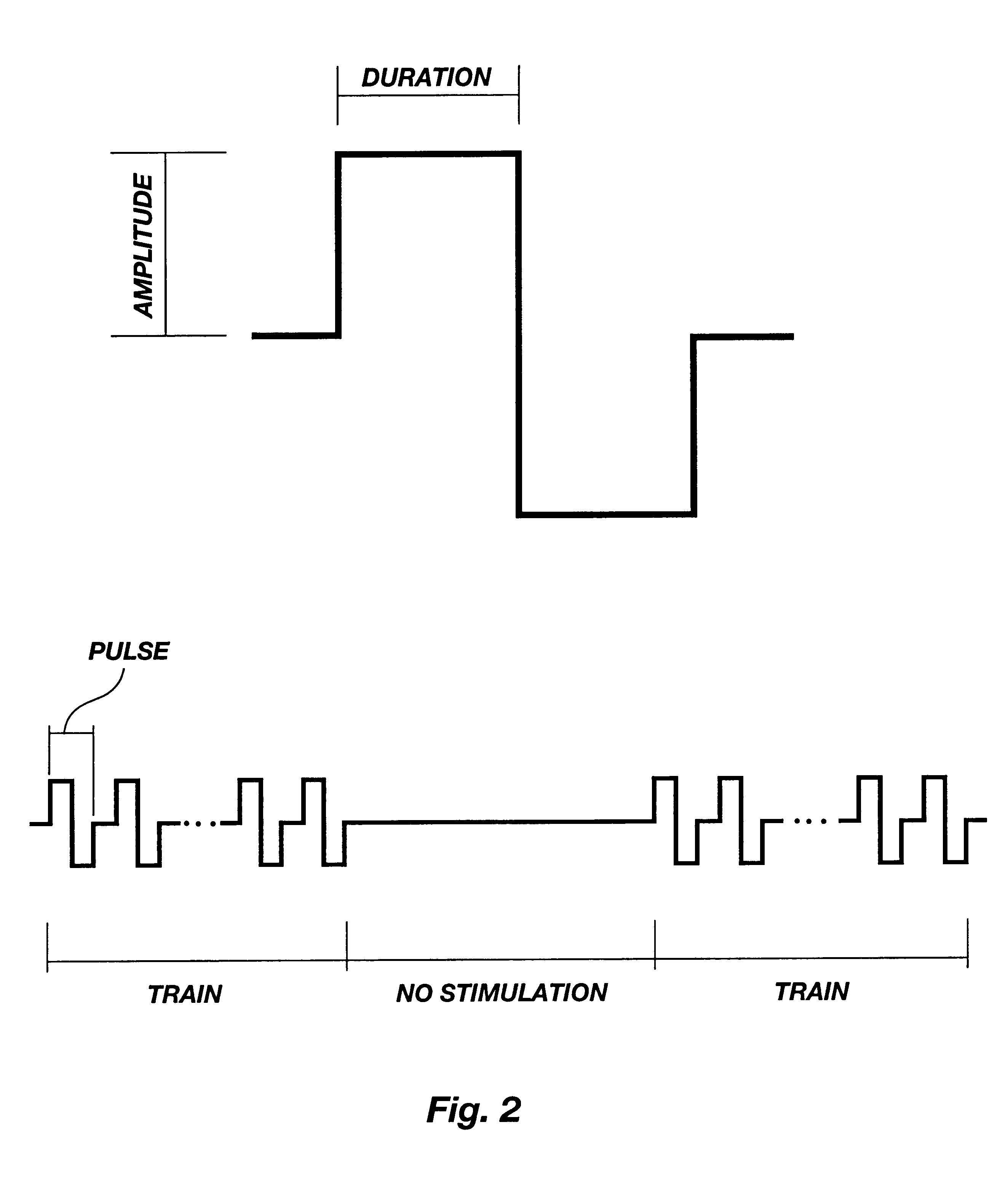
Method for genetic immunization and introduction of molecules into skeletal muscle and immune cells
InactiveUS6261281B1High transfection efficiencyGreat luciferace activityBacterial antigen ingredientsElectrotherapyVaccinationWhole body
A method is disclosed for enhanced vaccination and genetic vaccination of mammals. The vaccination is accomplished by delivering molecules such as proteins and nucleic acids into skeletal muscle and other cells residing in the skeletal muscle in vivo. The protein or nucleic acid is first injected into the muscle at one or multiple sites. Immediately or shortly after injection, electrodes are placed flanking the injection site and a specific amount of electrical current is passed through the muscle. The electrical current makes the muscle permeable, thus allowing the pharmaceutical drug or nucleic acid to enter the cell. The efficiency of transfer permits robust immune responses using DNA vaccines and produces sufficient secreted proteins for systemic biological activity to be observed.
Owner:INOVIO
Polyester polyether block copolymers
The present invention relates to novel bioabsorbable polymeric compositions based upon AB polyester polyether or related diblocks and triblocks. Compositions according to the present invention may be used in medical applications, for example, for reducing or preventing adhesion formation subsequent to medical procedures such as surgery, for producing surgical articles including stents and grafts, as coatings, sealants, lubricants, as transient barriers in the body, for materials which control the release of bioactive agents in the body, for wound and bum dressings and producing biodegradable articles, among numerous others.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
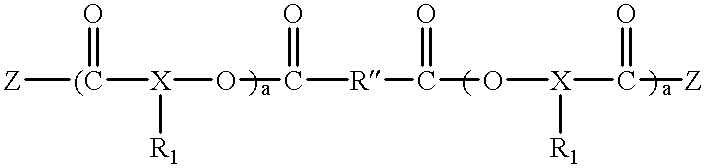
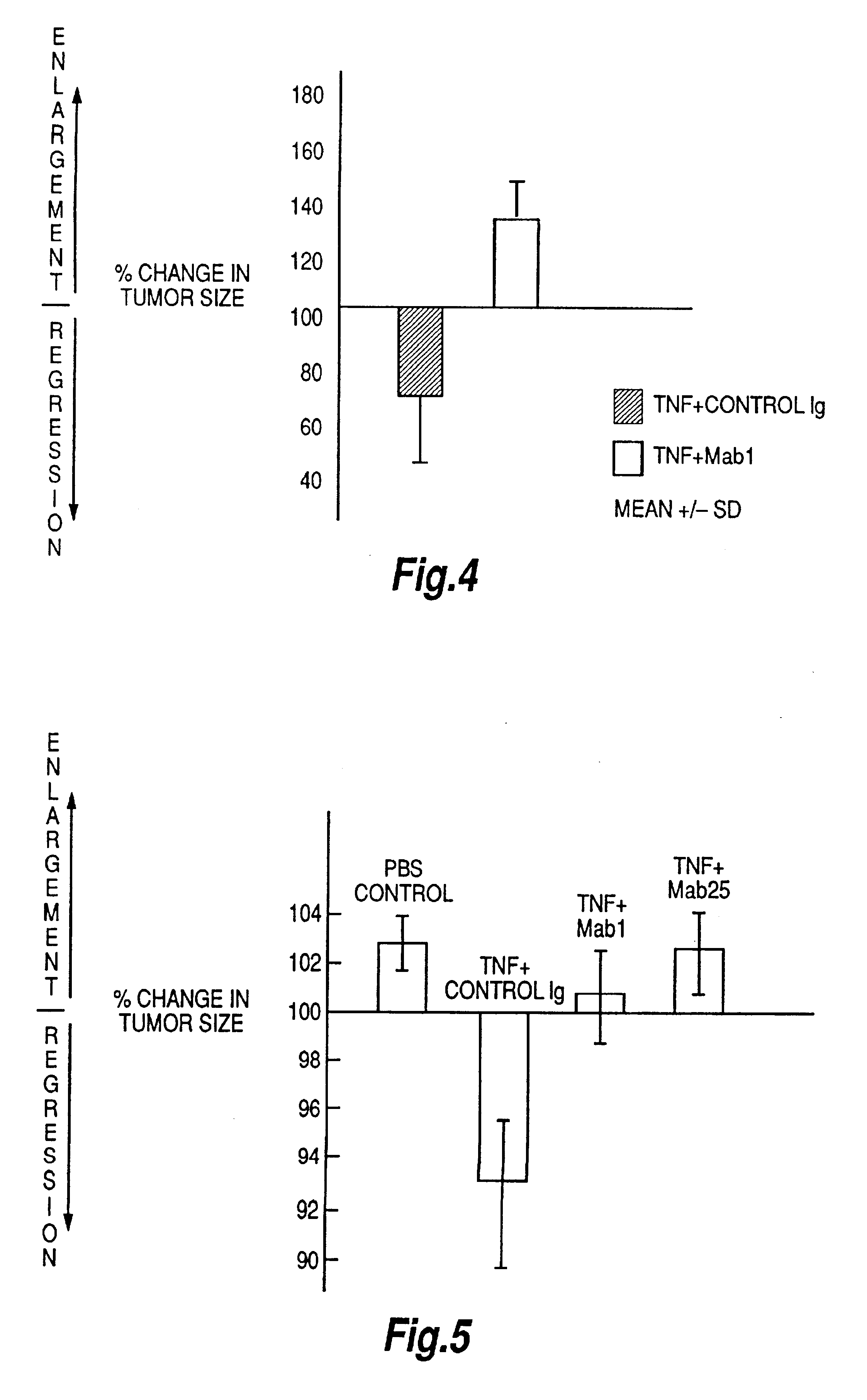
Tumour necrosis factor antibodies
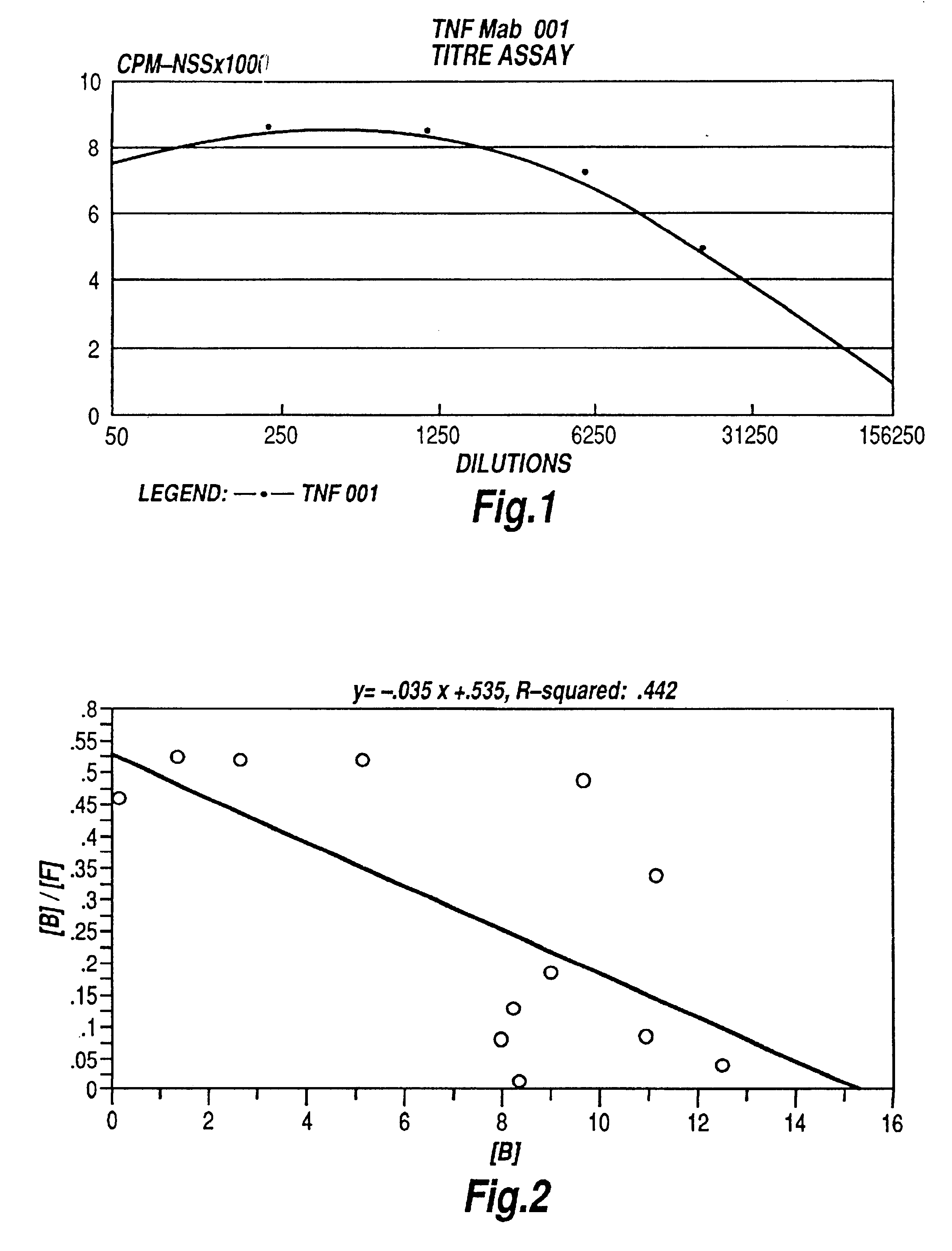
InactiveUS6451983B2Enhance or inhibit TNF alpha activityInduction of endothelial procoagulant activityPeptide/protein ingredientsAntibody mimetics/scaffoldsHuman tumorSingle-Chain Antibodies
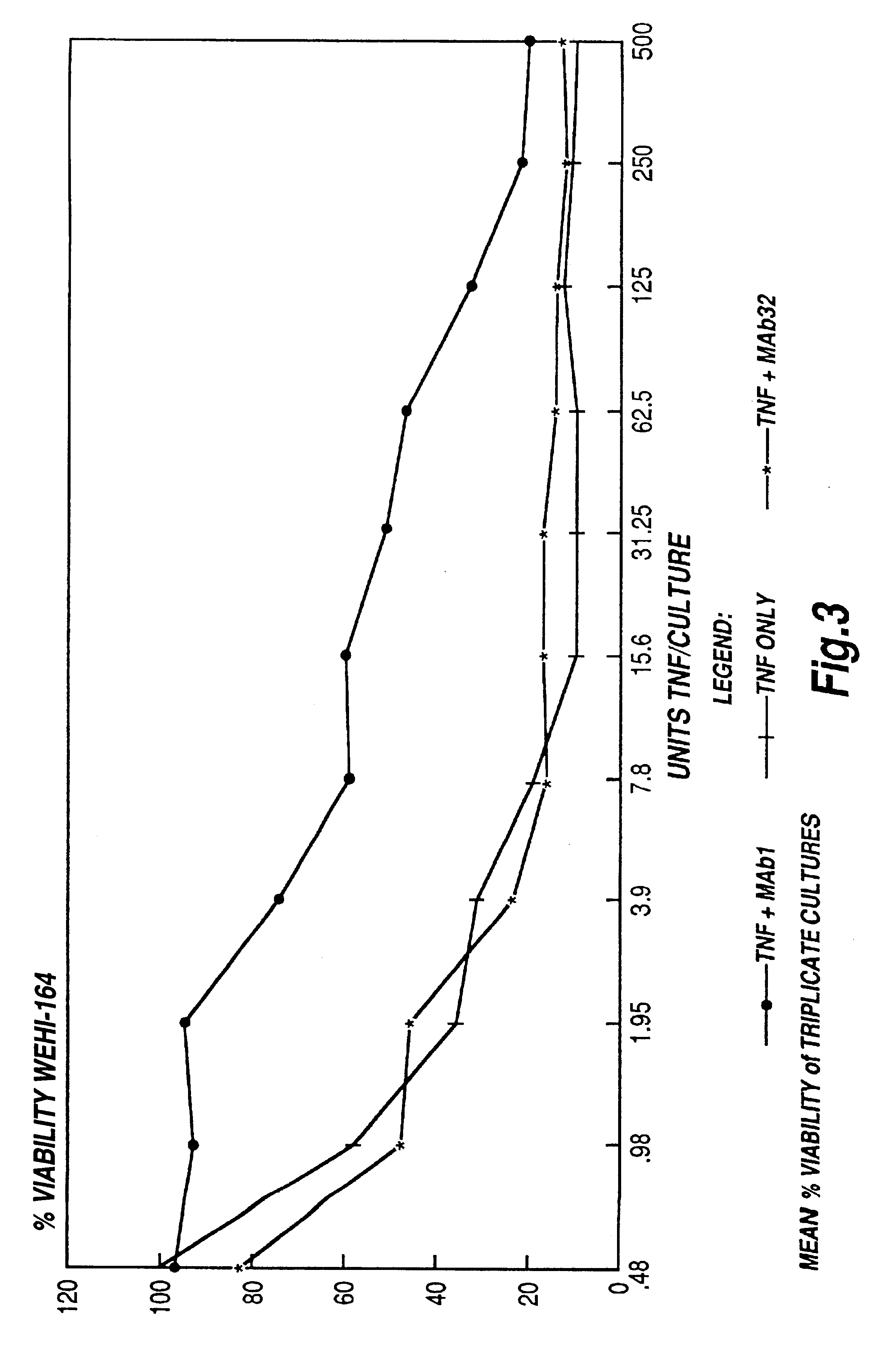
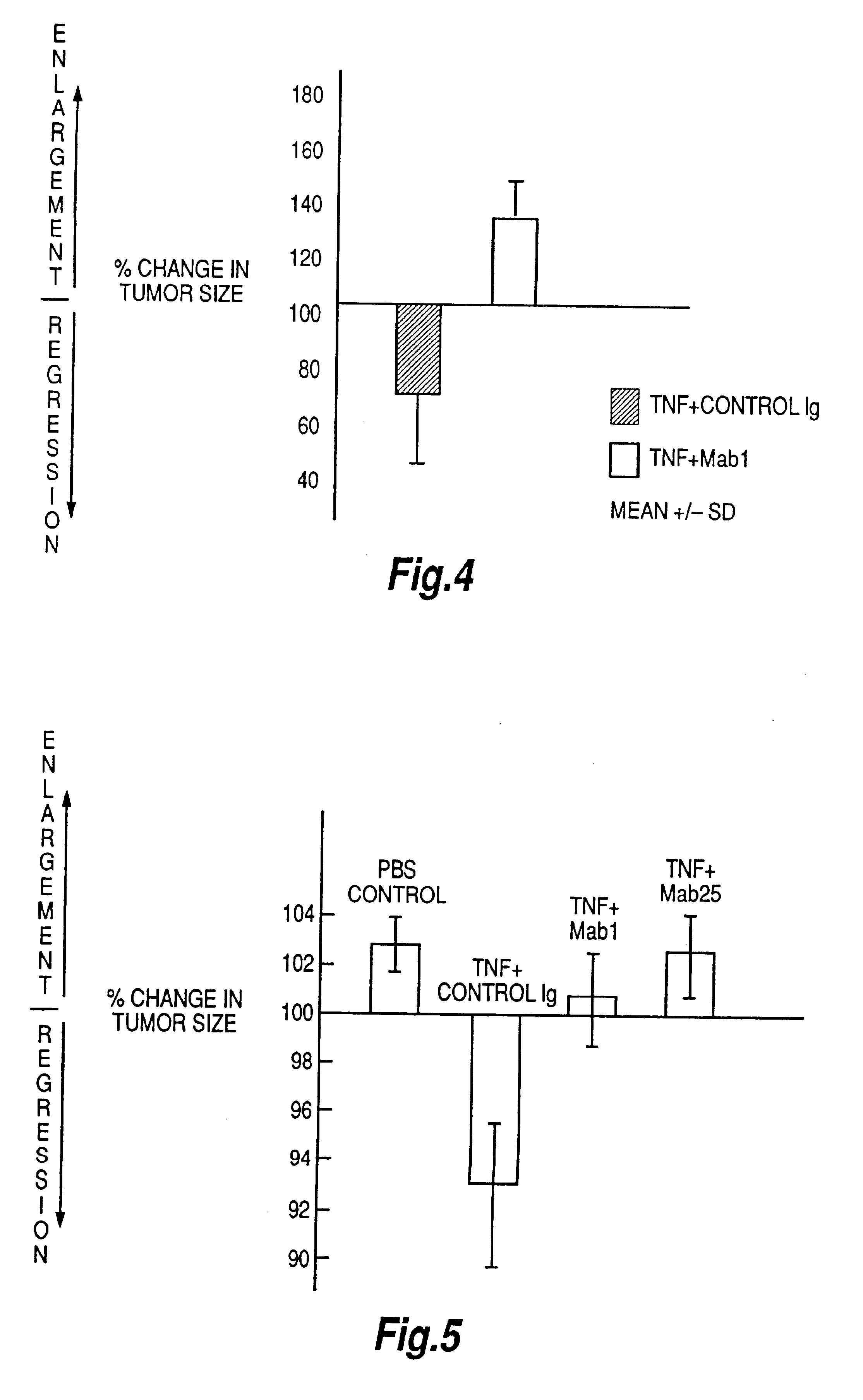
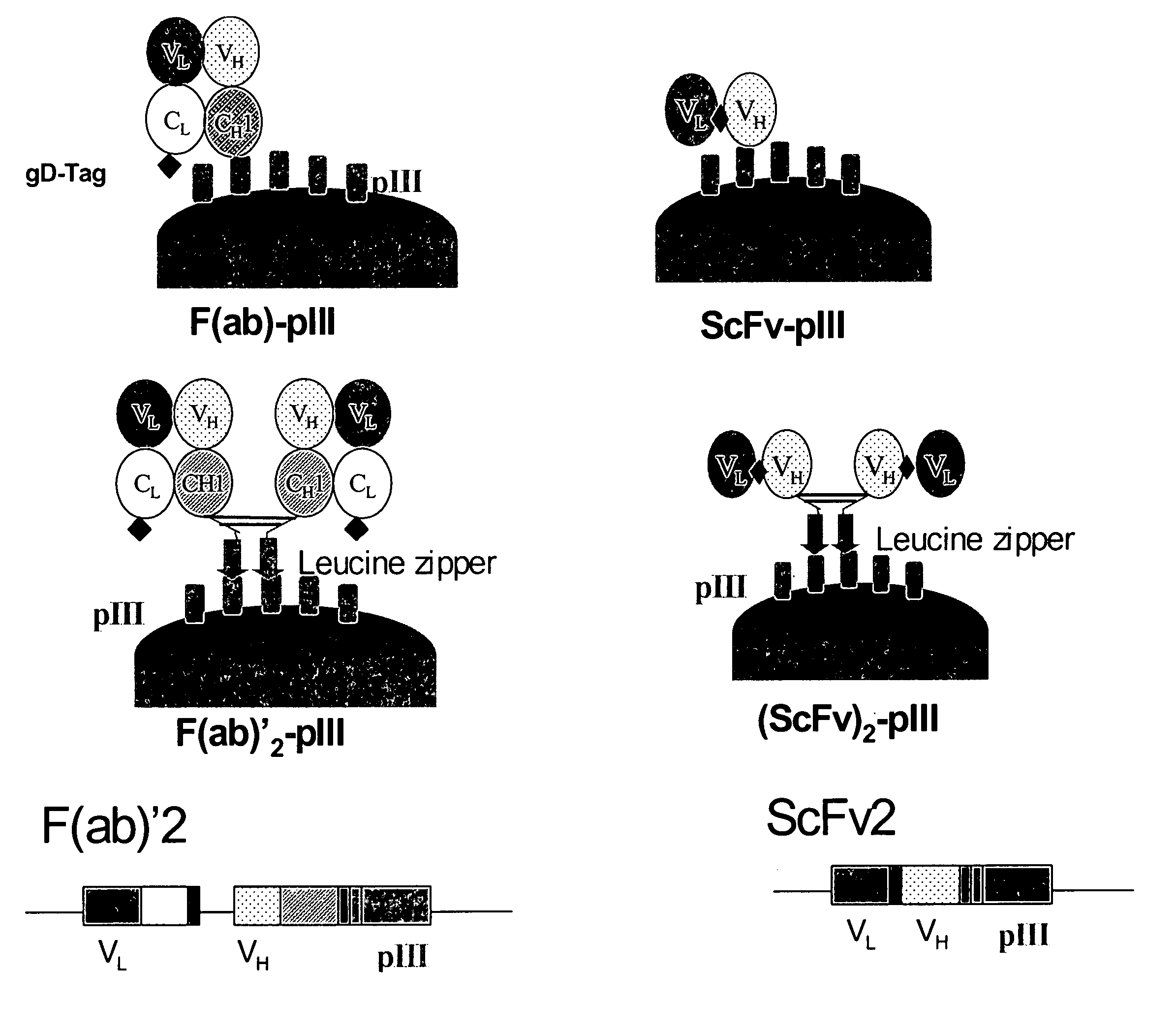
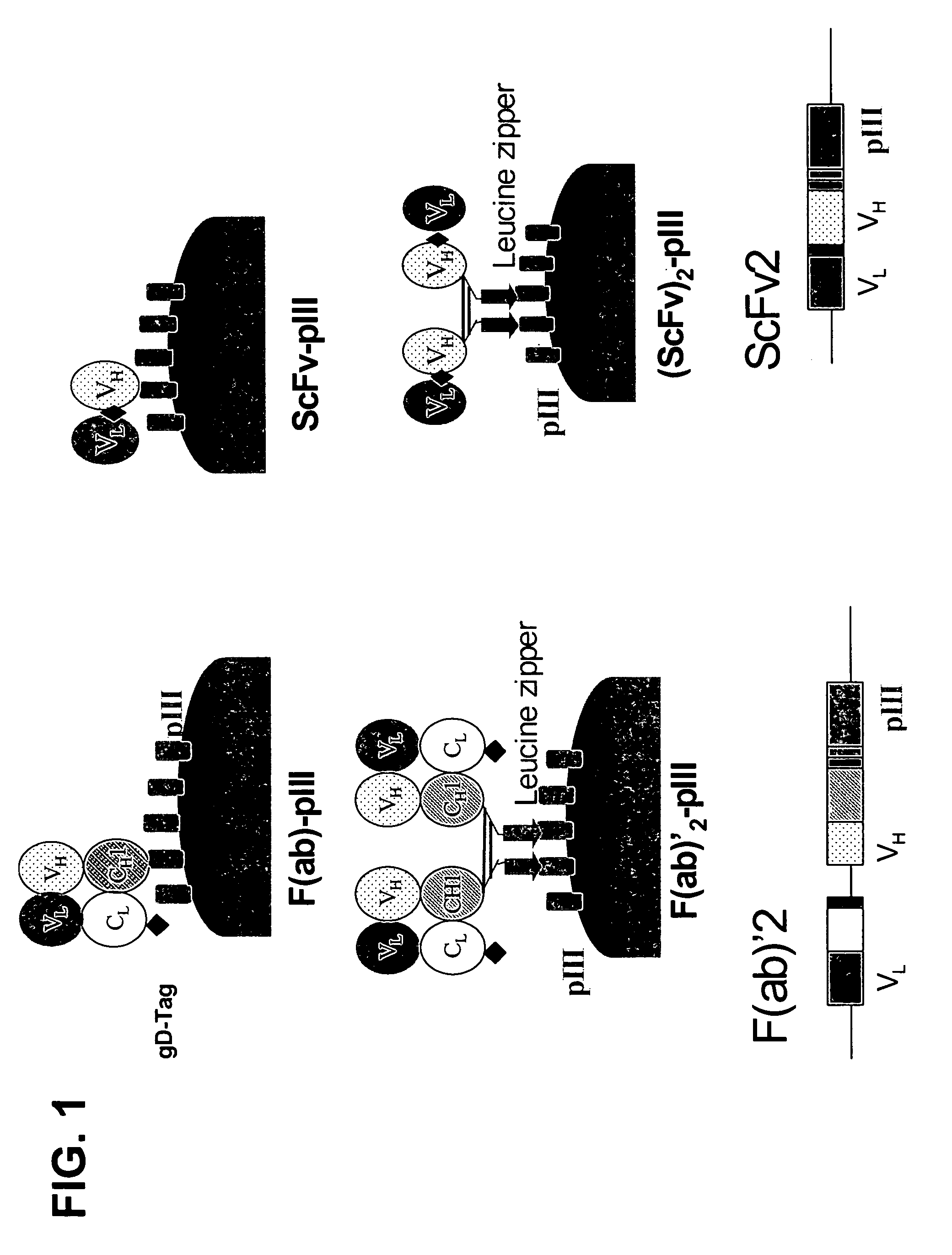
The present invention relates to ligands which bind to human tumor necrosis factor alpha (TNF) in a manner such that upon binding of these ligands to TNF the biological activity of TNF is modified. In preferred forms the ligand binds to TNF in a manner such that the induction of endothelial procoagulant activity of the TNF is inhibited; the binding of TNF to receptors on endothelial cells is inhibited; the induction of fibrin deposition in the tumor and tumor regression activities of the TNF are enhanced; and the cytotoxicity and receptor binding activities of the TNF are unaffected or enhanced on tumor cells. The ligand is preferably an antibody, F(ab) fragment, single domain antibody (dABs) single chain antibody or a serum binding protein. It is preferred, however, that the ligand is a monoclonal antibody or F(ab) fragment thereof.
Owner:CEPHALON AUSTRALIA
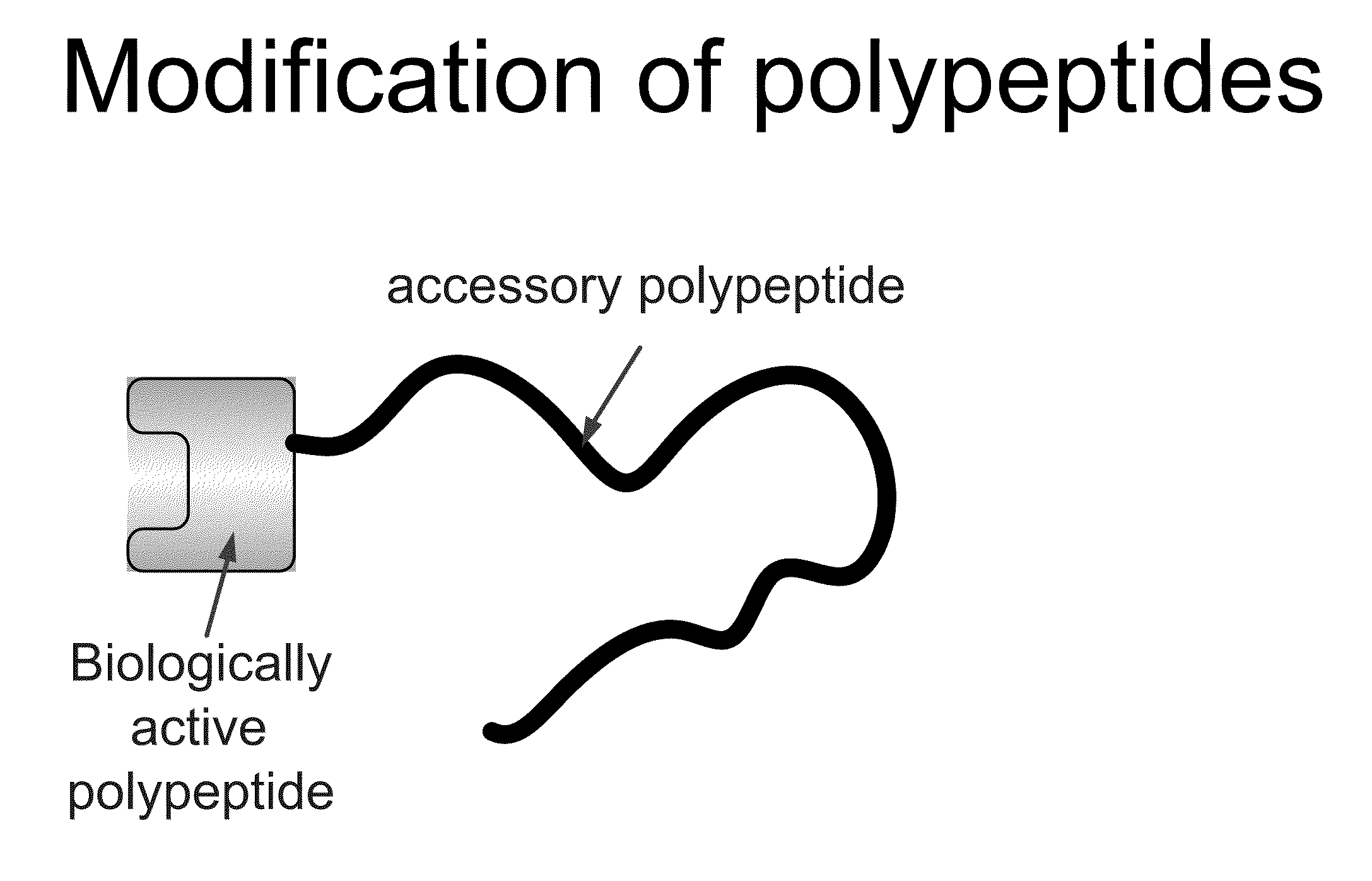
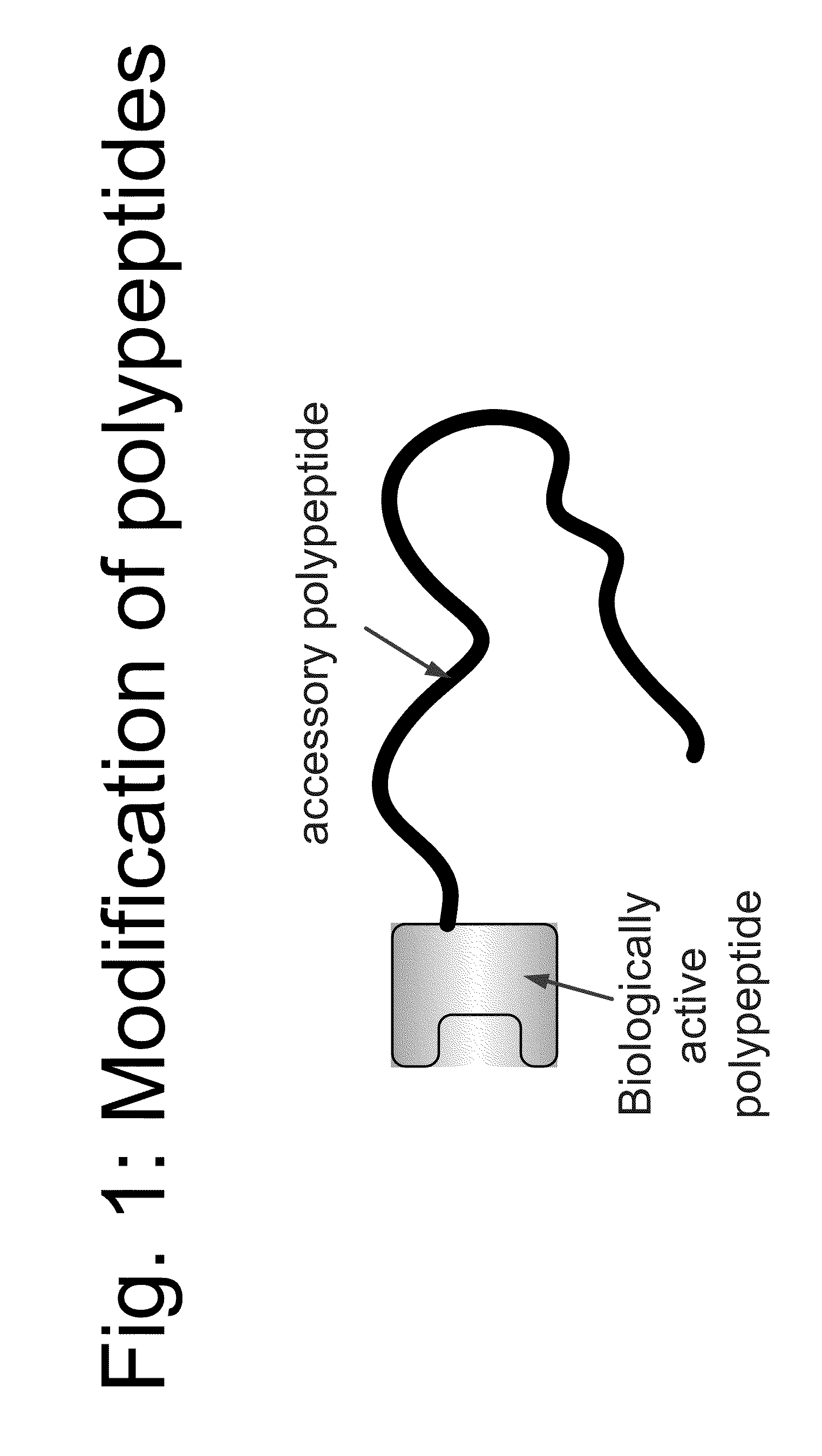
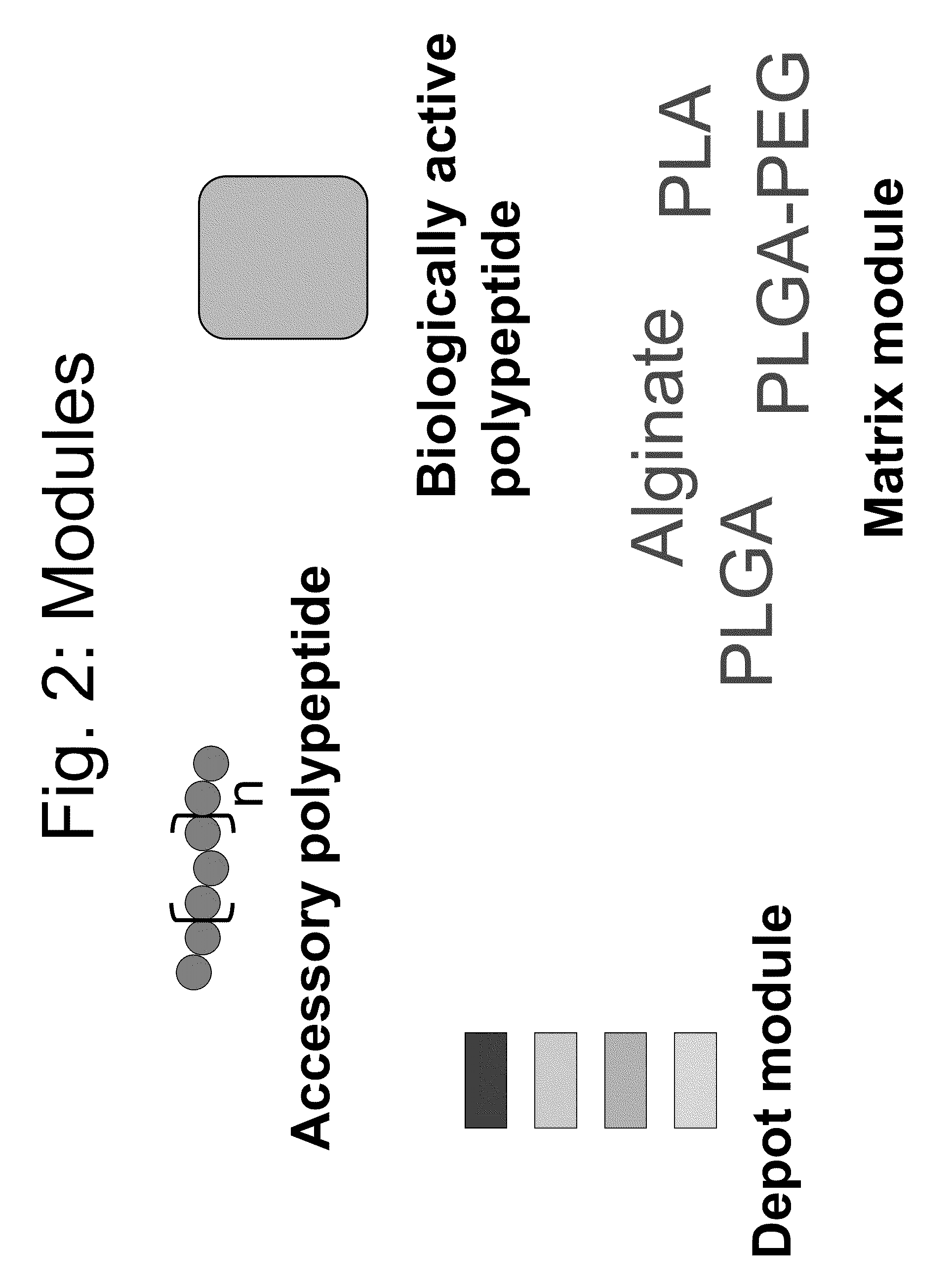
Compositions and methods for modifying properties of biologically active polypeptides
ActiveUS20090092582A1Improve protein solubilityReduce aggregationAntibacterial agentsSenses disorderDrug biological activityBiological activity
Owner:AMUNIX PHARMA INC
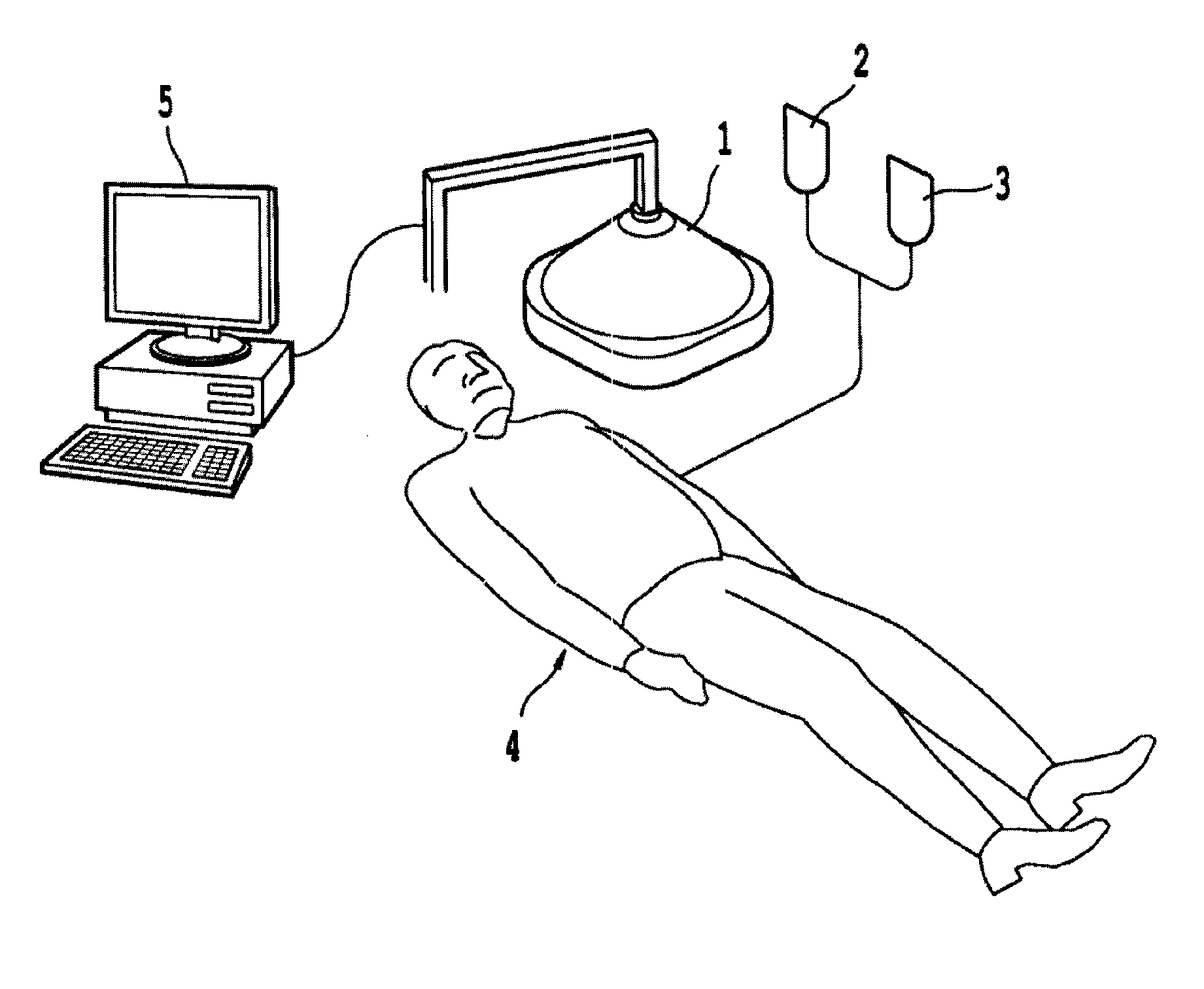
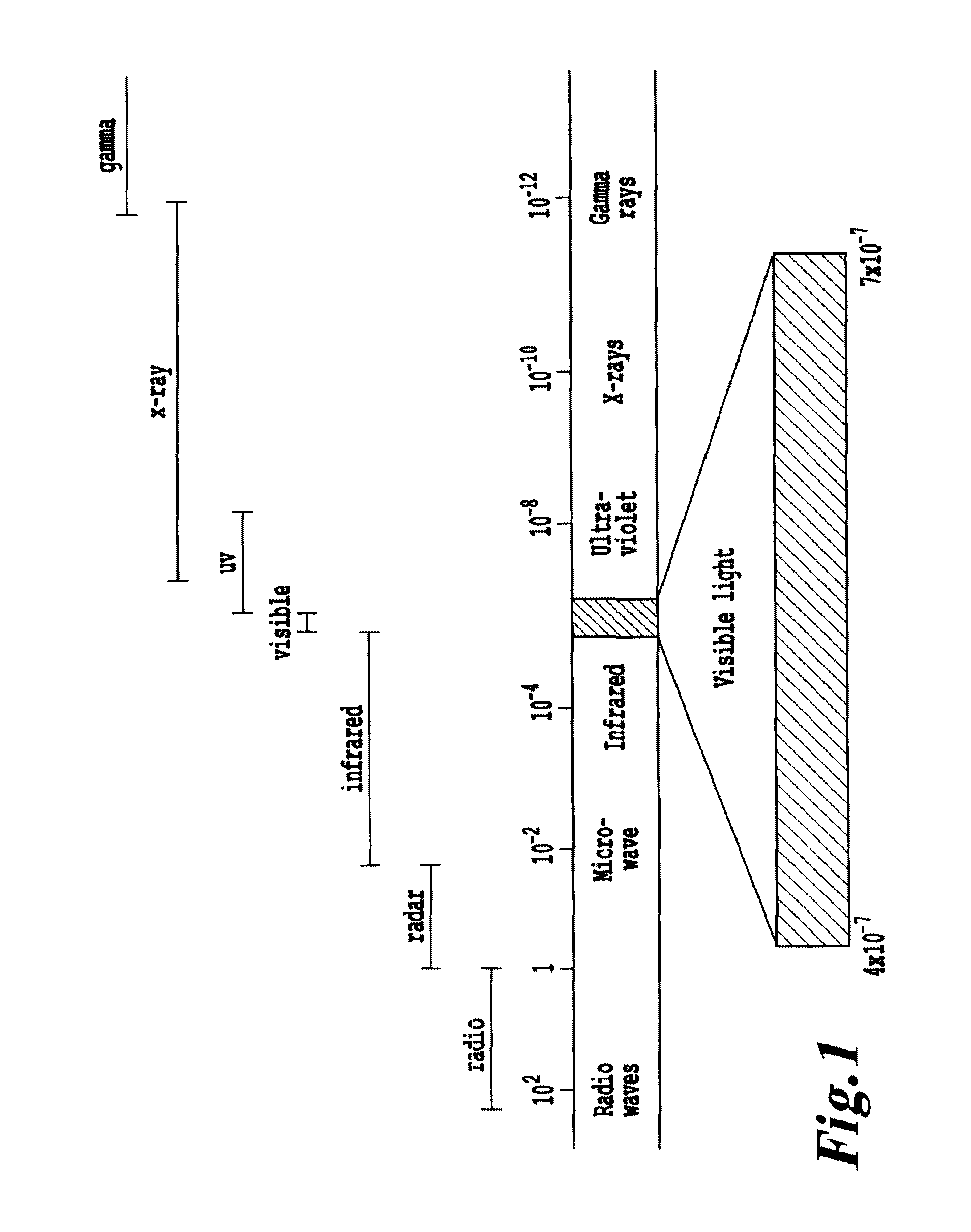
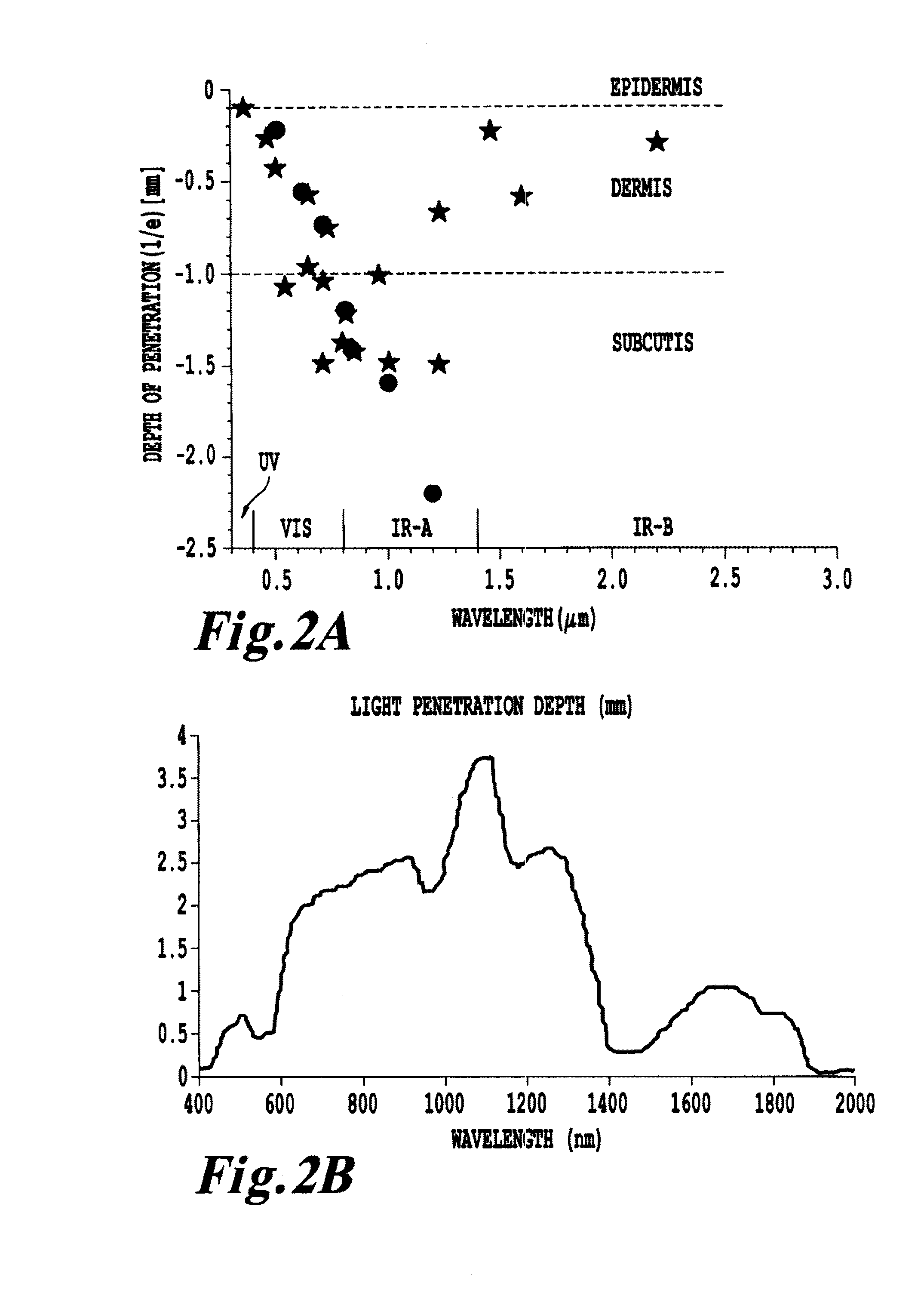
Non-invasive systems and methods for in-situ photobiomodulation
InactiveUS20100016783A1High selectivityAntibacterial agentsPowder deliveryDiseaseBiological regulation
Products, compositions, systems, and methods for modifying a target structure which mediates or is associated with a biological activity, including treatment of conditions, disorders, or diseases mediated by or associated with a target structure, such as a virus, cell, subcellular structure or extracellular structure. The methods may be performed in situ in a non-invasive manner by application of an initiation energy to a subject thus producing an effect on or change to the target structure directly or via a modulation agent. The methods may further be performed by application of an initiation energy to a subject in situ to activate a pharmaceutical agent directly or via an energy modulation agent, optionally in the presence of one or more plasmonics active agents, thus producing an effect on or change to the target structure. Kits containing products or compositions formulated or configured and systems for use in practicing these methods.
Owner:DUKE UNIV +1
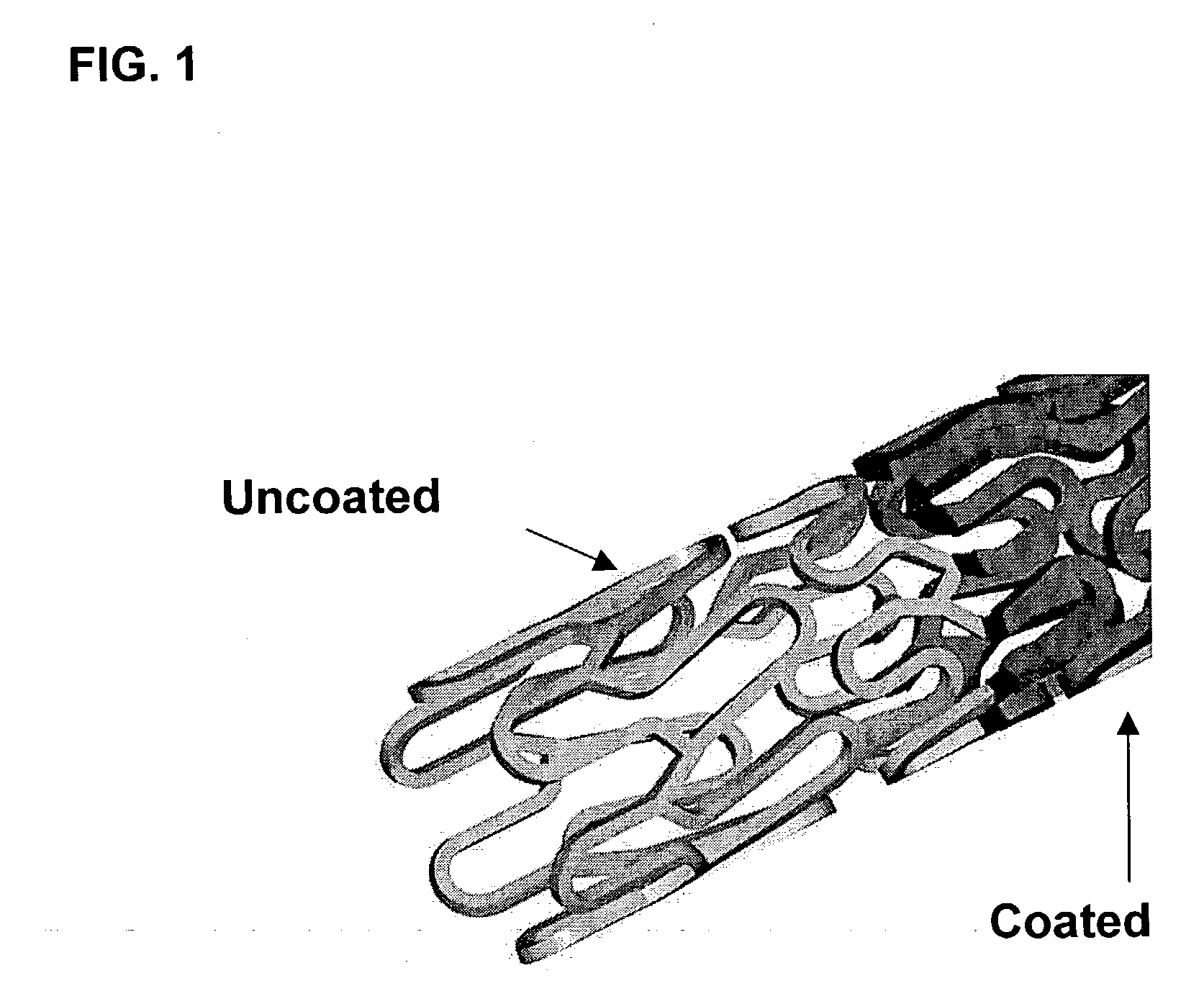
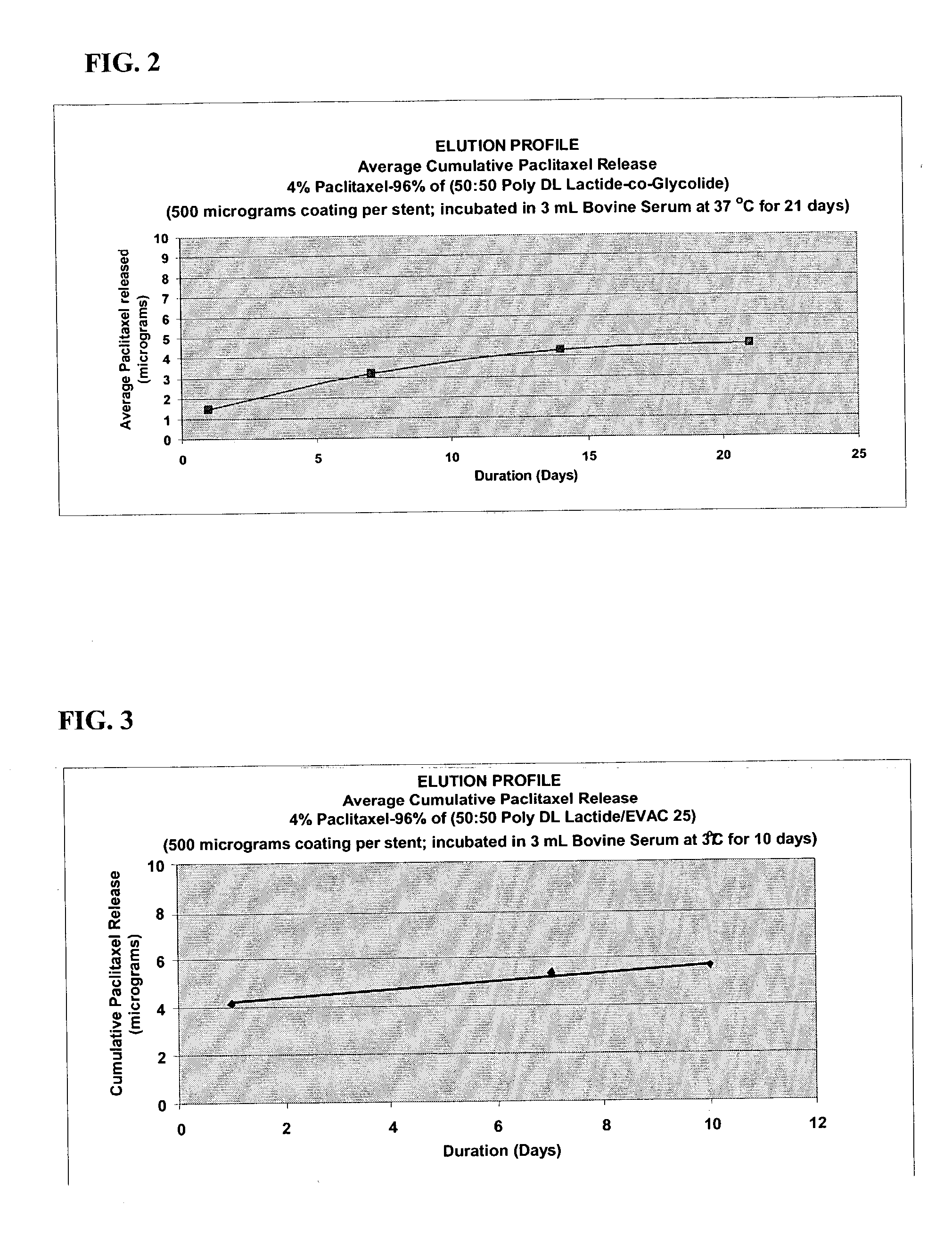
Drug eluting implantable medical device
A drug eluting medical device is provided for implanting into vessels or luminal structures within the body of a patient. The coated medical device, such as a stent, vascular, or synthetic graft comprises a coating consisting of a controlled-release matrix of a bioabsorbable, biocompatible, bioerodible, biodegradable, nontoxic material, such as a Poly(DL-Lactide-co-Glycolide) polymer, and at least one pharmaceutical substance, or bioactive agent incorporated within the matrix or layered within layers of matrix. In particular, the drug eluting medical device when implanted into a patient, delivers the drugs or bioactive agents within the matrix to adjacent tissues in a controlled and desired rate depending on the drug and site of implantation.
Owner:ORBUSNEICH MEDICAL PTE LTD
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS20050124045A1Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:SUN LEE HWEI K +2
Pentapeptide compounds and uses related thereto
Pentapeptide compounds are disclosed. The compounds have biological activity, e.g., cytotoxicity. Prodrugs having targeting groups and pentapeptide moieities, as well as precursors thereof are also disclosed. For example, precursors having a reactive linker that can serve as a reaction site for joining to a targeting agent, e.g., an antibody, as disclosed.
Owner:SEAGEN INC
Tumor necrosis factor peptide binding antibodies
InactiveUS6593458B1Enhance or inhibit TNF alpha activityInduction of endothelial procoagulant activityPeptide/protein ingredientsAntibody mimetics/scaffoldsDrug biological activityAntibody
Provided are isolated antibodies or fragments thereof which bind a peptide consisting of residues Leu63-Phe64-Lys65-Gly66-Gln67-Gly68-Cys69-Pro70-Ser71-Thr72-His73-Val74-Leu75-Leu76-Thr77-His78-Thr79-Ile80-Ser81-Arg82-Ile83 (peptide 304) of mature human TNF-alpha. The antibodies and fragments thereof may be used to identify antibodies capable of binding in the region of mature human TNF-alpha of amino acid resides 63-83. Antibodies or fragments thereof which bind particular regions of mature human TNF-alpha are shown to elicit particular biological activities dependent upon the particular region wherein binding occurs.
Owner:ARANA THERAPEUTIC LTD
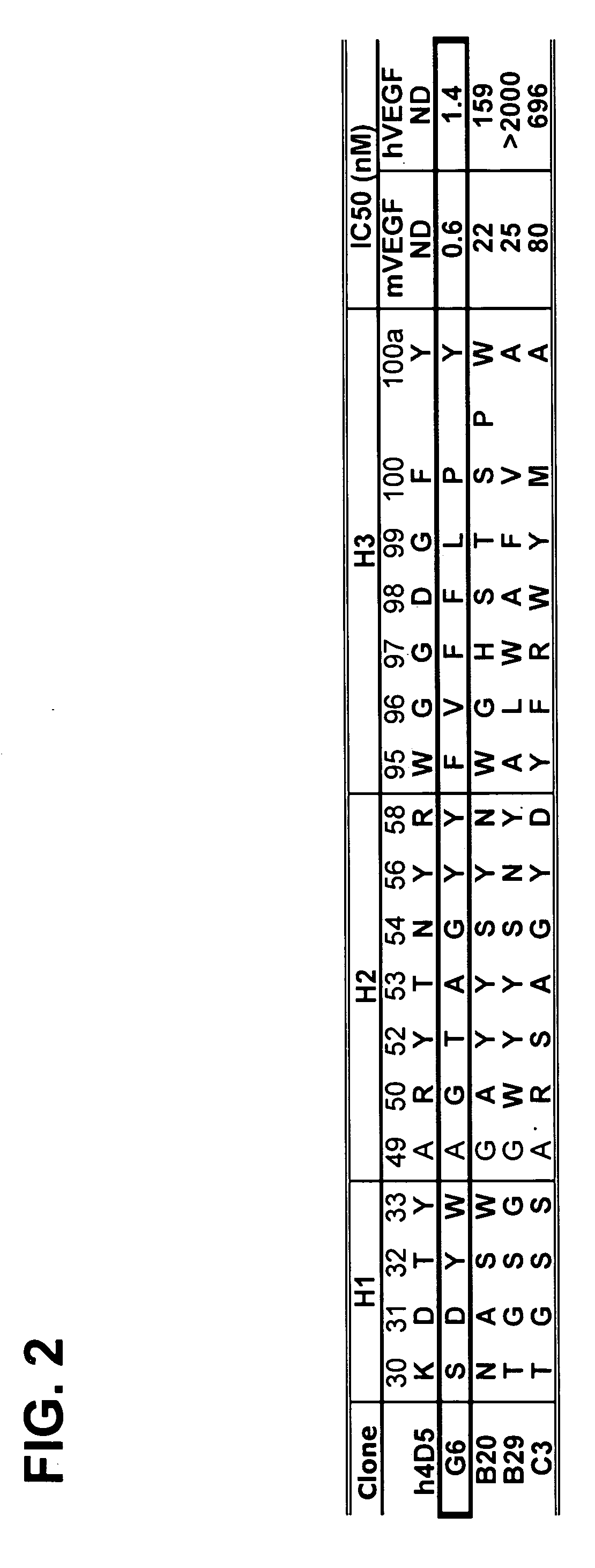
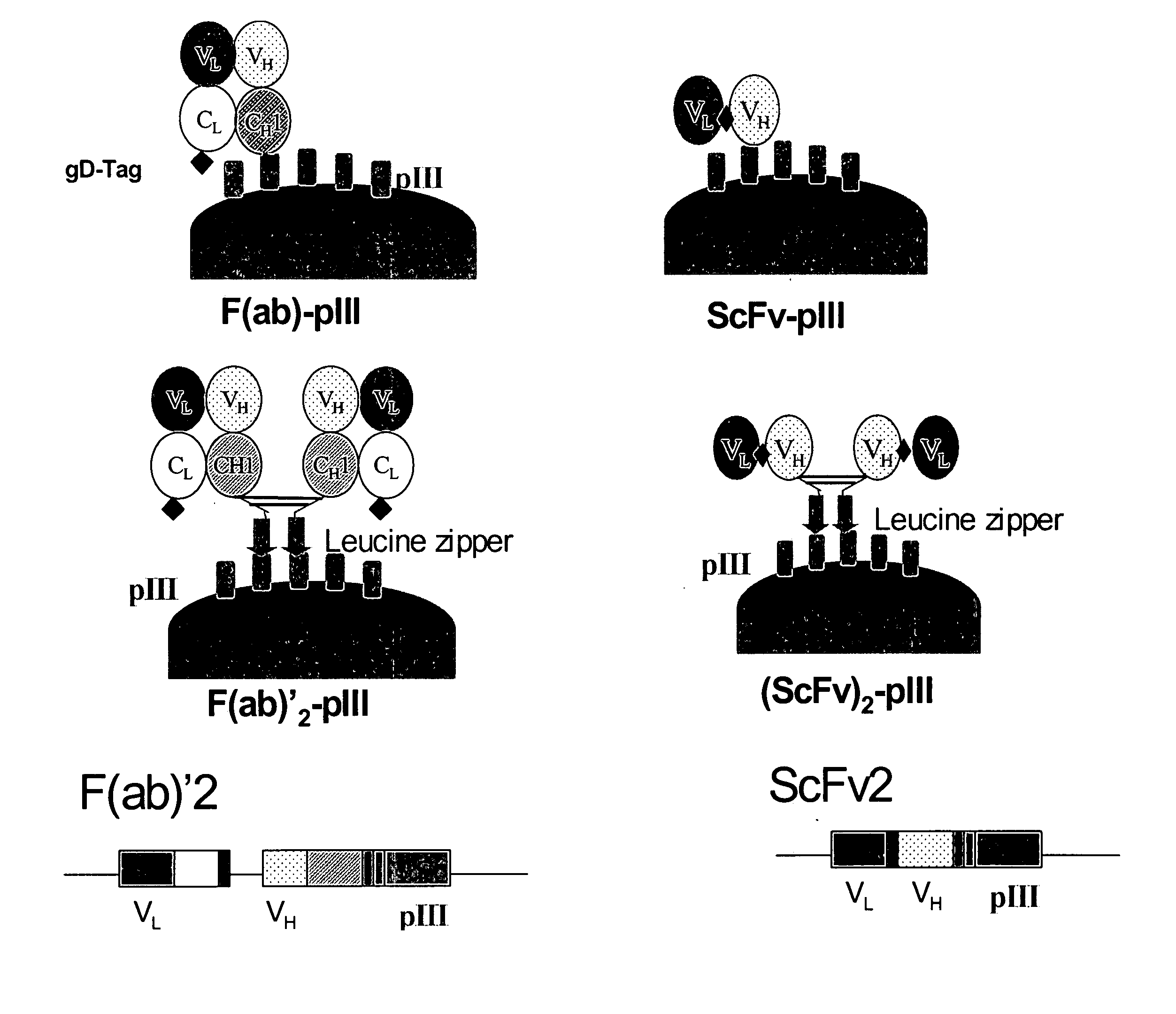
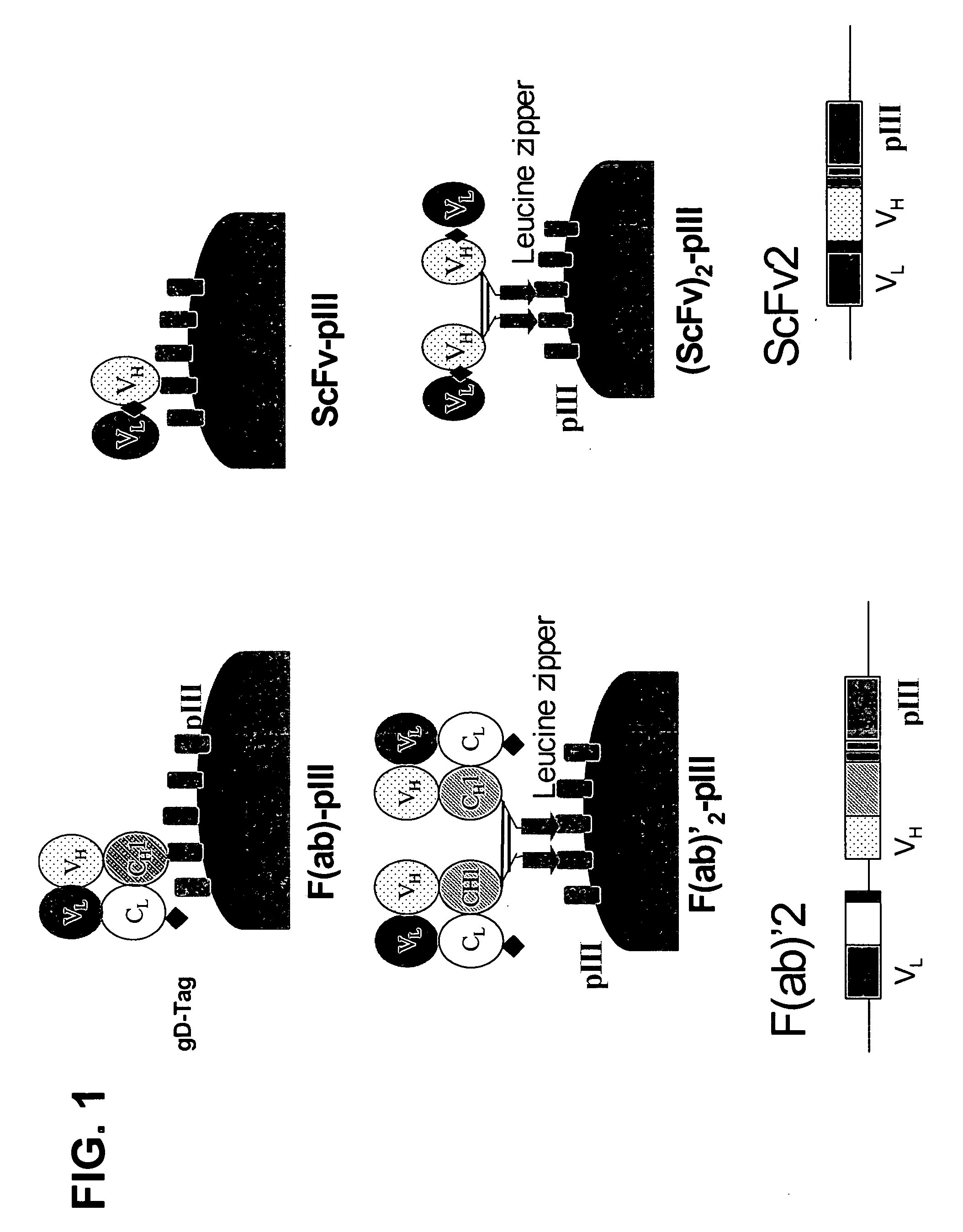
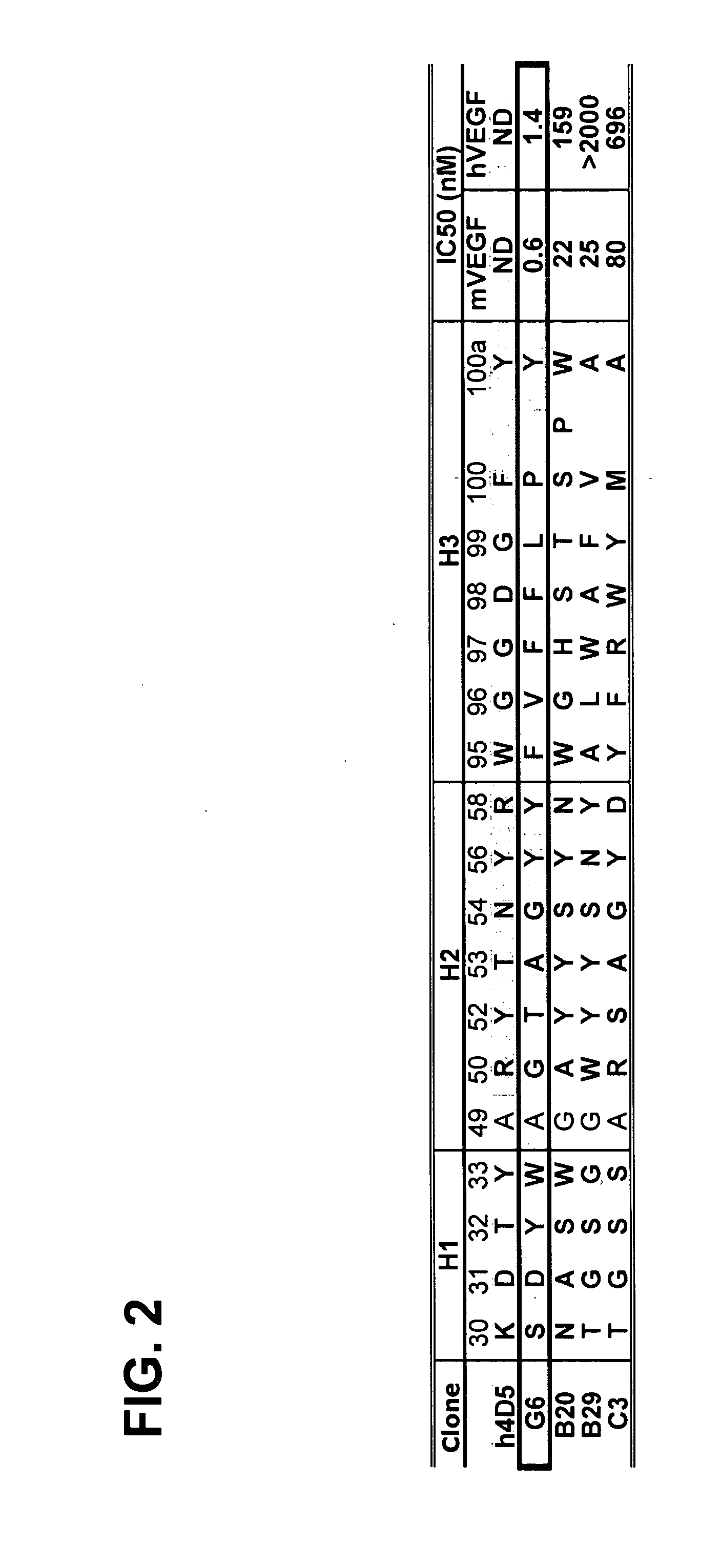
Anti-VEGF antibodies
Owner:GENENTECH INC
Adeno-associated virus vectors for expression of factor VIII by target cells
InactiveUS6200560B1Easily transfectedConvenient platformBiocideFactor VIIHigh level expressionHuman cell
The present invention provides improved viral vectors useful for the expression of genes at high levels in human cells. In particular, the present invention provides recombinant adeno-associated vectors (AAV) suitable for gene therapy. These vectors are capable of delivering nucleic acid containing constructs which result in the production of full-length therapeutic levels of biologically active Factor VIII in the recipient individual in vivo. The present invention also provides pharmaceutical compositions comprising such AAV vectors, as well as methods for making and using these constructs.
Owner:GENZYME CORP
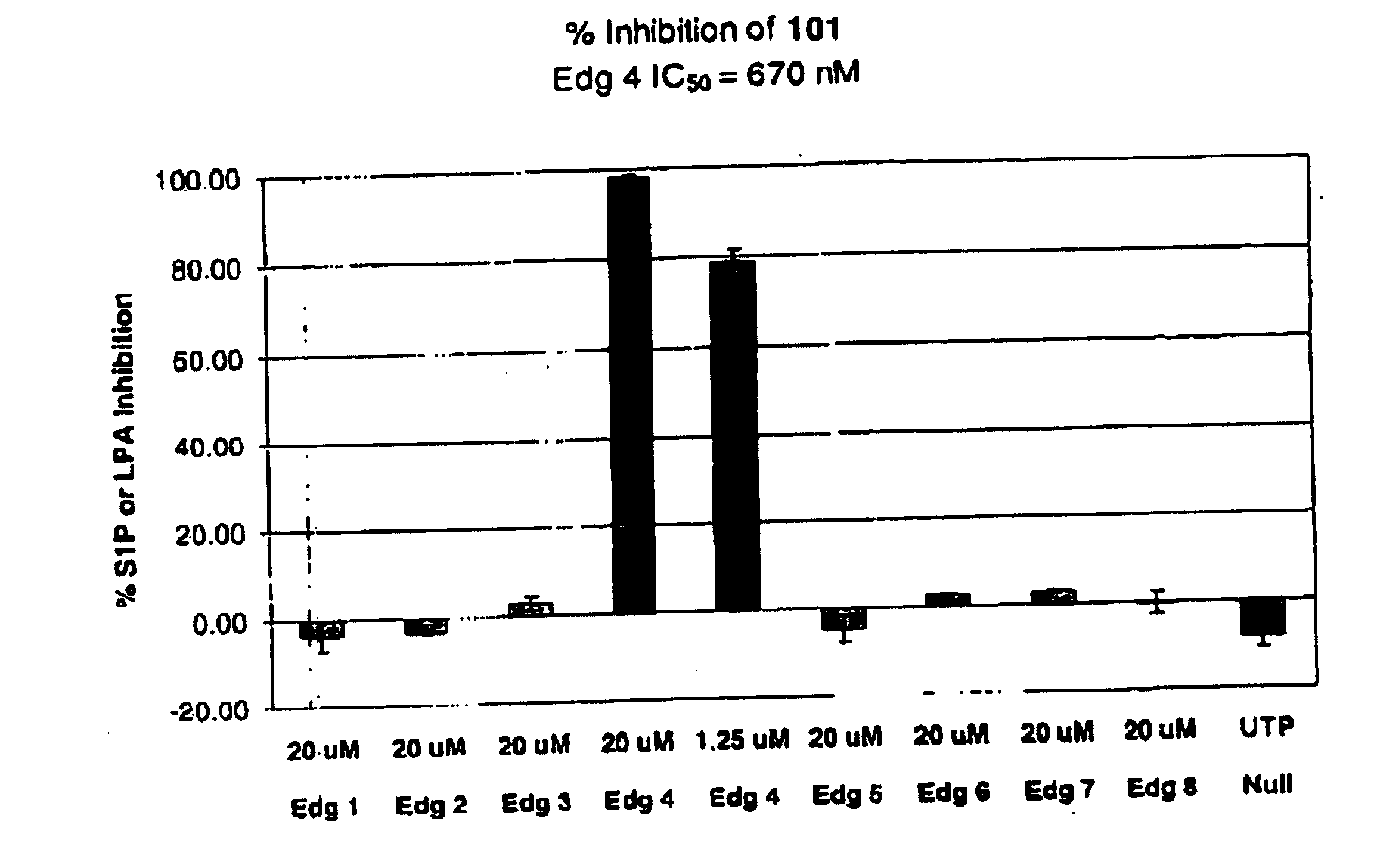
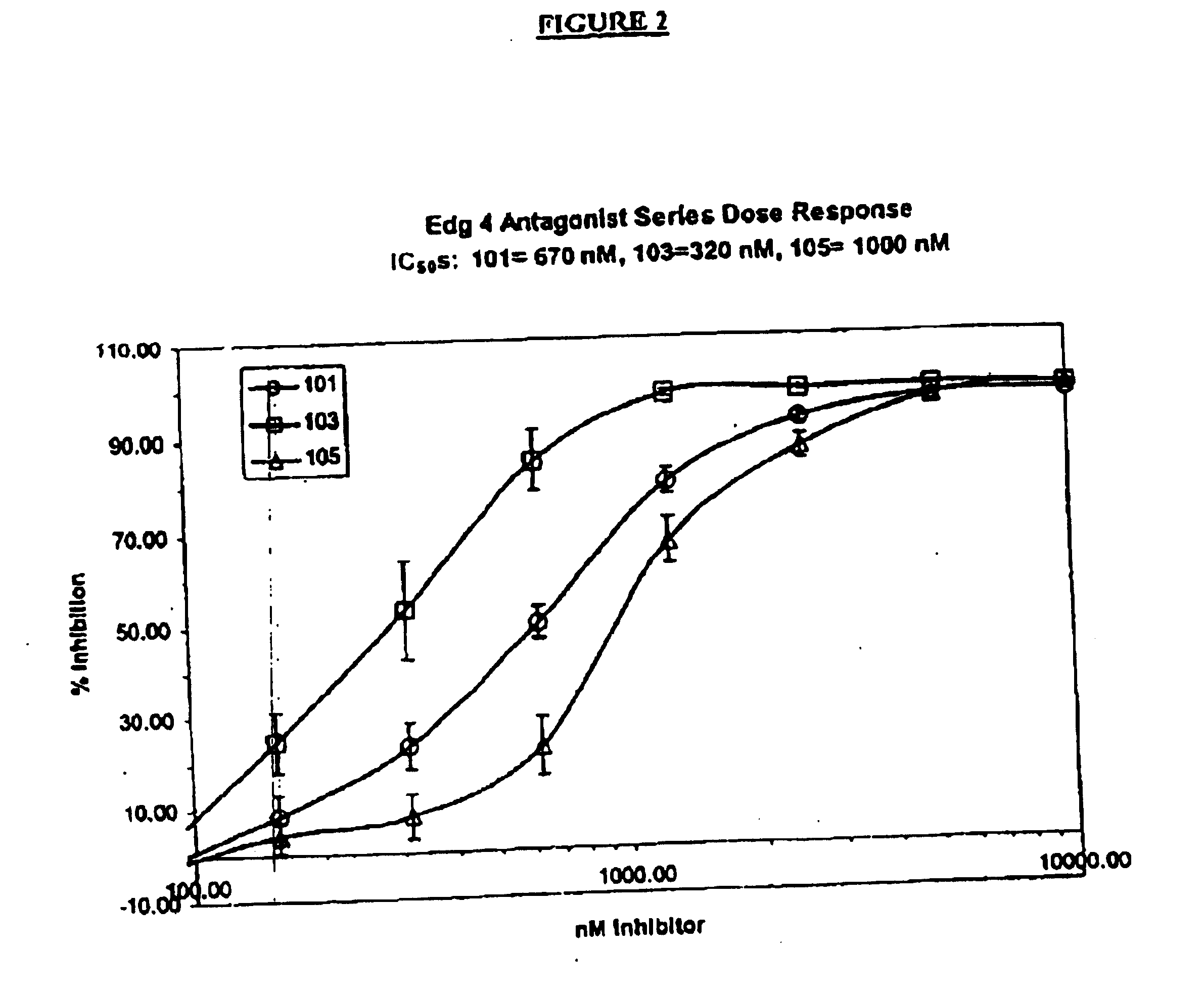
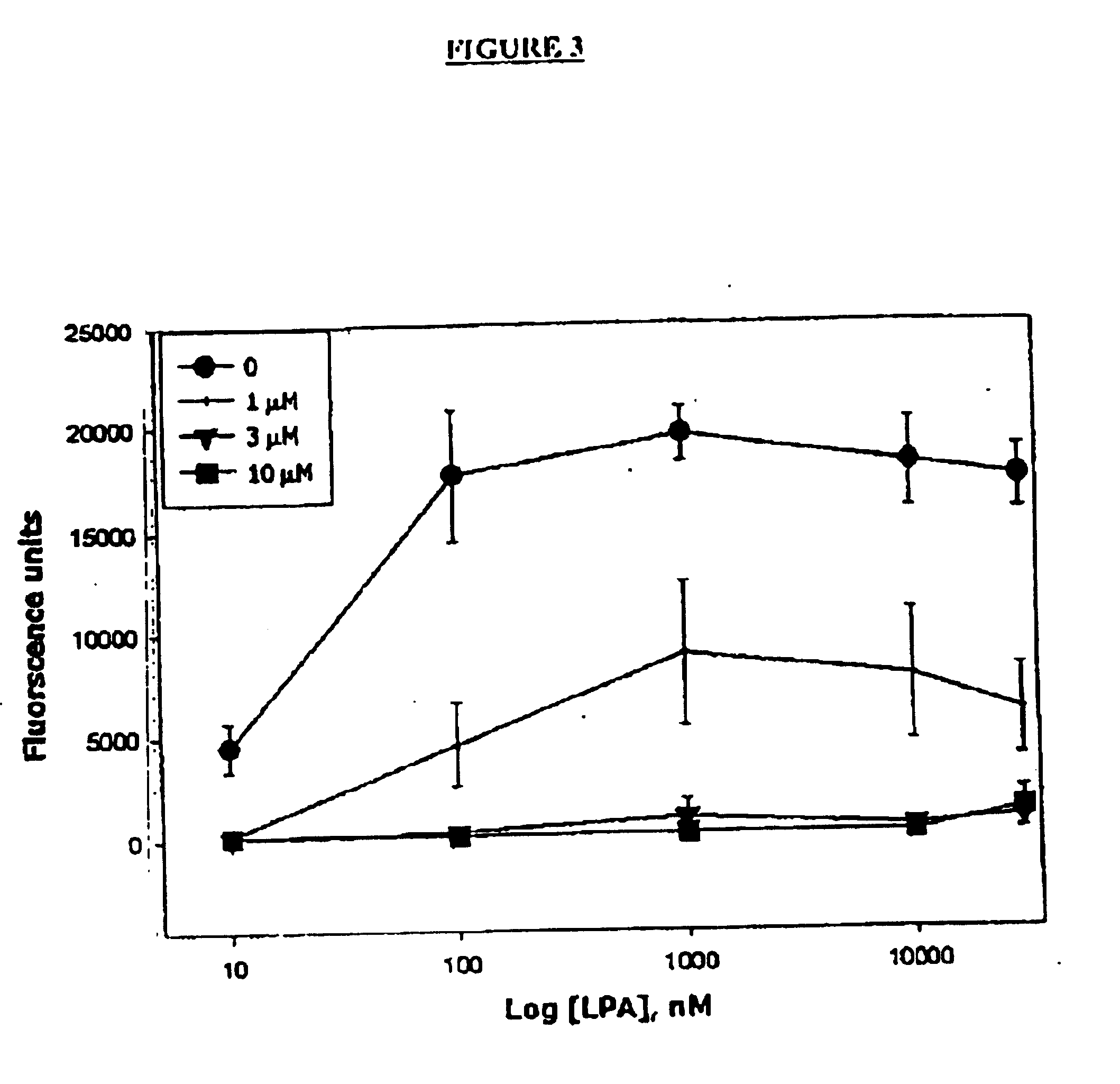
Methods of treating conditions associated with an EDG-4 receptor
InactiveUS20050113283A1Modulating biological activityBiocideAmide active ingredientsDrug biological activityReceptor modulator
The present invention provides a method of modulating an Edg-4 receptor mediated biological activity in a cell. A cell expressing the Edg-4 receptor is contacted with a modulator of an Edg-4 receptor sufficient to modulate the Edg-4 receptor mediated biological activity. In another aspect, the present invention provides a method for modulating an Edg-4 receptor mediated biological activity in a subject. A therapeutically effective amount of a modulator of the Edg-4 receptor is administered to the subject.
Owner:MANIV ENERGY CAPITAL
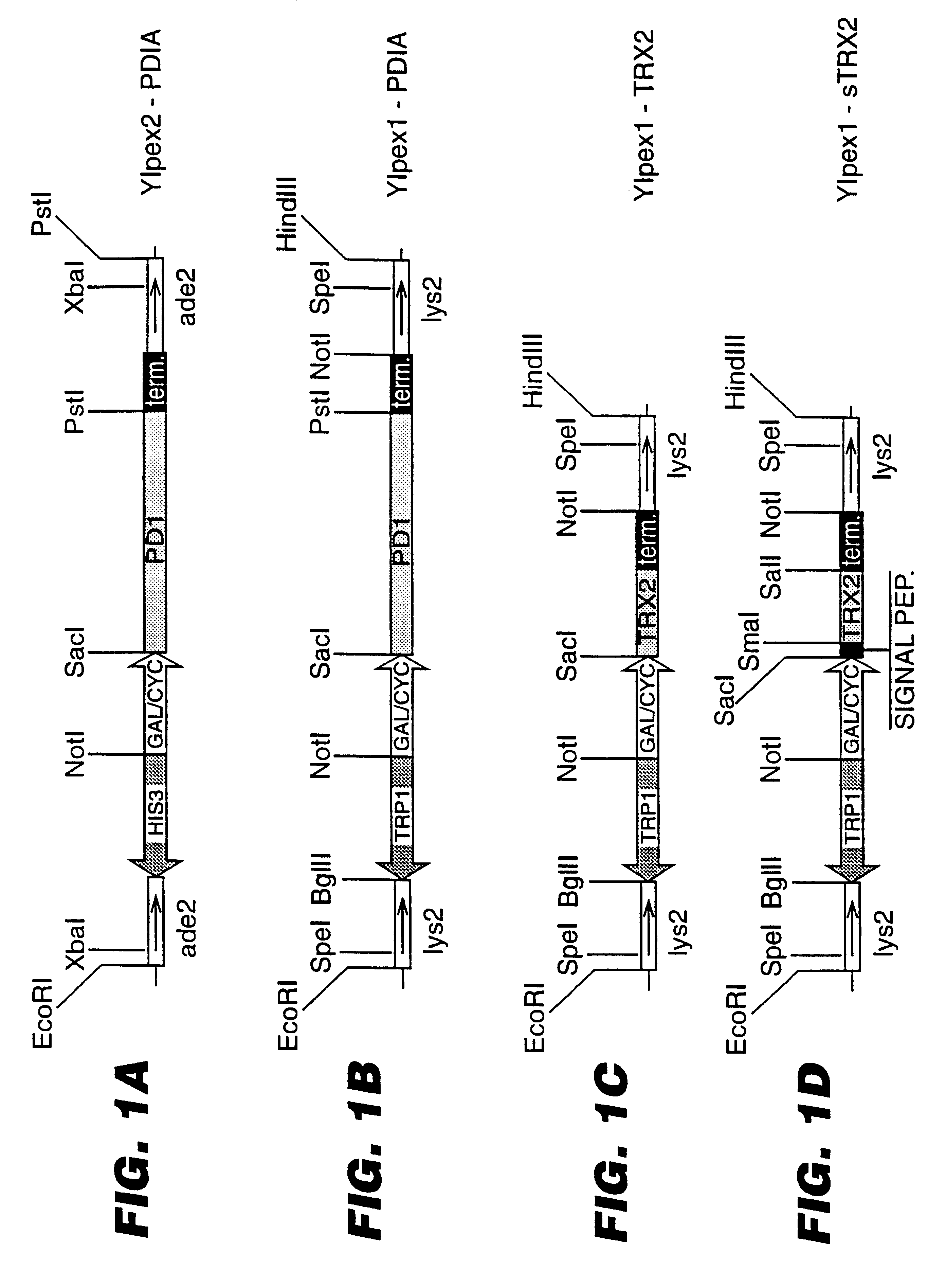
Expression of heterologous proteins
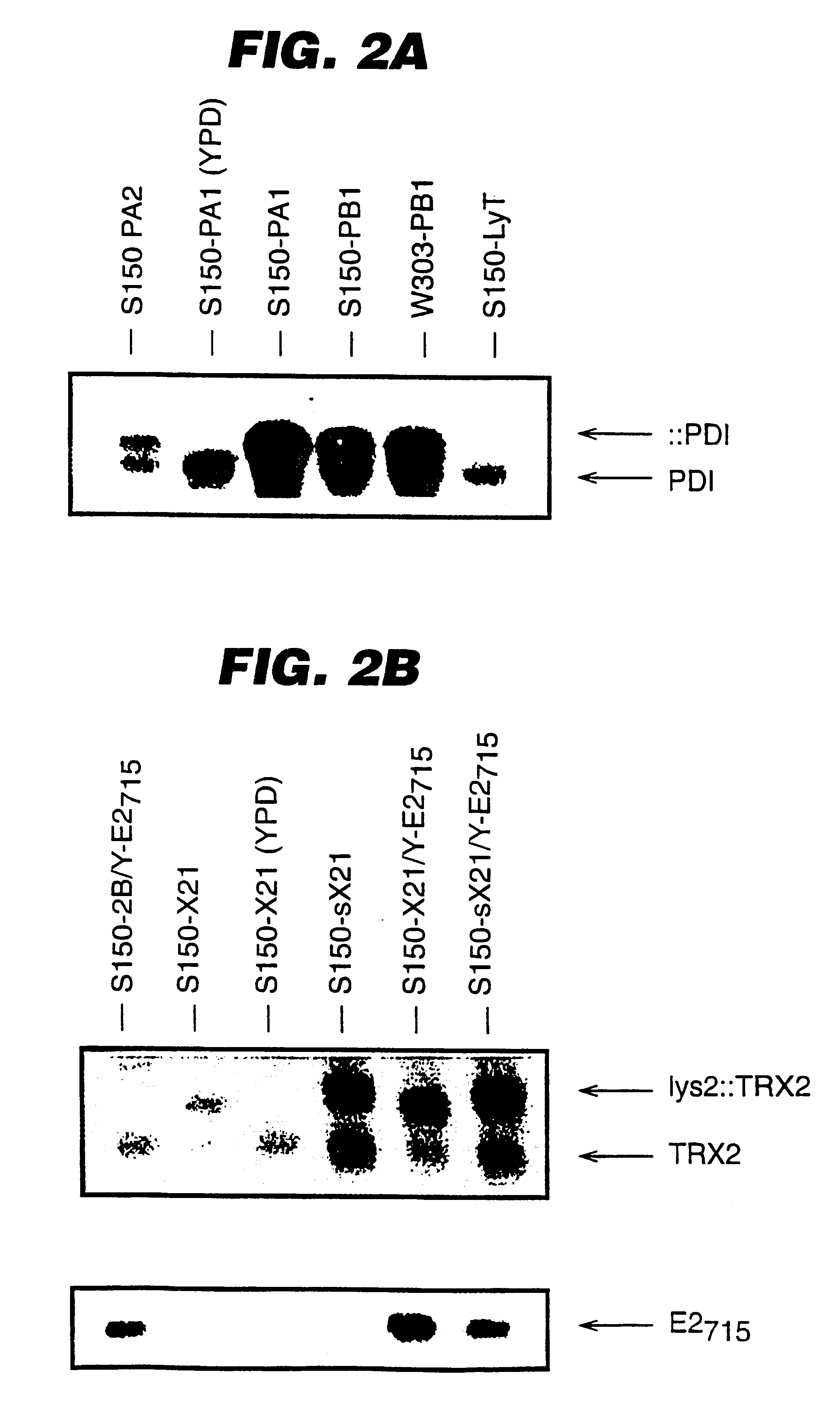
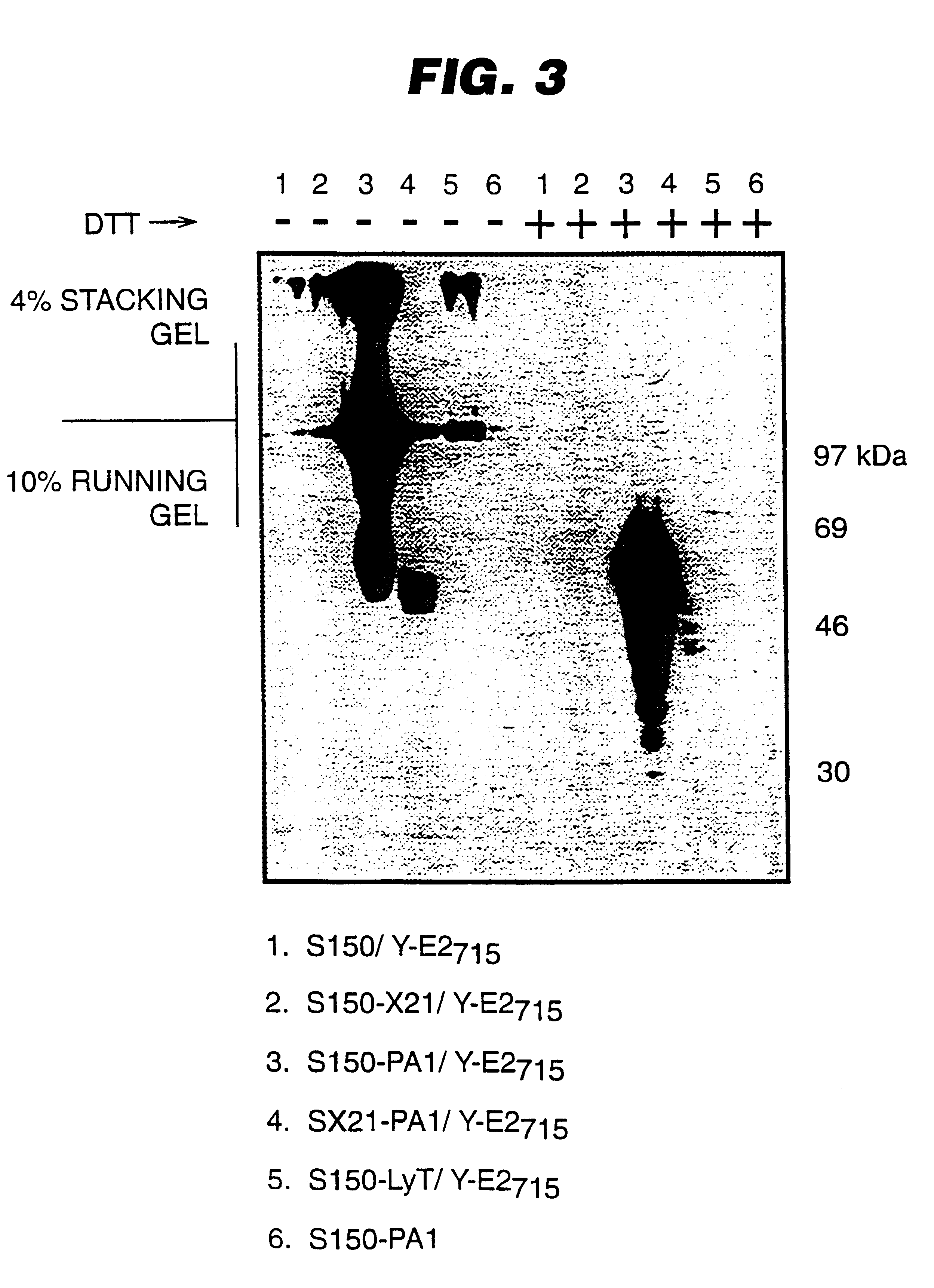
An expression system which provides heterologous proteins expressed by a non-native host organism but which have native-protein-like biological activity and / or structure. Disclosed are vectors, expression hosts and methods for expressing the heterologous proteins. The expression system involves co-expression of protein factor(s) which is / are capable of catalyzing disulphide bond formation and desired heterologous protein(s). The expression system is presented using yeast cells as the preferred host, protein disulphide isomerase (PDI) and thioredoxin (TRX) as the preferred examples of the protein factors and HCV-E2715 envelope glycoprotein and human FIGF as the preferred examples of the heterologous proteins.
Owner:NOVARTIS AG
42-O-alkoxyalkyl rapamycin derivatives and compositions comprising same
ActiveUS20050101624A1Improve adhesionSynthetically prepared with easeBiocideOrganic chemistryDrug biological activityRapastinel
42-O-alkoxyalkyl derivatives of rapamycin having biological activity are described. Compositions and delivery devices comprising the 42-O-alkoxyalkyl rapamycin derivatives are also disclosed.
Owner:BIOSENSORS INT GROUP
Biologically active dimerized and multimerized polypeptide fusions
InactiveUS6018026AImprove expression levelMass productionPeptide/protein ingredientsAntibody mimetics/scaffoldsExtracellular StructureA-DNA
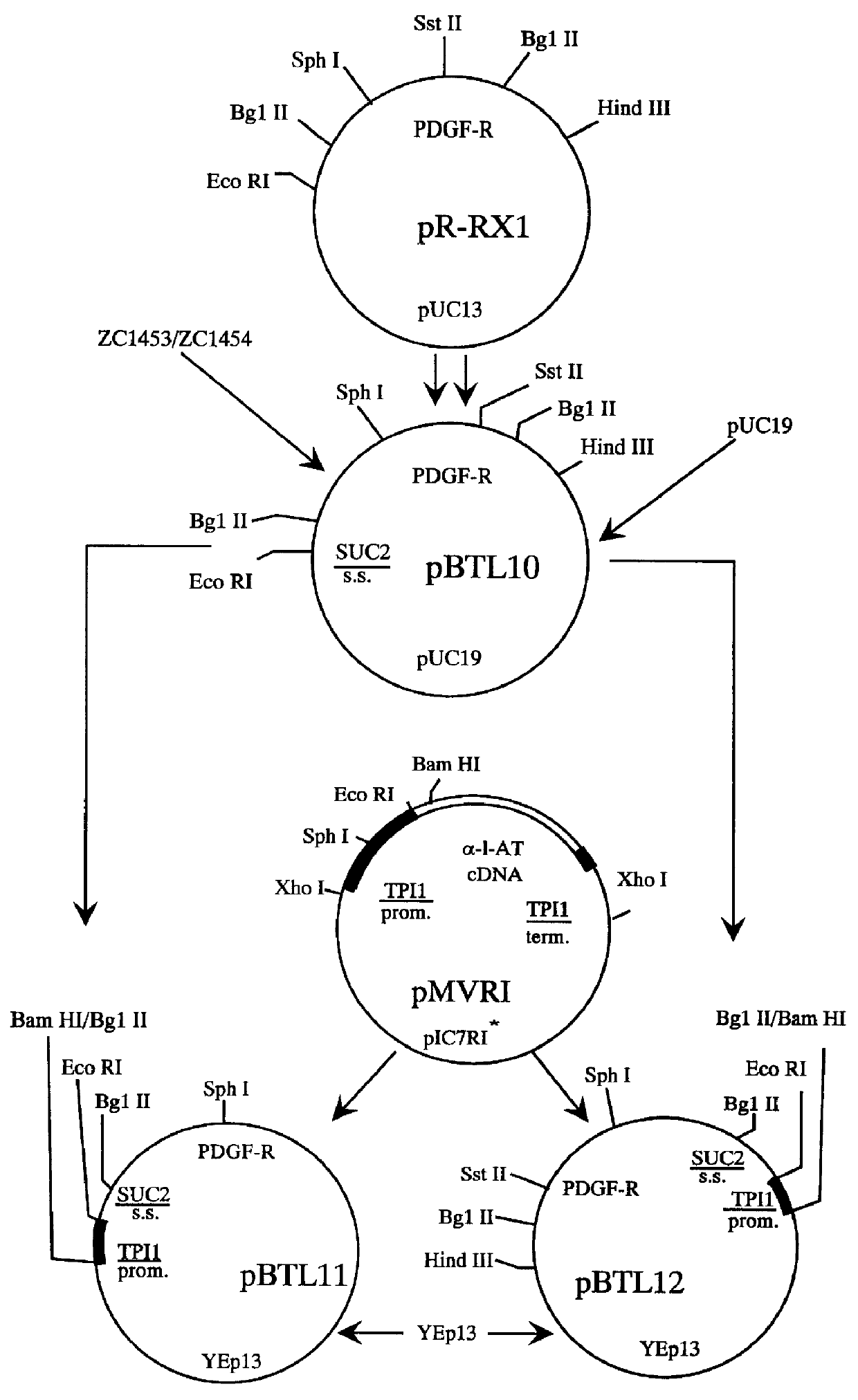
Methods for producing secreted receptor analogs and biologically active peptide dimers are disclosed. The methods for producing secreted receptor analogs and biologically active peptide dimers utilize a DNA sequence encoding a receptor analog or a peptide requiring dimerization for biological activity joined to a dimerizing protein. The receptor analog includes a ligand-binding domain. Polypeptides comprising essentially the extracellular domain of a human PDGF receptor fused to dimerizing proteins, the portion being capable of binding human PDGF or an isoform thereof, are also disclosed. The polypeptides may be used within methods for determining the presence of and for purifying human PDGF or isoforms thereof.
Owner:ZYMOGENETICS INC
Production of tetravalent antibodies
The present invention relates to a novel process for the preparation of biologically active antibody dimers in a pharmaceutically acceptable composition. The dimers can be composed of two antibody molecules having the same antigen binding specificity and linked through reducible, disulfide, or a non-reducible thioether, bond (homodimer). Alternatively, the dimers can be composed of two different antibody molecules having binding specificity for two distinct antigens (heterodimer). These dimers are useful for inducing hyper-cross-linking of membrane antigens. The present invention further relates to the use of biologically active antibody dimers for the preferential killing or inhibition of selected cell populations in the treatment of diseases such as cancer and autoimmune disorders.
Owner:BIOGEN INC
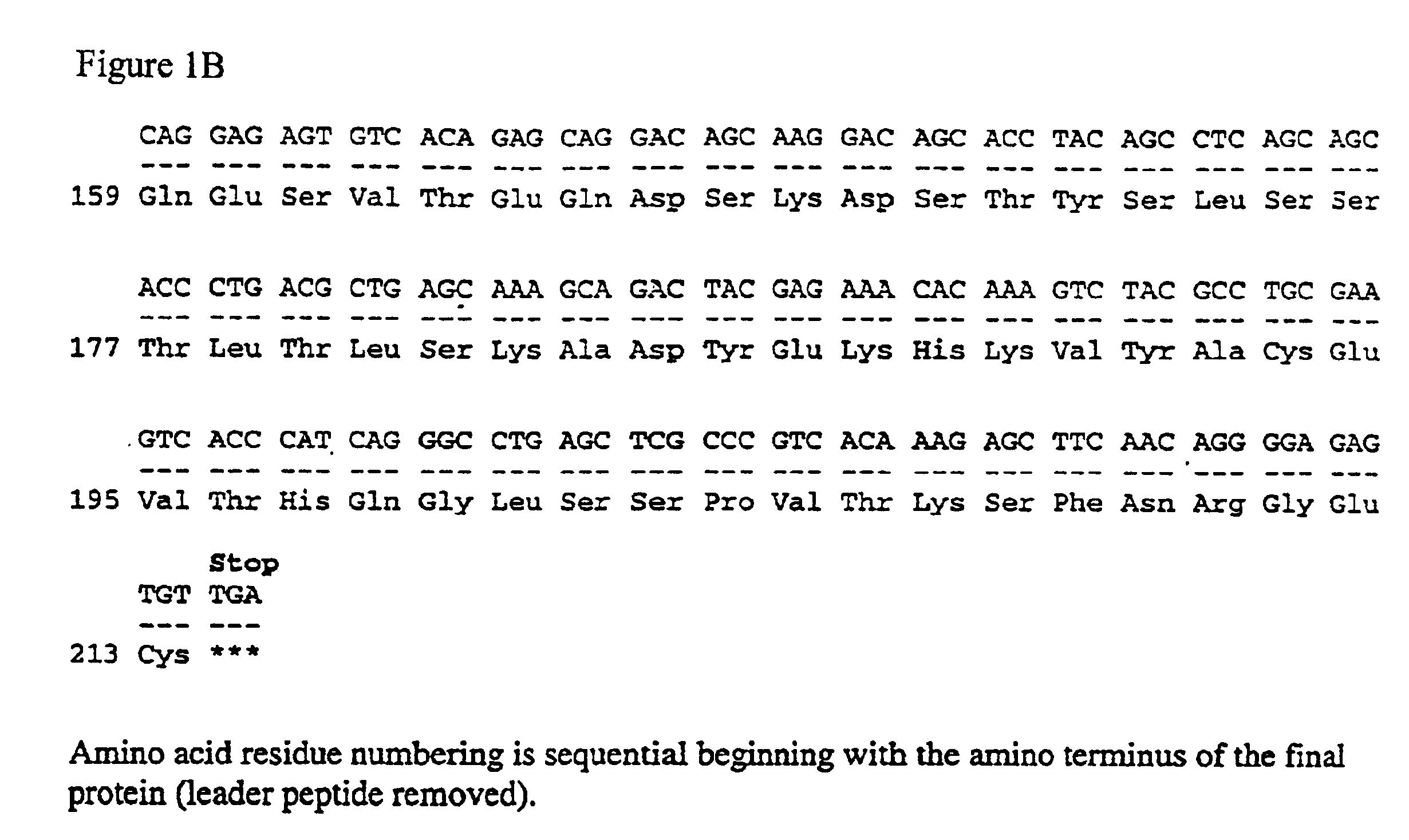
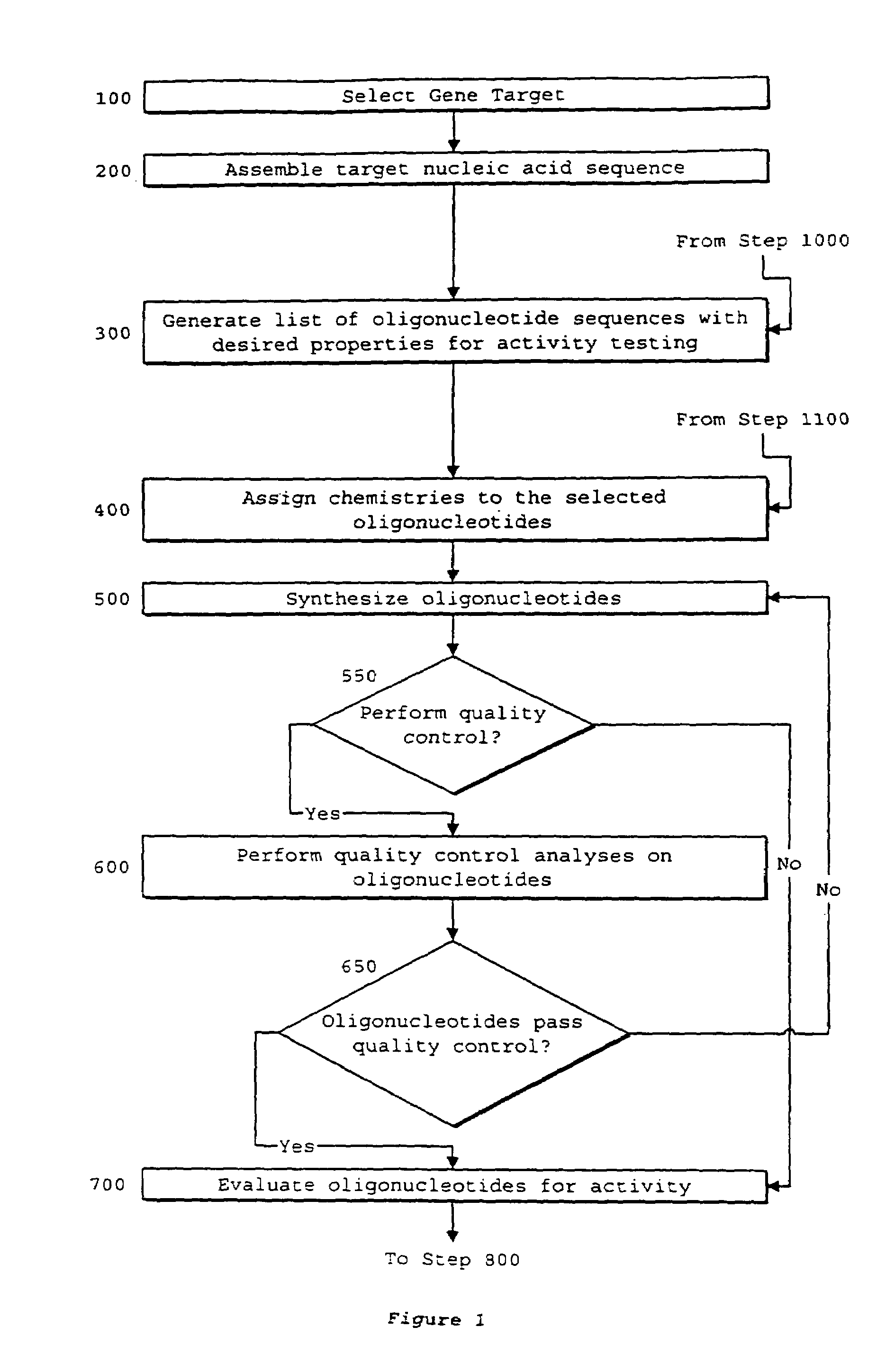
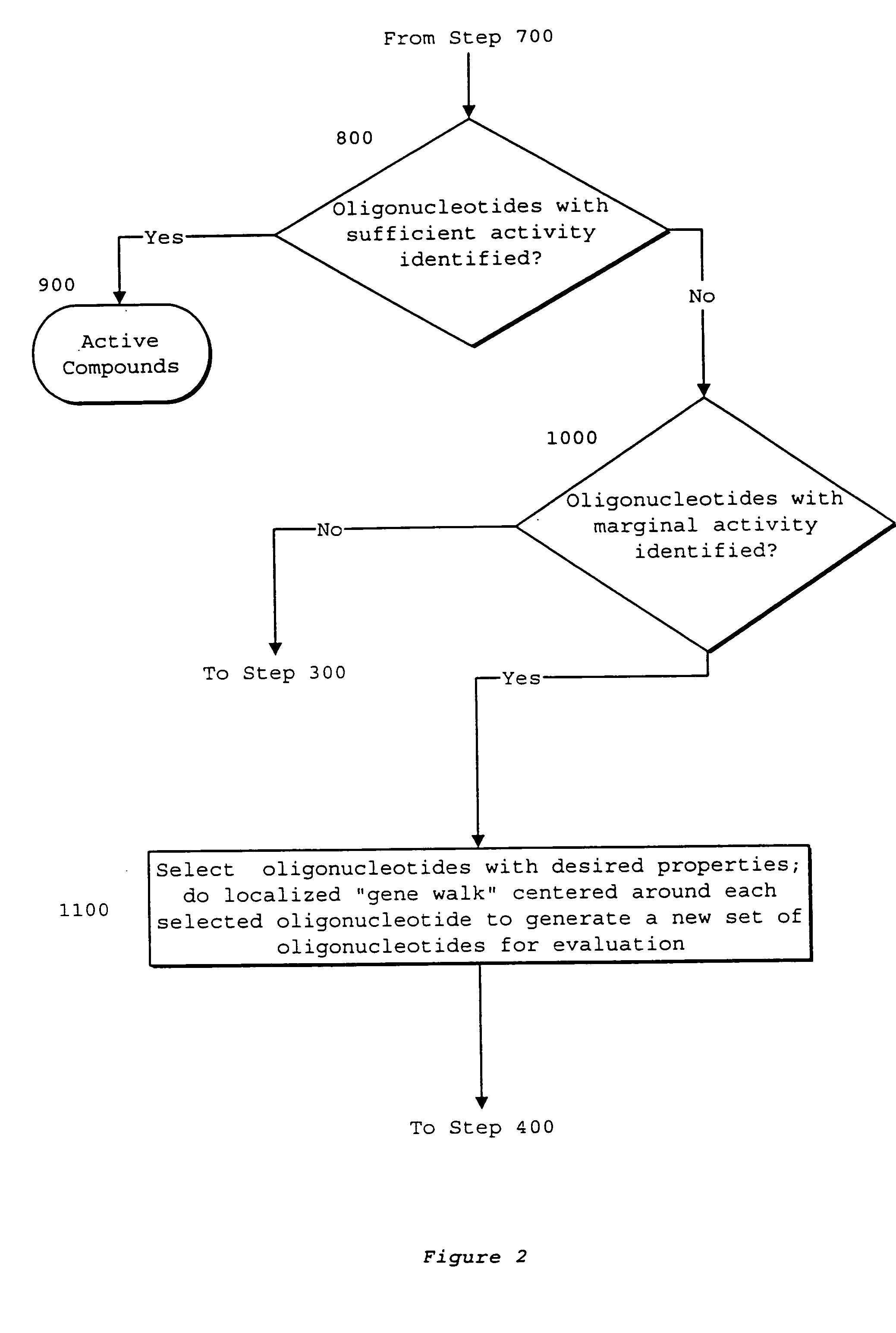
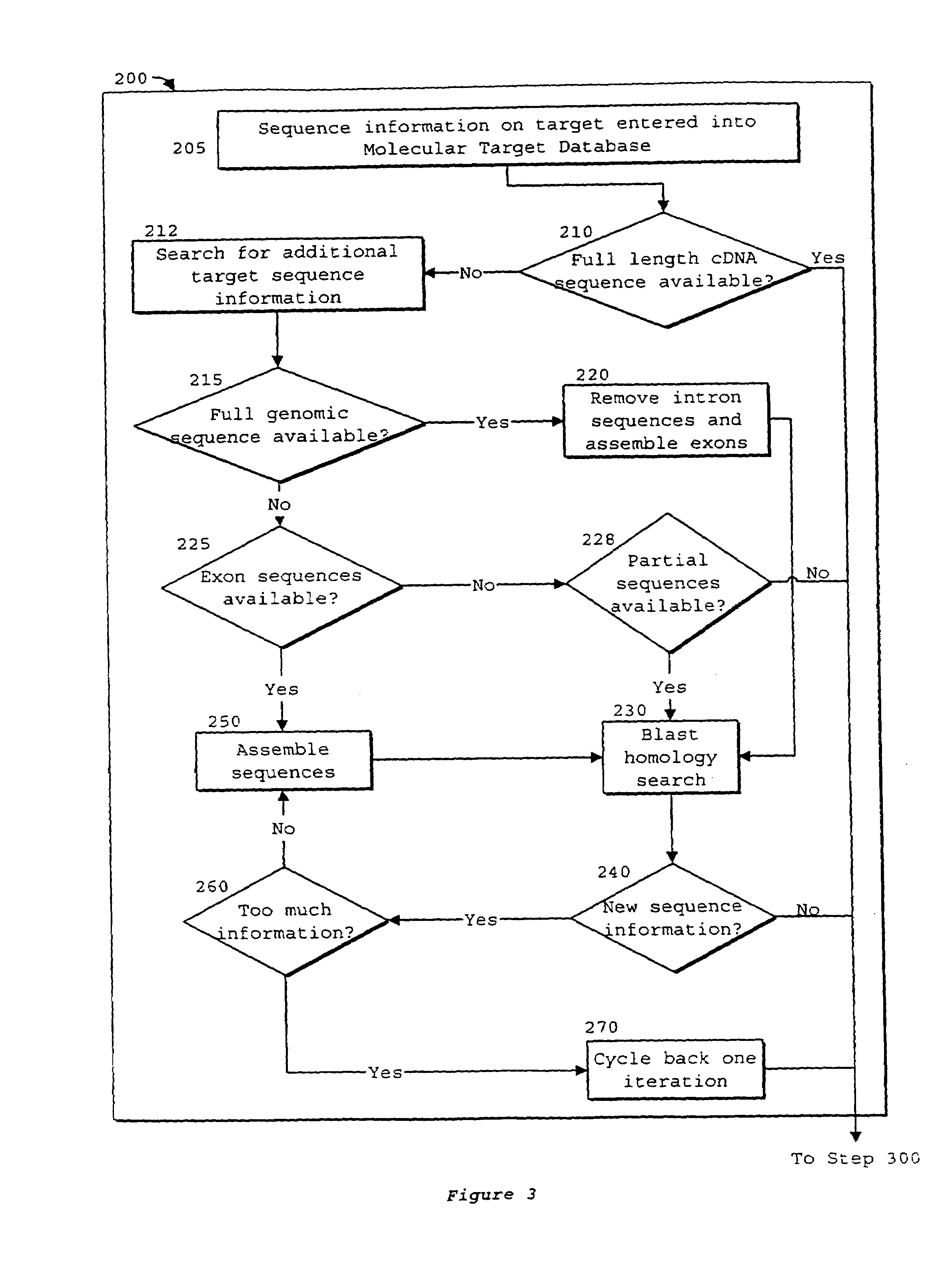
System of components for preparing oligonucleotides
Interative, preferably computer based iterative processes for generating synthetic compounds with desired physical, chemical and / or bioactive properties, i.e., active compounds, are provided. During iterations of the processes, a target nucleic acid sequence is provided or selected, and a library of candidate nucleobase sequences is generated in silico according to defined criteria. A “virtual” oligonucleotide chemistry is chosen and a library of virtual oligonucleotide compounds having the selected nucleobase sequences is generated. These virtual compounds are reviewed and compounds predicted to have particular properties are selected. The selected compounds are robotically synthesized and are preferably robotically assayed for a desired physical, chemical or biological activity. Active compounds are thus generated and, at the same time, preferred sequences and regions of the target nucleic acid that are amenable to oligonucleotide or sequence-based modulation are identified.
Owner:IONIS PHARMA INC
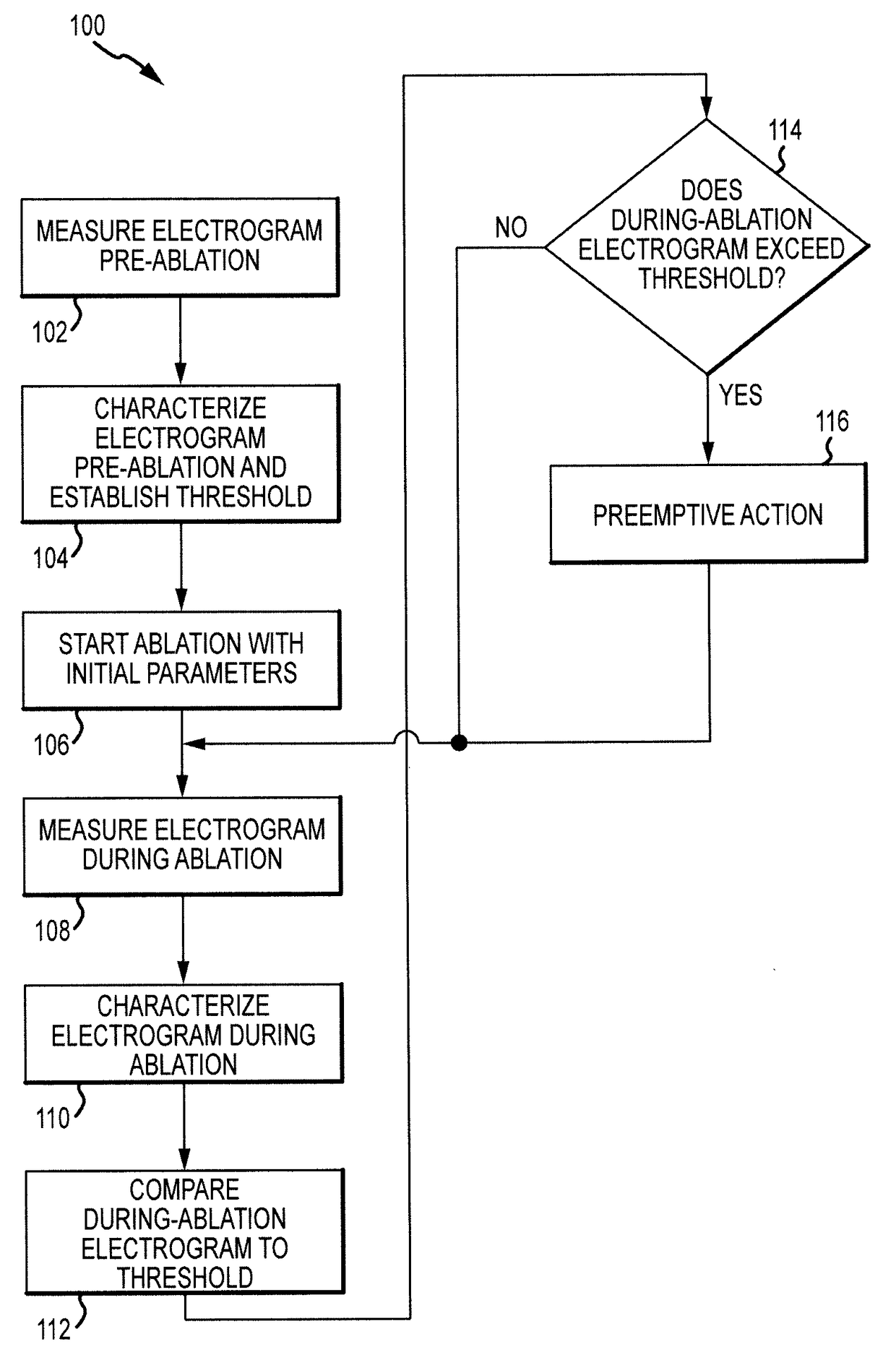
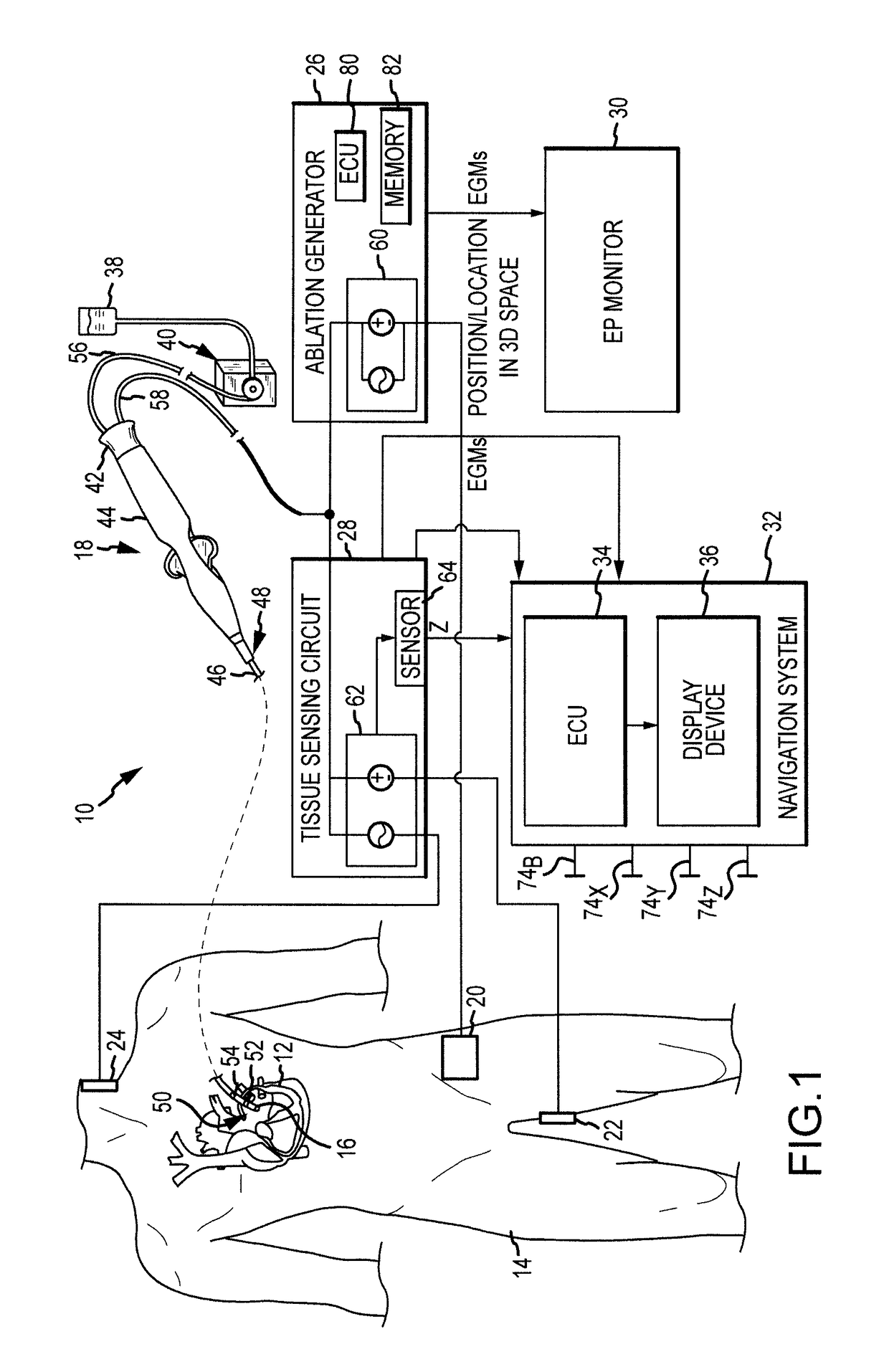
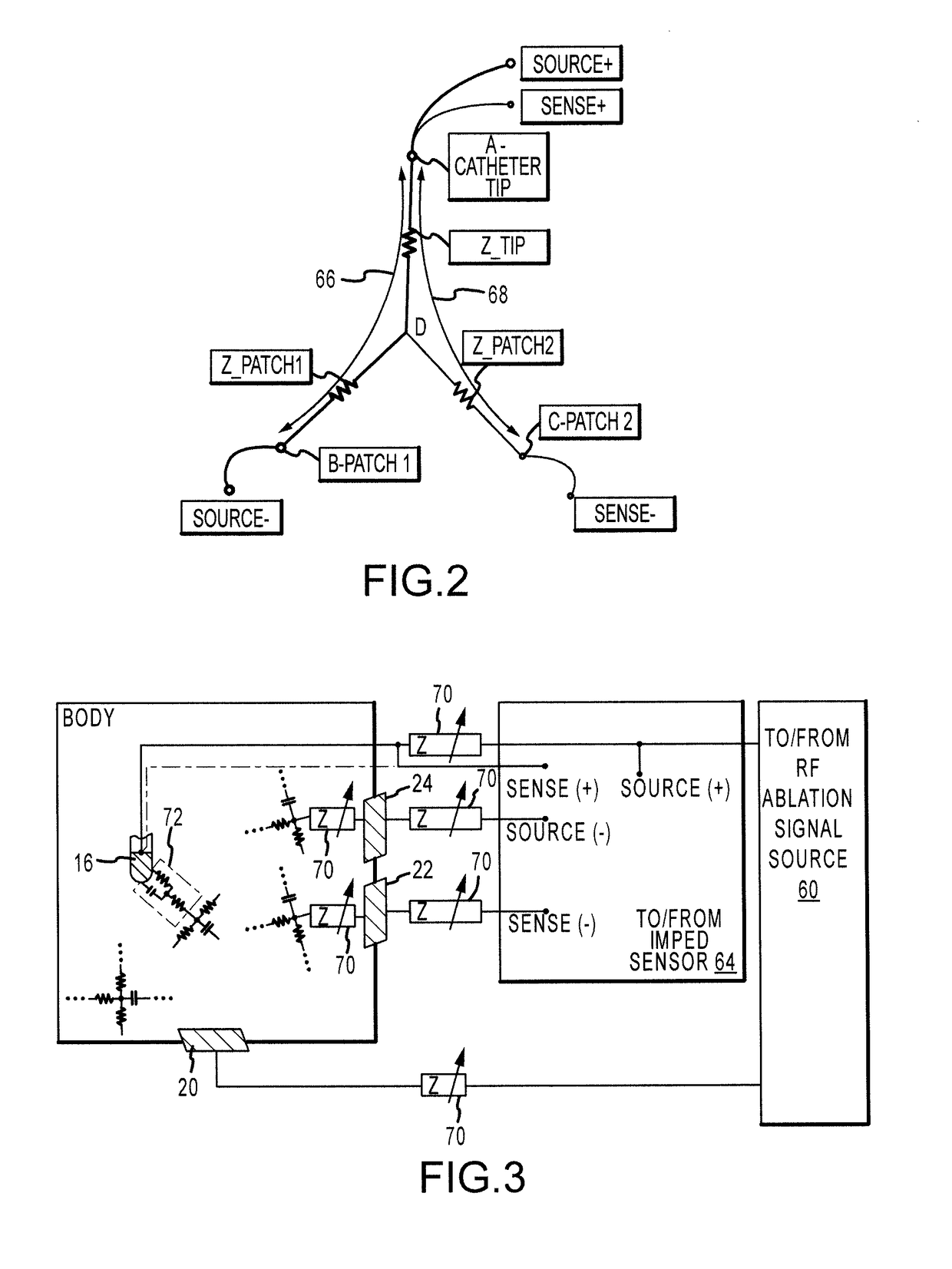
Electrogram-based ablation control
ActiveUS9918788B2Surgical navigation systemsSurgical instruments for heatingData setDrug biological activity
Methods, devices, and systems for predicting, diagnosing, and preventing adverse events during an ablation procedure are described. A method for providing ablation energy includes receiving a first signal based on biological activity of a tissue of a patient. The method further includes analyzing the first signal to yield a first data set, establishing a threshold parameter according to the first data set, and providing ablation energy for the ablation of a biological site.
Owner:ST JUDE MEDICAL ATRIAL FIBRILLATION DIV
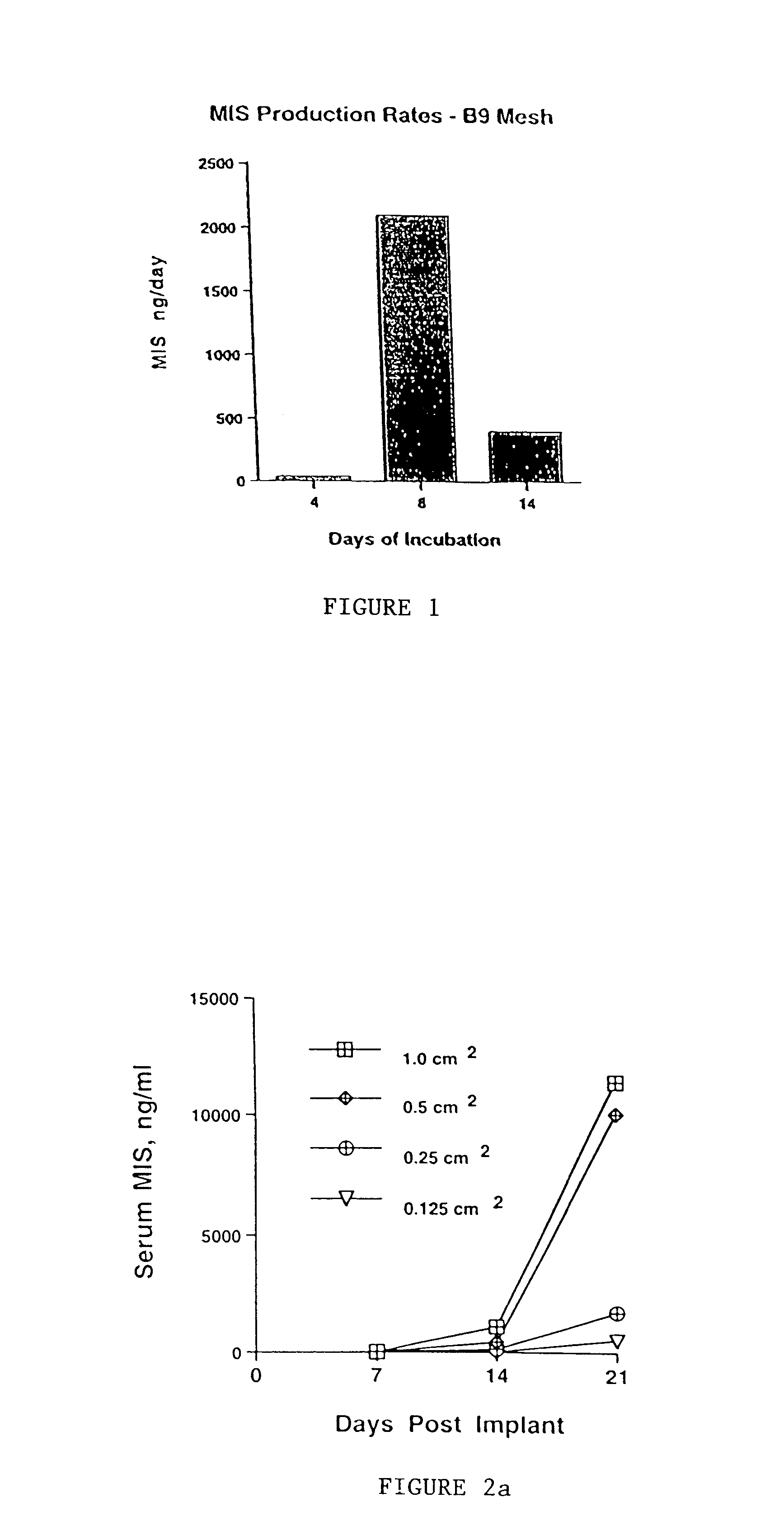
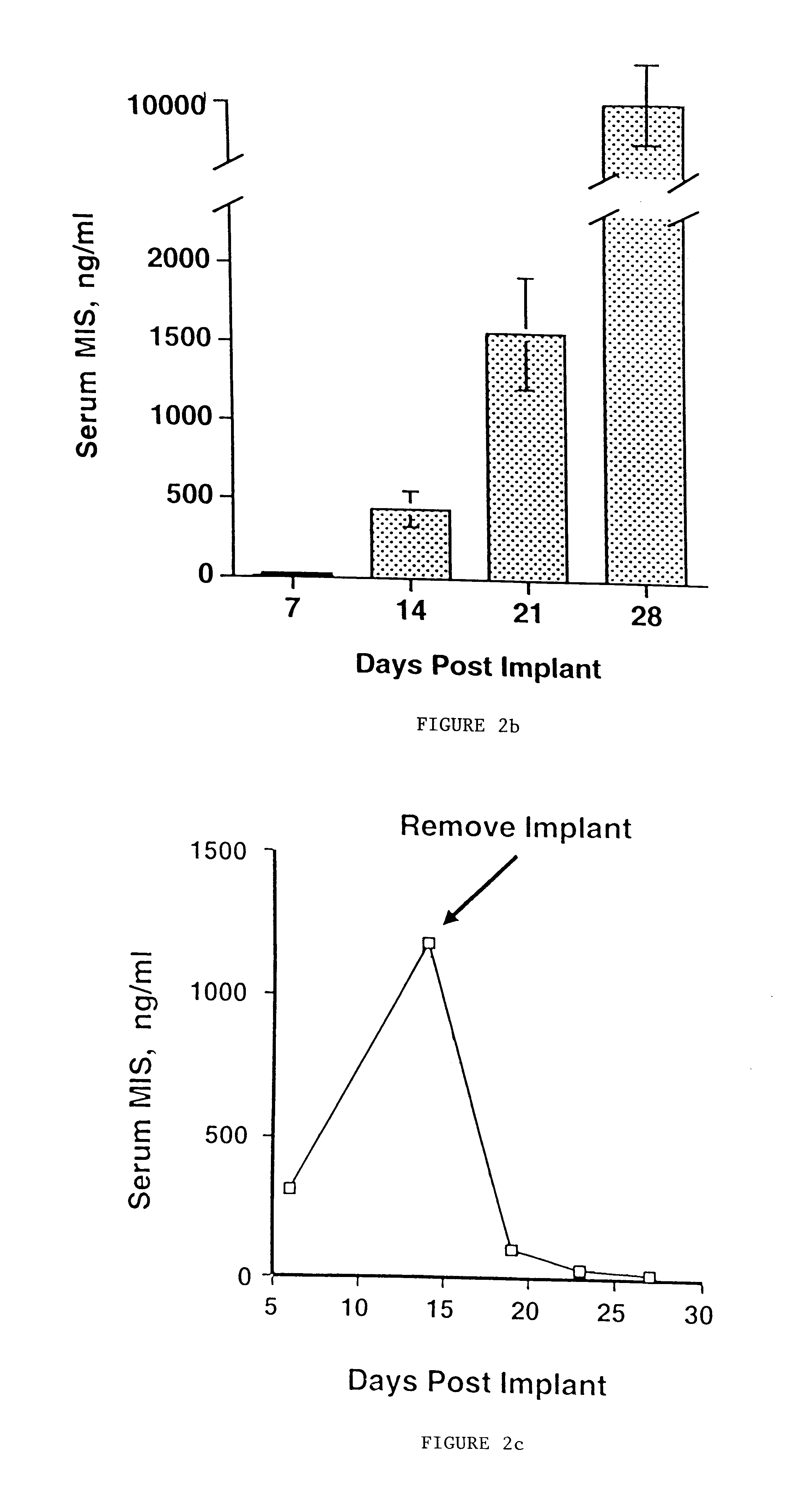
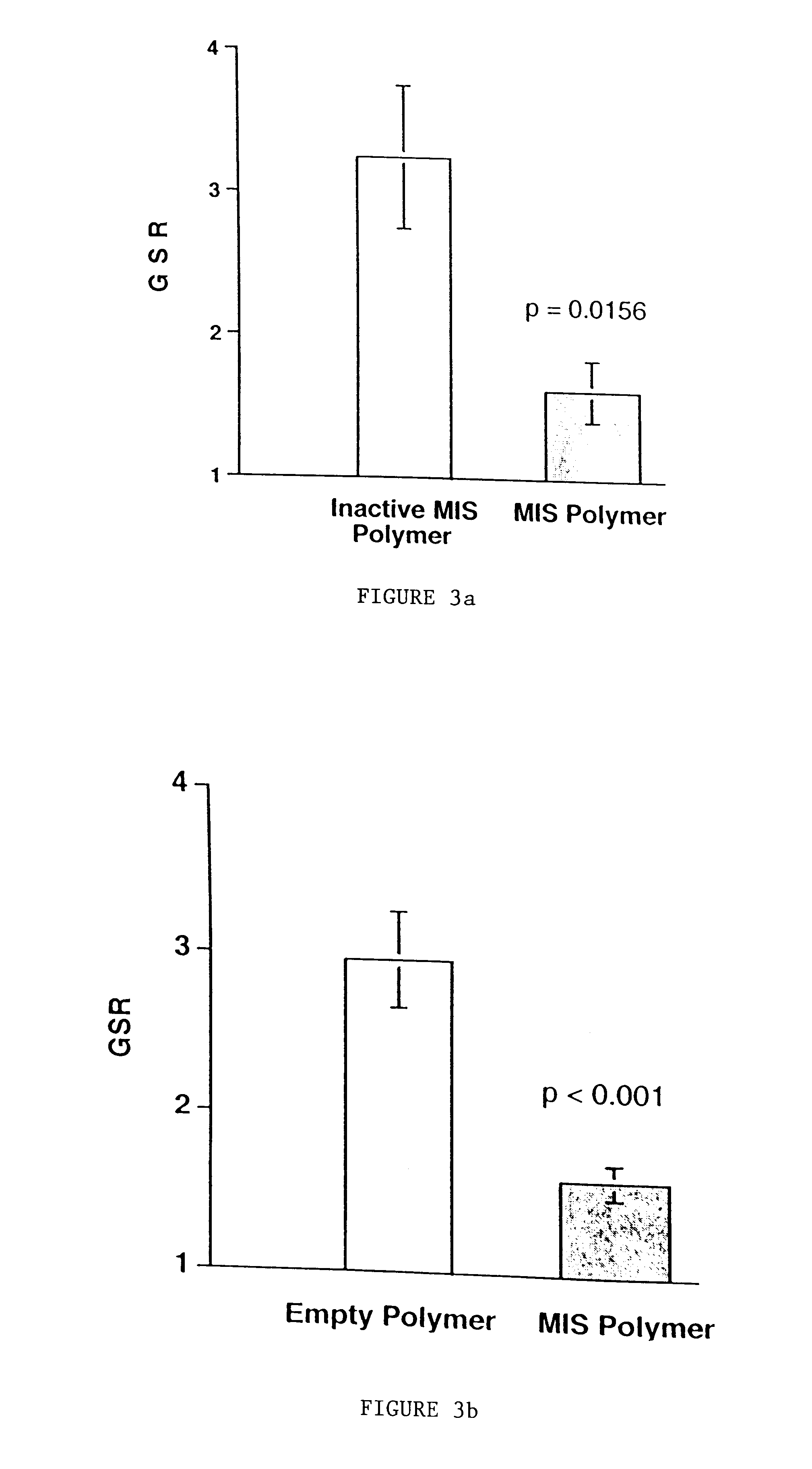
Delivery of therapeutic biologicals from implantable tissue matrices
Normal cells, such as fibroblasts or other tissue or organ cell types, are genetically engineered to express biologically active, therapeutic agents, such as proteins that are normally produced in small amounts, for example, MIS, or other members of the TGF-beta family Herceptin(TM), interferons, andanti-angiogenic factors. These cells are seeded into a matrix for implantation into the patient to be treated. Cells may also be engineered to include a lethal gene, so that implanted cells can be destroyed once treatment is completed. Cells can be implanted in a variety of different matrices. In a preferred embodiment, these matrices are implantable and biodegradable over a period of time equal to or less than the expected period of treatment, when cells engraft to form a functional tissue producing the desired biologically active agent. Implantation may be ectopic or in some cases orthotopic. Representative cell types include tissue specific cells, progenitor cells, and stem cells. Matrices can be formed of synthetic or natural materials, by chemical coupling at the time of implantation, using standard techniques for formation of fibrous matrices from polymeric fibers, and using micromachining or microfabrication techniques. These devices and strategies are used as delivery systems via standard or minimally invasive implantation techniques for any number of parenterally deliverable recombinant proteins, particularly those that are difficult to produce in large amounts and / or active forms using conventional methods of purification, for the treatment of a variety of conditions that produce abnormal growth, including treatment of malignant and benign neoplasias, vascular malformations (hemangiomas), inflammatory conditions, keloid formation, abdominal or plural adhesions, endometriosis, congenital or endocrine abnormalities, and other conditions that can produce abnormal growth such as infection. Efficacy of treatment with the therapeutic biologicals is detected by determining specific criteria, for example, cessation of cell proliferation, regression of abnormal tissue, or cell death, or expression of genes or proteins reflecting the above.
Owner:THE GENERAL HOSPITAL CORP
Biodegradable polyurethanes and use thereof
InactiveUS20050013793A1Improve responseIncrease ratingsCell culture supports/coatingSkeletal/connective tissue cellsPolymer scienceDrug biological activity
A biodegradable and biocompatible polyurethane composition synthesized by reacting isocyanate groups of at least one multifunctional isocyanate compound with at least one bioactive agent having at least one reactive group —X which is a hydroxyl group (—OH) or an amine group (—NH2). The polyurethane composition is biodegradable within a living organism to biocompatible degradation products including the bioactive agent. Preferably, the released bioactive agent affects at least one of biological activity or chemical activity in the host organism. A biodegradable polyurethane composition includes hard segments and soft segments. Each of the hard segments is preferably derived from a diurea diol or a diester diol and is preferably biodegradable into biomolecule degradation products or into biomolecule degradation products and a biocompatible diol. Another biodegradable polyurethane composition includes hard segments and soft segments. Each of the hard segments is derived from a diurethane diol and is biodegradable into biomolecule degradation products.
Owner:CARNEGIE MELLON UNIV +1
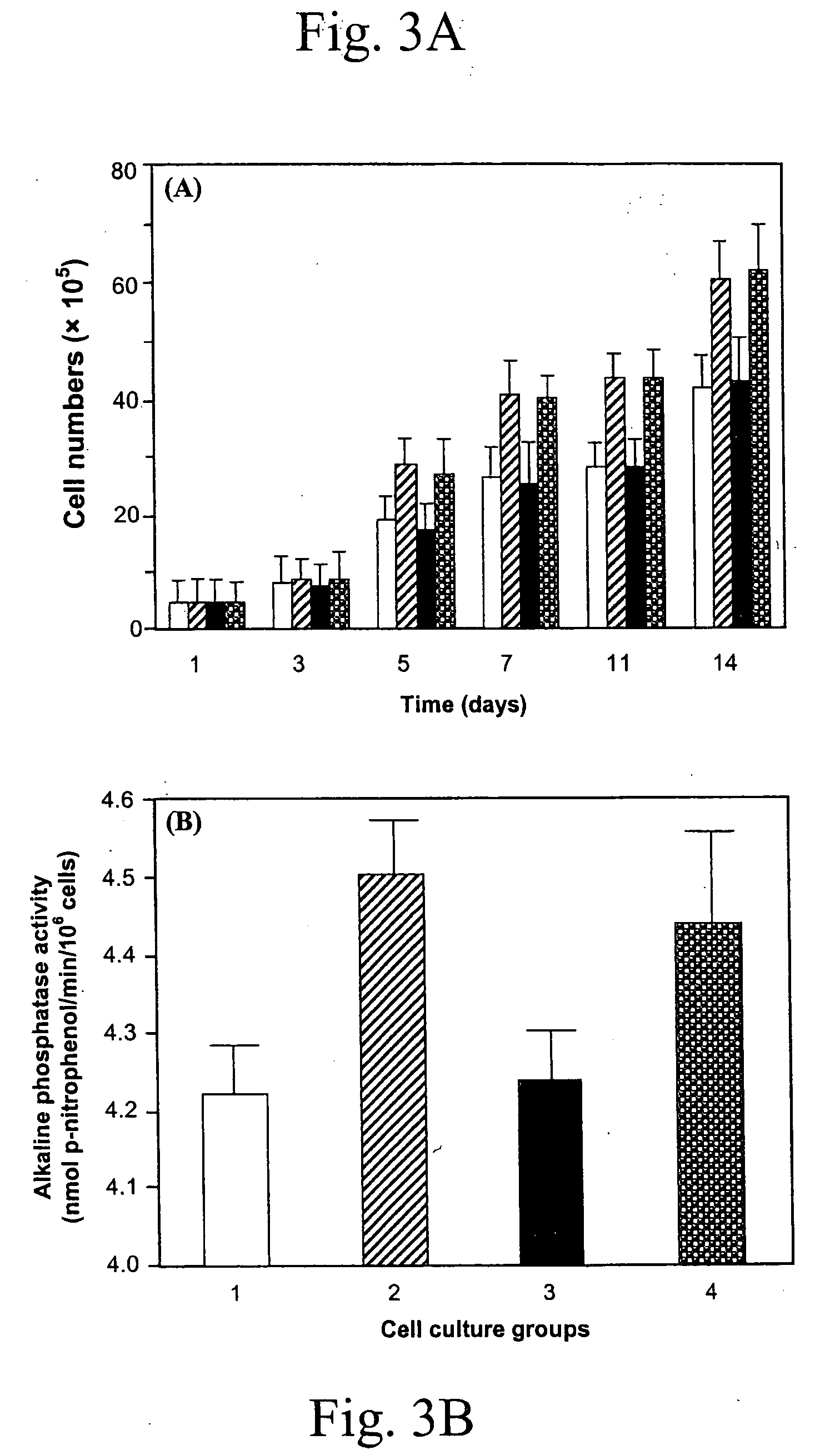
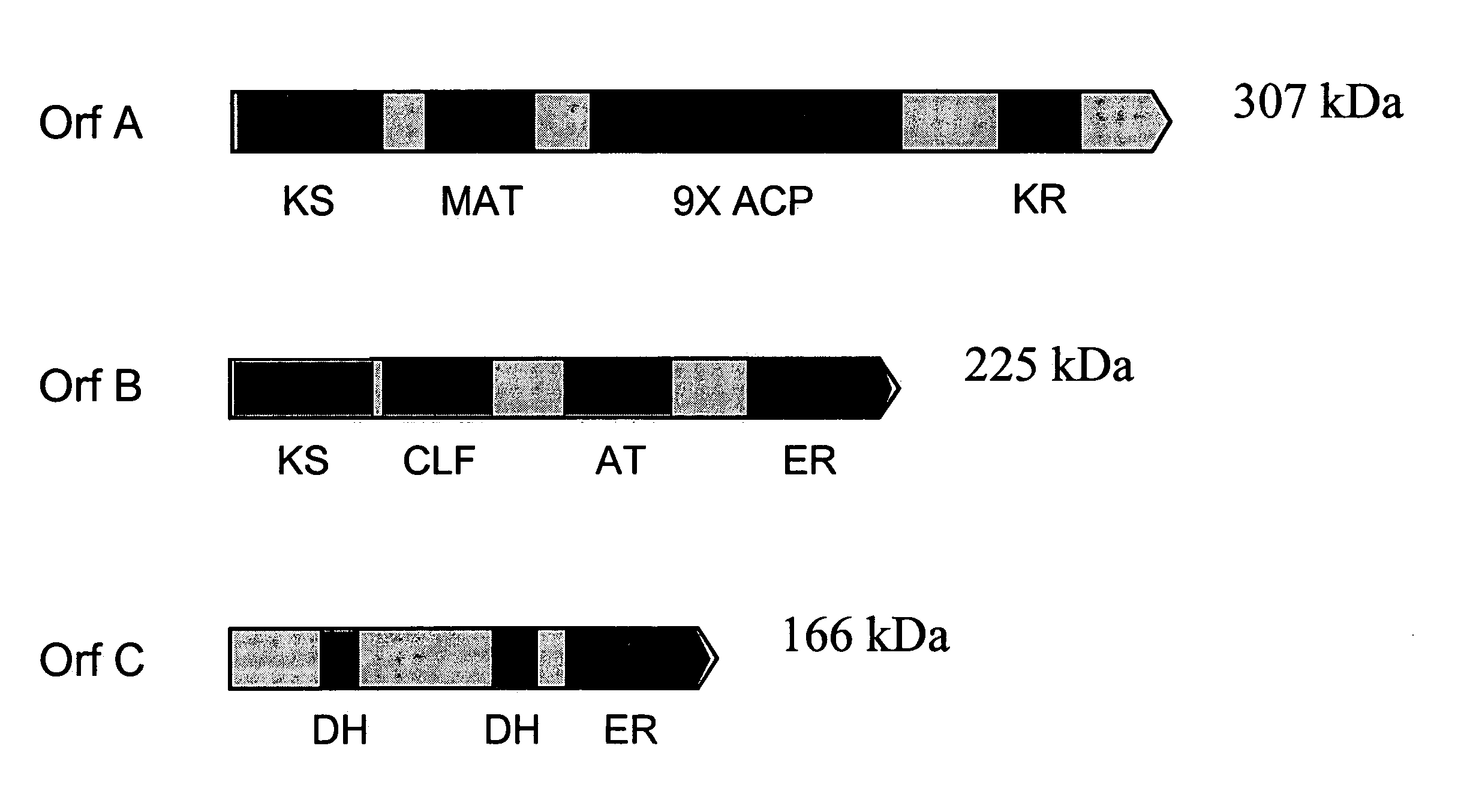
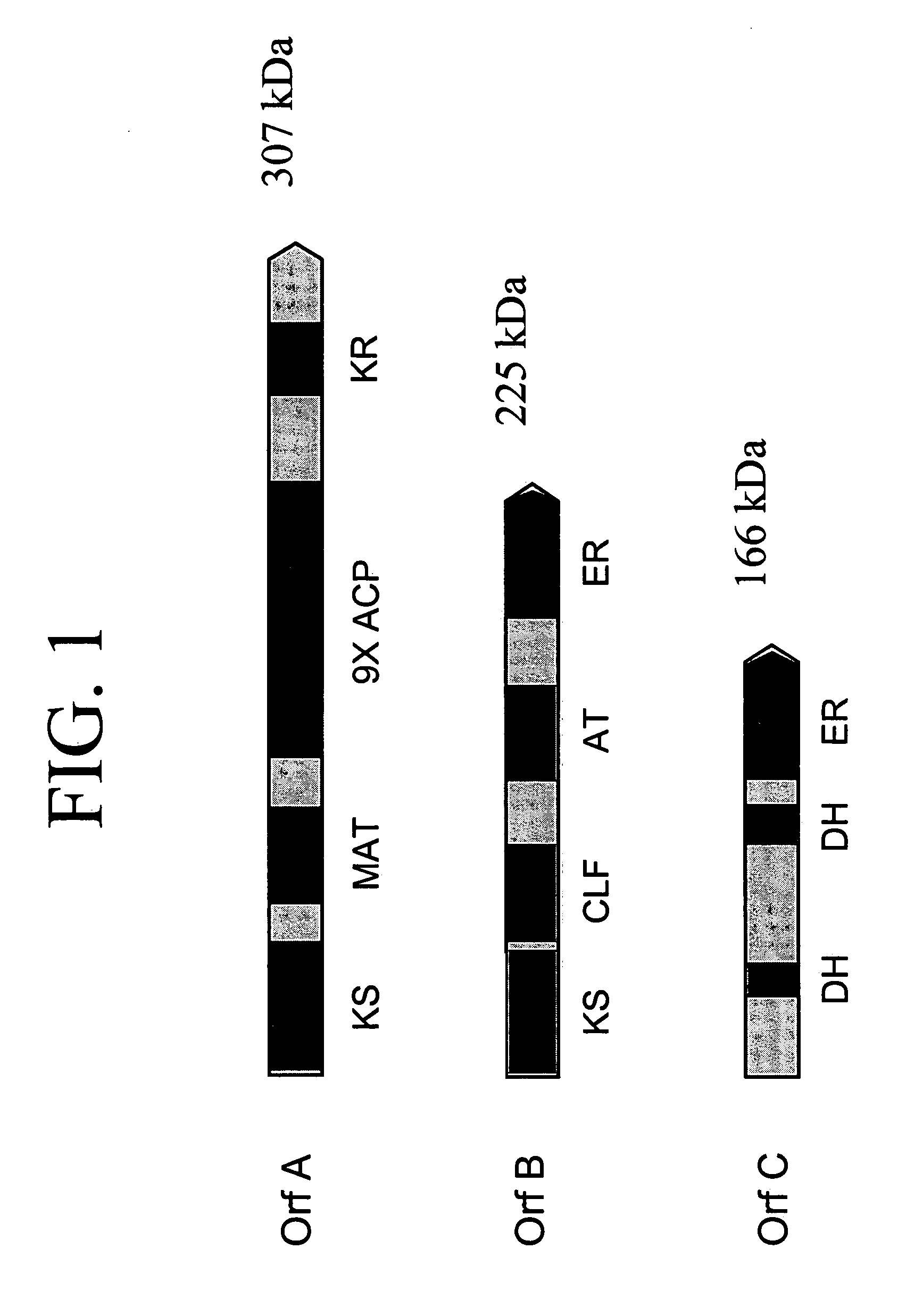
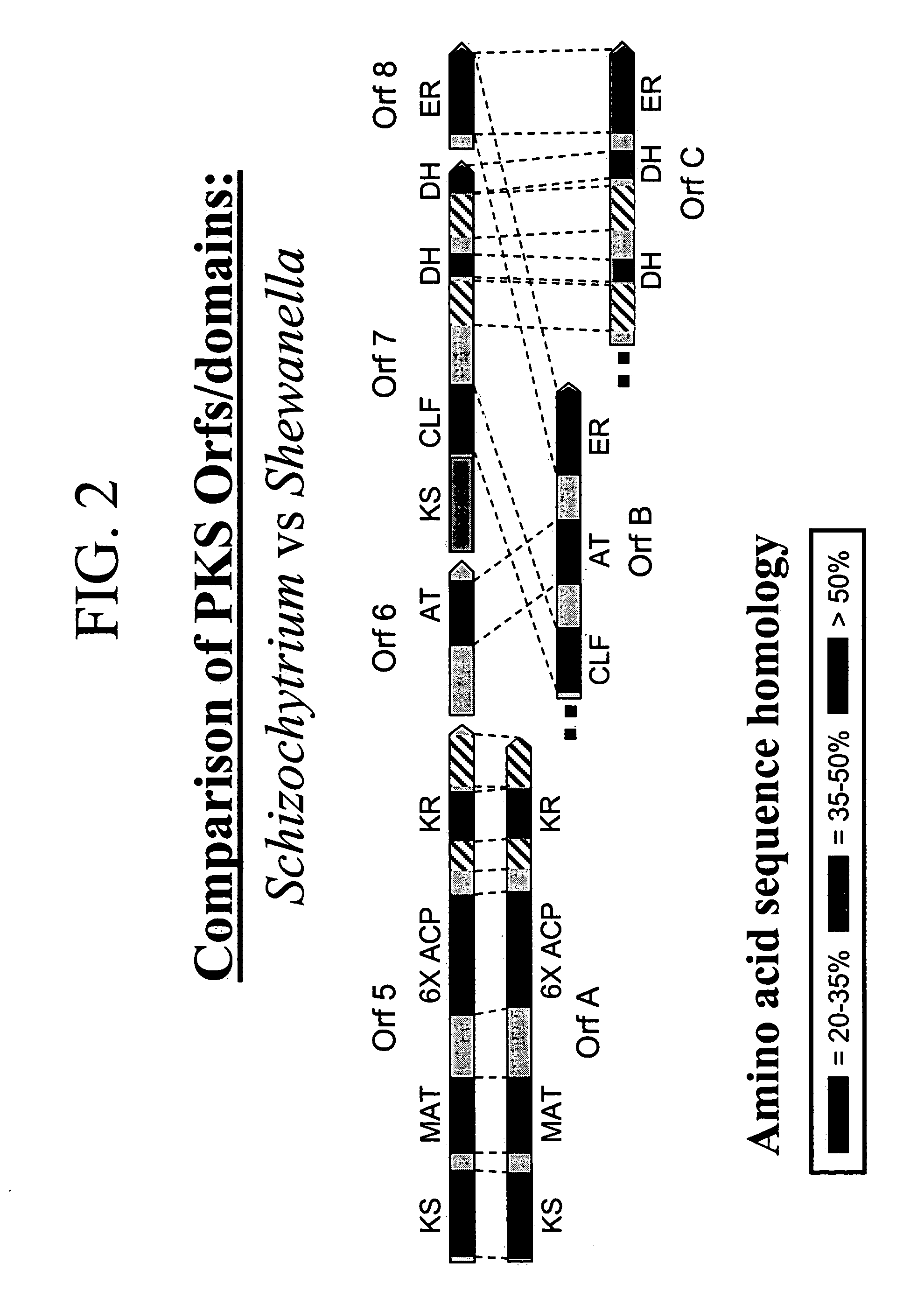
PUFA polyketide synthase systems and uses thereof
The invention generally relates to polyunsaturated fatty acid (PUFA) polyketide synthase (PKS) systems, to homologues thereof, to isolated nucleic acid molecules and recombinant nucleic acid molecules encoding biologically active domains of such a PUFA PKS system, to genetically modified organisms comprising PUFA PKS systems, to methods of making and using such systems for the production of bioactive molecules of interest, and to novel methods for identifying new bacterial and non-bacterial microorganisms having such a PUFA PKS system.
Owner:DSM IP ASSETS BV
Anti-VEGF antibodies
ActiveUS20070020267A1Inhibit bindingSenses disorderImmunoglobulins against growth factorsAnti vegf antibodyBacteriophage
Anti-VEGF antibodies and variants thereof, including those having high affinity for binding to VEGF, are disclosed. Also provided are methods of using phage display technology with naïve libraries to generate and select the anti-VEGF antibodies with desired binding and other biological activities. Further contemplated are uses of the antibodies in research, diagnostic and therapeutic applications.
Owner:GENENTECH INC
Self-assembling nanoparticle drug delivery system
InactiveUS20090226525A1High binding affinityHigh affinityPowder deliveryMicroencapsulation basedLipid formationMedicine
A self-assembling nanoparticle drug delivery system for the delivery of various bioactive agents including peptides, proteins, nucleic acids or synthetic chemical drugs is provided. The self-assembling nanoparticle drug delivery system described herein includes viral capsid proteins, such as Hepatitis B Virus core protein, encapsulating the bioactive agent, a lipid layer or lipid / cholesterol layer coat and targeting or facilitating molecules anchored in the lipid layer. A method for construction of the self-assembling nanoparticle drug delivery system is also provided.
Owner:CHIMEROS
Dimerized polypeptide fusions
InactiveUS6291646B1Mass productionImprove expression levelPeptide/protein ingredientsAntibody mimetics/scaffoldsExtracellular StructureA-DNA
Methods for producing secreted receptor analogs and biologically active peptide dimers are disclosed. The methods for producing secreted receptor analogs and biologically active peptide dimers utilize a DNA sequence encoding a receptor analog or a peptide requiring dimerization for biological activity joined to a dimerizing protein. The receptor analog includes a ligand-binding domain. Polypeptides comprising essentially the extracellular domain of a human PDGF receptor fused to dimerizing proteins, the portion being capable of binding human PDGF or an isoform thereof, are also disclosed. The polypeptides may be used within methods for determining the presence of and for purifying human PDGF or isoforms thereof.
Owner:ZYMOGENETICS INC
Methods and compositions for treating a disease condition in a subject
Methods for treating a disease condition in a subject are provided. The subject methods include selectively modulating at least one biological activity of advential tissue in a manner effective to treat the disease condition. Also provided are compositions, kits and systems for use in practicing the subject methods.
Owner:PALO ALTO INVESTORS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com