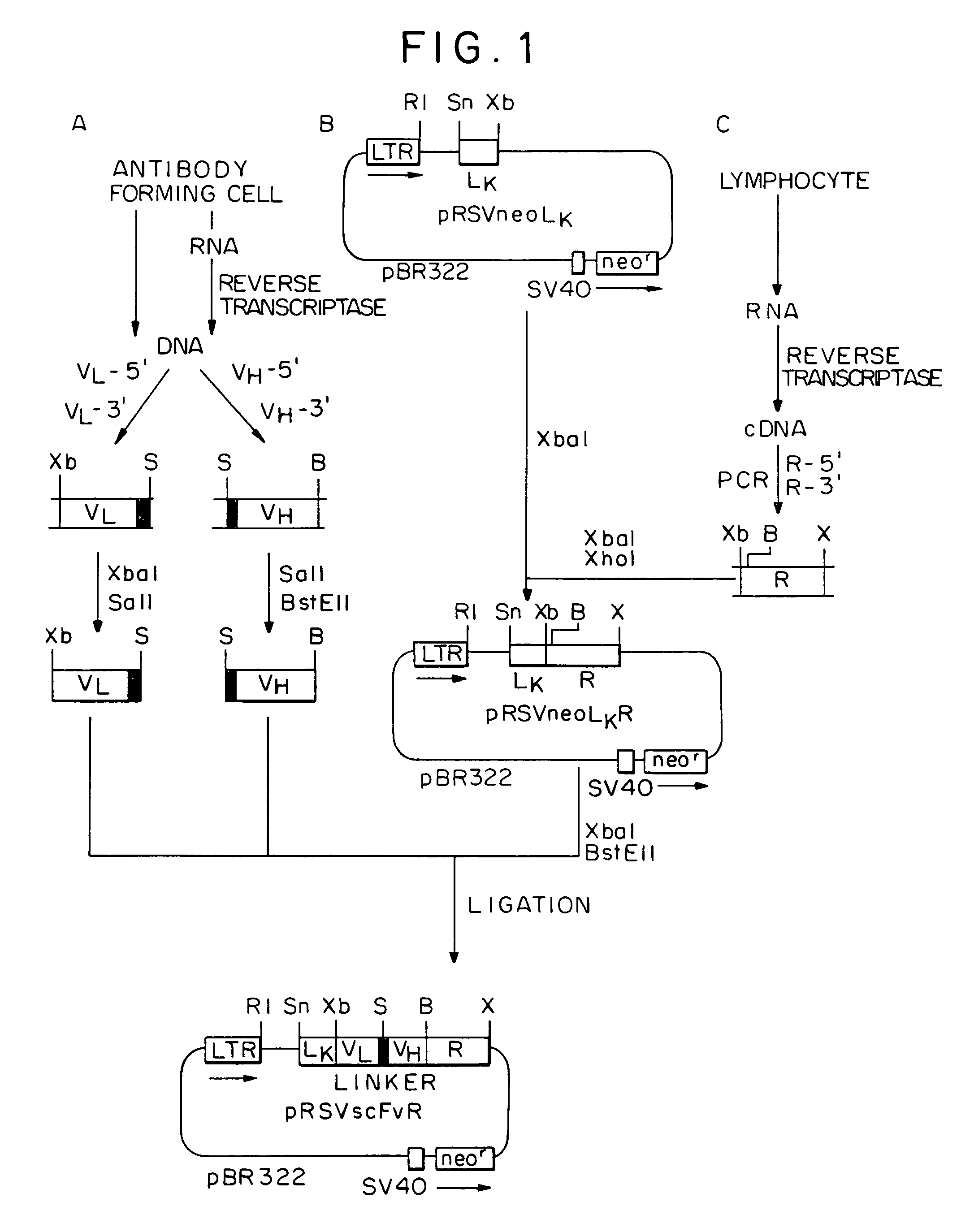
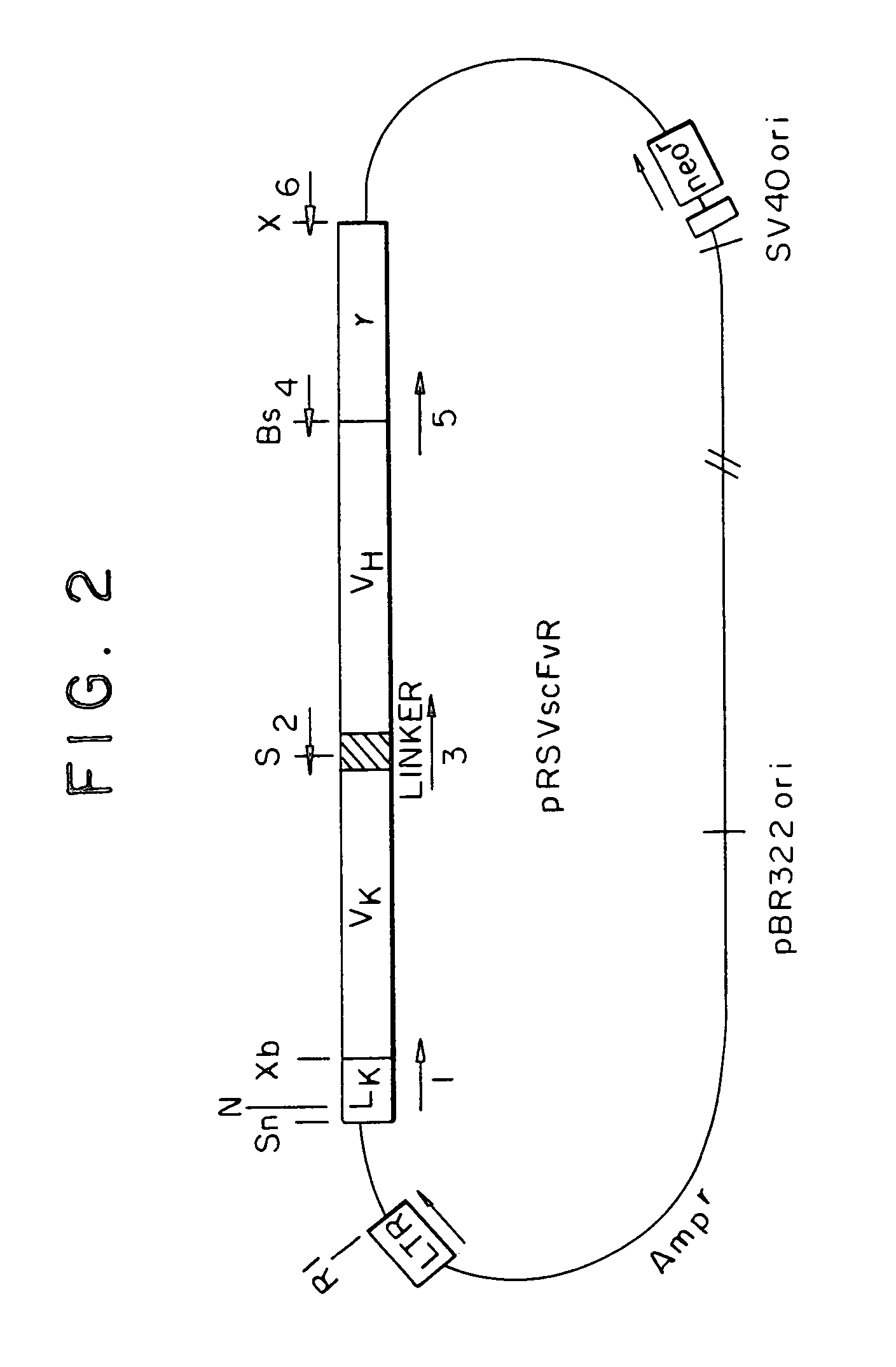
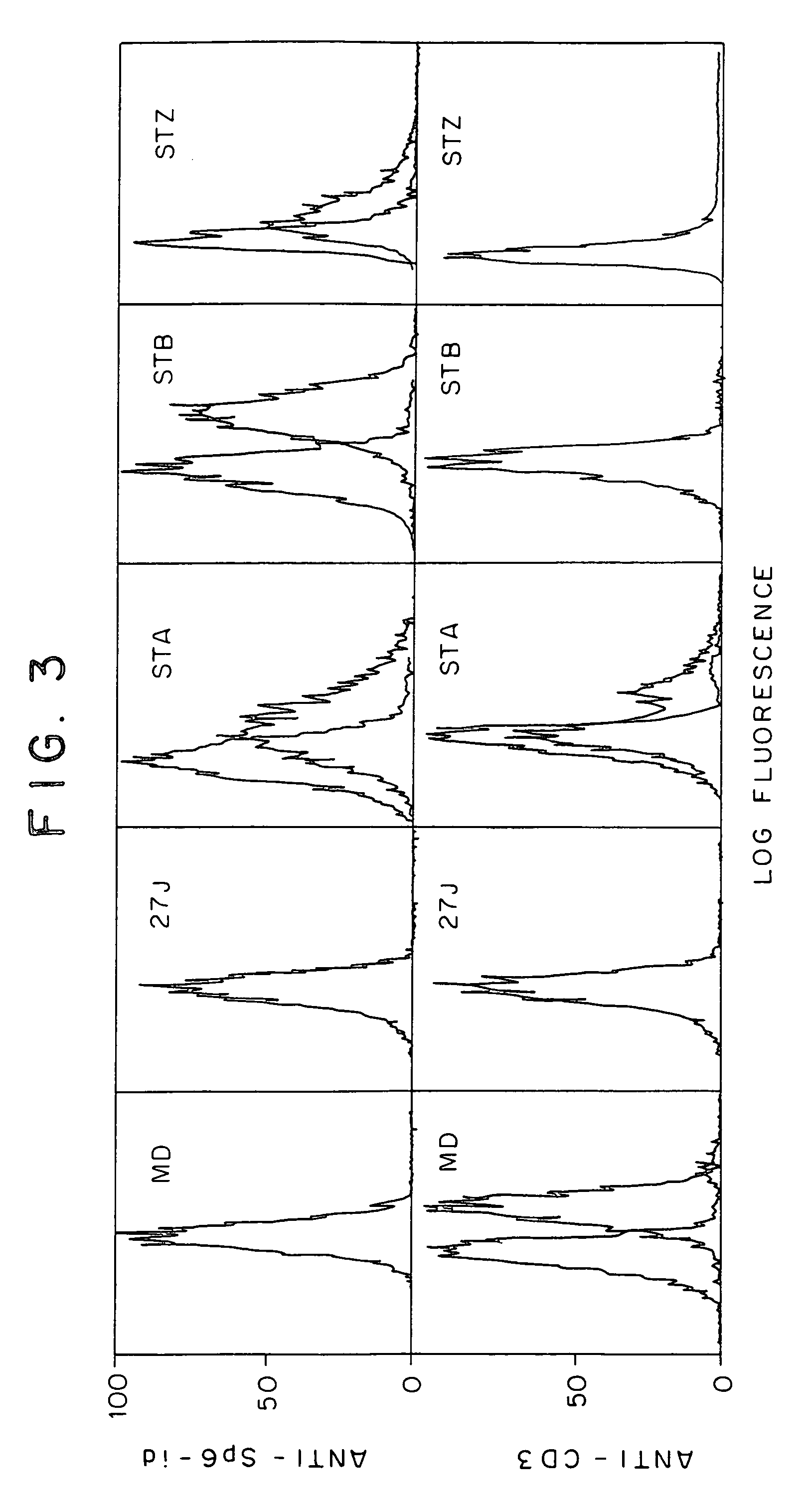
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
345 results about "Extracellular Structure" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
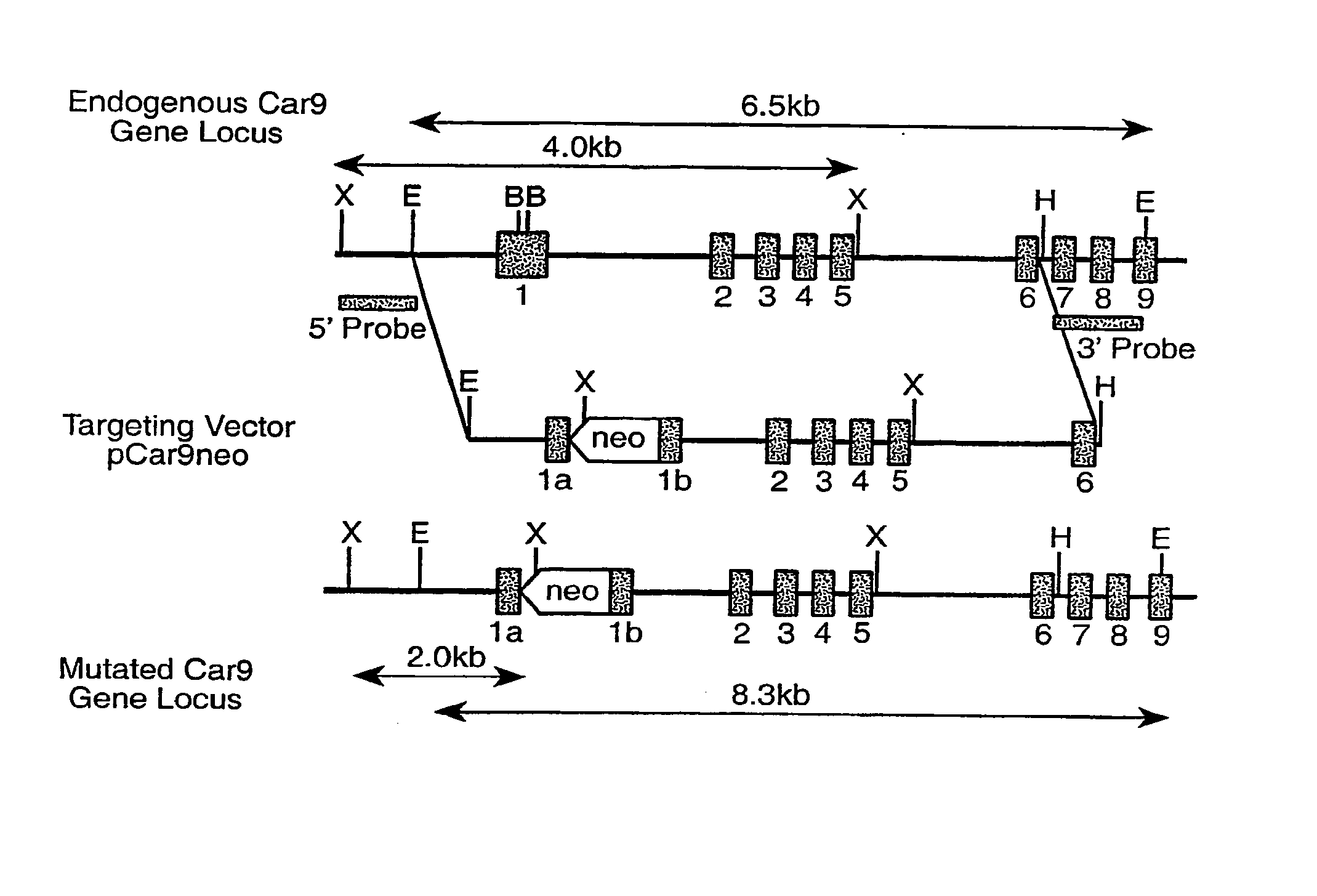
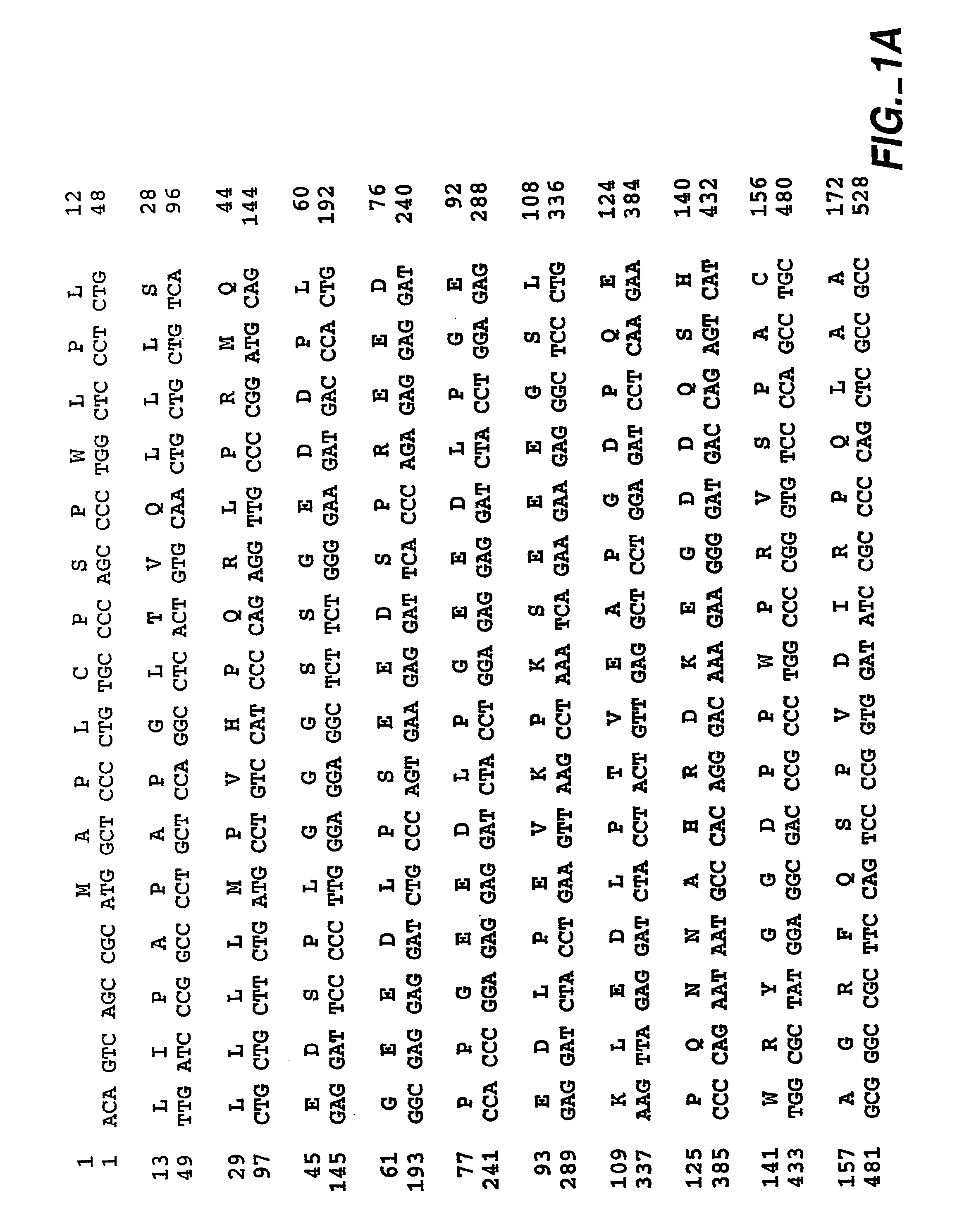
Extracellular matrix. A filamentous structure that is attached to the outer cell surface and provides anchorage, traction, and positional recognition to the cell.
Chimeric receptor genes and cells transformed therewith
ActiveUS7741465B1Limit acquisitionMicroorganismsGenetic material ingredientsAntibody typesLymphocyte
Chimeric receptor genes suitable for endowing lymphocytes with antibody-type specificity include a first gene segment encoding a single-chain Fv domain of a specific antibody and a second gene segment encoding all or part of the transmembrane and cytoplasmic domains, and optionally the extracellular domain, of an immune cell-triggering molecule. The chimeric receptor gene, when transfected to immune cells, expresses the antibody-recognition site and the immune cell-triggering moiety into one continuous chain. The transformed lymphocytes are useful in therapeutic treatment methods.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF +1
CE7-specific redirected immune cells
Genetically engineered, CE7-specific redirected immune cells expressing a cell surface protein having an extracellular domain comprising a receptor which is specific for CE7, an intracellular signaling domain, and a transmembrane domain, and methods of use for such cells for cellular immunotherapy of CE7+ neuroblastoma are disclosed. In one embodiment, the immune cell is a T cell and the cell surface protein is a single chain FvFc:ζ receptor where Fv designates the VH and VL chains of a single chain monoclonal antibody to CE7 linked by peptide, Fc represents a hinge —CH2—CH3 region of a human IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. DNA constructs encoding a chimeric T-cell receptor and a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor are also disclosed.
Owner:CITY OF HOPE
CD19-specific chimeric T cell receptor
InactiveUS7446179B2Peptide/protein ingredientsAntibody mimetics/scaffoldsIntracellular signallingTransmembrane domain
The present invention relates to a genetically engineered, CD19-specific chimeric T cell receptor and to immune cells expressing the chimeric receptor The present invention also relates to the use of such cells for cellular immunotherapy of CD9+ malignancies and for abrogating any untoward B cell function. The chimeric receptor is a single chain scFvFc:ζ receptor where scFvFc designates the extracellular domain, scFv designates the VH and VL chains of a single chain monoclonal antibody to CD19, Fc represents at least part of a constant region of an IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. The extracellular domain scFvFc and the intracellular domain ζ are linked by a transmembrane domain such as the transmembrane domain of CD4. In one aspect, the chimeric receptor comprises amino acids 23-634 of SEQ I DNO:2. The present invention further relates to a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor.
Owner:CITY OF HOPE
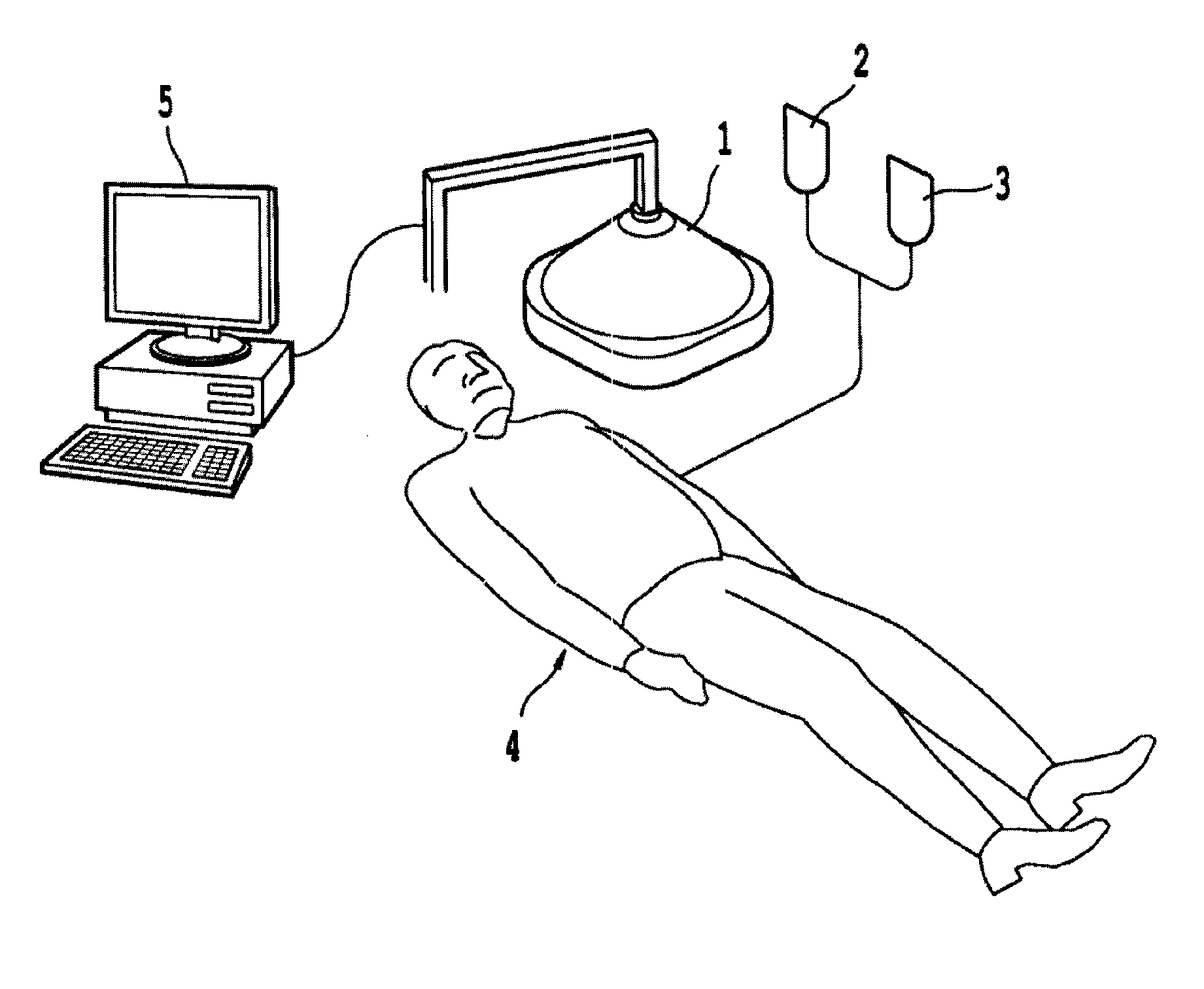
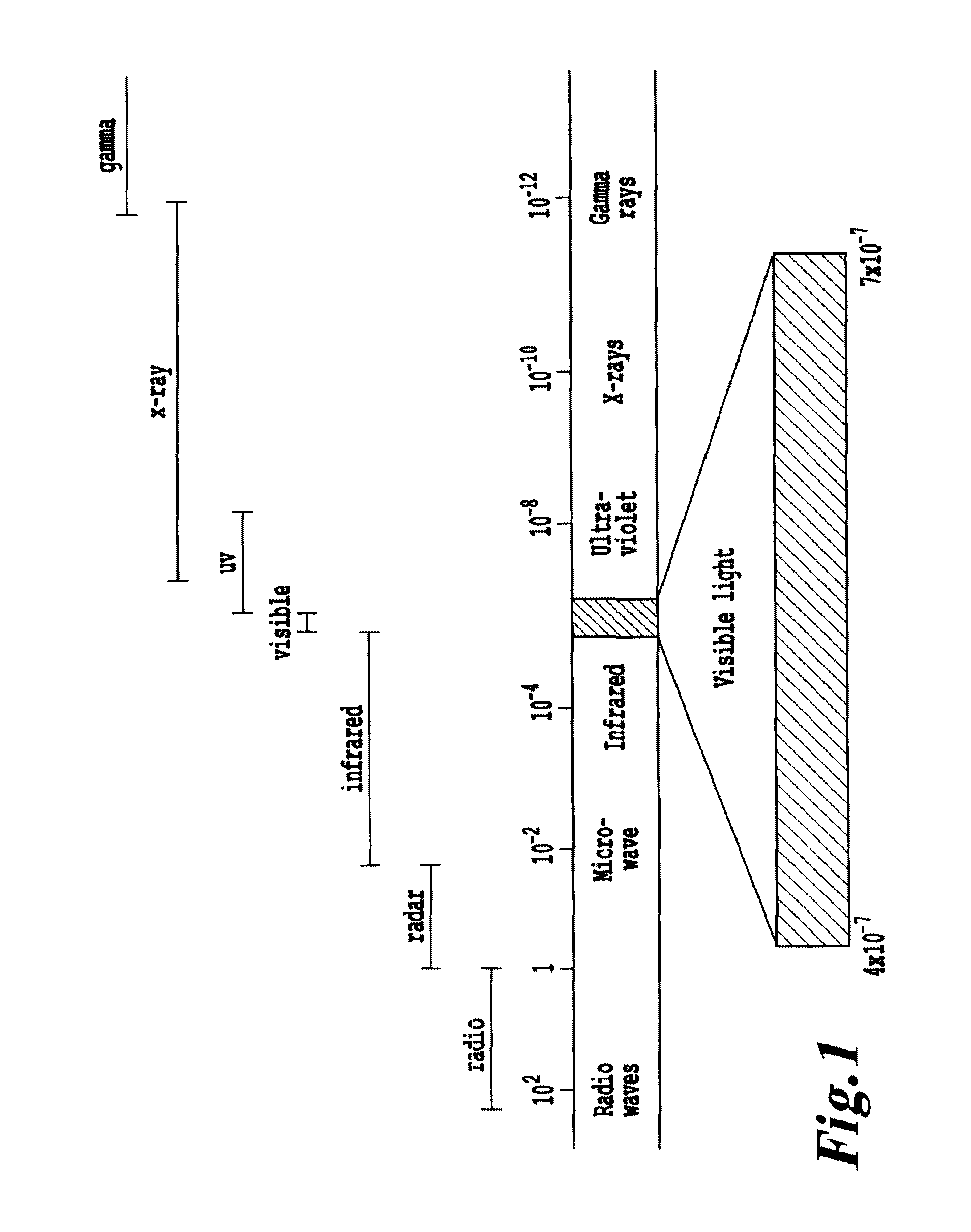
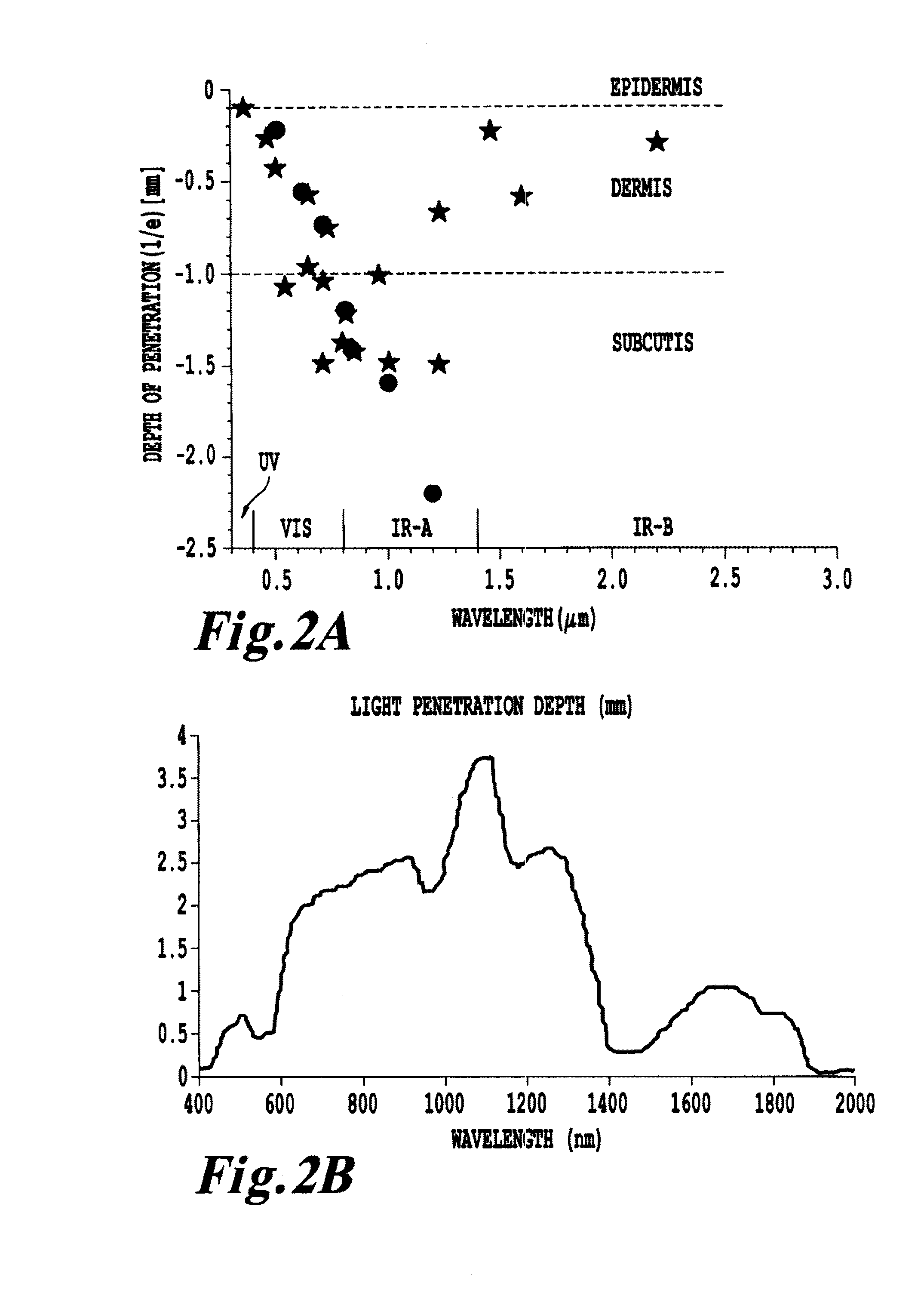
Non-invasive systems and methods for in-situ photobiomodulation
InactiveUS20100016783A1High selectivityAntibacterial agentsPowder deliveryDiseaseBiological regulation
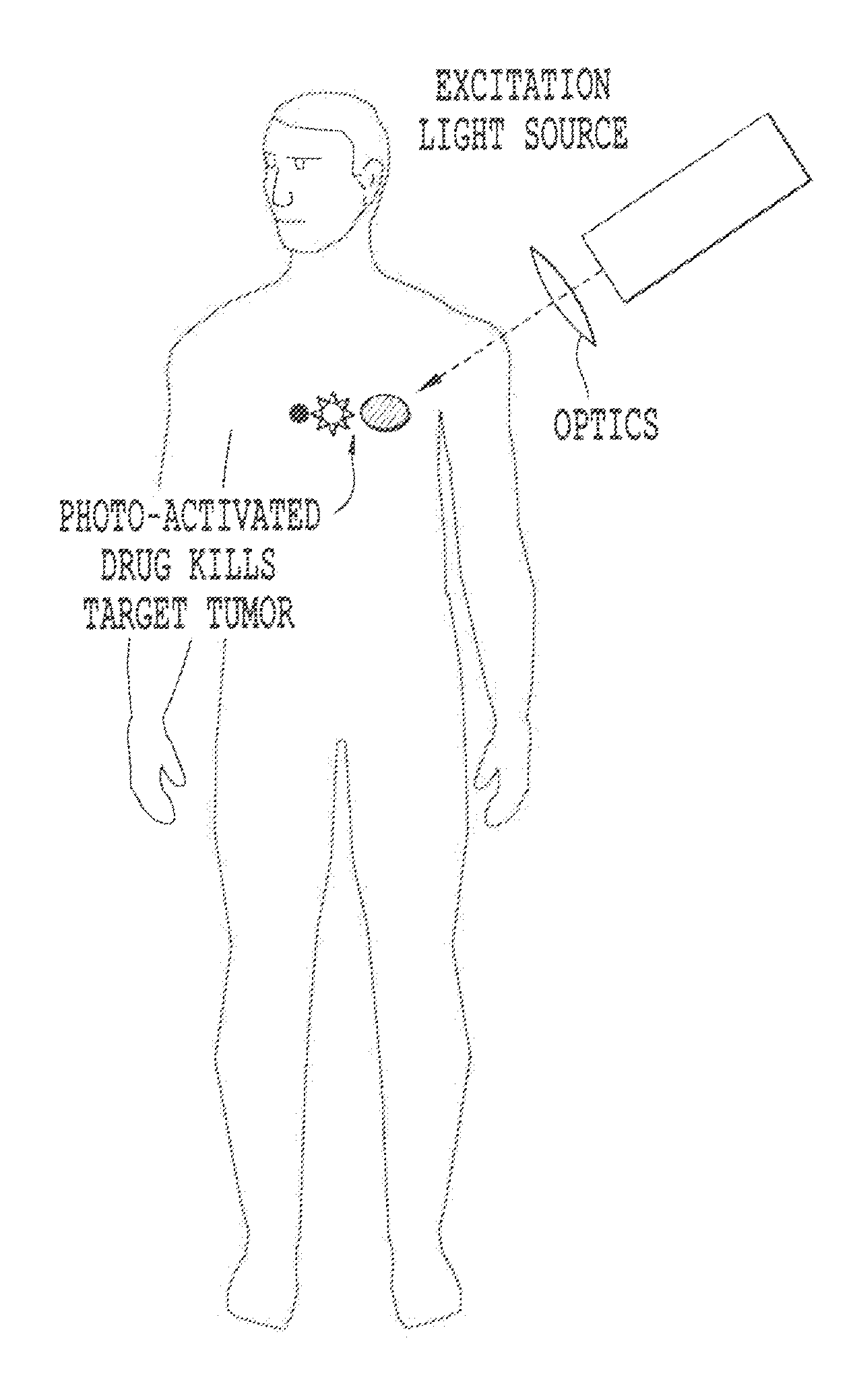
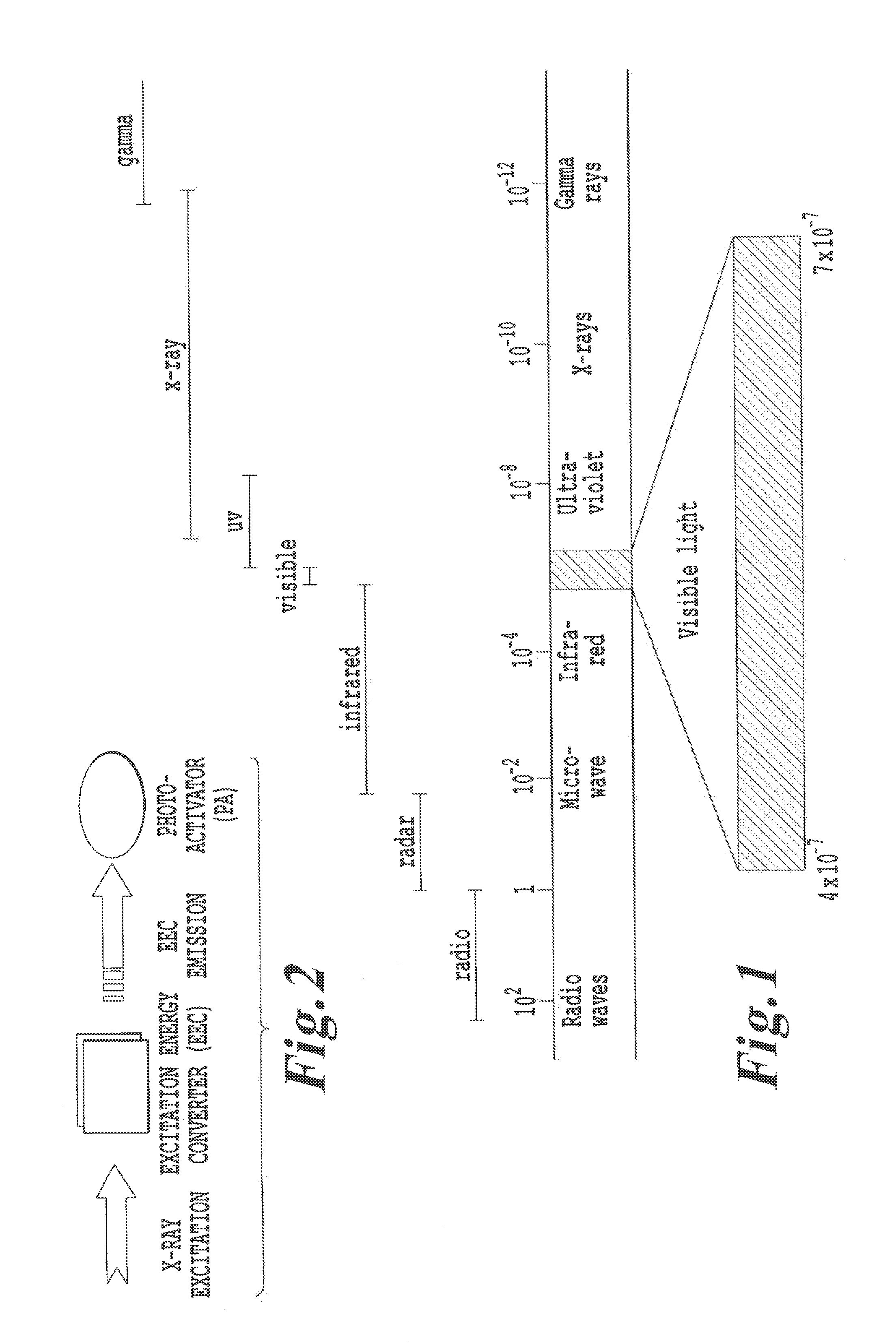
Products, compositions, systems, and methods for modifying a target structure which mediates or is associated with a biological activity, including treatment of conditions, disorders, or diseases mediated by or associated with a target structure, such as a virus, cell, subcellular structure or extracellular structure. The methods may be performed in situ in a non-invasive manner by application of an initiation energy to a subject thus producing an effect on or change to the target structure directly or via a modulation agent. The methods may further be performed by application of an initiation energy to a subject in situ to activate a pharmaceutical agent directly or via an energy modulation agent, optionally in the presence of one or more plasmonics active agents, thus producing an effect on or change to the target structure. Kits containing products or compositions formulated or configured and systems for use in practicing these methods.
Owner:DUKE UNIV +1
Antibody for 4-1BB
The present invention includes the receptor protein 4-1BB and the cDNA gene encoding for receptor protein 4-1BB. The nucleotide sequence of the isolated cDNA is disclosed herein along with the deduced amino acid sequence. The 4-1BB protein and fragments and derivatives can be used: 1) as a probe to isolate ligands to receptor protein 4-1BB, 2) to stimulate proliferation of B-cell's expressing 4-1BB, or 3) to block 4-1BB ligand binding. A monoclonal antibody against 4-1BB was developed which specifically recognizes an epitope on the extracellular domain of receptor protein 4-1BB. The monoclonal antibody can be used enhance T-cell proliferation and activation by treating T-cells that have expressed receptor protein 4-1BB with the monoclonal antibody. The effectiveness of the treatment was enhanced when conducted in the presence of protein tyrosinase kinase. A fusion protein for detecting cell membrane ligands to receptor protein 4-1BB was developed. It comprises the extracellular portion of the receptor protein 4-1BB and a detection protein bound to the portion of the receptor protein 4-1BB.
Owner:INDIANA UNIV RES & TECH CORP
Non-invasive energy upconversion methods and systems for in-situ photobiomodulation
ActiveUS20110021970A1High selectivityAvoid the needAntibacterial agentsSenses disorderDiseaseHigh energy
Products, compositions, systems, and methods for modifying a target structure which mediates or is associated with a biological activity, including treatment of conditions, disorders, or diseases mediated by or associated with a target structure, such as a virus, cell, subcellular structure or extracellular structure. The methods may be performed in situ in a non-invasive manner by placing a nanoparticle having a metallic shell on at least a fraction of a surface in a vicinity of a target structure in a subject and applying an initiation energy to a subject thus producing an effect on or change to the target structure directly or via a modulation agent. The nanoparticle is configured, upon exposure to a first wavelength λ1, to generate a second wavelength λ2 of radiation having a higher energy than the first wavelength λ1. The methods may further be performed by application of an initiation energy to a subject in situ to activate a pharmaceutical agent directly or via an energy modulation agent, optionally in the presence of one or more plasmonics active agents, thus producing an effect on or change to the target structure. Kits containing products or compositions formulated or configured and systems for use in practicing these methods.
Owner:DUKE UNIV +1
Biologically active dimerized and multimerized polypeptide fusions
InactiveUS6018026AImprove expression levelMass productionPeptide/protein ingredientsAntibody mimetics/scaffoldsExtracellular StructureA-DNA
Methods for producing secreted receptor analogs and biologically active peptide dimers are disclosed. The methods for producing secreted receptor analogs and biologically active peptide dimers utilize a DNA sequence encoding a receptor analog or a peptide requiring dimerization for biological activity joined to a dimerizing protein. The receptor analog includes a ligand-binding domain. Polypeptides comprising essentially the extracellular domain of a human PDGF receptor fused to dimerizing proteins, the portion being capable of binding human PDGF or an isoform thereof, are also disclosed. The polypeptides may be used within methods for determining the presence of and for purifying human PDGF or isoforms thereof.
Owner:ZYMOGENETICS INC
Antibodies to neutrokine-alpha
InactiveUS6403770B1Peptide/protein ingredientsGenetic material ingredientsScreening methodTnf family
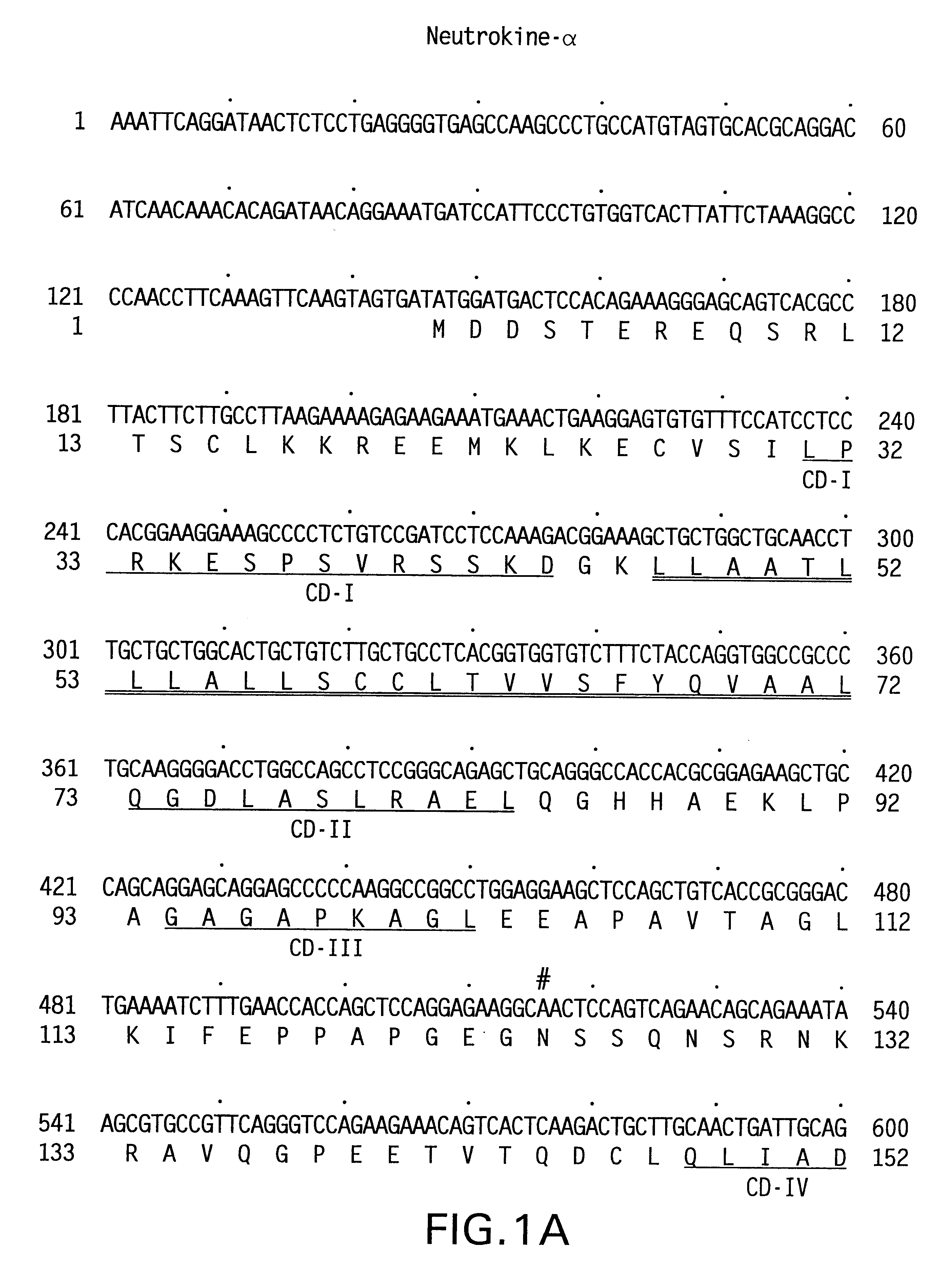
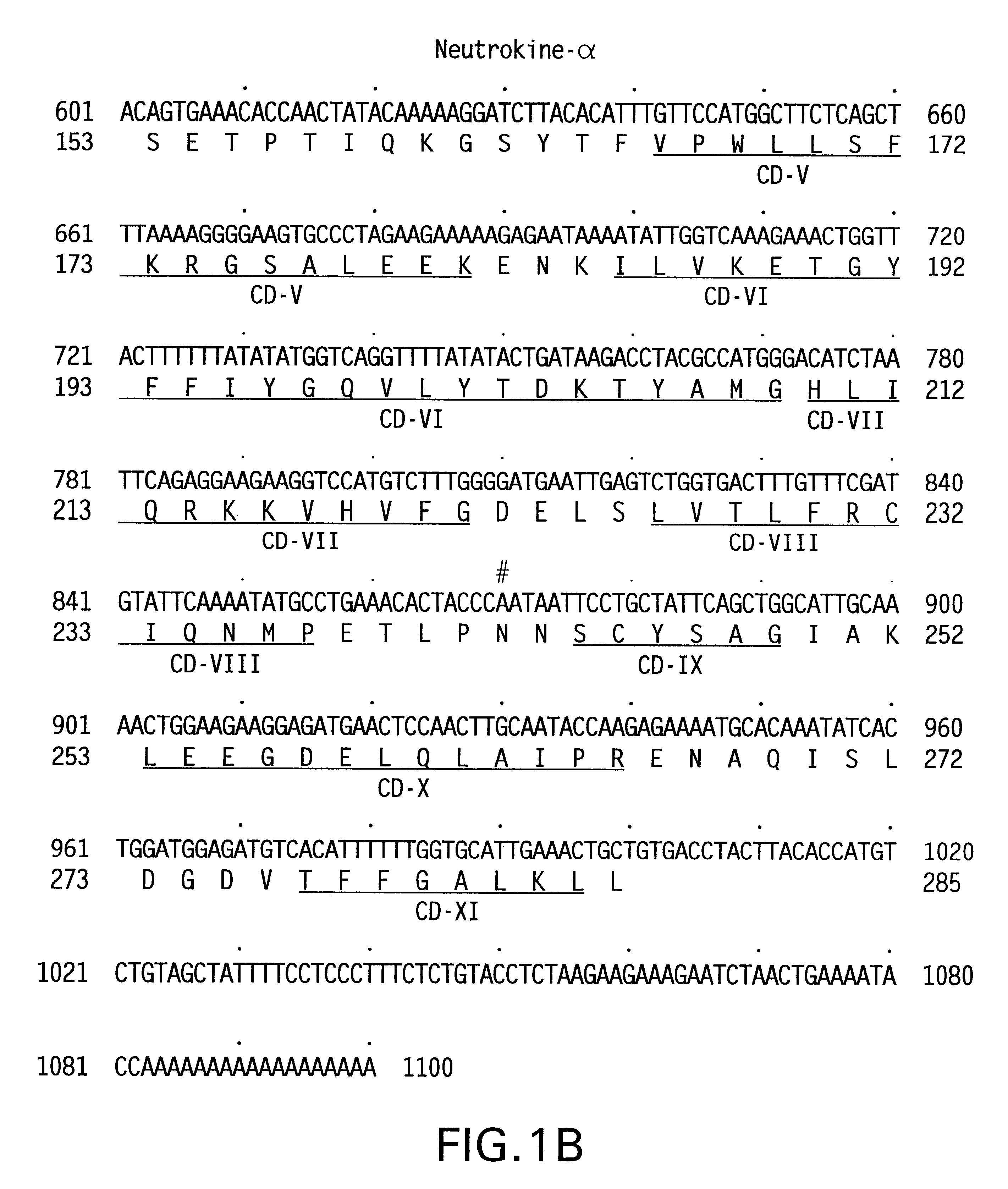
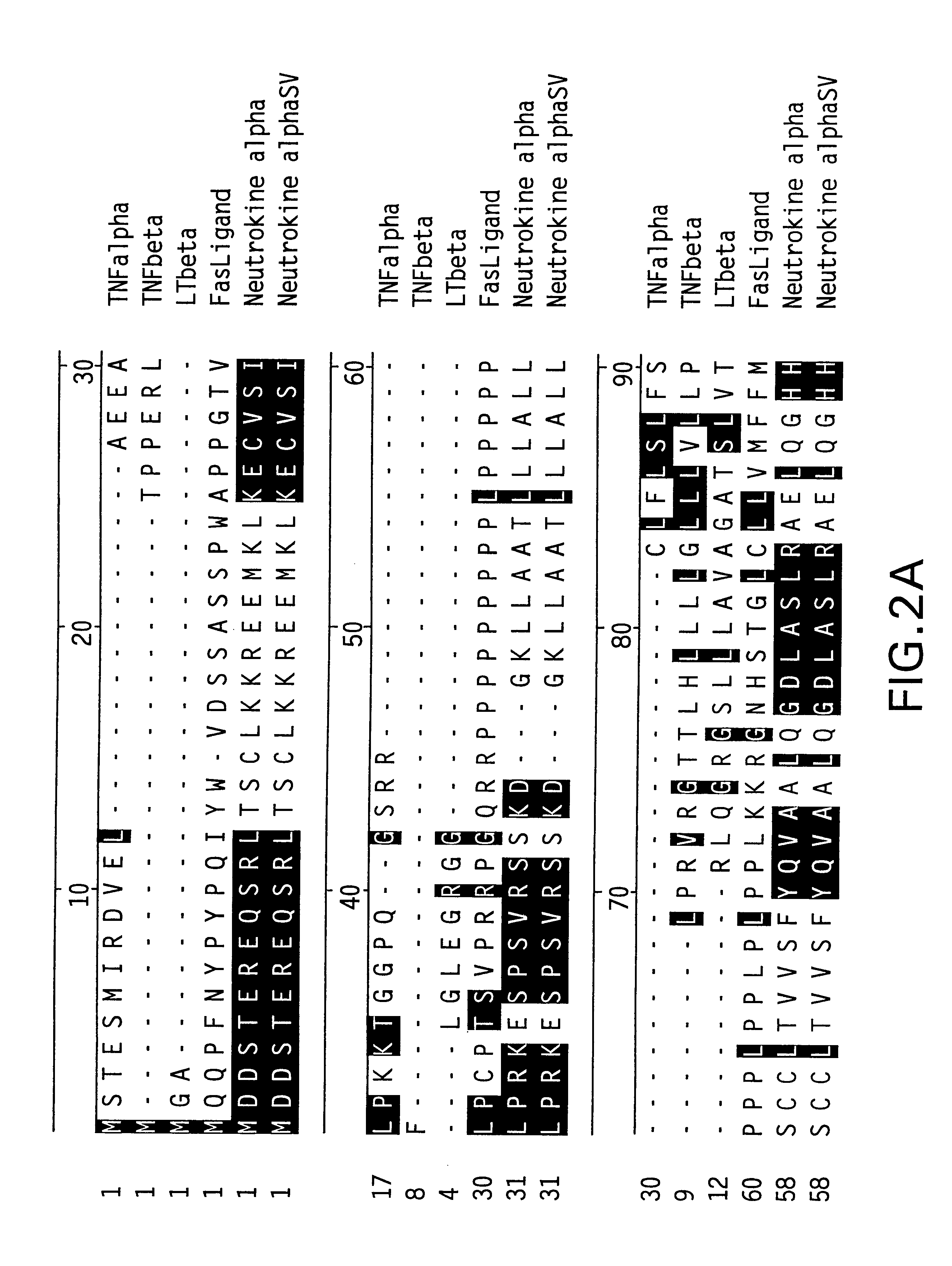
The present invention relates to a novel Neutrokine-alpha, and a splice variant thereof designated Neutrokine-alphaSV, polynucleotides and polypeptides which are members of the TNF family. In particular, isolated nucleic acid molecules are provided encoding the human Neutrokine-alpha and / or Neutrokine-alphaSV polypeptides, including soluble forms of the extracellular domain. Neutrokine-alpha and / or Neutrokine-alphaSV polypeptides are also provided as are vectors, host cells and recombinant methods for producing the same. The invention further relates to screening methods for identifying agonists and antagonists of Neutrokine-alpha and / or Neutrokine-alphaSV activity. Also provided are diagnostic methods for detecting immune system-related disorders and therapeutic methods for treating immune system-related disorders.
Owner:HUMAN GENOME SCI INC
Chimeric immunoreceptor useful in treating human gliomas
InactiveUS7514537B2Negligible toxicityPotent and selectiveBiocideAntibody mimetics/scaffoldsIntracellular signallingHuman glioma
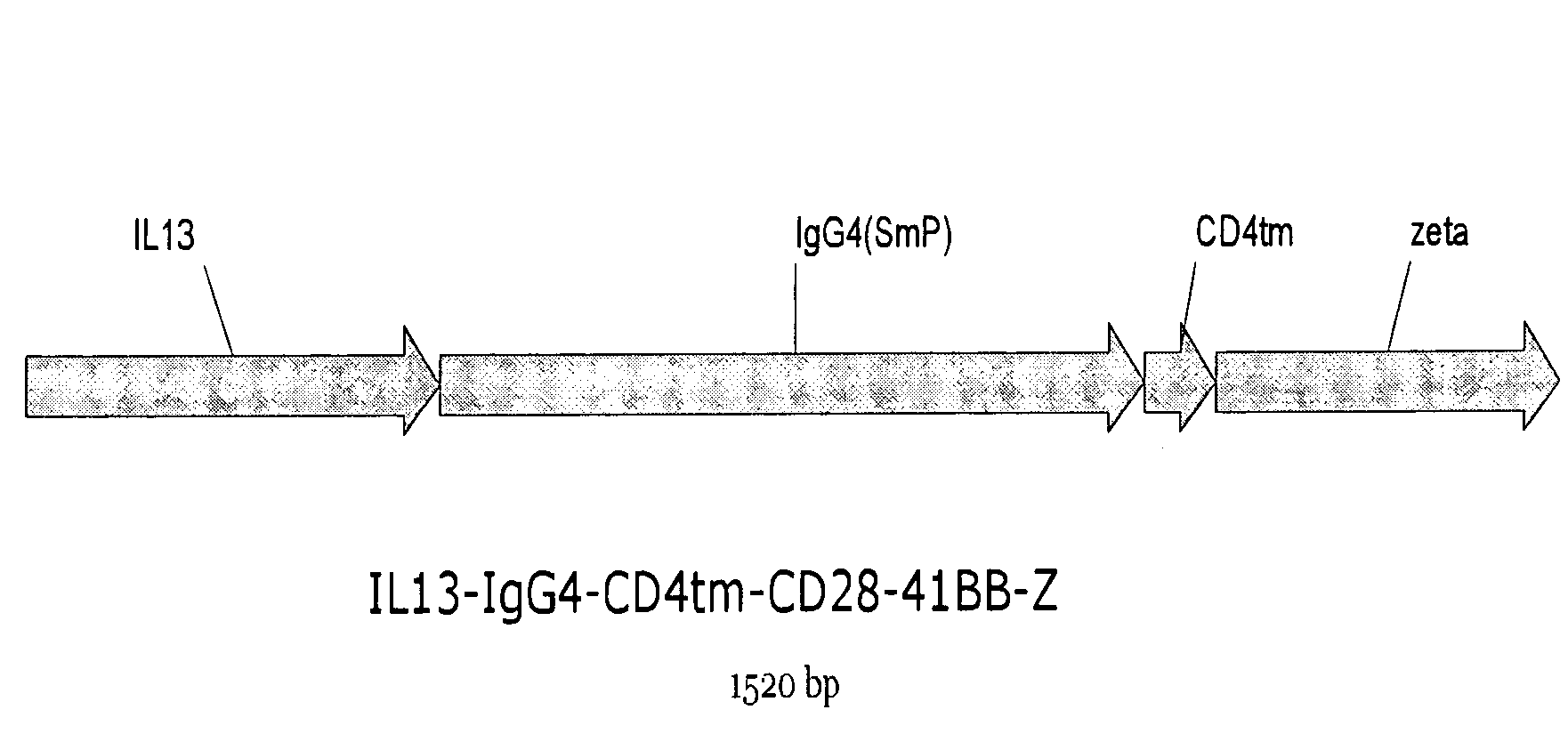
The present invention relates to chimeric transmembrane immunoreceptors, named “zetakines,” comprised of an extracellular domain comprising a soluble receptor ligand linked to a support region capable of tethering the extracellular domain to a cell surface, a transmembrane region and an intracellular signaling domain. Zetakines, when expressed on the surface of T lymphocytes, direct T cell activity to those specific cells expressing a receptor for which the soluble receptor ligand is specific. Zetakine chimeric immunoreceptors represent a novel extension of antibody-based immunoreceptors for redirecting the antigen specificity of T cells, with application to treatment of a variety of cancers, particularly via the autocrin / paracrine cytokine systems utilized by human maligancy. In a preferred embodiment is a glioma-specific immunoreceptor comprising the extracellular targeting domain of the IL-13Rα2-specific IL-13 mutant IL-13(E13Y) linked to the Fc region of IgG, the transmembrane domain of human CD4, and the human CD3 zeta chain.
Owner:CITY OF HOPE
Dimerized polypeptide fusions
InactiveUS6291646B1Mass productionImprove expression levelPeptide/protein ingredientsAntibody mimetics/scaffoldsExtracellular StructureA-DNA
Methods for producing secreted receptor analogs and biologically active peptide dimers are disclosed. The methods for producing secreted receptor analogs and biologically active peptide dimers utilize a DNA sequence encoding a receptor analog or a peptide requiring dimerization for biological activity joined to a dimerizing protein. The receptor analog includes a ligand-binding domain. Polypeptides comprising essentially the extracellular domain of a human PDGF receptor fused to dimerizing proteins, the portion being capable of binding human PDGF or an isoform thereof, are also disclosed. The polypeptides may be used within methods for determining the presence of and for purifying human PDGF or isoforms thereof.
Owner:ZYMOGENETICS INC
Monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen
InactiveUS6962981B1VirusesCell receptors/surface-antigens/surface-determinantsImmunoenzymatic assayAntigen
The present invention relates to monoclonal antibodies that bind to the extracellular domain of prostate-specific membrane antigen (PSMA), hybridoma cell lines producing the antibodies, and methods of using such antibodies for diagnosis and treatment of cancer. In particular, thirty-five monoclonal antibodies reactive with PSMA expressed on the cell surface are exemplified. Additionally, the present invention relates to a novel protein variant (PSM′) of the PSMA detected by a number of the antibodies of the invention. The hydrolase activity of PSMA and PSM′ allows the use of an immunoenzymatic assay for their detection.
Owner:ER SQUIBB & SONS INC
Chimeric receptors and uses thereof in immune therapy
ActiveUS20150139943A1Good curative effectEnhanced ADCC activityVirusesPeptide/protein ingredientsCytotoxicityCD8
Owner:COGENT BIOSCIENCES INC +2
Chimeric antigen receptor
ActiveUS20140242701A1High expressionHigh cytotoxic activityAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenAntigen receptor
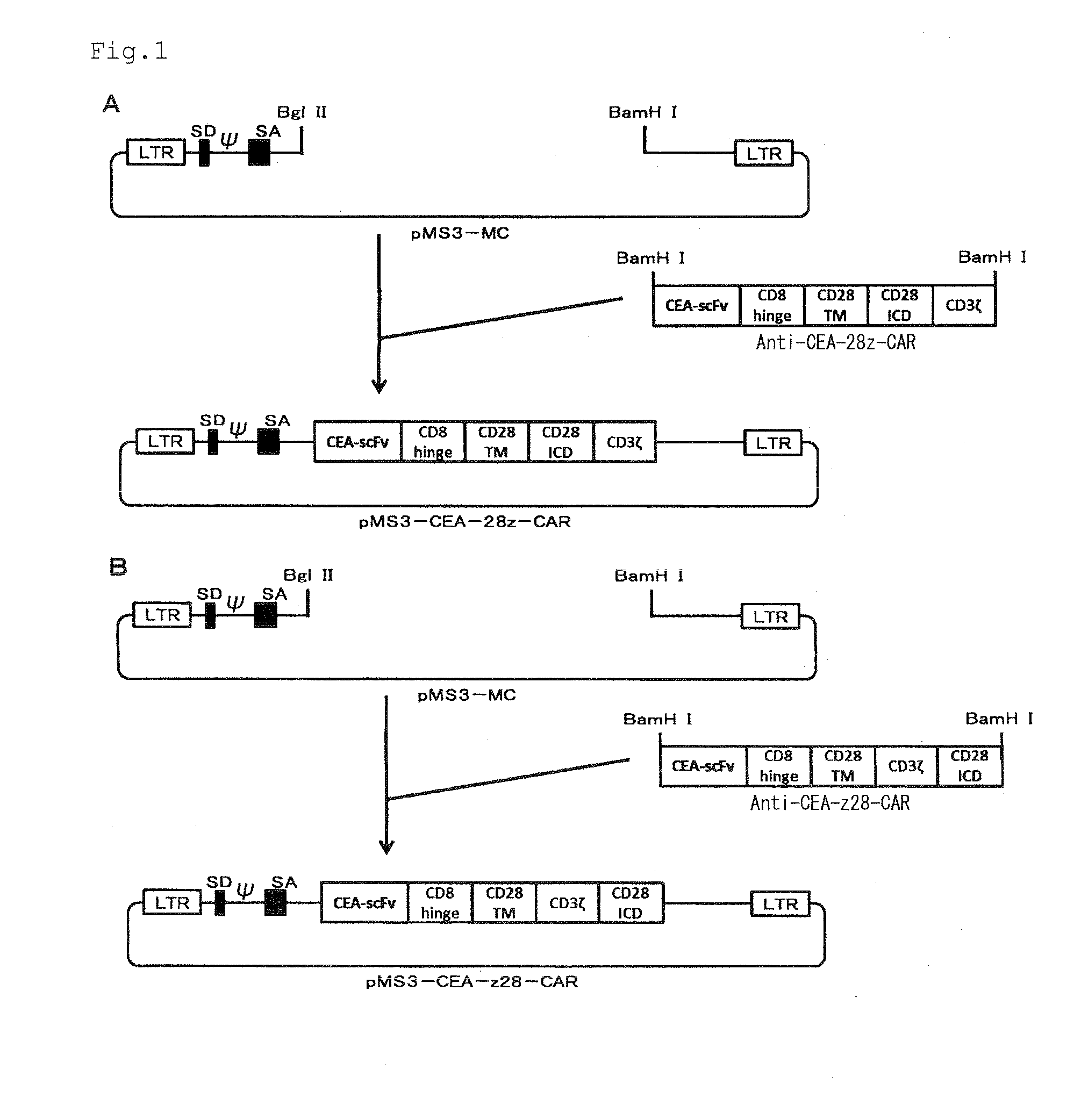
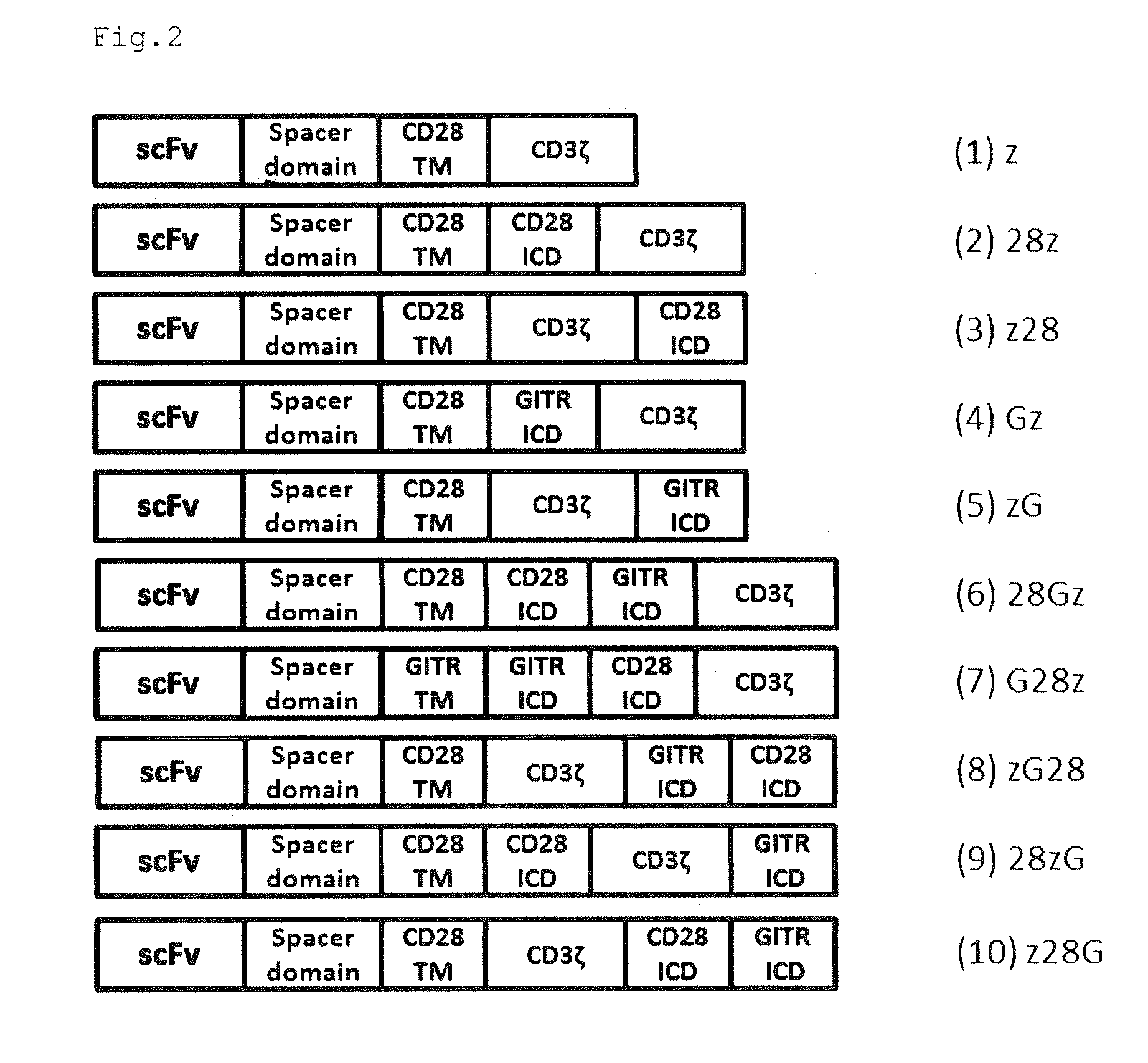
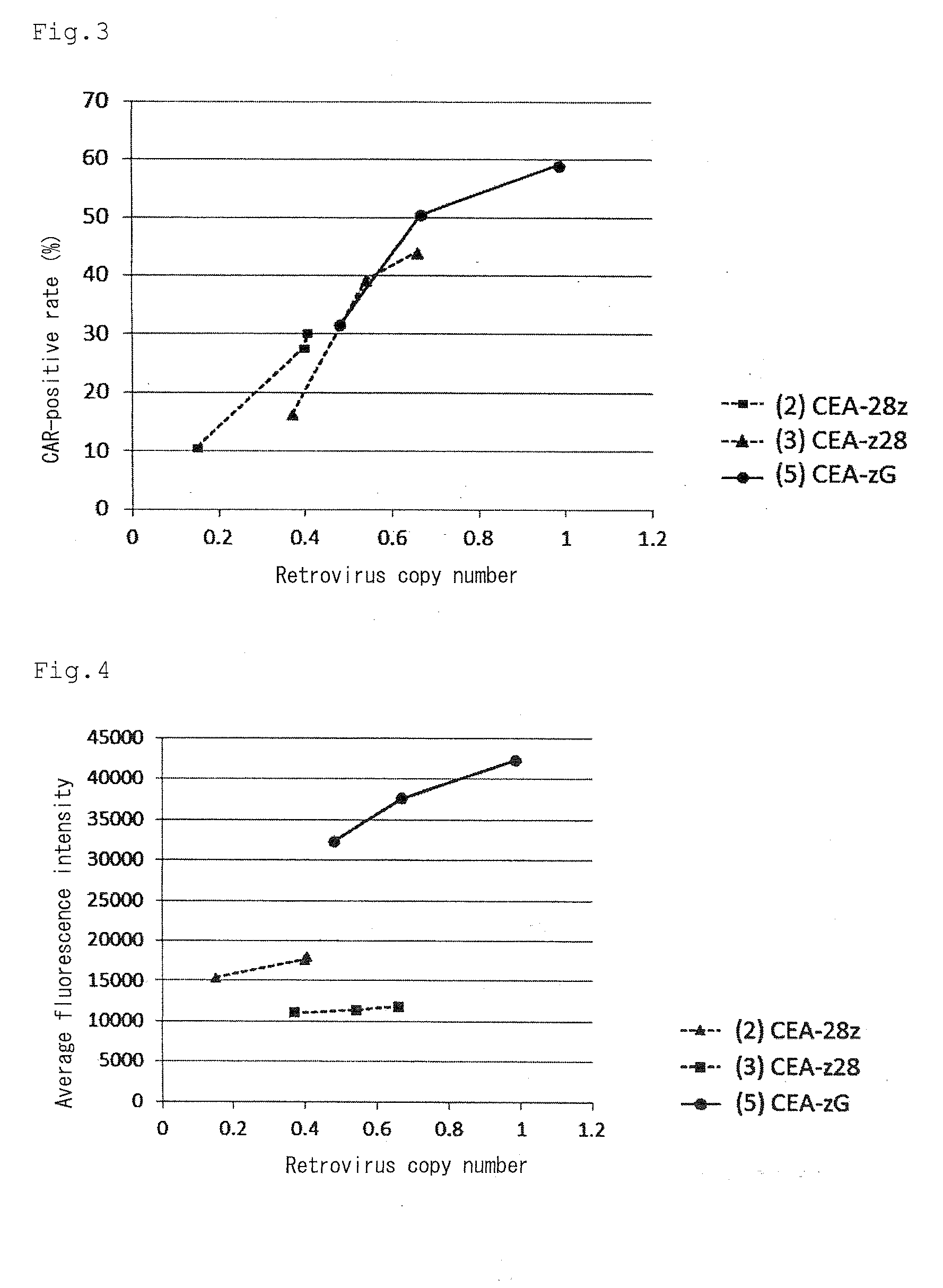
Provided are a chimeric antigen receptor comprising an extracellular domain capable of binding to an antigen, a transmembrane domain and at least one intracellular domain, the chimeric antigen receptor being characterized in that an intracellular domain of a glucocorticoid-induced tumor necrosis factor receptor (GITR) is contained as the intracellular domain; a nucleic acid encoding the chimeric antigen receptor; a cell expressing the chimeric antigen receptor; and a method for producing the cell.
Owner:MIE UNIVERSITY
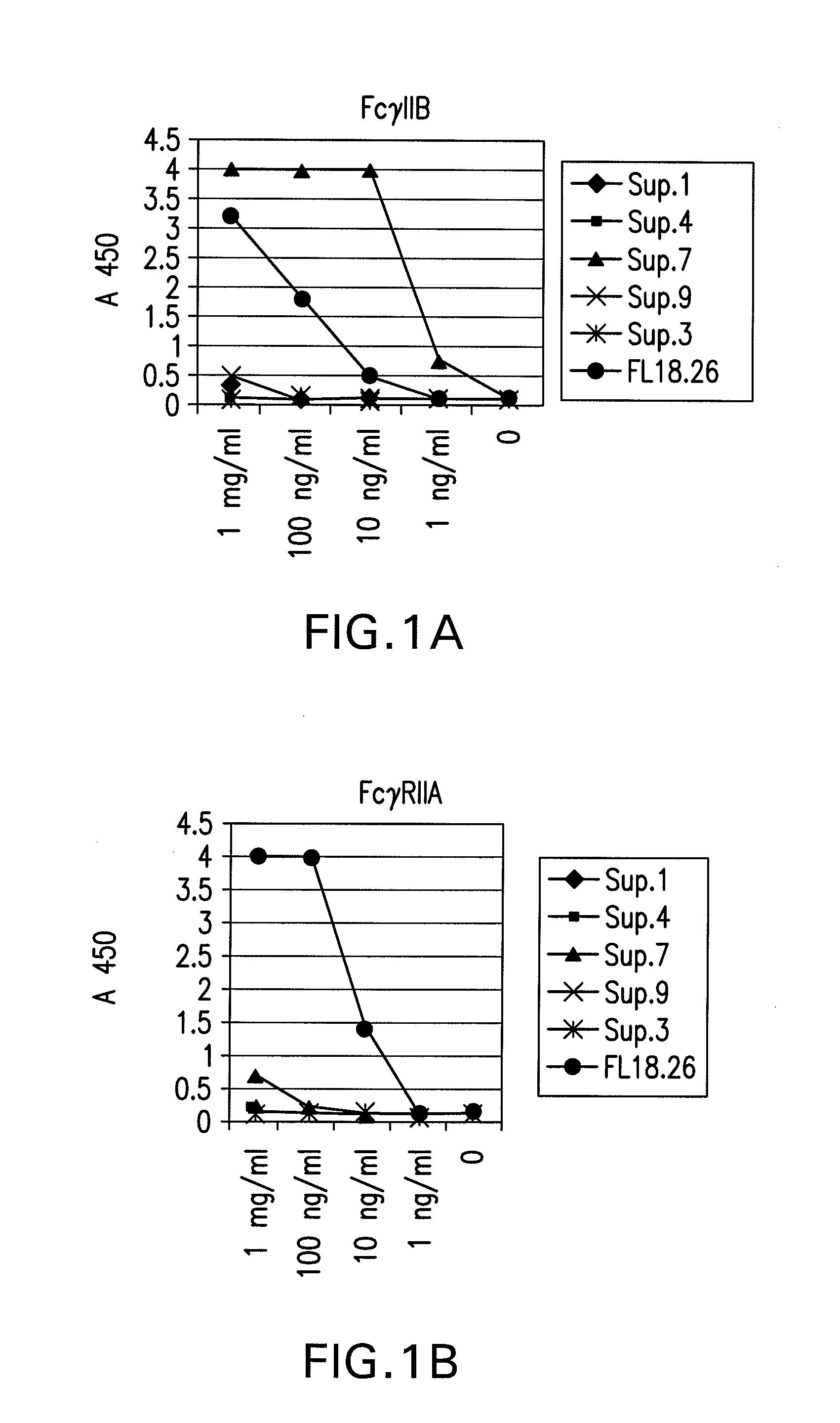
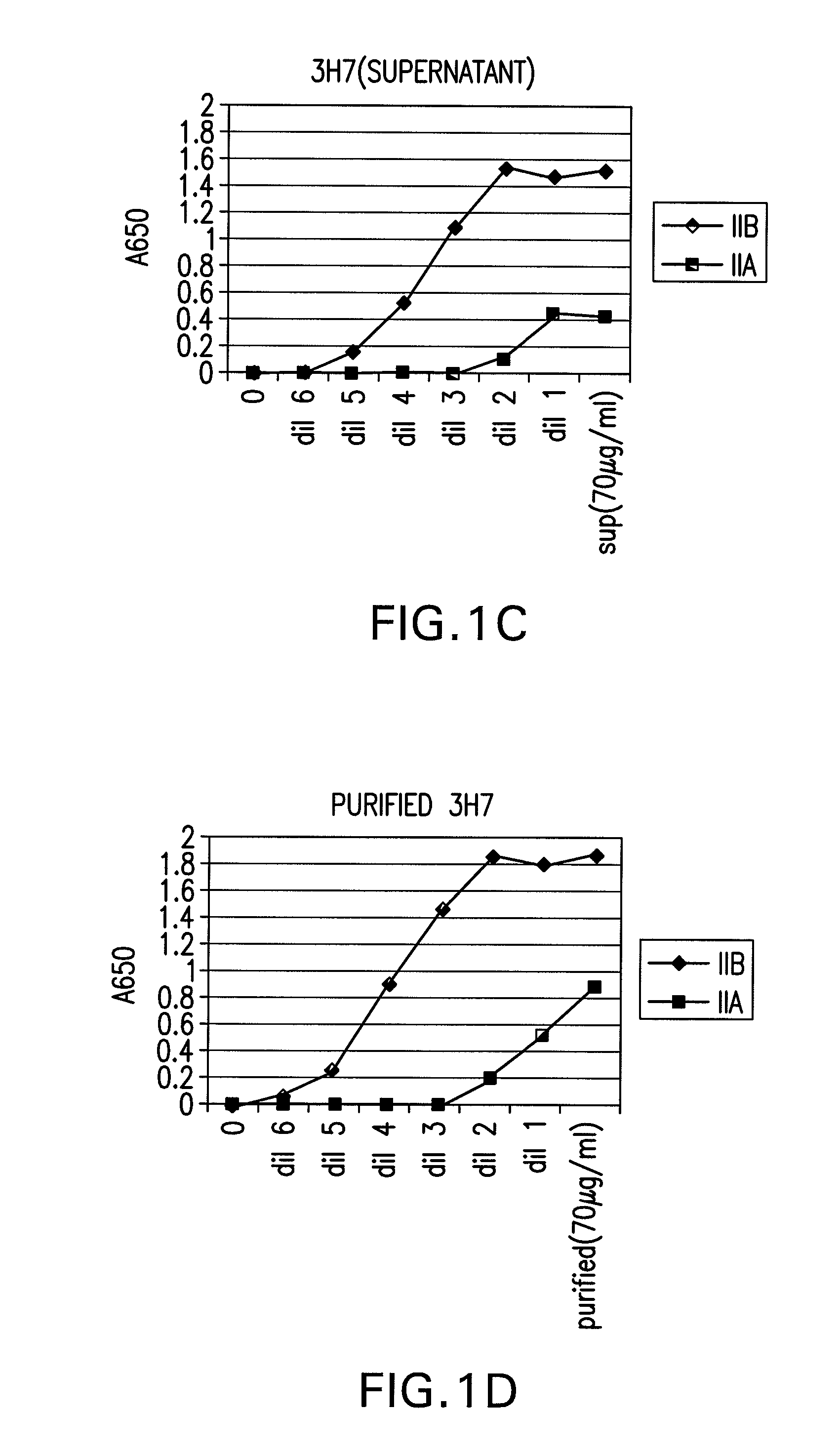
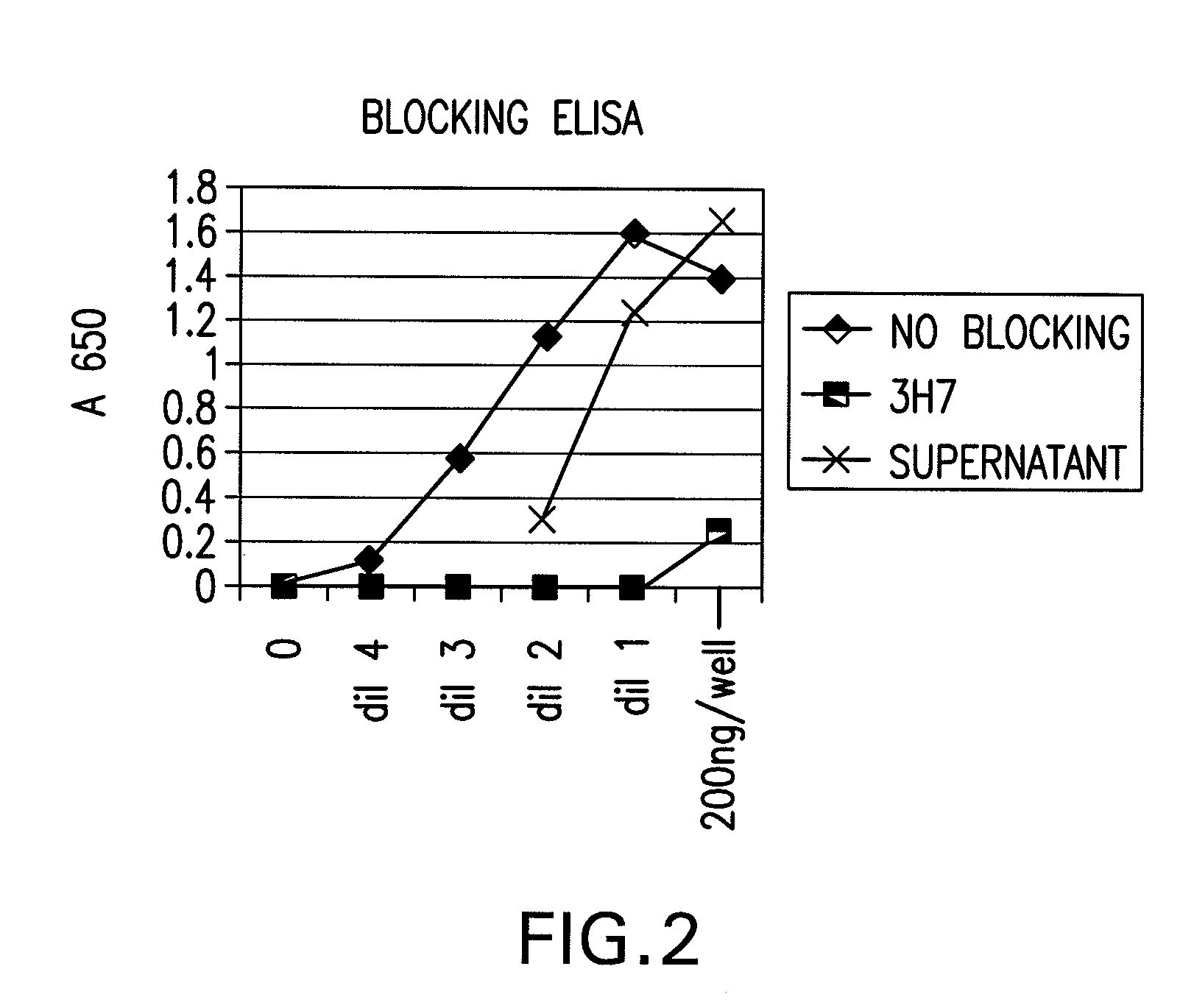
FcgammaRIIB-specific antibodies and methods of use thereof
InactiveUS20060177439A1Good curative effectConvenient treatmentImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCell activationImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind the extracellular domain of FcγRIIB, particularly human FcγRIIB, and block the Fc binding site of human FcγRIIB. The invention provides methods of treating cancer and / or regulating immune complex mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
Compositions and methods for diagnosing and treating cancer
The present invention relates to compositions and methods for characterizing, diagnosing, and treating cancer. In particular the invention provides the means and methods for the diagnosis, characterization, prognosis and treatment of cancer and specifically targeting cancer stem cells. The present invention provides an antibody that specifically binds to a non-ligand binding region of the extracellular domain of a human NOTCH receptor and inhibits growth of tumor cells. The present invention further provides a method of treating cancer, the method comprising administering a therapeutically effective amount of an antibody that specifically binds to a non-ligand binding region of the extracellular domain of a human NOTCH receptor protein and inhibits growth of tumor cells.
Owner:MEREO BIOPHARMA 5 INC
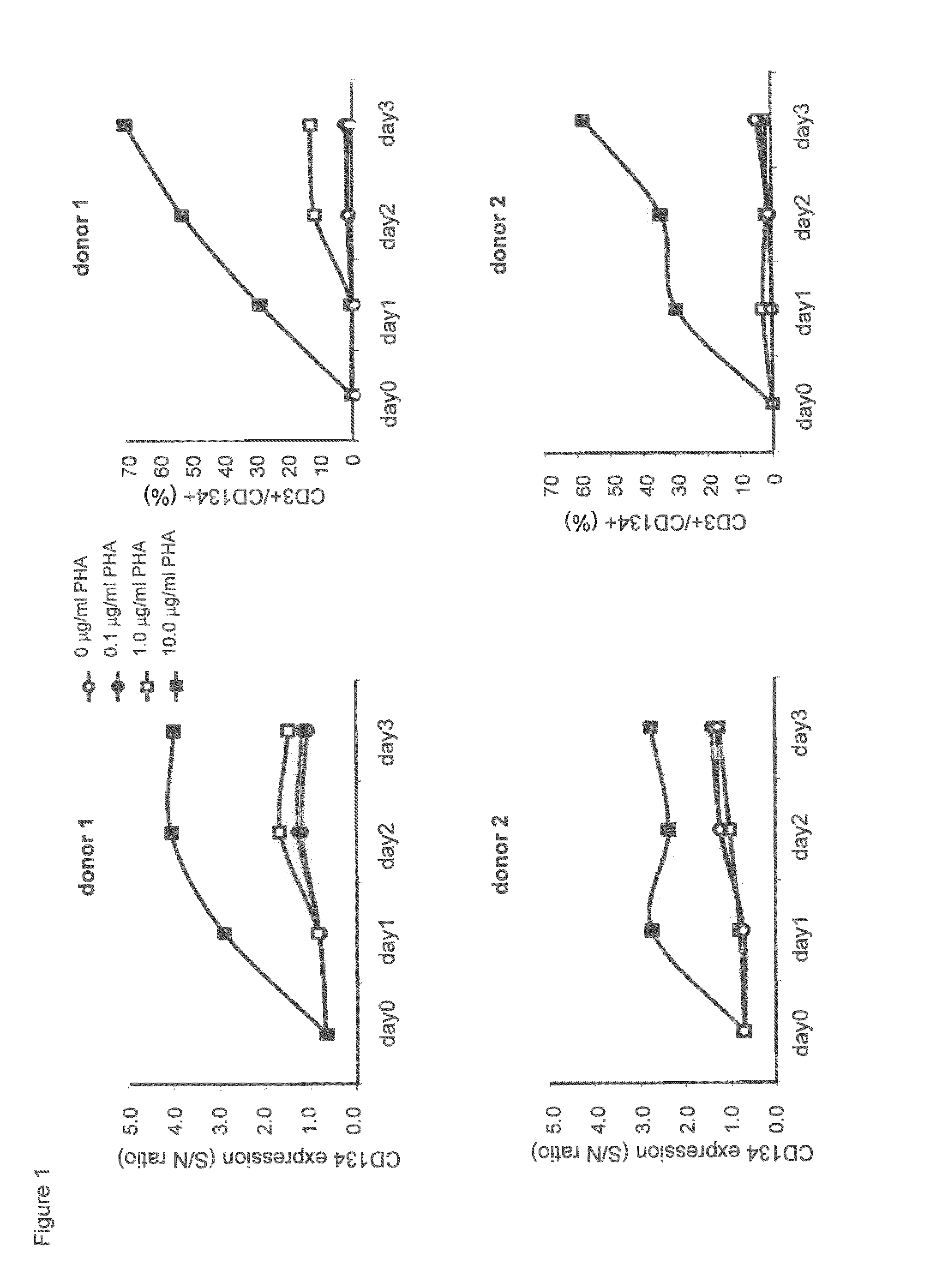
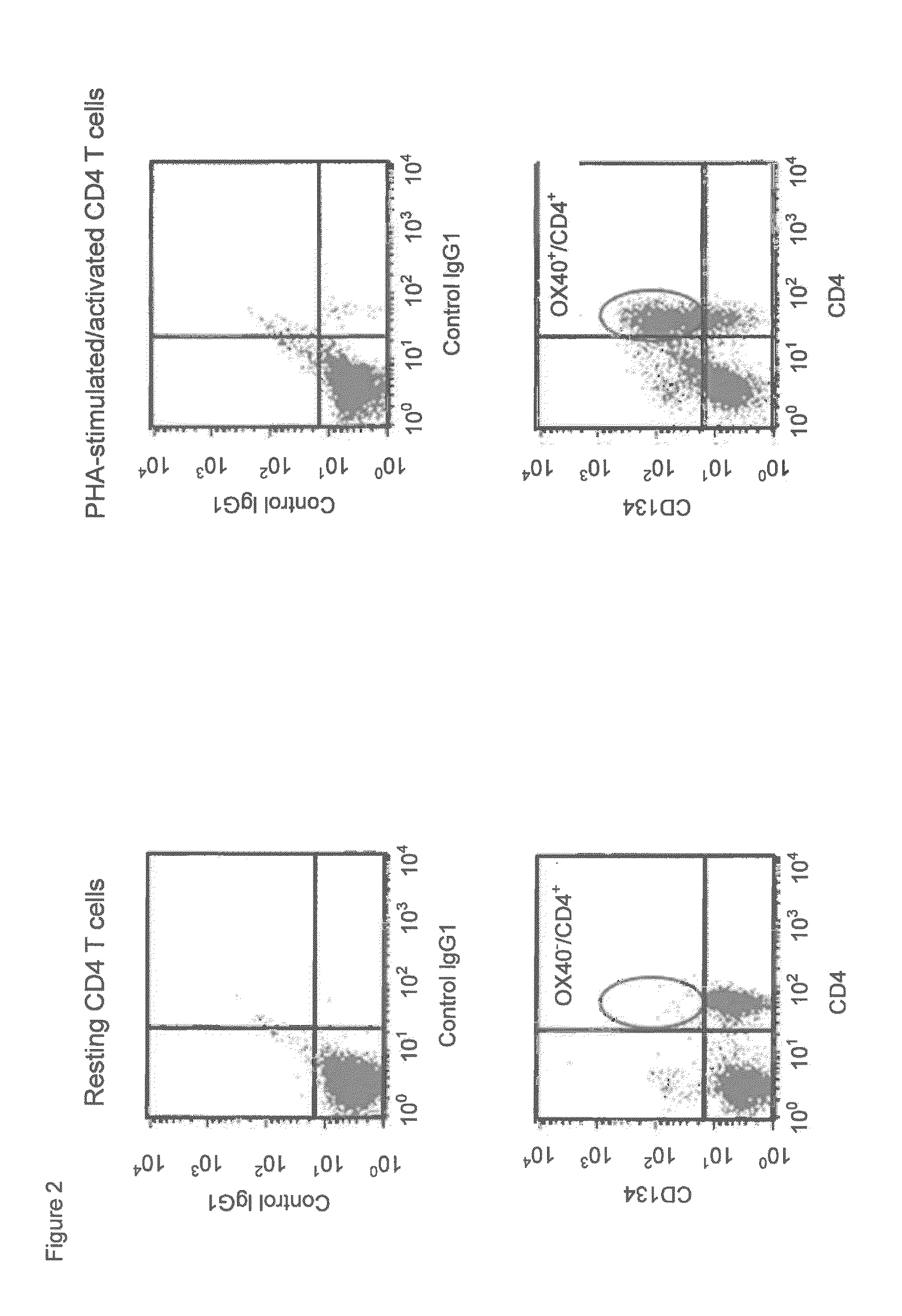
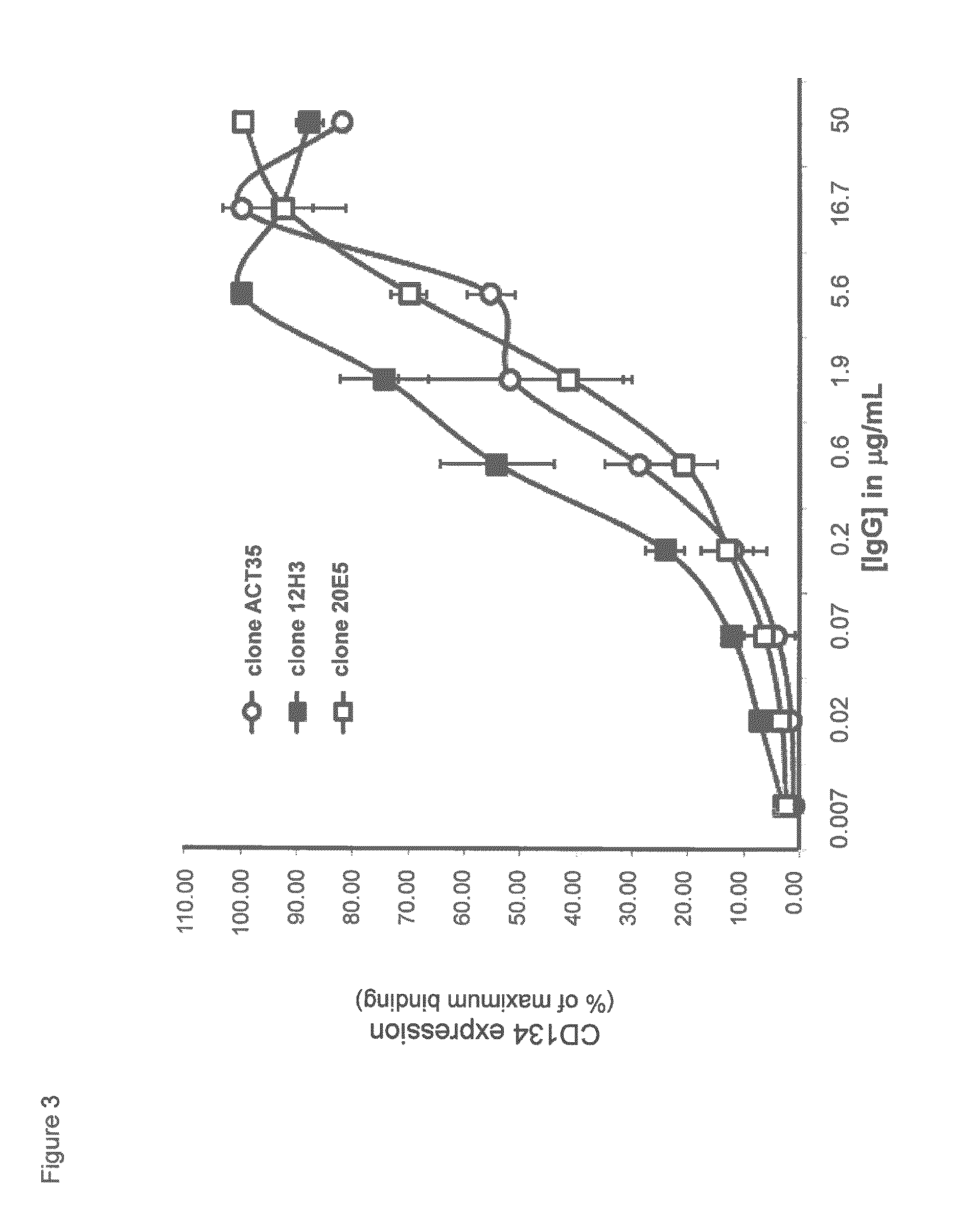
Humanized Anti-cd134 (OX40) antibodies and uses thereof
The invention provides antibodies that specifically bind to human CD134. Anti-human CD134 antibodies specifically bind to the extracellular domain of human CD134, including non-OX40 ligand (OX40L) binding domains on human CD134, which is expressed on e.g. activated human conventional effector CD4 and / or CD8 T lymphocytes (Teffs) and on activated human suppressive regulatory CD4 T lymphocytes (Tregs). Humanized anti-human CD134 antibodies are useful (e.g. to empower Teffs anti-cancer effector function and / or to inhibit Tregs suppressive function) for cancer treatment.
Owner:JANSSEN PHARMA INC
Recombinant antibody and antibody fragment
InactiveUS6989145B2Reduce in quantityHigh cytotoxic activitySenses disorderAntibody mimetics/scaffoldsDiseaseDiagnostic agent
A recombinant antibody or the antibody fragment thereof which specifically reacts with an extracellular domain of human CCR4; a DNA which encodes the recombinant antibody or the antibody fragment thereof; a method for producing the recombinant antibody or the antibody fragment thereof; a method for immunologically detecting CCR4, a method for immunologically detecting a cell which expressed CCR4 on the cell surface, a method for depleting a cell which expresses CCR4 on the cell surface, and a method for inhibiting production of Th2 cytokine, which comprise using the recombinant antibody according or antibody fragment thereof; a therapeutic or diagnostic agent for Th2-mediated immune diseases; and a therapeutic or diagnostic agent for a blood cancer.
Owner:KYOWA HAKKO KIRIN CO LTD
Modified antibodies to prostate-specific membrane antigen and uses thereof
InactiveUS7045605B2Less immunogenicHigh affinityNervous disorderHybrid cell preparationAntigen Binding FragmentAntigen binding
Modified antibodies, or antigen-binding fragments thereof, to the extracellular domain of human prostate specific membrane antigen (PSMA) are provided. The modified anti-PSMA antibodies, or antigen-binding fragments thereof, have been rendered less immunogenic compared to their unmodified counterparts to a given species, e.g., a human. Pharmaceutical compositions including the aforesaid antibodies, nucleic acids, recombinant expression vectors and host cells for making such antibodies and fragments are also disclosed. Methods of using the antibodies of the invention to detect human PSMA, or to ablate or kill a PSMA-expressing cell, e.g., a PSMA-expressing cancer or prostatic cell, either in vitro or in vivo, are also provided.
Owner:CORNELL RES FOUNDATION INC
Treatment and diagnosis of prostate cancer
InactiveUS7666425B1Effective diagnosisEffective treatmentPeptide/protein ingredientsMicrobiological testing/measurementAntigenProstatic epithelium
The present invention is directed to the use of antibodies or binding portions thereof, probes, ligands, or other biological agents which either recognize an extracellular domain of prostate specific membrane antigen or bind to and are internalized with prostate specific membrane antigen. These biological agents can be labeled and used for detection of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof. They also can be used alone or bound to a substance effective to ablate or kill such cells as a therapy for prostate cancer. Also disclosed are four hybridoma cell lines, each of which produces a monoclonal antibody recognizing extracellular domains of prostate specific membrane antigens of normal, benign hyperplastic, and cancerous prostate epithelial cells or portions thereof.
Owner:CORNELL RES FOUNDATION INC
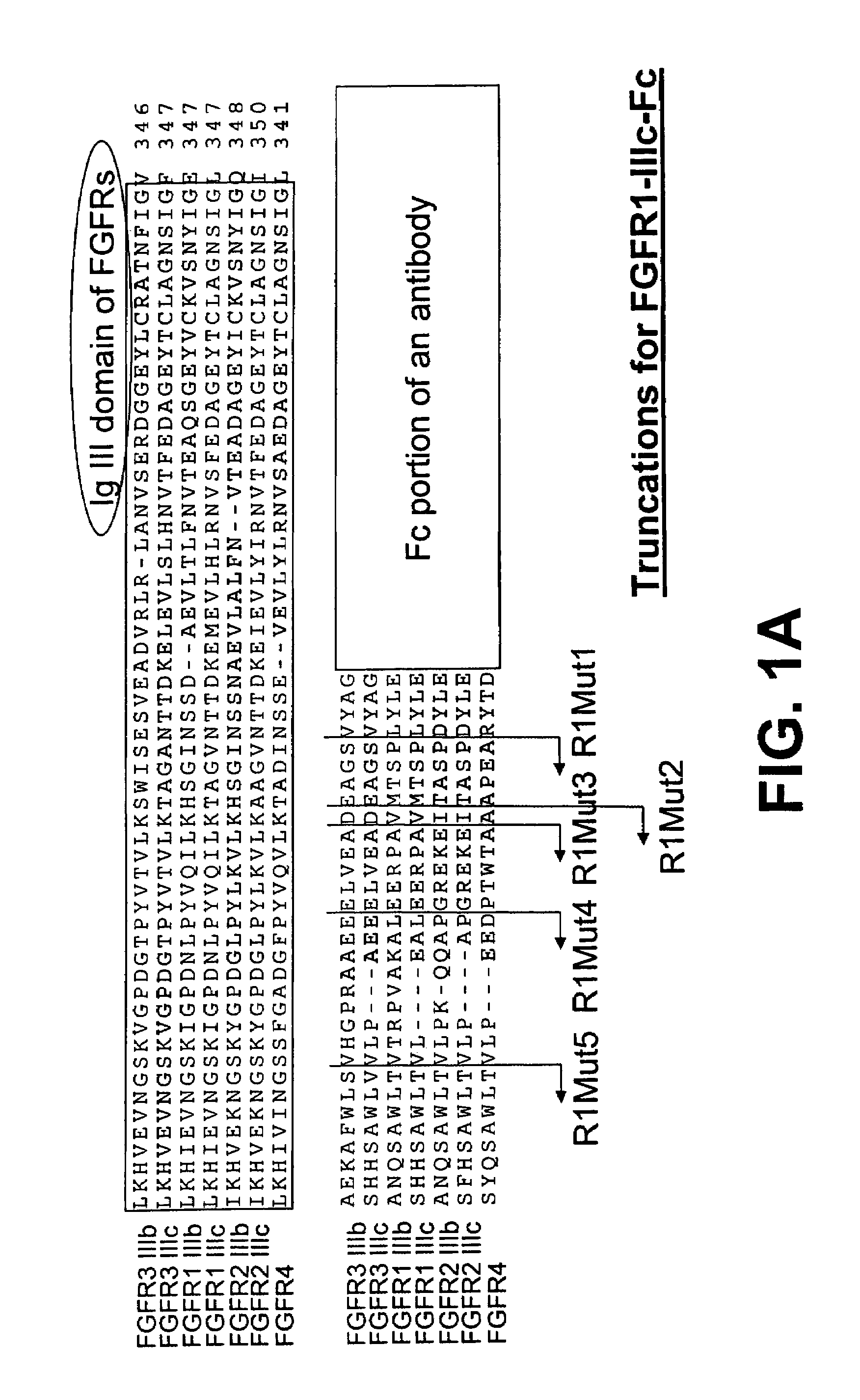
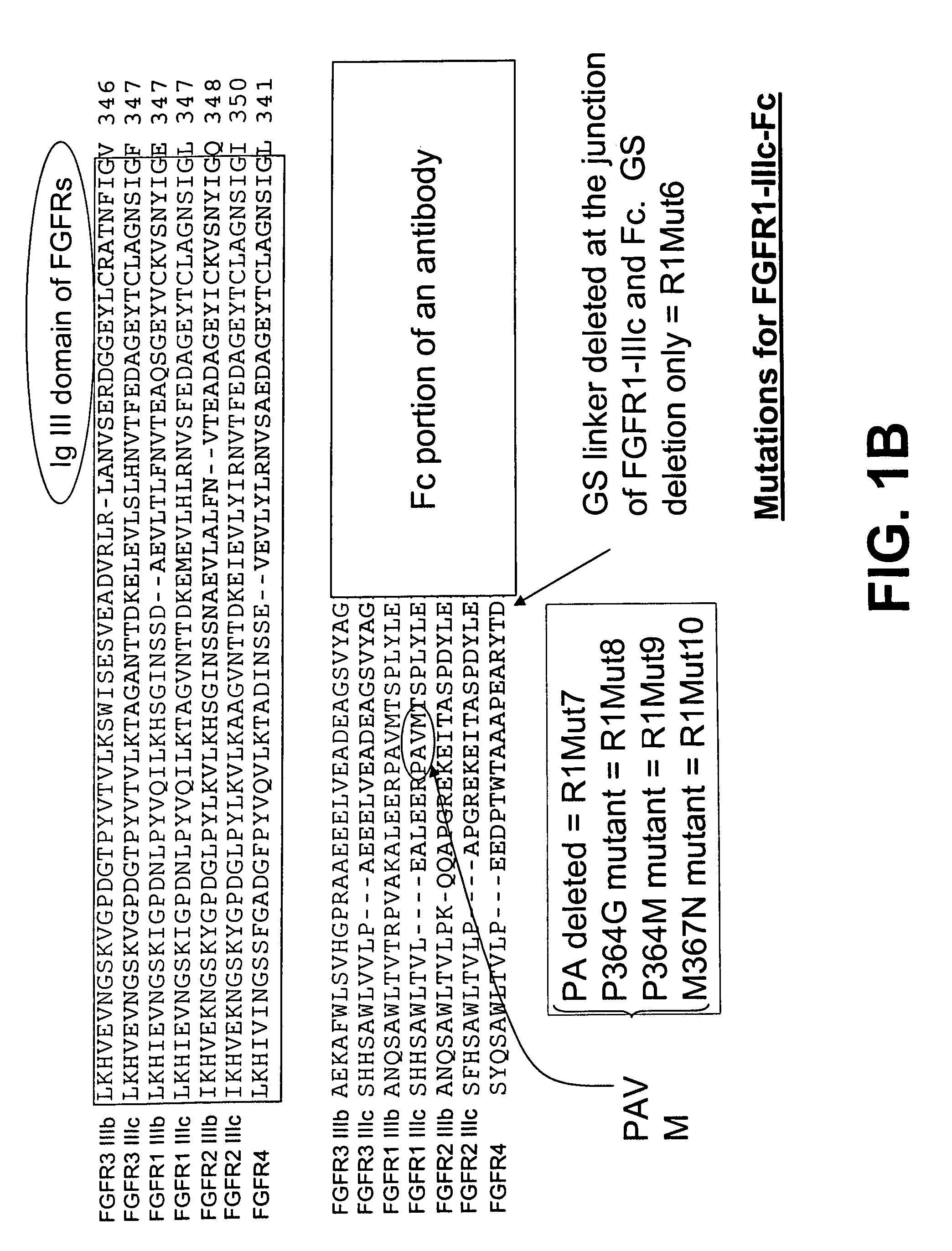
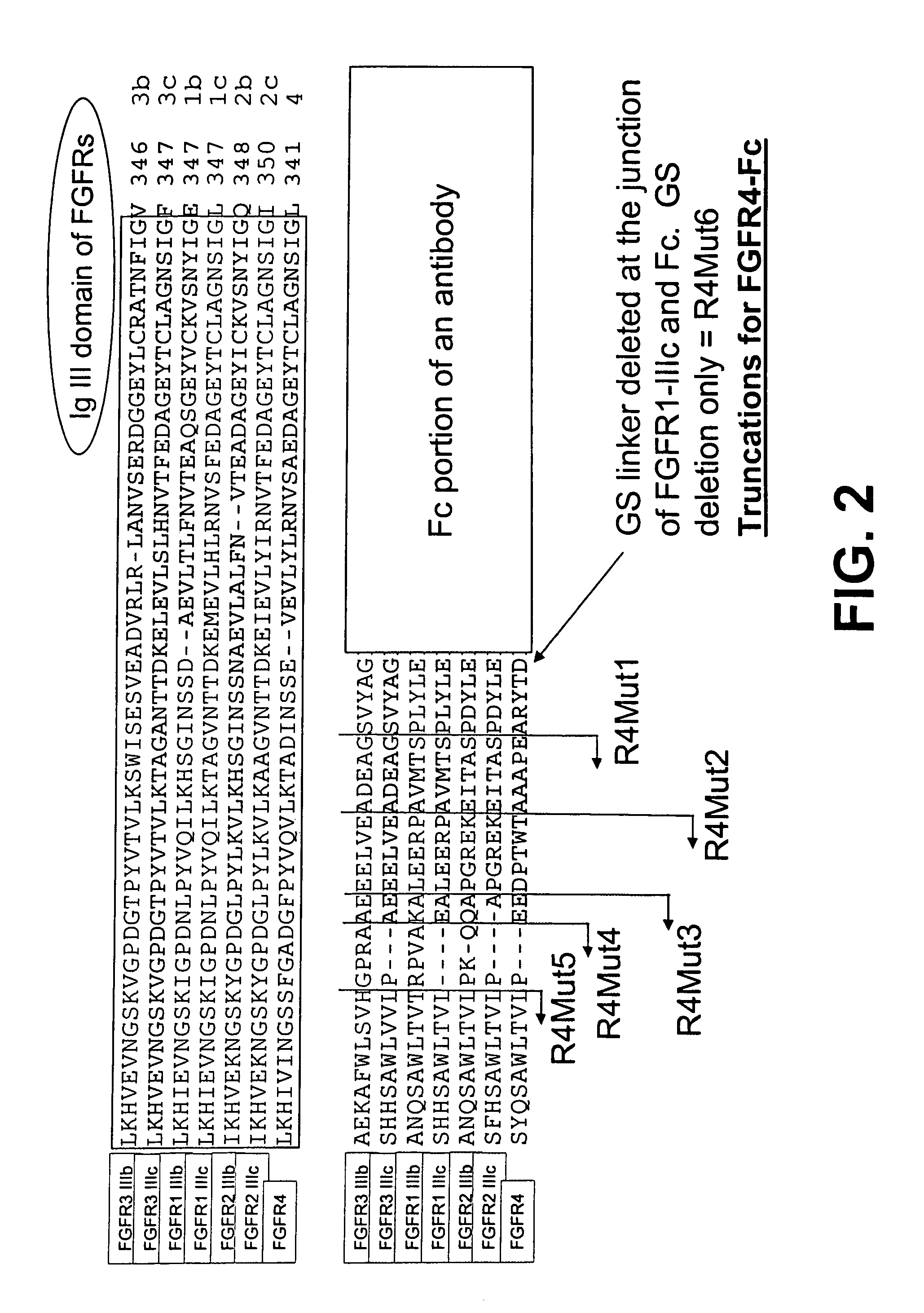
Compositions and methods of treating disease with FGFR fusion proteins
The invention provides FGFR fusion proteins, methods of making them, and methods of using them to treat proliferative disorders, including cancers and disorders of angiogenesis. The FGFR fusion molecules can be made in CHO cells and may comprise deletion mutations in the extracellular domains of the FGFRs which improve their stability. These fusion proteins inhibit the growth and viability of cancer cells in vitro and in vivo. The combination of the relatively high affinity of these receptors for their ligand FGFs and the demonstrated ability of these decoy receptors to inhibit tumor growth is an indication of the clinical value of the compositions and methods provided herein.
Owner:FIVE PRIME THERAPEUTICS
Treatment and diagnosis of cancer
InactiveUS7163680B2Effective diagnosisEffective treatmentBiological material analysisNanomedicineAntigenVascular endothelium
Owner:CORNELL RES FOUNDATION INC
Methods of treating prostate cancer with anti-prostate specific membrane antigen antibodies
ActiveUS7514078B2Relieve painReduce needSugar derivativesHybrid cell preparationAntigen Binding FragmentAnti-PSMA Antibody
Modified antibodies, or antigen-binding fragments thereof, to the extracellular domain of human prostate specific membrane antigen (PSMA) are provided. The modified anti-PSMA antibodies, or antigen-binding fragments thereof, have been rendered less immunogenic compared to their unmodified counterparts to a given species, e.g., a human. Pharmaceutical compositions including the aforesaid antibodies, nucleic acids, recombinant expression vectors and host cells for making such antibodies and fragments are also disclosed. Methods of using the antibodies of the invention to detect human PSMA, or to ablate or kill a PSMA-expressing cell, e.g., a PSMA-expressing cancer or prostatic cell, either in vitro or in vivo, are also provided.
Owner:CORNELL RES FOUNDATION INC
Anti-cd134 (OX40) antibodies and uses thereof
InactiveUS20150132288A1Enhance immune responseEnhancing immunostimulator/effector function of T-effectorLibrary screeningImmunoglobulins against cell receptors/antigens/surface-determinantsInhibitory modulationOX40 ligand
The invention provides antibodies that specifically bind to human CD134. Invention anti-human CD134 antibodies specifically bind to the extracellular domain of human CD134, including non-OX40 ligand (OX40L) binding domains on human CD134, which is expressed on e.g. activated human conventional effector CD4 and / or CD8 T lymphocytes (Teffs) and on activated human suppressive regulatory CD4 T lymphocytes (Tregs). Invention anti-human CD134 antibodies are useful (e.g. to empower Teffs anti-cancer effector function and / or to inhibit Tregs suppressive function) for cancer treatment.
Owner:JANSSEN PHARMA INC
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090074771A1Strong therapeutic activityEnhancing antibody-mediated effector functionAntibody ingredientsImmunoglobulinsTolerabilityImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
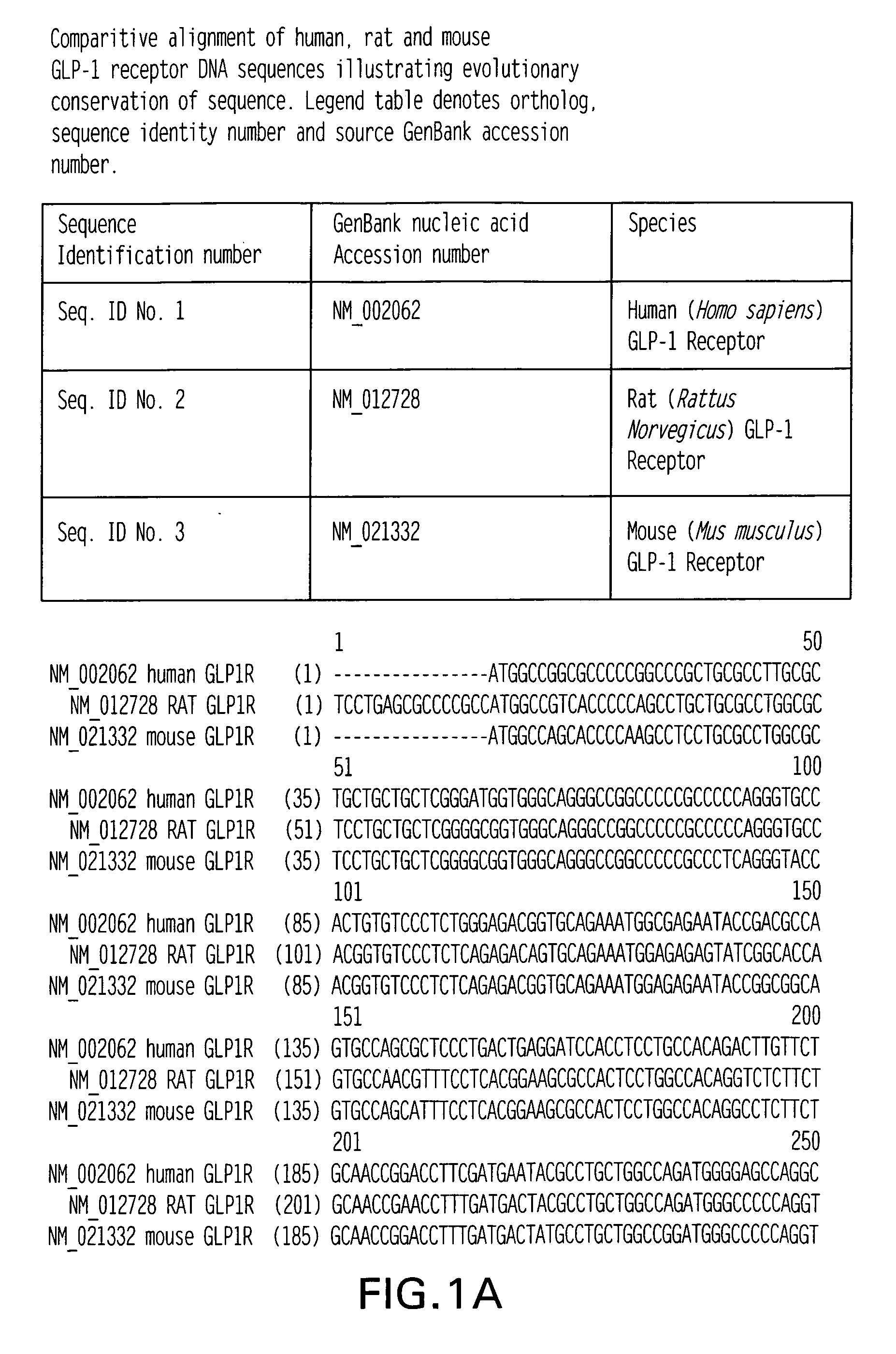
GLP-1 receptor agonist and allosteric modulator monoclonal antibodies and uses thereof
InactiveUS20060275288A1Induction of insulin secretionSuppression of glucagon releaseImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiabetes mellitusAllosteric modulator
The subject invention relates to monoclonal antibodies that have the ability to bolster the function of the GLP-1 receptor and may therefore have utility in the treatment of mammalian metabolic disorders such as, for example, diabetes. In particular, the invention describes the generation of fully human monoclonal antibodies made against extracellular domains of the human GLP-1 receptor which are capable of binding the intact receptor and activating it in a manner similar to the native ligand. Additionally, the invention describes methods used to generate and develop allosteric modulator antibodies of the human GLP-1 receptor with potential therapeutic uses.
Owner:ABBOTT LAB INC
Compositions and methods for inhibiting Wnt-dependent solid tumor cell growth
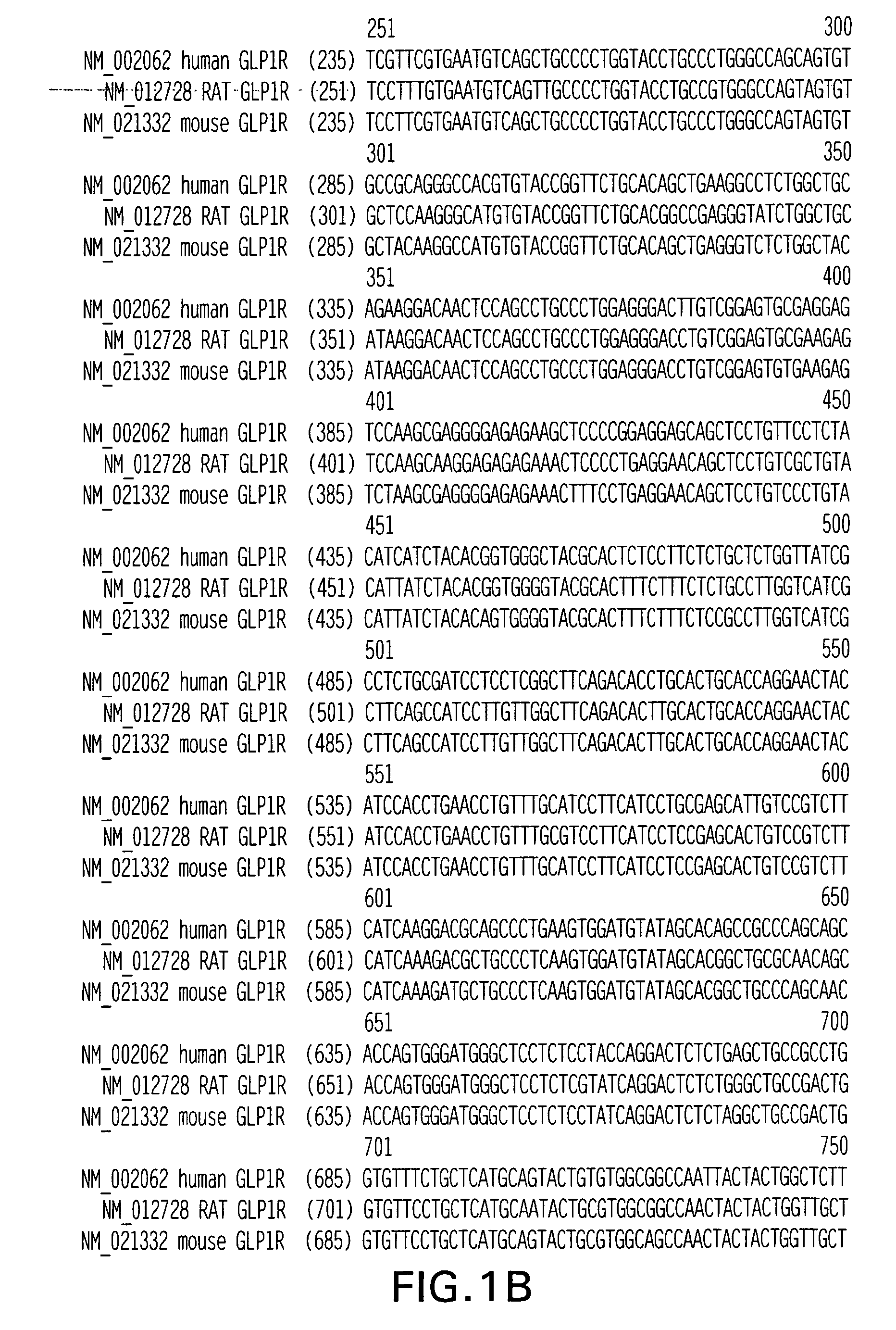
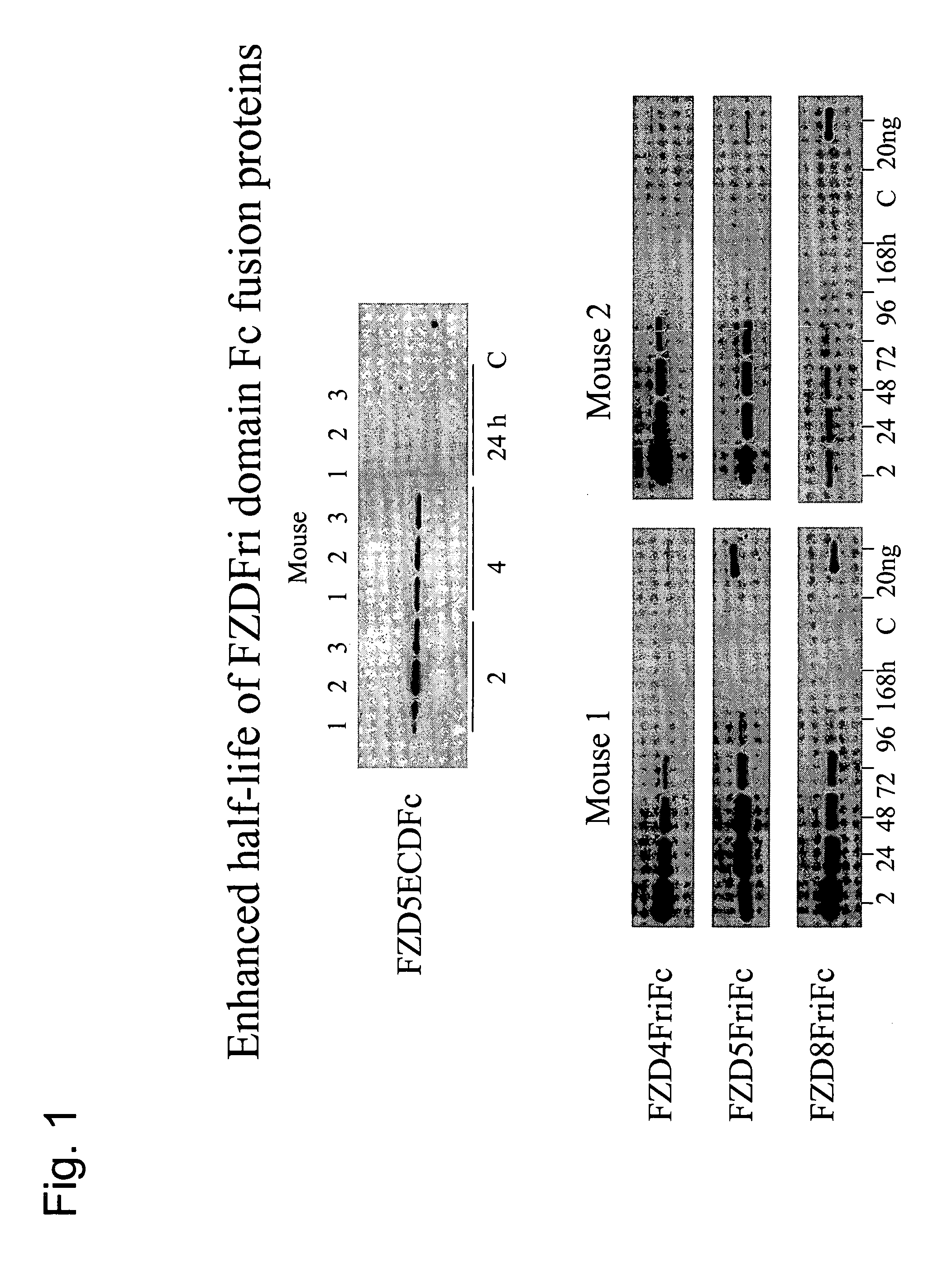
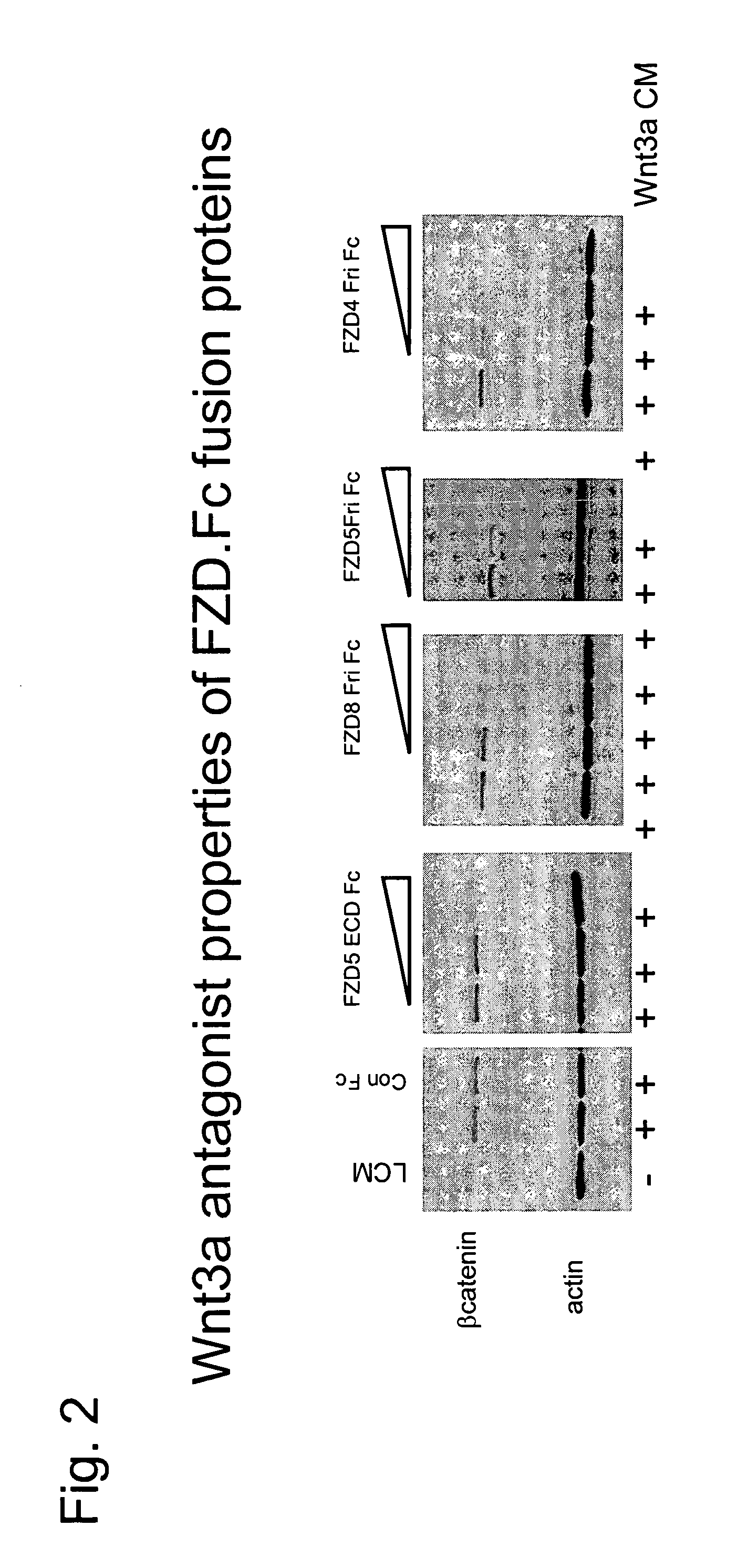
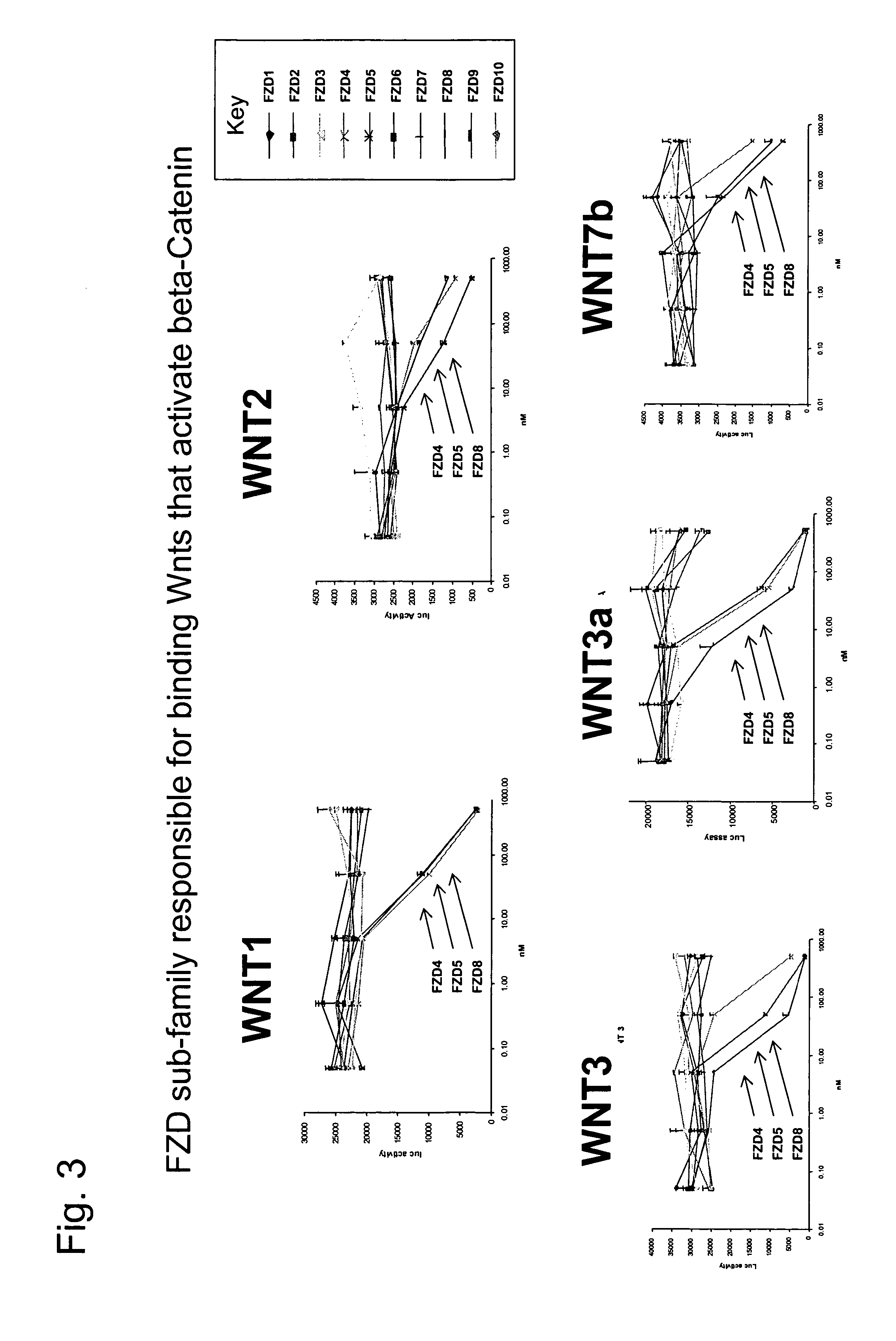
ActiveUS7723477B2Peptide/protein ingredientsAntibody mimetics/scaffoldsReceptorExtracellular Structure
The present invention relates to compositions and methods for characterizing, diagnosing, and treating cancer. In particular the invention provides the means and methods for the diagnosis, characterization, prognosis and treatment of cancer and specifically targeting cancer stem cells. The present invention provides a soluble FZD receptor comprising an extracellular domain of a human FZD receptor that inhibits growth of tumor cells. The present invention still further provides a soluble receptor comprising a Fri domain of a human FZD receptor that binds a ligand of a human FZD receptor and said soluble receptor is capable of inhibiting tumor growth. The present invention still further provides a method of treating cancer comprising administering a soluble FZD receptor comprising for example, either an extracellular domain of a human FZD receptor or a Fri domain of a human FZD receptor, in an amount effective to inhibit tumor growth.
Owner:MEREO BIOPHARMA 5 INC
Notch-based fusion proteins and uses thereof
InactiveUS20060030694A1Inhibit angiogenesisPeptide/protein ingredientsAntibody mimetics/scaffoldsAbnormal tissue growthVector system
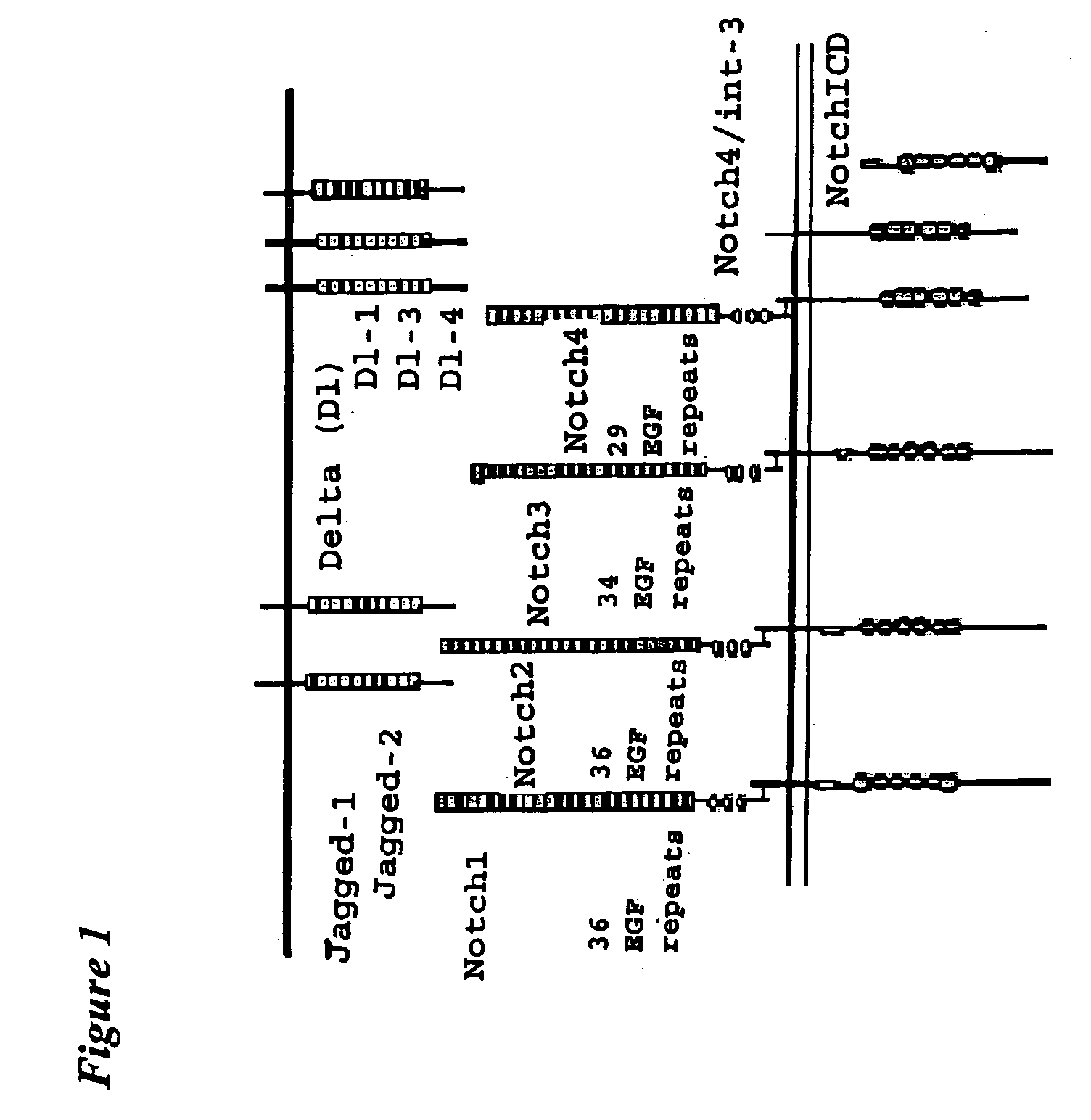
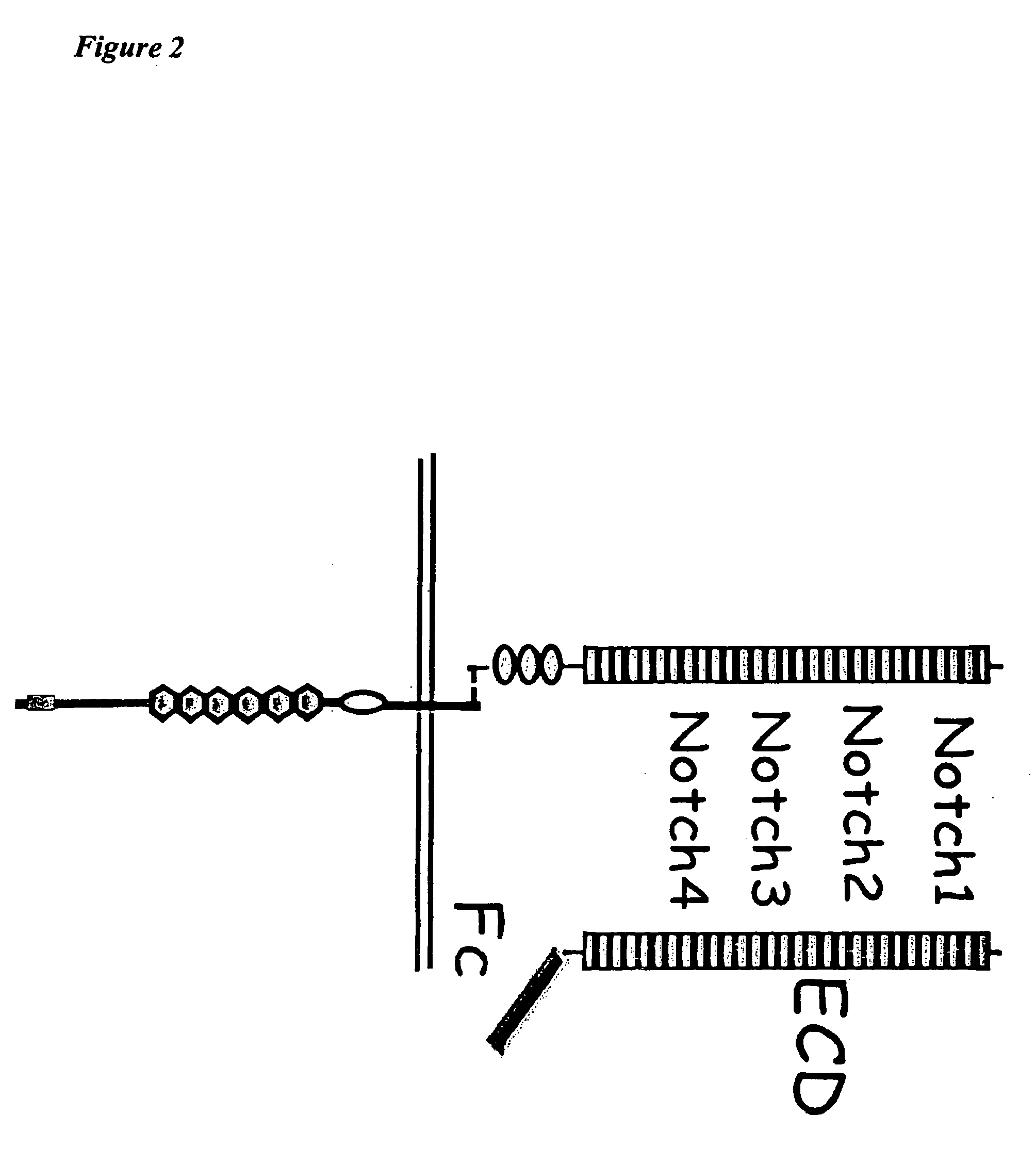
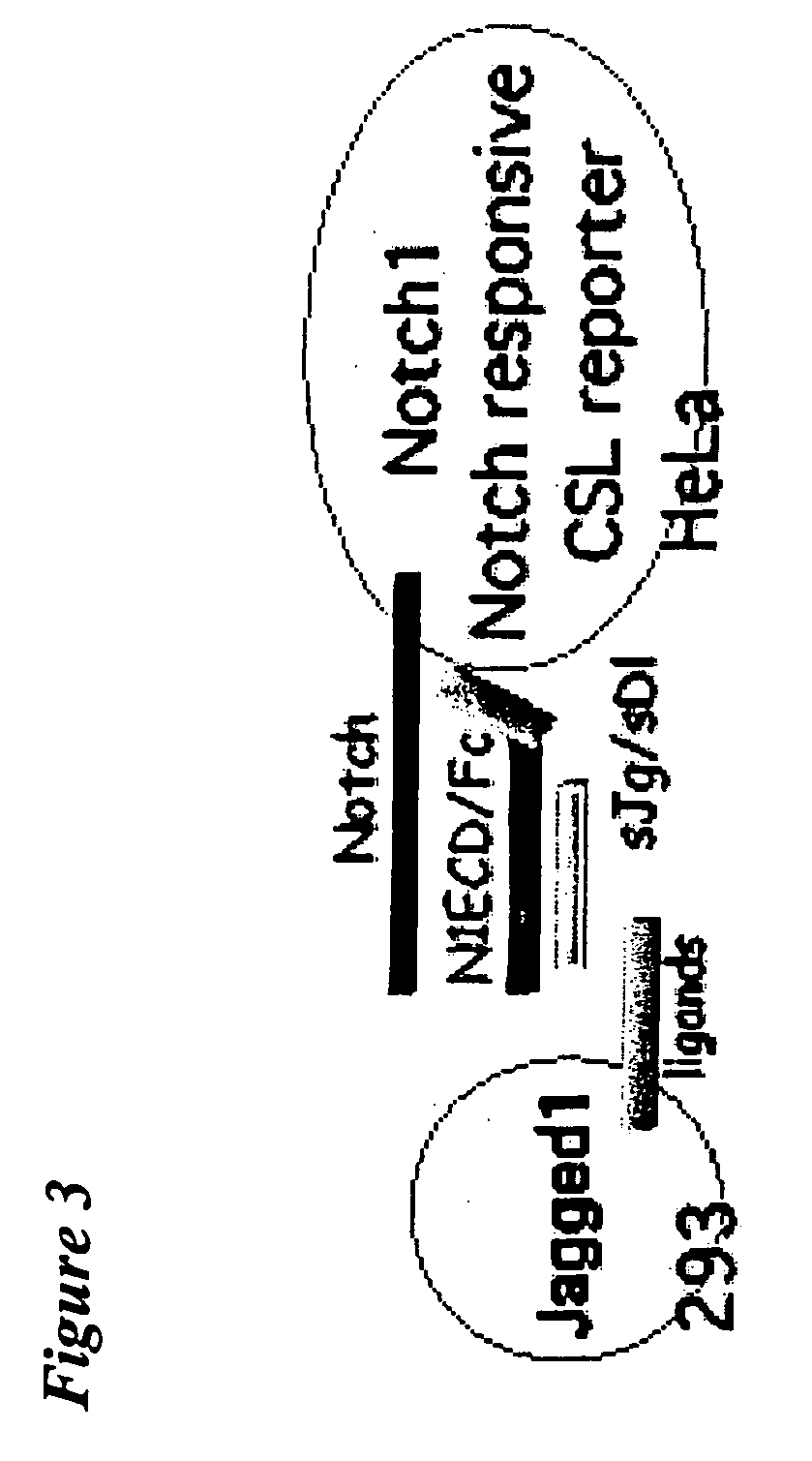
This invention provides a method for treating a subject having a tumor and a method for inhibiting angiogenesis in a subject, both comprising administering to the subject an effective amount of a composition of matter comprising the extracellular domain of a Notch receptor protein operably affixed to a half-life-increasing moiety. This invention also provides a composition of matter comprising the extracellular domain of Notch4 receptor protein operably affixed to a half-life-increasing moiety. This invention further provides an article of manufacture. Finally, this invention provides a replicable vector which encodes a polypeptide comprising the extracellular domain of a Notch receptor protein operably affixed to a half-life-increasing moiety, a host vector system which comprises such replicable vector and a method of producing such polypeptide.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
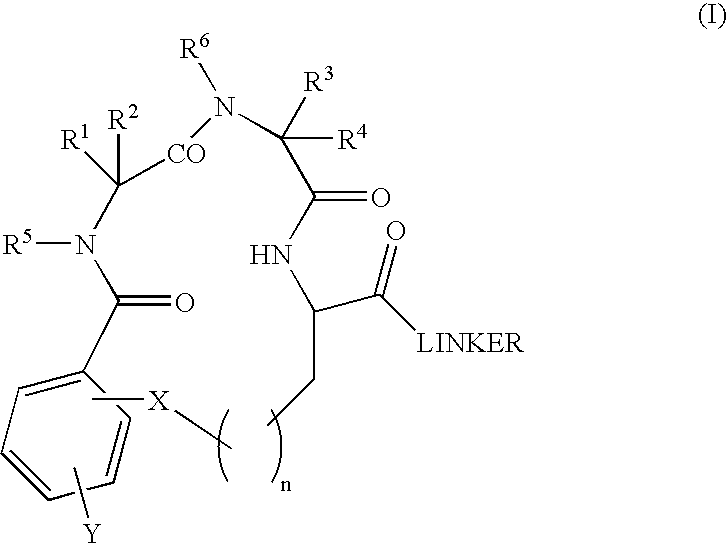
Β-turn peptidomimetic cyclic compounds
InactiveUS6881719B2Easy to manageReduce deliveryOrganic active ingredientsTetrapeptide ingredientsDiseaseNeurotrophin Receptor p75
Proteolytically stable small molecule β-turn peptidomimetic compounds have been identified as agonists or antagonists of neurotrophin receptors, such as TrkA. A compound of particular interest binds the immunoglobulin-like C2 region of the extracellular domain of TrkA, competes the binding of another TrkA ligand, affords selective trophic protection to TrkA-expressing cell lines and neuronal primary cultures, and induces the differentiation of primary neuronal cultures. The small β-turn peptidomimetic compounds of the invention can activate a tyrosine kinase neurotrophin receptor that normally binds a relatively large protein ligand. Such compounds that bind the extracellular domain of Trk receptors are useful pharmacological agents to address disorders where Trk receptors play a role, by targeting populations selectively.
Owner:TEXAS A&M UNIVERSITY +1
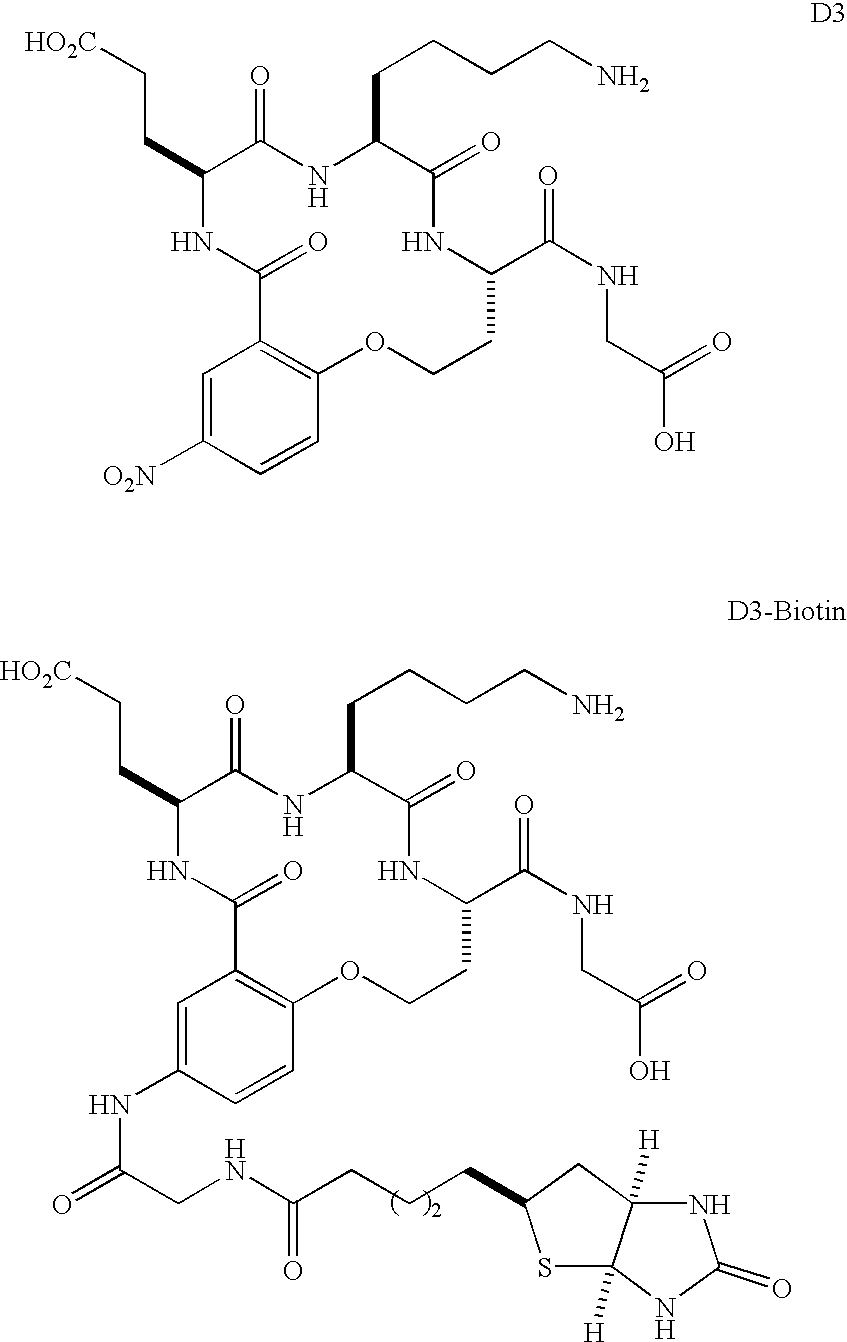
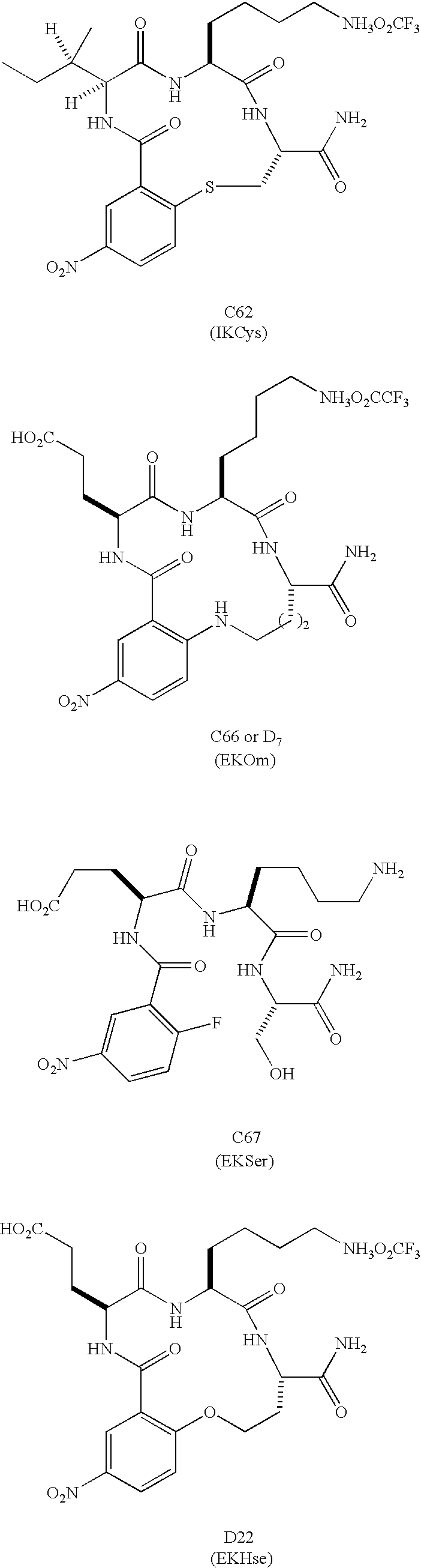
Soluble Form of Carbonic Anhydrase IX (s-CA IX), Assays to Detect s-CA IX, CA IX's Coexpression with HER-2/neu/c-erbB-2, and CA IX-Specific Monoclonal Antibodies to Non-Immunodominant Epitopes
InactiveUS20080176310A1Improve efficiencyIncrease resourcesOxidoreductasesFermentationKilodaltonWestern blot
Disclosed herein is the discovery of a soluble MN / CA IX (s-CA IX) in body fluids, such as, urine and serum. Said s-CA IX comprises the extracellular domain of CA IX or portions thereof. The predominant s-CA IX species is the extracellular domain comprising a proteoglycan-like (PG) domain and carbonic anhydrase (CA) domain, and having a molecular weight of about 50 / 54 kilodaltons (kd) upon Western blot. A smaller s-CA IX form of about 20 to about 30 kd comprising the CA domain or parts thereof, not linked to the PG domain, has also been found in body fluids. Diagnostic / prognostic methods for precancer and cancer that detect or detect and quantitate said s-CA IX in body fluids, are described. Also disclosed herein is the coexpression of CA IX and HER-2 / neu / c-erbB-2 that provides parallel, alternative and potentially synergistic diagnostic / prognostic and therapeutic strategies for precancer and cancer. Further disclosed are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, preferably monoclonal antibodies and immunoreactive fragments and engineered variants thereof. Such new MN / CA IX-specific antibodies, fragments and variants are useful diagnostically / prognostically and therapeutically for cancer and precancer. Particularly preferred are the new monoclonal antibodies, fragments and variants that are specific for the non-immunodominant epitopes of MN / CA IX, which antibodies are, among other uses, useful to detect soluble MN / CA IX (s-CA IX) in body fluids, alone but preferably in combination with antibodies specific to the immunodominant epitopes of MN / CA IX, for example, in a sandwich assay.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
Soluble Form of Carbonic Anhydrase IX (s-CA IX), Assays to Detect s-CA IX, CA IX's Coexpression with Her-2/neu/c-erbB-2, and CA IX-Specific Monoclonal Antibodies to Non-Immunodominant Epitopes
InactiveUS20080177046A1Good curative effectImprove efficiencyImmunoglobulins against animals/humansBiological material analysisKilodaltonWestern blot
Disclosed herein is the discovery of a soluble MN / CA IX (s-CA IX) in body fluids, such as, urine and serum. Said s-CA IX comprises the extracellular domain of CA IX or portions thereof. The Predominant s-CA IX species is the extracellular domain comprising a proteoglycan-like (PG) domain and carbonic anhydrase (CA) domain, and having a molecular weight of about 50 / 54 kilodaltons (kd) upon Western blot. A smaller s-CA IX form of about 20 to about 30 kd comprising the CA domain or parts thereof, not linked to the PG domain, has also been found in body fluids. Diagnostic / prognostic methods for precancer and cancer that detect or detect and quantitate said s-CA IX in body fluids, are described. Also disclosed herein is the coexpression of CA IX and HER-2 / neu / c-erbB-2 that provides parallel, alternative and potentially synergistic diagnostic / prognostic and therapeutic strategies for precancer and cancer. Further disclosed are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, preferably monoclonal antibodies and immunoreactive fragments and engineered variants thereof. Such new MN / CA IX-specific antibodies, fragments and variants are useful diagnostically / prognostically and therapeutically for cancer and precancer. Particularly preferred are the new monoclonal antibodies, fragments and variants that are specific for the non-immunodominant epitopes of MN / CA IX, which antibodies are, among other uses, useful to detect soluble MN / CA IX (s-CA IX) in body fluids, alone but preferably in combination with antibodies specific to the immunodominant epitopes of MN / CA IX, for example, in a sandwich assay.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com