Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
75 results about "Kilodalton" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A unit of atomic mass equal to one thousand daltons.
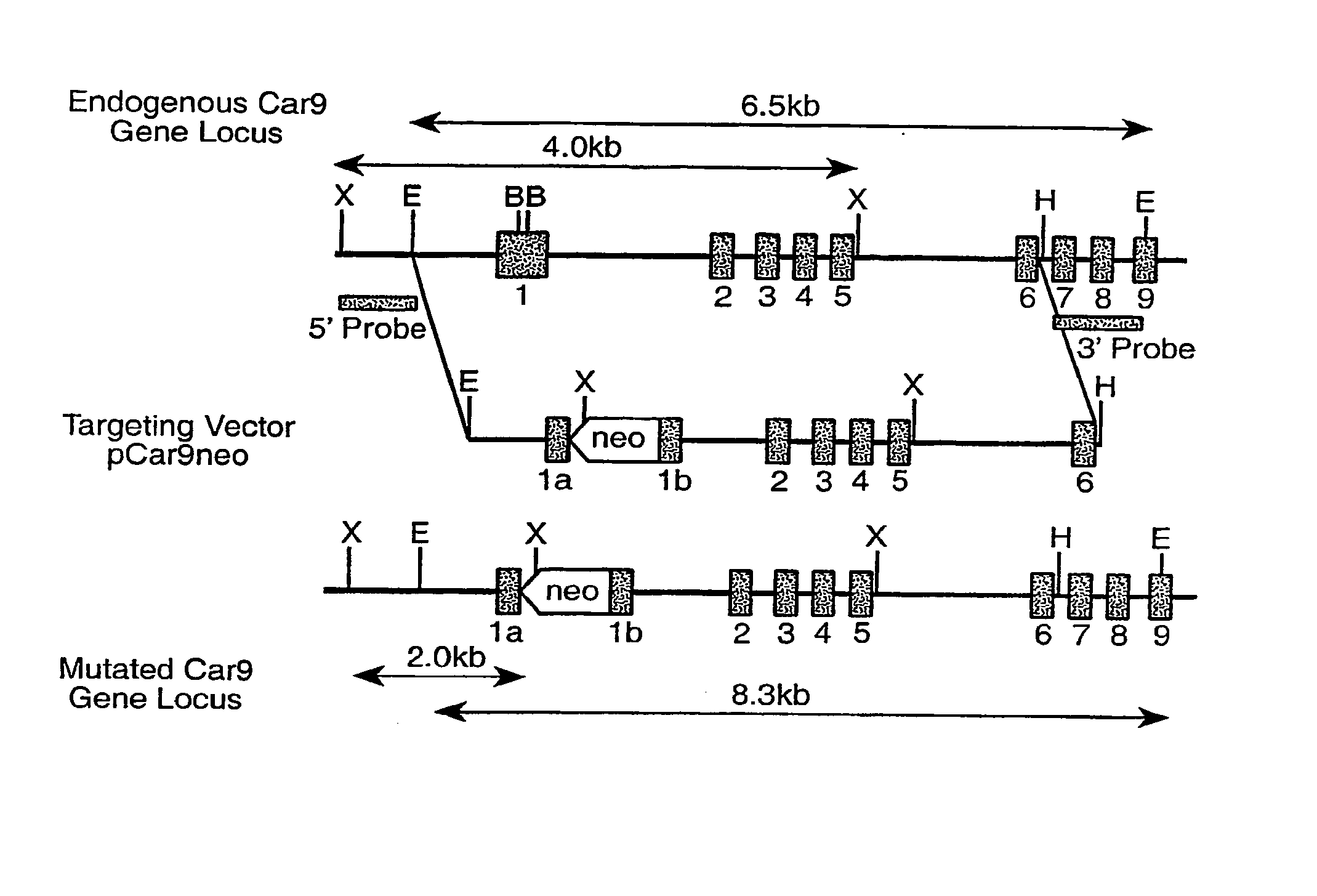
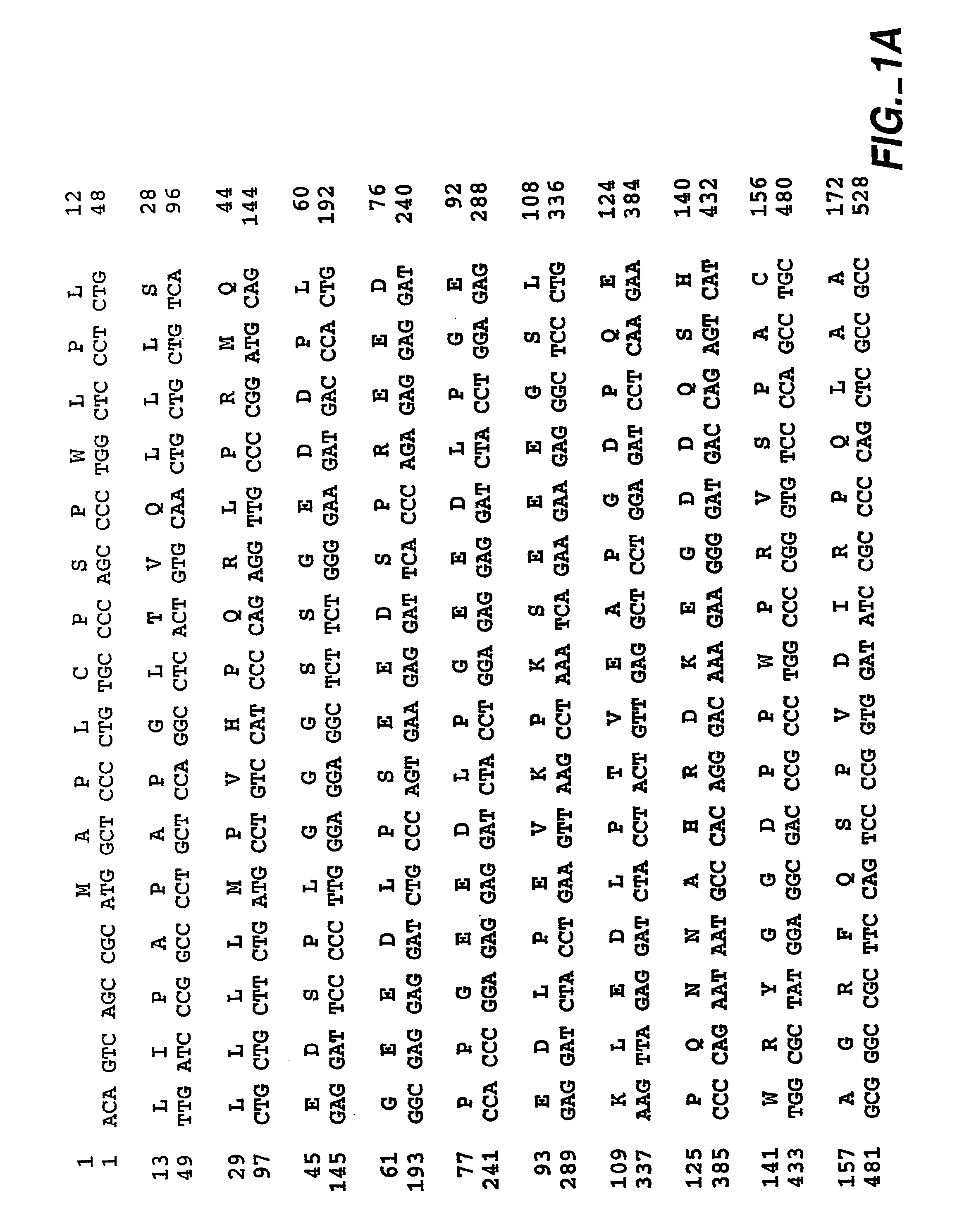
Homologous 28-kilodalton immunodominant protein genes of Ehrlicha canis and uses thereof
The present invention is directed to the cloning, sequencing and expression of homologous immunoreactive 28-kDa protein genes, p28-1, -2, -3, -5, -6, -7, -9, from a polymorphic multiple gene family of Ehrlichia canis. Further disclosed is a multigene locus encoding all nine homologous 28-kDa protein genes of Ehrlichia canis. Recombinant Ehrlichia canis 28-kDa proteins react with convalescent phase antiserum from an E. canis-infected dog, and may be useful in the development of vaccines and serodiagnostics that are particularly effective for disease prevention and serodiagnosis.
Owner:RES DEVMENT FOUND
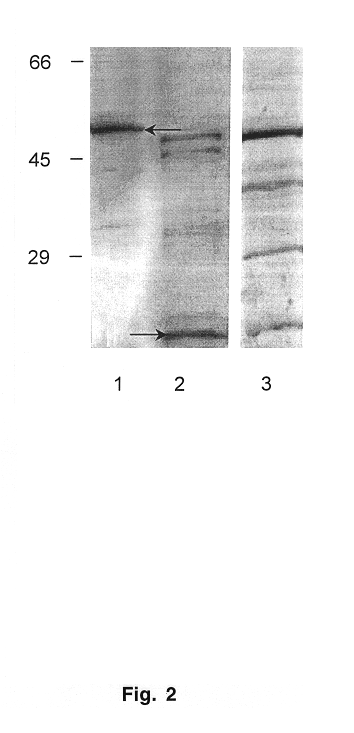
Soluble Form of Carbonic Anhydrase IX (s-CA IX), Assays to Detect s-CA IX, CA IX's Coexpression with HER-2/neu/c-erbB-2, and CA IX-Specific Monoclonal Antibodies to Non-Immunodominant Epitopes
InactiveUS20080176310A1Improve efficiencyIncrease resourcesOxidoreductasesFermentationKilodaltonWestern blot
Disclosed herein is the discovery of a soluble MN / CA IX (s-CA IX) in body fluids, such as, urine and serum. Said s-CA IX comprises the extracellular domain of CA IX or portions thereof. The predominant s-CA IX species is the extracellular domain comprising a proteoglycan-like (PG) domain and carbonic anhydrase (CA) domain, and having a molecular weight of about 50 / 54 kilodaltons (kd) upon Western blot. A smaller s-CA IX form of about 20 to about 30 kd comprising the CA domain or parts thereof, not linked to the PG domain, has also been found in body fluids. Diagnostic / prognostic methods for precancer and cancer that detect or detect and quantitate said s-CA IX in body fluids, are described. Also disclosed herein is the coexpression of CA IX and HER-2 / neu / c-erbB-2 that provides parallel, alternative and potentially synergistic diagnostic / prognostic and therapeutic strategies for precancer and cancer. Further disclosed are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, preferably monoclonal antibodies and immunoreactive fragments and engineered variants thereof. Such new MN / CA IX-specific antibodies, fragments and variants are useful diagnostically / prognostically and therapeutically for cancer and precancer. Particularly preferred are the new monoclonal antibodies, fragments and variants that are specific for the non-immunodominant epitopes of MN / CA IX, which antibodies are, among other uses, useful to detect soluble MN / CA IX (s-CA IX) in body fluids, alone but preferably in combination with antibodies specific to the immunodominant epitopes of MN / CA IX, for example, in a sandwich assay.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
Soluble Form of Carbonic Anhydrase IX (s-CA IX), Assays to Detect s-CA IX, CA IX's Coexpression with Her-2/neu/c-erbB-2, and CA IX-Specific Monoclonal Antibodies to Non-Immunodominant Epitopes
InactiveUS20080177046A1Good curative effectImprove efficiencyImmunoglobulins against animals/humansBiological material analysisKilodaltonWestern blot
Disclosed herein is the discovery of a soluble MN / CA IX (s-CA IX) in body fluids, such as, urine and serum. Said s-CA IX comprises the extracellular domain of CA IX or portions thereof. The Predominant s-CA IX species is the extracellular domain comprising a proteoglycan-like (PG) domain and carbonic anhydrase (CA) domain, and having a molecular weight of about 50 / 54 kilodaltons (kd) upon Western blot. A smaller s-CA IX form of about 20 to about 30 kd comprising the CA domain or parts thereof, not linked to the PG domain, has also been found in body fluids. Diagnostic / prognostic methods for precancer and cancer that detect or detect and quantitate said s-CA IX in body fluids, are described. Also disclosed herein is the coexpression of CA IX and HER-2 / neu / c-erbB-2 that provides parallel, alternative and potentially synergistic diagnostic / prognostic and therapeutic strategies for precancer and cancer. Further disclosed are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, preferably monoclonal antibodies and immunoreactive fragments and engineered variants thereof. Such new MN / CA IX-specific antibodies, fragments and variants are useful diagnostically / prognostically and therapeutically for cancer and precancer. Particularly preferred are the new monoclonal antibodies, fragments and variants that are specific for the non-immunodominant epitopes of MN / CA IX, which antibodies are, among other uses, useful to detect soluble MN / CA IX (s-CA IX) in body fluids, alone but preferably in combination with antibodies specific to the immunodominant epitopes of MN / CA IX, for example, in a sandwich assay.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
Cloned DNA polymerases from Thermotoga and mutants thereof
InactiveUS6506560B1Accurate sequence interpretationOptimization orderSugar derivativesBacteriaKilodaltonMutation
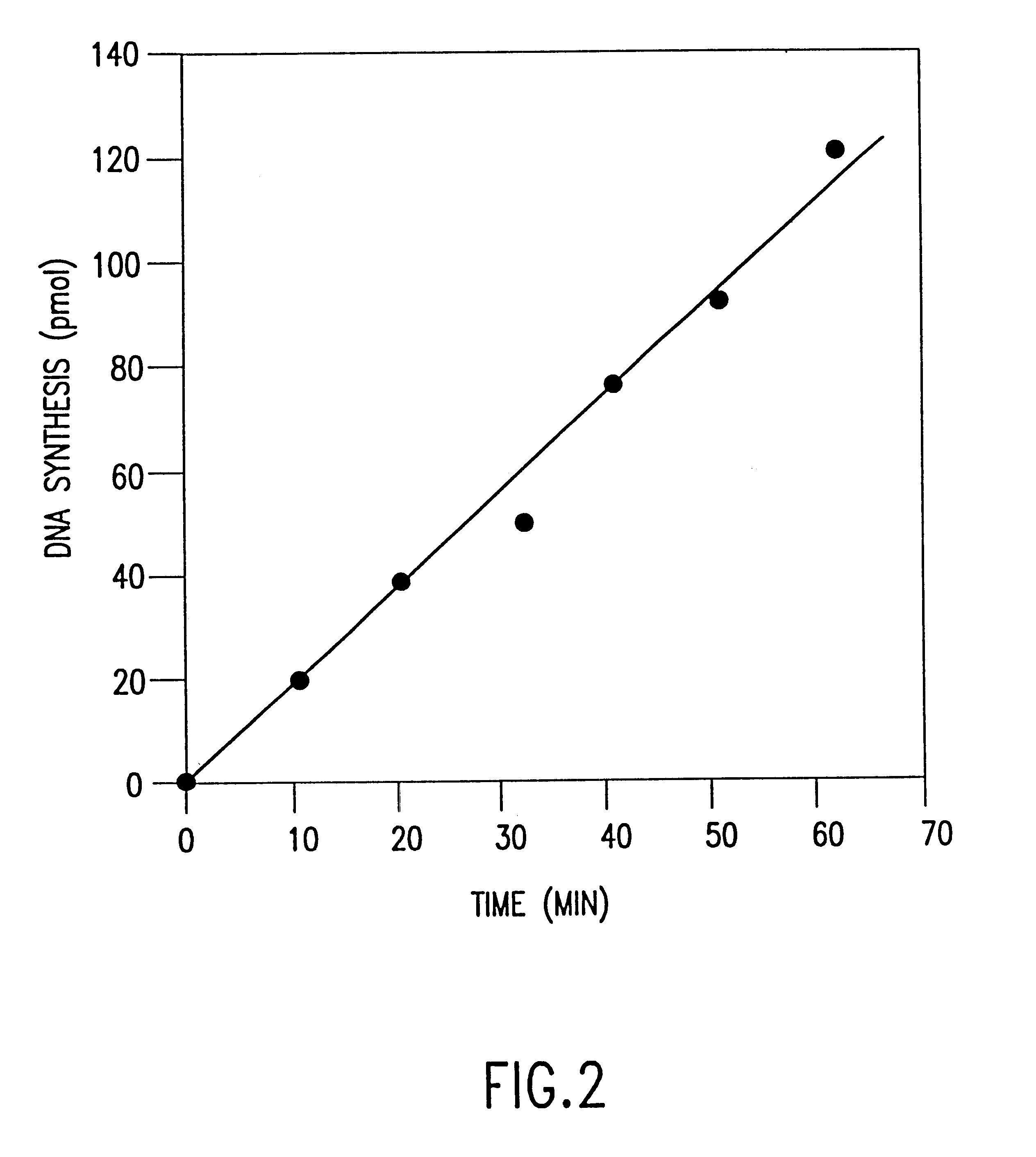
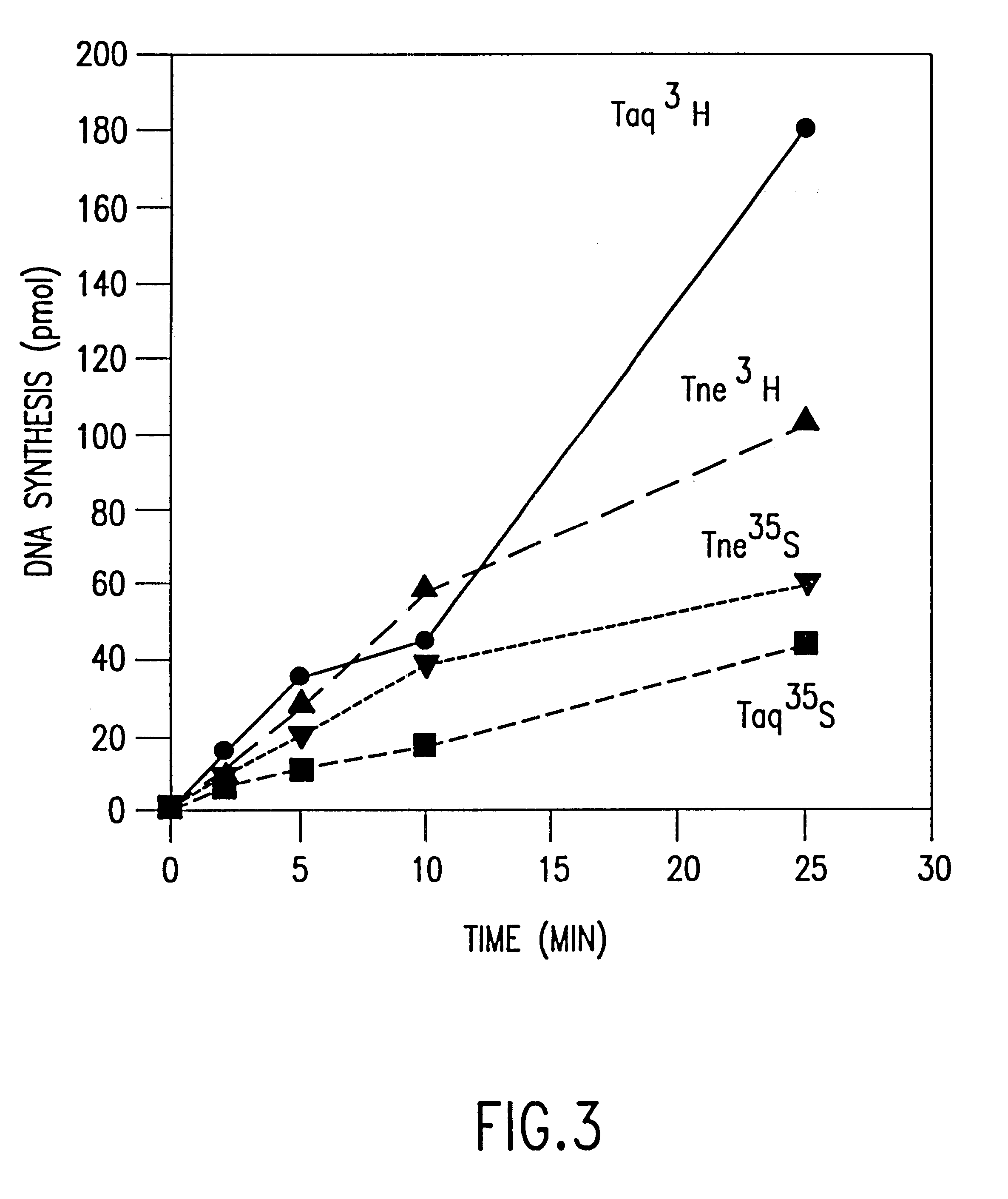
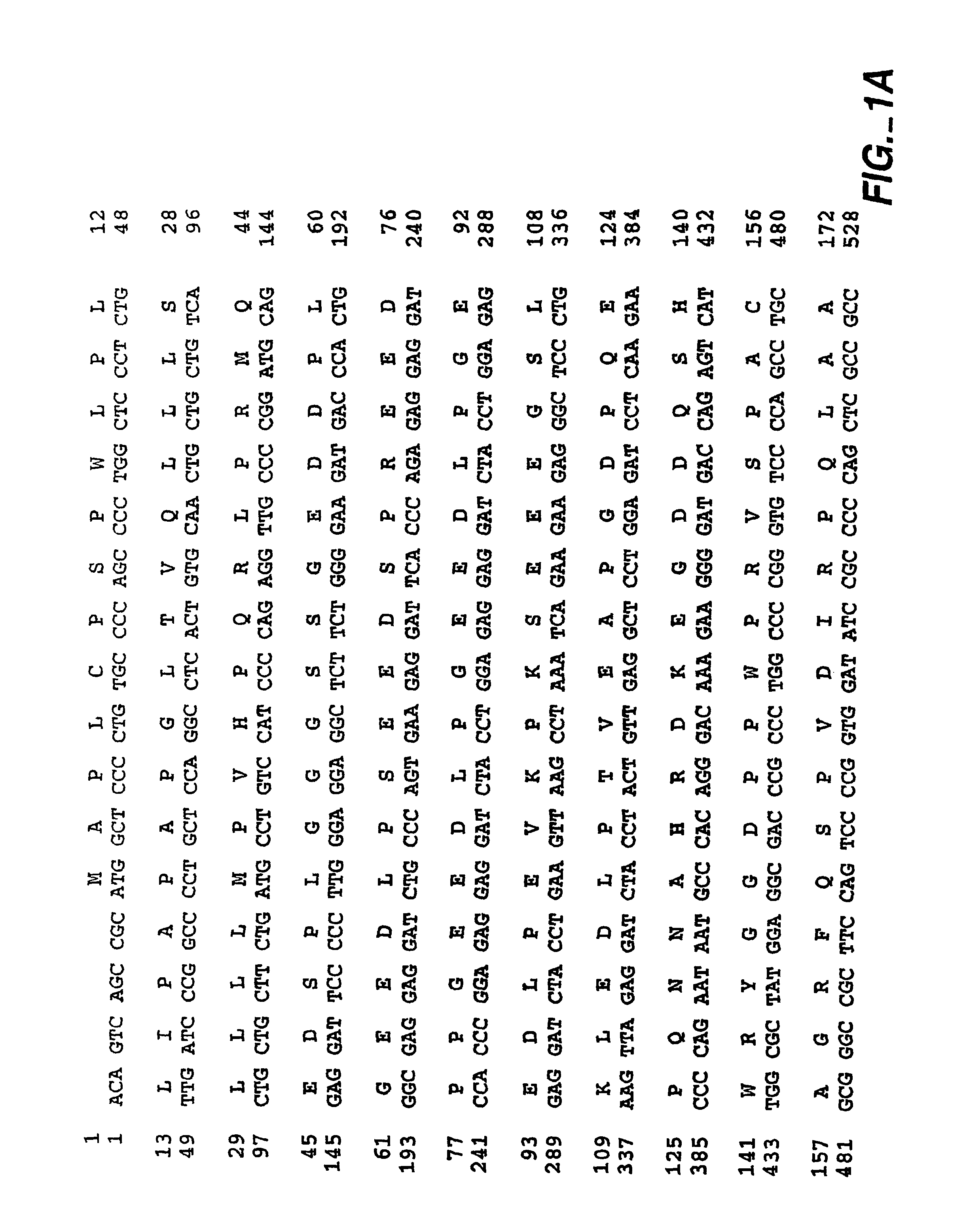
The invention relates to a substantially pure thermostable DNA polymerase from Thermotoga (Tne and Tma) and mutants thereof. The Tne DNA polymerase has a molecular weight of about 100 kilodaltons and is more thermostable than Taq DNA polymerase. The mutant DNA polymerase has at least one mutation selected from the group consisting of (1) a first mutation that substantially reduces or eliminates 3'->5' exonuclease activity of said DNA polymerase; (2) a second mutation that substantially reduces or eliminates 5'->3' exonuclease activity of said DNA polymerase; (3) a third mutation in the O helix of said DNA polymerase resulting in said DNA polymerase becoming non-discriminating against dideoxynucleotides. The present invention also relates to the cloning and expression of the wild type or mutant DNA polymerases in E. coli, to DNA molecules containing the cloned gene, and to host cells which express said genes. The DNA polymerases of the invention may be used in well-known DNA sequencing and amplification reactions.
Owner:INVITROGEN
Soluble form of carbonic anhydrase IX (S-CA IX), assays to detect s-CA IX, CA IX'S coexpression with HER-2/NEU/C-ERBB-2, and CA IX-specific monoclonal antibodies to non-immunodominant epitopes
InactiveUS7816493B2Good curative effectSugar derivativesBiological material analysisKilodaltonC erbb 2
Disclosed herein is the discovery of a soluble MN / CA IX (s-CA IX) found in body fluids, such as, urine and serum. Soluble CA IX comprises the extracellular domain of CA IX or portions thereof. The predominant s-CA IX species is the extracellular domain comprising a proteoglycan-like (PG) domain and carbonic anhydrase (CA) domain, and having a molecular weight of about 50 / 54 kilodaltons. Diagnostic / prognostic methods for precancer / cancer that detect or detect and quantitate s-CA IX in body fluids, are described. Also disclosed is the coexpression of CA IX and HER-2 that provides potentially synergistic diagnostic / prognostic and therapeutic strategies for precancer / cancer. Further disclosed are new MN / CA IX-specific antibodies generated from MN / CA IX-deficient mice, useful diagnostically / prognostically and therapeutically for cancer / precancer. Preferred are new antibodies, specific for non-immunodominant epitopes of MN / CA IX, useful to detect soluble CA IX (s-CA IX) in body fluids, preferably in combination with antibodies specific to immunodominant epitopes of MN / CA IX.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI
Glycosylation variants of iduronate 2-sulfatase
The present invention provides a highly glycosylated iduronate-2-sulfatase enzyme comprising an iduronate-2-sulfatase polypeptide with at least 5 kilodalton (kDa) more sugar than iduronate-2-sulfatase purified from a natural source, e.g. human liver. The present invention also provides an enzymatically active polypeptide fragment or variant of such a highly glycosylated iduronate-2-sulfatase. The present invention further provides an isolated nucleic acid encoding iduronate-2-sulfatase, as well as an expression vector, a host cell and a method for producing the present highly glycosylated iduronate-2-sulfatase enzyme. In one embodiment the present invention is directed to a method for producing a glycosylated iduronate-2-sulfatase enzyme which comprises culturing a host cell containing a nucleic acid encoding an enzymatically active iduronate-2-sulfatase polypeptide wherein the host cell glycosylates the polypeptide to a greater degree than a native iduronate-2-sulfatase polypeptide expressed by a natural human liver cell.
Owner:WOMENS & CHILDRENS HOSPITAL
Phytase-containing foodstuffs and methods of making and using them
InactiveUS6720014B1High nutritional valueImprove release efficiencyFungiBacteriaEscherichia coliPhytase activity
A purified recombinant phytase enzyme derived from Escherichia coli B. The enzyme has a molecular light of about 47.1 kilodaltons and has phytase activity. The enzyme can be produced from native or recombinant host cells and can be used to aid in the digestion of phytate where desired. In particular, the phytase of the present invention can be used in foodstuffs to improve the feeding value of phytate rich ingredients.
Owner:VERENIUM CORPORATION
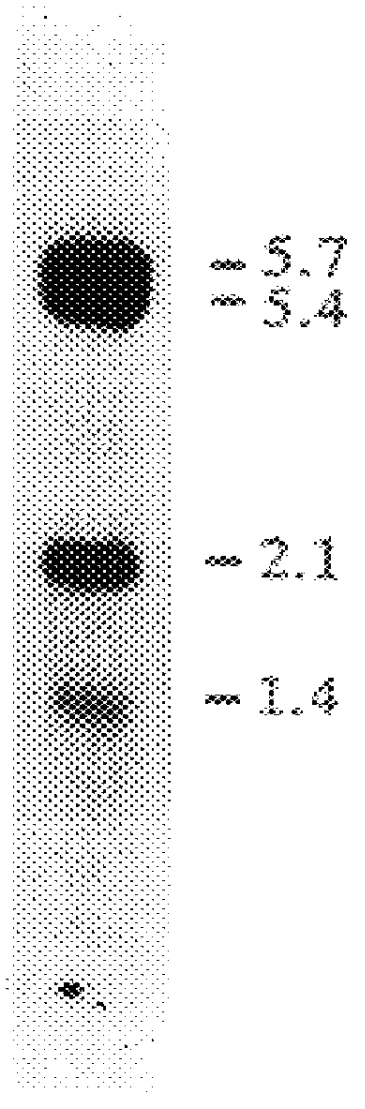
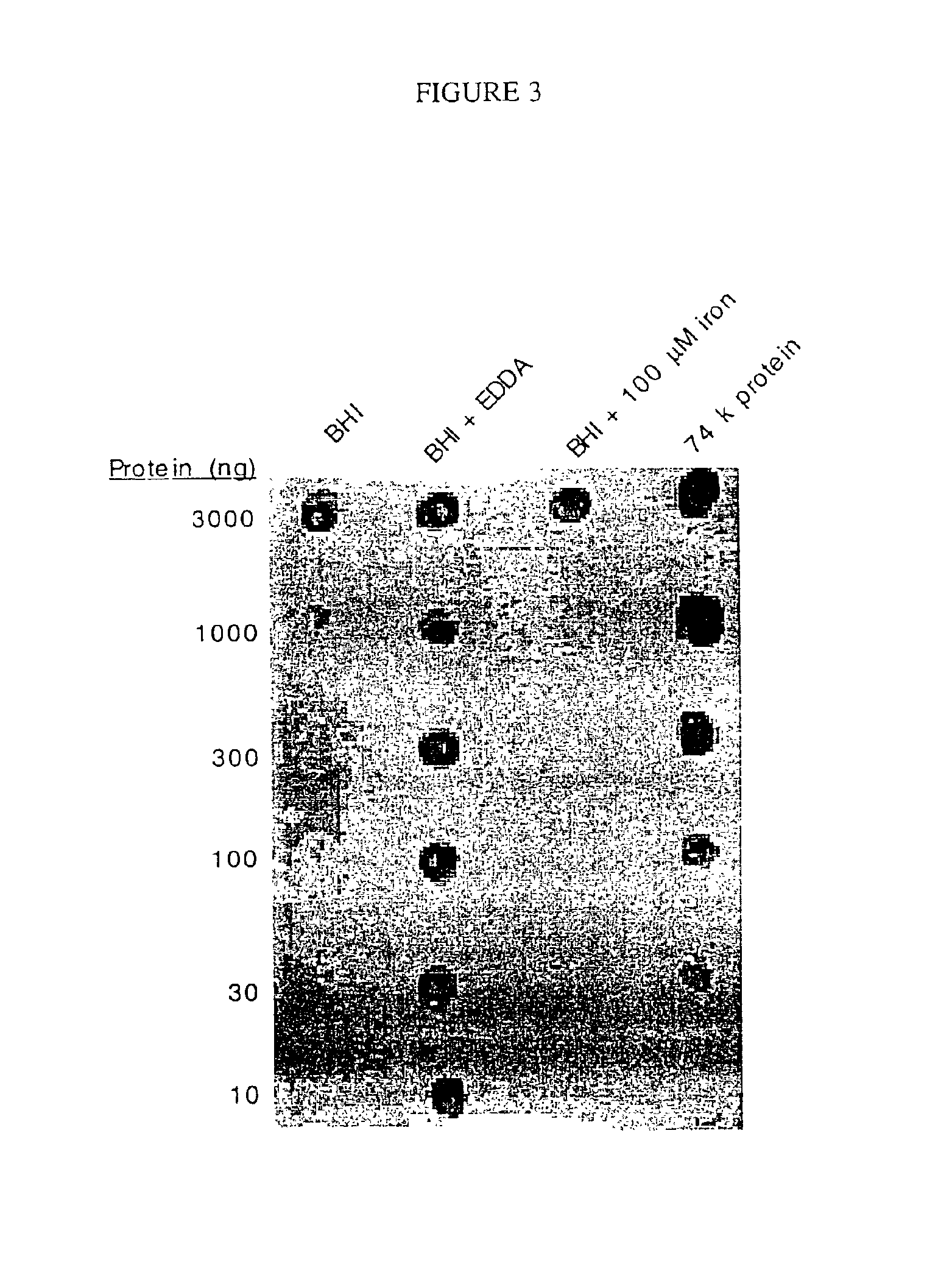
74-kilodalton outer membrane protein from Moraxella catarrhalis
InactiveUS6899885B1Enhance antibody responseLower titerBacterial antigen ingredientsBacteriaDiseaseKilodalton
A protein from M. catarrhalis, designated the 74 kD protein, is isolated and purified. The 74 kD protein has an amino-terminal amino acid sequence which is conserved among various strains of M. catarrhalis. The protein has a molecular weight of approximately 74.9 kD as measured on a 10% SDS-PAGE gel, while its molecular weight as measured by mass spectrometry is approximately 74 kD. The 74 kD protein is used to prepare a vaccine composition which elicits a protective immune response in a mammalian host, to protect the host against disease caused by M. catarrhalis.
Owner:WYETH HOLDINGS CORP
Rapid isolation of osteoinductive protein mixtures from mammalian bone tissue
InactiveUS7622562B2Improved and simplifiedRapid and high and isolationPeptide/protein ingredientsBone-inducing factorBones demineralizationMammalian tissue
A method for purifying bone-derived osteoinductive proteins including a demineralization process, a protein extraction process, a high molecular weight ultrafiltration process, a low molecular weight ultrafiltration process, and a recovery process. The high and low ultrafiltration processes preferably select proteins having a nominal molecular weight between approximately 8 kilodaltons and approximately 100 kilodaltons. Processes of the present invention may be used to recover osteoinductive proteins from bone demineralization waste streams.
Owner:ZIMMER ORTHOBIOLOGICS
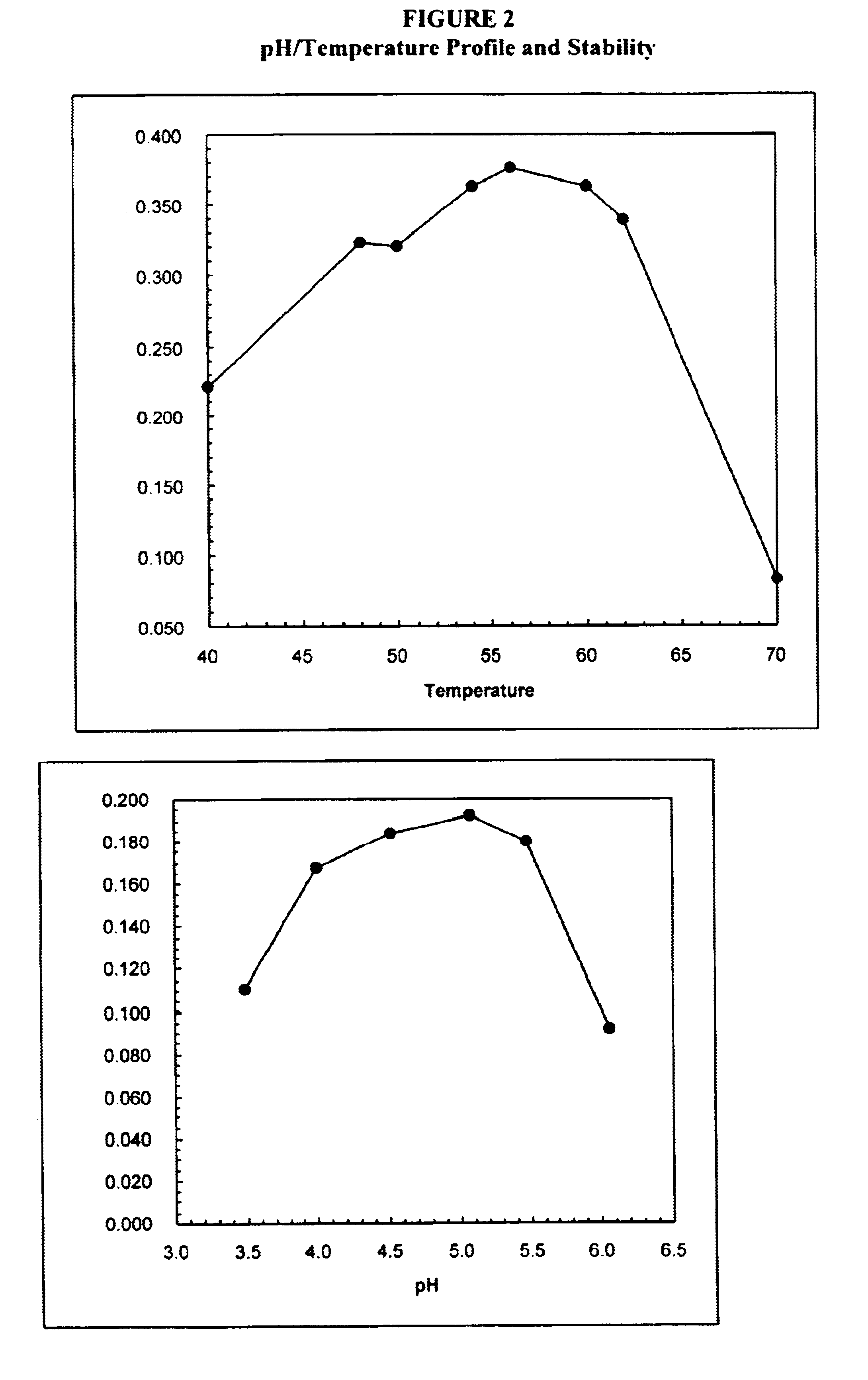
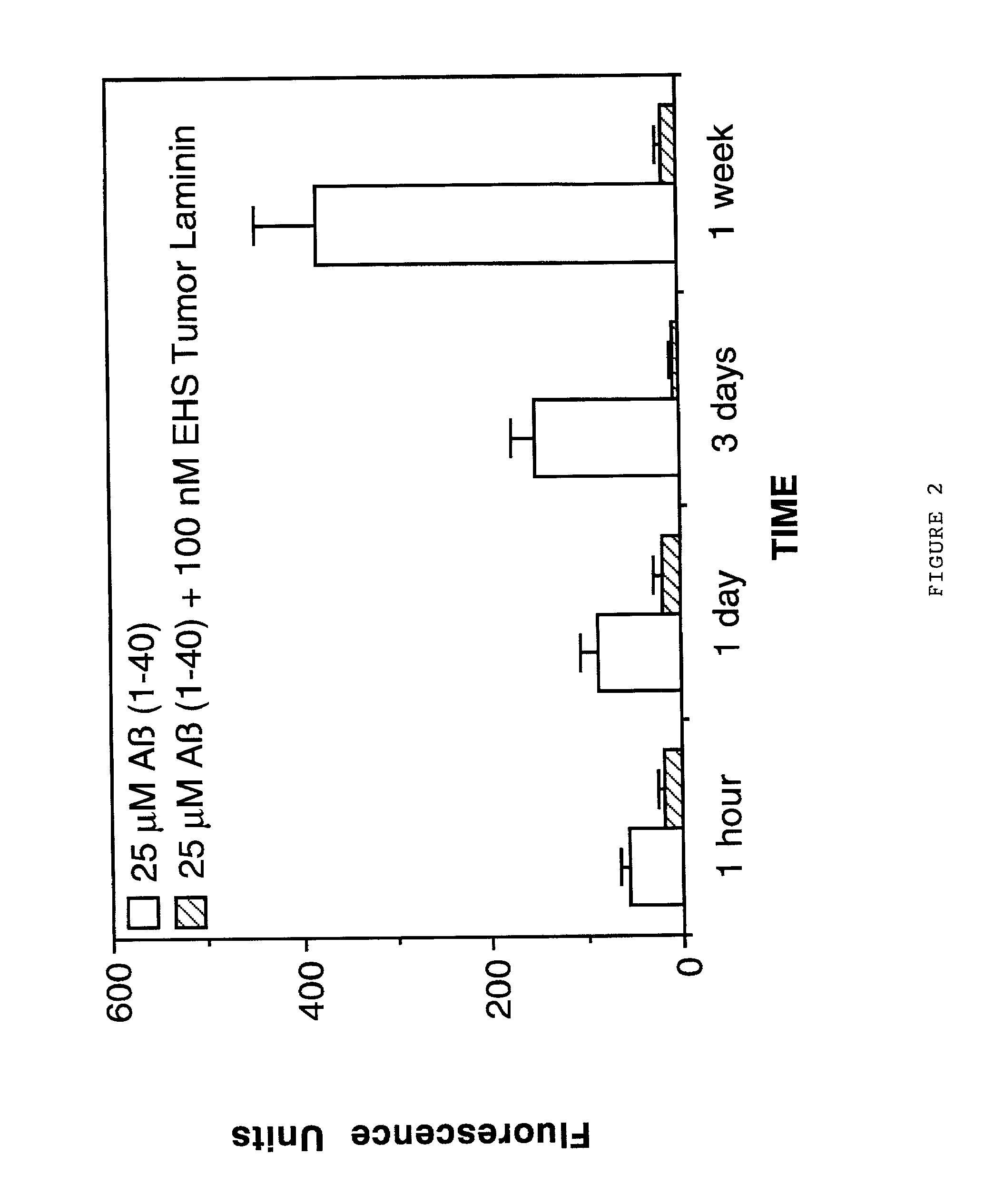
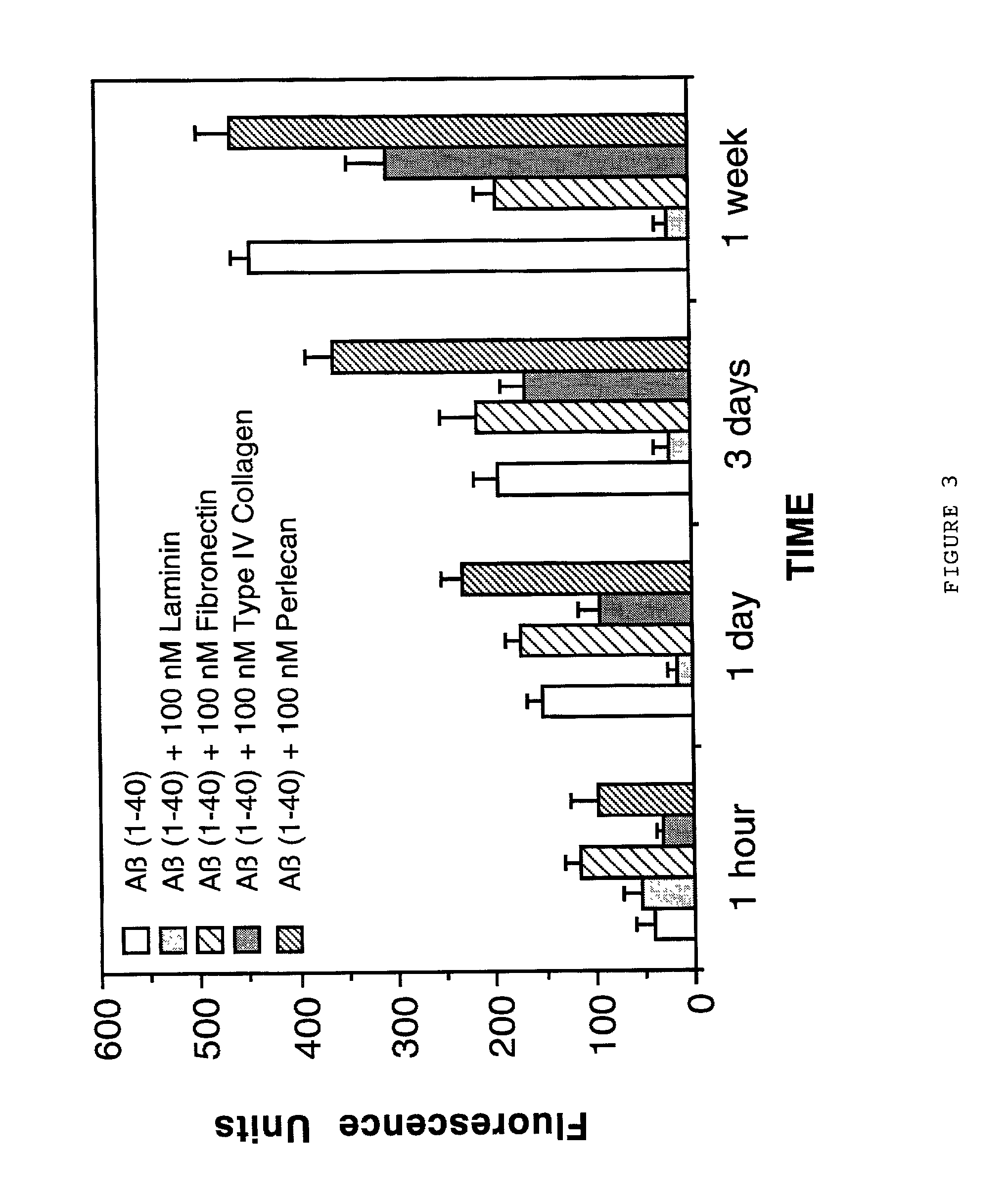
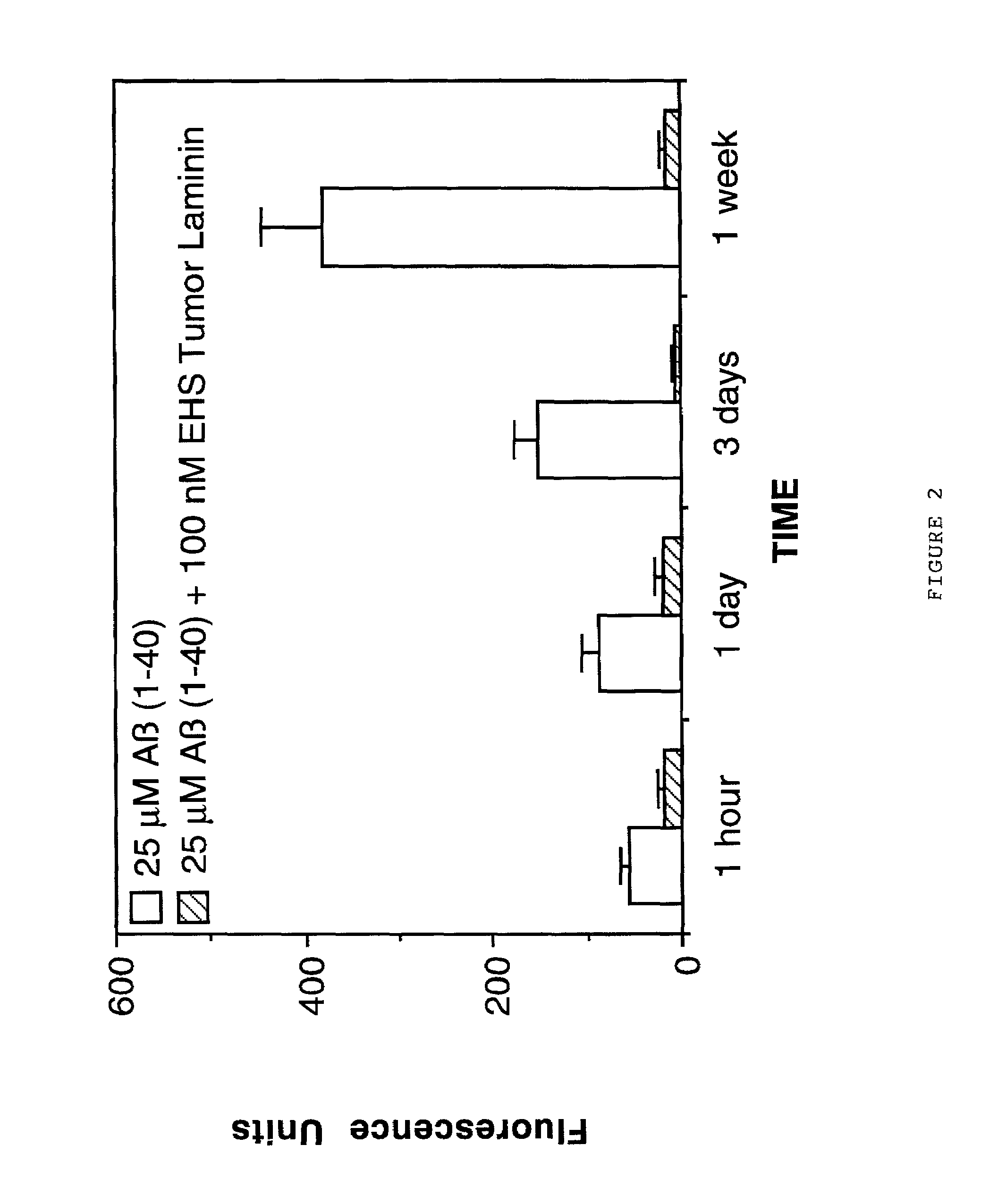
Therapeutic applications of laminin and laminin-derived protein fragments
InactiveUS20020111309A1Inhibiting amyloidosisPreventing amyloid depositionConnective tissue peptidesPeptide/protein ingredientsAmyloid betaKilodalton
Laminin and specific laminin-derived protein fragments are disclosed as potent inhibitors of Alzheimer's disease type amyloidoses. A specific region is identified within laminin which interacts with the Alzheimer's disease beta-amyloid protein and contributes to the observed inhibitory and therapeutic effects. A prominent ~130 kilodalton band in laminin was found in human serum and cerebrospinal fluid which primarily interacted with Abeta as determined by ligand blotting methodology. This ~130 kilodalton laminin fragment is known as the E8 fragment and is also believed to consist of the globular domains of the laminin A chain. The interaction of specific laminin fragments such as the newly discovered ~130 kDa protein is believed to bind Abeta in biological fluids and keep it in a soluble state.
Owner:UNIV OF WASHINGTON
Rapid isolation of osteoinductive protein mixtures from mammalian bone tissue
InactiveUS7241874B2Reduce lossesImproved and simplifiedHydrolasesBone-inducing factorBones demineralizationWaste stream
A method for purifying bone-derived osteogenic proteins including a demineralization process, a protein extraction process, a high molecular weight ultrafiltration process, a low molecular weight ultrafiltration process, and a recovery process. The high and low ultrafiltration processes preferably select proteins having a nominal molecular weight between approximately 8 kilodaltons and approximately 50 kilodaltons. Processes of the present invention may be used to recover osteogenic proteins from bone demineralization waste streams.
Owner:ZIMMER ORTHOBIOLOGICS
Use of poly-gamma-glutamic acid as fertilizer absorbefacient in agricultural planting
InactiveCN1827560AHigh environmental valueReduce the amount of applicationFertilizer mixturesKilodaltonPotassium
The invention relates to the application of the gamma-polyglutamic acid as the absorption promoting agent of the fertilizer in the agricultural husbandry. The said gamma-polyglutamic acid is a high molecular polymer of D-glutacid and L- glutacid formed by the alpha-amino and the gamma-carboxyl in the manner of peptide bond and there is also a dissociative carboxyl on the alpha-carbon atom of every repetitive unit with average molecular weight of 10-10,000 kilodaltons. The said gamma-polyglutamic acid can be used as the absorption promoting agent of the fertilizer made of single element of nitrogen, phosphor, kalium or their composition. The significance of the invention is: it can be degraded by edaphon and its environmental value is much better in relation to TPA; it can reduce by 10-30 % of the normal usage of the chemical fertilizer by applying 10-50 grams of fertilizer per area and increase by 10-30 % of the crop yield, at the same time it can improve the crop quality and increase the crop content of nitrogen, phosphor and kalium.
Owner:HUAZHONG AGRI UNIV
Electrochemical biosensor test strip
InactiveUSRE42924E1Easy to identifyEnsure correct executionImmobilised enzymesBioreactor/fermenter combinationsElectrochemical biosensorPolyethylene oxide
An electrochemical biosensor test strip with four new features. The test strip includes an indentation for tactile feel as to the location of the strips sample application port. The sample application port leads to a capillary test chamber, which includes a test reagent. The wet reagent includes from about 0.2% by weight to about 2% by weight polyethylene oxide from about 100 kilodaltons to about 900 kilodaltons mean molecular weight, which makes the dried reagent more hydrophilic and sturdier to strip processing steps, such as mechanical punching, and to mechanical manipulation by the test strip user. The roof of the capillary test chamber includes a transparent or translucent window which operates as a “fill to here” line, thereby identifying when enough test sample (a liquid sample, such as blood) has been added to the test chamber to accurately perform a test. The test strip may further include a notch located at the sample application port. The notch reduces a phenomenon called “dose hesitation”.
Owner:ROCHE OPERATIONS +1
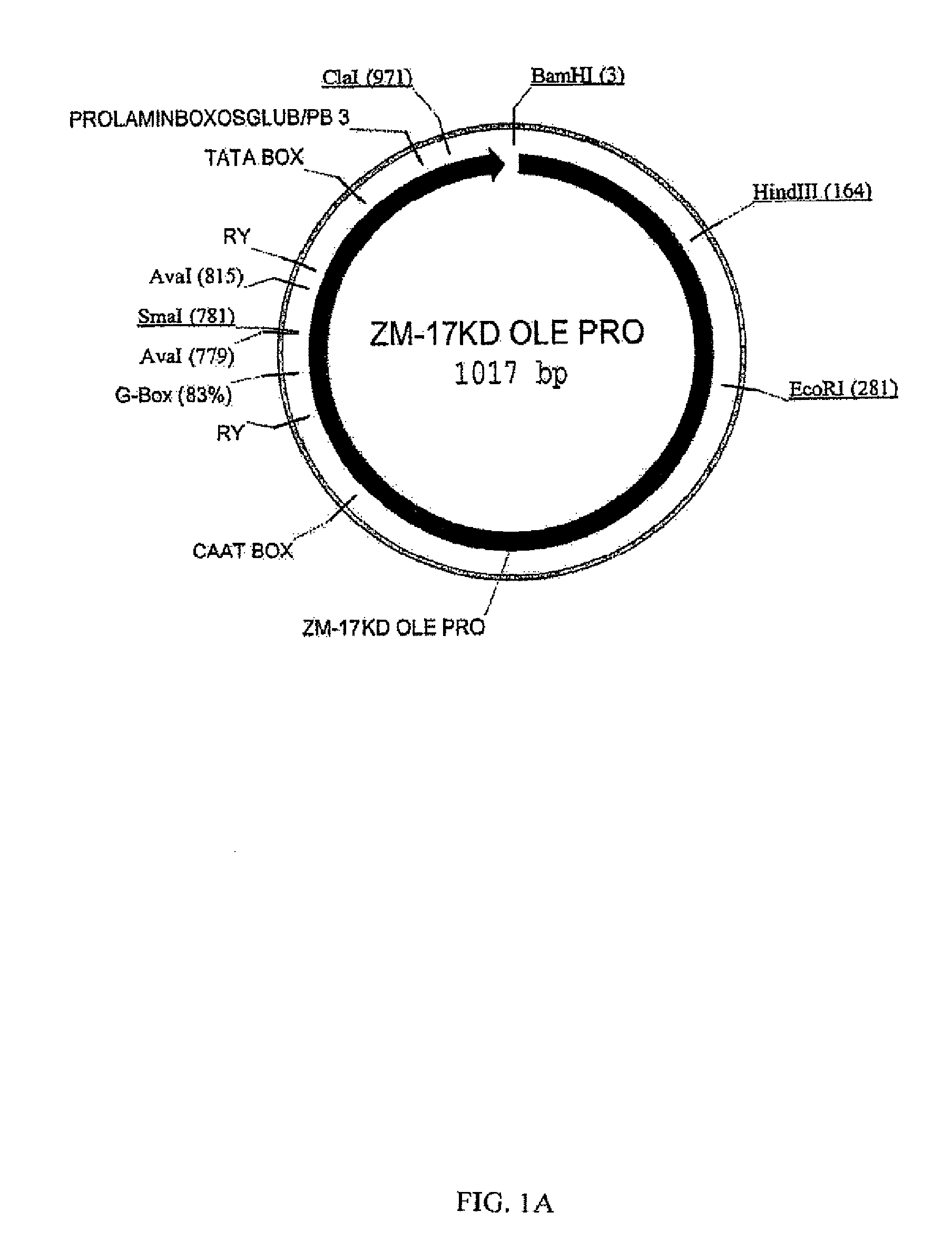
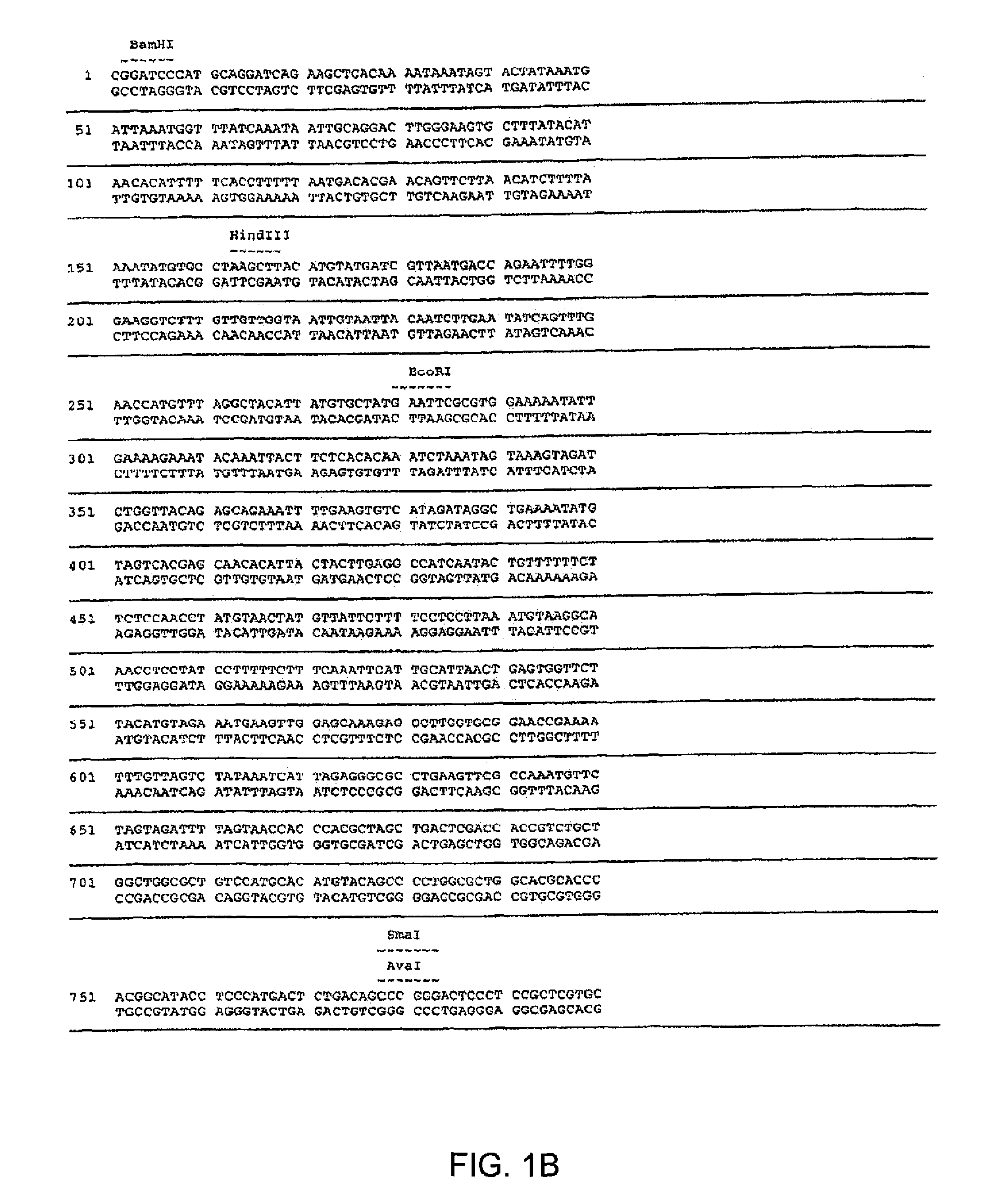
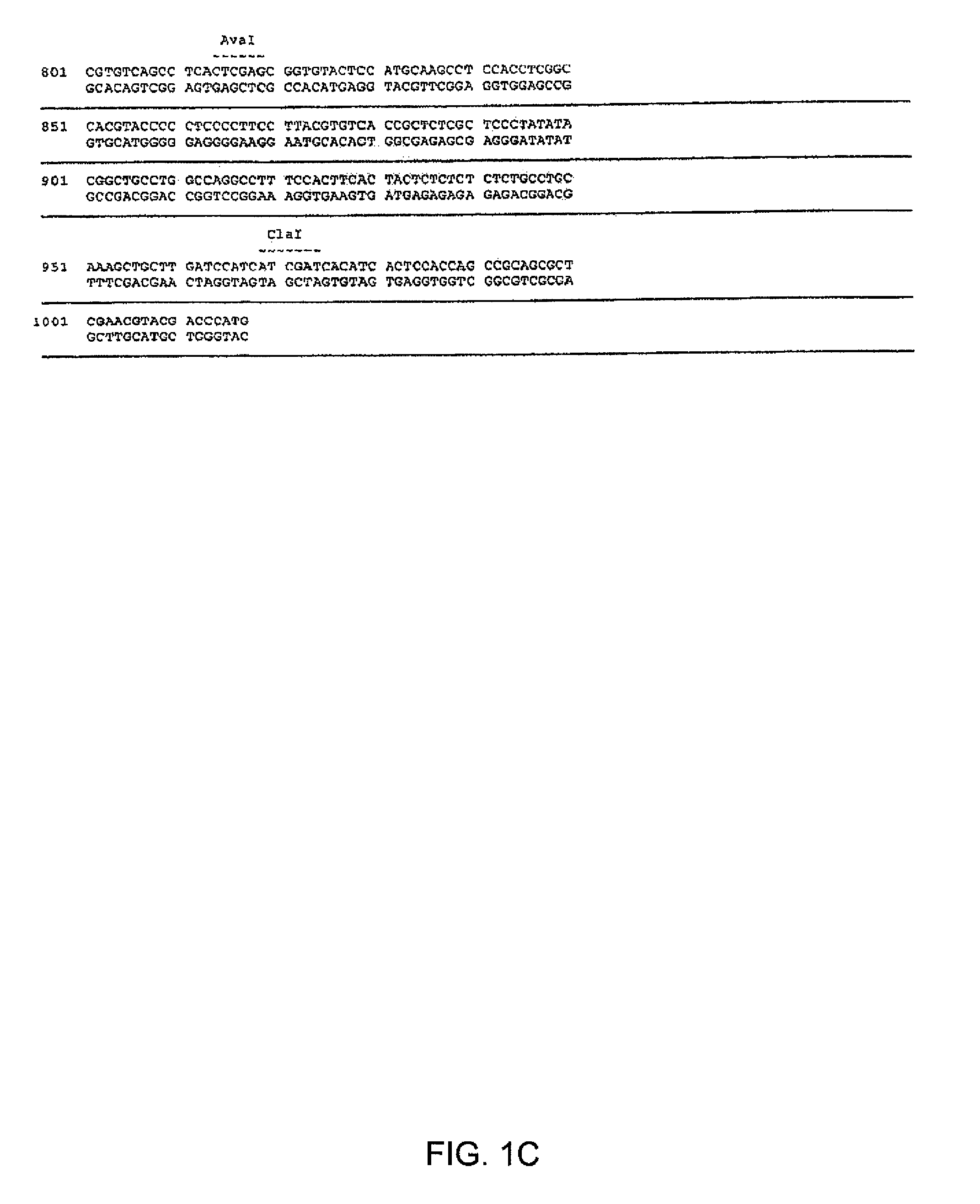
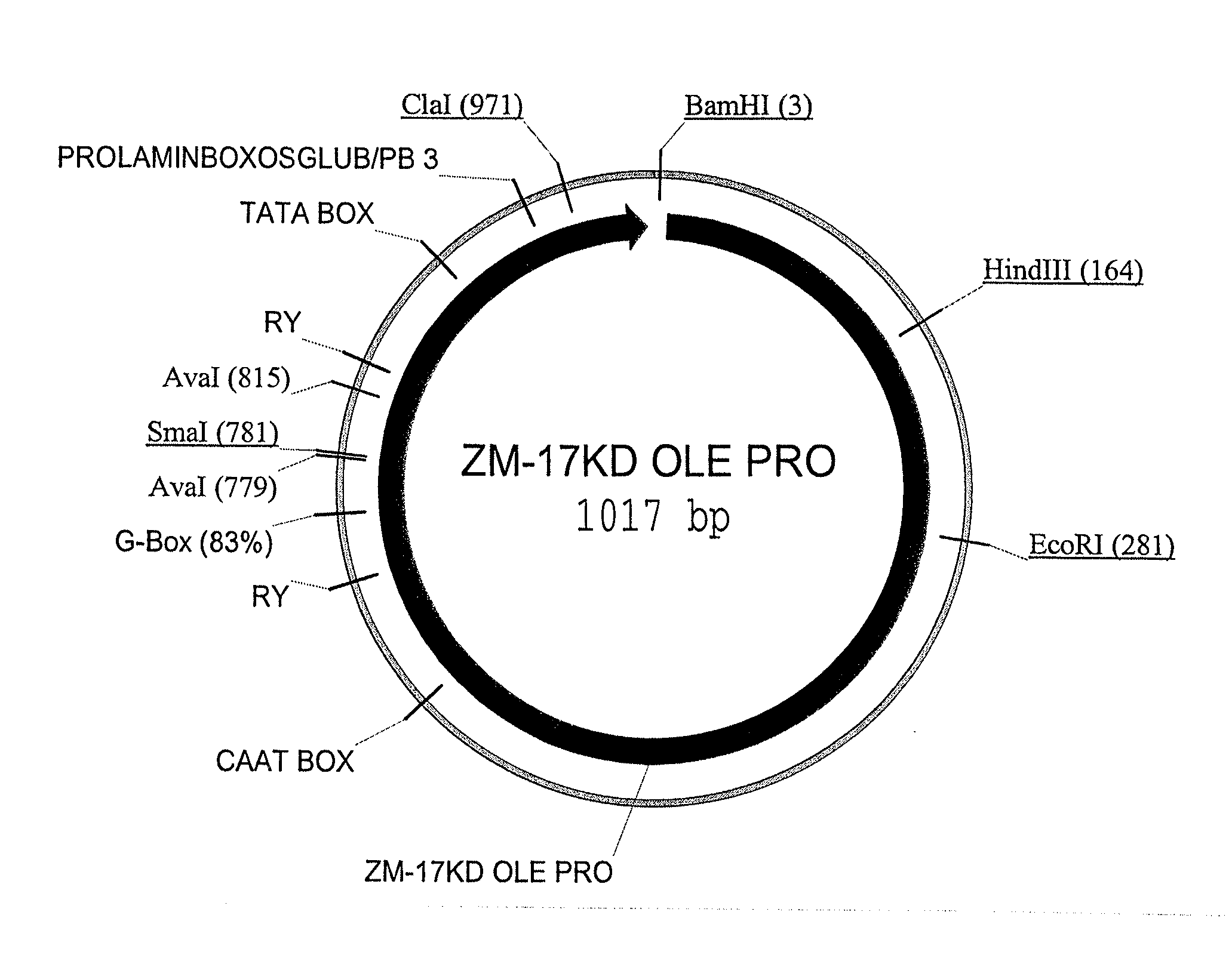
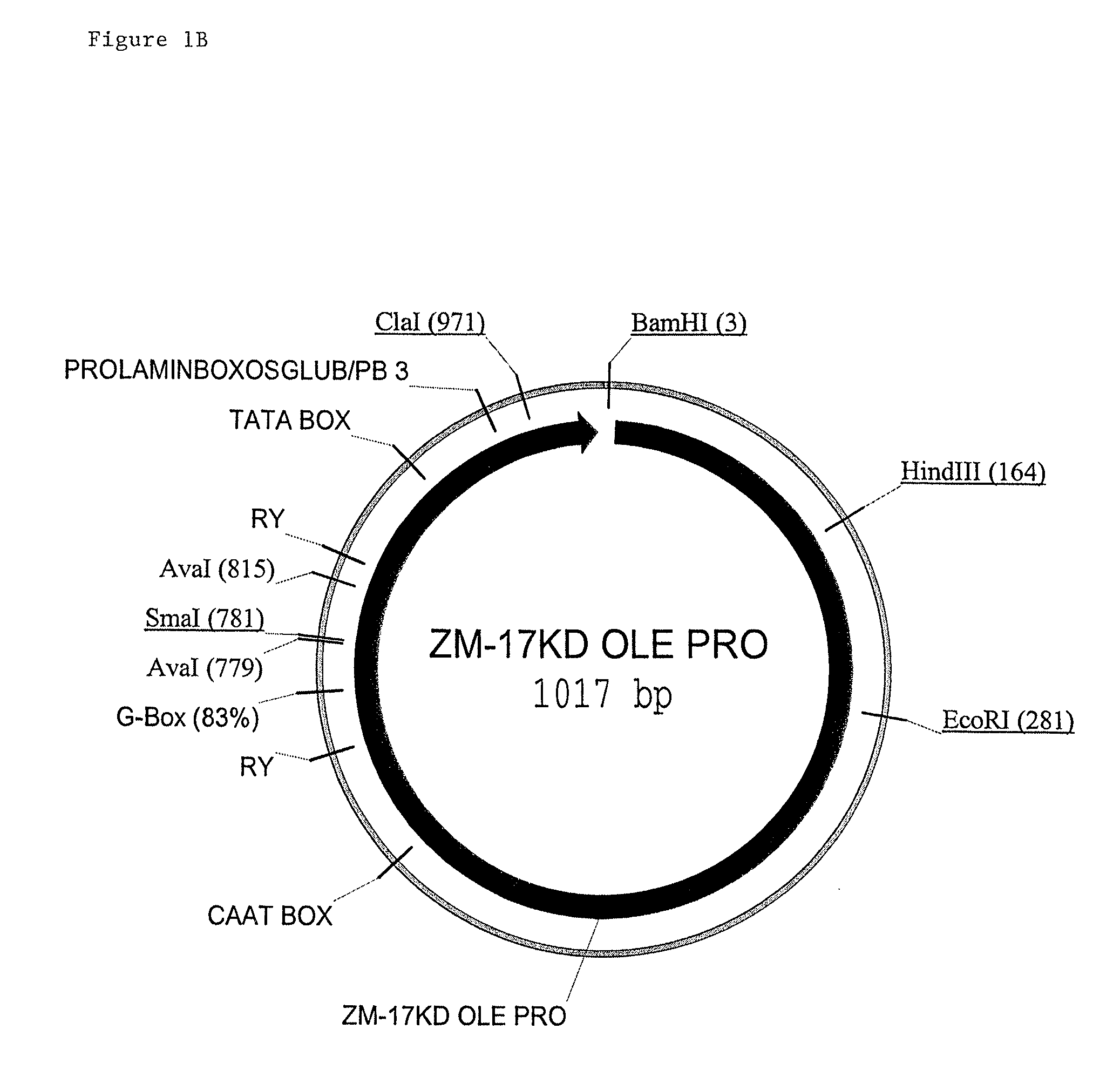
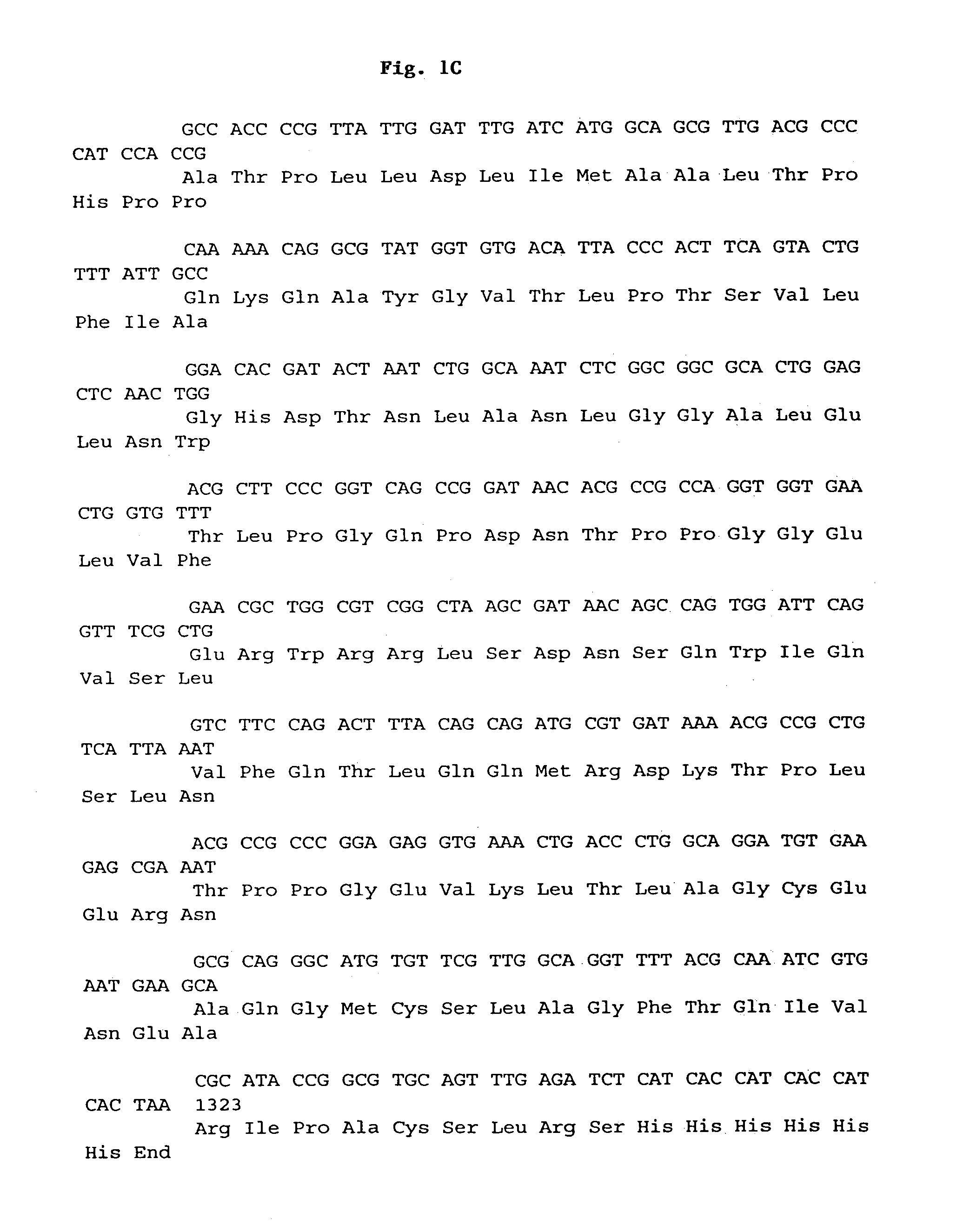
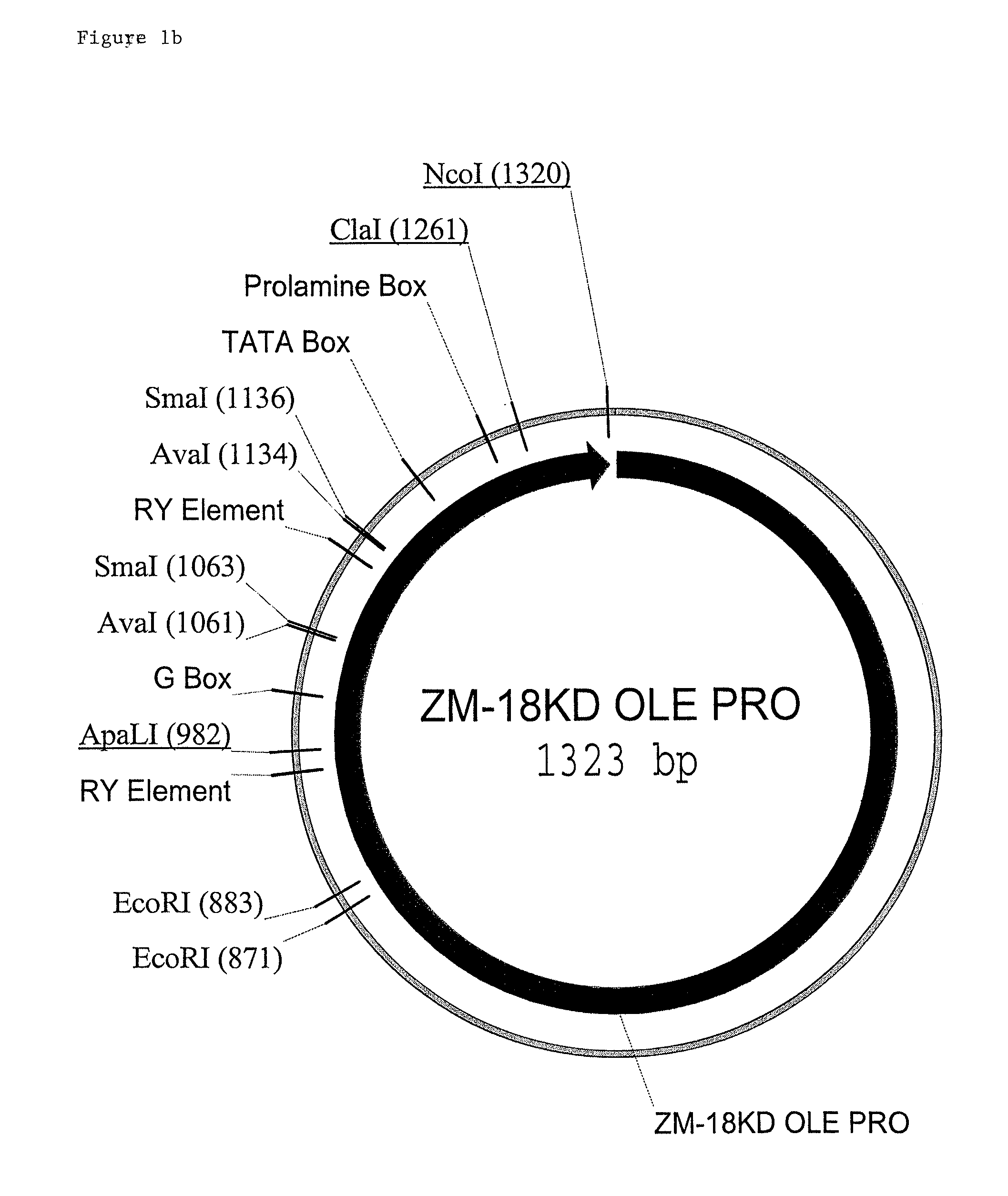
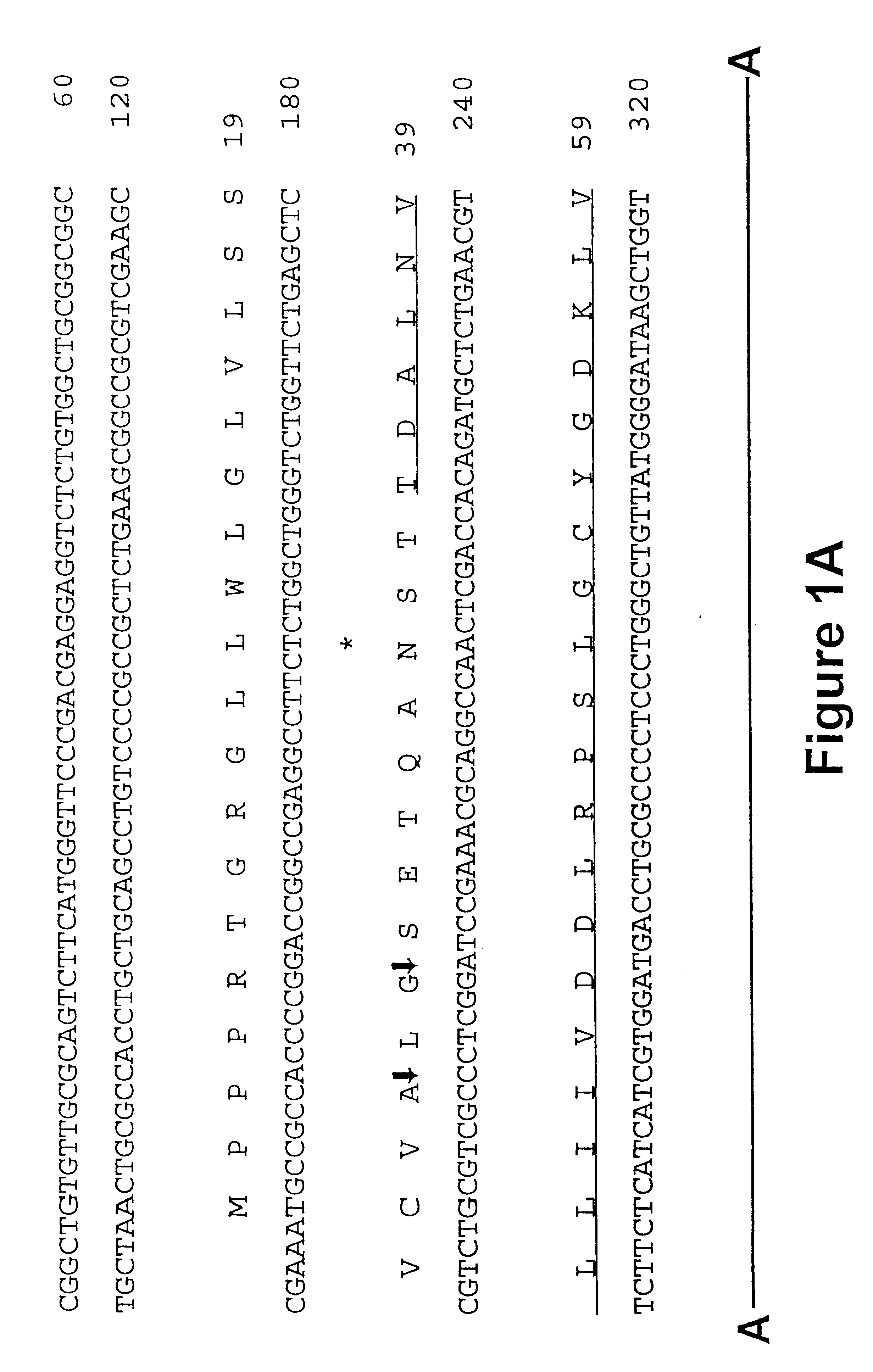
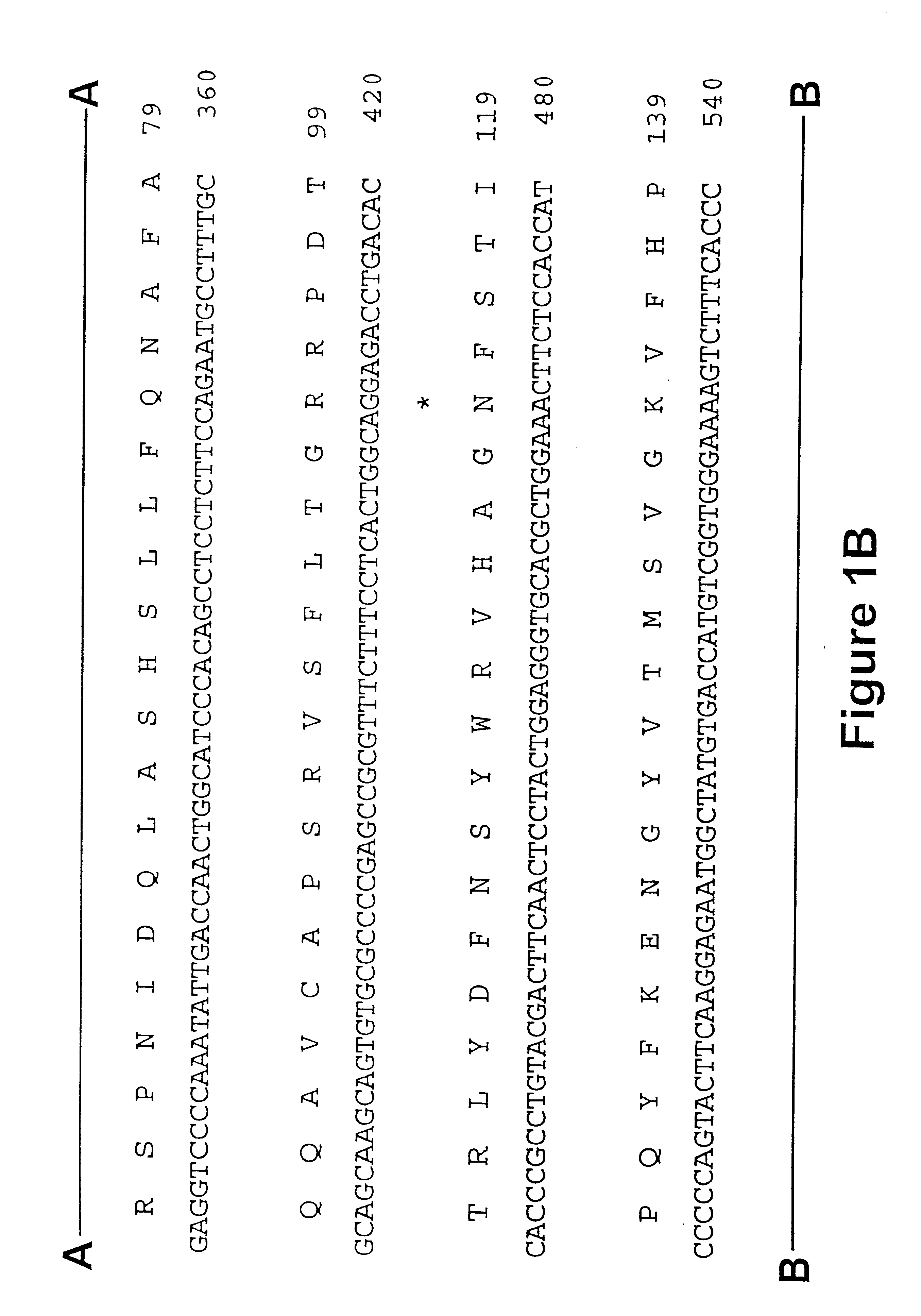
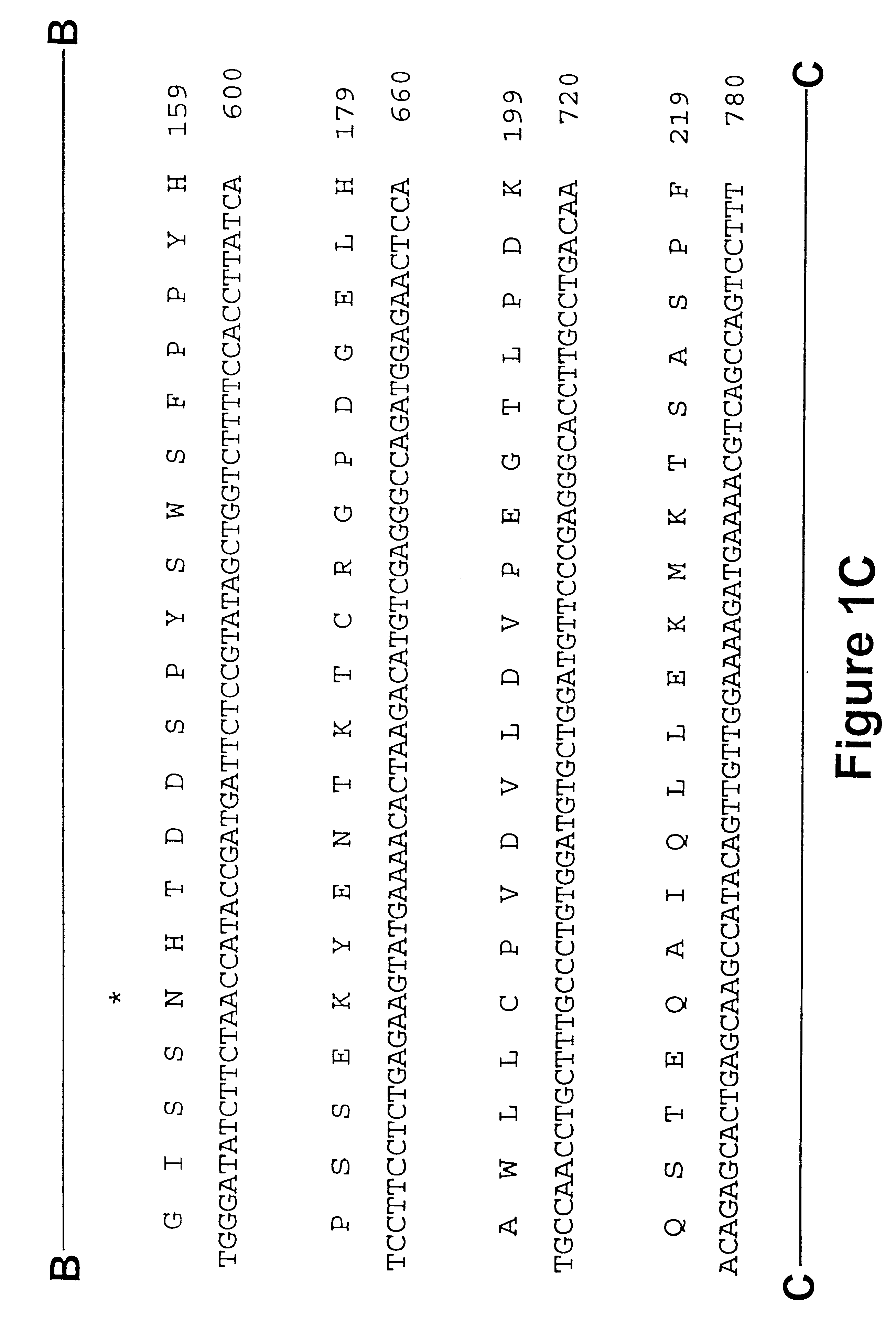
Maize 17KD oleosin seed-preferred regulatory element
The present invention provides compositions and methods for regulating expression of nucleotide sequences of interest in a plant. Compositions are novel nucleotide sequences for a seed-preferred promoter associated with the maize 17 KD OLE (17 kilodalton oleosin) coding region. A method for expressing a nucleotide sequence of interest in a plant using the regulatory sequence disclosed herein is provided.
Owner:PIONEER HI BRED INT INC
Electrochemical biosensor test strip
InactiveUSRE42560E1Easy to identifyEnsure correct executionImmobilised enzymesBioreactor/fermenter combinationsPunchingPolyethylene oxide
Owner:ROCHE DIABETES CARE INC +1
Maize 17kd oleosin seed-preferred regulatory element
The present invention provides compositions and methods for regulating expression of nucleotide sequences of interest in a plant. Compositions are novel nucleotide sequences for a seed-preferred promoter associated with the maize 17 KD OLE (17 kilodalton oleosin) coding region. A method for expressing a nucleotide sequence of interest in a plant using the regulatory sequence disclosed herein is provided.
Owner:PIONEER HI BRED INT INC
Phytase expression systems and methods of making and using them
InactiveUS7232677B2High nutritional valueImprove publishing efficiencyBioreactor/fermenter combinationsFungiEscherichia coliPhytase activity
The invention provides a purified or recombinant phytase enzyme (SEQ ID NO:2) initially derived from Escherichia coli B. The enzyme has a molecular weight of about 47.1 kilodaltons and has phytase activity (SEQ ID NO:2). The enzyme can be produced from native or recombinant host cells and can be used to aid in the digestion of phytate where desired. In particular, the phytase of the present invention can be used in foodstuffs to improve the feeding value of phytate rich ingredients.
Owner:VERENIUM CORPORATION
Method of quantifying cholesterol in high density lipoprotein and reagent compositions
ActiveUS20050095658A1Increasing degree of erasingDegree of erasingMicrobiological testing/measurementKilodaltonTest sample
A method for specifically quantifying HDL cholesterol in which cholesterol in lipoproteins other than HDL is erased in the first step, and HDL cholesterol is specifically quantified in the second step, by which accurate values can be obtained even in measurements of abnormal samples such as disorder of lipid metabolism and lipoprotein abnormality, is disclosed. The method for quantifying cholesterol in high density lipoprotein according to the present invention comprises a first step of erasing cholesterol in lipoproteins other than high density lipoprotein by treating a test sample with cholesterol esterase and cholesterol oxidase in the absence of a surfactant which acts on high density lipoprotein and removing generated hydrogen peroxide; and a second step of adding a surfactant which specifically acts on high density lipoprotein to the product of said first step and quantifying hydrogen peroxide generated from cholesterol in high density lipoprotein by actions of cholesterol esterase and cholesterol oxidase. As the cholesterol oxidase used in the first step, one having a molecular weight of not more than 60 kilodaltons is used.
Owner:DENKA CO LTD
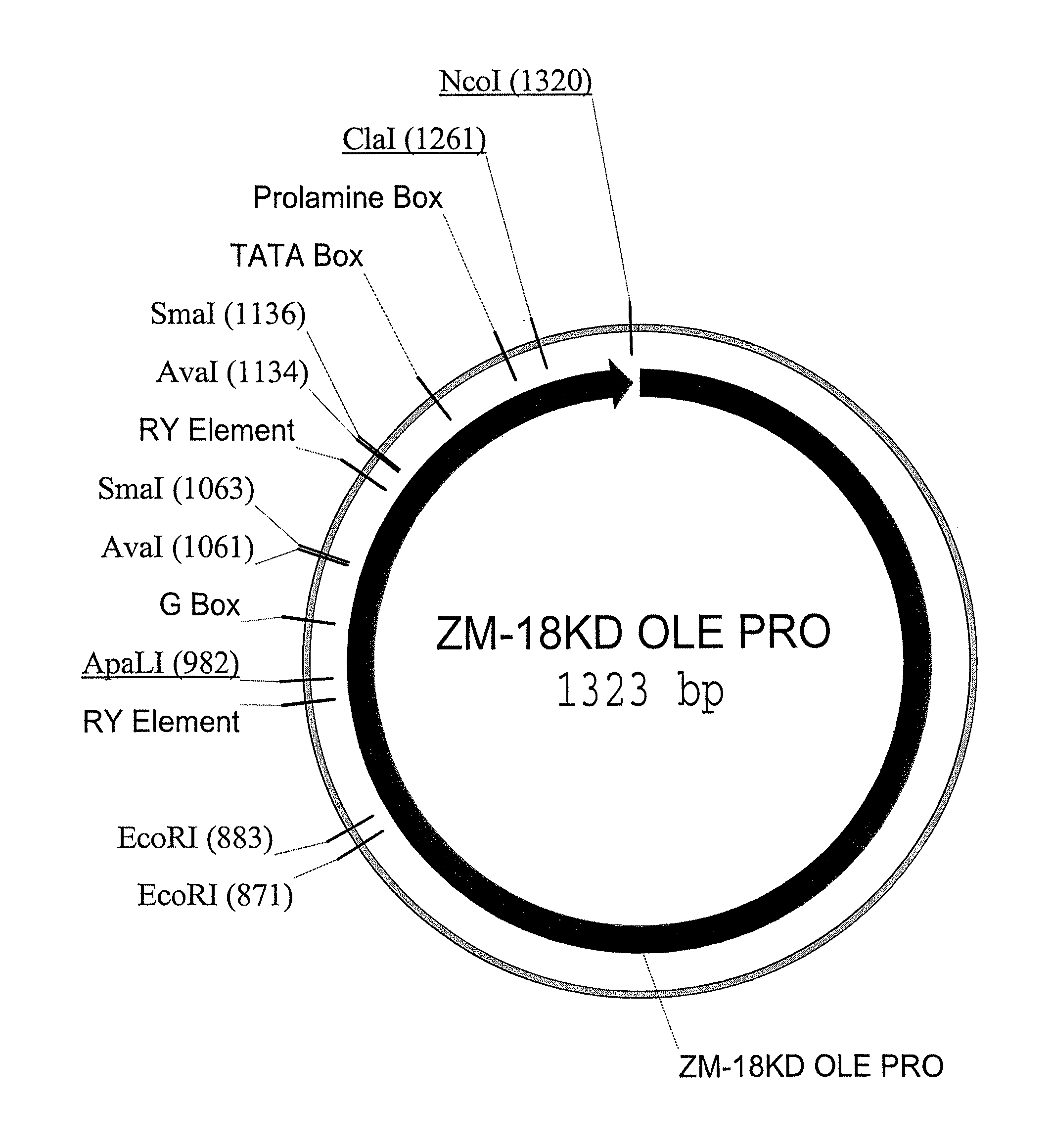
Maize 18kd oleosin seed-preferred regulatory element
InactiveUS20100281570A1Sugar derivativesOther foreign material introduction processesKilodaltonGenetics
The present invention provides compositions and methods for regulating expression of nucleotide sequences of interest in a plant. Compositions are novel nucleotide sequences for a seed-preferred promoter associated with the maize 18 KD OLE (18 kilodalton oleosin) coding region. A method for expressing a nucleotide sequence of interest in a plant using the regulatory sequence disclosed herein is provided.
Owner:PIONEER HI BRED INT INC
Glycosylation variants of iduronate 2-sulfatase
The present invention provides a highly glycosylated iduronate-2-sulfatase enzyme comprising an iduronate-2-sulfatase polypeptide with at least 5 kilodalton (kDa) more sugar than iduronate-2-sulfatase purified from a natural source, e.g. human liver. The present invention also provides an enzymatically active polypeptide fragment or variant of such a highly glycosylated iduronate-2-sulfatase. The present invention further provides an isolated nucleic acid encoding iduronate-2-sulfatase, as well as an expression vector, a host cell and a method for producing the present highly glycosylated iduronate-2-sulfatase enzyme. In one embodiment the present invention is directed to a method for producing a glycosylated iduronate-2-sulfatase enzyme which comprises culturing a host cell containing a nucleic acid encoding an enzymatically active iduronate-2-sulfatase polypeptide wherein the host cell glycosylates the polypeptide to a greater degree than a native iduronate-2-sulfatase polypeptide expressed by a natural human liver cell.
Owner:WOMENS & CHILDRENS HOSPITAL
Activity expression and application of thermobifida fusca xylose isomerase in wine brewing yeast
The invention discloses a method for extraction of xylose isomerases by taking brown hot crack loving spore fungus as sources and the activity expression and application of the brown hot crack loving spore fungi xylose isomerases in Saccharomyces cerevisiae. The molecule weight of the brown hot crack loving spore fungi xylose isomerases is 43 kilodaltons; the most suitable reaction temperature of the xylose isomerases is 80 to 85 DEG C; and the most suitable reaction pH of the xylose isomerases is 7.0. The xylose isomerases can convert xyloses into xyluloses under normal biochemical reaction conditions; genes of the xylose isomerases can be recombined into the Saccharomyces cerevisiae in which the activity expression is realized, and the xyloses or other carbon sources such as lignocellulose hydrolysate and so on can be utilized for production of various fermentation products such as alcohols and so on. The method utilizes the genetic engineering means to expand substrates which are converted by the alcohols and has important theoretical significance and application value on full utilization of lignocellulose resources.
Owner:GUANGXI ACAD OF SCI
Compound for enhancing immune response
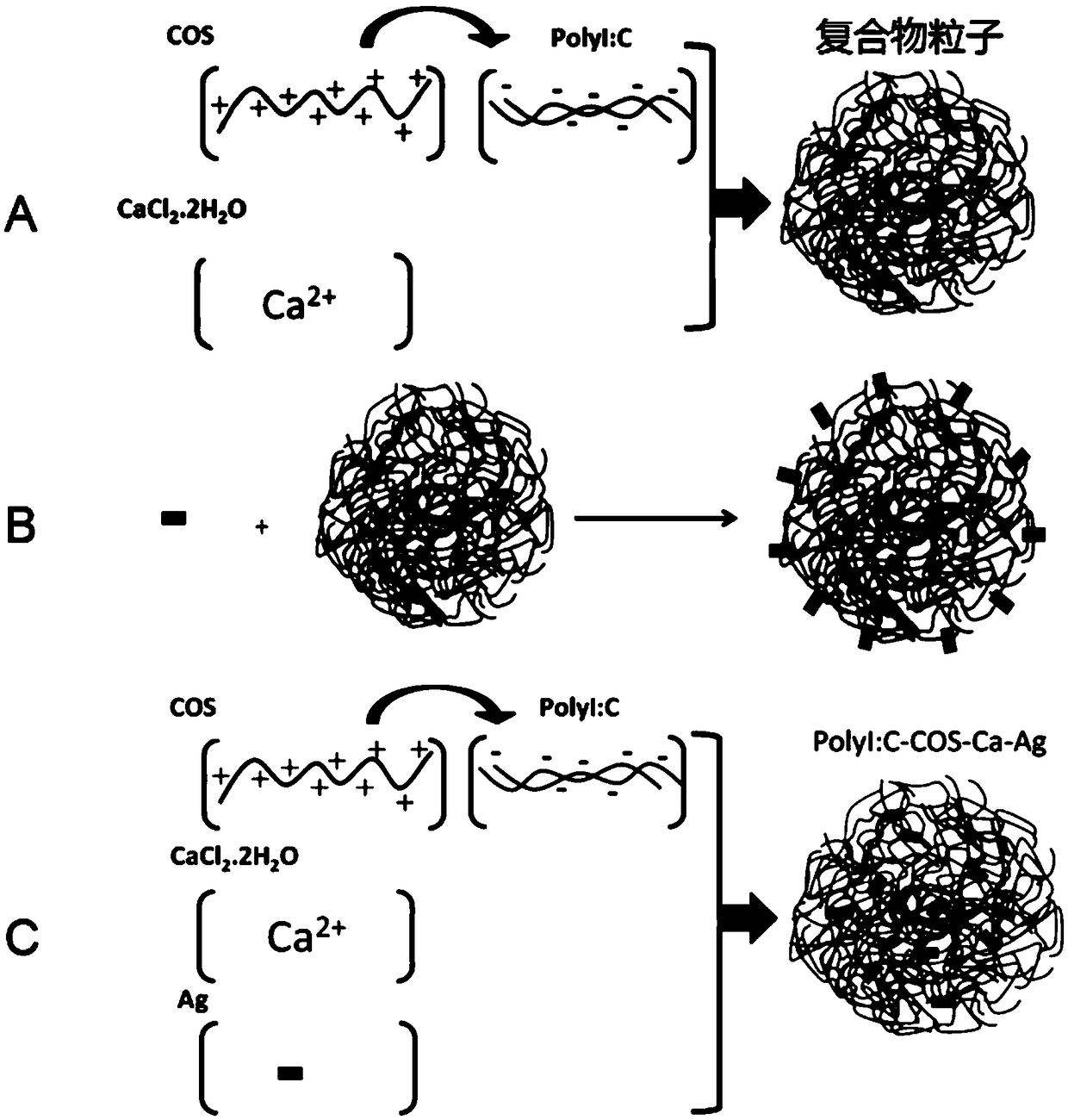
ActiveCN109078180AModerate viscosityModerate molecular massOrganic active ingredientsImmunological disordersDiseaseAnti virus
The invention relates to a novel compound, and researches the preparation, application and other aspects of the compound. The compound is prepared from at least the following components under suitableconditions: polyinosinic cells, cationic stabilizers and soluble calcium salts, wherein, the cationic stabilizer is a non-antibiotic amino compound with a molecular weight less than or equal to 5 kilodalton or a graft formed by the non-antibiotic amino compound and one or more of polyethylene glycol monomethyl ether, polyethylene glycol, polyethyleneimine, folic acid and galactose. The compound has moderate viscosity and molecular mass, convenient pharmacy, stable chemical properties, difficult degradation during long-term storage and safe use. The compound can significantly enhance the nonspecific immune response of the body when the compound is used alone to achieve the purpose of preventing and treating diseases, and has better anti-tumor, anti-virus and anti (super) bacteria functionswhen the compound is used in combination with other medicines and is easy to be absorbed by patients.
Owner:XINFU BEIJING MEDICAL TECH CO LTD
Recombinant protein containing a C-terminal fragment of plasmodium MSP-1
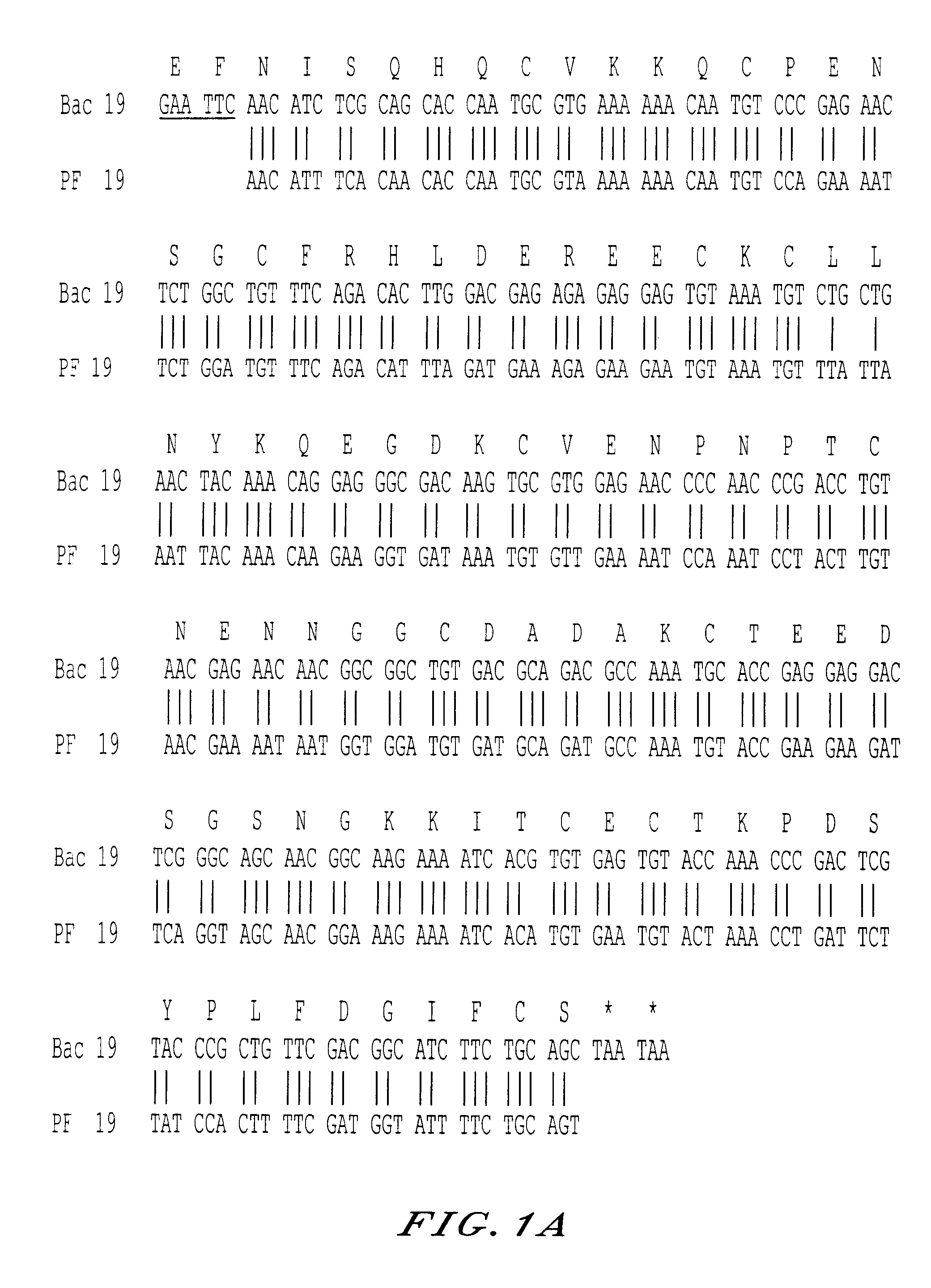
The invention relates to a recombinant protein fabricated in a baculovirus system, of which the essential constitutive polypeptide sequence is that of a C-terminal fragment of 19 kilodalton (p19) of the surface protein 1 (protein MSP-1) of the merozoite parasite of the Plasmodium type, particularly Plasmodium falciparum, which is infectious for humans, said C-terminal fragment remaining normally anchored at the surface of the parasite at the end of its penetration phase into human erythrocytes, in the occurrence of an infectious cycle. Said recombinant protein is applicable to the production of vaccines against malaria.
Owner:INST PASTEUR +1
Endoglucanases
In one aspect, the invention provides a purified thermostable enzyme derived from the archael bacterium AEPII 1a. In one aspect, the enzyme has a molecular weight of about 60.9 kilodaltons and has a cellulase activity. The enzyme can be produced from native or recombinant host cells, and can be used to aid in the digestion of cellulose. The invention also provides polypeptides having endoglucanase activity having homology to SEQ ID NO:2.
Owner:BP CORP NORTH AMERICA INC
In vitro formation of congophilic maltese-cross amyloid plaques to identify anti-plaque therapeutics for the treatment of Alzheimer's and Prion diseases
InactiveUS20020168753A1Test effectivenessCompounds screening/testingNervous disorderCongo redNeuroglycan C
Co-incubation of an amyloid protein with sulfated macromolecules as a method for the formation of amyloid plaques. The amyloid protein may be the beta-amyloid protein or the prion protein or the like. Amyoid plaque formation in one embodiment proceeds in vitro and desireably produces amyloid plaques that stain with Congo red and demonstrate a maltese-cross pattern when viewed under polarized light. The method also produces amyloid plaques that demonstrate an "amyloid star" appearance when viewed by transmission electron microscopy. Sulfated macromolecules include a sulfated proteoglycan selected from the group consisting of perlecan, ~220 kilodalton heparan sulfate proteoglyean, glypican, cerebroglycan, aggrecan, synaptoglycan (SV2PG), syndecan, N-syndecan (also known as syndecan-3), syndecan-1, syndecan-4, neurocan, phosphacan, decorin, biglycan, versican, amphiglycan, lumican, PG-M, PG-M (3), agrin, betaglycan, claustrin, brevican, appican, epican, neuroglycan-C, and fragments thereof. Thw sulfated macromolecule may be a sulfated glycosaminoglycan selected from the group consisting of heparin, heparan sulfate, dermatan sulfate, chondroitin sulfate, keratan sulfate, and fragments thereof. An in vivo assay is also presented for selecting a candidate therapeutic agent for inhibiting or disrupting amyloid plaque deposition or persistence. The assay includes a) pre-forming congophilic maltese-cross amyloid plaques in vitro following incubation of an amyloid protein and a selected sulfated macromolecule, b) using a first cannula and osmotic pump to continuously infuse for a selected duration the pre-formed congophilic maltese-cross amyloid plaques into a tissue or organ, c) changing the first cannulae and osmotic pump with a second cannulae and osmotic pump to administer the candidate therapeutic, and d) detecting the candidate therapeutic's ability to disrupt, reduce, or eliminate congophilic maltese-cross amyloid plaque deposition / persistence in the tissue or organ.
Owner:UNIV OF WASHINGTON
Rapid Isolation of Osteoinductive Protein Mixtures from Mammalian Bone Tissue
InactiveUS20070049731A1Improved and simplifiedRapid and high and isolationPeptide/protein ingredientsBone-inducing factorBones demineralizationUltrafiltration
A method for purifying bone-derived osteoinductive proteins including a demineralization process, a protein extraction process, a high molecular weight ultrafiltration process, a low molecular weight ultrafiltration process, and a recovery process. The high and low ultrafiltration processes preferably select proteins having a nominal molecular weight between approximately 8 kilodaltons and approximately 100 kilodaltons. Processes of the present invention may be used to recover osteoinductive proteins from bone demineralization waste streams.
Owner:ZIMMER ORTHOBIOLOGICS
Therapeutic applications of laminin and laminin-derived protein fragments
Laminin and specific laminin-derived protein fragments are disclosed as potent inhibitors of Alzheimer's disease type amyloidoses. A specific region is identified within laminin which interacts with the Alzheimer's disease beta-amyloid protein and contributes to the observed inhibitory and therapeutic effects.A prominent ˜130 kilodalton band in laminin was found in human serum and cerebrospinal fluid which primarily interacted with Aβ as determined by ligand blotting methodology. This ˜130 kilodalton laminin fragment is known as the E8 fragment and is also believed to consist of the globular domains of the laminin A chain. The interaction of specific laminin fragments such as the newly discovered ˜130 kDa protein is believed to bind Aβ in biological fluids and keep it in a soluble state.
Owner:UNIV OF WASHINGTON
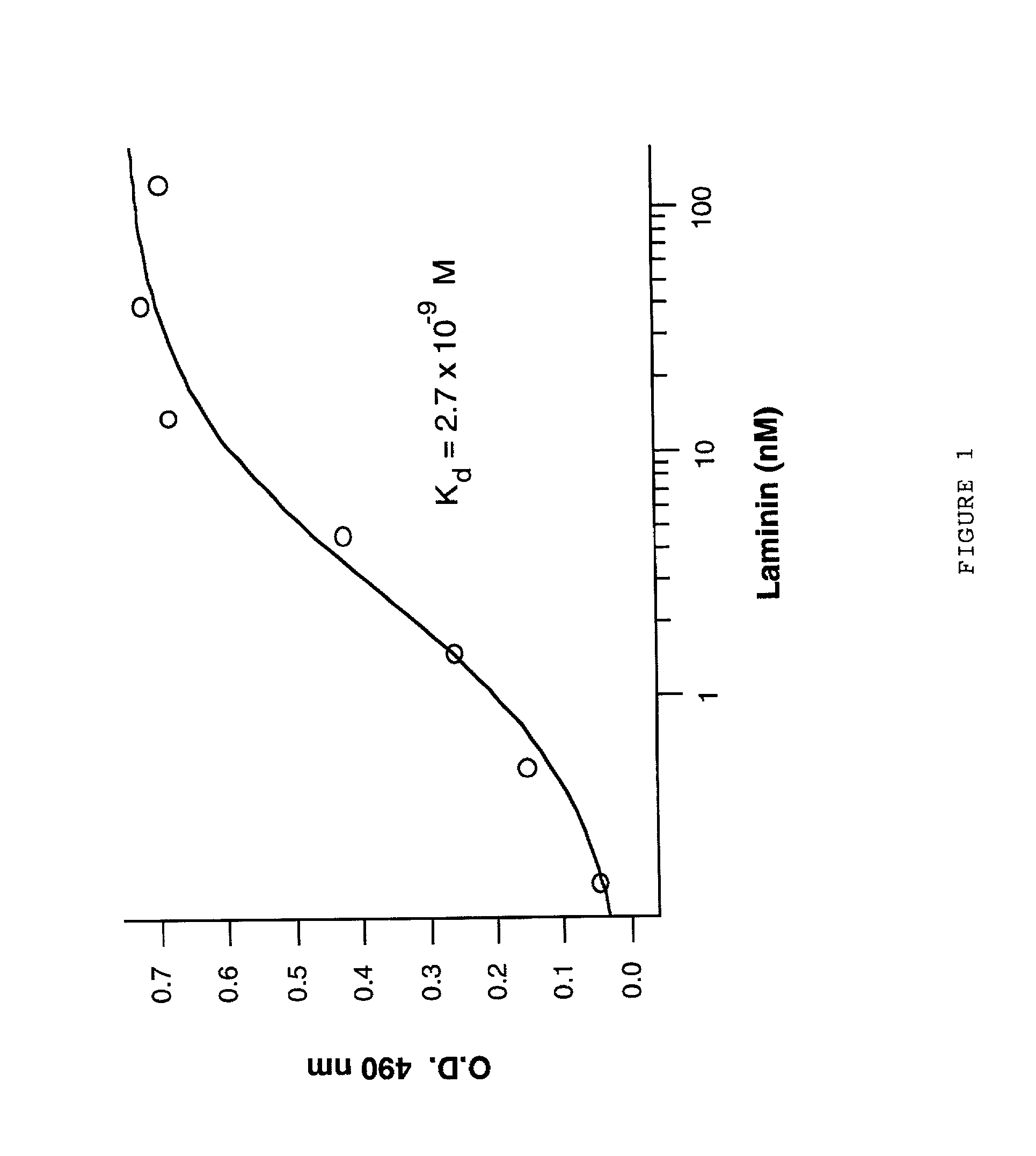
YIGSR peptidomimetics and methods for using the same
YIGSR peptidomimetics and methods for making and using the same are provided. The subject YIGSR peptidomimetics are non-peptidic, biodegradation-resistant molecules that mimic the pentapeptide tyrosine-isoleucine-glycine-serine-arginine (YIGSR) binding site for the 67 kiloDalton (kDa) laminin binding protein (LBP). The subject peptidomimetics include a rigid or substantially rigid non-peptidic scaffold that effectively presents or positions a tyrosine or tyrosine-like group, an isoleucine or isoleucine-like group, a serine or serine-like group, and an arginine or arginine-like group in substantially the same manner as occurs in the YIGSR peptide itself. The subject peptidomimetics find use in a variety of different applications, including diagnostic and therapeutic applications.
Owner:RGT UNIV OF CALIFORNIA
Recombinant bacillus phytases and uses thereof
The present invention clones and analyzes two phytase genes from microorganisms recognized as safe: Bacillus licheniformis and Bacillus subtilis 168. A method for overexpression and purification of phytase was established. These enzymes have a molecular weight of approximately 48 kDa (kilodaltons) and possess extracellular phytase hydrolytic activity. These recombinant enzymes can be used to enhance phytases in various commercial applications, including the production of animal feed and transgenic plants with increased rates of mature growth, flowering and fruiting.
Owner:THE UNIVERSITY OF HONG KONG
Rapid Isolation of Osteoinductive Protein Mixtures from Mammalian Bone Tissue
InactiveUS20070249815A1Improved and simplifiedRapid and high and isolationPeptide/protein ingredientsBone-inducing factorBones demineralizationUltrafiltration
A method for purifying bone-derived osteogenic proteins including a demineralization process, a protein extraction process, a high molecular weight ultrafiltration process, a low molecular weight ultrafiltration process, and a recovery process. The high and low ultrafiltration processes preferably select proteins having a nominal molecular weight between approximately 8 kilodaltons and approximately 50 kilodaltons. Processes of the present invention may be used to recover osteogenic proteins from bone demineralization waste streams.
Owner:ZIMMER ORTHOBIOLOGICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com