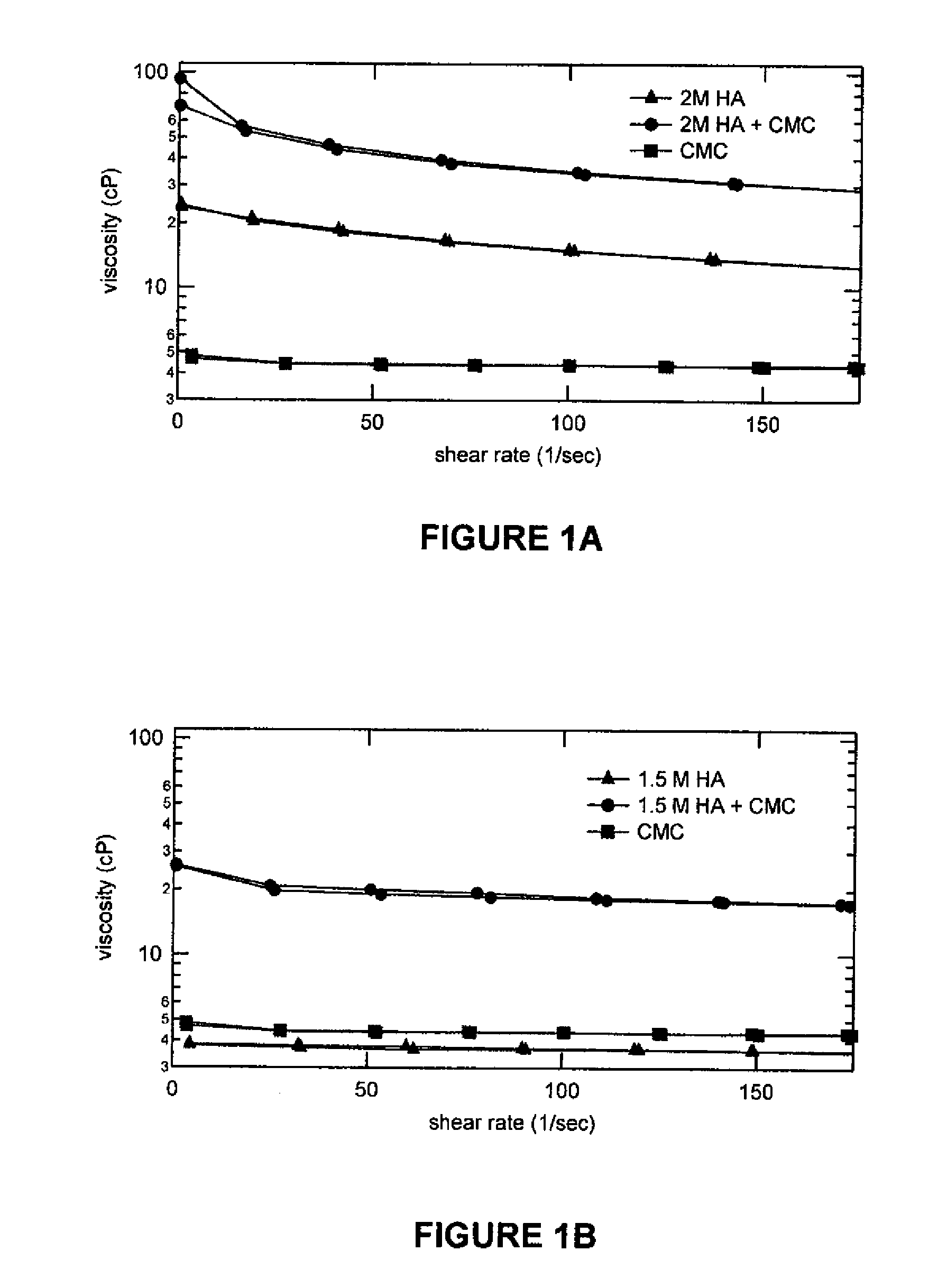
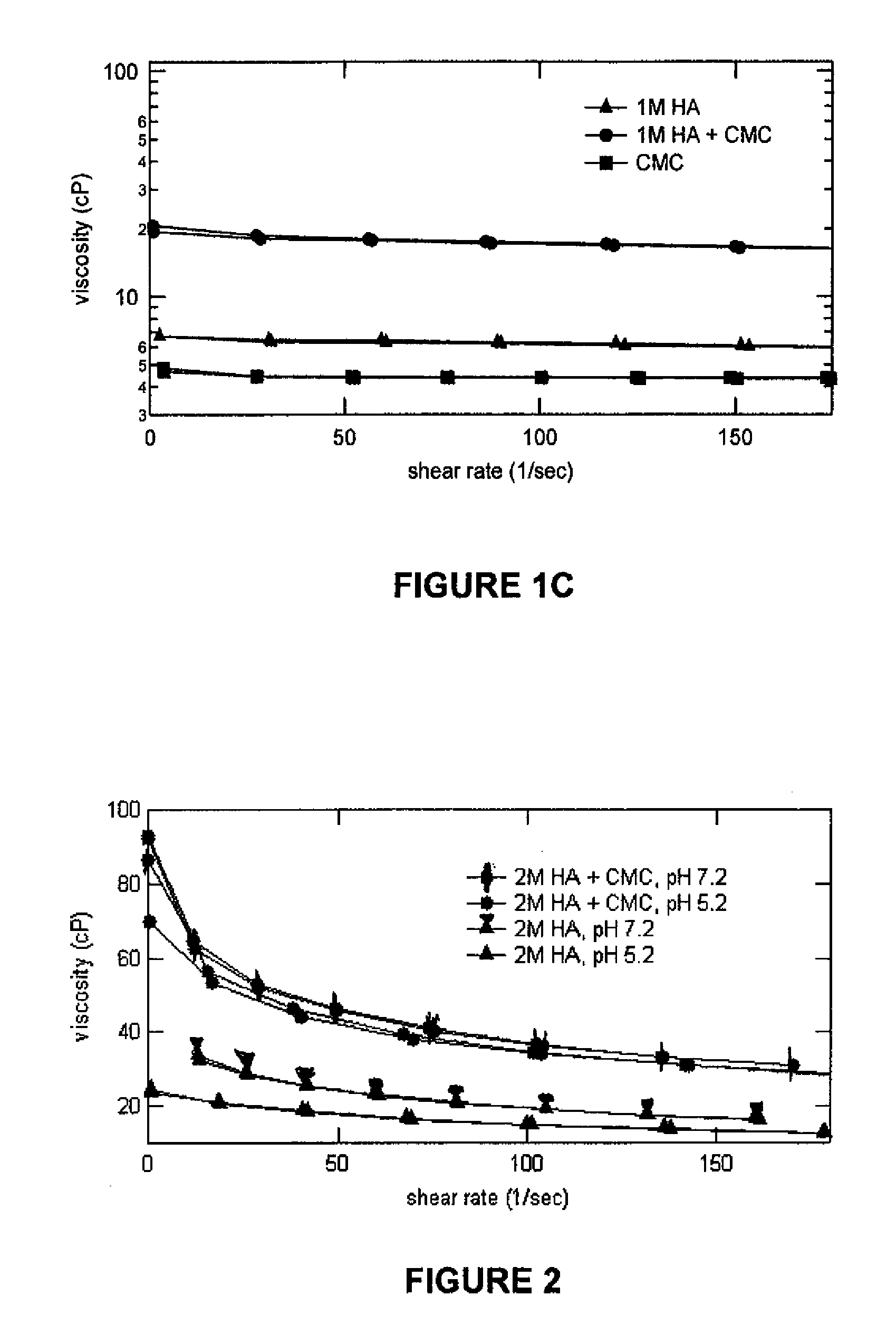
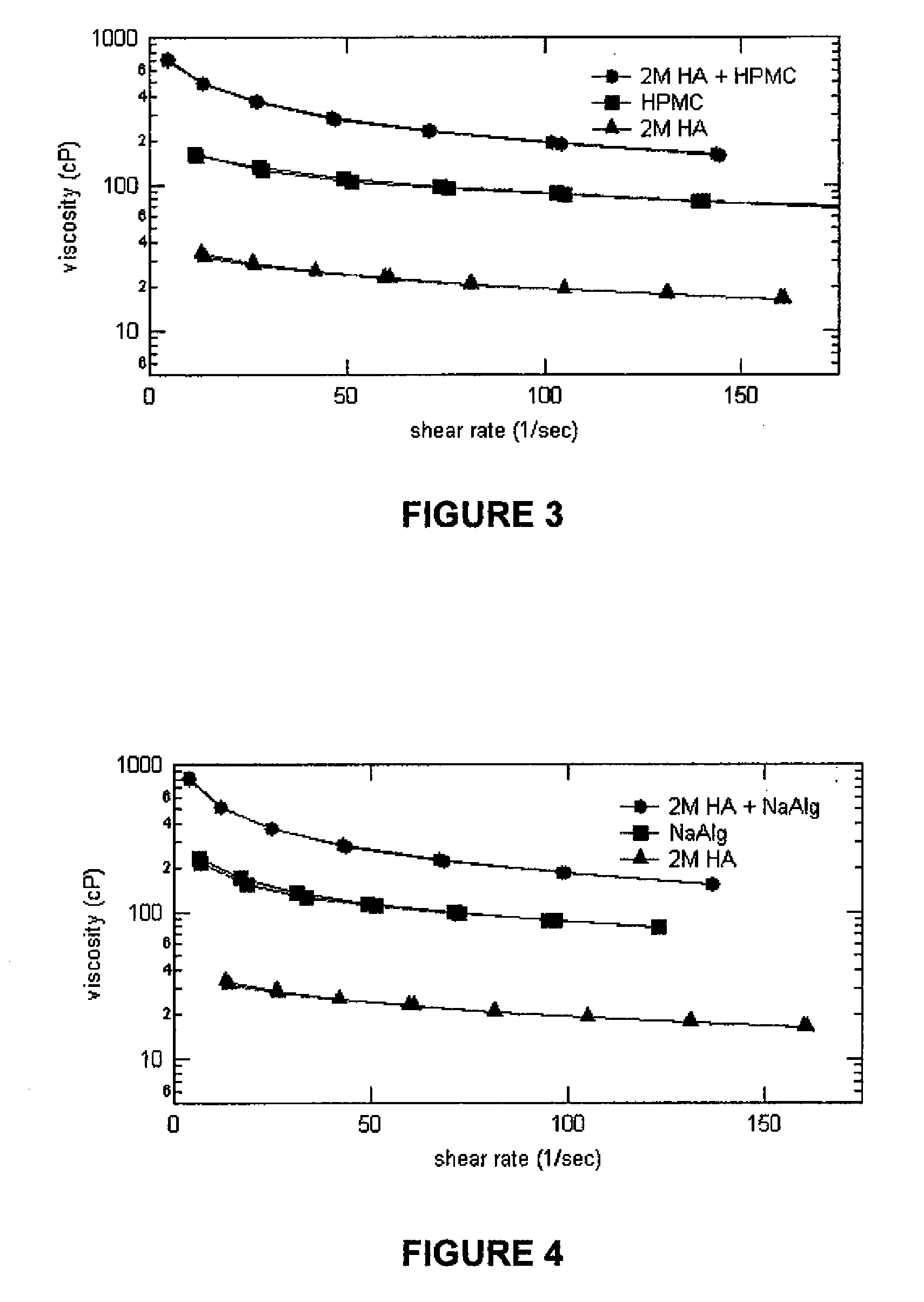
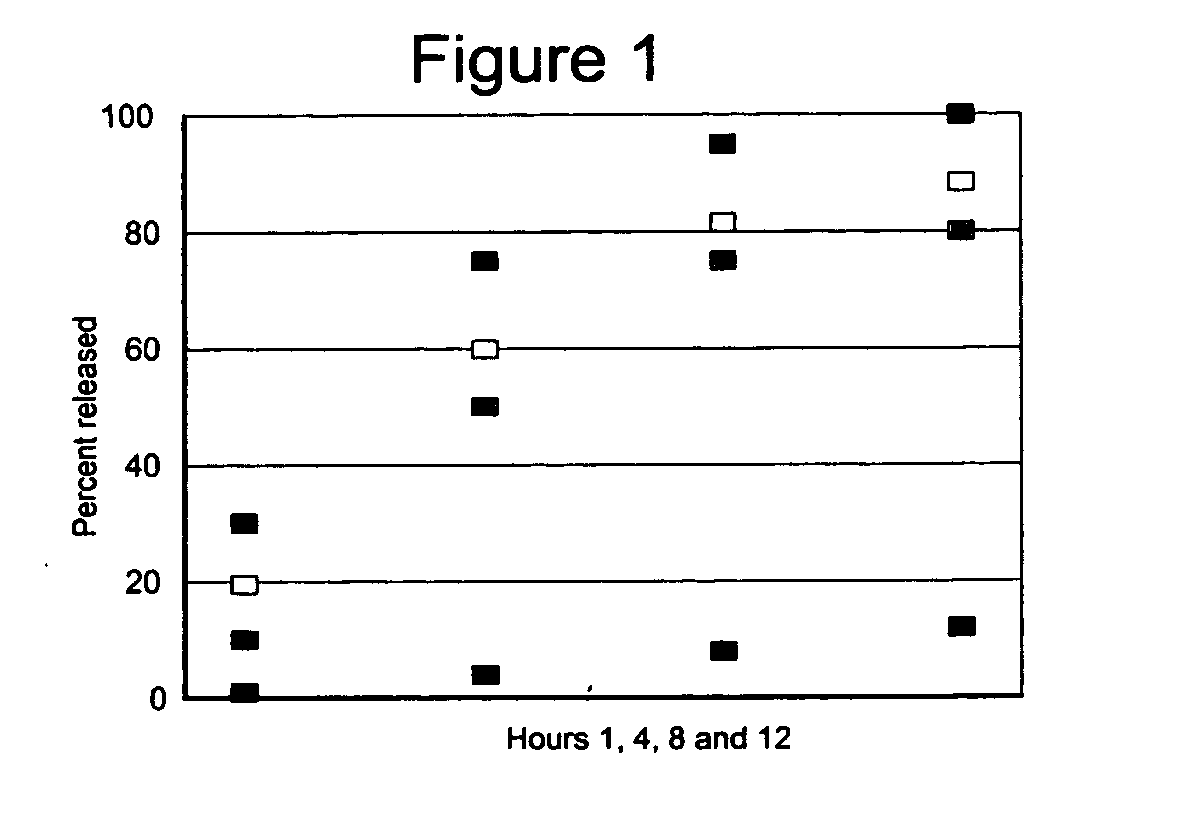
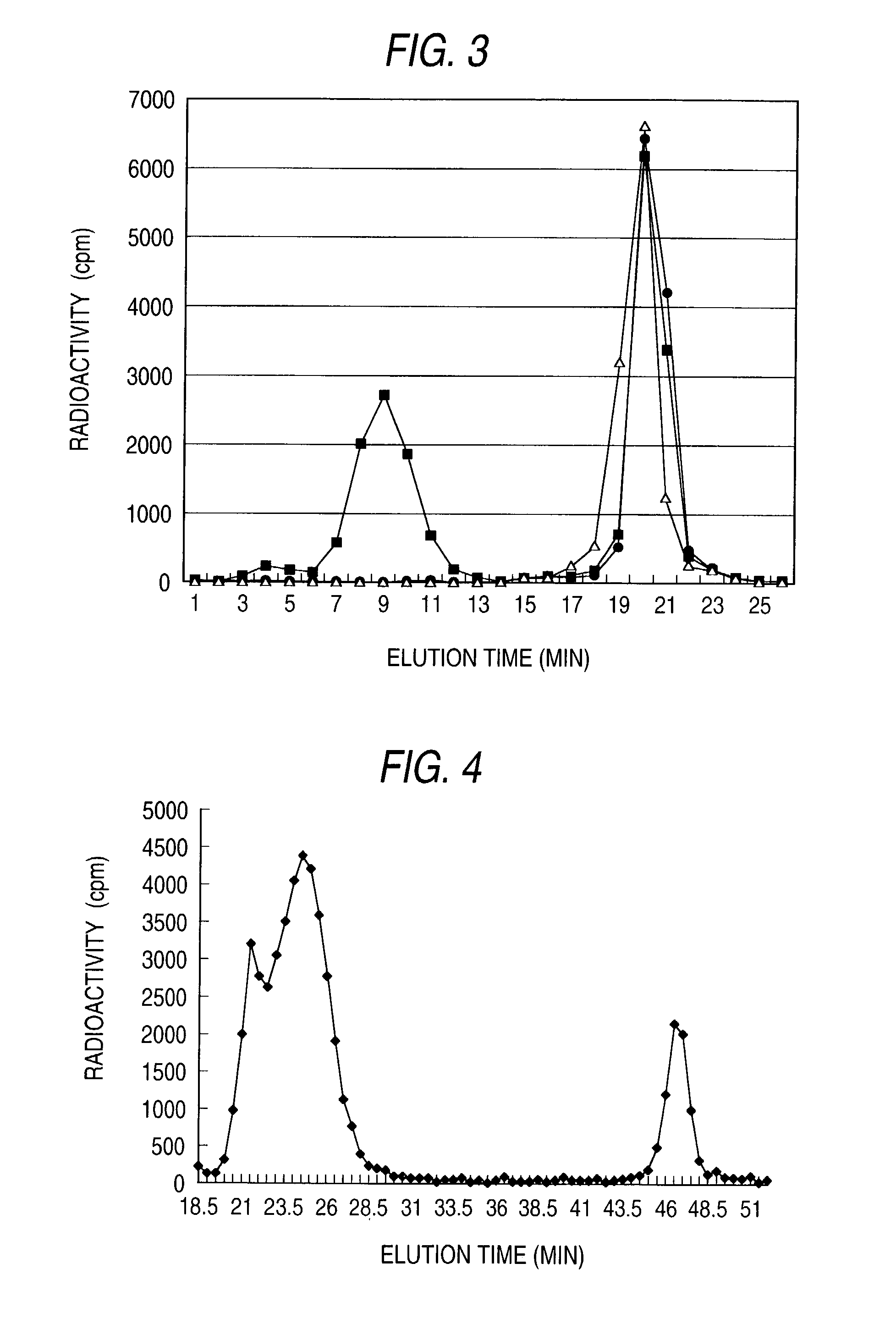
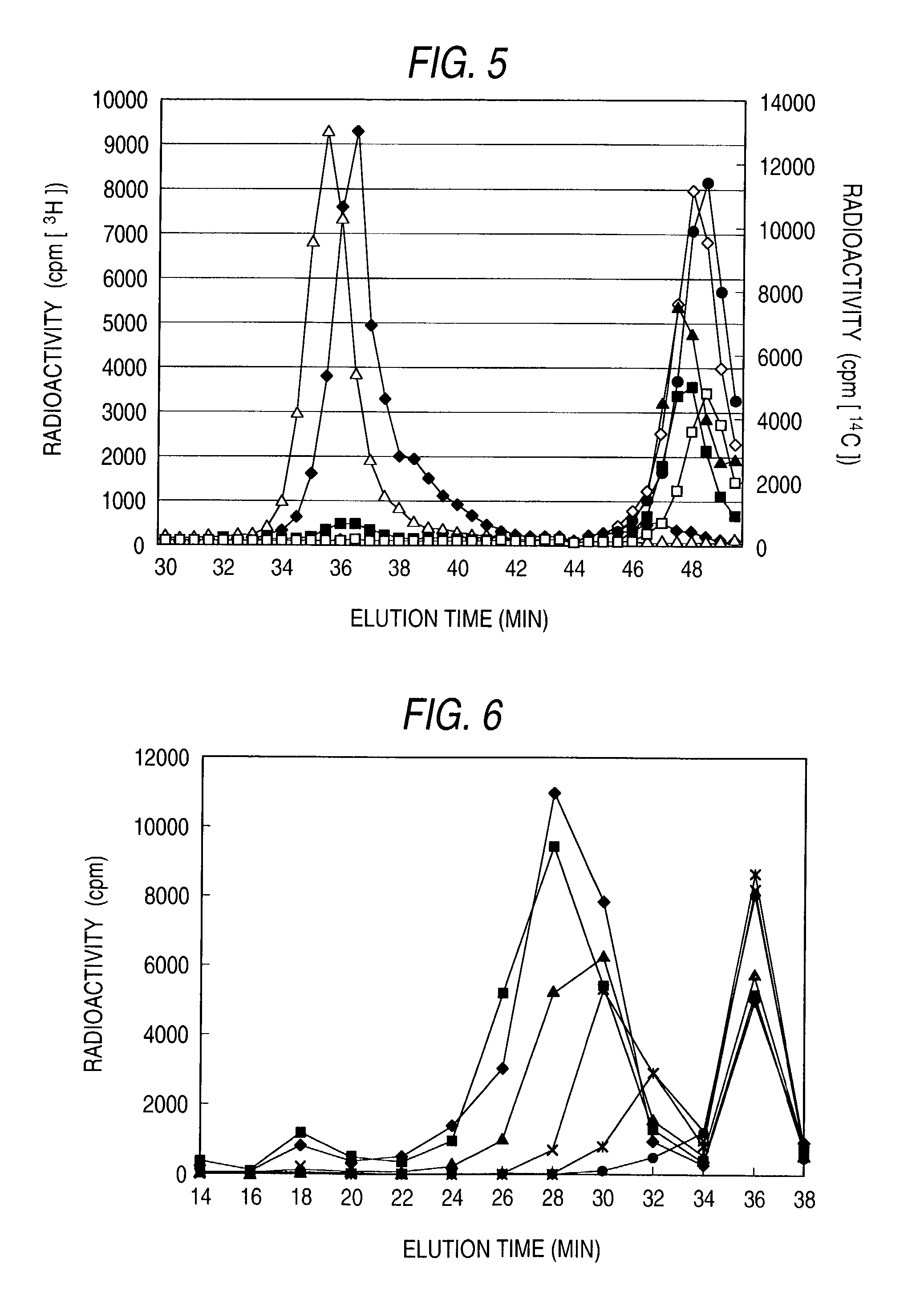
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
914 results about "Chondroitin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
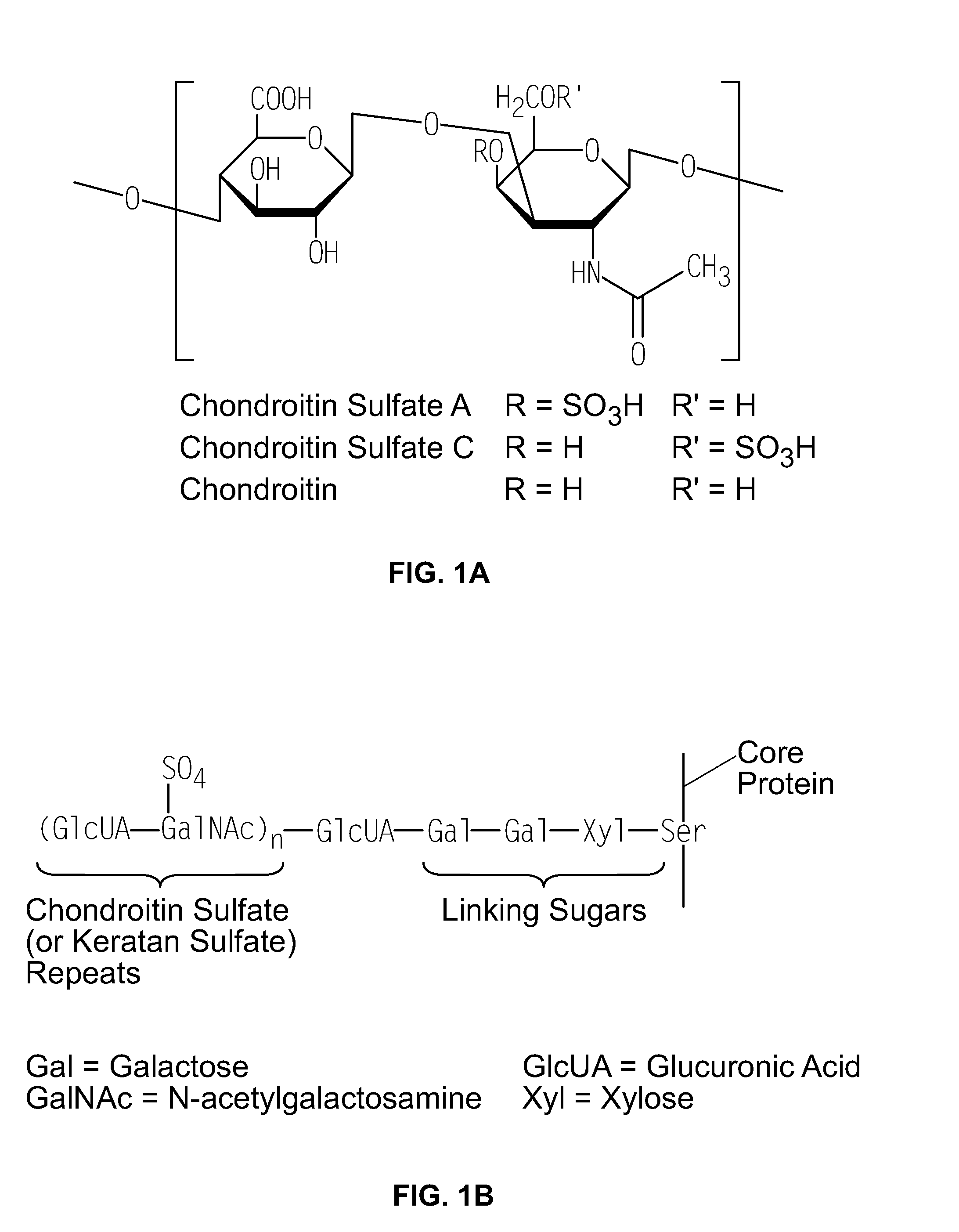
A chondroitin is a chondrin derivative.
Method for supplementing the diet
InactiveUS6579544B1Increased susceptibilityPrevent diseaseHeavy metal active ingredientsBiocideDietary supplementAlpha-Lipoic Acid
A dietary supplement blend composition is disclosed, the basic formulation of the composition containing vitamins, minerals, and carotenoids. The composition can also contain bioflavonoids, cartilage protectors such as glucosamine or chondroitin, alpha-lipoic acid, coenzyme Q10, and a source of omega-3 fatty acids such as flax seed oil. The composition is beneficial for improving health and preventing disease, particularly for degenerative conditions. A method for supplementing the diet is also disclosed, wherein the quantity of daily rations of the dietary supplement blend composition is determined based on the person's age, body weight, and quality of diet.
Owner:NUTRIEX
Method of using hydroxycarboxylic acids or related compounds for treating skin changes associated with intrinsic and extrinsic aging
A composition comprising an amphoteric or pseudo-amphoteric agent and a polyhydroxy alpha hydroxyacid existing as a free acid, lactone, or salt, and isomeric or non-isomeric forms thereof is provided. The amphoteric or pseudo-amphoteric agent can be selected from amino acids, dipeptides, aminoaldonic acid, aminouronic acid, lauryl aminoproplyglycine, aminoaldaric acid, neuraminic acid desulfated heparin, deacetylated hyaluronic acid, hyalobiuronic acid, chondrosine, deacetylated chondroitin, creatine, creatinine, hydroxyproline, homocysteine, homocystine, homoserine, ornithine, citrulline, phosphatidylserine, and sphingomyelin. The composition may contain other additives, including cosmetic or pharmaceutical agents for topical treatment of dermatological disorders.
Owner:TRISTRATA TECH
Stabilized Glycosaminoglycan Preparations and Related Methods
Compositions comprising a glycosaminoglycan (e.g., a hyaluronan, hyaluronic acid, hyaluronate, sodium hyaluronate, dermatan sulfate, karatan sulfate, chondroitin 6-sulfate, heparin, etc.) in combination with at least one component selected from; i) polyglycols (e.g., polyethylene glycol), ii) long chain hydroxy polyanionic polysaccharides (e.g., dextran, sodium alginate, alginic acid, propylene glycol alginate, carboxymethyl cellulose and carboxyethyl cellulose, hydroxyl ethyl starch, hydroxyl propyl methyl cellulose, hydroxy propyl ethyl cellulose, hydroxy propyl cellulose, methyl cellulose, polylysine, polyhistidine, polyhydroxy proline, poly ornithine, polyvinyl pyrolidone, polyvinyl alcohol, chitosan, etc.) and iii) long chain Nitrogen containing polymers (e.g., Polylysine, Polyvinylpyrrolidone, and polyvinyl alcohol). The invention also includes methods for using such compositions (e.g., as substance delivery materials, tissue fillers or bulking agents, as moistening or hydrating agents, etc.)
Owner:S K PHARMA INC
Pharmaceutical composition and method for the transdermal delivery of calcium
InactiveUS20070292493A1Reduce disadvantagesReduce and prevent likelihoodHalogenated hydrocarbon active ingredientsBiocideArginineTryptophan
The present invention relates to a method and transdermal pharmaceutical composition for preventing or reducing the likelihood of calcium deficiency or imbalances caused by calcium deficiency. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of calcium and a pharmaceutically acceptable carrier constituting a pluronic lecithin organogel. In addition to calcium, the transdermal pharmaceutical composition may also contain a therapeutically effective amount of: (1) a pharmaceutically acceptably salt of other minerals such as magnesium, zinc, selenium, manganese, or chromium; (2) a vitamin such as vitamin A, vitamin D, vitamin C, vitamin E or B-complex vitamins, choline, lecithin, inositol, PABA, biotin, or bioflavomoids; (3) a carotenoid such as lycopene or lutein; (4) a hormone such as dehydroepiandrosterone, progesterone, pregnenolone, or melatonin; (5) an amino acid such as arginine, glutamine, lysine, phenylalanine, tyrosine, GABA, tryptophan, carnitine, or acetyl-l-carnitine; (6) a fatty acid such as a fish oil or flax seed oil; (7) a vita-nutrient such as coenzyme Q10; (8) a cartilage building nutrient such as glucosamine, chondroitin, or MSM, (9) a herb such as ginkgo biloba, echinacea, 5-HTP, St. John's wort, or saw palmetto; or (9) any combination thereof. The transdermal pharmaceutical composition may be topically administered to a human to prevent or reduce the likelihood of calcium deficiency or imbalances caused by calcium deficiency such as hypertension, high cholesterol, colon and rectal cancer, osteomalacia, rickets, osteoporosis, cardiovascular disease, preeclampsia, tooth decay, and premenstrual syndrome.
Owner:BRIERRE BARBARA T
Compositions and methods for timed release of water-soluble nutritional supplements
InactiveUS20050181047A1Great flexibility in designingIncrease in plasma levelSulfur/selenium/tellurium active ingredientsFood shapingGreek letter betaGlucosamine Sulfate
The present invention relates to compositions of and methods for producing timed or retarded release formulations that contain glucosamine sulfate, beta-(1,4)-2-amino-2-deoxy-D-glucose, and chondroitin, (C14H19NO14SNa2)n; N-acetylchondrosanine (2-acetamide-2-deoxi-D-galactopiranose) and D-gluoronic acid copolymer and / or their dietary and nutraceutically acceptable salts of the same and / or hydrates of the active substance that provide a timed release formulation of the active substance.
Owner:OSMOPHARM USA
Treatment of central nervous system damage
InactiveUS20050118157A1Decreased inhibitory propertiesImprove plasticityCompound screeningNervous disorderNervous systemChondroitinase ABC
The invention provides a method of promoting neuronal plasticity in the CNS of a mammal, the method comprising administering to the CNS of the mammal an agent that reduces the inhibitory properties of chondroitin sulphate proteoglycans. Preferred agents are chondroitinases and sulfatases, e.g., chondroitinase ABC. Also provided are methods of identifying further agents.
Owner:KING'S COLLEGE LONDON +1
Human chondroitinase glycoprotein (CHASEGP), process for preparing the same, and pharmaceutical compositions comprising thereof
InactiveUS7544499B2BacteriaSugar derivativesChondroitinase activityChondroitin Sulfate Proteoglycans
Owner:HALOZYME
Regeneration of articular cartilage damaged by grade I and II osteoarthritis by means of the intraarticular application of a mixture of sodium hyaluronate and chondroitin sulfate in a gel vehicle
Methods of treating osteochondral lesions by the intraarticular application of a mixture of sodium hyaluronate and chondroitin sulfate are disclosed.
Owner:ALCON INC
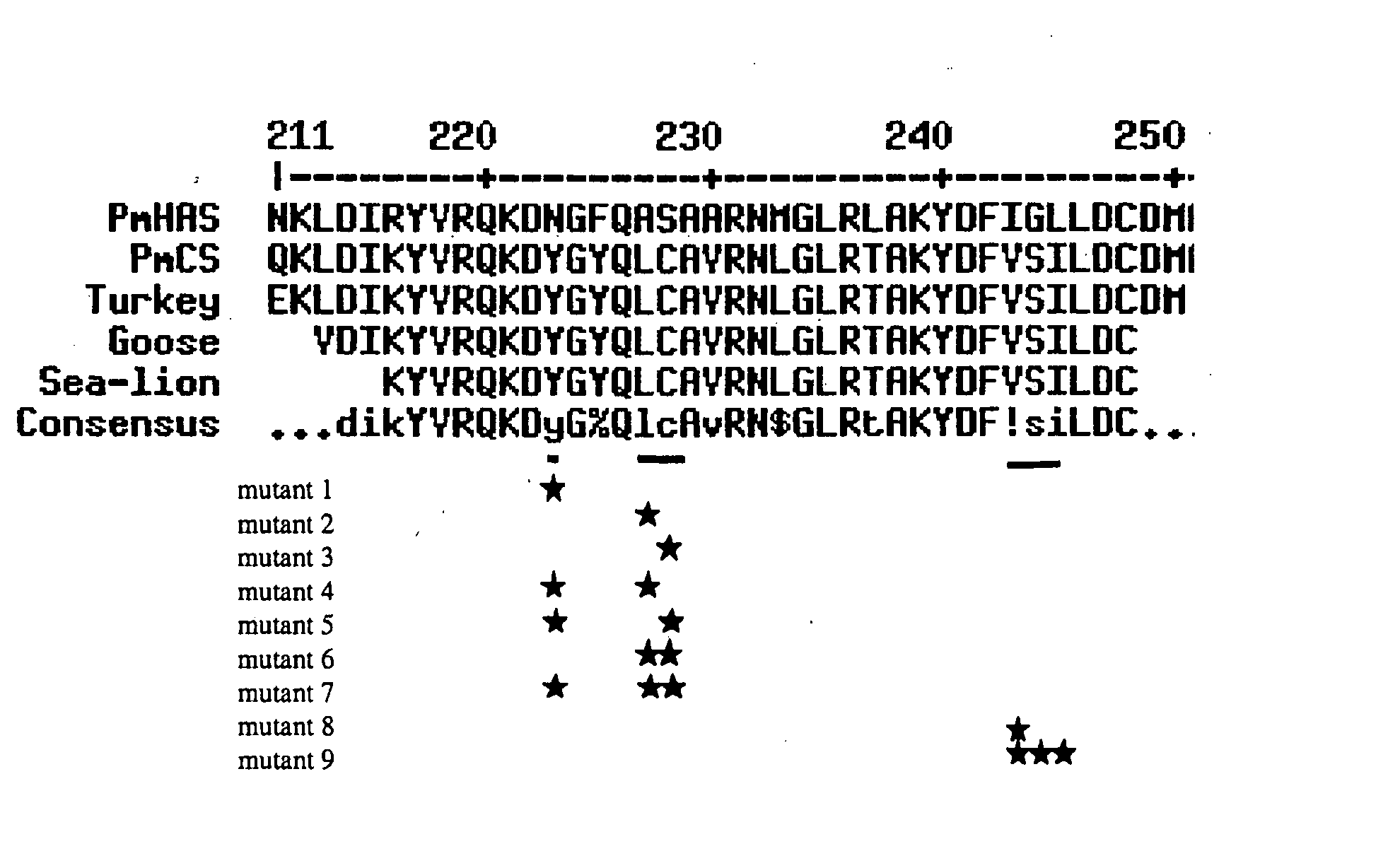
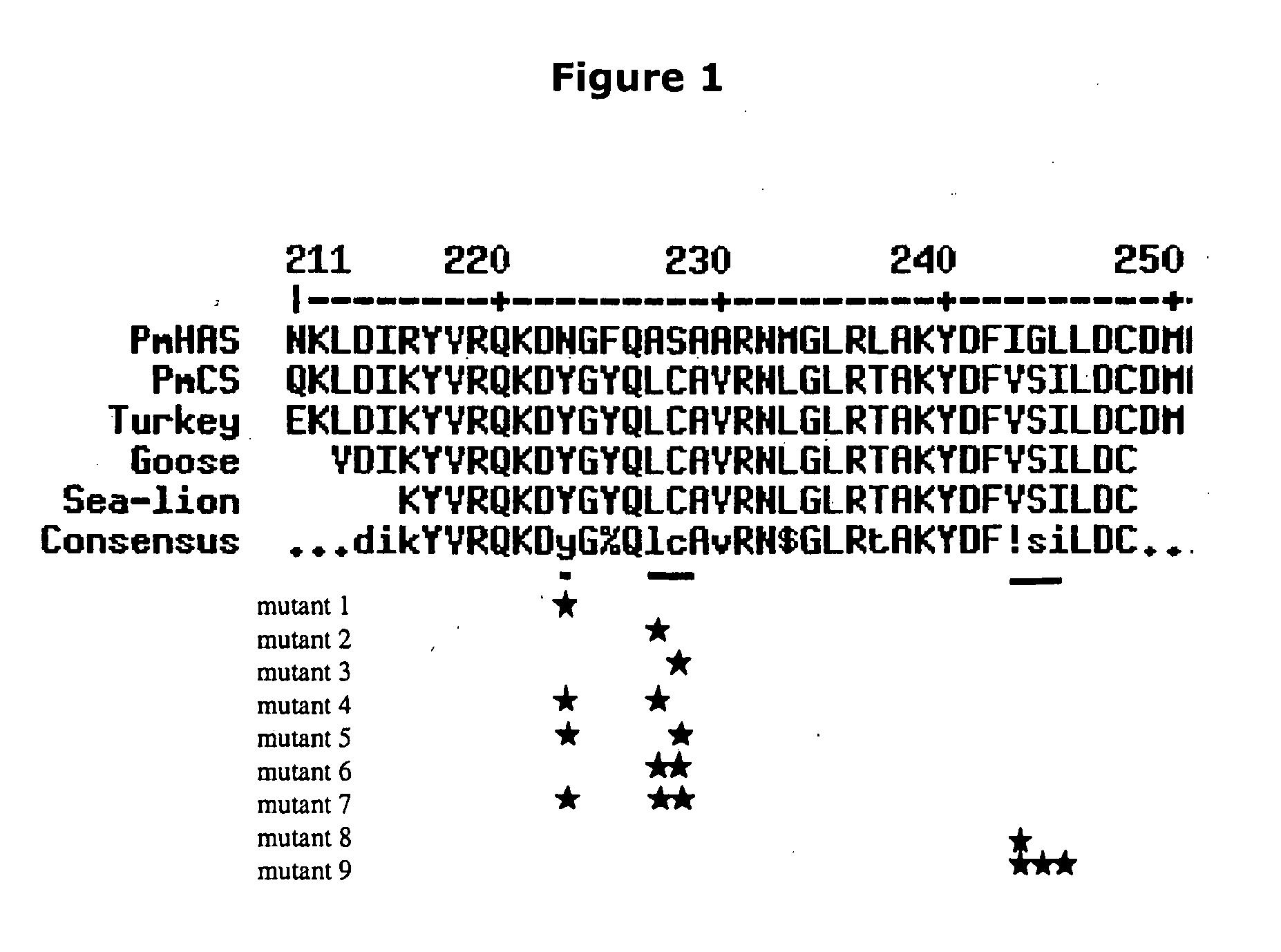
Chondroitin polymerase and DNA encoding the same
A chondroitin polymerase having such properties that it transfers GlcUA and GalNAc alternately to a non-reduced terminal of a sugar chain from a GlcUA donor and a GalNAc donor, respectively, and the like; and a process for producing the chondroitin polymerase.
Owner:SEIKAGAKU KOGYO CO LTD
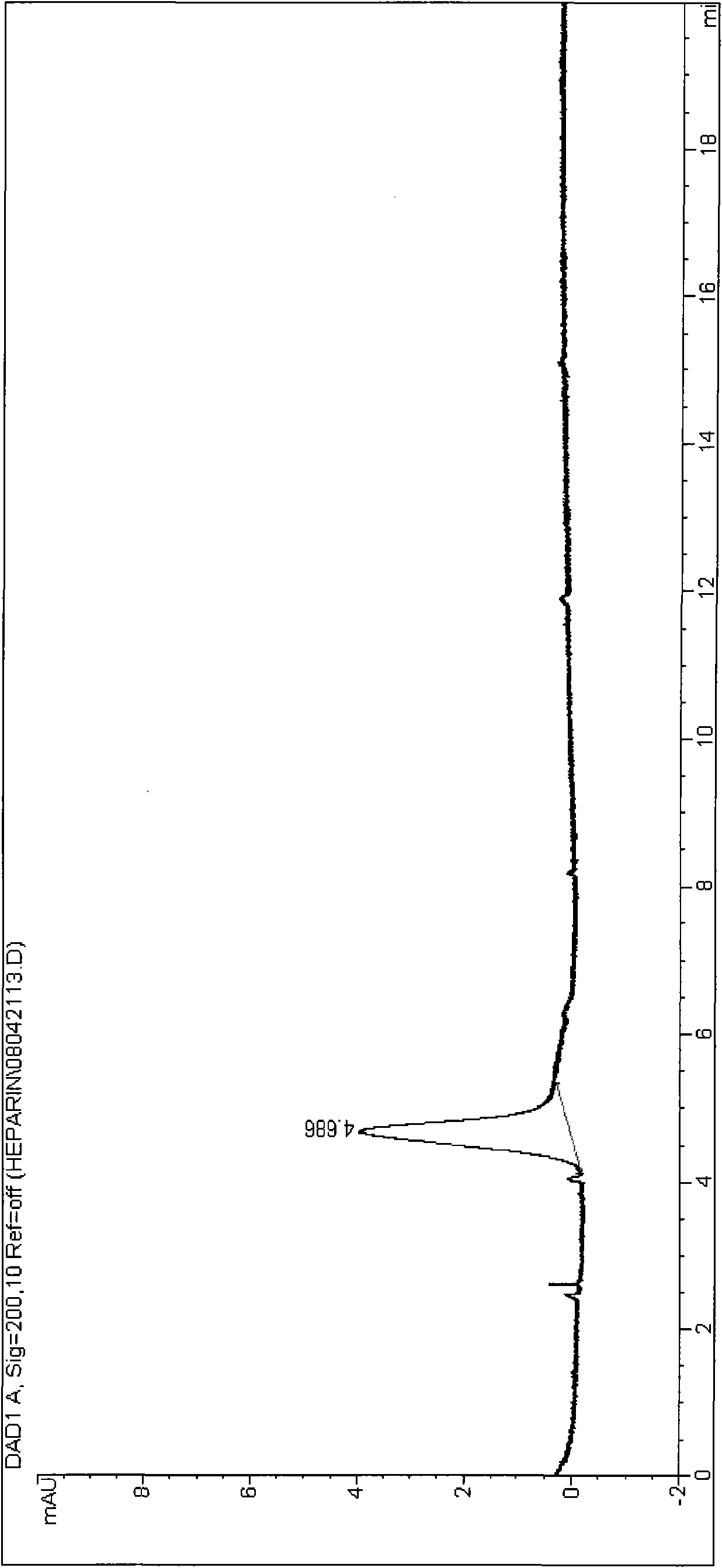
Method for separating chondroitin polysulfate from heparin sodium by extraction method
ActiveCN101575385APromote pig productionLiquid solutions solvent extractionFermentationCentrifugationDissolution
The invention discloses a method for separating chondroitin polysulfate from heparin sodium by an extraction method, breaking through the conclusion drawn by domestic and international experts, i.e., the chondroitin polysulfate in the heparin sodium is inseparable. The technical scheme is as follows: an acetone extraction method is adopted to remove the chondroitin polysulfate in the heparin sodium and thoroughly separate the chondroitin polysulfate from the heparin sodium by the refinement steps of crude product dissolution, enzymolysis, rapid heating, impurity centrifugation, a plurality of precipitation and oxidation so that the refined product rate of the heparin sodium reaches over 83 percent, the activity valence is over 180 usp / mg and over 200 iu / mg by being converted into the WHO international standard, and the quality standard accords with various indexes specified by Chinese pharmacopoeias, America pharmacopoeias, English pharmacopoeias and Western European pharmacopoeias.
Owner:QINGDAO JIULONG BIO PHARMA
Production of defined monodisperse heparosan polymers and unnatural polymers with polysaccharide synthases
InactiveUS20080109236A1Surgical adhesivesPharmaceutical delivery mechanismPolysaccharide synthesisSugar
The present invention relates to methodology for polymer grafting by a polysaccharide synthase and, more particularly, polymer grafting using the hyaluronate or chondroitin or heparin / heparosan synthases from Pasteurella, in order to create a variety of glycosaminoglycan oligosaccharides having a natural or chimeric or hybrid sugar structure with a targeted size that are substantially monodisperse in size. The present invention also relates to methodology for polymer grafting by a polysaccharide synthase to form glycosaminoglycan polymers having an unnatural structure.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Human chondroitinase glycoprotein (chasegp), process for preparing the same, and pharmaceutical compositions comprising thereof
ActiveUS20070148156A1Increase enzyme activityOptimize allocationBacteriaSugar derivativesChondroitinase activityChondroitin Sulfate Proteoglycans
The invention relates to the discovery of a novel Chondroitinase Glycoproteins (CHASEGP's), methods of manufacture, and potential uses in conditions where removal of chondroitin sulfates may be of therapeutic benefit. Chondroitinase Glycoproteins require both a substantial portion of the catalytic domain of the CHASEGP polypeptide and asparagine-linked glycosylation for optimal chondroitinase activity. The invention also includes carboxy-terminal deletion variants of CHASEGP that result in secreted variants of the protein to facilitate manufacture of a recombinant CHASEGP. Further described are suitable formulations of a substantially purified recombinant CHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity. CHASEGP is useful for the degradation of glycosaminoglycans and chondroitin sulfate proteoglycans under clinical conditions where their removal is of therapeutic value.
Owner:HALOZYME
Health food for enhancing human immunity and increasing bone mineral density and preparation method thereof
The invention relates to health food for enhancing human immunity and increasing bone mineral density and a preparation method thereof; the health food of the invention comprises the following components: hyaluronic acid, glucosamine, collagen, kudzu root extracts, chondroitin sulfate, vitamins, calcium, etc; because of the embedding processing of hyaluronic acid, the defect of property change of hyaluronic acid due to moisture absorption and influence of environmental factors is overcome; the health food prepared by the organic combination of the embedded hyaluronic acid with glucosamine, collagen, plant extracts, vitamins, mineral matter and the like has functions and effects which can supplement each other, has the effects of lubricating joints, increasing bone mineral density, and enhancing bone strength during long-term administration, and also has the functions of delaying aging, removing freckles and beautifying faces, and the like; the health food of the invention can be prepared into powder, granules, tablets, capsules, is convenient for carrying and taking, has stable and lasting efficacy, has no side effects, and is an ideal health food of patients with hypoimmunity and osteoporosis.
Owner:成都营养屋健康科技有限公司
Method and compositions for the treatment and prevention of pain and inflammation
InactiveUS20050101563A1Selective in their physiological impactBiocideSenses disorderCOX-2 inhibitorChondroitin
A method of preventing or treating pain or inflammation in a subject is provided by administering to the subject a Cox-2 inhibitor and a polyunsaturated fatty acid, or a prodrug thereof, wherein the amount of a Cox-2 inhibitor and polyunsaturated fatty acid or a pharmaceutically acceptable salt or prodrug thereof together constitute a pain or inflammation suppressing treatment or prevention effective amount. Glucosamine and / or chondroitin can optionally be present. Therapeutic compositions that contain the combination of Cox-2 inhibitor and polyunsaturated fatty acid and, optionally, the glucosamine and / or chondroitin, are disclosed, as are pharmaceutical compositions.
Owner:PHARMACIA CORP
Technology of animal high-calcium powder, chondrine and collagen by composite enzyme method
InactiveCN1896262AReduce pollution indexReduce acid and alkali consumptionFermentationFiltrationCollagen VI
The present invention relates to a biological fermentative production technology of extracting high-calcium powder, chondroitin sulfate and collagen from animal cartilage which includes the following steps: the animal cartilage is simply broken into 5-20mm fragments, mixed with 1-4 times weight of water in the reaction kettle, heated to 100DEG C and kept for 1-2 hours to denature the proteins, then cold water is passed over into the kettle jacket to cool down the kettle to 40-50DEG C, compound enzyme in mass ratio of 1:0.001 which is mainly collagenase is then added, the mixture is stirred and hydrolyzed for 8-12 hours under the condition of 40-50DEG C, pH 7-8, bone residue that contains calcium phosphate is obtained after simple filtration through 100-meshed sieves; the filtrate is clarificated after fine filtration with filter press, then ethanol is added to the final concentration of 60-70%, chondroitin will be sedimentated, while remaining liquid is the mixture of collagen and ethanol. The sedimentated calcium phosphate is washed and dried and becomes calcium phosphate powder. The crystal sediment of chondroitin is purified by ethanol-washing. Collagen is separated after the mixture of collagen and ethanol goes through the ethanol regenerating column. This technology can produce different products with high purity from animal cartilage and solves the problems of pollution and single product of traditional technology.
Owner:郭秀明
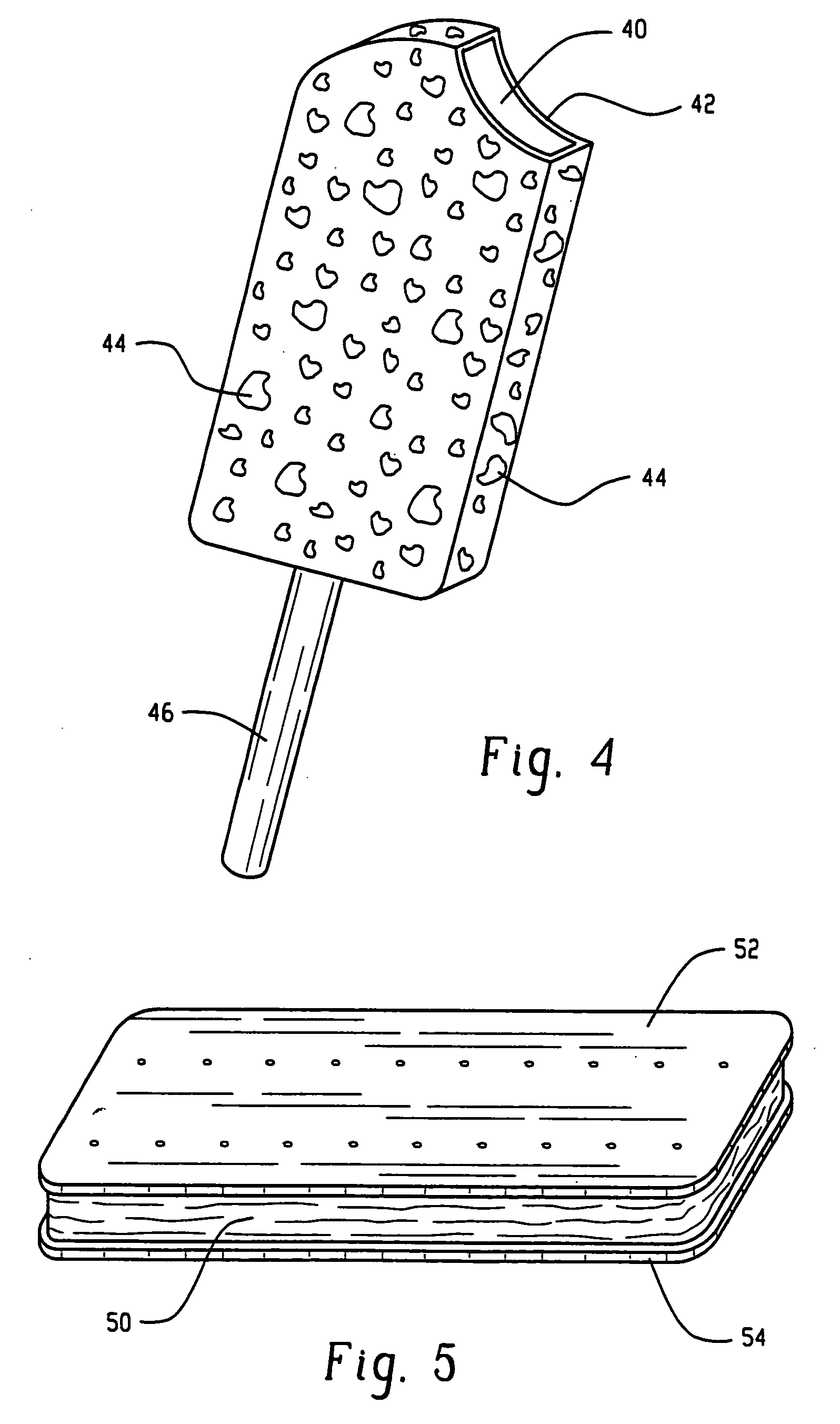
Cold canine treats
InactiveUS20050013899A1Easy to manageSafely chewableAnimal feeding devicesAnimal feeding stuffPush upsCompanion animal
A pet treat comprises a frozen mixture, the frozen mixture including protein, water, and a therapeutic substance such as glucosamine and / or chondroitin. The frozen mixture can be homogenized and further include entrained air bubbles, and optionally may have a meat flavor such as beef, chicken, lamb, or pork. A support structure, such as a stick, cone, push-up tube, or cup, can be provided to facilitate provision of the pet treat to the pet.
Owner:KOSTLAN GARY +1
Composition and methods for treating or preventing degenerative joint and cardiovascular conditions
A therapeutic composition and methods for the treatment and prevention of a degenerative joint disorder and / or a cardiovascular disease comprising polycosanols, glucosamine and chondroitin are disclosed.
Owner:TRX PHARMA
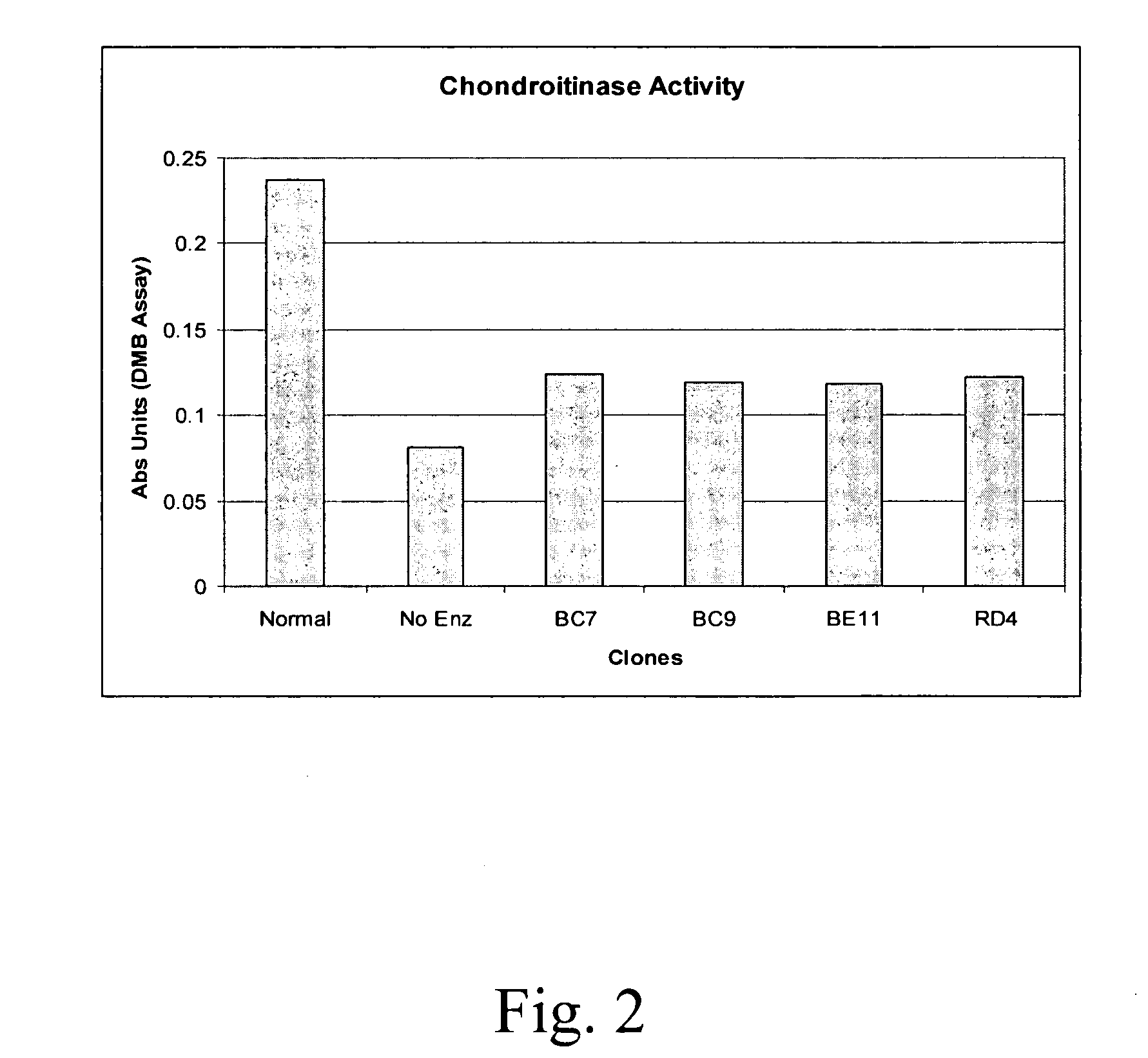
Compositions and methods of using chondroitinase ABCI mutants
One aspect of the present invention relates to mutants of chondroitinase ABCI. Such chondroitinase ABCI mutants exhibit altered chondroitin lyase activity or increased resistance to inactivation from stressors including exposure to UV light or heat. Methods of using chondroitinase ABCI mutant enzymes are also provided.
Owner:ACORDA THERAPEUTICS INC
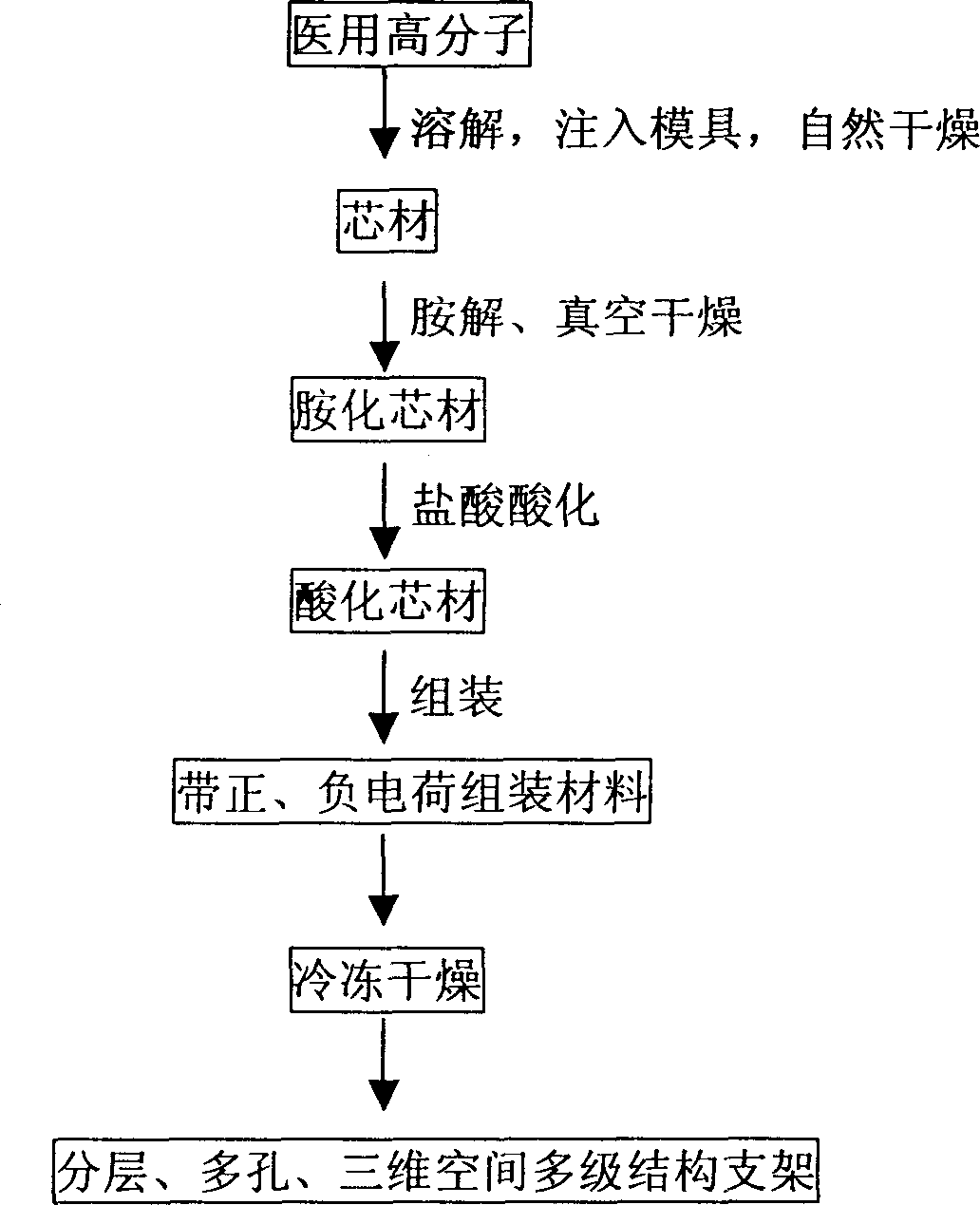
Porous, laminated, tri-dimensional multiple-grade structure tissue stent material and its preparation method
The present invention relates to porous laminated 3D tissue rack material and its preparation process. Polylactic aicd, polyglycolic acid, glycolic acid copolymer, polycaprolactone or other bioabsorbable material and chitosan, chondroitin sulfate, collegen, heparin sulfate, sodium alginate, hyaluronic acid, etc are used as the base material. The preparation process includes nanometer self-assembling to assemble chitosan, chondroitin sulfate or other positively or negatively charged medical polymer material to the surface of specific cylindrical core material, vacuum drying and freeze drying to form the highly bionic human body tubular tissue rack material with adjustable structure, size and shape.
Owner:WUHAN UNIV OF TECH
Tissue engineering artificial skin and preparation method thereof
ActiveCN104984407AWith mechanical strengthOrientational structureProsthesisCollagenanViral infection
The invention provides a tissue engineering artificial skin and a preparation method thereof. The artificial skin comprises an epidermal layer and a dermal layer, wherein the epidermal layer material is made of collagen or a mixed material of collagen and chitosan; the dermal layer material is made of collagen or a mixed material of collagen and chitosan, hyaluronic acid, chondroitin sulfate, alginate or polyving akohol. The epidermal layer comprises a semipermeable film; the dermal layer comprises two layers of three-dimensional porous supporting structures with different porosities. The used collagen is end-free collagen, so that the problems of immune rejection, foreign body reaction, viral infection and the like of the artificial skin during application are solved. The invention further provides the preparation method for the tissue engineering artificial skin.
Owner:BEIJING PAISHENG BIOTECH CO LTD
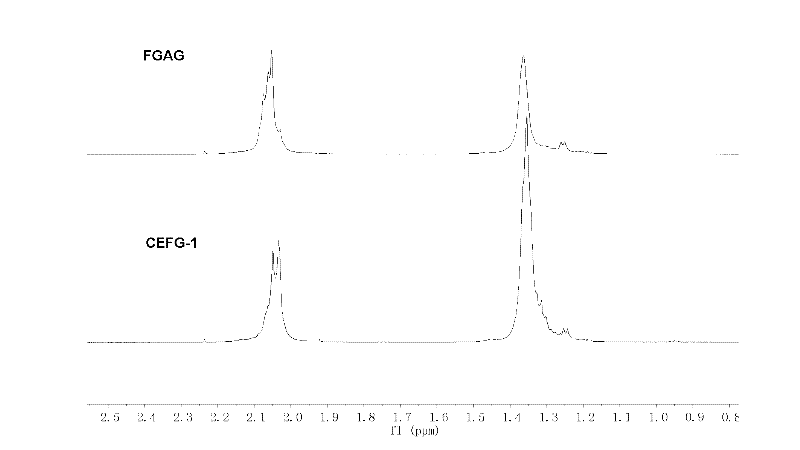
Fucosylated glycosaminoglycan derivative and preparation method thereof
InactiveCN102329397APotent anticoagulant activityOrganic active ingredientsBlood disorderOrganosulfateCarboxylic ester
The invention discloses a carboxylic ester of fucosylated glycosaminoglycan (CEFG) with anticoagulation activity, a pharmaceutically acceptable salt thereof, a preparation method of the CEFG and the pharmaceutically acceptable salt thereof, a pharmaceutical composition containing the CEFG or the salt thereof, and application of the pharmaceutical composition in preparation of anticoagulants. The monosaccharides for preparing the CEFG comprise D-glucuronic acid or D-glucuronate (D-GlcU), D-2-deoxy-2-acetyl galactosamine sulfate (D-GalNAcS) and L-fucose sulfate (L-FucS), wherein the molar ratio of D-GlcU to D-GalNAc to L-Fuc to -OSO3<-> is 1:(1+ / -0.3):(1+ / -0.3):(3.5+ / -0.5); the esterification degree of the D-GlcU is not lower than 20%; and the weight average molecular weight of the CEFG is 3000-20000 Da. The glycosylated chondroitin sulfate esterification derivative has strong anticoagulation activity, and can be applied in preparation of drugs for preventing and / or treating thrombotic diseases.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Multilayer films
InactiveUS20100062232A1Cosmetic preparationsMaterial analysis by observing effect on chemical indicatorCrosslinked chitosanMultilayer membrane
The present invention concerns multilayer films comprising a plurality of layers, at least some of the layers comprise (i) cross-linked chitosan, alginate, chondroitin sulfate, or hyaluronic acid and (ii) particles or void spaces; wherein the layers are 20-260 nm in thickness. Also disclosed are multilayer films comprising at least two of: a layer comprising a first polymer; a layer comprising a second polymer; a layer comprising particles, wherein said particles comprise ceramic material, metallic species, or both; and, a layer comprising a combination of said first polymer and said particles; wherein said multilayer film is capable of displaying structural color. Also provided are methods for making and using the inventive multilayer films and compositions comprising the multilayer films.
Owner:DREXEL UNIV
Preparation method of hyaluronic acid/gelatin/chondroitin sulfate bone repair bionic scaffold
The invention discloses a preparation method of hyaluronic acid / gelatin / chondroitin sulfate bone repair bionic scaffold. The method comprises the steps of adding activator and furfurylamine into MES buffer solution of hyaluronic acid to obtain modified hyaluronic acid solid; adding EDC / NHS (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide / N-hydroxysuccinimide) into gelatin aqueous solution, and adding furancarboxylic acid to obtain modified gelatin solid; dissolving the above two solids into MES buffer solution to obtain a mixed solution; dissolving MAL-PEG-MAL (maleimide-polyethylene glycol-maleimide) into MES buffer solution, adding into the mixed solution, and reacting in 37 DEG C water bath to form transparent cross-linked hydrogel; soaking the hydrogel in MES buffer solution of sodium chondroitin sulfate, adding EDC / NHS, and reacting under stirring to obtain the cartilage repair bionic scaffold. The bionic scaffold has the advantages of interpenetrating network structure, excellent biocompatibility and bioactivity, better compression strength, anti-washout property and degradability, simple preparation process, and easy operation.
Owner:SOUTH CHINA UNIV OF TECH
Method for co-production of de-boned raw chicken, chicken bone soup-stock and chondroitin sulfate using chicken skeleton
ActiveCN102690372AGood economic benefitsReduce waste dischargePoultry processingFood preparationChicken boneChicken Flavor
The invention discloses a method for co-production of de-boned raw chicken, chicken bone soup-stock and chondroitin sulfate using chicken skeletons. The method comprises the following steps: putting the chilled chicken skeleton in a deboner to separate a product A de-boned raw chicken and chicken bone residues; processing the separated chicken bone residues through hot-pressing extraction, vibrating residue removal and oil-water separation to obtain a bone soup and chicken bone oil, adsorbing and eluting the bone soup by using macroporous resins to obtain a product B chondroitin sulfate; processing the deoiled bone soup remained after the resin adsorption through double effect concentration or MVR concentration, homogenizing and blending the concentrated bone soup and the chicken bone oil to obtain a product C chicken bone soup-stock. The co-produced de-boned raw chicken has a fresh color, rich nutrition, a good emulsifying property of the de-boned chicken, and the de-boned raw chicken can be raw materials for meat product processing. The co-produced chicken bone soup-stock product has a delicate taste, a rich chicken flavor, rich nutrition, and can be used as a natural soup base in food. The purity of the co-produced chondroitin sulfate is 87.41%, and the molecular weight is 5.283e[+4].
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
Absorbable active tissue matter for repairing hard tissue and its prepn
The present invention relates to surgical material. The material includes biologically absorbable polymer 10-90 wt%, un-sintered calcium phosphate salt 5-80 wt%, and bone growth factor less than 10 wt%. The biologically absorbable polymer includes polyester, chitosan, chondroitin sulfate, collagen, alginate, etc.; and the calcium phosphate salt is un-sintered hydroxyapatite or tricalcium phosphate of granularity less than 100 microns. The hard tissue repairing material is prepared through mixing biologically absorbably polymer, un-sintered calcium phosphate salt and bone growth factor dispersed in solvent, molding, freeze drying to eliminate solvent and obtain the composition. The composition has the function of promoting bond healing and excellent biocompatibility.
Owner:SOUTHEAST UNIV
Palladium-mediated ketolization
ActiveUS20180155361A1Lower activation energyIncrease response rateOrganic chemistryChemical recyclingDiseaseNatural product
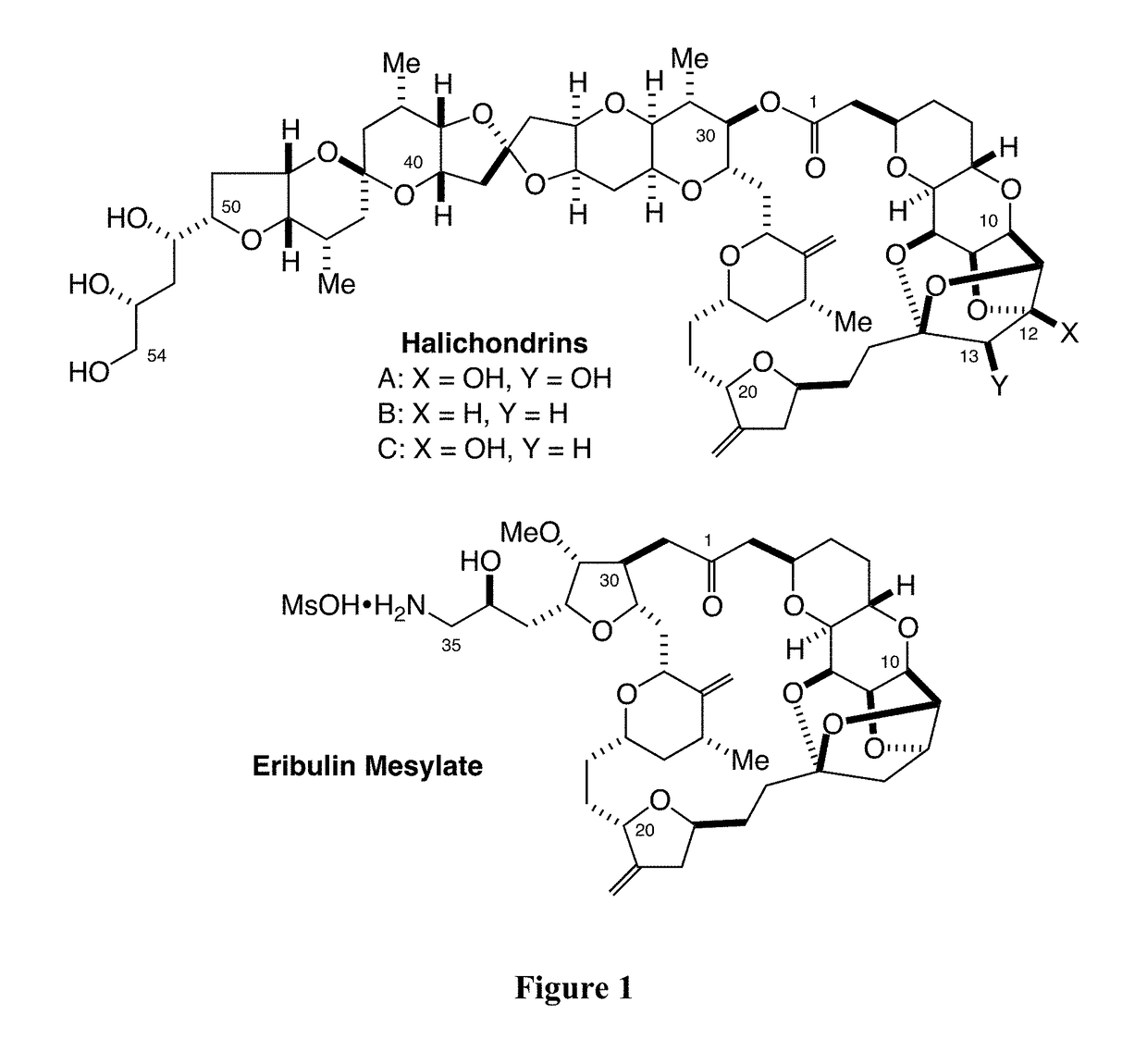
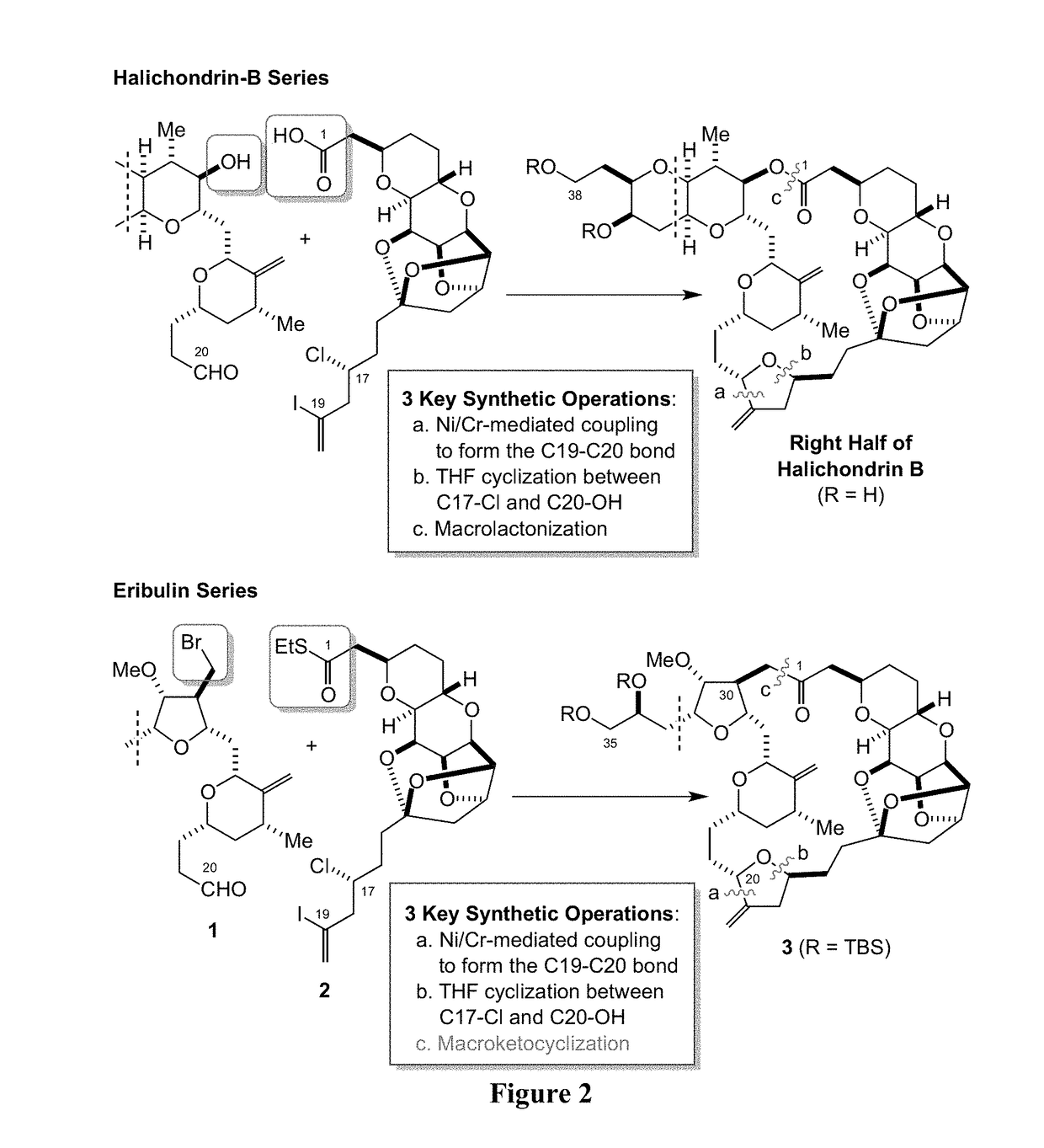
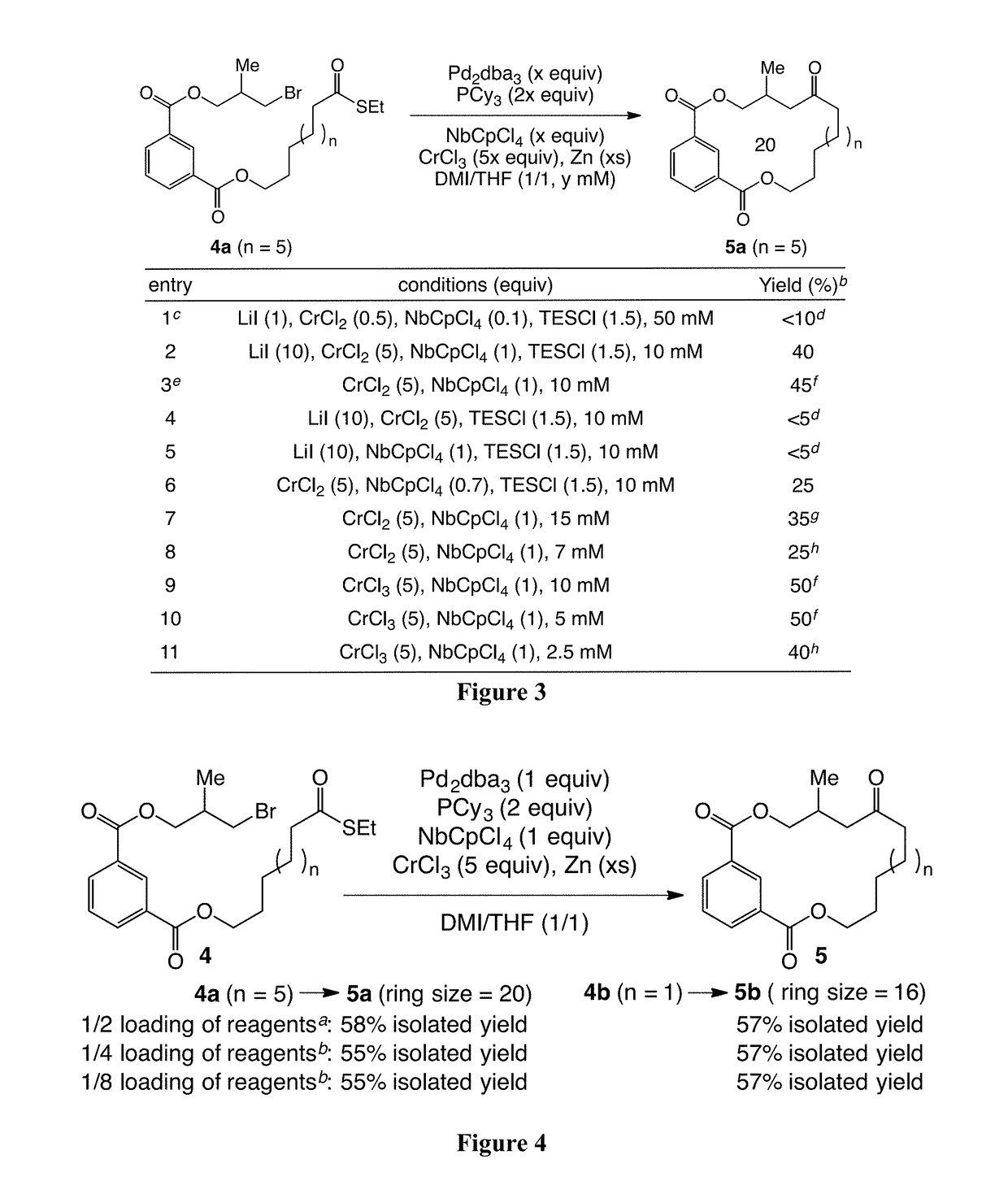
Provided herein are palladium-mediated coupling reactions useful in the preparation of ketone-containing organic molecules. The provided methods can be used for the preparation of natural products and pharmaceutical agents, including Eribulin, halichondrins, and analogs thereof. The present invention also provides novel halichondrin analogs which can be prepared via the palladium-mediated coupling reactions. The novel halichondrin analogs can be used in the prevention and / or treatment of diseases or conditions (e.g., proliferative diseases such as cancer).
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
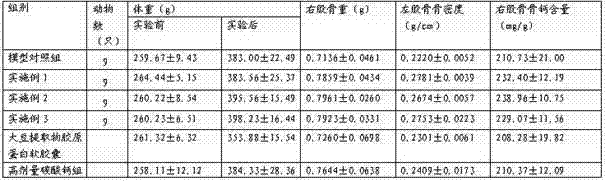
Composition capable of increasing bone density and improving functions of skeletons and joints and preparation method thereof
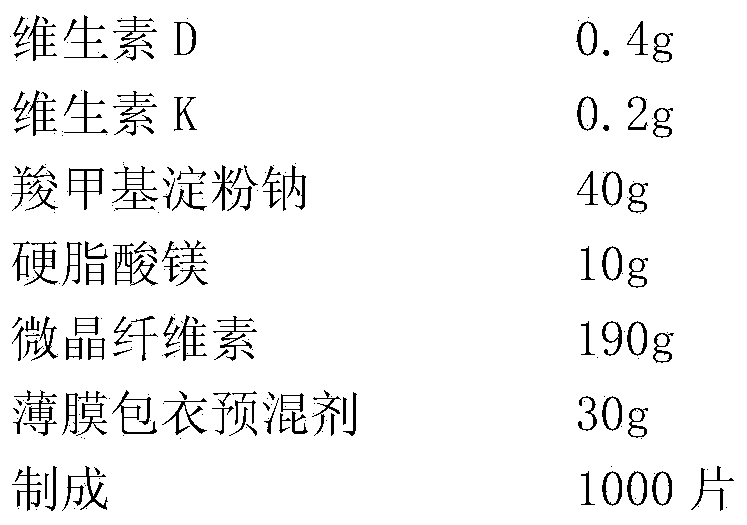
InactiveCN104188999APromote absorptionFacilitate depositionHydroxy compound active ingredientsPeptide/protein ingredientsPainful jointsIncreased Bone Density
The invention discloses a composition capable of increasing bone density and improving functions of skeletons and joints and a preparation method thereof. The composition capable of increasing the bone density and improving the functions of the skeletons and joints comprises the following main components in parts by mass: 1-400 parts of glucosamine, 1-400 parts of chondroitin sulfate, 1-400 parts of calcium medicinal preparation, 0.0001-1 part of vitamin D and 0.0001-1 part of vitamin K. The composition capable of increasing the bone density and improving the functions of the skeletons and joints has the beneficial effects that calcium is supplemented for young and old patients suffering from osteoporosis, absorption and deposition of calcium in skeleton are promoted, the bone density is effectively increased, and syndromes such as osteoarthritis and bone and arthrosis pain are also alleviated.
Owner:哈药集团制药六厂
Compositions and Methods for Bacterial Production of Chondroitin
The invention relates to the field of recombinant DNA technology for the production of chondroitin, including the production of chondroitin sulfate via a combination of recombinant bacterial fermentation and post-fermentation sulfation.
Owner:SEIKAGAKU KOGYO CO LTD
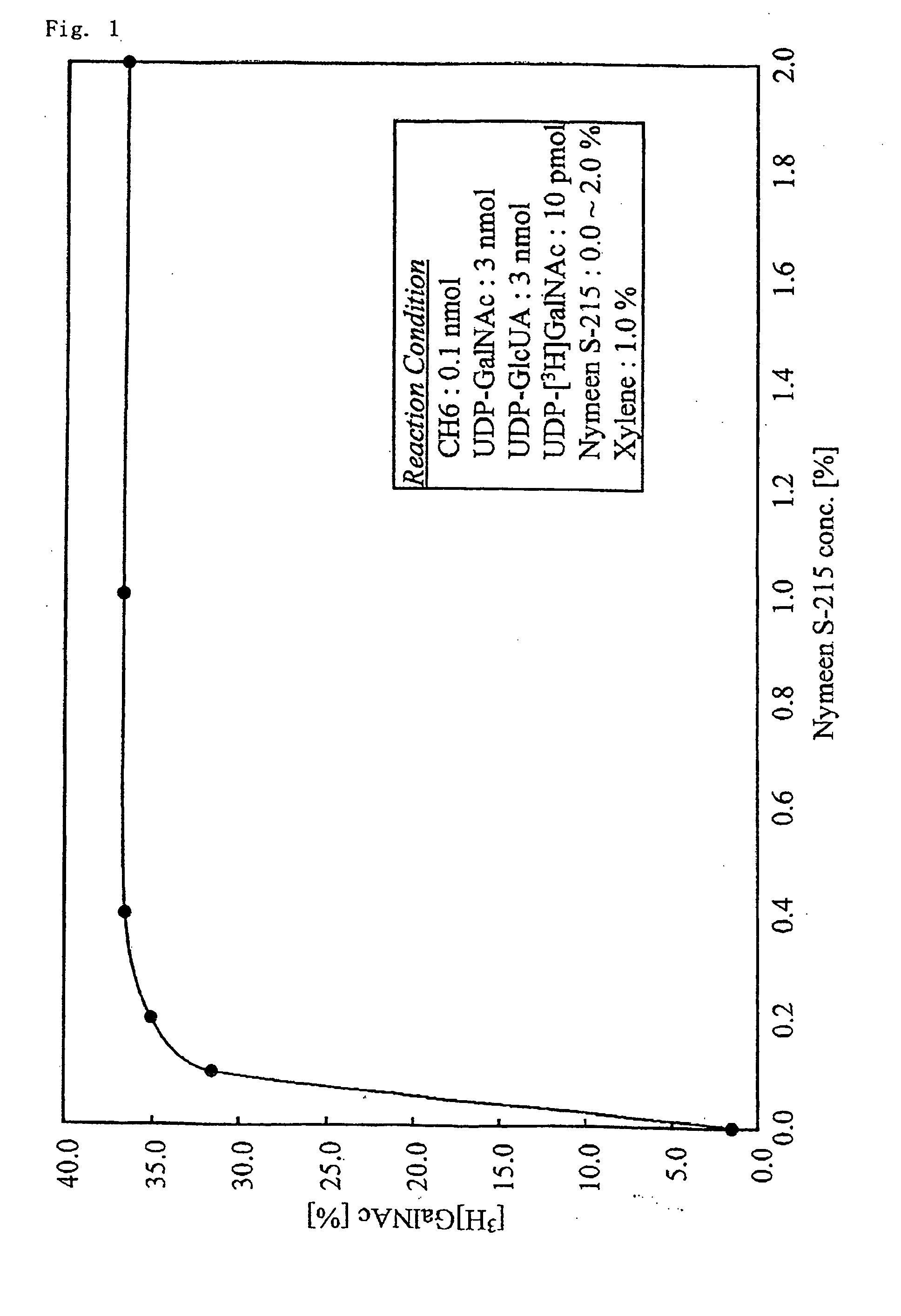
Long-chain chondroitin sugar chain and method for producing the same and method for promoting synthesis of chondroitin
ActiveUS20090263867A1Efficient productionEasy to produceBacteriaSugar derivativesFiltrationChondroitinase ABC
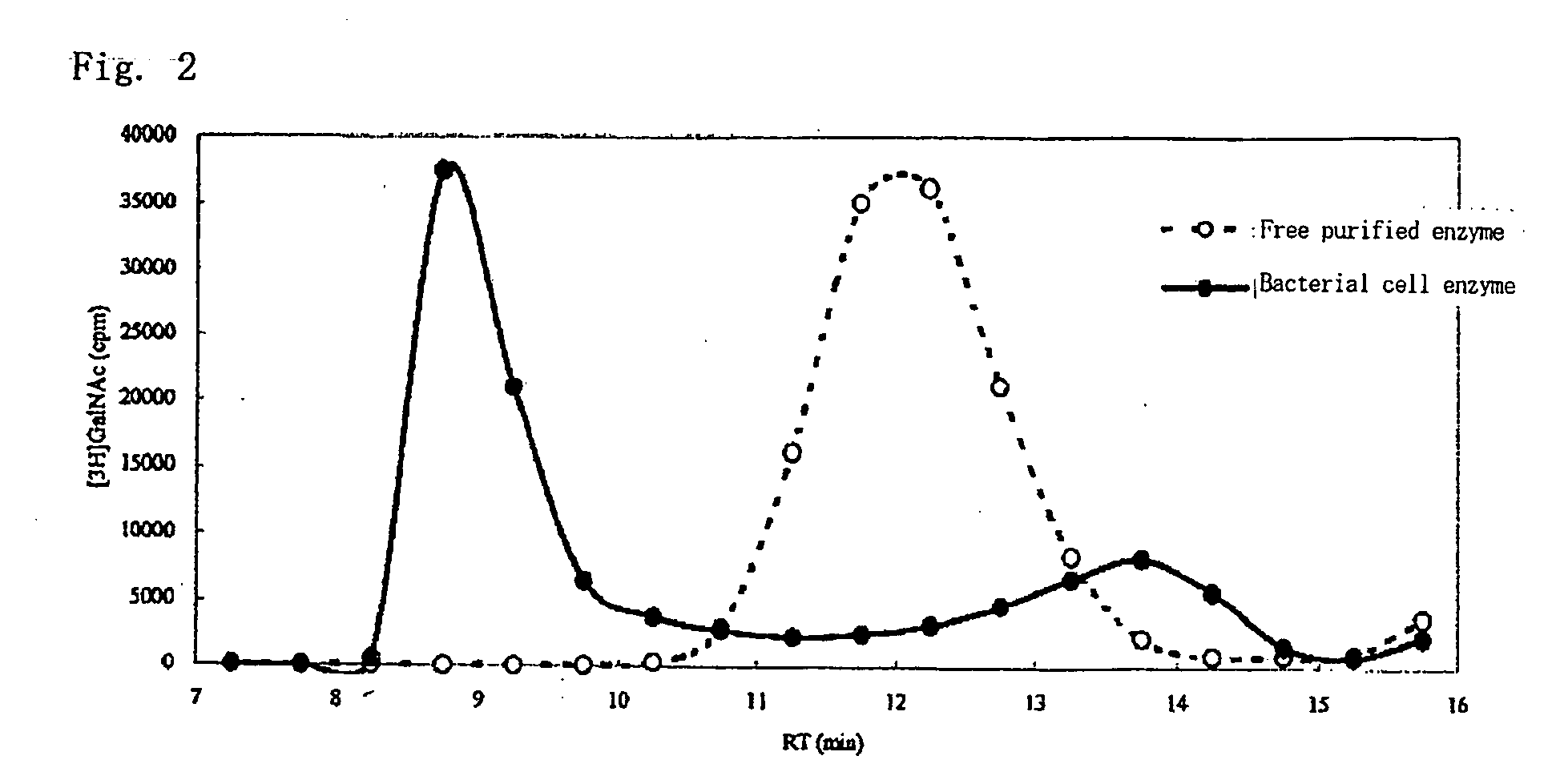
A method for producing a chondroitin sugar chain comprises at least the following step: a step of allowing “a glucuronic acid donor”, “an N-acetyl galactosamine donor”, “a sugar receptor” and “a bacterial cell enzyme which synthesizes chondroitin” to coexist in a reaction system in the presence of a surfactant. Here, the surfactant is preferably selected from n-nonyl-β-D-thiomaltopyranoside, sucrose monocaproate and sucrose monolaurate. The chondroitin sugar chain has all the following properties 1) to 3): 1) a weight average molecular weight: 50,000 or more when it is measured by gel filtration chromatography, 2) it is completely degraded to disaccharides with chondroitinase ABC, 3) when the sugar chain is decomposed with chondroitinase ABC and the decomposed products are subjected to a disaccharide analysis, substantially all of them correspond to an unsaturated disaccharide unit of chondroitin.
Owner:SEIKAGAKU KOGYO CO LTD
Composite bone tissue engineering rack material and its prepn
InactiveCN1526765AImprove performanceGood biological propertiesProsthesisApatiteBone tissue engineering
The composite bone tissue engineering rack material includes collagen, hydroxyapatite, chondroitin sulfate and bone morphogenetic protein; and has 3D porous structure of aperture 50-150 microns. The ratio between collagen and hydroxyapatite is 1 to 1-10, and the chondroitin sulfate is compounded with other components via cross-linking. The material may be constituted into tissue engineering artificial bone based on tissue engineering principle. The present invention has excellent physical, chemical and biological performance, is excellent bone tissue engineering rack material, and may be used in repairing bone defect caused by different reasons clinically.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com