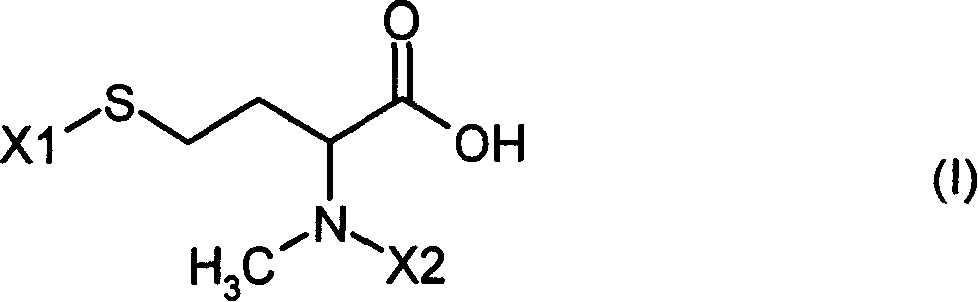
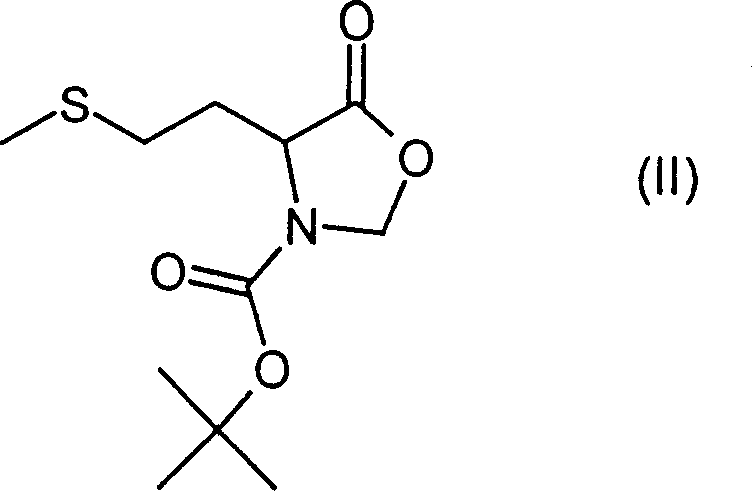
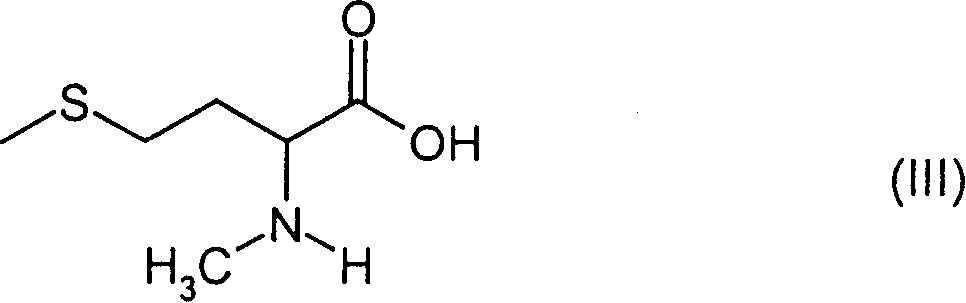
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
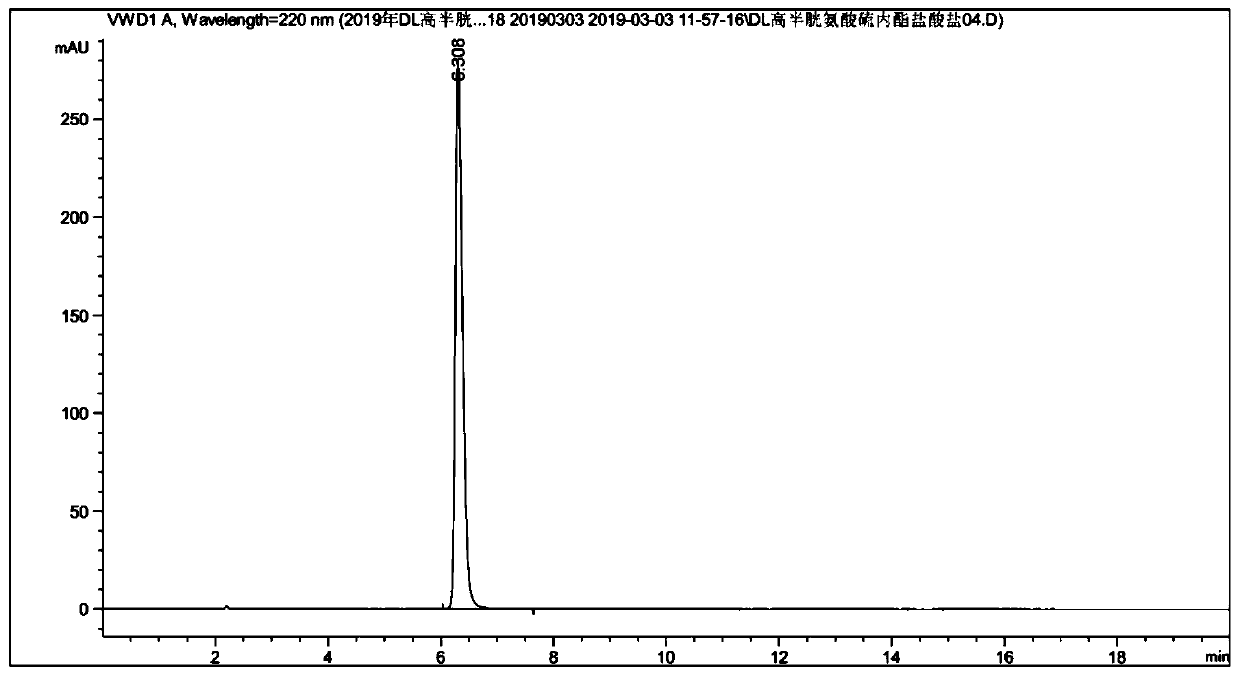
36 results about "Homocystine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
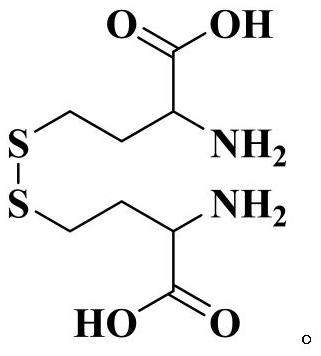
Homocystine is a chemical compound consisting of two homocysteine molecules joined by a disulfide bond. Its relationship with homocysteine is analogous to the relationship between cystine and cysteine.
Method of using hydroxycarboxylic acids or related compounds for treating skin changes associated with intrinsic and extrinsic aging
A composition comprising an amphoteric or pseudo-amphoteric agent and a polyhydroxy alpha hydroxyacid existing as a free acid, lactone, or salt, and isomeric or non-isomeric forms thereof is provided. The amphoteric or pseudo-amphoteric agent can be selected from amino acids, dipeptides, aminoaldonic acid, aminouronic acid, lauryl aminoproplyglycine, aminoaldaric acid, neuraminic acid desulfated heparin, deacetylated hyaluronic acid, hyalobiuronic acid, chondrosine, deacetylated chondroitin, creatine, creatinine, hydroxyproline, homocysteine, homocystine, homoserine, ornithine, citrulline, phosphatidylserine, and sphingomyelin. The composition may contain other additives, including cosmetic or pharmaceutical agents for topical treatment of dermatological disorders.
Owner:TRISTRATA TECH
Method of using hydroxycarboxylic acids or related compounds for treating skin changes asociated with intrinsic and extrinsic aging
A composition comprising an amphoteric or pseudo-amphoteric agent and a polyhydroxy alpha hydroxyacid existing as a free acid, lactone, or salt, and isomeric or non-isomeric forms thereof is provided. The amphoteric or pseudo-amphoteric agent can be selected from amino acids, dipeptides, aminoaldonic acid, aminouronic acid, lauryl aminoproplyglycine, aminoaldaric acid, neuraminic acid desulfated heparin, deacetylated hyaluronic acid, hyalobiuronic acid, chondrosine, deacetylated chondroitin, creatine, creatinine, hydroxyproline, homocysteine, homocystine, homoserine, ornithine, citrulline, phosphatidylserine, and sphingomyelin. The composition may contain other additives, including cosmetic or pharmaceutical agents for topical treatment of dermatological disorders.
Owner:TRISTRATA TECH
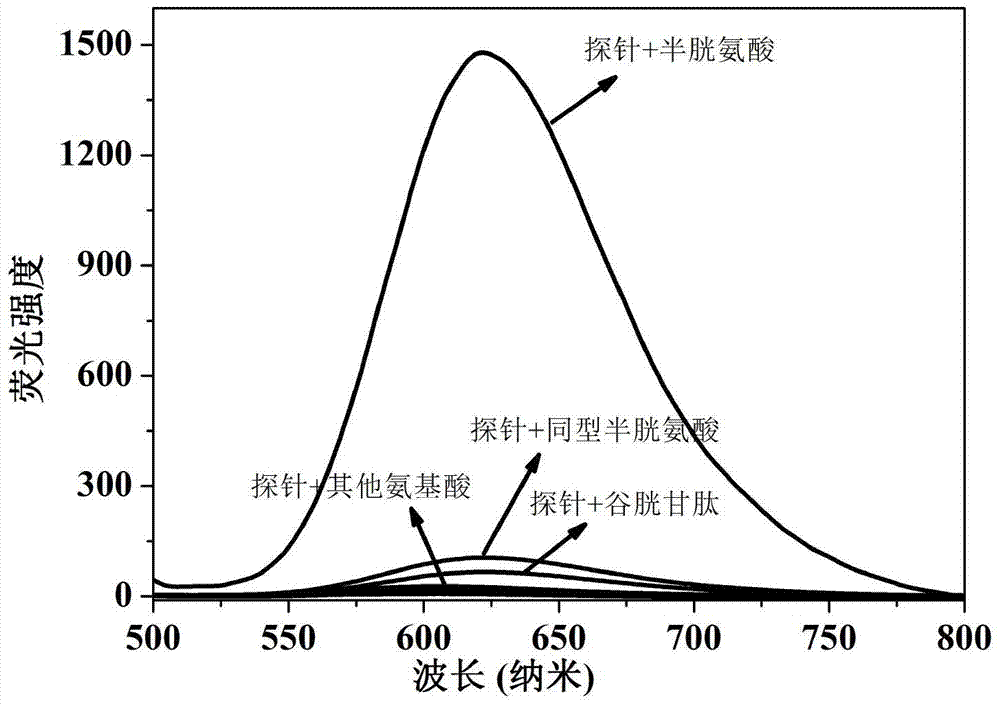
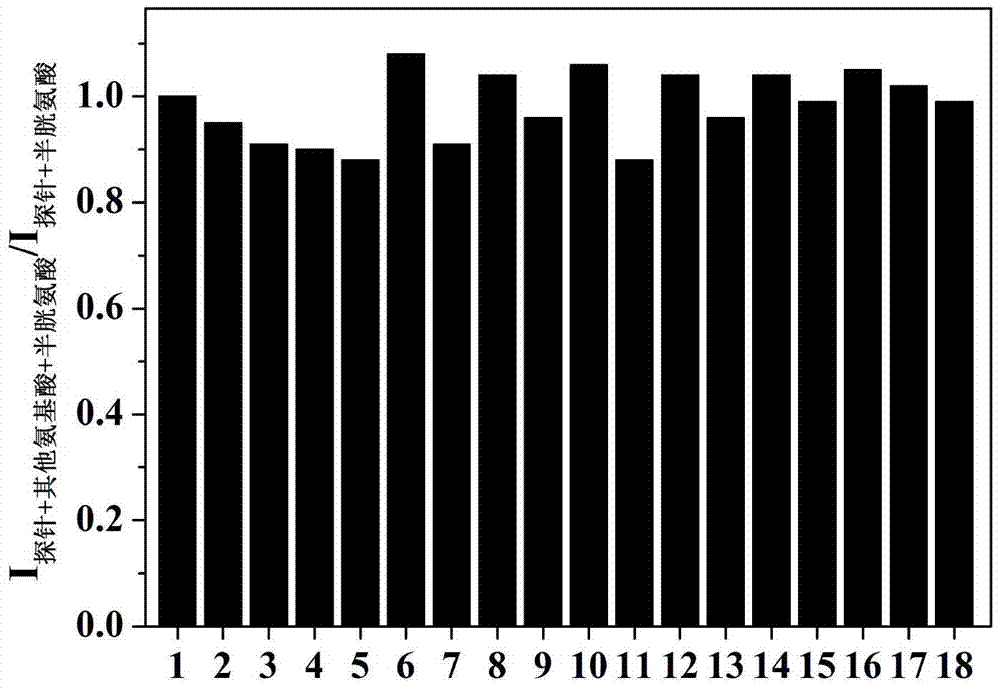
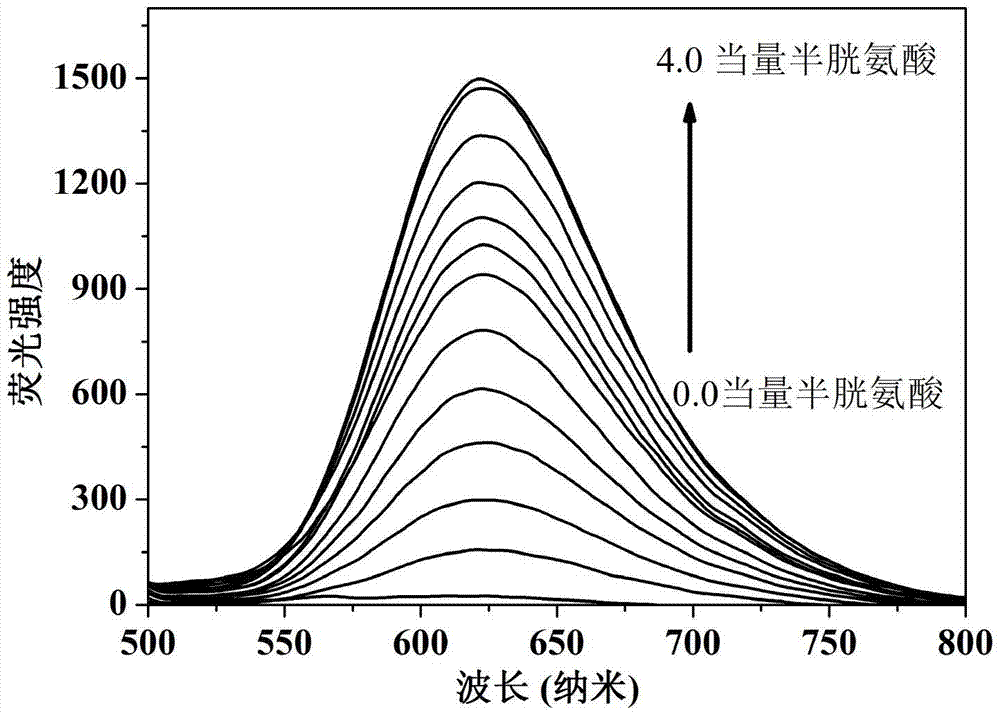
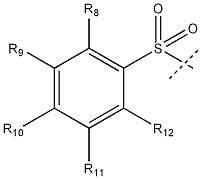
Preparation and applications of novel fluorescent probe capable of specifically recognizing cysteine
InactiveCN106946801AThe synthetic route is simpleLow costOrganic chemistryFluorescence/phosphorescenceInterference resistanceFluorescence sensing
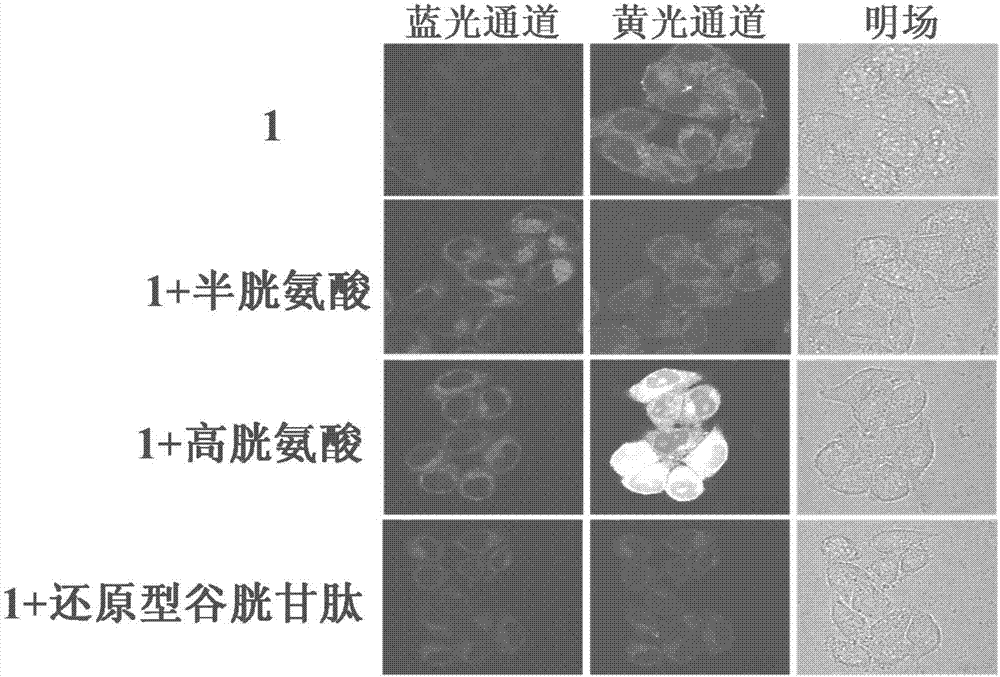
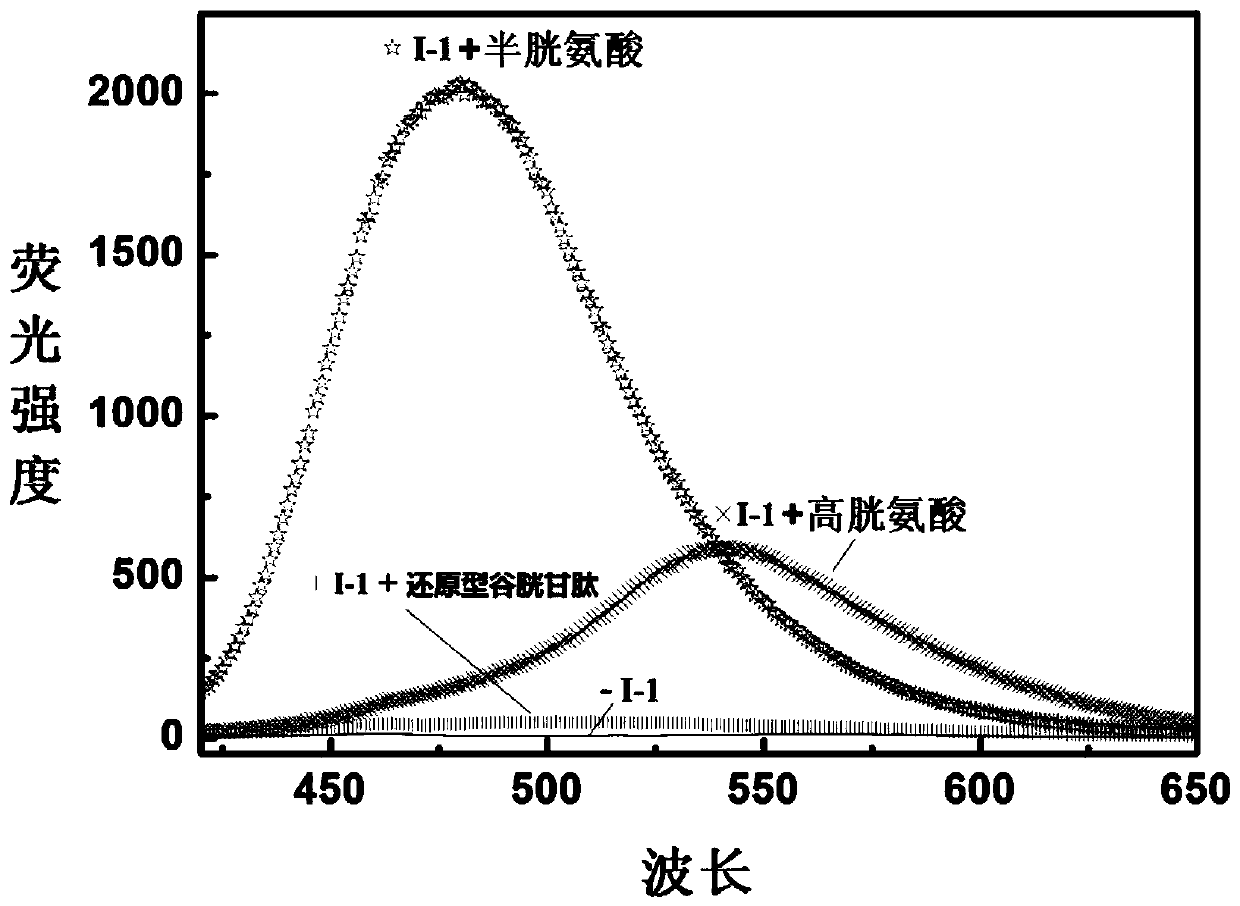
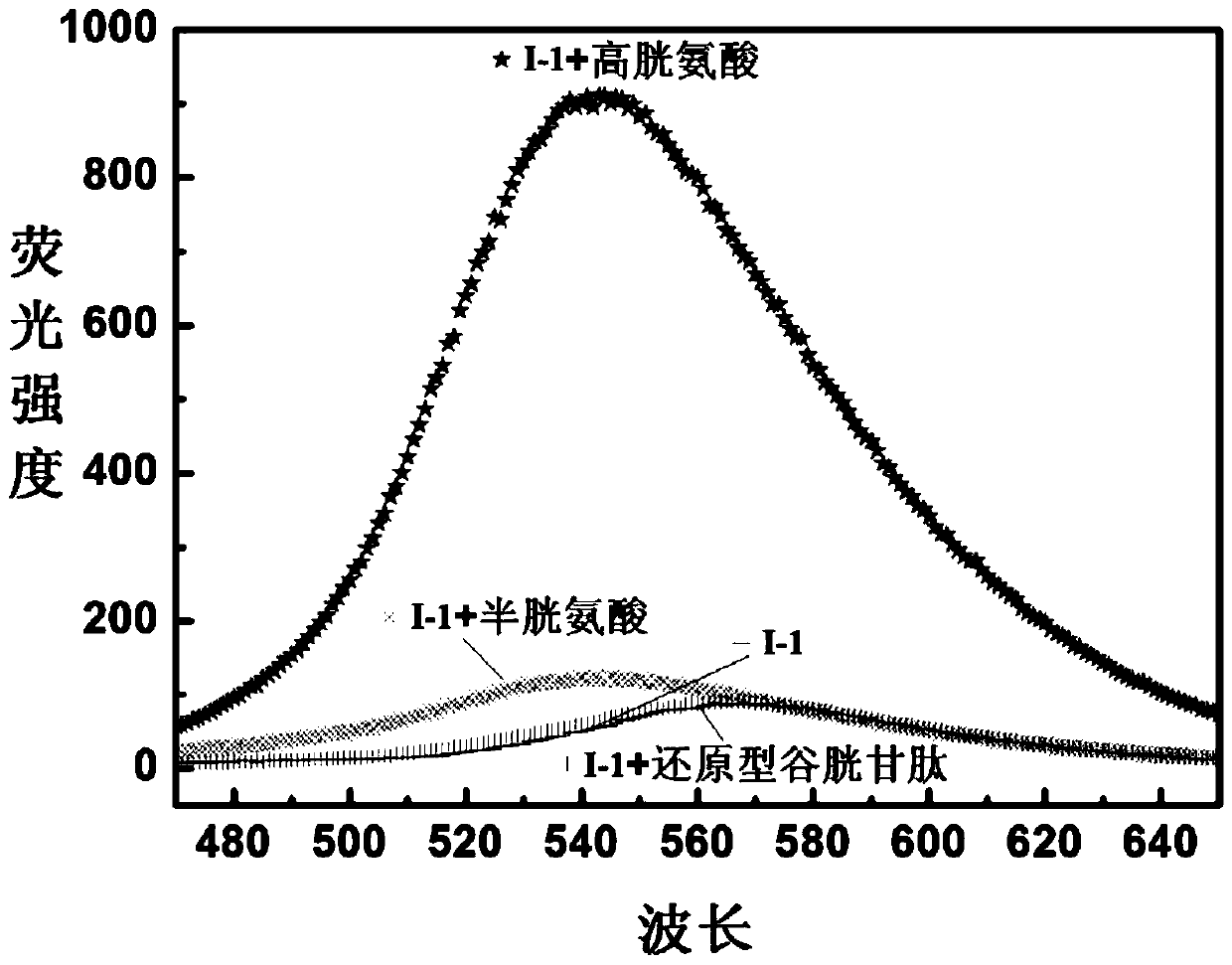
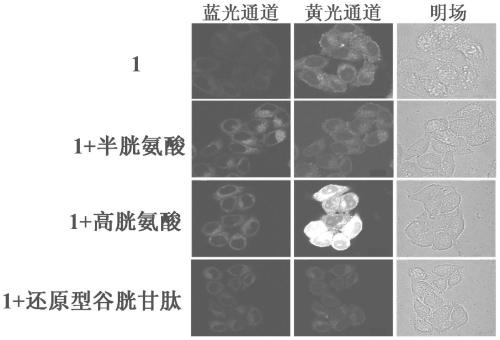
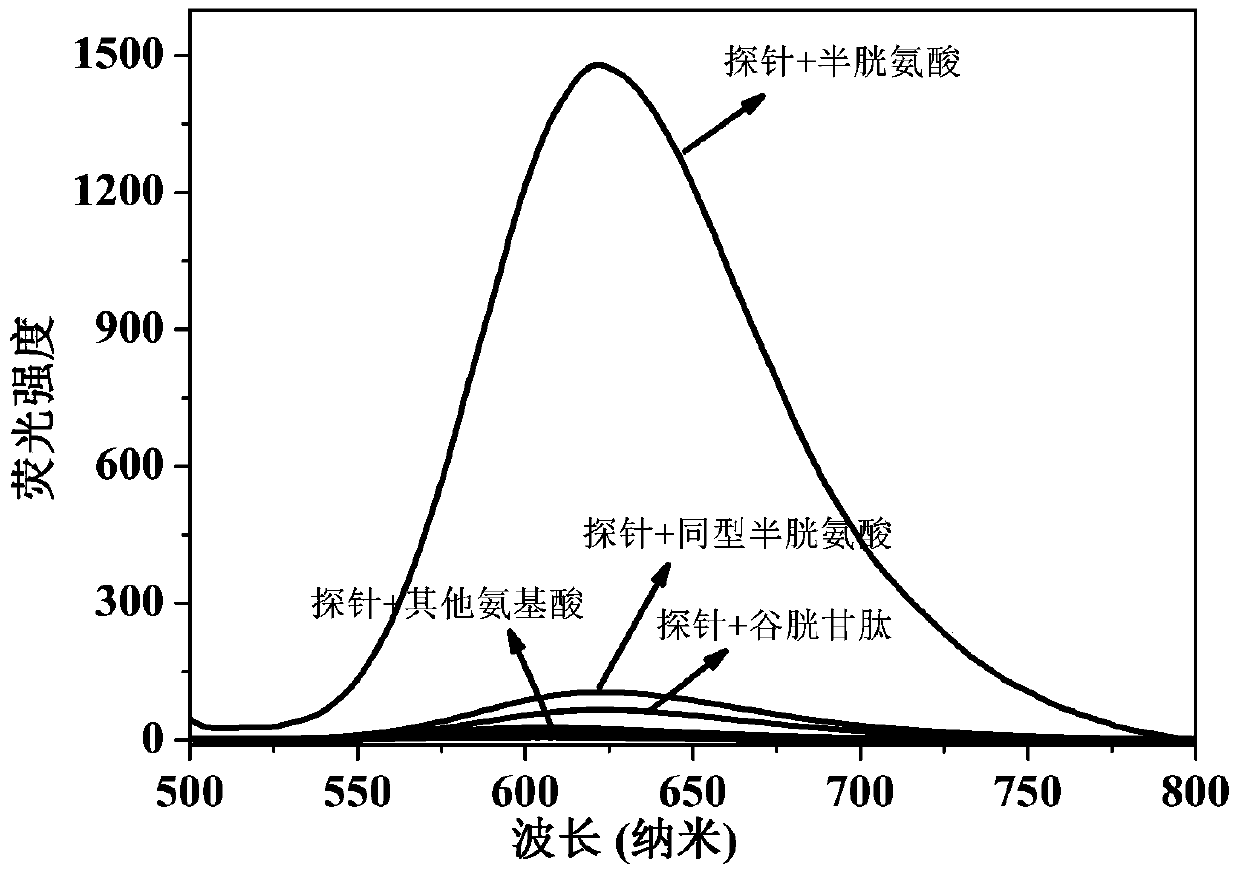
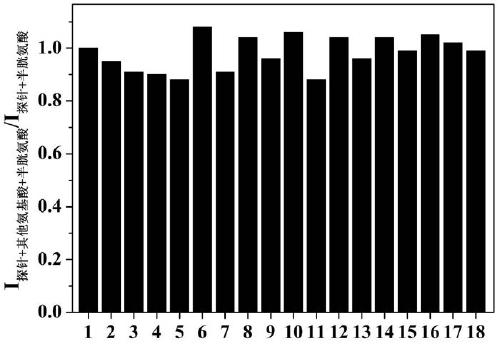
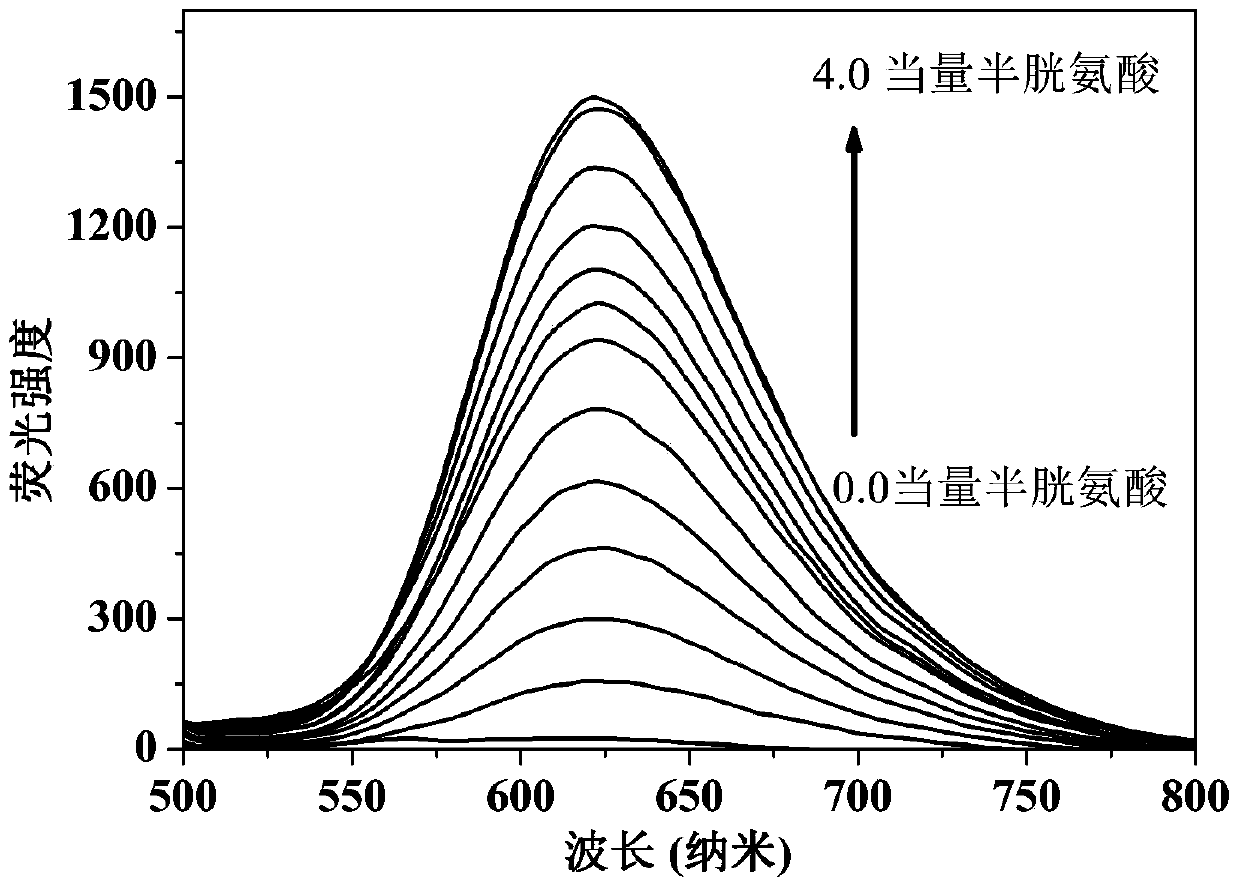
The present invention discloses a novel fluorescent probe capable of specifically recognizing cysteine, wherein the molecular structure formula is defined in the specification. According to the present invention, the fluorescent probe can distinguish the mercapto-containing cysteine from the mercapto-free amino acid region, can distinguish the cysteine from the homocystine and the glutathione having the similar structure, emits red light during cysteine detection, can reduce the background interference and the damage of light on tissue cells in biological applications, exhibits great Stokes shift, can reduce self-absorption so as to improve the sensitivity, can be used for the fluorescence sensing analysis of the cysteine in environments or biological samples, and has advantages of good selectivity to cysteine, high sensitivity, strong interference resistance, and good application prospect.
Owner:CENT SOUTH UNIV
High-sensitive blood-plasma total homocysteine detection reagent box
InactiveCN1979155AStrong detection specificityHigh sensitivityComponent separationOther chemical processesTotal homocysteineHomocysteine testing
The invention relates to a high sensitive blood plasma homocysteine testing kit that includes sample tube with stabilizer, reference substance and quality control serum of different Hcy thickness, DL- homocystine-D8 internal standard solution, reducer and trichloroacetic acid albumen precipitation. The invention has strong specificity for sample testing, and high sensitivity. It could be accurately and rapidly used in Hcy research.
Owner:上海特敏生物医药科技有限公司
Environmentally-friendly method for synthesizing selenomethionine
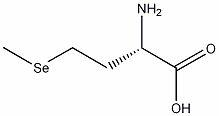
The invention relates to an environmentally-friendly method for synthesizing selenomethionine. The method comprises the following steps of: reducing alpha-amido-protected aspartate beta-alkyl grease into alpha-amido-protected homoserine, then carrying out acid catalysis for ring closing to obtain inner grease of the homoserine, then reacting with metal selenide to generate homocystine selenide, obtaining the alpha-amido-protected homoserine, and finally eliminating protection and obtaining a selenomethionine product. The environmentally-friendly method has the advantages that the highly-toxic and rotten dimethyl diselenide is prevented from being used, so that the damage to the environment and operators are greatly reduced; the yield is high, the production cost is lower, the product purity is high, and optical and pure selenomethionine can be produced by large batch; and the obtained product can be used for preparing the intermediate of a selenium-containing medicine, and solves the difficulty that the selenomethionine and the related medicines can not be popularized.
Owner:张家港阿拉宁生化技术有限公司
Method for producing DL-homocysteine lactone hydrochlorate
InactiveCN101144169AImprove qualityHigh yieldOrganic chemistryElectrolysis componentsElectrolysisDL-methionine
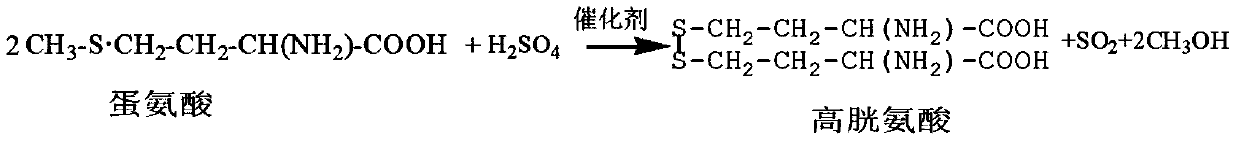
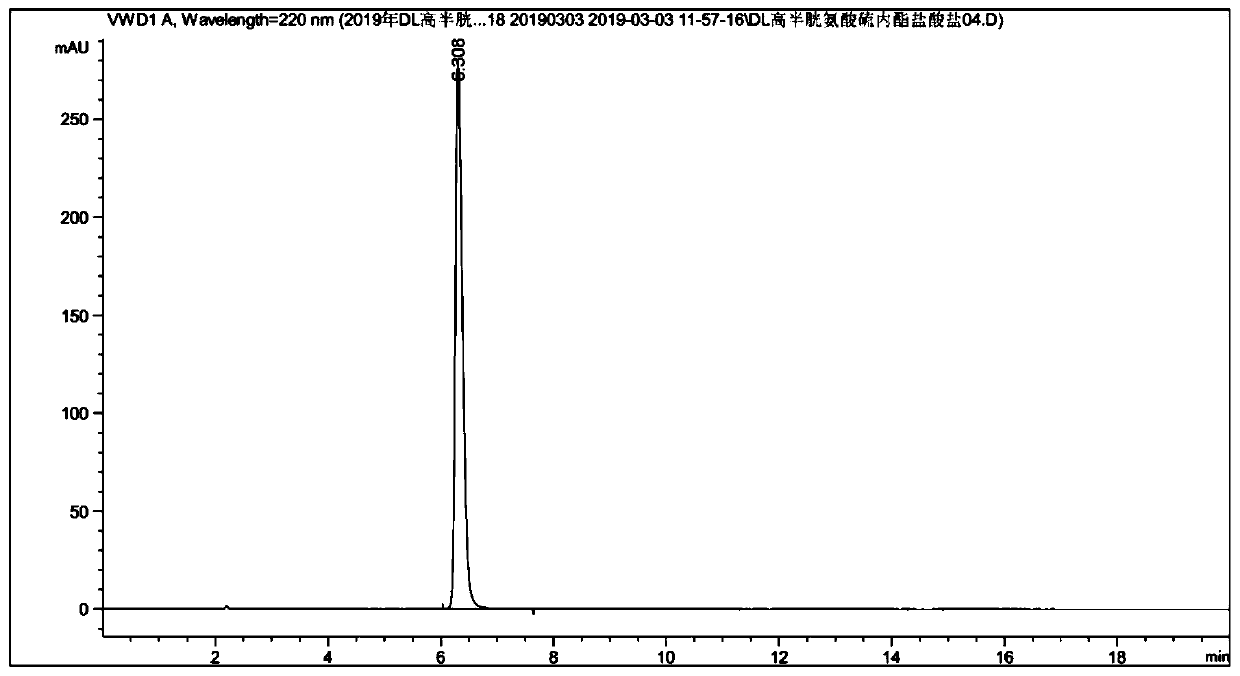
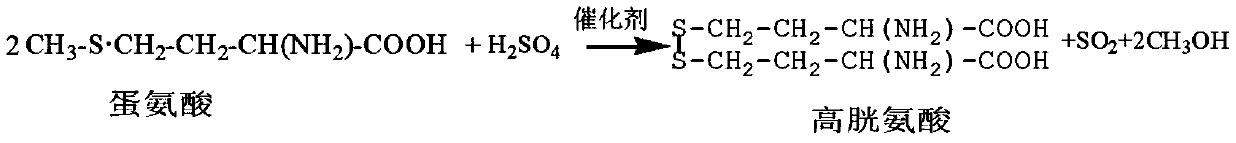
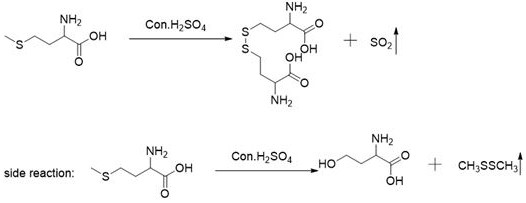
The present invention relates to a production method for DL-homocysteine lactone hydrochloride. The present invention adopts DL-methionine as the raw material, obtains DL-homocystine through the catalyzing and the hysrolyzing of concentrated sulfuric acid at a certain temperature and time, and electrolyzes the crystal of the obtained substance in chlorhydric acid medium to get the product. The present invention can realize that the content of the product is up to 99.8 percent-101.5 percent, the purity is over 99.5 percent, the yield is about 30 percent, and no water is discharged during the production process.
Owner:湖北新生源生物工程有限公司
Reactive-type fluorescence probe for distinguishing sulfydryl compounds, synthesis method and application thereof
ActiveCN107098915ARealize detectionImprove stabilityOrganic chemistryFluorescence/phosphorescenceQuantum yieldHydrogen
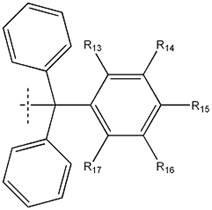
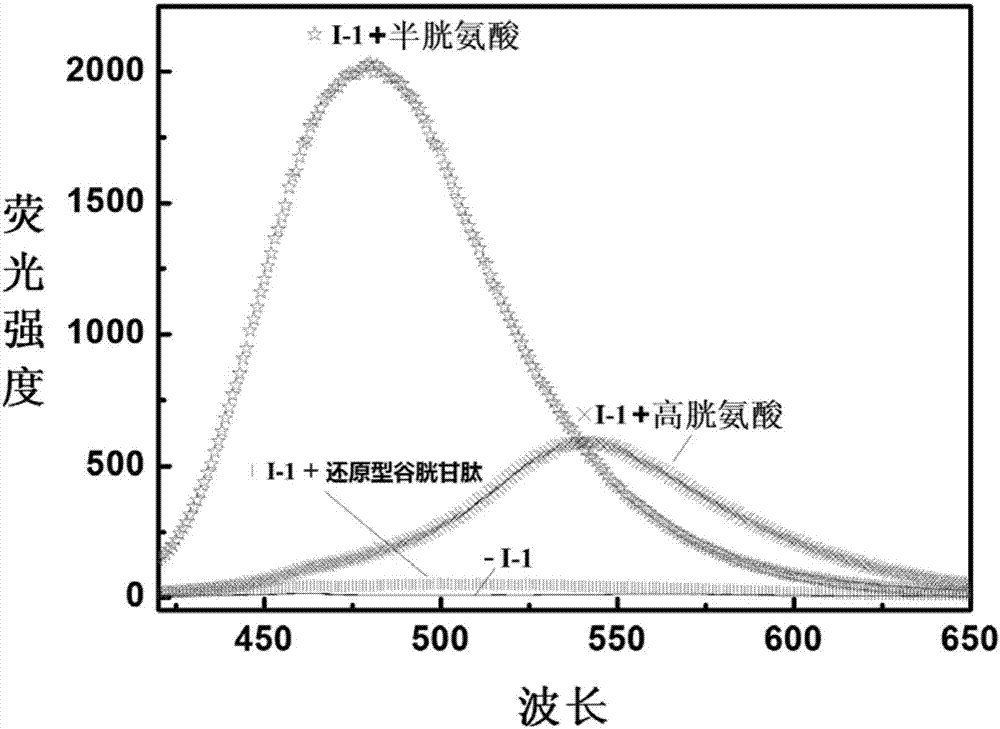
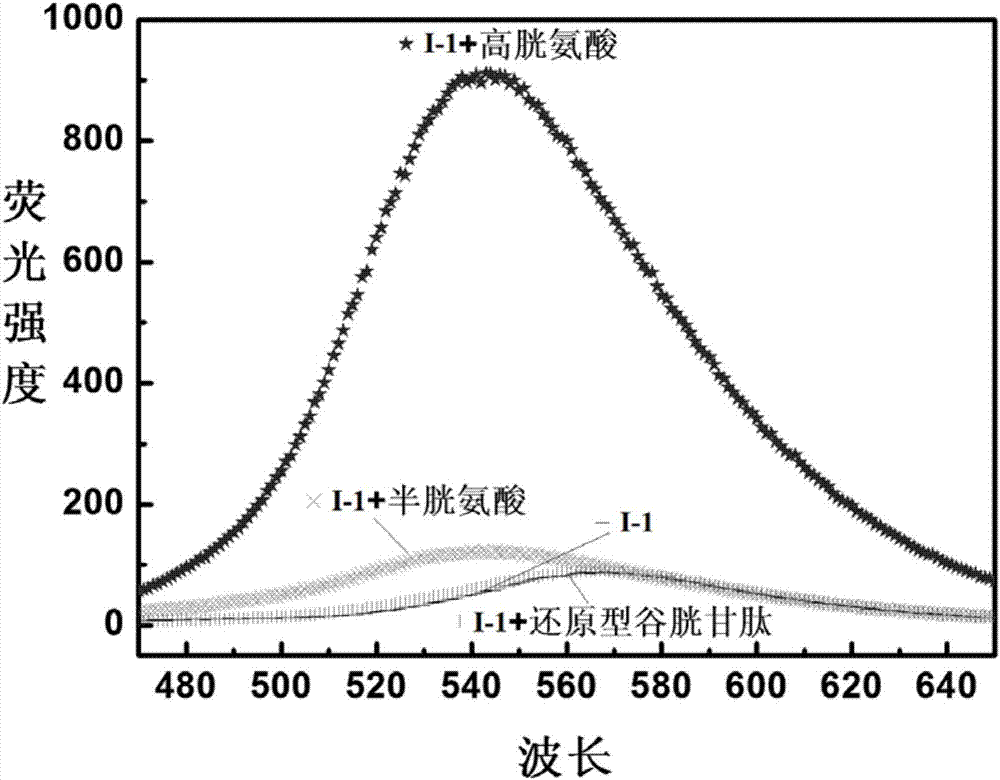
The invention discloses a reactive-type fluorescence probe for distinguishing sulfydryl compounds, wherein the structure of the fluorescence probe is represented as the formula (I). R1, R2, R3 and R4 are independently selected from one of hydrogen, a C1-C8 alkyl group and a C3-C8 cycloalkyl group, and X is independently selected from halogens; or the R1 and the R4 and / or the R2 and the R3 can form a ring. The invention also discloses a synthesis method and an application of the fluorescence probe. The fluorescence probe is low in molecular weight, is high in fluorescent quantum yield, has good cytocompatibility, can generate different fluorescence enhancement signals when being reacted respectively with cysteine and homocystine, can distinguish and recognize the cysteine and homocystine well, has good stability and is easy to prepare.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Therapeutic cell systems and methods for treating homocystinuria
InactiveUS20190309271A1Reduced immune reactionLower Level RequirementsCarbon-sulfur lyasesPeptide/protein ingredientsCysteine degradationRed blood cell
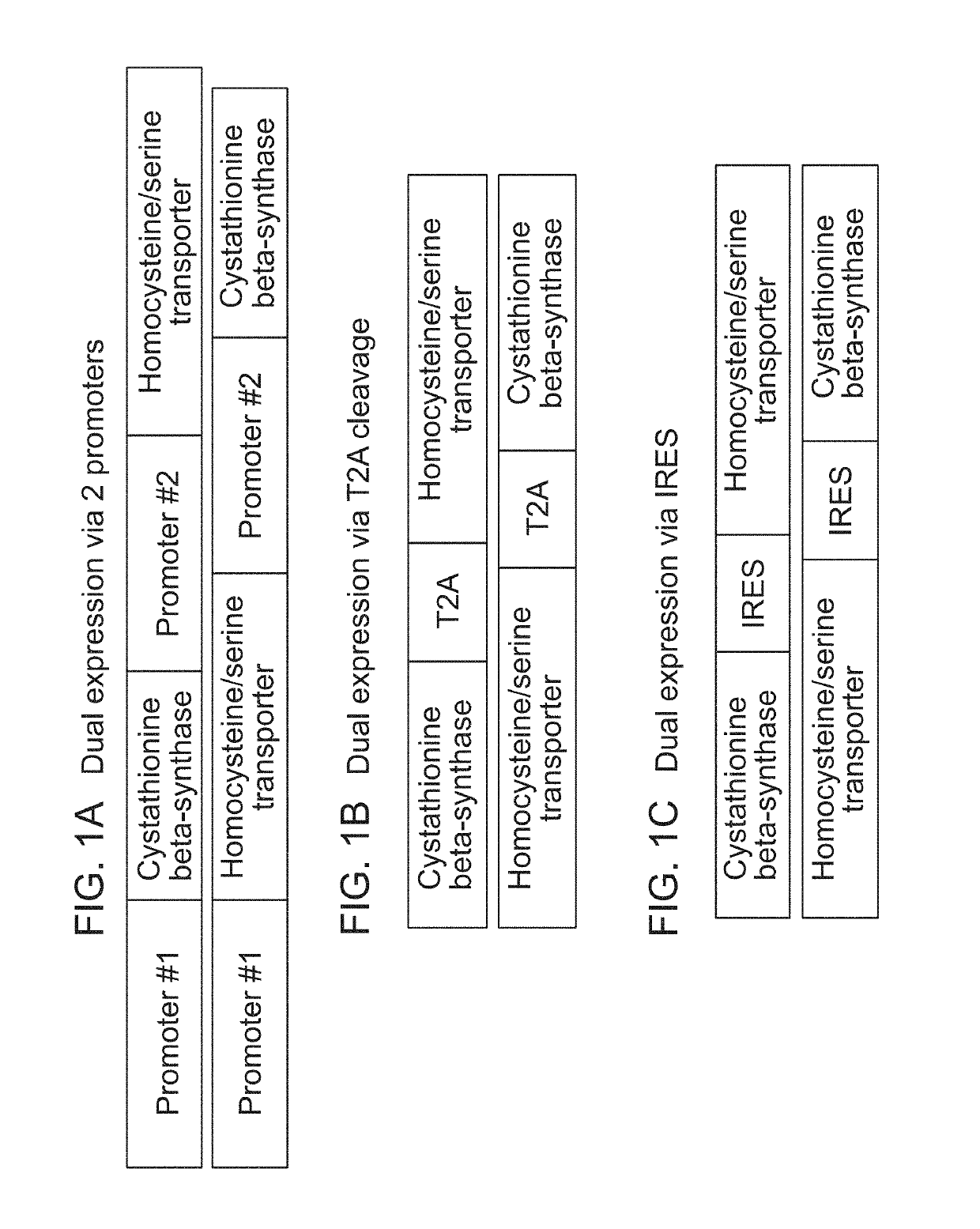
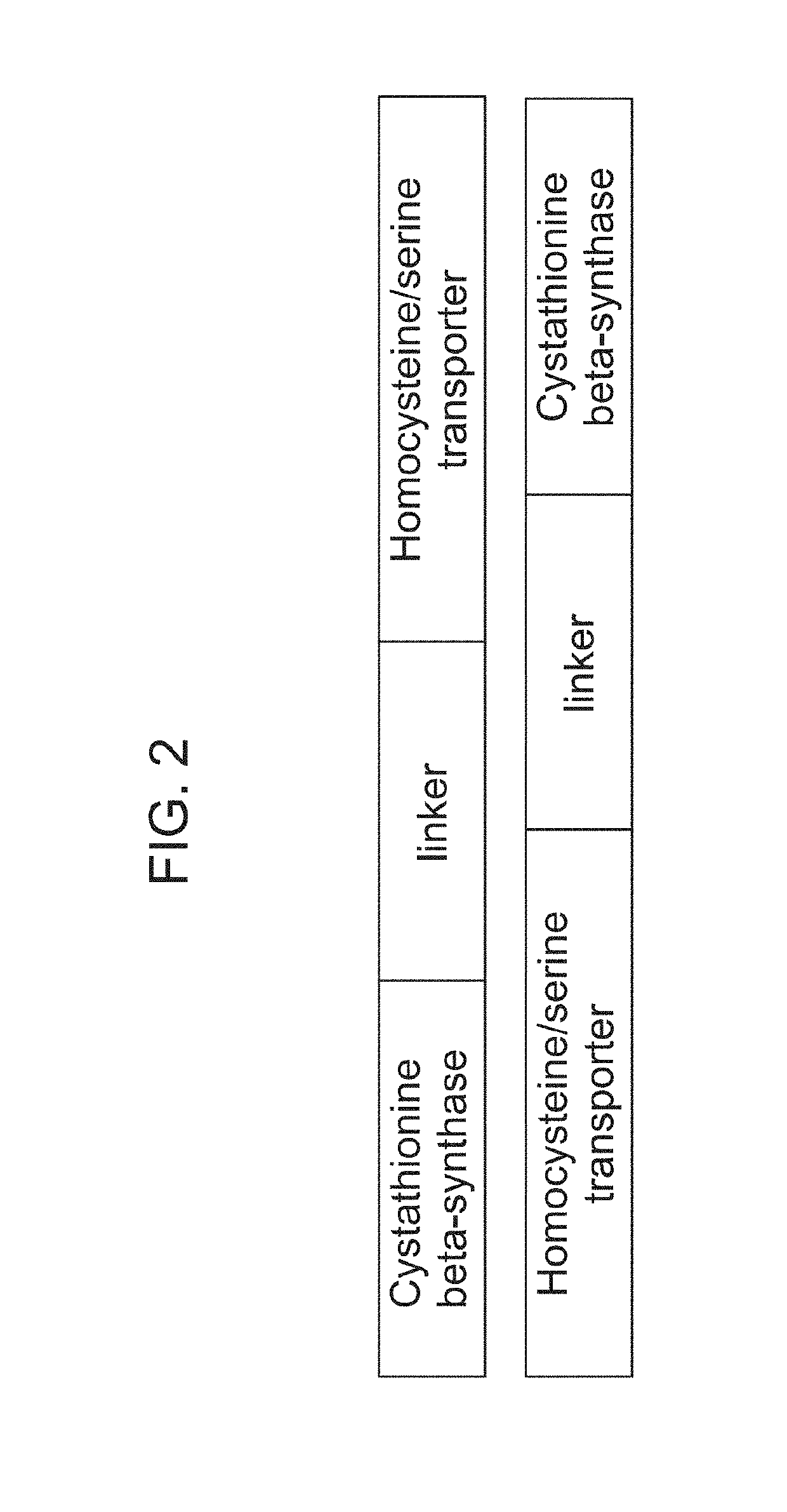
The present disclosure relates to erythroid cells that have been engineered to express a homocysteine reducing polypeptide, or a variant thereof, or a homocysteine degrading polypeptide, or a variant thereof. The engineered erythroid cells may further comprise an amino acid transporter, for example a homocysteine transporter or a serine transporter, or a cystathionine degrading polypeptide. The engineered erythroid cells of the present disclosure are useful in reducing the level of homocysteine in a subject. The engineered erythroid cells of the present disclosure are further useful in methods of treating homocystinuria.
Owner:RUBIUS THERAPEUTICS
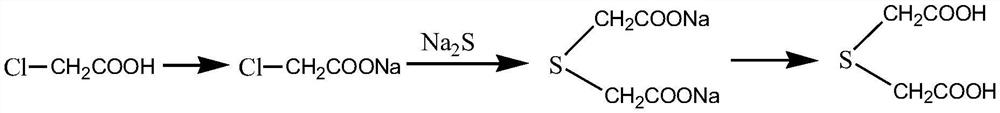
Preparation of homocystine
ActiveCN101348452AReduce manufacturing costHydropoly/poly sulfide preparationHalogenMethionine biosynthesis
A method for preparing homocystine is disclosed. The method capable of eliminating a great amount of pollutants, recycling useful materials, and reducing preparation cost comprises following steps that: methionine together with a 5 to 18mol / L sulfuric acid and a 0 to 50 percent (by weight) halogen acid is heated to and kept at a temperature of 100 to 150 DEG C for 0.5 to 20 hours, with the molar ratio of methionine to sulphuric acid to halogen acid being 1 to 2-6.5 to 0-4; after the reaction liquid is cooled down, the sulfuric acid in the reaction liquid is reclaimed through dialysis until the isoelectric point of homocystine is reached, and then the precipitate is filtrated, washed, and dried to obtain homocystine. The invention is suitable for the preparation of homocystine.
Owner:九江中星医药化工有限公司
Special high-yield and high-quality cultivation material formula and industrial cultivation method for pleurotus geesteranus
InactiveCN105917963AIncrease productionIncrease health and medicinal functionsCalcareous fertilisersBioloigcal waste fertilisersHuskThreonine
The invention discloses a special high-yield and high-quality cultivation material formula and industrial cultivation method for pleurotus geesteranus. The cultivation material formula is characterized in the a cultivation material is prepared from raw materials in parts by weight as follows: 29.5%-39.5% of mushroom dreg, 29.5%-39.5% of cottonseed hulls, 10 parts of wheat husk, 1 part of calcium carbonate and 10-30 parts of culturing packing. The industrial cultivation method comprises technological steps as follows: selection of a cultivation field; preparation of the raw materials; treatment of the raw materials; culture in a mushroom house as well as fruiting and harvesting. The development demands of efficient cultivation can be met, the yield of pleurotus geesteranus is increased by 19.54%-21.39%, the content of glutamic acid is increased by 12.04%-14.29%, the content of cysteine is increased by 20%-60%, the content of threonine is increased by 7.2%-9.6%, resource utilization of the culturing packing is realized, the raw materials are easy to obtain, the cost is low, no cow dung is added, the use amount of rice straw is small, harmless, high-quality and high-yield cultivation can be realized, and the problem of shortage of the raw materials is conveniently solved.
Owner:TECH CADRE TRAINING CENT OF FUJIAN ACAD OF AGRI SCI
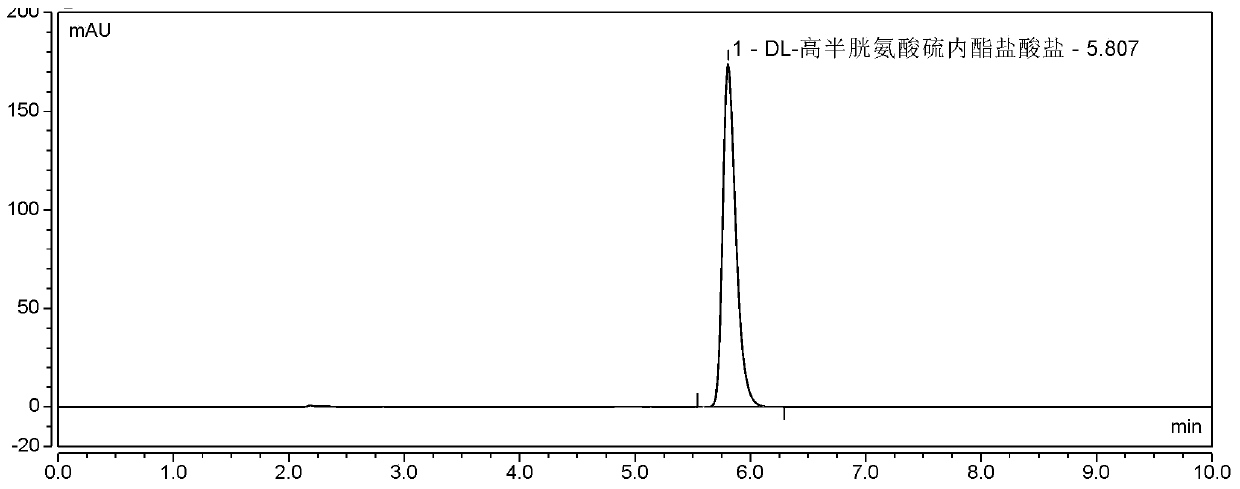
Continuous production method of DL-homocysteine thiolactone hydrochloride
InactiveCN111004209APromote circular economyLarge specific surface area volumeElectrolysis componentsElectrolytic organic productionHomocystineElectrolytic cell
The invention discloses a continuous production method of DL-homocysteine thiolactone hydrochloride. The method comprises the following steps: (1) continuously introducing DL-methionine as a raw material and 15-18 mol / L sulfuric acid into a liquid-liquid phase micro-channel reactor, carrying out a reaction to generate DL-homocystine, continuously introducing the DL-homocystine-containing reactionliquid flowing out of the liquid-liquid phase micro-channel reactor and hydrochloric acid into the cathode chamber of a plate-and-frame electrolytic cell, carrying out a reduction reaction on the DL-homocysteine in a hydrochloric acid system at a cathode by using graphite electrodes as the cathode and an anode to generate DL-homocysteine hydrochloride, and continuously flowing the DL-homocysteinehydrochloride-containing catholyte back to a the liquid-liquid phase micro-channel reactor, carrying out a reaction, continuously and circularly reacting until the reaction is complete, and collectingthe catholyte after the reaction is completed; and (2) carrying out impurity removal treatment on the catholyte collected in the step (1), and carrying out dehydration condensation to obtain the DL-homocysteine thiolactone hydrochloride. According to the method, the product yield is increased, the product purity is high, and the domestic medicine standard is met.
Owner:ZHEJIANG UNIV OF TECH
Selenium-enriched ecological bacterial fertilizer
InactiveCN108083901AIncrease productionHigh content of organic seleniumFungiBacteriaBenzoic acidPhosphate
The invention discloses selenium-enriched ecological bacterial fertilizer. The selenium-enriched ecological bacterial fertilizer is prepared from paddy rice straws, biogas slurry, mushroom residues, compound bacteria, potassium selenite, brown sugar, ammonium carbonate, monoammonium phosphate, potassium chloride, selenium humate, selenium-substituted homocystine, selenium ore powder, itaconic acid, dibutyl carboxyl toluene, a silane coupling agent KH-57, pentamethyl diethylene triamine, o-phenyl benzoic acid, polyvinyl alcohol, attapulgite, soya lecithin, sodium alginate and chitin through compound fertilizer granule preparation, coating material liquid preparation, fertilizer granule coating and drying. The ecological bacterial fertilizer can improve the yield of Chinese cabbage and the content of selenium.
Owner:广西胜荣科技集团有限公司
Synthesis method of DL-homocysteine thiolactone hydrochloride
ActiveCN109943860AHigh yieldHigh mechanical strengthOrganic chemistryElectrolytic organic productionLead bismuthElectrolysis
The invention discloses a synthesis method of DL-homocysteine thiolactone hydrochloride. A DL-homocystine is prepared into a catholyte with a hydrochloric acid solution as a medium, and electrolysis is conducted in an electrolysis tank to obtain the DL-homocysteine thiolactone hydrochloride, wherein according to a cathode electrode, a roughened glass carbon electrode serves as a base material, andthe surface of the base material is covered with lead-bismuth alloy. The method can be used for improving the yield of the DL-homocysteine thiolactone hydrochloride.
Owner:武汉本杰明医药股份有限公司
Colorimetric and fluorometric determination of homocysteine and cysteine
ActiveUS9201075B2Material analysis by observing effect on chemical indicatorBiological testingTotal homocysteineFluorescence
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
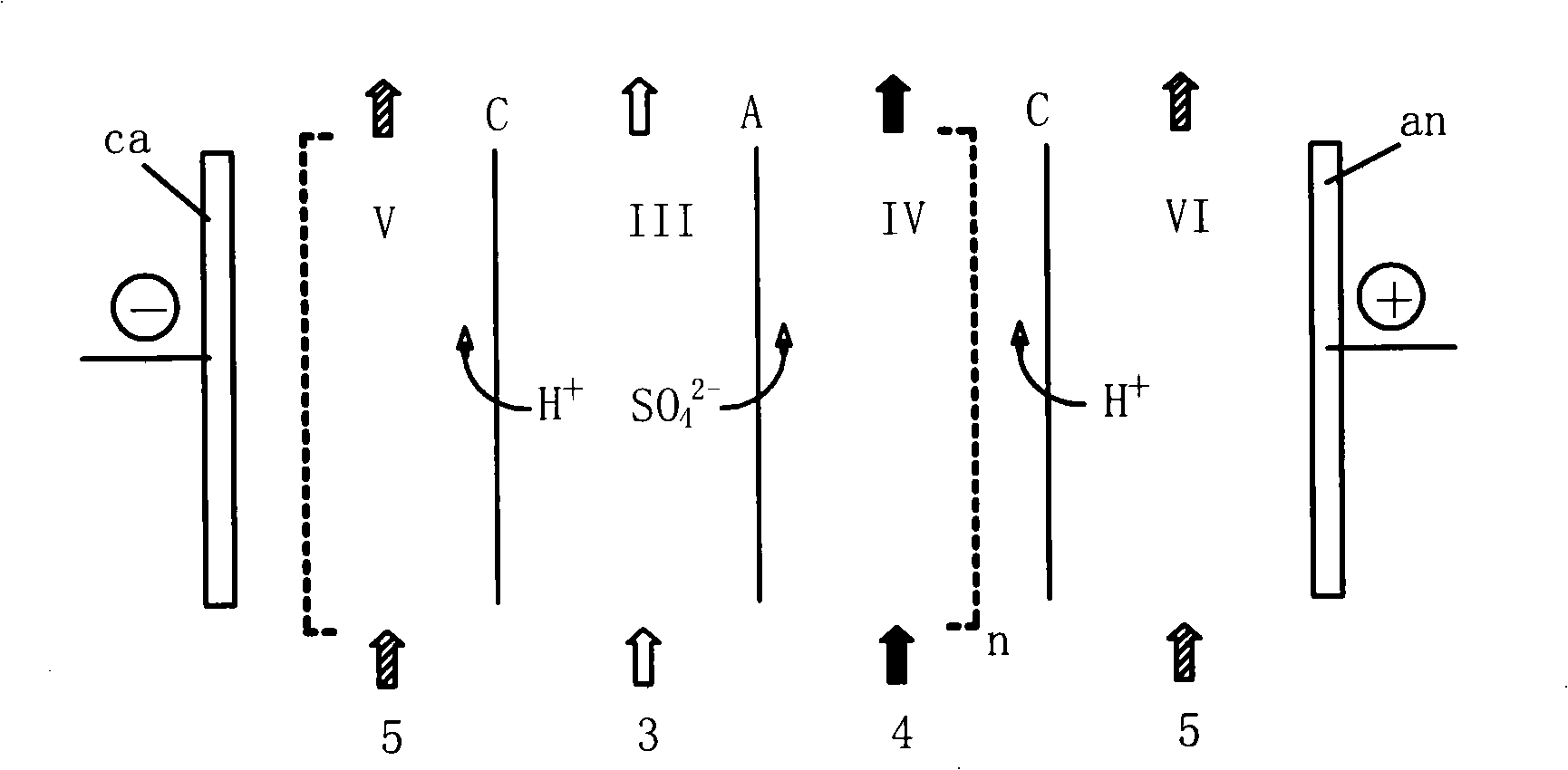
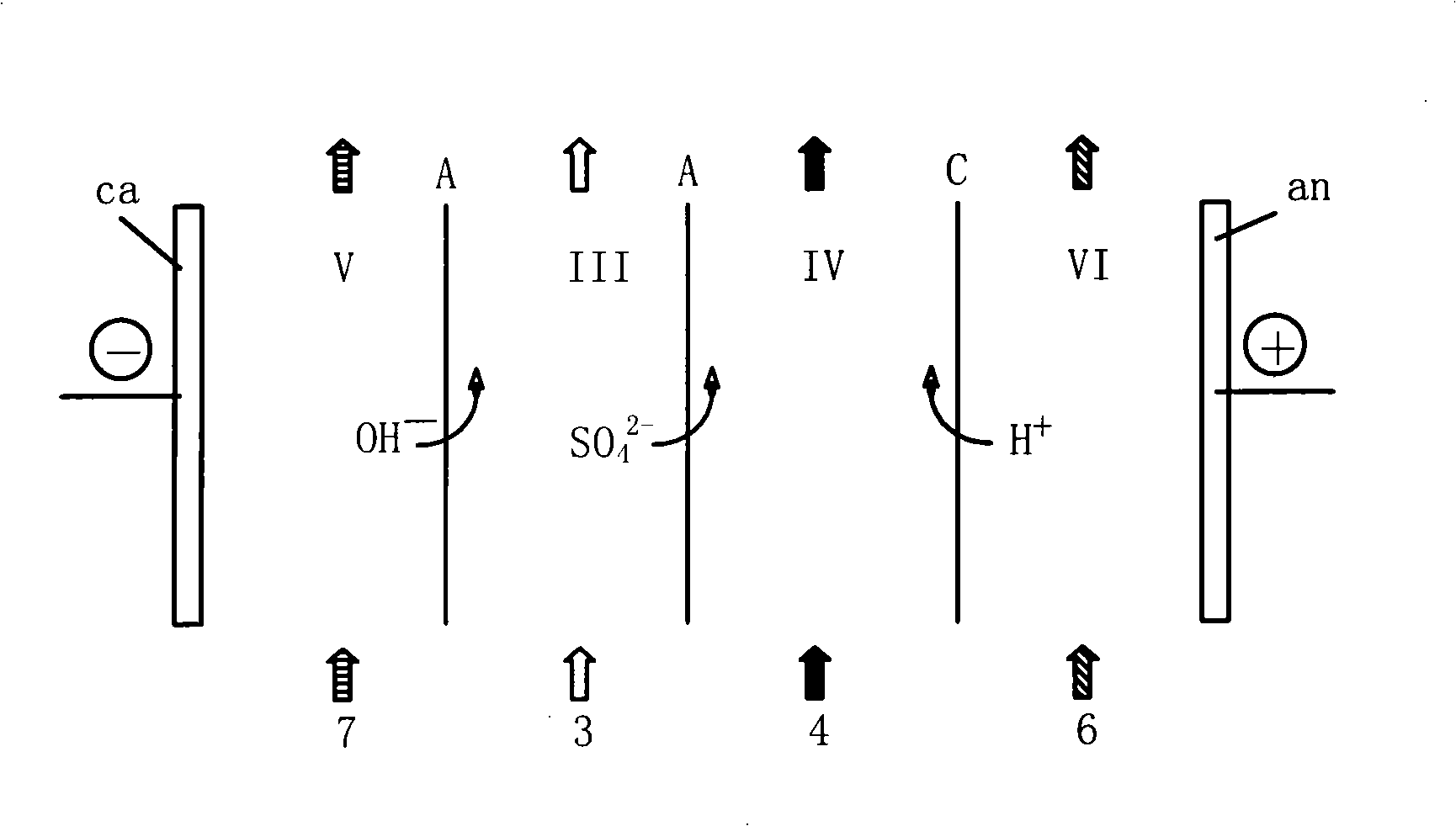
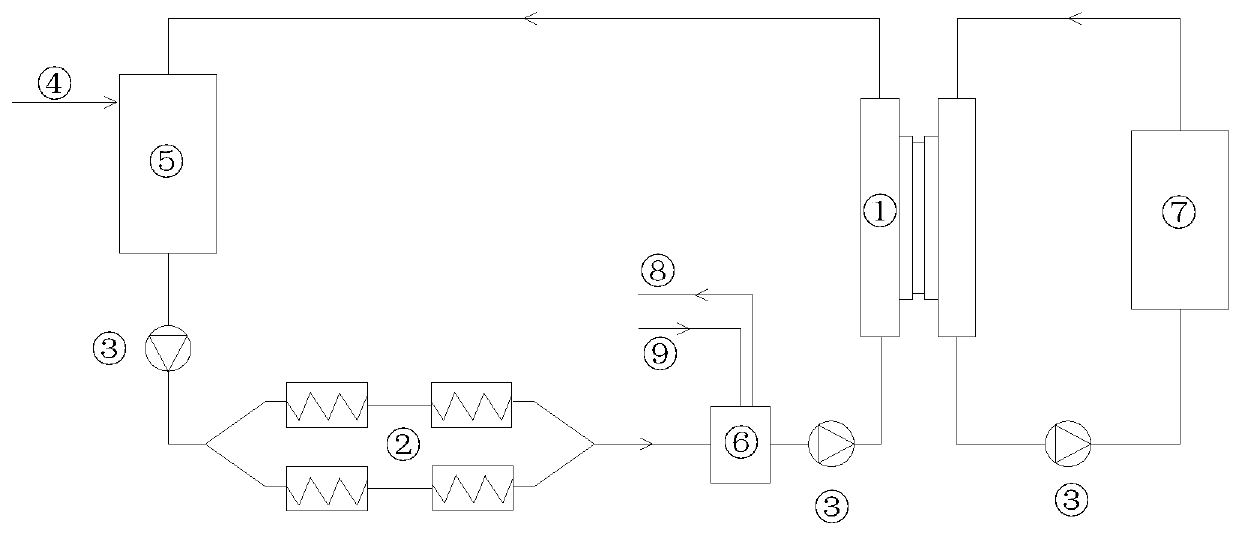
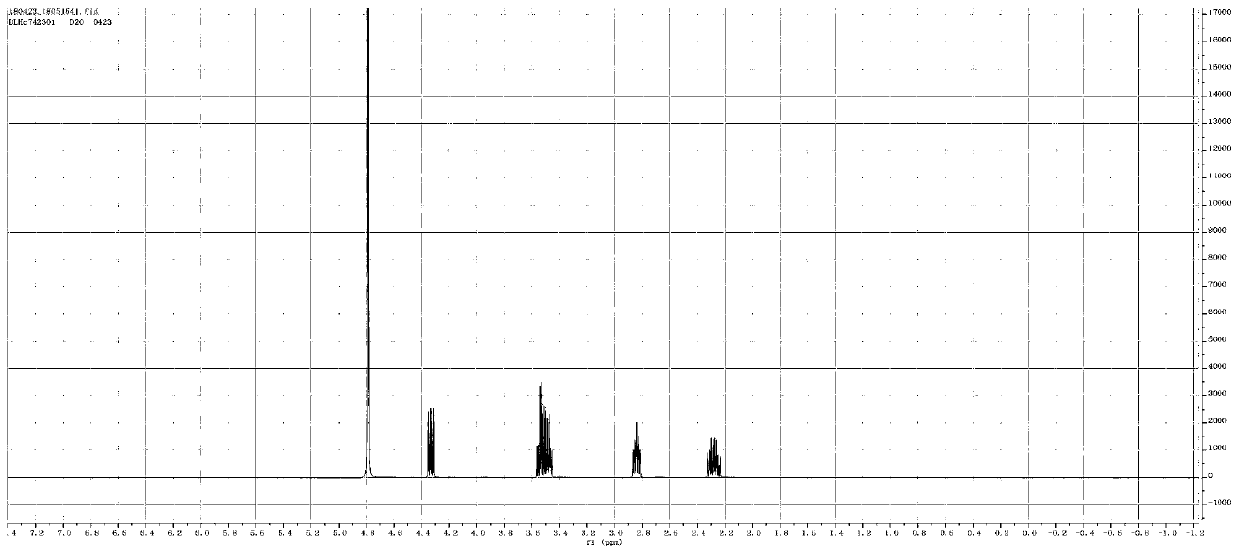
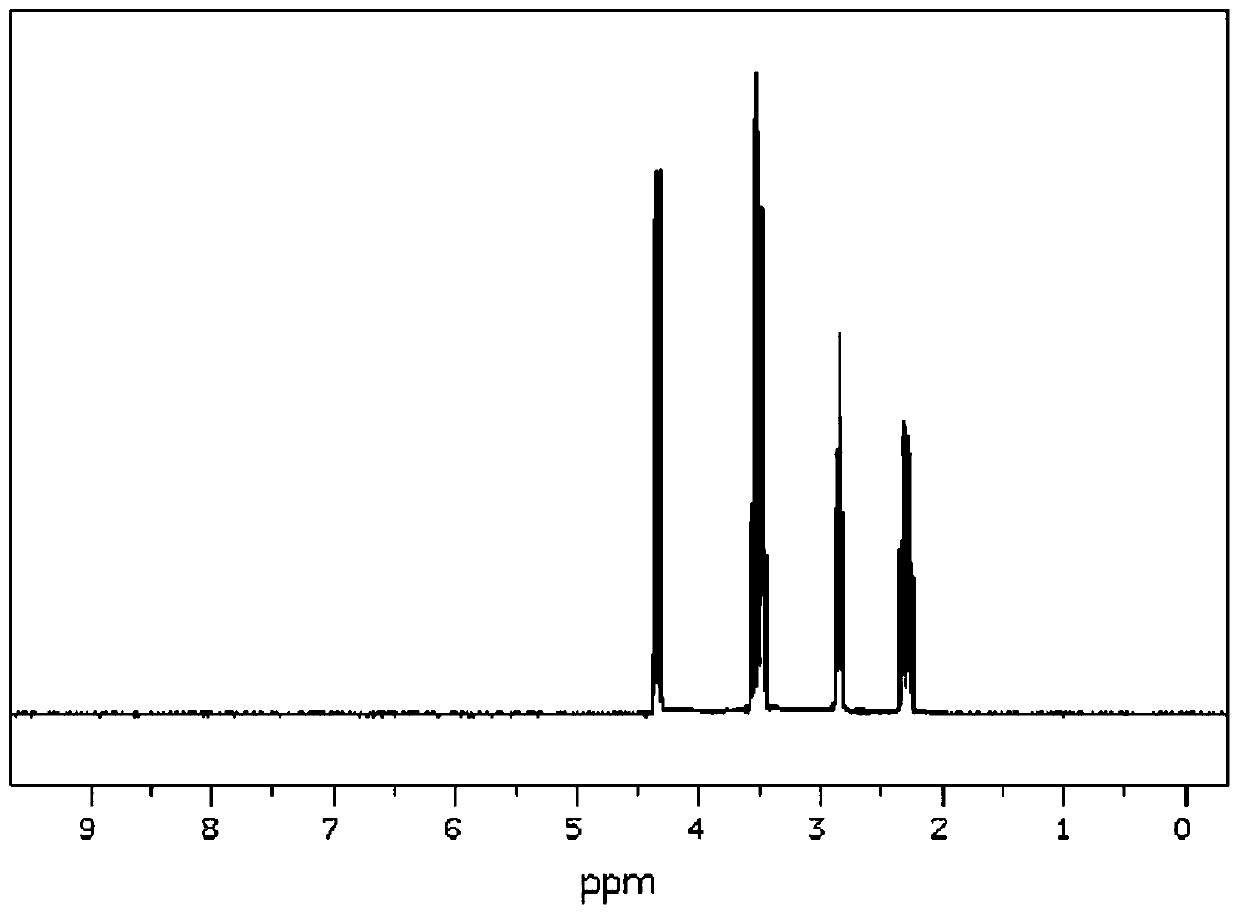
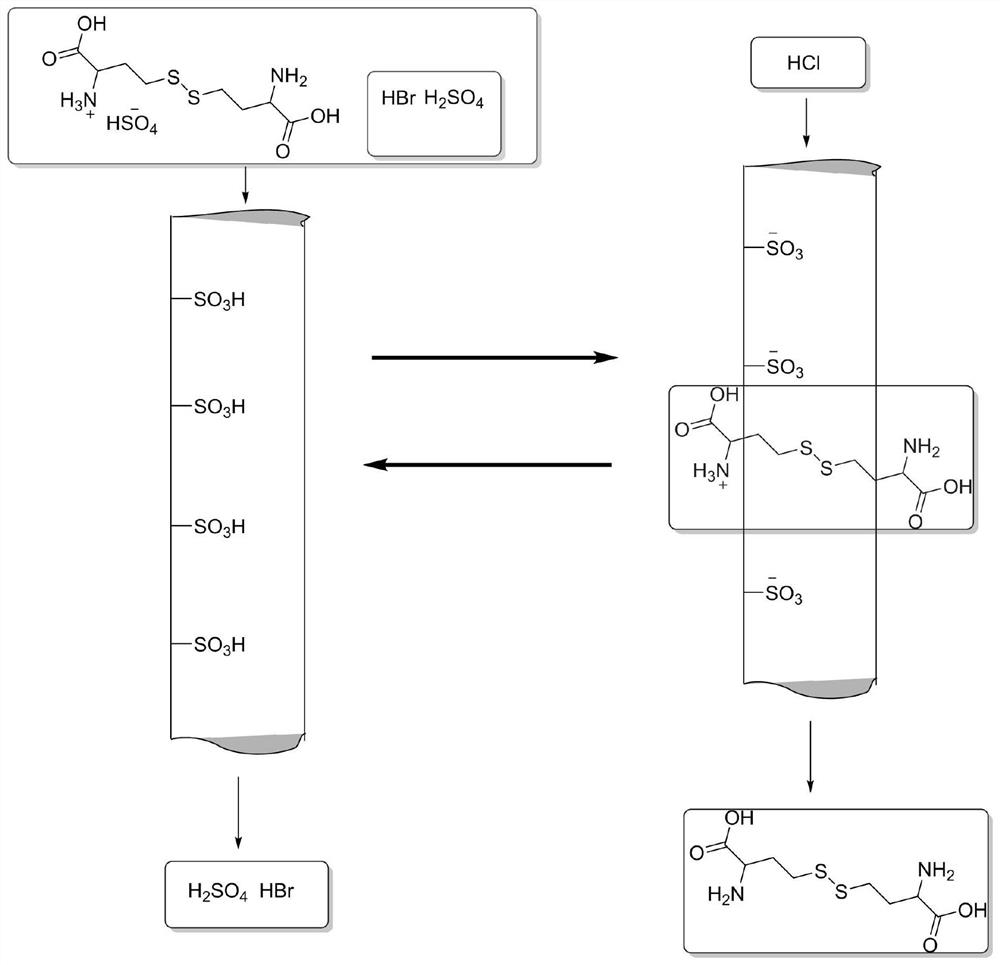
Post-treatment method of homocystine reaction solution
ActiveCN111635344AReduce manufacturing costAvoid it happening againHydrogen bromideOrganic compound preparationPhysical chemistryIon exchange
The invention provides a post-treatment method of a homocystine reaction solution, and relates to the technical field of homocystine preparation. The post-treatment method of the homocystine reactionsolution comprises the following steps: adsorbing the homocystine reaction solution in acid ion exchange resin, performing primary elution on the adsorption ion exchange resin through water, obtainingeluent and deacidified ion exchange resin, and wherein the eluent comprises recycled sulfuric acid and hydrobromic acid; and carrying out secondary elution on the deacidified ion exchange resin by using an acid eluent or an alkali eluent to obtain an acid eluent or an alkali eluent, the acid eluent or the alkali eluent containing homocystine. According to the post-treatment method, sulfuric acidand hydrobromic acid are weak in adsorption capacity in acidic ion resin, and are easily eluted with water to be separated from the reaction solution so that a large amount of waste salt generated byneutralizing the reaction liquid with alkali liquor is avoided, and the method is green and environment-friendly; complex devices are not needed, and the cost is low.
Owner:九江中星医药化工有限公司
Optimization of enzyme replacement therapy for treatment of homocystinuria
ActiveUS11324811B2Reduce the amount requiredDecrease in levelPeptide/protein ingredientsEnzyme stabilisationEnzyme proteinHomocystine
Owner:UNIV OF COLORADO THE REGENTS OF +1
Optimization of enzyme replacement therapy for treatment of homocystinuria
ActiveUS20200261555A1Reduce the amount requiredDecrease in levelPeptide/protein ingredientsEnzyme stabilisationEnzyme proteinHomocystine
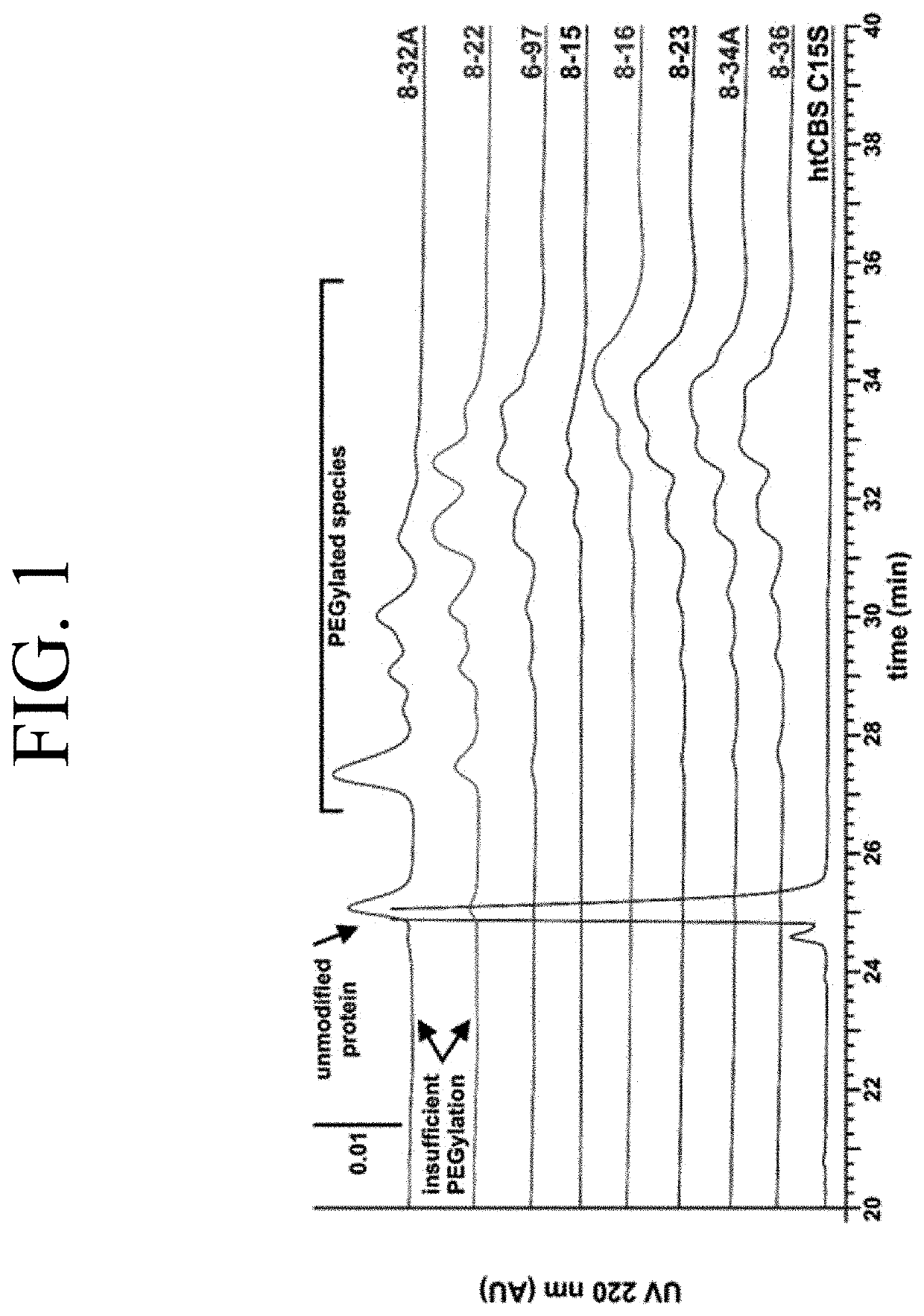
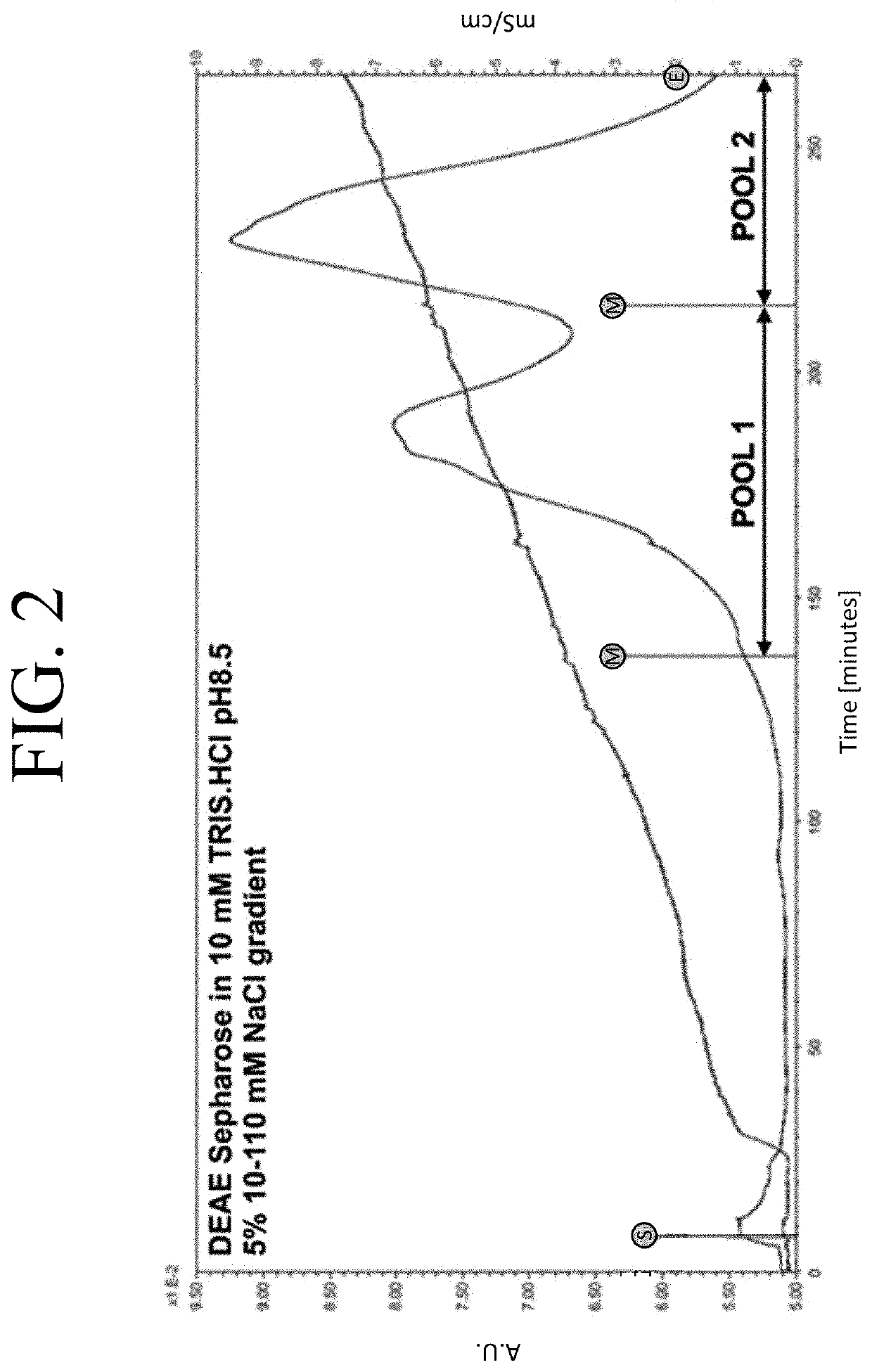
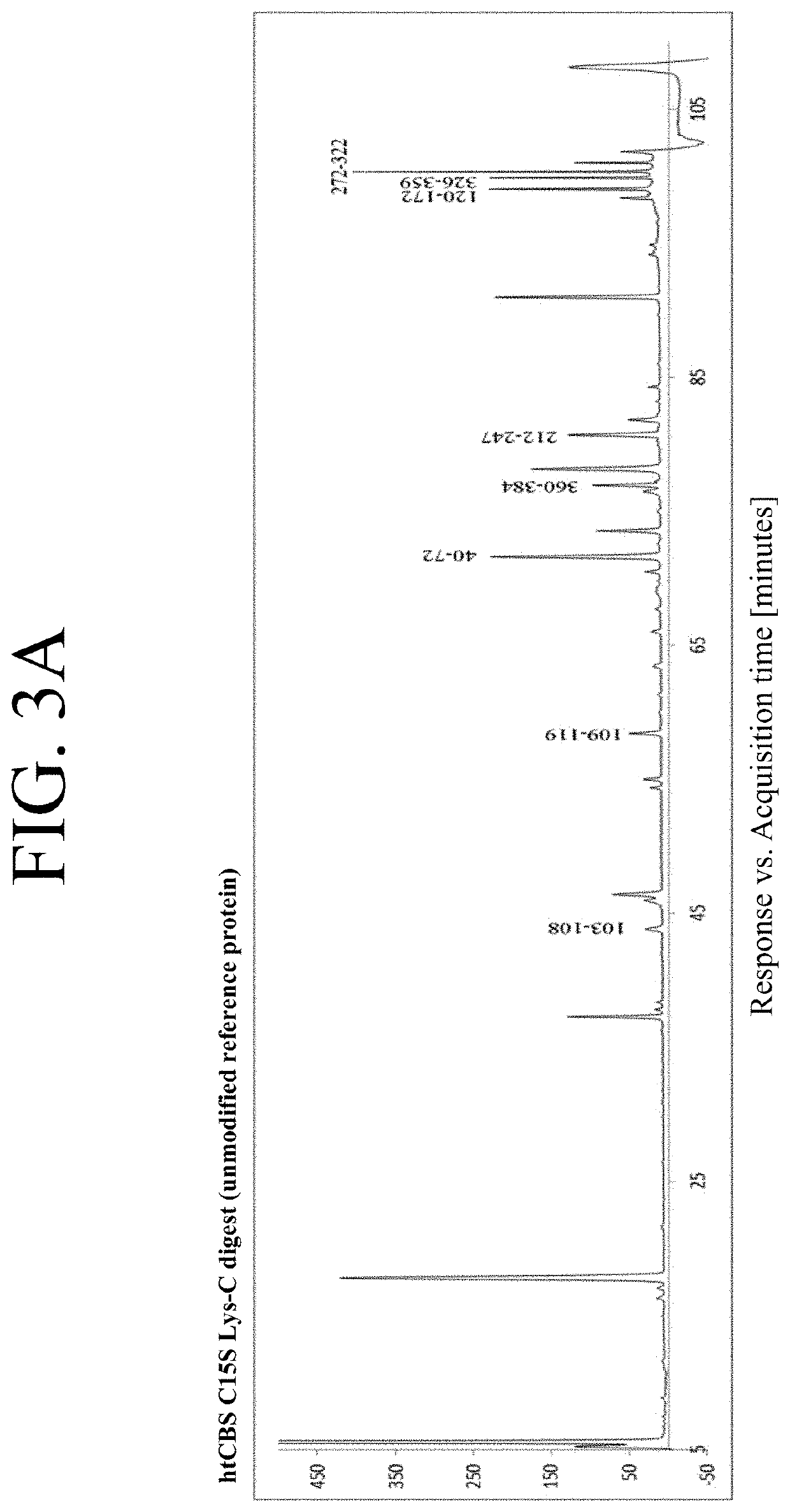
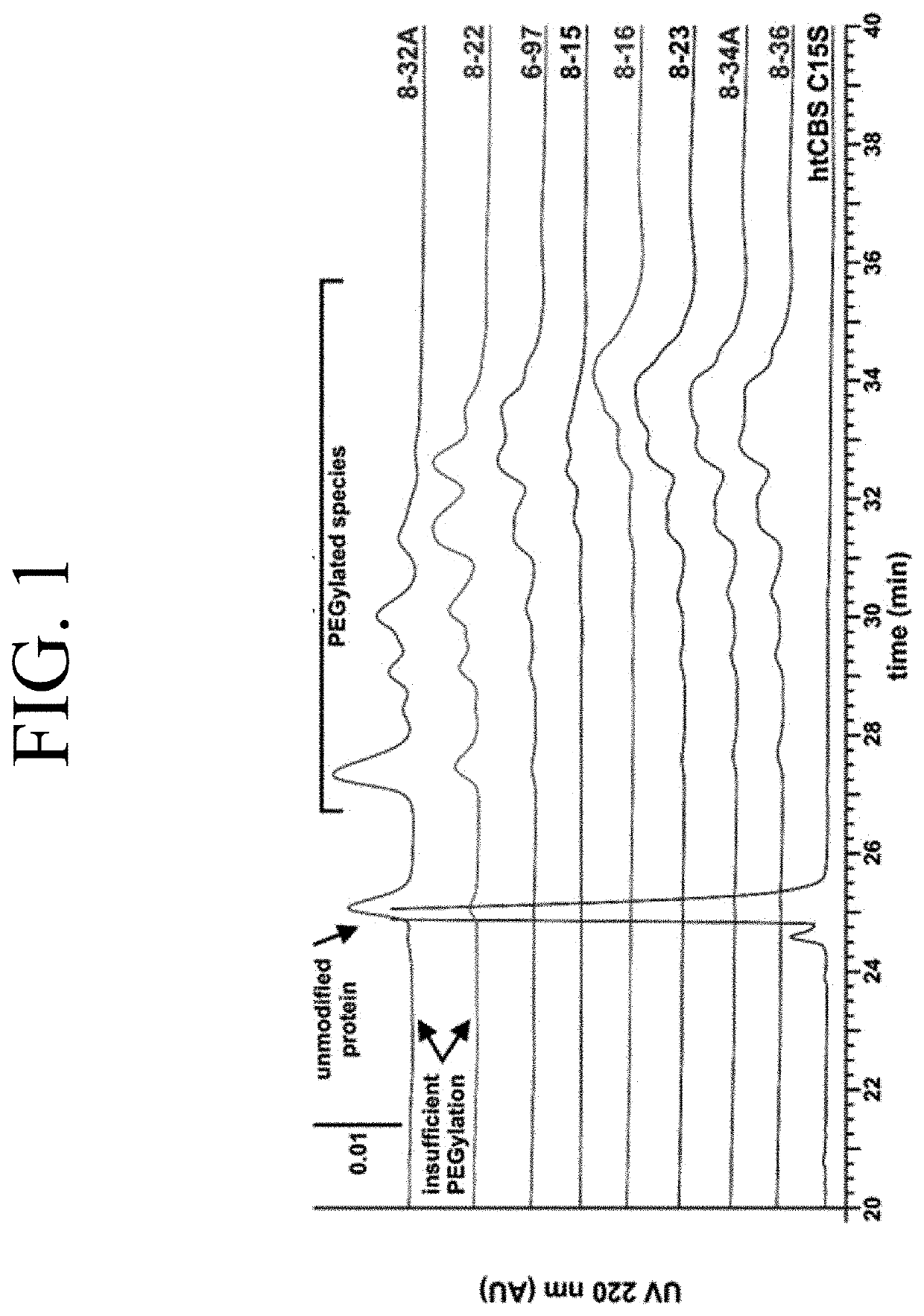
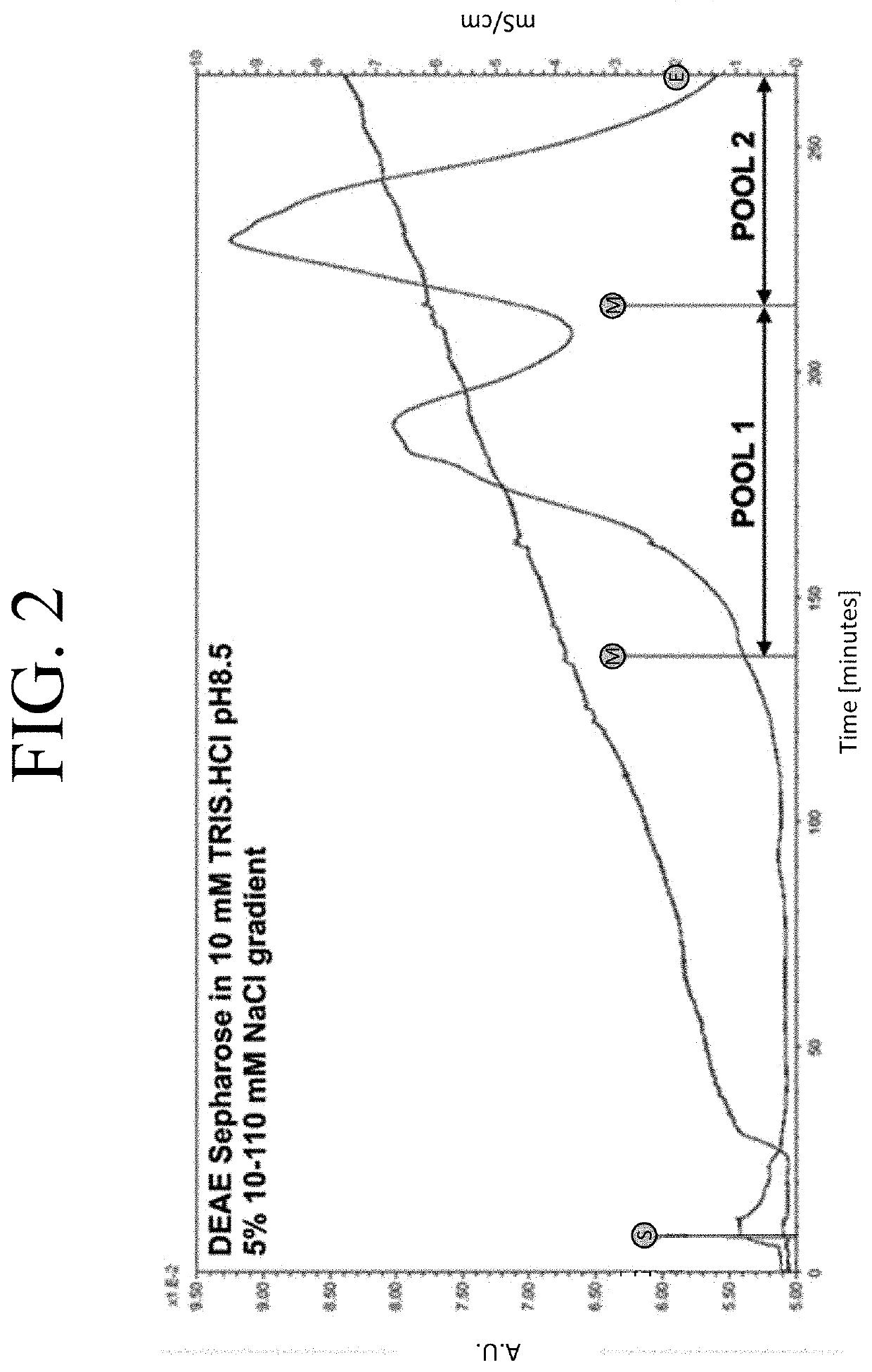
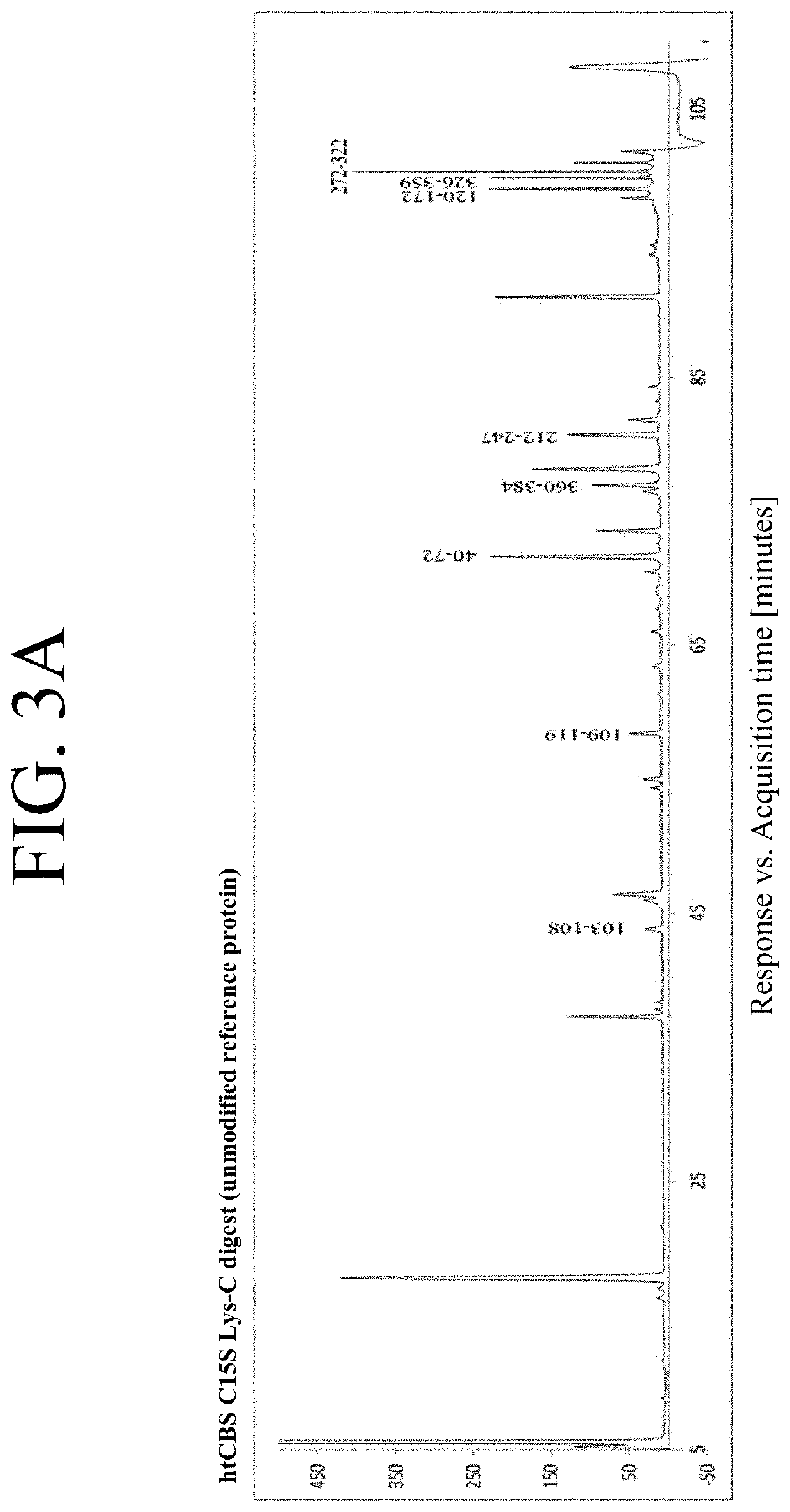
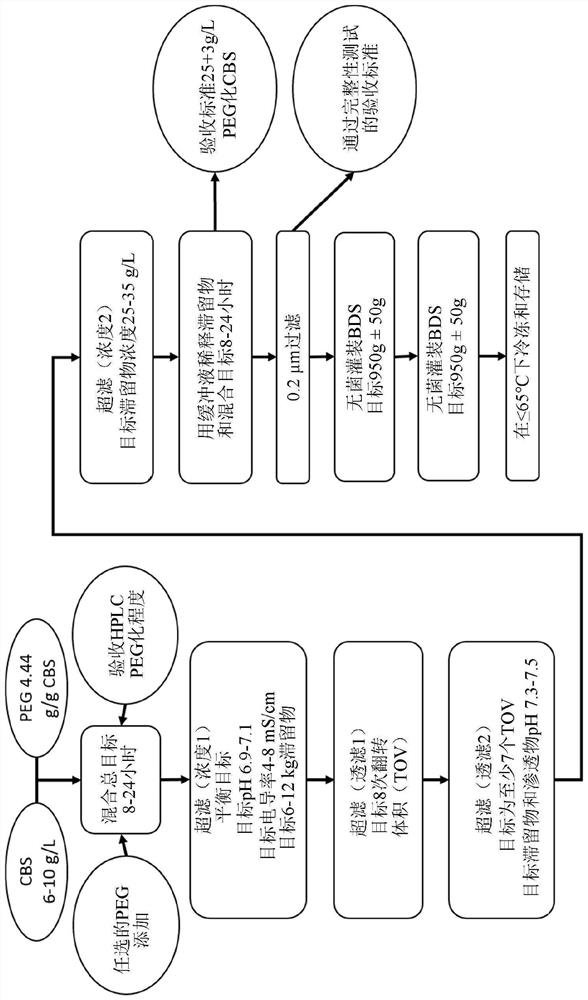
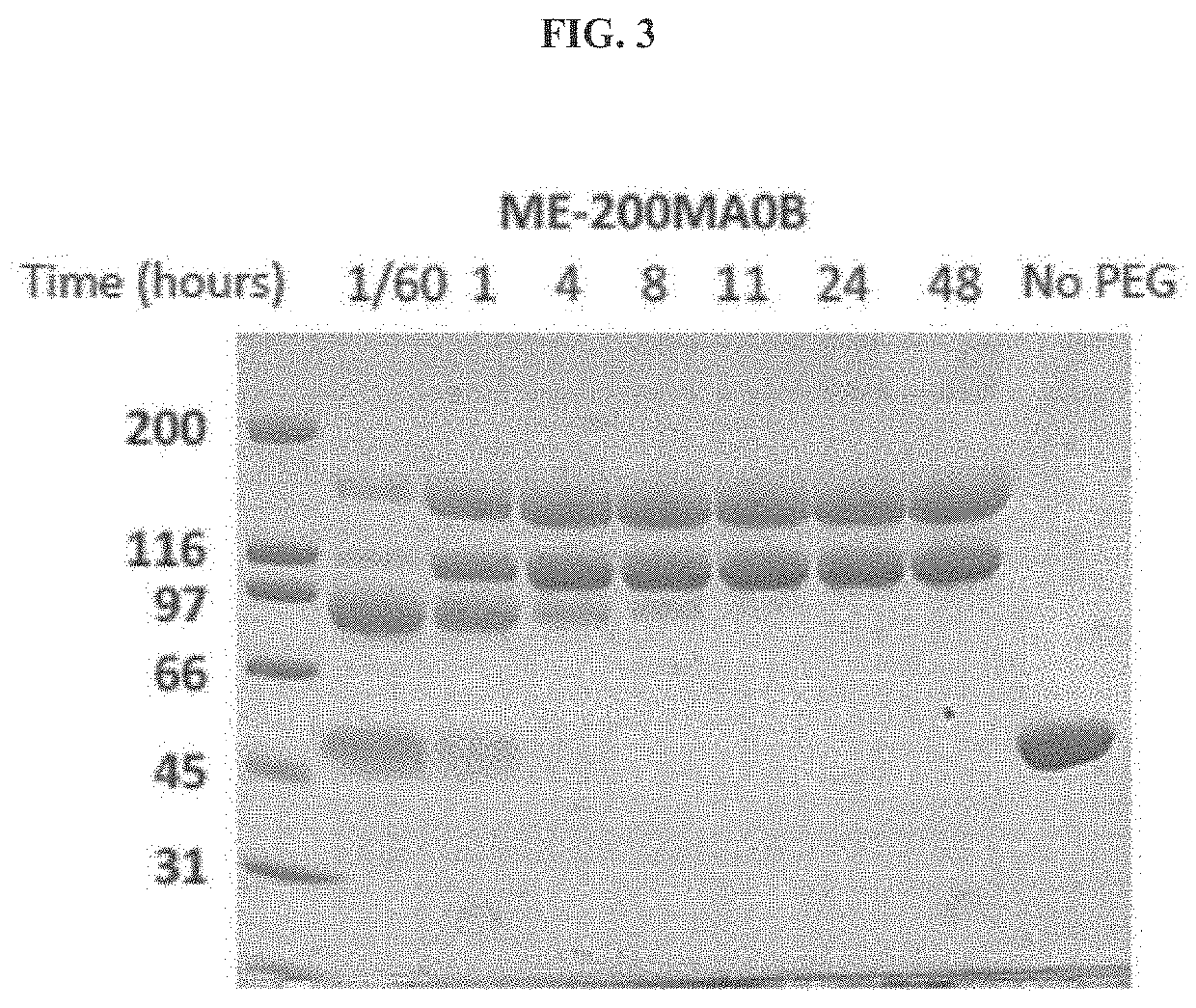
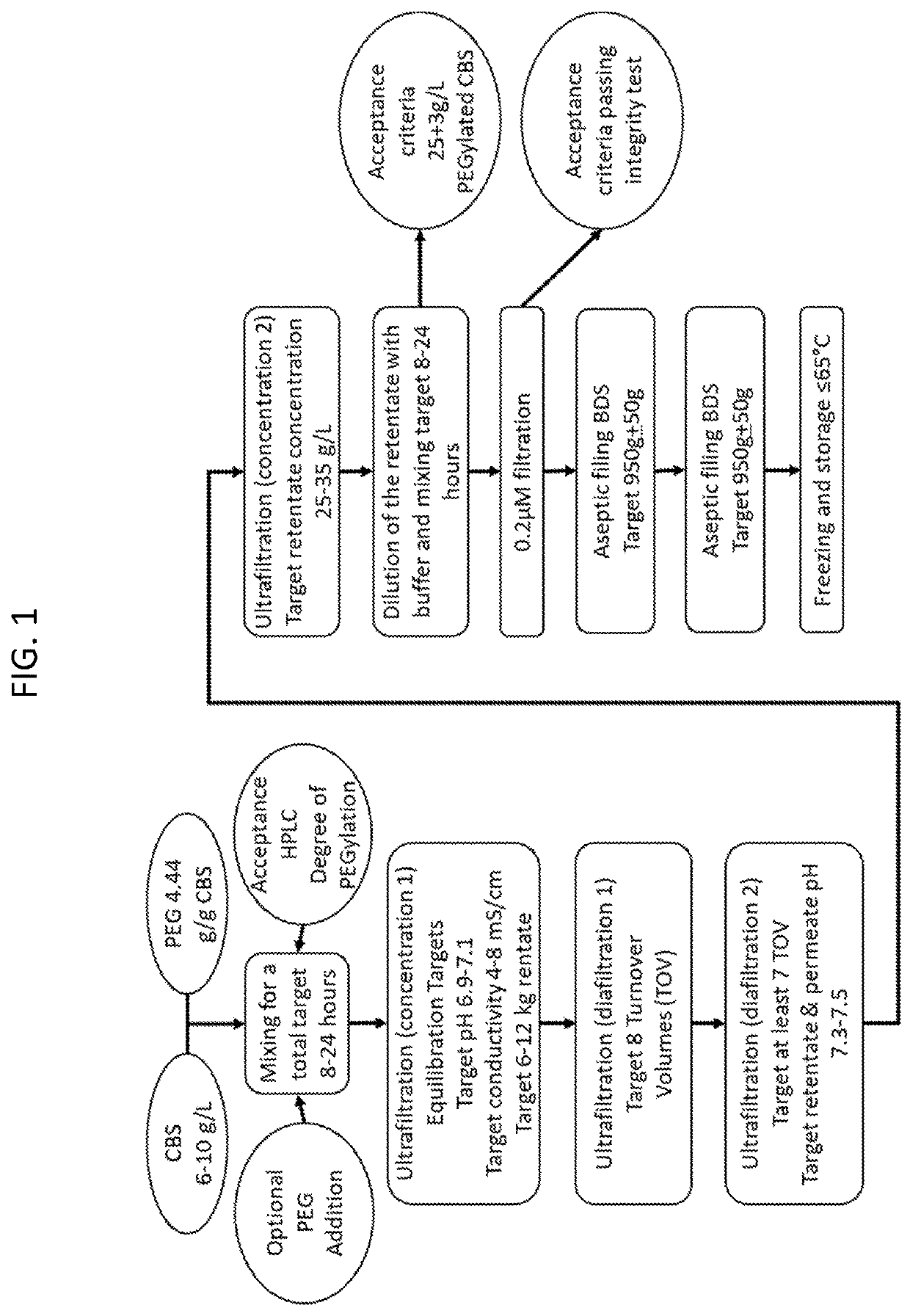
The present invention provides a method of PEGylating a human truncated cystathionine β-synthase protein containing a mutation of a cysteine to a serine at amino acid position 15 (htCBS C15S). The htCBS C15S was PEGylated with one of 5 kDa, 10 kDa, or 20 kDa NHS ester PEG molecules. In-process monitoring of the PEGylation process was used in the method to reduce levels of unPEGylated htCBS C15S and htCBS C15S with insufficient PEGylation. Administration of the PEGylated htCBS C15S had efficacy throughout the course of treatment for homocystinuria.
Owner:UNIV OF COLORADO THE REGENTS OF +1
PEGylated cystathionine beta synthase for enzyme therapy for treatment of homocystinuria
Owner:特拉维尔治疗瑞士有限公司 +1
Compositions and methods for treatment of homocystinuria
Provided are compositions and methods for enzyme replacement therapy using modified human cystathionine beta synthase (CBS) in the treatment of homocystinuria and related diseases and disorders.
Owner:UNIV OF COLORADO THE REGENTS OF
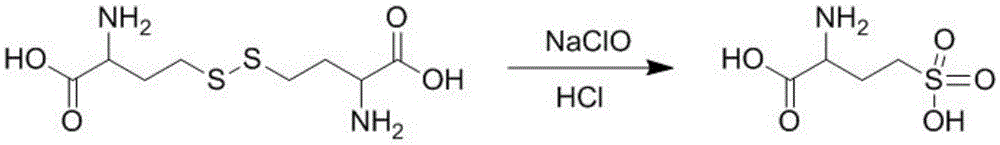
DL-homocysteic acid preparation method
ActiveCN105481729AAvoidAvoid pollutionOrganic chemistryOrganic compound preparationSodium chlorateBromine
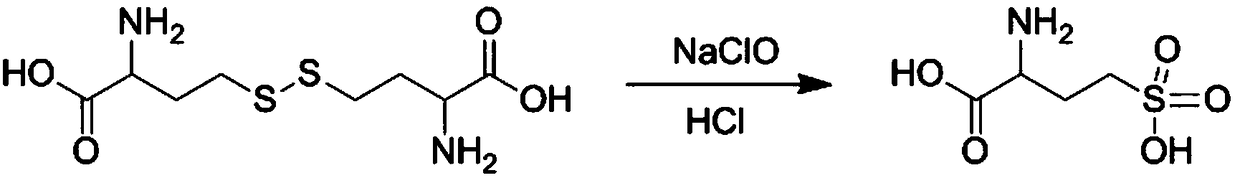
The present invention discloses a DL-homocysteic acid preparation method comprising the following steps: (1) dissolving DL-homocystine in an appropriate amount of hydrochloric acid, firstly heating to the temperature of 50 DEG C-70 DEG C, then adding dropwise sodium hypochlorite, after the addition is complete, then secondly heating to the temperature of 75 DEG C-85 DEG C for reaction for 1 to 3 hours to obtain a reaction solution; and (2) concentrating the reaction solution obtained in step (1) to a small volume, then adding an appropriate amount ethanol, crystallizing, then filtering to obtain a solid crystal, and drying thee solid crystal to obtain a DL-homocysteic acid desired product. The new method for preparation of the DL-homocysteic acid by reaction of the DL-homocystine and the sodium hypochlorite in the hydrochloric acid avoids use of bromine as a reactant, and avoids pollution generated by the use of bromine, and the DL-homocysteic acid product prepared by the method is high in quality and has the purity more than 98%.
Owner:CHONGQING TIANYI HENGHUA TECH CO LTD
The synthetic method of dl-homocysteine thiolactone hydrochloride
ActiveCN109943860BHigh yieldHigh mechanical strengthOrganic chemistryElectrolytic organic productionElectrolysisSynthesis methods
The invention discloses a synthesis method of DL-homocysteine thiolactone hydrochloride. A DL-homocystine is prepared into a catholyte with a hydrochloric acid solution as a medium, and electrolysis is conducted in an electrolysis tank to obtain the DL-homocysteine thiolactone hydrochloride, wherein according to a cathode electrode, a roughened glass carbon electrode serves as a base material, andthe surface of the base material is covered with lead-bismuth alloy. The method can be used for improving the yield of the DL-homocysteine thiolactone hydrochloride.
Owner:武汉本杰明医药股份有限公司
A kind of method adopting one pot method to synthesize selenomethionine
ActiveCN108794365BReduce pollutionReduce harmOrganic chemistryBulk chemical productionHomoserineEnvironmental engineering
The invention discloses a method for synthesizing selenomethionine by adopting a one-pot method. The method comprises the following steps: mixing selenium and a reducing agent and performing a reducing reaction to obtain metal dioxide M-2Se2; adding alpha-amino protected homoserine lactones in a solution containing metal dioxide M-2Se2 and generated when the reducing reaction is finished to perform a reaction to obtain alpha-amino protected homocysteine diselenide; and reducing the alpha-amino protected homocysteine diselenide, and then performing methylation to obtain alpha-amino protected selenomethionine; and performing deprotection on the alpha-amino protected selenomethionine to obtain selenomethionine. Through the adoption of the way, the method is low in cost, high in yield and highin product purity, can be used for large batch production, avoids toxicity pollution possibly generated in the process link, and greatly reduces environmental pollution and injury to operation personnel.
Owner:SUZHOU TOKIND CHEM CO LTD
A reactive fluorescent probe for distinguishing sulfhydryl compounds and its synthesis method and application
ActiveCN107098915BRealize detectionImprove stabilityOrganic chemistryFluorescence/phosphorescenceQuantum yieldHydrogen
The invention discloses a reactive-type fluorescence probe for distinguishing sulfydryl compounds, wherein the structure of the fluorescence probe is represented as the formula (I). R1, R2, R3 and R4 are independently selected from one of hydrogen, a C1-C8 alkyl group and a C3-C8 cycloalkyl group, and X is independently selected from halogens; or the R1 and the R4 and / or the R2 and the R3 can form a ring. The invention also discloses a synthesis method and an application of the fluorescence probe. The fluorescence probe is low in molecular weight, is high in fluorescent quantum yield, has good cytocompatibility, can generate different fluorescence enhancement signals when being reacted respectively with cysteine and homocystine, can distinguish and recognize the cysteine and homocystine well, has good stability and is easy to prepare.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
A kind of preparation method of dl-homosulfoalanine
ActiveCN105481729BAvoidAvoid pollutionOrganic chemistryOrganic compound preparationSodium chlorateBromine
Owner:CHONGQING TIANYI HENGHUA TECH CO LTD
Tail gas treatment method for preparing DL-homocystine
The invention discloses a tail gas treatment method for preparing DL-homocystine, and belongs to the field of medicines. The tail gas comprises sulfur dioxide gas and dimethyl disulfide gas, and oxidation absorption liquid is adopted to treat the tail gas. The method comprises the steps: treating tail gas through modified magnesium lithium silicate and then introducing the tail gas into oxidation absorption liquid to be treated, layering a solution obtained after treatment stands still, and taking an oil phase after water-oil separation; and finally, extracting, condensing and removing the oil phase by adopting an extracting agent. According to the tail gas treatment method for preparing the DL-homocystine, dimethyl disulfide can generate colorless and odorless dimethyl sulfoxide and sulfur dioxide can generate sulfuric acid respectively by adopting the oxidation absorption liquid, so emission of toxic and odorous gases is eliminated fundamentally; meanwhile, even if the oxidation absorption liquid enters a Fenton oxidation unit of a sewage treatment system for operation, toxic gas cannot overflow again due to re-acidification.
Owner:九江中星医药化工有限公司
Synthesis method of thionyl diacetic acid
ActiveCN112251769AImprove conversion rateIncrease conduction rateElectrolysis componentsThiol preparationPtru catalystDistillation
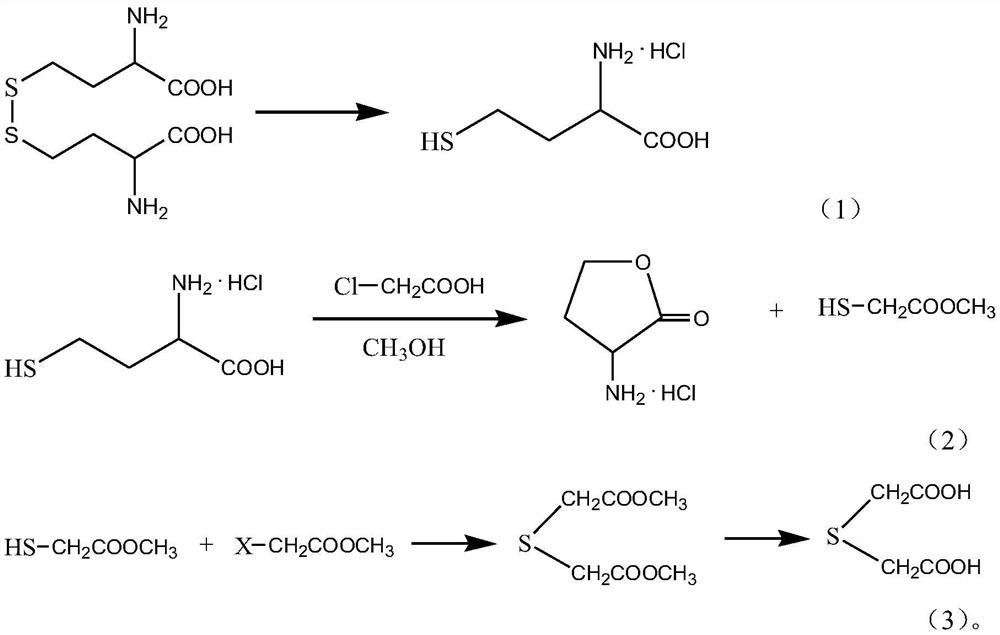
The invention discloses a synthesis method of thionyl diacetic acid. The method mainly comprises the following steps: dissolving homocystine in hydrochloric acid to obtain a catholyte, and carrying out an electroreduction reaction in an electrolytic cell to obtain homocysteine hydrochloride; dissolving the homocysteine hydrochloride and chloroacetic acid in water, adding methanol, dropwise addinga catalyst and a dehydrating agent, stirring to react for 2-5 hours, cooling the reaction product, filtering the reaction product to separate out homoserine lactone hydrochloride, and carrying out reduced pressure distillation on the filtrate to obtain methyl mercaptoacetate; and dissolving the methyl mercaptoacetate and the halogenated methyl acetate in methanol, controlling the temperature to be25-50 DEG C, reacting for 6-12 hours, carrying out reduced pressure distillation, adding dilute sulfuric acid, heating the mixture to 50-80 DEG C, and separating and purifying the mixture to obtain the thiodiacetic acid. According to the synthesis method of the thionyl diacetic acid, the yield can be effectively improved.
Owner:武汉本杰明医药股份有限公司
Pegylated cystathionine beta synthase for enzyme therapy for treatment of homocystinuria
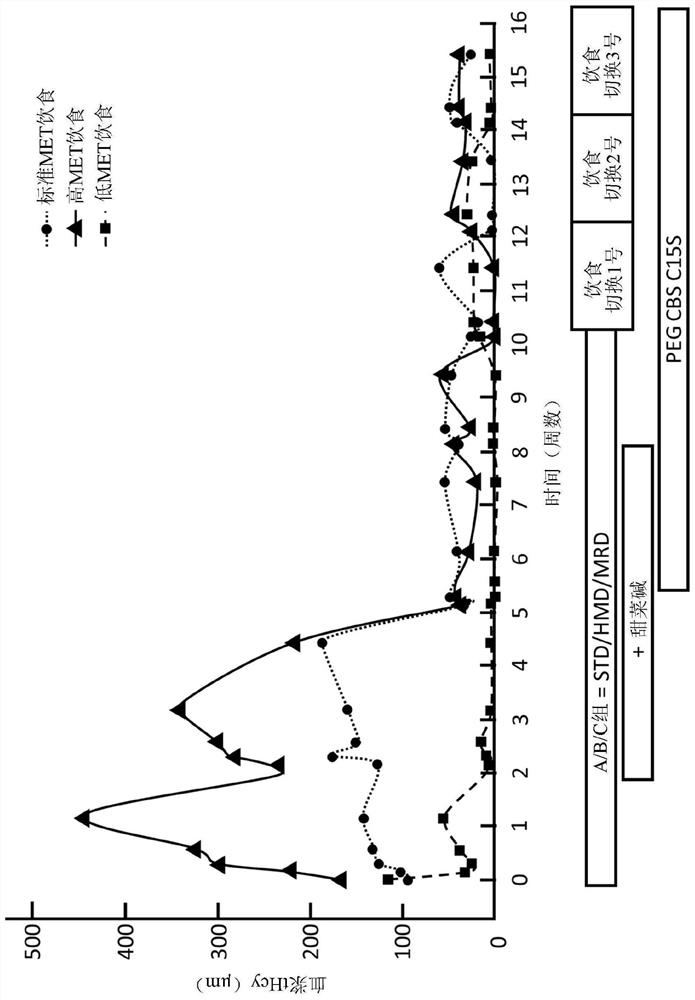
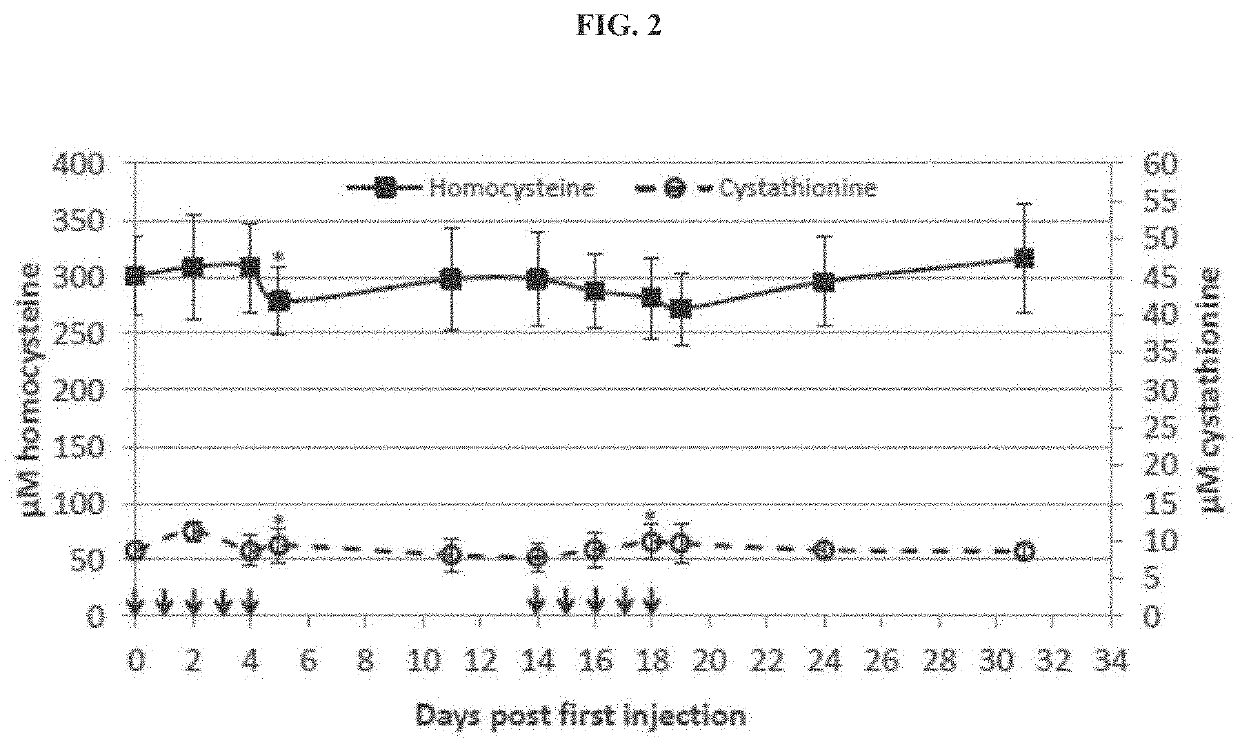
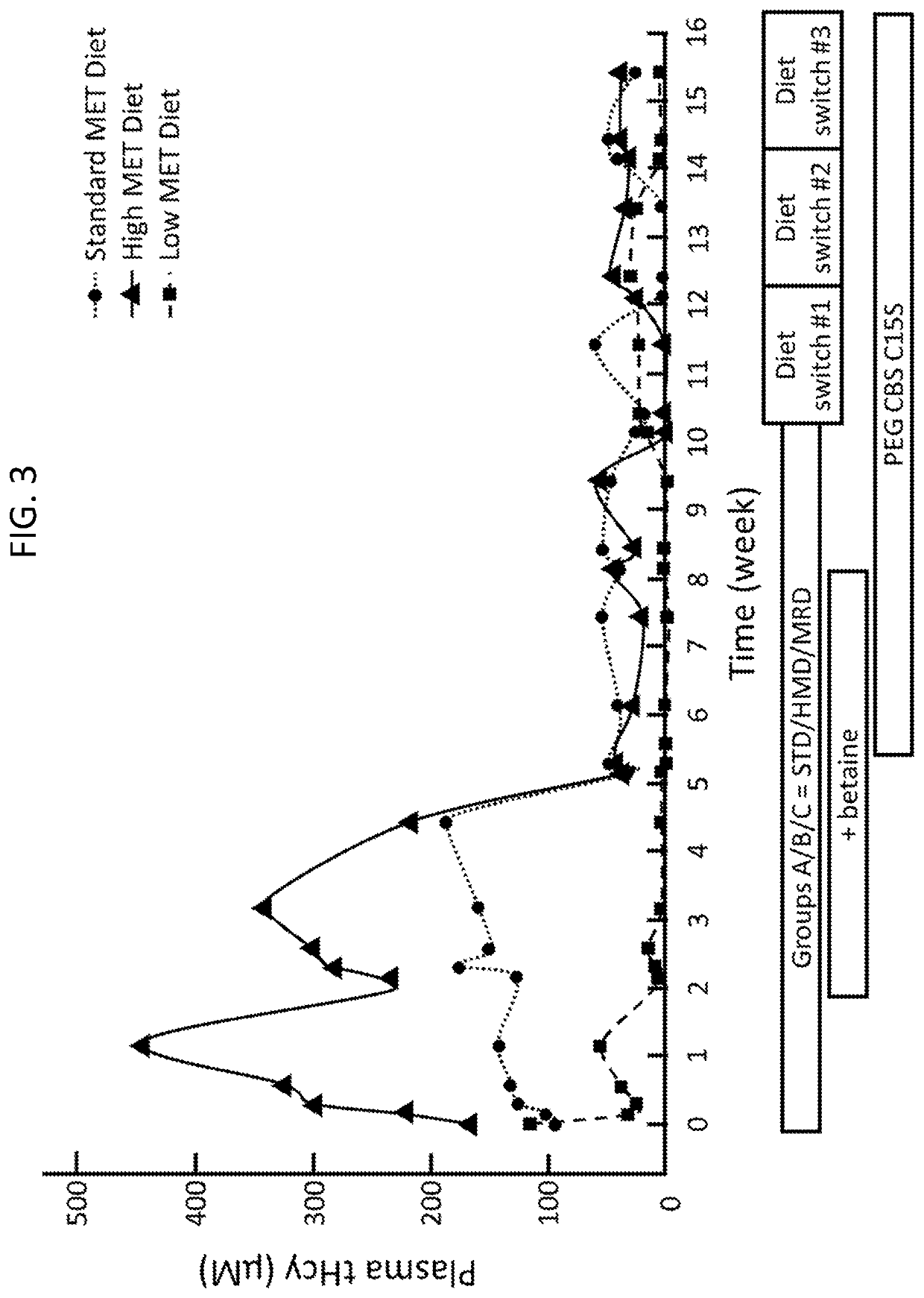
The present disclosure provides formulations for a drug product comprising a PEGylated CBS protein having the amino acid sequence of SEQ ID NO: 1. Dosages and dosing regimens are provided for treatment of homocystinuria in a subject in need thereof. Additionally, the dosages and dosing regimens are also provided to reduce the level of homocysteine (Hcy) or increase the levels of cysteine (Cys) and / or cystathionine (Cth) in a subject in need thereof.
Owner:TRAVERE THERAPEUTICS SWITZERLAND GMBH +1
Composition containing amlodipine, atorvastatin and folic acid as well as new application thereof
InactiveCN101590049AAvoid damageAddress clinical gapsBlood disorderHeterocyclic compound active ingredientsDiseaseLevamlodipine
The invention relates to a drug composition containing amlodipine, atorvastatin and folic acid and a new application thereof in preparing drugs for treating the HHCY-hyperhomocysteinemia and the elevated homocysteine. The composition of the amlodipine or a levoamlodipine, the atorvastatin and the folic acid can further reduce the Hcy level, and the effect is superior to that of single drug, so that the composition of the amlodipine or the levoamlodipine, the atorvastatin and the folic acid can not only lower the Hcy level, but also has synergetic effect on reducing the Hcy damages. The composition of the invention is a selection for treating Hcy, which provides a more effective treatment protocol for treating high homocysteine. The invention belongs to the pharmaceutical field.
Owner:北京奥萨医药研究中心有限公司 +1
Preparation and Application of a Novel Fluorescent Probe for Specific Recognition of Cysteine
InactiveCN106946801BThe synthetic route is simpleLow costOrganic chemistryFluorescence/phosphorescenceInterference resistanceFluorescence sensing
The present invention discloses a novel fluorescent probe capable of specifically recognizing cysteine, wherein the molecular structure formula is defined in the specification. According to the present invention, the fluorescent probe can distinguish the mercapto-containing cysteine from the mercapto-free amino acid region, can distinguish the cysteine from the homocystine and the glutathione having the similar structure, emits red light during cysteine detection, can reduce the background interference and the damage of light on tissue cells in biological applications, exhibits great Stokes shift, can reduce self-absorption so as to improve the sensitivity, can be used for the fluorescence sensing analysis of the cysteine in environments or biological samples, and has advantages of good selectivity to cysteine, high sensitivity, strong interference resistance, and good application prospect.
Owner:CENT SOUTH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com