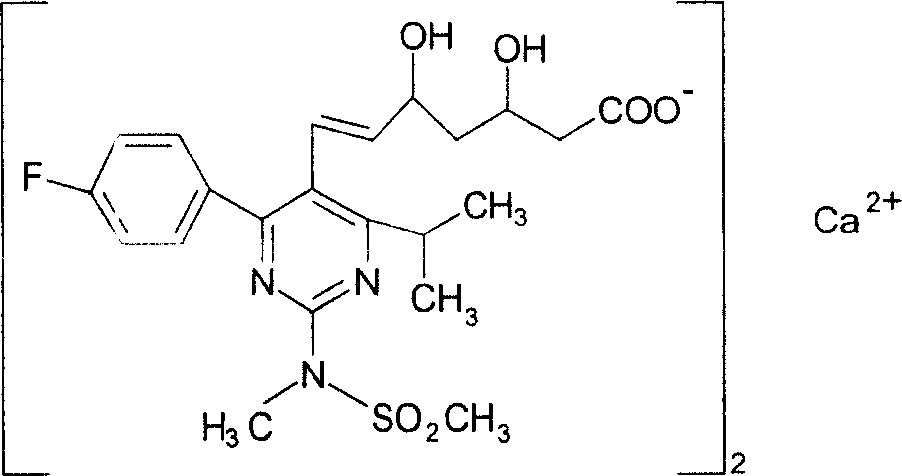
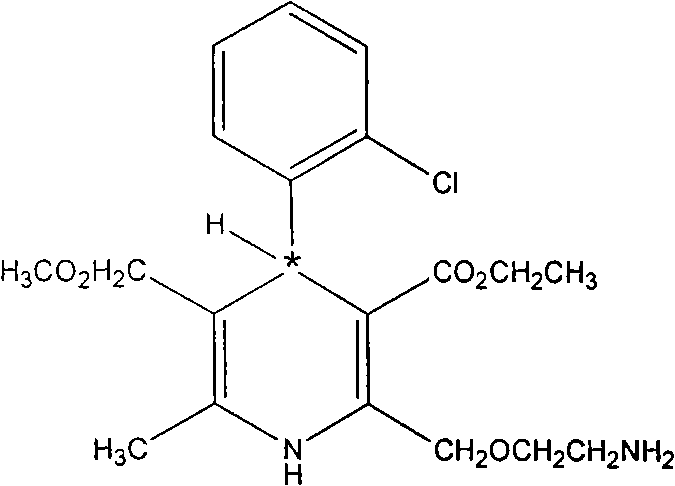
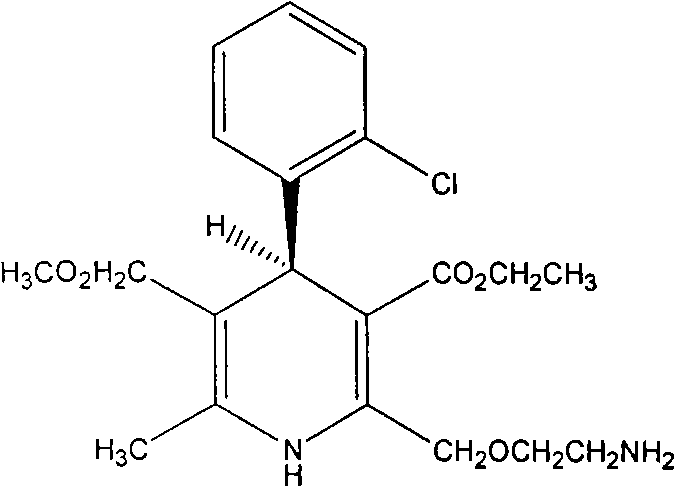
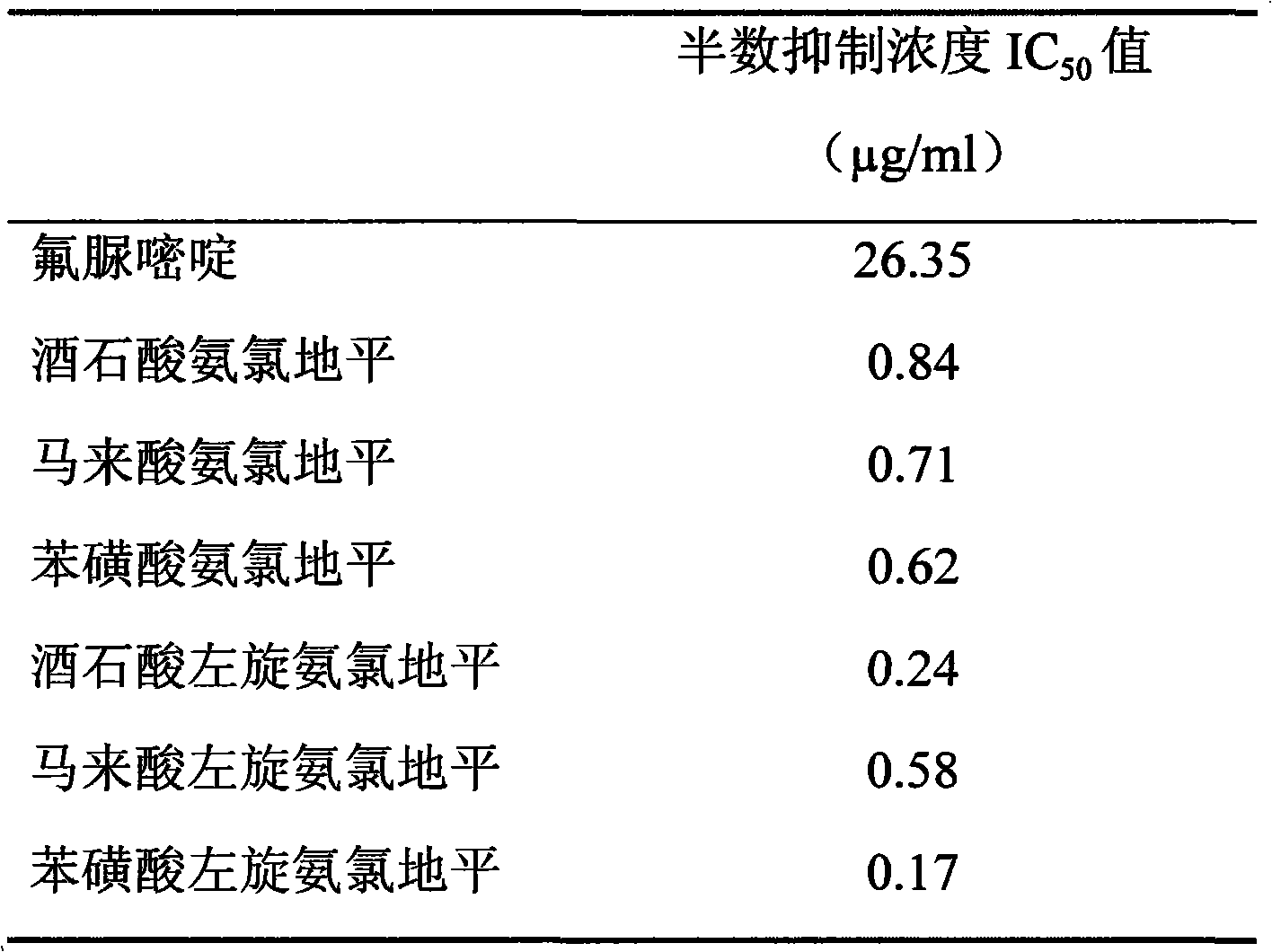
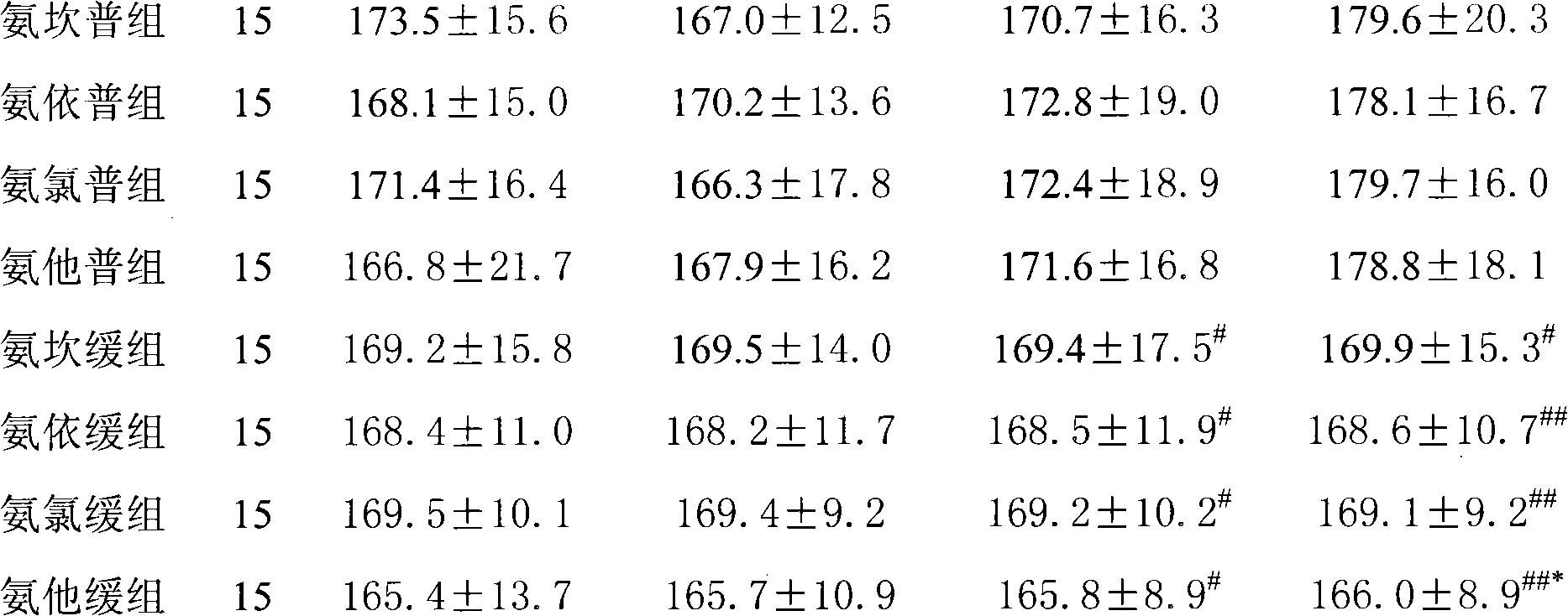
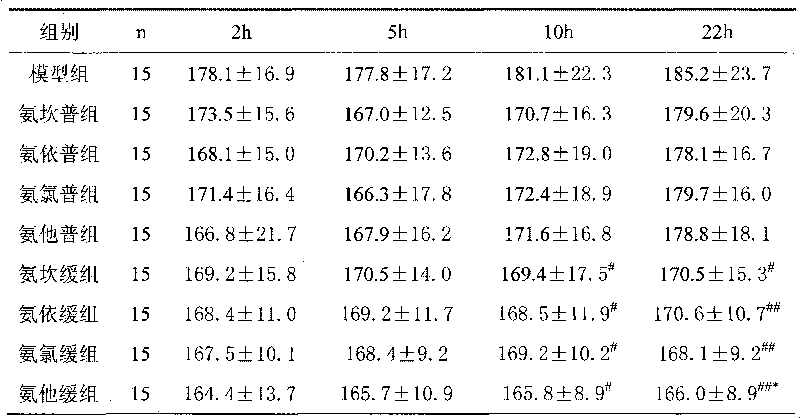
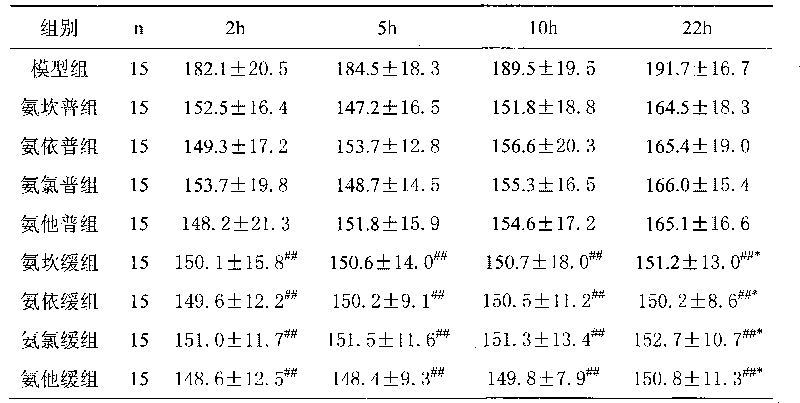
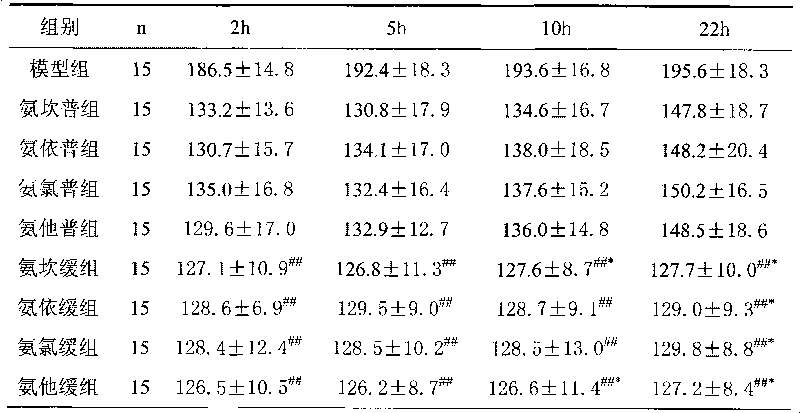
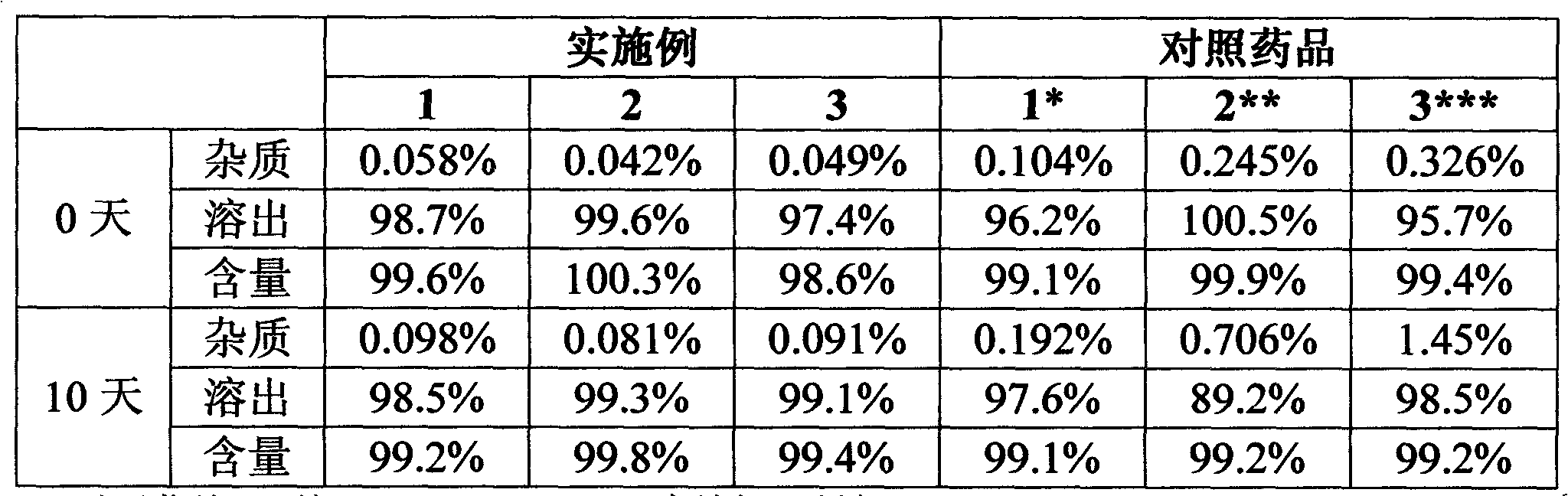
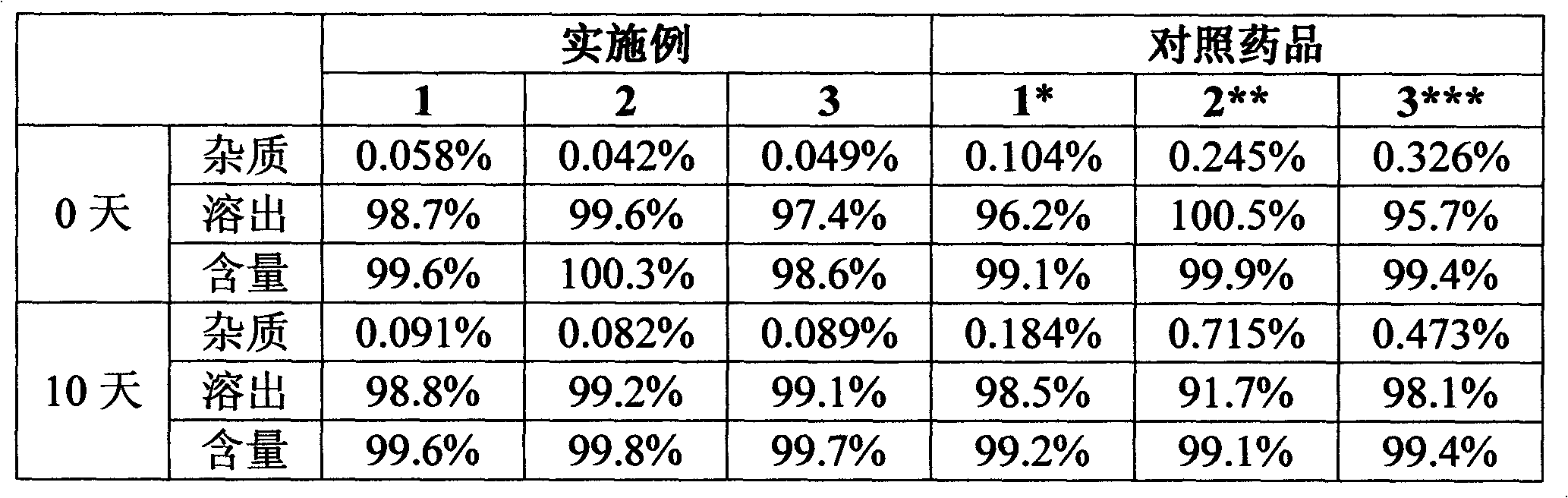
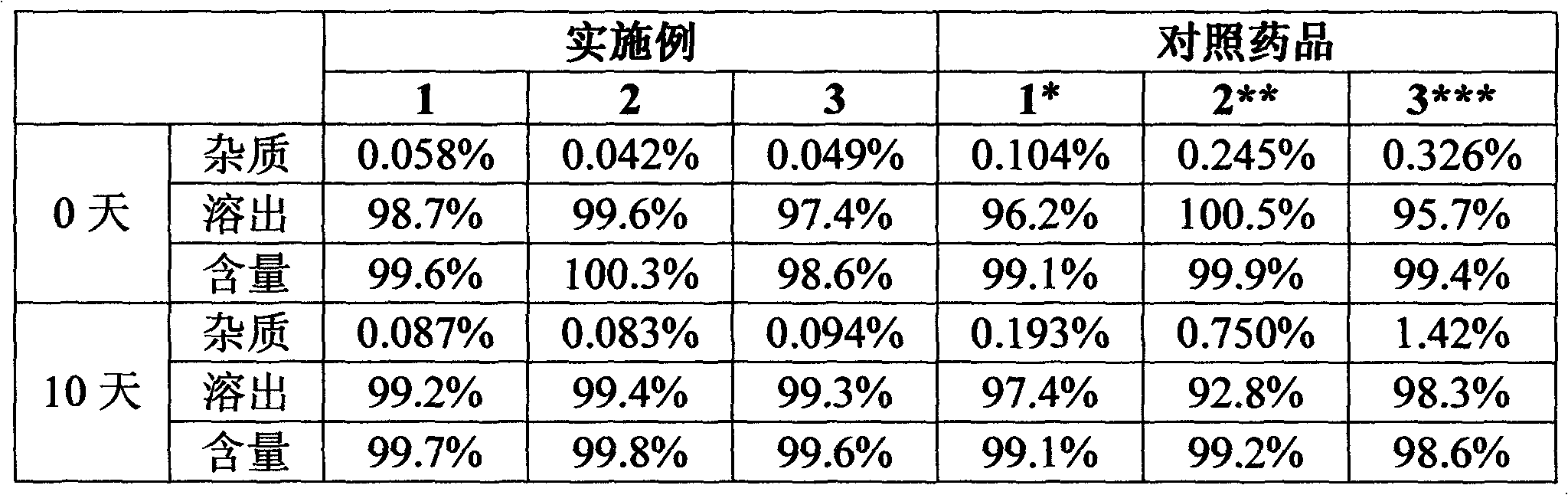
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
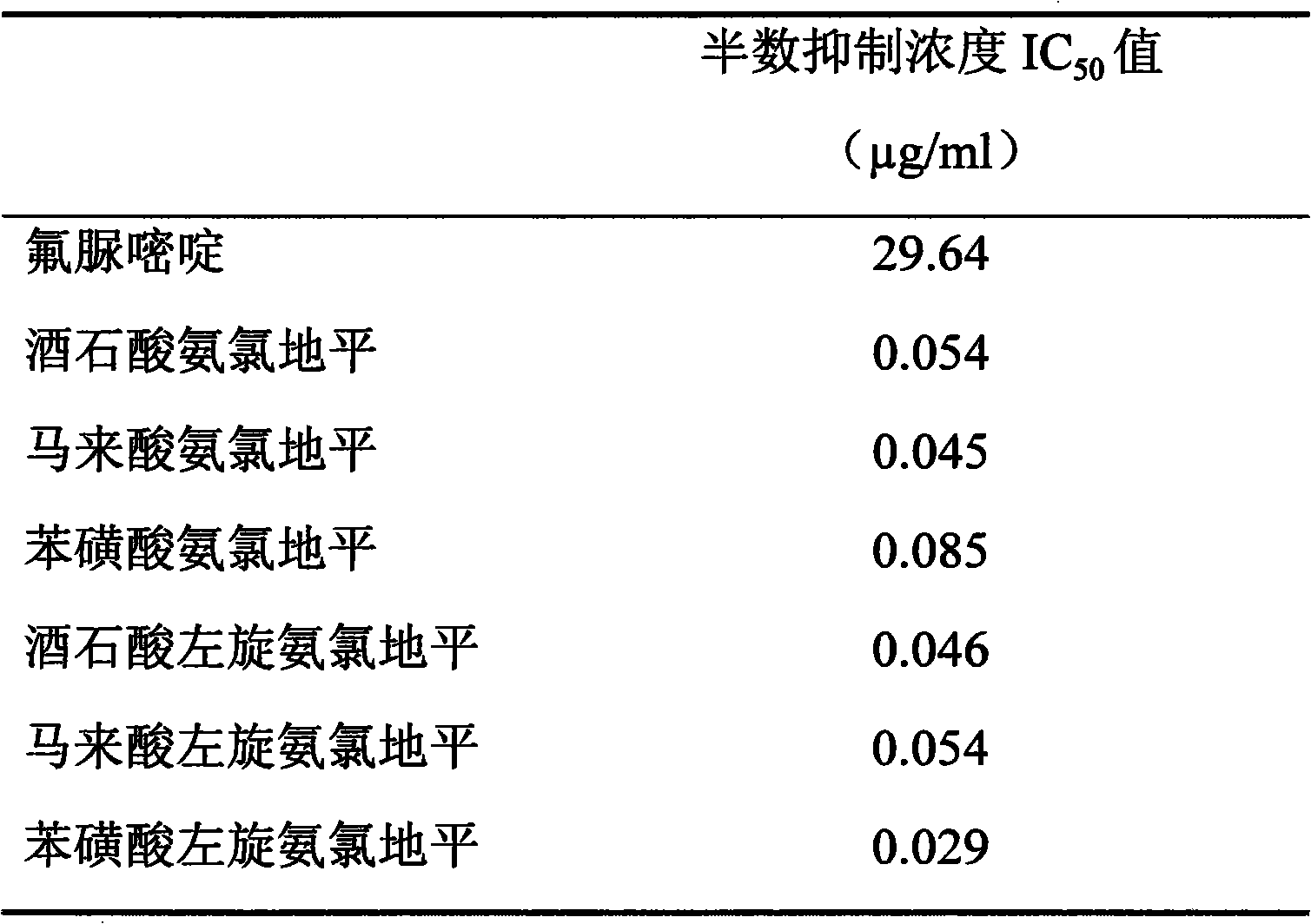
187 results about "Levamlodipine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
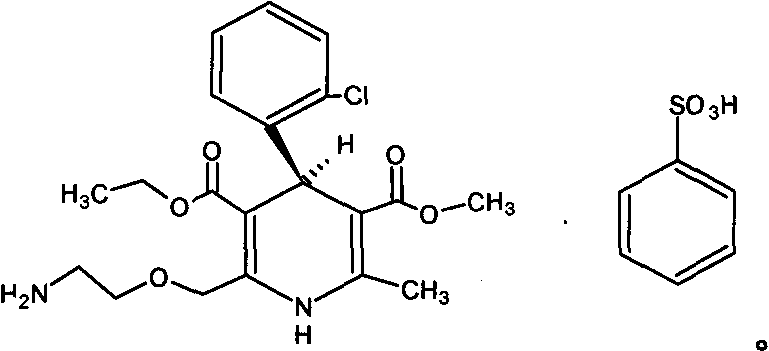
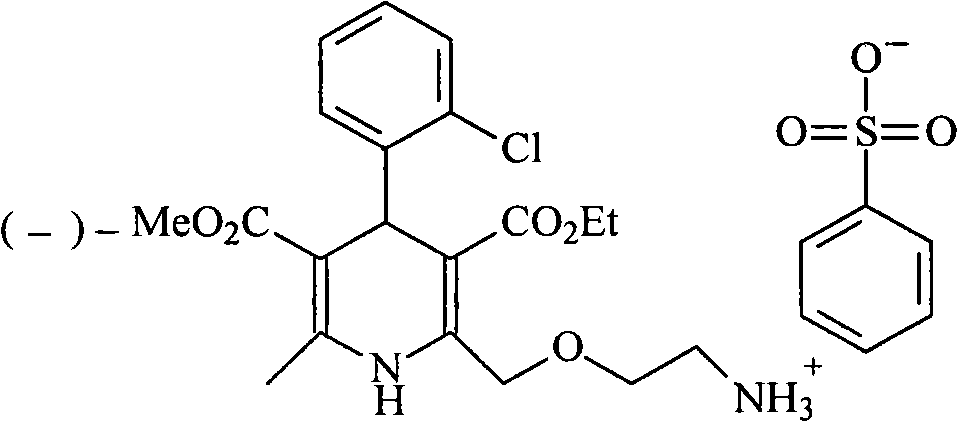
Levamlodipine (INN), also known as levoamlodipine or S-amlodipine is a pharmacologically active enantiomer of amlodipine. Amlodipine belongs to the dihydropyridine group of calcium channel blocker used as an antihypertensive and antianginal agent. Levamlodipine is currently marketed in Russia under the brand name EsCordi Cor (Actavis Pharma), in Brazil under the brand name Novanlo (Biolab Sanus) and in India under the trade names Eslo (Zuventus Healthcare Ltd.), Asomex (Emcure Pharmaceutical Ltd.), and Espin (Intas Pharmaceuticals Ltd.).
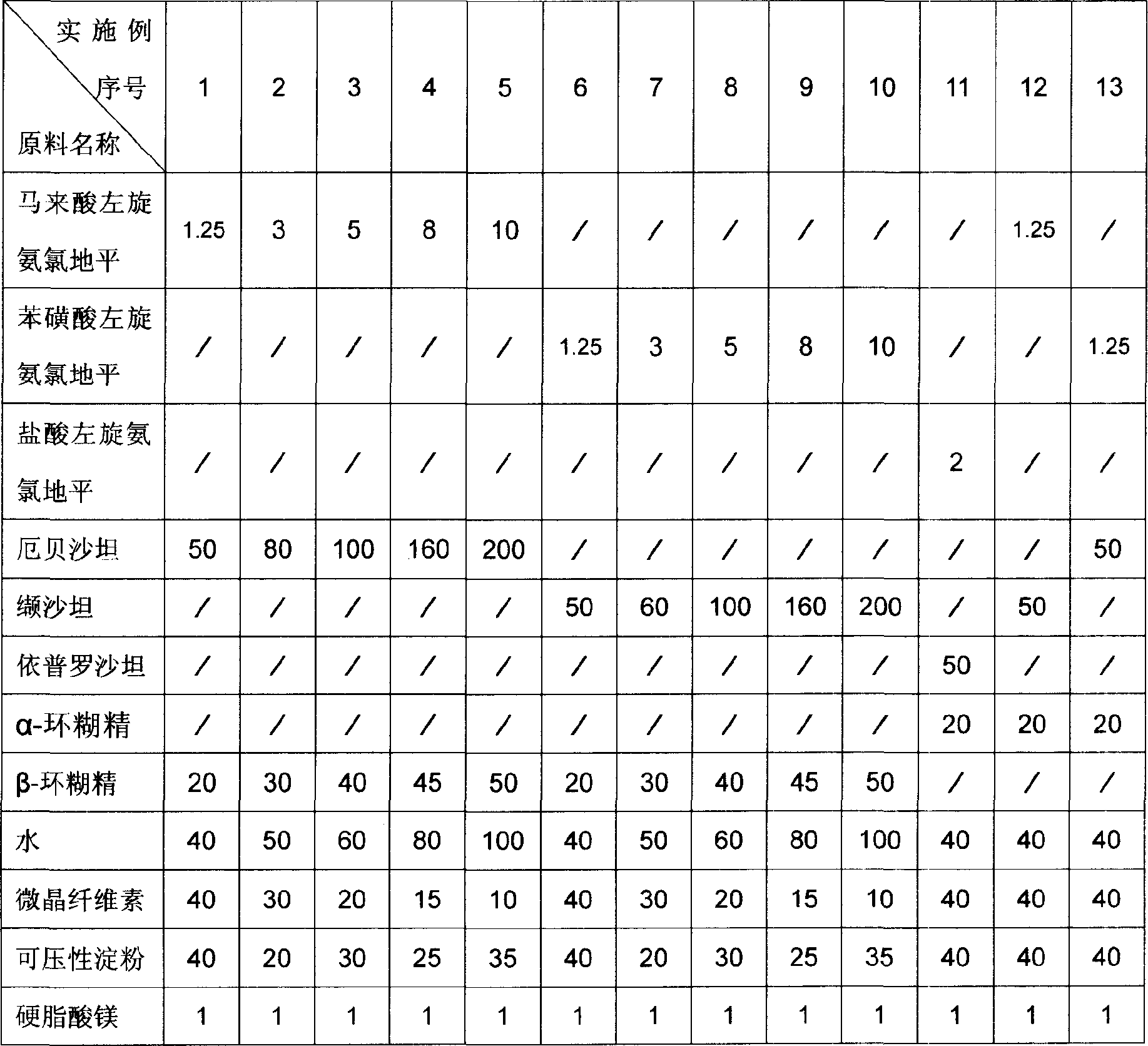
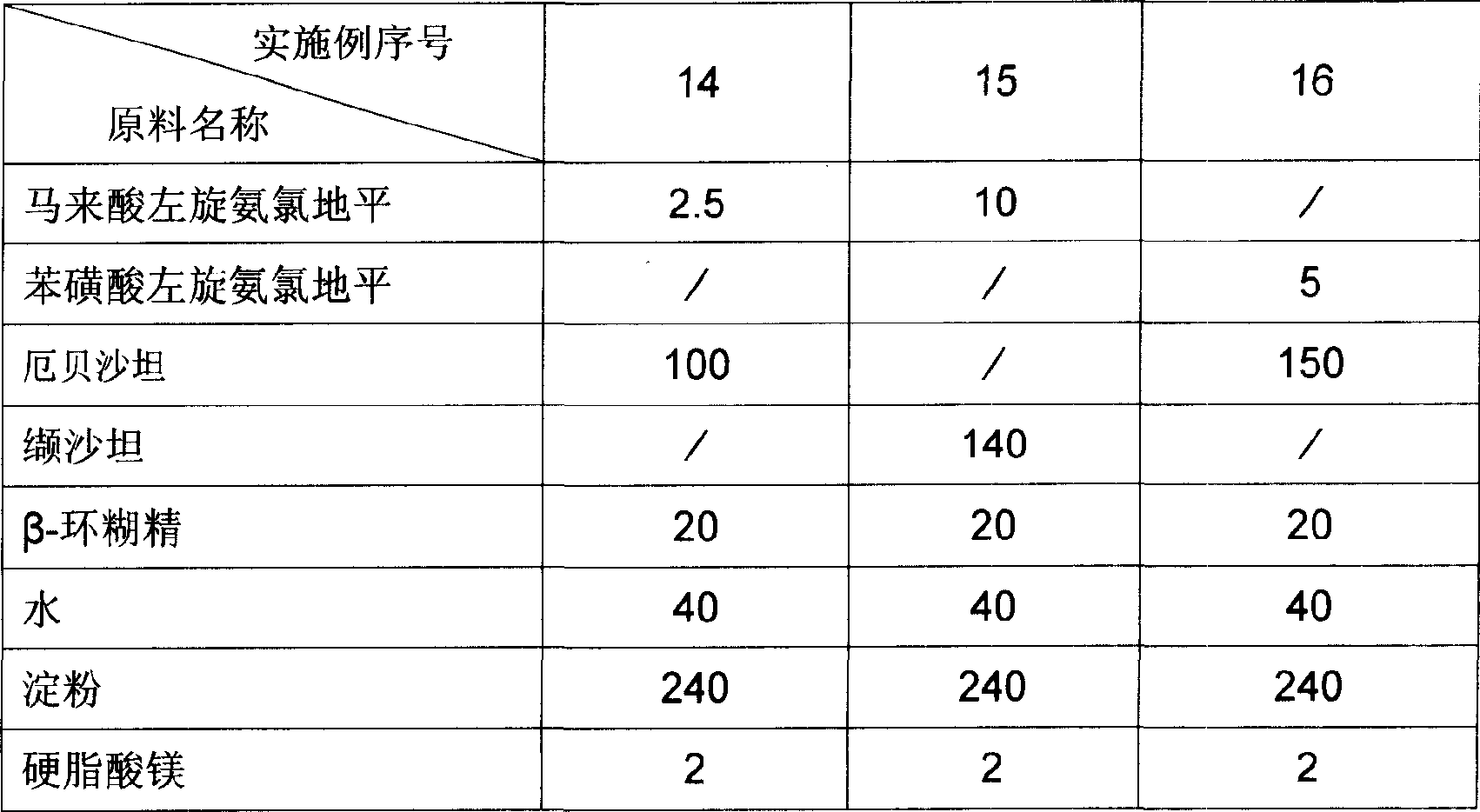
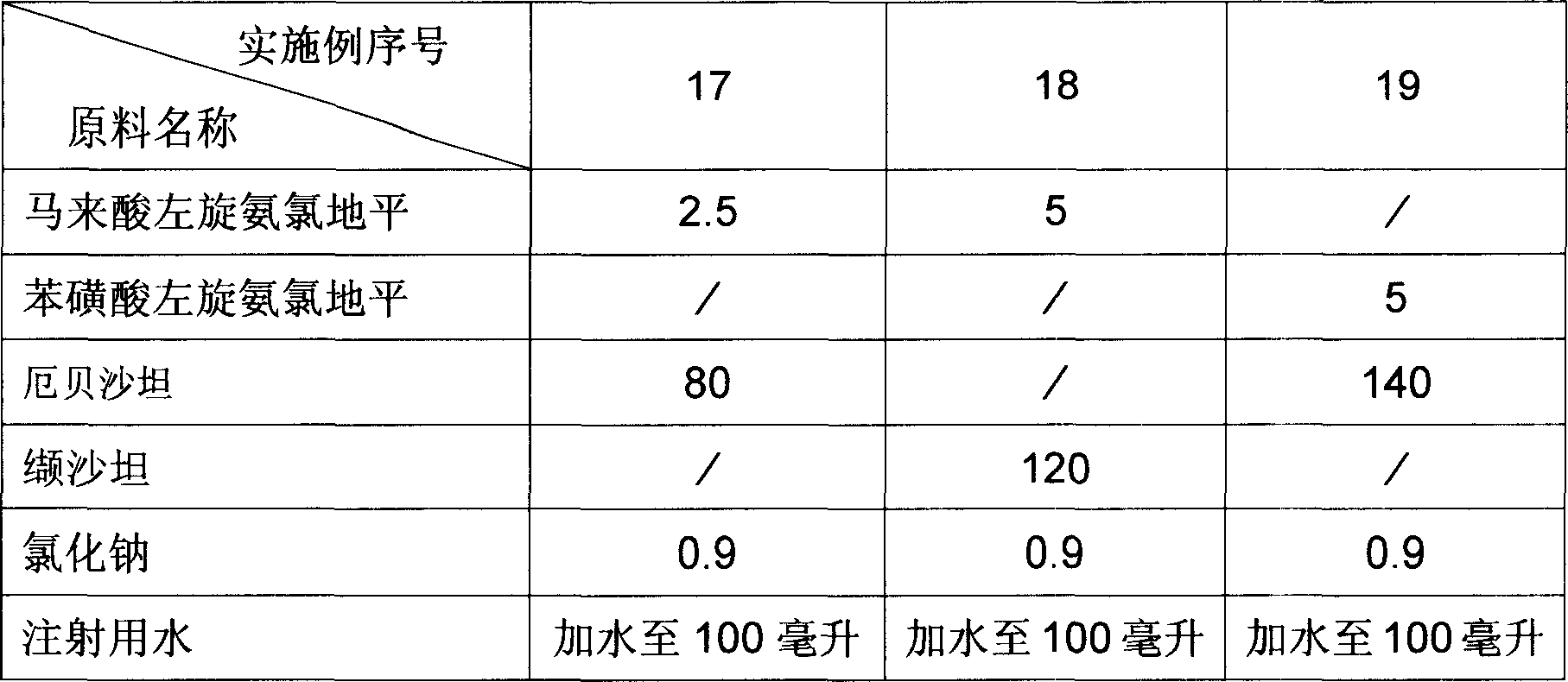
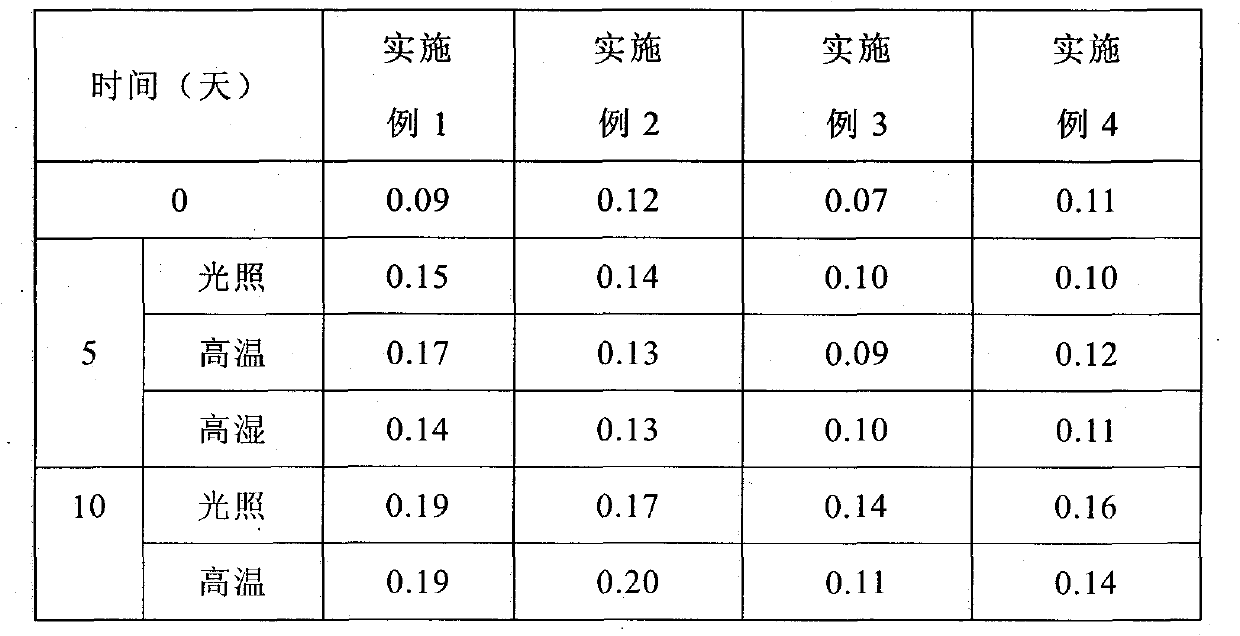
Pharmaceutical composition for treating hypertension and cardiovascular disease
InactiveCN1883478AImprove solubilityImprove bioavailabilityPill deliveryGranular deliveryVascular diseaseTreatment effect
Owner:CSPC OUYI PHARM CO LTD
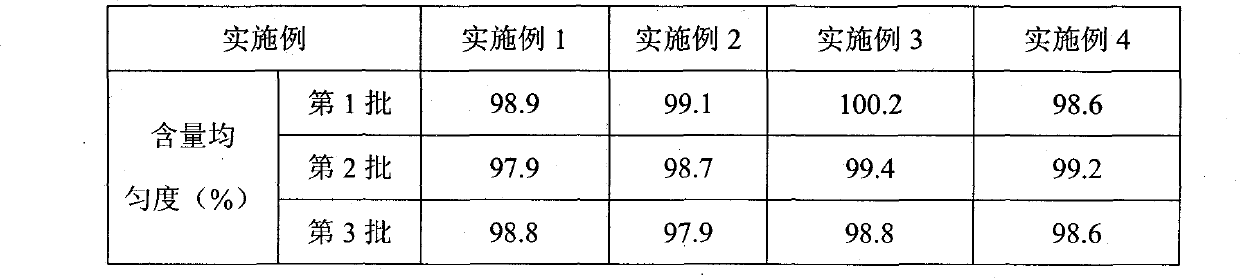
Levamlodipine beaylate tablets and preparation method thereof
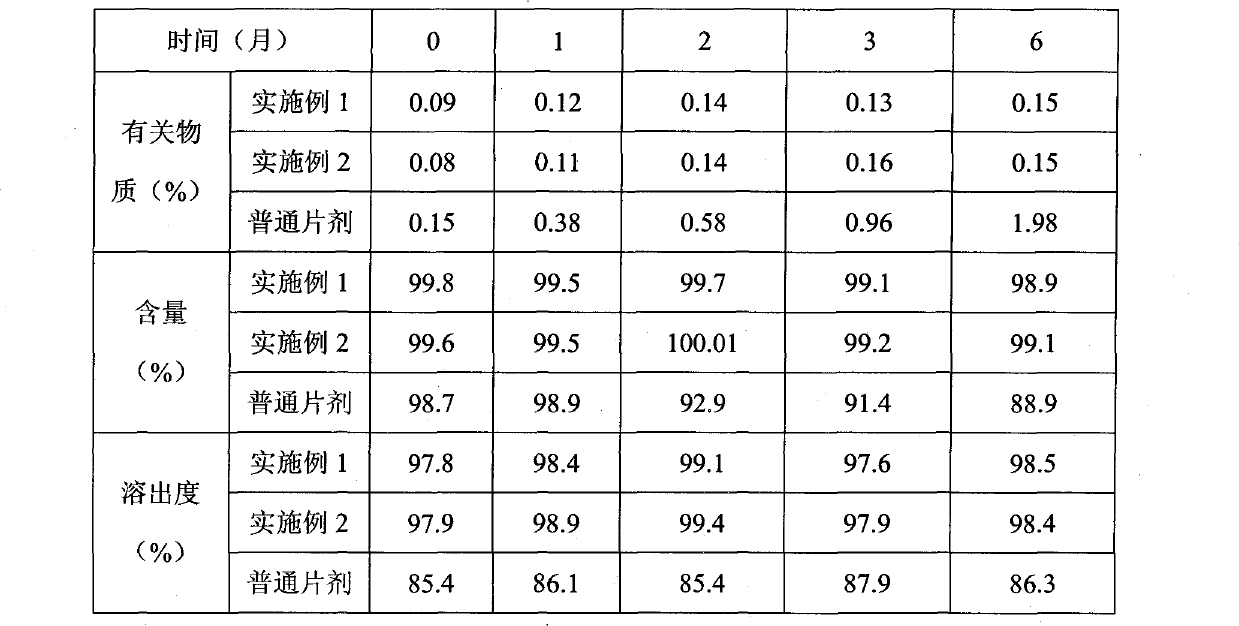
ActiveCN101766582AImprove stabilityRapid dissolutionOrganic active ingredientsPharmaceutical delivery mechanismActive componentLevamlodipine
The invention belongs to the technical field of medicament, and provides levamlodipine beaylate tablets and a preparation method thereof. The tablets consist of tablet cores using the levamlodipine beaylate as an active component and film coatings coated on the outer layer, wherein diluent in the tablet cores contains diatomite or aerosil, or a mixture of diatomite and aerosil, and contains other pharmaceutically acceptable supplementary materials; and the outer film coating accounts for 8 to 12 percent of the weight of the tablets, and can play a role in resisting humidity and avoiding light to ensure that the medicinal stability can be greatly improved, and related substances are obviously reduced. Furthermore, the tablets have small specification, so the tablets ensure uniform content, and improve dissolution; and the method is simple and controllable, and ensures that the hygroscopicity of the medicament is obviously reduced.
Owner:鲁南新时代生物技术有限公司
Levamlodipine besylate tablet, preparation process thereof and control method for relevant materials
ActiveCN102028662AGood treatment effectStable buckOrganic active ingredientsPill deliveryCross-linkSolubility
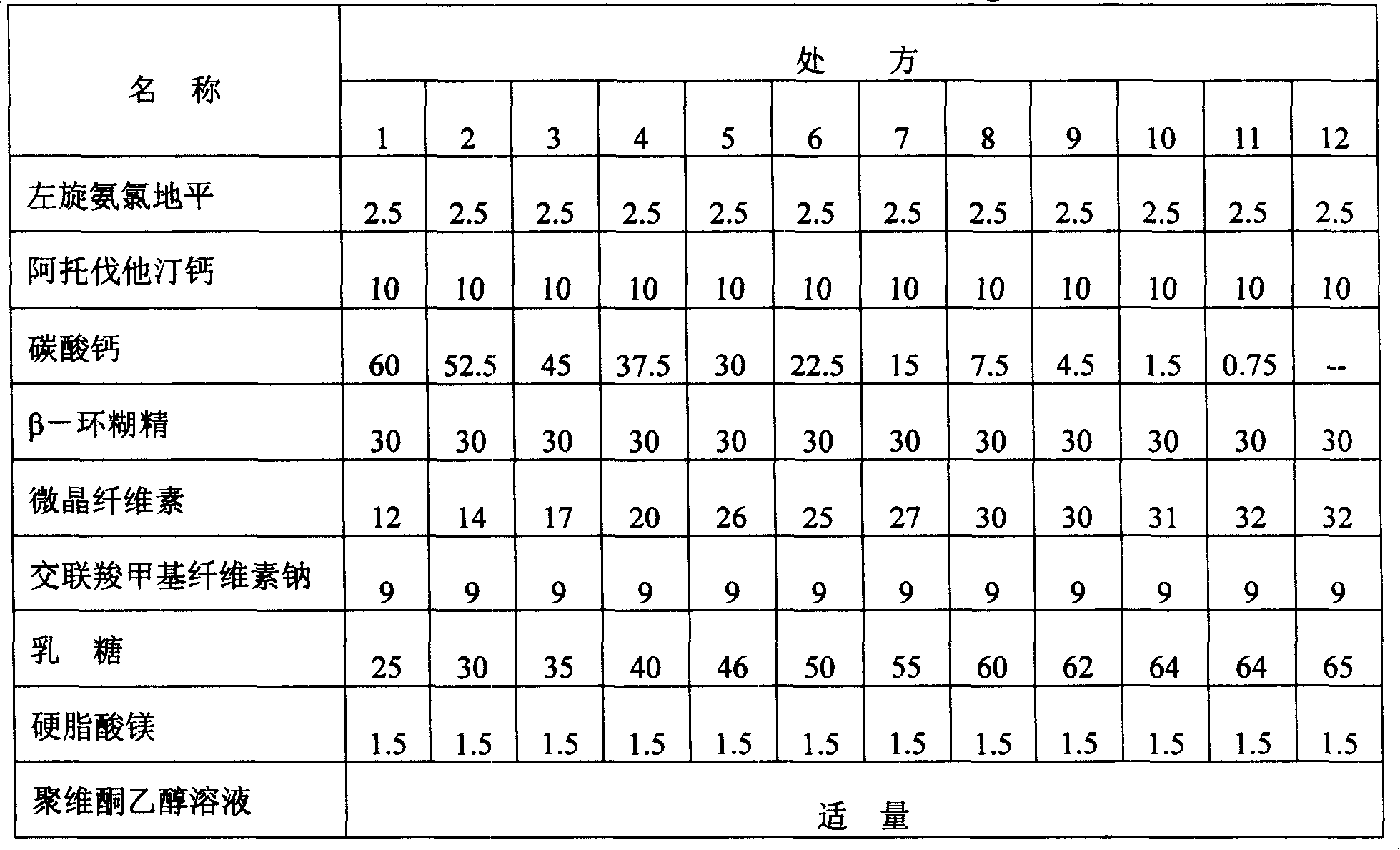
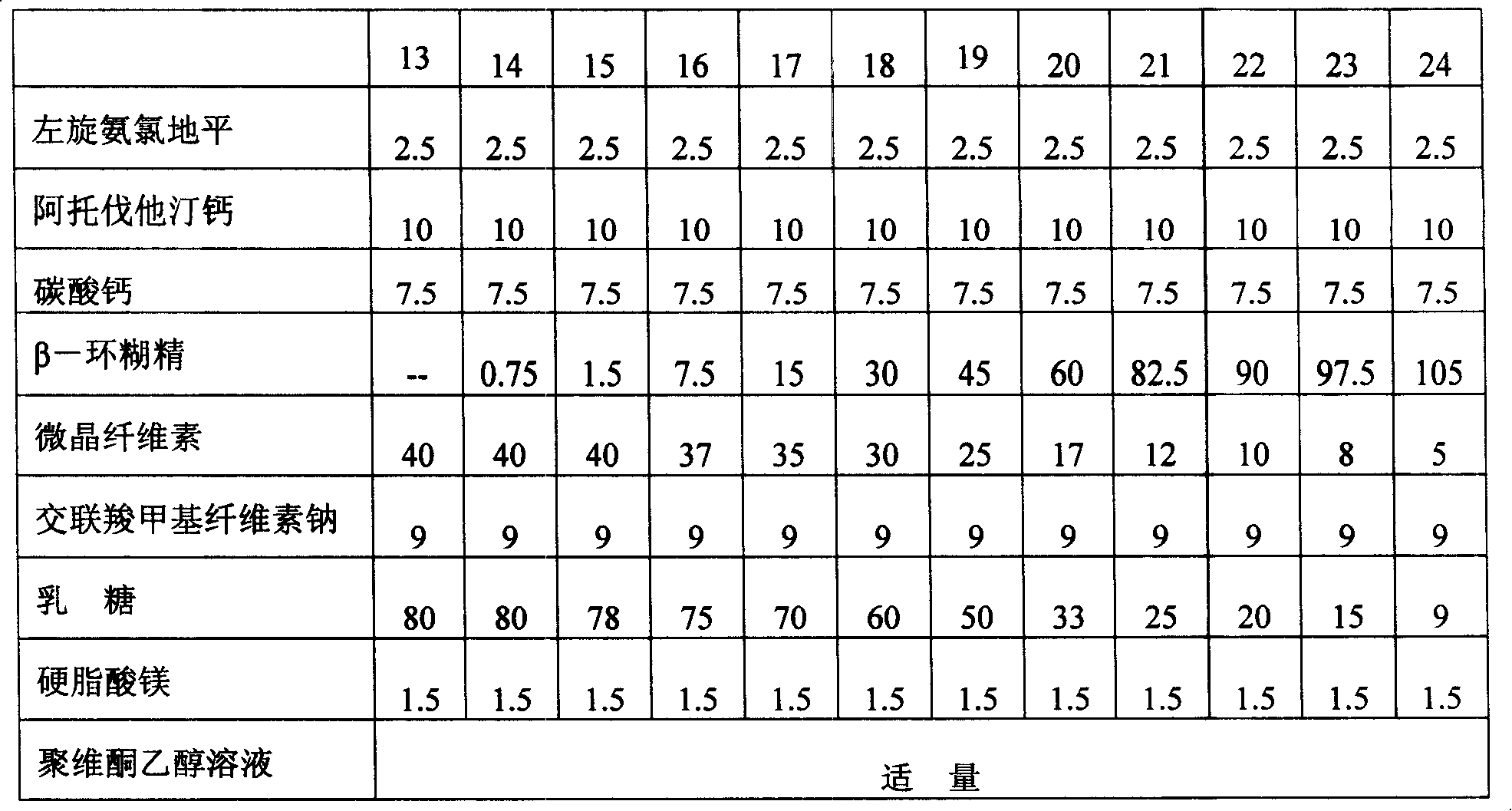
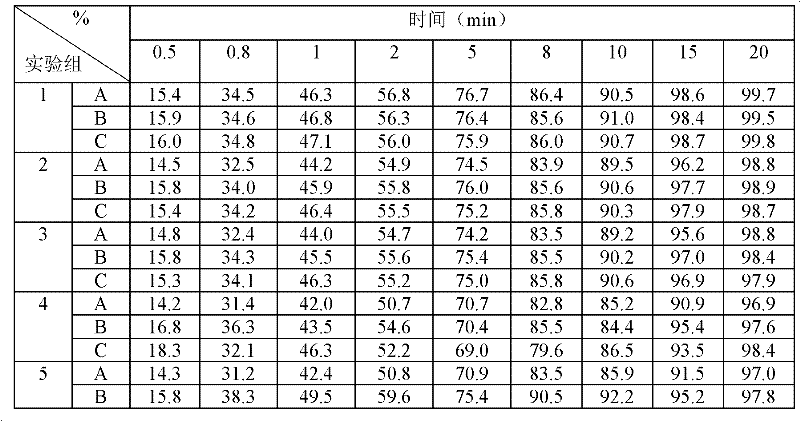
The invention relates to a levamlodipine besylate tablet, a preparation process thereof and a control method for relevant materials. Each 1000 levamlodipine besylate tablets provided by the invention comprise the following compositions: 2.5g of levamlodipine besylate (measured in besylate), 30 to 50g of lactose (as a filler), 20 to 40g of beta-cyclodextrin (as an inclusion agent), 25 to 45g of microcrystalline cellulose (as a disintegrating agent), 5 to 15g of cross-linked polyvinylpyrrolidone (as a disintegrating agent), 1 to 2g of magnesium stearate (as lubricant), and 50 to 80g of 2.5% HPMC (hydroxypropyl methylcellulose) and 50% ethanol (as an adhesive). The levamlodipine besylate tablet provided by the invention has the advantages of making multi-item improvements on the properties of the levamlodipine besylate, increasing the solubility and apparent dissolution rate of the tablet, improving the stability of the tablet, reducing the excitability of the tablet, significantly reducing the limit of the relevant material, and having better clinical treatment effect, so that the blood pressure lowering of the patient with hypertension is more stable.
Owner:JIANGXI SHIMEI PHARM CO LTD
Levamlodipine beaylate tablets and preparation method thereof
InactiveCN101721384AGood compressibilityPromote dissolutionOrganic active ingredientsPharmaceutical delivery mechanismMedicineFiller Excipient
The invention discloses levamlodipine beaylate tablets which is prepared by comprising the following raw materials in parts by weight: 1-20 parts of levamlodipine beaylate, 20-150 parts of filling agent, 10-100 parts of disintegrating agent and 1-10 parts of lubricant. The preparation method of the levamlodipine beaylate tablets comprises the following steps of: evenly mixing the raw materials, crushing, screening with a 60-100 mesh sieve, evenly mixing, and preparing the levamlodipine beaylate tablets in a novel powder feeder for a tablet machine by using a direct dry powder tablet compressing method. Compared with the traditional tablet production technology, the levamlodipine beaylate tablets of the invention have the advantages of favorable compressibility, friability and tablet weight variation, uniform content and stable quality.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Production method of levamlodipine besylate
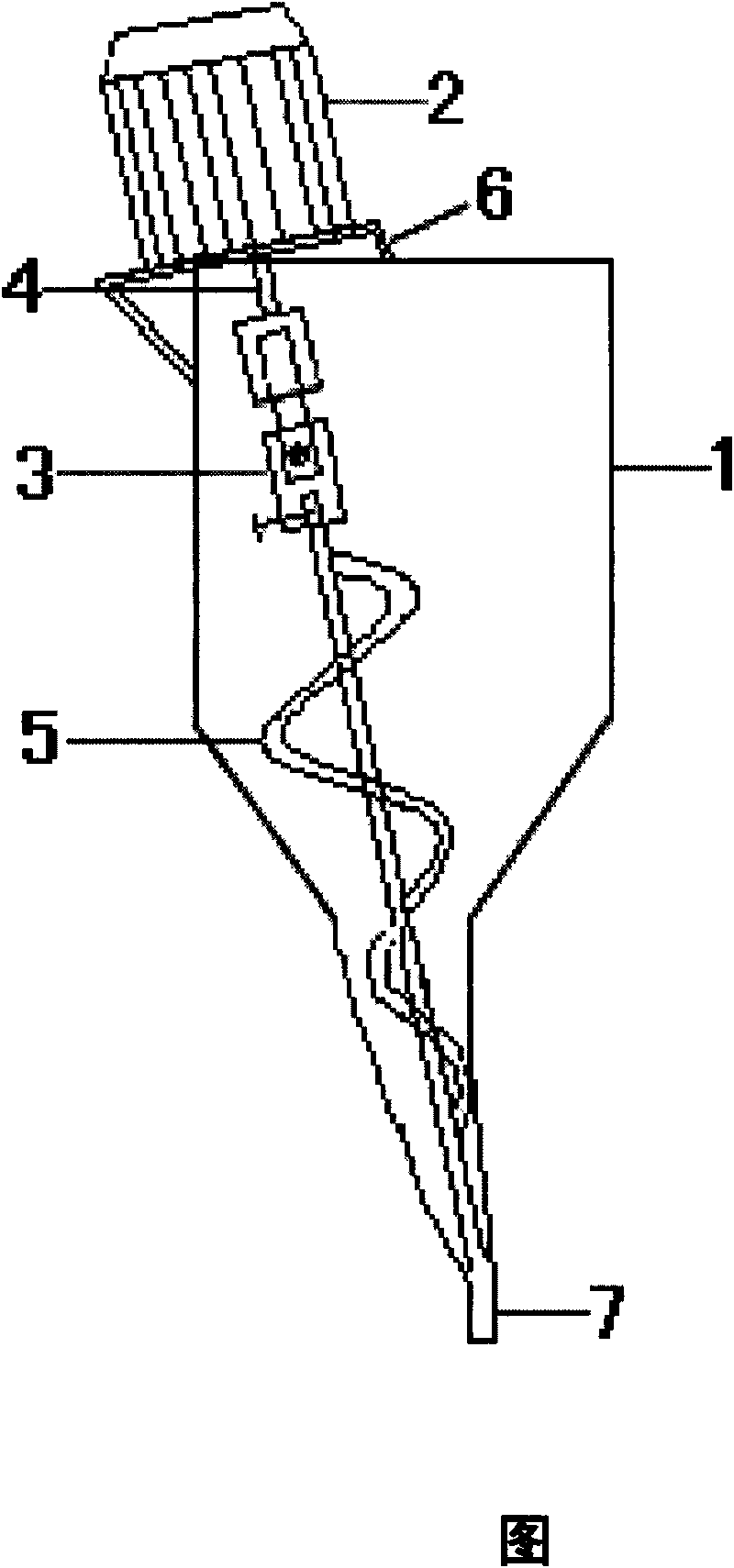
The invention takes N, N-dimethylformamide as a chiral auxiliary and separates amlodipine with tartaric acid resolution reagent to prepare l-amlodipine. In addition, benzene sulfonic acid and l-amlodipine alkali are directly salified and refined, filtered and dried via a special filter to produce levoamlodipine besylate. The upper part and the lower part of the special filter adopted by the invention are respectively provided with a hemispherical top cap and a hemispherical bottom cap, the middle part is provided with a lauter tank and ring groove filter plates are respectively arranged between the top cap and the lauter tank or the bottom cap and the lauter tank. A feed pipe, inlet and outlet of inert gases, an outlet of cooling fluid and a temperature meter are installed on the top cap of the filter; a discharge pipe and the outlet of cooling fluid are installed on the bottom cap. The filter is provided with an insulating layer and an interlayer, thus can control the temperature, avoid light, be filled with inert gases and protect the feed liquid and filtrate from oxidation, illumination and high temperature damage. The filtration efficiency is high, the effect is good and the structure is simple, the filter operation, disassembly, assembly and cleaning are convenient and the levoamlodipine besylate enjoys high synthesis and production yield and stable quality.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Levamlodipine besylate crystal and preparation method and application thereof
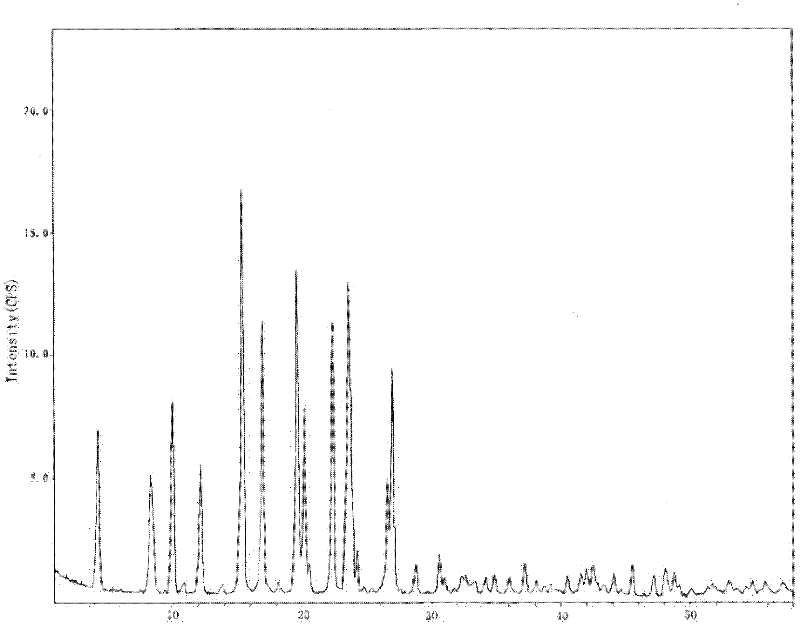
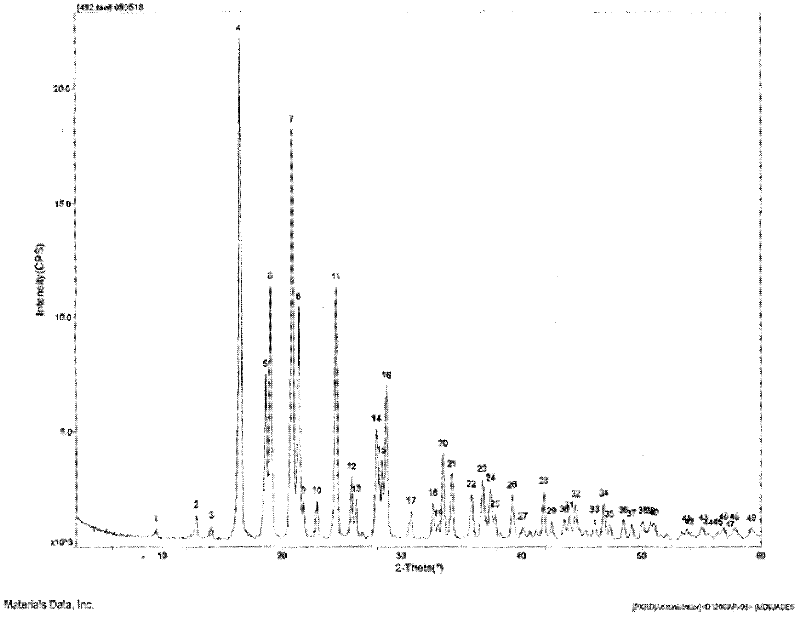
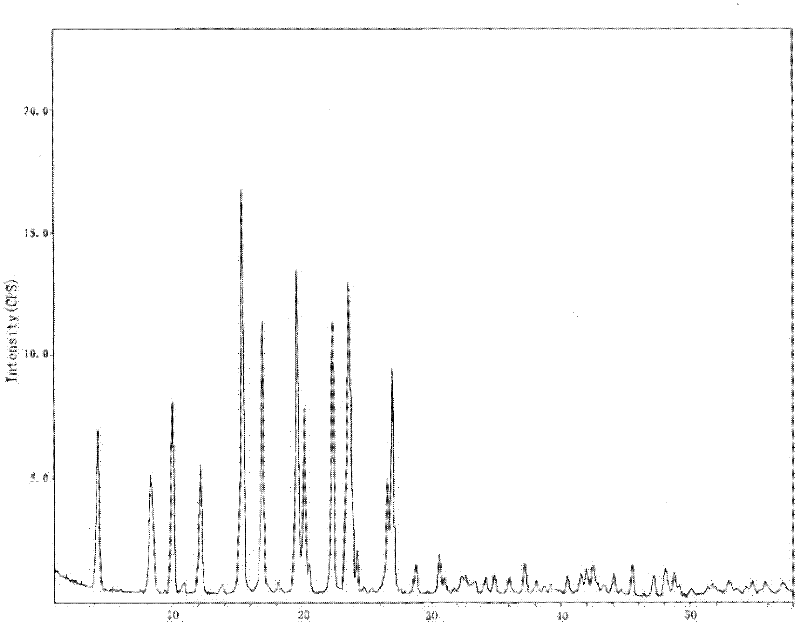
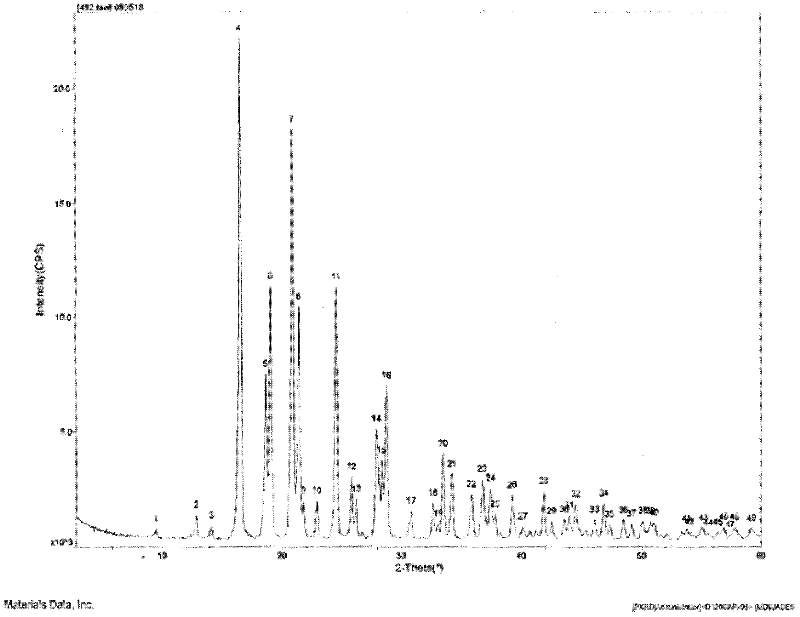
ActiveCN105111137AClarify the main parametersClear crystal formOrganic chemistry methodsSulfonic acids salts preparationSolubilitySpace group
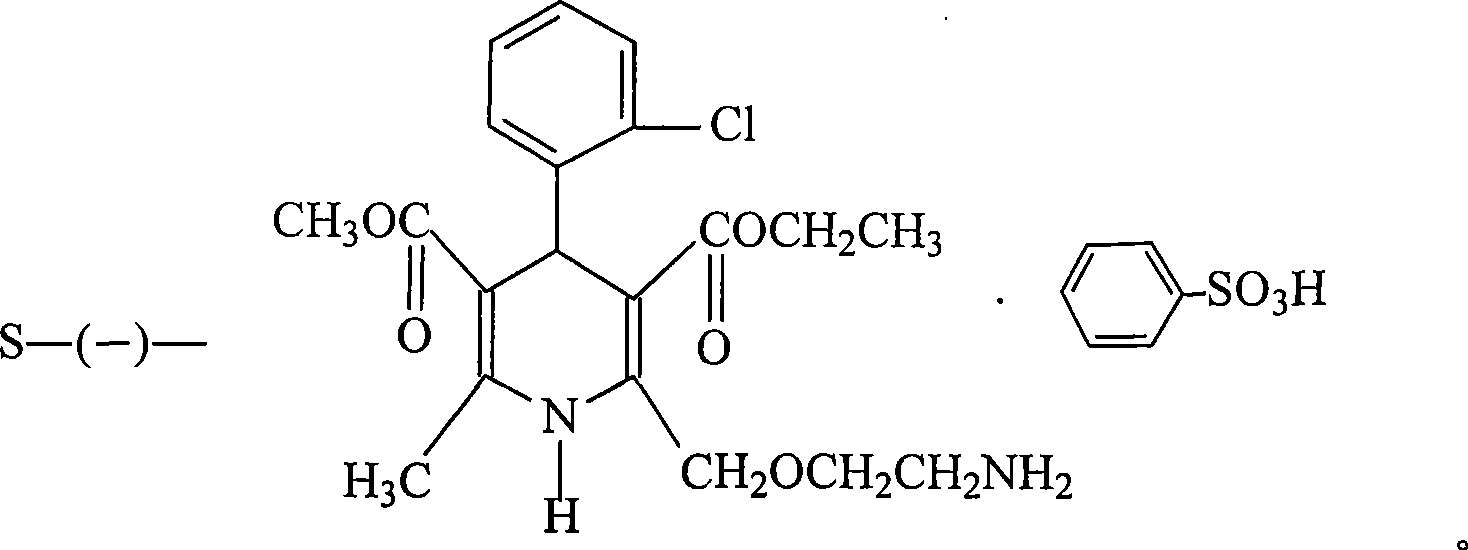
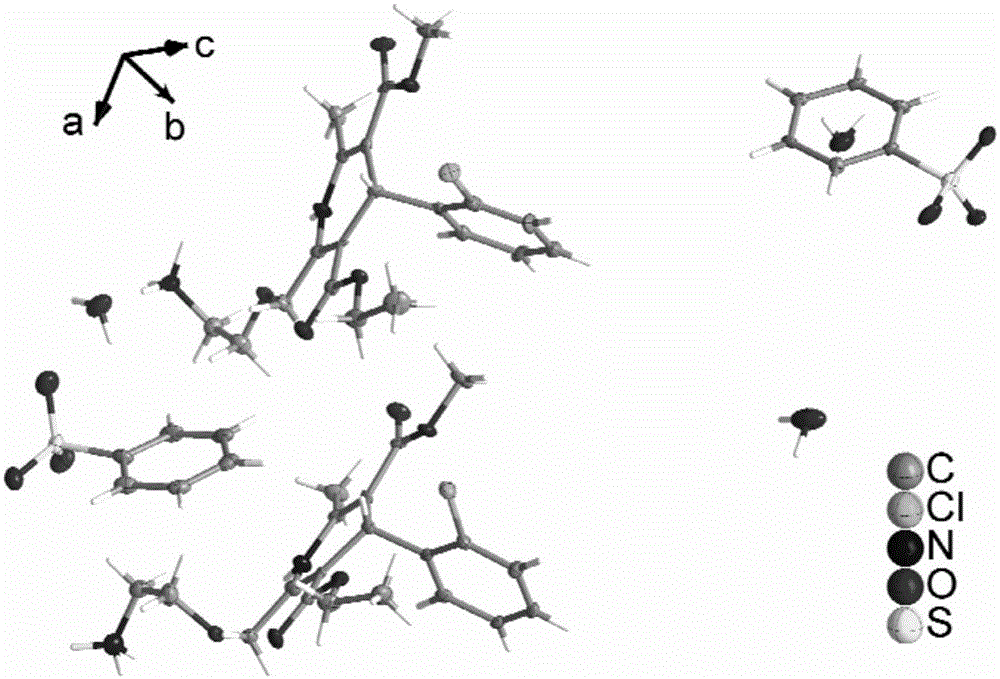
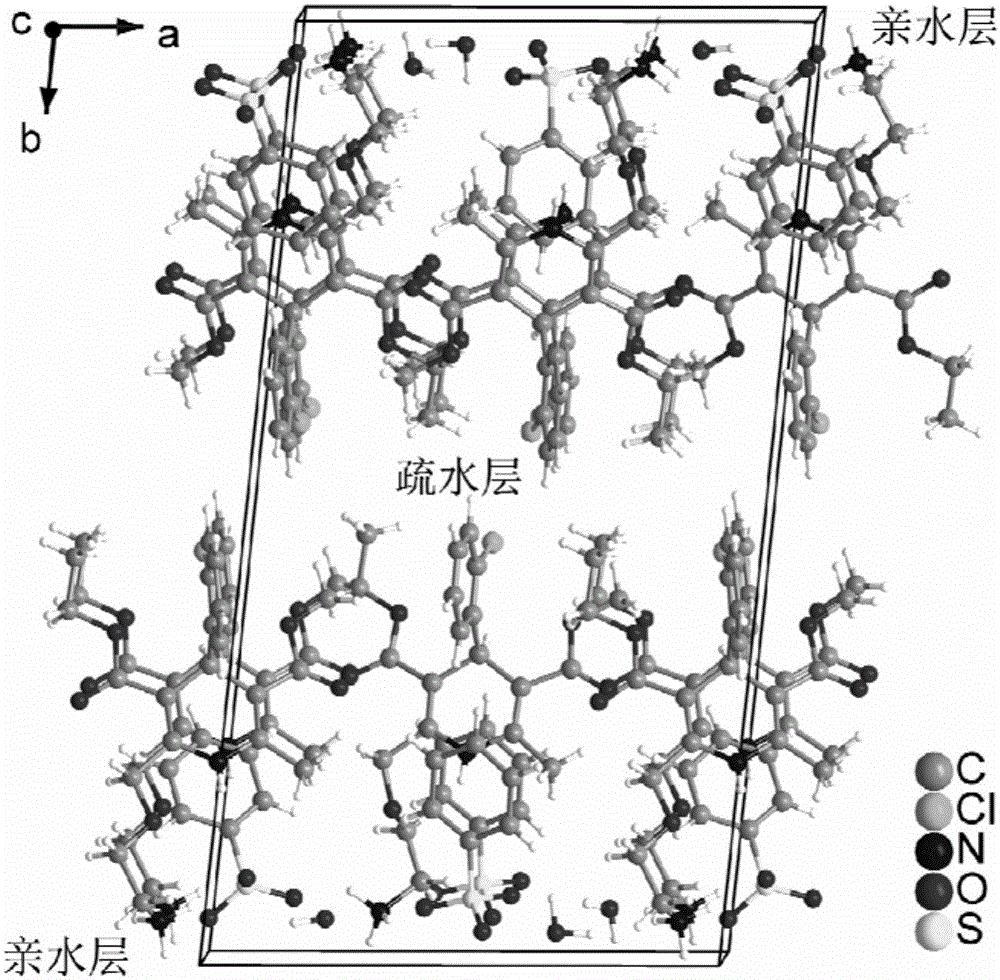
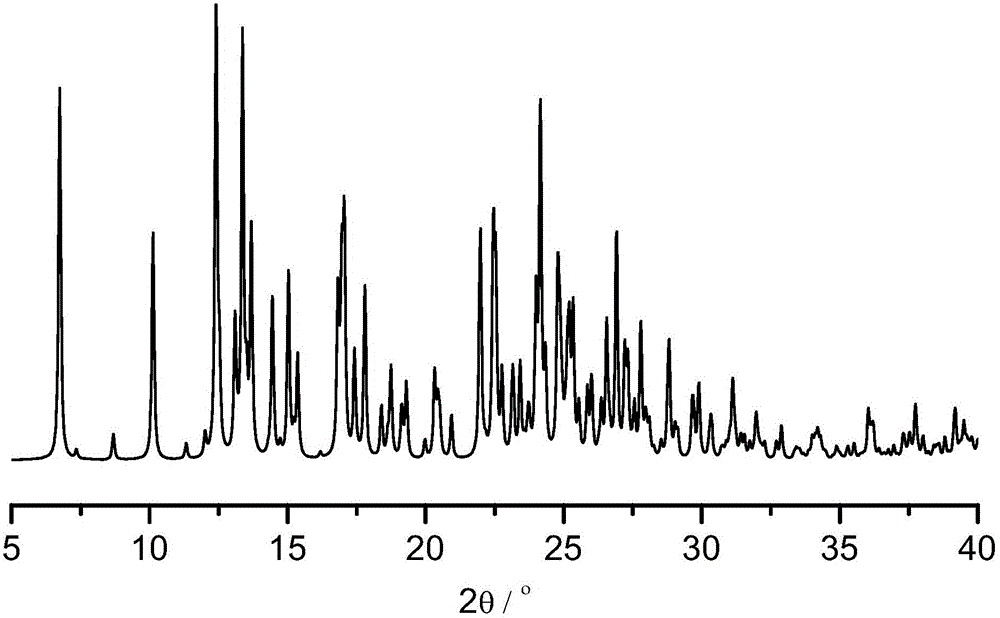
The invention provides a levamlodipine besylate crystal with the molecular formula being (C20H25ClN2O5) (C6H6O3S) (H2O) 1.5 and the molecular weight being 594.07. Crystallography measurement parameters are a monoclinic system and P21 chirality space groups, and the chirality absolute configuration is determined by crystallography Flack parameter being 0.08(6); the unit cell size is shown in the specification, wherein beta is 95.817(4) degrees, and V is 2880.1(11). The characteristic peak in an X-ray powder diffraction pattern (Cu-Kalpha) is displayed at 2thea being 6.70 degrees, 10.12 degrees, 12.40 degrees, 13.36 degrees, 13.68 degrees, 17.04 degrees, 22.46 degrees and 24.16 degrees. The invention further provides a preparation method and application of the levamlodipine besylate crystal. The levamlodipine besylate crystal has the specific crystal form and the amount of crystal water and specific crystallography main parameters and the exact atom spatial position, the solubleness and stability of existing levamlodipine besylate are improved, the stability and bioavailability of the levamlodipine besylate tablet can be improved, preparation is easy, the cost is low, and the obtained crystal is regular in form, uniform in particle size and suitable for large-scale application and popularization.
Owner:菲洋生物科技(吉林)有限公司
Preparation method of Levamlodipine besylate tablet
InactiveCN102846565AHigh dissolution rateConducive to the amount of controlOrganic active ingredientsPill deliveryLevamlodipineDissolution
A preparation method of a Levamlodipine besylate tablet includes pulverizing Levamlodipine besylate, a filler and a disintegrant, sieving, mixing, adding a lubricant, granulating, and tableting. The invention reduces the amount of related substance of the Levamlodipine besylate tablet, and improves stability. The method uses a fluidized bed granulation step to prepare the Levamlodipine besylate tablet, so as to simplify preparation step, shorten time, optimize process parameter, significantly increase the dissolution of the product and improve quality. The inventive method is suitable for industrial production.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA
Levamlodipine compound prepared in novel method
InactiveCN101805284AHigh purityNot easy to cause pollutionOrganic chemistryCamphoric acidLevamlodipine
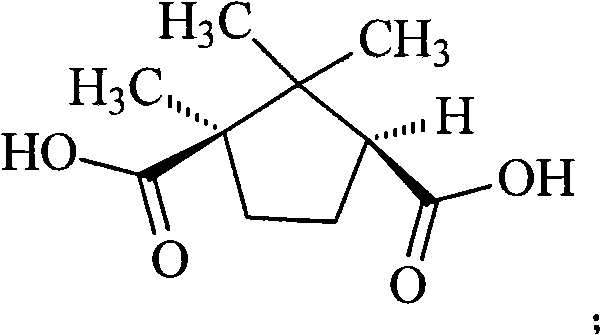
The invention provides a levamlodipine compound prepared in a novel method, which comprises the following steps: producing D-(plus)-levamlodipine camphorate through reaction of amlodipine and D-(plus)-camphanic acid, producing levamlodipine under the effect of sodium hydroxide, and producing the levamlodipine compound through salt forming reaction of the levamlodipine and benzenesulfonic acid. The method adopts a completely novel split reagent D-(plus)-camphanic acid, simplifies reaction procedures and is more suitable for industrial production. Moreover, the method has the advantage of high yield.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Compound of atorvastatin and levorotatory amlodipine and preparing method thereof
ActiveCN101224205AExcellent indicatorsHigh dissolution rateMetabolism disorderPill deliveryCyclodextrinLevamlodipine
The invention relates to a combination of atorvastatin and levamlodipine, mainly consists of levamlodipine or officinal salt thereof, atorvastatin or officinal salt thereof, alkali metal salt, and cyclodextrin and derivatives thereof and guarantees the stability and the bioavailability of atorvastatin and levamlodipine.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Medicine composition for treating hypertension complicated with hyperlipemia and cardiac and cerebral vascular diseases
The medicine composition for treating hypertension complicated with hyperlipemia and cardiac and cerebral vascular diseases belongs to the field of medicine technology, and aims at raising the comprehensive curative effect. Technologically, the medicine composition consists of levoamlodipine and its medicinal salt, HMG-COA reductase inhibitor and its medicinal salt and medicine carrier, with the content being levoamlodipine 1.25-20 mg and HMG-COA reductase inhibitor 2.5-100 mg. Owing to the adding or synergistic effect of the medicines, the present invention has obviously raised medicinal effect, reduced dosage and obviously lowered side effect.
Owner:CSPC OUYI PHARM CO LTD
Brand-new oral solid medicinal composition and preparation method thereof
ActiveCN102335176AGuaranteed curative effectHigh content of the main drugOrganic chemistryCapsule deliveryCross-linkValsartan
The invention discloses a brand-new oral solid medicinal composition. The medicinal composition is an oral preparation prepared from hydrochlorothiazide, levamlodipine, valsartan and pharmaceutically acceptable auxiliaries, and the oral preparation comprises but is not limited to tablets or capsules. The composition comprises the following raw materials in parts by weight: 5-25 parts of the hydrochlorothiazide, 2.5-5 parts of the levamlodipine, 80-160 parts of the valsartan, 40-120 parts of microcrystalline cellulose, 30-90 parts of compressible starch, 5-25 parts of cross-linked sodium carboxymethylcellulose, 3-8 parts of silicon dioxide and 1-2 parts of stearic acid. The medicinal composition disclosed by the invention has the advantages of scientific and reasonable prescription, low auxiliary content and high bioavailability, and is a first choice of medicine for treating hypertension.
Owner:HAINAN JINRUI PHARMA
Levamlodipine oral disintegrating tablet formulation and its preparing method
InactiveCN1813726APromote dissolutionUniform preparation contentOrganic active ingredientsPill deliveryMedicineAdditive ingredient
The present invention discloses a levoamlodipine oral disintegrating tablet preparation and its preparation method. It is made up by using levoamlodipine or its active salt as main medicine, using other ingredients as auxiliary material and adding stabilizing agent, for example citric acid, ascorbic acid, lactic acid, benzenesulfonic acid or maleic acid through a certain preparation process. Besides, said invention also provides the concrete steps of its preparation process.
Owner:SUZHOU DAWNRAYS PHARM CO LTD
Stable levamlodipine composition
ActiveCN102579440AImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsLevamlodipineBULK ACTIVE INGREDIENT
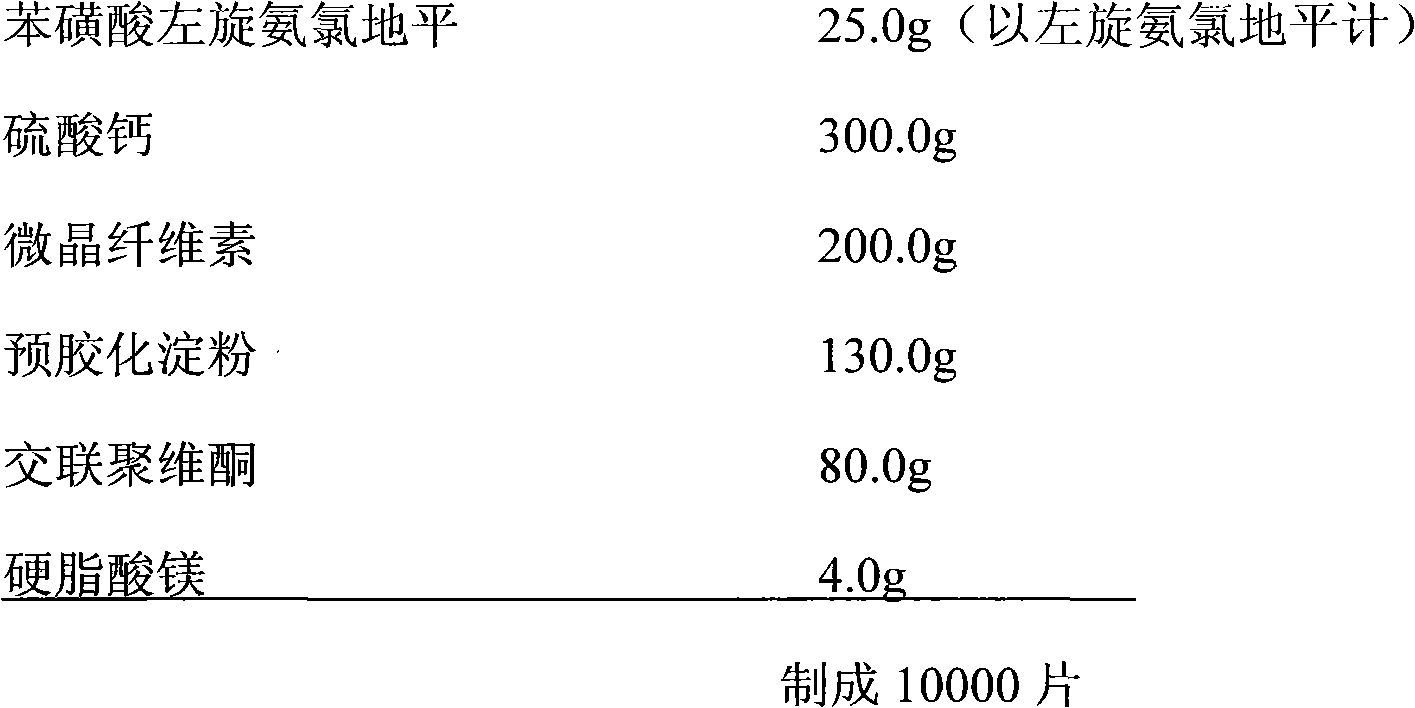
The invention relates to a stable levamlodipine medicinal composition, which comprises an active ingredient consisting of levamlodipine or a pharmaceutically-acceptable salt thereof, a stabilizer, a filler, a disintegrating agent and a lubricating agent. The stabilizer is one or a composition of two or more than two of calcium hydrophosphate, calcium phosphate, calcium sulfate, citric acid or sodium citrate. By selecting appropriate auxiliary materials, a weakly-acid micro environment is formed in a tablet, and degradation under the conditions of moisture, heat and light illumination is blocked, so that the stable quality of a medicament is ensured.
Owner:KANGYA OF NINGXIA PHARMA
Pharmaceutical compositions of levoamlodipine and atorvastatin
ActiveCN1843357AConvenient treatmentCombined use is excellentMetabolism disorderHeterocyclic compound active ingredientsLevamlodipineAtorvastatin
The invention relates to a pharmaceutical composition of Levamlodipine or its medicinal salts and addition salts with Atorvastatin or its medicinal salts, and the use of the pharmaceutical composition in preparing medicament for treating mixed type hypertension and lipidemia.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Composition of atorvastatin and L-amlodipine and preparation method thereof
ActiveCN101288670ASubstance reductionSimple preparation processMetabolism disorderInorganic non-active ingredientsCyclodextrinLevamlodipine
A combination of Atorvastatin and L-amlodipine is composed of L-amlodipine or medicated salt thereof, Atorvastatin or medicated salt thereof, alkaline metallic salt, cyclodextrin and derivant thereof, loading agent, disintegrant and lubricant; the combination improves the stability and bioavailability of Atorvastatin and L-amlodipine compound.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Pharmaceutical composition containing calcium blocker, AII receptor blocker and statins
InactiveCN101618215AReduce morbidityImprove complianceSenses disorderMetabolism disorderCandesartanLacidipine
The invention relates to a pharmaceutical composition containing a calcium channel blocker (CCB) or the mixture thereof, an angiotonin 3II receptor blocker (ARB) or the mixture thereof, statins or the mixture thereof and a pharmaceutically acceptable carrier, wherein the CCB is selected from l-amlodipine, amlodipine, lacidipine, nitrendipine or the mixture thereof; the angiotonin II receptor blocker is selected from telmisartan, losartan, irbesartan, candesartan or the mixture thereof; and the statins are selected from atorvastatin, simvastatin, ruishufatadine, fluvastatin or the mixture thereof. The pharmaceutical composition is used for treating various high blood pressures and preventing or treating cardiovascular and cerebrovascular diseases relevant to the hypertension, reduces the disease rate and / or mortality rate of the cardiovascular and cerebrovascular diseases and also improves the adaptability for a sufferer taking medicine.
Owner:王丽燕
Preparations containing rosuvastatin calcium and amlodipine and method for preparing the same
InactiveCN101095680AIt has the effect of lowering blood fat and blood pressureSynergisticOrganic active ingredientsMetabolism disorderLevamlodipineRosuvastatin Calcium
The invention provides a medical compound, which comprises effective amount of rosuvastatin calcium and amlodipine, which is characterized in that said two material are combined together and they can reduce blood fat and blood pressure, and they are cooperative.
Owner:SHANGHAI SINE PHARMA LAB
Levamlodipine besylate dropping balls and their making method
InactiveCN1981759ARapid dissolutionHigh dissolution rateOrganic active ingredientsPill deliveryAnginaLevamlodipine
A dripping pill of levamlodipine besylate for treating hypertension and angina pectoris is prepared from levamlodipine besylate and matrix. Its preparing process is also disclosed.
Owner:陈茜
Preparation method of levamlodipine and olmesartan medoxomil tablet
The invention relates to a preparation method of a compound tablet prepared from levamlodipine and olmesartan medoxomil as well as auxiliary materials. The preparation method is characterized in that the compound tablet is prepared by adopting a mode of non-wet granulation and has better quality and stability.
Owner:北京迈劲医药科技有限公司
Method for obtaining S-(-)-amlodipine in splitting manner
ActiveCN101654429AMoisture content no special requirementsShort reaction timeOptically-active compound separationOrganic racemisationEnantiomerLevamlodipine
The invention relates to a method for obtaining S-(-)-amlodipine in a splitting manner. The method comprises the following steps: dissolving racemic amlodipine and D-tartaric acid into a mixed cosolvent containg dimethylsulfoxide and urea to carry out a complex reaction, carrying out alkaline treatment, solvent-out crystallization and the like on the S-(-)-amlodipine, the D-tartaric acid and the urea complexes solid sedimentation obtained after the complex reaction is finished, and then obtaining S-(-)-amlodipine pure crystal. The method introduces the other chiral auxiliary reagent-urea in the prior dimethylsulfoxide solution, thus the racemic amlodipine can better react with a splitting agent D-tartaric acid in the cosolvent containing the dimethylsulfoxide and urea, the reaction time islargely shortened, and the reaction does not have special requirements on the water content of the used solvent; the enantiomeric purity of the obtained levamlodipine is over than 99% and the rate ofrecovery is over than 80%. The method can be applied to preparing the intermediate of other chiral medicaments basically meeting the standard of the medical industry, is simple and provides a betterprospect for the preparation of various agents by the amlodipine single enantiomer.
Owner:JIANGXI SHIMEI PHARM CO LTD
Application of amlodipine to preparation of medicaments for curing phoproliferative diseases
The invention relates to an application of amlodipine to the preparation of medicaments for curing phoproliferative diseases. The amlodipine is selected from racemic amlodipine, levorotatory amlodipine and pharmaceutically acceptable salts, and the phoproliferative diseases particularly refer to various malignant tumors, such as leukemia, lung cancer, gastric cancer, liver cancer, ovarian cancer, prostatic cancer, cervical cancer, breast cancer and squamous carcinoma.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Levamlodipine and telmisartan compound preparation
InactiveCN102058591AImprove toleranceSuppress heart rateOrganic active ingredientsPill deliveryHigh dosesLevamlodipine
The invention discloses a levamlodipine and telmisartan compound preparation and an optimal compound formulation. In the invention, the combination of the levamlodipine and the telmisartan is used for treating hypertension, good synergic antihypertension effect is obtained between the levamlodipine and the telmisartan, the doses of the levamlodipine and the telmisartan can be reduced while the equivalent and even better antihypertension effect is achieved, and the adverse effect caused by high dose of single drug is reduced. Besides, the telmisartan can obviously improve the tolerance of the levamlodipine, can inhibit heart rate increase caused by the levamlodipine and can reduce the incidence of peripheral edema.
Owner:SHIHUIDA PHARMA GRP (JILIN) LTD
Levamlodipine compound drug composition
InactiveCN101780079ATo overcome the characteristics of slow onset of antihypertensiveReduce doseOrganic active ingredientsPill deliveryCompounding drugsSide effect
The invention discloses a levamlodipine compound drug composition, which comprises levamlodipine or a pharmaceutically acceptable salt thereof and chlorthalidone. The combined drug of the levamlodipine and the chlorthalidone of the invention can be used for treating hypertension, the levamlodipine and the chlorthalidone have good synergic function for blood pressure reduction, and the invention can eliminate the side effect of edema caused by sodium water retention when the levamlodipine is separately used as the drug. In addition, the levamlodipine can enhance the insulin sensitivity of the body and resist the side effect of blood sugar rise caused by chlorthalidone. Moreover, the drug combination of the levamlodipine and the chlorthalidone can overcome the defect that the the levamlodipine is slow to become effective in blood pressure reduction.
Owner:SHIHUIDA PHARMA GRP (JILIN) LTD
Sustained and controlled release preparation for pharmaceutical composition for curing hypertension
ActiveCN101564536AImprove protectionReduce incidencePharmaceutical delivery mechanismCardiovascular disorderPharmaconLevamlodipine
The invention provides a sustained and controlled release preparation for a pharmaceutical composition for curing hypertension. The sustained and controlled release preparation composition includes a specific proportion of levamlodipine and angiotensin II receptor blocking pharmacon, wherein the levamlodipine is taken as the sustained and controlled release part and the angiotensin II receptor blocking pharmacon is taken as the common release part. The provided sustained and controlled release preparation enhances the therapeutic effect and reduces the treatment risk of the patients.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for preparing levamlodipine from racemic amlodipine maleate
ActiveCN101531629ALow toxicityImprove filtering effectOrganic chemistryCardiovascular disorderN dimethylformamideAmlodipine Maleate
The invention relates to a method for removing maleate of amlodipine maleate, which prepares free base of amlodipine from weak inorganic base, DMF and water as solvent by a settling method. The invention relates to a method for preparing levamlodipine from the amlodipine, which removes dextroisomer through salifying precipitation by using mixed solvent of N, N-dimethylformamide and ethanol and D(-)tartaric acid taken as a chiral reagent, and prepares the levamlodipine through precipitation by adding excessive water. The levamlodipine can be used for preparing levamlodipine besylate.
Owner:JIANGSU SIMCERE PHARMA +1
Pharmaceutical composition of atenolol/amlodipine/folacin compound and uses thereof
InactiveCN101406472AImprove antihypertensive effectTake a small doseOrganic active ingredientsCardiovascular disorderDiseaseSide effect
The invention relates to a pharmaceutical composition containing atenolol, amlodipine, or folic acid compounds and application thereof. The pharmaceutical composition contains an officinal dosage of the atenolol, an officinal dosage of the amlodipine or levamlodipine, an officinal dosage of the folic acid compounds, and a pharmaceutical acceptable carrier. The dosage of the atenolol is between 5 and 50 milligrams, the dosage of the amlodipine or the levamlodipine is between 0.5 and 5.0 milligrams, and the dosage of the folic acid type compounds is between 0.2 and 1.6 milligrams. The pharmaceutical composition has the following advantages: the pharmaceutical composition enhances the hypertension curative effect through multi-target synergistic hypotensive action, and reduces the taking dosage of the amlodipine simultaneously, that is, just about one fourth of the original dosage can achieve the same hypotensive effect and reduce the side effects and medical expenses; and more importantly, the pharmaceutical composition can effectively prevent and treat or delay various complications of high blood pressure cardiovascular and cerebrovascular diseases such as cerebrovascular disorder and the like through dual targets (Hcy and blood pressure) on the basis of reducing toxic side effects. Besides, the pharmaceutical composition ensures that patients can take medicine conveniently.
Owner:史克勇
Medicine application preparation for treating hypertension
InactiveCN101711747AImprove protectionReduce incidencePill deliveryCapsule deliveryInjury causeLevamlodipine
The invention provides a slow / controlled release preparation of a medicine composition for treating hypertension. The slow / controlled release preparation composition contains levorotatory amlodipine and an angiotensin II receptor blocking agent which are in a specific proportion. Compared with a common preparation, the slow / controlled release preparation provided by the invention can enhance the effect of lowering the pressure, lower the pressure more stably, improve the insulin resistance of a patient more effectively and better prevent organ injuries caused by the hypertension.
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation method of high-purity levamlodipine besylate
The invention belongs to the field of chemical pharmacy, and particularly relates to a preparation method of high-purity levamlodipine besylate, which comprises the following steps: (1) under the protection of nitrogen, dissolving levamlodipine in proportional hot water, adding a water solution of benzenesulfonic acid until the solid is completely dissolved, slowly cooling to precipitate a laminar solid, and filtering to obtain a crude levamlodipine besylate product; and (2) under the protection of nitrogen, dissolving the crude levamlodipine besylate product in proportional ethanol until the crude levamlodipine besylate product is completely dissolved, dropwisely adding proportional water to precipitate a solid, and filtering to obtain the refined levamlodipine besylate product. The invention successfully solves the problem of great filtration difficulty in the past patent technique; and thus, the quality of the end levamlodipine besylate product is excellent, and the HPLC content (area normalization) is almost 100%.
Owner:SHANDONG XINHUA PHARMA CO LTD
Levamlodipine besylate tablets
ActiveCN102670534AQuality improvementDissolution completeOrganic active ingredientsPharmaceutical non-active ingredientsMedicineLevamlodipine
The invention provides levamlodipine besylate tablets. The levamlodipine besylate tablets have the characteristics of high stability, low impurity content, complete dissolution and the like.
Owner:HEBEI RENHE YIKANG PHARMA
Novel oral solid medicinal composition and preparation method thereof
ActiveCN102342942AReduce dosageSolving Quality Control IssuesOrganic active ingredientsOrganic chemistryCandesartanLevamlodipine
The invention discloses a novel oral solid medicinal composition. The novel oral solid medicinal composition is an oral preparation prepared from hydrochlorothiazide, levamlodipine besylate, candesartan cilexetil and pharmaceutically acceptable auxiliary materials. The novel oral solid medicinal composition can be processed into tablets, capsules and the like. Specifically, the novel oral solid medicinal composition comprises: be weight, 5 to 25 parts of hydrochlorothiazide, 2.5 to 5 parts of levamlodipine besylate, 4 to 20 parts of candesartan cilexetil, 30 to 60 parts of microcrystalline cellulose, 30 to 60 parts of compressible starch, 30 to 50 parts of crosslinked polyvinylpyrrolidone, 1 to 2 parts of silica and 0.5 to 2 parts of magnesium stearate. The novel oral solid medicinal composition has a scientific and reasonable formula, low auxiliary material content and high bioavailability. Therefore, the novel oral solid medicinal composition is a drug of first choice for the treatment of hypertension.
Owner:HAINAN JINRUI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com