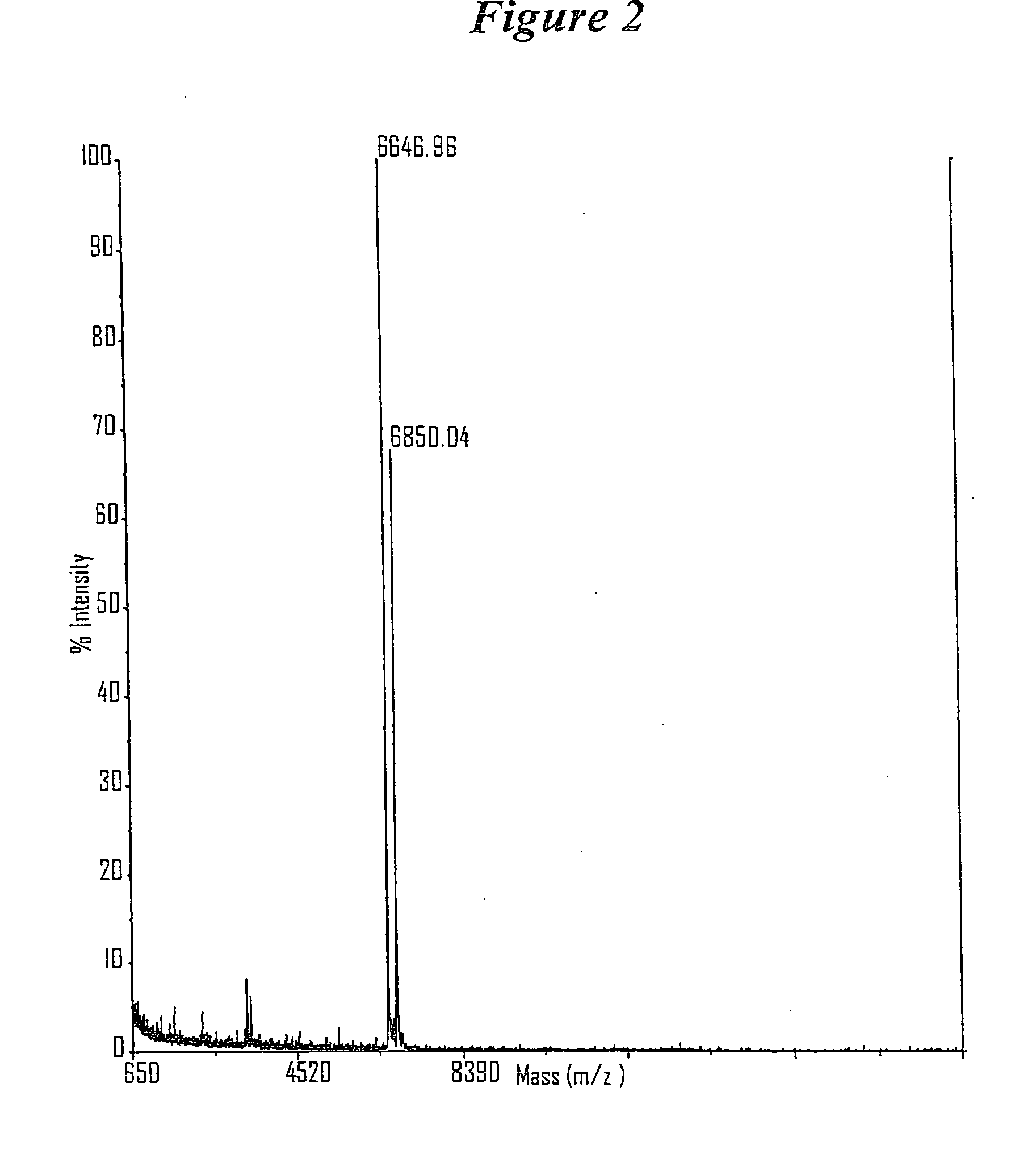
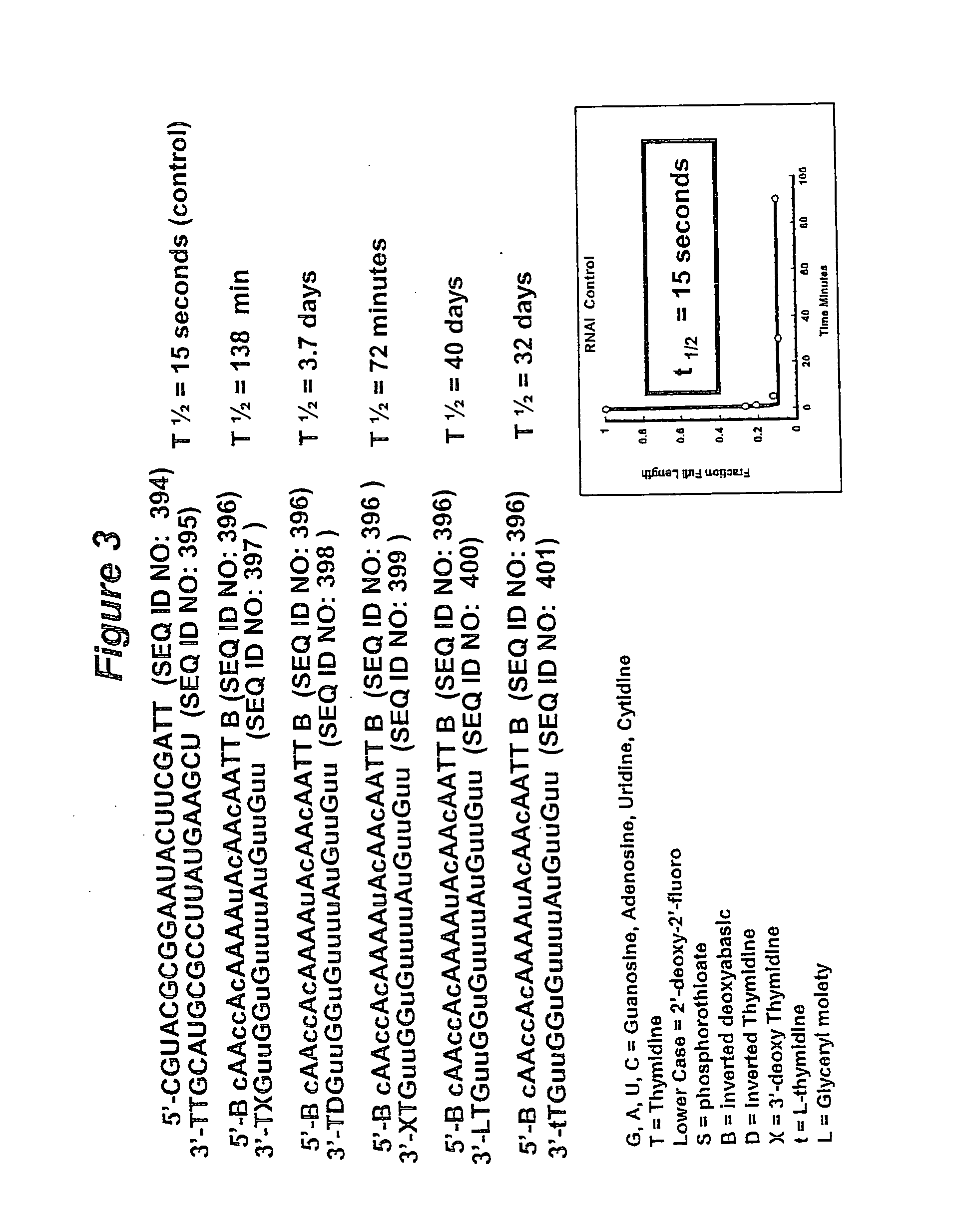
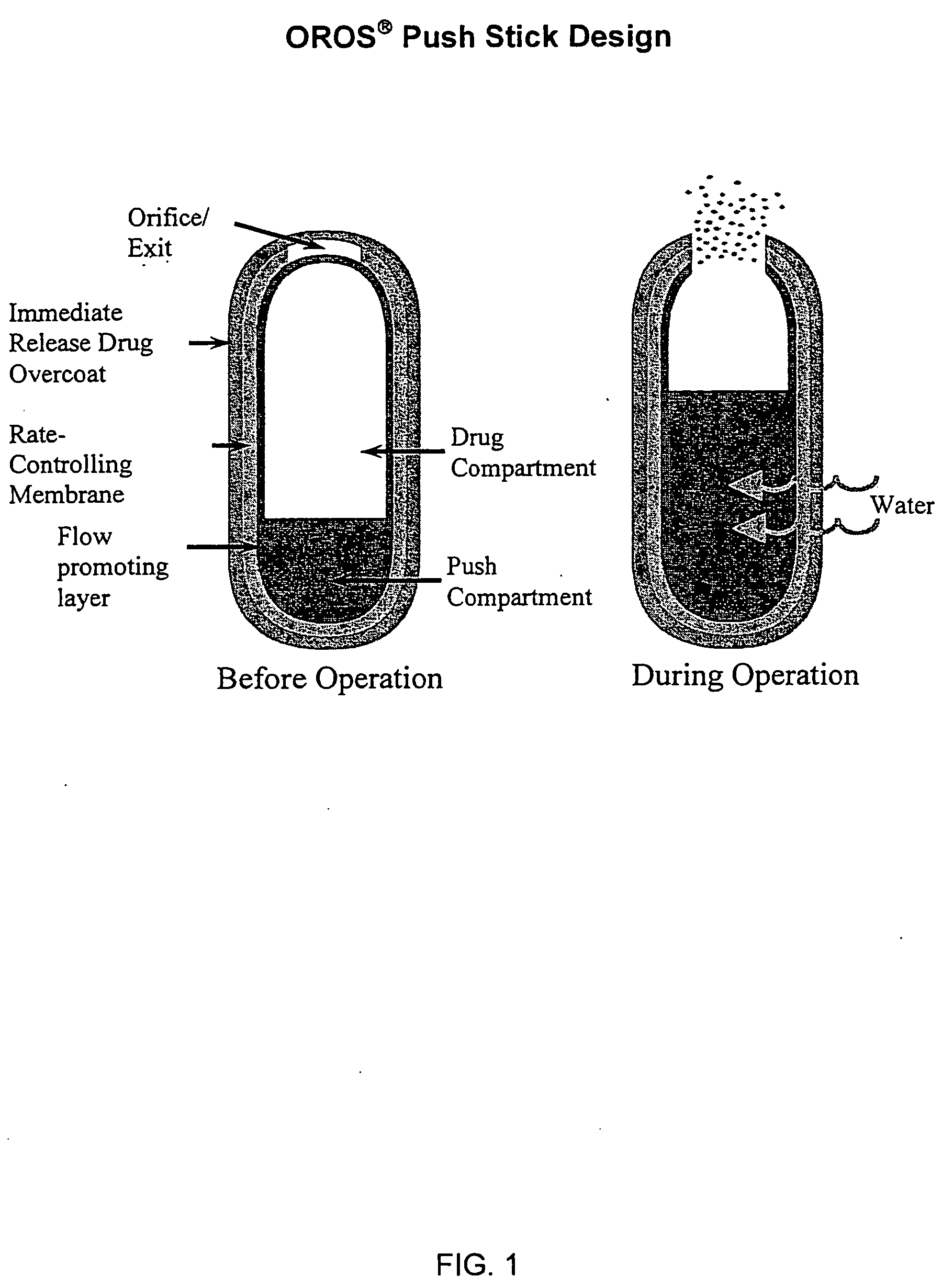
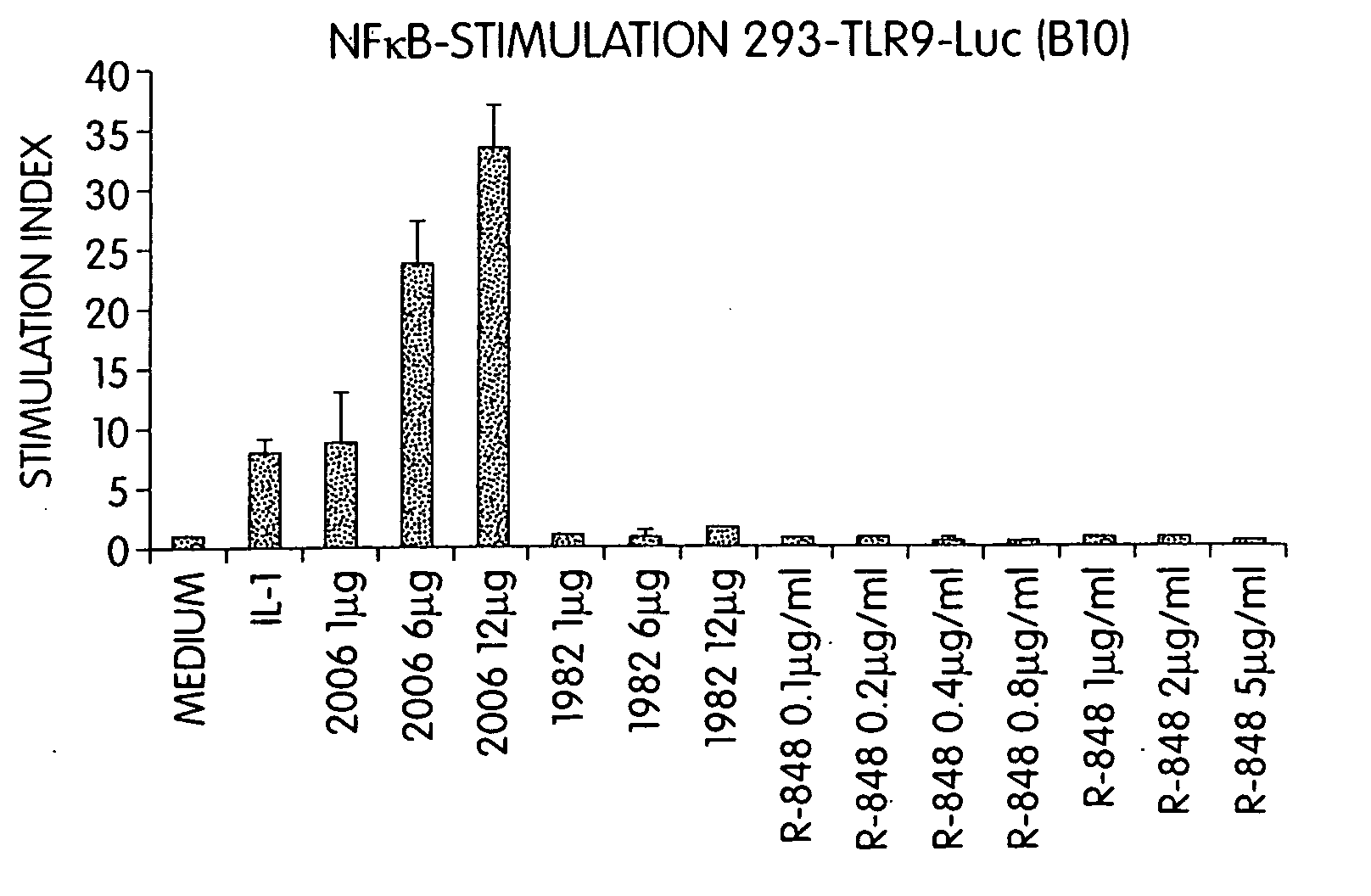
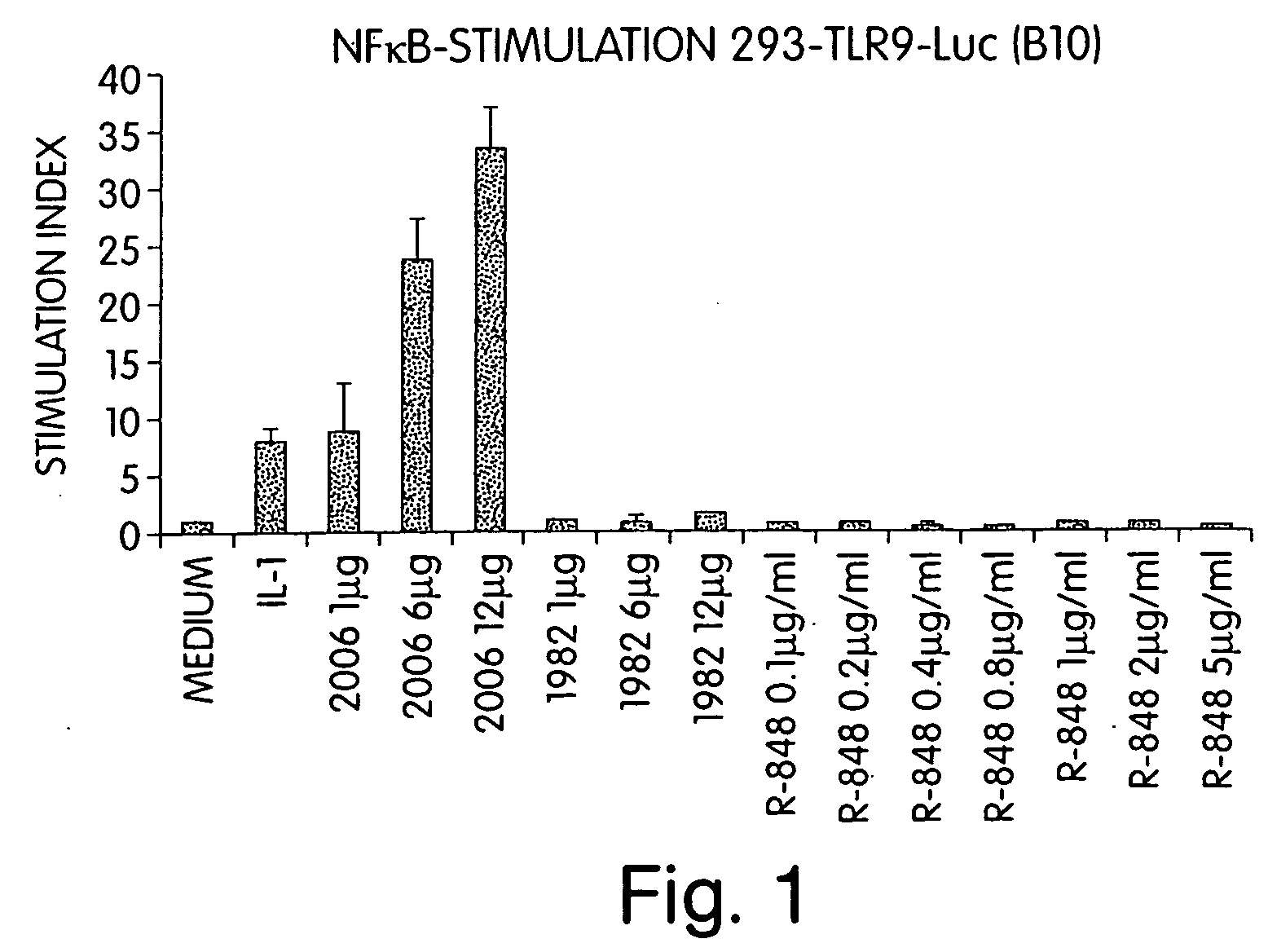
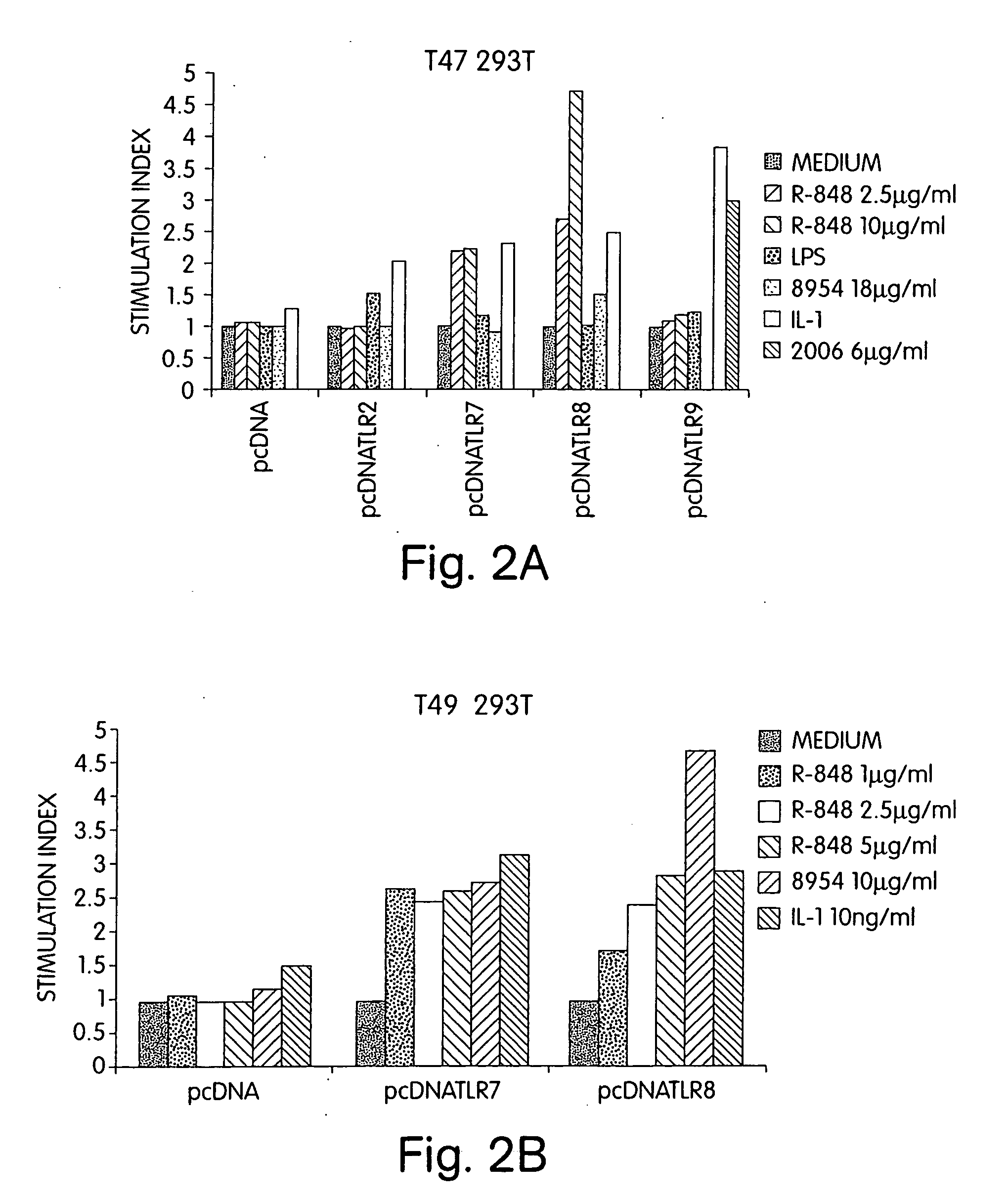
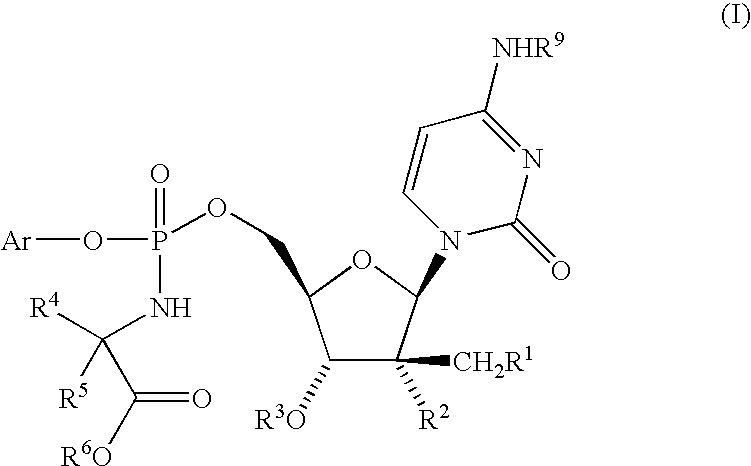
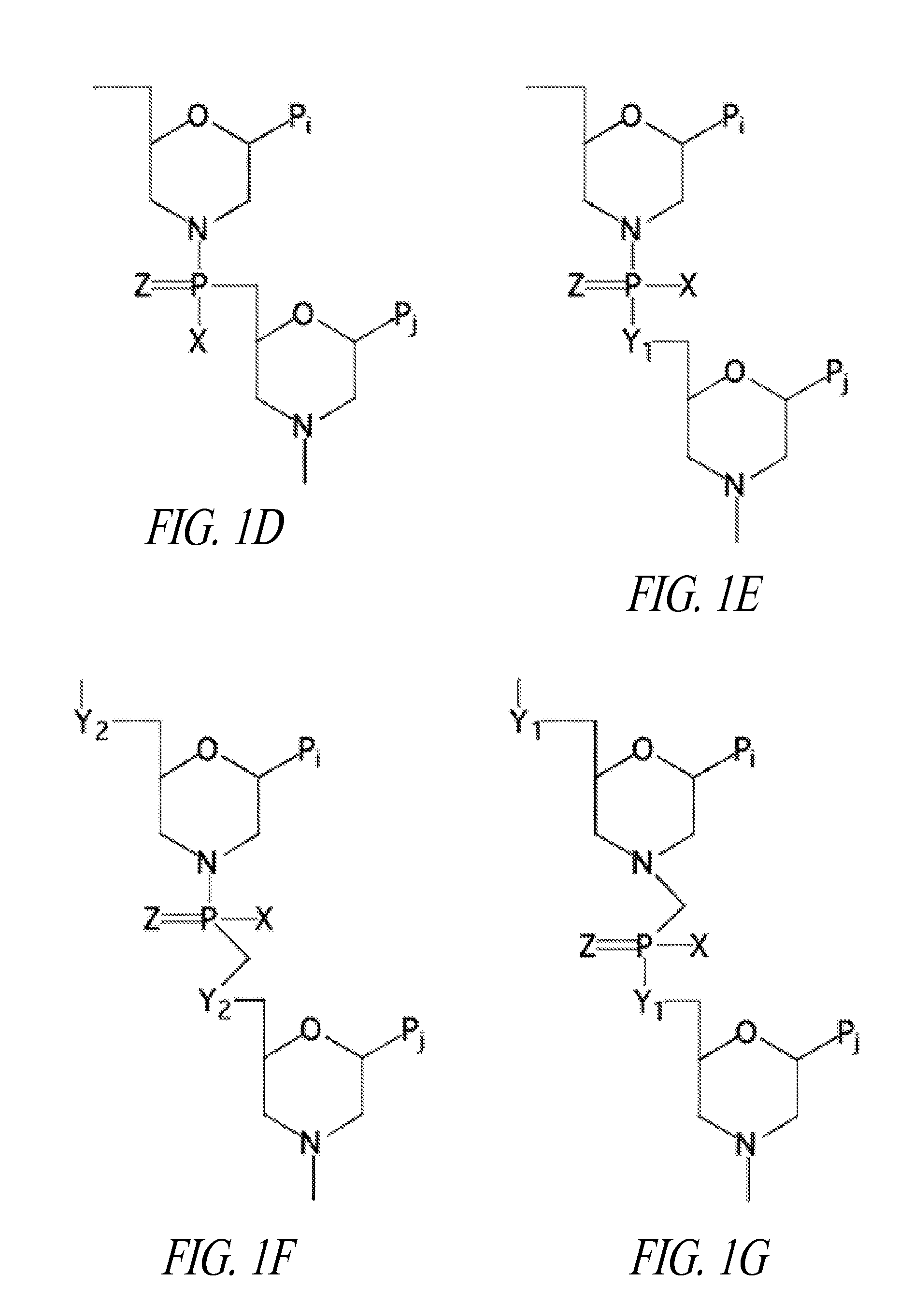
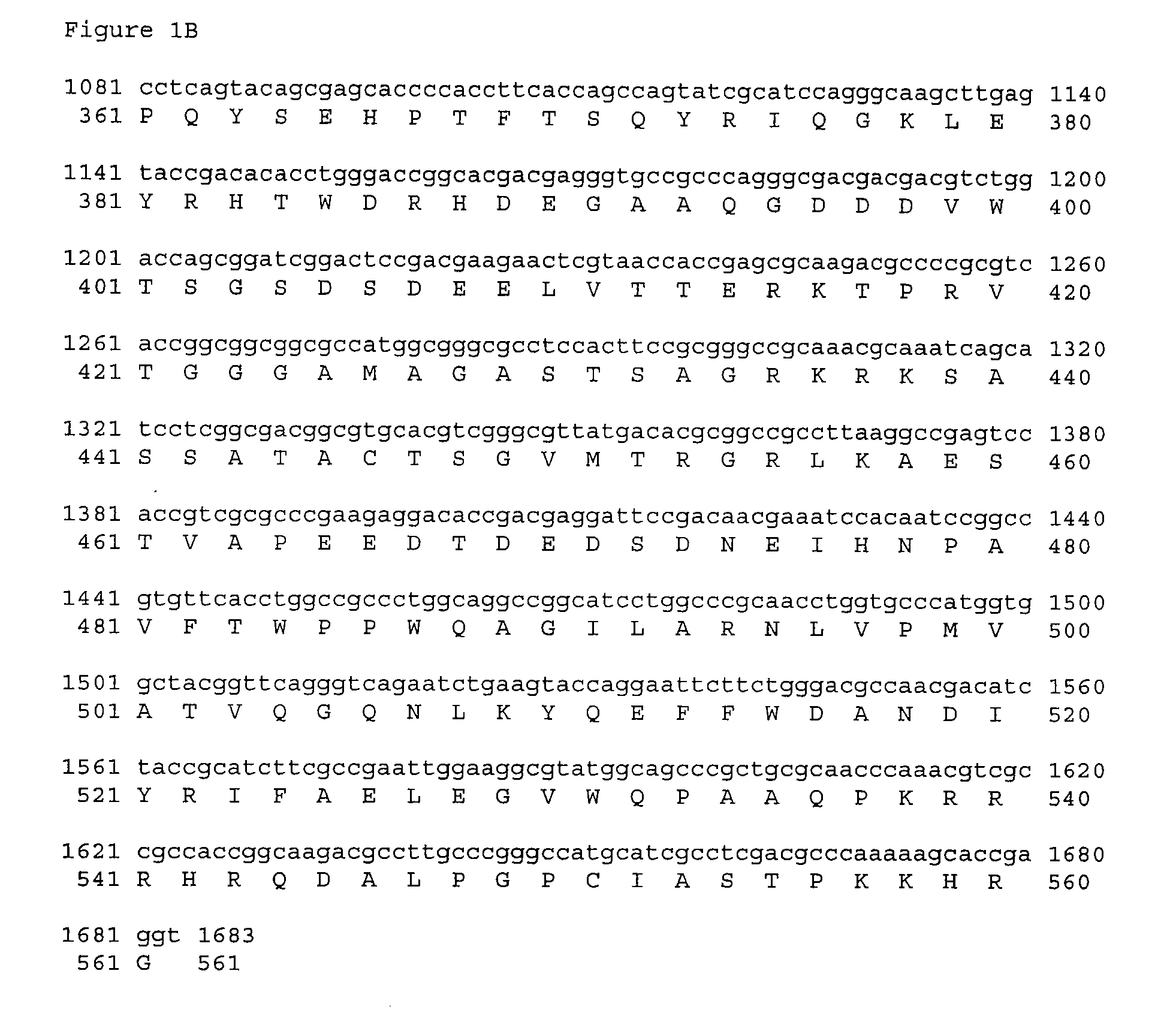
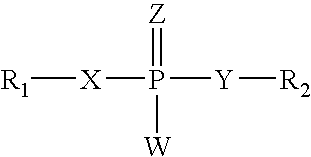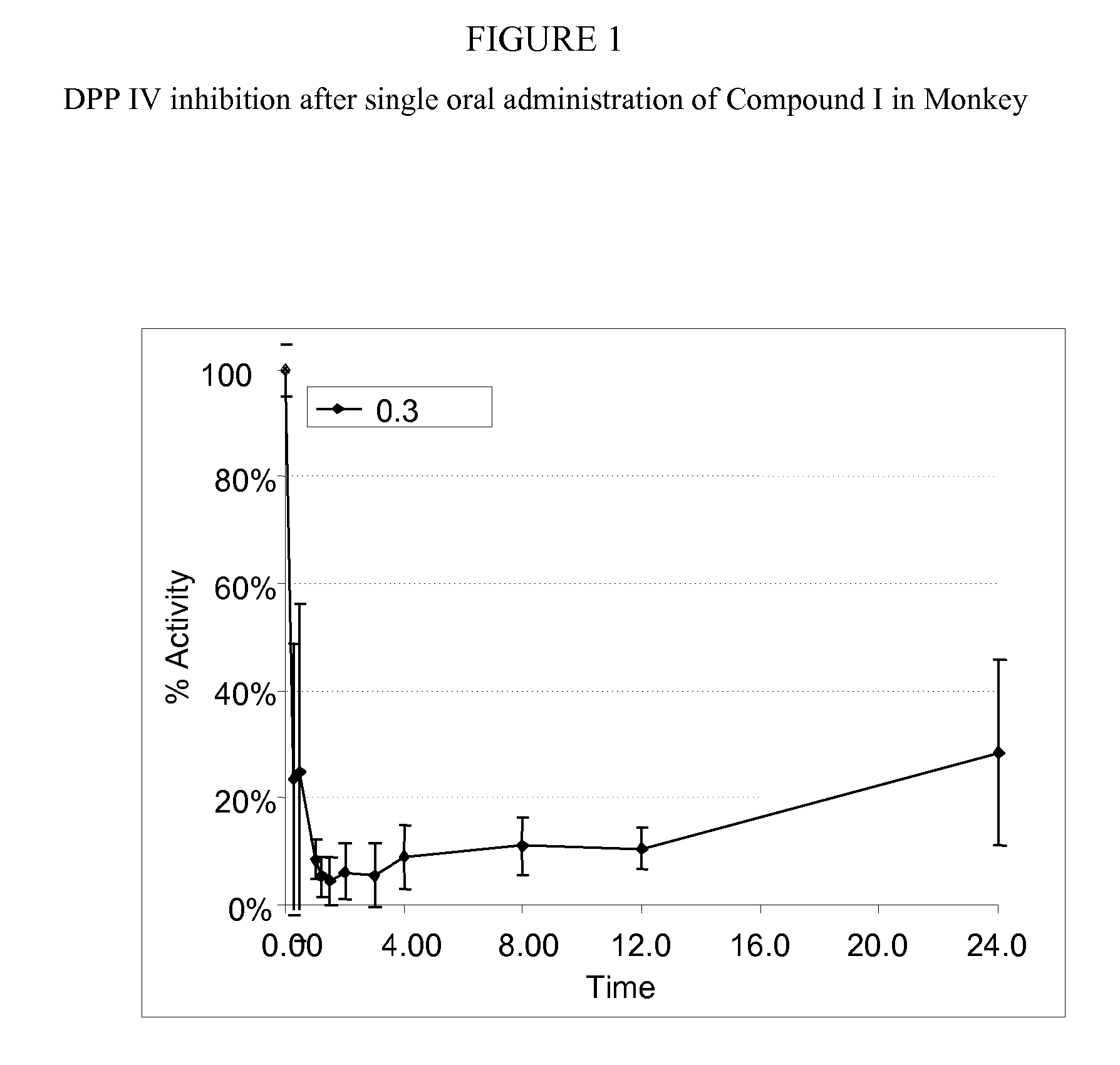Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3288results about How to "Reduce dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
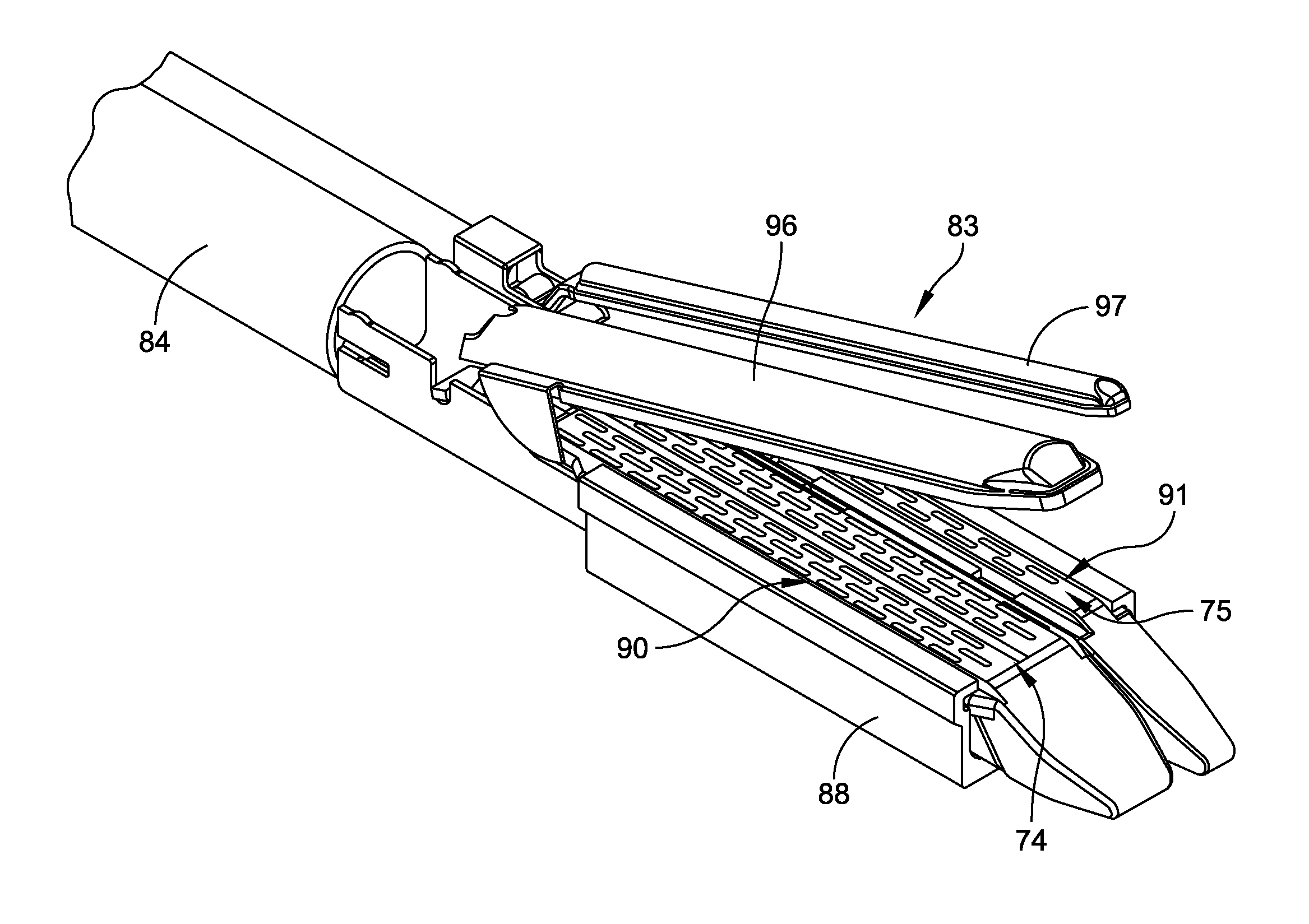
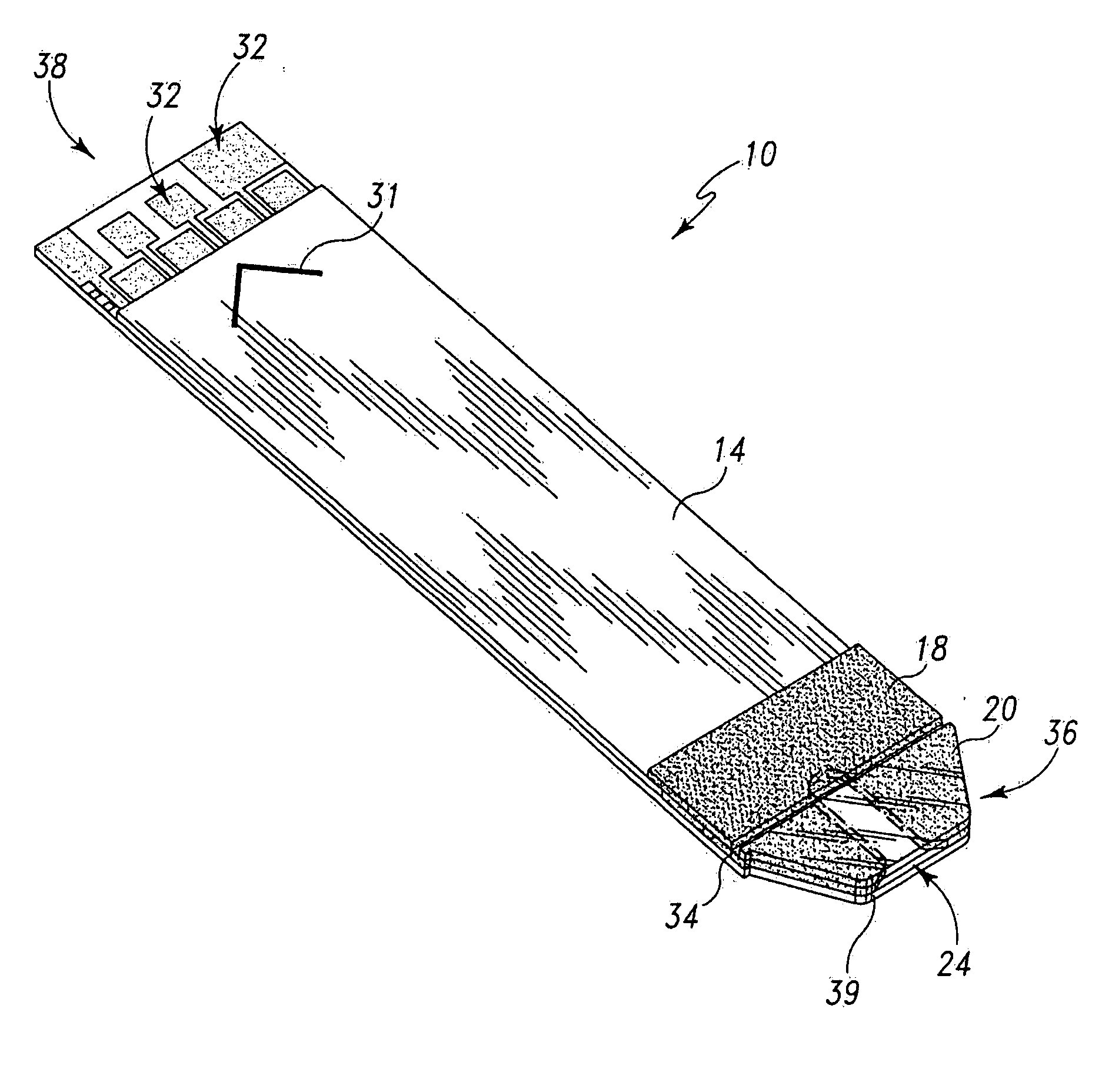
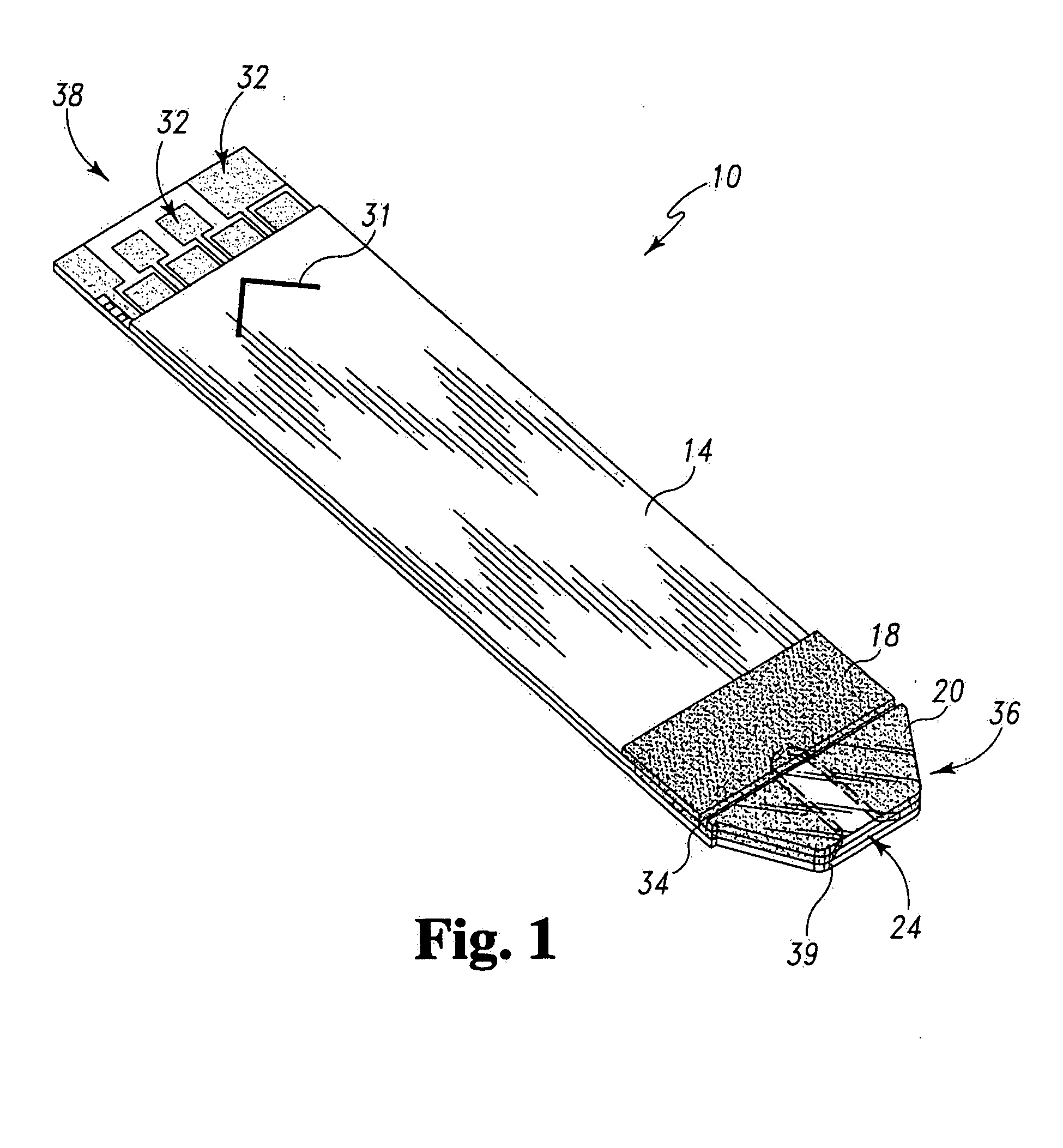
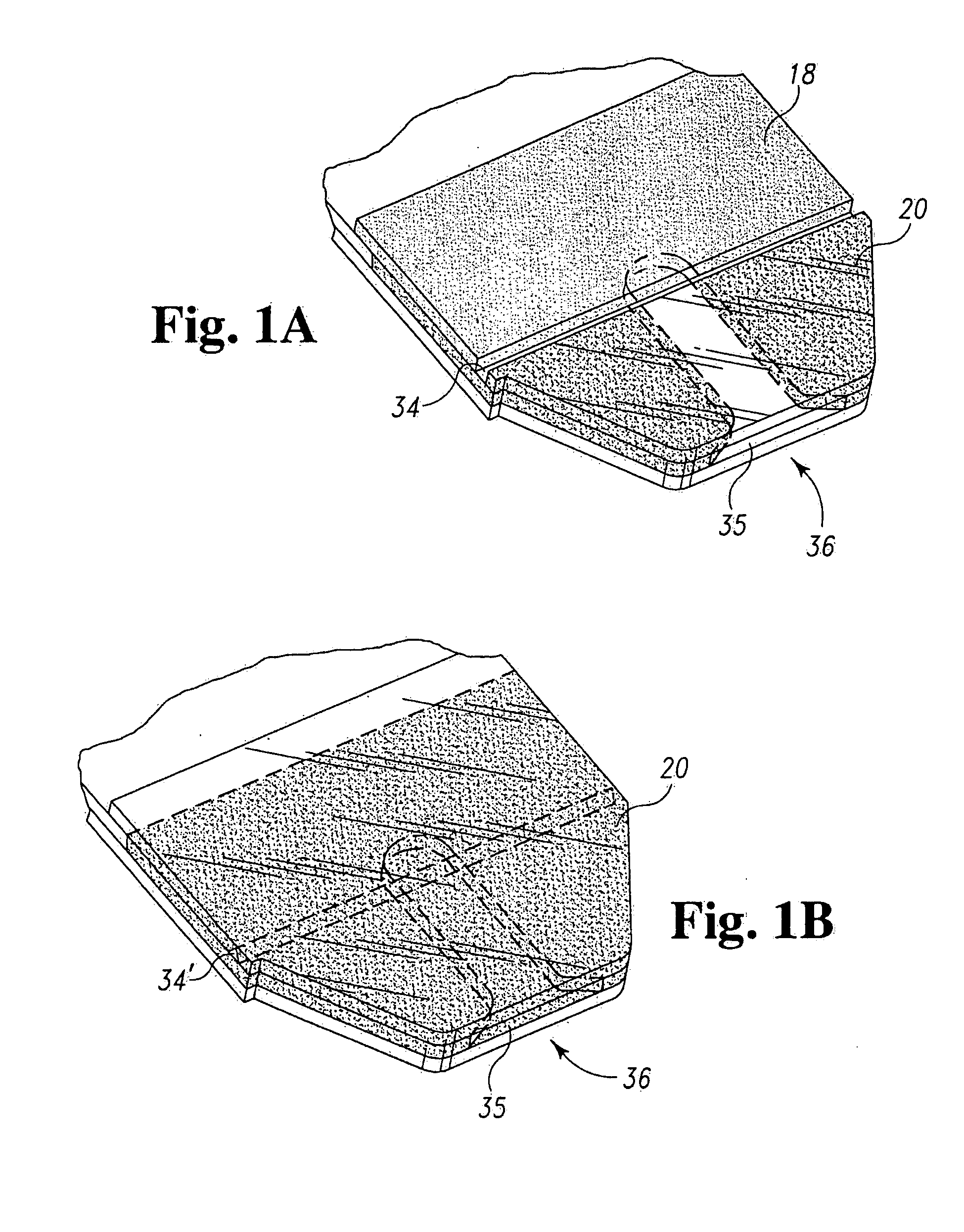
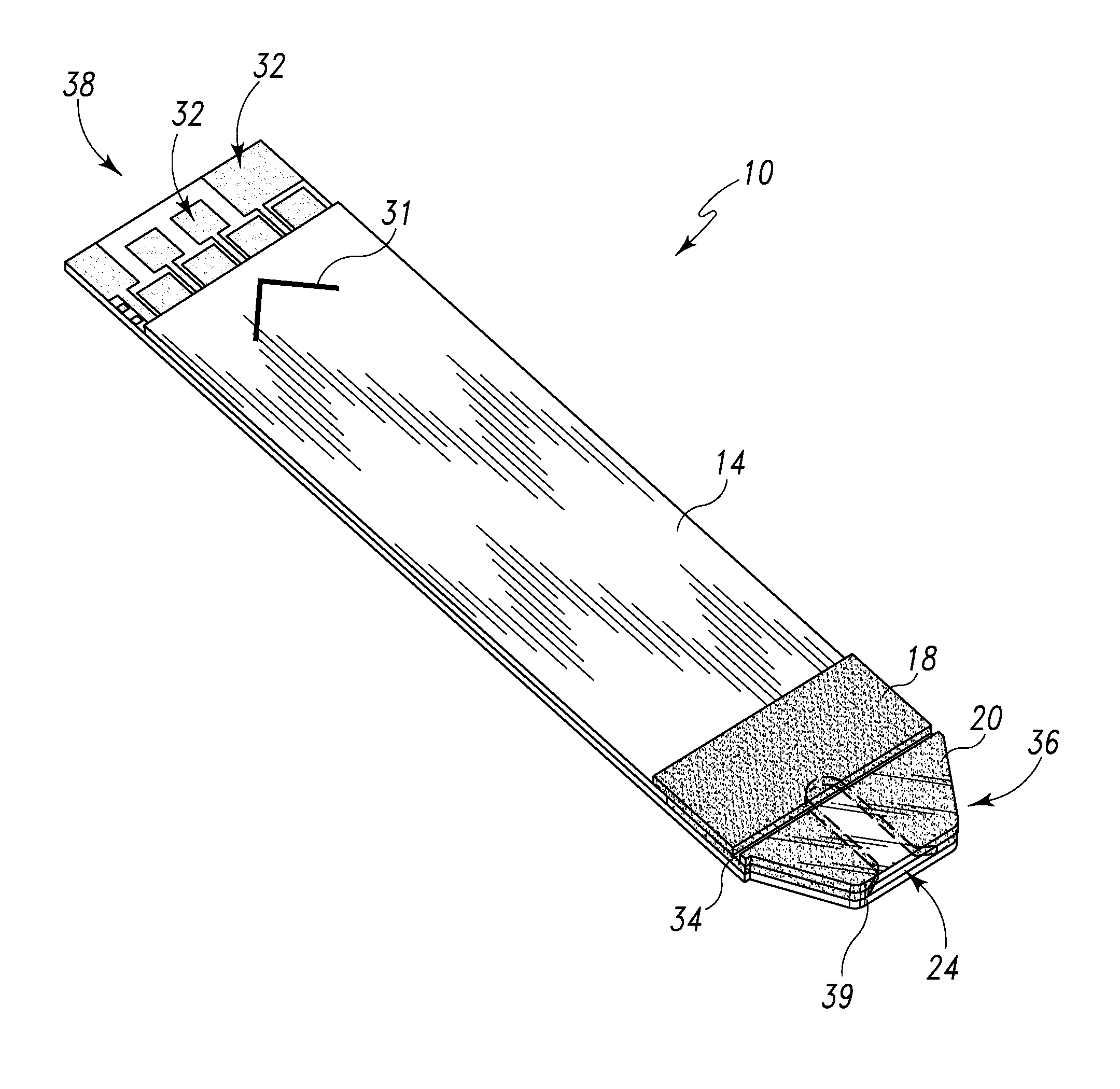
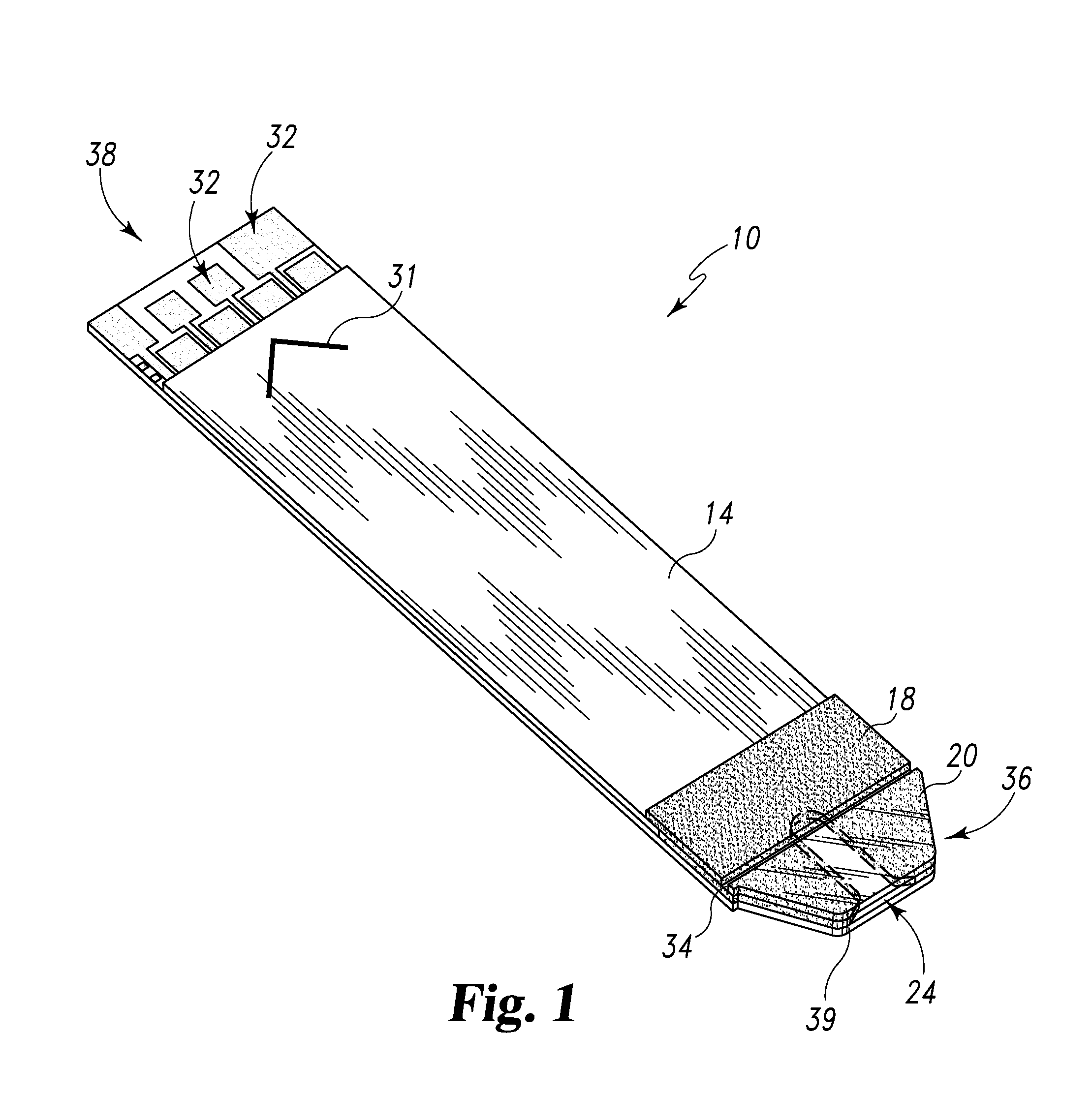
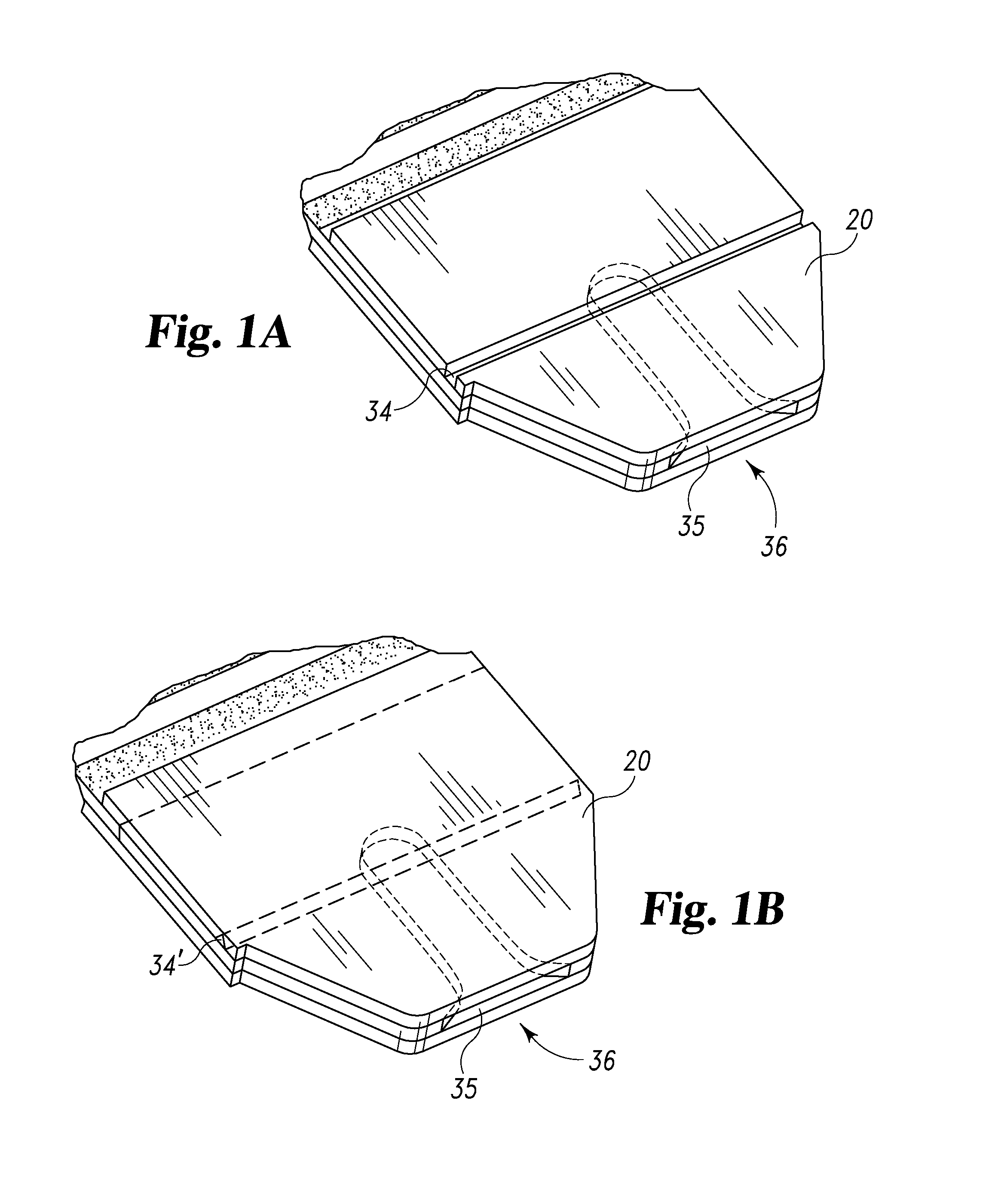
Radioactive therapeutic fastening instrument
ActiveUS8267849B2For accurate placementReduce doseSuture equipmentsStapling toolsBrachytherapyEngineering
An instrument used for brachytherapy delivery in the treatment of cancer by radiation therapy including a handle having first and second handle actuators; an end effector; and an instrument shaft that connects the handle with the end effector. The end effector has first and second adjacent disposed staple mechanisms that each retain a set of staples. The first mechanism is for holding standard staples in a first array, and dispensing the standard staples under control of the corresponding first handle actuator. The second mechanism is for holding radioactive source staples in a second array, and dispensing said radioactive source staples under control of the corresponding second handle actuator. A holder is for receiving the first and second mechanisms in a substantially parallel array so that the standard staples close the incision at a surgical margin while the source staples are secured adjacent thereto.
Owner:POINT SOURCE TECH
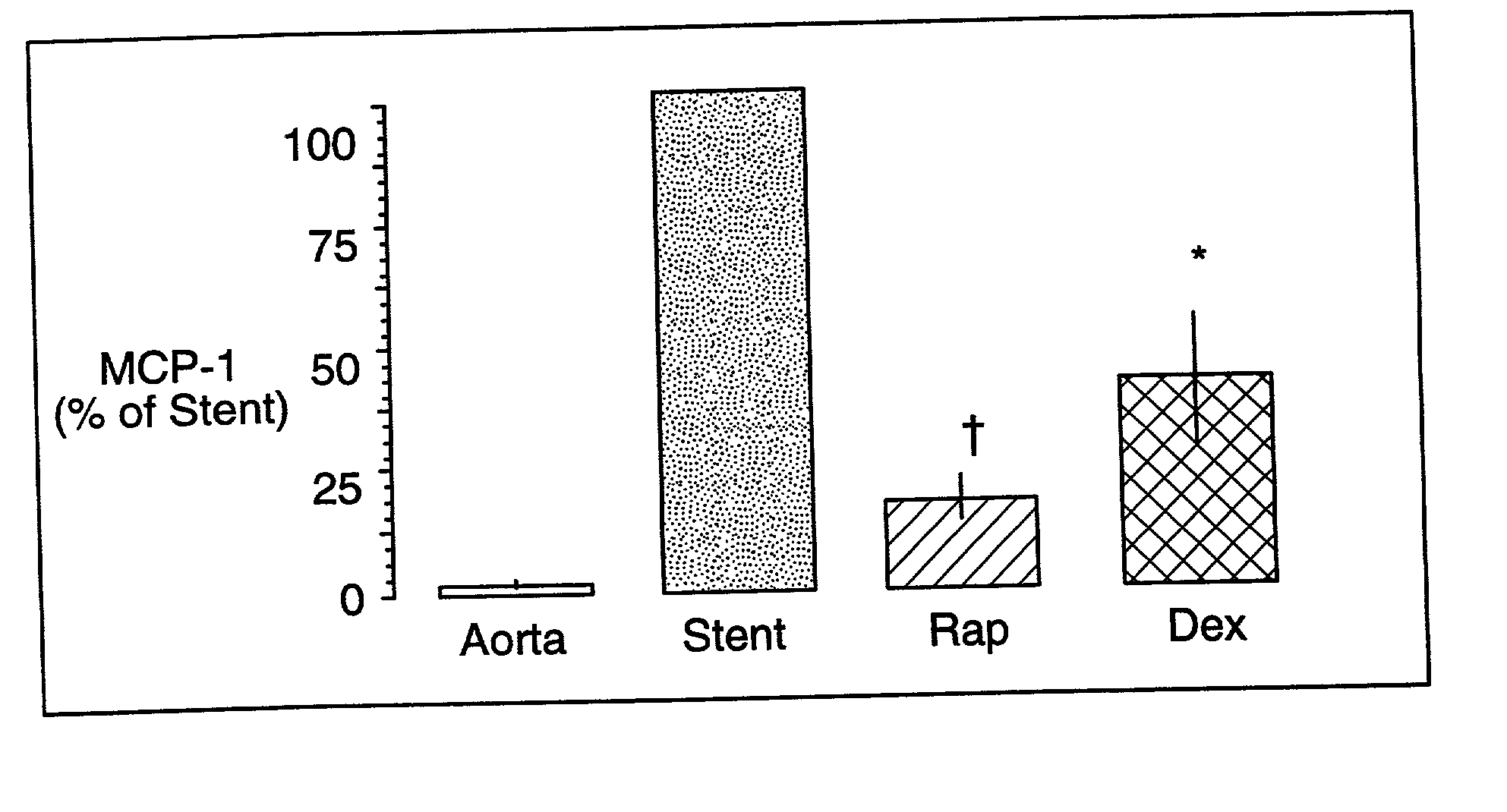
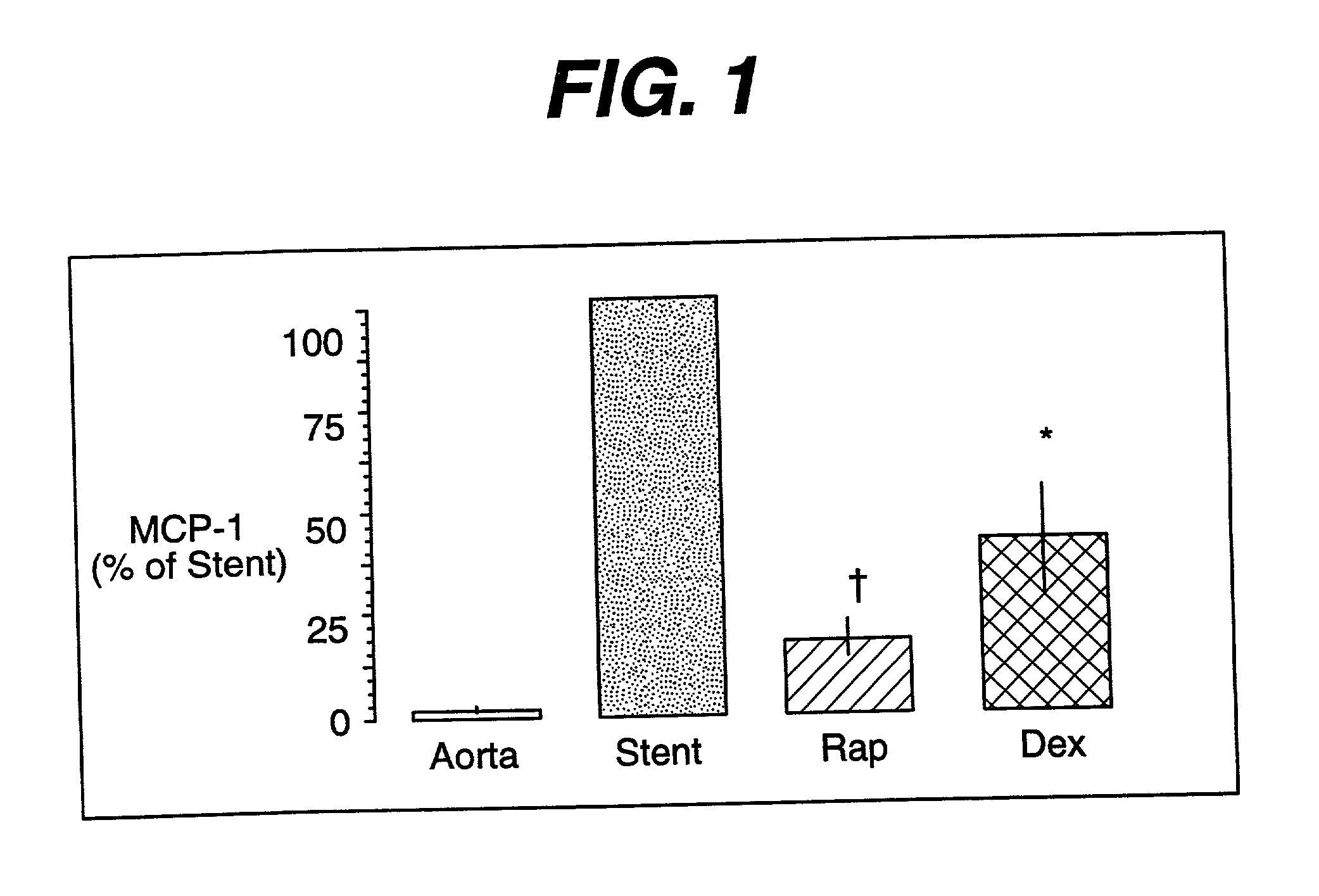
Drug/drug delivery systems for the prevention and treatment of vascular disease
InactiveUS20020007215A1Prevent proliferationGood effectOrganic active ingredientsStentsVascular diseaseWhole body
A drug and drug delivery system may be utilized in the treatment of vascular disease. A local delivery system is coated with rapamycin or other suitable drug, agent or compound and delivered intraluminally for the treatment and prevention of neointimal hyperplasia following percutaneous transluminal coronary angiography. The local delivery of the drugs or agents provides for increased effectiveness and lower systemic toxicity.
Owner:WYETH LLC
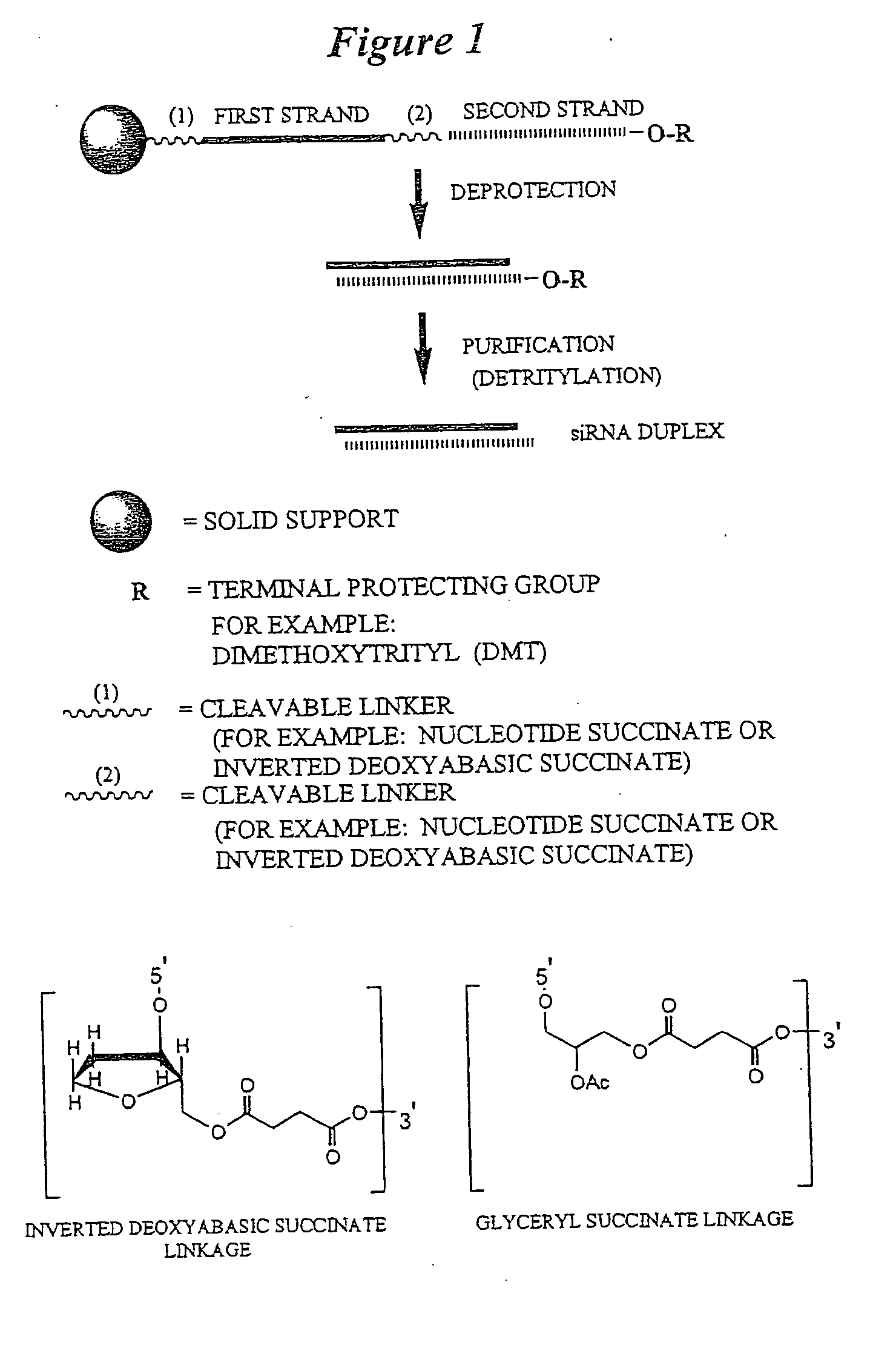
RNA interference mediated inhibition of B-cell CLL/Lymphoma-2 (BCL-2) gene expression using short interfering nucleic acid (siNA)
InactiveUS20050176025A1Improves various propertyImprove the immunityCompounds screening/testingSpecial deliveryAutoimmune conditionAutoimmune disease
This invention relates to compounds, compositions, and methods useful for modulating BCL2 gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of BCL2 gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of BCL2 genes (e.g., BCL2, BCL-XL, BCL2-L1, MCL-1 CED-9, BAG-1, E1B-194 and / or BCL-A1). The small nucleic acid molecules are useful in the treatment of cancer, malignant blood disease, polycytemia vera, idiopathic myelofibrosis, essential thrombocythemia, myelodysplastic syndromes, autoimmune disease, viral infection, and proliferative diseases and conditions
Owner:SIRNA THERAPEUTICS INC
Method and composition for the treatment of diabetes
InactiveUS6153632AIncrease uptakeImprove utilizationBiocidePeptide/protein ingredientsIGT - Impaired glucose toleranceGlycosidase inhibitor
This invention is directed to a novel method and composition for the treatment of diabetes mellitus (Type I, Impaired Glucose Tolerance ["IGT"]and Type II). More specifically, this invention pertains to a novel method of treating diabetes mellitus by incorporating a therapeutic amount of one or more insulin sensitizers along with one or more of an orally ingested insulin, an injected insulin, a sulfonylurea, a biguanide or an alpha-glucosidase inhibitor for the treatment of diabetes mellitus.
Owner:RIEVELEY CHERYL ANNE
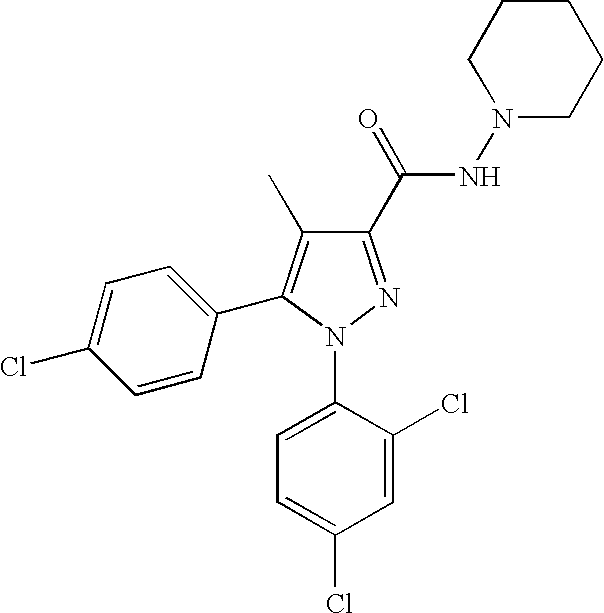
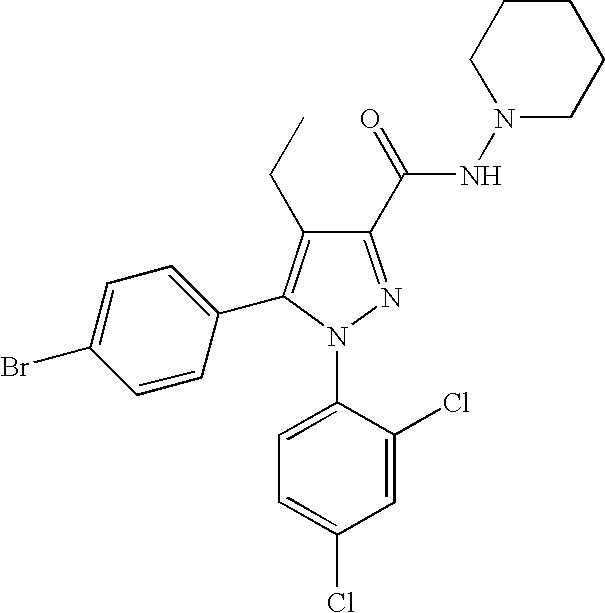
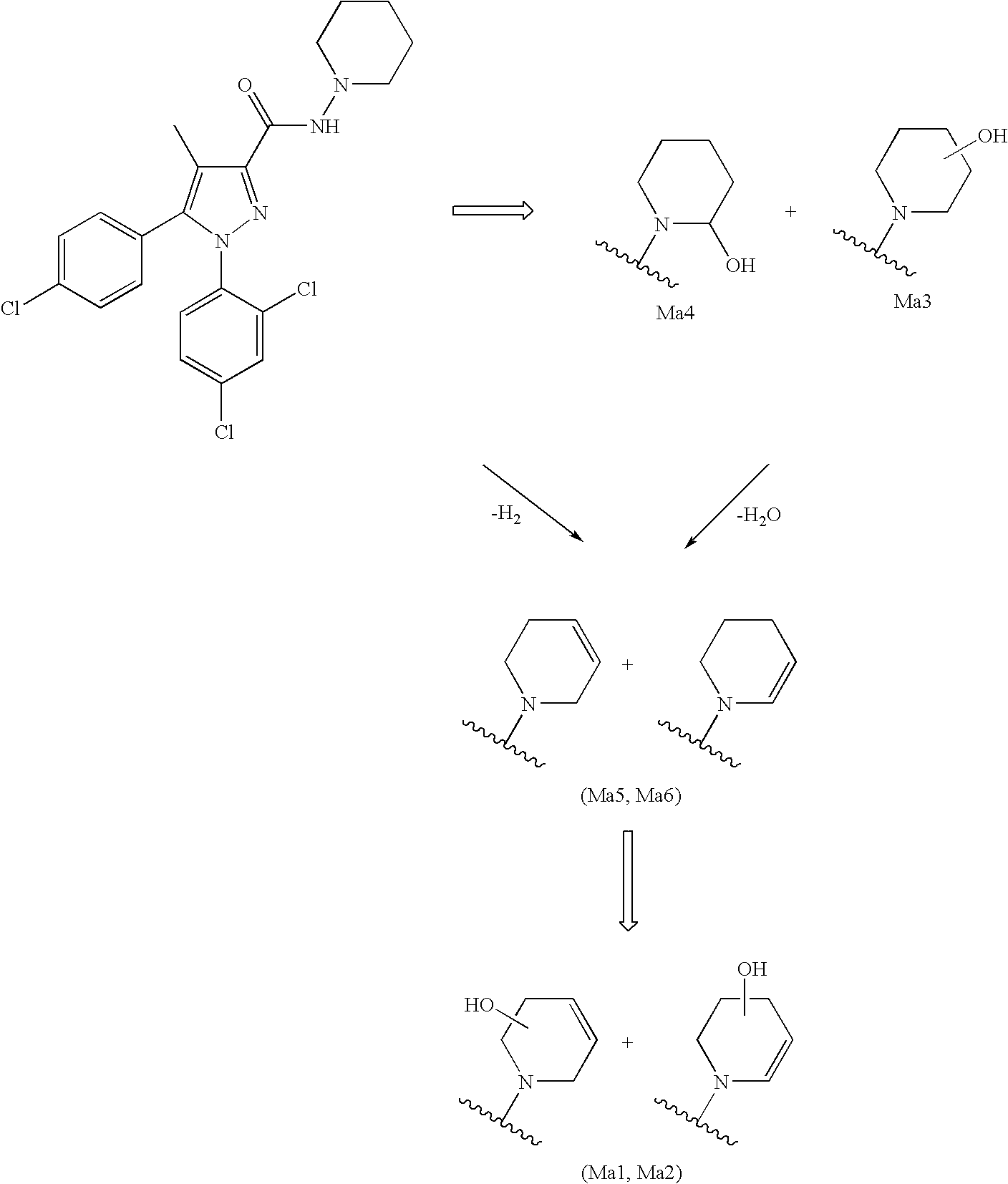
Biphenyl-pyrazolecarboxamide compounds
The present invention relates to biphenyl-pyrazole compounds and in particular biphenyl-pyrazolecarboxamides. The invention further provides compositions comprising a compound of this invention and the use of such compositions in methods of treating diseases and conditions beneficially treated by antagonism or inverse agonism of the CB1 receptor, such as obesity, smoking cessation, and normalization of blood lipid composition.
Owner:SUN PHARMA IND INC
RNA interference mediated inhibition of wingless gene expression using short interfering nucleic acid (siNA)
InactiveUS20050130181A1Improves various propertyImprove the immunityCompounds screening/testingSpecial deliveryWnt genesFhit gene
This invention relates to compounds, compositions, and methods useful for modulating wingless (WNT) gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of WNT gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of WNT genes such as WNT3A and WNT7A.
Owner:SIRNA THERAPEUTICS INC
Hepatitis b antiviral agents
Owner:NOVIRA THERAPEUTICS
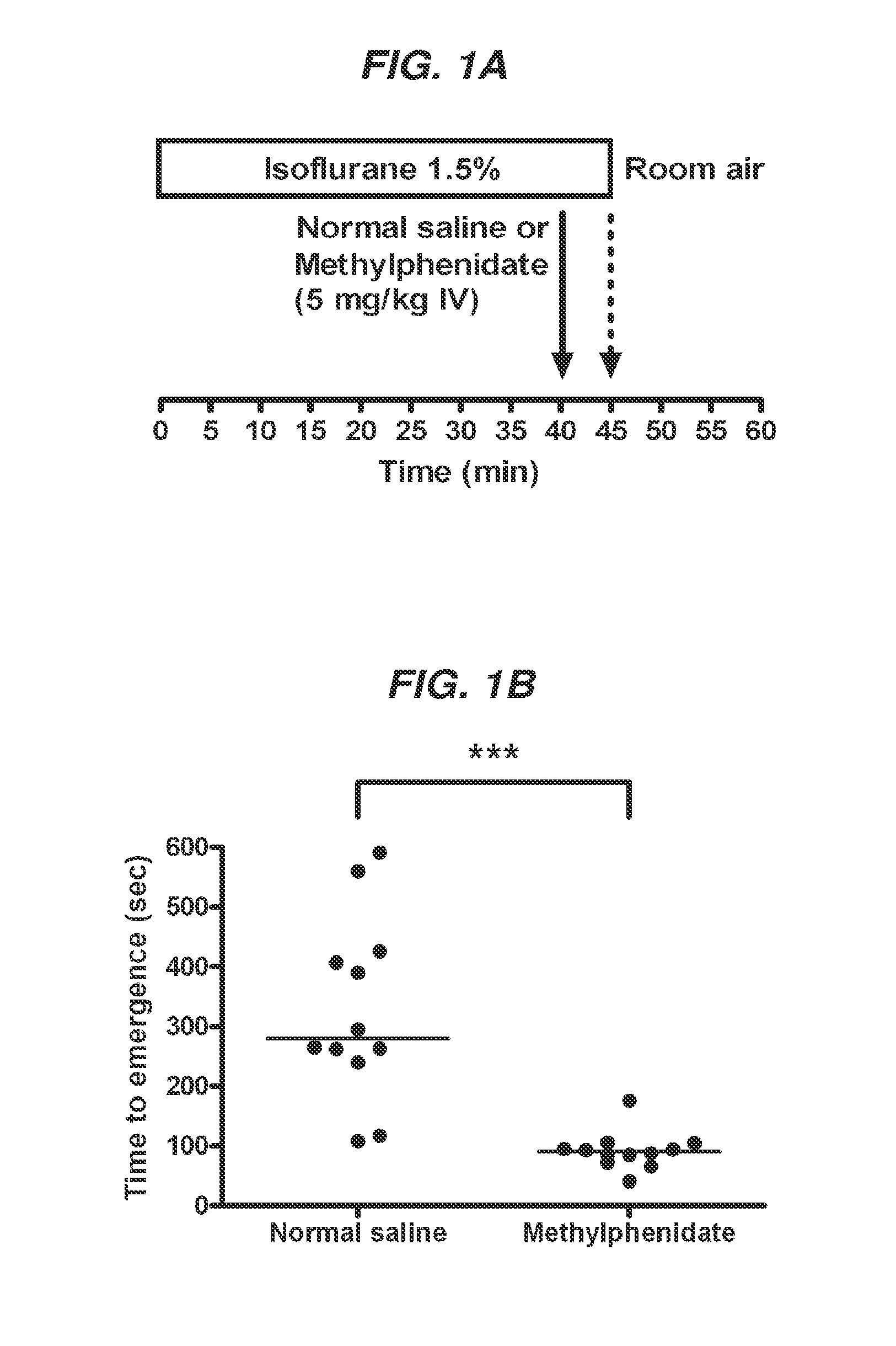
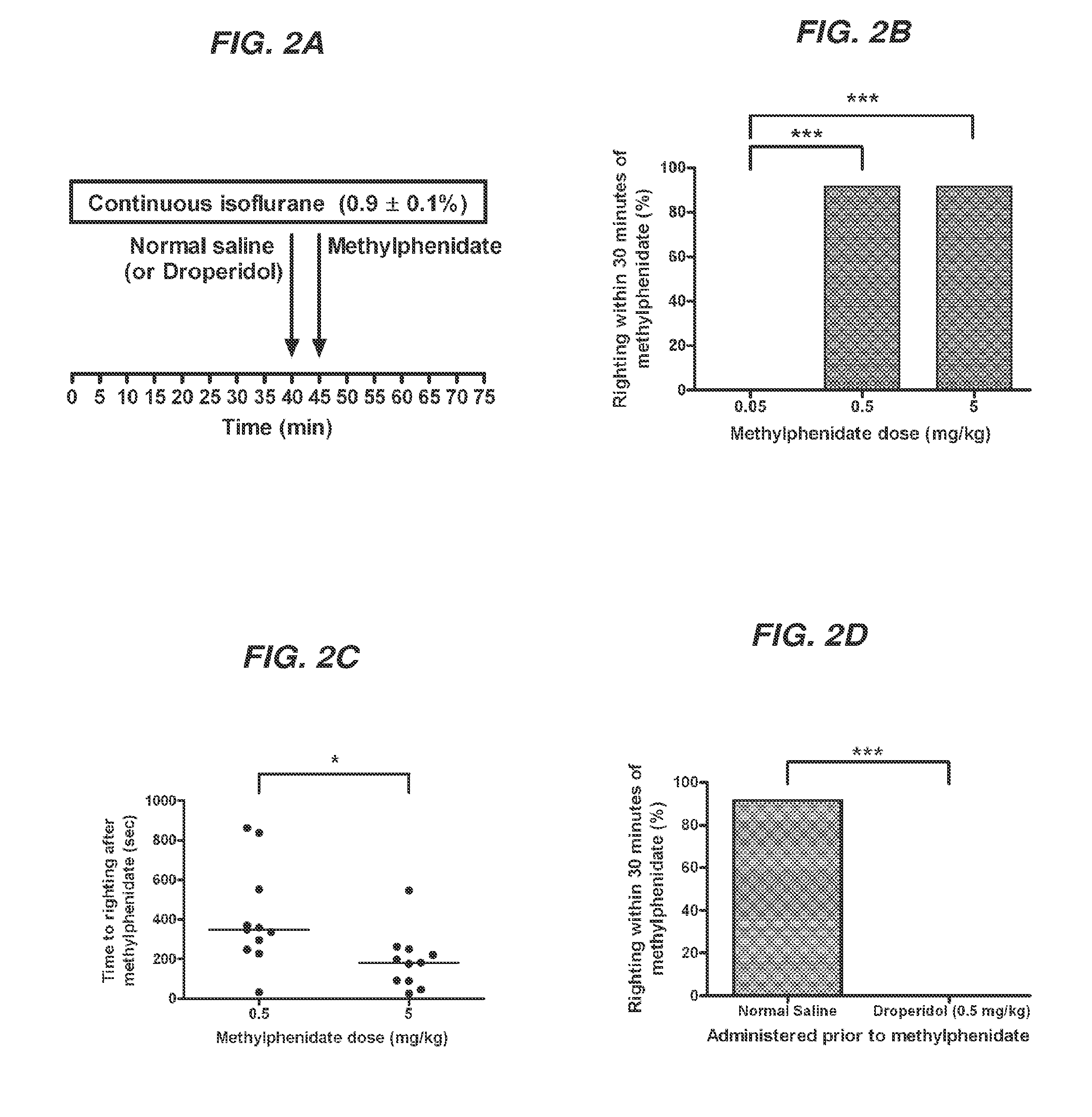
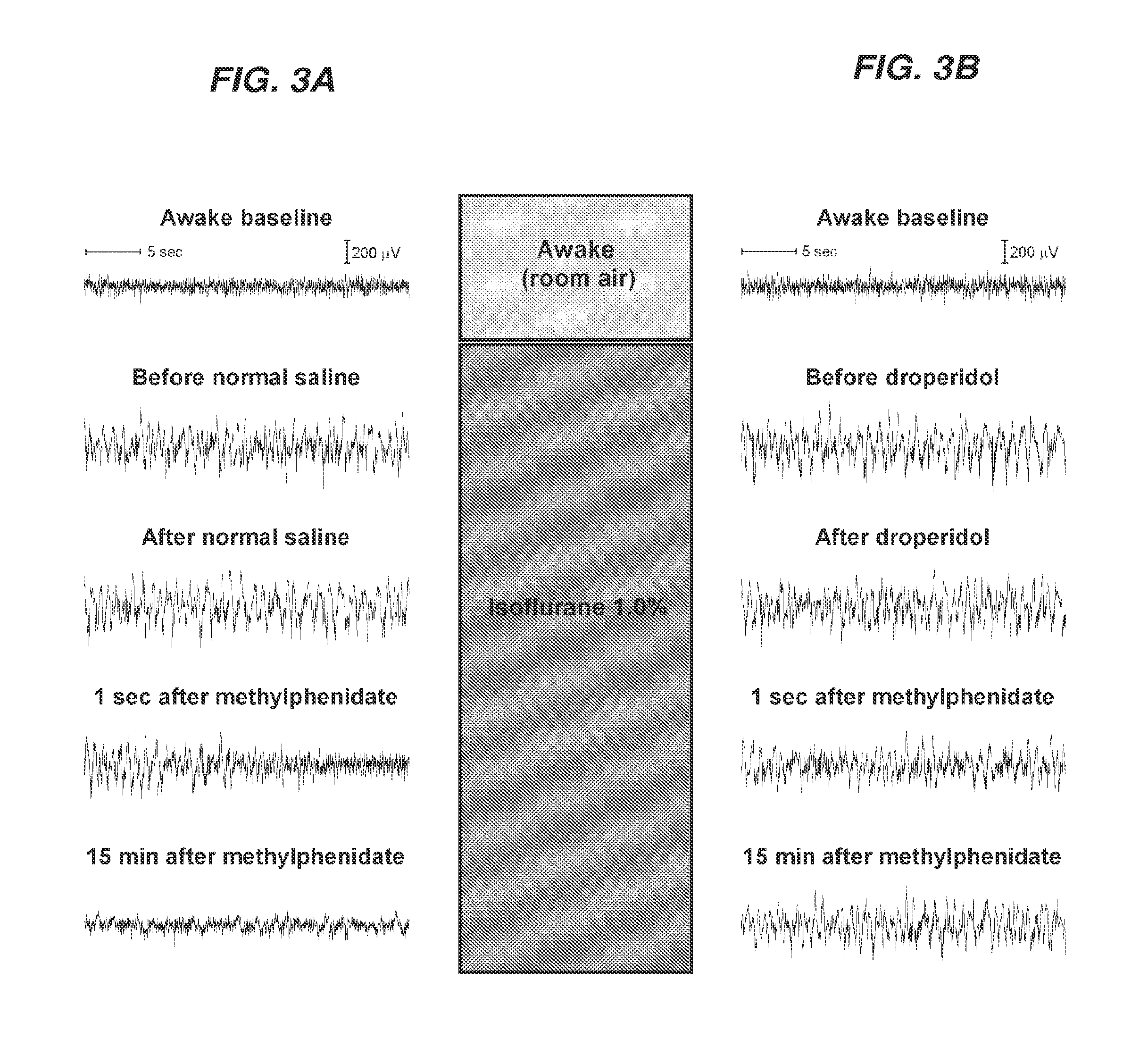
Reversal of General Anesthesia by Administration of Methylphenidate, Amphetamine, Modafinil, Amantadine, and/or Caffeine
ActiveUS20150196249A1High speedReduces and eliminates effectElectroencephalographyPharmaceutical delivery mechanismUnconsciousnessWhole body
Owner:THE GENERAL HOSPITAL CORP
RNA interference mediated inhibition of TNF and TNF receptor gene expression using short interfering nucleic acid (siNA)
InactiveUS20050227935A1Improves various propertyImprove the immunitySugar derivativesGenetic material ingredientsTumor necrosis factor receptorDouble strand
This invention relates to compounds, compositions, and methods useful for modulating tumor necrosis factor and / or tumor necrosis factor receptor gene expression using short interfering nucleic acid (siNA) molecules. This invention also relates to compounds, compositions, and methods useful for modulating the expression and activity of other genes involved in pathways of tumor necrosis factor and / or tumor necrosis factor receptor gene expression and / or activity by RNA interference (RNAi) using small nucleic acid molecules. In particular, the instant invention features small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules and methods used to modulate the expression of tumor necrosis factor and / or tumor necrosis factor receptor genes, (TNF and / or TNF receptor).
Owner:SIRNA THERAPEUTICS INC
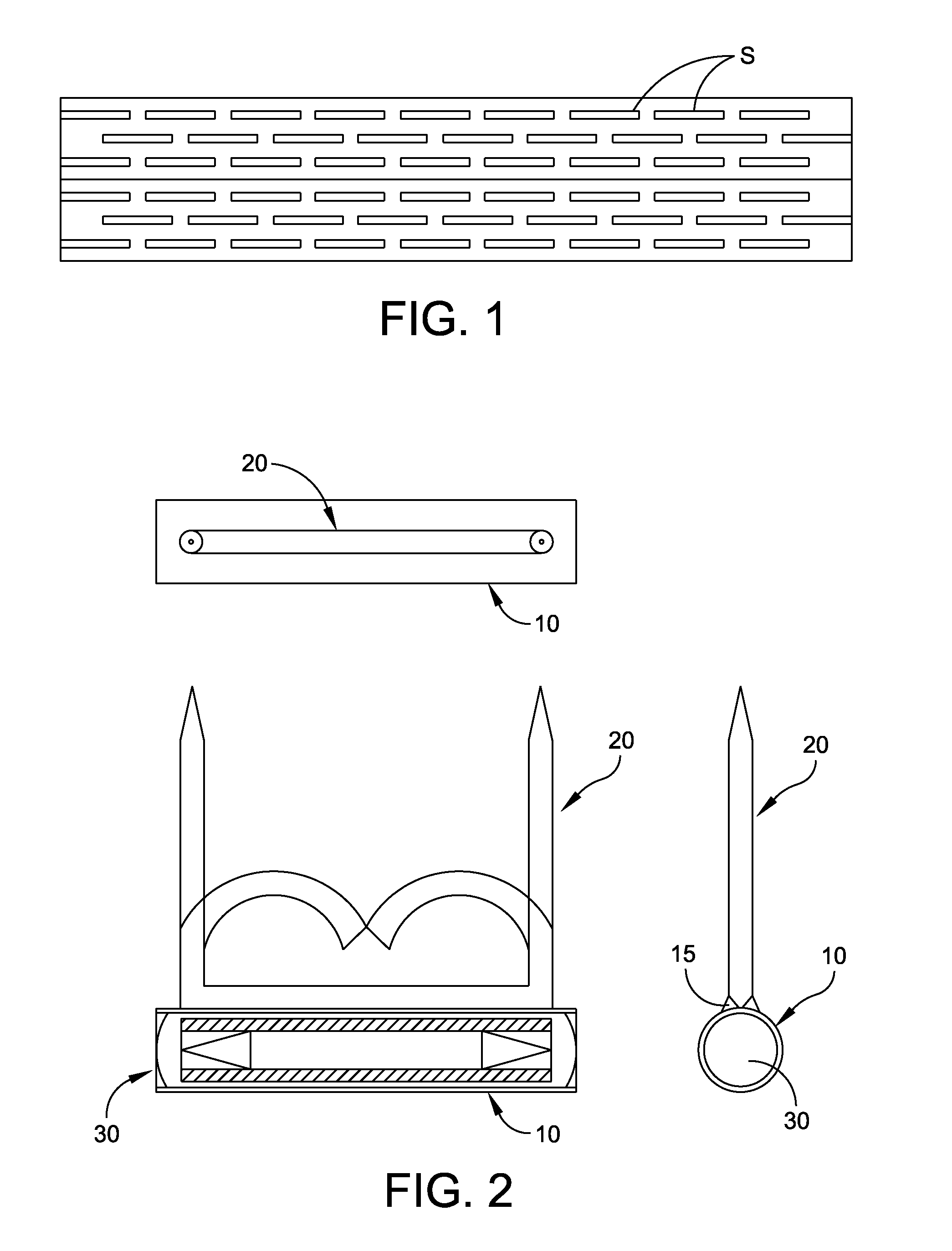
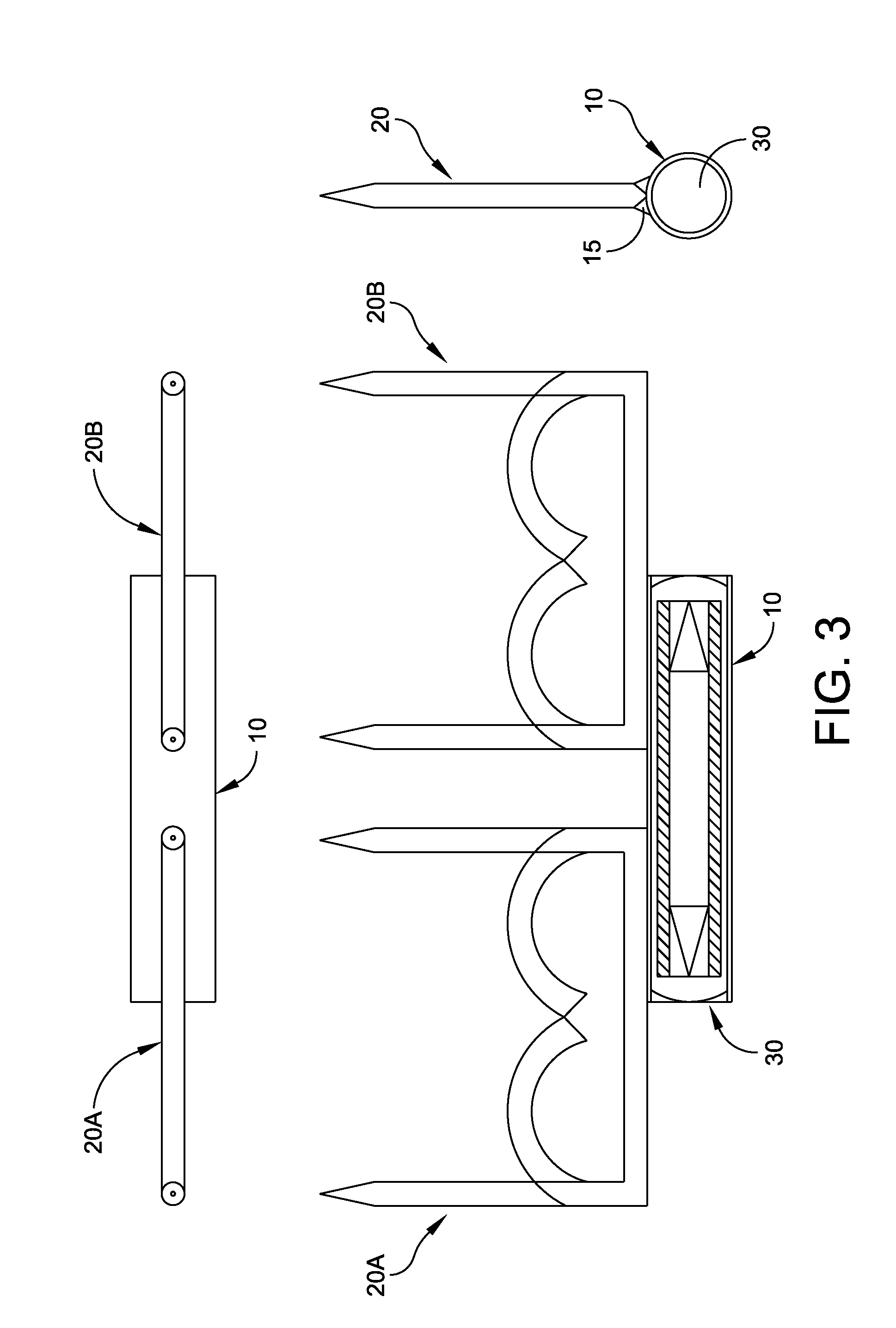
Test strip with flared sample receiving chamber
ActiveUS20050019212A1Promotes wickingReduce doseImmobilised enzymesBioreactor/fermenter combinationsReduced doseTest strips
A test strip with a sample receiving chamber having a novel flared portion that terminates in a sample receiving opening. The flared portion provides a reservoir from which sample fluid can be drawn into the capillary or sample receiving chamber. The wider opening provided by the present invention is easier to “target” with a sample fluid. In preferred embodiments, the hydrophilic reagent layer extends to the dosing end or side of the test strip and further promotes wicking of the sample into the sample receiving chamber and thus reduces dose hesitation. In other preferred embodiments, a tapered dosing end is provided on the test strip in combination with the flared portion, and this combination create a test strip that will draw sample fluid into the sample receiving chamber regardless of where along the dosing edge of the test strip the fluid sample makes contact.
Owner:ROCHE OPERATIONS +1
Oligonucleotides for genotyping thymidylate synthase gene
ActiveUS20070031829A1Sensitive and convenient detectionEasy to aimSugar derivativesMicrobiological testing/measurementGeneticsGenomic DNA
Oligonucleotides for genotyping the thymidylate synthase gene are provided. The number of tandem repeats in the promoter region of the thymidylate synthase gene can be identified based on the hybridization of an oligonucleotide of the invention to the genomic DNA of a subject. Therefore, the genotype of the thymidylate synthase gene can be identified based on the number of tandem repeats. The genotype relates to the responsiveness of a subject towards an antitumor agent.
Owner:F HOFFMANN LA ROCHE & CO AG
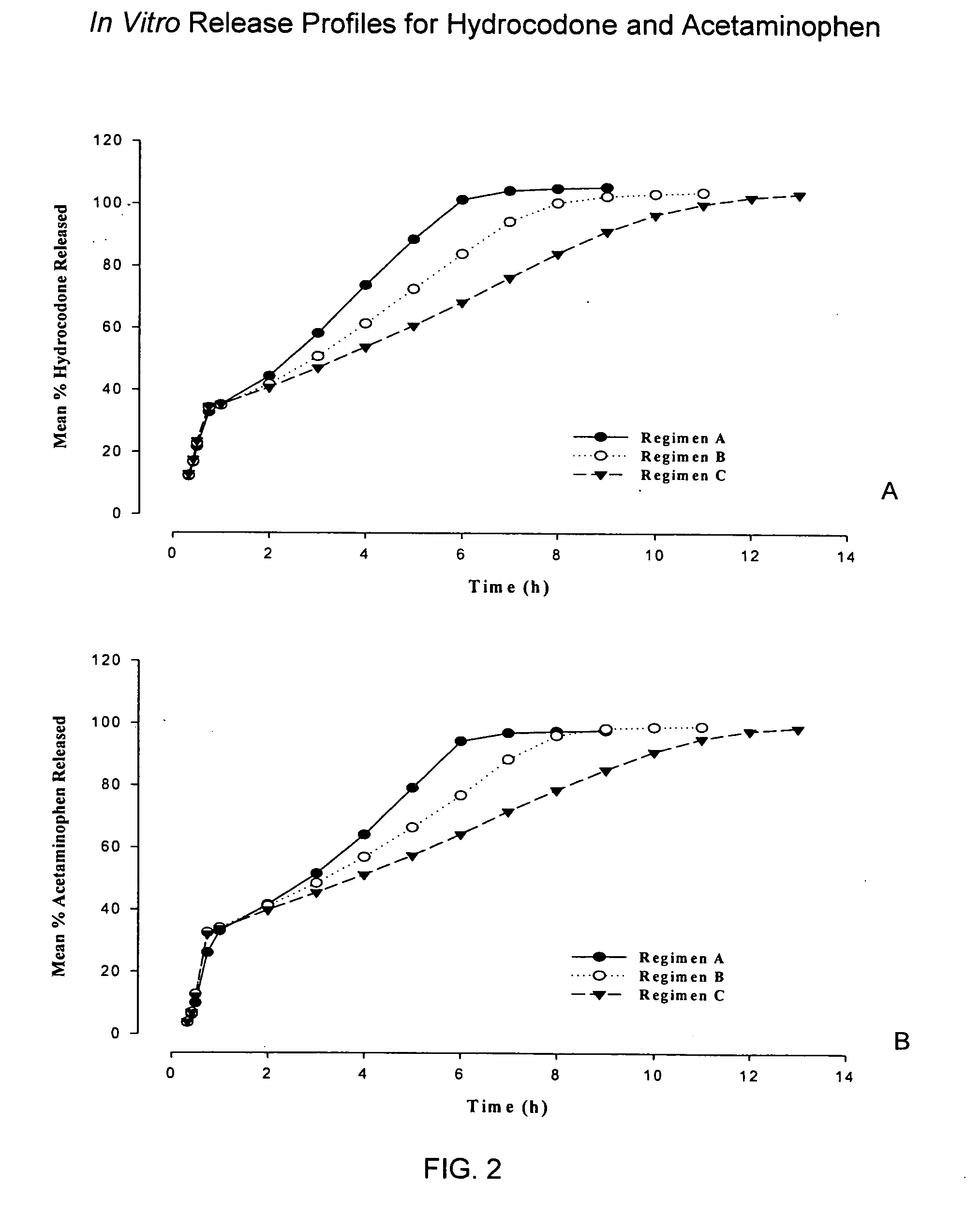
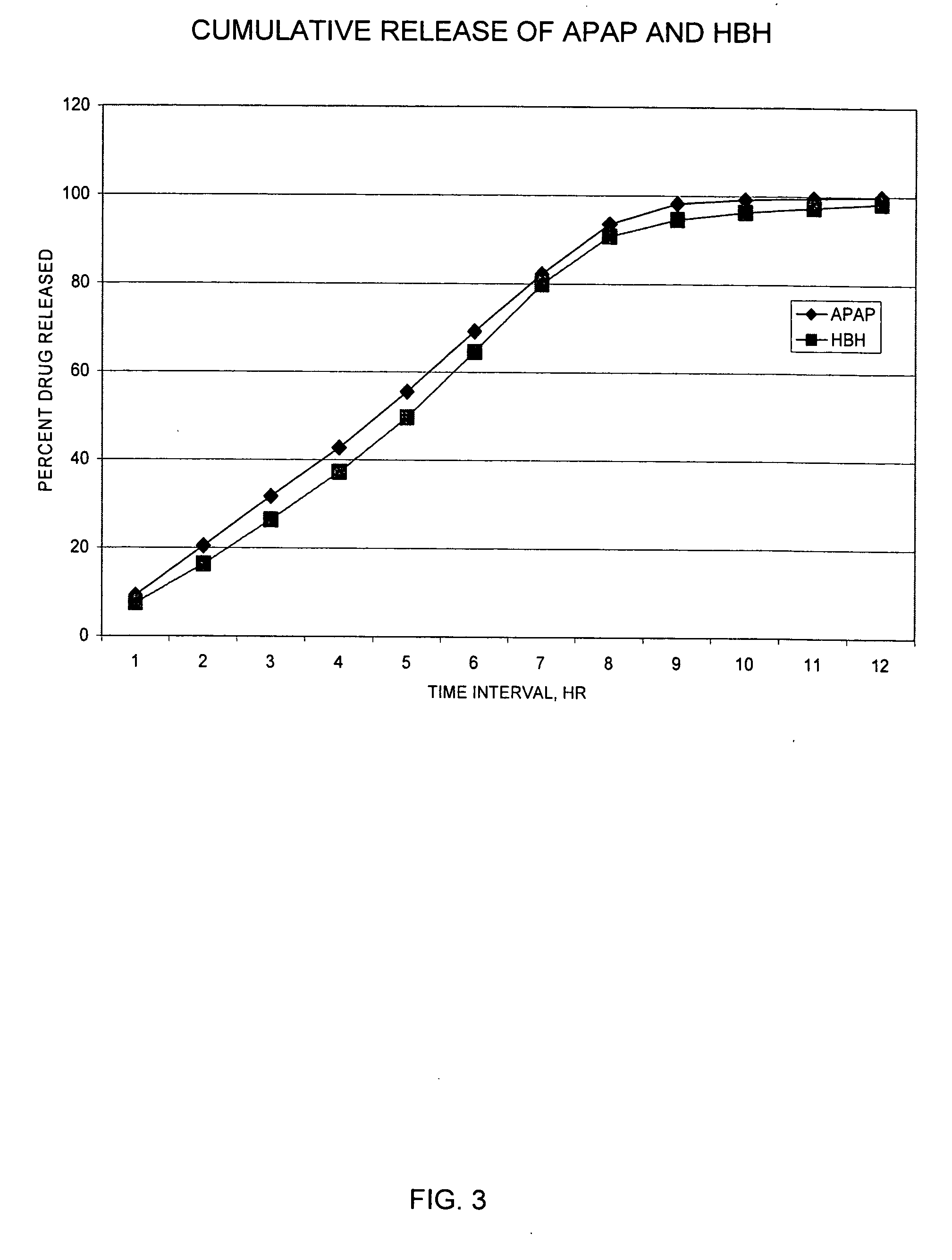
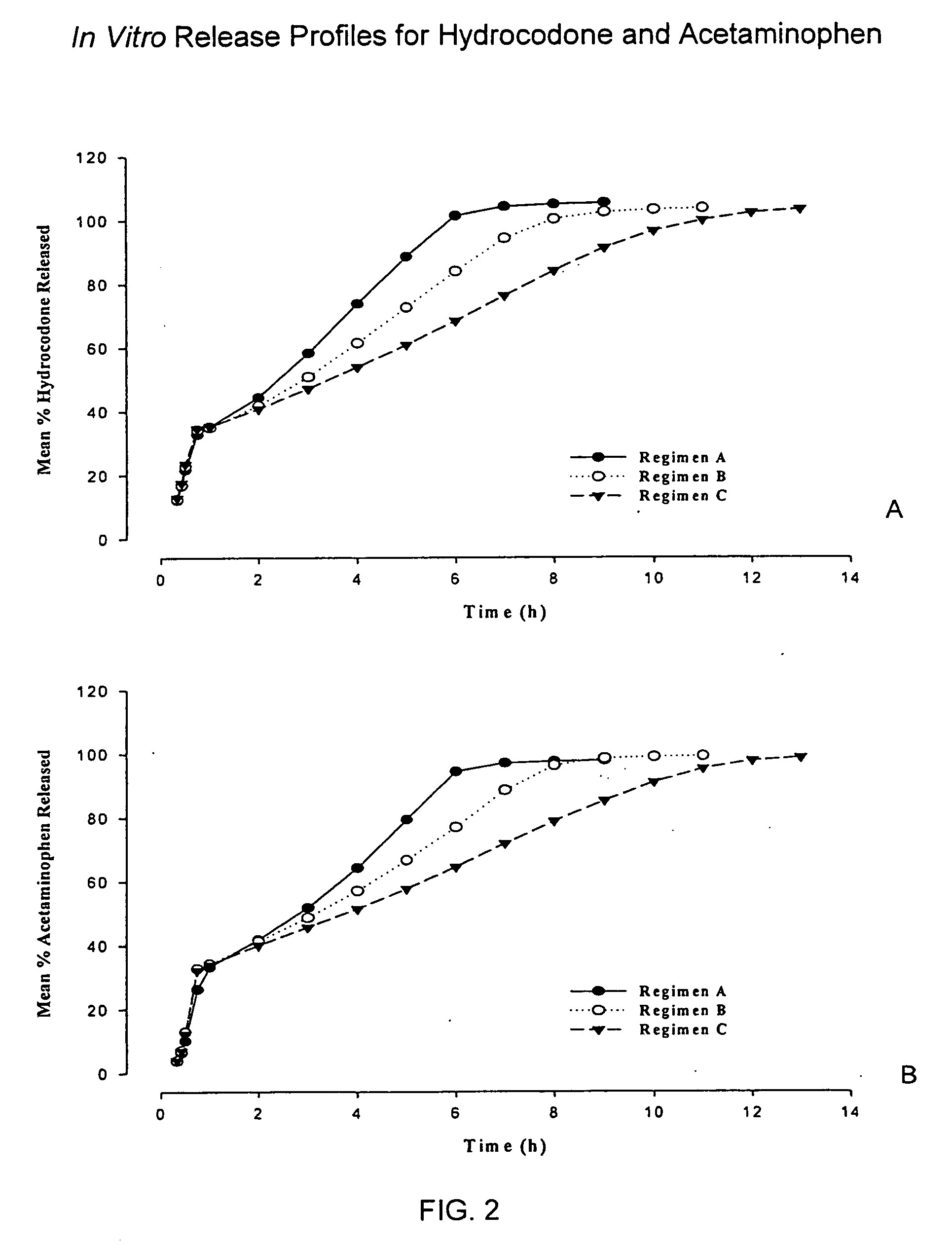
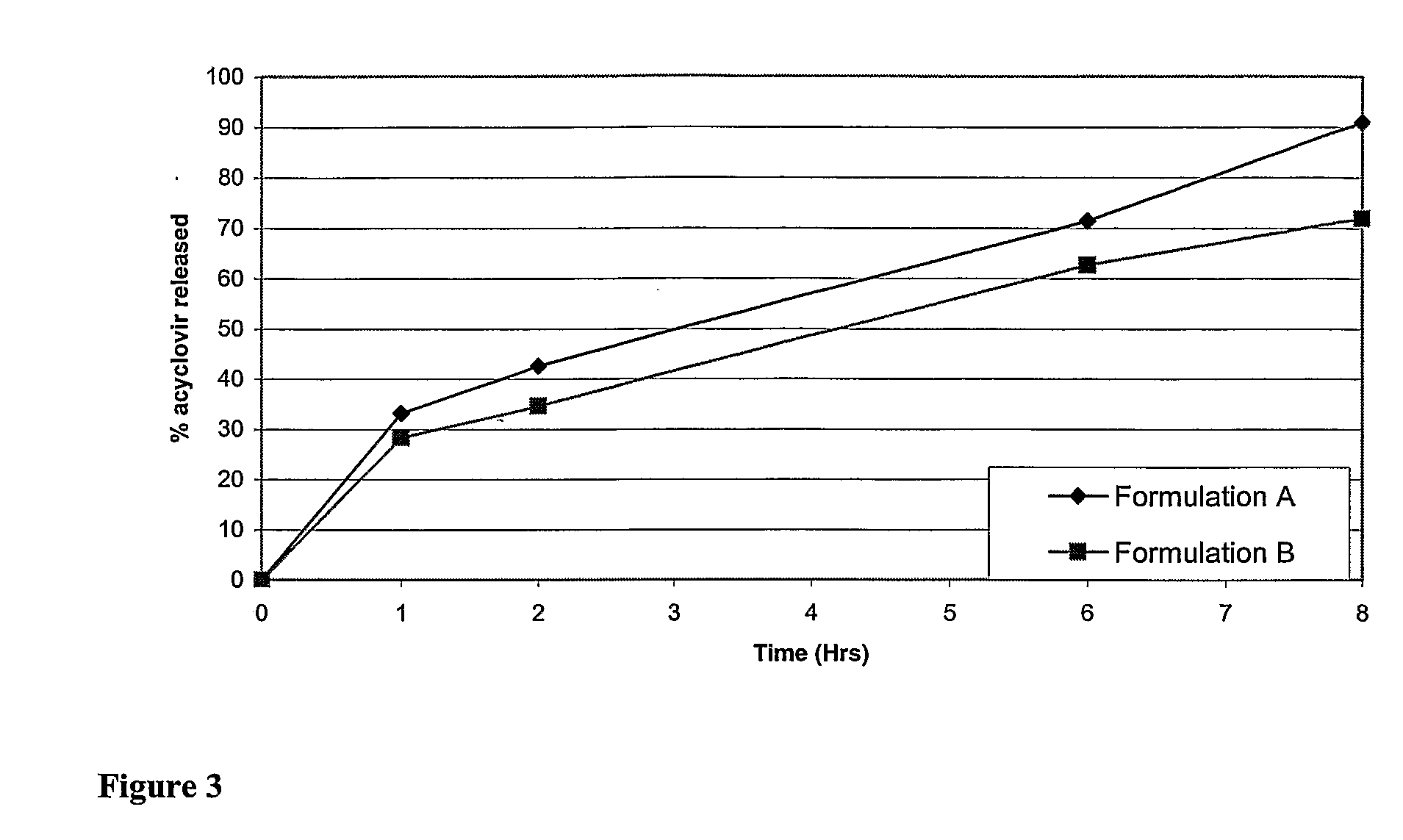
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
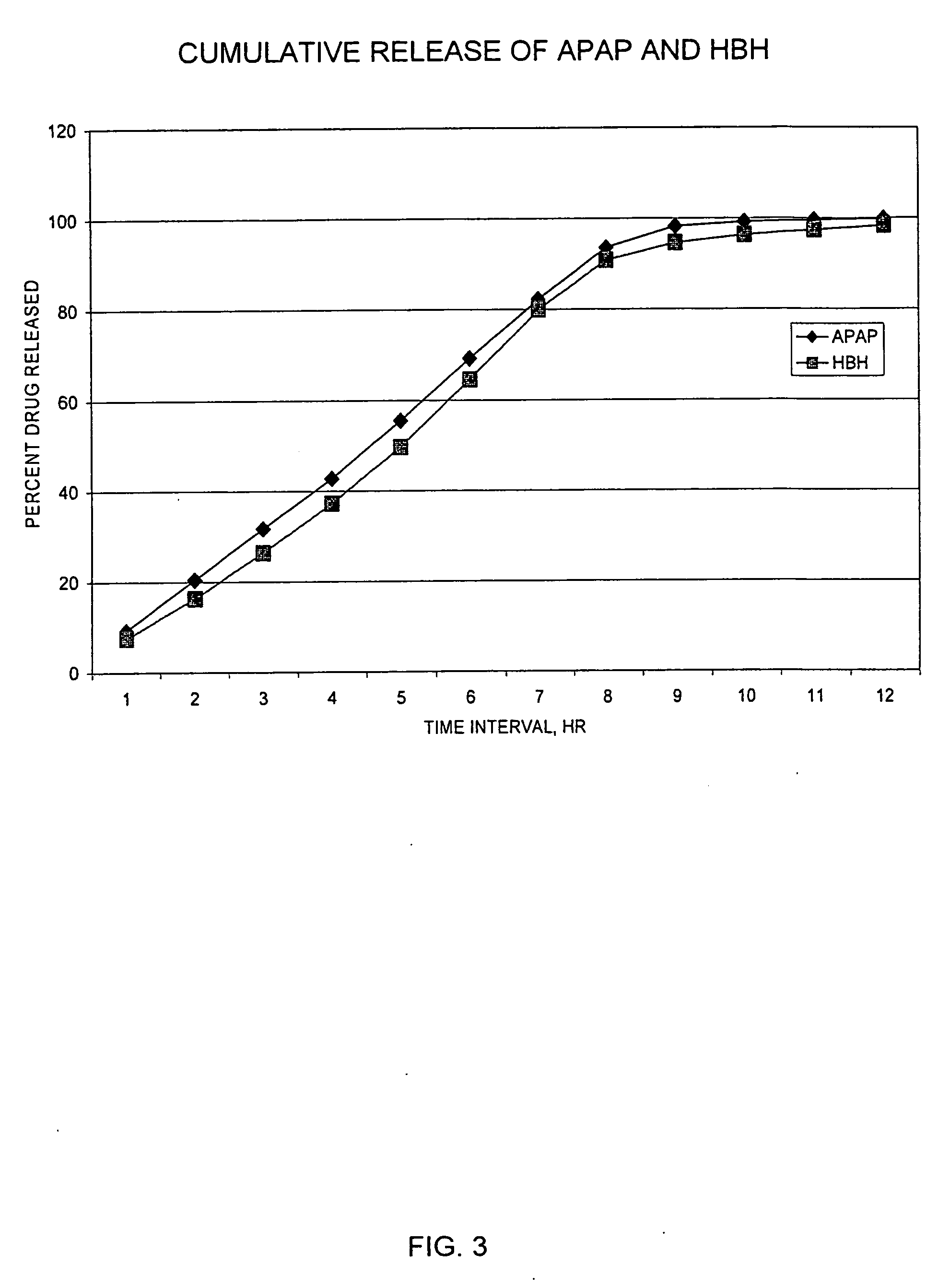
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
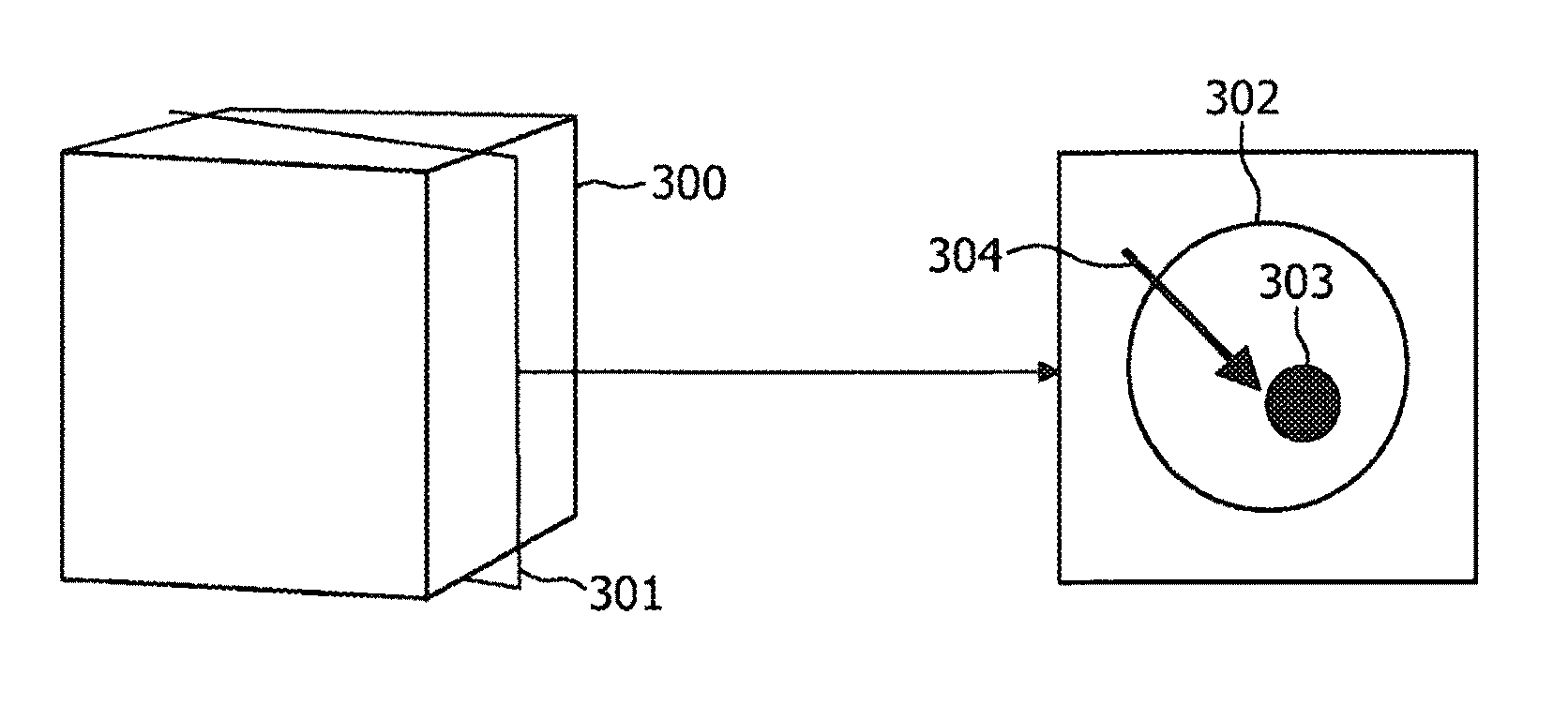
Targeting method, targeting device, computer readable medium and program element
ActiveUS8208708B2Efficient methodReduce doseMaterial analysis using wave/particle radiationRadiation/particle handlingFluoroscopic imageEntry point
According to an exemplary embodiment a targeting method for targeting a first object from an entry point to a target point in an object (110) under examination is provided, wherein the method comprises selecting a two-dimensional image (301) of the object under examination depicting the entry point (305) and the target point (303) and determining a planned path (304) from the entry point to the target point, wherein the planned path has a first direction. Furthermore, the method comprises recording data representing a fluoroscopic image of the object under examination, wherein the fluoroscopic image is recorded under a second direction so that a normal of the image coincide with the first direction and determining whether the first object is on the determined planned path based on shape and / or position of the projection of the first object in the fluoroscopic image.
Owner:KONINK PHILIPS ELECTRONICS NV
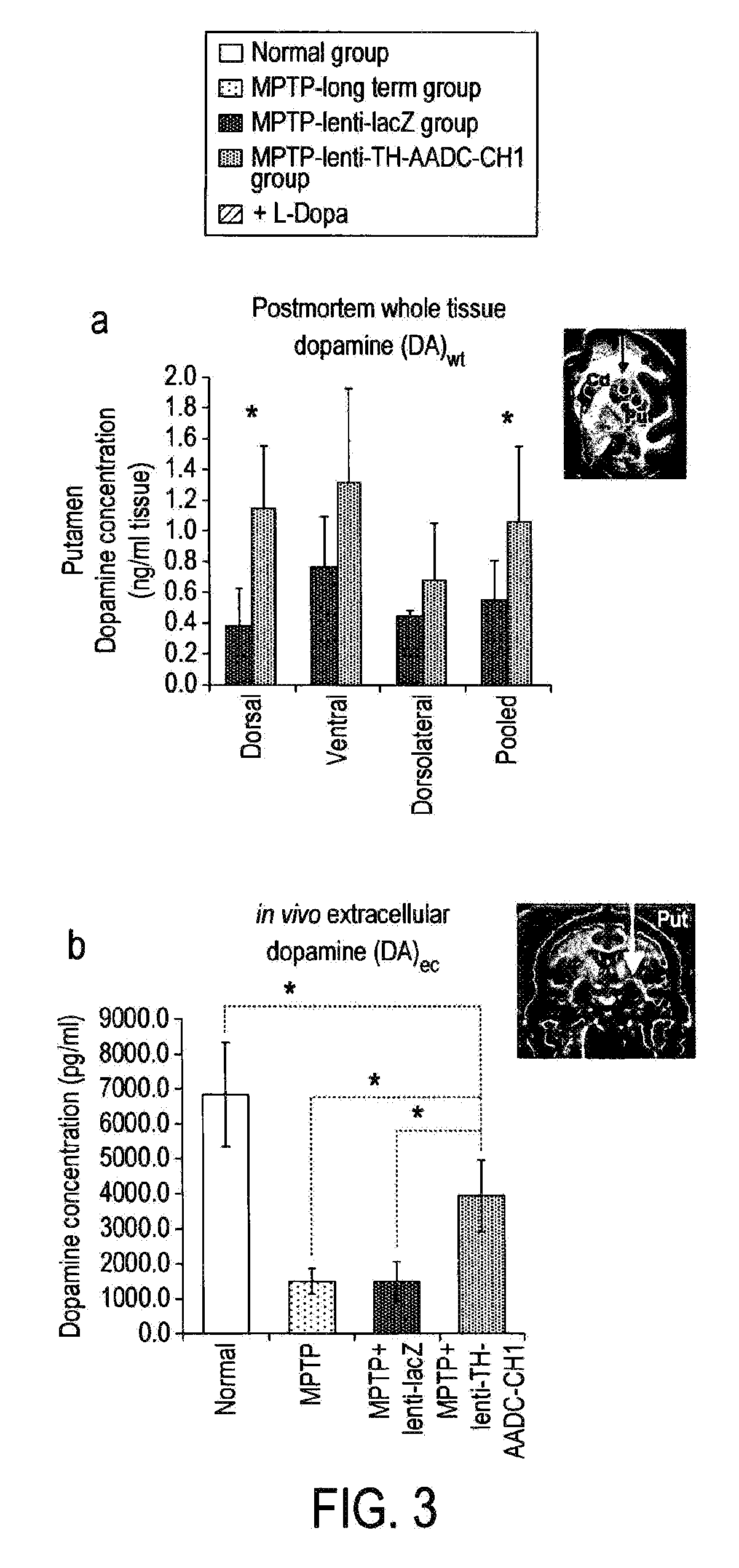
Treatment regimen for parkinson's disease
InactiveUS20120295960A1Reduce potential side effectsReduce maintenanceNervous disorderNucleic acid vectorSide effectCombination therapy
Provided is an improved treatment for Parkinson's Disease where the efficacy of L-Dopa treatment is increased by including gene therapy in the treatment regimen. The combination therapy results in long-term improvements in response to L-Dopa and diminished side effects caused by L-Dopa.
Owner:OXFORD BIOMEDICA (UK) LTD
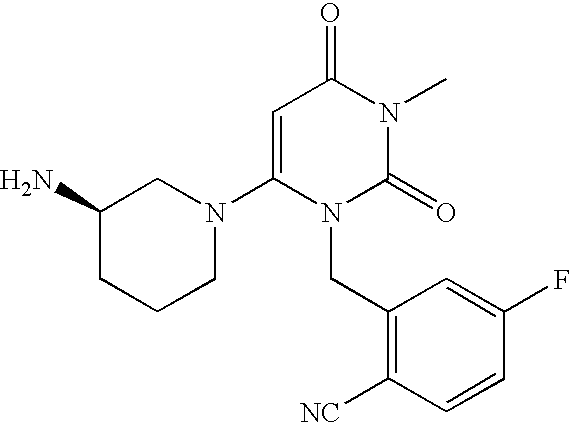
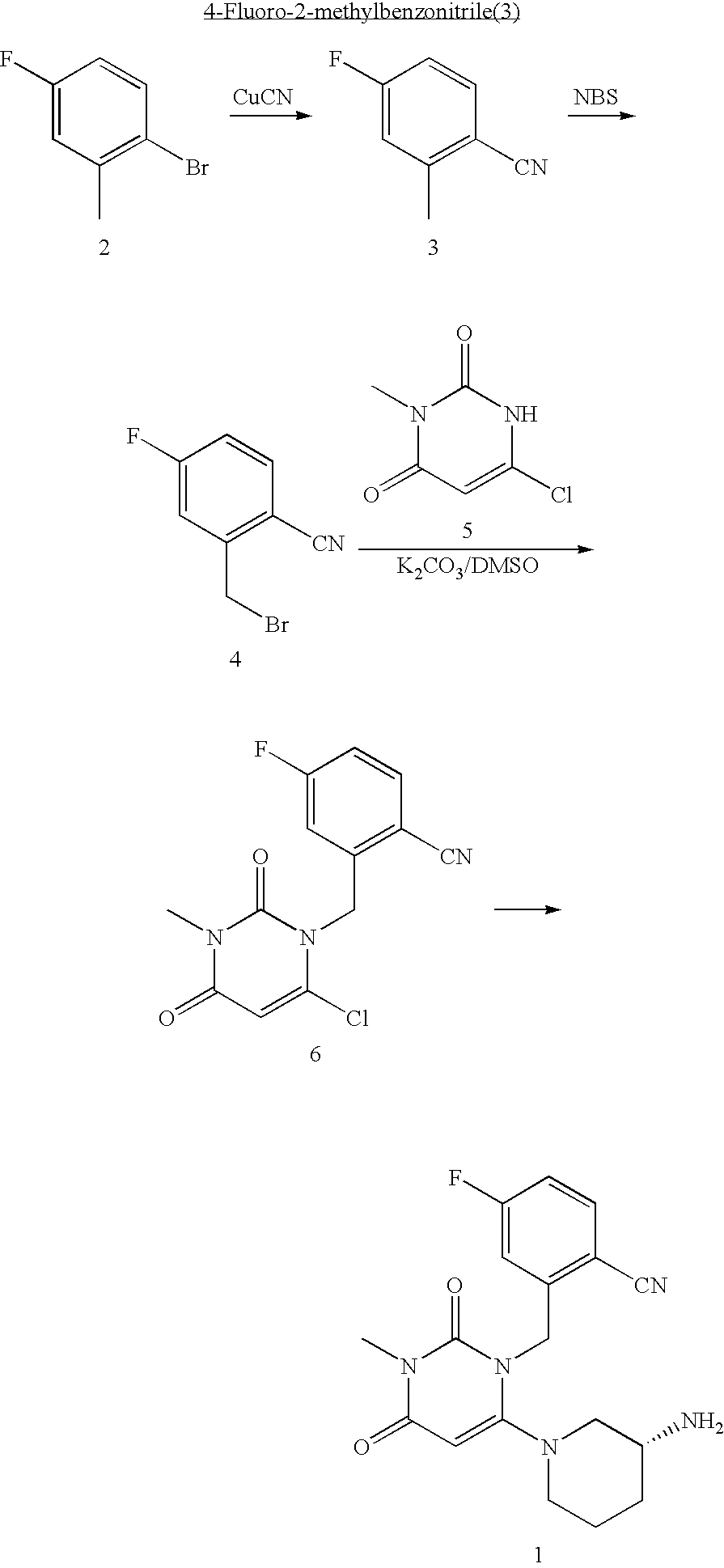
Administration of dipeptidyl peptidase inhibitors
InactiveUS20070060530A1Convenient treatmentEliminate side effectsBiocidePeptide/protein ingredientsDipeptidyl peptidaseBenzonitrile
Pharmaceutical compositions comprising 2-[6-(3-Amino-piperidin-1-yl)-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-ylmethyl]-4-fluoro-benzonitrile and pharmaceutically acceptable salts thereof are provided as well as kits and articles of manufacture comprising the pharmaceutical compositions as well as methods of using the pharmaceutical compositions.
Owner:TAKEDA PHARMA CO LTD
Biosensor with laser-sealed capillary space and method of making
ActiveUS20070278097A1Reduces dose hesitationPromotes wickingImmobilised enzymesBioreactor/fermenter combinationsEngineeringTest strips
A test strip or biosensor comprising a base substrate on which an electrode system is formed. One or more laminate layers overlie the base substrate to form a sample-receiving chamber in which a reagent is deposited. An opening is provided from the sample-receiving chamber to the exterior of the biosensor. The layers and the base substrate are laser welded to secure the biosensor. One of the layer and base substrate is light transmissive to allow laser welding at the interface therebetween. The biosensor may be formed from a series of continuous webs that are subsequently sliced to form individual biosensors.
Owner:ROCHE DIABETES CARE INC
RNA interference mediated inhibition of gene expression using chemically modified short interfering nucleic acid (siNA)
InactiveUS20070004664A1Improve various propertyModulating gene expressionBiocideOrganic active ingredientsBiological bodyNucleic acid sequencing
The present invention concerns methods and reagents useful in modulating gene expression in a variety of applications, including use in therapeutic, diagnostic, target validation, and genomic discovery applications. Specifically, the invention relates to synthetic chemically modified small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules capable of mediating RNA interference (RNAi) against target nucleic acid sequences. The small nucleic acid molecules are useful in the treatment of any disease or condition that responds to modulation of gene expression or activity in a cell, tissue, or organism.
Owner:SIRNA THERAPEUTICS INC
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20060251721A1Improved ability to treat painLess attentionBiocideNervous disorderImmediate releasePharmaceutical medicine
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
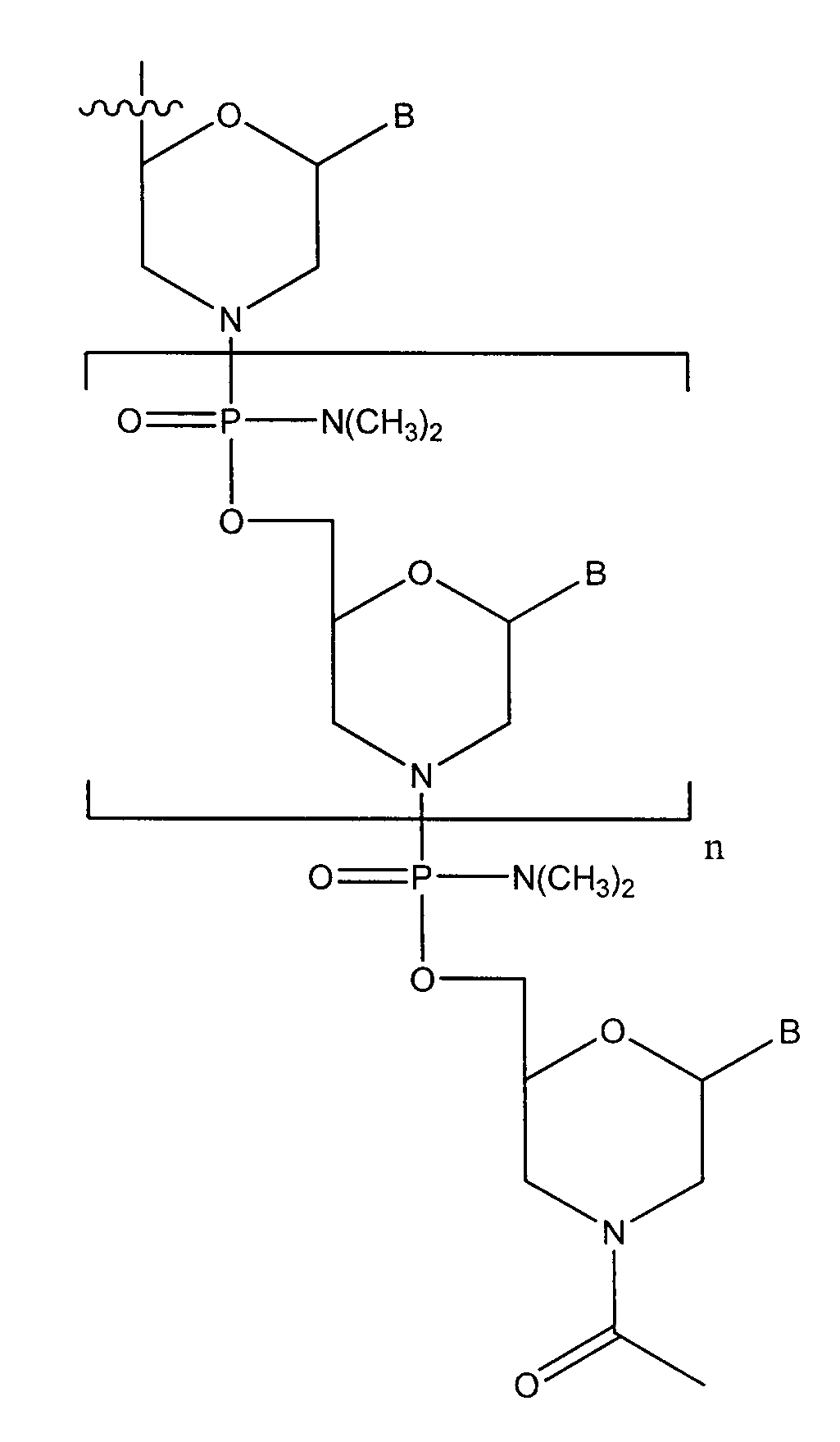
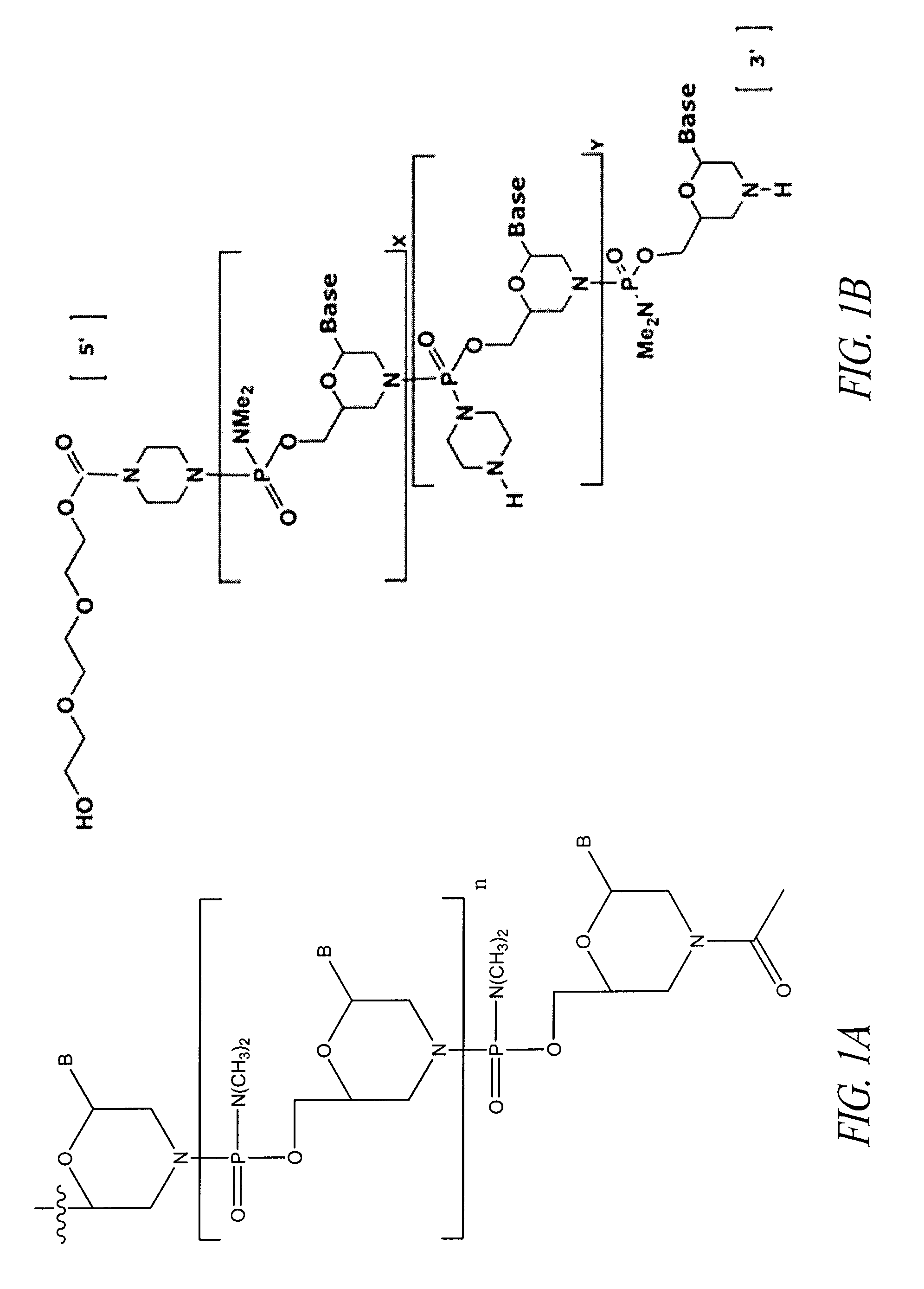
Oligonucleotide analogues having modified intersubunit linkages and/or terminal groups
ActiveUS20120065169A1Maintain good propertiesEnhanced cell deliveryAntibacterial agentsBiocideDiseaseEnd-group
Oligonucleotide analogues comprising modified intersubunit linkages and / or modified 3′ and / or 5′-end groups are provided. The disclosed compounds are useful for the treatment of diseases where inhibition of protein expression or correction of aberrant mRNA splice products produces beneficial therapeutic effects.
Owner:SAREPTA THERAPEUTICS INC
Methods and products for enhancing immune responses using imidazoquinoline compounds
InactiveUS20060188913A1Good curative effectReduce doseCompound screeningApoptosis detectionTime scheduleDisease
The invention involves administration of an imidazoquinoline agent in combination with another therapeutic agent. The combination of drugs may be administered in synergistic amounts or in various dosages or at various time schedules. The invention also relates to kits and compositions concerning the combination of drugs. The combinations can be used to enhance ADCC, stimulate immune responses and / or patient and treat certain disorders.
Owner:COLEY PHARM GRP INC +2
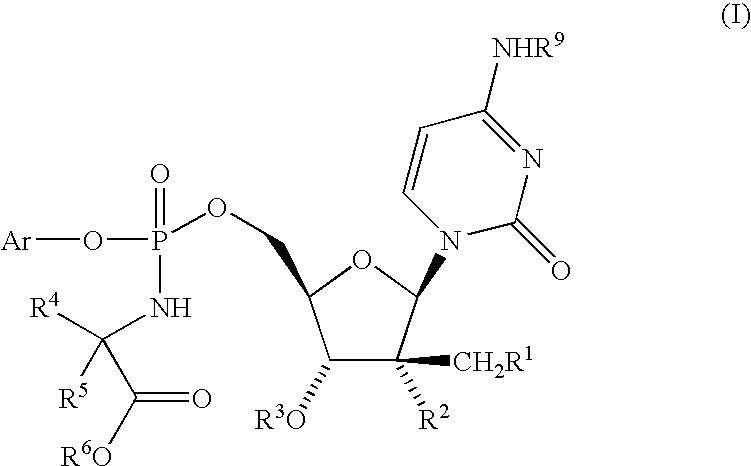
Nucleoside aryl phosphoramidates for the treatment of RNA-dependent RNA viral infection
InactiveUS7879815B2Effective penetrationLess susceptibleBiocideSugar derivativesHepatitis c viralPhosphoramidate
Owner:MSD ITAL +1
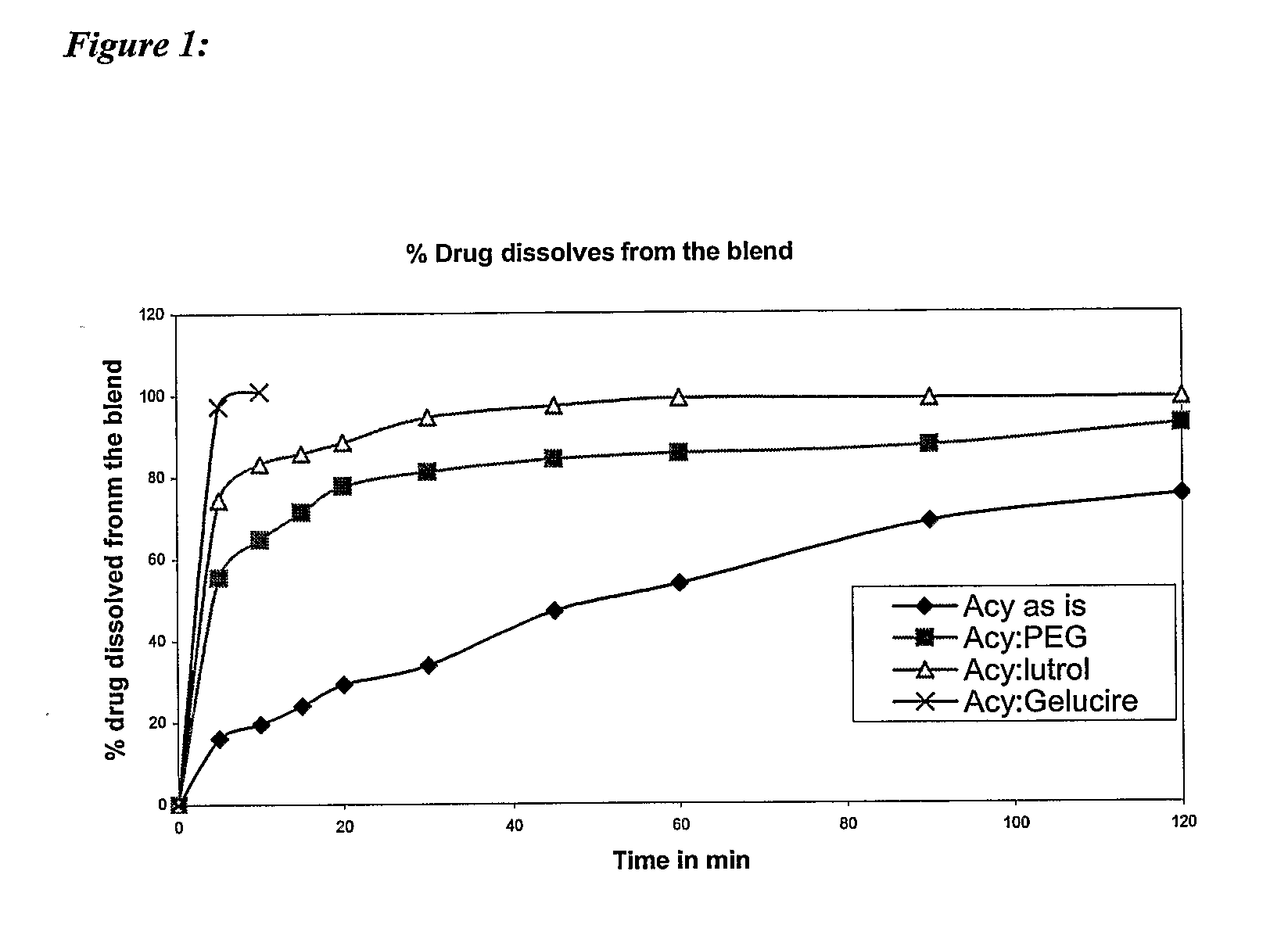
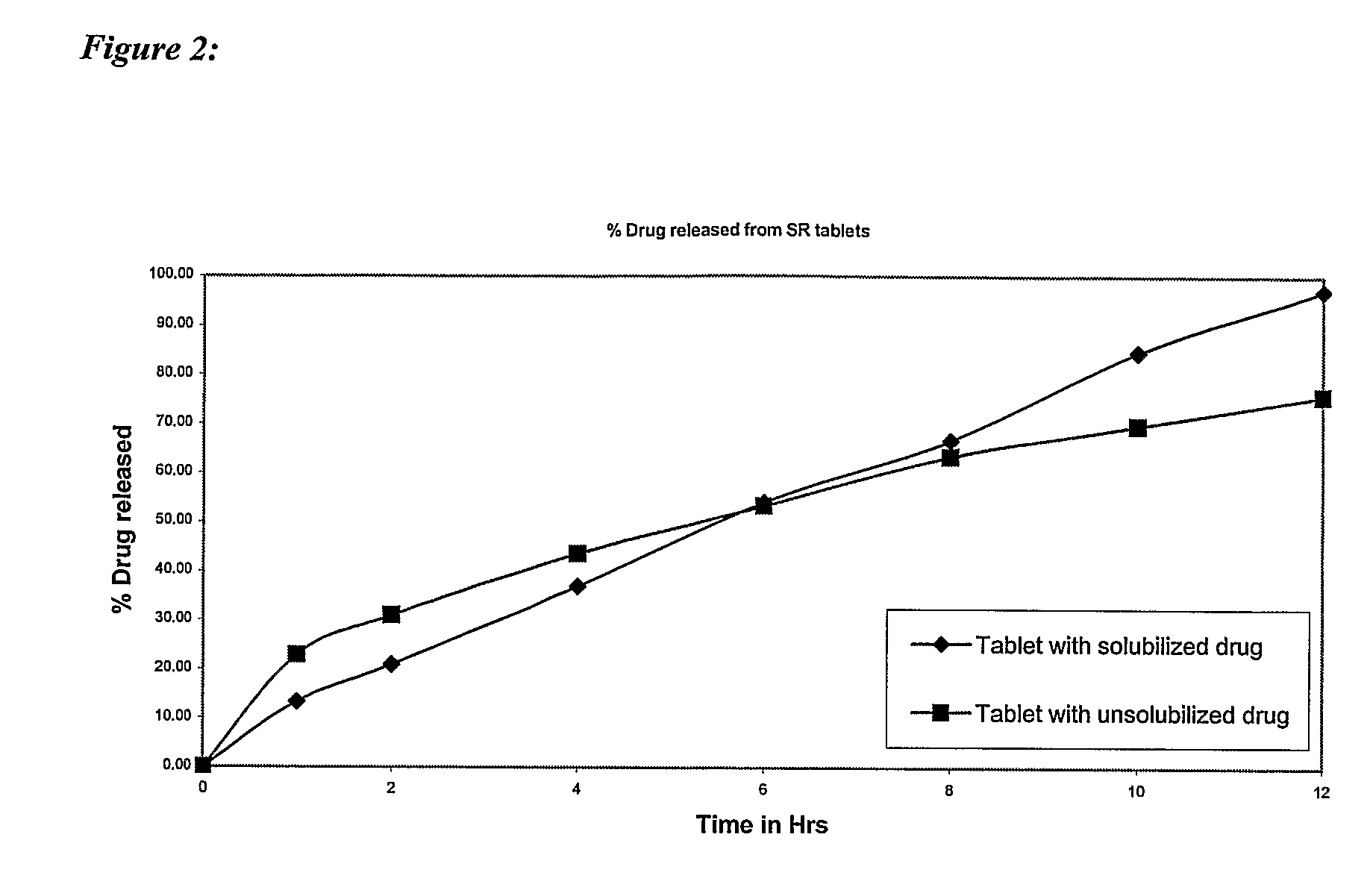
Controlled release pharmaceutical compositions with improved bioavailability
PendingUS20070196396A1Efficient retentionImprove bioavailabilityHeavy metal active ingredientsBiocideControlled releaseActive agent
The present invention provides a controlled release oral pharmaceutical composition having a therapeutically effective amount of one or more pharmacologically active agent having low bioavailability; one or more solubilizers; one or more biocompatible swelling agents; and a swelling enhancer. The swelling agent, in combination with swelling enhancer, swells in the presence of water in gastric fluid such that the size of the dosage form is sufficiently increased to provide retention of the dosage form in the stomach of a patient, which gradually erodes within the gastrointestinal tract over a prolonged time period.
Owner:RUBICON RES PTY LTD
Flat-panel detector with avalanche gain
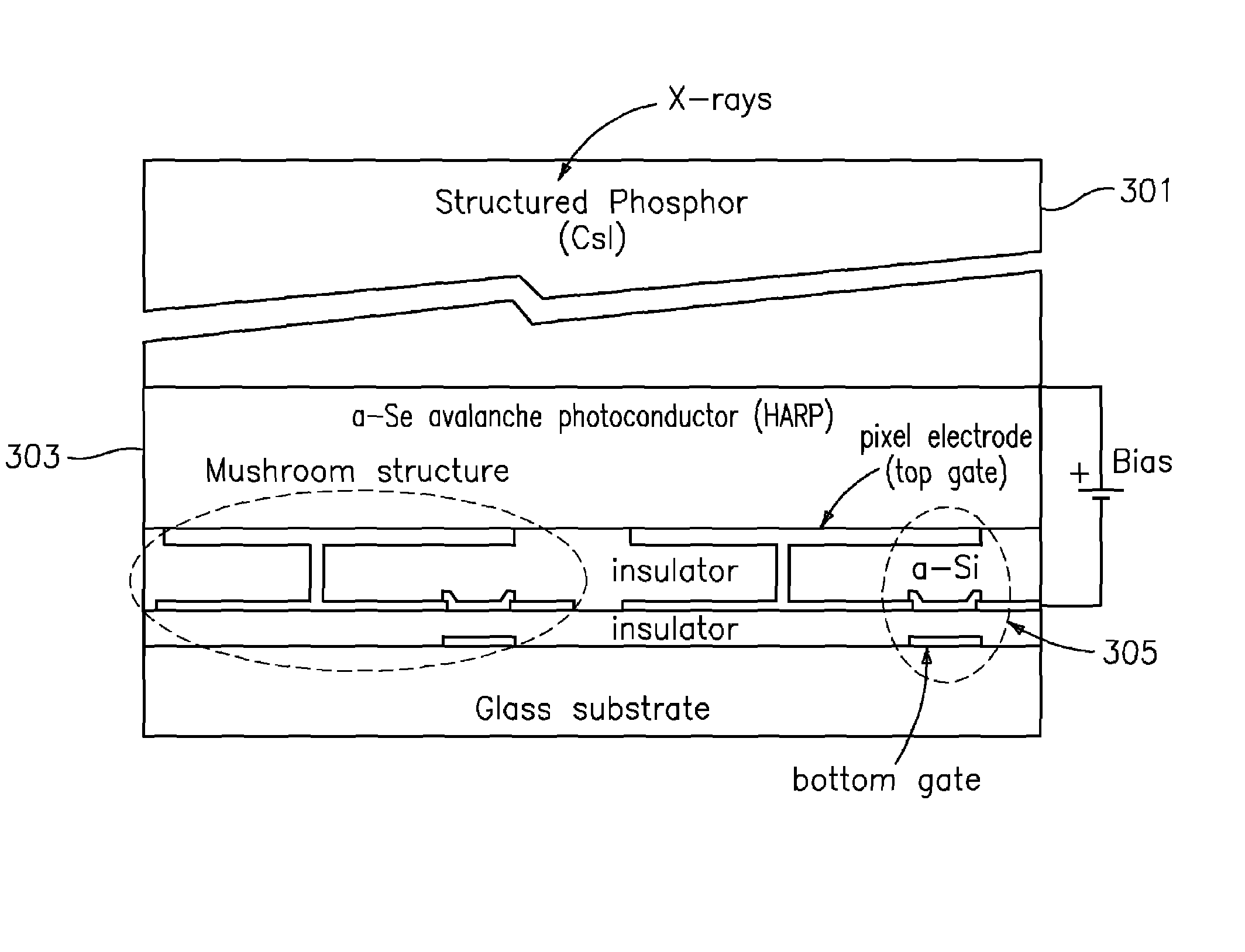
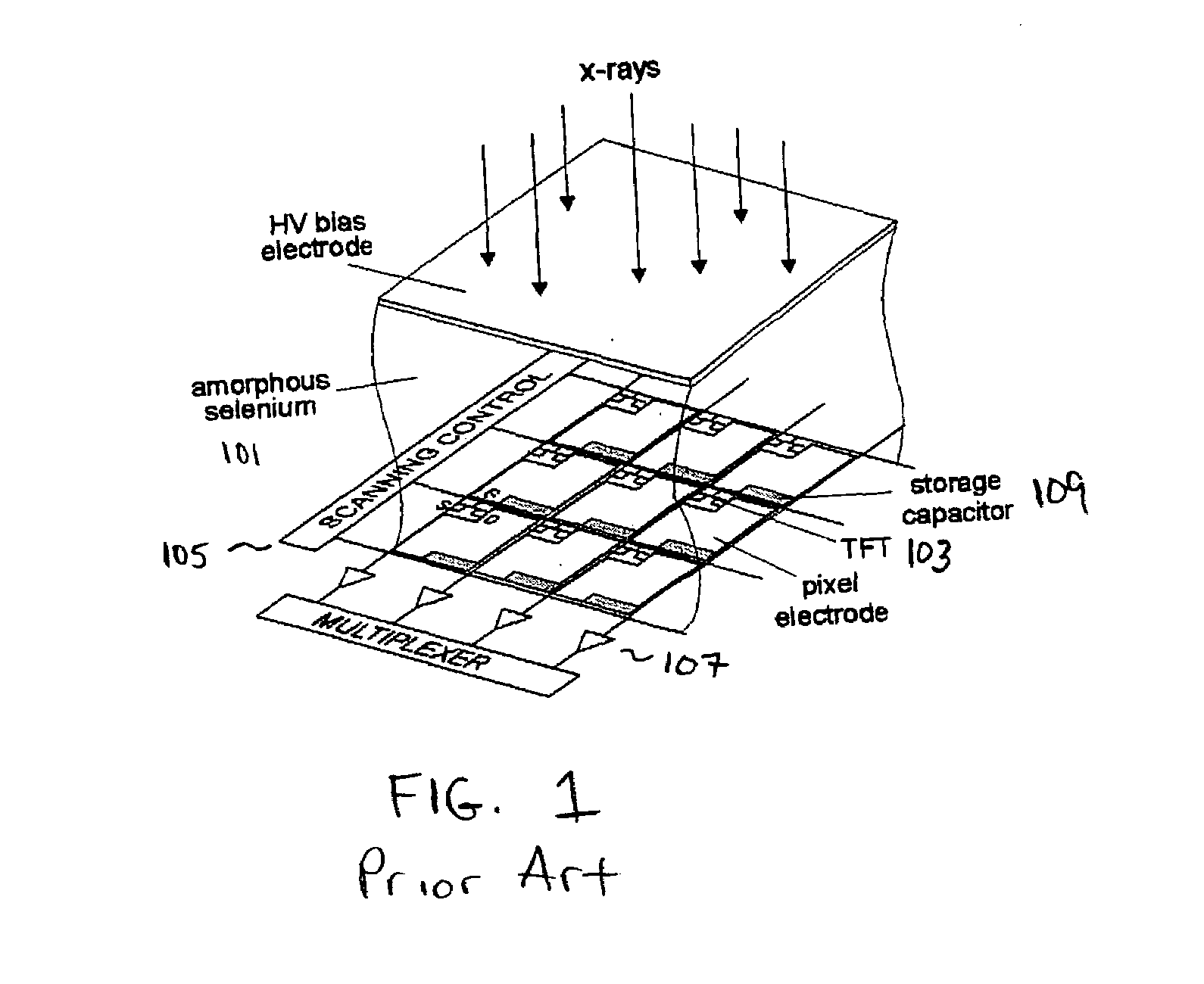
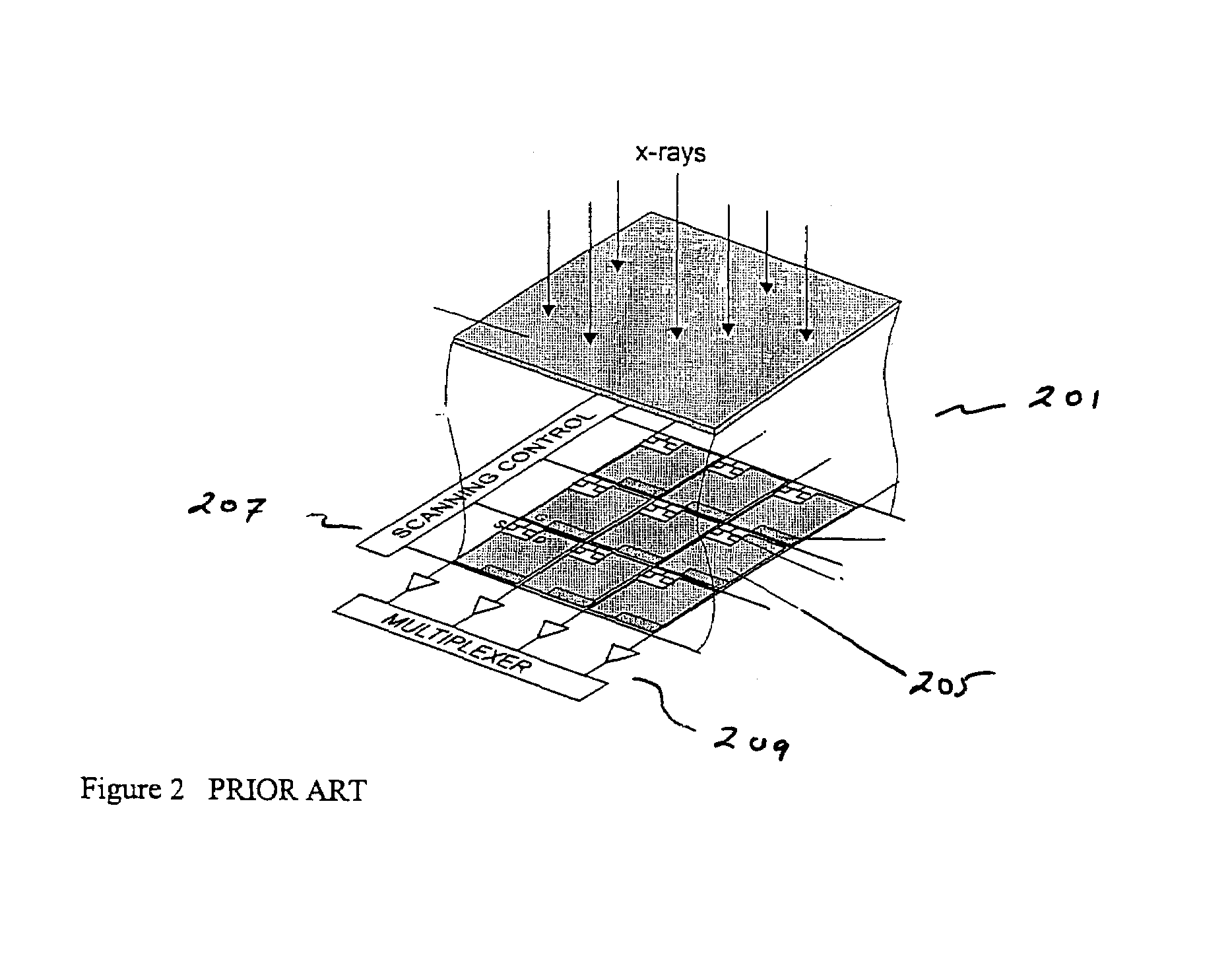
ActiveUS7323692B2Improve image qualityReduce doseSolid-state devicesMaterial analysis by optical meansFlat panel detectorAudio power amplifier
The present invention is an indirect AMFPI wherein a phosphor such as a structured cesium iodide (CsI) is used to convert x-ray energy to optical photons or a charge, which is then detected by a two-dimensional array of either thin-film transistors (TFTs) such as an amorphous a-Se TFTs or a photodiode array. A scanning control circuit generates pulses to turn on the TFTs one row at a time, and thus the charge in the individual arrays is transferred from the TFT to one or more external charge-sensitive amplifiers. The charge-sensitive amplifiers are shared by all the pixels in the same column. The two-dimensional array can be read in real time.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
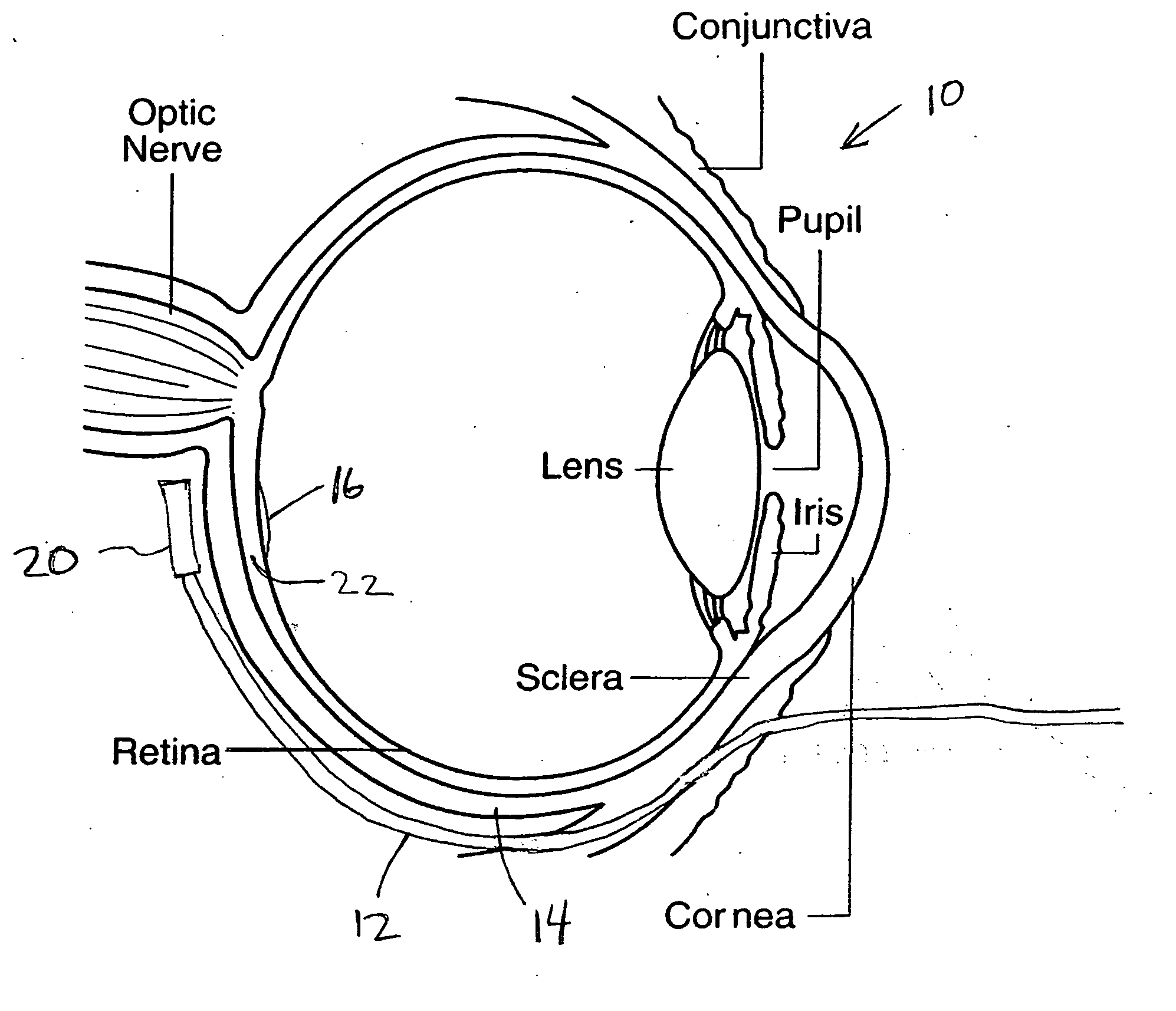
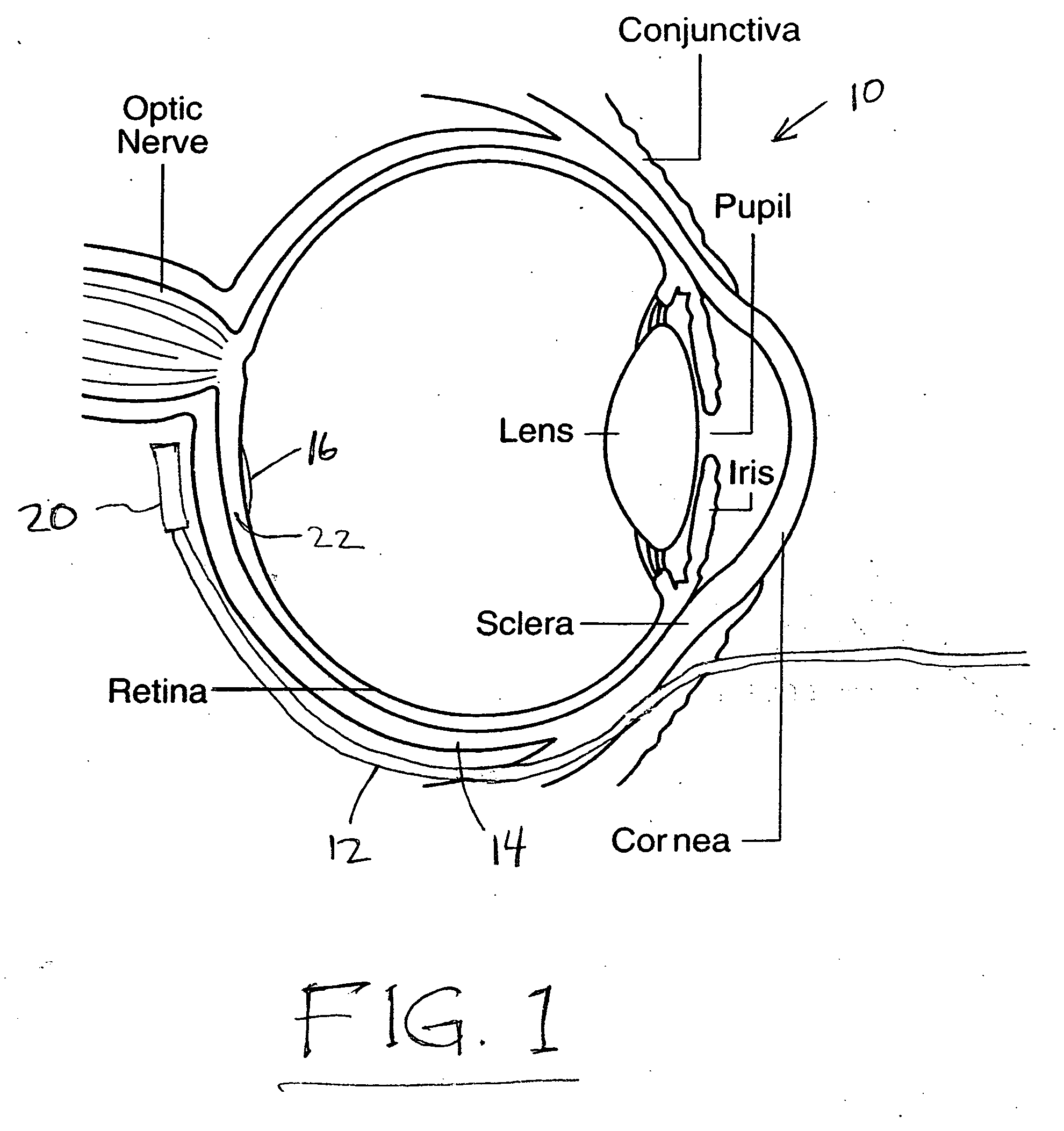
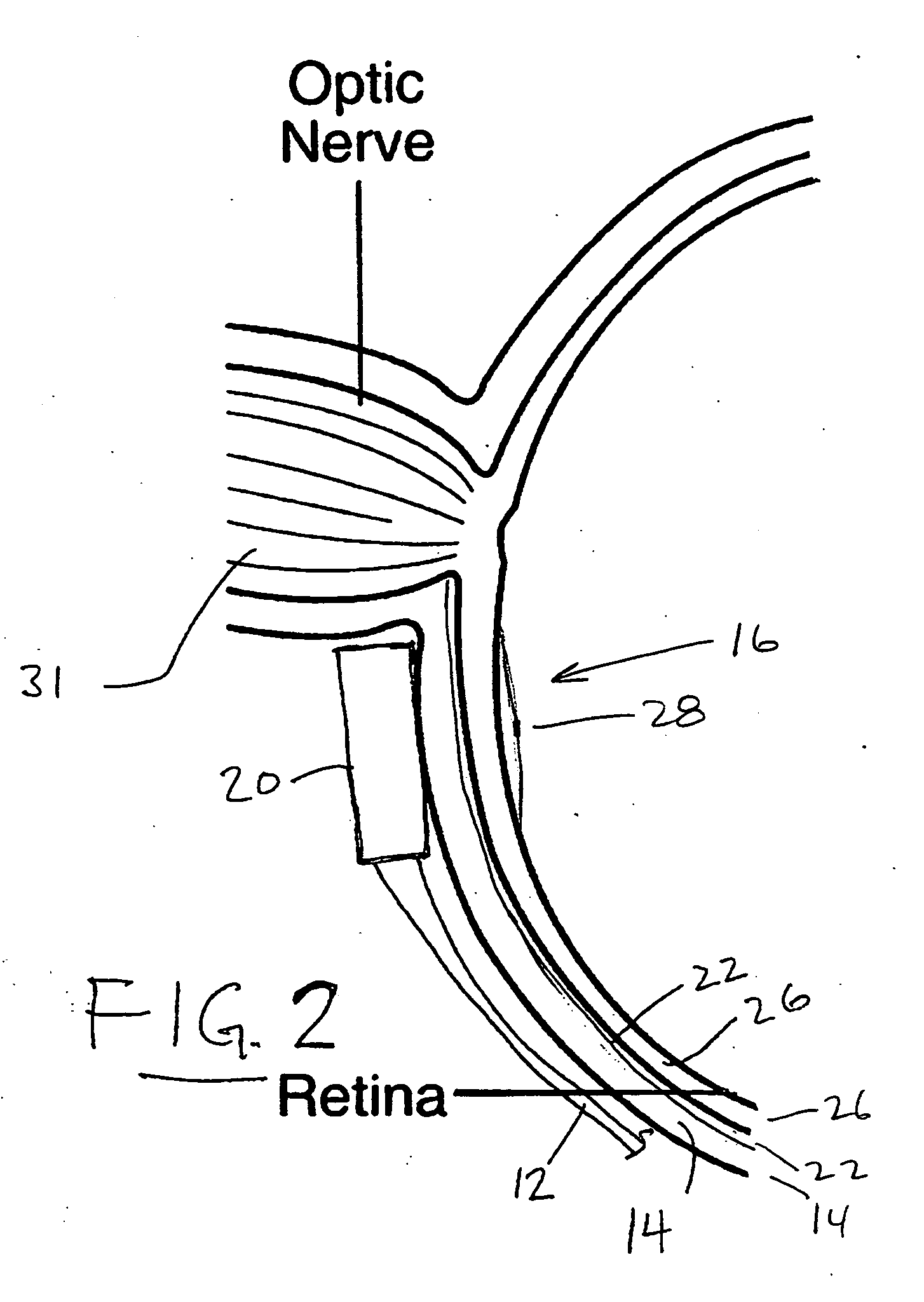
Treatment of age-related macular degeneration
Owner:XOFT INC +1
Azepane derivatives and methods of treating hepatitis B infections
ActiveUS9181288B2Reduce doseReduce frequencyPeptide/protein ingredientsGroup 5/15 element organic compoundsMedicineAzepane
Owner:NOVIRA THERAPEUTICS
Self-emulsifying composition of OMEGA3 fatty acid
ActiveUS8618168B2Maintain good propertiesAvoid high concentrationsBiocideNervous disorderHydrophilic-lipophilic balanceSelf emulsifying
This invention provides a self-emulsifying composition comprising 50 to 95% by weight in total of at least one compound selected from the group consisting of ω3 polyunsaturated fatty acids and their pharmaceutically acceptable salts and esters; and 5 to 50% by weight of an emulsifier having a hydrophilic lipophilic balance of at least 10. The composition has no or reduced ethanol content, and exhibits excellent self-emulsifying property, dispersibility in the composition, emulsion stability, and absorption property. The composition is adapted for use as a drug.
Owner:MOCHIDA PHARM CO LTD
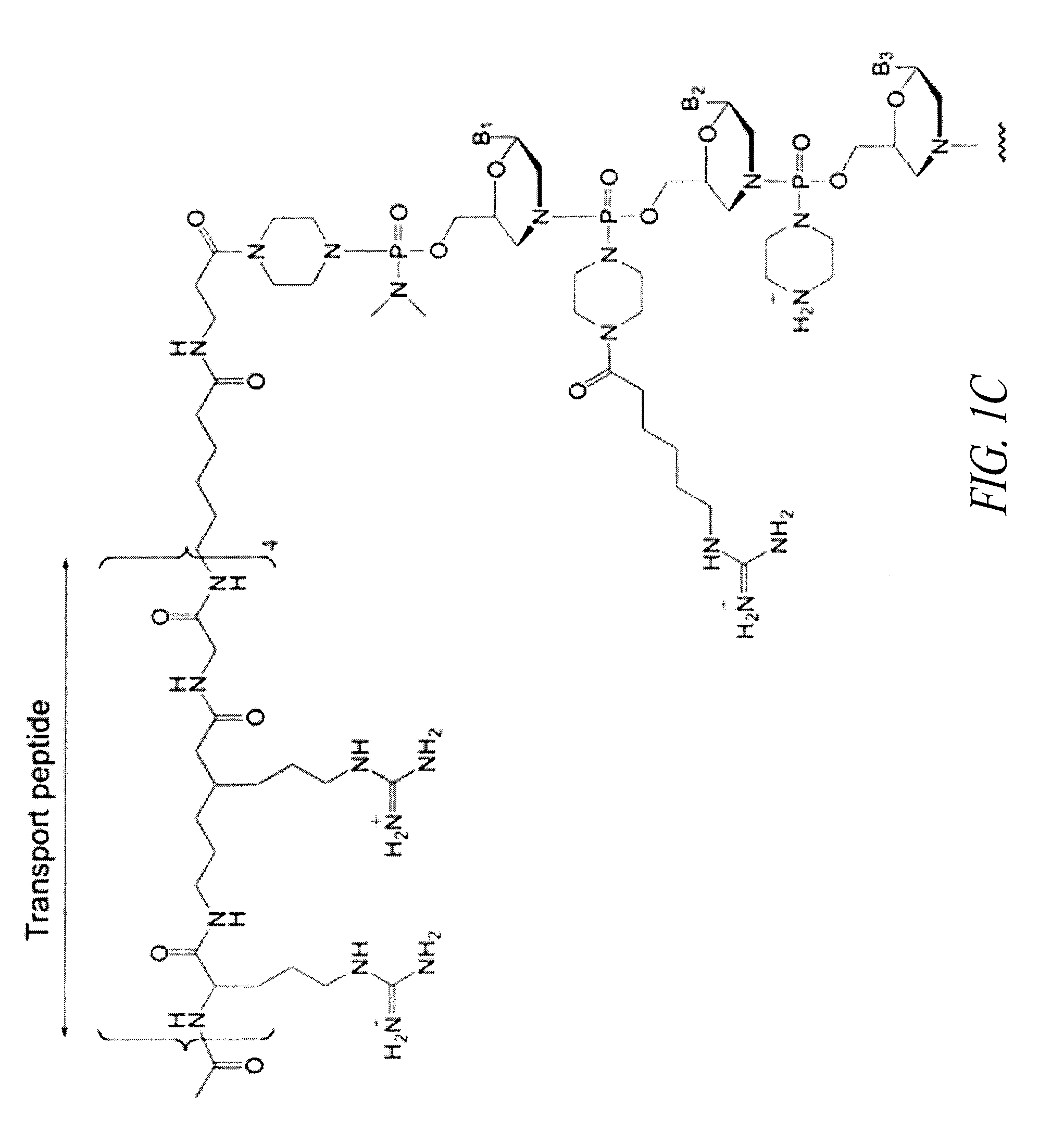
Peptide oligonucleotide conjugates
ActiveUS20120289457A1Easy to transportImprove propertiesAntibacterial agentsOrganic active ingredientsDiseaseADAMTS Proteins
Oligonucleotide analogues conjugated to carrier peptides are provided. The disclosed compounds are useful for the treatment of various diseases, for example diseases where inhibition of protein expression or correction of aberrant mRNA splice products produces beneficial therapeutic effects.
Owner:SAREPTA THERAPEUTICS INC
Codon-optimized polynucleotide-based vaccines against human cytomegalovirus infection
InactiveUS20080085870A1Reduce in quantityDecreased immunological responseOrganic active ingredientsPeptide/protein ingredientsAntigenAdjuvant
The invention is related to polynucleotide-based cytomegalovirus vaccines. In particular, the invention is plasmids operably encoding HCMV antigens, in which the naturally-occurring coding regions for the HCMV antigens have been modified for improved translation in human or other mammalian cells through codon optimization. HCMV antigens which are useful in the invention include, but are not limited to pp65, glycoprotein B (gB), IE1, and fragments, variants or derivatives of either of these antigens. In certain embodiments, sequences have been deleted, e.g., the Arg435-Lys438 putative kinase in pp65 and the membrane anchor and endocellular domains in gB. The invention is further directed to methods to induce an immune response to HCMV in a mammal, for example, a human, comprising delivering a plasmid encoding a codon-optimized HCMV antigen as described above. The invention is also directed to pharmaceutical compositions comprising plasmids encoding a codon-optimized HCMV antigen as described above, and further comprising adjuvants, excipients, or immune modulators.
Owner:VICAL INC
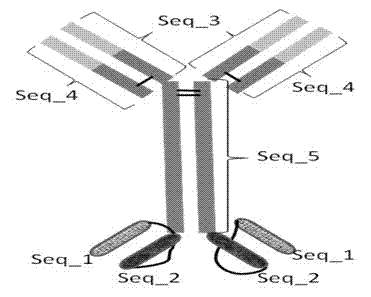
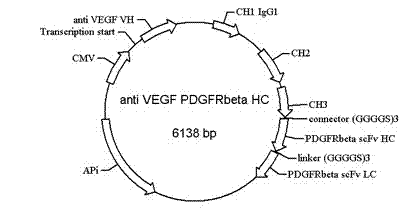
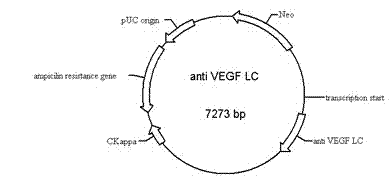
Bispecific antibody to VEGF/PDGFR beta and application thereof
InactiveCN102250246AInhibition of newbornsGood tumor activityHybrid immunoglobulinsAntibody ingredientsSingle-Chain AntibodiesBispecific monoclonal antibody
The invention relates to a medicine of a bispecific monoclonal antibody, and especially to a medicine of a bispecific monoclonal antibody to human vascular endothelial growth factor (VEGF / VEGF-A) and platelet-derived growth factor receptor (PDGFR) for resistance to angiogenesis of tumor. The bispecific antibody to VEGF / PDGFR beta provided in the invention is characterized in that: a monoclonal antibody to VEGF is used as the base for the antibody and a single chain antibody to PDGFR beta is connected with the terminal of FC segment of the monoclonal antibody to VEGF to form the bispecific antibody to VEGF / PDGFR beta. The bispecific antibody related to in the invention is obtained by employing technical means like gene engineering and constructing antibody segments which identify VEGF and PDGFR beta in a same antibody molecule that can be specifically bound with the two antibody segments; the effect of the bispecific antibody on inhibiting angiogenesis of tumor issue is obviously superior to that of a single antibody to VEGF; and the bispecific antibody has good activity in resisting tumors.
Owner:CHANGZHOU ADAM BIOTECH
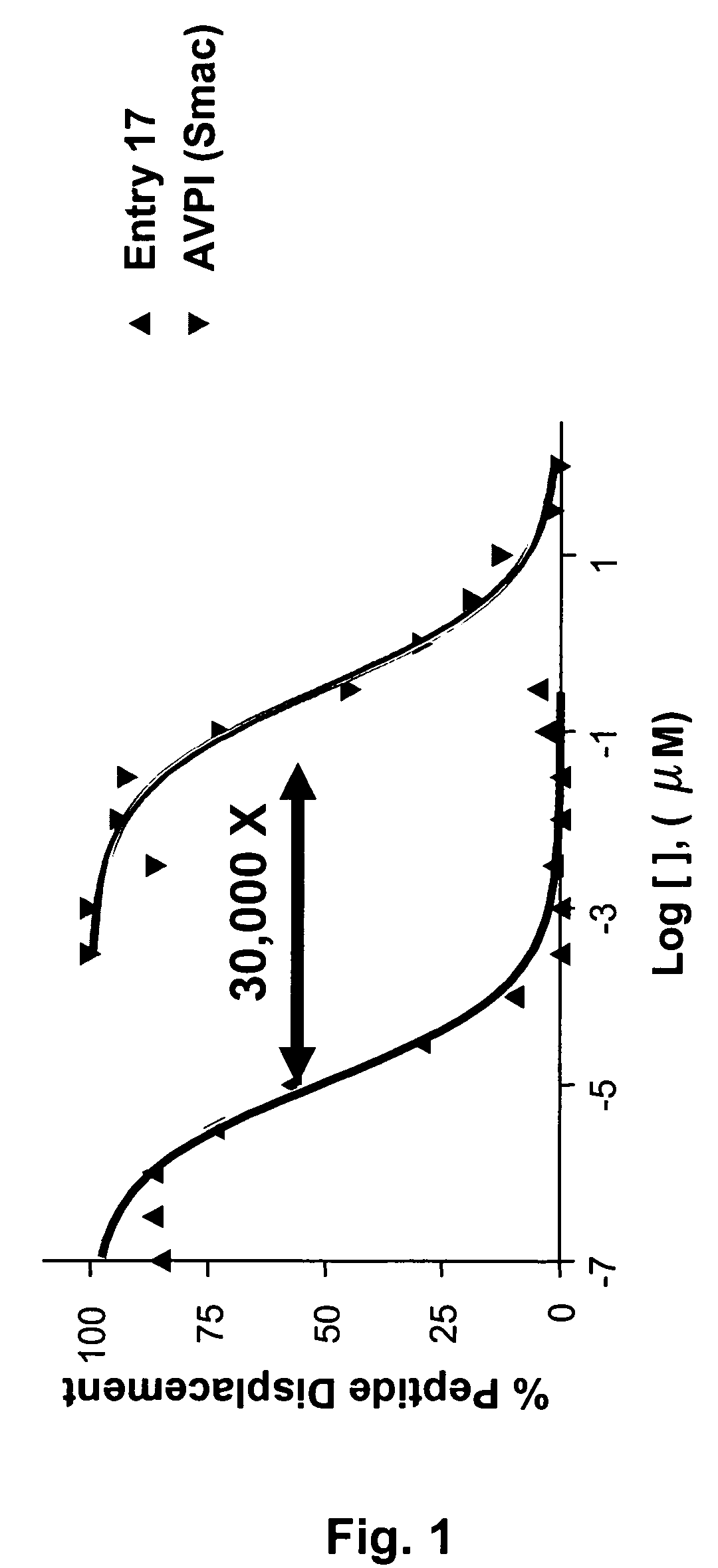
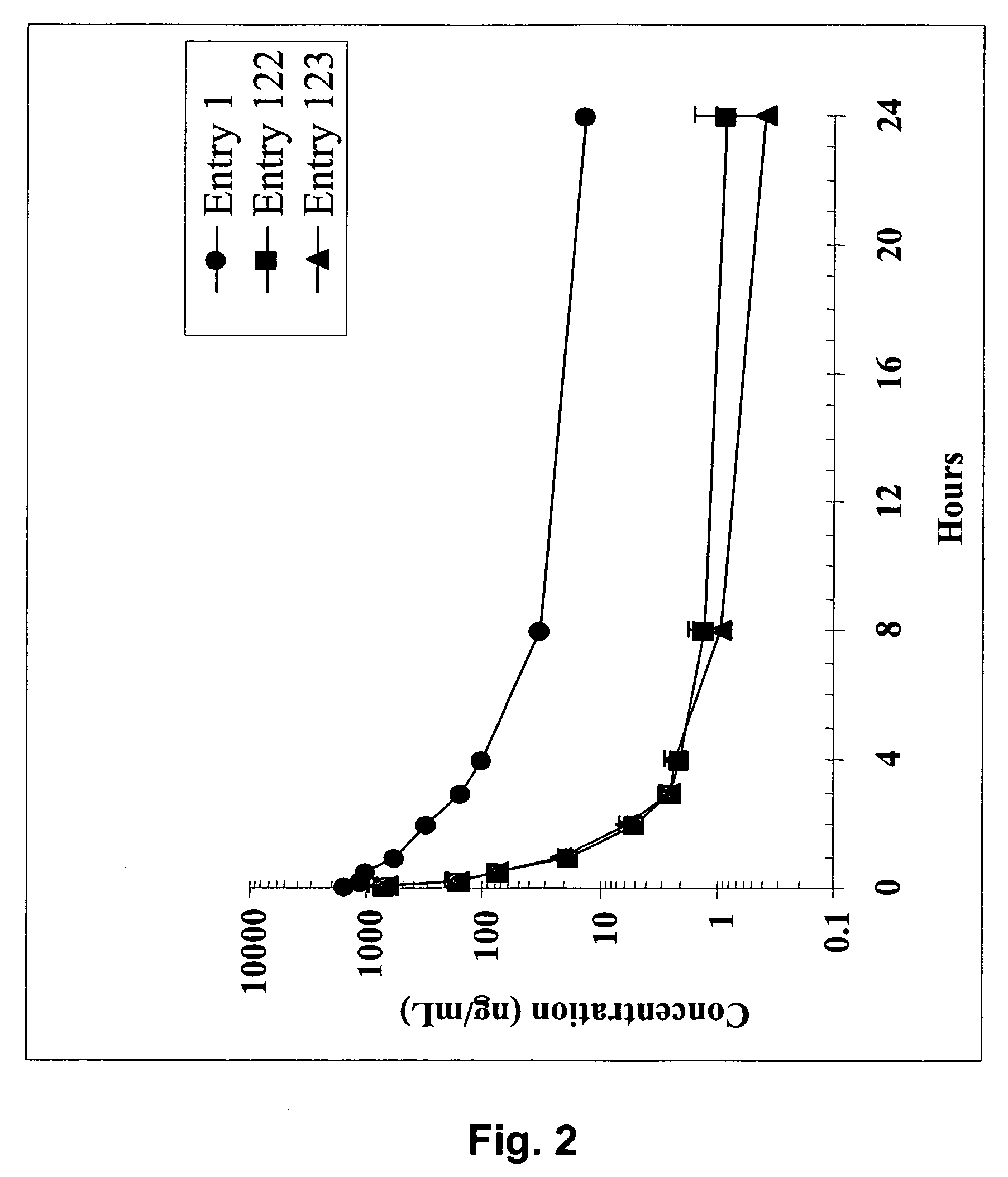
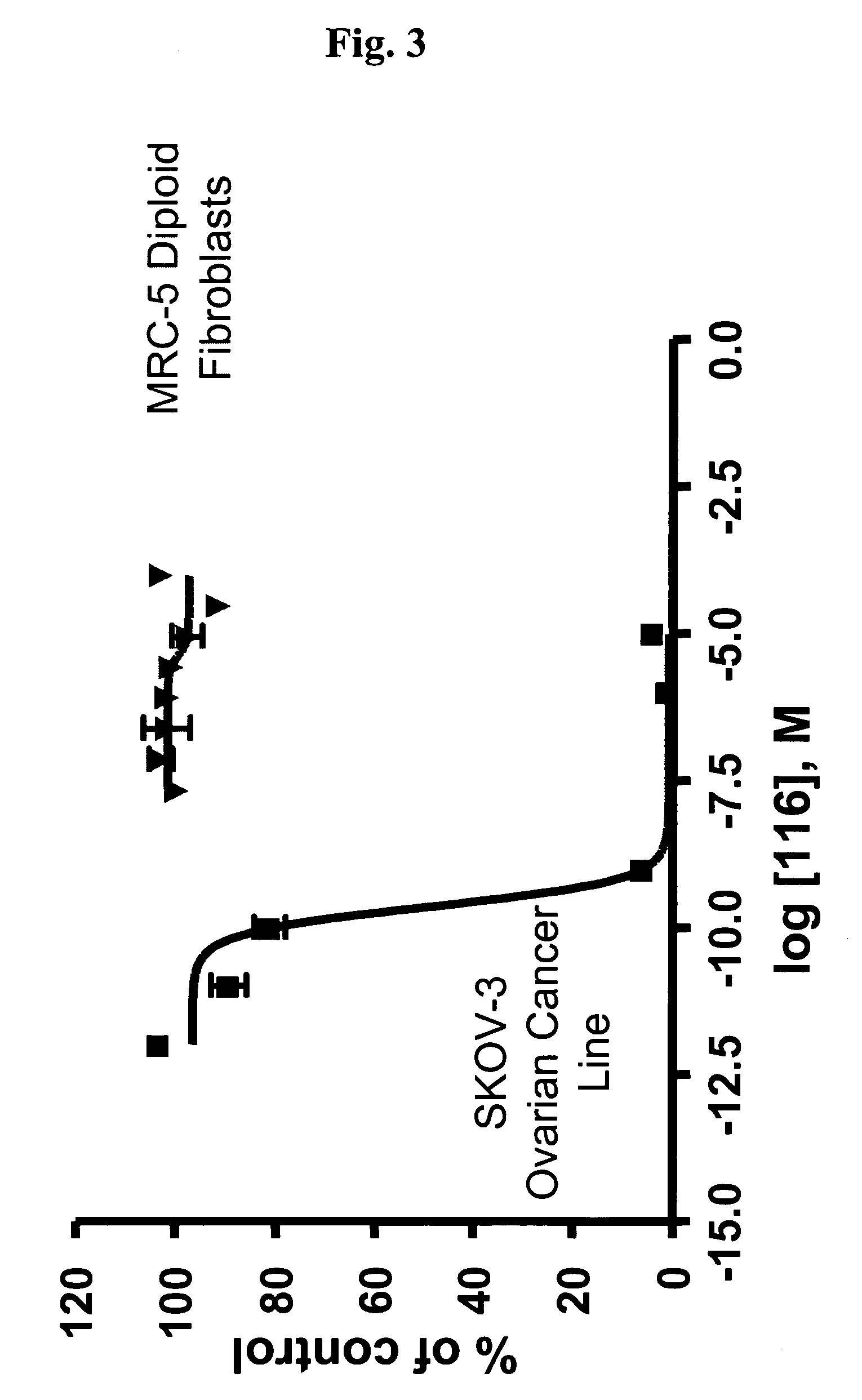
Dimeric IAP inhibitors
ActiveUS7517906B2High sensitivityAccelerated deathOrganic active ingredientsBiocideSmac mimeticsTopoisomerase inhibitor
Molecular mimics of Smac are capable of modulating apoptosis through their interaction with cellular IAPs (inhibitor of apoptosis proteins). The mimetics are based on a monomer or dimer of the N-terminal tetrapeptide of IAP-binding proteins, such as Smac / DIABLO, Hid, Grim and Reaper, which interact with a specific surface groove of IAP. Also disclosed are methods of using these peptidomimetics for therapeutic purposes. In various embodiments of the invention the Smac mimetics of the invention are combined with chemotherapeutic agents, including, but not limited to topoisomerase inhibitors, kinase inhibitors, NSAIDs, taxanes and platinum containing compounds use broader language
Owner:MEDIVIR AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com