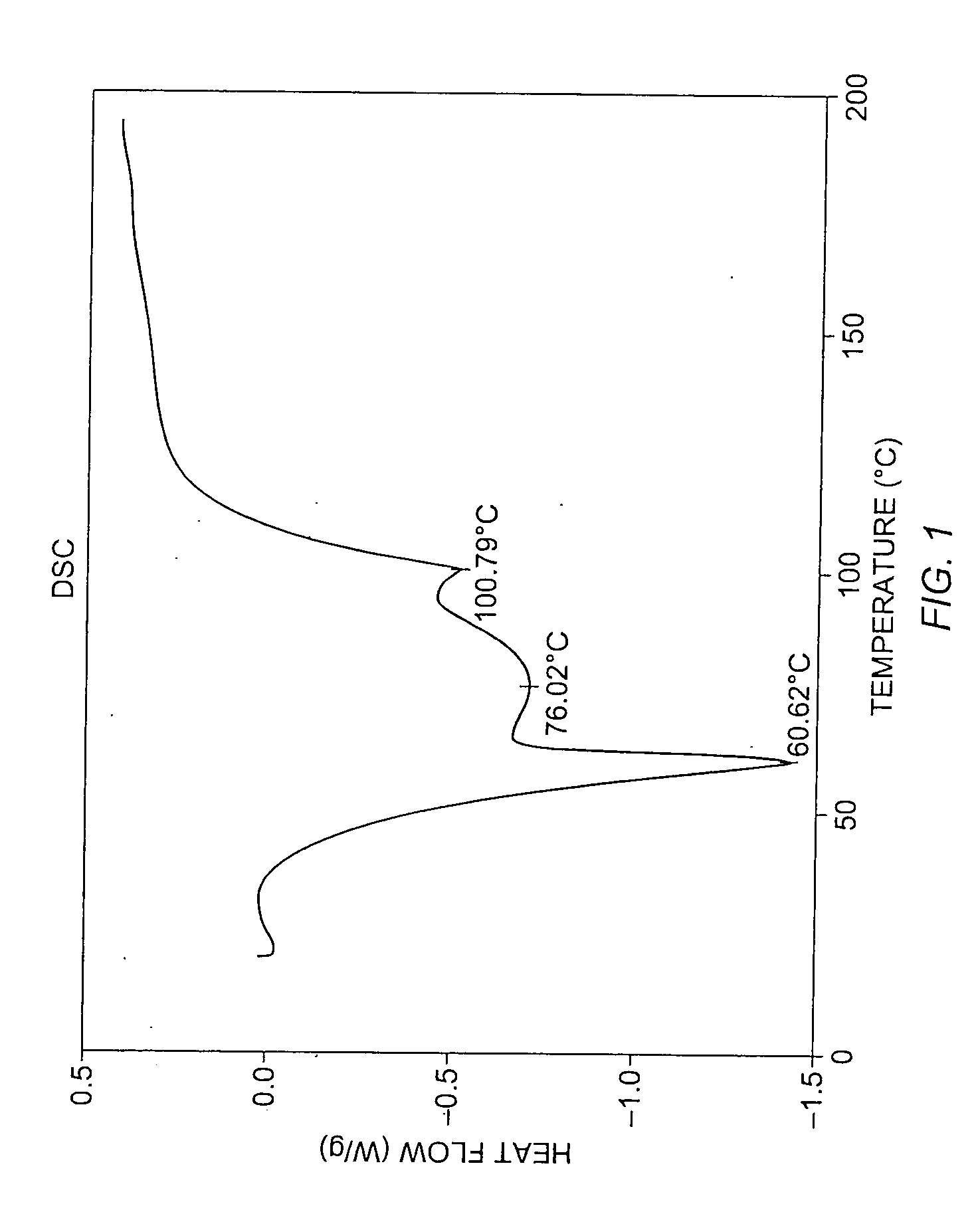
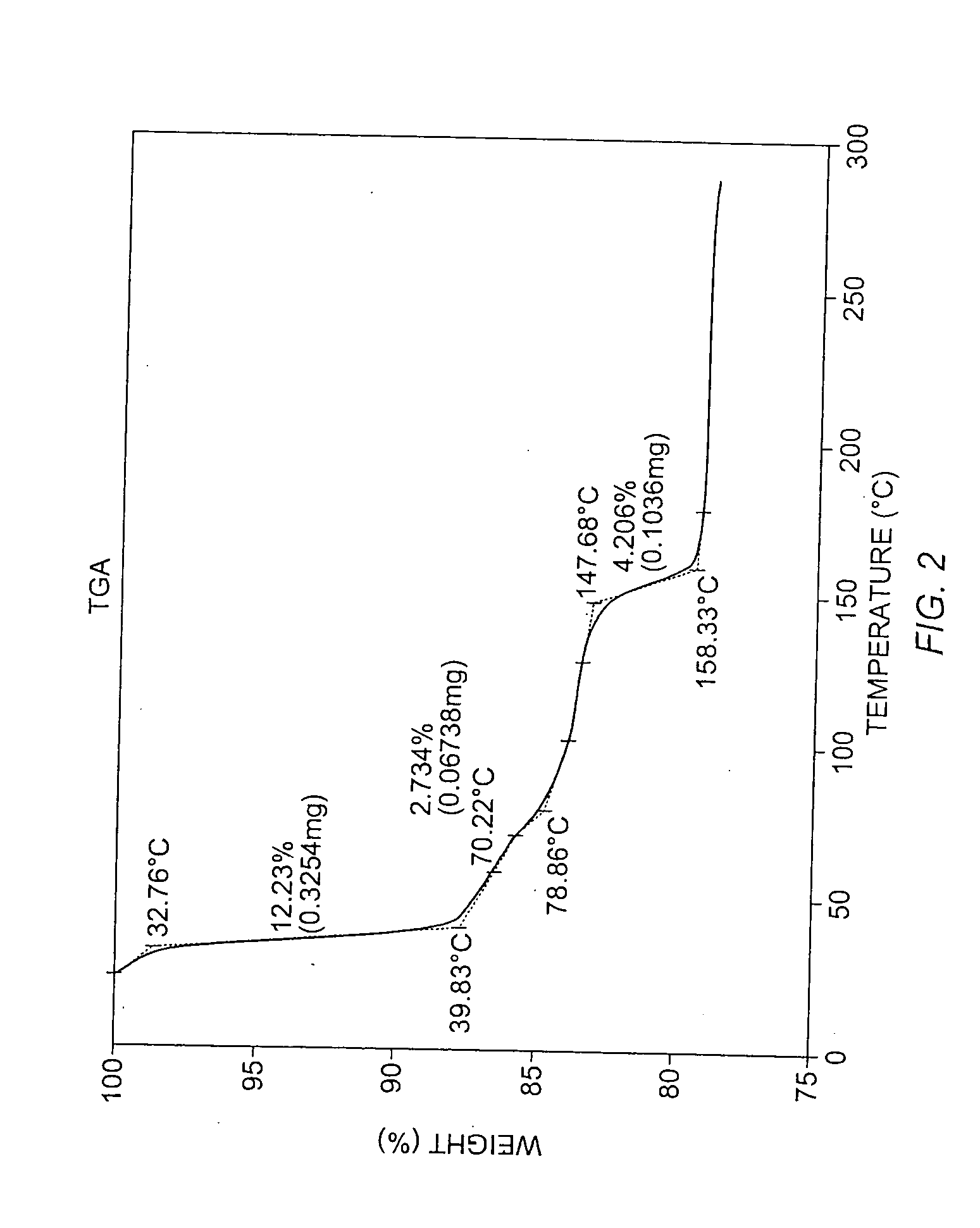
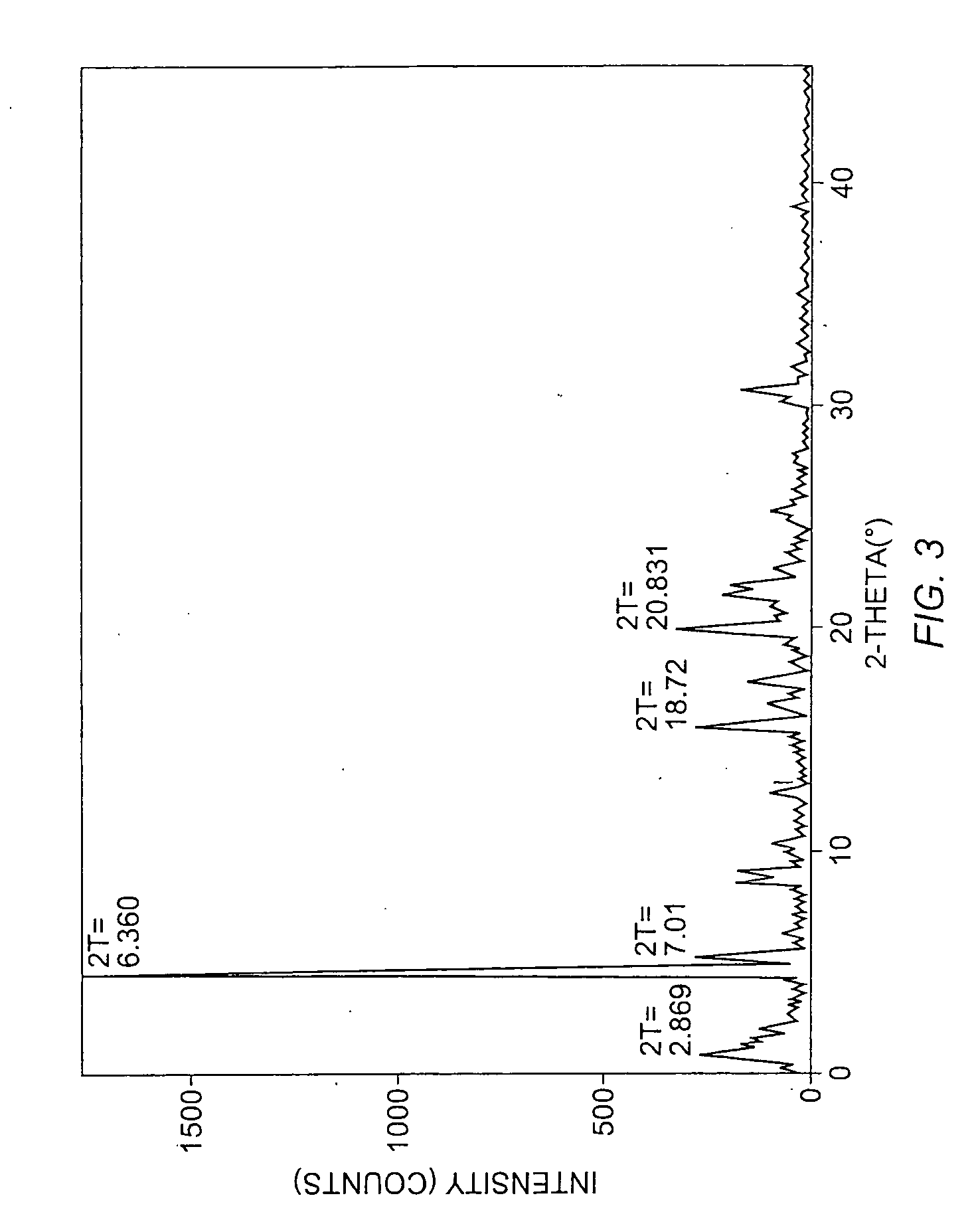
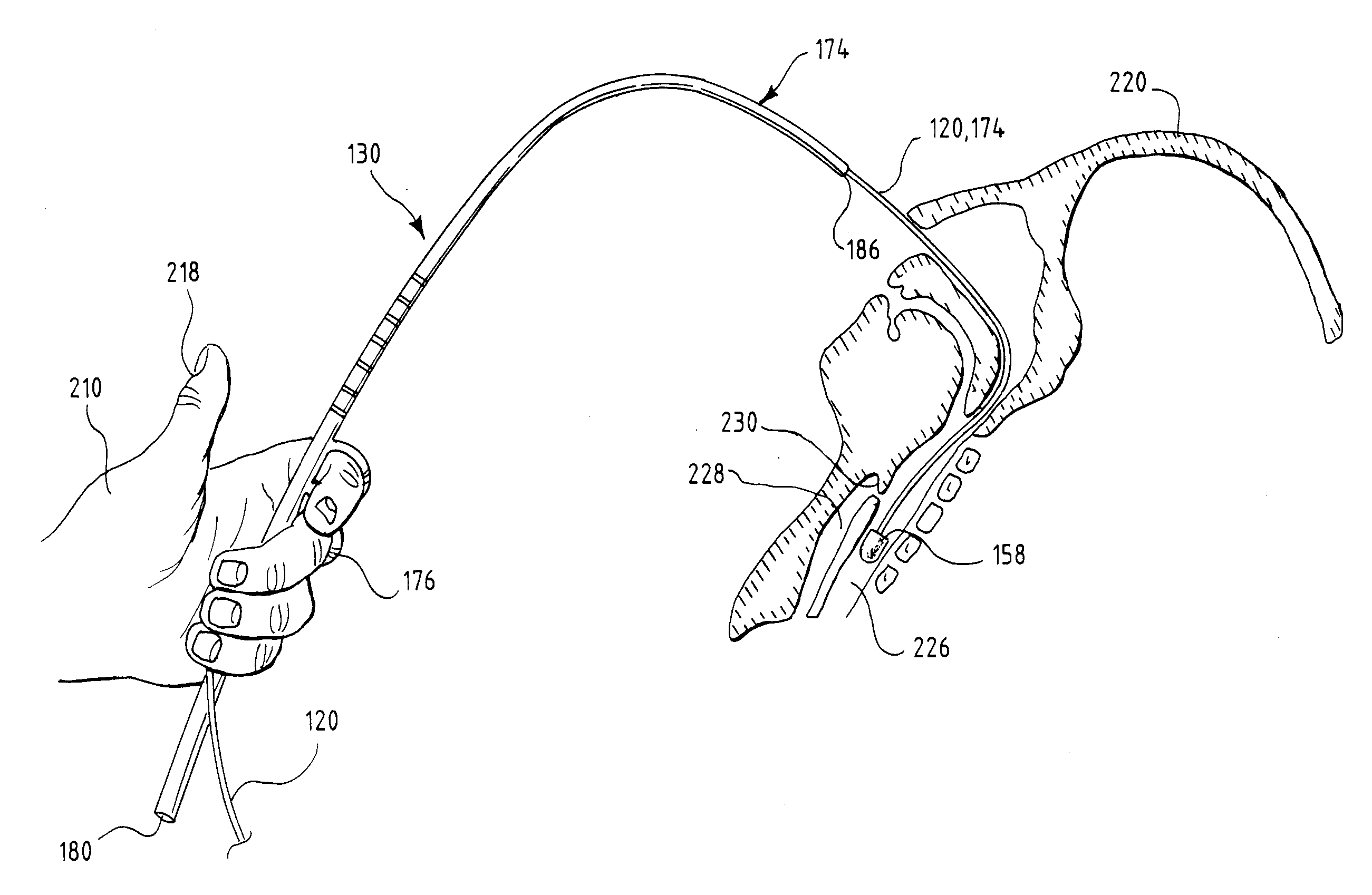
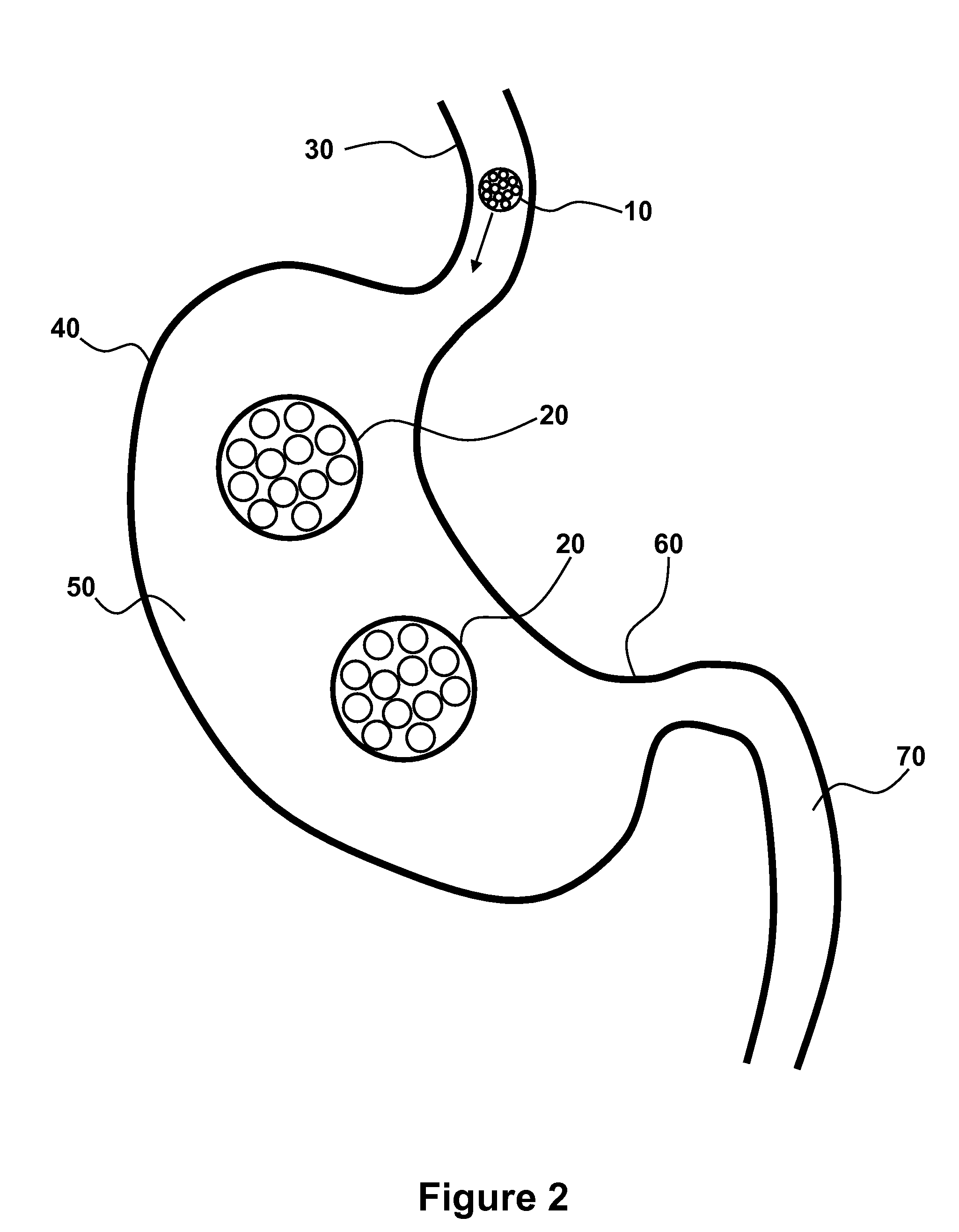
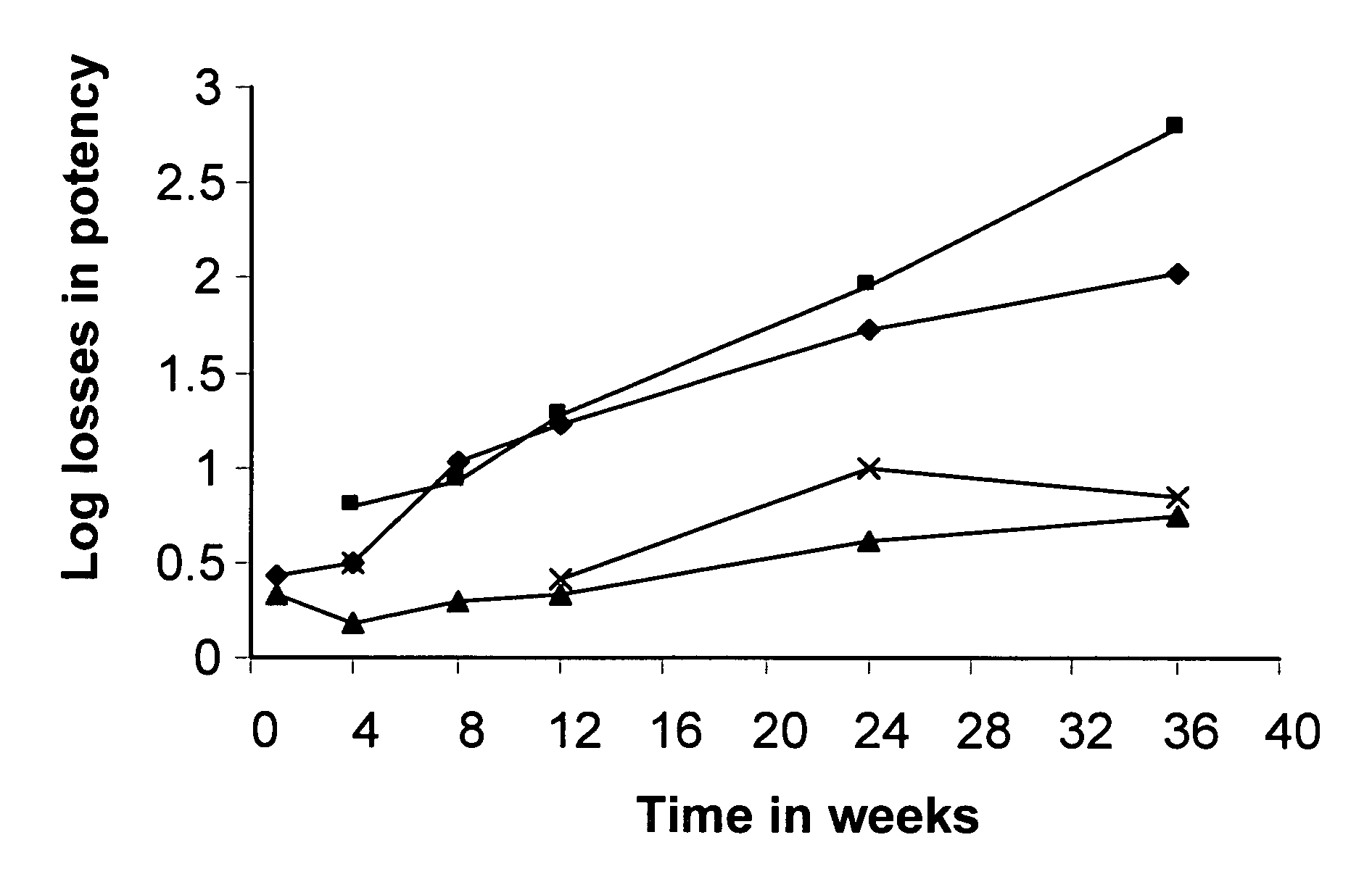
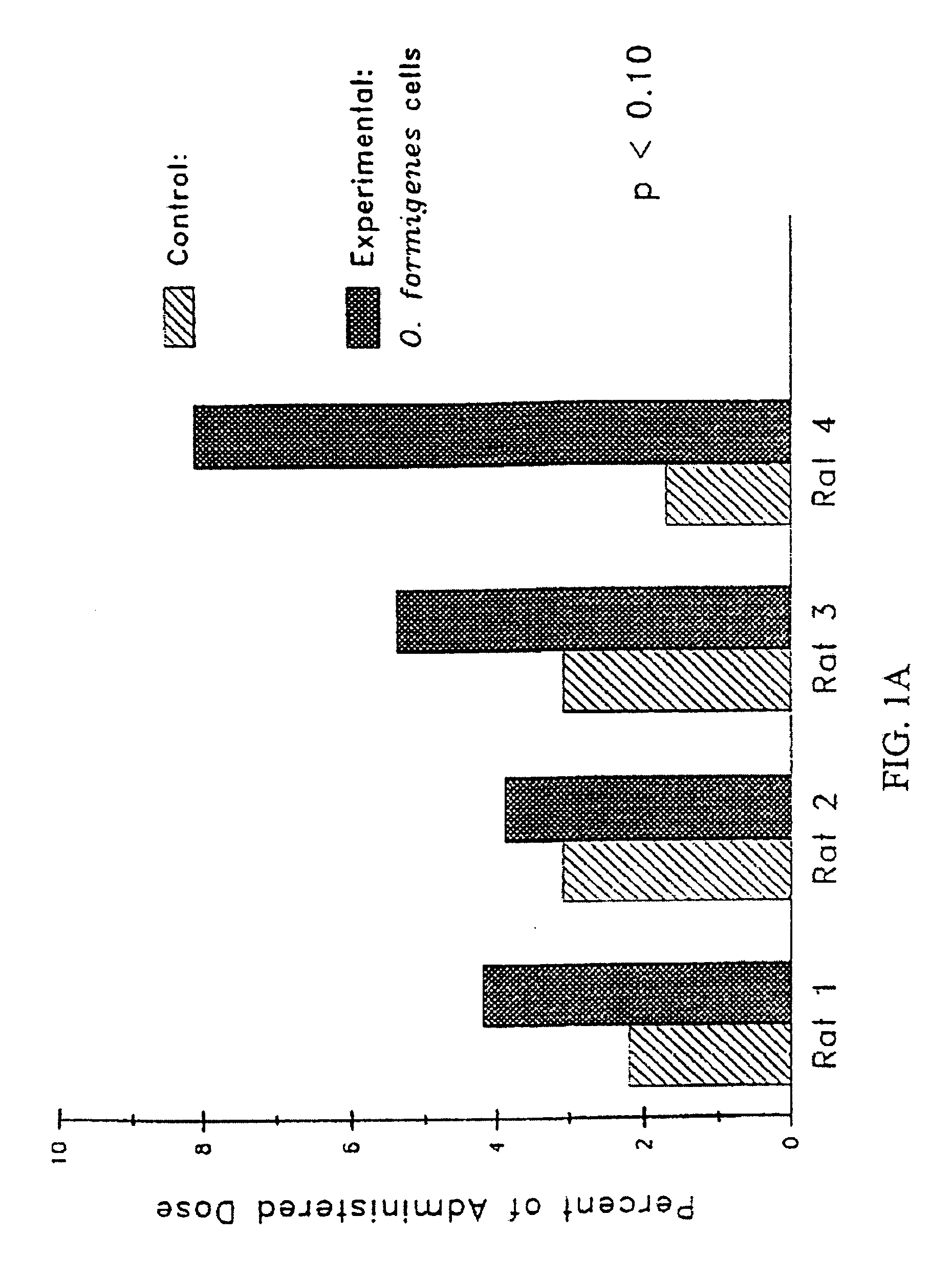
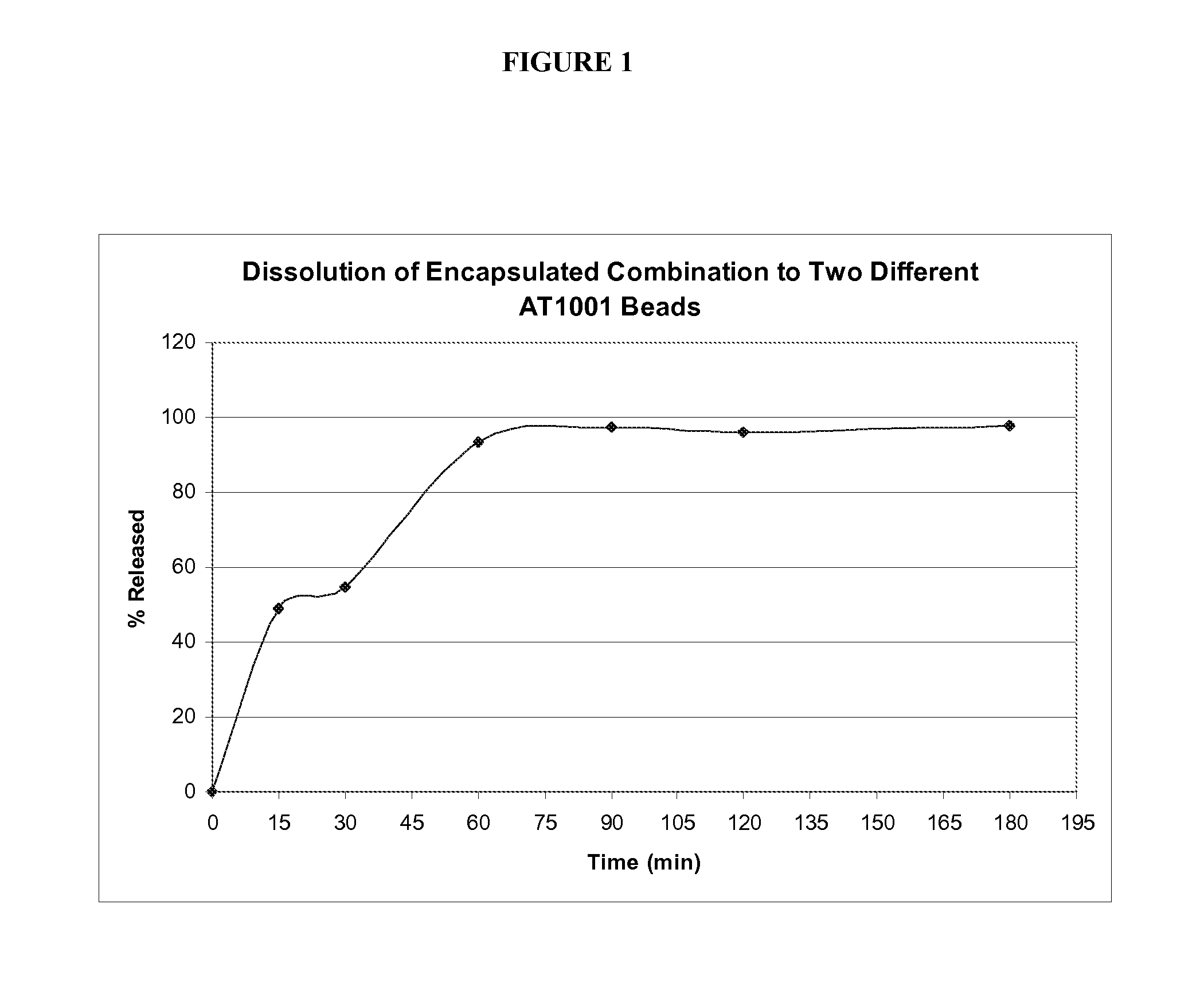
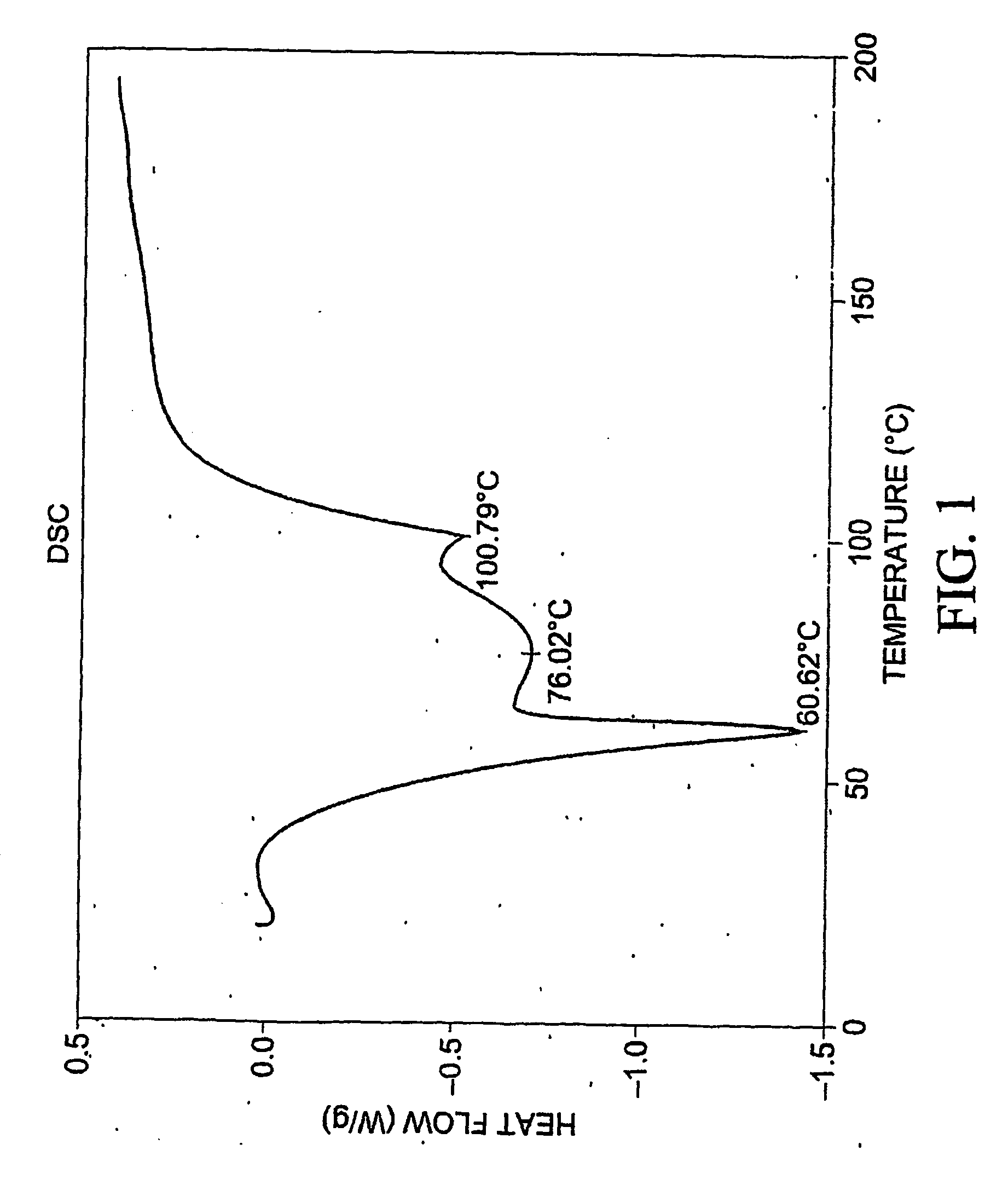
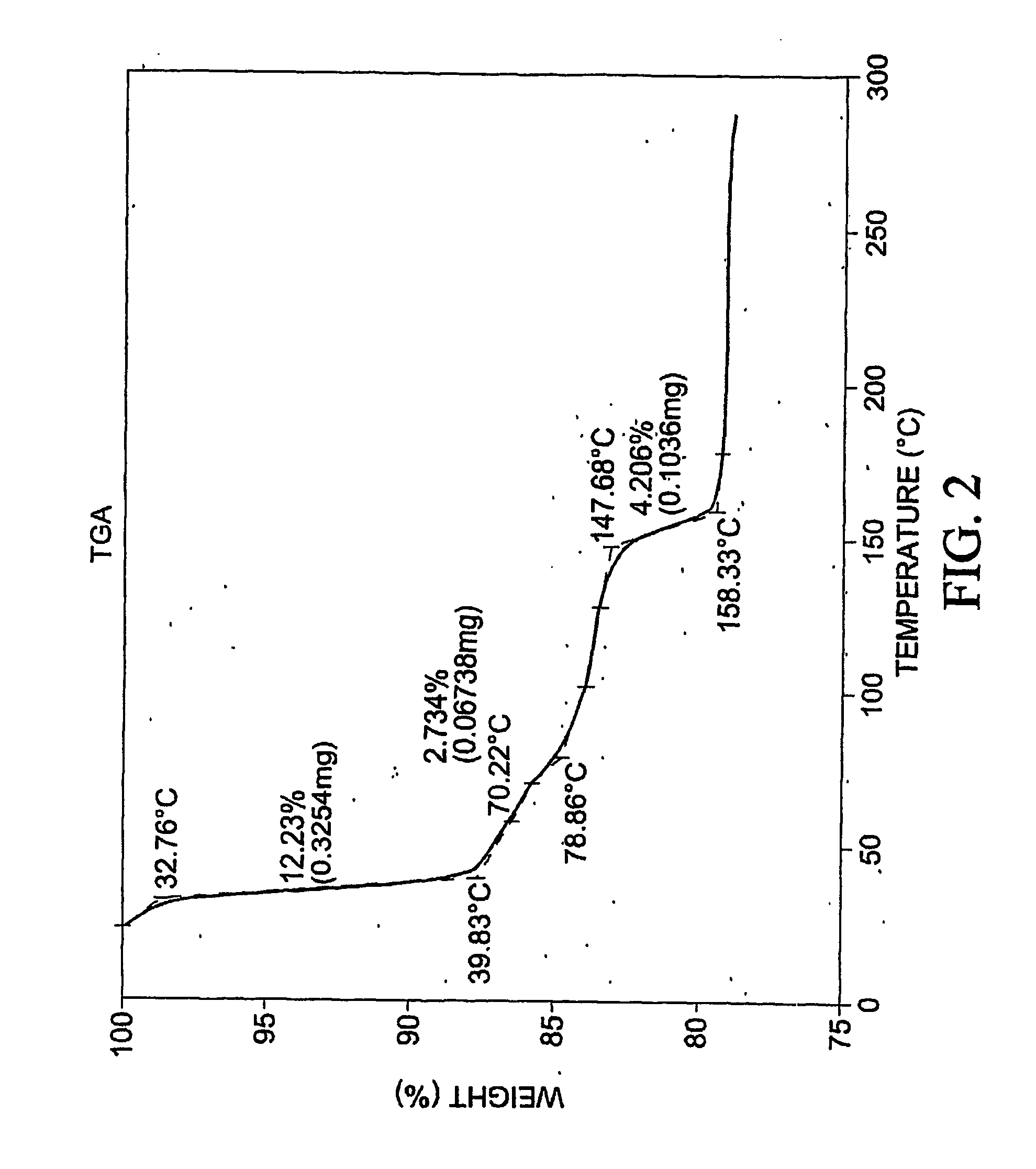
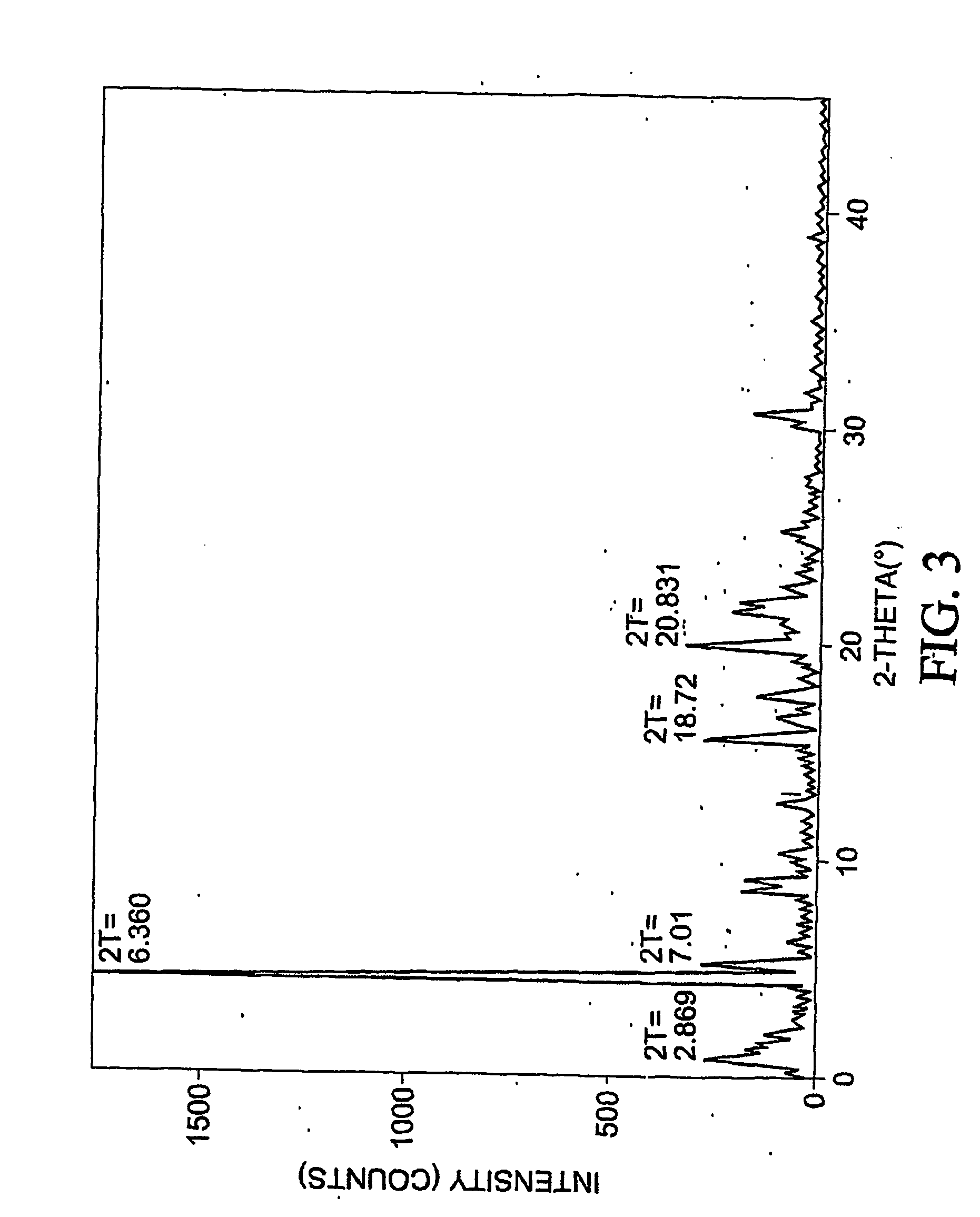
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
422 results about "Gastric fluid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
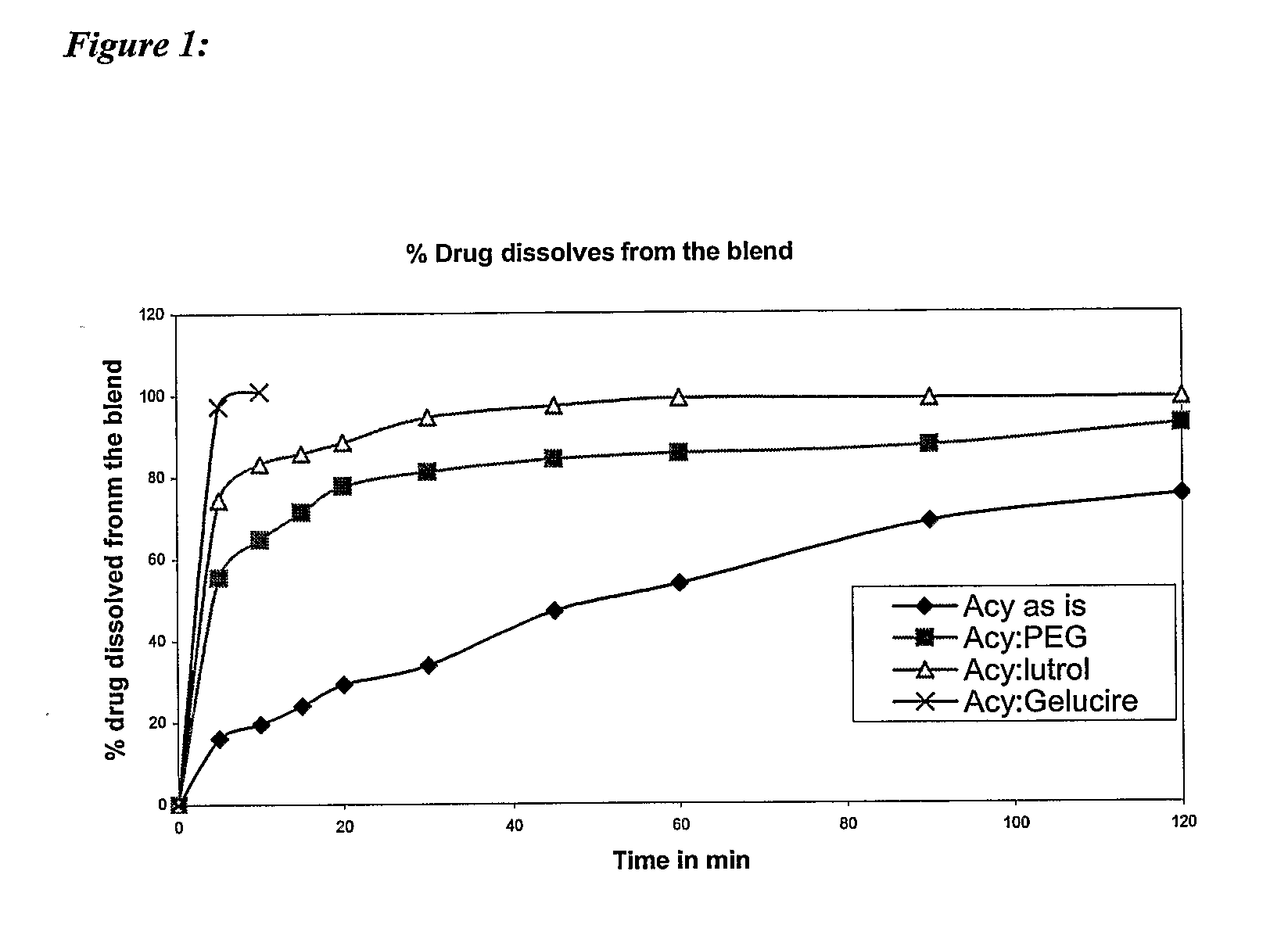
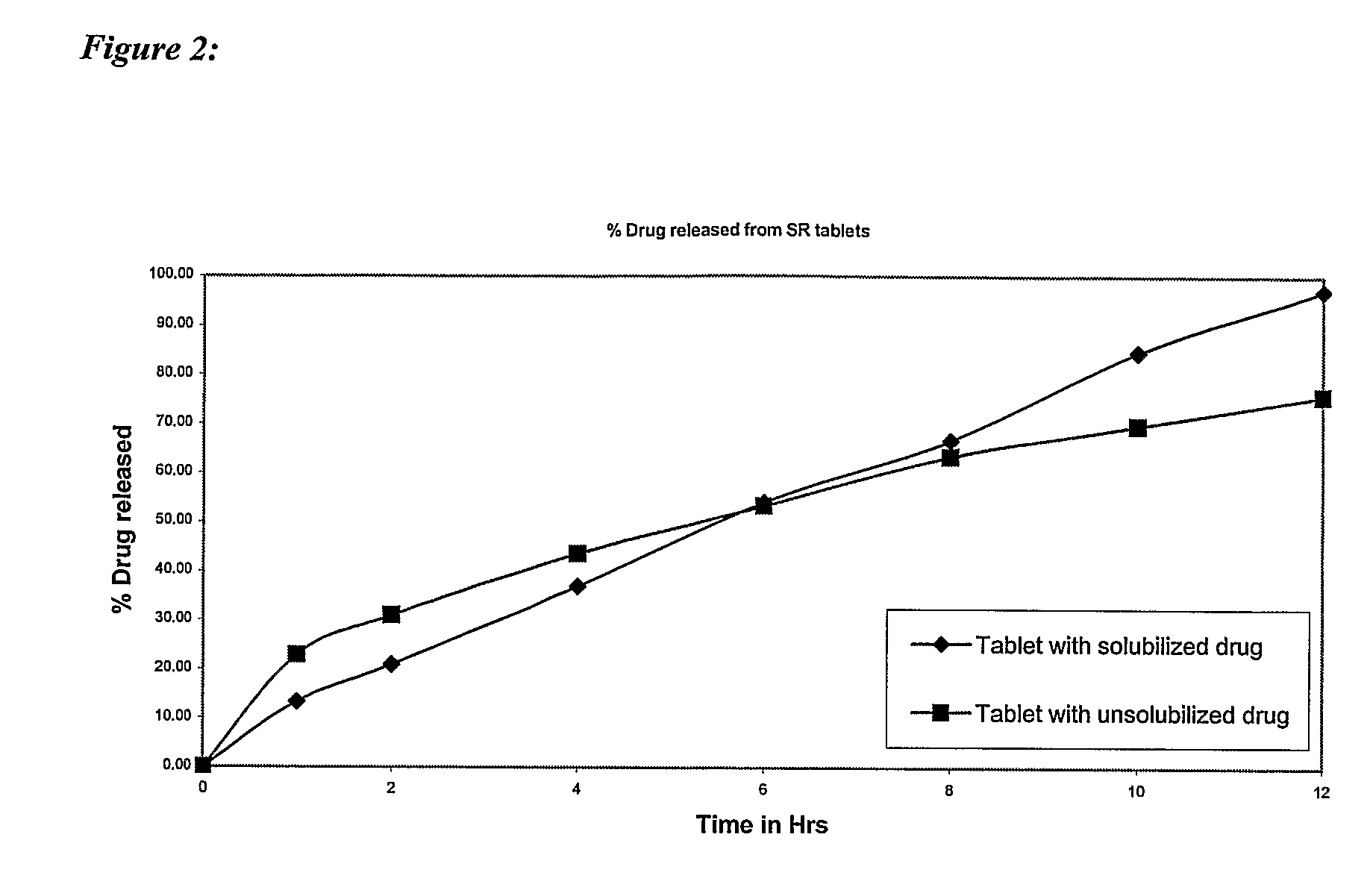
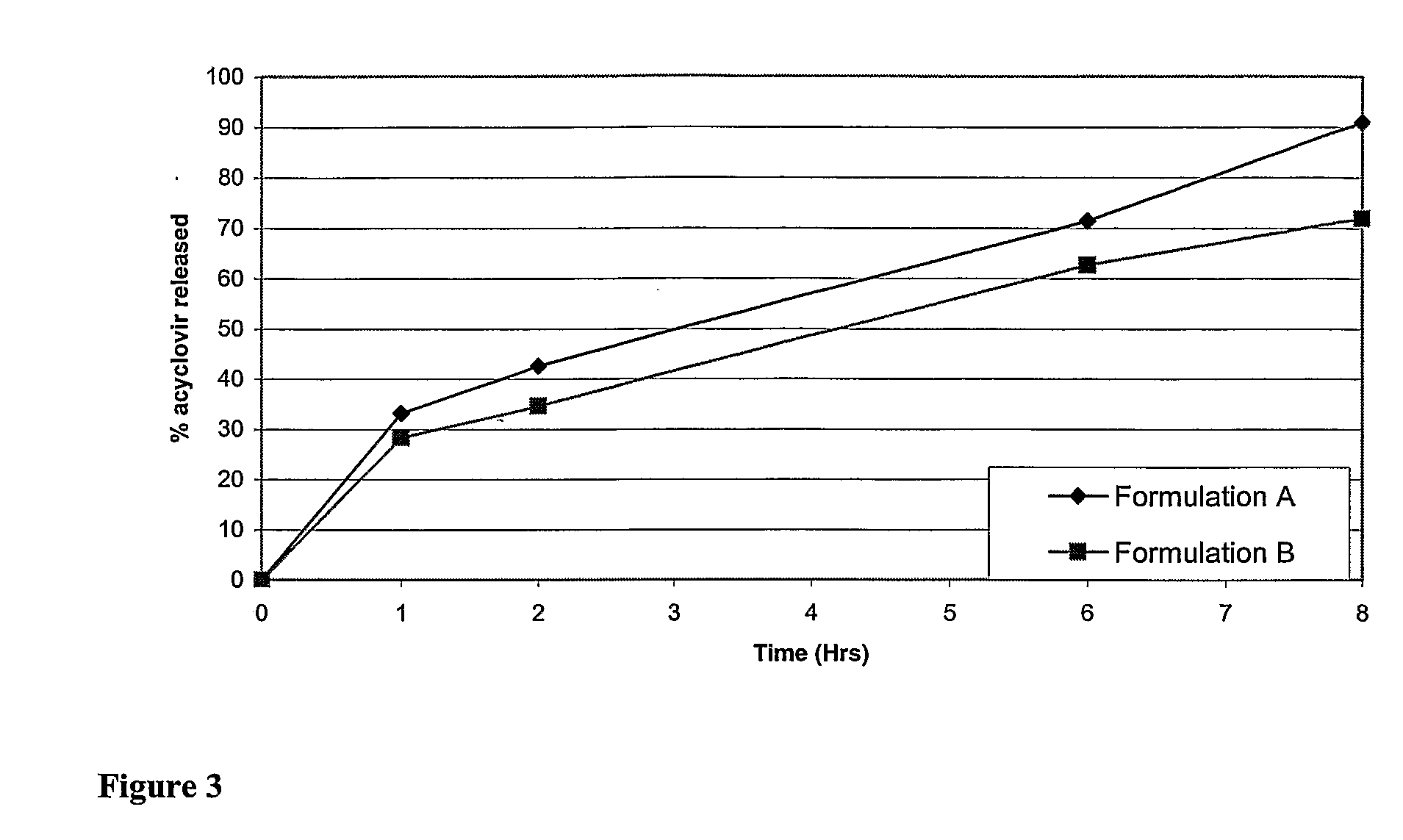
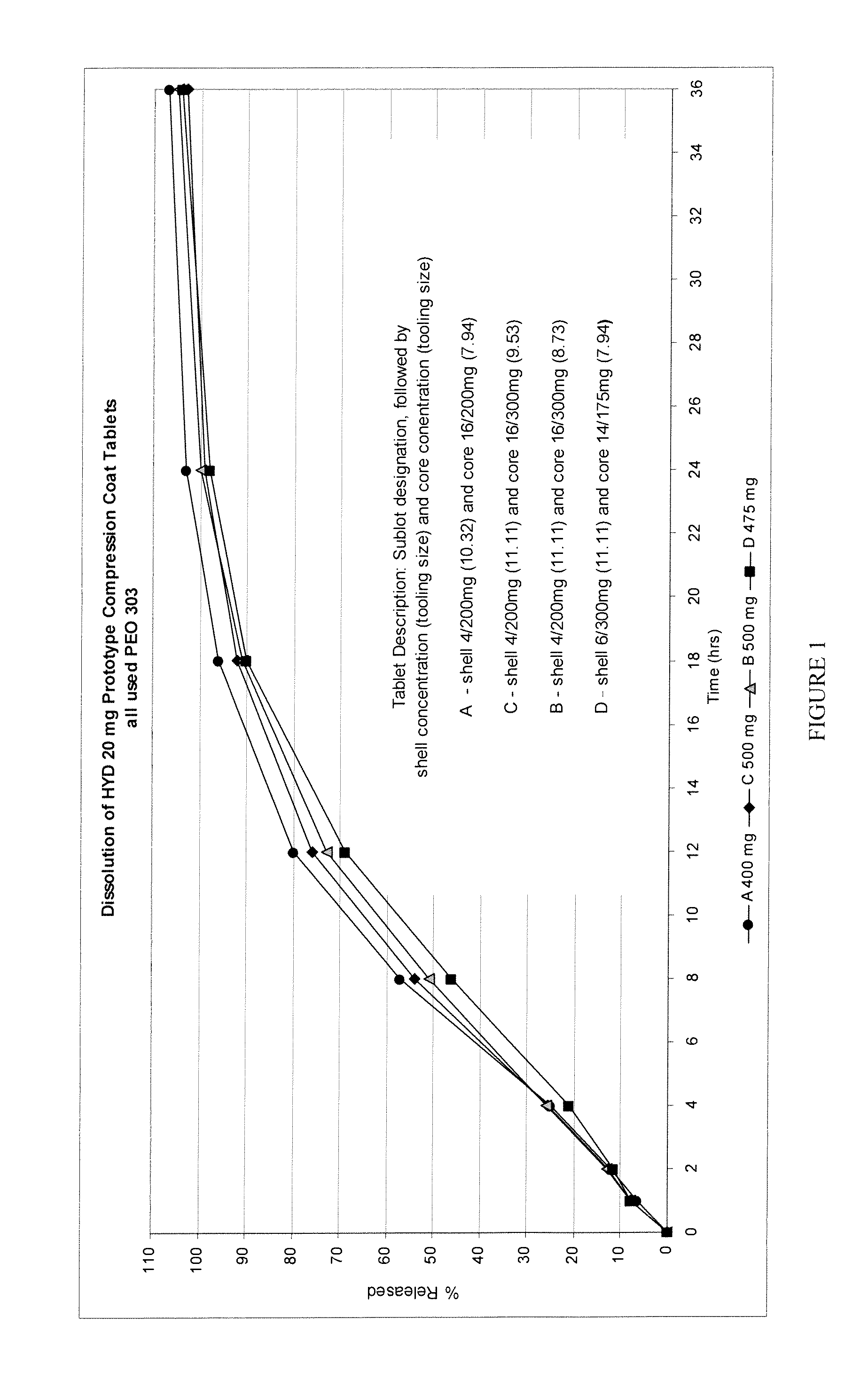
Simulated Gastric Fluid (SGF) is a synthetic form of the gastric fluid in the stomach. This fluid will show the effect of the gastric juice in the stomach on a particular drug in the stomach.
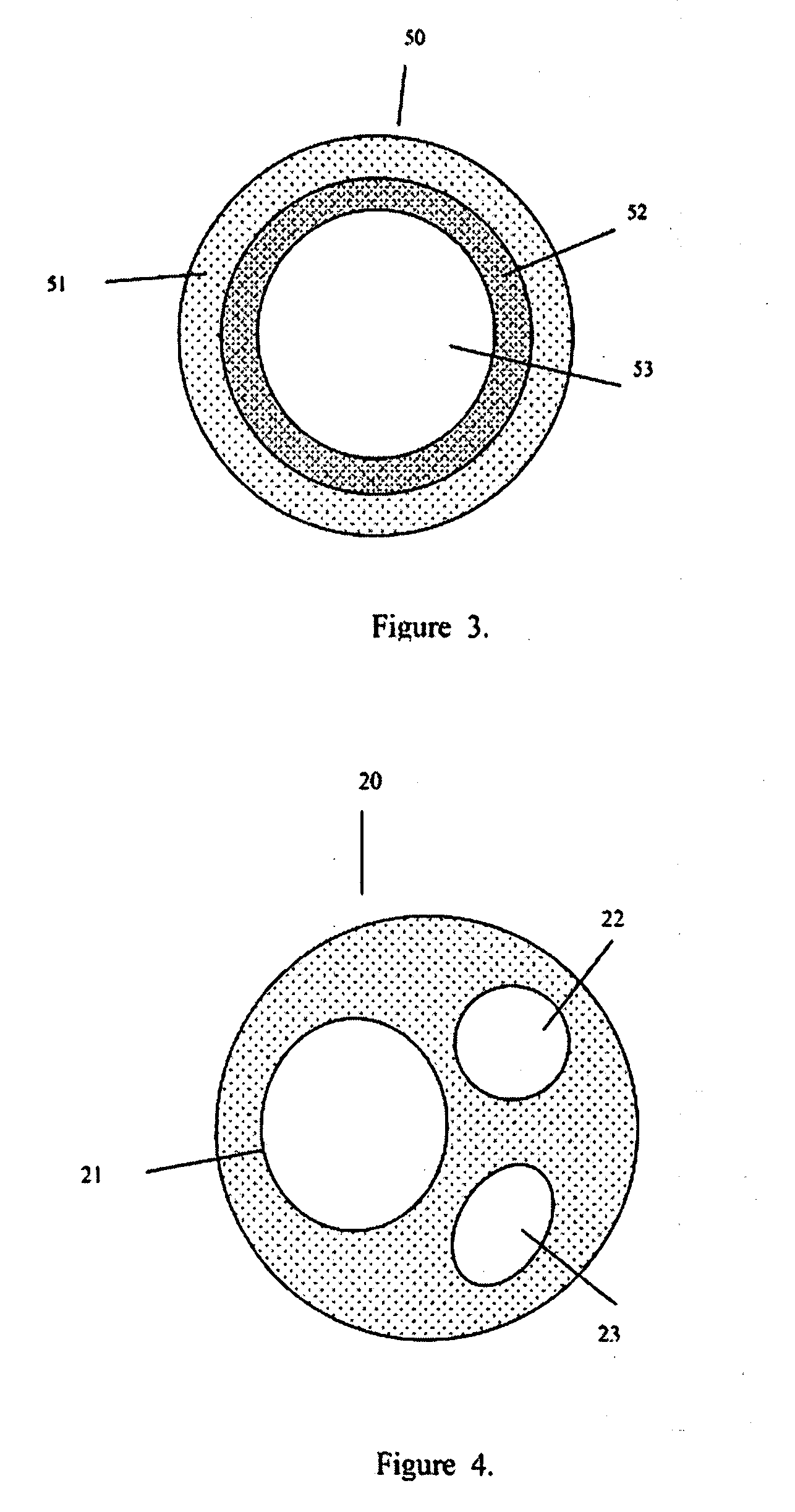
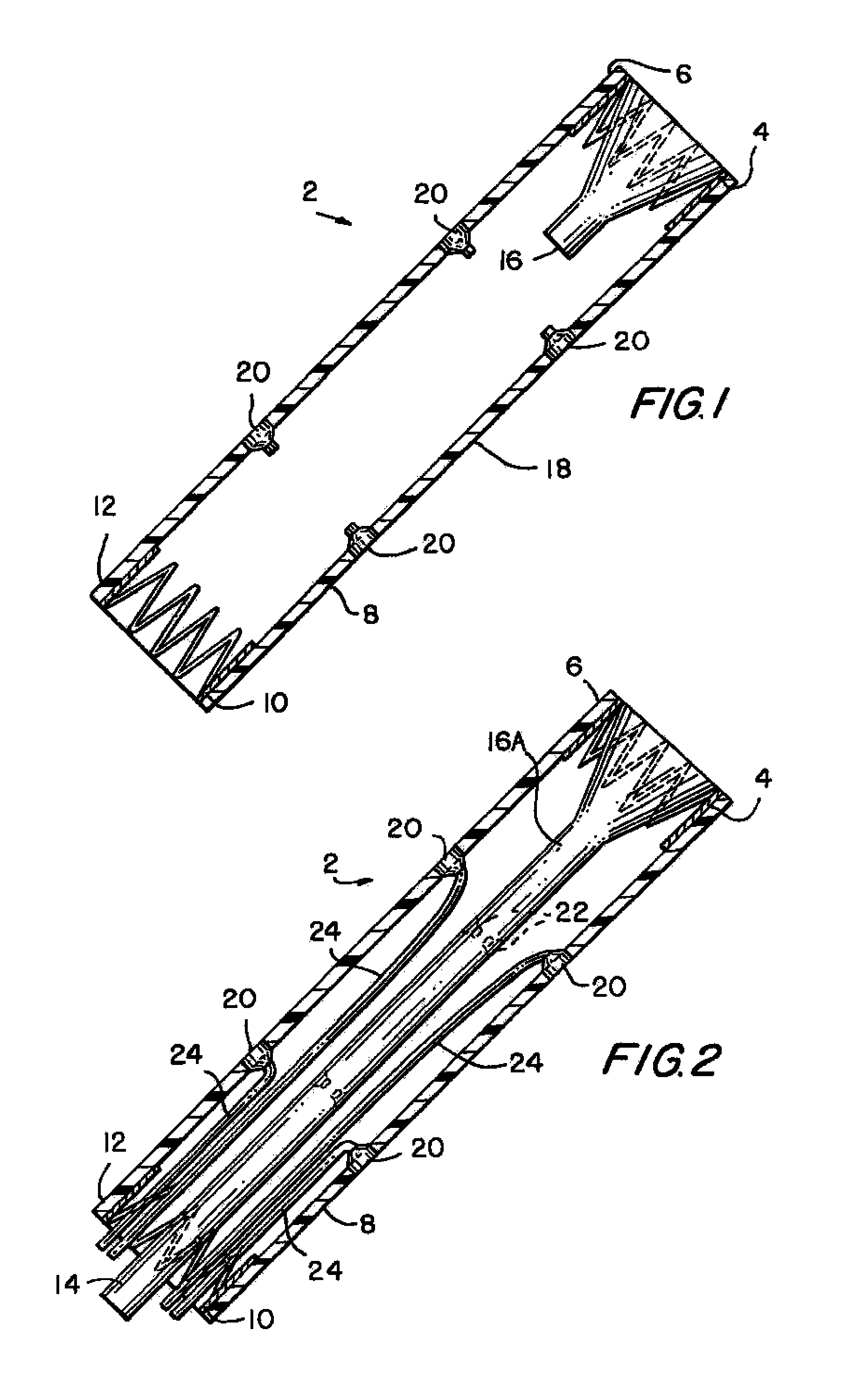
Tamper-resistant oral opioid agonist formulations
InactiveUS6696088B2Lower potentialReduce releasePowder deliveryNervous disorderOpioid AgonistOpioid antagonist
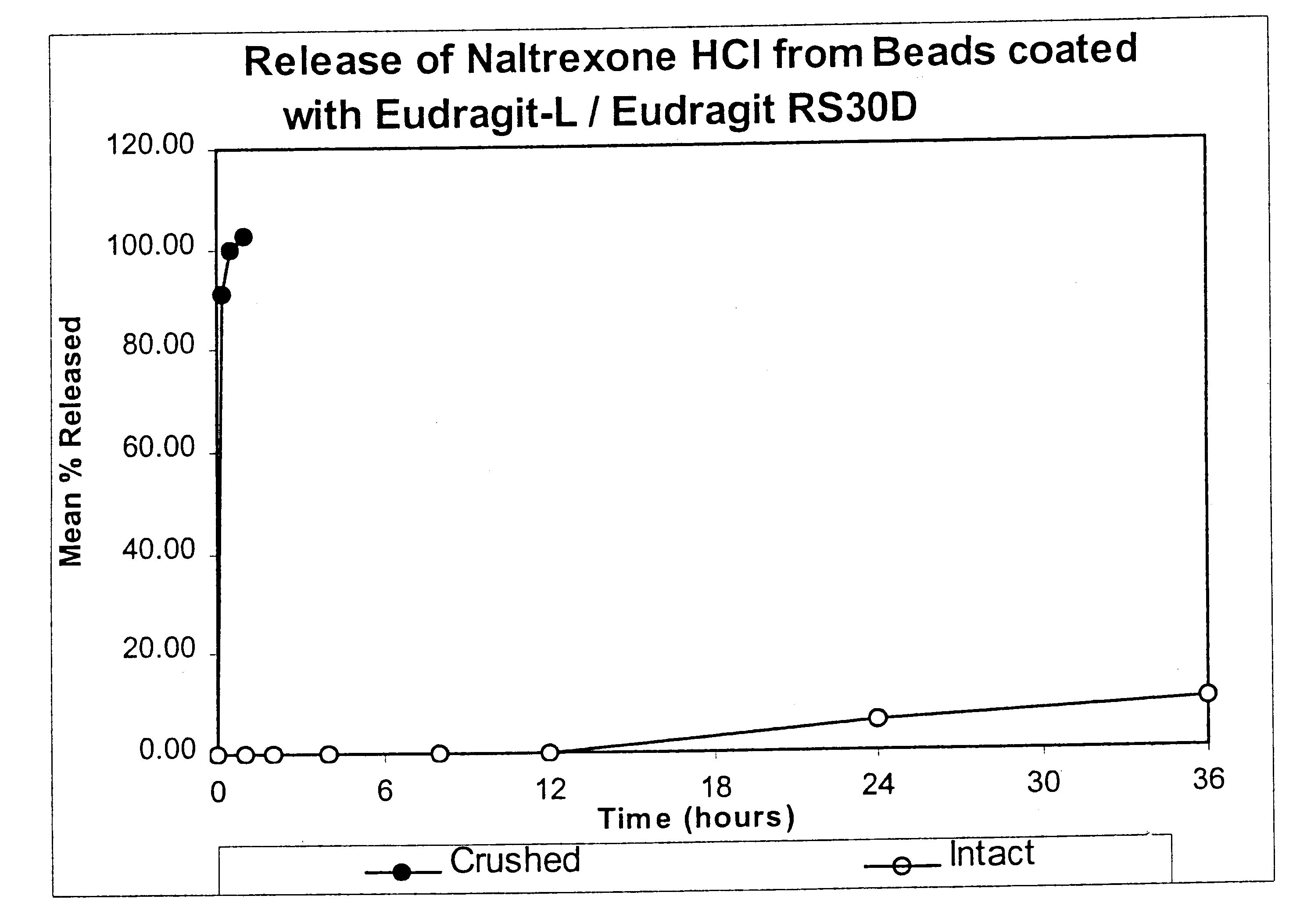
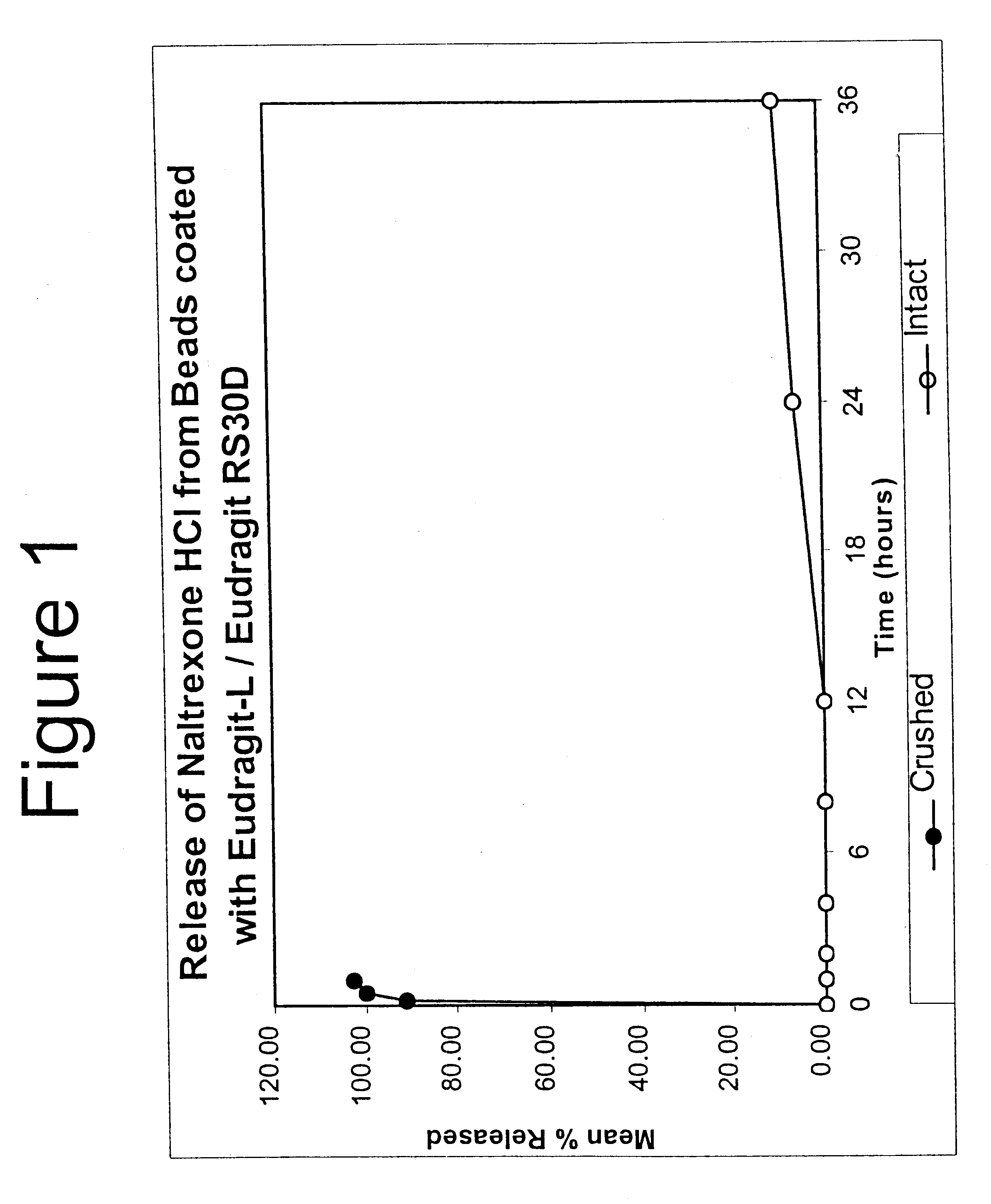
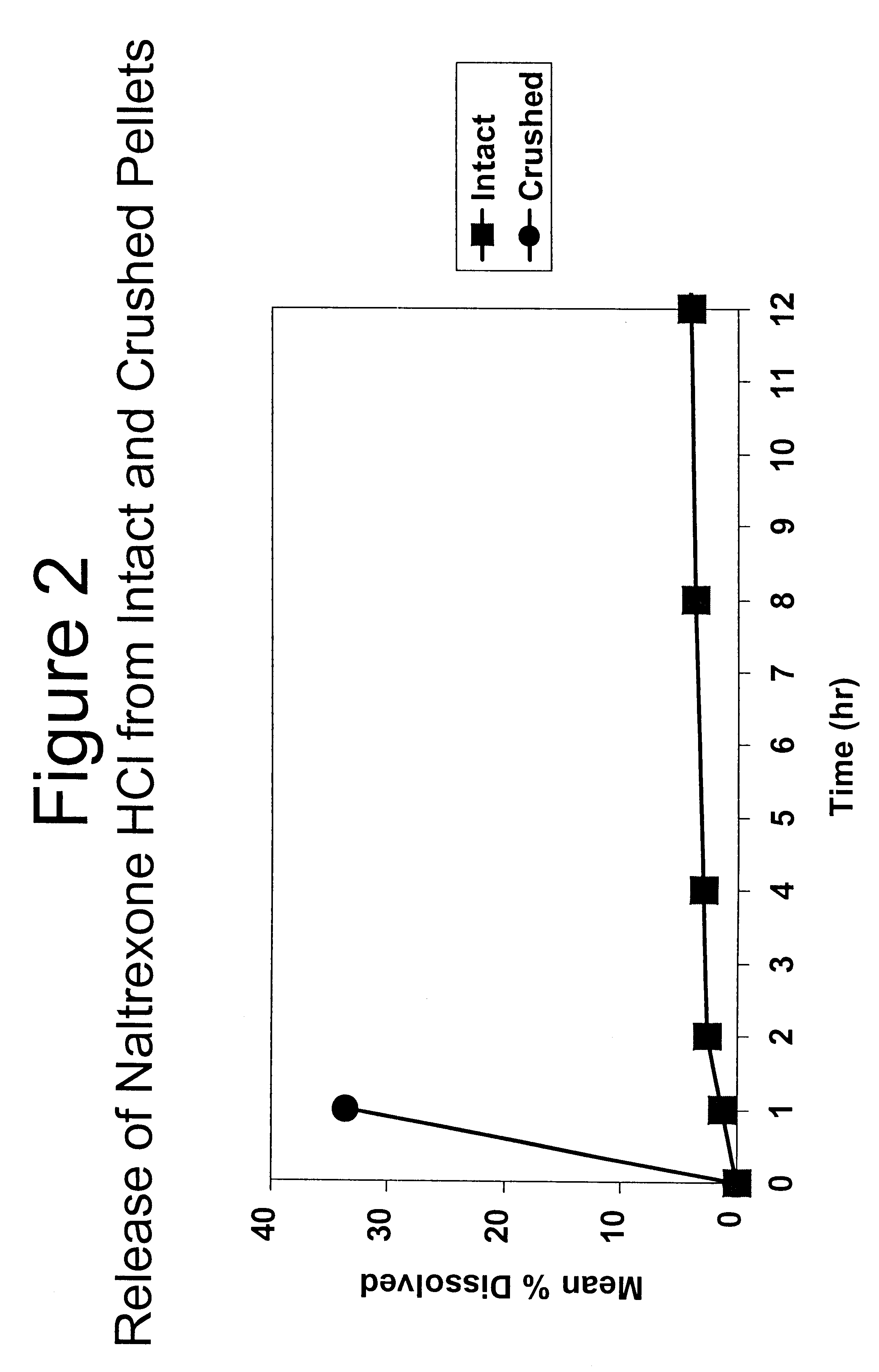
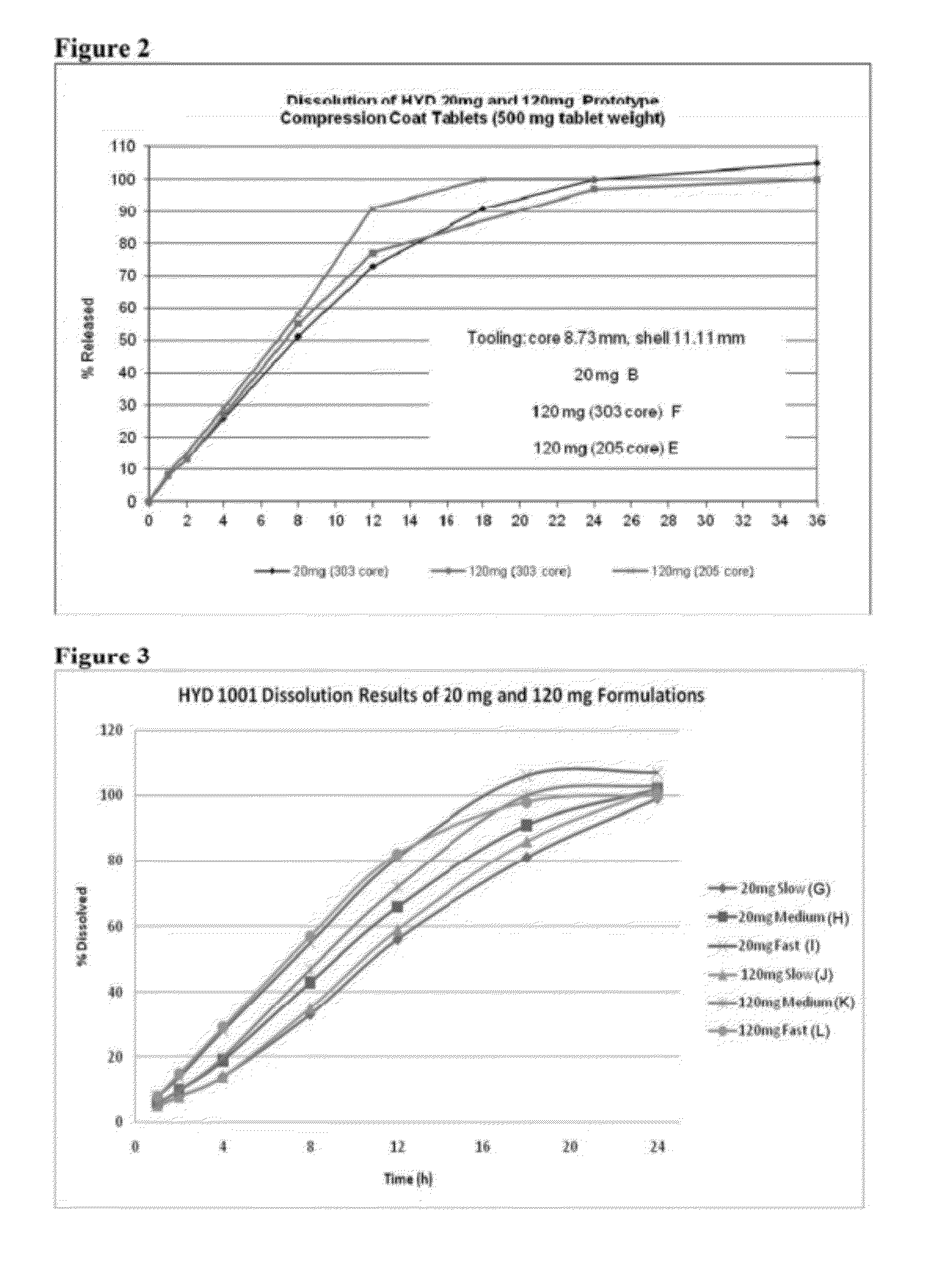
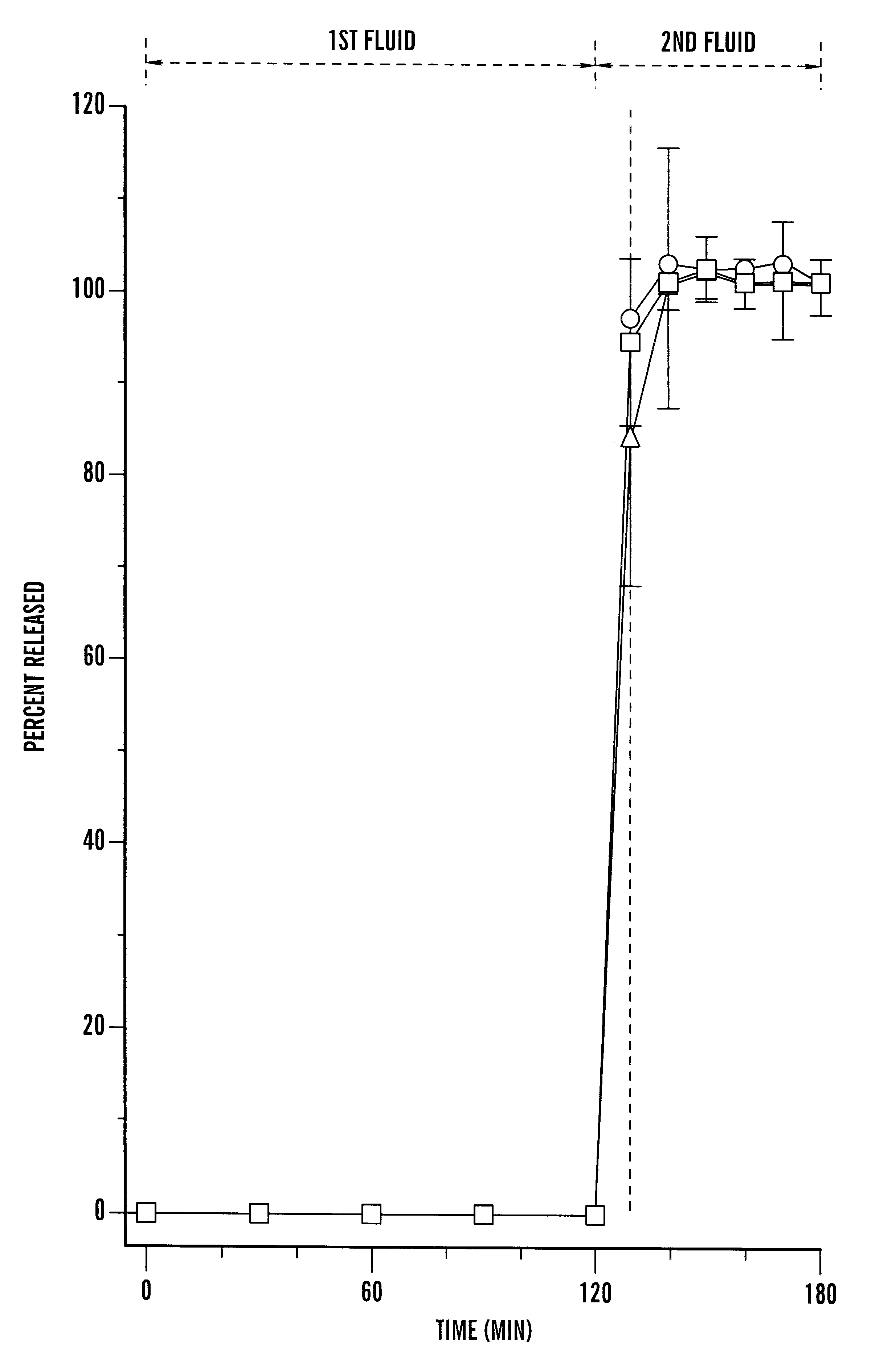
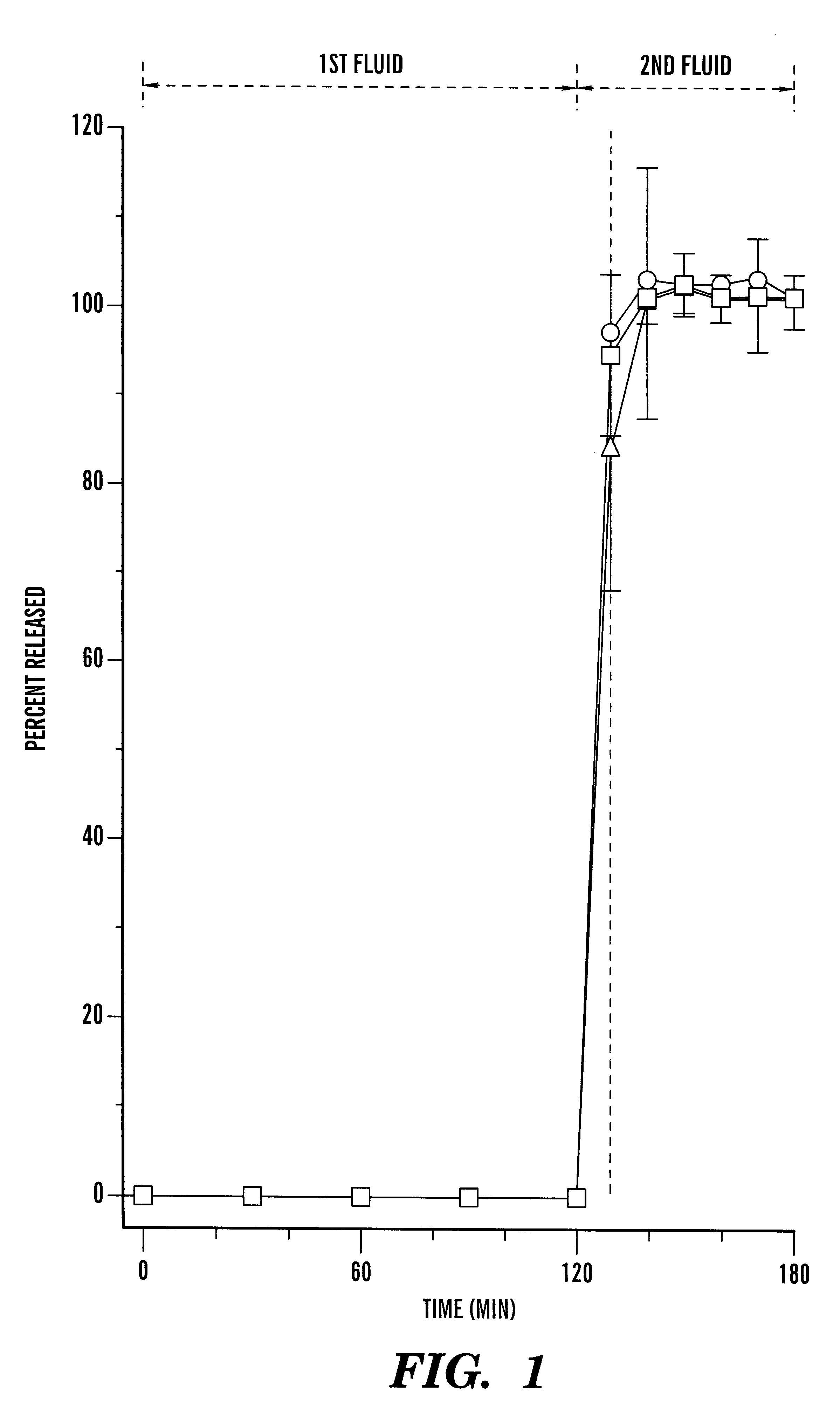
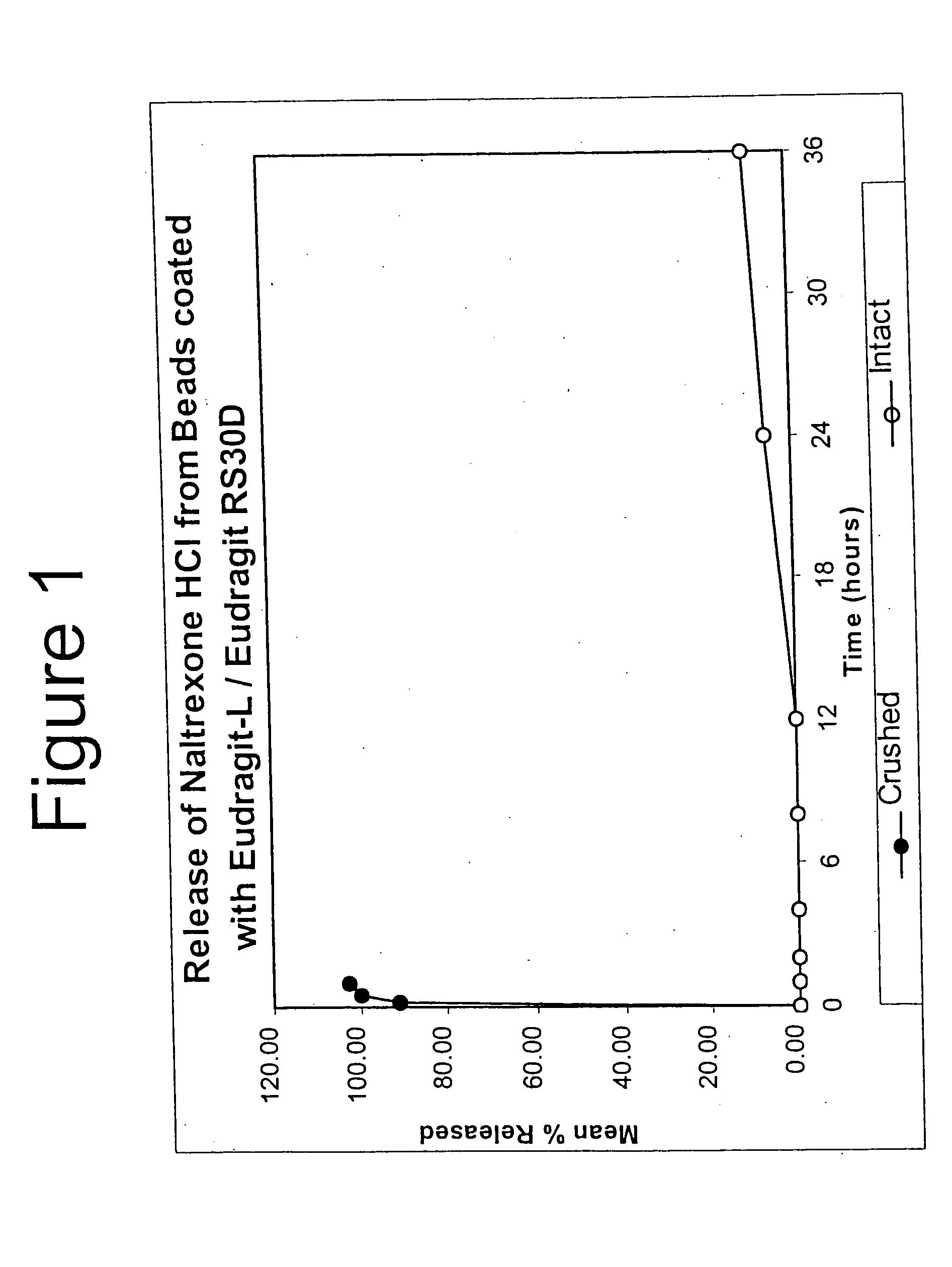
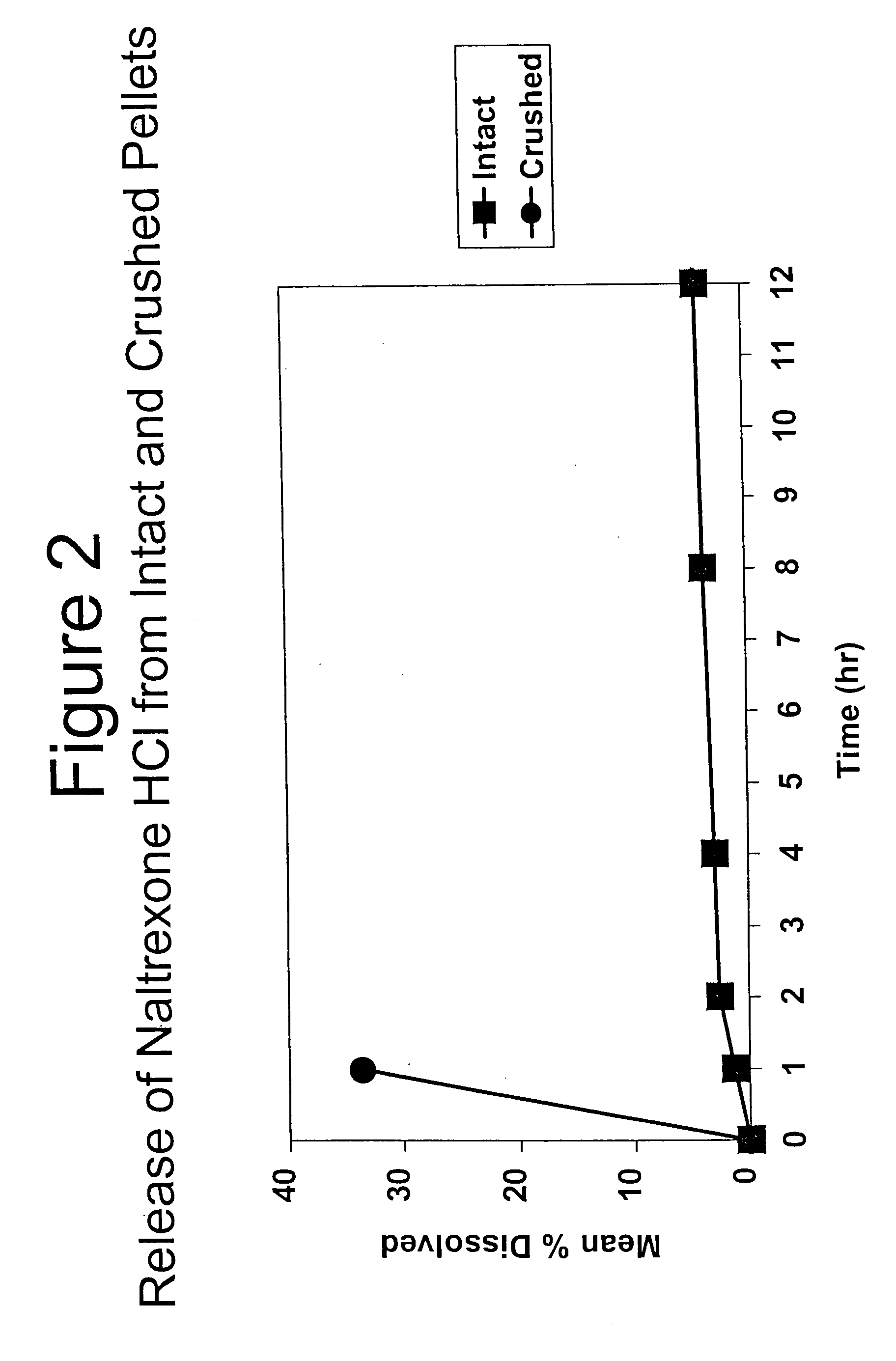
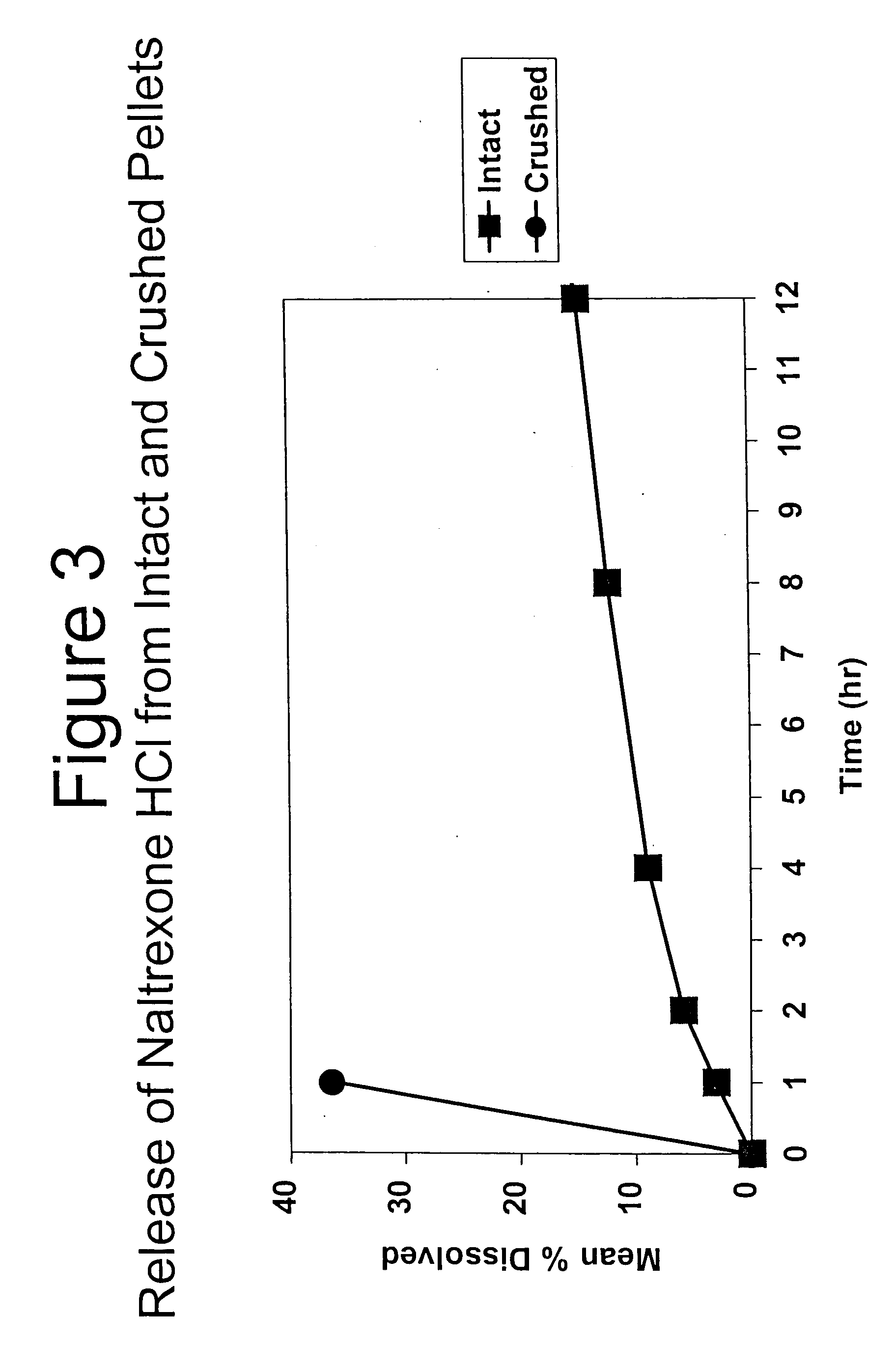
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
Endoscopic gastric bypass
An endoscopic device separates ingested food from gastric fluids or gastric fluids and digestive enzymes, to treat obesity. In a particular embodiment a gastric bypass stent comprises a tubular member and two or more stent members defining a lumen. The tubular member has a substantially liquid impervious coating or covering and one or more lateral openings to permit one-way liquid flow.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Endoscopic Gastric Bypass
An endoscopic device separates ingested food from gastric fluids or gastric fluids and digestive enzymes, to treat obesity. In a particular embodiment a gastric bypass stent comprises a tubular member and two or more stent members defining a lumen. The tubular member has a substantially liquid impervious coating or covering and one or more lateral openings to permit one-way liquid flow.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Stable probiotic microsphere compositions and their methods of preparation
The invention relates to viable and stable probiotic formulations for intestinal targeting made of microspheres comprising each a core of one or more probiotic bacteria, microcrystallline cellulose with a degree of polymerization from 165-365 and mean diameter from 45 to 180 μm, a disintegrant and a stabilizer, the core being coated with a non-enteric coating and further coated with an enteric coating. Each probiotic microsphere has a residual moisture level of less than 5% and a water activity (aw) between 0.1 and 0.5. Such a probiotic microsphere shows no reduction in viable bacteria after one hour in simulated gastric fluid. The present invention also relates to the process of preparing such formulation.
Owner:CANACURE CORP
Controlled release pharmaceutical compositions with improved bioavailability
PendingUS20070196396A1Efficient retentionImprove bioavailabilityHeavy metal active ingredientsBiocideControlled releaseActive agent
The present invention provides a controlled release oral pharmaceutical composition having a therapeutically effective amount of one or more pharmacologically active agent having low bioavailability; one or more solubilizers; one or more biocompatible swelling agents; and a swelling enhancer. The swelling agent, in combination with swelling enhancer, swells in the presence of water in gastric fluid such that the size of the dosage form is sufficiently increased to provide retention of the dosage form in the stomach of a patient, which gradually erodes within the gastrointestinal tract over a prolonged time period.
Owner:RUBICON RES PTY LTD
Encased Tamper Resistant Controlled Release Dosage Forms
ActiveUS20120164220A1Reducing abuse potential of dosage formBiocideNervous disorderGastric fluidEnzyme
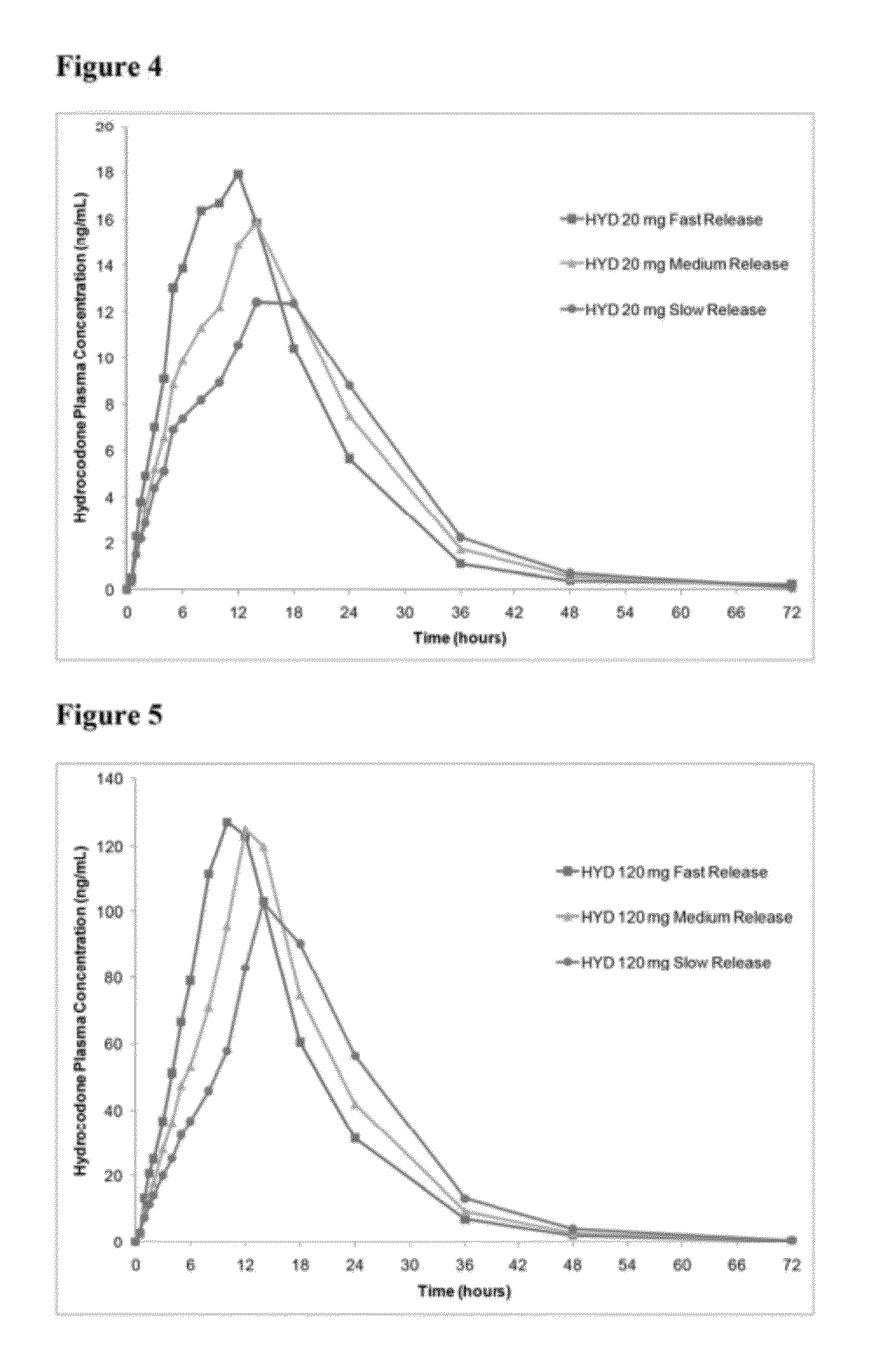
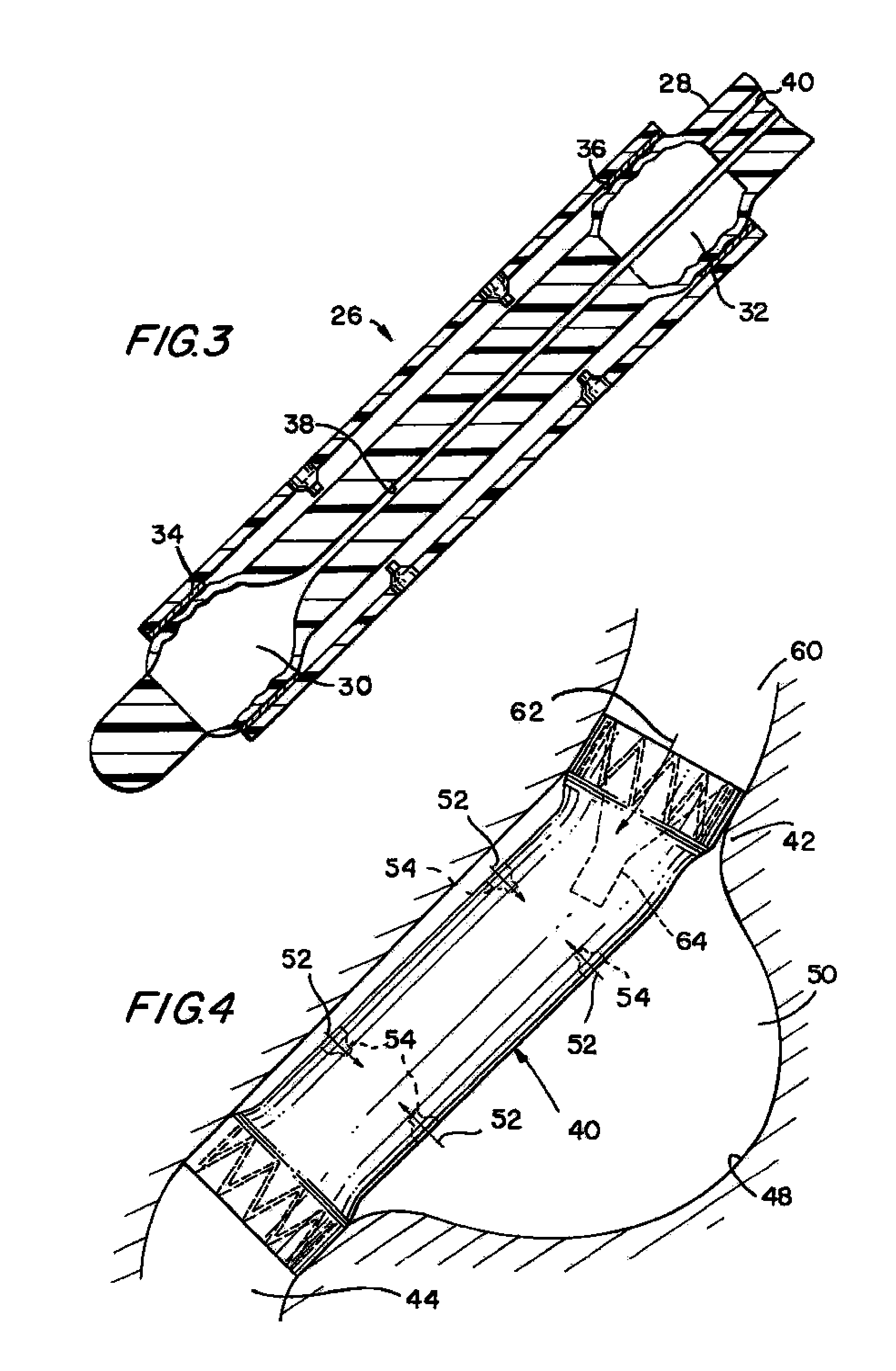
In certain embodiments, the present invention is directed to a solid controlled release dosage form comprising: a core comprising a first portion of an opioid analgesic dispersed in a first matrix material; and a shell encasing the core and comprising a second portion of the opioid analgesic dispersed in a second matrix material; wherein the amount of opioid analgesic released from the dosage form is proportional within 20% to elapsed time from 8 to 24 hours, as measured by an in-vitro dissolution in a USP Apparatus 1 (basket) at 100 rpm in 900 ml simulated gastric fluid without enzymes (SGF) at 37 C.
Owner:PURDUE PHARMA LP
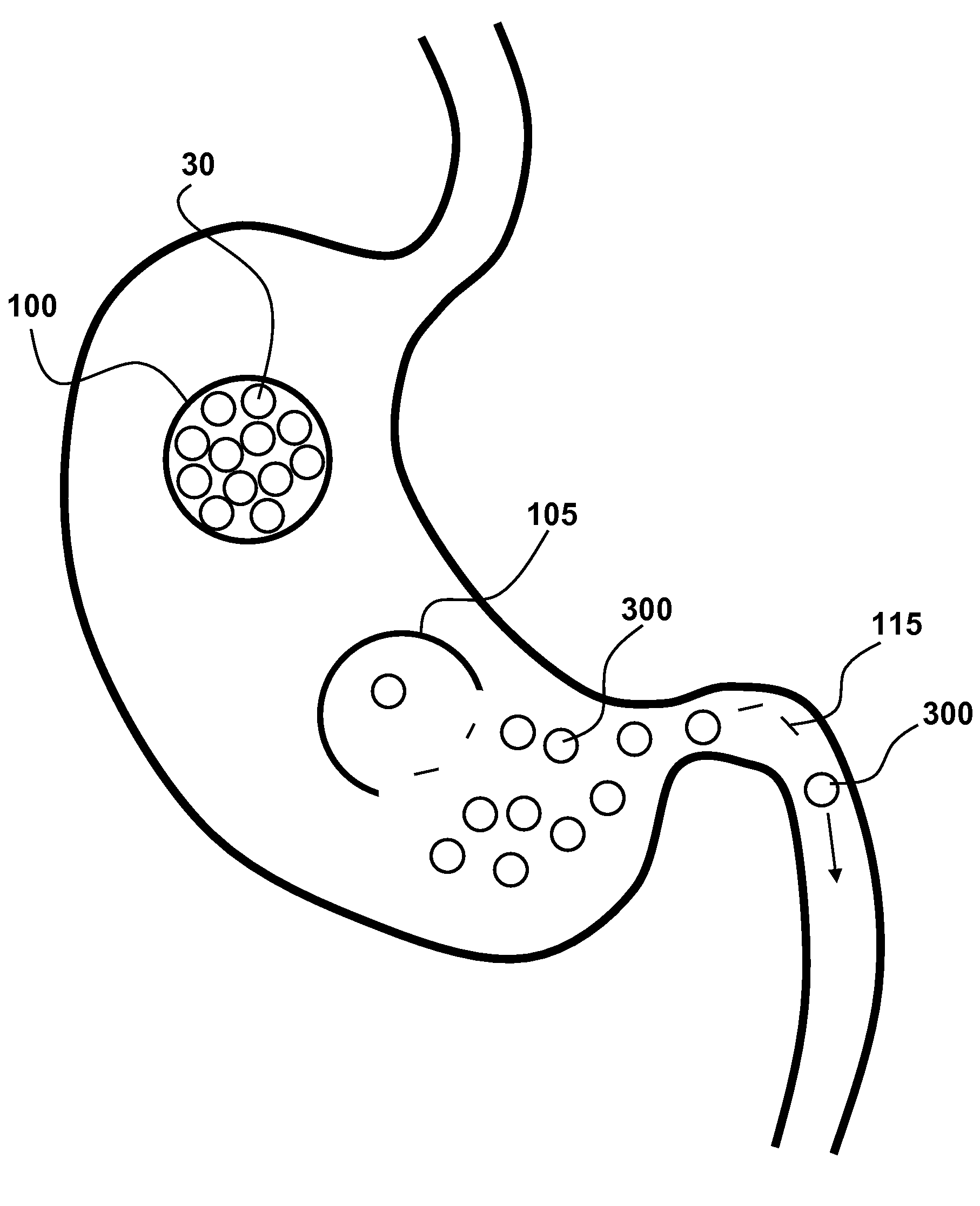
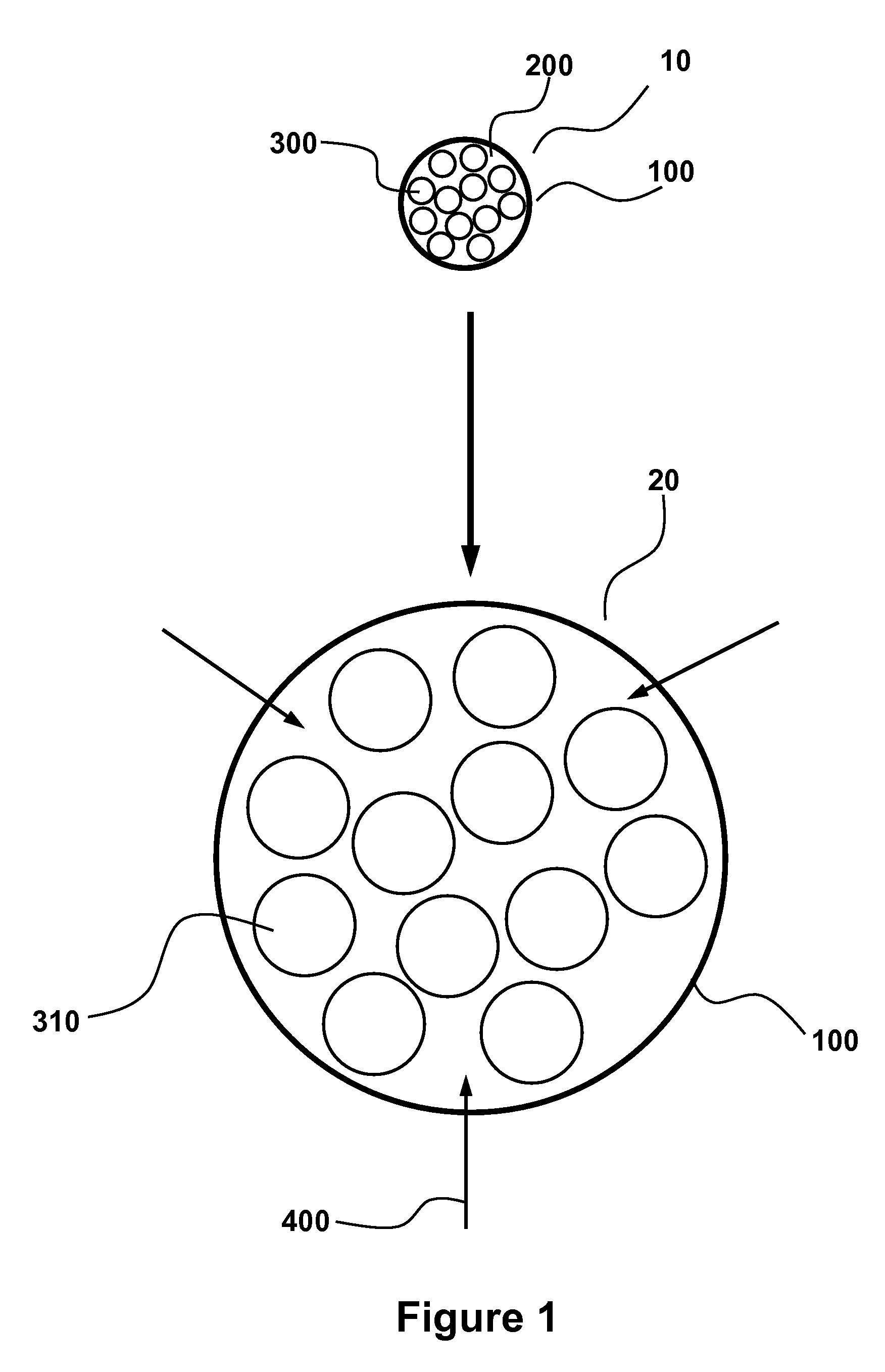
Injectable hollow tissue filler
The present invention comprises a plurality of injectable hollow particulate fillers suspended in a biocompatible fluid carrier to significantly improve the clumping resistance and injectability of the composition. The hollow particulate fillers have a lower effective density and are able to suspend in the carrier without precipitation. The loss of skin volume as a result of aging, diseases, weight loss, and injury can lead to uneven skin surface (e.g. wrinkle, etc.). The uneven skin can be repaired by injecting appropriate amount of hollow fillers underneath the skin. Some cases of urinary incontinence occur when the resistance to urine flow has decreased excessively. Continence is restored by injecting the present invention to the urethra tissue to increase resistance to urine outflow. Similarly, the present invention allows for the control of gastric fluid reflux by submucosal injections of the fillers to the esophageal-gastric and gastric-pyloric junction. For patients with vesicoureteral reflux, it can be treated by injection of the present invention into patients' ureteral tissue. This invention can also be used to repair defective or inadequately functioning muscles of the anal sphincter by administering an effective amount of injectable hollow fillers into the defect or anal sinuses.
Owner:CHU JACK FA DE
Alcohol Resistant Dosage Forms
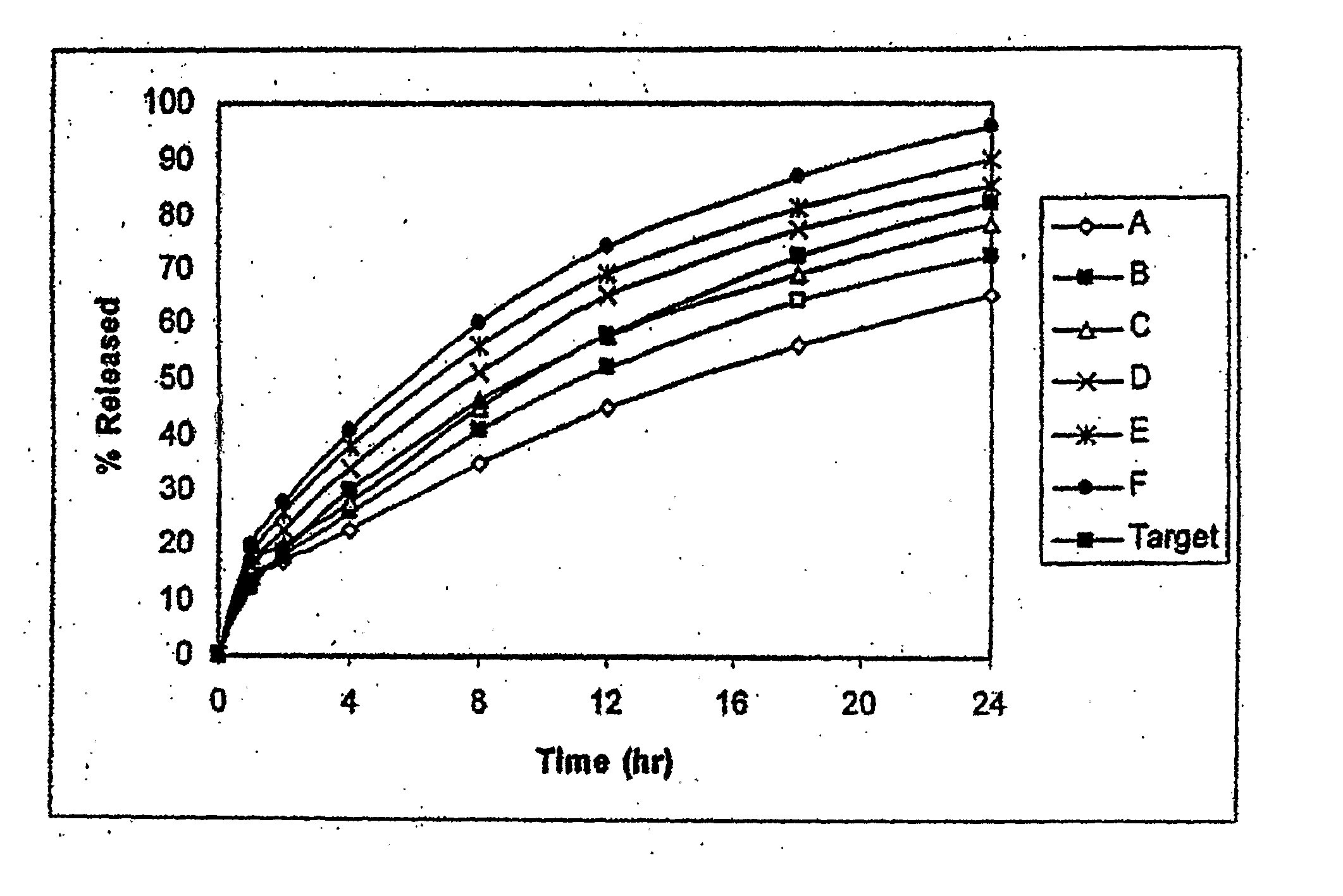
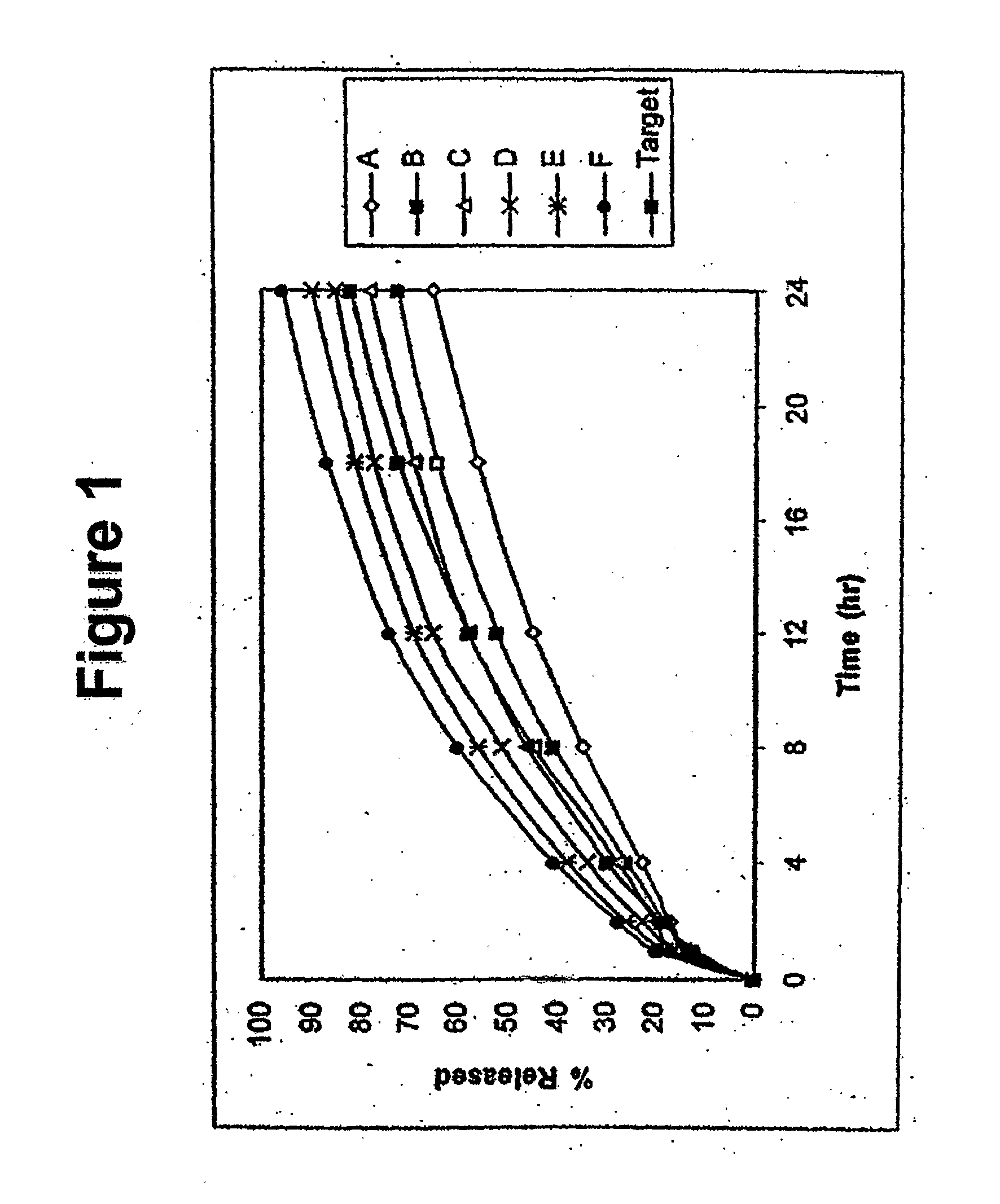
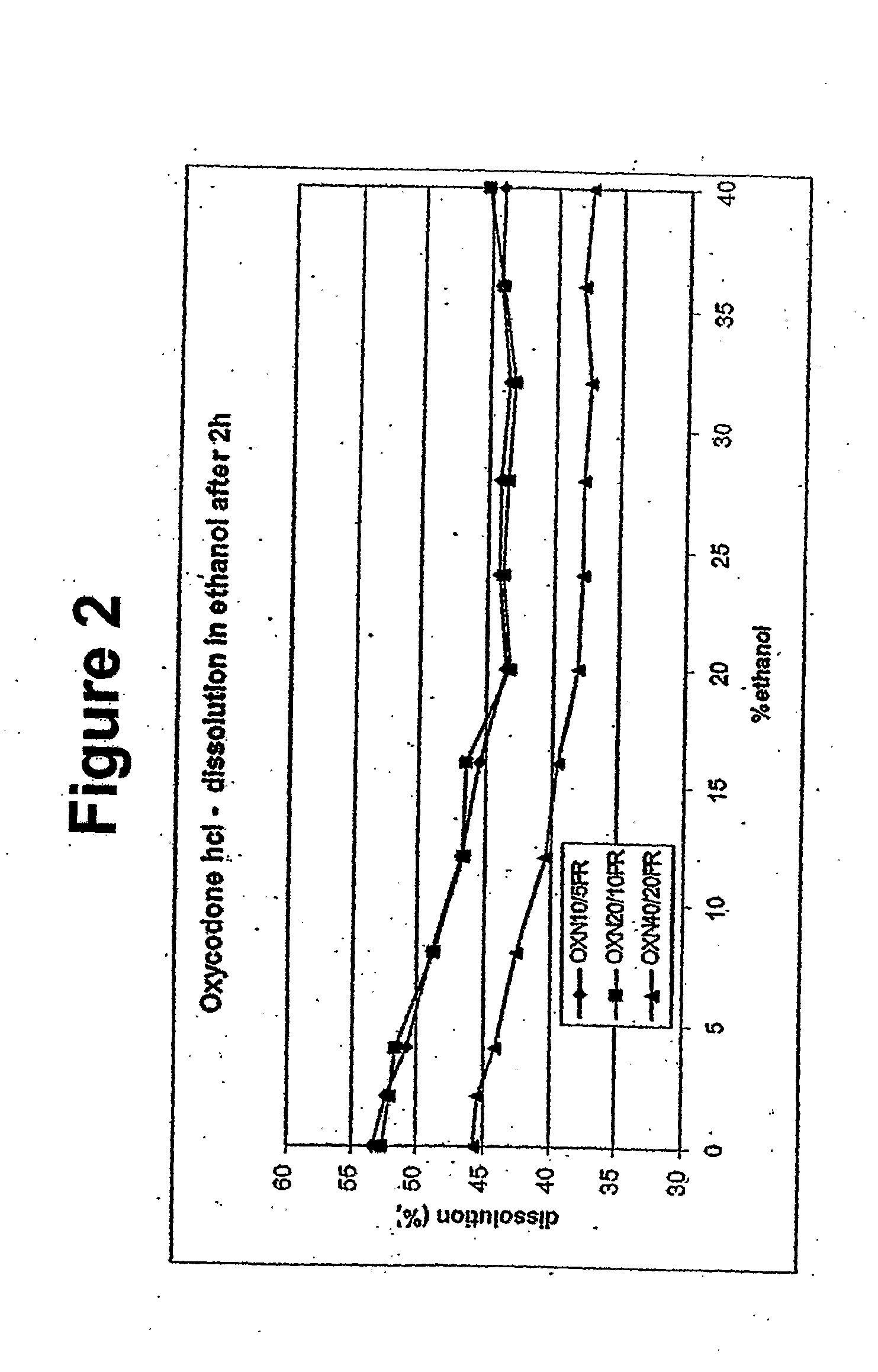
Disclosed in certain embodiments is a controlled release dosage form comprising a matrix comprising a pharmaceutically acceptable salt of an opioid analgesic in a controlled release material; wherein less than 25% of the opioid salt is released after 1 hour of in-vitro dissolution of the dosage form in 900 ml of Simulated Gastric Fluid with 20% ethanol using a USP Apparatus I (basket) apparatus at 100 rpm at 37 degrees C.°.
Owner:PURDUE PHARMA LP
Pharmaceutical composition comprising cyclosporin solid-state microemulsion
InactiveUS6306434B1Easy to controlMaintaining blood concentrationPowder deliveryCyclic peptide ingredientsIntestinal fluidBlood concentration
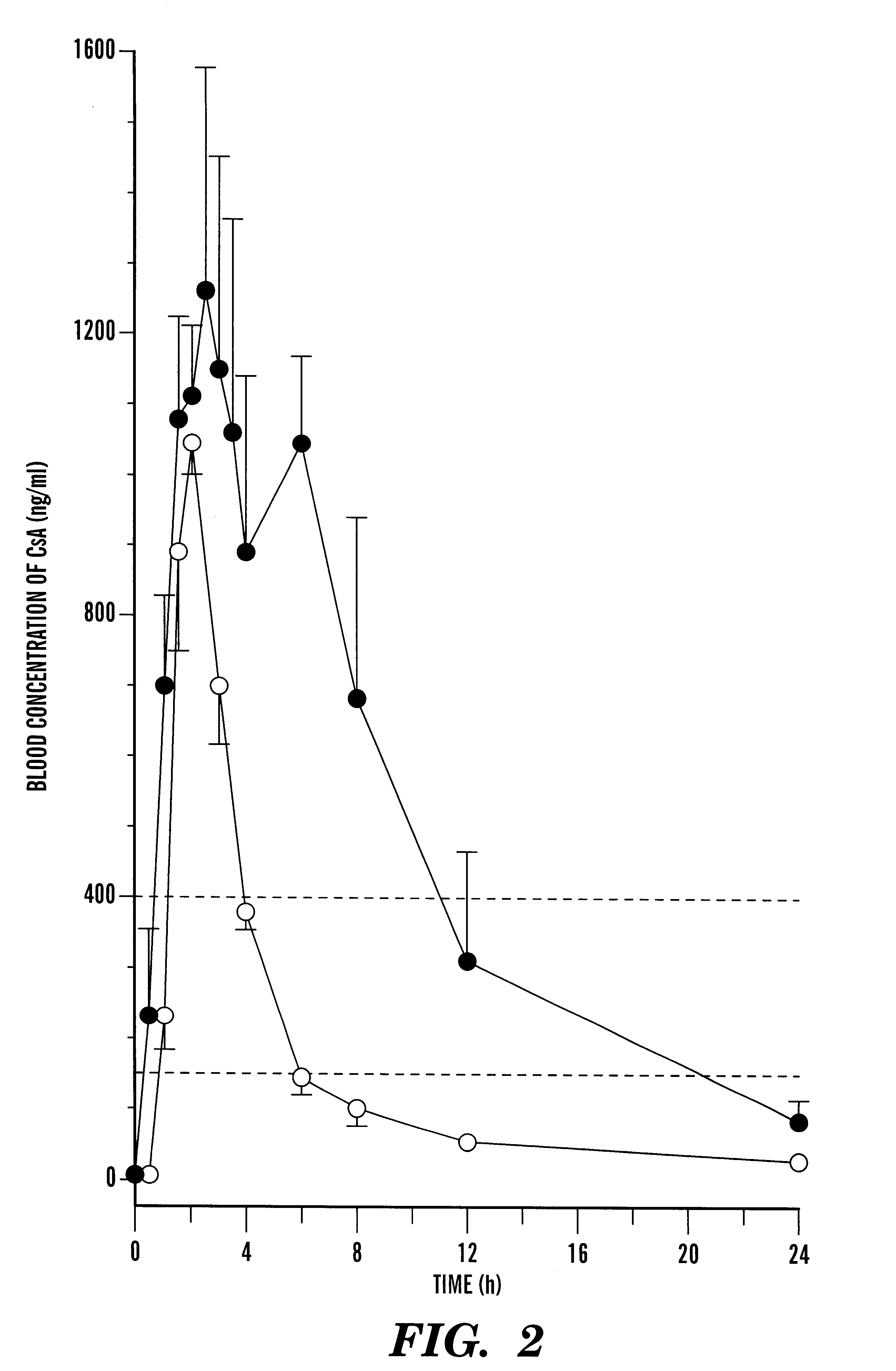
A pharmaceutical composition comprising a cyclosporin solid-state microemulsion is disclosed. In a preferred embodiment, the composition comprises a cyclosporin microemulsion dispersed in an enteric carrier. The composition does not dissolve in external phases such as artificial gastric fluid, but dissolves rapidly in artificial intestinal fluid, whereby it releases the cyclosporin microemulsion, providing rapid delivery of cyclosporin. The composition effectively maintains a therapeutic blood concentration of cyclosporin with once a day dosing, providing for convenience of administration and avoiding adverse effects induced by increasing peak blood cyclosporin concentrations associated with conventional cyclosporin formulations.
Owner:CHONG KUN DANG PHARMA CORP
Endoscopic gastric bypass
An endoscopic device separates ingested food from gastric fluids or gastric fluids and digestive enzymes, to treat obesity. In a particular embodiment a gastric bypass stent comprises a tubular member and two or more stent members defining a lumen. The tubular member has a substantially liquid impervious coating or covering and one or more lateral openings to permit one-way liquid flow.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Tamper-resistant oral opioid agonist formulations
Disclosed is an oral dosage form comprising (i) an opioid agonist in releasable form and (ii) a sequestered opioid antagonist which is substantially not released when the dosage form is administered intact, such that the ratio of the amount of antagonist released from said dosage form after tampering to the amount of said antagonist released from said intact dosage form is about 4:1 or greater, based on the in-vitro dissolution at 1 hour of said dosage form in 900 ml of Simulated Gastric Fluid using a USP Type II (paddle) apparatus at 75 rpm at 37 degrees C. wherein said agonist and antagonist are interdispersed and are not isolated from each other in two distinct layers.
Owner:PURDUE PHARMA LP
Superporous hydrogels for heavy-duty applications
InactiveUS7988992B2Improved strength and mechanical propertyPowder deliveryInorganic non-active ingredientsPolymer scienceGastric fluid
The present invention features modified superporous hydrogels (SPHs) and methods for their formation. The SPHs of the present invention are prepared by careful selection of the hydrophobic / hydrophilic reactive ingredients and by harmonizing the foaming and polymerization reactions, which results in the formation of SPHs having a homogeneous structure and favorable physical and mechanical properties, including swelling, strength, ruggedness, and resiliency. The SPHs of the present invention are particularly useful when employed in very harsh swelling environments, such as the low pH environment of the gastric fluid of the stomach, for extended periods of time.
Owner:KOS LIFE SCI
Swallowable Self-Expanding Gastric Space Occupying Device
InactiveUS20090192535A1Suppresses individual 's hungerFilling stomachDilatorsObesity treatmentGastric fluidMembrane configuration
Disclosed is a swallowable self-expanding gastric space occupying device and related methods of making and using the device to control obesity. The device has a membrane through which gastric liquid can pass, wherein the membrane provides an enclosure volume separated from the gastric environment by the membrane. A plurality of self-expanding components is contained in the enclosure volume, so that the components expand in volume upon contact with the gastric fluid, thereby expanding the device from an unexpanded volume to an expanded volume. Composite membranes provide the ability to precisely control how and when the membrane degrades in the stomach, to release the self-expanding components from the enclosure and to ensure that the released pieces are sufficiently small to not obstruct any portion of the gastrointestinal system.
Owner:7L LLC
Self emulsifying compositions for delivering lipophilic coenzyme Q10 and other dietary ingredients
InactiveUS20060051462A1Oral administration is convenientIncrease loadOrganic active ingredientsFood ingredientsSolubilityDietary supplement
The present invention provides novel dietary supplement compositions based on the use of a particular oil phase which comprises of Coenzyme Q10 and optionally other lipophilic dietary ingredients of low water solubility and a liquid mixture which comprises one or more emulsifiers, a fatty acid monoester formed between an short chain alcohol of C1 to C4 chain length and a saturated, or mono-unsaturated, or di-unsaturated (both conjugated and non-conjugated) fatty acid of C6 to C24 chain length, or medium chain mono- / di-esters, or the mixture of above. The composition is in a form of self-emulsifiable in the aqueous medium, for example, a simulated gastric fluid, which should provide a high oral bioavailability for the lipophilic dietary ingredients.
Owner:WANG JIMMY X
Rapidly expanding composition for gastric retention and controlled release of therapeutic agents, and dosage forms including the composition
InactiveUS20040234608A1Improved gastric retentionHigh retention ratePowder deliveryOrganic active ingredientsGastric fluidAttention deficits
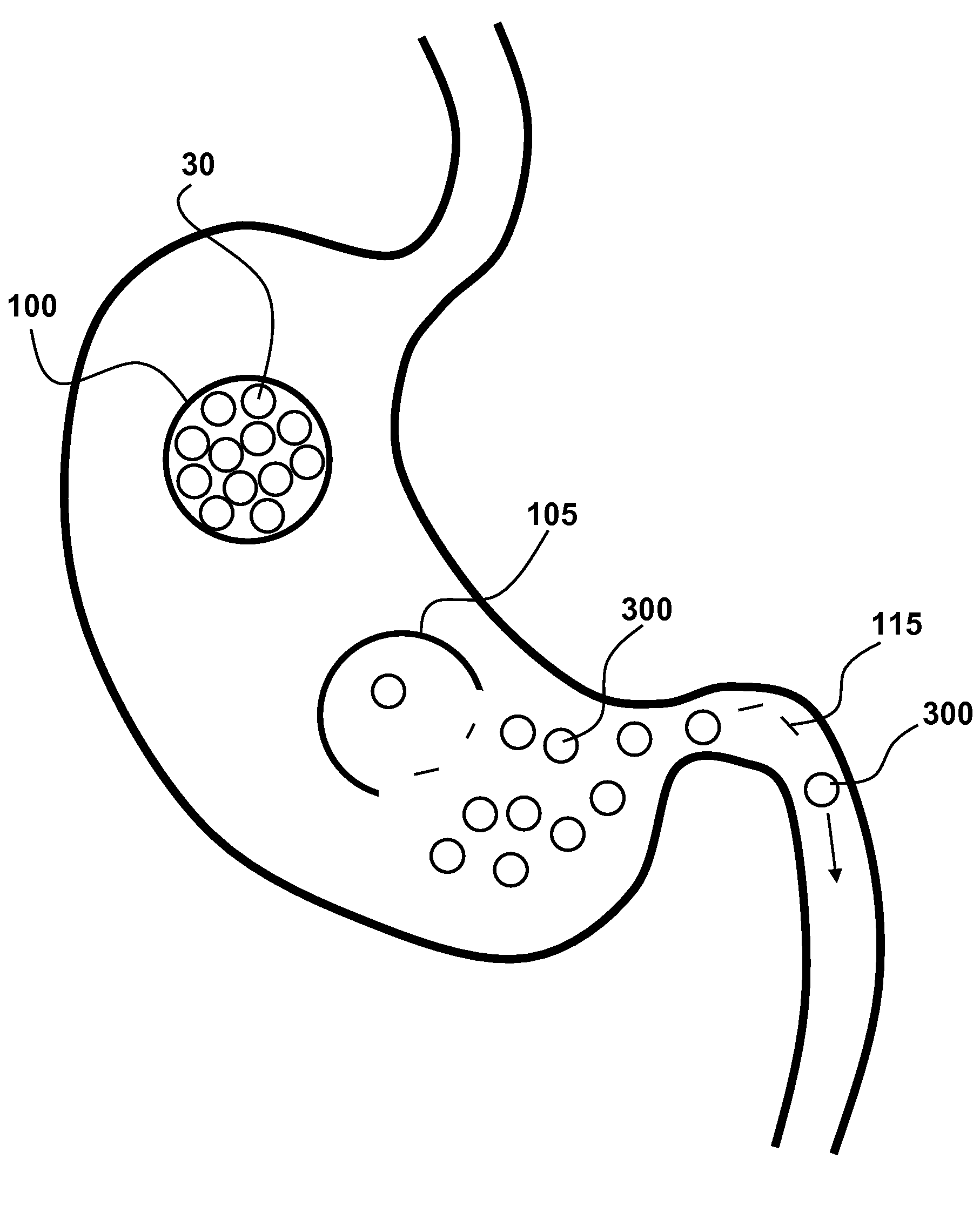
The present invention provides a pharmaceutical composition for use in a dosage form for oral administration to a patient. The composition expands upon contact with gastric fluid and promotes retention of the dosage form in the patient's stomach for a prolonged period of time. The present invention further provides pharmaceutical dosage forms containing an active ingredient, and the pharmaceutical composition. The forms are adapted for immediate or controlled release of the active ingredient. The dosage forms may be used advantageously in the treatment of Parkinson's disease with levodopa and hyperactivity and attention deficit disorder with methylphenidate.
Owner:TEVA PHARM USA INC
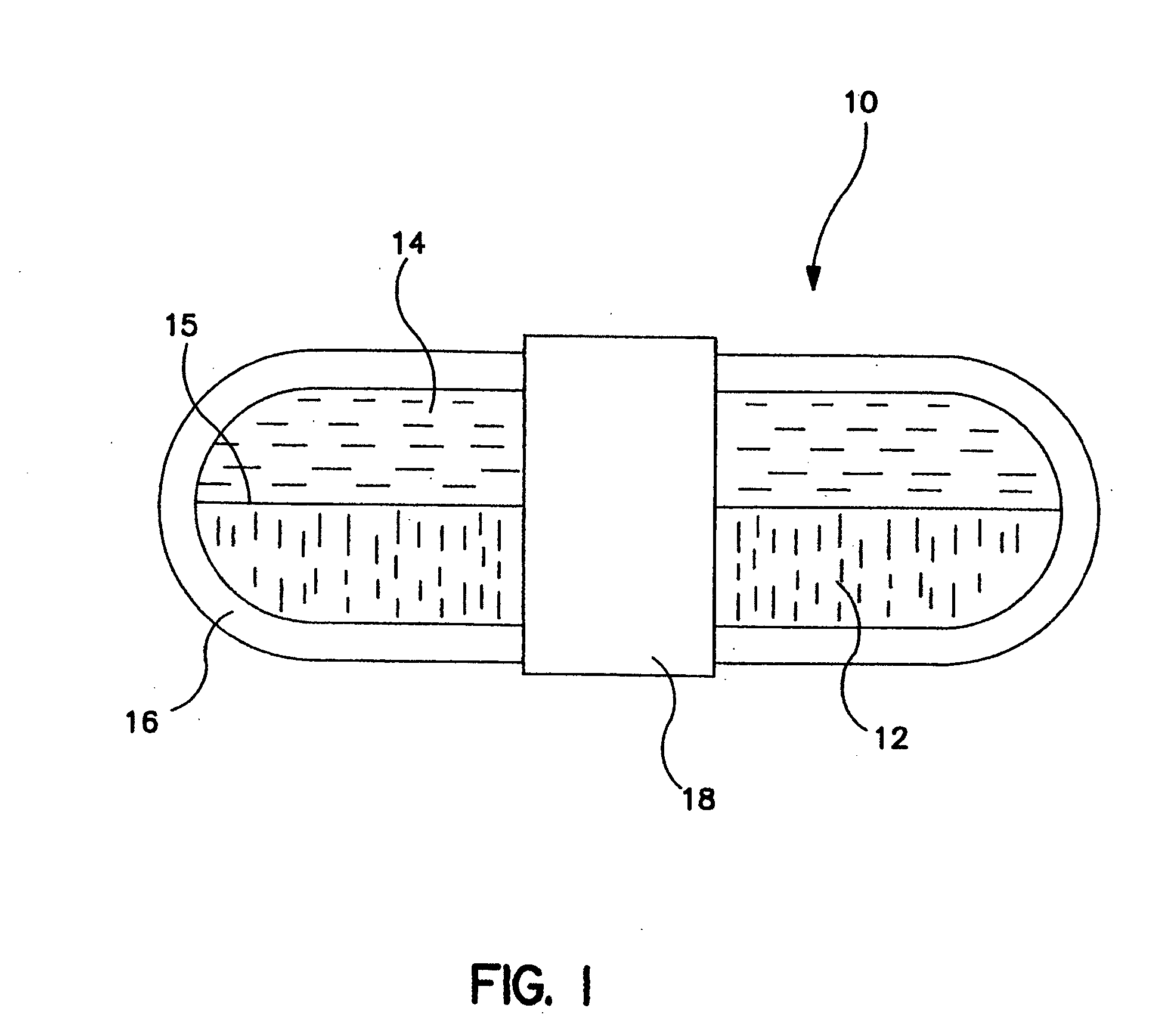
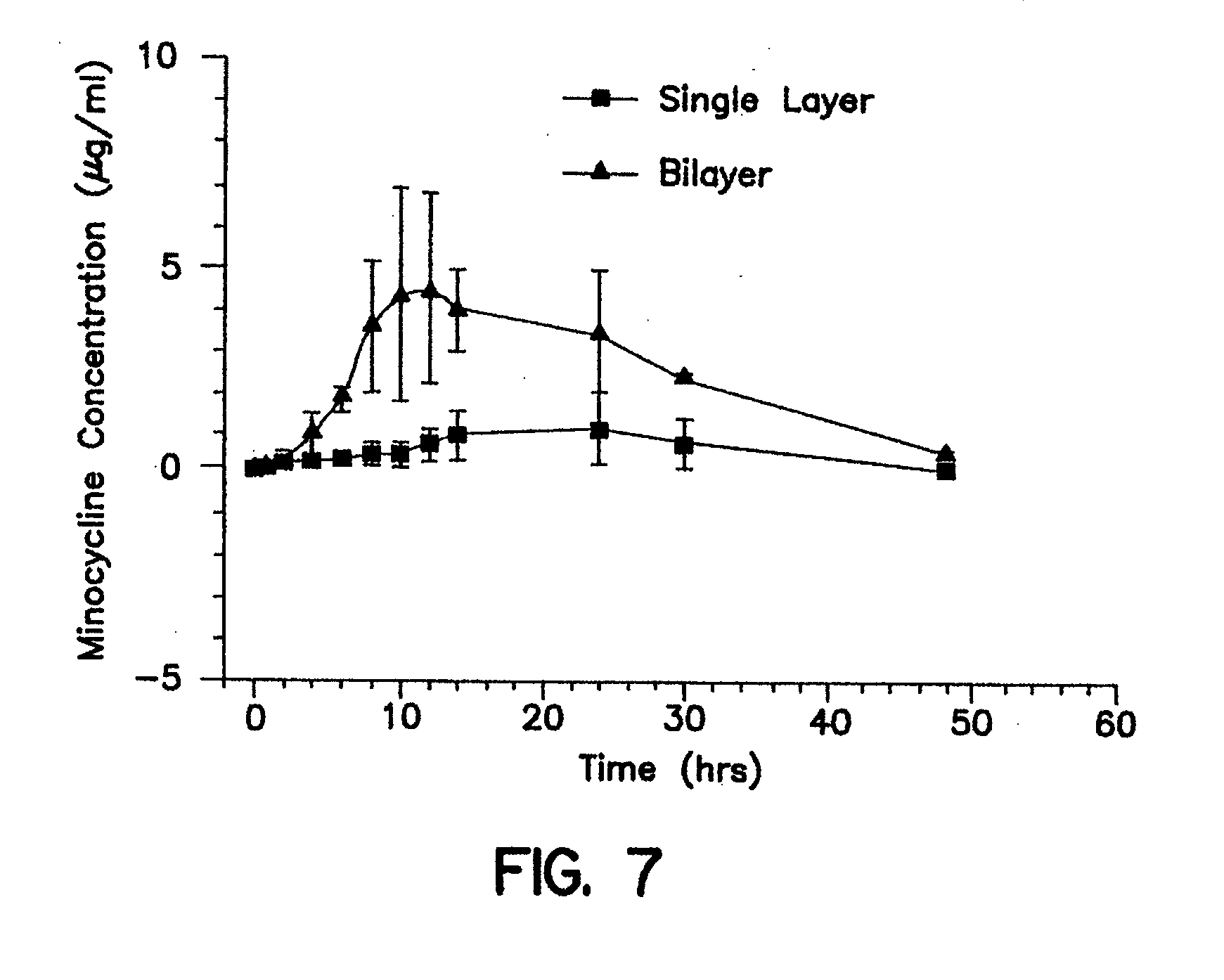
Gastric retention dosage form having multiple layers
The present invention is directed to a multilayered dosage form which is adapted for retention in the stomach and useful for the prolonged delivery of an active agent to a fluid environment of use. The active agent dosage form is a multilayer core, often bilayer, formed of polymer matrices that swell upon contact with the fluids of the stomach. At least one layer of the multilayered dosage form includes an active agent. A portion of the polymer matrices are surrounded by a band of insoluble material that prevents the covered portion of the polymer matrices from swelling and provides a segment of the dosage form that is of sufficient rigidity to withstand the contractions of the stomach and delay expulsion of the dosage form from the stomach until substantially all of the active agent has been dispensed.
Owner:ENCINAL PHARMA INVESTMENTS
Novel gastroretentive delivery system
InactiveUS20110268666A1High mechanical strengthIncrease valueAntibacterial agentsBiocideAdditive ingredientGastric fluid
A novel gastroretentive delivery system comprising a tablet comprising a pharmaceutical ingredient or diagnostic, which tablet comprises a gas releasing ingredient or a tandem of two gas releasing ingredients, an ingredient capable of unrestricted swelling in gastric fluid, an ingredient capable of limiting the unrestricted swelling and a hardening ingredient. The said system is based on the use of three different gastroretentive mechanisms: flotation, swelling and mechanical strength, the three mechanisms acting in a complimentary way. Processes for manufacturing same and methods of treatment are also disclosed.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
Pharmaceutical compositions with improved dissolution
InactiveUS20050025791A1Quantity minimizationIncrease speedPowder deliveryOrganic compounds purification/separation/stabilisationSolubilityDrug compound
The invention relates to methods of screening mixtures containing a pharmaceutical compound and an excipient to identify properties of the pharmaceutical compound / excipient combination that retard solid-state nucleation. The invention further relates to increasing the solubility, dissolution and bioavailability of a drug with low solubility in gastric fluids conditions by combining the drug with a recrystallization / precipitation retardant and an optional enhancer.
Owner:TRANSFORM PHARMACEUTICALS INC
Rosiglitazone formulations
Rosiglitazone is a drug used to treat type 2 diabetes. Methods for the formation of amorphous rosiglitazone and formulations comprising the amorphous rosiglitazone are described. Other formulations include pulsed-release formulations and formulations for retention in the stomach and upper gastrointestinal tract. Controlled-release dosage form include those wherein the maximum plasma concentration of rosiglitazone occurs greater than one hour after administration to a human and / or wherein less than 75 percent by weight of the rosiglitazone is released at 1 hour after immersion in simulated gastric fluid.
Owner:ACTAVIS GRP PTC EHF
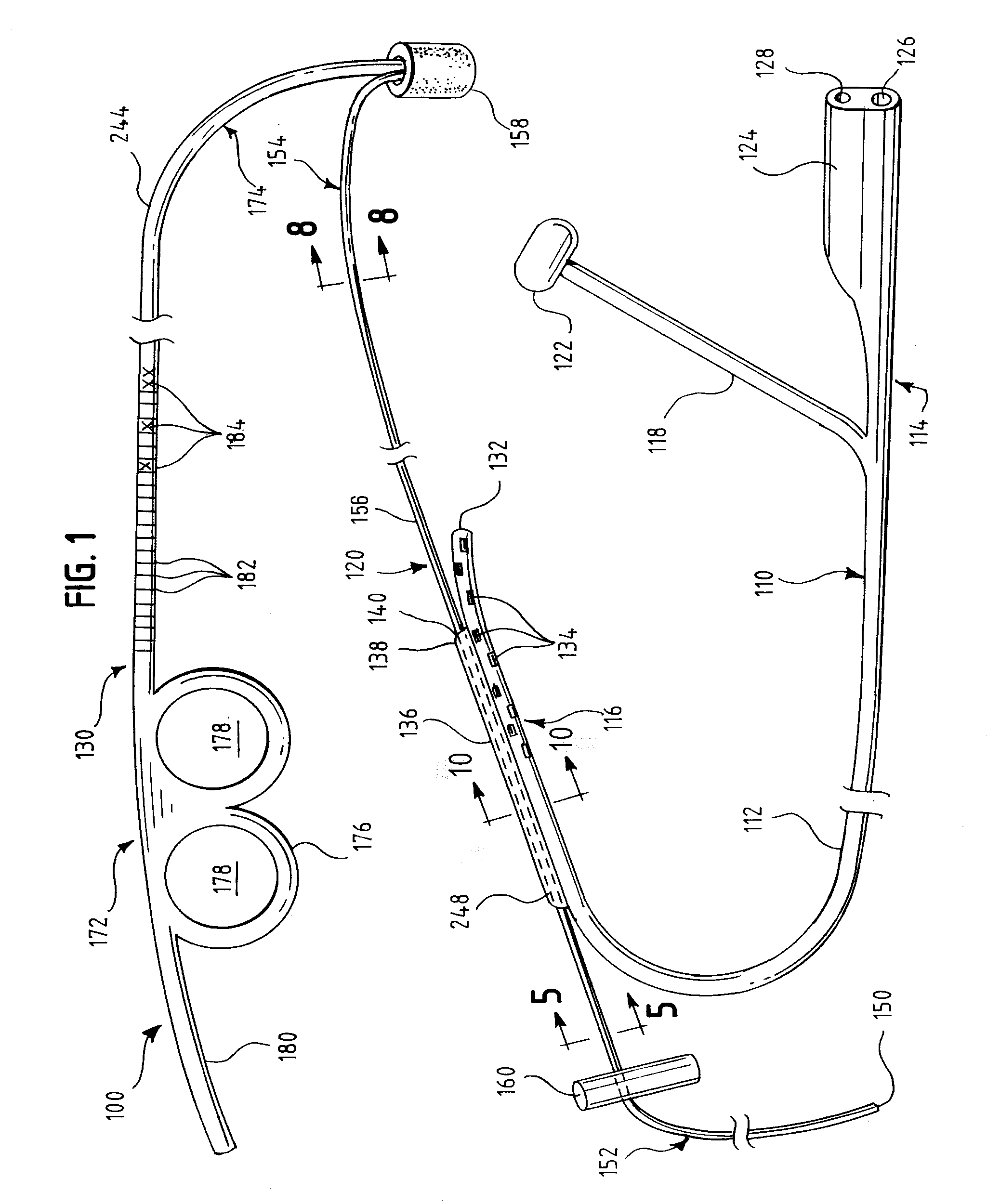
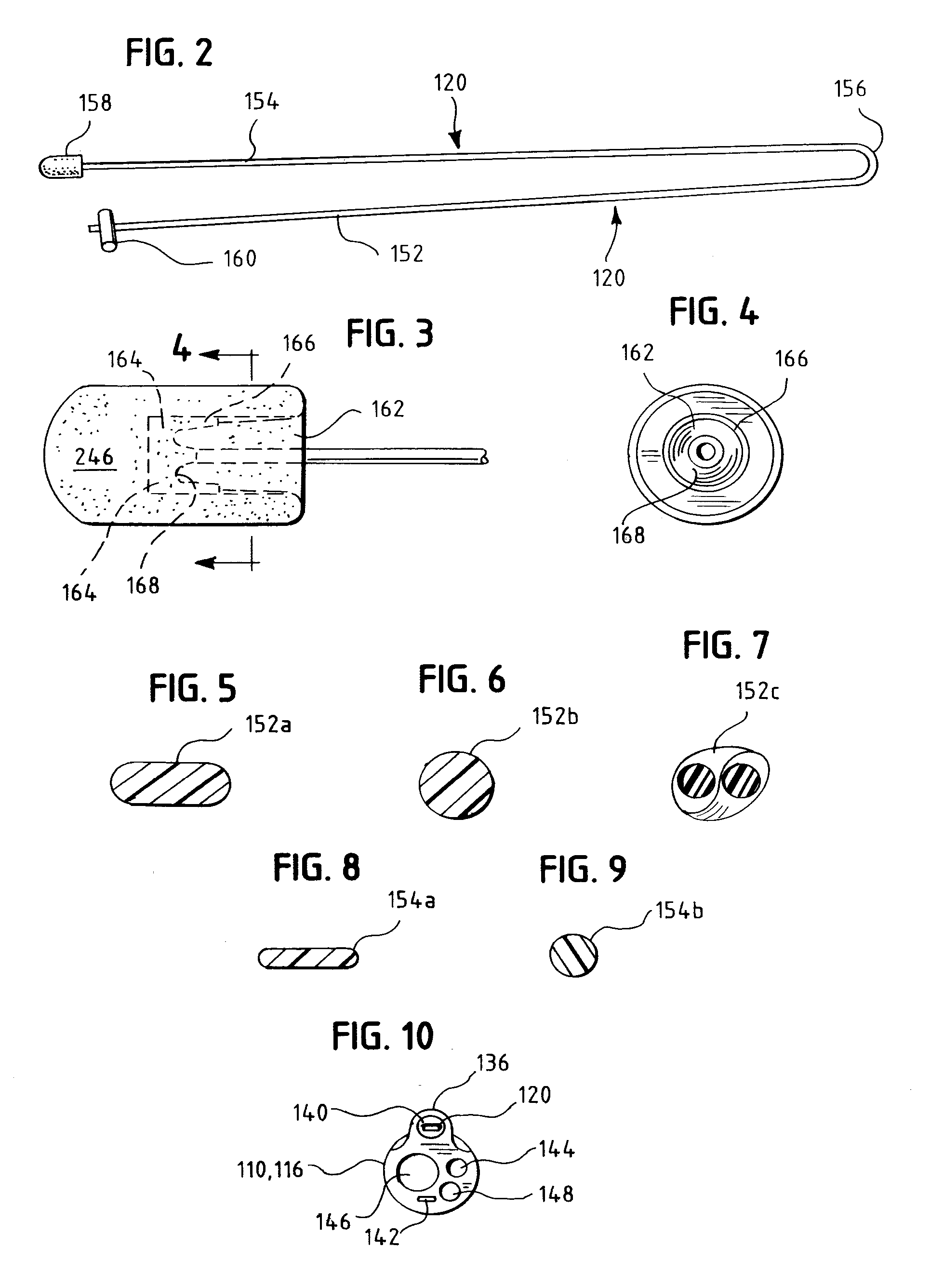
Insertion System and Methods for Nasogastric Tubes
ActiveUS20080004598A1Reduce disadvantagesEasy to controlDiagnosticsInfusion syringesNasal passageNasal passages
A nasogastric tube insertion system comprises a nasogastric tube, a guide element, and an inserter element. The inserter element has a slim, elongate main body, a handle attached to the body, and an anatomically curved insertion section. The guide element comprises a swallowable weight attached to a cord, string, monofilament line, tube, or other similar line. The swallowable weight may be ablative in the presence of stomach fluids or may be deflated to allow the guide element to be removed while the nasogastric tube remains in place. The inserter element is inserted through the patient's nasal passages and optionally into the oropharynx. The weight is released and the patient swallows it into the stomach. The guide element is threaded through the guide element retaining structure, and the nasogastric tube is safely inserted along the guide element into the patient's stomach. Chemical property indicators sensitive to fluids found in the stomach may be provided in the nasogastric tube or the guide element to verify correct placement of the nasogastric tube in the stomach.
Owner:CRITICAL DEVICE CORP
Swallowable self-expanding gastric space occupying device
InactiveUS8287562B2Filling stomachSuppresses the individual's hungerDilatorsObesity treatmentGastric fluidObesity
Owner:7L LLC
Pharmaceutical compositions and methods for treating or preventing oxalate-related disease
ActiveUS20070178070A1Suitable storage shelf-lifeAvoid any substantial degradationBiocideNervous disorderOral medicationDelivery vehicle
The present invention comprises methods and compositions for the reduction of oxalate in humans, animals and plants. For example, the invention provides methods and compositions for the delivery of one or more oxalate-reducing pharmaceutical compositions to the intestinal tracts of persons and animals. The methods and compositions can be used in treating and preventing oxalate-related conditions. A composition of the invention comprises an oral delivery vehicle comprising an oxalate degrading bacteria, one or more cryopreserving agents and one or more excipients. A composition of the invention is enteric coated and has a suitable shelf-life and acceptable properties to avoid negative impact from gastric fluid when it is orally administered.
Owner:OXTHERA INTPROP
Formulations for a tight junction effector
ActiveUS20070196501A1Stable in gastric fluidAntibacterial agentsBiocideIntestinal fluidSmall intestine
Enteric compositions comprising one or more tight junction agonists and / or one or more tight junction antagonists are provided. Compositions of the invention may comprise a delayed-release coating disposed over a tight junction agonist and / or tight junction antagonist layer which may be disposed over an inert core. Delayed-release coatings may be substantially stable in gastric fluid and substantially unstable in intestinal fluid, thus providing for substantial release of the tight junction agonist and / or antagonist from the composition in the duodenum or jejunum of the small intestine.
Owner:ALBA THERAPEUTICS CORP
Pharmaceutical compositions with improved dissolution
InactiveUS20060134198A1Quantity minimizationImprove throughputPowder deliveryBiocideSolubilityDrug compound
The invention relates to methods of screening mixtures containing a pharmaceutical compound and an excipient to identify properties of the pharmaceutical compound / excipient combination that retard solid-state nucleation. The invention further relates to increasing the solubility, dissolution and bioavailability of a drug with low solubility in gastric fluids conditions by combining the drug with a precipitation retardant and an optional enhancer.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
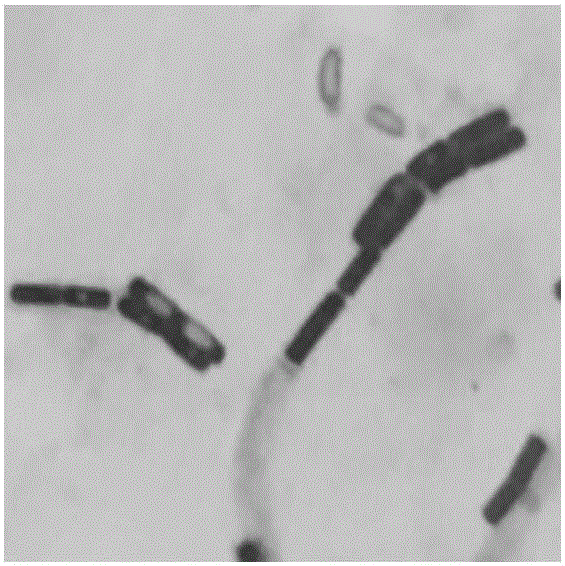
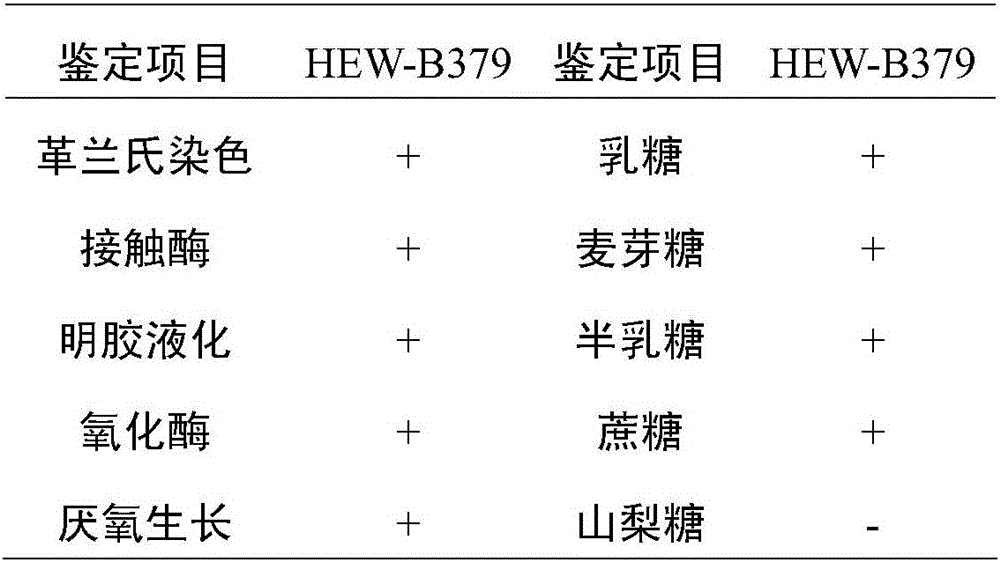
Bacillus coagulans HEW-B379 with probiotic effect, and application thereof
ActiveCN106011036AStrong heat resistanceStrong fermentation abilityAntibacterial agentsBacteriaEscherichia coliFeed conversion ratio
The invention provides a Bacillus coagulans HEW-B379 with a probiotic effect. The above strain is named as HEW-B379, and the preservation number of the strain is CGMCC No.12553. The Bacillus coagulans HEW-B379 has a substantial probiotic property, and can effectively inhibit growth breeding of enteropathogenic Escherichia coli, Staphylococcus aureus, Salmonella typhi, salmonella, Shigella, Proteus species, Shewanella putrefaciens and Pseudomonas aeruginosa. The Bacillus coagulans HEW-B379 has strong stress resistance, can resist high temperature and simulated gastric juice and simulate bile salt environment, can keep the survival rate of 99-100%, and can effectively adjust microbial balance of animal intestinal tracts, inhibit growth of harmful microbes, promote nutrition absorption of animals, improve the conversion rate of a feed and improve the productivity of the animals.
Owner:BEIJING HESWOF BIOTECH CO LTD
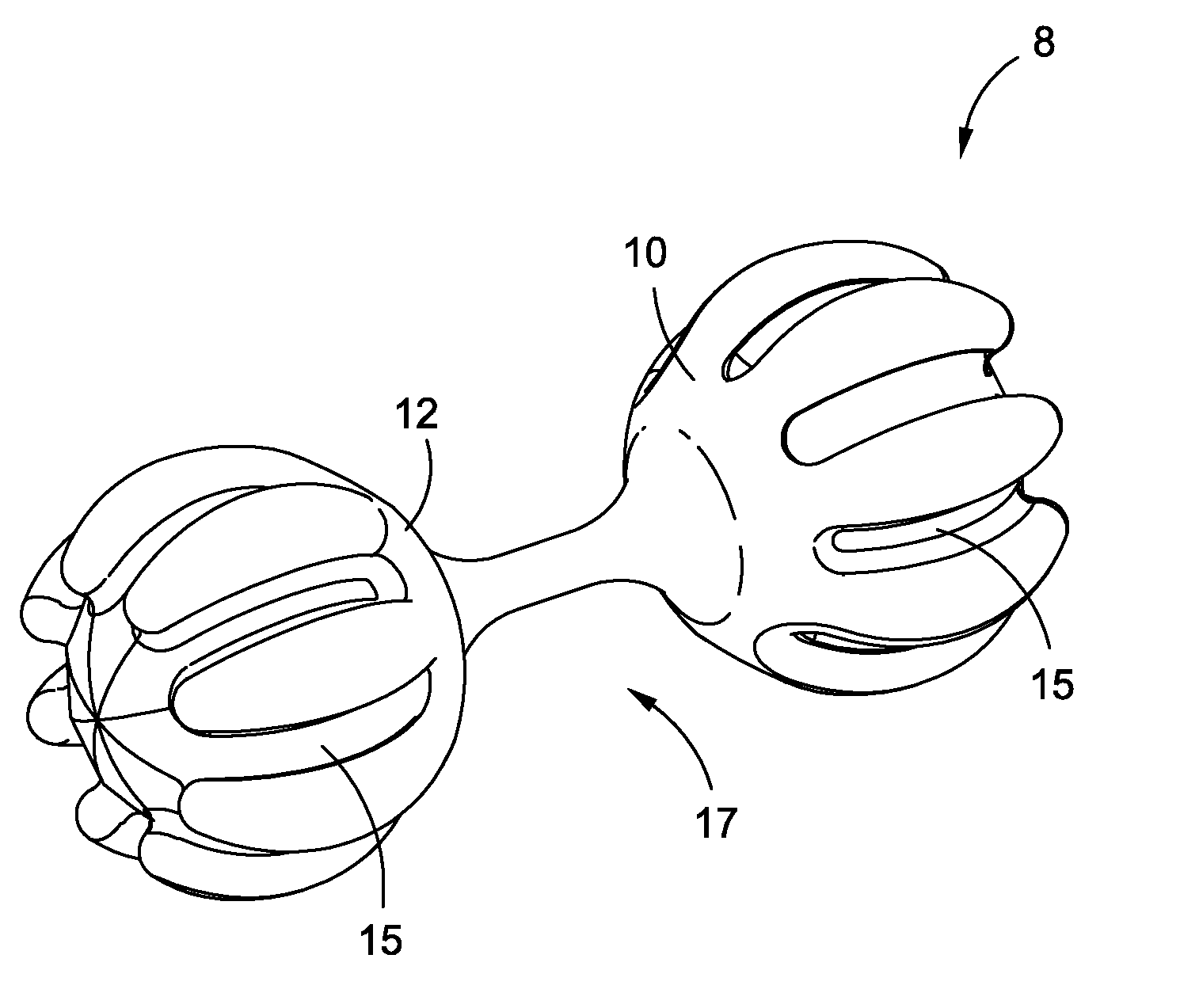
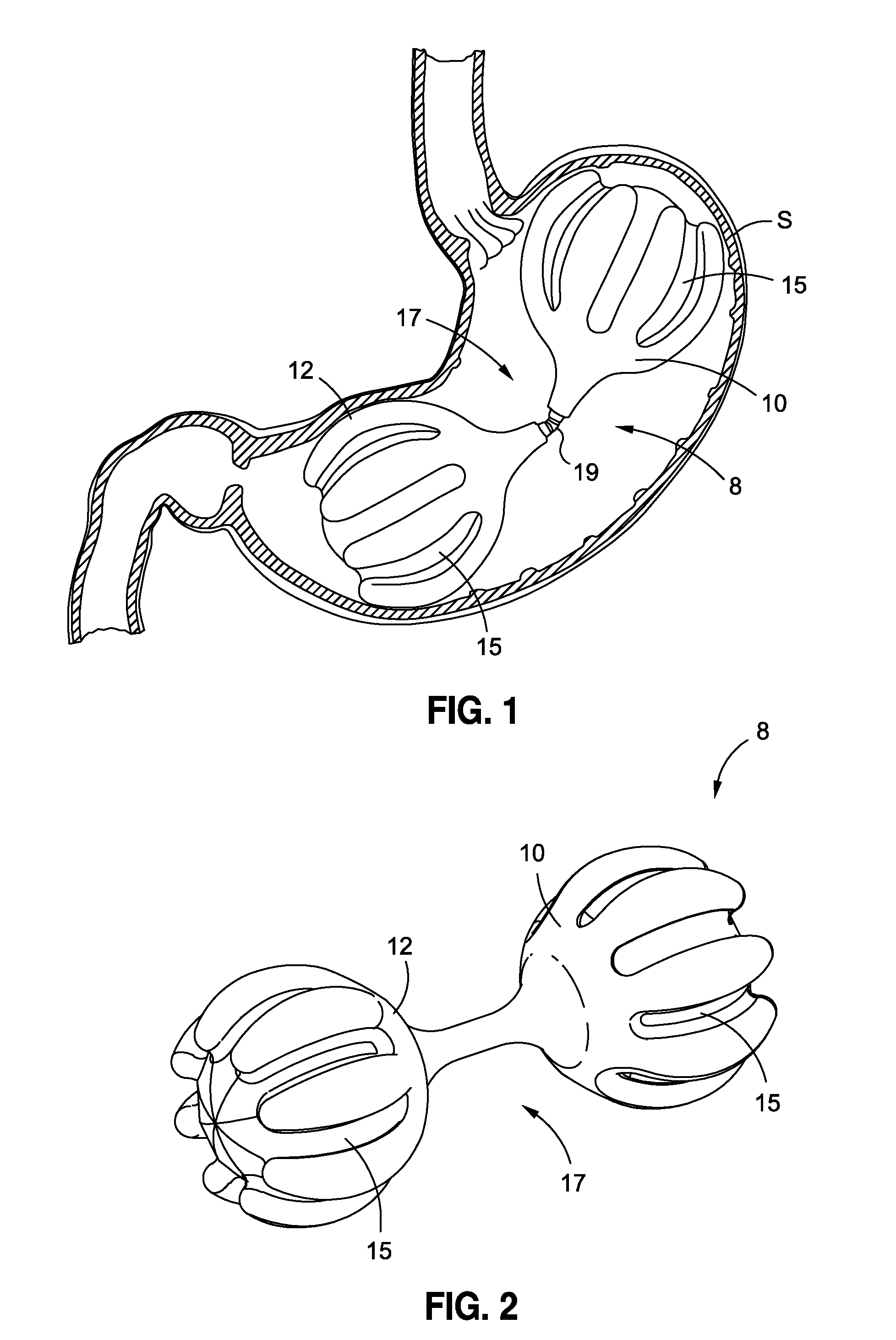
Intragastric implants with multiple fluid chambers
ActiveUS20120191125A1Inducing feeling of satietySmall internal volumeSurgeryDilatorsStomach wallsPump chamber
An intragastric obesity treatment implant promotes a feeling of satiety in the patient by contacting the insides of the stomach wall, reducing the space in the stomach, or otherwise reducing the amount of food consumed. One intragastric obesity treatment implant two inflatable balloons coupled via a flow restrictor through which fluid may flow in response to peristaltic motions of a patient's stomach. Additionally, one implant comprises a pumping chamber coupled to a reservoir, where the pumping chamber moves stomach fluids into the reservoir in response to peristaltic motions of the patient's stomach.
Owner:APOLLO ENDOSURGERY INC
Superporous Hydrogels for Heavy-Duty Applications
InactiveUS20080089940A1High strengthImprove mechanical propertiesPowder deliveryInorganic non-active ingredientsAdditive ingredientGastric fluid
The present invention features modified superporous hydrogels (SPHs) and methods for their formation. The SPHs of the present invention are prepared by careful selection of the hydrophobic / hydrophilic reactive ingredients and by harmonizing the foaming and polymerization reactions, which results in the formation of SPHs having a homogeneous structure and favorable physical and mechanical properties, including swelling, strength, ruggedness, and resiliency. The SPHs of the present invention are particularly useful when employed in very harsh swelling environments, such as the low pH environment of the gastric fluid of the stomach, for extended periods of time.
Owner:KOS LIFE SCI
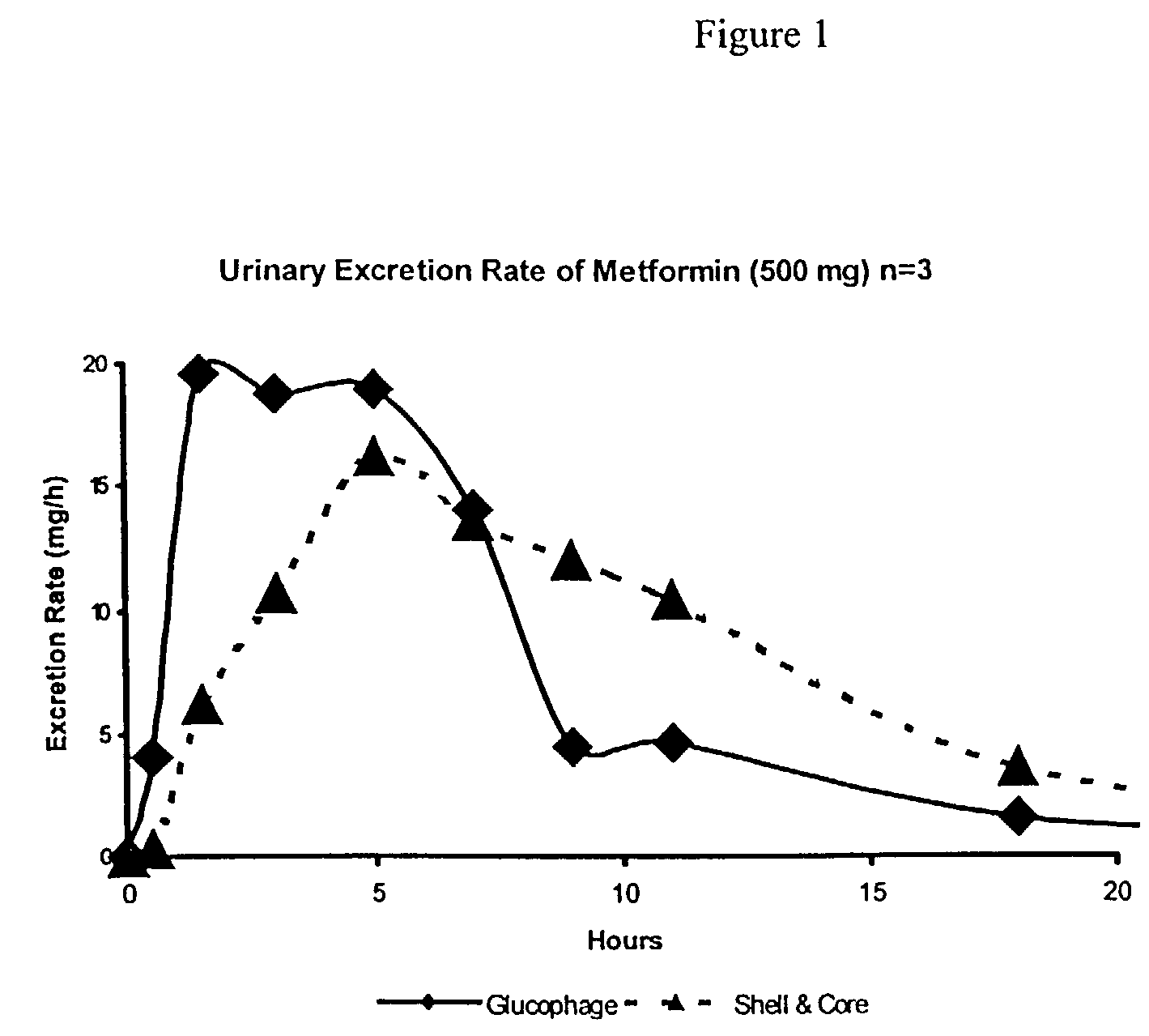
Shell-and-core dosage form approaching zero-order drug release
InactiveUS7736667B2Avoid insufficient thicknessHigh strengthSalicyclic acid active ingredientsPeptide/protein ingredientsControlled releaseRate limiting
Drugs are formulated as oral dosage forms for controlled release in which the release rate limiting portion is a shell surrounding the drug-containing core. The shell releases drug from the core by permitting diffusion of the drug from the core. The shell also promotes gastric retention of the dosage form by swelling upon imbibition of gastric fluid to a size that is retained in the stomach during the postprandial or fed mode.
Owner:DEPOMED SYST INC
Lactobacillus acidophilus and application thereof and feed additive thereof and premix compound thereof
ActiveCN102559539AStrong stress resistanceImprove immunityBacteriaMicroorganism based processesBiotechnologyHigh density
The invention belongs to the field of biotechnology, and relates to Lactobacillus acidophilus and application thereof and a feed additive thereof and a premix compound thereof. The Lactobacillus acidophilus of the invention has strong stress tolerance, and can tolerate simulated gastric fluid and simulated bile salt so as to guarantee that a great quantity of viable bacteria can smoothly enter the intestinal tract of an animal, and the viable bacteria play a probiotic role, so that the immunity of the animal is improved, and the breeding cost is reduced. The product obtained from the Lactobacillus acidophilus of the invention through culture, high-density fermentation and addition of protectant has a high content of viable Lactobacillus acidophilus, can be added as a biological feed additive in the drinking water or feed for breeding animals, and can be prepared into feed such as premix compound and so on, so as to reduce the addition of antibiotics, indeed improve the intestinal tract health and the immunity of animals, and increase the production performance of animals.
Owner:BEIJING DABEINONG TECH GRP CO LTD +2
Injectable hollow tissue filler
InactiveUS20110091564A1Equally distributedLow densityPowder deliverySkin implantsParticulatesEffective density
The present invention comprises a plurality of injectable hollow particulate fillers suspended in a biocompatible fluid carrier to significantly improve the clumping resistance and injectability of the composition. The hollow particulate fillers have a lower effective density and are able to suspend in the carrier without precipitation. The loss of skin volume as a result of aging, diseases, weight loss, and injury can lead to uneven skin surface (e.g. wrinkle, etc.). The uneven skin can be repaired by injecting appropriate amount of hollow fillers underneath the skin. Some cases of urinary incontinence occur when the resistance to urine flow has decreased excessively. Continence is restored by injecting the present invention to the urethra tissue to increase resistance to urine outflow. Similarly, the present invention allows for the control of gastric fluid reflux by submucosal injections of the fillers to the esophageal-gastric and gastric-pyloric junction. For patients with vesicoureteral reflux, it can be treated by injection of the present invention into patients' ureteral tissue. This invention can also be used to repair defective or inadequately functioning muscles of the anal sphincter by administering an effective amount of injectable hollow fillers into the defect or anal sinuses.
Owner:CHU JACK FA DE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com