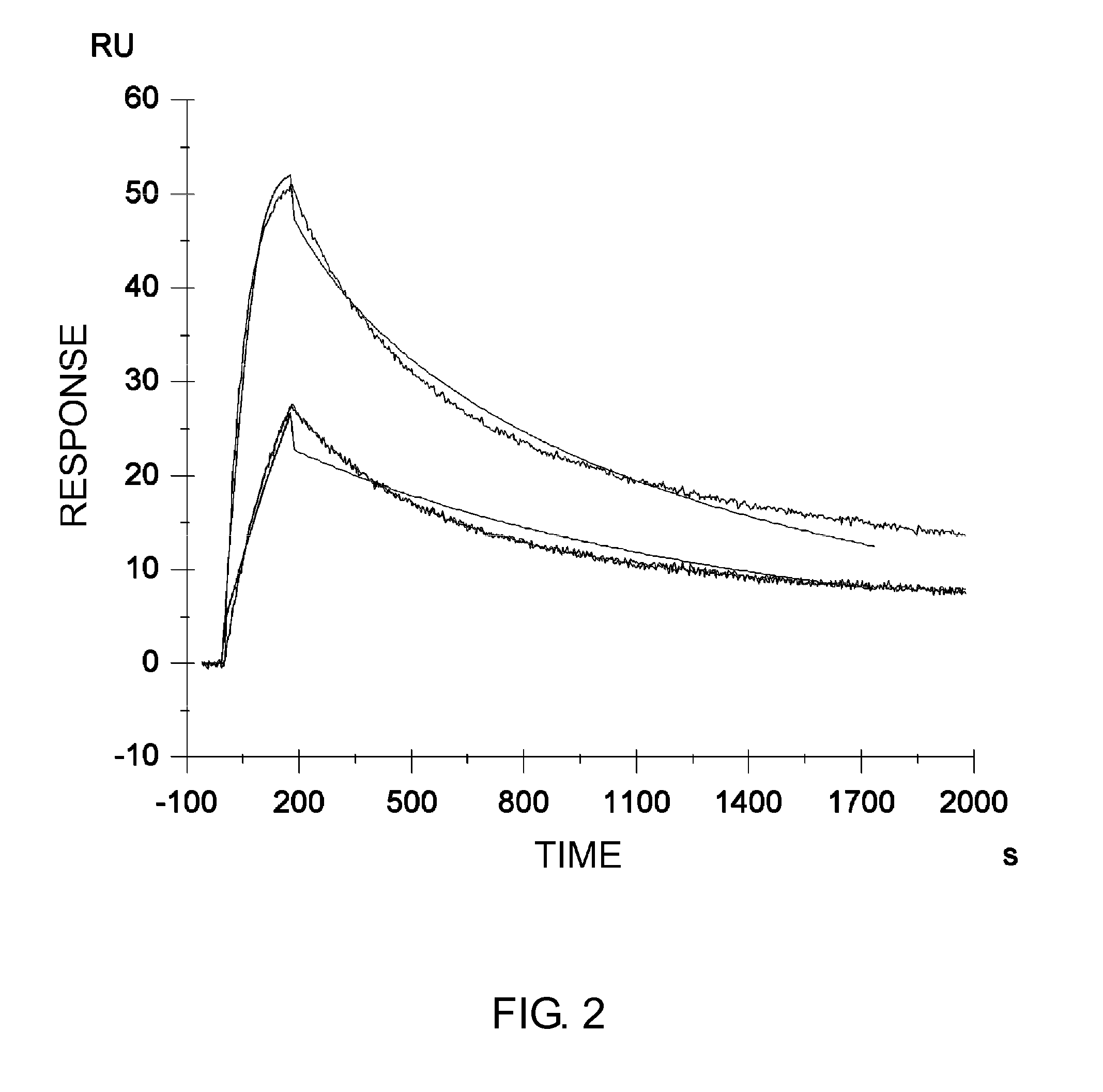
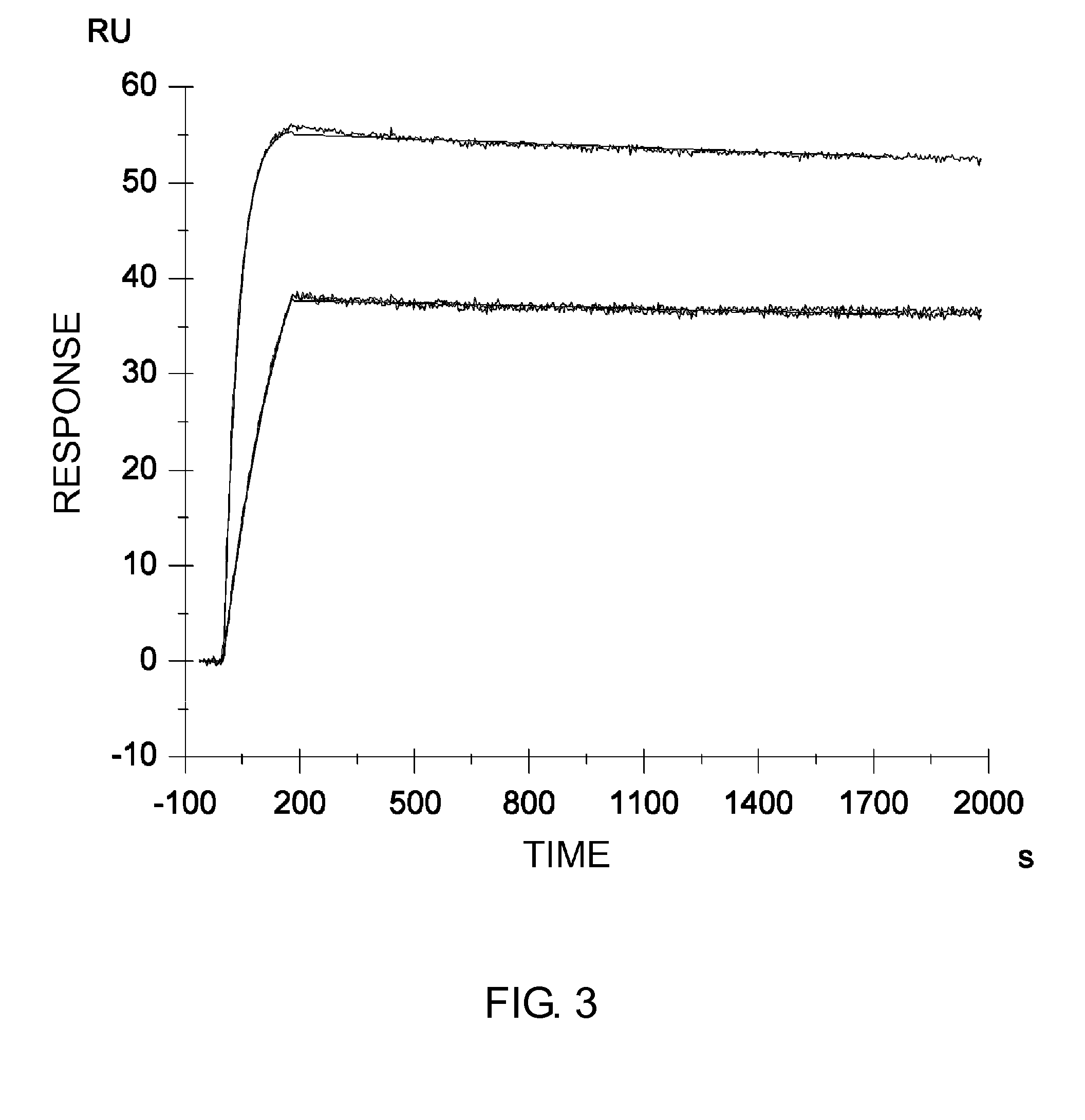
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3233results about How to "High retention rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
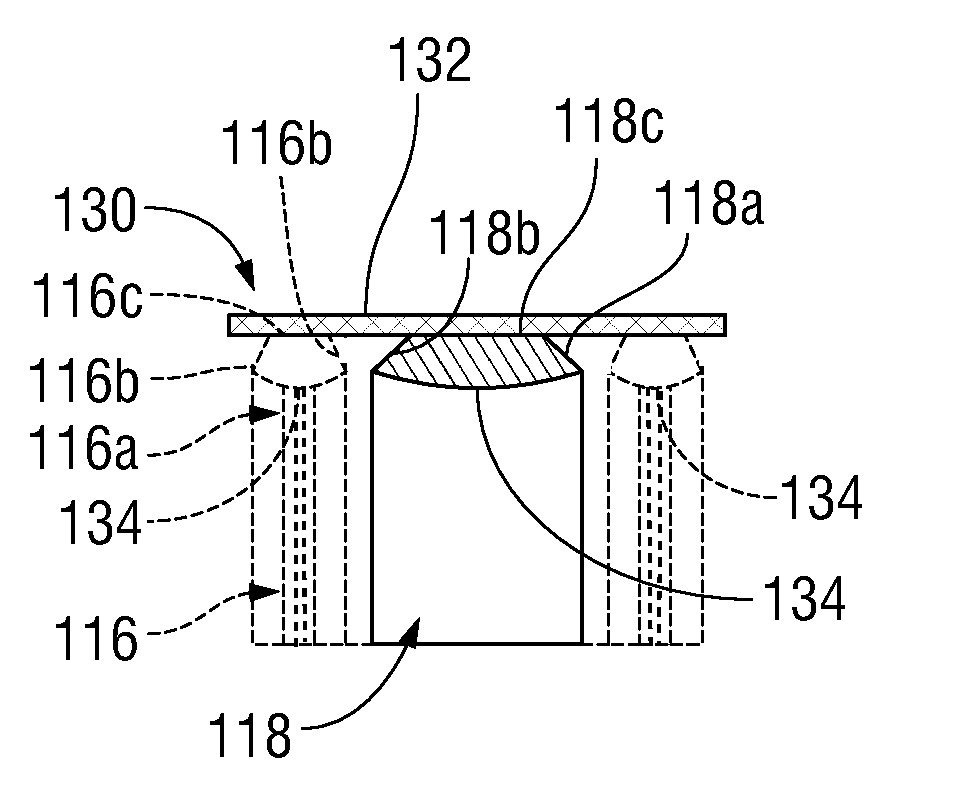
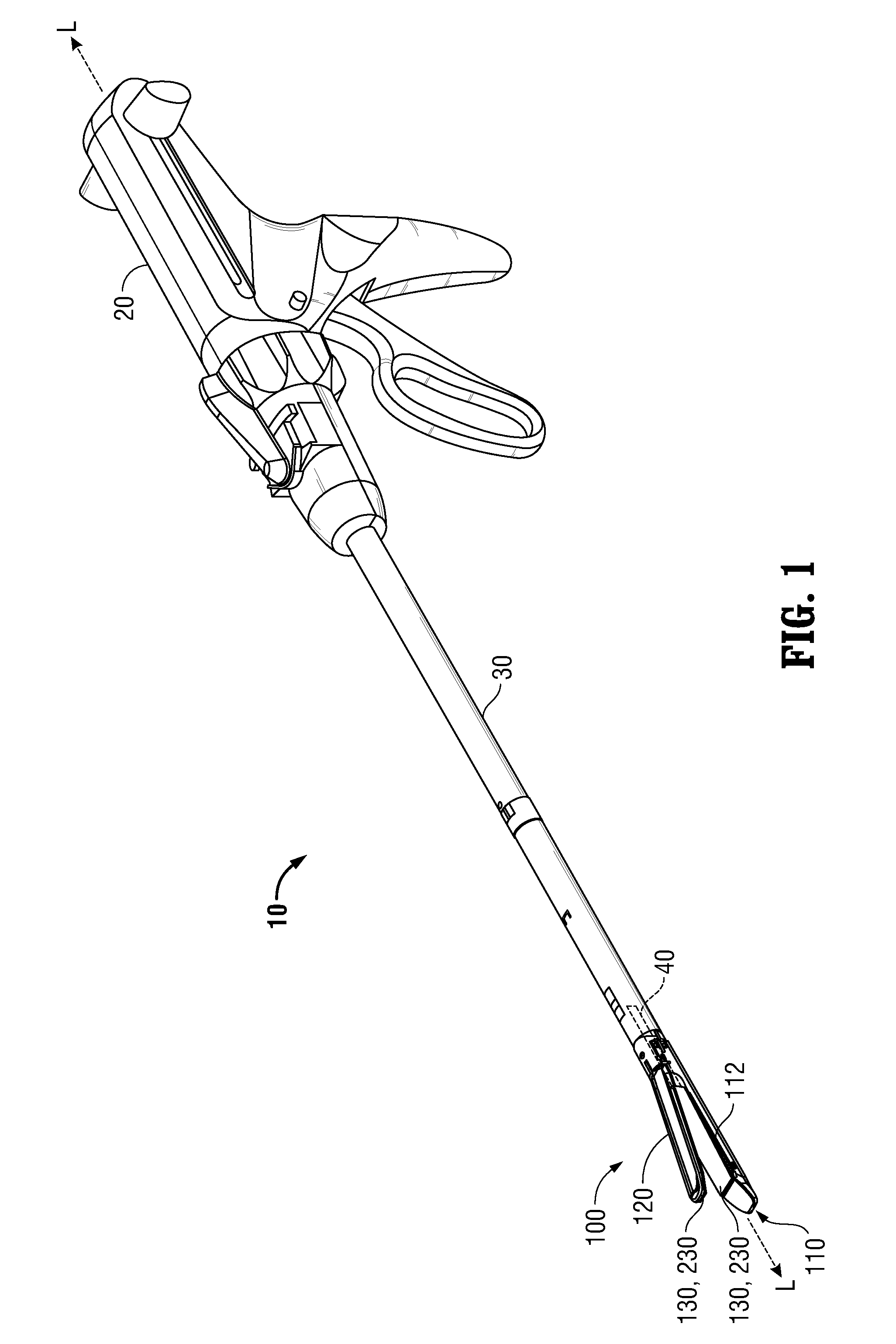
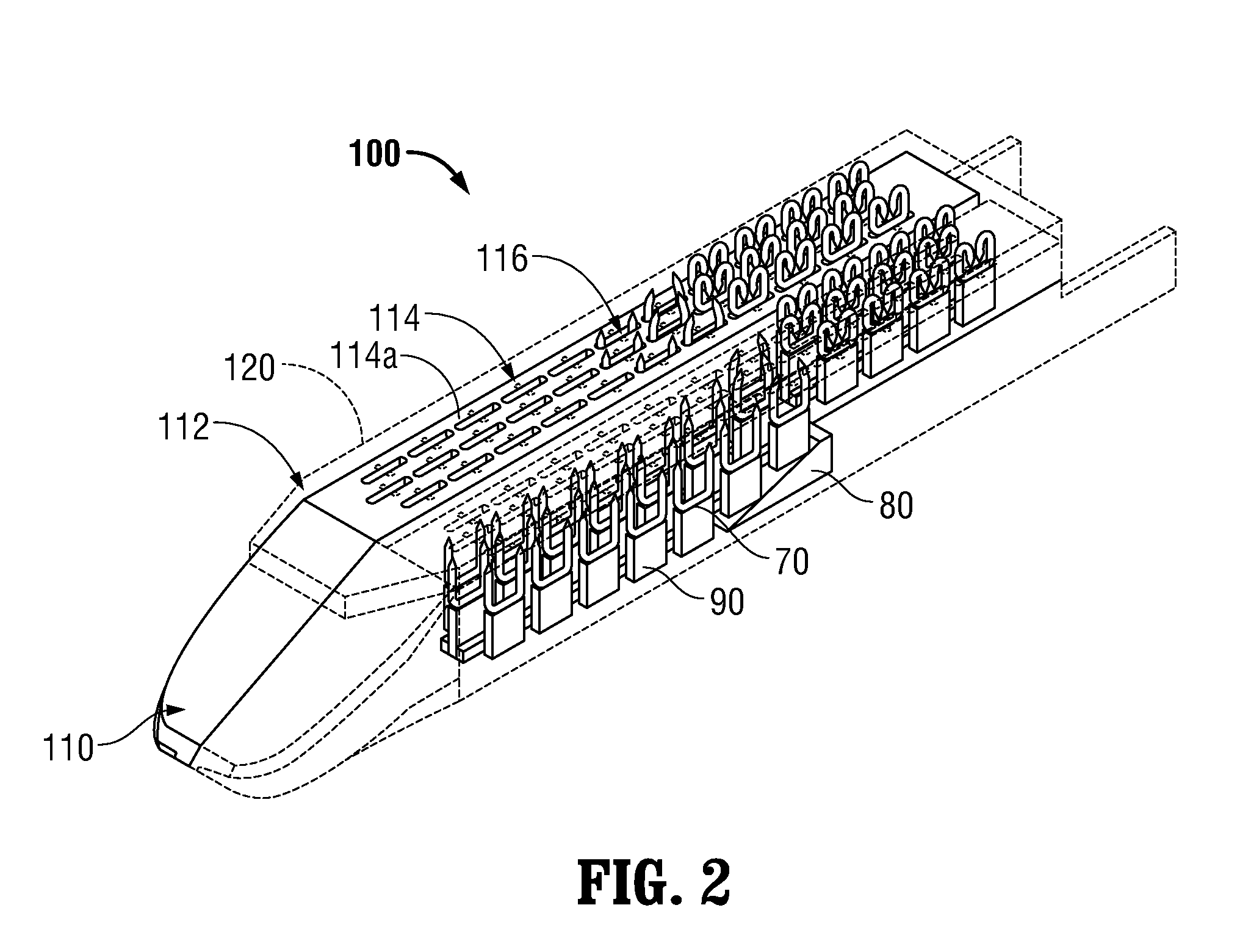
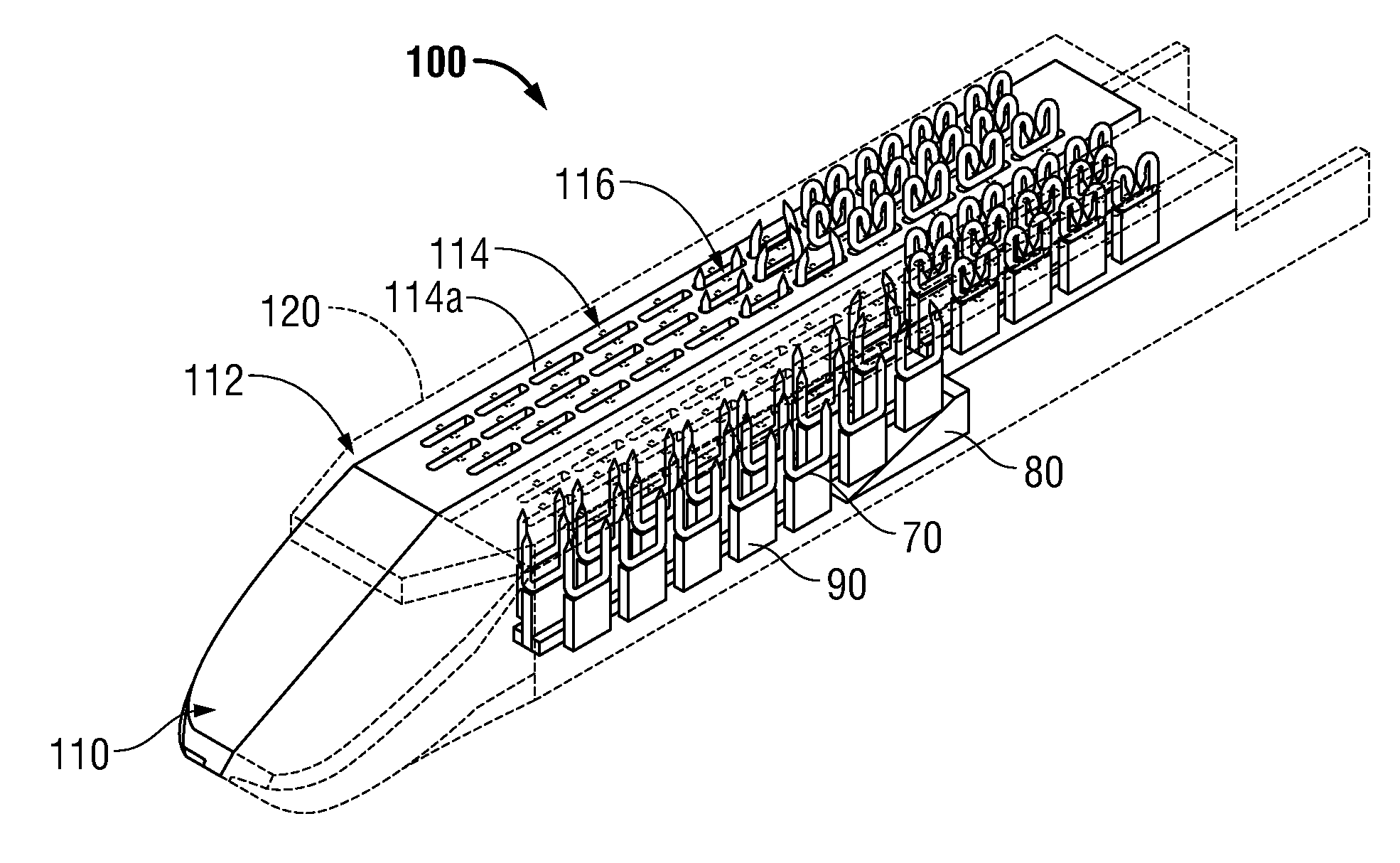
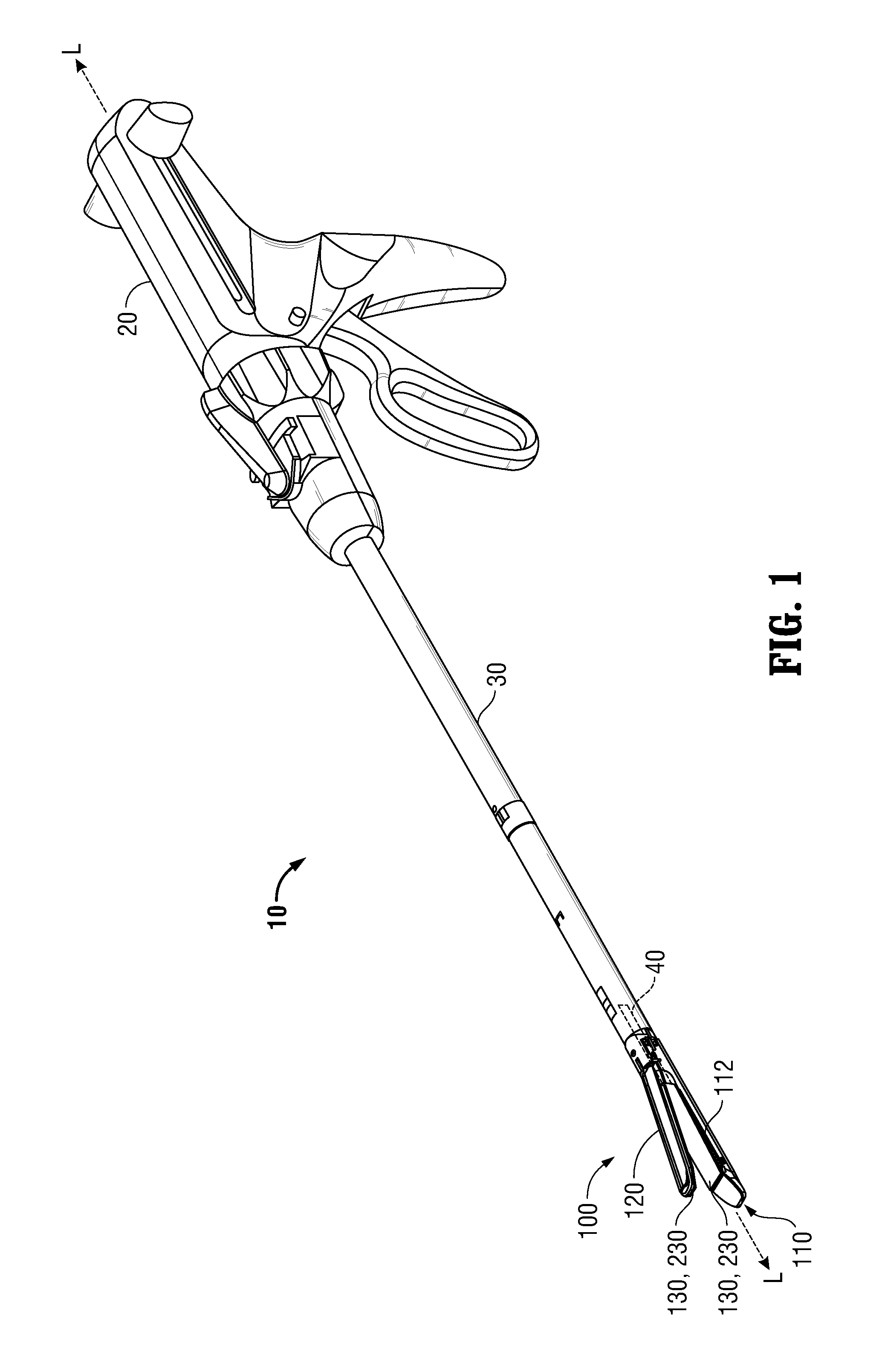
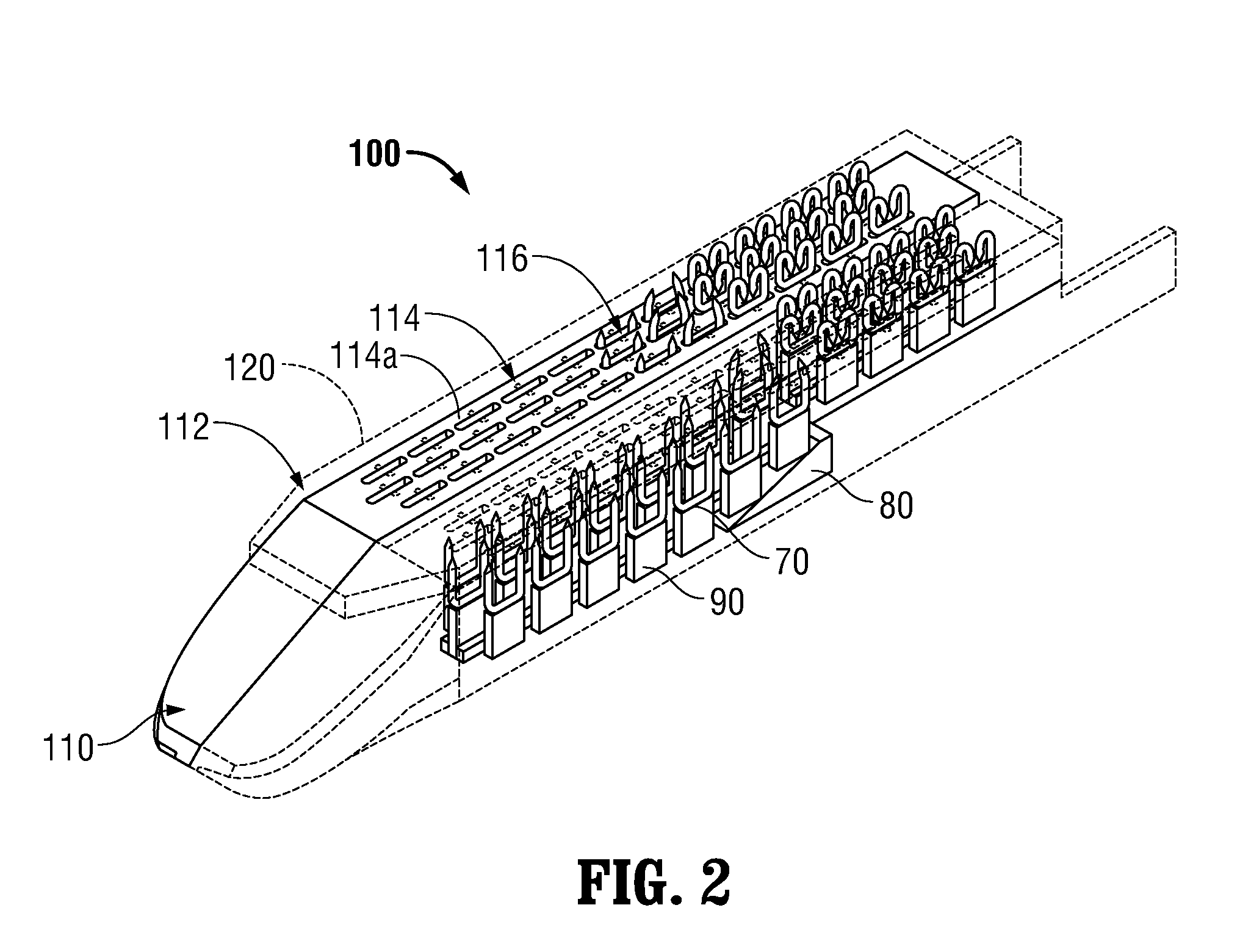
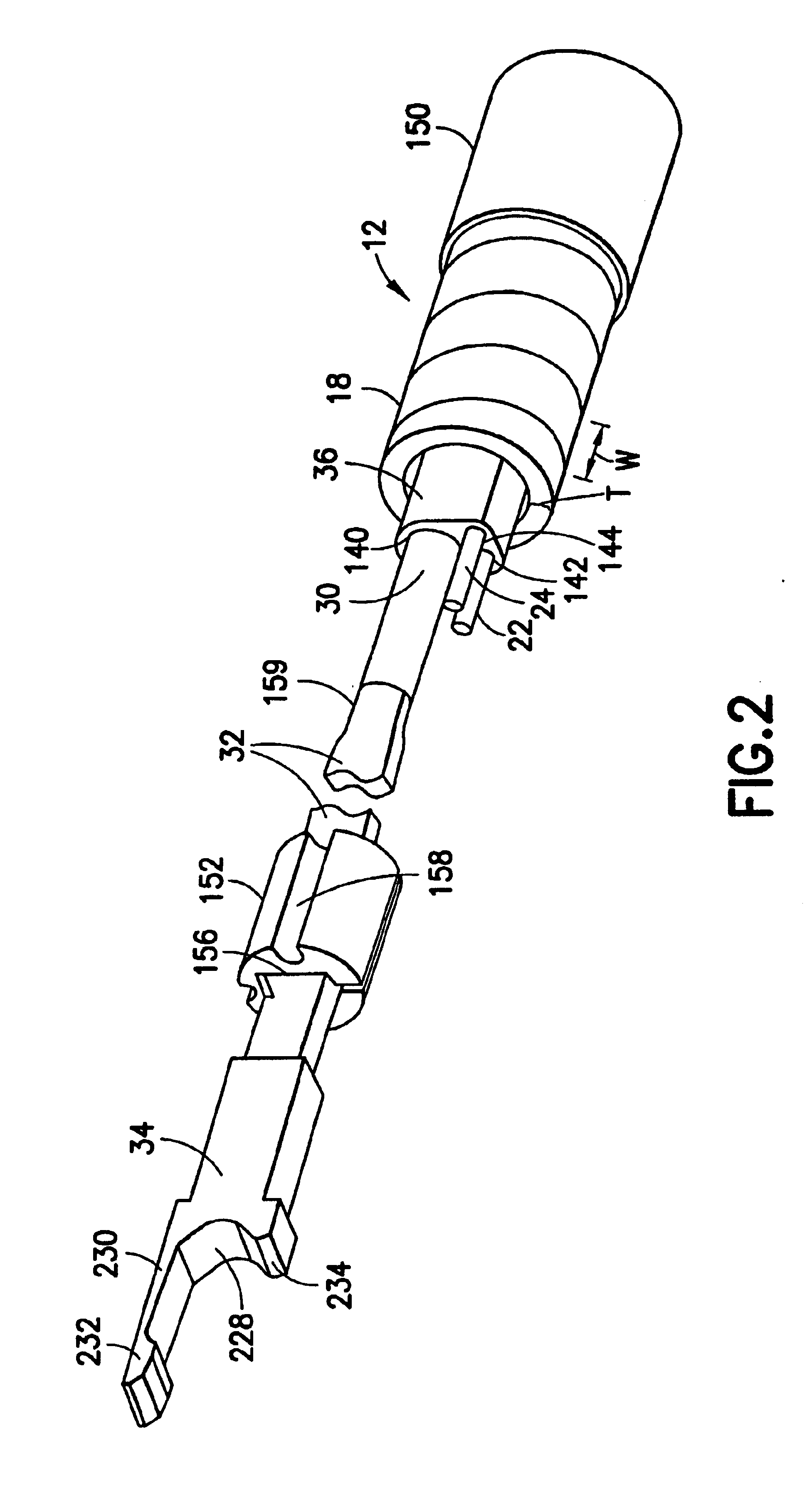
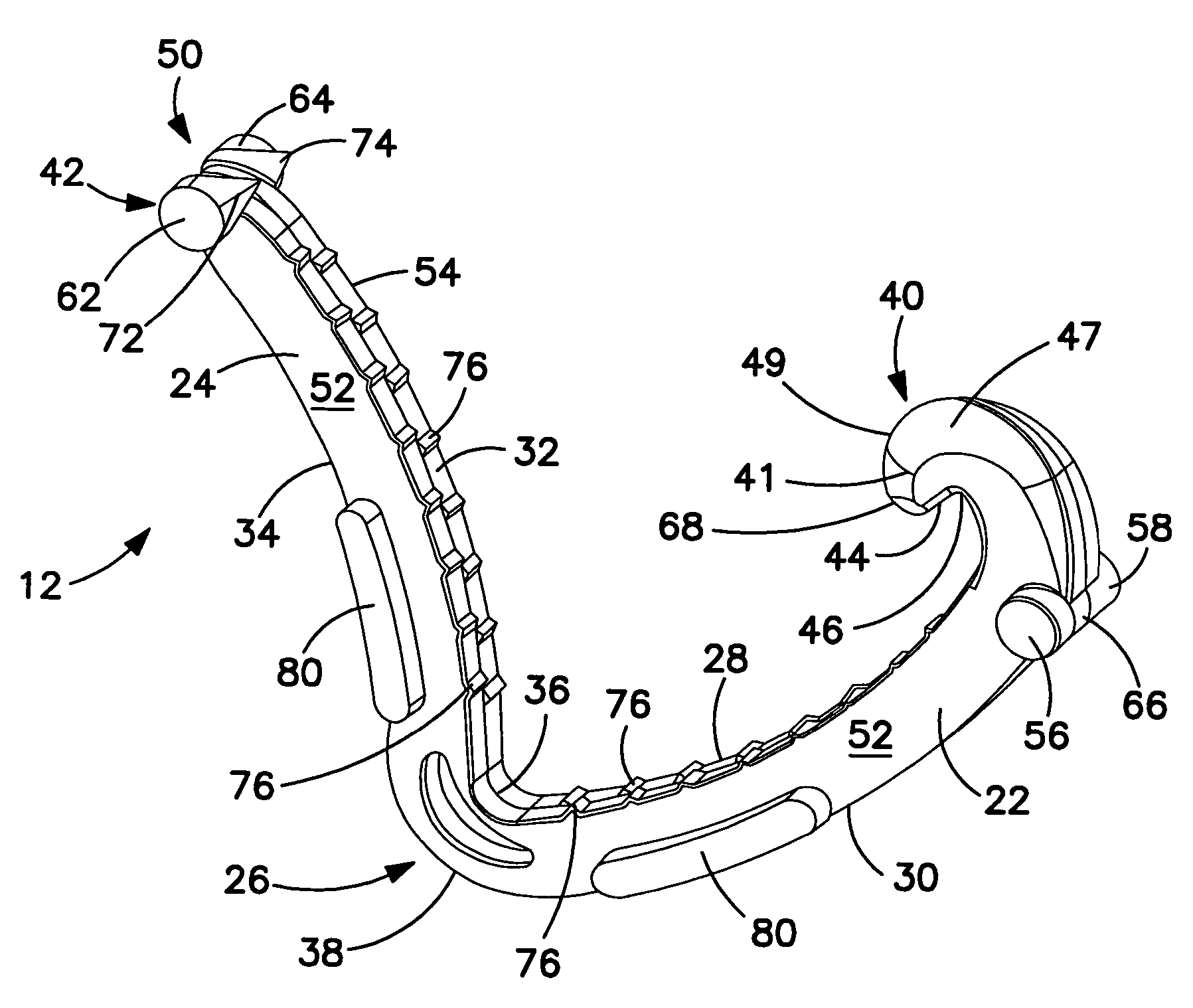
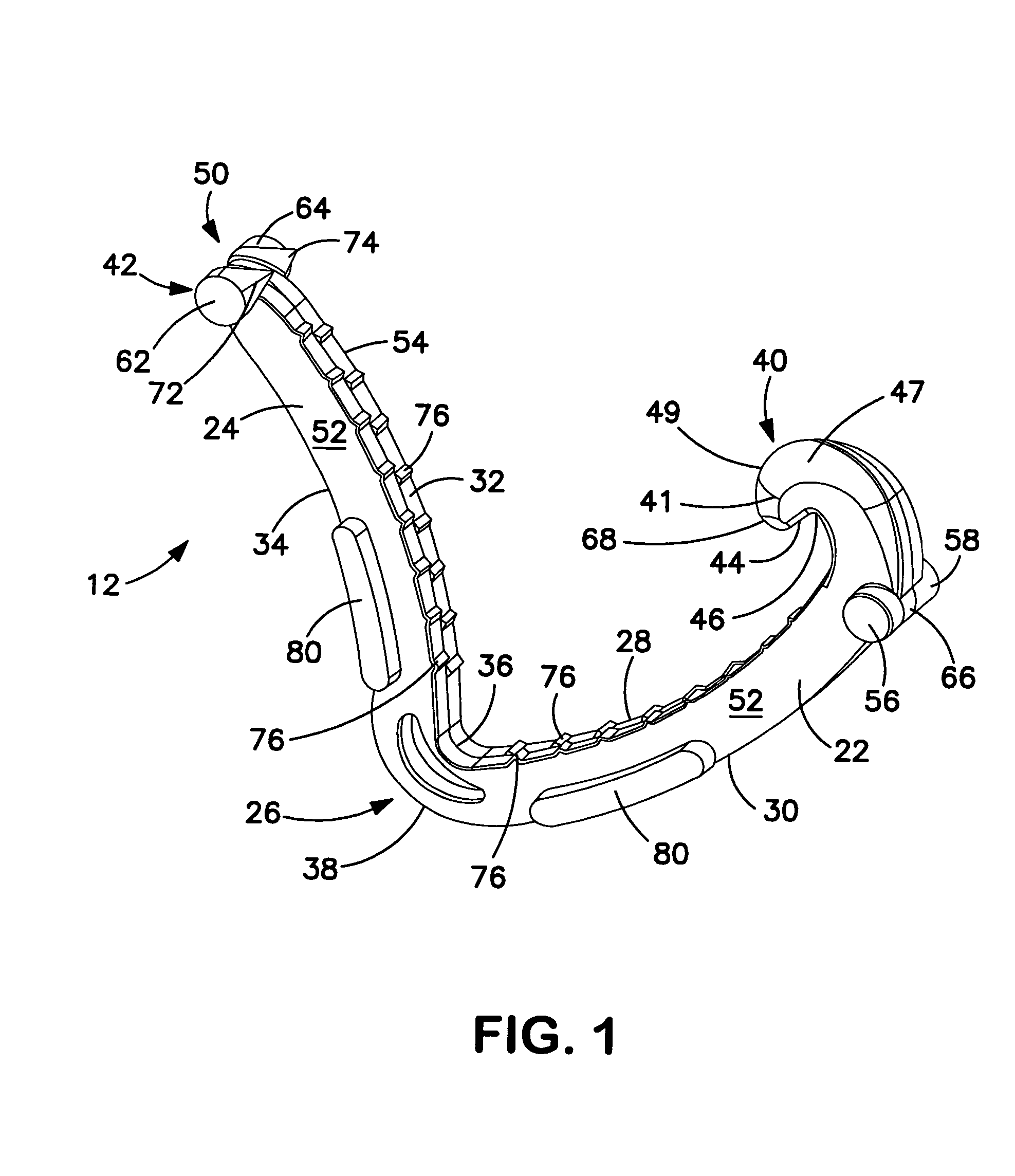
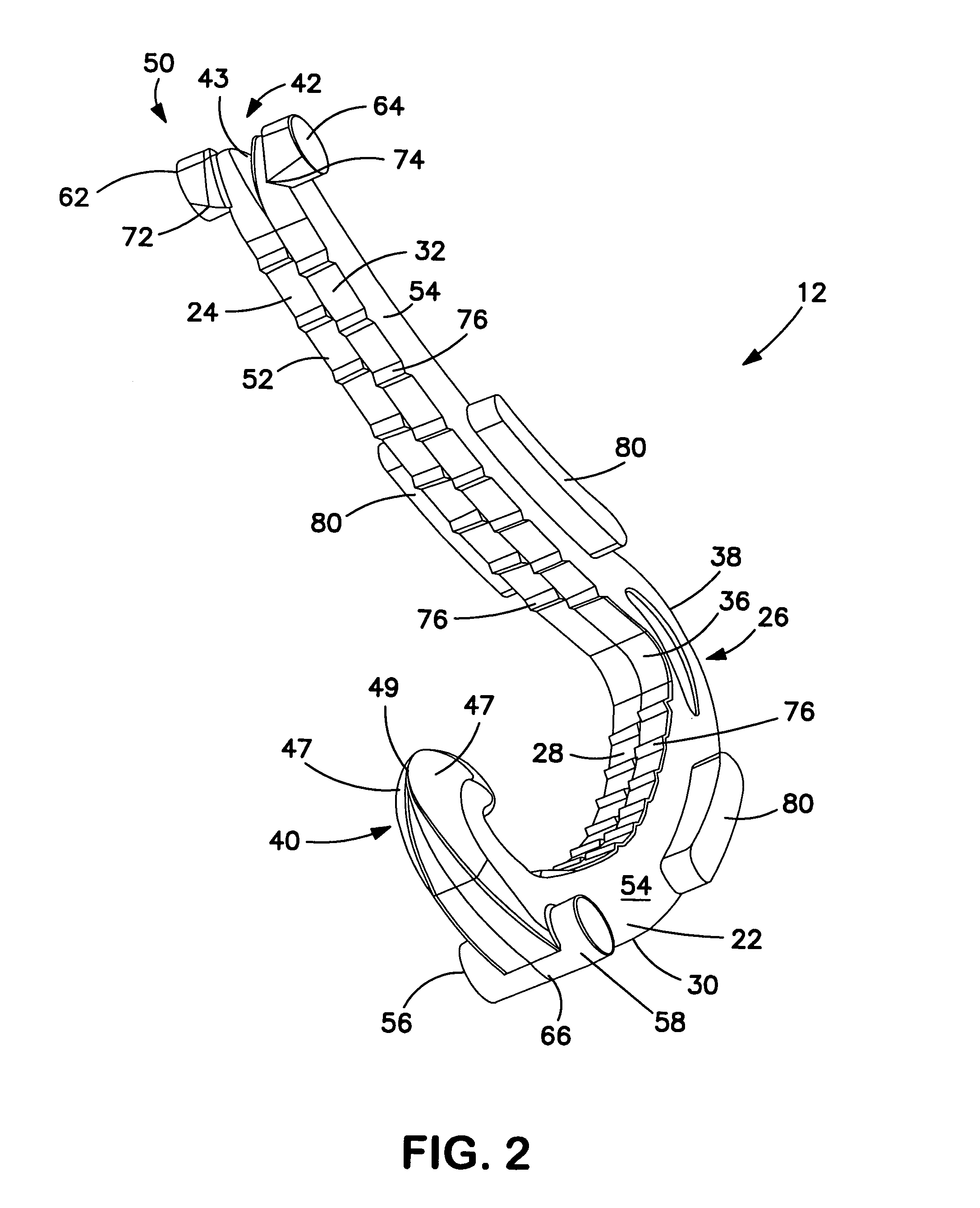
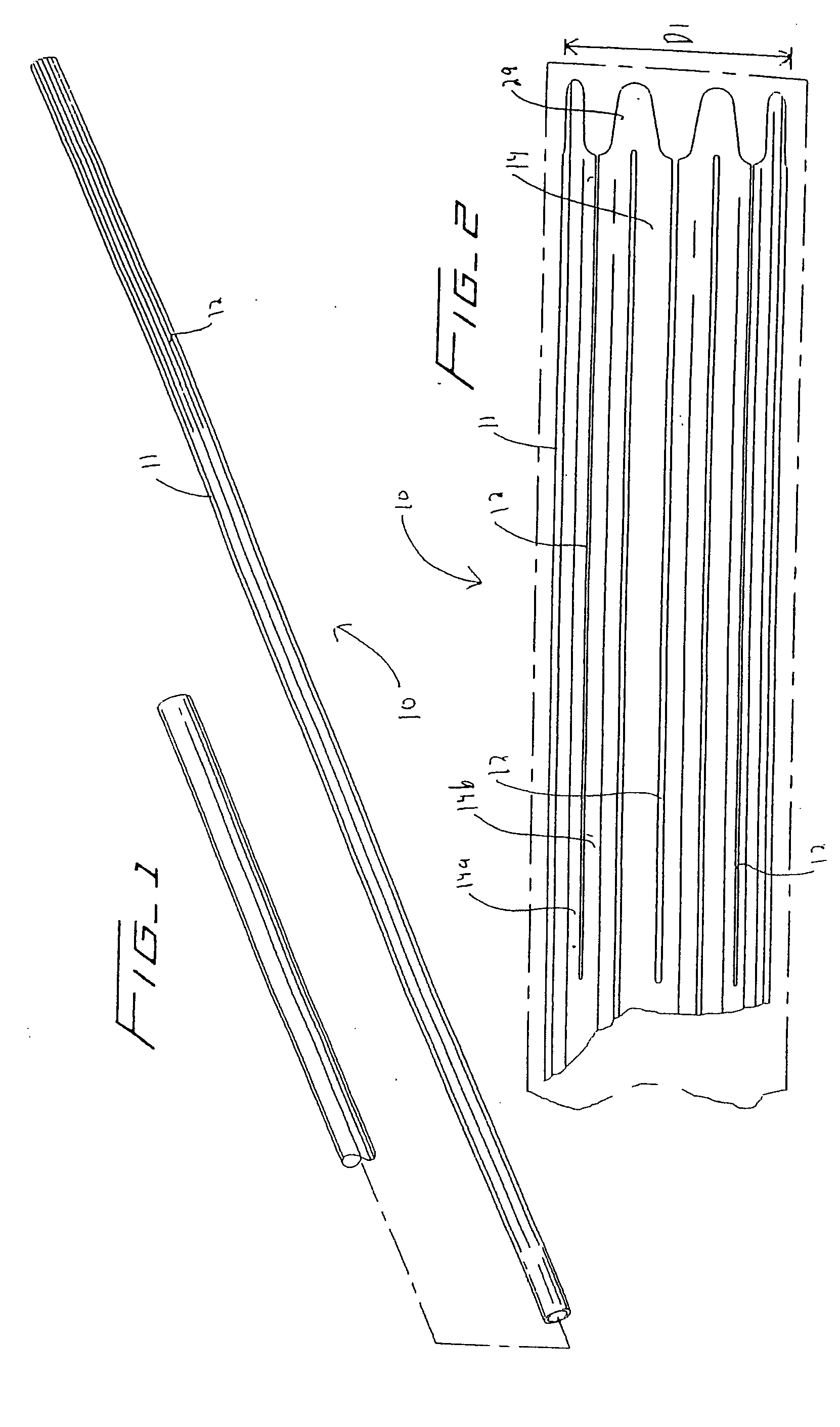
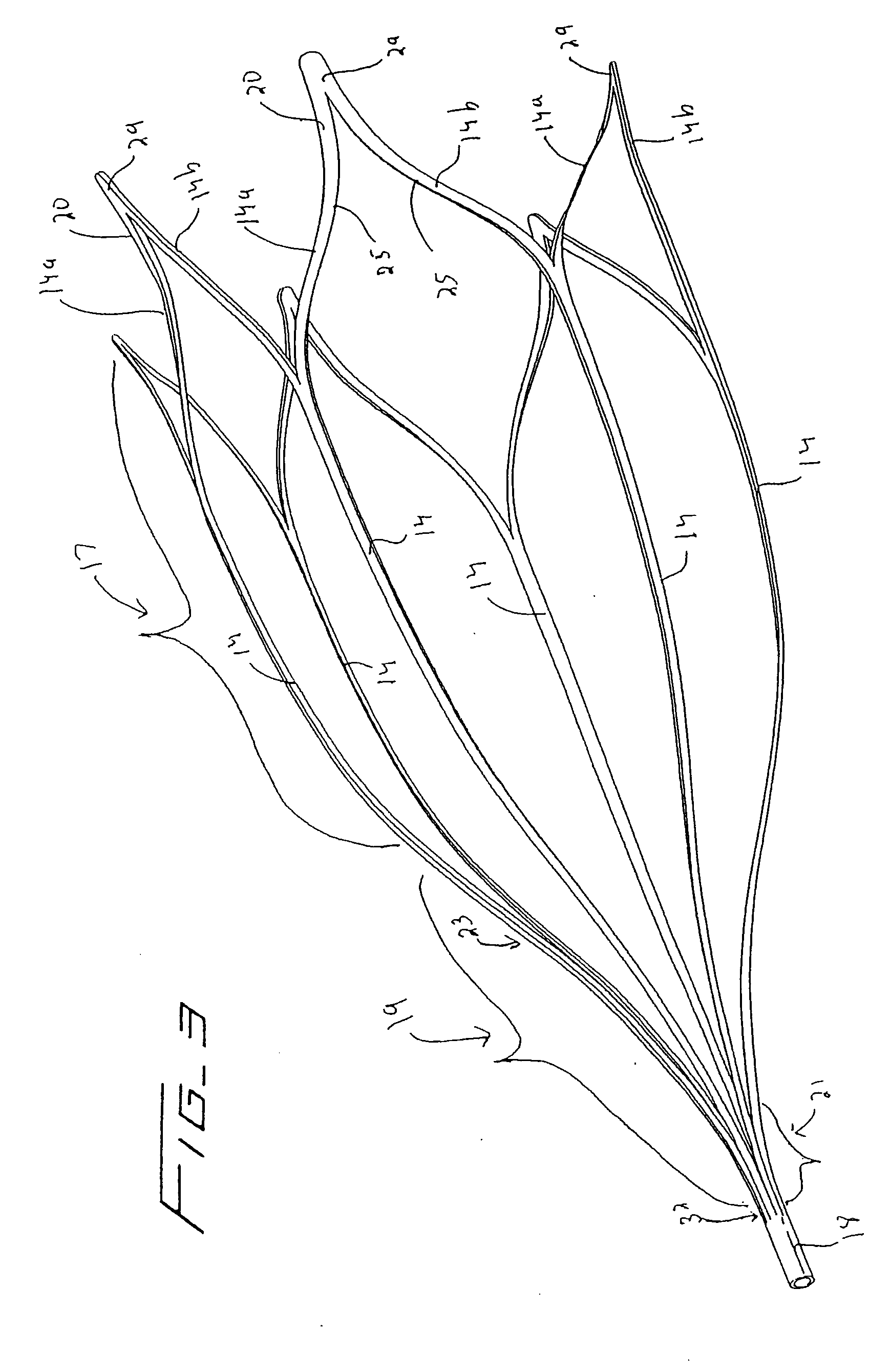
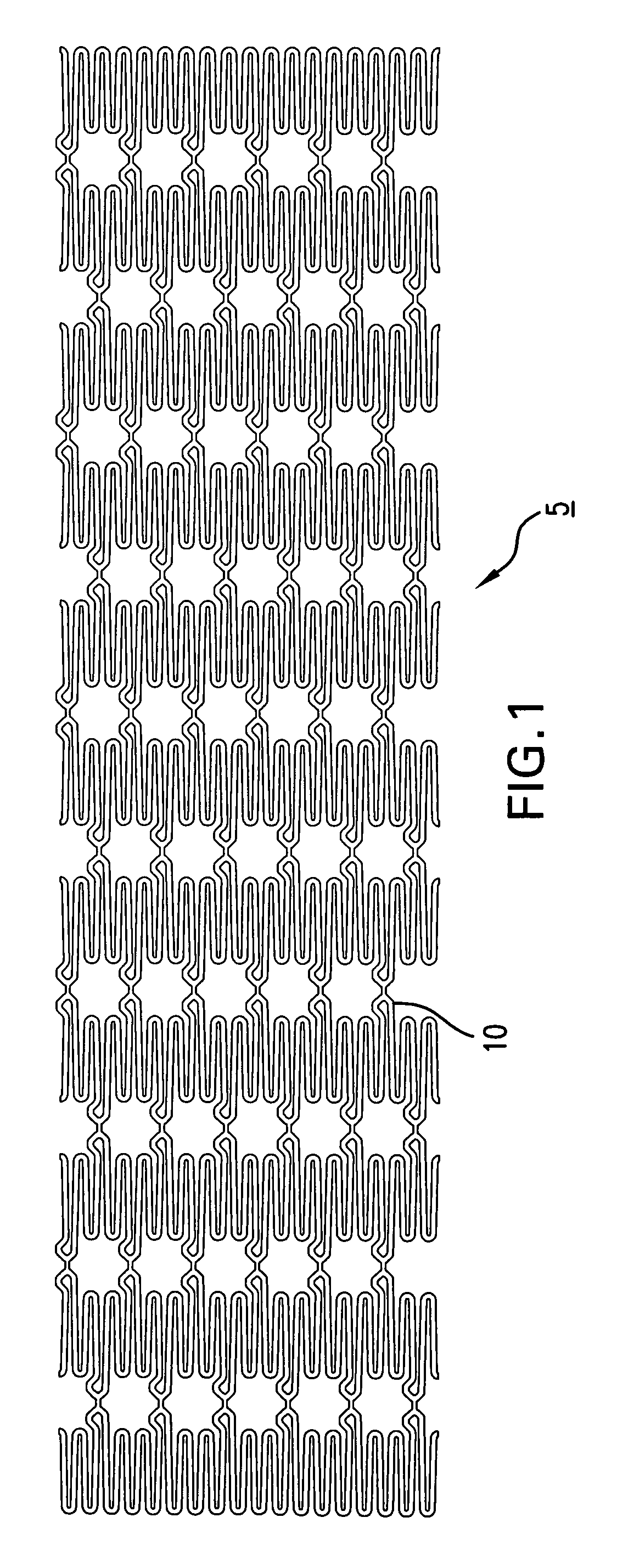
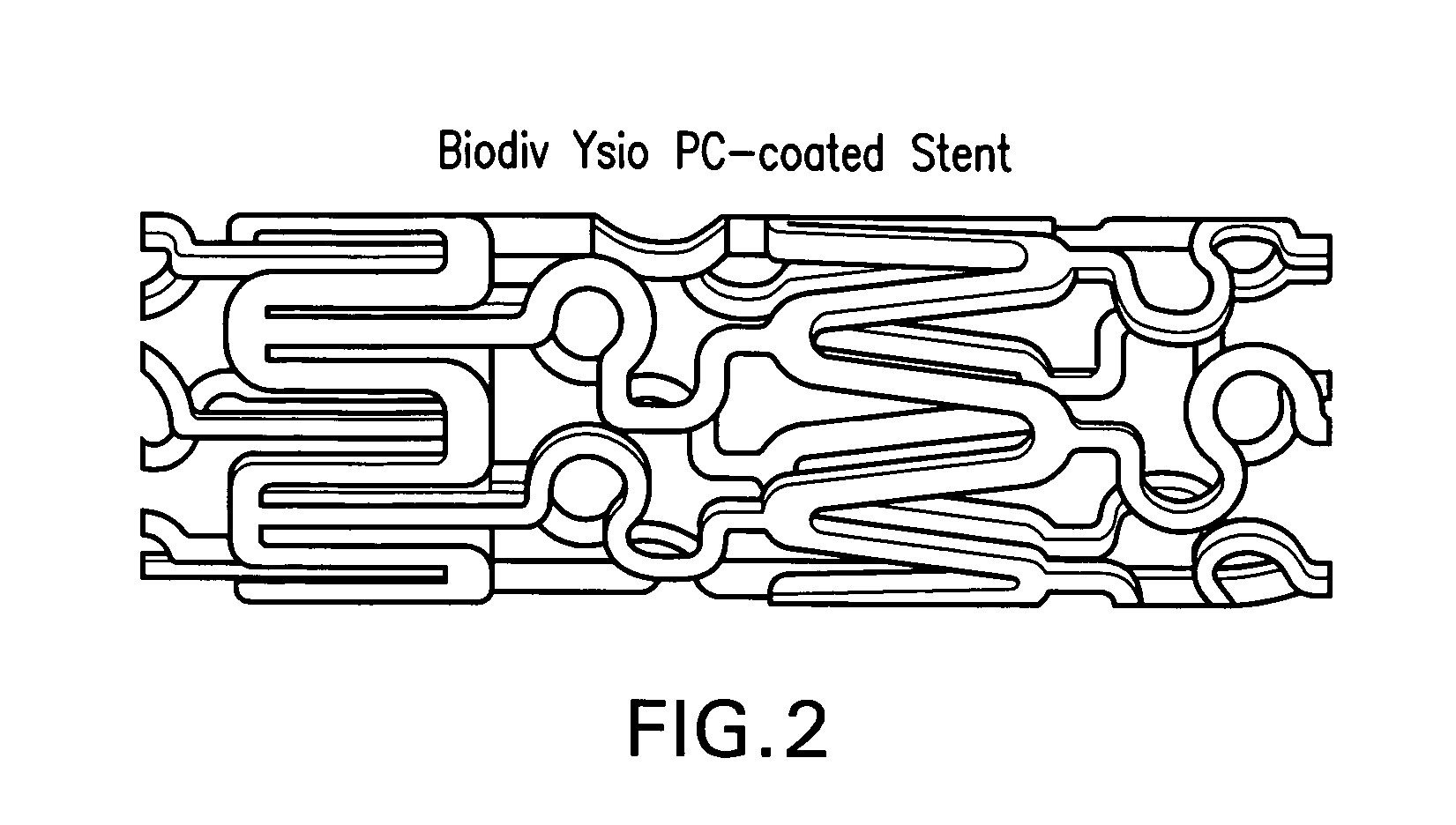
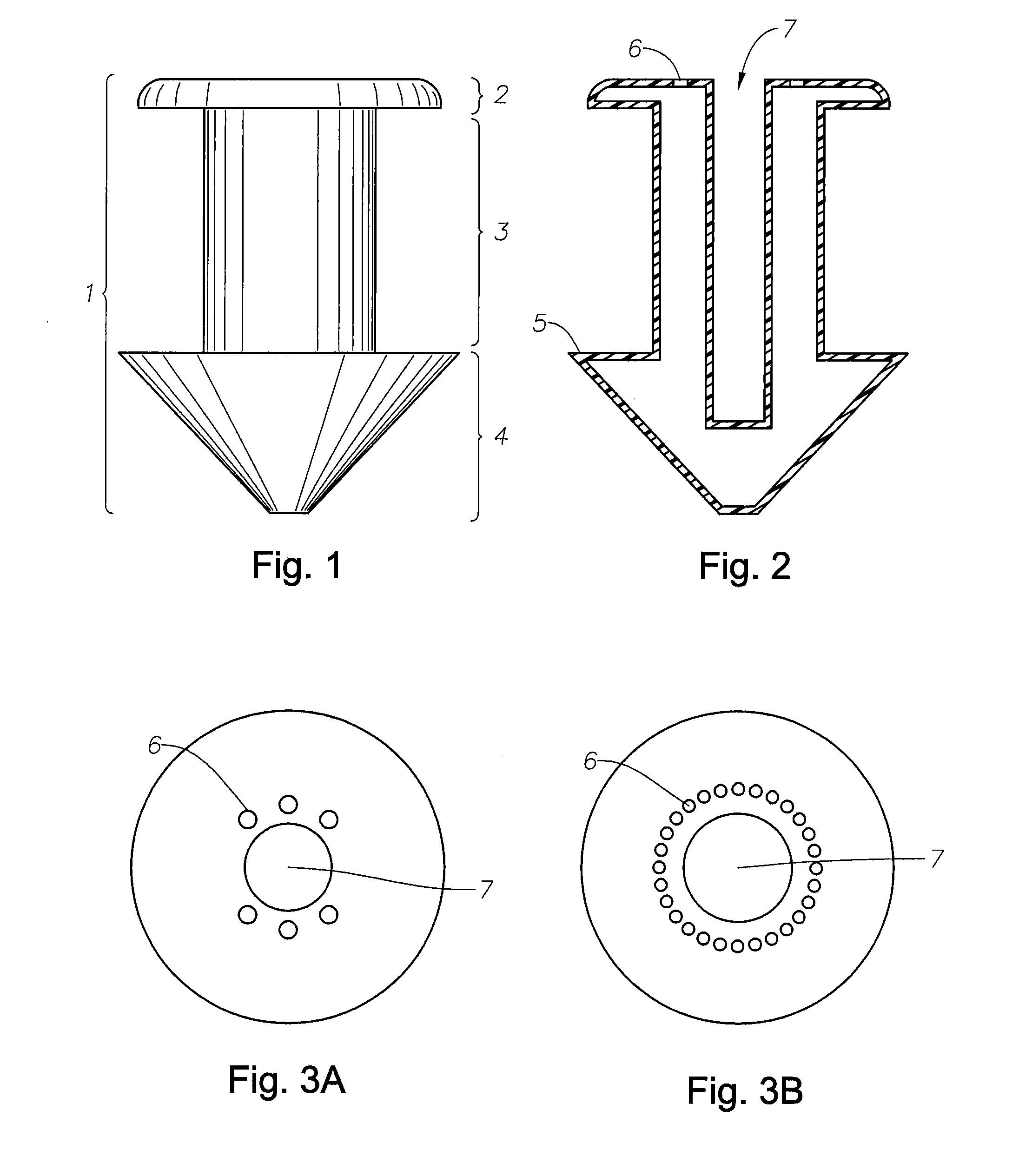
Surgical apparatus including surgical buttress
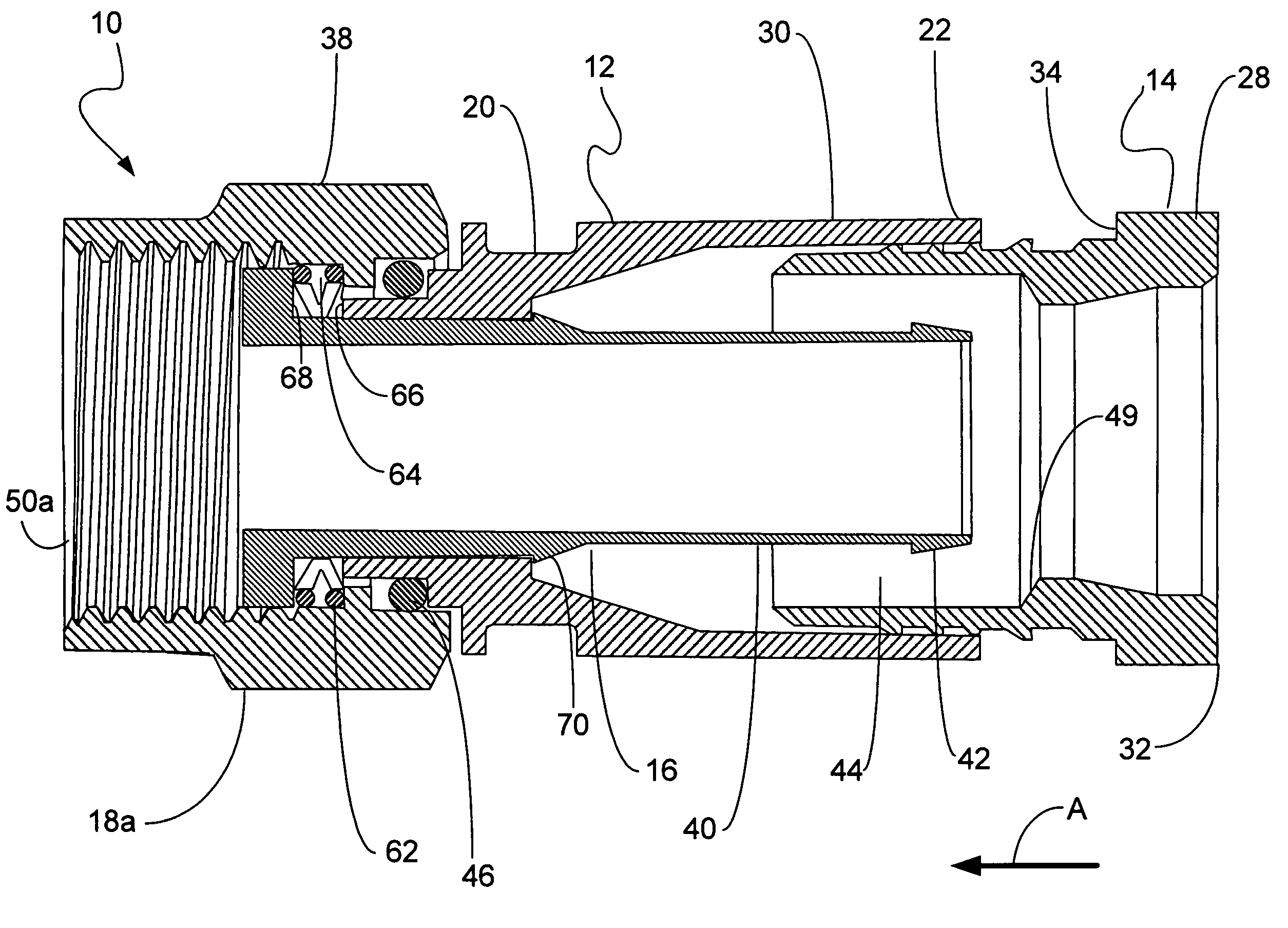
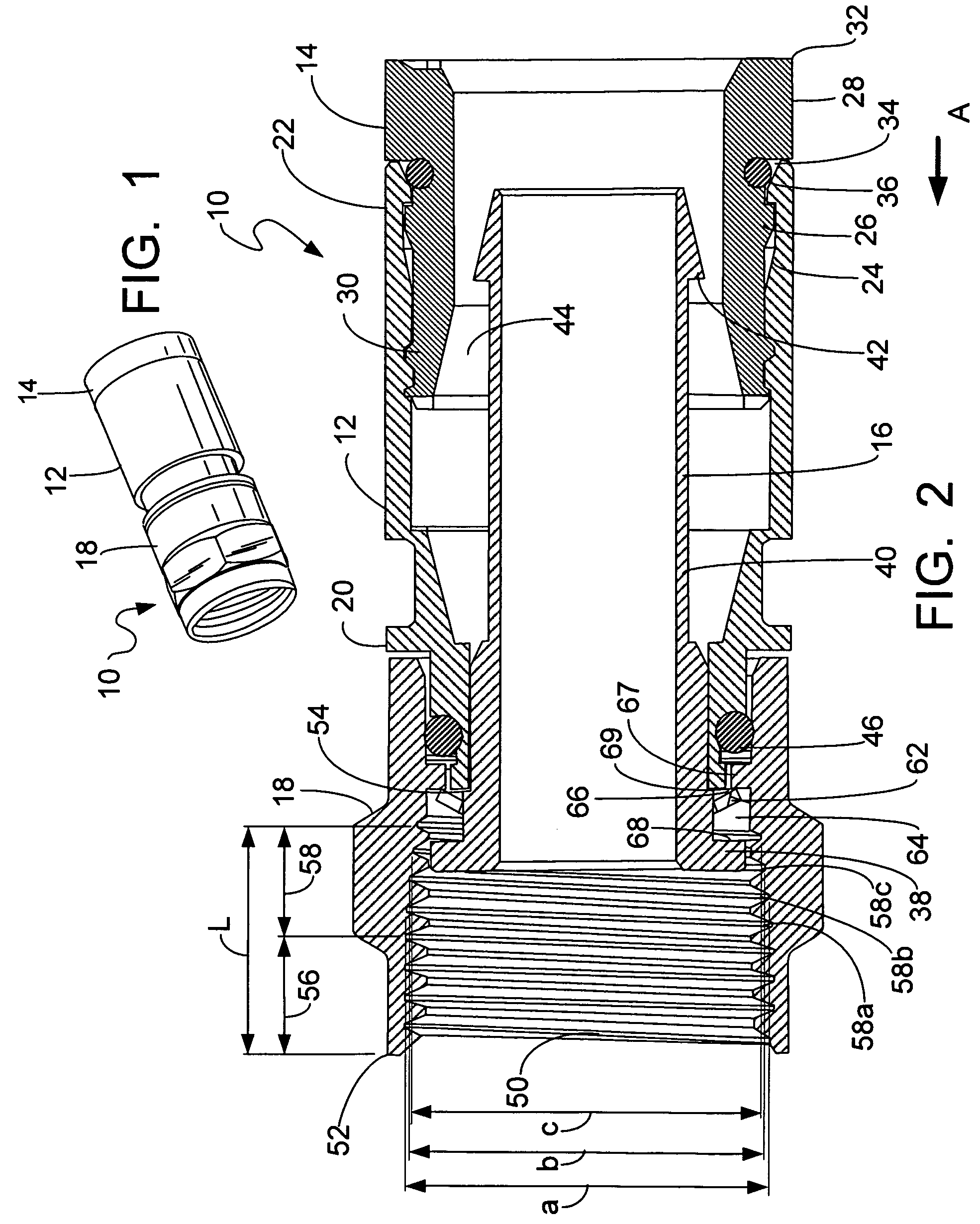
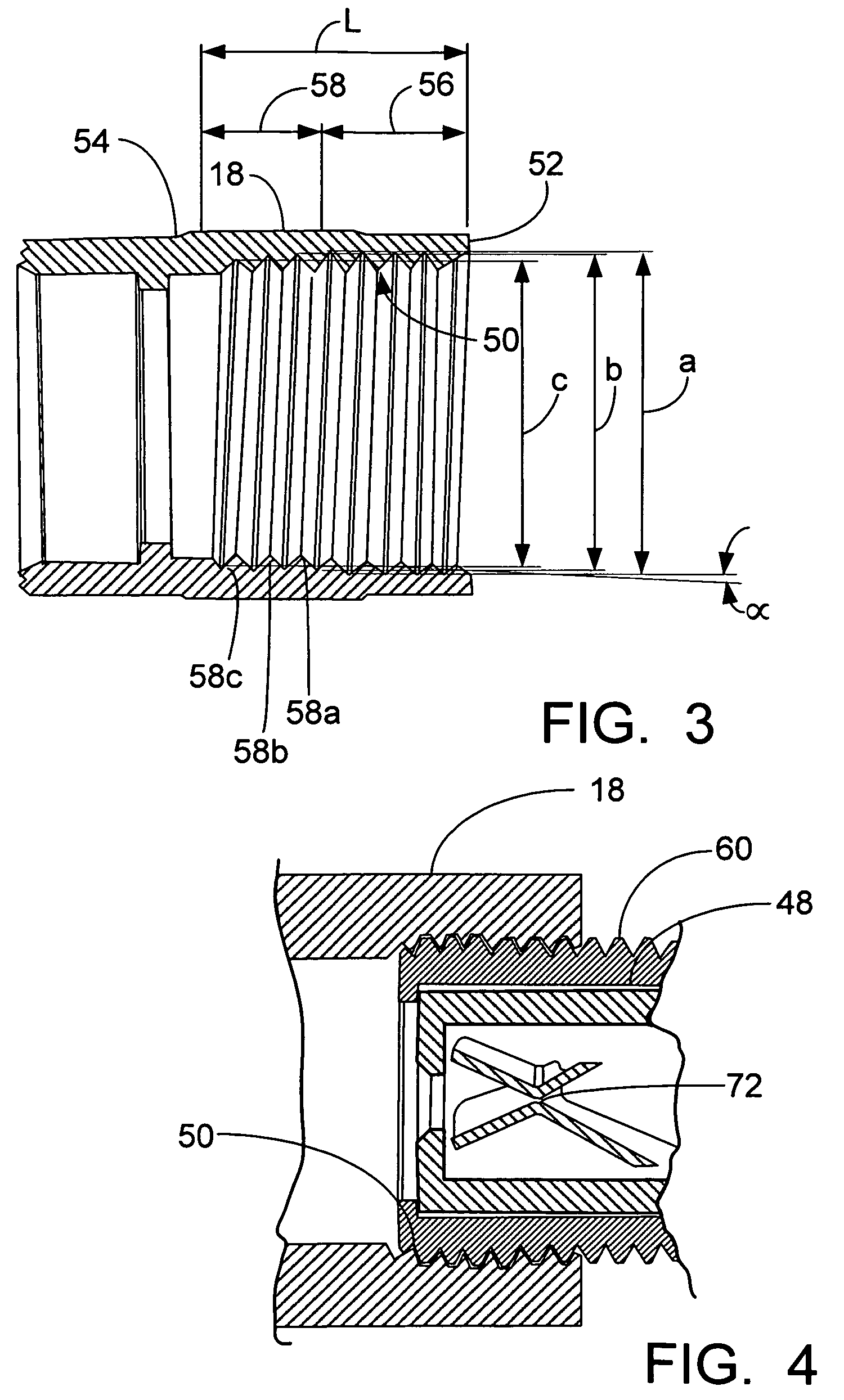
ActiveUS9295466B2High retention rateIncision instrumentsInternal osteosythesisButtressLateral recess
A surgical stapling apparatus includes a housing and an end effector secured to the housing. The housing supports a knife assembly including a knife. The end effector has first and second jaw members. One or both of the first and second jaw members define a knife channel. The first jaw member defines fastener slots and the second jaw member defines fastener pockets. One or more of the knife channel, the fastener slots, and the fastener pockets includes lateral recesses. Fasteners are disposed in the fastener slots and formed by the fastener pockets. One or more surgical buttresses have a body that overlies one or both of the fastener slots and the fastener pockets. Portions of the body of the one or more surgical buttresses are disposed within the lateral recesses to secure the one or more surgical buttresses to one or both of the first jaw member and the second jaw member.
Owner:TYCO HEALTHCARE GRP LP
Surgical Apparatus Including Surgical Buttress
A surgical stapling apparatus includes a housing and an end effector secured to the housing. The housing supports a knife assembly including a knife. The end effector has first and second jaw members. One or both of the first and second jaw members define a knife channel. The first jaw member defines fastener slots and the second jaw member defines fastener pockets. One or more of the knife channel, the fastener slots, and the fastener pockets includes lateral recesses. Fasteners are disposed in the fastener slots and formed by the fastener pockets. One or more surgical buttresses have a body that overlies one or both of the fastener slots and the fastener pockets. Portions of the body of the one or more surgical buttresses are disposed within the lateral recesses to secure the one or more surgical buttresses to one or both of the first jaw member and the second jaw member.
Owner:TYCO HEALTHCARE GRP LP
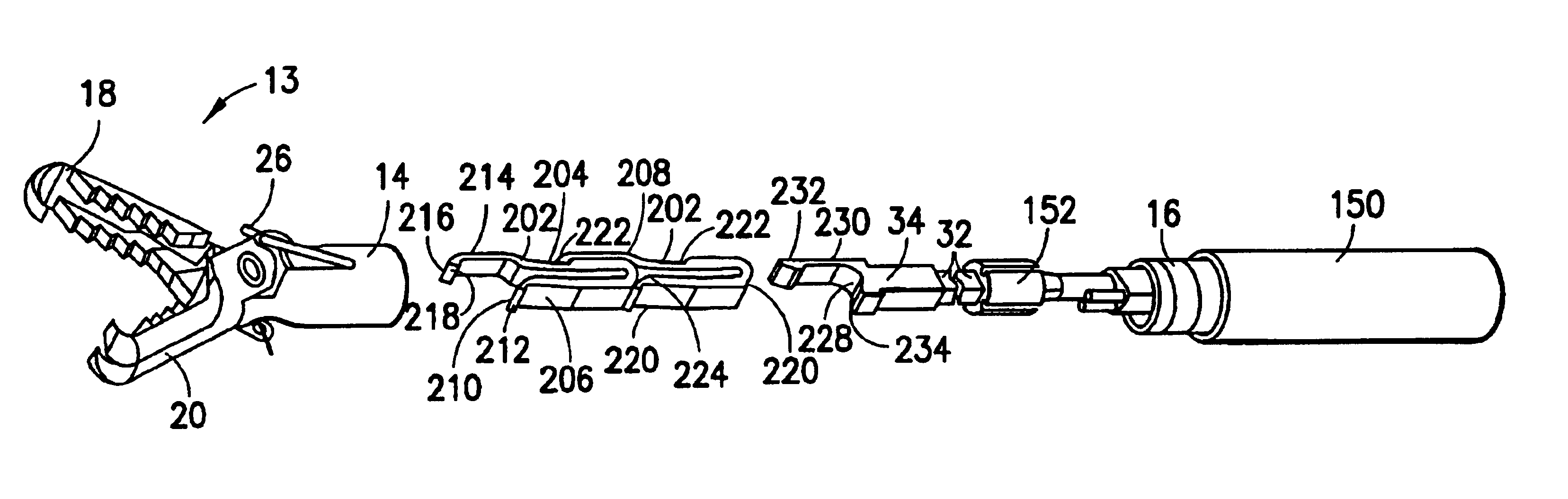
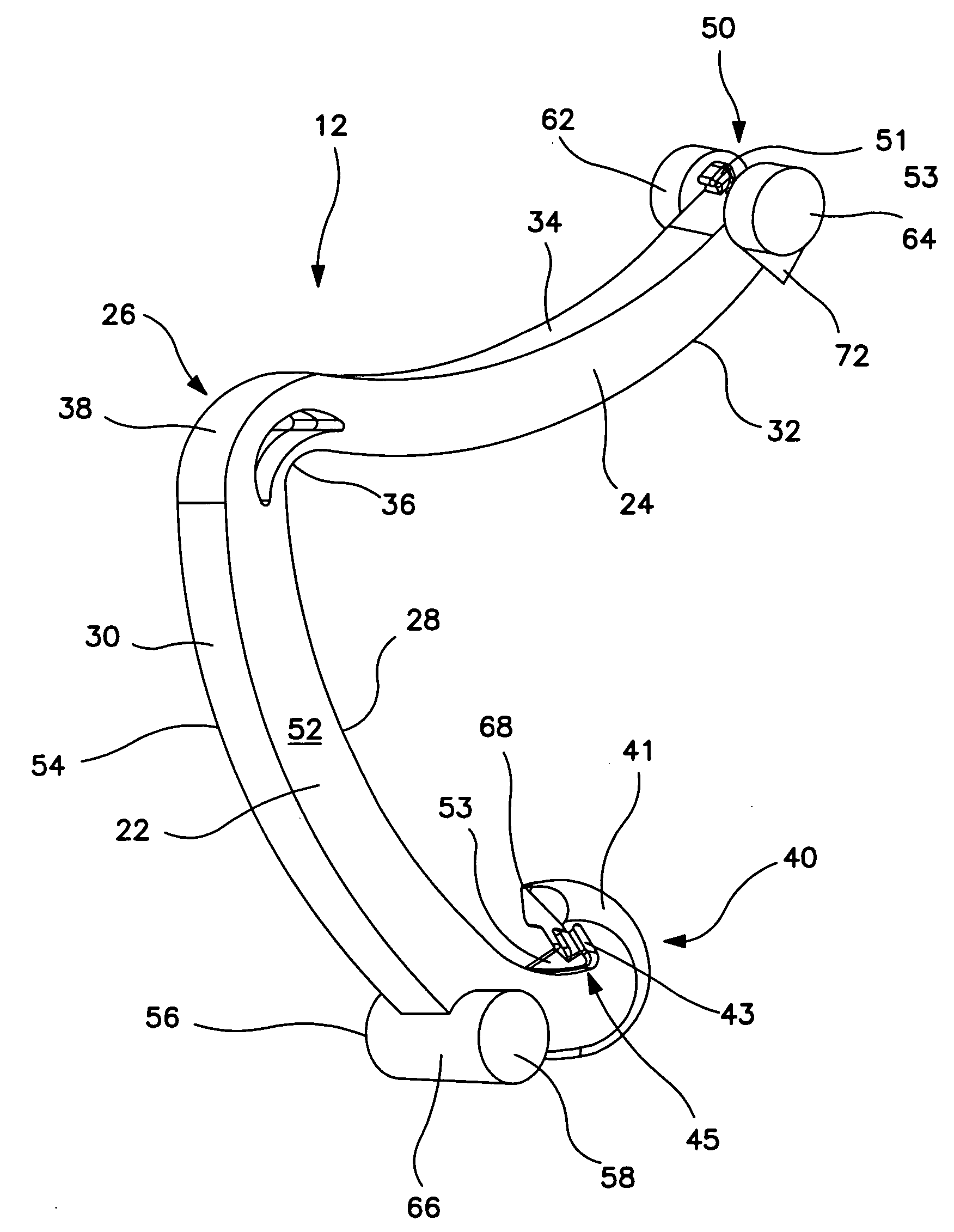
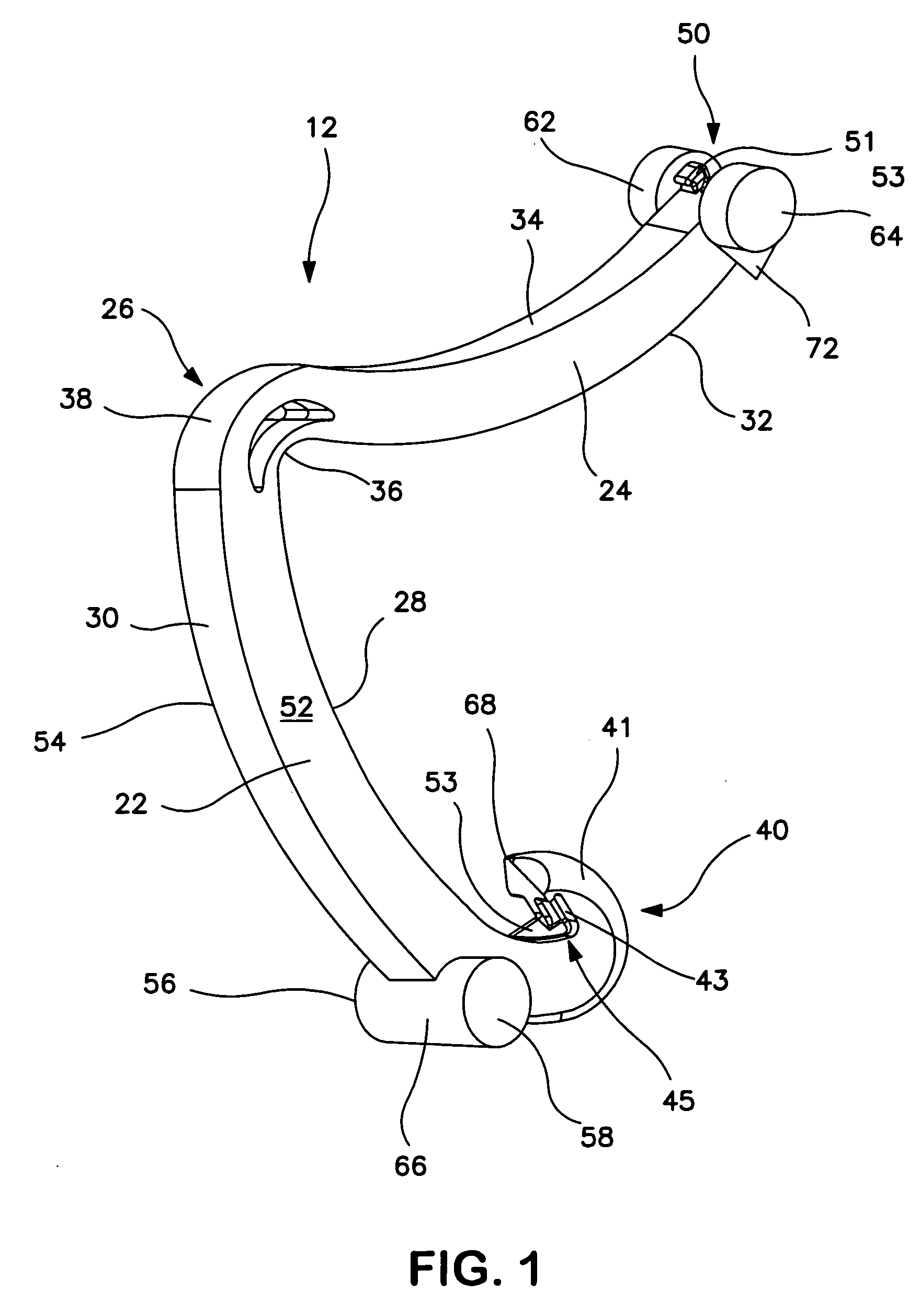
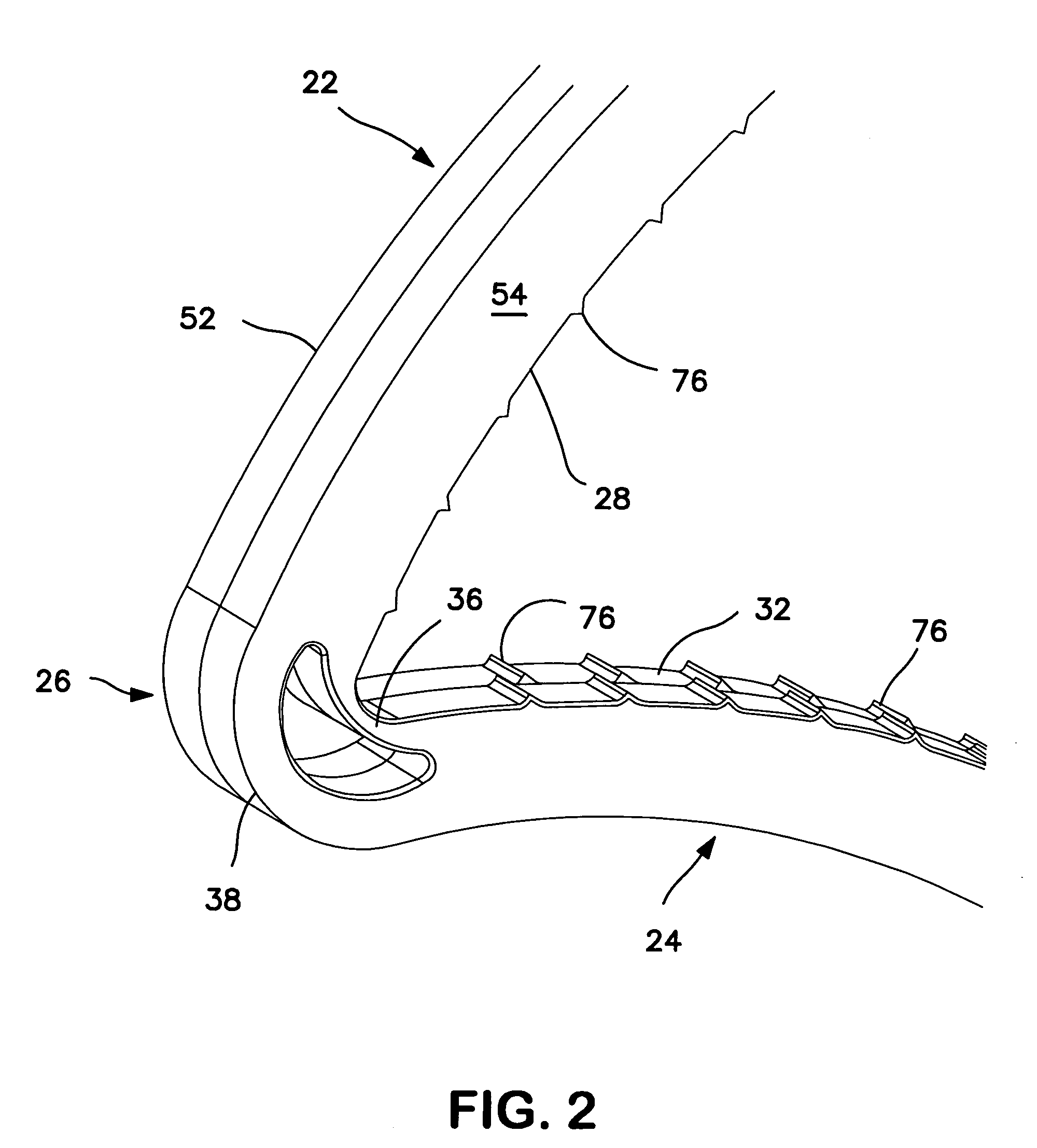
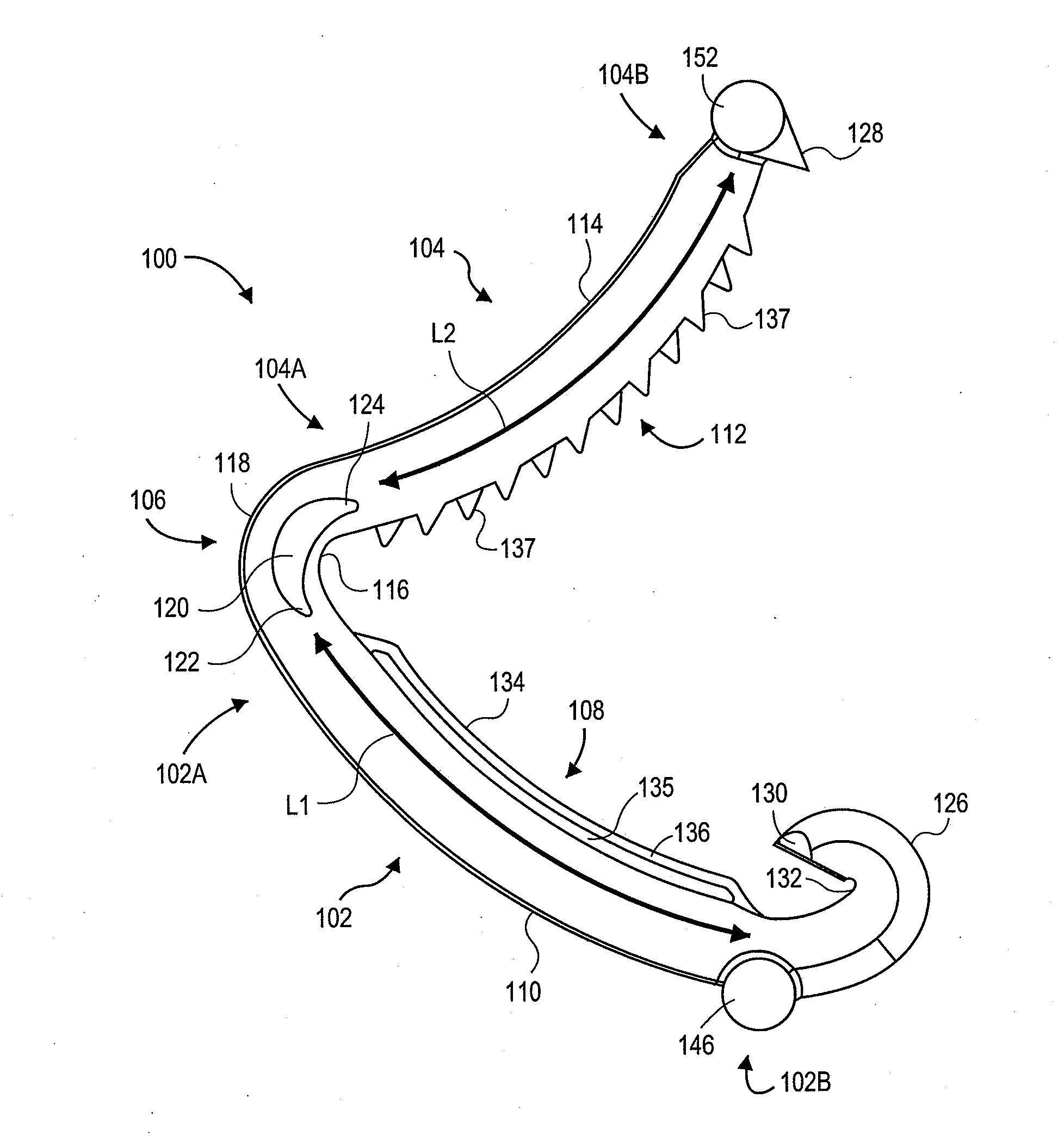
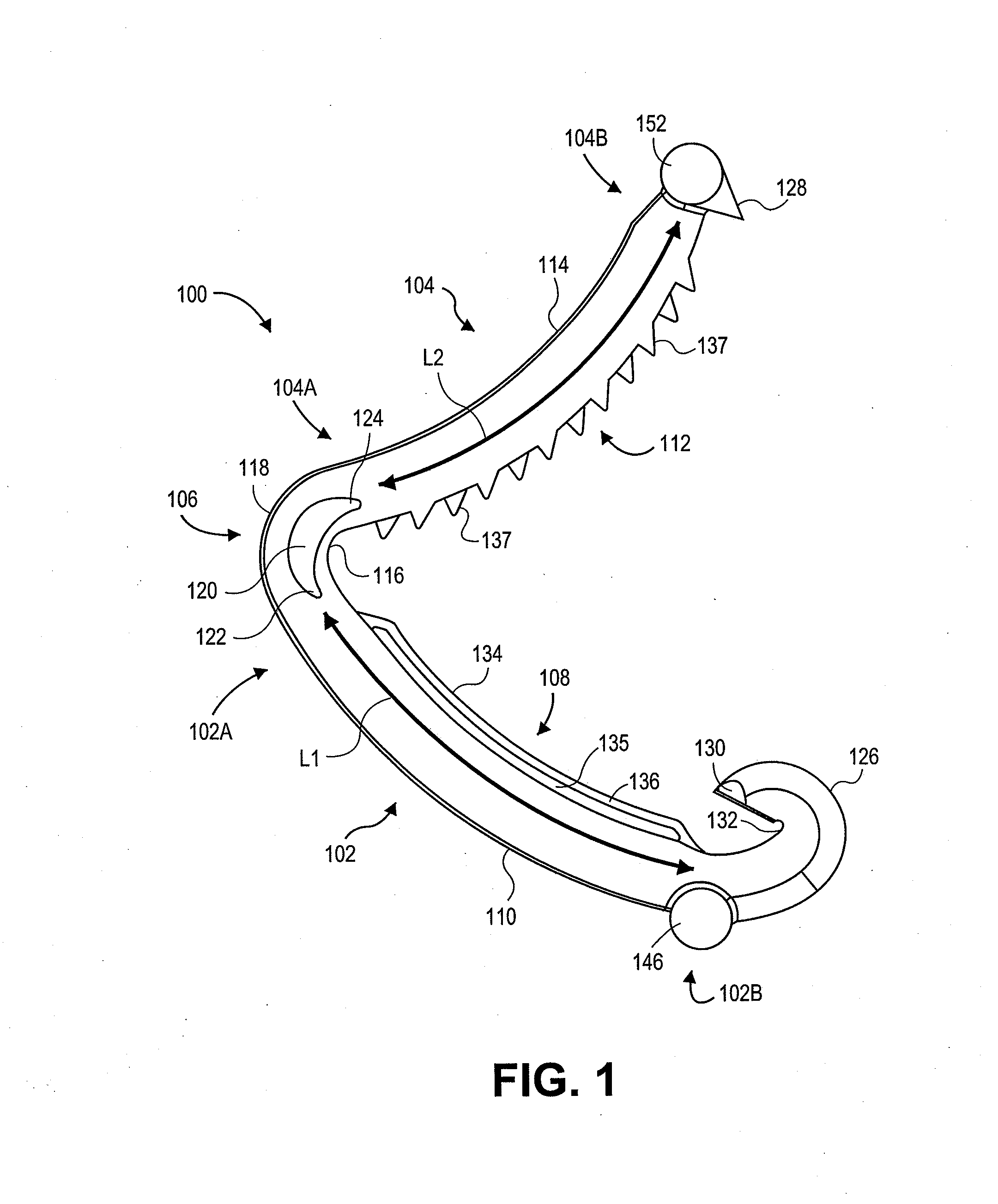
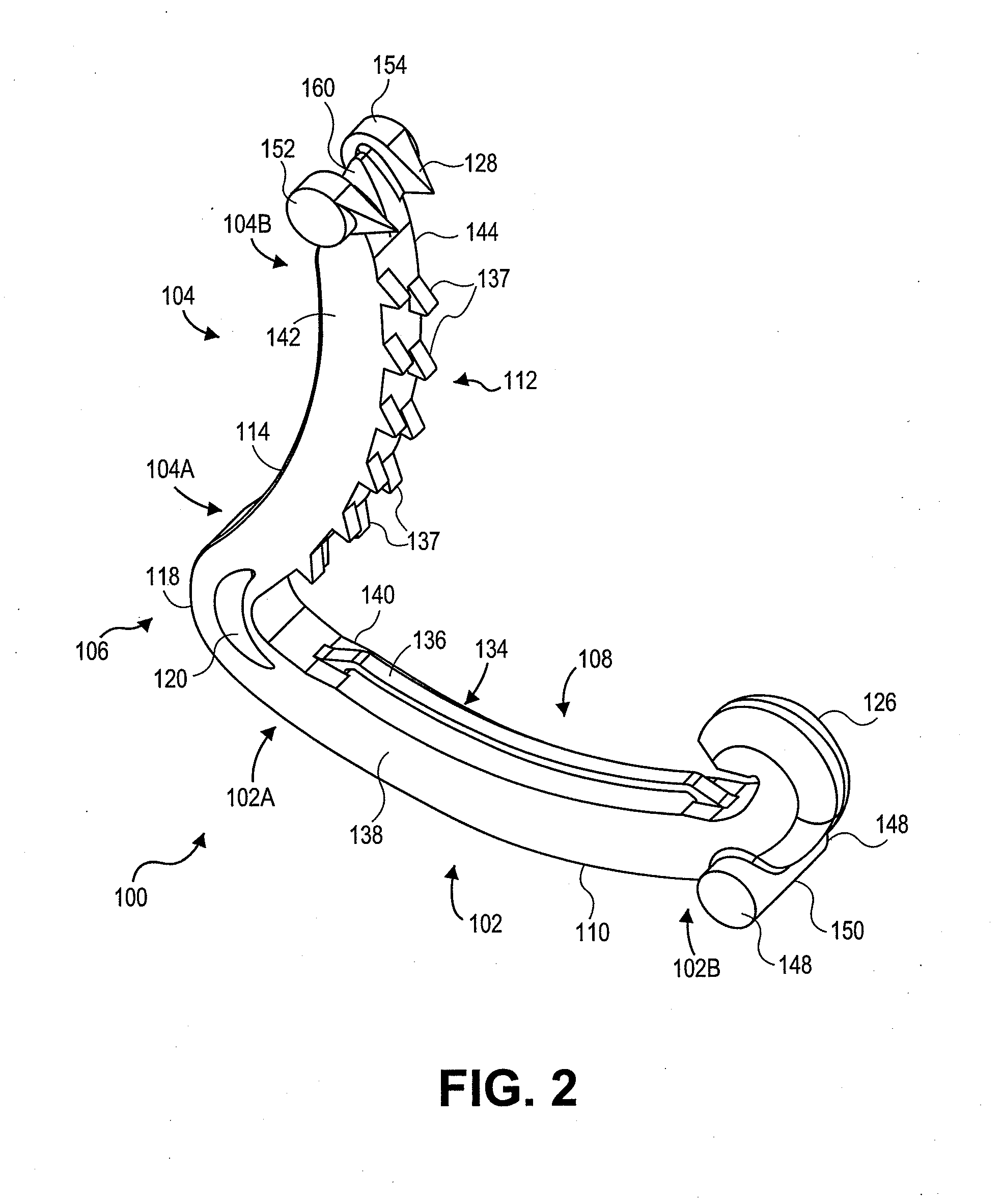
Surgical clip applier having jaws adapted to guide and deform a clip
A flexible clip applier includes a flat wire wound tubular coil, a pair of jaws at the end of the coil, end effector wires extending through the coil and coupled to the jaws, and a clip-advancing wire extending through the coil. A clip chamber is defined in the distal end of the coil. A clip pusher is provided at a distal end of the clip-advancing wire, and advances a clip into the jaws when the clip-advancing wire is moved distally. The jaws include channels in which a distalmost clip rides when the jaws are closed and the pusher is advanced, thereby causing the distalmost clip to be pushed over the tissue, and a distal anvil which operate to deform a portion of the clip to enhance its retention on the clamped tissue.
Owner:ETHICON ENDO SURGERY INC
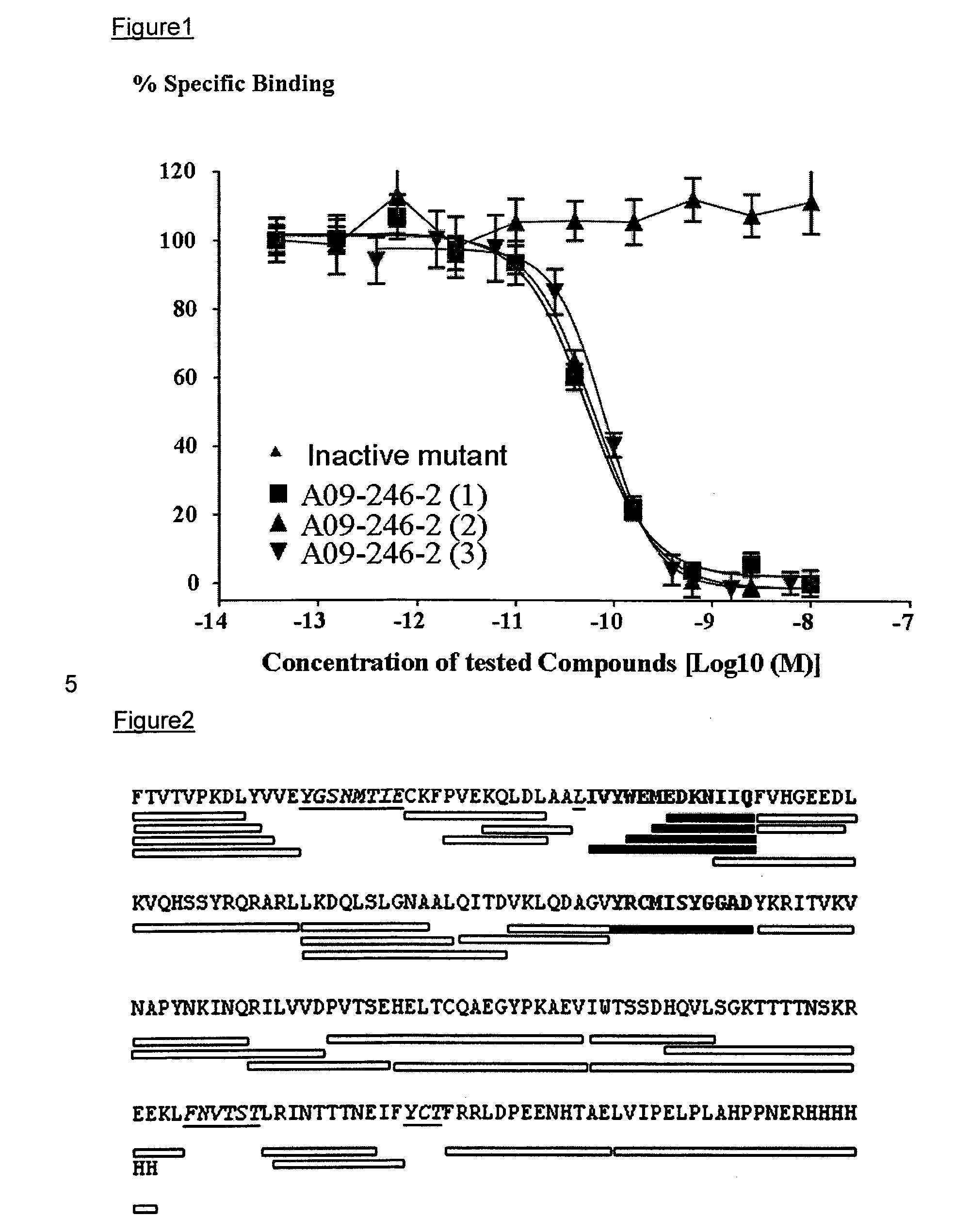
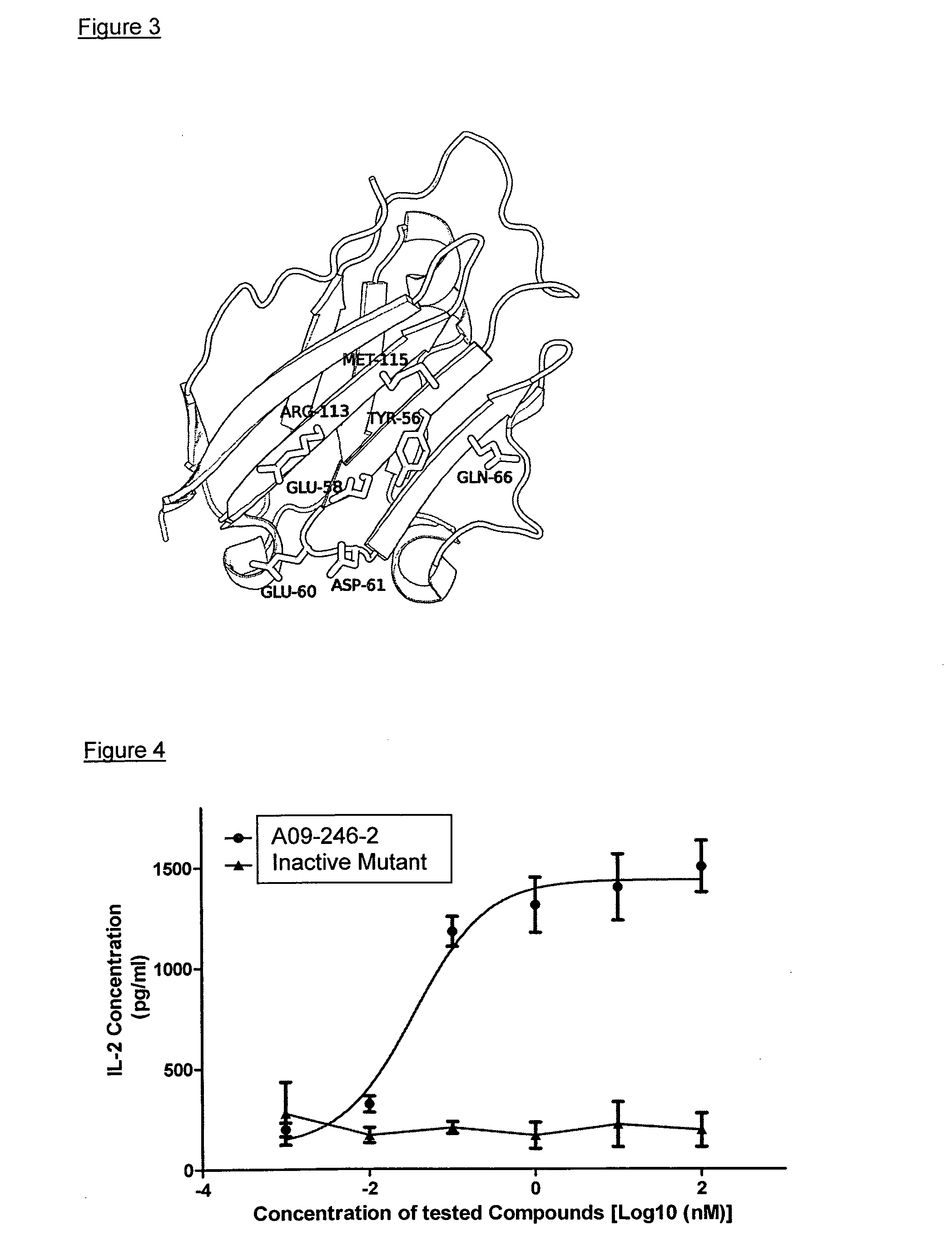
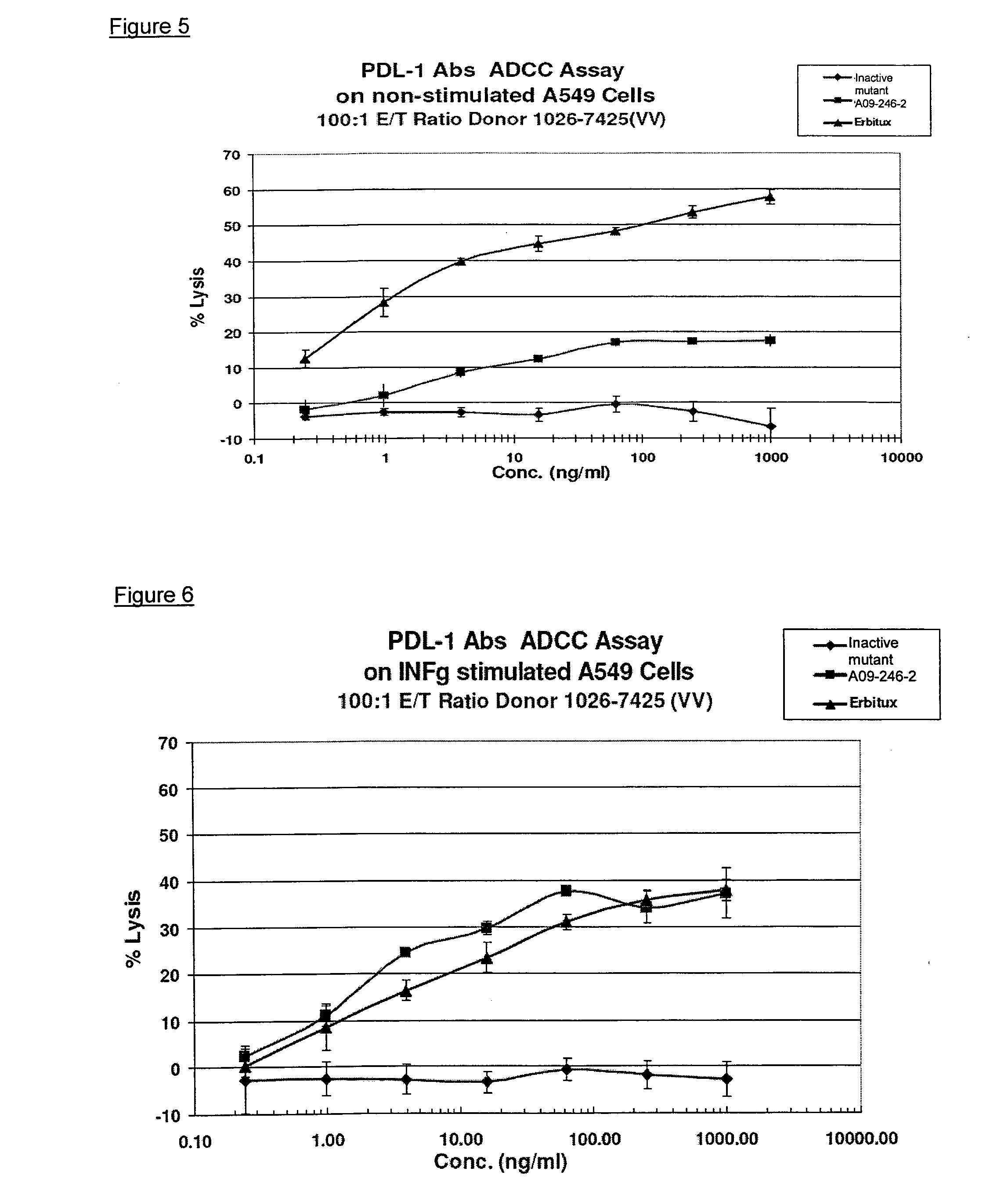
Anti-pd-l1 antibodies and uses thereof
ActiveUS20140341917A1Function increaseUpregulate cell-mediated immune responsesOrganic active ingredientsPeptide/protein ingredientsAntigen Binding FragmentAntigen binding
The present application relates to anti-PD-L1 antibodies or antigen binding fragments thereof, nucleic acid encoding the same, therapeutic compositions thereof, and their use to enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, such as tumor immunity, for the treatment of and cancer.
Owner:MERCK PATENT GMBH
Information display
InactiveUS20040239582A1Promote assimilationReading and comprehension speedAdvertisingCathode-ray tube indicatorsReal-time computing
Owner:PUREDEPTH
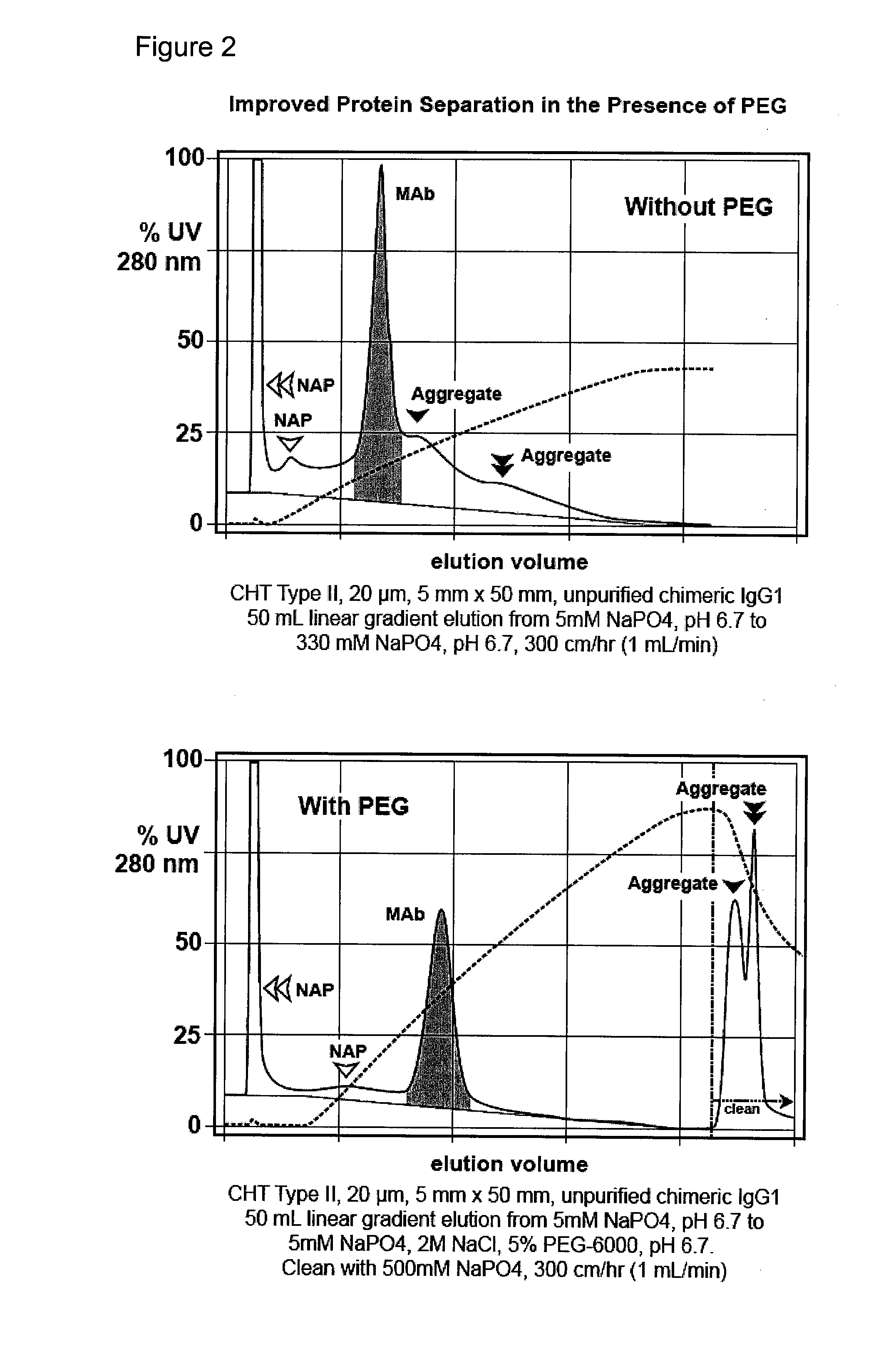
Enhanced capacity and purification of antibodies by mixed mode chromatography in the presence of aqueous-soluble nonionic organic polymers
ActiveUS20080177048A1High level of productivityEnhance binding capacityComponent separationSolid sorbent liquid separationChemistryWater soluble
This invention relates to the use of mixed mode chromatography for purification of at least one intact non-aggregated antibody from a mixture containing intact non-aggregated antibodies and undesirable materials, including fragmented or aggregated antibodies, host cell proteins, DNA, endotoxin, and / or virus. This invention further relates to the integration of such a method into a multi-step procedure with other fractionation methods for purification of antibodies suitable for in vivo applications.
Owner:BIO RAD LAB INC
Ligating clip with integral cutting guide
InactiveUS7326223B2Removably lock the clipHigh retention rateCurling devicesHair dryingEngineeringLigating clips
A polymeric, surgical clip having first and second curved legs with each having a pair of opposing side surfaces joined at their proximal ends by a flexible hinge section and movable from an open position to a closed position for clamping a vessel between curved opposing inner surfaces. The first leg terminates at its distal end in a female locking member, and the second leg member terminates in a male locking member complimentary to the female locking member such that when the first and second leg members are moved from an open position to a closed position about the hinge section the male member is lockingly engaged in the female locking member. The clip has at least one cutting guide extending outward from and disposed along at least a portion of the length of at least one of the side surfaces of at least one of the first and second legs. The cutting guide aids in cutting the ligated tissue a safe distance from the clip such that a tissue cuff is formed.
Owner:TECH HLDG CO II
Compositions comprising encapsulated material
InactiveUS20060039934A1High weight ratioImprove barrier propertiesAntibacterial agentsCosmetic preparationsWater basedSolvent
A composition such as a water-based consumer product comprises material (e.g. perfume) encapsulated within shell capsules, each capsule comprising an encapsulating wall having an inner surface and an outer surface, with a coating on the inner surface and / or outer surface of the shell wall, the composition further comprising surfactant and / or solvent. The coating can improve the barrier properties of the shell and can enhance retention of the encapsulated materials within the shell.
Owner:QUEST INTERNATIONAL
System and method for extended media retention
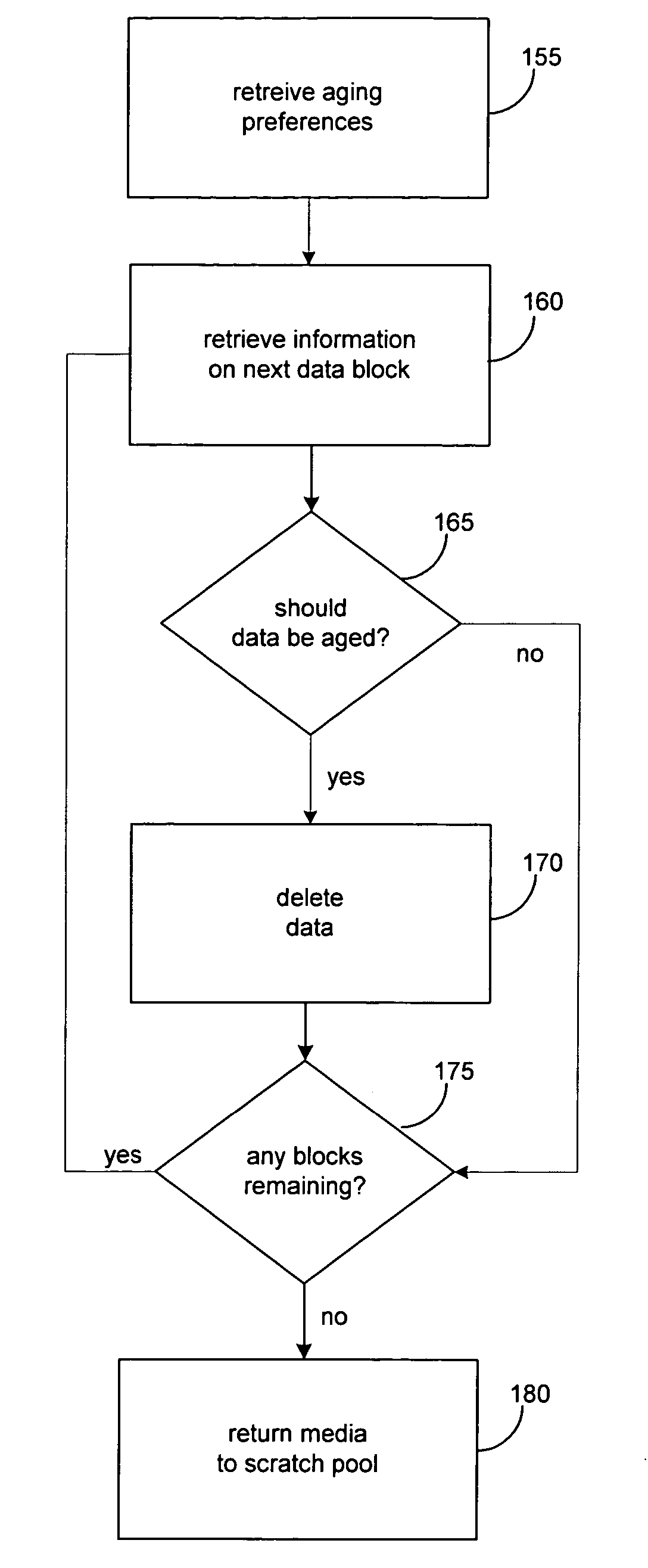
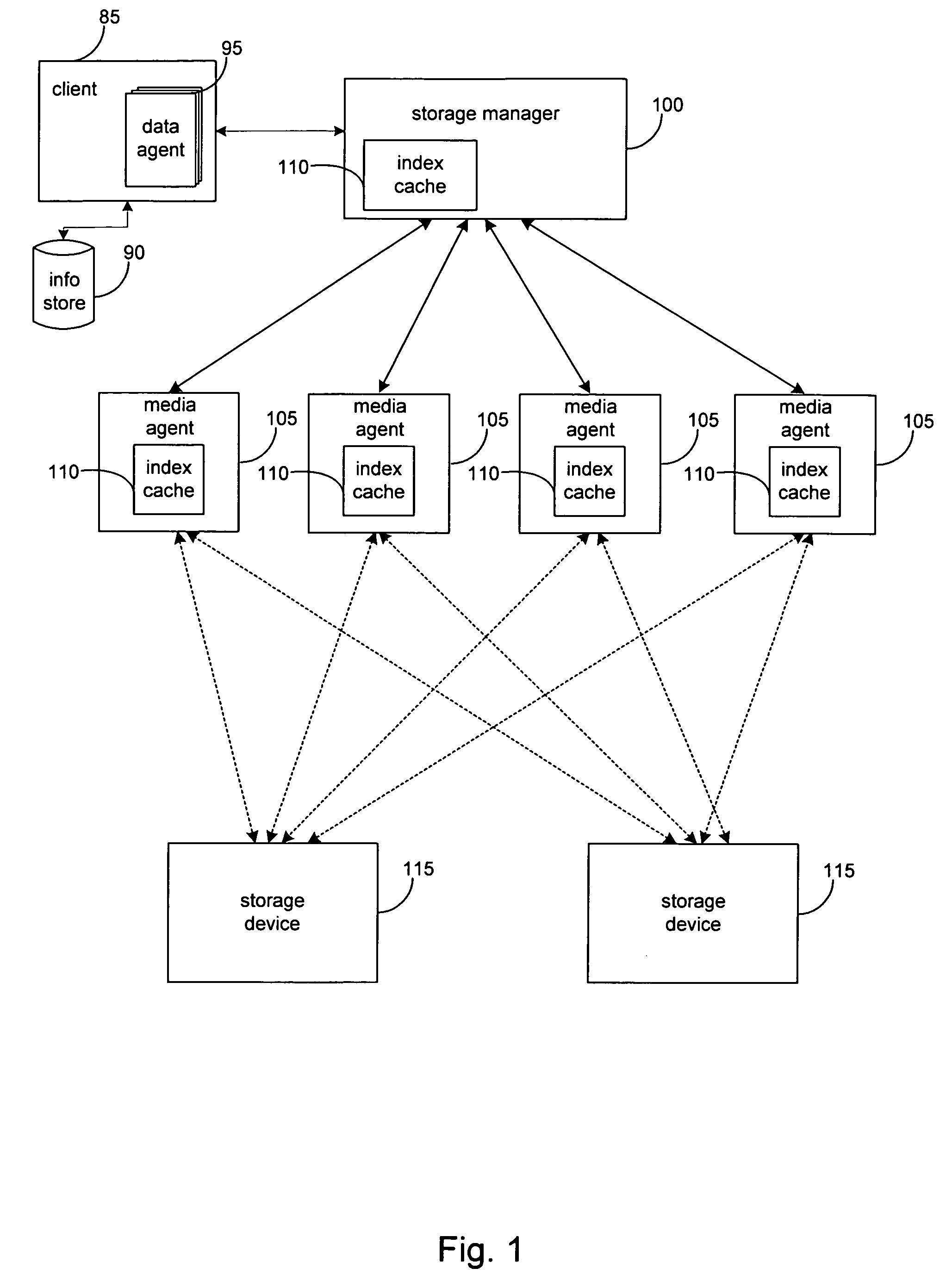
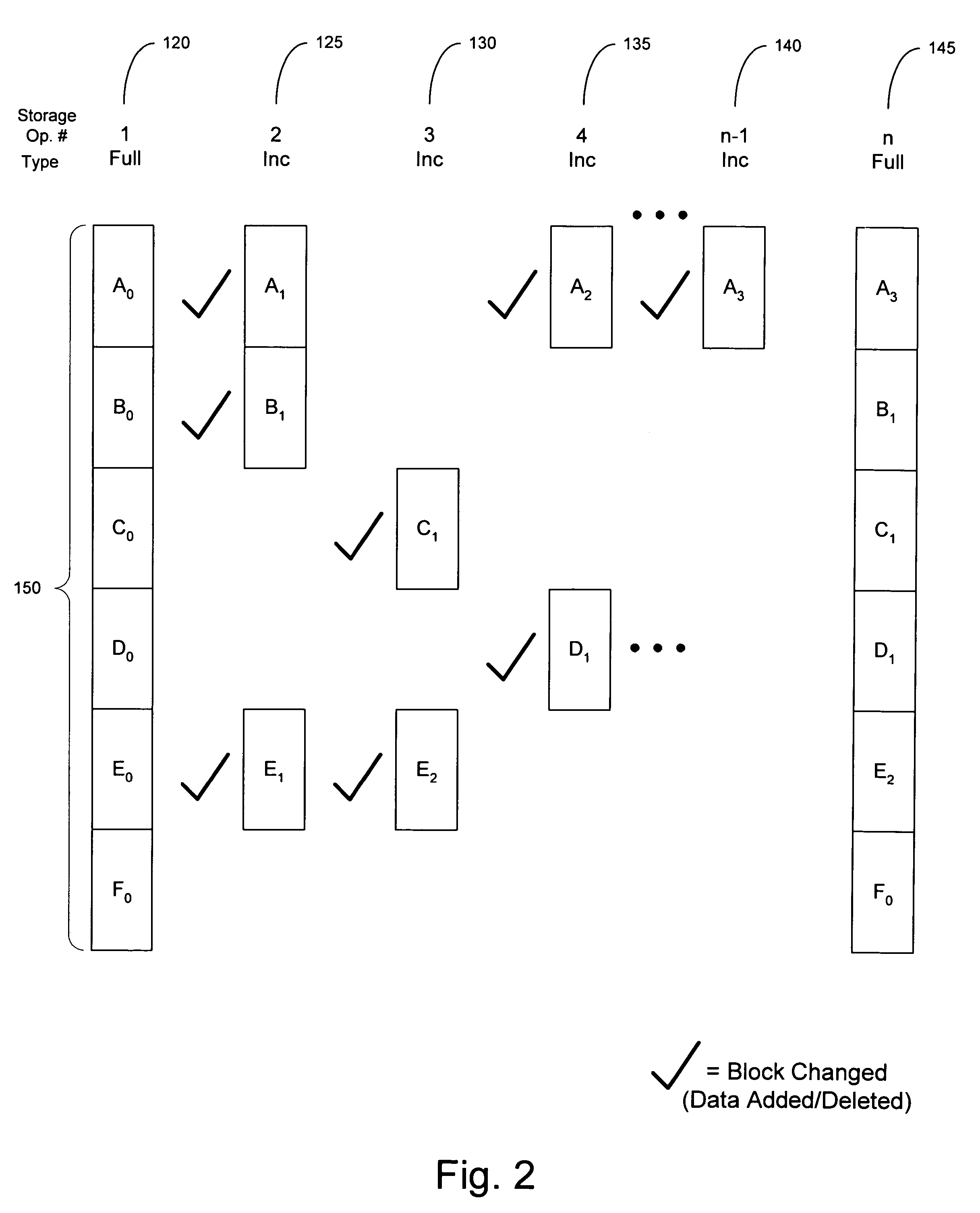
ActiveUS7596586B2High retention rateData processing applicationsInput/output to record carriersData setData element
Owner:COMMVAULT SYST INC
Ligating clip with integral interlocking latch mechanism
ActiveUS7316696B2High retention rateInterference minimizationCurling devicesIntravenous devicesDetentEngineering
A polymeric, surgical clip having first and second curved legs joined at their proximal ends by a flexible hinge section and movable from an open position to a closed position for clamping a vessel between curved opposing inner surfaces. The first leg terminates at its distal end in a female locking member comprising a resilient inwardly turned hook having a recess in the mouth of the hook, and the second leg member terminates in a male locking member complimentary to the female locking member and comprising a detent such that when the first and second leg members are moved from an open position to a closed position about the hinge section the hook member will deflect about the distal end of the second leg member thereby engulfing the male locking member in the mouth of the hook and matingly engaging the detent in the recess thereof, thus securely locking the clip in the closed position. The locking feature provides greater closure security to the clip when clamping large diameter or uncompressible tissue.
Owner:TELEFLEX MEDICAL INC
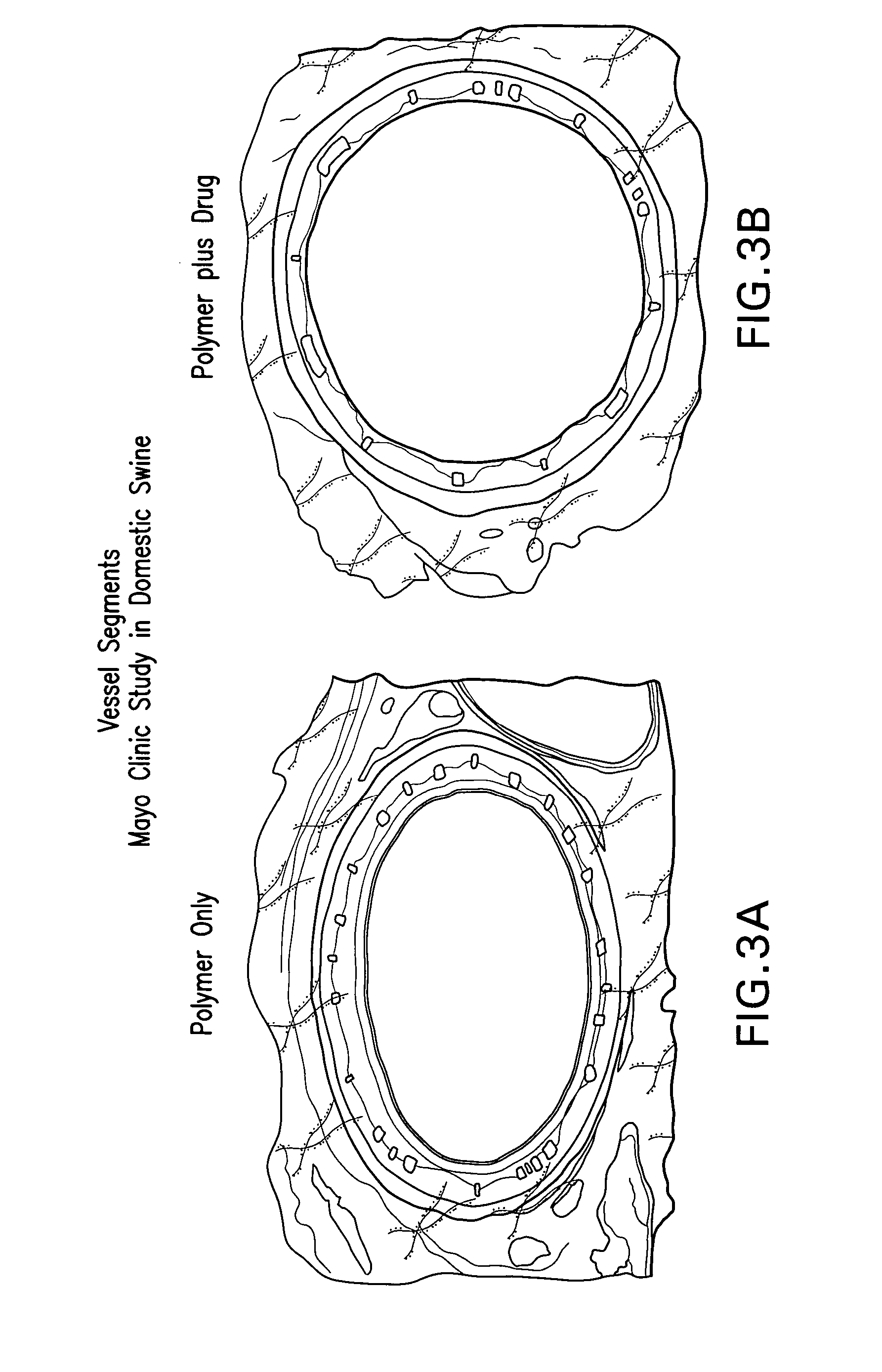
Delivery of Highly Lipophilic Agents Via Medical Devices
InactiveUS20090216317A1Easy to transportIncrease drug retentionAntibacterial agentsBiocideBiomedical engineeringDisease
An apparatus and system for delivering a lipophilic agent associated with a medical device including: a medical device, a first lipophilic agent capable of penetrating a body lumen, wherein the transfer coefficients of the first lipophilic agent is by an amount that is statistically significant of at least approximately 5,000, wherein the first lipophilic agent is associated with the medical device, wherein the first lipophilic agent / medical device is placed adjacent to said body lumen, and wherein a therapeutically effective amount of the first lipophilic agent is delivered to a desired area within a subject. Furthermore, the invention relates to a method for improving patency in a subject involving placement of a medical device in a body lumen for treating and / or preventing adjacent diseases or maintaining patency of the body lumen.
Owner:ABBOTT CARDIOVASCULAR +1
Radiolabeling kit and binding assay
InactiveUS20020102208A1No reduction in immunoreactivityNegligible lossIn-vivo radioactive preparationsDepsipeptidesTherapeutic antibodyAssay
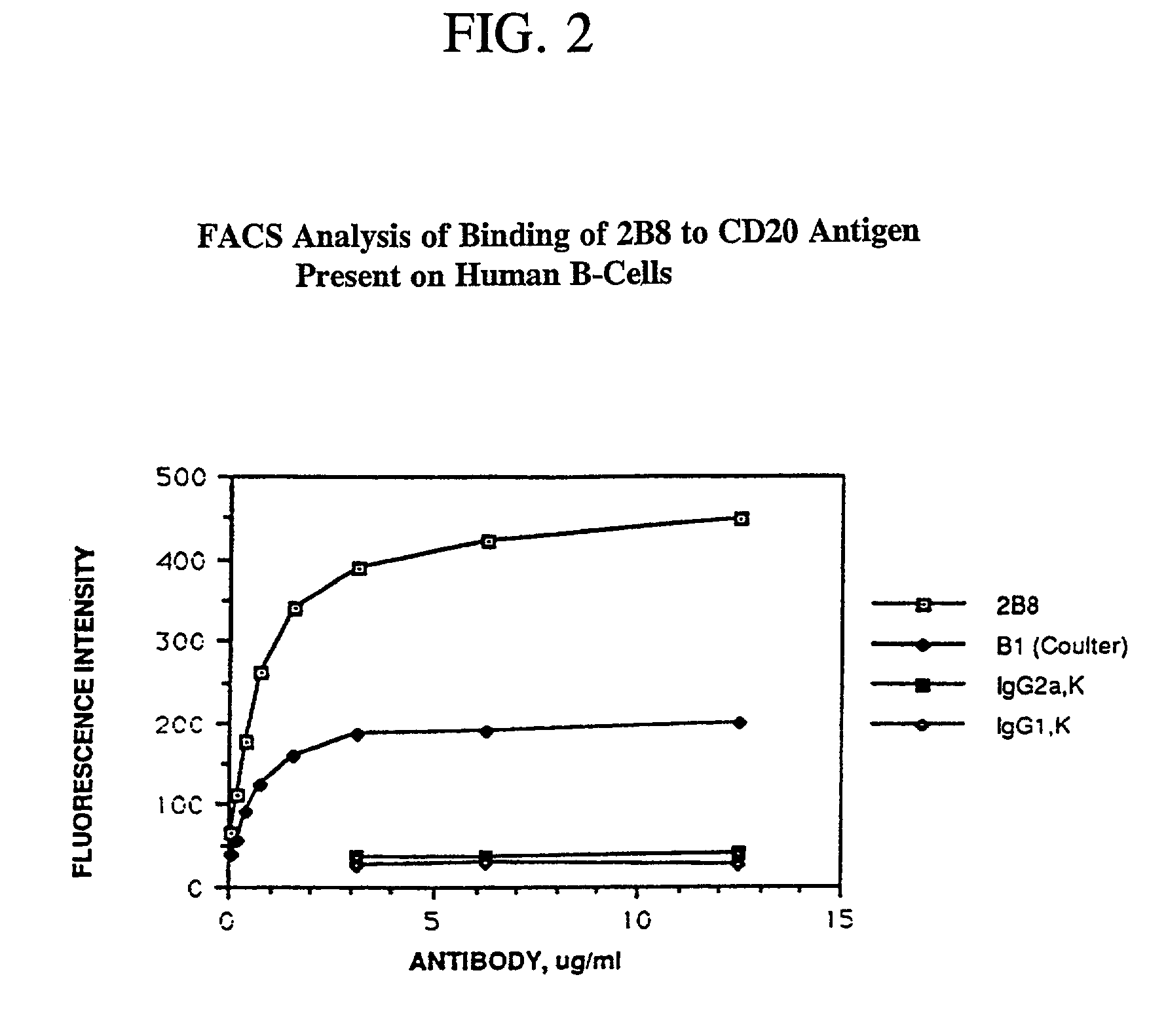
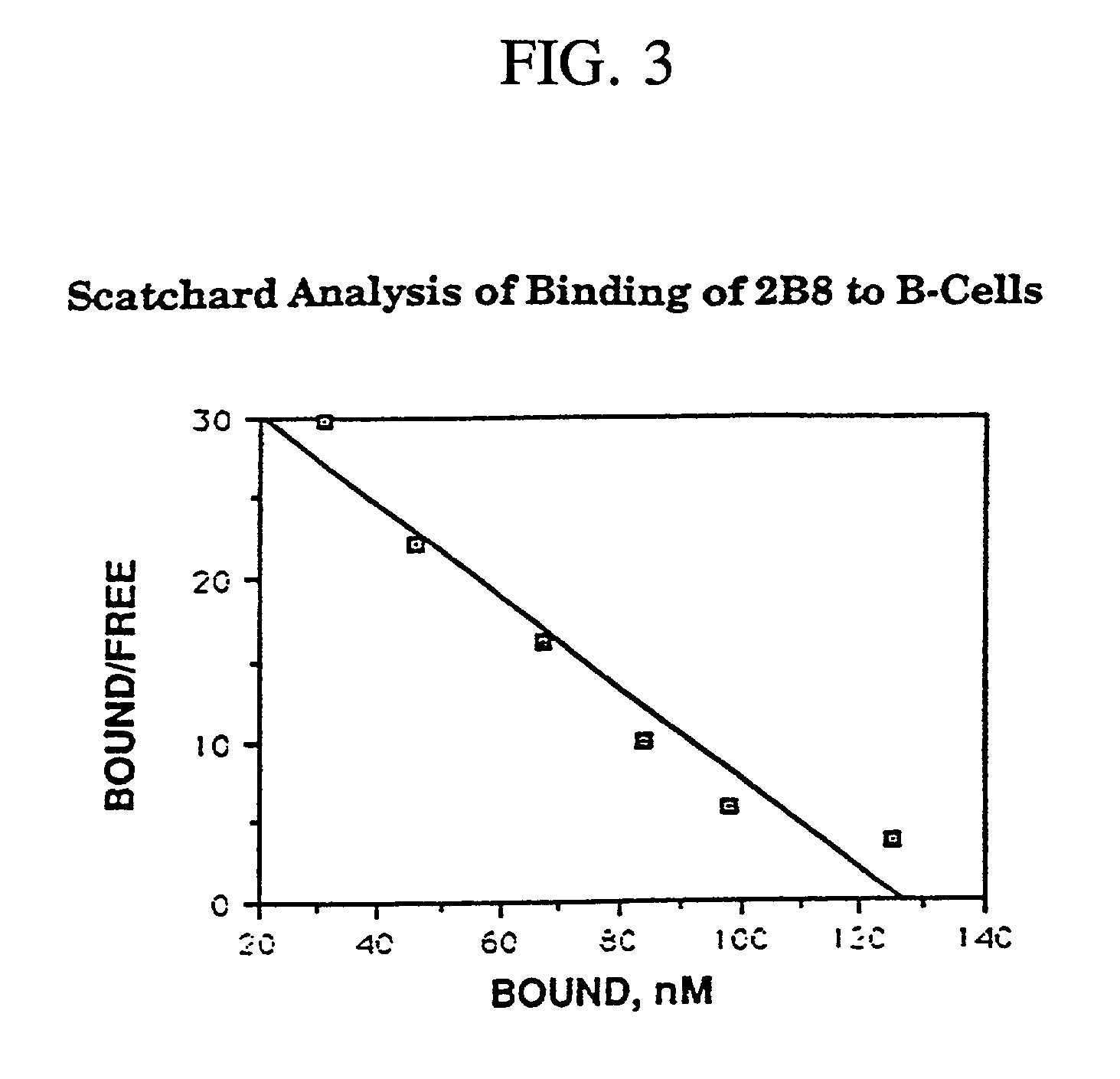
Antibody binding assays and radiolabeling kits are disclosed for radiolabeling and testing therapeutic antibodies in the commercial setting. In particular, the kits are designed for making and evaluating radiolabeled anti-CD20 conjugates to be used for the treatment and imaging of B cell lymphoma tumors. All kit reagents are sterile and are designed to achieve a high level of antibody radiolabeling and product stability with results which are highly reproducible.
Owner:BIOGEN INC
Delivery of highly lipophilic agents via medical devices
An apparatus and system for delivering a lipophilic agent associated with a medical device including: a medical device, a first lipophilic agent capable of penetrating a body lumen, wherein the transfer coefficients of the first lipophilic agent is by an amount that is statistically significant of at least approximately 5,000, wherein the first lipophilic agent is associated with the medical device, wherein the first lipophilic agent / medical device is placed adjacent to said body lumen, and wherein a therapeutically effective amount of the first lipophilic agent is delivered to a desired area within a subject. Furthermore, the invention relates to a method for improving patency in a subject involving placement of a medical device in a body lumen for treating and / or preventing adjacent diseases or maintaining patency of the body lumen.
Owner:ABBOTT LAB INC
Delivery of highly lipophilic agents via medical devices
Owner:ABBOTT LAB INC
Inserter device including rotor subassembly
InactiveUS20110106126A1Reduce manufacturing costImprove reliabilityCatheterSensorsLinear motionEngineering
An inserter subassembly including a rotor and drive member such that rotation of the rotor is translated to a linear motion including insertion and refraction paths.
Owner:ABBOTT DIABETES CARE INC
System and method for real time video production and multicasting
InactiveUS7024677B1Easy to controlCost efficientBroadcast information characterisationElectronic editing digitised analogue information signalsPersonalizationBroadcast time
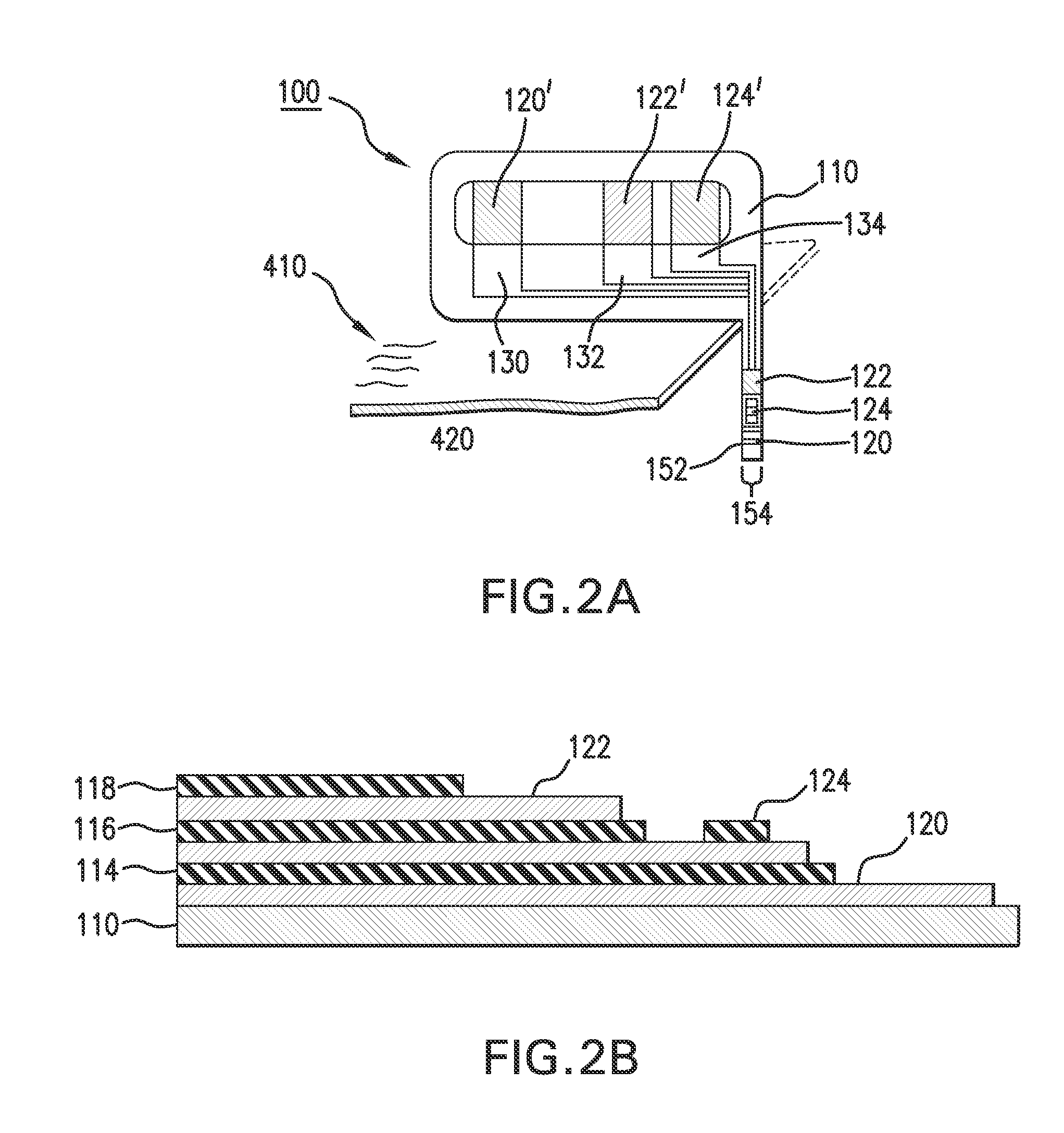
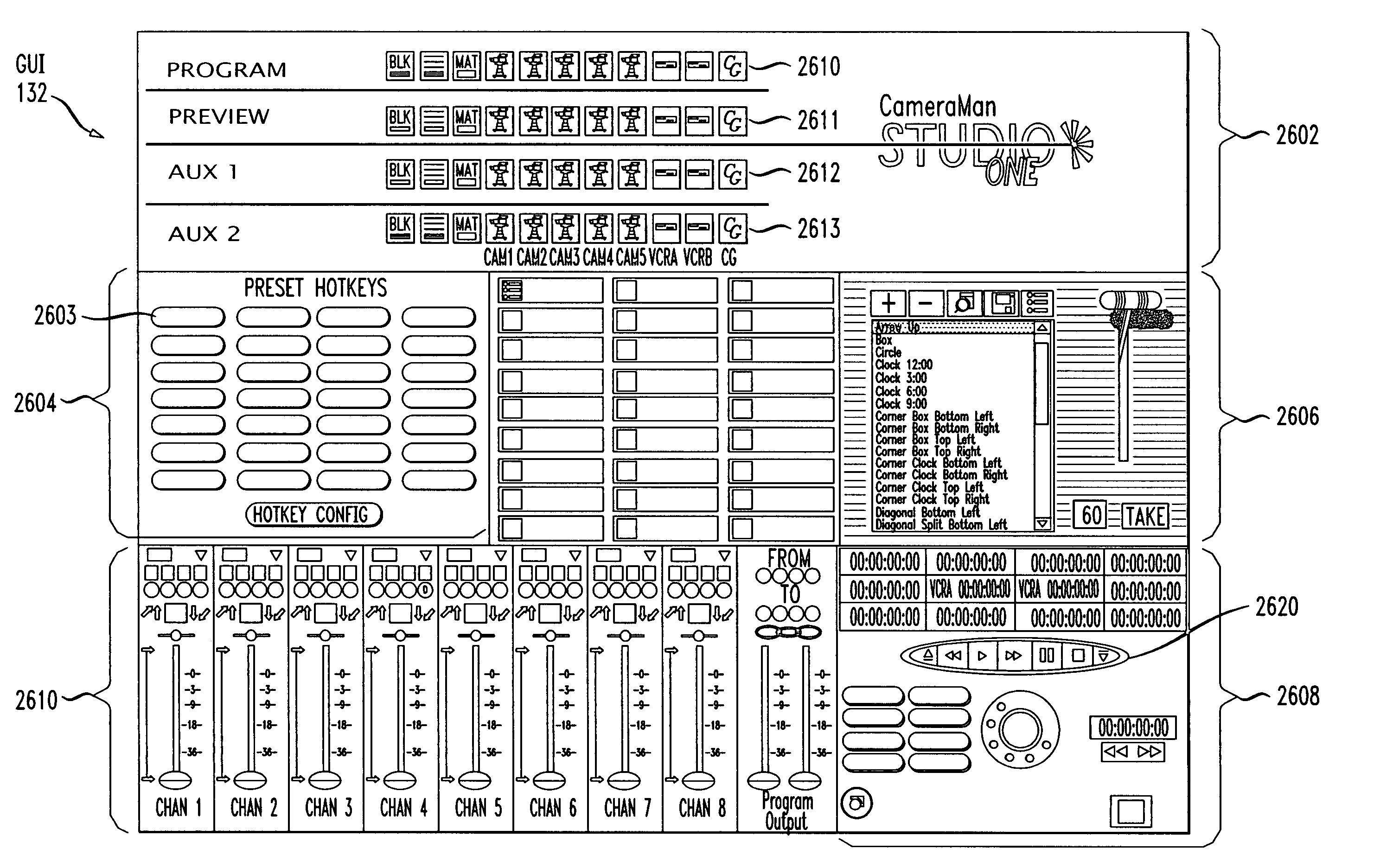
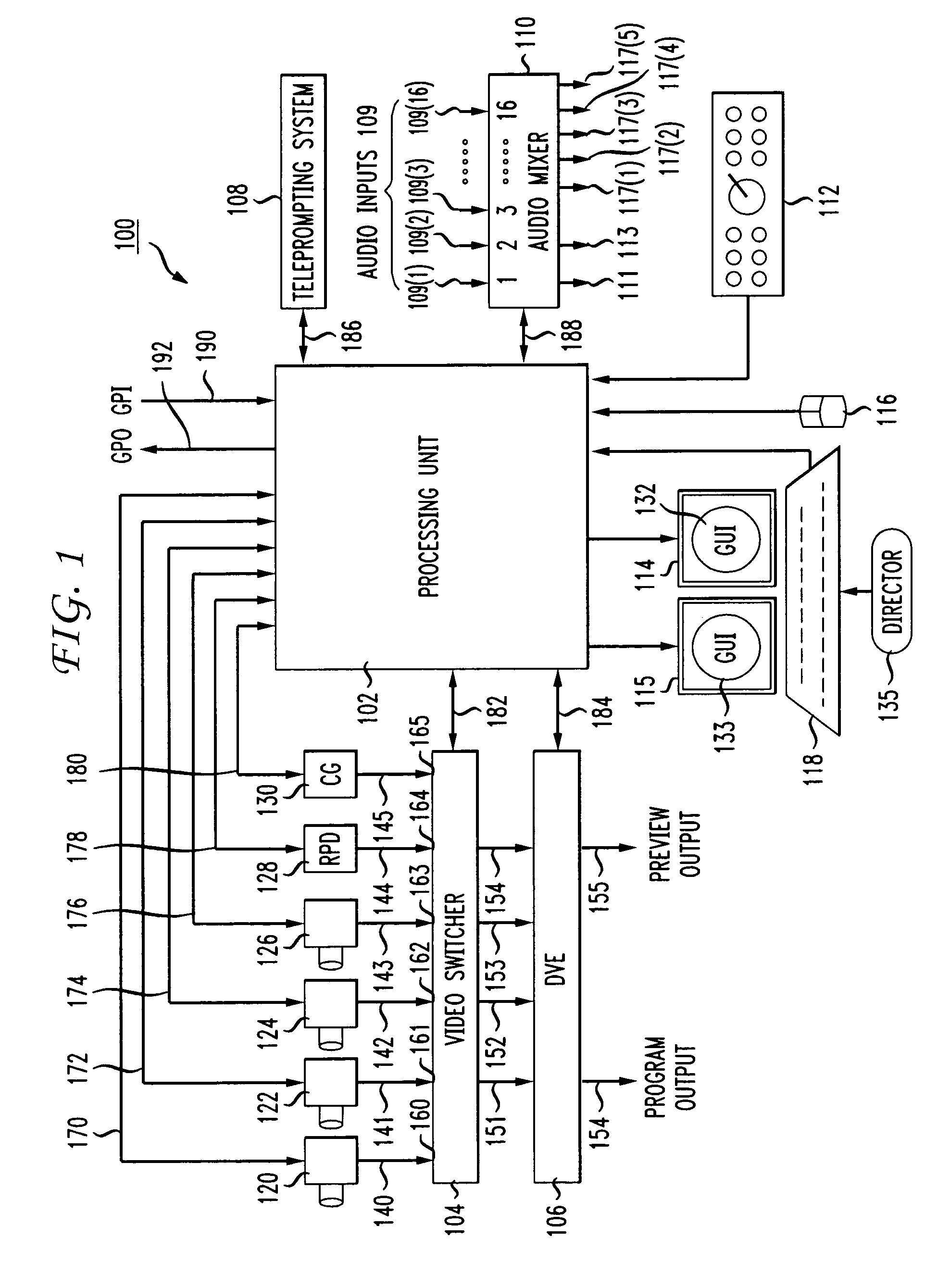
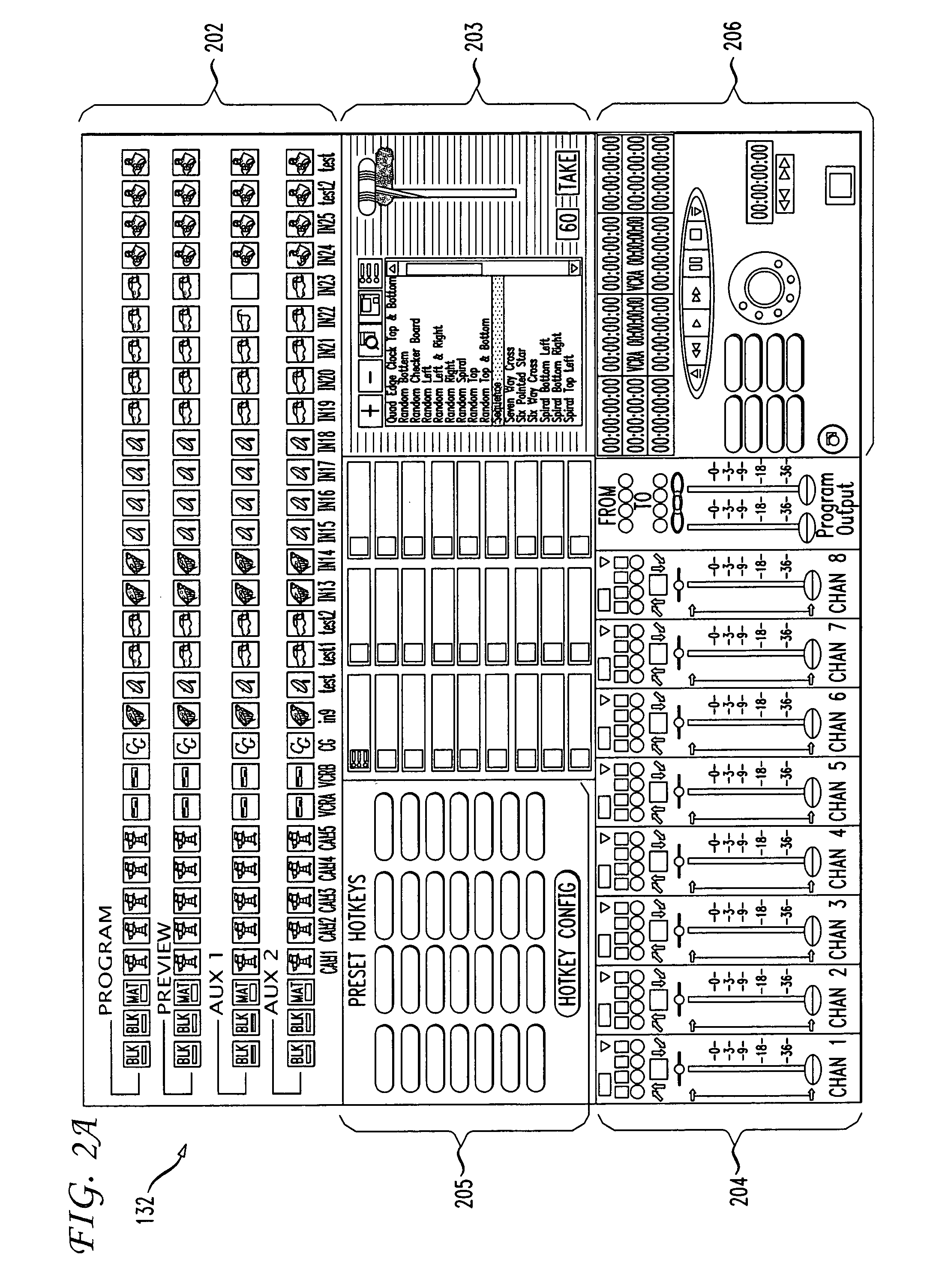
An integrated, fully automated video production system provides a video director with total control over all of the video production devices used to produce and broadcast a show. Such devices include, but are not limited to, cameras, robotic pan / tilt heads, video tape players and recorders (VTRs), video servers and virtual recorders, character generators, still stores, digital video disk players (DVDs), audio mixers, digital video effects (DVE), video switchers, and teleprompting systems. The video production system provides an automation capability that allows the video director to pre-produce a show, review the show in advance of “air time,” and then, with a touch of a button, produce the live show. In one embodiment, the invention provides a video production system having a processing unit in communication with one or more of the video production devices. The processing unit also records the show or elements from the show following its production. The show or elements can be recalled for subsequent broadcasts. An Internet interface supports live or on-demand requests for content from the video production. In an embodiment, an online user selects specific. elements and reorganizes the elements to personalize a broadcast. The processing unit receives and executes the online request to continuously stream or download the content to the user. In an embodiment, various supporting features enhance and extend the content of the video production, such as real time polling, hyperlinks to related web sites, video captions, and the like.
Owner:THOMSON LICENSING SA
Constant force coaxial cable connector
ActiveUS7566236B2High retention rateTrend downEngagement/disengagement of coupling partsTwo pole connectionsInterference fitMating connection
Owner:PPC BROADBAND INC
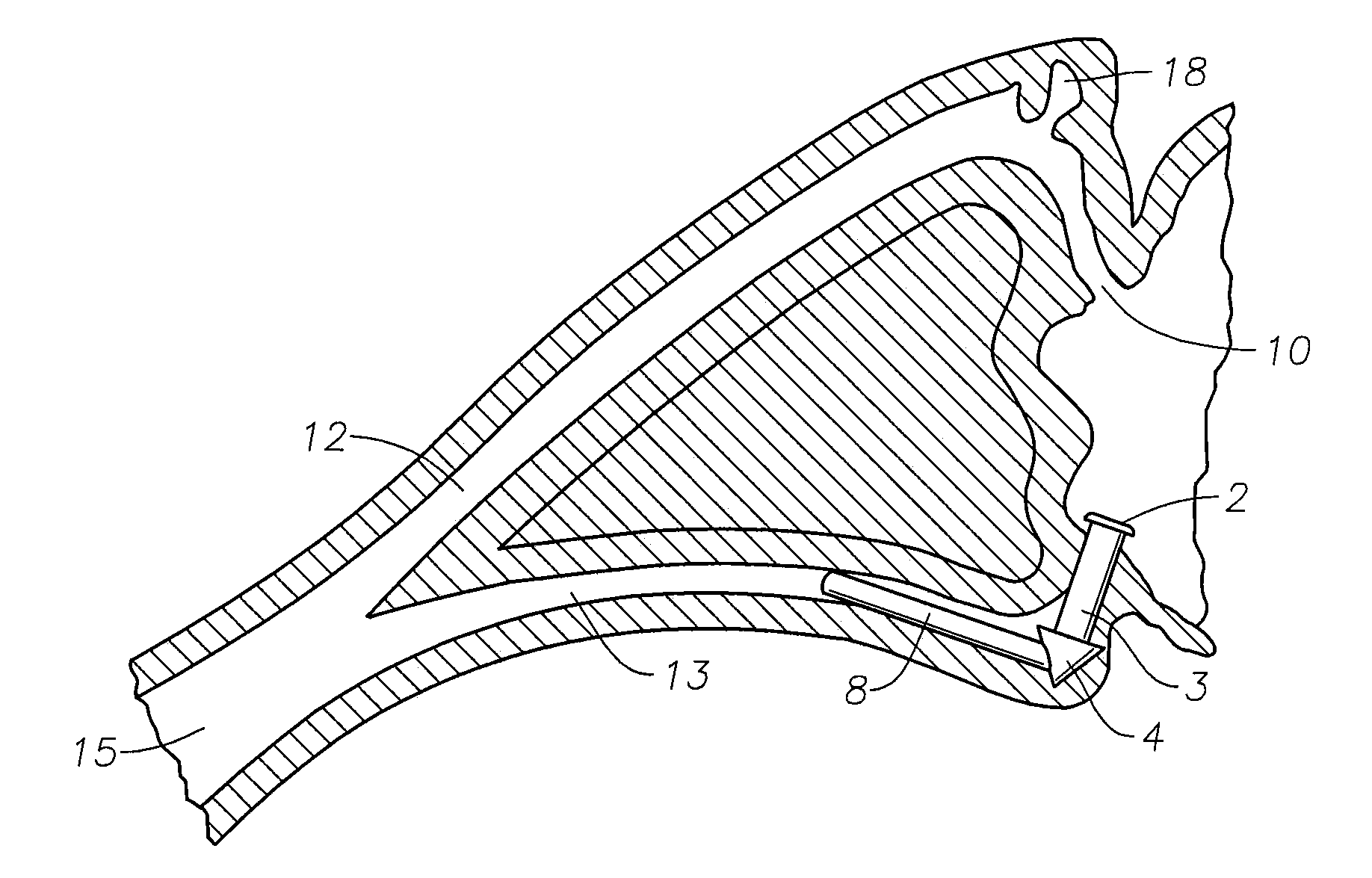
Vein filter
A vessel filter comprising a first region and a second region wherein the filter is movable between a collapsed position for delivery to the vessel and an expanded position for placement within the vessel. A first region has a filter portion having a converging region to direct particles toward the center of the filter and the second region is flared in the expanded position to have a transverse dimension increasing toward a second end portion opposite the first end portion. The second region includes a vessel engaging portion at the second end portion. The first region includes a plurality of spaced apart elongated struts with adjacent struts being joined.
Owner:ARGON MEDICAL DEVICES
User-retainable temperature and impedance monitoring methods and devices
InactiveUS6963772B2High retention rateDiagnostic recording/measuringSensorsMonitoring systemImpedance sensor
A user-retainable monitoring system is disclosed. At least a pair of sensors is provided in association with a support member. The support member is preferably of a type that may be worn by or at least temporarily implanted in a patient. Possible sensor types include temperature sensors and impedance sensors. Temperature sensors may be used to detect a temperature differential between areas of tissue indicative of pathology. Impedance sensors are used to detect subcutaneous fluid detection. The support member may take the form of a bandage, drain or other structure. Monitor structures as described may have stand-alone utility or be connected to a processor or data recorder to enable various functions.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Ligation clip with flexible clamping feature
ActiveUS20090171380A1Safe and robust retentionImproved vessel clampingWound clampsHinge angleBiomedical engineering
A polymeric surgical ligating clip is provided having curved leg members joined by a resilient hinge. A leg member includes a flexible rib protruding from a vessel clamping inner surface and extends longitudinally between the proximal and distal end portions of the leg member. The flexible rib defines a channel extending transversely through the rib along a majority of said length. The flexible rib can collapse and provides better retention of the clip on a vessel. The clip can contain a flexible rib on both legs in an interlocking fashion. Or a plurality of teeth can protrude from a second vessel clamping inner surface of a second leg member. The plurality of teeth can include first and second rows of teeth extending longitudinally on the second leg member, transversely separated from each other on opposite sides of a centerline of the clip.
Owner:TELEFLEX MEDICAL INC
Delivery of highly lipophilic agents via medical devices
InactiveUS20060240070A1Easy to transportIncrease drug retentionBiocideFibrinogenMedicineMedical device
An apparatus and system for delivering a lipophilic agent associated with a medical device including: a medical device, a first lipophilic agent capable of penetrating a body lumen, wherein the transfer coefficients of the first lipophilic agent is by an amount that is statistically significant of at least approximately 5,000, wherein the first lipophilic agent is associated with the medical device, wherein the first lipophilic agent / medical device is placed adjacent to said body lumen, and wherein a therapeutically effective amount of the first lipophilic agent is delivered to a desired area within a subject. Furthermore, the invention relates to a method for improving patency in a subject involving placement of a medical device in a body lumen for treating and / or preventing adjacent diseases or maintaining patency of the body lumen.
Owner:ABBOTT LAB INC
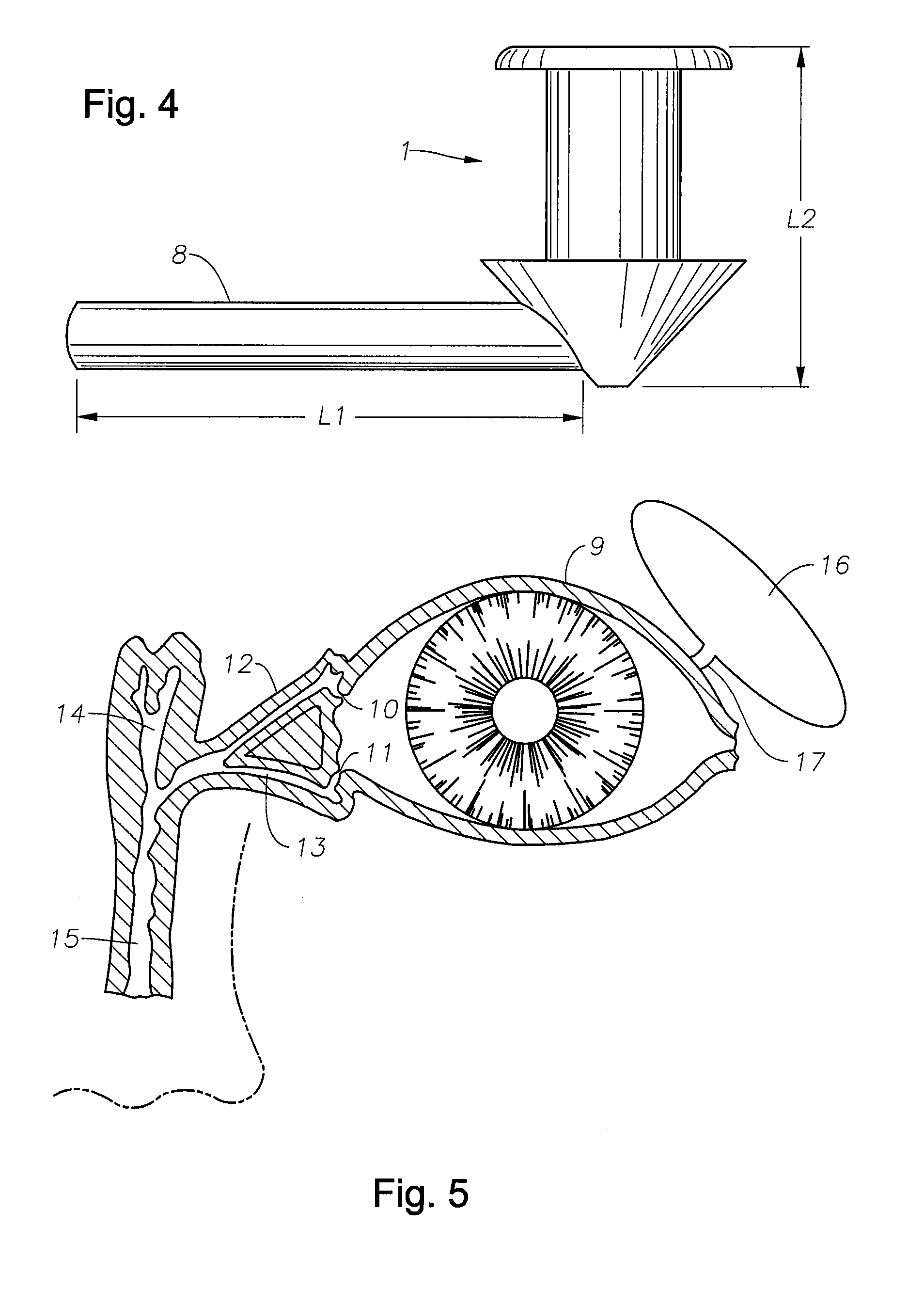
Punctal Plugs and Methods of Delivering Therapeutic Agents
ActiveUS20080181930A1High retention rateIncrease stiffnessBiocideSenses disorderCollagen Punctal PlugsParylene coating
The present invention concerns implantable ocular devices for the sustained release of medication to the eye, and methods for manufacturing and using such devices. In one embodiment, the present invention provides a device comprising: (a) a body comprising a matrix of a prostaglandin and a silicone; (b) a parylene coating on the outer surface of the body; and (c) one or more pores extending from the outer surface of the parylene coating to the outer surface of the body.
Owner:NOVARTIS AG
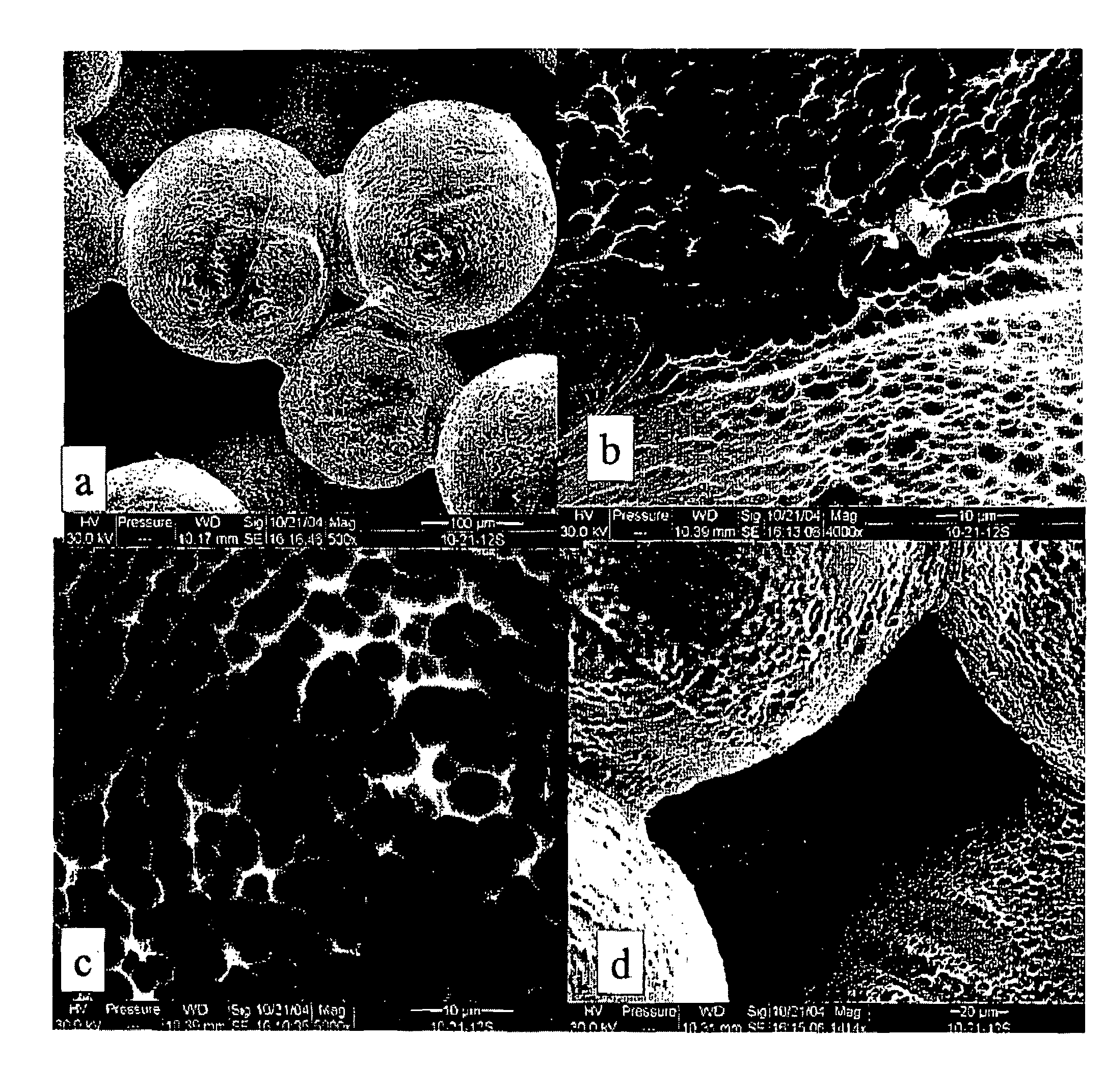
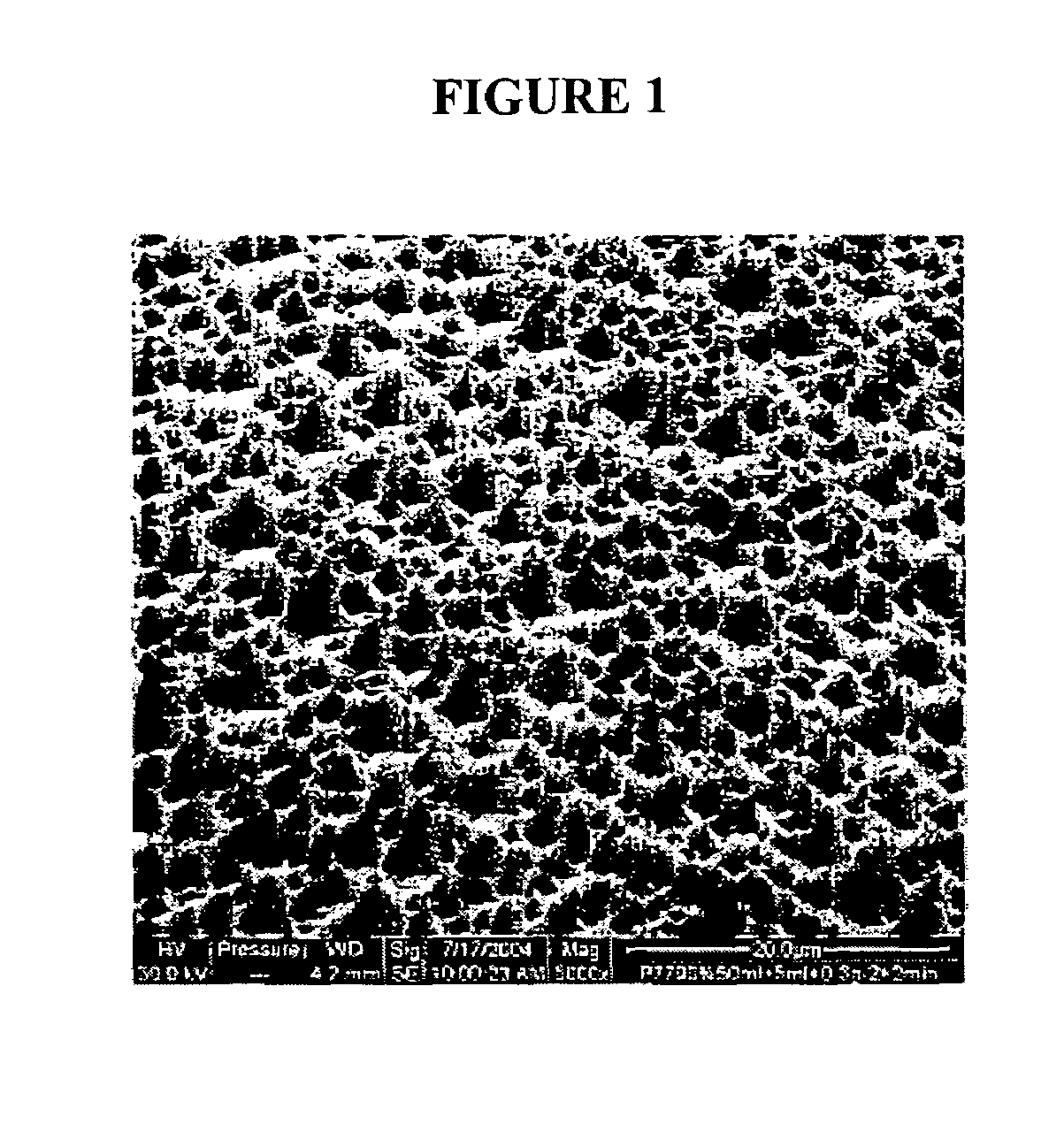
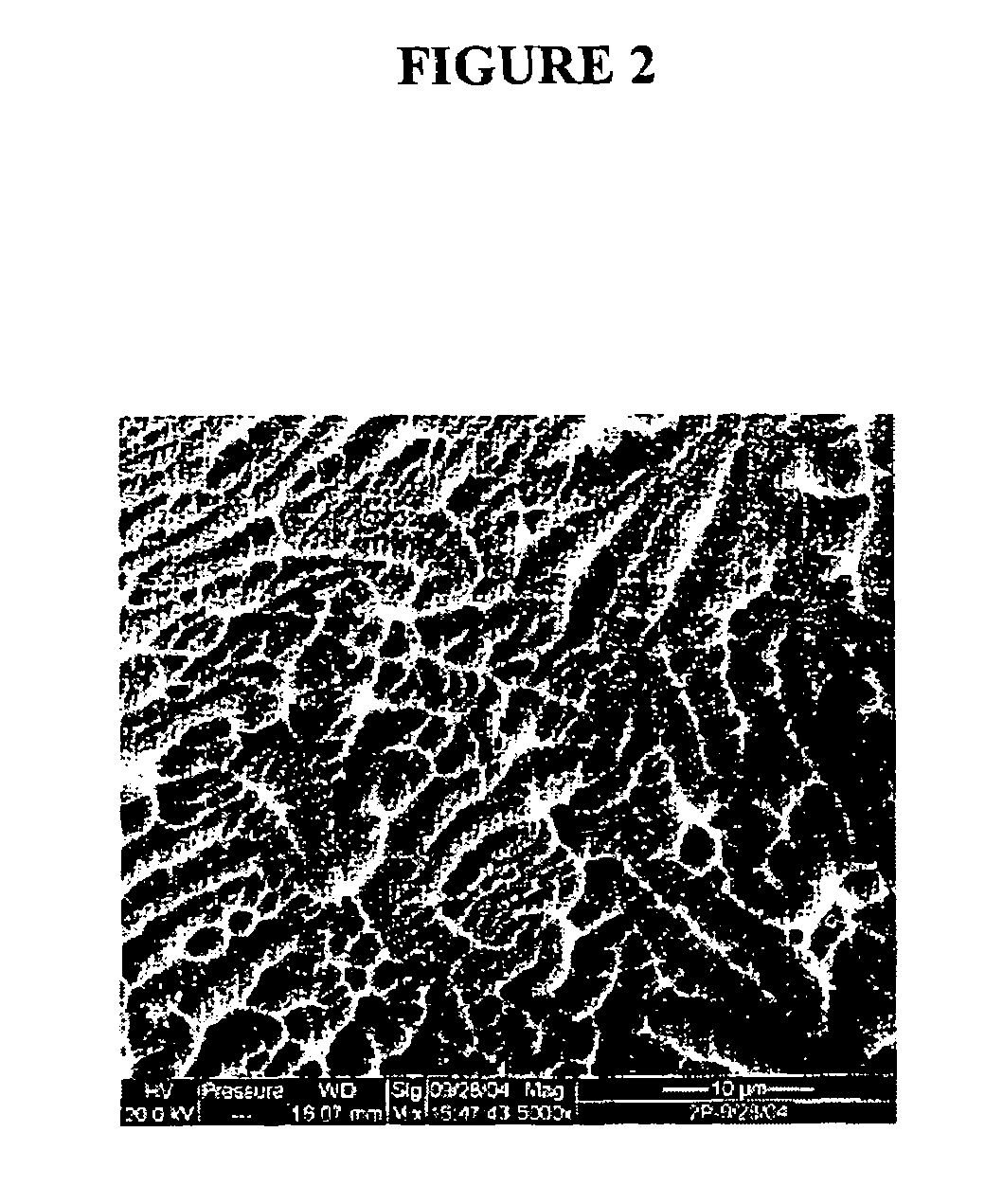
Implants with textured surface and methods for producing the same
ActiveUS7368065B2High retention rateConvenient coatingImpression capsDecorative surface effectsMicrometerChloride
Compositions and methods are provided for preparing a metal substrate having a uniform textured surface with a plurality of indentations with a diameter in the nanometer and micrometer range. The textured surface is produced by exposing the substrate to an etching fluid comprising a hydrohalic acid and a mixture of a hydrohalic acid and an oxyacid, a chloride containing compound, and an oxidant. The etching solution can be used at ambient temperature. This textured surface enhances adherence of coatings or cells onto the textured surface, improves the retention of proteins on the surface, and encourages bone in-growth.
Owner:DEPUY PROD INC
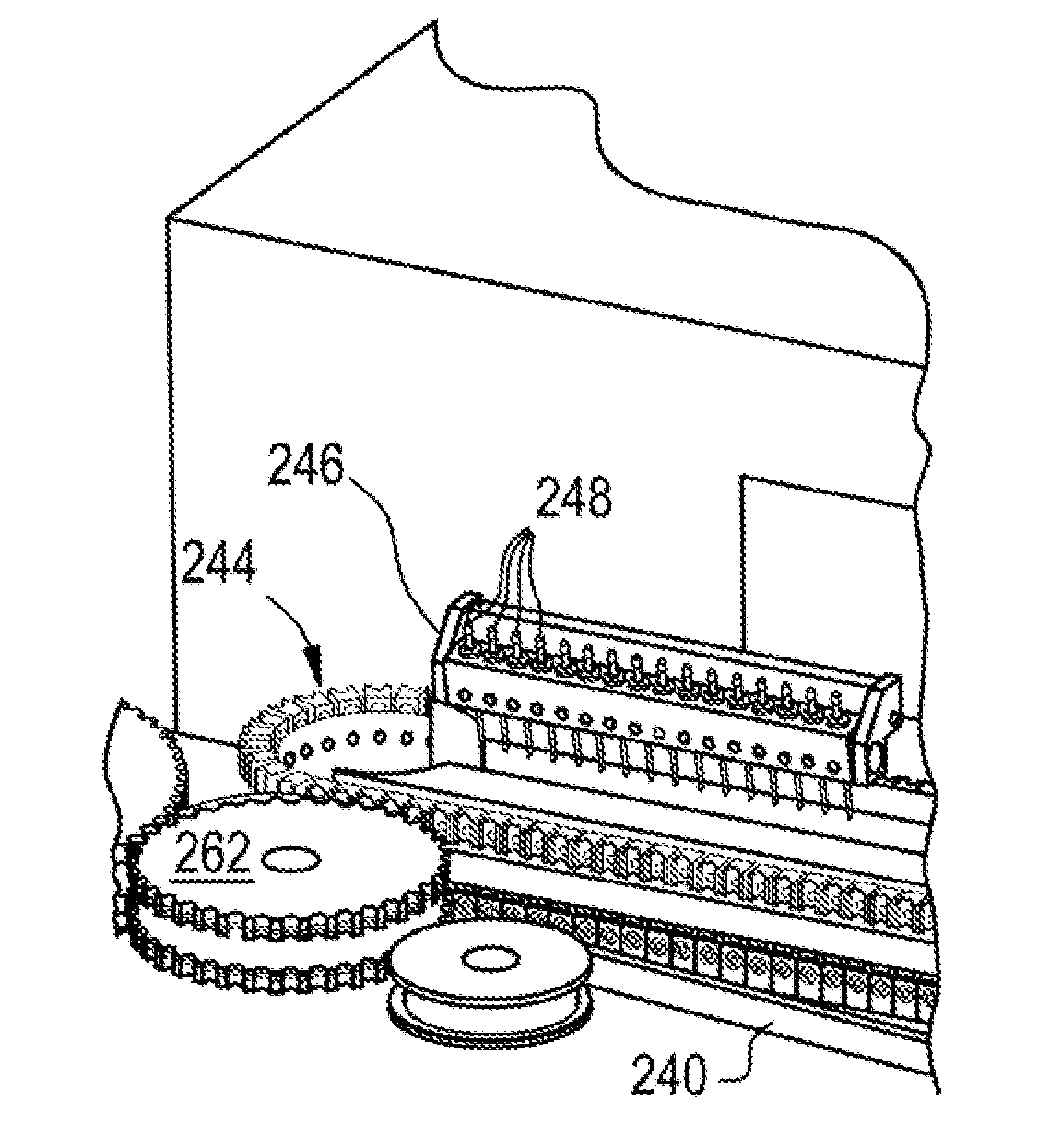
Method and system for the automated production of e-vapor devices
A method for automated manufacturing of e-vapor devices may include establishing a procession of partially assembled, oriented cartridge units of the e-vapor devices in an assembly path. The method may additionally include preparing the cartridge units for filling while the cartridge units are moving on a first drum-to-drum transport path of the assembly path. The method may also include adding liquid to the cartridge units while the cartridge units are moving in a filling workstation of the assembly path. The method may also include preparing the cartridge units for sealing while the cartridge units are moving on a second drum-to-drum transport path of the assembly path. The method further includes sealing the cartridge units while the cartridge units are moving in a sealing workstation of the assembly path.
Owner:AKRIA CLIENT SERVICES LLC
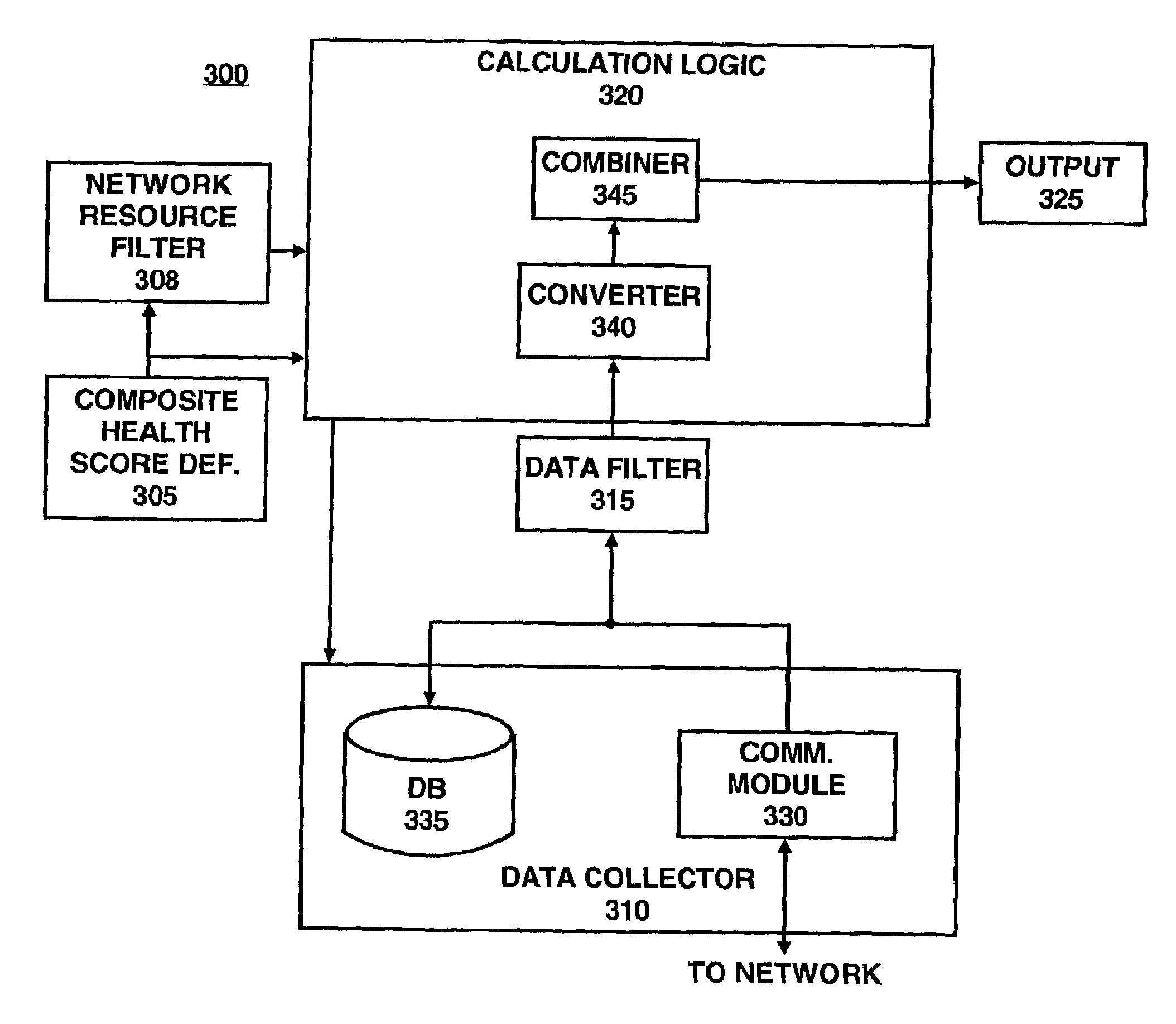
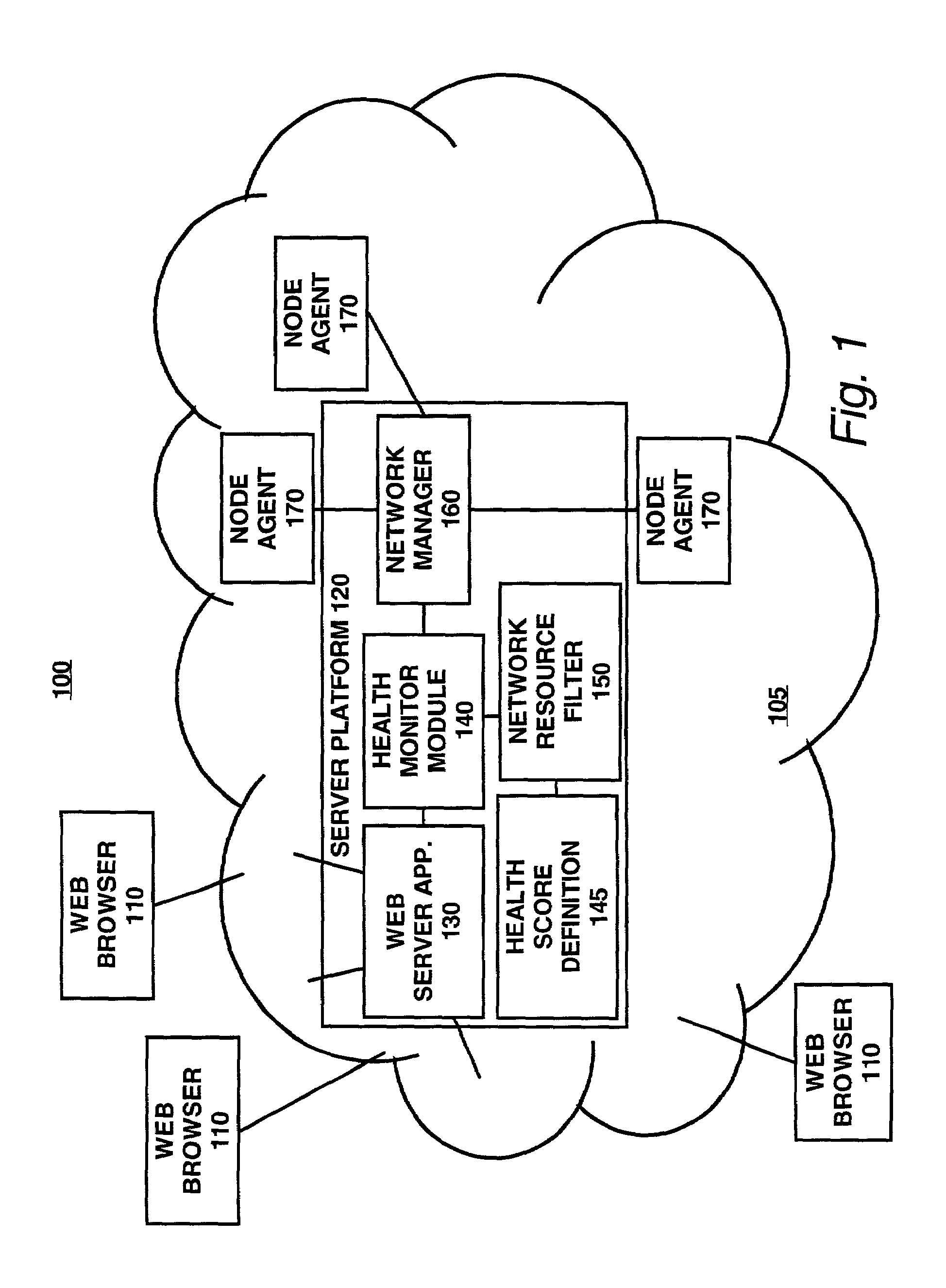
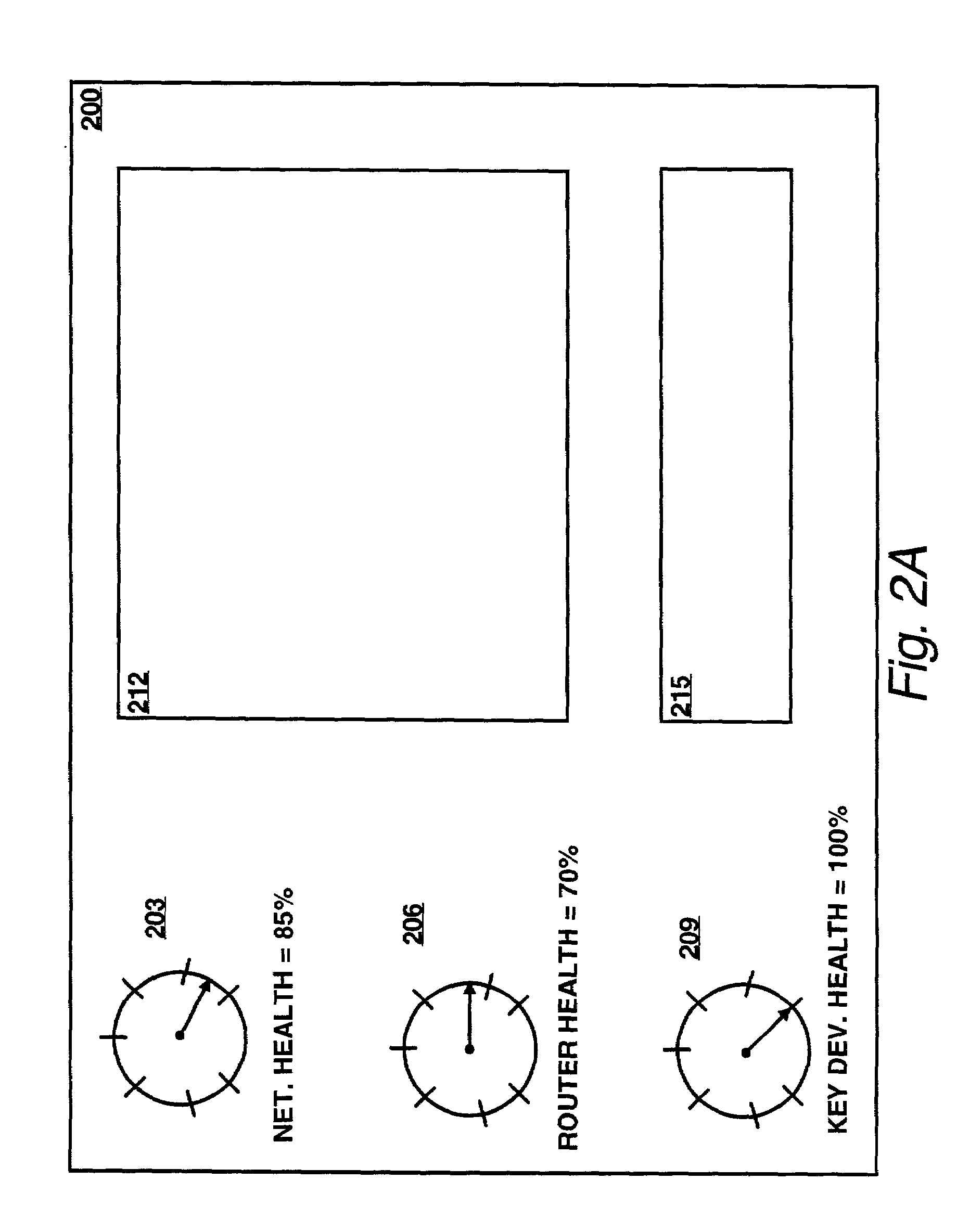
Method and apparatus for customizably calculating and displaying health of a computer network
InactiveUS7003564B2Easy to customizeFacilitates extensibleError preventionTransmission systemsGraphicsComputer module
Apparatus and methods facilitate customizable and extensible performance monitoring of a computer network. One method accepts a composite score definition in terms of N system variables, wherein N≧2; determines N raw data values, each raw data value corresponding to one of the N system variables; computes the composite score in accordance with the definition using the N raw data values as inputs; and outputs the composite score. The composite score definition is preferably in the form of a markup language, such as XML. The composite score definition preferably comprises, for each of the N system variables, a mapping and a weight. Preferably the composite score is displayed in at least one graphic form, such as a dial gauge, a bar indicator or a number, on a hypertext page. The hypertext page preferably contains one or more links to hypertext pages containing information regarding the scores and / or raw data values from which the composite score is derived. Another method accepts a mapping by which a raw data value associated with a corresponding system variable is mapped to a score, determines a raw data value corresponding to the system variable, converts the raw data value to a score in accordance with the mapping; and produces an output based on the score. One apparatus comprises a composite score definition, a data collector, a calculation logic and an output. The data collector collects a raw data value corresponding to one of the N system variables. The calculation logic is connected to the data collector and calculates the composite score in accordance with the definition using the N raw data values as inputs. The composite score is conveyed by way of the output. Preferably, the data collector comprises a database in which at least some of the raw data values are stored and a communication module by which at least some of the raw data values are transported, preferably according to the SNMP and / or the ICMP protocols. Another apparatus comprises a mapping, a data collector, a converter and an output. A raw data value associated with a corresponding system variable is mapped to a score, according to the mapping.
Owner:HEWLETT-PACKARD ENTERPRISE DEV LP
Anti-IL-6 Receptor Antibody
InactiveUS20110245473A1Enhanced antigen-neutralizing activity and pharmacokineticsGood treatment effectCompound screeningApoptosis detectionHigh concentrationHinge region
The present inventors succeeded in discovering specific amino acid mutations in the variable region, framework region, and constant region of TOCILIZUMAB, and this enables to reduce immunogenicity risk and the heterogeneity originated from disulfide bonds in the hinge region, as well as to improve antigen binding activity, pharmacokinetics, stability under acidic conditions, and stability in high concentration preparations.
Owner:CHUGAI PHARMA CO LTD
Percutaneous catheter directed intravascular occlusion devices
The present invention provides an improved vascular occlusion device having improved flexibility and retention of the type fabricated from braided tubular metal fabric having an expanded preset configuration and an elongated collapsed reduced diameter configuration for delivery through a catheter to a treatment site and shaped to create an occlusion of an abnormal opening in a body organ or vessel, the woven metal fabric having a memory property whereby the medical device tends to return to said expanded preset configuration when unconstrained. The device further including at least one disk portion adjacent a body cylindrical portion formed from the fabric and having a transition diameter between the disk and cylindrical portion, significantly smaller than the diameter of the disk and the diameter of the cylindrical portion.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
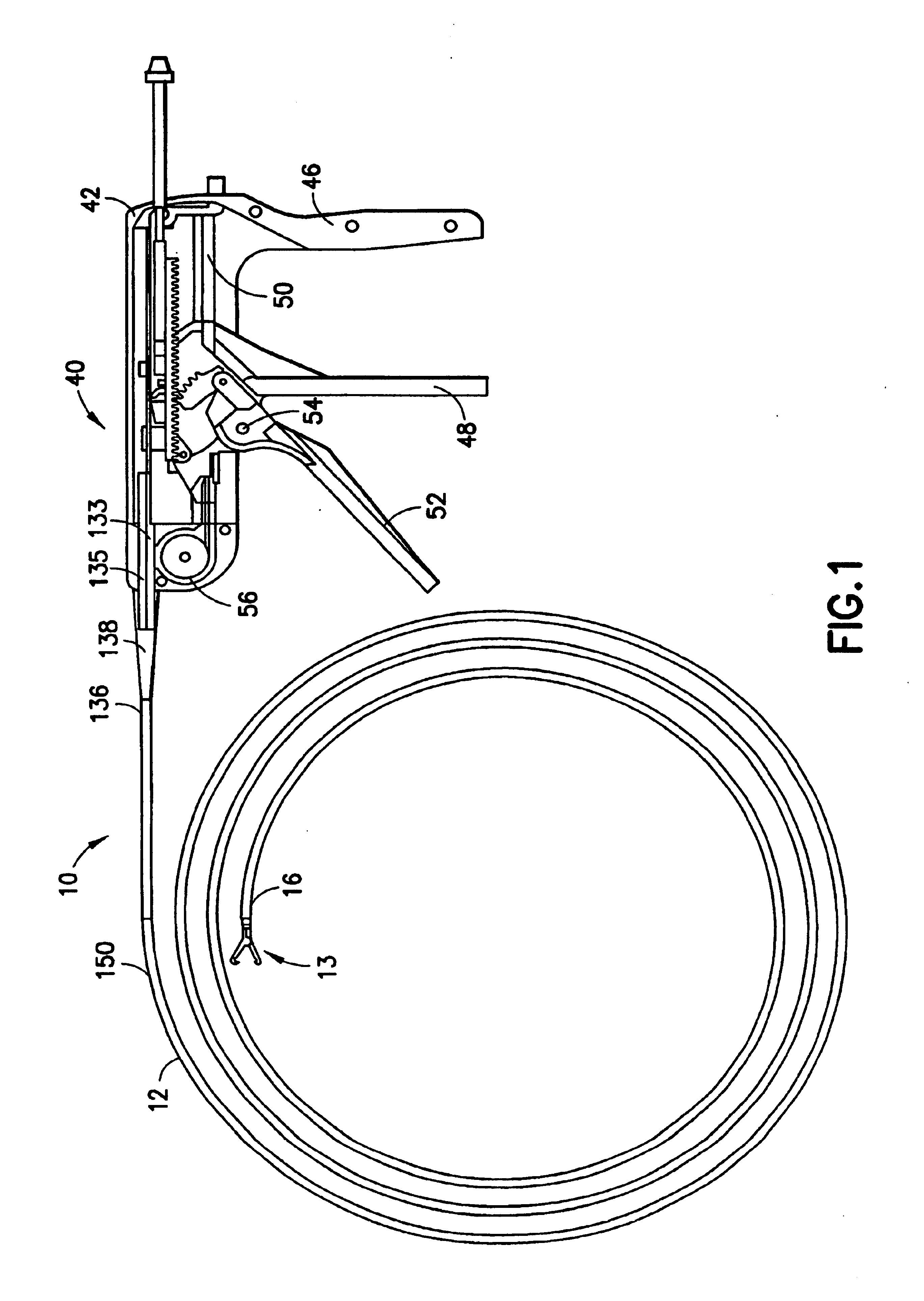
Surgical suturing apparatus with collapsible vacuum chamber
ActiveUS20060282095A1Broaden applicationHigh retention rateSuture equipmentsGastroscopesSurgical departmentSyringe needle
A surgical suturing apparatus includes a suture housing, a needle mounted within the suture housing for movement about an arcuate path, a drive assembly operably associated with the needle for controlling movement of the needle with a suture secured thereto about the arcuate path in a manner facilitating application of the suture to tissue, and a collapsible vacuum chamber containing the suture housing. The vacuum chamber is shaped and dimensioned for coupling to a vacuum line.
Owner:ETHICON ENDO SURGERY INC
High melt strength polypropylene
The present invention concerns a high melt strength propylene polymer or copolymer suitable for manufacturing foams and thermoformed product exhibiting a melt strength of at least 3 g and comprising a high molar mass portion and a low or medium molar mass portion. The polymers are produced by subjecting propylene and optionally other olefins to polymerization in a plurality of polymerization reactors connected in series, employing different amounts of hydrogen as a molar mass modifier in at least two of the reactors, and carrying out the polymerization reaction in the presence of a catalyst system capable of catalyzing the formation of a high molar mass polymerization product having a MFR2 of less than 0.1 g / l0 min and a low or medium molar mass polymerization product having a MFR2 of more than 0.5 g / 10 min.
Owner:BOREALIS TECH OY
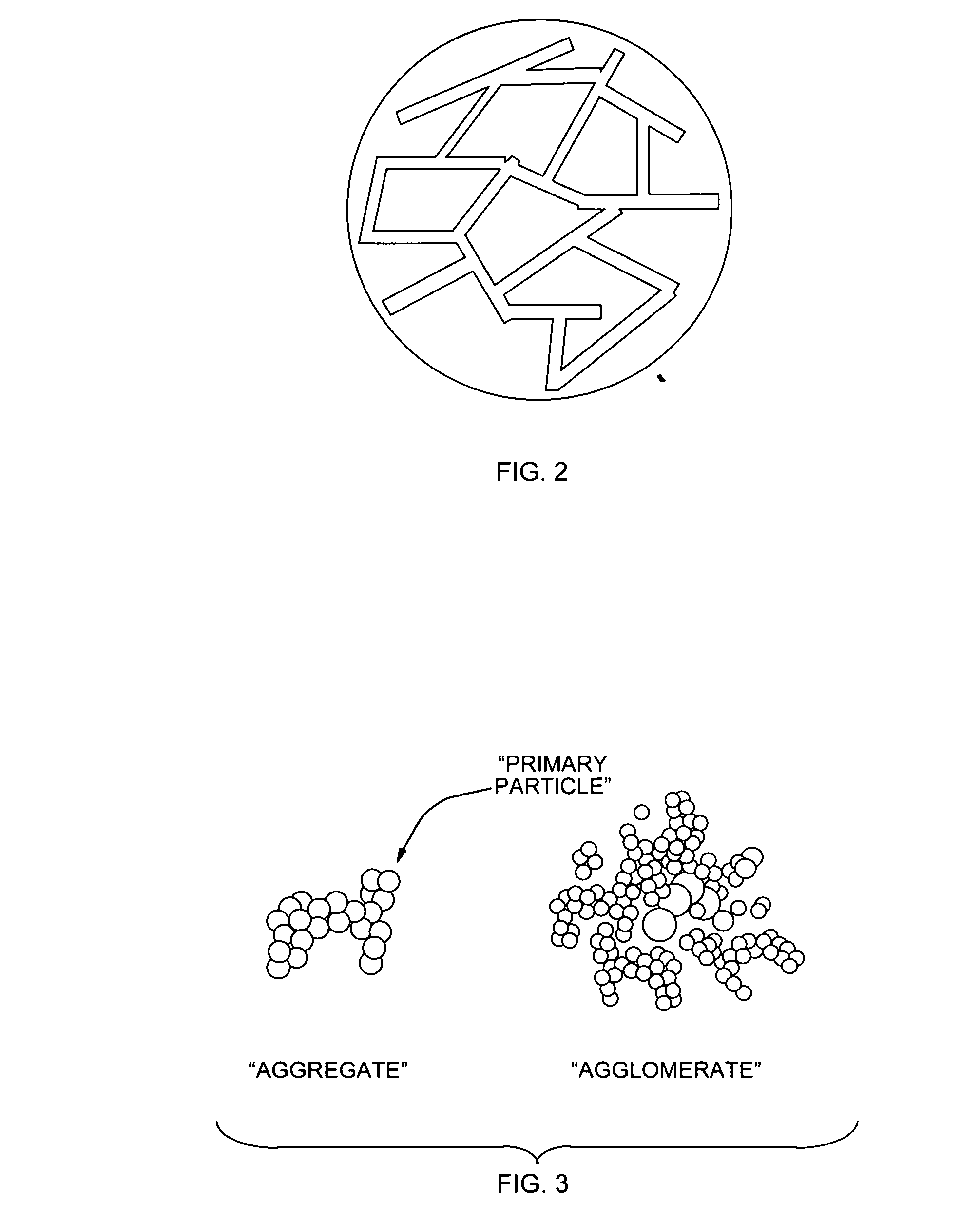
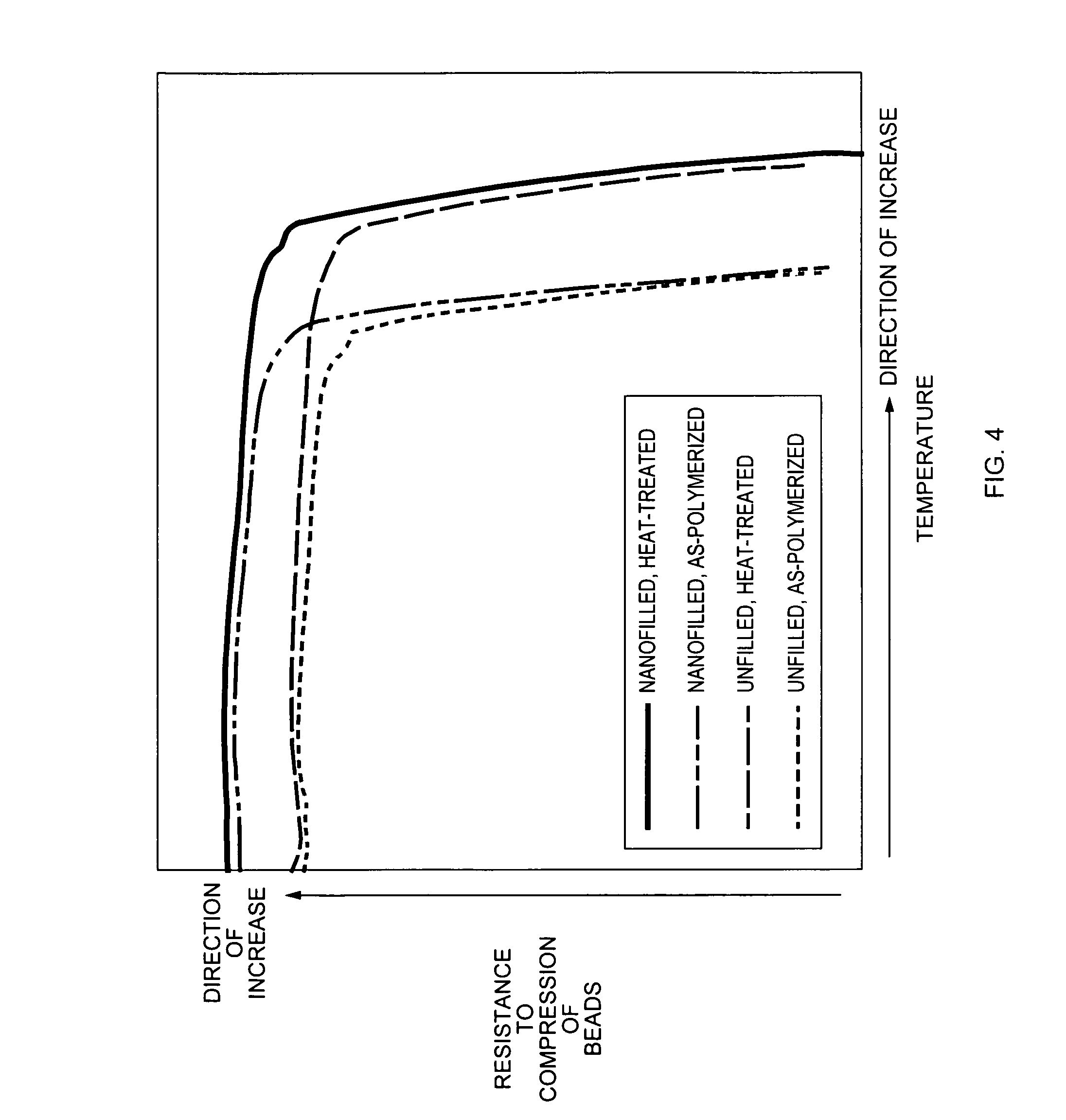
Thermoset nanocomposite particles, processing for their production, and their use in oil and natural gas drilling applications
ActiveUS20070066491A1Improve curingImprove heat transfer performanceMaterial nanotechnologySynthetic resin layered productsEnvironmental resistanceBall bearing
Thermoset polymer particles are used in many applications requiring lightweight particles possessing high stiffness, strength, temperature resistance, and / or resistance to aggressive environments. The present invention relates to the use of two different methods, either each by itself or in combination, to enhance the stiffness, strength, maximum possible use temperature, and environmental resistance of such particles. One method is the application of post-polymerization process steps (and especially heat treatment) to advance the curing reaction and to thus obtain a more densely crosslinked polymer network. In general, its main benefits are the enhancement of the maximum possible use temperature and the environmental resistance. The other method is the incorporation of nanofillers, resulting in a heterogeneous “nanocomposite” morphology. In general, its main benefits are increased stiffness and strength. Nanofiller incorporation and post-polymerization heat treatment can also be combined to obtain the benefits of both methods simultaneously. The present invention relates to the development of thermoset nanocomposite particles. It also relates to the optional further improvement of the heat resistance and environmental resistance of said particles via post-polymerization heat treatment. Furthermore, it also relates to processes for the manufacture of said particles. Finally, it also relates to the use of said particles in the construction, drilling, completion and / or fracture stimulation of oil and natural gas wells; for example, as a proppant partial monolayer, a proppant pack, an integral component of a gravel pack completion, a ball bearing, a solid lubricant, a drilling mud constituent, and / or a cement additive.
Owner:SUN DRILLING PRODS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com