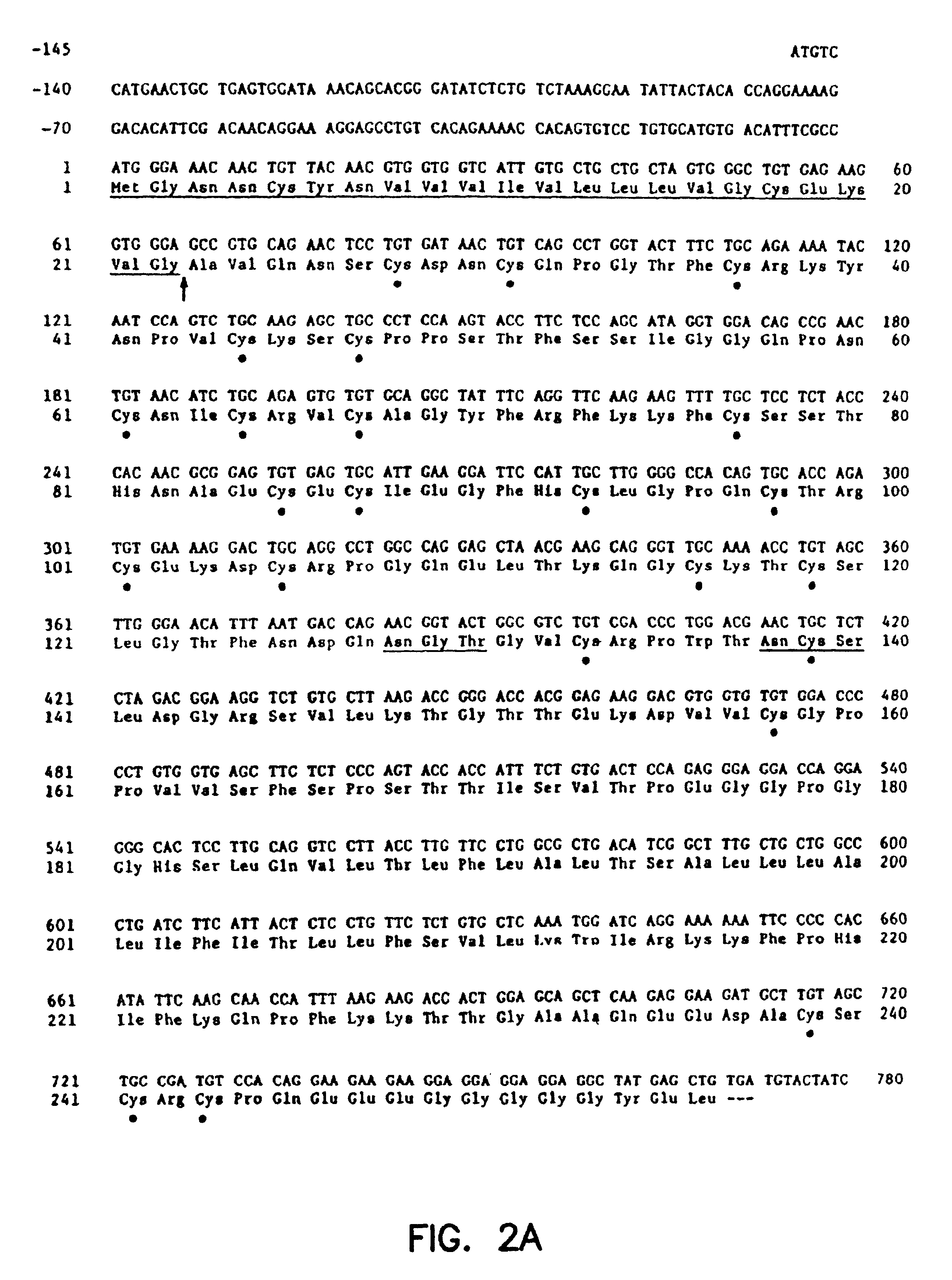
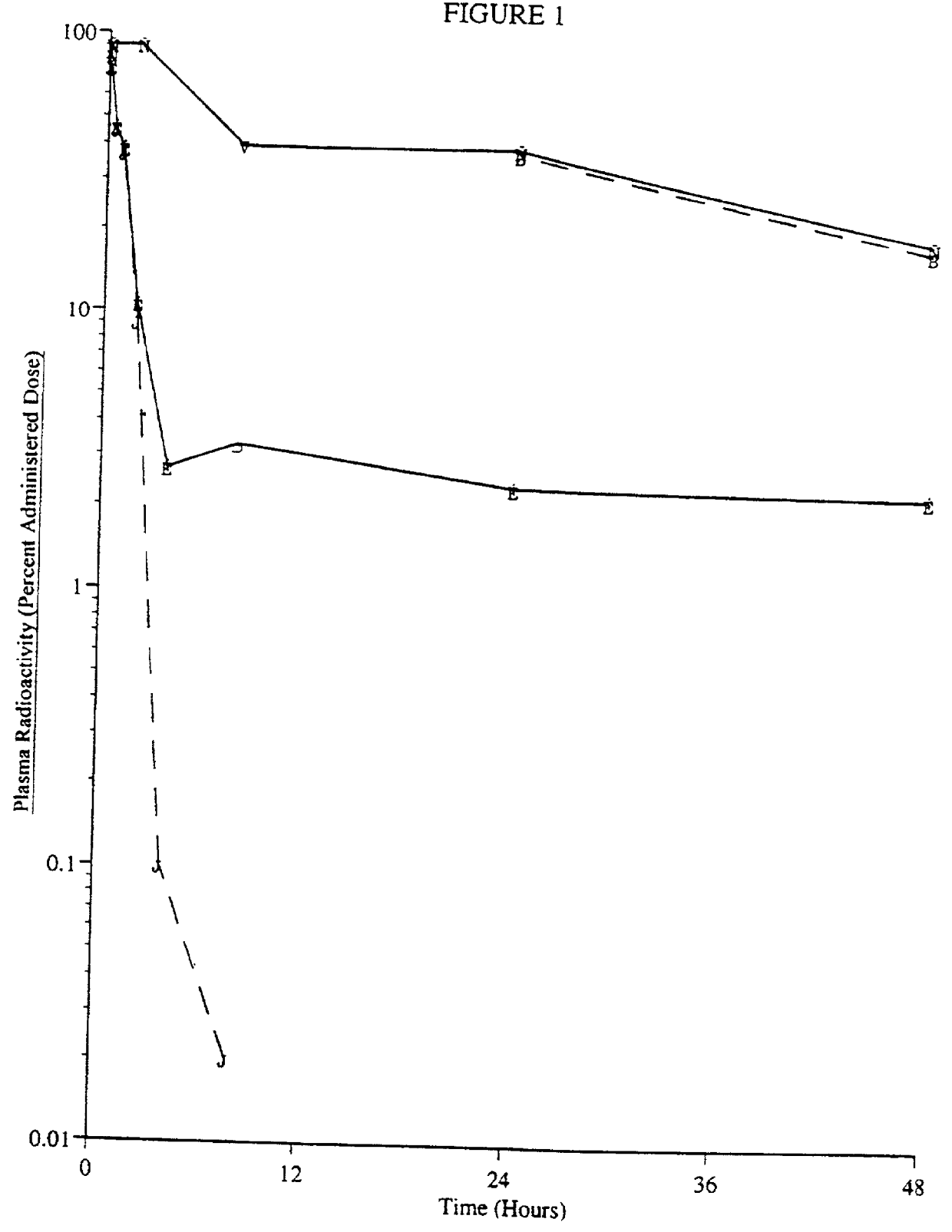
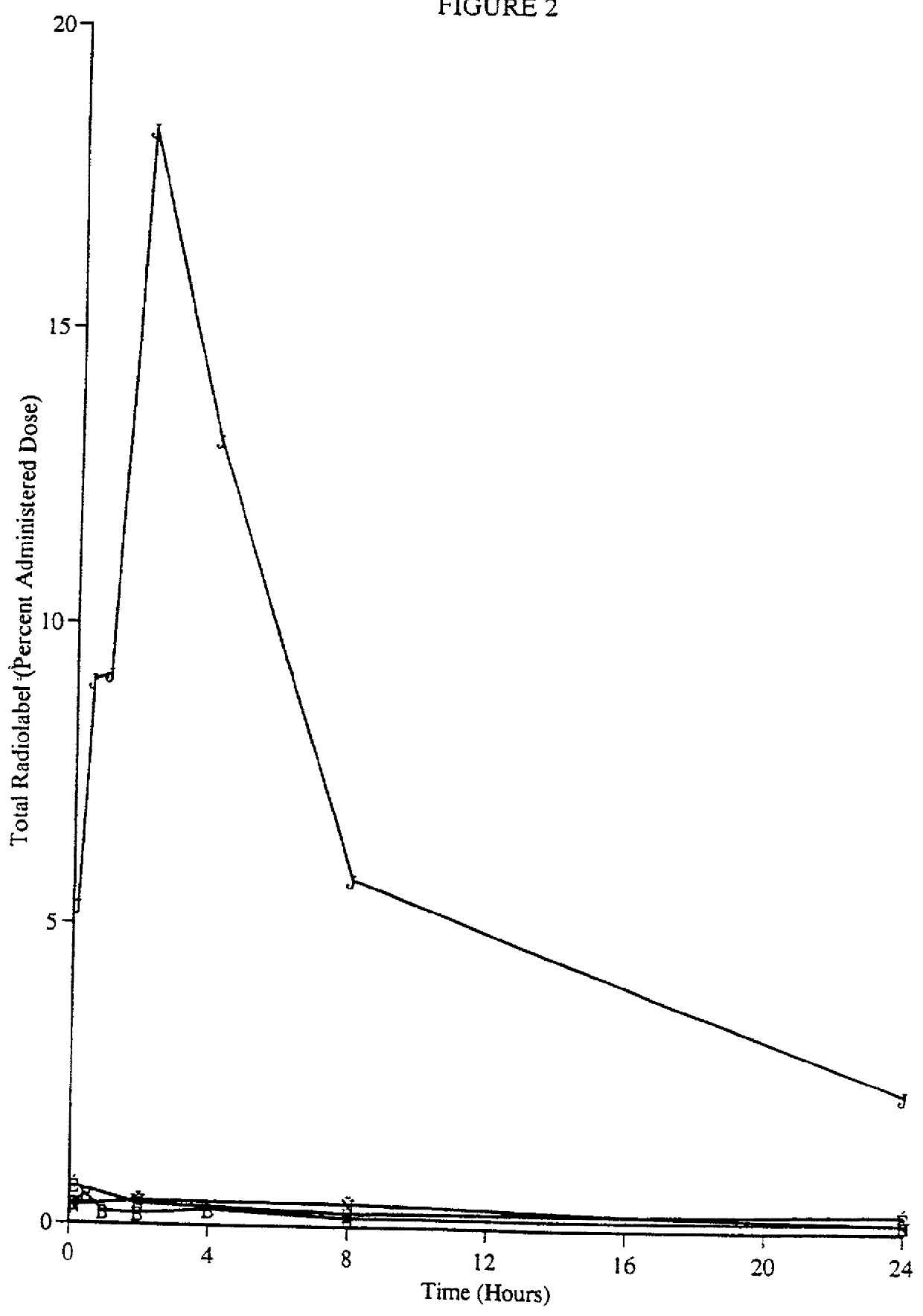
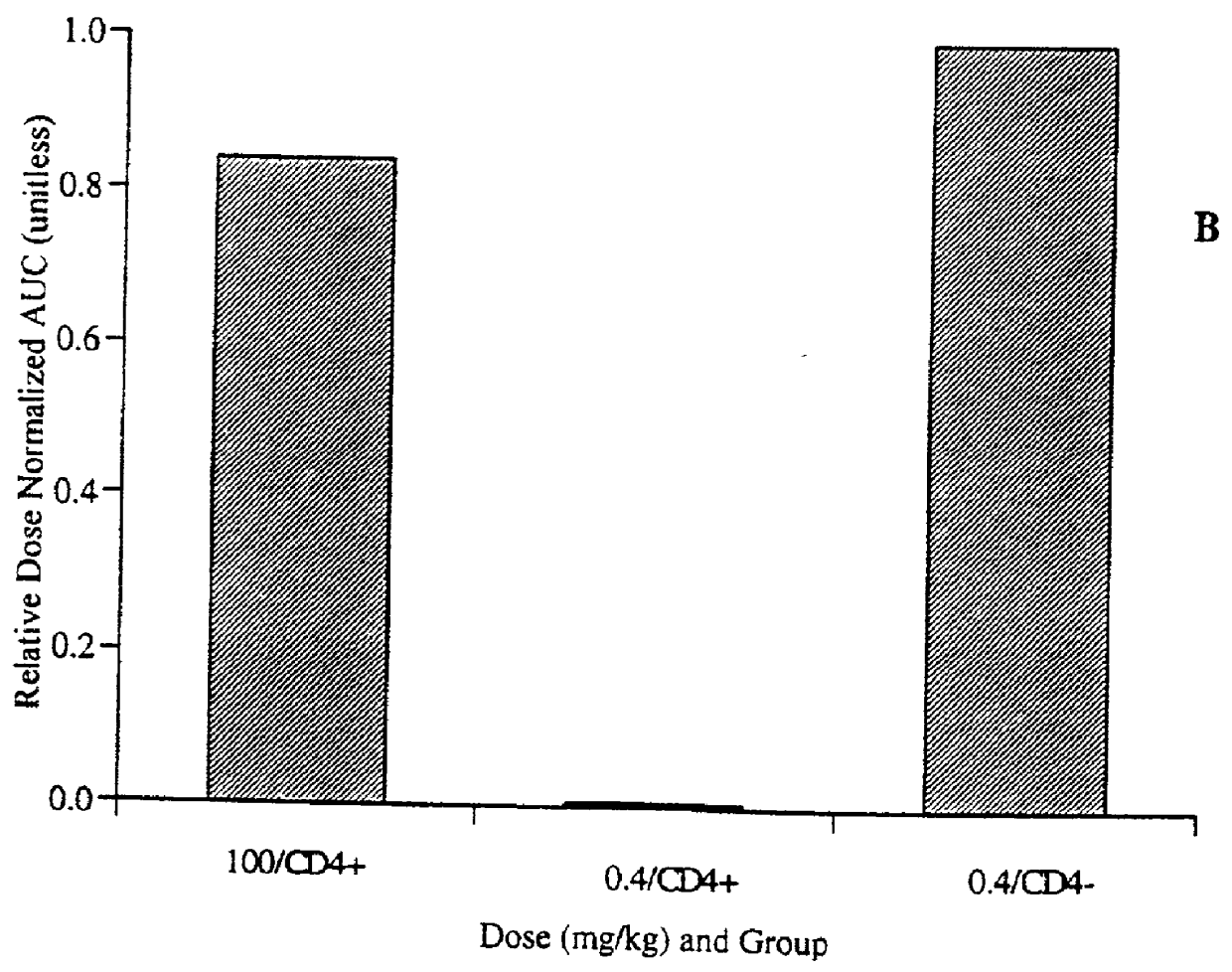
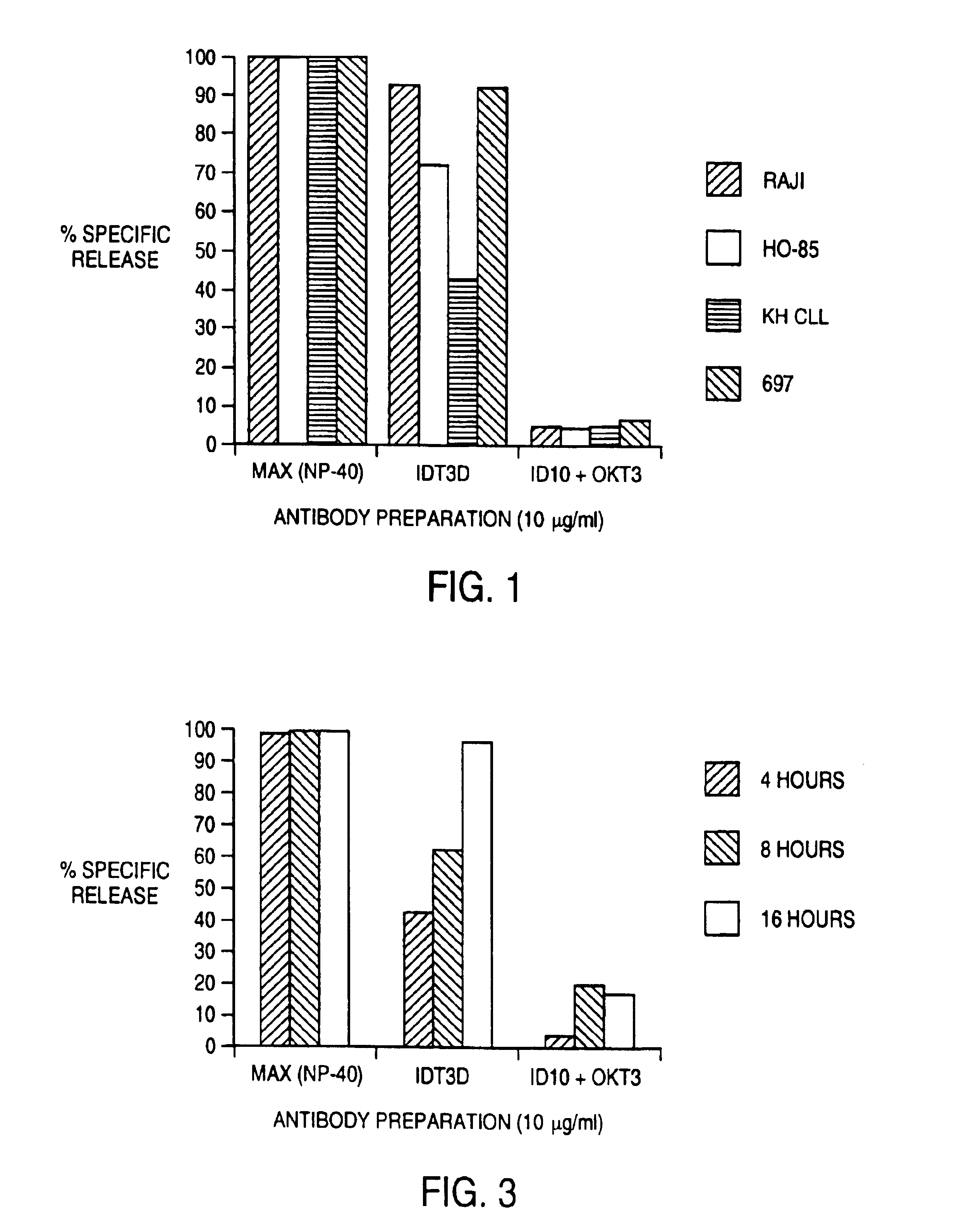
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1098 results about "B cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
B cells, also known as B lymphocytes, are a type of white blood cell of the small lymphocyte subtype. They function in the humoral immunity component of the adaptive immune system by secreting antibodies. Additionally, B cells present antigen (they are also classified as professional antigen-presenting cells (APCs)) and secrete cytokines. In mammals, B cells mature in the bone marrow, which is at the core of most bones. In birds, B cells mature in the bursa of Fabricius, a lymphoid organ. (The "B" from B cells comes from the name of this organ, where it was first discovered by Chang and Glick, and not from bone marrow as commonly believed).
CD19-specific chimeric T cell receptor
InactiveUS7446179B2Peptide/protein ingredientsAntibody mimetics/scaffoldsIntracellular signallingTransmembrane domain
The present invention relates to a genetically engineered, CD19-specific chimeric T cell receptor and to immune cells expressing the chimeric receptor The present invention also relates to the use of such cells for cellular immunotherapy of CD9+ malignancies and for abrogating any untoward B cell function. The chimeric receptor is a single chain scFvFc:ζ receptor where scFvFc designates the extracellular domain, scFv designates the VH and VL chains of a single chain monoclonal antibody to CD19, Fc represents at least part of a constant region of an IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. The extracellular domain scFvFc and the intracellular domain ζ are linked by a transmembrane domain such as the transmembrane domain of CD4. In one aspect, the chimeric receptor comprises amino acids 23-634 of SEQ I DNO:2. The present invention further relates to a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor.
Owner:CITY OF HOPE
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Owner:GLAXO SMITHKLINE LLC
Antibody for 4-1BB
The present invention includes the receptor protein 4-1BB and the cDNA gene encoding for receptor protein 4-1BB. The nucleotide sequence of the isolated cDNA is disclosed herein along with the deduced amino acid sequence. The 4-1BB protein and fragments and derivatives can be used: 1) as a probe to isolate ligands to receptor protein 4-1BB, 2) to stimulate proliferation of B-cell's expressing 4-1BB, or 3) to block 4-1BB ligand binding. A monoclonal antibody against 4-1BB was developed which specifically recognizes an epitope on the extracellular domain of receptor protein 4-1BB. The monoclonal antibody can be used enhance T-cell proliferation and activation by treating T-cells that have expressed receptor protein 4-1BB with the monoclonal antibody. The effectiveness of the treatment was enhanced when conducted in the presence of protein tyrosinase kinase. A fusion protein for detecting cell membrane ligands to receptor protein 4-1BB was developed. It comprises the extracellular portion of the receptor protein 4-1BB and a detection protein bound to the portion of the receptor protein 4-1BB.
Owner:INDIANA UNIV RES & TECH CORP
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Methods for the treatment of lymphoma by administration of a B cell-specific antibody are described. The invention encompasses providing to a patient both unlabeled antibodies and antibodies labeled with a radioisotope. A principal advantage of the method is that tumor responses can be obtained in a radiometric dose range that does not require hematopoietic stem cell replacement as an adjunct therapy also described is a composition useful in the treatment of lymphoma.
Owner:RGT UNIV OF MICHIGAN +2
Anti-cd3 antibodies, bispecific antigen-binding molecules that bind cd3 and cd20, and uses thereof
ActiveUS20140088295A1Useful in treatmentFacilitates directed killing (cell lysis) of the targetedImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD20Disease
The present invention provides antibodies that bind to CD3 and methods of using the same. According to certain embodiments, the antibodies of the invention bind human CD3 with high affinity and induce human T cell proliferation. The invention includes antibodies that bind CD3 and induce T cell-mediated killing of tumor cells. According to certain embodiments, the present invention provides bispecific antigen-binding molecules comprising a first antigen-binding domain that specifically binds human CD3, and a second antigen-binding molecule that specifically binds human CD20. In certain embodiments, the bispecific antigen-binding molecules of the present invention are capable of inhibiting the growth of B-cell tumors expressing CD20. The antibodies and bispecific antigen-binding molecules of the invention are useful for the treatment of diseases and disorders in which an upregulated or induced targeted immune response is desired and / or therapeutically beneficial. For example, the antibodies of the invention are useful for the treatment of various cancers as well as other CD20-related diseases and disorders.
Owner:REGENERON PHARM INC
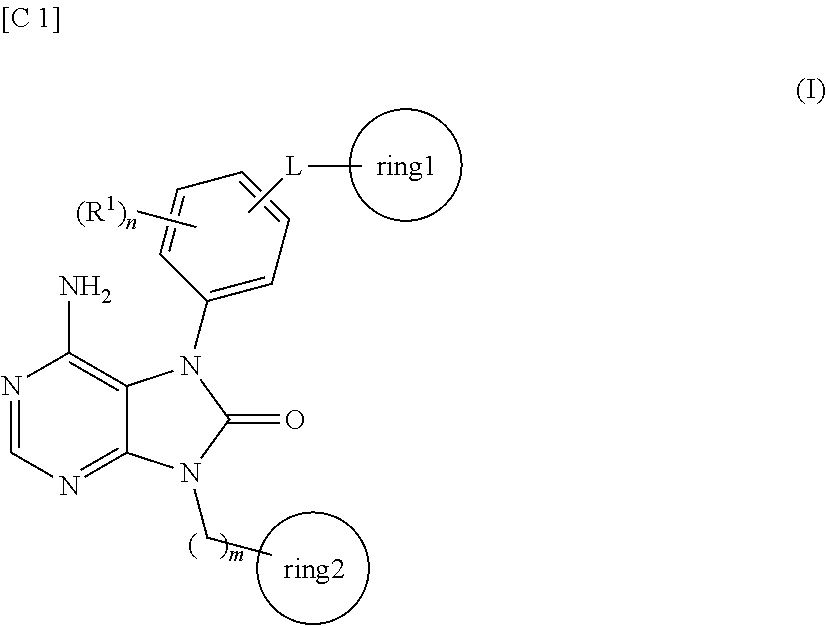
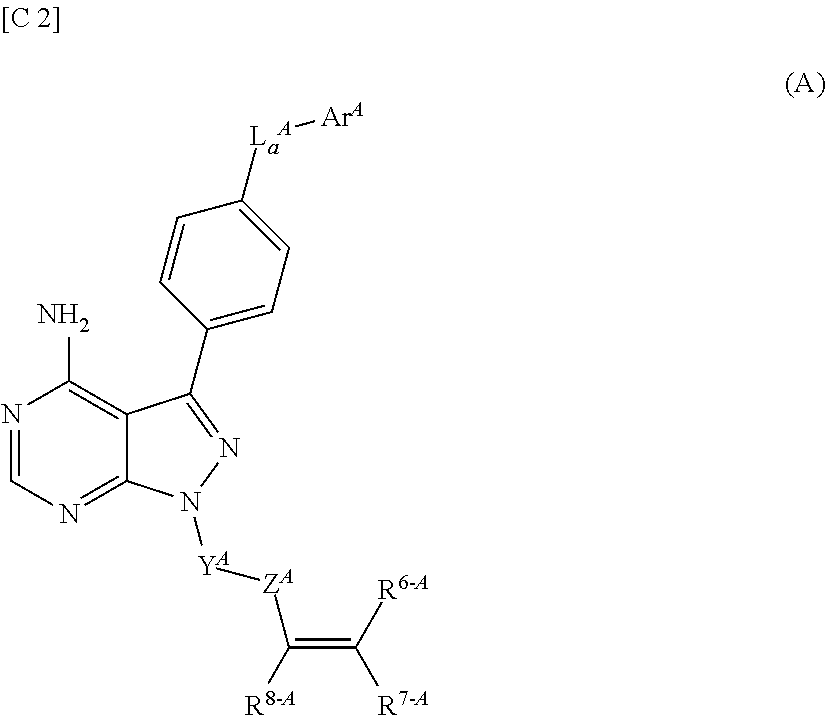
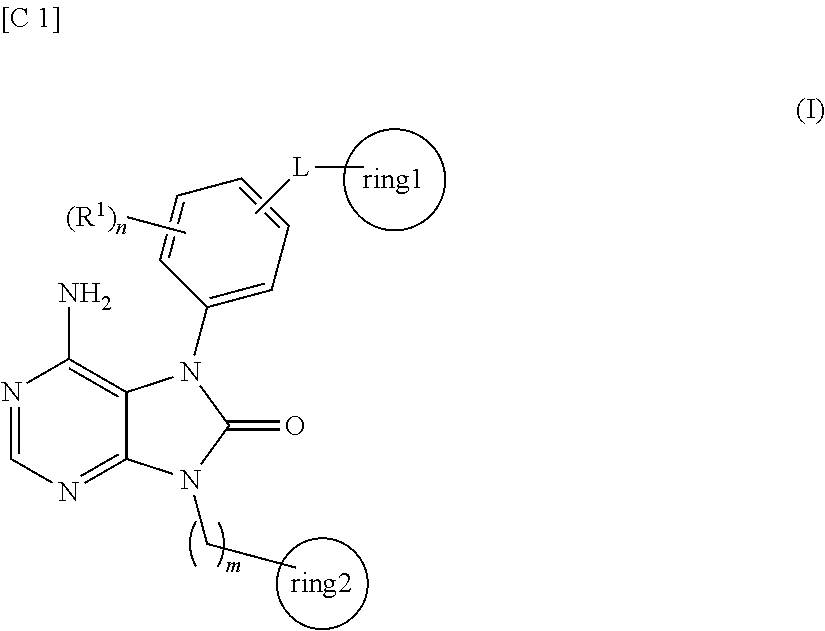
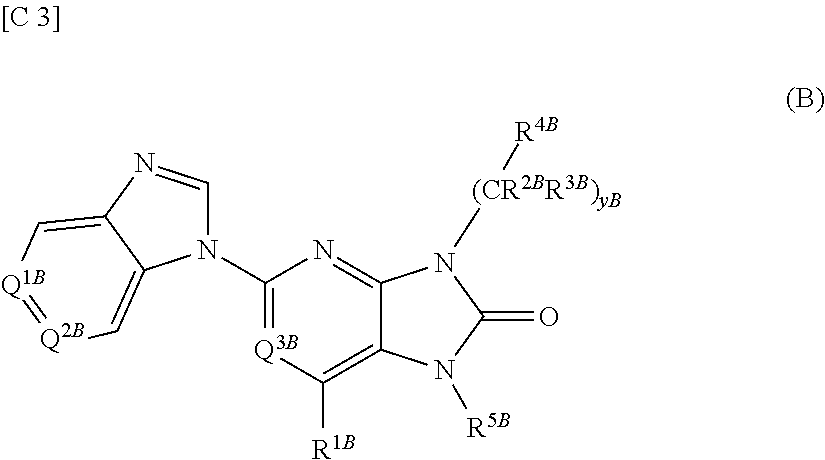
Purinone derivative
ActiveUS20130079327A1Good metabolic stabilityAvoid liver toxicityBiocideOrganic chemistryMast cellMetabolic stability
Compounds represented by general formula (I) (all of the symbols in the formula conform to the definitions in the Description) are compounds that, in addition to having a Btk-selective inhibitory activity, exhibit an excellent metabolic stability and can avoid hepatotoxicity or the like, and as a consequence can provide safe therapeutic agents for diseases in which B cells or mast cells participate.
Owner:ONO PHARMA CO LTD
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS20020012665A1Avoiding and decreasing and resistanceOrganic active ingredientsIn-vivo radioactive preparationsFactor iiBiological activation
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies. The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN(R).
Owner:BIOGEN INC
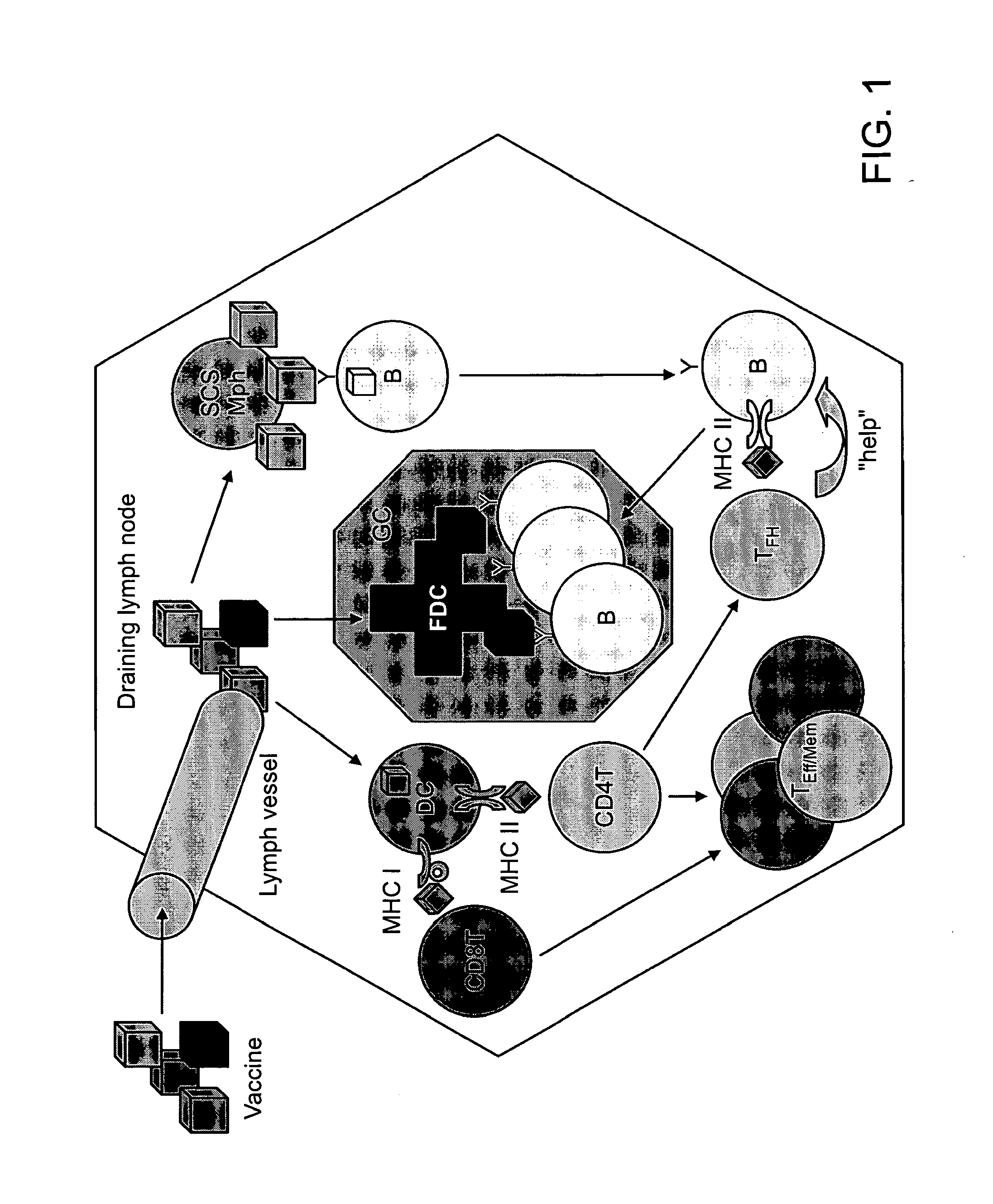
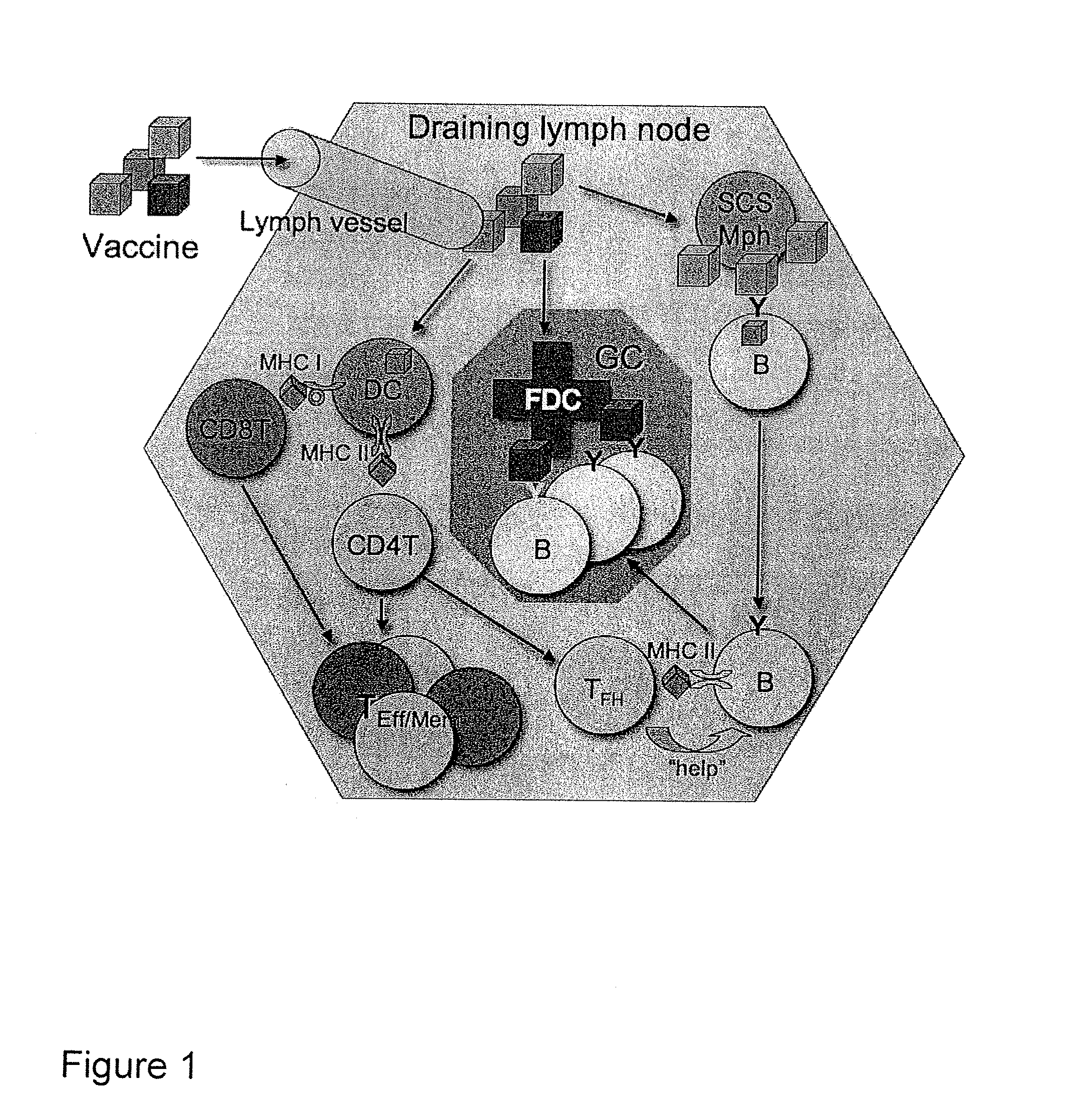
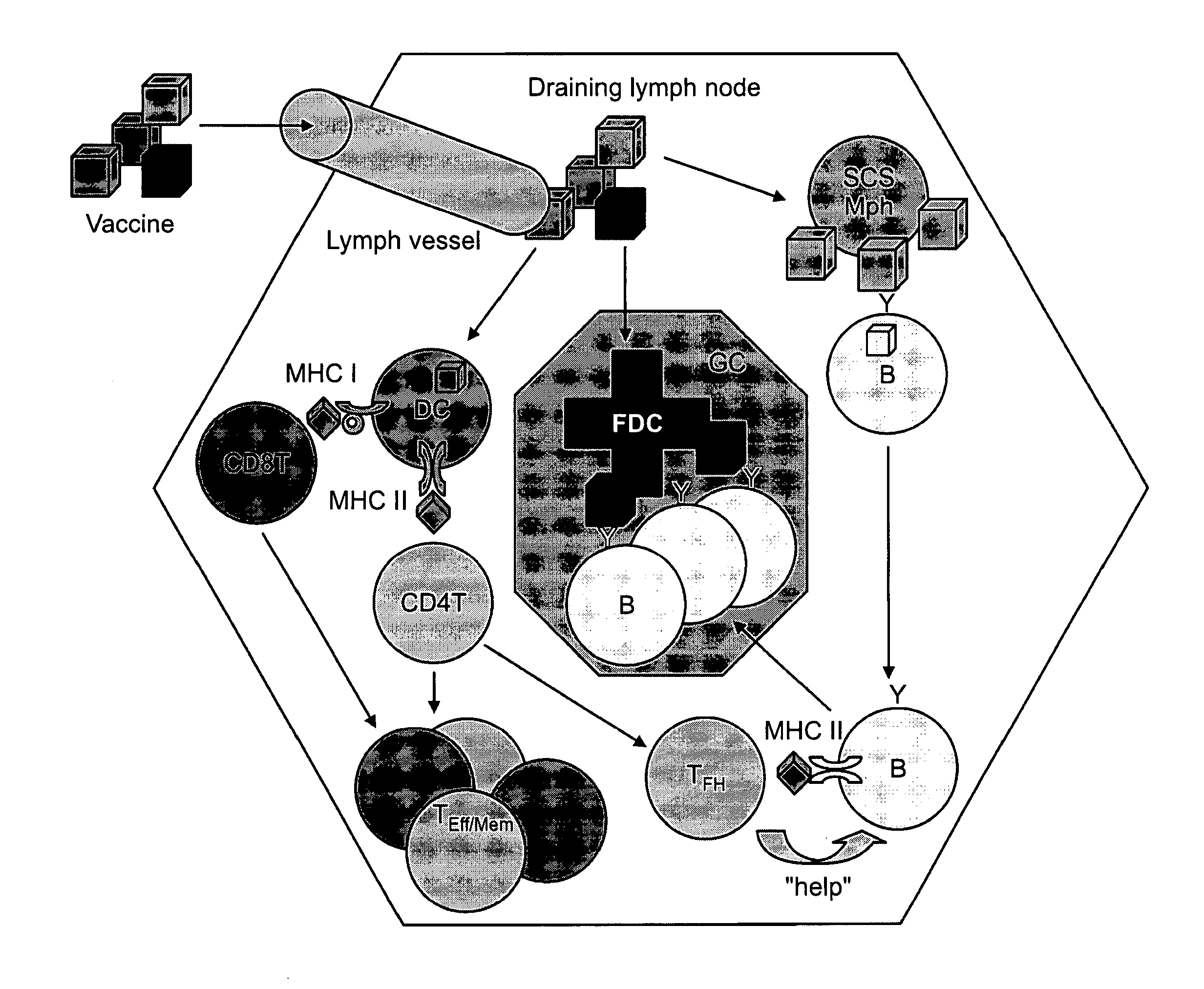
Vaccine Nanotechnology
ActiveUS20100233251A1Modulating immune systemEnhance and suppress and direct and immune responseNervous disorderAntipyreticDiseaseNanocarriers
Owner:MASSACHUSETTS INST OF TECH +4
Variant IgG3 Rituxan and therapeutic use thereof
InactiveUS20020128448A1Prevent and reduce proliferation of cellReduce and prevent proliferationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCD20Antigen binding
Monoclonal anti-human CD20 antigen binding antibodies containing human IgG3 constant domains are provided. These antibodies possess effector functions that render them well suited for use in therapeutic methods, especially treatments wherein inhibition of B cell function or B cell number is therapeutically desirable.
Owner:BIOGEN INC
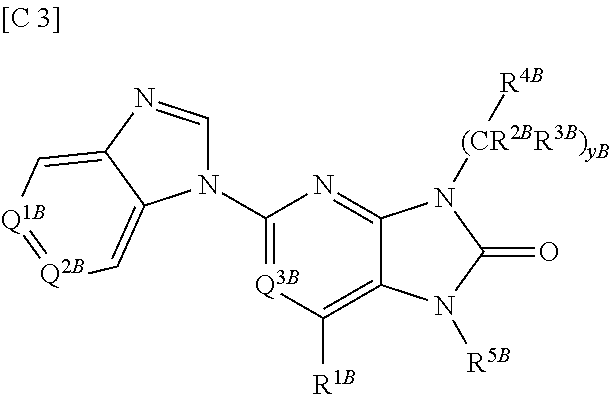
Purinone derivative
ActiveUS20130217880A1Good metabolic stabilityAvoid liver toxicityOrganic active ingredientsOrganic chemistryDiseaseMast cell
Compounds represented by general formula (I) (all of the symbols in the formula conform to the definitions in the Description) are compounds that, in addition to having a Btk-selective inhibitory activity, exhibit an excellent metabolic stability and can avoid hepatotoxicity or the like, and as a consequence can provide safe therapeutic agents for diseases in which B cells or mast cells participate.
Owner:ONO PHARMA CO LTD
Method for preparing monoclonal antibody
ActiveUS20060059575A1Improve productivityImprove expression efficiencyNucleic acid vectorImmunoglobulinsImmunoglobulin heavy chainMonoclonal antibody
A significantly increased amount of a monoclonal antibody is obtained from the culture medium of recombinant hybridoma prepared by introducing genes encoding a protein identical to the immunoglobulin heavy chain polypeptide of the specific monoclonal antibody into an immortalized B cell (hybridoma) producing the monoclonal antibody.
Owner:JAPAN TOBACCO INC +1
Targeting of Antigen Presenting Cells with Immunonanotherapeutics
ActiveUS20100129392A1Improve responsePowder deliverySnake antigen ingredientsNanocarriersImmunotherapeutic agent
The present invention provides compositions and systems for delivery of nanocarriers to cells of the immune system. The invention provides nanocarriers capable of stimulating an immune response in T cells and / or in B cells. The invention provides nanocarriers that comprise an immunofeature surface. The nanocarriers are capable of targeting antigen presenting cells when administered to a subject. The invention provides pharmaceutical compositions comprising inventive nanocarriers. The present invention provides methods of designing, manufacturing, and using inventive nanocarriers and pharmaceutical compositions thereof.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +2
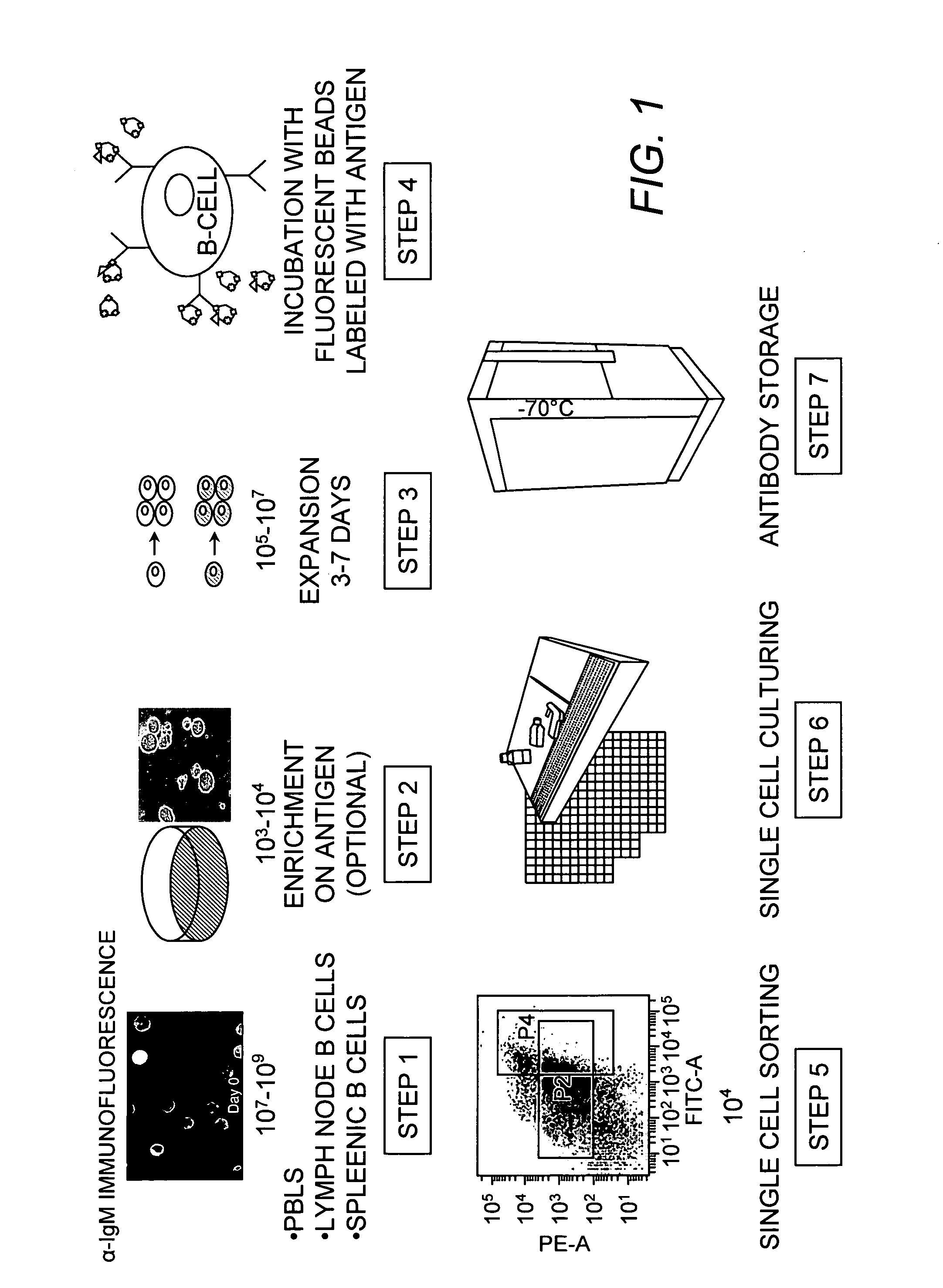
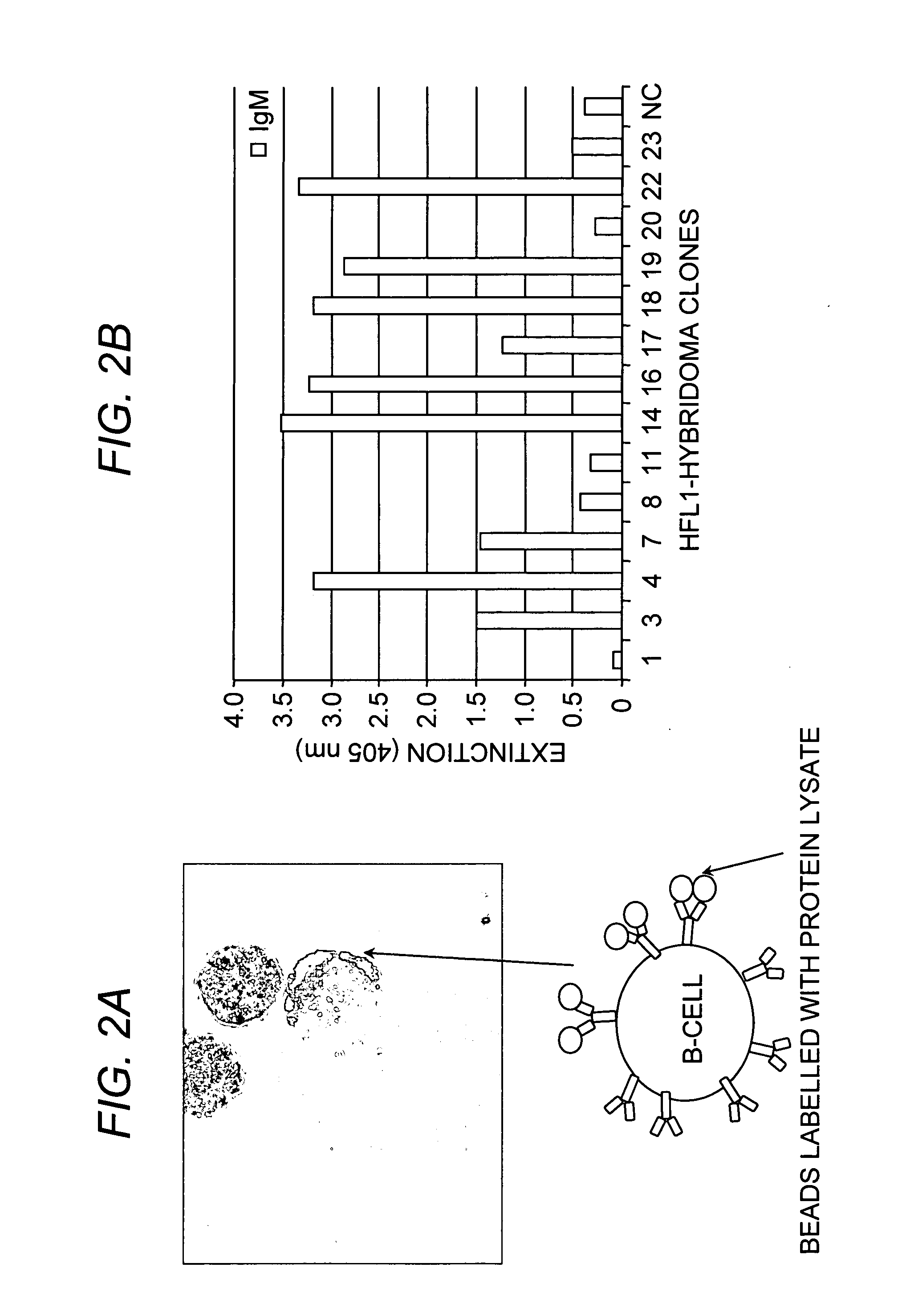
Method of producing a plurality of isolated antibodies to a plurality of cognate antigens
The present invention relates to a method for producing high affinity antibodies that are antigen-specific. The method involves binding a plurality of antibody-producing B-cells from a mammal to a plurality of cognate antigens; sorting the bound antibody-producing B-cell and cognate antigen; amplifying nucleic acid sequences encoding each antibody, or fragment thereof, from the B-cells; and expressing the each antibody in a protein expression system. Antibodies produced in this manner are useful in diagnostic and therapeutic applications.
Owner:MONTECITO BIO SCI
Vaccine Nanotechnology
ActiveUS20130236533A1Facilitate acquisitionModulating the immune systemNervous disorderAntipyreticDiseaseNanocarriers
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +3
Antagonist Anti-cd40 monoclonal antibodies and methods for their use
ActiveUS20080254026A1Improve effectivenessPositive therapeutic responseAntipyreticAnalgesicsAntigenDisease
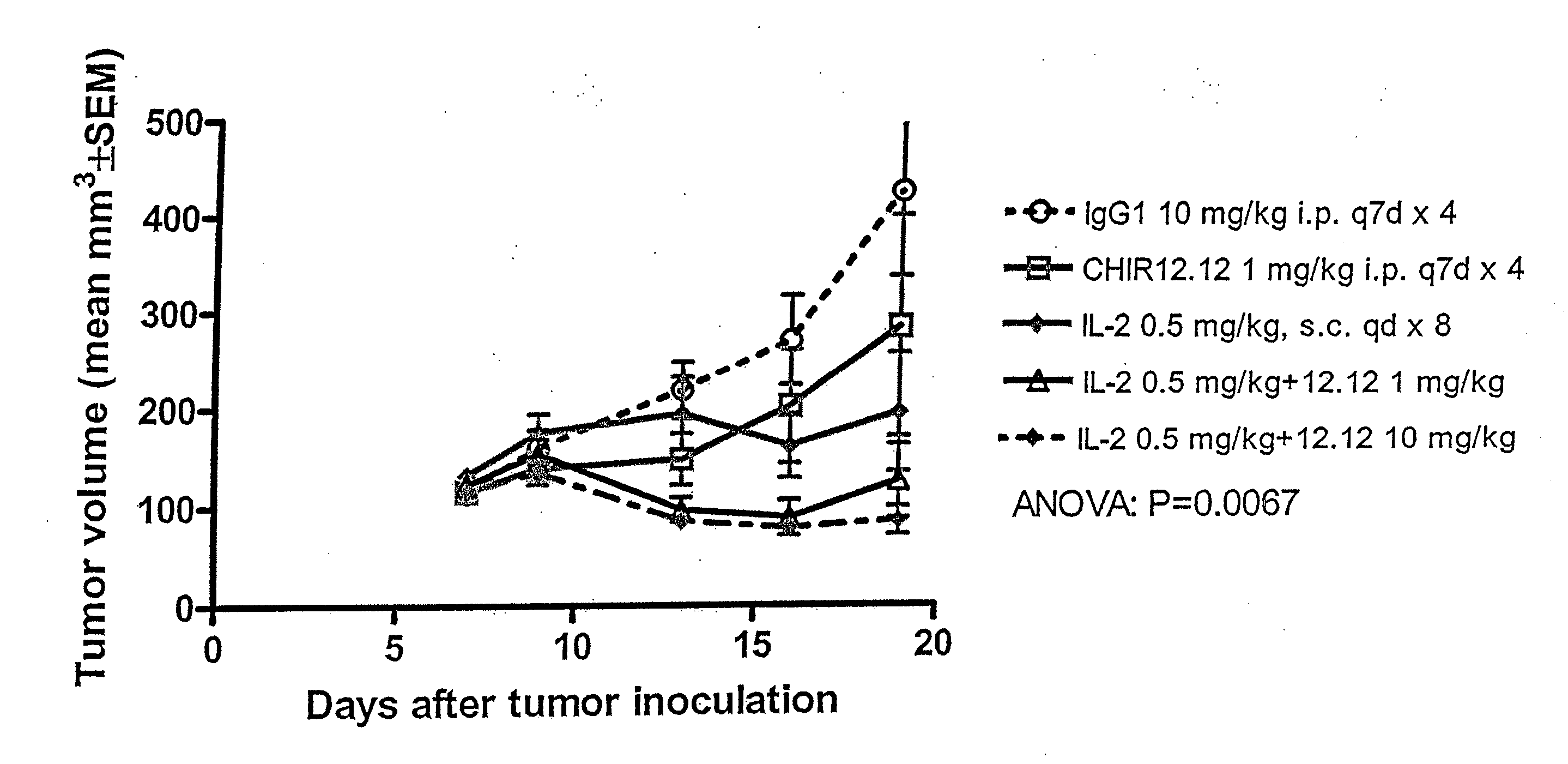
Compositions and methods of therapy for treating diseases mediated by stimulation of CD40 signaling on CD40-expressing cells are provided. The methods comprise administering a therapeutically effective amount of an antagonist anti-CD40 antibody or antigen-binding fragment thereof to a patient in need thereof. The antagonist anti-CD40 antibody or antigen-binding fragment thereof is free of significant agonist activity, but exhibits antagonist activity when the antibody binds a CD40 antigen on a human CD40-expressing cell. Antagonist activity of the anti-CD40 antibody or antigen-binding fragment thereof beneficially inhibits proliferation and / or differentiation of human CD40-expressing cells, such as B cells.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
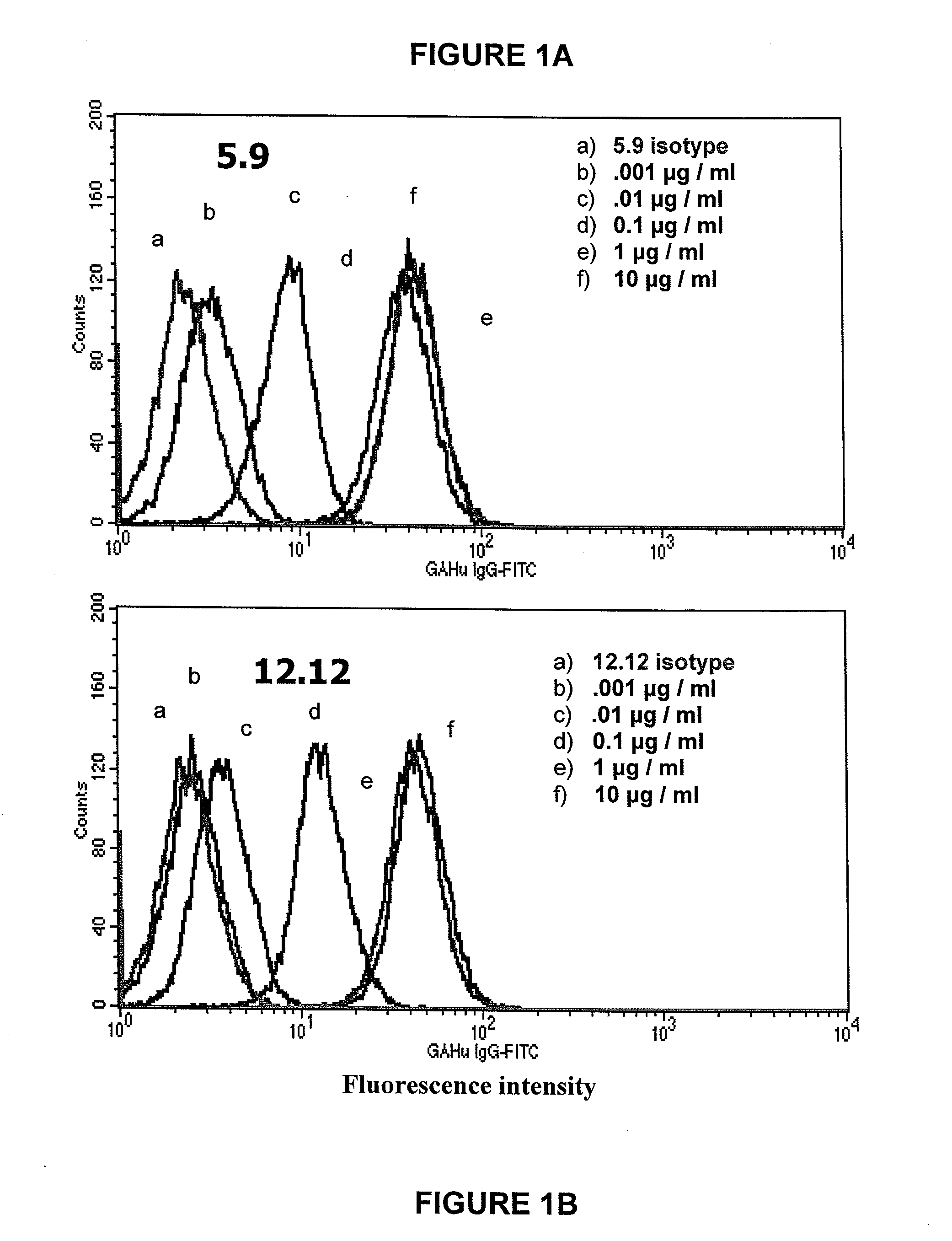
Monoclonal antibodies that suppress B cell growth and/or differentiation
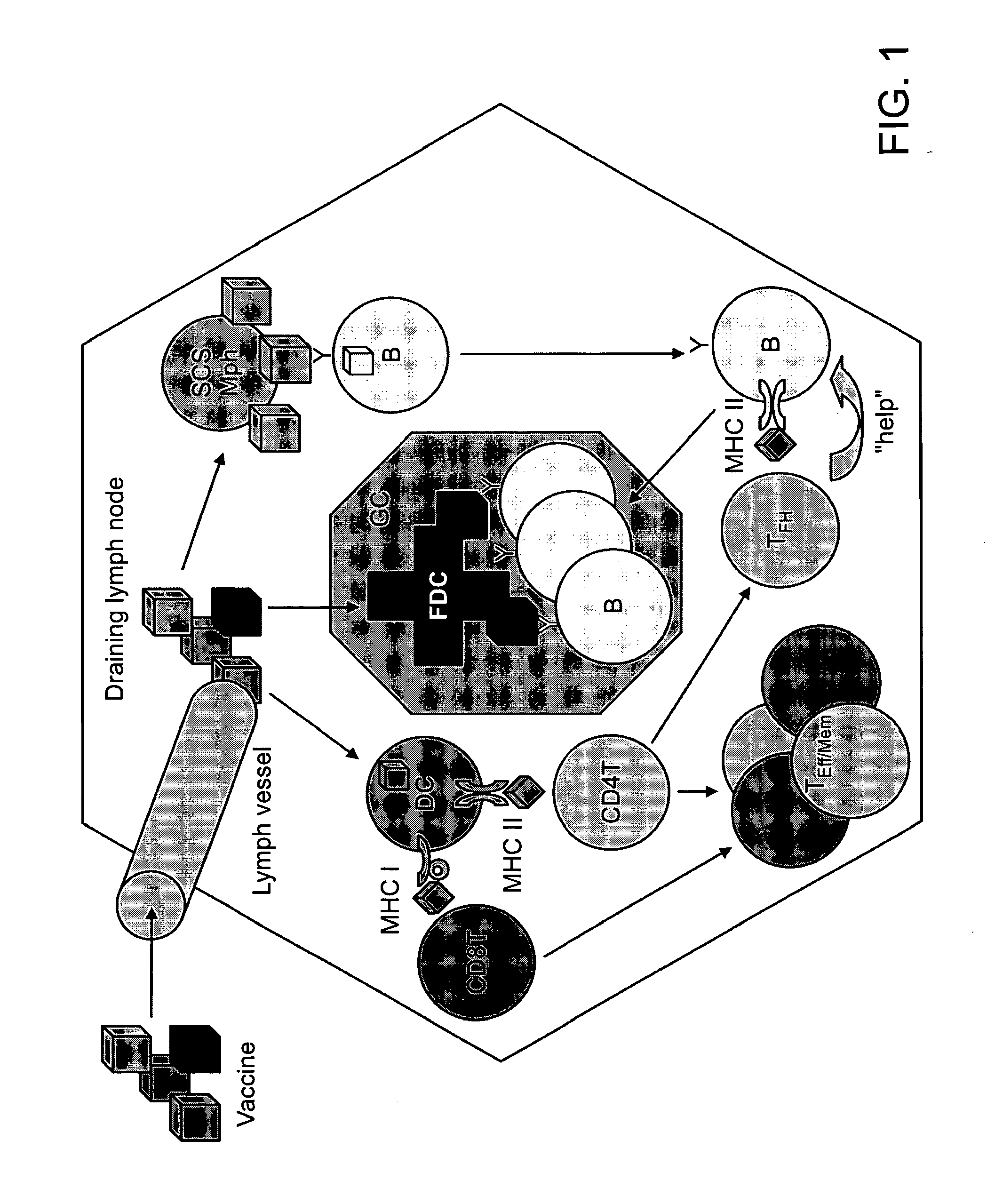
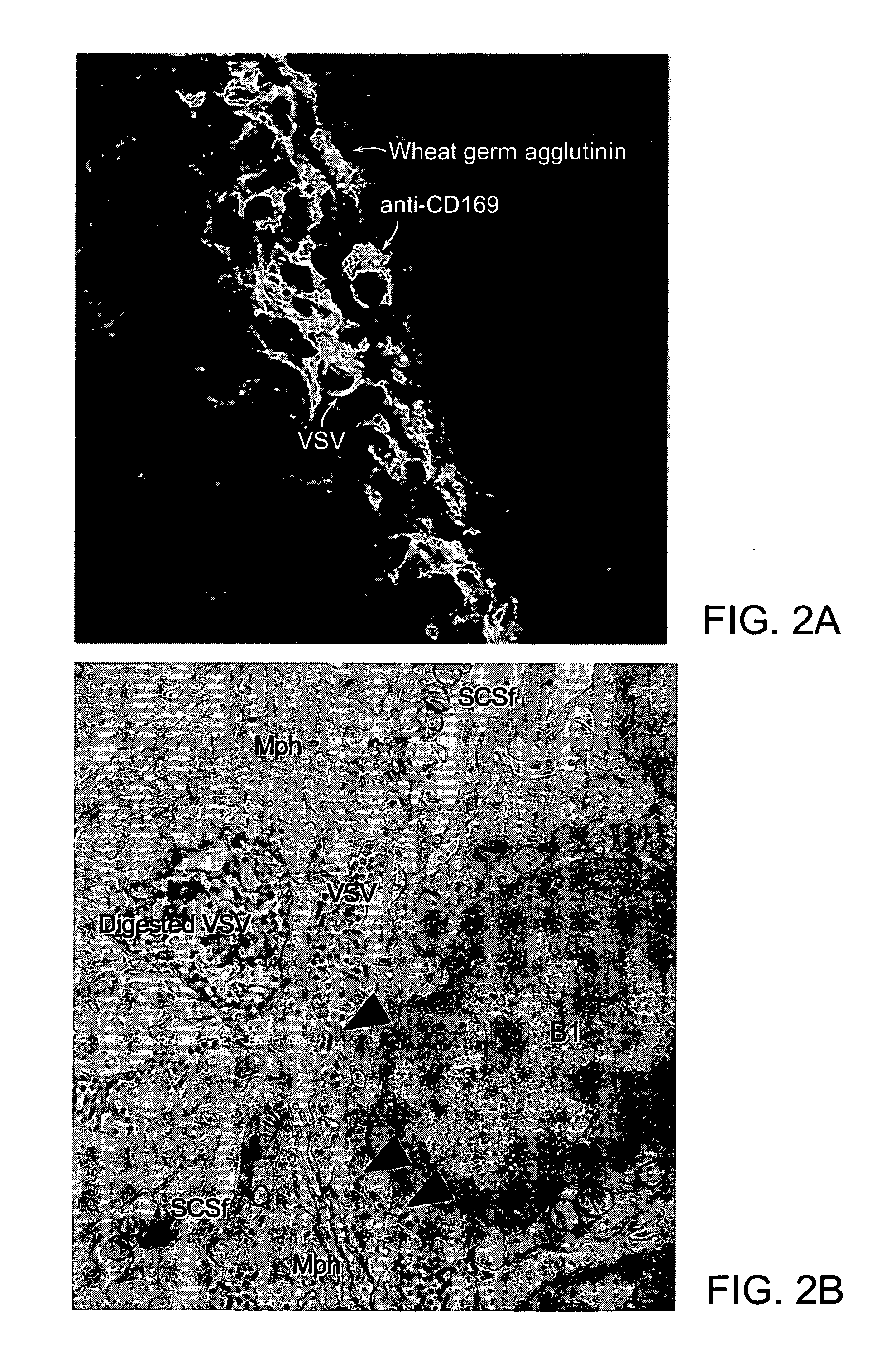
The present invention provides monoclonal antibodies which interfere with the interactions between FDCs and B cells, thereby suppressing the proliferation and / or differentiation of B cells in lymphoid follicles. The monoclonal antibodies of the present invention are useful for treating follicular lymphomas, multiple myeloma as well as autoimmune diseases.
Owner:OCHSNER CLINIC FOUND
Method of treating immune cell mediated systemic diseases
InactiveUS20010056066A1Prolonged systemic exposureEnhance and create propertyPeptide/protein ingredientsAntipyreticAntigenWhole body
An improved method of treating immune cell mediated systemic diseases, particularly T and B cell mediated diseases, is provided by increasing the systemic exposure, or bioavailibility, of a therapeutic protein. Such therapeutic protein is selected from the group consisting of a monoclonal antibody, a soluble receptor and a soluble ligand which binds to an antigen expressed on the surface of an immunce cell.
Owner:SMITHKLINE BECKMAN CORP
Humanized antibodies against CD3
Owner:ABBOTT BIOTHERAPEUTICS CORP
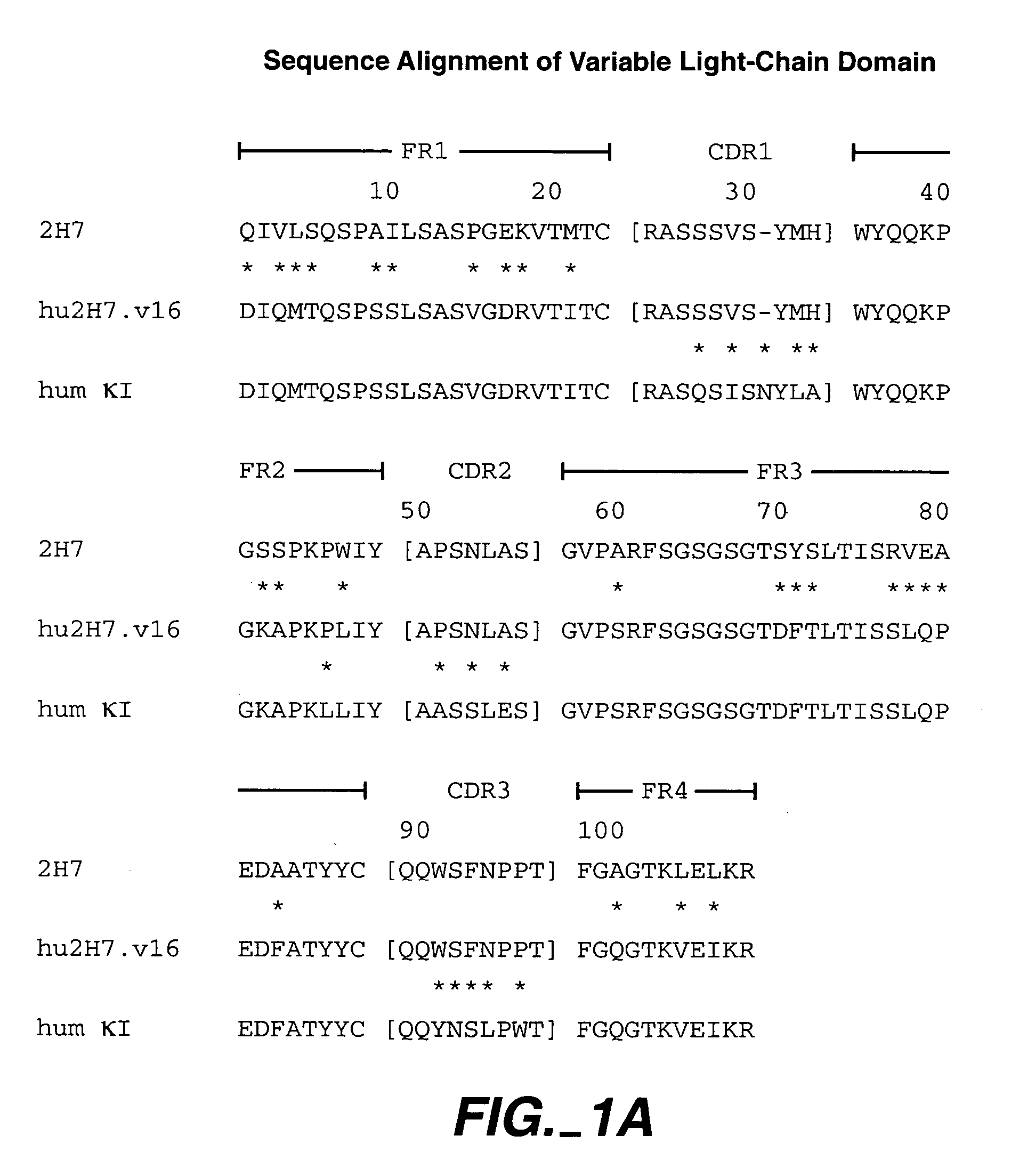
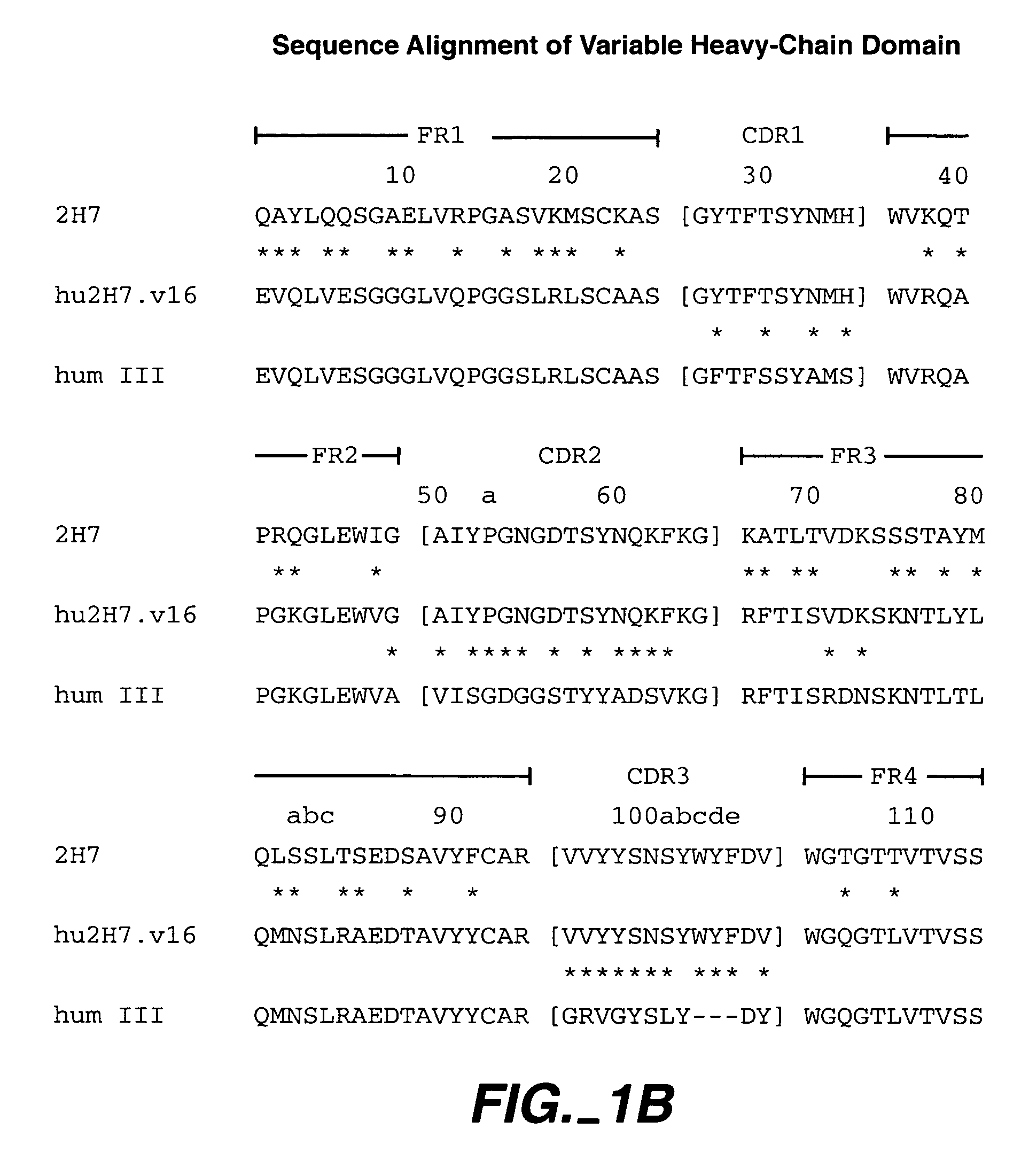
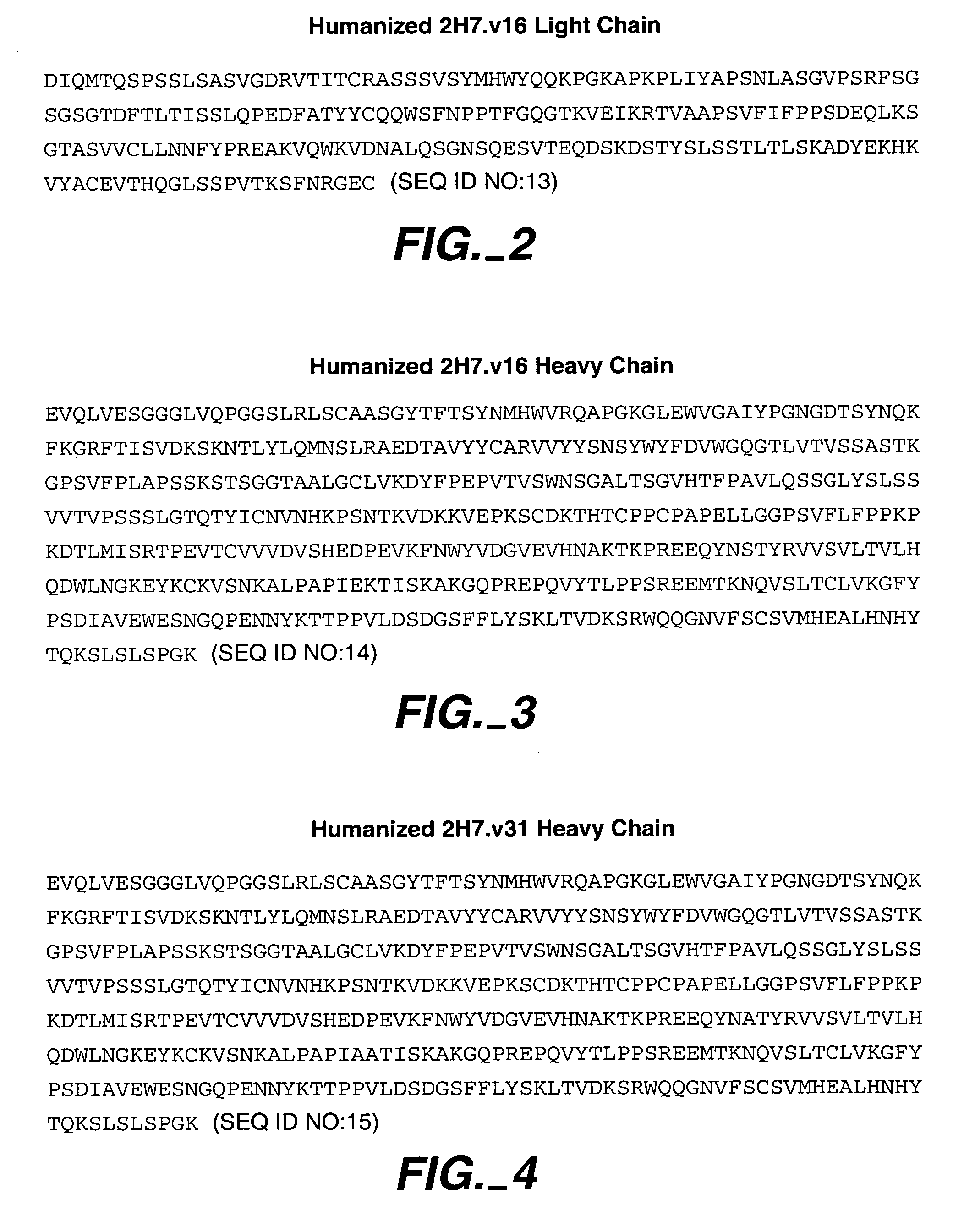
Immunoconjugates and humanized antibodies specific for B-cell lymphoma and leukemia cells
InactiveUS20050106108A1Lowered HAMA reactionReduced responsePeptide/protein ingredientsAntibody mimetics/scaffoldsHuman antimouse AntibodyComplementarity determining region
A chimeric LL2 monoclonal antibody is described in which the complementarity determining regions (CDRs) of the light and heavy chains of the murine LL2 anti-B-lymphoma, anti-leukemia cell monoclona lantibody has been recombinantly joined to the human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-cell lymphoma and leukemia cell internalization capacity of the parental murine LL2 monoclonal antibody, and which has the potential of exhibiting reduced human anti-mouse antibody production activity. A humanized LL2 monoclonal antibody is described in which the CDRs of the light and heavy chains have been recombinantly joined to a framework sequence of human light and heavy chains variable regions, respectively, and subsequently linked to human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-lymphoma and leukemia cell internalization capacities of the parental murine and chimeric LL2 monoclonal antibodies, and which has the potential for exhibiting reduced human anti-mouse antibody production activity. Vectors for producing recombinant chimeric and humanized chimeric monoclonal antibodies are provided. Isolated DNAs encoding the amino acid sequences of the LL2 variable light and heavy chain and CDR framework regions are described. Conjugates of chimeric and humanized chimeric LL2 antibodies with cytotoxic agents or labels find use in therapy and diagnosis of B-cell lymphomas and leukemias.
Owner:IMMUNOMEDICS INC
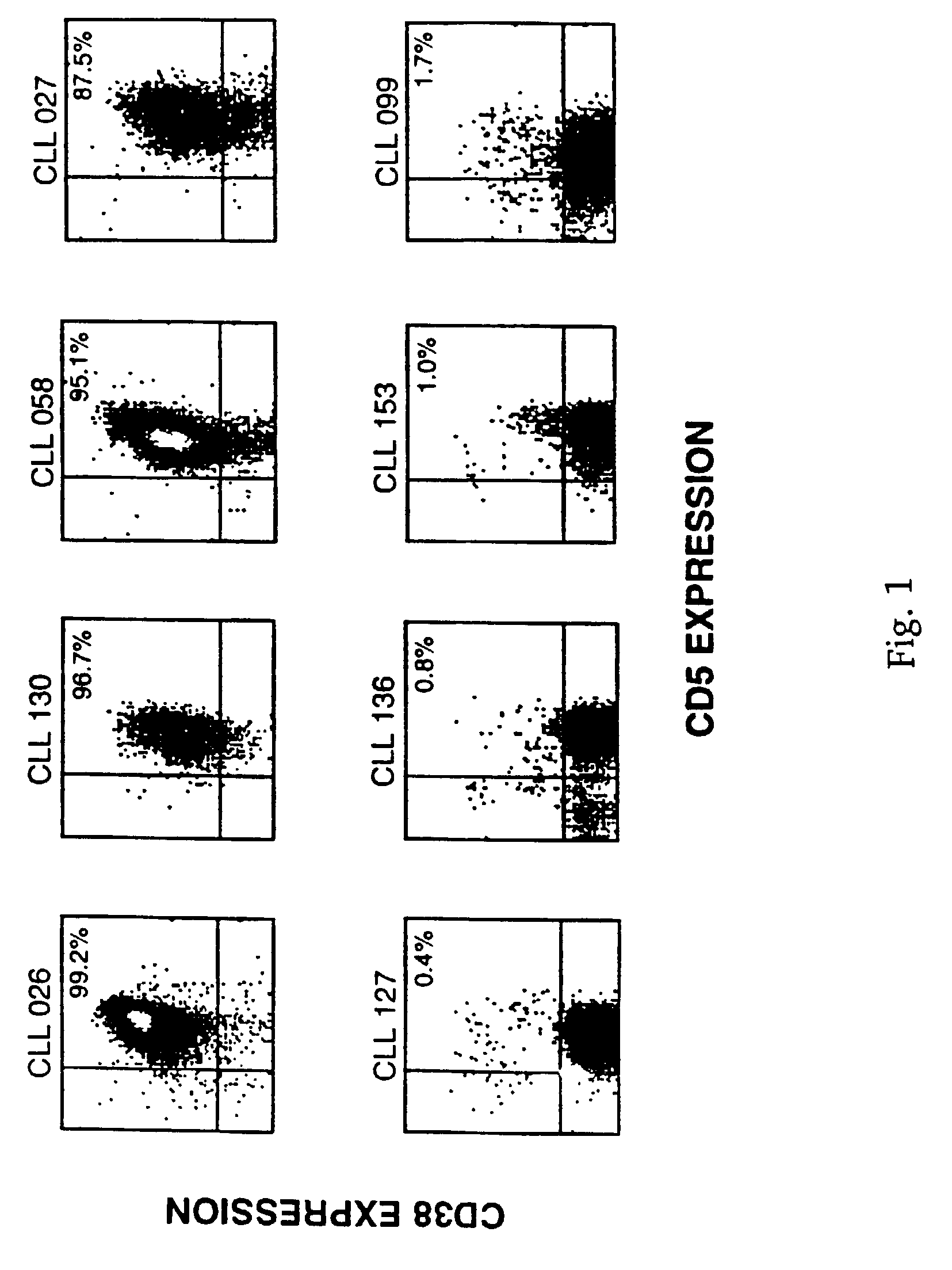
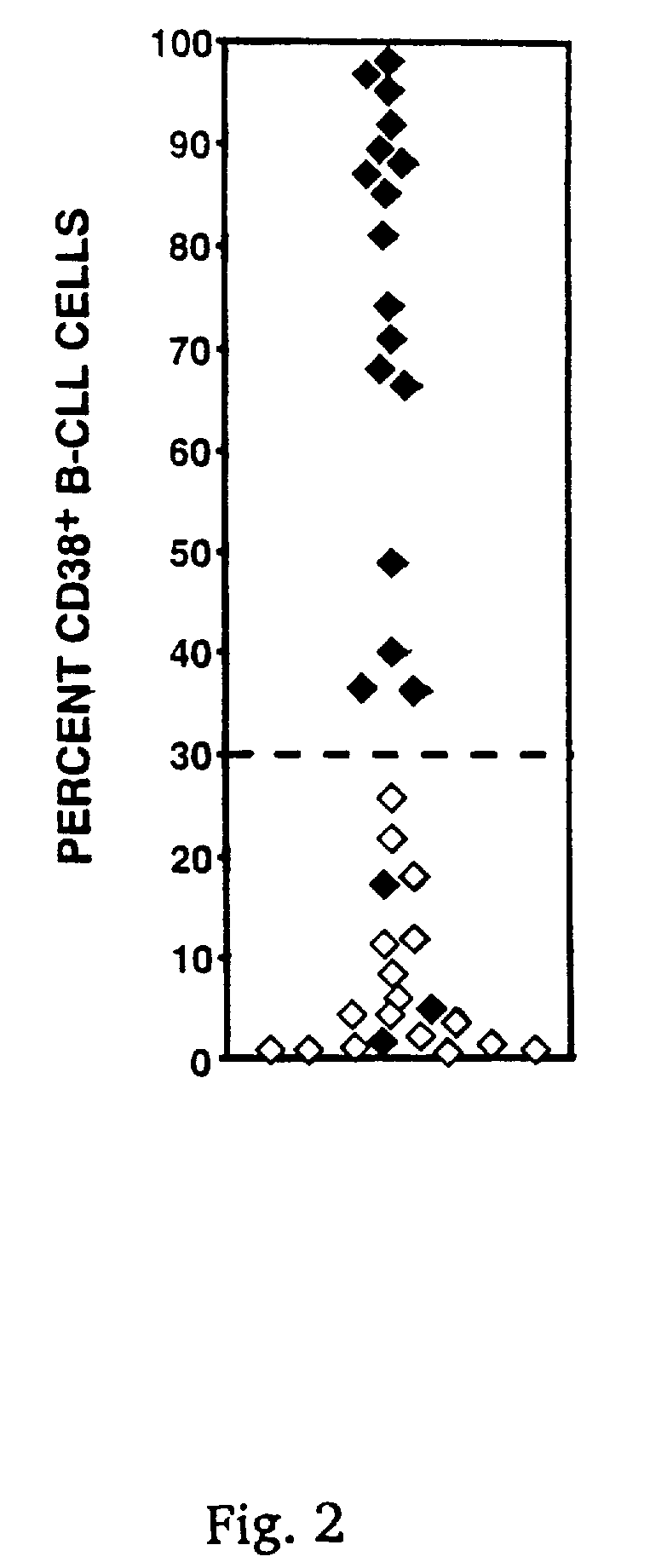
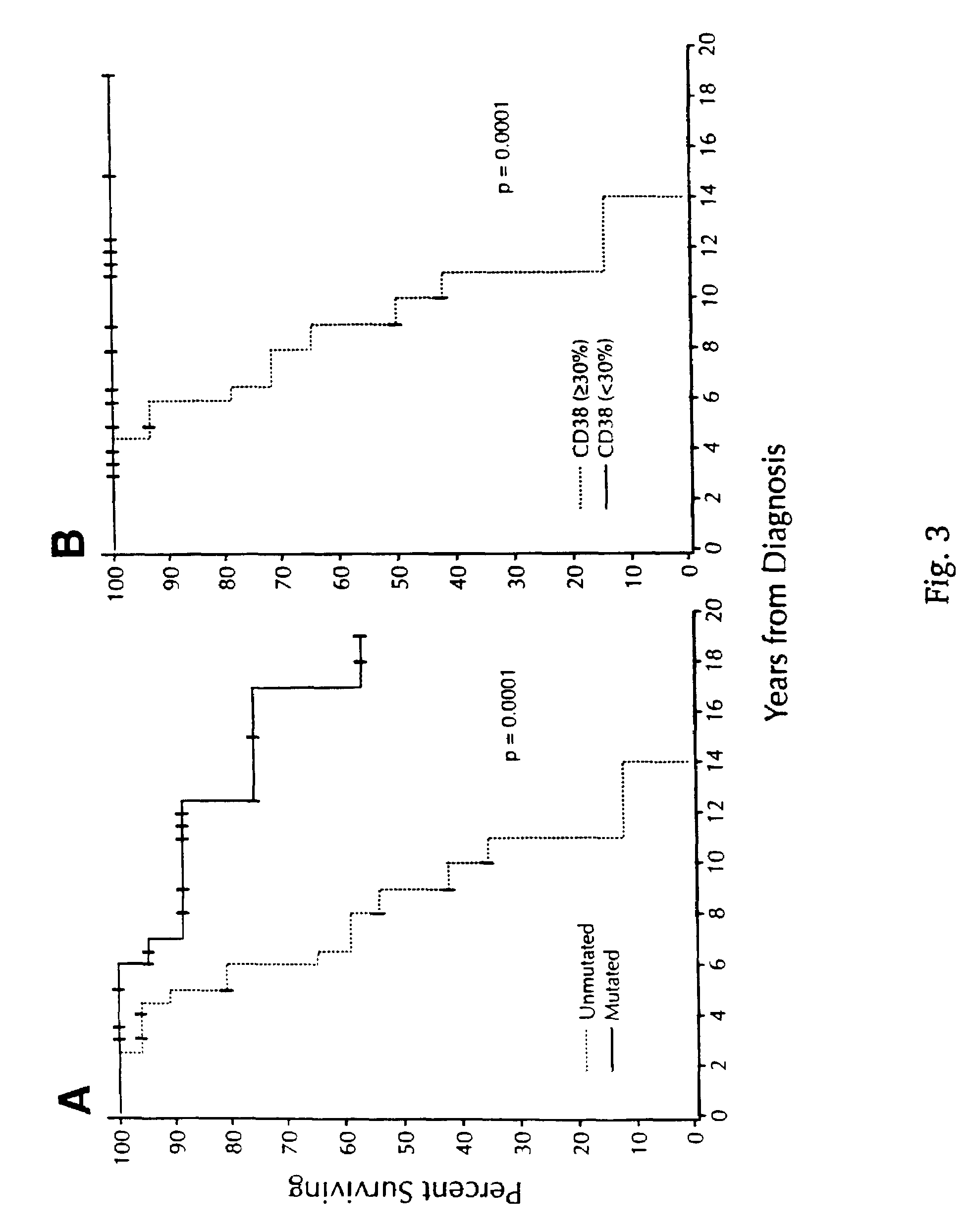
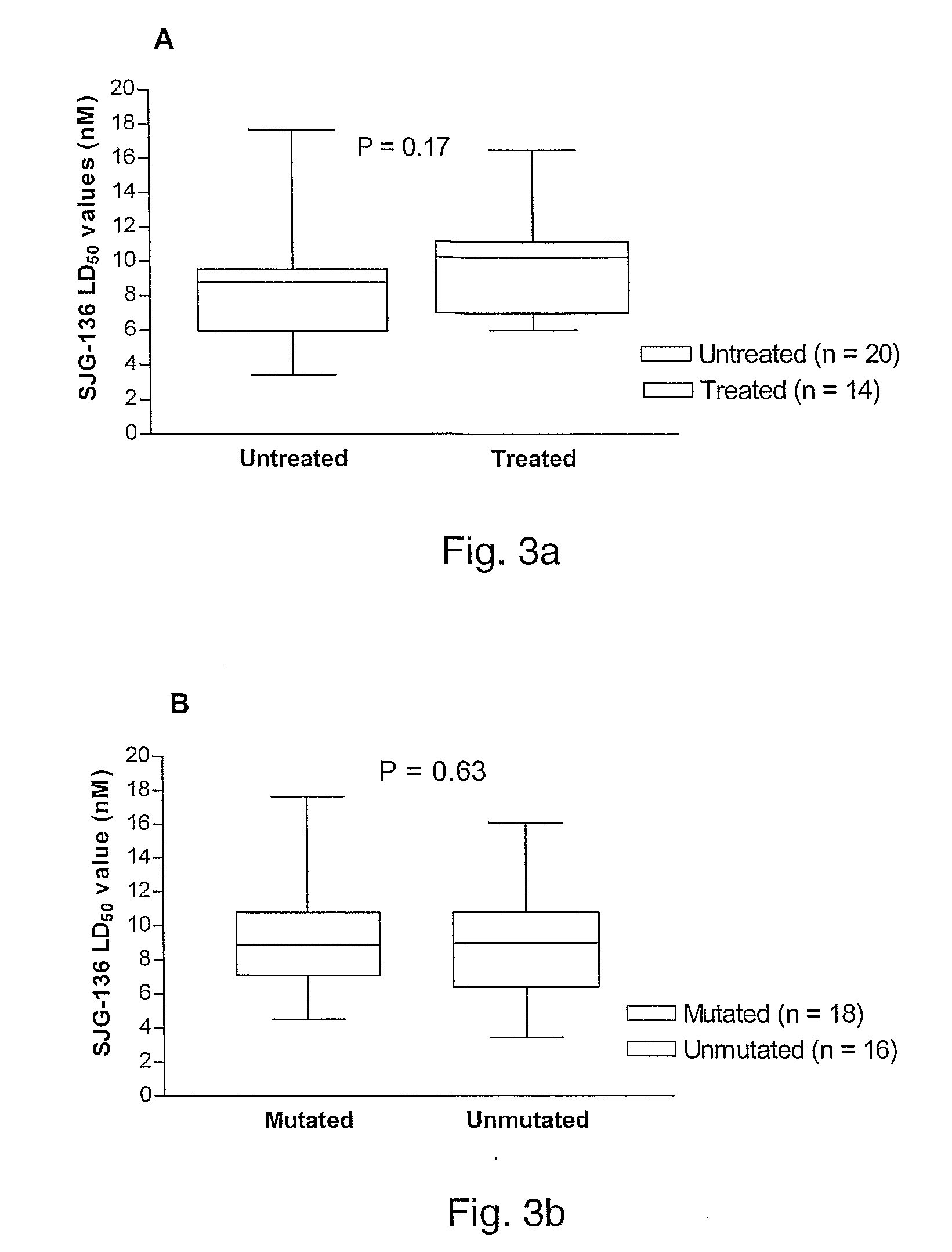
CD38 as a prognostic indicator in B cell chronic lymphocytic leukemia
The subject invention discloses a method for determining the prognosis and probable clinical course of a subject diagnosed with B-CLL. Specifically, the invention involves comparing CD38 expression in a biological sample from the subject containing B-CLL cells to a baseline level of CD38 expression, wherein an elevated level of CD38 expression in relation to the baseline level of CD38 expression may indicate poor prognosis or aggressive course of disease in the subject. Also disclosed is a method for determining whether the Ig V genes of the B-CLL cells of a B-CLL patient are mutated, comprising comparing CD38 expression in a biological sample from the subject containing B-CLL cells to a baseline level of CD38 expression, wherein a lower level of CD38 expression in relation to the baseline level indicates IG V gene mutation.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Combination of Anti-PD-1 Antibodies and Anti-CD20/Anti-CD3 Antibodies to Treat Cancer
InactiveUS20170174779A1Growth inhibitionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenCD20
The present invention provides methods for treating, reducing the severity, or inhibiting the growth of cancer (e.g., a B-cell cancer such as Hodgkin's lymphoma or acute lymphoblastic leukemia). The methods of the present invention comprise administering to a subject in need thereof a therapeutically effective amount of an antibody or antigen-binding fragment thereof that specifically binds to programmed death 1 (PD-1) receptor in combination with a therapeutically effective amount of a bispecific antibody that specifically binds to CD20 and CD3.
Owner:REGENERON PHARM INC
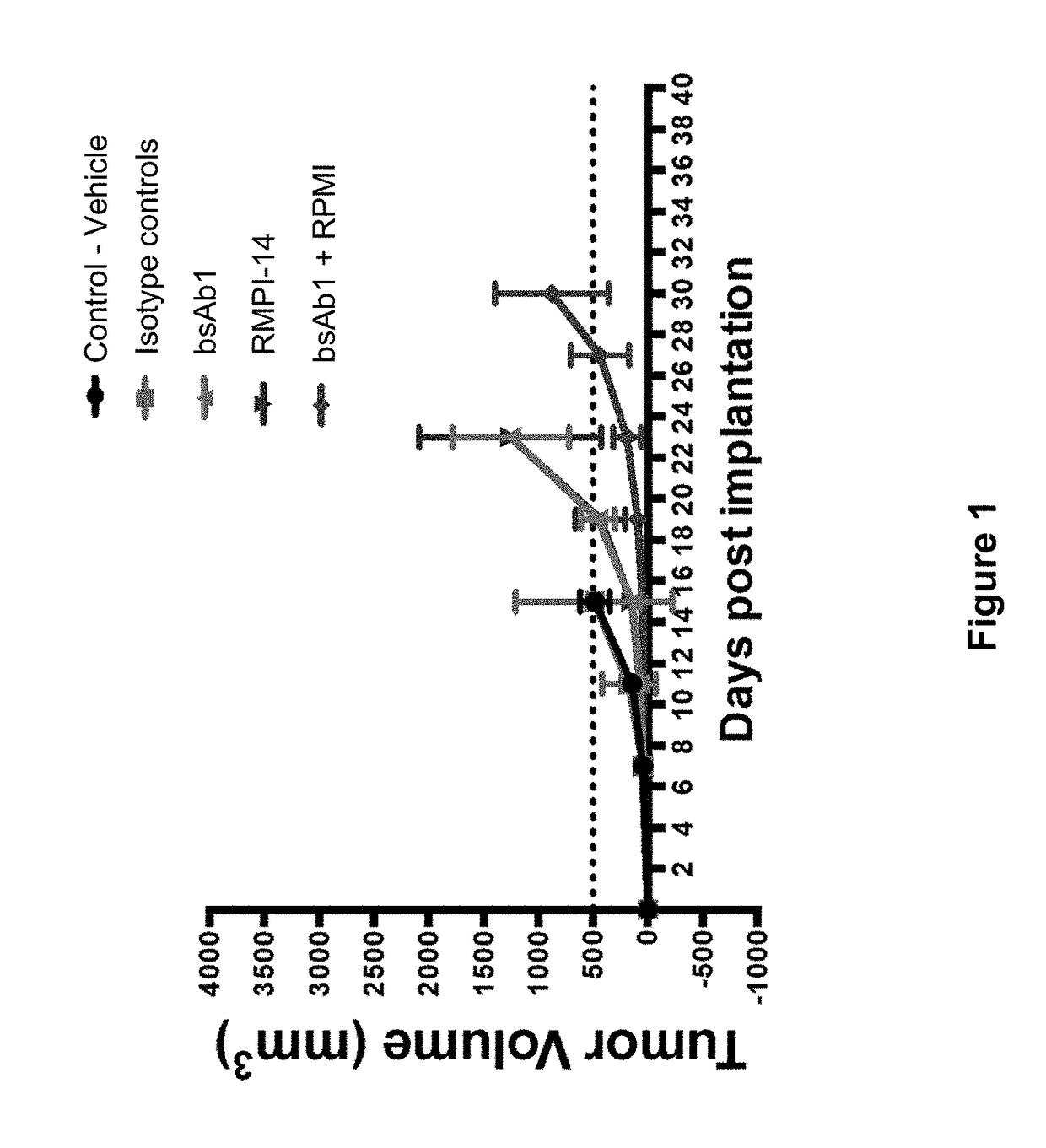
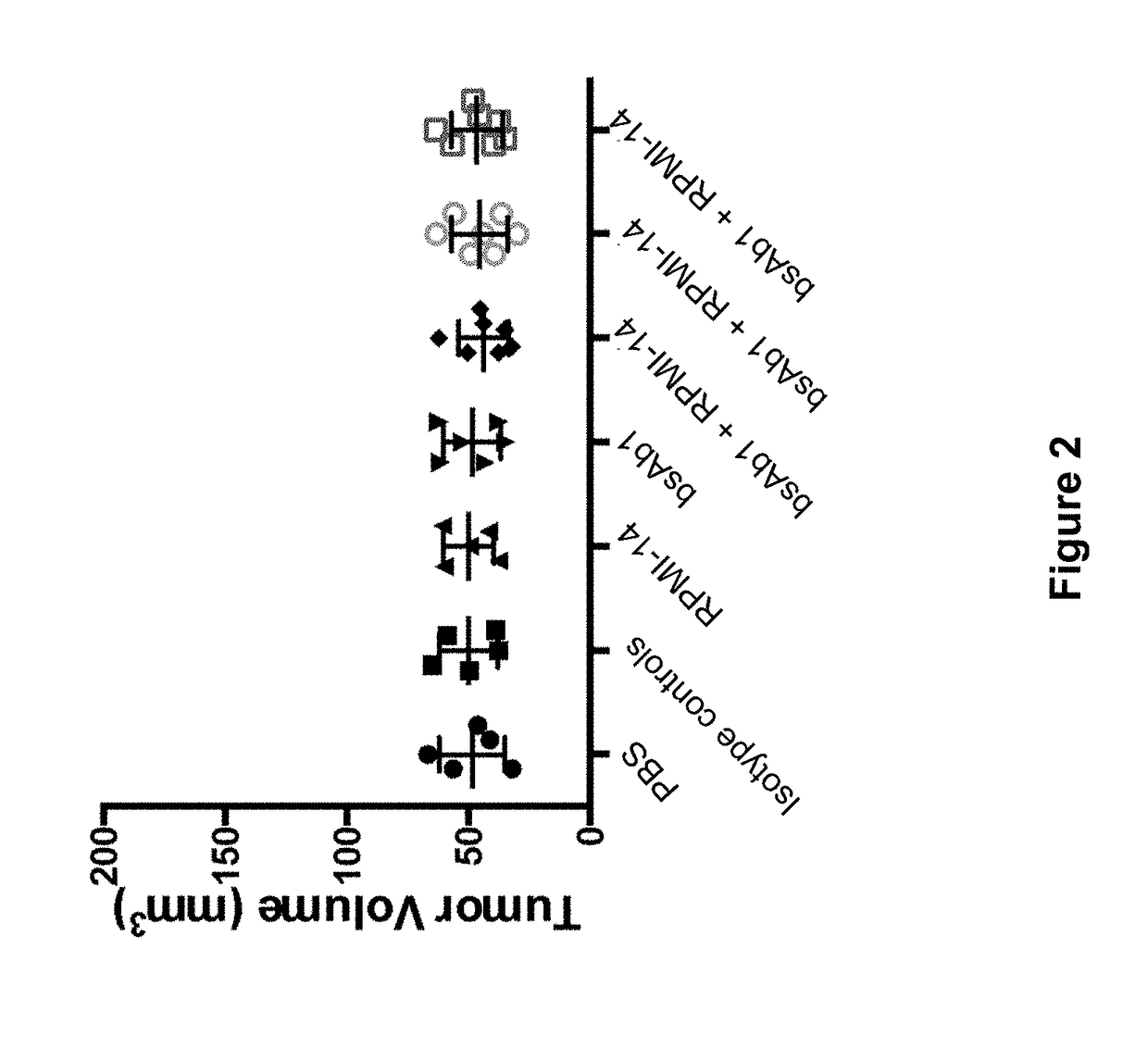
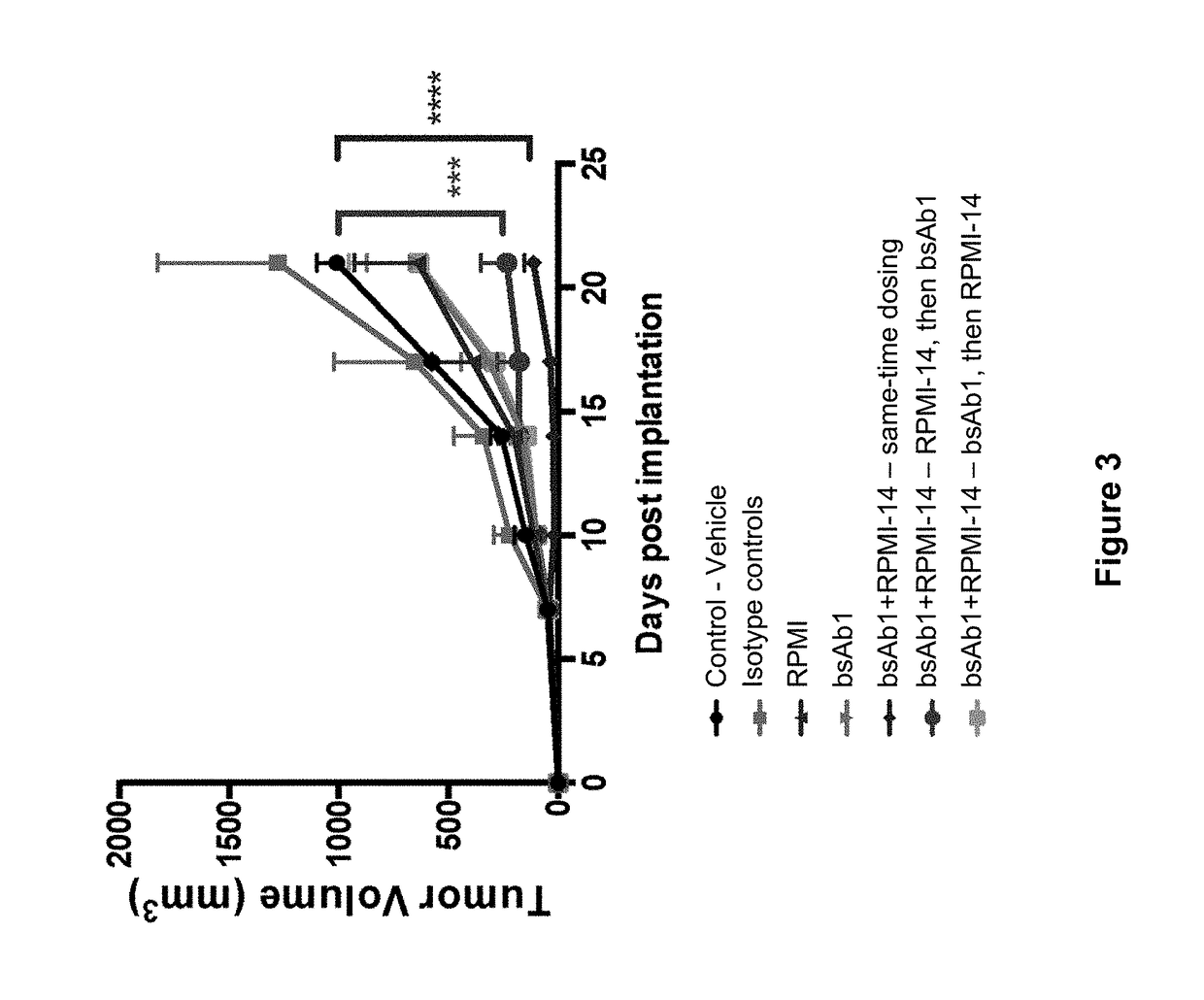
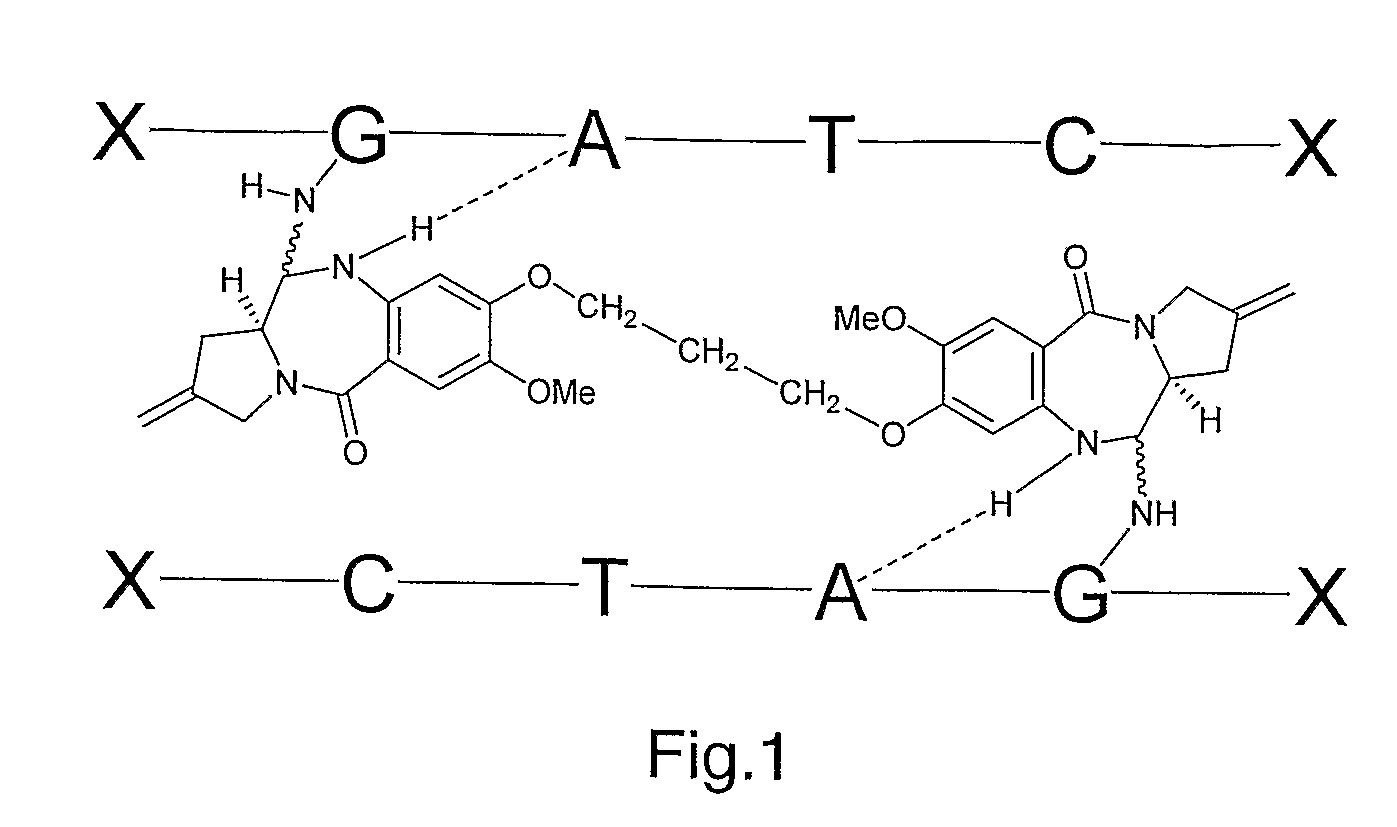
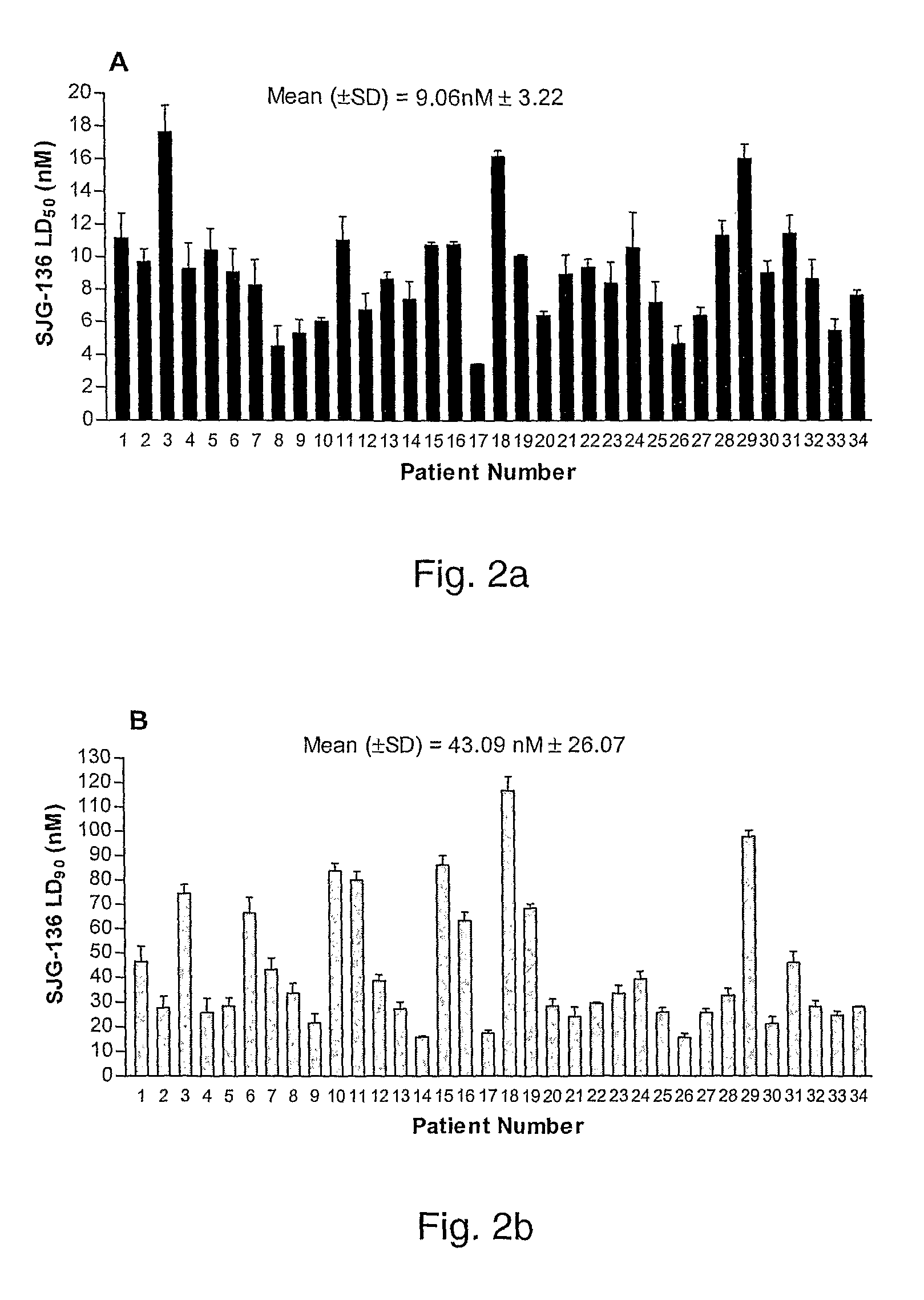
Pyrrolobenzodiazepine Therapeutic Agents Useful in the Treatment of Leukemias
A pyrrolobenzodiazepine dimer compound of Formula (I): or pharmaceutically acceptable salt or solvate thereof is useful as a therapeutic agent for the treatment of leukaemias, especially B-cell leukaemias, that exhibit resistance to other chemotherapeutic drugs, wherein: the dotted lines indicate the optional presence of a double bond between C1 and C2 or C2 and C3; R2 and R3 are independently selected from —H, ═O, ═CH2, —CN, —R, OR, halo, ═CH—R, O—SO2—R, CO2R and COR; R6, R7 and R9 are independently selected from II, R, OII, OR, SII, SR, NII2, NIIR, NRR′, nitro, Me3Sn and halo; where R and R′ are independently selected from optionally substituted C1-12 alkyl, C3-20 heterocyclyl and C5-20 aryl groups; R10 is a carbamate-based nitrogen protecting group and R15 is either O—R11, wherein R is an oxygen protecting group, or OH, or R10 and R15 together form a double bond between N10 and C11; R″ is a C3-12 alkylene group, which chain may be interrupted by one or more heteroatoms and / or aromatic rings, and each X is independently selected from 0, S, or NH; R2′, R3′, R6′, R7′, R9′, R10′ and R15′ are all independently selected from the same lists as previously defined for R2, R3, R6, R7, R9, R10 and R15 respectively.
Owner:SPIROGEN
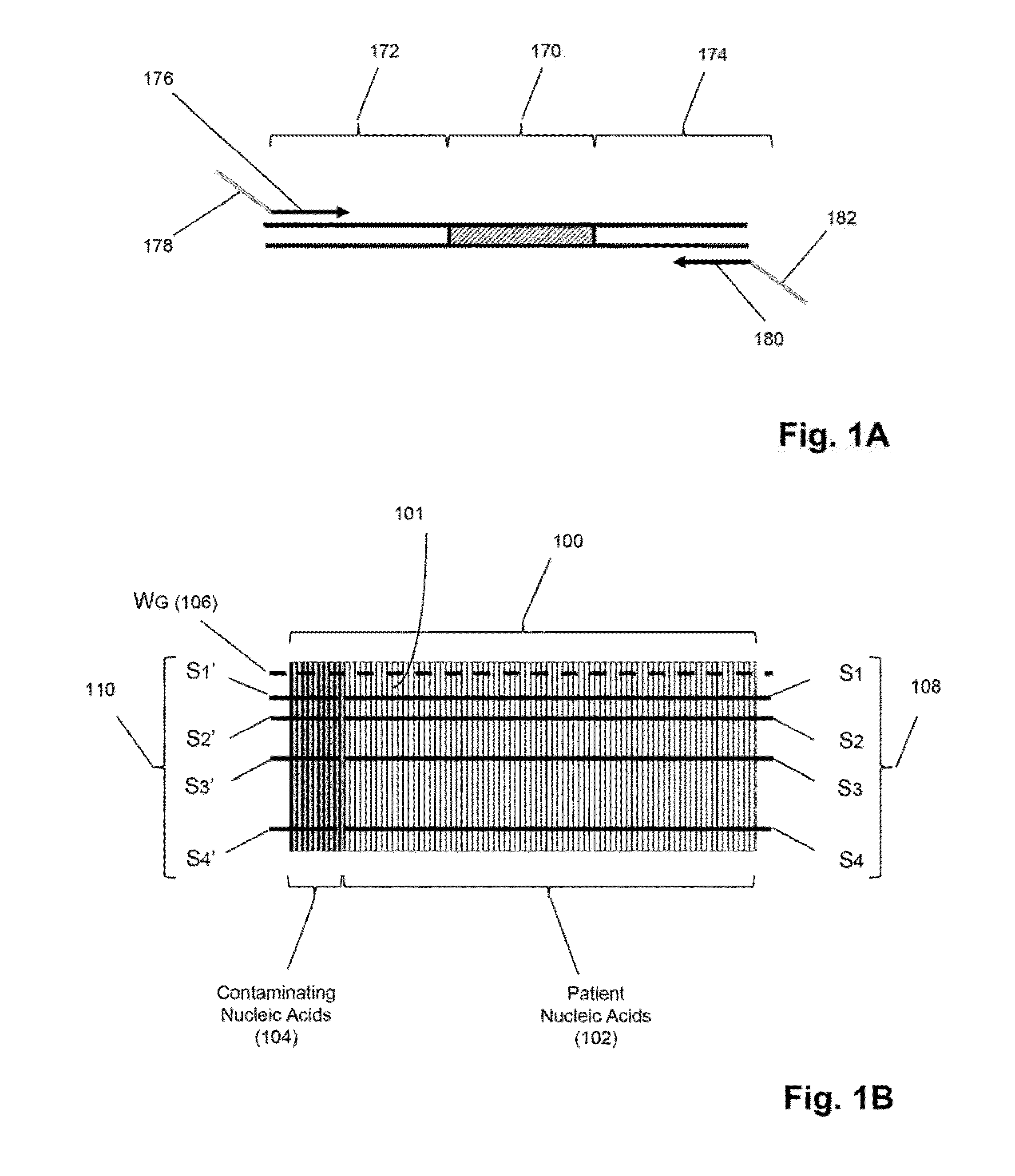
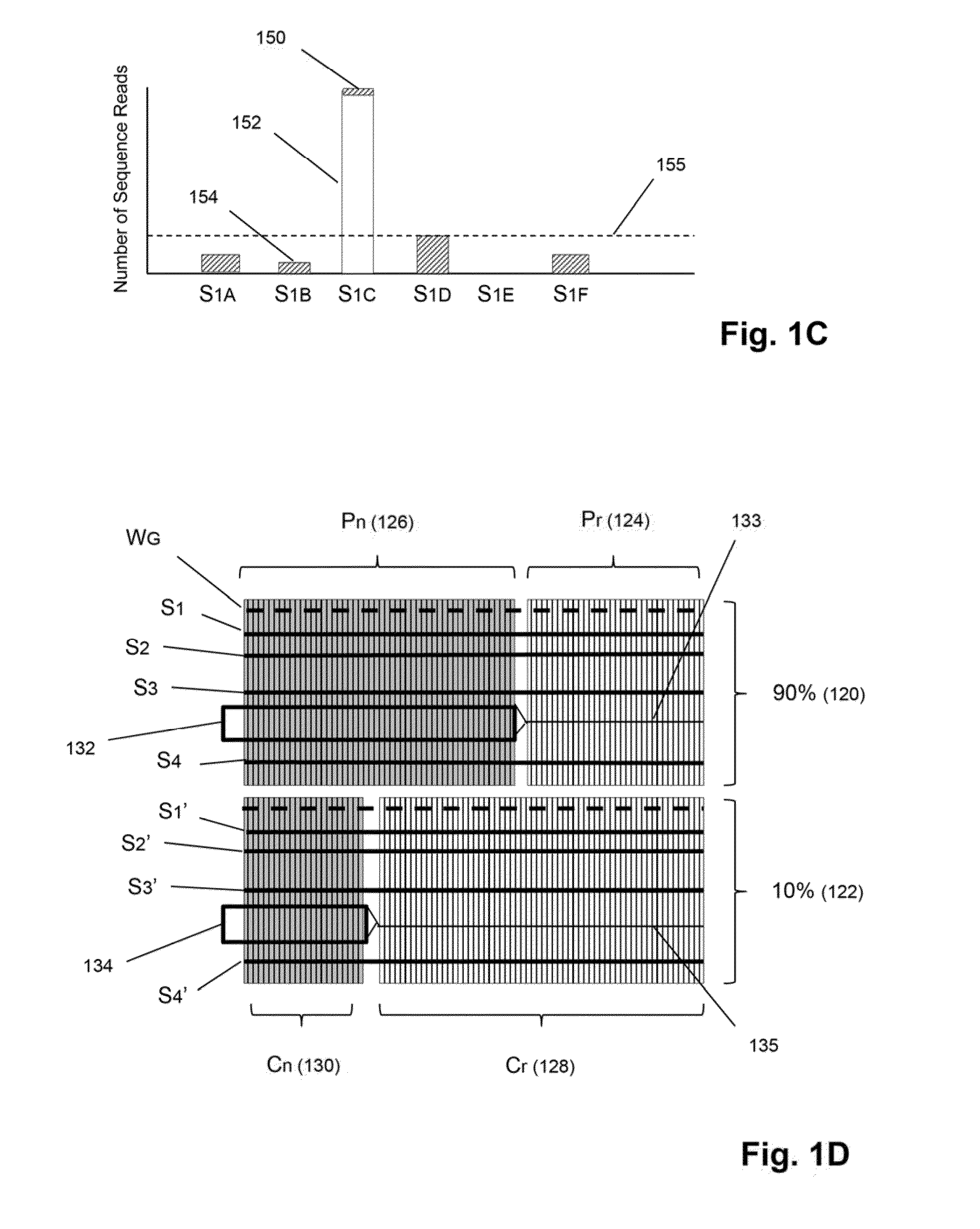
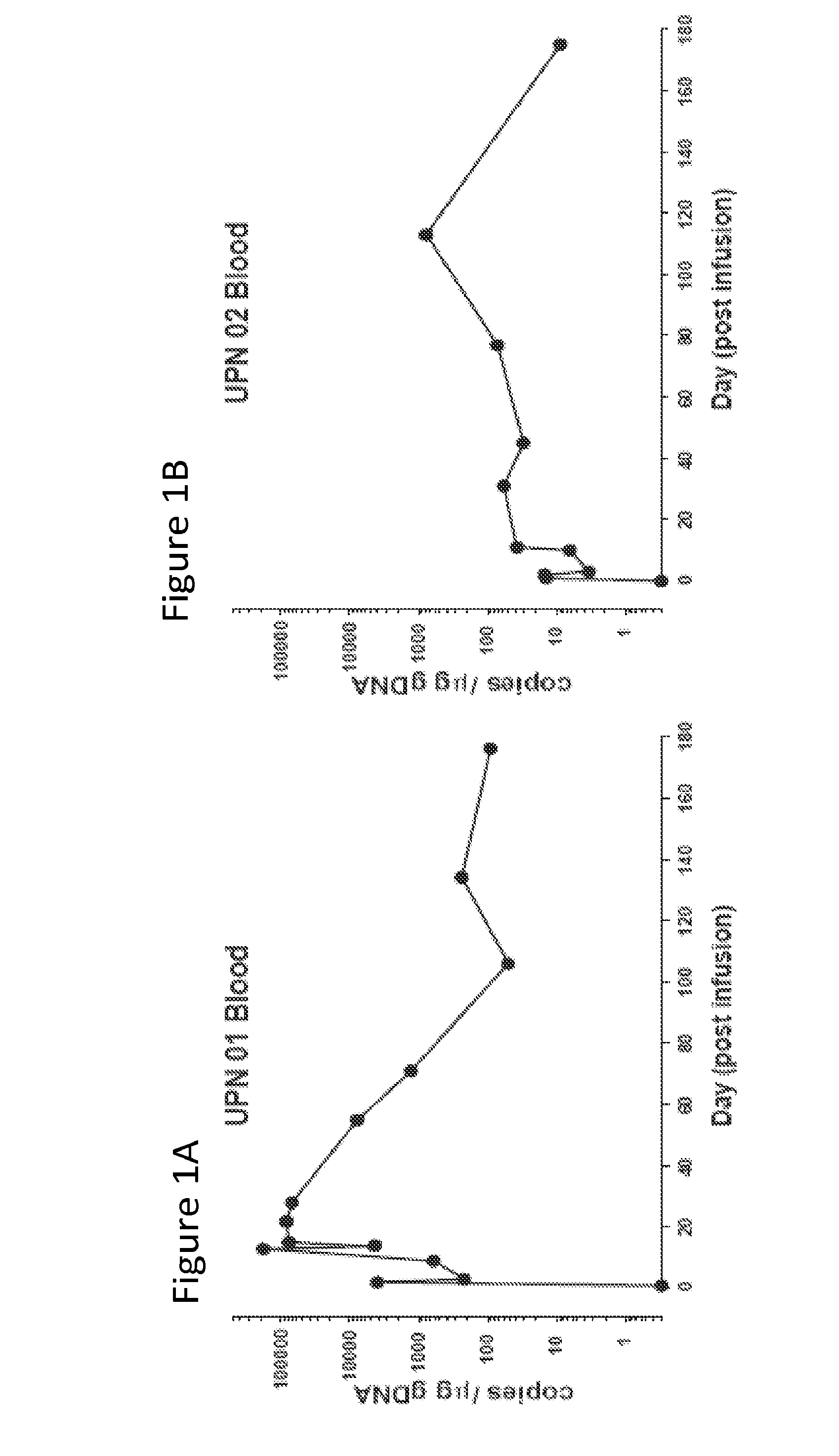
Detection and quantification of sample contamination in immune repertoire analysis
The invention is directed to methods for detecting and quantifying nucleic acid contamination in a tissue sample of an individual containing T cells and / or B cells, which is used for generating a sequence-based clonotype profile. In one aspect, the invention is implemented by measuring the presence and / or level of an endogenous or exogenous nucleic acid tag by which nucleic acid from an intended individual can be distinguished from that of unintended individuals. Endogenous tags include genetic identity markers, such as short tandem repeats, rare clonotypes or the like, and exogenous tags include sequence tags employed to determine clonotype sequences from sequence reads.
Owner:ADAPTIVE BIOTECH
Treatment of bone disorders
InactiveUS20060263355A1Enhance osteoblast formationImprove bone formationBiocidePeptide/protein ingredientsDiseaseCD20
Methods of treatment of various bone indications, such as osteoporosis, in a mammal are provided wherein an effective amount of an antagonist that binds to a B-cell surface marker, such as a CD20 antibody, is administered, optionally also with another medicament such as an agent that treats such disorders in an effective amount. Articles of manufacture are also provided. Further, a method of inhibiting osteolysis in a mammal is provided comprising introducing into said mammal an isolated odontoprogenitor or osteoprogenitor cell comprising a nucleic acid encoding an antibody that binds to a B-cell surface marker.
Owner:GENENTECH INC
Method for treating lupus
InactiveUS20060024295A1Reduce and minimize needAvoid much side effectAntipyreticAnalgesicsSurface markerRegimen
A method of treating lupus in a subject eligible for treatment is provided involving administering an effective amount of an antibody that binds to a B-cell surface marker to the subject to provide an initial exposure and a subsequent exposure to the antibody within certain dosing regimens and an article of manufacture therefor.
Owner:GENENTECH INC
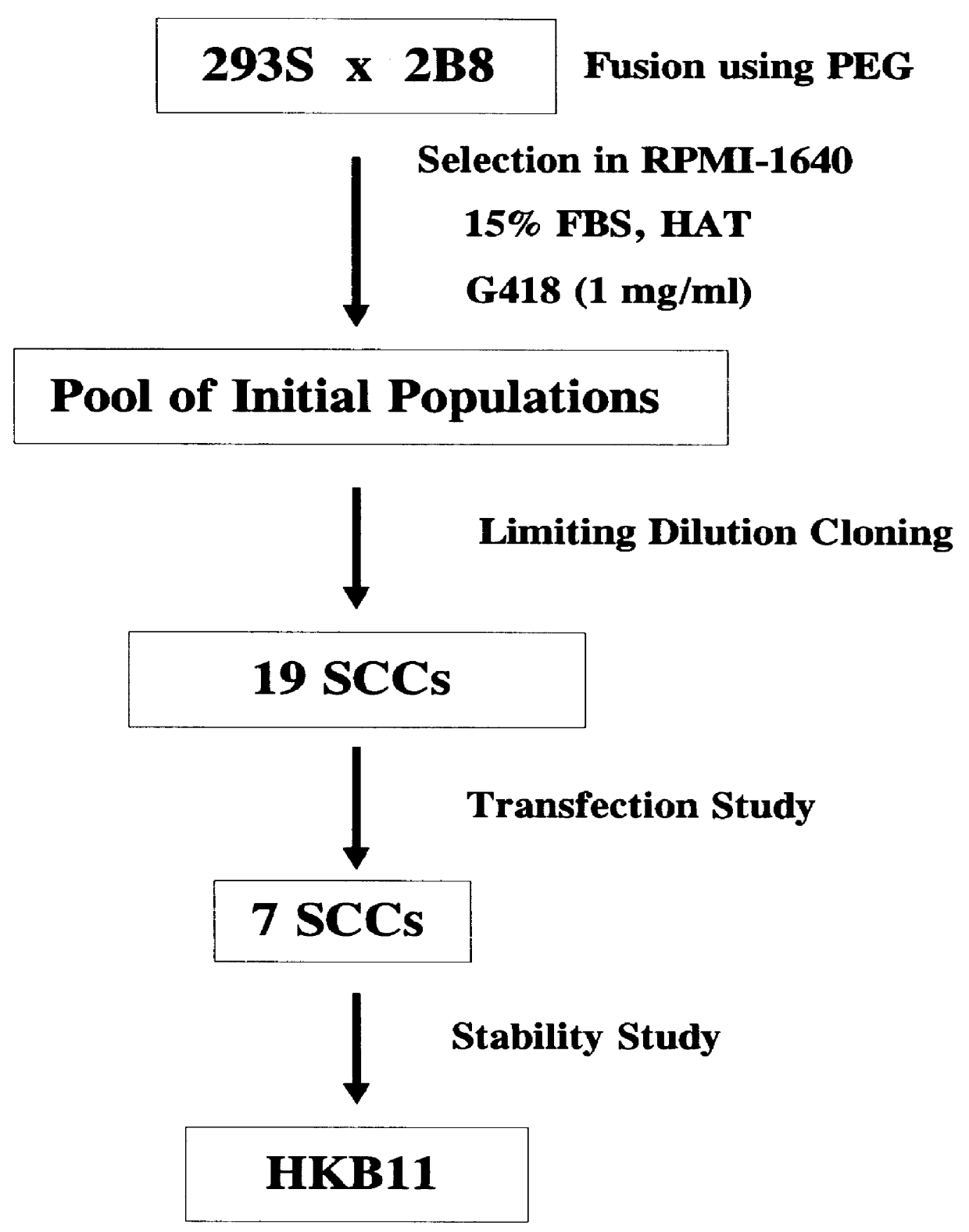
Human hybrid host cell for mammalian gene expression
InactiveUS6136599AEasily transfectedEasy to adaptGenetically modified cellsMutant preparationHeterologousMammal
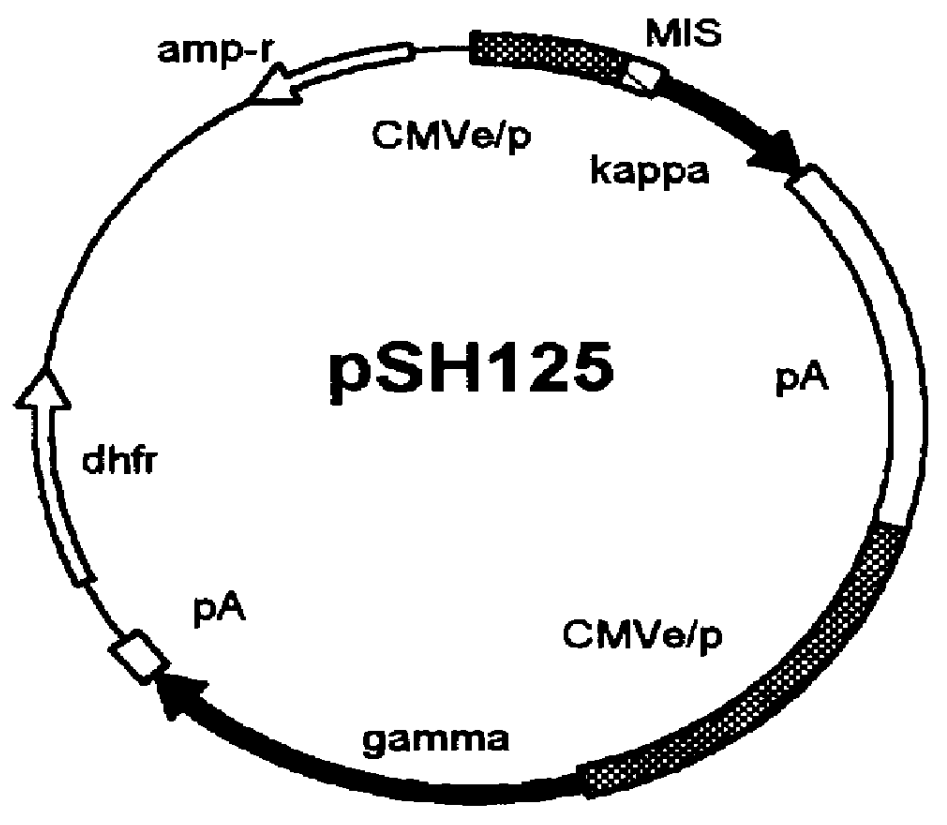
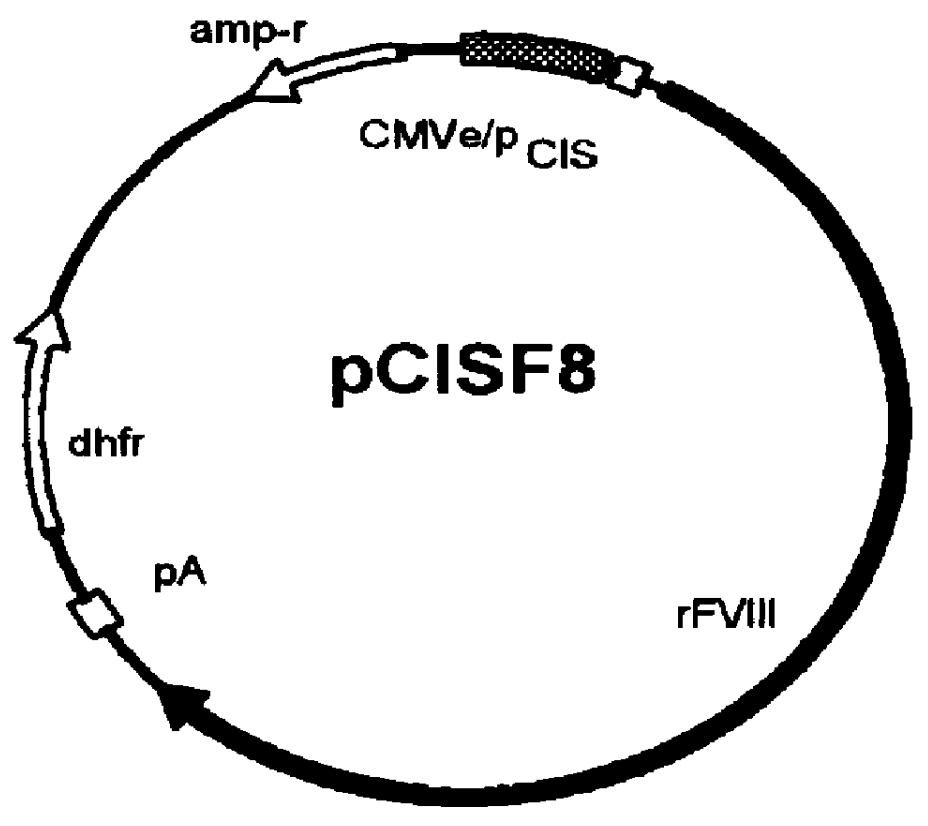
Human / human hybrid cells were made via fusion of human embryonic kidney cells (293S) and modified Burkitt's lymphoma cells (2B8). The fusion cells are useful as host cells for the recombinant expression of mammalian genes. The advantages of using these hybrid clones of human kidney- and B-cells, called HKBs, for mammalian gene expression, include (i) the cells are negative for immunoglobulin expression, (ii) the cells grow easily in plasma protein-free medium (with or without the addition of recombinant insulin) as suspension cultures in a shake flask or in a fermenter (iii) the cells are very susceptible for transfection of DNA, and (iv) the cells secrete high levels of heterologous recombinant proteins, such as recombinant monoclonal antibodies, soluble ICAM-1, rIL-4, and rFVIII.
Owner:BAYER HEALTHCARE LLC +1
Novel multi-oligosaccharide glycoconjugate bacterial meningitis vaccines
InactiveUS20010048929A1Inhibition effectWeight increaseAntibacterial agentsPeptide/protein ingredientsSerotypeTumor antigen
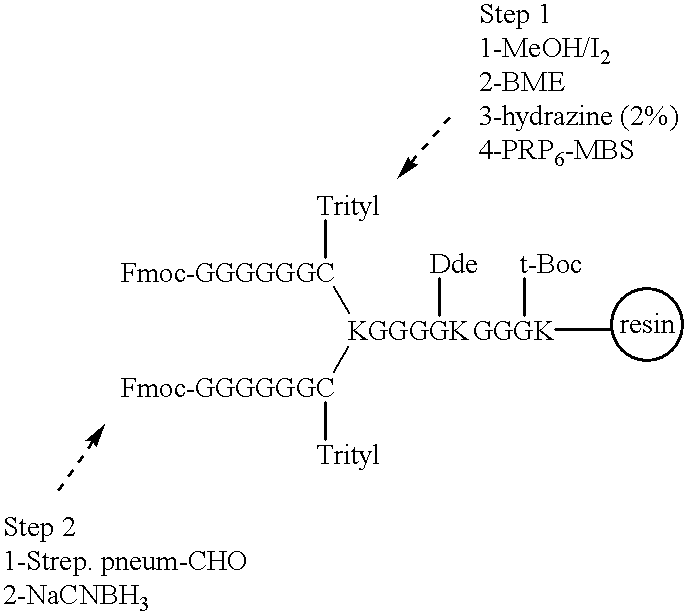
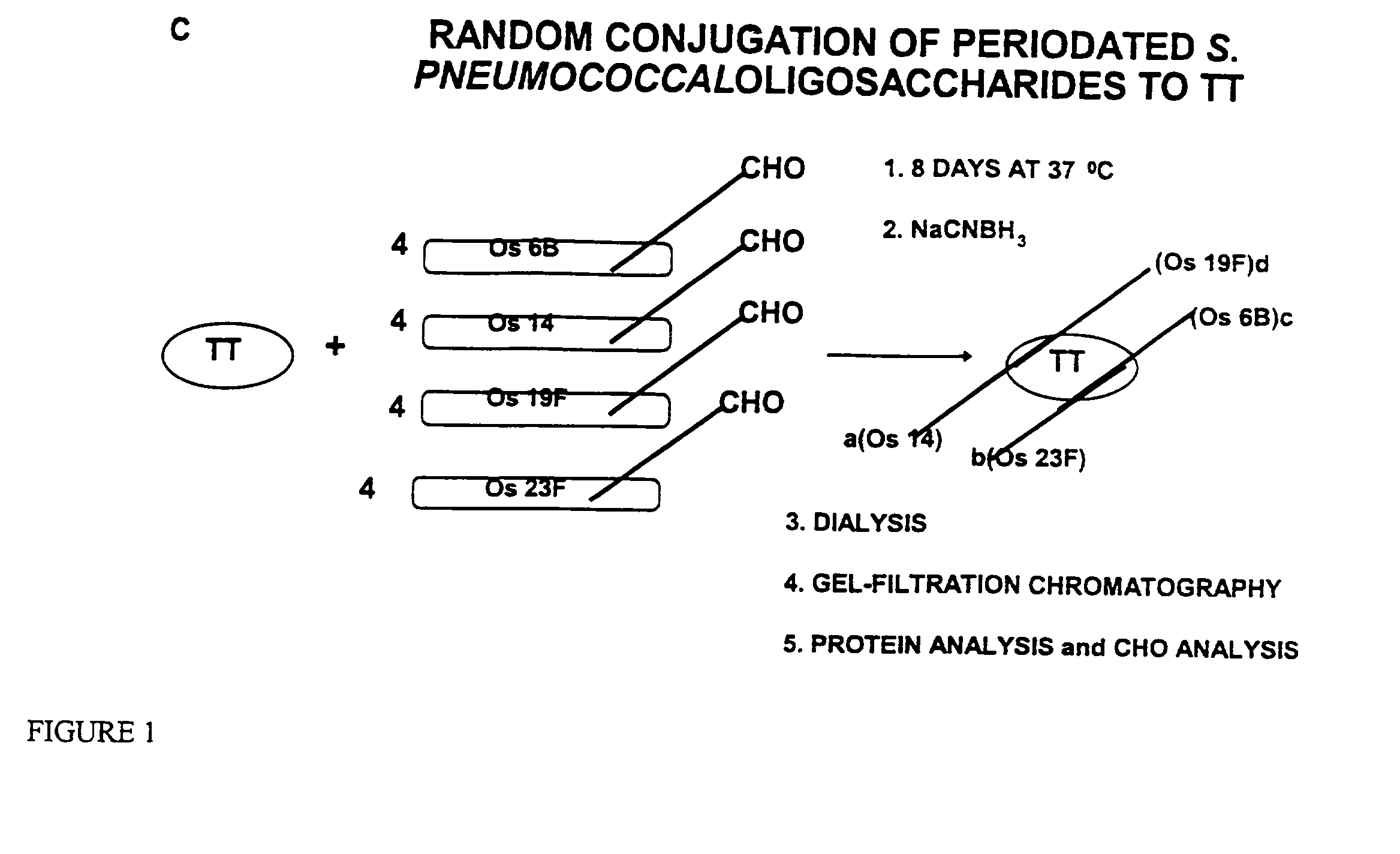
Multivalent immunogenic molecules comprise a carrier molecule containing at least one functional T-cell epitope and multiple different carbohydrate fragments each linker to the carrier molecule and each containing at least one functional B-cell epitope. The carrier molecule inputs enhanced immunogenicity to the multiple carbohydrate fragments. The carbohydrate fragments may be capsular oligosaccharide fragments from Streptococcus pneumoniae, which may be serotypes 1, 4, 5, 6B, 9V, 14, 18C, 19F or 23F, or Neisseria meningitidis, which may be serotype A, B, C, W-135 or Y. Such oligosaccharide fragments may be sized from 2 to 5 kDa. Alternatively, the carbohydrate fragments may be fragments of carbohydrate-based tumor antigens, such as Globo H, LeY or STn. The multivalent molecules may be produced by random conjugation or site-directed conjugation of the carbohydrate fragments to the carrier molecule. The multivalent molecules may be employed in vaccines or in the generation of antibodies for diagnostic application.
Owner:CONNAUGHT LAB
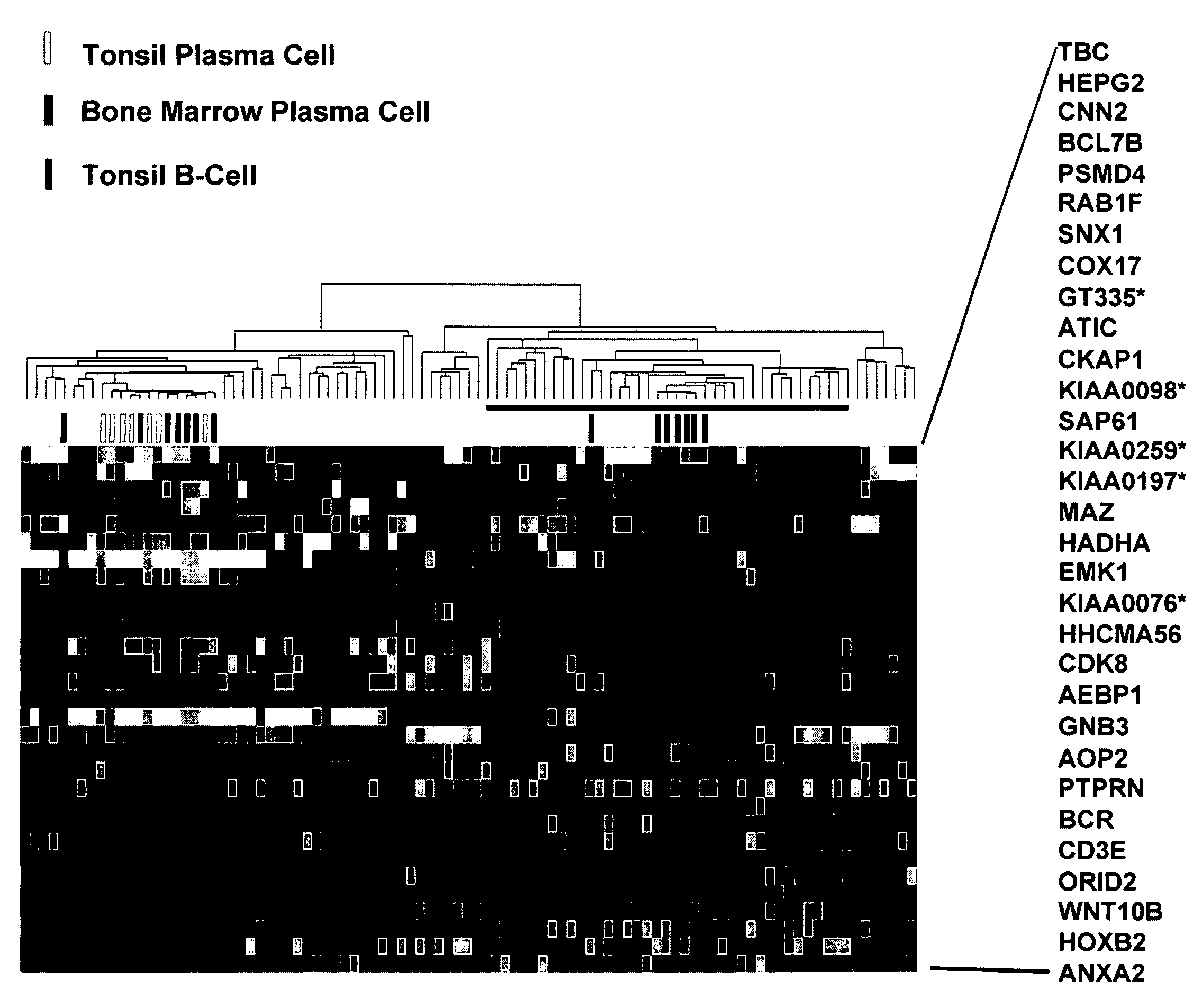
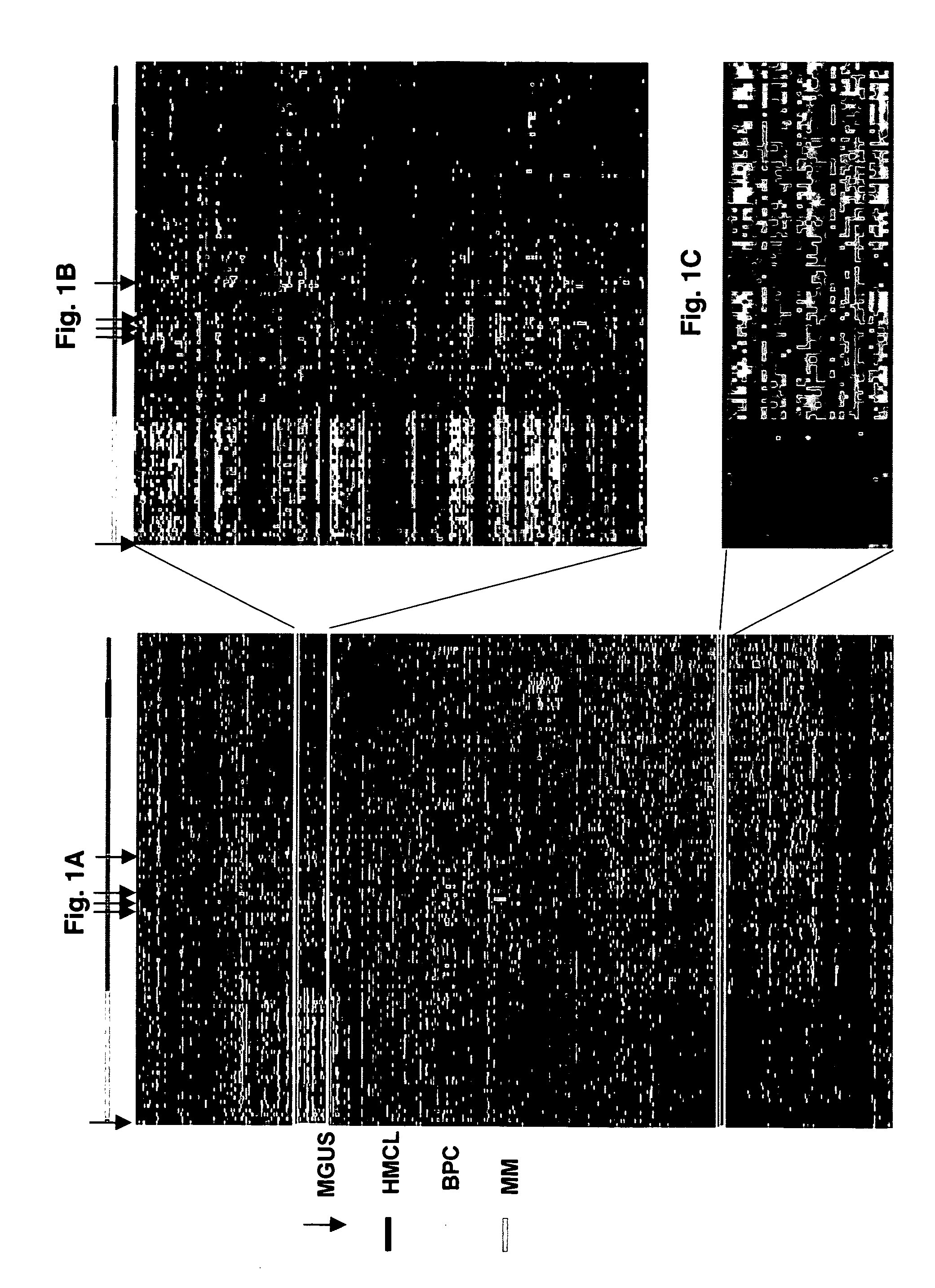
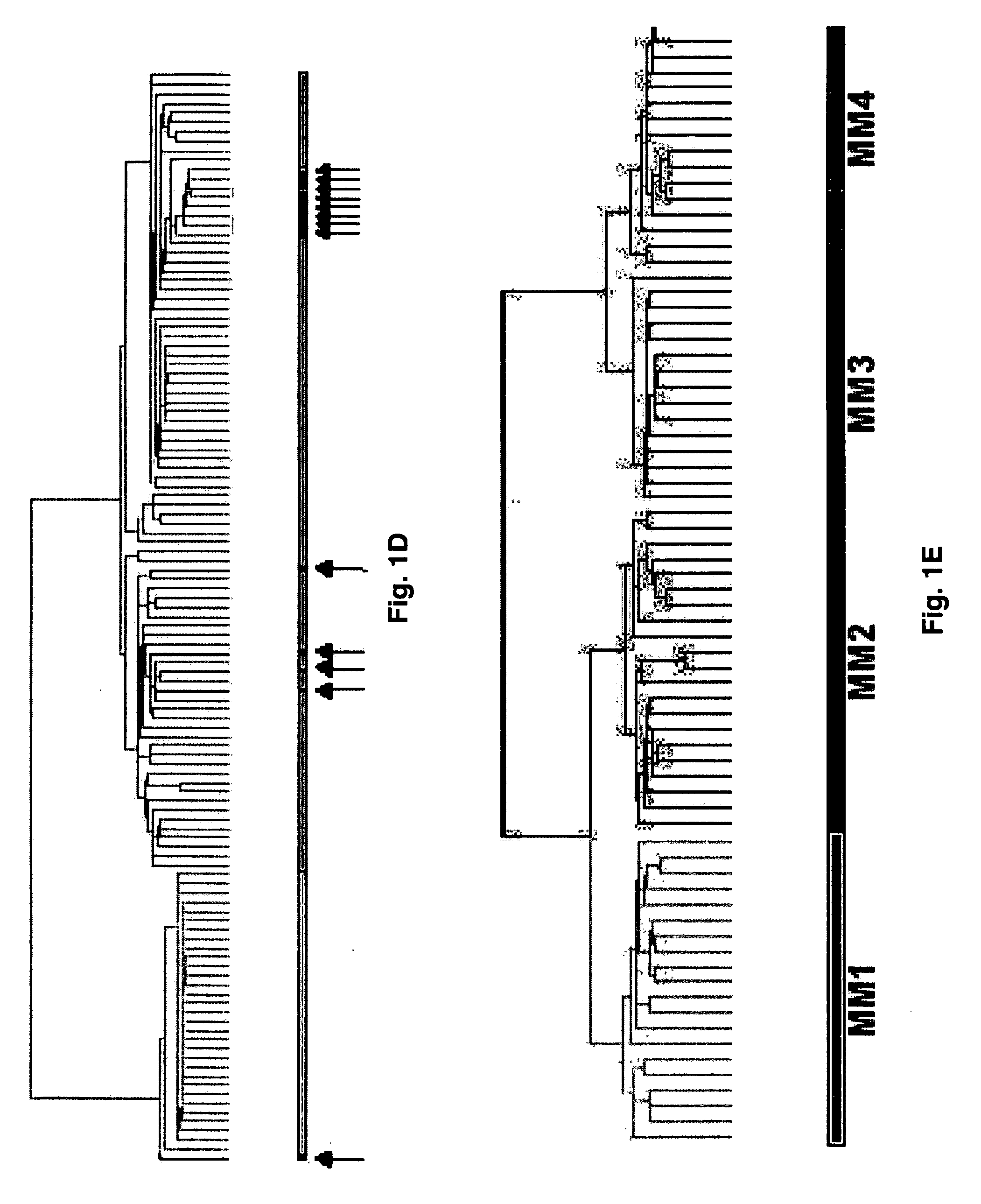
Diagnosis, prognosis and identification of potential therapeutic targets of multiple myeloma based on gene expression profiling
InactiveUS20080234139A1Microbiological testing/measurementLibrary screeningDevelopmental stagePlasma cell
Provided herein are methods for diagnosing and treating multiple myeloma based on statistical analysis of and subsequent increasing / inhibiting expression of subgroups of plasma cells and B cell genes. Also provided are methods for a developmental stage-based classification for multiple myeloma using hierarchical clustering analysis of plasma cell and B cell nucleic acids and for discriminating among normal, hyperplastic and malignant using gene expression array data and statistical analysis thereof. In addition methods for determining the risk of developing bone disease in a test individual by examining expression levels of a WNT signaling antagonist, such as DKK1, are provided. A kit comprising anti-DKK1 antibodies and detection reagents for measuring DKK1 protein levels also is provided.
Owner:BIOVENTURES LLC
Use of cart19 to deplete normal b cells to induce tolerance
InactiveUS20150290244A1Peptide/protein ingredientsSnake antigen ingredientsBinding domainAntigen binding
The present invention provides compositions and methods for inducing tolerance in a human. The invention includes administering a genetically modified T cell expressing a CAR wherein the CAR comprises an antigen binding domain, a transmembrane domain, a costimulatory signaling region, and a CD3 zeta signaling domain.
Owner:LG CHEM LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com