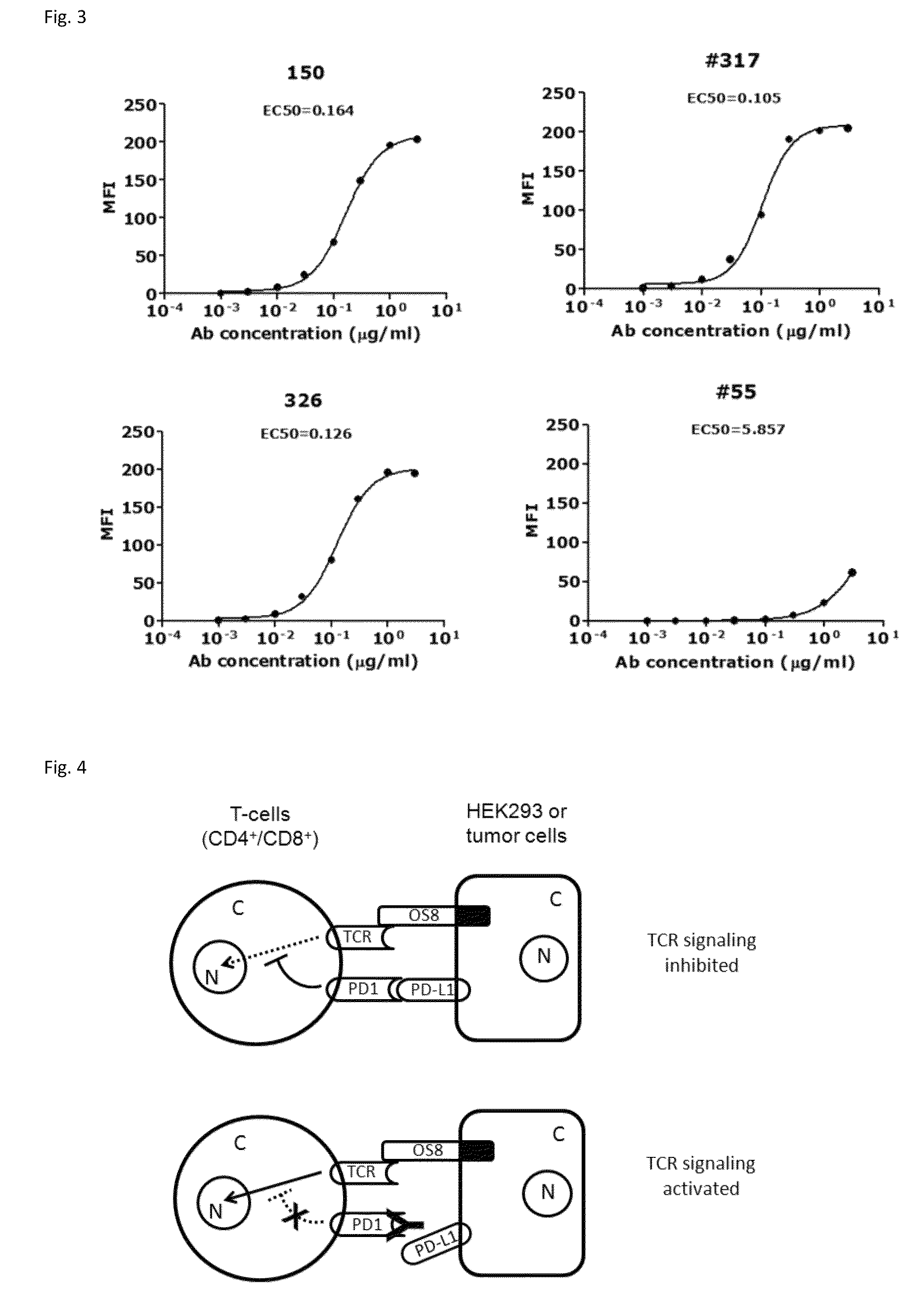
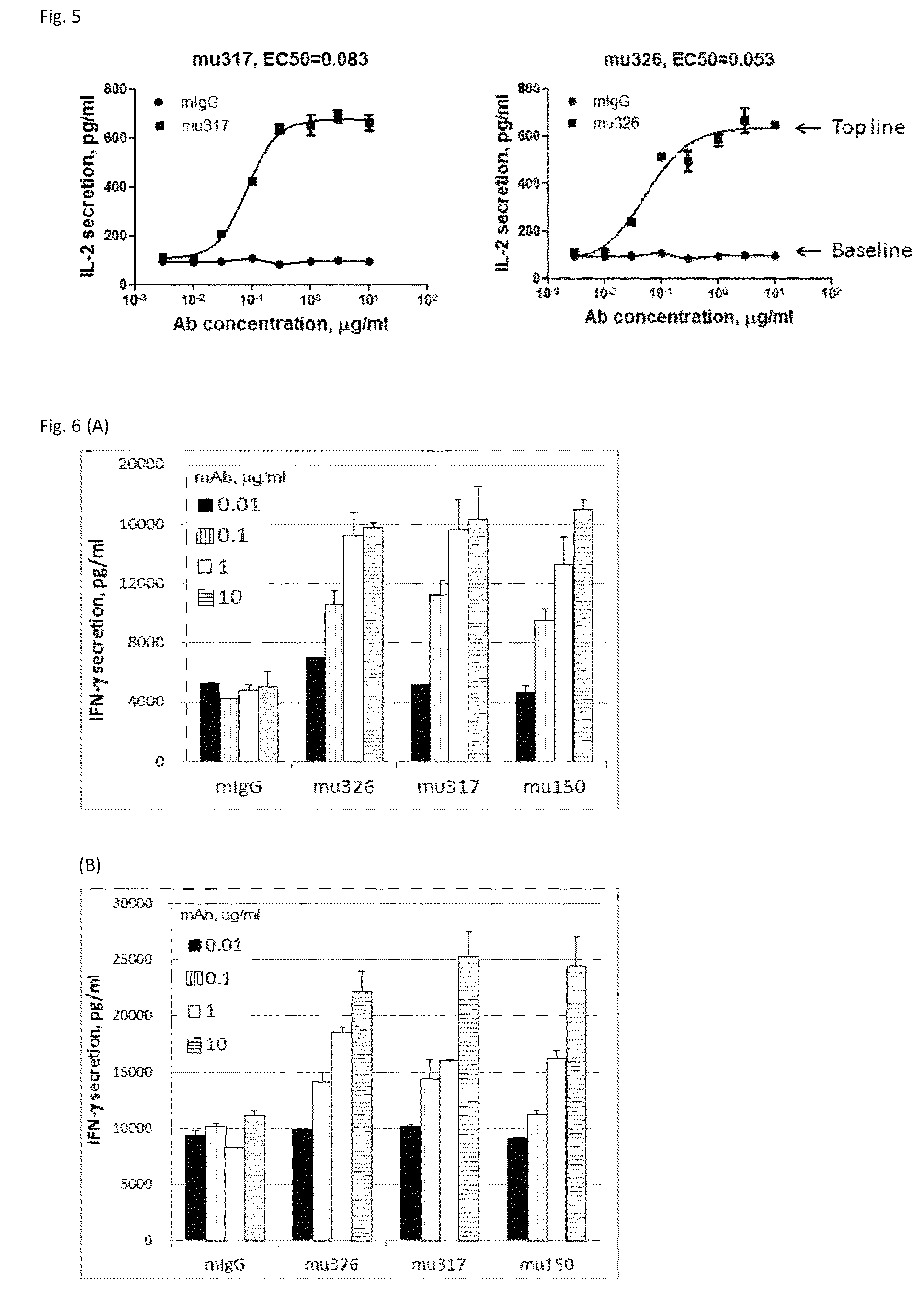
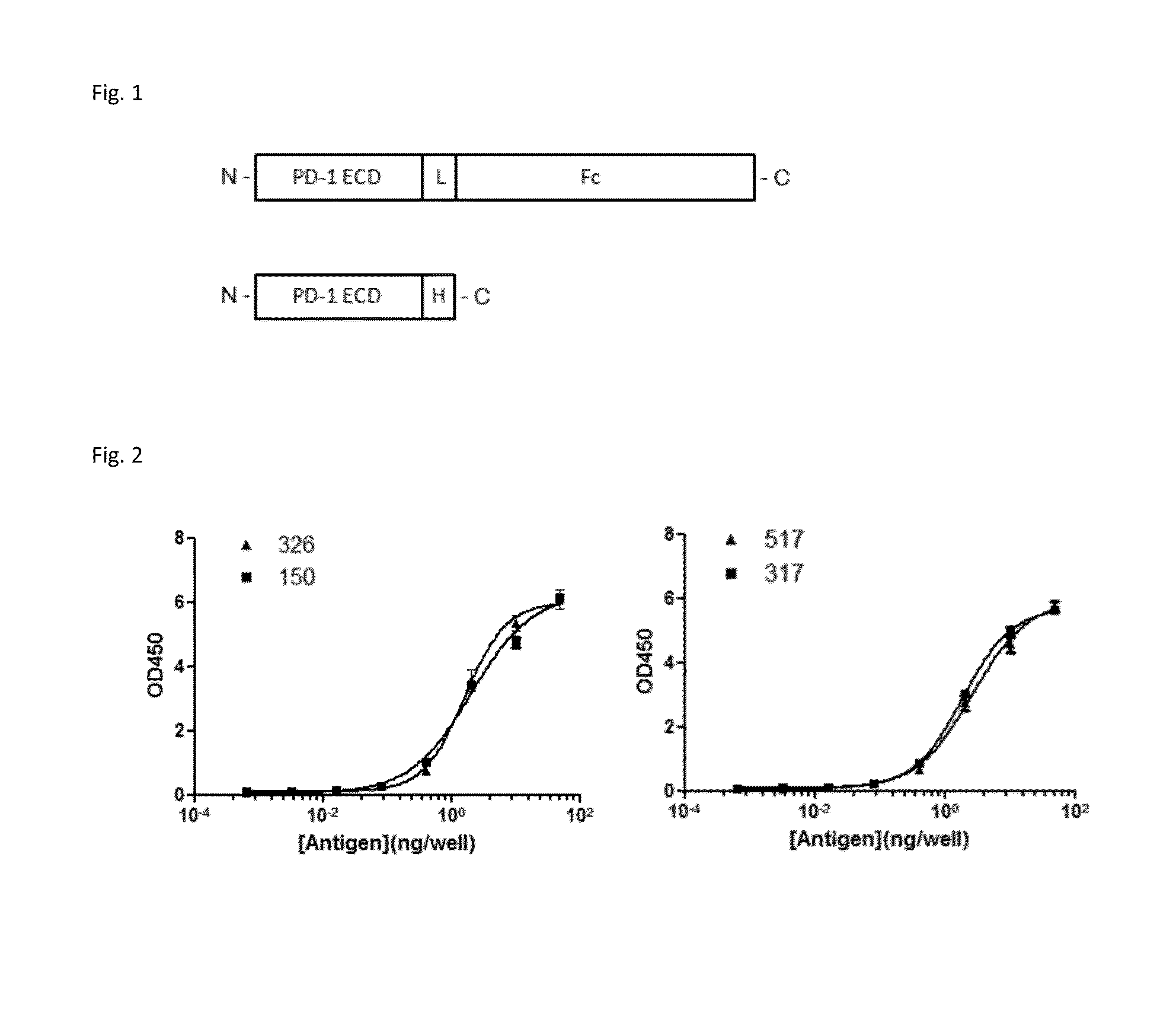
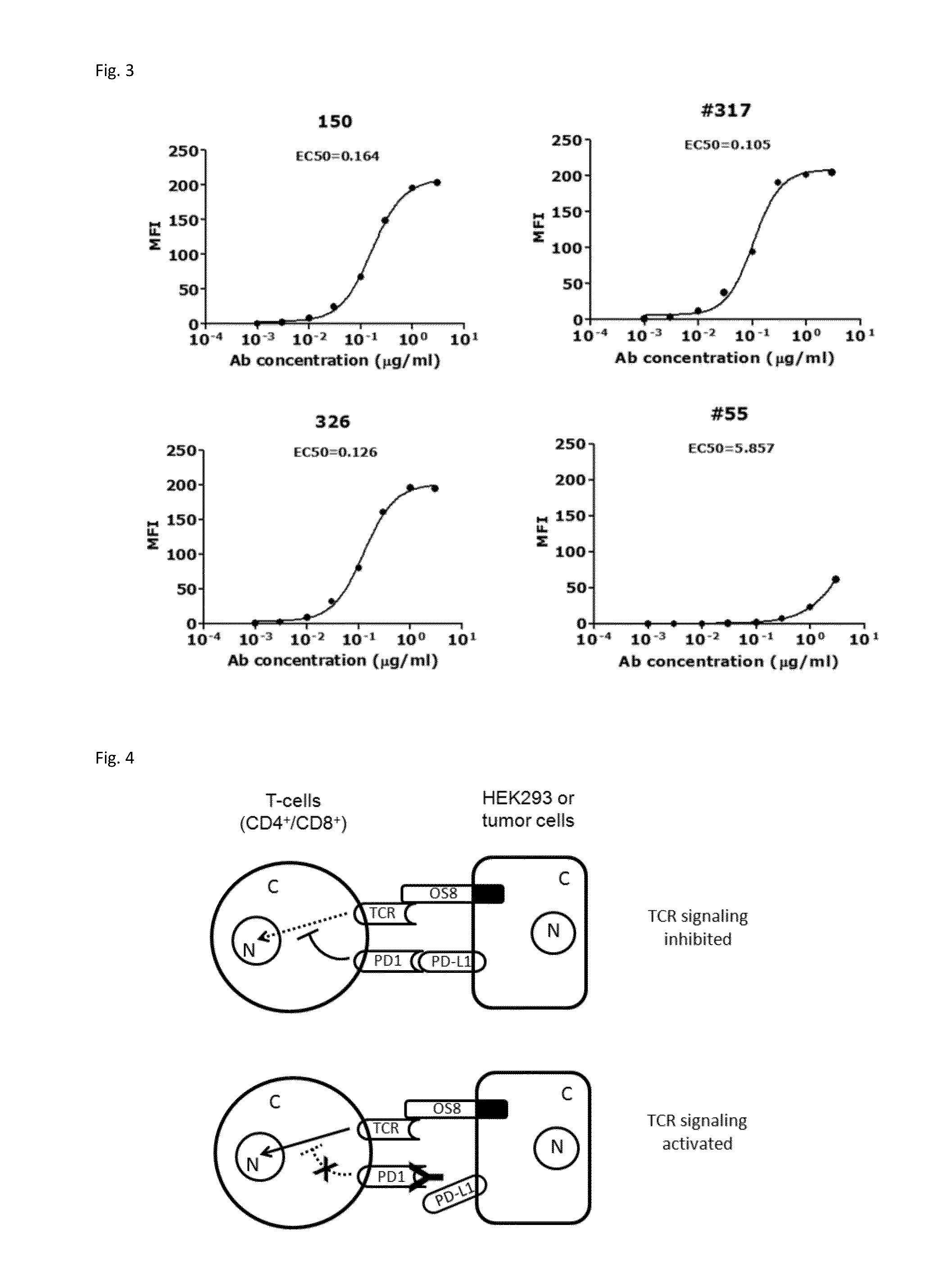
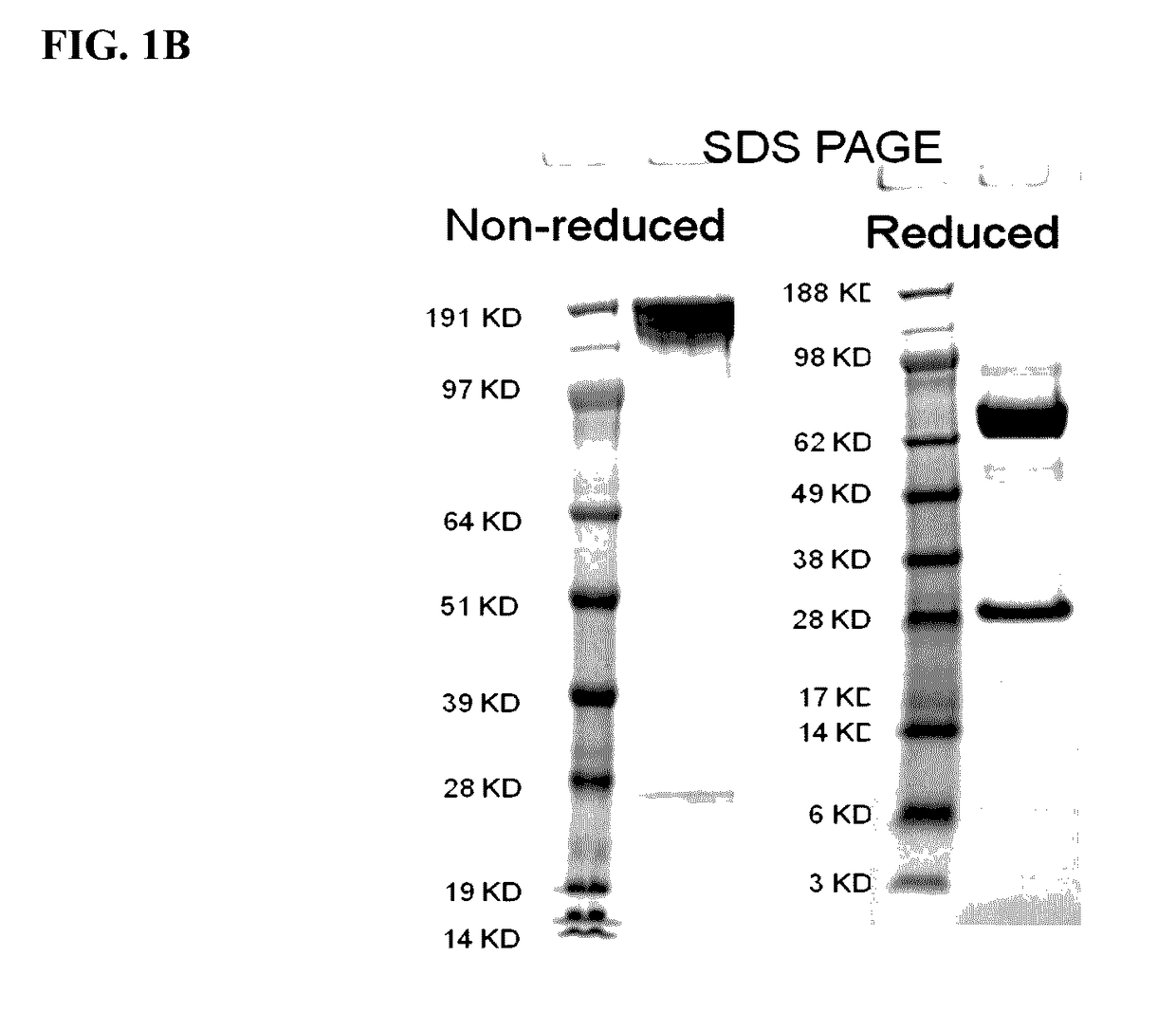
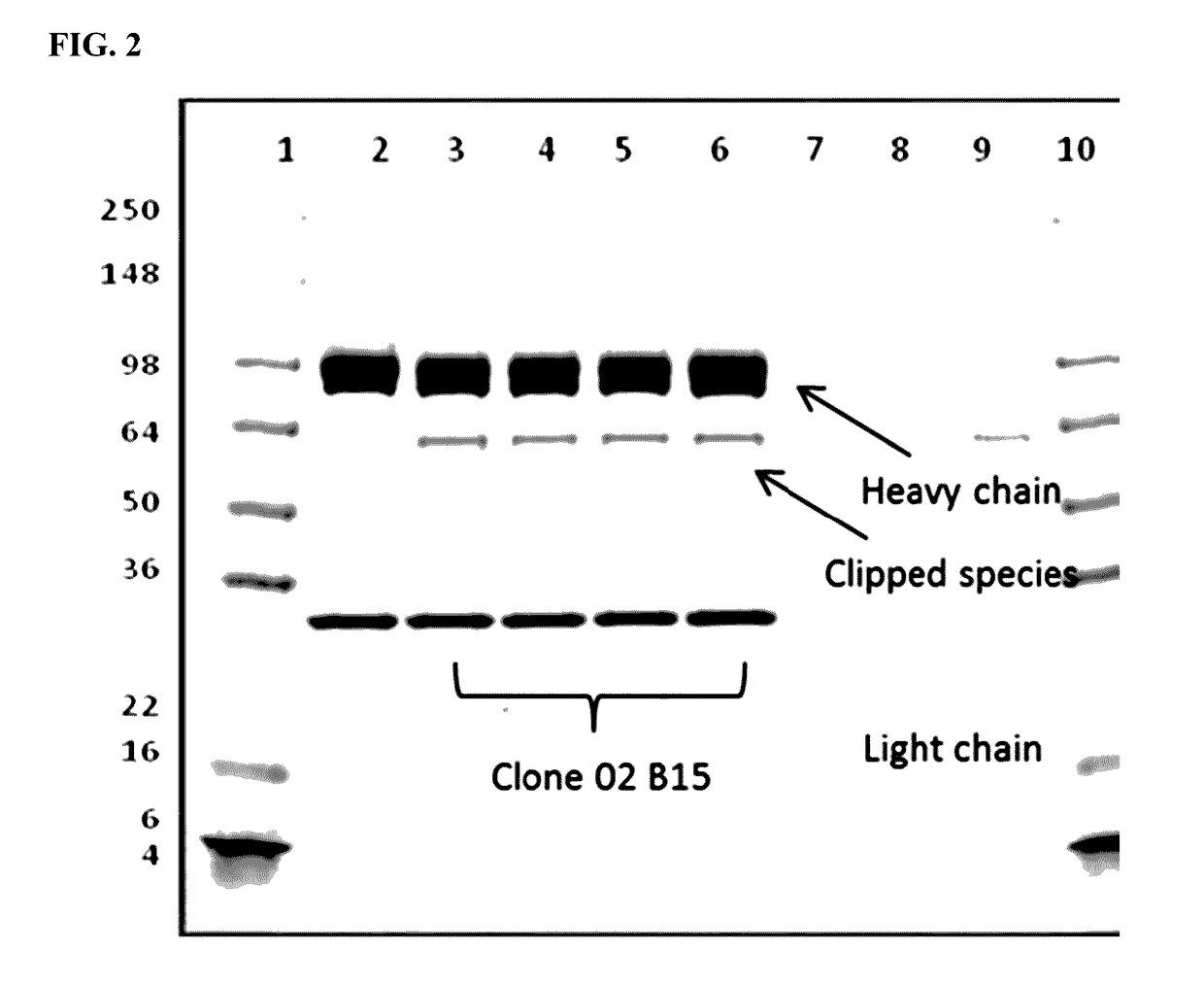
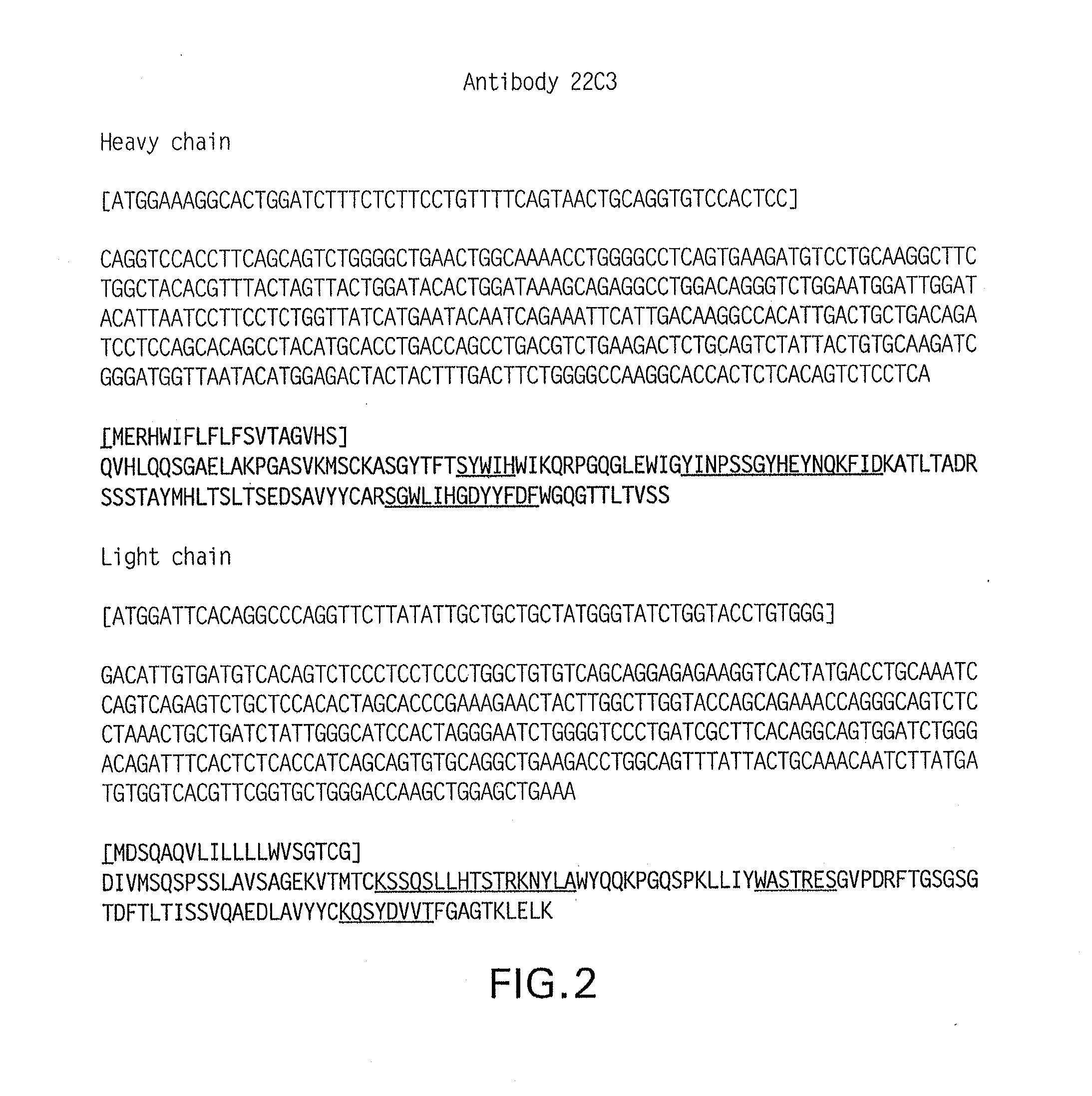
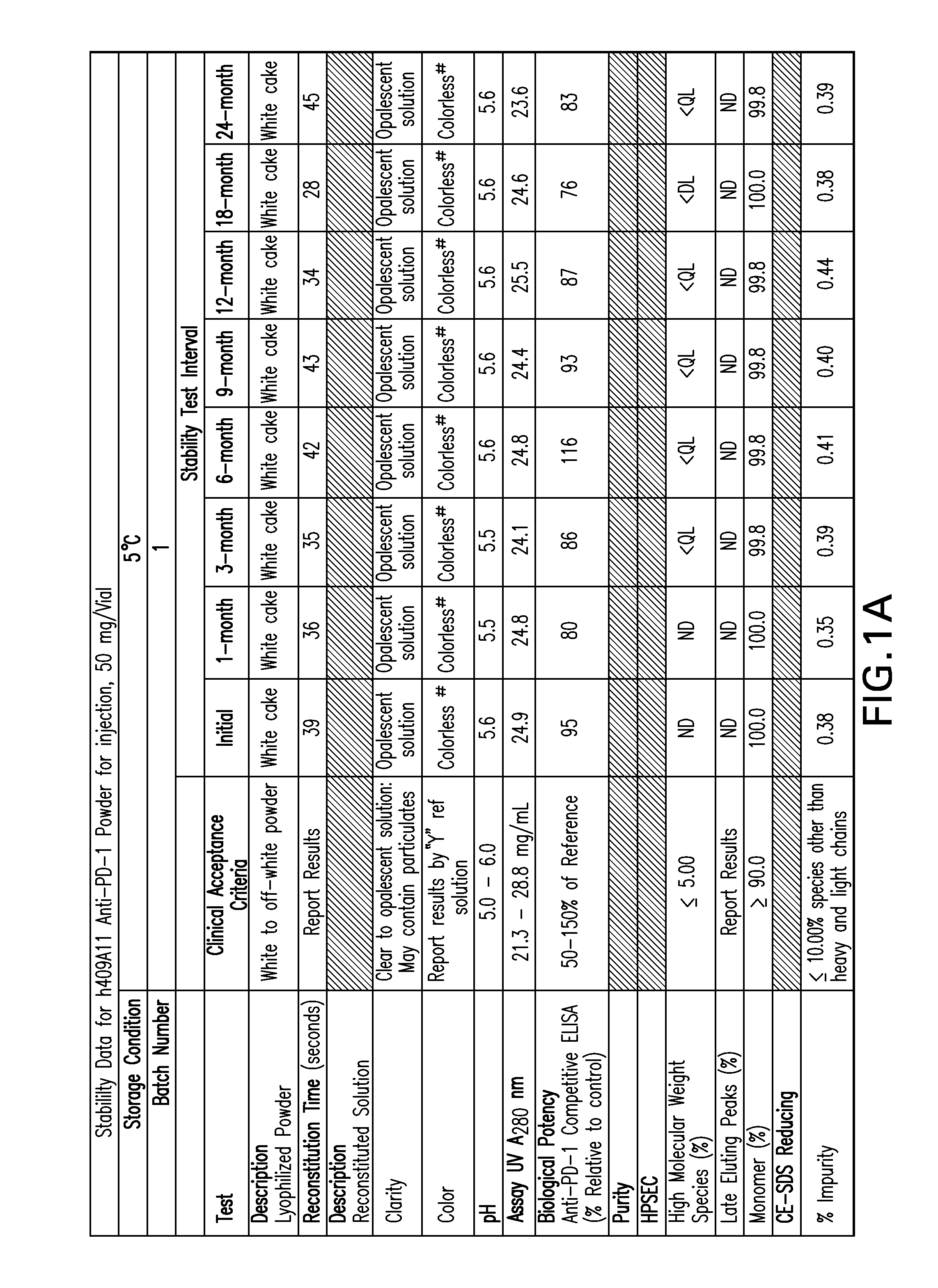
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
281 results about "Programmed death" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Programmed cell death. Programmed cell death (PCD) is the deliberate suicide of an unwanted cell in a multicellular organism. In contrast to necrosis, which is a form of cell death that results from acute tissue injury and provokes an inflammatory response, PCD is carried out in a regulated process that generally confers advantages...
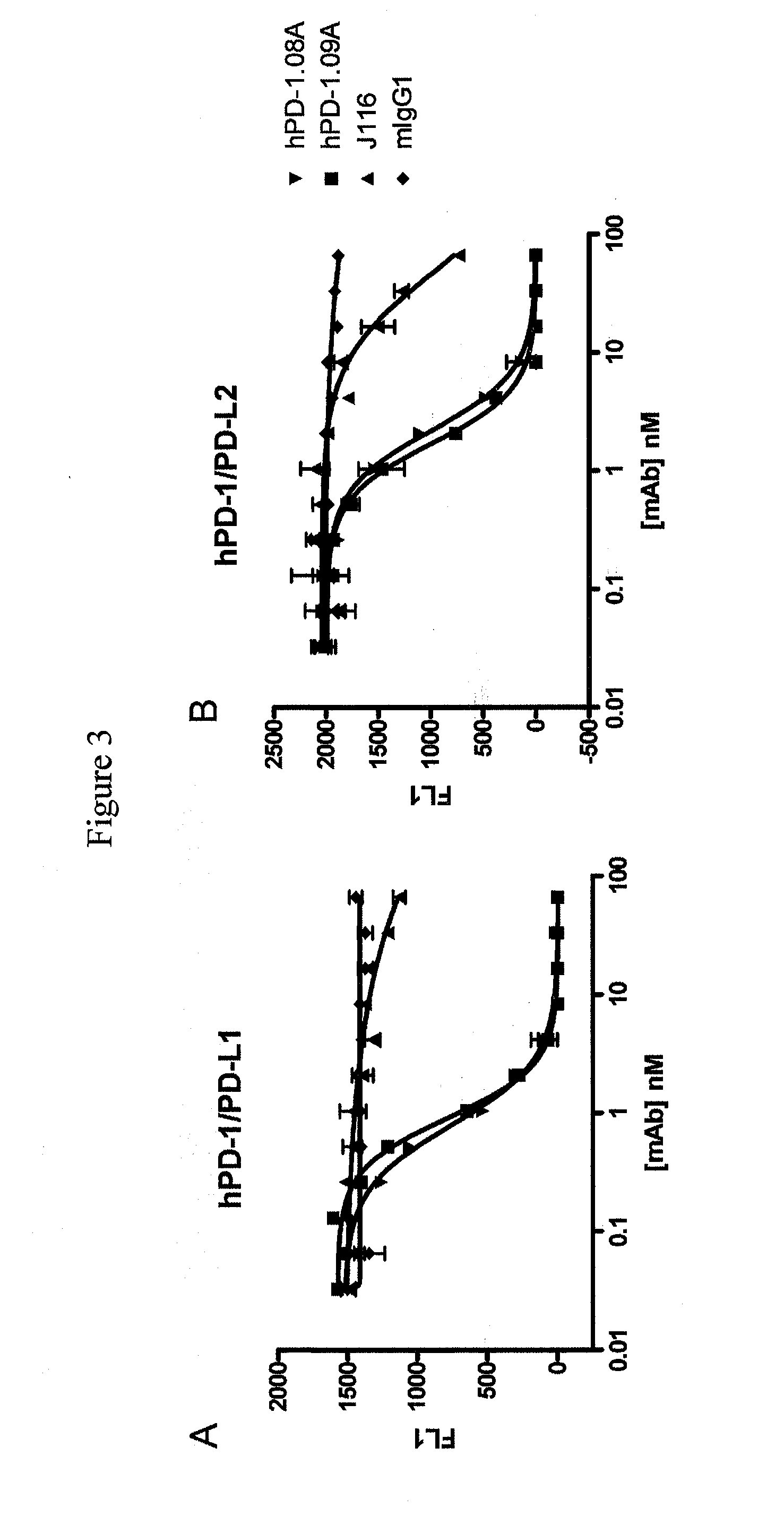
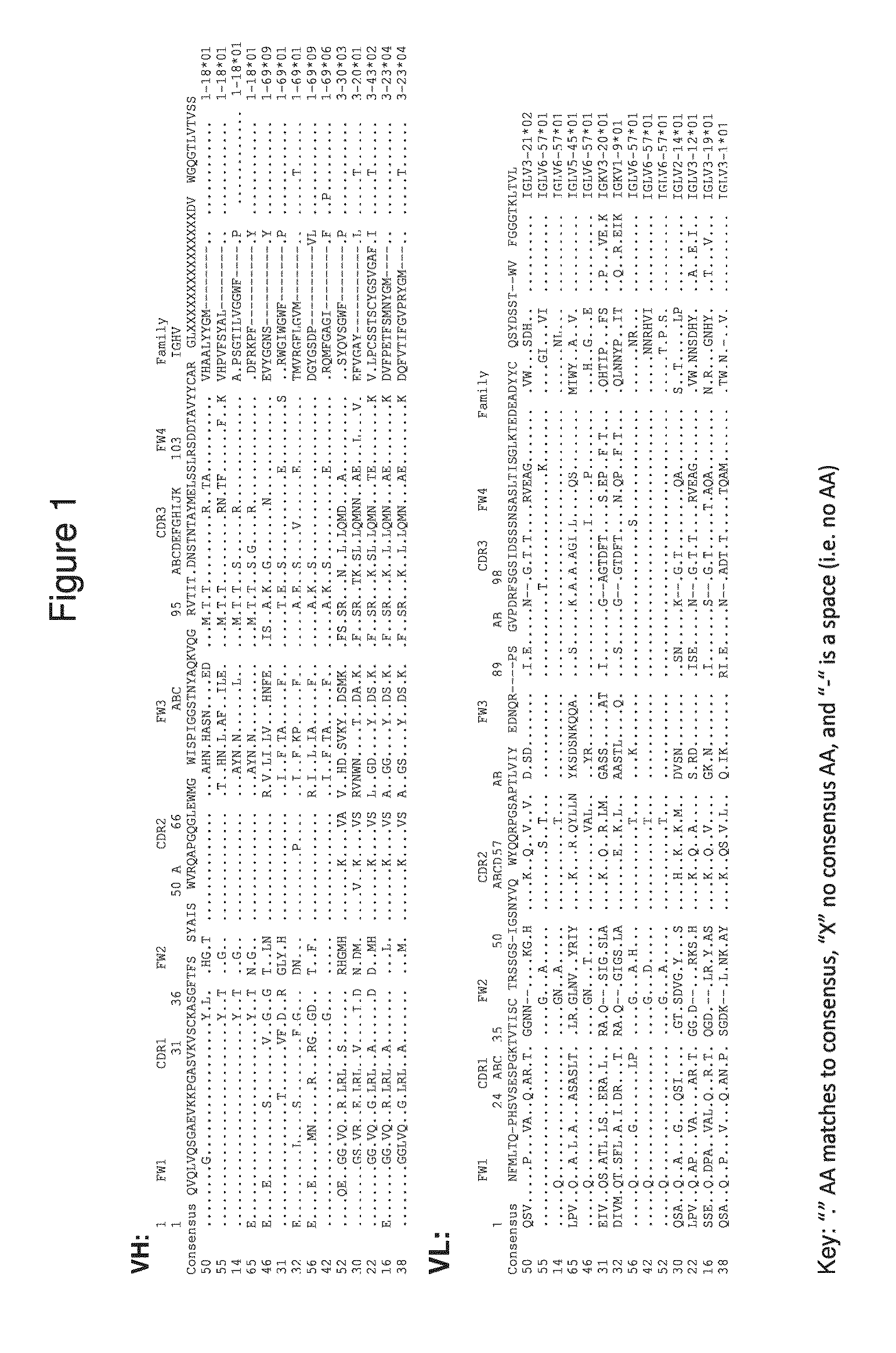
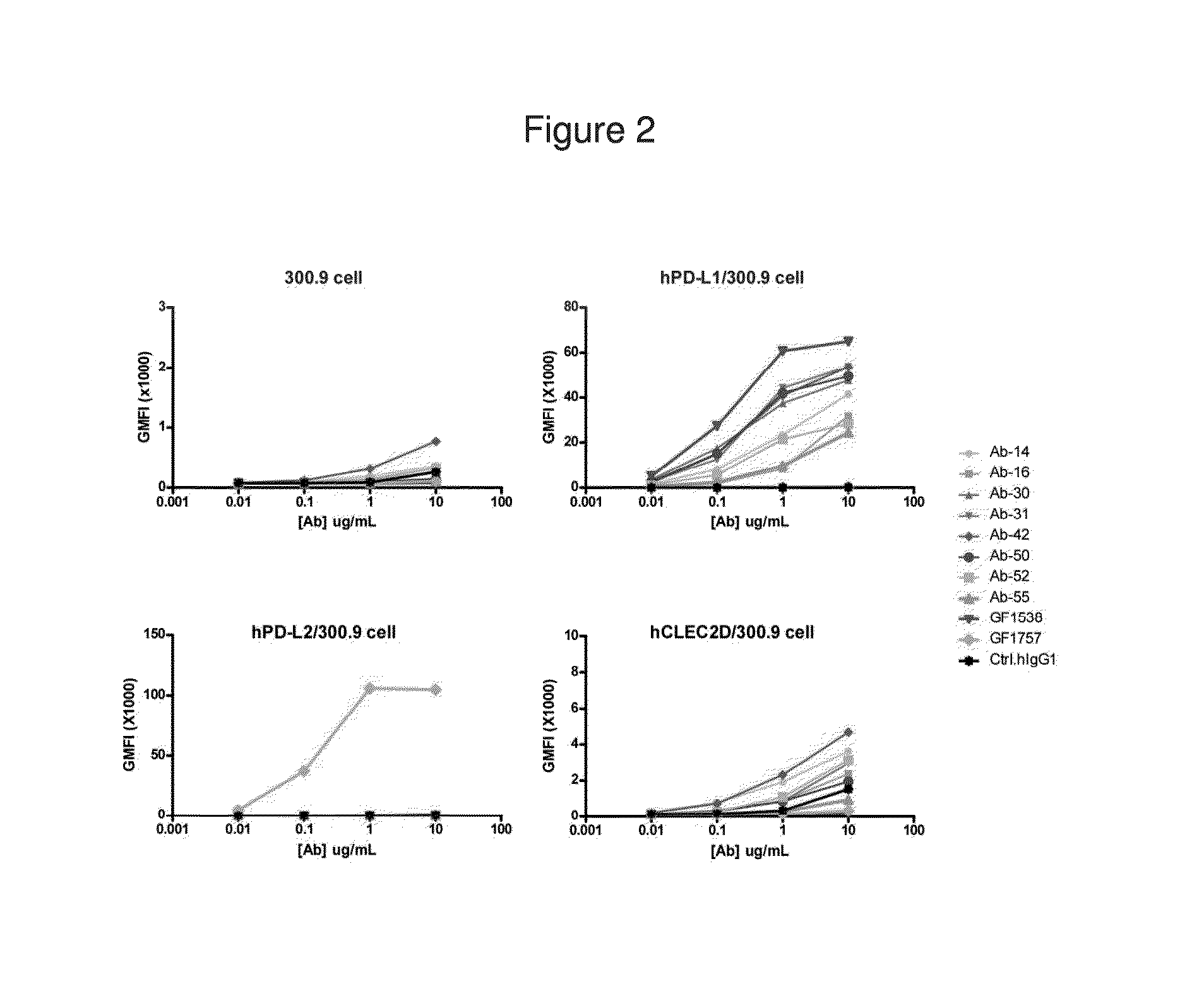
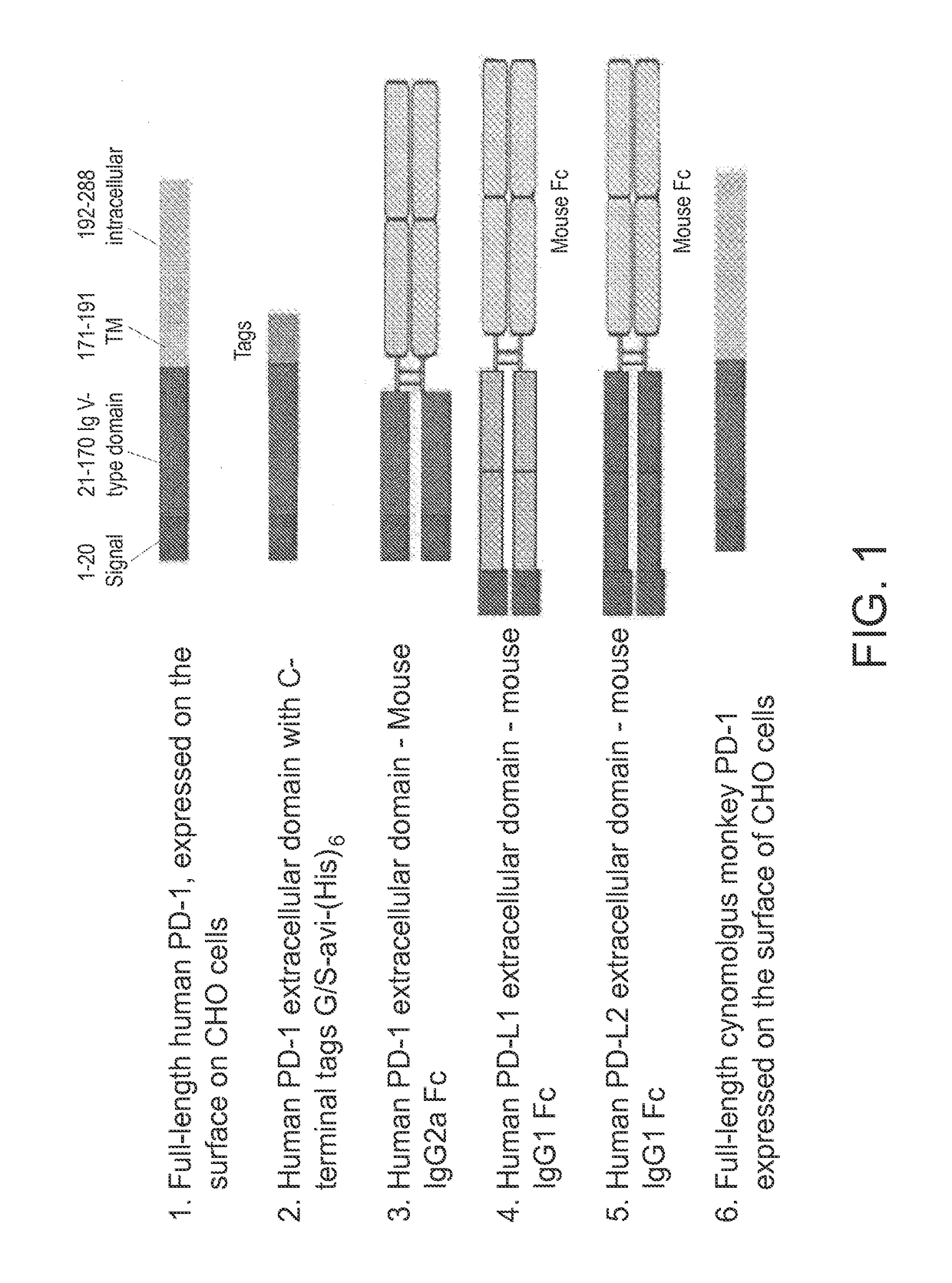
Antibodies to human programmed death receptor PD-1
ActiveUS8354509B2Increased activationIncreased proliferationSugar derivativesAntibody ingredientsProgrammed deathReceptor for activated C kinase 1
Antibodies which block the binding of human Programmed Death Receptor 1 (hPD-1) to its ligands (hPD-L1 or hPD-L2) and their variable region sequences are disclosed. A method of increasing the activity (or reducing downmodulation) of an immune response through the PD-I pathway is also disclosed.
Owner:MERCK SHARP & DOHME BV
Anti-PD1 antibodies and their use as therapeutics and diagnostics
ActiveUS8735553B1Inhibition of secretionNervous disorderImmunoglobulins against cell receptors/antigens/surface-determinantsProgrammed deathAnti pd1
Owner:BEIGENE SWITZERLAND GMBH
Anti-PD1 Antibodies and their Use as Therapeutics and Diagnostics
ActiveUS20150079109A1Improve the level ofHigh expressionNervous disorderAntiviralsAntiendomysial antibodiesInfectious Disorder
Provided are antibodies that specifically bind to Programmed Death-1 (PD1, Pdcd-1, or CD279) and inhibit PD1-mediated cellular signaling and activities in immune cells, antibodies binding to a set of amino acid residues required for its ligand binding, and uses of these antibodies to treat or diagnose cancer, infectious diseases or other pathological disorders modulated by PD1-mediated functions.
Owner:BEIGENE SWITZERLAND GMBH
Antibodies to human programmed death receptor pd-1
ActiveUS20100266617A1Increased activationIncreased proliferationSugar derivativesImmunoglobulins against animals/humansProgrammed deathAntibody
Antibodies which block the binding of human Programmed Death Receptor 1(hPD-1) to its ligands (hPD-L1 or hPD-L2) and their variable region sequences are disclosed. A method of increasing the activity (or reducing downmodulation) of an immune response through the PD-I pathway is also disclosed.
Owner:MERCK SHARP & DOHME BV
Human monoclonal antibodies to programmed death 1 (PD-1) and methods for treating cancer using anti-PD-1 antibodies alone or in combination with other immunotherapeutics
ActiveCN101213297AMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsImmunotherapeutic agentProgrammed death
The present invention provides isolated monoclonal antibodies, particularly human monoclonal antibodies, that specifically bind to PD-1 with high affinity. Nucleic acid molecules encoding the antibodies of the invention, expression vectors, host cells and methods for expressing the antibodies of the invention are also provided. Immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies of the invention are also provided. The invention also provides methods for detecting PD-1, as well as methods for treating various diseases, including cancer and infectious diseases, using anti-PD-1 antibodies. The present invention further provides methods for using a combination immunotherapy, such as the combination of anti-CTLA-4 and anti-PD-1 antibodies, to treat hyperproliferative disease, such as cancer. The invention also provides methods for altering adverse events related to treatment with such antibodies individually.
Owner:ONO PHARMA CO LTD +1
Antibodies to human programmed death receptor pd-1
ActiveUS20130109843A1Increased activationIncreased proliferationImmunoglobulins against cell receptors/antigens/surface-determinantsDisease diagnosisProgrammed deathAntibody
Antibodies which block binding of hPD-1 to hPD-L1 or hPD-L2 and their variable region sequences are disclosed. A method of increasing the activity (or reducing downmodulation) of an immune cell through the PD-1 pathway is also disclosed.
Owner:MERCK SHARP & DOHME BV
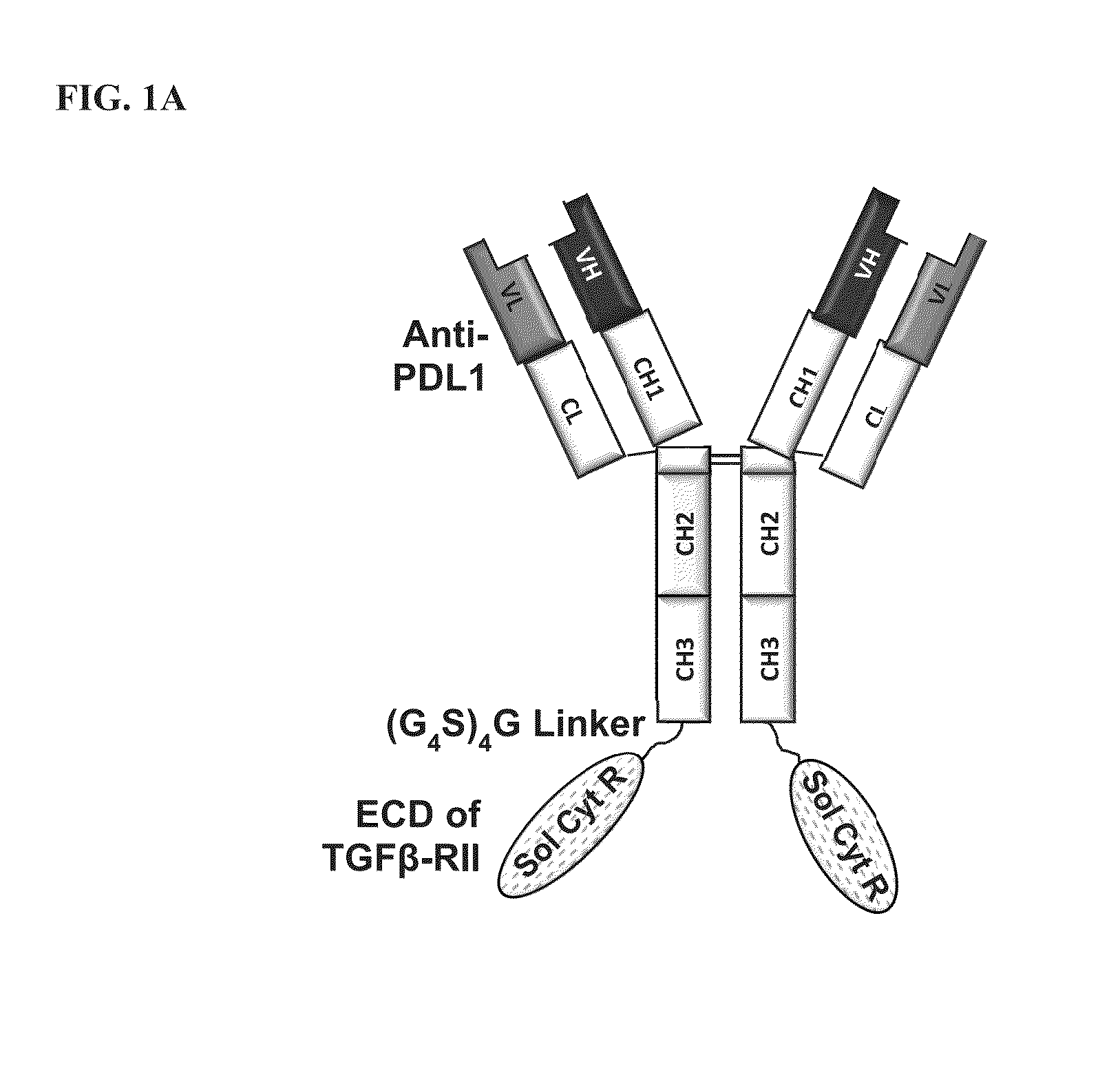
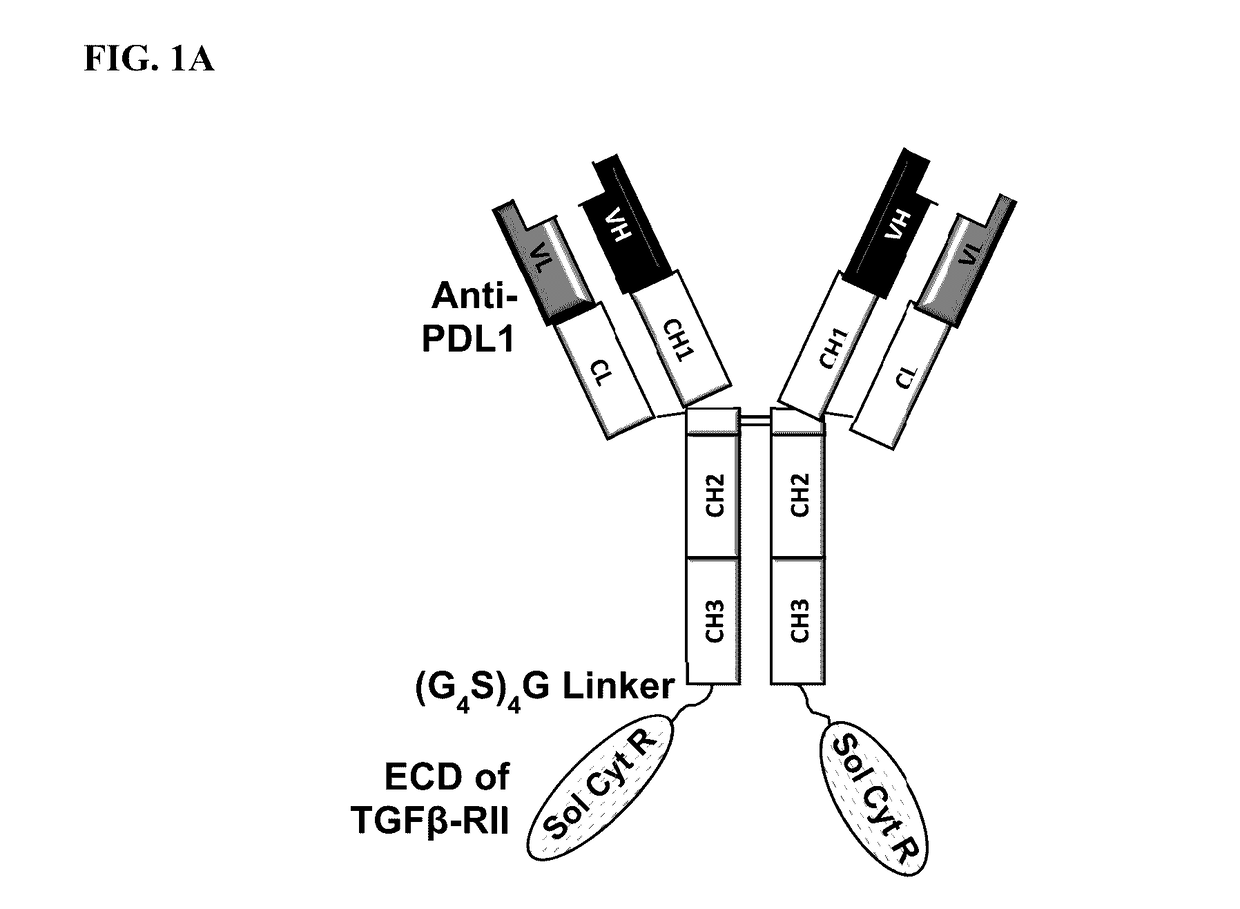
Targeted tgfß inhibition
ActiveUS20150225483A1Effective therapyPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigenAntigen Binding Fragment
This invention relates generally to bifunctional molecules including (a) a TGFβRII or fragment thereof capable of binding TGFβ and (b) an antibody, or antigen binding fragment thereof, that binds to an immune checkpoint protein, such as Programmed Death Ligand 1 (PD-L1), uses of such molecules (e.g., for treating cancer), and methods of making such molecules.
Owner:MERCK PATENT GMBH
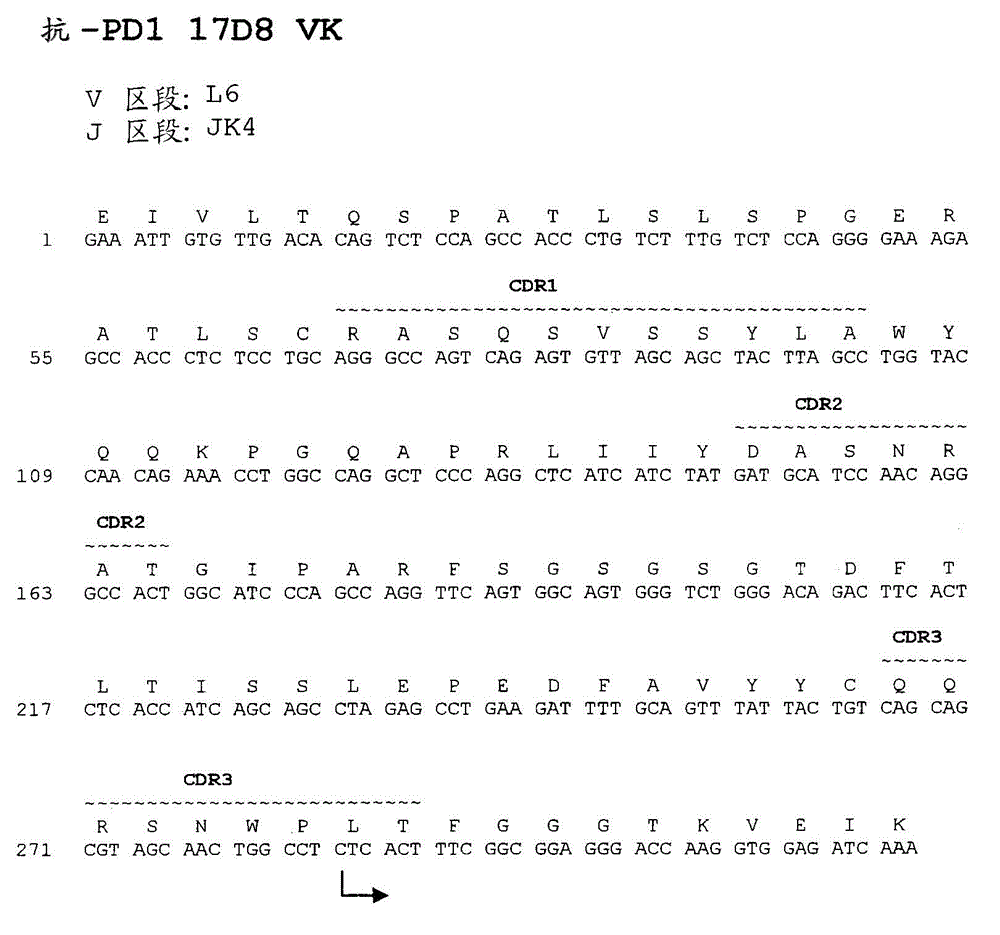
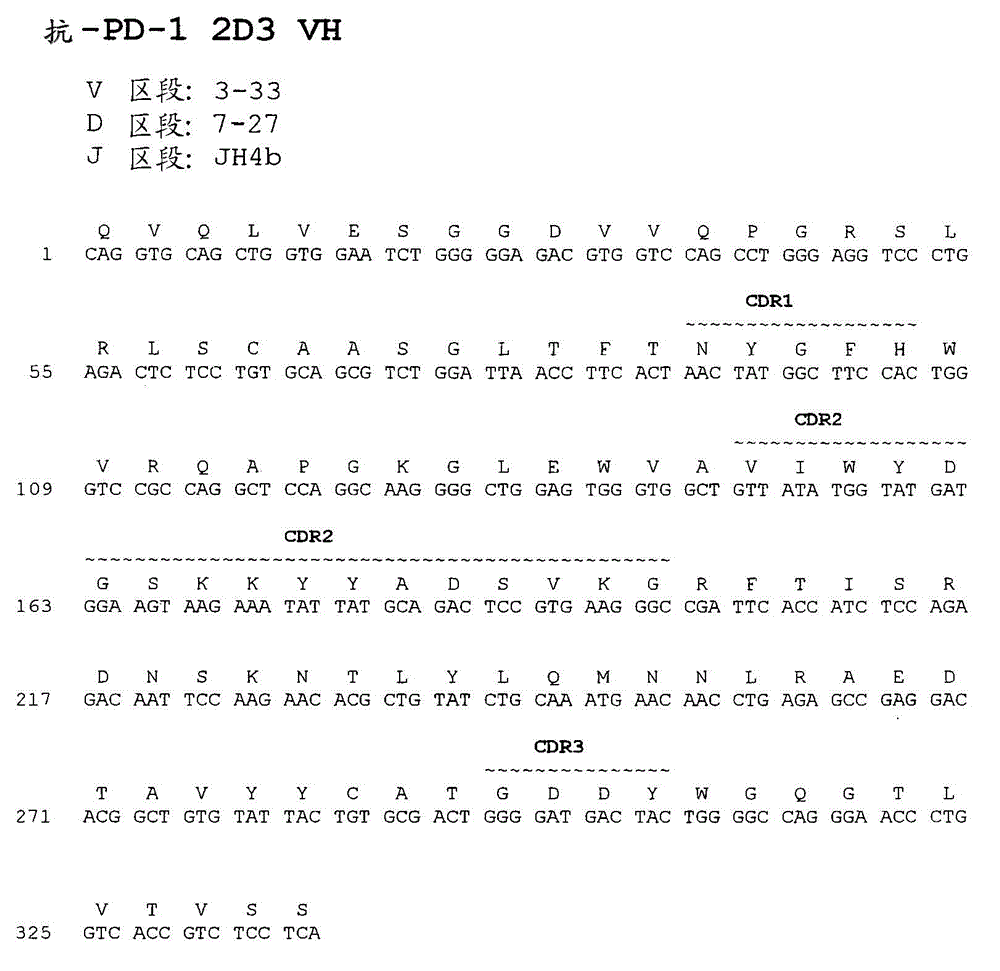
Antibodies directed against programmed death-1 (pd-1)
The invention relates to an isolated immunoglobulin heavy chain polypeptide and an isolated immunoglobulin light chain polypeptide that bind to a programmed death-1 (PD-1) protein. The invention provides a PD-1-binding agent that comprises the aforementioned immunoglobulin heavy chain polypeptide and immunoglobulin light chain polypeptide. The invention also provides related vectors, compositions, and methods of using the PD-1-binding agent to treat a cancer or an infectious disease.
Owner:ANAPTYSBIO INC
Targeted TGFβ inhibitors
ActiveUS9676863B2Effective therapyNervous disorderPeptide/protein ingredientsAntigenAntigen Binding Fragment
This invention relates generally to bifunctional molecules including (a) a TGFβRII or fragment thereof capable of binding TGFβ and (b) an antibody, or antigen binding fragment thereof, that binds to an immune checkpoint protein, such as Programmed Death Ligand 1 (PD-L1), uses of such molecules (e.g., for treating cancer), and methods of making such molecules.
Owner:MERCK PATENT GMBH
Antibodies that bind to human programmed death ligand 1 (pd-l1)
The present disclosure provides isolated antibodies that specifically bind to human PD-L1, as well as antigen binding fragments of such antibodies, and kits comprising the anti-PD-L1 antibodies or binding fragments and a set of reagents for detecting a complex of the antibody, or antigen binding fragment thereof, bound to human PD-L1. The antibodies and antigen binding fragments of this disclosure are useful for immunohistochemical detection of human PD-L1 expression in tissue samples. Nucleic acid molecules encoding the antibodies and antigen binding fragments of this disclosure, as well as expression vectors and host cells for expression thereof, are also provided.
Owner:MERCK SHARP & DOHME LLC
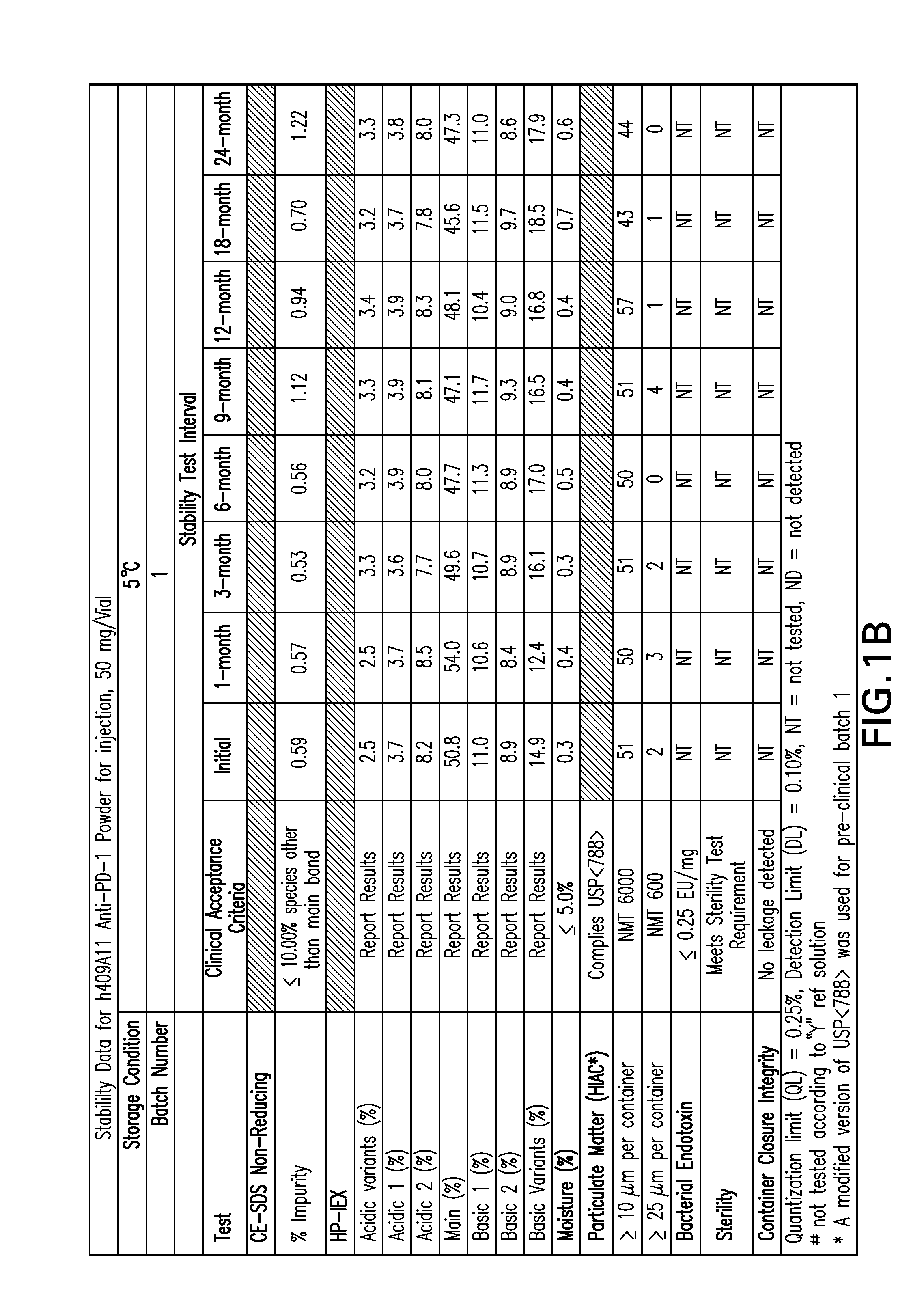
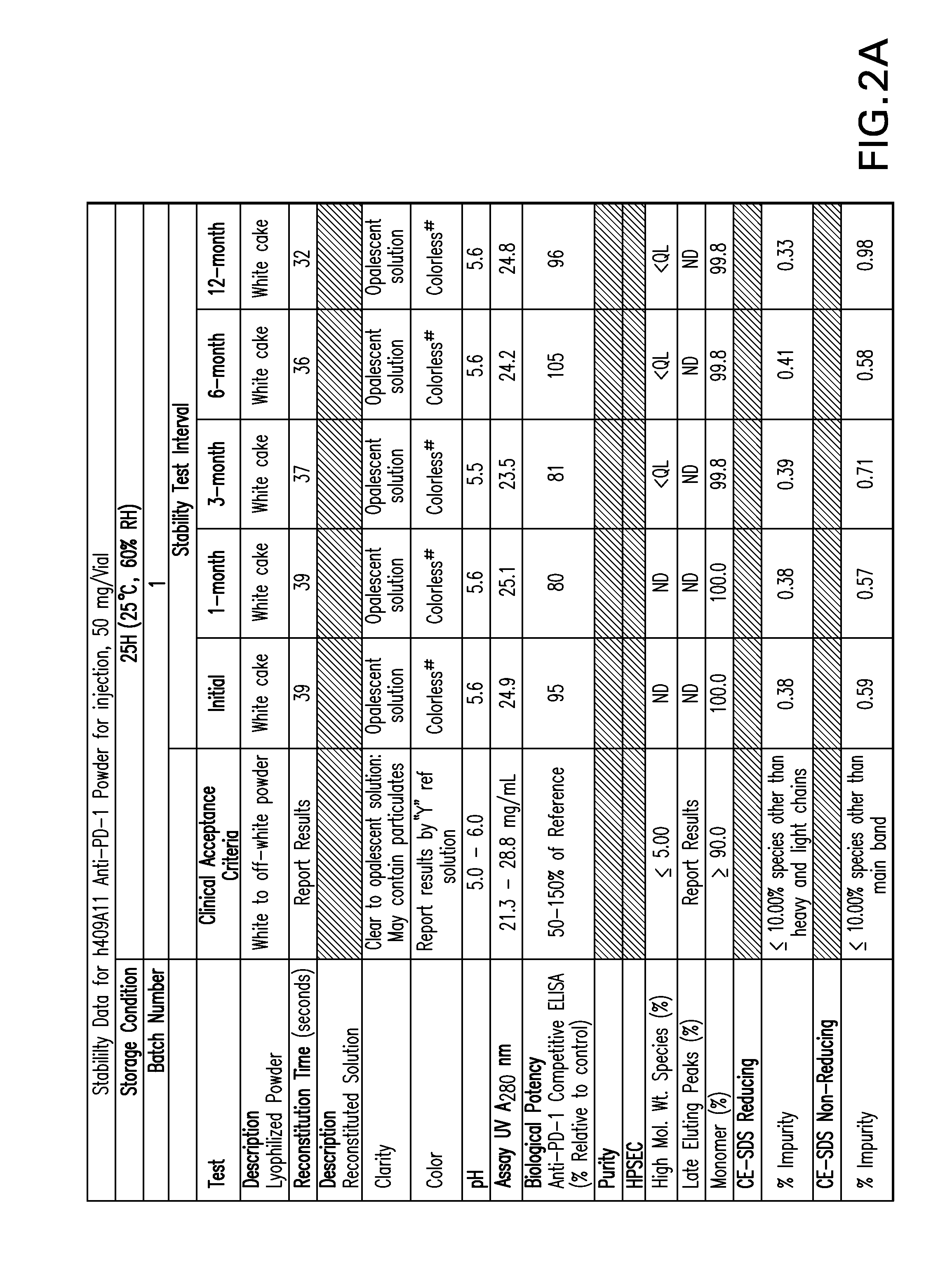
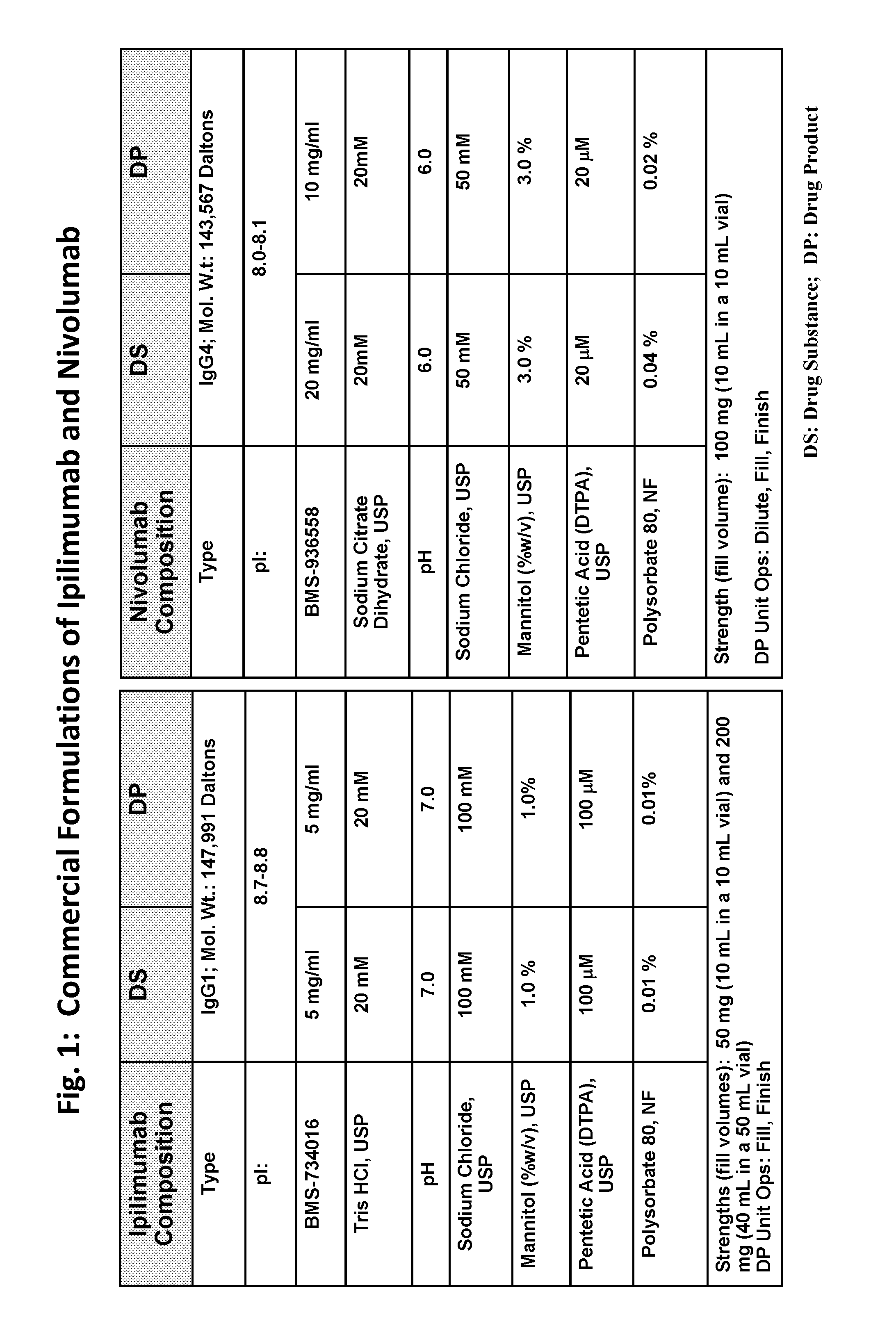
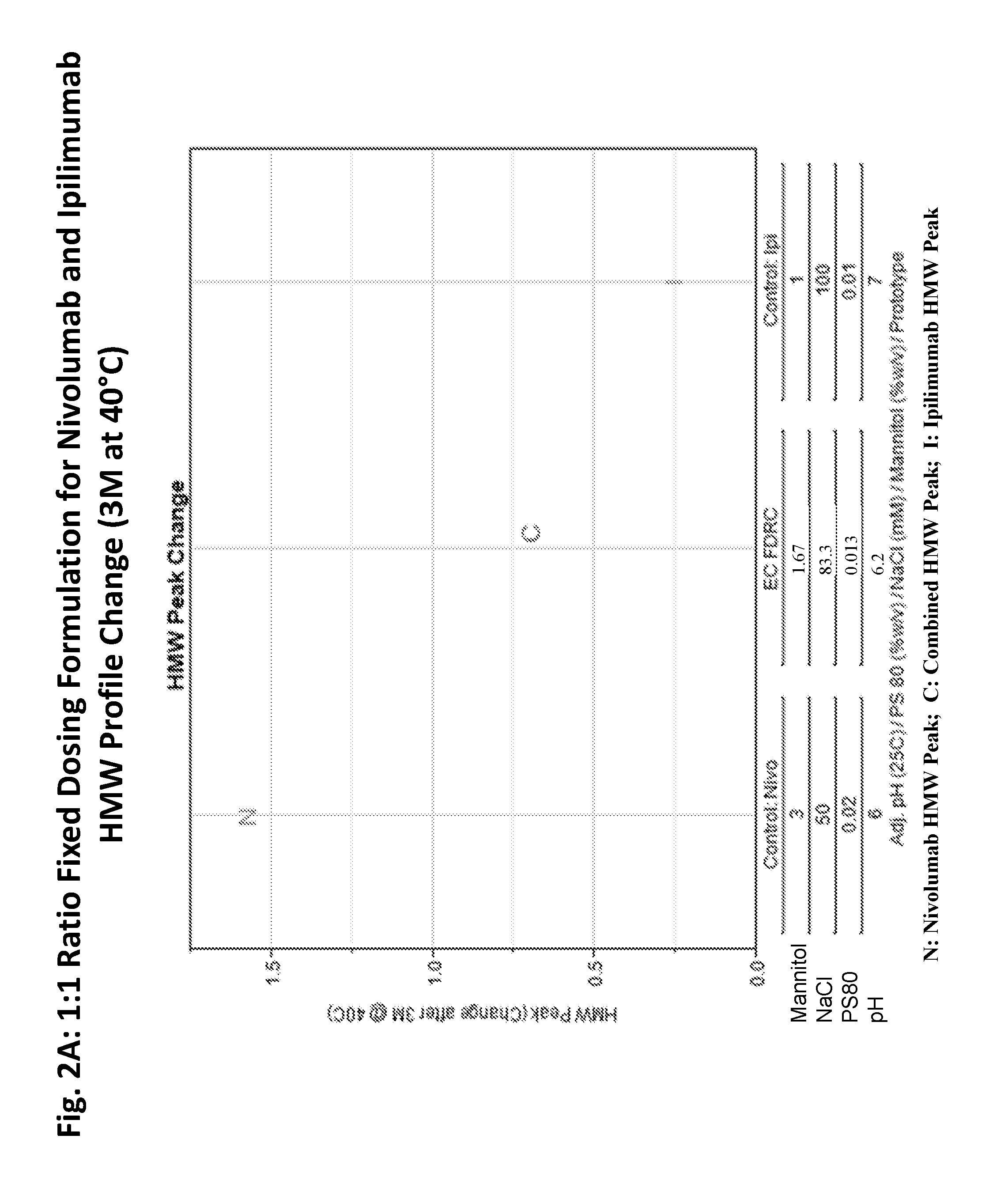
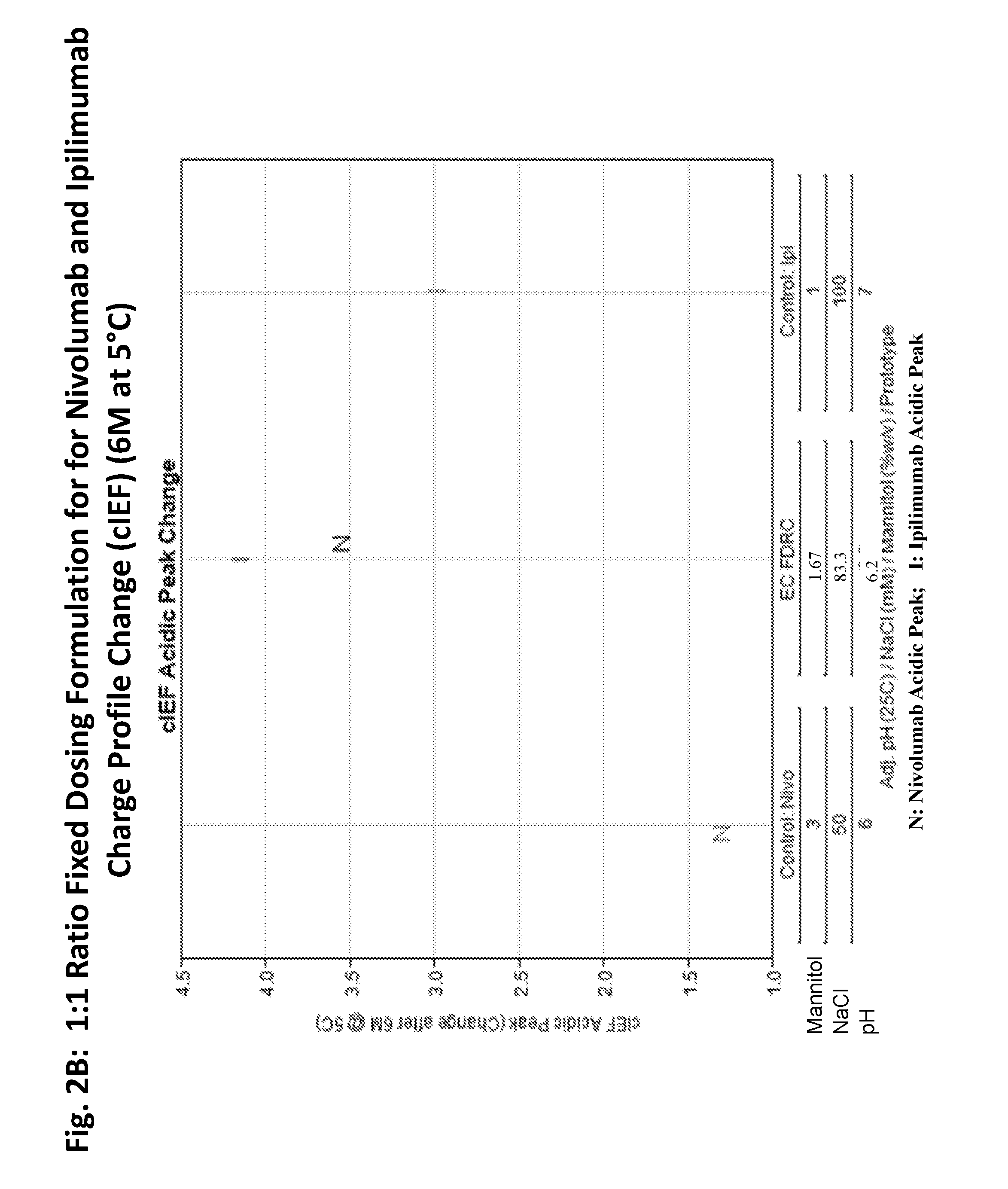
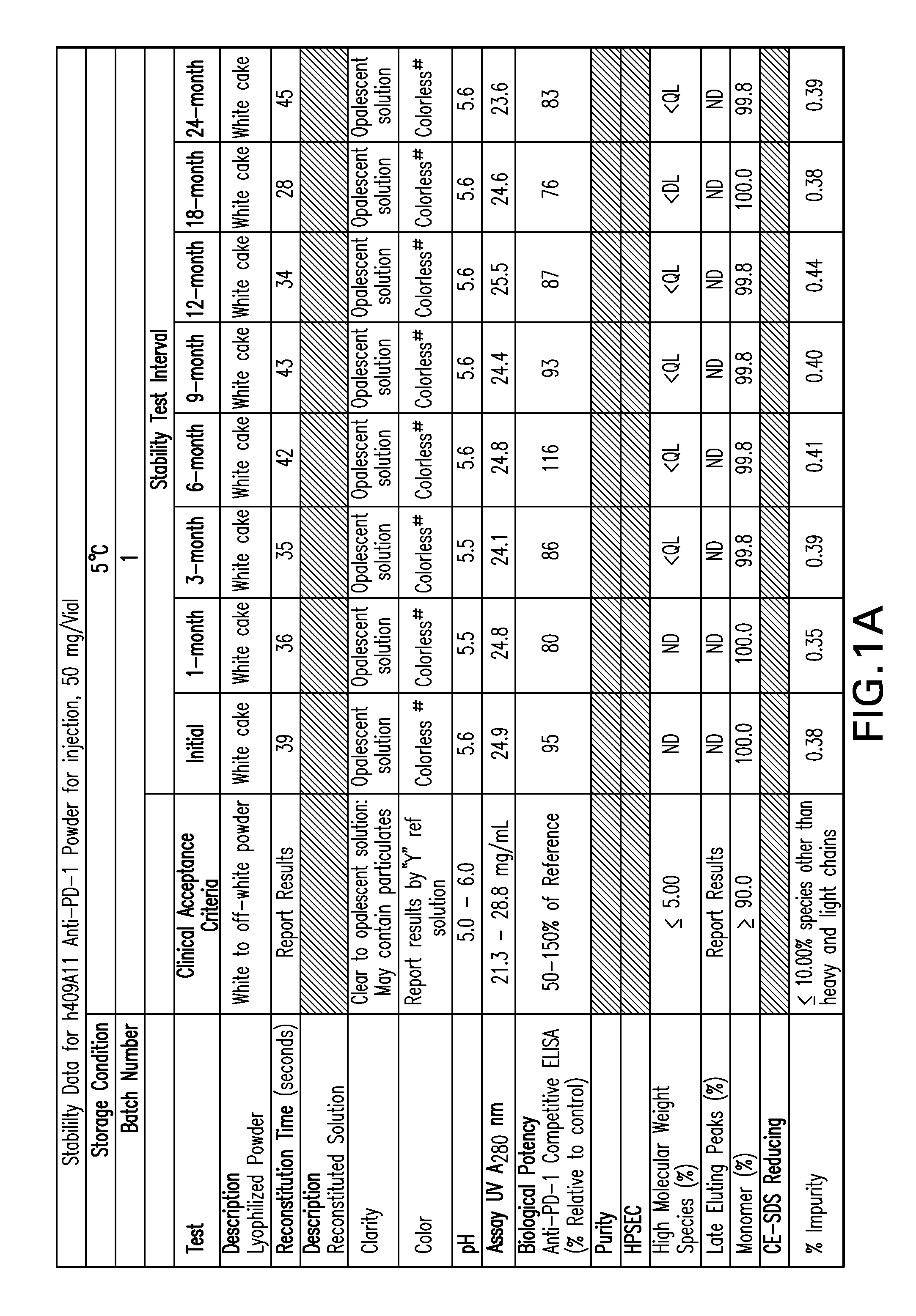
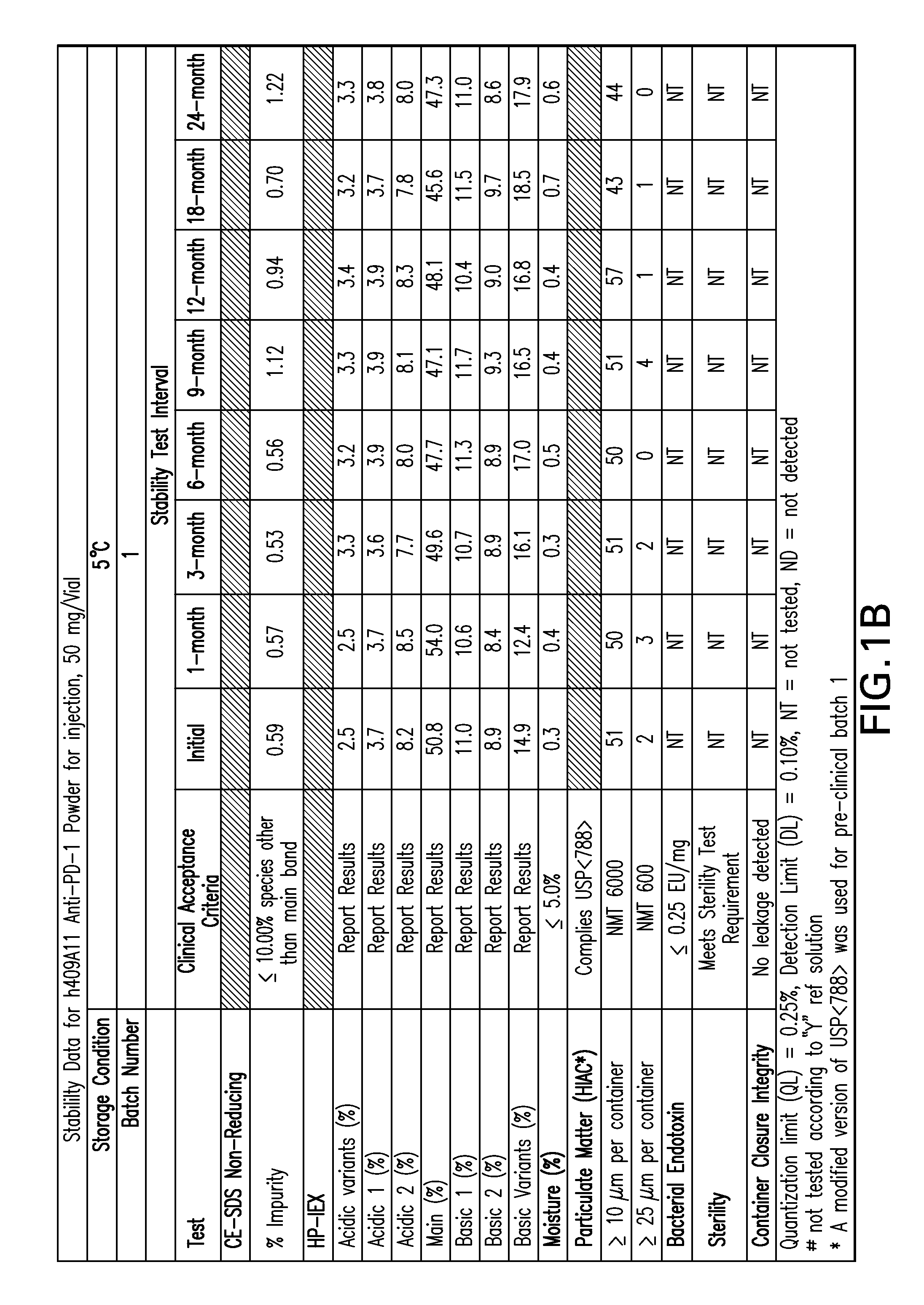
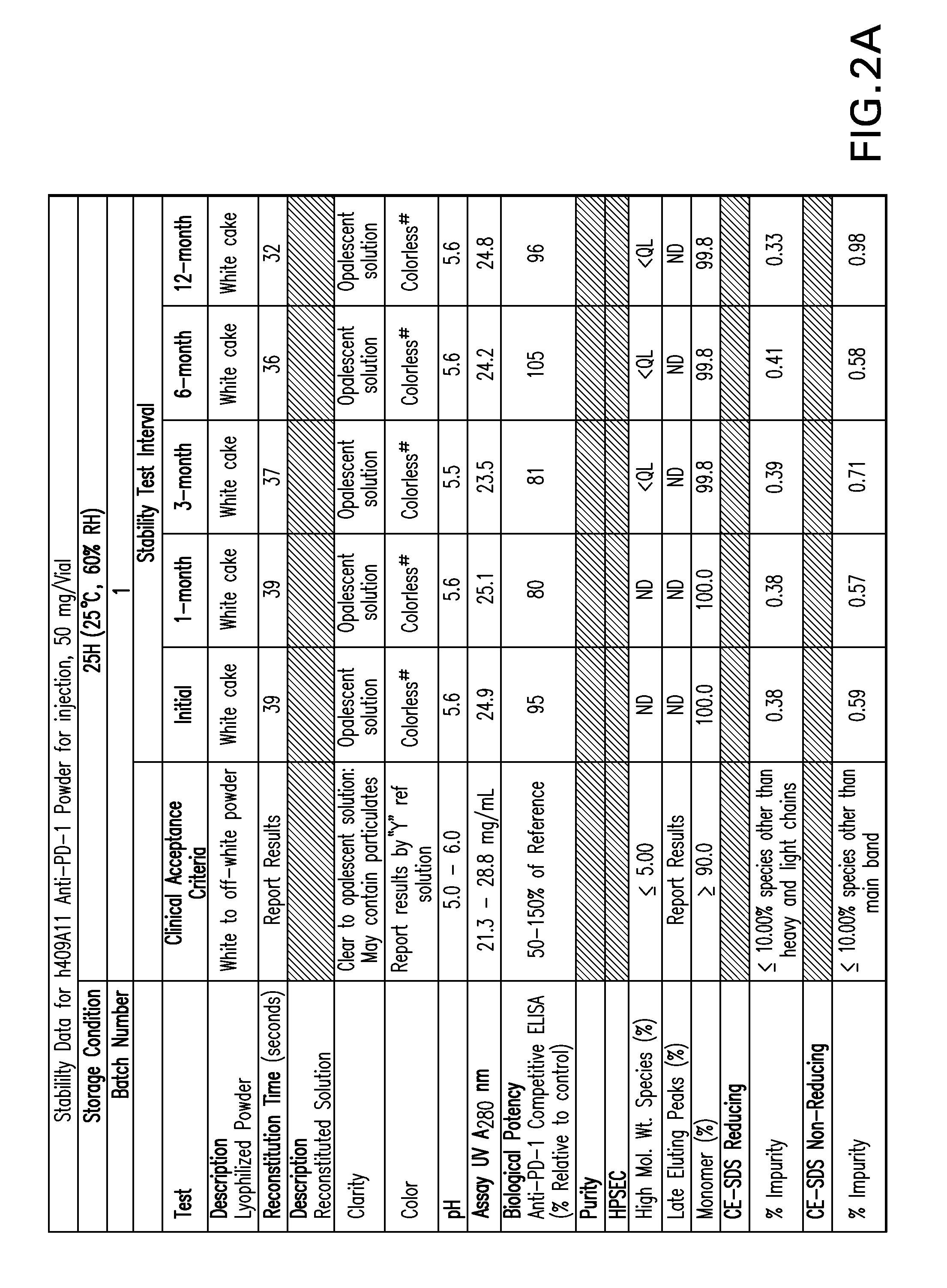
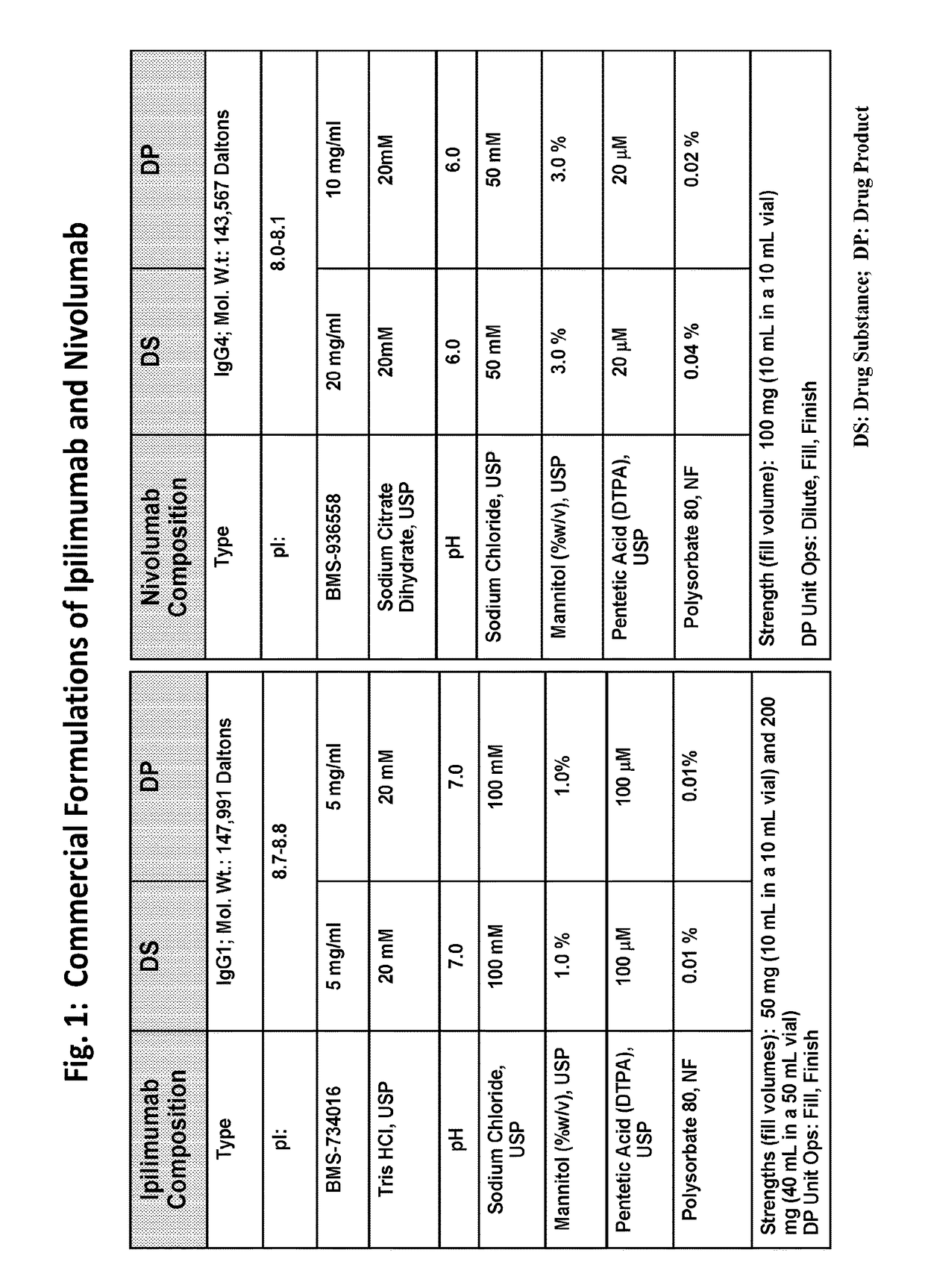
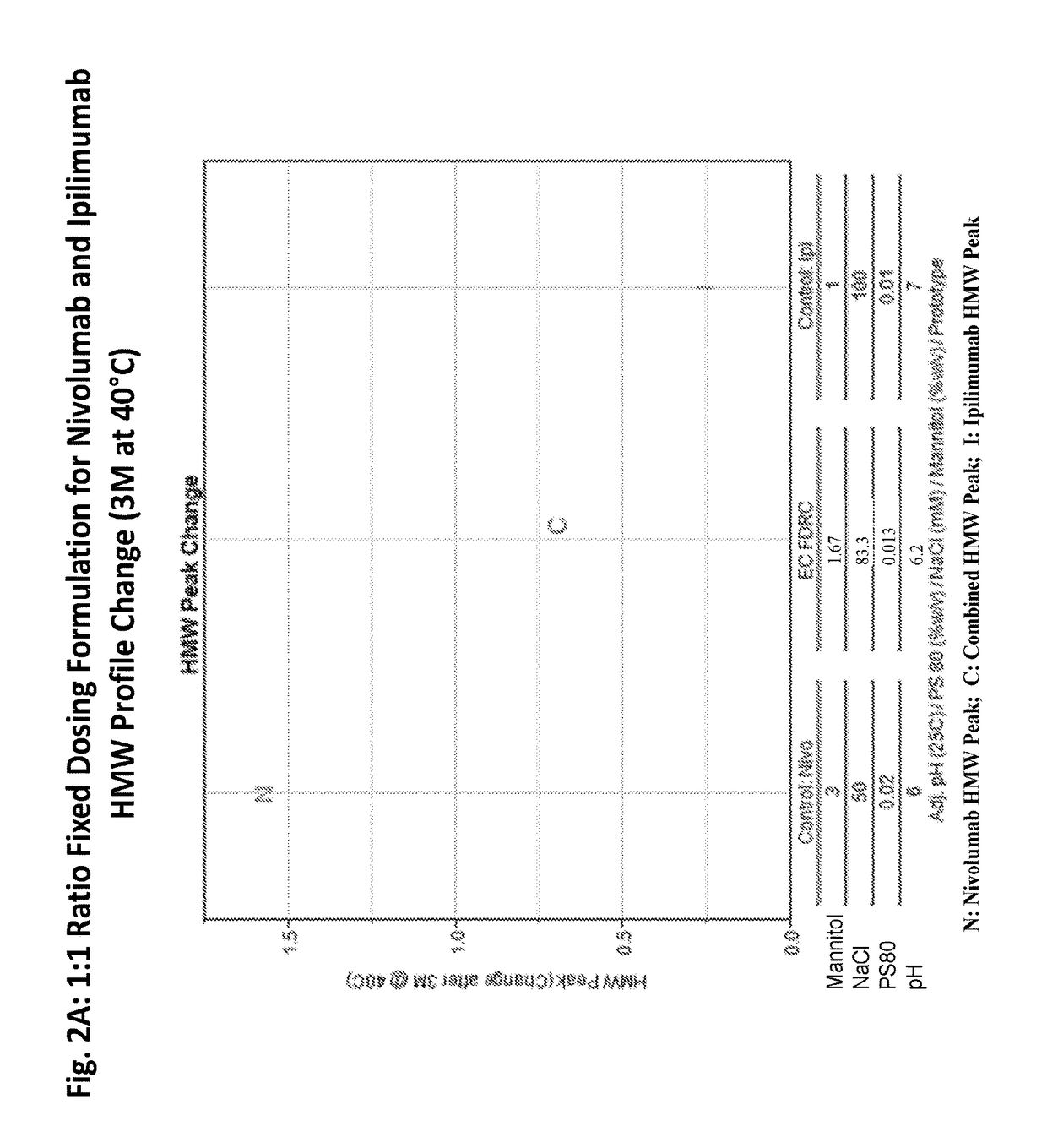
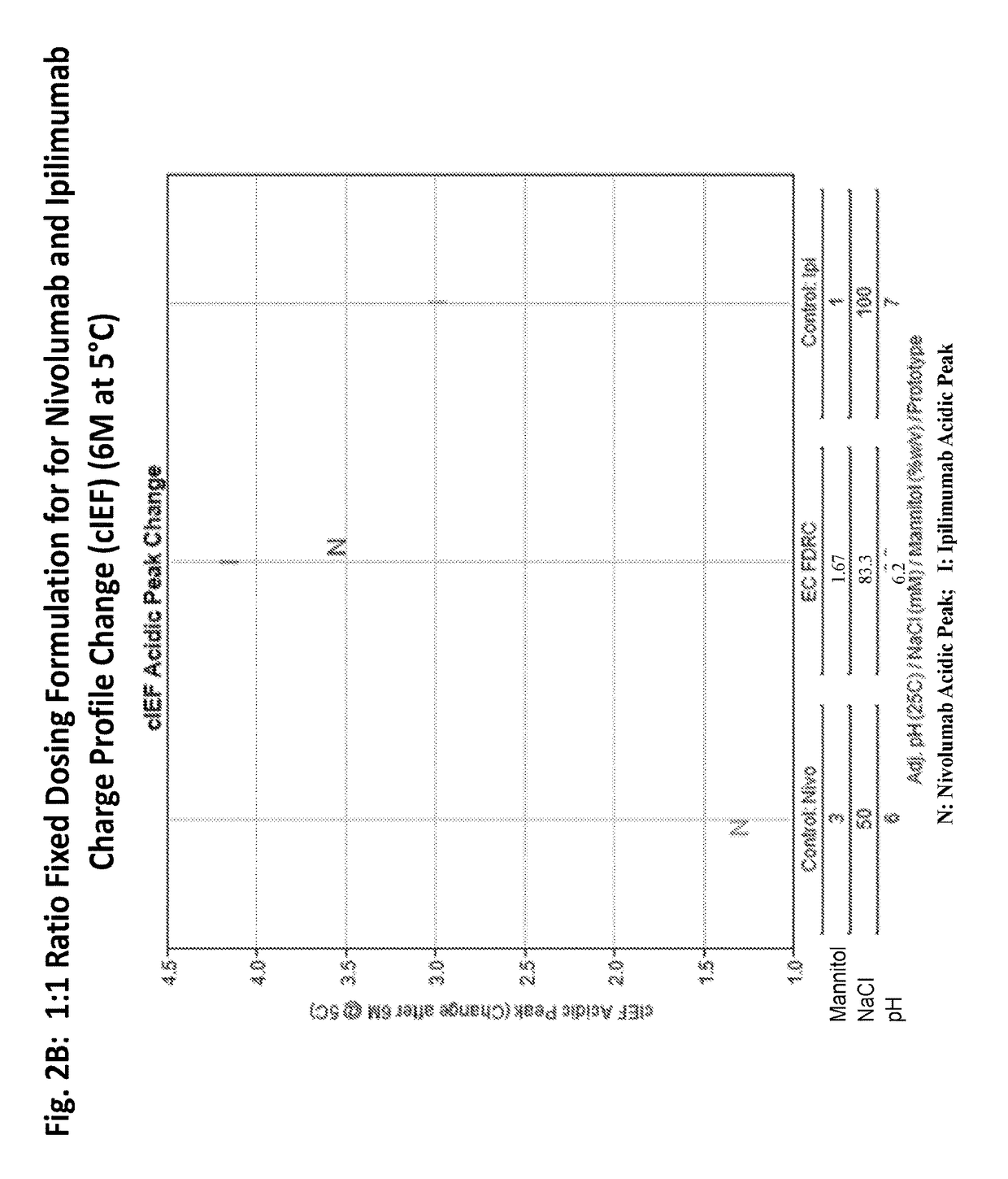
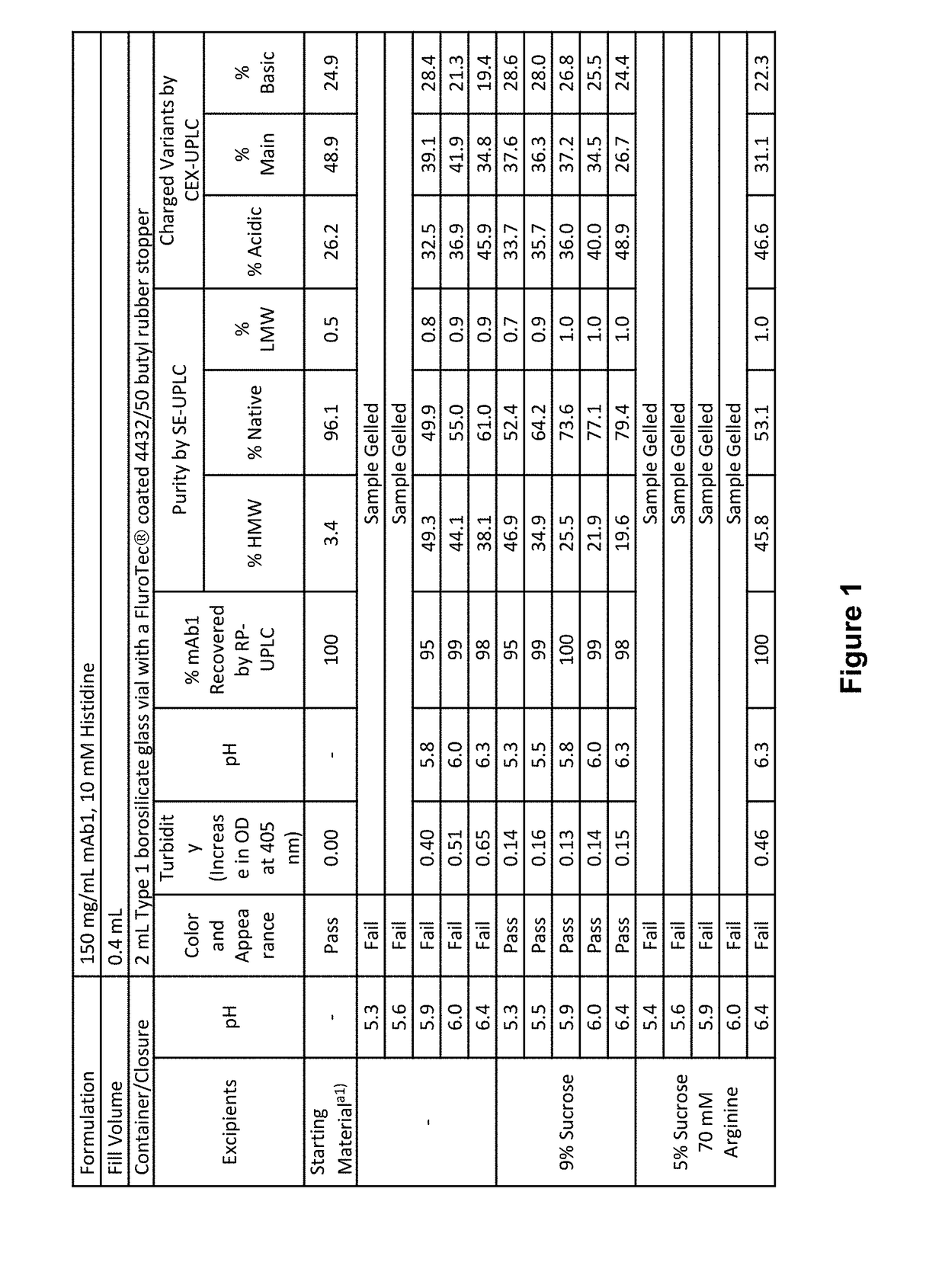
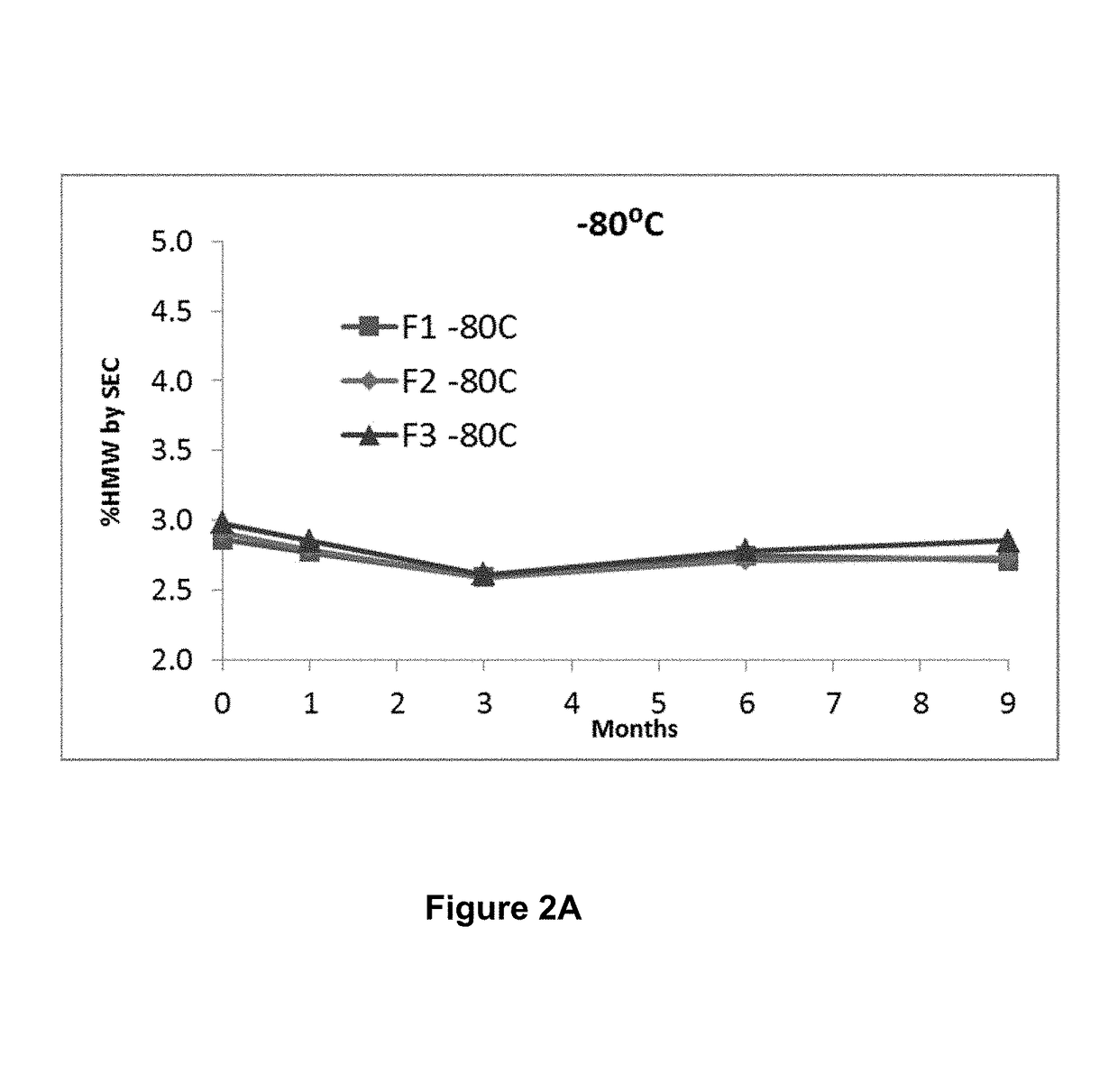
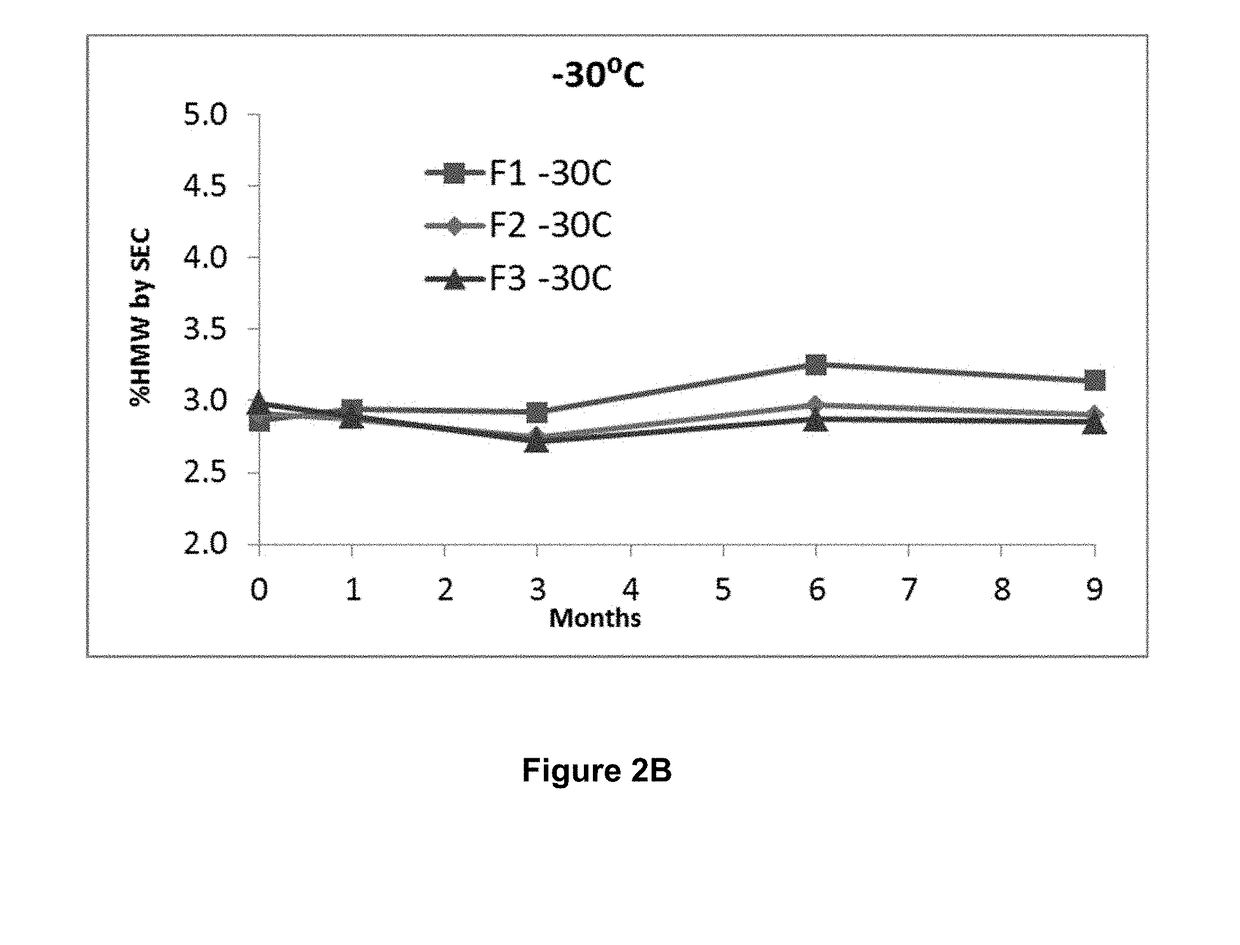
Stable formulations of antibodies to human programmed death receptor pd-1 and related treatments
ActiveUS20140234296A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntiviralsAntigen Binding FragmentProgrammed death
The present invention relates to stable formulations of antibodies against human programmed death receptor PD-1, or antigen binding fragments thereof. The present invention further provides methods for treating various cancers and chronic infections with stable formulations of antibodies against human programmed death receptor PD-1, or antigen binding fragments thereof.
Owner:MERCK SHARP & DOHME LLC
Human monoclonal Anti-pd-l1 antibodies and methods of use
ActiveUS20150274835A1Enhance immune responseIncrease in antigen specific T cell activityImmunoglobulins against cytokines/lymphokines/interferonsAntiviralsPD-L1Programmed death
The present invention comprises human monoclonal antibodies that bind to PD-L1 (also known as programmed death ligand 1 or B7H1). Binding of the invented antibody to PD-L1 inhibits binding to its receptor, PD1 (programmed death 1), and ligand-mediated activities and can be used to treat cancer and chronic viral infections.
Owner:DANA FARBER CANCER INST INC
Compositions comprising a combination of an Anti-pd-1 antibody and another antibody
InactiveUS20160304607A1Change is minimalSenses disorderNervous disorderAnticarcinogenProgrammed death
This provides pharmaceutical compositions that comprise a combination of an anti-cancer agent which is an first antibody and a second antibody. In some embodiments, the first antibody is an anti-Programmed Death-1 (PD-1) antibody. In certain embodiments, the composition is a fixed dose formulation. In certain embodiments, the composition is administered as a flat-dose. The disclosure also provides a kit for treating a subject afflicted with a disease, the kit comprising a dosage of any composition disclosed herein and instructions for using the composition in any of the disclosed methods for treating a disease.
Owner:BRISTOL MYERS SQUIBB CO
Antibodies against PD-1 and uses therefor
This disclosure provides antibodies and antigen-binding fragments that can act as agonists and / or antagonists of PD-1 (Programmed Death 1), thereby modulating immune responses in general, and those mediated by TcR and CD28, in particular. The disclosed compositions and methods may be used for example, in treating autoimmune diseases, inflammatory disorders, allergies, transplant rejection, cancer, and other immune system disorders.
Owner:WYETH LLC +1
Stable formulations of antibodies to human programmed death receptor PD-1 and related treatments
The present invention relates to stable formulations of antibodies against human programmed death receptor PD-1, or antigen binding fragments thereof. The present invention further provides methods for treating various cancers and chronic infections with stable formulations of antibodies against human programmed death receptor PD-1, or antigen binding fragments thereof.
Owner:MERCK SHARP & DOHME LLC
Human monoclonal antibodies to programmed death 1 (pd-1) and methods for treating cancer using anti-pd-1 antibodies alone or in combination with other immunotherapeutics
ActiveCN103059138ARadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesInfectious Disorder
The present invention provides isolated monoclonal antibodies, particularly human monoclonal antibodies, that specifically bind to PD-1 with high affinity. Nucleic acid molecules encoding the antibodies of the invention, expression vectors, host cells and methods for expressing the antibodies of the invention are also provided. Immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies of the invention are also provided. The invention also provides methods for detecting PD-1, as well as methods for treating various diseases, including cancer and infectious diseases, using anti-PD-1 antibodies. The present invention further provides methods for using a combination immunotherapy, such as the combination of anti-CTLA-4 and anti-PD-1 antibodies, to treat hyperproliferative disease, such as cancer. The invention also provides methods for altering adverse events related to treatment with such antibodies individually.
Owner:ONO PHARMA CO LTD +1
Treatment of lung cancer using a combination of an Anti-pd-1 antibody and another Anti-cancer agent
InactiveUS20170158776A1Durable clinical responseReliable responseHeavy metal active ingredientsImmunoglobulins against growth factorsAnticarcinogenTyrosine-kinase inhibitor
This disclosure provides a method for treating a subject afflicted with a lung cancer, which method comprises administering to the subject therapeutically effective amounts of: (a) an anti-cancer agent which is an antibody or an antigen-binding portion thereof that specifically binds to a Programmed Death-1 (PD-1) receptor and inhibits PD-1 activity; and (b) another anti-cancer agent. The other anti-cancer agent can be a platinum-based doublet chemotherapy, an EGFR-targeted tyrosine kinase inhibitor, bevacizumab, an anti-Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) antibody, or any other therapy used to treat lung cancer in the art or disclosed herein.
Owner:BRISTOL MYERS SQUIBB CO
Treatment of hodgkin's lymphoma using an Anti-pd-1 antibody
InactiveUS20160347836A1Inhibitory activityMicrobiological testing/measurementBiological material analysisAnticarcinogenCTLA4 Protein
This disclosure provides methods for treating Hodgkin's lymphoma in a subject comprising administering to the subject an anti-Programmed Death-1 (PD-1) antibody. In some embodiments, this invention relates to methods for treating Hodgkin's lymphoma in a subject comprising administering to the subject a combination of an anti-cancer agent which is an anti-Programmed Death-1 (PD-1) antibody and another anti-cancer agent such as an anti-Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) antibody.
Owner:BRISTOL MYERS SQUIBB CO
Monoclonal antibody with function of blocking human programmed death factors 1 (PD-1), encoding gene of monoclonal antibody and application thereof
ActiveCN105566496AHigh affinityImmunoglobulins against cell receptors/antigens/surface-determinantsAntiviralsAbnormal tissue growthAntigen
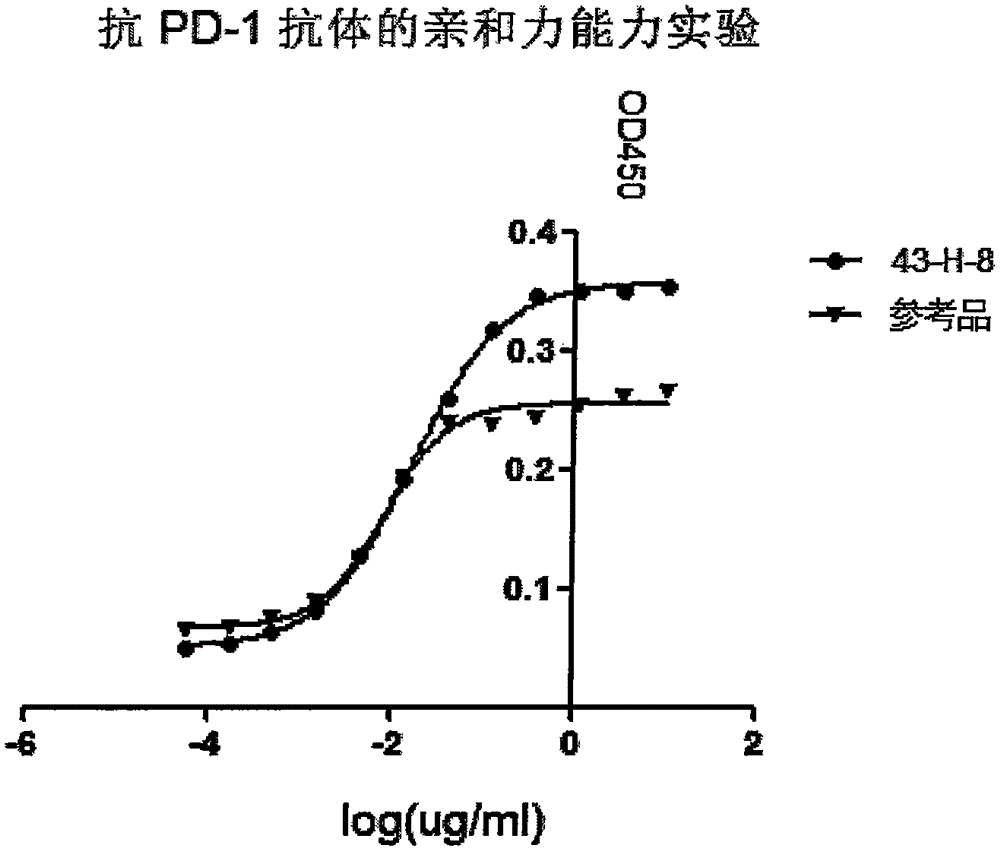
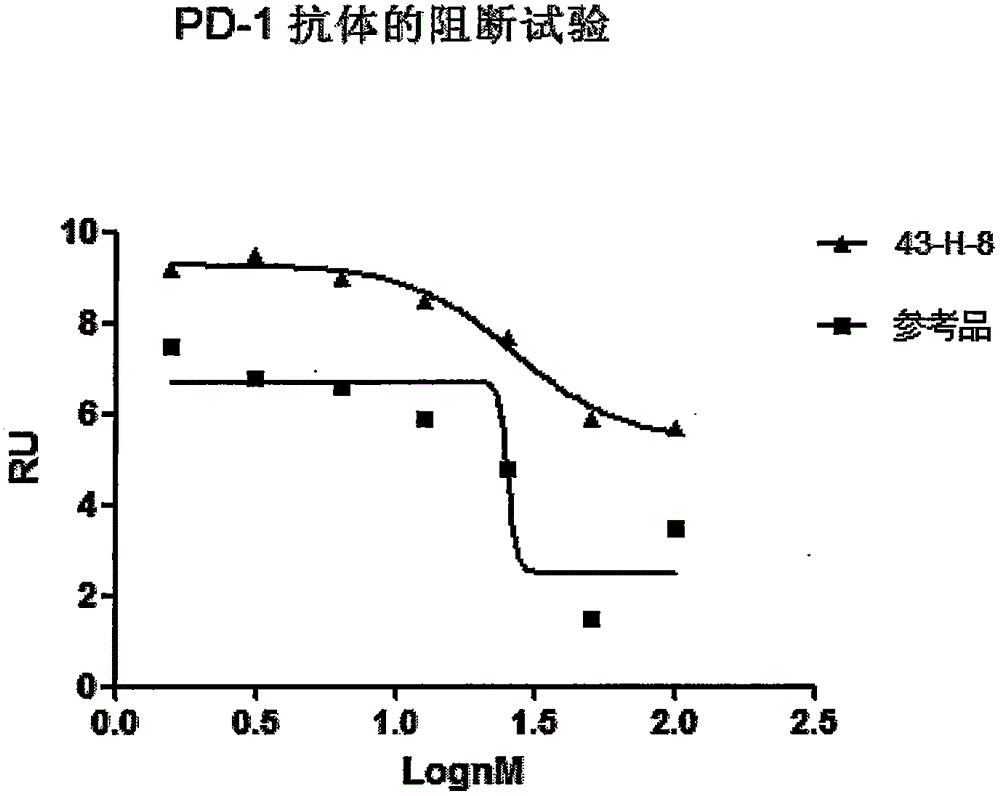
The invention discloses a monoclonal antibody with a function of blocking human programmed death factors 1 (PD-1), an encoding gene of the monoclonal antibody and application thereof. The monoclonal antibody comprises heavy chains and light chains. Amino acid sequences of the heavy chains are shown as 20 bits to 132 bits of SEQ ID NO.3, and amino acid sequences of the light chains are shown as 20 bits to 130 bits of SEQ ID NO.4. The monoclonal antibody 43-H-8 with the function of blocking the human programmed death factors PD-1, the encoding gene of the monoclonal antibody and the application have the advantages that the monoclonal antibody 43-H-8 can be specifically bound with human PD-1 antigens, the medium effective concentration EC50 (50% effective concentration) is 1.0665nM, accordingly, PD-1 / PD-L suppression signals can be specifically blocked, the monoclonal antibody 43-H-8 can be used as a blocker for PD-1 passages, and a novel medicine can be provided for tumor immunotherapy and treatment on chronic virus infectious diseases and autoimmune diseases.
Owner:大庆东竺明生物技术有限公司
Disruption of programmed death 1 (pd-1) ligand to adjuvant adeno-associated virus vector vaccines
InactiveUS20100035973A1Enhance immune responseImprove efficiencyPeptide/protein ingredientsGenetic material ingredientsVector vaccineProgrammed death
The invention provides for methods of modulating an immune response against a therapeutic polypeptide or an antigenic polypeptide delivered via rAAV comprising administering a modulator of programmed death-1 (PD-1) signaling.
Owner:NATIONWIDE CHILDRENS HOSPITAL
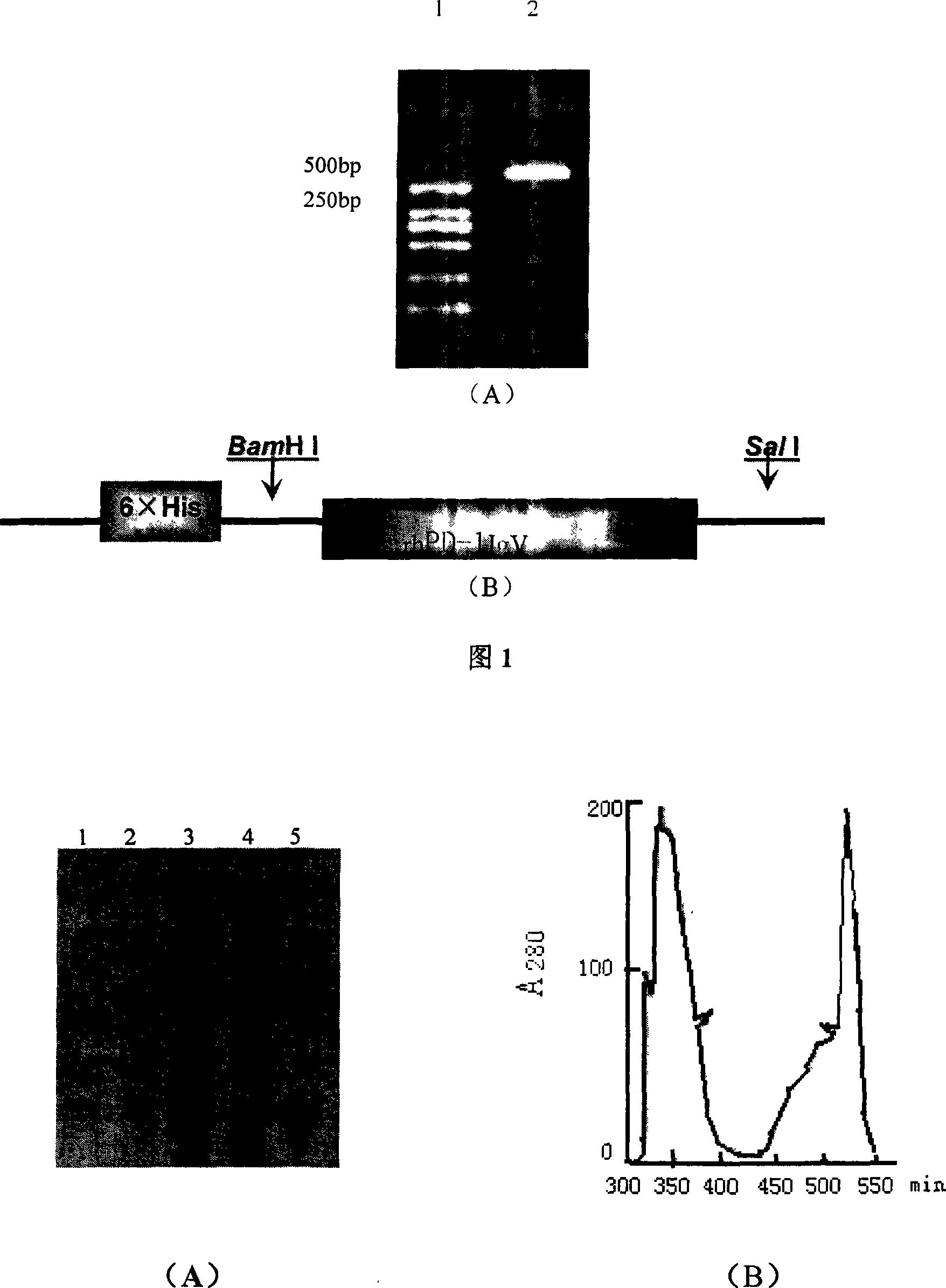
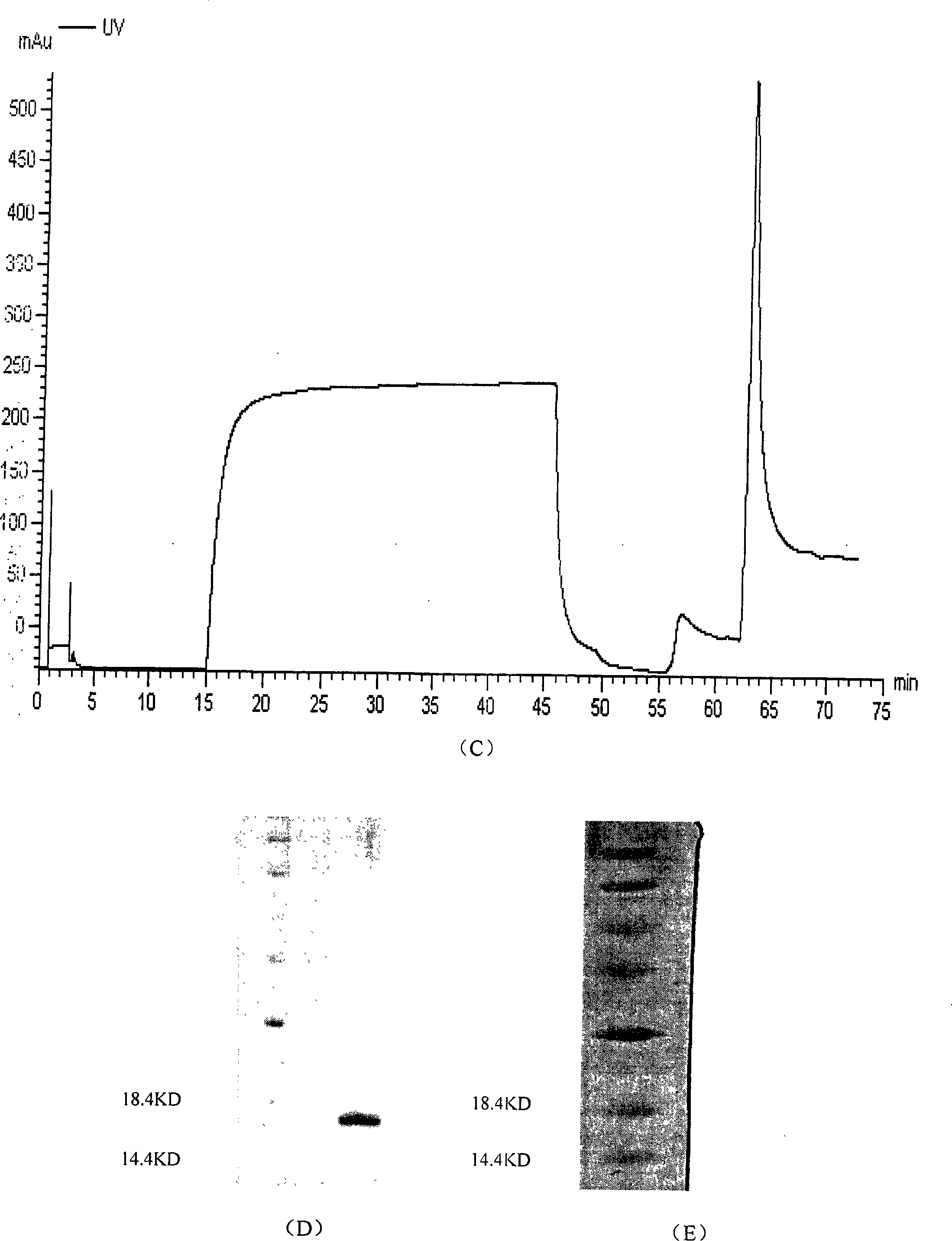
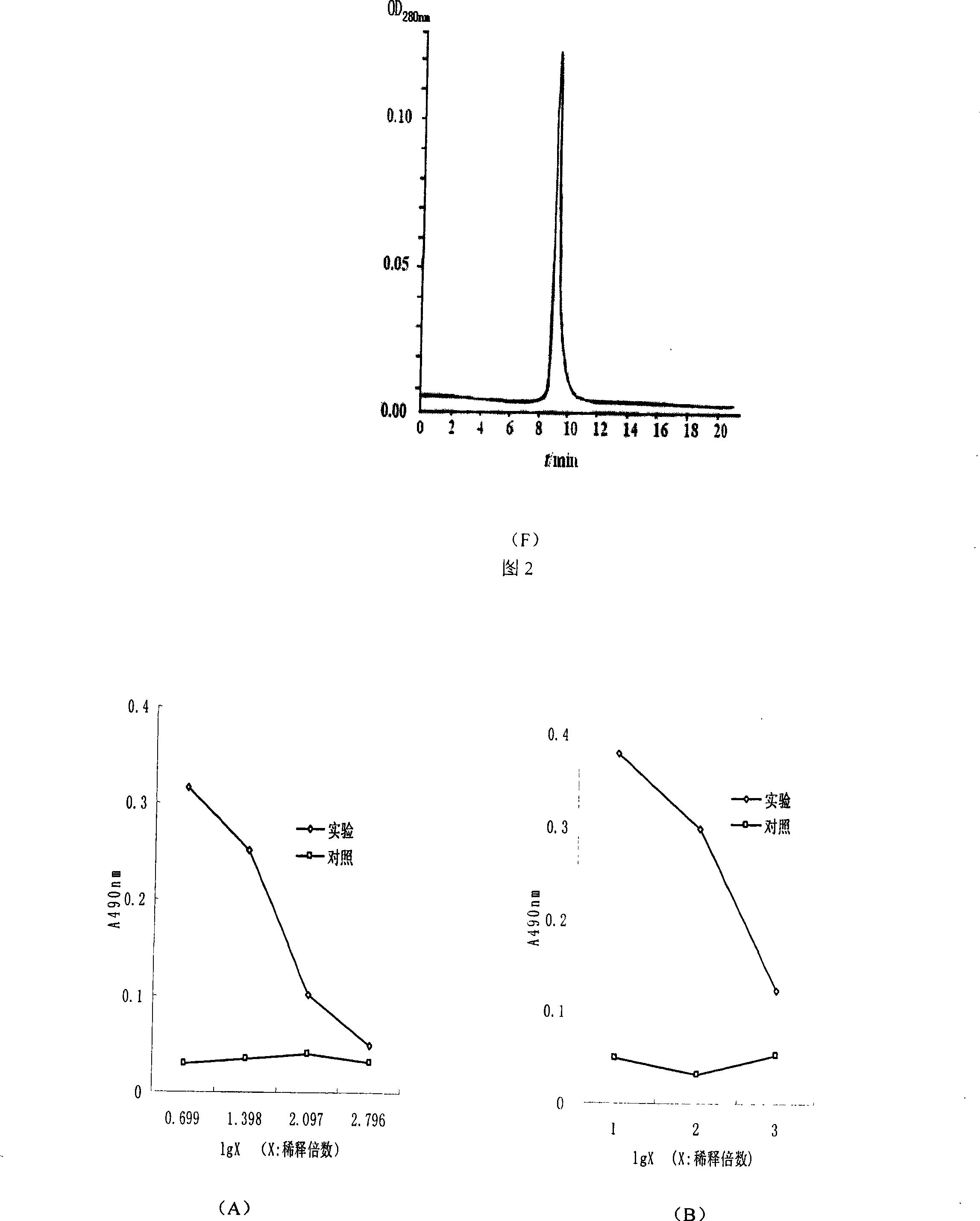
Soluble human program death protein-1-lgV and preparation method thereof
InactiveCN101215329ACell receptors/surface-antigens/surface-determinantsFermentationEscherichia coliProtein target
The invention discloses soluble programmed dead protein-1-IgV and a preparation process. The protein sequence of is showed as followed: PPTFFPAL LVVTEGDNAT FTCSFSNTSE SFVLNWYRMS PSNQTDKLAA FPEDRSQPGQ DSRFRVTQLP NGRDFHMSVV RARRNDSGTY LCGAISLAPK AQIKESLRAE LRVTERRAEV PTAHPSP. The invention obtains gene of Hpd-1-IgV through applying artificial synthesis according to the structure of human PD-1, target gene is cloned into pQE-30 pronucleus expression carrier to transform colibacillus and is expressed in colibacillus with high efficiency, bacterioclasis shows that target protein is existed in inclusion body form, and purified soluble programmed dead protein-1-IgV is finally obtained through inclusion body scouring, affinity chromatography, gel column chromatography and dialysis renaturation. The soluble programmed dead protein-1-IgV can respectively combine with mPD-L1 / Fc and hPD-L1 / Fc in extra-corporal shPD-1IgV and on cell level and molecular level, and has certain tumor killing effect in internal shPD-1IgV. Therefore, shPD-1IgV has excellent internal and external biological activity, provides experiment basis for curing tumor, establishes experiment foundation, and has potential application prospect.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Preparation method and application of PD-1/CTLA-4 (programmed death-1/cytotoxic T lymphocyte antigen-4) bispecific antibody
InactiveCN105754990ABlock immunosuppressive signalingEnhance the function of killing cancer cellsHybrid immunoglobulinsAntibody ingredientsPolymerase chain reactionImmunosuppression
The invention discloses a preparation method and application of a PD-1 / CTLA-4 (programmed death-1 / cytotoxic T lymphocyte antigen-4) bispecific antibody. The preparation method comprises the following steps of using a single-chain antibody gene as a template and introducing disulfide bond into a variable region of the antibody by a particular high-efficiency amplification primer through PCR (polymerase chain reaction) and Overlap PCR through a gene engineering technique, so as to prepare a PD-1 / CTLA-4 bispecific antibody fragment; cloning the gene fragment to a chronic virus expression carrier, and transfecting 293T cells to package the virus; transducing a CIK (cytokine induced killer) cell, separating PBMC (peripheral blood mononuclear cell) from peripheral blood of a health person, using CD3 / CD28 magnetic beads to stimulate the growth of the T cell, and co-culturing the chronic virus and the T cell, so as to obtain a novel anti-tumor T cell which can block an immunosuppression signal of the tumor and tumor micro environment source, and enhance the function of killing cancer cells.
Owner:杨晶
Compositions comprising a combination of an Anti-pd-1 antibody and another antibody
This provides pharmaceutical compositions that comprise a combination of an anti-cancer agent which is an first antibody and a second antibody. In some embodiments, the first antibody is an anti-Programmed Death-1 (PD-1) antibody. In certain embodiments, the composition is a fixed dose formulation. In certain embodiments, the composition is administered as a flat-dose. The disclosure also provides a kit for treating a subject afflicted with a disease, the kit comprising a dosage of any composition disclosed herein and instructions for using the composition in any of the disclosed methods for treating a disease.
Owner:BRISTOL MYERS SQUIBB CO
Immunohistochemical assay for detecting expression of programmed death ligand 1 (pd-l1) in tumor tissue
InactiveUS20160084839A1Improve standardizationAntibody ingredientsDisease diagnosisProgrammed deathPD-L1
The present disclosure provides processes for describing and quantifying the expression of human programmed death ligand-1 (PD-L1) in tumor tissue sections as detected by immunohistochemical assay using an antibody that specifically binds to PD-L1. The results generated using these processes have a variety of experimental, diagnostic and prognostic applications.
Owner:MERCK SHARP & DOHME CORP
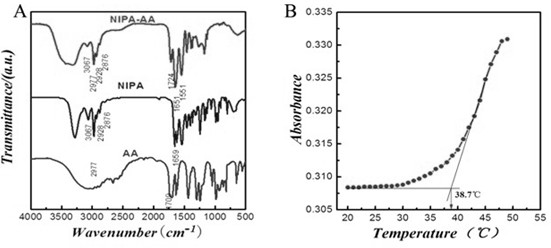
Tumor-targeted magnetic hydrogel nanoscale medicine delivery system and construction method and application thereof
InactiveCN102228425ARealize the mechanism of dual regulation inside and outside the cellOrganic active ingredientsPeptide/protein ingredientsCancer cellTumor targeting
The invention discloses a tumor-targeted magnetic hydrogel nanoscale medicine delivery system, a construction method and application thereof. The tumor-targeted magnetic hydrogel nanoscale medicine delivery system is prepared by the following steps of: grafting a hydrogel with temperature and pH sensitivity onto the surfaces of ferroferric oxide nanoparticles to form a magnetic hydrogel carrier by a photochemical immobilization method, grafting tumor necrosis factor-alpha, interferon-gamma and a targeted molecular ligand, namely folic acid on the surface of the magnetic hydrogel carrier, and absorbing adriamycin. The system can deliver an antitumor medicine to the periphery of tumor cells by utilizing active targeting, the tumor necrosis factor-alpha, the interferon-gamma and a tumor cellsurface receptor are combined and used for inducing the programmed death of the tumor cells, and the antitumor medicine, namely the adriamycin enters the cells along with the carrier and then is released. Through the experiment, the medicine delivery system has a negligible toxic or side effect, and efficiently inhibits the growth of cancer cells through the coaction mechanism of the tumor necrosis factor-alpha, the interferon-gamma and the antitumor medicine, namely the adriamycin inside and outside the tumor cells.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Stable antibody formulation
ActiveUS20190040137A1Pharmaceutical delivery mechanismImmunoglobulins against cell receptors/antigens/surface-determinantsProgrammed deathPharmaceutical formulation
The present invention provides stable pharmaceutical formulations comprising a human antibody that specifically binds to human programmed death-1 protein (PD-1). In certain embodiments, the formulations contain, in addition to an anti-PD-1 antibody, a buffer, an amino acid, a non-ionic surfactant, and a sugar. The pharmaceutical formulations of the present invention exhibit a substantial degree of antibody stability upon stress and storage.
Owner:REGENERON PHARM INC
Antibodies directed against programmed death-1 (PD-1)
The invention relates to an isolated immunoglobulin heavy chain polypeptide and an isolated immunoglobulin light chain polypeptide that bind to a programmed death-1 (PD-1) protein. The invention provides a PD-1-binding agent that comprises the aforementioned immunoglobulin heavy chain polypeptide and immunoglobulin light chain polypeptide. The invention also provides related vectors, compositions, and methods of using the PD-1-binding agent to treat a cancer or an infectious disease.
Owner:ANAPTYSBIO INC
Affinity peptide L8 of PD-1 (programmed death-1) protein extracellular domain and application thereof
ActiveCN103897036AEnhanced inhibitory effectProlong lifePeptide/protein ingredientsPeptide preparation methodsMelanomaSide effect
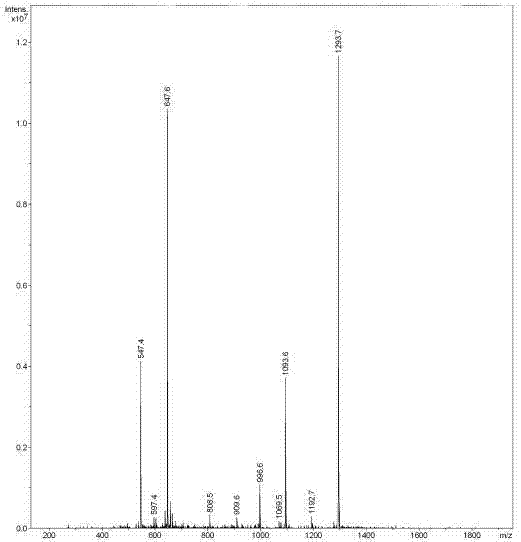
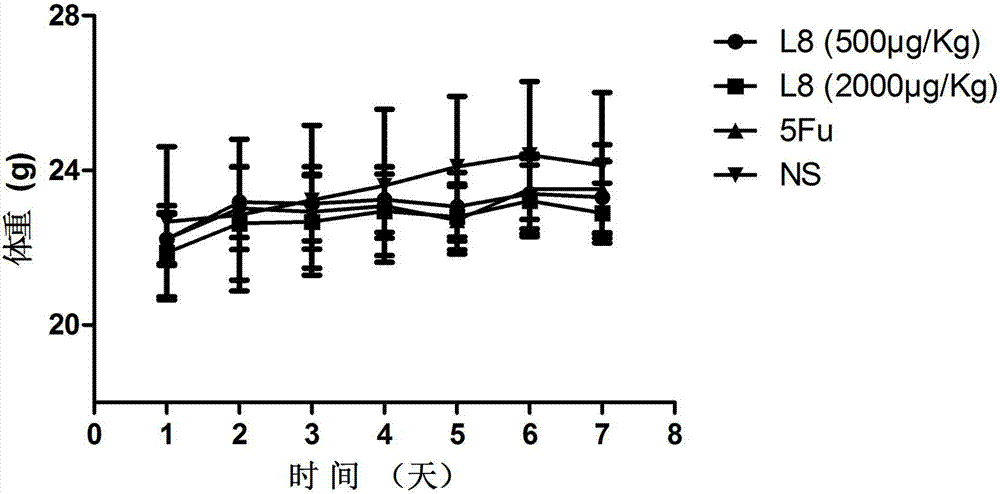
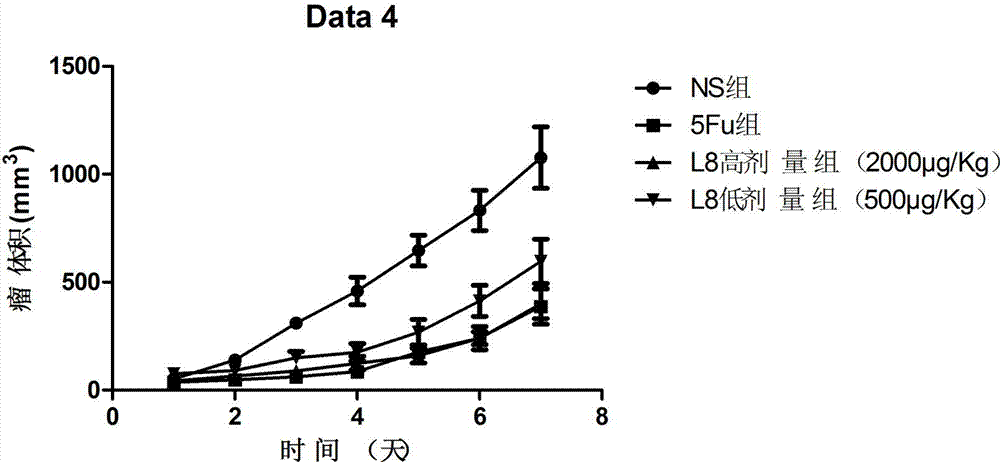
The invention belongs to the technical field of genetic engineering and medicine and in particular relates to an affinity peptide L8 of a PD-1 (programmed death-1) protein extracellular domain and an application thereof. The amino acid sequence of the affinity peptide L8 is Ser-Leu-Pro-Ser-Thr-Thr-Thr-Met-Arg-Leu-Thr-Ser, namely S-L-P-S-T-T-T-M-R-L-T-S, and the molecular weight of the affinity peptide L8 is 1293.7. The affinity peptide L8 is applied to preparation of antitumor related drugs and the tumor is colon cancer or melanoma. The affinity peptide L8 is synthesized by adopting a Fomc solid-phase peptide synthesis method. The obtained affinity peptide L8 of the PD-1 (programmed death-1) protein extracellular domain has clear effects and targets, has obvious inhibitory effects on tumors, especially on the colon cancer or melanoma, does not have obvious side effects and can obviously lengthen the lifetime of experimental animals; therefore the affinity peptide L8 has better medical application prospects.
Owner:ZHENGZHOU UNIV
Genetically engineered cell membrane nano vesicle and preparation and application thereof
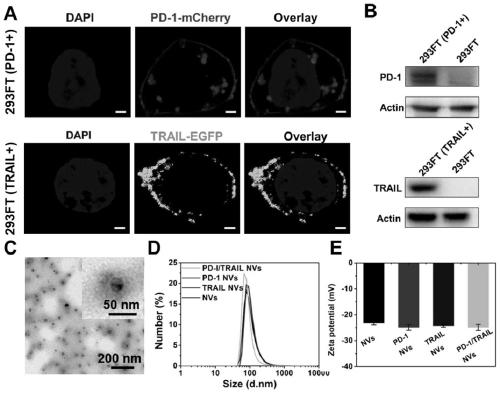
PendingCN110215514AGood biocompatibilitySmall toxicityPeptide/protein ingredientsNGF/TNF-superfamilyAbnormal tissue growthSignalling molecules
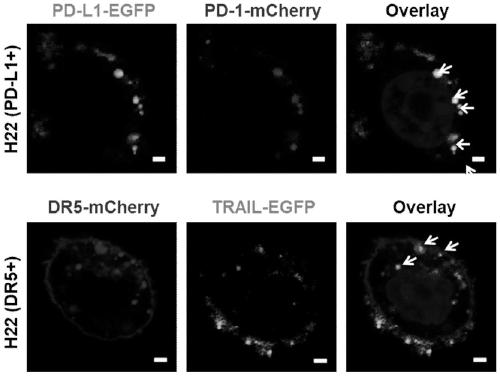
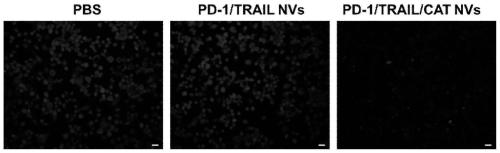
The invention provides a genetically engineered cell membrane nano vesicle and preparation and an application thereof. The nano vesicle is composed of biological cell membranes, and the surface is transferred with a tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), a programmed death receptor 1 (PD-1), and internal entrapment catalase (CAT). TRAIL can specifically induce tumor celldeath, release tumor antigens and "dangerous signal molecules", and triggers an immune response; the PD-1 can combines PD-L1 protein on cancer cells, and blocks related immunosuppressive pathways; andthe CAT can catalyze H2O2 of a tumor site to produce oxygen, improves a tumor hypoxic environment, and enhances infiltration of immune cells. Through organic integration of the functions, effective elimination of tumor cells and rapid activation of an autoimmune system are achieved, and a multi-point synergistic anti-tumor effect is exerted.
Owner:上海瑞可新生物科技有限公司 +1
Preparation and application of anti-human programmed death factor 1 (PD-1) monoclonal antibody
ActiveCN106046162AHigh affinityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenAutoimmune disease
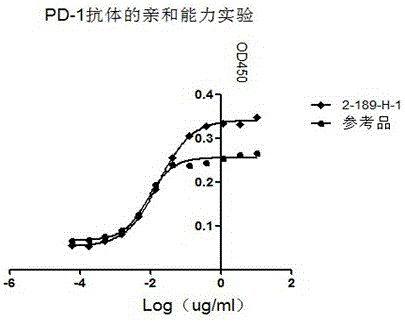
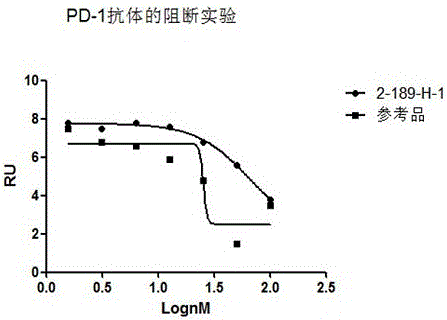
The invention discloses preparation, a variable-region sequence and application of an anti-human programmed death factor 1 (PD-1) monoclonal antibody. The invention provides the anti-human PD-1 monoclonal antibody which comprises gene sequences of heavy-chain variable regions of the monoclonal antibody and gene sequences of light-chain variable regions of the monoclonal antibody. The amino acid sequences of the heavy-chain variable regions are as shown in site 20th to site 134th of SEQ ID NO.3; the amino acid sequences of the light-chain variable regions are shown in site 20th to site 131th of SEQ ID NO.4. The invention discloses the monoclonal antibody 189-H-1 capable of blocking a human PD-1 function and a coding gene thereof. The monoclonal antibody 189-H-1 can be specifically combined with human PD-1 antigen, has median effective concentration EC50 being 1.0667 nM, can specifically block a PD-1 / PD-L inhibition signal, and therefore, the monoclonal antibody 189-H-1 can be used as a blocker of a PD-1 access, so that the anti-human PD-1 monoclonal antibody becomes a novel drug for tumor immune therapy, chronic virus infective disease treatment and autoimmune disease treatment.
Owner:大庆东竺明生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com