Genetically engineered cell membrane nano vesicle and preparation and application thereof
A technology of nanovesicles and genetic engineering, which is applied in the application field of preparing tumor immunotherapy drugs, can solve the problems such as difficulty in effectively activating the body's anti-tumor immune response, achieve low toxicity and side effects, achieve cascade synergy, and biocompatibility. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
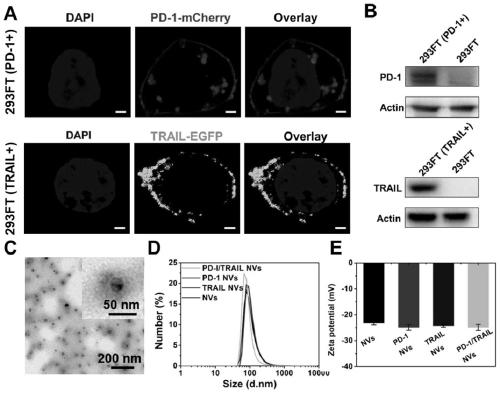
[0038] The gene sequences of PD-1 and TRAIL proteins were respectively connected with the mCherry red fluorescent protein gene sequence and the EGFP green fluorescent protein gene sequence through a flexible peptide sequence (ggaggttctggtggatctggtggaggttctggttctggatcaggtggt) to construct a new fusion protein gene sequence.
[0039] The PD-1-mCherry gene sequence is as follows:
[0040] GCCACCATGTGGGTCCGGCAGGTACCCTGGTCATTCACTTGGGCTGTGCTGCAGTTGAGCTGGCAATCAGGGTGGCTTCTAGAGGTCCCCAATGGGCCCTGGAGGTCCCTCACCTTCTACCCAGCCTGGCTCACAGTGTCAGAGGGAGCAAATGCCACCTTCACCTGCAGCTTGTCCAACTGGTCGGAGGATCTTATGCTGAACTGGAACCGCCTGAGTCCCAGCAACCAGACTGAAAAACAGGCCGCCTTCTGTAATGGTTTGAGCCAACCCGTCCAGGATGCCCGCTTCCAGATCATACAGCTGCCCAACAGGCATGACTTCCACATGAACATCCTTGACACACGGCGCAATGACAGTGGCATCTACCTCTGTGGGGCCATCTCCCTGCACCCCAAGGCAAAAATCGAGGAGAGCCCTGGAGCAGAGCTCGTGGTAACAGAGAGAATCCTGGAGACCTCAACAAGATATCCCAGCCCCTCGCCCAAACCAGAAGGCCGGTTTCAAGGCATGGTCATTGGTATCATGAGTGCCCTAGTGGGTATCCCTGTATTGCTGCTGCTGGCCTGGGCCCTAGCTGTCTTCTGCTCAACAAGTATGTCAG...
Embodiment 2
[0045] pCDH-CMV-PD-1-mCherry-Puro and pCDH-CMV-TRAIL-EGFP-Puro use 293FT cells (from ATCC) for lentiviral packaging, the packaging process is as follows:
[0046] 1. When 293FT cells are inoculated to a density of 95-99%, they can be used for lentivirus packaging;
[0047] 2. Preparation of Tube A: Using Opti-MEM TM Dilute Lipofectamine 3000 reagent (0.96ml Opti-MEM TM Medium I: 0.96ml Lipofectamine 3000: 0.04ml)——mix well;
[0048] 3. Prepare tube B: Dilute PLP / VSVG with Opti-MEM I medium TM Lentiviral packaging plasmid and target plasmid, then add P3000 reagent (0.96ml Opti-MEM TM I culture medium: 0.96ml, PLP1: 7.5μg, PLP2: 3μg, PLP / VSVG: 4μg, target plasmid: 10μg, P3000: 0.04ml)—mix well;
[0049] 4. Incubate the newly configured tubes A and B at room temperature for 5 minutes;
[0050] 5. Add tube A to tube B and mix well, incubate at room temperature for 10-20 minutes;
[0051] 6. Add the DNA-liposome complex to the cells and shake well;
[0052] 7. At 37°C, 5% CO...
Embodiment 3
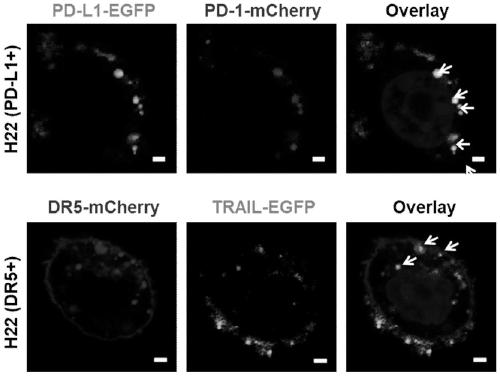
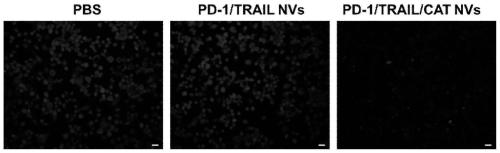
[0057] The successfully constructed stable cell lines were expanded and cultured (using DMEM medium containing 10% FBS in 5% CO 2 cultured at 37°C), the cells were scraped off with a cell scraper, collected by centrifugation, and the cell membrane was obtained according to the method provided by the cell membrane extraction kit. With PBS (0.0067M (PO 4 ) pH: 7.4) to dissolve the cell membrane, and after miscible with catalase (the cell membrane overexpressing PD1: the cell membrane overexpressing TRAIL: catalase mass ratio is 1:1:0.5), successively use 800nm, 400nm and 200nm The pore size was processed by a membrane extruder, centrifuged and washed 3 times, and nanovesicles with uniform particle size were finally obtained. Keep the whole process on ice or operate in a low temperature environment to prevent protein denaturation. The morphology of the product was characterized by TEM, and the results were as follows: figure 1 As shown in C, the results show that the particle ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Surface potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com