Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
7447 results about "Secretion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Secretion is the movement of material from one point to another, e.g. secreted chemical substance from a cell or gland. In contrast, excretion, is the removal of certain substances or waste products from a cell or organism. The classical mechanism of cell secretion is via secretory portals at the cell plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structure at the cell plasma membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell.
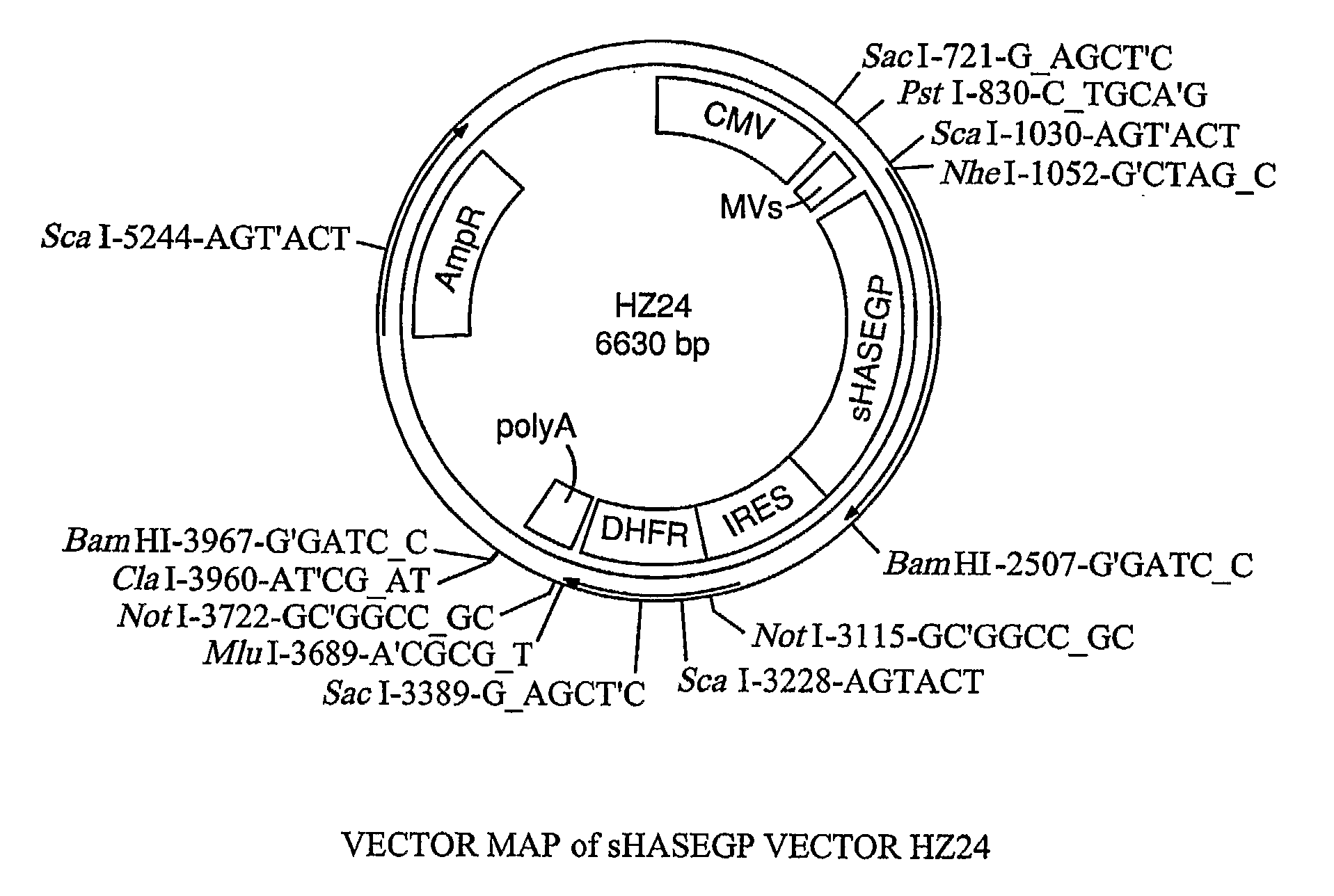
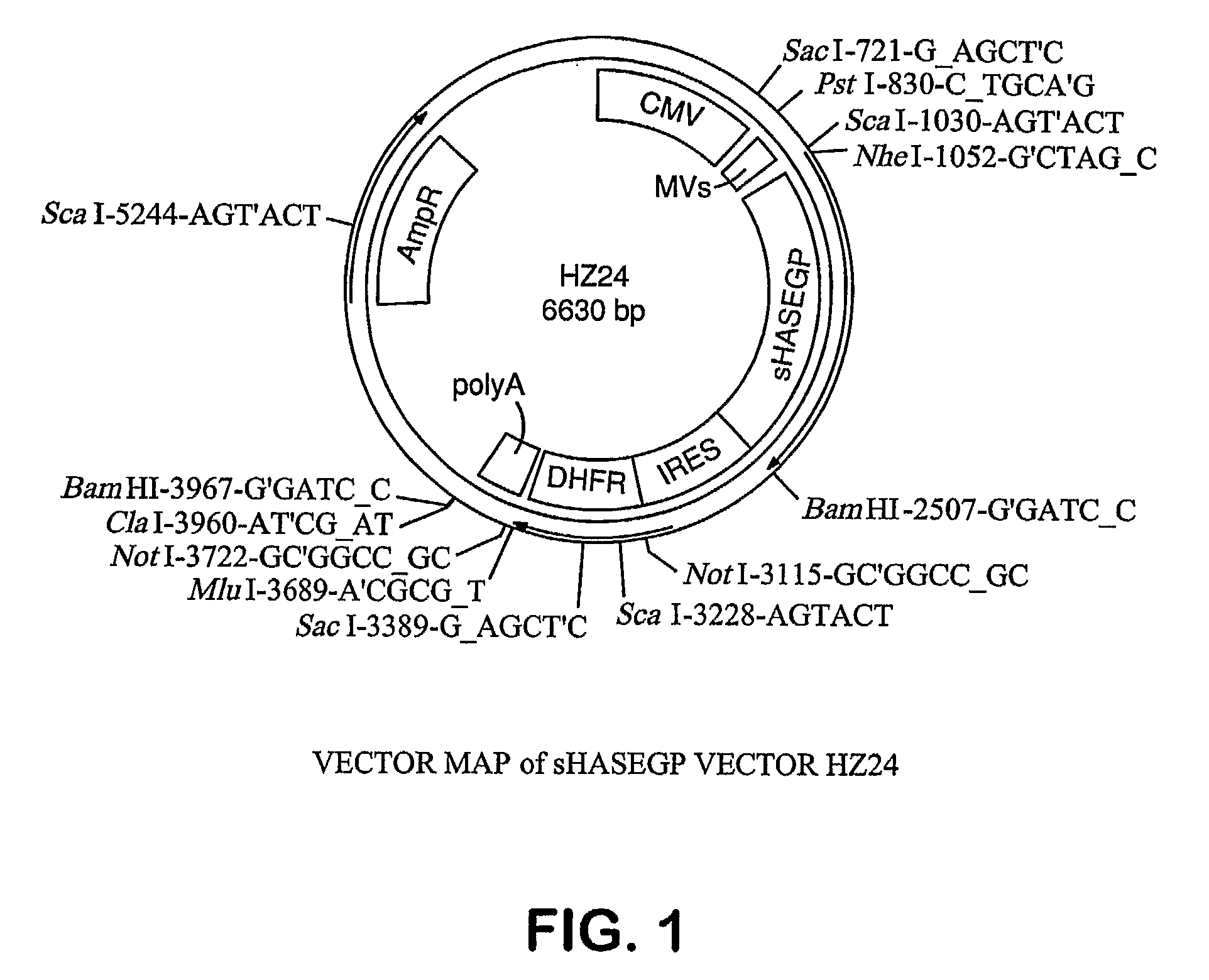
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminogly ycanases
PendingUS20060104968A1Improve extentIncrease ratingsSenses disorderNervous disorderHyaluronidaseRecombinant glycoprotein
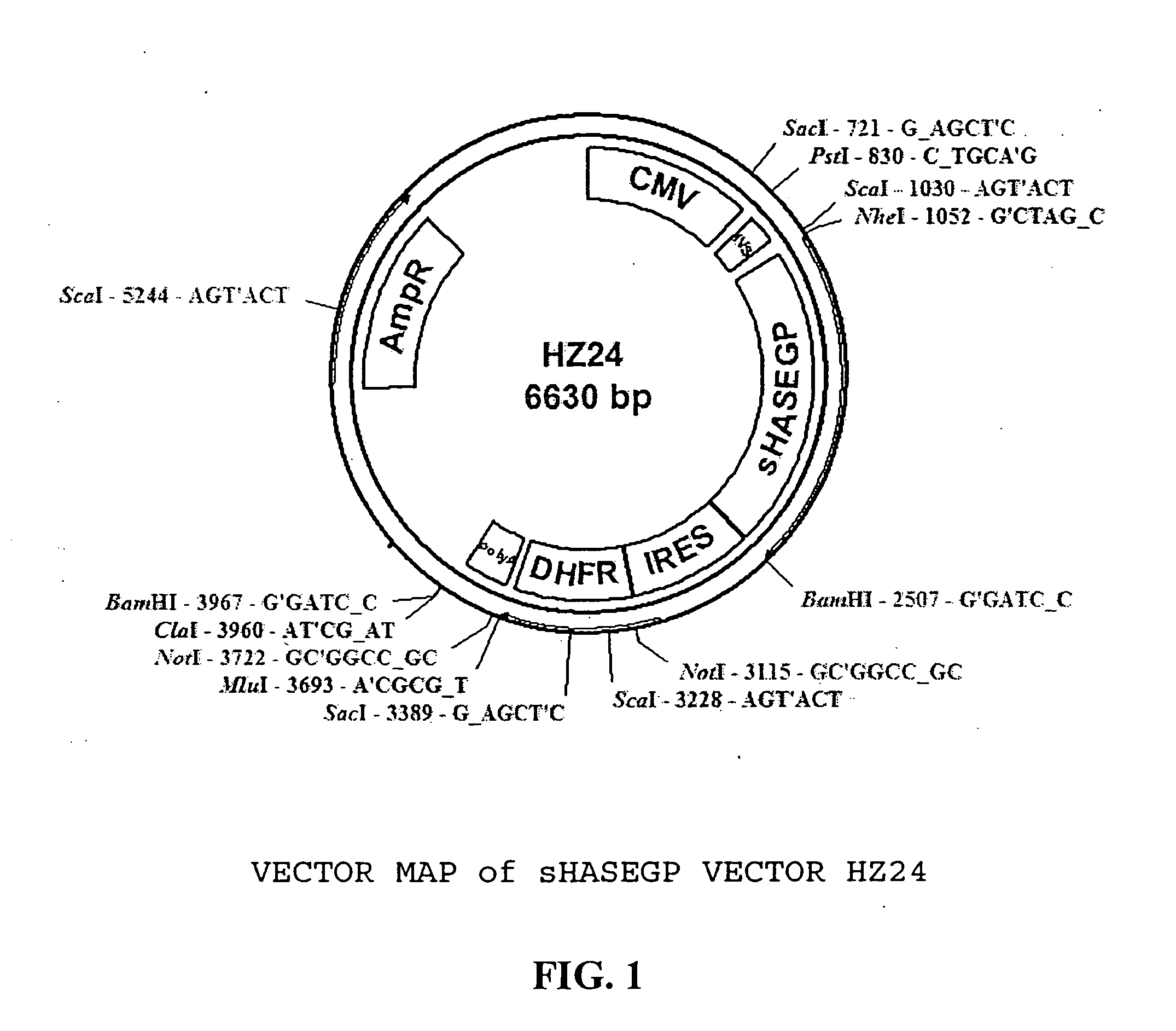
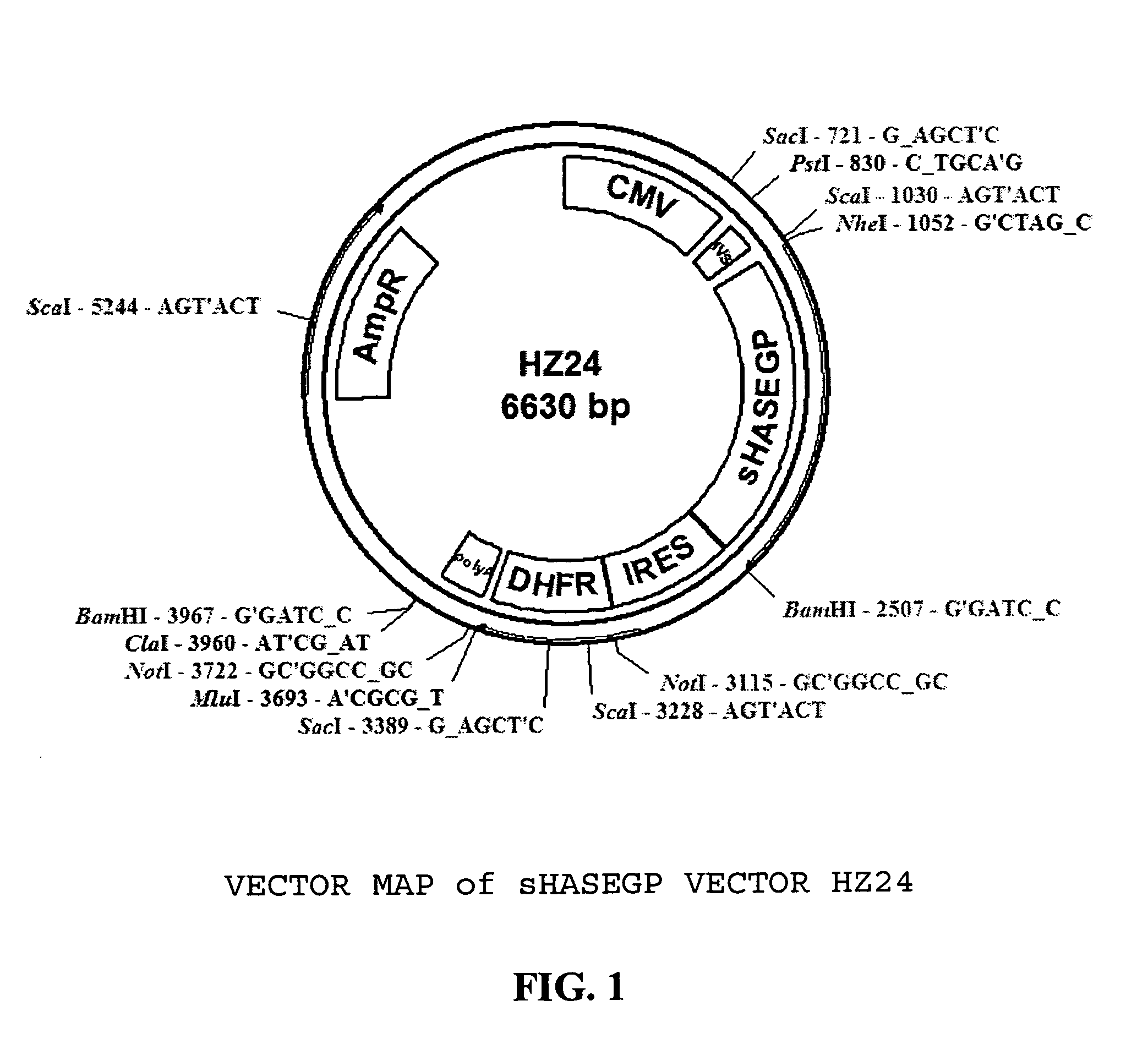
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminoglycanases
ActiveUS20050260186A1Improve extentIncrease ratingsAntibacterial agentsSenses disorderHyaluronidasePathology diagnosis
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Purine derivative
PCT No. PCT / JP97 / 02310 Sec. 371 Date Mar. 3, 1998 Sec. 102(e) Date Mar. 3, 1998 PCT Filed Jul. 3, 1997 PCT Pub. No. WO98 / 01448 PCT Pub. Date Jan. 15, 1998This invention relates to novel purine derivatives of formula (I): where R2 and R9 are hydrocarbon groups, R6 is an amino group and R8 is a hydroxyl, or acyloxy group. These purine derivatives are effective at promoting secretion of interferon in patients, and can be used to treat diseases against which interferon is effective.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Methods of synthesizing heteromultimeric polypeptides in yeast using a haploid mating strategy
ActiveUS20060270045A1Enhance full-length product generationIncrease secretionFungiMicrobiological testing/measurementBiotechnologyMating
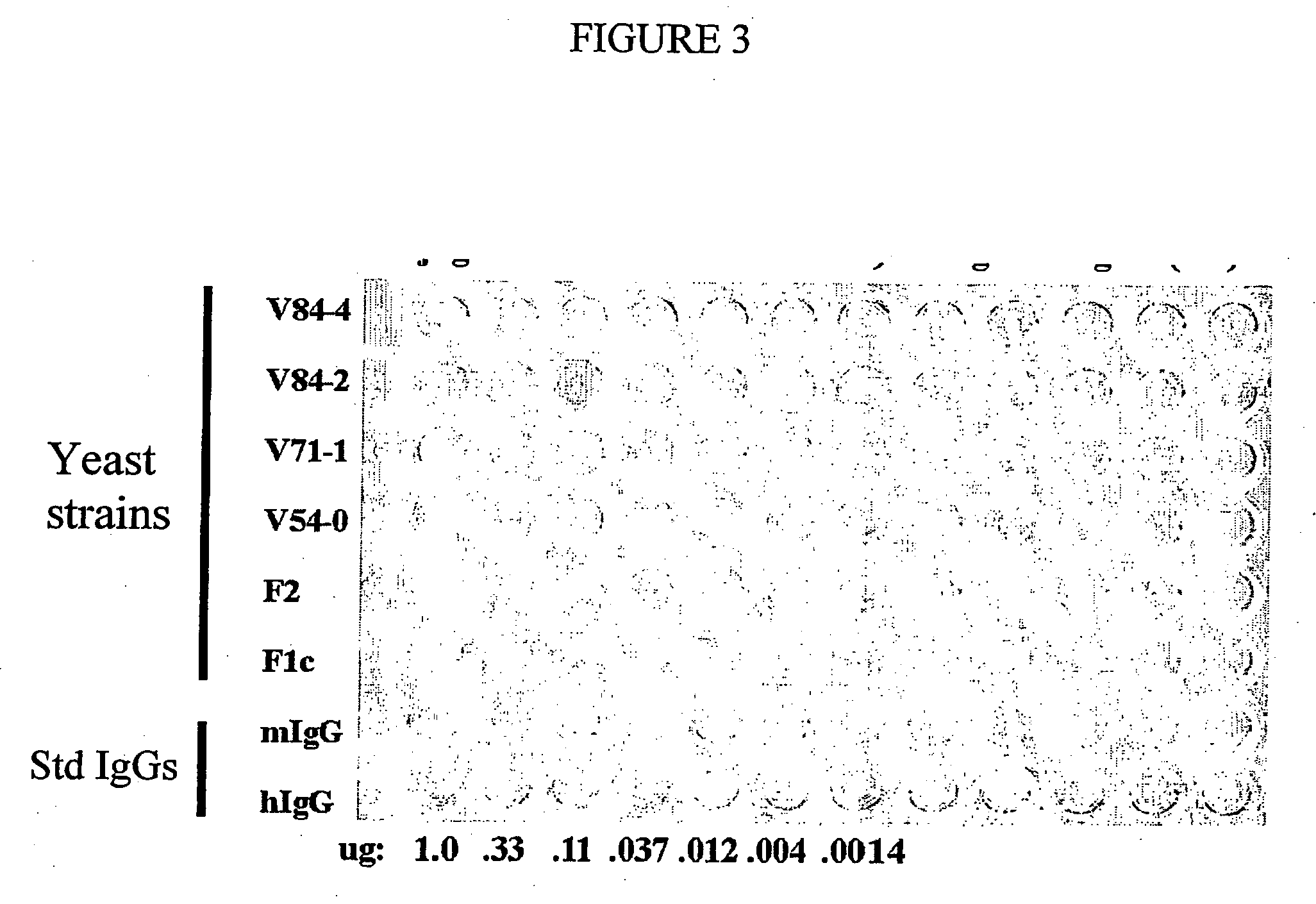
Methods are provided for the synthesis and secretion of recombinant proteins preferably large mammalian proteins or hetero-multimeric proteins at high levels and for prolonged time in polyploid, preferably diploid yeast. These methods use various mating competent yeast, including Pichia. In a preferred embodiment, a first expression vector is transformed into a first haploid cell; and a second expression vector is transformed into a second haploid cell. The transformed haploid cells, each individually synthesizing a non-identical polypeptide, are identified and then genetically crossed or fused. The resulting diploid strains are utilized to produce and secrete fully assembled and biologically functional hetero-multimeric protein.
Owner:KECK GRADUATE INST OF APPLIED LIFE SCI +1
Gastrointestinal methods and apparatus for use in treating disorders
InactiveUS7502649B2Reduce volumeCause a sensation of satiety felt by the patientElectrotherapyMetabolism disorderElectrical resistance and conductanceDisease
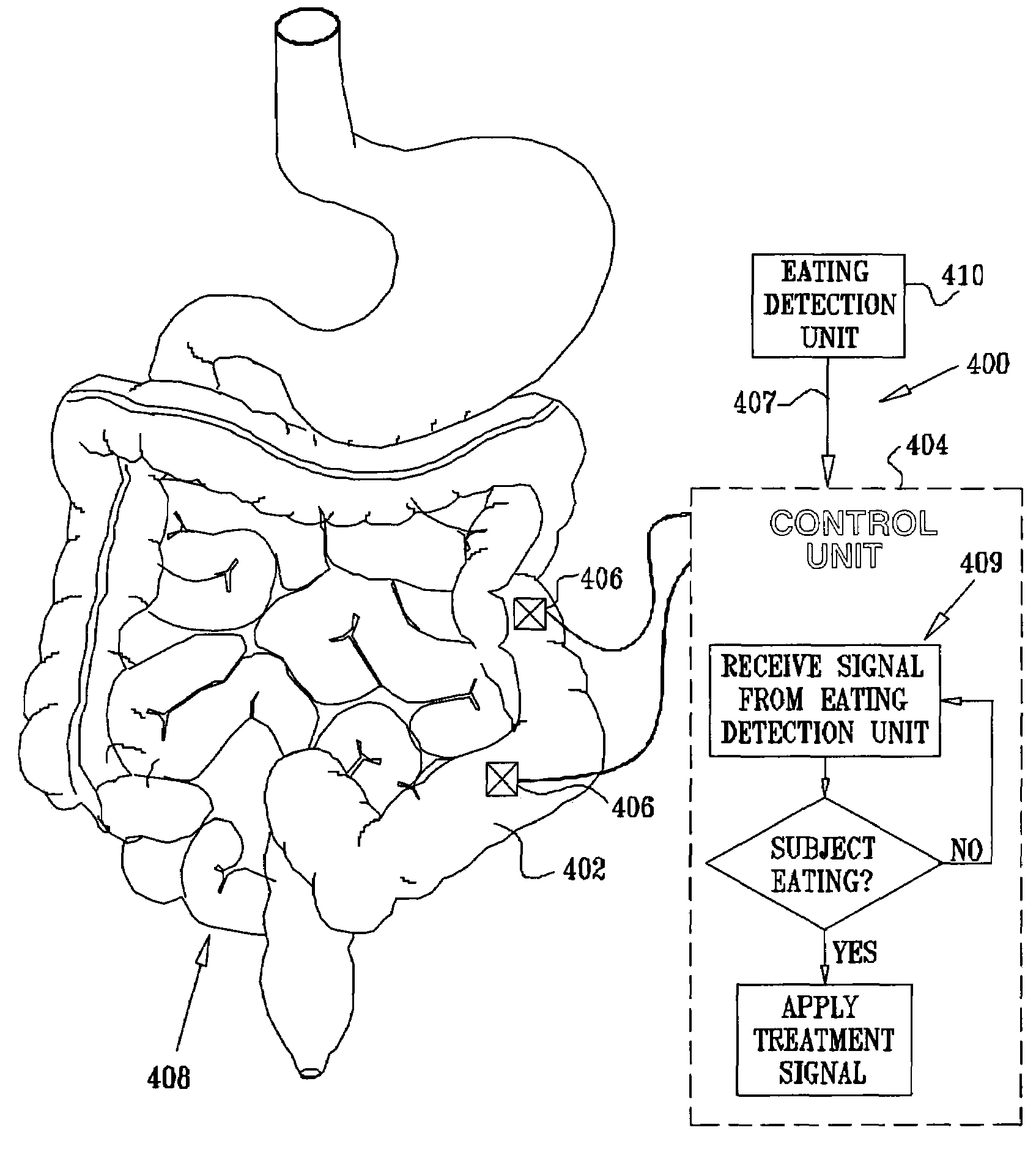
A method is provided for detecting a change in posture of a subject. An electrical impedance is measured between two or more sites on a stomach (20) of the subject, and an impedance signal is generated responsive thereto. The change in posture is detected by performing a posture analysis of the impedance signal. A method is also provided for treating a subject. The method includes applying an electrical signal to a site of the subject selected from the list consisting of: a colon (402) of the subject, and a distal small intestine (408) of the subject. The signal is configured to stimulate cells of the subject to increase secretion of glucagon-like-peptide-1 (GLP-1) or PYY, or to decrease secretion of ghrelin, in order to treat the subject.
Owner:TYLERTON INT INC
Drainage Device for the Treating Wounds Using a Reduced Pressure
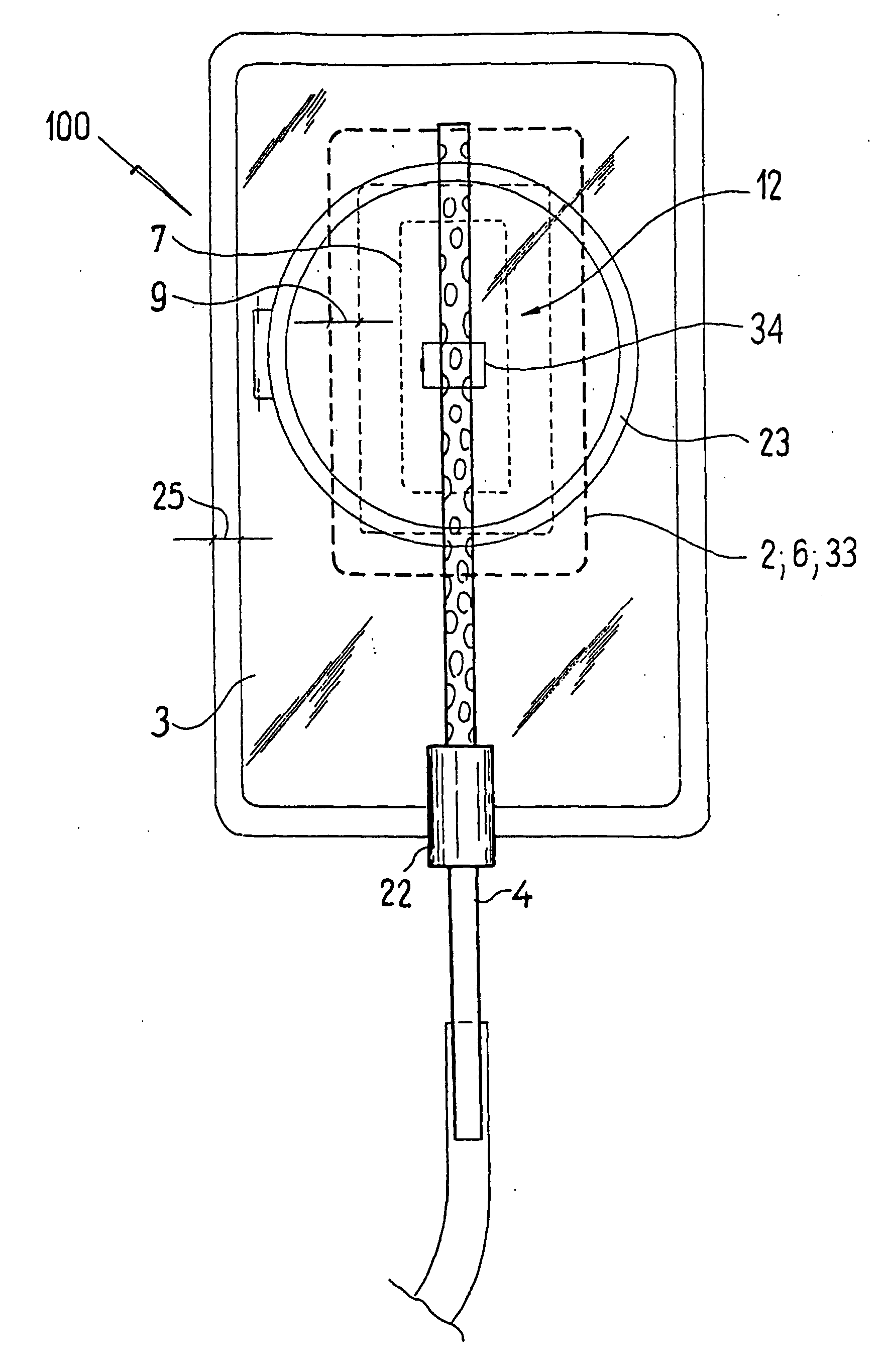
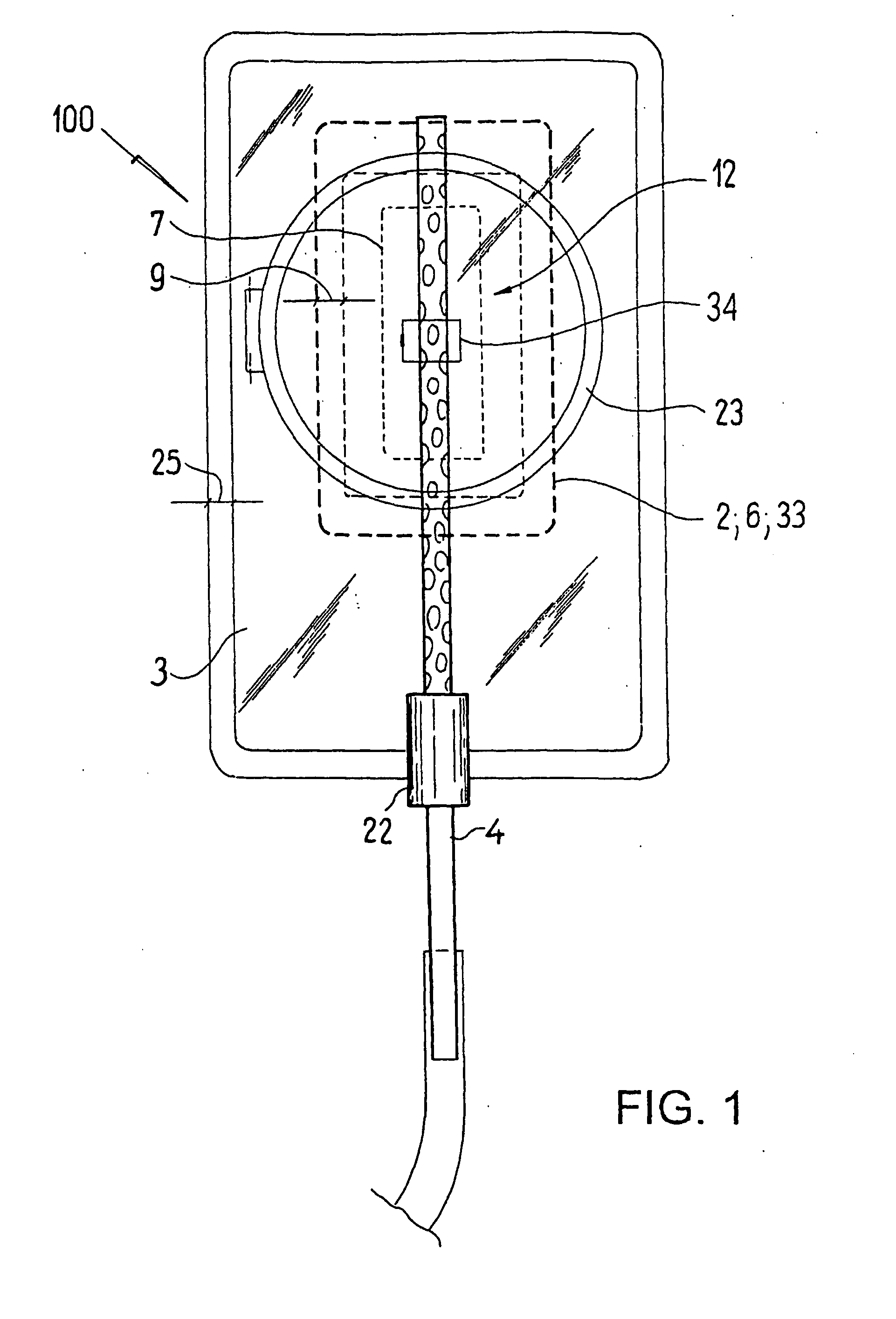
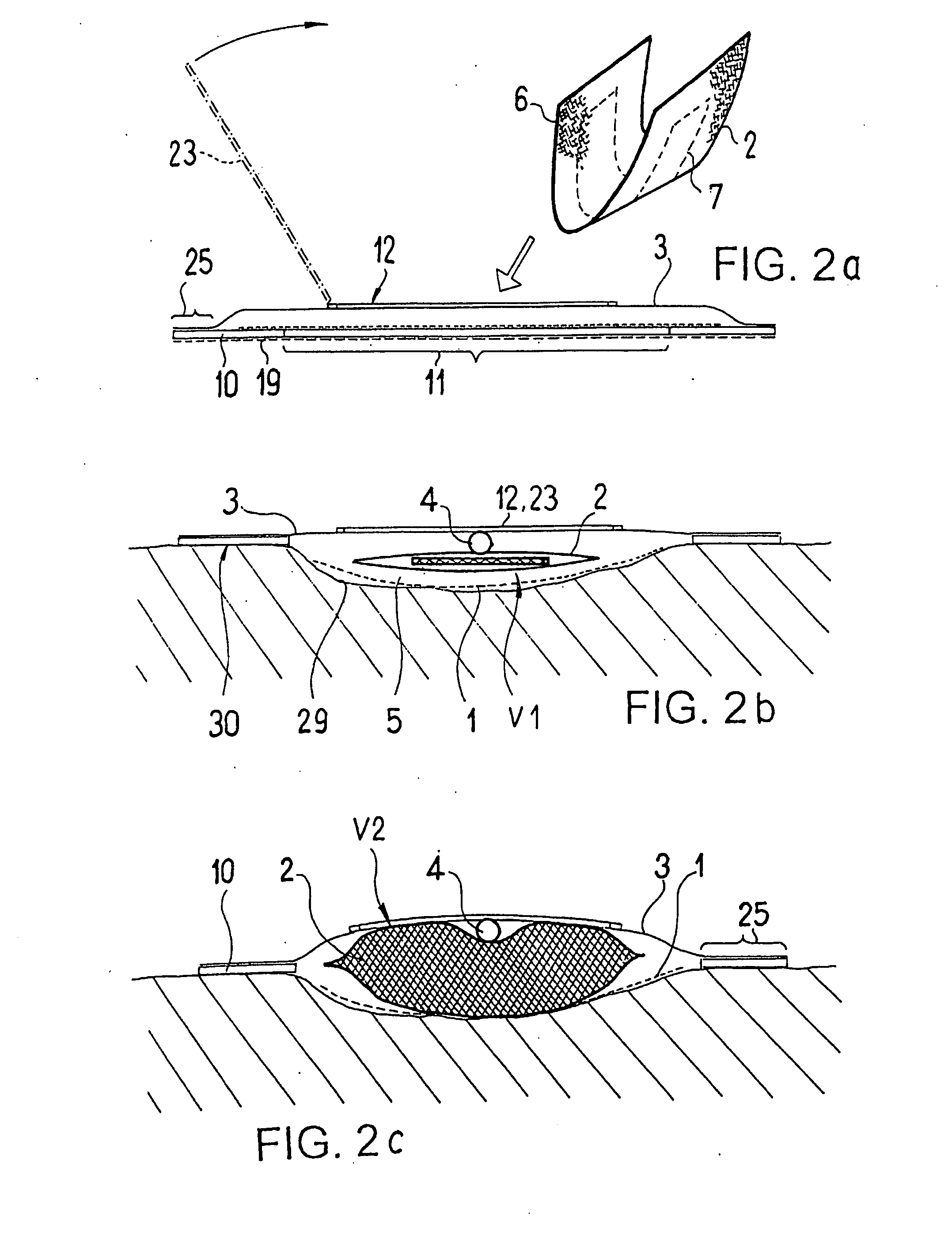
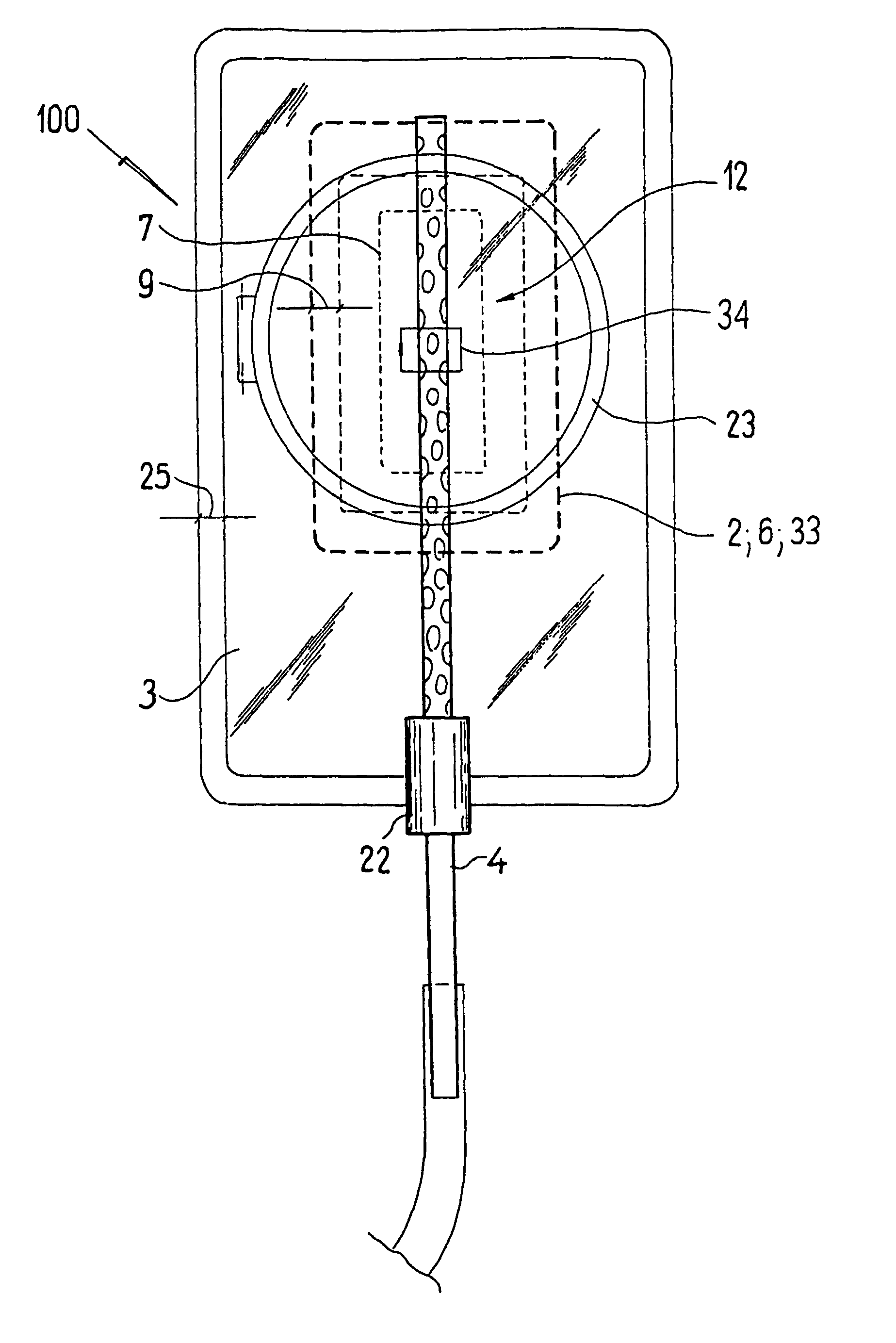
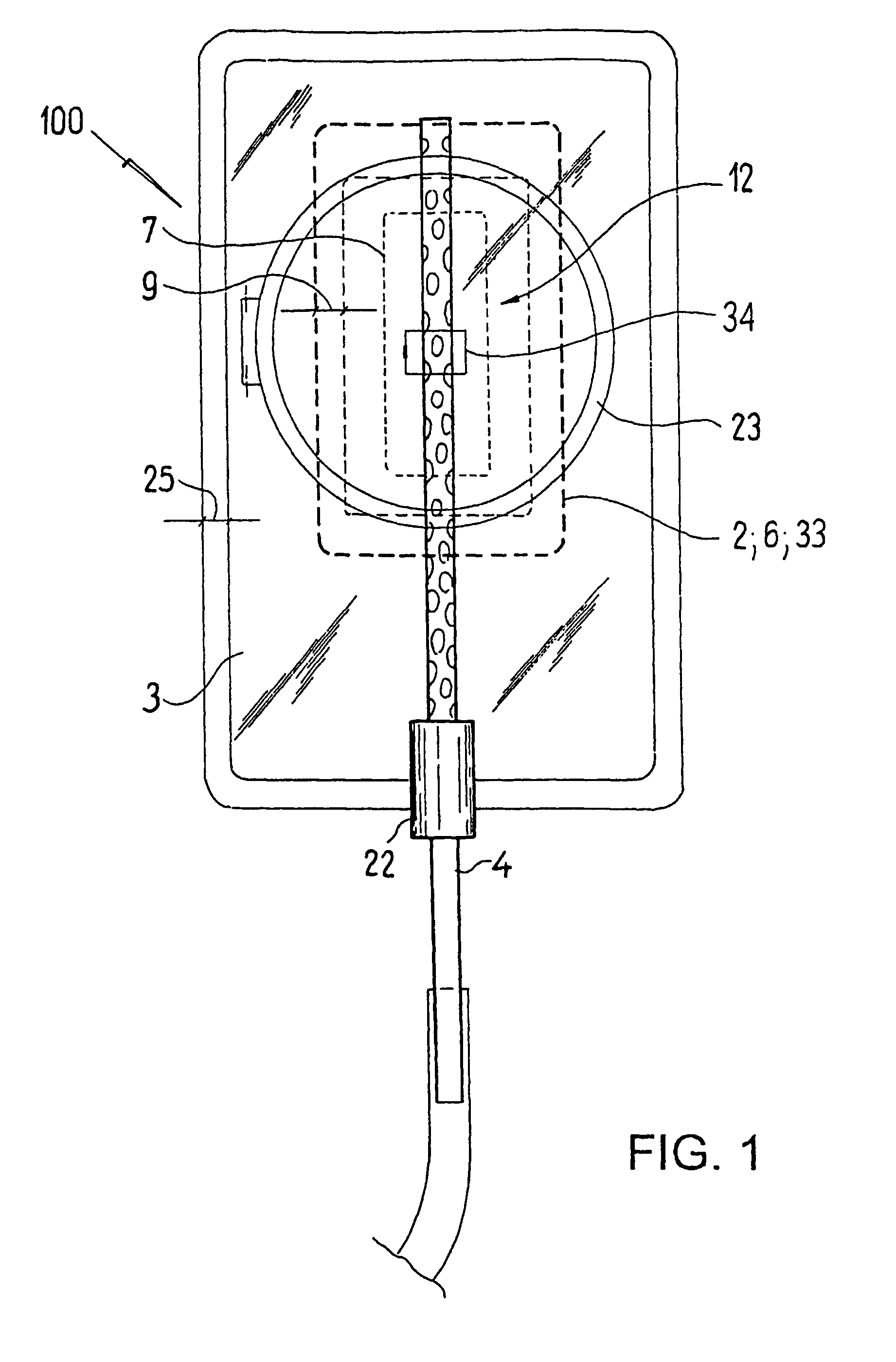
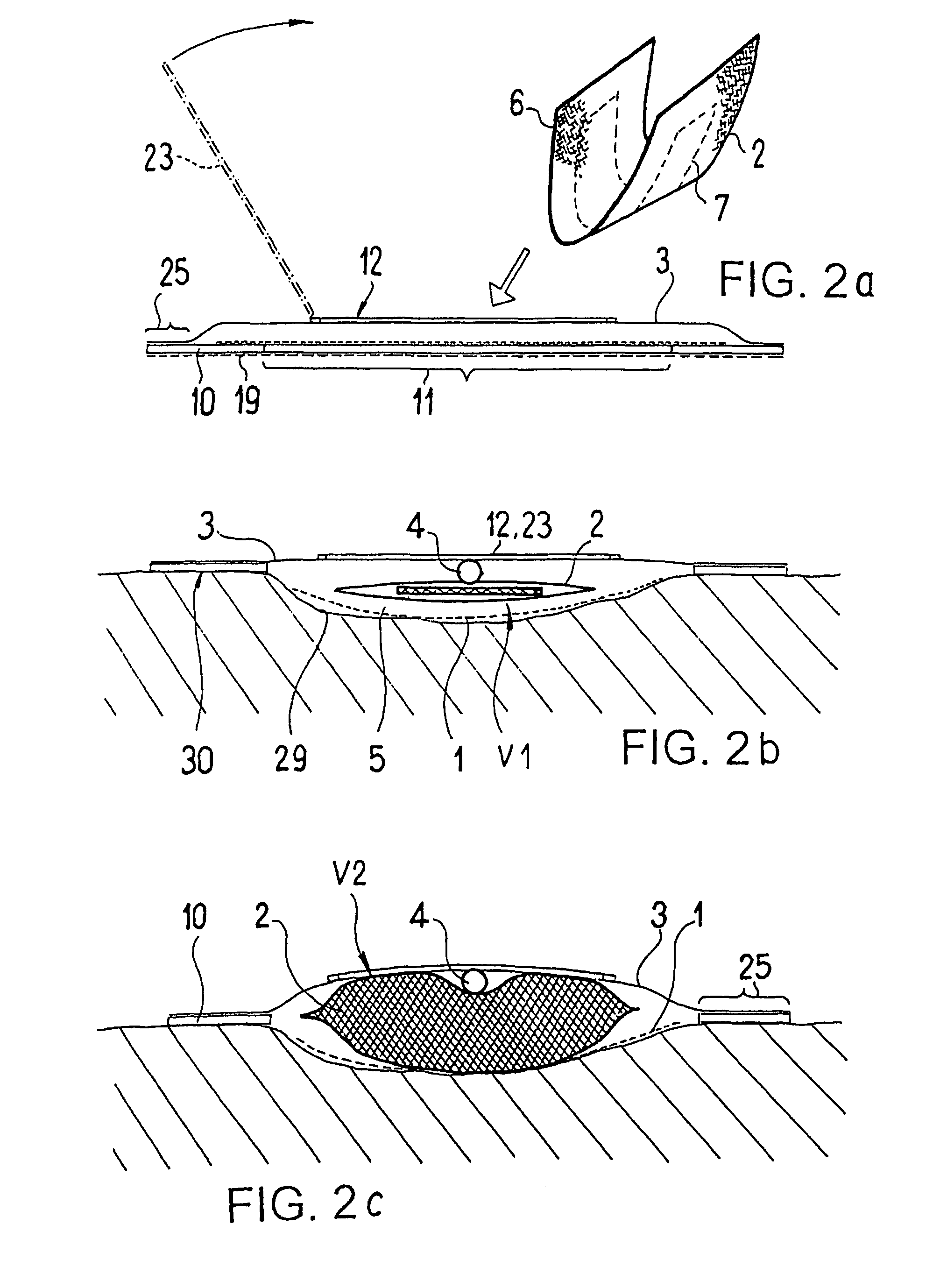
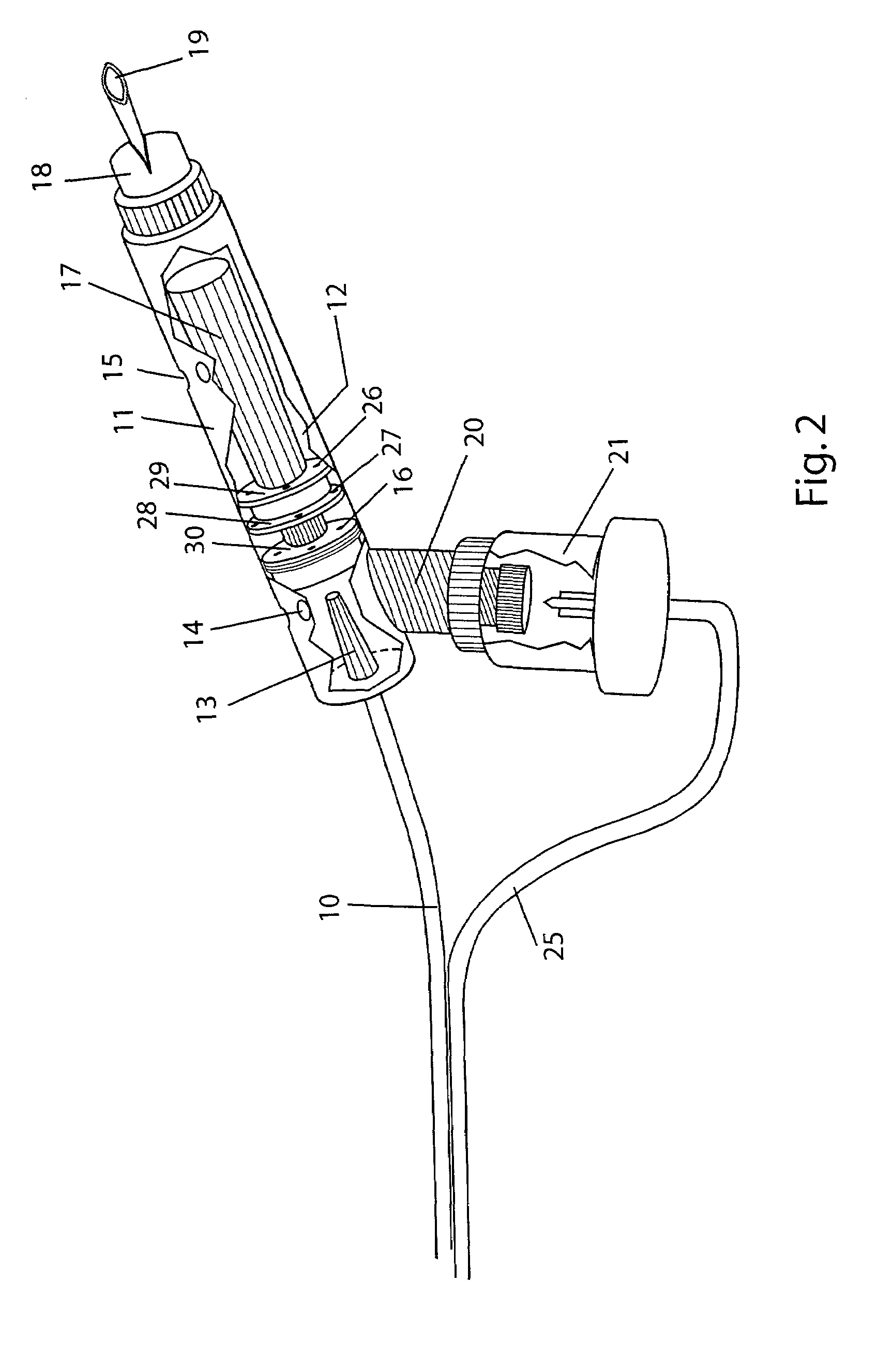
ActiveUS20080119802A1Cost-effectiveSimple designSurgical needlesWound drainsSkin surfaceDrainage tubes
The invention relates to a drainage device (100) for the treatment of wounds using reduced pressure, comprising (a) a gas-type wound-covering element (3), which, in the state in contact with the body of the patient, is fastened cohesively at the skin surface around the region of the wound and forms a sealed wound space, remaining between the respective wound and the wound-covering element (3); (b) at least one drainage tube (4), which can be connected to means generating the reduced pressure and can be inserted into the wound space approximately parallel to the wound-covering element (3); and (c) at least one absorption body (2) in the form of at least one layer (7) of a textile section (33), which is interspersed with super-absorbing particles and enclosed in an envelope. Due to the size of the pores of the envelope, the absorbed wound secretions remain within the absorption body (2) and, with that, underneath the wound-covering element (3) until the absorption body is removed from the wound space. An air opening (34) is provided for the wound-covering element (3).
Owner:BR ENTWICKLUNGS
Drainage device for treating wounds using a reduced pressure
The invention relates to a drainage device (100) for the treatment of wounds using reduced pressure, comprising (a) a gas-type wound-covering element (3), which, in the state in contact with the body of the patient, is fastened cohesively at the skin surface around the region of the wound and forms a sealed wound space, remaining between the respective wound and the wound-covering element (3); (b) at least one drainage tube (4), which can be connected to means generating the reduced pressure and can be inserted into the wound space approximately parallel to the wound-covering element (3); and (c) at least one absorption body (2) in the form of at least one layer (7) of a textile section (33), which is interspersed with super-absorbing particles and enclosed in an envelope. Due to the size of the pores of the envelope, the absorbed wound secretions remain within the absorption body (2) and, with that, underneath the wound-covering element (3) until the absorption body is removed from the wound space. An air opening (34) is provided for the wound-covering element (3).
Owner:BR ENTWICKLUNGS
Blood glucose level control
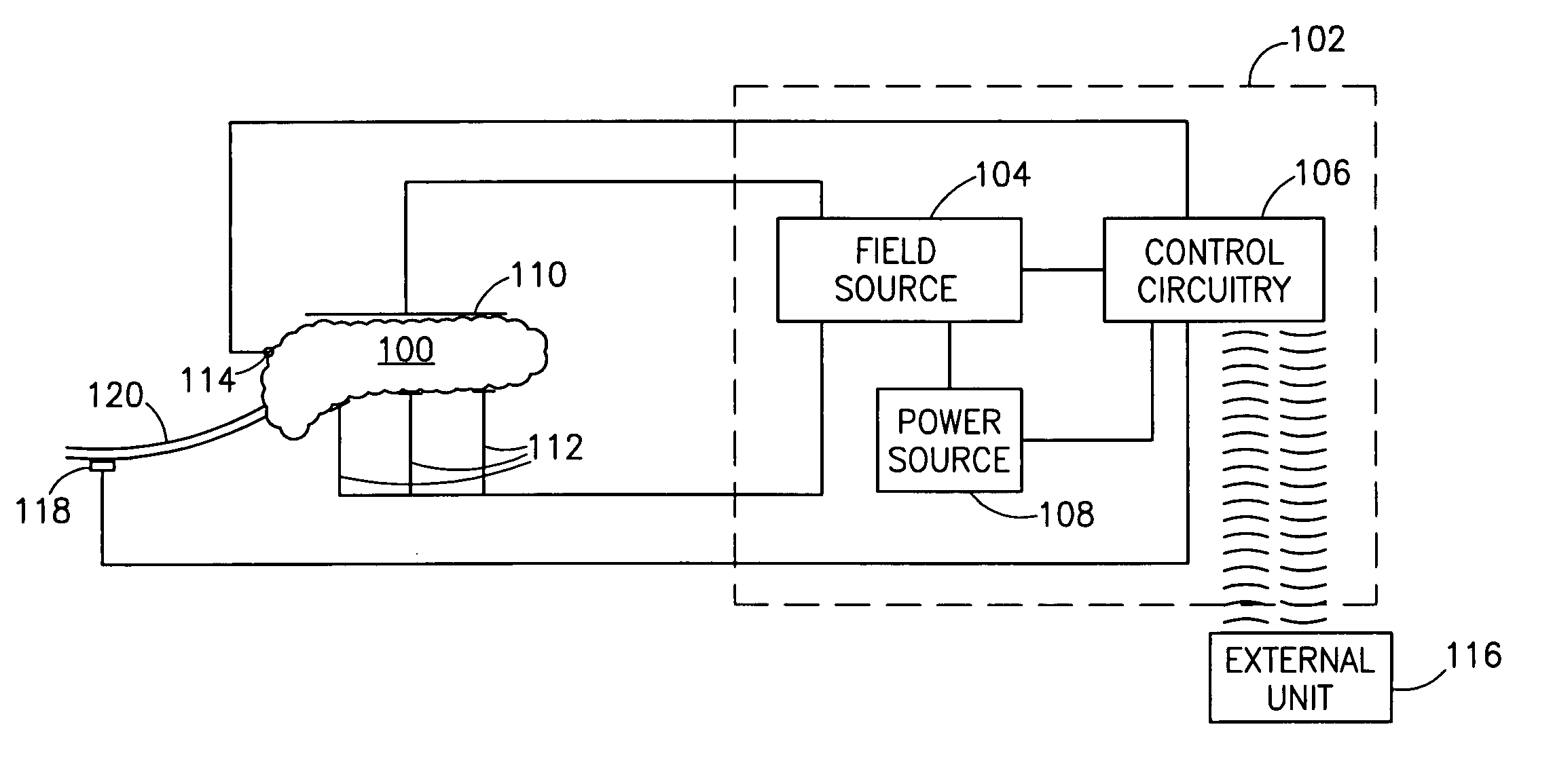
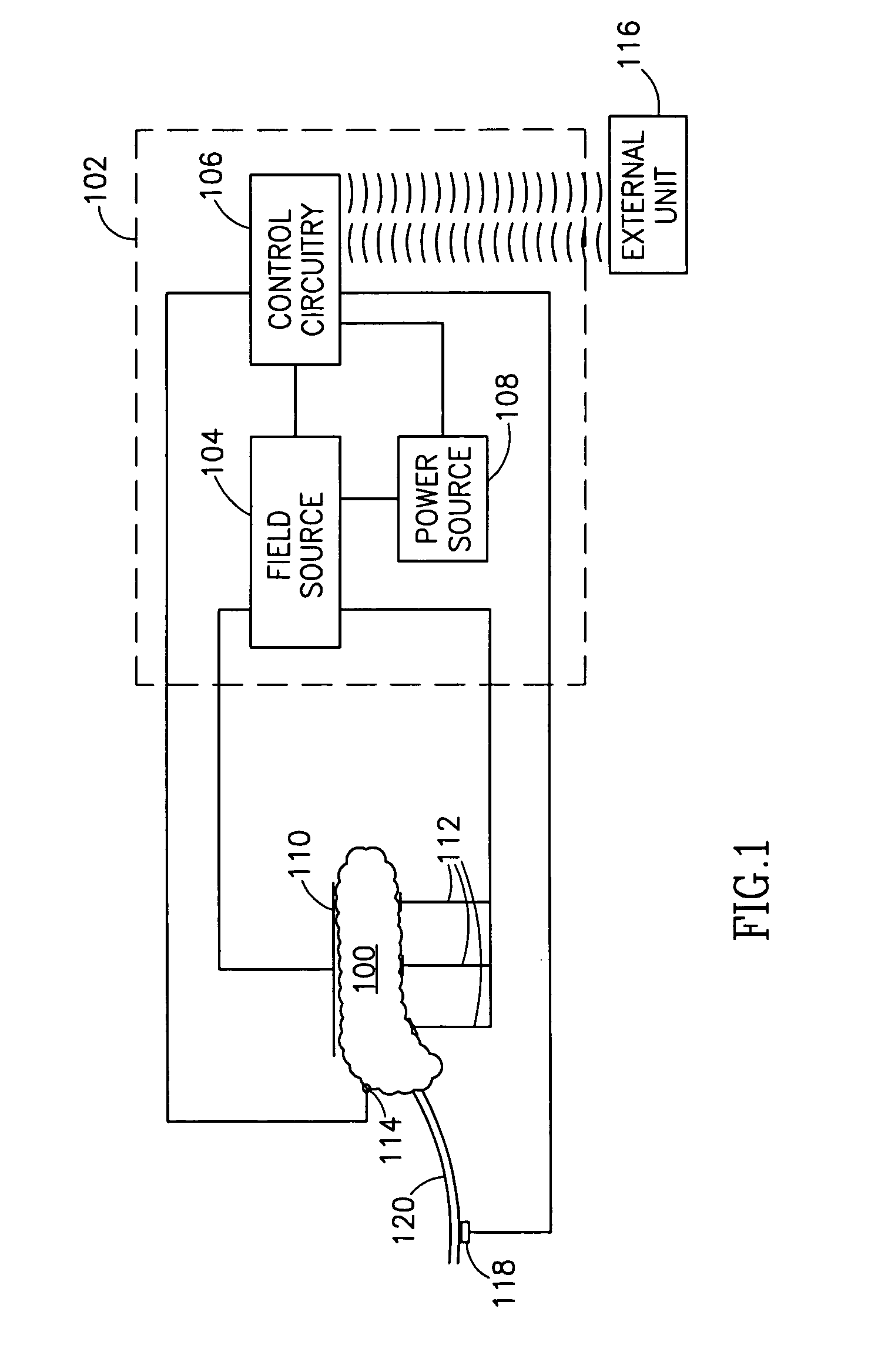
InactiveUS7006871B1Increased insulin secretionAvoiding unacceptable calcium level profileElectrotherapyDiagnostic recording/measuringGlucose sensorsLevel insulin
A pancreatic controller (102), comprising: a glucose sensor (118), for sensing a level of glucose or insulin in a body serum; at least one electrode (110, 112), for electrifying an insulin producing cell or group of cells; a power source (104) for electrifying said electrode with a pulse that does not initiate an action potential in said cell and has an effect of increasing insulin secretion; and a controller (106) which receives the sensed level and controls said power source to electrify said electrode to have a desired effect on said level.
Owner:METACURE
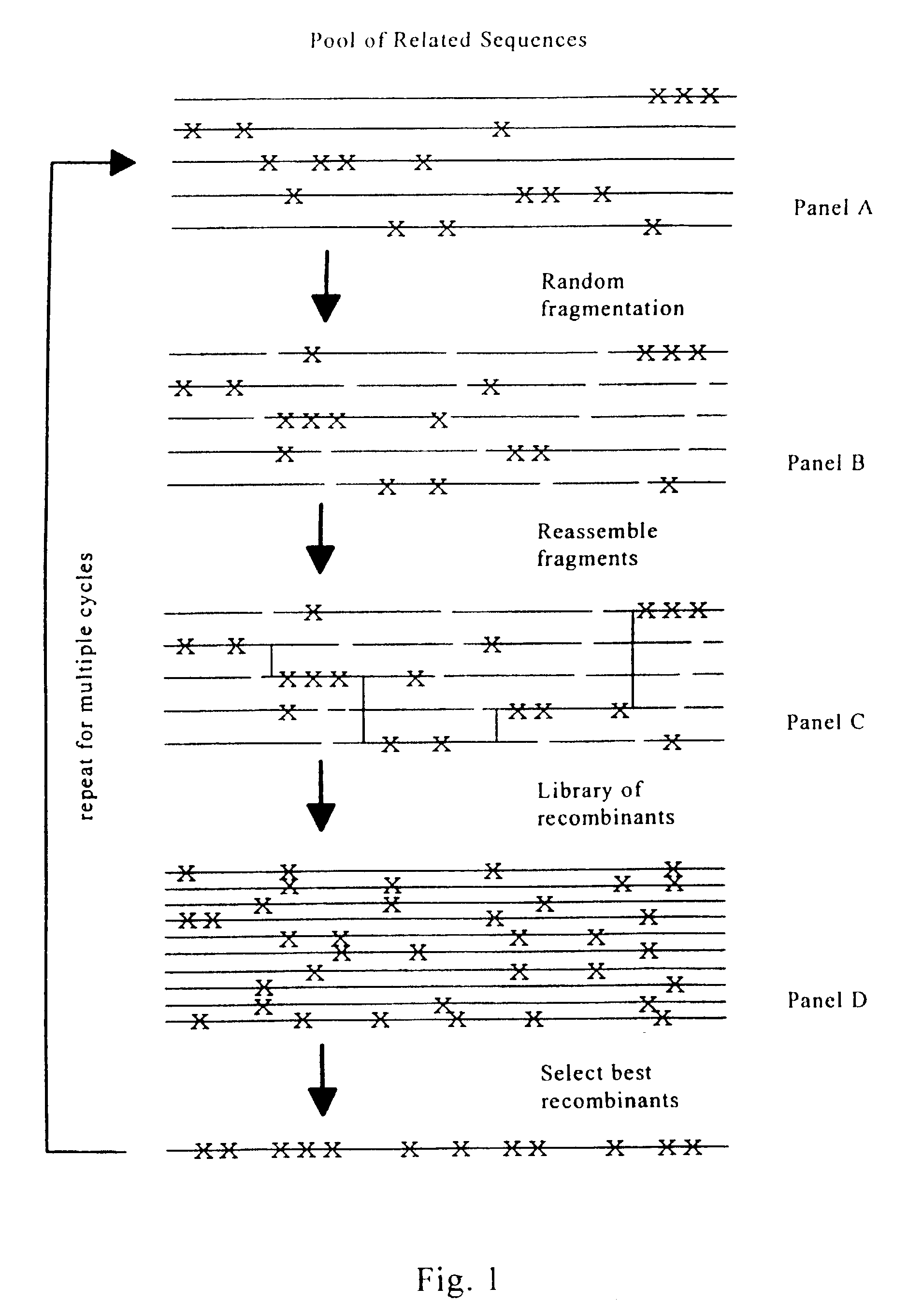
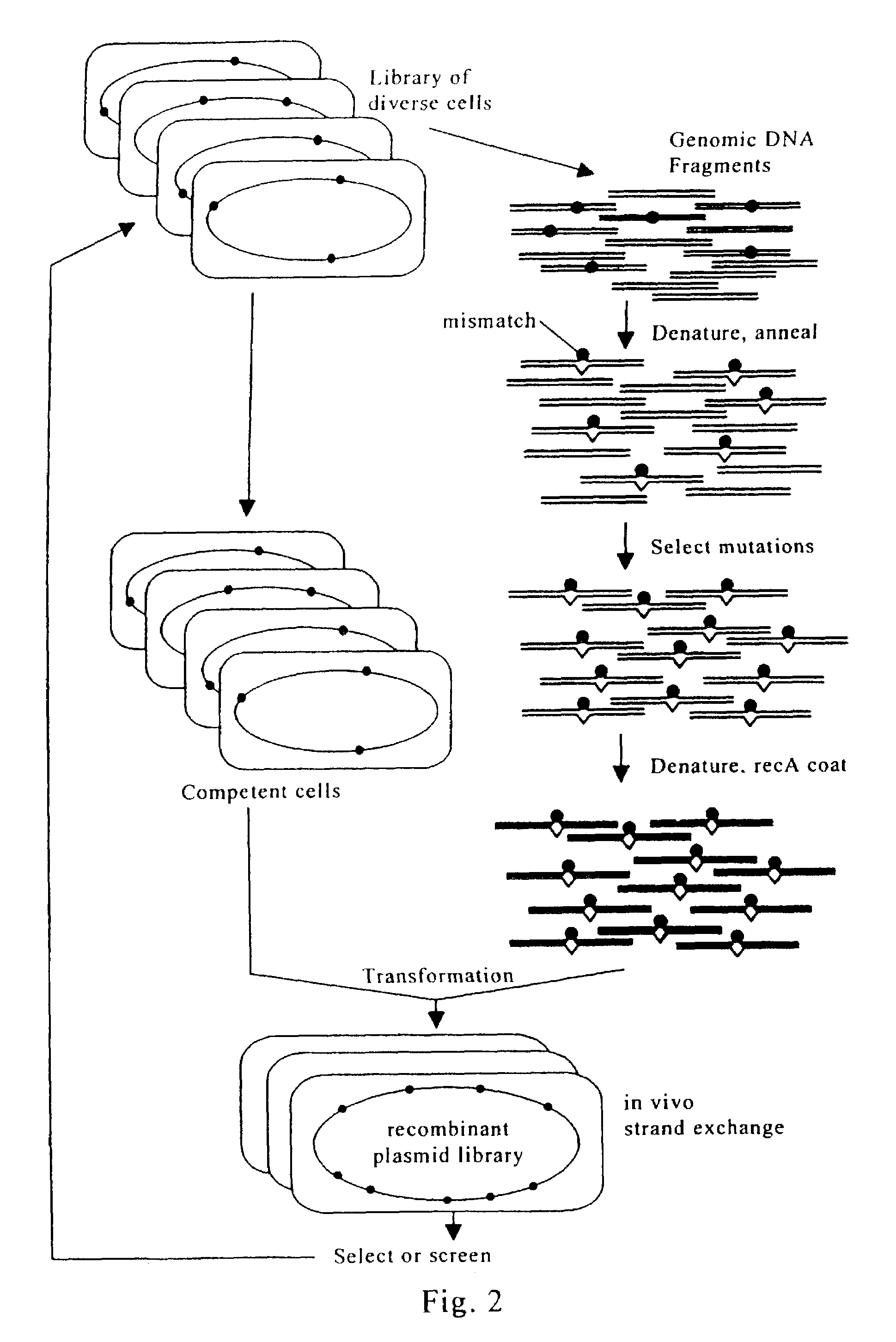
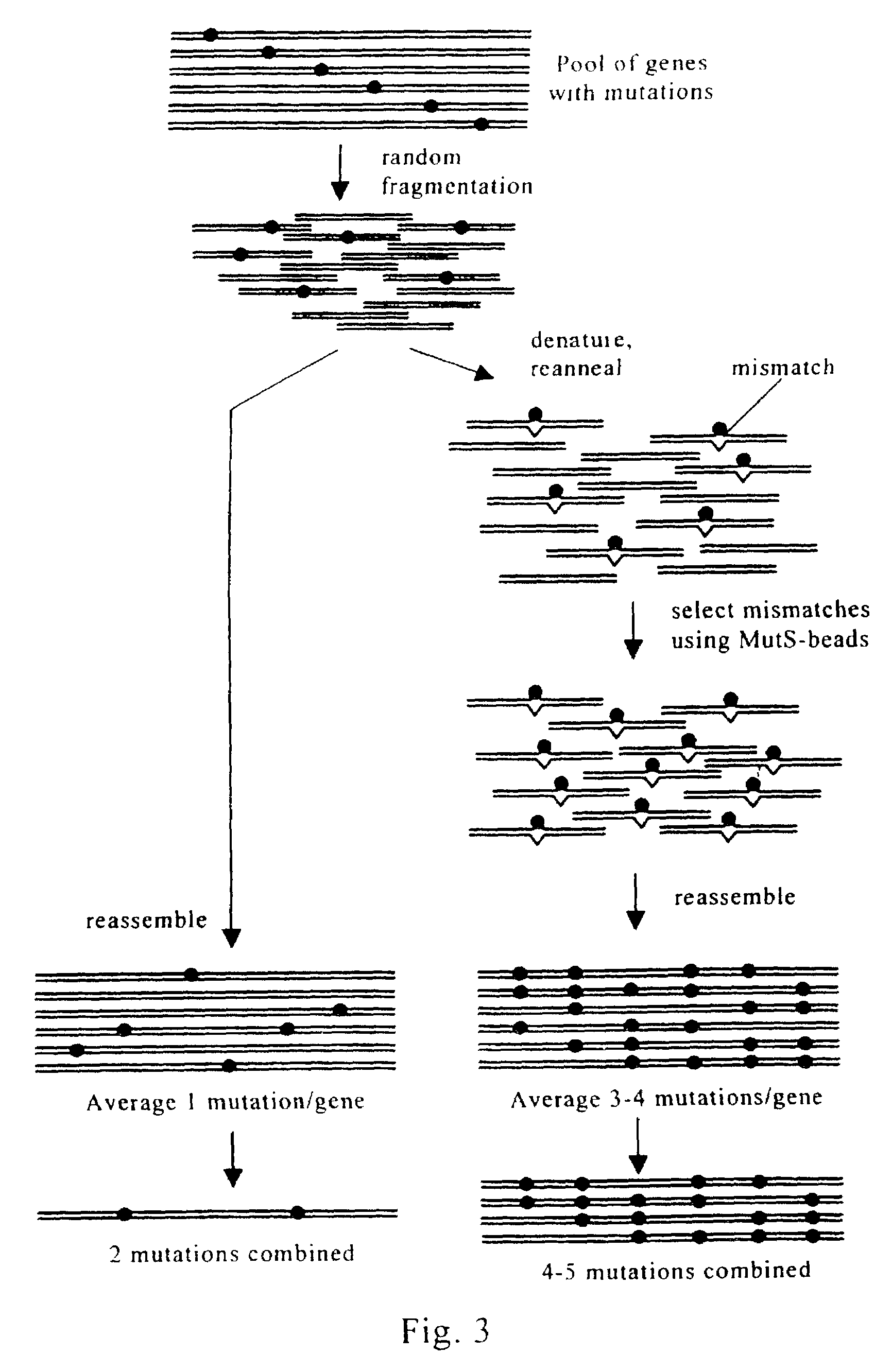
Evolution of whole cells and organisms by recursive sequence recombination
InactiveUS7148054B2Increase diversityFungiMicrobiological testing/measurementMetaboliteSecondary metabolite
The invention provides methods employing iterative cycles of recombination and selection / screening for evolution of whole cells and organisms toward acquisition of desired properties Examples of such properties include enhanced recombinogenicity, genome copy number, and capacity for expression and / or secretion of proteins and secondary metabolites.
Owner:CODEXIS MAYFLOWER HLDG LLC
Device for the Treatment of Wounds Using a Vacuum
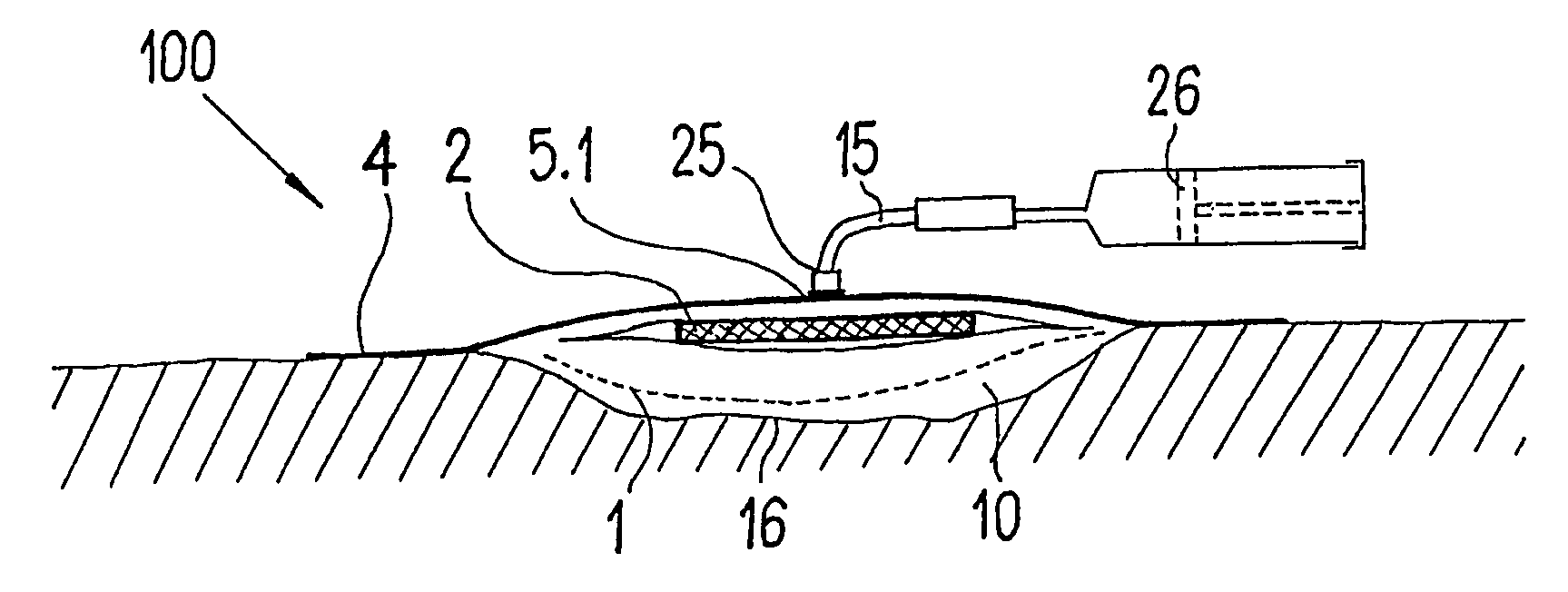
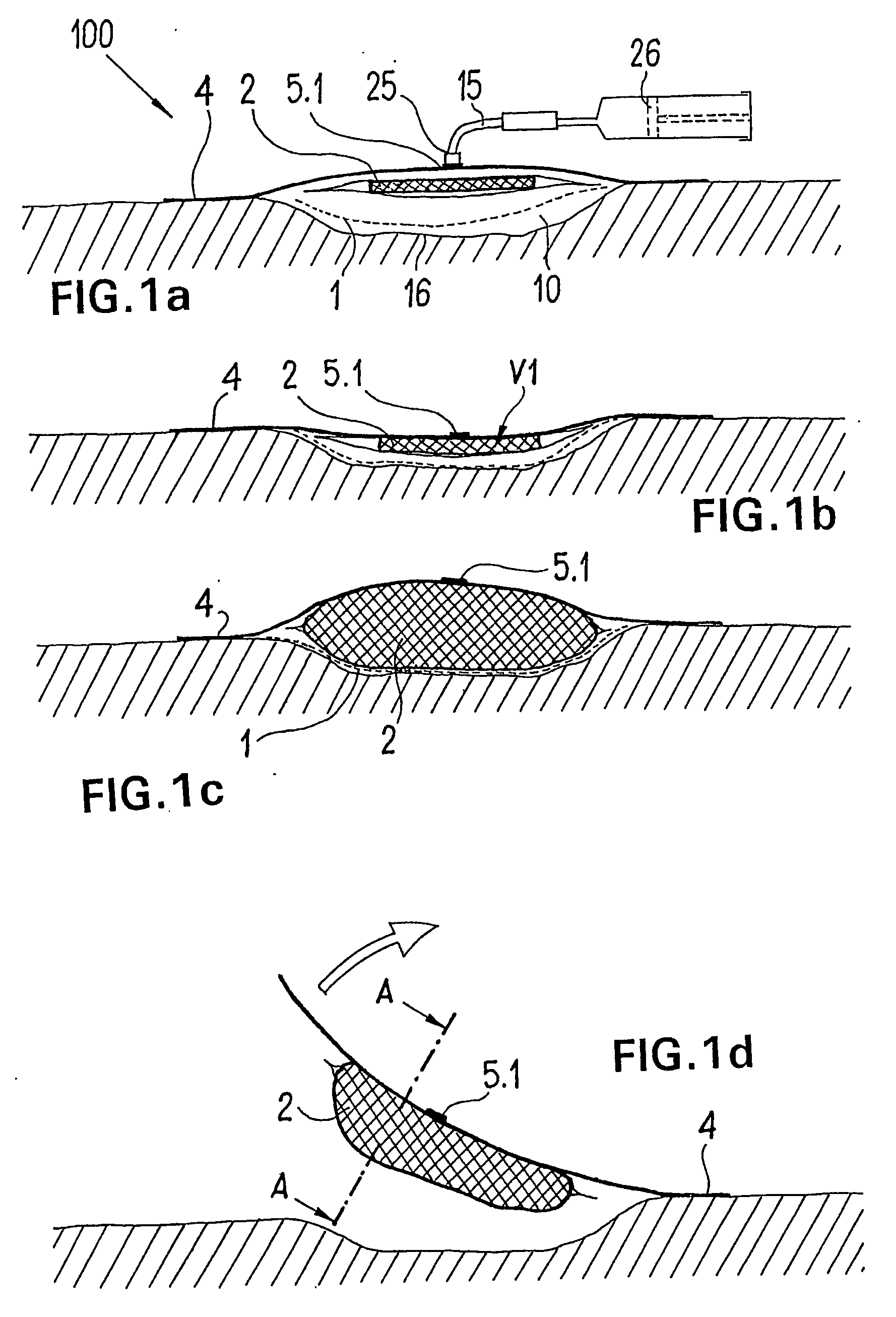
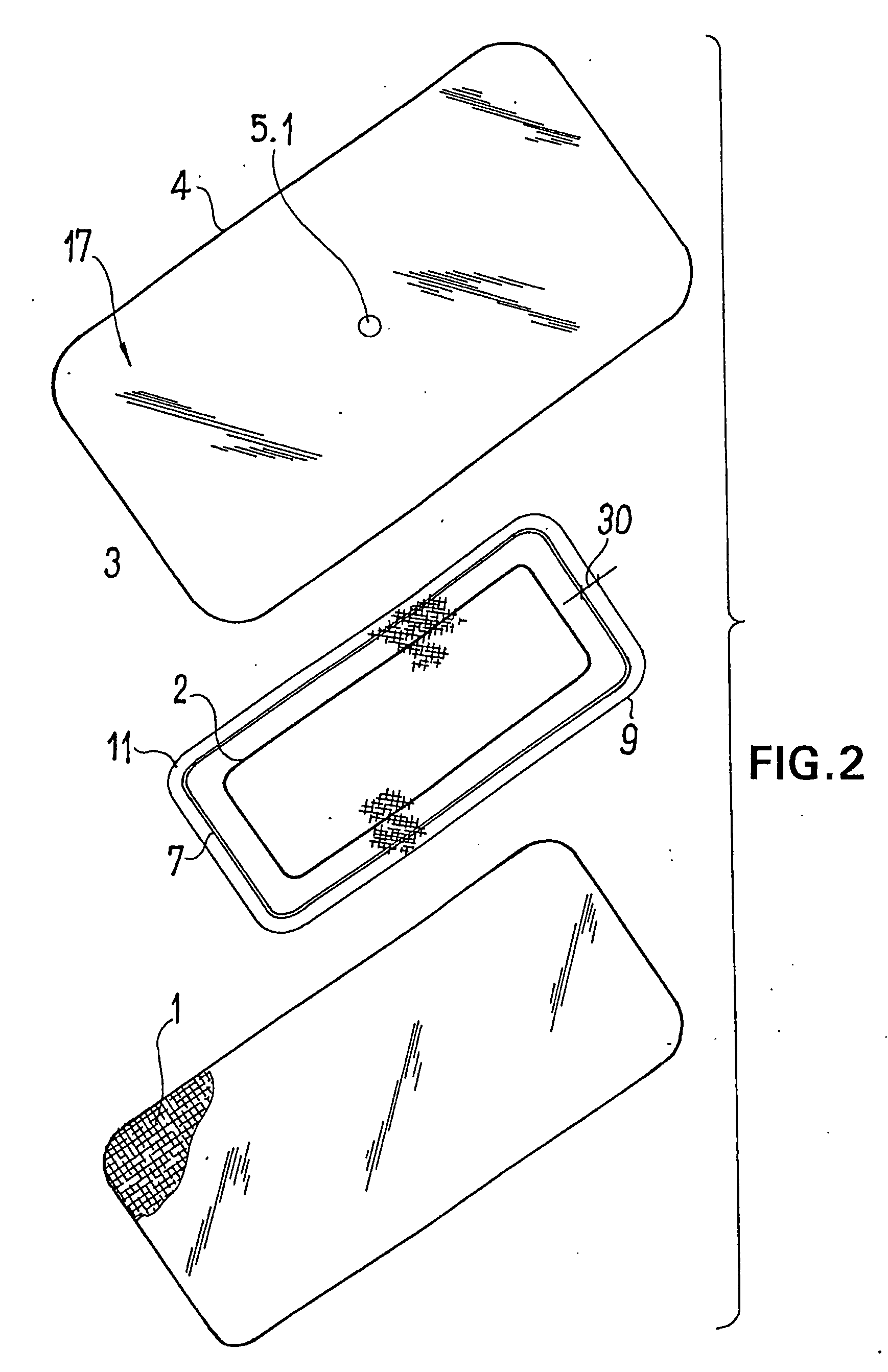
ActiveUS20080009812A1Cost effectiveSimple designNon-adhesive dressingsPlastersSecretionSecreted substance
The invention relates to a device (100) for treating wounds of the human or animal body using a vacuum and having a gas-tight wound-covering element (4), which, when placed in contact with the body of the patient, forms a wound space (10) between the respective wound and the wound-covering element, at least one connecting site (5.1; 5.2), which is in contact with the wound space (10), an absorption body (2), which is a layer, enclosed in an envelope, of a textile section, interspersed with super-absorbing particles, the envelope being permeable to liquids and having pores, the size of which does not exceed that of the super-absorbing particles. The absorption body (2), which is to be inserted in the wound space (10), has an initial volume, which enlarges in the course of the absorption process, and a final volume, so that, due to the size of the pores of the envelope, the absorbed wound secretions remain within the absorption body (2) and, with that, below the wound-covering element, until the absorption body is removed from the wound space.
Owner:BSN MEDICAL GMBH & CO KG
Inverse Sawtooth Pressure Wave Train Purging In Medical Ventilators
InactiveUS20100051029A1RespiratorsOperating means/releasing devices for valvesEngineeringGas release
This disclosure describes systems and methods for purging narrow diameter sensor tubing, occasionally referred to as “sensor lines”, in a ventilation system. The disclosure describes a novel approach in which a series of short, periodic releases of pressurized gas through the sensor tubes are used to clear any blockages due to condensation or patient secretions.
Owner:TYCO HEALTHCARE GRP LP
Apparatus and method of providing high frequency variable pressure to a patient
InactiveUS6581596B1Facilitates loosening and removalRespiratorsOperating means/releasing devices for valvesBreathing gasSecretion
A high frequency pressure oscillation device that selective restricts the flow of breathing gas to or from a patient to produce pressure spikes in the patient's airway that facilitate clearing secretions from the patient's airway. The device includes a patient circuit that defines a closed path between a source of breathing gas and the patient's airway. A valve is disposed in the patient circuit such that in an open position the path between the source of breathing gas and the airway of the patient is substantially unobstructed. When the valve is in a closed position, the path between the source of breathing gas and the patient's airway is at least partially obstructed to create the pressure spikes. An actuating system associated with the valve alternatively places the valve in the open position and in the closed position at a predetermined oscillation rate that is independent of patient effort.
Owner:RIC INVESTMENTS LLC
Postpartum cells derived from umbilical cord tissue, and methods of making and using the same
Cells derived from human umbilical cords are disclosed along with methods for their therapeutic use. Isolation techniques, culture methods and detailed characterization of the cells with respect to their cell surface markers, gene expression, and their secretion of trophic factors are described.
Owner:DEPUY SYNTHES PROD INC
Continuous high-frequency oscillation breathing treatment apparatus
ActiveUS7191780B2Assist in mucus secretionSimply and inexpensively manufacturingRespiratorsOperating means/releasing devices for valvesInhalationBreathing treatments
Owner:COMEDICA INC
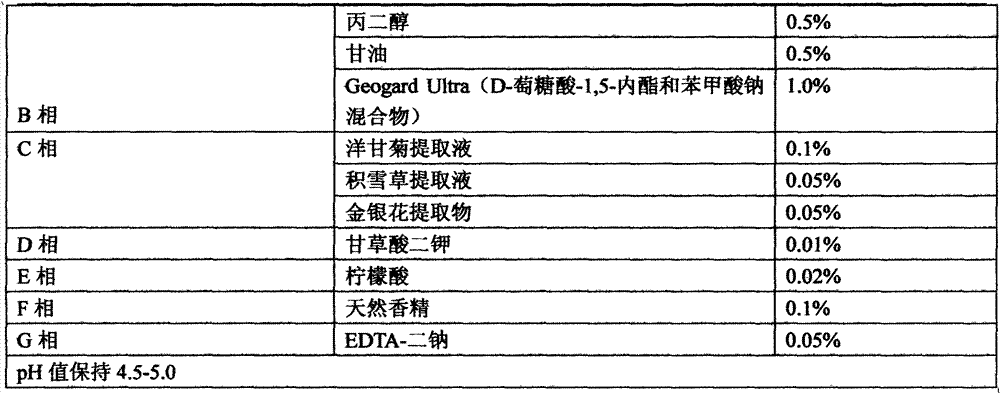
Natural plant extract composition and application thereof in cosmetics
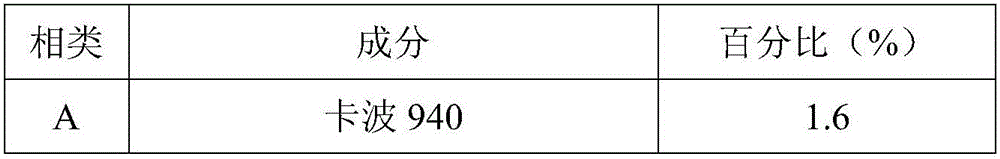
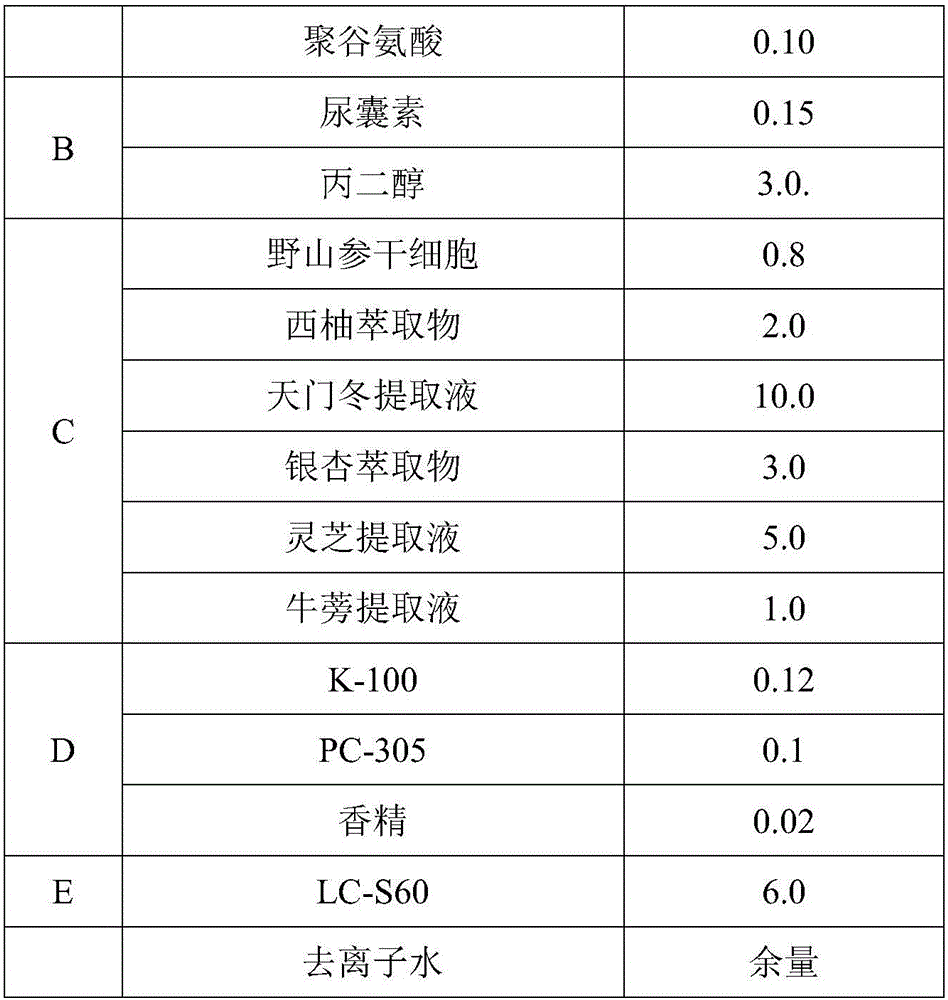
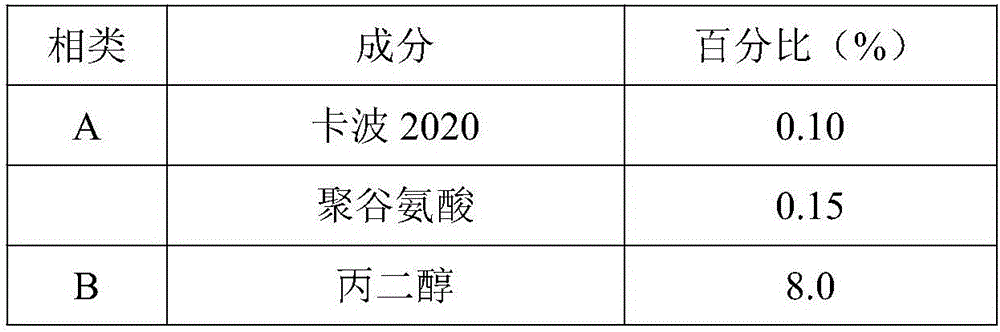
ActiveCN105748343ASpeed up the repair processIncrease elasticityCosmetic preparationsToilet preparationsBiologyInflammation
The invention relates to the technical field of cosmetics, in particular to a natural plant extract composition and application thereof in the cosmetics. The natural plant extract composition is prepared from the following raw materials in parts by weight: 0.1 to 10 parts of plant stem cells, 2 to 10 parts of grapefruit extracts, 1 to 10 parts of radix asparagi extracting solution, 1 to 10 parts of gingko extracts, 5 to 10 parts of lucid ganoderma extracting solution, and 1 to 5 parts of burdock extracting solution. The natural plant extract composition provided by the invention not only has remarkable moisturizing and anti-aging effect, but also has the effects in removing acnes, adjusting grease secretion, resisting inflammation and relieving, and can be used for preparing moisturizing, anti-aging or acne-removing cosmetics.
Owner:GUANGDONG COOWAY BIOTECH CO LTD
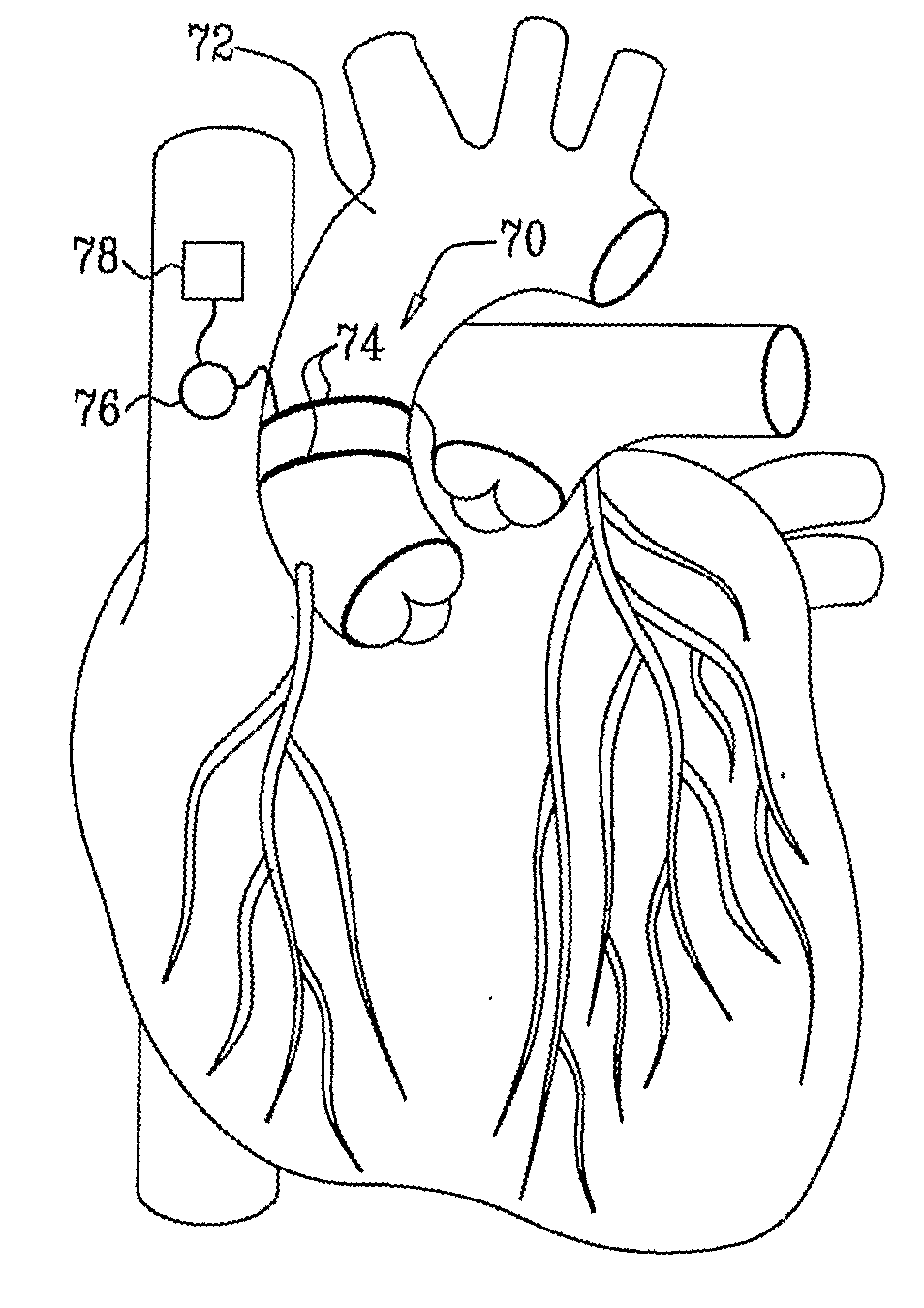
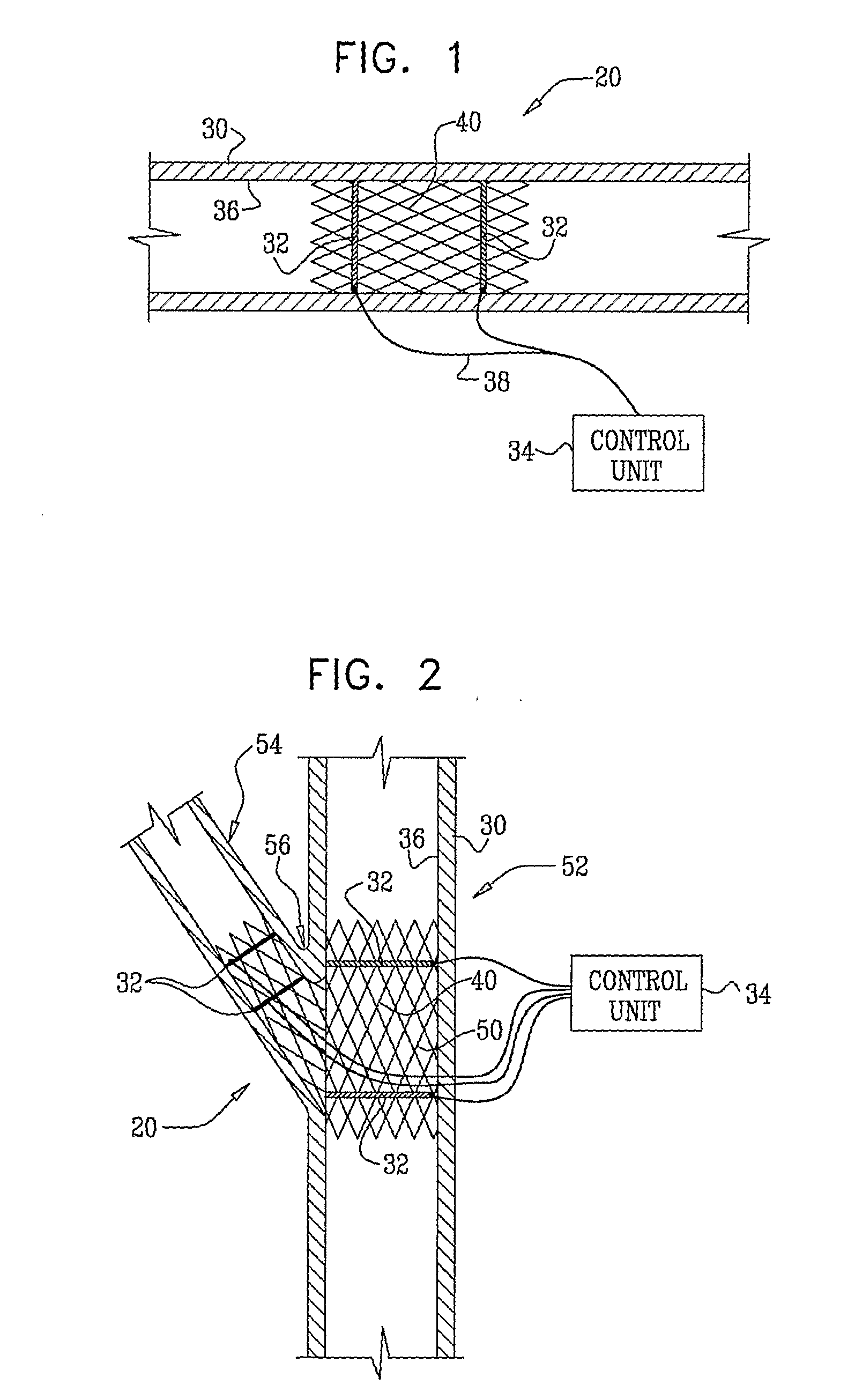
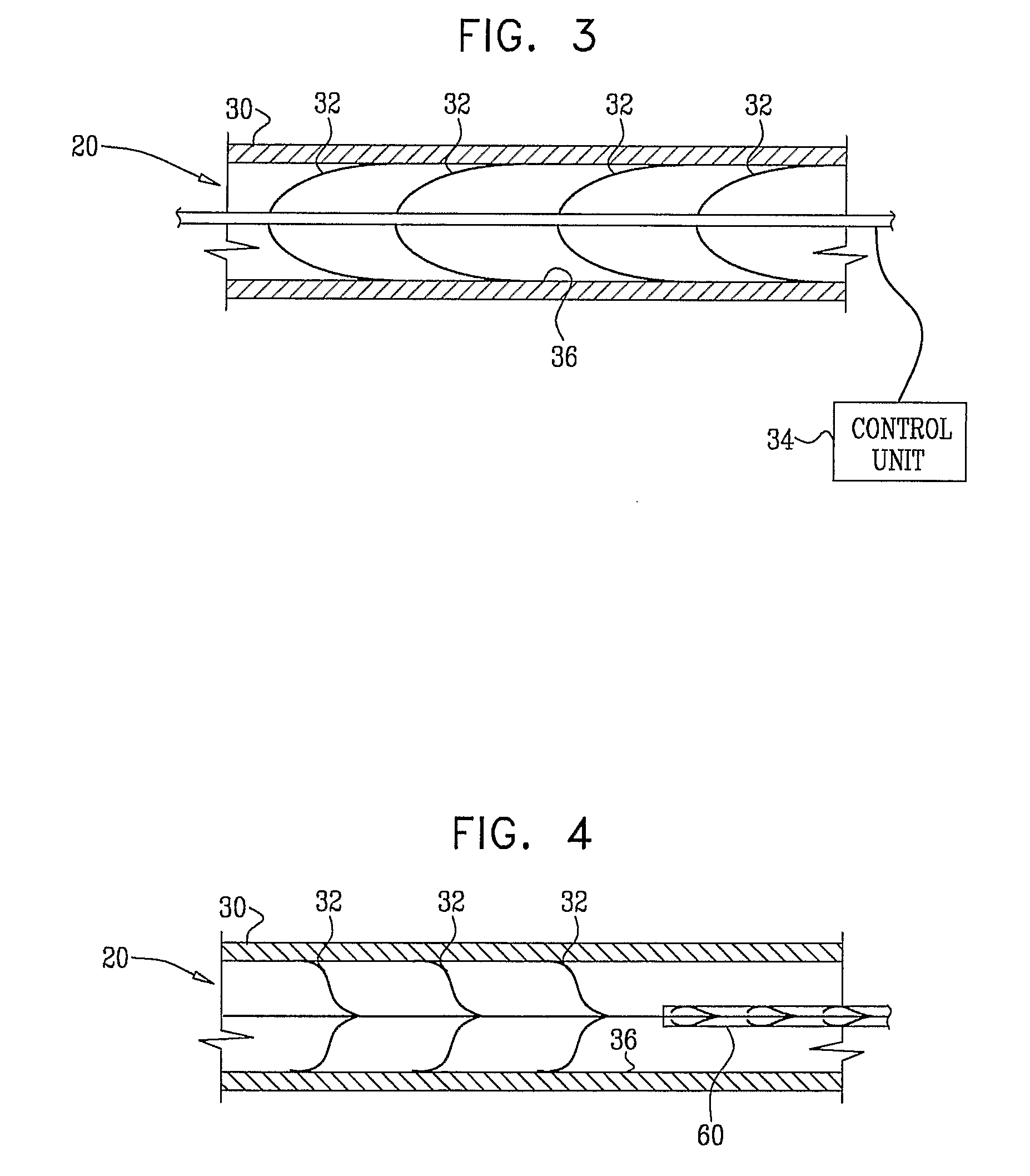
Electrical Stimulation of Blood Vessels
ActiveUS20080215117A1Increase heightReducing platelet aggregationStentsTransvascular endocardial electrodesNitric oxideSecretion
Apparatus (20) is provided, including a bifurcation stent (50) comprising one or more electrodes (32), the stent (50) configured to be placed in a primary passage (52) and a secondary passage (54) of a blood vessel (30), and a control unit (34), configured to drive the electrodes (32) to apply a signal to a wall (36) of the blood vessel (30), and to configure the signal to increase nitric oxide (NO) secretion by the wall (36). Other embodiments are also described.
Owner:ENOPACE BIOMEDICAL
Postpartum cells derived from umbilical cord tissue, and methods of making, culturing, and using the same
Cells derived from human umbilical cords are disclosed along with methods for their therapeutic use. Isolation techniques, culture methods and detailed characterization of the cells with respect to their cell surface markers, gene expression, and their secretion of trophic factors are described.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
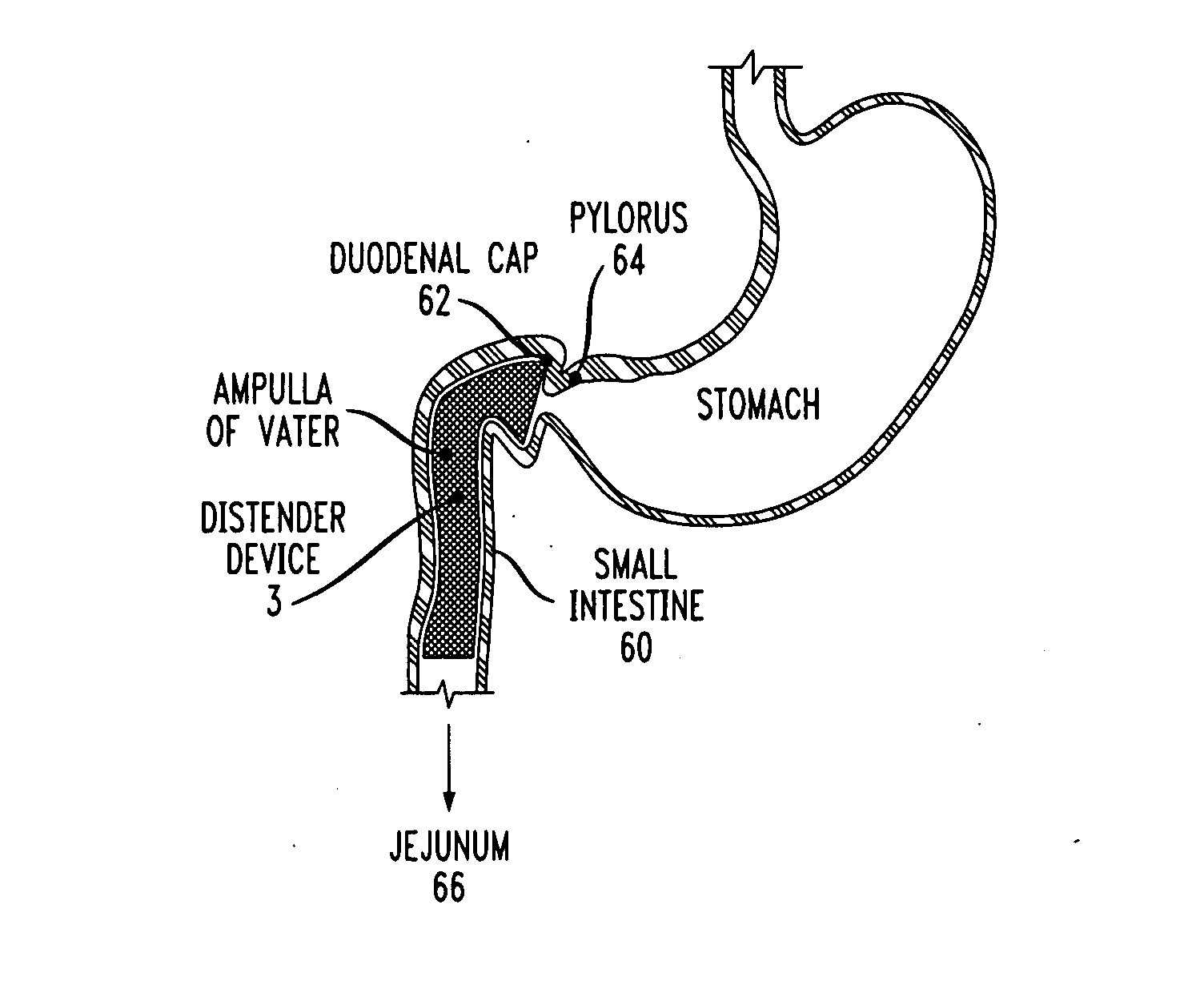
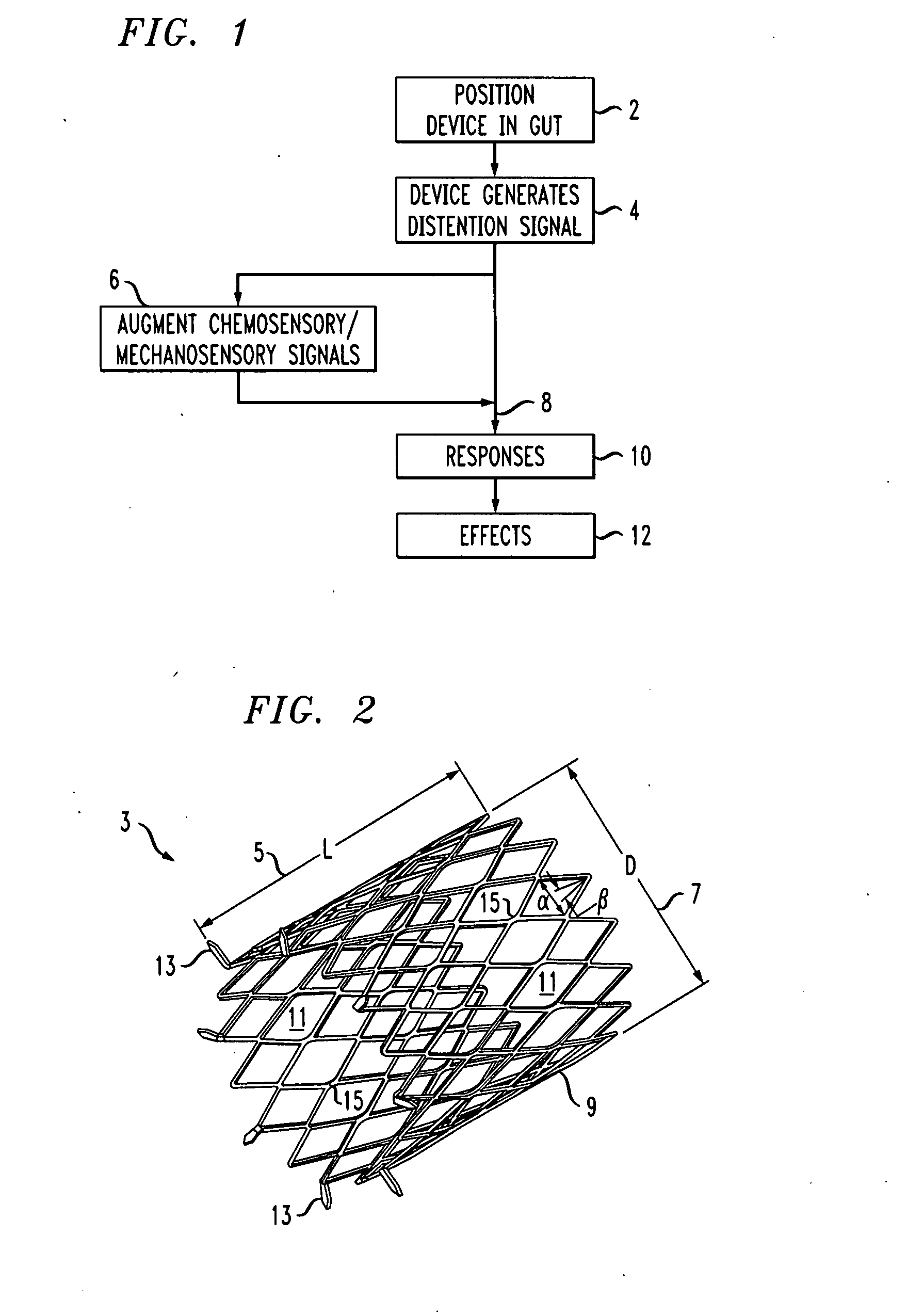
Distender device and method for treatment of obesity and metabolic and other diseases
A gastrointestinal implant device is positioned in a patient's small intestine or rectum and produces an outward force that itself produces a distension signal which is a therapeutically useful neural or humoral signal that evokes satiogenic or weight loss effects by itself. The device may advantageously be placed in the duodenum adjacent the pylorus or in the jejunum, ileum or rectum. The distension signals may amplify chemosensory or mechanosensory signals such as enteroendocrine secretions within the patient. The device may be a mesh and include a low material density that allows for unrestricted chyme absorption within the small intestine and unrestricted chyme flow through the gastrointestinal system. A method includes inserting the device into the patient then either retrieving the device after treatment is complete or allowing a device formed of a biodegradable material to degrade in time after treatment is complete.
Owner:ADVANCED NEUROMODULATION SYST INC
Wound drainage dressing
ActiveUS20100262091A1Easy constructionEasy to transportSurgical needlesPlastersSecretionBiomedical engineering
The invention relates to a wound drainage covering for covering, by means of low pressure, a wound that is to be treated. The covering comprises at least two layers that are superimposed. A first layer that is applied on the side of the wound is made of a functional textile material and second layer that is arranged thereon is dimensionally stable and permeable to liquid. The wound drainage covering has a simple design and due to the functional first layer, is effective and ensures, due to the dimensionally stable second layer, an optimal removal of wound secretion.
Owner:MEDELA HLDG AG
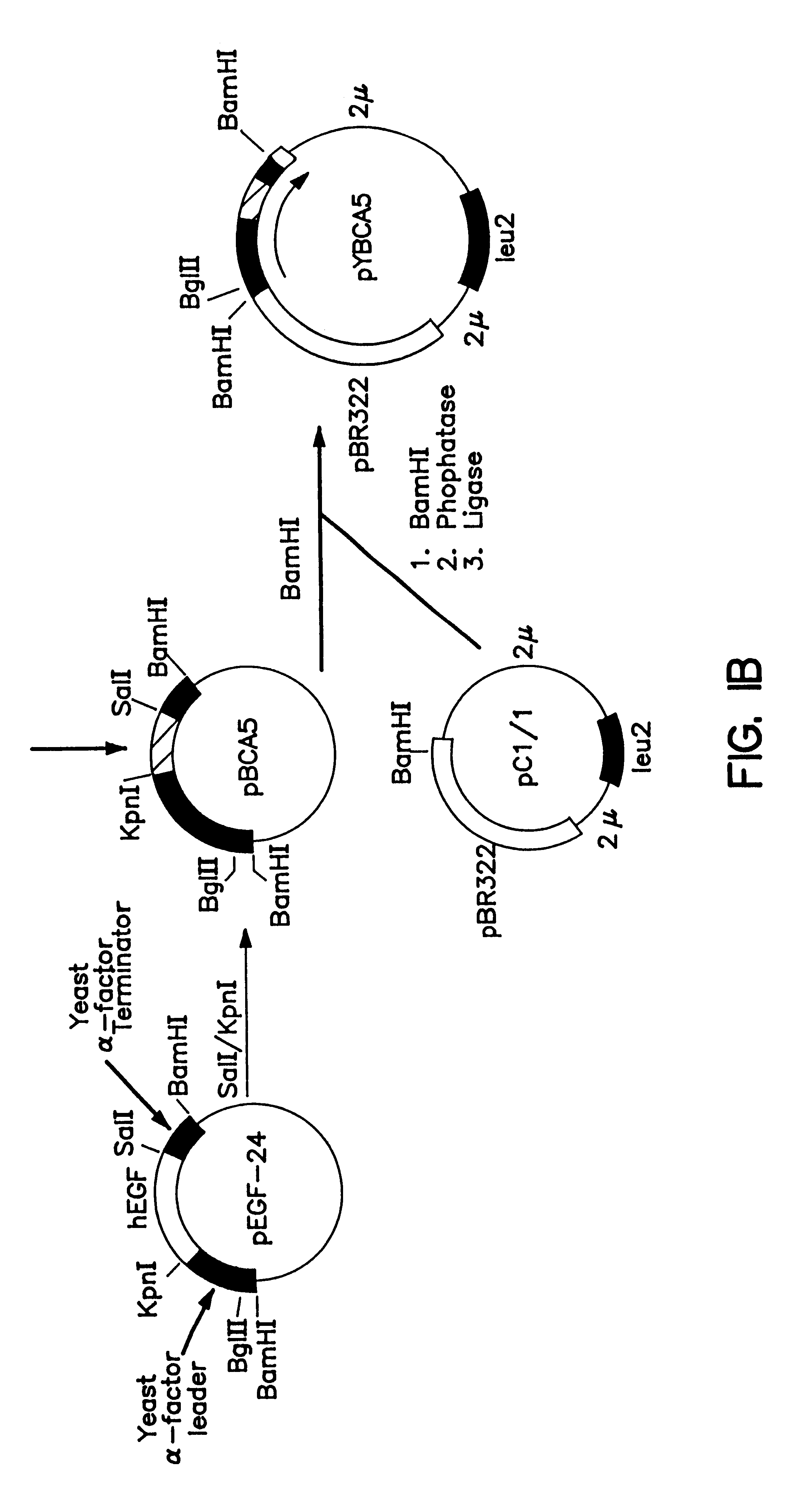
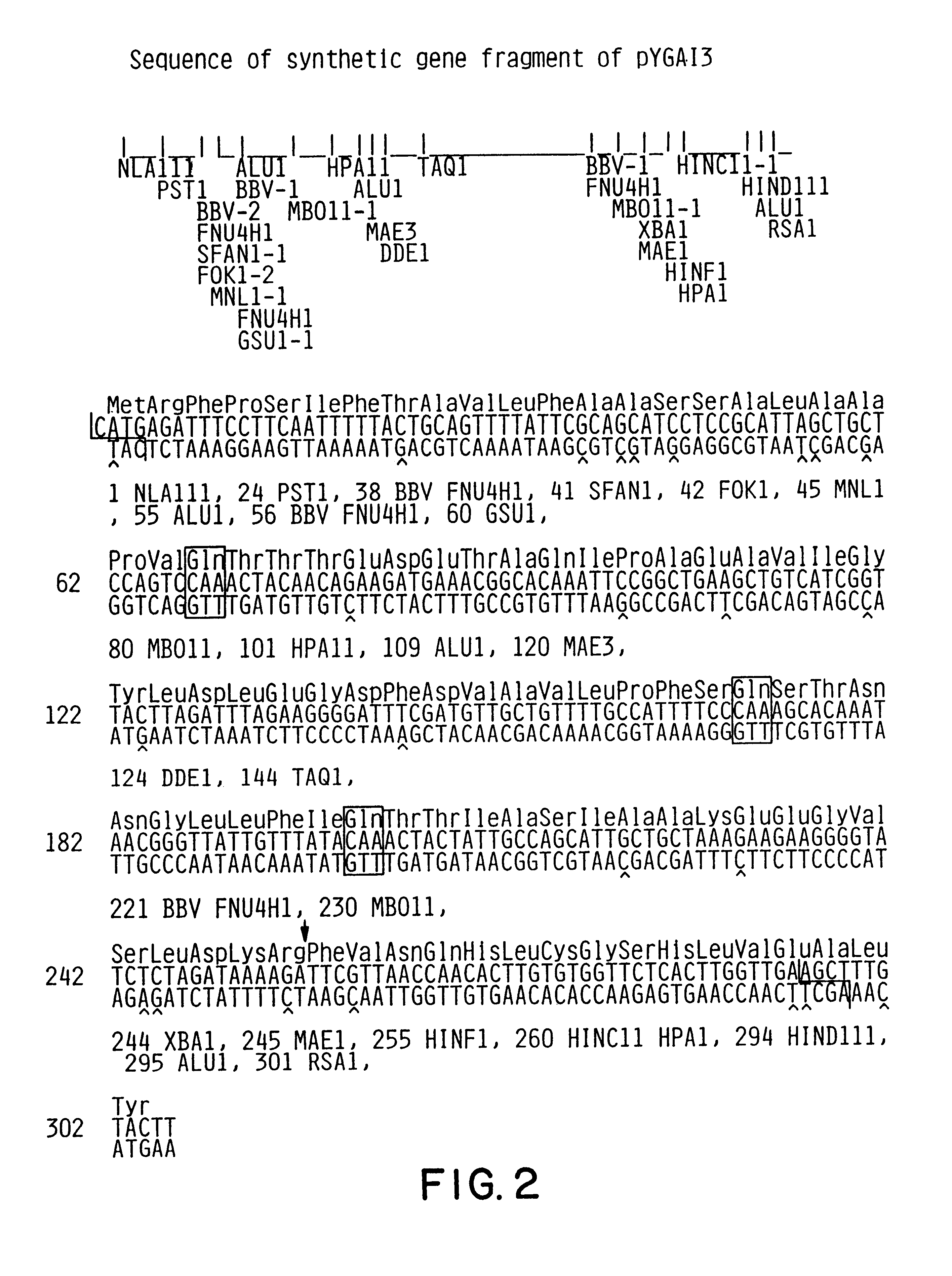
Expression and secretion of heterologous proteins in yeast employing truncated alpha-factor leader sequences
InactiveUSRE37343E1Efficiently direct expressionEfficient secretionPolypeptide with localisation/targeting motifFungiHeterologousBiotechnology
A yeast alpha-factor expression system is provided comprised of a truncated leader sequence, containing the alpha-factor signal peptide and one glycosylation site, linked by a processing site to a non-yeast protein-encoding sequence.
Owner:CHIRON CORP
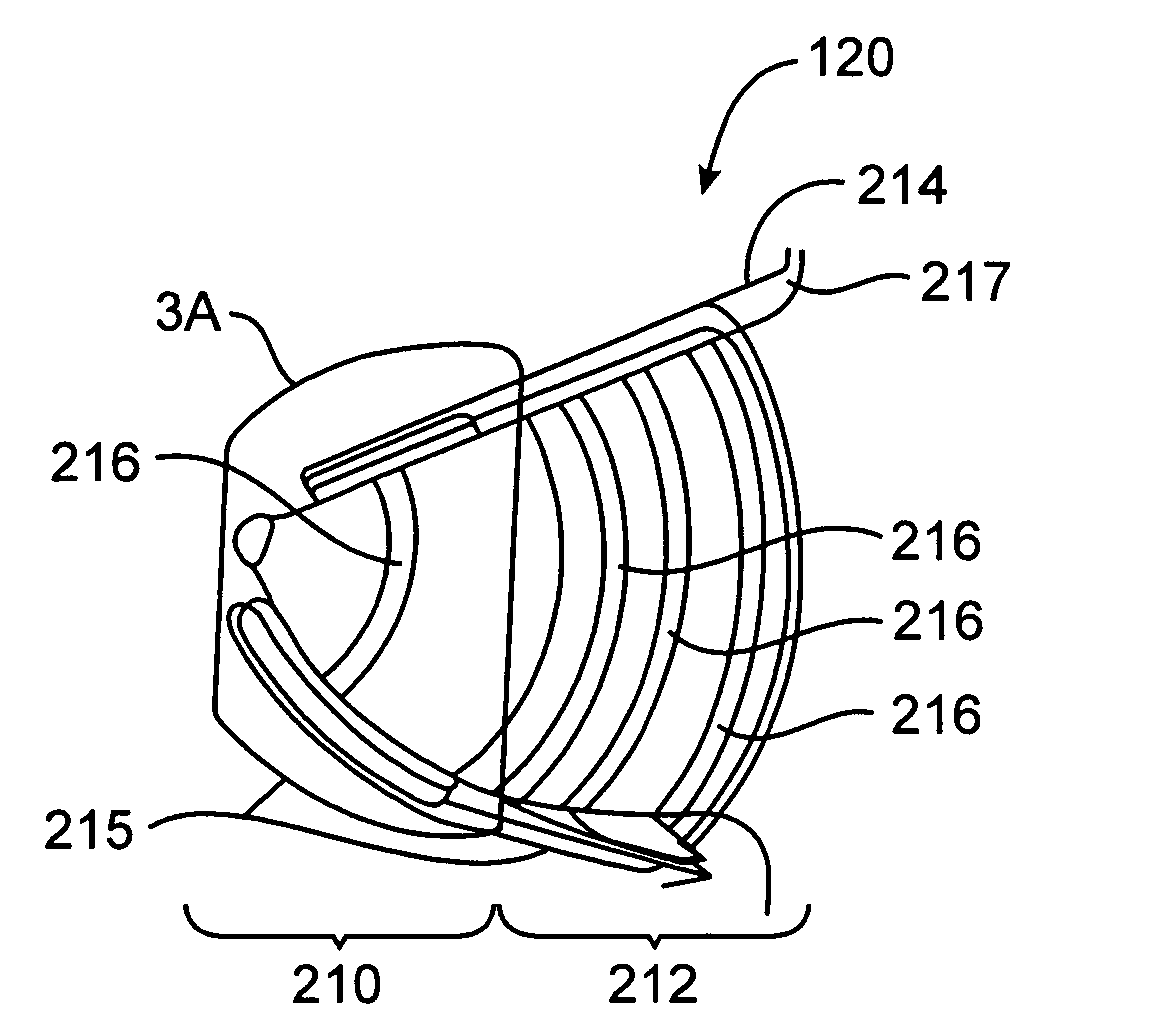
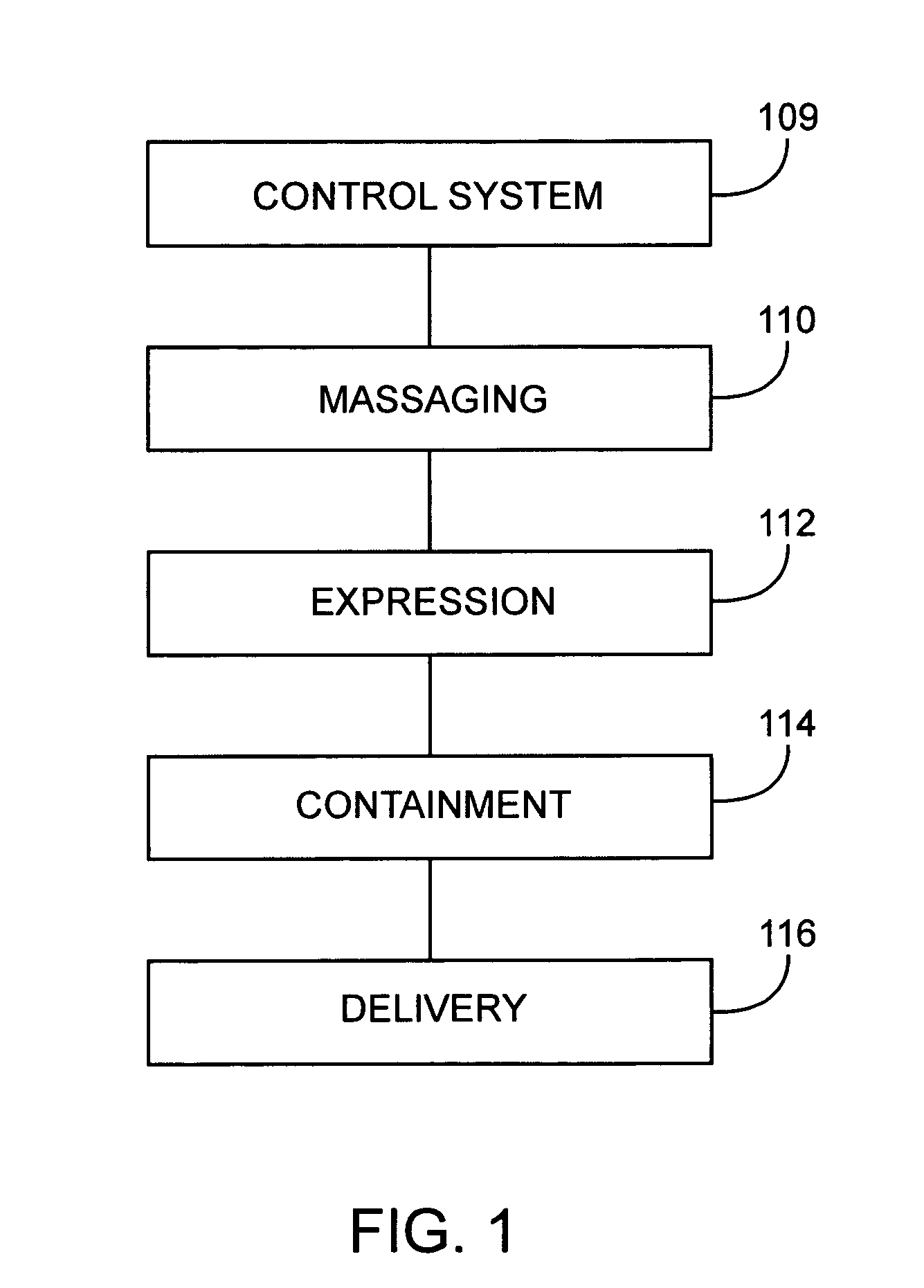
Breast milk expression system including massaging, expressing and containment features
InactiveUS20050234370A1Facilitating lifestyleHealthy and safe and efficient breast milk expression sessionPneumatic massageMilking pumpLactiferous sinusOxytocin
Disclosed is a breast pump that supports a mother's and infant's breastfeeding needs and facilitates the lifestyle of the mother's choice. The breast milk expression system disclosed allows breastfeeding mothers to obtain milk comfortably, hands-free, conveniently and with discretion. The breast milk expression system disclosed includes at least two contact points which mimic hand-expression as well as infant suckling and effectively stimulate the secretion of oxytocin and prolactin to provide a healthy, safe and efficient breastfeeding session. The system disclosed has a gentle rhythmic massage means and is located a distance from the base of the nipple and massages in all quadrants of the breast. The rhythmic forward pressure is provided by a plurality of opposing pairs of expression bellows that move the milk from the lactiferous sinuses through the nipple pores, bio-mimicking the techniques of hand expression.
Owner:PURONYX
Compositions and methods for administering Borrelia DNA
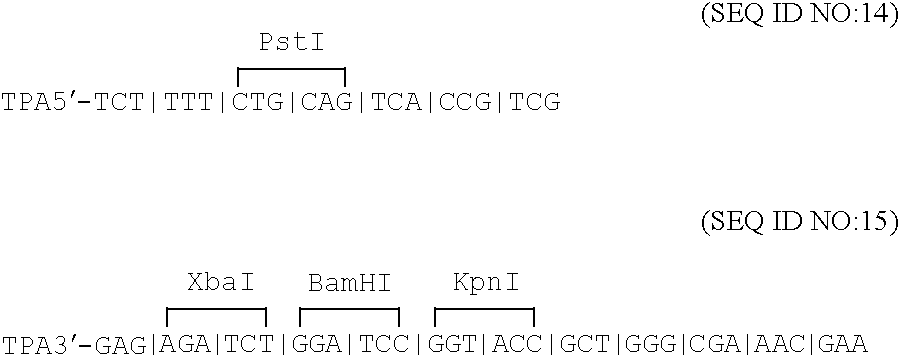
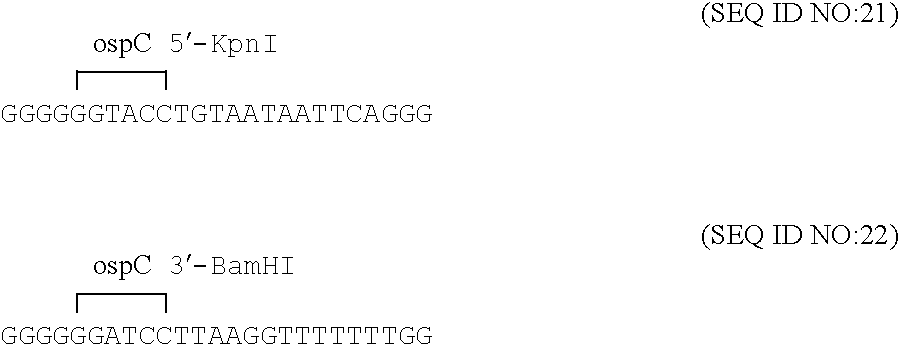
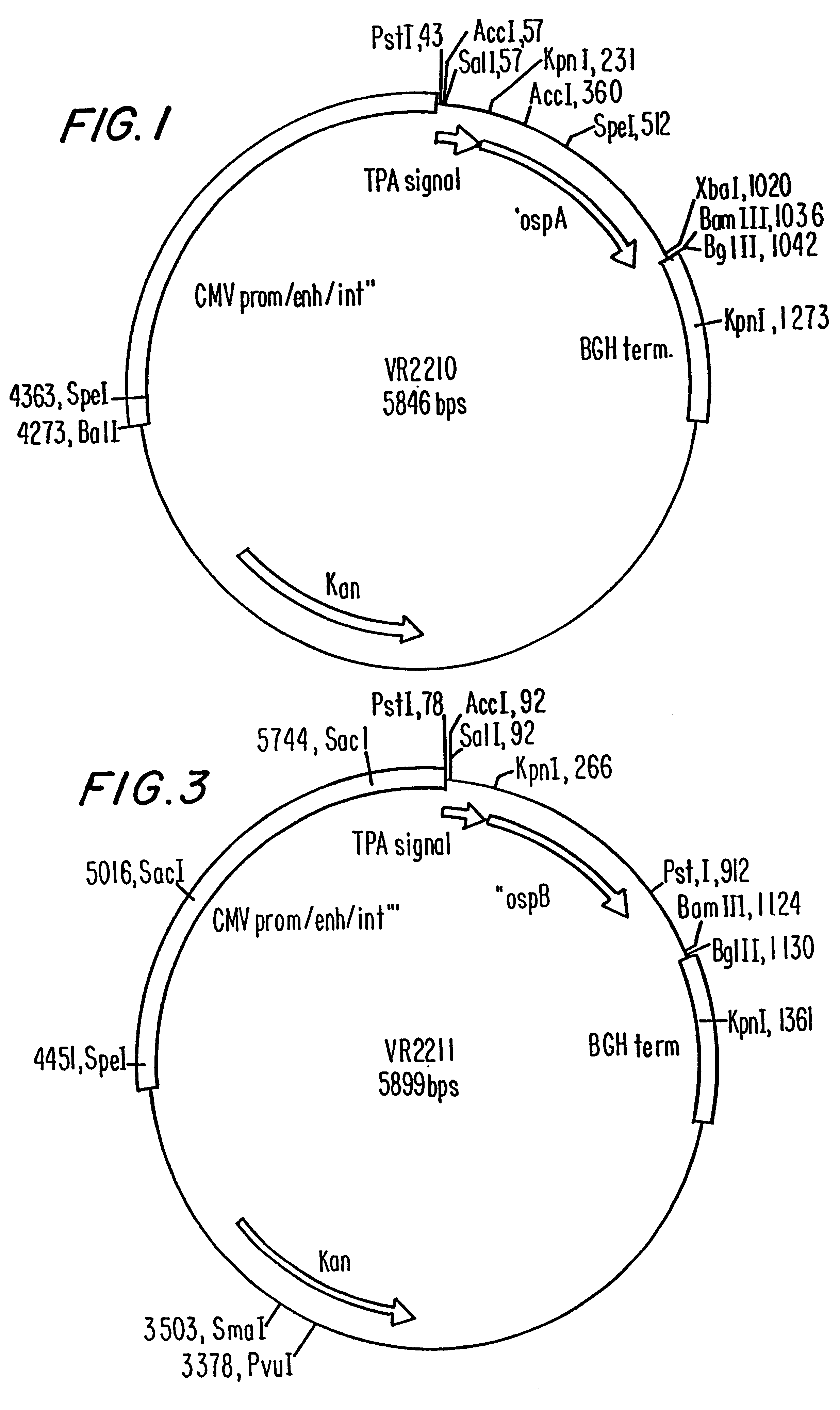
Disclosed is a vaccine against Lyme Disease or its causative agent Borrelia burgdorferi (sensu stricto or sensu lato) containing a plasmid a DNA encoding a promoter for driving expression in a mammalian cell, DNA encoding a leader peptide for facilitating secretion / release of a prokaryotic protein sequence from a mammalian cell, a DNA encoding Borrelia OspA or OspB, and a DNA encoding a terminator. Disclosed too is an immunogenic composition against Lyme Disease or its causative agent Borrelia burgdorferi (sensu stricto or sensu lato) containing a plasmid comprising a DNA encoding a promoter for driving expression in a mammalian cell, DNA encoding a leader peptide for facilitating secretion / release of a prokaryotic protein sequence from a mammalian cell, a DNA encoding a Borrelia OspC, and a DNA encoding a terminator. And, methods for making and using such vaccines and the immunogenic composition are also disclosed.
Owner:PASTEUR MERIEUX SERUMS & VACCINS SA
Spicy coarse grain dried bean curd and preparation method thereof
InactiveCN103444883AFull of nutritionHigh nutritional valueCheese manufactureFood scienceReady to eatLiver and kidney
The invention discloses spicy coarse grain dried bean curd. The spicy coarse grain dried bean curd is prepared from the following raw materials in parts by weight: 90 to 100 parts of dried soybeans, 3 to 4 parts of oat, 3 to 4 parts of millet, 4 to 5 parts of sorghum, 4 to 5 parts of corns, 2 to 3 parts of mulberries, 2 to 3 parts of hawthorn, 2 to 3 parts of jujubes, 1 to 2 parts of calamus leaves, 1 to 2 parts of platycladus orientalis leaves, 2 to 3 parts of prepared rehmannia root, 1 to 2 parts of fructus amomi, 1 to 2 parts of rehmannia flower, 1 to 2 parts of loofah sponge, 2 to 3 parts of cassia bark, and 2 to 3 parts of rhodiola rosea. A variety of grains are added into the spicy coarse grain dried bean curd, so that a nutritionally balanced structure system is formed; a plurality of seasonings are added, so that the dried bean curd tastes spicy and is easily preferred by consumers; the healthcare components of a plurality of traditional Chinese medicines are added, so that the nutrition value of the dried bean curd is high; the finished dried bean curd is ready to eat, spicy, unique, rich in nutrients and chewy, has the effects of stimulating appetite, improving digestion, tonifying liver and kidney, tonifying spleen and stomach, cooling blood to stop bleeding, promoting the secretion of saliva or body fluid and lubricating intestines, and is the best healthcare food catering to the market and consumers.
Owner:JINCAIDI FOOD CO LTD
Dual expression vector system for antibody expression in bacterial and mammalian cells
ActiveUS7112439B2Maintaining their functionalityEasy to identifyAnimal cellsBacteriaAntigenBacteroides
The present invention provides a dual expression vector, and methods for its use, for the expression and secretion of a full-length polypeptide of interest in eukaryotic cells, and a soluble domain or fragment of the polypeptide in bacteria. When expressed in bacteria, transcription from a bacterial promoter within a first intron and termination at the stop codon in a second intron results in expression of a fragment of the polypeptide, e.g., a Fab fragment, whereas in mammalian cells, splicing removes the bacterial regulatory sequences located in the two introns and generates the mammalian signal sequence, allowing expression of the full-length polypeptide, e.g., IgG heavy or light chain polypeptide. The dual expression vector system of the invention can be used to select and screen for new monoclonal antibodies, as well as to optimize monoclonal antibodies for binding to antigenic molecules of interest.
Owner:MACROGENICS INC
Device and method for treatment of wounds with nitric oxide
InactiveUS7122018B2Promote wound healingReduce the burden onBiocideOther blood circulation devicesHigh concentrationNitric oxide gas
Topical exposure of nitric oxide gas to wounds such as chronic non-healing wounds may be beneficial in promoting healing and preparing the wound bed for further treatment and recovery. Nitric oxide gas may be used to reduce the microbial infection, manage exudates secretion by reducing inflammation, upregulate expression of endogenous collagenase to locally debride the wound, and regulate the formation of collagen. High concentration of nitric oxide ranging from 160–400 ppm may be used without inducing toxicity in the healthy cells around a wound site. Exposure to the high concentration for a first treatment period reduces the microbial burden and inflammation, and increases collagenase expression to debride necrotic tissue at the wound site. After a first treatment period, a second treatment period at a lower concentration of nitric oxide, preferably ranging from 5–20 ppm may be used to restore the balance of nitric oxide and induce collagen expression aiding in the wound closure.
Owner:SENSORMEDICS +1
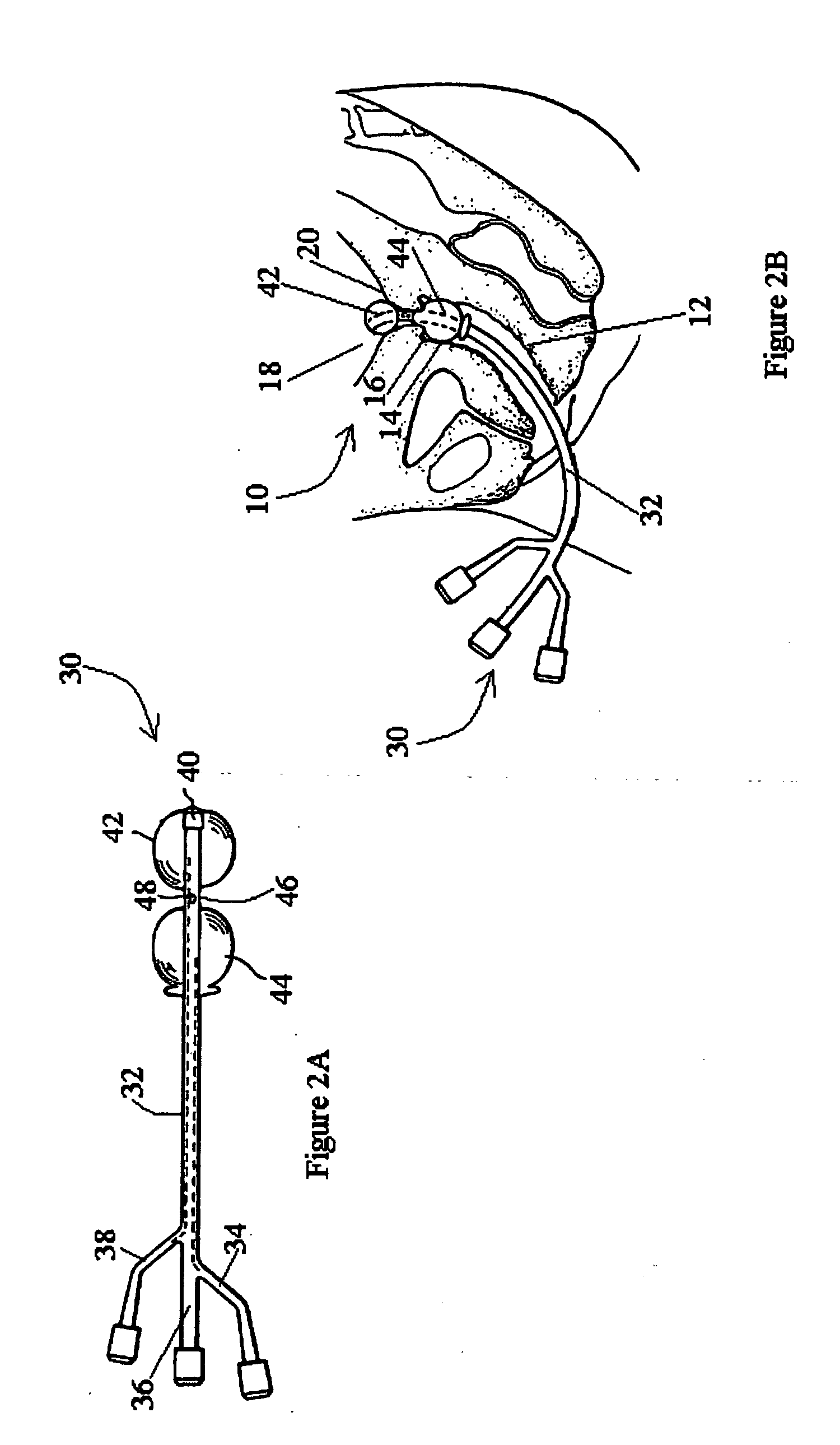
Inflatable system for cervical dilation and labor induction
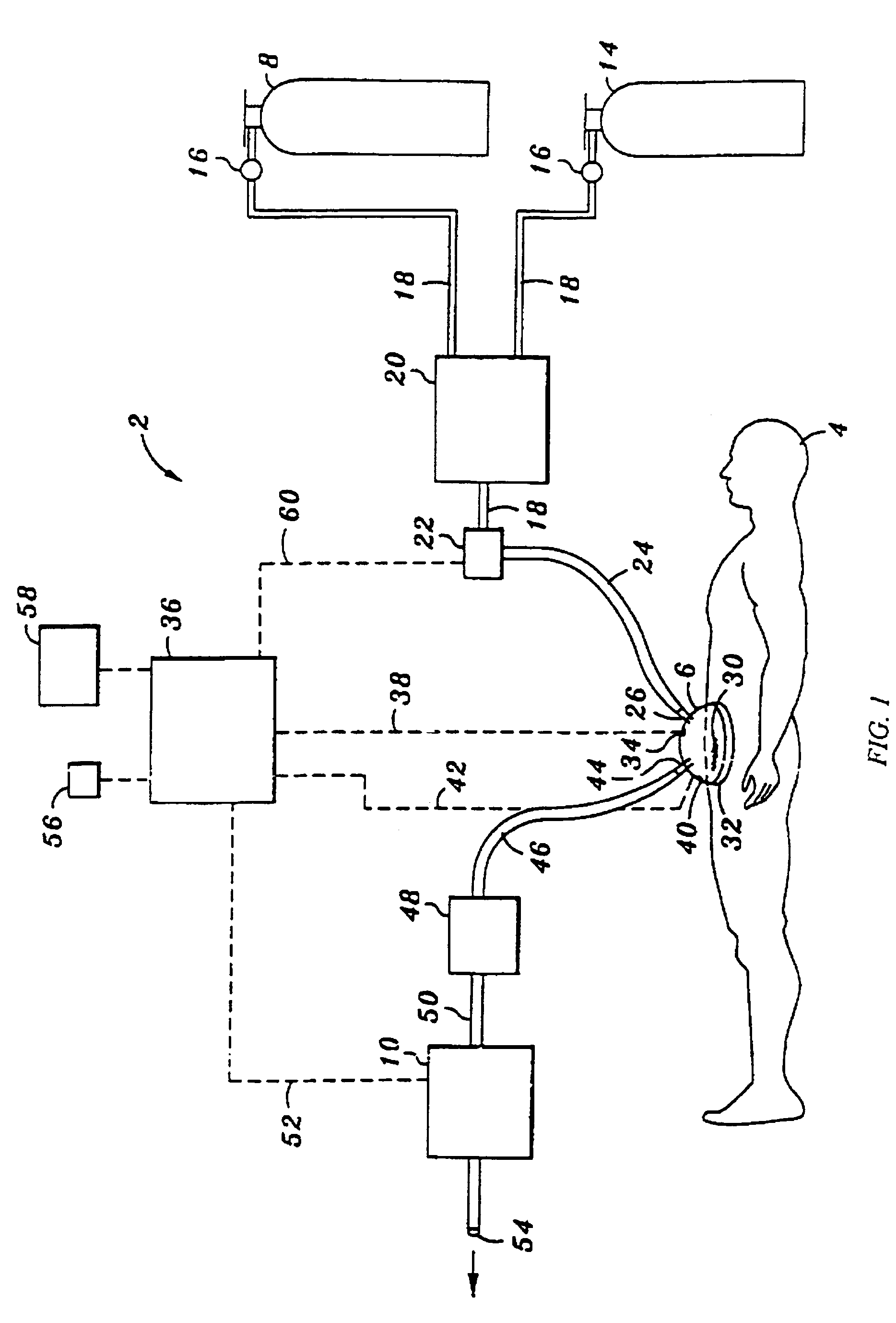
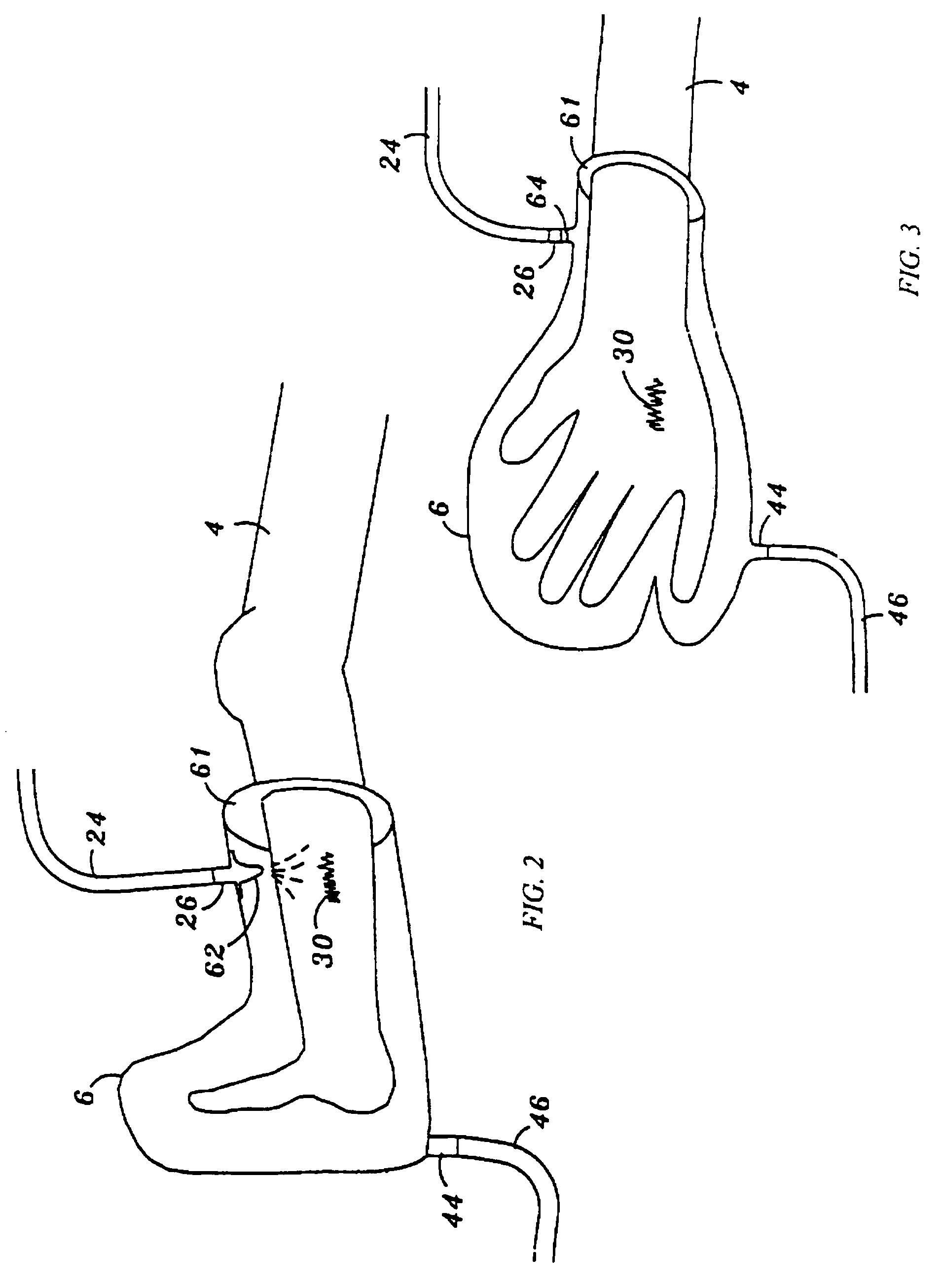
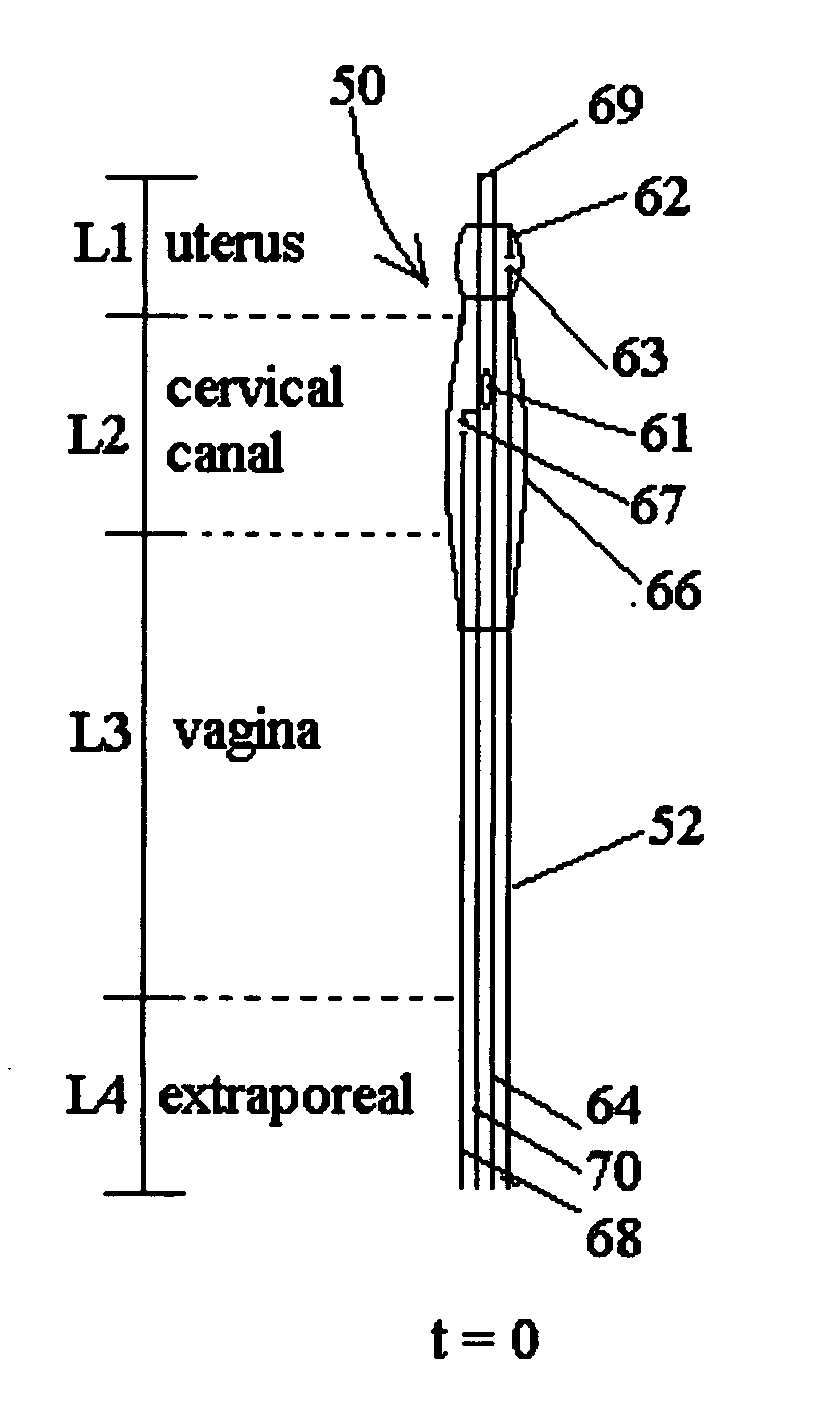
An inflatable system, of between one and three balloons, for cervical dilation and labor induction is provided. The inflatable system may have a uterine balloon, for positioning at a proximal portion of the uterus, with respect to an operator, adjacent to the cervical internal os, the uterine balloon being shaped so as to maximize the pressure against the decidua and the internal cervical os and so as to minimize the pressure on the fetal head. Additionally or alternatively, the inflatable system may have a vaginal balloon, for positioning in the vagina, for applying pressure on the external cervical os. Additionally or alternatively, the inflatable system may have a cervical balloon, for positioning in the cervical canal, the cervical balloon being shaped so as to maximize the contact area with the cervix. The balloons are operative to stimulate the secretion of hormone, by exerting pressure on the proximal decidual surfaces of the uterus and on the cervix, so as to soften and ripen the cervix, cause the cervix to dilate, and induce labor. The balloons, which may have rough external surfaces, in order to keep them anchored in place, may be inflated by the operator, directly after their insertion, or manually and gradually, by the woman herself. Various sensors and other instruments may be used with the inflatable system, to monitor cervical dilation, fetal well-being, and the woman's conditions.
Owner:ATAD - DEV & MEDICAL SERVICES
Hand-mouth baby wet tissue and preparation method thereof
InactiveCN103040688APromote secretionLower pHCosmetic preparationsToilet preparationsAdditive ingredientCleansing Agents
The invention relates to a hand-mouth baby wet tissue and a preparation method thereof. The hand-mouth baby wet tissue is composed of compound wet tissue liquid and non-woven fabrics for holding the compound wet tissue liquid, wherein every 100 parts of the compound wet tissue liquid comprises the following components in percent by weight: 0.01%-7.5% of a sweetening agent, 0.01-2.0% of a preservative, 0.01%-2.0% of a cleaning agent, 0.05%-5.0% of a solubilizer, 0-10.0% of a humectant, 0-5.0% of a skin care agent, 0-5.0% of an acidity regulator, 0-5.0% of essential oil, 0-5.0% of a stabilizer, 0-1.0% of natural essence, and the balance of pure water. The hand-mouth baby wet tissue has the advantages that edible sweetening agent is added into the compound wet tissue liquid to promote spittle secretion of babies, relieve the drop of pH value in the oral cavity and prevent the formation of decayed teeth. The product formula is stable and safe, and the material components are non-toxic. The formula is safe, non-irritant and non-corrosive, so that the allergic risks are reduced to be the lowest. The hand-mouth baby wet tissue can be used for cleaning the hands and mouths of an infant, infant tableware and other infant supplies and toys.
Owner:林建广
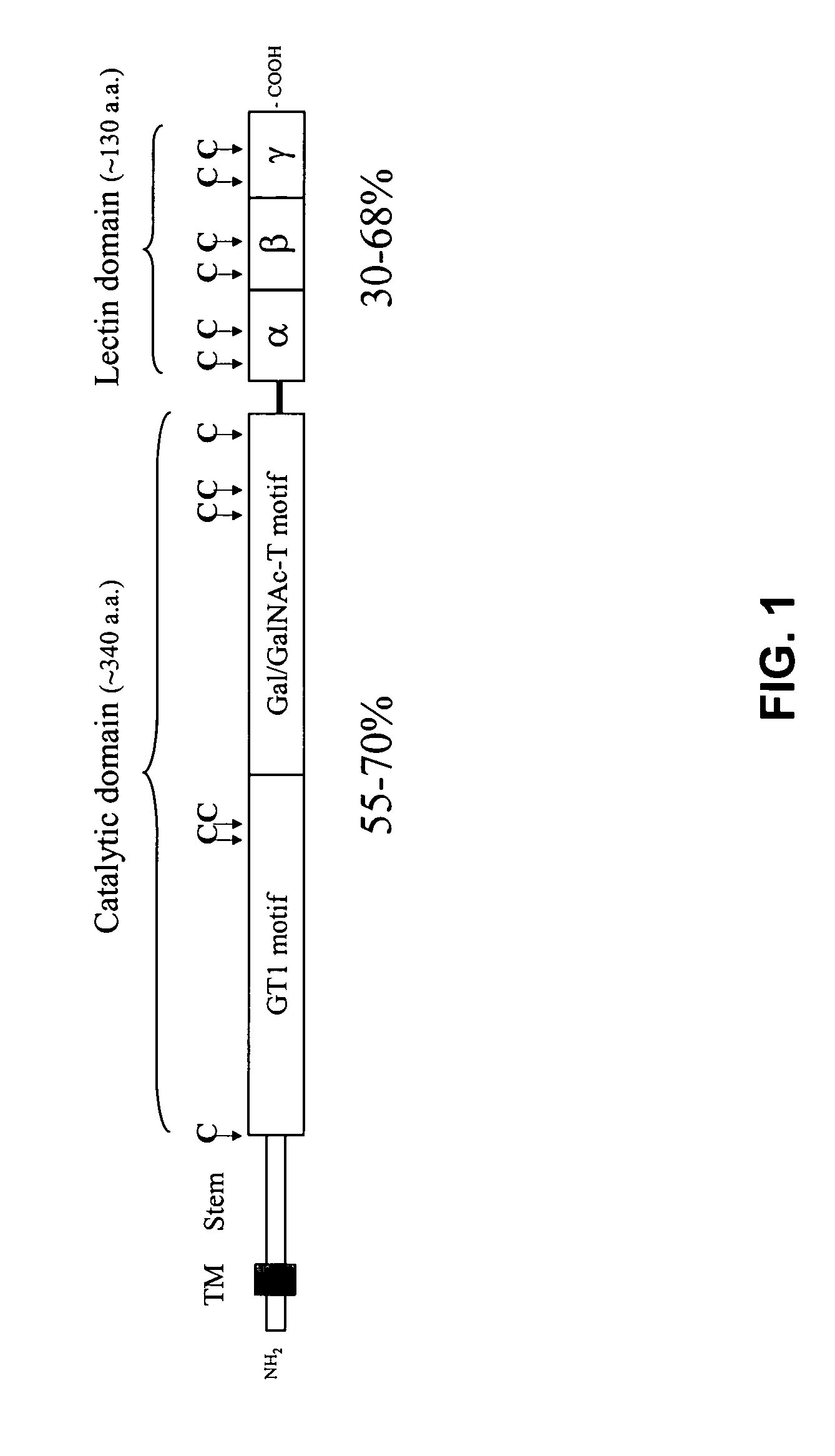
Methods to identify agents modulating functions of polypeptide galnac-transferases, pharmaceutical compositions comprising such agents and the use of such agents for preparing medicaments
Novel methods for identification of inhibitors or modulators of binding activities mediated by lectin domains of polypeptide GalNAc-transferases are disclosed. Direct binding activity of GalNAc-transferase lectins has been demonstrated for the first time and methods to measure lectin mediated binding of isolated lectins or enzymes with lectin domains are disclosed. The present invention specifically discloses a novel selective inhibitor of polypeptide GalNAc-transferase lectin domains, which provides a major advancement in that this inhibitor and related inhibitors sharing common characteristics of activity bind lectin domains without serving as acceptor substrate for glycosyltransferases involved in synthesis of O-glycans. This inhibitor is represented by the β-anomeric configuration of GalNAc-benzyl, GalNAcβ-benzyl. Methods for inhibiting intracellular transport, cell surface expression, and secretion of mucins and O-glycosylated glycoproteins without affecting O-glycosylation processing are disclosed using the novel selective inhibitor identified.
Owner:GLYCOZYM
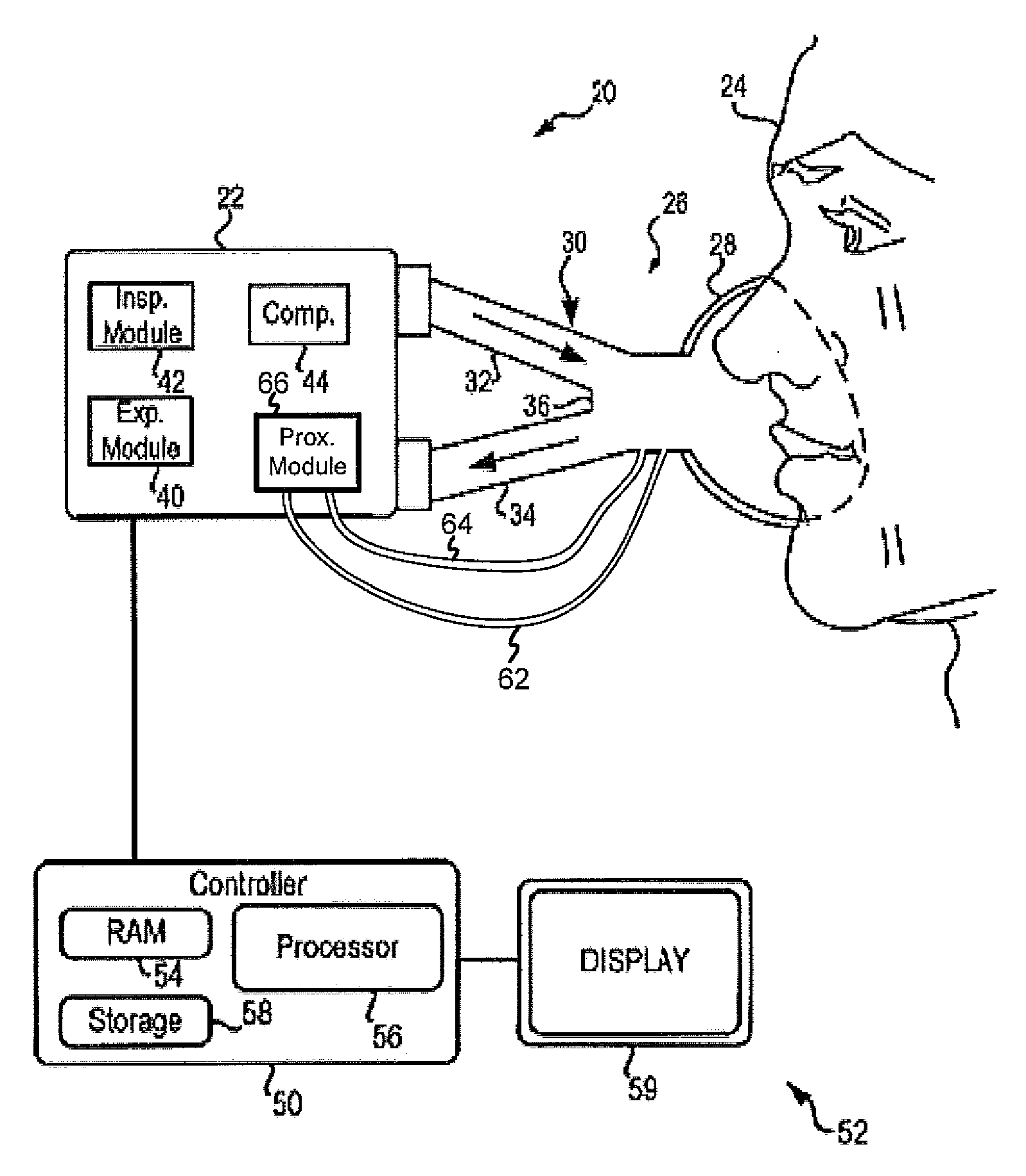
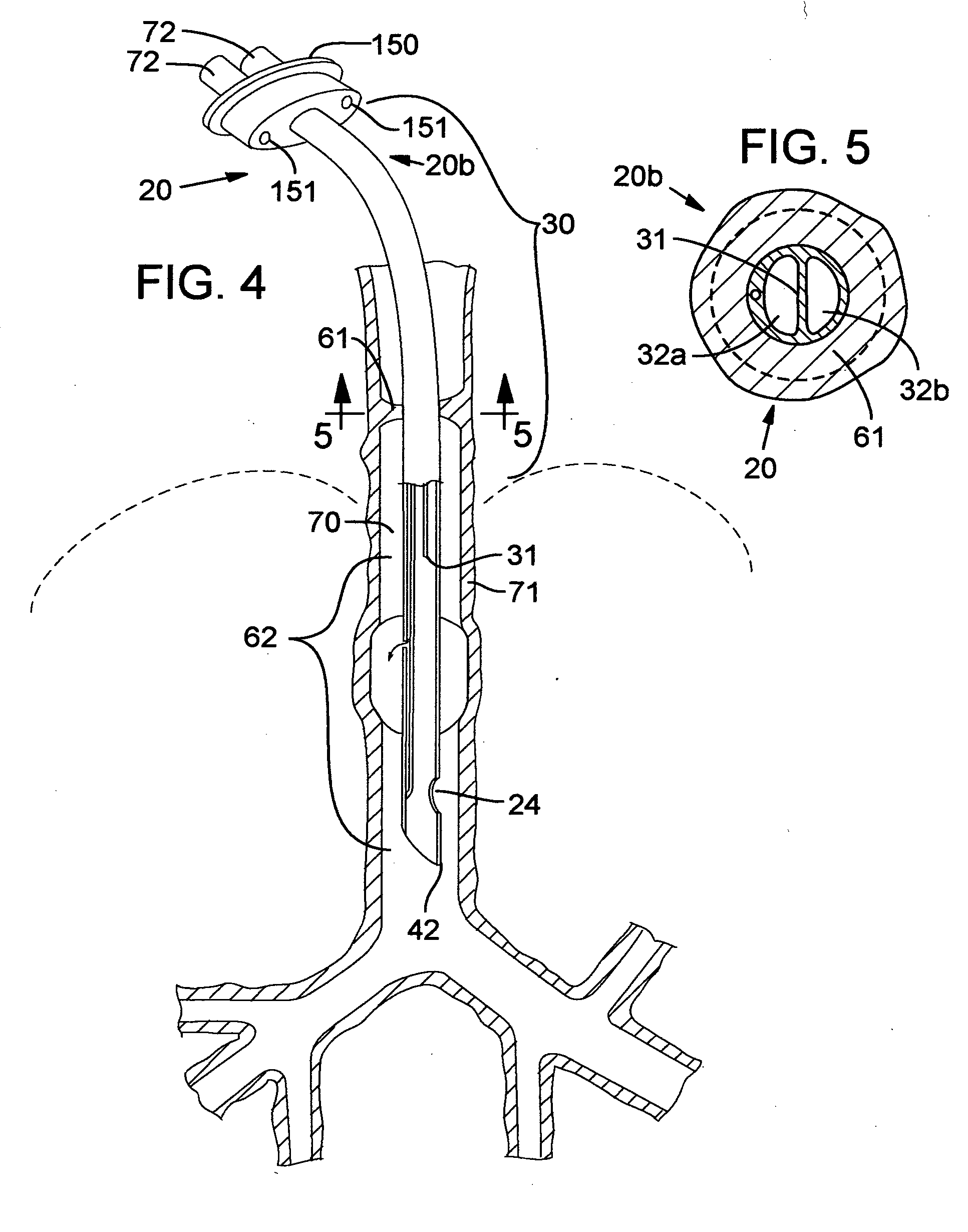
Secretion clearing ventilation catheter and airway management system
InactiveUS20070068530A1Easy to insertEasy to disassembleTracheal tubesBalloon catheterCollection systemWater vapor
A pulmonary secretion clearing ventilation catheter and related airway management system is disclosed. The ventilation catheter has a double lumen portion which each lumen of the double lumen portion operably secured to an airway management system so that inspiratory fluid (air / oxygen mixtures, with or without added water vapor) is delivered to the distal end of the ventilation catheter through one of the two lumens and expired inspiratory fluid, pulmonary secretions, and pulmonary fluids are removed from the patent through the other lumen. The expiratory fluid pathway preferably includes a secretion collection system for removing the pulmonary secretions and the like from the pathway, thereby improving operation and safety of the system. An improved inflatable cuff is also disclosed that permits a slight air leakage in the pneumatic seal between the patient's lungs and the environment, thereby facilitating removal of pulmonary secretions from the patient's lungs.
Owner:VERATHON MEDICAL CANADA ULC
Soluble Glycosaminoglycanases and Methods of Preparing and Using Soluble Glycosaminoglycanases
InactiveUS20090123367A1Facilitated DiffusionEnhance convective transportBacterial antigen ingredientsPeptide/protein ingredientsHyaluronidaseNuclear chemistry
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME +6
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com