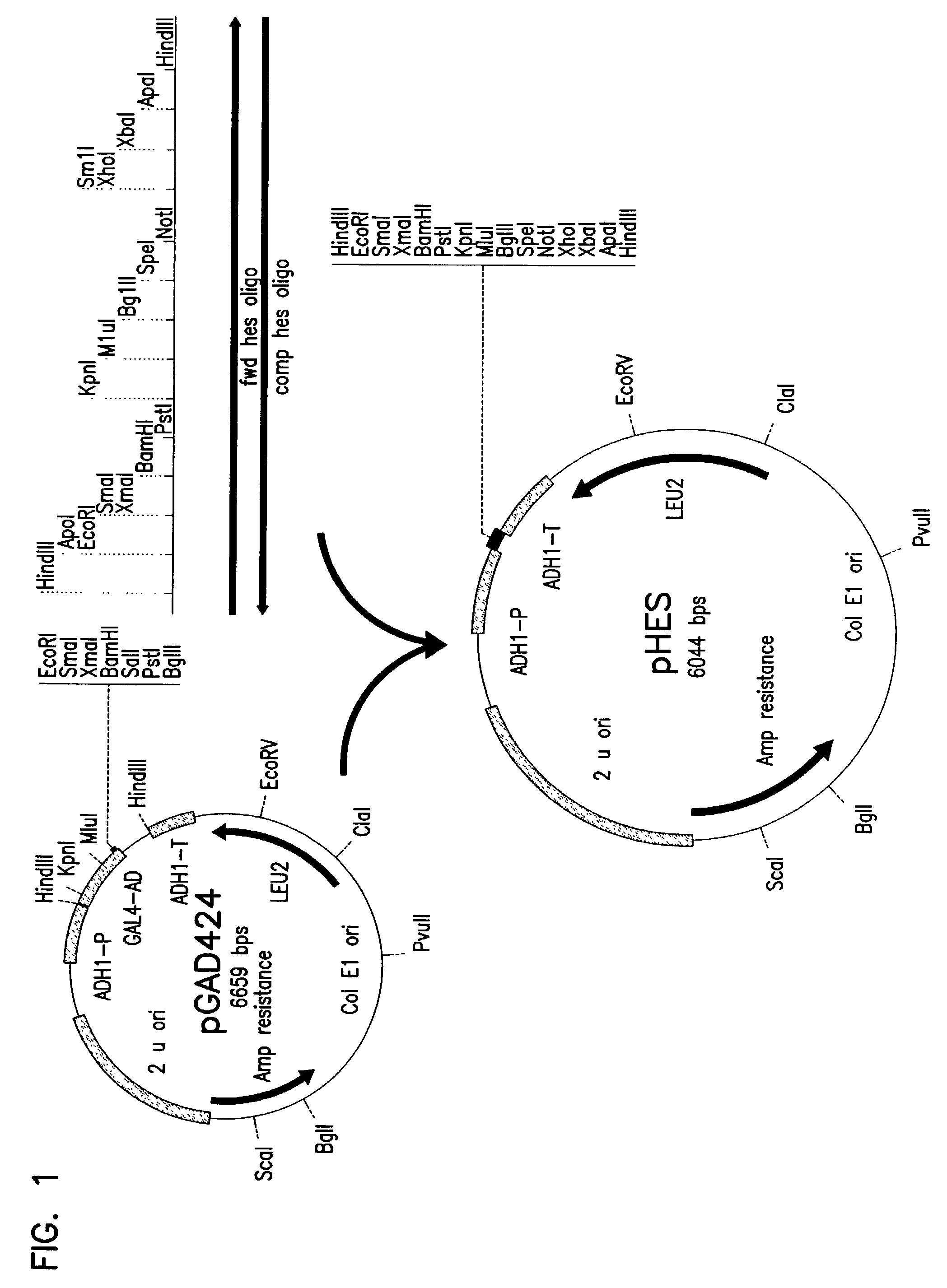
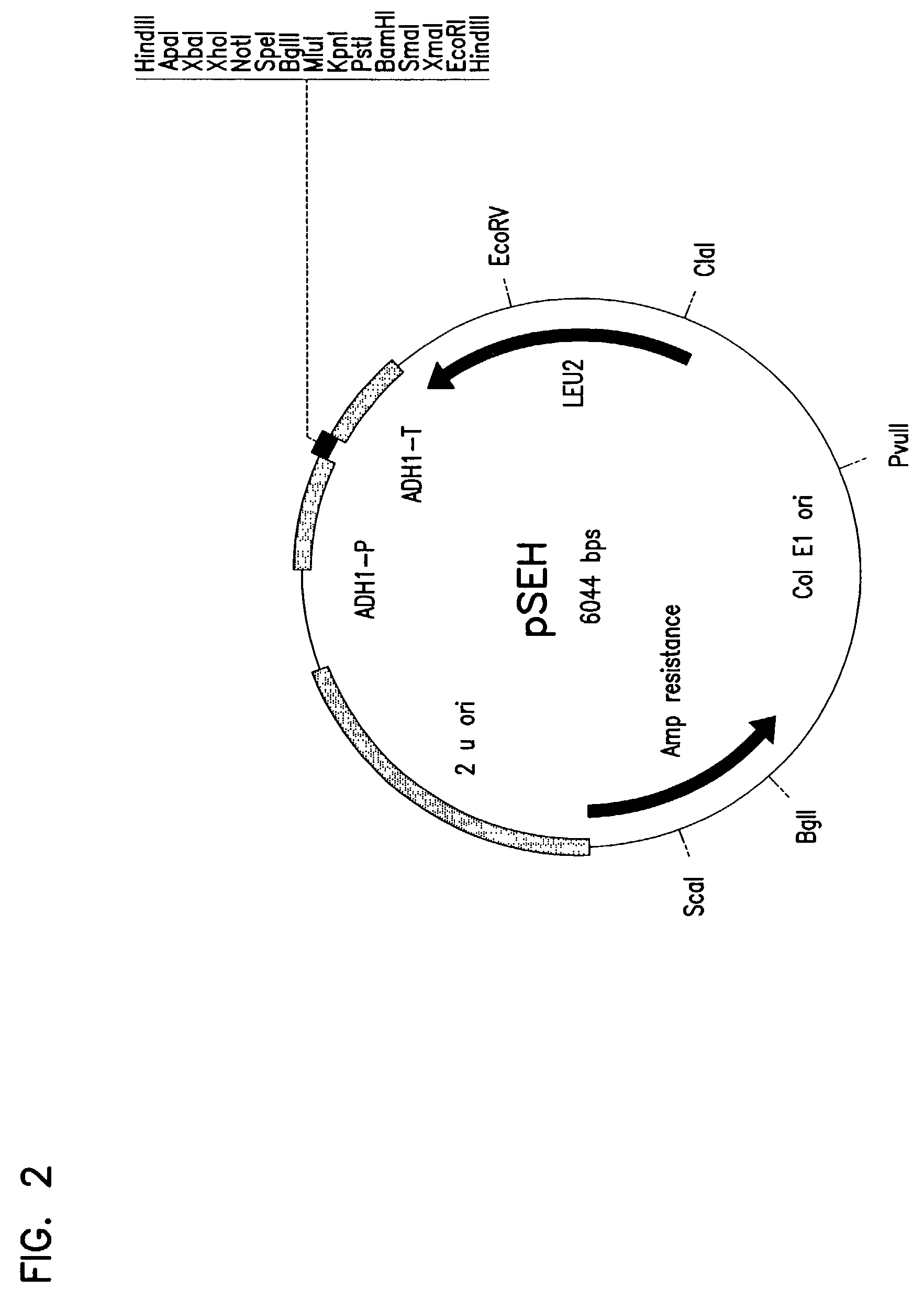
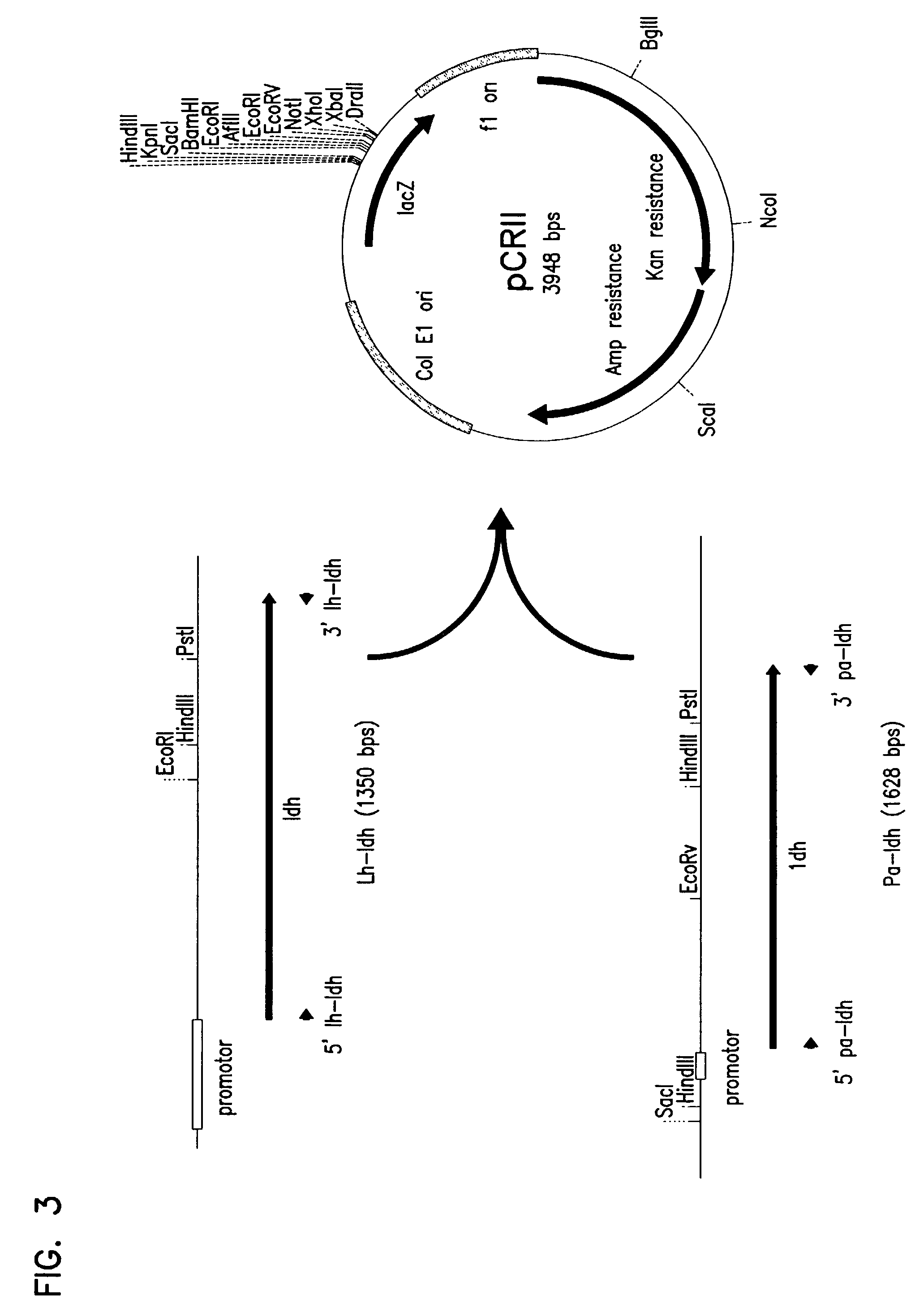
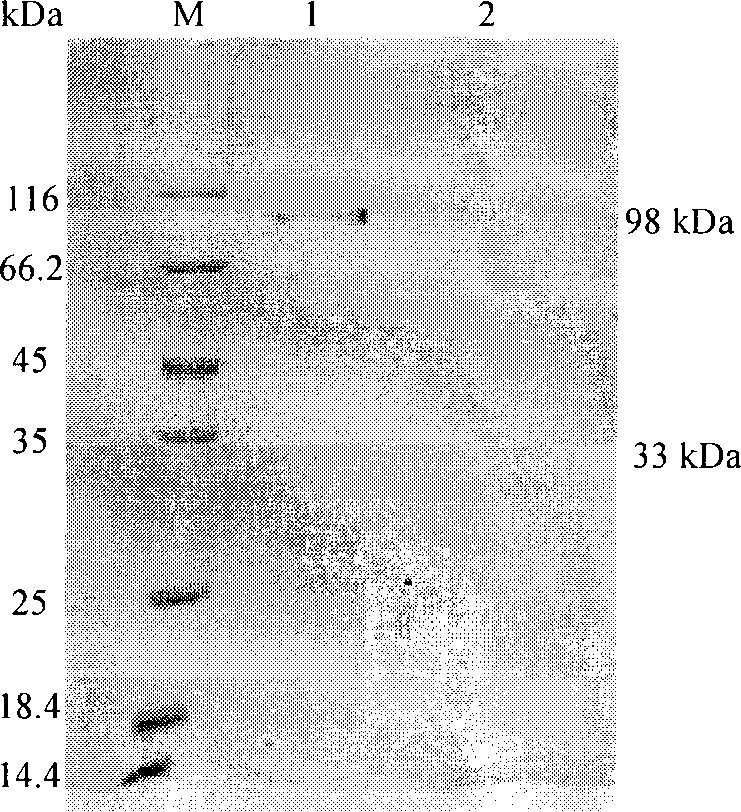
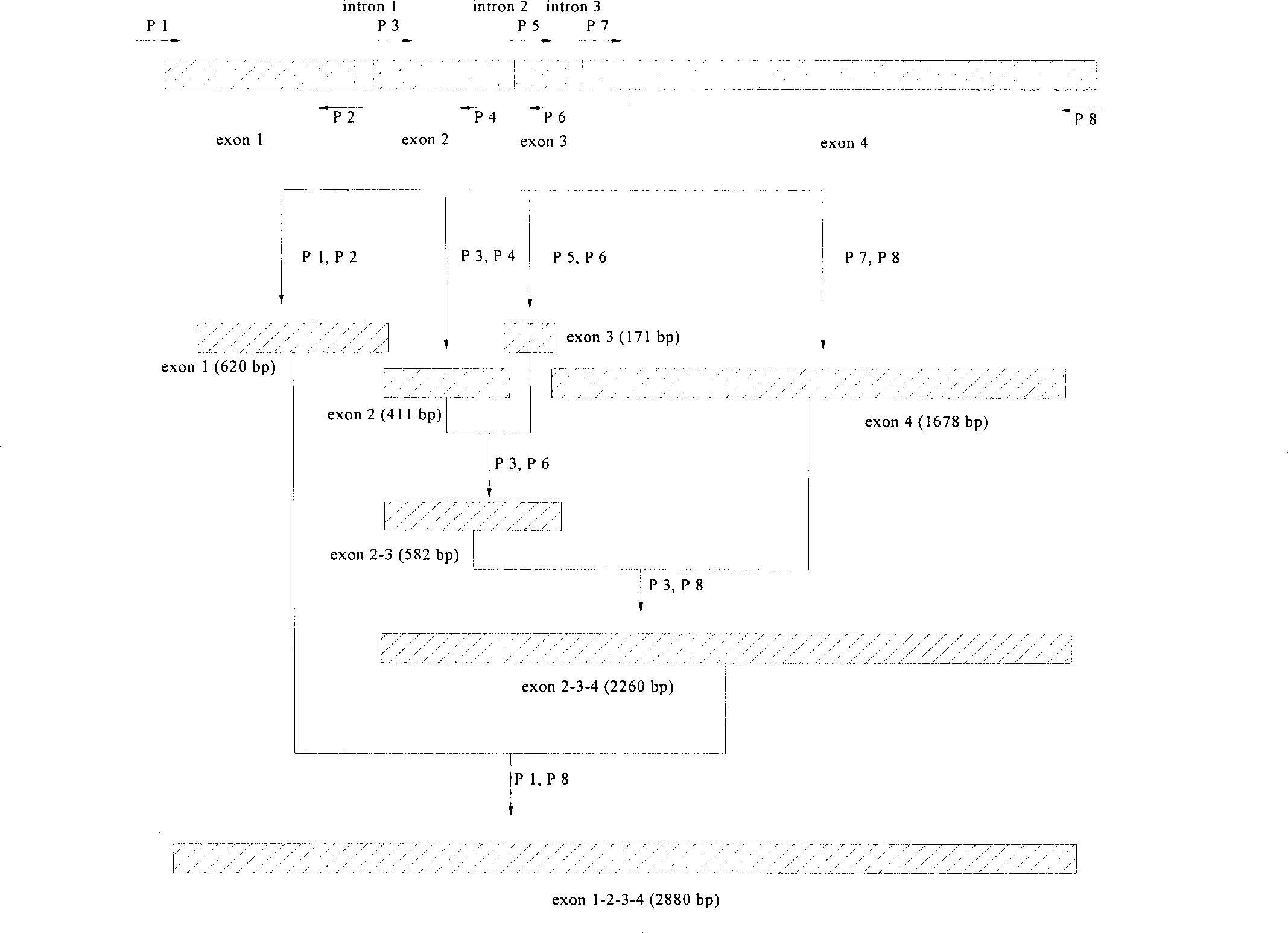
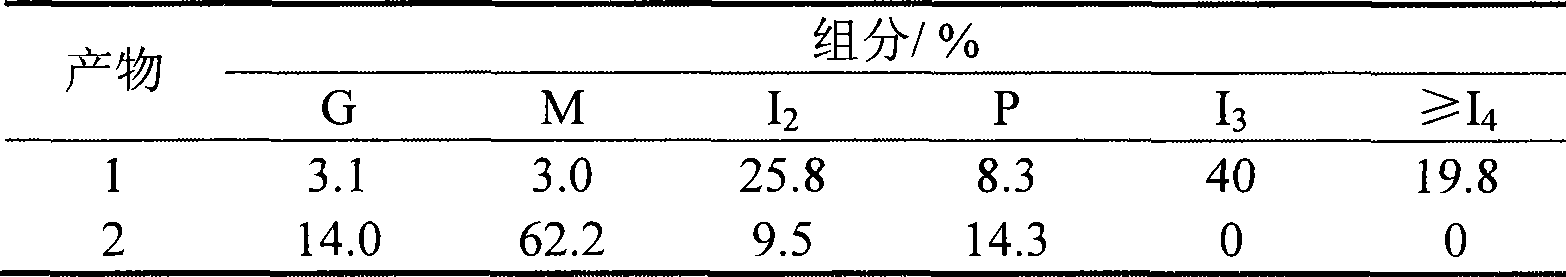
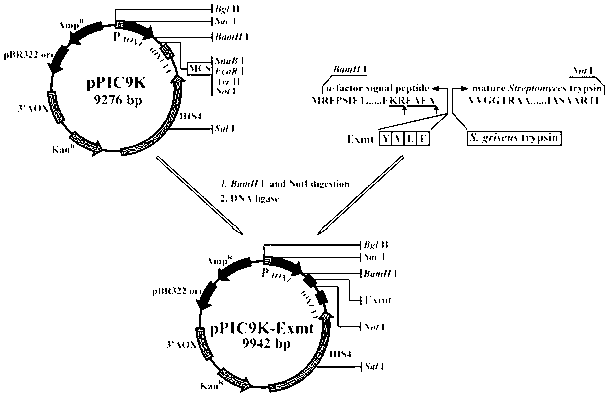
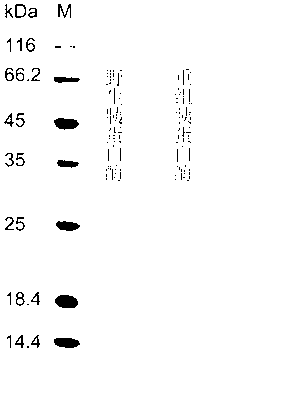
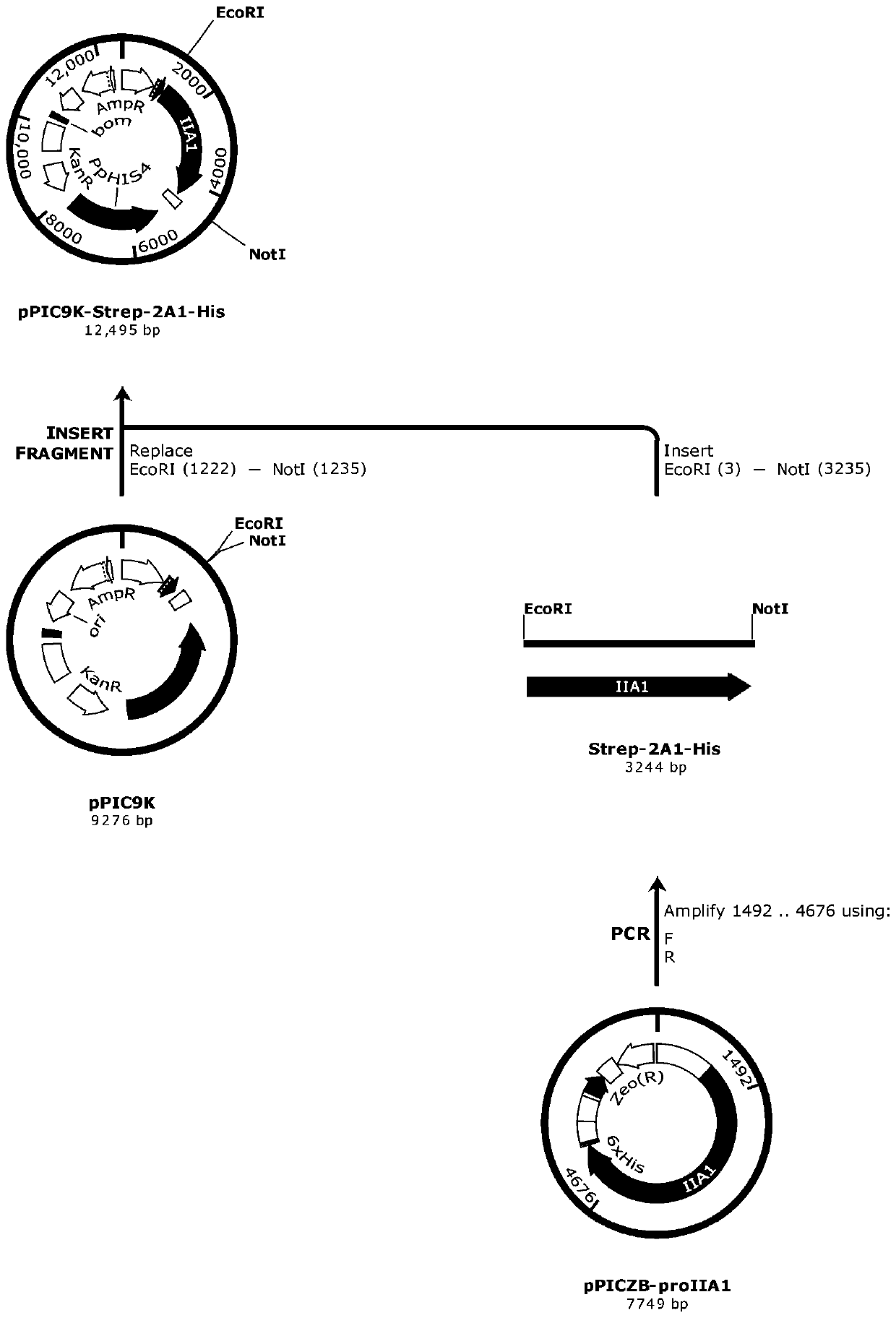
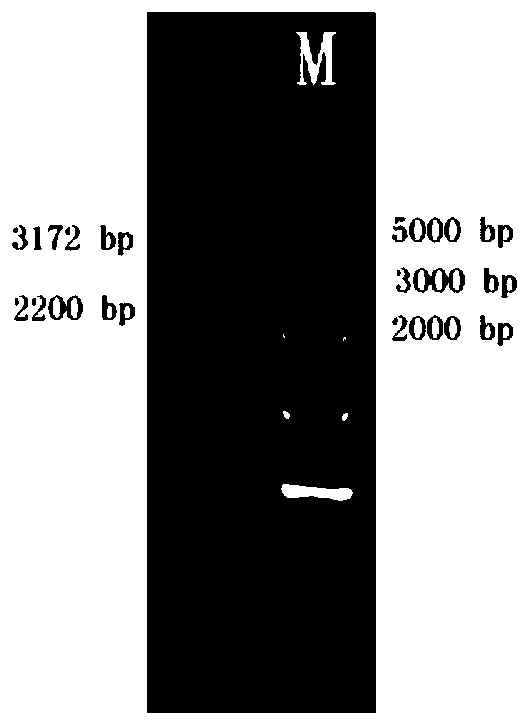
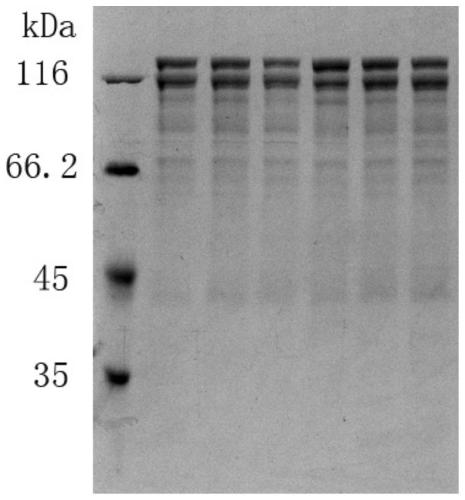
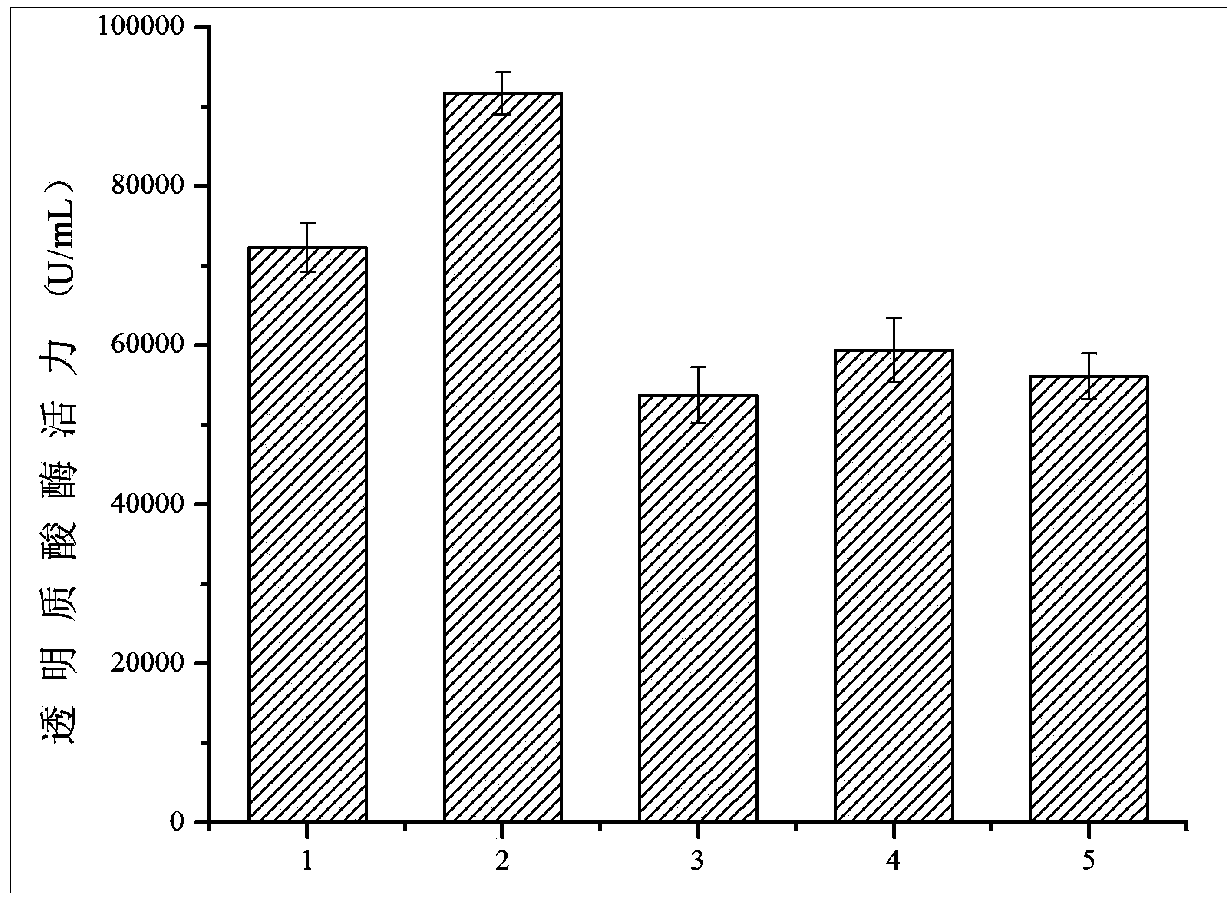
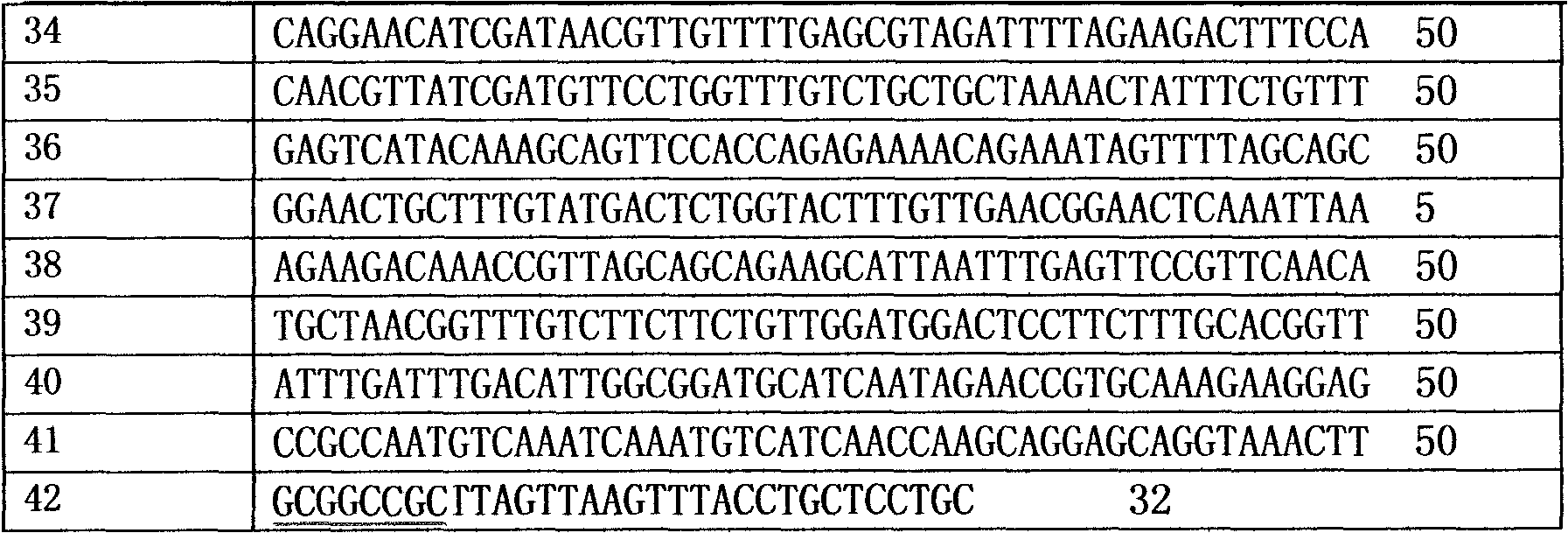Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
790 results about "Pichia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
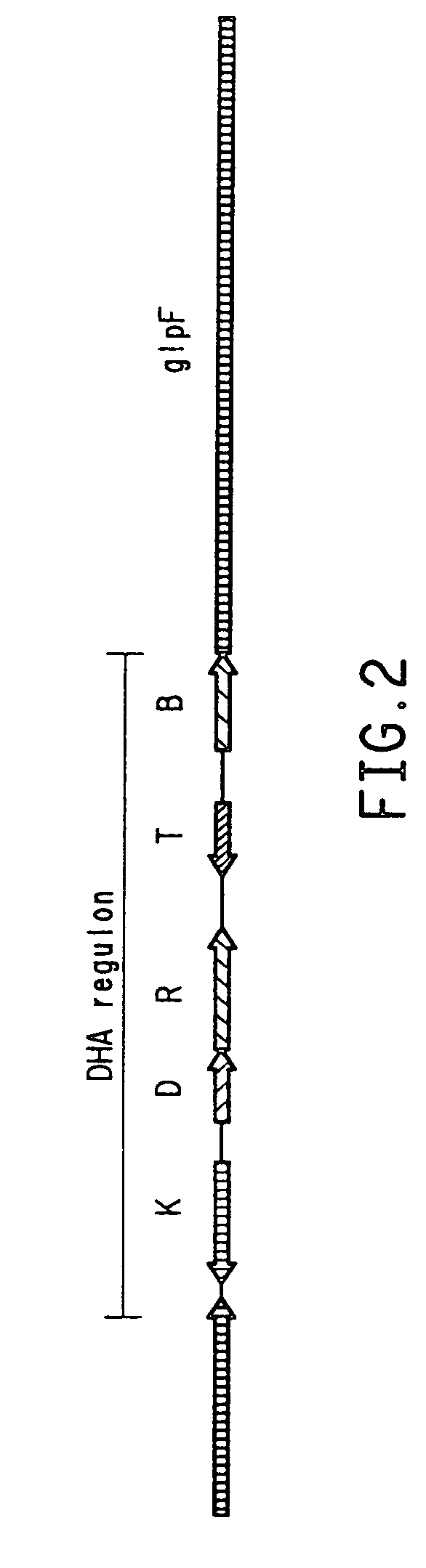
Pichia (Hansenula and Hyphopichia are obsolete synonyms) is a genus of yeasts in the family Saccharomycetaceae with spherical, elliptical, or oblong acuminate cells. Pichia is a teleomorph, and forms hat-shaped, hemispherical, or round ascospores during sexual reproduction. The anamorphs of some Pichia species are Candida species. The asexual reproduction is by multilateral budding.
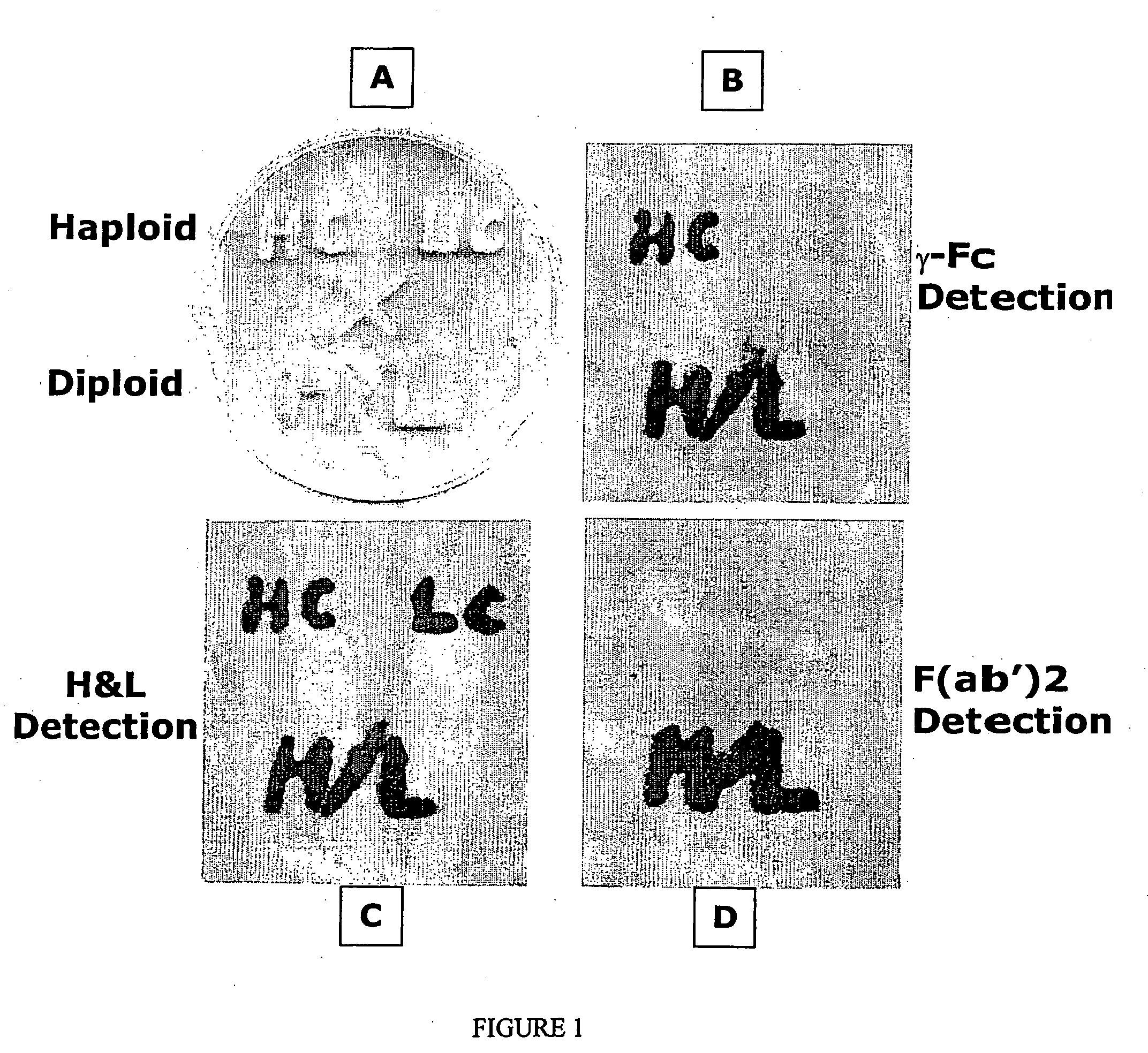
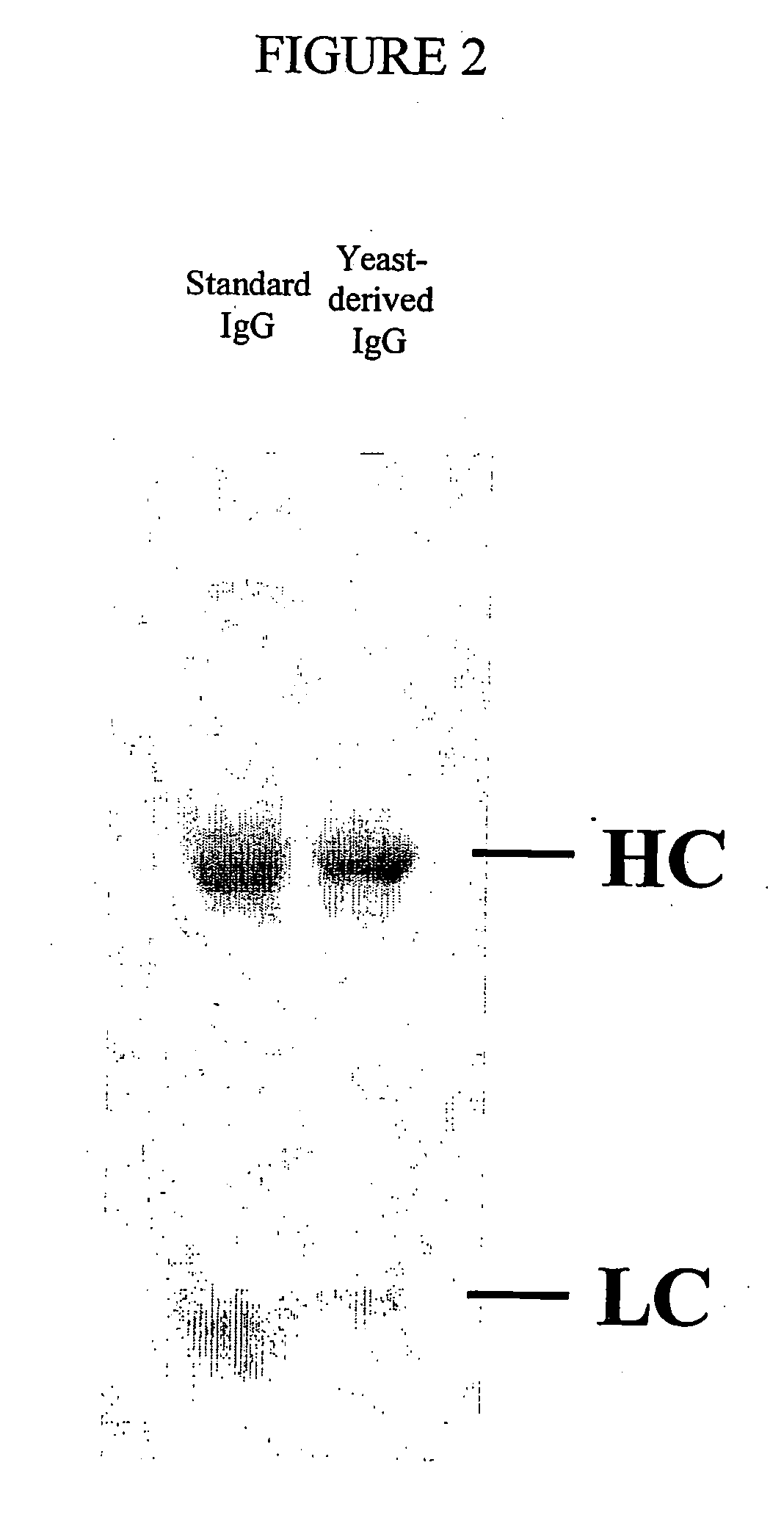
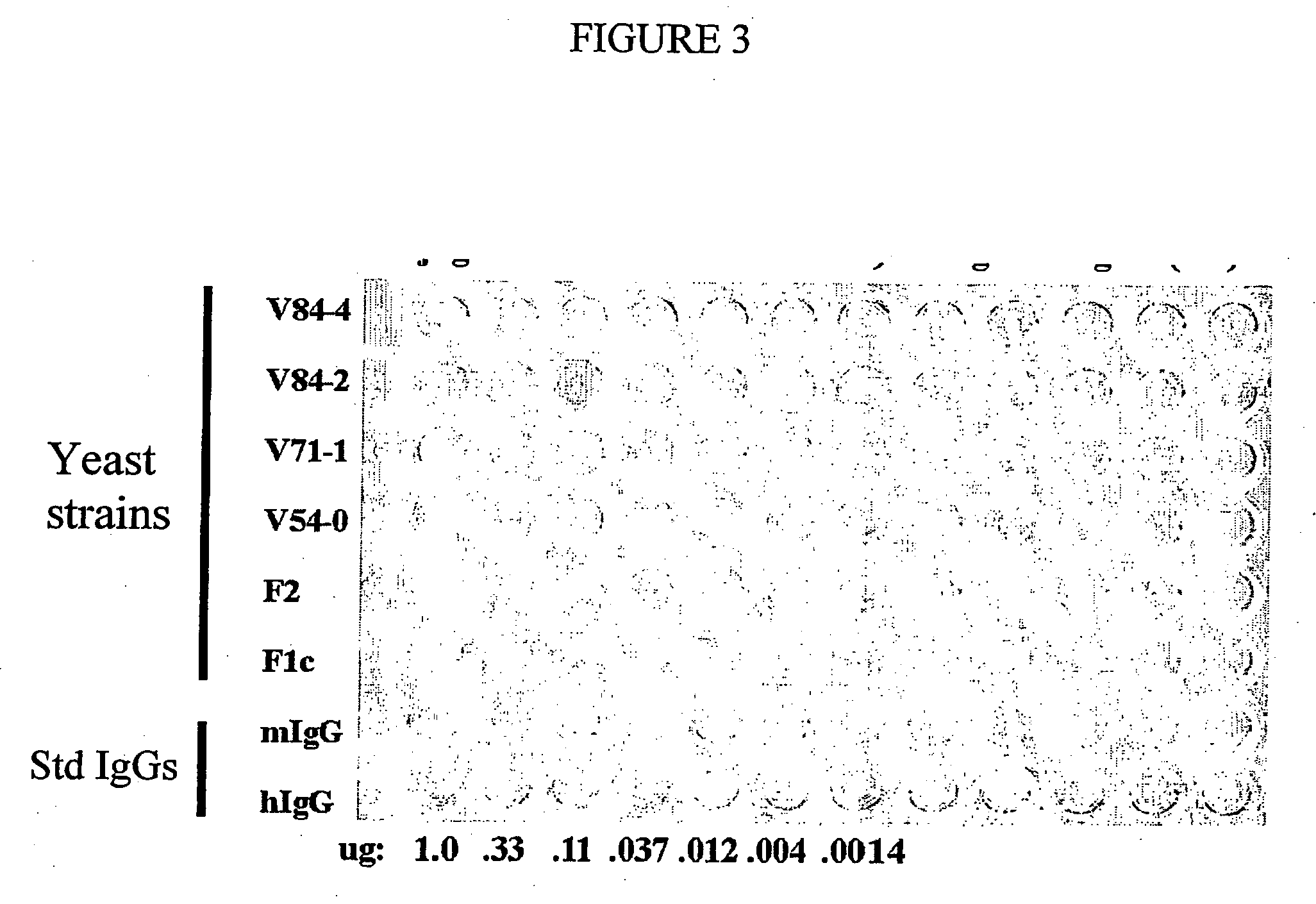
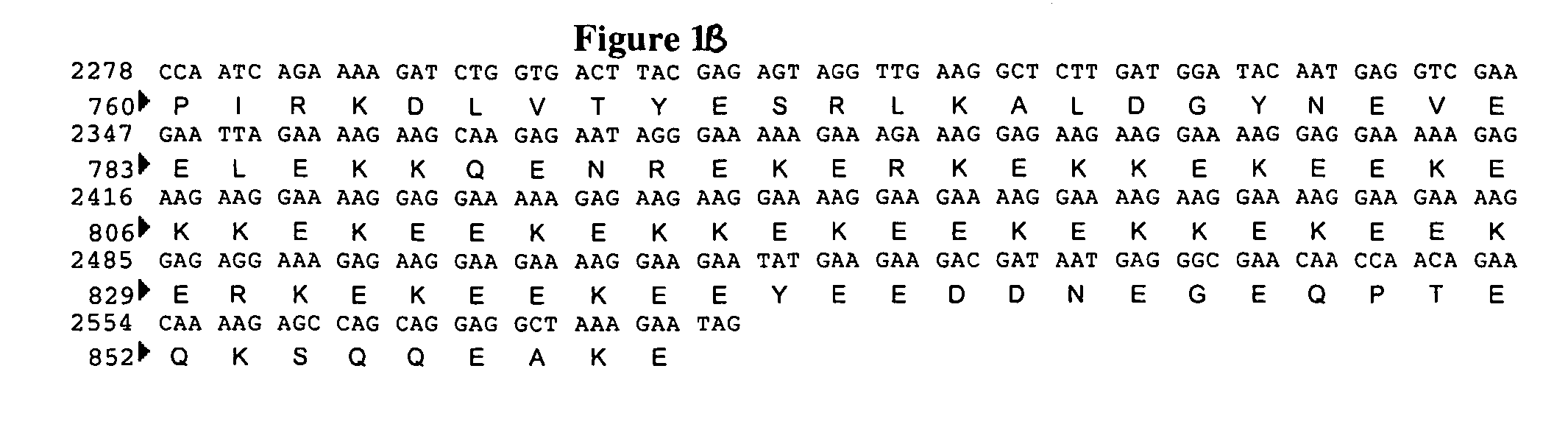
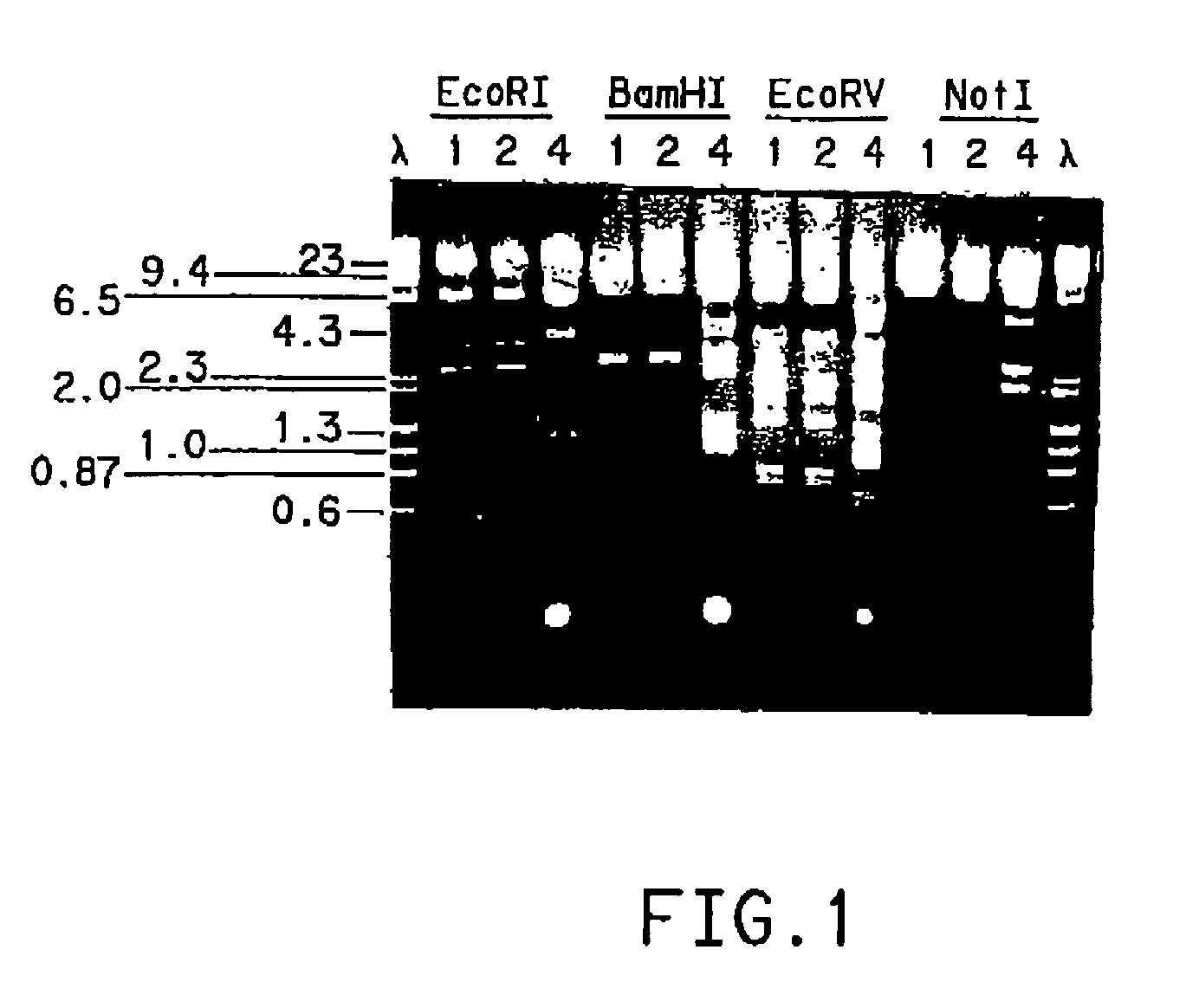
Methods of synthesizing heteromultimeric polypeptides in yeast using a haploid mating strategy
ActiveUS20060270045A1Enhance full-length product generationIncrease secretionFungiMicrobiological testing/measurementBiotechnologyMating
Methods are provided for the synthesis and secretion of recombinant proteins preferably large mammalian proteins or hetero-multimeric proteins at high levels and for prolonged time in polyploid, preferably diploid yeast. These methods use various mating competent yeast, including Pichia. In a preferred embodiment, a first expression vector is transformed into a first haploid cell; and a second expression vector is transformed into a second haploid cell. The transformed haploid cells, each individually synthesizing a non-identical polypeptide, are identified and then genetically crossed or fused. The resulting diploid strains are utilized to produce and secrete fully assembled and biologically functional hetero-multimeric protein.
Owner:KECK GRADUATE INST OF APPLIED LIFE SCI +1
Methods for eliminating mannosylphosphorylation of glycans in the production of glycoproteins
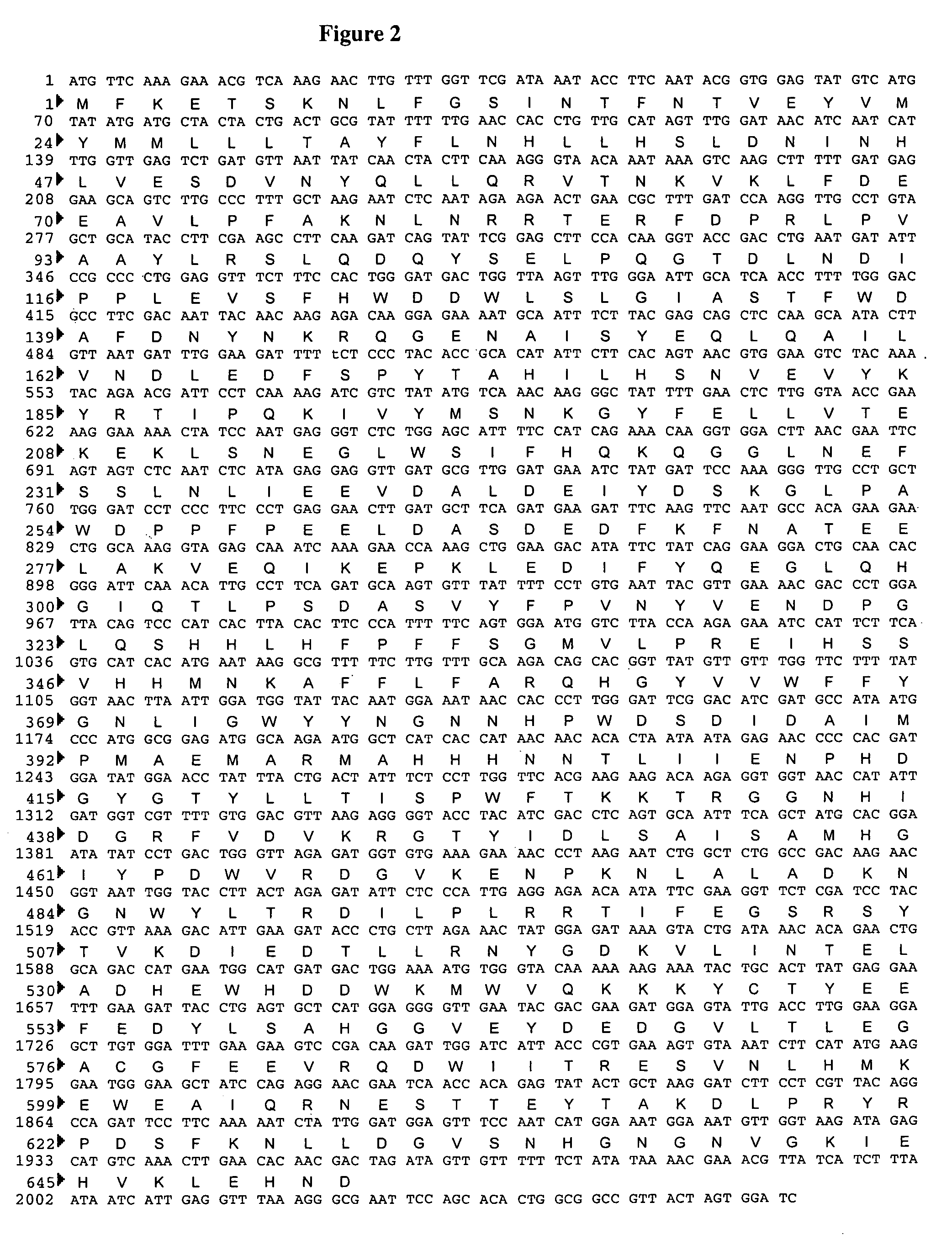
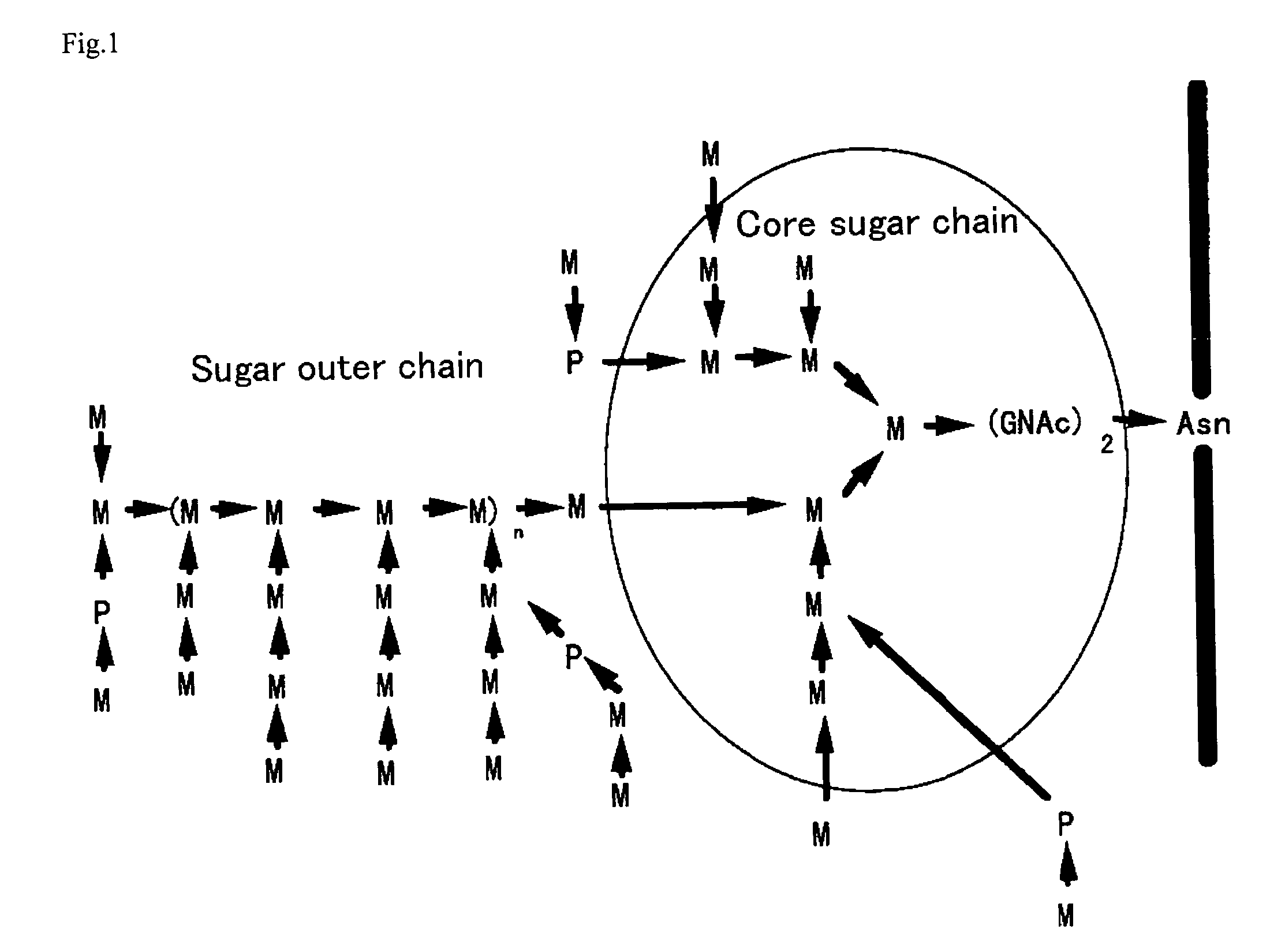
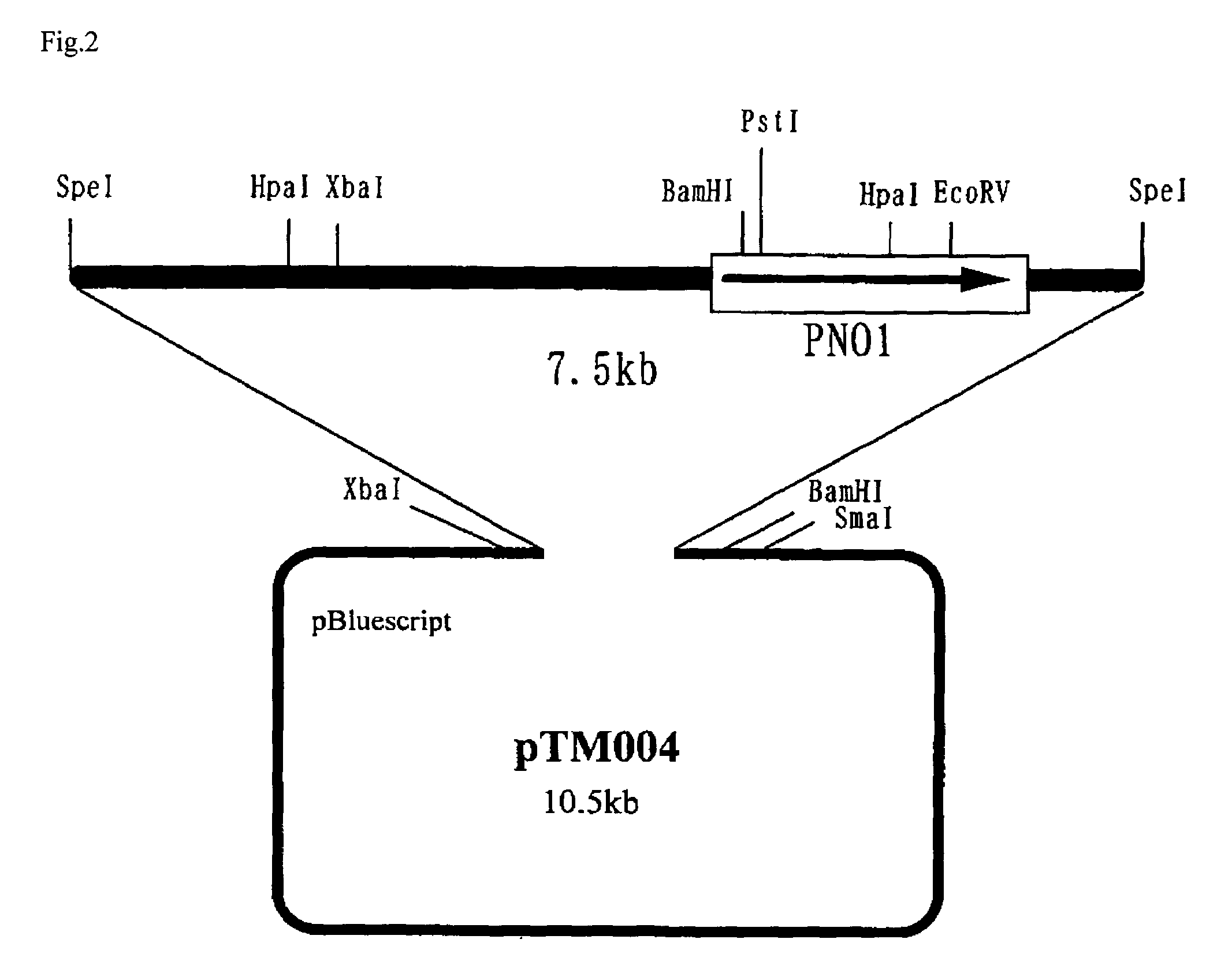
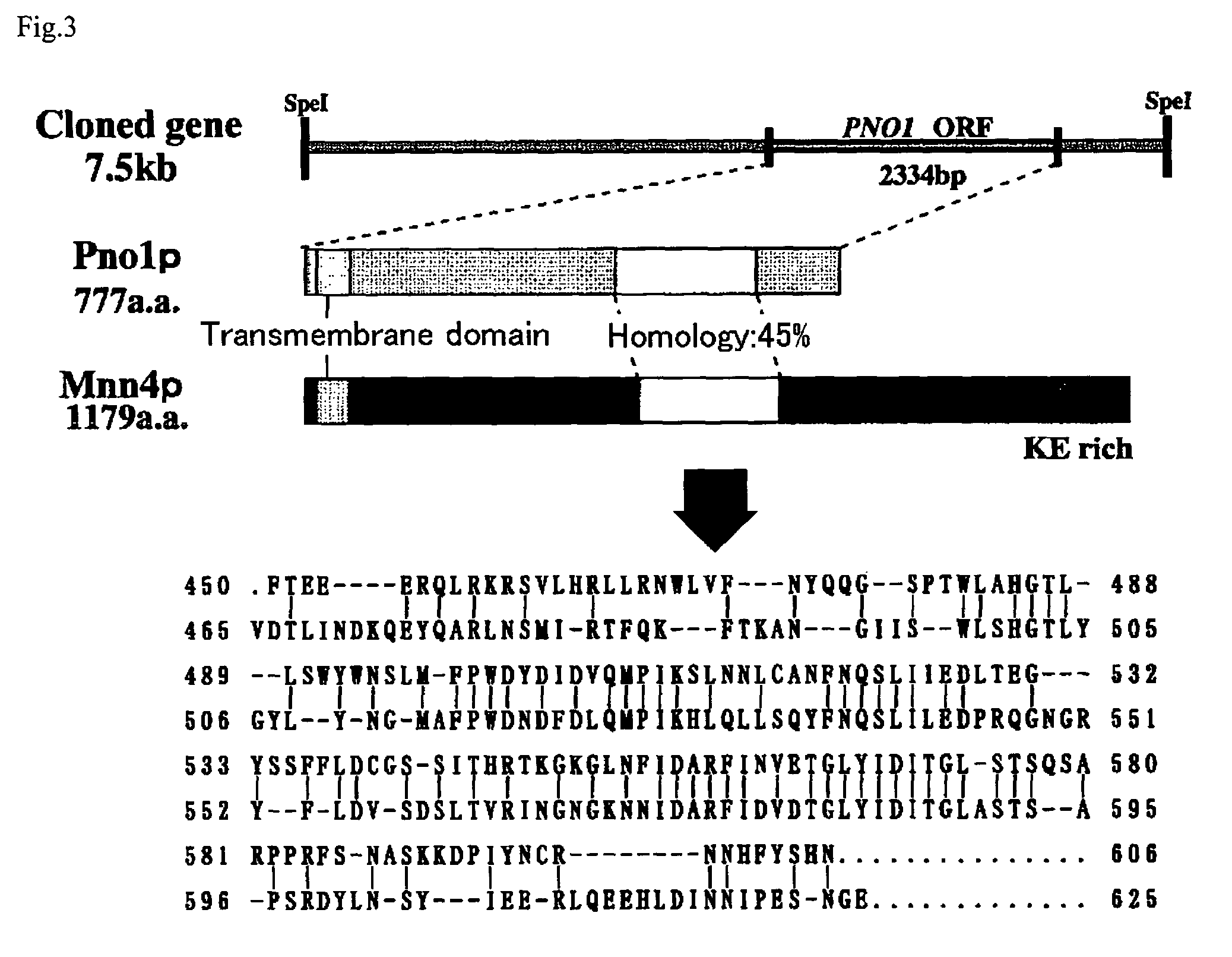
The present invention relates to the elimination of mannosylphosphorylation on the glycans of glycoproteins in the yeast genus Pichia. The elimination of mannosylphosphorylated glycoproteins results from the disruption of the PNO1 gene and the newly isolated P. pastoris MNN4B gene. The present invention further relates to methods for producing modified glycan structures in host cells that are free of glycan mannosylphosphorylation.
Owner:GLYCOFI
Process for producing protein with reduction of acidic sugar chain and glycoprotein produced thereby
The present invention intends to find out a gene participating in addition of mannose phosphate to a sugar chain of a glycoprotein originating in a yeast belonging to the genus Pichia and provide a means of controlling the same. The present invention also intends to provide a process for producing a protein with reduction of an acidic sugar chain by using the thus controlled yeast strain belonging to the genus Pichia. Namely, the present invention includes a protein participating in the addition of mannose phosphate to a sugar chain of a glycoprotein; a gene encoding this protein; a mutant of this gene; a vector carrying the mutant gene; a yeast strain belonging to the genus Pichia having been transformed by this vector; a process for producing a protein with reduction of an acidic sugar chain by using the transformed yeast strain; and a glycoprotein thus produced.
Owner:MITSUBISHI TANABE PHARMA CORP
Process for synthesizing ethyl caproate by yeast display lipase synthesis
InactiveCN101285078AImprove operational stabilityHigh biosecurityFungiHydrolasesBiotechnologySurface display
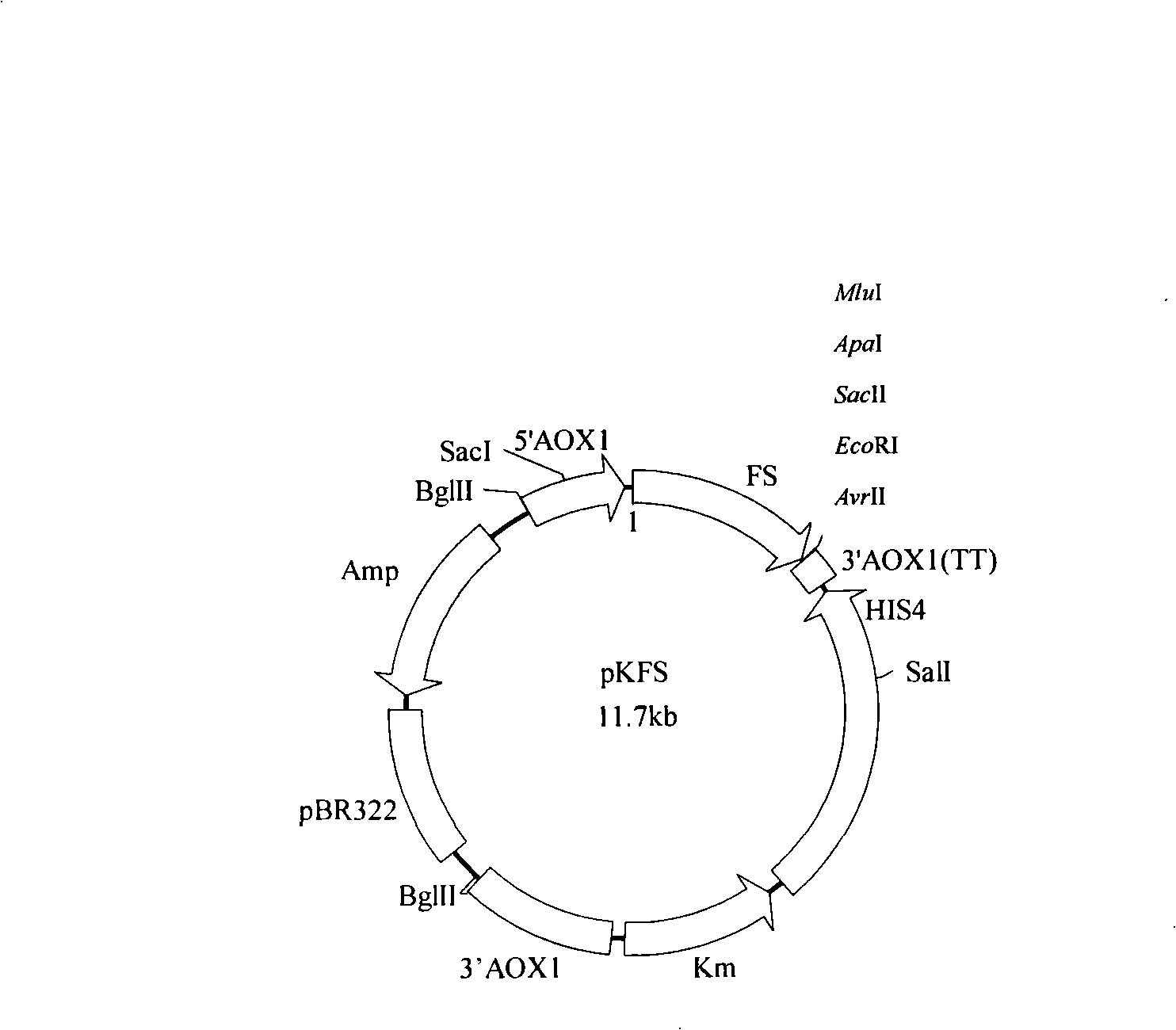
The invention discloses a method for synthesizing ethyl caproate under the catalysis of yeast display lipase. The method comprises two steps, namely the production of full cellular zymin and the synthesis of ethyl caproate. The method comprises the following: a step of cloning the lipase gene into the Pichia yeast surface display vector pKFS to construct the Pichia yeast surface display expression vector pKFS-lipase which is transformed into the host bacteria of Pichia yeast through linearization, a yeast genetic engineering bacteria capable of displaying active pheron on the surface of the Pichia yeast being obtained through screening, and the engineering bacteria being fermented in a rocking bottle to obtain a thallus which is used to make the full cellular zymin after 24 hours of vacuum freeze drying; and then a step of adopting caproic acid and alcohol as raw material which is esterified at the action of the biocatalyst of yeast full cellular zymin with the lipase displayed on the surface to obtain the ethyl caproate product. The method is capable of collecting thalli centrifugally for reuse after the reaction with short reaction time, high yield and good operational stability, thereby greatly reducing production costs.
Owner:SOUTH CHINA UNIV OF TECH
In vivo unnatural amino acid expression in the methylotrophic yeast pichia pastoris
InactiveUS20090197339A1Enhanced and novel stericEnhanced and novel and chemicalFungiSerum albuminYeastIn vivo
The invention provides orthogonal translation systems for the production of polypeptides comprising unnatural amino acids in methylotrophic yeast such as Pichia pastoris. Methods for producing polypeptides comprising unnatural amino acids in methylotrophic yeast such as Pichia pastoris are also provided.
Owner:THE SCRIPPS RES INST
Preparation method of recombinant porcine circovirus type 2 Cap antigen
InactiveCN102127533AEasy to operateSimple and fast operationViral antigen ingredientsMicroorganism based processesEscherichia coliTGE VACCINE
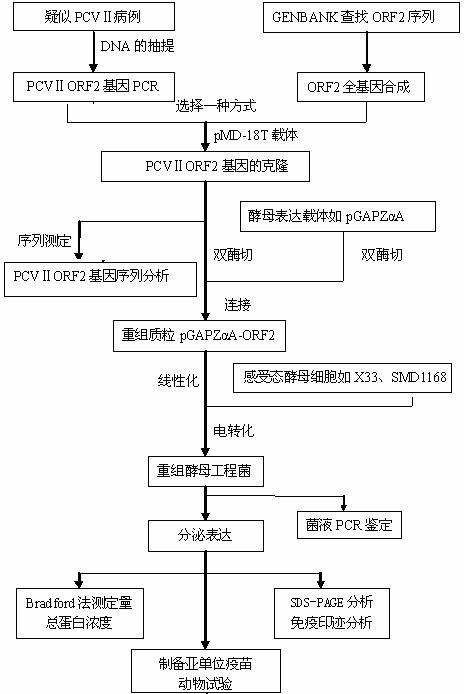
The invention discloses a preparation method of a recombinant porcine circovirus type 2 Cap antigen, which comprises the following steps: using a yeast expression system to amplify the PCV2-ORF2 gene; then, constructing an expression engineering bacterium: inserting an amplification sequence into a yeast expression vector to form a recombinant expression vector, linearizing, and transforming into a pichia pastoris host cell so as to finish the construction of the expression engineering bacterium; and finally, carrying out secretory expression on the recombinant microzyme to obtain the recombinant porcine circovirus type 2 Cap antigen protein. The yeast expression system can carry out processing, folding and posttranslational modification on the expressed protein, so that the expressed protein has biological activity. Compared with Escherichia coli, the yeast expression system has more advantages; and compared with a mammal cell expression system, the yeast expression system is more convenient to operate. Compared with Saccharomyces cerevisiae, the pichia pastoris can not be easily subjected to hyperglycosylation, thereby facilitating the separation and purification steps. By using the yeast expression system, the invention has the advantages of simple operation process, high expression quantity and low production cost, can implement large-scale production, and is beneficial to the popularization and application of the porcine circovirus subunit vaccine.
Owner:SOUTH CHINA AGRI UNIV
Acid beta-mannase, genes, engineering bacteria and structure thereof
InactiveCN101724614AWide pH rangeImprove heat resistanceFungiMicroorganism based processesPichia pastorisNucleotide
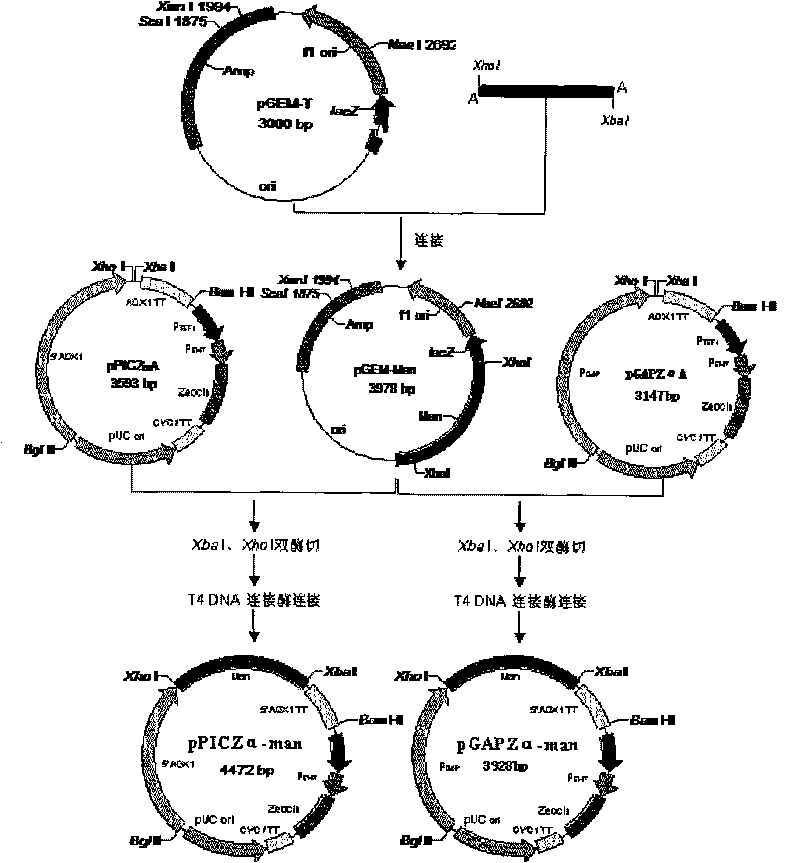
The invention discloses acid beta-mannase, genes, engineering bacteria and a structure thereof. A coding gene manAsp is from Aspergillus niger CBS 513.88, the coding gene has a nucleotide sequence expressed as SEQ ID NO.3 or 4, and the acid beta-mannase obtained by coding has an amino acid sequence expressed as SEQ ID NO.1 or 2. The structure of the engineering bacteria is obtained by guiding a pichia pastoris constitutive expression vector (pGAPZa-man) containing the coding gene sequence of the beta-mannase into pichia pastoris. The acid beta-mannase of the invention has the following properties: at the optimal pH of 5.0 and the optimal temperature of 40 DEG C, the beta-mannase has better stability when the pH is between 3.0 and 7.0, and the residual enzyme activity is 66.39 percent when the beta-mannase is treated for 1 hour at the temperature of 90 DEG C; the engineering bacteria achieve efficient expression of the acid beta-mannase; and the fermentation process is simple, has low extracting cost, is suitable for large-scale industrialized production, and has broad application prospect in the industries of feed, food, medicine, energy and the like.
Owner:CENT SOUTH UNIV
Glucose oxidase mutant gene, expression and application thereof
InactiveCN101955953AIncrease secreted expressionIncrease enzyme activityFungiBacteriaYeastGlucose polymers
The invention discloses a glucose oxidase mutant gene, expression and application thereof. In the invention, 272 basic groups are changed through codon optimization and GC content change and the content of GC is reduced to 48.44% from 55.54% so as to obtain the glucose oxidase mutant gene, wherein the basic group is represented by SEQ ID NO.2. The glucose oxidase mutant gene is transferred into pichia yeast to express; the experimental result shows that the secretory expression level of the glucose oxidase mutant gene in the pichia yeast is significantly improved by comparing with the same before mutation; compared with partial research at home and abroad, the final secretory expression of the glucose oxidase mutant gene achieves high expression level, thereby building the foundation for further expansion of industrial production. The determination of enzymatic properties of the glucose oxidase mutant gene shows that the recombinant glucose oxidase protein expressed by the glucose oxidase mutant gene has good thermal stability and high enzyme activity.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Human papilloma virus genes, vector, strain, and expression method thereof
ActiveCN104513826AImprove translationPromote research and developmentFungiViral antigen ingredientsPichia pastorisHuman Papillomavirus Major Capsid Protein L1
The present invention relates to genes of the Pichia pastoris expression codon-optimized main capsid protein L1 of human papilloma virus types 52,31 and 45, a vector containing the genes, a strain, a preparation method, and an expression method thereof.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Pichia pastoris strain with deletion of alpha-1,6-mannose transferase and construction method thereof
ActiveCN101195809ALow immunogenicityEasy to getFungiMicroorganism based processesBio engineeringPichia
The invention discloses Pichia pastoris strain with alpha-1, 6-mannosyl transferase absent and the establishing method, which belongs to the biological engineering field. The collection number of the Pichia pastoris strain with alpha-1, 6-mannosyl transferase absent is CGMCC No.1853. The invention has the advantages that the Pichia pastoris strain with alpha-1, 6-mannosyl transferase absent built by the invention can prevent the generation of excessive manna saccharify, when expressing extrinsic glucoprotein, reduce the immunogenicity of the pressed protein, and therefore, the invention has important application value in the biomedical field, and other fields; simultaneously, the strain can also be applied to the further gene knockout or the metabolic engineering reconstruction. The invention also builds a method for knocking out the alpha-1, 6-mannosyl transferase gene through secondary homologous recombination, mutant strain can be relatively easily obtained through the method, therefore the sieving workload is greatly reduced, and the success ratio is improved.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Prosuction of antibodies or (functionalized) fragments thereof derived from heavy chain immunoglobulins of camelidae
InactiveUS7794981B2High catalytic activityImprove efficiencyPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsNatural antibodyComplementarity determining region
A process is provided for the production of an antibody or a fragment or functionalized fragment thereof using a transformed lower eukaryotic host containing an example DNA sequence encoding the antibody or (functionalized) fragment thereof, wherein the antibody or (functionalized) fragment thereof is derived from a heavy chain immunoglobulin of Camelidae and is devoid of light chains, and wherein the lower eukaryotic host is a mould, preferably belonging to the genera Aspergillus or Trichoderma, or a yeast, preferably belonging to the yeast genera Saccharomyces, Kluyveromyces, Hansenula, or Pichia. The heavy chain fragment can contain at least the whole variable domain. A complementary determining region (CDR) different from the CDR belonging to the natural antibody ex Camelidae can be grafted on the framework of the variable domain of the heavy chain immunoglobulin. The catalytic antibodies can be raised in Camelidae against transition state molecules. The functionalized antibody or fragment thereof can comprise a fusion protein of both a heavy chain immunoglobulin from Camelidae or a fragment thereof and another polypeptide, e.g., an enzyme, preferably an oxido-reductase. Also provided are new products obtainable by a process as described, and compositions containing a product produced by a process as described, which composition may contain a new product as provided.
Owner:BAC IP
Gene recombination human collagen fusion peptide segment, preparation method and application thereof
ActiveCN102020716ANon-allergenicGood moisturizing effectCosmetic preparationsFungiGenetic engineeringChemistry
The invention relates to a gene recombination human collagen fusion peptide segment. The overall length of the peptide segment comprises 839 amino acids, the nitrogen end of the peptide segment comprises a type III human collagen peptide section of 908-1137, which totally contains 229 amino acids, and the carbon end of the peptide segment comprises a type I human collagen peptide segment of 497-1104, which total contains 608 amino acids, wherein the two peptide segments are connected by using glutamic acid and phenylalanine. The preparation method of the peptide segment comprises the following steps of: constructing cDNAs of clone type I (COL 1) and type III (COL 3) human collagen genes and a Pichia pastoris recombinant expression vector pPOC9K-COL3a1-COL1a1; constructing Pichia pastoris genetic engineering bacteria SMD1168-COL3a10COL1a1 of the gene recombination human collagen fusion peptide segment; preparing the gene recombination human collagen fusion peptide segment, and the like. Proved by tests, the gene recombination human collagen fusion peptide segment prepared by adopting the method can be used as a material for preparing cosmetics.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
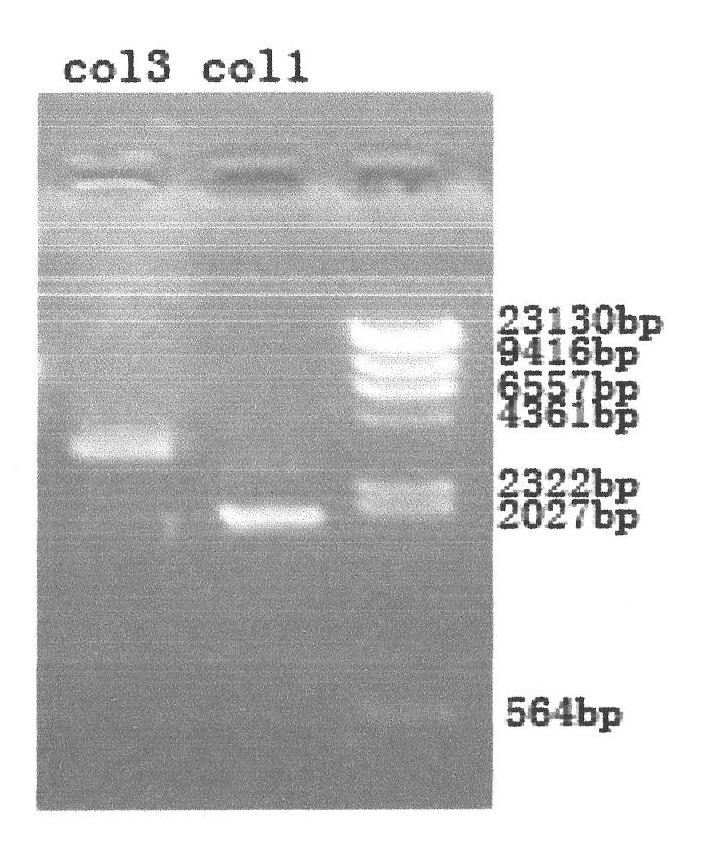
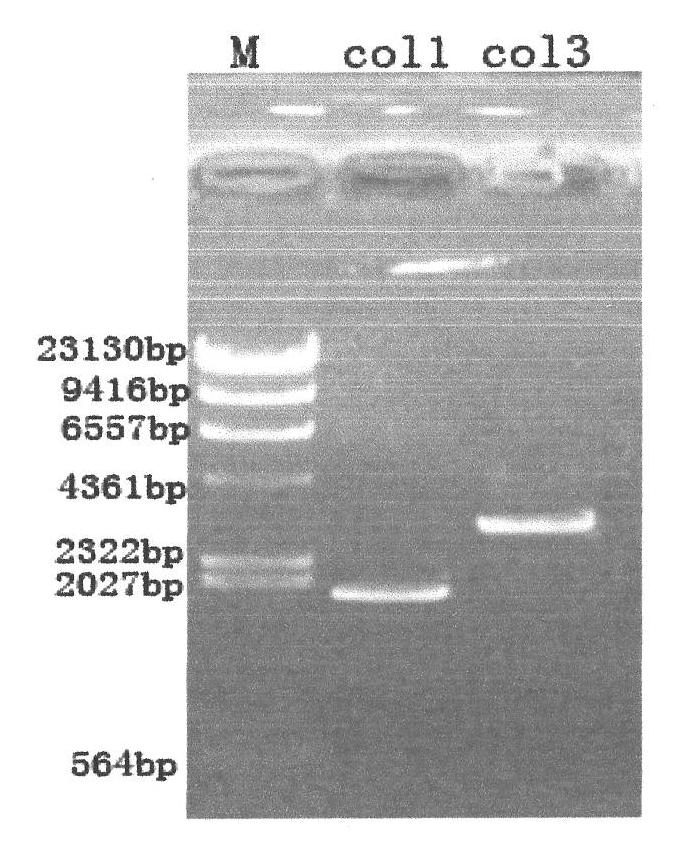
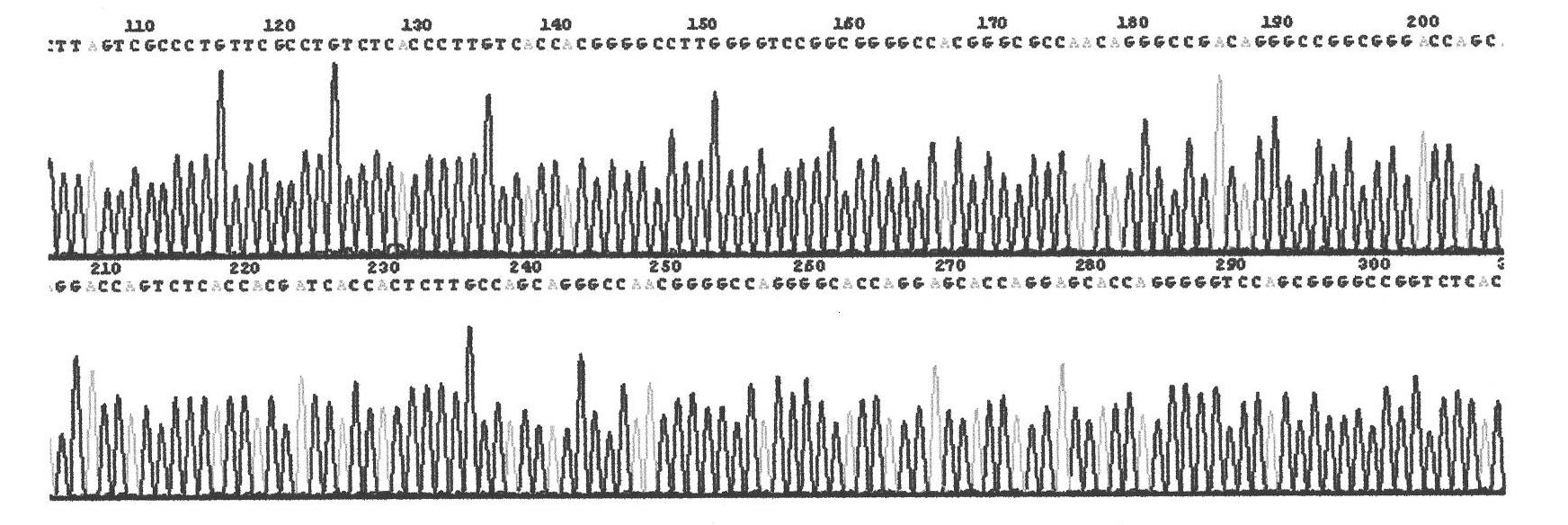
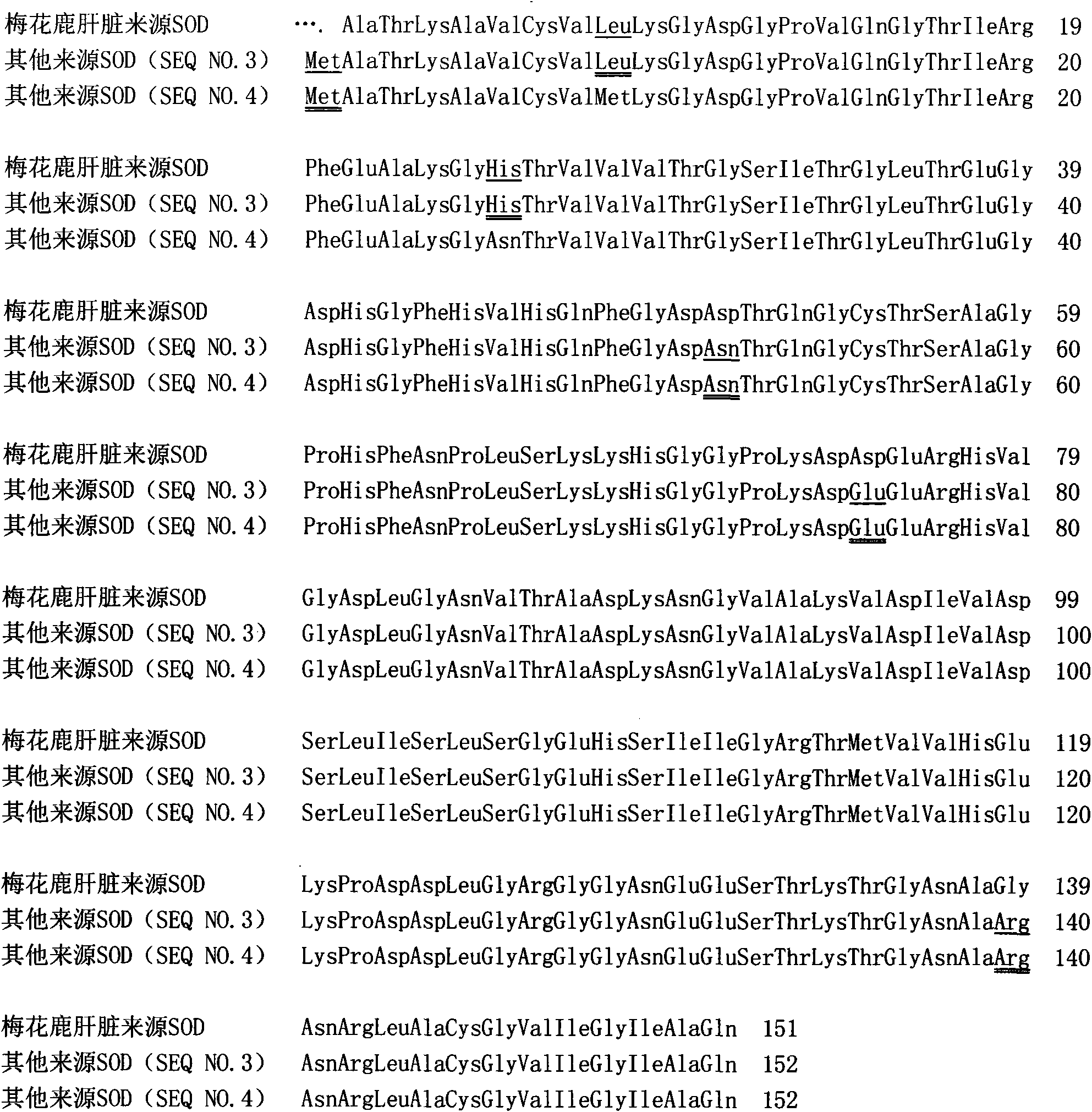
Superoxide dismutase and preparation method thereof
InactiveCN101633916ARealize the industrialization of productionGood pH stabilityFungiBacteriaDismutaseNucleotide
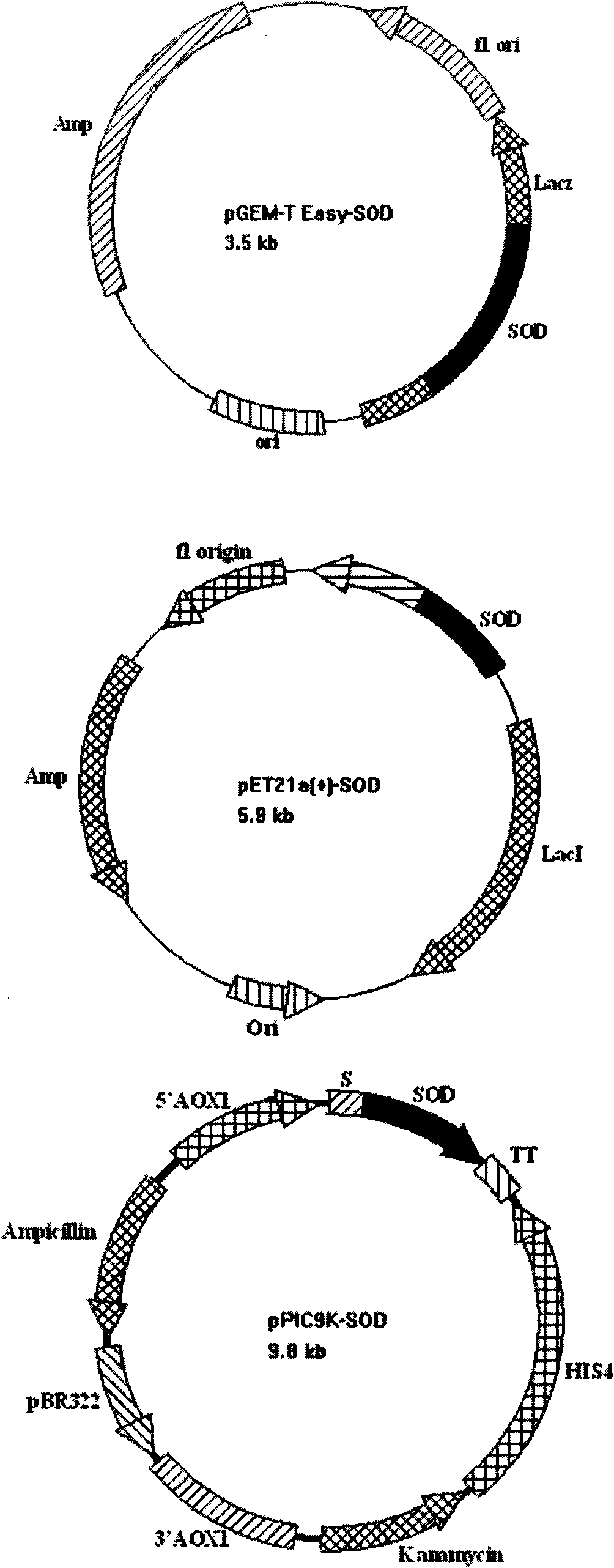
The invention provides a SOD gene from animal and a method for cloning and expressing the SOD gene in colibacillus and yeast cells, wherein a nucleotide sequence of superoxide dismutase is shown as SEQ NO.1; an amino acid sequence of the superoxide dismutase is shown as SEQ NO.2; carriers of nucleotide molecules are colibacillus plasmids or yeast plasmids; cells of the nucleotide molecules are formed by carrier conversion; and cells of the nucleotide molecules of the superoxide dismutase contain colibacillus containing the nucleotide molecules or converted by the carriers or pichia yeast containing the nucleotide molecules or converted by the carriers. The invention can prepare recombinant production strains which can efficiently express and secrete Cu / Zn-SOD, realizes the production industrialization of the Cu / Zn-SOD, and achieves good pH stability, favorable thermal stability and Cu / Zn-SOD products with anti-protease hydrolyzation capacity.
Owner:FUZHOU UNIV
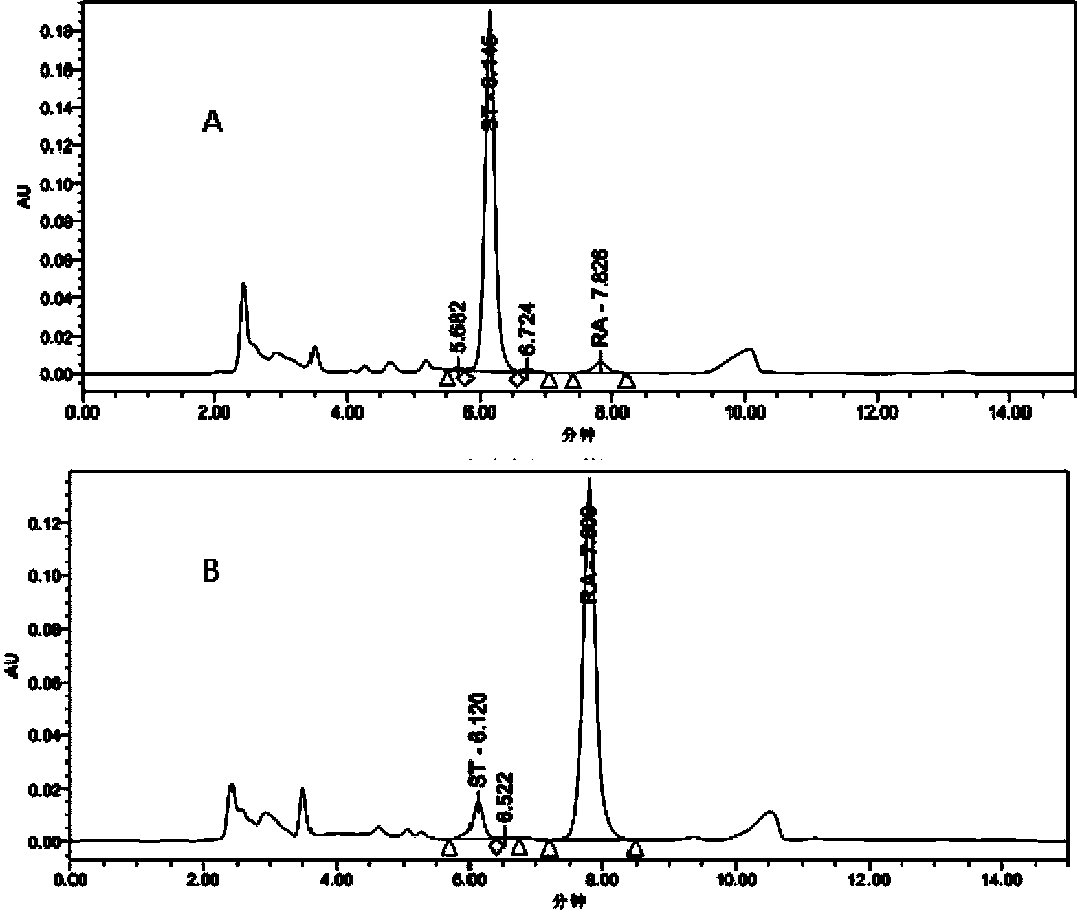
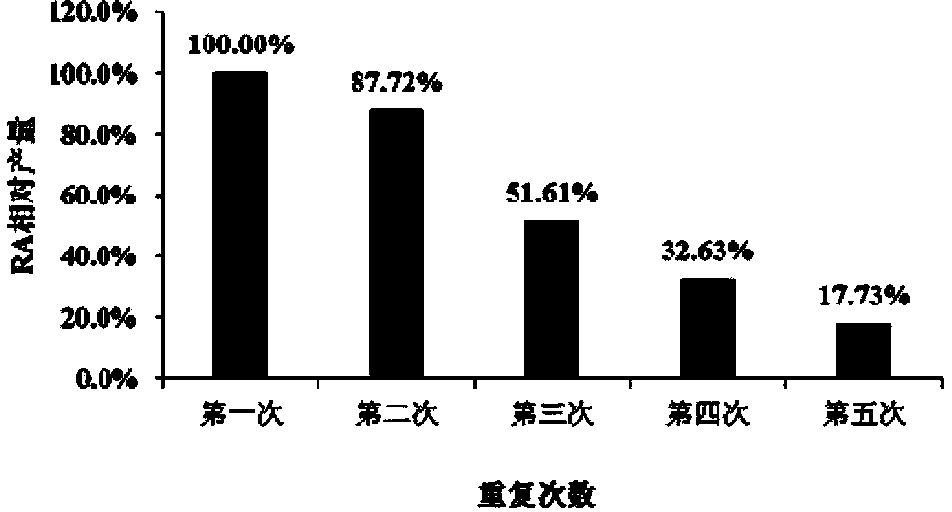
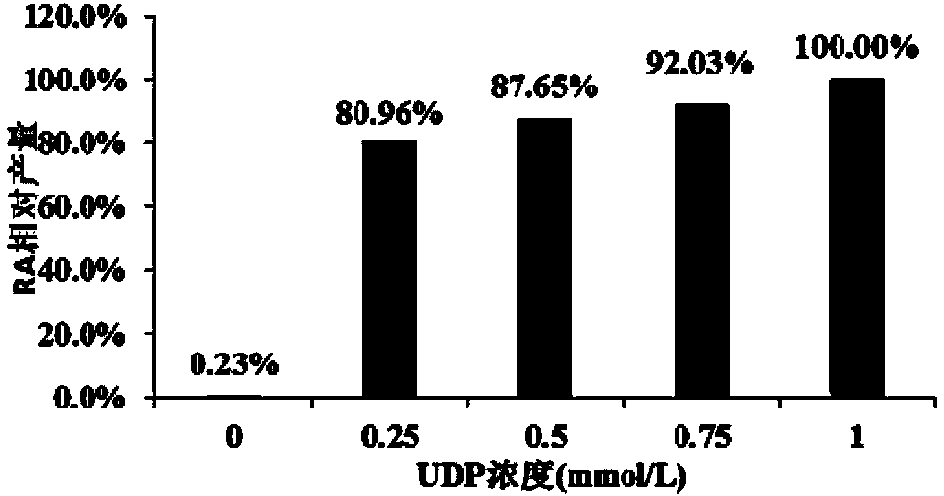
Recombinant pichia pastoris engineering bacterium and application thereof to synthesis of RA (rebaudioside A)
ActiveCN104232496AIncrease usageHigh synthesis efficiencyFungiMicroorganism based processesSucrose synthetasePtru catalyst
The invention discloses a recombinant pichia pastoris engineering bacterium and a method for using the recombinant pichia pastoris engineering bacterium as a whole-cell catalyst for synthesizing RA (rebaudioside A). The recombinant pichia pastoris engineering bacterium is obtained through conversion of pichia pastoris after linearization of an expression vector containing exogenous DNA as follows: the DNA sequence for coding sucrose synthetase Sus1 is represented by SEQ ID NO.3, and the DNA sequence for coding UDP-glycosyl transferase UGT76G1 is represented by SEQ ID NO.4. The intracellular expression of the Sus1 and the surface expression of the UGT76G1 are realized by the aid of the recombinant pichia pastoris engineering bacterium, and the recombinant pichia pastoris engineering bacterium is used as the whole-cell catalyst and can be effectively used for synthesizing the RA, so that the synthetic efficiency of the RA is improved, the raw material utilization rate is increased, the production cost is reduced, and a new industrial way is opened for production and processing of the RA.
Owner:GUANGZHOU KANGLINNAI BIOLOGICAL SCI & TECH CO LTD
Brewing method of improving aroma characteristics of prickly pear fruit wine by non-saccharomyces cerevisiae
InactiveCN111154593AGuaranteed AlcoholImprove aroma profileMicroorganism based processesAlcoholic beverage preparationBiotechnologyAroma aroma
The invention discloses a brewing method of improving aroma characteristics of prickly pear fruit wine by non-saccharomyces cerevisiae. The non-saccharomyces cerevisiae and saccharomyces cerevisiae are adopted for mixed fermentation. The non-saccharomyces cerevisiae is one or more species of Hanseniaspora uvarum strain F119, Wickerhamomyces anomalus strain C11 and Hyphopichia burtonii strain C30,and the non-saccharomyces cerevisiae is a wild non-saccharomyces cerevisiae isolated from Guinong No. 5 prickly pear. According to the present invention, the non-saccharomyces cerevisiae and saccharomyces cerevisiae are adopted for mixed fermentation, and the saccharomyces cerevisiae consumes sugar to produce alcohol; and the non-saccharomyces cerevisiae has low production of alcohol and high production of aroma substances, and the combination of the two yeasts not only ensures the alcohol content of fruit wine, but also improves the type, content and aroma characteristics of aroma substancesin fruit wine.
Owner:GUIZHOU INST OF TECH
Processes for the bioconversion of a fermentable carbon source to 1,3-propanediol by a single microorganism
A process is provided for the bioconversion of a carbon substrate to 1,3-propanediol by a single organism utilizing microorganisms, such as, Citrobacter, Enterobacter, Clostridium, Klebsiella, Aerobacter, Lactobacillus, Aspergillus, Saccharomyces, Zygosaccharomyces, Pichia, Kluyveromyces, Candida, Hansenula, Debaryomyces, Mucor, Torulopsis, Methylobacter, Escherichia, Salmonella, Bacillus, Streptomyces and Pseudomonas, containing the genes encoding for an active glycerol or diol dehydratase enzyme by contacting these organisms with a carbon substrate under the appropriate fermentation conditions. Specifically, Citrobacter and, Klebsiella provide the source of exogenous genes for such active dehydratase enzyme.
Owner:EI DU PONT DE NEMOURS & CO +1
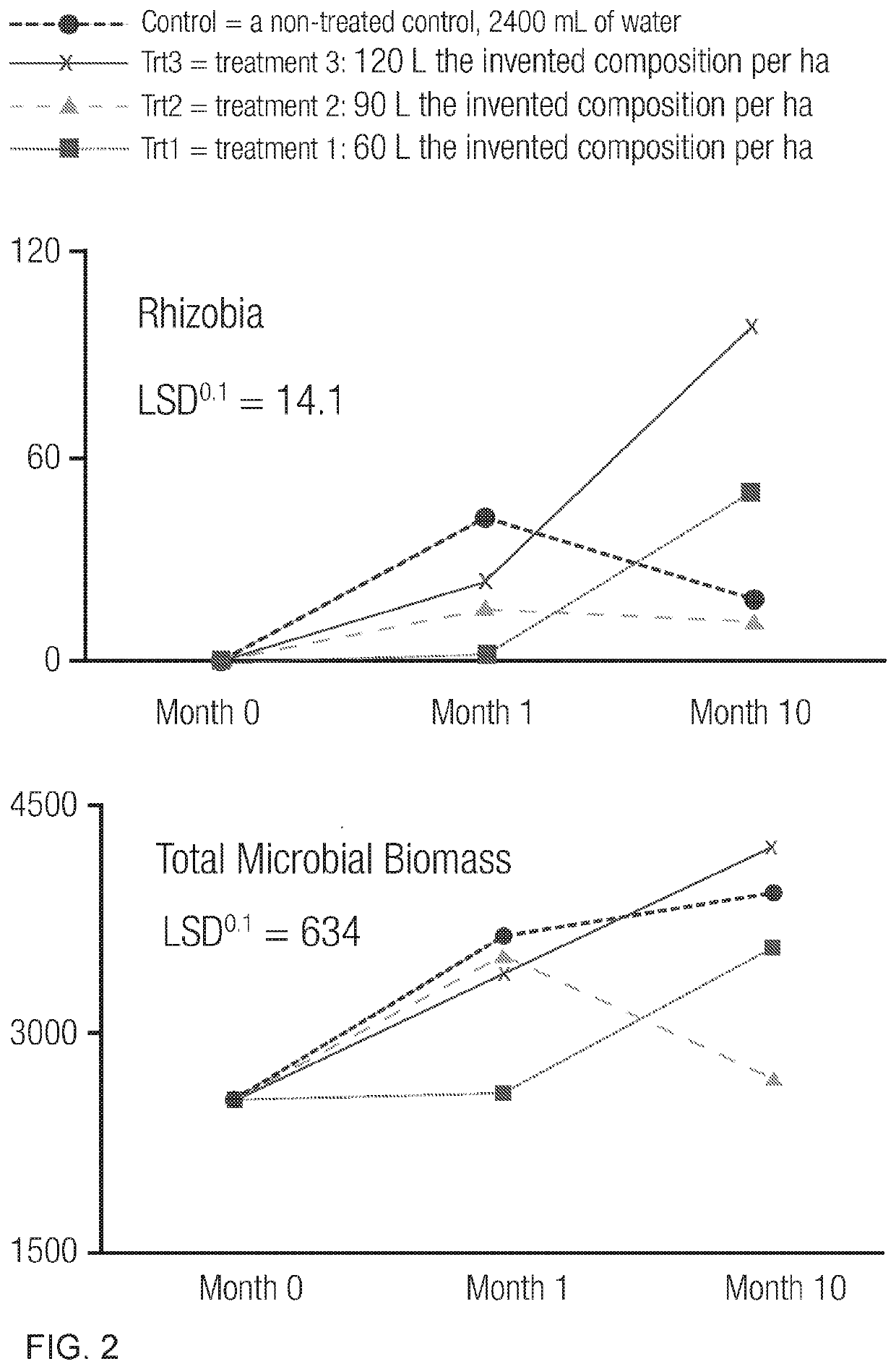
Microbial-based composition and method of use
A microbial-based composition provides a co-cultured microorganism consortium in culture medium that includes one or more Acetobacter sp., Bacillus sp., Bifidobacterium sp., Enter ococus sp., Gluconacetobacter sp., Lactobacillus sp., Rhodopseudomonas sp., Saccharomyces sp., Pichia sp., and Trichoderma sp., as well as a carbon source and chlorine-free water. In some embodiments, the microbial-based composition is useful in the agricultural industry as a plant growth promoting and silage-enhancing agent. For plant growth promotion applications, the microbial-based composition may be applied to the foliar surface of a plant or to the plant growth medium, such as soil or hydroponic solution, surrounding the plant. In silage operations, the microbial-based composition may be applied to a cut plant product during one or more stages in the silage process.
Owner:SUSTAINABLE COMMUNITY DEV LLC
Microbial bacterial strain for producing sphingolipid alkali
The invention relates to a microbial strain which can produce sphingosine, dihydrosphingosine, phytosphingosine and / or the derivatives thereof with improved level. In addition, the invention further discloses a method which is based on induced mutation or other selection technologies, and the strain is prepared accordingly. As an example, a mutant strain of pichia pastris is provided; and compared with the wild-type strain, compounds produced by the mutant strain are higher by about 50 percent.
Owner:科兹莫弗姆有限公司
Methods for the synthesis of lactic acid using crabtree-negative yeast transformed with the lactate dehydrogenase gene
InactiveUS7229805B2Considerable purificationSimpler and less-costlyFungiBiofuelsLactate dehydrogenaseTrichosporon
The invention provides methods and materials related to the production of lactic acid. Specifically, the invention provides methods for producing lactic acid using a crabtree-negative yeast, such as of the Kluyveromyces, Pichia, Candida, Trichosporon and Yamadazmya genera, which have been transformed with a lactate dehydrogenase gene.
Owner:CARGILL INC
Clone and expression of alpha-glucosidase gene
InactiveCN101434943AAchieve extracellular expressionHas transglycosidic activityFungiMicroorganism based processesIsomaltooligosaccharideEnzyme Gene
The invention discloses clone and expression of an Alpha-glucosidase (abbreviated as AGLU enzyme) gene and belongs to the fields of enzyme gene engineering and enzyme engineering. The invention obtains the aglu gene with SEQ ID NO of 1 through an Aspergillus niger (A.niger) WX-07 total DNA, and aglu cDNA uses an expression vector of plasmid pPIC9K and an expression parasitifer of pichia stipitis (P.pastoris), thus realizing extracellular dissolubility expression of the aglu gene; and the aglu cDNA totally has 2880 nucleic acid and encoding of 960 amino acid, constructs eukaryotic expression plasmid and transforms Pichia pastoris KM71 for AGLU enzyme expression. The recombinant enzyme has transnucleosidation activity, can generate isomalto oligosaccharide through maltose transnucleosidation, is applicable to the requirements of industrial application including food, feeding stuffs and medicaments and the like, and can be applied into the industrial production of isomalto oligosaccharide.
Owner:JIANGNAN UNIV
Method of cultivating biological flocs and application thereof in breeding litopenaeus vannamei
InactiveCN106754552AShort adaptabilityBacillus pumilus has strong vitality and adaptabilityFungiBacteriaBacillus pumilusPichia guilliermondii
The invention relates to a method of cultivating biological flocs and an application thereof in breeding litopenaeus vannamei and relates to cultivation of biological flocs. The cultivated biological flocs comprise bacillus pumilus, lactobacillus acidophilus and pichia guilliermondii. The method comprises the following steps: preparing a bacillus pumilus inoculant, a lactobacillus acidophilus inoculant and pichia guilliermondii powder; adopting a way of splashing bacillus pumilus powder, saccharomycetes powder and a lactic acid bacteria inoculant for a water body per mu every day, and arranging an nano aerating tube in the lower layer around a cultivation pond to aerate to form the flocs; adjusting the C / N weight of a cultivating water body and controlling the C / N at (12-14): 1; and after the biological flocs are cultivated, splashing the bacillus pumilus inoculant, pichia guilliermondii powder and a lactobacillus acidophilus inoculant to the water body per mu every day. The cultivated biological flocs can be applied to breeding litopenaeus vannamei.
Owner:THIRD INST OF OCEANOGRAPHY STATE OCEANIC ADMINISTATION
Engineered Saccharomyces cerevisiae producing heat-stability recombinant trypsin, and its application
InactiveCN103173367AHigh energy consumptionReduce energy consumptionFungiMicroorganism based processesPichia pastorisBiotechnology
The invention discloses an engineered Saccharomyces cerevisiae producing heat-stability recombinant trypsin, and its application, and belongs to the genetic engineering field. A trypsin gene obtained through in-vitro amplification is fused with a leading short peptide YVEF, and is wholly connected to a Pichia pastoris GS115 chromosome to construct engineered Saccharomyces cerevisiae efficiently secreting and expressing recombinant trypsin, and the recombinant trypsin having an improved stability is obtained after purification, and the heat stabilities of the recombinant trypsin at 40DEG C, 50DEG C and 60DEG C are 1.77, 2.6 and 31 times wild trypsin respectively, so the problem of the low stability of trypsin is solved. The production of trypsin through applying the engineered Saccharomyces cerevisiae has the advantages of high output, simple technology, intelligible heredity and application background of engineered Saccharomyces cerevisiae, and convenient industrial application.
Owner:JIANGNAN UNIV
Method for producing recombinant human type II collagen single chain by Pichia pastoris
InactiveCN110029111AOptimize secondary structureEliminate useConnective tissue peptidesPeptide preparation methodsPichia pastorisNucleotide
Owner:JIANGSU TRAUTEC MEDICAL TECH CO LTD
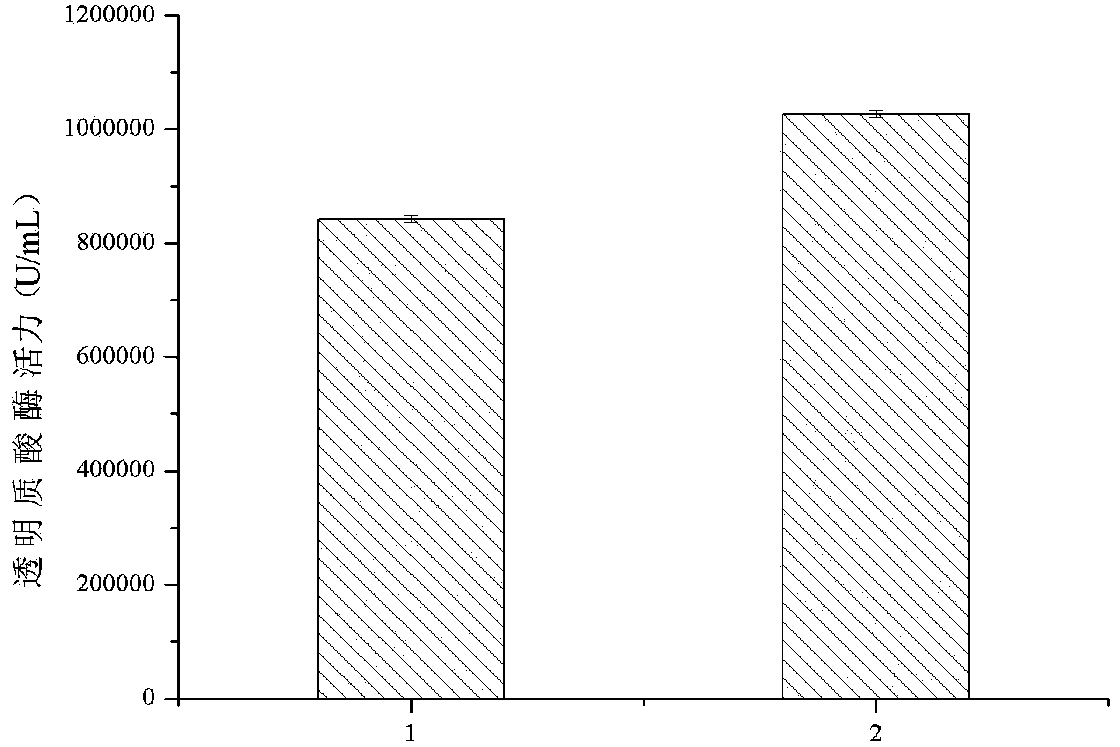
Method for improving pichia pastoris recombinant expression hyaluronidase
InactiveCN104263707AIncrease productionFungiMicroorganism based processesPichiaRecombinant Human Hyaluronidase
The invention discloses a method for improving pichia pastoris recombinant expression hyaluronidase, and belongs to the technical field of bioengineering. A sectional signal peptide sequence is fused at the front section of recombinant hyaluronidase gene, so that the problem of low yield of the original recombinant strain is solved, and the yield of the recombinant hyaluronidase in the pichia pastoris is obviously improved. The successful establishment of engineering lays a certain foundation for further reducing the production cost of hyaluronidase of leech and widening the application range, and the pichia pastoris recombinant expression hyaluronidase is suitable for industrial production.
Owner:JIANGNAN UNIV
Pichia engineering bacterial strain for expression of thermosacus aurantiancus miehe light spore variation gene Mn-sod
The invention relates to a pichia yeast engineering strain Pichia pastoris MS18 expressing thermostable manganese superoxide dismutase (Mn-SOD) gene Mn-sod obtained from Thermoascus aurantiacus var. levisporus. The manganese superoxide dismutase gene Mn-sod, which is obtainded from Thermoascus aurantiacus var. levisporus through the RT-PCR method, is cloned and inserted into pichia yeast integration expression vectorp PIC9K, and then the resulting manganese superoxide dismutase gene Mn-sod expression vector pPIC9K / Mn-sod is induced into pichia yeast GS115. After that, a yeast engineering bacteria MS18 expressing manganese superoxide dismutase is seleceted. The engineering bacteria is induced and cultured for six days, so the enzyme expression quantity is 0.92mg / mL, the enzyme activity is 2324 U / mL, and the molecular weight is 86.0 kDa. The engineering strain has stable activity at 50 DEG C and 60 DEG C, the enzyme activity remains 50% after heat preservation at 80 DEG C for 40 min and remains 20% after heat preservation at 90 DEG C for 60 min. The engineering strain of the invention has thermal stability, which can be used for production strain of thermostable manganese superoxide dismutase and has important economic value and social value.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
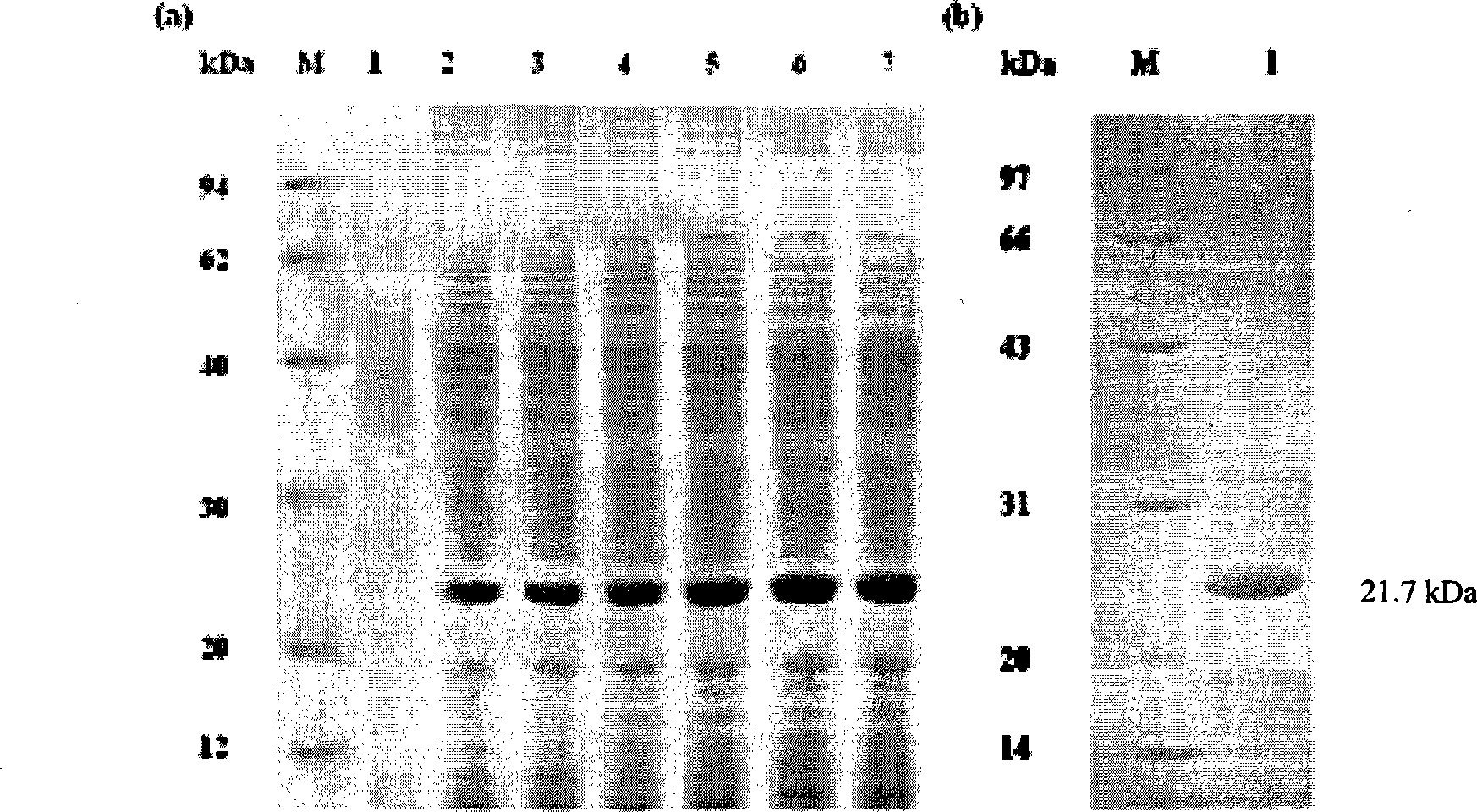
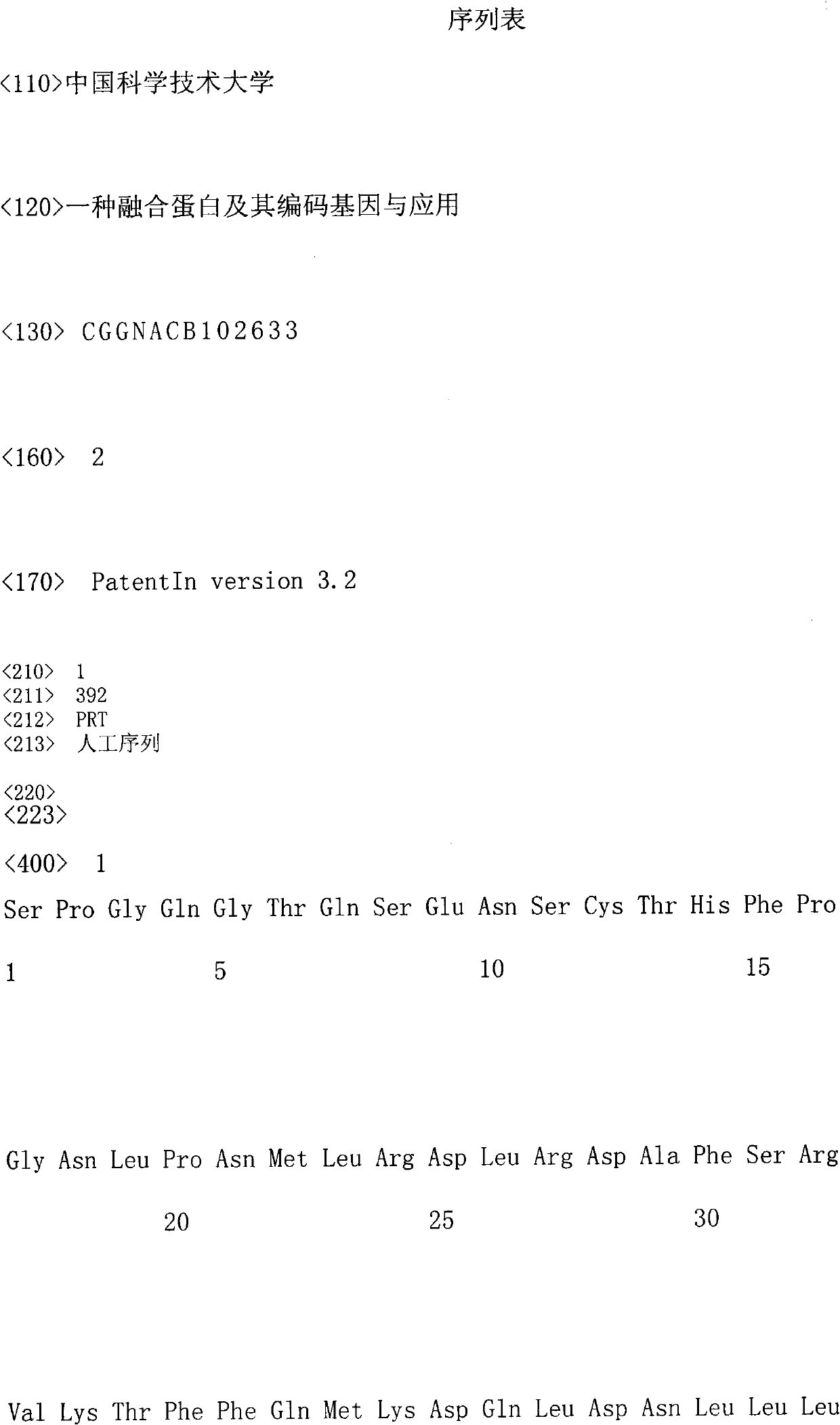
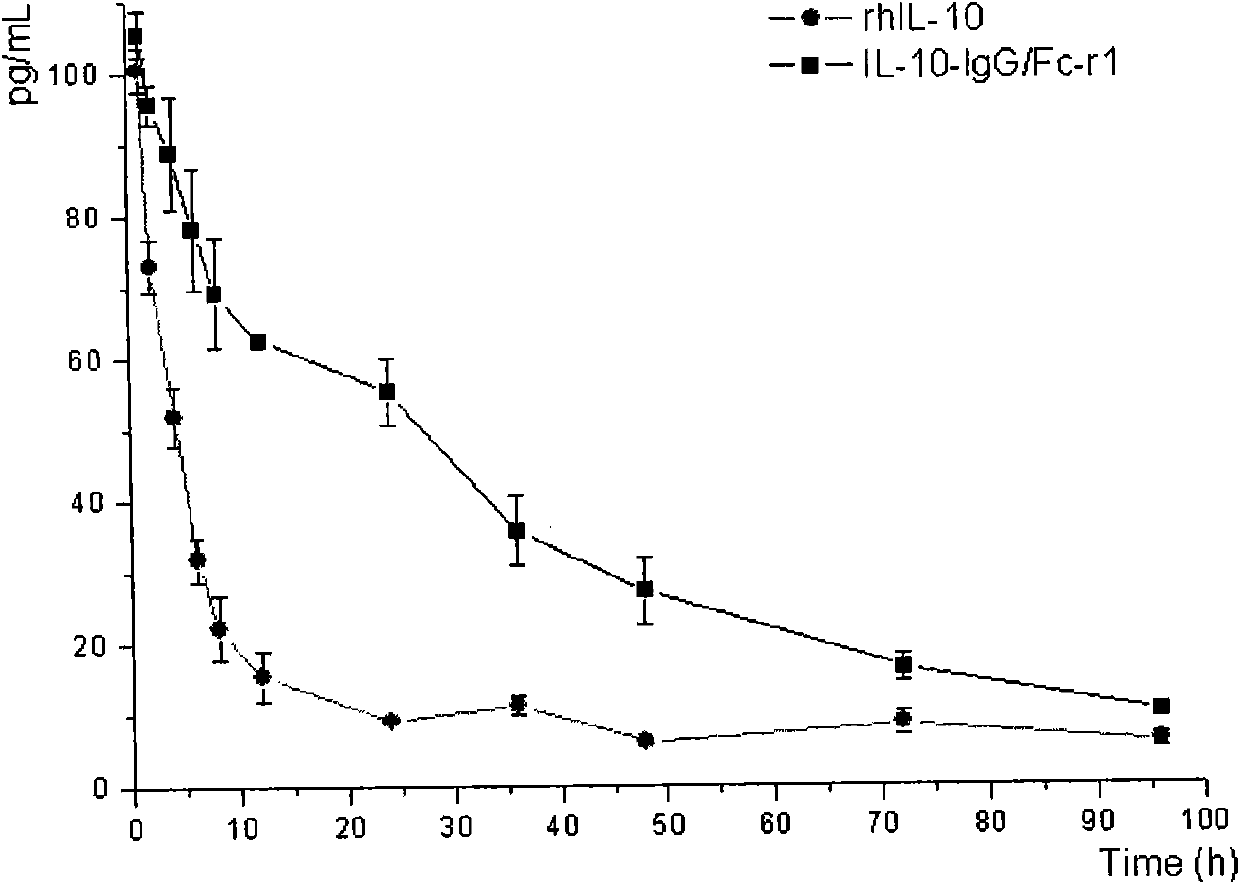
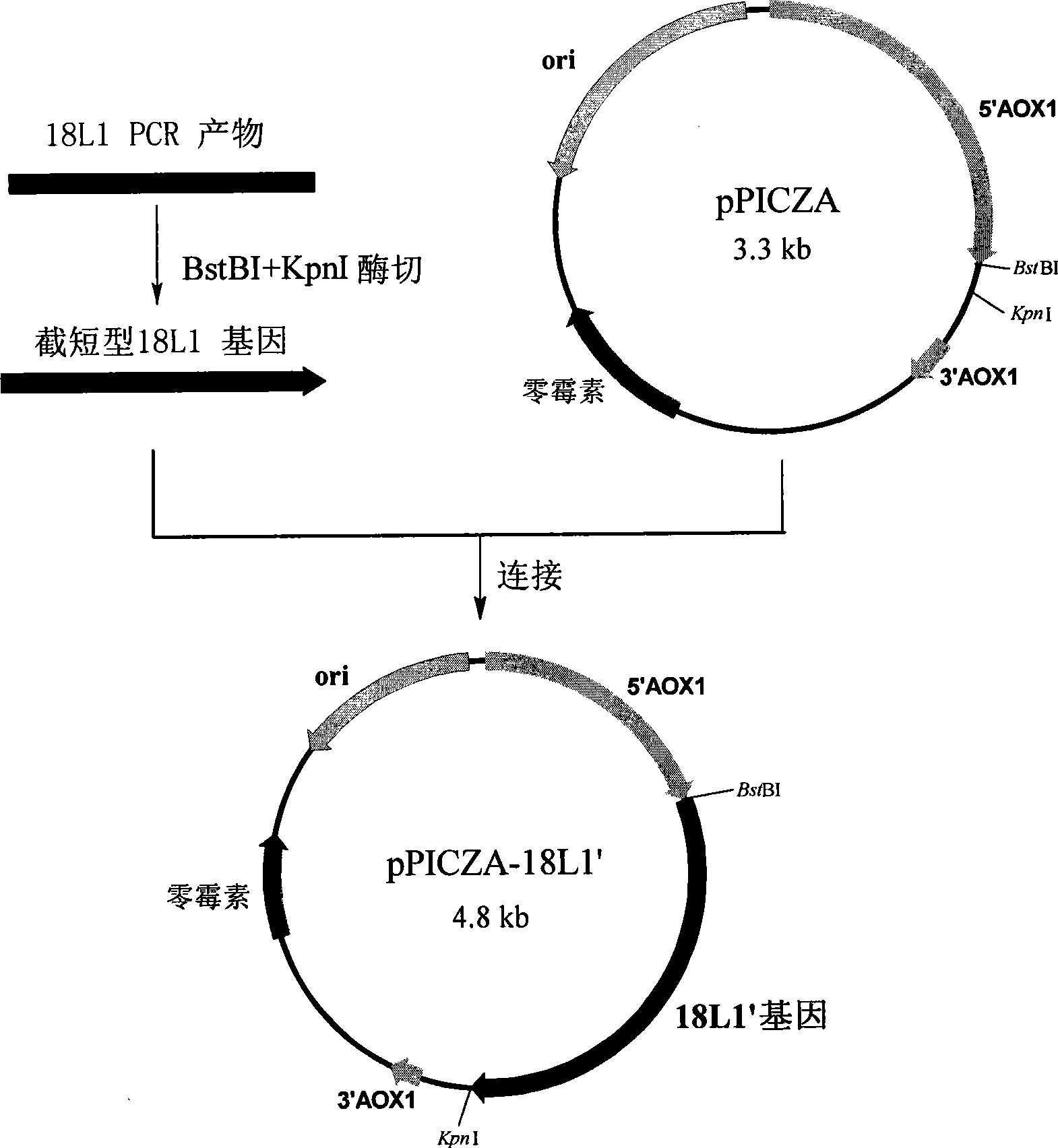
Fusion protein and coding gene and application thereof
The invention discloses a fusion protein and a coding gene and application thereof. The fusion protein provided by the invention is formed by connecting any one of the following proteins at the carboxyl terminal of human interleukin-10 protein: 1) human IgGFc-gamma 1 or mutant protein thereof, and 2) human IgGFc-gamma 2 or mutant protein thereof, wherein the amino acid sequence of the human interleukin-10 protein is an amino acid sequence from 1st to 160th site of the amino terminal of the sequence 1 in a sequence table. Proved by experiments, the IL-10-IgG / Fc fusion protein of interleukin-10activity is produced by using a pichia pastoris fermentation expression system, and a foundation for clinically researching the treatment effect of the IL-10-IgG / Fc fusion protein in the next step.
Owner:UNIV OF SCI & TECH OF CHINA
Method for preparing vaccine for anti-HPV 18 infection by pichia yeast expression system
ActiveCN101487010AGood neutralizing activityAvoid enteringViral antigen ingredientsAntiviralsYeastHuman papillomavirus
The invention discloses a method for preparing a recombinant human papillomavirus 18-typed L1 protein with a pichia pastoris expression system and comprises the steps of loading an HPV 18 L1 gene in accordance with optimal design into an expression carrier, transforming pichia pastoris and cultivating a transformant, thus obtaining the recombinant human papillomavirus 18-typed L1 protein, which is self-assembled into virus-like particles inside a pichia pastoris body; wherein, nucleotide sequences of the HPV 18 L1 gene in accordance with optimal design are shown in SEQ ID NO.1, SEQ ID NO.2, SEQ ID NO.3, SEQ ID NO.4, SEQ ID NO.5 or SEQ ID NO.6. The HPV 18 L1protein that is prepared by the method of the invention has high expressed quantity. The invention further discloses a method for preparing a papillomavirus 18-typed infection vaccine with better activity by utilizing the virus-like particles of the recombinant HPV 18 L1 protein.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Organic biology padding for efficient livestock and poultry and production method thereof
The invention discloses an organic biology padding for efficient livestock and poultry and a production method thereof, and belongs to the filed of fertilizer. The organic biology padding for efficient livestock and poultry comprises the following parts: 65 to 70 percent of plant fiber crushing materials, 12 to 15 percent of broken rice bran, 12 to 15 percent of clinker or fly ash, 3.5 to 4.5 percent of molasses, 0.6 to 0.8 percent of urea or diammonium phosphate, 0.2 to 0.4 percent of sodium chloride or potassium chloride, and 0.2 to 0.5 percent of inoculated strain, wherein the inoculated strain is the mixture of bacillus licheniformis, bacillus subtilis and pichia membranefaciens Hansen. According to the formula, a strain is inoculated for fermentation, and then the pH value is tested until the pH value is 5.0 and the final temperature is 60 DEG C; the pH value is kept within 5.0 to 6.0; and after 10 to 15 days, the fermentation is completed. The organic biology padding for efficient livestock and poultry and the production method thereof solve the problem of severe organic fecal pollution in the livestock and poultry farming industry of our country, can sustainably utilize and convert the livestock and poultry feces as resources into biological organic fertilizer, and achieve zero emission of livestock and poultry farming thoroughly.
Owner:GUANGZHOU DELI AGRI SCI & TECH
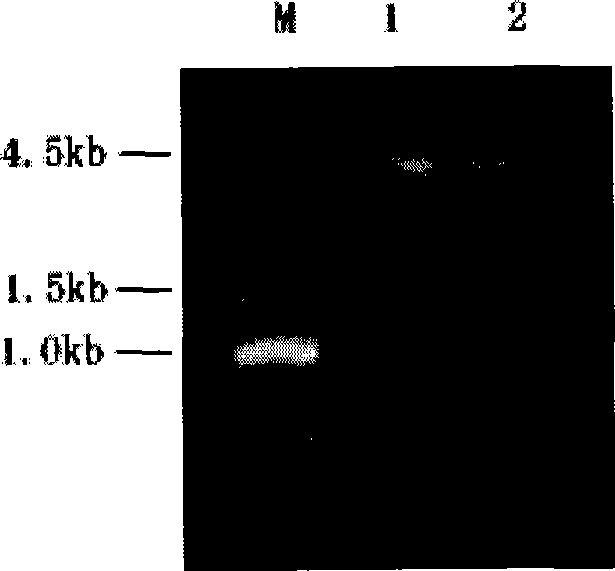
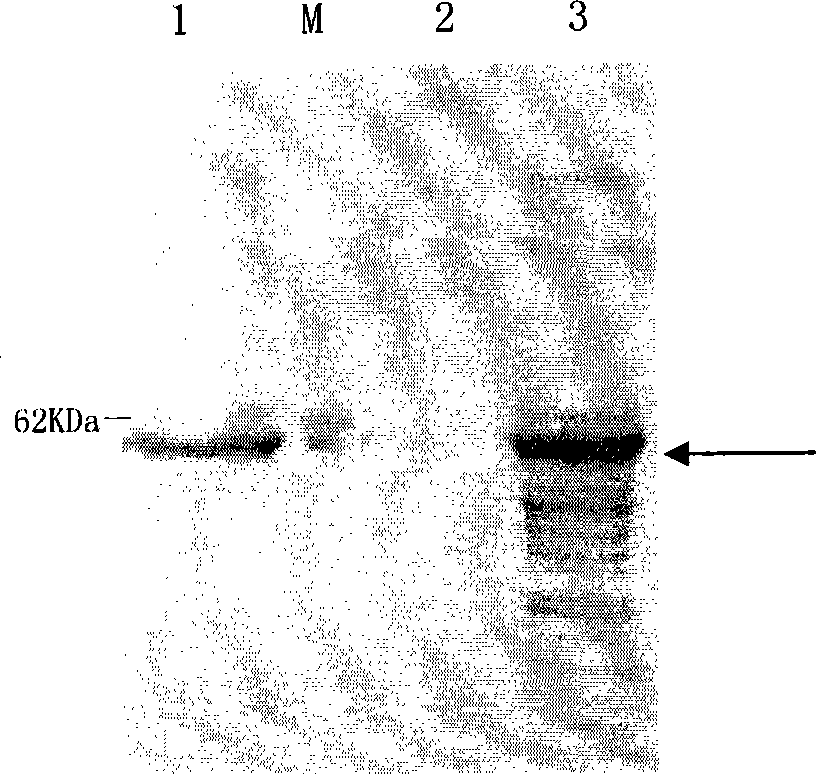
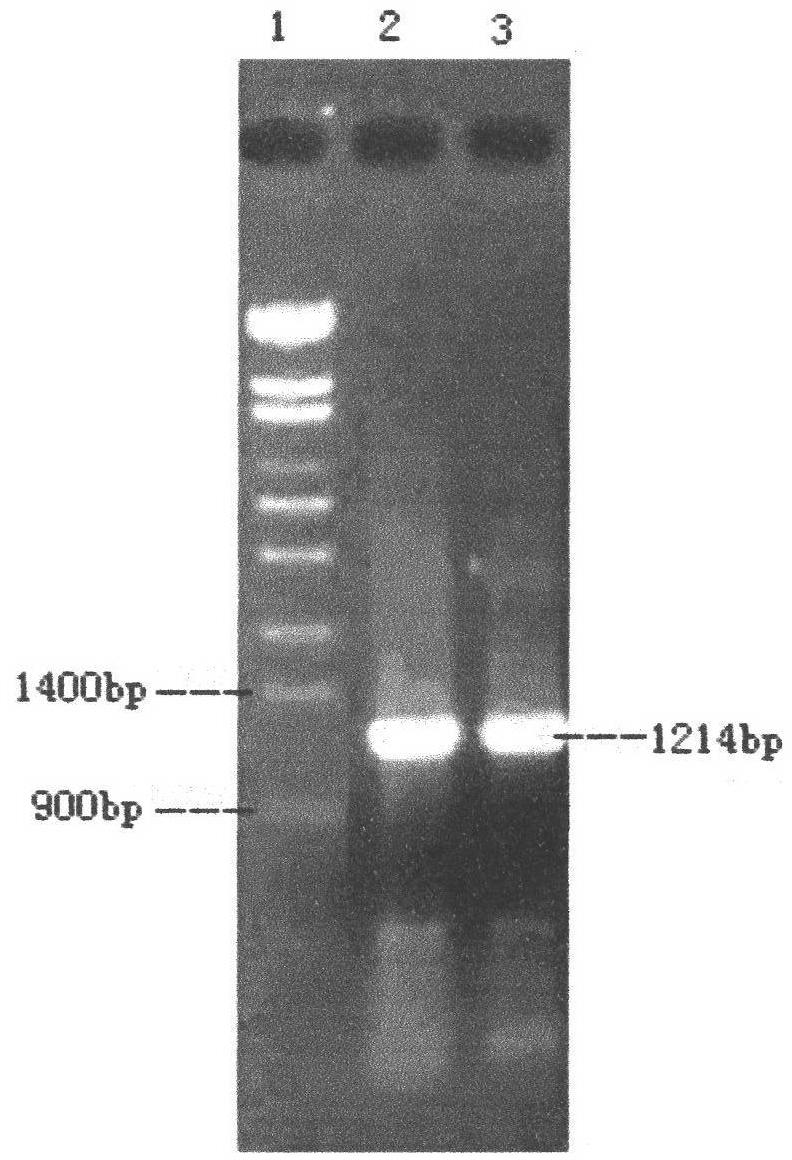
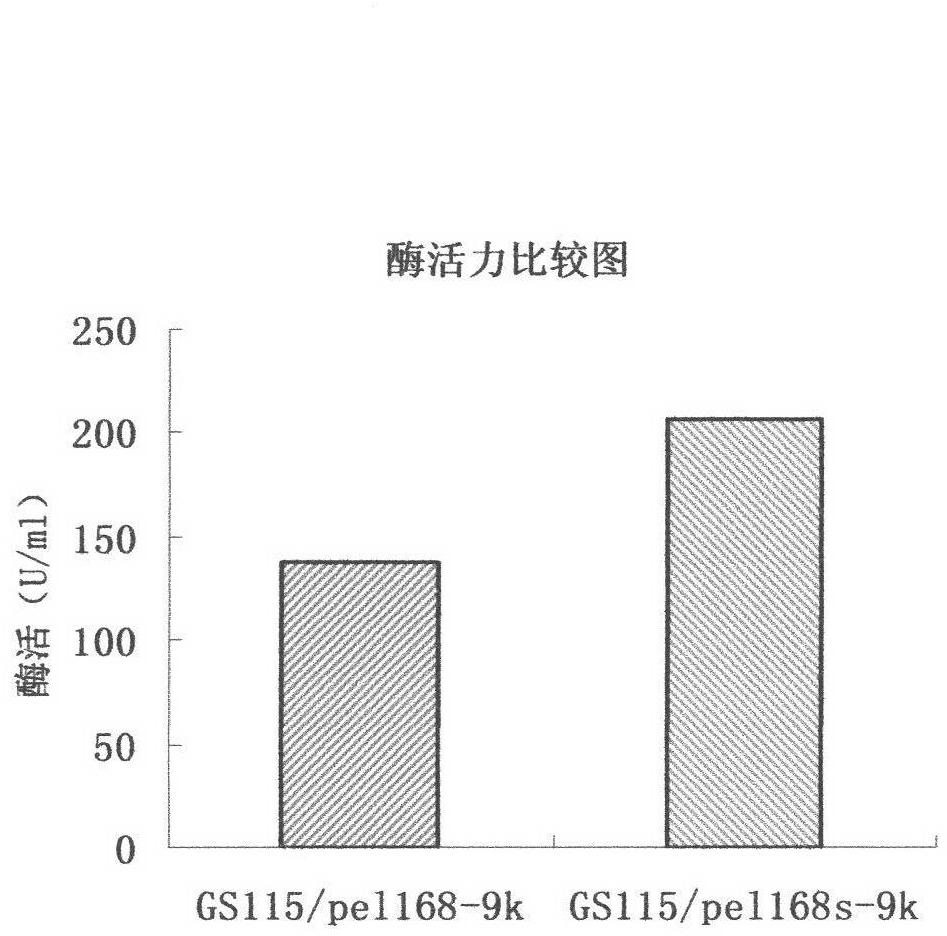
Optimized nucleotide sequence of alkaline pectinase pell68s and high-level expression method thereof
ActiveCN102604977AEasy to purifyHigh activityMicroorganism based processesEnzymesPectinaseBiotechnology
The invention provides and discloses an optimized nucleotide sequence of alkaline pectinase pel168s and a high-level expression method thereof. According to the method, a pel168 gene sequence (wherein the gene is Bacillus subtilis 168 the accession number of which in a GenBank is AL009126) is optimized by DNA works software, restriction enzyme cutting sites SalI and PmeI are shielded, a restriction enzyme cutting site EcoRI is added at the 3' end of a primer, and a restriction enzyme cutting site NotI is introduced at the 5' end of the primer. After the procedures of PCR (Polymerase Chain Reaction) amplification, connection transformation and sequencing verification, the optimized gene sequence of the alkaline pectinase pel168s is obtained. Recombinant plasmid pel168s-9k is constructed according to the sequence, and then is transformed into pichia yeast GS115, thereby obtaining a positive recombinant strain GS115 / pel168s-9k. According to the invention, when alkaline pectinase is produced by adopting the optimized nucleotide sequence of the alkaline pectinase pel168s and utilizing the pichia yeast, the target protein expression index is high, the purge process is simple, the production cost of the alkaline pectinase is reduced greatly, and the utilization rate of an enterprise on the alkaline pectinase is enhanced.
Owner:HUBEI UNIV
Reorganized mannase, genetically-engineered bacteria of recombined mannose and hydrolyzing preparation mannan oligosaccharide method
InactiveCN103146724AEasy to operateReduce manufacturing costFungiMicroorganism based processesEngineered geneticHydrolysis
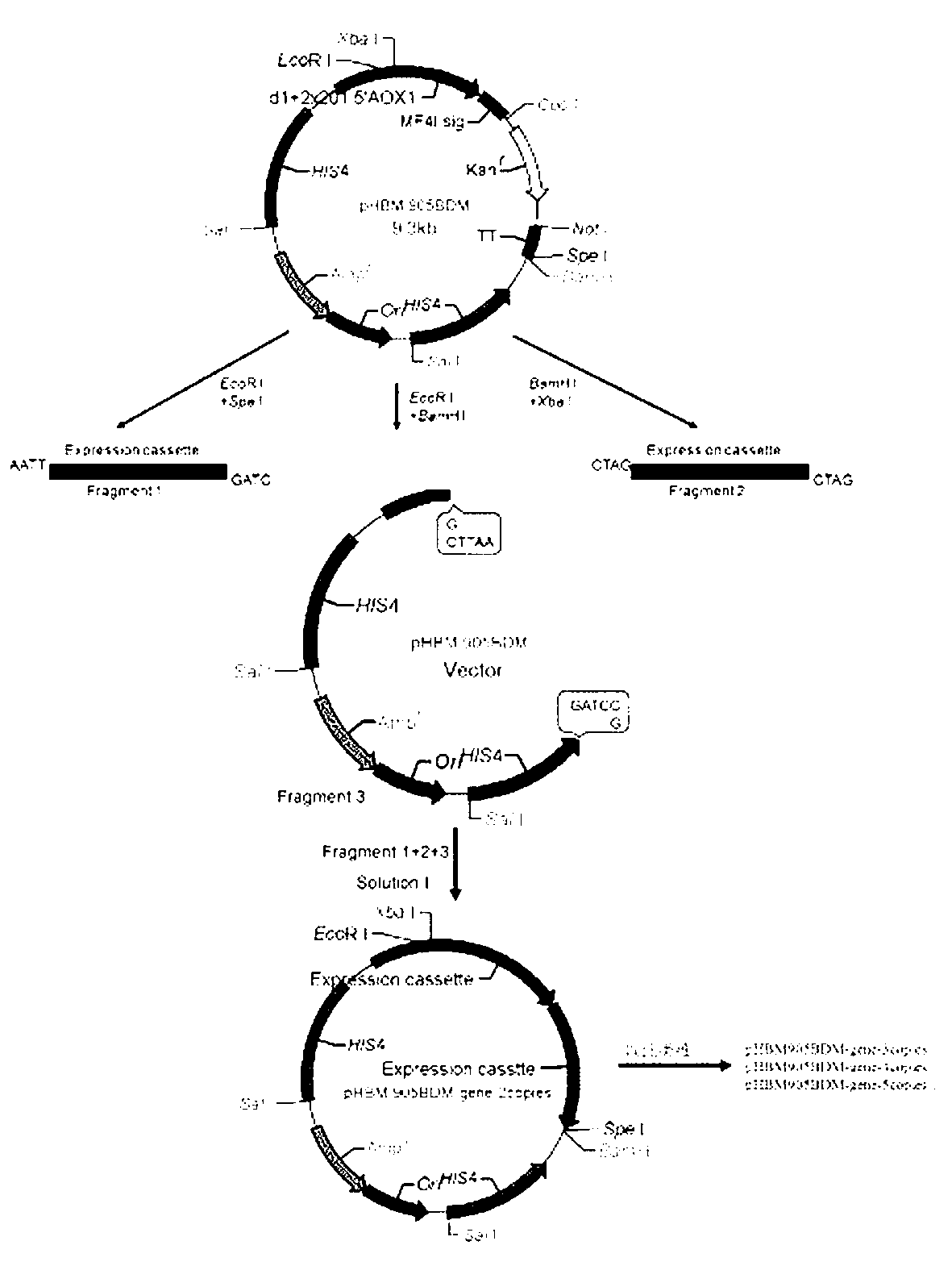
The invention provides reorganized mannase, genetically-engineered bacteria of the recombined mannose and a hydrolyzing preparation mannan oligosaccharide method. The hydrolyzing preparation mannan oligosaccharide method comprises the steps 1), optimizing and reorganizing beta-mannase genes according to pichia pastoris codon preference; 2), constructing expression vector pHBM905BDM-Man containing the beta-mannase genes, converting pitchia pastoris GS115 competence cells, and fostering and checking to obtain single-copy genetically-engineered bacteria; 3) constructing, fostering and checking to obtain multi-copy genetically-engineered bacterium; 4) using different-copy-number genetically-engineered bacteria to efficiently express and prepare reorganized beta-mannase; and 5) using reorganized beta-mannase hydrolysis mannan substrate to produce mannan oligosaccharide. Substrate hydrolysis concentration is 15-40%, temperature is 50 DEG C to 55 DEG C, and time is 0.5 hour to 2 hours. At present, two-copy beta-mannase genetically-engineered bacteria GS115 / MAN78(CCTCCNo:2012554) is high in expression level. The activity of the beta-mannase is 5000U / mL (a decimal number system method), hydrolysis substrate specificity is high, and hydrolysis substrate concentration is obviously higher than that of other methods.
Owner:HUBEI UNIV +1
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com