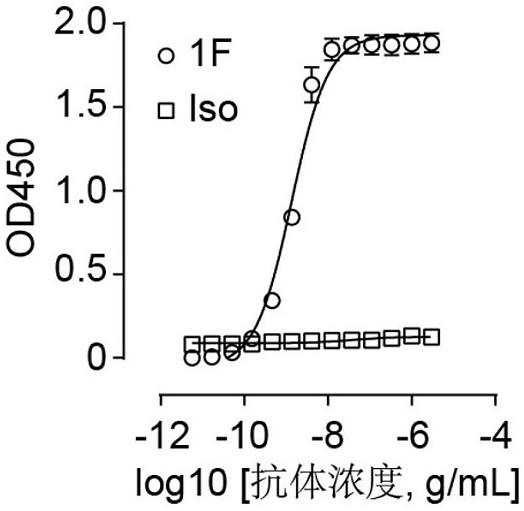
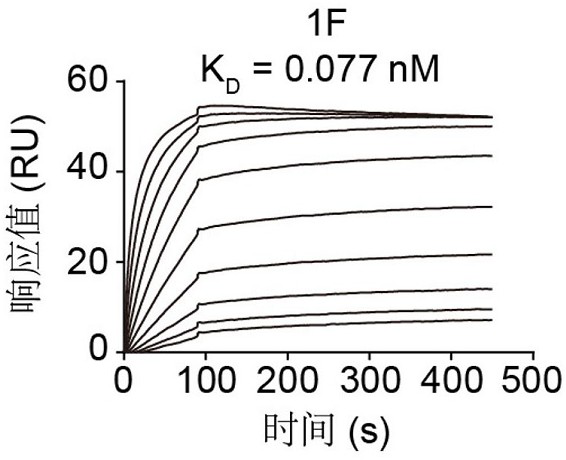
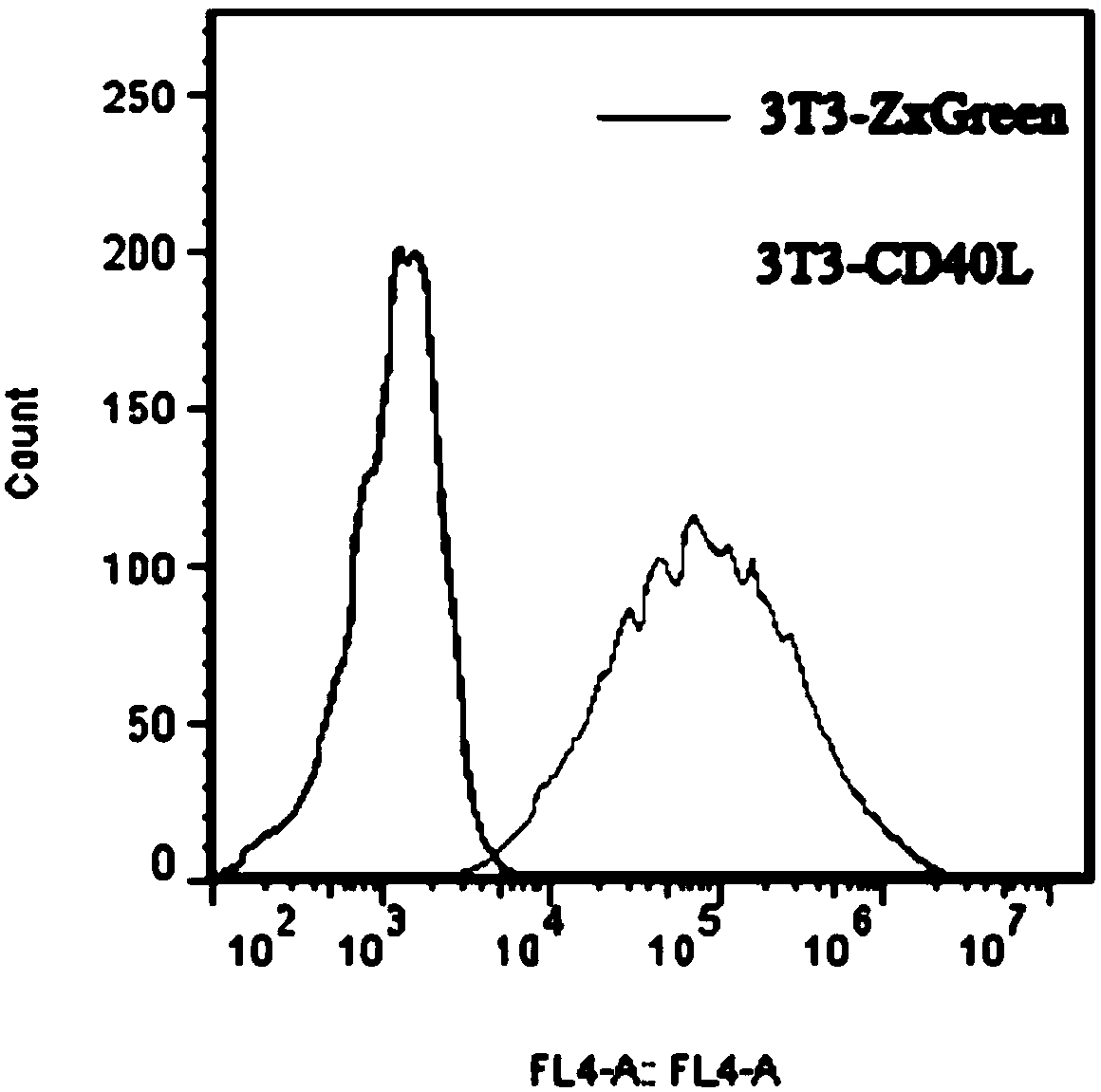
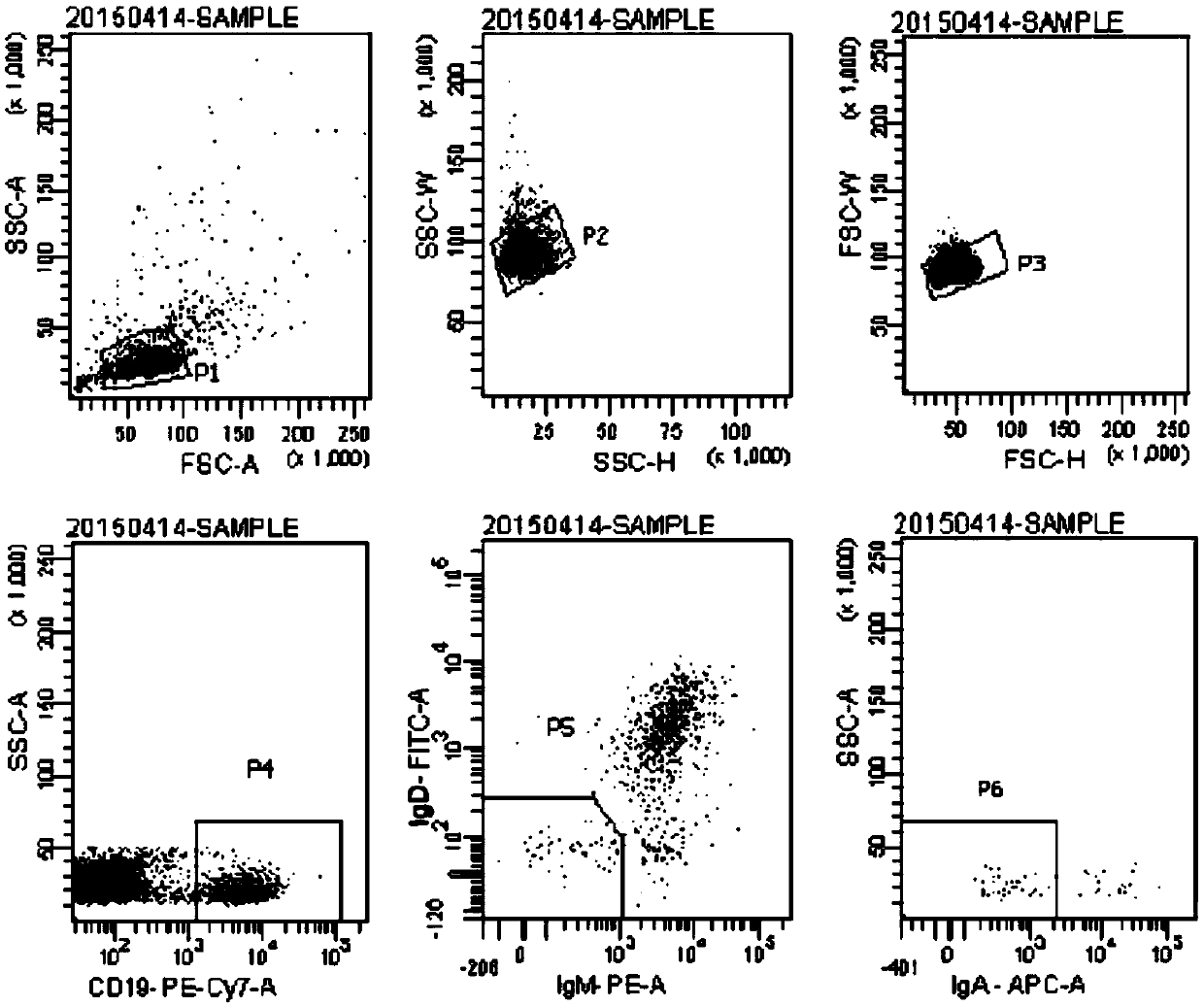
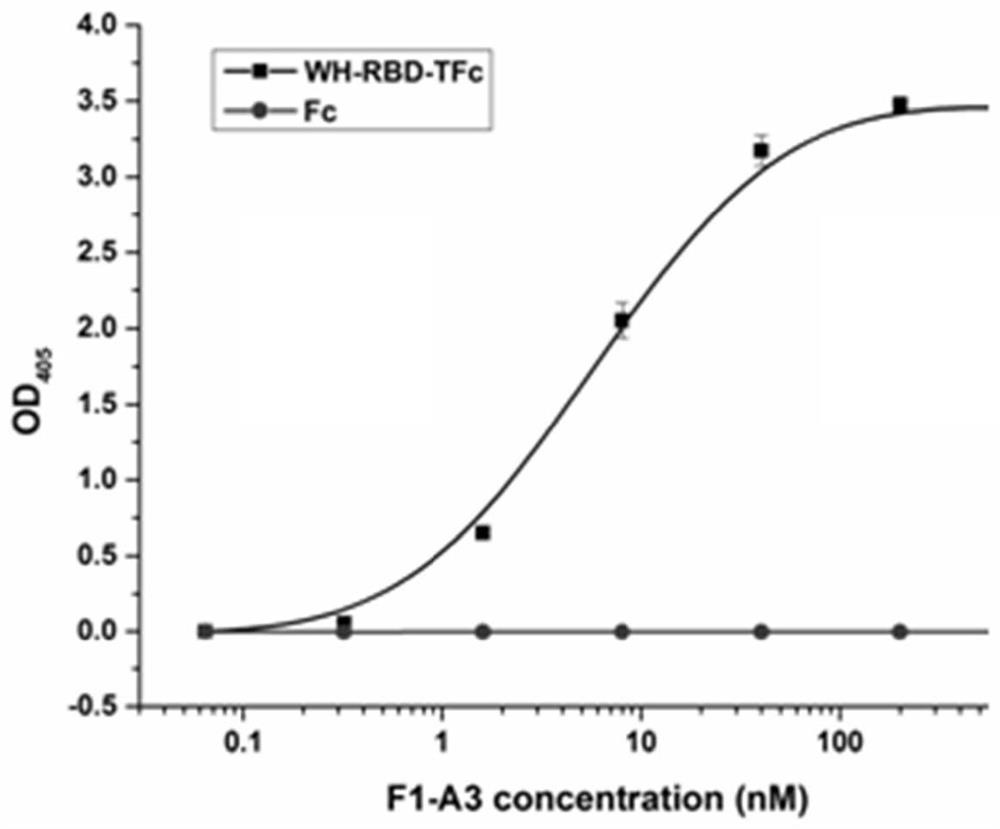
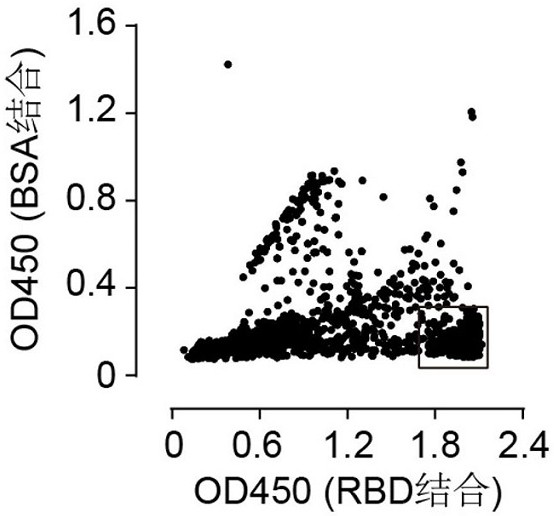
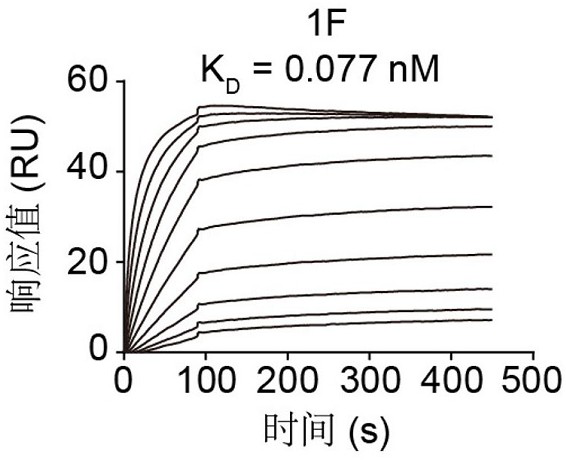
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
55results about How to "Good neutralizing activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hybridoma cell strain and secretion monoclonal antibody and application thereof
ActiveCN104694480AGood neutralizing activityHigh bonding strengthImmunoglobulins against virusesAntiviralsMaternal antibodyEpidemic diarrhea
The invention discloses a hybridoma cell strain and an anti-porcine-epidemic-diarrhea-virus monoclonal antibody produced by the hybridoma cell strain. The anti-porcine-epidemic-diarrhea-virus monoclonal antibody has the preventing and / or treating function for porcine epidemic diarrhea viruses, solves the problem of piglet epidemic diarrhea virus infection caused when existing vaccines and maternal antibodies are insufficient, overcomes the defect that an existing vaccine is single in prevention function and can also be used for developing diagnostic reagent products for the porcine epidemic diarrhea viruses.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Anti-H7N9 full-human-derived monoclonal antibody 5J13 and preparation method and application thereof
ActiveCN106519027AHigh affinityImprove featuresImmunoglobulins against virusesAntiviralsHemagglutininSide effect
The invention relates to an anti-H7N9 full-human-derived monoclonal antibody 5J13 and a preparation method and application thereof. Amino acid sequences of heavy and light chain CDR1, CDR2 and CDR3 of the antibody are GFSFSNYG in the heavy chain CDR1 area, ISYDGTNK in the heavy chain CDR2 area, AKGRGPYCSSSICYHGMDV in the heavy chain CDR3 area, QSVLSGSINMNY in the light chain CDR1 area, WAS in the light chain CDR2 area and QQYYSTPLT in the light chain CDR3 area correspondingly. The antibody can be combined with hemagglutinin A of the H7N9virus in a targeted mode and has remarkable neutralization activity in resisting H7N9 virus infection. Compared with a murine antibody, genes of the full-human-derived antibody are completely from human genes and have no components of other species, toxic and side effects such as the anti-mouse anti-antibody are avoided, the biocompatibility is better, and the anti-H7N9 full-human-derived monoclonal antibody 5J13 is more suitable and has more potential in becoming a macromolecular drug for treating influenza virus.
Owner:SHENZHEN INST OF ADVANCED TECH
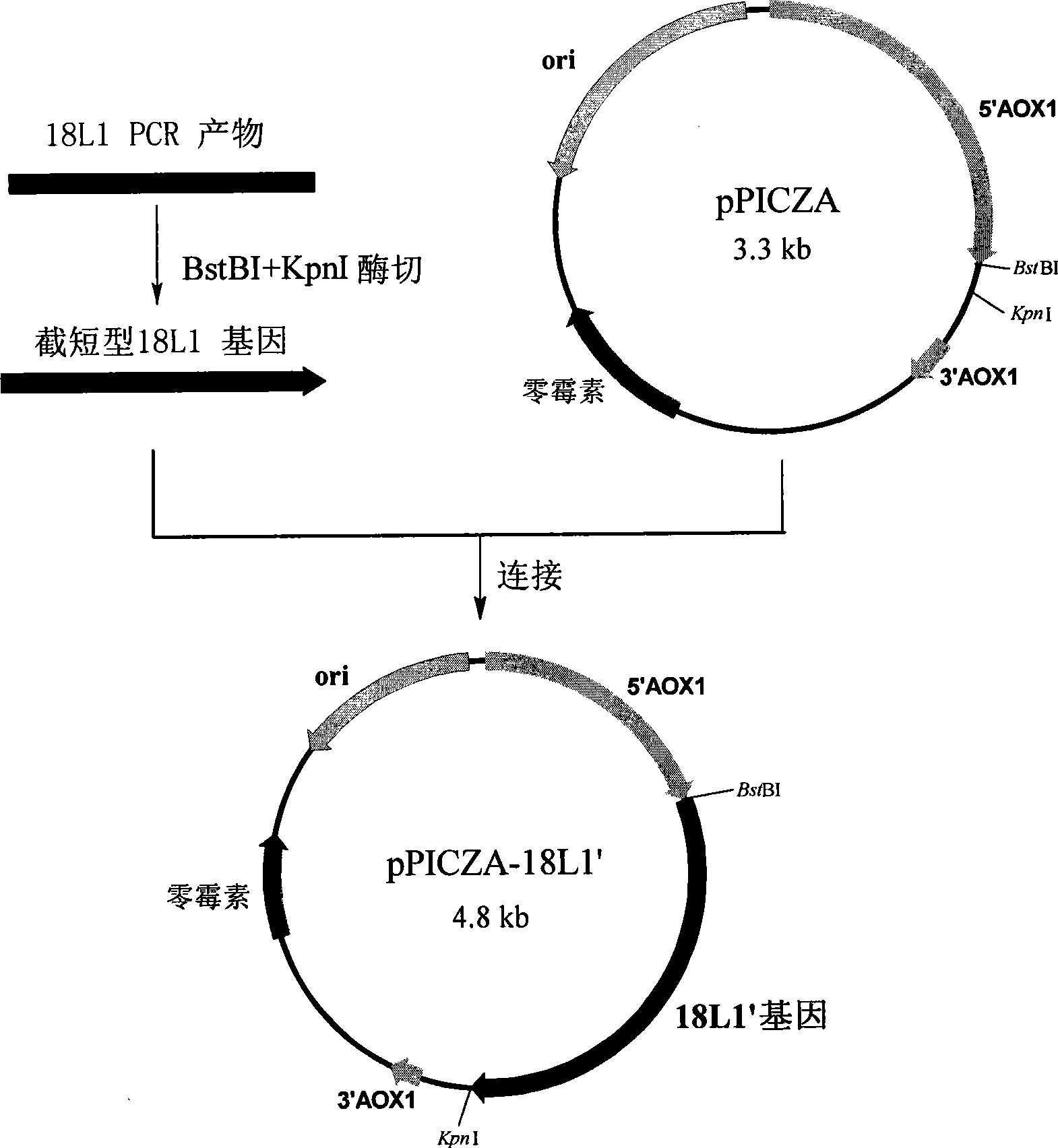
Method for preparing vaccine for anti-HPV 16 infection by pichia yeast expression system
ActiveCN101487009AGood neutralizing activityAvoid enteringViral antigen ingredientsAntiviralsPichiaHuman papillomavirus
The invention discloses a method for preparing a recombinant human papillomavirus 16-typed L1 protein with a pichia pastoris expression system and comprises the steps of loading an HPV 16 L1 gene in accordance with optimal design into an expression carrier, transforming pichia pastoris and cultivating a transformant, thus obtaining the recombinant human papillomavirus 16-typed L1 protein, which is self-assembled into virus-like particles inside a pichia pastoris body; wherein, nucleotide sequences of the HPV 16 L1 gene in accordance with optimal design are shown in SEQ ID NO.1, SEQ ID NO.2 or SEQ ID NO.3. The HPV 16 L1protein that is prepared by the method of the invention has high expressed quantity. The invention further discloses a method for preparing a papillomavirus 16-typed infection vaccine with better activity by utilizing the virus-like particles of the recombinant HPV 16 L1 protein.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Method for preparing vaccine for anti-HPV 18 infection by pichia yeast expression system
ActiveCN101487010AGood neutralizing activityAvoid enteringViral antigen ingredientsAntiviralsYeastHuman papillomavirus
The invention discloses a method for preparing a recombinant human papillomavirus 18-typed L1 protein with a pichia pastoris expression system and comprises the steps of loading an HPV 18 L1 gene in accordance with optimal design into an expression carrier, transforming pichia pastoris and cultivating a transformant, thus obtaining the recombinant human papillomavirus 18-typed L1 protein, which is self-assembled into virus-like particles inside a pichia pastoris body; wherein, nucleotide sequences of the HPV 18 L1 gene in accordance with optimal design are shown in SEQ ID NO.1, SEQ ID NO.2, SEQ ID NO.3, SEQ ID NO.4, SEQ ID NO.5 or SEQ ID NO.6. The HPV 18 L1protein that is prepared by the method of the invention has high expressed quantity. The invention further discloses a method for preparing a papillomavirus 18-typed infection vaccine with better activity by utilizing the virus-like particles of the recombinant HPV 18 L1 protein.
Owner:SHANGHAI ZERUN BIOTECHNOLOGY CO LTD
Antibody targeting SARS-CoV-2 as well as preparation method and application of antibody
ActiveCN112390879ARandom combinationInhibit bindingAntibody mimetics/scaffoldsImmunoglobulins against virusesDiseaseImmune escape
The invention provides an antibody targeting SARS-CoV-2 and a preparation method and application thereof. The antibody comprises VH and VL, wherein the VH comprises the following CDRs: VH CDR1, VH CDR2 and VH CDR3 with amino acid sequences as shown in SEQ ID NO: 1, 2 and 3; the VL comprises the following CDRs: VL CDR1, VL CDR2 and VL CDR3 with amino acid sequences as shown in SEQ ID NO: 4, 5 and 6. The antibody can be highly compatible and specifically combined with RBD of S proteins of SARS-CoV-2, inhibits combination of the RBD protein and receptor ACE2 protein, efficiently inhibits SARS-CoV-2 from infecting cells, and has very good neutralizing activity on potential pseudoviruses of immune escape mutation, so that the antibody can be effectively applied to diagnosis, prevention and treatment of SARS-CoV-2 viruses and related diseases.
Owner:SHANGHAI TECH UNIV
Anti-H7N9 full human derived monoclonal antibody hIg311 and preparation method and application thereof
ActiveCN110746503AGood neutralizing activityGood biocompatibilityImmunoglobulins against virusesAntiviralsHemagglutininAntiendomysial antibodies
The application relates to an anti-H7N9 full human derived monoclonal antibody hIg311 and a preparation method and application thereof. A memory B cell PCR method is used for quickly screening a fullhuman derived monoclonal antibody hIg311, and the anti-H7N9 human derived monoclonal antibody hIg311 is free from any mouse derived components. The antibody disclosed by the application can realize target combination of hemagglutinin HA of H7N9 viruses, and has notable anti H7N9 viral infection neutralization activity. The antibody disclosed by the application does not generate toxic and side effects for resisting mouse resisting antibodies, has good biocompatibility, and is suitable for becoming and has potential to become macromolecular drugs for treating influenza viruses.
Owner:SHENZHEN INST OF ADVANCED TECH
Monoclonal antibody of novel coronavirus and mutant thereof and application of monoclonal antibody
ActiveCN113943368AHigh affinityHigh neutralizing activityImmunoglobulins against virusesAntiviralsAntigenComplementarity determining region
The invention relates to the technical field of immunology and molecular virology, and particularly discloses a monoclonal antibody of novel coronavirus and a mutant thereof and application of the monoclonal antibody. Complementary determining regions CDR1, CDR2 and CDR3 of a heavy chain variable region of the monoclonal antibody or an antigen binding fragment thereof respectively have amino acid sequences as shown in SEQ ID NO: 1, SEQ ID NO: 2 and SEQ ID NO: 3; and complementary determining regions CDR1, CDR2 and CDR3 of a light chain variable region of the monoclonal antibody or the antigen binding fragment thereof respectively have amino acid sequences as shown in SEQ ID NO: 4, SEQ ID NO: 5 and SEQ ID NO: 6. The monoclonal antibody disclosed by the invention can be combined with the S protein RBD of the novel coronavirus and various mutant strains thereof with high affinity, is strong in neutralizing activity, and has ideal clinical application value for preventing and treating infection of the novel coronavirus and various mutant strains thereof.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Neutralizing human monoclonal antibody aiming at novel coronavirus and application thereof
ActiveCN113388029AGood neutralizing activityImmunoglobulins against virusesAntiviralsEpitopeReceptor
The invention discloses a neutralizing human monoclonal antibody aiming at a novel coronavirus. The antibody can specifically recognize a novel coronavirus antigen and has better neutralizing activity on a live virus. The epitope directed by the gene is mainly a receptor binding domain of a novel coronavirus. In addition, the antibody disclosed by the invention can be prepared into an antibody drug for preventing and treating the novel coronavirus, so that the antibody drug is clinically used for preventing and treating diseases caused by infection of the novel coronavirus.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Clostridium difficile exotoxin A carboxy-terminal gene sequence with optimized codon and nucleic acid vaccine thereof
InactiveCN102199611AStimulus expression levelGood immune protectionAntibacterial agentsBacterial antigen ingredientsPseudomonas exotoxinEscherichia coli
Owner:王世霞 +3
Compositions and methods related to prevention and treatment of rabies infection
ActiveCN104603149AReduce infectivityGood neutralizing activityImmunoglobulins against virusesAntiviralsAntibodyRabies virus
Owner:万机创科(香港)有限公司
Anti-human TSLP monoclonal antibody and application thereof
ActiveCN113683694AGood neutralizing activityQuite affinitySenses disorderDigestive systemDiseaseComplementarity determining region
The invention provides an anti-human thymic stromal lymphopoietin (TSLP) monoclonal antibody and an application thereof. The anti-human thymic stromal lymphopoietin (TSLP) monoclonal antibody comprises three heavy chain complementary determining regions (CDR-H1, CDR-H2 and CDR-H3) and three light chain complementary determining regions (CDR-L1, CDR-L2 and CDR-L3), and (a) the amino acid sequence of the CDR-H1 is as shown in SEQ ID NO: 1, and (b) the amino acid sequence of CDR-H2 is as shown in SEQ ID NO: 2; (c) the amino acid sequence of the CDR-H3 is as shown in SEQ ID NO: 3; (d) the amino acid sequence of the CDR-L1 is as shown in SEQ ID NO: 4; (e) the amino acid sequence of the CDR-L2 is as shown in SEQ ID NO: 5; and the amino acid sequence of the (f) CDR-L3 is as shown in SEQ ID NO: 6. Compared with an anti-human TSLP monoclonal antibody Tezepelumab (prepared according to sequence expression disclosed in the patent), the anti-human TSLP monoclonal antibody has the advantages that the affinity of the anti-human TSLP monoclonal antibody combined with the human TSLP is equivalent, the neutralizing activity of the anti-human TSLP monoclonal antibody at the cellular level is superior to that of the Tezepelumab, and the anti-human TSLP monoclonal antibody is expected to show a good clinical effect in the aspect of preventing and treating related diseases.
Owner:QYUNS THERAPEUTICS CO LTD
Humanized monoclonal antibody of novel coronavirus and application thereof
ActiveCN113292649ABinding blockGood neutralizing activityImmunoglobulins against virusesAntiviralsAntiendomysial antibodiesBinding inhibition
The invention relates to a novel human monoclonal antibody of coronavirus and application of the novel human monoclonal antibody. The antibody can be specifically combined with the 2019-nCoV RBD, the combination of the 2019-nCoV RBD and ACE2 is blocked, and the infection of the 2019-nCoV is inhibited.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
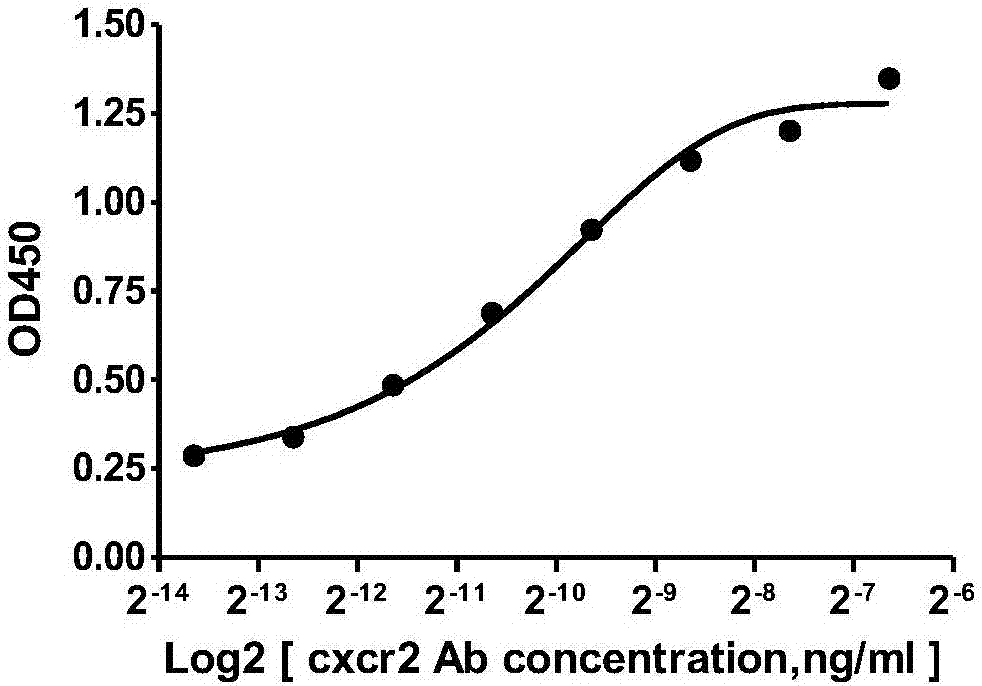
Human-mouse chimeric anti-CXCR2 full-molecule IgG and application thereof
ActiveCN107964045ABinding blockGood neutralizing activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHeavy chainNucleic acid sequencing
The invention discloses a human-mouse chimeric anti-CXCR2 full-molecule IgG and an application thereof and belongs to the field of biological pharmacy. The human-mouse chimeric anti-CXCR2 full-molecule IgG comprises a heavy chain variable region and a light chain variable region and is characterized in that the light chain variable region has a nucleic acid sequence shown in the formula of SEQ IDNO. 1 and the heavy chain variable region has a nucleic acid sequence shown in the formula of SEQ ID NO. 2. The mouse immunized by the specific recombinant CXCR2 protein is used, a mouse-derived anti-CXCR2 monoclonal antibody is prepared through a hybridoma technology, and the recombinant human-mouse chimeric anti-CXCR2 full-molecule IgG is prepared through genetic engineering and antibody engineering techniques. The chimeric antibody can effectively recognize the CXCR2 extracellular domain amino acid fragment and inhibit the binding of the CXCR2 protein to the GRO alpha protein.
Owner:NANJING MEDICAL UNIV
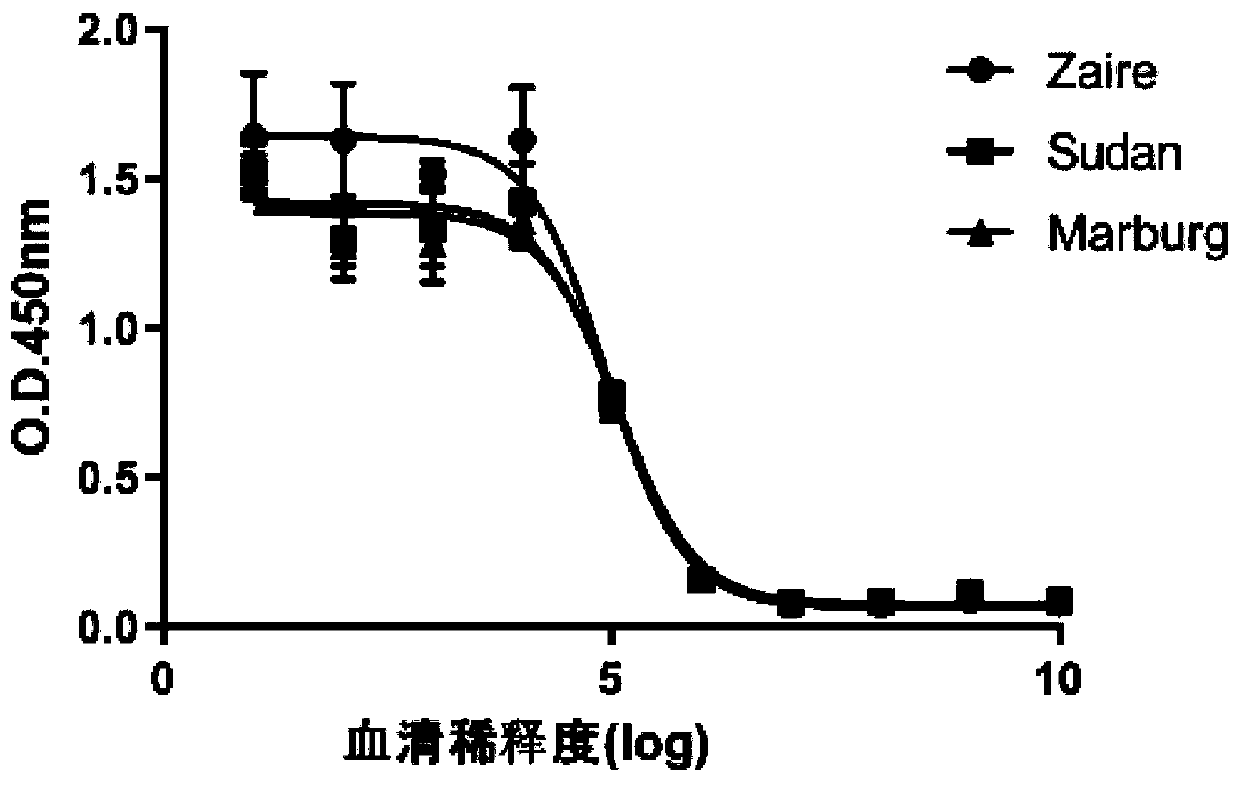
Anti-filovirus monoclonal neutralizing antibody as well as preparation method and application thereof
ActiveCN110551213AHigh homologyGood neutralizing activityImmunoglobulins against virusesFermentationHeavy chainAntigen Binding Fragment
The invention provides an anti-filovirus monoclonal neutralizing antibody as well as a preparation method and application thereof. A heavy chain variable region of an antigen binding segment of the monoclonal neutralizing antibody comprises amino acid sequences of SEQ ID NO:13 or SEQ ID NO:15 shown in the specification, and a light chain variable region of the antigen binding segment of the monoclonal neutralizing antibody comprises amino acid sequences of SEQ ID NO:14 or SEQ ID NO:16 shown in the specification. The antigen binding segment of the monoclonal neutralizing antibody is obtained from adult Chinese rhesus monkeys, after humanization, the antibody has good neutralizing activity upon three pseudoviruses of a Zaire type, a Sultan type and a Marburg type of filovirus, and has good affinity upon GP (glycoprotein); and the monoclonal neutralizing antibody is potentially applied to research on anti-filovirus treatment medicines, research on anti-filovirus detection kits, molecularbiological reagents for anti-filovirus antigen detection, and the like.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Anti-Ebola virus neutralizing monoclonal antibody
InactiveCN107417788AGood neutralizing activityImmunoglobulins against virusesAntibody ingredientsAntigenCross neutralization
The invention discloses a neutralizing monoclonal antibody against Ebola virus. Specifically, the invention discloses a monoclonal antibody against Ebola virus. The antibody has significant binding activity to Ebola virus antigen, and Has cross-neutralizing activity against Zaire and Sudan subtypes.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Anti-coronavirus bispecific neutralizing antibody and application
ActiveCN114149509AHigh affinityGood neutralizing activityHybrid immunoglobulinsImmunoglobulins against virusesEpitopeSingle-Chain Antibodies
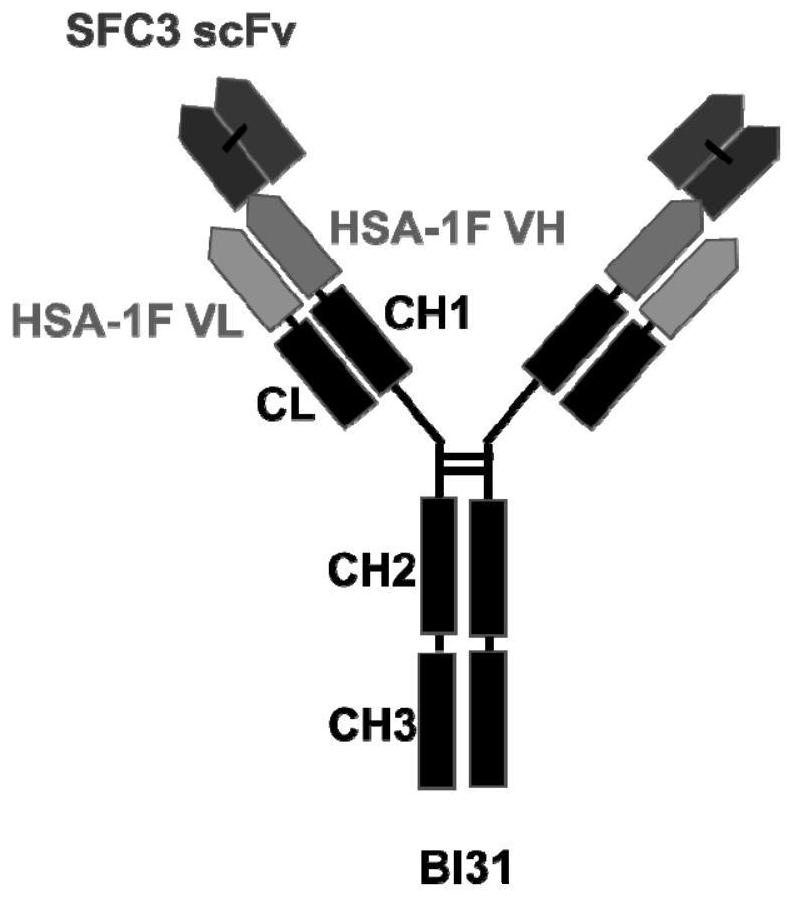
The invention discloses an anti-coronavirus bispecific neutralizing antibody and application thereof. Specifically, the invention discloses a bispecific antibody specifically combined with different epitopes of a novel coronavirus S protein receptor binding domain and application thereof. The bispecific antibody comprises a single-chain antibody SFC3scFv and a complete humanized antibody HSA-1F structure, has a highly stable symmetric structure, shows better neutralizing activity compared with a parent antibody and when the bispecific antibody is combined with the parent antibody, and also shows better neutralizing activity on an SARS-CoV-2 variant strain, an SARS-CoV virus and an MERS-CoV virus. According to the bispecific antibody, the neutralizing effect of the novel coronavirus is remarkably improved, the probability of generation of virus escape mutation is reduced, the treatment and prevention means of COVID-19 are enriched, and the bispecific antibody has a huge prospect of becoming a broad-spectrum antibody drug aiming at the coronavirus and has important significance on prevention and treatment of coronavirus infection.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Neutralizing antibody against novel coronavirus receptor binding regions and application thereof
ActiveCN113150135AHas a neutralizing effectGood neutralizing activityImmunoglobulins against virusesAntiviralsHeavy chainReceptor
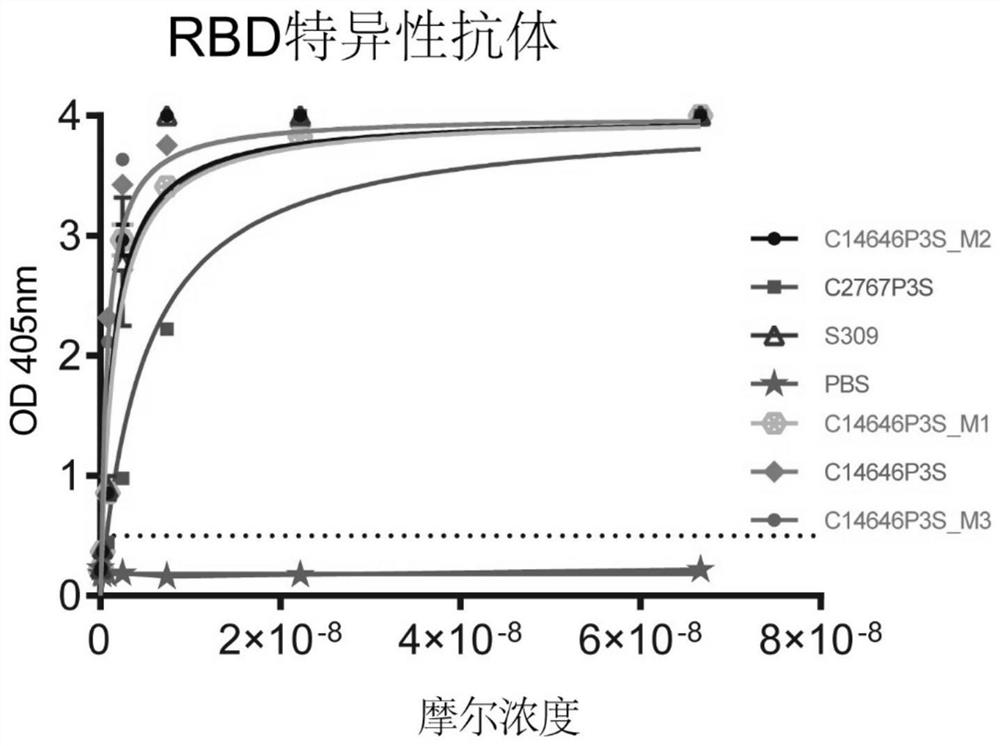
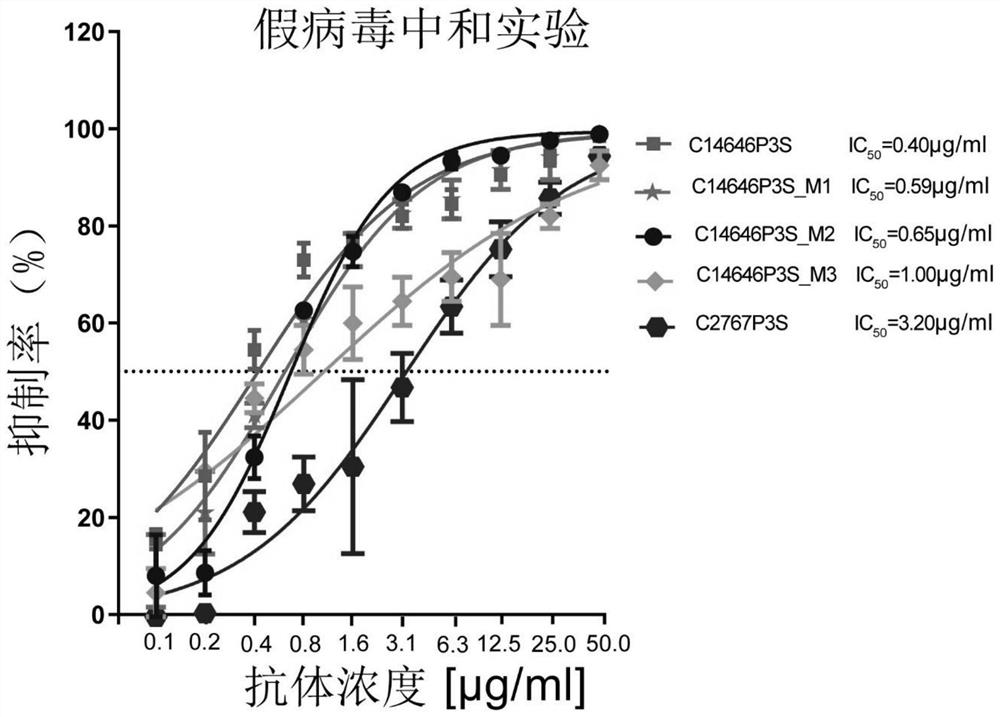
The invention provides a neutralizing antibody against novel coronavirus receptor binding regions and application thereof, and belongs to the technical field of biological products. The neutralizing antibody comprises a heavy chain variable region and a light chain variable region; and the heavy chain variable region comprises a heavy chain CDR3 as shown in SEQ ID NO: 3 or SEQ ID NO: 13, and the light chain variable region comprises a light chain CDR3 as shown in SEQ ID NO: 6 or SEQ ID NO: 16. According to the neutralizing antibody against the novel coronavirus receptor binding regions and the application thereof, the neutralizing antibodies C14646P3S and C2767P3S of the SARS-CoV-2 receptor binding region are obtained by utilizing LIBRA-seq high-throughput screening; and the neutralizing antibodies C14646P3S and C2767P3S have relatively strong neutralizing capability on SARS-CoV-2 pseudoviruses and live viruses in vitro, can well block the binding of a novel coronavirus receptor binding region and a receptor thereof, and have important application value in the aspects of prevention, treatment and diagnosis of SARS-CoV-2 infection.
Owner:SUN YAT SEN UNIV
Canine parvovirus resistant genetic engineering antibody and application thereof
PendingCN110845605AGood neutralizing activityInhibition of agglutinationImmunoglobulins against virusesAntiviralsGenetic engineeringHemagglutination
The invention provides a canine parvovirus resistant genetic engineering antibody and an application thereof, and relates to the technical field of antibody engineering. A variable region sequence ofan acquired canine parvovirus monoclonal antibody is assembled with a canine antibody constant region to obtain the canine parvovirus resistant genetic engineering chimeric antibody, according to experimental verification, the hemagglutination inhibition titer of cell supernatant of the genetic engineering antibody is 1:(24-25), and the neutralizing titer of the cell supernatant is 1:203. Obviously, the genetic engineering antibody shows good canine parvovirus neutralizing activity, agglutination of red blood cells by canine parvoviruses can be inhibited, and the canine parvovirus resistant genetic engineering antibody can be applied to the field of canine research of the monoclonal antibody, canine parvovirus control and the like, and is of great significance for promoting development ofcanine monoclonal antibody drugs.
Owner:CHANGCHUN UNIV OF TECH
Anti-H7N9 whole-human monoclonal antibody 9I17 and a preparation method therefor and application of the anti-H7N9 whole-human monoclonal antibody 9I17
ActiveCN111434685AGood neutralizing activityGood biocompatibilityImmunoglobulins against virusesAntiviralsHemagglutininAntiendomysial antibodies
The invention relates to an anti-H7N9 whole-human monoclonal antibody 9I17 and a preparation method therefor and application of the anti-H7N9 whole-human monoclonal antibody 9I17. The whole-human monoclonal antibody 9I17 is quickly screened by utilizing a memory B cell polymerase chain reaction (PCR) method, and does not include any murine component. The antibody disclosed by the invention can bebound to hemagglutinin HA of an H7N9 virus in a targeted manner, and has significant neutralizing activity of anti-H7N9 virus infection; and the antibody disclosed by the invention does not have toxicand side effects of anti-mouse anti-antibodies and the like, has better biocompatibility, and is more suitable and has more potential to become a macromolecular drug for treatment of influenza viruses.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
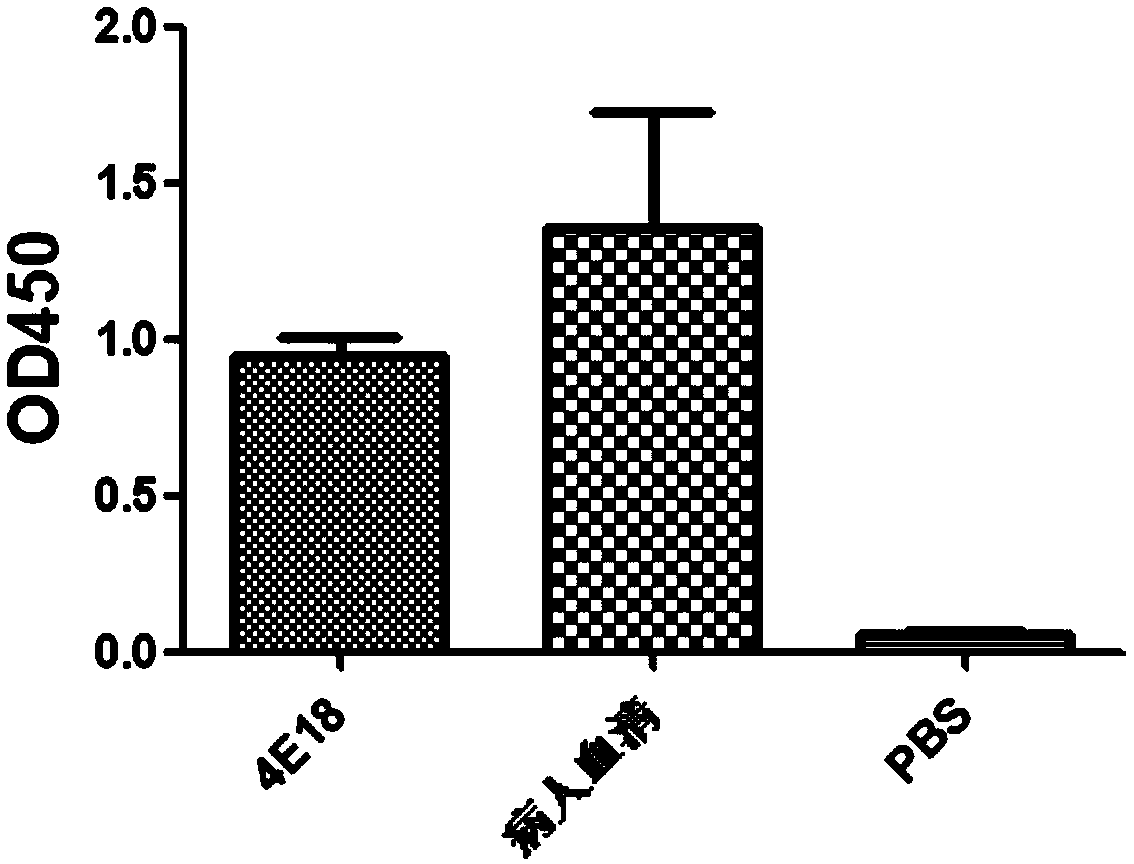
Anti-H7N9 fully humanized monoclonal antibody 4E18 and preparation method therefor and application of humanized monoclonal antibody
ActiveCN111320687AGood neutralizing activityGood biocompatibilityImmunoglobulins against virusesAntiviralsAntiendomysial antibodiesMacromolecular drug
The application relates to an anti-H7N9 fully humanized monoclonal antibody 4E18 and a preparation method therefor and application of the humanized monoclonal antibody. The fully humanized monoclonalantibody 4E18 is quickly screened from memory B cells by utilizing a polymerase chain reaction (PCR) method, and does not contain any murine component. The antibody of the application can be bound tohemagglutinin (HA) of an H7N9 virus in a targeted manner, and has a neutralizing activity of significantly resisting H7N9 virus infection; the antibody of the application does not have an anti-mouse anti-antibody toxic and side effect and the like, has higher biocompatibility, and is more suitable and has more potential to become a macromolecular drug for treating influenza viruses.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
SARS-CoV-2 Spike protein receptor binding domain dimer and application thereof
ActiveCN113583096AGood neutralizing activitySsRNA viruses positive-senseAntibody mimetics/scaffoldsDimerReceptor
The invention discloses an SARS-CoV-2 Spike protein receptor binding domain dimer and an application thereof, and particularly relates to an SARS-CoV-2 RBD dimer (SARS-CoV-2 RBD dimer) or an SARS-CoV-2 RBD monomer (SARS-CoV-2 RBD monomer) and an application of the SARS-CoV-2 RBD dimer or the SARS-CoV-2 RBD monomer or the SARS-CoV-2 RBD monomer or the SARS-CoV-2 RBD monomer or the SARS-CoV-2 RBD monomer in neutralizing the SARS-CoV-2, and the SARS-CoV-2 RBD dimer or the SARS-CoV-2 RBD monomer in neutralizing the SARS- the invention provides a dimer of SARS-CoV-2 RBD, and a preparation method of the dimer, a protein represented by Sequence 1 in the sequence table, a protein represented by Sequence 3 in the sequence table, and the protein shown as the 34th-272th amino acid residues in the sequence 3 of the sequence table. The invention has important value and wide application prospect in research and development of drugs, vaccines and the like for treating and preventing SARS-CoV-2.
Owner:TSINGHUA UNIV
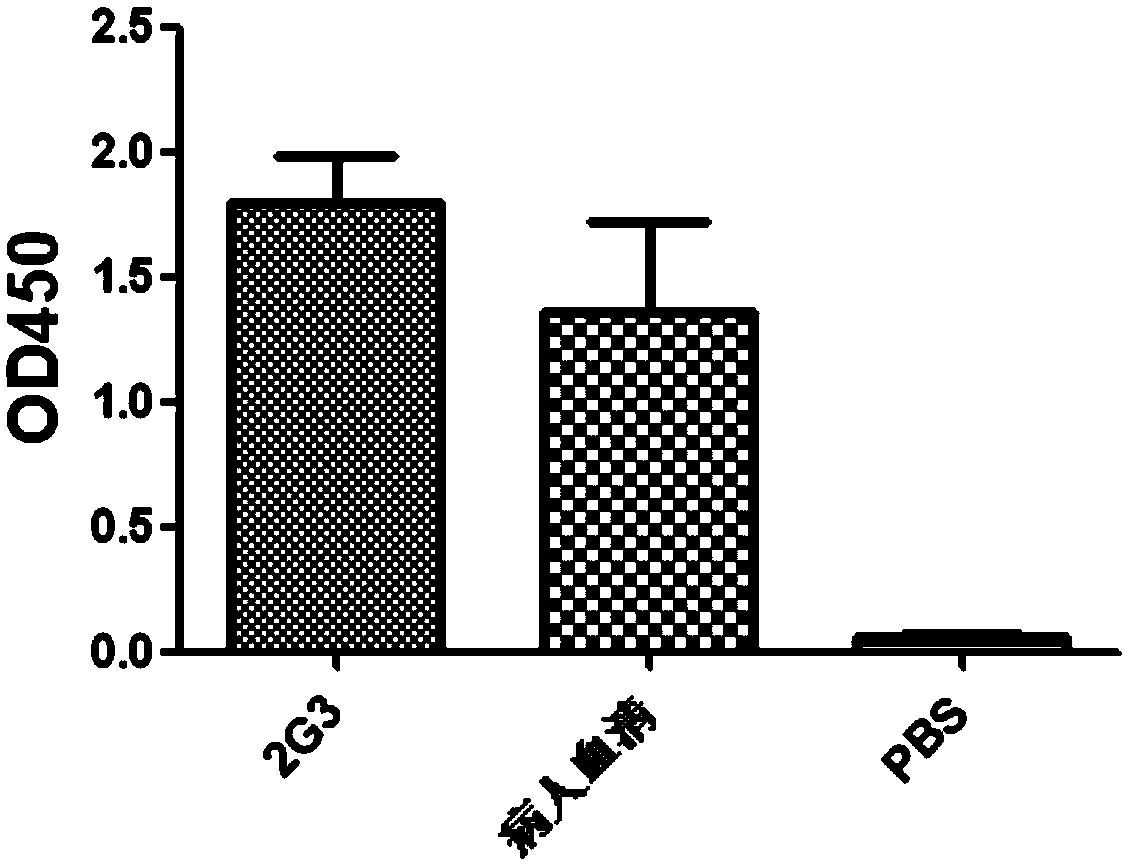
Anti-H7N9 fully humanized monoclonal antibody 2G3 and preparation method therefor and application of humanized monoclonal antibody
ActiveCN111320686AGood neutralizing activityGood biocompatibilityImmunoglobulins against virusesAntiviralsMacromolecular drugPolymerase chain reaction
The application relates to an anti-H7N9 fully humanized monoclonal antibody 2G3 and a preparation method therefor and application of the humanized monoclonal antibody. The fully humanized monoclonal antibody 2G3 is quickly screened from memory B cells by utilizing a polymerase chain reaction (PCR) method, and does not contain any murine component. The antibody of the application can be bound to hemagglutinin HA of an H7N9 virus in a targeted manner, and has a neutralizing activity of significantly resisting H7N9 virus infection; the antibody of the application does not have an anti-mouse anti-antibody toxic and side effect and the like, has higher biocompatibility, and is more suitable and has more potential to become a macromolecular drug for treating influenza viruses.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
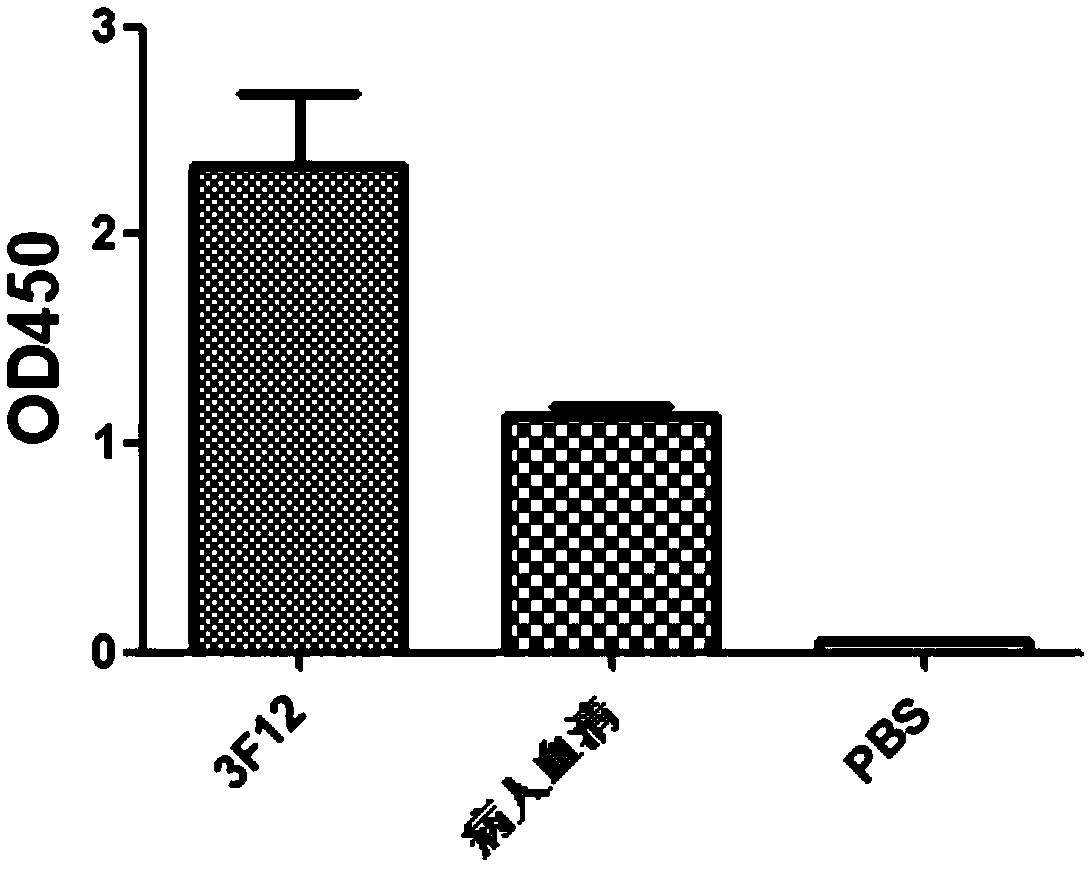
Anti-H7N9 full-human monoclonal antibody 3F12 as well as preparation method and application thereof
ActiveCN111320685AGood neutralizing activityGood biocompatibilityImmunoglobulins against virusesAntiviralsHemagglutininAntiendomysial antibodies
The invention relates to an anti-H7N9 full-human monoclonal antibody 3F12 as well as a preparation method and application thereof. The full-human monoclonal antibody 3F12 is rapidly screened by usinga memory B cell PCR (polymerase chain reaction) method, and no mouse-derived component is involved. The antibody provided by the invention is capable of achieving targeted combination with hemagglutinin HA of an H7N9 virus, and has remarkable anti-H7N9 virus infection neutralization activity. The antibody provided by the invention is free of toxic and side effect such as anti-mouse antiantibodies,has good biocompatibility and is suitable for and has the potential of being used as macromolecule medicines for treating influenza viruses.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Anti-H7N9 whole-human monoclonal antibody 7T33 and preparation method therefor and application of anti-H7N9 whole-human monoclonal antibody 7T33
ActiveCN111434682AGood neutralizing activityGood biocompatibilityImmunoglobulins against virusesAntiviralsHemagglutininAntiendomysial antibodies
The invention relates to an anti-H7N9 whole-human monoclonal antibody 7T33 and a preparation method therefor and application of the anti-H7N9 whole-human monoclonal antibody 7T33. The whole-human monoclonal antibody 7T33 is quickly screened by utilizing a memory B cell polymerase chain reaction (PCR) method, and does not include any murine component. The antibody disclosed by the invention can bebound to hemagglutinin HA of an H7N9 virus in a targeted manner, and has significant neutralizing activity of anti-H7N9 virus infection; and the antibody disclosed by the invention does not have toxicand side effects of anti-mouse anti-antibodies and the like, has better biocompatibility, and is more suitable and has more potential to become a macromolecular drug for treatment of influenza viruses.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Compositions and methods related to prevention and treatment of rabies infection
ActiveCN104603149BReduce infectivityGood neutralizing activityImmunoglobulins against virusesAntiviralsMedicineRabies virus
Owner:万机创科(香港)有限公司
Antibody targeting sars-cov-2 and its preparation method and application
ActiveCN112390879BRandom combinationInhibit bindingAntibody mimetics/scaffoldsImmunoglobulins against virusesDiseaseImmune escape
The present invention provides an antibody targeting SARS-CoV-2 and its preparation method and application. The antibody comprises VH and VL, and the VH comprises the following CDRs: VH with amino acid sequence as shown in SEQ ID NO: 1, 2, 3 CDR1, VH CDR2, VH CDR3; the VL comprises the following CDRs: VL CDR1, VL CDR2, VL CDR3 with amino acid sequences as shown in SEQ ID NO: 4, 5, 6. The antibody can bind with high affinity and specificity to the RBD of the S protein of SARS-CoV-2, inhibit the binding of the RBD protein to the receptor ACE2 protein, and efficiently inhibit SARS-CoV-2 from infecting cells, while preventing potential immune escape mutations The pseudovirus has good neutralizing activity, so it can be effectively applied to the diagnosis, prevention and treatment of SARS-CoV-2 virus and related diseases.
Owner:SHANGHAI TECH UNIV
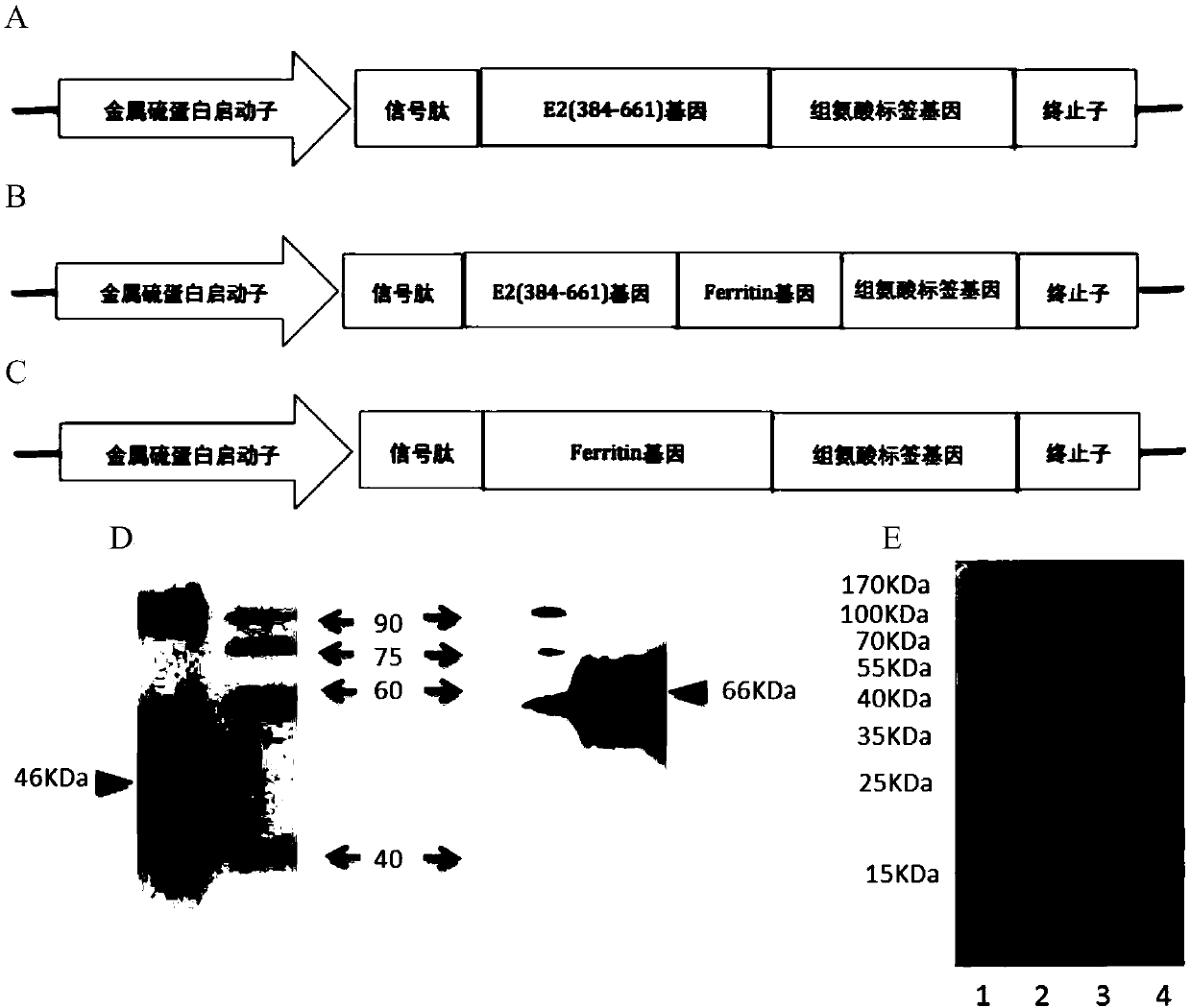
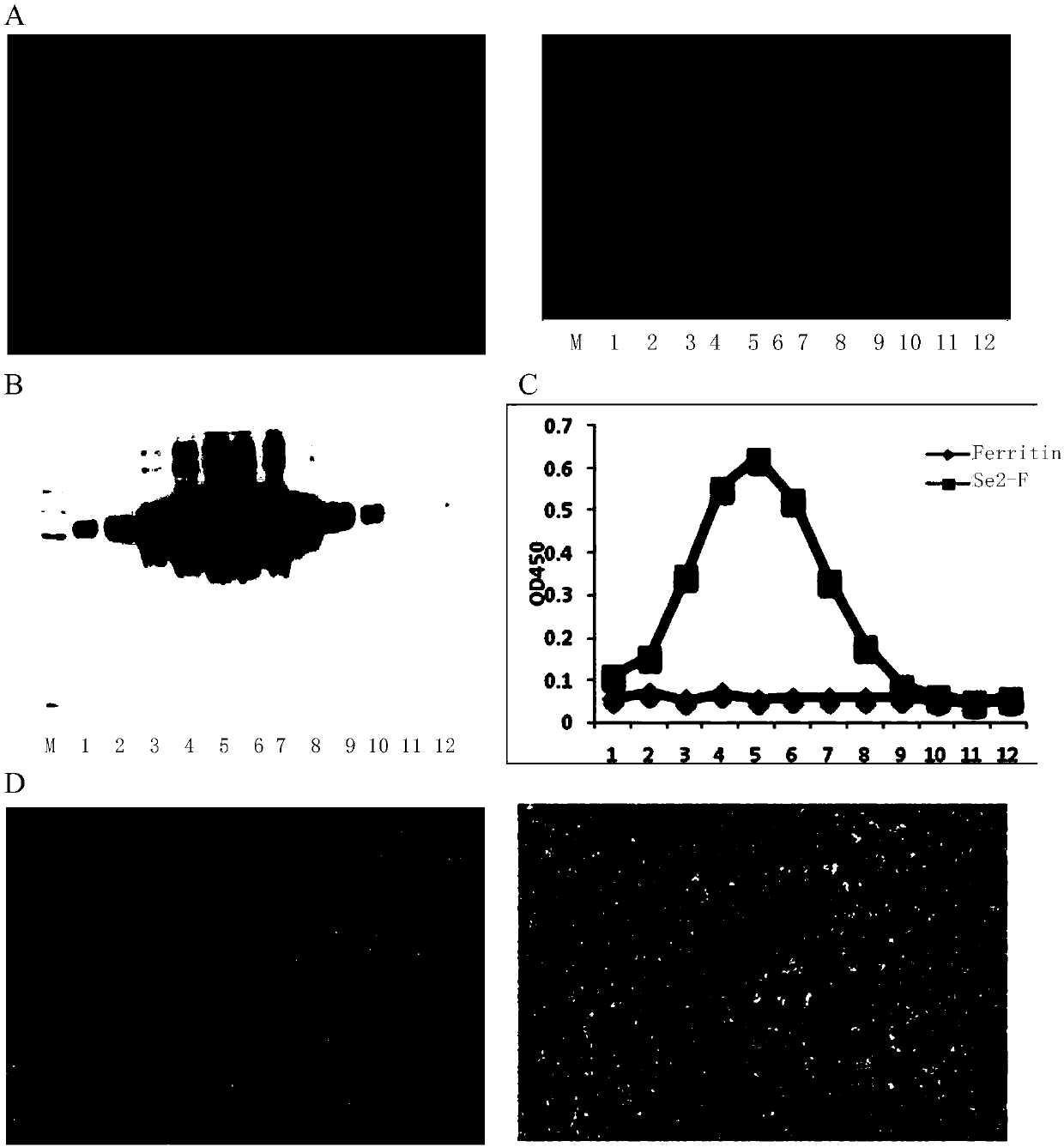
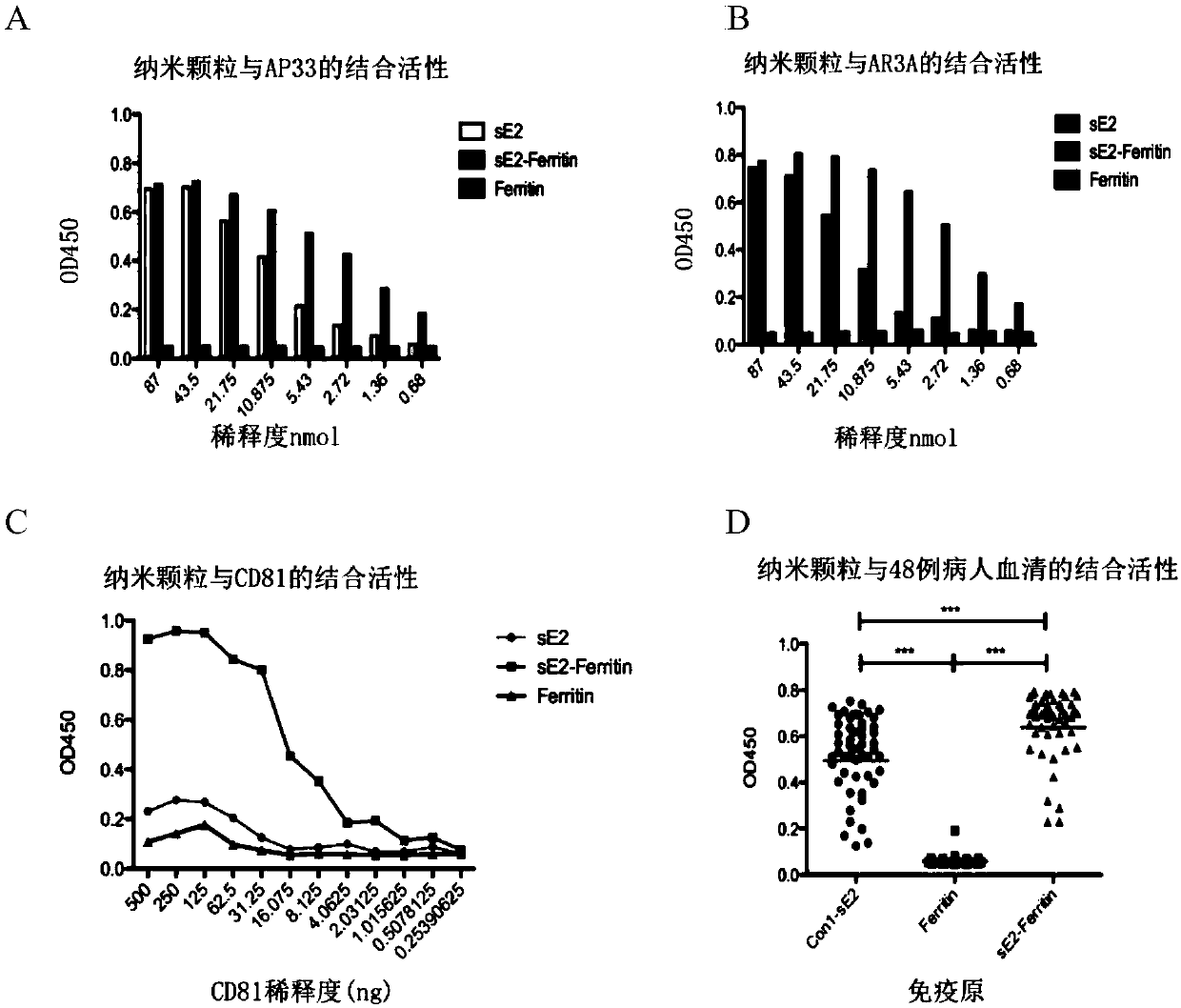
Preparation method and application of nanoparticle for showing hepatitis C virus envelope protein E2
InactiveCN107652366AGood neutralizing activitySsRNA viruses positive-senseAntibody mimetics/scaffoldsVaccine ImmunogenicityNonheme iron
The invention provides a preparation method and application of a nanoparticle for showing a hepatitis C virus envelope protein E2. Specifically, a truncated envelope protein sE2 (384-661) gene of an HCV 1B-type Con1 strain and a helicobacter pylori nonheme-iron protein gene are subjected to fusion expression, and sE2 can be shown on the surface of a nanoparticle in a correct conformation by self-assembling of ferroprotein monomers, so that the nanoparticle can be prepared into an HCV nanoparticle vaccine with good antigenicity and high safety, and the immunogenicity of a fusion protein is remarkably improved.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
A kind of anti-human tslp monoclonal antibody and application thereof
ActiveCN113683694BGood neutralizing activityQuite affinitySenses disorderDigestive systemDiseaseComplementarity determining region
This application provides an anti-human thymus stromal lymphopoietin (TSLP) monoclonal antibody and its application. The anti-human thymus stromal lymphopoietin (TSLP) monoclonal antibody of the present application comprises three heavy chain complementarity determining regions (CDR‑H1, CDR‑H2 and CDR‑H3) and three light chain complementarity determining regions (CDR‑L1 , CDR‑L2 and CDR‑L3), wherein, (a) the amino acid sequence of CDR‑H1 is shown in SEQ ID NO: 1; (b) the amino acid sequence of CDR‑H2 is shown in SEQ ID NO: 2; (c ) The amino acid sequence of CDR‑H3 is shown in SEQ ID NO: 3; (d) the amino acid sequence of CDR‑L1 is shown in SEQ ID NO: 4; (e) the amino acid sequence of CDR‑L2 is shown in SEQ ID NO: 5 and (f) the amino acid sequence of CDR-L3 is shown in SEQ ID NO:6. The anti-human thymus stromal lymphopoietin (TSLP) monoclonal antibody, compared with the anti-human TSLP monoclonal antibody Tezepelumab (expressed and prepared according to the sequence disclosed in the patent), has comparable affinity for binding human TSLP, and is in the cell level. It is more active than Tezepelumab and is expected to show good clinical effects in the prevention and treatment of related diseases.
Owner:QYUNS THERAPEUTICS CO LTD
Anti-h7n9 fully human monoclonal antibody 8d11 and its preparation method and application
ActiveCN111434683BGood neutralizing activityGood biocompatibilityImmunoglobulins against virusesAntiviralsAntiendomysial antibodiesMacromolecular drug
The invention relates to an anti-H7N9 fully human monoclonal antibody 8D11 and its preparation method and application. The fully human monoclonal antibody 8D11 was rapidly screened by memory B cell PCR method without any mouse components. The antibody of the present invention can target the hemagglutinin HA of H7N9 virus, and has a significant neutralizing activity against H7N9 virus infection; the antibody of the present invention does not produce toxic and side effects such as anti-mouse anti-antibody, has better biocompatibility, and is more It is suitable and has more potential to become a macromolecular drug for treating influenza virus.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Anti-h7n9 fully human monoclonal antibody 4e18 and its preparation method and application
ActiveCN111320687BGood neutralizing activityGood biocompatibilityImmunoglobulins against virusesAntiviralsHemagglutininAntiendomysial antibodies
This application relates to the anti-H7N9 fully human monoclonal antibody 4E18 and its preparation method and application. A fully human monoclonal antibody 4E18 was rapidly screened by memory B cell PCR without any mouse components. The antibody of the present application can target the hemagglutinin HA of the H7N9 virus, and has a significant neutralizing activity against H7N9 virus infection; the antibody of the present application does not cause toxic and side effects such as anti-mouse anti-antibody, has better biocompatibility, and is more It is suitable and has more potential to become a macromolecular drug for treating influenza virus.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com