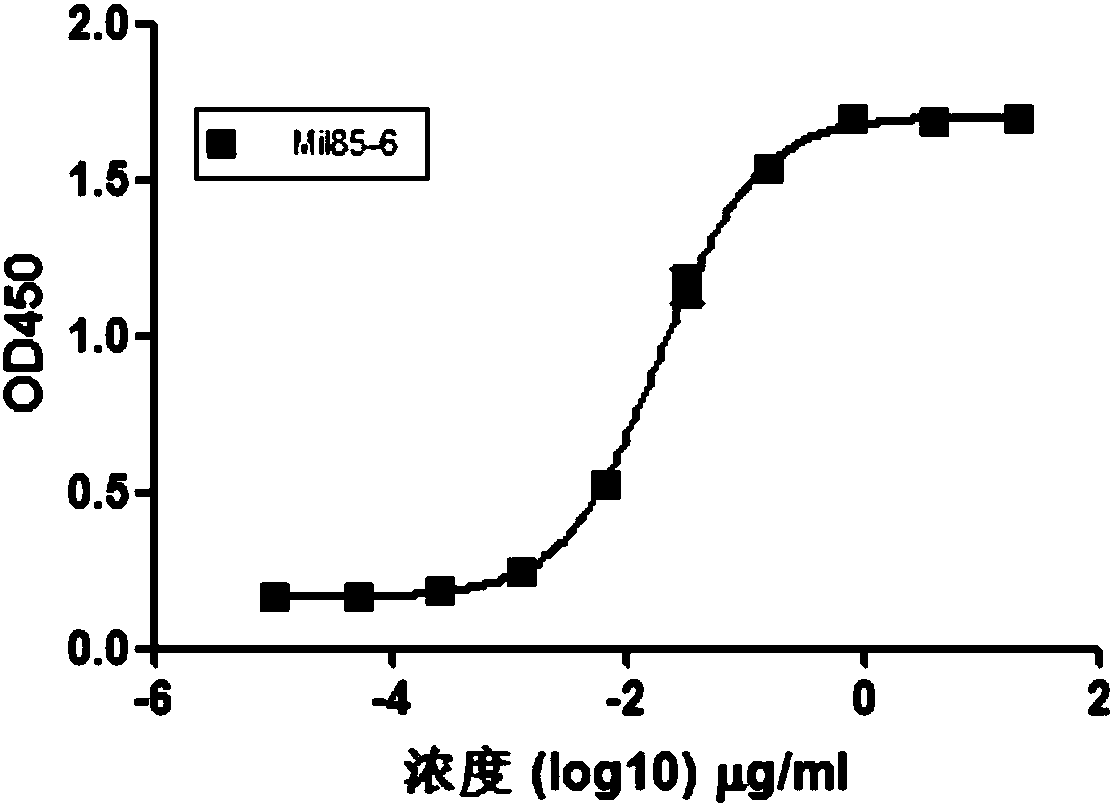
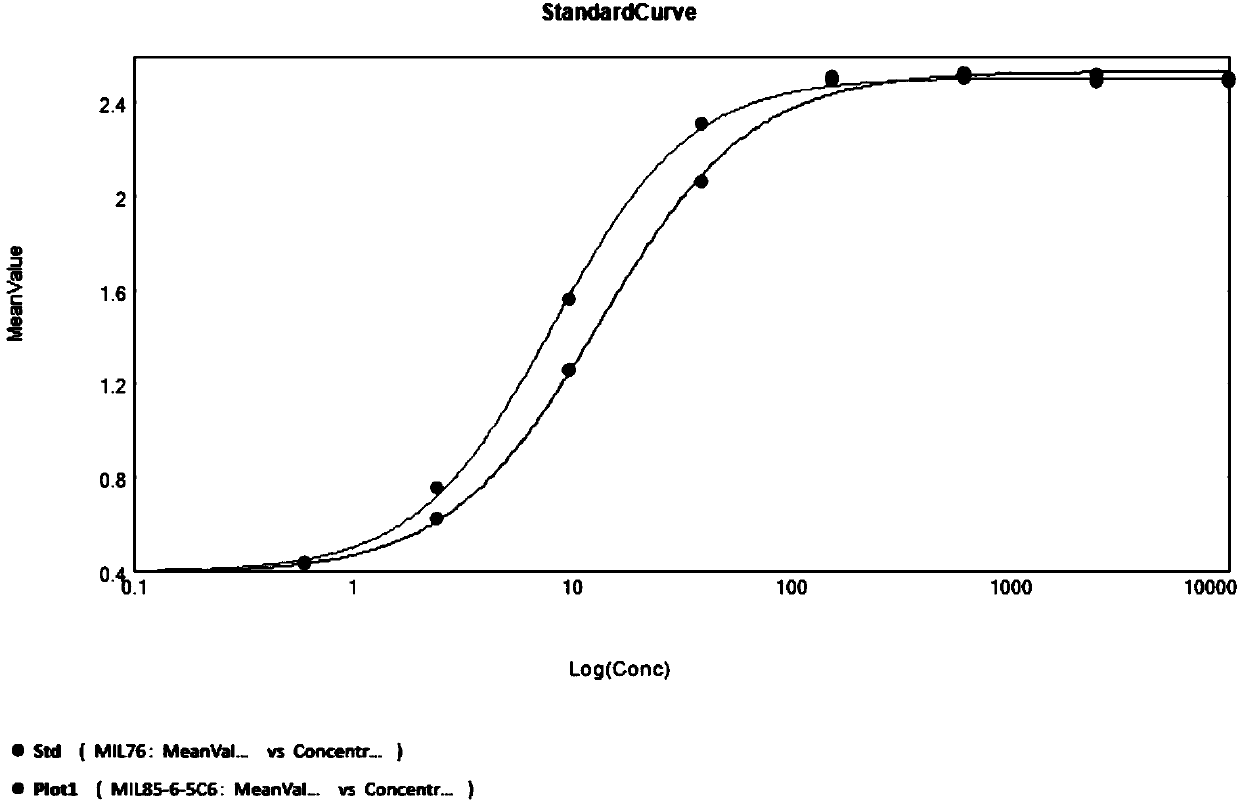
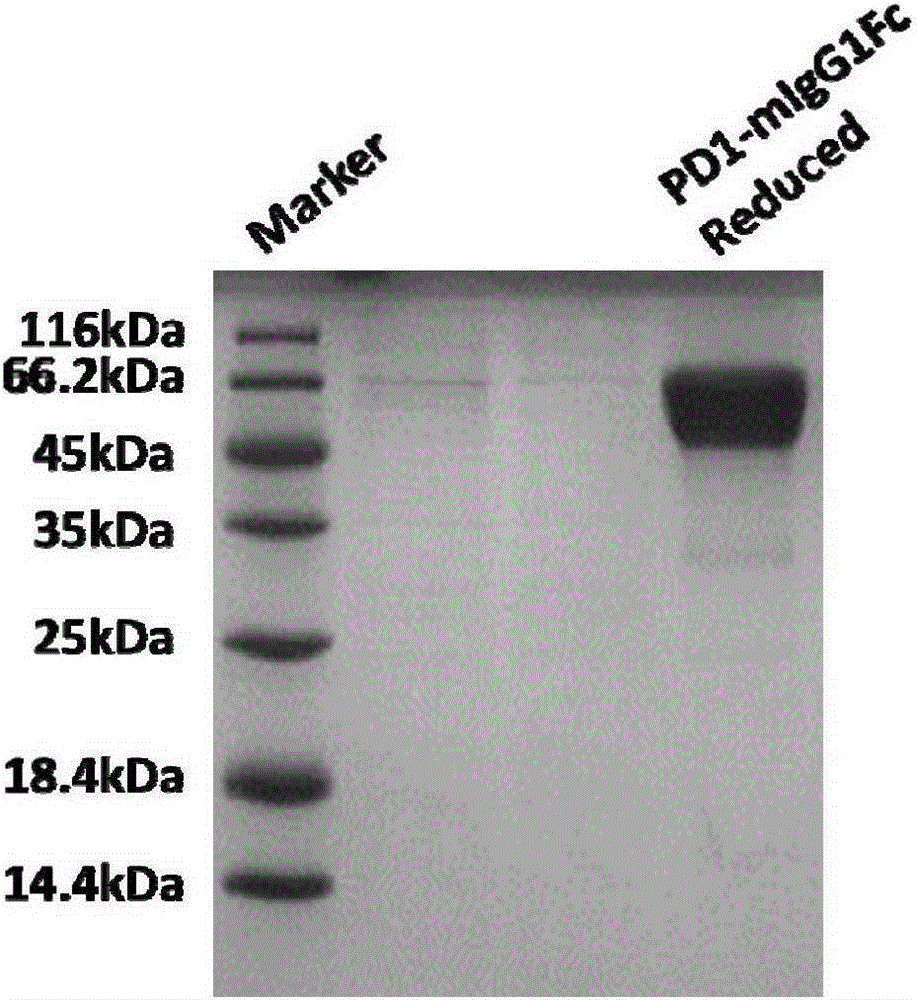
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
107results about How to "Binding block" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Anti-CTLA4-anti-PD-1 bifunctional antibody, pharmaceutical composition and use thereof
ActiveCN106967172ABinding blockRelief of immunosuppressionHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsMolecular ImmunologyT lymphocyte
Belonging to the field of tumor treatment and molecular immunology, the invention relates to an anti-CTLA4-anti-PD-1 bifunctional antibody, a pharmaceutical composition and use thereof. Specifically, the anti-CTLA4-anti-PD-1 bifunctional antibody comprises: a PD-1 (programmed death-1) targeted first protein functional region, and a CTLA4 (cytotoxic T lymphocyte sociated antigen 4) targeted second protein functional region. The bifunctional antibody provided by the invention can well bind to CTLA4 and PD-1 specifically, specifically remove the immunosuppression of CTLA4 and PD-1 on the body, and activate T lymphocytes, thus having good application prospect.
Owner:AKESO PHARMA INC
PDL-1 antibody, pharmaceutical composition thereof and application of PDL-1 antibody
ActiveCN107151269ABinding blockRelief of immunosuppressionAntibody mimetics/scaffoldsMicroorganism based processesMolecular ImmunologyHeavy chain
The invention belongs to the field of oncotherapy and molecular immunology, and relates to a PDL-1 antibody, a pharmaceutical composition thereof and application of the PDL-1 antibody, in particular to a monoclonal antibody of PDL-1 or an antigen binding fragment thereof. A variable region of the heavy chain of the monoclonal antibody comprises CDR of which the amino acid sequence is SEQ ID NO: 15-17; and a variable region of the light chain of the monoclonal antibody comprises CDR of which the amino acid sequence is SEQ ID NO: 18-20. The monoclonal antibody can be well combined to PDL-1 specifically, inhibition of PDL-1 to body immunity is relieved specifically, and T lymphocyte is activated.
Owner:SICHUAN KELUN BIOTECH BIOPHARMACEUTICAL CO LTD
Anti-PD1 monoclonal antibody, pharmaceutical composition and uses thereof
ActiveCN106977602ABinding blockRelief of immunosuppressionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsMolecular ImmunologyAntigen Binding Fragment
The present invention belongs to the field of tumor treatment and molecular immunology, and relates to an anti-PD1 monoclonal antibody, a pharmaceutical composition and uses thereof. In particularly, the present invention relates to a monoclonal antibody or an antigen-binding fragment thereof, wherein the heavy chain variable region of the monoclonal antibody comprises CDR having an amino acid sequence represented by SEQ ID NO:9-11, and / or the light chain variable region of the monoclonal antibody comprises CDR having an amino acid sequence represented by SEQ ID NO:12-14. According to the present invention, the monoclonal antibody can well and specifically bind to PD1, can specifically release the immunosuppression of PD1 on body, and can activate T lymphocytes.
Owner:CTTQ AKESO (SHANGHAI) BIOMED TECH CO LTD
Novel coronavirus (SARS-COV-2) spike protein binding molecule and applications thereof
ActiveCN111647077AAvoid infectionInhibition of amplificationComponent separationMaterial analysis by electric/magnetic meansReceptorIntravenous gammaglobulin
The invention relates to the technical field of medical biology, and specifically discloses a novel coronavirus (SARS-COV-2) spike protein binding molecule and applications thereof. The binding molecule can specifically bind the spike protein of SARS-COV-2 and includes at least one immunoglobulin single variable structural domain. The provided binding molecule can specifically bind the SARS-COV-2-Spike protein and effectively block the binding of the SARS-COV-2-Spike protein and human cell ACE2 receptors, so that the infection process of the SARS-COV-2 on cells can be further blocked, and theinfection and amplification of the SARS-COV-2 can be inhibited.
Owner:SHENZHEN IMMUNOTHERAPY BIOTECH CO LTD
Anti-PD-L1 antibody as well as pharmaceutical composition and application of anti-PD-L1 antibody
ActiveCN106699891ABinding blockRelief of immunosuppressionBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenMolecular Immunology
The invention belongs to the field of oncotherapy and molecular immunology, and relates to an anti-PD-L1 antibody as well as a pharmaceutical composition and an application of the anti-PD-L1 antibody. Particularly, the invention relates to a monoclonal antibody or an antigen binding fragment of the monoclonal antibody, wherein a heavy chain variable region of the monoclonal antibody comprises a CDR, the amino acid sequence of the CDR is SEQ ID NO:1-3, and / or a light chain variable region of the monoclonal antibody comprises a CDR, and the amino acid sequence of the CDR is SEQ ID NO:4-6. The monoclonal antibody is specifically bound with PD-L1 well, specifically relieves immunosuppression of PD-1 on the organism, and activates the T iymphocyte.
Owner:BEIJING MABWORKS BIOTECH
Anti-PD-1 (programmed cell death 1) monoclonal antibody as well as pharmaceutical composition and application thereof
InactiveCN106632674ABinding blockRelief of immunosuppressionRadioactive preparation carriersImmunoglobulins against cell receptors/antigens/surface-determinantsMolecular ImmunologyHeavy chain
The invention belongs to the fields of oncotherapy and molecular immunology, and relates to an anti-PD-1 (programmed cell death 1) monoclonal antibody as well as pharmaceutical composition and an application thereof, in particular to a monoclonal antibody or an antigen binding fragment thereof. The heavy chain variable region of the monoclonal antibody contains CDR with amino acid sequences represented as SEQ ID NO: 13-15; and / or the light chain variable region of the monoclonal antibody contains CDR with amino acid sequences represented as SEQ ID NO: 16-18. The monoclonal antibody can be specifically bound with PD-1 very well, specifically remove immunosuppression of PD-1 on an organism and activate T lymphocytes.
Owner:ZEDA BIOPHARMACEUTICALS INC
PDL-1 (programmed cell death-ligand 1) antibody and pharmaceutical composition and application thereof
ActiveCN108752476ABinding blockRelief of immunosuppressionAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenAntigen Binding Fragment
A monoclonal antibody for PDL-1 (programmed cell death-ligand 1) or its antigen binding fragment is provided. The monoclonal antibody can specifically bind with PDL-1, inhibition of PDL-1 upon body immunity is resolved, and T-lymphocytes are activated. A pharmaceutical composition containing the monoclonal antibody and application of the monoclonal antibody are also provided.
Owner:SICHUAN KELUN BIOTECH BIOPHARMACEUTICAL CO LTD
Anti-human PD-L1 antibody with high affinity, high specificity and multiple antigen recognition epitopes and having higher functionality
ActiveCN108239149ADeregulation of the immune systemActivate secretionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsEpitopeFactor ii
The invention discloses an anti-human PD-L1 antibody with high affinity, high specificity and multiple antigen recognition epitopes and having higher functionality. The PD-L1 monoclonal antibody can be specifically combined with PD-1, and can effectively block combination of PD-L1 and PD-1 protein, specifically relieve immune negative regulation of PD-L1, and activate T cell secretory cell factors. All the functions can reach a currently unique level of PD-L1 targeted drug Tecentriq (MPDL3280A), and partial antibodies have greater diversity by being different from epitope of Tecentriq. One PD-L1 antibody can promote combination of PD-L1 with PD-1 protein, but can still relieve immune negative regulation of PD-L1, and activate T cell secretory cell factors.
Owner:NANJING LEGEND BIOTECH CO LTD
Monoclonal antibody of anti-human PIGF (placental growth factor) protein as well as preparation method and application thereof
InactiveCN102219854ABinding blockImmunoglobulins against growth factorsAntibody ingredientsMonoclonal antibodyPlacental growth factor
The invention discloses a monoclonal antibody of anti-human PIGF (placental growth factor) protein, which can be specifically bonded with human PIGF and block PIGF from joining receptors of the PIGF. The invention further discloses a preparation method of the monoclonal antibody. Besides, the invention discloses an application of the monoclonal antibody in qualitative and quantitative detection to the human PIGF protein. Furthermore, the invention discloses an application of the monoclonal antibody in preparing drugs for treating tumors.
Owner:SUZHOU STAINWEI BIOTECH INC
Anti-PD-1-anti-VEGFA bispecific antibodies, pharmaceutical compositions and uses thereof
PendingCN112830972AReduced activityEliminate binding activityHybrid immunoglobulinsImmunoglobulins against growth factorsMolecular ImmunologyIntravenous gammaglobulin
The invention belongs to the field of tumor treatment and molecular immunology, and relates to an anti-VEGFA-anti-PD-1 bispecific antibody and an application thereof. Specifically, the anti-VEGFA-anti-PD-1 bispecific antibody comprises a PD-1-targeting first protein functional region and a VEGFA-targeting second protein functional region, according to an EU numbering system, a heavy chain constant region of immune globulin contained in the bispecific antibody mutates at two sites of 234 and 235, and after mutation, the affinity constant of the bispecific antibody is reduced compared to the affinity constant of Fc [gamma] RI, Fc [gamma] RIIa, Fc [gamma] RIIIa and / or C1q before mutation. The bispecific antibody disclosed by the invention can be specifically combined with VEGFA and PD-1, specifically relieve immunosuppression of VEGFA and PD-1 on an organism and inhibit angiogenesis caused by tumors at the same time, and has a good application prospect.
Owner:AKESO BIOPHARMA
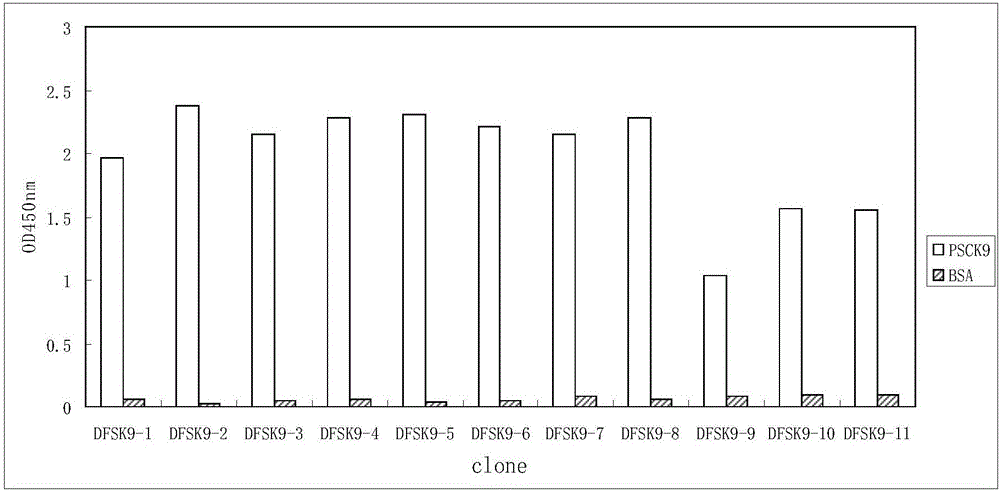
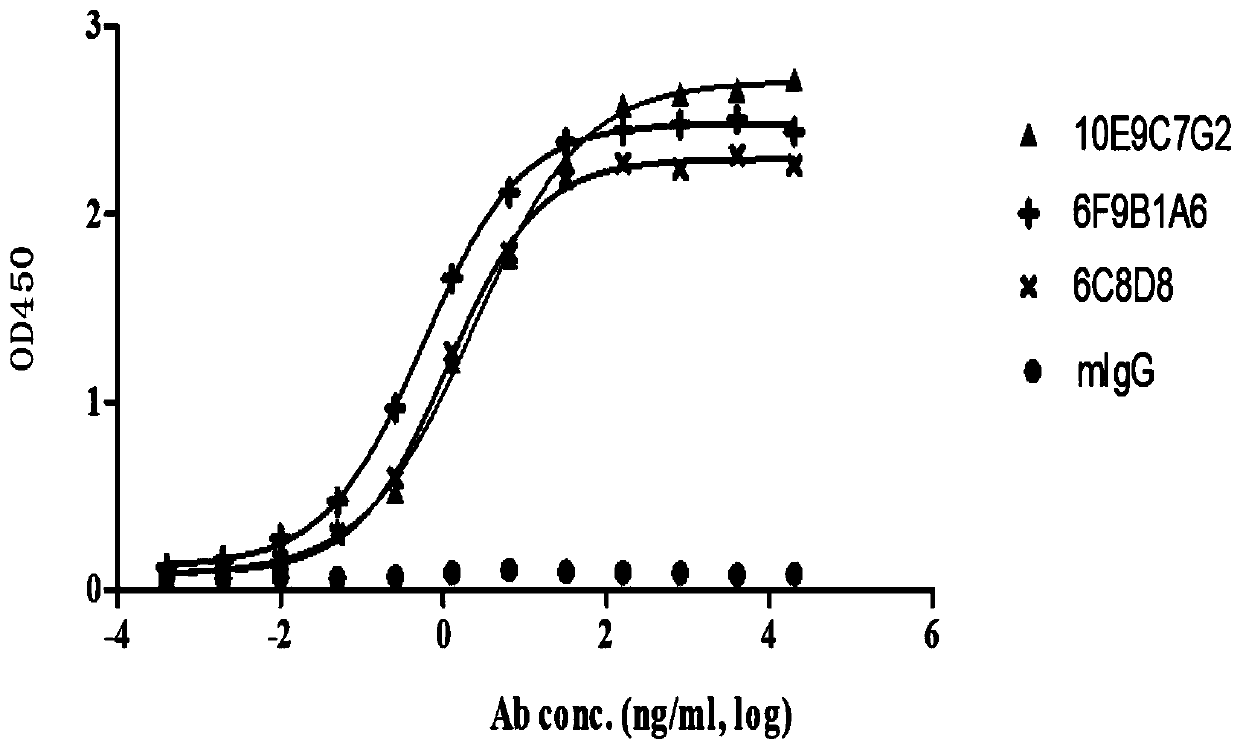
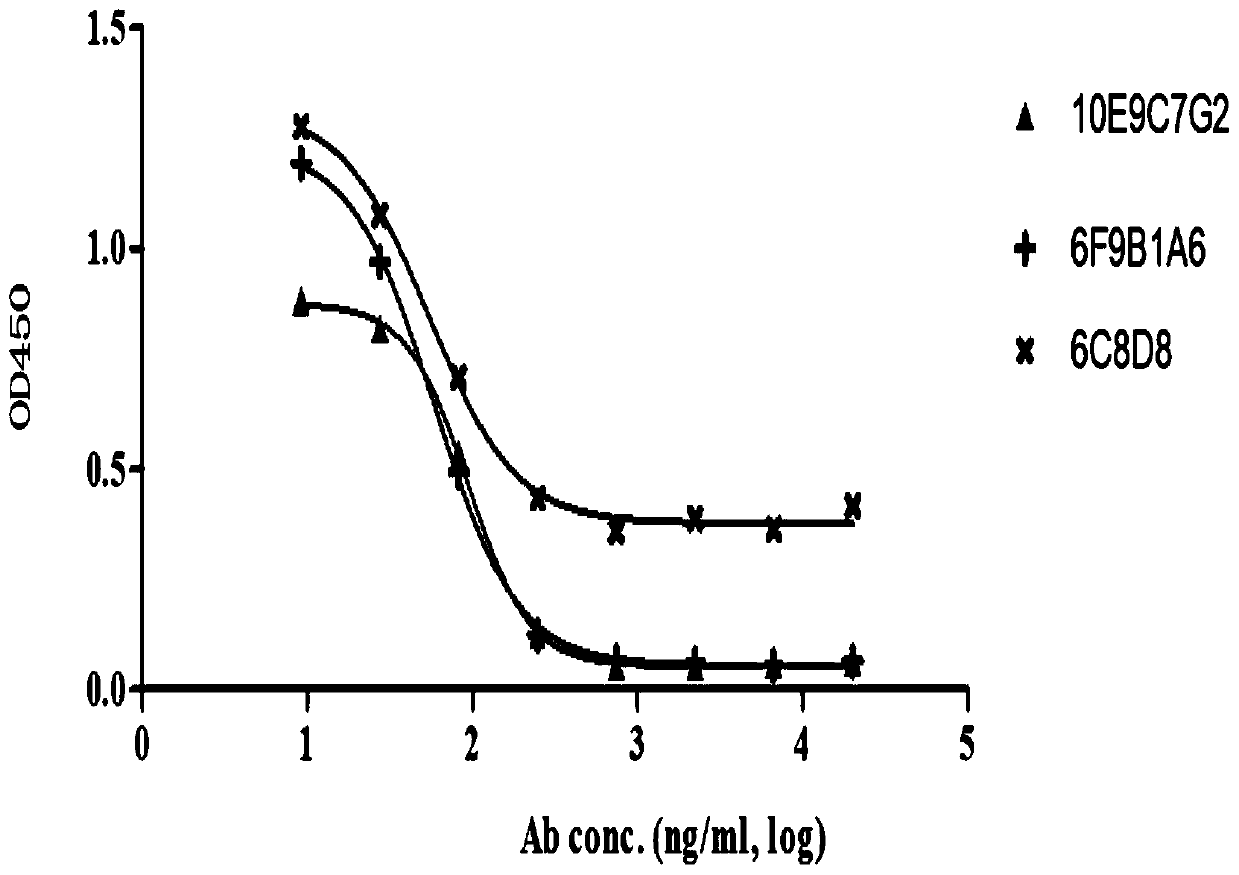
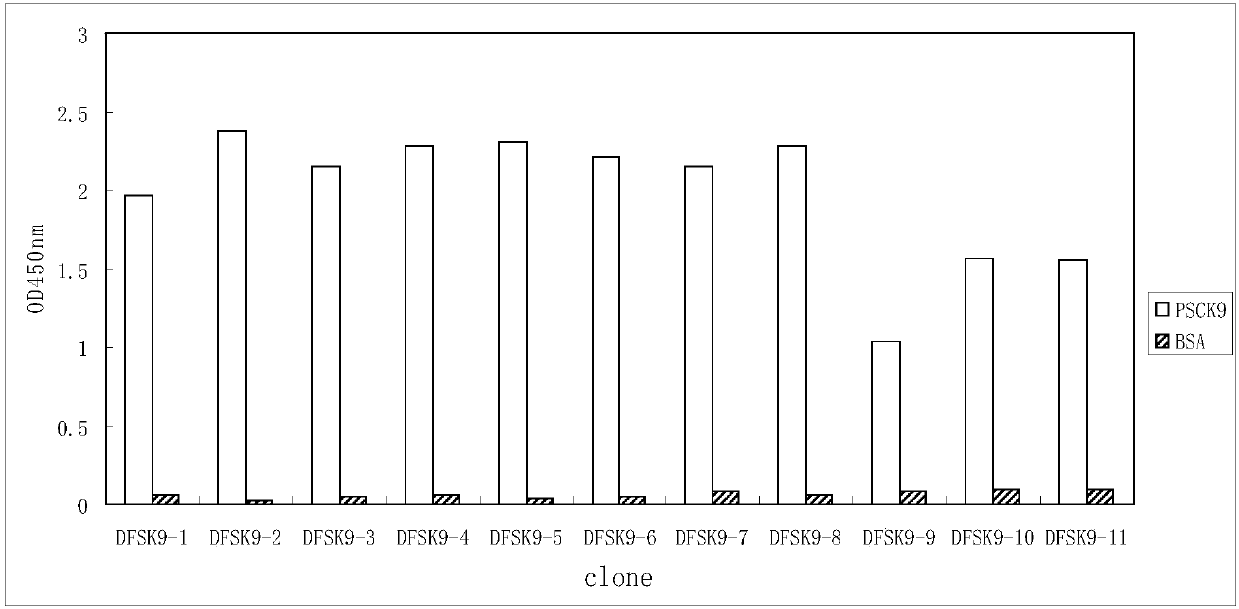
Anti-PCSK9 monoclonal antibody
ActiveCN106749670ABinding blockIncrease intakeMetabolism disorderMammal material medical ingredientsDyslipidemiaAmino acid
The invention relates to the technical field of antibody engineering and specifically discloses an anti-PCSK9 monoclonal antibody. The invention comprises an amino acid sequence coding an antibody variable region and a CDR region as well as an acquisition method and an application of the monoclonal antibody. The monoclonal antibody disclosed by the invention is prepared by the following steps: screening out anti-PCSK9 monoclonal antibodies from a phage library; performing affinity maturation by a method of establishing phage library through strand displacement; screening the mutant libraries of light-chain CDR1, 2, and 3 regions of the monoclonal antibody obtained by primary screening, and selecting the monoclonal antibodies with relatively high affinity; and screening the mutant libraries of heavy-chain CDR1, 2, and 3 to finally obtain the anti-PCSK9 monoclonal antibody with high affinity. In the invention, the obtained PCSK9 antibody has good affinity on PCSK9 and can inhibit combination of the PCSK9 with ligand thereof and can be used for treating dyslipidemia, cardiovascular and cerebrovascular diseases and thrombosis-obstructive diseases.
Owner:BEIJING DONGFANG BIOTECH
Group of PD-L1-resisting monoclonal antibodies and medical application thereof
ActiveCN110903391ACombined with effective blockingBinding blockImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsImmunopotencyMolecular Immunology
The invention discloses a group of PD-L1-resisting monoclonal antibodies and a medical application thereof and belongs to the field of oncotherapy and molecular immunology. The invention specificallyrelates to the group of PD-L1-resisting monoclonal antibodies and the medical application thereof. According to the invention, the group of PD-L1-resisting monoclonal antibodies, having an excellent effect of blocking the interaction between PD-L1 and PD-1, are obtained through a hybridoma technology. Humanized modification and affinity maturation are successfully performed on the antibodies. Theantibodies provided by the invention show a great application prospect of preparing medicines for blocking and adjusting the function and level of the PD-L1 and significantly enhancing organism immunity especially medicines for treating cancer.
Owner:DONGDA BIOSCIENCE INC (SUZHOU)
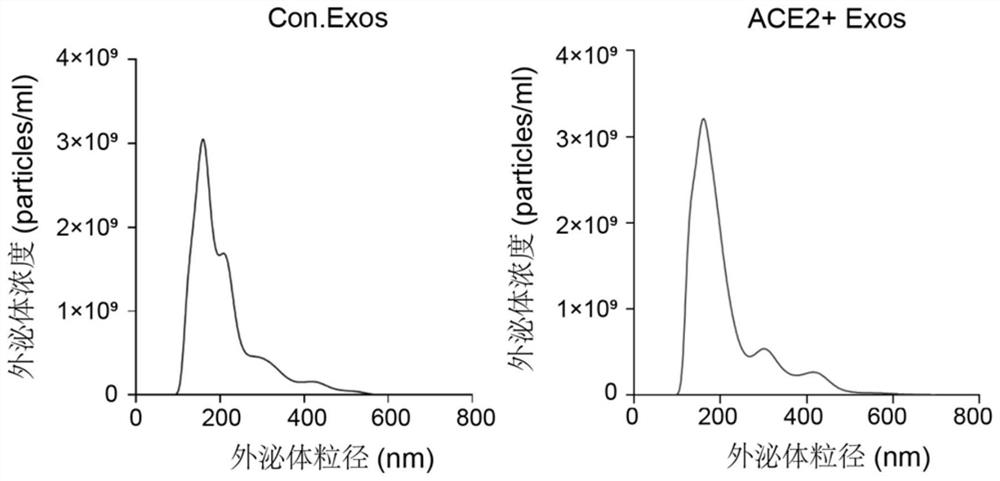
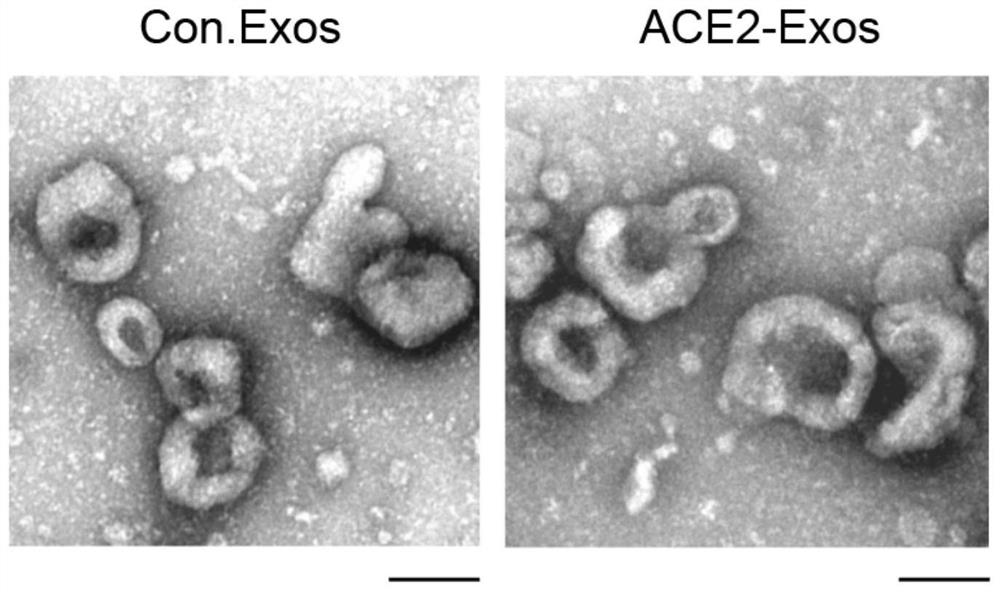
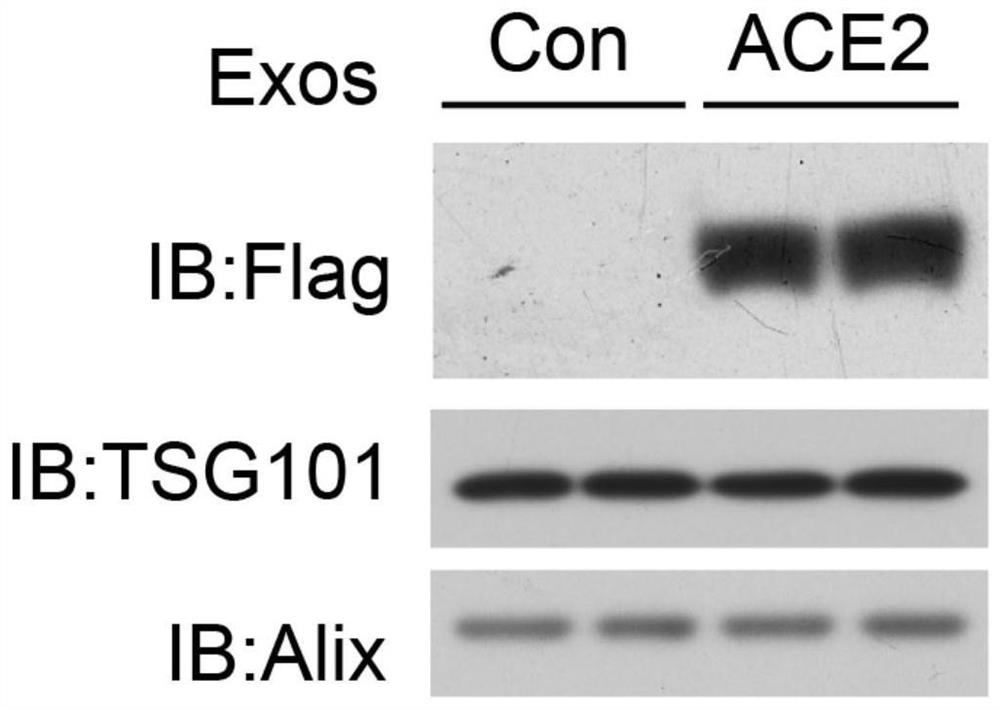
Preparation method and application of exosome for expressing ACE2 protein
ActiveCN112430581AAvoid accessPrevent intrusionGenetically modified cellsAntiviralsMesenchymal stem cellExosome
The invention discloses a preparation method of an exosome for expressing ACE2 protein. The preparation method comprises the following steps: constructing an hMSC human mesenchymal stem cell line forstably expressing human ACE2 protein; and extracting exosome rich in ACE2 protein from a culture supernatant of an hMSC human bone marrow mesenchymal stem cell line through an ultracentrifugation method. The exosome can neutralize new coronavirus, and has treatment and prevention potential on the novel coronavirus. Therefore, the exosome for expressing the ACE2 protein, which is prepared by the method, can be applied to the preparation of anti-novel coronavirus SARS-CoV2 medicines.
Owner:SUZHOU UNIV
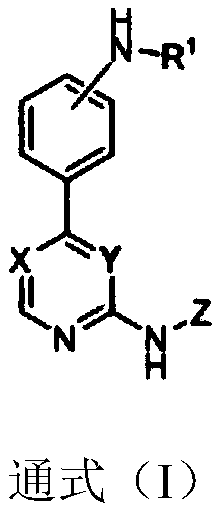
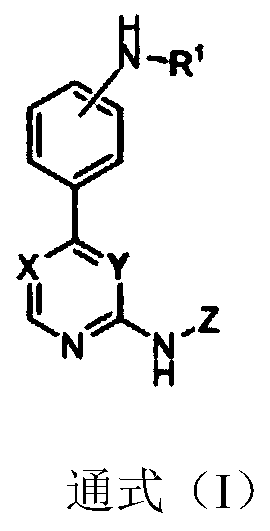
Substitutive phenyl pyrimidine derivative as JAK kinase inhibitor or medicinal salt, preparation method and application thereof
ActiveCN110330484ABlock hydrolysisInhibit bindingOrganic active ingredientsSenses disorderDiseaseMedicine
The invention discloses a substitutive phenyl pyrimidine derivative as shown in a formula (I) as a JAK kinase inhibitor or medicinal salt, a preparation method and application thereof. The compound has excellent JAK inhibiting action and is used for preparing a drug for preventing, treating or improving autoimmune disease, Sjogren's syndrome, Behcet's disease, multiple sclerosis, systemic lupus erythematosus and the like. The high activity JAK-3 inhibiting action IC50 shown by the compound can reach 1.7 n. The substitutive phenyl pyrimidine derivative is simple in synthetic route and high in implementation.
Owner:CHINA PHARM UNIV
CD47 antibody or immunocompetence fragment thereof and application
ActiveCN111454359ADoes not cause clearingSpecific target specificityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseRed cell agglutination
The invention provides a novel CD47 antibody or an immunocompetence fragment thereof, or a composition including the antibody / immunocompetence fragment. The CD47 antibody cannot cause red cell agglutination, and shows low combination at an extremely weak level or non-combination with red cells and blood platelets. The invention provides nucleic acid coding the CD47 antibody or the immunocompetencefragment, an expression vector and a host cell. In addition, the invention provides the purpose of the CD47 antibody or a medical composition containing the CD47 antibody / immunocompetence fragment for preparation of medicines for treating CD47 mediated diseases.
Owner:BETA PHARM SUZHOU LTD
c-MET kinase binding proteins
InactiveCN101018559ABinding blockBiologically activeCompound screeningApoptosis detectionKinase bindingC-Met
Owner:安姆根山景公司
Single-stranded aptamer and application thereof
InactiveCN104818280AAvoid destructionAchieve standardizationOrganic active ingredientsBiological testingDiseaseSingle strand
The invention discloses single-stranded aptamer. A general formula of the single-stranded aptamer is 5'-AGAGACGGACACAGGATGAGC-NCCTTCCCCAAGACAGCATCCA-3', with N referring to a random sequence 35 to 45 bp in length; the single-stranded aptamer can specifically neutralize RhD antibody; N is SEQ ID NO.2, SEQ ID NO.7 or SEQ ID NO.8. The single-stranded aptamer can specifically neutralize the RhD antibody and can be made into a composition, a kit or a chip for detection of the RhD antibody; therapeutic agent made from the single-stranded aptamer is applicable to treatment of hemolytic disease of the newborn, and auxiliary made from the single-stranded aptamer is suitable for emergency blood transfusion of patients with negative RhD.
Owner:SHENZHEN BLOOD CENT
EGFR-CD3 bifunctional antibody and application thereof
ActiveCN111848806APromote differentiation and proliferationLow immunogenicityHybrid immunoglobulinsAntibody ingredientsCD3Molecular biology
The invention provides an EGFR-CD3 bifunctional antibody. The bifunctional antibody comprises two same light chains and two same recombinant heavy chains, wherein the amino acid sequence of each lightchain is as shown in SEQ ID NO. 8, each recombinant heavy chain is formed by connecting an EGFR antibody heavy chain and a single-chain antibody scFv of CD3 protein through a linker sequence; the amino acid sequence of the EGFR antibody heavy chain is as shown in SEQ ID NO. 7; the amino acid sequence of the linker sequence linker is as shown in SEQ ID NO. 9; the amino acid sequence of the single-chain antibody scFv of the CD3 protein is shown as any one of SEQ ID NO.1, SEQ ID NO.2, SEQ ID NO.3, SEQ ID NO.4, SEQ ID NO.5 or SEQ ID NO.6. The EGFR-CD3 bifunctional antibody can be specifically combined with human CD3 protein and EGFR, the immunogenicity of the murine antibody can be reduced, T cells can be effectively activated, the binding of human EGFR and EGFR can be blocked, the bifunctional antibody can be anchored on the surface of tumor cells by binding with EGFR, and can activate T cells by binding with CD3 so as to achieve the tumor cell killing purpose.
Owner:广东安普泽生物医药股份有限公司
PCSK9 (Proprotein Convertase Subtilisin Kexin Type 9) resistant monoclonal antibody
ActiveCN107698680ABinding blockIncrease intakeMetabolism disorderMammal material medical ingredientsKexinPhage antibodies
The invention relates to the technical field of antibody engineering and in particular discloses a PCSK9 (Proprotein Convertase Subtilisin Kexin Type 9) resistant monoclonal antibody. The monoclonal antibody disclosed by the invention comprises an amino acid sequence coding an antibody variable region and a CDR region. The invention further discloses an acquiring method and application of the monoclonal antibody. The method comprises the following steps: screening a PCSK9 resistant monoclonal antibody from a phage antibody library, performing affinity maturation through a method for constructing the phage antibody library by virtue of strand displacement, performing mutant library-construction screening on light-chain CDR1, 2 and 3 regions of the monoclonal antibody obtained by preliminaryscreening, selecting a monoclonal antibody with high affinity, performing mutant library-construction screening on heavy-chain CDR1, 2 and 3 regions of the monoclonal antibody, and finally screeningthe PCSK9 resistant monoclonal antibody with high affinity. The PCSK9 resistant monoclonal antibody obtained in the invention has excellent affinity to PCSK9, is capable of inhibiting binding betweenthe PCSK9 and ligands thereof, and can be used for treating dyslipidemia, cardiovascular and cerebrovascular diseases and thrombosis-obstructive diseases.
Owner:BEIJING DONGFANG BIOTECH
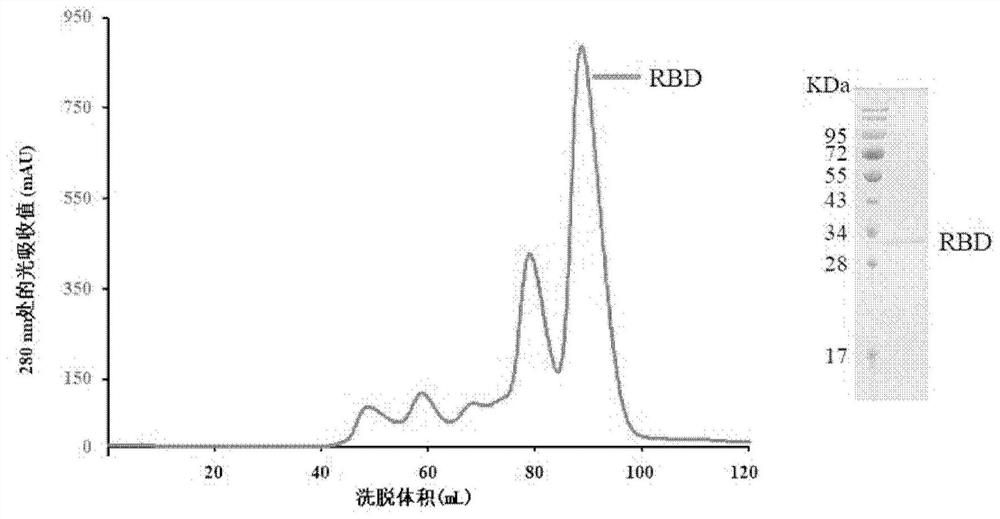
Humanized monoclonal antibody of novel coronavirus and application thereof
ActiveCN113292649ABinding blockGood neutralizing activityImmunoglobulins against virusesAntiviralsAntiendomysial antibodiesBinding inhibition
The invention relates to a novel human monoclonal antibody of coronavirus and application of the novel human monoclonal antibody. The antibody can be specifically combined with the 2019-nCoV RBD, the combination of the 2019-nCoV RBD and ACE2 is blocked, and the infection of the 2019-nCoV is inhibited.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
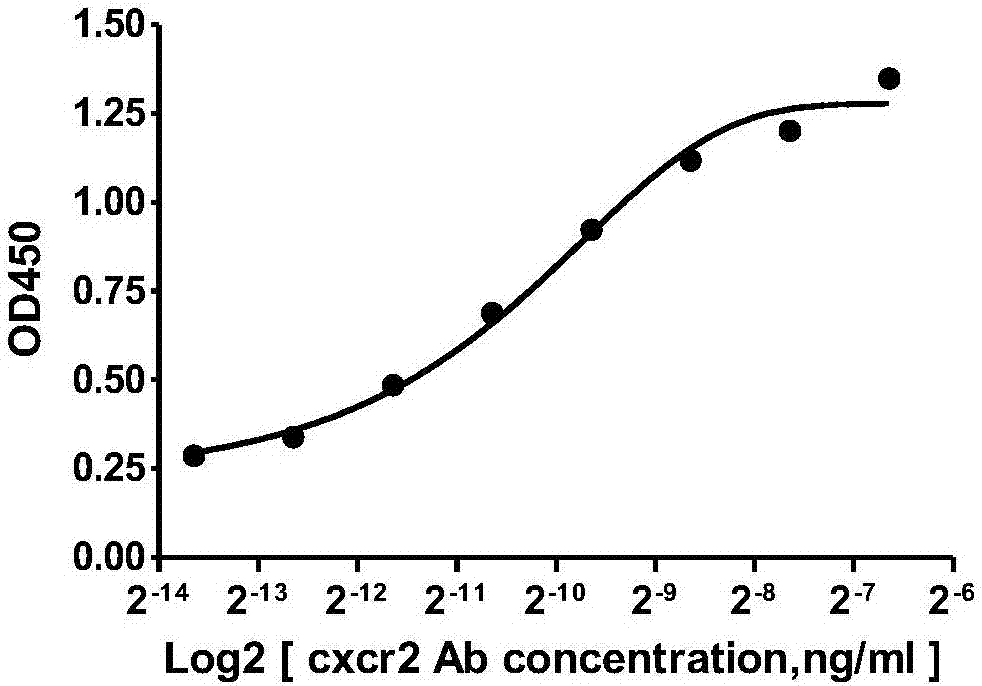
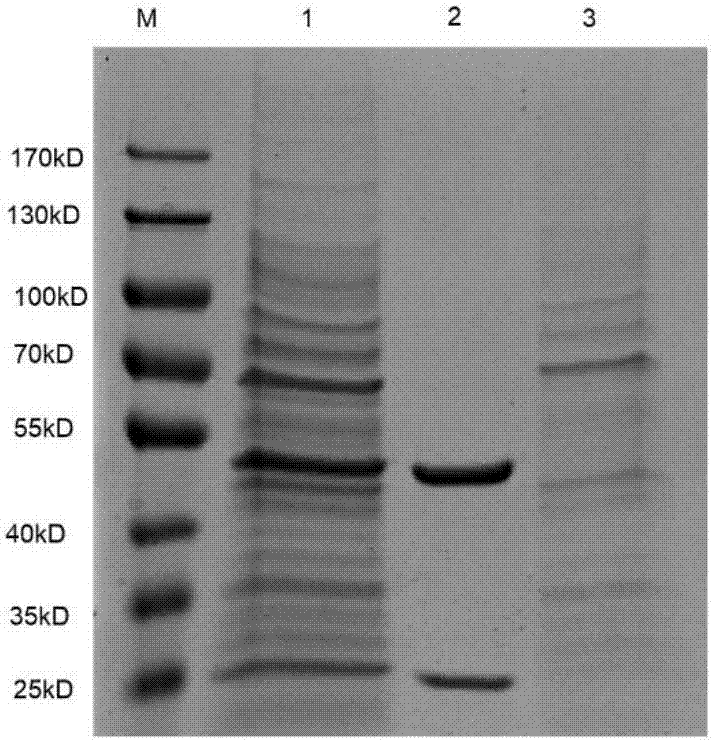
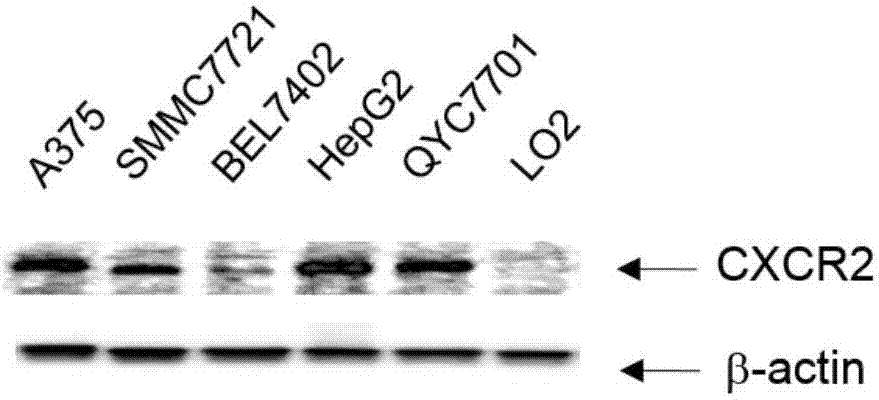
Human-mouse chimeric anti-CXCR2 full-molecule IgG and application thereof
ActiveCN107964045ABinding blockGood neutralizing activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHeavy chainNucleic acid sequencing
The invention discloses a human-mouse chimeric anti-CXCR2 full-molecule IgG and an application thereof and belongs to the field of biological pharmacy. The human-mouse chimeric anti-CXCR2 full-molecule IgG comprises a heavy chain variable region and a light chain variable region and is characterized in that the light chain variable region has a nucleic acid sequence shown in the formula of SEQ IDNO. 1 and the heavy chain variable region has a nucleic acid sequence shown in the formula of SEQ ID NO. 2. The mouse immunized by the specific recombinant CXCR2 protein is used, a mouse-derived anti-CXCR2 monoclonal antibody is prepared through a hybridoma technology, and the recombinant human-mouse chimeric anti-CXCR2 full-molecule IgG is prepared through genetic engineering and antibody engineering techniques. The chimeric antibody can effectively recognize the CXCR2 extracellular domain amino acid fragment and inhibit the binding of the CXCR2 protein to the GRO alpha protein.
Owner:NANJING MEDICAL UNIV
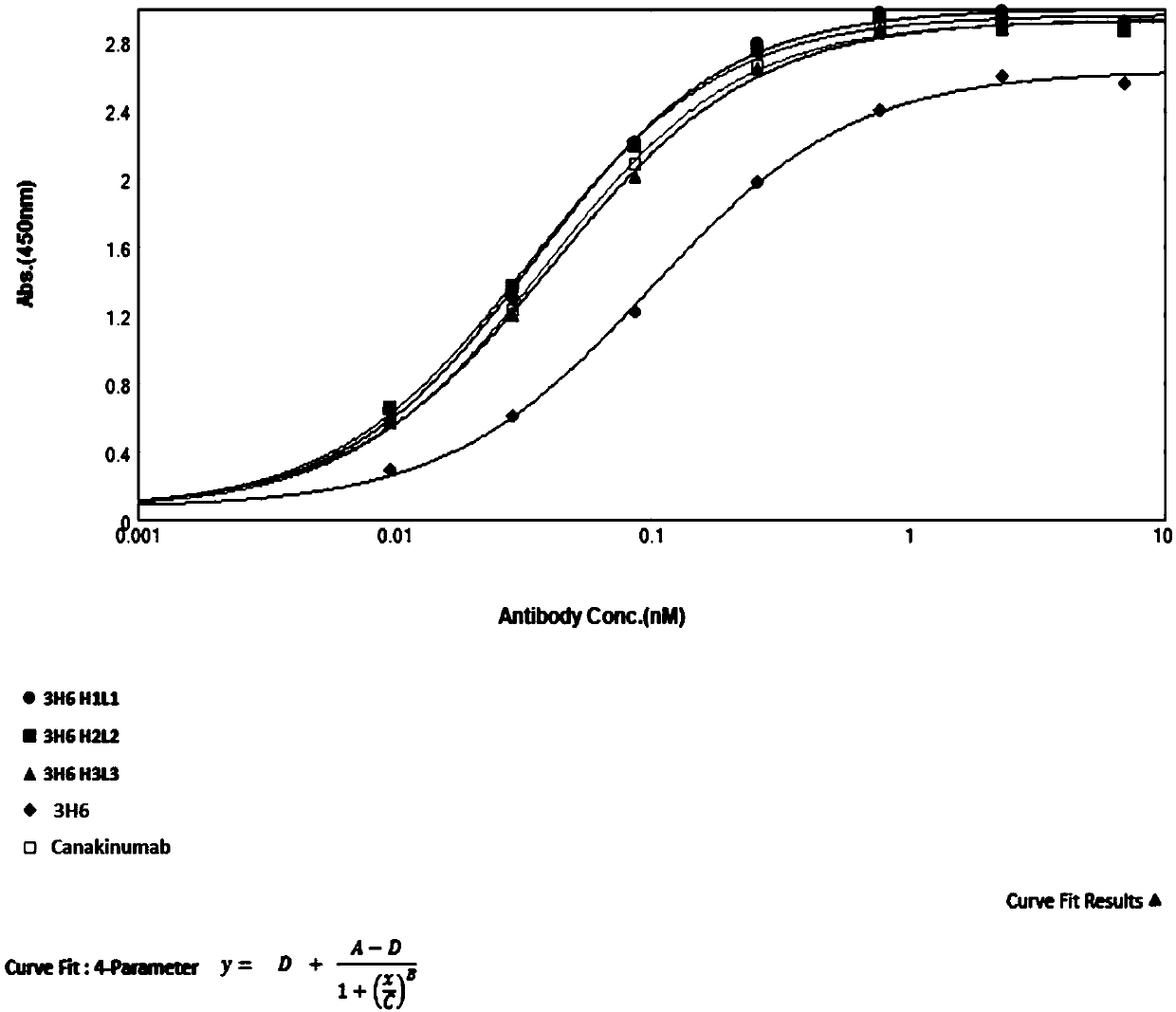
Anti-IL-1beta (anti-interleukin-1beta) antibody as well as medicine composition and application thereof
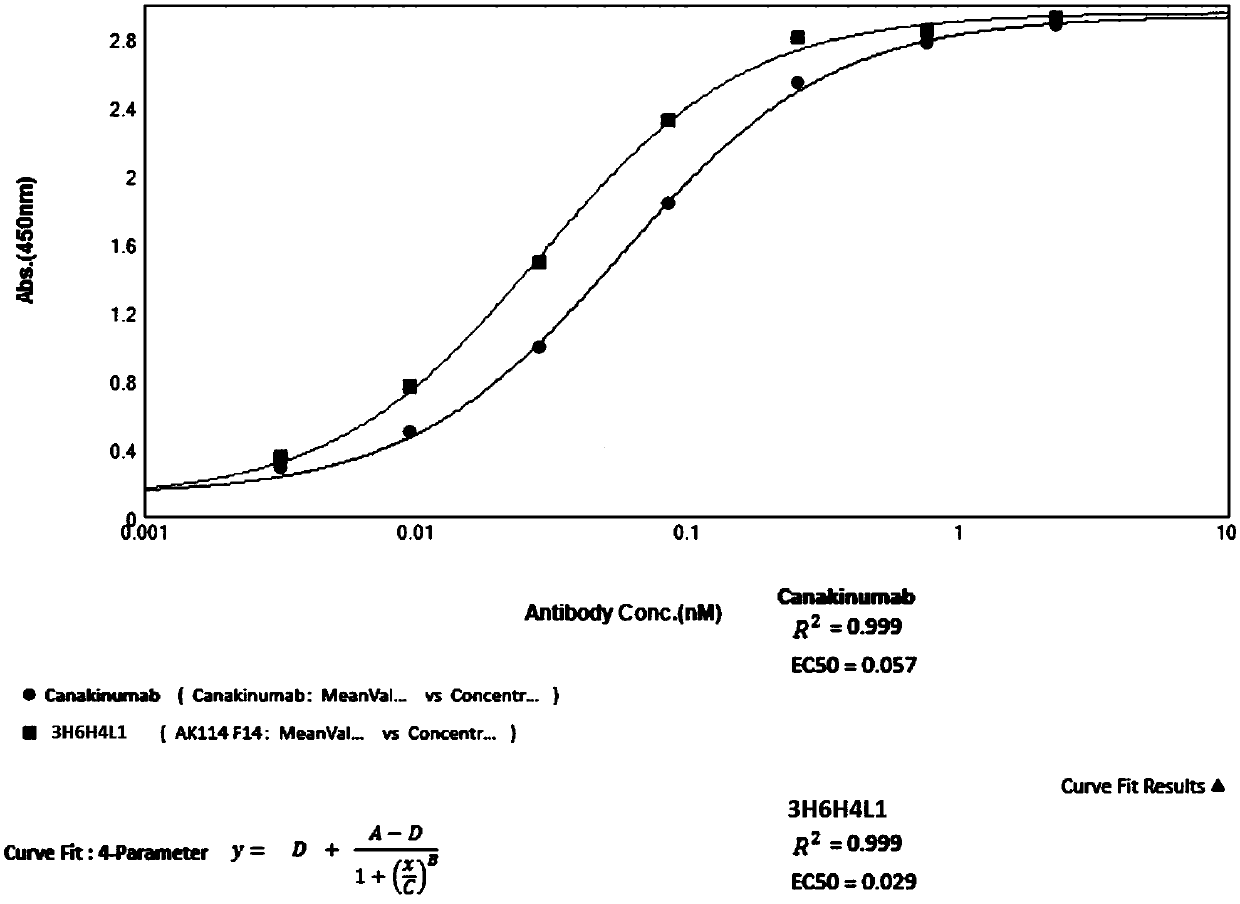
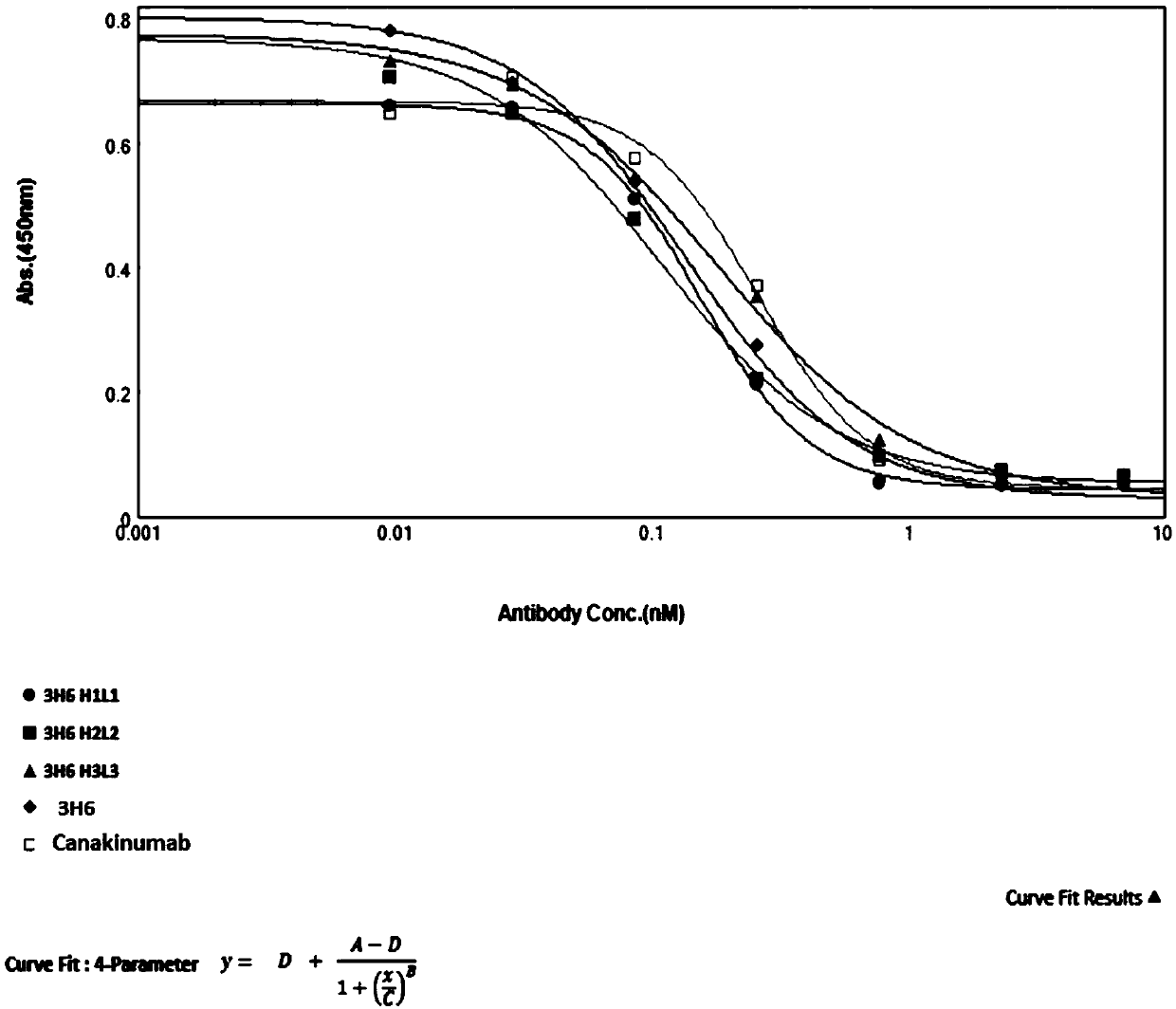
InactiveCN110818793AEffective combinationBinding blockAntipyreticAnalgesicsAntigenAutoimmune condition
The invention belongs to the field of immunology and relates to an anti-IL-1beta (anti-interleukin-1beta) antibody as well as a medicine composition and application thereof. Specially, the invention relates to the anti-IL-1beta antibody or an antigen binding fragment thereof. The heavy chain variable region of the antibody comprises HCDR1-3 (heavy chain complementary determining region 1-3) of which amino acid sequences are shown in SEQ ID NO:17-19 in the description respectively; and the light chain variable region of the antibody comprises LCDR1-3 (light chain complementary determining region 1-3) of which amino acid sequences are shown in SEQ ID NO:20-22 in the description respectively. The antibody provided by the invention is capable of effectively binding human IL-1beta, blocking binding of IL-1beta with a receptor IL-1R1 and inhibiting activation of a downstream signal channel of IL-1beta, and has the potential of preparing medicines for preventing and treating autoimmune diseases, cryopyrin-associated periodic syndromes of children and adults, systemic juvenile idiopathic arthritis, gouty arthritis, cardiovascular diseases or tumors.
Owner:AKESO BIOPHARMA
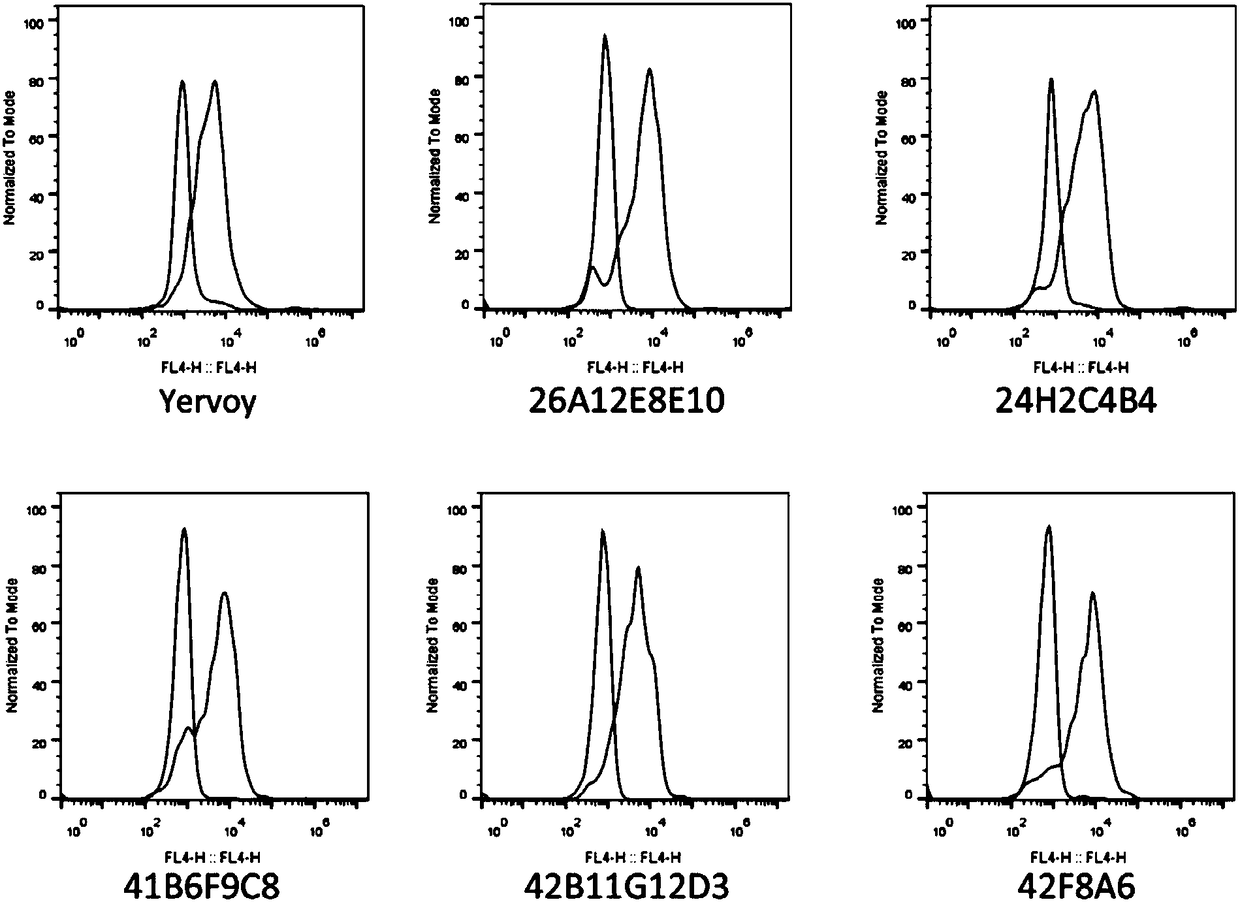
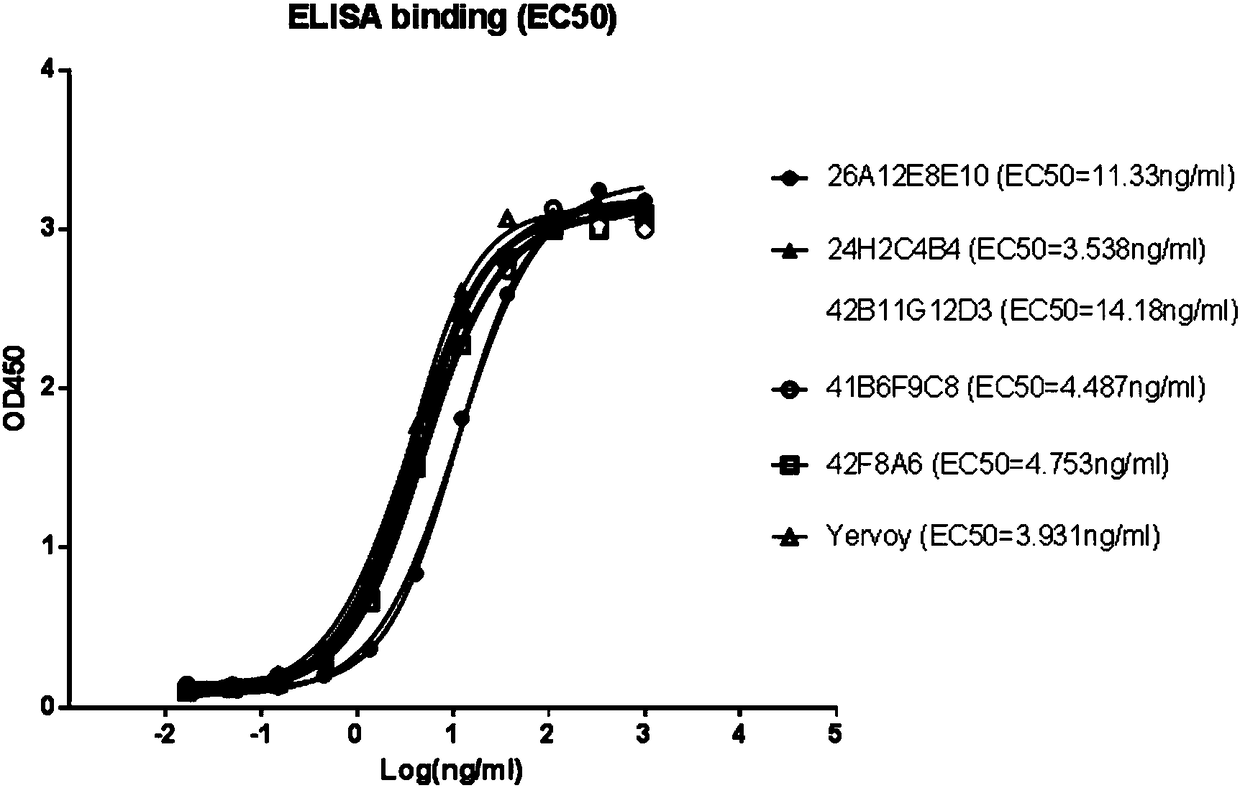
Higher-functionality antihuman CTLA4 antibody with high affinity and high specificity as well as multi-antigen recognition epitopes
ActiveCN108218987ADeregulation of the immune systemBinding blockImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesT cell
The invention discloses a higher-functionality antihuman CTLA4 antibody with high affinity and high specificity as well as multi-antigen recognition epitopes. The CTLA4 monoclonal antibody disclosed by the invention can be specifically combined with CTLA4 and has the advantages that the binding of the CTLA4 with B7 protein can be effectively blocked, down regulation of the CTLA4 can be specifically relieved and T cells are activated to secrete cytokines. All functions reach or exceed the level of a unique CTLA4 target drug Yervoy in the current market, and partial antibodies are different fromantigen epitopes of the Yervoy, so that greater diversity is achieved.
Owner:NANJING GENSCRIPT BIOTECH CO LTD
Epitope-specific antibody screening method and screened antibody
PendingCN109929036ASolve the problem of low hit rateSolve the problem of ineffective treatmentImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesDisease patient
The invention discloses an epitope-specific antibody screening method, and is used for solving the problem of low screening efficiency of functional antibodies having treatment function in an antibodylibrary screening technology; the method includes the steps of screening of functional epitope peptides and screening of living cells. The invention also provides a fully human anti-PD-1 monoclonal antibody obtained by screening a phage display antibody library by the method; for specific functional epitopes, the fully human anti-PD-1 monoclonal antibody can effectively block the binding of PD-1to a ligand of PD-1, has the characteristics of high affinity and low EC50, and can produce treatment effect on animal models at low dose. The screening efficiency of the functional antibody is greatly improved, and a potential, safer and more economical immunotherapy drug is provided for patients with malignant tumors and immune diseases.
Owner:TAIZHOU MABTECH PHARM CO LTD
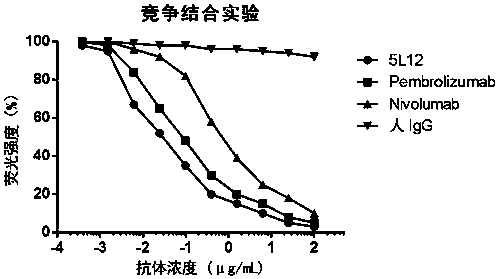
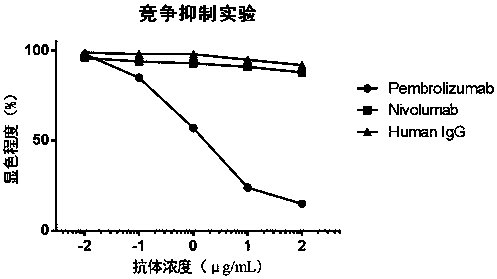
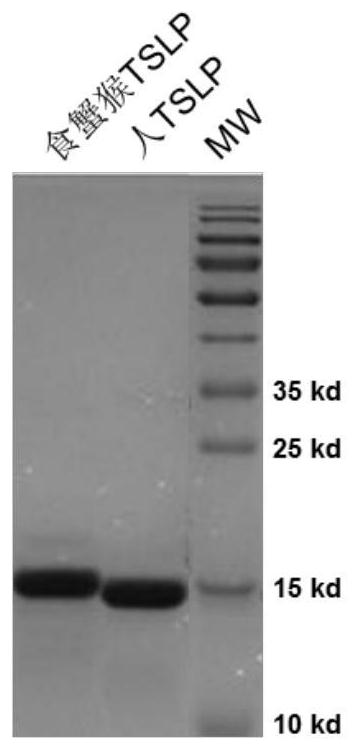
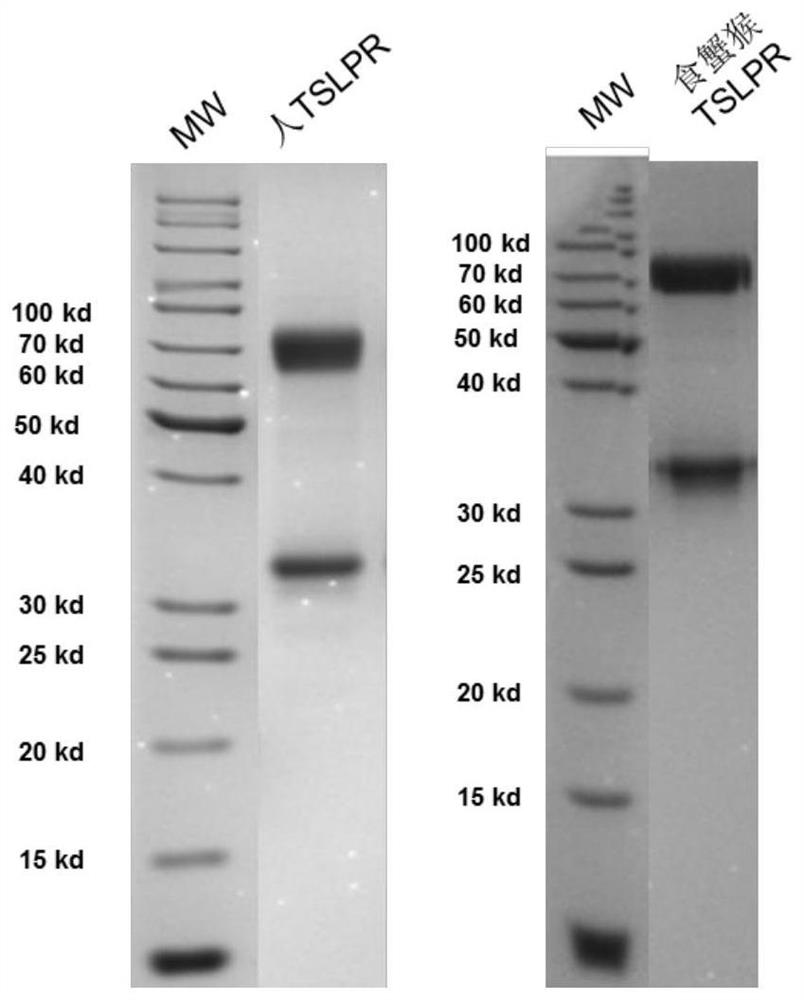
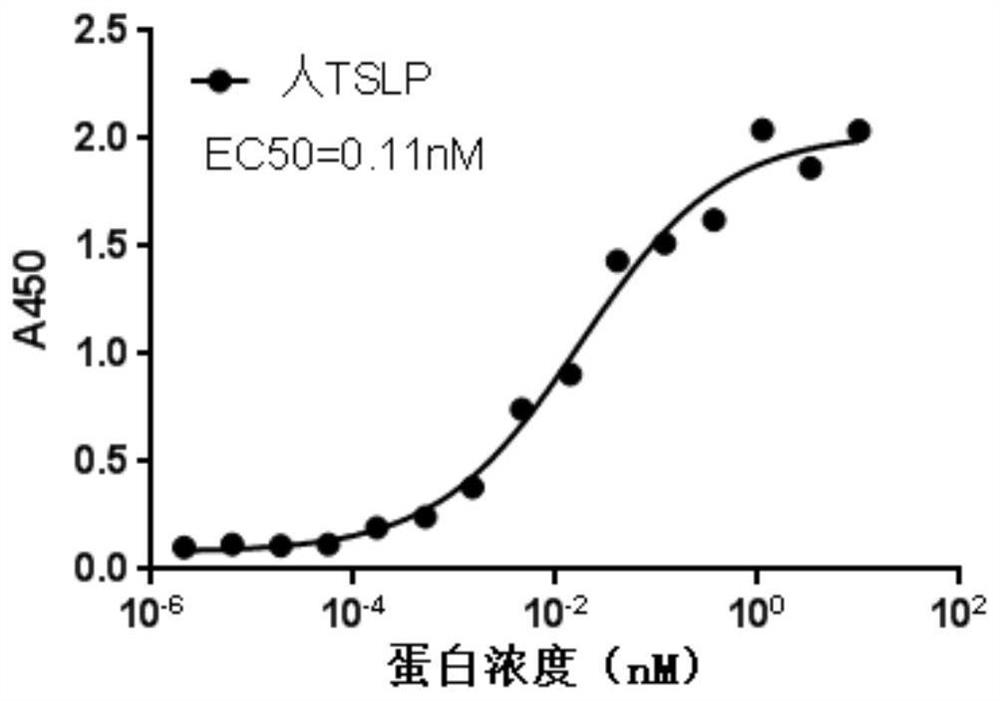
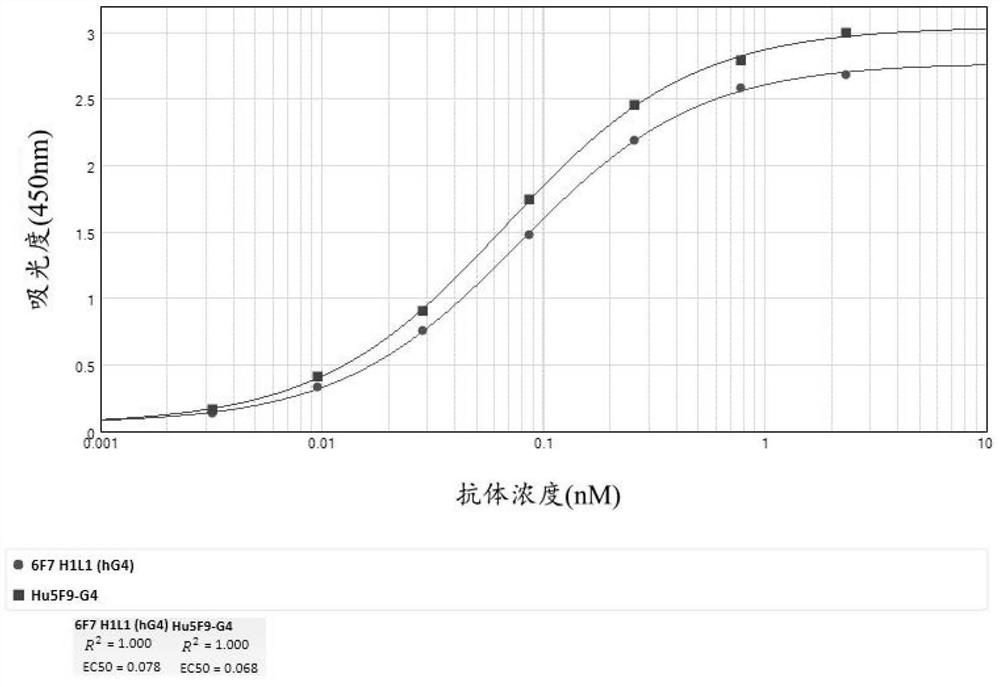
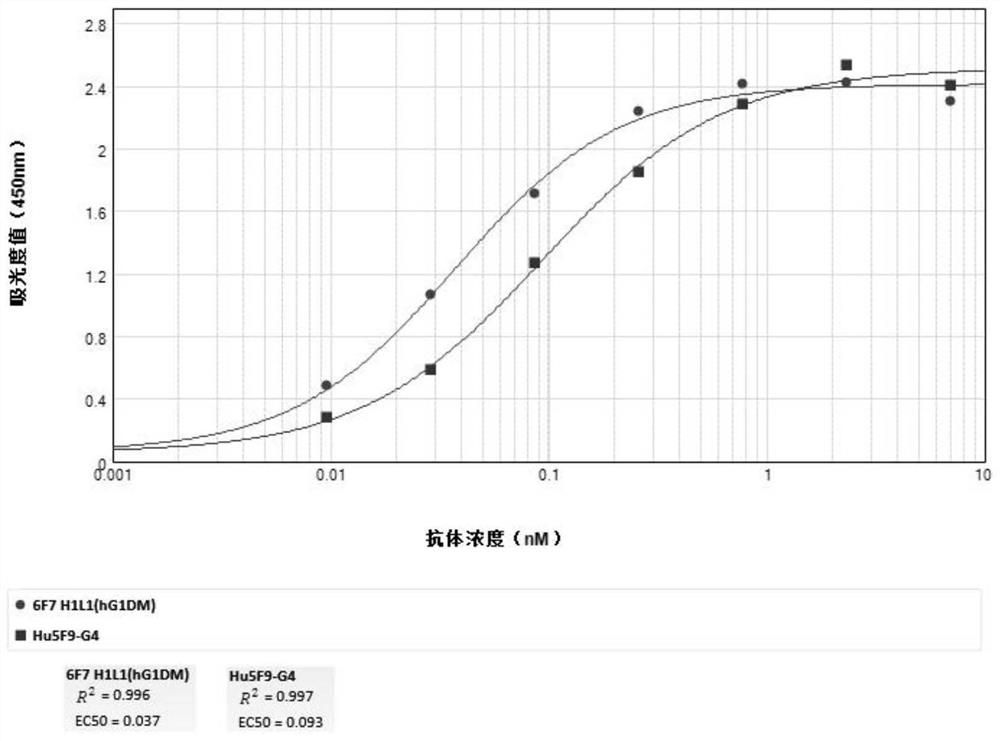
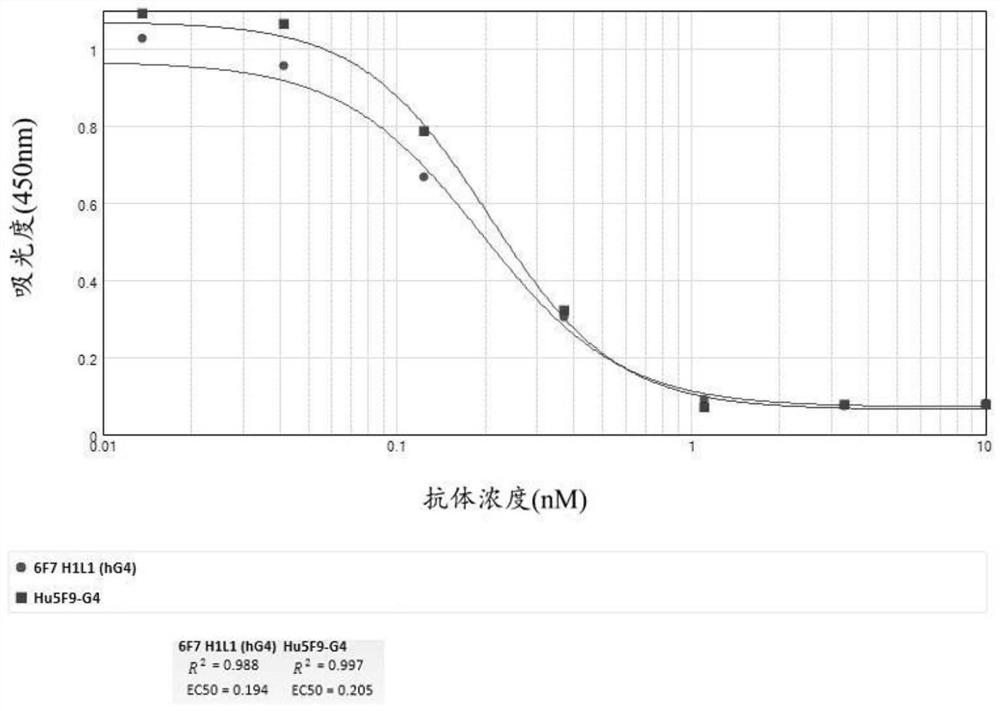
Development and application of therapeutic agent for TSLP-related diseases
The invention discloses development and an application of a therapeutic agent for TSLP-related diseases, and relates to an antibody binding to TSLP protein or an antigen binding part thereof as well as a preparation method and an application of the antibody. The antibody can be combined with human TSLP and / or cynomolgus monkey TSLP with high affinity, can block the combination of the TSLP and TSLPR, and can inhibit the transduction of a TSLP stimulating signal through an STAT5 pathway.
Owner:CHENGDU CONMED BIOSCI CO LTD +1
Anti-CD47 monoclonal antibody and use thereof
ActiveCN112442123ABinding blockEnhance phagocytosisBiological material analysisMammal material medical ingredientsAntiendomysial antibodiesBiochemistry
The invention relates to an anti-CD47 monoclonal antibody and use thereof. The anti-CD47 monoclonal antibody is secreted from a hybridoma cell strain with an accession number of CCTCC NO: C2018135.
Owner:AKESO BIOPHARMA
Injection preparation of anti-IL-17RA monoclonal antibody
ActiveCN111840217AHigh affinityImprove biological activityAntipyreticAnalgesicsSpondarthritisAutoimmune condition
The invention relates to the field of biological medicines, and particularly provides an injection preparation of an anti-IL-17RA monoclonal antibody. The preparation comprises pharmacodynamic molecules and a buffer solution, the pharmacodynamic molecules are the anti-IL-17RA monoclonal antibody, and the buffer solution comprises a buffer salt, a protein protective agent, an osmotic pressure regulator and a surfactant, wherein the pH value of the buffer solution is 5.5-6.5. According to the invention, all components in the buffer solution interact with each other for synergistic cooperation, astorage environment suitable for long-term storage is provided for the anti-IL-17RA monoclonal antibody, the degradation, aggregation or precipitation of the antibody caused in the long-term storageor transportation process of the preparation can be avoided, the antibody can be stored in the environment for 36 months or more, the activity and the purity of the antibody can be kept stable in thestorage period, and the drug effect of the biological drug is guaranteed; the injection preparation can be used for various autoimmune diseases including but not limited to psoriasis, rheumatoid arthritis, ankylosing spondylitis or scleroderma and the like.
Owner:BEIJING DONGFANG BIOTECH
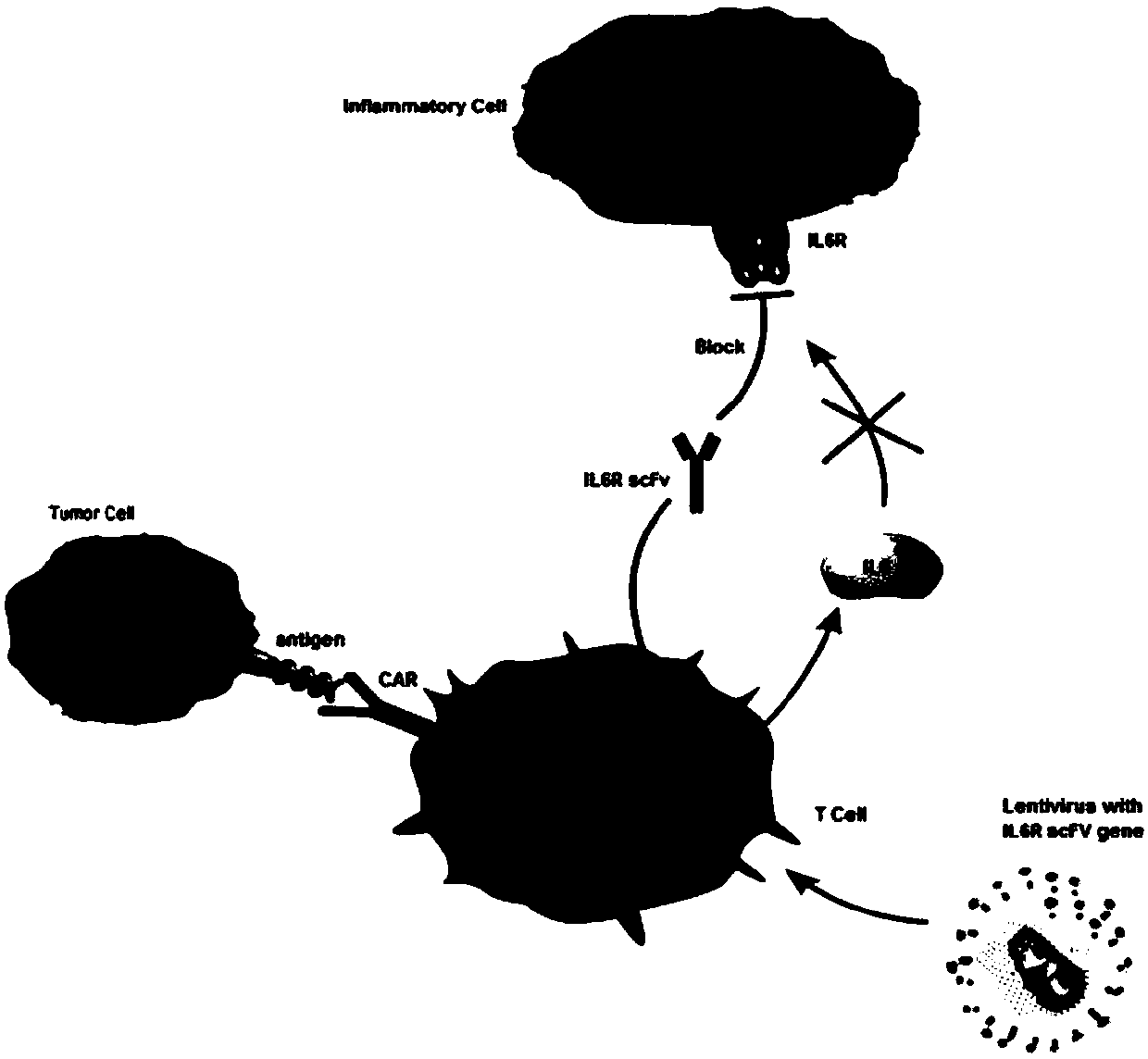
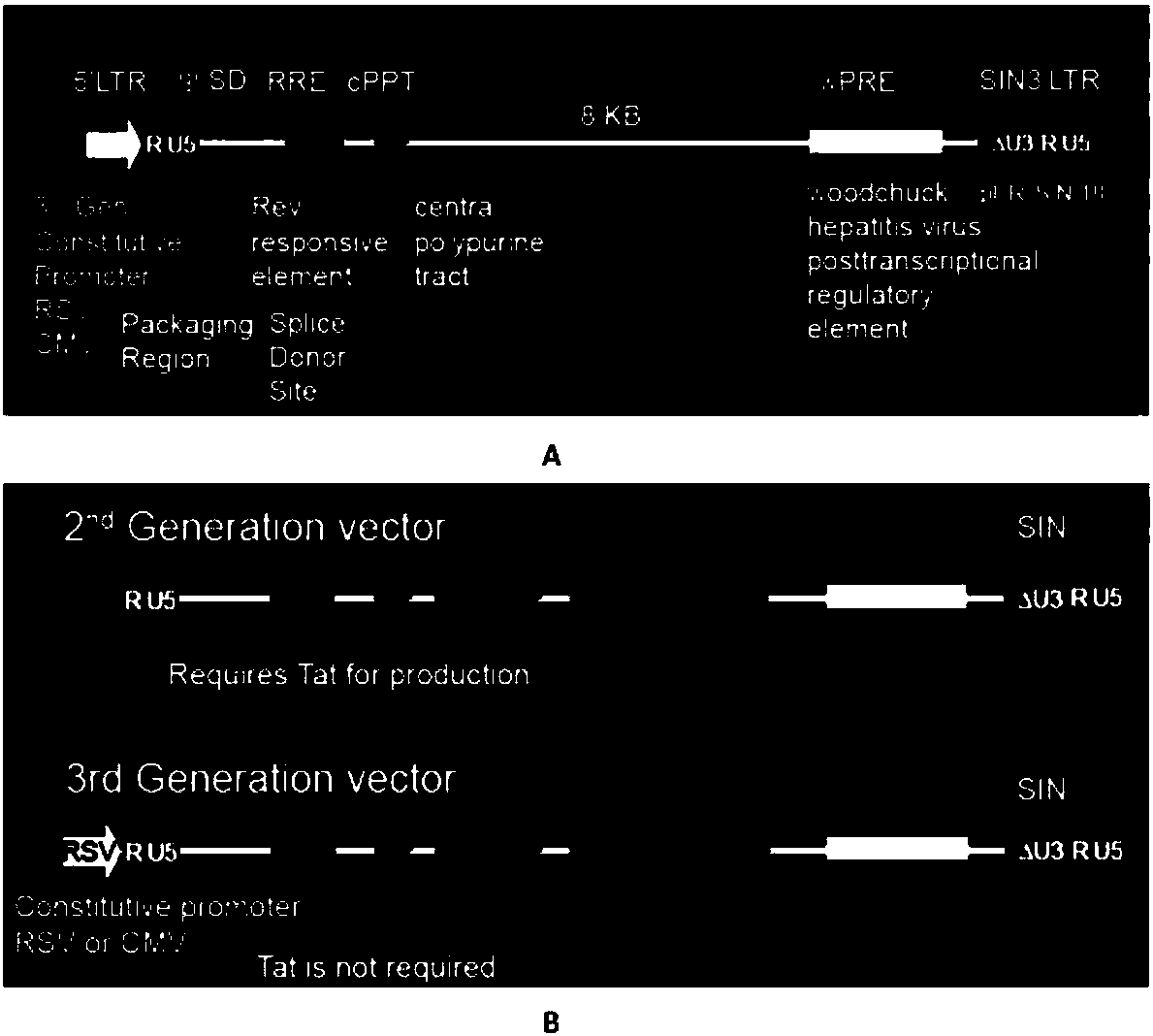
Closed IL6R CAR-T transgenic vector for relieving CRS as well as construction method and application of CAR-T transgenic vector
ActiveCN108148863AGood sealingAvoid the problem of low efficiency of in vivo deliveryVirusesPeptide/protein ingredientsSequence signalSingle-Chain Antibodies
The invention discloses a closed IL6R CAR-T transgenic vector for relieving CRS. The vector consists of an amicillin resistance gene AmpR sequence (SEQ ID NO.1), a prokaryotic replicon pUC Ori sequence (SEQ ID NO.2), a virus replicon SV40 Ori sequence (SEQ ID NO.3), an eWPRE-enhanced marmot hepatitis B virus post-transcriptional regulatory element (SEQ ID NO.11), a human EF1[alpha] promoter (SEQ ID NO.12), a lentivirus packaging cis element applied to lentivirus packaging, a human IL6R humanized single-chain antibody IL6RscFv1 (SEQ ID NO.21) or IL6RscFv2 (SEQ ID NO.22) or IL6RscFv3 (SEQ ID NO.23), an IRES ribosome bonding sequence (SEQ ID NO.25), an IL6 signal peptide (SEQ ID NO.26), a humanized antibody Fc segment (SEQ ID NO.27) and a chimeric antigen receptor for constituting a second-generation or third-generation CAR integrating recognition, transferring and promoting. In addition, the invention also discloses a construction method of the vector and an application of the vector inpreparing medicines for relieving CRS.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
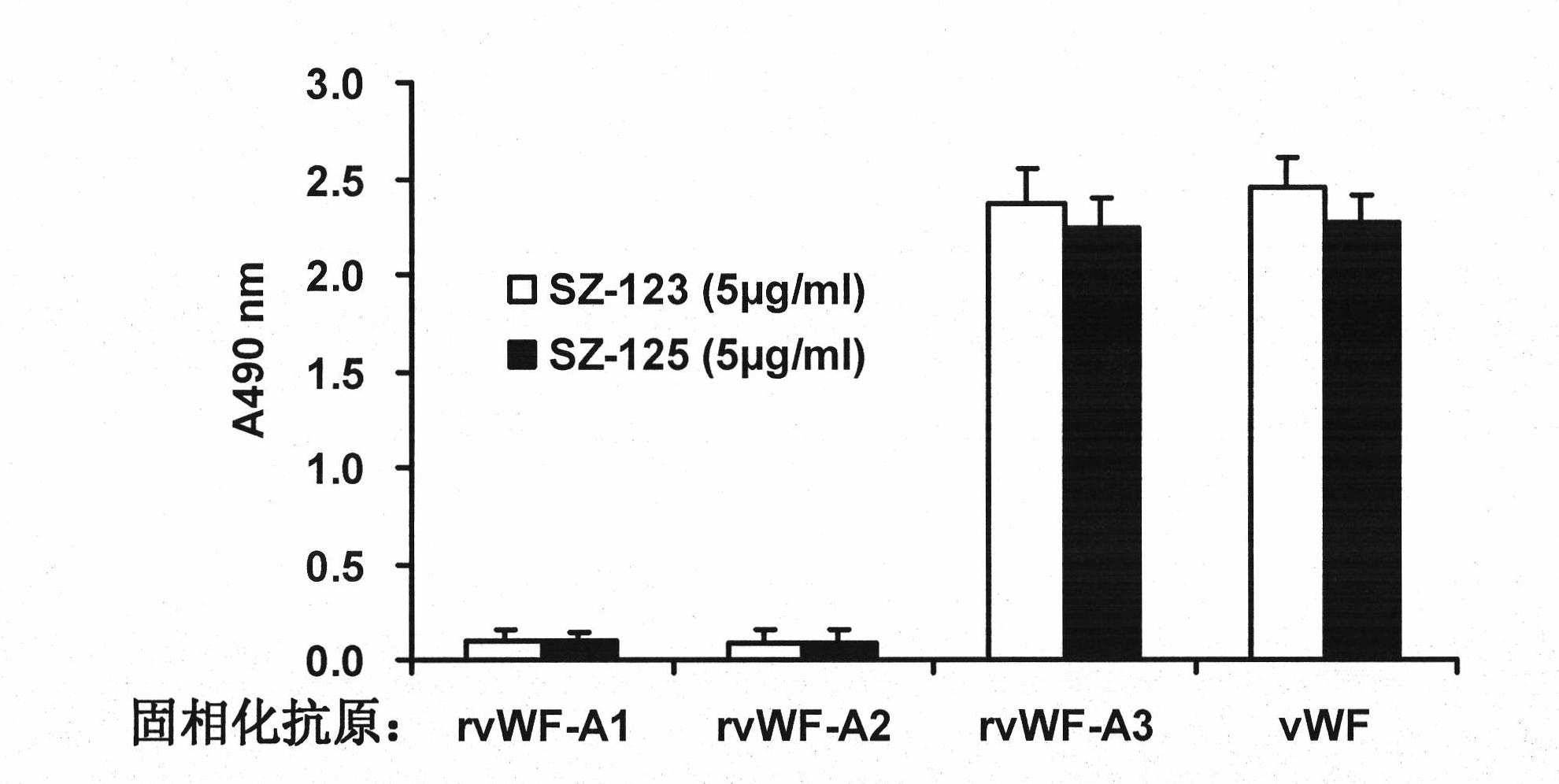
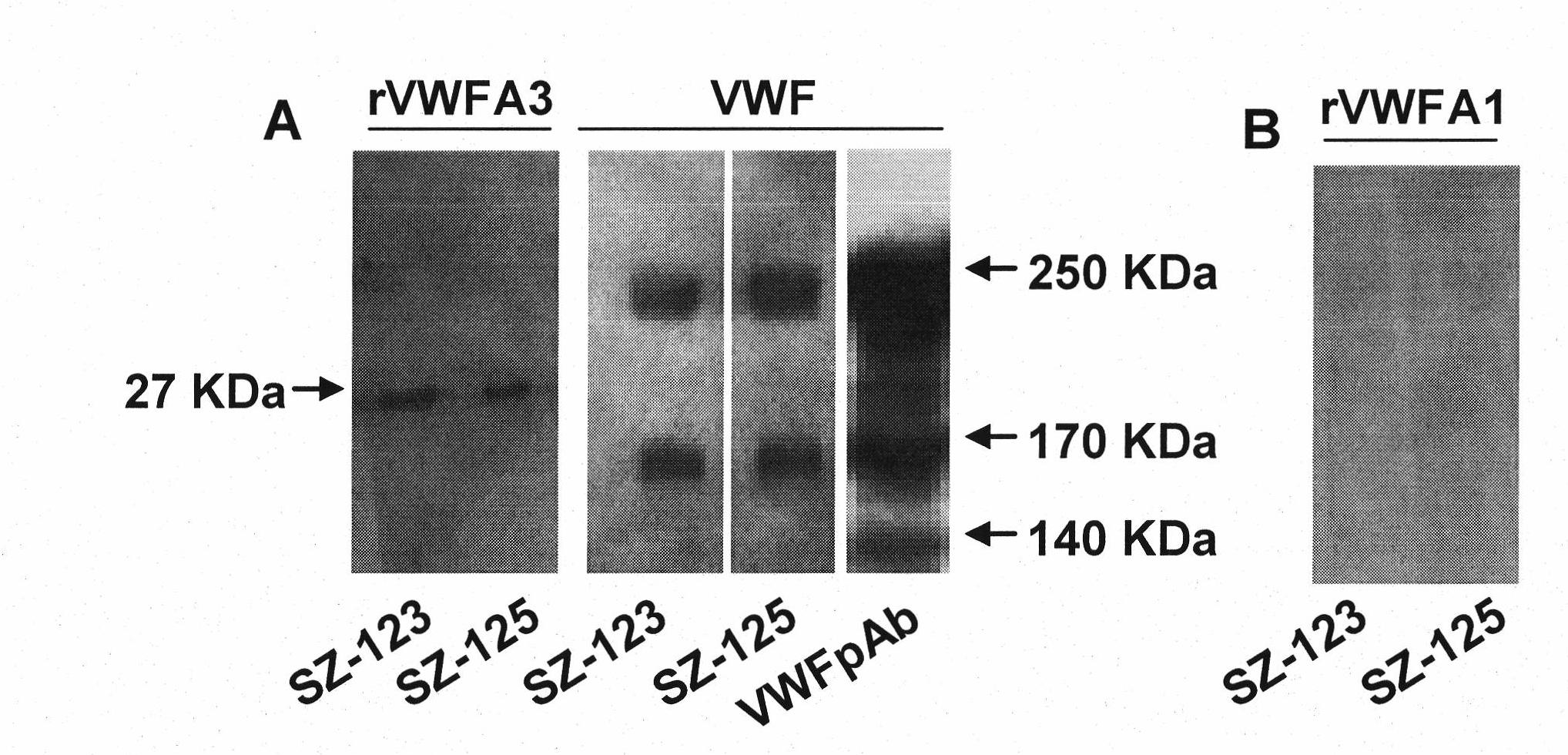
Bi-functional monoclonal antibody capable of anti-human von willbrand factor-A3
InactiveCN101597595BBinding blockBlocking interactionImmunoglobulins against animals/humansAntibody ingredientsVon Willebrand factorFactor ii
The invention relates to a hybridoma cell strain SZ-125(3D2) with the prevention number of CGMCC NO.2501 and a bi-functional monoclonal antibody capable of anti-human von willbrand factor-A3 secreted by the hybridoma cell strain. The monoclonal antibody is combined with the VWF A3 to block the combination of the VWF A3 and collagen and simultaneously influences the configuration of domain A1, thereby destroying the original domain A1 configuration capable of combining platelet GPIb alpha and achieving the aim of blocking the interaction of the VWF A1 and the GPIb alpha, so that not only combination of the VWF A3 and the collagen is blocked but also the combination of the VWF A1 and the platelet GPIb alpha is blocked. Thus, the monoclonal antibody can be used for preparing anti-artery thrombosis medicine with anti-platelet adhesion activity.
Owner:苏州苏大赛尔免疫生物技术有限公司
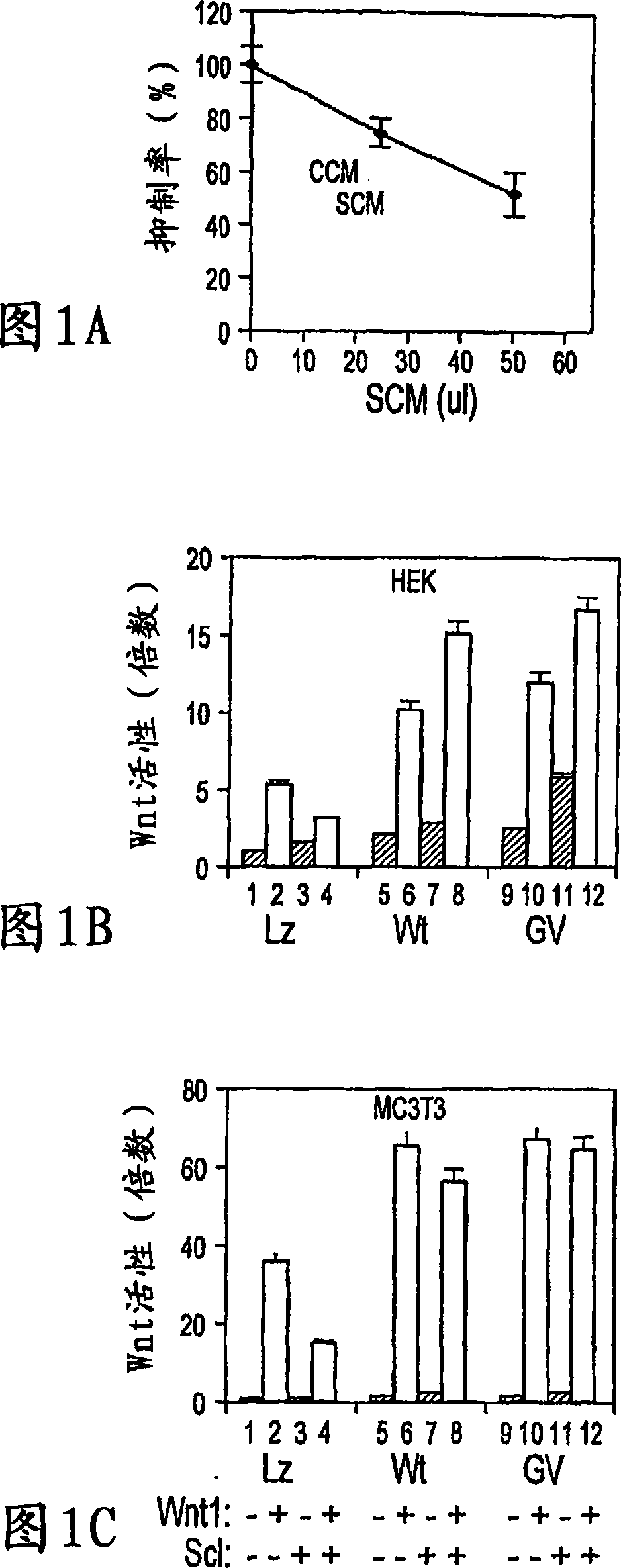
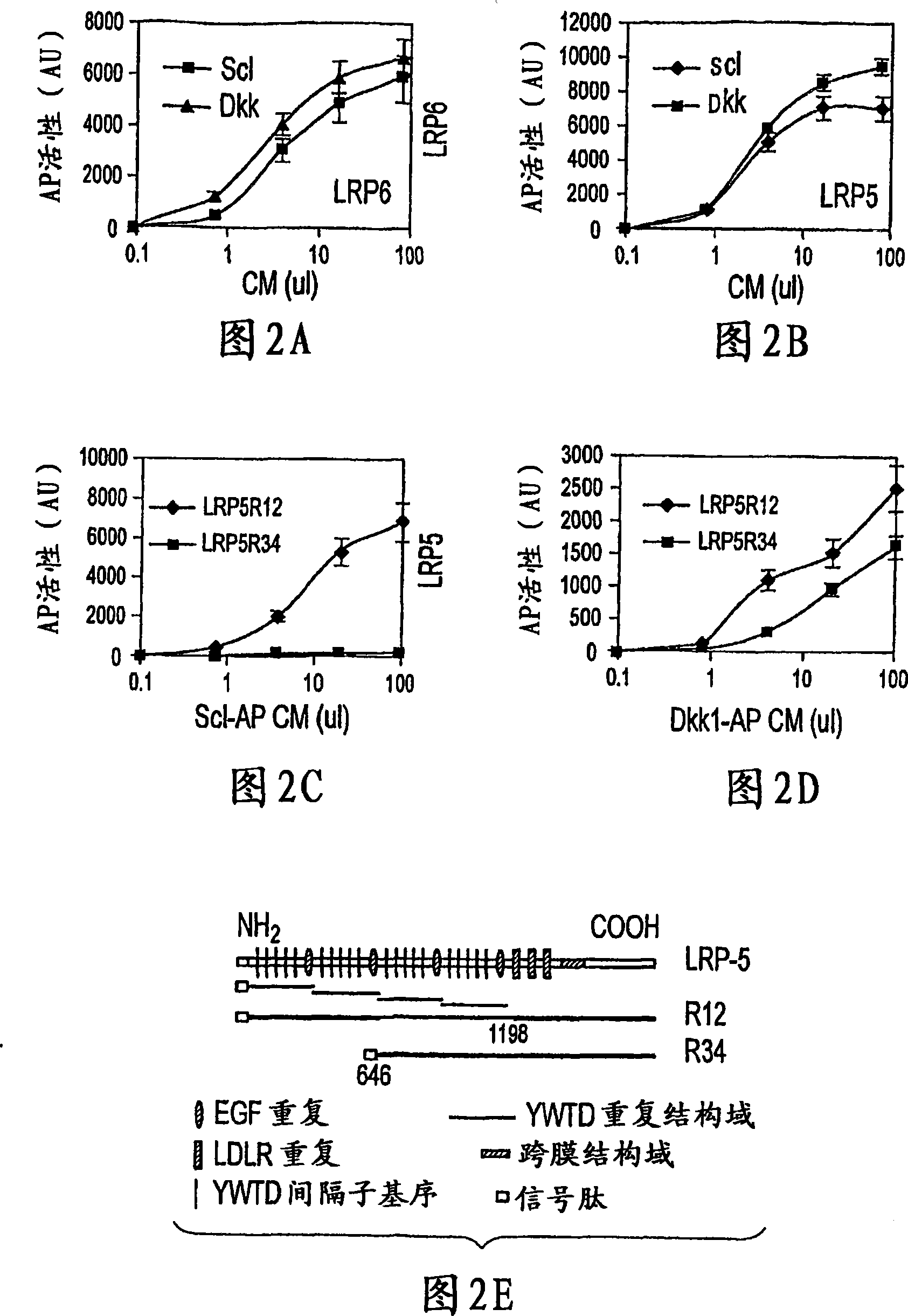
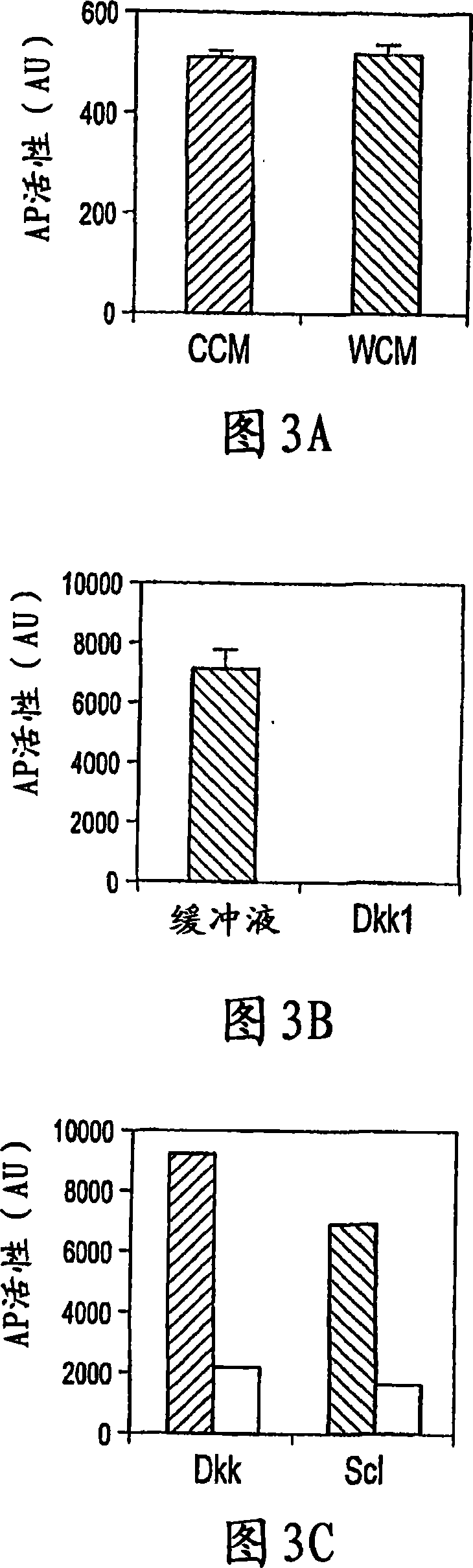
Sclerostin and the inhibition of wnt signaling and bone formation
InactiveCN101888849AAvoid combiningBinding blockCompound screeningApoptosis detectionLRP6Bone formation
The loss of the SOST gene product sclerostin leads to sclerosteosis characterized byihigh bone mass (HBM). In this report, we found that sclerostin could antagonize canonical, Wnt signaling in human embryonic kidney A293 cells and mouse osteoblastic MC3T3 cells. This sclerostin-mediated antagonism could be reversed by over-expression of Wnt coreceptor LRP5. In addition, we found that sclerostin bound to LRP5 as well as LRP6 and identified the first two YWTD-EGF repeat domains of LRP5 as being responsible for the binding. Although these two repeat domains are required for transducing canonical Wnt signals, canonical Wnt did not appear to compete with sclerostin for binding to LRP5. Examination of the expression of sclerostin and Wnt7b, an autocrine canonical Wnt, during primary calvarial osteoblast differentiation revealed that sclerostin is expressed at the late stages of osteoblast differentiation coinciding with the expression of osteogenic marker osteocalcin and trailing after the expression of Wnt7b. Given the plethora of evidence indicating that canonical Wnt signaling stimulates osteogenesis, we believe that the HBM phenotype associated with the loss of sclerostin may at least in part be attributed to an increase in canonical Wnt signaling resulting from the reduction in sclerostin-mediated Wnt antagonism.
Owner:UNIV OF CONNECTICUT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com