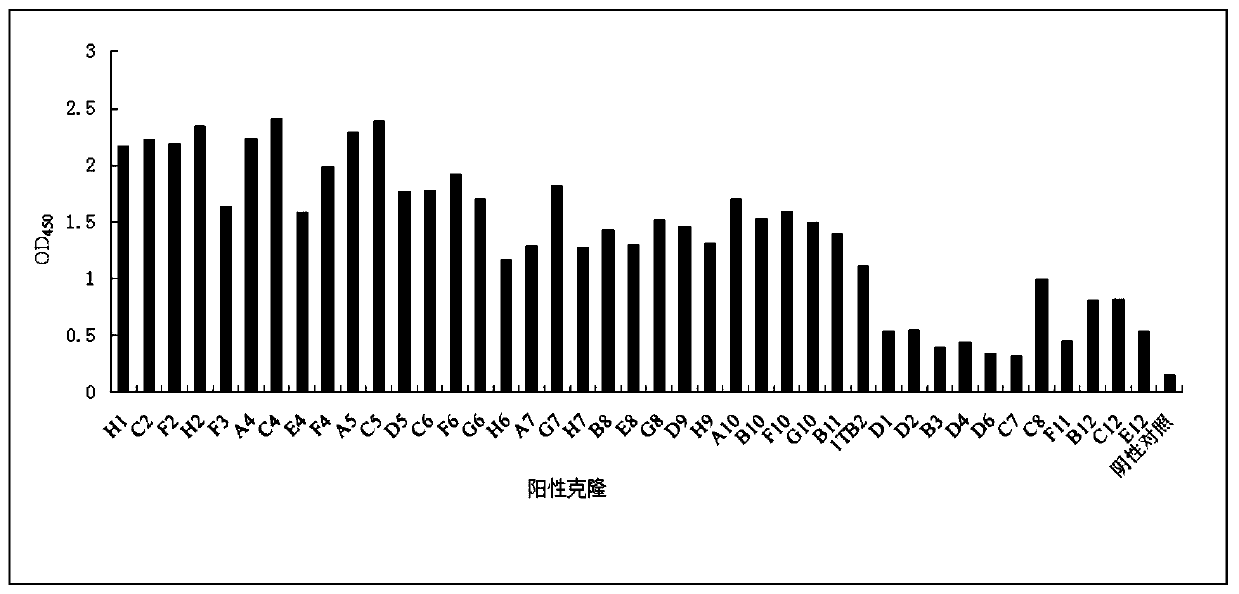
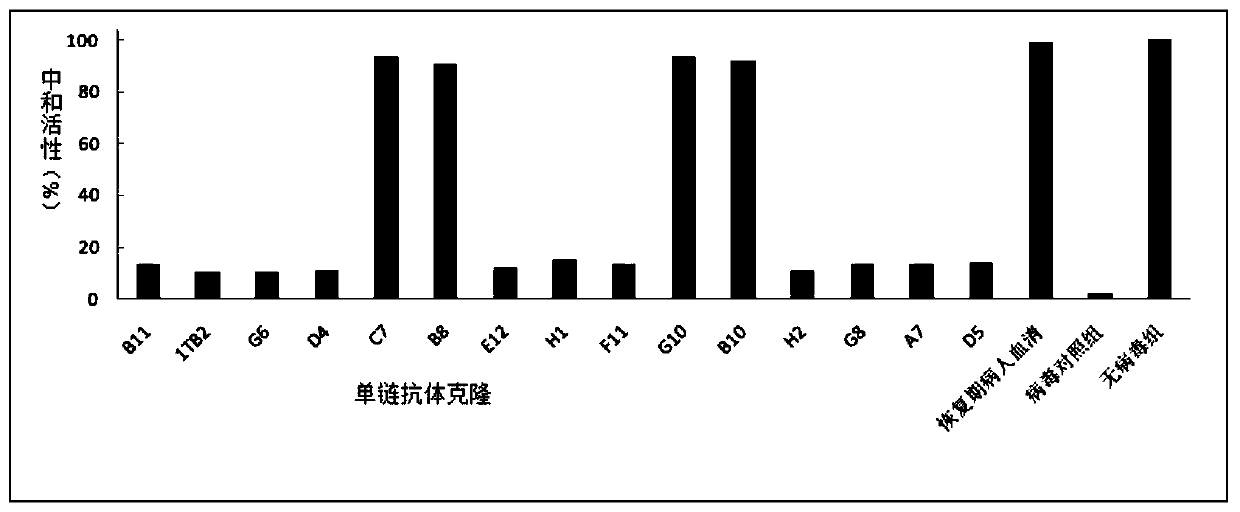
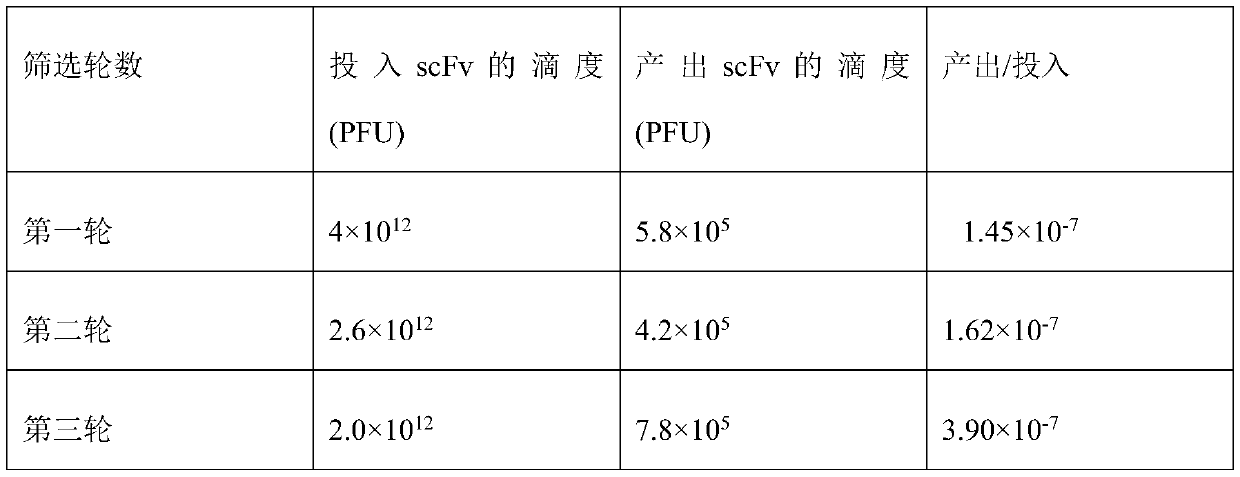
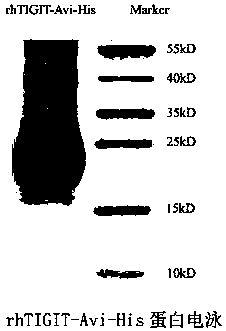
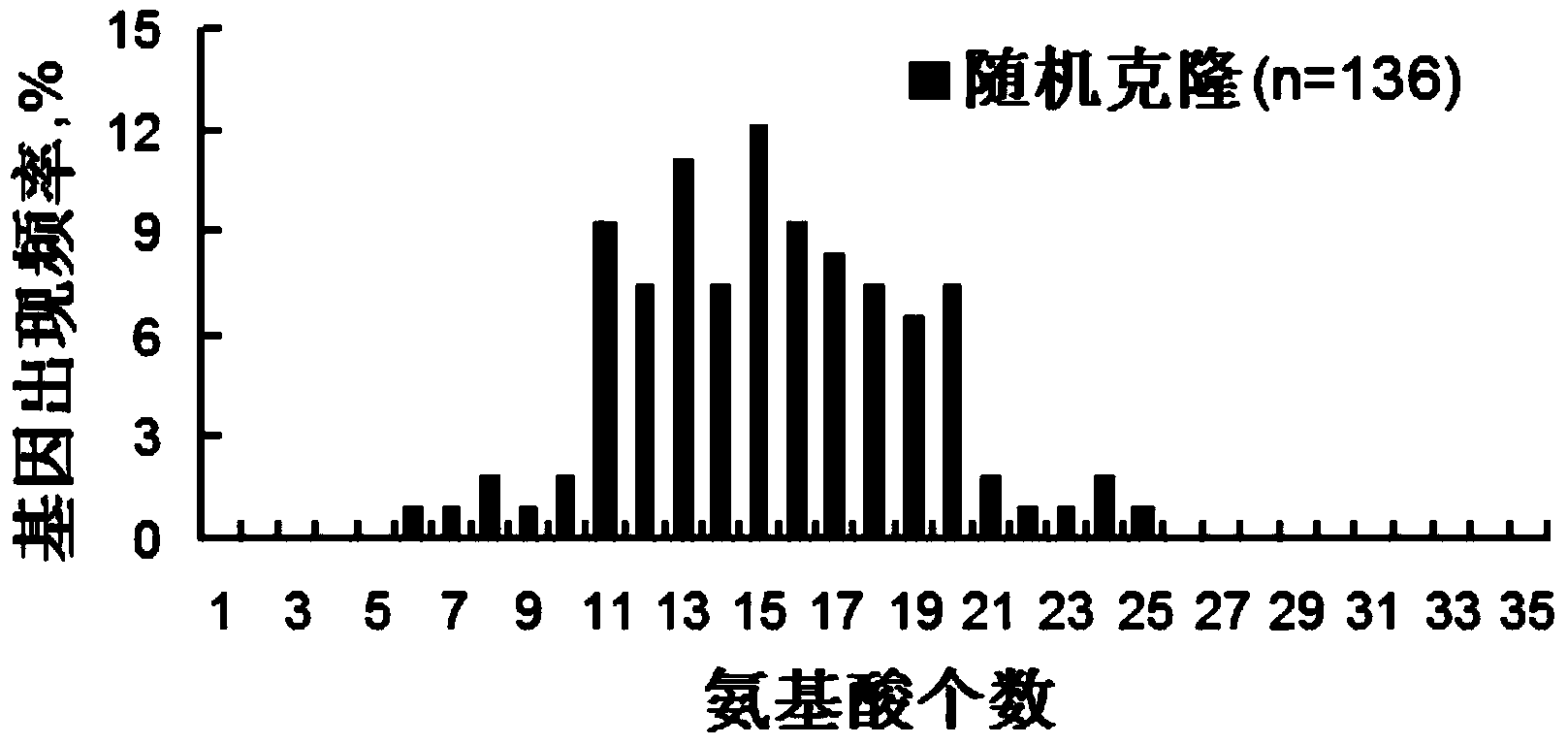
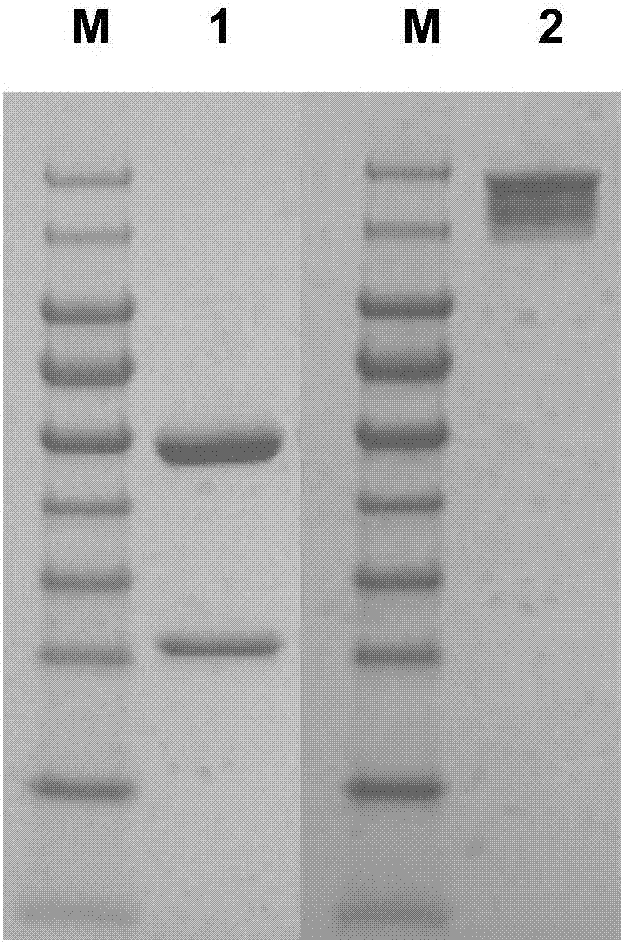
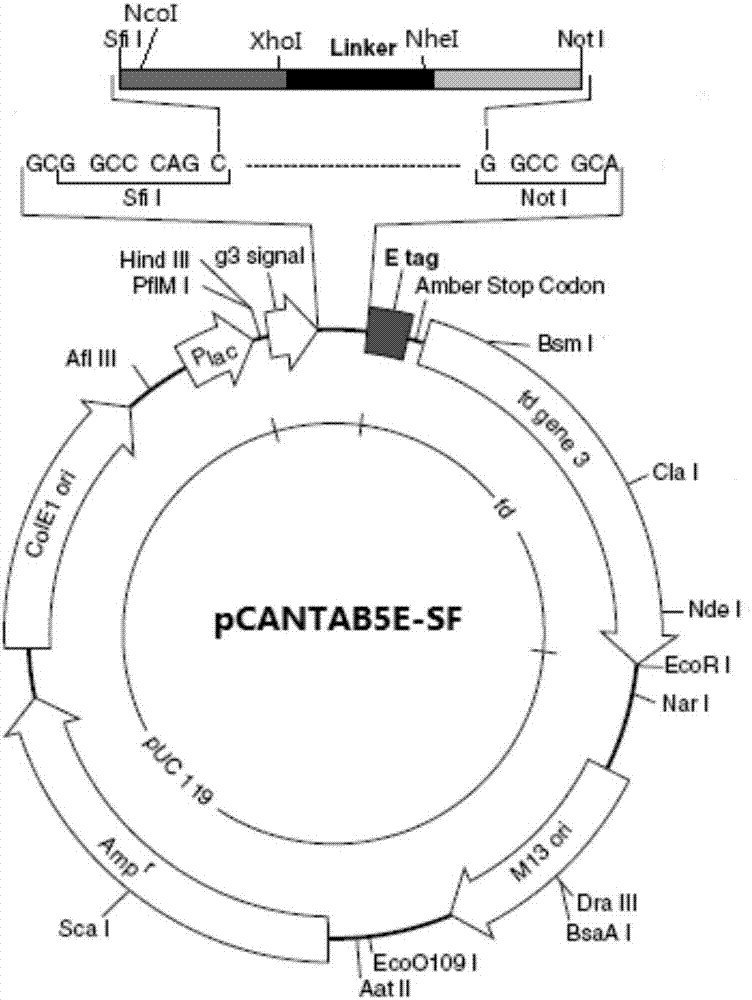
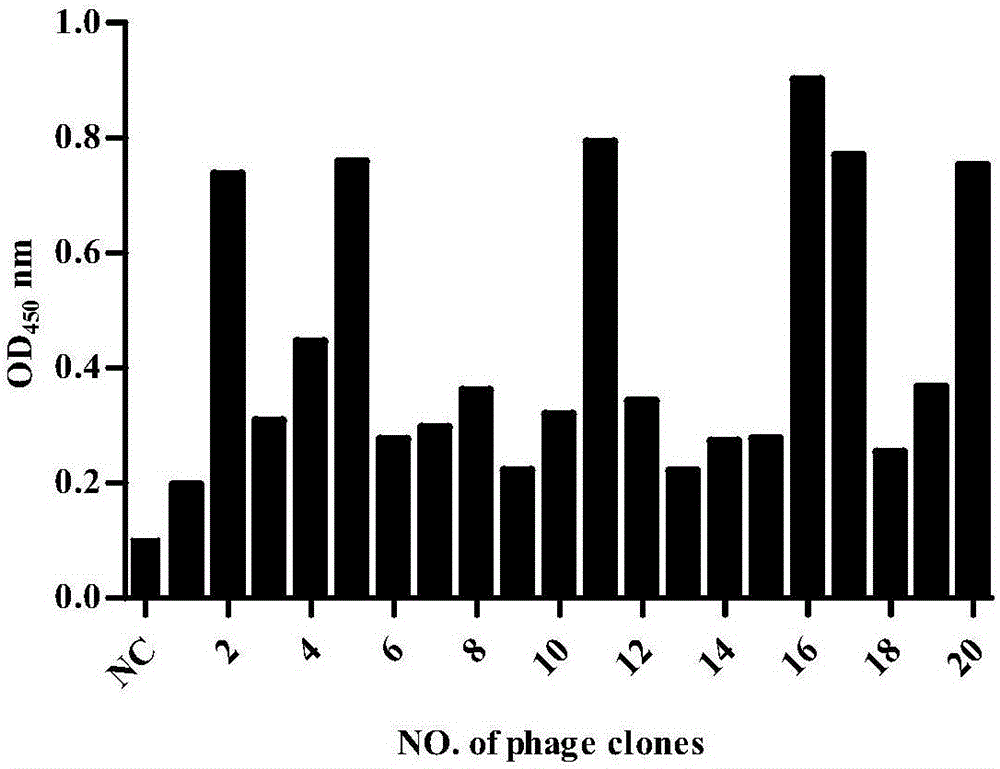
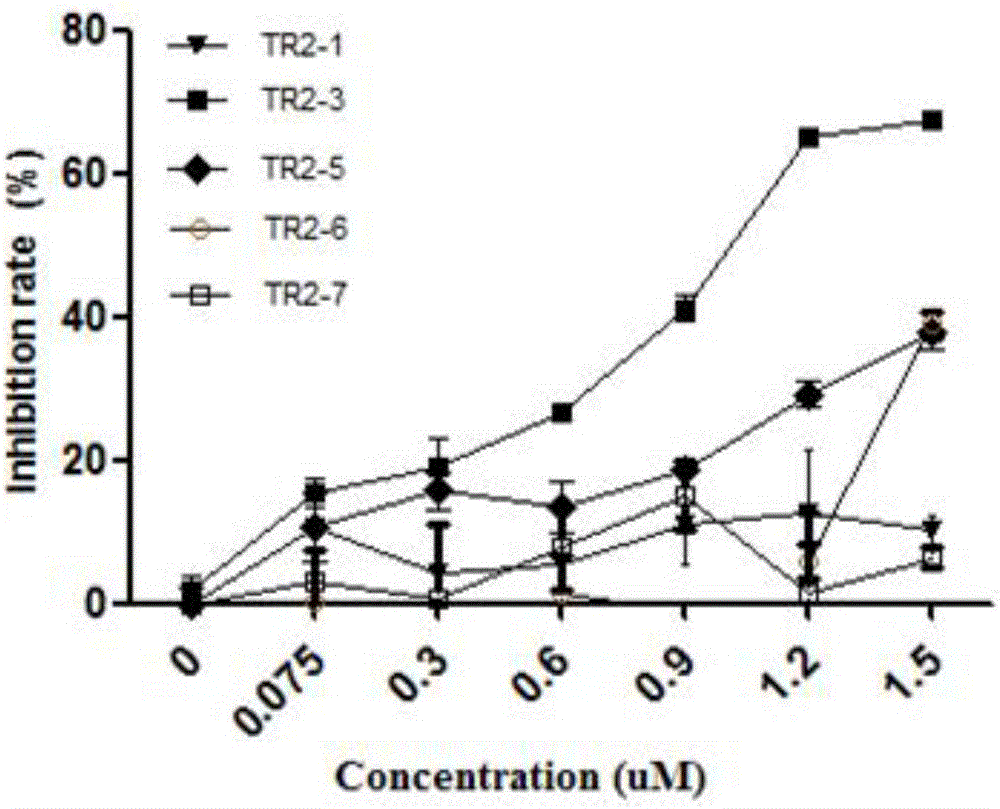
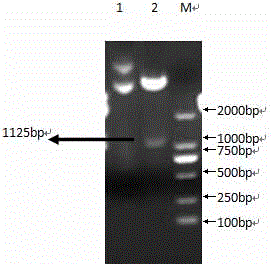
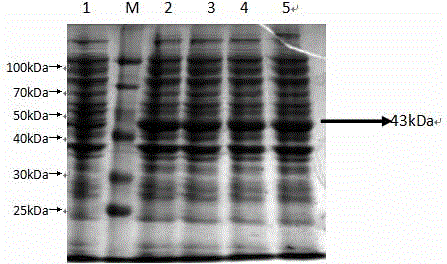
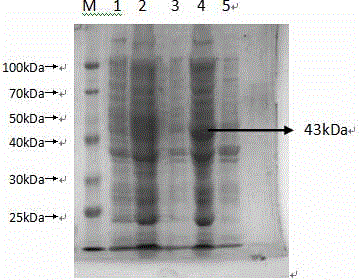
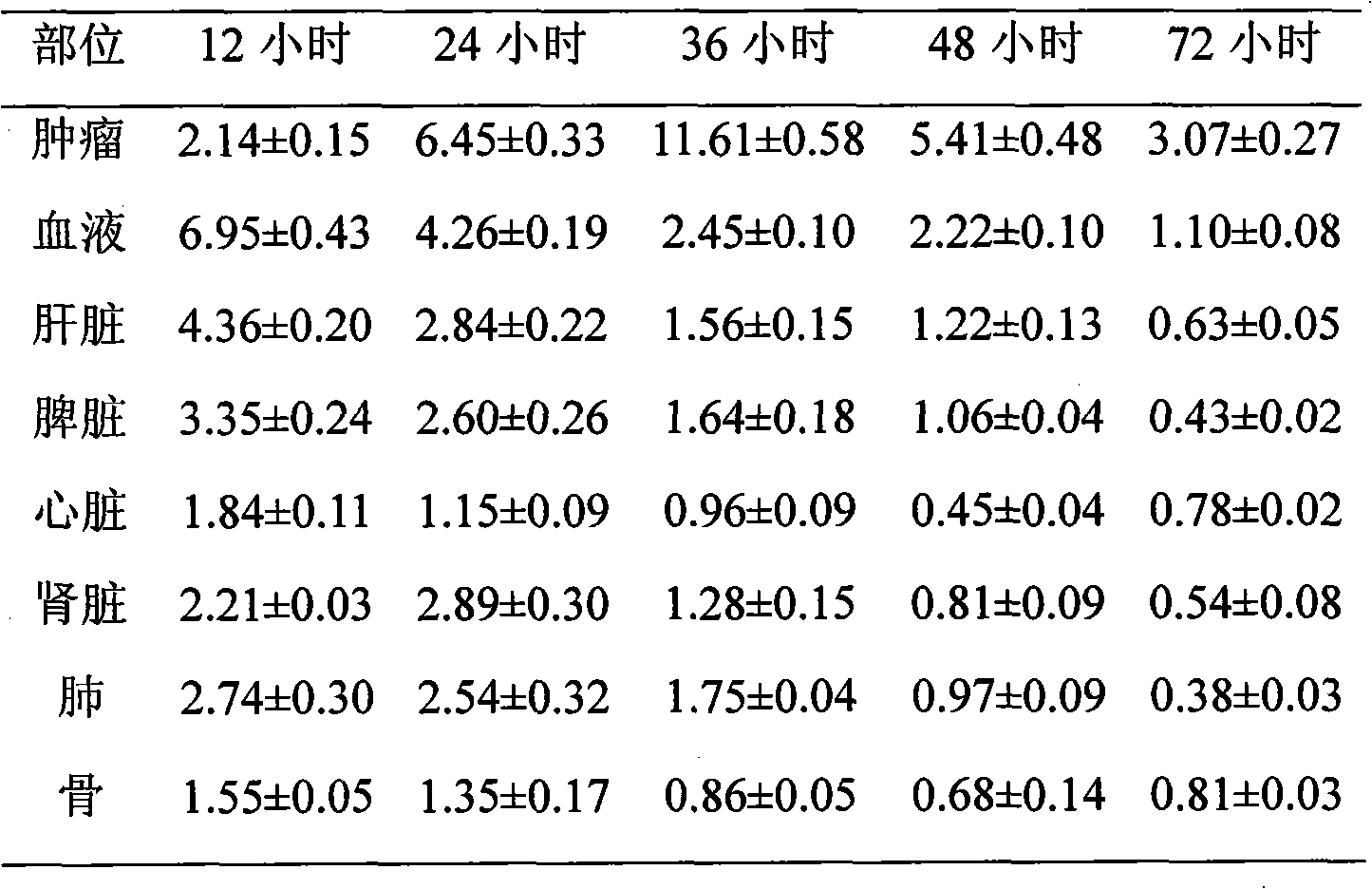
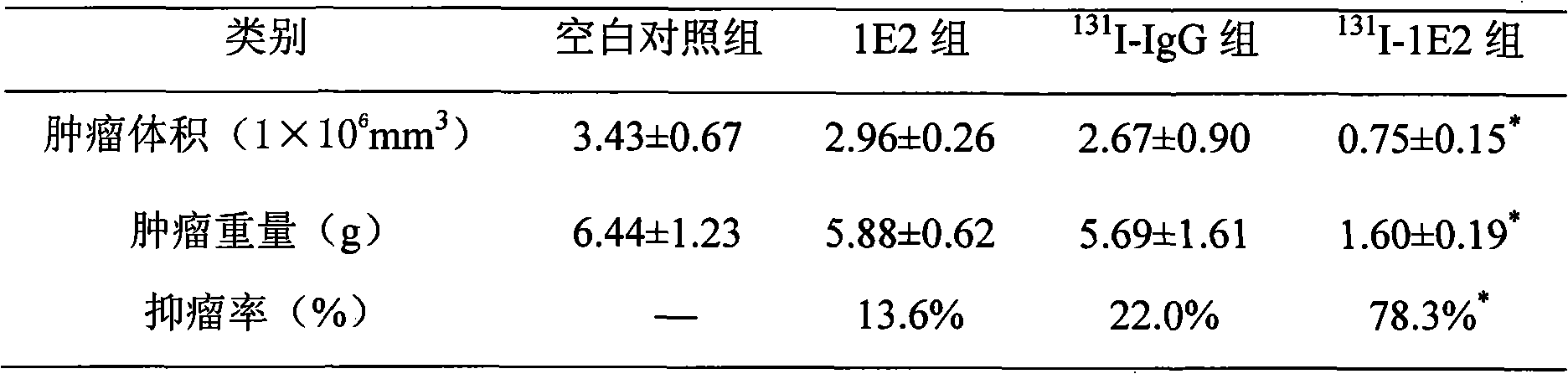
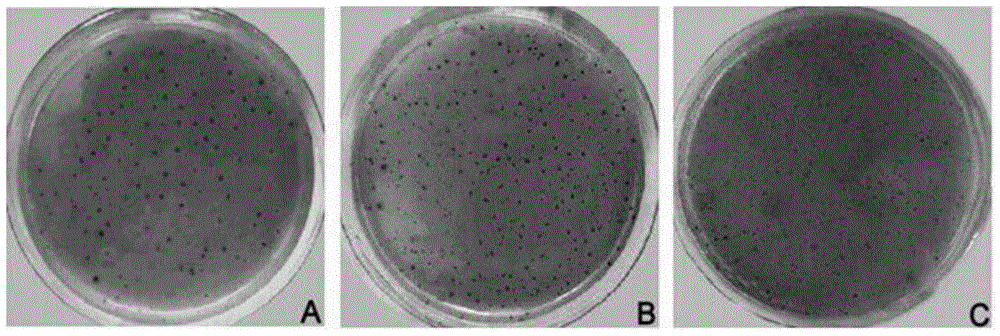
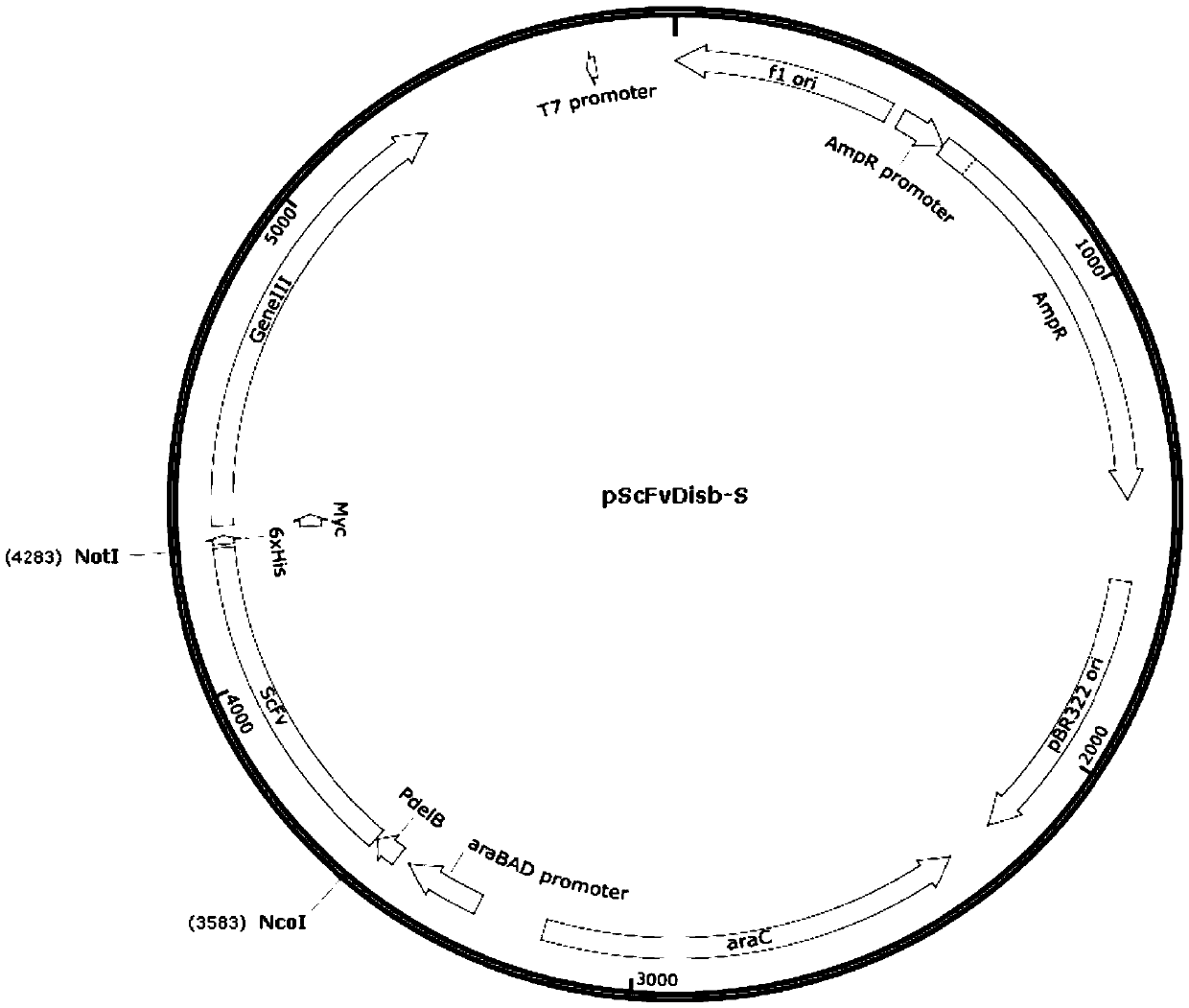
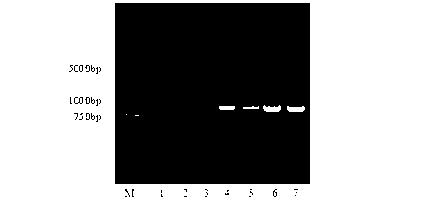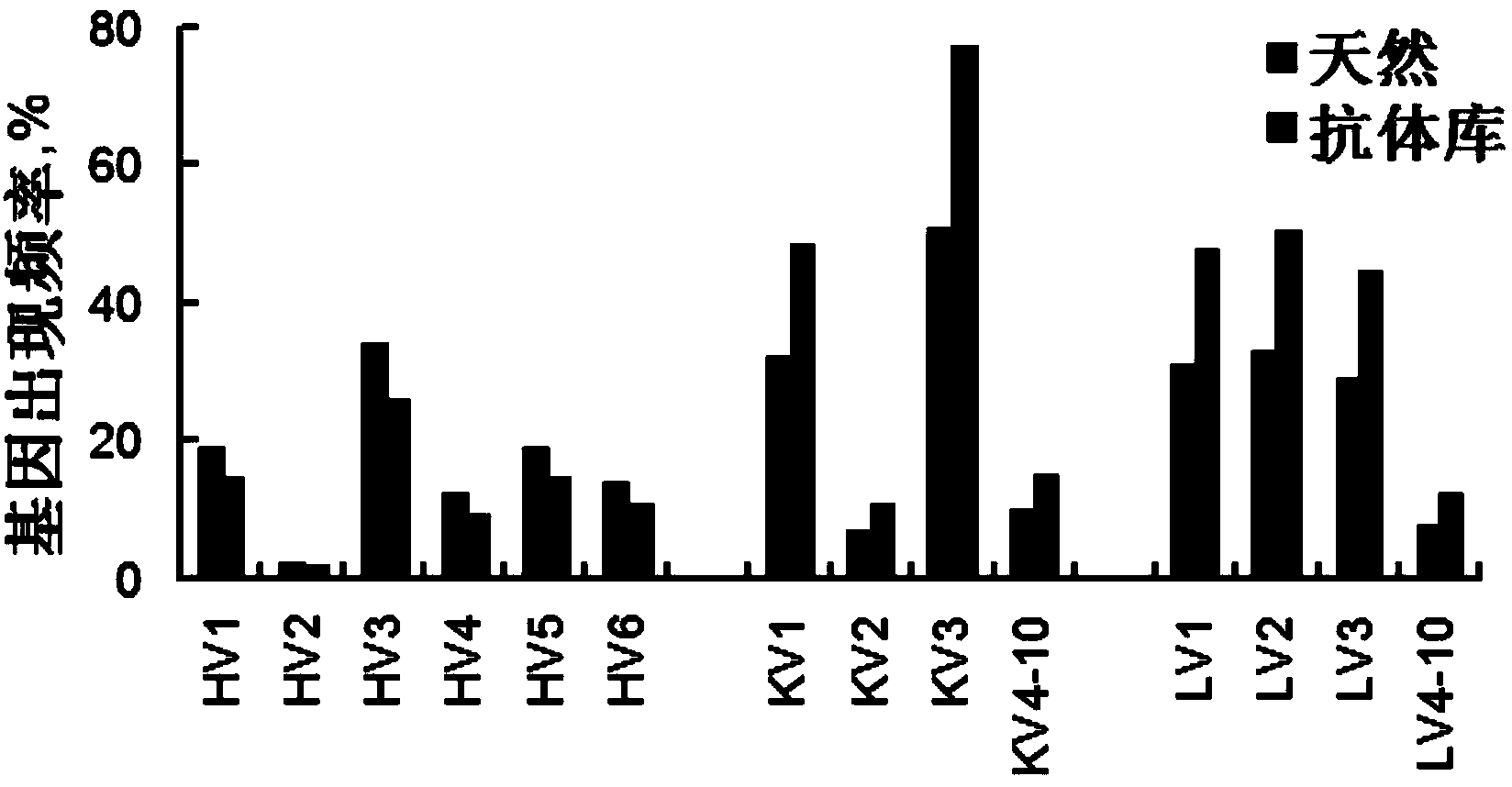Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
119 results about "Phage antibodies" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
SARS-CoV-2 (severe acute respiratory syndrome-corona virus disease-2) inhibitor and application thereof
InactiveCN111333722AHigh affinityRealize standardized productionBiological material analysisImmunoglobulins against virusesPhage antibodiesAntibody fragments
The invention relates to an SARS-CoV-2 (severe acute respiratory syndrome-corona virus disease-2) inhibitor and application thereof and in particularly relates to a neutralizing antibody for SARS-CoV-2 and application of the neutralizing antibody. According to the antibody, a phage display technology is adopted to construct a high-capacity human immunity phage antibody library, and an SARS-CoV-2 protein is adopted a target, human antibody single-chain antibody fragments are screened, and an antibody with a good neutralizing function on SARS-CoV-2 viruses is obtained. The antibody provided by the invention can be used for treating diseases caused by infection of novel corona viruses, and has significant clinical application value.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE CONTROL & PREVENTION PUBLIC HEALTH RES INST OF JIANGSU PROVINCE
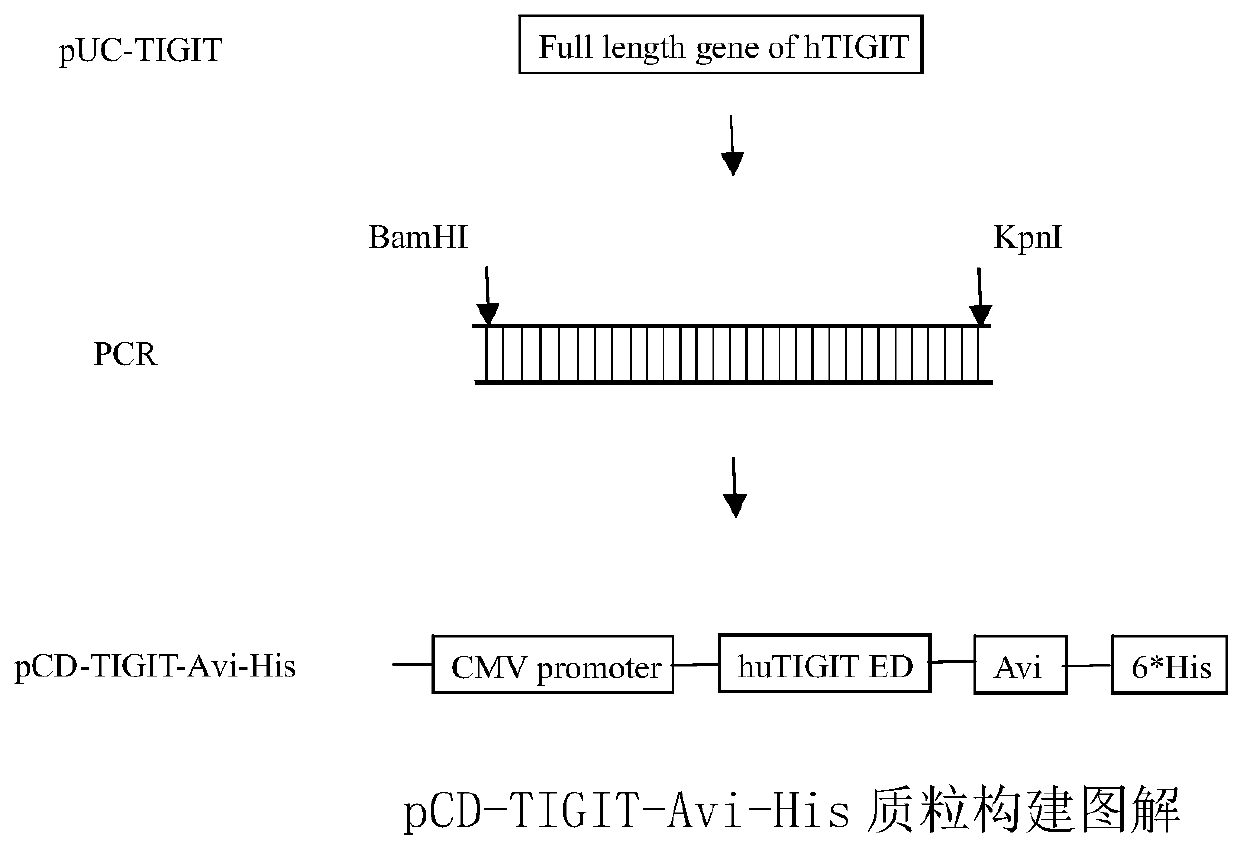
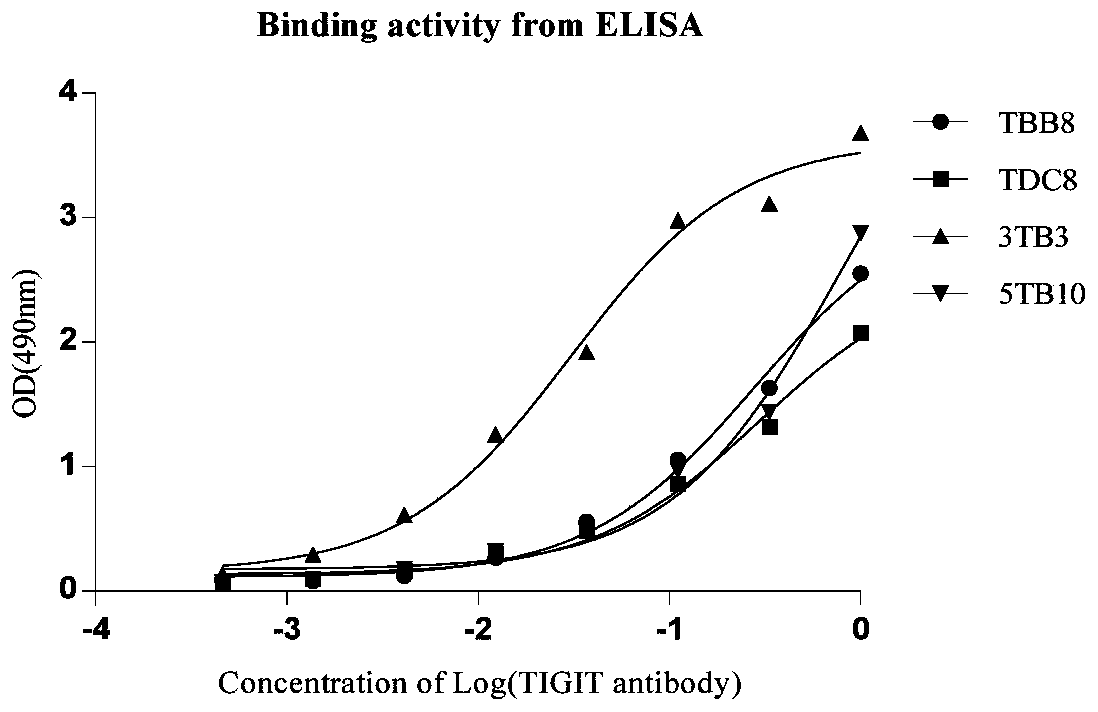
Full-human huTIGIT monoclonal antibody and application thereof
The invention relates to an anti-human TIGIT monoclonal antibody which is particularly related to high-affinity anti-human TIGIT monoclonal antibody with blocking activity. A large-capacity fully-synthetic human phage antibody library is constructed, the specific anti-human TIGIT monoclonal antibody and the high-affinity anti-human TIGIT monoclonal antibody which is optimized after molecular evolution are screened from the human phage antibody library, and the monoclonal antibody comprises a full-human frame region and variable regions of a full-human light chain and a heavy chain.
Owner:ANHUI ANKE BIOTECHNOLOGY (GRP) CO LTD
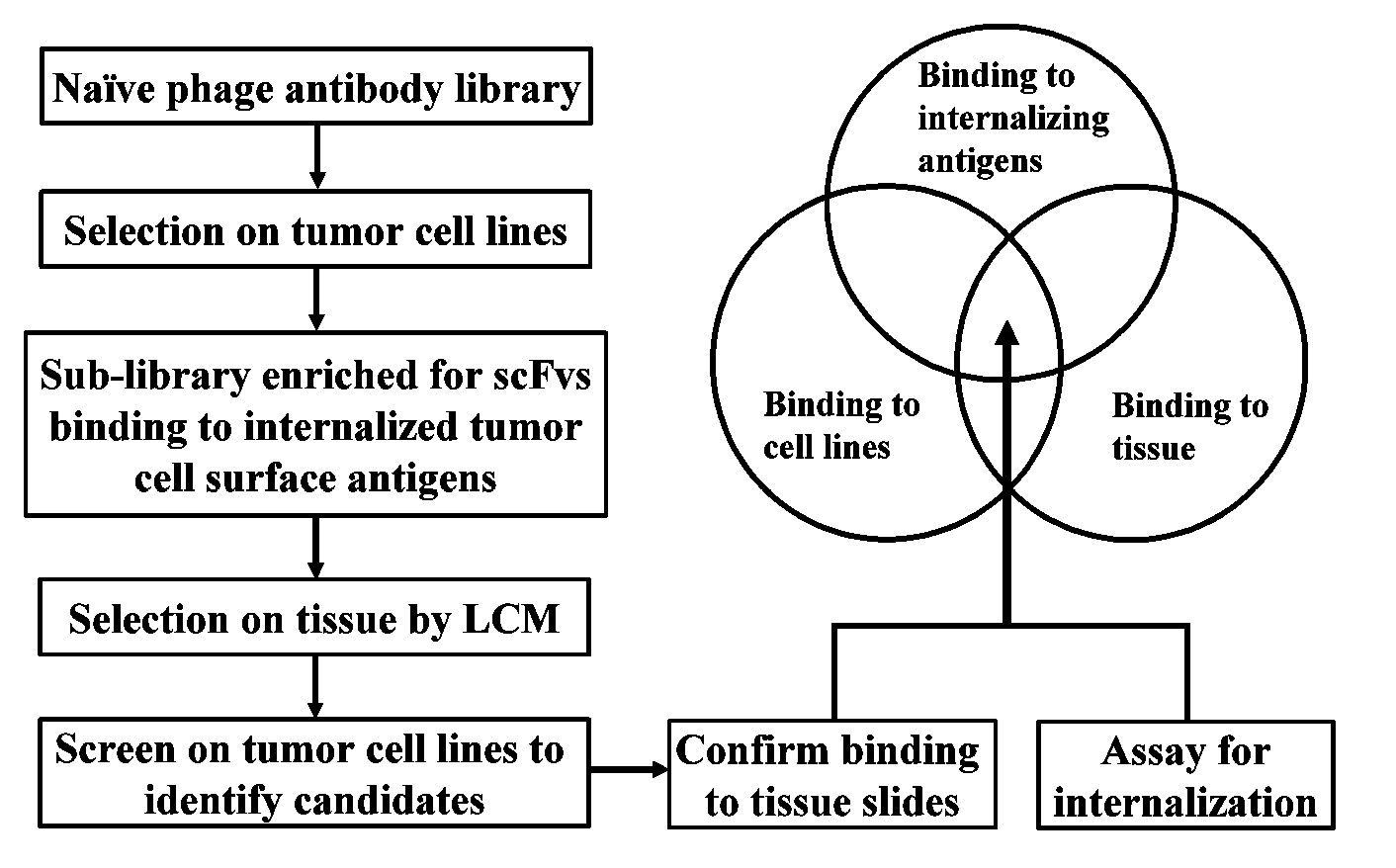
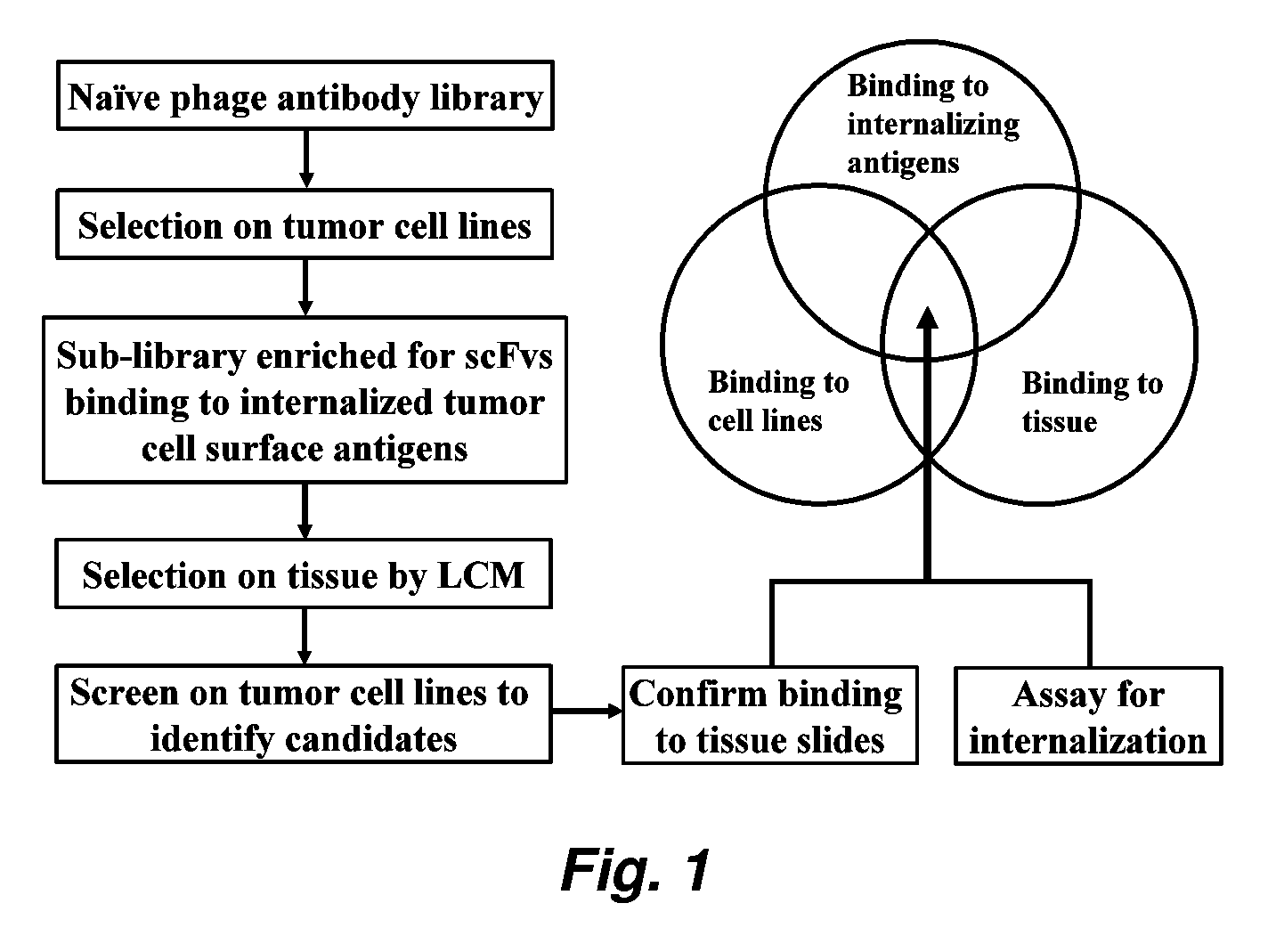
Internalizing human monoclonal antibodies targeting prostate cancer cells in situ
ActiveUS20100233165A1Easy to addImprove stabilityIn-vivo radioactive preparationsSugar derivativesProstate cancer cellPhage antibodies
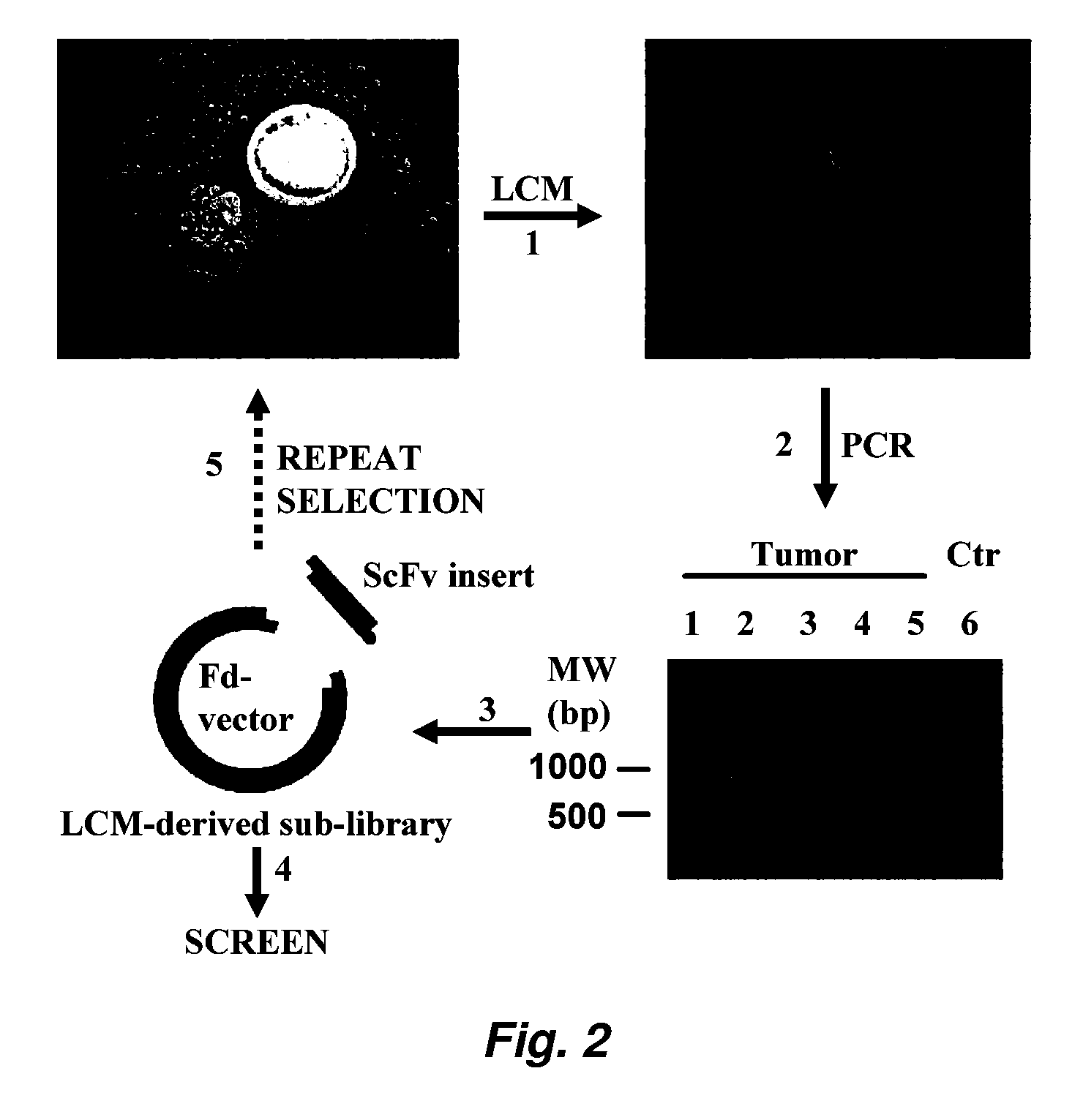
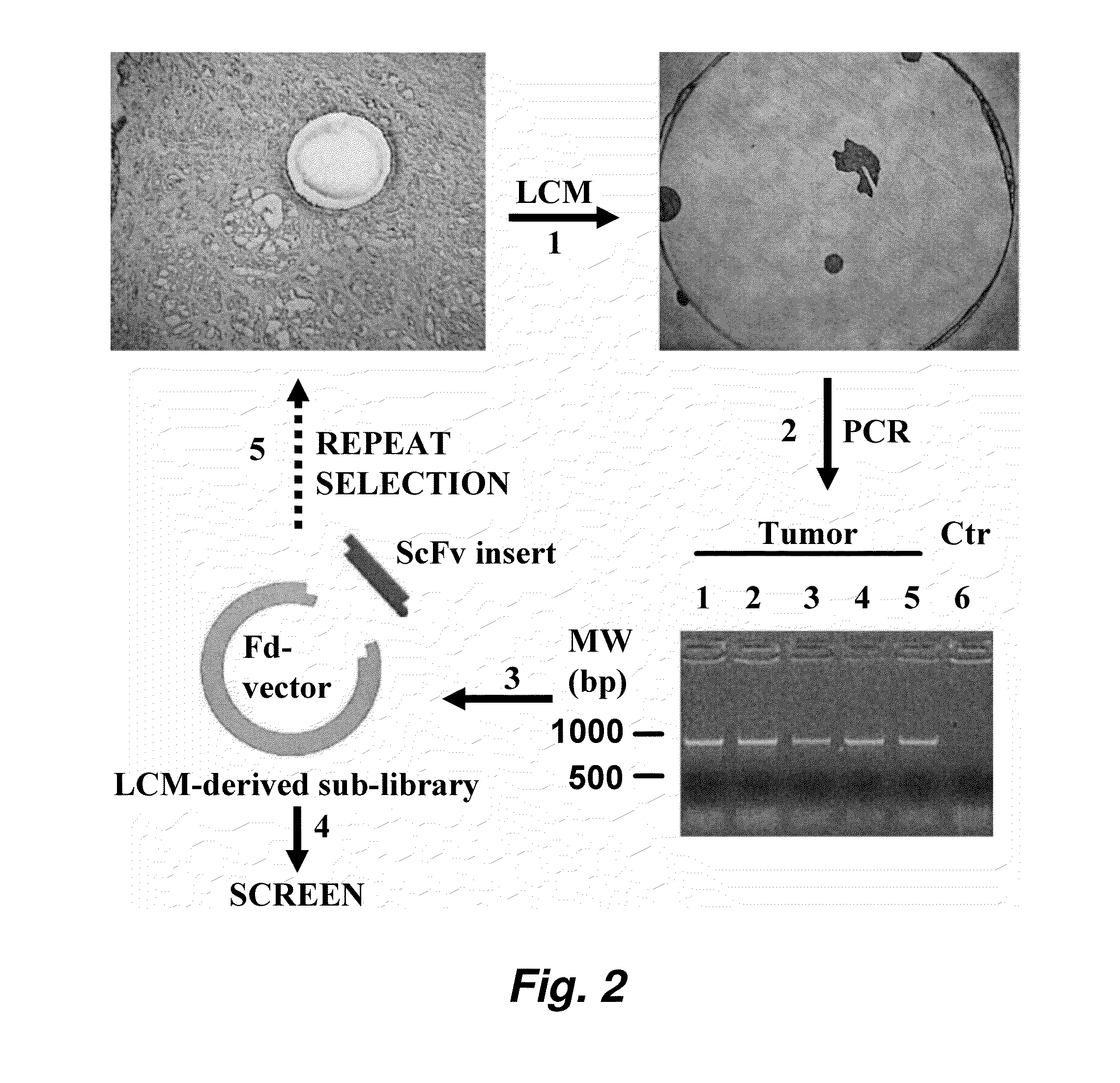
This invention provides a method that allows selection of antibodies against cells (e.g., tumor cells) in situ using laser capture microdissection. By restricting antibody selection to binders of internalizing epitopes, a panel of phage antibodies was generated that targets clinically represented prostate cancer antigens.
Owner:RGT UNIV OF CALIFORNIA
Internalizing human monoclonal antibodies targeting prostate cancer cells in situ
ActiveUS8865873B2Easy to addImprove stabilitySugar derivativesMicrobiological testing/measurementEpitopeProstate cancer cell
This invention provides a method that allows selection of antibodies against cells (e.g., tumor cells) in situ using laser capture microdissection. By restricting antibody selection to binders of internalizing epitopes, a panel of phage antibodies was generated that targets clinically represented prostate cancer antigens.
Owner:RGT UNIV OF CALIFORNIA
Coding gene of anti-Cry1Ac toxin single-chain variable fragments (scFv) and immuno-polymerase chain reaction (PCR) detection method
InactiveCN102936598ACoding sequences are not readily availableEnhanced detection signalMicrobiological testing/measurementImmunoglobulins against bacteriaPhage antibodiesSingle-Chain Antibodies
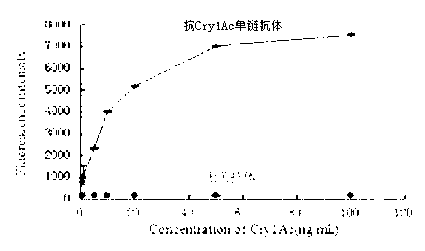
The invention relates to a coding sequence of anti-Cry1Ac scFv and an immuno-PCR detection method for detecting Cry1Ac through the phage scFv. The scFv are obtained through elutriation in a natural phage antibody base according to the method. The method is characterized in that the specificity of the scFv is determined by a CDR region sequence in a heavy chain and light chain variable region sequence, immuno-PCR results indicate that a fluorescence detection system can be used for quantitative detection of the Cry1Ac, and the detection range is 0.2-100ng / mL. The method is fast, simple and high in flexibility; a step for marking reporter DNA molecules during an existing immuno-PCR process is avoided, the experimental operation is simplified, and potential practical values are provided.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
A completely humanized anti-VEGFR-2 monoclonal antibody and a preparing method thereof
ActiveCN105646710AHigh affinityPrevent proliferationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsMetastatic renal cell cancerDisease
The invention discloses a completely humanized anti-VEGFR-2 monoclonal antibody, and discloses a method of screening by adopting a phage antibody library technique and preparing the novel anti-VEGFR-2 monoclonal antibody or a segment thereof by utilizing a genetic engineering manner. The antibody can be a univalent antibody or a bivalent antibody. The antibody can interdict VEGF-A, VEGF-C, VEGF-D and VEGF-E simultaneously so that the antibody has more effective anti-angiogenesis effects. The antibody can be applied for treating diseases caused by tumor angiogenesis. The diseases comprise, but not limited to, gastric cancer, adenocarcinoma of esophagogastric junction, non-small cell lung cancer, metastatic non-small cell lung cancer, hepatocellular carcinoma, metastatic hepatocellular carcinoma, HER2-negtive metastatic breast cancer, metastatic colorectal cancer, metastatic melanoma, metastatic renal cell carcinoma, glioblastoma, ovarian cancer, prostate cancer and solid tumor.
Owner:SICHUAN KELUN BIOTECH BIOPHARMACEUTICAL CO LTD
Antibody library of bacteriophages and applications in immunoassay of pesticide residue
InactiveCN101289760AWide range of affinity optionsIncrease screening throughputPeptide librariesMicroorganism librariesSingle-Chain AntibodiesPesticide residue
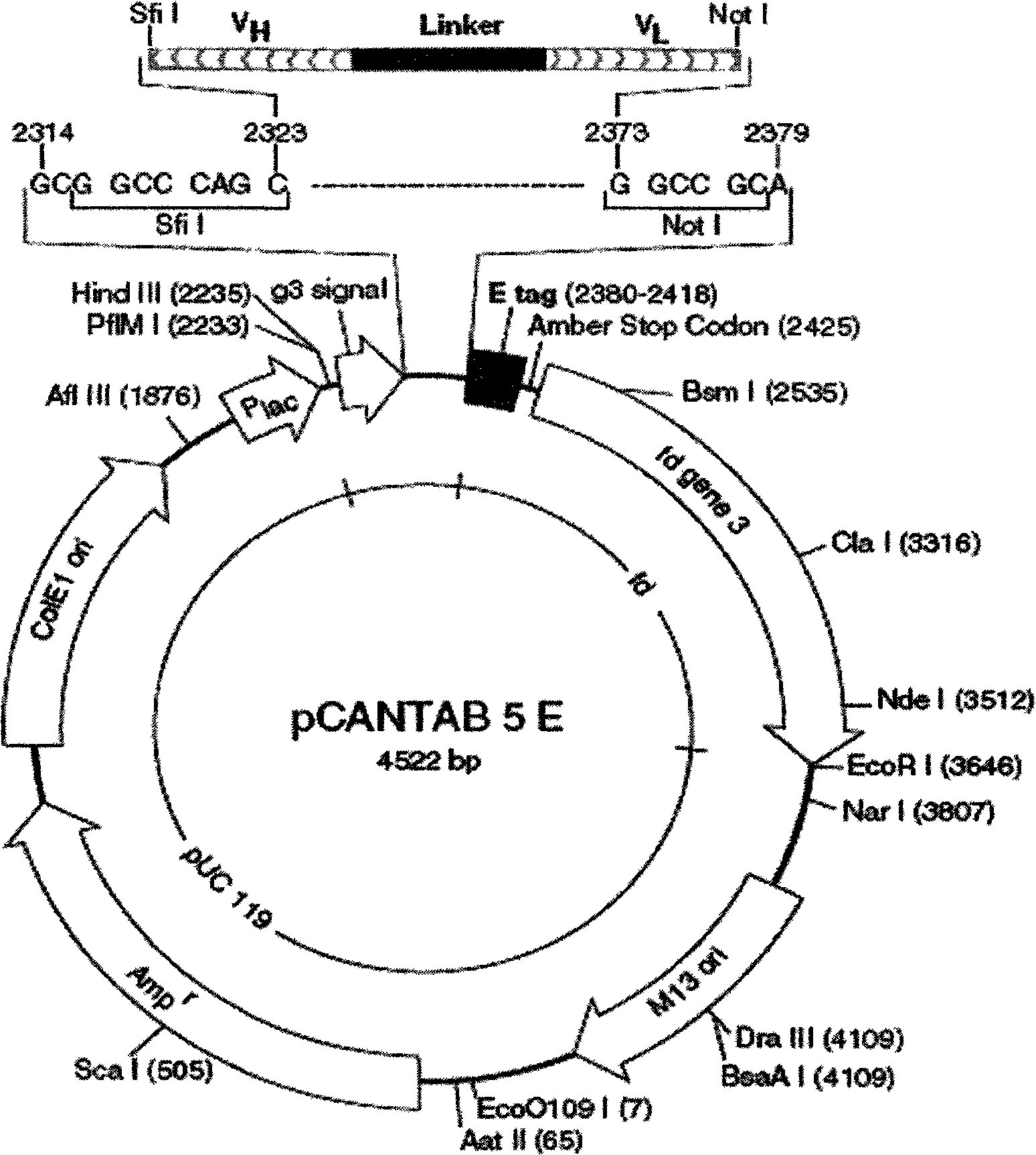
The invention relates to a phage antibody library which assembles an ScFv gene fragment between Restriction Enzyme cutting sites of SfiI and NotI of a pCANTAB5E carrier. The phage antibody library is characterized in that the ScFv gene fragment can be affinitive and enriched with antigens of 16-membered macrolide agricultural chemicals to form a soluble single-chain antibody and the phage antibody library is applied to immunoassay of pesticide residue. The phage antibody library has the advantages that the antibody with high affinity can be obtained without animal immunization, the test period is short, the antibody library is the phage antibody library which takes small molecular milbemycin oxime of a 16-membered macrolide generic structure as immunogen to construct, the antibody library theoretically can directly obtain a specific antibody library of 16-membered macrolide compound through screening, the screening flux is high, the efficiency is high, the specificity is strong, and the affinity selecting range is wide, so that the phage antibody library has wide application prospect in the aspects such as agricultural chemical antibody preparation, testing technique development and the like.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Fully-humanized anti-human interleukin 17A single-chain antibody
The invention discloses a fully-humanized anti-human interleukin 17A single-chain antibody, relates to the technical field of genetically engineered antibodies and specifically relates to a fully-humanized antibody fragment which is genetically engineered, screened, expressed and provided with special affinity with interleukin 17A (IL-17A). The genetically engineered antibody fragment is connected with a heavy chain region and a light chain region end to end by virtue of a soft connecting fragment. By constructing a great-capacity natural phage antibody library, an antibody fragment with special adsorption capacity on IL-17A is obtained by biological elutriation. The antibody fragment is constructed into a full-length antibody, so that the reaction of IL-17A on interleukin 6(IL-6) released by a human fibrosarcoma cell HT1080 can be inhibited by virtue of eukaryotic secretory expression and affinity purification. The antibody fragment disclosed by the invention can be used for detecting and treating rheumatoid arthritis.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI +1
Anti-VEGFR (vascular endothelial growth factor receptor) 2 monoclonal antibody and application thereof
ActiveCN106892980AIncrease calcium ion concentrationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesVascular proliferation
The invention belongs to the field of antibody drug, and discloses a full human-derived anti-human VEGFR2 monoclonal antibody screened by a phage antibody base technology and prepared by a gene engineering method, and further discloses a carrier including polynucleotide for encoding the monoclonal antibody, a host cell and an application. Through combining with VEGFR2, the monoclonal antibody stops the combination of VEGF with VEGR2, and does not induce receptor dimerization, cause the following tyrosine residue phosphorylation in intracellular tyrosine kinase structure and activate downstream signal access including activation of phospholipase C and increase of calcium ion concentration in the cell and others; the antibody drug triggers endothelial proliferation, survival, cytoskeleton rearrangement, cell migration, gene expression and others, and finally stops the vascular proliferation caused by combination of VEGF and VEGFR2 and induction of dimerization. The monoclonal antibody can be used for treating diseases caused by tumor angiogenesis.
Owner:CHANGCHUN GENESCIENCE PHARM CO LTD
Fully human anti-VEGF (Vascular Endothelial Growth Factor) monoclonal antibody and preparation method as well as application thereof
ActiveCN102167740AAntibody affinity is highInhibition of proliferative abilityDigestive systemImmunoglobulins against growth factorsPhage antibodiesAntiendomysial antibodies
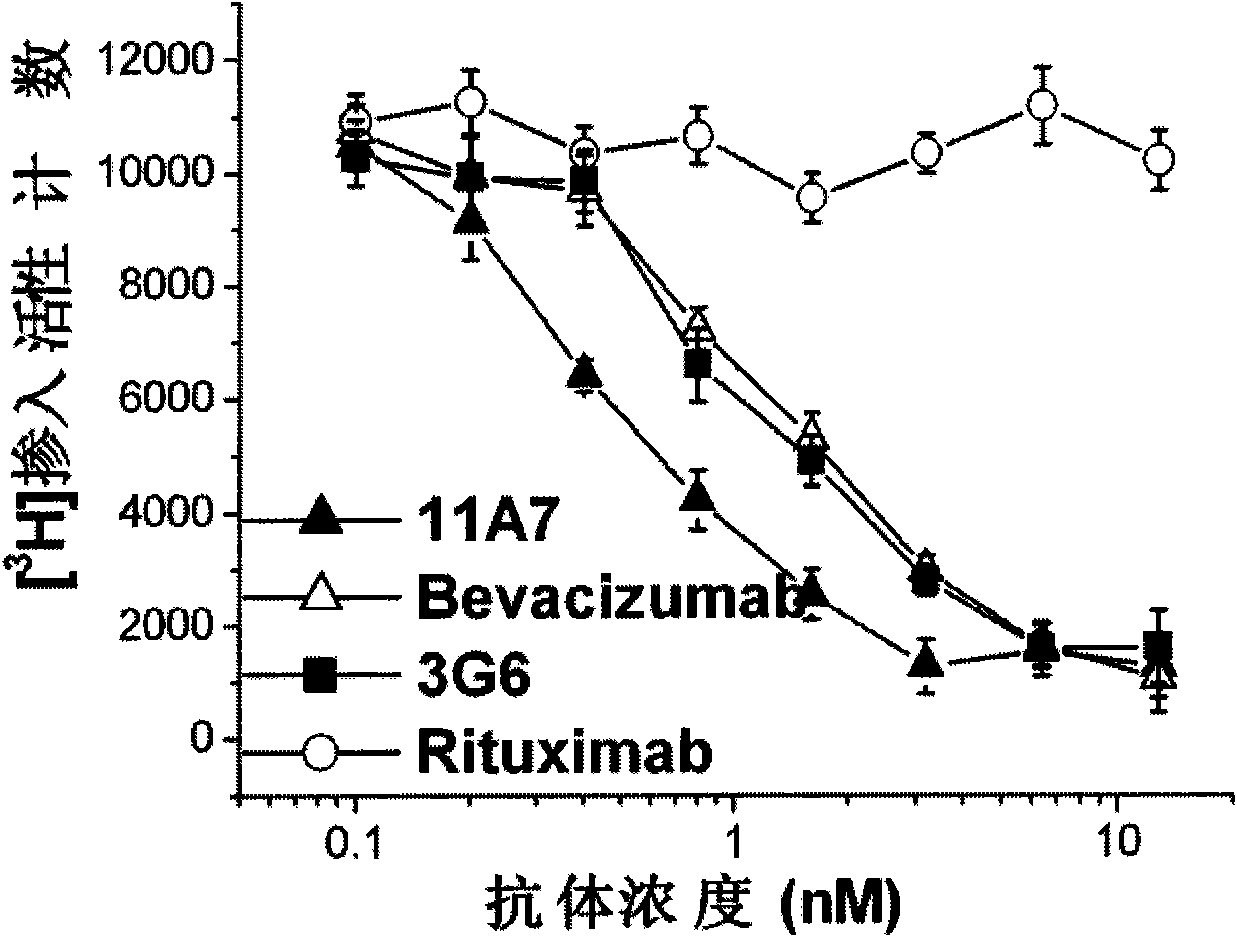
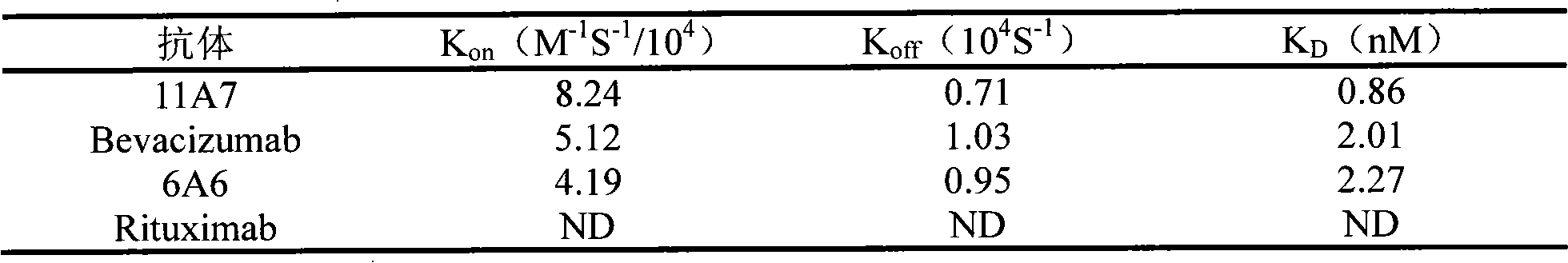
The invention relates to the field of biotechnology, in particular to a fully human anti-VEGF (Vascular Endothelial Growth Factor) monoclonal antibody and a preparation method as well as application thereof. In the invention, a large-capacity natural human phage antibody library is constructed; a fully human anti-VEGF antibody 11A7 is screened from the library; an amino acid sequence of a heavy chain variable region of the antibody is shown as SEQ ID NO: 6; and an amino acid sequence of a light chain variable region of the antibody is shown as SEQ ID NO: 8. The invention also discloses the preparation method of the 11A7 antibody, a nucleotide sequence encoding the 11A7 antibody, a nucleic acid sequence-containing expression vector and a host cell. Compared with a humanized antibody bevacizumab, the 11A7 antibody has higher affinity of the antibody and higher capacity of suppressing proliferation of tumor cells and can be used for obviously suppressing growth of tumors and preparing anti-tumor medicaments.
Owner:YUEHAI BIOPHARM SHAOXING LTD SHAOXING CITY
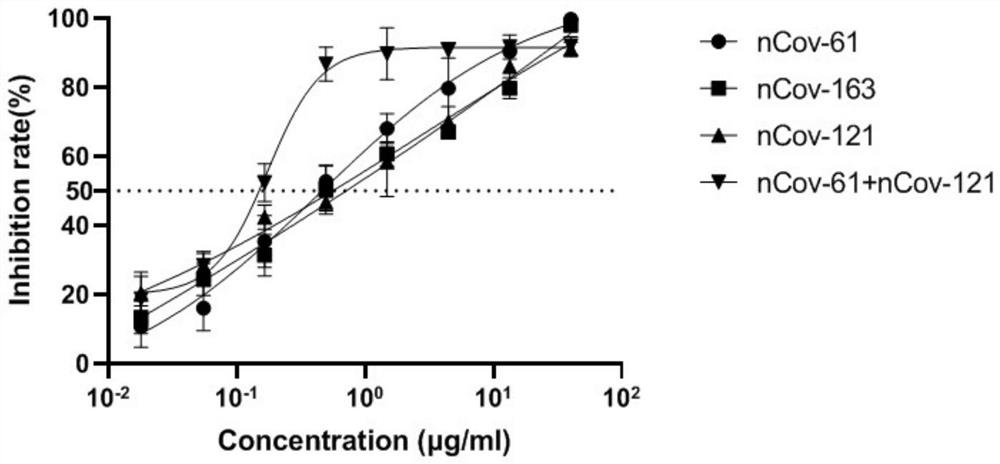
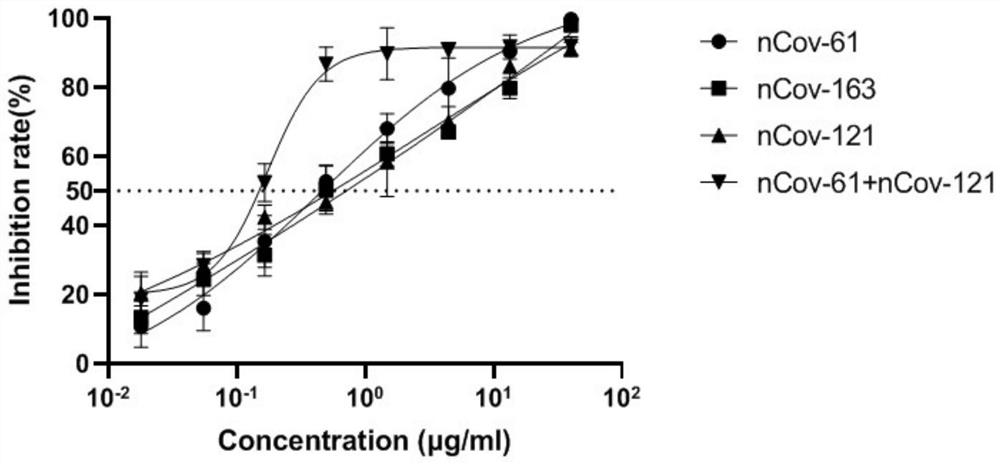
Humanized anti-novel coronavirus neutralizing antibody nCoV-61 and application thereof
ActiveCN112430265AHigh affinityImmunoglobulins against virusesAntiviralsPhage antibodiesNeutralizing antibody
The invention discloses a humanized anti-novel coronavirus neutralizing antibody nCoV-61 and an application thereof. The humanized neutralizing antibody nCoV-61 specific to the novel coronavirus surface antigen is successfully obtained by utilizing a phage antibody library technology, has a neutralizing function of preventing the novel coronavirus from infecting sensitive cells in vitro, and is high in antigen affinity, and the antibody is expected to be prepared into specific antibody drugs for preventing and treating novel coronavirus pneumonia clinically.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Methods of preventing adhesions following laminectomies and other surgical procedures
InactiveUS20050074453A1Good treatment effectImprove effectivenessPeptide/protein ingredientsImmunoglobulins against growth factorsPhage antibodiesSpinal nerve
Adhesions and scar formation following a surgical procedure are controlled by providing a human recombinant phage antibody, and introducing the antibody onto or into an area of the body following the procedure to inhibit adhesions, or scar formation. According to one embodiment, the antibody is used to prevent the formation of scar tissue following spinal surgery. This may be carried out by placing the antibody over the dura lining the spinal nerves and spinal cord. Alternatively, the antibody may be used to inhibit adhesions following abdominal surgery, or placed around the great vessels following an anterior approach to the spine or other regions. Importantly, the antibody may be used to inhibit adhesion formation adjacent to areas where growth factors are used to stimulate healing. The invention may further include the step of protecting the growth factors and / or the area of the body where stimulated healing is desired from the antibodies to the growth factors. As a further option, other medications or therapeutic substances may be added to the antibody(ies) to enhance healing or effectiveness.
Owner:FERREE BRET A
Humanized anti-novel coronavirus neutralizing antibody nCoV-121 and application thereof
ActiveCN112409479AHigh affinityImmunoglobulins against virusesAntiviralsPhage antibodiesBacteriophage
The invention discloses a humanized anti-novel coronavirus neutralizing antibody nCoV-121 and application thereof. The humanized neutralizing antibody nCoV-121 specifically aiming at a novel coronavirus surface antigen is successfully obtained with a phage antibody library technology, has a neutralizing function of preventing the novel coronavirus from infecting sensitive cells in vitro and is high in antigen affinity, thereby being expected to be prepared into specific antibody drugs for preventing and treating novel coronavirus pneumonia clinically.
Owner:中国疾病预防控制中心病毒病预防控制所
Phage antibodies to radiation-inducible neoantigens
InactiveUS20080187488A1Ultrasonic/sonic/infrasonic diagnosticsPowder deliveryAntigenPhage antibodies
A method for identifying a molecule that binds an irradiated tumor in a subject and molecules identified thereby. The method includes the steps of: (a) exposing a tumor to ionizing radiation; (b) administering to a subject a library of diverse molecules; and (c) isolating from the tumor one or more molecules of the library of diverse molecules, whereby a molecule that binds an irradiated tumor is identified. Also provided are therapeutic and diagnostic methods using targeting ligands that bind an irradiated tumor.
Owner:VANDERBILT UNIV
Rabies virus resistant specific humanized antibody and application thereof
ActiveCN104193823AStrong neutralizing activityImmunoglobulins against virusesAntiviralsPhage antibodiesBacteriophage
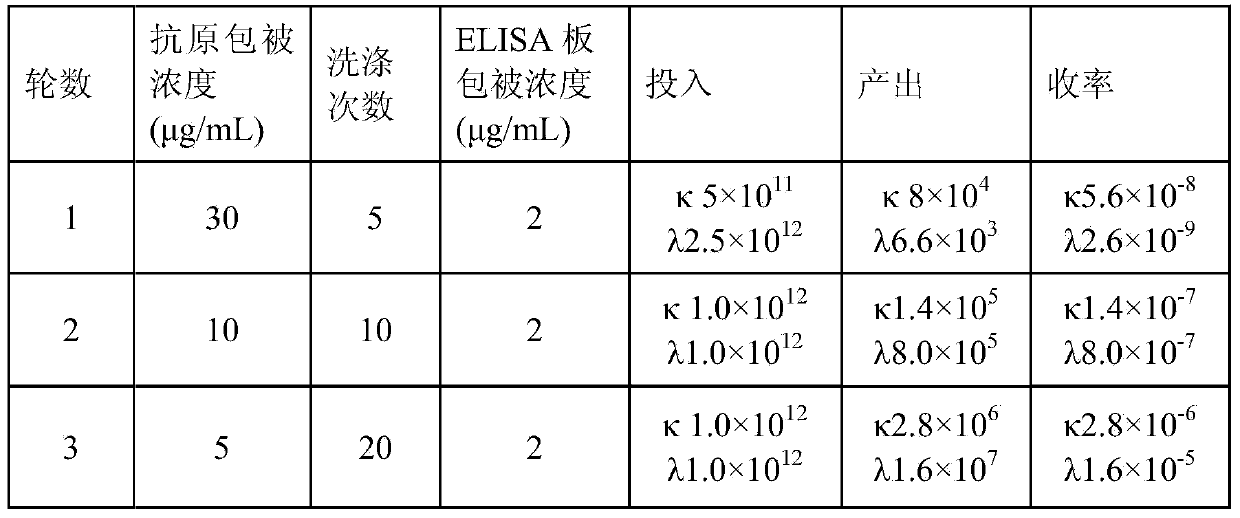
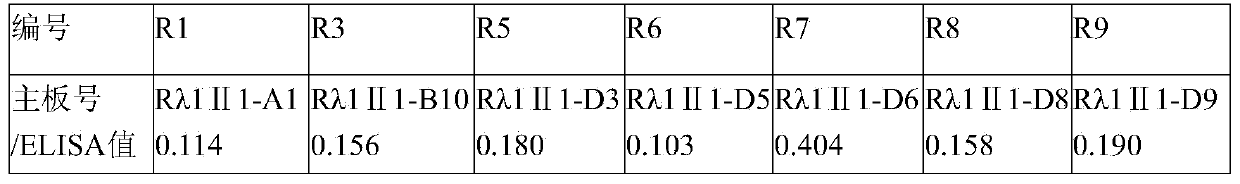
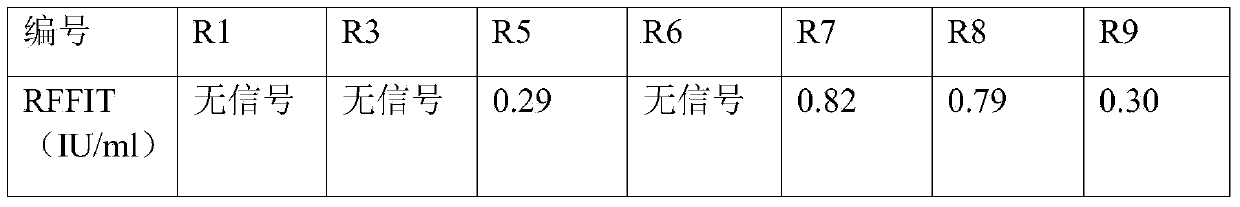
The invention aims at providing a rabies virus resistant neutralizing antibody, and particularly provides a humanized or completely humanized monoclonal antibody to meet the requirement for clinically diagnosing and / or treating rabies. A phage antibody library is prepared by adopting a phage antibody library technology and taking 32 parts of high-potency healthy human peripheral blood inoculated with rabies vaccines as a raw material; 7 ELISA positive antibodies are obtained through three rounds of screening from the phage antibody library; and furthermore, the neutralizing activity of the 7 ELISA positive antibodies is measured through an RFFIT method, wherein four ELISA positive antibodies, namely R5, R7, R8 and R9, have higher neutralizing activity in all. The rabies virus resistant neutralizing antibody with high affinity, which is provided by the invention, can be used for substituting ERIG and HRIG to carry out active and / or passive immune therapy on rabies virus seriously-exposed persons.
Owner:LANZHOU INST OF BIOLOGICAL PROD
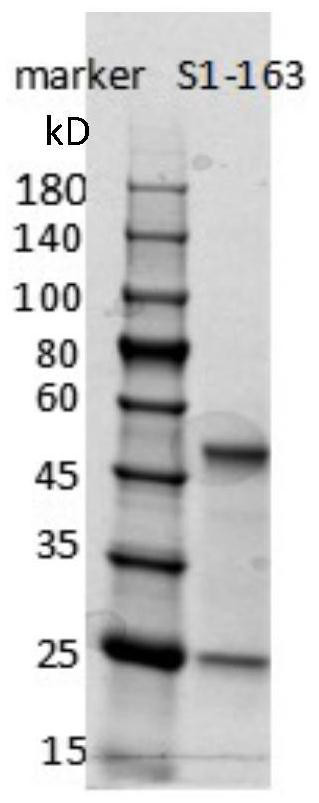
Humanized anti-novel coronavirus neutralizing antibody nCoV-163 and application thereof
ActiveCN112341541AHigh affinityImmunoglobulins against virusesAntiviralsPhage antibodiesPharmaceutical drug
The invention discloses a humanized anti-novel coronavirus neutralizing antibody nCoV-163 and application thereof. According to the humanized anti-novel coronavirus neutralizing antibody nCoV-163 andthe application thereof, the humanized neutralizing antibody nCoV-163 specifically aiming at a novel coronavirus surface antigen is successfully obtained by utilizing a phage antibody library technology, the antibody nCoV-163 is provided with a neutralizing function of preventing novel coronavirus from infecting sensitive cells in vitro and is high in antigen affinity, and the antibody is expectedto be prepared into specific antibody drugs for preventing and treating novel coronavirus pneumonia clinically.
Owner:中国疾病预防控制中心病毒病预防控制所
Anti-IL-1beta monoclonal antibody and application thereof
The invention provides the technical field of antibody medicine, discloses a fully-humanized human anti-IL-1beta monoclonal antibody screened through the phage antibody library technology and prepared through the genetic engineering technology, and discloses a DNA molecule for encoding the monoclonal antibody, a DNA molecule expression carrier for encoding the monoclonal antibody, a host cell and application. The anti-IL-1beta monoclonal antibody can prevent bonding of human IL-1beta and human IL-1R. By means of bonding of IL-1beta, blocking of IL-1beta and a receptor signal channel of the IL-1beta, unnecessary IL-1beta initial induced heat transfer, amplified lymphocyte responses and stimulation acute stage responses can be reduced. The anti-IL-1beta monoclonal antibody can be used for detecting IL-1beta expressions and used for prevention and treatment of cryopyrin-associated periodic syndromes.
Owner:CHANGCHUN GENESCIENCE PHARM CO LTD
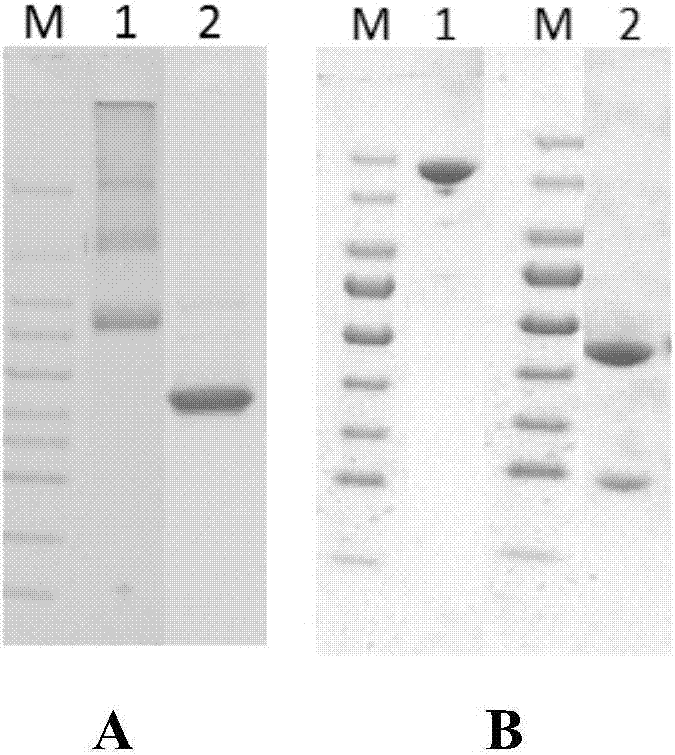
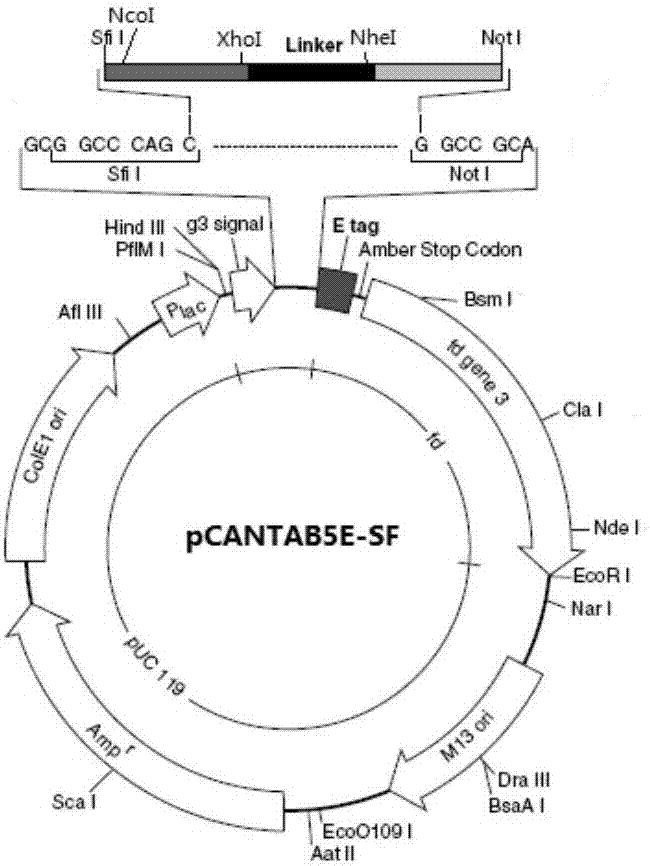
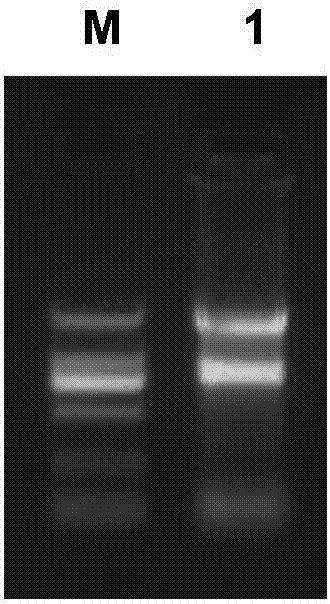
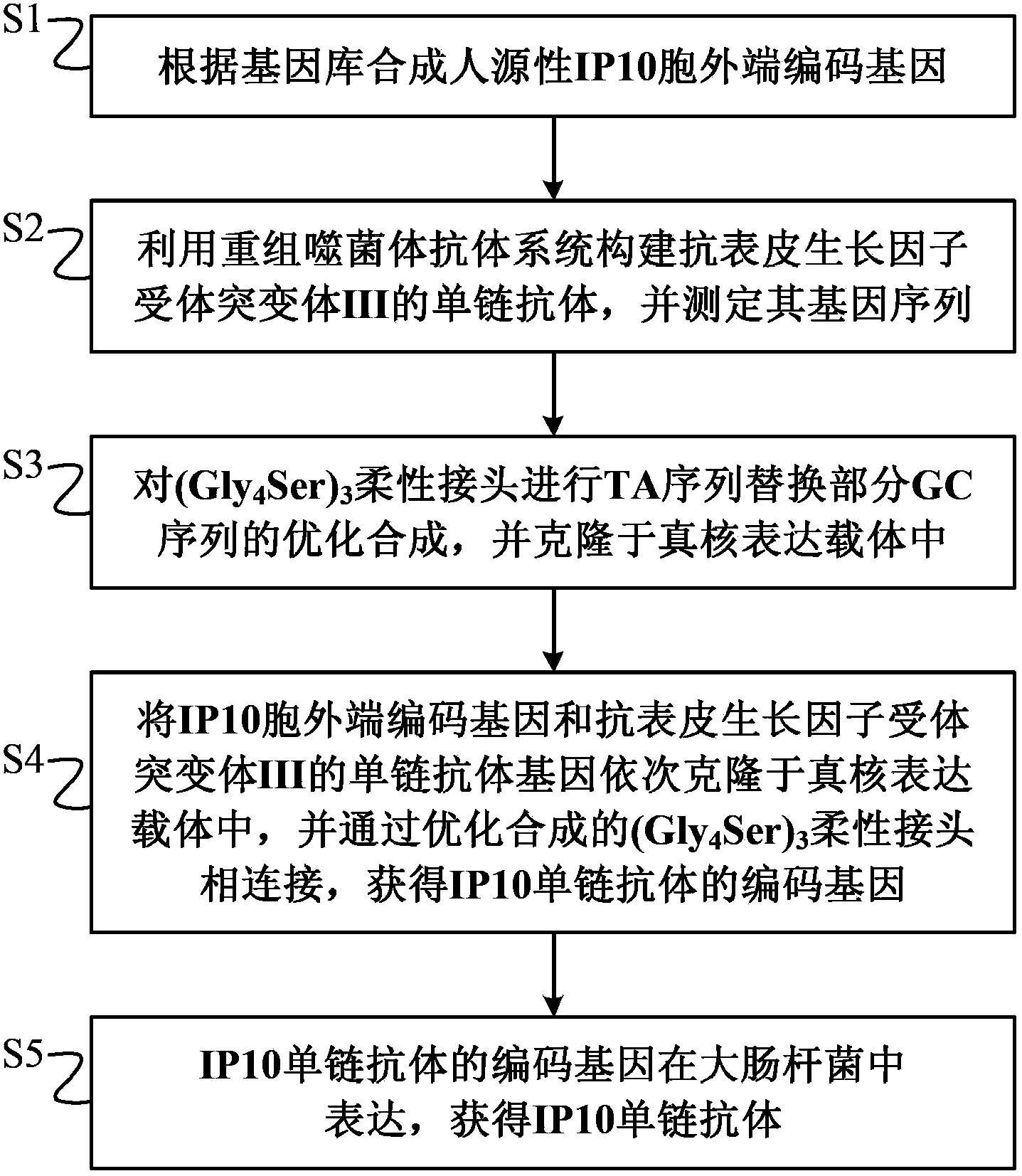
Preparation method of novel cytokine fusion protein IP10 single-chain antibody
InactiveCN103451218AEnhance targeted killing activityImprove targetingMicroorganism based processesIn-vivo testing preparationsEscherichia coliPhage antibodies
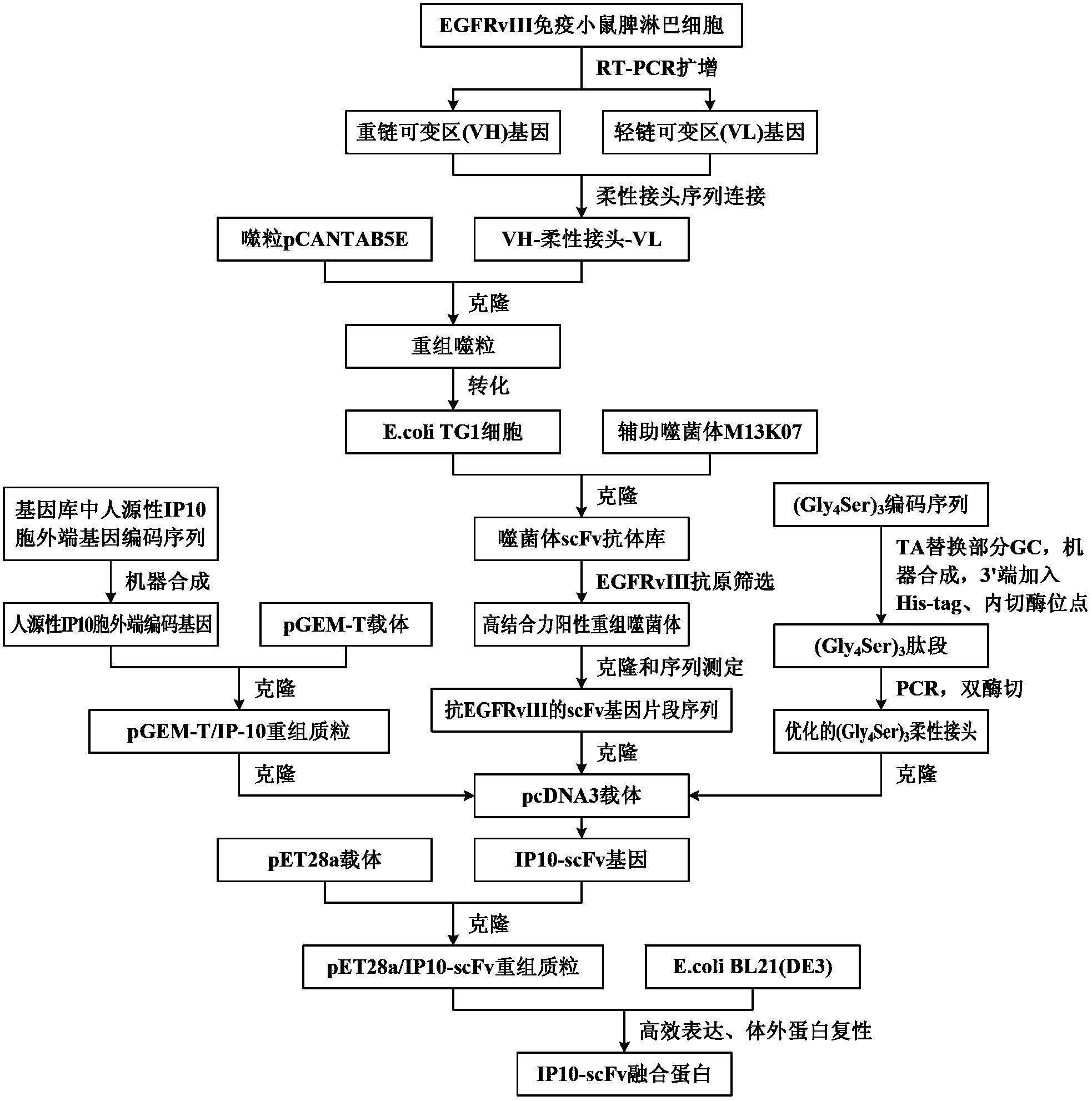
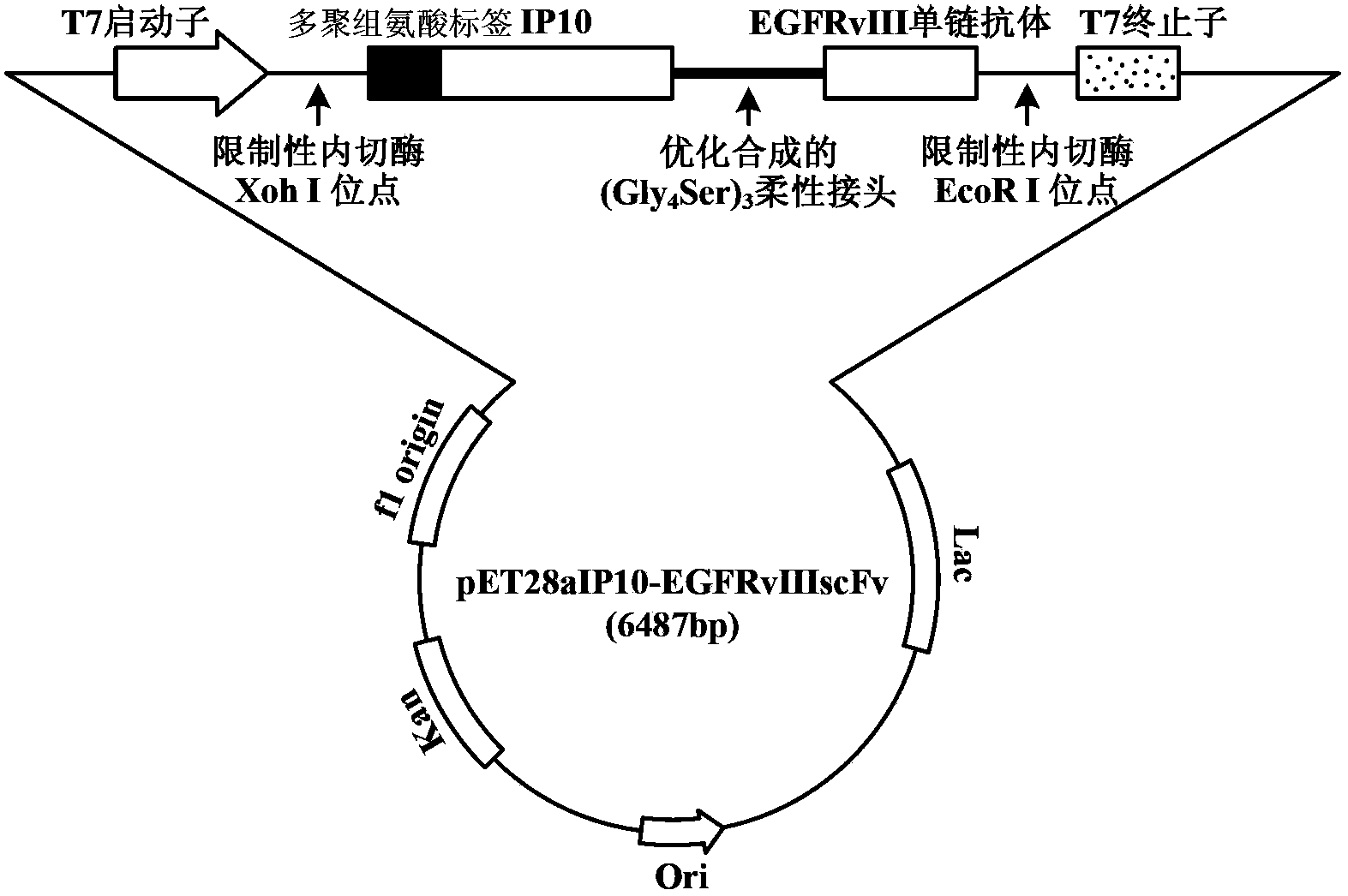
The invention discloses a preparation method of a novel cytokine fusion protein-chemokine interferon inducible protein 10 single-chain antibody (IP10-scFv). The method comprises the steps of: S1. synthesizing a human IP10 extracellular terminal gene; S2. utilizing a recombinant phage antibody system to construct the single-chain antibody of an anti-epidermal growth factor receptor mutant III (EGFRvIII); S3. conducting optimization synthesis on a (Gly4Ser)3 flexible joint; S4. cloning IP10 and the anti-EGFRvIII single-chain antibody into an eukaryotic expression vector, and conducting connection through the optimized (Gly4Ser)3 flexible joint, thus obtaining an IP10-scFv gene; and S5. subjecting the gene to high expression in Escherichia coli, thus obtaining IP10-scFv with biological activity. The IP10-scFv provided in the invention has a higher antigen-antibody binding rate, can reduce the dosage of IP10 clinically and reduce the toxic and side effects, and further can reduce the adoptive input amount of glioma specific cytotoxic T lymphocyte (CTL) and increase the activity of CTL.
Owner:卢小玲 +1
Anti-Staphylococcus aureus alpha hemolysin monoclonal antibody and applications thereof
ActiveCN109384844AInhibit hemolytic activityHigh affinityAntibacterial agentsImmunoglobulins against bacteriaPhage antibodiesStaphylococcus cohnii
The invention discloses an anti-Staphylococcus aureus alpha hemolysin monoclonal antibody and applications thereof, wherein the monoclonal antibody comprises a heavy chain and a light chain, the aminoacid sequence of the variable region of the heavy chain is represented by the sequence 2 in the sequence listing, and the amino acid sequence of the variable region of the light chain is representedby the sequence 4 in the sequence listing. According to the present invention, the full human source anti-alpha hemolysin monoclonal antibody is rapidly screened from the phage antibody library by using the alpha hemolysin as the target antigen; and the obtained monoclonal antibody is the completely-new antibody, has high affinity, can highly inhibit the hemolytic activity of alpha-hemolysin, canimprove the survival rate of mice infected with Staphylococcus aureus, and further has important application value in the prevention and control of Staphylococcus aureus.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Human monoclonal antibody against IgE, preparation method and purpose thereof
ActiveCN102167745AAntibody affinity is highInhibition of degranulationAntibody ingredientsImmunoglobulinsNucleotideAllergic asthma
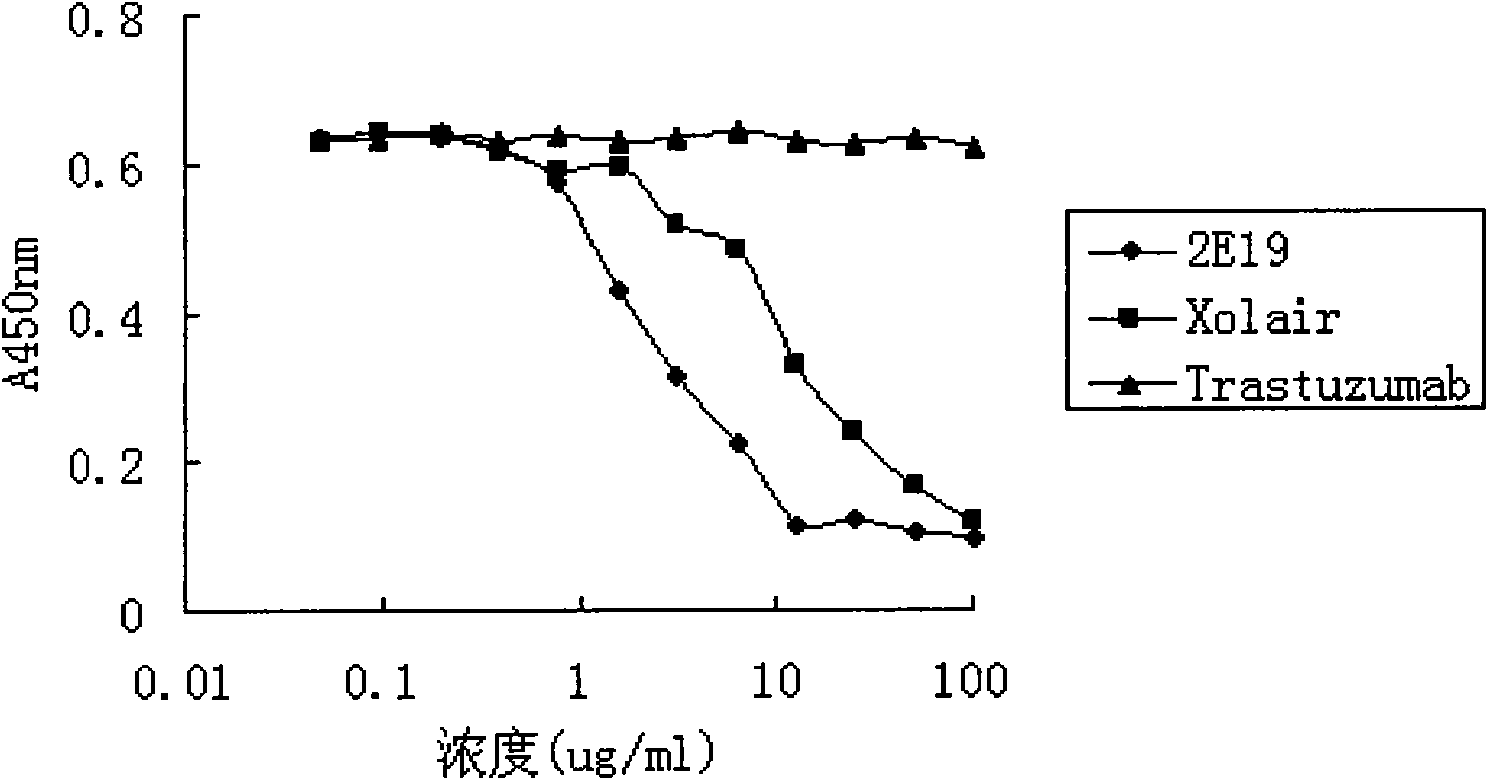
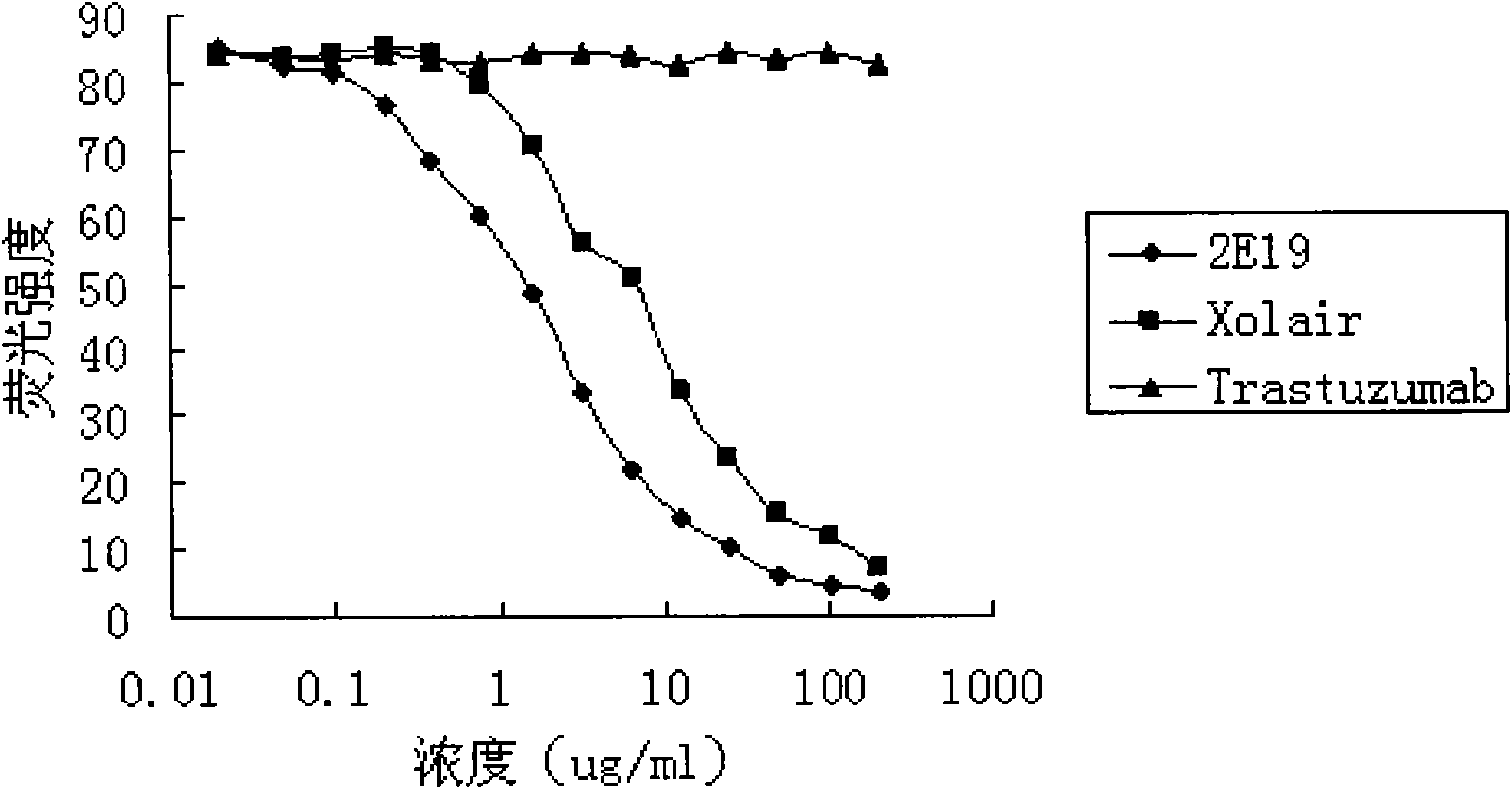
The invention relates to the biotechnology field, and more specifically, the invention discloses a human monoclonal antibody against IgE, a preparation method and a purpose thereof. According to the invention, a natural human phage antibody library of large capacity is constructed;; a human antibody 2E19 strain against IgE is obtained through screening from the library; an amino acid sequence of a heavy chain variable region is expressed as SEQ ID NO: 6; and the amino acid sequence of a light chain variable region is expressed as SEQ ID NO: 8. Besides, the invention also discloses the preparation method of the antibody 2E19, as well as a nucleotide sequence for encoding the antibody 2E19 and an expression vector and a host cell comprising the nucleotide sequence. The antibody 2E19 of the invention has a higher antibody affinity, so as to be able to obviously inhibit degranulation caused by the combination of IgE and FC epsilon RI on a cell surface, and the antibody 2E19 does not combine with the IgE combined with the FC epsilon RI on the cell surface. The antibody 2E19 of the invention could be used for the preparation of a medicine for treating hypersensitivity diseases, especially for treating allergic asthma.
Owner:越海百奥药业(绍兴)有限公司
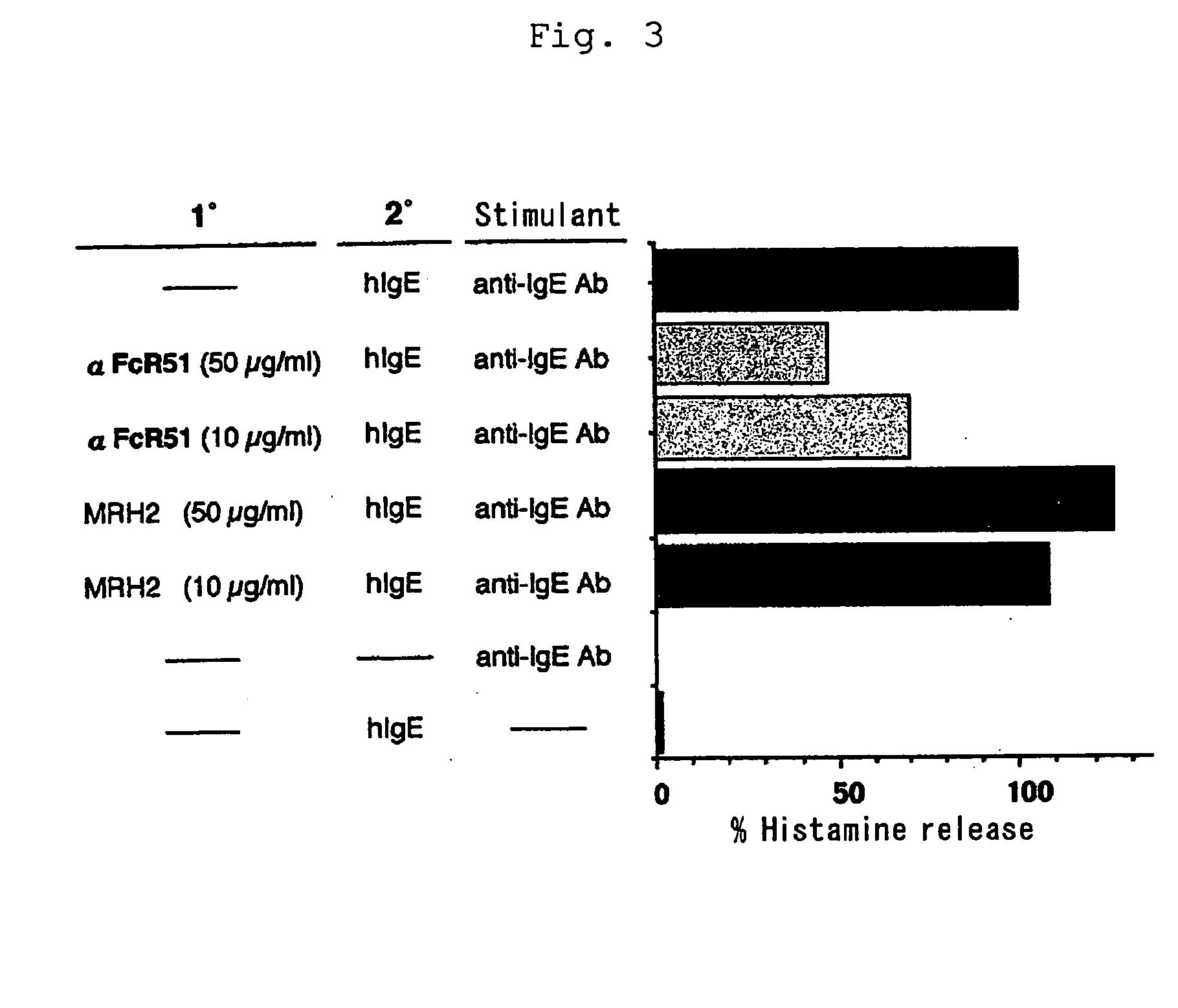
Human type antihuman ige receptor antibody and fragment
InactiveUS20050170451A1Specific and fundamental efficacyLack of efficacyAnimal cellsSugar derivativesPhage antibodiesAntibody fragments
An antibody or an antibody fragment is provided that is efficacious for treating allergic diseases in which IgE is involved. A human antibody and a fragment thereof to a receptor (FcεRI) having high affinity to Fc portion of IgE was obtained using phage antibody display technique. The human anti-IgE receptor antibody and a fragment thereof of the invention has an activity to inhibit the binding between IgE and IgE receptor and hence is expected to be useful as a medicament for treating allergic diseases caused by the binding between IgE and IgE receptor.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES INST
Anti-phosphatidylinositol proteoglycan 3 complete humanized antibody
InactiveCN105037540AImmunoglobulins against animals/humansAntibody ingredientsPhage antibodiesAntigen
The invention discloses a complete humanized antibody which is selected from humanized high-capacity phage antibody library and is high-affinity-combined with phosphatidylinositol proteoglycan 3. The invention includes a selection method of the antibody, an antibody coding sequence and corresponding amino acid residue sequence, and especially includes three CDR-zone sequences respectively at a heavy chain and a light chain, combination characters of an antigen and the construction method of the complete antibody. The antibody is a complete humanized antibody, is used for treatment in human body, is low in immunogenicity, is less in toxic and side effects, and has a potential value of treating liver cancer and melanin tumor.
Owner:BEIJING BIYANG BIOTECH
Full-humanized agonist single-chain antibody resistant to human death receptor 5 and application thereof
ActiveCN106397594AEnhanced inhibitory effectGood antitumor activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsEscherichia coliTumor target
The invention relates to a single-chain antibody capable of specific binding and capable of activating human death receptor 5 (DR5), and belongs to a full-humanized antibody; the single-chain antibody is obtained by performing quintuple screening in a high-capacity full-humanized phage antibody library by using human death receptor DR5 extracellular protein as a target antigen; the single-chain antibody may be acquired by induced expression in Escherichia coli, nickel ion affinity chromatography and molecular-exclusion chromatographic purification; pharmacodynamic experiments prove that the single-chain antibody is efficient in suppressing the growth of DR5 positive colon cancer cell COLO205 and breast cancer cell MDA-MB-231; the single-chain antibody and other antibodies modified therefrom, including full-length, bispecific or fusion protein forms, may act as drugs for positive tumor targeting therapy of death receptor DR5; the single-chain antibody has the advantages such as low immunogenicity, high specificity and high penetrability and is an ideal targeting tumor therapeutic drug.
Owner:CHINA PHARM UNIV
Humanized single-chain antibody 8B of clostridium perfringens alpha-toxin
ActiveCN104531730AAchieve therapeutic effectExempt from humanizationAntinoxious agentsAntibody ingredientsPhage antibodiesSingle-Chain Antibodies
The invention discloses a humanized single-chain antibody 8B of clostridium perfringens alpha-toxin. The humanized single-chain antibody 8B is screened from a phage antibody library Source bioscience, is a full-humanized antibody of the clostridium perfringens alpha-toxin and can be used for achieving a toxin neutralization treating effect, overcoming various side effects of a heterologous antibody and eliminating complex steps and high cost of humanization modification on the heterologous antibody; and the single-chain antibody is small in molecular weight, strong in in-vivo penetrability and capable of rapidly reaching to damaged tissues and cells to exert an antitoxin effect, so that the double aims of economy and high efficiency are achieved. The alpha-toxin has a certain homology with other types of toxins of clostridium perfringens, so that the antibody drug possibly generates inhibiting and treating effects for other types of toxins and can also be used as a detecting reagent to detect the clostridium perfringens alpha-toxin.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
131I labeled anti-tumor humanized monoclonal antibody 1E2 and use thereof
InactiveCN101407545AImprove targetingGood inhibitory effectIn-vivo radioactive preparationsImmunoglobulins against animals/humansAntigenPhage antibodies
The invention provides an anti-lung cancer human resource monoclonal antibody 1E2 marked by <131>I. The human resource 1E2 antibody, Na <131>I and chloramine T are used as raw materials, and purified 1E2 antibody marked by the <131>I can be prepared by using the chloramine T method, wherein, the human source 1E2 antibody is obtained by taking paracanser lymph nodes of a non-small cell lung cancer patient, extracting total RNA in lymph node tissues, and then a phage antibody library containing the 1E2 antibody is obtained by using the constructing technology of the phage antibody library, and the phage antibody 1E2 is adsorbed by using a carbamyl phosphate synthetase antigen, and a human source 1E2 antibody which is specific to the carbamyl phosphate synthetase is finally obtained by washing, screening and amplifying. The invention applies radiation immunity treatment technology, combines advantages of radionuclide and anti-tumor cell human source monoclonal antibody to one, improves the targeting ability and curative effect of medicines to tumor cells, reduces side effects and poor immune reactions, and is wider in application range and suitable for treatments of the non-small cell lung cancer of each stage.
Owner:CHONGQING MEDICAL UNIVERSITY
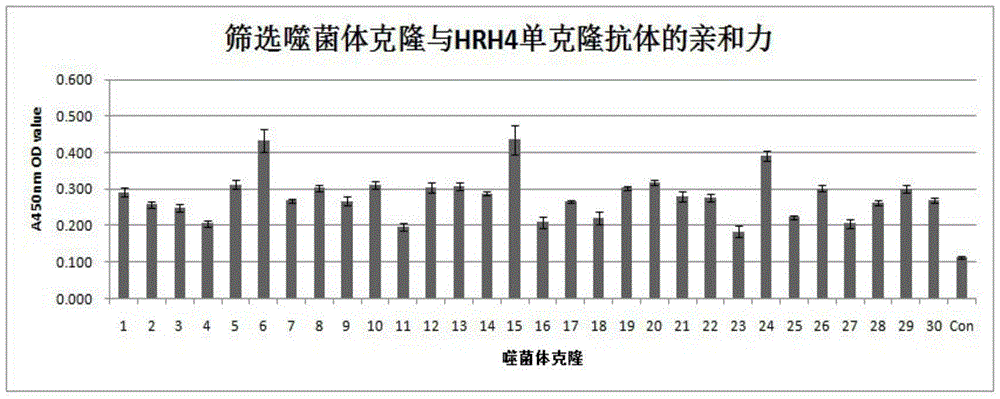
Method utilizing phage antibody library to screen human histamine receptor 4 (HR4) epitope mimic peptide and vaccine construction method thereof
The invention discloses a method utilizing phage antibody library to screen human histamine receptor 4 (HR4) epitope mimic peptide and a vaccine construction method thereof. A phage random dodecapeptide library is utilized to screen one polypeptide section, and the amino acid sequence of the polypeptide section is FNKWMDCLSVTH. The provided method utilizes a phage random dodecapeptide library technology to screen the polypeptide sequence of HR4 epitope mimic peptide; ELISA identifies the affinity of phage monoclone on HR4 mono-antibody, then sequencing is carried out to obtain one polypeptide sequence, which is names as PT1; the targeting result of phage positive clone is further identified to indicate that phage positive clone can specifically combine the human HR4 monoclonal antibody; the human HR4 monoclonal antibody specific polypeptide can be obtained through screening; thus the HR4 epitope mimic peptide vaccine can be constructed; and the vaccine on the basis of HR4 mimic epitope can be used to treat allergic rhinitis in clinic, can also be applied to the research on HR4 antagonist, and provides primary experimental references for the construction of HR4 antagonist.
Owner:JILIN UNIV
Phage antibody library and application thereof in content determination of clenbuterol hydrochloride
ActiveCN103361741AHigh application valueThe detection process is fastMicroorganism librariesBiological testingSingle-Chain AntibodiesEnzyme
The invention provides a phage antibody library and application thereof in content determination of clenbuterol hydrochloride. A gene VL of an antibody light chain in a variable field is assembled between the enzyme cutting sites of Sall and NotI of phage plasmid pIT2, a gene VH construction of an antibody heavy chain in the variable field is assembled between the enzyme cutting sites of SfiI and XhoI, and the gene of the antibody is obtained after bovine serum albumin and conjugate of clenbuterol hydrochloride are used for immunization of mice. The antibody is the affinity and concentration of single chain antibody library, the bovine serum albumin and the conjugate of the clenbuterol hydrochloride, monoclonal antibody of the clenbuterol hydrochloride is developed to apply to test of residual quantity of the clenbuterol hydrochloride. By using the antibody library, high affinity anti-clenbuterol hydrochloride and chemical substance antibodies with similar structure of the clenbuterol hydrochloride can be obtained directly, and the development and test of the clenbuterol hydrochloride and the chemical substance antibodies with similar structure of the clenbuterol hydrochloride have wide application prospect.
Owner:WEIFANG UNIV OF SCI & TECH +1
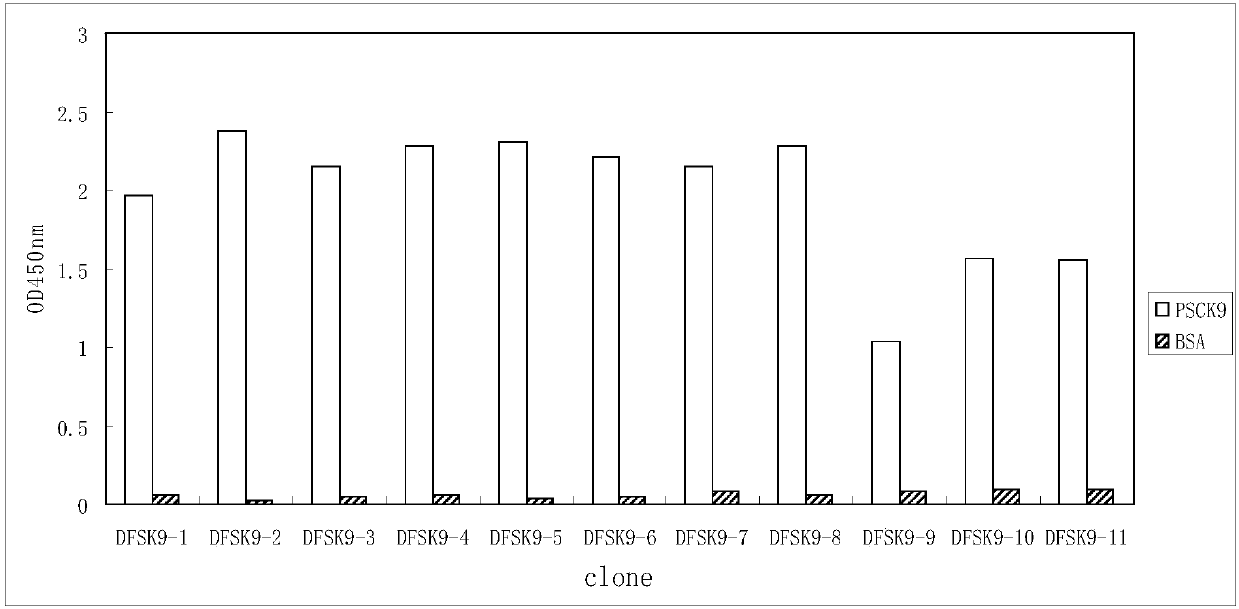
PCSK9 (Proprotein Convertase Subtilisin Kexin Type 9) resistant monoclonal antibody
ActiveCN107698680ABinding blockIncrease intakeMetabolism disorderMammal material medical ingredientsKexinPhage antibodies
The invention relates to the technical field of antibody engineering and in particular discloses a PCSK9 (Proprotein Convertase Subtilisin Kexin Type 9) resistant monoclonal antibody. The monoclonal antibody disclosed by the invention comprises an amino acid sequence coding an antibody variable region and a CDR region. The invention further discloses an acquiring method and application of the monoclonal antibody. The method comprises the following steps: screening a PCSK9 resistant monoclonal antibody from a phage antibody library, performing affinity maturation through a method for constructing the phage antibody library by virtue of strand displacement, performing mutant library-construction screening on light-chain CDR1, 2 and 3 regions of the monoclonal antibody obtained by preliminaryscreening, selecting a monoclonal antibody with high affinity, performing mutant library-construction screening on heavy-chain CDR1, 2 and 3 regions of the monoclonal antibody, and finally screeningthe PCSK9 resistant monoclonal antibody with high affinity. The PCSK9 resistant monoclonal antibody obtained in the invention has excellent affinity to PCSK9, is capable of inhibiting binding betweenthe PCSK9 and ligands thereof, and can be used for treating dyslipidemia, cardiovascular and cerebrovascular diseases and thrombosis-obstructive diseases.
Owner:BEIJING DONGFANG BIOTECH
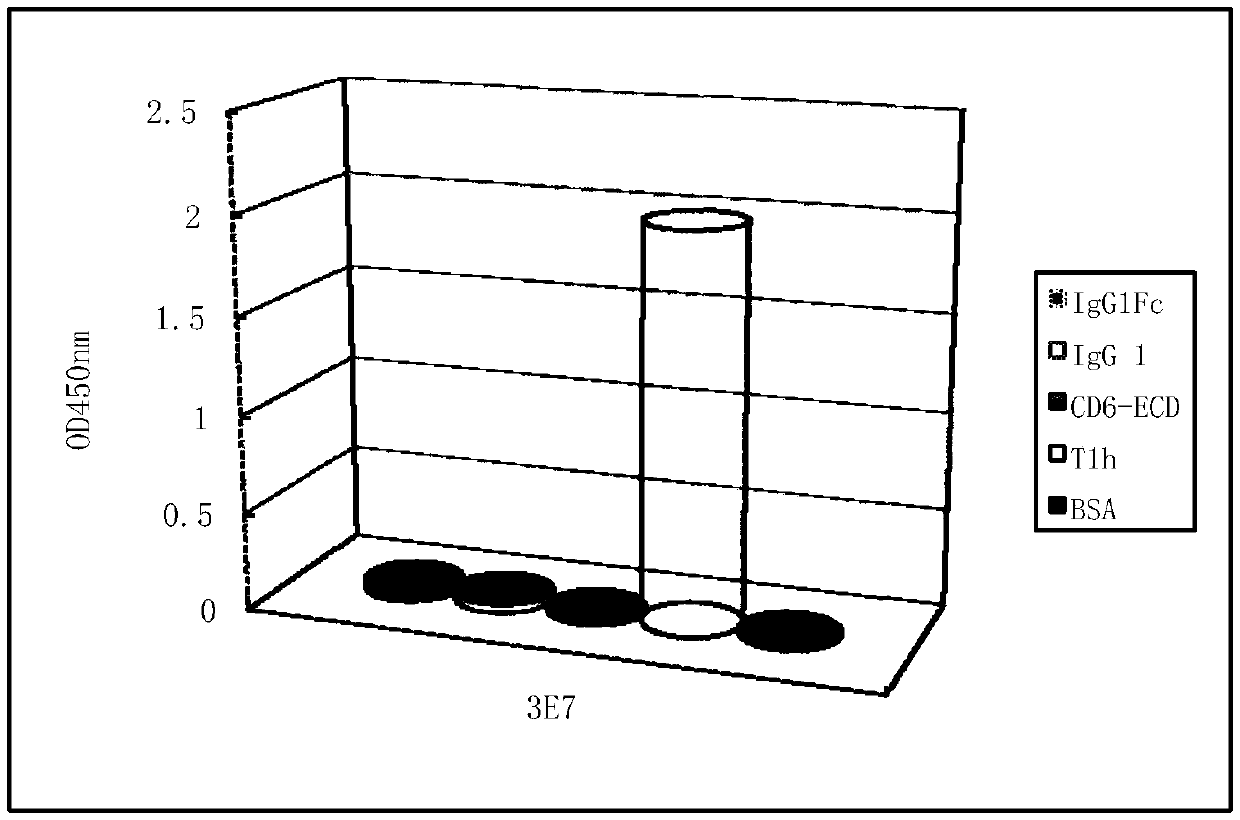
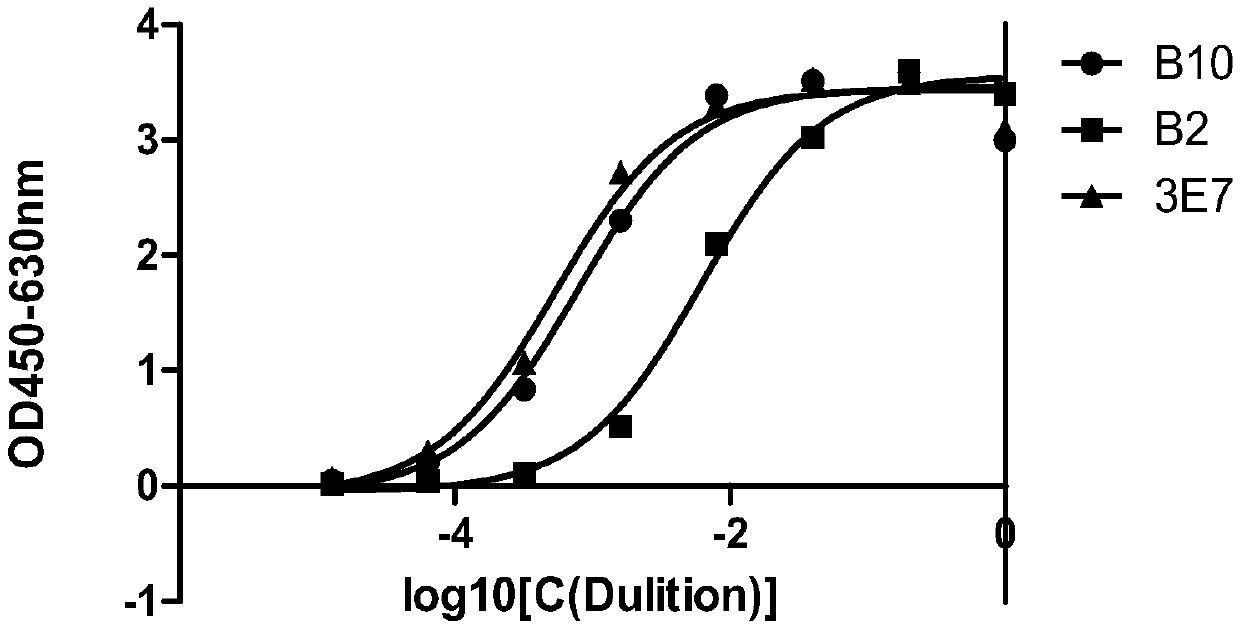
Detecting antibody for CD6-resistant monoclonal antibody T1h and application
ActiveCN105504062ASolve the technical problems of concentration detectionSensitive detectionFungiBacteriaSingle-Chain AntibodiesBlood concentration
The invention relates to the technical field of biological pharmacy, in particular to an obtaining method, a preparing method and a detecting method of a detecting antibody for a CD6-resistant monoclonal antibody T1h. According to the screening technology of a total-synthesis phage antibody library, single-chain antibodies of the antibody T1h are subjected to biopanning, single-chain antibodies of bacteriophage of the antibody T1h are subjected to positive clone identification, gradient-dilution phage ELISA is carried out, the affinity of the single-chain antibodies of the antibody T1h is compared, then all antibodies of the antibody T1h are prepared, and a new specificity monoclonal antibody of the antibody T1h is obtained. A heavy-chain variable region of the antibody comprises the amino acid sequence of SEQ.1, and a light-chain variable region of the antibody comprises the amino acid sequence of SEQ.2. The antibody is used for detecting the concentration of T1h in the blood and other body liquid in the clinical test, the blood concentration of the T1h can be sensitively and rapidly detected, a reliable research method is provided for the clinical test pharmacology and medicine generation analyzing of the T1h, and meanwhile the antibody can be used for neutralizing the T1h antibody.
Owner:BIOTECH PHARMA CO LTD
BP180 single chain antibody of humanized anti-bullous pemphigoid antigen
InactiveCN101333255ABlock inflammatory responseImmunoglobulins against animals/humansAntibody ingredientsAntigenSingle-Chain Antibodies
The invention discloses a human-derived anti-bullous pemphigoid antigen BP180 ScFv; the gene sequence of the BP180 ScFv structurally belongs to the single-chain antibody (scFv) and is composed of a light chain variable region gene and a heavy chain variable region gene. The antibody preparation method mainly comprises the following steps: a, separating the peripheral blood mononuclear cells of bullous pemphigoid patients, extracting the RNA and constructing a phage antibody library through RT-PCR amplification of the antibody light and heavy chain variable region genes; b, selecting the BP180 phage antibodies from the phage antibody library; c, obtaining the human-derived anti-BP180 ScFv of soluble expression through genetic engineering means; d, identifying the binding activity, specificity, affinity and other immunological characteristics of the obtained antibody. The antibody of the invention can block the binding activity between the bullous pemphigoid autoantibody and the corresponding self-antigen and can block the activation of the complement-mediated inflammatory response as a result of the combination of the autoantibodies, and can be used in bullous pemphigoid treatment.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com