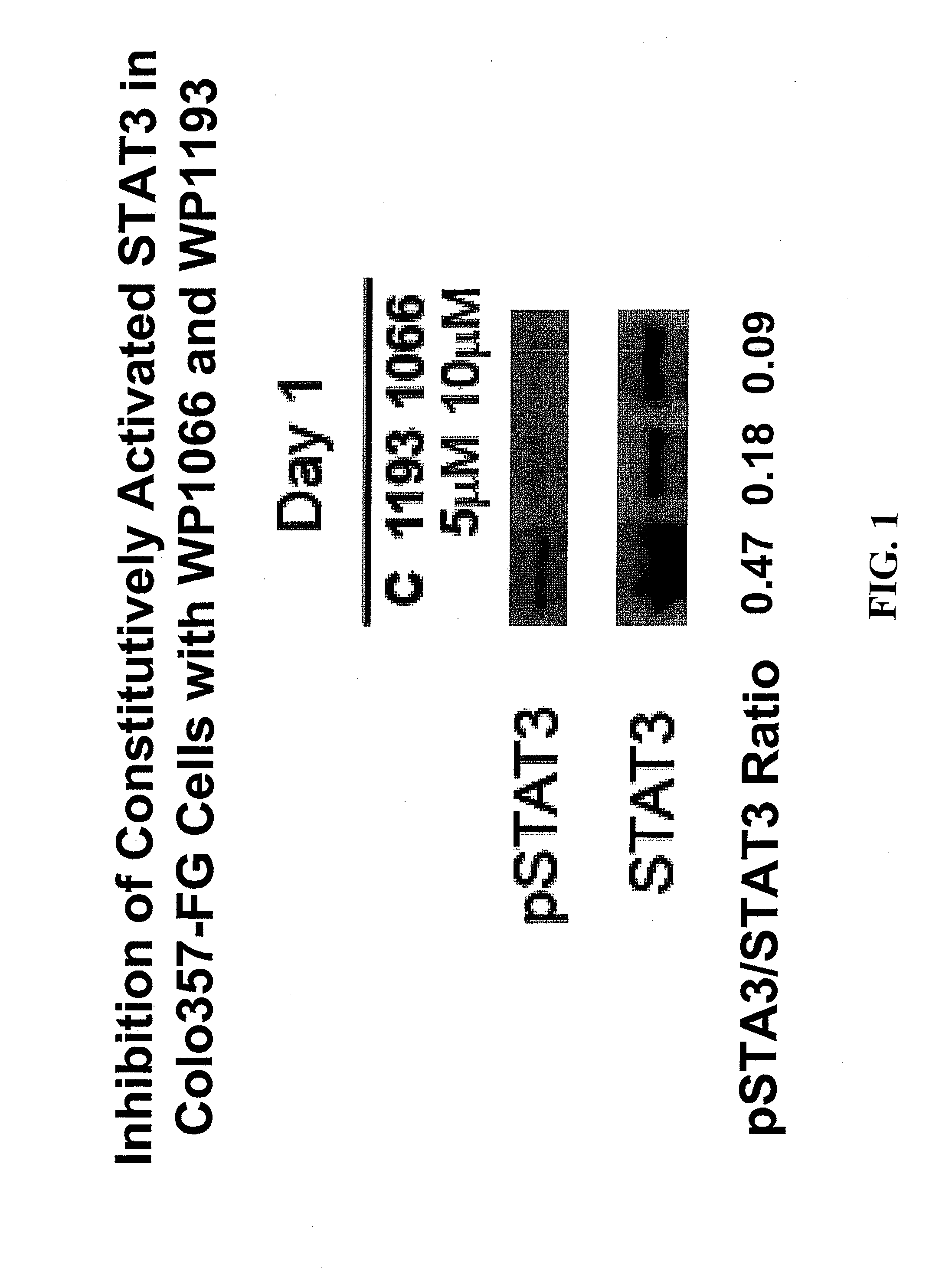
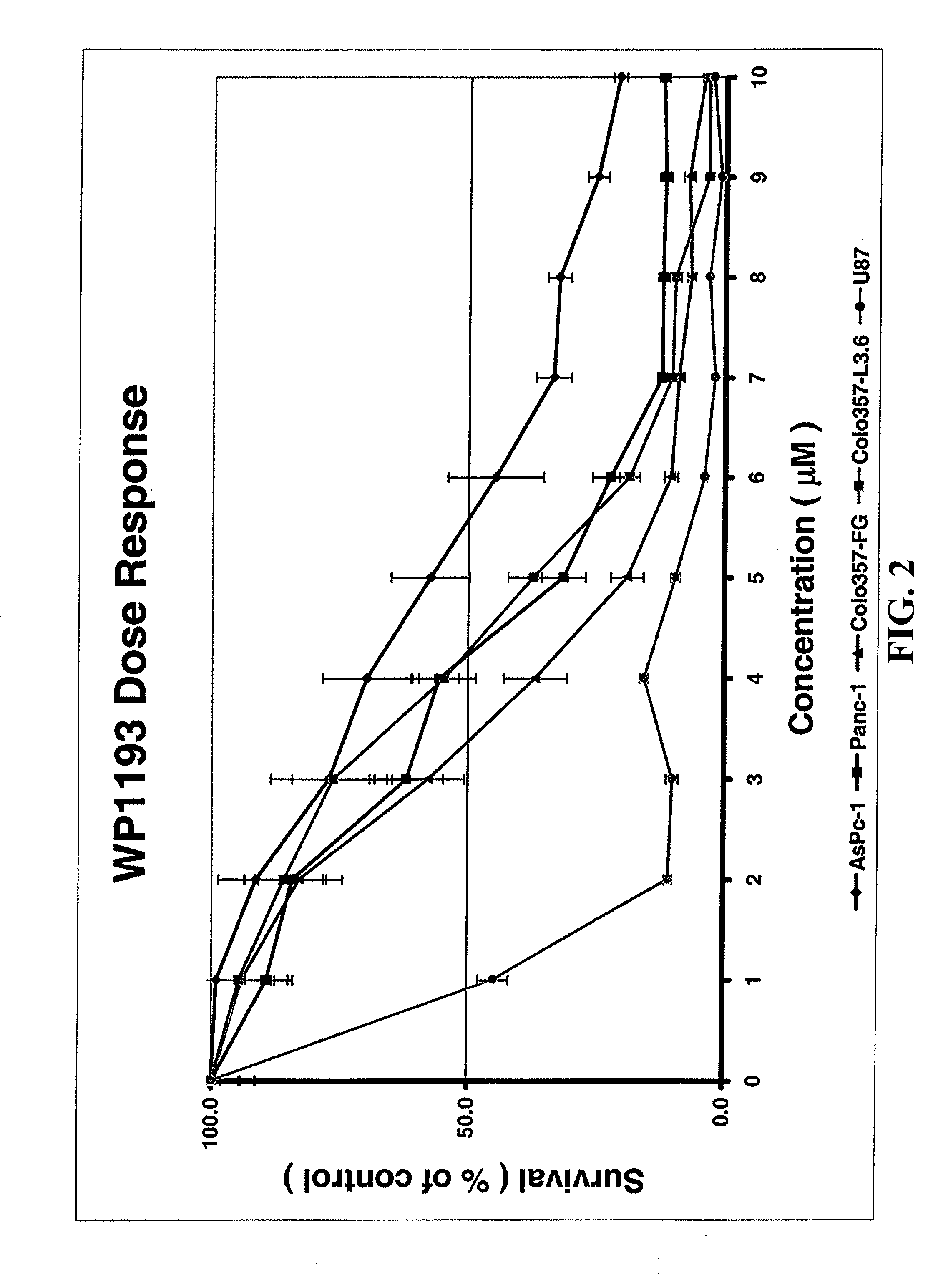
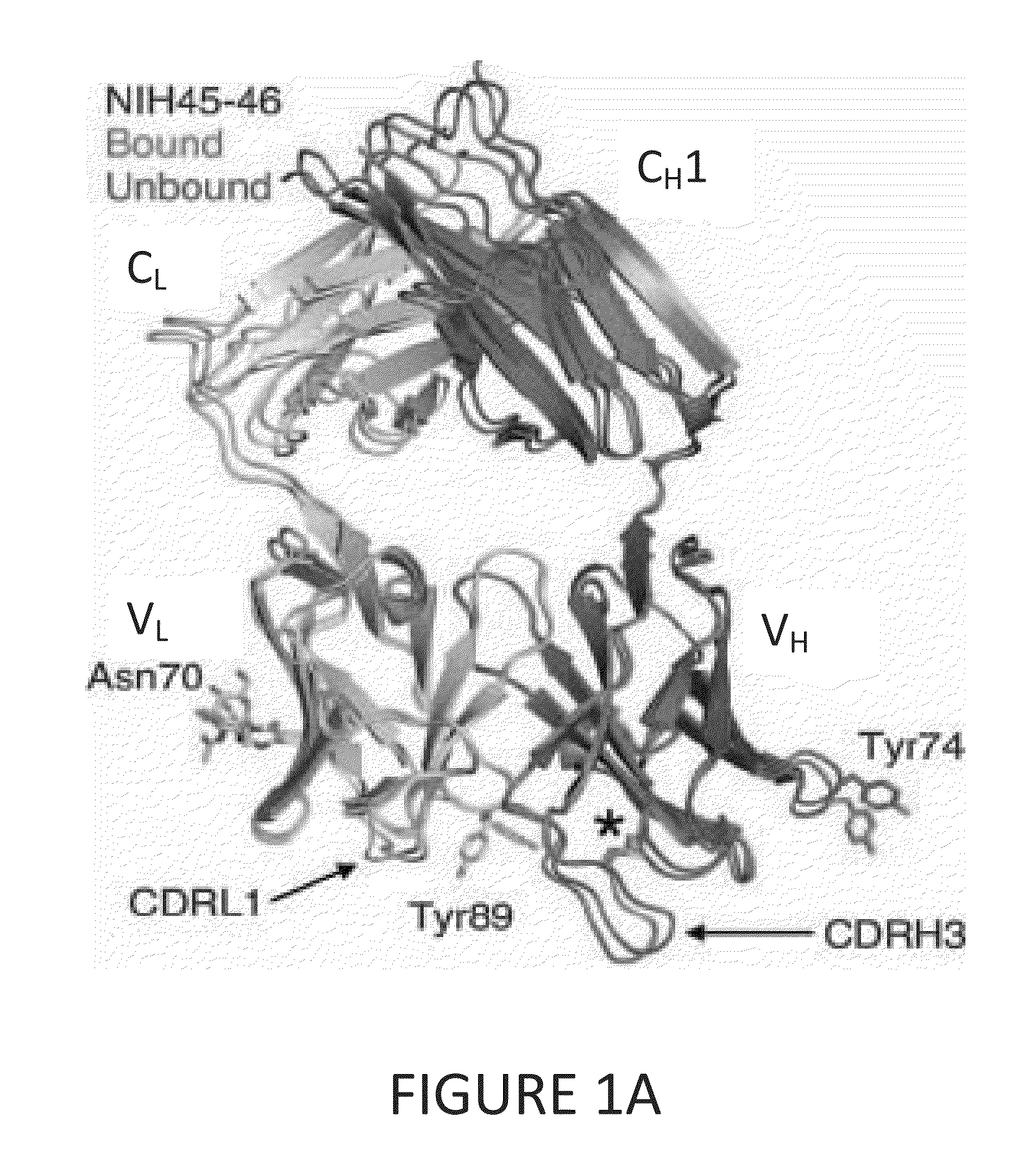
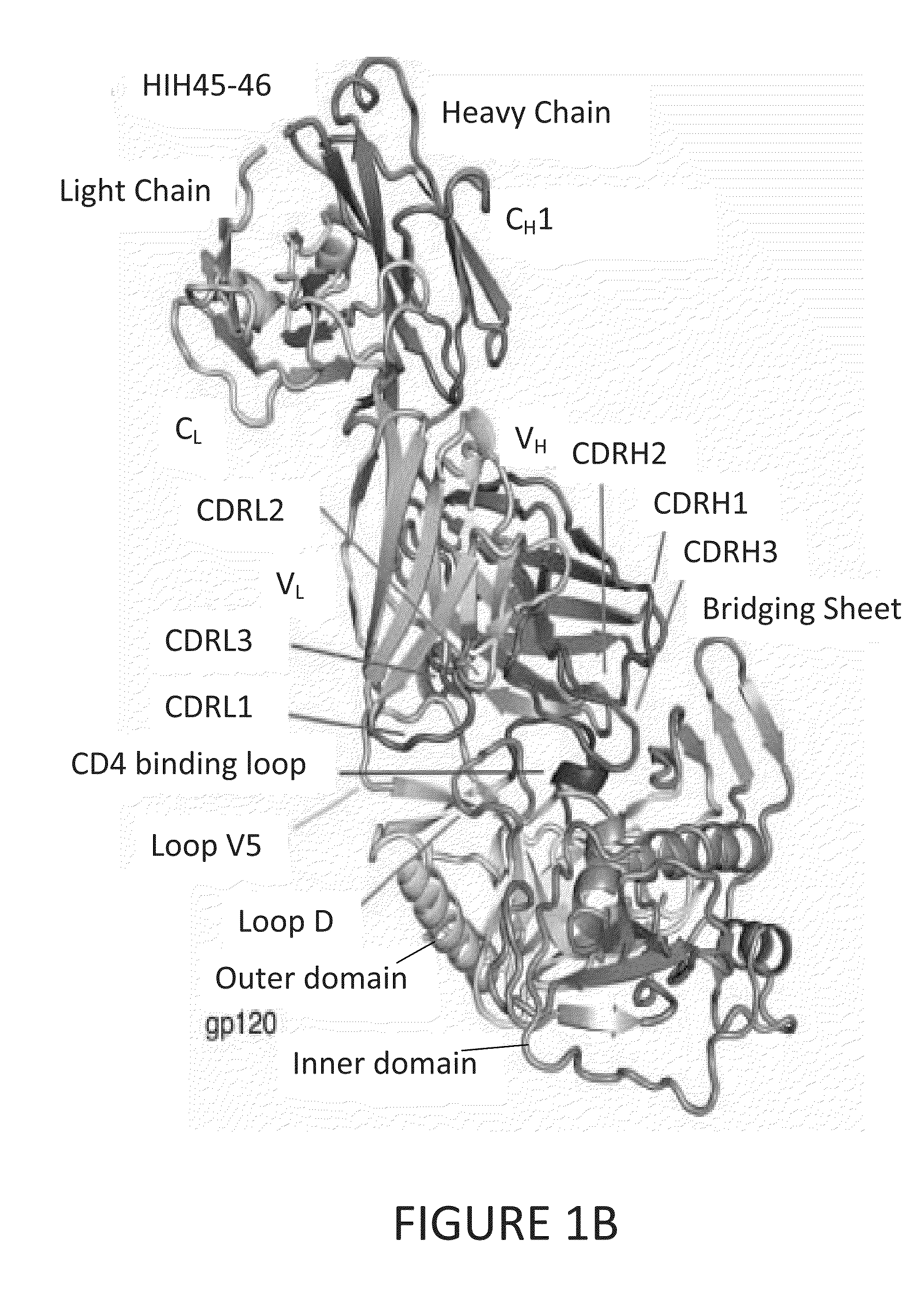
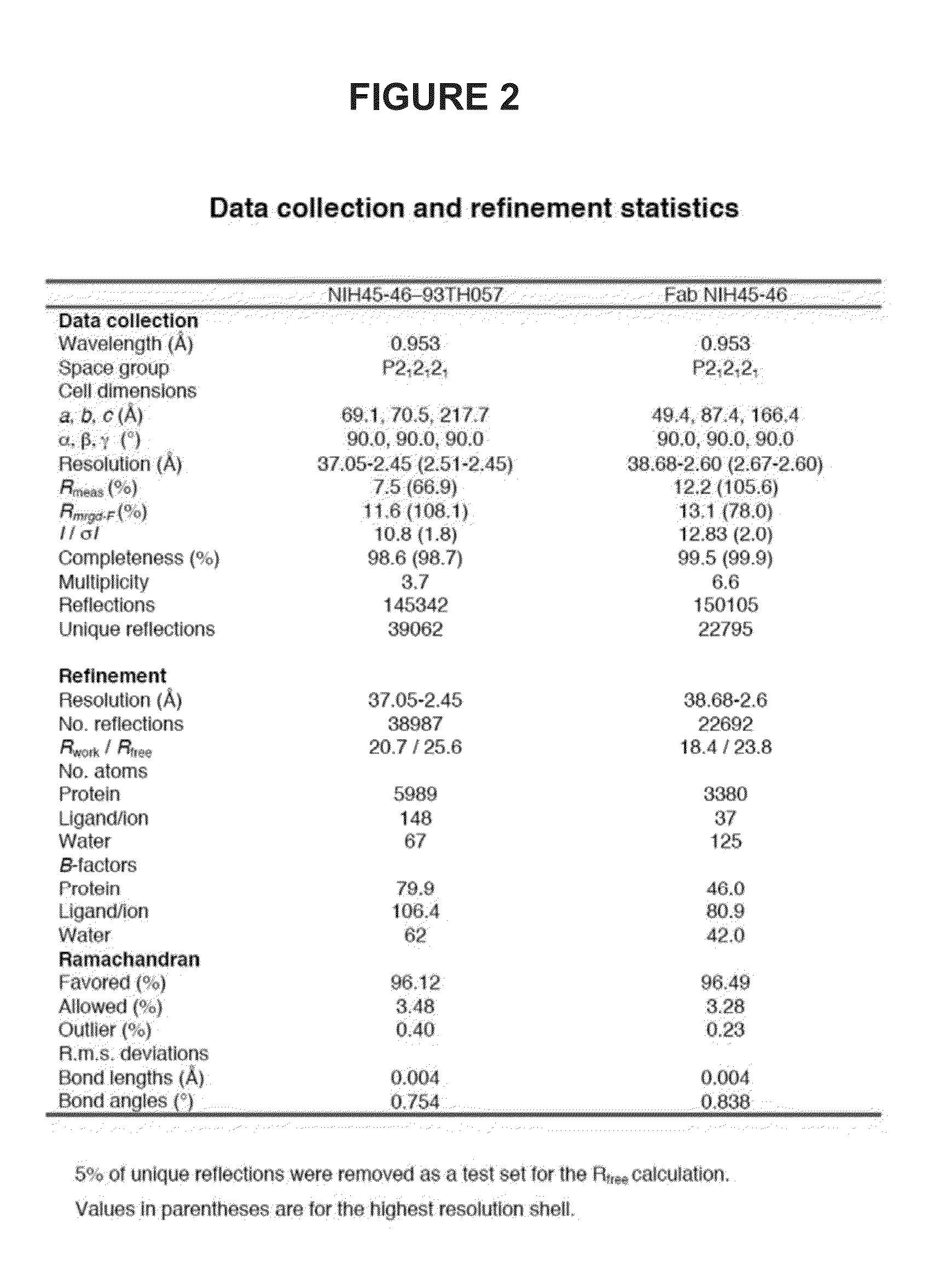
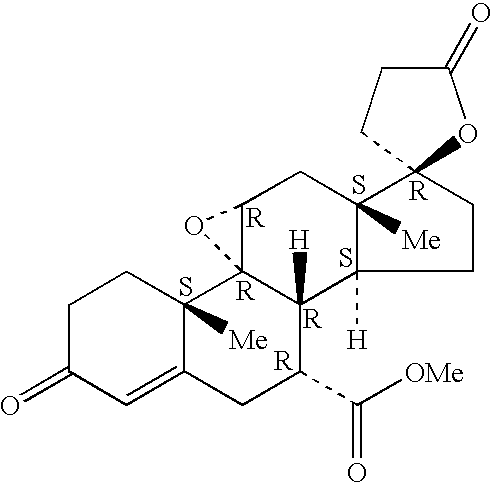
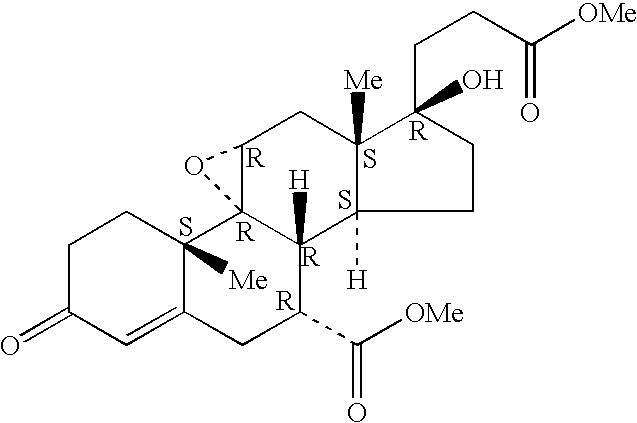
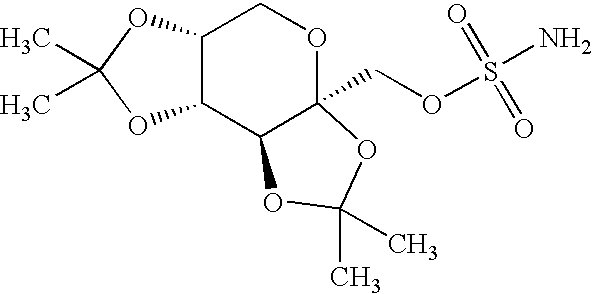
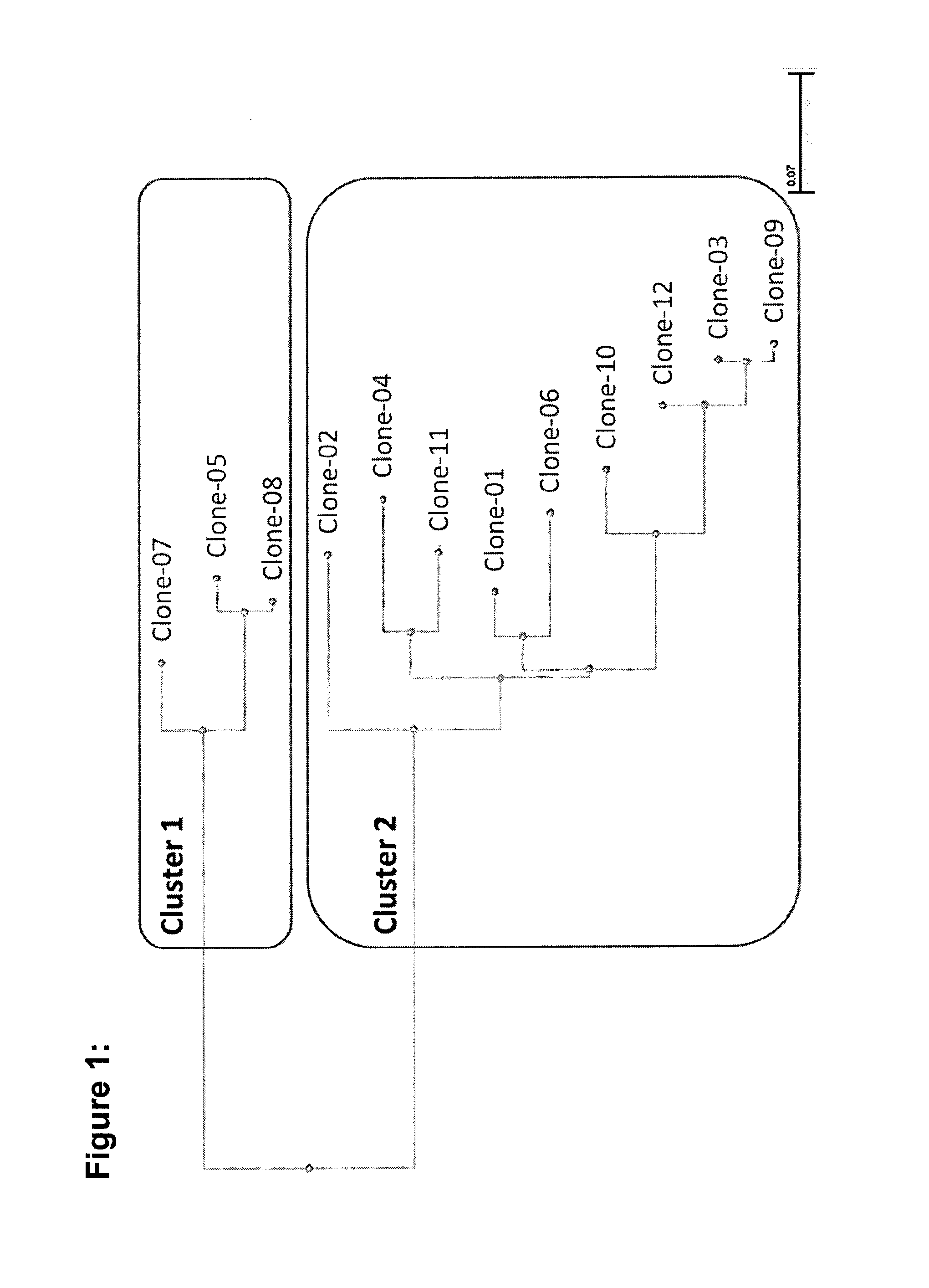
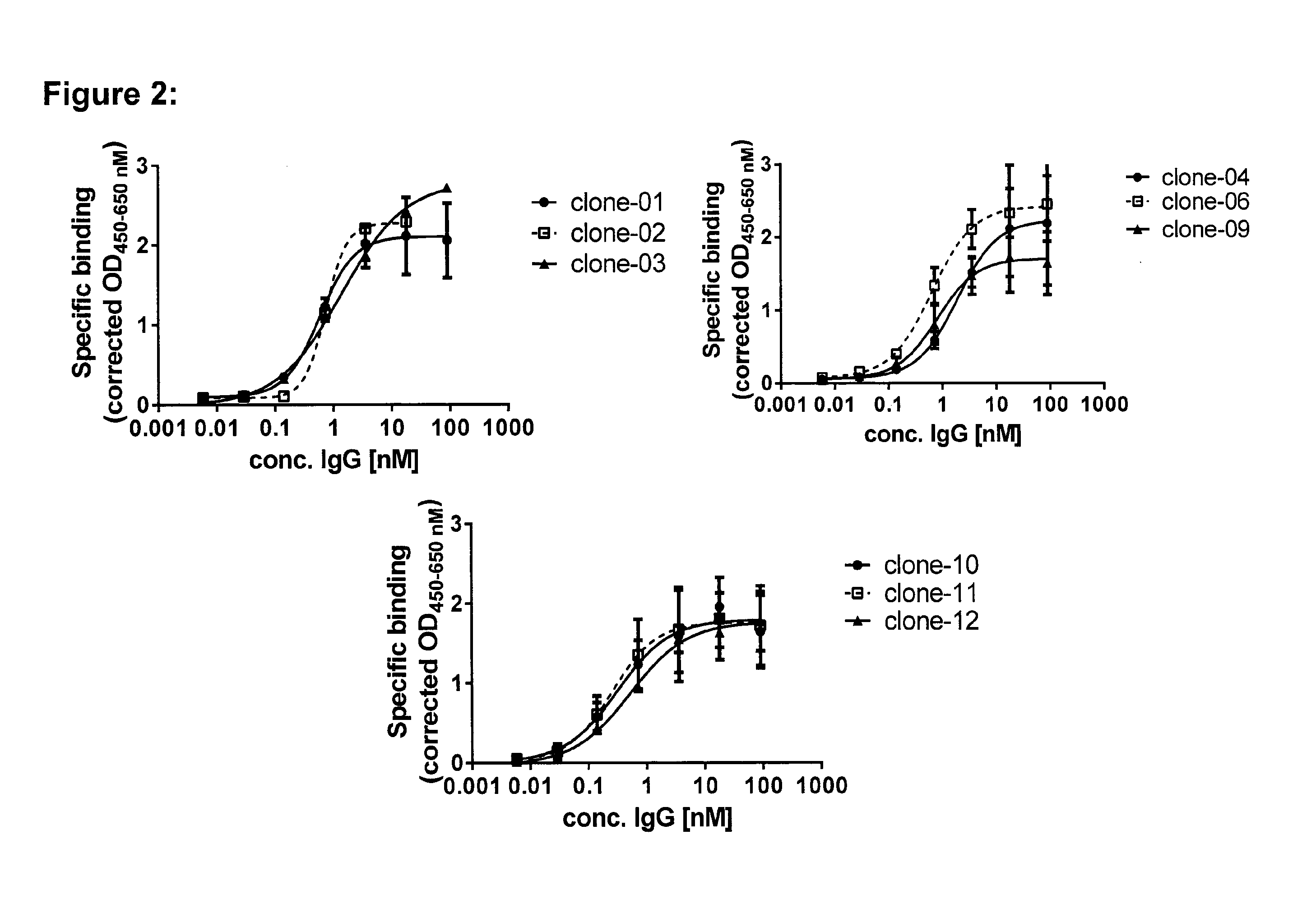
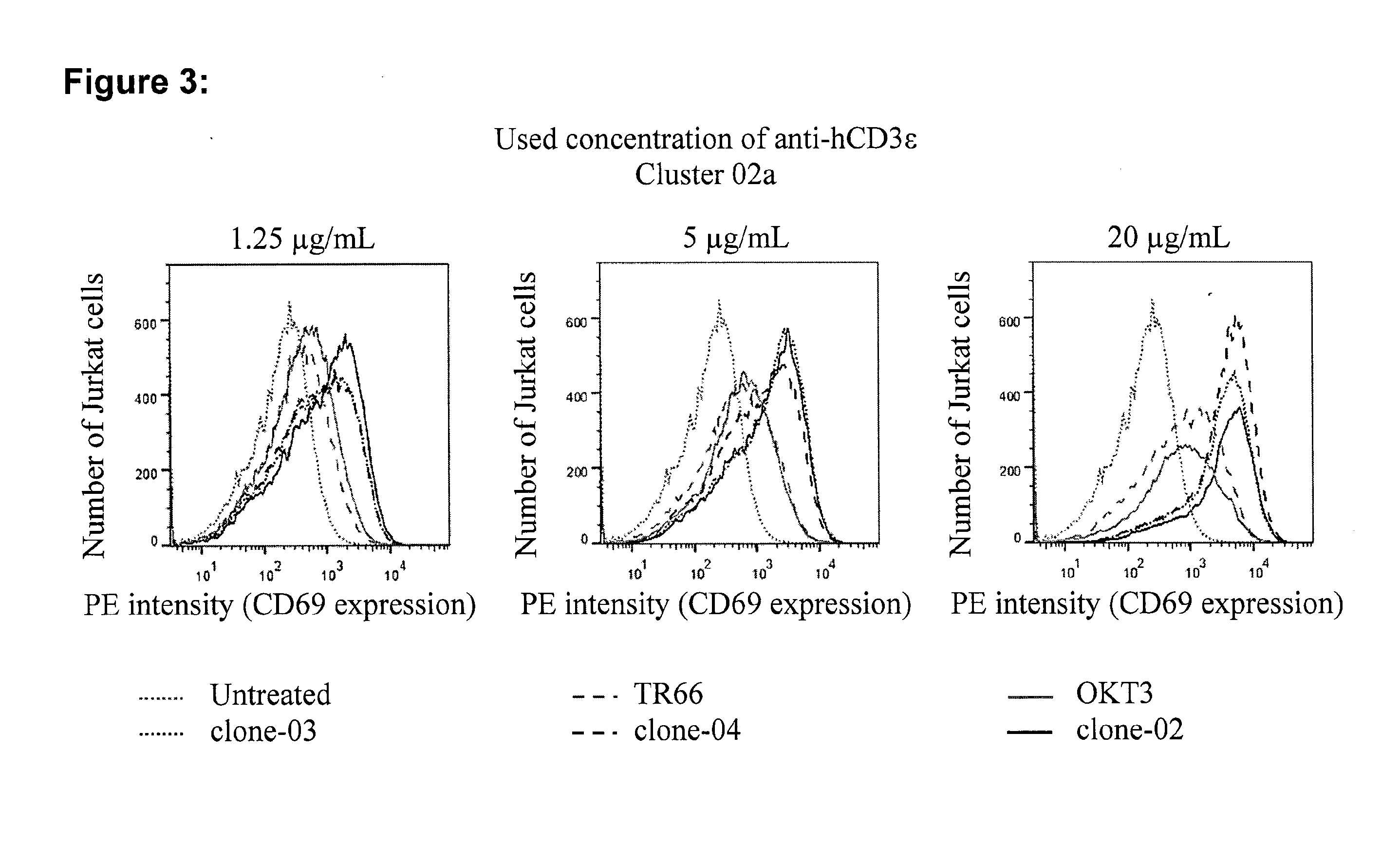
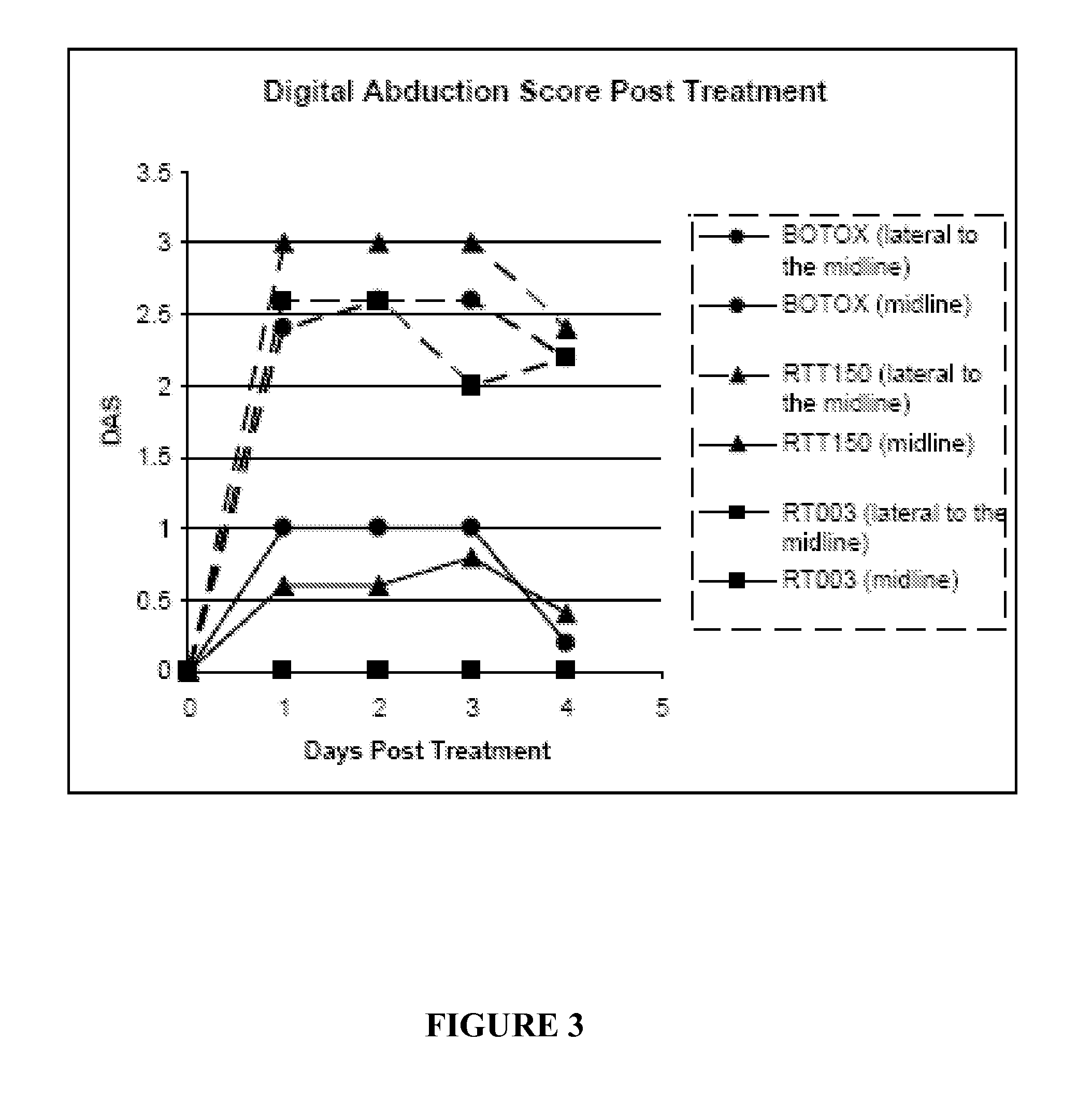
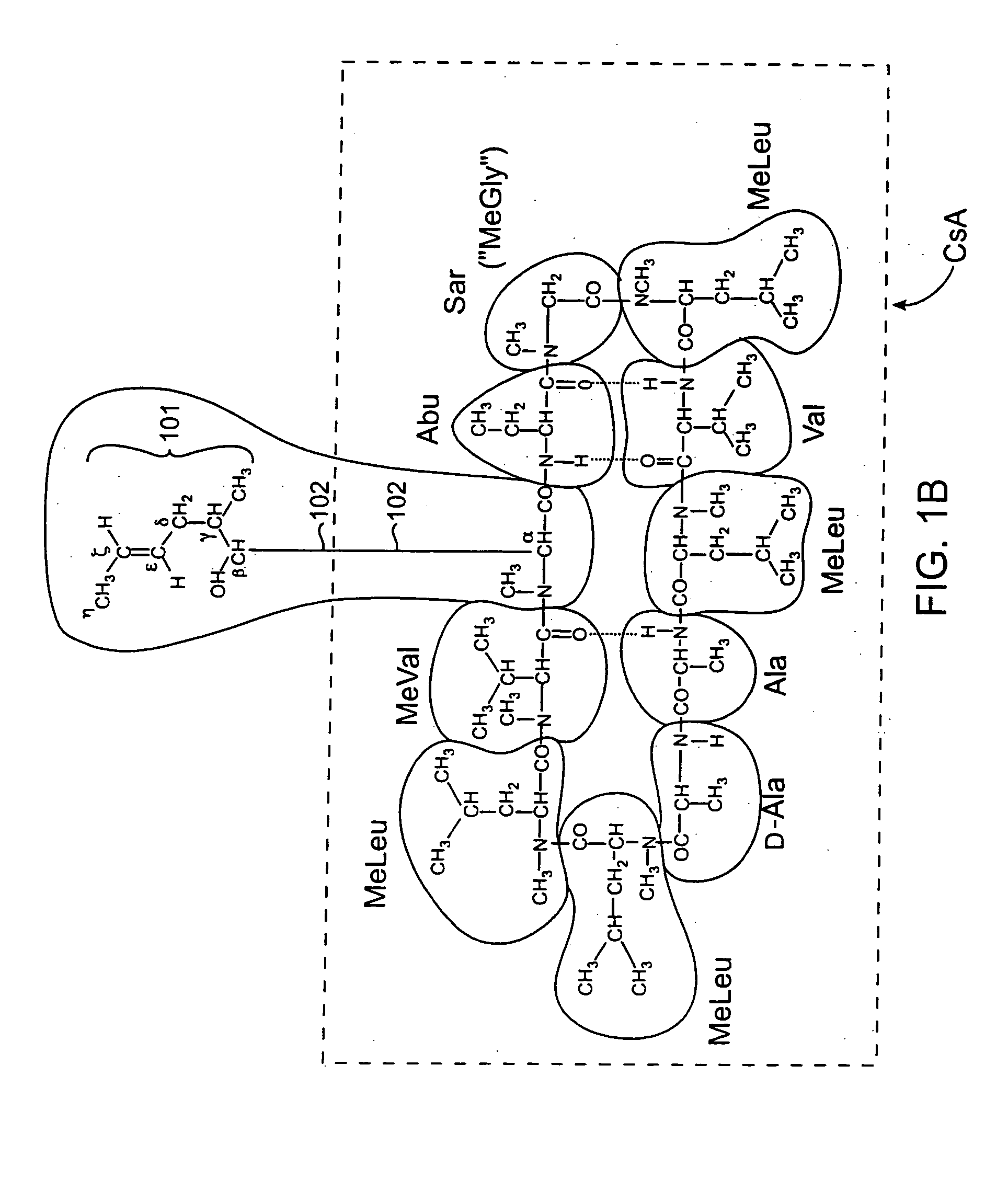
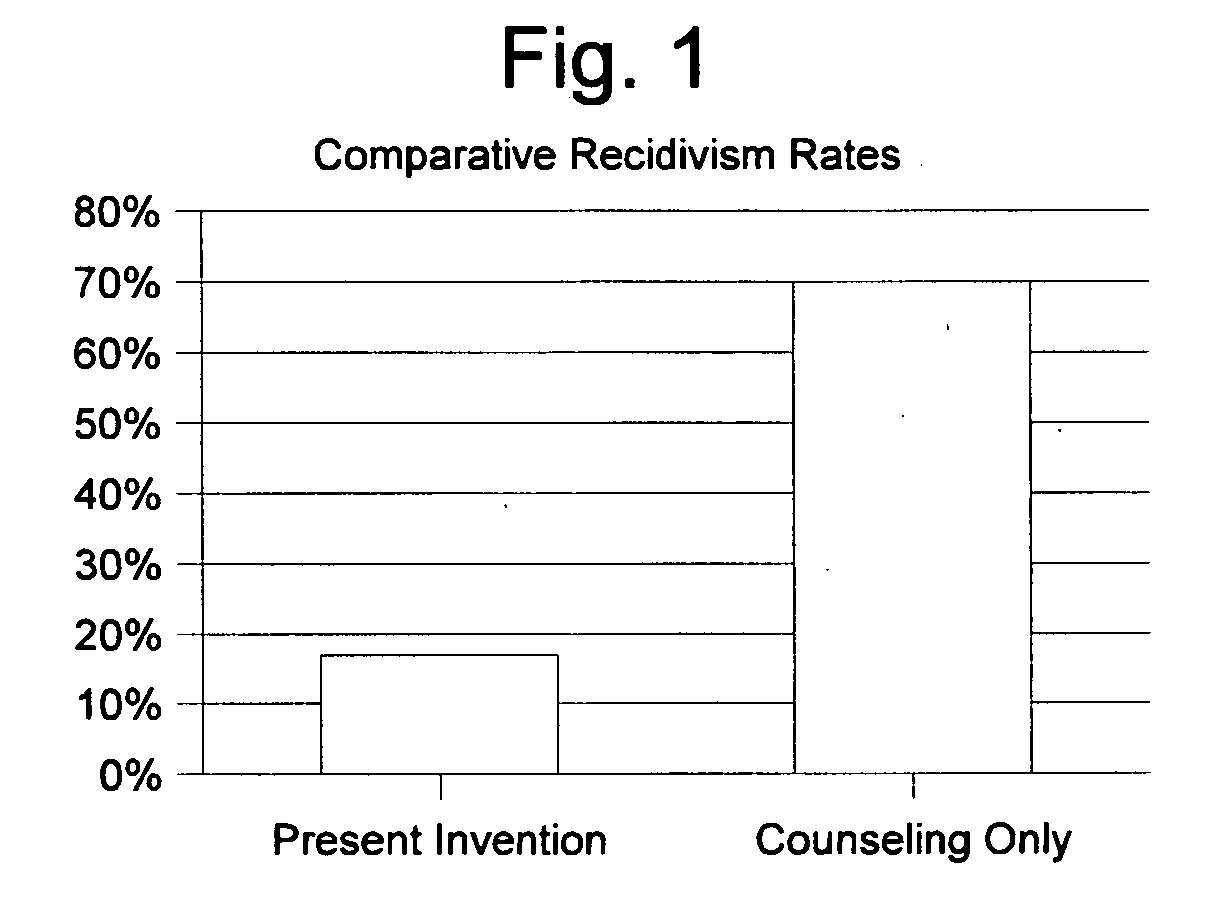
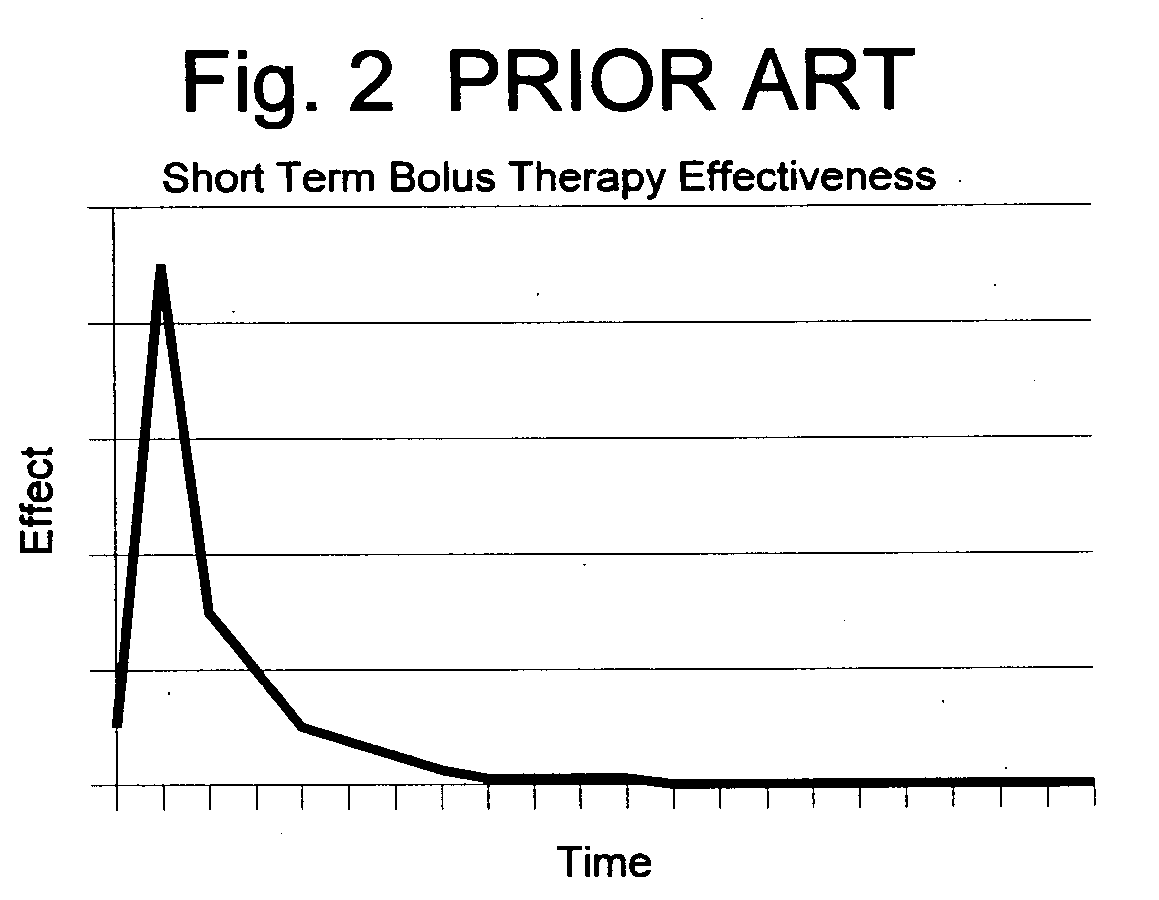
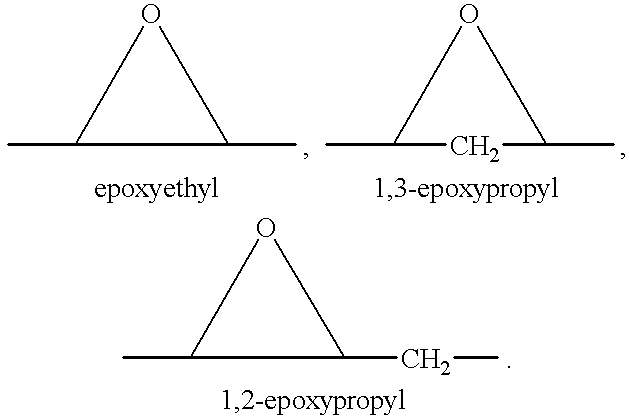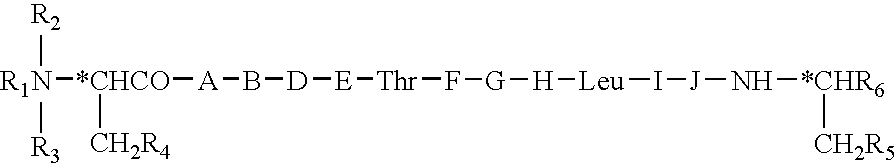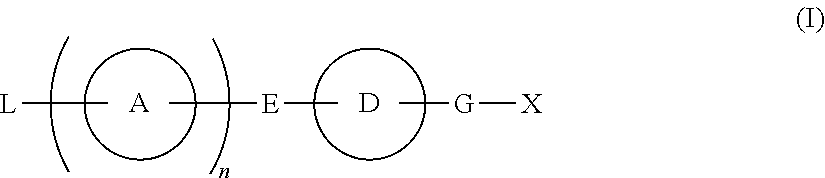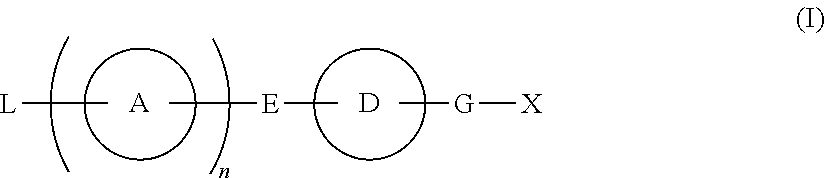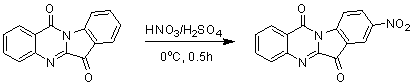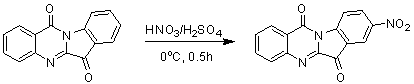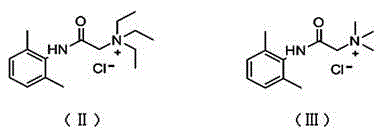Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
249 results about "Potency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In the field of pharmacology, potency is a measure of drug activity expressed in terms of the amount required to produce an effect of given intensity. A highly potent drug (e.g., fentanyl, alprazolam, risperidone) evokes a given response at low concentrations, while a drug of lower potency (meperidine, diazepam, ziprasidone) evokes the same response only at higher concentrations. Higher potency does not necessarily mean more side effects.
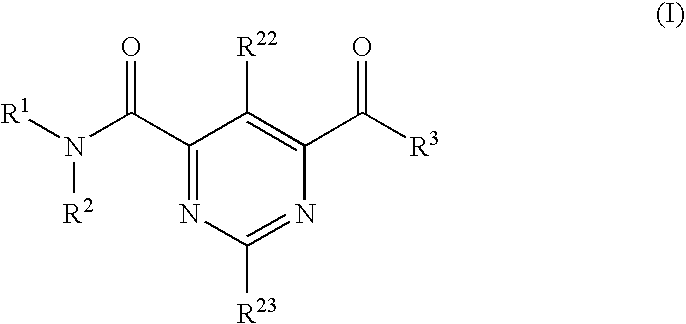
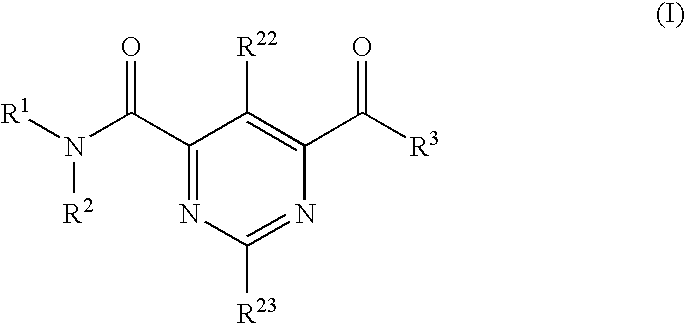
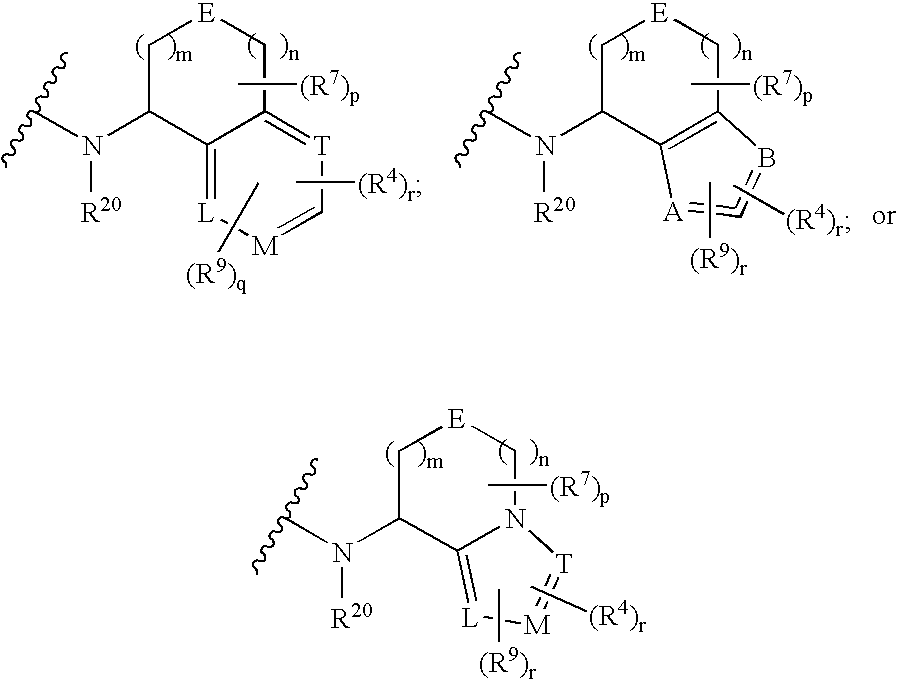
Compound Having S1P Receptor Binding Potency and Use Thereof
InactiveUS20080207584A1Easy to optimizeEasy to separateBiocideNervous disorderAutoimmune conditionS1P Receptor
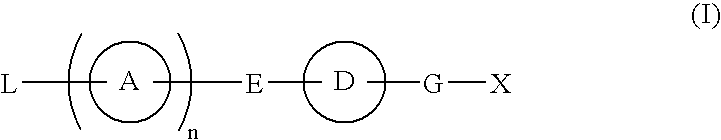
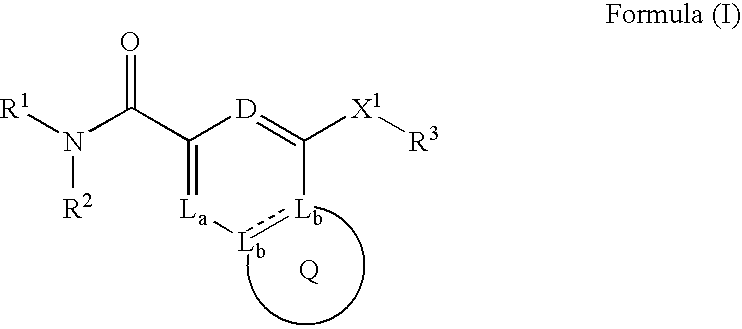
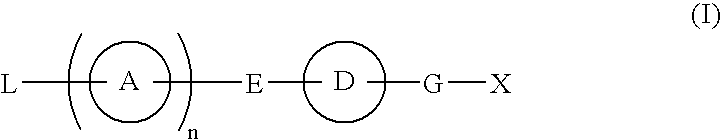
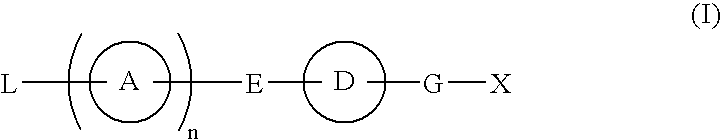
Provided are: a compound represented by formula (I):(wherein ring A and ring D each represent a cyclic group which may have a substituent(s); E and G each represent a bond or a spacer having 1 to 8 atoms in its main chain; L represents a hydrogen atom or a substituent; X represents amino which may have a substituent(s), or a heterocyclic group which contains at least one nitrogen atom and which may have a substituent(s); n represents 0 to 3, in which when n is 2 or more, a plurality of ring A's may be the same or different from one another); a salt thereof; an N-oxide form thereof; a solvate thereof, a prodrug thereof; and a medicament which includes those. The compound represented by formula (I) is capable of binding S1P receptors (in particular, EDG-1 and / or EDG-6), and useful for preventing and / or treating rejection in transplantation, autoimmune diseases, allergic diseases, etc.
Owner:ONO PHARMA CO LTD
Methods and materials for the treatment of pain comprising opioid antagonists
InactiveUS20050038062A1Enhance neuropathic pain-alleviating potencyEnhancing the potency of opioid agonistsBiocideNervous disorderOpioid AgonistOpioid antagonist
Methods and compositions for treating subjects with pain, including neuropathic pain, using opioid antagonists or combinations of opioid antagonists and opioid agonists, including, for example, wherein the amount of an opioid antagonist enhances the neuropathic pain-alleviating potency of an opioid agonist.
Owner:PAIN THERAPEUTICS INC
Acetamide derivative having defined particle size
InactiveUSRE37516E1Reduce and eliminate symptomIncrease alertnessPowder deliveryBiocideMedicineSafety profile
Pharmaceutical compositions comprising modafinil in the form of particles of defined size. The particle size of modafinil can have a significant effect on the potency and safety profile of the drug.
Owner:CEPHALON INC
Heterobicyclic metalloprotease inhibitors
The present invention relates generally to amide group containing pharmaceutical agents, and in particular, to amide containing heterobicyclic metalloprotease inhibitor compounds. More particularly, the present invention provides a new class of heterobicyclic MMP-13 inhibiting and MMP-3 inhibiting compounds, that exhibit an increased potency in relation to currently known MMP-13 and MMP-3 inhibitors.
Owner:ALANTOS PHARMA HLDG INC
Orally Bioavailable Caffeic Acid Related Anticancer Drugs
ActiveUS20070232668A1Improved pharmacological profileImproved tissue penetrationBiocideSenses disorderCancer cellMedicine
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Anti-hiv antibodies having increased potency and breadth
Embodiments of the present invention are directed to compositions and methods for anti-HIV (anti-CD4 binding site) antibodies having improved potency and breadth.
Owner:CALIFORNIA INST OF TECH +1
Prostatic hormonal implants treatment of prostate cancer
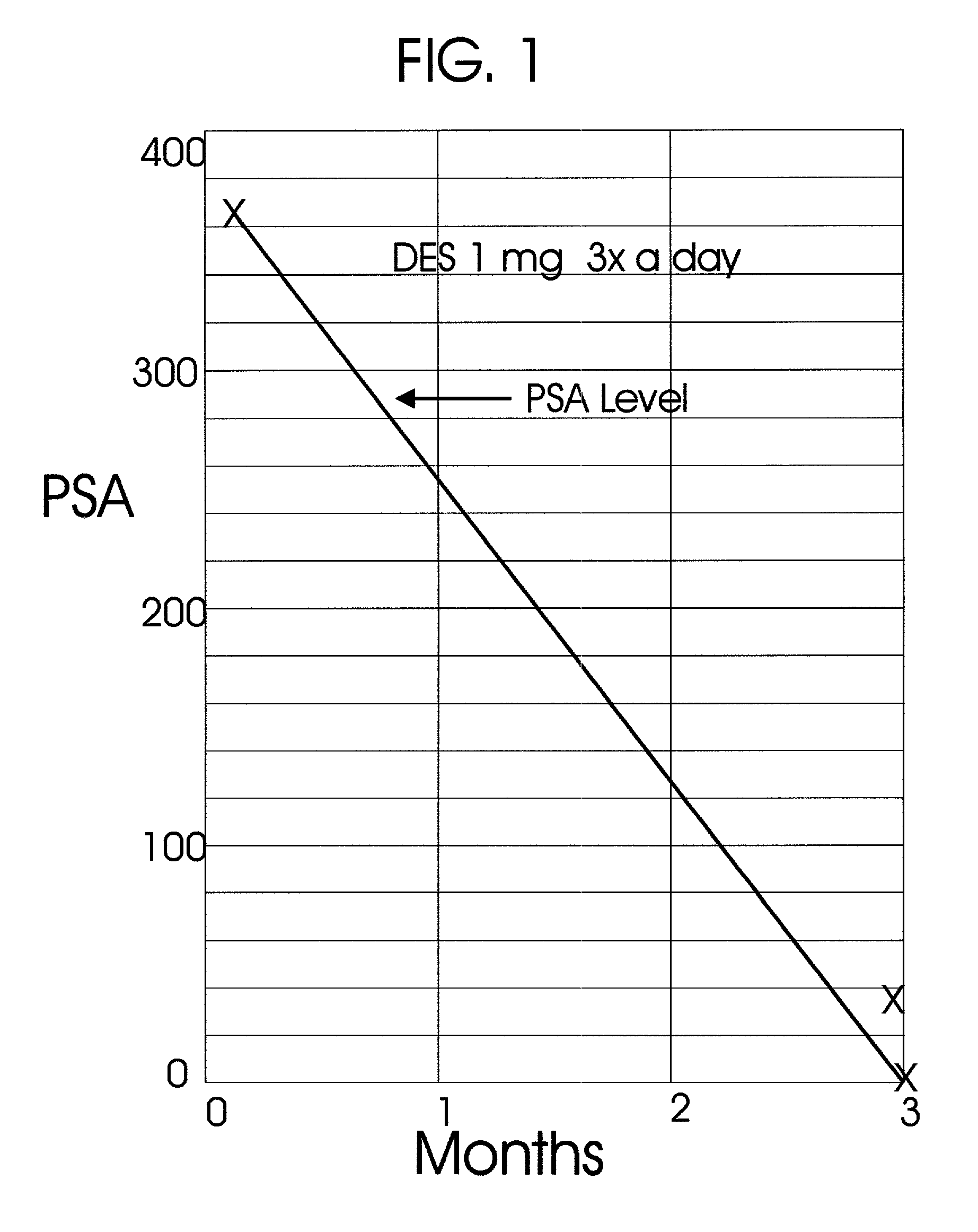
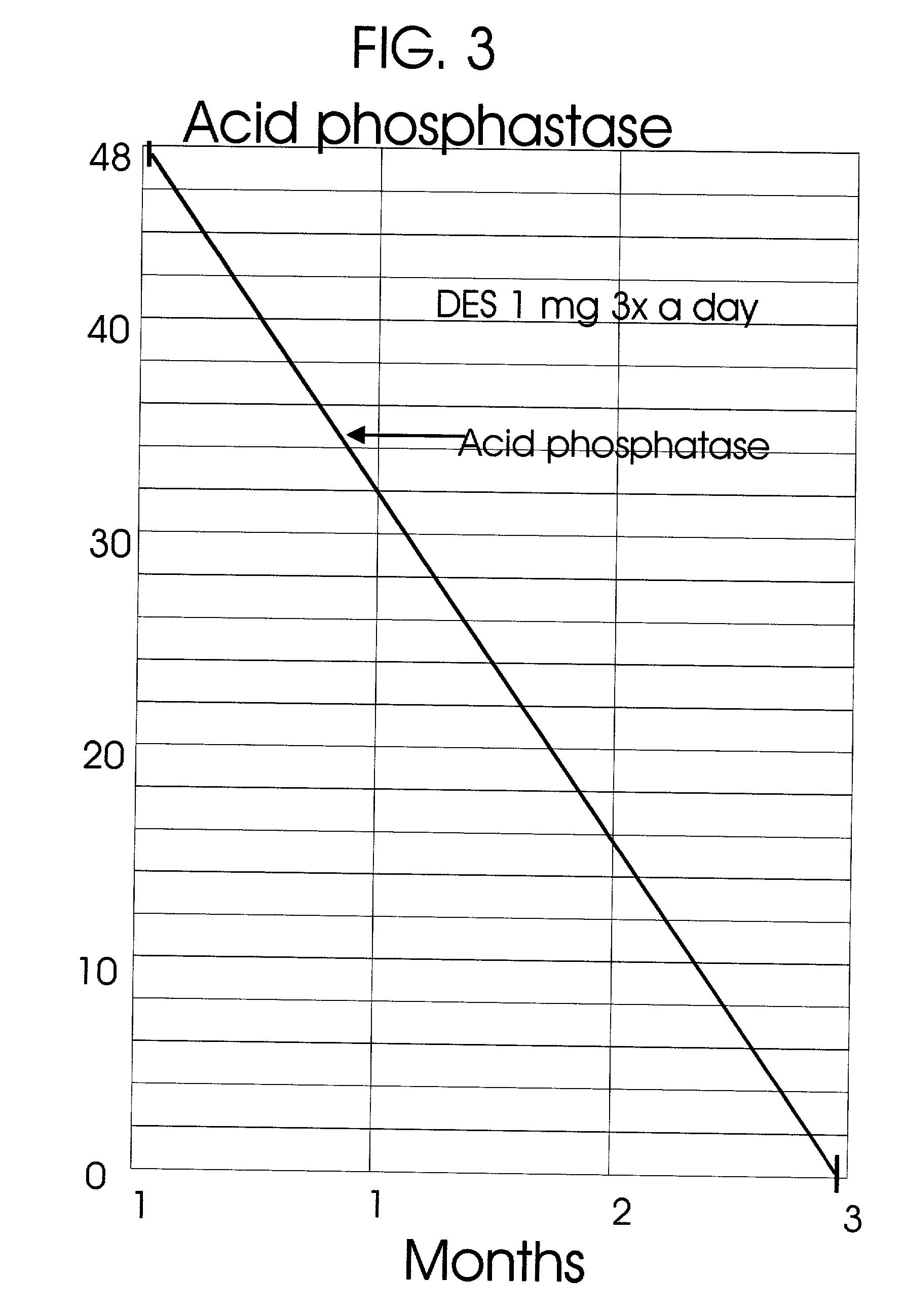
An improved method and products for the primary hormonal treatment of early stage, low and intermediate risk prostate cancers by prostatic implants of androgen suppressive drugs formulated as fused with a lipoid carrier or encapsulated in microcapsules or in Silastic capsules is provided. Such prostatic implants renders a constant slow-release of their contents to the prostate for extended periods by biodegradation and diffusion. It facilitates higher prostatic and lower systemic concentrations of androgen suppressive hormones. Because of their high prostatic and lower systemic concentrations, tumor control is much improved and the their systemic toxicity is minimized. Tumor control after such primary hormonal implant treatment is followed by clinical examinations and the biochemical tumor control is followed by periodic estimations of serum levels of PSA and acid phosphatase. More complex and expensive surgery or radiation therapy for this group of good prognostic early stage prostate cancer is reserved for those patients failing to this primary hormonal treatment. It will preserve potency more than by surgery or radiation therapy. Furthermore, it would reduce the cost of treatment for early stage prostate cancer significantly. Androgen suppressive hormonal implants to the prostate before, during or after lower dose conventional radiation therapy would also facilitate equal or better cure rates of localized prostate cancer as compared to the more complex and toxic higher dose radiation therapy.
Owner:SAHADEVAN VELAYUDHAN
Epoxy-steroidal aldosterone antagonist and calcium channel blocker combination therapy for treatment of congestive heart failure
InactiveUS20020042405A1Reduce pathogenicityImprove the level ofMetabolism disorderBlood disorderCalcium channel blockerAldosterone
A combination therapy comprising a therapeutically-effective amount of an epoxy-steroidal aldosterone receptor antagonist and a therapeutically-effective amount of a calcium channel blocker is described for treatment of circulatory disorders, including cardiovascular disorders such as hypertension, congestive heart failure, cirrhosis and ascites. Preferred calcium channel blockers are those compounds having high potency and bioavailability. Preferred epoxy-steroidal aldosterone receptor antagonists are 20-spiroxane steroidal compounds characterized by the presence of a 9alpha,11alpha-substituted epoxy moiety. A preferred combination therapy includes the calcium channel blocker verapamil HCl (Benzenacetonitrile, (±)-alpha[3[[2-(3,4-dimethoxyphenyl) ethyl]methylamino]propyl]-3,4-dimethoxy-alpha-(1-methylethyl)hydrochloride) and the aldosterone receptor antagonist epoxymexrenone.
Owner:SCHUH JOSEPH R
Multicyclic bis-amide MMP inhibitors
The present invention relates generally to bis-amide group containing pharmaceutical agents, and in particular, to multicyclic bis-amide MMP-13 inhibitor compounds. More particularly, the present invention provides a new class of MMP-13 inhibiting compounds, containing a pyrimidinyl bis-amide group in combination with a heterocyclic moiety, that exhibit an increased potency and solubility in relation to currently known bis-amide group containing MMP-13 inhibitors.
Owner:ALANTOS PHARMA HLDG INC
Salinosporamides and methods for use thereof
InactiveUS7176232B2Advantageous in treating neoplastic disorderEffective inhibitors of hyperproliferative mammalian cellsBiocideOrganic chemistryCancer cellMammal
Owner:RGT UNIV OF CALIFORNIA
Heterobicyclic metalloprotease inhibitors
The present invention relates generally to heterobicyclic containing pharmaceutical agents, and in particular, to heterobicyclic metalloprotease inhibitor compounds. More particularly, the present invention provides a new class of heterobicyclic metalloprotease inhibiting compounds that exhibit an increased potency in relation to currently known metalloprotease inhibitors.
Owner:NOLTE BERT +9
Stable, buffered, pharmaceutical compositions including motilin-like peptides
Stable, pharmaceutical compositions including a synthetic motilin-like peptide in a buffered solution are disclosed. The composition provides for a peptide that remains stable and substantially retains its initial potency during extended storage and after steam sterilization.
Owner:BAXTER INT INC +1
Nutrient compositions and methods for enhanced effectiveness of the immune system
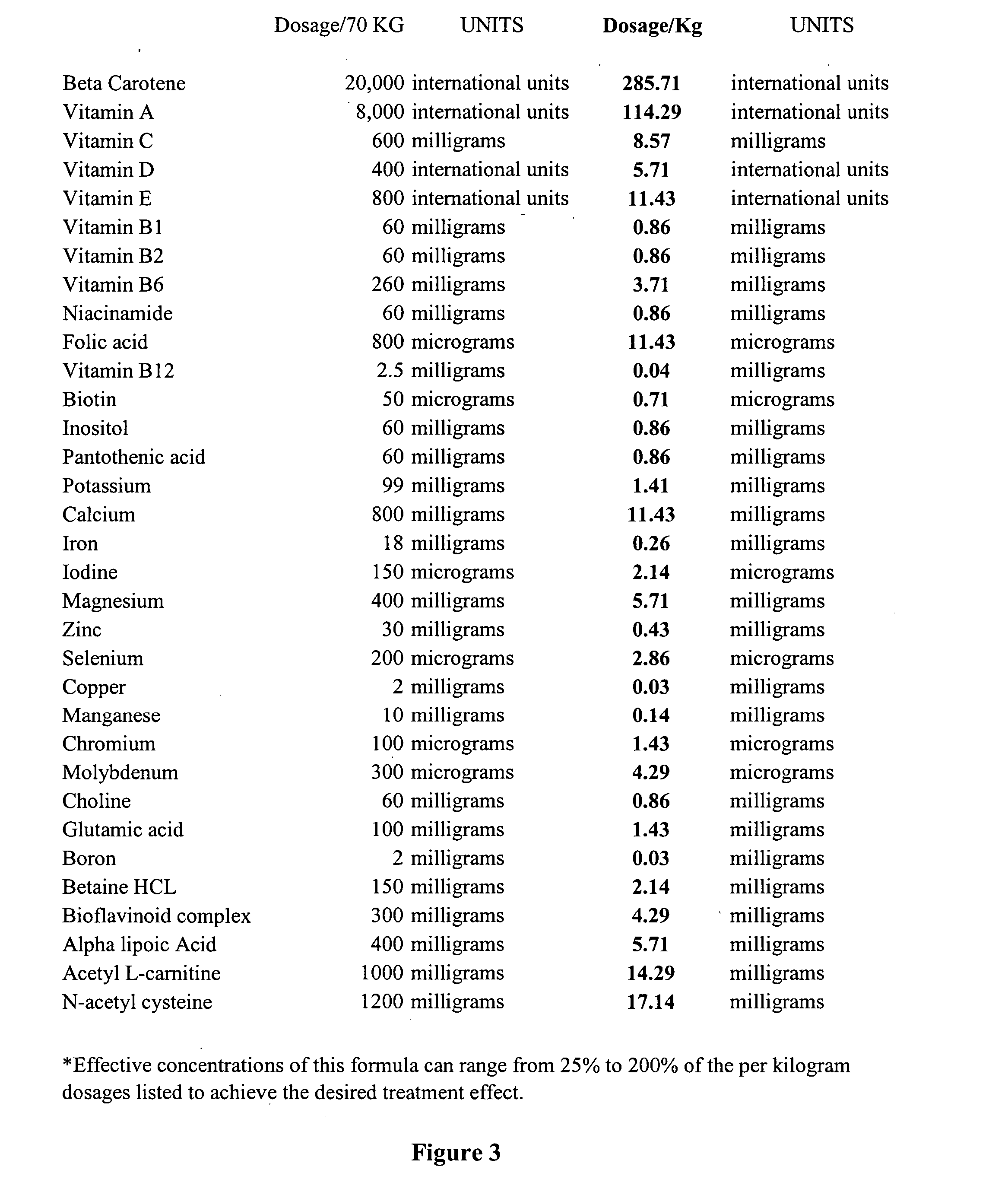
InactiveUS20050142124A1Reduce the burden onTimelyOrganic active ingredientsAntimycoticsDiseaseBeta-Carotene
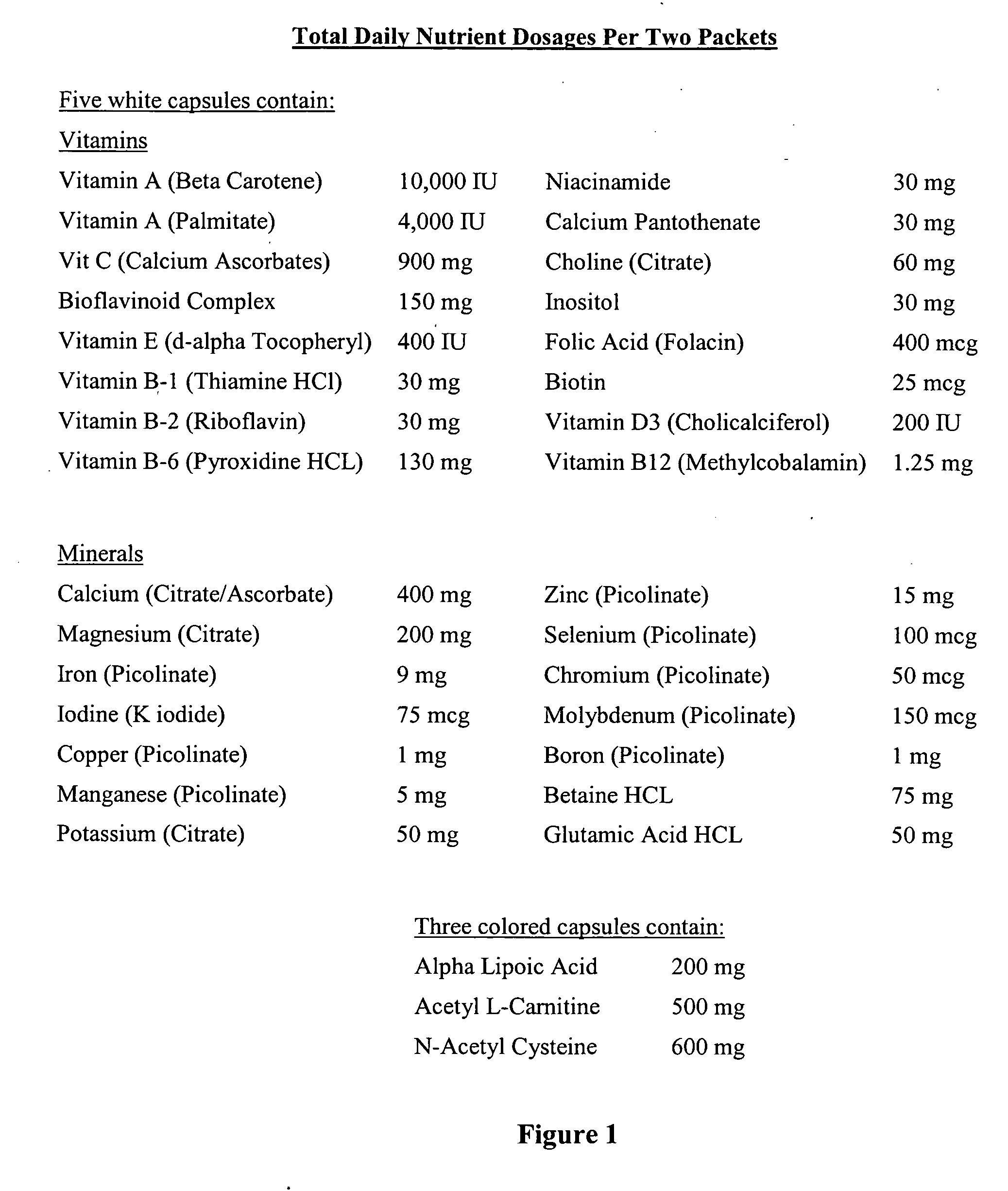
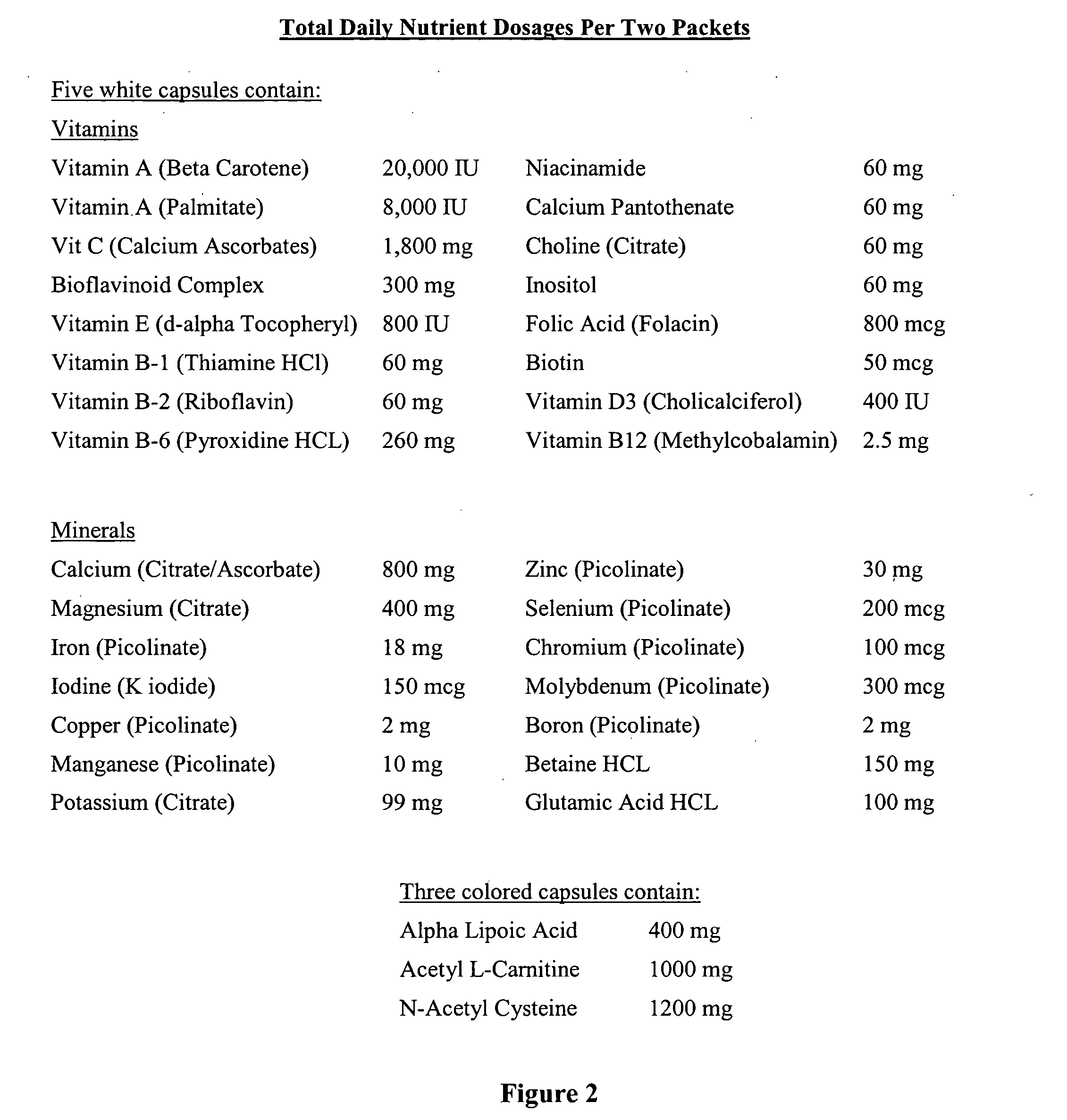
The invention provides a nutrient composition for augmenting immune strength or physiological detoxification. The nutrient composition consists of an optimal combination of an effective amount of at least one vitamin antioxidant, at least one mineral antioxidant and a highly saturable amount of at least three high potency antioxidants. The at least one vitamin antioxidant can be vitamin C, bioflavonoid complex, vitamin E, vitamin B6 or beta-carotene and the at least one mineral antioxidant can be zinc or selenium. The at least three high potency antioxidants can be alpha lipoic acid, acetyl L-carnitine, N-acetyl-cysteine, co-enzyme Q10 or glutathione. Also provided is a nutrient composition for augmenting immune strength or physiological detoxification that consists of an optimal combination of an effective amount of at least three vitamin antioxidants, at least two mineral antioxidants and a highly saturable amount of at least three high potency antioxidants. Further provided is a method of stimulating immune system function or a method of augmenting a therapeutic treatment of a disease. The method consists of administering to an individual a nutrient composition of the invention one or more times a day over a period of about 5-7 weeks, the immune system function being stimulated to result in an increase of CD4+ cells of at least about 15% compared to pre-administration levels. A method of stimulating a physiological detoxification function of an individual or a method of augmenting a therapeutic treatment of a disease is also provided. The method consists of administering to an individual a nutrient composition of the invention one or more times a day over a period of about 5-7 weeks, the physiological detoxification function being stimulated to result in a decrease of one or more free radical markers by about 20% compared to pre-administration levels.
Owner:INTEGRATIVE HEALTH CONSULTING INC
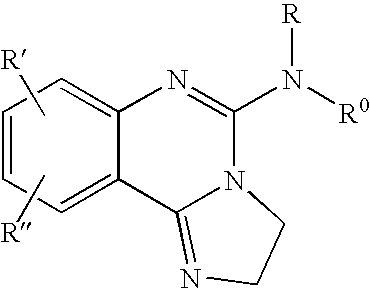
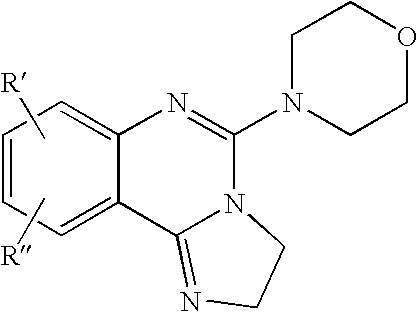
Fused azole-pyrimidine derivatives
The present invention relates to hovel fused azolepyrimidine derivatives, processes for preparing them and pharmaceutical preparations containing them. The fused azolepyrimidine derivatives of the present invention exhibit enhanced potency for phosphotidylinositol-3-kinase (PI3K) inhibition, especially for PI3K-γ inhibition and can be used for the prophylaxis and treatment of diseases associated with PI3K and particularly with PI3K-γ activity. More specifically, the azole derivatives of the present invention are useful for treatment and prophylaxis of diseases as follows: inflammatory and immunoregulatory disorders, such as asthma, atopic dermatitis, rhinitis, allergic diseases, chronic obstructive pulmonary disease (COPD), septic shock, joint diseases, autoixnmune pathologies such as rheumatoid arthritis, and Graves' disease, cancer, myocardial contractility disorders, heart failure, thromboembolism, ischemia, and atherosclerosis. The compounds of the present invention are also useful for pulmonary hypertension, renal failure, cardiac hypertrophy, as well as neurodegenerative disorders such as Parkinson's disease, Alzheimer's disease, diabetes and focal ischemia, since the diseases also relate to PI3K activity in a human or animal subject.
Owner:BAYER INTELLECTUAL PROPERTY GMBH +1
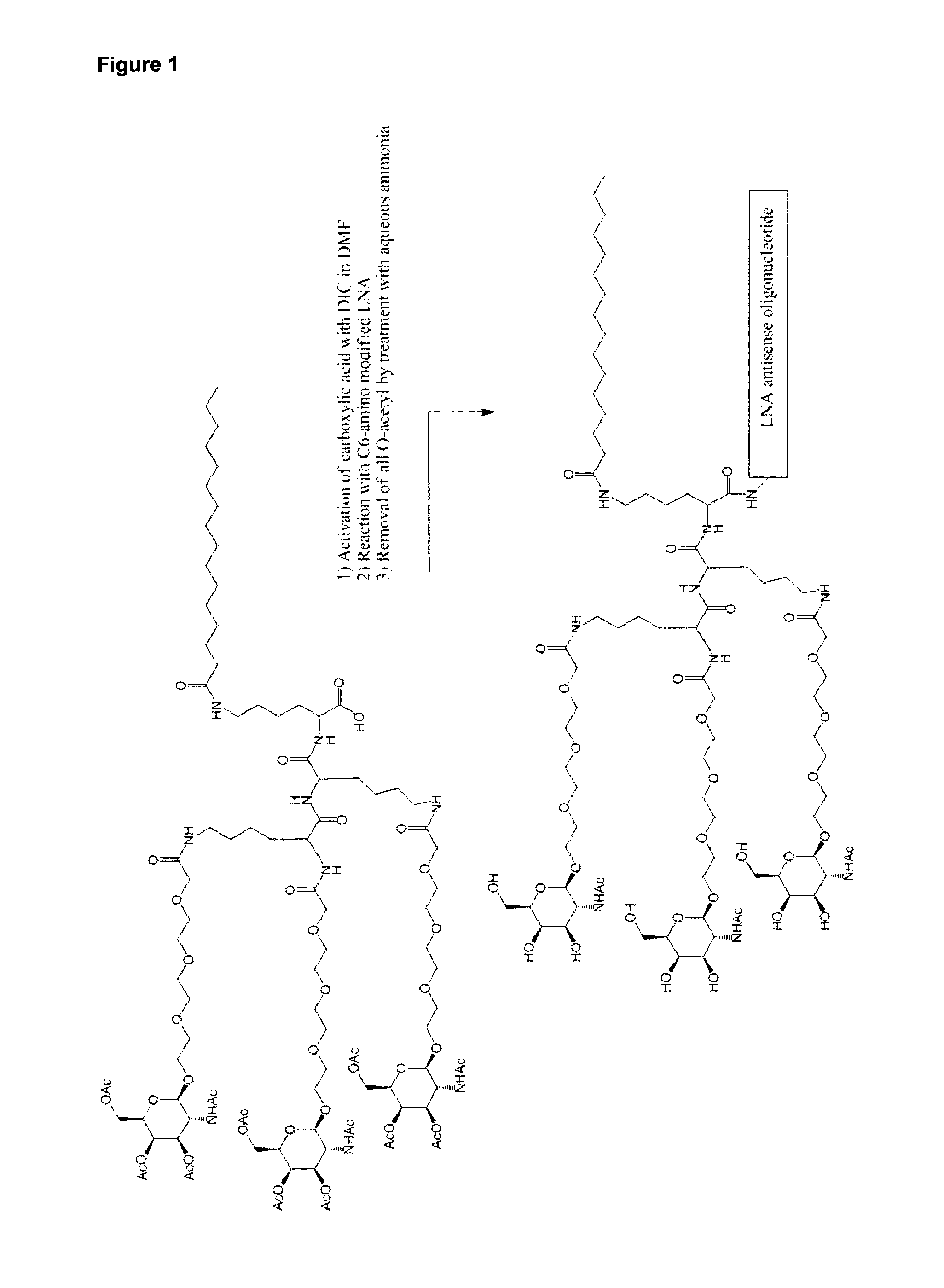
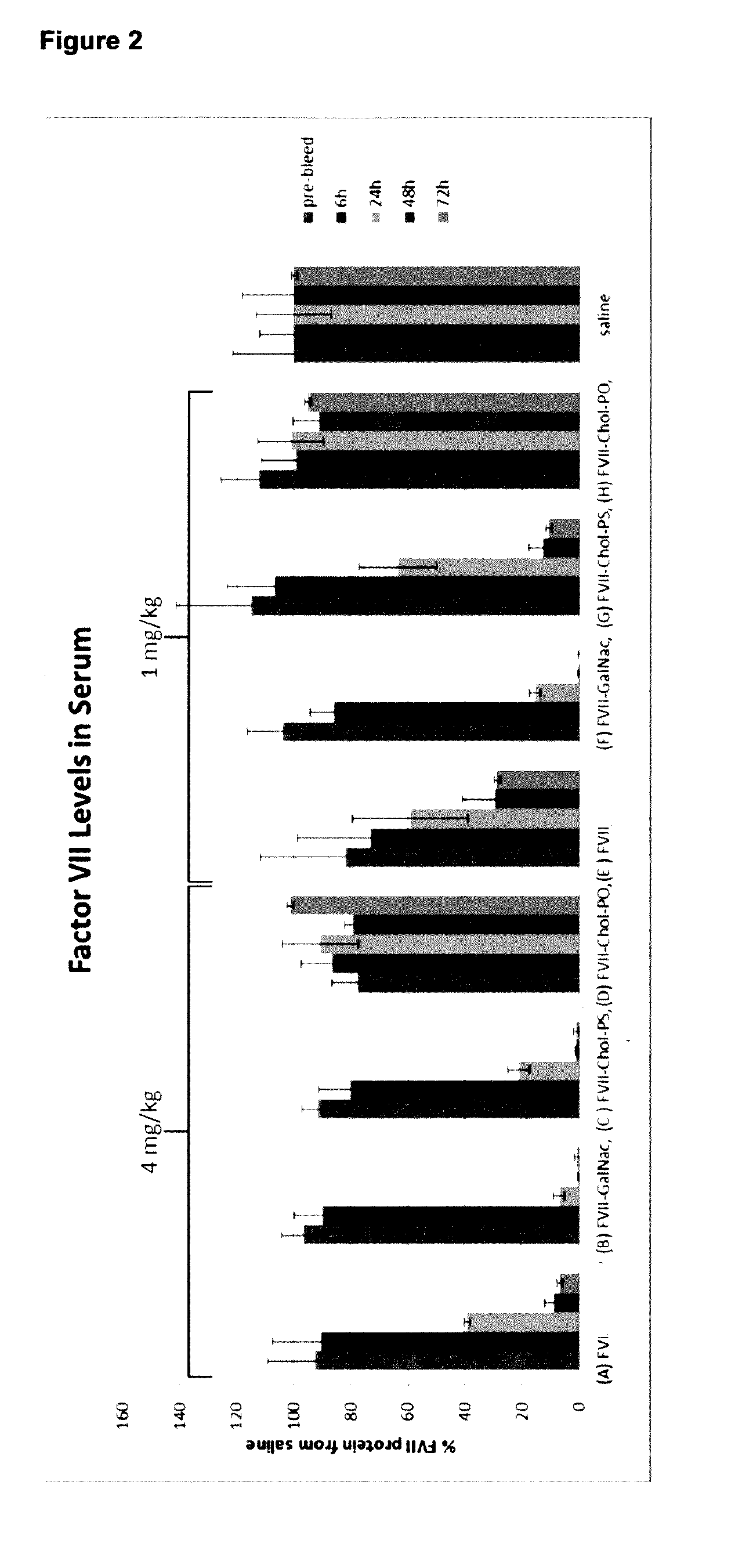
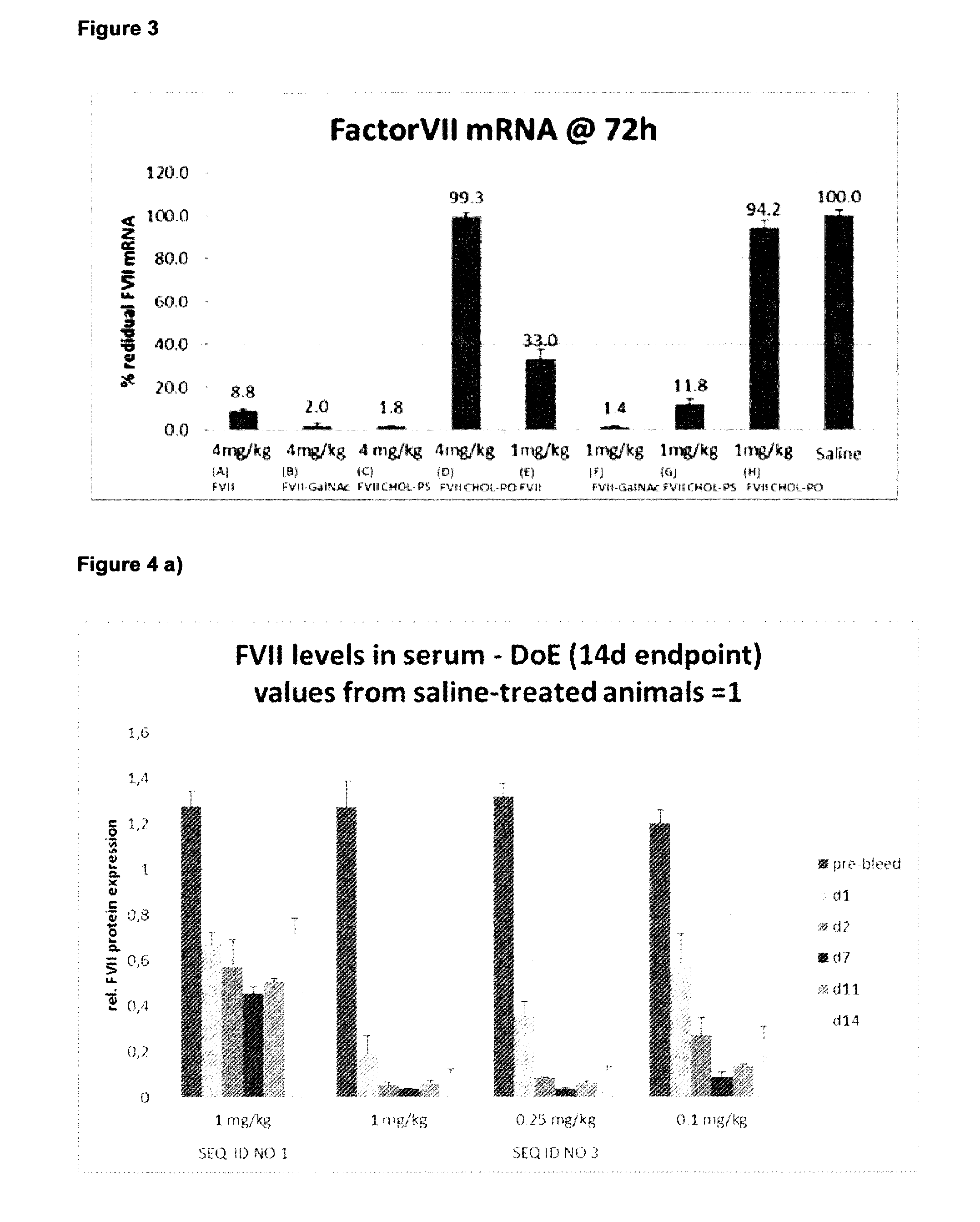
Lna oligonucleotide carbohydrate conjugates
The invention provides LNA therapeutics oligonucleotide carbohydrate conjugates with considerably enhanced potency, extended therapeutic index and reduced toxicity.
Owner:F HOFFMANN LA ROCHE & CO AG +1
Compound having s1p receptor binding potency and use thereof
Provided are: a compound represented by formula (I):(wherein ring A and ring D each represent a cyclic group which may have a substituent(s); E and G each represent a bond or a spacer having 1 to 8 atoms in its main chain; L represents a hydrogen atom or a substituent; X represents amino which may have a substituent(s), or a heterocylic group which contains at least one nitrogen atom and which may have a substituent(s); n represents 0 to 3, and when n is 2 or more, a plurality of ring A's may be the same or different from one another); a salt, an N-oxide form, a solvate, or a prodrug thereof; and a medicament which includes those. The compound of formula (I) is capable of binding S1P receptors (in particular, EDG-1 and / or EDG-6), and useful for preventing and / or treating rejection in transplantation, autoimmune diseases, allergic diseases, etc.
Owner:ONO PHARMA CO LTD
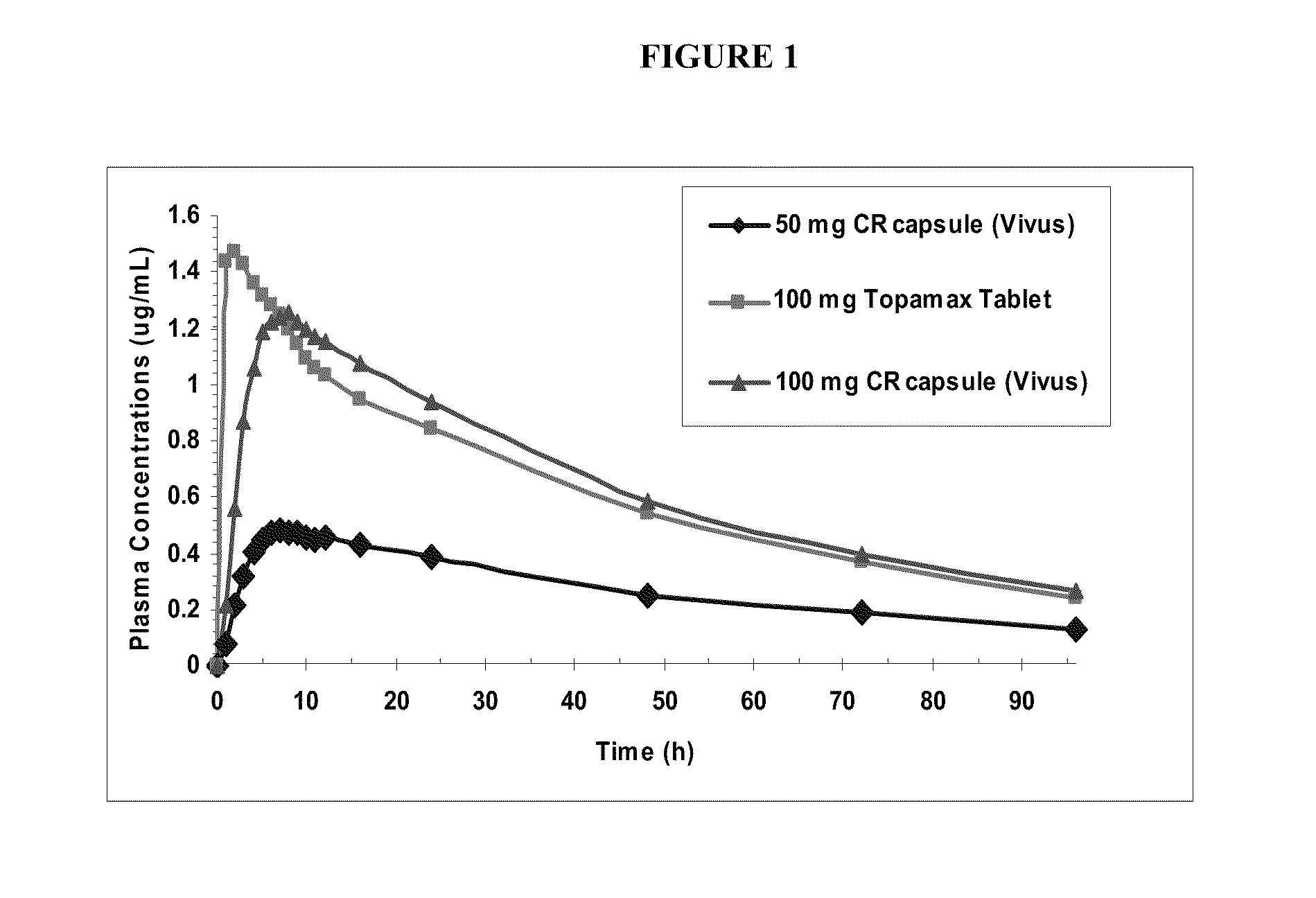
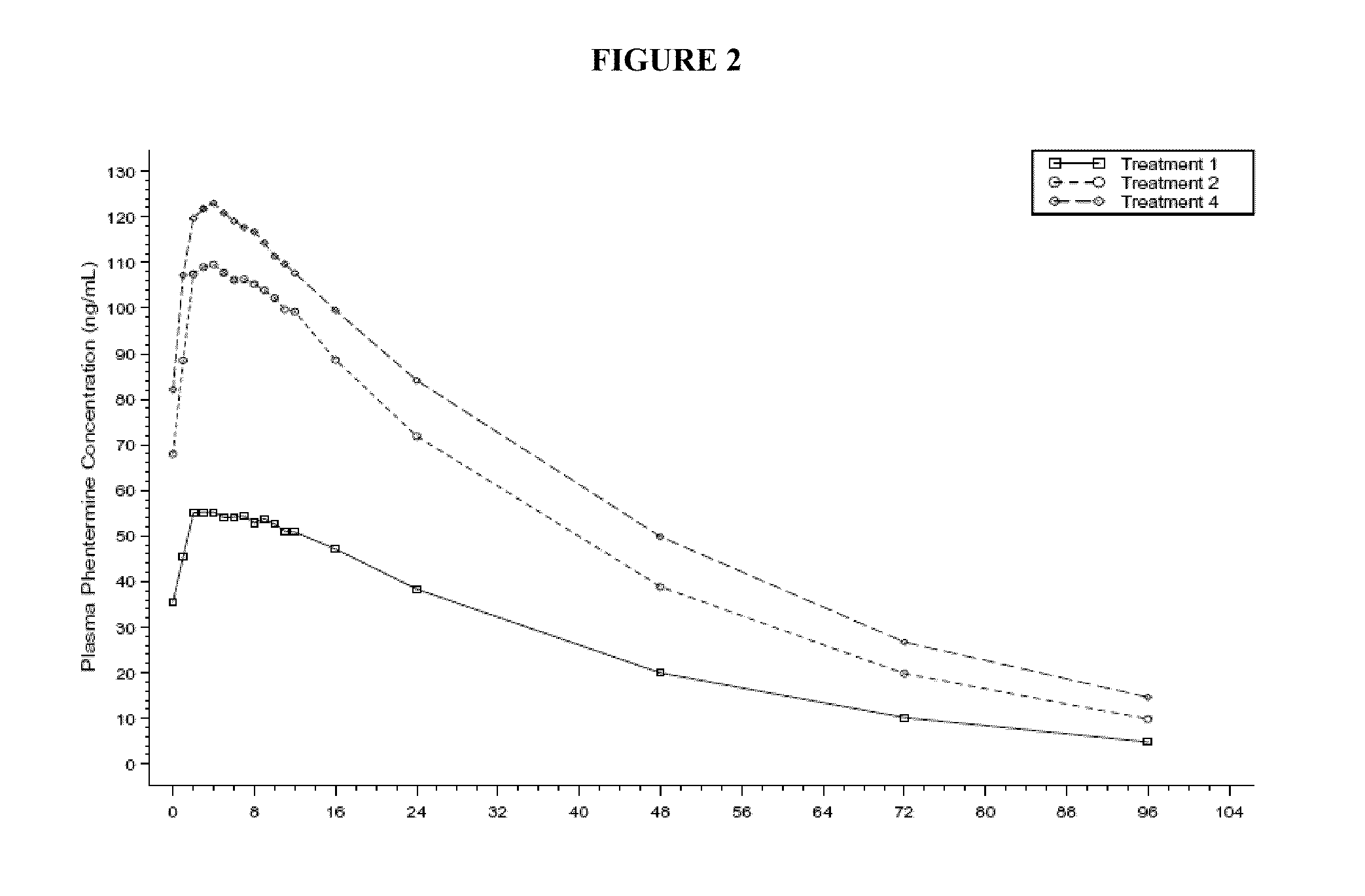
Low dose topiramate / phentermine composition and methods of use thereof
A method for effecting weight loss by administering a combination of topiramate and phentermine is provided. The phentermine is generally administered in immediate release form, in a daily dose in the range of 2 mg to 8 mg, in combination with a daily dose of topiramate selected to prevent the loss of effectiveness of phentermine alone. Methods for treating obesity, conditions associated with obesity, and other indications are also provided, as are compositions and dosage forms containing low doses of phentermine and topiramate, e.g., 3.75 mg phentermine and 23 mg topiramate.
Owner:VIVUS LLC
Anti-platelet thrombolysin and preparation method thereof
ActiveCN101838323ASpecific site of actionLow bleeding tendencyPeptide/protein ingredientsPeptide preparation methodsVascular endotheliumPurification methods
The invention relates to the field of medicaments, and in particular provides anti-platelet thrombolysin. The anti-platelet thrombolysin of the invention consists of two peptide chains, namely an alpha chain and a beta chain, wherein an amino acid sequence of the alpha chain is shown as SEQ ID NO.1; the amino acid sequence of the beta chain is shown as SEQ ID NO.2; and the specific activity of the anti-platelet thrombolysin is greater than 1,000 activity units in each milligram of protein. Platelet membrane glycoprotein GPIb protein of a key component is aggregated mainly by mediated platelets; and the combination of the platelet membrane glycoprotein GPIb protein and blood plasma vWF is interrupted so as to prevent platelets from adhering to a vascular endothelial cell wall so that the aggregation of the platelets is inhibited. The invention also provides a method for purifying the anti-platelet thrombolysin, which has the characteristics of high specificity, high yield and short period. Pharmacology, toxicology, potency, pharmacokinetic experiments show that the anti-platelet thrombolysin of the invention is a safe and high-efficiency medicament for inhibiting the aggregation of the platelets and has a broad clinical application prospect.
Owner:ZHAOKE PHARMA HEFEI
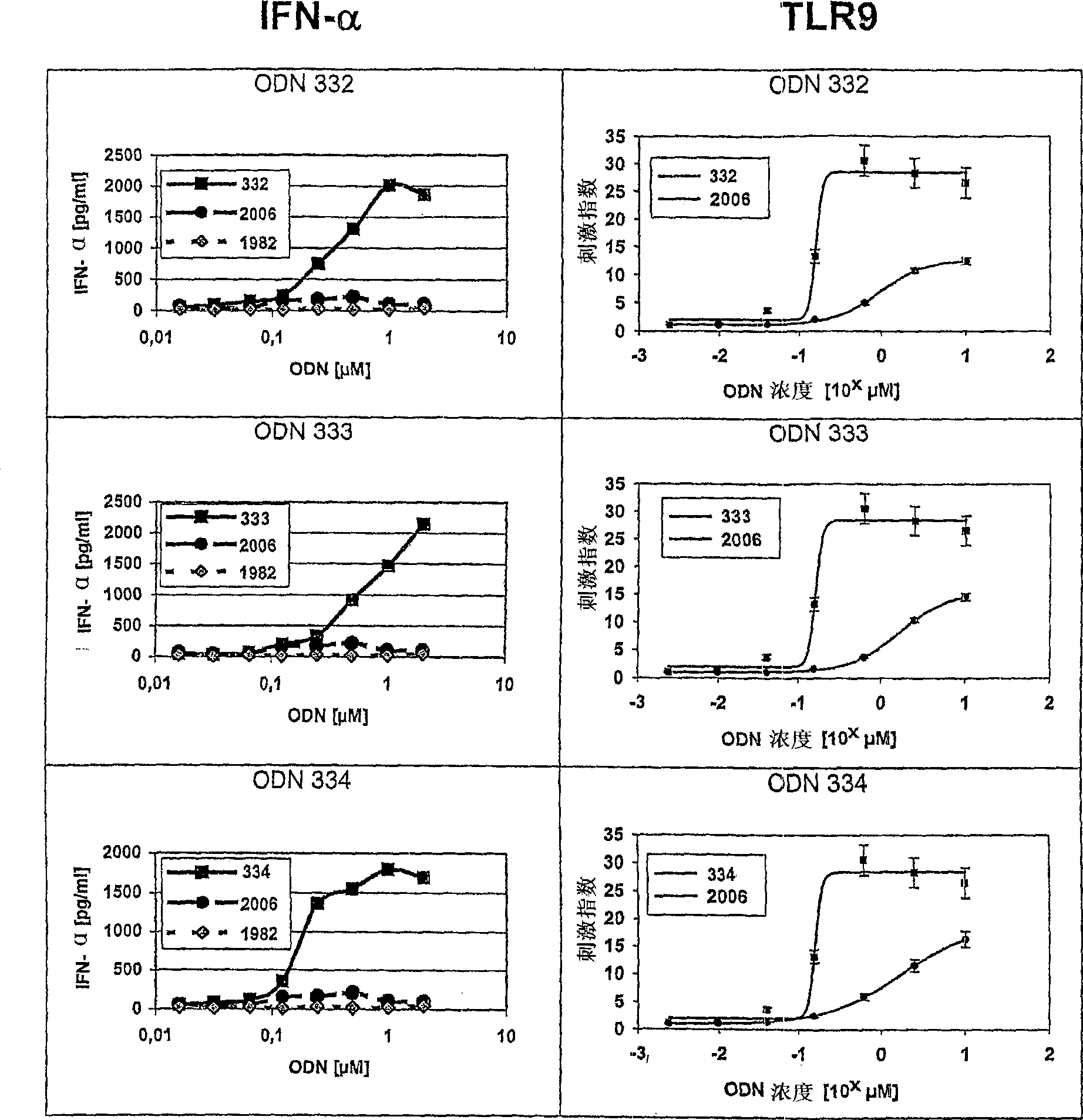
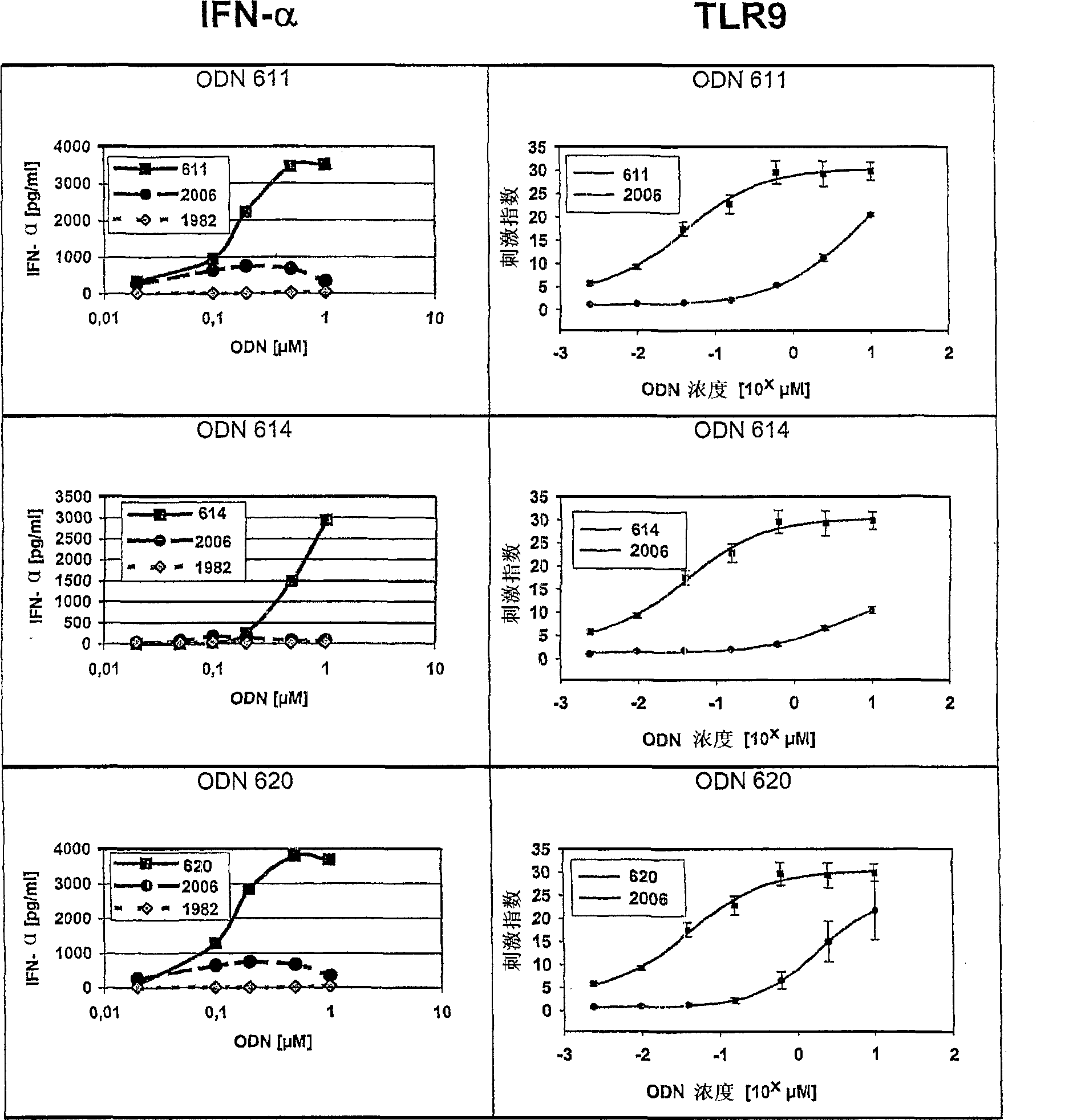
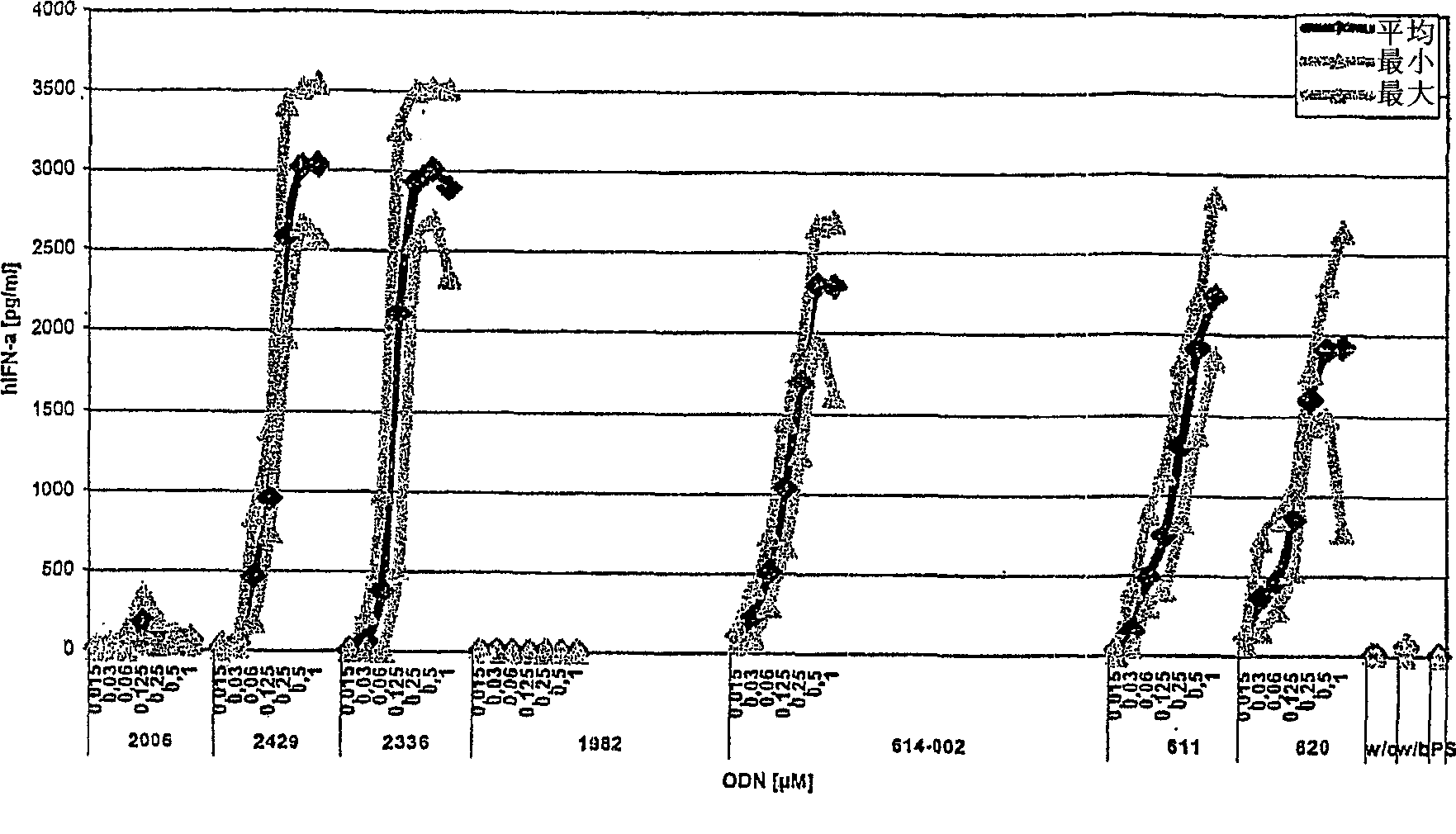
C-class oligonucleotide analogs with enhanced immunostimulatory potency
The invention relates to a class of CpG immunostimulatory oligonucleotides containing a CpG immunostimulatory motif and a second motif which is capable of forming secondary structure, including duplex and higher order structures, in vitro and in vivo. The oligonucleotides of the invention are useful as adjuvants in vaccination. The oligonucleotides are also useful for inducing an immune response, inducing expression of a type I interferon (IFN), inducing expression of gamma interferon (IFN-gamma), and for treating a variety of conditions, including allergy, asthma, infection, and cancer.
Owner:科勒制药有限公司 +1
Compound having S1P receptor binding potency and use thereof
InactiveUS8039674B2Improve permeabilityLess side effectsBiocideNervous disorderHydrogen atomS1P Receptor
Provided are: a compound represented by formula (I):(wherein ring A and ring D each represent a cyclic group which may have a substituent(s); E and G each represent a bond or a spacer having 1 to 8 atoms in its main chain; L represents a hydrogen atom or a substituent; X represents amino which may have a substituent(s), or a heterocyclic group which contains at least one nitrogen atom and which may have a substituent(s); n represents 0 to 3, in which when n is 2 or more, a plurality of ring A's may be the same or different from one another); a salt thereof; an N-oxide form thereof; a solvate thereof, a prodrug thereof; and a medicament which includes those. The compound represented by formula (I) is capable of binding S1P receptors (in particular, EDG-1 and / or EDG-6), and useful for preventing and / or treating rejection in transplantation, autoimmune diseases, allergic diseases, etc.
Owner:ONO PHARMA CO LTD
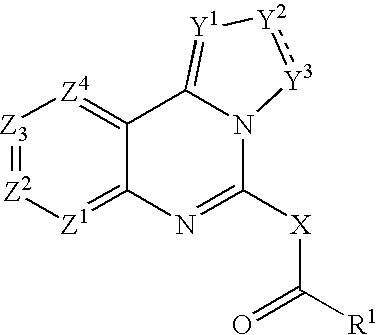
Use of tryptanthrin compound as indoleamine 2,3-dioxygenase (IDO) inhibitor
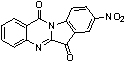
InactiveCN103054870AExcellent inhibitory effectOrganic active ingredientsNervous disorderDiseaseInhibition constant
The invention belongs to the medicinal field, and concretely relates to a use of a 8-nitrotryptanthrin compound as an IDO inhibitor. The 8-nitrotryptanthrin compound is a reversible IDO inhibitor, has an inhibition constant Ki of 0.054muM, has in-vitro and cell-based median effective inhibition concentrations IC50 of 0.103muM and 1.80*10<-5>muM respectively, and has an inhibition effectiveness obviously better than a present inhibitor 1-methyltryptophan (Ki of 34muM and IC50 of 340muM). 8-nitrotryptanthrin disclosed in the invention can effectively lower the abnormally-increasing IDO activity in a tumor animal model as the IDO inhibitor, and also has tumor treatment effects comprising tumor growth delaying, tumor volume reduction and in-vitro tumor cell killing. 8-nitrotryptanthrin disclosed in the invention has a wide application prospect, and can be used for treating serious diseases having the IDO mediated tryptophan metabolism approach pathology characteristics, such as cancers, the Alzheimer disease, tristimania, cataract and the like as the IDO inhibitor.
Owner:FUDAN UNIV
N-terminally modified GLP-1 receptor modulators
InactiveUS20070021346A1Improve efficacyMaintain good propertiesSenses disorderNervous disorderGlucagon-like peptide-1Drug biological activity
The subject matter described herein provides novel human glucagon-like peptide-1 (GLP-1) receptor modulators that have biological activity similar or superior to native GLP-1 peptide and thus are useful for the treatment or prevention of diseases or disorders associated with GLP activity. The described compounds include chemically modified peptides that not only stimulate insulin secretion in type II diabetics, but also produce other beneficial insulinotropic responses. These synthetic peptide GLP-1 receptor modulators exhibit increased stability to proteolytic cleavage making them ideal therapeutic candidates for oral or parenteral administration. The disclosed and claimed peptides show desirable pharmacokinetic properties and desirable potency in efficacy models of diabetes.
Owner:BRISTOL MYERS SQUIBB CO
Novel antibodies
InactiveUS20160090416A1High affinityHigh potencyAnimal cellsBacteriaEpitopeAntiendomysial antibodies
Owner:NUMAB INNOVATION AG
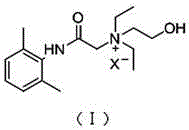
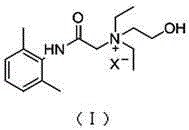
Application of N-acetanilide cationic compound in preparation of local nerve blocking drug
The invention discloses an application of an N-acetanilide cationic compound in preparation of a local nerve blocking drug. The cationic compound is a quaternary ammonium salt compound and has a structure as shown in a formula (I), wherein X is a halogen atom. When used alone, the compound has the characteristics of good safety performance, strong nerve blocking effect and the like, can take a reversible and durable local anaesthesia effect in vivo and can be used as a local anaesthesia drug or an analgesic drug which is long-acting and / or capable of realizing selective blocking. Particularly, a composition composed of the compound and other local anaesthesia drugs has the remarkable characteristics of high effect taking speed, strong effect, long acting time, little nerve injury and the like when used for nerve blocking.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
Injectable Botulinum Toxin Formulations
ActiveUS20110268765A1Rapid onsetLow antigenicityCosmetic preparationsSenses disorderClinical efficacyEfficacy
This invention provides novel injectable compositions comprising botulinum toxin that may be administered to a subject for various therapeutic, aesthetic and / or cosmetic purposes. The injectable compositions contemplated by the invention exhibit one or more advantages over conventional botulinum toxin formulations, including reduced antigenicity, a reduced tendency to undergo unwanted localized diffusion following injection, increased duration of clinical efficacy or enhanced potency relative, faster onset of clinical efficacy, and / or improved stability.
Owner:REVANCE THERAPEUTICS INC
Method for obtaining high-purity 17α-acetoxy-11β-(4-n,n-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione
The invention discloses a method of acquiring high-purity 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione, comprising the following steps: a) putting the acquired crude 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione in a proper solvent system to generate a pure 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione solid; b) separating the 17 alpha-acetoxy-11 beta-(4-N, N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione solid; and c) carrying out recrystallization on the acquired solid. The compound used as a new oral emergency contraception can be taken in 120 h after unprotected sexual intercourse of women without a reduction of emergency contraception effect with the delay of the time of using drugs, and has good safety and survivability simultaneously.
Owner:SICHUAN UNIV
Cyclosporine analogue mixtures and their use as immunomodulating agents
ActiveUS20050192214A1Improve effectivenessLow toxicitySenses disorderDispersion deliveryCyclosporinsImmunomodulating Agent
The invention is directed to isomeric mixtures of cyclosporine analogues that are structurally similar to cyclosporine A. The mixtures possess enhanced efficacy and reduced toxicity over the individual isomers and over naturally occurring and other presently known cyclosporines and cyclosporine derivatives. Embodiments of the present invention are directed toward cis and trans-isomers of cyclosporin A analogs referred to as ISATX247, and derivatives thereof. Mixtures of ISATX247 isomers exhibit a combination of enhanced potency and reduced toxicity over the naturally occurring and presently known cyclosporins. ISATX247 isomers and alkylated, arylated, and deuterated derivatives are synthesized by stereoselective pathways where the particular conditions of a reaction determine the degree of siereoselectivity. The ratio of isomers in a mixture may range from about 10 to 90 percent by weight of the (E)-isomer to about 90 to 10 percent by weight of the (Z)-isomer, based on the total weight of the mixture.
Owner:AURINIA PHARMA
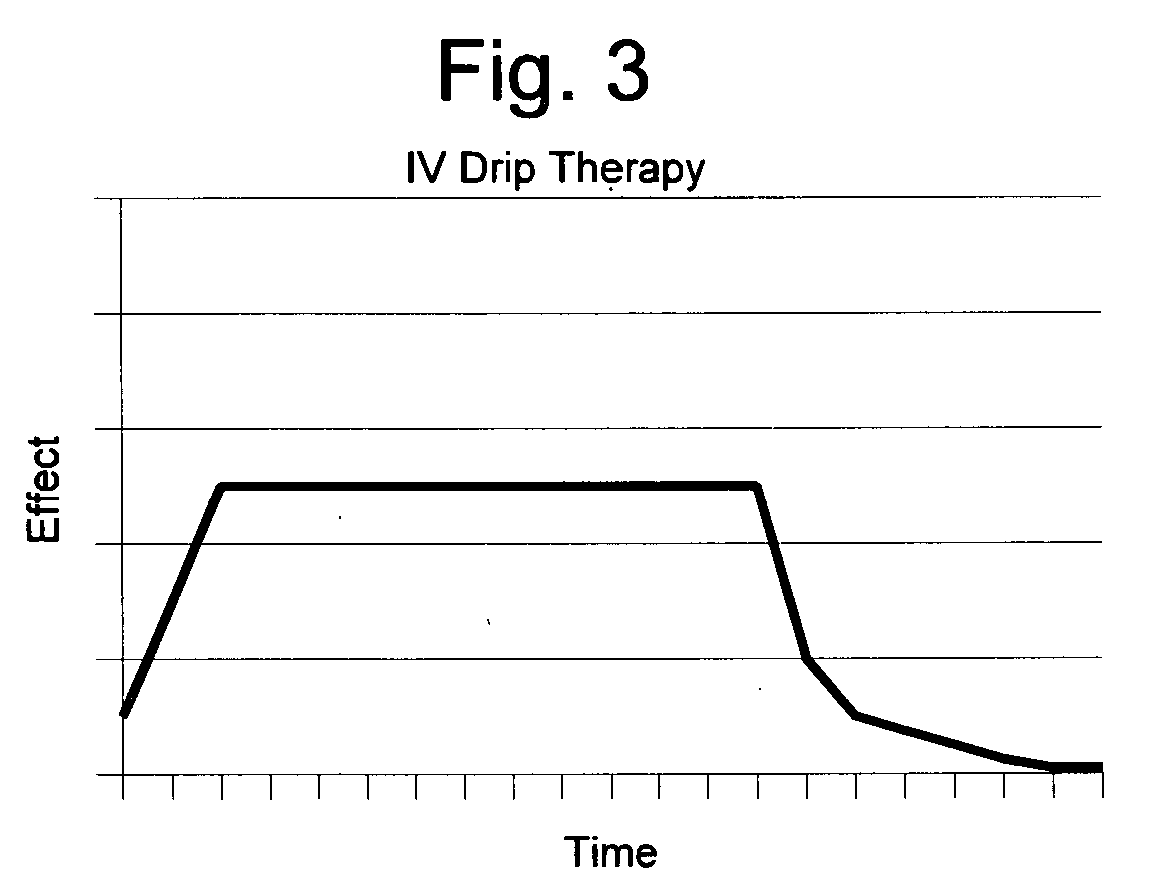
High potency clinical anti-craving treatment and method of use
ActiveUS20050287226A1Quick effectReduce cravingsHeavy metal active ingredientsBiocideSubstance abuserActive agent
A practical high potency anti-craving medication is disclosed which comprises three components: an amino-acid component, a vitamin component, and a mineral component, wherein each component is selected for maximum efficacy in the body of an individual suffering from substance abuse disorder as opposed to the body of a healthy individual. Additionally, the active agents are received by means of a prolonged administration, preferably by means of an IV drip, thus assuring a period of time in which the active agents are present in desired concentrations, and more preferably a prolonged time during which they are simultaneously present in desired concentrations. The agents of each component are also selected so as to allow easy administration of the medication to patients in three vials of medication rather than as a large number of individual vials.
Owner:REAL SUBSTANCE SOLUTIONS
Antagonistic analogs of gh rh (2003)
InactiveUS20070042950A1Inhibition releaseEnhanced inhibitory effectPeptide preparation methodsDepsipeptidesHuman cancerCancer cell
There is provided a novel series of synthetic antagonistic analogs of hGH-RH(1-29)NH2. These analogs inhibit the activity of endogenous hGH-RH on the pituitary GH-RH receptors, and therefore prevent the release of growth hormone. The analogs also inhibit the proliferation of human cancers through a direct effect on the cancer cells. The higher inhibitory potencies of the new analogs, as compared to previously described ones, results from replacement of various amino acids.
Owner:THE ADMINISTRATORS OF THE TULANE EDUCATIONAL FUND +1
High potency recombinant antibodies, methods for producing them and use in cancer therapy
InactiveUS20100166746A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsPenetranceCancer therapy
The present invention contemplates improved recombinant anti-tumor antibodies having faster Kon and faster Koff rates, resulting in a uniform tumor penetrance, as compared to the same recombinant anti-tumor antibody without said faster Kon and faster Koff rates, and methods of improving the same.
Owner:MEDIMMUNE LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com