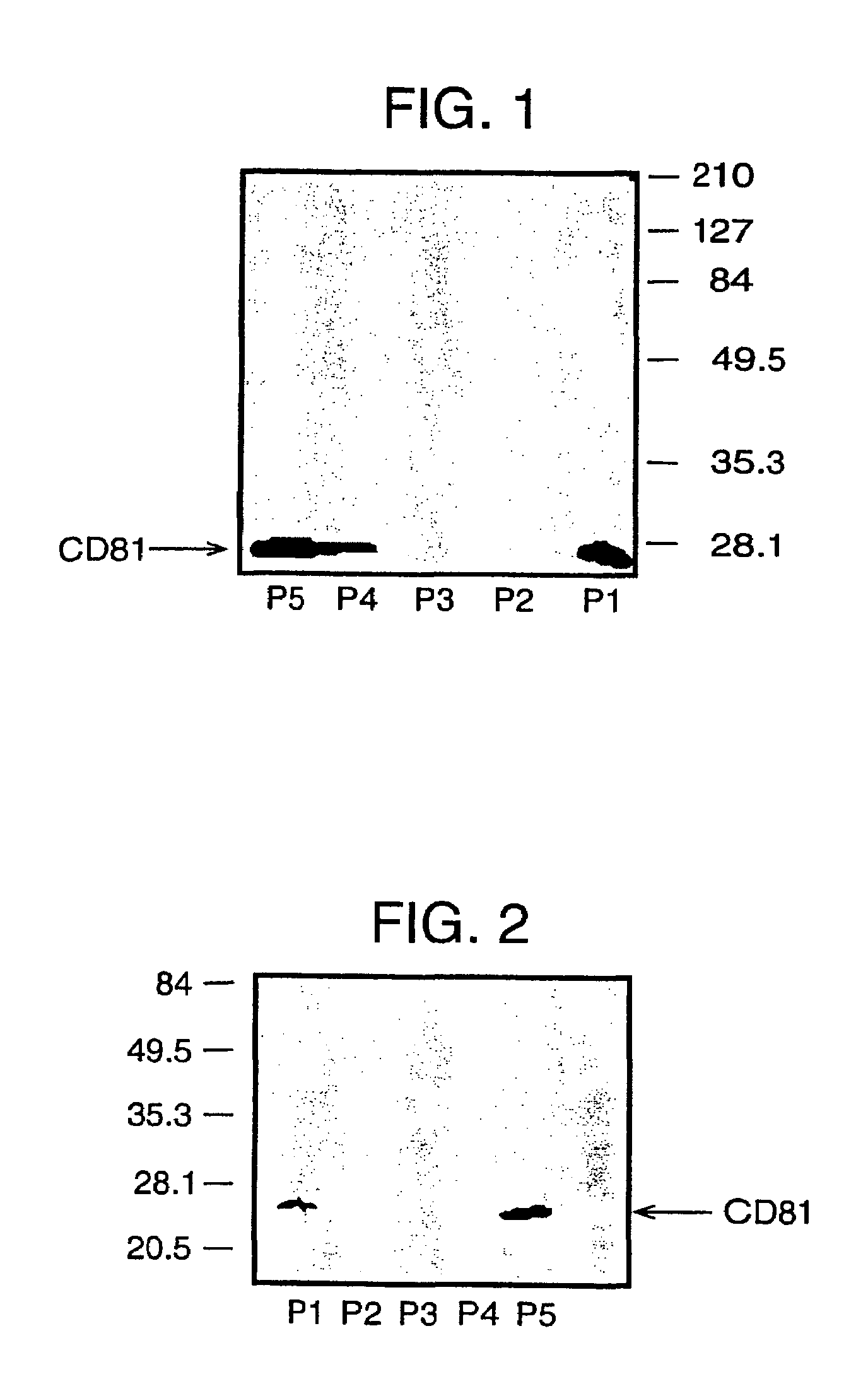
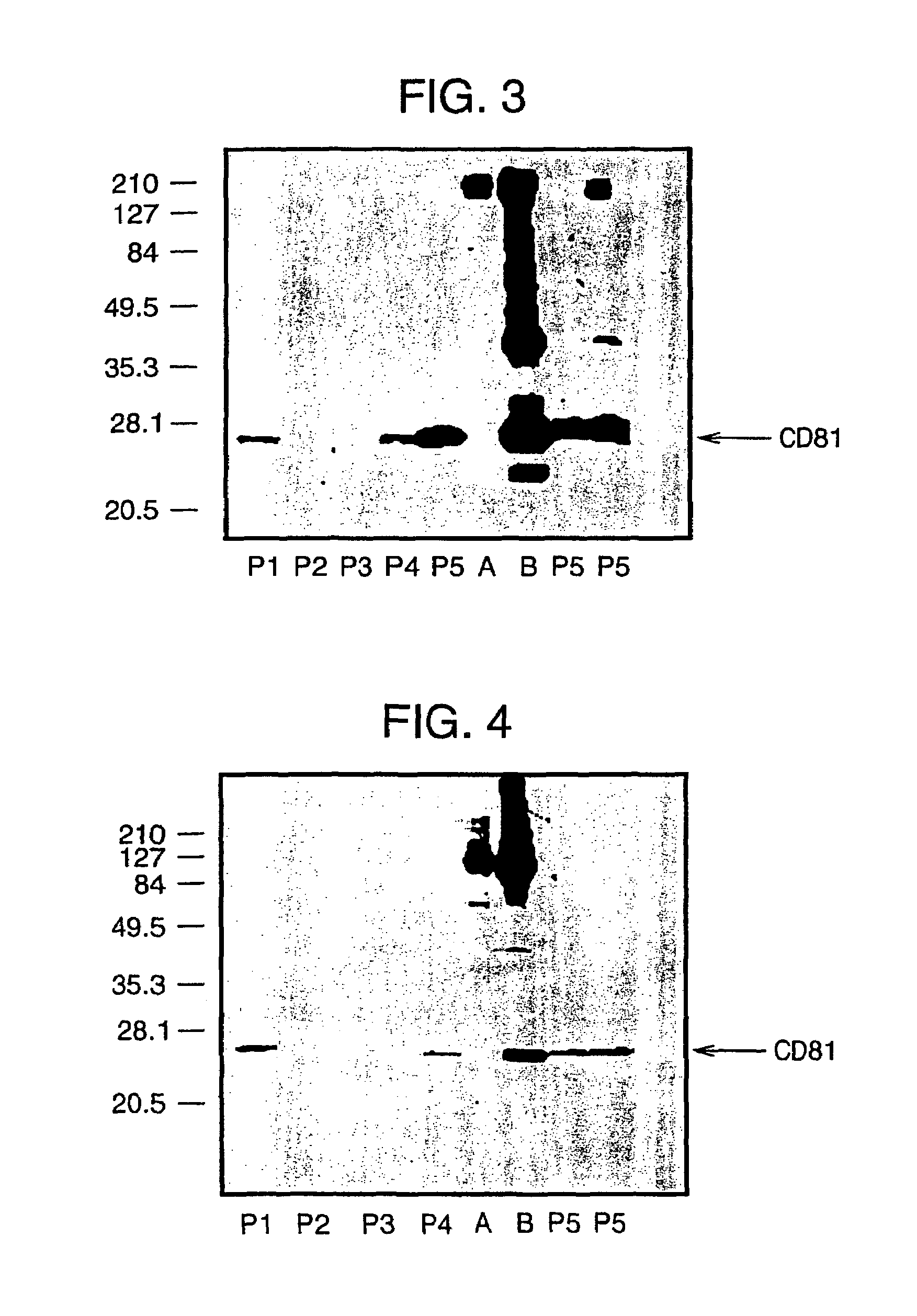
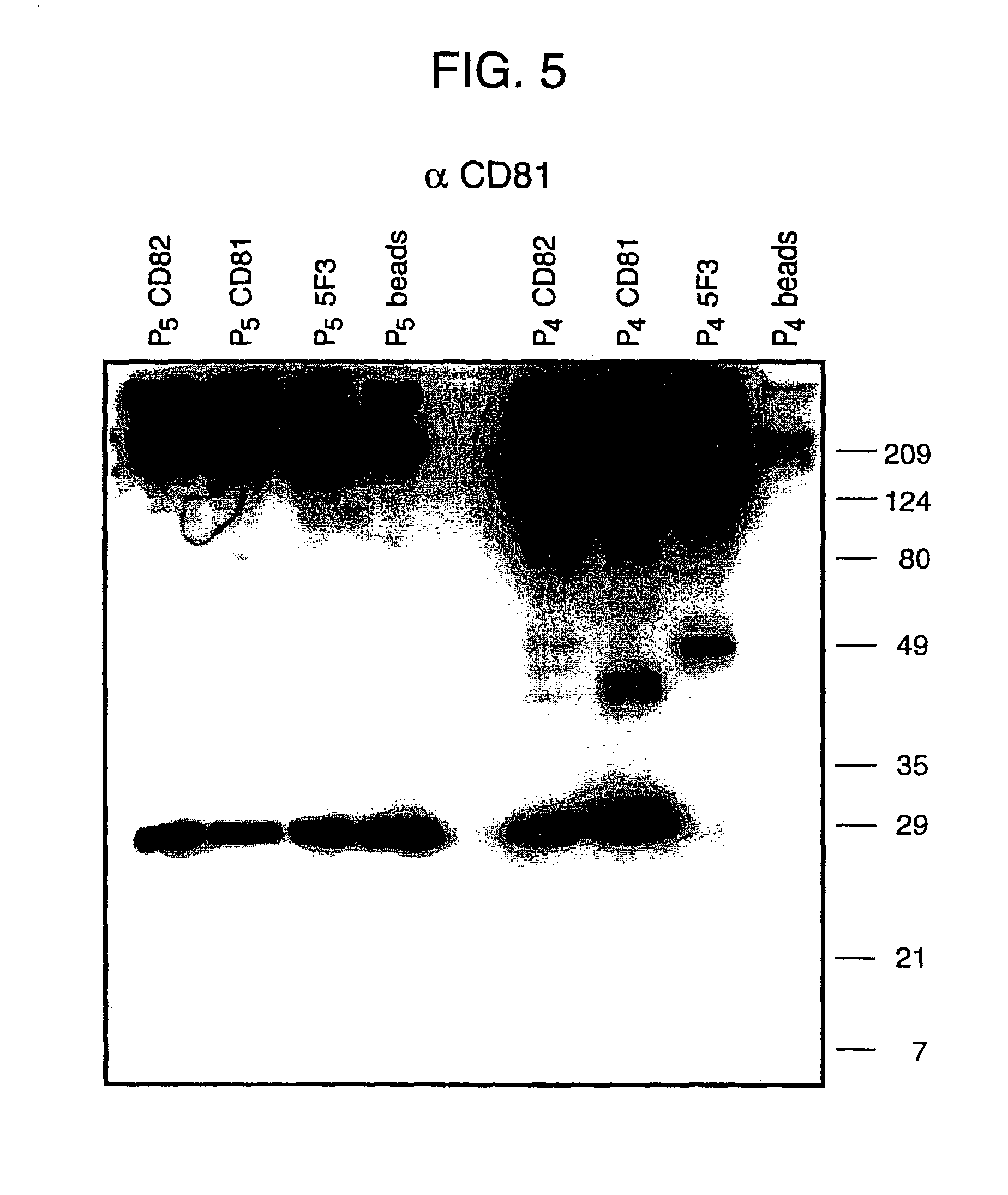
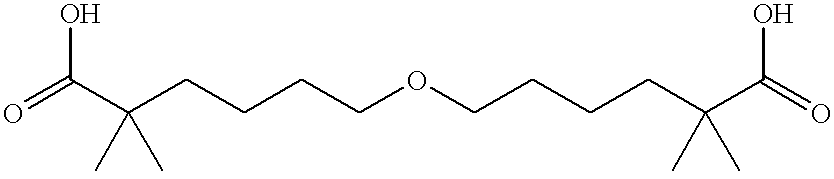
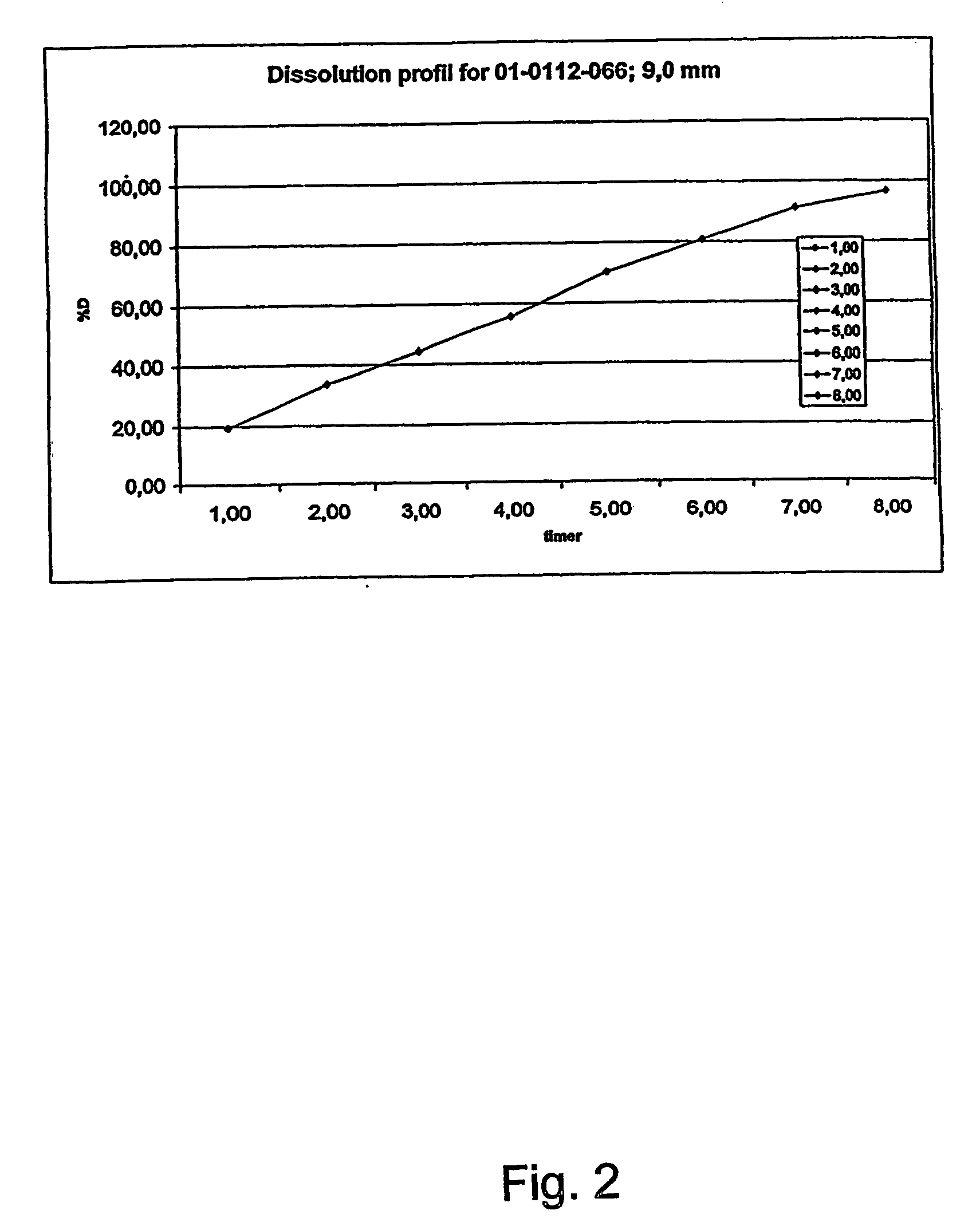
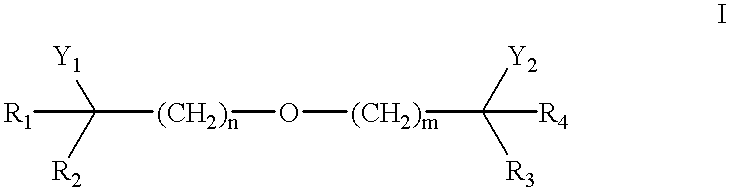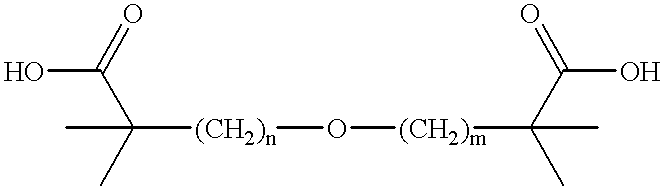Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
9358 results about "Blood plasma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Blood plasma is a yellowish liquid component of blood that holds the blood cells in whole blood in suspension. It is the liquid part of the blood that carries cells and proteins throughout the body. It makes up about 55% of the body's total blood volume. It is the intravascular fluid part of extracellular fluid (all body fluid outside cells). It is mostly water (up to 95% by volume), and contains dissolved proteins (6–8%) (e.g. serum albumins, globulins, and fibrinogen), glucose, clotting factors, electrolytes (Na⁺, Ca²⁺, Mg²⁺, HCO₃⁻, Cl⁻, etc.), hormones, carbon dioxide (plasma being the main medium for excretory product transportation) and oxygen. It plays a vital role in an intravascular osmotic effect that keeps electrolyte concentration balanced and protects the body from infection and other blood disorders.
Tissue resurfacing
InactiveUS7335199B2Easy to getRapid treatment at the tissue surfaceInstrument handpiecesSurgical instruments for heatingSkin treatmentsSkin surface
Owner:ENERGIST
Non-invasive prenatal diagnosis
InactiveUS6258540B1% accurate detection rateIncrease the amount of foetal nucleic acid materialMicrobiological testing/measurementRecombinant DNA-technologyPrenatal diagnosisBlood typing
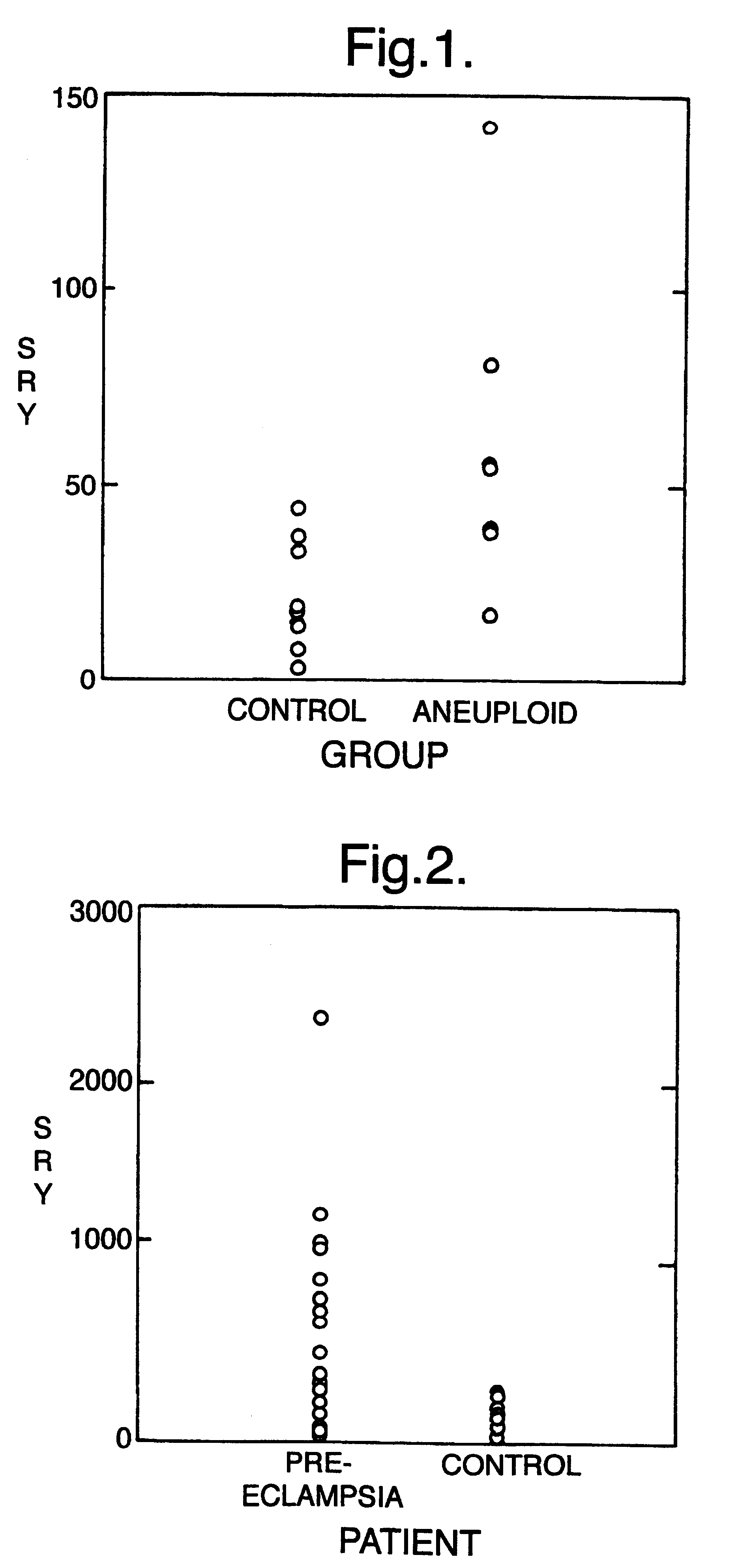
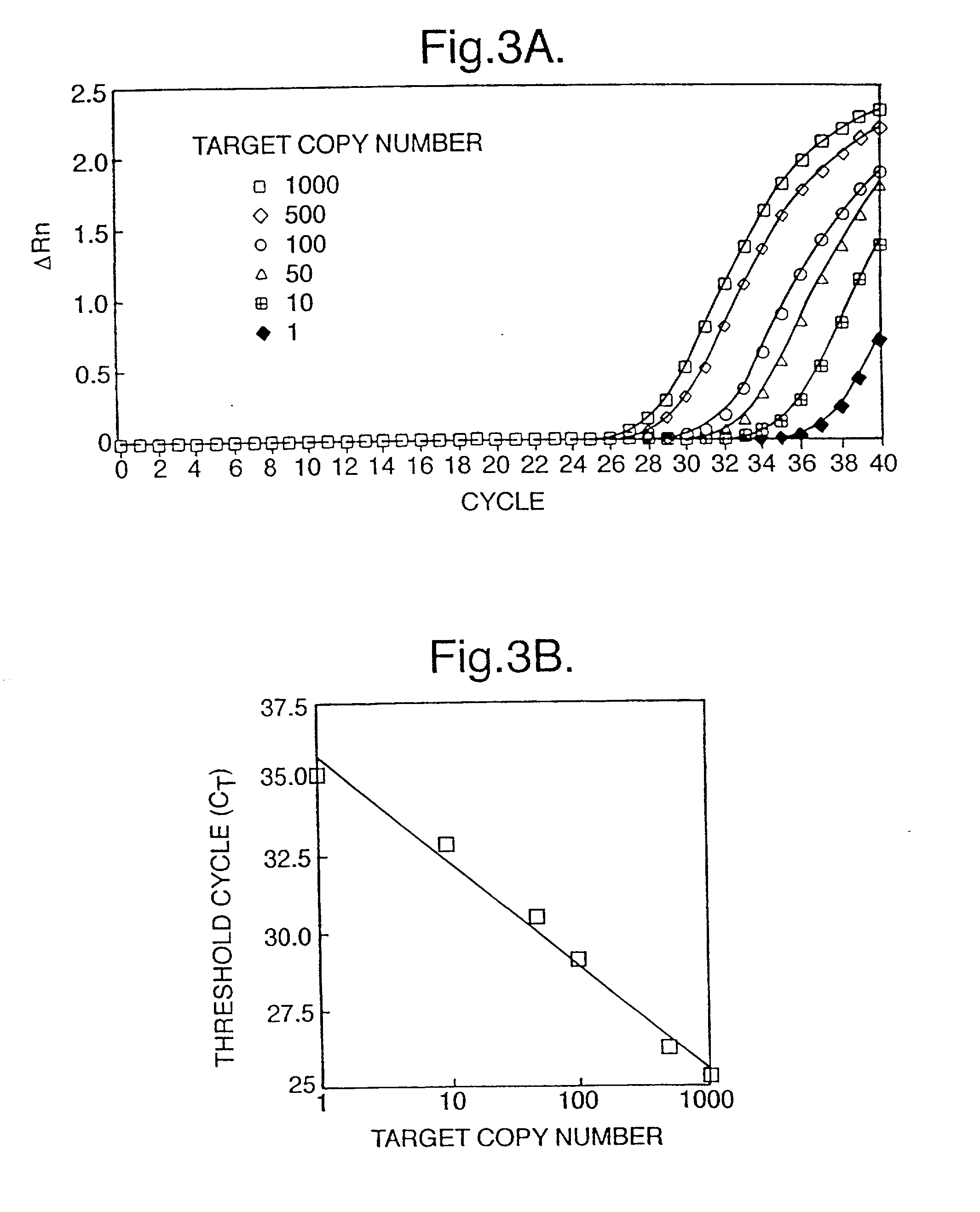
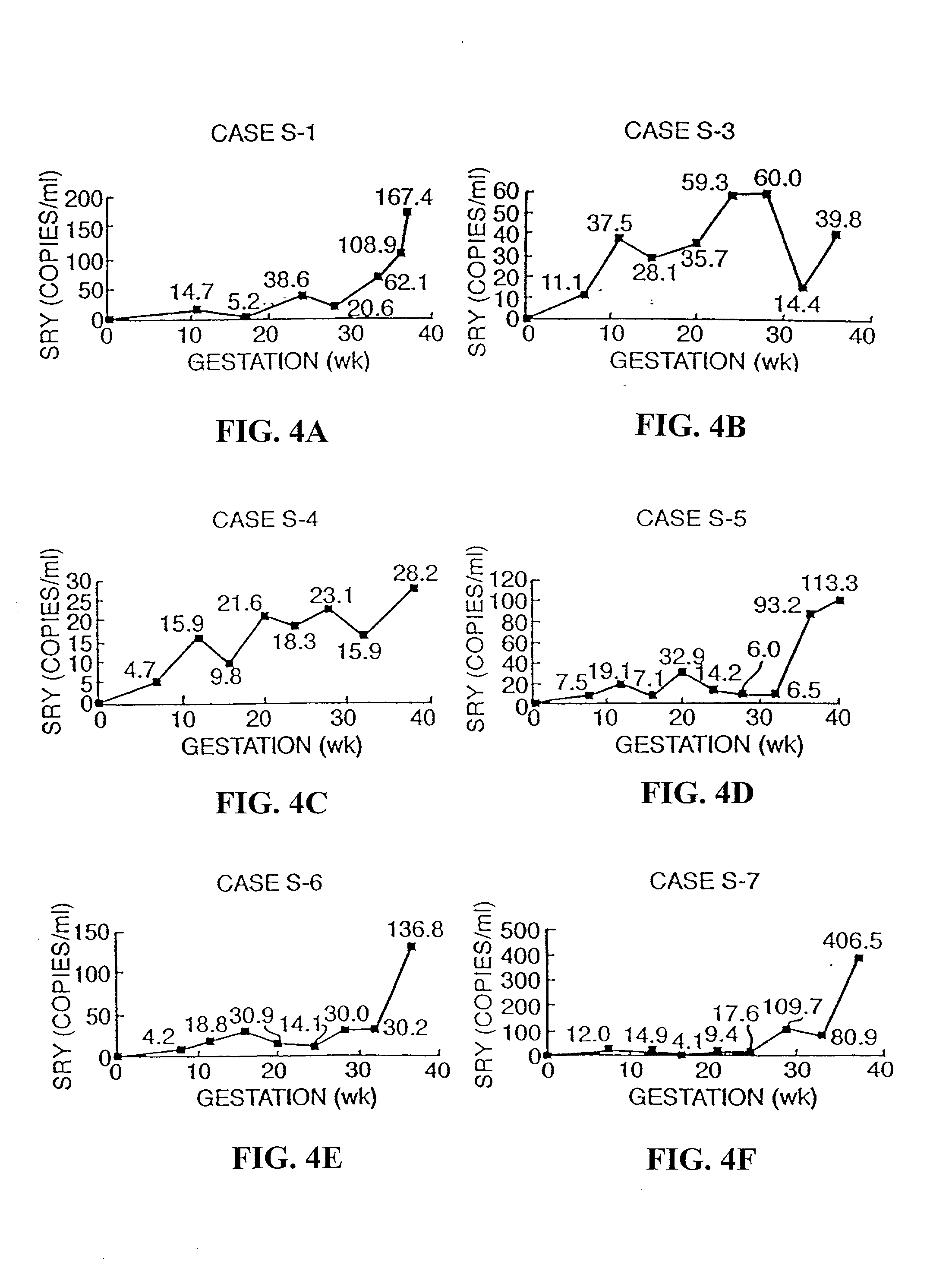
The invention relates to a detection method performed on a maternal serum or plasma sample from a pregnant female, which method comprises detecting the presence of a nucleic acid of foetal origin in the sample. The invention enables non-invasive prenatal diagnosis including for example sex determination, blood typing and other genotyping, and detection of pre-eclampsia in the mother.
Owner:SEQUENOM INC
Multiparticulate modified release composition
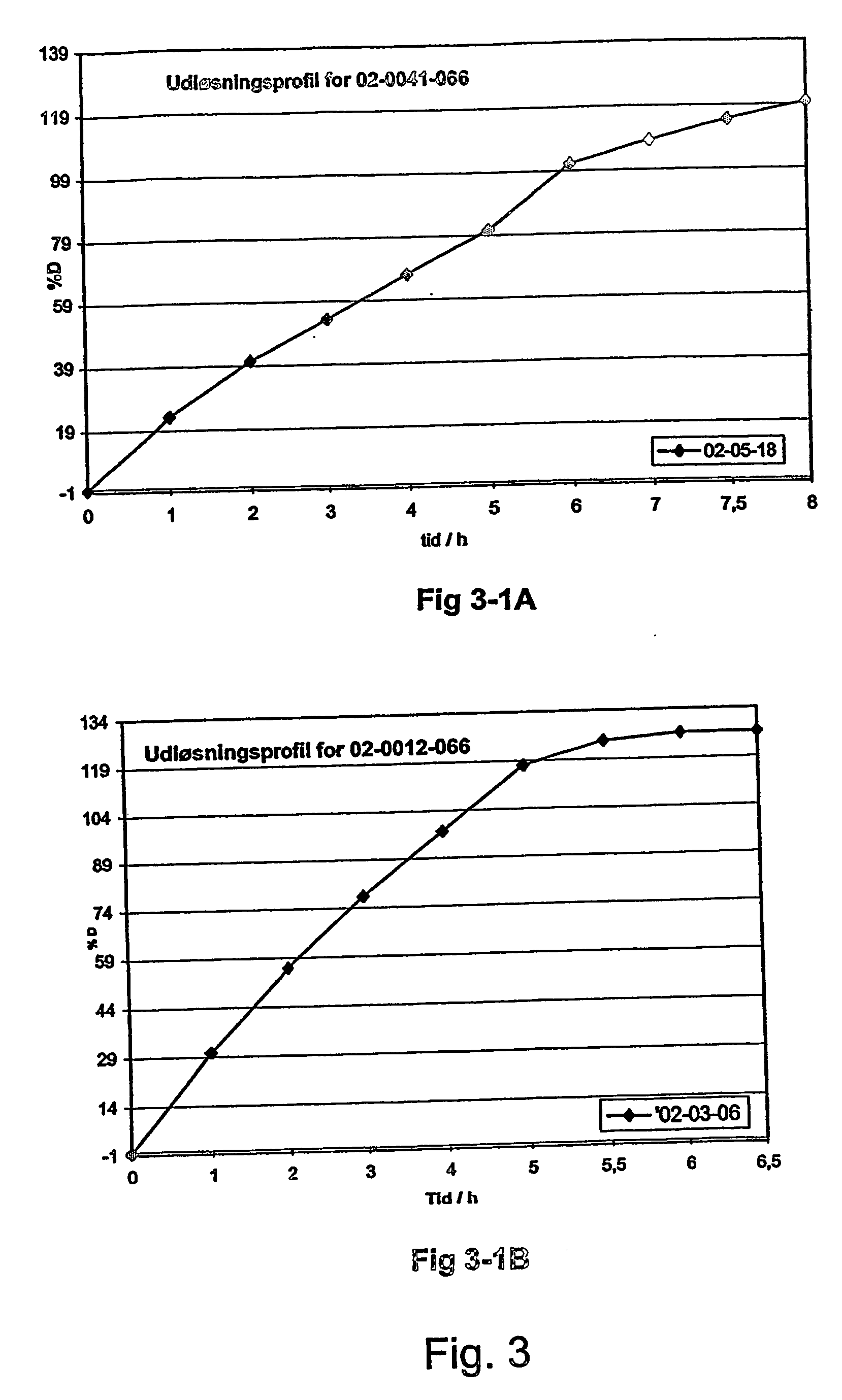
The invention relates to a multiparticulate modified release composition that in operation delivers an active ingredient in a pulsed or bimodal manner. The multiparticulate modified release composition comprises an immediate release component and a modified release component; the immediate release component comprising a first population of active ingredient containing particles and the modified release component compnsimg a second population of active ingredient containing particles coated with a controlled release coating; wherein the combination of the immediate release and modified release components in operation deliver the active ingredient in a pulsed or a bimodal manner. The invention also relates to a solid oral dosage form containing such a multiparticulate modified release composition. The plasma profile achieved by the multiparticulate modified release composition is advantageous in reducing patient tolerance to the active ingredient and in increasing patient compliance by reducing dosage frequency.
Owner:ALKERMES PHARMA IRELAND LTD +1
Non-invasive detection of fetal genetic traits
InactiveUS20050164241A1Facilitates non-invasive detectionComponent separationOther chemical processesPregnancyNon invasive
Blood plasma of pregnant women contains fetal and (generally>90%) maternal circulatory extracellular DNA. Most of said fetal DNA contains ≦500 base pairs, said maternal DNA having a greater size. Separation of circulatory extracellular DNA of <500 base pairs results in separation of fetal from maternal DNA. A fraction of a blood plasma or serum sample of a pregnant woman containing, due to size separation (e.g. by chromatography, density gradient centrifugation or nanotechnological methods), extracellular DNA substantially comprising ≦500 base pairs is useful for non-invasive detection of fetal genetic traits (including the fetal RhD gene in pregnancies at risk for HDN; fetal Y chromosome-specific sequences in pregnancies at risk for X chromosome-linked disorders; chromosomal aberrations; hereditary Mendelian genetic disorders and corresponding genetic markers; and traits decisive for paternity determination) by e.g. PCR, ligand chain reaction or probe hybridization techniques, or nucleic acid arrays.
Owner:SEQUENOM INC
Method and apparatus of distributed plasma processing system for conformal ion stimulated nanoscale deposition process
InactiveUS20080026574A1Good coverageHigh aspect ratio (HAR) featuresVacuum evaporation coatingSputtering coatingPlasma densityHigh density
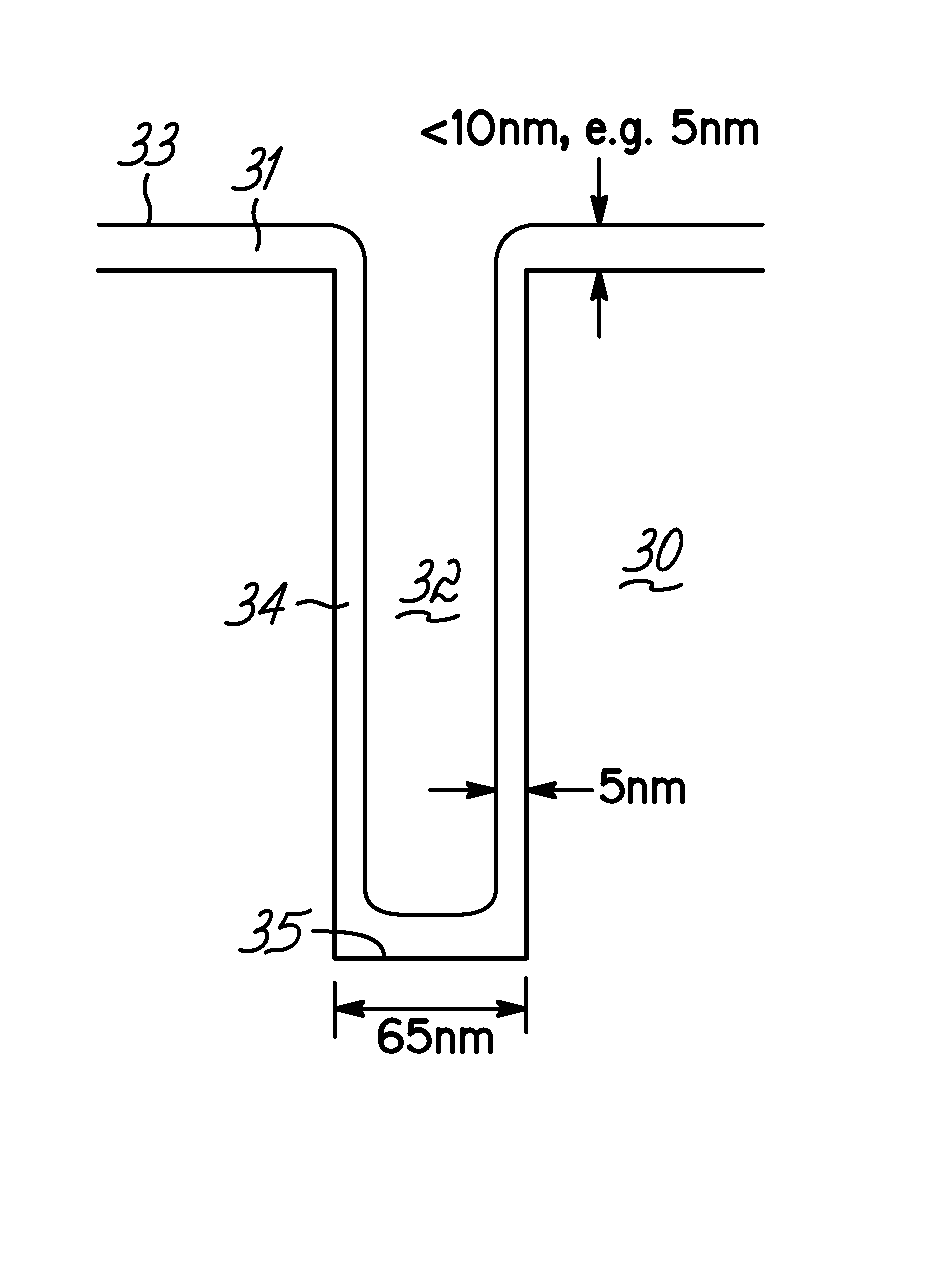
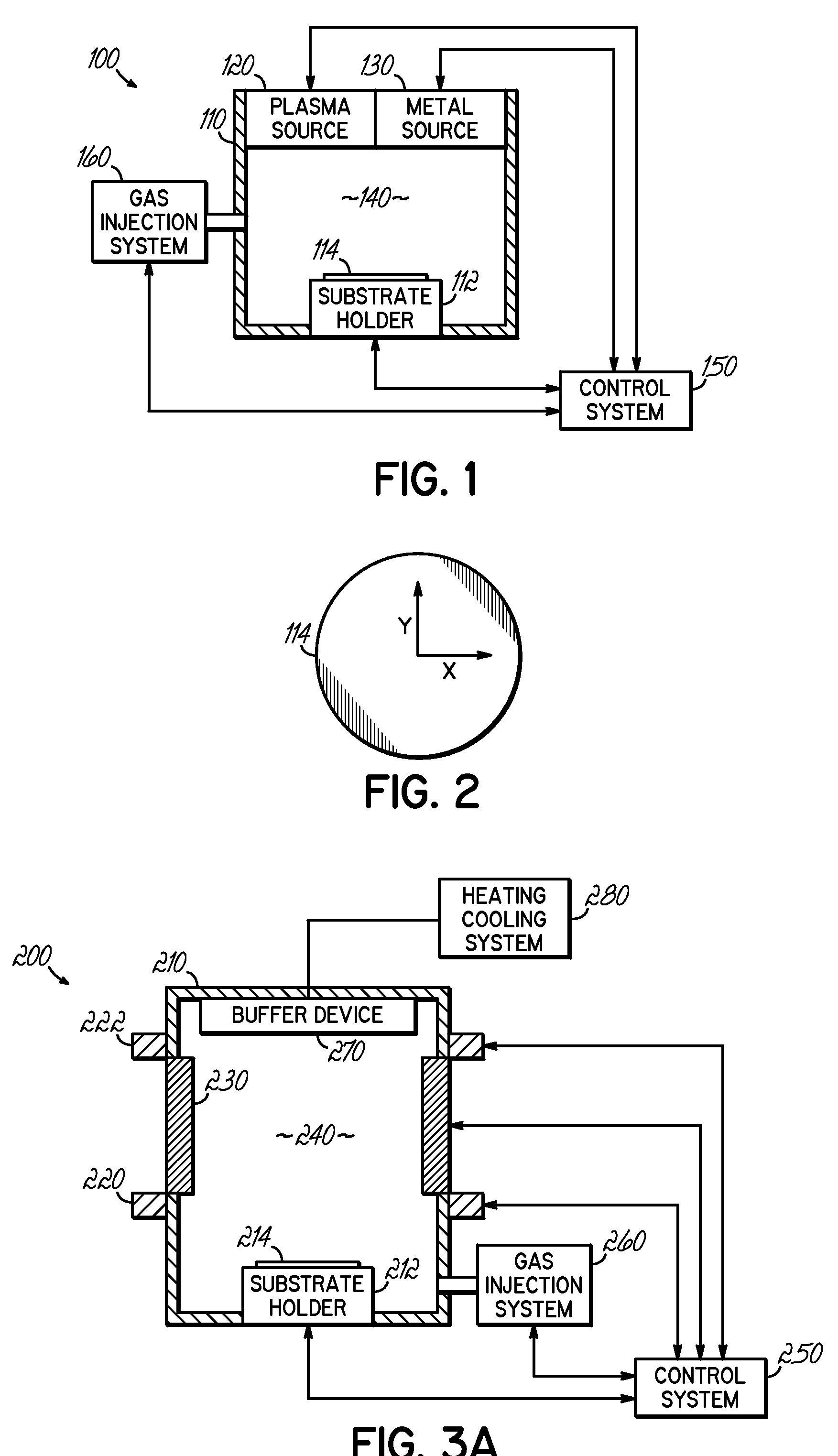
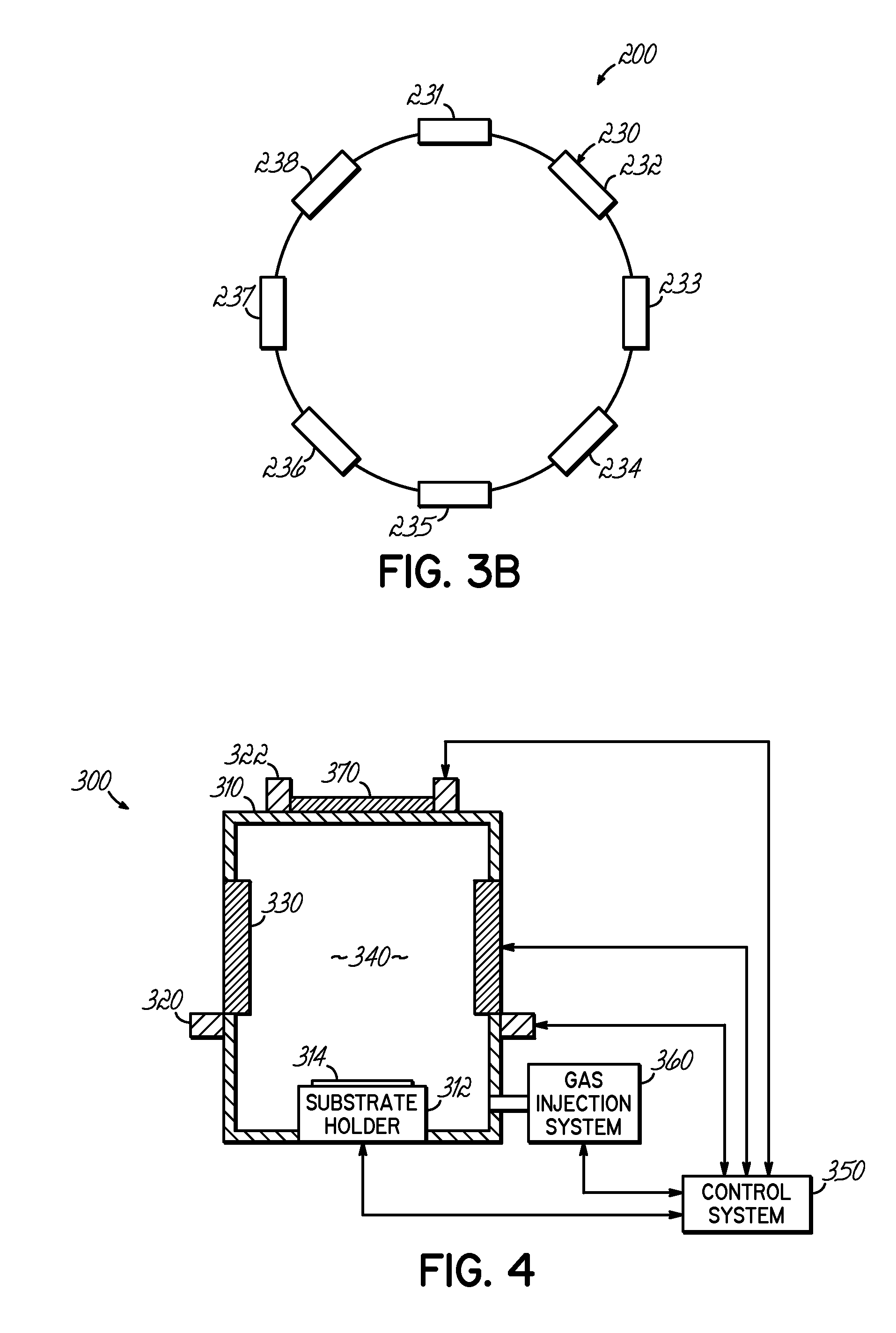
A deposition system and method of operating thereof is described for depositing a conformal metal or other similarly responsive coating material film in a high aspect ratio feature using a high density plasma is described. The deposition system includes a plasma source, and a distributed metal source for forming plasma and introducing metal vapor to the deposition system, respectively. The deposition system is configured to form a plasma having a plasma density and generate metal vapor having a metal density, wherein the ratio of the metal density to the plasma density proximate the substrate is less than or equal to unity. This ratio should exist at least within a distance from the surface of the substrate that is about twenty percent of the diameter of the substrate. A ratio that is uniform within plus or minus twenty-five percent substantially across the surface of said substrate is desirable. The ratio is particularly effective for plasma density exceeding 1012 cm−3, and for depositing film on substrates having nanoscale features with maximum film thickness less than half of the feature width, for example, at ten percent of the feature width.
Owner:TOKYO ELECTRON LTD
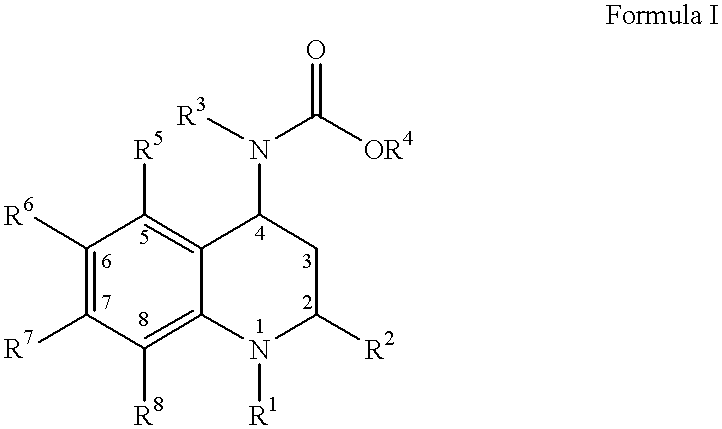
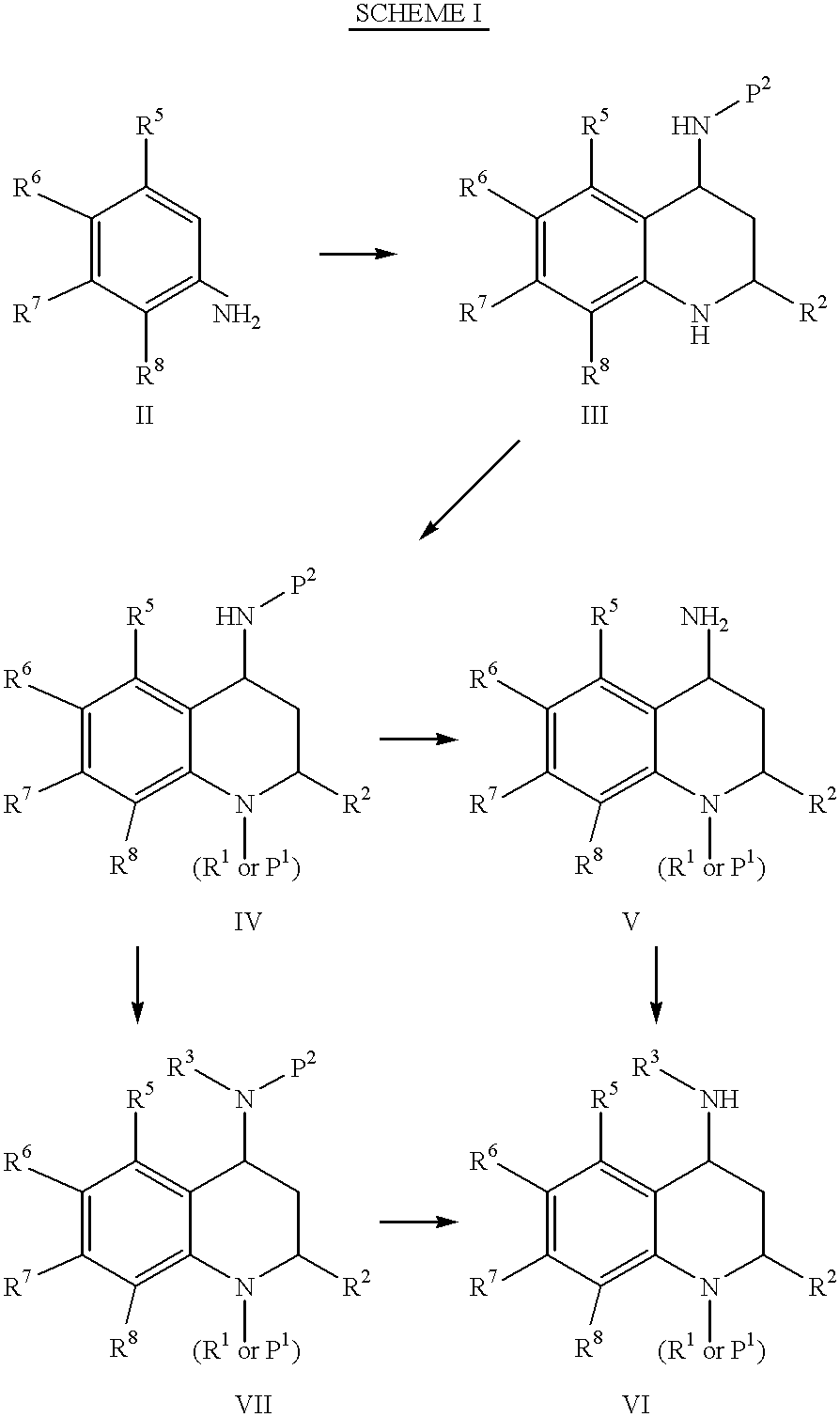
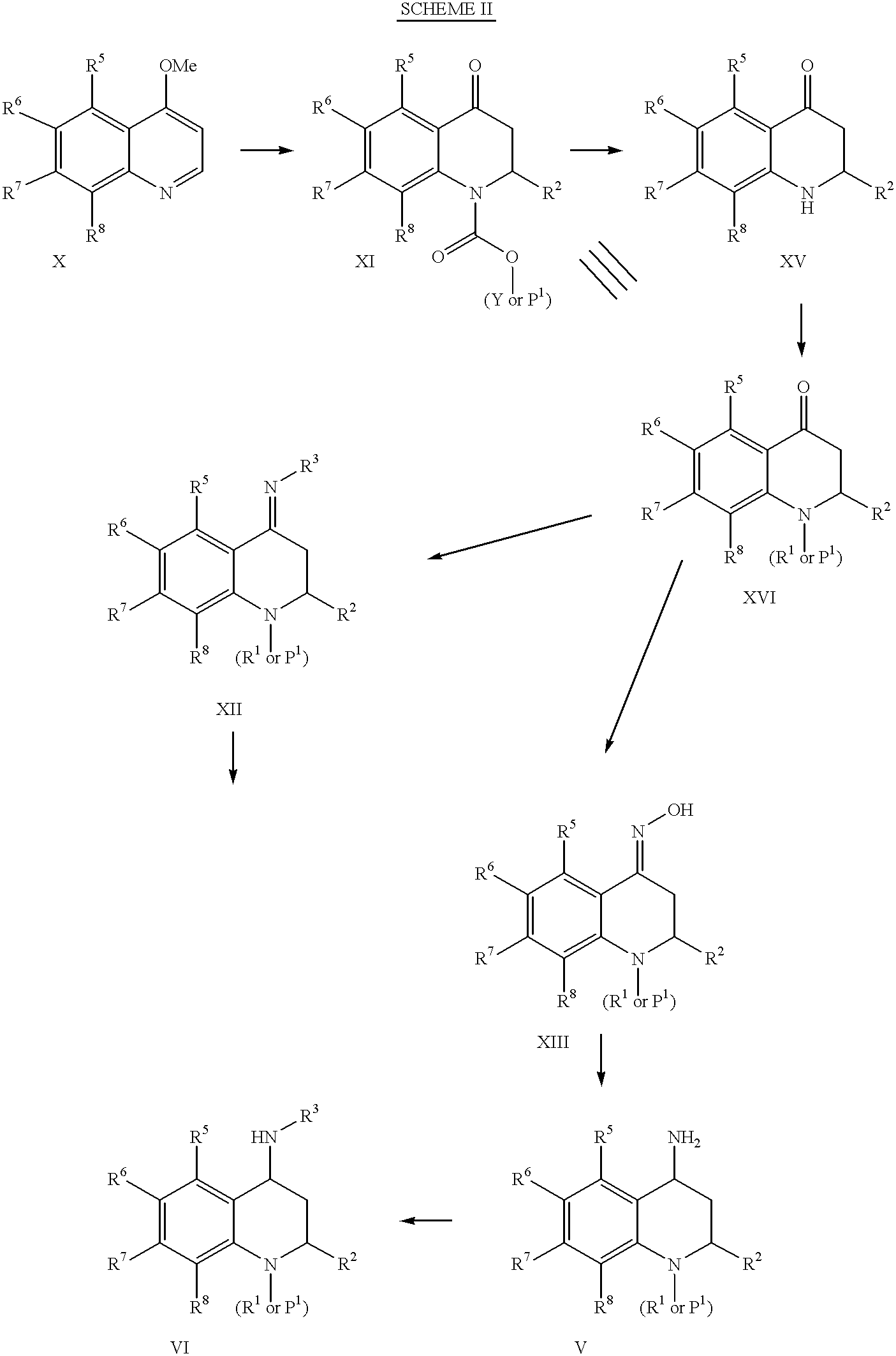
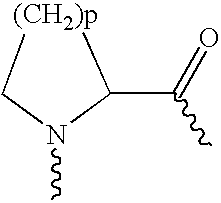
4-Carboxyamino-2-substituted-1,2,3,4-tetrahydroquinolines
Cholesteryl ester transfer protein inhibitors, pharmaceutical compositions containing such inhibitors and the use of such inhibitors to elevate certain plasma lipid levels, including high density lipoprotein-cholesterol and to lower certain other plasma lipid levels, such as LDL-cholesterol and triglycerides and accordingly to treat diseases which are exacerbated by low levels of HDL cholesterol and / or high levels of LDL-cholesterol and triglycerides, such as atherosclerosis and cardiovascular diseases in some mammals, including humans.
Owner:PFIZER INC
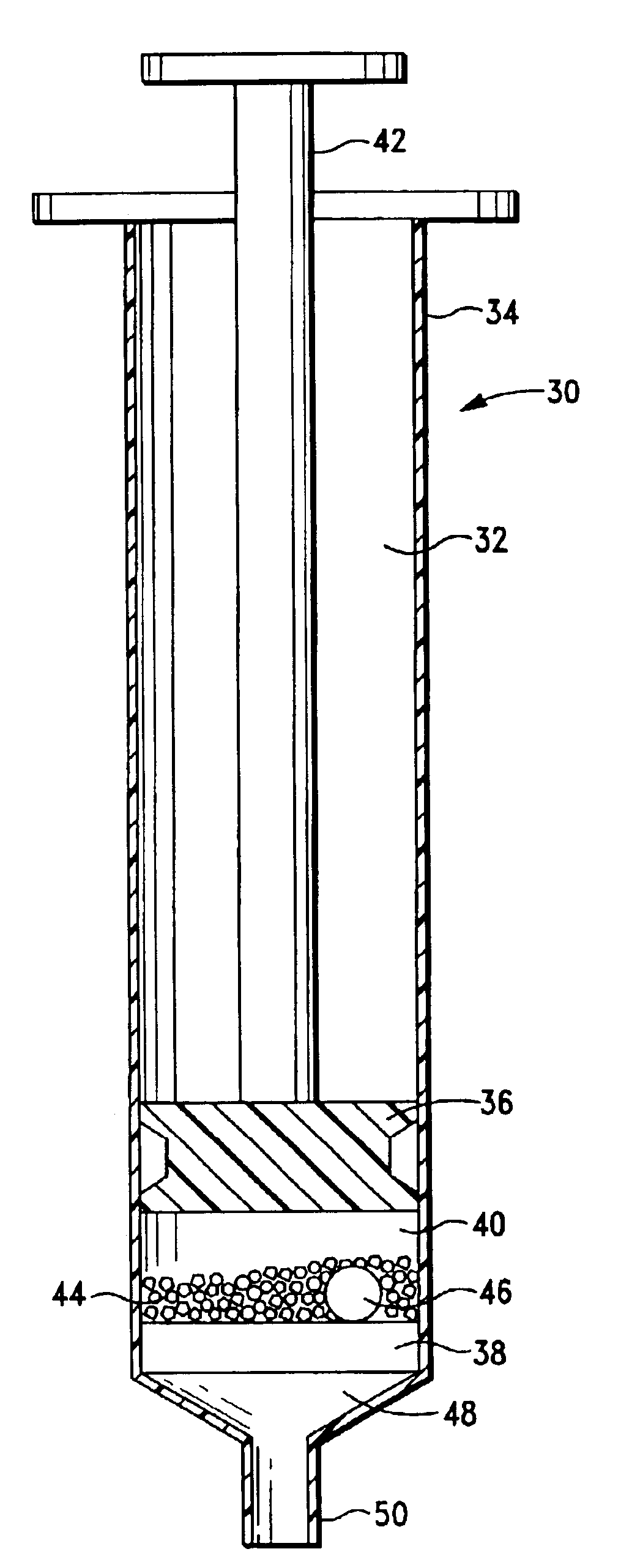
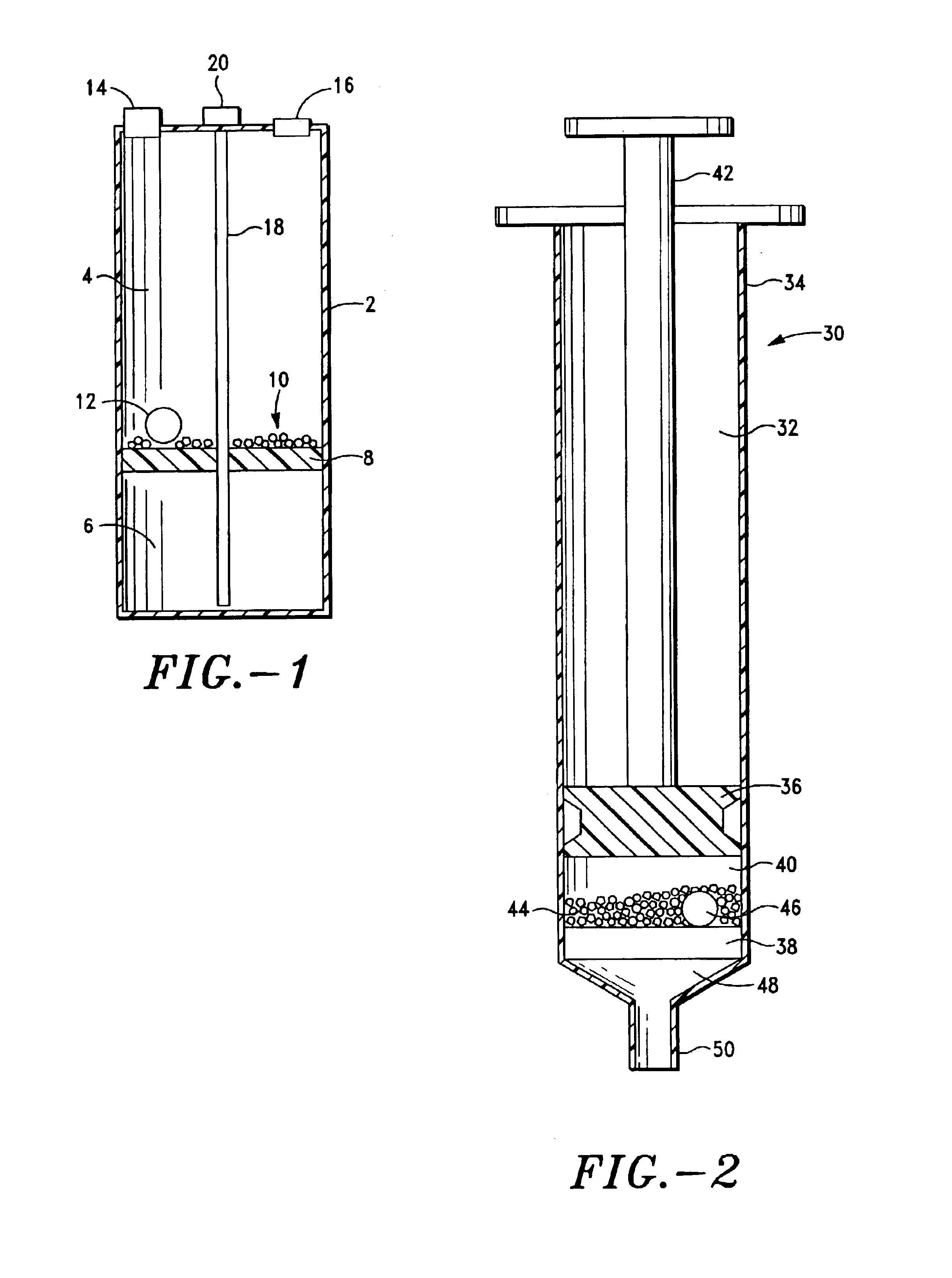
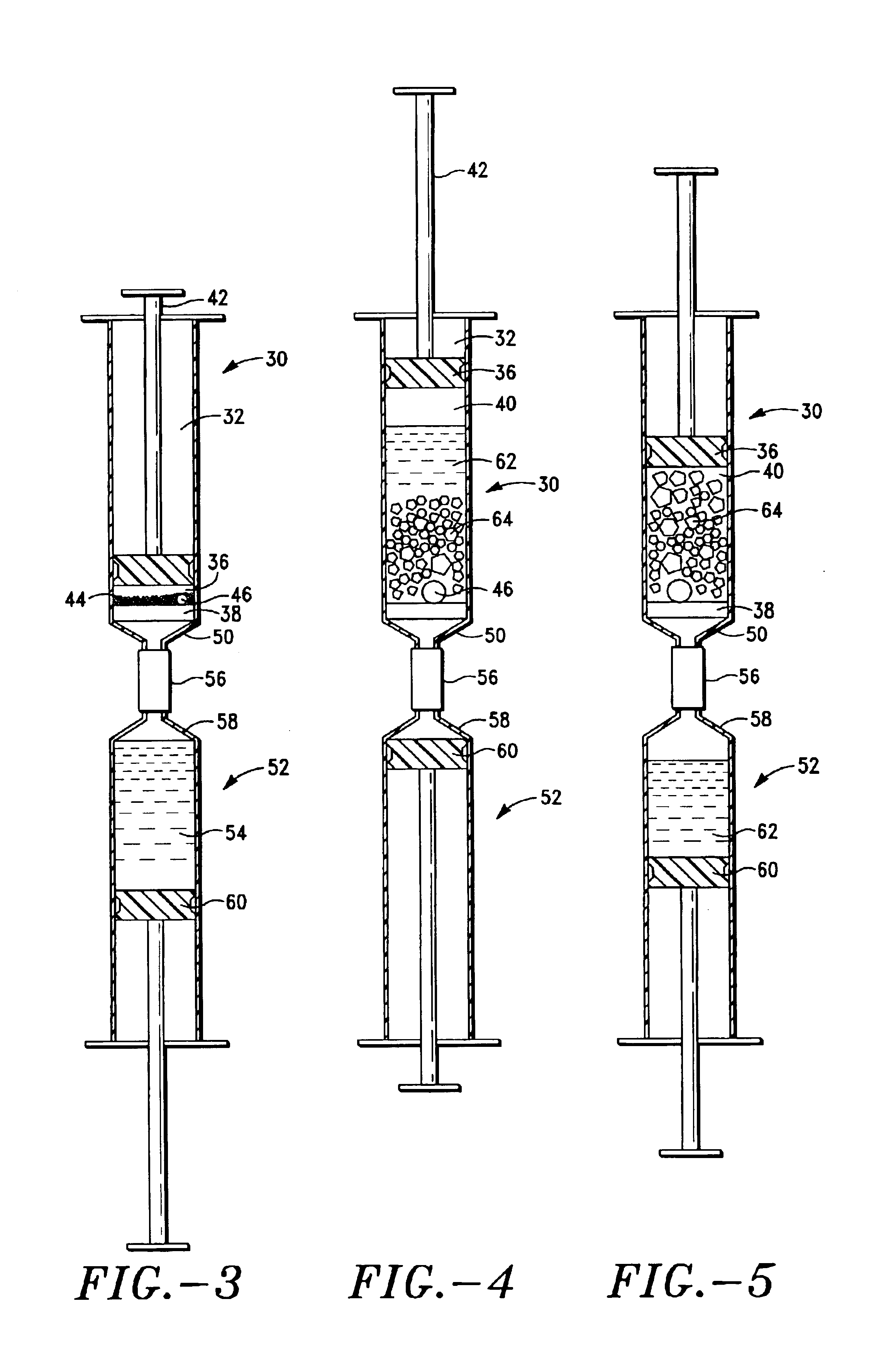
Plasma concentrate apparatus and method
ActiveUS6905612B2Effective absorptionPromote absorptionMixing methodsDead animal preservationRed blood cellAbsorption of water
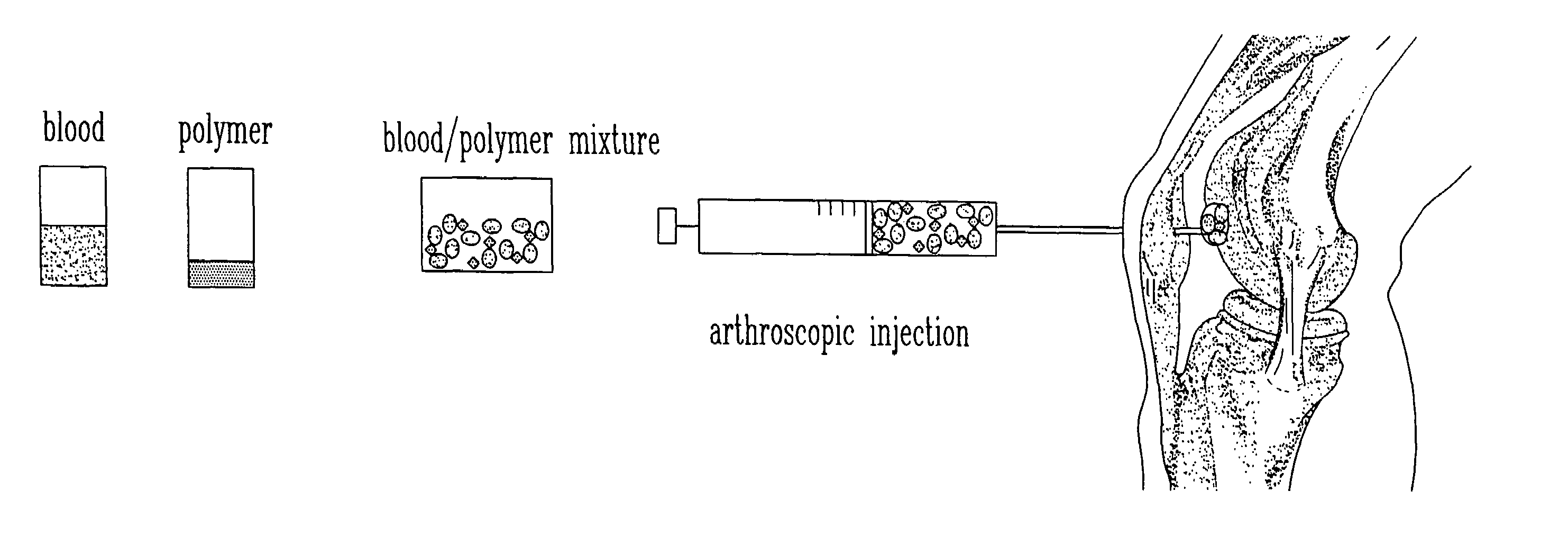
A plasma concentrator for producing plasma concentrate from a plasma from which erythrocytes have been substantially removed. The device comprises a concentrating chamber having an inlet port and an concentrate outlet, the concentrating chamber containing hydrogel beads and at least one inert agitator; and a concentrate chamber having an inlet communicating with the concentrator outlet through a filter, and having an plasma concentrate outlet port. A process for producing plasma concentrate from plasma from which erythrocytes have been substantially removed, comprising the steps of a) moving the plasma into a concentrating chamber containing hydrogel beads and an agitator to form a hydrogel bead-plasma mixture; b) causing the agitator to stir the hydrogel bead-plasma mixture, facilitating absorption of water by the beads from the plasma, until a hydrogel bead-plasma concentrate is formed; and c) separating the plasma concentrate from the hydrogel beads by passing the plasma concentrate through a filter. The concentrator can be one or more syringe devices coupled for multiple concentrations.
Owner:HANUMAN
4-amino substituted-2-substituted-1,2,3,4-tetrahydroquinolines
Cholesteryl ester transfer protein inhibitors, pharmaceutical compositions containing such inhibitors and the use of such inhibitors to elevate certain plasma lipid levels, including high density lipoprotein-cholesterol and to lower certain other plasma lipid levels, such as LDL-cholesterol and triglycerides and accordingly to treat diseases which are exacerbated by low levels of HDL cholesterol and / or high levels of LDL-cholesterol and triglycerides, such as atherosclerosis and cardiovascular diseases in some mammals, including humans.
Owner:PFIZER INC
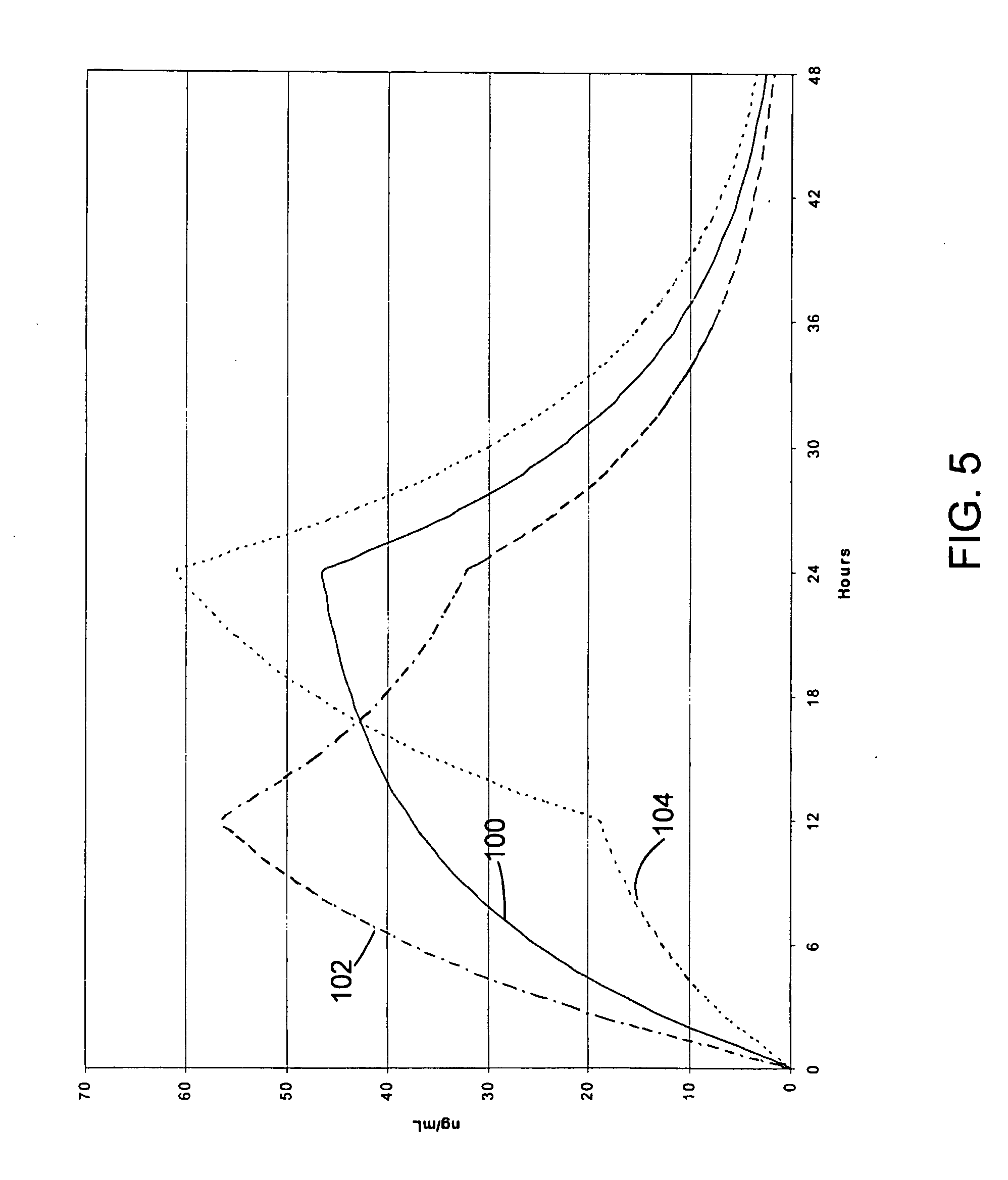
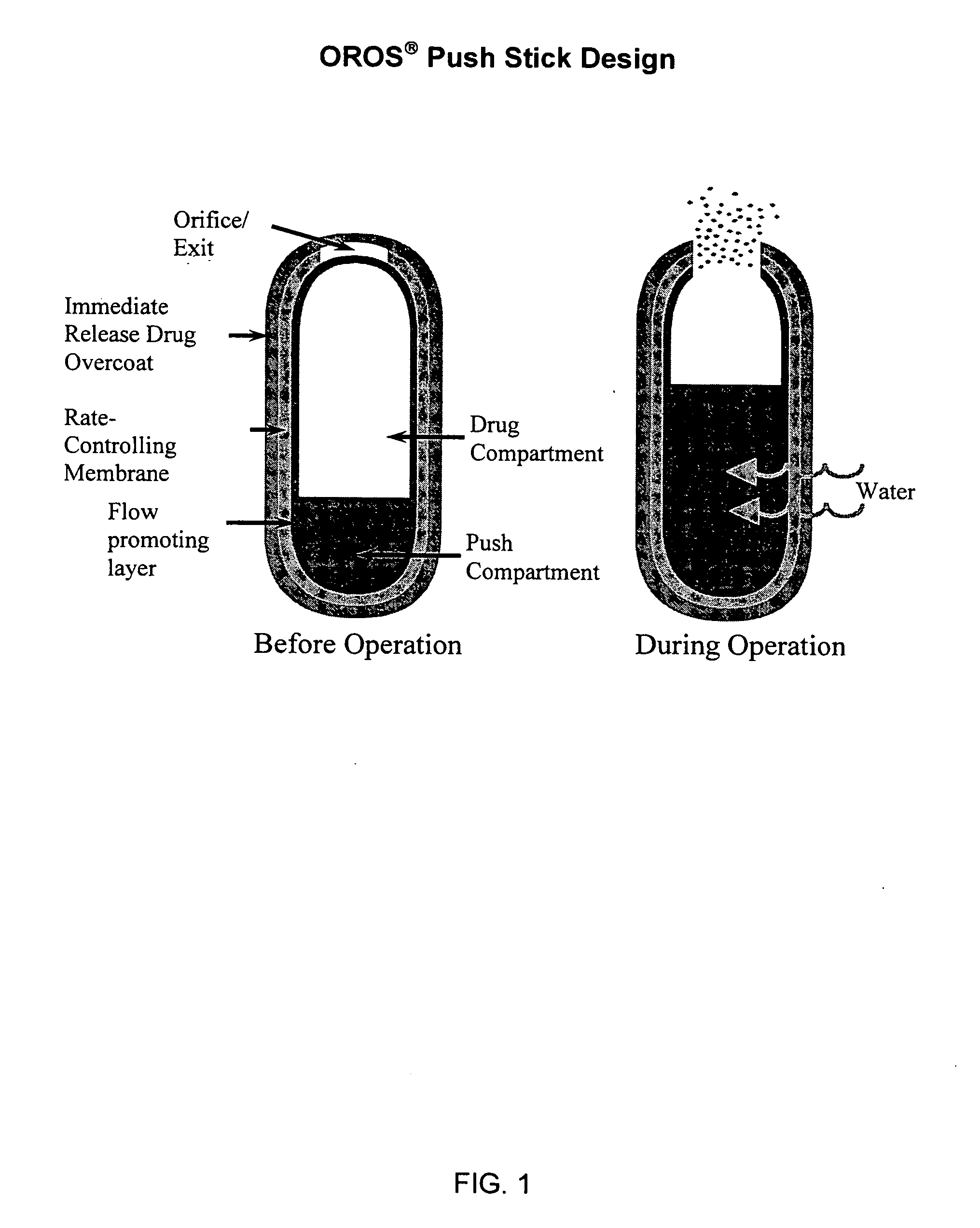
Once-a-day, oral, controlled-release, oxycodone dosage forms
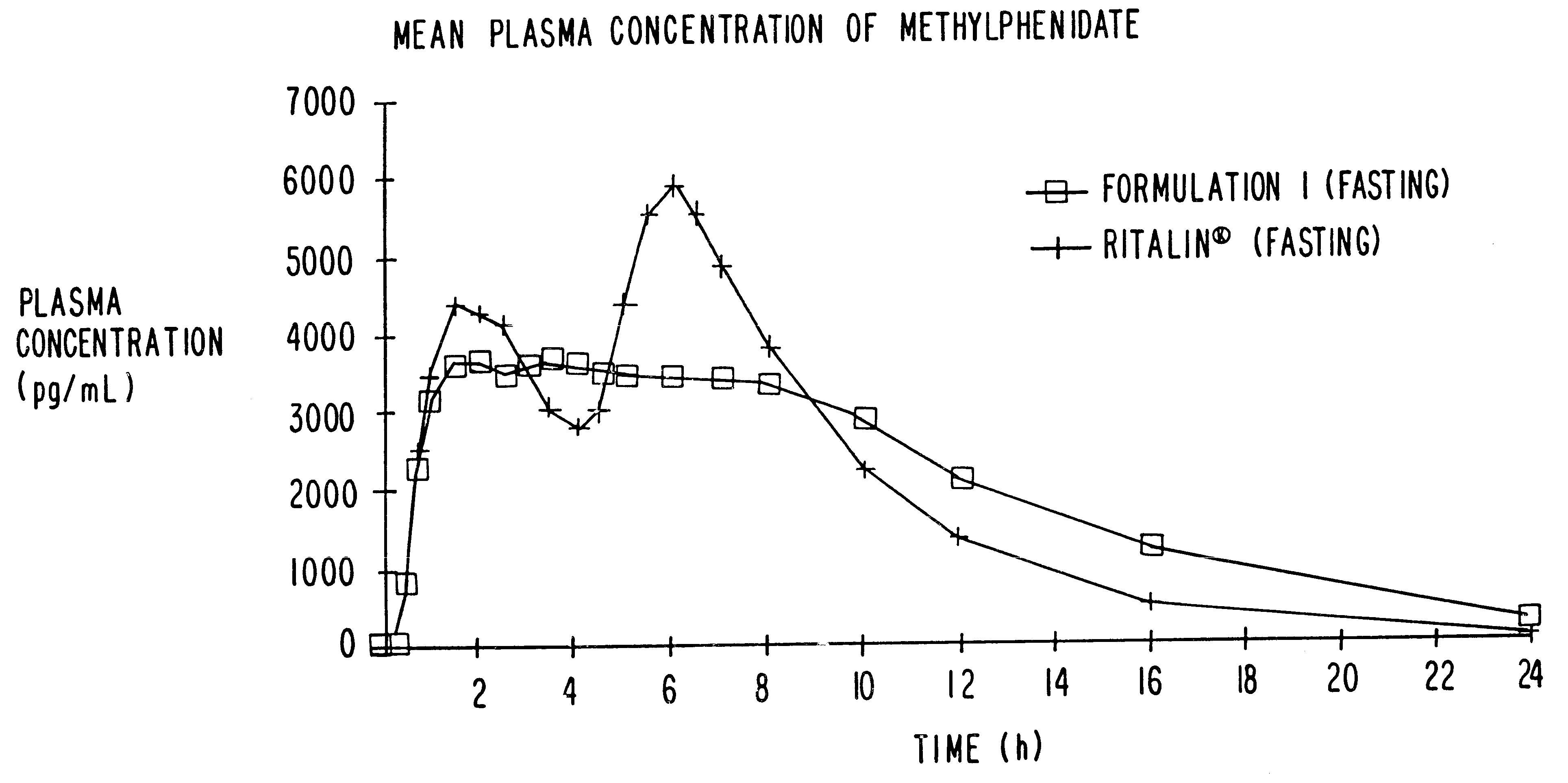
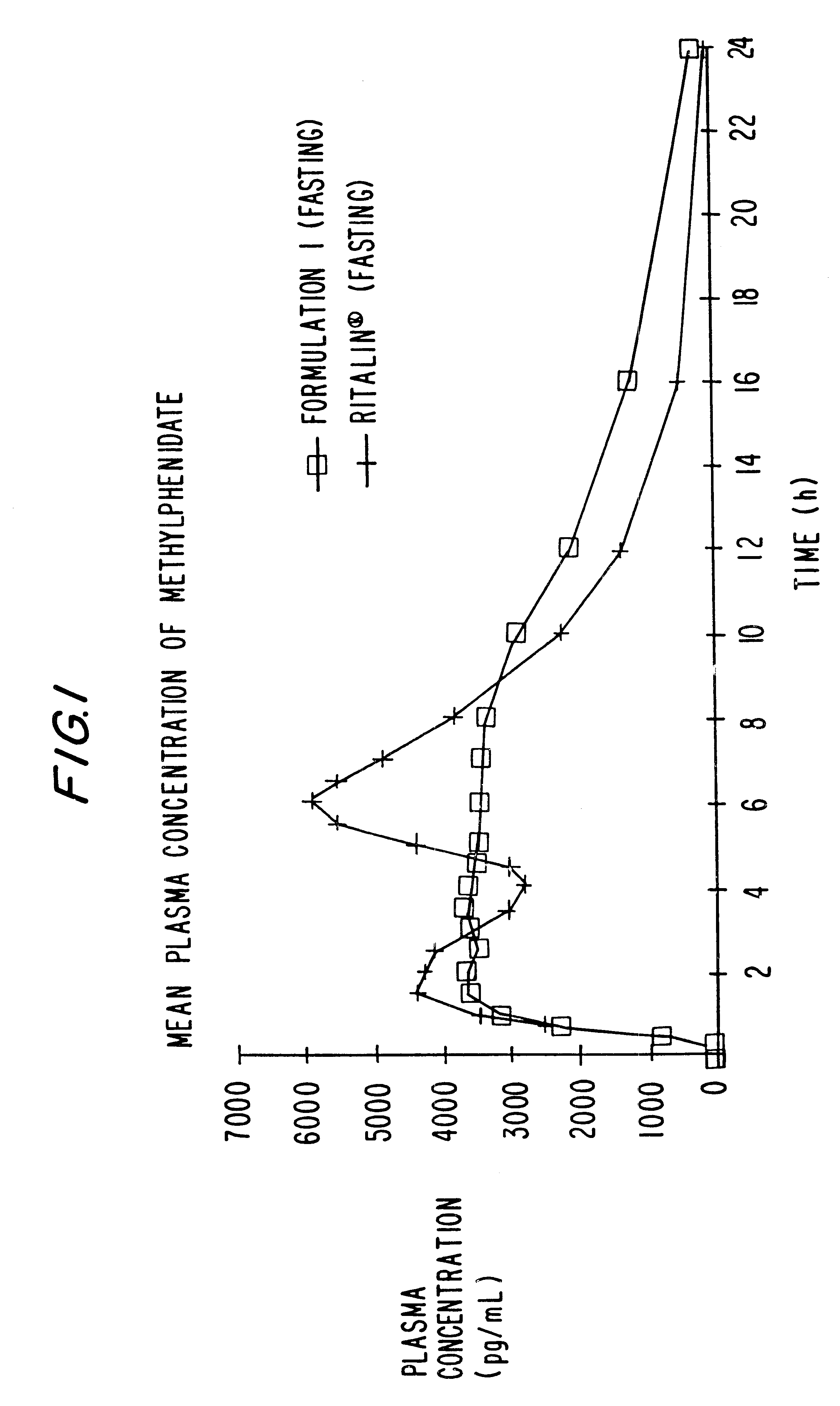
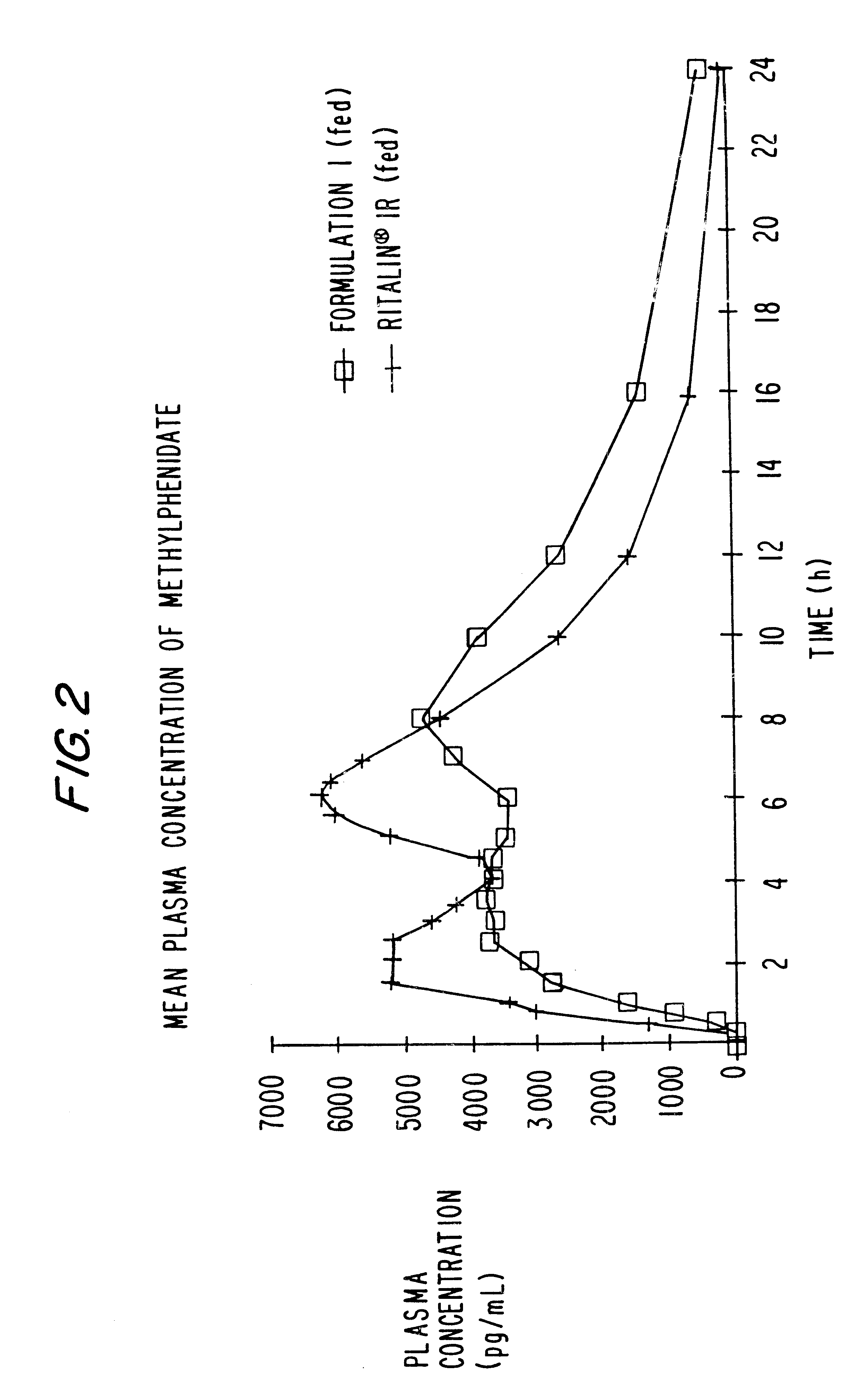
Oxycodone formulations are provided which produce substantially flat in vivo steady state plasma profiles. Tolerance levels associated with such profiles and tolerance levels associated with biphasic profiles are shown not to be statistically different. The substantially flat in vivo steady state plasma profiles are produced by dosage forms having substantially zero order in vitro release profiles. Such release profiles produce low single dose in vivo Cmax levels which can reduce the probability of adverse side effects.
Owner:ALZA CORP
Lyophilization process and products obtained thereby
ActiveUS20070116729A1Dissolve fastSuitable for useBiocidePowder deliveryHigh concentrationFreeze-drying
A lyophilization process which comprises dissolving a material in one or more solvents for said material to form a solution; forcing said material at least partially out of solution by combining the solution and a non-solvent for the material, which non-solvent is miscible with the solvent or solvents used and wherein said non-solvent is volatilizable under freeze-drying conditions. In addition, for hydrophobic and / or lipophilic materials, the anti-solvent can be omitted, and the solution of the material in the solvent can be subjected directly to freeze drying. The lyophilizates can then be reconstituted with typical aqueous diluent in the case of hydrophilic materials. Hydrophobic and or lipophilic materials can be initially reconstituted with propylene glycol and / or polyethyleneglycol to form a high concentration solution therein and this is further diluted for use with a diluent of Intralipid, plasma, serum, or even whole blood.
Owner:SCIDOSE PHARMA +1
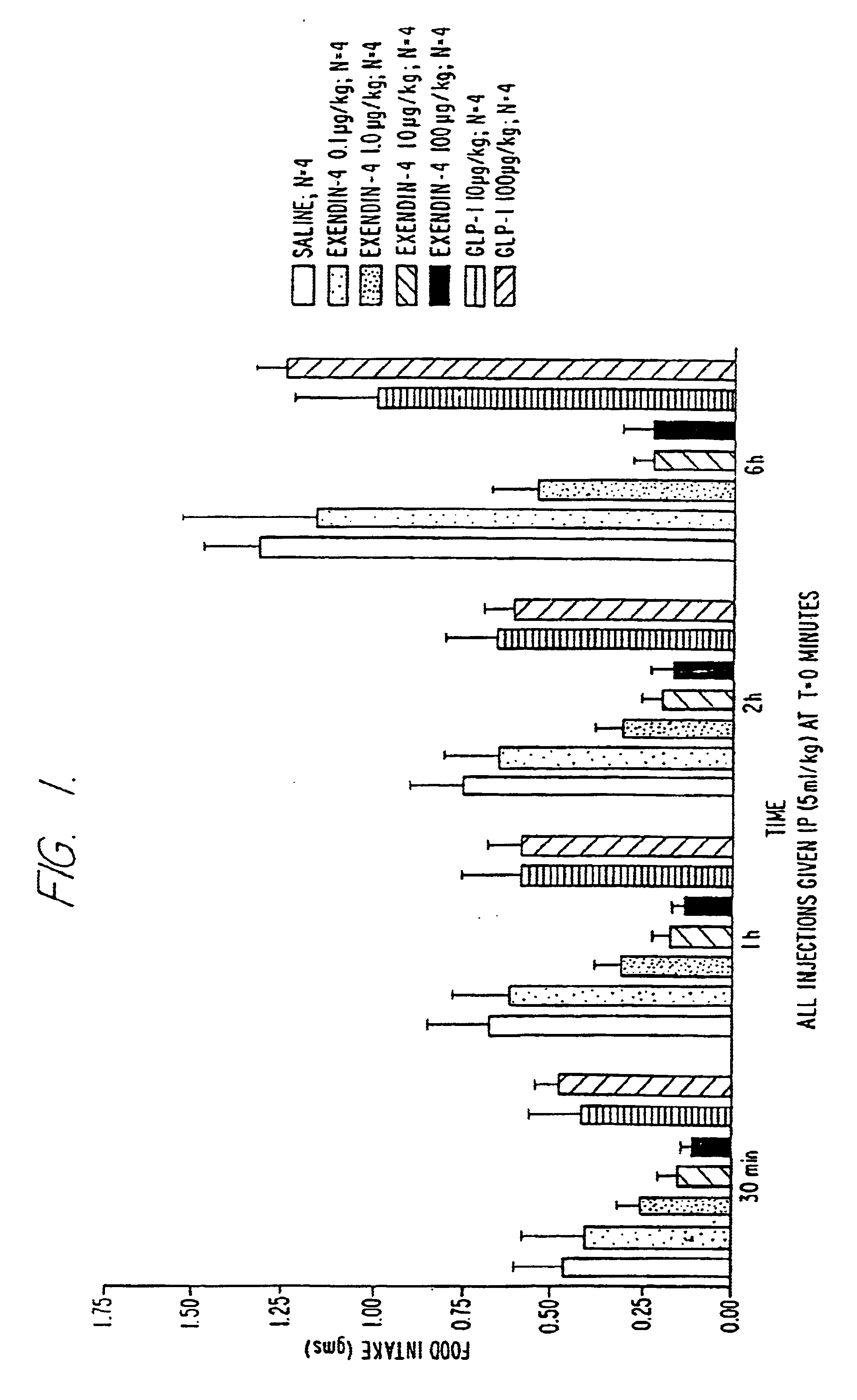
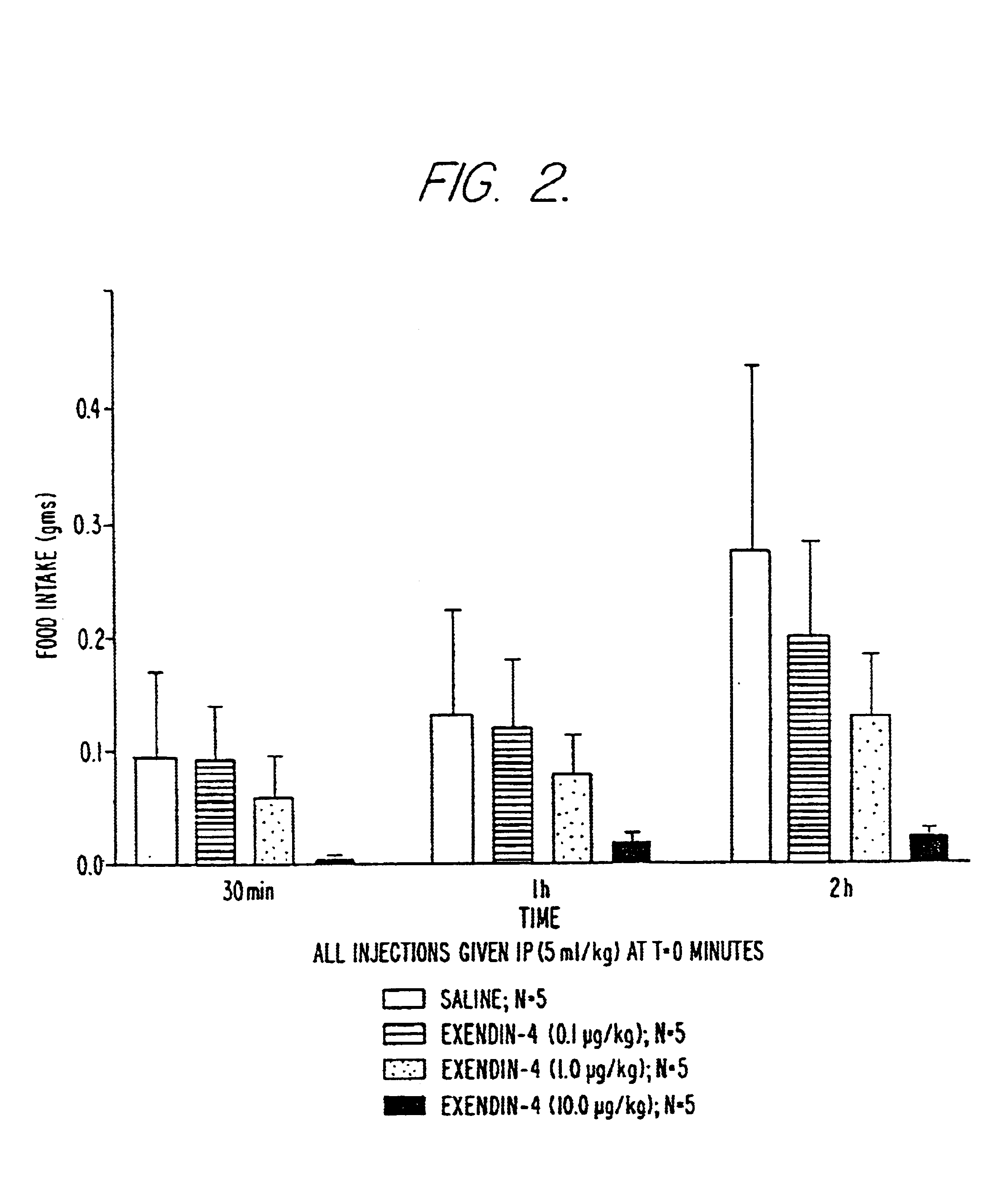
Use of exendins for the reduction of food intake
InactiveUS6956026B2Reduce appetiteReduce cardiac riskPeptide/protein ingredientsPharmaceutical delivery mechanismFeeding disabilityCvd risk
Methods for treating conditions or disorders which can be alleviated by reducing food intake are disclosed which comprise administration of an effective amount of an exendin or an exendin agonist, alone or in conjunction with other compounds or compositions that affect satiety. The methods are useful for treating conditions or disorders, including obesity, Type II diabetes, eating disorders, and insulin-resistance syndrome. The methods are also useful for lowering the plasma glucose level, lowering the plasma lipid level, reducing the cardiac risk, reducing the appetite, and reducing the weight of subjects. Pharmaceutical compositions for use in the methods of the invention are also disclosed.
Owner:ASTRAZENECA PHARMA LP
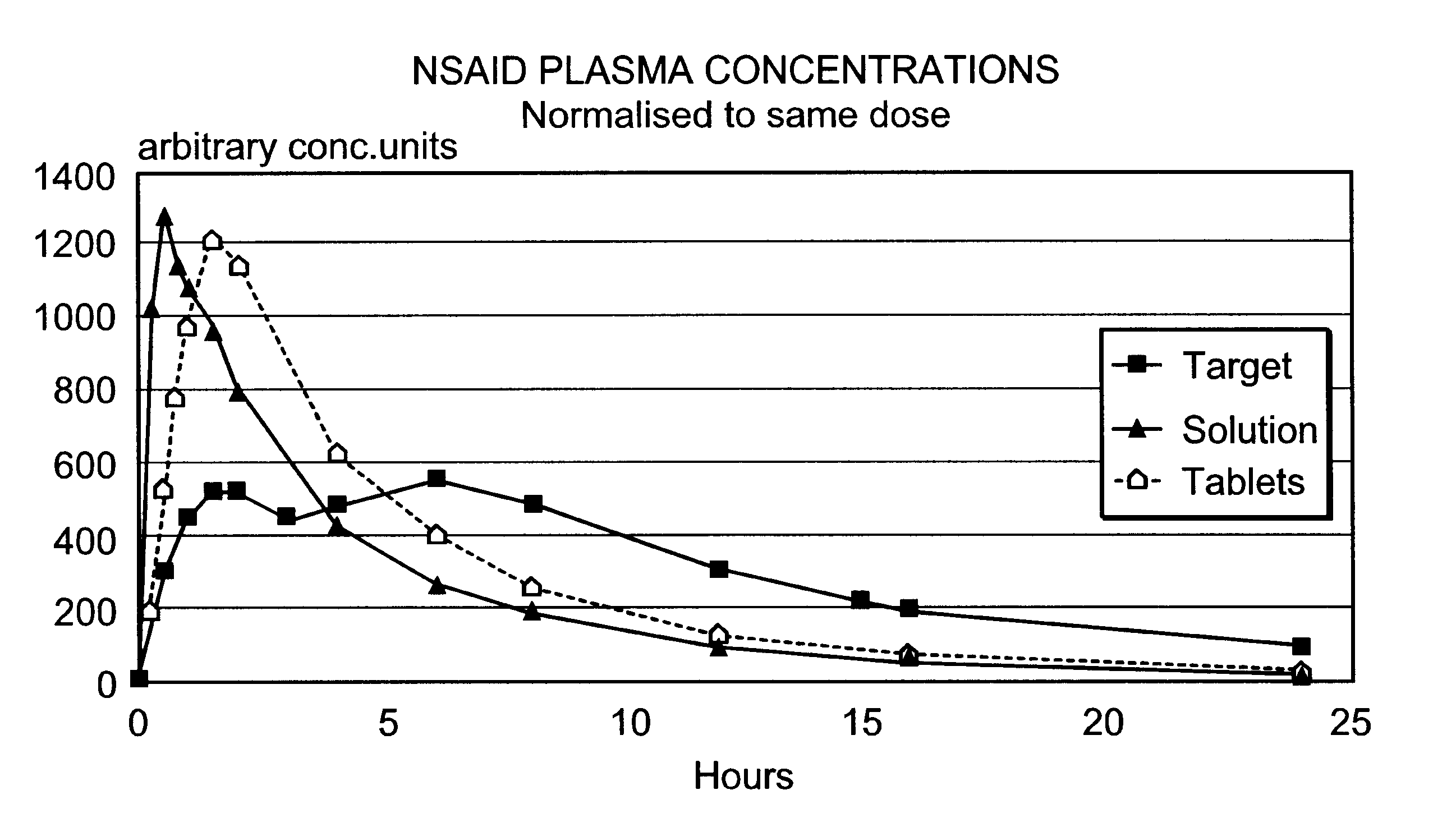
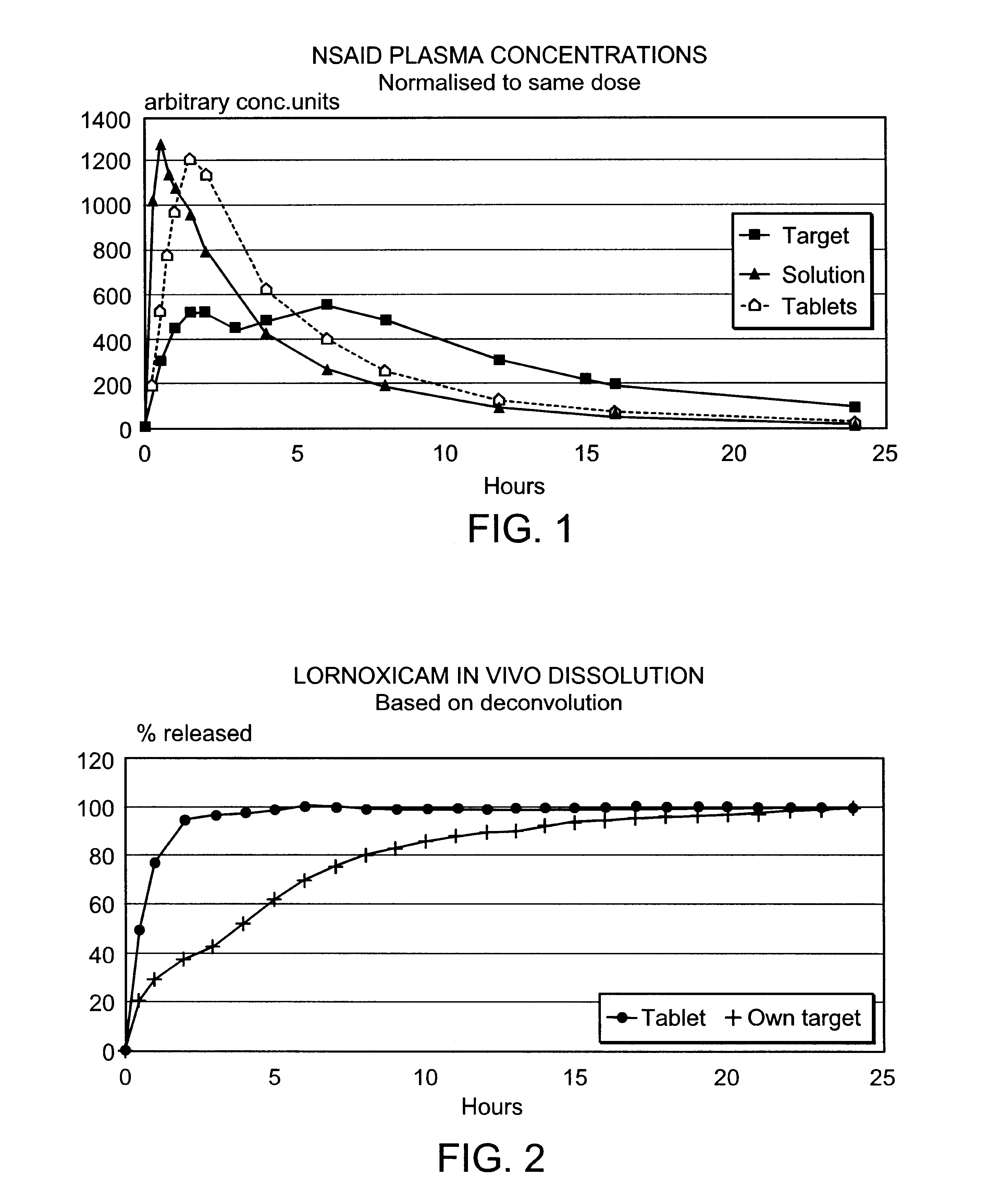
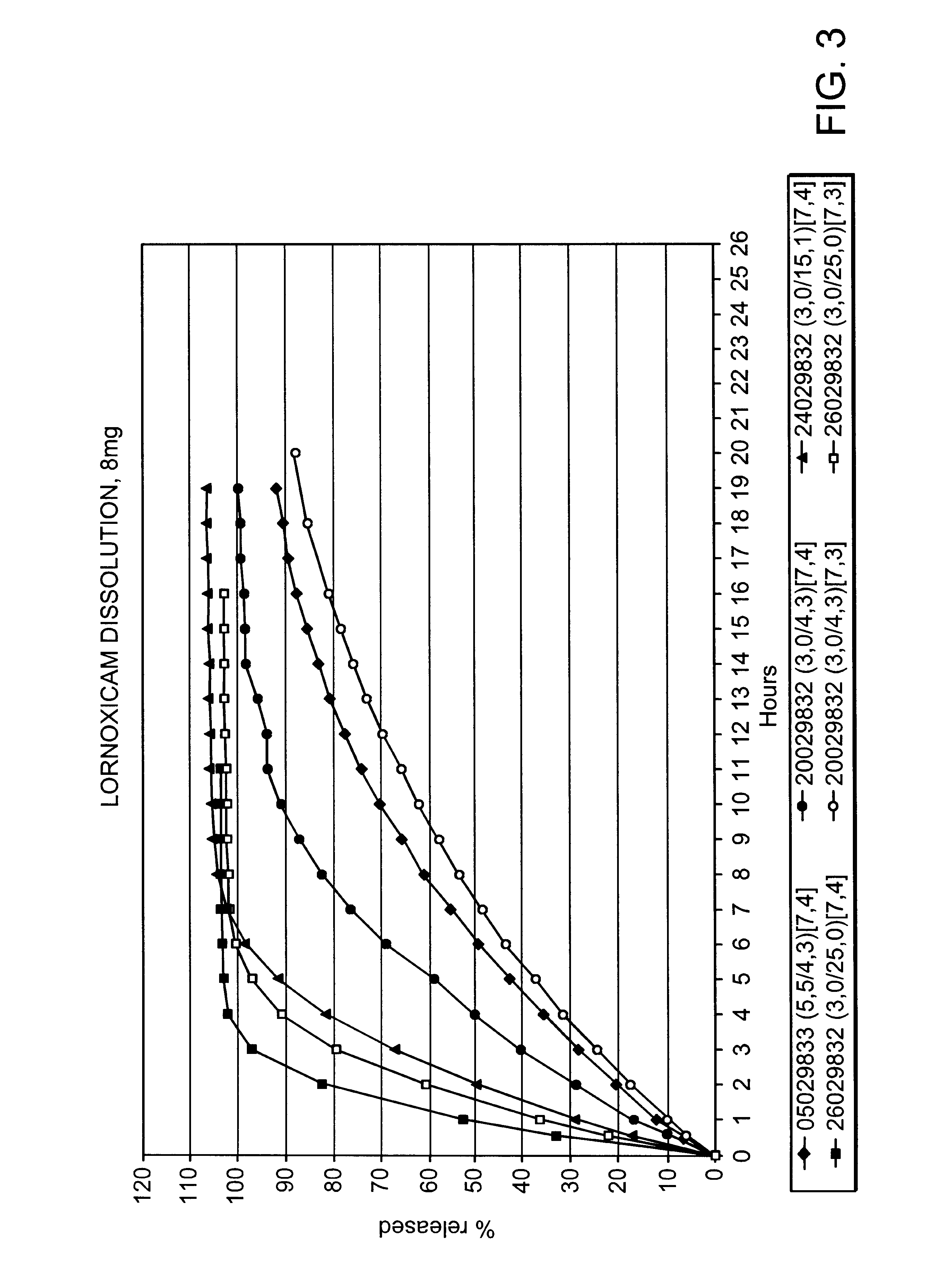
Modified release multiple-units compositions of non-steroid anti-inflammatory drug substances (NSAIDs)
InactiveUS6599529B1Keep low levelQuick releasePowder deliveryNervous disorderNon steroid anti inflammatory drugTherapeutic effect
An oral pharmaceutical modified release multiple-units composition for the administration of a therapeutically and / or prophylactically effective amount of a non-steroid anti-inflammatory drug substance to obtain both a relatively fast onset of the therapeutic effect and the maintenance of a therapeutically active plasma concentration for a relatively long period of time is disclosed.
Owner:TAKEDA PHARMA AS +1
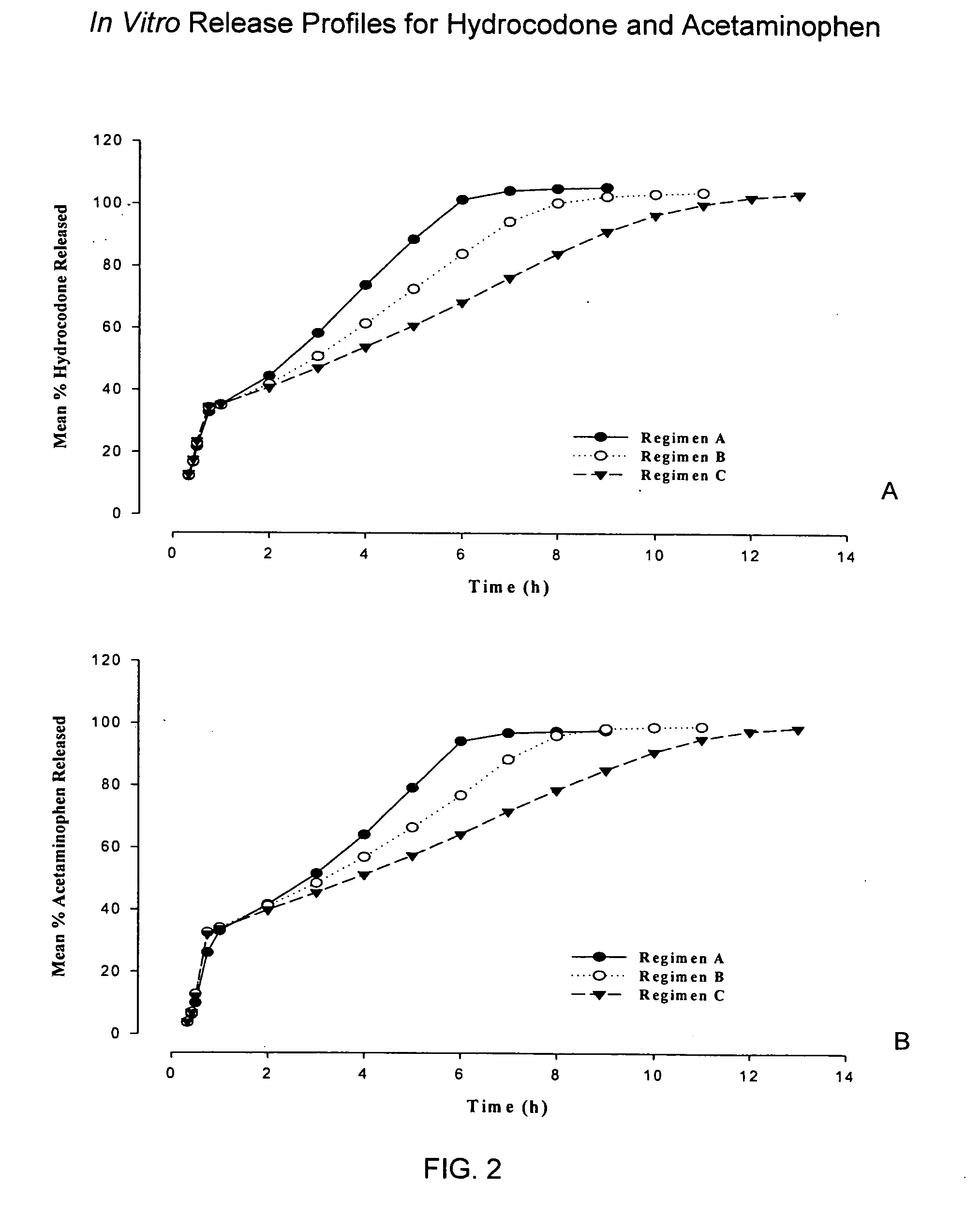
Controlled release formulations of opioid and nonopioid analgesics
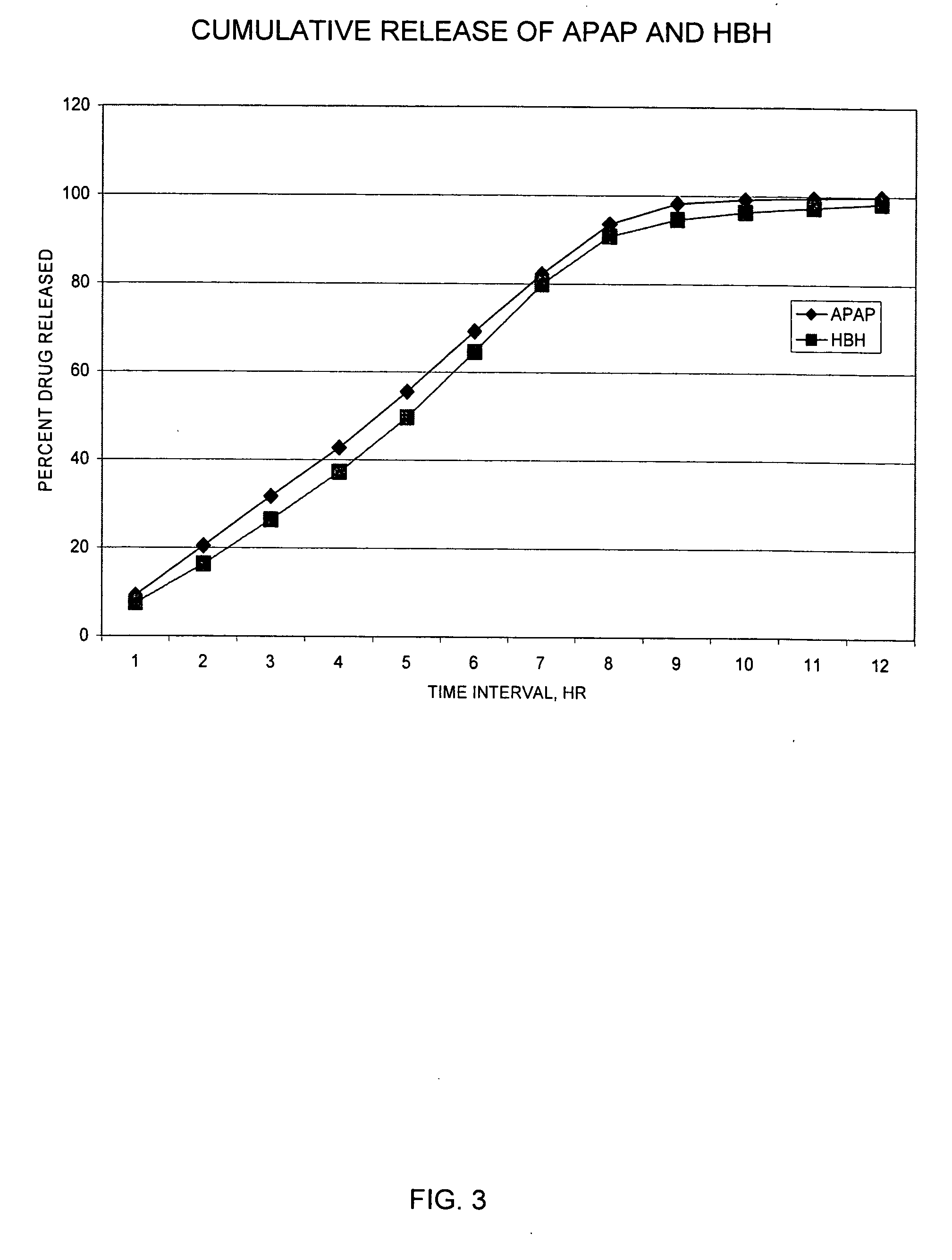
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Method for preparing thrombin for use in a biological glue
InactiveUS6472162B1Derive fast acting, stable autologous thrombinSimple preparatory procedureBioreactor/fermenter combinationsBiological substance pretreatmentsTissue sealantDonors plasma
A sterile method for preparing stable thrombin component from a single donor's plasma in which the thrombin component and the clotting and adhesive proteins component are harvested simultaneously from the same donor plasma in less than one hour. The combined components provide an improved biological hemostatic agent and tissue sealant by virtue of its freedom from the risk of contaminating viruses or bacteria from allogenic human or bovine blood sources. The thrombin provides polymerization of the clotting and adhesive proteins in less than five seconds, and is sufficiently stable to provide that fast clotting over a six hour period. Further, the clotting times can be predictably lengthened by diluting the thrombin with saline.
Owner:ASAHI KASEI MEDICAL CO LTD
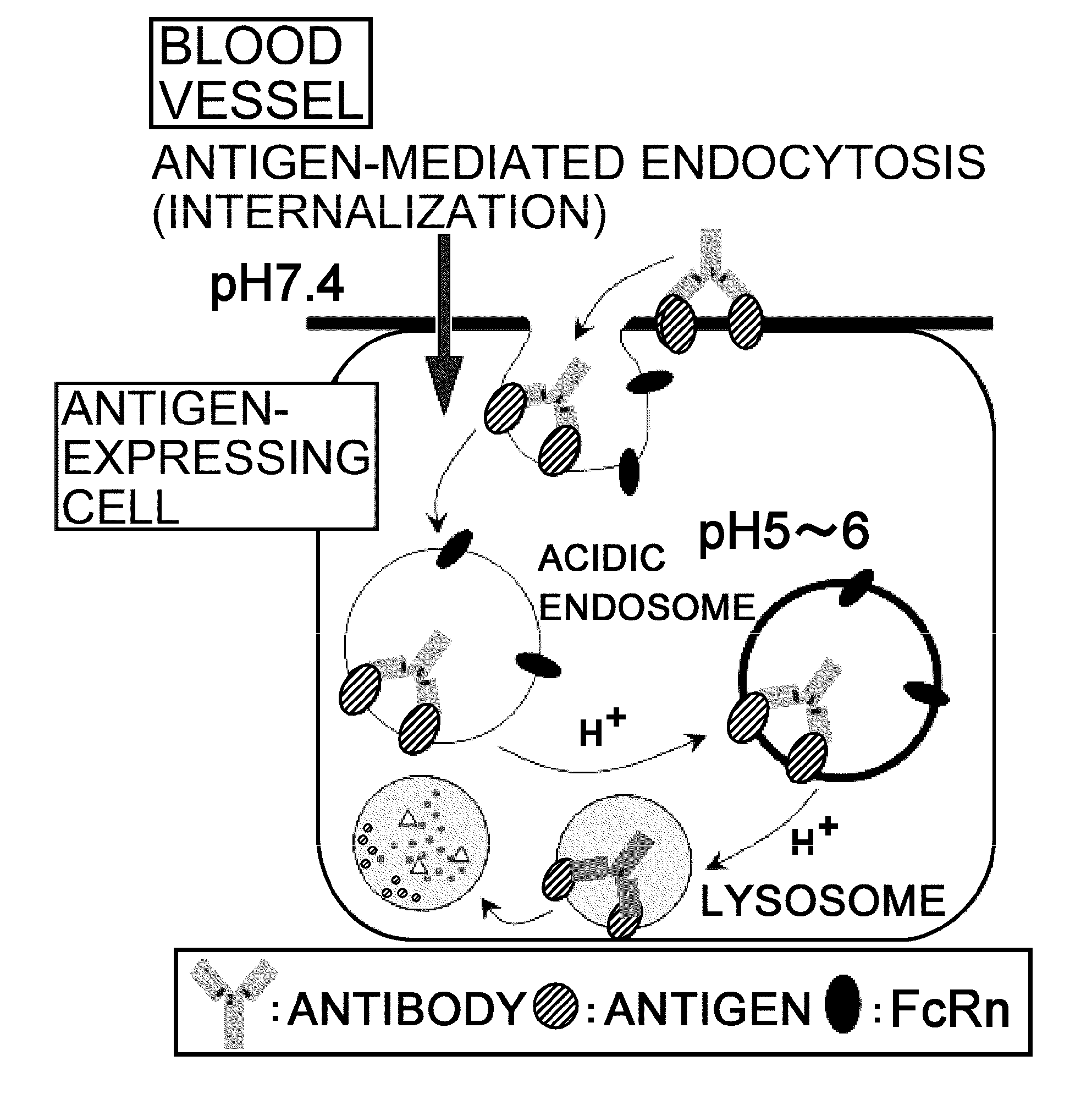
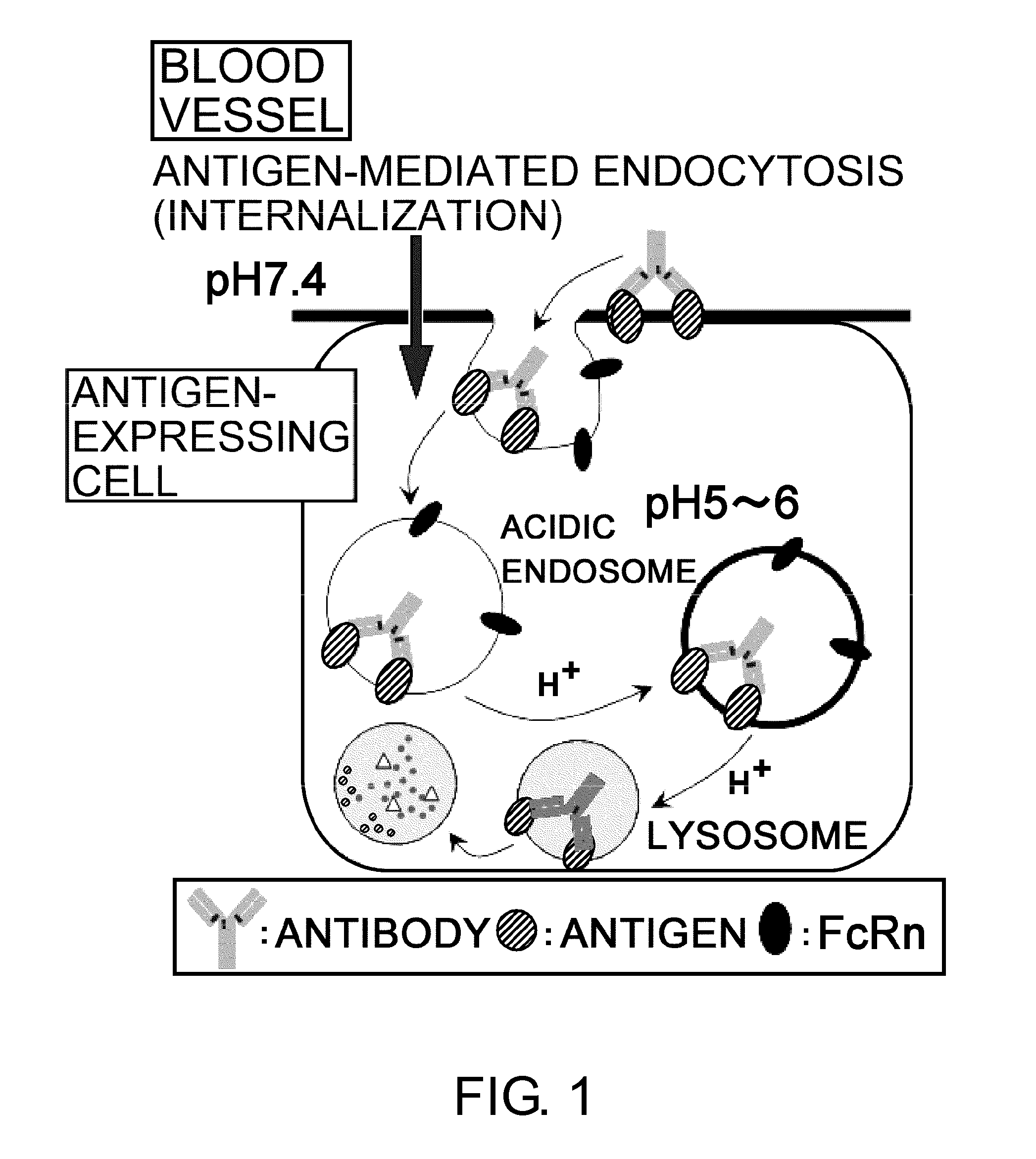
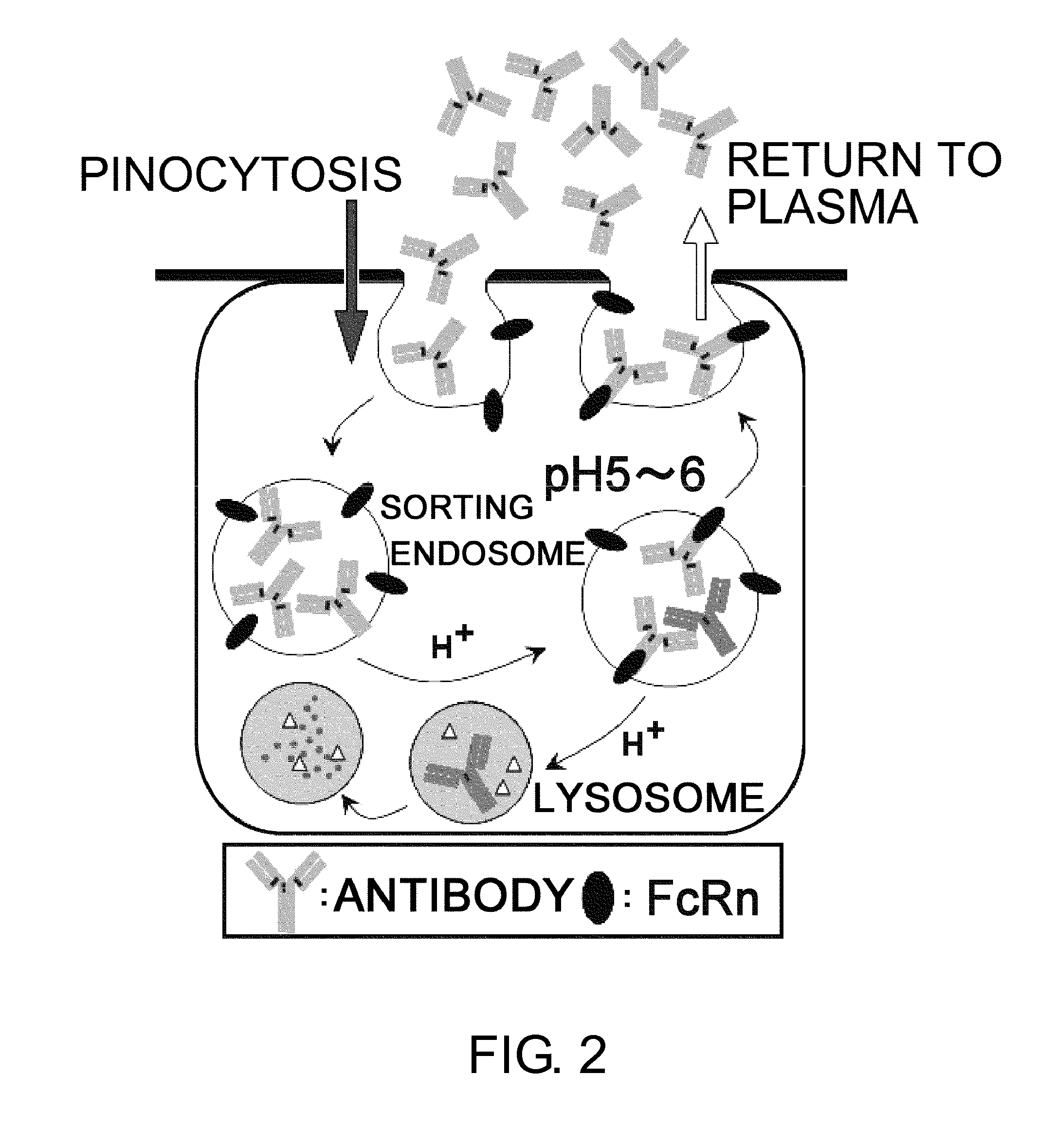
Antigen-binding molecule capable of binding to two or more antigen molecules repeatedly
InactiveUS20110111406A1Pharmacokinetics of the antigen-binding molecule can be improvedGood effectSsRNA viruses negative-senseCompound screeningHalf-lifeAntigen binding
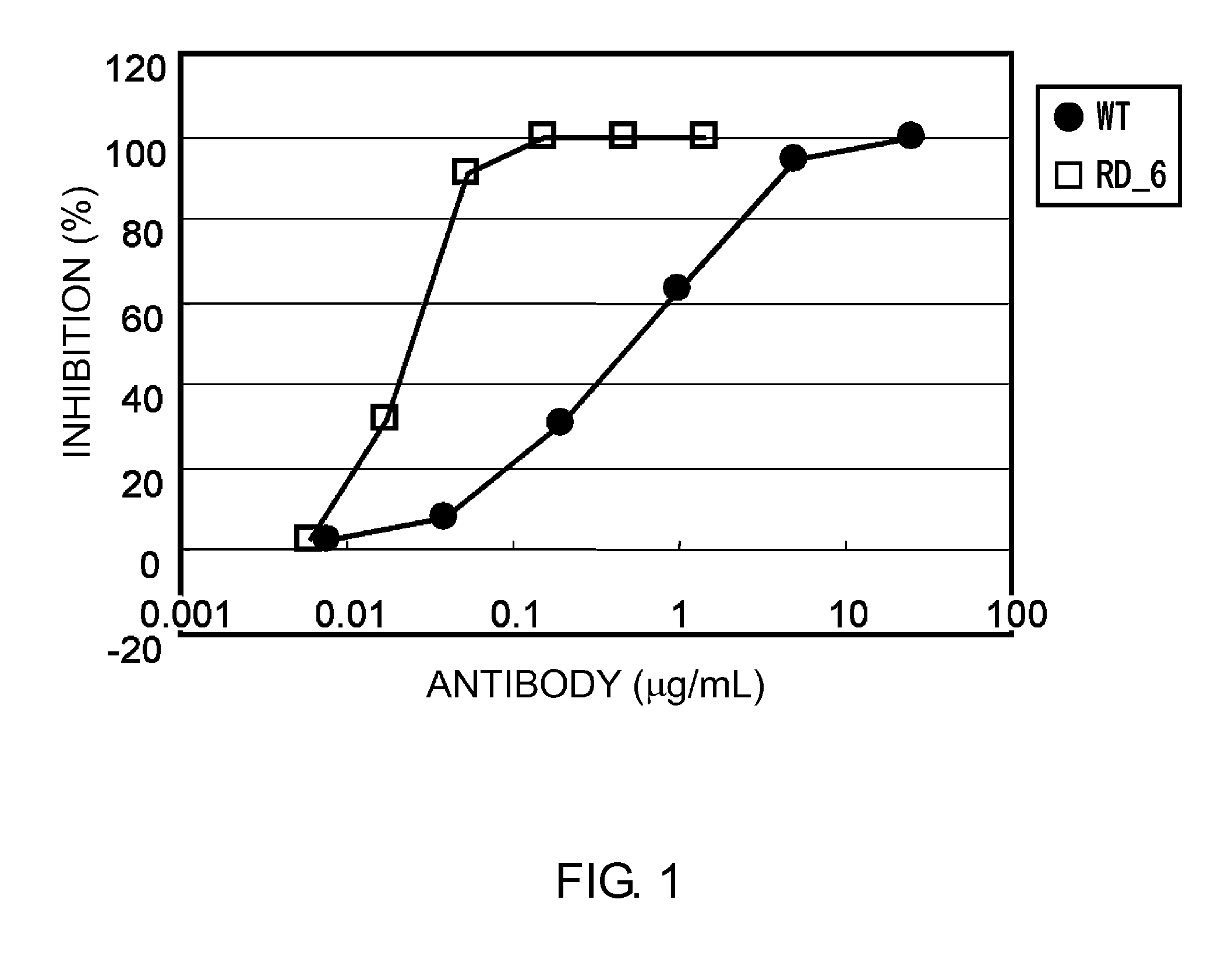
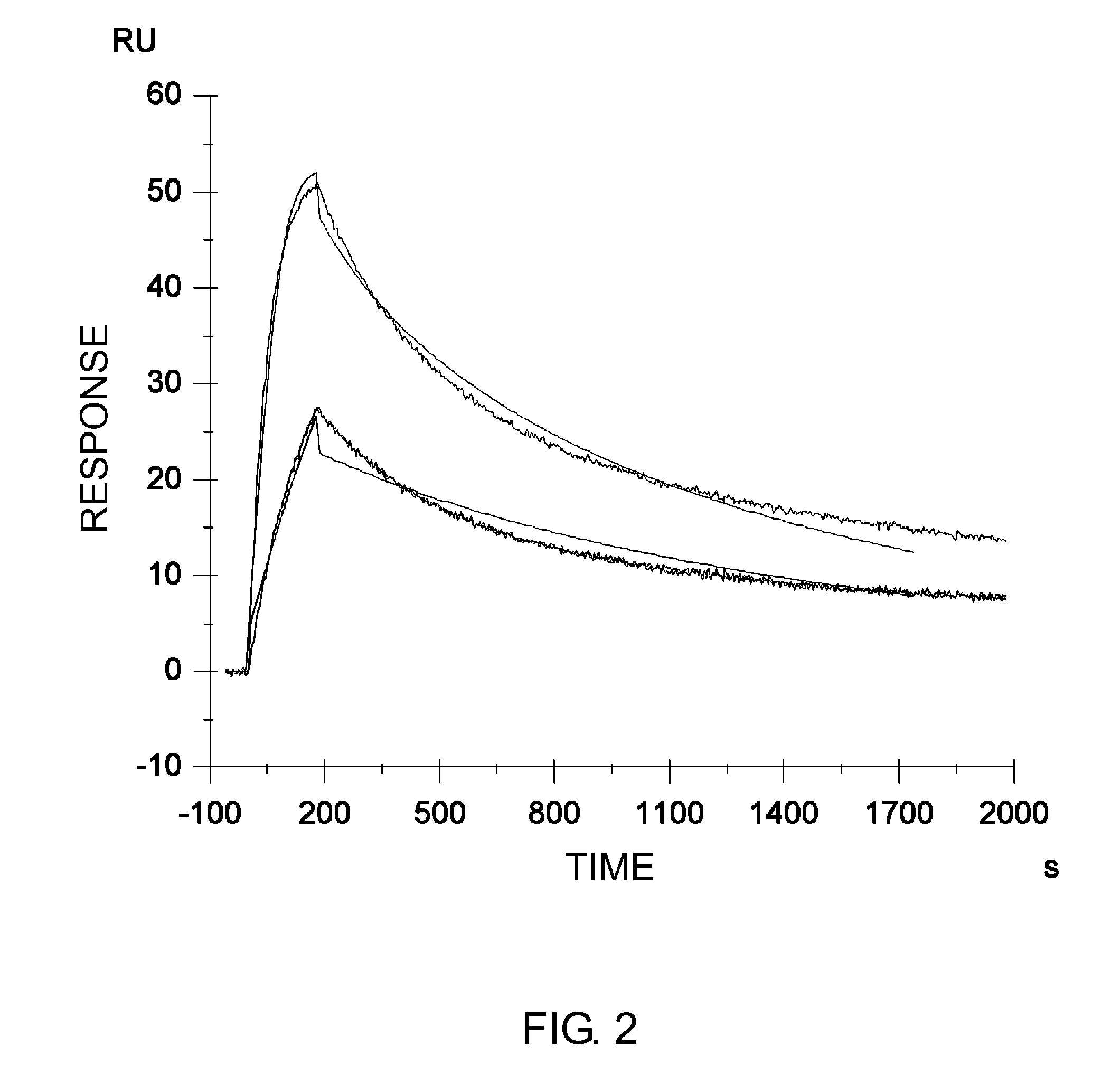
The present inventors discovered that antibodies having weaker antigen-binding activity at the early endosomal pH in comparison with that at the pH of plasma are capable of binding to multiple antigen molecules with a single antibody molecule, have long half-lives in plasma, and have improved durations of time in which they can bind to antigen.
Owner:CHUGAI PHARMA CO LTD
Non-invasive prenatal diagnosis
InactiveUS20010051341A1Improve accuracy100% accurate detection rateMicrobiological testing/measurementFermentationPrenatal diagnosisBlood typing
The invention relates to a detection method performed on a maternal serum or plasma from a pregnant female, which method comprises the presence of a nucleic acid of fetal origin in the sample. The invention enables non-invasive prenatal diagnosis including, for example, sex determination, blood typing and other genotyping, and detection of pre-eclampsia in the mother.
Owner:ISIS INNOVATION LTD
Tissue-and serum-derived glycoproteins and methods of their use
InactiveUS20070099251A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBlood plasmaBiology
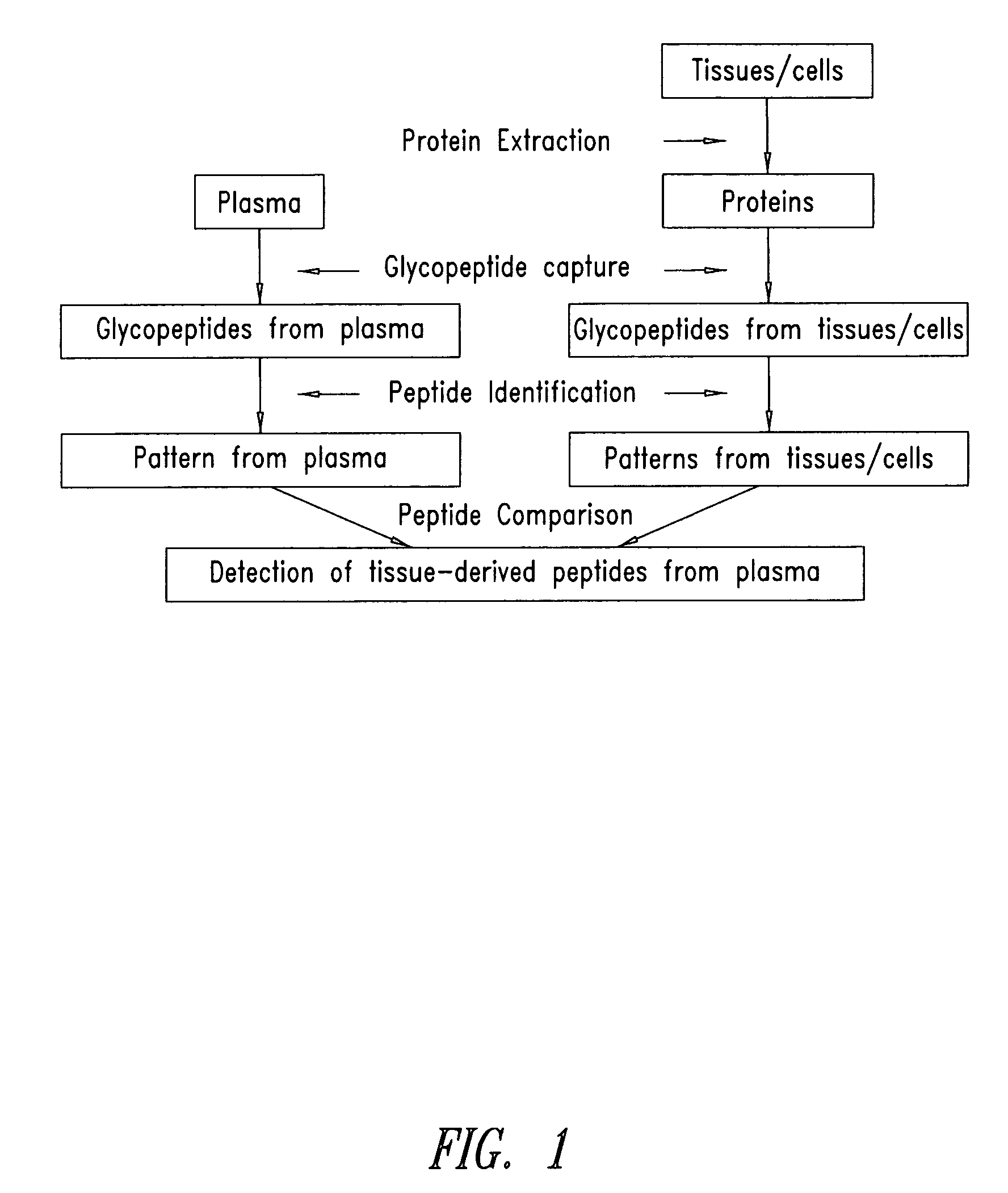
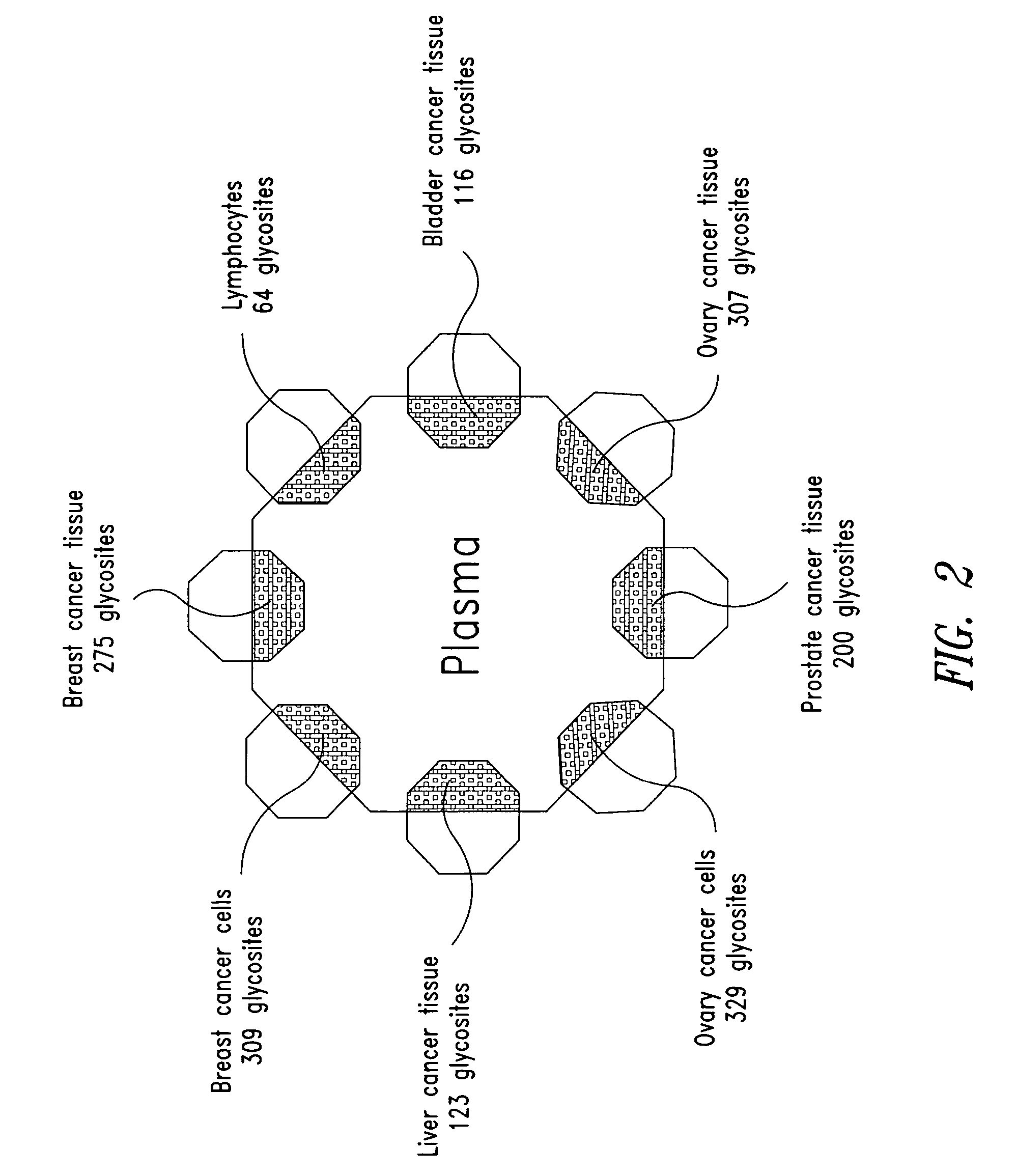
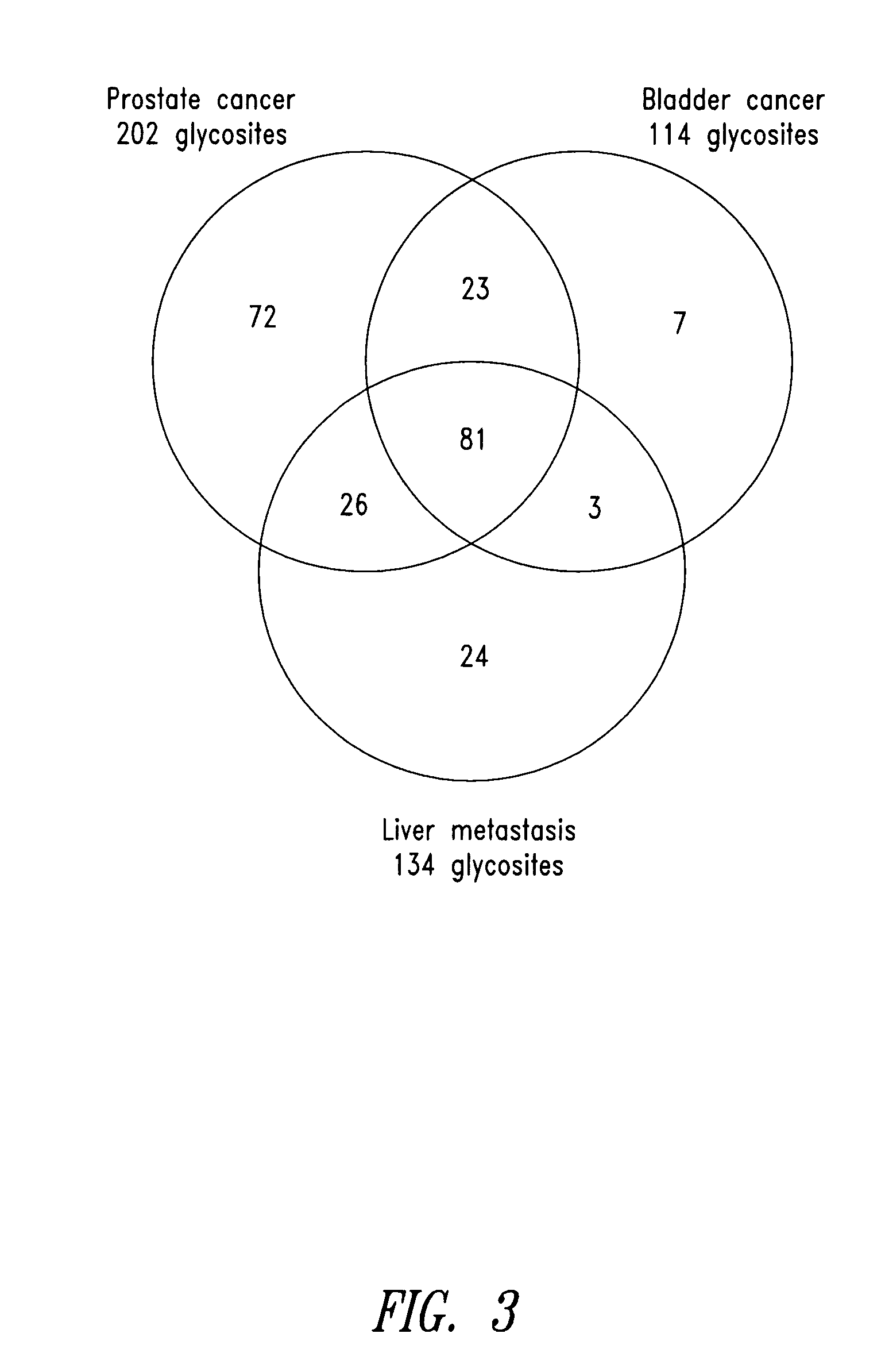
The present invention is directed generally to tissue-derived glycoproteins and glycosites detectable in plasma via mass spectrometric analysis of glycoproteins from both tissues and blood. The invention also provides methods for identifying tissue-derived glycoproteins and glycosites in plasma, panels of detection reagents for detecting same, as well methods for detecting disease using such panels. The invention further provides a database of tissue-derived glycoproteins and glycosites detectable in plasma.
Owner:INSTITUTE FOR SYSTEMS BIOLOGY
Process for discriminating between biological states based on hidden patterns from biological data
The invention describes a process for determining a biological state through the discovery and analysis of hidden or non-obvious, discriminatory biological data patterns. The biological data can be from health data, clinical data, or from a biological sample, (e.g., a biological sample from a human, e.g., serum, blood, saliva, plasma, nipple aspirants, synovial fluids, cerebrospinal fluids, sweat, urine, fecal matter, tears, bronchial lavage, swabbings, needle aspirantas, semen, vaginal fluids, pre-ejaculate.), etc. which is analyzed to determine the biological state of the donor. The biological state can be a pathologic diagnosis, toxicity state, efficacy of a drug, prognosis of a disease, etc. Specifically, the invention concerns processes that discover hidden discriminatory biological data patterns (e.g., patterns of protein expression in a serum sample that classify the biological state of an organ) that describe biological states.
Owner:ASPIRA WOMENS HEALTH INC +1
Composition and method for the repair and regeneration of cartilage and other tissues
InactiveUS7148209B2Add supportImprove coagulation/solidificationBiocidePeptide/protein ingredientsAbnormal tissue growthRepair tissue
Owner:SMITH & NEPHEW ORTHOPAEDICS
Surface coating for chamber components used in plasma systems
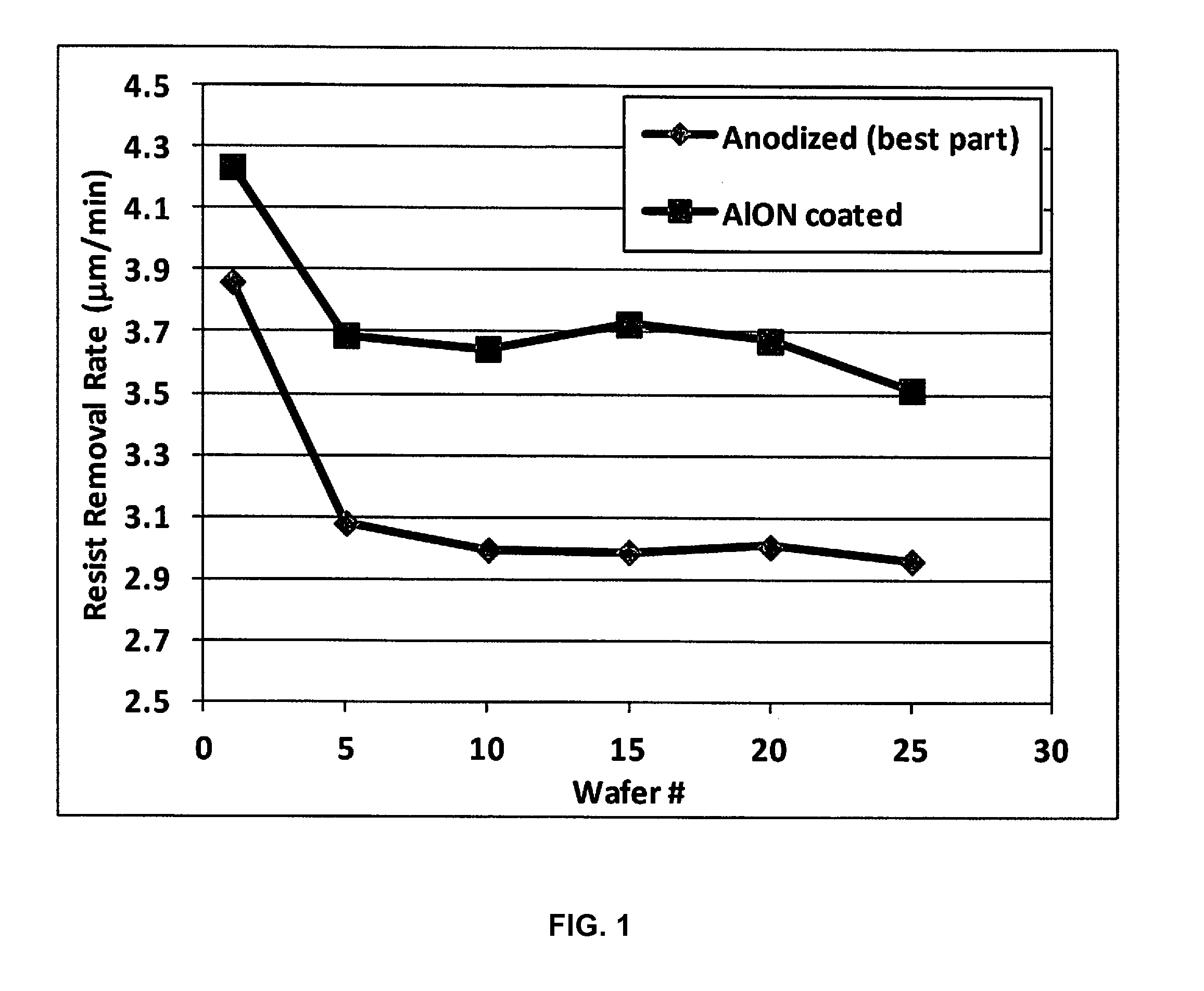
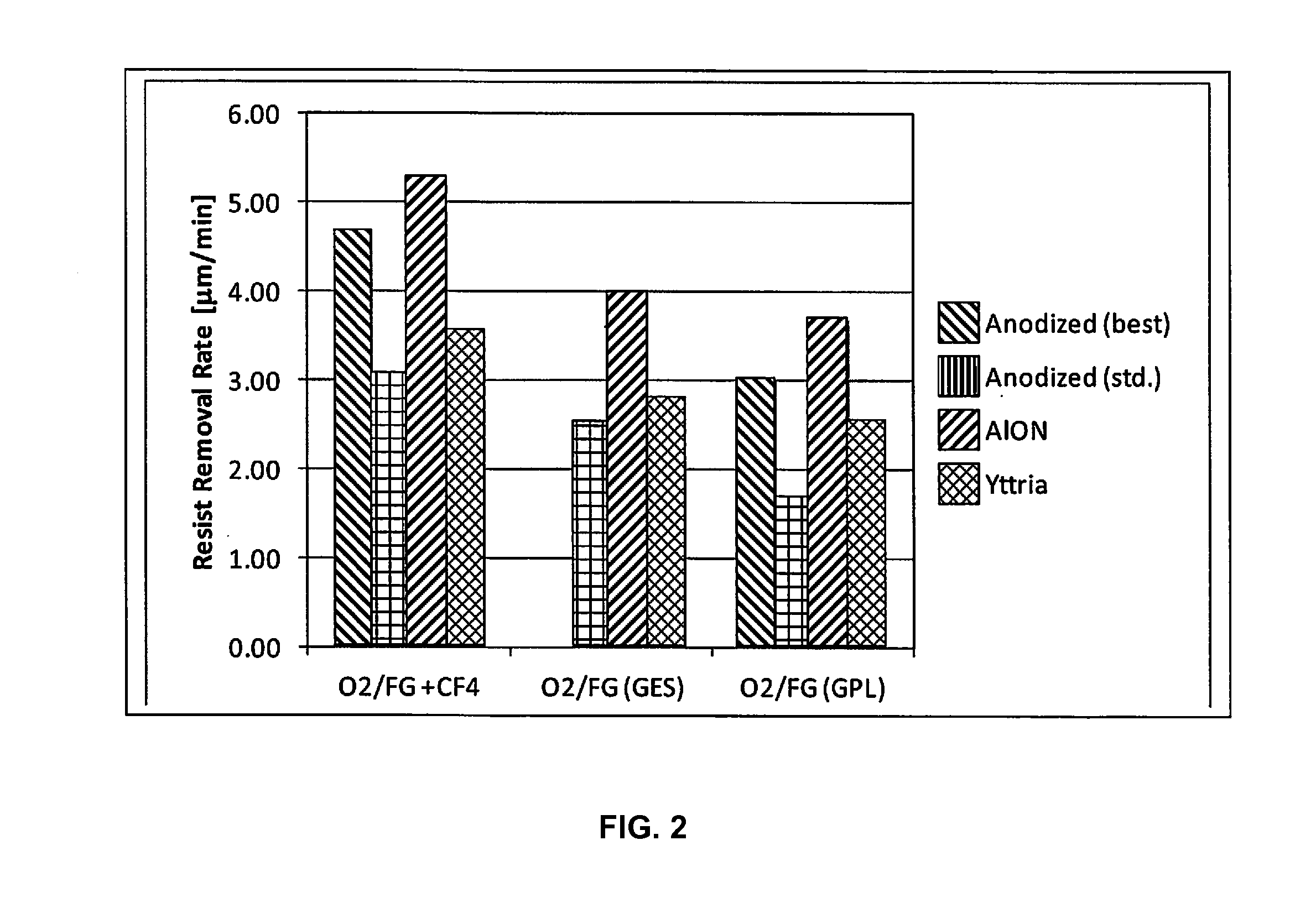
ActiveUS20170032942A1Low plasma surface recombination rateHigh trafficElectric discharge tubesVacuum evaporation coatingBlood plasmaPlasma chamber
Disclosed herein are surface coatings for plasma components that have the benefit of being robust against chemical and plasma physical attack in aggressive (e.g., fluorine-based) plasma environments. The coatings also provide low plasma surface recombination rates for active oxygen, nitrogen, fluorine, and hydrogen species when compared with other known surface treatments. The coatings can be applied to any plasma system component not requiring etching or plasma cleaning including but not limited to materials like quartz, aluminum, or anodized aluminum. Additionally, the efficiency of the system is increased by applying a non-reactive coating to system components thereby increasing the flow of excited plasma species to the plasma chamber of the system.
Owner:ENTEGRIS INC
Antibodies with modified isoelectric points
ActiveUS20120028304A1Increased serum half-lifeMinimize the possibilityAnimal cellsSugar derivativesHalf-lifeBlood plasma
The invention relates generally to compositions and methods for altering the isoelectric point of an antibody, and in some cases, resulting in improved plasma pharmacokinetics, e.g. increased serum half-life in vivo.
Owner:XENCOR
Method of Modifying Isoelectric Point of Antibody Via Amino Acid Substitution in CDR
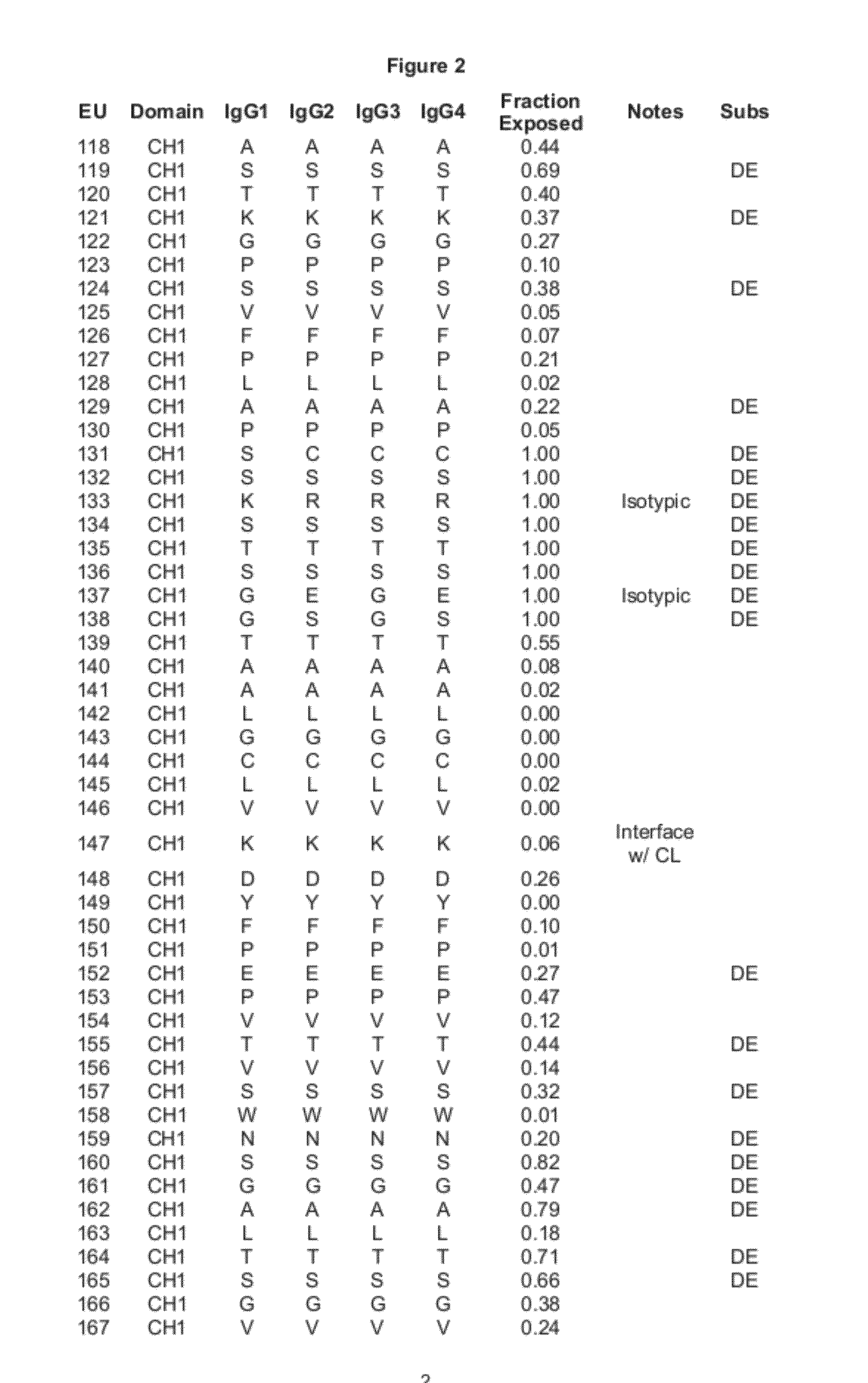
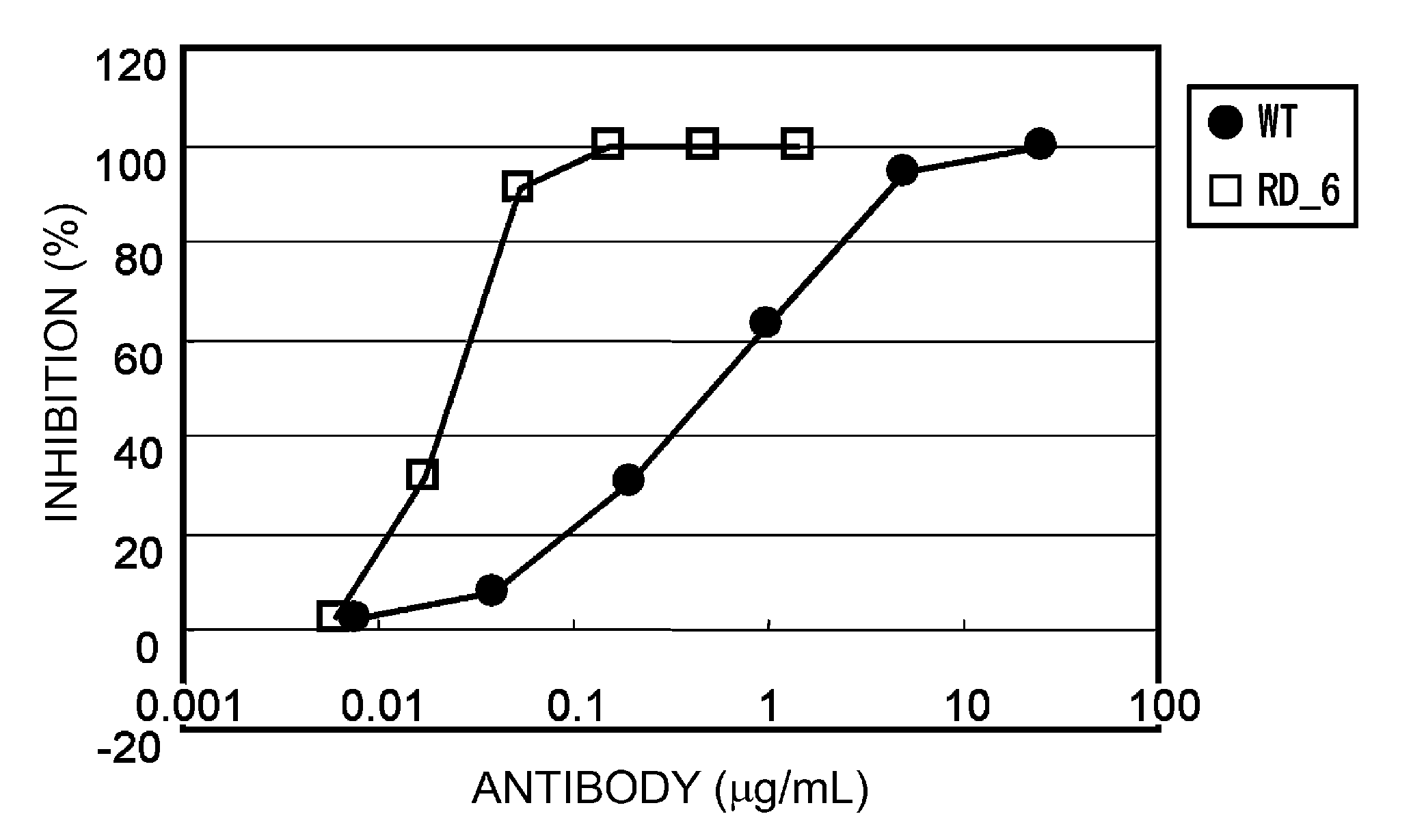
ActiveUS20110076275A1Enhanced antigen-neutralizing activityImprove retentionSugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsComplementarity determining regionAmino acid substitution
The present inventors provide methods for modifying the isoelectric point of an antibody while retaining its antigen-binding activity, comprising modifying the charge of at least one exposable amino acid residue on the surface of the complementarity determining region (CDR). The present invention also provides methods for purifying multispecific antibodies, comprising modifying isoelectric point, and methods for improving the plasma pharmacokinetics of antibodies, comprising modifying isoelectric point. The present invention further provides antibodies with a modified isoelectric point, pharmaceutical compositions comprising the antibodies as an active ingredient, and methods for producing the antibodies and compositions.
Owner:CHUGAI PHARMA CO LTD
Method to generate a plasma stream for performing electrosurgery
InactiveUS8057468B2Control areaEnhance flow-assisted removalSurgical instruments for heatingSurgical instruments for aspiration of substancesNoble gasSurgical site
Owner:BOVIE MEDICAL CORP
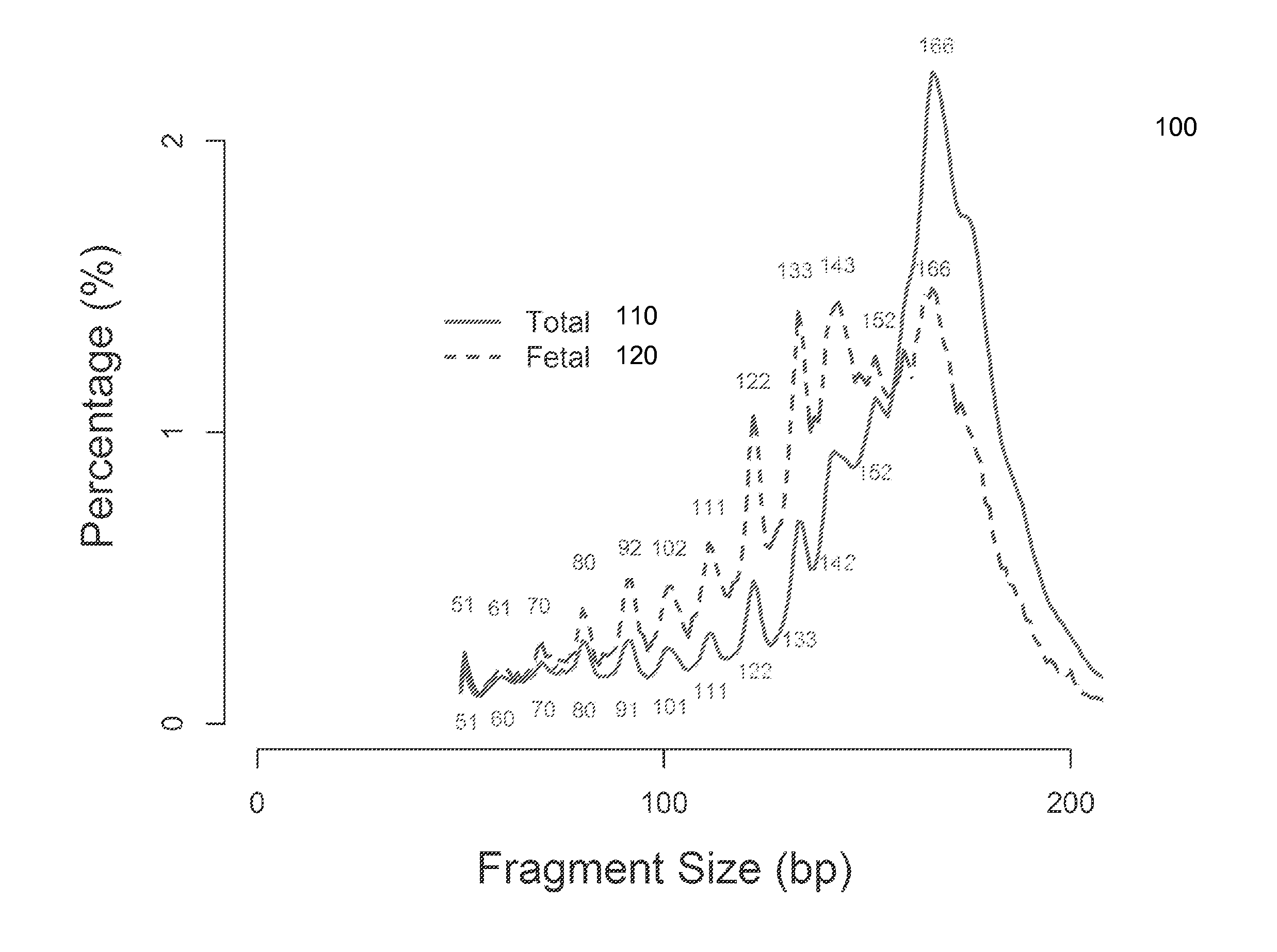
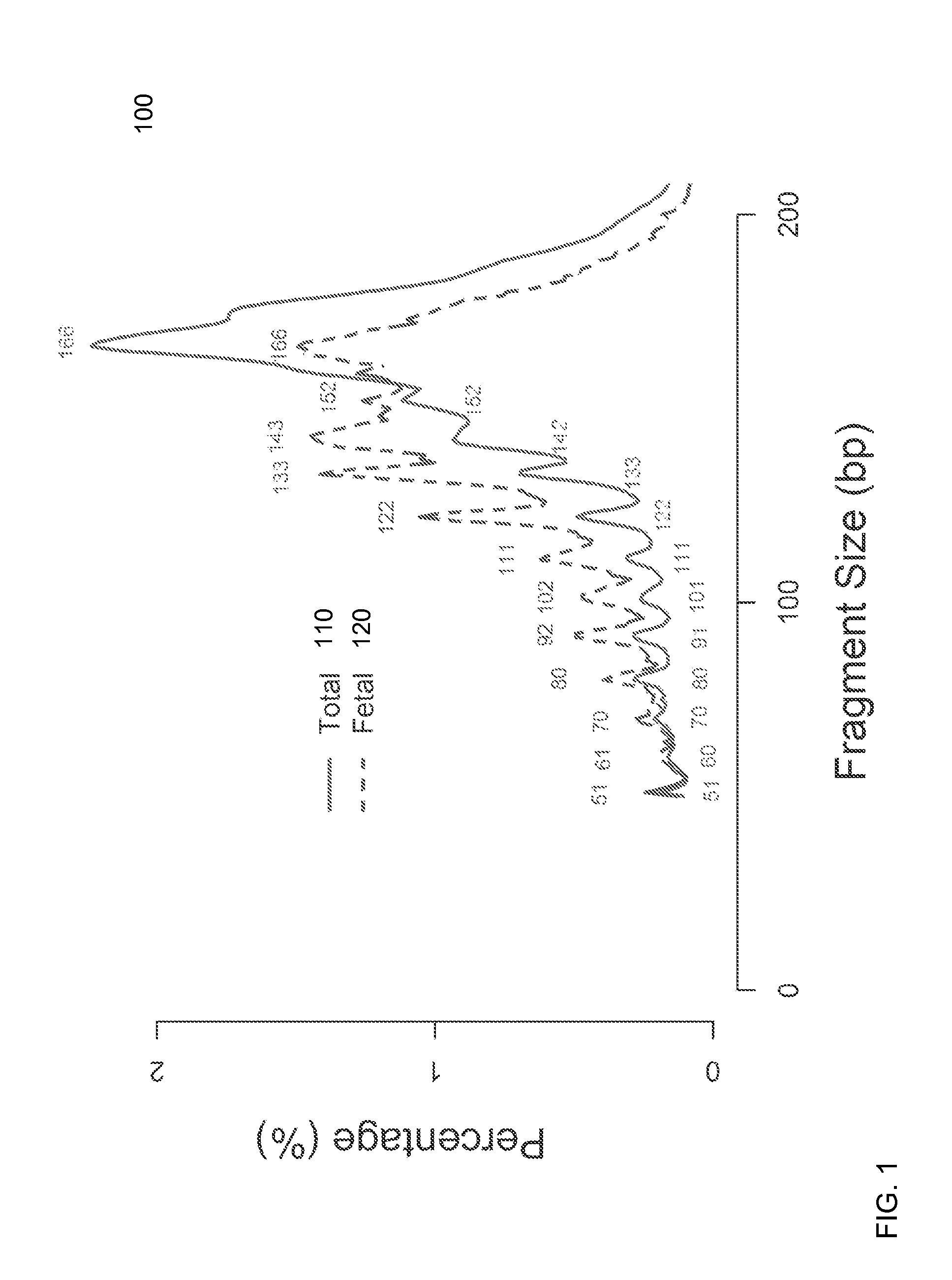
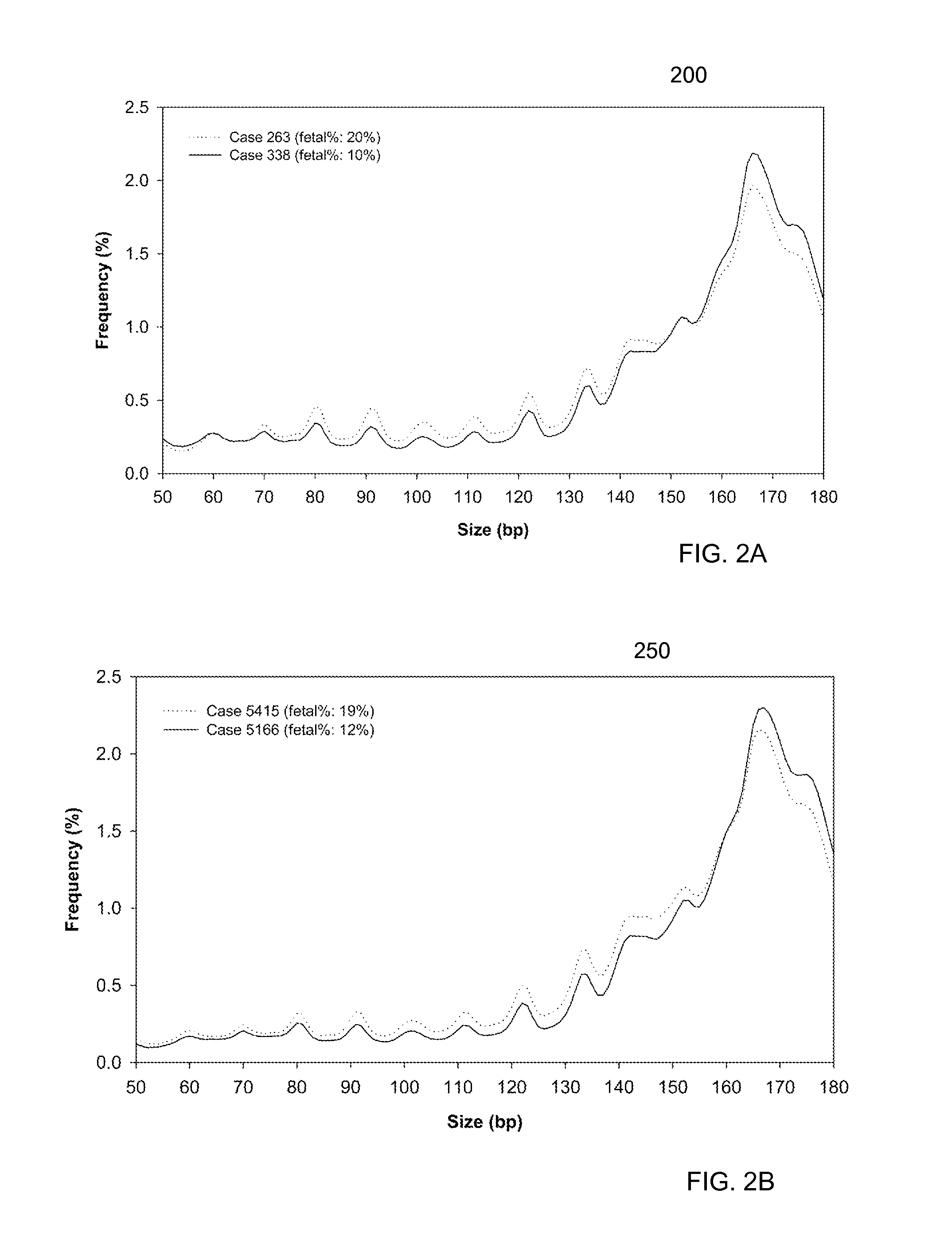
Size-based analysis of fetal DNA fraction in maternal plasma
ActiveUS20130237431A1Health-index calculationMicrobiological testing/measurementAbnormal tissue growthBlood plasma
A fractional concentration of clinically-relevant DNA in a mixture of DNA from a biological sample is determined based on amounts of DNA fragments at multiple sizes. For example, the fractional concentration of fetal DNA in maternal plasma or tumor DNA in a patient's plasma can be determined. The size of DNA fragments in a sample is shown to be correlated with a proportion of fetal DNA and a proportion of tumor DNA, respectively. Calibration data points (e.g., as a calibration function) indicate a correspondence between values of a size parameter and the fractional concentration of the clinically-relevant DNA. For a given sample, a first value of a size parameter can be determined from the sizes of DNA fragments in a sample. A comparison of the first value to the calibration data points can provide the estimate of the fractional concentration of the clinically-relevant DNA.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Method for the preparation of purified HCV RNA by exosome separation
InactiveUS7198923B1Simple preparation processPromote progressSsRNA viruses positive-senseMicrobiological testing/measurementExosomeBlood plasma
Owner:GRIFOLS WORLDWIDE OPERATIONS +1
Method for treating alzheimer's disease
InactiveUS20020055529A1Reduce vascular and cardiac diseaseBiocideAnimal repellantsCholesterolPlasma triglyceride level
The present invention provides a method for treating or preventing the onset of Alzheimer's Disease comprising administering to a mammal in need thereof an Alzheimer's Disease-preventing or treating amount of a plasma-triglyceride level-lowering agent. Optionally, the plasma-triglyceride level-lowering agent can be co-administered with a cholesterol level-lowering agent.
Owner:WARNER LAMBERT CO LLC
Morphine controlled release system
InactiveUS20070003617A1Low administration frequencyAffecting extent of drug bioavailabilityBiocideNervous disorderMorphineDissolution
A composition for controlled release of an opioid from a pharmaceutical composition, the method comprises controlling the release of at least one opioid into an aqueous medium by erosion of at least one surface of a pharmaceutical composition comprising I) a matrix composition comprising a) polymer or a mixture of polymers, b) an opioid and, optionally, c) one or more pharmaceutically acceptable excipients, and (i) a coating. The matrix composition has a conus-like shape so the surface area exposed to the aqueous medium increases at least during initial erosion of the matrix composition, and the dissolution of the opioid-when tested in a Dissolution Test as described herein with or without application of sinkers-results in a zero order release of at least 80% of the opioid contained in the composition. Such compositions are especially suitable for controlled release of an opioid to obtain a delayed pead concentration and a prolonged therapeutically effective plasma concentration upon oral administration. Once or twice daily administration is possible. The matrix typically comprises PEO and the active substance is typically an opioid such as morphine or a glucuronide thereof.
Owner:EGALET LTD
Controlled release formulations having rapid onset and rapid decline of effective plasma drug concentrations
InactiveUS6419960B1Patient compliance is goodGood retarding effectPowder deliveryOrganic active ingredientsImmediate releasePlasma drug concentration
The invention is directed to oral modified / controlled release drug formulations which provide a rapid initial onset of effect and a prolonged duration of effect. Preferably, the peak concentration is lower than that provided by the reference standard for immediate release formulations of the drug, and the duration of effect falls rapidly at the end of the dosing interval.
Owner:RHODES PHARMA LP
Dosage form containing multiple drugs
A pharmaceutical dosage form comprising a first drug and a second drug, both of which are selected from decongestants, antitussives, expectorants, analgesics and antihistamines. The dosage form provides a plasma concentration within a therapeutic range of the second drug over a period which is coextensive with at least about 70% of a period over which the dosage form provides a plasma concentration within a therapeutic range of the first drug. This Abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
System and method for treating biological tissue with a plasma gas discharge
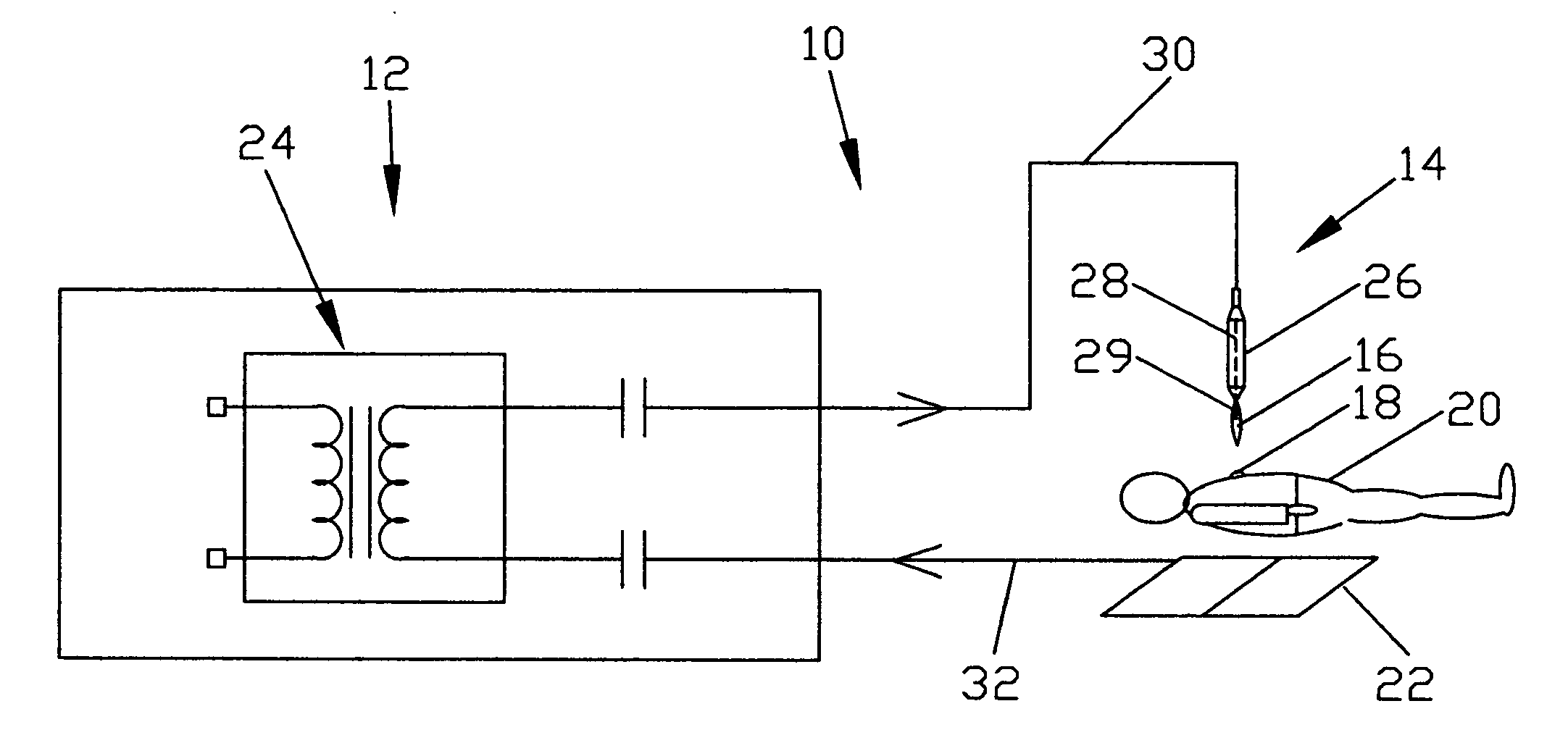
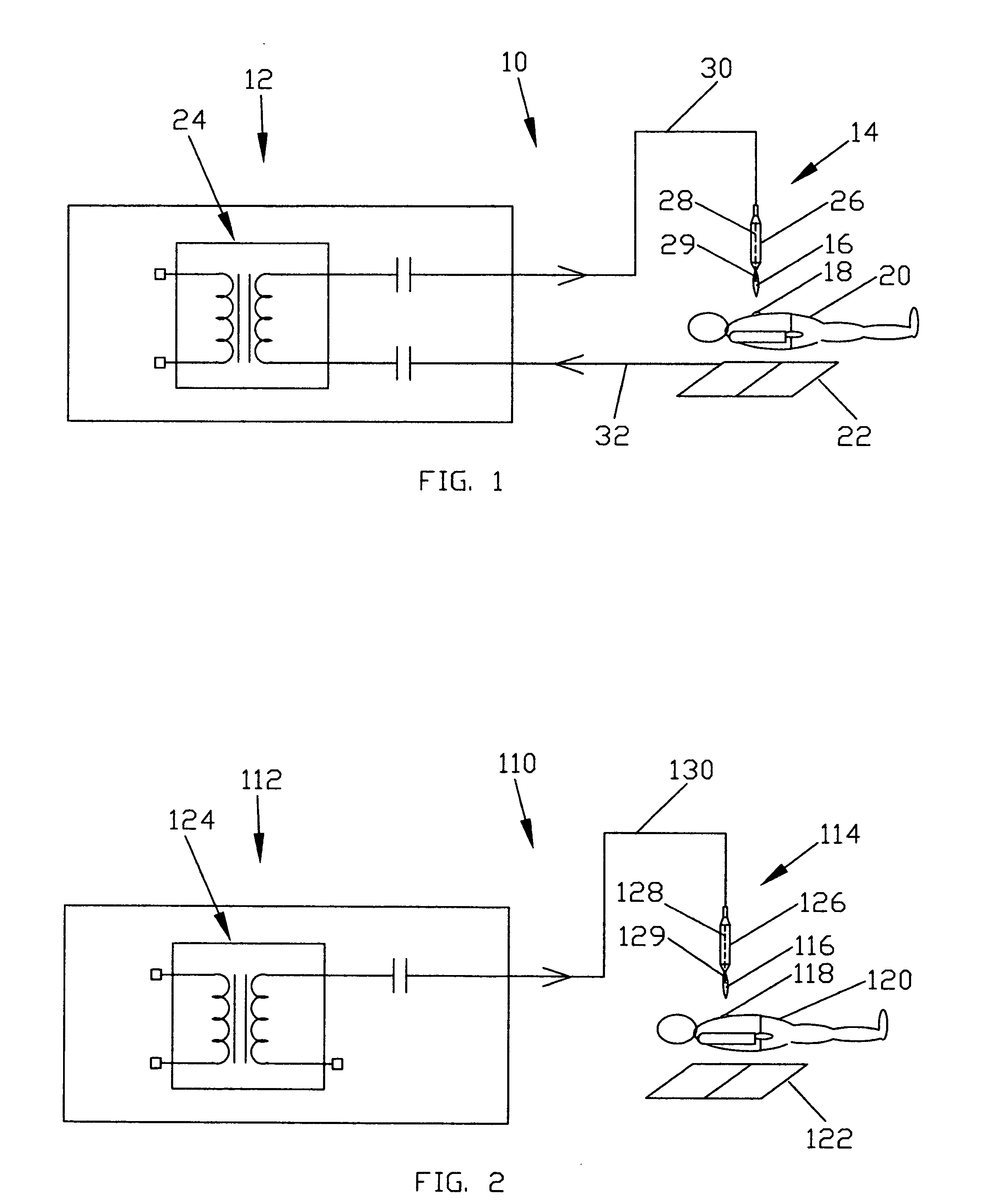
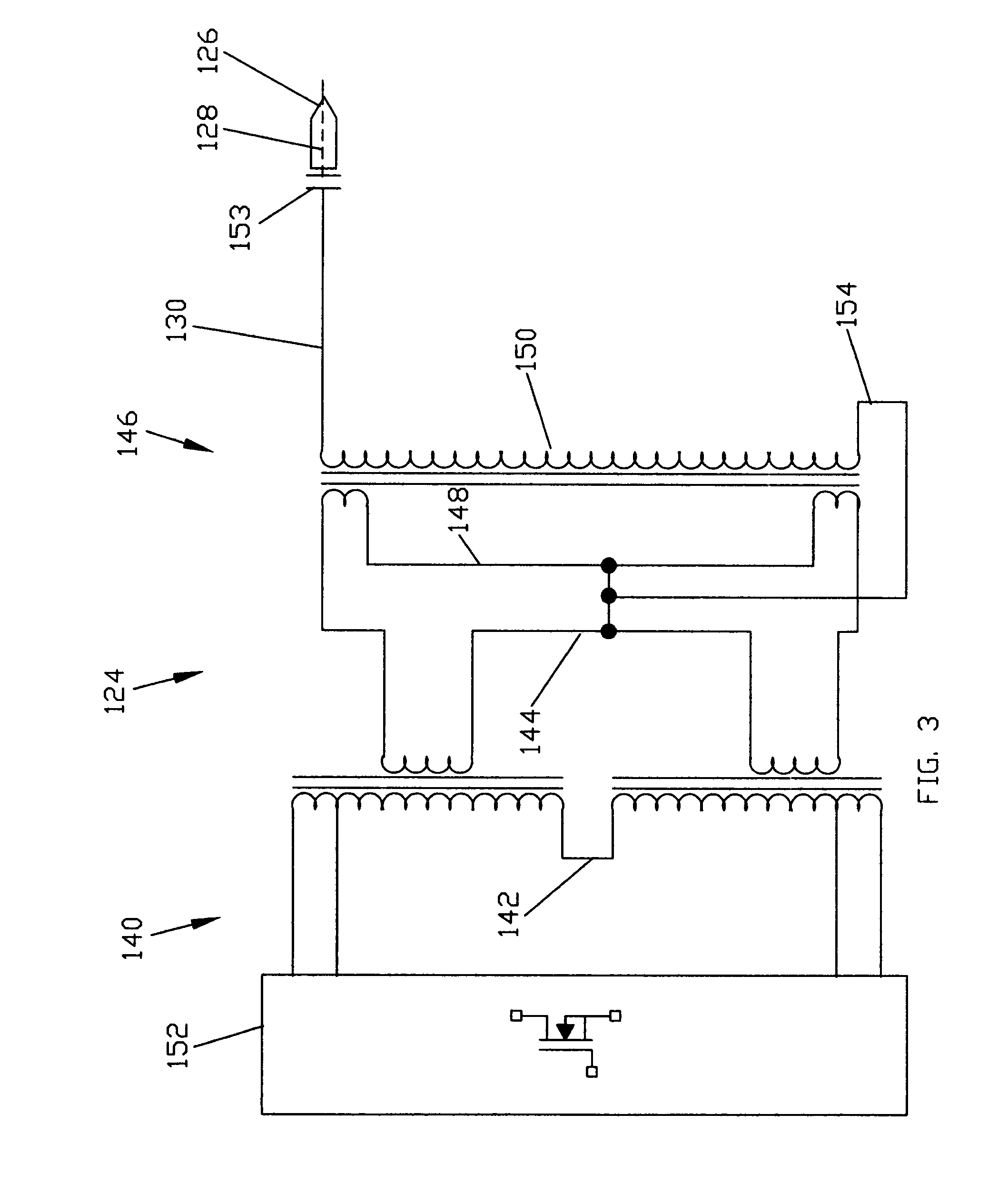
ActiveUS20060189976A1Effective treatmentSurgical instrument detailsPlasma techniqueMedicineProduct gas
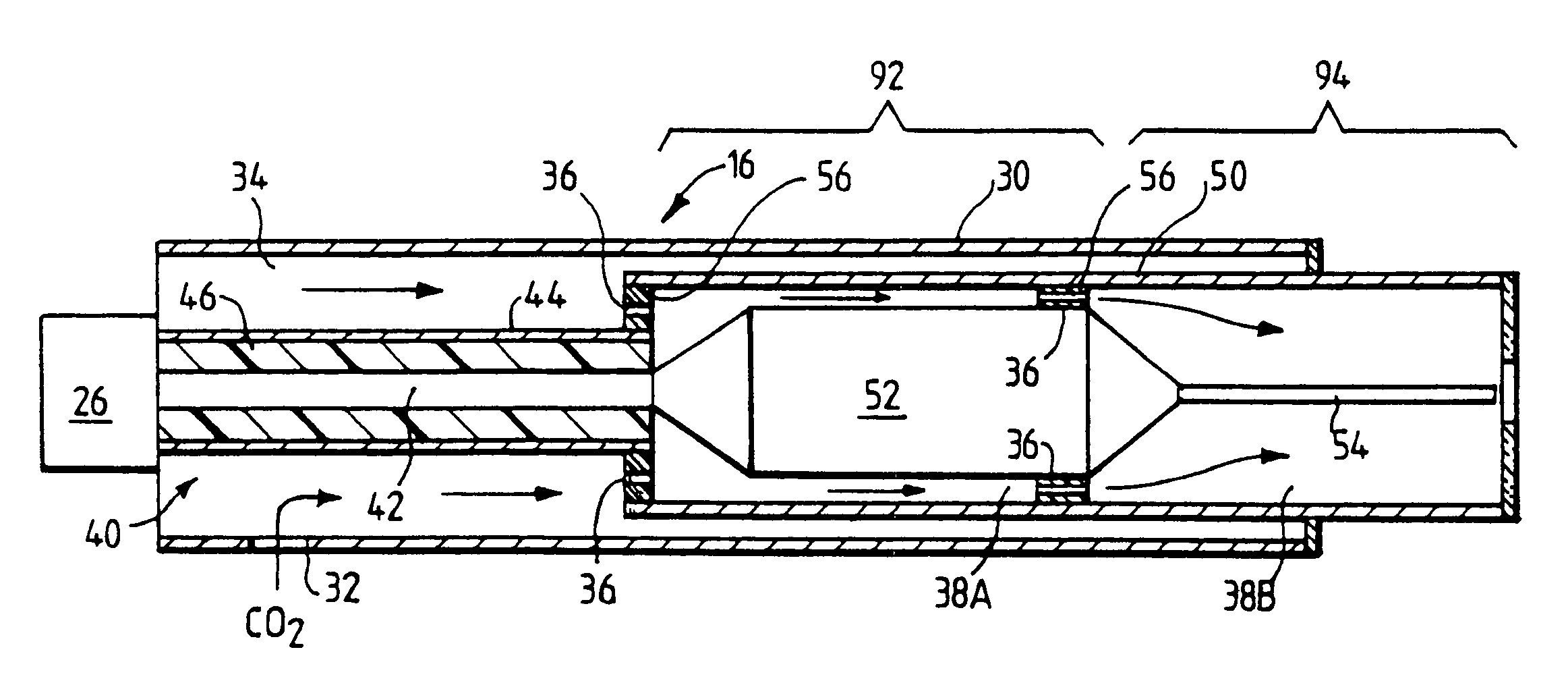
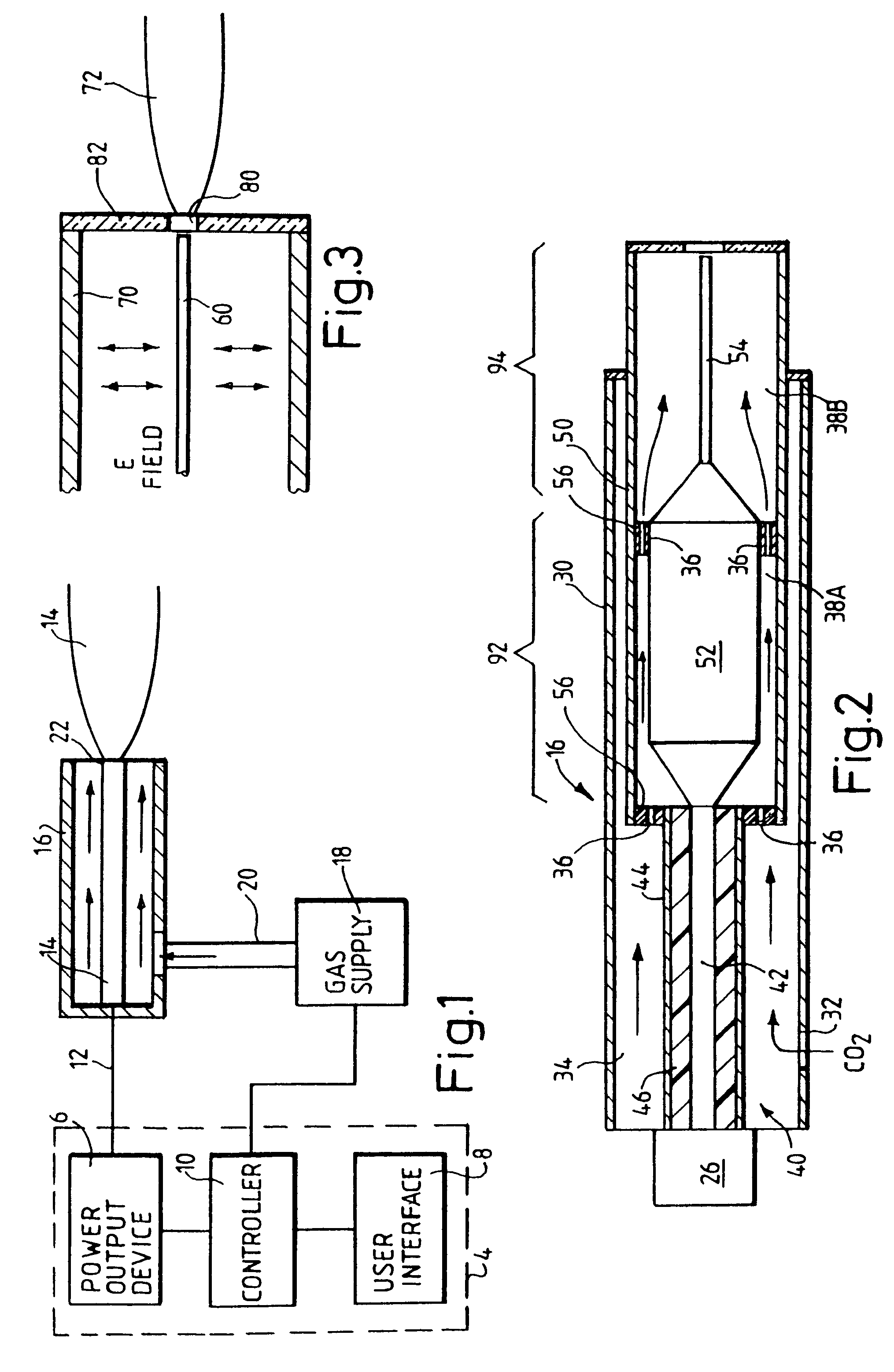
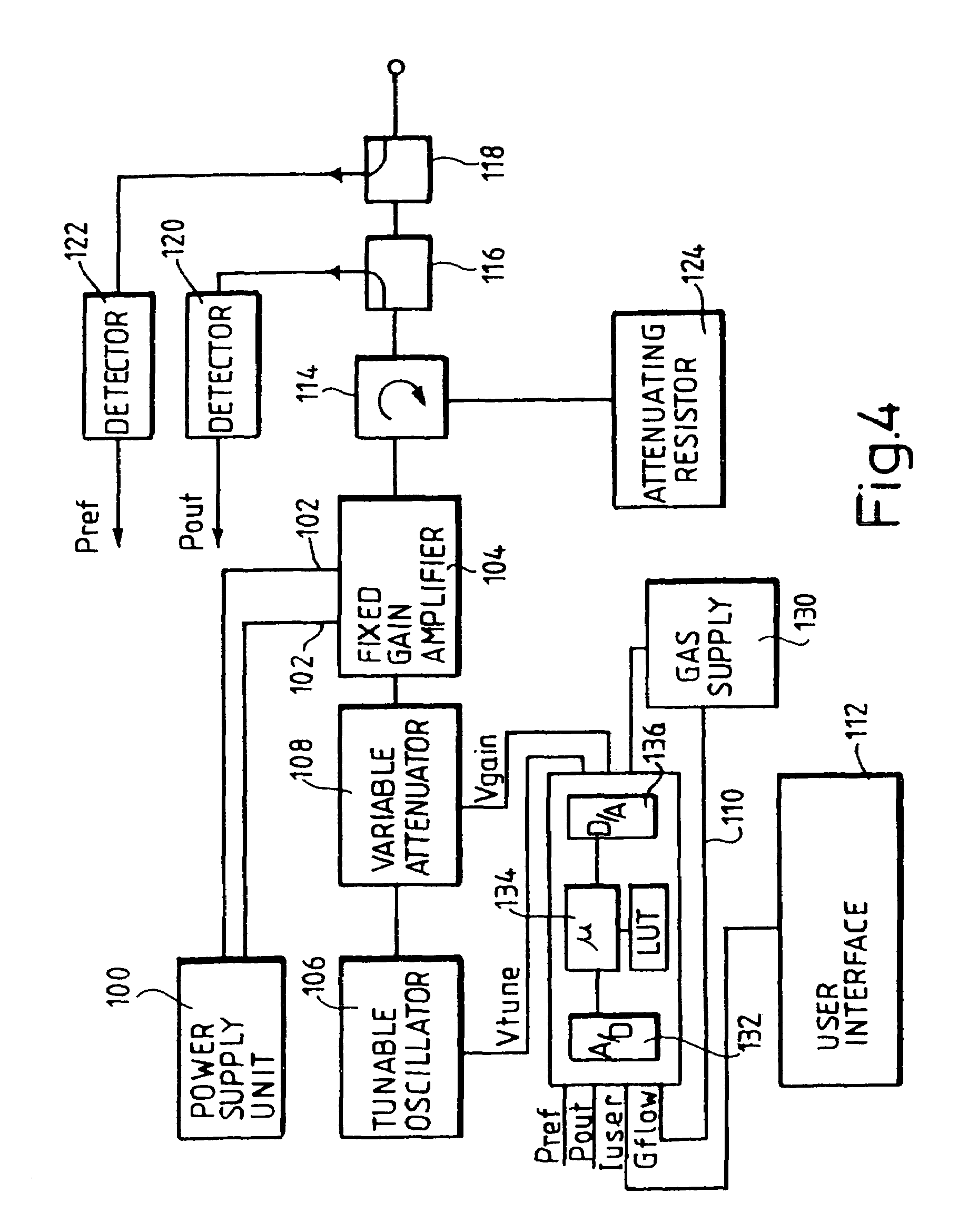
Devices and methods for treating biological tissue using a plasma gas-discharge are disclosed herein. An electrode for igniting a gas flow to form a plasma gas-discharge, wherein the electrode is configured within the device such that upon encountering a surface of the biological tissue by the electrode, a path of current from the electrode to the surface of the biological tissue is formed, thereby igniting the gas flow and forming the plasma gas-discharge. In some embodiments, electromagnetic interactions between the treated biological tissue and the plasma gas discharge traversing the electromagnetic interaction gap shape the profile of the plasma gas discharge. According to some embodiments, the device includes an electrode for igniting gas of the gas flow, and electromagnetic interactions between the electrode and the skin determine, at least in part, the electromagnetic interactions that shape the profile of the plasma gas discharge. In some embodiments, the device further includes a housing for providing support for the electrode, wherein the electrode is disposed relative to the housing such that the electrode is substantially electrically unshielded by the housing, and the electrode is positioned to electromagnetically interact with a surface of the biological tissue to shape, at least in part, the plasma profile. According to some embodiments, the presently disclosed device includes a dual-purpose nozzle-electrode for gas delivery and for igniting the gas flow. A method of transdermal ion delivery of a plasma flux to biological tissue as a means of treating the biological tissue is also provided.
Owner:ALMA LASERS LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com