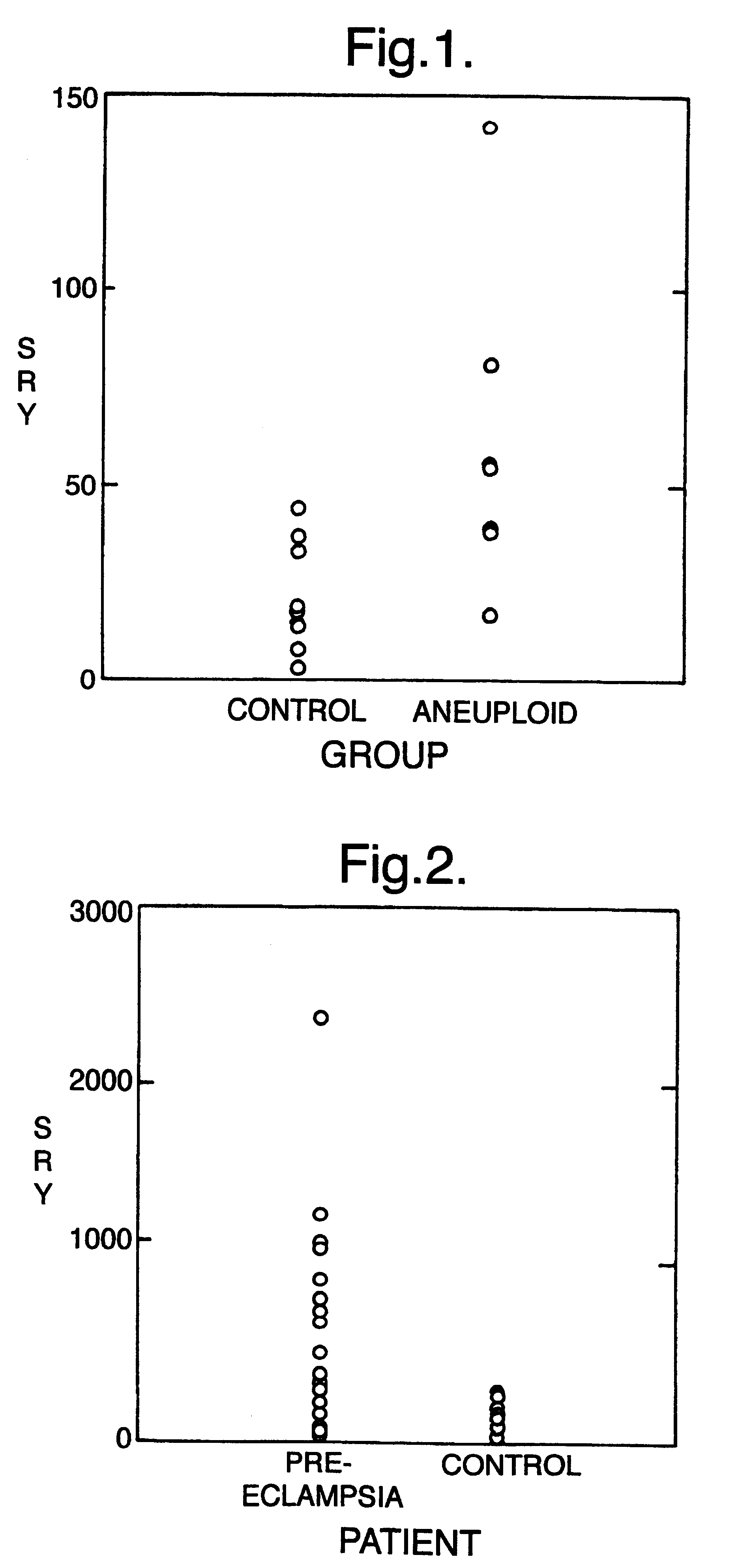
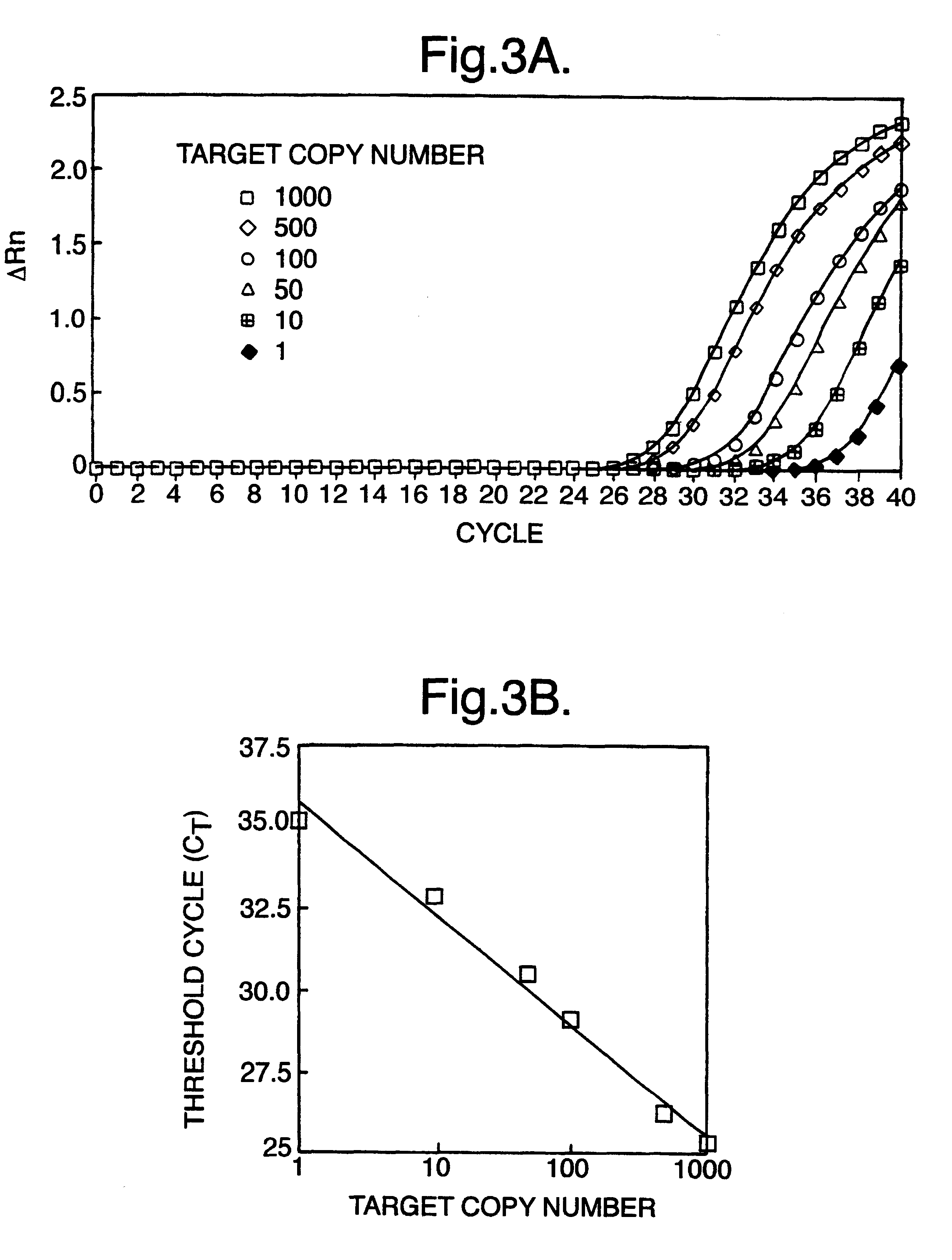
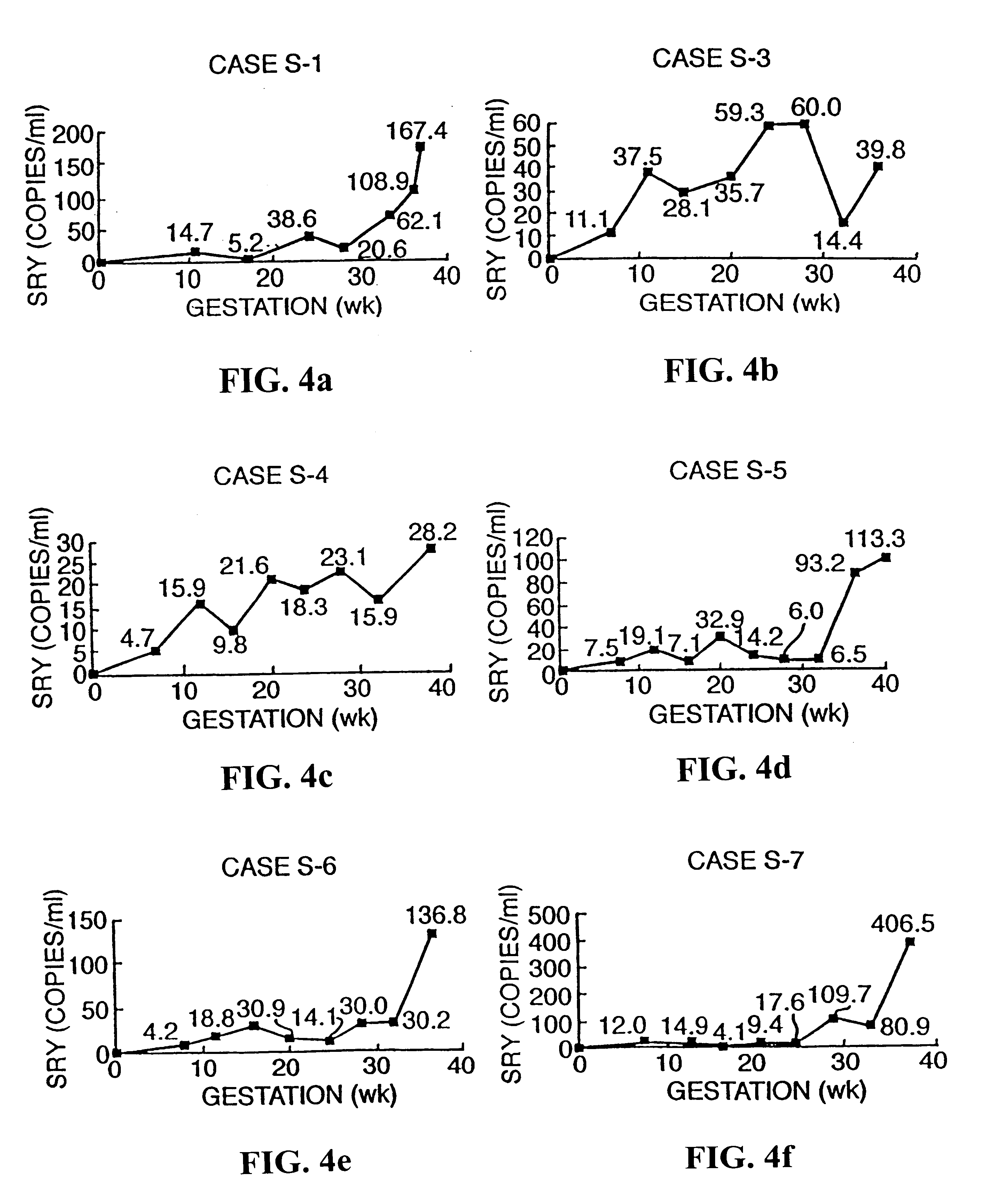
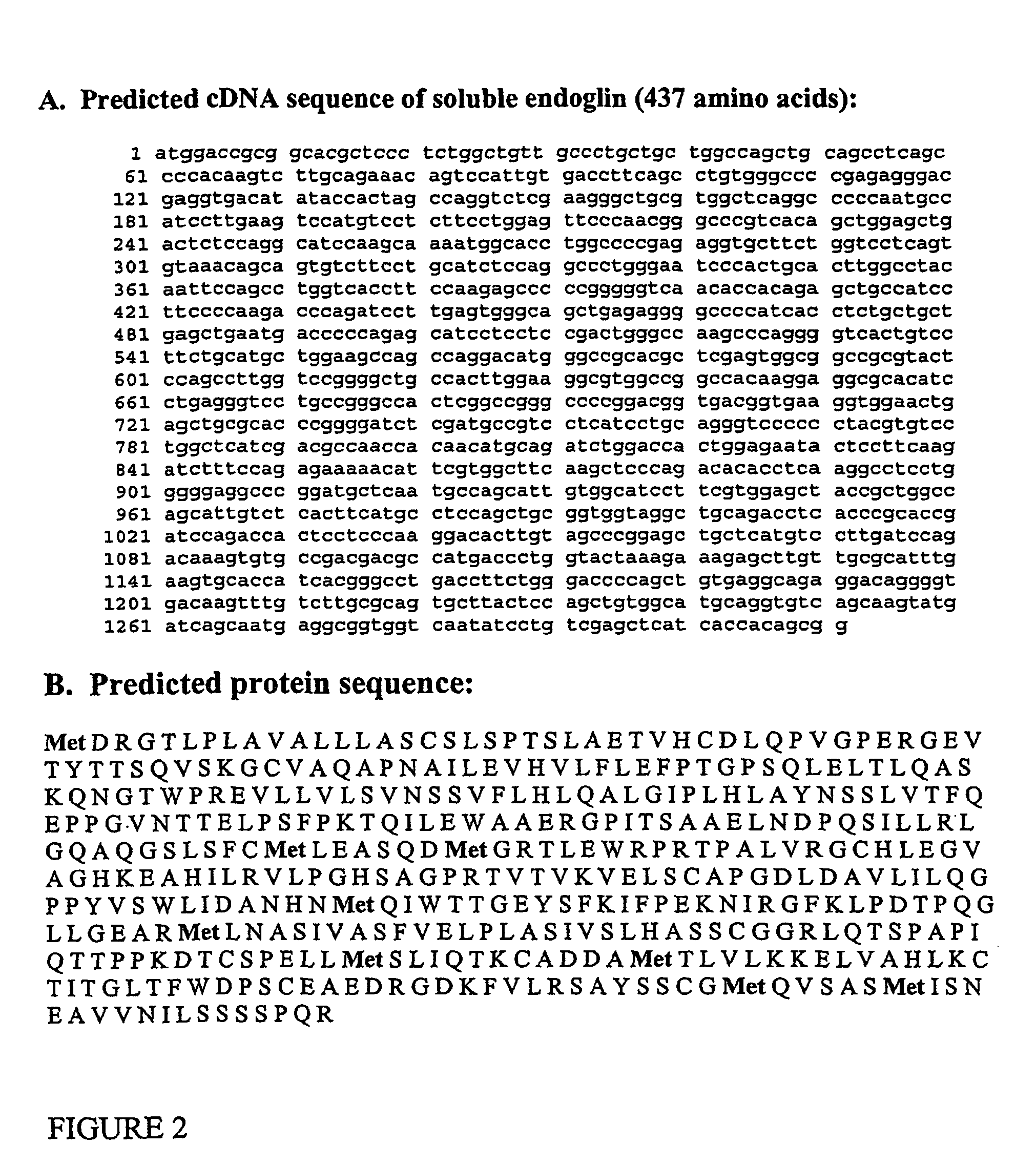
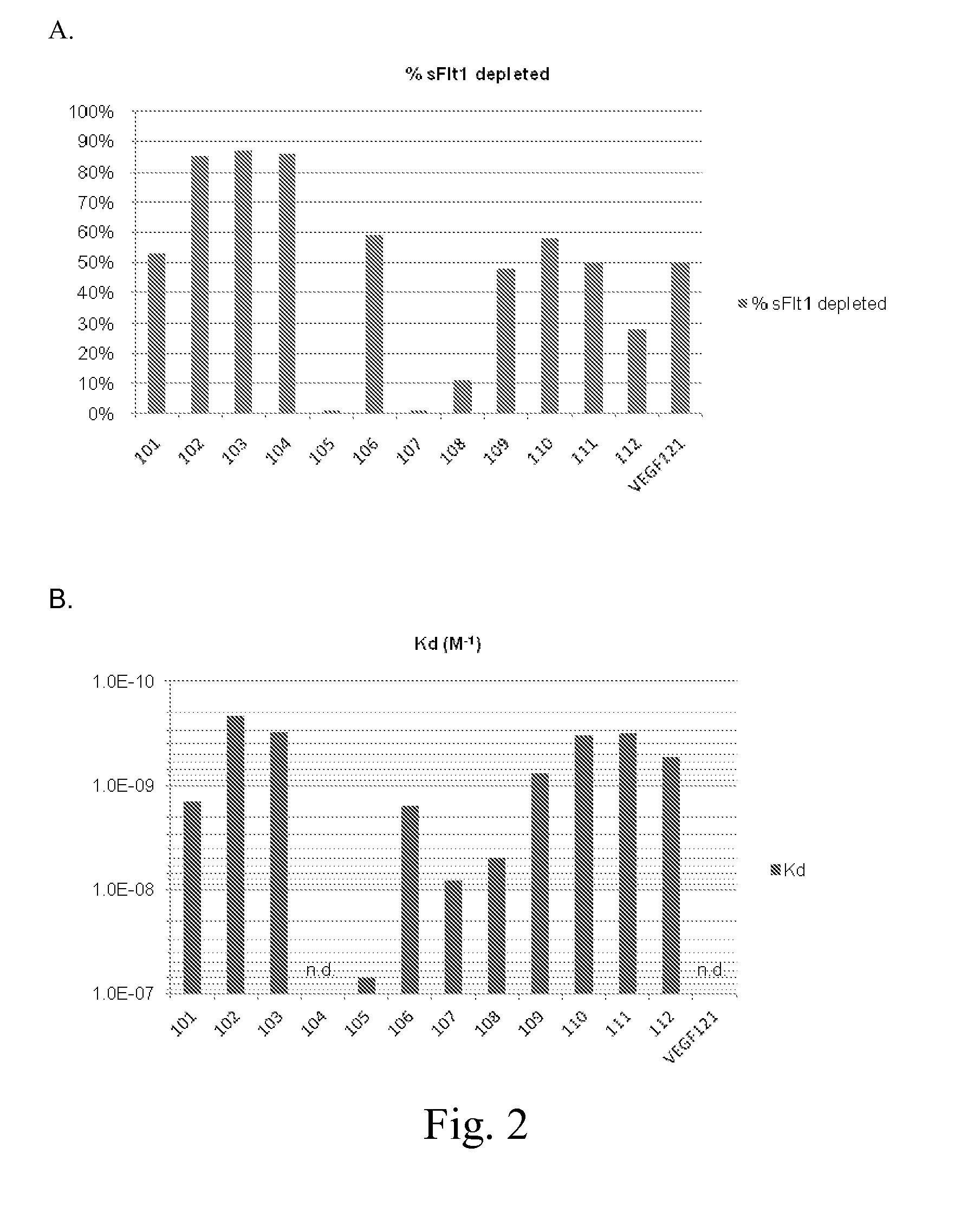Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
130 results about "Eclampsia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A life threatening condition during pregnancy or shortly after giving birth characterized by the development of seizures.
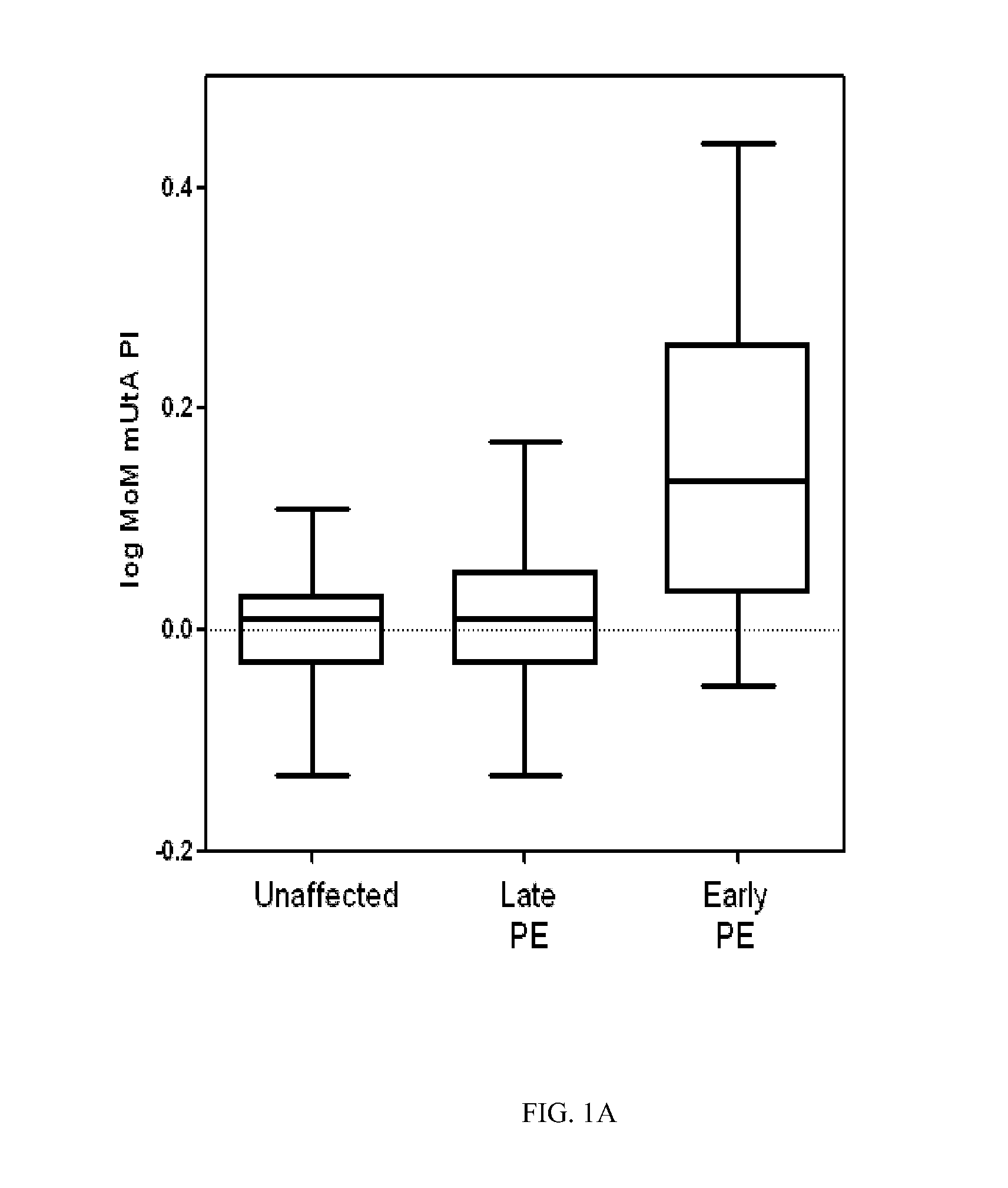
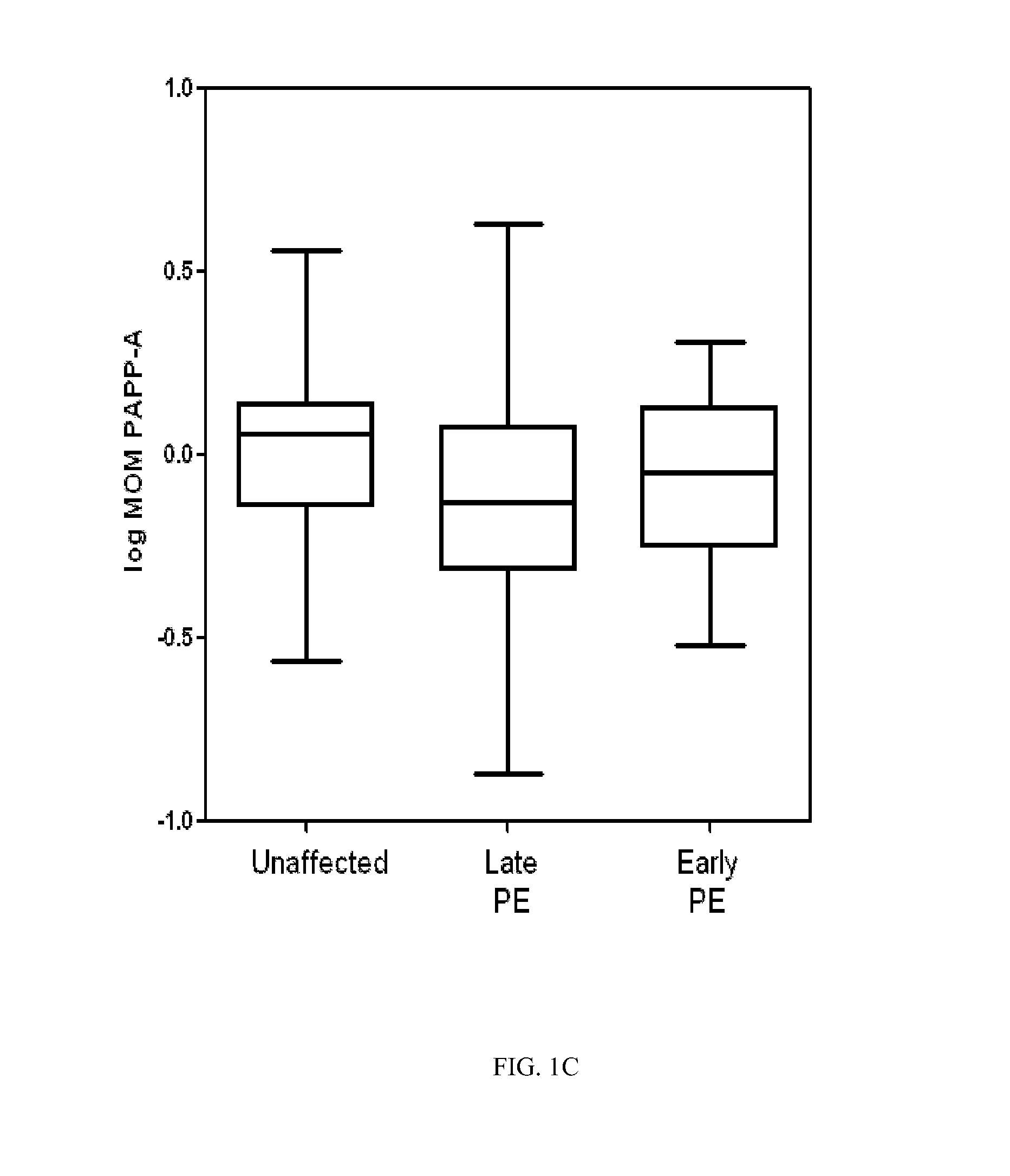
Non-invasive prenatal diagnosis
InactiveUS6258540B1% accurate detection rateIncrease the amount of foetal nucleic acid materialMicrobiological testing/measurementRecombinant DNA-technologyPrenatal diagnosisBlood typing
The invention relates to a detection method performed on a maternal serum or plasma sample from a pregnant female, which method comprises detecting the presence of a nucleic acid of foetal origin in the sample. The invention enables non-invasive prenatal diagnosis including for example sex determination, blood typing and other genotyping, and detection of pre-eclampsia in the mother.
Owner:SEQUENOM INC
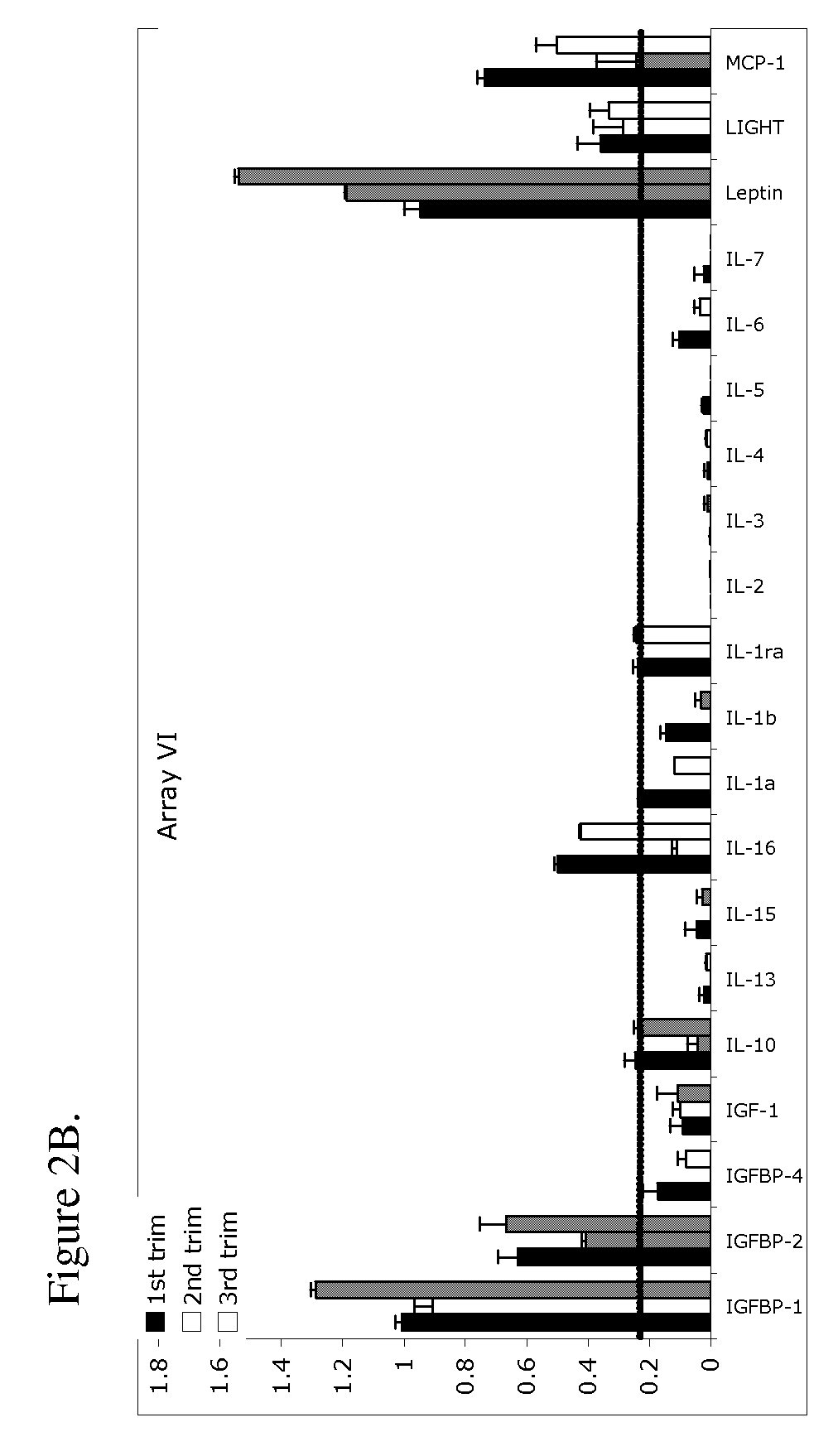
Pregnancy biomarker profiles, methods and compositions related thereto
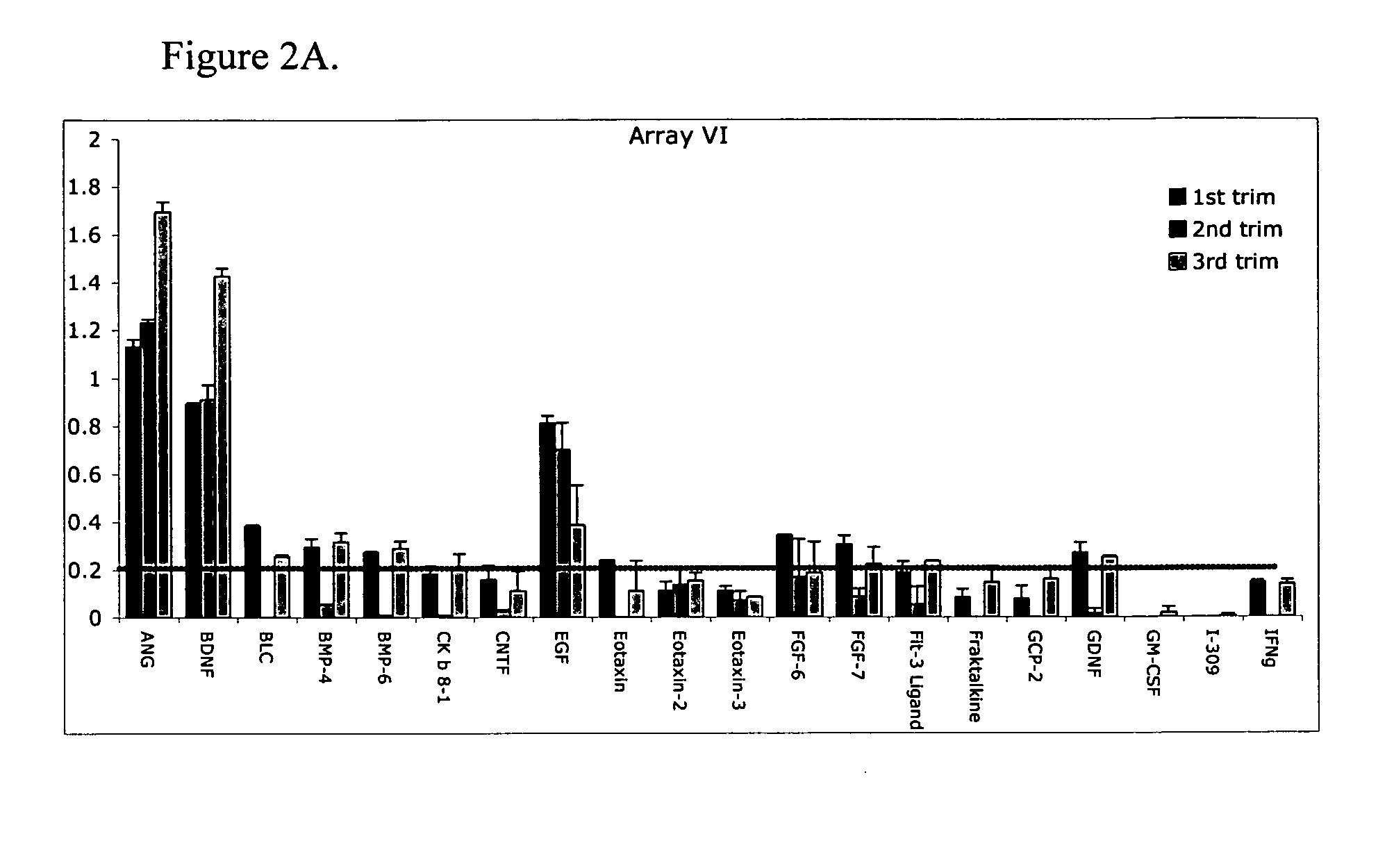
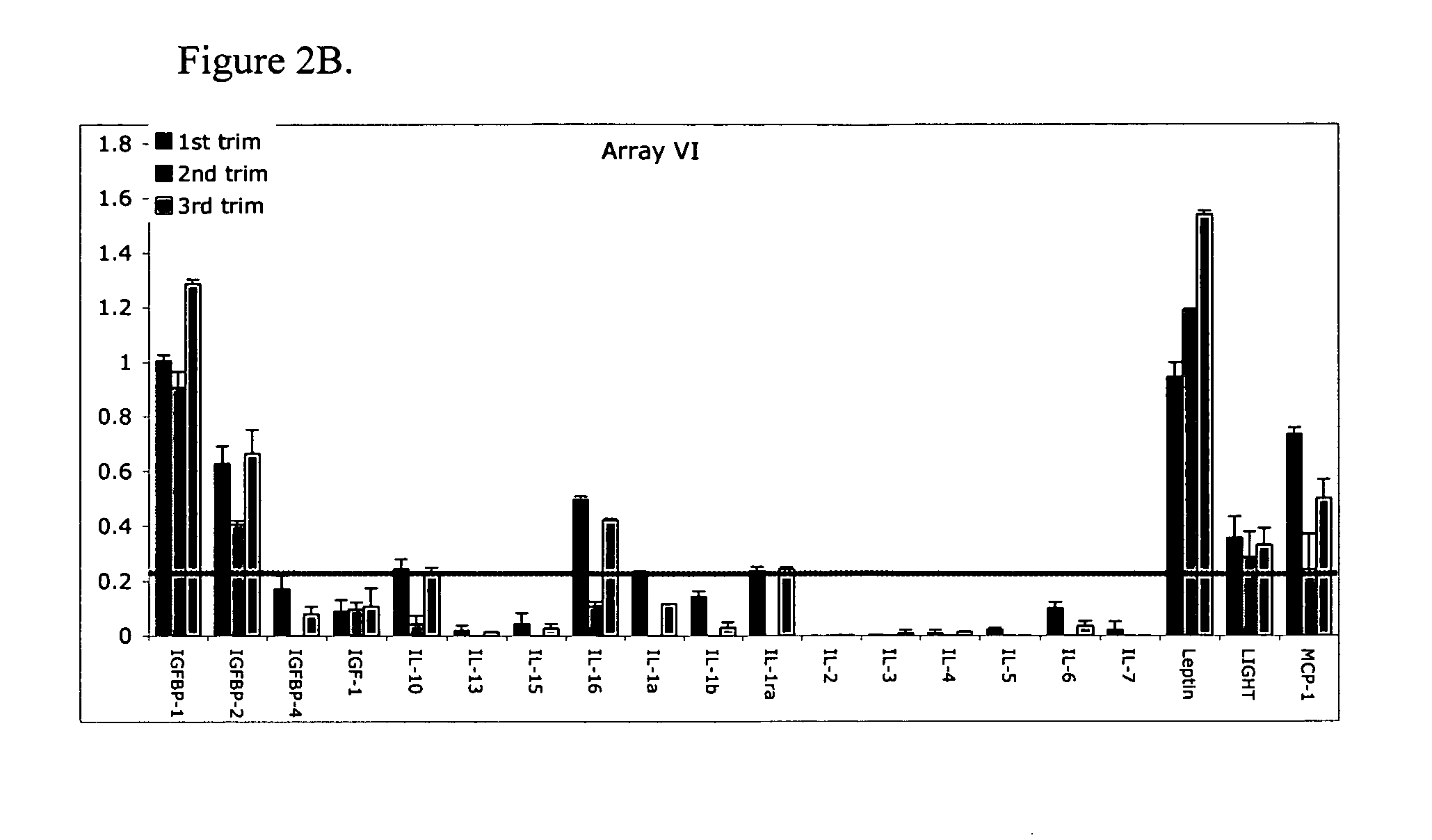
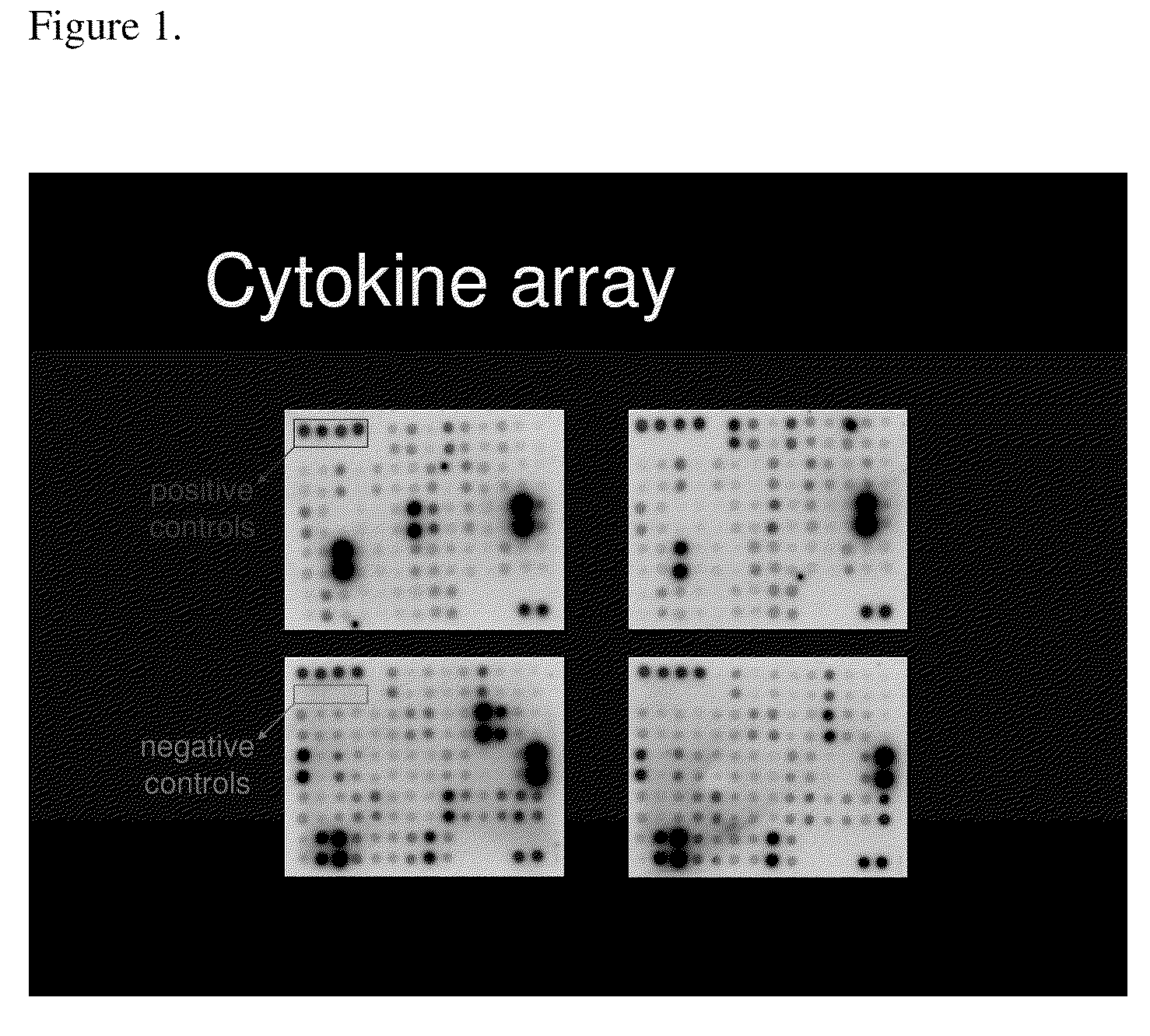
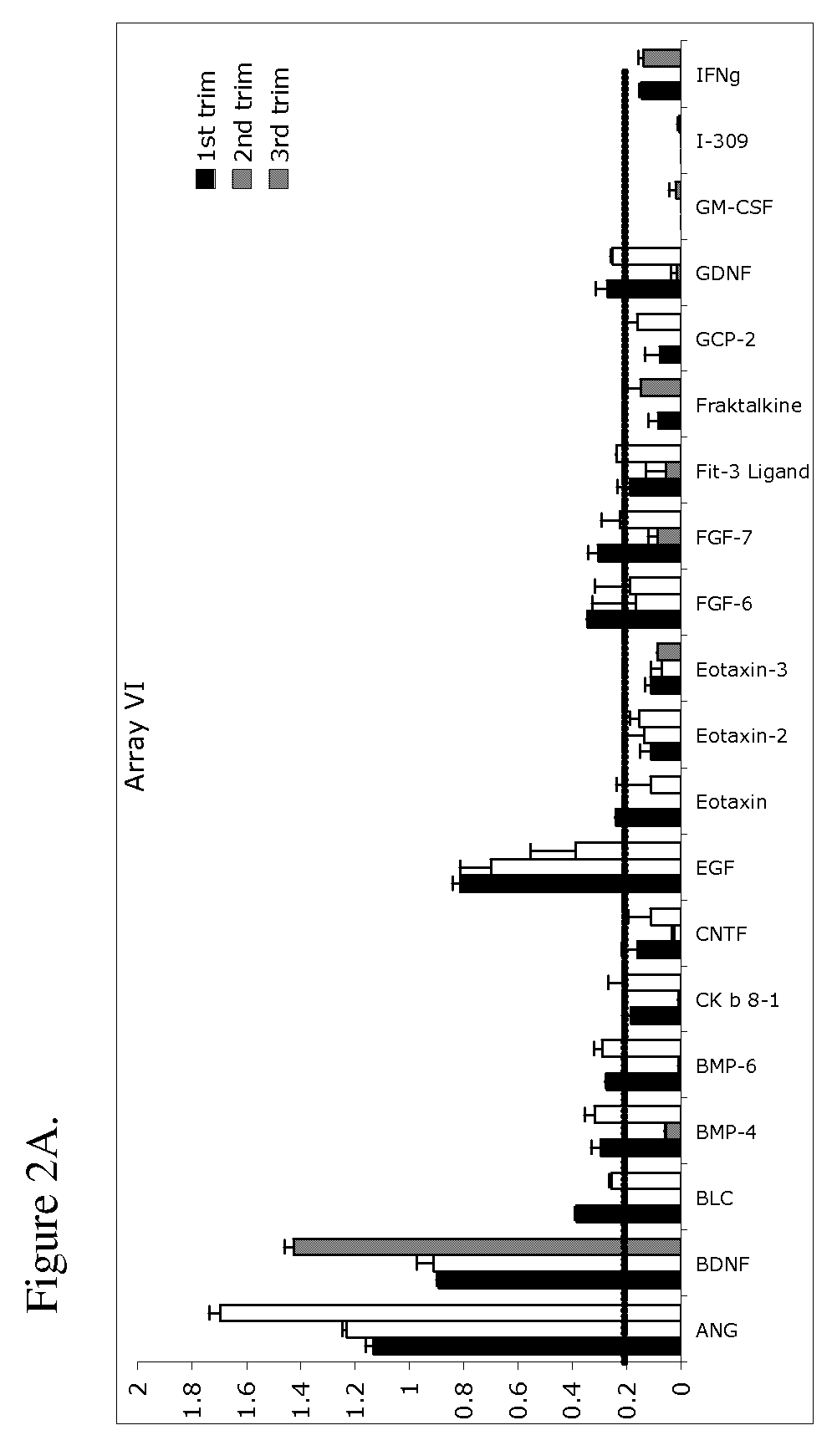
ActiveUS20070178605A1Reduction in trophoblast viabilityFunctional impairmentCompound screeningApoptosis detectionComplicated pregnancyObstetrics
The present invention provides methods and compositions related to biomarker profiles for each trimester of pregnancy. The present invention also provides methods for identifying patients at risk of developing a complication of pregnancy, such as preeclampsia. In further embodiments, the present invention relates to methods for the diagnosis of patients with preeclampsia.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
Methods of determining whether a pregnant woman is at risk of developing preeclampsia
ActiveUS7790463B2Reduced viabilityFunctional impairmentCompound screeningApoptosis detectionComplicated pregnancyGestational period
The present invention provides methods and compositions related to biomarker profiles for each trimester of pregnancy. The present invention also provides methods for identifying patients at risk of developing a complication of pregnancy, such as preeclampsia. In further embodiments, the present invention relates to methods for the diagnosis of patients with preeclampsia.
Owner:UNITED STATES OF AMERICA +1
Methods for determining the risk of prenatal complications
The disclosure relates to methods, medical profiles, kits and apparatus for use in determining the risk that a pregnant individual has for developing pre-eclampsia based on amounts of certain biochemical markers in a biological sample from the individual and biophysical markers. The disclosure also relates to methods, medical profiles, kits and apparatus for use in determining the risk that a pregnant individual is carrying a fetus having a chromosomal abnormality based on amounts of certain biochemical markers in a biological sample from the individual and biophysical markers.
Owner:WALLAC
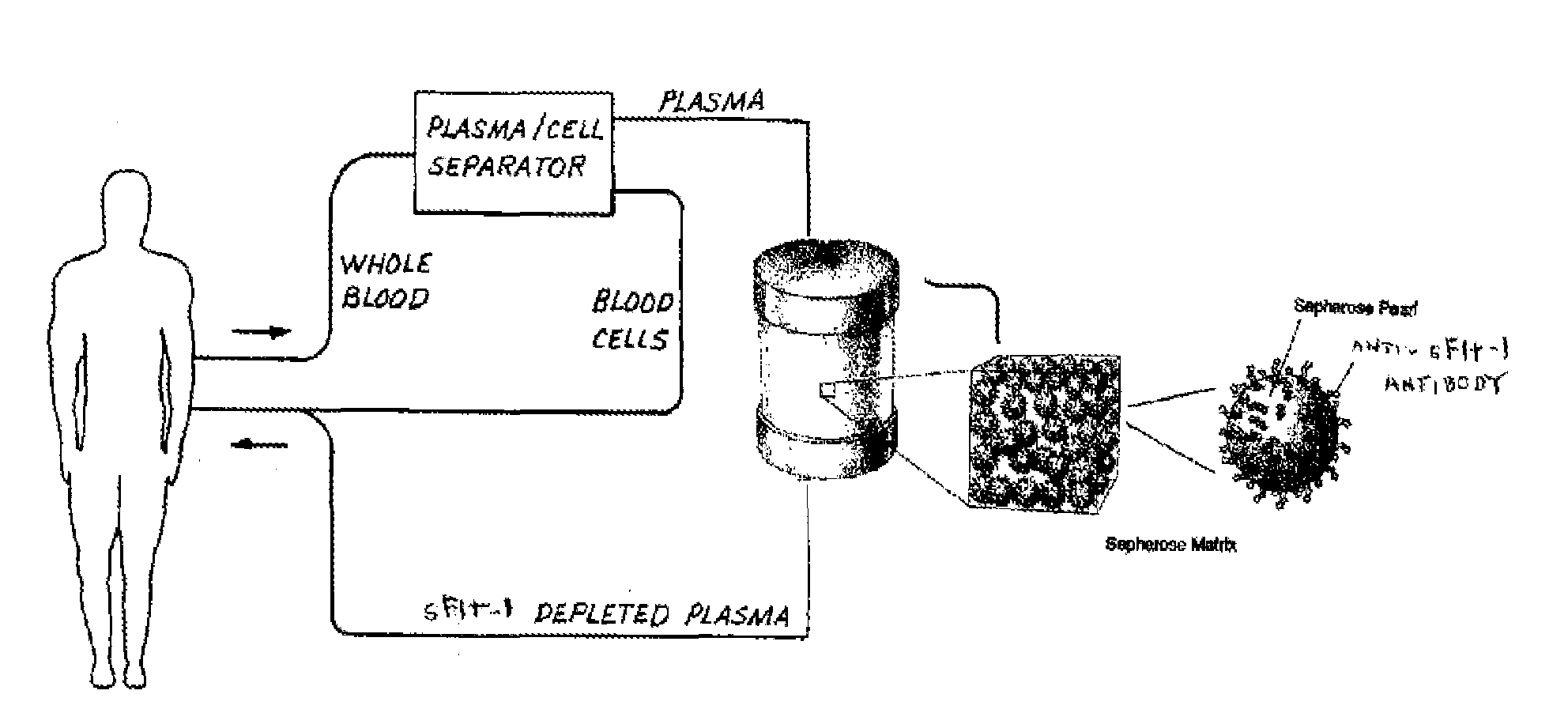
Treatment of pre-eclampsia in pregnant women using targeted apheresis
InactiveUS20060153835A1Reduce development riskAntibody ingredientsImmunoglobulinsDiseaseAntiendomysial antibodies
This invention uses “targeted apheresis” to treat pregnant women who are at risk of developing eclampsia. “Targeted Apheresis” is a process whereby the sFlt-1 receptors responsible for causing the disease symptoms are selectively removed from the blood by passing the blood through a cartridge containing either immobilized PIGF, and / or through a cartridge containing immobilized anti-sFlt-1 antibody. The sFlt-1 receptor is bound out and the cleaned blood is returned to the patient Removal of circulating sFlt-1 receptors will diminish the risk of developing eclampsia during pregnancy.
Owner:SMITH HENRY J +1
Screening for gestational disorders
InactiveUS20080213794A1Simple and inexpensiveDisease diagnosisBiological testingDiseaseDiabetes mellitus
The invention relates to methods and compositions for identifying subjects having, or predisposed to having, gestational diabetes, preeclampsia, and gestational hypertension. The methods are applicable to urine and / or blood samples and can be conducted prior to the third trimester of pregnancy.
Owner:THE GENERAL HOSPITAL CORP +1
Markers for the prognosis and risk assessment of pregnancy-induced hypertension and preeclampsia
InactiveUS20130177901A1Microbiological testing/measurementHeart/pulse rate measurement devicesGestational hypertensionRisk evaluation
The present invention relates to the prognosis and risk assessment in pregnant women to develop pregnancy-induced hypertension and / or preeclampsia by the determination of marker levels.
Owner:CEZANNE
Methods
The present invention relates to the use of selective aquaporin inhibitors, e.g., of aquaporin-4 or aquaporin-2, e.g., certain phenylbenzamide compounds, for the prophylaxis, treatment and control of aquaporin-mediated conditions, e.g., diseases of water imbalance, for example edema (particularly edema of the brain and spinal cord, e.g., following trauma or ischemic stroke, as well as the edema associated with glioma, meningitis, acute mountain sickness, epileptic seizures, infections, metabolic disorders, hypoxia, water intoxication, hepatic failure, hepatic encephalopathy, diabetic ketoacidosis, abscess, eclampsia, Creutzfeldt-Jakob disease, and lupus cerebritis, as well as edema consequent to microgravity and / or radiation exposure, as well as edema consequent to invasive central nervous system procedures, e.g., neurosurgery, endovascular clot removal, spinal tap, aneurysm repair, or deep brain stimulation, as well as retinal edema), as well as hyponatremia and excess fluid retention, and diseases such as epilepsy, retinal ischemia and other diseases of the eye associated with abnormalities in intraocular pressure and / or tissue hydration, myocardial ischemia, myocardial ischemia / reperfusion injury, myocardial infarction, myocardial hypoxia, congestive heart failure, sepsis, and neuromyelitis optica, as well as migraines, as well as to novel assays for identifying aquaporin inhibitors.
Owner:AEROMICS
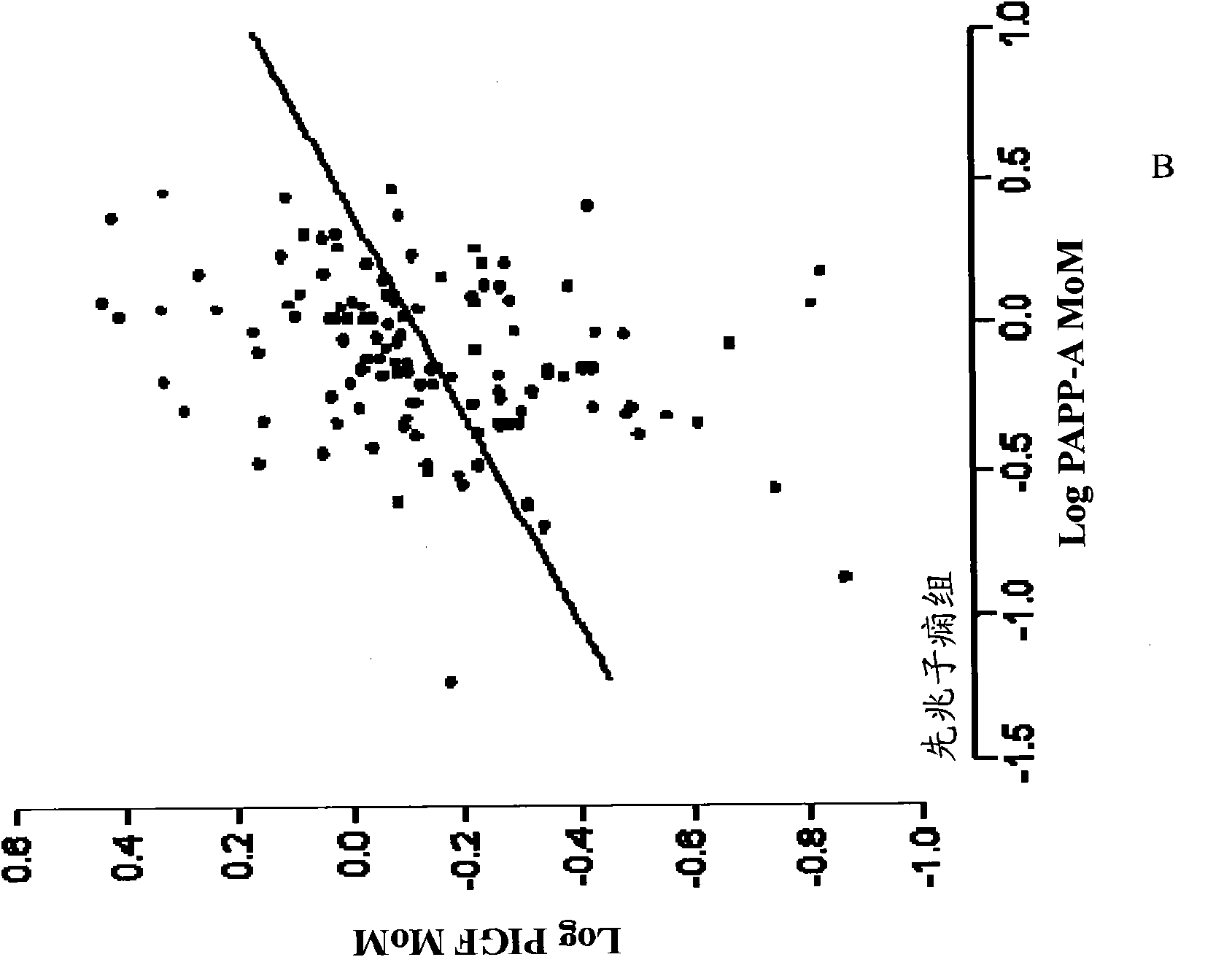
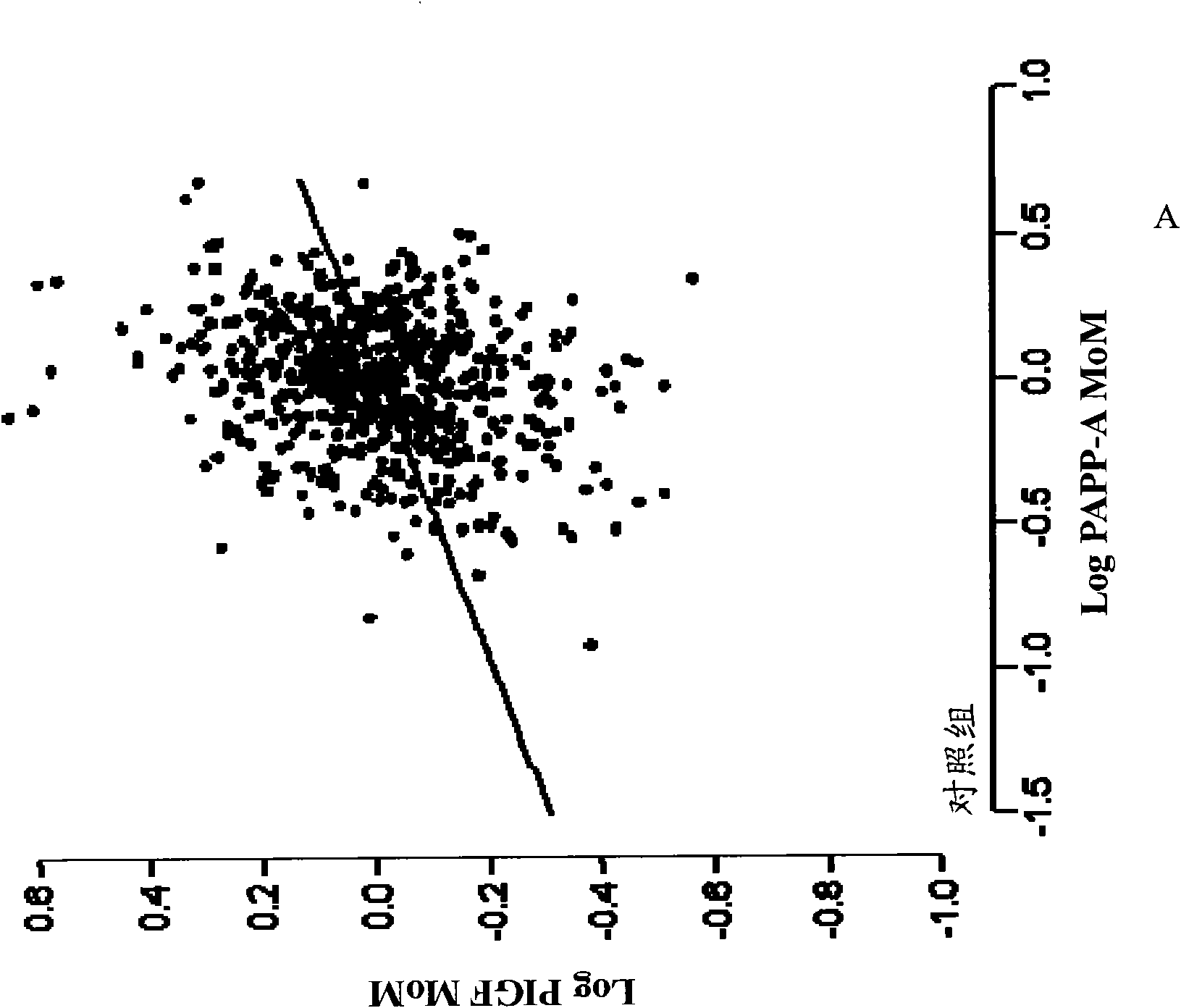
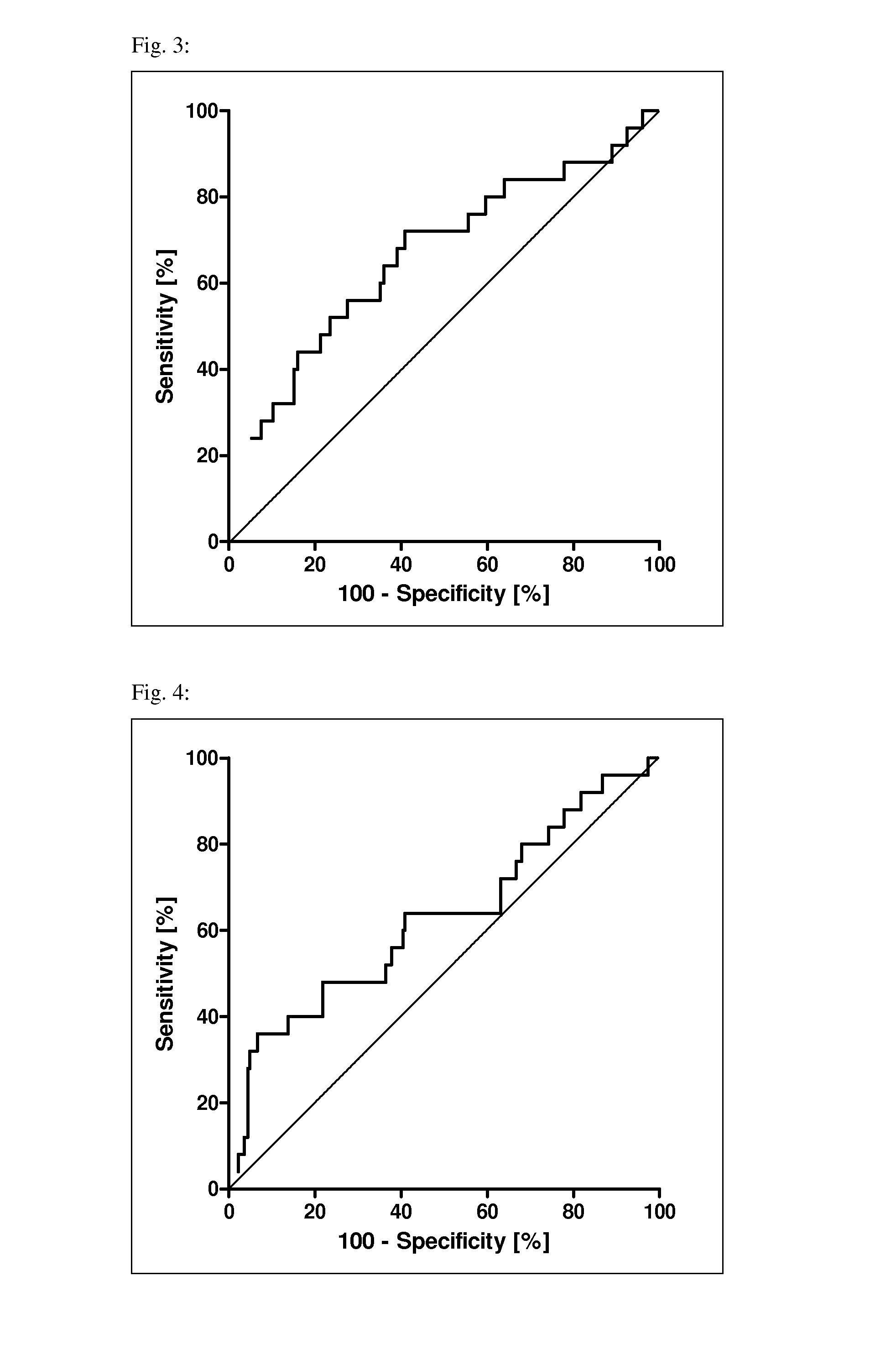
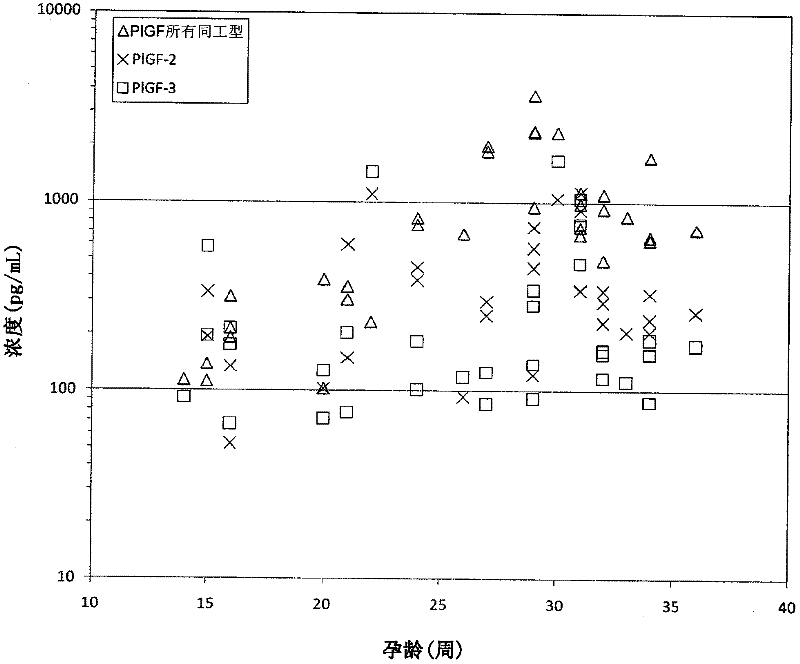
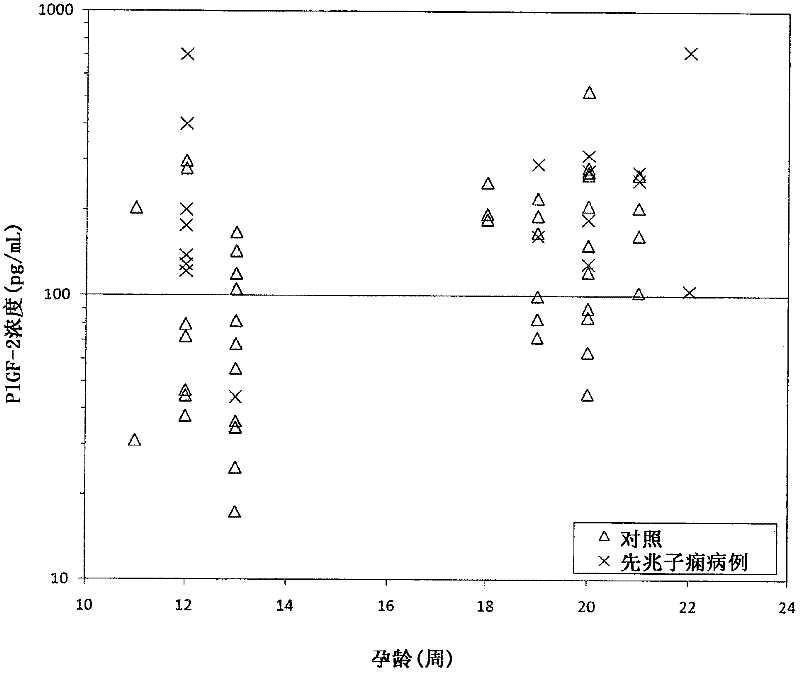
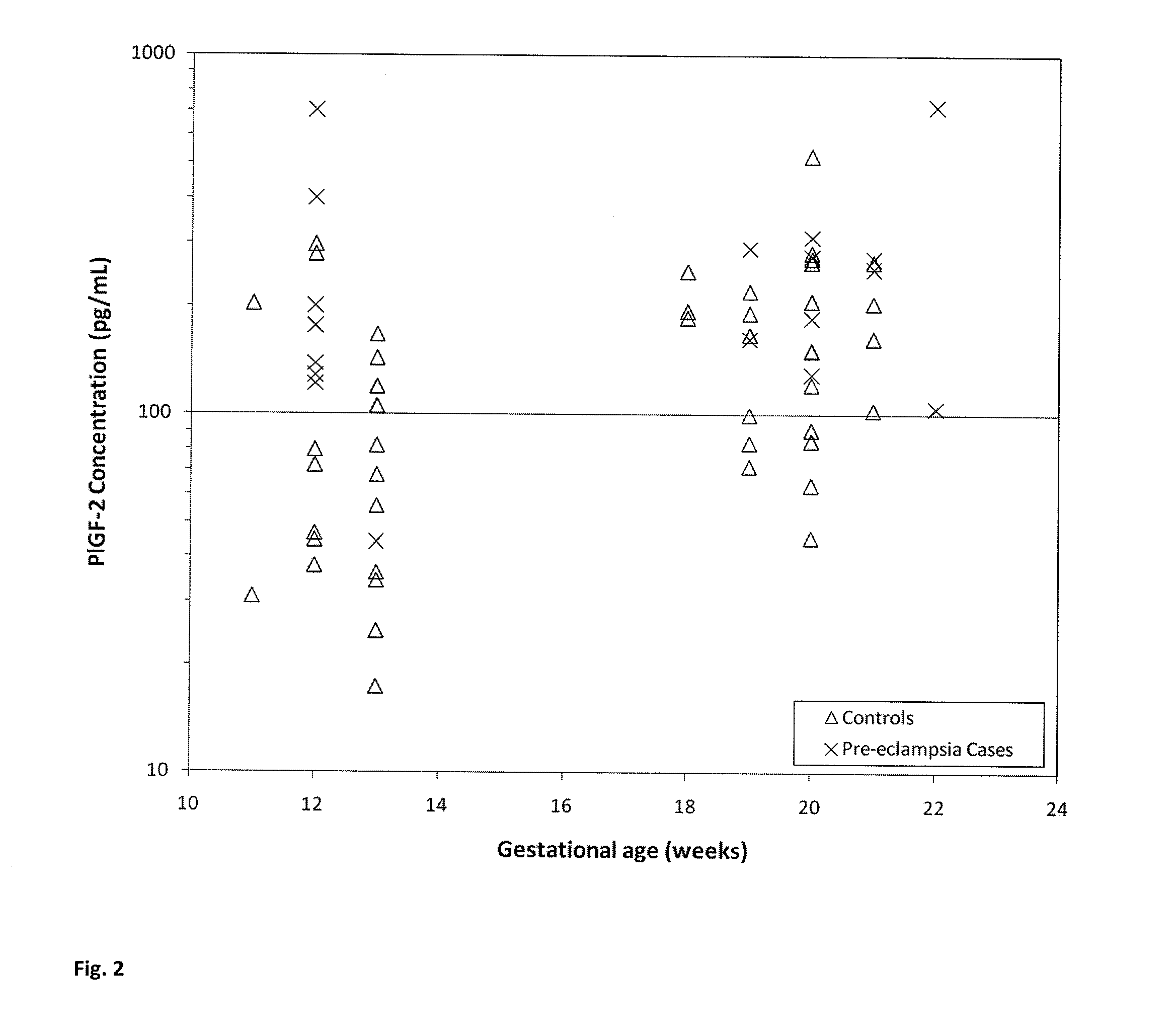
Method for determining the risk of preeclampsia using pigf-2 and pigf-3 markers
The present invention relates to a method for determining the risk of a pregnant woman developing pre-eclampsia. The method comprises i) determining the level of one or more biochemical markers in a sample obtained from a pregnant woman, and ii) comparing the level of the at least one biochemical marker in the sample with the level of the same biochemical marker in a control sample. A difference in the level of the biochemical marker in the sample relative to the control sample is indicative of an increased risk of developing pre-eclampsia. The isoform biochemical markers are preferably PlGF-2 and PlGF-3. The present invention relates also to a method for determining whether a pregnant woman has pre-eclampsia and as well as a kit for assessing the risk or presence of pre-eclampsia. In addition, the invention relates also to a computer program used in these determinations.
Owner:PERKINELMER HEALTH SCIENCES INC +2
Use of compounds that bind soluble endoglin and SFLT-1 for the treatment of pregnancy related hypertensive disorders
ActiveUS7740849B2Increasing expression level and biological activityIncreases dephosphorylationOrganic active ingredientsDisease diagnosisPhysiologyNitric oxide
Owner:HOSPITAL FOR SICK CHILDREN +1
Biomarkers and parameters for preeclampsia
The application discloses new test panels comprising biomarkers and clinical parameters, for the prediction, diagnosis, prognosis and / or monitoring of hypertensive disorders of pregnancy and particularly preeclampsia; and related methods, uses, kits and devices.
Owner:MYCARTIS
Multifactorial telehealth care pregnancy and birth monitoring
ActiveUS20150018635A1Low-cost implementationElectromyographyAuscultation instrumentsAccelerometerObstetrics
A system for monitoring a fetus in a pregnant woman, and / or the maternal health risk for pregnancies complicated by such as pre-eclampsia and hypertensive disorders is configured to be worn by the pregnant woman, preferably so as to allow monitoring during daily life, e.g. in the form of an adhesive patch. The unit has a sound sensor, e.g. a microphone or accelerometer, to be positioned on the skin of the abdominal area so as to detect a vascular sound from umbilical arteries of the fetus or from the uterine arteries of the pregnant woman. The sound sensor is functionally connected to a processing unit which executes a processing algorithm on the captured vascular sound and extracts a signal parameter accordingly. The processing unit then communicates the signal parameter, e.g. using an audio signal, a visual display or by means of a wired or a wireless data signal.
Owner:VIEWCARE TECH 1 APS
Novel serine protease
InactiveUS20050059002A1Sure easyOrganic active ingredientsPeptide/protein ingredientsDiseaseObstetrics
The invention relates to a enzyme predicted to be a serine protease, which is specifically expressed in association with embryo implantation and placentation in pregnant uterus. The enzyme of the invention is useful in the evaluation of fertility and monitoring of early pregnancy, placental development and function, fetal development, parturition, and conditions such as pre-eclampsia, intrauterine growth restriction, early abortion, abnormal uterine bleeding, endometriosis, and cancers, and may provide a potential target for contraception. It may also be important in diseases of the heart, testis or ovary, and may play a role in muscle function, including cardiac muscle, skeletal muscle, lung and the diaphragm. In addition the enzyme of the invention is useful in the screening of candidate drugs for fertility control or for treatment of fertility-related disorders.
Owner:PRINCE HENRYS INST OF MEDICAL RES
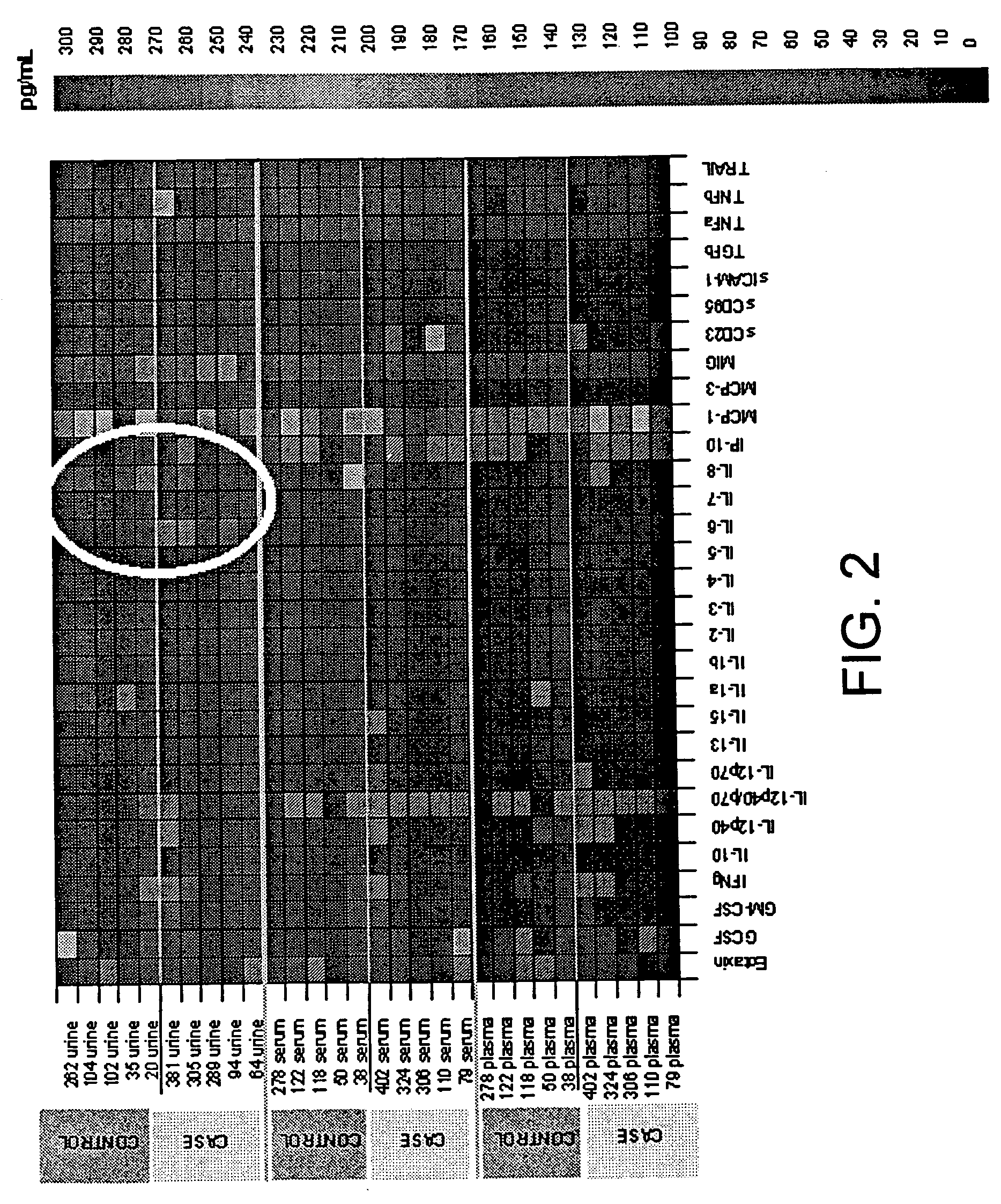
Serum-based, diagnostic, biological assay to predict pregnancy disorders
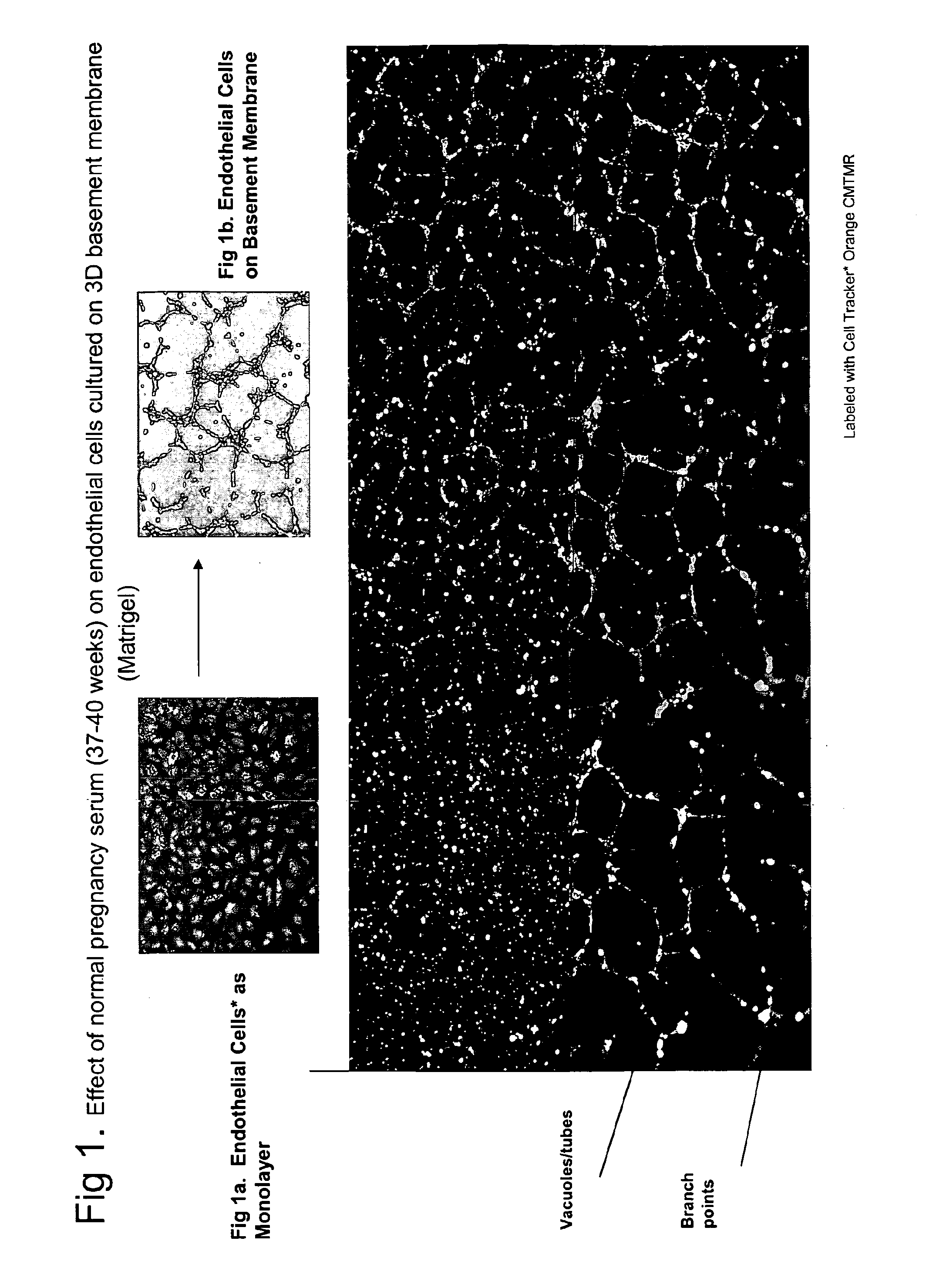
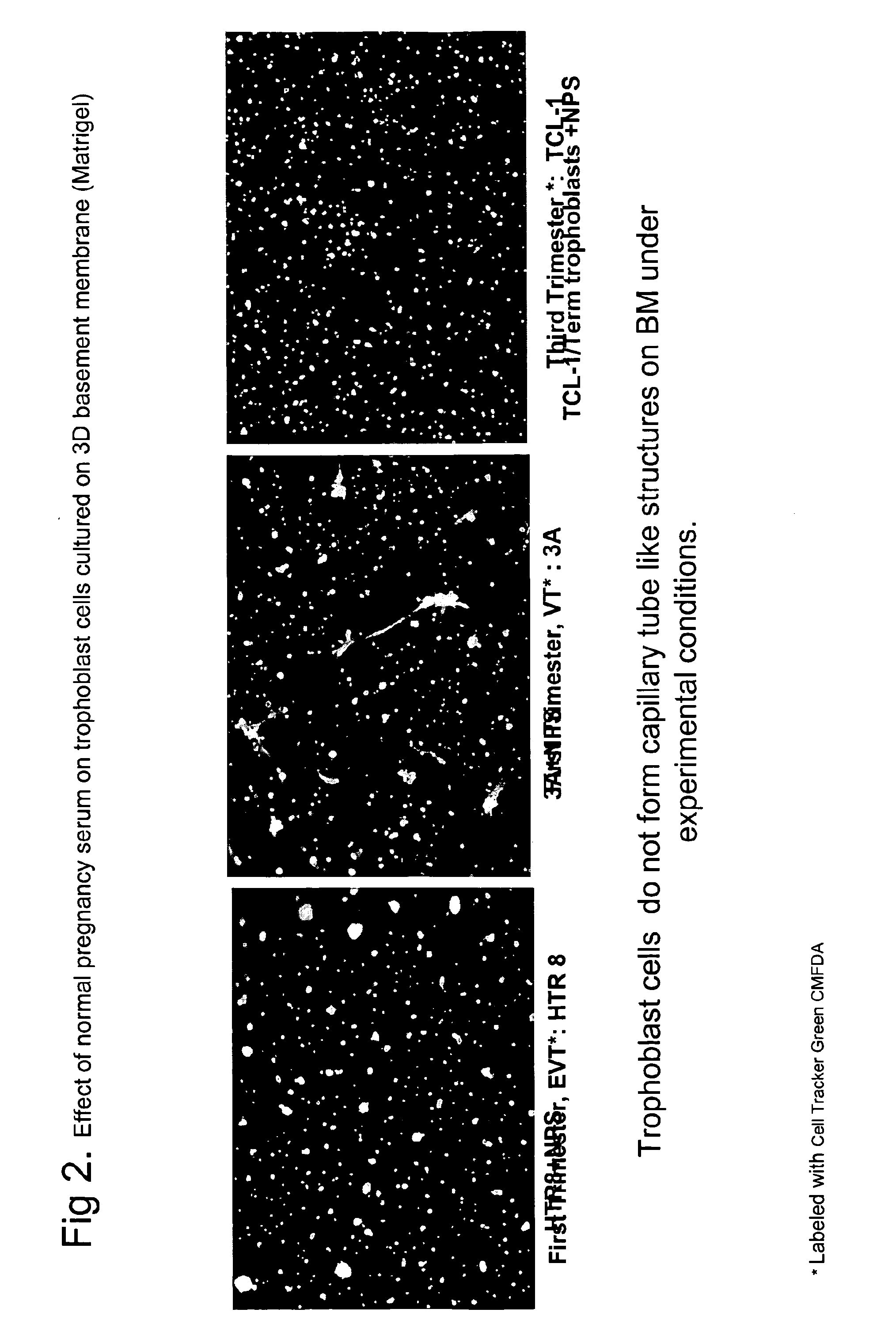
ActiveUS20110059904A1Simple, non-invasiveCost effectiveMicrobiological testing/measurementDisease diagnosisTrophoblastBiology
The invention provides serum-based, diagnostic, biological assays for predicting disorders of pregnancy resulting from poor trophoblast and / or placental ischemia, including preeclampsia. Serum samples from such subjects exhibit an ability to disrupt the architecture involving fetal trophoblasts and maternal endothelial cells in a three-dimensional, dual cell co-culture system provided herein, in contrast to normal pregnancy serum samples. Based on these distinctions, the assays are employed to predict pregnancy outcomes as early as first trimester.
Owner:WOMEN & INFANTS HOSPITAL OF RHODE ISLAND
Method for determining the risk of preeclampsia using PIGF-2 and PIGF-3 markers
InactiveUS20110294227A1Increased riskDisease diagnosisFluorescence/phosphorescenceEclampsiaIncreased risk
The present invention relates to a method for determining the risk of a pregnant woman developing pre-eclampsia. The method comprises i) determining the level of one or more biochemical markers in a sample obtained from a pregnant woman, and ii) comparing the level of the at least one biochemical marker in the sample with the level of the same biochemical marker in a control sample. A difference in the level of the biochemical marker in the sample relative to the control sample is indicative of an increased risk of developing pre-eclampsia. The isoform biochemical markers are preferably P1GF-2 and P1GF-3. The present invention relates also to a method for determining whether a pregnant woman has pre-eclampsia and as well as a kit for assessing the risk or presence of pre-eclampsia. In addition, the invention relates also to a computer program used in these determinations.
Owner:PERKINELMER HEALTH SCIENCES INC +2
Screen for pre-eclampsia
It has been demonstrated that the level of asymmetric dimethylarginine (ADMA) increases in women that subsequently develop pre-eclampsia or whose fetus subsequently develops intrauterine growth restriction (IUGR) and that ADMA plays a key role in the development of maternal hypertension. Accordingly, the level of ADMA in a pregnant woman can be used to determine whether or not a pregnant woman is at risk of developing pre-eclampsia or whether or not a fetus is at risk of developing IUGR. Furthermore, antagonists of ADMA activity are useful in the inhibition or prevention of pre-eclampsia or inhibition or prevention of IUGR.
Owner:UNIV COLLEGE OF LONDON
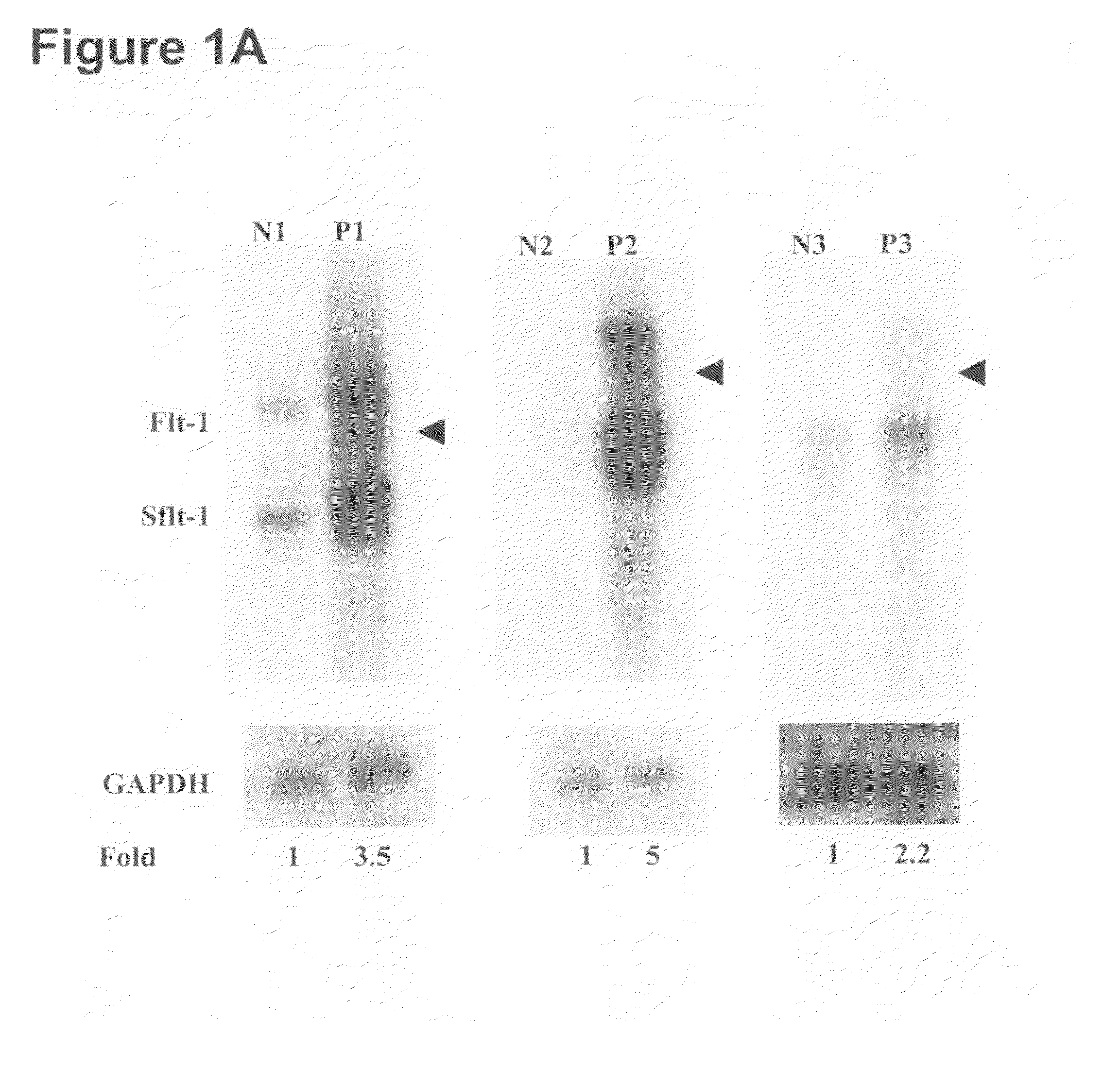
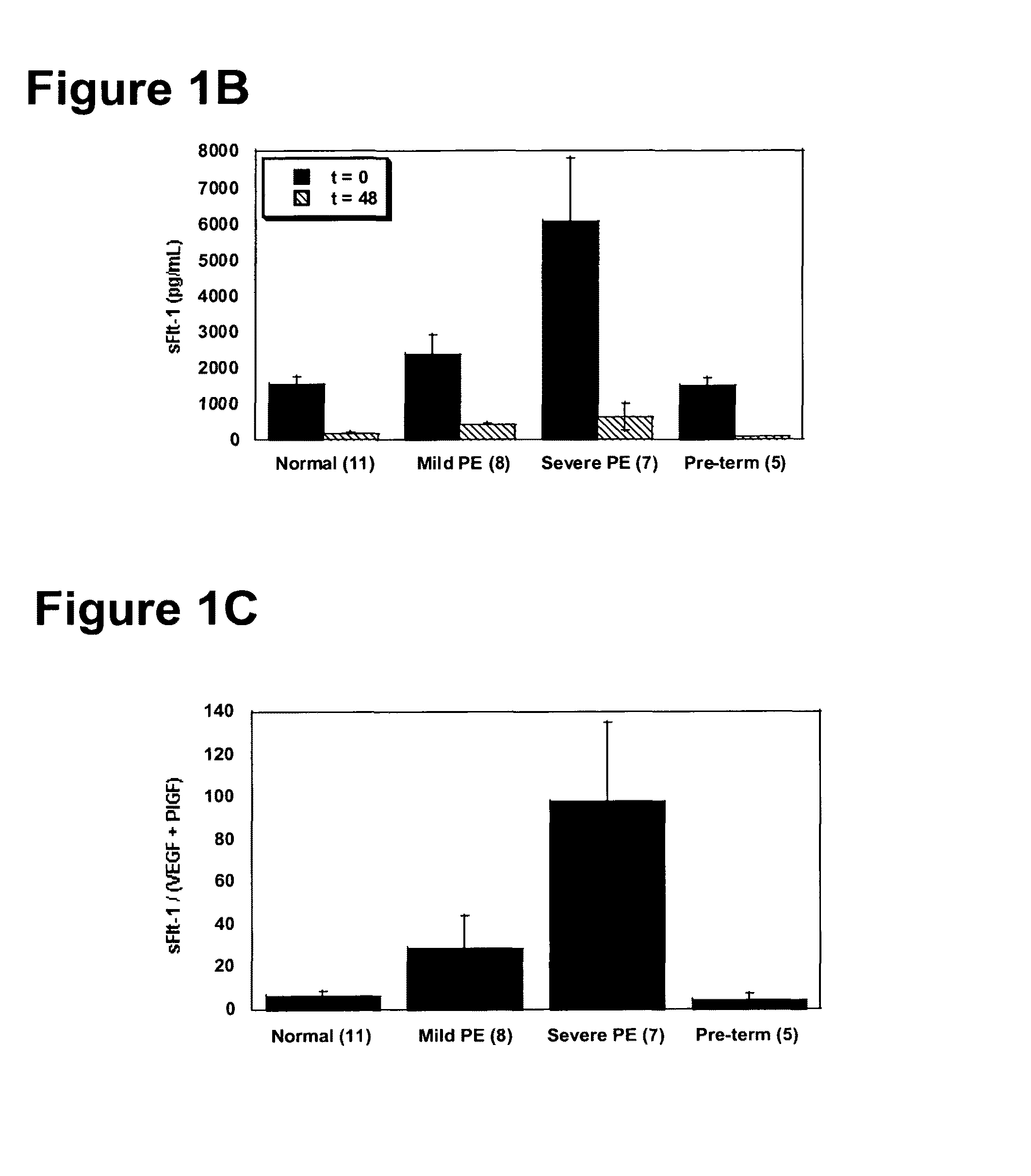
Methods of diagnosing pre-eclampsia or eclampsia
Disclosed herein are methods for diagnosing pre-eclampsia and eclampsia. Also disclosed herein are methods for treating pre-eclampsia and eclampsia using compounds that increase VEGF or PlGF levels or compounds that decrease sFlt-1 levels. Compounds that inhibit the binding of VEGF or PlGF to sFlt1- are also disclosed herein for the treatment of pre-eclampsia or eclampsia.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Use of antithrombin in the treatment of pre-eclampsia
In one aspect, the disclosure provides methods for the treatment of pre-eclampsia and severe pre-eclampsia comprising administering antithrombin. In some embodiments, the antithrombin used in the methods disclosed herein is ATryn®.
Owner:GTC BIOTHERAPEUTICS INC
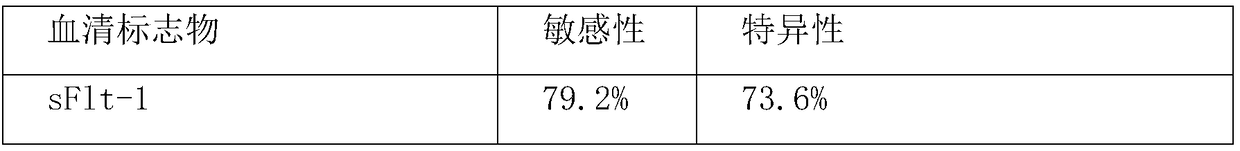
Method for jointly detecting sFlt-1/PLGF and HLA-G for detecting pre-eclampsia
InactiveCN108717123AIncreased sensitivityImprove featuresDisease diagnosisBiological testingWater bathsPositive control
The invention relates to a method for jointly detecting sFlt-1 / PLGF and HLA-G for predicting pre-eclampsia. The method comprises the following steps: collecting serum specimen: respectively collecting5-10 ml of venous blood from pregnant women in a pre-eclampsia group and normal pregnant women with same gestational weeks, centrifuging all specimens in a low temperature centrifuge at 3000 rpm for10 min within 4 h, taking the serum and subpackaging in EP tubes, and storing in a refrigerator at minus 80 DEG C for testing; coating: using a 0.05 M PH9.0 carbonate coated buffer solution to diluteantibodies to the protein content of 1-10[mu]g / ml, adding 0.1ml of the diluted antibodies into reaction holes of each polystyrene board, staying overnight at 4 DEG C, discarding the solution in the holes the next day, and washing for 3 times; setting up standard holes and sample holes, washing, and setting up blank holes, negative control holes and positive control holes at the same time; adding enzyme-labeled antibodies; adding 100 [mu]L of horseradish peroxidase-labeled detection antibodies into each of the standard holes and the sample holes except for the blank holes, sealing the reactionholes with microplate sealers, and incubating in a water bath kettle or an incubator at 37 DEG C for 60 min; performing board-washing; adding 50 [mu]L of a substrate A and a substrate B into each of the holes, and measuring OD values of each hole. The method is high in detection specificity and sensitivity.
Owner:卢英
Fermented feed capable of promoting growth of live pigs, as well as preparation method and use method of fermented feed
InactiveCN107535713AGreat tasteFull of nutritionFood processingAnimal feeding stuffBiotechnologyPhosphate
The invention relates to the technical field of pig feeds, in particular to a fermented feed capable of promoting growth of live pigs, and a preparation method and use method of the fermented feed. The fermented feed is mainly prepared from raw materials of distillers' grains, watermelon peel, peanut vines, helianthus annuus, bulbo-phylulm ambrosirm, polygonum aviculare, pennisetum giganteum, humulus scandens, populus tomentosa, beta vulgaris, corydalis yanhusuo, polygala tenuifolia, scirpus triqueter, eutrema yunnanense, hyssopus officinalis, uncaria rhynchophylla, bombyx mori Linnaeus, crataegus pinnatifida, pinus armandi franch, stone powder, dicalcium phosphate, brown sugar, an enzyme preparation, table salt, microorganisms and the like through steps of treating the raw materials, performing blending and mixing, adopting a lighting stir-frying with bran method, performing fermentation and the like. The formula of the fermented feed is most scientific and reasonable; the fermented feed is rich in nutrition and good in palatability, and has the health-care efficacy of clearing heat, removing toxicity, invigorating the stomach, promoting digestion, resisting bacteria, disinfectinginsects, calming and soothing the nerves, resisting eclampsia, stimulating appetite and the like; the constitutions of the live pigs can be effectively strengthened, and the quick growth of the livepigs can be promoted, so that the average weight gain of the live pigs can be increased, the prevalence rate is reduced, the time that the live pigs become full-grown and ready for slaughter is shortened, and further the breeding benefits of the live pigs are effectively increased; and the preparation method of the fermented feed is simple, and the fermented feed is easy to produce.
Owner:贵港正邦农牧科技有限公司
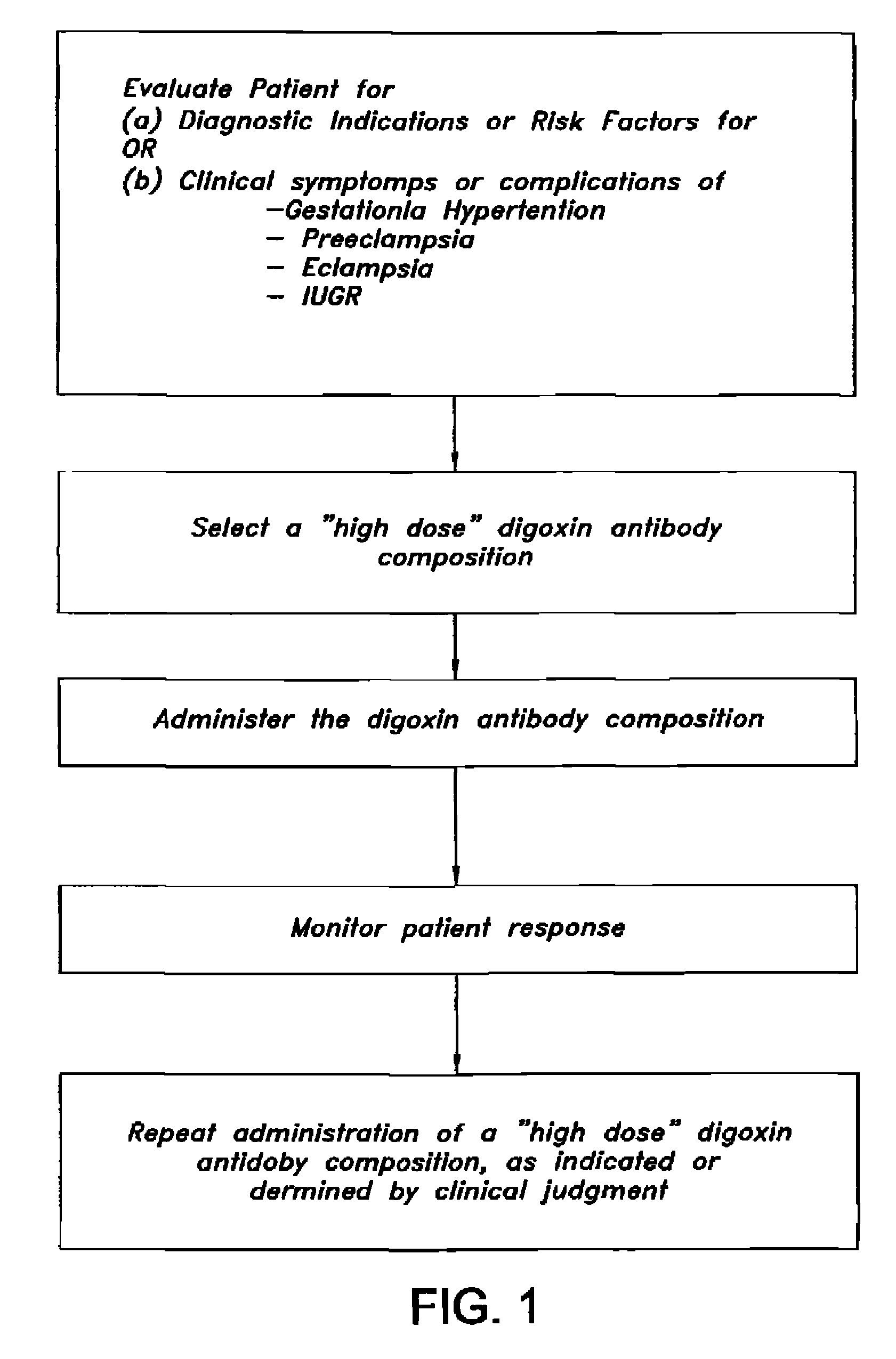
Antibody composition and passive immunization against pregnancy-induced hypertension
A composition is provided to prevent, limit the effects of, delay the onset of, or treat one or more of the causes, symptoms or complications of gestational hypertension, preeclampsia, eclampsia and / or intrauterine growth restriction. The composition comprises a therapeutically effective amount of an antibody that reacts immunologically with or binds digoxin and has a high dose of digoxin binding capacity as the active ingredient. There is also provided a method of preventing, limiting the effects of, delaying the onset of, or treating a cause, symptom or complication of gestational hypertension, preeclampsia, eclampsia or intrauterine growth restriction, comprising the step of administering to a mammal a composition comprising a therapeutically effective amount of an antibody that reacts immunologically with or binds digoxin and has a high dose of digoxin binding capacity.
Owner:VELO BIO
Application of miR-155 as molecular marker in diagnosis of preeclampsia
InactiveCN107400728AReliable indicatorReliable basisMicrobiological testing/measurementBacteriuriaEclampsia
The invention discloses application of miR-155 as a molecular marker in diagnosis of preeclampsia (PE). The nucleotide sequence of miR-155 is shown as SEQ. ID. NO.1. By means of experiments, the invention finds that the expression of miR-155 in sera of PE patients is significantly enhanced compared with normal pregnant women, and the ratio of PE patients' serum miRNA to urine miRNA is significantly increased compared with a normal pregnancy group, and ROC mapping shows that miR-155 has medium diagnostic value on preeclampsia. Therefore, through determination of patients' peripheral circulation miR-155, and combination of the clinicopathological features thereof, the invention provides a reliable index and basis for diagnosis, treatment and disease assessment of preeclampsia.
Owner:XIAN MEDICAL UNIV
Method for screening severe preeclampsia patient in early pregnancy
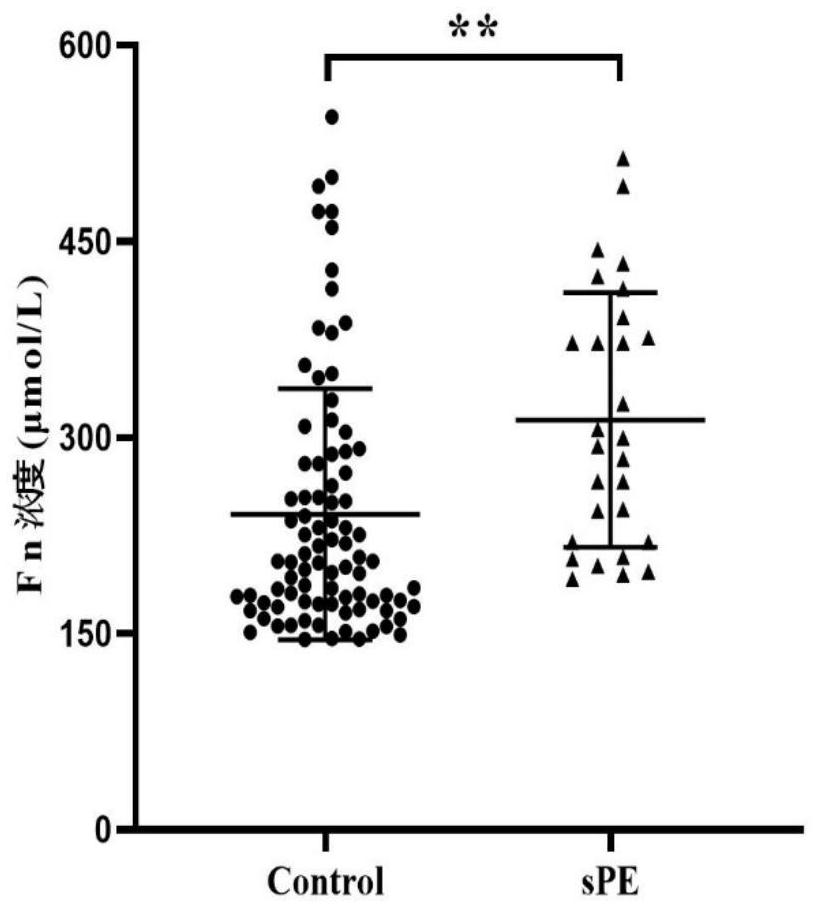
PendingCN113092777AImprove forecast accuracyReduce morbidityComponent separationDisease diagnosisProteomics methodsElisa kit
The invention relates to a method for screening a severe preeclampsia patient in an early pregnancy, which adopts plasma fibronectin FN and complement 4b of a pregnant woman in the early pregnancy as early pregnancy plasma markers for predicting sPE, and establishes a prediction model for predicting or assisting in predicting whether the pregnant woman progresses to the sPE patient, so that the sPE can be accurately predicted. The method has the beneficial effects that the plasma biological marker of the sPE pregnant woman at the early pregnancy stage is searched by adopting an MS-based proteomics method, and the differential expression plasma marker obtained by proteomics analysis is verified through a commercially available ELISA kit;through proteomics analysis and ELISA verification, based on the difference indexes in the pregnant woman baseline features and the difference indexes in the pregnant woman plasma biomarkers in the prospective queue, an early pregnancy prediction model of sPE is constructed, the prediction accuracy of sPE is improved, clinical early intervention is guided, the incidence rate of PE is reduced as much as possible, and adverse pregnancy events are reduced.
Owner:TEDA INT CARDIOVASCULAR HOSPITAL
Biomarkers for preeclampsia
InactiveUS20100190181A1Lower blood lipid levelsMicrobiological testing/measurementDisease diagnosisObstetricsPregnancy early
The present invention relates to novel biomarkers such as histidine and ketone bodies and methods of use thereof for detecting preeclampsia in pregnant individuals and for identifying individuals at risk at an early stage of pregnancy.
Owner:UNIV OF LEEDS
Application of reagent for detecting expression levels of two biomarkers in sample in preparation of kit for detecting preeclampsia
PendingCN113567687AReduce morbidityReduce mortalityComponent separationDisease diagnosisObstetricsMortality rate
According to the invention, the ratio of biomarkers leptin to ceramide related to preeclampsia is found for the first time, and early diagnosis of preeclampsia is realized by detecting the level change of the ratio of leptin to ceramide of a subject. The method can be used for evaluating the preeclampsia disease risk during pregnancy, has high accuracy and specificity, and provides an effective method for early prediction and precise prevention of preeclampsia. In the early stage of morbidity, timely and accurate auxiliary diagnosis is carried out on pregnant women with preeclampsia risks, clinical close monitoring on patients is helped, effective intervention is timely carried out to control the illness state and prolong the gestational week, and the method has important significance in reducing the preeclampsia morbidity and mortality rate and guaranteeing the safety of mothers and infants.
Owner:天津云检医疗器械有限公司 +1
Pre-eclampsia screening methods
InactiveUS20150157276A1Reduce severityHigh frequencyEvaluation of blood vesselsCatheterPatient specificEclampsia
The present invention relates generally to methods for treating early and late onset pre-eclampsia, as well as to methods of screening for and predicting the likelihood that a pregnant female patient will develop early and / or late pre-eclampsia, by assessing specific combinations of factors. In the methods of the invention, the a priori risk of developing early preeclampsia may be calculated utilizing coefficients for each of the maternal factors (binary variables), the coefficients being generated utilizing logistic regression analysis. The a posteriori risk of developing early preeclampsia may be calculated utilizing coefficients for each of the patient-specific factors, the coefficients being generated utilizing logistic regression analysis.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Eclampsia detection method based on newborn egg signals
ActiveCN111938631AImprove diagnostic efficiencyDiagnostic recording/measuringSensorsData ingestionPhysiology
The invention discloses an eclampsia detection method based on newborn egg signals. The eclampsia detection method comprises a training part and a test part, wherein the training part comprises: performing 0.5-40Hz filtering on data, performing 32Hz down-sampling, performing 0.5-12.5 Hz filtering, segmenting all the data into 24s sections, extracting characteristic values, performing standardizingand performing classification marking on the characteristic values; and performing training by using an SVM to obtain a model. The test part comprises: performing 0.5-40Hz filtering on the data, thenperforming 32Hz down-sampling, then performing 0.5-12.5 Hz filtering, extracting characteristic values from every 24s of data, excluding non-convulsion data according to a threshold condition, standardizing the data which cannot be excluded, performing predicting by using the model, and judging whether the data is convulsion data or not. According to the method, the newborn eeg signals can be subjected to eclampsia detection, medical staff can be assisted in diagnosing neonatal convulsion, and the diagnosis efficiency is improved.
Owner:NANJING VISHEE MEDICAL TECH
Methods and systems for treating or preventing pregnancy-related hypertensive disorders
ActiveUS20140065150A1Decrease in amountReduce the amount requiredHaemofiltrationMammal material medical ingredientsEclamptic seizureHeavy chain
Disclosed are methods and apparatuses for treating a pregnancy related hypertensive disorder, such as pre-eclampsia and eclampsia, using ex vivo treatment with an anti-sFlt-1 receptor (sFlt-1) antibody bound to a solid support in order to reduce blood levels of sFlt-1. Further disclosed are the sequences of the heavy chain and light chain CDRs of the anti-sFlt-1 antibodies.
Owner:AGGAMIN LLC
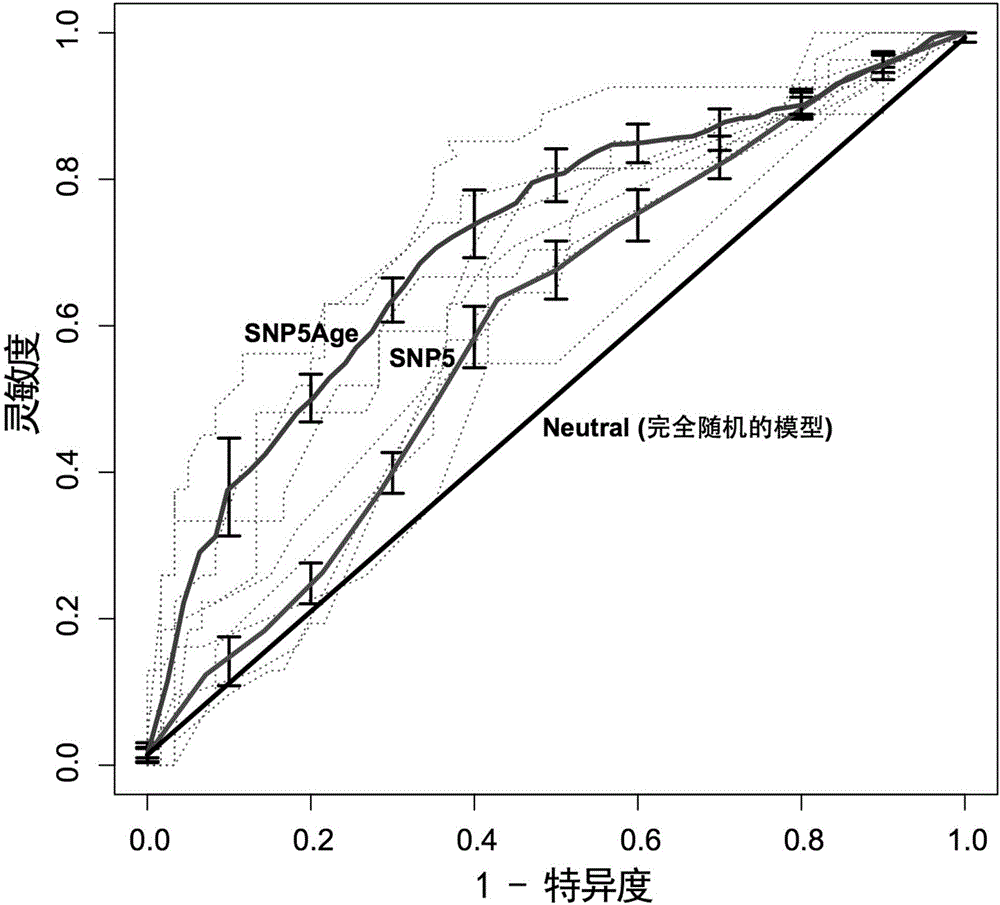
Whole set SNP for predicting preeclampsia and application thereof
ActiveCN106755492AImprove accuracyStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationEclampsiaRisk evaluation
The invention discloses a whole set SNP for predicting preeclampsia and an application thereof. The whole set SNP provided by the invention is composed of rs7412, rs2070744, rs1800896, rs1799724, rs4762, rs2549782, rs1800629 and rs1695. The whole set SNP can be used for predicting the risk of PE of Chinese. Experiments verify that combination of the whole set SNP and combination of the whole set SNP and age for predicting the risk of PE of a to-be-detected object have a high accuracy, specificity, sensitivity and an area under the curve. The whole set SNP provided by the invention can evaluate risk of illness of the preeclampsia so as to guide intervention as soon as possible and prevention of preeclampsia, so that the morbidity of morbidity and harm to mothers and infants by preeclampsia are reduced.
Owner:深圳金蕊科技有限公司
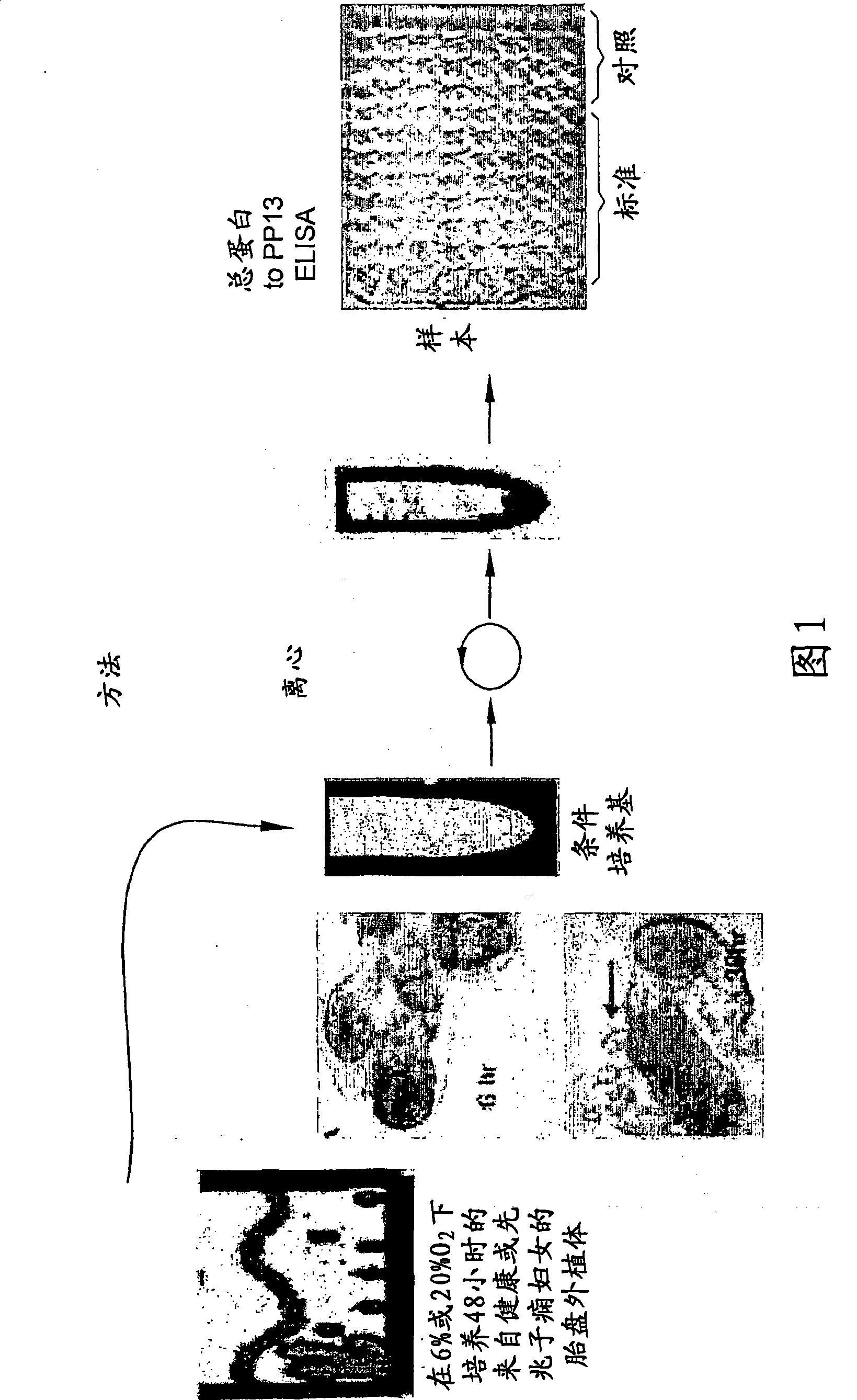
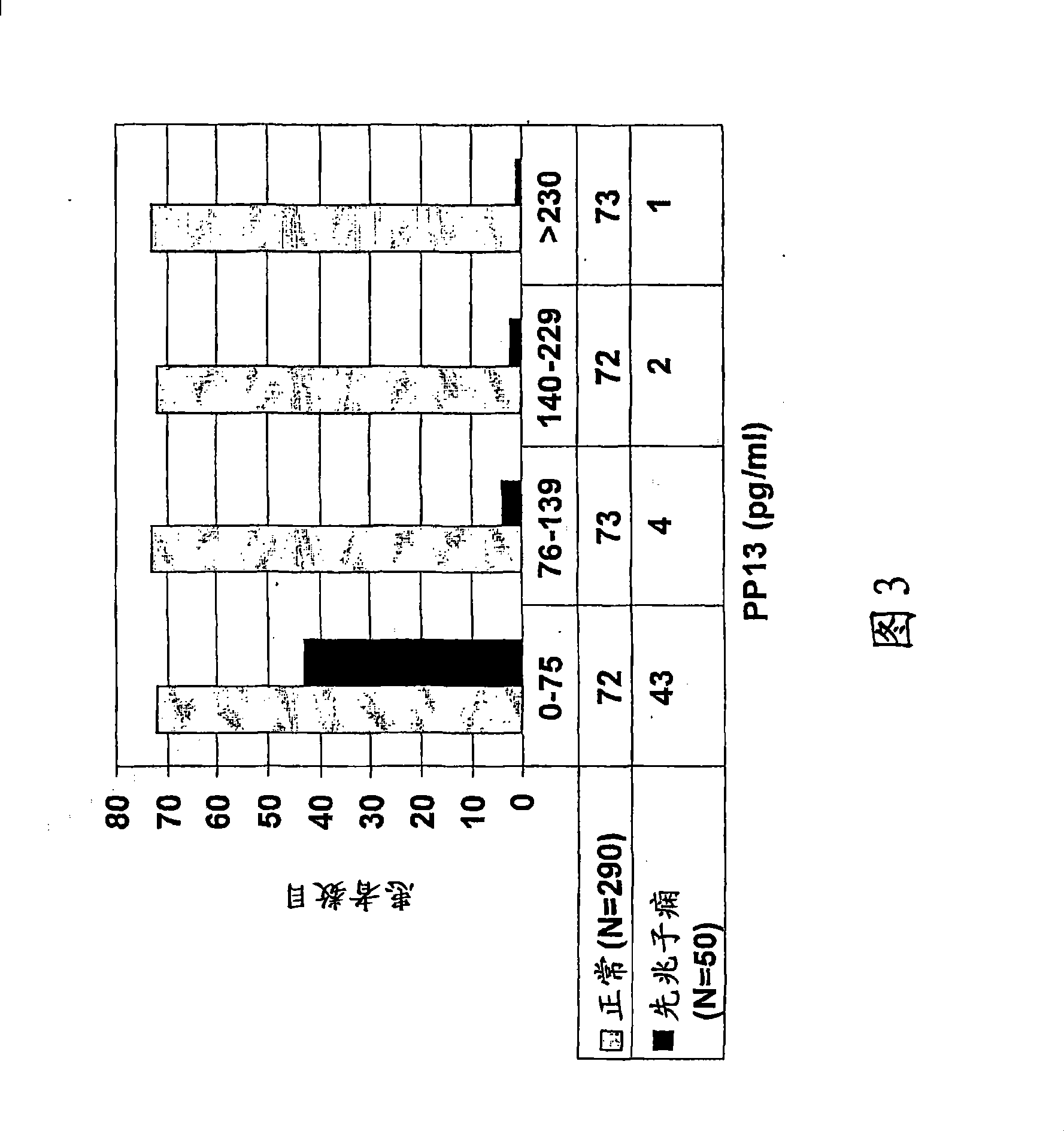
Method for determining the effectiveness of a treatment for preeclampsia
A method for determining the effectiveness of a treatment for preeclampsia of a pregnant woman at risk for preeclampsia, the method comprising: (a) determining a first concentration of placental protein 13 (PP13) in a bodily substance of the woman obtained prior to the treatment; (b) determining a second concentration of PP13 in a bodily substance of the woman obtained after initiation of the treatment; and (c) comparing the first and second concentrations to a corresponding normal level of PP13 and, based on the comparison, determining the effectiveness of the treatment. Diagnostic kits for practicing the method are also disclosed.
Owner:DIAGNOSTIC TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com