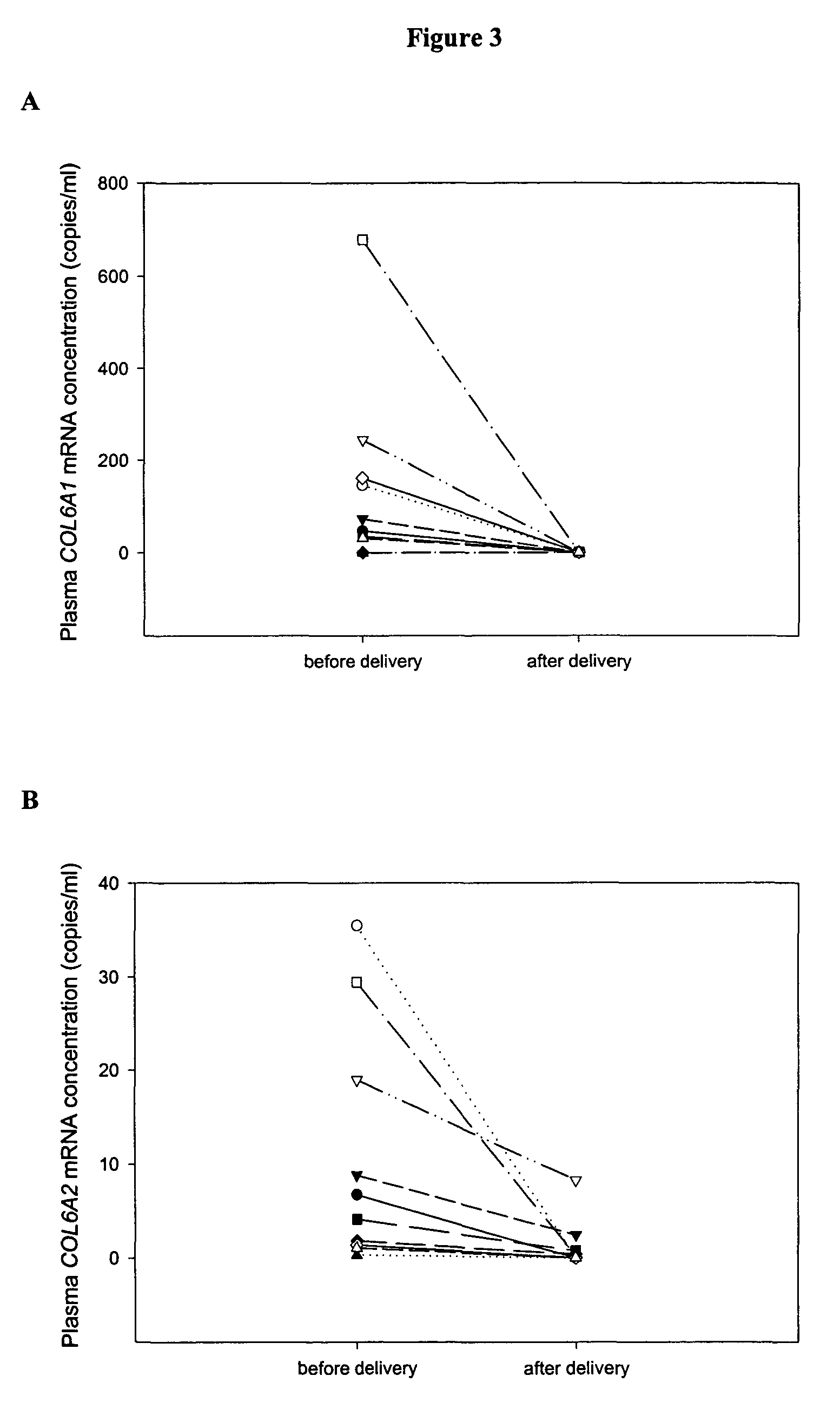
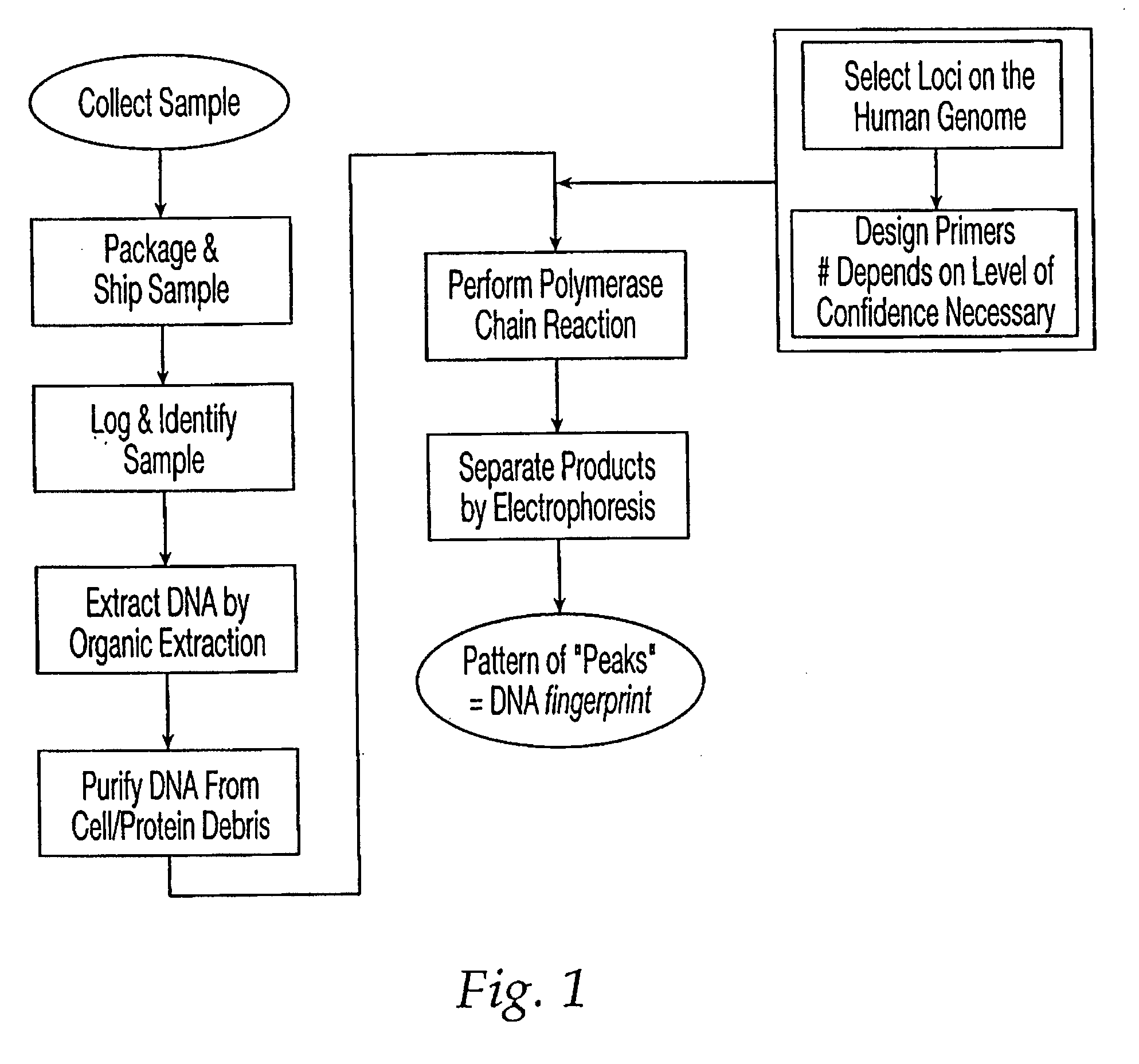
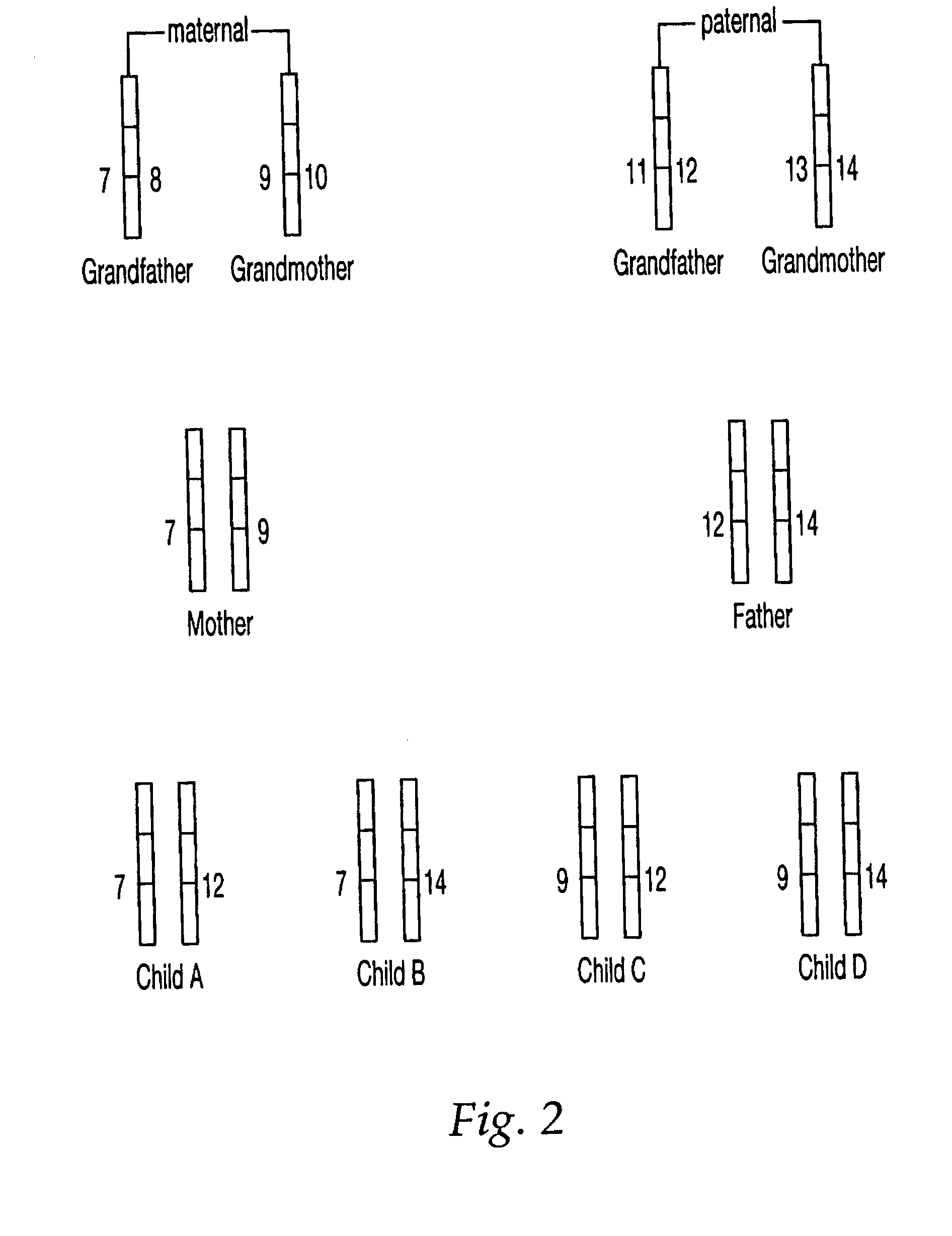Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
251 results about "Prenatal diagnosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
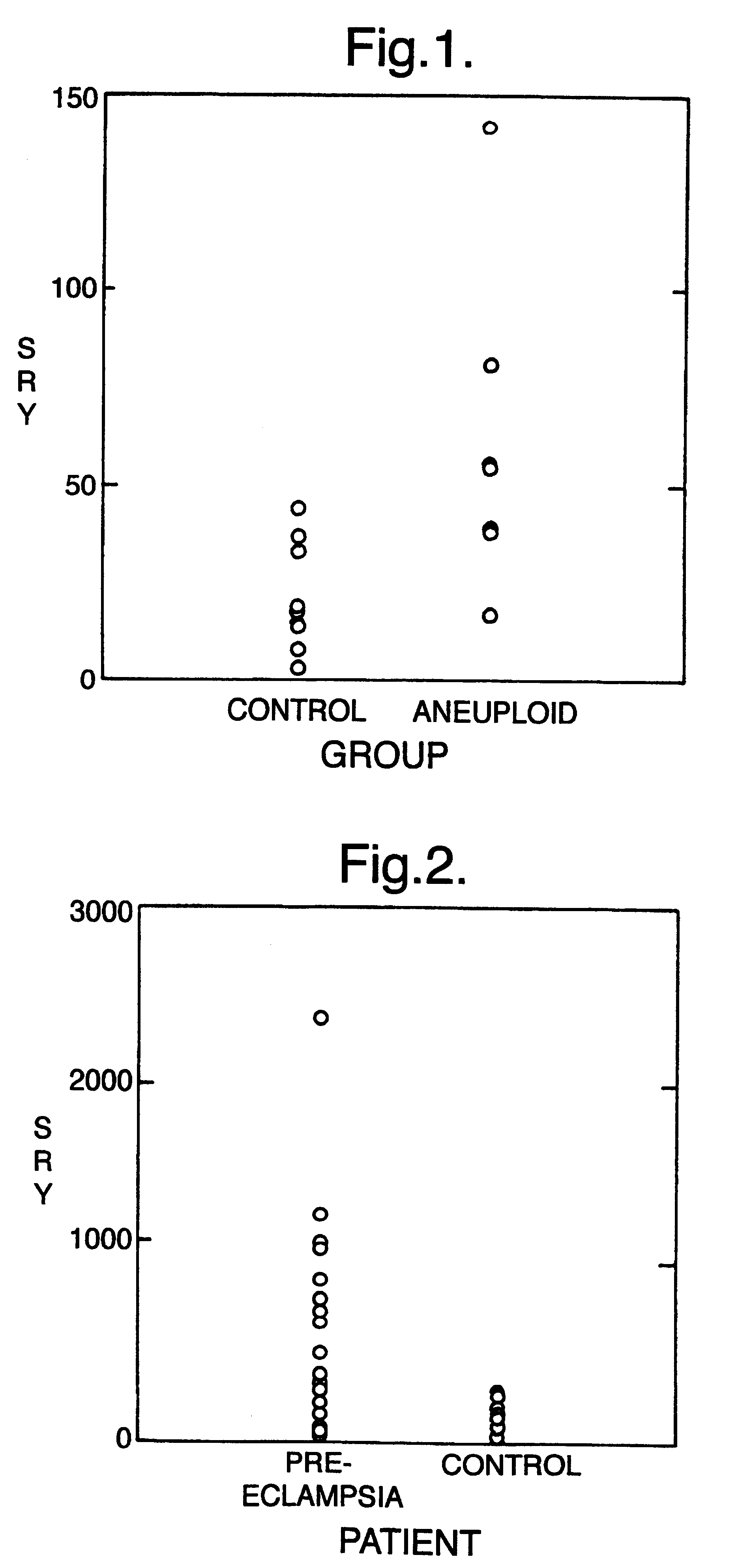
Prenatal testing consists of prenatal screening and prenatal diagnosis, which are aspects of prenatal care that focus on detecting problems with the pregnancy as early as possible. These may be anatomic and physiologic problems with the health of the zygote, embryo, or fetus, either before gestation even starts (as in preimplantation genetic diagnosis) or as early in gestation as practicable. Screening can detect problems such as neural tube defects, chromosome abnormalities, and gene mutations that would lead to genetic disorders and birth defects, such as spina bifida, cleft palate, Downs Syndrome, Tay–Sachs disease, sickle cell anemia, thalassemia, cystic fibrosis, muscular dystrophy, and fragile X syndrome. Some tests are designed to discover problems which primarily affect the health of the mother, such as PAPP-A to detect pre-eclampsia or glucose tolerance tests to diagnose gestational diabetes. Screening can also detect anatomical defects such as hydrocephalus, anencephaly, heart defects, and amniotic band syndrome.
Non-invasive prenatal diagnosis
InactiveUS6258540B1% accurate detection rateIncrease the amount of foetal nucleic acid materialMicrobiological testing/measurementRecombinant DNA-technologyPrenatal diagnosisBlood typing
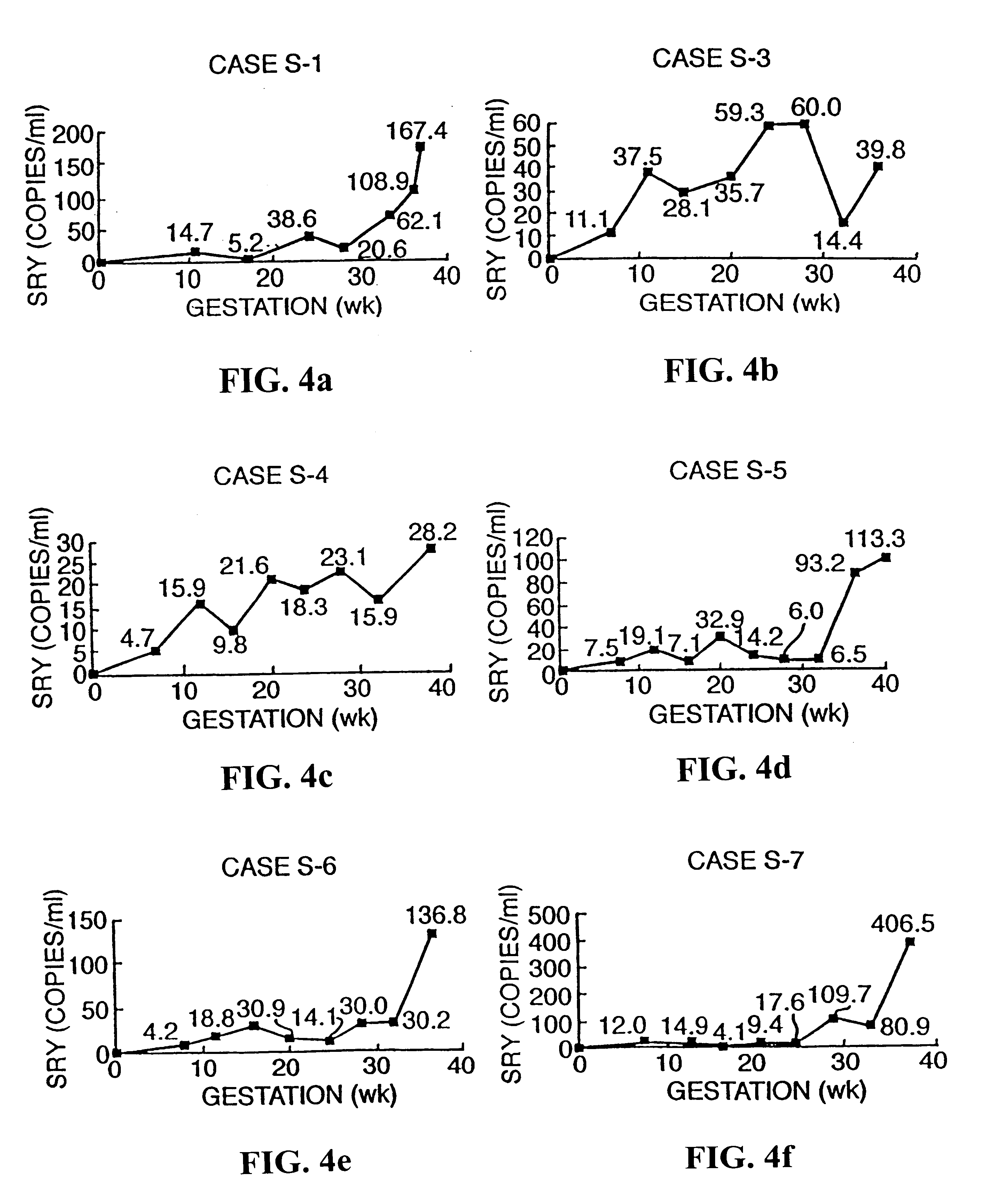
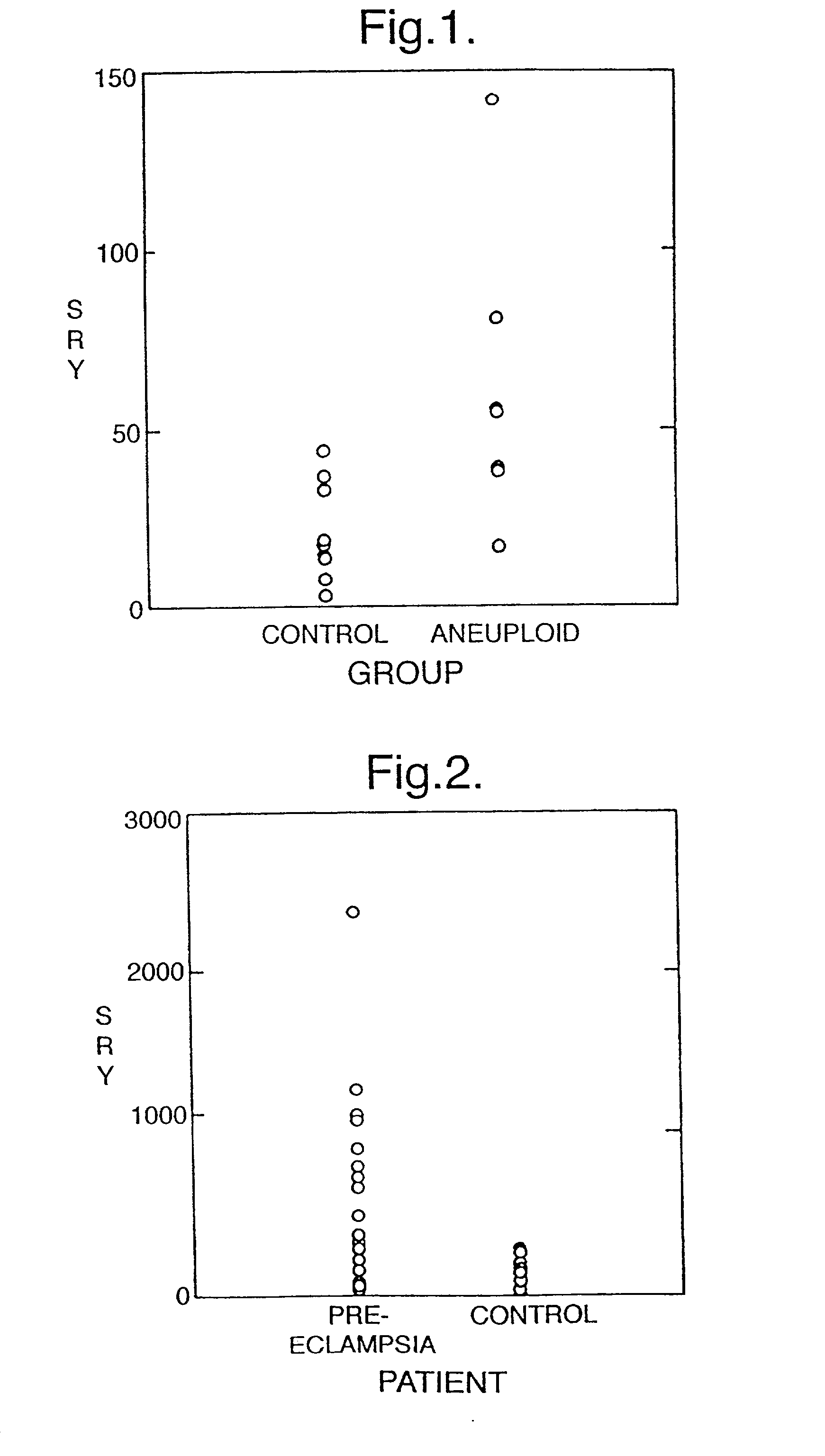
The invention relates to a detection method performed on a maternal serum or plasma sample from a pregnant female, which method comprises detecting the presence of a nucleic acid of foetal origin in the sample. The invention enables non-invasive prenatal diagnosis including for example sex determination, blood typing and other genotyping, and detection of pre-eclampsia in the mother.
Owner:SEQUENOM INC
Sequencing methods and compositions for prenatal diagnoses
ActiveUS20110201507A1Quality improvementEasy to analyzeMicrobiological testing/measurementLibrary screeningPrenatal diagnosisGenetics
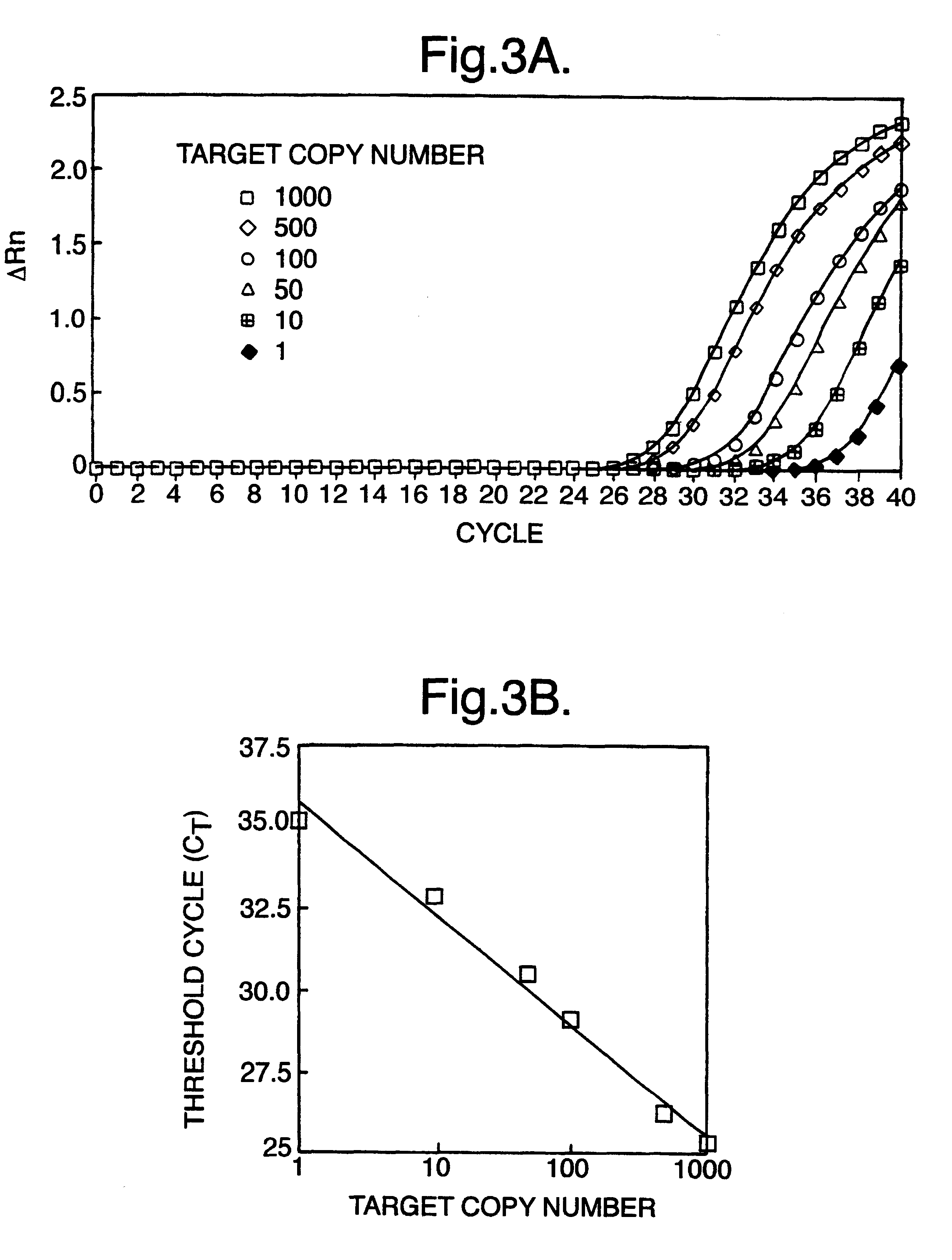
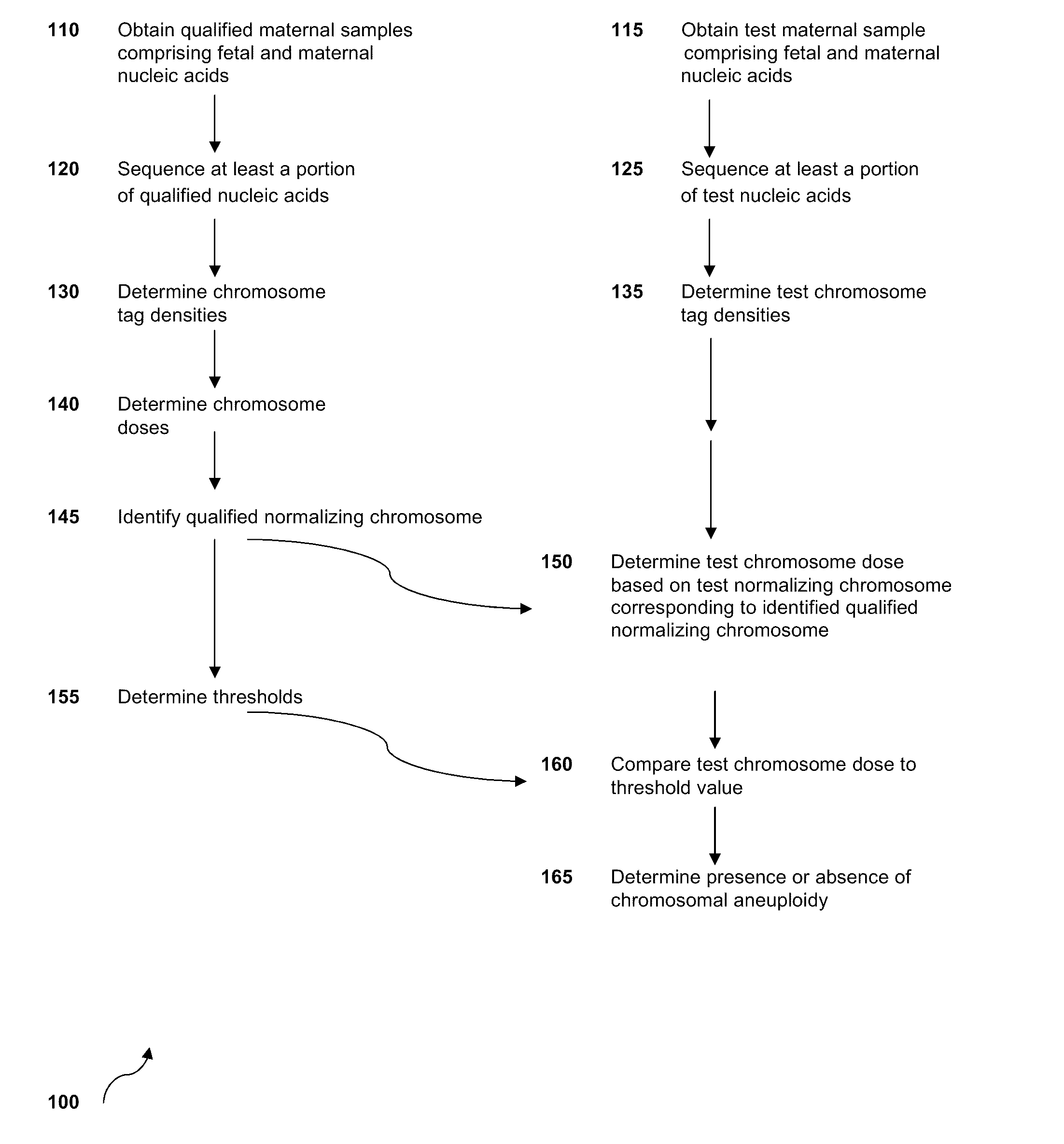
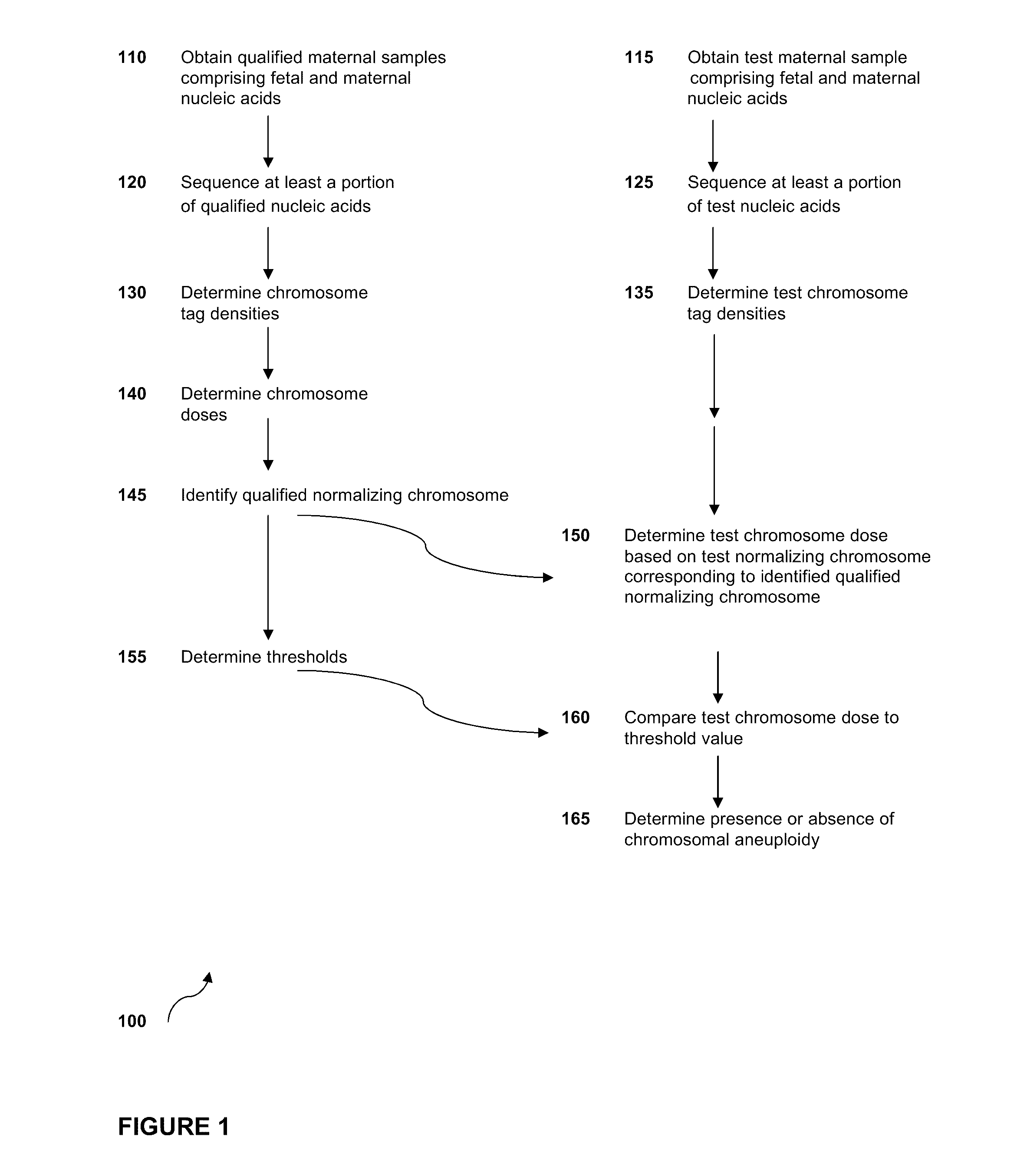
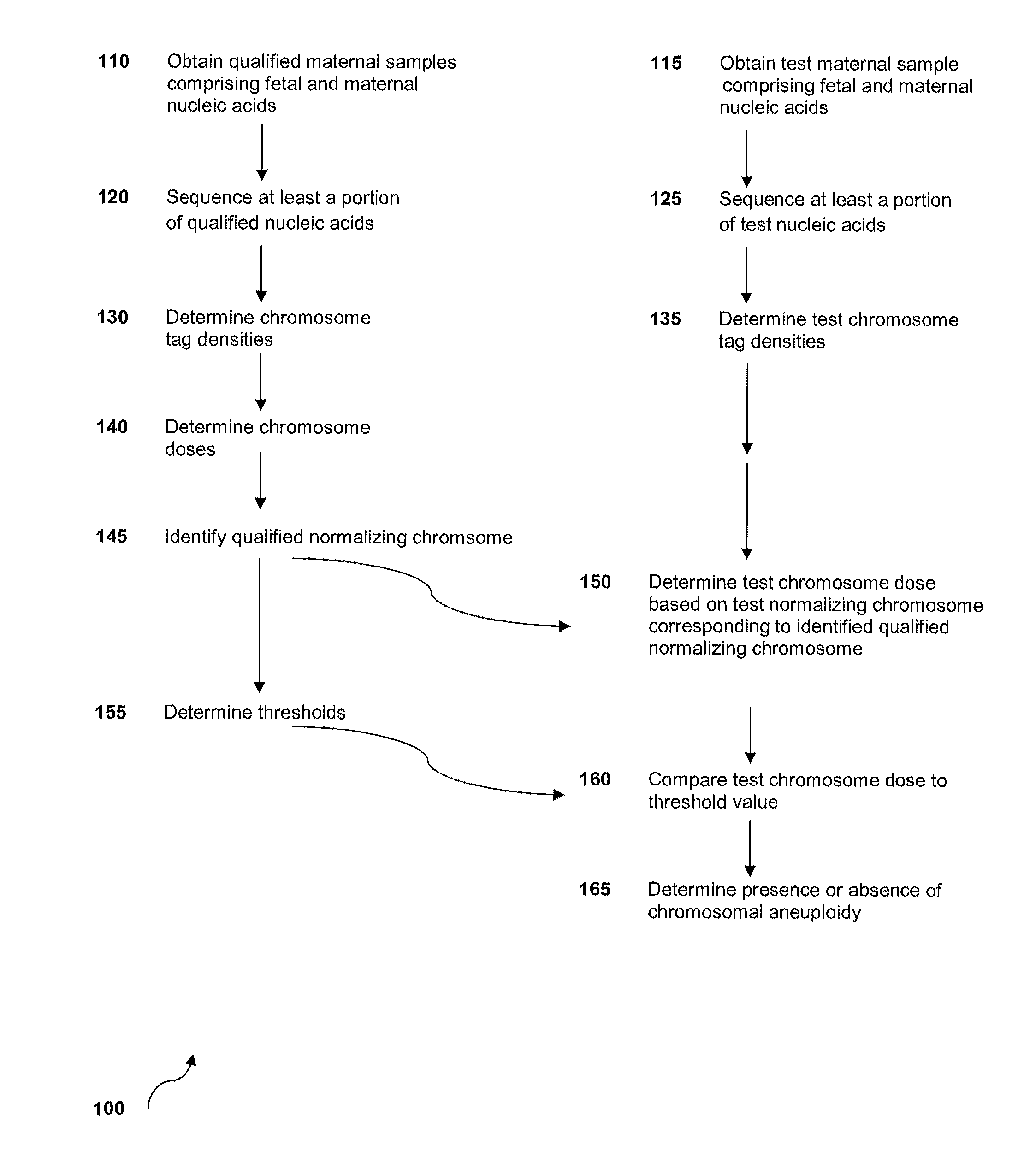
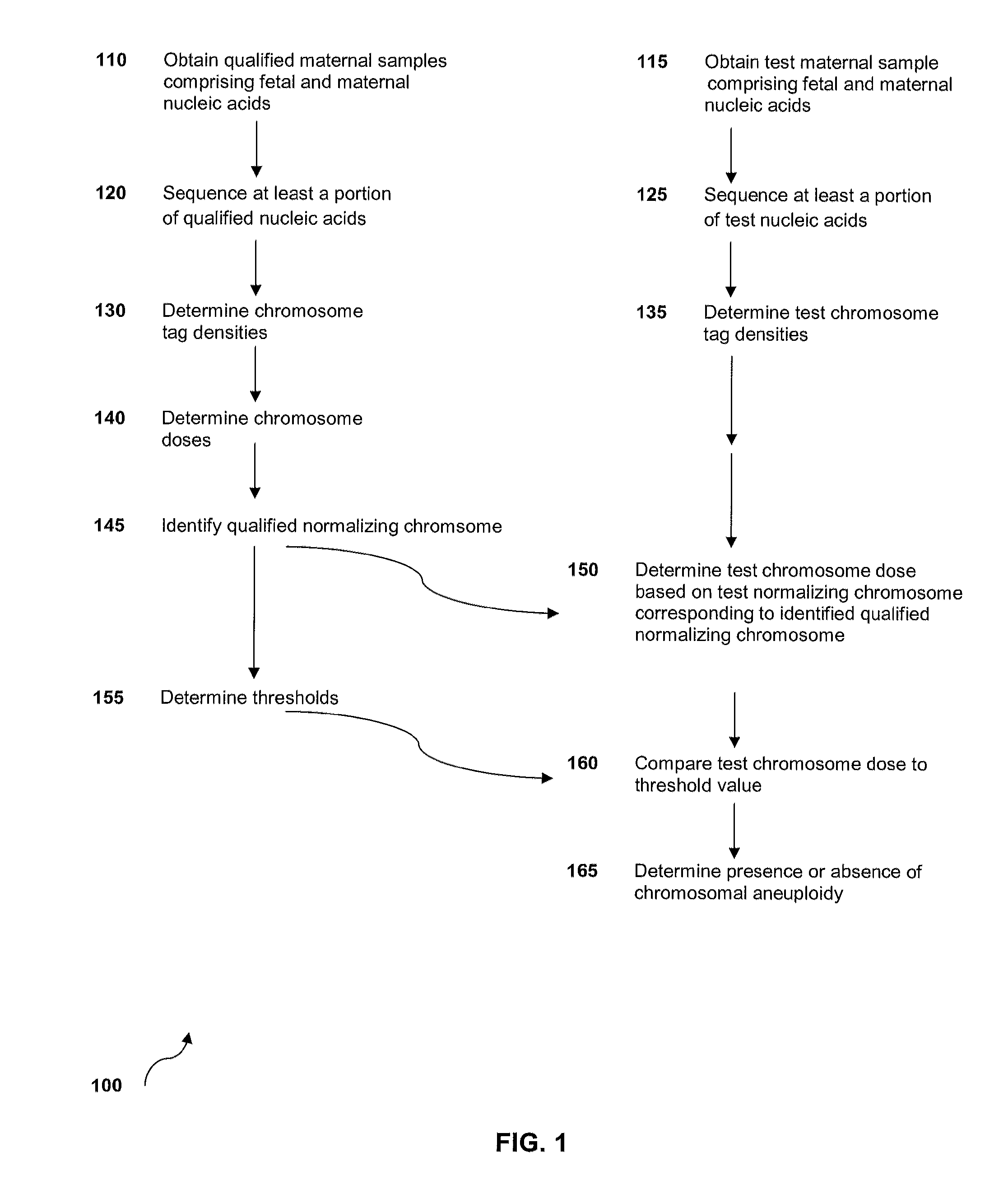
The invention provides methods for determining aneuploidy and / or fetal fraction in maternal samples comprising fetal and maternal cfDNA by massively parallel sequencing. The method comprises a novel protocol for preparing sequencing libraries that unexpectedly improves the quality of library DNA while expediting the process of analysis of samples for prenatal diagnoses.
Owner:VERINATA HEALTH INC
Non-invasive prenatal diagnosis
InactiveUS20010051341A1Improve accuracy100% accurate detection rateMicrobiological testing/measurementFermentationPrenatal diagnosisBlood typing
The invention relates to a detection method performed on a maternal serum or plasma from a pregnant female, which method comprises the presence of a nucleic acid of fetal origin in the sample. The invention enables non-invasive prenatal diagnosis including, for example, sex determination, blood typing and other genotyping, and detection of pre-eclampsia in the mother.
Owner:ISIS INNOVATION LTD
Method for sample analysis of aneuploidies in maternal samples
InactiveUS20120270739A1Decreases sequencing biasQuality improvementMicrobiological testing/measurementLibrary member identificationPrenatal diagnosisAnalysis method
The invention provides methods for determining aneuploidy and / or fetal fraction in maternal samples comprising fetal and maternal cfDNA by massively parallel sequencing. The method comprises a novel protocol for preparing sequencing libraries that unexpectedly improves the quality of library DNA while expediting the process of analysis of samples for prenatal diagnoses. The novel protocol can be performed in solution or on a solid surface.
Owner:VERINATA HEALTH INC
Methods and apparatus for sample tracking
InactiveUS20040157220A1Reduce chanceMicrobiological testing/measurementFermentationPrenatal diagnosisGenotype
A method and apparatus are provided for identifying a biological sample obtained during either paternity screening, genetic screening, prenatal diagnosis, presymptomatic diagnosis, diagnosis to detect the presence of a target microorganism carrier detection analysis, forensic chemical analysis, or diagnosis of a subject to determine whether a subject is afflicted with a particular disease or disorder, or is at risk of developing a particular disorder, wherein the result obtained from the analysis is associated with the unique DNA fingerprint biological barcode of the genotype of the subject being analyzed. The methods and apparatus of the invention have application in the fields of diagnostic medicine, disease diagnosis in animals and plants, identification of genetically inherited diseases in humans, family relationship analysis, forensic analysis, and microbial typing.
Owner:HANDYLAB
Novel markers for prenatal diagnosis and monitoring
ActiveUS20070275402A1Sugar derivativesMicrobiological testing/measurementObstetricsPrenatal diagnosis
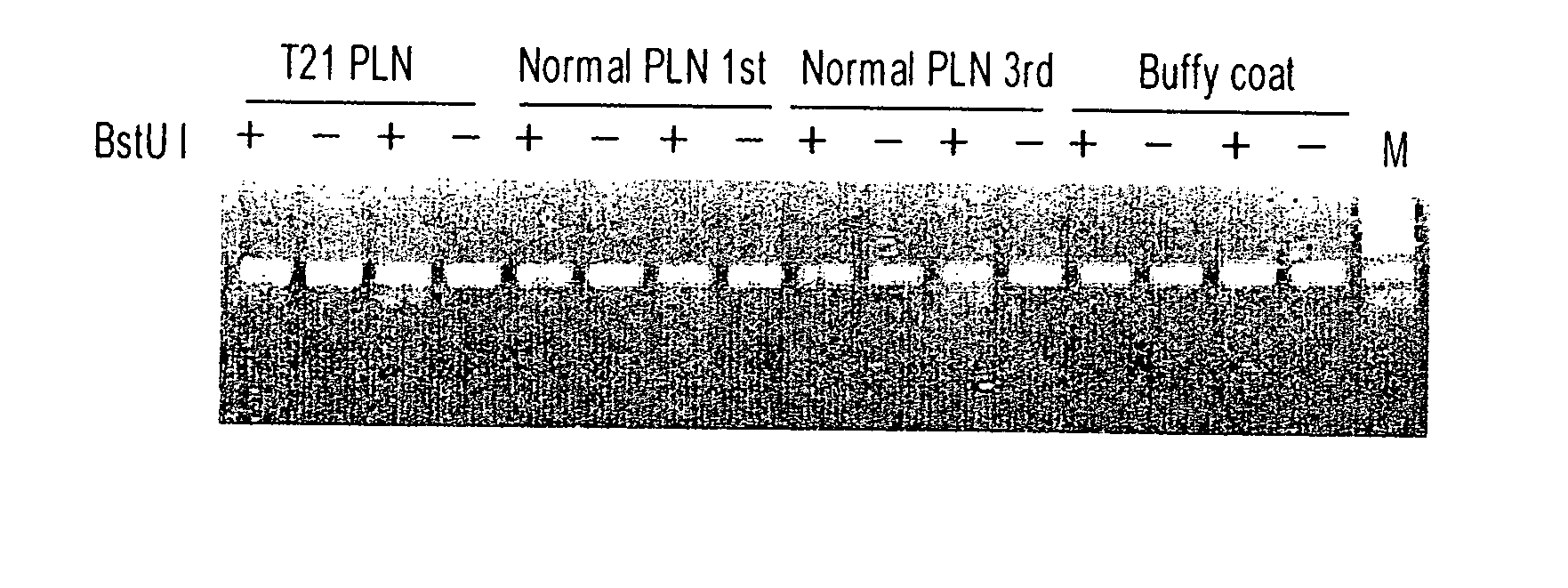
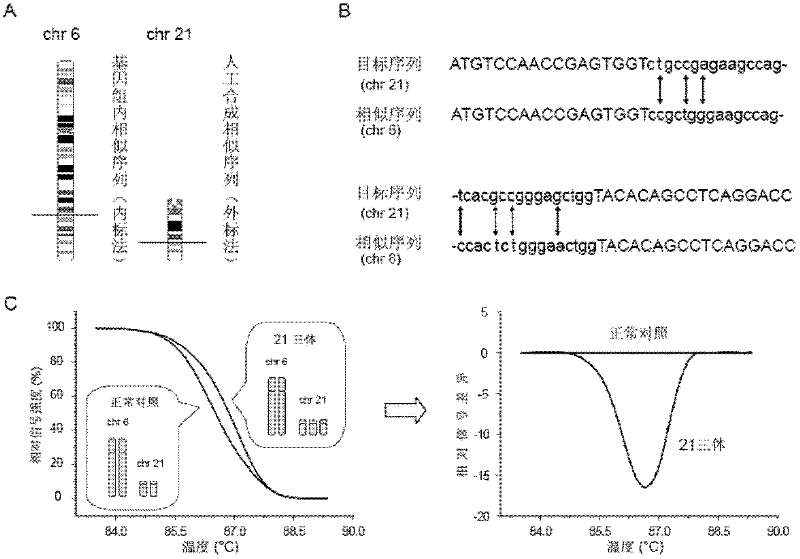
This application provides the use of novel fetal markers for prenatal diagnosis and monitoring of certain pregnancy-related conditions. More specifically, the invention resides in the discovery that certain CpG islands located on fetal chromosome 21 demonstrate a methylation profile that is distinct from that of the corresponding CpG islands located on maternal chromosome 21. This application also provides kits for diagnosing or monitoring of the relevant conditions.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Markers for prenatal diagnosis and monitoring
Methods and kits are provided for diagnosing, monitoring, or predicting preeclaimpsia in a pregnant woman, trisomy 18 and trisomy 21 in a fetus, as well as for detecting pregnancy in a woman, by quantitatively measuring in the maternal blood the amount of one or more RNA species derived from a set of genetic loci and comparing the amount of the RNA species with a standard control.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Kits for Prenatal Testing
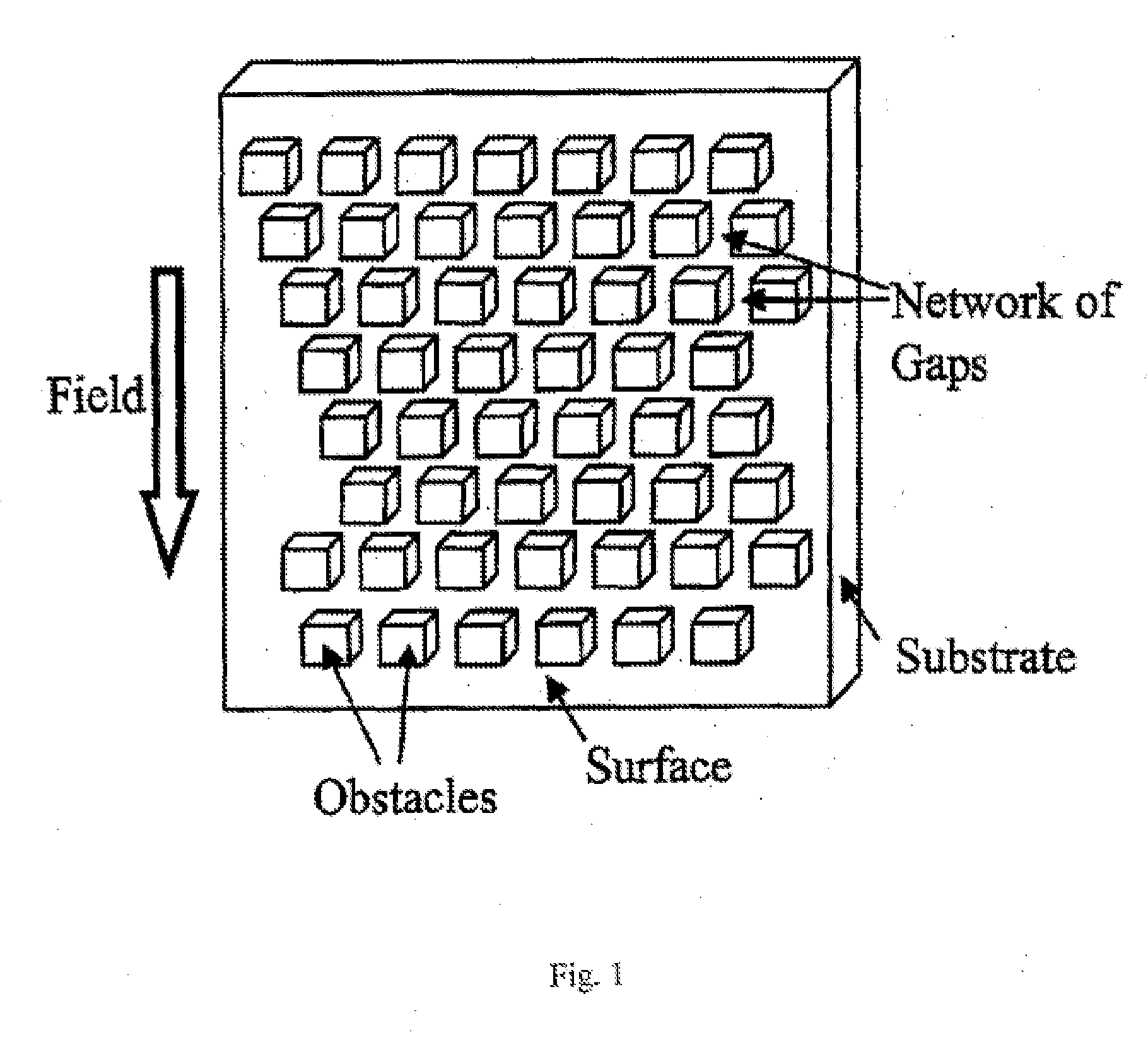
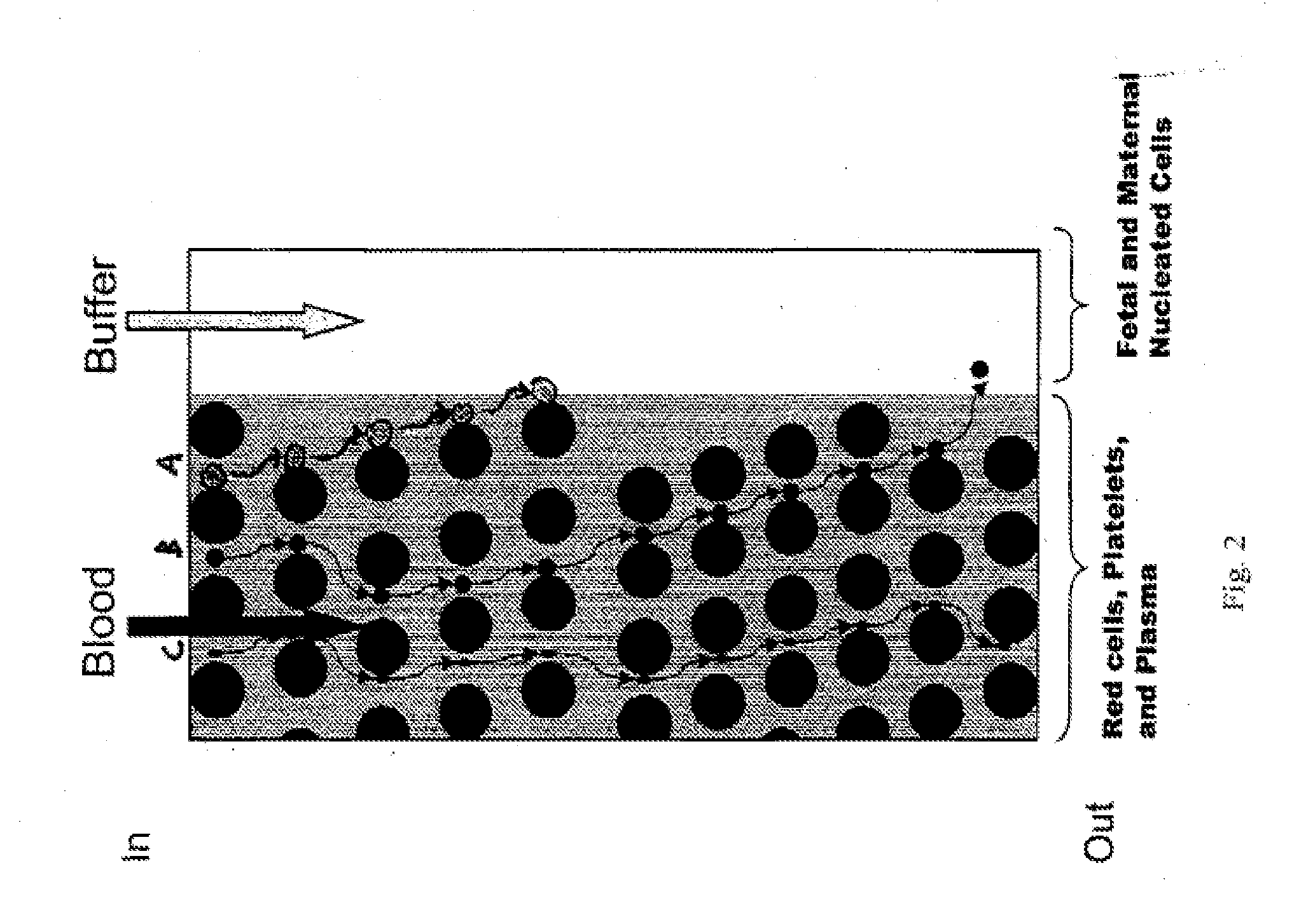
The invention relates to a kit for prenatal testing comprising a size-based separation module which enriches a first cell type from a maternal blood sample found in vivo in a pregnant female at a concentration of less than 1% of all blood cells, and a set of instructions for analyzing said one or more enriched cells to make a prenatal diagnosis. In some embodiments, the size-based separation module can comprise a plurality of obstacles to selectively direct the one or more cells of the first cell type in a first direction away from one or more cells of a second cell type.
Owner:THE GENERAL HOSPITAL CORP +2
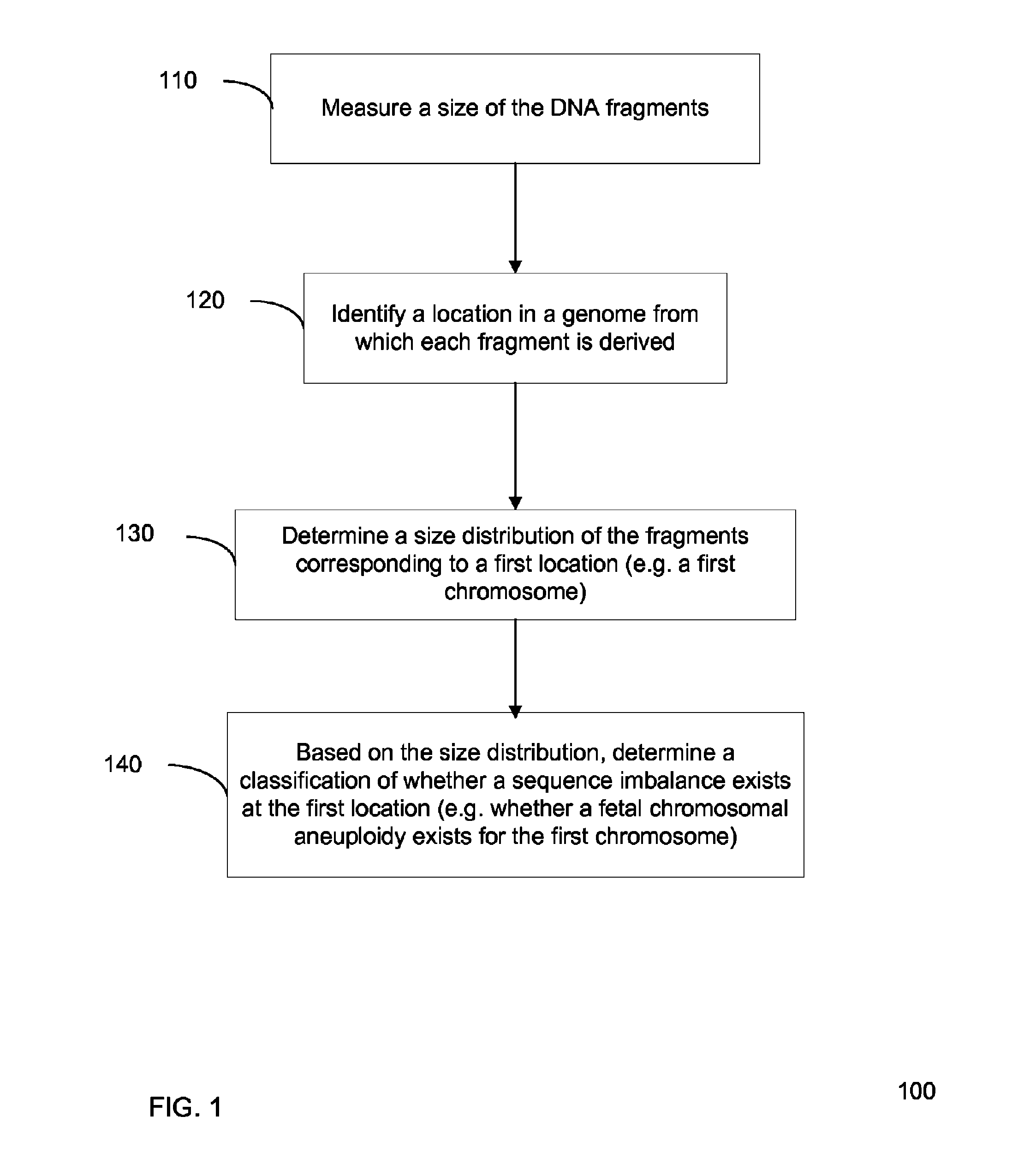
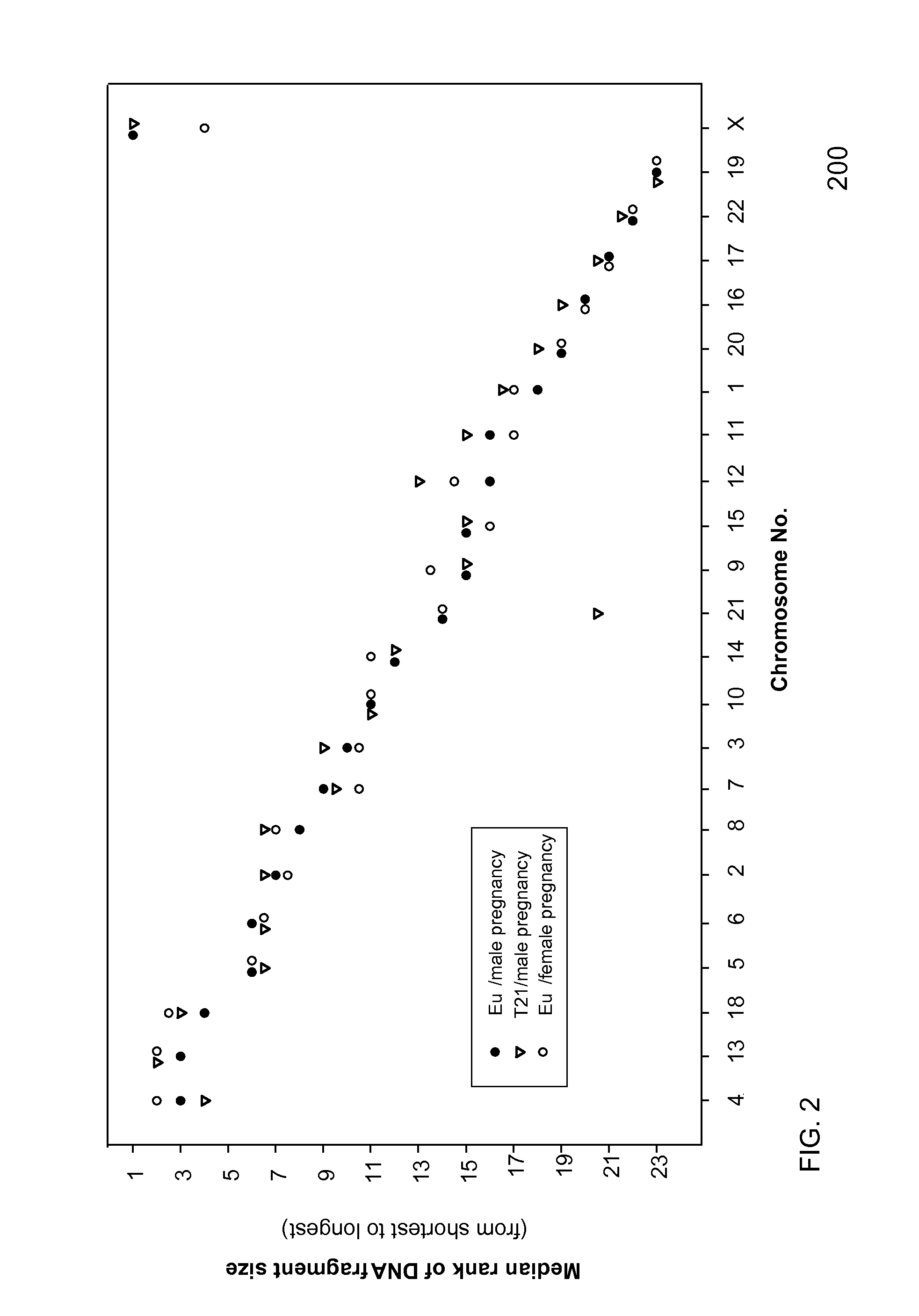
Size-based genomic analysis
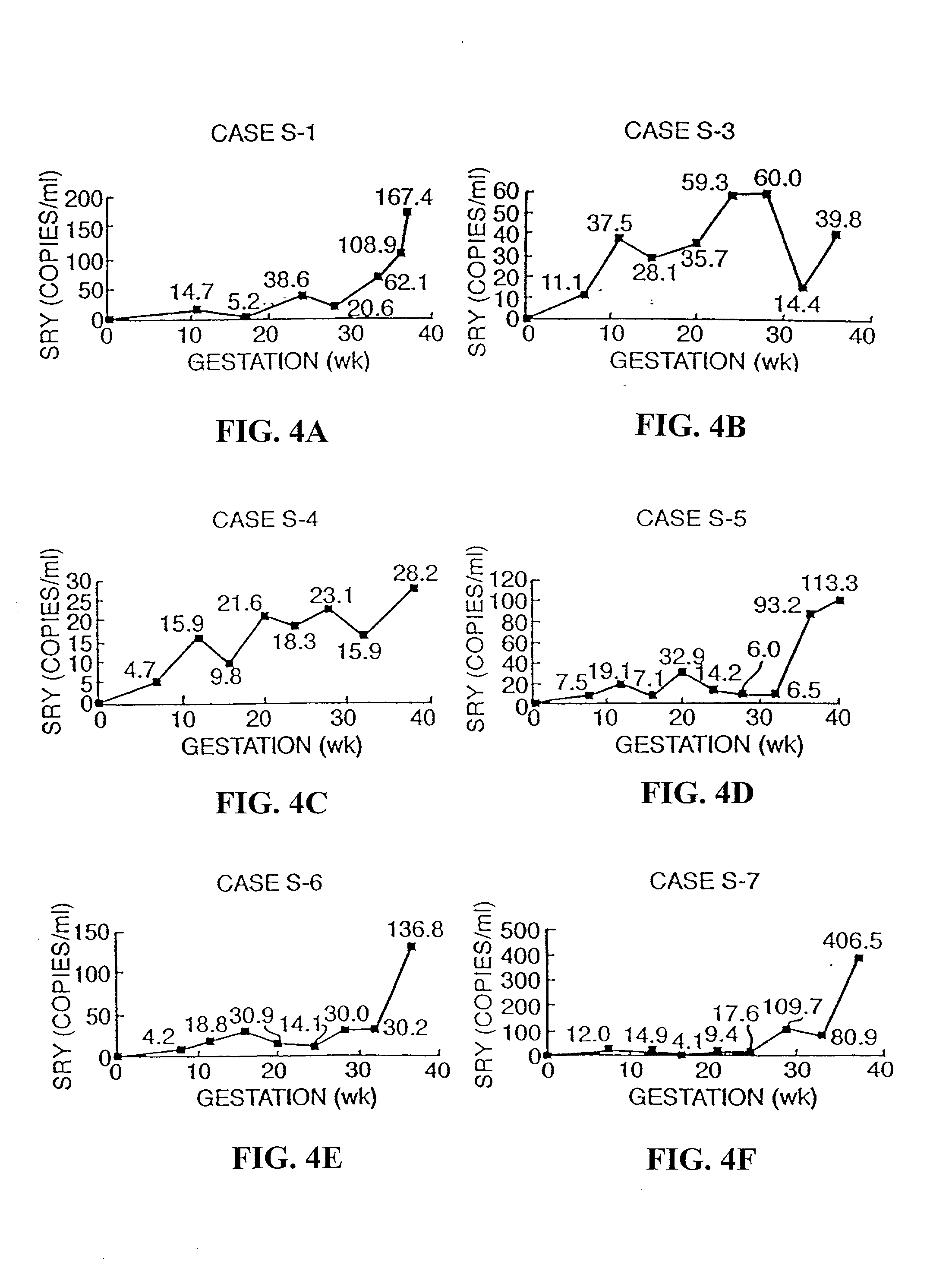
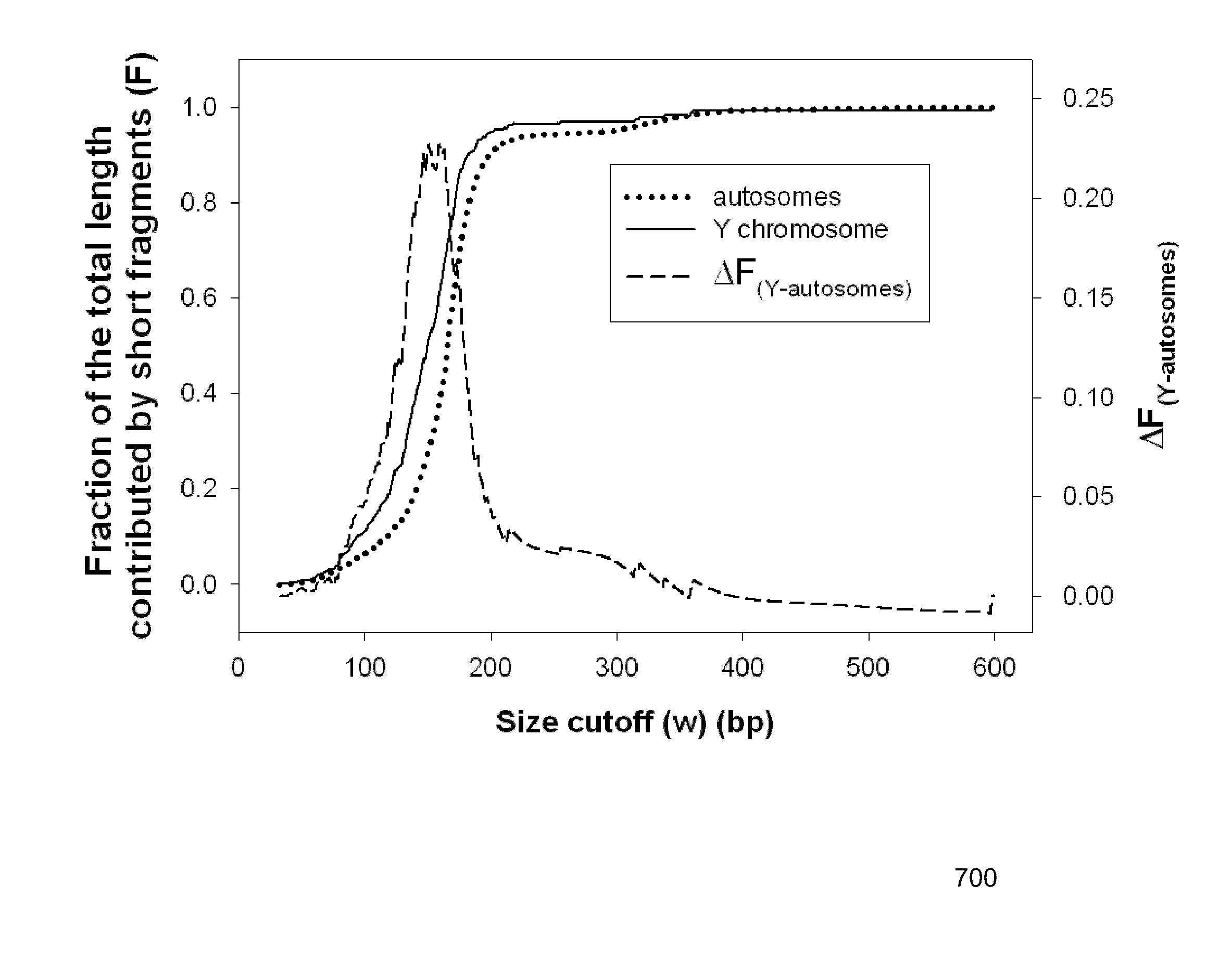
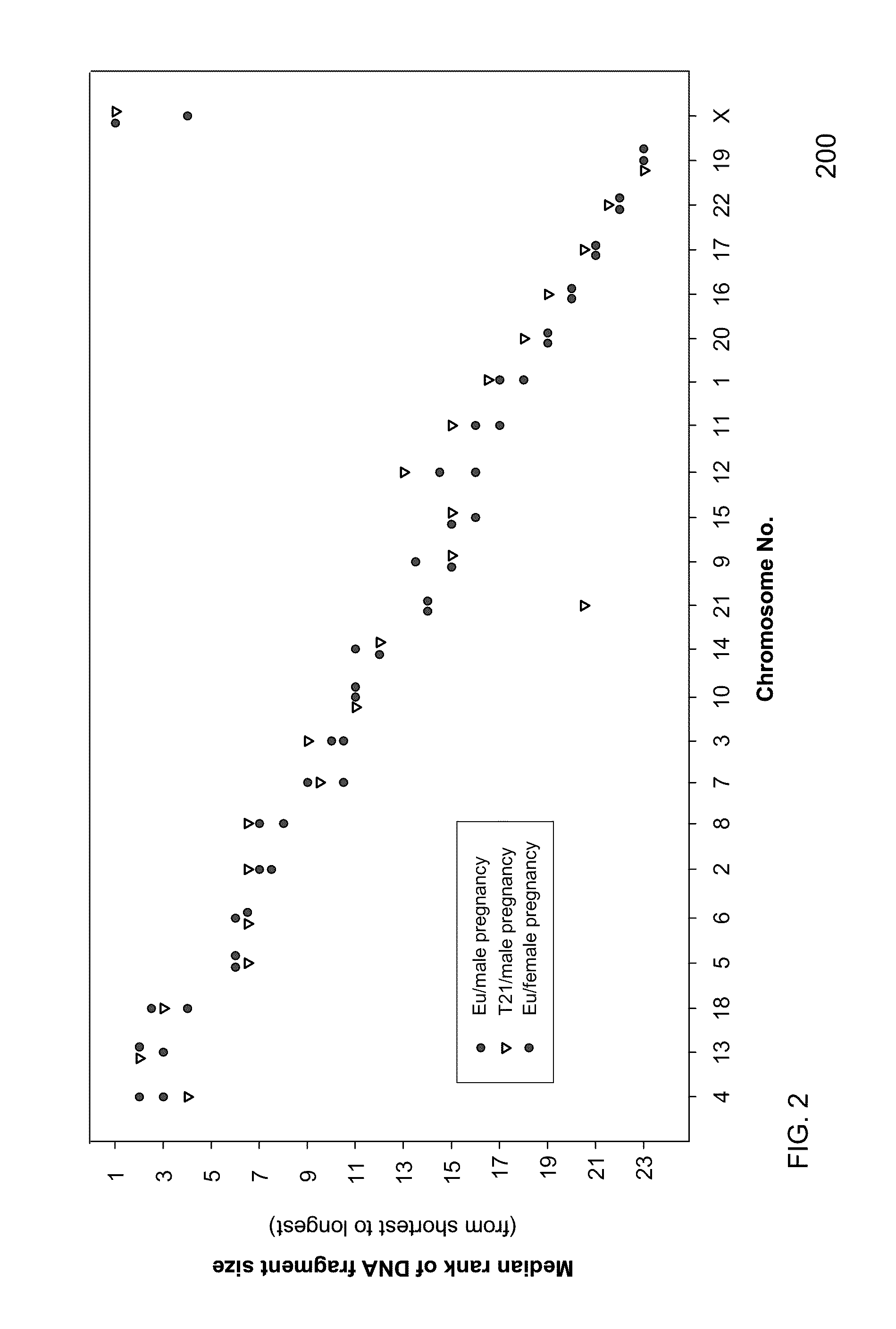
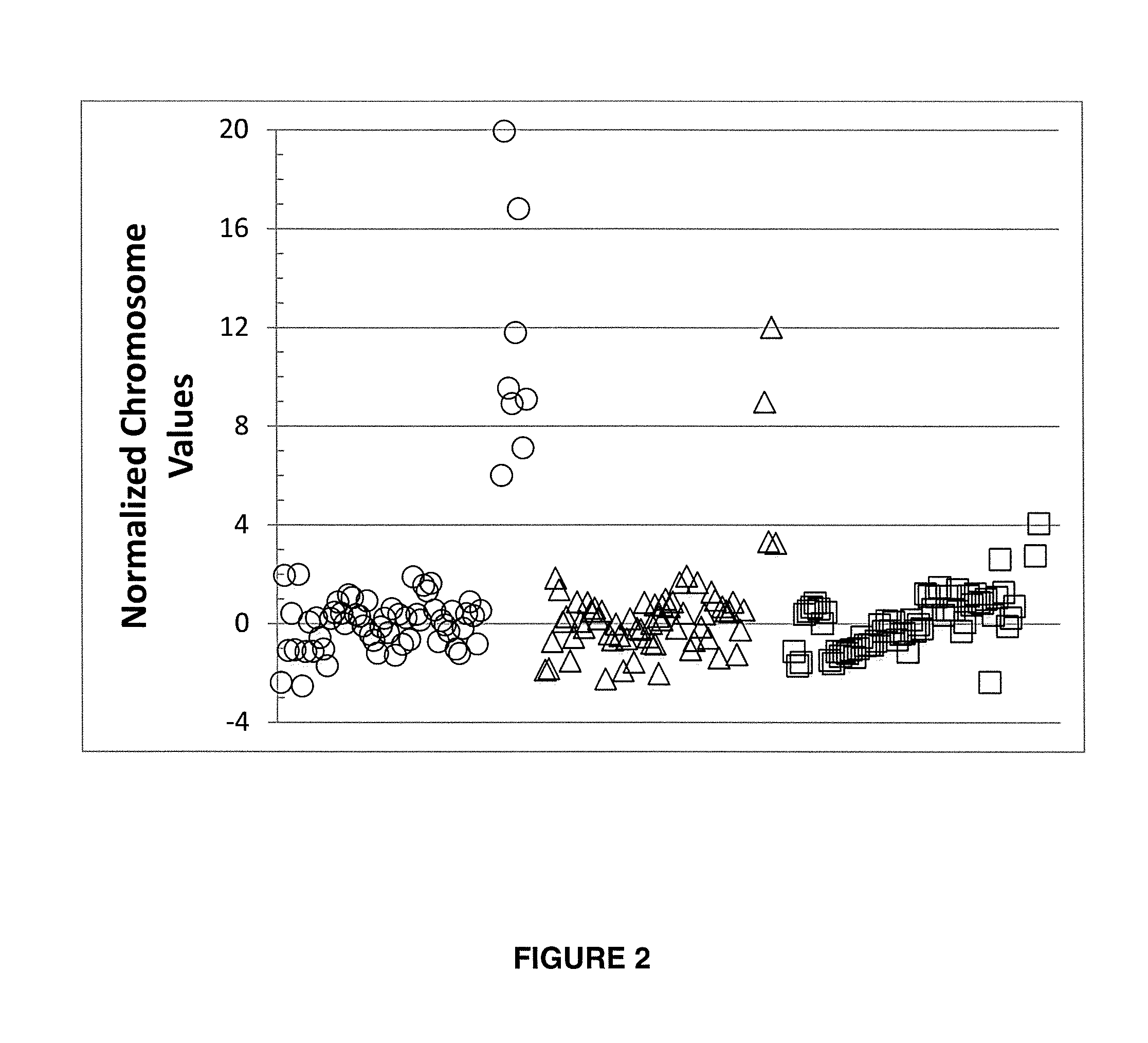
Systems, methods, and apparatuses for performing a prenatal diagnosis of a sequence imbalance are provided. A shift (e.g. to a smaller size distribution) can signify an imbalance in certain circumstances. For example, a size distribution of fragments of nucleic acids from an at-risk chromosome can be used to determine a fetal chromosomal aneuploidy. A size ranking of different chromosomes can be used to determine changes of a rank of an at-risk chromosome from an expected ranking. Also, a difference between a statistical size value for one chromosome can be compared to a statistical size value of another chromosome to identify a significant shift in size. A genotype and haplotype of the fetus may also be determined using a size distribution to determine whether a sequence imbalance occurs in a maternal sample relative to a genotypes or haplotype of the mother, thereby providing a genotype or haplotype of the fetus.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
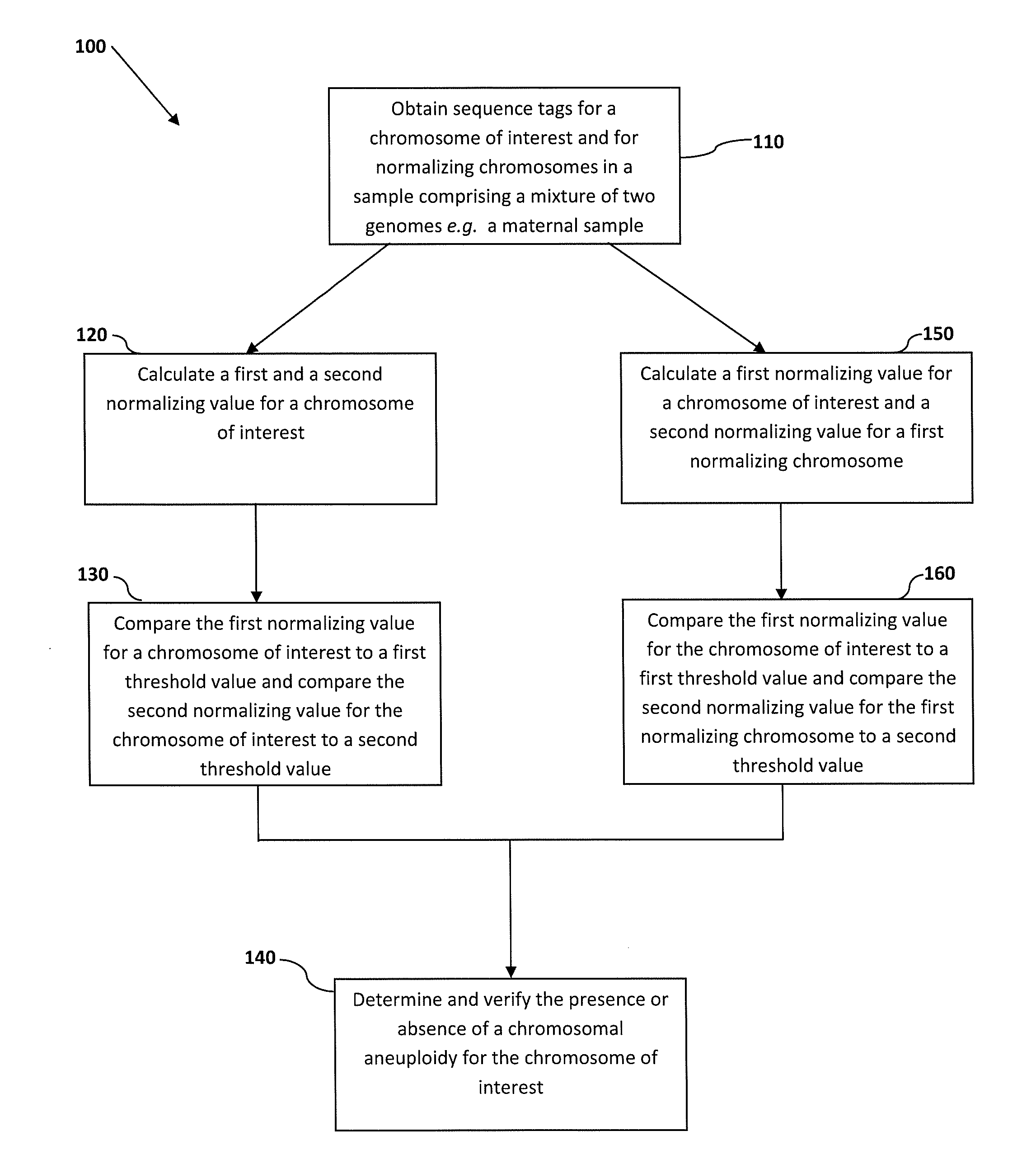
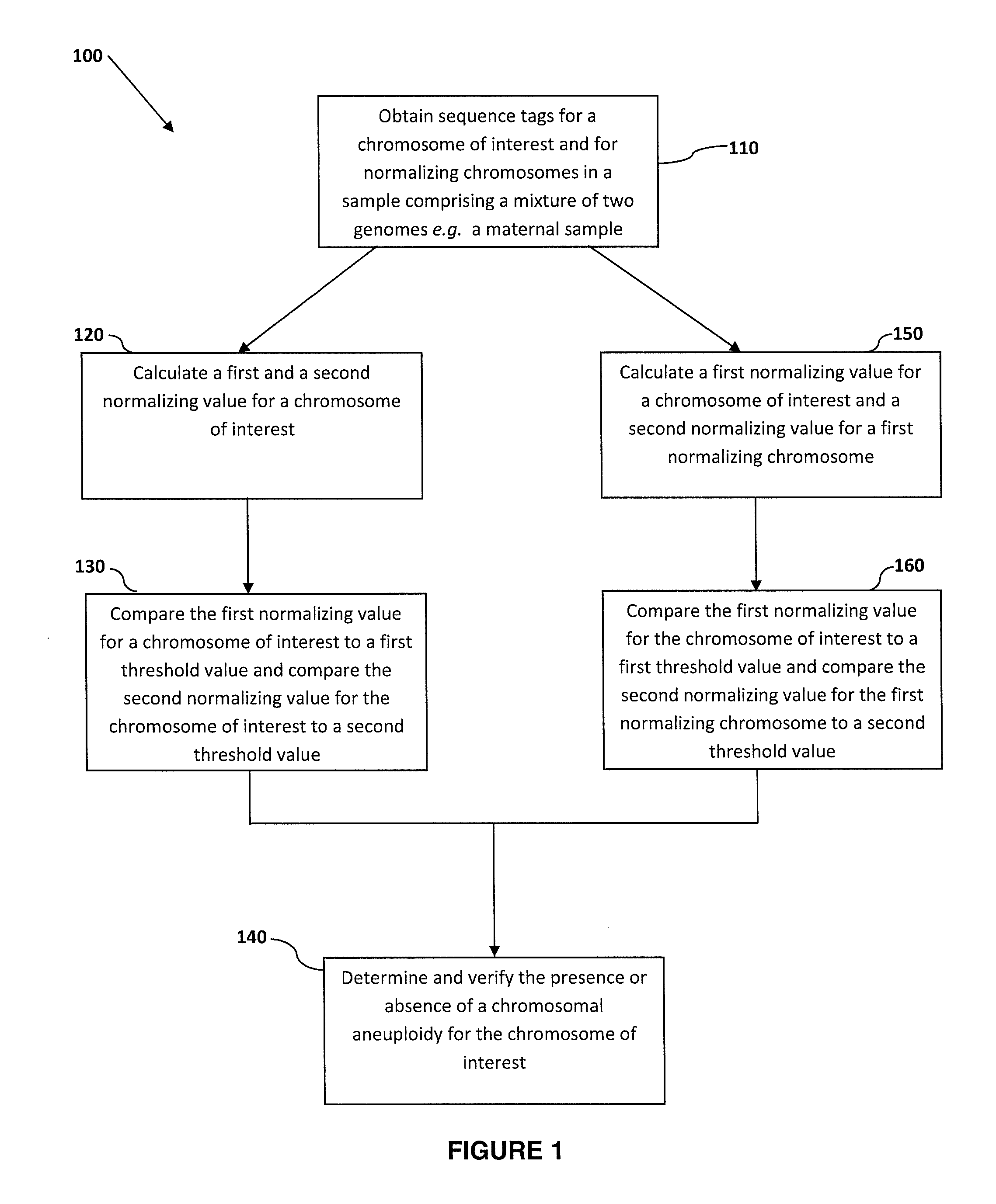
Normalizing chromosomes for the determination and verification of common and rare chromosomal aneuploidies
Owner:VERINATA HEALTH INC
Size-based genomic analysis
Systems, methods, and apparatuses for performing a prenatal diagnosis of a sequence imbalance are provided. A shift (e.g. to a smaller size distribution) can signify an imbalance in certain circumstances. For example, a size distribution of fragments of nucleic acids from an at-risk chromosome can be used to determine a fetal chromosomal aneuploidy. A size ranking of different chromosomes can be used to determine changes of a rank of an at-risk chromosome from an expected ranking. Also, a difference between a statistical size value for one chromosome can be compared to a statistical size value of another chromosome to identify a significant shift in size. A genotype and haplotype of the fetus may also be determined using a size distribution to determine whether a sequence imbalance occurs in a maternal sample relative to a genotypes or haplotype of the mother, thereby providing a genotype or haplotype of the fetus.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Non-invasive prenatal genetic diagnosis using transcervical cells
InactiveUS20050181429A1Microbiological testing/measurementDisease diagnosisPrenatal diagnosisStaining
A non-invasive, risk-free method of prenatal diagnosis is provided. According to the method of the present invention transcervical specimens are subjected to trophoblast-specific immuno-staining followed by FISH, PRINS, Q-FISH and / or MCB analyses and / or other DNA-based genetic analysis in order to determine fetal gender and / or identify chromosomal and / or DNA abnormalities in a fetus. Also provided is a method of in situ chromosomal, DNA and / or RNA analysis of a prestained specimen by incubating the prestained specimen in ammonium hydroxide. Also provided is a method of identifying embryonic cells according to a nucleus / cytoplasm ratio of at least 1:1 and the presence of at least variably condensed chromatin.
Owner:MONALIZA MEDICAL
Markers for prenatal diagnosis and monitoring
This application provides the use of novel fetal markers for prenatal diagnosis and monitoring of certain pregnancy-related conditions. More specifically, the invention resides in the discovery that certain CpG islands located on fetal chromosome 21 demonstrate a methylation profile that is distinct from that of the corresponding CpG islands located on maternal chromosome 21. This application also provides kits for diagnosing or monitoring of the relevant conditions.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
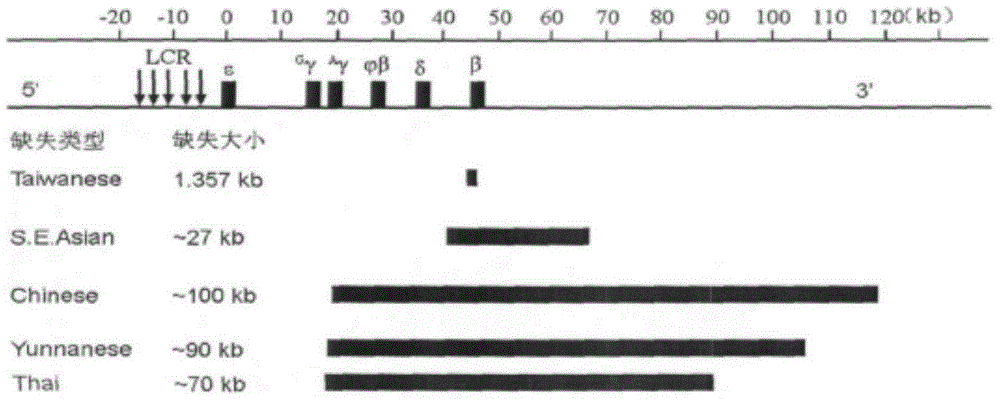
Nucleic acid film tape and kit for diagnosing alpha mediterranean anemia
ActiveCN101092647AReduce financial burdenRich reference informationMicrobiological testing/measurementPrenatal diagnosisThalassemia
This invention relates to test kit and test paper with oligonucleotide probes for diagnosing alpha-thalassemia. The test paper comprises a base, and specific oligonucleotide probes immobilized on the base. The oligonucleotide probes comprise: specific oligonucleotide probes for detecting mutation of alpha-globin gene, specific oligonucleotide probes for detecting deletion of alpha-globin gene, and base sequence complementary with the above sequences. The test kit comprises specific primer for amplifying alpha-globin gene or transcript, and specific oligonucleotide probes for detecting mutation / deletion of alpha-globin gene. The test kit and the test paper have such advantages as low cost, rapid detection, and stable results. The test kit and the test paper can be used in detection of mutation / deletion of alpha-globin gene, and prenatal diagnosis of severe thalassemia (alphaTalpha) caused by point mutation, and do not have error diagnosis.
Owner:亚能生物技术(深圳)有限公司
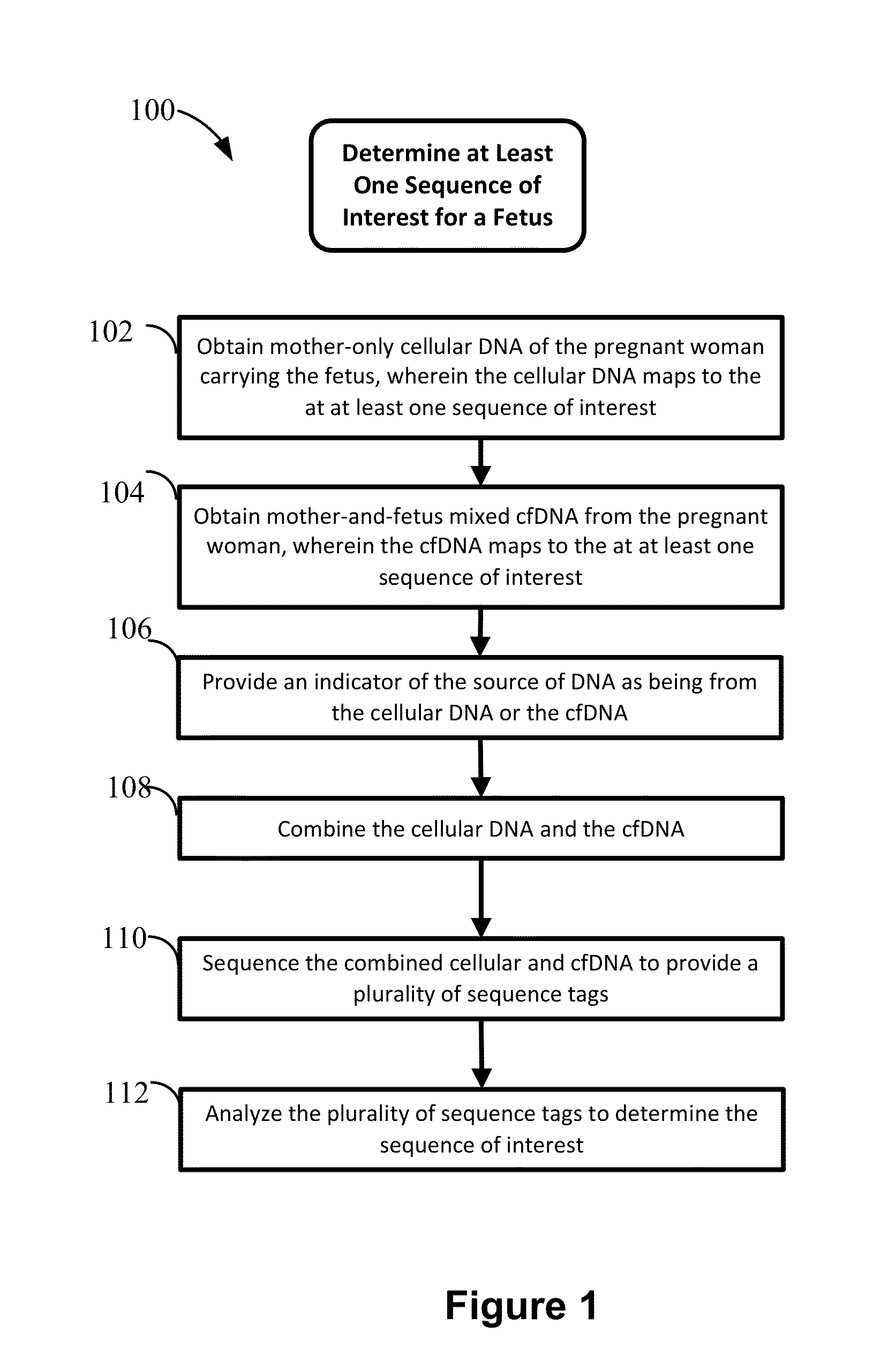
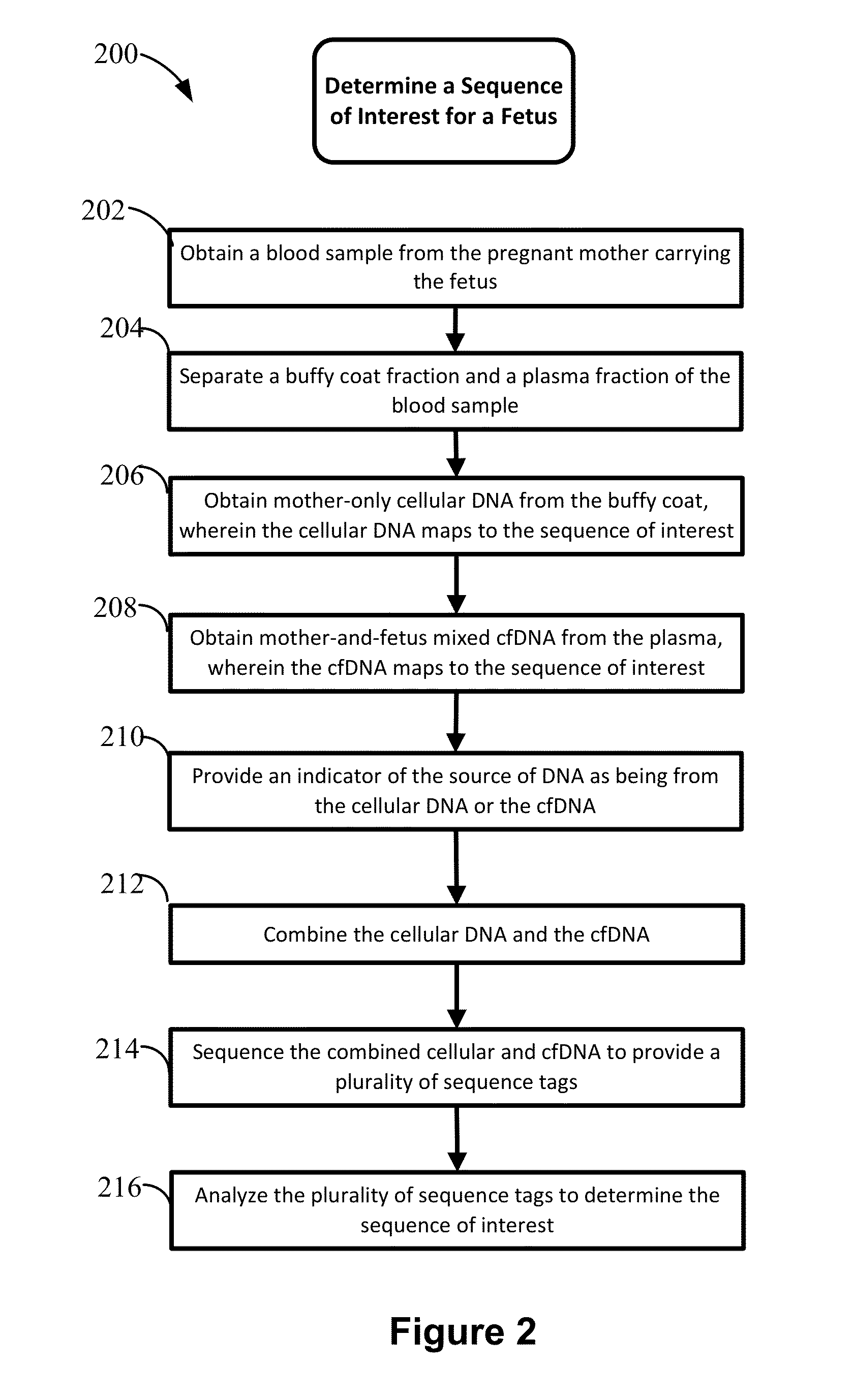
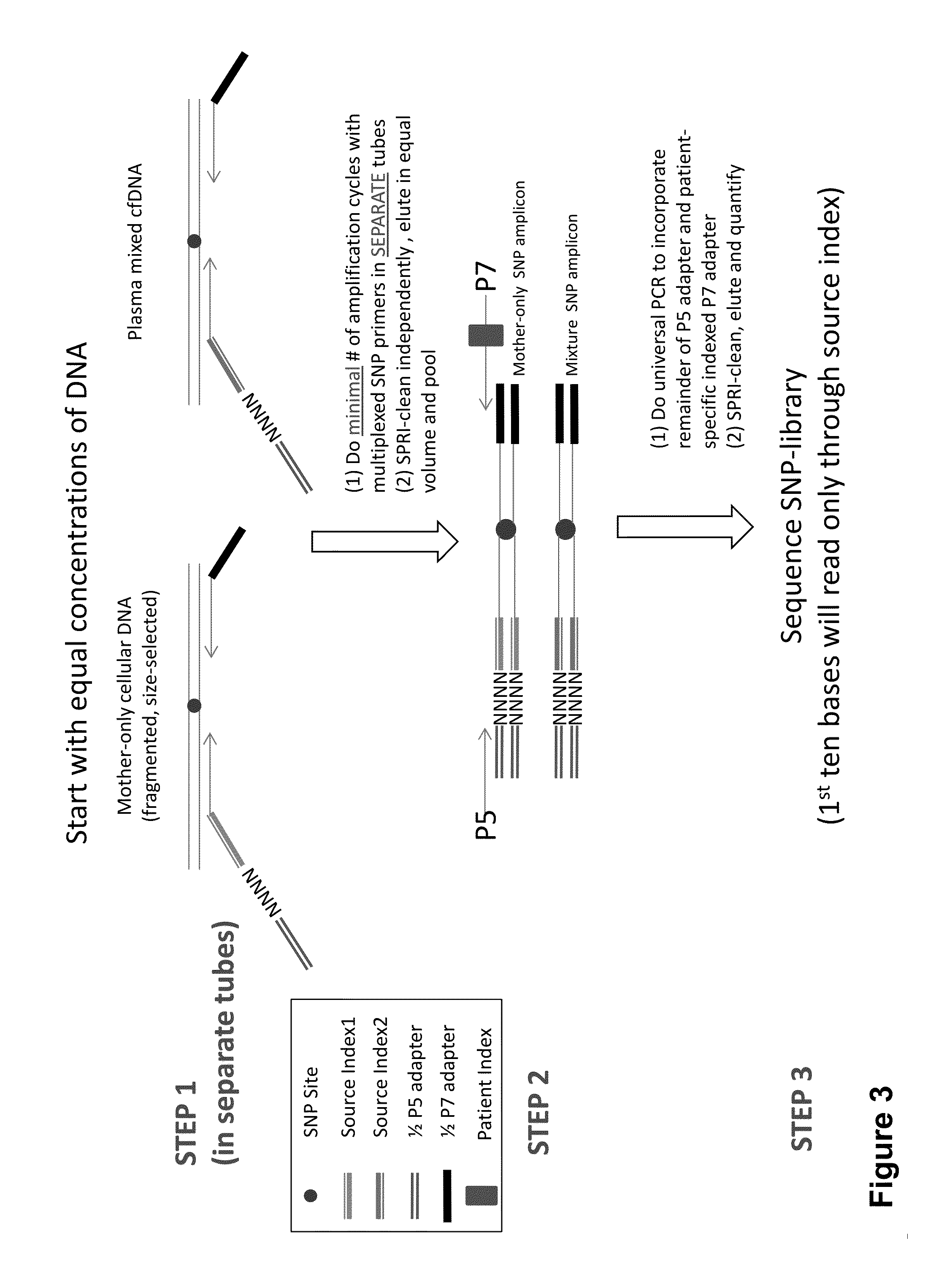
Non-invasive prenatal diagnosis of fetal genetic condition using cellular DNA and cell free DNA
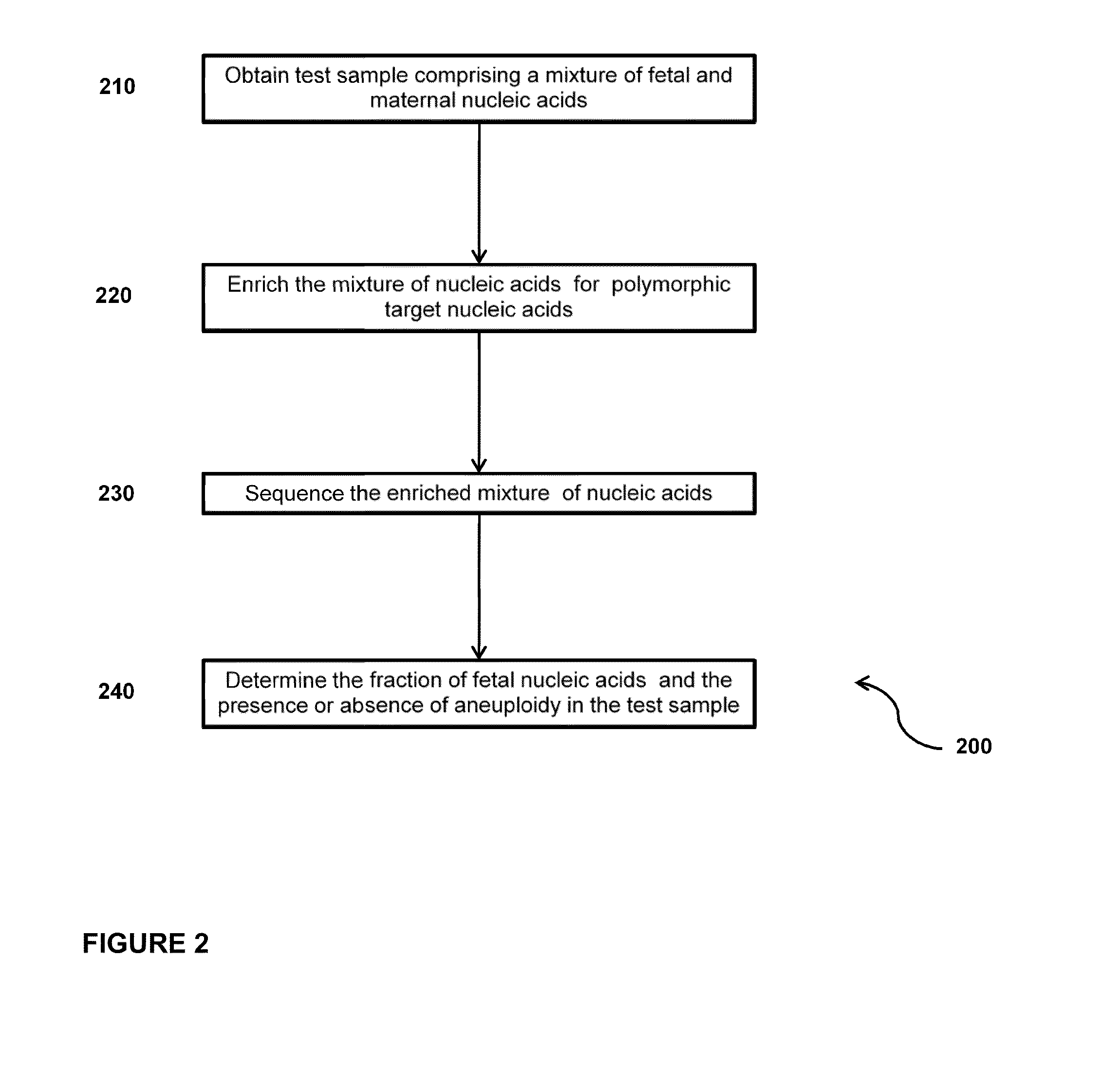
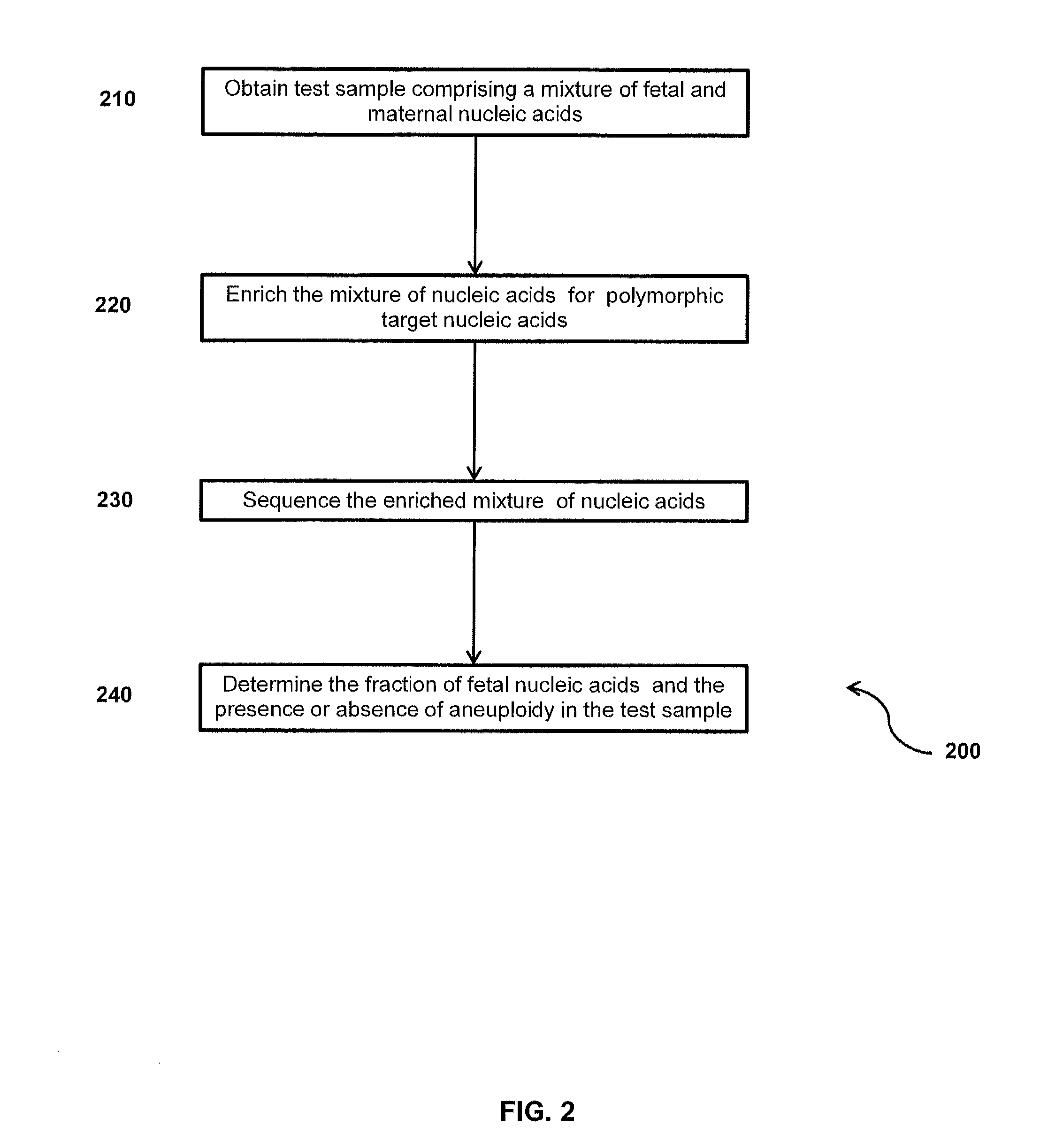
Disclosed are methods for determining at least one sequence of interest of a fetus of a pregnant mother. In various embodiments, the method can determine one or more sequences of interest in a test sample that comprises a mixture of maternal cellular DNA and mother-and-fetus cfDNA. In some embodiments, methods are provided for determining whether the fetus has a genetic disease. In some embodiments, methods are provided for determining whether the fetus is homozygous in a disease causing allele when the mother is heterozygous of the same allele. In some embodiments, methods are provided for determining whether the fetus has a copy number variation (CNV) or a non-CNV genetic sequence anomaly.
Owner:ILLUMINA INC
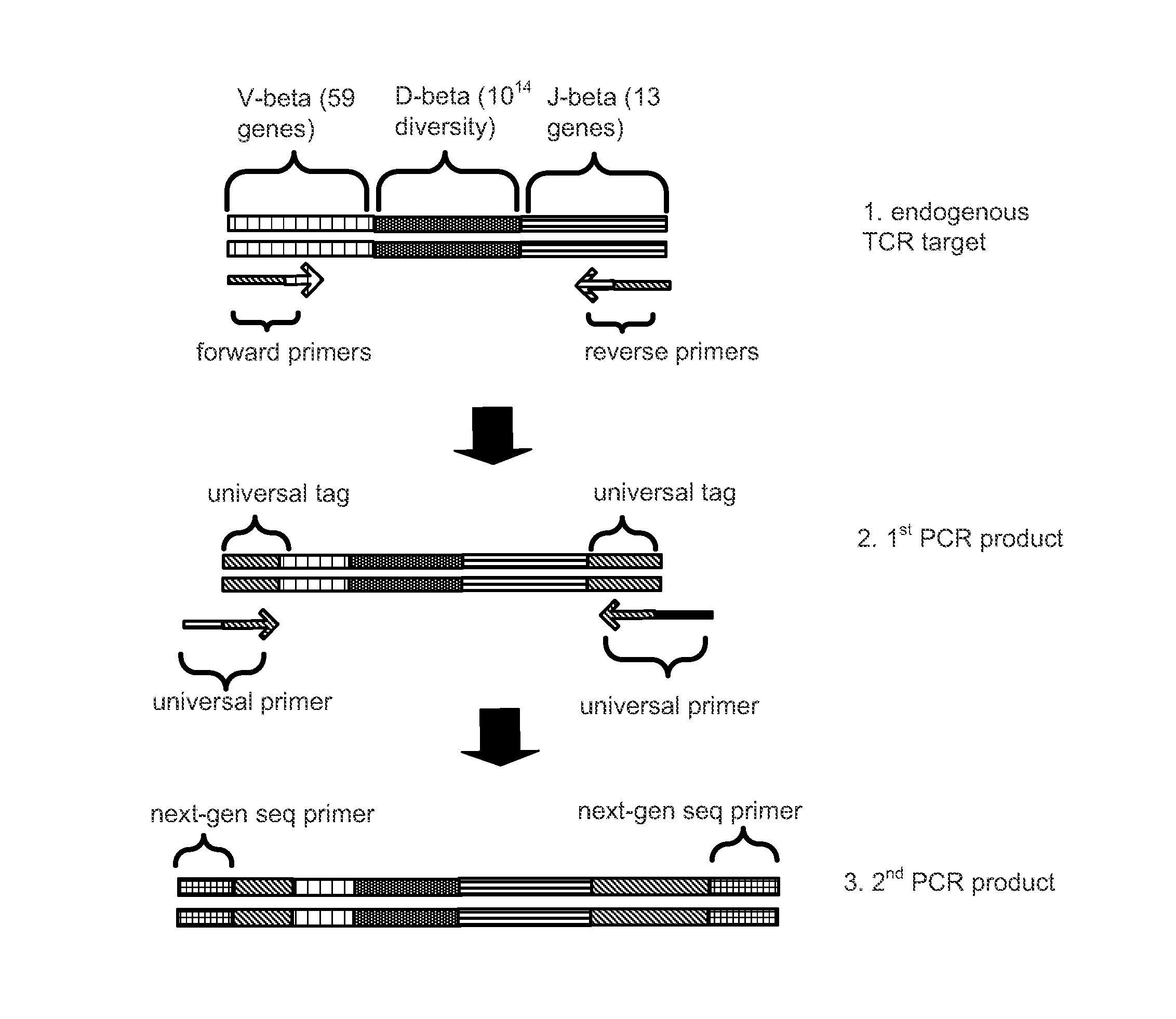
System and Methods for Genetic Analysis of Mixed Cell Populations
InactiveUS20150154352A1Reduce error rateMicrobiological testing/measurementBiological testingMixed cellPrenatal diagnosis
Methods and systems are provided for massively parallel genetic analysis of single cells in emulsions or droplets. A biological sample is divided into subsamples of single cells or cell supbopulations, and a fusion complex is formed by molecular linkage and amplification techniques. Methods, apparatuses, and systems are provided for high-throughput, massively parallel analysis of the subsamples. These methods integrate molecular, algorithmic, and engineering approaches. They have broad and useful application in a number of biological and medical fields, including immunology, noninvasive prenatal diagnosis, and noninvasive cancer diagnosis.
Owner:GIGAGEN
Clinical antenatal diagnosis inspection instrument in obstetrics and gynecology department
The invention discloses a clinical antenatal diagnosis inspection instrument in an obstetrics and gynecology department. The clinical antenatal diagnosis inspection instrument comprises a fixed bed board, wherein legs are fixedly connected with the bottom surface of the fixed bed board, and an end part of the fixed bed board is connected with a moving bed board through a rotating shaft, side surfaces of the two legs close to the moving bed board are fixedly connected with struts, and the struts are perpendicular to the legs and are arranged below the moving bed board. The clinical antenatal diagnosis inspection instrument in the obstetrics and gynecology department has the advantages that the angle of the moving bed board can be adjusted through extension and retraction of first electric telescopic links, so as to help a pregnant woman to adjust the lying angle and receive inspection more comfortably; the distance between a B-ultrasonic probe and an inspection part of the pregnant woman can be adjusted through a second electric telescopic link; the B-ultrasonic probe can be moved to the inspection part of the pregnant woman through a first motor, and the angle between the B-ultrasonic probe and an inspection part of the pregnant woman can be adjusted through a second motor, so that an inspection operator can use the clinical antenatal diagnosis inspection instrument conveniently; the usage is convenient.
Owner:孙梅玲
Clinical antenatal diagnosing and examining device for obstetric and gynecologic department
InactiveCN107361980ASimple structureSimple and fast operationPatient positioningOperating tablesPrenatal diagnosisEngineering
The invention discloses a clinical antenatal diagnosing and examining device for the obstetric and gynecologic department. The clinical antenatal diagnosing and examining device comprises a bed board, the bed board is composed of three support plates which are connected through rotating shafts, support legs are arranged at four corners of the lower surface of the support plate in the middle, each of the two support legs on the right is provided with a support frame, and first electric telescopic rods are connected between the support frames and the lower surface of the support plate on the right through hinge seats. The clinical antenatal diagnosing and examining device has the advantages that through an arc pathway, a pregnant woman can be examined from multiple angles, and examination is enabled to be more accurate; through an inflated cushion, the pregnant woman is enabled to feel more comfortable; through telescoping of the first electric telescopic rods, angles of an upper body and feet of the pregnant woman can be adjusted, and more convenience is brought to the pregnant women during examination; to sum up, the clinical antenatal diagnosing and examining device is simple in structure, simple to operate, capable of enabling examination to be more accurate by examining the pregnant woman in multiple angles, and capable of adjusting angles of two ends of a ward bed so as to enable the pregnant women to feed more comfortable when lying down.
Owner:郑敏
Method for detecting gene copy number variation
ActiveCN102409088ASimple designThe result is accurateMicrobiological testing/measurementPrenatal diagnosisWild type
Owner:郭奇伟 +1
Method for detecting COL7A1 gene mutation and uses thereof
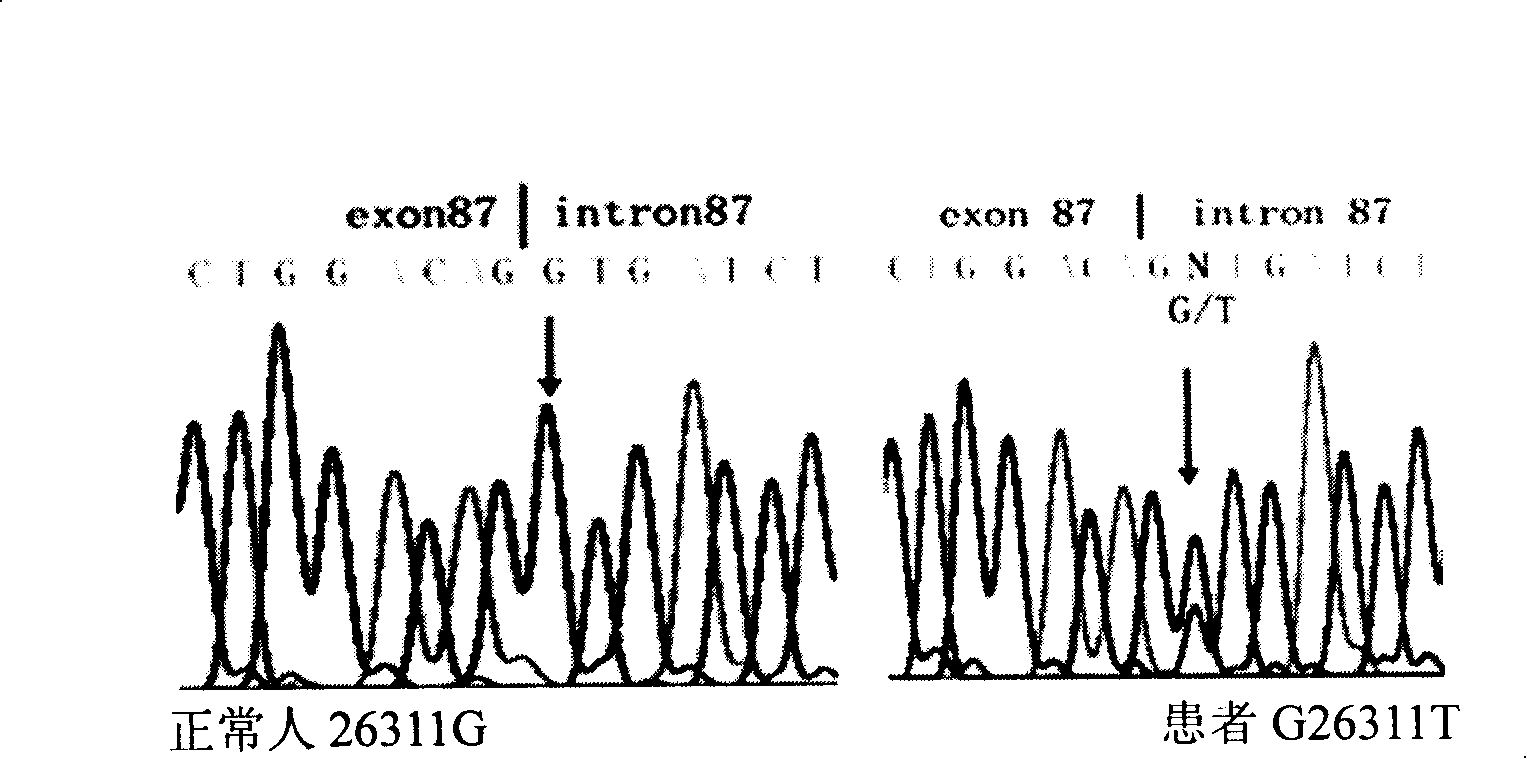
The invention discloses a method of detecting 26311 Mutation of COL7A1 gene and a usage thereof. The method adopts primers of p87F (TGGGCCTGAAATATGAGGAG) and P87R (TAGGCCACTGGAGAGACAGG) to PCR-amplify COL7A1 gene including the section of 26251-26350, implements the sequence after the purification of products, or uses BslI for the endonuclease detection after using p87Fa (TCCCACGGGGGCCCCTGGACAG) and P87Ra (TCCCCCGCCCCCACCCT GCCA) for the nest amplification of the PCR products. The usage is the application in preparations or gene chips for detecting the diagnosis of dominant dystrophic epidemolysis bullosa genealogy. The invention provides a novel way for the prenatal diagnosis of the bullosa and provides a basis for the gene therapy.
Owner:HUAZHONG UNIV OF SCI & TECH
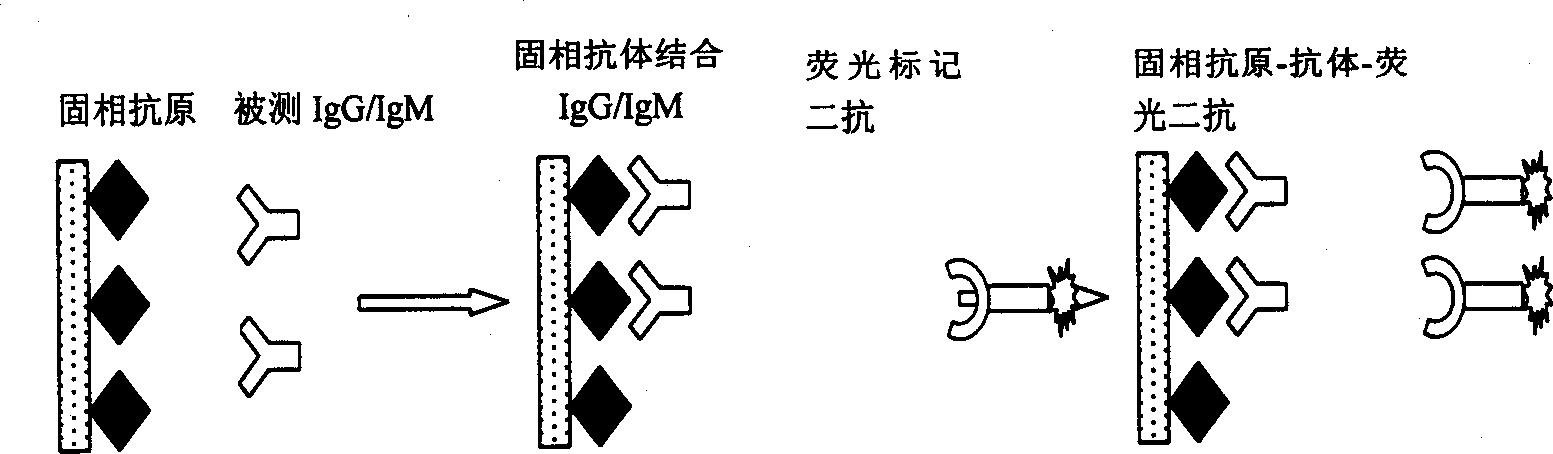
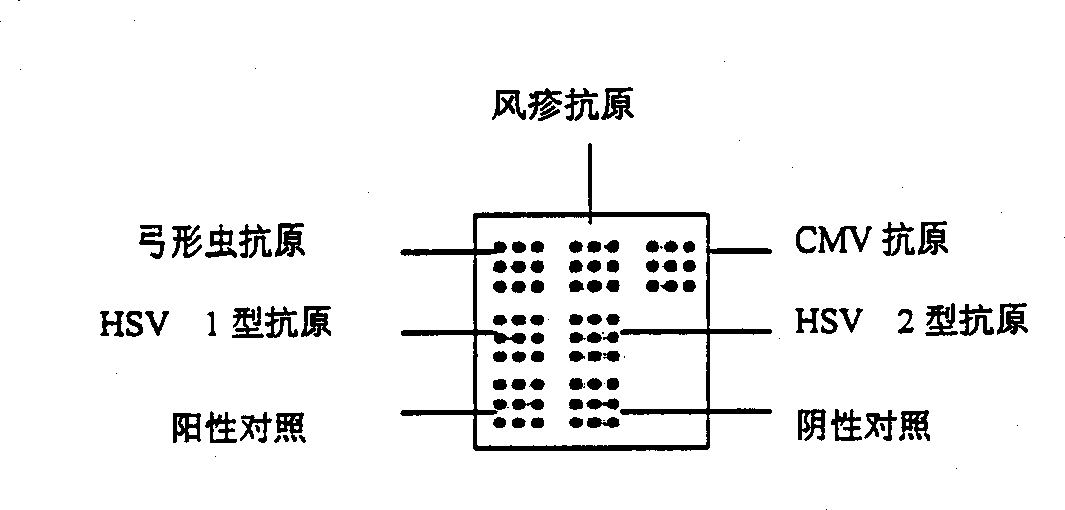
Protein-chip for prenated diagnosis and its preparing process
A protein chip for prenatal diagnosis features that the protein or polypeptide array is sequentially arranged on solid substrate for testing, recognizing, discriminating and diagnosing the protein. It is prepared by loading the dot matrix of the antigens of Toxoplasma, rubella, cytomegalovirus and herpes simplex viruses A and B, positive reference and negative reference in a same cavity on biochip. Its advantages are one-step reaction for different results, high speed and sensitivity, and high stability and reliability.
Owner:GENETECH BIOTECH SHANGHAI
Processes and compositions for methylation-based enrichment of fetal nucleic acid from a maternal sample useful for non invasive prenatal diagnoses
InactiveCN102648292AMicrobiological testing/measurementDisease diagnosisPrenatal diagnosisNon invasive
Owner:SEQUENOM INC +1
Primer set and kit for detecting rare deletion type thalassemia
ActiveCN103602752AEasy to operateLow costMicrobiological testing/measurementDNA/RNA fragmentationBeta thalassemiaPrenatal diagnosis
The invention belongs to the technical field of biology and particularly relates to a primer set and a kit for detecting rare deletion type thalassemia. The primer set and the kit can be directly used for rapidly and stably detecting domestic 11 types of known rare deletion type thalassemia. The primer set for detecting the rare deletion type thalassemia comprises a primer set A and a primer set B, wherein the primer set A comprises 12 primers for detecting alpha-thalassemia deletion gene types; the primer set B comprises 10 primers for detecting beta-thalassemia deletion gene types. The kit can adopt a multiplex Gap-PCR (Polymerase Chain Reaction) technology to detect 11 rare deletion gene types and can be used for directly detecting 11 rare deletion thalassemia gene types for one time. The kit is simple to operate and saves time and cost when being compared with a manner of combining a nested PCR with a genetic analysis in antenatal diagnosis; conditions are created for comprehensively carrying out thalassemia screening and scientific evidences are provided for thalassemia diagnosis of premarital detection, antenatal detection and fetuses of the pregnancy.
Owner:亚能生物技术(深圳)有限公司
Method for detecting GJB2 gene mutation by fluorescence quantitative PCR technology, and kit thereof
InactiveCN102465174AMicrobiological testing/measurementFluorescence/phosphorescencePrenatal diagnosisFluorescence
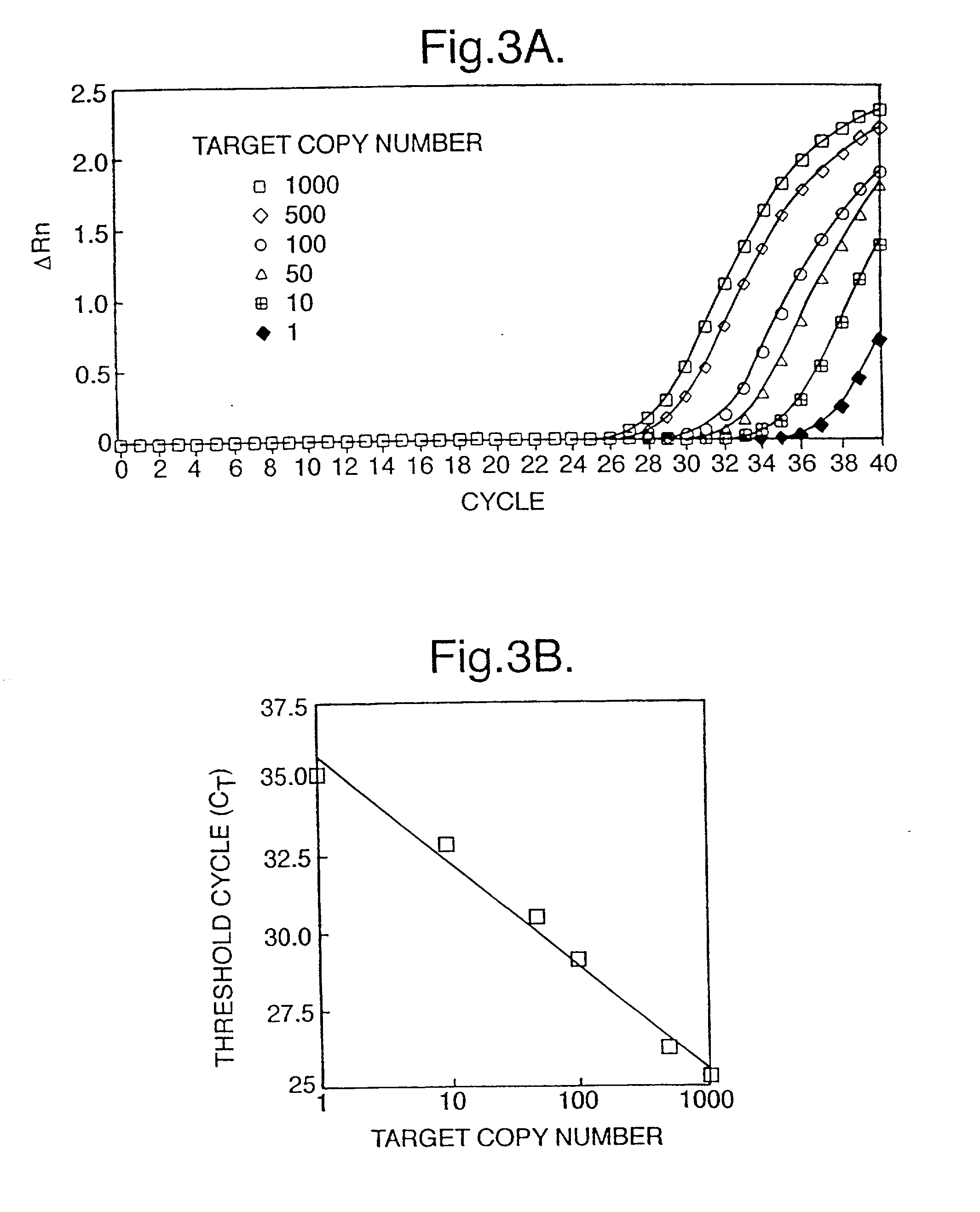
The invention provides a method for detecting 235delC mutation of deafness related gene GJB2 by a fluorescence quantitative PCR technology, and a kit thereof. According to the kit, specific oligonucleotide primers are designed according to the 235delC mutational site of the GJB2 gene; a fluorescent substance is adopted, and a sample requiring detection is detected respectively by a wild type fluorescent PCR system and a mutant type fluorescent PCR system; the Ct values of the two reactions and the different value (DeltaCt) of the two Ct values are compared to judge whether the 235delC mutation of the GJB2 gene exists. With adopting the method and the kit of the present invention, the valuable clinical reference information can be provided for the etiological diagnosis of the deafness patient, and the pregnant couple with the possibility of deafness carriers can be screened so as to provide assistance for prenatal diagnosis of the deafness. The kit of the present invention adopts the special PCR reaction system and the special reaction conditions, and specifically detects the 235delC mutational site of the GJB2 gene, such that the kit has advantages of high throughput and automatic tube closing operation, and is applicable for the clinical laboratories.
Owner:王宝恒
Joint detection kit for alpha,beta-thalassemia associated mutant genes
ActiveCN102181560AHigh detection specificityLow costMicrobiological testing/measurementPrenatal diagnosisBeta thalassemia
The invention discloses a joint detection kit for detecting alpha,beta-thalassemia associated with mutant genes, which comprises (1) a gene chip and (2) a primer, wherein the gene chip is provided with probes, and the probes are a sequence SEQ ID Nos:1-31 and a sequence which is complementary to the sequence SEQ ID Nos:1-31; and the primer is a sequence SEQ ID Nos:32-42. The thalassemia gene detection kit provides a platform for detecting 16 mutant genes associated with alpha-thalassemia (three deletion types and two mutant types) and beta-thalassemia, can perform synchronous joint detection,improve the specificity of detection, reduce cost and shorten detection time, and has a great significance for the screening of patients suffering from thalassemia, genetic counseling and prenatal diagnosis.
Owner:潮州凯普生物化学有限公司 +1
Methods for sample tracking
InactiveUS20090298049A1Reduce chanceMicrobiological testing/measurementLibrary screeningPrenatal diagnosisGenotype
A method and apparatus are provided for identifying a biological sample obtained during either paternity screening, genetic screening, prenatal diagnosis, presymptomatic diagnosis, diagnosis to detect the presence of a target microorganism carrier detection analysis, forensic chemical analysis, or diagnosis of a subject to determine whether a subject is afflicted with a particular disease or disorder, or is at risk of developing a particular disorder, wherein the result obtained from the analysis is associated with the unique DNA fingerprint biological barcode of the genotype of the subject being analyzed. The methods and apparatus of the invention have application in the fields of diagnostic medicine, disease diagnosis in animals and plants, identification of genetically inherited diseases in humans, family relationship analysis, forensic analysis, and microbial typing.
Owner:HANDYLAB
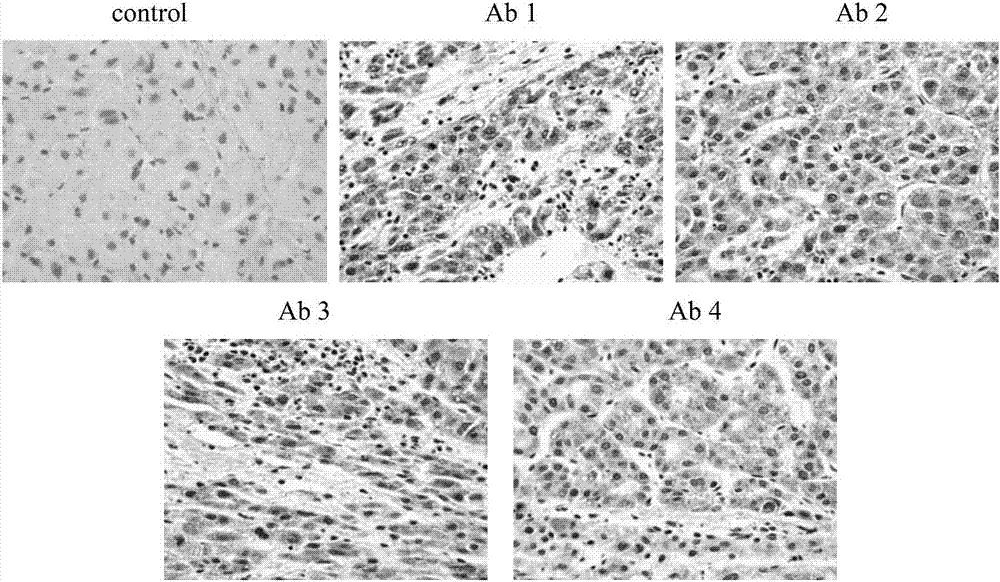
Monoclonal antibody for detecting human alpha-fetoprotein, kit and application
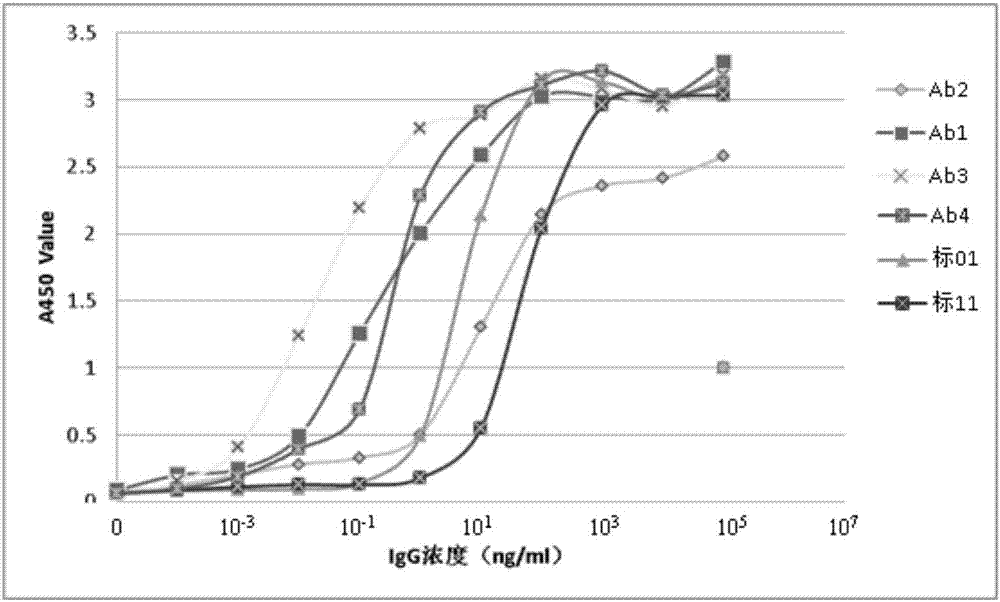
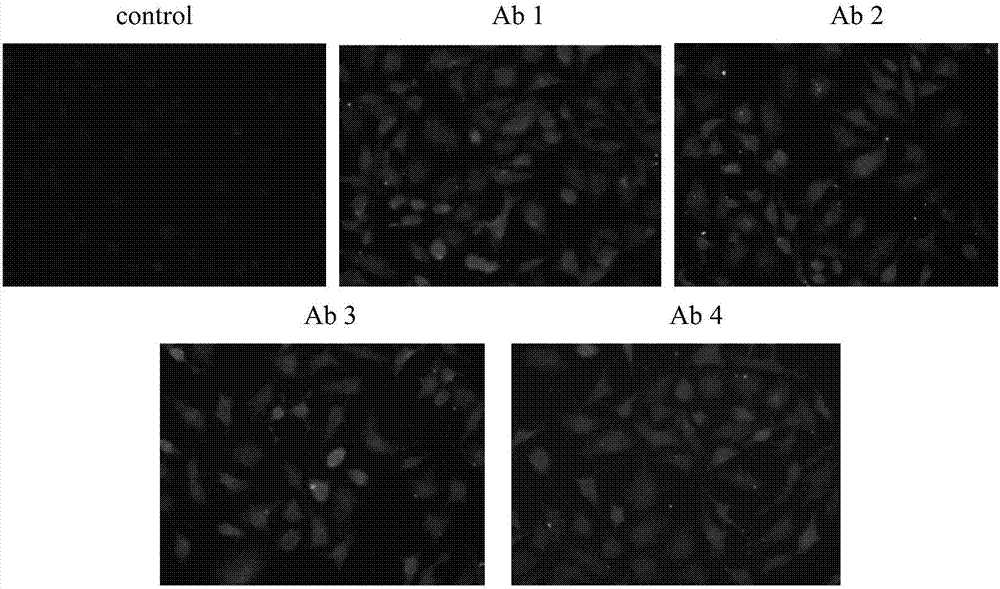
ActiveCN107022030ARealize quantitative detectionStrong specificityImmunoglobulins against animals/humansBiological material analysisSerum igeEpitope
The invention discloses a monoclonal antibody for detecting human alpha-fetoprotein, a kit and application. Three monoclonal antibodies including monoclonal antibodies Ab1, Ab3 and Ab4 can be used for specifically identifying human-derived natural hAFP (human Alpha-fetoprotein) in human hepatocellular carcinoma cells and hepatocellular carcinoma tissues and have very good specificity. The invention discloses a mouse-derived monoclonal antibody capable of being specifically combined with the human alpha-fetoprotein; the antibody can be combined with different epitopes of the human alpha-fetoprotein to form a double-antibody sandwich mode, so that quantitative detection of the alpha-fetoprotein in human blood serum is realized; the monoclonal antibody has a certain effect on prenatal diagnosis and early diagnosis of hepatocellular carcinoma and has very good popularization and application value.
Owner:广东谷盈生物科技产业研究院有限公司
Method of prenatal gene screen for down's syndrome using nucleated erythrocyte and kit
InactiveCN101358241ASimple and fast operationAccurate Screening ResultsMicrobiological testing/measurementFluorescence/phosphorescencePrenatal diagnosisFluorescence
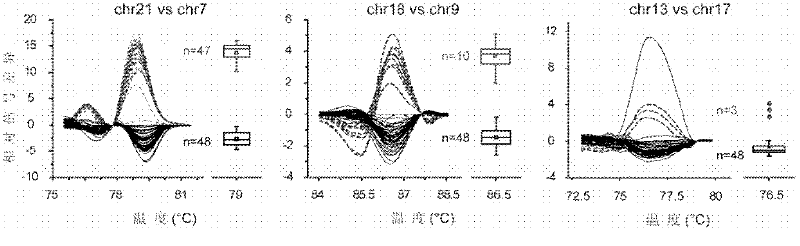
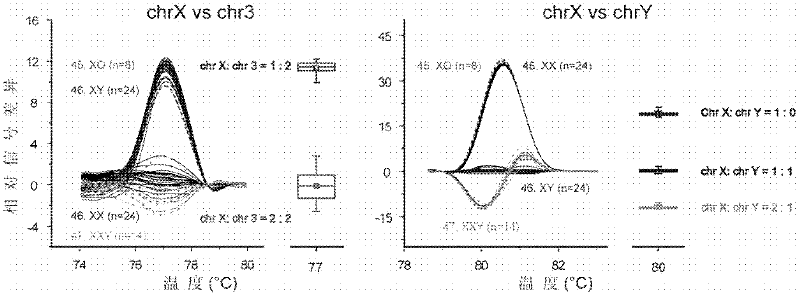
The invention relates to a prenatal gene screening method for Down's syndrome by using nucleated erythrocyte and a kit thereof. The method is that a kit which comprises a nucleated erythrocyte purification reagent, primers and a bichrome Taqman fluorescent probe which are synthesized by the specific sequence DSCR section sequence of chromosome 21 and the GAPDH gene sequence on chromosome 16, and an amplification buffering action reagent of PCR action. The invention realizes that through the following steps: (a) the purification of fetus NRBC and the extraction of DNA; (b) the synthesis of the primers and the bichrome Taqman fluorescent probe respectively by using the specific sequence DSCR section sequence of chromosome 21 and the GAPDH gene sequence on chromosome 16; (c) the co-amplification of two pairs of the primers to obtain the curve of fluorescence quantitative PCR by the bichrome fluorescence quantitative PCR reaction in the amplification buffering reaction system. The invention has simple and convenient operation and high testing throughput, and belongs to a non-invasive prenatal diagnostic method.
Owner:SHANDONG YADA PHARMA
Kit for detecting disease-causing genic mutation of neural tube defect of neonatus and application thereof
InactiveCN102181569AReduce morbidityQuick and easy detectionMicrobiological testing/measurementFluorescence/phosphorescenceDiseasePrenatal diagnosis
The invention discloses a kit for detecting whether mutation occurs at a relevant single nucleotide polymorphism (SNP) locus on a disease-causing gene of a neural tube defect of a neonatus. The kit mainly comprises a specific primer pair and a specific fluorescent probe pair which are used for detecting a No.rs1801133 SNP locus polymorphism genotype and a No.rs1801131 SNP locus polymorphism genotype on a methylenetetrahydrofolate reductase (MTHFR) gene and a No.rs1801394 SNP locus polymorphism genotype on a methionine synthase reductase (MTRR) gene, and the conventional fluorescent quantitative PCR reaction reagent. The kit is used for detecting a mutant of the disease-causing gene of the neural tube defect of the neonatus, can be used for quickly and conveniently searching a carrier with the disease-causing gene, can be applied to antenatal diagnosis and timely treatment, and reduces the morbidity of the neutral tube defect of the neonatus so as to fulfill the aims of promoting good prenatal and postnatal care.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
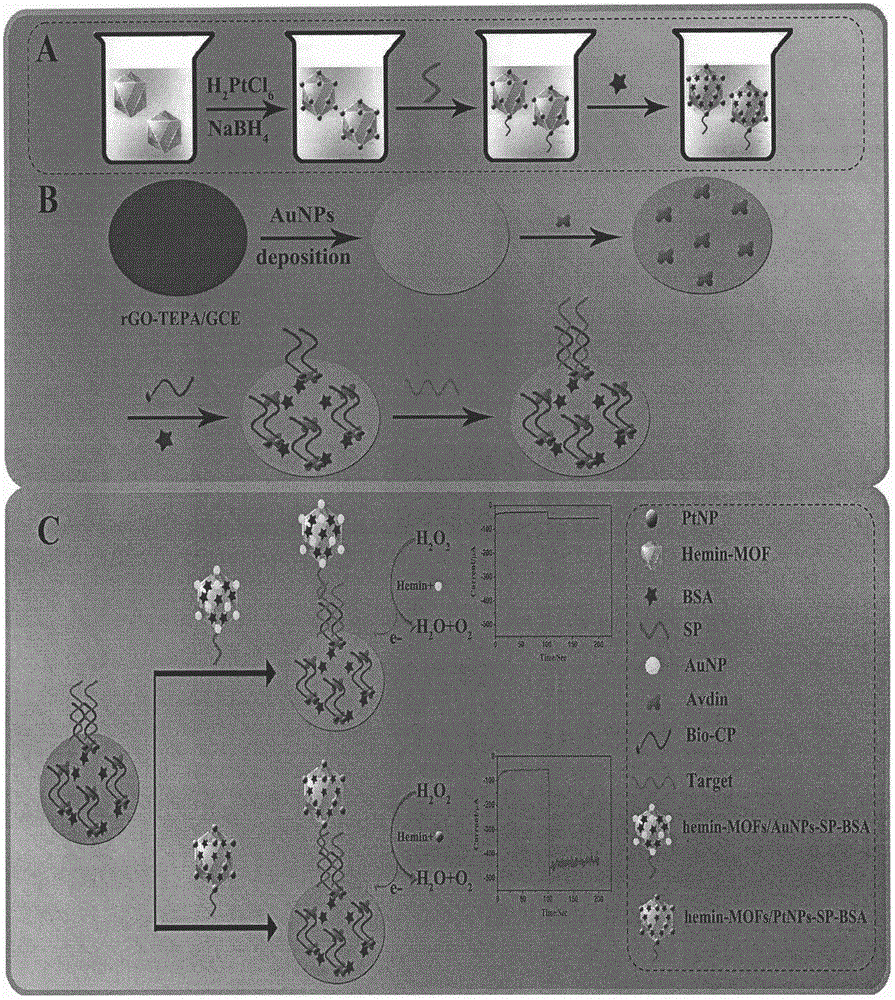
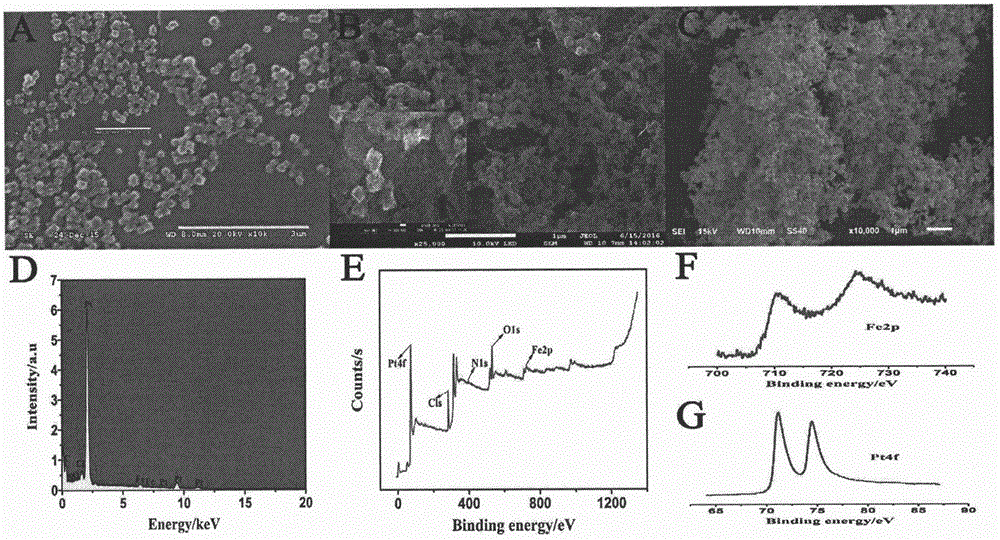
Preparation method of electrochemical sensor for FGFR3 (fibroblast growth factor receptor 3)-1138G>A gene polymorphism detection
ActiveCN106483176AImprove catalytic performanceHigh sensitivityMaterial analysis by electric/magnetic meansPrenatal diagnosisHemin
The invention relates to a preparation method and application of an electrochemical sensor for achondroplasia prenatal diagnosis gene-fibroblast growth factor receptor 3 (FGFR3) gene polymorphism detection, belonging to the technical field of electrochemical detection. The preparation method is characterized by comprising the following steps: synthesizing a Hemin-MOFs composite material, reducing platinum nanoparticles onto the Hemin-MOFs composite material, and mixing a single-strand DNA signal probe with the composite material to obtain a biological signal probe; and carrying out layer-by-layer self-assembly by using reducible graphene oxide tetraethylenepentamine, nano gold and avidin to fix the biotinylated DNA capture probe, thereby preparing the electrochemical sensor for FGFR3-1138G>A gene polymorphism detection. The sensor is successfully used for detecting FGFR3-gene single base mutation. The sensor has the advantages of high sensitivity and high specificity, and is quick and convenient for detection. The invention provides a new detection method for prenatal noninvasive diagnosis of achondroplasia.
Owner:CHONGQING MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com